User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
How to explain physician compounding to legislators
In Ohio, new limits on drug compounding in physicians’ offices went into effect in April and have become a real hindrance to care for dermatology patients. The State of Ohio Board of Pharmacy has defined compounding as combining two or more prescription drugs and has required that physicians who perform this “compounding” must obtain a “Terminal Distributor of Dangerous Drugs” license. Ohio is the “test state,” and these rules, unless vigorously opposed, will be coming to your state.
[polldaddy:9779752]
The rules state that “compounded” drugs used within 6 hours of preparation must be prepared in a designated clean medication area with proper hand hygiene and the use of powder-free gloves. “Compounded” drugs that are used more than 6 hours after preparation, require a designated clean room with access limited to authorized personnel, environmental control devices such as a laminar flow hood, and additional equipment and training of personnel to maintain an aseptic environment. A separate license is required for each office location.
The state pharmacy boards are eager to restrict physicians – as well as dentists and veterinarians – and to collect annual licensing fees. Additionally, according to an article from the Ohio State Medical Association, noncompliant physicians can be fined by the pharmacy board.
We are talking big money, power, and dreams of clinical relevancy (and billable activities) here.
What can dermatologists do to prevent this regulatory overreach? I encourage you to plan a visit to your state representative, where you can demonstrate how these restrictions affect you and your patients – an exercise that should be both fun and compelling. All you need to illustrate your case is a simple kit that includes a syringe (but no needles in the statehouse!), a bottle of lidocaine with epinephrine, a bottle of 8.4% bicarbonate, alcohol pads, and gloves.
First, explain to your audience that there is a skin cancer epidemic with more than 5.4 million new cases a year and that, over the past 20 years, the incidence of skin cancer has doubled and is projected to double again over the next 20 years. Further, explain that dermatologists treat more than 70% of these cases in the office setting, under local anesthesia, at a huge cost savings to the public and government (it costs an average of 12 times as much to remove these cancers in the outpatient department at the hospital). Remember, states foot most of the bill for Medicaid and Medicare gap indigent coverage.
Take the bottle of lidocaine with epinephrine and open the syringe pack (Staffers love this demonstration; everyone is fascinated with shots.). Put on your gloves, wipe the top of the lidocaine bottle with an alcohol swab, and explain that this medicine is the anesthetic preferred for skin cancer surgery. Explain how it not only numbs the skin, but also causes vasoconstriction, so that the cancer can be easily and safely removed in the office.
Then explain that, in order for the epinephrine to be stable, the solution has to be very acidic (a pH of 4.2, in fact). Explain that this makes it burn like hell unless you add 0.1 cc per cc of 8.4% bicarbonate, in which case the perceived pain on a 10-point scale will drop from 8 to 2. Then pick up the bottle of bicarbonate and explain that you will no longer be able to mix these two components anymore without a “Terminal Distributor of Dangerous Drugs” license because your state pharmacy board considers this compounding. Your representative is likely to give you looks of astonishment, disbelief, and then a dawning realization of the absurdity of the situation.
Follow-up questions may include “Why can’t you buy buffered lidocaine with epinephrine from the compounding pharmacy?” Easy answer: because each patient needs an individual prescription, and you may not know in advance which patient will need it, and how much the patient will need, and it becomes unstable once it has been buffered. It also will cost the patient $45 per 5-cc syringe, and it will be degraded by the time the patient returns from the compounding pharmacy. Explain further that it costs you only 84 cents to make a 5-cc syringe of buffered lidocaine; that some patients may need as many as 10 syringes; and that these costs are all included in the surgery (free!) if the physician draws it up in the office.
A simple summary is – less pain, less cost – and no history of infections or complications.
It is an eye-opener when you demonstrate how ridiculous the compounding rules being imposed are for physicians and patients. I’ve used this demonstration at the state and federal legislative level, and more recently, at the Food and Drug Administration.
If you get the chance, when a state legislator is in your office, become an advocate for your patients and fellow physicians. Make sure physician offices are excluded from these definitions of com
This column was updated June 22, 2017.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at dermnews@frontlinemedcom.com.
In Ohio, new limits on drug compounding in physicians’ offices went into effect in April and have become a real hindrance to care for dermatology patients. The State of Ohio Board of Pharmacy has defined compounding as combining two or more prescription drugs and has required that physicians who perform this “compounding” must obtain a “Terminal Distributor of Dangerous Drugs” license. Ohio is the “test state,” and these rules, unless vigorously opposed, will be coming to your state.
[polldaddy:9779752]
The rules state that “compounded” drugs used within 6 hours of preparation must be prepared in a designated clean medication area with proper hand hygiene and the use of powder-free gloves. “Compounded” drugs that are used more than 6 hours after preparation, require a designated clean room with access limited to authorized personnel, environmental control devices such as a laminar flow hood, and additional equipment and training of personnel to maintain an aseptic environment. A separate license is required for each office location.
The state pharmacy boards are eager to restrict physicians – as well as dentists and veterinarians – and to collect annual licensing fees. Additionally, according to an article from the Ohio State Medical Association, noncompliant physicians can be fined by the pharmacy board.
We are talking big money, power, and dreams of clinical relevancy (and billable activities) here.
What can dermatologists do to prevent this regulatory overreach? I encourage you to plan a visit to your state representative, where you can demonstrate how these restrictions affect you and your patients – an exercise that should be both fun and compelling. All you need to illustrate your case is a simple kit that includes a syringe (but no needles in the statehouse!), a bottle of lidocaine with epinephrine, a bottle of 8.4% bicarbonate, alcohol pads, and gloves.
First, explain to your audience that there is a skin cancer epidemic with more than 5.4 million new cases a year and that, over the past 20 years, the incidence of skin cancer has doubled and is projected to double again over the next 20 years. Further, explain that dermatologists treat more than 70% of these cases in the office setting, under local anesthesia, at a huge cost savings to the public and government (it costs an average of 12 times as much to remove these cancers in the outpatient department at the hospital). Remember, states foot most of the bill for Medicaid and Medicare gap indigent coverage.
Take the bottle of lidocaine with epinephrine and open the syringe pack (Staffers love this demonstration; everyone is fascinated with shots.). Put on your gloves, wipe the top of the lidocaine bottle with an alcohol swab, and explain that this medicine is the anesthetic preferred for skin cancer surgery. Explain how it not only numbs the skin, but also causes vasoconstriction, so that the cancer can be easily and safely removed in the office.
Then explain that, in order for the epinephrine to be stable, the solution has to be very acidic (a pH of 4.2, in fact). Explain that this makes it burn like hell unless you add 0.1 cc per cc of 8.4% bicarbonate, in which case the perceived pain on a 10-point scale will drop from 8 to 2. Then pick up the bottle of bicarbonate and explain that you will no longer be able to mix these two components anymore without a “Terminal Distributor of Dangerous Drugs” license because your state pharmacy board considers this compounding. Your representative is likely to give you looks of astonishment, disbelief, and then a dawning realization of the absurdity of the situation.
Follow-up questions may include “Why can’t you buy buffered lidocaine with epinephrine from the compounding pharmacy?” Easy answer: because each patient needs an individual prescription, and you may not know in advance which patient will need it, and how much the patient will need, and it becomes unstable once it has been buffered. It also will cost the patient $45 per 5-cc syringe, and it will be degraded by the time the patient returns from the compounding pharmacy. Explain further that it costs you only 84 cents to make a 5-cc syringe of buffered lidocaine; that some patients may need as many as 10 syringes; and that these costs are all included in the surgery (free!) if the physician draws it up in the office.
A simple summary is – less pain, less cost – and no history of infections or complications.
It is an eye-opener when you demonstrate how ridiculous the compounding rules being imposed are for physicians and patients. I’ve used this demonstration at the state and federal legislative level, and more recently, at the Food and Drug Administration.
If you get the chance, when a state legislator is in your office, become an advocate for your patients and fellow physicians. Make sure physician offices are excluded from these definitions of com
This column was updated June 22, 2017.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at dermnews@frontlinemedcom.com.
In Ohio, new limits on drug compounding in physicians’ offices went into effect in April and have become a real hindrance to care for dermatology patients. The State of Ohio Board of Pharmacy has defined compounding as combining two or more prescription drugs and has required that physicians who perform this “compounding” must obtain a “Terminal Distributor of Dangerous Drugs” license. Ohio is the “test state,” and these rules, unless vigorously opposed, will be coming to your state.
[polldaddy:9779752]
The rules state that “compounded” drugs used within 6 hours of preparation must be prepared in a designated clean medication area with proper hand hygiene and the use of powder-free gloves. “Compounded” drugs that are used more than 6 hours after preparation, require a designated clean room with access limited to authorized personnel, environmental control devices such as a laminar flow hood, and additional equipment and training of personnel to maintain an aseptic environment. A separate license is required for each office location.
The state pharmacy boards are eager to restrict physicians – as well as dentists and veterinarians – and to collect annual licensing fees. Additionally, according to an article from the Ohio State Medical Association, noncompliant physicians can be fined by the pharmacy board.
We are talking big money, power, and dreams of clinical relevancy (and billable activities) here.
What can dermatologists do to prevent this regulatory overreach? I encourage you to plan a visit to your state representative, where you can demonstrate how these restrictions affect you and your patients – an exercise that should be both fun and compelling. All you need to illustrate your case is a simple kit that includes a syringe (but no needles in the statehouse!), a bottle of lidocaine with epinephrine, a bottle of 8.4% bicarbonate, alcohol pads, and gloves.
First, explain to your audience that there is a skin cancer epidemic with more than 5.4 million new cases a year and that, over the past 20 years, the incidence of skin cancer has doubled and is projected to double again over the next 20 years. Further, explain that dermatologists treat more than 70% of these cases in the office setting, under local anesthesia, at a huge cost savings to the public and government (it costs an average of 12 times as much to remove these cancers in the outpatient department at the hospital). Remember, states foot most of the bill for Medicaid and Medicare gap indigent coverage.
Take the bottle of lidocaine with epinephrine and open the syringe pack (Staffers love this demonstration; everyone is fascinated with shots.). Put on your gloves, wipe the top of the lidocaine bottle with an alcohol swab, and explain that this medicine is the anesthetic preferred for skin cancer surgery. Explain how it not only numbs the skin, but also causes vasoconstriction, so that the cancer can be easily and safely removed in the office.
Then explain that, in order for the epinephrine to be stable, the solution has to be very acidic (a pH of 4.2, in fact). Explain that this makes it burn like hell unless you add 0.1 cc per cc of 8.4% bicarbonate, in which case the perceived pain on a 10-point scale will drop from 8 to 2. Then pick up the bottle of bicarbonate and explain that you will no longer be able to mix these two components anymore without a “Terminal Distributor of Dangerous Drugs” license because your state pharmacy board considers this compounding. Your representative is likely to give you looks of astonishment, disbelief, and then a dawning realization of the absurdity of the situation.
Follow-up questions may include “Why can’t you buy buffered lidocaine with epinephrine from the compounding pharmacy?” Easy answer: because each patient needs an individual prescription, and you may not know in advance which patient will need it, and how much the patient will need, and it becomes unstable once it has been buffered. It also will cost the patient $45 per 5-cc syringe, and it will be degraded by the time the patient returns from the compounding pharmacy. Explain further that it costs you only 84 cents to make a 5-cc syringe of buffered lidocaine; that some patients may need as many as 10 syringes; and that these costs are all included in the surgery (free!) if the physician draws it up in the office.
A simple summary is – less pain, less cost – and no history of infections or complications.
It is an eye-opener when you demonstrate how ridiculous the compounding rules being imposed are for physicians and patients. I’ve used this demonstration at the state and federal legislative level, and more recently, at the Food and Drug Administration.
If you get the chance, when a state legislator is in your office, become an advocate for your patients and fellow physicians. Make sure physician offices are excluded from these definitions of com
This column was updated June 22, 2017.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at dermnews@frontlinemedcom.com.
Best Practices: Protecting Dry Vulnerable Skin with CeraVe® Healing Ointment
A supplement to Dermatology News. This advertising supplement is sponsored by Valeant Pharmaceuticals.
- Reinforcing the Skin Barrier
- NEA Seal of Acceptance
- A Preventative Approach to Dry, Cracked Skin
- CeraVe Ointment in the Clinical Setting
Faculty/Faculty Disclosure
Sheila Fallon Friedlander, MD
Professor of Clinical Dermatology & Pediatrics
Director, Pediatric Dermatology Fellowship Training Program
University of California at San Diego School of Medicine
Rady Children’s Hospital,
San Diego, California
Dr. Friedlander was compensated for her participation in the development of this article.
CeraVe is a registered trademark of Valeant Pharmaceuticals International, Inc. or its affiliates.
A supplement to Dermatology News. This advertising supplement is sponsored by Valeant Pharmaceuticals.
- Reinforcing the Skin Barrier
- NEA Seal of Acceptance
- A Preventative Approach to Dry, Cracked Skin
- CeraVe Ointment in the Clinical Setting
Faculty/Faculty Disclosure
Sheila Fallon Friedlander, MD
Professor of Clinical Dermatology & Pediatrics
Director, Pediatric Dermatology Fellowship Training Program
University of California at San Diego School of Medicine
Rady Children’s Hospital,
San Diego, California
Dr. Friedlander was compensated for her participation in the development of this article.
CeraVe is a registered trademark of Valeant Pharmaceuticals International, Inc. or its affiliates.
A supplement to Dermatology News. This advertising supplement is sponsored by Valeant Pharmaceuticals.
- Reinforcing the Skin Barrier
- NEA Seal of Acceptance
- A Preventative Approach to Dry, Cracked Skin
- CeraVe Ointment in the Clinical Setting
Faculty/Faculty Disclosure
Sheila Fallon Friedlander, MD
Professor of Clinical Dermatology & Pediatrics
Director, Pediatric Dermatology Fellowship Training Program
University of California at San Diego School of Medicine
Rady Children’s Hospital,
San Diego, California
Dr. Friedlander was compensated for her participation in the development of this article.
CeraVe is a registered trademark of Valeant Pharmaceuticals International, Inc. or its affiliates.
When Does Different Types of Organ Damage From Lupus Occur? Long-Term Study Sheds Light
TOPLINE:
The first year after the diagnosis of systemic lupus erythematosus (SLE) is crucial, with the highest percentage of patients experiencing organ damage. Cardiovascular issues are the second most prevalent after musculoskeletal damage in both early and later stages of SLE.
METHODOLOGY:
- Researchers assessed organ damage persisting at least 6 months over different stages of lupus in 4219 patients with SLE (mean age, 35.9 years; 89.6% women) from the Spanish Society of Rheumatology Lupus Registry.
- Damage was assessed using the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index (SDI).
- Longitudinal analysis was conducted globally and by each SDI domain on 1274 patients with recorded damage event dates.
- Follow-up data were available out to 10 years in 1113 patients and to 20 years in 601.
TAKEAWAY:
- New damage was recorded in 20% of the patients with SLE within the first year after diagnosis, with the annual percentage of patients with new damage decreasing to 5% after the first 5 years of follow-up.
- In the first year, musculoskeletal damage was reported by the highest proportion of patients (21%), followed by cardiovascular damage inclusive of cerebrovascular accidents and claudication for 6 months (19%).
- The cardiovascular system remained the second most affected system even during the later stages of the diseases at years 10 and 20 of follow-up (20%-25%).
- Apart from musculoskeletal and cardiovascular damage, patients with lupus also showed renal and ocular damage in the early and later stages of the disease, respectively.
IN PRACTICE:
“Our study highlights the importance of cardiovascular damage and the need for its prevention during the earliest stages of the disease,” the authors wrote.
SOURCE:
The study was led by Irene Altabás-González, MD, PhD, Rheumatology Department, Vigo University Hospital Group, Vigo, Spain. It was published online in Lupus Science & Medicine.
LIMITATIONS:
The retrospective collection of data in the study may have led to missing items; for example, the dates of damage events for the whole cohort were not available.
DISCLOSURES:
The registry was supported by the Spanish Society of Rheumatology. No specific funding was received for the study. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
TOPLINE:
The first year after the diagnosis of systemic lupus erythematosus (SLE) is crucial, with the highest percentage of patients experiencing organ damage. Cardiovascular issues are the second most prevalent after musculoskeletal damage in both early and later stages of SLE.
METHODOLOGY:
- Researchers assessed organ damage persisting at least 6 months over different stages of lupus in 4219 patients with SLE (mean age, 35.9 years; 89.6% women) from the Spanish Society of Rheumatology Lupus Registry.
- Damage was assessed using the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index (SDI).
- Longitudinal analysis was conducted globally and by each SDI domain on 1274 patients with recorded damage event dates.
- Follow-up data were available out to 10 years in 1113 patients and to 20 years in 601.
TAKEAWAY:
- New damage was recorded in 20% of the patients with SLE within the first year after diagnosis, with the annual percentage of patients with new damage decreasing to 5% after the first 5 years of follow-up.
- In the first year, musculoskeletal damage was reported by the highest proportion of patients (21%), followed by cardiovascular damage inclusive of cerebrovascular accidents and claudication for 6 months (19%).
- The cardiovascular system remained the second most affected system even during the later stages of the diseases at years 10 and 20 of follow-up (20%-25%).
- Apart from musculoskeletal and cardiovascular damage, patients with lupus also showed renal and ocular damage in the early and later stages of the disease, respectively.
IN PRACTICE:
“Our study highlights the importance of cardiovascular damage and the need for its prevention during the earliest stages of the disease,” the authors wrote.
SOURCE:
The study was led by Irene Altabás-González, MD, PhD, Rheumatology Department, Vigo University Hospital Group, Vigo, Spain. It was published online in Lupus Science & Medicine.
LIMITATIONS:
The retrospective collection of data in the study may have led to missing items; for example, the dates of damage events for the whole cohort were not available.
DISCLOSURES:
The registry was supported by the Spanish Society of Rheumatology. No specific funding was received for the study. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
TOPLINE:
The first year after the diagnosis of systemic lupus erythematosus (SLE) is crucial, with the highest percentage of patients experiencing organ damage. Cardiovascular issues are the second most prevalent after musculoskeletal damage in both early and later stages of SLE.
METHODOLOGY:
- Researchers assessed organ damage persisting at least 6 months over different stages of lupus in 4219 patients with SLE (mean age, 35.9 years; 89.6% women) from the Spanish Society of Rheumatology Lupus Registry.
- Damage was assessed using the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index (SDI).
- Longitudinal analysis was conducted globally and by each SDI domain on 1274 patients with recorded damage event dates.
- Follow-up data were available out to 10 years in 1113 patients and to 20 years in 601.
TAKEAWAY:
- New damage was recorded in 20% of the patients with SLE within the first year after diagnosis, with the annual percentage of patients with new damage decreasing to 5% after the first 5 years of follow-up.
- In the first year, musculoskeletal damage was reported by the highest proportion of patients (21%), followed by cardiovascular damage inclusive of cerebrovascular accidents and claudication for 6 months (19%).
- The cardiovascular system remained the second most affected system even during the later stages of the diseases at years 10 and 20 of follow-up (20%-25%).
- Apart from musculoskeletal and cardiovascular damage, patients with lupus also showed renal and ocular damage in the early and later stages of the disease, respectively.
IN PRACTICE:
“Our study highlights the importance of cardiovascular damage and the need for its prevention during the earliest stages of the disease,” the authors wrote.
SOURCE:
The study was led by Irene Altabás-González, MD, PhD, Rheumatology Department, Vigo University Hospital Group, Vigo, Spain. It was published online in Lupus Science & Medicine.
LIMITATIONS:
The retrospective collection of data in the study may have led to missing items; for example, the dates of damage events for the whole cohort were not available.
DISCLOSURES:
The registry was supported by the Spanish Society of Rheumatology. No specific funding was received for the study. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
US Experience With Infliximab Biosimilars Suggests Need for More Development Incentives
TOPLINE:
Uptake of infliximab biosimilars rose slowly across private insurance, Medicaid, and Medicare when two were available in the United States during 2016-2020 but increased significantly through 2022 after the third biosimilar became available in July 2020. However, prescriptions in Medicare still lagged behind those in private insurance and Medicaid.
METHODOLOGY:
- Researchers analyzed electronic health records from over 1100 US rheumatologists who participated in a national registry, the Rheumatology Informatics System for Effectiveness (RISE), for all infliximab administrations (bio-originator or biosimilar) to patients older than 18 years from April 2016 to September 2022.
- They conducted an interrupted time series to account for autocorrelation and model the effect of each infliximab biosimilar release (infliximab-dyyb in November 2016, infliximab-abda in July 2017, and infliximab-axxq in July 2020) on uptake across Medicare, Medicaid, and private insurers.
TAKEAWAY:
- The researchers identified 659,988 infliximab administrations for 37,560 unique patients, with 52% on Medicare, 4.8% on Medicaid, and 43% on private insurance.
- Biosimilar uptake rose slowly with average annual increases < 5% from 2016 to June 2020 (Medicare, 3.2%; Medicaid, 5.2%; private insurance, 1.8%).
- After the third biosimilar release in July 2020, the average annual increase reached 13% for Medicaid and 16.4% for private insurance but remained low for Medicare (5.6%).
- By September 2022, biosimilar uptake was higher for Medicaid (43.8%) and private insurance (38.5%) than for Medicare (24%).
IN PRACTICE:
“Our results suggest policymakers may need to do more to allow biosimilars to get a foothold in the market by incentivizing the development and entry of multiple biosimilars, address anticompetitive pricing strategies, and may need to amend Medicare policy to [incentivize] uptake in order to ensure a competitive and sustainable biosimilar market that gradually reduces total drug expenditures and out-of-pocket costs over time,” wrote the authors of the study.
SOURCE:
The study was led by Eric T. Roberts, PhD, University of California, San Francisco. It was published online on July 30, 2024, in Arthritis & Rheumatology.
LIMITATIONS:
First, while the biosimilar introductions are likely catalysts for many changes in the market, some changes in slopes may also be attributable to the natural growth of the market over time. Second, this study may neither be generalizable to academic medical centers, which are underrepresented in RISE, nor be generalizable to infliximab prescriptions from other specialties. Third, uptake among privately insured patients changed shortly after November-December 2020, raising the possibility that the delay reflected negotiations between insurance companies and relevant entities regarding formulary coverage.
DISCLOSURES:
This study was funded by grants from the Agency for Healthcare Research and Quality and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. One author disclosed receiving consulting fees from Pfizer, AstraZeneca, and Bristol-Myers Squibb and grant funding from AstraZeneca, the Bristol-Myers Squibb Foundation, and Aurinia.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Uptake of infliximab biosimilars rose slowly across private insurance, Medicaid, and Medicare when two were available in the United States during 2016-2020 but increased significantly through 2022 after the third biosimilar became available in July 2020. However, prescriptions in Medicare still lagged behind those in private insurance and Medicaid.
METHODOLOGY:
- Researchers analyzed electronic health records from over 1100 US rheumatologists who participated in a national registry, the Rheumatology Informatics System for Effectiveness (RISE), for all infliximab administrations (bio-originator or biosimilar) to patients older than 18 years from April 2016 to September 2022.
- They conducted an interrupted time series to account for autocorrelation and model the effect of each infliximab biosimilar release (infliximab-dyyb in November 2016, infliximab-abda in July 2017, and infliximab-axxq in July 2020) on uptake across Medicare, Medicaid, and private insurers.
TAKEAWAY:
- The researchers identified 659,988 infliximab administrations for 37,560 unique patients, with 52% on Medicare, 4.8% on Medicaid, and 43% on private insurance.
- Biosimilar uptake rose slowly with average annual increases < 5% from 2016 to June 2020 (Medicare, 3.2%; Medicaid, 5.2%; private insurance, 1.8%).
- After the third biosimilar release in July 2020, the average annual increase reached 13% for Medicaid and 16.4% for private insurance but remained low for Medicare (5.6%).
- By September 2022, biosimilar uptake was higher for Medicaid (43.8%) and private insurance (38.5%) than for Medicare (24%).
IN PRACTICE:
“Our results suggest policymakers may need to do more to allow biosimilars to get a foothold in the market by incentivizing the development and entry of multiple biosimilars, address anticompetitive pricing strategies, and may need to amend Medicare policy to [incentivize] uptake in order to ensure a competitive and sustainable biosimilar market that gradually reduces total drug expenditures and out-of-pocket costs over time,” wrote the authors of the study.
SOURCE:
The study was led by Eric T. Roberts, PhD, University of California, San Francisco. It was published online on July 30, 2024, in Arthritis & Rheumatology.
LIMITATIONS:
First, while the biosimilar introductions are likely catalysts for many changes in the market, some changes in slopes may also be attributable to the natural growth of the market over time. Second, this study may neither be generalizable to academic medical centers, which are underrepresented in RISE, nor be generalizable to infliximab prescriptions from other specialties. Third, uptake among privately insured patients changed shortly after November-December 2020, raising the possibility that the delay reflected negotiations between insurance companies and relevant entities regarding formulary coverage.
DISCLOSURES:
This study was funded by grants from the Agency for Healthcare Research and Quality and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. One author disclosed receiving consulting fees from Pfizer, AstraZeneca, and Bristol-Myers Squibb and grant funding from AstraZeneca, the Bristol-Myers Squibb Foundation, and Aurinia.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Uptake of infliximab biosimilars rose slowly across private insurance, Medicaid, and Medicare when two were available in the United States during 2016-2020 but increased significantly through 2022 after the third biosimilar became available in July 2020. However, prescriptions in Medicare still lagged behind those in private insurance and Medicaid.
METHODOLOGY:
- Researchers analyzed electronic health records from over 1100 US rheumatologists who participated in a national registry, the Rheumatology Informatics System for Effectiveness (RISE), for all infliximab administrations (bio-originator or biosimilar) to patients older than 18 years from April 2016 to September 2022.
- They conducted an interrupted time series to account for autocorrelation and model the effect of each infliximab biosimilar release (infliximab-dyyb in November 2016, infliximab-abda in July 2017, and infliximab-axxq in July 2020) on uptake across Medicare, Medicaid, and private insurers.
TAKEAWAY:
- The researchers identified 659,988 infliximab administrations for 37,560 unique patients, with 52% on Medicare, 4.8% on Medicaid, and 43% on private insurance.
- Biosimilar uptake rose slowly with average annual increases < 5% from 2016 to June 2020 (Medicare, 3.2%; Medicaid, 5.2%; private insurance, 1.8%).
- After the third biosimilar release in July 2020, the average annual increase reached 13% for Medicaid and 16.4% for private insurance but remained low for Medicare (5.6%).
- By September 2022, biosimilar uptake was higher for Medicaid (43.8%) and private insurance (38.5%) than for Medicare (24%).
IN PRACTICE:
“Our results suggest policymakers may need to do more to allow biosimilars to get a foothold in the market by incentivizing the development and entry of multiple biosimilars, address anticompetitive pricing strategies, and may need to amend Medicare policy to [incentivize] uptake in order to ensure a competitive and sustainable biosimilar market that gradually reduces total drug expenditures and out-of-pocket costs over time,” wrote the authors of the study.
SOURCE:
The study was led by Eric T. Roberts, PhD, University of California, San Francisco. It was published online on July 30, 2024, in Arthritis & Rheumatology.
LIMITATIONS:
First, while the biosimilar introductions are likely catalysts for many changes in the market, some changes in slopes may also be attributable to the natural growth of the market over time. Second, this study may neither be generalizable to academic medical centers, which are underrepresented in RISE, nor be generalizable to infliximab prescriptions from other specialties. Third, uptake among privately insured patients changed shortly after November-December 2020, raising the possibility that the delay reflected negotiations between insurance companies and relevant entities regarding formulary coverage.
DISCLOSURES:
This study was funded by grants from the Agency for Healthcare Research and Quality and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. One author disclosed receiving consulting fees from Pfizer, AstraZeneca, and Bristol-Myers Squibb and grant funding from AstraZeneca, the Bristol-Myers Squibb Foundation, and Aurinia.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Nonmelanoma Skin Cancer: Encouraging Data on Laser Treatment
TOPLINE:
Published
METHODOLOGY:
- Using MEDLINE, the Cochrane Library, and www.clinicaltrials.gov, researchers systematically reviewed 50 unique published articles that evaluated the role of laser therapy for NMSC.
- Of the 50 studies, 37 focused on lasers for the treatment of basal cell carcinoma (BCC), 10 on lasers for the treatment of squamous cell carcinoma (SCC), and three on the treatment of both tumor types.
- The analysis was limited to studies published in English from the first data available through May 1, 2023.
TAKEAWAY:
- Data was strongest for the use of lasers for treating BCC, especially pulsed-dye lasers (PDL). Of 11 unique studies on PDL as monotherapy for managing BCCs, clearance rates ranged from 14.3% to 90.0%.
- For SCCs, 13 studies were identified that evaluated the use of lasers alone or in combination with PDL for treating SCC in situ. Among case reports that used PDL and thulium lasers separately, clearance rates of 100% were reported, while several case series that used the CO2 laser reported response rates that ranged from 61.5% to 100%.
- The best evidence for clearing both BCC and SCC tumors was observed when ablative lasers such as the CO2 or erbium yttrium aluminum garnet are combined with methyl aminolevulinate–photodynamic therapy (PDT) or 5-aminolevulinic acid–PDT, “likely due to increased delivery of the photosensitizing compound to neoplastic cells,” the authors wrote.
IN PRACTICE:
“Additional investigations with longer follow-up periods are needed to determine optimal laser parameters, number of treatment sessions required, and recurrence rates (using complete histologic analysis through step sectioning) before lasers can fully be adopted into clinical practice,” the authors wrote. “Surgical excision, specifically Mohs micrographic surgery,” they added, “persists as the gold standard for high-risk and cosmetically sensitive tumors, offering the highest cure rates in a single office visit.”
SOURCE:
Amanda Rosenthal, MD, of the Department of Dermatology, Kaiser Permanente Los Angeles Medical Center in California, and colleagues conducted the review. The study was published in the August 2024 issue of Dermatologic Surgery.
LIMITATIONS:
Laser therapy is not FDA approved for the treatment of NMSC and remains an alternative treatment option. Also, most published studies focus on BCCs, while studies on cutaneous SCCs are more limited.
DISCLOSURES:
The researchers reported having no financial disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
Published
METHODOLOGY:
- Using MEDLINE, the Cochrane Library, and www.clinicaltrials.gov, researchers systematically reviewed 50 unique published articles that evaluated the role of laser therapy for NMSC.
- Of the 50 studies, 37 focused on lasers for the treatment of basal cell carcinoma (BCC), 10 on lasers for the treatment of squamous cell carcinoma (SCC), and three on the treatment of both tumor types.
- The analysis was limited to studies published in English from the first data available through May 1, 2023.
TAKEAWAY:
- Data was strongest for the use of lasers for treating BCC, especially pulsed-dye lasers (PDL). Of 11 unique studies on PDL as monotherapy for managing BCCs, clearance rates ranged from 14.3% to 90.0%.
- For SCCs, 13 studies were identified that evaluated the use of lasers alone or in combination with PDL for treating SCC in situ. Among case reports that used PDL and thulium lasers separately, clearance rates of 100% were reported, while several case series that used the CO2 laser reported response rates that ranged from 61.5% to 100%.
- The best evidence for clearing both BCC and SCC tumors was observed when ablative lasers such as the CO2 or erbium yttrium aluminum garnet are combined with methyl aminolevulinate–photodynamic therapy (PDT) or 5-aminolevulinic acid–PDT, “likely due to increased delivery of the photosensitizing compound to neoplastic cells,” the authors wrote.
IN PRACTICE:
“Additional investigations with longer follow-up periods are needed to determine optimal laser parameters, number of treatment sessions required, and recurrence rates (using complete histologic analysis through step sectioning) before lasers can fully be adopted into clinical practice,” the authors wrote. “Surgical excision, specifically Mohs micrographic surgery,” they added, “persists as the gold standard for high-risk and cosmetically sensitive tumors, offering the highest cure rates in a single office visit.”
SOURCE:
Amanda Rosenthal, MD, of the Department of Dermatology, Kaiser Permanente Los Angeles Medical Center in California, and colleagues conducted the review. The study was published in the August 2024 issue of Dermatologic Surgery.
LIMITATIONS:
Laser therapy is not FDA approved for the treatment of NMSC and remains an alternative treatment option. Also, most published studies focus on BCCs, while studies on cutaneous SCCs are more limited.
DISCLOSURES:
The researchers reported having no financial disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
Published
METHODOLOGY:
- Using MEDLINE, the Cochrane Library, and www.clinicaltrials.gov, researchers systematically reviewed 50 unique published articles that evaluated the role of laser therapy for NMSC.
- Of the 50 studies, 37 focused on lasers for the treatment of basal cell carcinoma (BCC), 10 on lasers for the treatment of squamous cell carcinoma (SCC), and three on the treatment of both tumor types.
- The analysis was limited to studies published in English from the first data available through May 1, 2023.
TAKEAWAY:
- Data was strongest for the use of lasers for treating BCC, especially pulsed-dye lasers (PDL). Of 11 unique studies on PDL as monotherapy for managing BCCs, clearance rates ranged from 14.3% to 90.0%.
- For SCCs, 13 studies were identified that evaluated the use of lasers alone or in combination with PDL for treating SCC in situ. Among case reports that used PDL and thulium lasers separately, clearance rates of 100% were reported, while several case series that used the CO2 laser reported response rates that ranged from 61.5% to 100%.
- The best evidence for clearing both BCC and SCC tumors was observed when ablative lasers such as the CO2 or erbium yttrium aluminum garnet are combined with methyl aminolevulinate–photodynamic therapy (PDT) or 5-aminolevulinic acid–PDT, “likely due to increased delivery of the photosensitizing compound to neoplastic cells,” the authors wrote.
IN PRACTICE:
“Additional investigations with longer follow-up periods are needed to determine optimal laser parameters, number of treatment sessions required, and recurrence rates (using complete histologic analysis through step sectioning) before lasers can fully be adopted into clinical practice,” the authors wrote. “Surgical excision, specifically Mohs micrographic surgery,” they added, “persists as the gold standard for high-risk and cosmetically sensitive tumors, offering the highest cure rates in a single office visit.”
SOURCE:
Amanda Rosenthal, MD, of the Department of Dermatology, Kaiser Permanente Los Angeles Medical Center in California, and colleagues conducted the review. The study was published in the August 2024 issue of Dermatologic Surgery.
LIMITATIONS:
Laser therapy is not FDA approved for the treatment of NMSC and remains an alternative treatment option. Also, most published studies focus on BCCs, while studies on cutaneous SCCs are more limited.
DISCLOSURES:
The researchers reported having no financial disclosures.
A version of this article first appeared on Medscape.com.
Nemolizumab Benefits Seen in Adults, Teens With Atopic Dermatitis
TOPLINE:
(AD).
METHODOLOGY:
- The researchers conducted two 48-week randomized, double-blind, placebo-controlled phase 3 trials, ARCADIA 1 (n = 941; 47% women) and ARCADIA 2 (n = 787; 52% women), involving patients aged 12 and older with moderate to severe AD.
- Participants were randomly assigned in a 2:1 ratio to receive either 30 mg nemolizumab (with a 60-mg loading dose) or placebo, along with background topical corticosteroids with or without topical calcineurin inhibitors. The mean age range was 33.3-35.2 years.
- The coprimary endpoints were Investigator’s Global Assessment (IGA) success (score of 0 or 1 with at least a two-point improvement from baseline) and at least a 75% improvement in the Eczema Area and Severity Index (EASI-75) at week 16.
TAKEAWAY:
- At week 16, significantly more patients receiving nemolizumab vs placebo achieved IGA success in both the ARCADIA 1 (36% vs 25%; P = .0003) and ARCADIA 2 (38% vs 26%; P = .0006) trials.
- EASI-75 response rates were also significantly higher in the nemolizumab group than in the placebo group in both trials: ARCADIA 1 (44% vs 29%; P < .0001) and 2 (42% vs 30%; P = .0006).
- Significant improvements in pruritus were observed as early as week 1, with a greater proportion of participants in the nemolizumab vs placebo group achieving at least a four-point reduction in the Peak Pruritus Numerical Rating Scale score in both trials.
- Rates of adverse events were similar between the nemolizumab and placebo groups, with severe treatment-emergent adverse events occurring in 2%-4% of patients.
IN PRACTICE:
“Nemolizumab showed statistically and clinically significant improvements in inflammation and pruritus in adults and adolescents with moderate to severe atopic dermatitis and a rapid effect in reducing pruritus, as one of the primary complaints of patients. As such, nemolizumab might offer a valuable extension of the therapeutic armament if approved,” the authors concluded.
SOURCE:
The study was led by Jonathan Silverberg, MD, PhD, from the Department of Dermatology, George Washington University, Washington, DC. It was published online in The Lancet.
LIMITATIONS:
The study’s limitations included the absence of longer-term safety data. Additionally, the predominantly White population of the trials may limit the generalizability of the findings to other racial and ethnic groups. The use of concomitant topical therapy might have influenced the placebo response.
DISCLOSURES:
This study was funded by Galderma. Dr. Silverberg received honoraria from pharmaceutical companies, including Galderma, and his institution also received grants from Galderma, Incyte, and Pfizer. Four authors were employees of Galderma. Other authors also declared having ties with pharmaceutical companies, including Galderma, outside this work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
(AD).
METHODOLOGY:
- The researchers conducted two 48-week randomized, double-blind, placebo-controlled phase 3 trials, ARCADIA 1 (n = 941; 47% women) and ARCADIA 2 (n = 787; 52% women), involving patients aged 12 and older with moderate to severe AD.
- Participants were randomly assigned in a 2:1 ratio to receive either 30 mg nemolizumab (with a 60-mg loading dose) or placebo, along with background topical corticosteroids with or without topical calcineurin inhibitors. The mean age range was 33.3-35.2 years.
- The coprimary endpoints were Investigator’s Global Assessment (IGA) success (score of 0 or 1 with at least a two-point improvement from baseline) and at least a 75% improvement in the Eczema Area and Severity Index (EASI-75) at week 16.
TAKEAWAY:
- At week 16, significantly more patients receiving nemolizumab vs placebo achieved IGA success in both the ARCADIA 1 (36% vs 25%; P = .0003) and ARCADIA 2 (38% vs 26%; P = .0006) trials.
- EASI-75 response rates were also significantly higher in the nemolizumab group than in the placebo group in both trials: ARCADIA 1 (44% vs 29%; P < .0001) and 2 (42% vs 30%; P = .0006).
- Significant improvements in pruritus were observed as early as week 1, with a greater proportion of participants in the nemolizumab vs placebo group achieving at least a four-point reduction in the Peak Pruritus Numerical Rating Scale score in both trials.
- Rates of adverse events were similar between the nemolizumab and placebo groups, with severe treatment-emergent adverse events occurring in 2%-4% of patients.
IN PRACTICE:
“Nemolizumab showed statistically and clinically significant improvements in inflammation and pruritus in adults and adolescents with moderate to severe atopic dermatitis and a rapid effect in reducing pruritus, as one of the primary complaints of patients. As such, nemolizumab might offer a valuable extension of the therapeutic armament if approved,” the authors concluded.
SOURCE:
The study was led by Jonathan Silverberg, MD, PhD, from the Department of Dermatology, George Washington University, Washington, DC. It was published online in The Lancet.
LIMITATIONS:
The study’s limitations included the absence of longer-term safety data. Additionally, the predominantly White population of the trials may limit the generalizability of the findings to other racial and ethnic groups. The use of concomitant topical therapy might have influenced the placebo response.
DISCLOSURES:
This study was funded by Galderma. Dr. Silverberg received honoraria from pharmaceutical companies, including Galderma, and his institution also received grants from Galderma, Incyte, and Pfizer. Four authors were employees of Galderma. Other authors also declared having ties with pharmaceutical companies, including Galderma, outside this work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
(AD).
METHODOLOGY:
- The researchers conducted two 48-week randomized, double-blind, placebo-controlled phase 3 trials, ARCADIA 1 (n = 941; 47% women) and ARCADIA 2 (n = 787; 52% women), involving patients aged 12 and older with moderate to severe AD.
- Participants were randomly assigned in a 2:1 ratio to receive either 30 mg nemolizumab (with a 60-mg loading dose) or placebo, along with background topical corticosteroids with or without topical calcineurin inhibitors. The mean age range was 33.3-35.2 years.
- The coprimary endpoints were Investigator’s Global Assessment (IGA) success (score of 0 or 1 with at least a two-point improvement from baseline) and at least a 75% improvement in the Eczema Area and Severity Index (EASI-75) at week 16.
TAKEAWAY:
- At week 16, significantly more patients receiving nemolizumab vs placebo achieved IGA success in both the ARCADIA 1 (36% vs 25%; P = .0003) and ARCADIA 2 (38% vs 26%; P = .0006) trials.
- EASI-75 response rates were also significantly higher in the nemolizumab group than in the placebo group in both trials: ARCADIA 1 (44% vs 29%; P < .0001) and 2 (42% vs 30%; P = .0006).
- Significant improvements in pruritus were observed as early as week 1, with a greater proportion of participants in the nemolizumab vs placebo group achieving at least a four-point reduction in the Peak Pruritus Numerical Rating Scale score in both trials.
- Rates of adverse events were similar between the nemolizumab and placebo groups, with severe treatment-emergent adverse events occurring in 2%-4% of patients.
IN PRACTICE:
“Nemolizumab showed statistically and clinically significant improvements in inflammation and pruritus in adults and adolescents with moderate to severe atopic dermatitis and a rapid effect in reducing pruritus, as one of the primary complaints of patients. As such, nemolizumab might offer a valuable extension of the therapeutic armament if approved,” the authors concluded.
SOURCE:
The study was led by Jonathan Silverberg, MD, PhD, from the Department of Dermatology, George Washington University, Washington, DC. It was published online in The Lancet.
LIMITATIONS:
The study’s limitations included the absence of longer-term safety data. Additionally, the predominantly White population of the trials may limit the generalizability of the findings to other racial and ethnic groups. The use of concomitant topical therapy might have influenced the placebo response.
DISCLOSURES:
This study was funded by Galderma. Dr. Silverberg received honoraria from pharmaceutical companies, including Galderma, and his institution also received grants from Galderma, Incyte, and Pfizer. Four authors were employees of Galderma. Other authors also declared having ties with pharmaceutical companies, including Galderma, outside this work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Recommendations From a Pediatric Dermatologist on Using AI in Daily Practice
TORONTO — with the various AI models.
He reminds doctors that many of their colleagues and patients and their families are already using these systems, “and you don’t want to be left behind.”
In an interview following his presentation on AI at the annual meeting of the Society for Pediatric Dermatology (SPD), Dr. Yan discussed his tips for using AI.
Changing Fast
From the outset, most generative AI systems have been very good at processing language — for example, generating letters of medical necessity and summarizing disease processes into lay terms. But now they’re becoming “truly multimodal,” said Dr. Yan. “You can enter images; you could have it process audio; you can even start to have it refine video.”
To get started, he recommends signing up for a free account with ChatGPT, Gemini, Perplexity, Claude, and/or Microsoft Copilot. “To make the best choice, you have to try them out yourself because they each have their own kind of flavor and strengths and weaknesses,” said Dr. Yan.
Personally, he finds that ChatGPT is the most versatile, Gemini perhaps a little better in terms of image generation, and Perplexity probably the best at references because it was designed as an online library.
Once you figure out which platforms you prefer, consider signing up for a premium subscription, which is typically month to month and can be canceled at any time, Dr. Yan said. “This will allow you to get the most out of the AI model.”
As these AI systems are based on large language models, they are excellent at text, Dr. Yan noted. He suggests asking one to generate a letter or patient instruction sheet. “If you have a premium model, give it a PDF to summarize an article or take a photo of something that you want its opinion on.”
Privacy Critical
Always pay attention to privacy issues and avoid entering any private health information that would violate the Health Insurance Portability and Accountability Act (HIPAA), he said.
“We have to be very careful about how we interact with AI,” said Dr. Yan. “We can’t be posting private patient health information into these systems, no matter how useful these systems are.” Many academic institutions are creating “walled gardens” — private areas of AI access that don’t allow patient information to “leak out,” he said. “These AI models may have HIPAA protections in place and come with specific guidelines of use.”
The AI “scribe,” which helps with electronic health record documentation, is one of the most useful tools for clinicians, he said. He referred to a recent study showing that an AI scribe saved users an average of 1 hour at the keyboard every day, and a small patient survey showing 71% reported that it led to spending more time with their physician.
When entering requests into a prompt line with an AI system, Dr. Yan stressed that these prompts need to be clear and concise. For a complicated calculation or multistep problem, try adding the words “let’s do this step by step,” he said. “This is a technique invoking a ‘chain of thought’ that allows the system to enhance its accuracy when solving problems.”
If the response is not satisfactory, try being more detailed in the request, he advised, and consider giving the system examples of what you’re looking for and telling it what you don’t want in the output.
“For instance, if you’re asking for a differential diagnosis of rashes that affect the hands and feet, you can stipulate that you only want rashes that are vesicular or that arise in neonates, so you can get a more focused answer,” said Dr. Yan.
If there are “long-winded verbose” responses, add the phrase “be concise,” and it will shorten the response by about 50%, he added.
AI Hallucinations
Dr. Yan broached an issue that occasionally comes up, AI hallucinations, which refer to inaccurate or misleading responses on the basis of incomplete training or intrinsic biases within the model. He pointed to the case of a doctor discussing issues related to a patient’s hands, feet, and mouth, which the AI-generated model summarized as “the patient being diagnosed with hand, foot, and mouth disease.”
Another example he provided was a request to generate a letter of medical necessity for using ustekinumab (Stelara) for treating hidradenitis suppurative in a child that included references for its effectiveness and safety in children. The AI system generated “false references that sounded like they should be real because the authors are often people who have written in that field or on that subject,” said Dr. Yan.
When pressed, the system did acknowledge the references were hypothetical but were meant to illustrate the types of studies that would typically support the use of this drug in pediatric patients with HS. “ It’s well meaning, in the sense that it’s trying to help you achieve your goals using this training system,” said Dr. Yan.
“If you’re skeptical about a response, double-check the answer with a Google search or run the response through another AI [tool] asking it to check if the response is accurate,” he added.
While AI systems won’t replace the clinician, they are continuing to improve and becoming more sophisticated. Dr. Yan advises keeping up with emerging developments and engaging and adapting the most appropriate AI tool for an individual clinician’s work.
Asked to comment on the presentation at the SPD meeting, Sheilagh Maguiness, MD, director of the Division of Pediatric Dermatology at the University of Minnesota, Minneapolis, who, like other doctors, is increasingly testing AI, said she foresees a time when AI scribes fully replace humans for completing tasks during patient interactions.
“The hope is that if the AI scribes get good enough, we can just open our phone, have them translate the interaction, and create the notes for us.”
While she likes the idea of using ChatGPT to help with tasks like letters of recommendation for medications, Dr. Yan’s comments reiterated the importance of “checking and double-checking ChatGPT because it’s not correct all the time.” She particularly welcomed the advice “that we can just go back and ask it again to clarify, and that may improve its answers.”
Dr. Yan’s disclosures included an investment portfolio that includes companies working in the AI space, including Google, Apple, Nvidia, Amazon, Microsoft, and Arm. Dr. Maguiness had no relevant disclosures.
A version of this article first appeared on Medscape.com.
TORONTO — with the various AI models.
He reminds doctors that many of their colleagues and patients and their families are already using these systems, “and you don’t want to be left behind.”
In an interview following his presentation on AI at the annual meeting of the Society for Pediatric Dermatology (SPD), Dr. Yan discussed his tips for using AI.
Changing Fast
From the outset, most generative AI systems have been very good at processing language — for example, generating letters of medical necessity and summarizing disease processes into lay terms. But now they’re becoming “truly multimodal,” said Dr. Yan. “You can enter images; you could have it process audio; you can even start to have it refine video.”
To get started, he recommends signing up for a free account with ChatGPT, Gemini, Perplexity, Claude, and/or Microsoft Copilot. “To make the best choice, you have to try them out yourself because they each have their own kind of flavor and strengths and weaknesses,” said Dr. Yan.
Personally, he finds that ChatGPT is the most versatile, Gemini perhaps a little better in terms of image generation, and Perplexity probably the best at references because it was designed as an online library.
Once you figure out which platforms you prefer, consider signing up for a premium subscription, which is typically month to month and can be canceled at any time, Dr. Yan said. “This will allow you to get the most out of the AI model.”
As these AI systems are based on large language models, they are excellent at text, Dr. Yan noted. He suggests asking one to generate a letter or patient instruction sheet. “If you have a premium model, give it a PDF to summarize an article or take a photo of something that you want its opinion on.”
Privacy Critical
Always pay attention to privacy issues and avoid entering any private health information that would violate the Health Insurance Portability and Accountability Act (HIPAA), he said.
“We have to be very careful about how we interact with AI,” said Dr. Yan. “We can’t be posting private patient health information into these systems, no matter how useful these systems are.” Many academic institutions are creating “walled gardens” — private areas of AI access that don’t allow patient information to “leak out,” he said. “These AI models may have HIPAA protections in place and come with specific guidelines of use.”
The AI “scribe,” which helps with electronic health record documentation, is one of the most useful tools for clinicians, he said. He referred to a recent study showing that an AI scribe saved users an average of 1 hour at the keyboard every day, and a small patient survey showing 71% reported that it led to spending more time with their physician.
When entering requests into a prompt line with an AI system, Dr. Yan stressed that these prompts need to be clear and concise. For a complicated calculation or multistep problem, try adding the words “let’s do this step by step,” he said. “This is a technique invoking a ‘chain of thought’ that allows the system to enhance its accuracy when solving problems.”
If the response is not satisfactory, try being more detailed in the request, he advised, and consider giving the system examples of what you’re looking for and telling it what you don’t want in the output.
“For instance, if you’re asking for a differential diagnosis of rashes that affect the hands and feet, you can stipulate that you only want rashes that are vesicular or that arise in neonates, so you can get a more focused answer,” said Dr. Yan.
If there are “long-winded verbose” responses, add the phrase “be concise,” and it will shorten the response by about 50%, he added.
AI Hallucinations
Dr. Yan broached an issue that occasionally comes up, AI hallucinations, which refer to inaccurate or misleading responses on the basis of incomplete training or intrinsic biases within the model. He pointed to the case of a doctor discussing issues related to a patient’s hands, feet, and mouth, which the AI-generated model summarized as “the patient being diagnosed with hand, foot, and mouth disease.”
Another example he provided was a request to generate a letter of medical necessity for using ustekinumab (Stelara) for treating hidradenitis suppurative in a child that included references for its effectiveness and safety in children. The AI system generated “false references that sounded like they should be real because the authors are often people who have written in that field or on that subject,” said Dr. Yan.
When pressed, the system did acknowledge the references were hypothetical but were meant to illustrate the types of studies that would typically support the use of this drug in pediatric patients with HS. “ It’s well meaning, in the sense that it’s trying to help you achieve your goals using this training system,” said Dr. Yan.
“If you’re skeptical about a response, double-check the answer with a Google search or run the response through another AI [tool] asking it to check if the response is accurate,” he added.
While AI systems won’t replace the clinician, they are continuing to improve and becoming more sophisticated. Dr. Yan advises keeping up with emerging developments and engaging and adapting the most appropriate AI tool for an individual clinician’s work.
Asked to comment on the presentation at the SPD meeting, Sheilagh Maguiness, MD, director of the Division of Pediatric Dermatology at the University of Minnesota, Minneapolis, who, like other doctors, is increasingly testing AI, said she foresees a time when AI scribes fully replace humans for completing tasks during patient interactions.
“The hope is that if the AI scribes get good enough, we can just open our phone, have them translate the interaction, and create the notes for us.”
While she likes the idea of using ChatGPT to help with tasks like letters of recommendation for medications, Dr. Yan’s comments reiterated the importance of “checking and double-checking ChatGPT because it’s not correct all the time.” She particularly welcomed the advice “that we can just go back and ask it again to clarify, and that may improve its answers.”
Dr. Yan’s disclosures included an investment portfolio that includes companies working in the AI space, including Google, Apple, Nvidia, Amazon, Microsoft, and Arm. Dr. Maguiness had no relevant disclosures.
A version of this article first appeared on Medscape.com.
TORONTO — with the various AI models.
He reminds doctors that many of their colleagues and patients and their families are already using these systems, “and you don’t want to be left behind.”
In an interview following his presentation on AI at the annual meeting of the Society for Pediatric Dermatology (SPD), Dr. Yan discussed his tips for using AI.
Changing Fast
From the outset, most generative AI systems have been very good at processing language — for example, generating letters of medical necessity and summarizing disease processes into lay terms. But now they’re becoming “truly multimodal,” said Dr. Yan. “You can enter images; you could have it process audio; you can even start to have it refine video.”
To get started, he recommends signing up for a free account with ChatGPT, Gemini, Perplexity, Claude, and/or Microsoft Copilot. “To make the best choice, you have to try them out yourself because they each have their own kind of flavor and strengths and weaknesses,” said Dr. Yan.
Personally, he finds that ChatGPT is the most versatile, Gemini perhaps a little better in terms of image generation, and Perplexity probably the best at references because it was designed as an online library.
Once you figure out which platforms you prefer, consider signing up for a premium subscription, which is typically month to month and can be canceled at any time, Dr. Yan said. “This will allow you to get the most out of the AI model.”
As these AI systems are based on large language models, they are excellent at text, Dr. Yan noted. He suggests asking one to generate a letter or patient instruction sheet. “If you have a premium model, give it a PDF to summarize an article or take a photo of something that you want its opinion on.”
Privacy Critical
Always pay attention to privacy issues and avoid entering any private health information that would violate the Health Insurance Portability and Accountability Act (HIPAA), he said.
“We have to be very careful about how we interact with AI,” said Dr. Yan. “We can’t be posting private patient health information into these systems, no matter how useful these systems are.” Many academic institutions are creating “walled gardens” — private areas of AI access that don’t allow patient information to “leak out,” he said. “These AI models may have HIPAA protections in place and come with specific guidelines of use.”
The AI “scribe,” which helps with electronic health record documentation, is one of the most useful tools for clinicians, he said. He referred to a recent study showing that an AI scribe saved users an average of 1 hour at the keyboard every day, and a small patient survey showing 71% reported that it led to spending more time with their physician.
When entering requests into a prompt line with an AI system, Dr. Yan stressed that these prompts need to be clear and concise. For a complicated calculation or multistep problem, try adding the words “let’s do this step by step,” he said. “This is a technique invoking a ‘chain of thought’ that allows the system to enhance its accuracy when solving problems.”
If the response is not satisfactory, try being more detailed in the request, he advised, and consider giving the system examples of what you’re looking for and telling it what you don’t want in the output.
“For instance, if you’re asking for a differential diagnosis of rashes that affect the hands and feet, you can stipulate that you only want rashes that are vesicular or that arise in neonates, so you can get a more focused answer,” said Dr. Yan.
If there are “long-winded verbose” responses, add the phrase “be concise,” and it will shorten the response by about 50%, he added.
AI Hallucinations
Dr. Yan broached an issue that occasionally comes up, AI hallucinations, which refer to inaccurate or misleading responses on the basis of incomplete training or intrinsic biases within the model. He pointed to the case of a doctor discussing issues related to a patient’s hands, feet, and mouth, which the AI-generated model summarized as “the patient being diagnosed with hand, foot, and mouth disease.”
Another example he provided was a request to generate a letter of medical necessity for using ustekinumab (Stelara) for treating hidradenitis suppurative in a child that included references for its effectiveness and safety in children. The AI system generated “false references that sounded like they should be real because the authors are often people who have written in that field or on that subject,” said Dr. Yan.
When pressed, the system did acknowledge the references were hypothetical but were meant to illustrate the types of studies that would typically support the use of this drug in pediatric patients with HS. “ It’s well meaning, in the sense that it’s trying to help you achieve your goals using this training system,” said Dr. Yan.
“If you’re skeptical about a response, double-check the answer with a Google search or run the response through another AI [tool] asking it to check if the response is accurate,” he added.
While AI systems won’t replace the clinician, they are continuing to improve and becoming more sophisticated. Dr. Yan advises keeping up with emerging developments and engaging and adapting the most appropriate AI tool for an individual clinician’s work.
Asked to comment on the presentation at the SPD meeting, Sheilagh Maguiness, MD, director of the Division of Pediatric Dermatology at the University of Minnesota, Minneapolis, who, like other doctors, is increasingly testing AI, said she foresees a time when AI scribes fully replace humans for completing tasks during patient interactions.
“The hope is that if the AI scribes get good enough, we can just open our phone, have them translate the interaction, and create the notes for us.”
While she likes the idea of using ChatGPT to help with tasks like letters of recommendation for medications, Dr. Yan’s comments reiterated the importance of “checking and double-checking ChatGPT because it’s not correct all the time.” She particularly welcomed the advice “that we can just go back and ask it again to clarify, and that may improve its answers.”
Dr. Yan’s disclosures included an investment portfolio that includes companies working in the AI space, including Google, Apple, Nvidia, Amazon, Microsoft, and Arm. Dr. Maguiness had no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM SPD 2024
Study Identifies Oral Antibiotics Linked to Severe Cutaneous Reactions
according to a large, population-based, nested case-control study of older adults, spanning two decades.
The findings, published online in JAMA, “underscore the importance of judicious prescribing, with preferential use of antibiotics associated with a lower risk when clinically appropriate,” noted senior author David Juurlink, MD, PhD, professor of medicine; pediatrics; and health policy, management and evaluation at the University of Toronto, and head of the Clinical Pharmacology and Toxicology Division at Sunnybrook Health Sciences Centre, also in Toronto, Ontario, Canada, and coauthors.
“We hope our study raises awareness about the importance of drug allergy and gains support for future studies to improve drug allergy care,” lead author Erika Lee, MD, clinical immunology and allergy lecturer at the University of Toronto’s Drug Allergy Clinic, Sunnybrook Health Sciences Centre, said in an interview. “It is important to recognize symptoms and signs of a severe drug rash and promptly stop culprit drugs to prevent worsening reaction.”
Serious cADRs are “a group of rare but potentially life-threatening drug hypersensitivity reactions involving the skin and, frequently, internal organs,” the authors wrote. “Typically delayed in onset, these reactions include drug reaction with eosinophilia and systemic symptoms, Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN) — the most severe cADR, which has a reported mortality of 20%-40%,” they noted.
Speculation Without Data
Although it has been speculated that some oral antibiotics are more likely than others to be associated with serious cADRs, there have been no population-based studies examining this, they added.
The study included adults aged 66 years or older and used administrative health databases in Ontario, spanning from April 1, 2002, to March 31, 2022. Data on antibiotic use were taken from the Ontario Drug Benefit database. The Canadian Institute for Health Information (CIHI) National Ambulatory Care Reporting System was used to obtain data on emergency department (ED) visits for cADRs, while the CIHI Discharge Abstract Database was used to identify hospitalizations for cADRs. Finally, demographic information and outpatient healthcare utilization data were obtained from the Registered Persons Database and the Ontario Health Insurance Plan database, respectively.
A cohort of 21,758 older adults (median age, 75 years; 64.1% women) who had an ED visit or hospitalization for serious cADRs within 60 days of receiving antibiotic therapy was matched by age and sex with 87,025 antibiotic-treated controls who did not have a cutaneous reaction.
The median duration of antibiotic prescription was 7 days among cases and controls, and among the cases, the median latency period between antibiotic prescriptions and hospital visits for cADRs was 14 days. Most of the case patients went to the ED only (86.9%), and the rest were hospitalized.
The most commonly prescribed antibiotic class was penicillins (28.9%), followed by cephalosporins (18.2%), fluoroquinolones (16.5%), macrolides (14.8%), nitrofurantoin (8.6%), and sulfonamides (6.2%). Less commonly used antibiotics (“other” antibiotics) accounted for 6.9%.
Macrolide antibiotics were used as the reference because they are rarely associated with serious cADRs, noted the authors, and the multivariable analysis, adjusted for risk factors associated with serious cADRs, including malignancy, chronic liver disease, chronic kidney disease, and HIV.
After multivariable adjustment, relative to macrolides, sulfonamides were most strongly associated with serious cADRs (adjusted odds ratio [aOR], 2.9) but so were all other antibiotic classes, including cephalosporins (aOR, 2.6), “other” antibiotics (aOR, 2.3), nitrofurantoin (aOR, 2.2), penicillins (aOR, 1.4), and fluoroquinolones (aOR,1.3).
In the secondary analysis, the crude rate of ED visits or hospitalizations for cADRs was highest for cephalosporins (4.92 per 1000 prescriptions), followed by sulfonamides (3.22 per 1000 prescriptions). Among hospitalized patients, the median length of stay was 6 days, with 9.6% requiring transfer to a critical care unit and 5.3% dying in the hospital.
Hospitalizations, ED Visits Not Studied Previously
“Notably, the rate of antibiotic-associated serious cADRs leading to an ED visit or hospitalization has not been previously studied,” noted the authors. “We found that at least two hospital encounters for serious cADRs ensued for every 1000 antibiotic prescriptions. This rate is considerably higher than suggested by studies that examine only SJS/TEN and drug reaction with eosinophilia and systemic symptoms.”
Dr. Lee also emphasized the previously unreported findings about nitrofurantoin. “It is surprising to find that nitrofurantoin, a commonly prescribed antibiotic for urinary tract infection, is also associated with an increased risk of severe drug rash,” she said in an interview.
“This finding highlights a potential novel risk at a population-based level and should be further explored in other populations to verify this association,” the authors wrote.
Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security in Baltimore, Maryland, and a spokesperson for the Infectious Diseases Society of America, who was not involved in the study, agreed that the nitrofurantoin finding was surprising, but he was not surprised that sulfonamides were high on the list.
“The study reinforces that antibiotics are not benign medications to be dispensed injudiciously,” he said in an interview. “Antibiotics have risks, including serious skin reactions, as well as the fostering of antibiotic resistance. Clinicians should always first ask themselves if their patient actually merits an antibiotic and then assess what is the safest antibiotic for the purpose, bearing in mind that certain antibiotics are more likely to result in adverse reactions than others.”
The study was supported by the Canadian Institutes of Health Research. The study was conducted at ICES, which is funded in part by an annual grant from the Ontario Ministry of Health and Long-Term Care. One coauthor reported receiving compensation from the British Journal of Dermatology as reviewer and section editor, the American Academy of Dermatology as guidelines writer, Canadian Dermatology Today as manuscript writer, and the National Eczema Association and the Canadian Agency for Drugs and Technologies in Health as consultant; as well as receiving research grants to the coauthor’s institution from the National Eczema Association, Eczema Society of Canada, Canadian Dermatology Foundation, Canadian Institutes of Health Research, US National Institutes of Health, and PSI Foundation. Another coauthor reported receiving grants from AbbVie, Bausch Health, Celgene, Lilly, Incyte, Janssen, LEO Pharma, L’Oréal, Novartis, Organon, Pfizer, Sandoz, Amgen, and Boehringer Ingelheim; receiving payment or honoraria for speaking from Sanofi China; participating on advisory boards for LEO Pharma, Novartis, Sanofi, and Union Therapeutics; and receiving equipment donation from L’Oréal. Dr. Adalja reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
according to a large, population-based, nested case-control study of older adults, spanning two decades.
The findings, published online in JAMA, “underscore the importance of judicious prescribing, with preferential use of antibiotics associated with a lower risk when clinically appropriate,” noted senior author David Juurlink, MD, PhD, professor of medicine; pediatrics; and health policy, management and evaluation at the University of Toronto, and head of the Clinical Pharmacology and Toxicology Division at Sunnybrook Health Sciences Centre, also in Toronto, Ontario, Canada, and coauthors.
“We hope our study raises awareness about the importance of drug allergy and gains support for future studies to improve drug allergy care,” lead author Erika Lee, MD, clinical immunology and allergy lecturer at the University of Toronto’s Drug Allergy Clinic, Sunnybrook Health Sciences Centre, said in an interview. “It is important to recognize symptoms and signs of a severe drug rash and promptly stop culprit drugs to prevent worsening reaction.”
Serious cADRs are “a group of rare but potentially life-threatening drug hypersensitivity reactions involving the skin and, frequently, internal organs,” the authors wrote. “Typically delayed in onset, these reactions include drug reaction with eosinophilia and systemic symptoms, Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN) — the most severe cADR, which has a reported mortality of 20%-40%,” they noted.
Speculation Without Data
Although it has been speculated that some oral antibiotics are more likely than others to be associated with serious cADRs, there have been no population-based studies examining this, they added.
The study included adults aged 66 years or older and used administrative health databases in Ontario, spanning from April 1, 2002, to March 31, 2022. Data on antibiotic use were taken from the Ontario Drug Benefit database. The Canadian Institute for Health Information (CIHI) National Ambulatory Care Reporting System was used to obtain data on emergency department (ED) visits for cADRs, while the CIHI Discharge Abstract Database was used to identify hospitalizations for cADRs. Finally, demographic information and outpatient healthcare utilization data were obtained from the Registered Persons Database and the Ontario Health Insurance Plan database, respectively.
A cohort of 21,758 older adults (median age, 75 years; 64.1% women) who had an ED visit or hospitalization for serious cADRs within 60 days of receiving antibiotic therapy was matched by age and sex with 87,025 antibiotic-treated controls who did not have a cutaneous reaction.
The median duration of antibiotic prescription was 7 days among cases and controls, and among the cases, the median latency period between antibiotic prescriptions and hospital visits for cADRs was 14 days. Most of the case patients went to the ED only (86.9%), and the rest were hospitalized.
The most commonly prescribed antibiotic class was penicillins (28.9%), followed by cephalosporins (18.2%), fluoroquinolones (16.5%), macrolides (14.8%), nitrofurantoin (8.6%), and sulfonamides (6.2%). Less commonly used antibiotics (“other” antibiotics) accounted for 6.9%.
Macrolide antibiotics were used as the reference because they are rarely associated with serious cADRs, noted the authors, and the multivariable analysis, adjusted for risk factors associated with serious cADRs, including malignancy, chronic liver disease, chronic kidney disease, and HIV.
After multivariable adjustment, relative to macrolides, sulfonamides were most strongly associated with serious cADRs (adjusted odds ratio [aOR], 2.9) but so were all other antibiotic classes, including cephalosporins (aOR, 2.6), “other” antibiotics (aOR, 2.3), nitrofurantoin (aOR, 2.2), penicillins (aOR, 1.4), and fluoroquinolones (aOR,1.3).
In the secondary analysis, the crude rate of ED visits or hospitalizations for cADRs was highest for cephalosporins (4.92 per 1000 prescriptions), followed by sulfonamides (3.22 per 1000 prescriptions). Among hospitalized patients, the median length of stay was 6 days, with 9.6% requiring transfer to a critical care unit and 5.3% dying in the hospital.
Hospitalizations, ED Visits Not Studied Previously
“Notably, the rate of antibiotic-associated serious cADRs leading to an ED visit or hospitalization has not been previously studied,” noted the authors. “We found that at least two hospital encounters for serious cADRs ensued for every 1000 antibiotic prescriptions. This rate is considerably higher than suggested by studies that examine only SJS/TEN and drug reaction with eosinophilia and systemic symptoms.”
Dr. Lee also emphasized the previously unreported findings about nitrofurantoin. “It is surprising to find that nitrofurantoin, a commonly prescribed antibiotic for urinary tract infection, is also associated with an increased risk of severe drug rash,” she said in an interview.
“This finding highlights a potential novel risk at a population-based level and should be further explored in other populations to verify this association,” the authors wrote.
Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security in Baltimore, Maryland, and a spokesperson for the Infectious Diseases Society of America, who was not involved in the study, agreed that the nitrofurantoin finding was surprising, but he was not surprised that sulfonamides were high on the list.
“The study reinforces that antibiotics are not benign medications to be dispensed injudiciously,” he said in an interview. “Antibiotics have risks, including serious skin reactions, as well as the fostering of antibiotic resistance. Clinicians should always first ask themselves if their patient actually merits an antibiotic and then assess what is the safest antibiotic for the purpose, bearing in mind that certain antibiotics are more likely to result in adverse reactions than others.”
The study was supported by the Canadian Institutes of Health Research. The study was conducted at ICES, which is funded in part by an annual grant from the Ontario Ministry of Health and Long-Term Care. One coauthor reported receiving compensation from the British Journal of Dermatology as reviewer and section editor, the American Academy of Dermatology as guidelines writer, Canadian Dermatology Today as manuscript writer, and the National Eczema Association and the Canadian Agency for Drugs and Technologies in Health as consultant; as well as receiving research grants to the coauthor’s institution from the National Eczema Association, Eczema Society of Canada, Canadian Dermatology Foundation, Canadian Institutes of Health Research, US National Institutes of Health, and PSI Foundation. Another coauthor reported receiving grants from AbbVie, Bausch Health, Celgene, Lilly, Incyte, Janssen, LEO Pharma, L’Oréal, Novartis, Organon, Pfizer, Sandoz, Amgen, and Boehringer Ingelheim; receiving payment or honoraria for speaking from Sanofi China; participating on advisory boards for LEO Pharma, Novartis, Sanofi, and Union Therapeutics; and receiving equipment donation from L’Oréal. Dr. Adalja reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
according to a large, population-based, nested case-control study of older adults, spanning two decades.
The findings, published online in JAMA, “underscore the importance of judicious prescribing, with preferential use of antibiotics associated with a lower risk when clinically appropriate,” noted senior author David Juurlink, MD, PhD, professor of medicine; pediatrics; and health policy, management and evaluation at the University of Toronto, and head of the Clinical Pharmacology and Toxicology Division at Sunnybrook Health Sciences Centre, also in Toronto, Ontario, Canada, and coauthors.
“We hope our study raises awareness about the importance of drug allergy and gains support for future studies to improve drug allergy care,” lead author Erika Lee, MD, clinical immunology and allergy lecturer at the University of Toronto’s Drug Allergy Clinic, Sunnybrook Health Sciences Centre, said in an interview. “It is important to recognize symptoms and signs of a severe drug rash and promptly stop culprit drugs to prevent worsening reaction.”
Serious cADRs are “a group of rare but potentially life-threatening drug hypersensitivity reactions involving the skin and, frequently, internal organs,” the authors wrote. “Typically delayed in onset, these reactions include drug reaction with eosinophilia and systemic symptoms, Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN) — the most severe cADR, which has a reported mortality of 20%-40%,” they noted.
Speculation Without Data
Although it has been speculated that some oral antibiotics are more likely than others to be associated with serious cADRs, there have been no population-based studies examining this, they added.
The study included adults aged 66 years or older and used administrative health databases in Ontario, spanning from April 1, 2002, to March 31, 2022. Data on antibiotic use were taken from the Ontario Drug Benefit database. The Canadian Institute for Health Information (CIHI) National Ambulatory Care Reporting System was used to obtain data on emergency department (ED) visits for cADRs, while the CIHI Discharge Abstract Database was used to identify hospitalizations for cADRs. Finally, demographic information and outpatient healthcare utilization data were obtained from the Registered Persons Database and the Ontario Health Insurance Plan database, respectively.
A cohort of 21,758 older adults (median age, 75 years; 64.1% women) who had an ED visit or hospitalization for serious cADRs within 60 days of receiving antibiotic therapy was matched by age and sex with 87,025 antibiotic-treated controls who did not have a cutaneous reaction.
The median duration of antibiotic prescription was 7 days among cases and controls, and among the cases, the median latency period between antibiotic prescriptions and hospital visits for cADRs was 14 days. Most of the case patients went to the ED only (86.9%), and the rest were hospitalized.
The most commonly prescribed antibiotic class was penicillins (28.9%), followed by cephalosporins (18.2%), fluoroquinolones (16.5%), macrolides (14.8%), nitrofurantoin (8.6%), and sulfonamides (6.2%). Less commonly used antibiotics (“other” antibiotics) accounted for 6.9%.
Macrolide antibiotics were used as the reference because they are rarely associated with serious cADRs, noted the authors, and the multivariable analysis, adjusted for risk factors associated with serious cADRs, including malignancy, chronic liver disease, chronic kidney disease, and HIV.
After multivariable adjustment, relative to macrolides, sulfonamides were most strongly associated with serious cADRs (adjusted odds ratio [aOR], 2.9) but so were all other antibiotic classes, including cephalosporins (aOR, 2.6), “other” antibiotics (aOR, 2.3), nitrofurantoin (aOR, 2.2), penicillins (aOR, 1.4), and fluoroquinolones (aOR,1.3).
In the secondary analysis, the crude rate of ED visits or hospitalizations for cADRs was highest for cephalosporins (4.92 per 1000 prescriptions), followed by sulfonamides (3.22 per 1000 prescriptions). Among hospitalized patients, the median length of stay was 6 days, with 9.6% requiring transfer to a critical care unit and 5.3% dying in the hospital.
Hospitalizations, ED Visits Not Studied Previously
“Notably, the rate of antibiotic-associated serious cADRs leading to an ED visit or hospitalization has not been previously studied,” noted the authors. “We found that at least two hospital encounters for serious cADRs ensued for every 1000 antibiotic prescriptions. This rate is considerably higher than suggested by studies that examine only SJS/TEN and drug reaction with eosinophilia and systemic symptoms.”
Dr. Lee also emphasized the previously unreported findings about nitrofurantoin. “It is surprising to find that nitrofurantoin, a commonly prescribed antibiotic for urinary tract infection, is also associated with an increased risk of severe drug rash,” she said in an interview.
“This finding highlights a potential novel risk at a population-based level and should be further explored in other populations to verify this association,” the authors wrote.
Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security in Baltimore, Maryland, and a spokesperson for the Infectious Diseases Society of America, who was not involved in the study, agreed that the nitrofurantoin finding was surprising, but he was not surprised that sulfonamides were high on the list.
“The study reinforces that antibiotics are not benign medications to be dispensed injudiciously,” he said in an interview. “Antibiotics have risks, including serious skin reactions, as well as the fostering of antibiotic resistance. Clinicians should always first ask themselves if their patient actually merits an antibiotic and then assess what is the safest antibiotic for the purpose, bearing in mind that certain antibiotics are more likely to result in adverse reactions than others.”
The study was supported by the Canadian Institutes of Health Research. The study was conducted at ICES, which is funded in part by an annual grant from the Ontario Ministry of Health and Long-Term Care. One coauthor reported receiving compensation from the British Journal of Dermatology as reviewer and section editor, the American Academy of Dermatology as guidelines writer, Canadian Dermatology Today as manuscript writer, and the National Eczema Association and the Canadian Agency for Drugs and Technologies in Health as consultant; as well as receiving research grants to the coauthor’s institution from the National Eczema Association, Eczema Society of Canada, Canadian Dermatology Foundation, Canadian Institutes of Health Research, US National Institutes of Health, and PSI Foundation. Another coauthor reported receiving grants from AbbVie, Bausch Health, Celgene, Lilly, Incyte, Janssen, LEO Pharma, L’Oréal, Novartis, Organon, Pfizer, Sandoz, Amgen, and Boehringer Ingelheim; receiving payment or honoraria for speaking from Sanofi China; participating on advisory boards for LEO Pharma, Novartis, Sanofi, and Union Therapeutics; and receiving equipment donation from L’Oréal. Dr. Adalja reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM JAMA
Xanthelasma Not Linked to Heart Diseases, Study Finds
TOPLINE:
Xanthelasma palpebrarum, characterized by yellowish plaques on the eyelids, is not associated with increased rates of dyslipidemia or cardiovascular disease.
METHODOLOGY:
- Researchers conducted a case-control study at a single tertiary care center in Israel and analyzed data from 35,452 individuals (mean age, 52.2 years; 69% men) who underwent medical screening from 2001 to 2020.
- They compared 203 patients with xanthelasma palpebrarum with 2030 individuals without the disease (control).
- Primary outcomes were prevalence of dyslipidemia and cardiovascular disease between the two groups.
TAKEAWAY:
- Lipid profiles were similar between the two groups, with no difference in total cholesterol, high- and low-density lipoprotein, and triglyceride levels (all P > .05).
- The prevalence of dyslipidemia was similar for patients with xanthelasma palpebrarum and controls (46% vs 42%, respectively; P = .29), as was the incidence of cardiovascular disease (8.9% vs 10%, respectively; P = .56).
- The incidence of diabetes (P = .13), cerebrovascular accidents (P > .99), ischemic heart disease (P = .73), and hypertension (P = .56) were not significantly different between the two groups.
IN PRACTICE:
“Our study conducted on a large population of individuals undergoing comprehensive ophthalmic and systemic screening tests did not find a significant association between xanthelasma palpebrarum and an increased prevalence of lipid abnormalities or cardiovascular disease,” the authors wrote.
SOURCE:
The study was led by Yael Lustig, MD, of the Goldschleger Eye Institute at Sheba Medical Center, in Ramat Gan, Israel. It was published online on August 5, 2024, in Ophthalmology.
LIMITATIONS:
The retrospective nature of the study and the single-center design may have limited the generalizability of the findings. The study population was self-selected, potentially introducing selection bias. Lack of histopathologic examination could have affected the accuracy of the diagnosis.
DISCLOSURES:
No funding sources were disclosed for this study. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Xanthelasma palpebrarum, characterized by yellowish plaques on the eyelids, is not associated with increased rates of dyslipidemia or cardiovascular disease.
METHODOLOGY:
- Researchers conducted a case-control study at a single tertiary care center in Israel and analyzed data from 35,452 individuals (mean age, 52.2 years; 69% men) who underwent medical screening from 2001 to 2020.
- They compared 203 patients with xanthelasma palpebrarum with 2030 individuals without the disease (control).
- Primary outcomes were prevalence of dyslipidemia and cardiovascular disease between the two groups.
TAKEAWAY:
- Lipid profiles were similar between the two groups, with no difference in total cholesterol, high- and low-density lipoprotein, and triglyceride levels (all P > .05).
- The prevalence of dyslipidemia was similar for patients with xanthelasma palpebrarum and controls (46% vs 42%, respectively; P = .29), as was the incidence of cardiovascular disease (8.9% vs 10%, respectively; P = .56).
- The incidence of diabetes (P = .13), cerebrovascular accidents (P > .99), ischemic heart disease (P = .73), and hypertension (P = .56) were not significantly different between the two groups.
IN PRACTICE:
“Our study conducted on a large population of individuals undergoing comprehensive ophthalmic and systemic screening tests did not find a significant association between xanthelasma palpebrarum and an increased prevalence of lipid abnormalities or cardiovascular disease,” the authors wrote.
SOURCE:
The study was led by Yael Lustig, MD, of the Goldschleger Eye Institute at Sheba Medical Center, in Ramat Gan, Israel. It was published online on August 5, 2024, in Ophthalmology.
LIMITATIONS:
The retrospective nature of the study and the single-center design may have limited the generalizability of the findings. The study population was self-selected, potentially introducing selection bias. Lack of histopathologic examination could have affected the accuracy of the diagnosis.
DISCLOSURES:
No funding sources were disclosed for this study. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Xanthelasma palpebrarum, characterized by yellowish plaques on the eyelids, is not associated with increased rates of dyslipidemia or cardiovascular disease.
METHODOLOGY:
- Researchers conducted a case-control study at a single tertiary care center in Israel and analyzed data from 35,452 individuals (mean age, 52.2 years; 69% men) who underwent medical screening from 2001 to 2020.
- They compared 203 patients with xanthelasma palpebrarum with 2030 individuals without the disease (control).
- Primary outcomes were prevalence of dyslipidemia and cardiovascular disease between the two groups.
TAKEAWAY:
- Lipid profiles were similar between the two groups, with no difference in total cholesterol, high- and low-density lipoprotein, and triglyceride levels (all P > .05).
- The prevalence of dyslipidemia was similar for patients with xanthelasma palpebrarum and controls (46% vs 42%, respectively; P = .29), as was the incidence of cardiovascular disease (8.9% vs 10%, respectively; P = .56).
- The incidence of diabetes (P = .13), cerebrovascular accidents (P > .99), ischemic heart disease (P = .73), and hypertension (P = .56) were not significantly different between the two groups.
IN PRACTICE:
“Our study conducted on a large population of individuals undergoing comprehensive ophthalmic and systemic screening tests did not find a significant association between xanthelasma palpebrarum and an increased prevalence of lipid abnormalities or cardiovascular disease,” the authors wrote.
SOURCE:
The study was led by Yael Lustig, MD, of the Goldschleger Eye Institute at Sheba Medical Center, in Ramat Gan, Israel. It was published online on August 5, 2024, in Ophthalmology.
LIMITATIONS:
The retrospective nature of the study and the single-center design may have limited the generalizability of the findings. The study population was self-selected, potentially introducing selection bias. Lack of histopathologic examination could have affected the accuracy of the diagnosis.
DISCLOSURES:
No funding sources were disclosed for this study. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Association Between Pruritus and Fibromyalgia: Results of a Population-Based, Cross-Sectional Study
Pruritus, which is defined as an itching sensation that elicits a desire to scratch, is the most common cutaneous condition. Pruritus is considered chronic when it lasts for more than 6 weeks.1 Etiologies implicated in chronic pruritus include but are not limited to primary skin diseases such as atopic dermatitis, systemic causes, neuropathic disorders, and psychogenic reasons.2 In approximately 8% to 35% of patients, the cause of pruritus remains elusive despite intensive investigation.3 The mechanisms of itch are multifaceted and include complex neural pathways.4 Although itch and pain share many similarities, they have distinct pathways based on their spinal connections.5 Nevertheless, both conditions show a wide overlap of receptors on peripheral nerve endings and activated brain parts.6,7 Fibromyalgia, the third most common musculoskeletal condition, affects 2% to 3% of the population worldwide and is at least 7 times more common in females.8,9 Its pathogenesis is not entirely clear but is thought to involve neurogenic inflammation, aberrations in peripheral nerves, and central pain mechanisms. Fibromyalgia is characterized by a plethora of symptoms including chronic widespread pain, autonomic disturbances, persistent fatigue and sleep disturbances, and hyperalgesia, as well as somatic and psychiatric symptoms.10
Fibromyalgia is accompanied by altered skin features including increased counts of mast cells and excessive degranulation,11 neurogenic inflammation with elevated cytokine expression,12 disrupted collagen metabolism,13 and microcirculation abnormalities.14 There has been limited research exploring the dermatologic manifestations of fibromyalgia. One retrospective study that included 845 patients with fibromyalgia reported increased occurrence of “neurodermatoses,” including pruritus, neurotic excoriations, prurigo nodules, and lichen simplex chronicus (LSC), among other cutaneous comorbidities.15 Another small study demonstrated an increased incidence of xerosis and neurotic excoriations in females with fibromyalgia.16 A paucity of large epidemiologic studies demonstrating the fibromyalgia-pruritus connection may lead to misdiagnosis, misinterpretation, and undertreatment of these patients.
Up to 49% of fibromyalgia patients experience small-fiber neuropathy.17 Electrophysiologic measurements, quantitative sensory testing, pain-related evoked potentials, and skin biopsies showed that patients with fibromyalgia have compromised small-fiber function, impaired pathways carrying fiber pain signals, and reduced skin innervation and regenerating fibers.18,19 Accordingly, pruritus that has been reported in fibromyalgia is believed to be of neuropathic origin.15 Overall, it is suspected that the same mechanism that causes hypersensitivity and pain in fibromyalgia patients also predisposes them to pruritus. Similar systemic treatments (eg, analgesics, antidepressants, anticonvulsants) prescribed for both conditions support this theory.20-25
Our large cross-sectional study sought to establish the association between fibromyalgia and pruritus as well as related pruritic conditions.
Methods
Study Design and Setting—We conducted a cross-sectional retrospective study using data-mining techniques to access information from the Clalit Health Services (CHS) database. Clalit Health Services is the largest health maintenance organization in Israel. It encompasses an extensive database with continuous real-time input from medical, administrative, and pharmaceutical computerized operating systems, which helps facilitate data collection for epidemiologic studies. A chronic disease register is gathered from these data sources and continuously updated and validated through logistic checks. The current study was approved by the institutional review board of the CHS (approval #0212-17-com2). Informed consent was not required because the data were de-identified and this was a noninterventional observational study.
Study Population and Covariates—Medical records of CHS enrollees were screened for the diagnosis of fibromyalgia, and data on prevalent cases of fibromyalgia were retrieved. The diagnosis of fibromyalgia was based on the documentation of a fibromyalgia-specific diagnostic code registered by a board-certified rheumatologist. A control group of individuals without fibromyalgia was selected through 1:2 matching based on age, sex, and primary care clinic. The control group was randomly selected from the list of CHS members frequency-matched to cases, excluding case patients with fibromyalgia. Age matching was grounded on the exact year of birth (1-year strata).
Other covariates in the analysis included pruritus-related skin disorders, including prurigo nodularis, neurotic excoriations, and LSC. There were 3 socioeconomic status categories according to patients' poverty index: low, intermediate, and high.26
Statistical Analysis—The distribution of sociodemographic and clinical features was compared between patients with fibromyalgia and controls using the χ2 test for sex and socioeconomic status and the t test for age. Conditional logistic regression then was used to calculate adjusted odds ratio (OR) and 95% CI to compare patients with fibromyalgia and controls with respect to the presence of pruritic comorbidities. All statistical analyses were performed using SPSS software (version 26). P<.05 was considered statistically significant in all tests.
Results
Our study population comprised 4971 patients with fibromyalgia and 9896 age- and sex-matched controls. Proportional to the reported female predominance among patients with fibromyalgia,27 4479 (90.1%) patients with fibromyalgia were females and a similar proportion was documented among controls (P=.99). There was a slightly higher proportion of unmarried patients among those with fibromyalgia compared with controls (41.9% vs 39.4%; P=.004). Socioeconomic status was matched between patients and controls (P=.99). Descriptive characteristics of the study population are presented in Table 1.
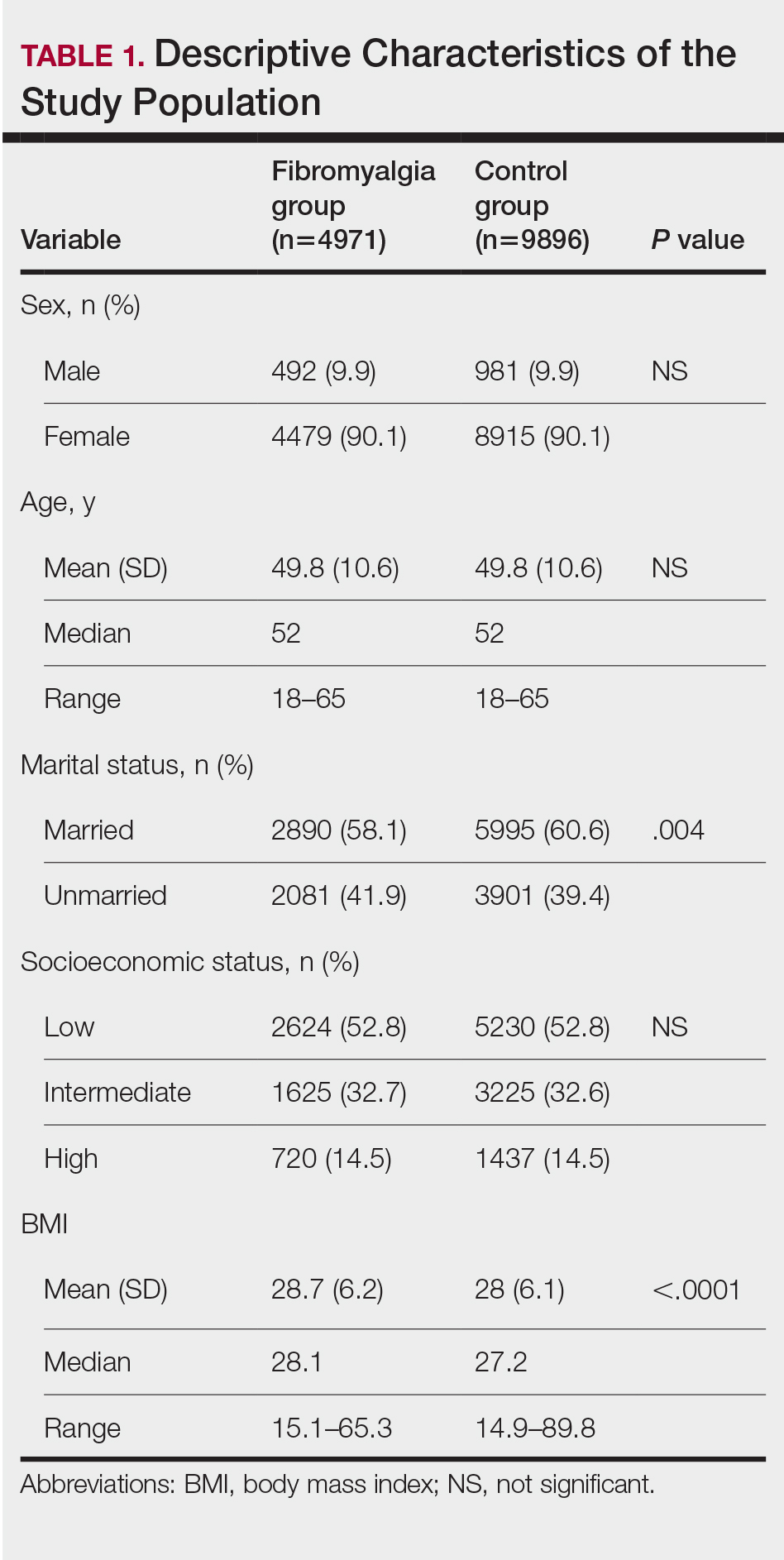
We assessed the presence of pruritus as well as 3 other pruritus-related skin disorders—prurigo nodularis, neurotic excoriations, and LSC—among patients with fibromyalgia and controls. Logistic regression was used to evaluate the independent association between fibromyalgia and pruritus. Table 2 presents the results of multivariate logistic regression models and summarizes the adjusted ORs for pruritic conditions in patients with fibromyalgia and different demographic features across the entire study sample. Fibromyalgia demonstrated strong independent associations with pruritus (OR, 1.8; 95% CI, 1.8-2.4; P<.001), prurigo nodularis (OR, 2.9; 95% CI, 1.1-8.4; P=.038), and LSC (OR, 1.5; 95% CI, 1.1-2.1; P=.01); the association with neurotic excoriations was not significant. Female sex significantly increased the risk for pruritus (OR 1.3; 95% CI, 1.0-1.6; P=.039), while age slightly increased the odds for pruritus (OR, 1.0; 95% CI, 1.0-1.04; P<.001), neurotic excoriations (OR, 1.0; 95% CI, 1.0-1.1; P=.046), and LSC (OR, 1.0; 95% CI, 1.01-1.04; P=.006). Finally, socioeconomic status was inversely correlated with pruritus (OR, 1.1; 95% CI, 1.1-1.5; P=.002).
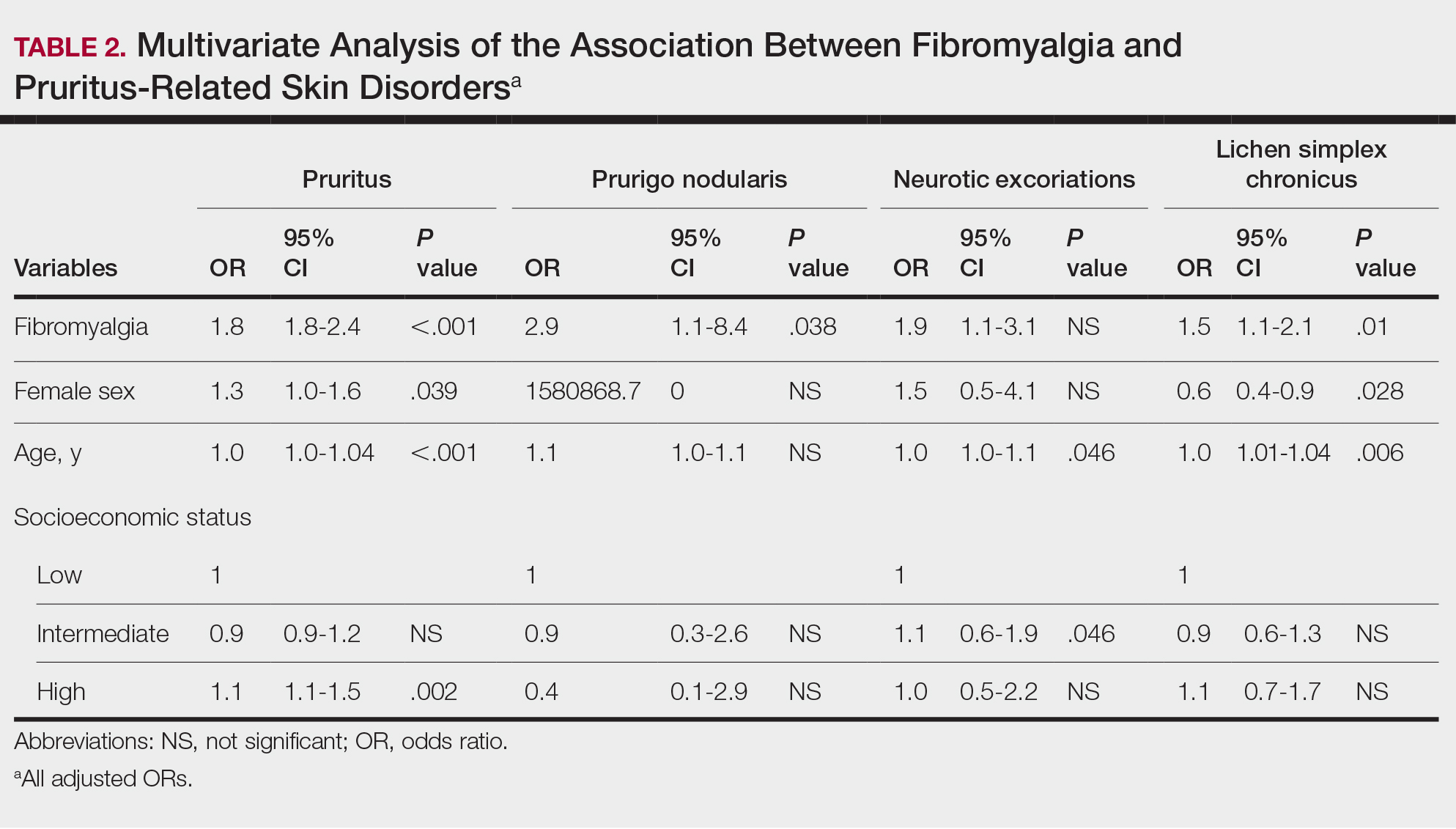
Frequencies and ORs for the association between fibromyalgia and pruritus with associated pruritic disorders stratified by exclusion of pruritic dermatologic and/or systemic diseases that may induce itch are presented in the eTable. Analyzing the entire study cohort, significant increases were observed in the odds of all 4 pruritic disorders analyzed. The frequency of pruritus was almost double in patients with fibromyalgia compared with controls (11.7% vs 6.0%; OR, 2.1; 95% CI, 1.8-2.3; P<.0001). Prurigo nodularis (0.2% vs 0.1%; OR, 2.9; 95% CI, 1.1-8.4; P=.05), neurotic excoriations (0.6% vs 0.3%; OR, 1.9; 95% CI, 1.1-3.1; P=.018), and LSC (1.3% vs 0.8%; OR, 1.5; 95% CI, 1.1-2.1; P=.01) frequencies were all higher in patients with fibromyalgia than controls. When primary skin disorders that may cause itch (eg, pemphigus vulgaris, Darier disease, dermatitis, eczema, ichthyosis, psoriasis, parapsoriasis, urticaria, xerosis, atopic dermatitis, dermatitis herpetiformis, lichen planus) were excluded, the prevalence of pruritus in patients with fibromyalgia was still 1.97 times greater than in the controls (6.9% vs. 3.5%; OR, 2.0; 95% CI, 1.7-2.4; P<.0001). These results remained unchanged even when excluding pruritic dermatologic disorders as well as systemic diseases associated with pruritus (eg, chronic renal failure, dialysis, hyperthyroidism, hyperparathyroidism/hypoparathyroidism, hypothyroidism). Patients with fibromyalgia still displayed a significantly higher prevalence of pruritus compared with the control group (6.6% vs 3.3%; OR, 2.1; 95% CI, 1.7-2.6; P<.0001).
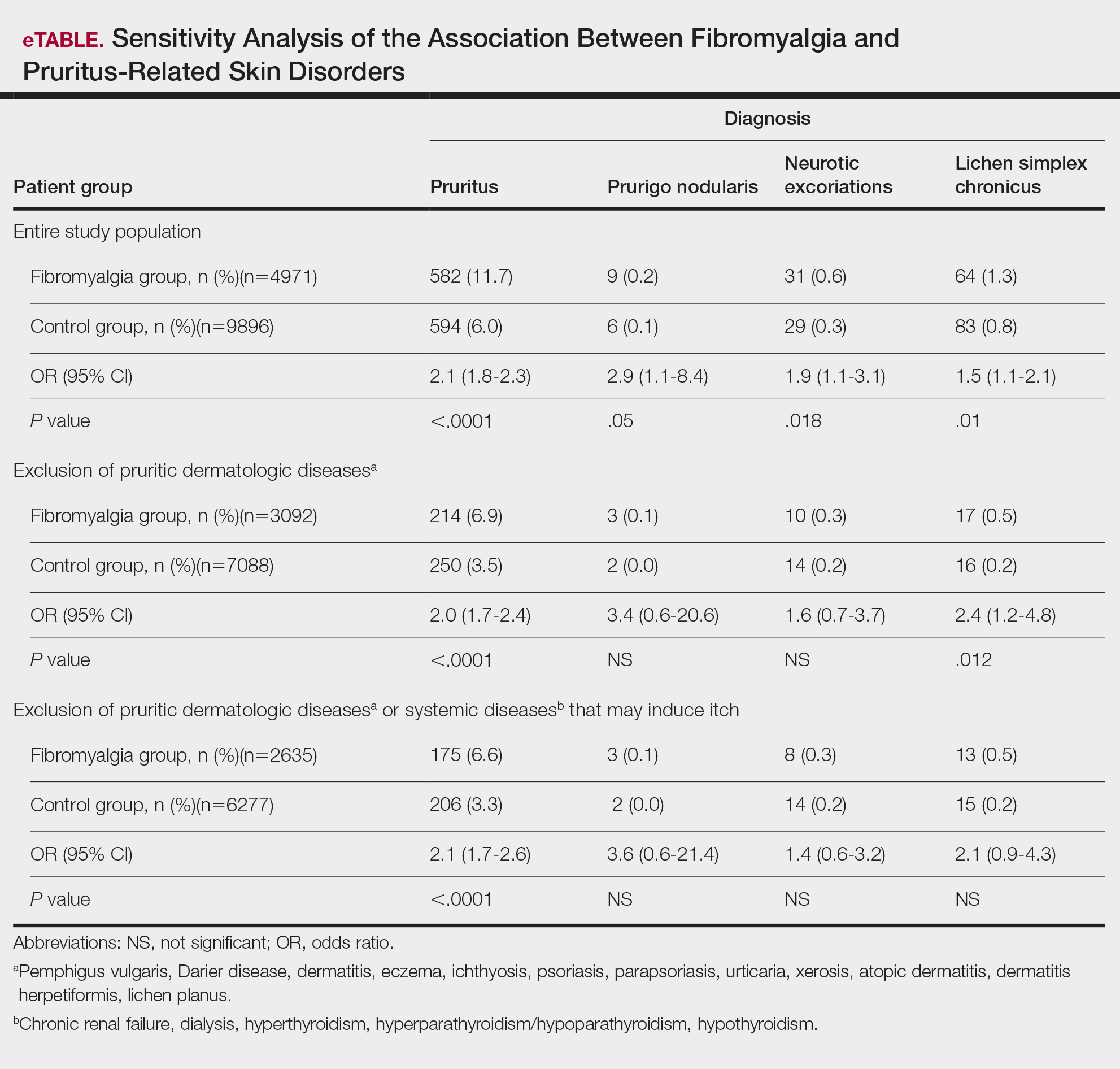
Comment
A wide range of skin manifestations have been associated with fibromyalgia, but the exact mechanisms remain unclear. Nevertheless, it is conceivable that autonomic nervous system dysfunction,28-31 amplified cutaneous opioid receptor activity,32 and an elevated presence of cutaneous mast cells with excessive degranulation may partially explain the frequent occurrence of pruritus and related skin disorders such as neurotic excoriations, prurigo nodularis, and LSC in individuals with fibromyalgia.15,16 In line with these findings, our study—which was based on data from the largest health maintenance organization in Israel—demonstrated an increased prevalence of pruritus and related pruritic disorders among individuals diagnosed with fibromyalgia.
This cross-sectional study links pruritus with fibromyalgia. Few preliminary epidemiologic studies have shown an increased occurrence of cutaneous manifestations in patients with fibromyalgia. One chart review that looked at skin findings in patients with fibromyalgia revealed 32 distinct cutaneous manifestations, and pruritus was the major concern in 3.3% of 845 patients.15
A focused cross-sectional study involving only women (66 with fibromyalgia and 79 healthy controls) discovered 14 skin conditions that were more common in those with fibromyalgia. Notably, xerosis and neurotic excoriations were more prevalent compared to the control group.16
The brain and the skin—both derivatives of the embryonic ectoderm33,34—are linked by pruritus. Although itch has its dedicated neurons, there is a wide-ranging overlap of brain-activated areas between pain and itch,6 and the neural anatomy of pain and itch are closely related in both the peripheral and central nervous systems35-37; for example, diseases of the central nervous system are accompanied by pruritus in as many as 15% of cases, while postherpetic neuralgia can result in chronic pain, itching, or a combination of both.38,39 Other instances include notalgia paresthetica and brachioradial pruritus.38 Additionally, there is a noteworthy psychologic impact associated with both itch and pain,40,41 with both psychosomatic and psychologic factors implicated in chronic pruritus and in fibromyalgia.42 Lastly, the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system are altered in both fibromyalgia and pruritus.43-45
Tey et al45 characterized the itch experienced in fibromyalgia as functional, which is described as pruritus associated with a somatoform disorder. In our study, we found a higher prevalence of pruritus among patients with fibromyalgia, and this association remained significant (P<.05) even when excluding other pruritic skin conditions and systemic diseases that can trigger itching. In addition, our logistic regression analyses revealed independent associations between fibromyalgia and pruritus, prurigo nodularis, and LSC.
According to Twycross et al,46 there are 4 clinical categories of itch, which may coexist7: pruritoceptive (originating in the skin), neuropathic (originating in pathology located along the afferent pathway), neurogenic (central origin but lacks a neural pathology), and psychogenic.47 Skin biopsy findings in patients with fibromyalgia include increased mast cell counts11 and degranulation,48 increased expression of δ and κ opioid receptors,32 vasoconstriction within tender points,49 and elevated IL-1β, IL-6, or tumor necrosis factor α by reverse transcriptase-polymerase chain reaction.12 A case recently was presented by Görg et al50 involving a female patient with fibromyalgia who had been experiencing chronic pruritus, which the authors attributed to small-fiber neuropathy based on evidence from a skin biopsy indicating a reduced number of intraepidermal nerves and the fact that the itching originated around tender points. Altogether, the observed alterations may work together to make patients with fibromyalgia more susceptible to various skin-related comorbidities in general, especially those related to pruritus. Eventually, it might be the case that several itch categories and related pathomechanisms are involved in the pruritus phenotype of patients with fibromyalgia.
Age-related alterations in nerve fibers, lower immune function, xerosis, polypharmacy, and increased frequency of systemic diseases with age are just a few of the factors that may predispose older individuals to pruritus.51,52 Indeed, our logistic regression model showed that age was significantly and independently associated with pruritus (P<.001), neurotic excoriations (P=.046), and LSC (P=.006). Female sex also was significantly linked with pruritus (P=.039). Intriguingly, high socioeconomic status was significantly associated with the diagnosis of pruritus (P=.002), possibly due to easier access to medical care.
There is a considerable overlap between the therapeutic approaches used in pruritus, pruritus-related skin disorders, and fibromyalgia. Antidepressants, anxiolytics, analgesics, and antiepileptics have been used to address both conditions.45 The association between these conditions advocates for a multidisciplinary approach in patients with fibromyalgia and potentially supports the rationale for unified therapeutics for both conditions.
Conclusion
Our findings indicate an association between fibromyalgia and pruritus as well as associated pruritic skin disorders. Given the convoluted and largely undiscovered mechanisms underlying fibromyalgia, managing patients with this condition may present substantial challenges.53 The data presented here support the implementation of a multidisciplinary treatment approach for patients with fibromyalgia. This approach should focus on managing fibromyalgia pain as well as addressing its concurrent skin-related conditions. It is advisable to consider treatments such as antiepileptics (eg, pregabalin, gabapentin) that specifically target neuropathic disorders in affected patients. These treatments may hold promise for alleviating fibromyalgia-related pain54 and mitigating its related cutaneous comorbidities, especially pruritus.
- Stander S, Weisshaar E, Mettang T, et al. Clinical classification of itch: a position paper of the International Forum for the Study of Itch. Acta Derm Venereol. 2007; 87:291-294.
- Yosipovitch G, Bernhard JD. Clinical practice. chronic pruritus. N Engl J Med. 2013;368:1625-1634.
- Song J, Xian D, Yang L, et al. Pruritus: progress toward pathogenesis and treatment. Biomed Res Int. 2018;2018:9625936.
- Potenzieri C, Undem BJ. Basic mechanisms of itch. Clin Exp Allergy. 2012;42:8-19.
- McMahon SB, Koltzenburg M. Itching for an explanation. Trends Neurosci. 1992;15:497-501.
- Drzezga A, Darsow U, Treede RD, et al. Central activation by histamine-induced itch: analogies to pain processing: a correlational analysis of O-15 H2O positron emission tomography studies. Pain. 2001; 92:295-305.
- Yosipovitch G, Greaves MW, Schmelz M. Itch. Lancet. 2003;361:690-694.
- Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. part I. Arthritis Rheum. 2008; 58:15-25.
- Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. part II. Arthritis Rheum. 2008; 58:26-35.
- Sarzi-Puttini P, Giorgi V, Marotto D, et al. Fibromyalgia: an update on clinical characteristics, aetiopathogenesis and treatment. Nat Rev Rheumatol. 2020;16:645-660.
- Blanco I, Beritze N, Arguelles M, et al. Abnormal overexpression of mastocytes in skin biopsies of fibromyalgia patients. Clin Rheumatol. 2010;29:1403-1412.
- Salemi S, Rethage J, Wollina U, et al. Detection of interleukin 1beta (IL-1beta), IL-6, and tumor necrosis factor-alpha in skin of patients with fibromyalgia. J Rheumatol. 2003;30:146-150.
- Sprott H, Muller A, Heine H. Collagen cross-links in fibromyalgia syndrome. Z Rheumatol. 1998;57(suppl 2):52-55.
- Morf S, Amann-Vesti B, Forster A, et al. Microcirculation abnormalities in patients with fibromyalgia—measured by capillary microscopy and laser fluxmetry. Arthritis Res Ther. 2005;7:R209-R216.
- Laniosz V, Wetter DA, Godar DA. Dermatologic manifestations of fibromyalgia. Clin Rheumatol. 2014;33:1009-1013.
- Dogramaci AC, Yalcinkaya EY. Skin problems in fibromyalgia. Nobel Med. 2009;5:50-52.
- Grayston R, Czanner G, Elhadd K, et al. A systematic review and meta-analysis of the prevalence of small fiber pathology in fibromyalgia: implications for a new paradigm in fibromyalgia etiopathogenesis. Semin Arthritis Rheum. 2019;48:933-940.
- Uceyler N, Zeller D, Kahn AK, et al. Small fibre pathology in patients with fibromyalgia syndrome. Brain. 2013;136:1857-1867.
- Devigili G, Tugnoli V, Penza P, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain. 2008; 131:1912- 1925.
- Reed C, Birnbaum HG, Ivanova JI, et al. Real-world role of tricyclic antidepressants in the treatment of fibromyalgia. Pain Pract. 2012; 12:533-540.
- Moret C, Briley M. Antidepressants in the treatment of fibromyalgia. Neuropsychiatr Dis Treat. 2006;2:537-548.
- Arnold LM, Keck PE Jr, Welge JA. Antidepressant treatment of fibromyalgia. a meta-analysis and review. Psychosomatics. 2000;41:104-113.
- Moore A, Wiffen P, Kalso E. Antiepileptic drugs for neuropathic pain and fibromyalgia. JAMA. 2014;312:182-183.
- Shevchenko A, Valdes-Rodriguez R, Yosipovitch G. Causes, pathophysiology, and treatment of pruritus in the mature patient. Clin Dermatol. 2018;36:140-151.
- Scheinfeld N. The role of gabapentin in treating diseases with cutaneous manifestations and pain. Int J Dermatol. 2003;42:491-495.
- Points Location Intelligence. Accessed July 30, 2024. https://points.co.il/en/points-location-intelligence/
- Yunus MB. The role of gender in fibromyalgia syndrome. Curr Rheumatol Rep. 2001;3:128-134.
- Cakir T, Evcik D, Dundar U, et al. Evaluation of sympathetic skin response and f wave in fibromyalgia syndrome patients. Turk J Rheumatol. 2011;26:38-43.
- Ozkan O, Yildiz M, Koklukaya E. The correlation of laboratory tests and sympathetic skin response parameters by using artificial neural networks in fibromyalgia patients. J Med Syst. 2012;36:1841-1848.
- Ozkan O, Yildiz M, Arslan E, et al. A study on the effects of sympathetic skin response parameters in diagnosis of fibromyalgia using artificial neural networks. J Med Syst. 2016;40:54.
- Ulas UH, Unlu E, Hamamcioglu K, et al. Dysautonomia in fibromyalgia syndrome: sympathetic skin responses and RR interval analysis. Rheumatol Int. 2006;26:383-387.
- Salemi S, Aeschlimann A, Wollina U, et al. Up-regulation of delta-opioid receptors and kappa-opioid receptors in the skin of fibromyalgia patients. Arthritis Rheum. 2007;56:2464-2466.
- Elshazzly M, Lopez MJ, Reddy V, et al. Central nervous system. StatPearls. StatPearls Publishing; 2022.
- Hu MS, Borrelli MR, Hong WX, et al. Embryonic skin development and repair. Organogenesis. 2018;14:46-63.
- Davidson S, Zhang X, Yoon CH, et al. The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci. 2007;27:10007-10014.
- Sikand P, Shimada SG, Green BG, et al. Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain. 2009;144:66-75.
- Davidson S, Giesler GJ. The multiple pathways for itch and their interactions with pain. Trends Neurosci. 2010;33:550-558.
- Dhand A, Aminoff MJ. The neurology of itch. Brain. 2014;137:313-322.
- Binder A, Koroschetz J, Baron R. Disease mechanisms in neuropathic itch. Nat Clin Pract Neurol. 2008;4:329-337.
- Fjellner B, Arnetz BB. Psychological predictors of pruritus during mental stress. Acta Derm Venereol. 1985;65:504-508.
- Papoiu AD, Wang H, Coghill RC, et al. Contagious itch in humans: a study of visual ‘transmission’ of itch in atopic dermatitis and healthy subjects. Br J Dermatol. 2011;164:1299-1303.
- Stumpf A, Schneider G, Stander S. Psychosomatic and psychiatric disorders and psychologic factors in pruritus. Clin Dermatol. 2018;36:704-708.
- Herman JP, McKlveen JM, Ghosal S, et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. 2016;6:603-621.
- Brown ED, Micozzi MS, Craft NE, et al. Plasma carotenoids in normal men after a single ingestion of vegetables or purified beta-carotene. Am J Clin Nutr. 1989;49:1258-1265.
- Tey HL, Wallengren J, Yosipovitch G. Psychosomatic factors in pruritus. Clin Dermatol. 2013;31:31-40.
- Twycross R, Greaves MW, Handwerker H, et al. Itch: scratching more than the surface. QJM. 2003;96:7-26.
- Bernhard JD. Itch and pruritus: what are they, and how should itches be classified? Dermatol Ther. 2005;18:288-291.
- Enestrom S, Bengtsson A, Frodin T. Dermal IgG deposits and increase of mast cells in patients with fibromyalgia—relevant findings or epiphenomena? Scand J Rheumatol. 1997;26:308-313.
- Jeschonneck M, Grohmann G, Hein G, et al. Abnormal microcirculation and temperature in skin above tender points in patients with fibromyalgia. Rheumatology (Oxford). 2000;39:917-921.
- Görg M, Zeidler C, Pereira MP, et al. Generalized chronic pruritus with fibromyalgia. J Dtsch Dermatol Ges. 2021;19:909-911.
- Garibyan L, Chiou AS, Elmariah SB. Advanced aging skin and itch: addressing an unmet need. Dermatol Ther. 2013;26:92-103.
- Cohen KR, Frank J, Salbu RL, et al. Pruritus in the elderly: clinical approaches to the improvement of quality of life. P T. 2012;37:227-239.
- Tzadok R, Ablin JN. Current and emerging pharmacotherapy for fibromyalgia. Pain Res Manag. 2020; 2020:6541798.
- Wiffen PJ, Derry S, Moore RA, et al. Antiepileptic drugs for neuropathic pain and fibromyalgia—an overview of Cochrane reviews. Cochrane Database Syst Rev. 2013:CD010567.
Pruritus, which is defined as an itching sensation that elicits a desire to scratch, is the most common cutaneous condition. Pruritus is considered chronic when it lasts for more than 6 weeks.1 Etiologies implicated in chronic pruritus include but are not limited to primary skin diseases such as atopic dermatitis, systemic causes, neuropathic disorders, and psychogenic reasons.2 In approximately 8% to 35% of patients, the cause of pruritus remains elusive despite intensive investigation.3 The mechanisms of itch are multifaceted and include complex neural pathways.4 Although itch and pain share many similarities, they have distinct pathways based on their spinal connections.5 Nevertheless, both conditions show a wide overlap of receptors on peripheral nerve endings and activated brain parts.6,7 Fibromyalgia, the third most common musculoskeletal condition, affects 2% to 3% of the population worldwide and is at least 7 times more common in females.8,9 Its pathogenesis is not entirely clear but is thought to involve neurogenic inflammation, aberrations in peripheral nerves, and central pain mechanisms. Fibromyalgia is characterized by a plethora of symptoms including chronic widespread pain, autonomic disturbances, persistent fatigue and sleep disturbances, and hyperalgesia, as well as somatic and psychiatric symptoms.10
Fibromyalgia is accompanied by altered skin features including increased counts of mast cells and excessive degranulation,11 neurogenic inflammation with elevated cytokine expression,12 disrupted collagen metabolism,13 and microcirculation abnormalities.14 There has been limited research exploring the dermatologic manifestations of fibromyalgia. One retrospective study that included 845 patients with fibromyalgia reported increased occurrence of “neurodermatoses,” including pruritus, neurotic excoriations, prurigo nodules, and lichen simplex chronicus (LSC), among other cutaneous comorbidities.15 Another small study demonstrated an increased incidence of xerosis and neurotic excoriations in females with fibromyalgia.16 A paucity of large epidemiologic studies demonstrating the fibromyalgia-pruritus connection may lead to misdiagnosis, misinterpretation, and undertreatment of these patients.
Up to 49% of fibromyalgia patients experience small-fiber neuropathy.17 Electrophysiologic measurements, quantitative sensory testing, pain-related evoked potentials, and skin biopsies showed that patients with fibromyalgia have compromised small-fiber function, impaired pathways carrying fiber pain signals, and reduced skin innervation and regenerating fibers.18,19 Accordingly, pruritus that has been reported in fibromyalgia is believed to be of neuropathic origin.15 Overall, it is suspected that the same mechanism that causes hypersensitivity and pain in fibromyalgia patients also predisposes them to pruritus. Similar systemic treatments (eg, analgesics, antidepressants, anticonvulsants) prescribed for both conditions support this theory.20-25
Our large cross-sectional study sought to establish the association between fibromyalgia and pruritus as well as related pruritic conditions.
Methods
Study Design and Setting—We conducted a cross-sectional retrospective study using data-mining techniques to access information from the Clalit Health Services (CHS) database. Clalit Health Services is the largest health maintenance organization in Israel. It encompasses an extensive database with continuous real-time input from medical, administrative, and pharmaceutical computerized operating systems, which helps facilitate data collection for epidemiologic studies. A chronic disease register is gathered from these data sources and continuously updated and validated through logistic checks. The current study was approved by the institutional review board of the CHS (approval #0212-17-com2). Informed consent was not required because the data were de-identified and this was a noninterventional observational study.
Study Population and Covariates—Medical records of CHS enrollees were screened for the diagnosis of fibromyalgia, and data on prevalent cases of fibromyalgia were retrieved. The diagnosis of fibromyalgia was based on the documentation of a fibromyalgia-specific diagnostic code registered by a board-certified rheumatologist. A control group of individuals without fibromyalgia was selected through 1:2 matching based on age, sex, and primary care clinic. The control group was randomly selected from the list of CHS members frequency-matched to cases, excluding case patients with fibromyalgia. Age matching was grounded on the exact year of birth (1-year strata).
Other covariates in the analysis included pruritus-related skin disorders, including prurigo nodularis, neurotic excoriations, and LSC. There were 3 socioeconomic status categories according to patients' poverty index: low, intermediate, and high.26
Statistical Analysis—The distribution of sociodemographic and clinical features was compared between patients with fibromyalgia and controls using the χ2 test for sex and socioeconomic status and the t test for age. Conditional logistic regression then was used to calculate adjusted odds ratio (OR) and 95% CI to compare patients with fibromyalgia and controls with respect to the presence of pruritic comorbidities. All statistical analyses were performed using SPSS software (version 26). P<.05 was considered statistically significant in all tests.
Results
Our study population comprised 4971 patients with fibromyalgia and 9896 age- and sex-matched controls. Proportional to the reported female predominance among patients with fibromyalgia,27 4479 (90.1%) patients with fibromyalgia were females and a similar proportion was documented among controls (P=.99). There was a slightly higher proportion of unmarried patients among those with fibromyalgia compared with controls (41.9% vs 39.4%; P=.004). Socioeconomic status was matched between patients and controls (P=.99). Descriptive characteristics of the study population are presented in Table 1.

We assessed the presence of pruritus as well as 3 other pruritus-related skin disorders—prurigo nodularis, neurotic excoriations, and LSC—among patients with fibromyalgia and controls. Logistic regression was used to evaluate the independent association between fibromyalgia and pruritus. Table 2 presents the results of multivariate logistic regression models and summarizes the adjusted ORs for pruritic conditions in patients with fibromyalgia and different demographic features across the entire study sample. Fibromyalgia demonstrated strong independent associations with pruritus (OR, 1.8; 95% CI, 1.8-2.4; P<.001), prurigo nodularis (OR, 2.9; 95% CI, 1.1-8.4; P=.038), and LSC (OR, 1.5; 95% CI, 1.1-2.1; P=.01); the association with neurotic excoriations was not significant. Female sex significantly increased the risk for pruritus (OR 1.3; 95% CI, 1.0-1.6; P=.039), while age slightly increased the odds for pruritus (OR, 1.0; 95% CI, 1.0-1.04; P<.001), neurotic excoriations (OR, 1.0; 95% CI, 1.0-1.1; P=.046), and LSC (OR, 1.0; 95% CI, 1.01-1.04; P=.006). Finally, socioeconomic status was inversely correlated with pruritus (OR, 1.1; 95% CI, 1.1-1.5; P=.002).

Frequencies and ORs for the association between fibromyalgia and pruritus with associated pruritic disorders stratified by exclusion of pruritic dermatologic and/or systemic diseases that may induce itch are presented in the eTable. Analyzing the entire study cohort, significant increases were observed in the odds of all 4 pruritic disorders analyzed. The frequency of pruritus was almost double in patients with fibromyalgia compared with controls (11.7% vs 6.0%; OR, 2.1; 95% CI, 1.8-2.3; P<.0001). Prurigo nodularis (0.2% vs 0.1%; OR, 2.9; 95% CI, 1.1-8.4; P=.05), neurotic excoriations (0.6% vs 0.3%; OR, 1.9; 95% CI, 1.1-3.1; P=.018), and LSC (1.3% vs 0.8%; OR, 1.5; 95% CI, 1.1-2.1; P=.01) frequencies were all higher in patients with fibromyalgia than controls. When primary skin disorders that may cause itch (eg, pemphigus vulgaris, Darier disease, dermatitis, eczema, ichthyosis, psoriasis, parapsoriasis, urticaria, xerosis, atopic dermatitis, dermatitis herpetiformis, lichen planus) were excluded, the prevalence of pruritus in patients with fibromyalgia was still 1.97 times greater than in the controls (6.9% vs. 3.5%; OR, 2.0; 95% CI, 1.7-2.4; P<.0001). These results remained unchanged even when excluding pruritic dermatologic disorders as well as systemic diseases associated with pruritus (eg, chronic renal failure, dialysis, hyperthyroidism, hyperparathyroidism/hypoparathyroidism, hypothyroidism). Patients with fibromyalgia still displayed a significantly higher prevalence of pruritus compared with the control group (6.6% vs 3.3%; OR, 2.1; 95% CI, 1.7-2.6; P<.0001).

Comment
A wide range of skin manifestations have been associated with fibromyalgia, but the exact mechanisms remain unclear. Nevertheless, it is conceivable that autonomic nervous system dysfunction,28-31 amplified cutaneous opioid receptor activity,32 and an elevated presence of cutaneous mast cells with excessive degranulation may partially explain the frequent occurrence of pruritus and related skin disorders such as neurotic excoriations, prurigo nodularis, and LSC in individuals with fibromyalgia.15,16 In line with these findings, our study—which was based on data from the largest health maintenance organization in Israel—demonstrated an increased prevalence of pruritus and related pruritic disorders among individuals diagnosed with fibromyalgia.
This cross-sectional study links pruritus with fibromyalgia. Few preliminary epidemiologic studies have shown an increased occurrence of cutaneous manifestations in patients with fibromyalgia. One chart review that looked at skin findings in patients with fibromyalgia revealed 32 distinct cutaneous manifestations, and pruritus was the major concern in 3.3% of 845 patients.15
A focused cross-sectional study involving only women (66 with fibromyalgia and 79 healthy controls) discovered 14 skin conditions that were more common in those with fibromyalgia. Notably, xerosis and neurotic excoriations were more prevalent compared to the control group.16
The brain and the skin—both derivatives of the embryonic ectoderm33,34—are linked by pruritus. Although itch has its dedicated neurons, there is a wide-ranging overlap of brain-activated areas between pain and itch,6 and the neural anatomy of pain and itch are closely related in both the peripheral and central nervous systems35-37; for example, diseases of the central nervous system are accompanied by pruritus in as many as 15% of cases, while postherpetic neuralgia can result in chronic pain, itching, or a combination of both.38,39 Other instances include notalgia paresthetica and brachioradial pruritus.38 Additionally, there is a noteworthy psychologic impact associated with both itch and pain,40,41 with both psychosomatic and psychologic factors implicated in chronic pruritus and in fibromyalgia.42 Lastly, the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system are altered in both fibromyalgia and pruritus.43-45
Tey et al45 characterized the itch experienced in fibromyalgia as functional, which is described as pruritus associated with a somatoform disorder. In our study, we found a higher prevalence of pruritus among patients with fibromyalgia, and this association remained significant (P<.05) even when excluding other pruritic skin conditions and systemic diseases that can trigger itching. In addition, our logistic regression analyses revealed independent associations between fibromyalgia and pruritus, prurigo nodularis, and LSC.
According to Twycross et al,46 there are 4 clinical categories of itch, which may coexist7: pruritoceptive (originating in the skin), neuropathic (originating in pathology located along the afferent pathway), neurogenic (central origin but lacks a neural pathology), and psychogenic.47 Skin biopsy findings in patients with fibromyalgia include increased mast cell counts11 and degranulation,48 increased expression of δ and κ opioid receptors,32 vasoconstriction within tender points,49 and elevated IL-1β, IL-6, or tumor necrosis factor α by reverse transcriptase-polymerase chain reaction.12 A case recently was presented by Görg et al50 involving a female patient with fibromyalgia who had been experiencing chronic pruritus, which the authors attributed to small-fiber neuropathy based on evidence from a skin biopsy indicating a reduced number of intraepidermal nerves and the fact that the itching originated around tender points. Altogether, the observed alterations may work together to make patients with fibromyalgia more susceptible to various skin-related comorbidities in general, especially those related to pruritus. Eventually, it might be the case that several itch categories and related pathomechanisms are involved in the pruritus phenotype of patients with fibromyalgia.
Age-related alterations in nerve fibers, lower immune function, xerosis, polypharmacy, and increased frequency of systemic diseases with age are just a few of the factors that may predispose older individuals to pruritus.51,52 Indeed, our logistic regression model showed that age was significantly and independently associated with pruritus (P<.001), neurotic excoriations (P=.046), and LSC (P=.006). Female sex also was significantly linked with pruritus (P=.039). Intriguingly, high socioeconomic status was significantly associated with the diagnosis of pruritus (P=.002), possibly due to easier access to medical care.
There is a considerable overlap between the therapeutic approaches used in pruritus, pruritus-related skin disorders, and fibromyalgia. Antidepressants, anxiolytics, analgesics, and antiepileptics have been used to address both conditions.45 The association between these conditions advocates for a multidisciplinary approach in patients with fibromyalgia and potentially supports the rationale for unified therapeutics for both conditions.
Conclusion
Our findings indicate an association between fibromyalgia and pruritus as well as associated pruritic skin disorders. Given the convoluted and largely undiscovered mechanisms underlying fibromyalgia, managing patients with this condition may present substantial challenges.53 The data presented here support the implementation of a multidisciplinary treatment approach for patients with fibromyalgia. This approach should focus on managing fibromyalgia pain as well as addressing its concurrent skin-related conditions. It is advisable to consider treatments such as antiepileptics (eg, pregabalin, gabapentin) that specifically target neuropathic disorders in affected patients. These treatments may hold promise for alleviating fibromyalgia-related pain54 and mitigating its related cutaneous comorbidities, especially pruritus.
Pruritus, which is defined as an itching sensation that elicits a desire to scratch, is the most common cutaneous condition. Pruritus is considered chronic when it lasts for more than 6 weeks.1 Etiologies implicated in chronic pruritus include but are not limited to primary skin diseases such as atopic dermatitis, systemic causes, neuropathic disorders, and psychogenic reasons.2 In approximately 8% to 35% of patients, the cause of pruritus remains elusive despite intensive investigation.3 The mechanisms of itch are multifaceted and include complex neural pathways.4 Although itch and pain share many similarities, they have distinct pathways based on their spinal connections.5 Nevertheless, both conditions show a wide overlap of receptors on peripheral nerve endings and activated brain parts.6,7 Fibromyalgia, the third most common musculoskeletal condition, affects 2% to 3% of the population worldwide and is at least 7 times more common in females.8,9 Its pathogenesis is not entirely clear but is thought to involve neurogenic inflammation, aberrations in peripheral nerves, and central pain mechanisms. Fibromyalgia is characterized by a plethora of symptoms including chronic widespread pain, autonomic disturbances, persistent fatigue and sleep disturbances, and hyperalgesia, as well as somatic and psychiatric symptoms.10
Fibromyalgia is accompanied by altered skin features including increased counts of mast cells and excessive degranulation,11 neurogenic inflammation with elevated cytokine expression,12 disrupted collagen metabolism,13 and microcirculation abnormalities.14 There has been limited research exploring the dermatologic manifestations of fibromyalgia. One retrospective study that included 845 patients with fibromyalgia reported increased occurrence of “neurodermatoses,” including pruritus, neurotic excoriations, prurigo nodules, and lichen simplex chronicus (LSC), among other cutaneous comorbidities.15 Another small study demonstrated an increased incidence of xerosis and neurotic excoriations in females with fibromyalgia.16 A paucity of large epidemiologic studies demonstrating the fibromyalgia-pruritus connection may lead to misdiagnosis, misinterpretation, and undertreatment of these patients.
Up to 49% of fibromyalgia patients experience small-fiber neuropathy.17 Electrophysiologic measurements, quantitative sensory testing, pain-related evoked potentials, and skin biopsies showed that patients with fibromyalgia have compromised small-fiber function, impaired pathways carrying fiber pain signals, and reduced skin innervation and regenerating fibers.18,19 Accordingly, pruritus that has been reported in fibromyalgia is believed to be of neuropathic origin.15 Overall, it is suspected that the same mechanism that causes hypersensitivity and pain in fibromyalgia patients also predisposes them to pruritus. Similar systemic treatments (eg, analgesics, antidepressants, anticonvulsants) prescribed for both conditions support this theory.20-25
Our large cross-sectional study sought to establish the association between fibromyalgia and pruritus as well as related pruritic conditions.
Methods
Study Design and Setting—We conducted a cross-sectional retrospective study using data-mining techniques to access information from the Clalit Health Services (CHS) database. Clalit Health Services is the largest health maintenance organization in Israel. It encompasses an extensive database with continuous real-time input from medical, administrative, and pharmaceutical computerized operating systems, which helps facilitate data collection for epidemiologic studies. A chronic disease register is gathered from these data sources and continuously updated and validated through logistic checks. The current study was approved by the institutional review board of the CHS (approval #0212-17-com2). Informed consent was not required because the data were de-identified and this was a noninterventional observational study.
Study Population and Covariates—Medical records of CHS enrollees were screened for the diagnosis of fibromyalgia, and data on prevalent cases of fibromyalgia were retrieved. The diagnosis of fibromyalgia was based on the documentation of a fibromyalgia-specific diagnostic code registered by a board-certified rheumatologist. A control group of individuals without fibromyalgia was selected through 1:2 matching based on age, sex, and primary care clinic. The control group was randomly selected from the list of CHS members frequency-matched to cases, excluding case patients with fibromyalgia. Age matching was grounded on the exact year of birth (1-year strata).
Other covariates in the analysis included pruritus-related skin disorders, including prurigo nodularis, neurotic excoriations, and LSC. There were 3 socioeconomic status categories according to patients' poverty index: low, intermediate, and high.26
Statistical Analysis—The distribution of sociodemographic and clinical features was compared between patients with fibromyalgia and controls using the χ2 test for sex and socioeconomic status and the t test for age. Conditional logistic regression then was used to calculate adjusted odds ratio (OR) and 95% CI to compare patients with fibromyalgia and controls with respect to the presence of pruritic comorbidities. All statistical analyses were performed using SPSS software (version 26). P<.05 was considered statistically significant in all tests.
Results
Our study population comprised 4971 patients with fibromyalgia and 9896 age- and sex-matched controls. Proportional to the reported female predominance among patients with fibromyalgia,27 4479 (90.1%) patients with fibromyalgia were females and a similar proportion was documented among controls (P=.99). There was a slightly higher proportion of unmarried patients among those with fibromyalgia compared with controls (41.9% vs 39.4%; P=.004). Socioeconomic status was matched between patients and controls (P=.99). Descriptive characteristics of the study population are presented in Table 1.

We assessed the presence of pruritus as well as 3 other pruritus-related skin disorders—prurigo nodularis, neurotic excoriations, and LSC—among patients with fibromyalgia and controls. Logistic regression was used to evaluate the independent association between fibromyalgia and pruritus. Table 2 presents the results of multivariate logistic regression models and summarizes the adjusted ORs for pruritic conditions in patients with fibromyalgia and different demographic features across the entire study sample. Fibromyalgia demonstrated strong independent associations with pruritus (OR, 1.8; 95% CI, 1.8-2.4; P<.001), prurigo nodularis (OR, 2.9; 95% CI, 1.1-8.4; P=.038), and LSC (OR, 1.5; 95% CI, 1.1-2.1; P=.01); the association with neurotic excoriations was not significant. Female sex significantly increased the risk for pruritus (OR 1.3; 95% CI, 1.0-1.6; P=.039), while age slightly increased the odds for pruritus (OR, 1.0; 95% CI, 1.0-1.04; P<.001), neurotic excoriations (OR, 1.0; 95% CI, 1.0-1.1; P=.046), and LSC (OR, 1.0; 95% CI, 1.01-1.04; P=.006). Finally, socioeconomic status was inversely correlated with pruritus (OR, 1.1; 95% CI, 1.1-1.5; P=.002).

Frequencies and ORs for the association between fibromyalgia and pruritus with associated pruritic disorders stratified by exclusion of pruritic dermatologic and/or systemic diseases that may induce itch are presented in the eTable. Analyzing the entire study cohort, significant increases were observed in the odds of all 4 pruritic disorders analyzed. The frequency of pruritus was almost double in patients with fibromyalgia compared with controls (11.7% vs 6.0%; OR, 2.1; 95% CI, 1.8-2.3; P<.0001). Prurigo nodularis (0.2% vs 0.1%; OR, 2.9; 95% CI, 1.1-8.4; P=.05), neurotic excoriations (0.6% vs 0.3%; OR, 1.9; 95% CI, 1.1-3.1; P=.018), and LSC (1.3% vs 0.8%; OR, 1.5; 95% CI, 1.1-2.1; P=.01) frequencies were all higher in patients with fibromyalgia than controls. When primary skin disorders that may cause itch (eg, pemphigus vulgaris, Darier disease, dermatitis, eczema, ichthyosis, psoriasis, parapsoriasis, urticaria, xerosis, atopic dermatitis, dermatitis herpetiformis, lichen planus) were excluded, the prevalence of pruritus in patients with fibromyalgia was still 1.97 times greater than in the controls (6.9% vs. 3.5%; OR, 2.0; 95% CI, 1.7-2.4; P<.0001). These results remained unchanged even when excluding pruritic dermatologic disorders as well as systemic diseases associated with pruritus (eg, chronic renal failure, dialysis, hyperthyroidism, hyperparathyroidism/hypoparathyroidism, hypothyroidism). Patients with fibromyalgia still displayed a significantly higher prevalence of pruritus compared with the control group (6.6% vs 3.3%; OR, 2.1; 95% CI, 1.7-2.6; P<.0001).

Comment
A wide range of skin manifestations have been associated with fibromyalgia, but the exact mechanisms remain unclear. Nevertheless, it is conceivable that autonomic nervous system dysfunction,28-31 amplified cutaneous opioid receptor activity,32 and an elevated presence of cutaneous mast cells with excessive degranulation may partially explain the frequent occurrence of pruritus and related skin disorders such as neurotic excoriations, prurigo nodularis, and LSC in individuals with fibromyalgia.15,16 In line with these findings, our study—which was based on data from the largest health maintenance organization in Israel—demonstrated an increased prevalence of pruritus and related pruritic disorders among individuals diagnosed with fibromyalgia.
This cross-sectional study links pruritus with fibromyalgia. Few preliminary epidemiologic studies have shown an increased occurrence of cutaneous manifestations in patients with fibromyalgia. One chart review that looked at skin findings in patients with fibromyalgia revealed 32 distinct cutaneous manifestations, and pruritus was the major concern in 3.3% of 845 patients.15
A focused cross-sectional study involving only women (66 with fibromyalgia and 79 healthy controls) discovered 14 skin conditions that were more common in those with fibromyalgia. Notably, xerosis and neurotic excoriations were more prevalent compared to the control group.16
The brain and the skin—both derivatives of the embryonic ectoderm33,34—are linked by pruritus. Although itch has its dedicated neurons, there is a wide-ranging overlap of brain-activated areas between pain and itch,6 and the neural anatomy of pain and itch are closely related in both the peripheral and central nervous systems35-37; for example, diseases of the central nervous system are accompanied by pruritus in as many as 15% of cases, while postherpetic neuralgia can result in chronic pain, itching, or a combination of both.38,39 Other instances include notalgia paresthetica and brachioradial pruritus.38 Additionally, there is a noteworthy psychologic impact associated with both itch and pain,40,41 with both psychosomatic and psychologic factors implicated in chronic pruritus and in fibromyalgia.42 Lastly, the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system are altered in both fibromyalgia and pruritus.43-45
Tey et al45 characterized the itch experienced in fibromyalgia as functional, which is described as pruritus associated with a somatoform disorder. In our study, we found a higher prevalence of pruritus among patients with fibromyalgia, and this association remained significant (P<.05) even when excluding other pruritic skin conditions and systemic diseases that can trigger itching. In addition, our logistic regression analyses revealed independent associations between fibromyalgia and pruritus, prurigo nodularis, and LSC.
According to Twycross et al,46 there are 4 clinical categories of itch, which may coexist7: pruritoceptive (originating in the skin), neuropathic (originating in pathology located along the afferent pathway), neurogenic (central origin but lacks a neural pathology), and psychogenic.47 Skin biopsy findings in patients with fibromyalgia include increased mast cell counts11 and degranulation,48 increased expression of δ and κ opioid receptors,32 vasoconstriction within tender points,49 and elevated IL-1β, IL-6, or tumor necrosis factor α by reverse transcriptase-polymerase chain reaction.12 A case recently was presented by Görg et al50 involving a female patient with fibromyalgia who had been experiencing chronic pruritus, which the authors attributed to small-fiber neuropathy based on evidence from a skin biopsy indicating a reduced number of intraepidermal nerves and the fact that the itching originated around tender points. Altogether, the observed alterations may work together to make patients with fibromyalgia more susceptible to various skin-related comorbidities in general, especially those related to pruritus. Eventually, it might be the case that several itch categories and related pathomechanisms are involved in the pruritus phenotype of patients with fibromyalgia.
Age-related alterations in nerve fibers, lower immune function, xerosis, polypharmacy, and increased frequency of systemic diseases with age are just a few of the factors that may predispose older individuals to pruritus.51,52 Indeed, our logistic regression model showed that age was significantly and independently associated with pruritus (P<.001), neurotic excoriations (P=.046), and LSC (P=.006). Female sex also was significantly linked with pruritus (P=.039). Intriguingly, high socioeconomic status was significantly associated with the diagnosis of pruritus (P=.002), possibly due to easier access to medical care.
There is a considerable overlap between the therapeutic approaches used in pruritus, pruritus-related skin disorders, and fibromyalgia. Antidepressants, anxiolytics, analgesics, and antiepileptics have been used to address both conditions.45 The association between these conditions advocates for a multidisciplinary approach in patients with fibromyalgia and potentially supports the rationale for unified therapeutics for both conditions.
Conclusion
Our findings indicate an association between fibromyalgia and pruritus as well as associated pruritic skin disorders. Given the convoluted and largely undiscovered mechanisms underlying fibromyalgia, managing patients with this condition may present substantial challenges.53 The data presented here support the implementation of a multidisciplinary treatment approach for patients with fibromyalgia. This approach should focus on managing fibromyalgia pain as well as addressing its concurrent skin-related conditions. It is advisable to consider treatments such as antiepileptics (eg, pregabalin, gabapentin) that specifically target neuropathic disorders in affected patients. These treatments may hold promise for alleviating fibromyalgia-related pain54 and mitigating its related cutaneous comorbidities, especially pruritus.
- Stander S, Weisshaar E, Mettang T, et al. Clinical classification of itch: a position paper of the International Forum for the Study of Itch. Acta Derm Venereol. 2007; 87:291-294.
- Yosipovitch G, Bernhard JD. Clinical practice. chronic pruritus. N Engl J Med. 2013;368:1625-1634.
- Song J, Xian D, Yang L, et al. Pruritus: progress toward pathogenesis and treatment. Biomed Res Int. 2018;2018:9625936.
- Potenzieri C, Undem BJ. Basic mechanisms of itch. Clin Exp Allergy. 2012;42:8-19.
- McMahon SB, Koltzenburg M. Itching for an explanation. Trends Neurosci. 1992;15:497-501.
- Drzezga A, Darsow U, Treede RD, et al. Central activation by histamine-induced itch: analogies to pain processing: a correlational analysis of O-15 H2O positron emission tomography studies. Pain. 2001; 92:295-305.
- Yosipovitch G, Greaves MW, Schmelz M. Itch. Lancet. 2003;361:690-694.
- Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. part I. Arthritis Rheum. 2008; 58:15-25.
- Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. part II. Arthritis Rheum. 2008; 58:26-35.
- Sarzi-Puttini P, Giorgi V, Marotto D, et al. Fibromyalgia: an update on clinical characteristics, aetiopathogenesis and treatment. Nat Rev Rheumatol. 2020;16:645-660.
- Blanco I, Beritze N, Arguelles M, et al. Abnormal overexpression of mastocytes in skin biopsies of fibromyalgia patients. Clin Rheumatol. 2010;29:1403-1412.
- Salemi S, Rethage J, Wollina U, et al. Detection of interleukin 1beta (IL-1beta), IL-6, and tumor necrosis factor-alpha in skin of patients with fibromyalgia. J Rheumatol. 2003;30:146-150.
- Sprott H, Muller A, Heine H. Collagen cross-links in fibromyalgia syndrome. Z Rheumatol. 1998;57(suppl 2):52-55.
- Morf S, Amann-Vesti B, Forster A, et al. Microcirculation abnormalities in patients with fibromyalgia—measured by capillary microscopy and laser fluxmetry. Arthritis Res Ther. 2005;7:R209-R216.
- Laniosz V, Wetter DA, Godar DA. Dermatologic manifestations of fibromyalgia. Clin Rheumatol. 2014;33:1009-1013.
- Dogramaci AC, Yalcinkaya EY. Skin problems in fibromyalgia. Nobel Med. 2009;5:50-52.
- Grayston R, Czanner G, Elhadd K, et al. A systematic review and meta-analysis of the prevalence of small fiber pathology in fibromyalgia: implications for a new paradigm in fibromyalgia etiopathogenesis. Semin Arthritis Rheum. 2019;48:933-940.
- Uceyler N, Zeller D, Kahn AK, et al. Small fibre pathology in patients with fibromyalgia syndrome. Brain. 2013;136:1857-1867.
- Devigili G, Tugnoli V, Penza P, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain. 2008; 131:1912- 1925.
- Reed C, Birnbaum HG, Ivanova JI, et al. Real-world role of tricyclic antidepressants in the treatment of fibromyalgia. Pain Pract. 2012; 12:533-540.
- Moret C, Briley M. Antidepressants in the treatment of fibromyalgia. Neuropsychiatr Dis Treat. 2006;2:537-548.
- Arnold LM, Keck PE Jr, Welge JA. Antidepressant treatment of fibromyalgia. a meta-analysis and review. Psychosomatics. 2000;41:104-113.
- Moore A, Wiffen P, Kalso E. Antiepileptic drugs for neuropathic pain and fibromyalgia. JAMA. 2014;312:182-183.
- Shevchenko A, Valdes-Rodriguez R, Yosipovitch G. Causes, pathophysiology, and treatment of pruritus in the mature patient. Clin Dermatol. 2018;36:140-151.
- Scheinfeld N. The role of gabapentin in treating diseases with cutaneous manifestations and pain. Int J Dermatol. 2003;42:491-495.
- Points Location Intelligence. Accessed July 30, 2024. https://points.co.il/en/points-location-intelligence/
- Yunus MB. The role of gender in fibromyalgia syndrome. Curr Rheumatol Rep. 2001;3:128-134.
- Cakir T, Evcik D, Dundar U, et al. Evaluation of sympathetic skin response and f wave in fibromyalgia syndrome patients. Turk J Rheumatol. 2011;26:38-43.
- Ozkan O, Yildiz M, Koklukaya E. The correlation of laboratory tests and sympathetic skin response parameters by using artificial neural networks in fibromyalgia patients. J Med Syst. 2012;36:1841-1848.
- Ozkan O, Yildiz M, Arslan E, et al. A study on the effects of sympathetic skin response parameters in diagnosis of fibromyalgia using artificial neural networks. J Med Syst. 2016;40:54.
- Ulas UH, Unlu E, Hamamcioglu K, et al. Dysautonomia in fibromyalgia syndrome: sympathetic skin responses and RR interval analysis. Rheumatol Int. 2006;26:383-387.
- Salemi S, Aeschlimann A, Wollina U, et al. Up-regulation of delta-opioid receptors and kappa-opioid receptors in the skin of fibromyalgia patients. Arthritis Rheum. 2007;56:2464-2466.
- Elshazzly M, Lopez MJ, Reddy V, et al. Central nervous system. StatPearls. StatPearls Publishing; 2022.
- Hu MS, Borrelli MR, Hong WX, et al. Embryonic skin development and repair. Organogenesis. 2018;14:46-63.
- Davidson S, Zhang X, Yoon CH, et al. The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci. 2007;27:10007-10014.
- Sikand P, Shimada SG, Green BG, et al. Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain. 2009;144:66-75.
- Davidson S, Giesler GJ. The multiple pathways for itch and their interactions with pain. Trends Neurosci. 2010;33:550-558.
- Dhand A, Aminoff MJ. The neurology of itch. Brain. 2014;137:313-322.
- Binder A, Koroschetz J, Baron R. Disease mechanisms in neuropathic itch. Nat Clin Pract Neurol. 2008;4:329-337.
- Fjellner B, Arnetz BB. Psychological predictors of pruritus during mental stress. Acta Derm Venereol. 1985;65:504-508.
- Papoiu AD, Wang H, Coghill RC, et al. Contagious itch in humans: a study of visual ‘transmission’ of itch in atopic dermatitis and healthy subjects. Br J Dermatol. 2011;164:1299-1303.
- Stumpf A, Schneider G, Stander S. Psychosomatic and psychiatric disorders and psychologic factors in pruritus. Clin Dermatol. 2018;36:704-708.
- Herman JP, McKlveen JM, Ghosal S, et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. 2016;6:603-621.
- Brown ED, Micozzi MS, Craft NE, et al. Plasma carotenoids in normal men after a single ingestion of vegetables or purified beta-carotene. Am J Clin Nutr. 1989;49:1258-1265.
- Tey HL, Wallengren J, Yosipovitch G. Psychosomatic factors in pruritus. Clin Dermatol. 2013;31:31-40.
- Twycross R, Greaves MW, Handwerker H, et al. Itch: scratching more than the surface. QJM. 2003;96:7-26.
- Bernhard JD. Itch and pruritus: what are they, and how should itches be classified? Dermatol Ther. 2005;18:288-291.
- Enestrom S, Bengtsson A, Frodin T. Dermal IgG deposits and increase of mast cells in patients with fibromyalgia—relevant findings or epiphenomena? Scand J Rheumatol. 1997;26:308-313.
- Jeschonneck M, Grohmann G, Hein G, et al. Abnormal microcirculation and temperature in skin above tender points in patients with fibromyalgia. Rheumatology (Oxford). 2000;39:917-921.
- Görg M, Zeidler C, Pereira MP, et al. Generalized chronic pruritus with fibromyalgia. J Dtsch Dermatol Ges. 2021;19:909-911.
- Garibyan L, Chiou AS, Elmariah SB. Advanced aging skin and itch: addressing an unmet need. Dermatol Ther. 2013;26:92-103.
- Cohen KR, Frank J, Salbu RL, et al. Pruritus in the elderly: clinical approaches to the improvement of quality of life. P T. 2012;37:227-239.
- Tzadok R, Ablin JN. Current and emerging pharmacotherapy for fibromyalgia. Pain Res Manag. 2020; 2020:6541798.
- Wiffen PJ, Derry S, Moore RA, et al. Antiepileptic drugs for neuropathic pain and fibromyalgia—an overview of Cochrane reviews. Cochrane Database Syst Rev. 2013:CD010567.
- Stander S, Weisshaar E, Mettang T, et al. Clinical classification of itch: a position paper of the International Forum for the Study of Itch. Acta Derm Venereol. 2007; 87:291-294.
- Yosipovitch G, Bernhard JD. Clinical practice. chronic pruritus. N Engl J Med. 2013;368:1625-1634.
- Song J, Xian D, Yang L, et al. Pruritus: progress toward pathogenesis and treatment. Biomed Res Int. 2018;2018:9625936.
- Potenzieri C, Undem BJ. Basic mechanisms of itch. Clin Exp Allergy. 2012;42:8-19.
- McMahon SB, Koltzenburg M. Itching for an explanation. Trends Neurosci. 1992;15:497-501.
- Drzezga A, Darsow U, Treede RD, et al. Central activation by histamine-induced itch: analogies to pain processing: a correlational analysis of O-15 H2O positron emission tomography studies. Pain. 2001; 92:295-305.
- Yosipovitch G, Greaves MW, Schmelz M. Itch. Lancet. 2003;361:690-694.
- Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. part I. Arthritis Rheum. 2008; 58:15-25.
- Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. part II. Arthritis Rheum. 2008; 58:26-35.
- Sarzi-Puttini P, Giorgi V, Marotto D, et al. Fibromyalgia: an update on clinical characteristics, aetiopathogenesis and treatment. Nat Rev Rheumatol. 2020;16:645-660.
- Blanco I, Beritze N, Arguelles M, et al. Abnormal overexpression of mastocytes in skin biopsies of fibromyalgia patients. Clin Rheumatol. 2010;29:1403-1412.
- Salemi S, Rethage J, Wollina U, et al. Detection of interleukin 1beta (IL-1beta), IL-6, and tumor necrosis factor-alpha in skin of patients with fibromyalgia. J Rheumatol. 2003;30:146-150.
- Sprott H, Muller A, Heine H. Collagen cross-links in fibromyalgia syndrome. Z Rheumatol. 1998;57(suppl 2):52-55.
- Morf S, Amann-Vesti B, Forster A, et al. Microcirculation abnormalities in patients with fibromyalgia—measured by capillary microscopy and laser fluxmetry. Arthritis Res Ther. 2005;7:R209-R216.
- Laniosz V, Wetter DA, Godar DA. Dermatologic manifestations of fibromyalgia. Clin Rheumatol. 2014;33:1009-1013.
- Dogramaci AC, Yalcinkaya EY. Skin problems in fibromyalgia. Nobel Med. 2009;5:50-52.
- Grayston R, Czanner G, Elhadd K, et al. A systematic review and meta-analysis of the prevalence of small fiber pathology in fibromyalgia: implications for a new paradigm in fibromyalgia etiopathogenesis. Semin Arthritis Rheum. 2019;48:933-940.
- Uceyler N, Zeller D, Kahn AK, et al. Small fibre pathology in patients with fibromyalgia syndrome. Brain. 2013;136:1857-1867.
- Devigili G, Tugnoli V, Penza P, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain. 2008; 131:1912- 1925.
- Reed C, Birnbaum HG, Ivanova JI, et al. Real-world role of tricyclic antidepressants in the treatment of fibromyalgia. Pain Pract. 2012; 12:533-540.
- Moret C, Briley M. Antidepressants in the treatment of fibromyalgia. Neuropsychiatr Dis Treat. 2006;2:537-548.
- Arnold LM, Keck PE Jr, Welge JA. Antidepressant treatment of fibromyalgia. a meta-analysis and review. Psychosomatics. 2000;41:104-113.
- Moore A, Wiffen P, Kalso E. Antiepileptic drugs for neuropathic pain and fibromyalgia. JAMA. 2014;312:182-183.
- Shevchenko A, Valdes-Rodriguez R, Yosipovitch G. Causes, pathophysiology, and treatment of pruritus in the mature patient. Clin Dermatol. 2018;36:140-151.
- Scheinfeld N. The role of gabapentin in treating diseases with cutaneous manifestations and pain. Int J Dermatol. 2003;42:491-495.
- Points Location Intelligence. Accessed July 30, 2024. https://points.co.il/en/points-location-intelligence/
- Yunus MB. The role of gender in fibromyalgia syndrome. Curr Rheumatol Rep. 2001;3:128-134.
- Cakir T, Evcik D, Dundar U, et al. Evaluation of sympathetic skin response and f wave in fibromyalgia syndrome patients. Turk J Rheumatol. 2011;26:38-43.
- Ozkan O, Yildiz M, Koklukaya E. The correlation of laboratory tests and sympathetic skin response parameters by using artificial neural networks in fibromyalgia patients. J Med Syst. 2012;36:1841-1848.
- Ozkan O, Yildiz M, Arslan E, et al. A study on the effects of sympathetic skin response parameters in diagnosis of fibromyalgia using artificial neural networks. J Med Syst. 2016;40:54.
- Ulas UH, Unlu E, Hamamcioglu K, et al. Dysautonomia in fibromyalgia syndrome: sympathetic skin responses and RR interval analysis. Rheumatol Int. 2006;26:383-387.
- Salemi S, Aeschlimann A, Wollina U, et al. Up-regulation of delta-opioid receptors and kappa-opioid receptors in the skin of fibromyalgia patients. Arthritis Rheum. 2007;56:2464-2466.
- Elshazzly M, Lopez MJ, Reddy V, et al. Central nervous system. StatPearls. StatPearls Publishing; 2022.
- Hu MS, Borrelli MR, Hong WX, et al. Embryonic skin development and repair. Organogenesis. 2018;14:46-63.
- Davidson S, Zhang X, Yoon CH, et al. The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci. 2007;27:10007-10014.
- Sikand P, Shimada SG, Green BG, et al. Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain. 2009;144:66-75.
- Davidson S, Giesler GJ. The multiple pathways for itch and their interactions with pain. Trends Neurosci. 2010;33:550-558.
- Dhand A, Aminoff MJ. The neurology of itch. Brain. 2014;137:313-322.
- Binder A, Koroschetz J, Baron R. Disease mechanisms in neuropathic itch. Nat Clin Pract Neurol. 2008;4:329-337.
- Fjellner B, Arnetz BB. Psychological predictors of pruritus during mental stress. Acta Derm Venereol. 1985;65:504-508.
- Papoiu AD, Wang H, Coghill RC, et al. Contagious itch in humans: a study of visual ‘transmission’ of itch in atopic dermatitis and healthy subjects. Br J Dermatol. 2011;164:1299-1303.
- Stumpf A, Schneider G, Stander S. Psychosomatic and psychiatric disorders and psychologic factors in pruritus. Clin Dermatol. 2018;36:704-708.
- Herman JP, McKlveen JM, Ghosal S, et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. 2016;6:603-621.
- Brown ED, Micozzi MS, Craft NE, et al. Plasma carotenoids in normal men after a single ingestion of vegetables or purified beta-carotene. Am J Clin Nutr. 1989;49:1258-1265.
- Tey HL, Wallengren J, Yosipovitch G. Psychosomatic factors in pruritus. Clin Dermatol. 2013;31:31-40.
- Twycross R, Greaves MW, Handwerker H, et al. Itch: scratching more than the surface. QJM. 2003;96:7-26.
- Bernhard JD. Itch and pruritus: what are they, and how should itches be classified? Dermatol Ther. 2005;18:288-291.
- Enestrom S, Bengtsson A, Frodin T. Dermal IgG deposits and increase of mast cells in patients with fibromyalgia—relevant findings or epiphenomena? Scand J Rheumatol. 1997;26:308-313.
- Jeschonneck M, Grohmann G, Hein G, et al. Abnormal microcirculation and temperature in skin above tender points in patients with fibromyalgia. Rheumatology (Oxford). 2000;39:917-921.
- Görg M, Zeidler C, Pereira MP, et al. Generalized chronic pruritus with fibromyalgia. J Dtsch Dermatol Ges. 2021;19:909-911.
- Garibyan L, Chiou AS, Elmariah SB. Advanced aging skin and itch: addressing an unmet need. Dermatol Ther. 2013;26:92-103.
- Cohen KR, Frank J, Salbu RL, et al. Pruritus in the elderly: clinical approaches to the improvement of quality of life. P T. 2012;37:227-239.
- Tzadok R, Ablin JN. Current and emerging pharmacotherapy for fibromyalgia. Pain Res Manag. 2020; 2020:6541798.
- Wiffen PJ, Derry S, Moore RA, et al. Antiepileptic drugs for neuropathic pain and fibromyalgia—an overview of Cochrane reviews. Cochrane Database Syst Rev. 2013:CD010567.
Practice Points
- Dermatologists should be aware of the connection between fibromyalgia, pruritus, and related conditions to improve patient care.
- The association between fibromyalgia and pruritus underscores the importance of employing multidisciplinary treatment strategies for managing these conditions.



