User login
Study Links Newer Shingles Vaccine to Delayed Dementia Diagnosis
The study builds on previous observations of a reduction in dementia risk with the older live shingles vaccine and reports a delay in dementia diagnosis of 164 days with the newer recombinant version, compared with the live vaccine.
“Given the prevalence of dementia, a delay of 164 days in diagnosis would not be a trivial effect at the public health level. It’s a big enough effect that if there is a causality it feels meaningful,” said senior author Paul Harrison, DM, FRCPsych, professor of psychiatry at the University of Oxford, Oxford, England.
But Dr. Harrison stressed that the study had not proven that the shingles vaccine reduced dementia risk.
“The design of the study allows us to do away with many of the confounding effects we usually see in observational studies, but this is still an observational study, and as such it cannot prove a definite causal effect,” he said.
The study was published online on July 25 in Nature Medicine.
‘Natural Experiment’
Given the risk for deleterious consequences of shingles, vaccination is now recommended for older adults in many countries. The previously used live shingles vaccine (Zostavax) is being replaced in most countries with the new recombinant shingles vaccine (Shingrix), which is more effective at preventing shingles infection.
The current study made use of a “natural experiment” in the United States, which switched over from use of the live vaccine to the recombinant vaccine in October 2017.
Researchers used electronic heath records to compare the incidence of a dementia diagnosis in individuals who received the live shingles vaccine prior to October 2017 with those who received the recombinant version after the United States made the switch.
They also used propensity score matching to further control for confounding factors, comparing 103,837 individuals who received a first dose of the live shingles vaccine between October 2014 and September 2017 with the same number of matched people who received the recombinant vaccine between November 2017 and October 2020.
Results showed that within the 6 years after vaccination, the recombinant vaccine was associated with a delay in the diagnosis of dementia, compared with the live vaccine. Specifically, receiving the recombinant vaccine was associated with a 17% increase in diagnosis-free time, translating to 164 additional days lived without a diagnosis of dementia in those subsequently affected.
As an additional control, the researchers also found significantly lower risks for dementia in individuals receiving the new recombinant shingles vaccine vs two other vaccines commonly used in older people: influenza and tetanus/diphtheria/pertussis vaccines, with increases in diagnosis-free time of 14%-27%.
Reduced Risk or Delayed Diagnosis?
Speaking at a Science Media Centre press conference on the study, lead author Maxime Taquet, PhD, FRCPsych, clinical lecturer in psychiatry at the University of Oxford, noted that the total number of dementia cases were similar in the two shingles vaccine groups by the end of the 6-year follow-up period but there was a difference in the time at which they received a diagnosis of dementia.
“The study suggests that rather than actually reducing dementia risk, the recombinant vaccine delays the onset of dementia compared to the live vaccine in patients who go on to develop the condition,” he explained.
But when comparing the recombinant vaccine with the influenza and tetanus/diphtheria/pertussis vaccines there was a clear reduction in dementia risk itself, Dr. Taquet reported.
“It might well be that the live vaccine has a potential effect on the risk of dementia itself and therefore the recombinant vaccine only shows a delay in dementia compared to the live vaccine, but both of them might decrease the overall risk of dementia,” he suggested.
But the researchers cautioned that this study could not prove causality.
“While the two groups were very carefully matched in terms of factors that might influence the development of dementia, we still have to be cautious before assuming that the vaccine is indeed causally reducing the risk of onset of dementia,” Dr. Harrison warned.
The researchers say the results would need to be confirmed in a randomized trial, which may have to be conducted in a slightly younger age group, as currently shingles vaccine is recommended for all older individuals in the United Kingdom.
Vaccine recommendations vary from country to country, Dr. Harrison added. In the United States, the Centers for Disease Control and Prevention recommends the recombinant shingles vaccine for all adults aged 50 years or older.
In the meantime, it would be interesting to see whether further observational studies in other countries find similar results as this US study, Dr. Harrison said.
Mechanism Uncertain
Speculating on a possible mechanism behind the findings, Dr. Harrison suggested two plausible explanations.
“First, it is thought that the herpes virus could be one of many factors that could promote dementia, so a vaccine that stops reactivation of this virus might therefore be delaying that process,” he noted.
The other possibility is that adjuvants included in the recombinant vaccine to stimulate the immune system might have played a role.
“We don’t have any data on the mechanism, and thus study did not address that, so further studies are needed to look into this,” Dr. Harrison said.
Stronger Effect in Women
Another intriguing finding is that the association with the recombinant vaccine and delayed dementia diagnosis seemed to be stronger in women vs men.
In the original study of the live shingles vaccine, a protective effect against dementia was shown only in women.
In the current study, the delay in dementia diagnosis was seen in both sexes but was stronger in women, showing a 22% increased time without dementia in women versus a 13% increased time in men with the recombinant versus the live vaccine.
As expected, the recombinant vaccine was associated with a lower risk for shingles disease vs the live vaccine (2.5% versus 3.5%), but women did not have a better response than men did in this respect.
“The better protection against shingles with the recombinant vaccine was similar in men and women, an observation that might be one reason to question the possible mechanism behind the dementia effect being better suppression of the herpes zoster virus by the recombinant vaccine,” Dr. Harrison commented.
Though these findings are not likely to lead to any immediate changes in policy regarding the shingles vaccine, Dr. Harrison said it would be interesting to see whether uptake of the vaccine increased after this study.
He estimated that, currently in the United Kingdom, about 60% of older adults choose to have the shingles vaccine. A 2020 study in the United States found that only about one-third of US adults over 60 had received the vaccine.
“It will be interesting to see if that figure increases after these data are publicized, but I am not recommending that people have the vaccine specifically to lower their risk of dementia because of the caveats about the study that we have discussed,” he commented.
Outside Experts Positive
Outside experts, providing comment to the Science Media Centre, welcomed the new research.
“ The study is very well-conducted and adds to previous data indicating that vaccination against shingles is associated with lower dementia risk. More research is needed in future to determine why this vaccine is associated with lower dementia risk,” said Tara Spires-Jones, FMedSci, president of the British Neuroscience Association.
The high number of patients in the study and the adjustments for potential confounders are also strong points, noted Andrew Doig, PhD, professor of biochemistry, University of Manchester, Manchester, England.
“This is a significant result, comparable in effectiveness to the recent antibody drugs for Alzheimer’s disease,” Dr. Doig said. “Administering the recombinant shingles vaccine could well be a simple and cheap way to lower the risk of Alzheimer’s disease.”
Dr. Doig noted that a link between herpes zoster infection and the onset of dementia has been suspected for some time, and a trial of the antiviral drug valacyclovir against Alzheimer’s disease is currently underway.
In regard to the shingles vaccine, he said a placebo-controlled trial would be needed to prove causality.
“We also need to see how many years the effect might last and whether we should vaccinate people at a younger age. We know that the path to Alzheimer’s can start decades before any symptoms are apparent, so the vaccine might be even more effective if given to people in their 40s or 50s,” he said.
Dr. Harrison and Dr. Taquet reported no disclosures. Dr. Doig is a founder, director, and consultant for PharmaKure, which works on Alzheimer’s drugs and diagnostics. Other commentators declared no disclosures.
A version of this article first appeared on Medscape.com.
The study builds on previous observations of a reduction in dementia risk with the older live shingles vaccine and reports a delay in dementia diagnosis of 164 days with the newer recombinant version, compared with the live vaccine.
“Given the prevalence of dementia, a delay of 164 days in diagnosis would not be a trivial effect at the public health level. It’s a big enough effect that if there is a causality it feels meaningful,” said senior author Paul Harrison, DM, FRCPsych, professor of psychiatry at the University of Oxford, Oxford, England.
But Dr. Harrison stressed that the study had not proven that the shingles vaccine reduced dementia risk.
“The design of the study allows us to do away with many of the confounding effects we usually see in observational studies, but this is still an observational study, and as such it cannot prove a definite causal effect,” he said.
The study was published online on July 25 in Nature Medicine.
‘Natural Experiment’
Given the risk for deleterious consequences of shingles, vaccination is now recommended for older adults in many countries. The previously used live shingles vaccine (Zostavax) is being replaced in most countries with the new recombinant shingles vaccine (Shingrix), which is more effective at preventing shingles infection.
The current study made use of a “natural experiment” in the United States, which switched over from use of the live vaccine to the recombinant vaccine in October 2017.
Researchers used electronic heath records to compare the incidence of a dementia diagnosis in individuals who received the live shingles vaccine prior to October 2017 with those who received the recombinant version after the United States made the switch.
They also used propensity score matching to further control for confounding factors, comparing 103,837 individuals who received a first dose of the live shingles vaccine between October 2014 and September 2017 with the same number of matched people who received the recombinant vaccine between November 2017 and October 2020.
Results showed that within the 6 years after vaccination, the recombinant vaccine was associated with a delay in the diagnosis of dementia, compared with the live vaccine. Specifically, receiving the recombinant vaccine was associated with a 17% increase in diagnosis-free time, translating to 164 additional days lived without a diagnosis of dementia in those subsequently affected.
As an additional control, the researchers also found significantly lower risks for dementia in individuals receiving the new recombinant shingles vaccine vs two other vaccines commonly used in older people: influenza and tetanus/diphtheria/pertussis vaccines, with increases in diagnosis-free time of 14%-27%.
Reduced Risk or Delayed Diagnosis?
Speaking at a Science Media Centre press conference on the study, lead author Maxime Taquet, PhD, FRCPsych, clinical lecturer in psychiatry at the University of Oxford, noted that the total number of dementia cases were similar in the two shingles vaccine groups by the end of the 6-year follow-up period but there was a difference in the time at which they received a diagnosis of dementia.
“The study suggests that rather than actually reducing dementia risk, the recombinant vaccine delays the onset of dementia compared to the live vaccine in patients who go on to develop the condition,” he explained.
But when comparing the recombinant vaccine with the influenza and tetanus/diphtheria/pertussis vaccines there was a clear reduction in dementia risk itself, Dr. Taquet reported.
“It might well be that the live vaccine has a potential effect on the risk of dementia itself and therefore the recombinant vaccine only shows a delay in dementia compared to the live vaccine, but both of them might decrease the overall risk of dementia,” he suggested.
But the researchers cautioned that this study could not prove causality.
“While the two groups were very carefully matched in terms of factors that might influence the development of dementia, we still have to be cautious before assuming that the vaccine is indeed causally reducing the risk of onset of dementia,” Dr. Harrison warned.
The researchers say the results would need to be confirmed in a randomized trial, which may have to be conducted in a slightly younger age group, as currently shingles vaccine is recommended for all older individuals in the United Kingdom.
Vaccine recommendations vary from country to country, Dr. Harrison added. In the United States, the Centers for Disease Control and Prevention recommends the recombinant shingles vaccine for all adults aged 50 years or older.
In the meantime, it would be interesting to see whether further observational studies in other countries find similar results as this US study, Dr. Harrison said.
Mechanism Uncertain
Speculating on a possible mechanism behind the findings, Dr. Harrison suggested two plausible explanations.
“First, it is thought that the herpes virus could be one of many factors that could promote dementia, so a vaccine that stops reactivation of this virus might therefore be delaying that process,” he noted.
The other possibility is that adjuvants included in the recombinant vaccine to stimulate the immune system might have played a role.
“We don’t have any data on the mechanism, and thus study did not address that, so further studies are needed to look into this,” Dr. Harrison said.
Stronger Effect in Women
Another intriguing finding is that the association with the recombinant vaccine and delayed dementia diagnosis seemed to be stronger in women vs men.
In the original study of the live shingles vaccine, a protective effect against dementia was shown only in women.
In the current study, the delay in dementia diagnosis was seen in both sexes but was stronger in women, showing a 22% increased time without dementia in women versus a 13% increased time in men with the recombinant versus the live vaccine.
As expected, the recombinant vaccine was associated with a lower risk for shingles disease vs the live vaccine (2.5% versus 3.5%), but women did not have a better response than men did in this respect.
“The better protection against shingles with the recombinant vaccine was similar in men and women, an observation that might be one reason to question the possible mechanism behind the dementia effect being better suppression of the herpes zoster virus by the recombinant vaccine,” Dr. Harrison commented.
Though these findings are not likely to lead to any immediate changes in policy regarding the shingles vaccine, Dr. Harrison said it would be interesting to see whether uptake of the vaccine increased after this study.
He estimated that, currently in the United Kingdom, about 60% of older adults choose to have the shingles vaccine. A 2020 study in the United States found that only about one-third of US adults over 60 had received the vaccine.
“It will be interesting to see if that figure increases after these data are publicized, but I am not recommending that people have the vaccine specifically to lower their risk of dementia because of the caveats about the study that we have discussed,” he commented.
Outside Experts Positive
Outside experts, providing comment to the Science Media Centre, welcomed the new research.
“ The study is very well-conducted and adds to previous data indicating that vaccination against shingles is associated with lower dementia risk. More research is needed in future to determine why this vaccine is associated with lower dementia risk,” said Tara Spires-Jones, FMedSci, president of the British Neuroscience Association.
The high number of patients in the study and the adjustments for potential confounders are also strong points, noted Andrew Doig, PhD, professor of biochemistry, University of Manchester, Manchester, England.
“This is a significant result, comparable in effectiveness to the recent antibody drugs for Alzheimer’s disease,” Dr. Doig said. “Administering the recombinant shingles vaccine could well be a simple and cheap way to lower the risk of Alzheimer’s disease.”
Dr. Doig noted that a link between herpes zoster infection and the onset of dementia has been suspected for some time, and a trial of the antiviral drug valacyclovir against Alzheimer’s disease is currently underway.
In regard to the shingles vaccine, he said a placebo-controlled trial would be needed to prove causality.
“We also need to see how many years the effect might last and whether we should vaccinate people at a younger age. We know that the path to Alzheimer’s can start decades before any symptoms are apparent, so the vaccine might be even more effective if given to people in their 40s or 50s,” he said.
Dr. Harrison and Dr. Taquet reported no disclosures. Dr. Doig is a founder, director, and consultant for PharmaKure, which works on Alzheimer’s drugs and diagnostics. Other commentators declared no disclosures.
A version of this article first appeared on Medscape.com.
The study builds on previous observations of a reduction in dementia risk with the older live shingles vaccine and reports a delay in dementia diagnosis of 164 days with the newer recombinant version, compared with the live vaccine.
“Given the prevalence of dementia, a delay of 164 days in diagnosis would not be a trivial effect at the public health level. It’s a big enough effect that if there is a causality it feels meaningful,” said senior author Paul Harrison, DM, FRCPsych, professor of psychiatry at the University of Oxford, Oxford, England.
But Dr. Harrison stressed that the study had not proven that the shingles vaccine reduced dementia risk.
“The design of the study allows us to do away with many of the confounding effects we usually see in observational studies, but this is still an observational study, and as such it cannot prove a definite causal effect,” he said.
The study was published online on July 25 in Nature Medicine.
‘Natural Experiment’
Given the risk for deleterious consequences of shingles, vaccination is now recommended for older adults in many countries. The previously used live shingles vaccine (Zostavax) is being replaced in most countries with the new recombinant shingles vaccine (Shingrix), which is more effective at preventing shingles infection.
The current study made use of a “natural experiment” in the United States, which switched over from use of the live vaccine to the recombinant vaccine in October 2017.
Researchers used electronic heath records to compare the incidence of a dementia diagnosis in individuals who received the live shingles vaccine prior to October 2017 with those who received the recombinant version after the United States made the switch.
They also used propensity score matching to further control for confounding factors, comparing 103,837 individuals who received a first dose of the live shingles vaccine between October 2014 and September 2017 with the same number of matched people who received the recombinant vaccine between November 2017 and October 2020.
Results showed that within the 6 years after vaccination, the recombinant vaccine was associated with a delay in the diagnosis of dementia, compared with the live vaccine. Specifically, receiving the recombinant vaccine was associated with a 17% increase in diagnosis-free time, translating to 164 additional days lived without a diagnosis of dementia in those subsequently affected.
As an additional control, the researchers also found significantly lower risks for dementia in individuals receiving the new recombinant shingles vaccine vs two other vaccines commonly used in older people: influenza and tetanus/diphtheria/pertussis vaccines, with increases in diagnosis-free time of 14%-27%.
Reduced Risk or Delayed Diagnosis?
Speaking at a Science Media Centre press conference on the study, lead author Maxime Taquet, PhD, FRCPsych, clinical lecturer in psychiatry at the University of Oxford, noted that the total number of dementia cases were similar in the two shingles vaccine groups by the end of the 6-year follow-up period but there was a difference in the time at which they received a diagnosis of dementia.
“The study suggests that rather than actually reducing dementia risk, the recombinant vaccine delays the onset of dementia compared to the live vaccine in patients who go on to develop the condition,” he explained.
But when comparing the recombinant vaccine with the influenza and tetanus/diphtheria/pertussis vaccines there was a clear reduction in dementia risk itself, Dr. Taquet reported.
“It might well be that the live vaccine has a potential effect on the risk of dementia itself and therefore the recombinant vaccine only shows a delay in dementia compared to the live vaccine, but both of them might decrease the overall risk of dementia,” he suggested.
But the researchers cautioned that this study could not prove causality.
“While the two groups were very carefully matched in terms of factors that might influence the development of dementia, we still have to be cautious before assuming that the vaccine is indeed causally reducing the risk of onset of dementia,” Dr. Harrison warned.
The researchers say the results would need to be confirmed in a randomized trial, which may have to be conducted in a slightly younger age group, as currently shingles vaccine is recommended for all older individuals in the United Kingdom.
Vaccine recommendations vary from country to country, Dr. Harrison added. In the United States, the Centers for Disease Control and Prevention recommends the recombinant shingles vaccine for all adults aged 50 years or older.
In the meantime, it would be interesting to see whether further observational studies in other countries find similar results as this US study, Dr. Harrison said.
Mechanism Uncertain
Speculating on a possible mechanism behind the findings, Dr. Harrison suggested two plausible explanations.
“First, it is thought that the herpes virus could be one of many factors that could promote dementia, so a vaccine that stops reactivation of this virus might therefore be delaying that process,” he noted.
The other possibility is that adjuvants included in the recombinant vaccine to stimulate the immune system might have played a role.
“We don’t have any data on the mechanism, and thus study did not address that, so further studies are needed to look into this,” Dr. Harrison said.
Stronger Effect in Women
Another intriguing finding is that the association with the recombinant vaccine and delayed dementia diagnosis seemed to be stronger in women vs men.
In the original study of the live shingles vaccine, a protective effect against dementia was shown only in women.
In the current study, the delay in dementia diagnosis was seen in both sexes but was stronger in women, showing a 22% increased time without dementia in women versus a 13% increased time in men with the recombinant versus the live vaccine.
As expected, the recombinant vaccine was associated with a lower risk for shingles disease vs the live vaccine (2.5% versus 3.5%), but women did not have a better response than men did in this respect.
“The better protection against shingles with the recombinant vaccine was similar in men and women, an observation that might be one reason to question the possible mechanism behind the dementia effect being better suppression of the herpes zoster virus by the recombinant vaccine,” Dr. Harrison commented.
Though these findings are not likely to lead to any immediate changes in policy regarding the shingles vaccine, Dr. Harrison said it would be interesting to see whether uptake of the vaccine increased after this study.
He estimated that, currently in the United Kingdom, about 60% of older adults choose to have the shingles vaccine. A 2020 study in the United States found that only about one-third of US adults over 60 had received the vaccine.
“It will be interesting to see if that figure increases after these data are publicized, but I am not recommending that people have the vaccine specifically to lower their risk of dementia because of the caveats about the study that we have discussed,” he commented.
Outside Experts Positive
Outside experts, providing comment to the Science Media Centre, welcomed the new research.
“ The study is very well-conducted and adds to previous data indicating that vaccination against shingles is associated with lower dementia risk. More research is needed in future to determine why this vaccine is associated with lower dementia risk,” said Tara Spires-Jones, FMedSci, president of the British Neuroscience Association.
The high number of patients in the study and the adjustments for potential confounders are also strong points, noted Andrew Doig, PhD, professor of biochemistry, University of Manchester, Manchester, England.
“This is a significant result, comparable in effectiveness to the recent antibody drugs for Alzheimer’s disease,” Dr. Doig said. “Administering the recombinant shingles vaccine could well be a simple and cheap way to lower the risk of Alzheimer’s disease.”
Dr. Doig noted that a link between herpes zoster infection and the onset of dementia has been suspected for some time, and a trial of the antiviral drug valacyclovir against Alzheimer’s disease is currently underway.
In regard to the shingles vaccine, he said a placebo-controlled trial would be needed to prove causality.
“We also need to see how many years the effect might last and whether we should vaccinate people at a younger age. We know that the path to Alzheimer’s can start decades before any symptoms are apparent, so the vaccine might be even more effective if given to people in their 40s or 50s,” he said.
Dr. Harrison and Dr. Taquet reported no disclosures. Dr. Doig is a founder, director, and consultant for PharmaKure, which works on Alzheimer’s drugs and diagnostics. Other commentators declared no disclosures.
A version of this article first appeared on Medscape.com.
FROM NATURE MEDICINE
Mycobacterium interjectum Infection in an Immunocompetent Host Following Contact With Aquarium Fish
To the Editor:
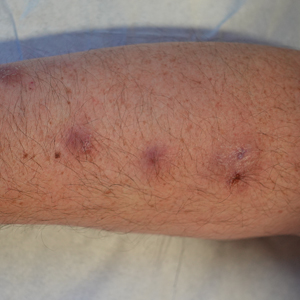
A 48-year-old man presented with nodular lesions in a sporotrichoid pattern on the right hand and forearm of 3 months’ duration (Figure). There were no lymphadeno-pathies, and he had no notable medical history. He denied fever and other systemic symptoms. The patient recently had manipulated a warm water fish aquarium. Although he did not recall a clear injury, inadvertent mild trauma was a possibility. He denied other contact or trauma in relation to animals or vegetables.
Histopathology from a punch biopsy of the forearm revealed a granulomatous infiltrate with necrosis at the deep dermis level at the interface with the subcutaneous cellular tissue that was composed of mainly epithelioid cells with a few multinucleated giant cells. No acid-fast bacilli or fungi were observed with special stains.
A polymerase chain reaction assay for atypical mycobacteria was positive for Mycobacterium interjectum. The culture of the skin biopsy was negative for fungi and mycobacteria after long incubation (6 weeks) on 2 occasions, and an antibiogram was not available. Complementary tests including hemogram, HIV serology, and chest and upper extremity radiographs did not reveal any abnormalities.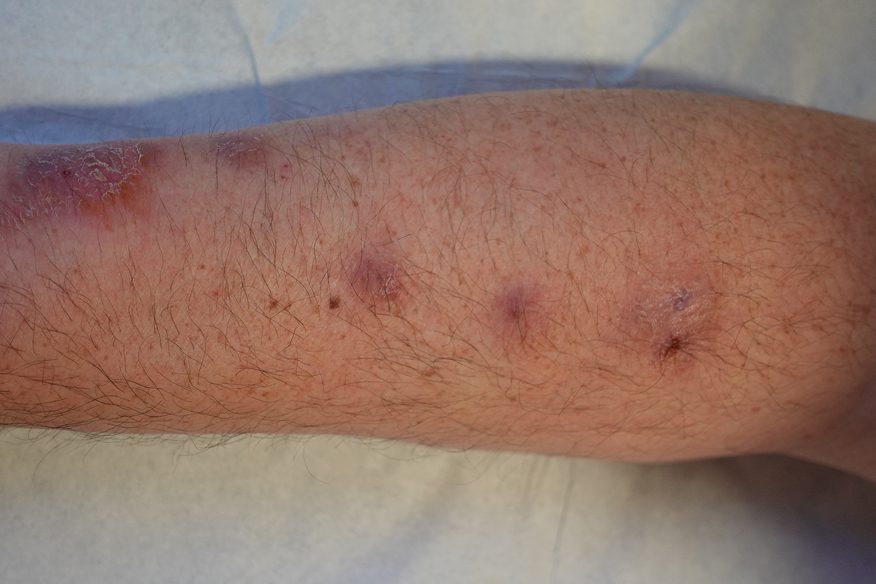
The patient was treated with rifampicin 600 mg/d, clarithromycin 500 mg every 12 hours, and co-trimoxazole 160/800 mg every 12 hours for 9 months with some resolution but persistence of some residual scarring lesions. There was no recurrence at 6-month follow-up.
Mycobacterium interjectum is a rare, slow-growing, scotochromogenic mycobacteria. Case reports usually refer to lymphadenitis in healthy children and pulmonary infections in immunocompromised or immunocompetent adults.1,2 A case of M interjectum with cutaneous involvement was reported by Fukuoka et al,3 with ulcerated nodules and abscesses on the leg identified in an immunocompromised patient. Our patient did not present with any cause of immunosuppression or clear injury predisposing him to infection. This microorganism has been detected in water, soil,3 and aquarium fish,4 the latter being the most likely source of infection in our patient. Given its slow growth rate and the need for a specific polymerase chain reaction assay, which is not widely available, M interjectum infection may be underdiagnosed.
No standard antibiotic regimen has been established, but M interjectum has proven to be a multidrug-resistant bacterium with frequent therapy failures. Treatment options have ranged from standard tuberculostatic therapy to combination therapy with medications such as amikacin, levofloxacin, rifampicin, and co-trimoxazole.1 Because an antibiogram was not available for our patient, empiric treatment with rifampicin, clarithromycin, and co-trimoxazole was prescribed for 9 months, with satisfactory response and tolerance. These drugs were selected because of their susceptibility profile in the literature.1,5
- Sotello D, Hata DJ, Reza M, et al. Disseminated Mycobacterium interjectum infection with bacteremia, hepatic and pulmonary involvement associated with a long-term catheter infection. Case Rep Infect Dis. 2017;2017:1-5.
- Dholakia YN. Mycobacterium interjectum isolated from an immunocompetent host with lung infection. Int J Mycobacteriol. 2017;6:401-403.
- Fukuoka M, Matsumura Y, Kore-eda S, et al. Cutaneous infection due to Mycobacterium interjectum in an immunosuppressed patient with microscopic polyangiitis. Br J Dermatol. 2008;159:1382-1384.
- Zanoni RG, Florio D, Fioravanti ML, et al. Occurrence of Mycobacterium spp. in ornamental fish in Italy. J Fish Dis. 2008;31:433-441.
- Emler S, Rochat T, Rohner P, et al. Chronic destructive lung disease associated with a novel mycobacterium. Am J Respir Crit Care Med. 1994;150:261-265.
To the Editor:
A 48-year-old man presented with nodular lesions in a sporotrichoid pattern on the right hand and forearm of 3 months’ duration (Figure). There were no lymphadeno-pathies, and he had no notable medical history. He denied fever and other systemic symptoms. The patient recently had manipulated a warm water fish aquarium. Although he did not recall a clear injury, inadvertent mild trauma was a possibility. He denied other contact or trauma in relation to animals or vegetables.
Histopathology from a punch biopsy of the forearm revealed a granulomatous infiltrate with necrosis at the deep dermis level at the interface with the subcutaneous cellular tissue that was composed of mainly epithelioid cells with a few multinucleated giant cells. No acid-fast bacilli or fungi were observed with special stains.
A polymerase chain reaction assay for atypical mycobacteria was positive for Mycobacterium interjectum. The culture of the skin biopsy was negative for fungi and mycobacteria after long incubation (6 weeks) on 2 occasions, and an antibiogram was not available. Complementary tests including hemogram, HIV serology, and chest and upper extremity radiographs did not reveal any abnormalities.
The patient was treated with rifampicin 600 mg/d, clarithromycin 500 mg every 12 hours, and co-trimoxazole 160/800 mg every 12 hours for 9 months with some resolution but persistence of some residual scarring lesions. There was no recurrence at 6-month follow-up.
Mycobacterium interjectum is a rare, slow-growing, scotochromogenic mycobacteria. Case reports usually refer to lymphadenitis in healthy children and pulmonary infections in immunocompromised or immunocompetent adults.1,2 A case of M interjectum with cutaneous involvement was reported by Fukuoka et al,3 with ulcerated nodules and abscesses on the leg identified in an immunocompromised patient. Our patient did not present with any cause of immunosuppression or clear injury predisposing him to infection. This microorganism has been detected in water, soil,3 and aquarium fish,4 the latter being the most likely source of infection in our patient. Given its slow growth rate and the need for a specific polymerase chain reaction assay, which is not widely available, M interjectum infection may be underdiagnosed.
No standard antibiotic regimen has been established, but M interjectum has proven to be a multidrug-resistant bacterium with frequent therapy failures. Treatment options have ranged from standard tuberculostatic therapy to combination therapy with medications such as amikacin, levofloxacin, rifampicin, and co-trimoxazole.1 Because an antibiogram was not available for our patient, empiric treatment with rifampicin, clarithromycin, and co-trimoxazole was prescribed for 9 months, with satisfactory response and tolerance. These drugs were selected because of their susceptibility profile in the literature.1,5
To the Editor:
A 48-year-old man presented with nodular lesions in a sporotrichoid pattern on the right hand and forearm of 3 months’ duration (Figure). There were no lymphadeno-pathies, and he had no notable medical history. He denied fever and other systemic symptoms. The patient recently had manipulated a warm water fish aquarium. Although he did not recall a clear injury, inadvertent mild trauma was a possibility. He denied other contact or trauma in relation to animals or vegetables.
Histopathology from a punch biopsy of the forearm revealed a granulomatous infiltrate with necrosis at the deep dermis level at the interface with the subcutaneous cellular tissue that was composed of mainly epithelioid cells with a few multinucleated giant cells. No acid-fast bacilli or fungi were observed with special stains.
A polymerase chain reaction assay for atypical mycobacteria was positive for Mycobacterium interjectum. The culture of the skin biopsy was negative for fungi and mycobacteria after long incubation (6 weeks) on 2 occasions, and an antibiogram was not available. Complementary tests including hemogram, HIV serology, and chest and upper extremity radiographs did not reveal any abnormalities.
The patient was treated with rifampicin 600 mg/d, clarithromycin 500 mg every 12 hours, and co-trimoxazole 160/800 mg every 12 hours for 9 months with some resolution but persistence of some residual scarring lesions. There was no recurrence at 6-month follow-up.
Mycobacterium interjectum is a rare, slow-growing, scotochromogenic mycobacteria. Case reports usually refer to lymphadenitis in healthy children and pulmonary infections in immunocompromised or immunocompetent adults.1,2 A case of M interjectum with cutaneous involvement was reported by Fukuoka et al,3 with ulcerated nodules and abscesses on the leg identified in an immunocompromised patient. Our patient did not present with any cause of immunosuppression or clear injury predisposing him to infection. This microorganism has been detected in water, soil,3 and aquarium fish,4 the latter being the most likely source of infection in our patient. Given its slow growth rate and the need for a specific polymerase chain reaction assay, which is not widely available, M interjectum infection may be underdiagnosed.
No standard antibiotic regimen has been established, but M interjectum has proven to be a multidrug-resistant bacterium with frequent therapy failures. Treatment options have ranged from standard tuberculostatic therapy to combination therapy with medications such as amikacin, levofloxacin, rifampicin, and co-trimoxazole.1 Because an antibiogram was not available for our patient, empiric treatment with rifampicin, clarithromycin, and co-trimoxazole was prescribed for 9 months, with satisfactory response and tolerance. These drugs were selected because of their susceptibility profile in the literature.1,5
- Sotello D, Hata DJ, Reza M, et al. Disseminated Mycobacterium interjectum infection with bacteremia, hepatic and pulmonary involvement associated with a long-term catheter infection. Case Rep Infect Dis. 2017;2017:1-5.
- Dholakia YN. Mycobacterium interjectum isolated from an immunocompetent host with lung infection. Int J Mycobacteriol. 2017;6:401-403.
- Fukuoka M, Matsumura Y, Kore-eda S, et al. Cutaneous infection due to Mycobacterium interjectum in an immunosuppressed patient with microscopic polyangiitis. Br J Dermatol. 2008;159:1382-1384.
- Zanoni RG, Florio D, Fioravanti ML, et al. Occurrence of Mycobacterium spp. in ornamental fish in Italy. J Fish Dis. 2008;31:433-441.
- Emler S, Rochat T, Rohner P, et al. Chronic destructive lung disease associated with a novel mycobacterium. Am J Respir Crit Care Med. 1994;150:261-265.
- Sotello D, Hata DJ, Reza M, et al. Disseminated Mycobacterium interjectum infection with bacteremia, hepatic and pulmonary involvement associated with a long-term catheter infection. Case Rep Infect Dis. 2017;2017:1-5.
- Dholakia YN. Mycobacterium interjectum isolated from an immunocompetent host with lung infection. Int J Mycobacteriol. 2017;6:401-403.
- Fukuoka M, Matsumura Y, Kore-eda S, et al. Cutaneous infection due to Mycobacterium interjectum in an immunosuppressed patient with microscopic polyangiitis. Br J Dermatol. 2008;159:1382-1384.
- Zanoni RG, Florio D, Fioravanti ML, et al. Occurrence of Mycobacterium spp. in ornamental fish in Italy. J Fish Dis. 2008;31:433-441.
- Emler S, Rochat T, Rohner P, et al. Chronic destructive lung disease associated with a novel mycobacterium. Am J Respir Crit Care Med. 1994;150:261-265.
Practice Points
- Mycobacterium interjectum can cause cutaneous nodules in a sporotrichoid or lymphocutaneous pattern and may affect immunocompromised and immunocompetent patients.
- This mycobacteria has been detected in water, soil, and aquarium fish. The latter could be a source of infection and should be taken into account in the anamnesis.
- There is no established therapeutic regimen for M interjectum infection. Combination therapy with rifampicin, clarithromycin, and co-trimoxazole could be an option, though it must always be adapted to an antibiogram if results are available.
Disruptive Sleep Linked to Increased Susceptibility to COVID-19
Individuals with preexisting sleep disturbances including obstructive sleep apnea (OSA), insomnia, and abnormal sleep duration showed significantly increased vulnerability to COVID-19, as well as an increased risk for hospitalization, mortality, and long COVID, according to new data from more than 8 million individuals.
, wrote Jiawei Zhou, MD, of The First Hospital of China Medical University, Shenyang, China, and colleagues. Most previous research has focused on the impact of COVID-19 on sleep disturbances, not the impact of sleep disturbances on COVID-19, and most studies on the latter topic have focused only on OSA, the researchers wrote.
In a meta-analysis published in eClinicalMedicine, part of The Lancet Discovery Science, the researchers identified 48 observational studies published between October 27, 2023, and May 8, 2024, that involved COVID-19 and sleep disturbances including OSA, insomnia, abnormal sleep duration, and night shift work, among others. The study population included 8,664,026 adults.
The primary outcomes were COVID-19 susceptibility, hospitalization, mortality, and long COVID. Overall, the presence of preexisting sleep disturbances was associated with a significantly increased risk for each of these outcomes, with odds ratios (ORs) of 1.12, 1.25, 1.45, and 1.36, respectively.
In subgroup analyses, the association between preexisting sleep disturbances and greater susceptibility and hospitalization was higher in younger adults (younger than 60 years) than in older adults (aged 60 years and older), but the risk for death was lower in younger adults with sleep disturbances than in older adults with sleep disturbances (OR, 1.22 vs OR, 2.07, respectively). Men with sleep disturbances had a higher risk for COVID-19 mortality than women with sleep disturbances.
Preexisting sleep disturbances overall were significantly associated with long COVID and more so in a subgroup analysis of patients whose definition of long COVID was symptoms lasting 3 or more months vs those lasting 1 month (P = .029).
When the researchers broke down associations with COVID-19 outcomes and specific sleep disturbances, they found significant associations between OSA and all four primary outcomes. Abnormal sleep duration was associated with an increased risk for COVID-19 susceptibility, hospitalization, and long COVID. Night shift work was associated with an increased risk for COVID-19 susceptibility and hospitalization, and insomnia was associated with an increased risk for long COVID.
Although the exact mechanism behind the associations between preexisting sleep disturbances and COVID-19 outcomes is uncertain, persistent sleep deprivation could set the stage in various ways, including the promotion of elevated C-reactive protein and interleukin-6 levels, the researchers wrote.
“Overall, the compromised innate and adaptive immune functions combined with a persistent inflammatory state may explain the higher risk of susceptibility, severity, and longer recovery time observed in patients with sleep disturbances. Fortunately, early intervention for sleep disturbances could attenuate the adverse effects of COVID-19,” they noted in their discussion.
The findings were limited by several factors including the observational nature of the studies and the heterogeneity of outcomes, the researchers wrote. Looking ahead, randomized, controlled trials are needed to examine the effect of interventions for sleep disturbances in the prevention and course of COVID-19, they said.
However, the study is the first known to examine multiple types of sleep disturbances and their possible influences on the full clinical course of COVID-19 and support the need for early evaluation and intervention for individuals with sleep disturbances to reduce short-term and long-term effects of the disease, the researchers concluded.
Findings Reflect the Need to Address Sleep Issues Early
Although the results of the current study were not surprising, “it is always worth doing meta-analyses to see if there is a potential signal in the published data to suggest a need for a new study,” Arun Chatterjee, MD, professor of pulmonary, critical care, allergy, and immunologic diseases at Wake Forest University, Winston-Salem, North Carolina, said in an interview.
“Lack of sleep, whether acute active deprivation (zero sleep for one night) or subacute/chronic sleep debt, such as only 5 hours per night, has been demonstrated to affect lymphocyte proliferation, reduce immune globulin levels, increase inflammatory markers, shorten telomeres, and affect the immune system in various ways,” said Dr. Chatterjee, who was not involved in the meta-analysis.
The clinical takeaway from the current meta-analysis is that adequate sleep is important for various reasons, Dr. Chatterjee said. “Sleep disruption affects health across a spectrum of systems; adding an annual sleep wellness and screening event to healthcare visits is probably worth the investment,” he noted.
Much more is needed in the way of additional research, Dr. Chatterjee told this news organization. Notably, studies are needed to examine what sleep disruption does to immune status, as well as all other physiologic and mental health systems, he said.
The study was supported by the National Natural Science Foundation of China and the Key Laboratory of Respiratory Diseases of Liaoning Province. The researchers had no financial conflicts to disclose. Chatterjee had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Individuals with preexisting sleep disturbances including obstructive sleep apnea (OSA), insomnia, and abnormal sleep duration showed significantly increased vulnerability to COVID-19, as well as an increased risk for hospitalization, mortality, and long COVID, according to new data from more than 8 million individuals.
, wrote Jiawei Zhou, MD, of The First Hospital of China Medical University, Shenyang, China, and colleagues. Most previous research has focused on the impact of COVID-19 on sleep disturbances, not the impact of sleep disturbances on COVID-19, and most studies on the latter topic have focused only on OSA, the researchers wrote.
In a meta-analysis published in eClinicalMedicine, part of The Lancet Discovery Science, the researchers identified 48 observational studies published between October 27, 2023, and May 8, 2024, that involved COVID-19 and sleep disturbances including OSA, insomnia, abnormal sleep duration, and night shift work, among others. The study population included 8,664,026 adults.
The primary outcomes were COVID-19 susceptibility, hospitalization, mortality, and long COVID. Overall, the presence of preexisting sleep disturbances was associated with a significantly increased risk for each of these outcomes, with odds ratios (ORs) of 1.12, 1.25, 1.45, and 1.36, respectively.
In subgroup analyses, the association between preexisting sleep disturbances and greater susceptibility and hospitalization was higher in younger adults (younger than 60 years) than in older adults (aged 60 years and older), but the risk for death was lower in younger adults with sleep disturbances than in older adults with sleep disturbances (OR, 1.22 vs OR, 2.07, respectively). Men with sleep disturbances had a higher risk for COVID-19 mortality than women with sleep disturbances.
Preexisting sleep disturbances overall were significantly associated with long COVID and more so in a subgroup analysis of patients whose definition of long COVID was symptoms lasting 3 or more months vs those lasting 1 month (P = .029).
When the researchers broke down associations with COVID-19 outcomes and specific sleep disturbances, they found significant associations between OSA and all four primary outcomes. Abnormal sleep duration was associated with an increased risk for COVID-19 susceptibility, hospitalization, and long COVID. Night shift work was associated with an increased risk for COVID-19 susceptibility and hospitalization, and insomnia was associated with an increased risk for long COVID.
Although the exact mechanism behind the associations between preexisting sleep disturbances and COVID-19 outcomes is uncertain, persistent sleep deprivation could set the stage in various ways, including the promotion of elevated C-reactive protein and interleukin-6 levels, the researchers wrote.
“Overall, the compromised innate and adaptive immune functions combined with a persistent inflammatory state may explain the higher risk of susceptibility, severity, and longer recovery time observed in patients with sleep disturbances. Fortunately, early intervention for sleep disturbances could attenuate the adverse effects of COVID-19,” they noted in their discussion.
The findings were limited by several factors including the observational nature of the studies and the heterogeneity of outcomes, the researchers wrote. Looking ahead, randomized, controlled trials are needed to examine the effect of interventions for sleep disturbances in the prevention and course of COVID-19, they said.
However, the study is the first known to examine multiple types of sleep disturbances and their possible influences on the full clinical course of COVID-19 and support the need for early evaluation and intervention for individuals with sleep disturbances to reduce short-term and long-term effects of the disease, the researchers concluded.
Findings Reflect the Need to Address Sleep Issues Early
Although the results of the current study were not surprising, “it is always worth doing meta-analyses to see if there is a potential signal in the published data to suggest a need for a new study,” Arun Chatterjee, MD, professor of pulmonary, critical care, allergy, and immunologic diseases at Wake Forest University, Winston-Salem, North Carolina, said in an interview.
“Lack of sleep, whether acute active deprivation (zero sleep for one night) or subacute/chronic sleep debt, such as only 5 hours per night, has been demonstrated to affect lymphocyte proliferation, reduce immune globulin levels, increase inflammatory markers, shorten telomeres, and affect the immune system in various ways,” said Dr. Chatterjee, who was not involved in the meta-analysis.
The clinical takeaway from the current meta-analysis is that adequate sleep is important for various reasons, Dr. Chatterjee said. “Sleep disruption affects health across a spectrum of systems; adding an annual sleep wellness and screening event to healthcare visits is probably worth the investment,” he noted.
Much more is needed in the way of additional research, Dr. Chatterjee told this news organization. Notably, studies are needed to examine what sleep disruption does to immune status, as well as all other physiologic and mental health systems, he said.
The study was supported by the National Natural Science Foundation of China and the Key Laboratory of Respiratory Diseases of Liaoning Province. The researchers had no financial conflicts to disclose. Chatterjee had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Individuals with preexisting sleep disturbances including obstructive sleep apnea (OSA), insomnia, and abnormal sleep duration showed significantly increased vulnerability to COVID-19, as well as an increased risk for hospitalization, mortality, and long COVID, according to new data from more than 8 million individuals.
, wrote Jiawei Zhou, MD, of The First Hospital of China Medical University, Shenyang, China, and colleagues. Most previous research has focused on the impact of COVID-19 on sleep disturbances, not the impact of sleep disturbances on COVID-19, and most studies on the latter topic have focused only on OSA, the researchers wrote.
In a meta-analysis published in eClinicalMedicine, part of The Lancet Discovery Science, the researchers identified 48 observational studies published between October 27, 2023, and May 8, 2024, that involved COVID-19 and sleep disturbances including OSA, insomnia, abnormal sleep duration, and night shift work, among others. The study population included 8,664,026 adults.
The primary outcomes were COVID-19 susceptibility, hospitalization, mortality, and long COVID. Overall, the presence of preexisting sleep disturbances was associated with a significantly increased risk for each of these outcomes, with odds ratios (ORs) of 1.12, 1.25, 1.45, and 1.36, respectively.
In subgroup analyses, the association between preexisting sleep disturbances and greater susceptibility and hospitalization was higher in younger adults (younger than 60 years) than in older adults (aged 60 years and older), but the risk for death was lower in younger adults with sleep disturbances than in older adults with sleep disturbances (OR, 1.22 vs OR, 2.07, respectively). Men with sleep disturbances had a higher risk for COVID-19 mortality than women with sleep disturbances.
Preexisting sleep disturbances overall were significantly associated with long COVID and more so in a subgroup analysis of patients whose definition of long COVID was symptoms lasting 3 or more months vs those lasting 1 month (P = .029).
When the researchers broke down associations with COVID-19 outcomes and specific sleep disturbances, they found significant associations between OSA and all four primary outcomes. Abnormal sleep duration was associated with an increased risk for COVID-19 susceptibility, hospitalization, and long COVID. Night shift work was associated with an increased risk for COVID-19 susceptibility and hospitalization, and insomnia was associated with an increased risk for long COVID.
Although the exact mechanism behind the associations between preexisting sleep disturbances and COVID-19 outcomes is uncertain, persistent sleep deprivation could set the stage in various ways, including the promotion of elevated C-reactive protein and interleukin-6 levels, the researchers wrote.
“Overall, the compromised innate and adaptive immune functions combined with a persistent inflammatory state may explain the higher risk of susceptibility, severity, and longer recovery time observed in patients with sleep disturbances. Fortunately, early intervention for sleep disturbances could attenuate the adverse effects of COVID-19,” they noted in their discussion.
The findings were limited by several factors including the observational nature of the studies and the heterogeneity of outcomes, the researchers wrote. Looking ahead, randomized, controlled trials are needed to examine the effect of interventions for sleep disturbances in the prevention and course of COVID-19, they said.
However, the study is the first known to examine multiple types of sleep disturbances and their possible influences on the full clinical course of COVID-19 and support the need for early evaluation and intervention for individuals with sleep disturbances to reduce short-term and long-term effects of the disease, the researchers concluded.
Findings Reflect the Need to Address Sleep Issues Early
Although the results of the current study were not surprising, “it is always worth doing meta-analyses to see if there is a potential signal in the published data to suggest a need for a new study,” Arun Chatterjee, MD, professor of pulmonary, critical care, allergy, and immunologic diseases at Wake Forest University, Winston-Salem, North Carolina, said in an interview.
“Lack of sleep, whether acute active deprivation (zero sleep for one night) or subacute/chronic sleep debt, such as only 5 hours per night, has been demonstrated to affect lymphocyte proliferation, reduce immune globulin levels, increase inflammatory markers, shorten telomeres, and affect the immune system in various ways,” said Dr. Chatterjee, who was not involved in the meta-analysis.
The clinical takeaway from the current meta-analysis is that adequate sleep is important for various reasons, Dr. Chatterjee said. “Sleep disruption affects health across a spectrum of systems; adding an annual sleep wellness and screening event to healthcare visits is probably worth the investment,” he noted.
Much more is needed in the way of additional research, Dr. Chatterjee told this news organization. Notably, studies are needed to examine what sleep disruption does to immune status, as well as all other physiologic and mental health systems, he said.
The study was supported by the National Natural Science Foundation of China and the Key Laboratory of Respiratory Diseases of Liaoning Province. The researchers had no financial conflicts to disclose. Chatterjee had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Study Detects Bacteria in Tattoo, Permanent Makeup Inks
When US researchers tested 75 unopened and sealed tattoo and permanent makeup inks from 14 different manufacturers, they discovered that about 35% of the products were contaminated with bacteria.
They detected both aerobic bacteria and anaerobic bacteria, which thrive in low-oxygen environments like the dermal layer of the skin.
“This suggests that contaminated tattoo inks could be a source of infection from both types of bacteria,” Seong-Jae Peter Kim, PhD, a microbiologist with the Division of Microbiology, National Center for Toxicological Research, US Food and Drug Administration, who worked on the study, said in a news release.
The findings “are concerning,” said Waleed Javaid, MD, professor of medicine and director of infection prevention and control for the Mount Sinai Health System in New York City. “This contamination poses a significant health risk, as these inks are injected into the dermal layer of the skin, creating an environment conducive to bacterial infections,” said Dr. Javaid, who wasn’t involved in the study, which was published online in Applied and Environmental Microbiology.
New Body Art Culture
Tattoos are more popular than ever, and it is estimated that at least 32% of people in the United States have at least one tattoo. And the rise in popularity has coincided with an increase in ink-related infections.
This new research joins previous studies that have demonstrated that commercial tattoo and permanent makeup inks are often contaminated with pathogenic microorganisms.
Of the 75 ink samples that Dr. Kim and colleagues tested, 26 were contaminated with 34 bacterial isolates classified into 14 genera and 22 species. Among the 34 bacterial isolates, 19 were identified as possibly pathogenic bacterial strains.
Two species — Cutibacterium acnes (four strains) and Staphylococcus epidermidis (two strains) — were isolated under anaerobic conditions.
Two possibly pathogenic bacterial strains — Staphylococcus saprophyticus and C acnes — were isolated from the same two ink samples, indicating that tattoo and permanent makeup inks can harbor both aerobic (S saprophyticus) and anaerobic (C acnes) bacteria.
There was no significant association between sterility claims on the ink label and the absence of bacterial contamination.
“The presence of bacteria like Cutibacterium acnes and Staphylococcus epidermidis, which can cause skin infections and other complications, underscores the potential danger to individuals receiving tattoos or permanent makeup,” Dr. Javaid explained.
The results “emphasize the importance of monitoring these products for both aerobic and anaerobic bacteria, including possibly pathogenic microorganisms,” Dr. Kim said in the news release.
The next steps, according to the researchers, include developing more efficient and accurate microbial detection methods for tattoo inks to streamline the monitoring process and examining the occurrence, co-occurrence, and diversity of microbial contaminants in tattoo inks to prevent future contamination.
Counseling Patients
Healthcare professionals play a “crucial role in counseling patients about the risks associated with tattoos. They should inform patients about the potential for infections, allergic reactions, and other complications related to tattooing and permanent ink,” said Dr. Javaid.
Specific advice can include ensuring that the tattoo parlor adheres to strict hygiene practices and verifying that tattoo inks are from reputable sources and, if possible, have undergone sterilization.
Clinicians should discuss the importance of proper aftercare to minimize the risk for infection, recommend patients with compromised immune systems or skin conditions to reconsider getting a tattoo, and encourage patients to be aware of the signs of infection and to seek medical attention promptly if any symptoms arise.
“Enhanced regulatory measures would help reduce the risk of infections and ensure safer tattooing practices for consumers,” Dr. Javaid said. The findings of Dr. Kim and colleagues “indicate that current manufacturing and sterilization processes are inadequate.”
Regulations could include stricter manufacturing standards to ensure sterility, the mandatory testing of inks for microbial contamination before they reach the market, clear labeling requirements that accurately reflect the sterility and safety of products, and regular inspections and audits of tattoo ink manufacturers, he said, which could encourage the development of more effective sterilization techniques to eliminate bacterial contamination.
The FDA has created a document — Think Before You Ink: Tattoo Safety — for consumers who are considering getting a tattoo.
A version of this article first appeared on Medscape.com.
When US researchers tested 75 unopened and sealed tattoo and permanent makeup inks from 14 different manufacturers, they discovered that about 35% of the products were contaminated with bacteria.
They detected both aerobic bacteria and anaerobic bacteria, which thrive in low-oxygen environments like the dermal layer of the skin.
“This suggests that contaminated tattoo inks could be a source of infection from both types of bacteria,” Seong-Jae Peter Kim, PhD, a microbiologist with the Division of Microbiology, National Center for Toxicological Research, US Food and Drug Administration, who worked on the study, said in a news release.
The findings “are concerning,” said Waleed Javaid, MD, professor of medicine and director of infection prevention and control for the Mount Sinai Health System in New York City. “This contamination poses a significant health risk, as these inks are injected into the dermal layer of the skin, creating an environment conducive to bacterial infections,” said Dr. Javaid, who wasn’t involved in the study, which was published online in Applied and Environmental Microbiology.
New Body Art Culture
Tattoos are more popular than ever, and it is estimated that at least 32% of people in the United States have at least one tattoo. And the rise in popularity has coincided with an increase in ink-related infections.
This new research joins previous studies that have demonstrated that commercial tattoo and permanent makeup inks are often contaminated with pathogenic microorganisms.
Of the 75 ink samples that Dr. Kim and colleagues tested, 26 were contaminated with 34 bacterial isolates classified into 14 genera and 22 species. Among the 34 bacterial isolates, 19 were identified as possibly pathogenic bacterial strains.
Two species — Cutibacterium acnes (four strains) and Staphylococcus epidermidis (two strains) — were isolated under anaerobic conditions.
Two possibly pathogenic bacterial strains — Staphylococcus saprophyticus and C acnes — were isolated from the same two ink samples, indicating that tattoo and permanent makeup inks can harbor both aerobic (S saprophyticus) and anaerobic (C acnes) bacteria.
There was no significant association between sterility claims on the ink label and the absence of bacterial contamination.
“The presence of bacteria like Cutibacterium acnes and Staphylococcus epidermidis, which can cause skin infections and other complications, underscores the potential danger to individuals receiving tattoos or permanent makeup,” Dr. Javaid explained.
The results “emphasize the importance of monitoring these products for both aerobic and anaerobic bacteria, including possibly pathogenic microorganisms,” Dr. Kim said in the news release.
The next steps, according to the researchers, include developing more efficient and accurate microbial detection methods for tattoo inks to streamline the monitoring process and examining the occurrence, co-occurrence, and diversity of microbial contaminants in tattoo inks to prevent future contamination.
Counseling Patients
Healthcare professionals play a “crucial role in counseling patients about the risks associated with tattoos. They should inform patients about the potential for infections, allergic reactions, and other complications related to tattooing and permanent ink,” said Dr. Javaid.
Specific advice can include ensuring that the tattoo parlor adheres to strict hygiene practices and verifying that tattoo inks are from reputable sources and, if possible, have undergone sterilization.
Clinicians should discuss the importance of proper aftercare to minimize the risk for infection, recommend patients with compromised immune systems or skin conditions to reconsider getting a tattoo, and encourage patients to be aware of the signs of infection and to seek medical attention promptly if any symptoms arise.
“Enhanced regulatory measures would help reduce the risk of infections and ensure safer tattooing practices for consumers,” Dr. Javaid said. The findings of Dr. Kim and colleagues “indicate that current manufacturing and sterilization processes are inadequate.”
Regulations could include stricter manufacturing standards to ensure sterility, the mandatory testing of inks for microbial contamination before they reach the market, clear labeling requirements that accurately reflect the sterility and safety of products, and regular inspections and audits of tattoo ink manufacturers, he said, which could encourage the development of more effective sterilization techniques to eliminate bacterial contamination.
The FDA has created a document — Think Before You Ink: Tattoo Safety — for consumers who are considering getting a tattoo.
A version of this article first appeared on Medscape.com.
When US researchers tested 75 unopened and sealed tattoo and permanent makeup inks from 14 different manufacturers, they discovered that about 35% of the products were contaminated with bacteria.
They detected both aerobic bacteria and anaerobic bacteria, which thrive in low-oxygen environments like the dermal layer of the skin.
“This suggests that contaminated tattoo inks could be a source of infection from both types of bacteria,” Seong-Jae Peter Kim, PhD, a microbiologist with the Division of Microbiology, National Center for Toxicological Research, US Food and Drug Administration, who worked on the study, said in a news release.
The findings “are concerning,” said Waleed Javaid, MD, professor of medicine and director of infection prevention and control for the Mount Sinai Health System in New York City. “This contamination poses a significant health risk, as these inks are injected into the dermal layer of the skin, creating an environment conducive to bacterial infections,” said Dr. Javaid, who wasn’t involved in the study, which was published online in Applied and Environmental Microbiology.
New Body Art Culture
Tattoos are more popular than ever, and it is estimated that at least 32% of people in the United States have at least one tattoo. And the rise in popularity has coincided with an increase in ink-related infections.
This new research joins previous studies that have demonstrated that commercial tattoo and permanent makeup inks are often contaminated with pathogenic microorganisms.
Of the 75 ink samples that Dr. Kim and colleagues tested, 26 were contaminated with 34 bacterial isolates classified into 14 genera and 22 species. Among the 34 bacterial isolates, 19 were identified as possibly pathogenic bacterial strains.
Two species — Cutibacterium acnes (four strains) and Staphylococcus epidermidis (two strains) — were isolated under anaerobic conditions.
Two possibly pathogenic bacterial strains — Staphylococcus saprophyticus and C acnes — were isolated from the same two ink samples, indicating that tattoo and permanent makeup inks can harbor both aerobic (S saprophyticus) and anaerobic (C acnes) bacteria.
There was no significant association between sterility claims on the ink label and the absence of bacterial contamination.
“The presence of bacteria like Cutibacterium acnes and Staphylococcus epidermidis, which can cause skin infections and other complications, underscores the potential danger to individuals receiving tattoos or permanent makeup,” Dr. Javaid explained.
The results “emphasize the importance of monitoring these products for both aerobic and anaerobic bacteria, including possibly pathogenic microorganisms,” Dr. Kim said in the news release.
The next steps, according to the researchers, include developing more efficient and accurate microbial detection methods for tattoo inks to streamline the monitoring process and examining the occurrence, co-occurrence, and diversity of microbial contaminants in tattoo inks to prevent future contamination.
Counseling Patients
Healthcare professionals play a “crucial role in counseling patients about the risks associated with tattoos. They should inform patients about the potential for infections, allergic reactions, and other complications related to tattooing and permanent ink,” said Dr. Javaid.
Specific advice can include ensuring that the tattoo parlor adheres to strict hygiene practices and verifying that tattoo inks are from reputable sources and, if possible, have undergone sterilization.
Clinicians should discuss the importance of proper aftercare to minimize the risk for infection, recommend patients with compromised immune systems or skin conditions to reconsider getting a tattoo, and encourage patients to be aware of the signs of infection and to seek medical attention promptly if any symptoms arise.
“Enhanced regulatory measures would help reduce the risk of infections and ensure safer tattooing practices for consumers,” Dr. Javaid said. The findings of Dr. Kim and colleagues “indicate that current manufacturing and sterilization processes are inadequate.”
Regulations could include stricter manufacturing standards to ensure sterility, the mandatory testing of inks for microbial contamination before they reach the market, clear labeling requirements that accurately reflect the sterility and safety of products, and regular inspections and audits of tattoo ink manufacturers, he said, which could encourage the development of more effective sterilization techniques to eliminate bacterial contamination.
The FDA has created a document — Think Before You Ink: Tattoo Safety — for consumers who are considering getting a tattoo.
A version of this article first appeared on Medscape.com.
FROM APPLIED AND ENVIRONMENTAL MICROBIOLOGY
Flu May Increase MI Risk Sixfold, More If No CVD History
“Our study results confirm previous findings of an increased risk of MI during or immediately following acute severe flu infection and raises the idea of giving prophylactic anticoagulation to these patients,” reported Patricia Bruijning-Verhagen, MD, University Medical Center Utrecht, the Netherlands, who is the senior author of the study, which was published online in NEJM Evidence.
“Our results also change things — in that we now know the focus should be on people without a history of cardiovascular disease — and highlight the importance of flu vaccination, particularly for this group,” she pointed out.
The observational, self-controlled, case-series study linked laboratory records on respiratory virus polymerase chain reaction (PCR) testing from 16 laboratories in the Netherlands to national mortality, hospitalization, medication, and administrative registries. Investigators compared the incidence of acute MI during the risk period — days 1-7 after influenza infection — with that in the control period — 1 year before and 51 weeks after the risk period.
The researchers found 26,221 positive PCR tests for influenza, constituting 23,405 unique influenza illness episodes. Of the episodes of acute MI occurring in the year before or the year after confirmed influenza infection and included in the analysis, 25 cases of acute MI occurred on days 1-7 after influenza infection and 394 occurred during the control period.
The adjusted relative incidence of acute MI during the risk period compared with during the control period was 6.16 (95% CI, 4.11-9.24).
The relative incidence of acute MI in individuals with no previous hospitalization for coronary artery disease was 16.60 (95% CI, 10.45-26.37); for those with a previous hospital admission for coronary artery disease, the relative incidence was 1.43 (95% CI, 0.53-3.84).
A temporary increase in the risk for MI has been reported in several previous studies. A 2018 Canadian study by Kwong and colleagues showed a sixfold elevation in the risk for acute MI after influenza infection, which was subsequently confirmed in studies from the United States, Denmark, and Scotland.
In their study, Dr. Bruijning-Verhagen and colleagues aimed to further quantify the association between laboratory-confirmed influenza infection and acute MI and to look at specific subgroups that might have the potential to guide a more individualized approach to prevention.
They replicated the Canadian study using a self-controlled case-series design that corrects for time-invariant confounding and found very similar results: A sixfold increase in the risk for acute MI in the first week after laboratory-confirmed influenza infection.
“The fact that we found similar results to Kwong et al. strengthens the finding that acute flu infection is linked to increased MI risk. This is becoming more and more clear now. It also shows that this effect is generalizable to other countries,” Dr. Bruijning-Verhagen said.
People Without Cardiovascular Disease at Highest Risk
The researchers moved the field ahead by also looking at whether there is a difference in risk between individuals with flu who already had cardiovascular disease and those who did not.
“Most previous studies of flu and MI didn’t stratify between individuals with and without existing cardiovascular disease. And the ones that did look at this weren’t able to show a difference with any confidence,” Dr. Bruijning-Verhagen explained. “There have been suggestions before of a higher risk of MI in individuals with acute flu infection who do not have existing known cardiovascular disease, but this was uncertain.”
The current study showed a large difference between the two groups, with a much higher risk for MI linked to flu in individuals without any known cardiovascular disease.
“You would think patients with existing cardiovascular disease would be more at risk of MI with flu infection, so this was a surprising result,” reported Dr. Bruijning-Verhagen. “But I think the result is real. The difference between the two groups was too big for it not to be.”
Influenza can cause a hypercoagulable state, systemic inflammation, and vascular changes that can trigger MI, even in patients not thought to be at risk before, she pointed out. And this is on top of high cardiac demands because of the acute infection.
Patients who already have cardiovascular disease may be protected to some extent by the cardiovascular medications that they are taking, she added.
These results could justify the use of short-term anticoagulation in patients with severe flu infection to cover the high-risk period, Dr. Bruijning-Verhagen suggested. “We give short-term anticoagulation as prophylaxis to patients when they have surgery. This would not be that different. But obviously, this approach would have to be tested.”
Clinical studies looking at such a strategy are currently underway.
‘Get Your Flu Shot’
The results reinforce the need for anyone who is eligible to get the flu vaccine. “These results should give extra weight to the message to get your flu shot,” she said. “Even if you do not consider yourself someone at risk of cardiovascular disease, our study shows that you can still have an increased risk of MI as a result of severe flu infection.”
In many countries, the flu vaccine is recommended for everyone older than 60 or 65 years and for younger people with a history of cardiovascular disease. Data on flu vaccination was not available in the current study, but the average age of patients infected with flu was 74 years, so most patients would have been eligible to receive vaccination, she said.
In the Netherlands where the research took place, flu vaccination is recommended for everyone older than 60 years, and uptake is about 60%.
“There will be some cases in younger people, but the number needed to vaccinate to show a benefit would be much larger in younger people, and that may not be cost-effective,” reported Dr. Bruijning-Verhagen.
Flu vaccination policies vary across the world, with many factors being taken into account; some countries already advocate for universal vaccination every year.
Extend Flu Vaccination to Prevent ACS
This study “provides further impetus to policy makers to review and update guidelines on prevention of acute coronary syndromes,” Raina MacIntyre, MBBS, Zubair Akhtar, MPH, and Aye Moa, MPH, University of New South Wales, Sydney, Australia, wrote in an accompanying editorial.
“Although vaccination to prevent influenza is recommended and funded in many countries for people 65 years of age and older, the additional benefits of prevention of ACS [acute coronary syndromes] have not been adopted universally into policy and practice nor have recommendations considered prevention of ACS in people 50-64 years of age,” they added.
“Vaccination is low-hanging fruit for people at risk of acute myocardial infarction who have not yet had a first event. It is time that we viewed influenza vaccine as a routine preventive measure for ACS and for people with coronary artery disease risk factors, along with statins, blood pressure control, and smoking cessation,” she explained.
The question of whether the link found between elevated MI risk and severe flu infection might be the result of MI being more likely to be detected in patients hospitalized with severe flu infection, who would undergo a thorough workup, was raised in a second editorial by Lori E. Dodd, PhD, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland.
“I think this would be very unlikely to account for the large effect we found,” responded Dr. Bruijning-Verhagen. “There may be the occasional silent MI that gets missed in patients who are not hospitalized, but, in general, acute MI is not something that goes undetected.”
A version of this article appeared on Medscape.com.
“Our study results confirm previous findings of an increased risk of MI during or immediately following acute severe flu infection and raises the idea of giving prophylactic anticoagulation to these patients,” reported Patricia Bruijning-Verhagen, MD, University Medical Center Utrecht, the Netherlands, who is the senior author of the study, which was published online in NEJM Evidence.
“Our results also change things — in that we now know the focus should be on people without a history of cardiovascular disease — and highlight the importance of flu vaccination, particularly for this group,” she pointed out.
The observational, self-controlled, case-series study linked laboratory records on respiratory virus polymerase chain reaction (PCR) testing from 16 laboratories in the Netherlands to national mortality, hospitalization, medication, and administrative registries. Investigators compared the incidence of acute MI during the risk period — days 1-7 after influenza infection — with that in the control period — 1 year before and 51 weeks after the risk period.
The researchers found 26,221 positive PCR tests for influenza, constituting 23,405 unique influenza illness episodes. Of the episodes of acute MI occurring in the year before or the year after confirmed influenza infection and included in the analysis, 25 cases of acute MI occurred on days 1-7 after influenza infection and 394 occurred during the control period.
The adjusted relative incidence of acute MI during the risk period compared with during the control period was 6.16 (95% CI, 4.11-9.24).
The relative incidence of acute MI in individuals with no previous hospitalization for coronary artery disease was 16.60 (95% CI, 10.45-26.37); for those with a previous hospital admission for coronary artery disease, the relative incidence was 1.43 (95% CI, 0.53-3.84).
A temporary increase in the risk for MI has been reported in several previous studies. A 2018 Canadian study by Kwong and colleagues showed a sixfold elevation in the risk for acute MI after influenza infection, which was subsequently confirmed in studies from the United States, Denmark, and Scotland.
In their study, Dr. Bruijning-Verhagen and colleagues aimed to further quantify the association between laboratory-confirmed influenza infection and acute MI and to look at specific subgroups that might have the potential to guide a more individualized approach to prevention.
They replicated the Canadian study using a self-controlled case-series design that corrects for time-invariant confounding and found very similar results: A sixfold increase in the risk for acute MI in the first week after laboratory-confirmed influenza infection.
“The fact that we found similar results to Kwong et al. strengthens the finding that acute flu infection is linked to increased MI risk. This is becoming more and more clear now. It also shows that this effect is generalizable to other countries,” Dr. Bruijning-Verhagen said.
People Without Cardiovascular Disease at Highest Risk
The researchers moved the field ahead by also looking at whether there is a difference in risk between individuals with flu who already had cardiovascular disease and those who did not.
“Most previous studies of flu and MI didn’t stratify between individuals with and without existing cardiovascular disease. And the ones that did look at this weren’t able to show a difference with any confidence,” Dr. Bruijning-Verhagen explained. “There have been suggestions before of a higher risk of MI in individuals with acute flu infection who do not have existing known cardiovascular disease, but this was uncertain.”
The current study showed a large difference between the two groups, with a much higher risk for MI linked to flu in individuals without any known cardiovascular disease.
“You would think patients with existing cardiovascular disease would be more at risk of MI with flu infection, so this was a surprising result,” reported Dr. Bruijning-Verhagen. “But I think the result is real. The difference between the two groups was too big for it not to be.”
Influenza can cause a hypercoagulable state, systemic inflammation, and vascular changes that can trigger MI, even in patients not thought to be at risk before, she pointed out. And this is on top of high cardiac demands because of the acute infection.
Patients who already have cardiovascular disease may be protected to some extent by the cardiovascular medications that they are taking, she added.
These results could justify the use of short-term anticoagulation in patients with severe flu infection to cover the high-risk period, Dr. Bruijning-Verhagen suggested. “We give short-term anticoagulation as prophylaxis to patients when they have surgery. This would not be that different. But obviously, this approach would have to be tested.”
Clinical studies looking at such a strategy are currently underway.
‘Get Your Flu Shot’
The results reinforce the need for anyone who is eligible to get the flu vaccine. “These results should give extra weight to the message to get your flu shot,” she said. “Even if you do not consider yourself someone at risk of cardiovascular disease, our study shows that you can still have an increased risk of MI as a result of severe flu infection.”
In many countries, the flu vaccine is recommended for everyone older than 60 or 65 years and for younger people with a history of cardiovascular disease. Data on flu vaccination was not available in the current study, but the average age of patients infected with flu was 74 years, so most patients would have been eligible to receive vaccination, she said.
In the Netherlands where the research took place, flu vaccination is recommended for everyone older than 60 years, and uptake is about 60%.
“There will be some cases in younger people, but the number needed to vaccinate to show a benefit would be much larger in younger people, and that may not be cost-effective,” reported Dr. Bruijning-Verhagen.
Flu vaccination policies vary across the world, with many factors being taken into account; some countries already advocate for universal vaccination every year.
Extend Flu Vaccination to Prevent ACS
This study “provides further impetus to policy makers to review and update guidelines on prevention of acute coronary syndromes,” Raina MacIntyre, MBBS, Zubair Akhtar, MPH, and Aye Moa, MPH, University of New South Wales, Sydney, Australia, wrote in an accompanying editorial.
“Although vaccination to prevent influenza is recommended and funded in many countries for people 65 years of age and older, the additional benefits of prevention of ACS [acute coronary syndromes] have not been adopted universally into policy and practice nor have recommendations considered prevention of ACS in people 50-64 years of age,” they added.
“Vaccination is low-hanging fruit for people at risk of acute myocardial infarction who have not yet had a first event. It is time that we viewed influenza vaccine as a routine preventive measure for ACS and for people with coronary artery disease risk factors, along with statins, blood pressure control, and smoking cessation,” she explained.
The question of whether the link found between elevated MI risk and severe flu infection might be the result of MI being more likely to be detected in patients hospitalized with severe flu infection, who would undergo a thorough workup, was raised in a second editorial by Lori E. Dodd, PhD, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland.
“I think this would be very unlikely to account for the large effect we found,” responded Dr. Bruijning-Verhagen. “There may be the occasional silent MI that gets missed in patients who are not hospitalized, but, in general, acute MI is not something that goes undetected.”
A version of this article appeared on Medscape.com.
“Our study results confirm previous findings of an increased risk of MI during or immediately following acute severe flu infection and raises the idea of giving prophylactic anticoagulation to these patients,” reported Patricia Bruijning-Verhagen, MD, University Medical Center Utrecht, the Netherlands, who is the senior author of the study, which was published online in NEJM Evidence.
“Our results also change things — in that we now know the focus should be on people without a history of cardiovascular disease — and highlight the importance of flu vaccination, particularly for this group,” she pointed out.
The observational, self-controlled, case-series study linked laboratory records on respiratory virus polymerase chain reaction (PCR) testing from 16 laboratories in the Netherlands to national mortality, hospitalization, medication, and administrative registries. Investigators compared the incidence of acute MI during the risk period — days 1-7 after influenza infection — with that in the control period — 1 year before and 51 weeks after the risk period.
The researchers found 26,221 positive PCR tests for influenza, constituting 23,405 unique influenza illness episodes. Of the episodes of acute MI occurring in the year before or the year after confirmed influenza infection and included in the analysis, 25 cases of acute MI occurred on days 1-7 after influenza infection and 394 occurred during the control period.
The adjusted relative incidence of acute MI during the risk period compared with during the control period was 6.16 (95% CI, 4.11-9.24).
The relative incidence of acute MI in individuals with no previous hospitalization for coronary artery disease was 16.60 (95% CI, 10.45-26.37); for those with a previous hospital admission for coronary artery disease, the relative incidence was 1.43 (95% CI, 0.53-3.84).
A temporary increase in the risk for MI has been reported in several previous studies. A 2018 Canadian study by Kwong and colleagues showed a sixfold elevation in the risk for acute MI after influenza infection, which was subsequently confirmed in studies from the United States, Denmark, and Scotland.
In their study, Dr. Bruijning-Verhagen and colleagues aimed to further quantify the association between laboratory-confirmed influenza infection and acute MI and to look at specific subgroups that might have the potential to guide a more individualized approach to prevention.
They replicated the Canadian study using a self-controlled case-series design that corrects for time-invariant confounding and found very similar results: A sixfold increase in the risk for acute MI in the first week after laboratory-confirmed influenza infection.
“The fact that we found similar results to Kwong et al. strengthens the finding that acute flu infection is linked to increased MI risk. This is becoming more and more clear now. It also shows that this effect is generalizable to other countries,” Dr. Bruijning-Verhagen said.
People Without Cardiovascular Disease at Highest Risk
The researchers moved the field ahead by also looking at whether there is a difference in risk between individuals with flu who already had cardiovascular disease and those who did not.
“Most previous studies of flu and MI didn’t stratify between individuals with and without existing cardiovascular disease. And the ones that did look at this weren’t able to show a difference with any confidence,” Dr. Bruijning-Verhagen explained. “There have been suggestions before of a higher risk of MI in individuals with acute flu infection who do not have existing known cardiovascular disease, but this was uncertain.”
The current study showed a large difference between the two groups, with a much higher risk for MI linked to flu in individuals without any known cardiovascular disease.
“You would think patients with existing cardiovascular disease would be more at risk of MI with flu infection, so this was a surprising result,” reported Dr. Bruijning-Verhagen. “But I think the result is real. The difference between the two groups was too big for it not to be.”
Influenza can cause a hypercoagulable state, systemic inflammation, and vascular changes that can trigger MI, even in patients not thought to be at risk before, she pointed out. And this is on top of high cardiac demands because of the acute infection.
Patients who already have cardiovascular disease may be protected to some extent by the cardiovascular medications that they are taking, she added.
These results could justify the use of short-term anticoagulation in patients with severe flu infection to cover the high-risk period, Dr. Bruijning-Verhagen suggested. “We give short-term anticoagulation as prophylaxis to patients when they have surgery. This would not be that different. But obviously, this approach would have to be tested.”
Clinical studies looking at such a strategy are currently underway.
‘Get Your Flu Shot’
The results reinforce the need for anyone who is eligible to get the flu vaccine. “These results should give extra weight to the message to get your flu shot,” she said. “Even if you do not consider yourself someone at risk of cardiovascular disease, our study shows that you can still have an increased risk of MI as a result of severe flu infection.”
In many countries, the flu vaccine is recommended for everyone older than 60 or 65 years and for younger people with a history of cardiovascular disease. Data on flu vaccination was not available in the current study, but the average age of patients infected with flu was 74 years, so most patients would have been eligible to receive vaccination, she said.
In the Netherlands where the research took place, flu vaccination is recommended for everyone older than 60 years, and uptake is about 60%.
“There will be some cases in younger people, but the number needed to vaccinate to show a benefit would be much larger in younger people, and that may not be cost-effective,” reported Dr. Bruijning-Verhagen.
Flu vaccination policies vary across the world, with many factors being taken into account; some countries already advocate for universal vaccination every year.
Extend Flu Vaccination to Prevent ACS
This study “provides further impetus to policy makers to review and update guidelines on prevention of acute coronary syndromes,” Raina MacIntyre, MBBS, Zubair Akhtar, MPH, and Aye Moa, MPH, University of New South Wales, Sydney, Australia, wrote in an accompanying editorial.
“Although vaccination to prevent influenza is recommended and funded in many countries for people 65 years of age and older, the additional benefits of prevention of ACS [acute coronary syndromes] have not been adopted universally into policy and practice nor have recommendations considered prevention of ACS in people 50-64 years of age,” they added.
“Vaccination is low-hanging fruit for people at risk of acute myocardial infarction who have not yet had a first event. It is time that we viewed influenza vaccine as a routine preventive measure for ACS and for people with coronary artery disease risk factors, along with statins, blood pressure control, and smoking cessation,” she explained.
The question of whether the link found between elevated MI risk and severe flu infection might be the result of MI being more likely to be detected in patients hospitalized with severe flu infection, who would undergo a thorough workup, was raised in a second editorial by Lori E. Dodd, PhD, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland.
“I think this would be very unlikely to account for the large effect we found,” responded Dr. Bruijning-Verhagen. “There may be the occasional silent MI that gets missed in patients who are not hospitalized, but, in general, acute MI is not something that goes undetected.”
A version of this article appeared on Medscape.com.
FROM NEJM EVIDENCE
Long COVID & Chronic Fatigue: The Similarities are Uncanny
An estimated two million people in England and Scotland were experiencing symptoms of long COVID as of March 2024, according to the Office for National Statistics. Of these, 1.5 million said the condition was adversely affecting their day-to-day activities.
As more research emerges about long COVID, some experts are noticing that its trigger factors, symptoms, and causative mechanisms overlap with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS).
ME/CFS is characterized by severe fatigue that does not improve with rest, in addition to pain and cognitive problems. One in four patients are bed- or house-bound with severe forms of the condition, sometimes experiencing atypical seizures, and speech and swallowing difficulties.
Despite affecting around 250,000 people in the UK and around 2 million people in the European Union (EU), it is a relatively poorly funded disease research area. Increased research into long COVID is thus providing a much-needed boost to ME/CFS research.
“What we already know about the possible causation of ME/CFS is helping research into the causes of long COVID. At the same time, research into long COVID is opening up new avenues of research that may also be relevant to ME/CFS. It is becoming a two-way process,” Dr. Charles Shepherd, honorary medical adviser to the UK-based ME Association, told this news organization.
While funding remains an issue, promising research is currently underway in the UK to improve diagnosis, treatment, and understanding of the pathology of ME/CFS.
Viral Reactivation
Dr. David Newton is research director at ME Research UK. “Viral infection is commonly reported as a trigger for [ME/CFS, meaning that the disease] may be caused by reactivation of latent viruses, including human herpes viruses and enteroviruses,” he said.
Herpes viruses can lie dormant in their host’s immune system for long periods of time. They can be reactivated by factors including infections, stress, and a weakened immune system, and may cause temporary symptoms or persistent disease.
A 2021 pilot study found that people with ME/CFS have a higher concentration of human herpesvirus 6B (HHV-6B) DNA in their saliva, and that concentration correlates with symptom severity. HHV-6B is a common virus typically contracted during infancy and childhood.
A continuation of this research is now underway at Brunel University to improve understanding of HHV-6B’s role in the onset and progression of ME/CFS, and to support the development of diagnostic and prognostic markers, as well as therapeutics such as antiviral therapies.
Mitochondrial Dysfunction
Dr. Shepherd explained that there is now sound evidence demonstrating that biochemical abnormalities in ME/CFS affect how mitochondria produce energy after physical exertion. Research is thus underway to see if treating mitochondrial dysfunction improves ME/CFS symptoms.
A phase 2a placebo-controlled clinical trial from 2023 found that AXA1125, a drug that works by modulating energy metabolism, significantly improved symptoms of fatigue in patients with fatigue-dominant long COVID, although it did not improve mitochondrial respiration.
“[The findings suggest] that improving mitochondrial health may be one way to restore normal functioning among people with long COVID, and by extension CFS,” study author Betty Raman, associate professor of cardiovascular medicine at the University of Oxford, told this news organization. She noted, however, that plans for a phase III trial have stalled due to insufficient funding.
Meanwhile, researchers from the Quadram Institute in Norwich and the University of East Anglia are conducting a pilot study to see if red light therapy can relieve symptoms of ME/CFS. Red light can be absorbed by mitochondria and is used to boost energy production. The trial will monitor patients remotely from their homes and will assess cognitive function and physical activity levels.
Gut Dysbiosis
Many studies have found that people with ME/CFS have altered gut microbiota, which suggests that changes in gut bacteria may contribute to the condition. Researchers at the Quadram Institute will thus conduct a clinical trial called RESTORE-ME to see whether fecal microbiota transplants (FMT) can treat the condition.
Rik Haagmans is a research scientist and PhD candidate at the Quadram Institute. He told this news organization: “Our FMT studies, if effective, could provide a longer lasting or even permanent relief of ME/CFS, as restoring the gut microbial composition wouldn’t require continuous medication,” he said.
Biobank and Biomarkers
Europe’s first ME/CFS-specific biobank is in the UK and is called UKMEB. It now has more than 30,000 blood samples from patients with ME/CFS, multiple sclerosis, and healthy controls. Uniquely, it includes samples from people with ME/CFS who are house- and bed-bound. Caroline Kingdon, RN, MSc, a research fellow and biobank lead at the London School of Hygiene and Tropical Medicine, told this news organization that samples and data from the UKMEB have been provided to research groups all over the world and have contributed to widely cited literature.
One group making use of these samples is led by Fatima Labeed, PhD, senior lecturer in human biology at the University of Surrey. Dr. Labeed and her team are developing a diagnostic test for ME/CFS based on electrical properties in white blood cells.
“To date, studies of ME/CFS have focused on the biochemical behavior of cells: the amount and type of proteins that cells use. We have taken a different approach, studying the electrical properties,” she explained to this news organization.
Her research builds on initial observations from 2019 that found differences in the electrical impedance of white blood cells between people with ME/CFS and controls. While the biological implications remain unknown, the findings may represent a biomarker for the condition.
Using blood samples from the UKMEB, the researchers are now investigating this potential biomarker with improved techniques and a larger patient cohort, including those with mild/moderate and severe forms of ME/CFS. So far, they have received more than 100 blood samples and have analyzed the electrical properties of 42.
“Based on the results we have so far, we are very close to having a biomarker for diagnosis. Our results so far show a high degree of accuracy and are able to distinguish between ME/CFS and other diseases,” said Dr. Labeed.
Genetic Test
Another promising avenue for diagnostics comes from a research team at the University of Edinburgh led by Professor Chris Ponting at the university’s Institute of Genetics and Cancer. They are currently working on DecodeMe, a large genetic study of ME using data from more than 26,000 people.
“We are studying blood-based biomarkers that distinguish people with ME from population controls. We’ve found a large number — including some found previously in other studies — and are writing these results up for publication,” said Ponting. The results should be published in early 2025.
The Future
While research into ME/CFS has picked up pace in recent years, funding remains a key bottleneck.
“Over the last 10 years, only £8.05m has been spent on ME research,” Sonya Chowdhury, chief executive of UK charity Action for ME told this news organization. She believes this amount is not equitably comparable to research funding allocated to other diseases.
In 2022, the UK government announced its intention to develop a cross-government interim delivery plan on ME/CFS for England, however publication of the final plan has been delayed numerous times.
Dr. Shepherd agreed that increased funding is crucial for progress to be made. He said the biggest help to ME/CFS research would be to end the disparity in government research funding for the disease, and match what is given for many other disabling long-term conditions.
“It’s not fair to continue to rely on the charity sector to fund almost all of the biomedical research into ME/CFS here in the UK,” he said.
A version of this article appeared on Medscape.com.
An estimated two million people in England and Scotland were experiencing symptoms of long COVID as of March 2024, according to the Office for National Statistics. Of these, 1.5 million said the condition was adversely affecting their day-to-day activities.
As more research emerges about long COVID, some experts are noticing that its trigger factors, symptoms, and causative mechanisms overlap with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS).
ME/CFS is characterized by severe fatigue that does not improve with rest, in addition to pain and cognitive problems. One in four patients are bed- or house-bound with severe forms of the condition, sometimes experiencing atypical seizures, and speech and swallowing difficulties.
Despite affecting around 250,000 people in the UK and around 2 million people in the European Union (EU), it is a relatively poorly funded disease research area. Increased research into long COVID is thus providing a much-needed boost to ME/CFS research.
“What we already know about the possible causation of ME/CFS is helping research into the causes of long COVID. At the same time, research into long COVID is opening up new avenues of research that may also be relevant to ME/CFS. It is becoming a two-way process,” Dr. Charles Shepherd, honorary medical adviser to the UK-based ME Association, told this news organization.
While funding remains an issue, promising research is currently underway in the UK to improve diagnosis, treatment, and understanding of the pathology of ME/CFS.
Viral Reactivation
Dr. David Newton is research director at ME Research UK. “Viral infection is commonly reported as a trigger for [ME/CFS, meaning that the disease] may be caused by reactivation of latent viruses, including human herpes viruses and enteroviruses,” he said.
Herpes viruses can lie dormant in their host’s immune system for long periods of time. They can be reactivated by factors including infections, stress, and a weakened immune system, and may cause temporary symptoms or persistent disease.
A 2021 pilot study found that people with ME/CFS have a higher concentration of human herpesvirus 6B (HHV-6B) DNA in their saliva, and that concentration correlates with symptom severity. HHV-6B is a common virus typically contracted during infancy and childhood.
A continuation of this research is now underway at Brunel University to improve understanding of HHV-6B’s role in the onset and progression of ME/CFS, and to support the development of diagnostic and prognostic markers, as well as therapeutics such as antiviral therapies.
Mitochondrial Dysfunction
Dr. Shepherd explained that there is now sound evidence demonstrating that biochemical abnormalities in ME/CFS affect how mitochondria produce energy after physical exertion. Research is thus underway to see if treating mitochondrial dysfunction improves ME/CFS symptoms.
A phase 2a placebo-controlled clinical trial from 2023 found that AXA1125, a drug that works by modulating energy metabolism, significantly improved symptoms of fatigue in patients with fatigue-dominant long COVID, although it did not improve mitochondrial respiration.
“[The findings suggest] that improving mitochondrial health may be one way to restore normal functioning among people with long COVID, and by extension CFS,” study author Betty Raman, associate professor of cardiovascular medicine at the University of Oxford, told this news organization. She noted, however, that plans for a phase III trial have stalled due to insufficient funding.
Meanwhile, researchers from the Quadram Institute in Norwich and the University of East Anglia are conducting a pilot study to see if red light therapy can relieve symptoms of ME/CFS. Red light can be absorbed by mitochondria and is used to boost energy production. The trial will monitor patients remotely from their homes and will assess cognitive function and physical activity levels.
Gut Dysbiosis
Many studies have found that people with ME/CFS have altered gut microbiota, which suggests that changes in gut bacteria may contribute to the condition. Researchers at the Quadram Institute will thus conduct a clinical trial called RESTORE-ME to see whether fecal microbiota transplants (FMT) can treat the condition.
Rik Haagmans is a research scientist and PhD candidate at the Quadram Institute. He told this news organization: “Our FMT studies, if effective, could provide a longer lasting or even permanent relief of ME/CFS, as restoring the gut microbial composition wouldn’t require continuous medication,” he said.
Biobank and Biomarkers
Europe’s first ME/CFS-specific biobank is in the UK and is called UKMEB. It now has more than 30,000 blood samples from patients with ME/CFS, multiple sclerosis, and healthy controls. Uniquely, it includes samples from people with ME/CFS who are house- and bed-bound. Caroline Kingdon, RN, MSc, a research fellow and biobank lead at the London School of Hygiene and Tropical Medicine, told this news organization that samples and data from the UKMEB have been provided to research groups all over the world and have contributed to widely cited literature.
One group making use of these samples is led by Fatima Labeed, PhD, senior lecturer in human biology at the University of Surrey. Dr. Labeed and her team are developing a diagnostic test for ME/CFS based on electrical properties in white blood cells.
“To date, studies of ME/CFS have focused on the biochemical behavior of cells: the amount and type of proteins that cells use. We have taken a different approach, studying the electrical properties,” she explained to this news organization.
Her research builds on initial observations from 2019 that found differences in the electrical impedance of white blood cells between people with ME/CFS and controls. While the biological implications remain unknown, the findings may represent a biomarker for the condition.
Using blood samples from the UKMEB, the researchers are now investigating this potential biomarker with improved techniques and a larger patient cohort, including those with mild/moderate and severe forms of ME/CFS. So far, they have received more than 100 blood samples and have analyzed the electrical properties of 42.
“Based on the results we have so far, we are very close to having a biomarker for diagnosis. Our results so far show a high degree of accuracy and are able to distinguish between ME/CFS and other diseases,” said Dr. Labeed.
Genetic Test
Another promising avenue for diagnostics comes from a research team at the University of Edinburgh led by Professor Chris Ponting at the university’s Institute of Genetics and Cancer. They are currently working on DecodeMe, a large genetic study of ME using data from more than 26,000 people.
“We are studying blood-based biomarkers that distinguish people with ME from population controls. We’ve found a large number — including some found previously in other studies — and are writing these results up for publication,” said Ponting. The results should be published in early 2025.
The Future
While research into ME/CFS has picked up pace in recent years, funding remains a key bottleneck.
“Over the last 10 years, only £8.05m has been spent on ME research,” Sonya Chowdhury, chief executive of UK charity Action for ME told this news organization. She believes this amount is not equitably comparable to research funding allocated to other diseases.
In 2022, the UK government announced its intention to develop a cross-government interim delivery plan on ME/CFS for England, however publication of the final plan has been delayed numerous times.
Dr. Shepherd agreed that increased funding is crucial for progress to be made. He said the biggest help to ME/CFS research would be to end the disparity in government research funding for the disease, and match what is given for many other disabling long-term conditions.
“It’s not fair to continue to rely on the charity sector to fund almost all of the biomedical research into ME/CFS here in the UK,” he said.
A version of this article appeared on Medscape.com.
An estimated two million people in England and Scotland were experiencing symptoms of long COVID as of March 2024, according to the Office for National Statistics. Of these, 1.5 million said the condition was adversely affecting their day-to-day activities.
As more research emerges about long COVID, some experts are noticing that its trigger factors, symptoms, and causative mechanisms overlap with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS).
ME/CFS is characterized by severe fatigue that does not improve with rest, in addition to pain and cognitive problems. One in four patients are bed- or house-bound with severe forms of the condition, sometimes experiencing atypical seizures, and speech and swallowing difficulties.
Despite affecting around 250,000 people in the UK and around 2 million people in the European Union (EU), it is a relatively poorly funded disease research area. Increased research into long COVID is thus providing a much-needed boost to ME/CFS research.
“What we already know about the possible causation of ME/CFS is helping research into the causes of long COVID. At the same time, research into long COVID is opening up new avenues of research that may also be relevant to ME/CFS. It is becoming a two-way process,” Dr. Charles Shepherd, honorary medical adviser to the UK-based ME Association, told this news organization.
While funding remains an issue, promising research is currently underway in the UK to improve diagnosis, treatment, and understanding of the pathology of ME/CFS.
Viral Reactivation
Dr. David Newton is research director at ME Research UK. “Viral infection is commonly reported as a trigger for [ME/CFS, meaning that the disease] may be caused by reactivation of latent viruses, including human herpes viruses and enteroviruses,” he said.
Herpes viruses can lie dormant in their host’s immune system for long periods of time. They can be reactivated by factors including infections, stress, and a weakened immune system, and may cause temporary symptoms or persistent disease.
A 2021 pilot study found that people with ME/CFS have a higher concentration of human herpesvirus 6B (HHV-6B) DNA in their saliva, and that concentration correlates with symptom severity. HHV-6B is a common virus typically contracted during infancy and childhood.
A continuation of this research is now underway at Brunel University to improve understanding of HHV-6B’s role in the onset and progression of ME/CFS, and to support the development of diagnostic and prognostic markers, as well as therapeutics such as antiviral therapies.
Mitochondrial Dysfunction
Dr. Shepherd explained that there is now sound evidence demonstrating that biochemical abnormalities in ME/CFS affect how mitochondria produce energy after physical exertion. Research is thus underway to see if treating mitochondrial dysfunction improves ME/CFS symptoms.
A phase 2a placebo-controlled clinical trial from 2023 found that AXA1125, a drug that works by modulating energy metabolism, significantly improved symptoms of fatigue in patients with fatigue-dominant long COVID, although it did not improve mitochondrial respiration.
“[The findings suggest] that improving mitochondrial health may be one way to restore normal functioning among people with long COVID, and by extension CFS,” study author Betty Raman, associate professor of cardiovascular medicine at the University of Oxford, told this news organization. She noted, however, that plans for a phase III trial have stalled due to insufficient funding.
Meanwhile, researchers from the Quadram Institute in Norwich and the University of East Anglia are conducting a pilot study to see if red light therapy can relieve symptoms of ME/CFS. Red light can be absorbed by mitochondria and is used to boost energy production. The trial will monitor patients remotely from their homes and will assess cognitive function and physical activity levels.
Gut Dysbiosis
Many studies have found that people with ME/CFS have altered gut microbiota, which suggests that changes in gut bacteria may contribute to the condition. Researchers at the Quadram Institute will thus conduct a clinical trial called RESTORE-ME to see whether fecal microbiota transplants (FMT) can treat the condition.
Rik Haagmans is a research scientist and PhD candidate at the Quadram Institute. He told this news organization: “Our FMT studies, if effective, could provide a longer lasting or even permanent relief of ME/CFS, as restoring the gut microbial composition wouldn’t require continuous medication,” he said.
Biobank and Biomarkers
Europe’s first ME/CFS-specific biobank is in the UK and is called UKMEB. It now has more than 30,000 blood samples from patients with ME/CFS, multiple sclerosis, and healthy controls. Uniquely, it includes samples from people with ME/CFS who are house- and bed-bound. Caroline Kingdon, RN, MSc, a research fellow and biobank lead at the London School of Hygiene and Tropical Medicine, told this news organization that samples and data from the UKMEB have been provided to research groups all over the world and have contributed to widely cited literature.
One group making use of these samples is led by Fatima Labeed, PhD, senior lecturer in human biology at the University of Surrey. Dr. Labeed and her team are developing a diagnostic test for ME/CFS based on electrical properties in white blood cells.
“To date, studies of ME/CFS have focused on the biochemical behavior of cells: the amount and type of proteins that cells use. We have taken a different approach, studying the electrical properties,” she explained to this news organization.
Her research builds on initial observations from 2019 that found differences in the electrical impedance of white blood cells between people with ME/CFS and controls. While the biological implications remain unknown, the findings may represent a biomarker for the condition.
Using blood samples from the UKMEB, the researchers are now investigating this potential biomarker with improved techniques and a larger patient cohort, including those with mild/moderate and severe forms of ME/CFS. So far, they have received more than 100 blood samples and have analyzed the electrical properties of 42.
“Based on the results we have so far, we are very close to having a biomarker for diagnosis. Our results so far show a high degree of accuracy and are able to distinguish between ME/CFS and other diseases,” said Dr. Labeed.
Genetic Test
Another promising avenue for diagnostics comes from a research team at the University of Edinburgh led by Professor Chris Ponting at the university’s Institute of Genetics and Cancer. They are currently working on DecodeMe, a large genetic study of ME using data from more than 26,000 people.
“We are studying blood-based biomarkers that distinguish people with ME from population controls. We’ve found a large number — including some found previously in other studies — and are writing these results up for publication,” said Ponting. The results should be published in early 2025.
The Future
While research into ME/CFS has picked up pace in recent years, funding remains a key bottleneck.
“Over the last 10 years, only £8.05m has been spent on ME research,” Sonya Chowdhury, chief executive of UK charity Action for ME told this news organization. She believes this amount is not equitably comparable to research funding allocated to other diseases.
In 2022, the UK government announced its intention to develop a cross-government interim delivery plan on ME/CFS for England, however publication of the final plan has been delayed numerous times.
Dr. Shepherd agreed that increased funding is crucial for progress to be made. He said the biggest help to ME/CFS research would be to end the disparity in government research funding for the disease, and match what is given for many other disabling long-term conditions.
“It’s not fair to continue to rely on the charity sector to fund almost all of the biomedical research into ME/CFS here in the UK,” he said.
A version of this article appeared on Medscape.com.
How Common Are Life-Threatening Infections In Infants with Pustules, Vesicles?
TOPLINE:
, according to the findings from a retrospective study.
METHODOLOGY:
- Researchers reviewed the electronic medical records of infants aged ≤ 60 days who received a pediatric dermatology consultation at six US academic institutions between September 2013 and August 2019.
- Among 879 consults, 183 afebrile infants were identified as having presented with pustules, vesicles, and/or bullae.
- Infectious disease workups included blood cultures, urine cultures, lumbar punctures, and HSV testing using viral skin culture, direct immunofluorescence assay, and/or polymerase chain reaction.
- Patients were categorized by gestational age as preterm (< 37 weeks), full-term (37-42 weeks), and post-term (≥ 42 weeks).
- Overall, 67.8% of infants had pustules, 31.1% had vesicles, and 10.4% had bullae.
TAKEAWAY:
- None of the cases showed positive cerebrospinal fluid or pathogenic blood cultures. In 122 of the cases (66.6%), a noninfectious cause was diagnosed, and an infectious cause was diagnosed in 71 cases (38.8%; some patients had more than one diagnosis).
- Of the 127 newborns evaluated for HSV infection, nine (7.1%) tested positive, of whom seven (5.5%) had disease affecting the skin, eye, and mouth and were full- term infants, and two (1.6%) had disseminated HSV and were preterm infants.
- Angioinvasive fungal infection was diagnosed in five infants (2.7%), all of whom were preterm infants (< 28 weeks gestational age).
- The risk for life-threatening disease was higher in preterm infants born before 32 weeks of gestational age (P < .01) compared with those born after 32 weeks.
IN PRACTICE:
“Full-term, well-appearing, afebrile infants ≤ 60 days of age presenting with pustules or vesicles may not require full SBI [serious bacterial infection] work-up, although larger studies are needed,” the authors concluded. Testing for HSV, they added, “is recommended in all infants with vesicles, grouped pustules, or pustules accompanied by punched out or grouped erosions,” and preterm infants “should be assessed for disseminated fungal infection and HSV in the setting of fluid-filled skin lesions.”
SOURCE:
The study was led by Sonora Yun, BA, Columbia University, New York City, and was published online in Pediatrics.
LIMITATIONS:
The data were limited by the sample size and very low incidence of serious infections. Infants probably had atypical or severe presentations that warranted pediatric dermatology consultation, which may have led to overrepresentation of infectious disease rates. The study inclusion was restricted to those who received a dermatology consult; therefore, the findings may not be generalizable to outpatient primary care.
DISCLOSURES:
This study did not receive any external funding. The authors declared that they had no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
, according to the findings from a retrospective study.
METHODOLOGY:
- Researchers reviewed the electronic medical records of infants aged ≤ 60 days who received a pediatric dermatology consultation at six US academic institutions between September 2013 and August 2019.
- Among 879 consults, 183 afebrile infants were identified as having presented with pustules, vesicles, and/or bullae.
- Infectious disease workups included blood cultures, urine cultures, lumbar punctures, and HSV testing using viral skin culture, direct immunofluorescence assay, and/or polymerase chain reaction.
- Patients were categorized by gestational age as preterm (< 37 weeks), full-term (37-42 weeks), and post-term (≥ 42 weeks).
- Overall, 67.8% of infants had pustules, 31.1% had vesicles, and 10.4% had bullae.
TAKEAWAY:
- None of the cases showed positive cerebrospinal fluid or pathogenic blood cultures. In 122 of the cases (66.6%), a noninfectious cause was diagnosed, and an infectious cause was diagnosed in 71 cases (38.8%; some patients had more than one diagnosis).
- Of the 127 newborns evaluated for HSV infection, nine (7.1%) tested positive, of whom seven (5.5%) had disease affecting the skin, eye, and mouth and were full- term infants, and two (1.6%) had disseminated HSV and were preterm infants.
- Angioinvasive fungal infection was diagnosed in five infants (2.7%), all of whom were preterm infants (< 28 weeks gestational age).
- The risk for life-threatening disease was higher in preterm infants born before 32 weeks of gestational age (P < .01) compared with those born after 32 weeks.
IN PRACTICE:
“Full-term, well-appearing, afebrile infants ≤ 60 days of age presenting with pustules or vesicles may not require full SBI [serious bacterial infection] work-up, although larger studies are needed,” the authors concluded. Testing for HSV, they added, “is recommended in all infants with vesicles, grouped pustules, or pustules accompanied by punched out or grouped erosions,” and preterm infants “should be assessed for disseminated fungal infection and HSV in the setting of fluid-filled skin lesions.”
SOURCE:
The study was led by Sonora Yun, BA, Columbia University, New York City, and was published online in Pediatrics.
LIMITATIONS:
The data were limited by the sample size and very low incidence of serious infections. Infants probably had atypical or severe presentations that warranted pediatric dermatology consultation, which may have led to overrepresentation of infectious disease rates. The study inclusion was restricted to those who received a dermatology consult; therefore, the findings may not be generalizable to outpatient primary care.
DISCLOSURES:
This study did not receive any external funding. The authors declared that they had no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
, according to the findings from a retrospective study.
METHODOLOGY:
- Researchers reviewed the electronic medical records of infants aged ≤ 60 days who received a pediatric dermatology consultation at six US academic institutions between September 2013 and August 2019.
- Among 879 consults, 183 afebrile infants were identified as having presented with pustules, vesicles, and/or bullae.
- Infectious disease workups included blood cultures, urine cultures, lumbar punctures, and HSV testing using viral skin culture, direct immunofluorescence assay, and/or polymerase chain reaction.
- Patients were categorized by gestational age as preterm (< 37 weeks), full-term (37-42 weeks), and post-term (≥ 42 weeks).
- Overall, 67.8% of infants had pustules, 31.1% had vesicles, and 10.4% had bullae.
TAKEAWAY:
- None of the cases showed positive cerebrospinal fluid or pathogenic blood cultures. In 122 of the cases (66.6%), a noninfectious cause was diagnosed, and an infectious cause was diagnosed in 71 cases (38.8%; some patients had more than one diagnosis).
- Of the 127 newborns evaluated for HSV infection, nine (7.1%) tested positive, of whom seven (5.5%) had disease affecting the skin, eye, and mouth and were full- term infants, and two (1.6%) had disseminated HSV and were preterm infants.
- Angioinvasive fungal infection was diagnosed in five infants (2.7%), all of whom were preterm infants (< 28 weeks gestational age).
- The risk for life-threatening disease was higher in preterm infants born before 32 weeks of gestational age (P < .01) compared with those born after 32 weeks.
IN PRACTICE:
“Full-term, well-appearing, afebrile infants ≤ 60 days of age presenting with pustules or vesicles may not require full SBI [serious bacterial infection] work-up, although larger studies are needed,” the authors concluded. Testing for HSV, they added, “is recommended in all infants with vesicles, grouped pustules, or pustules accompanied by punched out or grouped erosions,” and preterm infants “should be assessed for disseminated fungal infection and HSV in the setting of fluid-filled skin lesions.”
SOURCE:
The study was led by Sonora Yun, BA, Columbia University, New York City, and was published online in Pediatrics.
LIMITATIONS:
The data were limited by the sample size and very low incidence of serious infections. Infants probably had atypical or severe presentations that warranted pediatric dermatology consultation, which may have led to overrepresentation of infectious disease rates. The study inclusion was restricted to those who received a dermatology consult; therefore, the findings may not be generalizable to outpatient primary care.
DISCLOSURES:
This study did not receive any external funding. The authors declared that they had no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
What Are the Ethics of Sex and Romance for Older Adults in Nursing Homes?
This transcript has been edited for clarity.
I had a case a couple years ago in which I found myself completely at odds with the person complaining. A daughter came to me and said [paraphrasing], look, my dad is in a nursing home, and he’s just there for care that he needs, but he’s mentally competent. He’s enjoying watching television, playing games. He plays bridge and does many things. The nursing home is letting him have a romantic relationship with a woman who’s also in the nursing home. I think you, ethicist, should both intervene and try to stop that, and write more about the immorality of facilities like nursing homes or other long-term care settings permitting romance or sexual relations to take place.
I was reminded of that case because a report recently appeared that sexually transmitted diseases are on the rise among the elderly, both in nursing homes and in other settings. This obviously is linked up to another technological advance: the erectile dysfunction drugs.
I’m sure there are many men who, at one point in their lives, could not engage in sexual activity due to impotence. We have found a treatment for erectile dysfunction. Loads and loads of men are using it, and we forget that some of them are going to be older. The rate of impotence goes up directly with aging. If you’re in a nursing home, home care, or wherever you are, you may find yourself able to engage in sex in a way that your dad or your granddad may not have been.
We also know — and I found this out when I was tracking sales of erectile dysfunction drugs — that some of these older men are going to visit prostitutes. That’s another route, unsafe sex, for sexual diseases to be spreading into various older communities.
Morally, I think every individual who is competent and wishes to engage in a romantic or sexual relationship should be able to do so. If they’re within a marriage and they want to resume sexual activity because they get better or they can use these drugs, well, that’s great. If they’re single and they’re just living with others and they form an interesting romantic relationship, why shouldn’t they be allowed to engage in sex?
It is not only something that I didn’t agree with the complaining daughter about, but also I think some of these facilities should make more rooms for privacy and more opportunity for intimacy. It’s not like we should tell granddad that he’s living in a college dorm and try to make sure that his roommate doesn’t come in if he’s going to have his girlfriend over.
Are there ethical issues? Sure. Obviously, we should remember, if we have older patients, to talk to them about sexually transmitted diseases as part of a discussion of their sex life. We shouldn’t presume that they’re not doing something. We should presume that they might be, and then remind them about safe sex, particularly if they’re going to use third parties like prostitutes.
Competency becomes important. It’s one thing to have a mutually agreed upon romantic relationship. It’s another thing if somebody is taking advantage of someone who has Alzheimer’s or severe mental dysfunction and they’re not consenting.
How do we determine that and how do we manage that? I think people who are incompetent need to be protected from sexual advances unless they have a relative or someone who says they can engage if they enjoy it and it brings them pleasure. I wouldn’t just have people who are vulnerable, exploited, or acting in a predatory way toward others.
As I said, we need to rethink the design of where older people are living, whether it’s assisted living, nursing home living, or wherever, just to give them the opportunity to have a full life, as any individual would have once they’re past the age of majority, no matter who they want to have romance with and what they want to do in terms of how far that intimacy goes.
Sadly, I didn’t agree with the daughter who came to me and asked me to stop it. I wouldn’t stop it nor would I publish against it. There are risks that we ought to be aware of, including exploiting vulnerable people if they can’t consent, and the danger of transmission of disease, as would be true in any group that might engage in high-risk behavior.
Another risk may be injury if someone is frail and can’t physically sustain sexual intimacy because they’re just too frail to do it. We also need to be sure to address the issue of sexuality with patients to make sure they know what’s going on, what risks there are, what rights they have, and so on.
At the end of the day, I’m not in the camp that says, “Just say no” when it comes to sex among the elderly.
Dr. Caplan is director, Division of Medical Ethics, New York University Langone Medical Center, New York. He has served as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position); he also serves as a contributing author and advisor for Medscape.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I had a case a couple years ago in which I found myself completely at odds with the person complaining. A daughter came to me and said [paraphrasing], look, my dad is in a nursing home, and he’s just there for care that he needs, but he’s mentally competent. He’s enjoying watching television, playing games. He plays bridge and does many things. The nursing home is letting him have a romantic relationship with a woman who’s also in the nursing home. I think you, ethicist, should both intervene and try to stop that, and write more about the immorality of facilities like nursing homes or other long-term care settings permitting romance or sexual relations to take place.
I was reminded of that case because a report recently appeared that sexually transmitted diseases are on the rise among the elderly, both in nursing homes and in other settings. This obviously is linked up to another technological advance: the erectile dysfunction drugs.
I’m sure there are many men who, at one point in their lives, could not engage in sexual activity due to impotence. We have found a treatment for erectile dysfunction. Loads and loads of men are using it, and we forget that some of them are going to be older. The rate of impotence goes up directly with aging. If you’re in a nursing home, home care, or wherever you are, you may find yourself able to engage in sex in a way that your dad or your granddad may not have been.
We also know — and I found this out when I was tracking sales of erectile dysfunction drugs — that some of these older men are going to visit prostitutes. That’s another route, unsafe sex, for sexual diseases to be spreading into various older communities.
Morally, I think every individual who is competent and wishes to engage in a romantic or sexual relationship should be able to do so. If they’re within a marriage and they want to resume sexual activity because they get better or they can use these drugs, well, that’s great. If they’re single and they’re just living with others and they form an interesting romantic relationship, why shouldn’t they be allowed to engage in sex?
It is not only something that I didn’t agree with the complaining daughter about, but also I think some of these facilities should make more rooms for privacy and more opportunity for intimacy. It’s not like we should tell granddad that he’s living in a college dorm and try to make sure that his roommate doesn’t come in if he’s going to have his girlfriend over.
Are there ethical issues? Sure. Obviously, we should remember, if we have older patients, to talk to them about sexually transmitted diseases as part of a discussion of their sex life. We shouldn’t presume that they’re not doing something. We should presume that they might be, and then remind them about safe sex, particularly if they’re going to use third parties like prostitutes.
Competency becomes important. It’s one thing to have a mutually agreed upon romantic relationship. It’s another thing if somebody is taking advantage of someone who has Alzheimer’s or severe mental dysfunction and they’re not consenting.
How do we determine that and how do we manage that? I think people who are incompetent need to be protected from sexual advances unless they have a relative or someone who says they can engage if they enjoy it and it brings them pleasure. I wouldn’t just have people who are vulnerable, exploited, or acting in a predatory way toward others.
As I said, we need to rethink the design of where older people are living, whether it’s assisted living, nursing home living, or wherever, just to give them the opportunity to have a full life, as any individual would have once they’re past the age of majority, no matter who they want to have romance with and what they want to do in terms of how far that intimacy goes.
Sadly, I didn’t agree with the daughter who came to me and asked me to stop it. I wouldn’t stop it nor would I publish against it. There are risks that we ought to be aware of, including exploiting vulnerable people if they can’t consent, and the danger of transmission of disease, as would be true in any group that might engage in high-risk behavior.
Another risk may be injury if someone is frail and can’t physically sustain sexual intimacy because they’re just too frail to do it. We also need to be sure to address the issue of sexuality with patients to make sure they know what’s going on, what risks there are, what rights they have, and so on.
At the end of the day, I’m not in the camp that says, “Just say no” when it comes to sex among the elderly.
Dr. Caplan is director, Division of Medical Ethics, New York University Langone Medical Center, New York. He has served as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position); he also serves as a contributing author and advisor for Medscape.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I had a case a couple years ago in which I found myself completely at odds with the person complaining. A daughter came to me and said [paraphrasing], look, my dad is in a nursing home, and he’s just there for care that he needs, but he’s mentally competent. He’s enjoying watching television, playing games. He plays bridge and does many things. The nursing home is letting him have a romantic relationship with a woman who’s also in the nursing home. I think you, ethicist, should both intervene and try to stop that, and write more about the immorality of facilities like nursing homes or other long-term care settings permitting romance or sexual relations to take place.
I was reminded of that case because a report recently appeared that sexually transmitted diseases are on the rise among the elderly, both in nursing homes and in other settings. This obviously is linked up to another technological advance: the erectile dysfunction drugs.
I’m sure there are many men who, at one point in their lives, could not engage in sexual activity due to impotence. We have found a treatment for erectile dysfunction. Loads and loads of men are using it, and we forget that some of them are going to be older. The rate of impotence goes up directly with aging. If you’re in a nursing home, home care, or wherever you are, you may find yourself able to engage in sex in a way that your dad or your granddad may not have been.
We also know — and I found this out when I was tracking sales of erectile dysfunction drugs — that some of these older men are going to visit prostitutes. That’s another route, unsafe sex, for sexual diseases to be spreading into various older communities.
Morally, I think every individual who is competent and wishes to engage in a romantic or sexual relationship should be able to do so. If they’re within a marriage and they want to resume sexual activity because they get better or they can use these drugs, well, that’s great. If they’re single and they’re just living with others and they form an interesting romantic relationship, why shouldn’t they be allowed to engage in sex?
It is not only something that I didn’t agree with the complaining daughter about, but also I think some of these facilities should make more rooms for privacy and more opportunity for intimacy. It’s not like we should tell granddad that he’s living in a college dorm and try to make sure that his roommate doesn’t come in if he’s going to have his girlfriend over.
Are there ethical issues? Sure. Obviously, we should remember, if we have older patients, to talk to them about sexually transmitted diseases as part of a discussion of their sex life. We shouldn’t presume that they’re not doing something. We should presume that they might be, and then remind them about safe sex, particularly if they’re going to use third parties like prostitutes.
Competency becomes important. It’s one thing to have a mutually agreed upon romantic relationship. It’s another thing if somebody is taking advantage of someone who has Alzheimer’s or severe mental dysfunction and they’re not consenting.
How do we determine that and how do we manage that? I think people who are incompetent need to be protected from sexual advances unless they have a relative or someone who says they can engage if they enjoy it and it brings them pleasure. I wouldn’t just have people who are vulnerable, exploited, or acting in a predatory way toward others.
As I said, we need to rethink the design of where older people are living, whether it’s assisted living, nursing home living, or wherever, just to give them the opportunity to have a full life, as any individual would have once they’re past the age of majority, no matter who they want to have romance with and what they want to do in terms of how far that intimacy goes.
Sadly, I didn’t agree with the daughter who came to me and asked me to stop it. I wouldn’t stop it nor would I publish against it. There are risks that we ought to be aware of, including exploiting vulnerable people if they can’t consent, and the danger of transmission of disease, as would be true in any group that might engage in high-risk behavior.
Another risk may be injury if someone is frail and can’t physically sustain sexual intimacy because they’re just too frail to do it. We also need to be sure to address the issue of sexuality with patients to make sure they know what’s going on, what risks there are, what rights they have, and so on.
At the end of the day, I’m not in the camp that says, “Just say no” when it comes to sex among the elderly.
Dr. Caplan is director, Division of Medical Ethics, New York University Langone Medical Center, New York. He has served as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position); he also serves as a contributing author and advisor for Medscape.
A version of this article first appeared on Medscape.com.
New Mid-Year Vaccine Recommendations From ACIP
This transcript has been edited for clarity.
ACIP, the CDC’s Advisory Committee on Immunization Practices, met for 3 days in June. New vaccines and new recommendations for respiratory syncytial virus (RSV), flu, COVID, and a new pneumococcal vaccine were revealed.
RSV Protection
We’ll begin with RSV vaccines for adults aged 60 or older. For this group, shared clinical decision-making is out; it no longer applies. New, more specific recommendations from ACIP for RSV vaccines are both age based and risk based. The age-based recommendation applies to those aged 75 or older, who should receive a single RSV vaccine dose. If they have already received a dose under the old recommendation, they don’t need another one, at least for now.
The risk-based recommendation applies to adults from age 60 up to 75, but only for those with risk factors for severe RSV. These risk factors include lung disease, heart disease, immunocompromise, diabetes, obesity with a BMI of 40 or more, neurologic conditions, neuromuscular conditions, chronic kidney disease, liver disorders, hematologic disorders, frailty, and living in a nursing home or other long-term care facility. Those aged 60-75 with these risk factors should receive the RSV vaccine, and those without them should not receive it. The best time to get the RSV vaccine is late summer, but early fall administration with other adult vaccines is allowed and is acceptable.
Vaccine safety concerns were top of mind as ACIP members began their deliberations. Possible safety concerns for RSV vaccines have been detected for Guillain-Barré syndrome, atrial fibrillation, and idiopathic thrombocytopenic purpura. Safety surveillance updates are still interim and inconclusive. These signals still need further study and clarification.
Two RSV vaccines have been on the market: one by Pfizer, called Abrysvo, which does not contain an adjuvant; and another one by GSK, called Arexvy, which does contain an adjuvant. With the recent FDA approval of Moderna’s new mRNA RSV vaccine, mRESVIA, there are now three RSV vaccines licensed for those 60 or older. Arexvy is now FDA approved for adults in their 50s. That just happened in early June, but ACIP doesn’t currently recommend it for this fifty-something age group, even for those at high risk for severe RSV disease. This may change with greater clarification of potential vaccine safety concerns.
There is also news about protecting babies from RSV. RSV is the most common cause of hospitalization for infants in the United States, and most hospitalizations for RSV are in healthy, full-term infants. We now have two ways to protect babies: a dose of RSV vaccine given to mom, or a dose of the long-acting monoclonal antibody nirsevimab given to the baby. ACIP clarified that those who received a dose of maternal RSV vaccine during a previous pregnancy are not recommended to receive additional doses during future pregnancies, but infants born to those who were vaccinated for RSV during a prior pregnancy can receive nirsevimab, which is recommended for infants up to 8 months of age during their first RSV season, and for high-risk infants and toddlers aged 8-19 months during their second RSV season.
Last RSV season, supplies of nirsevimab were limited and doses had to be prioritized. No supply problems are anticipated for the upcoming season. A study published in March showed that nirsevimab was 90% effective at preventing RSV-associated hospitalization for infants in their first RSV season.
COVID
Here’s what’s new for COVID vaccines. A new-formula COVID vaccine will be ready for fall. ACIP voted unanimously to recommend a dose of the updated 2024-2025 COVID vaccine for everyone aged 6 months or older. This is a universal recommendation, just like the one we have for flu. But understand that even though COVID has waned, it’s still more deadly than flu. Most Americans now have some immunity against COVID, but this immunity wanes with time, and it also wanes as the virus keeps changing. These updated vaccines provide an incremental boost to our immunity for the new formula for fall. FDA has directed manufacturers to use a monovalent JN.1 lineage formula, with a preference for the KP.2 strain.
Older adults (aged 75 or older) and children under 6 months old are hit hardest by COVID. The littlest ones are too young to be vaccinated, but they can get protection from maternal vaccination. The uptake for last year’s COVID vaccine has been disappointing. Only 22.5% of adults and 14% of children received a dose of the updated shot. Focus-group discussions highlight the importance of a physician recommendation. Adults and children who receive a healthcare provider’s recommendation to get the COVID vaccine are more likely to get vaccinated.
Pneumococcal Vaccines
On June 17, 2024, a new pneumococcal vaccine, PCV21, was FDA approved for those aged 18 or older under an accelerated-approval pathway. ACIP voted to keep it simple and recommends PCV21 as an option for adults aged 19 or older who currently have an indication to receive a dose of PCV. This new PCV21 vaccine is indicated for prevention of both invasive pneumococcal disease (IPD) and pneumococcal pneumonia. Its brand name is Capvaxive and it’s made by Merck. IPD includes bacteremia, pneumonia, pneumococcal bacteremia, and meningitis.
There are two basic types of pneumococcal vaccines: polysaccharide vaccines (PPSV), which do not produce memory B cells; and PCV conjugate vaccines, which do trigger memory B-cell production and therefore induce greater long-term immunity. PCV21 covers 11 unique serotypes not in PCV20. This is important because many cases of adult disease are caused by subtypes not covered by other FDA-approved pneumococcal vaccines. PCV21 has greater coverage of the serotypes that cause invasive disease in adults as compared with PCV20. PCV20 covers up to 58% of those strains, while PCV21 covers up to 84% of strains responsible for invasive disease in adults. But there’s one serotype missing in PCV21, which may limit the groups who receive it. PCV21 does not cover serotype 4, a major cause of IPD in certain populations. Adults experiencing homelessness are 100-300 times more likely to develop IPD due to serotype 4. So are adults in Alaska, especially Alaska Natives. They have an 88-fold increase in serotype 4 invasive disease. Serotype 4 is covered by other pneumococcal vaccines, so for these patients, PCV20 is likely a better high-valent conjugate vaccine option than PCV21.
Flu Vaccines
What’s new for flu? Everyone aged 6 months or older needs a seasonal flu vaccination every year. That’s not new, but there are two new things coming this fall: (1) The seasonal flu vaccine is going trivalent. FDA has removed the Yamagata flu B strain because it no longer appears to be circulating. (2) ACIP made a special off-label recommendation to boost flu protection for solid organ transplant recipients ages 18-64 who are on immunosuppressive medications. These high-risk patients now have the off-label option of receiving one of the higher-dose flu vaccines, including high-dose and adjuvanted flu vaccines, which are FDA approved only for those 65 or older.
Sandra Adamson Fryhofer, Adjunct Clinical Associate Professor of Medicine, Emory University School of Medicine, Atlanta, Georgia, has disclosed the following relevant financial relationships: Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for American Medical Association; Medical Association of Atlanta; ACIP liaison. Received income in an amount equal to or greater than $250 from American College of Physicians; Medscape; American Medical Association.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
ACIP, the CDC’s Advisory Committee on Immunization Practices, met for 3 days in June. New vaccines and new recommendations for respiratory syncytial virus (RSV), flu, COVID, and a new pneumococcal vaccine were revealed.
RSV Protection
We’ll begin with RSV vaccines for adults aged 60 or older. For this group, shared clinical decision-making is out; it no longer applies. New, more specific recommendations from ACIP for RSV vaccines are both age based and risk based. The age-based recommendation applies to those aged 75 or older, who should receive a single RSV vaccine dose. If they have already received a dose under the old recommendation, they don’t need another one, at least for now.
The risk-based recommendation applies to adults from age 60 up to 75, but only for those with risk factors for severe RSV. These risk factors include lung disease, heart disease, immunocompromise, diabetes, obesity with a BMI of 40 or more, neurologic conditions, neuromuscular conditions, chronic kidney disease, liver disorders, hematologic disorders, frailty, and living in a nursing home or other long-term care facility. Those aged 60-75 with these risk factors should receive the RSV vaccine, and those without them should not receive it. The best time to get the RSV vaccine is late summer, but early fall administration with other adult vaccines is allowed and is acceptable.
Vaccine safety concerns were top of mind as ACIP members began their deliberations. Possible safety concerns for RSV vaccines have been detected for Guillain-Barré syndrome, atrial fibrillation, and idiopathic thrombocytopenic purpura. Safety surveillance updates are still interim and inconclusive. These signals still need further study and clarification.
Two RSV vaccines have been on the market: one by Pfizer, called Abrysvo, which does not contain an adjuvant; and another one by GSK, called Arexvy, which does contain an adjuvant. With the recent FDA approval of Moderna’s new mRNA RSV vaccine, mRESVIA, there are now three RSV vaccines licensed for those 60 or older. Arexvy is now FDA approved for adults in their 50s. That just happened in early June, but ACIP doesn’t currently recommend it for this fifty-something age group, even for those at high risk for severe RSV disease. This may change with greater clarification of potential vaccine safety concerns.
There is also news about protecting babies from RSV. RSV is the most common cause of hospitalization for infants in the United States, and most hospitalizations for RSV are in healthy, full-term infants. We now have two ways to protect babies: a dose of RSV vaccine given to mom, or a dose of the long-acting monoclonal antibody nirsevimab given to the baby. ACIP clarified that those who received a dose of maternal RSV vaccine during a previous pregnancy are not recommended to receive additional doses during future pregnancies, but infants born to those who were vaccinated for RSV during a prior pregnancy can receive nirsevimab, which is recommended for infants up to 8 months of age during their first RSV season, and for high-risk infants and toddlers aged 8-19 months during their second RSV season.
Last RSV season, supplies of nirsevimab were limited and doses had to be prioritized. No supply problems are anticipated for the upcoming season. A study published in March showed that nirsevimab was 90% effective at preventing RSV-associated hospitalization for infants in their first RSV season.
COVID
Here’s what’s new for COVID vaccines. A new-formula COVID vaccine will be ready for fall. ACIP voted unanimously to recommend a dose of the updated 2024-2025 COVID vaccine for everyone aged 6 months or older. This is a universal recommendation, just like the one we have for flu. But understand that even though COVID has waned, it’s still more deadly than flu. Most Americans now have some immunity against COVID, but this immunity wanes with time, and it also wanes as the virus keeps changing. These updated vaccines provide an incremental boost to our immunity for the new formula for fall. FDA has directed manufacturers to use a monovalent JN.1 lineage formula, with a preference for the KP.2 strain.
Older adults (aged 75 or older) and children under 6 months old are hit hardest by COVID. The littlest ones are too young to be vaccinated, but they can get protection from maternal vaccination. The uptake for last year’s COVID vaccine has been disappointing. Only 22.5% of adults and 14% of children received a dose of the updated shot. Focus-group discussions highlight the importance of a physician recommendation. Adults and children who receive a healthcare provider’s recommendation to get the COVID vaccine are more likely to get vaccinated.
Pneumococcal Vaccines
On June 17, 2024, a new pneumococcal vaccine, PCV21, was FDA approved for those aged 18 or older under an accelerated-approval pathway. ACIP voted to keep it simple and recommends PCV21 as an option for adults aged 19 or older who currently have an indication to receive a dose of PCV. This new PCV21 vaccine is indicated for prevention of both invasive pneumococcal disease (IPD) and pneumococcal pneumonia. Its brand name is Capvaxive and it’s made by Merck. IPD includes bacteremia, pneumonia, pneumococcal bacteremia, and meningitis.
There are two basic types of pneumococcal vaccines: polysaccharide vaccines (PPSV), which do not produce memory B cells; and PCV conjugate vaccines, which do trigger memory B-cell production and therefore induce greater long-term immunity. PCV21 covers 11 unique serotypes not in PCV20. This is important because many cases of adult disease are caused by subtypes not covered by other FDA-approved pneumococcal vaccines. PCV21 has greater coverage of the serotypes that cause invasive disease in adults as compared with PCV20. PCV20 covers up to 58% of those strains, while PCV21 covers up to 84% of strains responsible for invasive disease in adults. But there’s one serotype missing in PCV21, which may limit the groups who receive it. PCV21 does not cover serotype 4, a major cause of IPD in certain populations. Adults experiencing homelessness are 100-300 times more likely to develop IPD due to serotype 4. So are adults in Alaska, especially Alaska Natives. They have an 88-fold increase in serotype 4 invasive disease. Serotype 4 is covered by other pneumococcal vaccines, so for these patients, PCV20 is likely a better high-valent conjugate vaccine option than PCV21.
Flu Vaccines
What’s new for flu? Everyone aged 6 months or older needs a seasonal flu vaccination every year. That’s not new, but there are two new things coming this fall: (1) The seasonal flu vaccine is going trivalent. FDA has removed the Yamagata flu B strain because it no longer appears to be circulating. (2) ACIP made a special off-label recommendation to boost flu protection for solid organ transplant recipients ages 18-64 who are on immunosuppressive medications. These high-risk patients now have the off-label option of receiving one of the higher-dose flu vaccines, including high-dose and adjuvanted flu vaccines, which are FDA approved only for those 65 or older.
Sandra Adamson Fryhofer, Adjunct Clinical Associate Professor of Medicine, Emory University School of Medicine, Atlanta, Georgia, has disclosed the following relevant financial relationships: Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for American Medical Association; Medical Association of Atlanta; ACIP liaison. Received income in an amount equal to or greater than $250 from American College of Physicians; Medscape; American Medical Association.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
ACIP, the CDC’s Advisory Committee on Immunization Practices, met for 3 days in June. New vaccines and new recommendations for respiratory syncytial virus (RSV), flu, COVID, and a new pneumococcal vaccine were revealed.
RSV Protection
We’ll begin with RSV vaccines for adults aged 60 or older. For this group, shared clinical decision-making is out; it no longer applies. New, more specific recommendations from ACIP for RSV vaccines are both age based and risk based. The age-based recommendation applies to those aged 75 or older, who should receive a single RSV vaccine dose. If they have already received a dose under the old recommendation, they don’t need another one, at least for now.
The risk-based recommendation applies to adults from age 60 up to 75, but only for those with risk factors for severe RSV. These risk factors include lung disease, heart disease, immunocompromise, diabetes, obesity with a BMI of 40 or more, neurologic conditions, neuromuscular conditions, chronic kidney disease, liver disorders, hematologic disorders, frailty, and living in a nursing home or other long-term care facility. Those aged 60-75 with these risk factors should receive the RSV vaccine, and those without them should not receive it. The best time to get the RSV vaccine is late summer, but early fall administration with other adult vaccines is allowed and is acceptable.
Vaccine safety concerns were top of mind as ACIP members began their deliberations. Possible safety concerns for RSV vaccines have been detected for Guillain-Barré syndrome, atrial fibrillation, and idiopathic thrombocytopenic purpura. Safety surveillance updates are still interim and inconclusive. These signals still need further study and clarification.
Two RSV vaccines have been on the market: one by Pfizer, called Abrysvo, which does not contain an adjuvant; and another one by GSK, called Arexvy, which does contain an adjuvant. With the recent FDA approval of Moderna’s new mRNA RSV vaccine, mRESVIA, there are now three RSV vaccines licensed for those 60 or older. Arexvy is now FDA approved for adults in their 50s. That just happened in early June, but ACIP doesn’t currently recommend it for this fifty-something age group, even for those at high risk for severe RSV disease. This may change with greater clarification of potential vaccine safety concerns.
There is also news about protecting babies from RSV. RSV is the most common cause of hospitalization for infants in the United States, and most hospitalizations for RSV are in healthy, full-term infants. We now have two ways to protect babies: a dose of RSV vaccine given to mom, or a dose of the long-acting monoclonal antibody nirsevimab given to the baby. ACIP clarified that those who received a dose of maternal RSV vaccine during a previous pregnancy are not recommended to receive additional doses during future pregnancies, but infants born to those who were vaccinated for RSV during a prior pregnancy can receive nirsevimab, which is recommended for infants up to 8 months of age during their first RSV season, and for high-risk infants and toddlers aged 8-19 months during their second RSV season.
Last RSV season, supplies of nirsevimab were limited and doses had to be prioritized. No supply problems are anticipated for the upcoming season. A study published in March showed that nirsevimab was 90% effective at preventing RSV-associated hospitalization for infants in their first RSV season.
COVID
Here’s what’s new for COVID vaccines. A new-formula COVID vaccine will be ready for fall. ACIP voted unanimously to recommend a dose of the updated 2024-2025 COVID vaccine for everyone aged 6 months or older. This is a universal recommendation, just like the one we have for flu. But understand that even though COVID has waned, it’s still more deadly than flu. Most Americans now have some immunity against COVID, but this immunity wanes with time, and it also wanes as the virus keeps changing. These updated vaccines provide an incremental boost to our immunity for the new formula for fall. FDA has directed manufacturers to use a monovalent JN.1 lineage formula, with a preference for the KP.2 strain.
Older adults (aged 75 or older) and children under 6 months old are hit hardest by COVID. The littlest ones are too young to be vaccinated, but they can get protection from maternal vaccination. The uptake for last year’s COVID vaccine has been disappointing. Only 22.5% of adults and 14% of children received a dose of the updated shot. Focus-group discussions highlight the importance of a physician recommendation. Adults and children who receive a healthcare provider’s recommendation to get the COVID vaccine are more likely to get vaccinated.
Pneumococcal Vaccines
On June 17, 2024, a new pneumococcal vaccine, PCV21, was FDA approved for those aged 18 or older under an accelerated-approval pathway. ACIP voted to keep it simple and recommends PCV21 as an option for adults aged 19 or older who currently have an indication to receive a dose of PCV. This new PCV21 vaccine is indicated for prevention of both invasive pneumococcal disease (IPD) and pneumococcal pneumonia. Its brand name is Capvaxive and it’s made by Merck. IPD includes bacteremia, pneumonia, pneumococcal bacteremia, and meningitis.
There are two basic types of pneumococcal vaccines: polysaccharide vaccines (PPSV), which do not produce memory B cells; and PCV conjugate vaccines, which do trigger memory B-cell production and therefore induce greater long-term immunity. PCV21 covers 11 unique serotypes not in PCV20. This is important because many cases of adult disease are caused by subtypes not covered by other FDA-approved pneumococcal vaccines. PCV21 has greater coverage of the serotypes that cause invasive disease in adults as compared with PCV20. PCV20 covers up to 58% of those strains, while PCV21 covers up to 84% of strains responsible for invasive disease in adults. But there’s one serotype missing in PCV21, which may limit the groups who receive it. PCV21 does not cover serotype 4, a major cause of IPD in certain populations. Adults experiencing homelessness are 100-300 times more likely to develop IPD due to serotype 4. So are adults in Alaska, especially Alaska Natives. They have an 88-fold increase in serotype 4 invasive disease. Serotype 4 is covered by other pneumococcal vaccines, so for these patients, PCV20 is likely a better high-valent conjugate vaccine option than PCV21.
Flu Vaccines
What’s new for flu? Everyone aged 6 months or older needs a seasonal flu vaccination every year. That’s not new, but there are two new things coming this fall: (1) The seasonal flu vaccine is going trivalent. FDA has removed the Yamagata flu B strain because it no longer appears to be circulating. (2) ACIP made a special off-label recommendation to boost flu protection for solid organ transplant recipients ages 18-64 who are on immunosuppressive medications. These high-risk patients now have the off-label option of receiving one of the higher-dose flu vaccines, including high-dose and adjuvanted flu vaccines, which are FDA approved only for those 65 or older.
Sandra Adamson Fryhofer, Adjunct Clinical Associate Professor of Medicine, Emory University School of Medicine, Atlanta, Georgia, has disclosed the following relevant financial relationships: Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for American Medical Association; Medical Association of Atlanta; ACIP liaison. Received income in an amount equal to or greater than $250 from American College of Physicians; Medscape; American Medical Association.
A version of this article first appeared on Medscape.com.
Summer Is Not Over: Let's Talk About Recreational Water–Associated Illnesses
Recently I was in Wyoming. As I rode down the Snake River, the guide pointed out tree trunks that had been chewed on by beavers. Days later I joined a local friend for a hike to Taggart Lake. Upon reaching the end of the trail as I began to cast my eyes on the magnificent scenery, I could not help but notice several children, including toddlers, playing in the fresh warm water. The next thing out of my friend’s mouth was “You know there is Giardia in there.” Little did she know, she and the guide had just helped me select a topic for ID Consult.
Giardia, aka ”beaver fever,” was discussed in detail in this column as part of the differential of a diarrheal illness by Christopher J. Harrison, MD. However, it is the perfect time of year to revisit other recreational water–associated illnesses.
Infections acquired during recreational water activity can lead to illnesses involving the gastrointestinal tract, central nervous system, respiratory tract, skin, eyes, and ears. Pathogens, chemicals, and toxins are transmitted by ingestion, contact with contaminated water or a sick individual or animal, and inhalation of aerosols. The National Waterborne Disease and Outbreak Surveillance System (WBDOSS) collects data on waterborne disease and outbreaks associated with recreational water, drinking water, and environmental and undetermined exposures to water. All reporting to the Centers for Disease Control and Prevention (CDC) is voluntary. However, mandatory pathogen reporting requirements can vary by state. Ideally, once an agency has completed the outbreak investigation, the definitive cause and source will be determined, and interventions to prevent future outbreaks implemented.
Treated Versus Untreated Water
One useful way to help narrow the etiology of a patient’s symptoms is to consider those illnesses associated with treated water venues (e.g., pools, hot tubs, water parks) versus untreated water venues (e.g., rivers, lakes, oceans). Parents may forget to offer that information since they may not perceive a connection between water exposure and the illness, especially if they traveled within the US.
In 2021, the CDC reported results of data submitted between 2015 and 2019 from treated recreational water facilities. Of the 208 outbreaks, most (96%) were associated with public pools, hot tubs, or water playgrounds. These outbreaks resulted in at least 3,646 cases of illness, 286 hospitalizations, and 13 deaths. Overall infectious etiologies were the primary cause of illness. Of the 155 outbreaks with a confirmed etiology, Cryptosporidium was the causative pathogen in 49% of the outbreaks and accounted for 84% (2,492) of cases, while Legionella caused 42% of outbreaks, accounted for 13% (354) of cases, and was responsible for all 13 deaths. Slightly more than half (107 of 208) of the outbreaks started between June-August with Cryptosporidium accounting for 63 of the outbreaks during that period. A little more than one-third were associated with a hotel or resort. The majority of hotel recreational water–associated illnesses was associated with hot tubs. Of the 53 outbreaks without a confirmed etiology, 20 were suspected to have a chemical related etiology (excess chlorine, altered pool chemistry).
In contrast, there were 140 untreated recreational water outbreaks reported between 2000 and 2014 from 35 states and Guam involving 4,958 cases and 2 deaths. The etiology was confirmed for 103 (74%) outbreaks including 5 that had multiple etiologies and 8 due to toxins or chemicals; 7 of 8 toxins were from harmful algal blooms. Enteric pathogens were the etiology in 84% of outbreaks including: Norovirus (n = 1459), Shigella (n = 362) Avian schistosomes (n = 345), Cryptosporidium (n = 314) and Escherichia coli (n = 155).There were 24 cases of Giardia. The two deaths were due to Naegleria fowleri. The top 2 settings for these outbreaks were public parks (36%) and beaches (32%) with most outbreaks (n = 117) being associated with a lake /pond venue. Most outbreaks began between June and August.
The major differences between the two types of recreational water–associated illnesses are their most common settings and etiologies. With that in mind, let us briefly review the most common etiology from each venue.
Treated Water Venue: Cryptosporidiosis
Cryptosporidium is an oocyst-forming protozoa that causes a self-limited watery, nonbloody diarrhea which usually resolves within 10-14 days. Most patients have associated abdominal cramps, fever, and vomiting although infected persons can be asymptomatic. Infection in the immunocompromised potentially can lead to profuse and prolonged diarrhea. Oocysts are excreted in the feces of infected hosts and as little as 10 can cause infection. They can survive extreme environmental conditions in water and soil for several months and even survive up to 7 days in a properly chlorinated pool. Transmission occurs between humans via contaminated food and water or from infected animals. Oocysts have been isolated in raw or unpasteurized milk and apple cider. Incidence is highest in children 1 through 4 years of age.
Diagnosis today is usually via molecular methods (nucleic acid amplification tests, aka NAATs), due to their high sensitivity and specificity and is the preferred method. These tests can identify multiple gastrointestinal tract pathogens with a single assay. Diagnosis by microscopy or fecal immunoassay antigens are still available. Treatment is supportive in most cases. If needed, a 3-day course of nitazoxanide can be prescribed. Immunocompromised patients should be managed in consultation with an infectious disease specialist.
Untreated Water Venue: Norovirus
Norovirus is a viral illness characterized by the abrupt onset of vomiting and/or watery diarrhea, usually associated with nausea and abdominal cramps. Symptoms persist 24-72 hours, however they may be prolonged in the immunocompromised and persons at the extremes of the age spectrum. Norovirus has replaced rotavirus as the major cause of medically attended gastroenteritis. While a major cause of recreational water–associated illnesses, high attack rates also occur in semi closed communities including cruise ships, childcare centers, and schools. Transmission is fecal-oral, vomitus oral, person to person, by ingestion of contaminated food and water or touching contaminated surfaces with subsequent touching of the mouth. Asymptomatic viral shedding may occur, especially in children. Prolonged shedding (> 6 mos.) has been reported in immunocompromised hosts.
Molecular diagnosis with stool is utilized most often. Treatment is supportive.
Take Home Message
When evaluating your patients for an acute gastrointestinal illness, consider water-related activities and their potential for being the source. Encourage patients not to ignore posted advisories on beaches, to not swim if they have diarrhea, not to swallow the water they swim in and to minimize water entering their nose while swimming in warm freshwater. If you start seeing several patients with similar symptoms and/or etiology, consider contacting your local or state health department. It could be the beginning of an outbreak.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She has no relevant financial disclosures.
Suggested Readings
Graciaa DS et al. Outbreaks Associated with Untreated Recreational Water — United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2018 Jun 29;67(25):701-706. doi: 10.15585/mmwr.mm6725a1.
Hlavsa MC et al. Outbreaks Associated with Treated Recreational Water — United States, 2015–2019. MMWR Morb Mortal Wkly Rep. 2021;70:733–738. doi: 10.15585/mmwr.mm7020a1.
Kimberlin DW et al., eds. Red Book Report of the Committee on Infectious Diseases. 33rd ed. American Academy of Pediatrics. 2024. Cryptosporidiosis, p 338-40 and Norovirus, p 622-624.Waterborne Outbreaks Summary Reports. CDC. 2024 April 18.
Recently I was in Wyoming. As I rode down the Snake River, the guide pointed out tree trunks that had been chewed on by beavers. Days later I joined a local friend for a hike to Taggart Lake. Upon reaching the end of the trail as I began to cast my eyes on the magnificent scenery, I could not help but notice several children, including toddlers, playing in the fresh warm water. The next thing out of my friend’s mouth was “You know there is Giardia in there.” Little did she know, she and the guide had just helped me select a topic for ID Consult.
Giardia, aka ”beaver fever,” was discussed in detail in this column as part of the differential of a diarrheal illness by Christopher J. Harrison, MD. However, it is the perfect time of year to revisit other recreational water–associated illnesses.
Infections acquired during recreational water activity can lead to illnesses involving the gastrointestinal tract, central nervous system, respiratory tract, skin, eyes, and ears. Pathogens, chemicals, and toxins are transmitted by ingestion, contact with contaminated water or a sick individual or animal, and inhalation of aerosols. The National Waterborne Disease and Outbreak Surveillance System (WBDOSS) collects data on waterborne disease and outbreaks associated with recreational water, drinking water, and environmental and undetermined exposures to water. All reporting to the Centers for Disease Control and Prevention (CDC) is voluntary. However, mandatory pathogen reporting requirements can vary by state. Ideally, once an agency has completed the outbreak investigation, the definitive cause and source will be determined, and interventions to prevent future outbreaks implemented.
Treated Versus Untreated Water
One useful way to help narrow the etiology of a patient’s symptoms is to consider those illnesses associated with treated water venues (e.g., pools, hot tubs, water parks) versus untreated water venues (e.g., rivers, lakes, oceans). Parents may forget to offer that information since they may not perceive a connection between water exposure and the illness, especially if they traveled within the US.
In 2021, the CDC reported results of data submitted between 2015 and 2019 from treated recreational water facilities. Of the 208 outbreaks, most (96%) were associated with public pools, hot tubs, or water playgrounds. These outbreaks resulted in at least 3,646 cases of illness, 286 hospitalizations, and 13 deaths. Overall infectious etiologies were the primary cause of illness. Of the 155 outbreaks with a confirmed etiology, Cryptosporidium was the causative pathogen in 49% of the outbreaks and accounted for 84% (2,492) of cases, while Legionella caused 42% of outbreaks, accounted for 13% (354) of cases, and was responsible for all 13 deaths. Slightly more than half (107 of 208) of the outbreaks started between June-August with Cryptosporidium accounting for 63 of the outbreaks during that period. A little more than one-third were associated with a hotel or resort. The majority of hotel recreational water–associated illnesses was associated with hot tubs. Of the 53 outbreaks without a confirmed etiology, 20 were suspected to have a chemical related etiology (excess chlorine, altered pool chemistry).
In contrast, there were 140 untreated recreational water outbreaks reported between 2000 and 2014 from 35 states and Guam involving 4,958 cases and 2 deaths. The etiology was confirmed for 103 (74%) outbreaks including 5 that had multiple etiologies and 8 due to toxins or chemicals; 7 of 8 toxins were from harmful algal blooms. Enteric pathogens were the etiology in 84% of outbreaks including: Norovirus (n = 1459), Shigella (n = 362) Avian schistosomes (n = 345), Cryptosporidium (n = 314) and Escherichia coli (n = 155).There were 24 cases of Giardia. The two deaths were due to Naegleria fowleri. The top 2 settings for these outbreaks were public parks (36%) and beaches (32%) with most outbreaks (n = 117) being associated with a lake /pond venue. Most outbreaks began between June and August.
The major differences between the two types of recreational water–associated illnesses are their most common settings and etiologies. With that in mind, let us briefly review the most common etiology from each venue.
Treated Water Venue: Cryptosporidiosis
Cryptosporidium is an oocyst-forming protozoa that causes a self-limited watery, nonbloody diarrhea which usually resolves within 10-14 days. Most patients have associated abdominal cramps, fever, and vomiting although infected persons can be asymptomatic. Infection in the immunocompromised potentially can lead to profuse and prolonged diarrhea. Oocysts are excreted in the feces of infected hosts and as little as 10 can cause infection. They can survive extreme environmental conditions in water and soil for several months and even survive up to 7 days in a properly chlorinated pool. Transmission occurs between humans via contaminated food and water or from infected animals. Oocysts have been isolated in raw or unpasteurized milk and apple cider. Incidence is highest in children 1 through 4 years of age.
Diagnosis today is usually via molecular methods (nucleic acid amplification tests, aka NAATs), due to their high sensitivity and specificity and is the preferred method. These tests can identify multiple gastrointestinal tract pathogens with a single assay. Diagnosis by microscopy or fecal immunoassay antigens are still available. Treatment is supportive in most cases. If needed, a 3-day course of nitazoxanide can be prescribed. Immunocompromised patients should be managed in consultation with an infectious disease specialist.
Untreated Water Venue: Norovirus
Norovirus is a viral illness characterized by the abrupt onset of vomiting and/or watery diarrhea, usually associated with nausea and abdominal cramps. Symptoms persist 24-72 hours, however they may be prolonged in the immunocompromised and persons at the extremes of the age spectrum. Norovirus has replaced rotavirus as the major cause of medically attended gastroenteritis. While a major cause of recreational water–associated illnesses, high attack rates also occur in semi closed communities including cruise ships, childcare centers, and schools. Transmission is fecal-oral, vomitus oral, person to person, by ingestion of contaminated food and water or touching contaminated surfaces with subsequent touching of the mouth. Asymptomatic viral shedding may occur, especially in children. Prolonged shedding (> 6 mos.) has been reported in immunocompromised hosts.
Molecular diagnosis with stool is utilized most often. Treatment is supportive.
Take Home Message
When evaluating your patients for an acute gastrointestinal illness, consider water-related activities and their potential for being the source. Encourage patients not to ignore posted advisories on beaches, to not swim if they have diarrhea, not to swallow the water they swim in and to minimize water entering their nose while swimming in warm freshwater. If you start seeing several patients with similar symptoms and/or etiology, consider contacting your local or state health department. It could be the beginning of an outbreak.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She has no relevant financial disclosures.
Suggested Readings
Graciaa DS et al. Outbreaks Associated with Untreated Recreational Water — United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2018 Jun 29;67(25):701-706. doi: 10.15585/mmwr.mm6725a1.
Hlavsa MC et al. Outbreaks Associated with Treated Recreational Water — United States, 2015–2019. MMWR Morb Mortal Wkly Rep. 2021;70:733–738. doi: 10.15585/mmwr.mm7020a1.
Kimberlin DW et al., eds. Red Book Report of the Committee on Infectious Diseases. 33rd ed. American Academy of Pediatrics. 2024. Cryptosporidiosis, p 338-40 and Norovirus, p 622-624.Waterborne Outbreaks Summary Reports. CDC. 2024 April 18.
Recently I was in Wyoming. As I rode down the Snake River, the guide pointed out tree trunks that had been chewed on by beavers. Days later I joined a local friend for a hike to Taggart Lake. Upon reaching the end of the trail as I began to cast my eyes on the magnificent scenery, I could not help but notice several children, including toddlers, playing in the fresh warm water. The next thing out of my friend’s mouth was “You know there is Giardia in there.” Little did she know, she and the guide had just helped me select a topic for ID Consult.
Giardia, aka ”beaver fever,” was discussed in detail in this column as part of the differential of a diarrheal illness by Christopher J. Harrison, MD. However, it is the perfect time of year to revisit other recreational water–associated illnesses.
Infections acquired during recreational water activity can lead to illnesses involving the gastrointestinal tract, central nervous system, respiratory tract, skin, eyes, and ears. Pathogens, chemicals, and toxins are transmitted by ingestion, contact with contaminated water or a sick individual or animal, and inhalation of aerosols. The National Waterborne Disease and Outbreak Surveillance System (WBDOSS) collects data on waterborne disease and outbreaks associated with recreational water, drinking water, and environmental and undetermined exposures to water. All reporting to the Centers for Disease Control and Prevention (CDC) is voluntary. However, mandatory pathogen reporting requirements can vary by state. Ideally, once an agency has completed the outbreak investigation, the definitive cause and source will be determined, and interventions to prevent future outbreaks implemented.
Treated Versus Untreated Water
One useful way to help narrow the etiology of a patient’s symptoms is to consider those illnesses associated with treated water venues (e.g., pools, hot tubs, water parks) versus untreated water venues (e.g., rivers, lakes, oceans). Parents may forget to offer that information since they may not perceive a connection between water exposure and the illness, especially if they traveled within the US.
In 2021, the CDC reported results of data submitted between 2015 and 2019 from treated recreational water facilities. Of the 208 outbreaks, most (96%) were associated with public pools, hot tubs, or water playgrounds. These outbreaks resulted in at least 3,646 cases of illness, 286 hospitalizations, and 13 deaths. Overall infectious etiologies were the primary cause of illness. Of the 155 outbreaks with a confirmed etiology, Cryptosporidium was the causative pathogen in 49% of the outbreaks and accounted for 84% (2,492) of cases, while Legionella caused 42% of outbreaks, accounted for 13% (354) of cases, and was responsible for all 13 deaths. Slightly more than half (107 of 208) of the outbreaks started between June-August with Cryptosporidium accounting for 63 of the outbreaks during that period. A little more than one-third were associated with a hotel or resort. The majority of hotel recreational water–associated illnesses was associated with hot tubs. Of the 53 outbreaks without a confirmed etiology, 20 were suspected to have a chemical related etiology (excess chlorine, altered pool chemistry).
In contrast, there were 140 untreated recreational water outbreaks reported between 2000 and 2014 from 35 states and Guam involving 4,958 cases and 2 deaths. The etiology was confirmed for 103 (74%) outbreaks including 5 that had multiple etiologies and 8 due to toxins or chemicals; 7 of 8 toxins were from harmful algal blooms. Enteric pathogens were the etiology in 84% of outbreaks including: Norovirus (n = 1459), Shigella (n = 362) Avian schistosomes (n = 345), Cryptosporidium (n = 314) and Escherichia coli (n = 155).There were 24 cases of Giardia. The two deaths were due to Naegleria fowleri. The top 2 settings for these outbreaks were public parks (36%) and beaches (32%) with most outbreaks (n = 117) being associated with a lake /pond venue. Most outbreaks began between June and August.
The major differences between the two types of recreational water–associated illnesses are their most common settings and etiologies. With that in mind, let us briefly review the most common etiology from each venue.
Treated Water Venue: Cryptosporidiosis
Cryptosporidium is an oocyst-forming protozoa that causes a self-limited watery, nonbloody diarrhea which usually resolves within 10-14 days. Most patients have associated abdominal cramps, fever, and vomiting although infected persons can be asymptomatic. Infection in the immunocompromised potentially can lead to profuse and prolonged diarrhea. Oocysts are excreted in the feces of infected hosts and as little as 10 can cause infection. They can survive extreme environmental conditions in water and soil for several months and even survive up to 7 days in a properly chlorinated pool. Transmission occurs between humans via contaminated food and water or from infected animals. Oocysts have been isolated in raw or unpasteurized milk and apple cider. Incidence is highest in children 1 through 4 years of age.
Diagnosis today is usually via molecular methods (nucleic acid amplification tests, aka NAATs), due to their high sensitivity and specificity and is the preferred method. These tests can identify multiple gastrointestinal tract pathogens with a single assay. Diagnosis by microscopy or fecal immunoassay antigens are still available. Treatment is supportive in most cases. If needed, a 3-day course of nitazoxanide can be prescribed. Immunocompromised patients should be managed in consultation with an infectious disease specialist.
Untreated Water Venue: Norovirus
Norovirus is a viral illness characterized by the abrupt onset of vomiting and/or watery diarrhea, usually associated with nausea and abdominal cramps. Symptoms persist 24-72 hours, however they may be prolonged in the immunocompromised and persons at the extremes of the age spectrum. Norovirus has replaced rotavirus as the major cause of medically attended gastroenteritis. While a major cause of recreational water–associated illnesses, high attack rates also occur in semi closed communities including cruise ships, childcare centers, and schools. Transmission is fecal-oral, vomitus oral, person to person, by ingestion of contaminated food and water or touching contaminated surfaces with subsequent touching of the mouth. Asymptomatic viral shedding may occur, especially in children. Prolonged shedding (> 6 mos.) has been reported in immunocompromised hosts.
Molecular diagnosis with stool is utilized most often. Treatment is supportive.
Take Home Message
When evaluating your patients for an acute gastrointestinal illness, consider water-related activities and their potential for being the source. Encourage patients not to ignore posted advisories on beaches, to not swim if they have diarrhea, not to swallow the water they swim in and to minimize water entering their nose while swimming in warm freshwater. If you start seeing several patients with similar symptoms and/or etiology, consider contacting your local or state health department. It could be the beginning of an outbreak.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She has no relevant financial disclosures.
Suggested Readings
Graciaa DS et al. Outbreaks Associated with Untreated Recreational Water — United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2018 Jun 29;67(25):701-706. doi: 10.15585/mmwr.mm6725a1.
Hlavsa MC et al. Outbreaks Associated with Treated Recreational Water — United States, 2015–2019. MMWR Morb Mortal Wkly Rep. 2021;70:733–738. doi: 10.15585/mmwr.mm7020a1.
Kimberlin DW et al., eds. Red Book Report of the Committee on Infectious Diseases. 33rd ed. American Academy of Pediatrics. 2024. Cryptosporidiosis, p 338-40 and Norovirus, p 622-624.Waterborne Outbreaks Summary Reports. CDC. 2024 April 18.