User login
Sensory comeback: New findings show the path to smell and taste recovery after COVID
Good news for people struggling with sensory problems after a bout of COVID-19. Although mild cases of the disease often impair the ability to taste and smell, and the problem can drag on for months, a new study from Italy shows that most people return to their senses, as it were, within 3 years.
said Paolo Boscolo-Rizzo, MD, a professor of medicine, surgery, and health sciences at the University of Trieste (Italy), and a co-author of the study, published as a research letter in JAMA Otolaryngology–Head & Neck Surgery.
Dr. Boscolo-Rizzo and his colleagues analyzed data from 88 adults with mild COVID-19, which was defined as having no lower respiratory disease and blood oxygen saturation of 94% or greater. Another group of 88 adults who never contracted the virus but sometimes had difficulties with smell and taste were also studied. In both groups, the average age was 49 years, all participants were White, and 58% were women.
The researchers tested participants’ sense of smell with sticks that contained different odors and checked their sense of taste with strips that had different tastes. Over time, fewer people had difficulty distinguishing odors. Three years after developing COVID-19, only 12 people had impaired smell, compared with 36 people at year 1 and 24 people at year 2. And at the 3-year mark, all participants had at least a partial ability to smell.
The story was similar with sense of taste, with 10 of 88 people reporting impairments 3 years later. By then, people with COVID-19 were no more likely to have trouble with smell or taste than people who did not get the virus.
A study this past June showed a strong correlation between severity of COVID-19 symptoms and impaired sense of taste and smell and estimated that millions of Americans maintained altered senses. More than 10% of people in the Italian study still had trouble with smell or taste 3 years later.
Emerging treatments, psychological concerns
“We’re seeing fewer people with this problem, but there are still people suffering from it,” said Fernando Carnavali, MD, an internal medicine physician and a site director for the Center for Post-COVID Care at the Icahn School of Medicine at Mount Sinai, New York City.
Dr. Carnavali wasn’t part of this study, but he did find the new results encouraging, and he called for similar studies in diverse populations that have experienced COVID-19. He also noted that an impaired sense of smell is distressing.
“It really has a significant psychological impact,” Dr. Carnavali said.
He recalled a patient crying in his office because her inability to smell made it impossible for her to cook. Dr. Carnavali recommended clinicians refer patients facing protracted loss of smell or taste to mental health professionals for support.
Treatments are emerging for COVID-19 smell loss. One approach is to inject platelet-rich plasma into a patient’s nasal cavities to help neurons related to smell repair themselves.
A randomized trial showed platelet-rich plasma significantly outperformed placebo in patients with smell loss up to a year after getting COVID-19.
“I wish more people would do it,” said Zara Patel, MD, an otolaryngologist at Stanford (Calif.) Medicine, who helped conduct that trial. She said some physicians may be nervous about injecting plasma so close to the skull and are therefore hesitant to try this approach.
Another technique may help to address the olfactory condition known as parosmia, in which patients generally experience a benign odor as rancid, according to otolaryngologist Nyssa Farrell, MD, of Washington University School of Medicine, St. Louis. Dr. Farrell said around two-thirds of patients who contract COVID-19 develop the condition, and the rates of long-term parosmia range from 10%-50% depending on various studies.
“It is almost always foul; this can profoundly affect someone’s quality of life,” impairing their ability to eat or to be intimate with a partner who now smells unpleasant, said Dr. Farrell, who wasn’t associated with this research.
The treatment, called a stellate ganglion block, is provided through a shot into nerves in the neck. People with parosmia associated with COVID-19 often report that this method cures them. Dr. Patel said that may be because their psychological health is improving, not their sense of smell, because the area of the body where the stellate ganglion block is applied is not part of the olfactory system.
Earlier this year, Dr. Farrell and colleagues reported that parosmia linked to COVID-19 is associated with an increased risk for depression, anxiety, and suicidal ideation.
One coauthor reported receiving grants from Smell and Taste Lab, Takasago, Baia Foods, and Frequency Therapeutics. The other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Good news for people struggling with sensory problems after a bout of COVID-19. Although mild cases of the disease often impair the ability to taste and smell, and the problem can drag on for months, a new study from Italy shows that most people return to their senses, as it were, within 3 years.
said Paolo Boscolo-Rizzo, MD, a professor of medicine, surgery, and health sciences at the University of Trieste (Italy), and a co-author of the study, published as a research letter in JAMA Otolaryngology–Head & Neck Surgery.
Dr. Boscolo-Rizzo and his colleagues analyzed data from 88 adults with mild COVID-19, which was defined as having no lower respiratory disease and blood oxygen saturation of 94% or greater. Another group of 88 adults who never contracted the virus but sometimes had difficulties with smell and taste were also studied. In both groups, the average age was 49 years, all participants were White, and 58% were women.
The researchers tested participants’ sense of smell with sticks that contained different odors and checked their sense of taste with strips that had different tastes. Over time, fewer people had difficulty distinguishing odors. Three years after developing COVID-19, only 12 people had impaired smell, compared with 36 people at year 1 and 24 people at year 2. And at the 3-year mark, all participants had at least a partial ability to smell.
The story was similar with sense of taste, with 10 of 88 people reporting impairments 3 years later. By then, people with COVID-19 were no more likely to have trouble with smell or taste than people who did not get the virus.
A study this past June showed a strong correlation between severity of COVID-19 symptoms and impaired sense of taste and smell and estimated that millions of Americans maintained altered senses. More than 10% of people in the Italian study still had trouble with smell or taste 3 years later.
Emerging treatments, psychological concerns
“We’re seeing fewer people with this problem, but there are still people suffering from it,” said Fernando Carnavali, MD, an internal medicine physician and a site director for the Center for Post-COVID Care at the Icahn School of Medicine at Mount Sinai, New York City.
Dr. Carnavali wasn’t part of this study, but he did find the new results encouraging, and he called for similar studies in diverse populations that have experienced COVID-19. He also noted that an impaired sense of smell is distressing.
“It really has a significant psychological impact,” Dr. Carnavali said.
He recalled a patient crying in his office because her inability to smell made it impossible for her to cook. Dr. Carnavali recommended clinicians refer patients facing protracted loss of smell or taste to mental health professionals for support.
Treatments are emerging for COVID-19 smell loss. One approach is to inject platelet-rich plasma into a patient’s nasal cavities to help neurons related to smell repair themselves.
A randomized trial showed platelet-rich plasma significantly outperformed placebo in patients with smell loss up to a year after getting COVID-19.
“I wish more people would do it,” said Zara Patel, MD, an otolaryngologist at Stanford (Calif.) Medicine, who helped conduct that trial. She said some physicians may be nervous about injecting plasma so close to the skull and are therefore hesitant to try this approach.
Another technique may help to address the olfactory condition known as parosmia, in which patients generally experience a benign odor as rancid, according to otolaryngologist Nyssa Farrell, MD, of Washington University School of Medicine, St. Louis. Dr. Farrell said around two-thirds of patients who contract COVID-19 develop the condition, and the rates of long-term parosmia range from 10%-50% depending on various studies.
“It is almost always foul; this can profoundly affect someone’s quality of life,” impairing their ability to eat or to be intimate with a partner who now smells unpleasant, said Dr. Farrell, who wasn’t associated with this research.
The treatment, called a stellate ganglion block, is provided through a shot into nerves in the neck. People with parosmia associated with COVID-19 often report that this method cures them. Dr. Patel said that may be because their psychological health is improving, not their sense of smell, because the area of the body where the stellate ganglion block is applied is not part of the olfactory system.
Earlier this year, Dr. Farrell and colleagues reported that parosmia linked to COVID-19 is associated with an increased risk for depression, anxiety, and suicidal ideation.
One coauthor reported receiving grants from Smell and Taste Lab, Takasago, Baia Foods, and Frequency Therapeutics. The other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Good news for people struggling with sensory problems after a bout of COVID-19. Although mild cases of the disease often impair the ability to taste and smell, and the problem can drag on for months, a new study from Italy shows that most people return to their senses, as it were, within 3 years.
said Paolo Boscolo-Rizzo, MD, a professor of medicine, surgery, and health sciences at the University of Trieste (Italy), and a co-author of the study, published as a research letter in JAMA Otolaryngology–Head & Neck Surgery.
Dr. Boscolo-Rizzo and his colleagues analyzed data from 88 adults with mild COVID-19, which was defined as having no lower respiratory disease and blood oxygen saturation of 94% or greater. Another group of 88 adults who never contracted the virus but sometimes had difficulties with smell and taste were also studied. In both groups, the average age was 49 years, all participants were White, and 58% were women.
The researchers tested participants’ sense of smell with sticks that contained different odors and checked their sense of taste with strips that had different tastes. Over time, fewer people had difficulty distinguishing odors. Three years after developing COVID-19, only 12 people had impaired smell, compared with 36 people at year 1 and 24 people at year 2. And at the 3-year mark, all participants had at least a partial ability to smell.
The story was similar with sense of taste, with 10 of 88 people reporting impairments 3 years later. By then, people with COVID-19 were no more likely to have trouble with smell or taste than people who did not get the virus.
A study this past June showed a strong correlation between severity of COVID-19 symptoms and impaired sense of taste and smell and estimated that millions of Americans maintained altered senses. More than 10% of people in the Italian study still had trouble with smell or taste 3 years later.
Emerging treatments, psychological concerns
“We’re seeing fewer people with this problem, but there are still people suffering from it,” said Fernando Carnavali, MD, an internal medicine physician and a site director for the Center for Post-COVID Care at the Icahn School of Medicine at Mount Sinai, New York City.
Dr. Carnavali wasn’t part of this study, but he did find the new results encouraging, and he called for similar studies in diverse populations that have experienced COVID-19. He also noted that an impaired sense of smell is distressing.
“It really has a significant psychological impact,” Dr. Carnavali said.
He recalled a patient crying in his office because her inability to smell made it impossible for her to cook. Dr. Carnavali recommended clinicians refer patients facing protracted loss of smell or taste to mental health professionals for support.
Treatments are emerging for COVID-19 smell loss. One approach is to inject platelet-rich plasma into a patient’s nasal cavities to help neurons related to smell repair themselves.
A randomized trial showed platelet-rich plasma significantly outperformed placebo in patients with smell loss up to a year after getting COVID-19.
“I wish more people would do it,” said Zara Patel, MD, an otolaryngologist at Stanford (Calif.) Medicine, who helped conduct that trial. She said some physicians may be nervous about injecting plasma so close to the skull and are therefore hesitant to try this approach.
Another technique may help to address the olfactory condition known as parosmia, in which patients generally experience a benign odor as rancid, according to otolaryngologist Nyssa Farrell, MD, of Washington University School of Medicine, St. Louis. Dr. Farrell said around two-thirds of patients who contract COVID-19 develop the condition, and the rates of long-term parosmia range from 10%-50% depending on various studies.
“It is almost always foul; this can profoundly affect someone’s quality of life,” impairing their ability to eat or to be intimate with a partner who now smells unpleasant, said Dr. Farrell, who wasn’t associated with this research.
The treatment, called a stellate ganglion block, is provided through a shot into nerves in the neck. People with parosmia associated with COVID-19 often report that this method cures them. Dr. Patel said that may be because their psychological health is improving, not their sense of smell, because the area of the body where the stellate ganglion block is applied is not part of the olfactory system.
Earlier this year, Dr. Farrell and colleagues reported that parosmia linked to COVID-19 is associated with an increased risk for depression, anxiety, and suicidal ideation.
One coauthor reported receiving grants from Smell and Taste Lab, Takasago, Baia Foods, and Frequency Therapeutics. The other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA OTOLARYNGOLOGY–HEAD & NECK SURGERY
PCSK9 inhibitors for severe COVID? Pilot trial signals of benefit
PCSK9 inhibitors may best be known for their powerful LDL-lowering effects but are less appreciated as anti-inflammatory agents with potential beyond cardiovascular health.
In a small pilot trial, for example, patients hospitalized with severe COVID-19 who received a single injection of PCSK9 inhibitor became less sick and more likely to survive than those given a placebo. Their 30-day risk of death or intubation fell significantly, as did their levels of the inflammatory cytokine interleukin 6 (IL-6).
Indeed, survival gains in the PCSK9-inhibitor group were greatest among patients with higher baseline concentrations of IL-6. Although the trial wasn’t powered for clinical outcomes, it suggests the drugs’ efficacy in COVID-19 tracks with intensity of inflammation, proposes a report published in the Journal of the American College of Cardiology.
Therefore, “PCSK9 inhibition may represent a novel therapeutic pathway in addition to currently recommended therapeutic approaches for severe COVID-19,” conclude the authors, led by Eliano P. Navarese, MD, PhD, Nicolaus Copernicus University, Bydgoszcz, Poland.
PCSK9 inhibitors as anti-inflammatories
Although the study was small and only hypothesis-generating, the fact that outcomes for actively treated patients were proportional to baseline IL-6 levels “strongly suggests that PCSK9 inhibition can directly modulate inflammation in COVID-19,” argues an editorial accompanying the report.
and likely sheds light on “mechanisms through which PCSK9 inhibition dually modulates lipoprotein metabolism and inflammation,” write Sascha N. Goonewardena, MD, University of Michigan, Ann Arbor, and Robert S. Rosenson, MD, Icahn School of Medicine at Mount Sinai, New York.
The results are consistent with prior evidence that the drugs are anti-inflammatory at least partly because of their interference with inflammatory pathways triggered by PCSK9 and mediated by IL-6, as described by Dr. Navarese and colleagues.
Indeed, they write, PCSK9 inhibitors may improve COVID outcomes mostly through mechanisms unrelated to LDL-receptor expression, “including direct inhibition of PCSK9-triggered inflammation.”
If true, the authors observe, it might explain “why the positive findings of the present study have not been consistently observed in trials involving other lipid-lowering agents, such as statins.” Those drugs are well-known to decrease levels of the inflammatory biomarker C-reactive protein.
In patients with stable coronary disease, in whom inflammation is typically tracked by measuring CRP, “the PCSK9 inhibitors have not been shown to have an anti-inflammatory effect,” Dr. Rosenson further explained.
But the current study’s patients with acute, severe COVID-19, a “profound inflammatory insult” with upregulation of IL-6, were “a good population” for evaluating the drugs’ potential anti-inflammatory effects, Dr. Rosenson said in an interview. The results “are quite enticing but require corroboration in a larger trial.”
A single injection
The IMPACT-SIRIO 5 trial entered 60 adults hospitalized with severe COVID-19 and elevated IL-6 at four centers in Poland. Patients with other known active infections were excluded.
They were randomly assigned double-blind to receive a 140 mg injection of evolocumab (Repatha) or placebo. The 2 groups were similar with respect to demographics, body-mass index, time since symptom onset, and treatments for managing COVID-19 and its complications.
Rates of death or need for intubation at 30 days, the primary endpoint, were 23.3% in the PCSK9-inhibitor group and 53.3% for controls, a risk difference of 30% (95% confidence interval –53.4% to –6.6%). The median durations of oxygen therapy were significantly different at 13 days and 20 days, respectively, the report states.
Serum IL-6 levels fell further over 30 days in the PCSK9-inhibitor group (–56% vs. –21% among controls). A drop by more than 90% was seen in 60% of patients in the PCSK9-inhibitor group and in 27% of controls.
The average hospital stay was shorter for those getting the PCSK9 inhibitor, compared with placebo, 16 days versus 22 days, and their 30-day mortality was numerically lower, 16% versus 33.3%.
Patients’ baseline IL-6 levels above the median, the report states, had a lower mortality on the PCSK9 inhibitor versus placebo (risk difference –37.5%; 95% CI –68.2% to –6.70%).
A larger trial to corroborate these results would potentially enter similar patients hospitalized with COVID-19 with reproducible evidence of an ongoing cytokine storm, such as elevated levels of IL-6, who would be assigned to either a PCSK9 inhibitor or placebo, Dr. Rosenson proposed.
Although the current primary endpoint that combines mortality and intubation was “reasonable” for a small pilot trial, he said, if the researchers embark on a larger study, “they’ll want to look at those events separately.”
Dr. Navarese discloses receiving speaker and consultancy fees from Amgen, Sanofi-Regeneron, Bayer; and grants from Abbott. Disclosures for the other authors are in the report. Rosenson discloses receiving research funding to his institution from Amgen, Arrowhead, Eli Lilly, Novartis, and Regeneron; consulting fees from Amgen, Arrowhead, CRISPR Therapeutics, Eli Lilly, Lipigon, Novartis, Precision Biosciences, Regeneron, Ultragenyx, and Verve; speaking fees from Amgen, Kowa, and Regeneron; and royalties from Wolters Kluwer; and owning stock in MediMergent. Dr. Goonewardena reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
PCSK9 inhibitors may best be known for their powerful LDL-lowering effects but are less appreciated as anti-inflammatory agents with potential beyond cardiovascular health.
In a small pilot trial, for example, patients hospitalized with severe COVID-19 who received a single injection of PCSK9 inhibitor became less sick and more likely to survive than those given a placebo. Their 30-day risk of death or intubation fell significantly, as did their levels of the inflammatory cytokine interleukin 6 (IL-6).
Indeed, survival gains in the PCSK9-inhibitor group were greatest among patients with higher baseline concentrations of IL-6. Although the trial wasn’t powered for clinical outcomes, it suggests the drugs’ efficacy in COVID-19 tracks with intensity of inflammation, proposes a report published in the Journal of the American College of Cardiology.
Therefore, “PCSK9 inhibition may represent a novel therapeutic pathway in addition to currently recommended therapeutic approaches for severe COVID-19,” conclude the authors, led by Eliano P. Navarese, MD, PhD, Nicolaus Copernicus University, Bydgoszcz, Poland.
PCSK9 inhibitors as anti-inflammatories
Although the study was small and only hypothesis-generating, the fact that outcomes for actively treated patients were proportional to baseline IL-6 levels “strongly suggests that PCSK9 inhibition can directly modulate inflammation in COVID-19,” argues an editorial accompanying the report.
and likely sheds light on “mechanisms through which PCSK9 inhibition dually modulates lipoprotein metabolism and inflammation,” write Sascha N. Goonewardena, MD, University of Michigan, Ann Arbor, and Robert S. Rosenson, MD, Icahn School of Medicine at Mount Sinai, New York.
The results are consistent with prior evidence that the drugs are anti-inflammatory at least partly because of their interference with inflammatory pathways triggered by PCSK9 and mediated by IL-6, as described by Dr. Navarese and colleagues.
Indeed, they write, PCSK9 inhibitors may improve COVID outcomes mostly through mechanisms unrelated to LDL-receptor expression, “including direct inhibition of PCSK9-triggered inflammation.”
If true, the authors observe, it might explain “why the positive findings of the present study have not been consistently observed in trials involving other lipid-lowering agents, such as statins.” Those drugs are well-known to decrease levels of the inflammatory biomarker C-reactive protein.
In patients with stable coronary disease, in whom inflammation is typically tracked by measuring CRP, “the PCSK9 inhibitors have not been shown to have an anti-inflammatory effect,” Dr. Rosenson further explained.
But the current study’s patients with acute, severe COVID-19, a “profound inflammatory insult” with upregulation of IL-6, were “a good population” for evaluating the drugs’ potential anti-inflammatory effects, Dr. Rosenson said in an interview. The results “are quite enticing but require corroboration in a larger trial.”
A single injection
The IMPACT-SIRIO 5 trial entered 60 adults hospitalized with severe COVID-19 and elevated IL-6 at four centers in Poland. Patients with other known active infections were excluded.
They were randomly assigned double-blind to receive a 140 mg injection of evolocumab (Repatha) or placebo. The 2 groups were similar with respect to demographics, body-mass index, time since symptom onset, and treatments for managing COVID-19 and its complications.
Rates of death or need for intubation at 30 days, the primary endpoint, were 23.3% in the PCSK9-inhibitor group and 53.3% for controls, a risk difference of 30% (95% confidence interval –53.4% to –6.6%). The median durations of oxygen therapy were significantly different at 13 days and 20 days, respectively, the report states.
Serum IL-6 levels fell further over 30 days in the PCSK9-inhibitor group (–56% vs. –21% among controls). A drop by more than 90% was seen in 60% of patients in the PCSK9-inhibitor group and in 27% of controls.
The average hospital stay was shorter for those getting the PCSK9 inhibitor, compared with placebo, 16 days versus 22 days, and their 30-day mortality was numerically lower, 16% versus 33.3%.
Patients’ baseline IL-6 levels above the median, the report states, had a lower mortality on the PCSK9 inhibitor versus placebo (risk difference –37.5%; 95% CI –68.2% to –6.70%).
A larger trial to corroborate these results would potentially enter similar patients hospitalized with COVID-19 with reproducible evidence of an ongoing cytokine storm, such as elevated levels of IL-6, who would be assigned to either a PCSK9 inhibitor or placebo, Dr. Rosenson proposed.
Although the current primary endpoint that combines mortality and intubation was “reasonable” for a small pilot trial, he said, if the researchers embark on a larger study, “they’ll want to look at those events separately.”
Dr. Navarese discloses receiving speaker and consultancy fees from Amgen, Sanofi-Regeneron, Bayer; and grants from Abbott. Disclosures for the other authors are in the report. Rosenson discloses receiving research funding to his institution from Amgen, Arrowhead, Eli Lilly, Novartis, and Regeneron; consulting fees from Amgen, Arrowhead, CRISPR Therapeutics, Eli Lilly, Lipigon, Novartis, Precision Biosciences, Regeneron, Ultragenyx, and Verve; speaking fees from Amgen, Kowa, and Regeneron; and royalties from Wolters Kluwer; and owning stock in MediMergent. Dr. Goonewardena reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
PCSK9 inhibitors may best be known for their powerful LDL-lowering effects but are less appreciated as anti-inflammatory agents with potential beyond cardiovascular health.
In a small pilot trial, for example, patients hospitalized with severe COVID-19 who received a single injection of PCSK9 inhibitor became less sick and more likely to survive than those given a placebo. Their 30-day risk of death or intubation fell significantly, as did their levels of the inflammatory cytokine interleukin 6 (IL-6).
Indeed, survival gains in the PCSK9-inhibitor group were greatest among patients with higher baseline concentrations of IL-6. Although the trial wasn’t powered for clinical outcomes, it suggests the drugs’ efficacy in COVID-19 tracks with intensity of inflammation, proposes a report published in the Journal of the American College of Cardiology.
Therefore, “PCSK9 inhibition may represent a novel therapeutic pathway in addition to currently recommended therapeutic approaches for severe COVID-19,” conclude the authors, led by Eliano P. Navarese, MD, PhD, Nicolaus Copernicus University, Bydgoszcz, Poland.
PCSK9 inhibitors as anti-inflammatories
Although the study was small and only hypothesis-generating, the fact that outcomes for actively treated patients were proportional to baseline IL-6 levels “strongly suggests that PCSK9 inhibition can directly modulate inflammation in COVID-19,” argues an editorial accompanying the report.
and likely sheds light on “mechanisms through which PCSK9 inhibition dually modulates lipoprotein metabolism and inflammation,” write Sascha N. Goonewardena, MD, University of Michigan, Ann Arbor, and Robert S. Rosenson, MD, Icahn School of Medicine at Mount Sinai, New York.
The results are consistent with prior evidence that the drugs are anti-inflammatory at least partly because of their interference with inflammatory pathways triggered by PCSK9 and mediated by IL-6, as described by Dr. Navarese and colleagues.
Indeed, they write, PCSK9 inhibitors may improve COVID outcomes mostly through mechanisms unrelated to LDL-receptor expression, “including direct inhibition of PCSK9-triggered inflammation.”
If true, the authors observe, it might explain “why the positive findings of the present study have not been consistently observed in trials involving other lipid-lowering agents, such as statins.” Those drugs are well-known to decrease levels of the inflammatory biomarker C-reactive protein.
In patients with stable coronary disease, in whom inflammation is typically tracked by measuring CRP, “the PCSK9 inhibitors have not been shown to have an anti-inflammatory effect,” Dr. Rosenson further explained.
But the current study’s patients with acute, severe COVID-19, a “profound inflammatory insult” with upregulation of IL-6, were “a good population” for evaluating the drugs’ potential anti-inflammatory effects, Dr. Rosenson said in an interview. The results “are quite enticing but require corroboration in a larger trial.”
A single injection
The IMPACT-SIRIO 5 trial entered 60 adults hospitalized with severe COVID-19 and elevated IL-6 at four centers in Poland. Patients with other known active infections were excluded.
They were randomly assigned double-blind to receive a 140 mg injection of evolocumab (Repatha) or placebo. The 2 groups were similar with respect to demographics, body-mass index, time since symptom onset, and treatments for managing COVID-19 and its complications.
Rates of death or need for intubation at 30 days, the primary endpoint, were 23.3% in the PCSK9-inhibitor group and 53.3% for controls, a risk difference of 30% (95% confidence interval –53.4% to –6.6%). The median durations of oxygen therapy were significantly different at 13 days and 20 days, respectively, the report states.
Serum IL-6 levels fell further over 30 days in the PCSK9-inhibitor group (–56% vs. –21% among controls). A drop by more than 90% was seen in 60% of patients in the PCSK9-inhibitor group and in 27% of controls.
The average hospital stay was shorter for those getting the PCSK9 inhibitor, compared with placebo, 16 days versus 22 days, and their 30-day mortality was numerically lower, 16% versus 33.3%.
Patients’ baseline IL-6 levels above the median, the report states, had a lower mortality on the PCSK9 inhibitor versus placebo (risk difference –37.5%; 95% CI –68.2% to –6.70%).
A larger trial to corroborate these results would potentially enter similar patients hospitalized with COVID-19 with reproducible evidence of an ongoing cytokine storm, such as elevated levels of IL-6, who would be assigned to either a PCSK9 inhibitor or placebo, Dr. Rosenson proposed.
Although the current primary endpoint that combines mortality and intubation was “reasonable” for a small pilot trial, he said, if the researchers embark on a larger study, “they’ll want to look at those events separately.”
Dr. Navarese discloses receiving speaker and consultancy fees from Amgen, Sanofi-Regeneron, Bayer; and grants from Abbott. Disclosures for the other authors are in the report. Rosenson discloses receiving research funding to his institution from Amgen, Arrowhead, Eli Lilly, Novartis, and Regeneron; consulting fees from Amgen, Arrowhead, CRISPR Therapeutics, Eli Lilly, Lipigon, Novartis, Precision Biosciences, Regeneron, Ultragenyx, and Verve; speaking fees from Amgen, Kowa, and Regeneron; and royalties from Wolters Kluwer; and owning stock in MediMergent. Dr. Goonewardena reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Is it time for yet another COVID booster? It’s complicated
For some people who have received a two-dose primary series and all the recommended boosters, that could mean a sixth shot since COVID-19 vaccines became available. But is even that enough (or too much)?
At this point, no one knows for sure, but new guidance may be on the docket.
On Jan. 26, the FDA’s Vaccines and Related Biological Products Advisory Committee is meeting. On the agenda is discussion about plans for future vaccinations for COVID-19.The committee, made up of external advisers, evaluates data on vaccines and other products for the agency.
According to the FDA announcement, after the meeting, “the FDA will consider whether to recommend adjustments to the current authorizations and approvals, and the FDA will consider the most efficient and transparent process to use for selection of strains for inclusion in the primary and booster vaccines.”
From there, the CDC will take up the issue and decide on recommendations.
The issue is important, as more than 550 Americans a day are still dying from COVID-19, as of the week ending Jan. 13, the CDC reported. That’s up from 346 a day for the week ending Dec. 28.
Yet, uptake of the newest vaccine, the bivalent booster, has been slow. As of Jan. 11, just 15.9% of the population 5 years and up has gotten it; for those most vulnerable to COVID19 – those 65 and up – the number is just 39%.
COVID vaccines, 2023 and beyond
Meanwhile, infectious disease experts have widely differing views on what the vaccination landscape of 2023 and beyond should look like. Among the areas of disagreement are how effective the bivalent vaccine is, which people most need another shot, and what type of vaccine is best.
“I think we probably will need another booster,” says Peter Hotez, MD, PhD, dean of the National School of Tropical Medicine at Baylor College of Medicine, and codirector of the Center for Vaccine Development at Texas Children’s Hospital in Houston. “The question is, what is it going to be? Is it going to be the same bivalent that we just got, or will it be a new bivalent or even a trivalent?”
The trivalent booster, he suggested, might include something more protective against XBB.1.5.
The bivalent booster gives “broadened immunity” that is improved from the original booster shots, says Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape, WebMD’s sister site for health professionals.
In his publication Ground Truths, Dr. Topol on Jan. 11 explained how new data caused him to reverse his previously skeptical view of how the FDA authorized the bivalent vaccine in September without data on how it affected humans at the time.
Paul Offit, MD, director of the Vaccine Education Center and a professor of pediatrics at the Children’s Hospital of Philadelphia, is a member of the FDA advisory committee for vaccines. He still takes a dimmer view of more bivalent booster vaccines, at least as a blanket recommendation.
While he acknowledges that boosters can help some groups – such as older adults, people with multiple health conditions, and those with compromised immune systems – he opposes a recommendation that’s population-wide.
“People who fall into those three groups do benefit,” he says, “but the recommendation is everyone over 6 months get the bivalent, and what I’m asking is, ‘Where is the data that a healthy 12-year-old boy needs a booster to stay out of the hospital?’ ”
Evolving research
“We are trying to understand how to stay one step ahead rather than several steps behind [the virus],“ says Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota.
Among the key questions: How well can a vaccine work against a single subvariant, when no one can say for sure what the next predominant subvariant will be?
Much more research has become available recently about the bivalent vaccine and its effectiveness, Dr. Osterholm says. “The bivalent vaccine is working as well as we could have expected,” he says, especially in high-risk people and in those over age 65. “The challenge we have is, what does that mean going forward?”
In his review, Dr. Topol concludes: “There is now more than ample, highly consistent evidence via lab studies and clinical outcomes to support the bivalent’s benefit over the original booster.”
Among other evidence, he looked at eight studies, including four that used a live virus as part of the research. Six of the eight studies showed the bivalent booster is more effective against the BA.5 variant, compared with the original booster shots. Two others showed no real difference.
“The four live virus studies offer consistent evidence of broadened immunity for the BA.5 vaccine that is improved over the original booster shots,” Dr. Topol wrote. The evidence also found the bivalent antibody response superior against XBB, he wrote.
Dr. Topol also cited CDC data that supports the benefits of the bivalent shot on hospitalization in older adults. During November, hospitalization of adults 65 and above was 2.5 times higher for those vaccinated who did not get the booster, compared to those who got the updated bivalent booster.
Boosters do matter, Dr. Offit says. “But not for all.” In a perspective published Jan. 11 in the New England Journal of Medicine – the same issue that published the two studies finding few differences between the original and bivalent – Dr. Offit wrote that boosting is best reserved for vulnerable groups.
Chasing the variants with a bivalent vaccine, he says, “has not panned out. There remains no evidence that a bivalent vaccine is any better than what we had. Please, show me the data that one is better than the other.”
Dr. Offit believes the goal should not be to prevent all symptomatic infections in healthy, young people by boosting them “with vaccines containing mRNA from strains that might disappear a few months later.”
The CDC needs to parse the data by subgroups, Dr. Offit says. “The critical question is, ‘Who gets hospitalized and who is dying? Who are they?’ ”
That data should take into account age, ethnicity, vaccine history, and other factors, Dr. Offit says, because right now, there is no great data to say, “OK, everyone gets a boost.”
Future vaccine costs
Another debate – for not only current boosters but future ones, too – centers on cost. Without congressional action to fund more vaccines, vaccine makers have suggested their prices may reach $130 a dose, compared with the average $20-per-dose cost the federal government pays now, according to a Kaiser Family Foundation report.
The government has spent more than $30 billion on COVID-19 vaccines, including the bivalent, to provide them free of charge.
The suggested price increase infuriated many. On Jan. 10, Sen. Bernie Sanders (I-Vt.), incoming chair of the Senate Committee on Health, Education, Labor and Pensions, sent a letter to Moderna CEO Stéphane Bancel, urging him to reconsider and refrain from any price increase.
“The huge increase in price that you have proposed will have a significantly negative impact on the budgets of Medicaid, Medicare and other government programs that will continue covering the vaccine without cost-sharing for patients.”
He pointed out, too, the $19 billion in profits Moderna has made over the past 2 years.
While most people with health insurance would likely still get the vaccines and booster for free, according to the Kaiser analysis, will a higher price discourage people from keeping up with recommended vaccinations, including a possible new booster?
“I think so, yes,” Dr. Hotez says, noting that vaccine reluctance is high as it is, even with free vaccinations and easy access.
“The government is balking at paying for the boosters,” he says. “I think it’s very tone deaf from the pharmaceutical companies [to increase the price]. Given all the help they’ve gotten from the American people, I think they should not be gouging at this point.”
He noted that the federal government provided not just money to the companies for the vaccines, but a “glide path” through the FDA for the vaccine approvals.
Are new, variant-specific boosters coming?
Are Moderna, Pfizer-BioNTech, and others developing more variant-specific vaccines, boosters, or other advances?
Novavax, approved in July 2022 as a primary series and in some cases as a booster, is “also developing an Omicron-containing bivalent vaccine at the direction of public health agencies,” says spokesperson Alison Chartan.
Pfizer responded: “When and if we have something to share we will let you know.”
Moderna did not respond.
A version of this article first appeared on WebMD.com.
For some people who have received a two-dose primary series and all the recommended boosters, that could mean a sixth shot since COVID-19 vaccines became available. But is even that enough (or too much)?
At this point, no one knows for sure, but new guidance may be on the docket.
On Jan. 26, the FDA’s Vaccines and Related Biological Products Advisory Committee is meeting. On the agenda is discussion about plans for future vaccinations for COVID-19.The committee, made up of external advisers, evaluates data on vaccines and other products for the agency.
According to the FDA announcement, after the meeting, “the FDA will consider whether to recommend adjustments to the current authorizations and approvals, and the FDA will consider the most efficient and transparent process to use for selection of strains for inclusion in the primary and booster vaccines.”
From there, the CDC will take up the issue and decide on recommendations.
The issue is important, as more than 550 Americans a day are still dying from COVID-19, as of the week ending Jan. 13, the CDC reported. That’s up from 346 a day for the week ending Dec. 28.
Yet, uptake of the newest vaccine, the bivalent booster, has been slow. As of Jan. 11, just 15.9% of the population 5 years and up has gotten it; for those most vulnerable to COVID19 – those 65 and up – the number is just 39%.
COVID vaccines, 2023 and beyond
Meanwhile, infectious disease experts have widely differing views on what the vaccination landscape of 2023 and beyond should look like. Among the areas of disagreement are how effective the bivalent vaccine is, which people most need another shot, and what type of vaccine is best.
“I think we probably will need another booster,” says Peter Hotez, MD, PhD, dean of the National School of Tropical Medicine at Baylor College of Medicine, and codirector of the Center for Vaccine Development at Texas Children’s Hospital in Houston. “The question is, what is it going to be? Is it going to be the same bivalent that we just got, or will it be a new bivalent or even a trivalent?”
The trivalent booster, he suggested, might include something more protective against XBB.1.5.
The bivalent booster gives “broadened immunity” that is improved from the original booster shots, says Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape, WebMD’s sister site for health professionals.
In his publication Ground Truths, Dr. Topol on Jan. 11 explained how new data caused him to reverse his previously skeptical view of how the FDA authorized the bivalent vaccine in September without data on how it affected humans at the time.
Paul Offit, MD, director of the Vaccine Education Center and a professor of pediatrics at the Children’s Hospital of Philadelphia, is a member of the FDA advisory committee for vaccines. He still takes a dimmer view of more bivalent booster vaccines, at least as a blanket recommendation.
While he acknowledges that boosters can help some groups – such as older adults, people with multiple health conditions, and those with compromised immune systems – he opposes a recommendation that’s population-wide.
“People who fall into those three groups do benefit,” he says, “but the recommendation is everyone over 6 months get the bivalent, and what I’m asking is, ‘Where is the data that a healthy 12-year-old boy needs a booster to stay out of the hospital?’ ”
Evolving research
“We are trying to understand how to stay one step ahead rather than several steps behind [the virus],“ says Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota.
Among the key questions: How well can a vaccine work against a single subvariant, when no one can say for sure what the next predominant subvariant will be?
Much more research has become available recently about the bivalent vaccine and its effectiveness, Dr. Osterholm says. “The bivalent vaccine is working as well as we could have expected,” he says, especially in high-risk people and in those over age 65. “The challenge we have is, what does that mean going forward?”
In his review, Dr. Topol concludes: “There is now more than ample, highly consistent evidence via lab studies and clinical outcomes to support the bivalent’s benefit over the original booster.”
Among other evidence, he looked at eight studies, including four that used a live virus as part of the research. Six of the eight studies showed the bivalent booster is more effective against the BA.5 variant, compared with the original booster shots. Two others showed no real difference.
“The four live virus studies offer consistent evidence of broadened immunity for the BA.5 vaccine that is improved over the original booster shots,” Dr. Topol wrote. The evidence also found the bivalent antibody response superior against XBB, he wrote.
Dr. Topol also cited CDC data that supports the benefits of the bivalent shot on hospitalization in older adults. During November, hospitalization of adults 65 and above was 2.5 times higher for those vaccinated who did not get the booster, compared to those who got the updated bivalent booster.
Boosters do matter, Dr. Offit says. “But not for all.” In a perspective published Jan. 11 in the New England Journal of Medicine – the same issue that published the two studies finding few differences between the original and bivalent – Dr. Offit wrote that boosting is best reserved for vulnerable groups.
Chasing the variants with a bivalent vaccine, he says, “has not panned out. There remains no evidence that a bivalent vaccine is any better than what we had. Please, show me the data that one is better than the other.”
Dr. Offit believes the goal should not be to prevent all symptomatic infections in healthy, young people by boosting them “with vaccines containing mRNA from strains that might disappear a few months later.”
The CDC needs to parse the data by subgroups, Dr. Offit says. “The critical question is, ‘Who gets hospitalized and who is dying? Who are they?’ ”
That data should take into account age, ethnicity, vaccine history, and other factors, Dr. Offit says, because right now, there is no great data to say, “OK, everyone gets a boost.”
Future vaccine costs
Another debate – for not only current boosters but future ones, too – centers on cost. Without congressional action to fund more vaccines, vaccine makers have suggested their prices may reach $130 a dose, compared with the average $20-per-dose cost the federal government pays now, according to a Kaiser Family Foundation report.
The government has spent more than $30 billion on COVID-19 vaccines, including the bivalent, to provide them free of charge.
The suggested price increase infuriated many. On Jan. 10, Sen. Bernie Sanders (I-Vt.), incoming chair of the Senate Committee on Health, Education, Labor and Pensions, sent a letter to Moderna CEO Stéphane Bancel, urging him to reconsider and refrain from any price increase.
“The huge increase in price that you have proposed will have a significantly negative impact on the budgets of Medicaid, Medicare and other government programs that will continue covering the vaccine without cost-sharing for patients.”
He pointed out, too, the $19 billion in profits Moderna has made over the past 2 years.
While most people with health insurance would likely still get the vaccines and booster for free, according to the Kaiser analysis, will a higher price discourage people from keeping up with recommended vaccinations, including a possible new booster?
“I think so, yes,” Dr. Hotez says, noting that vaccine reluctance is high as it is, even with free vaccinations and easy access.
“The government is balking at paying for the boosters,” he says. “I think it’s very tone deaf from the pharmaceutical companies [to increase the price]. Given all the help they’ve gotten from the American people, I think they should not be gouging at this point.”
He noted that the federal government provided not just money to the companies for the vaccines, but a “glide path” through the FDA for the vaccine approvals.
Are new, variant-specific boosters coming?
Are Moderna, Pfizer-BioNTech, and others developing more variant-specific vaccines, boosters, or other advances?
Novavax, approved in July 2022 as a primary series and in some cases as a booster, is “also developing an Omicron-containing bivalent vaccine at the direction of public health agencies,” says spokesperson Alison Chartan.
Pfizer responded: “When and if we have something to share we will let you know.”
Moderna did not respond.
A version of this article first appeared on WebMD.com.
For some people who have received a two-dose primary series and all the recommended boosters, that could mean a sixth shot since COVID-19 vaccines became available. But is even that enough (or too much)?
At this point, no one knows for sure, but new guidance may be on the docket.
On Jan. 26, the FDA’s Vaccines and Related Biological Products Advisory Committee is meeting. On the agenda is discussion about plans for future vaccinations for COVID-19.The committee, made up of external advisers, evaluates data on vaccines and other products for the agency.
According to the FDA announcement, after the meeting, “the FDA will consider whether to recommend adjustments to the current authorizations and approvals, and the FDA will consider the most efficient and transparent process to use for selection of strains for inclusion in the primary and booster vaccines.”
From there, the CDC will take up the issue and decide on recommendations.
The issue is important, as more than 550 Americans a day are still dying from COVID-19, as of the week ending Jan. 13, the CDC reported. That’s up from 346 a day for the week ending Dec. 28.
Yet, uptake of the newest vaccine, the bivalent booster, has been slow. As of Jan. 11, just 15.9% of the population 5 years and up has gotten it; for those most vulnerable to COVID19 – those 65 and up – the number is just 39%.
COVID vaccines, 2023 and beyond
Meanwhile, infectious disease experts have widely differing views on what the vaccination landscape of 2023 and beyond should look like. Among the areas of disagreement are how effective the bivalent vaccine is, which people most need another shot, and what type of vaccine is best.
“I think we probably will need another booster,” says Peter Hotez, MD, PhD, dean of the National School of Tropical Medicine at Baylor College of Medicine, and codirector of the Center for Vaccine Development at Texas Children’s Hospital in Houston. “The question is, what is it going to be? Is it going to be the same bivalent that we just got, or will it be a new bivalent or even a trivalent?”
The trivalent booster, he suggested, might include something more protective against XBB.1.5.
The bivalent booster gives “broadened immunity” that is improved from the original booster shots, says Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape, WebMD’s sister site for health professionals.
In his publication Ground Truths, Dr. Topol on Jan. 11 explained how new data caused him to reverse his previously skeptical view of how the FDA authorized the bivalent vaccine in September without data on how it affected humans at the time.
Paul Offit, MD, director of the Vaccine Education Center and a professor of pediatrics at the Children’s Hospital of Philadelphia, is a member of the FDA advisory committee for vaccines. He still takes a dimmer view of more bivalent booster vaccines, at least as a blanket recommendation.
While he acknowledges that boosters can help some groups – such as older adults, people with multiple health conditions, and those with compromised immune systems – he opposes a recommendation that’s population-wide.
“People who fall into those three groups do benefit,” he says, “but the recommendation is everyone over 6 months get the bivalent, and what I’m asking is, ‘Where is the data that a healthy 12-year-old boy needs a booster to stay out of the hospital?’ ”
Evolving research
“We are trying to understand how to stay one step ahead rather than several steps behind [the virus],“ says Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota.
Among the key questions: How well can a vaccine work against a single subvariant, when no one can say for sure what the next predominant subvariant will be?
Much more research has become available recently about the bivalent vaccine and its effectiveness, Dr. Osterholm says. “The bivalent vaccine is working as well as we could have expected,” he says, especially in high-risk people and in those over age 65. “The challenge we have is, what does that mean going forward?”
In his review, Dr. Topol concludes: “There is now more than ample, highly consistent evidence via lab studies and clinical outcomes to support the bivalent’s benefit over the original booster.”
Among other evidence, he looked at eight studies, including four that used a live virus as part of the research. Six of the eight studies showed the bivalent booster is more effective against the BA.5 variant, compared with the original booster shots. Two others showed no real difference.
“The four live virus studies offer consistent evidence of broadened immunity for the BA.5 vaccine that is improved over the original booster shots,” Dr. Topol wrote. The evidence also found the bivalent antibody response superior against XBB, he wrote.
Dr. Topol also cited CDC data that supports the benefits of the bivalent shot on hospitalization in older adults. During November, hospitalization of adults 65 and above was 2.5 times higher for those vaccinated who did not get the booster, compared to those who got the updated bivalent booster.
Boosters do matter, Dr. Offit says. “But not for all.” In a perspective published Jan. 11 in the New England Journal of Medicine – the same issue that published the two studies finding few differences between the original and bivalent – Dr. Offit wrote that boosting is best reserved for vulnerable groups.
Chasing the variants with a bivalent vaccine, he says, “has not panned out. There remains no evidence that a bivalent vaccine is any better than what we had. Please, show me the data that one is better than the other.”
Dr. Offit believes the goal should not be to prevent all symptomatic infections in healthy, young people by boosting them “with vaccines containing mRNA from strains that might disappear a few months later.”
The CDC needs to parse the data by subgroups, Dr. Offit says. “The critical question is, ‘Who gets hospitalized and who is dying? Who are they?’ ”
That data should take into account age, ethnicity, vaccine history, and other factors, Dr. Offit says, because right now, there is no great data to say, “OK, everyone gets a boost.”
Future vaccine costs
Another debate – for not only current boosters but future ones, too – centers on cost. Without congressional action to fund more vaccines, vaccine makers have suggested their prices may reach $130 a dose, compared with the average $20-per-dose cost the federal government pays now, according to a Kaiser Family Foundation report.
The government has spent more than $30 billion on COVID-19 vaccines, including the bivalent, to provide them free of charge.
The suggested price increase infuriated many. On Jan. 10, Sen. Bernie Sanders (I-Vt.), incoming chair of the Senate Committee on Health, Education, Labor and Pensions, sent a letter to Moderna CEO Stéphane Bancel, urging him to reconsider and refrain from any price increase.
“The huge increase in price that you have proposed will have a significantly negative impact on the budgets of Medicaid, Medicare and other government programs that will continue covering the vaccine without cost-sharing for patients.”
He pointed out, too, the $19 billion in profits Moderna has made over the past 2 years.
While most people with health insurance would likely still get the vaccines and booster for free, according to the Kaiser analysis, will a higher price discourage people from keeping up with recommended vaccinations, including a possible new booster?
“I think so, yes,” Dr. Hotez says, noting that vaccine reluctance is high as it is, even with free vaccinations and easy access.
“The government is balking at paying for the boosters,” he says. “I think it’s very tone deaf from the pharmaceutical companies [to increase the price]. Given all the help they’ve gotten from the American people, I think they should not be gouging at this point.”
He noted that the federal government provided not just money to the companies for the vaccines, but a “glide path” through the FDA for the vaccine approvals.
Are new, variant-specific boosters coming?
Are Moderna, Pfizer-BioNTech, and others developing more variant-specific vaccines, boosters, or other advances?
Novavax, approved in July 2022 as a primary series and in some cases as a booster, is “also developing an Omicron-containing bivalent vaccine at the direction of public health agencies,” says spokesperson Alison Chartan.
Pfizer responded: “When and if we have something to share we will let you know.”
Moderna did not respond.
A version of this article first appeared on WebMD.com.
Covid vax prevents death in children regardless of variant
The vaccine’s effectiveness against infection in the short term has been established, as has the waning effectiveness of the vaccine over time, wrote Juan Manuel Castelli, MD, of the Ministry of Health of Argentina, Buenos Aires, and colleagues, in the British Medical Journal.
However, data on the impact of vaccine effectiveness on mortality in children and adolescents are limited, especially during periods of omicron variant dominance, the researchers said.
In their new study, the researchers reviewed data from 844,460 children and adolescents aged 3-17 years from the National Surveillance System and the Nominalized Federal Vaccination Registry of Argentina, during a time that included a period of omicron dominance.
Argentina began vaccinating adolescents aged 12-17 years against COVID-19 in August 2021 and added children aged 3-11 years in October 2021. Those aged 12-17 years who were considered fully vaccinated received two doses of either Pfizer-BioNTech and/or Moderna vaccines, and fully-vaccinated 3- to 11-year-olds received two doses of Sinopharm vaccine.
The average time from the second vaccine dose to a COVID-19 test was 66 days for those aged 12-17 years and 54 days for 3- to 11-year-olds. The researchers matched COVID-19 cases with uninfected controls, and a total of 139,321 cases were included in the analysis.
Overall, the estimated vaccine effectiveness against COVID-19 was 64.2% during a period of delta dominance (61.2% in children aged 3-11 years and 66.8% in adolescents aged 12-17 years).
During a period of omicron dominance, estimated vaccine effectiveness was 19.9% across all ages (15.9% and 26.0% for younger and older age groups, respectively).
Effectiveness of the vaccine decreased over time, regardless of the dominant variant, but the decline was greater during the omicron dominant period, the researchers noted. During the omicron period, effectiveness in children aged 3-11 years decreased from 37.6% at 15-30 days postvaccination to 2.0% at 60 days or longer after vaccination. In adolescents aged 12-17 years, vaccine effectiveness during the omicron period decreased from 55.8% at 15-30 days postvaccination to 12.4% at 60 days or longer after vaccination.
Despite the waning protection against infection, the vaccine’s effectiveness against death from COVID-19 was 66.9% in children aged 3-11 years and 97.6% in adolescents aged 12-17 during the period of omicron dominance, the researchers noted.
The results are consistent with similar studies showing a decreased vaccine effectiveness against infection but a persistent effectiveness against deaths over time, the researchers wrote in the discussion section of their paper.
“Our results suggest that the primary vaccination schedule is effective in preventing mortality in children and adolescents with COVID-19 regardless of the circulating SARS-CoV-2 variant,” the researchers said.
Study limitations and strengths
The study was limited by several factors including the incomplete data on symptoms and hospital admissions, the possible impact of unmeasured confounding variables, and the observational design that prevents conclusions of causality, the researchers noted. However, the results were strengthened by the large sample size and access to detailed vaccination records, they said.
Both heterologous and homologous mRNA vaccine schedules showed similar effectiveness in preventing short-term infection and mortality from COVID-19 during periods of differing dominant variants, they noted.
The study findings support the vaccination of children against COVID-19 as an important public health measure to prevent mortality in children and adolescents, they concluded.
Data support value of vaccination, outside experts say
“COVID vaccines may not be as effective over time as the gene variants in the SARS-CoV-2 virus change,” Adrienne G. Randolph, MD, a pediatrician at Harvard Medical School and Boston Children’s Hospital, said in an interview. “Therefore, it is essential to assess vaccine effectiveness over time to look at effectiveness against variants and duration of effectiveness.” Dr. Randolph, who was not involved in the study, said she was not surprised by the findings, which she described as consistent with data from the United States. “COVID vaccines are very effective against preventing life-threatening disease, but the effectiveness against less severe illness for COVID vaccines is not as effective against Omicron,” she noted.
The take-home message for clinicians is that it’s important to get children vaccinated against COVID to prevent severe and life-threatening illness, said Dr. Randolph. “Although these cases are uncommon in children, it is not possible to predict which children will be the most severely affected by COVID,” she emphasized.
However, “we need more data on the new COVID booster vaccines in children that are designed to be more effective against Omicron’s newer variants,” Dr. Randolph said in an interview. “We also need more data on COVID vaccine effectiveness in the youngest children, under 5 years of age, and data on vaccinating mothers to prevent COVID in infants,” she said.
Tim Joos, MD, a Seattle-based clinician who practices a combination of internal medicine and pediatrics, agreed that future research should continue to assess how the new COVID boosters are faring against new variants, noting that the current study did not include data from children who received the new bivalent vaccine.
“The methodology of this study uses a test negative case control design which is common for estimating vaccine effectiveness post-release of a vaccine, but is subject to biases,” Dr. Joos explained. “These are not the clean effectiveness numbers of the prospective randomized control trials that we are used to hearing about when a vaccine is first being approved.”
“Nevertheless, the study reinforces the initial manufacturers’ studies that the vaccines are effective at preventing infection in the pediatric population,” Dr. Joos said in an interview. The current study also reinforces the effectiveness of vaccines in preventing “the rare but devastating mortality from COVID-19 in the pediatric population.”
Commenting on other research showing an increasing ratio of COVID deaths among vaccinated individuals compared to total COVID deaths, he noted that this finding is “likely reflecting a denominator effect of rapidly declining COVID deaths overall,” partly from the vaccines and partly from immunity after previous natural infection.
The study received no outside funding. The researchers, Dr. Randolph, and Dr. Joos had no financial conflicts to disclose. Dr. Joos serves on the Editorial Advisory Board of Pediatric News.
The vaccine’s effectiveness against infection in the short term has been established, as has the waning effectiveness of the vaccine over time, wrote Juan Manuel Castelli, MD, of the Ministry of Health of Argentina, Buenos Aires, and colleagues, in the British Medical Journal.
However, data on the impact of vaccine effectiveness on mortality in children and adolescents are limited, especially during periods of omicron variant dominance, the researchers said.
In their new study, the researchers reviewed data from 844,460 children and adolescents aged 3-17 years from the National Surveillance System and the Nominalized Federal Vaccination Registry of Argentina, during a time that included a period of omicron dominance.
Argentina began vaccinating adolescents aged 12-17 years against COVID-19 in August 2021 and added children aged 3-11 years in October 2021. Those aged 12-17 years who were considered fully vaccinated received two doses of either Pfizer-BioNTech and/or Moderna vaccines, and fully-vaccinated 3- to 11-year-olds received two doses of Sinopharm vaccine.
The average time from the second vaccine dose to a COVID-19 test was 66 days for those aged 12-17 years and 54 days for 3- to 11-year-olds. The researchers matched COVID-19 cases with uninfected controls, and a total of 139,321 cases were included in the analysis.
Overall, the estimated vaccine effectiveness against COVID-19 was 64.2% during a period of delta dominance (61.2% in children aged 3-11 years and 66.8% in adolescents aged 12-17 years).
During a period of omicron dominance, estimated vaccine effectiveness was 19.9% across all ages (15.9% and 26.0% for younger and older age groups, respectively).
Effectiveness of the vaccine decreased over time, regardless of the dominant variant, but the decline was greater during the omicron dominant period, the researchers noted. During the omicron period, effectiveness in children aged 3-11 years decreased from 37.6% at 15-30 days postvaccination to 2.0% at 60 days or longer after vaccination. In adolescents aged 12-17 years, vaccine effectiveness during the omicron period decreased from 55.8% at 15-30 days postvaccination to 12.4% at 60 days or longer after vaccination.
Despite the waning protection against infection, the vaccine’s effectiveness against death from COVID-19 was 66.9% in children aged 3-11 years and 97.6% in adolescents aged 12-17 during the period of omicron dominance, the researchers noted.
The results are consistent with similar studies showing a decreased vaccine effectiveness against infection but a persistent effectiveness against deaths over time, the researchers wrote in the discussion section of their paper.
“Our results suggest that the primary vaccination schedule is effective in preventing mortality in children and adolescents with COVID-19 regardless of the circulating SARS-CoV-2 variant,” the researchers said.
Study limitations and strengths
The study was limited by several factors including the incomplete data on symptoms and hospital admissions, the possible impact of unmeasured confounding variables, and the observational design that prevents conclusions of causality, the researchers noted. However, the results were strengthened by the large sample size and access to detailed vaccination records, they said.
Both heterologous and homologous mRNA vaccine schedules showed similar effectiveness in preventing short-term infection and mortality from COVID-19 during periods of differing dominant variants, they noted.
The study findings support the vaccination of children against COVID-19 as an important public health measure to prevent mortality in children and adolescents, they concluded.
Data support value of vaccination, outside experts say
“COVID vaccines may not be as effective over time as the gene variants in the SARS-CoV-2 virus change,” Adrienne G. Randolph, MD, a pediatrician at Harvard Medical School and Boston Children’s Hospital, said in an interview. “Therefore, it is essential to assess vaccine effectiveness over time to look at effectiveness against variants and duration of effectiveness.” Dr. Randolph, who was not involved in the study, said she was not surprised by the findings, which she described as consistent with data from the United States. “COVID vaccines are very effective against preventing life-threatening disease, but the effectiveness against less severe illness for COVID vaccines is not as effective against Omicron,” she noted.
The take-home message for clinicians is that it’s important to get children vaccinated against COVID to prevent severe and life-threatening illness, said Dr. Randolph. “Although these cases are uncommon in children, it is not possible to predict which children will be the most severely affected by COVID,” she emphasized.
However, “we need more data on the new COVID booster vaccines in children that are designed to be more effective against Omicron’s newer variants,” Dr. Randolph said in an interview. “We also need more data on COVID vaccine effectiveness in the youngest children, under 5 years of age, and data on vaccinating mothers to prevent COVID in infants,” she said.
Tim Joos, MD, a Seattle-based clinician who practices a combination of internal medicine and pediatrics, agreed that future research should continue to assess how the new COVID boosters are faring against new variants, noting that the current study did not include data from children who received the new bivalent vaccine.
“The methodology of this study uses a test negative case control design which is common for estimating vaccine effectiveness post-release of a vaccine, but is subject to biases,” Dr. Joos explained. “These are not the clean effectiveness numbers of the prospective randomized control trials that we are used to hearing about when a vaccine is first being approved.”
“Nevertheless, the study reinforces the initial manufacturers’ studies that the vaccines are effective at preventing infection in the pediatric population,” Dr. Joos said in an interview. The current study also reinforces the effectiveness of vaccines in preventing “the rare but devastating mortality from COVID-19 in the pediatric population.”
Commenting on other research showing an increasing ratio of COVID deaths among vaccinated individuals compared to total COVID deaths, he noted that this finding is “likely reflecting a denominator effect of rapidly declining COVID deaths overall,” partly from the vaccines and partly from immunity after previous natural infection.
The study received no outside funding. The researchers, Dr. Randolph, and Dr. Joos had no financial conflicts to disclose. Dr. Joos serves on the Editorial Advisory Board of Pediatric News.
The vaccine’s effectiveness against infection in the short term has been established, as has the waning effectiveness of the vaccine over time, wrote Juan Manuel Castelli, MD, of the Ministry of Health of Argentina, Buenos Aires, and colleagues, in the British Medical Journal.
However, data on the impact of vaccine effectiveness on mortality in children and adolescents are limited, especially during periods of omicron variant dominance, the researchers said.
In their new study, the researchers reviewed data from 844,460 children and adolescents aged 3-17 years from the National Surveillance System and the Nominalized Federal Vaccination Registry of Argentina, during a time that included a period of omicron dominance.
Argentina began vaccinating adolescents aged 12-17 years against COVID-19 in August 2021 and added children aged 3-11 years in October 2021. Those aged 12-17 years who were considered fully vaccinated received two doses of either Pfizer-BioNTech and/or Moderna vaccines, and fully-vaccinated 3- to 11-year-olds received two doses of Sinopharm vaccine.
The average time from the second vaccine dose to a COVID-19 test was 66 days for those aged 12-17 years and 54 days for 3- to 11-year-olds. The researchers matched COVID-19 cases with uninfected controls, and a total of 139,321 cases were included in the analysis.
Overall, the estimated vaccine effectiveness against COVID-19 was 64.2% during a period of delta dominance (61.2% in children aged 3-11 years and 66.8% in adolescents aged 12-17 years).
During a period of omicron dominance, estimated vaccine effectiveness was 19.9% across all ages (15.9% and 26.0% for younger and older age groups, respectively).
Effectiveness of the vaccine decreased over time, regardless of the dominant variant, but the decline was greater during the omicron dominant period, the researchers noted. During the omicron period, effectiveness in children aged 3-11 years decreased from 37.6% at 15-30 days postvaccination to 2.0% at 60 days or longer after vaccination. In adolescents aged 12-17 years, vaccine effectiveness during the omicron period decreased from 55.8% at 15-30 days postvaccination to 12.4% at 60 days or longer after vaccination.
Despite the waning protection against infection, the vaccine’s effectiveness against death from COVID-19 was 66.9% in children aged 3-11 years and 97.6% in adolescents aged 12-17 during the period of omicron dominance, the researchers noted.
The results are consistent with similar studies showing a decreased vaccine effectiveness against infection but a persistent effectiveness against deaths over time, the researchers wrote in the discussion section of their paper.
“Our results suggest that the primary vaccination schedule is effective in preventing mortality in children and adolescents with COVID-19 regardless of the circulating SARS-CoV-2 variant,” the researchers said.
Study limitations and strengths
The study was limited by several factors including the incomplete data on symptoms and hospital admissions, the possible impact of unmeasured confounding variables, and the observational design that prevents conclusions of causality, the researchers noted. However, the results were strengthened by the large sample size and access to detailed vaccination records, they said.
Both heterologous and homologous mRNA vaccine schedules showed similar effectiveness in preventing short-term infection and mortality from COVID-19 during periods of differing dominant variants, they noted.
The study findings support the vaccination of children against COVID-19 as an important public health measure to prevent mortality in children and adolescents, they concluded.
Data support value of vaccination, outside experts say
“COVID vaccines may not be as effective over time as the gene variants in the SARS-CoV-2 virus change,” Adrienne G. Randolph, MD, a pediatrician at Harvard Medical School and Boston Children’s Hospital, said in an interview. “Therefore, it is essential to assess vaccine effectiveness over time to look at effectiveness against variants and duration of effectiveness.” Dr. Randolph, who was not involved in the study, said she was not surprised by the findings, which she described as consistent with data from the United States. “COVID vaccines are very effective against preventing life-threatening disease, but the effectiveness against less severe illness for COVID vaccines is not as effective against Omicron,” she noted.
The take-home message for clinicians is that it’s important to get children vaccinated against COVID to prevent severe and life-threatening illness, said Dr. Randolph. “Although these cases are uncommon in children, it is not possible to predict which children will be the most severely affected by COVID,” she emphasized.
However, “we need more data on the new COVID booster vaccines in children that are designed to be more effective against Omicron’s newer variants,” Dr. Randolph said in an interview. “We also need more data on COVID vaccine effectiveness in the youngest children, under 5 years of age, and data on vaccinating mothers to prevent COVID in infants,” she said.
Tim Joos, MD, a Seattle-based clinician who practices a combination of internal medicine and pediatrics, agreed that future research should continue to assess how the new COVID boosters are faring against new variants, noting that the current study did not include data from children who received the new bivalent vaccine.
“The methodology of this study uses a test negative case control design which is common for estimating vaccine effectiveness post-release of a vaccine, but is subject to biases,” Dr. Joos explained. “These are not the clean effectiveness numbers of the prospective randomized control trials that we are used to hearing about when a vaccine is first being approved.”
“Nevertheless, the study reinforces the initial manufacturers’ studies that the vaccines are effective at preventing infection in the pediatric population,” Dr. Joos said in an interview. The current study also reinforces the effectiveness of vaccines in preventing “the rare but devastating mortality from COVID-19 in the pediatric population.”
Commenting on other research showing an increasing ratio of COVID deaths among vaccinated individuals compared to total COVID deaths, he noted that this finding is “likely reflecting a denominator effect of rapidly declining COVID deaths overall,” partly from the vaccines and partly from immunity after previous natural infection.
The study received no outside funding. The researchers, Dr. Randolph, and Dr. Joos had no financial conflicts to disclose. Dr. Joos serves on the Editorial Advisory Board of Pediatric News.
FROM THE BMJ
U.S. flu activity already at mid-season levels
according to the Centers of Disease Control and Prevention.
Nationally, 6% of all outpatient visits were because of flu or flu-like illness for the week of Nov. 13-19, up from 5.8% the previous week, the CDC’s Influenza Division said in its weekly FluView report.
Those figures are the highest recorded in November since 2009, but the peak of the 2009-10 flu season occurred even earlier – the week of Oct. 18-24 – and the rate of flu-like illness had already dropped to just over 4.0% by Nov. 15-21 that year and continued to drop thereafter.
Although COVID-19 and respiratory syncytial virus (RSV) are included in the data from the CDC’s Outpatient Influenza-like Illness Surveillance Network, the agency did note that “seasonal influenza activity is elevated across the country” and estimated that “there have been at least 6.2 million illnesses, 53,000 hospitalizations, and 2,900 deaths from flu” during the 2022-23 season.
Total flu deaths include 11 reported in children as of Nov. 19, and children ages 0-4 had a higher proportion of visits for flu like-illness than other age groups.
The agency also said the cumulative hospitalization rate of 11.3 per 100,000 population “is higher than the rate observed in [the corresponding week of] every previous season since 2010-2011.” Adults 65 years and older have the highest cumulative rate, 25.9 per 100,000, for this year, compared with 20.7 for children 0-4; 11.1 for adults 50-64; 10.3 for children 5-17; and 5.6 for adults 18-49 years old, the CDC said.
A version of this article first appeared on WebMD.com.
according to the Centers of Disease Control and Prevention.
Nationally, 6% of all outpatient visits were because of flu or flu-like illness for the week of Nov. 13-19, up from 5.8% the previous week, the CDC’s Influenza Division said in its weekly FluView report.
Those figures are the highest recorded in November since 2009, but the peak of the 2009-10 flu season occurred even earlier – the week of Oct. 18-24 – and the rate of flu-like illness had already dropped to just over 4.0% by Nov. 15-21 that year and continued to drop thereafter.
Although COVID-19 and respiratory syncytial virus (RSV) are included in the data from the CDC’s Outpatient Influenza-like Illness Surveillance Network, the agency did note that “seasonal influenza activity is elevated across the country” and estimated that “there have been at least 6.2 million illnesses, 53,000 hospitalizations, and 2,900 deaths from flu” during the 2022-23 season.
Total flu deaths include 11 reported in children as of Nov. 19, and children ages 0-4 had a higher proportion of visits for flu like-illness than other age groups.
The agency also said the cumulative hospitalization rate of 11.3 per 100,000 population “is higher than the rate observed in [the corresponding week of] every previous season since 2010-2011.” Adults 65 years and older have the highest cumulative rate, 25.9 per 100,000, for this year, compared with 20.7 for children 0-4; 11.1 for adults 50-64; 10.3 for children 5-17; and 5.6 for adults 18-49 years old, the CDC said.
A version of this article first appeared on WebMD.com.
according to the Centers of Disease Control and Prevention.
Nationally, 6% of all outpatient visits were because of flu or flu-like illness for the week of Nov. 13-19, up from 5.8% the previous week, the CDC’s Influenza Division said in its weekly FluView report.
Those figures are the highest recorded in November since 2009, but the peak of the 2009-10 flu season occurred even earlier – the week of Oct. 18-24 – and the rate of flu-like illness had already dropped to just over 4.0% by Nov. 15-21 that year and continued to drop thereafter.
Although COVID-19 and respiratory syncytial virus (RSV) are included in the data from the CDC’s Outpatient Influenza-like Illness Surveillance Network, the agency did note that “seasonal influenza activity is elevated across the country” and estimated that “there have been at least 6.2 million illnesses, 53,000 hospitalizations, and 2,900 deaths from flu” during the 2022-23 season.
Total flu deaths include 11 reported in children as of Nov. 19, and children ages 0-4 had a higher proportion of visits for flu like-illness than other age groups.
The agency also said the cumulative hospitalization rate of 11.3 per 100,000 population “is higher than the rate observed in [the corresponding week of] every previous season since 2010-2011.” Adults 65 years and older have the highest cumulative rate, 25.9 per 100,000, for this year, compared with 20.7 for children 0-4; 11.1 for adults 50-64; 10.3 for children 5-17; and 5.6 for adults 18-49 years old, the CDC said.
A version of this article first appeared on WebMD.com.
Sexual dysfunction, hair loss linked with long COVID
according to findings of a large study.
Anuradhaa Subramanian, PhD, with the Institute of Applied Health Research at the University of Birmingham (England), led the research published online in Nature Medicine.
The team analyzed 486,149 electronic health records from adult patients with confirmed COVID in the United Kingdom, compared with 1.9 million people with no history of COVID, from January 2020 to April 2021. Researchers matched both groups closely in terms of demographic, social, and clinical traits.
New symptoms
The team identified 62 symptoms, including the well-known indicators of long COVID, such as fatigue, loss of sense of smell, shortness of breath, and brain fog, but also hair loss, sexual dysfunction, chest pain, fever, loss of control of bowel movements, and limb swelling.
“These differences in symptoms reported between the infected and uninfected groups remained even after we accounted for age, sex, ethnic group, socioeconomic status, body mass index, smoking status, the presence of more than 80 health conditions, and past reporting of the same symptom,” Dr. Subramanian and coresearcher Shamil Haroon, PhD, wrote in a summary of their research in The Conversation.
They pointed out that only 20 of the symptoms they found are included in the World Health Organization’s clinical case definition for long COVID.
They also found that people more likely to have persistent symptoms 3 months after COVID infection were also more likely to be young, female, smokers, to belong to certain minority ethnic groups, and to have lower socioeconomic status. They were also more likely to be obese and have a wide range of health conditions.
Dr. Haroon, an associate clinical professor at the University of Birmingham, said that one reason it appeared that younger people were more likely to get symptoms of long COVID may be that older adults with COVID were more likely to be hospitalized and weren’t included in this study.
“Since we only considered nonhospitalized adults, the older adults we included in our study may have been relatively healthier and thus had a lower symptom burden,” he said.
Dr. Subramania noted that older patients were more likely to report lasting COVID-related symptoms in the study, but when researchers accounted for a wide range of other conditions that patients had before infection (which generally more commonly happen in older adults), they found younger age as a risk factor for long-term COVID-related symptoms.
In the study period, most patients were unvaccinated, and results came before the widespread Delta and Omicron variants.
More than half (56.6%) of the patients infected with the virus that causes COVID had been diagnosed in 2020, and 43.4% in 2021. Less than 5% (4.5%) of the patients infected with the virus and 4.7% of the patients with no recorded evidence of a COVID infection had received at least a single dose of a COVID vaccine before the study started.
Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape, said more studies need to be done to see whether results would be different with vaccination status and evolving variants.
But he noted that this study has several strengths: “The hair loss, libido loss, and ejaculation difficulty are all new symptoms,” and the study – large and carefully controlled – shows these issues were among those more likely to occur.
A loss of sense of smell – which is not a new observation – was still the most likely risk shown in the study, followed by hair loss, sneezing, ejaculation difficulty, and reduced sex drive; followed by shortness of breath, fatigue, chest pain associated with breathing difficulties, hoarseness, and fever.
Three main clusters of symptoms
Given the wide range of symptoms, long COVID likely represents a group of conditions, the authors wrote.
They found three main clusters. The largest, with roughly 80% of people with long COVID in the study, faced a broad spectrum of symptoms, ranging from fatigue to headache and pain. The second-largest group, (15%) mostly had symptoms having to do with mental health and thinking skills, including depression, anxiety, brain fog, and insomnia. The smallest group (5%) had mainly respiratory symptoms such as shortness of breath, coughing, and wheezing.
Putting symptoms in clusters will be important to start understanding what leads to long COVID, said Farha Ikramuddin, MD, a rehabilitation specialist at the University of Minnesota, Minneapolis.
She added that, while the symptoms listed in this paper are new in published research, she has certainly been seeing them over time in her long COVID clinic. (The researchers also used only coded health care data, so they were limited in what symptoms they could discover, she notes.)
Dr. Ikramuddin said a strength of the paper is its large size, but she also cautioned that it’s difficult to determine whether members of the comparison group truly had no COVID infection when the information is taken from their medical records. Often, people test at home or assume they have COVID and don’t test; therefore the information wouldn’t be recorded.
Evaluating nonhospitalized patients is also important, she said, as much of the research on long COVID has come from hospitalized patients, so little has been known about the symptoms of those with milder infections.
“Patients who have been hospitalized and have long COVID look very different from the patients who were not hospitalized,” Dr. Ikramuddin said.
One clear message from the paper, she said, is that listening and asking extensive questions about symptoms are important with patients who have had COVID.
“Counseling has also become very important for our patients in the pandemic,” she said.
It will also be important to do studies on returning to work for patients with long COVID to see how many are able to return and at what capacity, Dr. Ikramuddin said.
A version of this article first appeared on WebMD.com.
according to findings of a large study.
Anuradhaa Subramanian, PhD, with the Institute of Applied Health Research at the University of Birmingham (England), led the research published online in Nature Medicine.
The team analyzed 486,149 electronic health records from adult patients with confirmed COVID in the United Kingdom, compared with 1.9 million people with no history of COVID, from January 2020 to April 2021. Researchers matched both groups closely in terms of demographic, social, and clinical traits.
New symptoms
The team identified 62 symptoms, including the well-known indicators of long COVID, such as fatigue, loss of sense of smell, shortness of breath, and brain fog, but also hair loss, sexual dysfunction, chest pain, fever, loss of control of bowel movements, and limb swelling.
“These differences in symptoms reported between the infected and uninfected groups remained even after we accounted for age, sex, ethnic group, socioeconomic status, body mass index, smoking status, the presence of more than 80 health conditions, and past reporting of the same symptom,” Dr. Subramanian and coresearcher Shamil Haroon, PhD, wrote in a summary of their research in The Conversation.
They pointed out that only 20 of the symptoms they found are included in the World Health Organization’s clinical case definition for long COVID.
They also found that people more likely to have persistent symptoms 3 months after COVID infection were also more likely to be young, female, smokers, to belong to certain minority ethnic groups, and to have lower socioeconomic status. They were also more likely to be obese and have a wide range of health conditions.
Dr. Haroon, an associate clinical professor at the University of Birmingham, said that one reason it appeared that younger people were more likely to get symptoms of long COVID may be that older adults with COVID were more likely to be hospitalized and weren’t included in this study.
“Since we only considered nonhospitalized adults, the older adults we included in our study may have been relatively healthier and thus had a lower symptom burden,” he said.
Dr. Subramania noted that older patients were more likely to report lasting COVID-related symptoms in the study, but when researchers accounted for a wide range of other conditions that patients had before infection (which generally more commonly happen in older adults), they found younger age as a risk factor for long-term COVID-related symptoms.
In the study period, most patients were unvaccinated, and results came before the widespread Delta and Omicron variants.
More than half (56.6%) of the patients infected with the virus that causes COVID had been diagnosed in 2020, and 43.4% in 2021. Less than 5% (4.5%) of the patients infected with the virus and 4.7% of the patients with no recorded evidence of a COVID infection had received at least a single dose of a COVID vaccine before the study started.
Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape, said more studies need to be done to see whether results would be different with vaccination status and evolving variants.
But he noted that this study has several strengths: “The hair loss, libido loss, and ejaculation difficulty are all new symptoms,” and the study – large and carefully controlled – shows these issues were among those more likely to occur.
A loss of sense of smell – which is not a new observation – was still the most likely risk shown in the study, followed by hair loss, sneezing, ejaculation difficulty, and reduced sex drive; followed by shortness of breath, fatigue, chest pain associated with breathing difficulties, hoarseness, and fever.
Three main clusters of symptoms
Given the wide range of symptoms, long COVID likely represents a group of conditions, the authors wrote.
They found three main clusters. The largest, with roughly 80% of people with long COVID in the study, faced a broad spectrum of symptoms, ranging from fatigue to headache and pain. The second-largest group, (15%) mostly had symptoms having to do with mental health and thinking skills, including depression, anxiety, brain fog, and insomnia. The smallest group (5%) had mainly respiratory symptoms such as shortness of breath, coughing, and wheezing.
Putting symptoms in clusters will be important to start understanding what leads to long COVID, said Farha Ikramuddin, MD, a rehabilitation specialist at the University of Minnesota, Minneapolis.
She added that, while the symptoms listed in this paper are new in published research, she has certainly been seeing them over time in her long COVID clinic. (The researchers also used only coded health care data, so they were limited in what symptoms they could discover, she notes.)
Dr. Ikramuddin said a strength of the paper is its large size, but she also cautioned that it’s difficult to determine whether members of the comparison group truly had no COVID infection when the information is taken from their medical records. Often, people test at home or assume they have COVID and don’t test; therefore the information wouldn’t be recorded.
Evaluating nonhospitalized patients is also important, she said, as much of the research on long COVID has come from hospitalized patients, so little has been known about the symptoms of those with milder infections.
“Patients who have been hospitalized and have long COVID look very different from the patients who were not hospitalized,” Dr. Ikramuddin said.
One clear message from the paper, she said, is that listening and asking extensive questions about symptoms are important with patients who have had COVID.
“Counseling has also become very important for our patients in the pandemic,” she said.
It will also be important to do studies on returning to work for patients with long COVID to see how many are able to return and at what capacity, Dr. Ikramuddin said.
A version of this article first appeared on WebMD.com.
according to findings of a large study.
Anuradhaa Subramanian, PhD, with the Institute of Applied Health Research at the University of Birmingham (England), led the research published online in Nature Medicine.
The team analyzed 486,149 electronic health records from adult patients with confirmed COVID in the United Kingdom, compared with 1.9 million people with no history of COVID, from January 2020 to April 2021. Researchers matched both groups closely in terms of demographic, social, and clinical traits.
New symptoms
The team identified 62 symptoms, including the well-known indicators of long COVID, such as fatigue, loss of sense of smell, shortness of breath, and brain fog, but also hair loss, sexual dysfunction, chest pain, fever, loss of control of bowel movements, and limb swelling.
“These differences in symptoms reported between the infected and uninfected groups remained even after we accounted for age, sex, ethnic group, socioeconomic status, body mass index, smoking status, the presence of more than 80 health conditions, and past reporting of the same symptom,” Dr. Subramanian and coresearcher Shamil Haroon, PhD, wrote in a summary of their research in The Conversation.
They pointed out that only 20 of the symptoms they found are included in the World Health Organization’s clinical case definition for long COVID.
They also found that people more likely to have persistent symptoms 3 months after COVID infection were also more likely to be young, female, smokers, to belong to certain minority ethnic groups, and to have lower socioeconomic status. They were also more likely to be obese and have a wide range of health conditions.
Dr. Haroon, an associate clinical professor at the University of Birmingham, said that one reason it appeared that younger people were more likely to get symptoms of long COVID may be that older adults with COVID were more likely to be hospitalized and weren’t included in this study.
“Since we only considered nonhospitalized adults, the older adults we included in our study may have been relatively healthier and thus had a lower symptom burden,” he said.
Dr. Subramania noted that older patients were more likely to report lasting COVID-related symptoms in the study, but when researchers accounted for a wide range of other conditions that patients had before infection (which generally more commonly happen in older adults), they found younger age as a risk factor for long-term COVID-related symptoms.
In the study period, most patients were unvaccinated, and results came before the widespread Delta and Omicron variants.
More than half (56.6%) of the patients infected with the virus that causes COVID had been diagnosed in 2020, and 43.4% in 2021. Less than 5% (4.5%) of the patients infected with the virus and 4.7% of the patients with no recorded evidence of a COVID infection had received at least a single dose of a COVID vaccine before the study started.
Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape, said more studies need to be done to see whether results would be different with vaccination status and evolving variants.
But he noted that this study has several strengths: “The hair loss, libido loss, and ejaculation difficulty are all new symptoms,” and the study – large and carefully controlled – shows these issues were among those more likely to occur.
A loss of sense of smell – which is not a new observation – was still the most likely risk shown in the study, followed by hair loss, sneezing, ejaculation difficulty, and reduced sex drive; followed by shortness of breath, fatigue, chest pain associated with breathing difficulties, hoarseness, and fever.
Three main clusters of symptoms
Given the wide range of symptoms, long COVID likely represents a group of conditions, the authors wrote.
They found three main clusters. The largest, with roughly 80% of people with long COVID in the study, faced a broad spectrum of symptoms, ranging from fatigue to headache and pain. The second-largest group, (15%) mostly had symptoms having to do with mental health and thinking skills, including depression, anxiety, brain fog, and insomnia. The smallest group (5%) had mainly respiratory symptoms such as shortness of breath, coughing, and wheezing.
Putting symptoms in clusters will be important to start understanding what leads to long COVID, said Farha Ikramuddin, MD, a rehabilitation specialist at the University of Minnesota, Minneapolis.
She added that, while the symptoms listed in this paper are new in published research, she has certainly been seeing them over time in her long COVID clinic. (The researchers also used only coded health care data, so they were limited in what symptoms they could discover, she notes.)
Dr. Ikramuddin said a strength of the paper is its large size, but she also cautioned that it’s difficult to determine whether members of the comparison group truly had no COVID infection when the information is taken from their medical records. Often, people test at home or assume they have COVID and don’t test; therefore the information wouldn’t be recorded.
Evaluating nonhospitalized patients is also important, she said, as much of the research on long COVID has come from hospitalized patients, so little has been known about the symptoms of those with milder infections.
“Patients who have been hospitalized and have long COVID look very different from the patients who were not hospitalized,” Dr. Ikramuddin said.
One clear message from the paper, she said, is that listening and asking extensive questions about symptoms are important with patients who have had COVID.
“Counseling has also become very important for our patients in the pandemic,” she said.
It will also be important to do studies on returning to work for patients with long COVID to see how many are able to return and at what capacity, Dr. Ikramuddin said.
A version of this article first appeared on WebMD.com.
FROM NATURE MEDICINE
Advisory on youth mental health crisis gets mixed reviews
The advisory on youth mental health from Surgeon General Vivek Murthy, MD, casts a necessary spotlight on the crisis, clinical psychiatrists say. But some think it could have produced more specifics about funding and payment parity for reimbursement.
The 53-page advisory says that about one in five U.S. children and adolescents aged 3-17 suffer from a mental, emotional, developmental, or behavioral disorder. In the decade before COVID, feelings of sadness and hopelessness, as well as suicidal behaviors, were on the rise. The pandemic has exacerbated symptoms of anxiety, depression, and other mental health issues in young people. Compared with 2019, ED visits in early 2021 for suspected suicide attempts rose 51% in adolescent girls and 4% in boys. “Depressive and anxiety symptoms doubled during the pandemic,” the advisory said.
Scope of the advisory
The advisory, released Dec. 7, covers all sectors and considers all social and policy factors that might be contributing to this crisis, said Jessica (Jessi) Gold, MD, MS, an assistant professor in the department of psychiatry at Washington University, St. Louis.
“It is always possible to reimagine health care to be more patient centered and mental health forward.” But changes of this magnitude take time, Dr. Gold, also director of wellness, engagement, and outreach at the university, said in an interview.
She has seen the impact of the pandemic firsthand in her clinic among students and frontline health care workers aged 18-30. People in that age group “feel everything deeply,” Dr. Gold said. Emotions tied to COVID-19 are just a part of it. Confounding factors, such as climate change, racism, and school shootings all contribute to their overall mental health.
Some children and adolescents with social anxiety have fared better during the pandemic, but those who are part of demographic groups such as racial and ethnic minorities, LGBTQ individuals, low-income youth, and those involved in juvenile justice or welfare systems face a higher risk of mental health challenges, the pandemic notwithstanding.
In her work with schools, Denese Shervington, MD, MPH, has witnessed more mental health challenges related to isolation and separation. “There’s an overall worry about the loss of what used to be, the seeming predictability and certainty of prepandemic life,” said Dr. Shervington, clinical professor of psychiatry at Tulane University, and president and CEO of the Institute for Women and Ethnic Studies, both in New Orleans.
A systems of care plan
The advisory lists actionable items for health care and 10 other industry sectors to improve mental health of children and young adults.
Health care organizations and professionals were advised to take the following six steps:
- Implement trauma-informed care principles and other prevention strategies. This may involve referring patients to resources such as economic and legal supports, school enrichment programs, and educating families on healthy child development in the clinic.
- Routinely screen children for mental health challenges and risk factors such as adverse childhood experiences during primary care well-visits or annual physicals, or at schools or EDs. Primary care physicians should use principles of trauma-informed care to conduct these screenings.
- Screen parents, caregivers, and other family members for depression, intimate partner violence, substance use, and other challenges. These can be done in tandem with broader assessments of social determinants of health such as food or housing insecurity.
- Combine efforts of clinical staff with trusted community partners and child welfare and juvenile justice. Hospital-based violence intervention programs, for example, identify patients at risk of repeat violent injury and refer them to hospital- and community-based resources.
- Build multidisciplinary teams, enlisting children and families to develop services that are tailored to their needs for screening and treatment. Such services should reflect cultural diversity and offered in multiple languages.
- Support the well-being of mental health workers and community leaders to foster their ability to help youth and their families.
Dr. Murthy is talking about a “systems of care” approach, in which all sectors that touch children and youth – not just health care – must work together and do their jobs effectively but collaboratively to address this public health crisis, said Aradhana (Bela) Sood MD, MSHA, FAACAP, senior professor of child mental health policy at Virginia Commonwealth University, Richmond. “An investment in infrastructure support of positive mental health in early childhood, be it in schools, communities, or family well-being will lead to a future where illness is not the result of major preventable societal factors, such as a lack of social supports and trauma.”
Changes will ‘take a lot of buy-in’
The recommendations are actionable in the real world – but there are a lot of them, said Dr. Gold. Dr. Murthy doesn’t specify what the plan is to accomplish these metrics or fund them, she added. He “has money and funders like foundations as steps, but foundations have also suffered in the pandemic, so it is not that simple.” Many of these changes are wide in scope and will take a lot of buy-in.
Dr. Shervington would like to have seen more of a focus on educator well-being, given that young people spend a lot of time in educational settings.
“My organization just completed a study in New Orleans that showed teachers having elevated levels of trauma-based conditions since the pandemic,” she said. Schools are indeed a key place to support holistic mental health by focusing on school climate, Dr. Sood added. “If school administrators became uniformly consistent with recognizing the importance of psychological wellness as a prerequisite of good learning, they will create environments where teachers are keenly aware of a child’s mental wellness and make reduction of bullying, wellness check-ins, [and] school-based mental health clinics a priority.
“These are ways nonmedical, community-based supports can enhance student well-being, and reduce depression and other mental health conditions,” Dr. Sood added.
Child psychiatrists stretched ‘even thinner’
Despite mental health parity rules, health plans have not been held accountable. That failure, combined with excessive demands for prior authorization for mental health treatments “have led to dangerous shortages of psychiatrists able to accept insurance,” said Paul S. Nestadt, MD, an assistant professor and public mental health researcher at Johns Hopkins University, Baltimore.
“This is particularly true for child psychiatrists, who are stretched even thinner than those of us in general practice,” Dr. Nestadt said.
While he doesn’t address it head on, Dr. Murthy uses classic parity language when he states that “mental health is no less important than physical health,” said Dr. Nestadt, who consulted with the surgeon general on developing this advisory. “While many of us would have liked to see parity highlighted more directly, this advisory was designed to be an overview.”
Highlighting social media, gun violence
Dr. Nestadt said he was pleased that the advisory emphasized the importance of restricting access to lethal means in preventing youth suicide.
“With youth suicide rates rising faster than in other age groups, and suicide mortality tied so closely to method availability, the surgeon general made the right choice in highlighting the role of guns in suicide,” he said.
The advisory also discussed the role of media and social media companies in addressing the crisis, which is important, said Dr. Gold.
“I believe very strongly that the way we talk about and portray mental health in the media matters,” she said. “I have seen it matter in the clinic with patients. They’ll wonder if someone will think they are now violent if they are diagnosed with a mental illness. Stories change the narrative.”
While the advisory isn’t perfect, the state of youth mental health “will only get worse if we don’t do something,” noted Dr. Gold. “It is critical that this is validated and discussed at the highest level and messages like Dr. Murthy’s get heard.”
Dr. Gold, Dr. Shervington, and Dr. Sood had no disclosures. Dr. Nestadt disclosed serving as a consultant to the surgeon general advisory.
The advisory on youth mental health from Surgeon General Vivek Murthy, MD, casts a necessary spotlight on the crisis, clinical psychiatrists say. But some think it could have produced more specifics about funding and payment parity for reimbursement.
The 53-page advisory says that about one in five U.S. children and adolescents aged 3-17 suffer from a mental, emotional, developmental, or behavioral disorder. In the decade before COVID, feelings of sadness and hopelessness, as well as suicidal behaviors, were on the rise. The pandemic has exacerbated symptoms of anxiety, depression, and other mental health issues in young people. Compared with 2019, ED visits in early 2021 for suspected suicide attempts rose 51% in adolescent girls and 4% in boys. “Depressive and anxiety symptoms doubled during the pandemic,” the advisory said.
Scope of the advisory
The advisory, released Dec. 7, covers all sectors and considers all social and policy factors that might be contributing to this crisis, said Jessica (Jessi) Gold, MD, MS, an assistant professor in the department of psychiatry at Washington University, St. Louis.
“It is always possible to reimagine health care to be more patient centered and mental health forward.” But changes of this magnitude take time, Dr. Gold, also director of wellness, engagement, and outreach at the university, said in an interview.
She has seen the impact of the pandemic firsthand in her clinic among students and frontline health care workers aged 18-30. People in that age group “feel everything deeply,” Dr. Gold said. Emotions tied to COVID-19 are just a part of it. Confounding factors, such as climate change, racism, and school shootings all contribute to their overall mental health.
Some children and adolescents with social anxiety have fared better during the pandemic, but those who are part of demographic groups such as racial and ethnic minorities, LGBTQ individuals, low-income youth, and those involved in juvenile justice or welfare systems face a higher risk of mental health challenges, the pandemic notwithstanding.
In her work with schools, Denese Shervington, MD, MPH, has witnessed more mental health challenges related to isolation and separation. “There’s an overall worry about the loss of what used to be, the seeming predictability and certainty of prepandemic life,” said Dr. Shervington, clinical professor of psychiatry at Tulane University, and president and CEO of the Institute for Women and Ethnic Studies, both in New Orleans.
A systems of care plan
The advisory lists actionable items for health care and 10 other industry sectors to improve mental health of children and young adults.
Health care organizations and professionals were advised to take the following six steps:
- Implement trauma-informed care principles and other prevention strategies. This may involve referring patients to resources such as economic and legal supports, school enrichment programs, and educating families on healthy child development in the clinic.
- Routinely screen children for mental health challenges and risk factors such as adverse childhood experiences during primary care well-visits or annual physicals, or at schools or EDs. Primary care physicians should use principles of trauma-informed care to conduct these screenings.
- Screen parents, caregivers, and other family members for depression, intimate partner violence, substance use, and other challenges. These can be done in tandem with broader assessments of social determinants of health such as food or housing insecurity.
- Combine efforts of clinical staff with trusted community partners and child welfare and juvenile justice. Hospital-based violence intervention programs, for example, identify patients at risk of repeat violent injury and refer them to hospital- and community-based resources.
- Build multidisciplinary teams, enlisting children and families to develop services that are tailored to their needs for screening and treatment. Such services should reflect cultural diversity and offered in multiple languages.
- Support the well-being of mental health workers and community leaders to foster their ability to help youth and their families.
Dr. Murthy is talking about a “systems of care” approach, in which all sectors that touch children and youth – not just health care – must work together and do their jobs effectively but collaboratively to address this public health crisis, said Aradhana (Bela) Sood MD, MSHA, FAACAP, senior professor of child mental health policy at Virginia Commonwealth University, Richmond. “An investment in infrastructure support of positive mental health in early childhood, be it in schools, communities, or family well-being will lead to a future where illness is not the result of major preventable societal factors, such as a lack of social supports and trauma.”
Changes will ‘take a lot of buy-in’
The recommendations are actionable in the real world – but there are a lot of them, said Dr. Gold. Dr. Murthy doesn’t specify what the plan is to accomplish these metrics or fund them, she added. He “has money and funders like foundations as steps, but foundations have also suffered in the pandemic, so it is not that simple.” Many of these changes are wide in scope and will take a lot of buy-in.
Dr. Shervington would like to have seen more of a focus on educator well-being, given that young people spend a lot of time in educational settings.
“My organization just completed a study in New Orleans that showed teachers having elevated levels of trauma-based conditions since the pandemic,” she said. Schools are indeed a key place to support holistic mental health by focusing on school climate, Dr. Sood added. “If school administrators became uniformly consistent with recognizing the importance of psychological wellness as a prerequisite of good learning, they will create environments where teachers are keenly aware of a child’s mental wellness and make reduction of bullying, wellness check-ins, [and] school-based mental health clinics a priority.
“These are ways nonmedical, community-based supports can enhance student well-being, and reduce depression and other mental health conditions,” Dr. Sood added.
Child psychiatrists stretched ‘even thinner’
Despite mental health parity rules, health plans have not been held accountable. That failure, combined with excessive demands for prior authorization for mental health treatments “have led to dangerous shortages of psychiatrists able to accept insurance,” said Paul S. Nestadt, MD, an assistant professor and public mental health researcher at Johns Hopkins University, Baltimore.
“This is particularly true for child psychiatrists, who are stretched even thinner than those of us in general practice,” Dr. Nestadt said.
While he doesn’t address it head on, Dr. Murthy uses classic parity language when he states that “mental health is no less important than physical health,” said Dr. Nestadt, who consulted with the surgeon general on developing this advisory. “While many of us would have liked to see parity highlighted more directly, this advisory was designed to be an overview.”
Highlighting social media, gun violence
Dr. Nestadt said he was pleased that the advisory emphasized the importance of restricting access to lethal means in preventing youth suicide.
“With youth suicide rates rising faster than in other age groups, and suicide mortality tied so closely to method availability, the surgeon general made the right choice in highlighting the role of guns in suicide,” he said.
The advisory also discussed the role of media and social media companies in addressing the crisis, which is important, said Dr. Gold.
“I believe very strongly that the way we talk about and portray mental health in the media matters,” she said. “I have seen it matter in the clinic with patients. They’ll wonder if someone will think they are now violent if they are diagnosed with a mental illness. Stories change the narrative.”
While the advisory isn’t perfect, the state of youth mental health “will only get worse if we don’t do something,” noted Dr. Gold. “It is critical that this is validated and discussed at the highest level and messages like Dr. Murthy’s get heard.”
Dr. Gold, Dr. Shervington, and Dr. Sood had no disclosures. Dr. Nestadt disclosed serving as a consultant to the surgeon general advisory.
The advisory on youth mental health from Surgeon General Vivek Murthy, MD, casts a necessary spotlight on the crisis, clinical psychiatrists say. But some think it could have produced more specifics about funding and payment parity for reimbursement.
The 53-page advisory says that about one in five U.S. children and adolescents aged 3-17 suffer from a mental, emotional, developmental, or behavioral disorder. In the decade before COVID, feelings of sadness and hopelessness, as well as suicidal behaviors, were on the rise. The pandemic has exacerbated symptoms of anxiety, depression, and other mental health issues in young people. Compared with 2019, ED visits in early 2021 for suspected suicide attempts rose 51% in adolescent girls and 4% in boys. “Depressive and anxiety symptoms doubled during the pandemic,” the advisory said.
Scope of the advisory
The advisory, released Dec. 7, covers all sectors and considers all social and policy factors that might be contributing to this crisis, said Jessica (Jessi) Gold, MD, MS, an assistant professor in the department of psychiatry at Washington University, St. Louis.
“It is always possible to reimagine health care to be more patient centered and mental health forward.” But changes of this magnitude take time, Dr. Gold, also director of wellness, engagement, and outreach at the university, said in an interview.
She has seen the impact of the pandemic firsthand in her clinic among students and frontline health care workers aged 18-30. People in that age group “feel everything deeply,” Dr. Gold said. Emotions tied to COVID-19 are just a part of it. Confounding factors, such as climate change, racism, and school shootings all contribute to their overall mental health.
Some children and adolescents with social anxiety have fared better during the pandemic, but those who are part of demographic groups such as racial and ethnic minorities, LGBTQ individuals, low-income youth, and those involved in juvenile justice or welfare systems face a higher risk of mental health challenges, the pandemic notwithstanding.
In her work with schools, Denese Shervington, MD, MPH, has witnessed more mental health challenges related to isolation and separation. “There’s an overall worry about the loss of what used to be, the seeming predictability and certainty of prepandemic life,” said Dr. Shervington, clinical professor of psychiatry at Tulane University, and president and CEO of the Institute for Women and Ethnic Studies, both in New Orleans.
A systems of care plan
The advisory lists actionable items for health care and 10 other industry sectors to improve mental health of children and young adults.
Health care organizations and professionals were advised to take the following six steps:
- Implement trauma-informed care principles and other prevention strategies. This may involve referring patients to resources such as economic and legal supports, school enrichment programs, and educating families on healthy child development in the clinic.
- Routinely screen children for mental health challenges and risk factors such as adverse childhood experiences during primary care well-visits or annual physicals, or at schools or EDs. Primary care physicians should use principles of trauma-informed care to conduct these screenings.
- Screen parents, caregivers, and other family members for depression, intimate partner violence, substance use, and other challenges. These can be done in tandem with broader assessments of social determinants of health such as food or housing insecurity.
- Combine efforts of clinical staff with trusted community partners and child welfare and juvenile justice. Hospital-based violence intervention programs, for example, identify patients at risk of repeat violent injury and refer them to hospital- and community-based resources.
- Build multidisciplinary teams, enlisting children and families to develop services that are tailored to their needs for screening and treatment. Such services should reflect cultural diversity and offered in multiple languages.
- Support the well-being of mental health workers and community leaders to foster their ability to help youth and their families.
Dr. Murthy is talking about a “systems of care” approach, in which all sectors that touch children and youth – not just health care – must work together and do their jobs effectively but collaboratively to address this public health crisis, said Aradhana (Bela) Sood MD, MSHA, FAACAP, senior professor of child mental health policy at Virginia Commonwealth University, Richmond. “An investment in infrastructure support of positive mental health in early childhood, be it in schools, communities, or family well-being will lead to a future where illness is not the result of major preventable societal factors, such as a lack of social supports and trauma.”
Changes will ‘take a lot of buy-in’
The recommendations are actionable in the real world – but there are a lot of them, said Dr. Gold. Dr. Murthy doesn’t specify what the plan is to accomplish these metrics or fund them, she added. He “has money and funders like foundations as steps, but foundations have also suffered in the pandemic, so it is not that simple.” Many of these changes are wide in scope and will take a lot of buy-in.
Dr. Shervington would like to have seen more of a focus on educator well-being, given that young people spend a lot of time in educational settings.
“My organization just completed a study in New Orleans that showed teachers having elevated levels of trauma-based conditions since the pandemic,” she said. Schools are indeed a key place to support holistic mental health by focusing on school climate, Dr. Sood added. “If school administrators became uniformly consistent with recognizing the importance of psychological wellness as a prerequisite of good learning, they will create environments where teachers are keenly aware of a child’s mental wellness and make reduction of bullying, wellness check-ins, [and] school-based mental health clinics a priority.
“These are ways nonmedical, community-based supports can enhance student well-being, and reduce depression and other mental health conditions,” Dr. Sood added.
Child psychiatrists stretched ‘even thinner’
Despite mental health parity rules, health plans have not been held accountable. That failure, combined with excessive demands for prior authorization for mental health treatments “have led to dangerous shortages of psychiatrists able to accept insurance,” said Paul S. Nestadt, MD, an assistant professor and public mental health researcher at Johns Hopkins University, Baltimore.
“This is particularly true for child psychiatrists, who are stretched even thinner than those of us in general practice,” Dr. Nestadt said.
While he doesn’t address it head on, Dr. Murthy uses classic parity language when he states that “mental health is no less important than physical health,” said Dr. Nestadt, who consulted with the surgeon general on developing this advisory. “While many of us would have liked to see parity highlighted more directly, this advisory was designed to be an overview.”
Highlighting social media, gun violence
Dr. Nestadt said he was pleased that the advisory emphasized the importance of restricting access to lethal means in preventing youth suicide.
“With youth suicide rates rising faster than in other age groups, and suicide mortality tied so closely to method availability, the surgeon general made the right choice in highlighting the role of guns in suicide,” he said.
The advisory also discussed the role of media and social media companies in addressing the crisis, which is important, said Dr. Gold.
“I believe very strongly that the way we talk about and portray mental health in the media matters,” she said. “I have seen it matter in the clinic with patients. They’ll wonder if someone will think they are now violent if they are diagnosed with a mental illness. Stories change the narrative.”
While the advisory isn’t perfect, the state of youth mental health “will only get worse if we don’t do something,” noted Dr. Gold. “It is critical that this is validated and discussed at the highest level and messages like Dr. Murthy’s get heard.”
Dr. Gold, Dr. Shervington, and Dr. Sood had no disclosures. Dr. Nestadt disclosed serving as a consultant to the surgeon general advisory.
A pandemic silver lining? Dramatic drop in teen drug use
Illicit drug use among U.S. teenagers dropped sharply in 2021, likely because of stay-at-home orders and other restrictions on social activities due to the COVID-19 pandemic.
The latest findings, from the Monitoring the Future survey, represent the largest 1-year decrease in overall illicit drug use reported since the survey began in 1975.
“We have never seen such dramatic decreases in drug use among teens in just a 1-year period,” Nora Volkow, MD, director of the National Institute on Drug Abuse (NIDA), said in a news release.
“These data are unprecedented and highlight one unexpected potential consequence of the COVID-19 pandemic, which caused seismic shifts in the day-to-day lives of adolescents,” said Dr. Volkow.
The annual Monitoring the Future survey is conducted by researchers at the University of Michigan, Ann Arbor, and funded by NIDA, to assess drug and alcohol use and related attitudes among adolescent students across the United States.
This year’s self-reported survey included 32,260 students in grades 8, 10, and 12 across 319 public and private schools.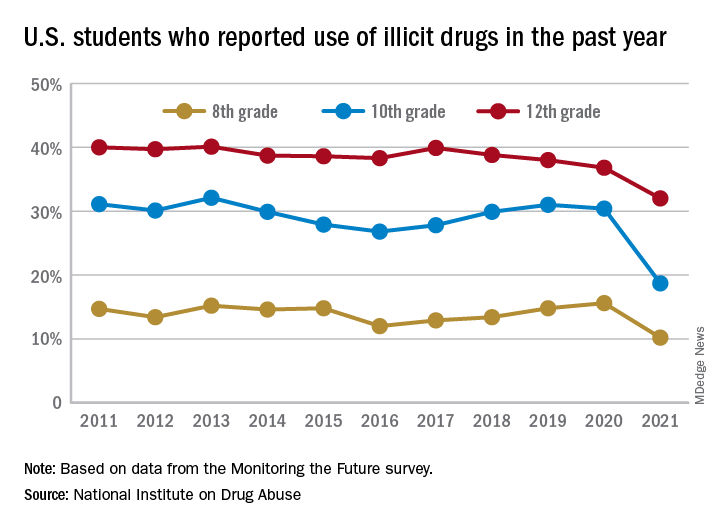
Compared with 2020, the percentage of students reporting any illicit drug use (other than marijuana) in 2021 decreased significantly for 8th graders (down 5.4%), 10th graders (down 11.7%), and 12th graders (down 4.8%).
For alcohol, about 47% of 12th graders and 29% of 10th graders said they drank alcohol in 2021, down significantly from 55% and 41%, respectively, in 2020. The percentage of 8th graders who said they drank alcohol remained stable (17% in 2021 and 20% in 2020).
For teen vaping, about 27% of 12th graders and 20% of 10th graders said they had vaped nicotine in 2021, down significantly from nearly 35% and 31%, respectively, in 2020. Fewer 8th graders also vaped nicotine in 2021 compared with 2020 (12% vs. 17%).
For marijuana, use dropped significantly for all three grades in 2021 compared with 2020. About 31% of 12th graders and 17% of 10th graders said they used marijuana in 2021, down from 35% and 28% in 2020. Among 8th graders, 7% used marijuana in 2021, down from 11% in 2020.
The latest survey also shows significant declines in use of a range of other drugs for many of the age cohorts, including cocaine, hallucinogens, and nonmedical use of amphetamines, tranquilizers, and prescription opioids.
“We knew that this year’s data would illuminate how the COVID-19 pandemic may have impacted substance use among young people, and in the coming years, we will find out whether those impacts are long-lasting as we continue tracking the drug use patterns of these unique cohorts of adolescents,” Richard A. Miech, PhD, who heads the Monitoring the Future study at the University of Michigan, said in the news release.
“Moving forward, it will be crucial to identify the pivotal elements of this past year that contributed to decreased drug use – whether related to drug availability, family involvement, differences in peer pressure, or other factors – and harness them to inform future prevention efforts,” Dr. Volkow added.
In 2021, students across all age groups reported moderate increases in feelings of boredom, anxiety, depression, loneliness, worry, difficulty sleeping, and other negative mental health indicators since the beginning of the pandemic.
A version of this article first appeared on Medscape.com.
Illicit drug use among U.S. teenagers dropped sharply in 2021, likely because of stay-at-home orders and other restrictions on social activities due to the COVID-19 pandemic.
The latest findings, from the Monitoring the Future survey, represent the largest 1-year decrease in overall illicit drug use reported since the survey began in 1975.
“We have never seen such dramatic decreases in drug use among teens in just a 1-year period,” Nora Volkow, MD, director of the National Institute on Drug Abuse (NIDA), said in a news release.
“These data are unprecedented and highlight one unexpected potential consequence of the COVID-19 pandemic, which caused seismic shifts in the day-to-day lives of adolescents,” said Dr. Volkow.
The annual Monitoring the Future survey is conducted by researchers at the University of Michigan, Ann Arbor, and funded by NIDA, to assess drug and alcohol use and related attitudes among adolescent students across the United States.
This year’s self-reported survey included 32,260 students in grades 8, 10, and 12 across 319 public and private schools.
Compared with 2020, the percentage of students reporting any illicit drug use (other than marijuana) in 2021 decreased significantly for 8th graders (down 5.4%), 10th graders (down 11.7%), and 12th graders (down 4.8%).
For alcohol, about 47% of 12th graders and 29% of 10th graders said they drank alcohol in 2021, down significantly from 55% and 41%, respectively, in 2020. The percentage of 8th graders who said they drank alcohol remained stable (17% in 2021 and 20% in 2020).
For teen vaping, about 27% of 12th graders and 20% of 10th graders said they had vaped nicotine in 2021, down significantly from nearly 35% and 31%, respectively, in 2020. Fewer 8th graders also vaped nicotine in 2021 compared with 2020 (12% vs. 17%).
For marijuana, use dropped significantly for all three grades in 2021 compared with 2020. About 31% of 12th graders and 17% of 10th graders said they used marijuana in 2021, down from 35% and 28% in 2020. Among 8th graders, 7% used marijuana in 2021, down from 11% in 2020.
The latest survey also shows significant declines in use of a range of other drugs for many of the age cohorts, including cocaine, hallucinogens, and nonmedical use of amphetamines, tranquilizers, and prescription opioids.
“We knew that this year’s data would illuminate how the COVID-19 pandemic may have impacted substance use among young people, and in the coming years, we will find out whether those impacts are long-lasting as we continue tracking the drug use patterns of these unique cohorts of adolescents,” Richard A. Miech, PhD, who heads the Monitoring the Future study at the University of Michigan, said in the news release.
“Moving forward, it will be crucial to identify the pivotal elements of this past year that contributed to decreased drug use – whether related to drug availability, family involvement, differences in peer pressure, or other factors – and harness them to inform future prevention efforts,” Dr. Volkow added.
In 2021, students across all age groups reported moderate increases in feelings of boredom, anxiety, depression, loneliness, worry, difficulty sleeping, and other negative mental health indicators since the beginning of the pandemic.
A version of this article first appeared on Medscape.com.
Illicit drug use among U.S. teenagers dropped sharply in 2021, likely because of stay-at-home orders and other restrictions on social activities due to the COVID-19 pandemic.
The latest findings, from the Monitoring the Future survey, represent the largest 1-year decrease in overall illicit drug use reported since the survey began in 1975.
“We have never seen such dramatic decreases in drug use among teens in just a 1-year period,” Nora Volkow, MD, director of the National Institute on Drug Abuse (NIDA), said in a news release.
“These data are unprecedented and highlight one unexpected potential consequence of the COVID-19 pandemic, which caused seismic shifts in the day-to-day lives of adolescents,” said Dr. Volkow.
The annual Monitoring the Future survey is conducted by researchers at the University of Michigan, Ann Arbor, and funded by NIDA, to assess drug and alcohol use and related attitudes among adolescent students across the United States.
This year’s self-reported survey included 32,260 students in grades 8, 10, and 12 across 319 public and private schools.
Compared with 2020, the percentage of students reporting any illicit drug use (other than marijuana) in 2021 decreased significantly for 8th graders (down 5.4%), 10th graders (down 11.7%), and 12th graders (down 4.8%).
For alcohol, about 47% of 12th graders and 29% of 10th graders said they drank alcohol in 2021, down significantly from 55% and 41%, respectively, in 2020. The percentage of 8th graders who said they drank alcohol remained stable (17% in 2021 and 20% in 2020).
For teen vaping, about 27% of 12th graders and 20% of 10th graders said they had vaped nicotine in 2021, down significantly from nearly 35% and 31%, respectively, in 2020. Fewer 8th graders also vaped nicotine in 2021 compared with 2020 (12% vs. 17%).
For marijuana, use dropped significantly for all three grades in 2021 compared with 2020. About 31% of 12th graders and 17% of 10th graders said they used marijuana in 2021, down from 35% and 28% in 2020. Among 8th graders, 7% used marijuana in 2021, down from 11% in 2020.
The latest survey also shows significant declines in use of a range of other drugs for many of the age cohorts, including cocaine, hallucinogens, and nonmedical use of amphetamines, tranquilizers, and prescription opioids.
“We knew that this year’s data would illuminate how the COVID-19 pandemic may have impacted substance use among young people, and in the coming years, we will find out whether those impacts are long-lasting as we continue tracking the drug use patterns of these unique cohorts of adolescents,” Richard A. Miech, PhD, who heads the Monitoring the Future study at the University of Michigan, said in the news release.
“Moving forward, it will be crucial to identify the pivotal elements of this past year that contributed to decreased drug use – whether related to drug availability, family involvement, differences in peer pressure, or other factors – and harness them to inform future prevention efforts,” Dr. Volkow added.
In 2021, students across all age groups reported moderate increases in feelings of boredom, anxiety, depression, loneliness, worry, difficulty sleeping, and other negative mental health indicators since the beginning of the pandemic.
A version of this article first appeared on Medscape.com.
Pfizer says its COVID-19 pill is highly effective
, according to the drug’s maker, Pfizer.
The drug -- called Paxlovid -- was 89% effective, compared to a placebo, at preventing hospitalization or death in patients with COVID-19 who were at high risk of severe complications. The company says it plans to ask the FDA to authorize the drug for emergency use.
The medication appears to work so well that Pfizer has stopped enrollment in the trial of the drug, which works by blocking an enzyme called a protease that the new coronavirus needs to make more copies of itself.
Stopping a clinical trial is a rare action that’s typically taken when a therapy appears to be very effective or clearly dangerous. In both those cases, it’s considered unethical to continue a clinical trial where people are randomly assigned either an active drug or a placebo, when safer or more effective options are available to them.
In this case, the company said in a news release that the move was recommended by an independent panel of advisers who are overseeing the trial, called a data safety monitoring committee, and done in consultation with the FDA.
“Today’s news is a real game-changer in the global efforts to halt the devastation of this pandemic,” said Albert Bourla, PhD, Pfizer chairman and chief executive officer. “These data suggest that our oral antiviral candidate, if approved or authorized by regulatory authorities, has the potential to save patients’ lives, reduce the severity of COVID-19 infections, and eliminate up to nine out of ten hospitalizations.”
In a randomized clinical trial that included more than 1,900 patients who tested positive for COVID-19 and were at risk for having severe complications for their infections, those who received Paxlovid within 3 days of the start of their symptoms were 89% less likely to be hospitalized than those who got a placebo pill -- three patients out of 389 who got the drug were hospitalized, compared with 27 out of 385 who got the placebo. Among patients who got the drug within 5 days of the start of their symptoms, six out of 607 were hospitalized within 28 days, compared to 41 out of 612 who got the placebo.
There were no deaths over the course of a month in patients who took Paxlovid, but 10 deaths in the group that got the placebo.
The news comes on the heels of an announcement in October by the drug company Merck that its experimental antiviral pill, molnupiravir, reduced the risk of hospitalization or death by 50% in patients with mild to moderate COVID, compared to a placebo.
The United Kingdom became the first country to authorize the use of molnupiravir, which is brand-named Lagevrio.
Stephen Griffin, PhD, an associate professor of medicine at the University of Leeds, hailed the success of both new antiviral pills.
“They both demonstrate that, with appropriate investment, the development of bespoke direct-acting antiviral drugs targeting SARS-CoV2 was eminently feasible and has ultimately proven far more successful than repurposing other drugs with questionable antiviral effects,” said Dr. Griffin, who was not involved in the development of either drug.
“The success of these antivirals potentially marks a new era in our ability to prevent the severe consequences of SARS-CoV2 infection, and is also a vital element for the care of clinically vulnerable people who may be unable to either receive or respond to vaccines,” he said.
A version of this article first appeared on WebMD.com.
, according to the drug’s maker, Pfizer.
The drug -- called Paxlovid -- was 89% effective, compared to a placebo, at preventing hospitalization or death in patients with COVID-19 who were at high risk of severe complications. The company says it plans to ask the FDA to authorize the drug for emergency use.
The medication appears to work so well that Pfizer has stopped enrollment in the trial of the drug, which works by blocking an enzyme called a protease that the new coronavirus needs to make more copies of itself.
Stopping a clinical trial is a rare action that’s typically taken when a therapy appears to be very effective or clearly dangerous. In both those cases, it’s considered unethical to continue a clinical trial where people are randomly assigned either an active drug or a placebo, when safer or more effective options are available to them.
In this case, the company said in a news release that the move was recommended by an independent panel of advisers who are overseeing the trial, called a data safety monitoring committee, and done in consultation with the FDA.
“Today’s news is a real game-changer in the global efforts to halt the devastation of this pandemic,” said Albert Bourla, PhD, Pfizer chairman and chief executive officer. “These data suggest that our oral antiviral candidate, if approved or authorized by regulatory authorities, has the potential to save patients’ lives, reduce the severity of COVID-19 infections, and eliminate up to nine out of ten hospitalizations.”
In a randomized clinical trial that included more than 1,900 patients who tested positive for COVID-19 and were at risk for having severe complications for their infections, those who received Paxlovid within 3 days of the start of their symptoms were 89% less likely to be hospitalized than those who got a placebo pill -- three patients out of 389 who got the drug were hospitalized, compared with 27 out of 385 who got the placebo. Among patients who got the drug within 5 days of the start of their symptoms, six out of 607 were hospitalized within 28 days, compared to 41 out of 612 who got the placebo.
There were no deaths over the course of a month in patients who took Paxlovid, but 10 deaths in the group that got the placebo.
The news comes on the heels of an announcement in October by the drug company Merck that its experimental antiviral pill, molnupiravir, reduced the risk of hospitalization or death by 50% in patients with mild to moderate COVID, compared to a placebo.
The United Kingdom became the first country to authorize the use of molnupiravir, which is brand-named Lagevrio.
Stephen Griffin, PhD, an associate professor of medicine at the University of Leeds, hailed the success of both new antiviral pills.
“They both demonstrate that, with appropriate investment, the development of bespoke direct-acting antiviral drugs targeting SARS-CoV2 was eminently feasible and has ultimately proven far more successful than repurposing other drugs with questionable antiviral effects,” said Dr. Griffin, who was not involved in the development of either drug.
“The success of these antivirals potentially marks a new era in our ability to prevent the severe consequences of SARS-CoV2 infection, and is also a vital element for the care of clinically vulnerable people who may be unable to either receive or respond to vaccines,” he said.
A version of this article first appeared on WebMD.com.
, according to the drug’s maker, Pfizer.
The drug -- called Paxlovid -- was 89% effective, compared to a placebo, at preventing hospitalization or death in patients with COVID-19 who were at high risk of severe complications. The company says it plans to ask the FDA to authorize the drug for emergency use.
The medication appears to work so well that Pfizer has stopped enrollment in the trial of the drug, which works by blocking an enzyme called a protease that the new coronavirus needs to make more copies of itself.
Stopping a clinical trial is a rare action that’s typically taken when a therapy appears to be very effective or clearly dangerous. In both those cases, it’s considered unethical to continue a clinical trial where people are randomly assigned either an active drug or a placebo, when safer or more effective options are available to them.
In this case, the company said in a news release that the move was recommended by an independent panel of advisers who are overseeing the trial, called a data safety monitoring committee, and done in consultation with the FDA.
“Today’s news is a real game-changer in the global efforts to halt the devastation of this pandemic,” said Albert Bourla, PhD, Pfizer chairman and chief executive officer. “These data suggest that our oral antiviral candidate, if approved or authorized by regulatory authorities, has the potential to save patients’ lives, reduce the severity of COVID-19 infections, and eliminate up to nine out of ten hospitalizations.”
In a randomized clinical trial that included more than 1,900 patients who tested positive for COVID-19 and were at risk for having severe complications for their infections, those who received Paxlovid within 3 days of the start of their symptoms were 89% less likely to be hospitalized than those who got a placebo pill -- three patients out of 389 who got the drug were hospitalized, compared with 27 out of 385 who got the placebo. Among patients who got the drug within 5 days of the start of their symptoms, six out of 607 were hospitalized within 28 days, compared to 41 out of 612 who got the placebo.
There were no deaths over the course of a month in patients who took Paxlovid, but 10 deaths in the group that got the placebo.
The news comes on the heels of an announcement in October by the drug company Merck that its experimental antiviral pill, molnupiravir, reduced the risk of hospitalization or death by 50% in patients with mild to moderate COVID, compared to a placebo.
The United Kingdom became the first country to authorize the use of molnupiravir, which is brand-named Lagevrio.
Stephen Griffin, PhD, an associate professor of medicine at the University of Leeds, hailed the success of both new antiviral pills.
“They both demonstrate that, with appropriate investment, the development of bespoke direct-acting antiviral drugs targeting SARS-CoV2 was eminently feasible and has ultimately proven far more successful than repurposing other drugs with questionable antiviral effects,” said Dr. Griffin, who was not involved in the development of either drug.
“The success of these antivirals potentially marks a new era in our ability to prevent the severe consequences of SARS-CoV2 infection, and is also a vital element for the care of clinically vulnerable people who may be unable to either receive or respond to vaccines,” he said.
A version of this article first appeared on WebMD.com.