User login
How Safe is Anti–IL-6 Therapy During Pregnancy?
TOPLINE:
The maternal and neonatal outcomes in pregnant women treated with anti–interleukin (IL)-6 therapy for COVID-19 are largely favorable, with transient neonatal cytopenia observed in around one third of the babies being the only possible adverse outcome that could be related to anti–IL-6 therapy.
METHODOLOGY:
- Despite guidance, very few pregnant women with COVID-19 are offered evidence-based therapies such as anti–IL-6 due to concerns regarding fetal safety in later pregnancy.
- In this retrospective study, researchers evaluated maternal and neonatal outcomes in 25 pregnant women with COVID-19 (mean age at admission, 33 years) treated with anti–IL-6 (tocilizumab or sarilumab) at two tertiary hospitals in London.
- Most women (n = 16) received anti–IL-6 in the third trimester of pregnancy, whereas nine received it during the second trimester.
- Maternal and neonatal outcomes were assessed through medical record reviews and maternal medicine networks, with follow-up for 12 months.
- The women included in the study constituted a high-risk population with severe COVID-19; 24 required level two or three critical care. All women were receiving at least three concomitant medications due to their critical illness.
TAKEAWAY:
- Overall, 24 of 25 women treated with IL-6 receptor antibodies survived until hospital discharge.
- The sole death occurred in a woman with severe COVID-19 pneumonitis who later developed myocarditis and cardiac arrest. The physicians believed that these complications were more likely due to severe COVID-19 rather than anti–IL-6 therapy.
- All pregnancies resulted in live births; however, 16 babies had to be delivered preterm due to COVID-19 complications.
- Transient cytopenia was observed in 6 of 19 babies in whom a full blood count was performed. All the six babies were premature, with cytopenia resolving within 7 days in four babies; one baby died from complications associated with extreme prematurity.
IN PRACTICE:
“Although the authors found mild, transitory cytopenia in some (6 of 19) exposed infants, most had been delivered prematurely due to progressive COVID-19–related morbidity, and distinguishing drug effects from similar prematurity-related effects is difficult,” wrote Steven L. Clark, MD, from the Department of Obstetrics and Gynecology, Baylor College of Medicine, Houston, in an accompanying editorial.
SOURCE:
The study was led by Melanie Nana, MRCP, from the Department of Obstetric Medicine, St Thomas’ Hospital, London, England. It was published online in The Lancet Rheumatology.
LIMITATIONS:
The study was retrospective in design, which may have introduced bias. The small sample size of 25 women may have limited the generalizability of the findings. Additionally, the study did not include a control group, which made it difficult to attribute outcomes solely to anti–IL-6 therapy. The lack of long-term follow-up data on the neonates also limited the understanding of potential long-term effects.
DISCLOSURES:
This study did not receive any funding. Some authors, including the lead author, received speaker fees, grants, or consultancy fees from academic institutions or pharmaceutical companies or had other ties with various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The maternal and neonatal outcomes in pregnant women treated with anti–interleukin (IL)-6 therapy for COVID-19 are largely favorable, with transient neonatal cytopenia observed in around one third of the babies being the only possible adverse outcome that could be related to anti–IL-6 therapy.
METHODOLOGY:
- Despite guidance, very few pregnant women with COVID-19 are offered evidence-based therapies such as anti–IL-6 due to concerns regarding fetal safety in later pregnancy.
- In this retrospective study, researchers evaluated maternal and neonatal outcomes in 25 pregnant women with COVID-19 (mean age at admission, 33 years) treated with anti–IL-6 (tocilizumab or sarilumab) at two tertiary hospitals in London.
- Most women (n = 16) received anti–IL-6 in the third trimester of pregnancy, whereas nine received it during the second trimester.
- Maternal and neonatal outcomes were assessed through medical record reviews and maternal medicine networks, with follow-up for 12 months.
- The women included in the study constituted a high-risk population with severe COVID-19; 24 required level two or three critical care. All women were receiving at least three concomitant medications due to their critical illness.
TAKEAWAY:
- Overall, 24 of 25 women treated with IL-6 receptor antibodies survived until hospital discharge.
- The sole death occurred in a woman with severe COVID-19 pneumonitis who later developed myocarditis and cardiac arrest. The physicians believed that these complications were more likely due to severe COVID-19 rather than anti–IL-6 therapy.
- All pregnancies resulted in live births; however, 16 babies had to be delivered preterm due to COVID-19 complications.
- Transient cytopenia was observed in 6 of 19 babies in whom a full blood count was performed. All the six babies were premature, with cytopenia resolving within 7 days in four babies; one baby died from complications associated with extreme prematurity.
IN PRACTICE:
“Although the authors found mild, transitory cytopenia in some (6 of 19) exposed infants, most had been delivered prematurely due to progressive COVID-19–related morbidity, and distinguishing drug effects from similar prematurity-related effects is difficult,” wrote Steven L. Clark, MD, from the Department of Obstetrics and Gynecology, Baylor College of Medicine, Houston, in an accompanying editorial.
SOURCE:
The study was led by Melanie Nana, MRCP, from the Department of Obstetric Medicine, St Thomas’ Hospital, London, England. It was published online in The Lancet Rheumatology.
LIMITATIONS:
The study was retrospective in design, which may have introduced bias. The small sample size of 25 women may have limited the generalizability of the findings. Additionally, the study did not include a control group, which made it difficult to attribute outcomes solely to anti–IL-6 therapy. The lack of long-term follow-up data on the neonates also limited the understanding of potential long-term effects.
DISCLOSURES:
This study did not receive any funding. Some authors, including the lead author, received speaker fees, grants, or consultancy fees from academic institutions or pharmaceutical companies or had other ties with various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The maternal and neonatal outcomes in pregnant women treated with anti–interleukin (IL)-6 therapy for COVID-19 are largely favorable, with transient neonatal cytopenia observed in around one third of the babies being the only possible adverse outcome that could be related to anti–IL-6 therapy.
METHODOLOGY:
- Despite guidance, very few pregnant women with COVID-19 are offered evidence-based therapies such as anti–IL-6 due to concerns regarding fetal safety in later pregnancy.
- In this retrospective study, researchers evaluated maternal and neonatal outcomes in 25 pregnant women with COVID-19 (mean age at admission, 33 years) treated with anti–IL-6 (tocilizumab or sarilumab) at two tertiary hospitals in London.
- Most women (n = 16) received anti–IL-6 in the third trimester of pregnancy, whereas nine received it during the second trimester.
- Maternal and neonatal outcomes were assessed through medical record reviews and maternal medicine networks, with follow-up for 12 months.
- The women included in the study constituted a high-risk population with severe COVID-19; 24 required level two or three critical care. All women were receiving at least three concomitant medications due to their critical illness.
TAKEAWAY:
- Overall, 24 of 25 women treated with IL-6 receptor antibodies survived until hospital discharge.
- The sole death occurred in a woman with severe COVID-19 pneumonitis who later developed myocarditis and cardiac arrest. The physicians believed that these complications were more likely due to severe COVID-19 rather than anti–IL-6 therapy.
- All pregnancies resulted in live births; however, 16 babies had to be delivered preterm due to COVID-19 complications.
- Transient cytopenia was observed in 6 of 19 babies in whom a full blood count was performed. All the six babies were premature, with cytopenia resolving within 7 days in four babies; one baby died from complications associated with extreme prematurity.
IN PRACTICE:
“Although the authors found mild, transitory cytopenia in some (6 of 19) exposed infants, most had been delivered prematurely due to progressive COVID-19–related morbidity, and distinguishing drug effects from similar prematurity-related effects is difficult,” wrote Steven L. Clark, MD, from the Department of Obstetrics and Gynecology, Baylor College of Medicine, Houston, in an accompanying editorial.
SOURCE:
The study was led by Melanie Nana, MRCP, from the Department of Obstetric Medicine, St Thomas’ Hospital, London, England. It was published online in The Lancet Rheumatology.
LIMITATIONS:
The study was retrospective in design, which may have introduced bias. The small sample size of 25 women may have limited the generalizability of the findings. Additionally, the study did not include a control group, which made it difficult to attribute outcomes solely to anti–IL-6 therapy. The lack of long-term follow-up data on the neonates also limited the understanding of potential long-term effects.
DISCLOSURES:
This study did not receive any funding. Some authors, including the lead author, received speaker fees, grants, or consultancy fees from academic institutions or pharmaceutical companies or had other ties with various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
New First-Line Therapies for Migraine Prevention
This transcript has been edited for clarity.
Today I am going to talk about the position statement from the American Headache Society (AHS) “Calcitonin gene-related peptide [CGRP]–targeting therapies are a first-line option for the prevention of migraine”. This update is of critical importance because about three fourths of people with migraine get their care from a primary care clinician, not from a neurologist or a headache specialist. CGRP-targeting therapies have transformed migraine care at the specialty level, but many in primary care are not yet familiar with this class of medicines. Until this new statement was released, CGRPs were not viewed as first-line agents for migraine. That has now changed.
Two main types of therapy for people with migraine headache are: (1) acute or abortive therapy (when a headache develops, it is treated), and (2) preventive therapy. Preventive therapy is typically used when the patient has headaches on 4 or more days per month. Preventive therapy is aimed at reducing the frequency and severity of headaches. About 40% of patients with migraine qualify for preventive therapy, but only a minority are receiving it.
The armamentarium for preventive therapy of migraines had not changed in a long time — until now. First-line preventive therapy has traditionally consisted of three classes of agents: beta-blockers, tricyclic antidepressants, and topiramate. These medicines were developed for different therapeutic purposes, yet they work for migraines. These drugs may have off-target effects that can make them difficult to tolerate.
Based on new evidence, candesartan — an angiotensin receptor blocker (ARB) — is now also a first-line drug for migraine. This is good news, because ARBs are a drug class that we have a lot of experience with, are easy to use, and could be an excellent choice for people with concomitant hypertension or chronic kidney disease. The serotonin-norepinephrine reuptake inhibitors (venlafaxine and duloxetine) are also considered first-line agents for migraine treatment.
In the AHS’s new position statement, the two main drug classes are small-molecule CGRP receptor antagonists and monoclonal antibodies.
The role of the neuropeptide CGRP in migraine was originally discovered after finding that blood levels of CGRP were elevated during migraine attacks. This led to the discovery of agents that blocked CGRP, initially for acute treatment of migraine, and then for preventive therapy. Multiple clinical studies show the CGRP targeting therapies to be as or even more effective than traditional first-line agents at decreasing the number of migraine days per month.
The efficacy and safety of these agents have been demonstrated in both randomized trials and in real-world studies. Other important positive endpoints include fewer days of migraine, reduced acute medication use, and improvements in many quality-of-life outcomes. Studies also have shown that CGRP-targeting therapies are well tolerated and safe, with very few serious adverse events.
Furthermore, studies have shown the CGRP targeting therapies are effective in individuals who have failed multiple other first-line therapies. They fit now both as first-line agents and as agents that can be used in difficult-to-treat patients as well as in patients who struggle with acute medication overuse, which is often very challenging.
To quote from the AHS statement,
Side effects are uncommon and can include hypertension, constipation, and Raynaud phenomenon.
The position statement is strong and is based on a lot of evidence and clinical experience. CGRP-targeting therapies are now first-line agents for the prevention of migraine headache. We should learn more about and begin to feel comfortable using this class of agents because they stand to benefit our patients greatly. I’d suggest looking at the table below and picking one new agent to become familiar with so that you can add that agent to your toolbox. 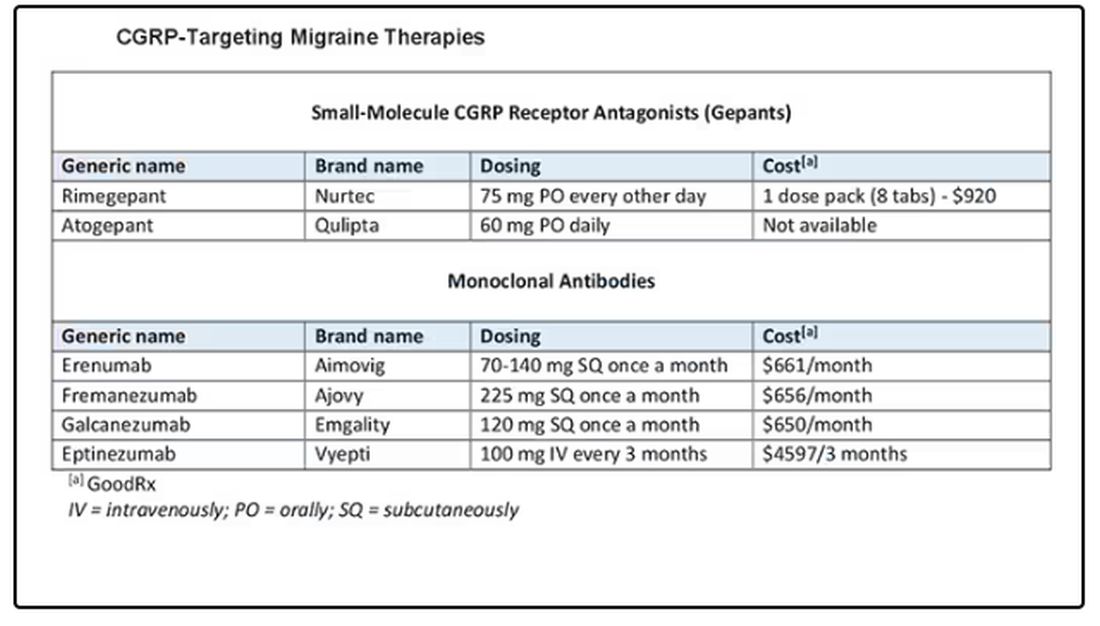
Dr. Skolnik, professor, Department of Family Medicine, Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, Pennsylvania, and associate director, Department of Family Medicine, Abington Jefferson Health, Abington, Pennsylvania, disclosed ties with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, Bayer, and Teva.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Today I am going to talk about the position statement from the American Headache Society (AHS) “Calcitonin gene-related peptide [CGRP]–targeting therapies are a first-line option for the prevention of migraine”. This update is of critical importance because about three fourths of people with migraine get their care from a primary care clinician, not from a neurologist or a headache specialist. CGRP-targeting therapies have transformed migraine care at the specialty level, but many in primary care are not yet familiar with this class of medicines. Until this new statement was released, CGRPs were not viewed as first-line agents for migraine. That has now changed.
Two main types of therapy for people with migraine headache are: (1) acute or abortive therapy (when a headache develops, it is treated), and (2) preventive therapy. Preventive therapy is typically used when the patient has headaches on 4 or more days per month. Preventive therapy is aimed at reducing the frequency and severity of headaches. About 40% of patients with migraine qualify for preventive therapy, but only a minority are receiving it.
The armamentarium for preventive therapy of migraines had not changed in a long time — until now. First-line preventive therapy has traditionally consisted of three classes of agents: beta-blockers, tricyclic antidepressants, and topiramate. These medicines were developed for different therapeutic purposes, yet they work for migraines. These drugs may have off-target effects that can make them difficult to tolerate.
Based on new evidence, candesartan — an angiotensin receptor blocker (ARB) — is now also a first-line drug for migraine. This is good news, because ARBs are a drug class that we have a lot of experience with, are easy to use, and could be an excellent choice for people with concomitant hypertension or chronic kidney disease. The serotonin-norepinephrine reuptake inhibitors (venlafaxine and duloxetine) are also considered first-line agents for migraine treatment.
In the AHS’s new position statement, the two main drug classes are small-molecule CGRP receptor antagonists and monoclonal antibodies.
The role of the neuropeptide CGRP in migraine was originally discovered after finding that blood levels of CGRP were elevated during migraine attacks. This led to the discovery of agents that blocked CGRP, initially for acute treatment of migraine, and then for preventive therapy. Multiple clinical studies show the CGRP targeting therapies to be as or even more effective than traditional first-line agents at decreasing the number of migraine days per month.
The efficacy and safety of these agents have been demonstrated in both randomized trials and in real-world studies. Other important positive endpoints include fewer days of migraine, reduced acute medication use, and improvements in many quality-of-life outcomes. Studies also have shown that CGRP-targeting therapies are well tolerated and safe, with very few serious adverse events.
Furthermore, studies have shown the CGRP targeting therapies are effective in individuals who have failed multiple other first-line therapies. They fit now both as first-line agents and as agents that can be used in difficult-to-treat patients as well as in patients who struggle with acute medication overuse, which is often very challenging.
To quote from the AHS statement,
Side effects are uncommon and can include hypertension, constipation, and Raynaud phenomenon.
The position statement is strong and is based on a lot of evidence and clinical experience. CGRP-targeting therapies are now first-line agents for the prevention of migraine headache. We should learn more about and begin to feel comfortable using this class of agents because they stand to benefit our patients greatly. I’d suggest looking at the table below and picking one new agent to become familiar with so that you can add that agent to your toolbox. 
Dr. Skolnik, professor, Department of Family Medicine, Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, Pennsylvania, and associate director, Department of Family Medicine, Abington Jefferson Health, Abington, Pennsylvania, disclosed ties with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, Bayer, and Teva.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Today I am going to talk about the position statement from the American Headache Society (AHS) “Calcitonin gene-related peptide [CGRP]–targeting therapies are a first-line option for the prevention of migraine”. This update is of critical importance because about three fourths of people with migraine get their care from a primary care clinician, not from a neurologist or a headache specialist. CGRP-targeting therapies have transformed migraine care at the specialty level, but many in primary care are not yet familiar with this class of medicines. Until this new statement was released, CGRPs were not viewed as first-line agents for migraine. That has now changed.
Two main types of therapy for people with migraine headache are: (1) acute or abortive therapy (when a headache develops, it is treated), and (2) preventive therapy. Preventive therapy is typically used when the patient has headaches on 4 or more days per month. Preventive therapy is aimed at reducing the frequency and severity of headaches. About 40% of patients with migraine qualify for preventive therapy, but only a minority are receiving it.
The armamentarium for preventive therapy of migraines had not changed in a long time — until now. First-line preventive therapy has traditionally consisted of three classes of agents: beta-blockers, tricyclic antidepressants, and topiramate. These medicines were developed for different therapeutic purposes, yet they work for migraines. These drugs may have off-target effects that can make them difficult to tolerate.
Based on new evidence, candesartan — an angiotensin receptor blocker (ARB) — is now also a first-line drug for migraine. This is good news, because ARBs are a drug class that we have a lot of experience with, are easy to use, and could be an excellent choice for people with concomitant hypertension or chronic kidney disease. The serotonin-norepinephrine reuptake inhibitors (venlafaxine and duloxetine) are also considered first-line agents for migraine treatment.
In the AHS’s new position statement, the two main drug classes are small-molecule CGRP receptor antagonists and monoclonal antibodies.
The role of the neuropeptide CGRP in migraine was originally discovered after finding that blood levels of CGRP were elevated during migraine attacks. This led to the discovery of agents that blocked CGRP, initially for acute treatment of migraine, and then for preventive therapy. Multiple clinical studies show the CGRP targeting therapies to be as or even more effective than traditional first-line agents at decreasing the number of migraine days per month.
The efficacy and safety of these agents have been demonstrated in both randomized trials and in real-world studies. Other important positive endpoints include fewer days of migraine, reduced acute medication use, and improvements in many quality-of-life outcomes. Studies also have shown that CGRP-targeting therapies are well tolerated and safe, with very few serious adverse events.
Furthermore, studies have shown the CGRP targeting therapies are effective in individuals who have failed multiple other first-line therapies. They fit now both as first-line agents and as agents that can be used in difficult-to-treat patients as well as in patients who struggle with acute medication overuse, which is often very challenging.
To quote from the AHS statement,
Side effects are uncommon and can include hypertension, constipation, and Raynaud phenomenon.
The position statement is strong and is based on a lot of evidence and clinical experience. CGRP-targeting therapies are now first-line agents for the prevention of migraine headache. We should learn more about and begin to feel comfortable using this class of agents because they stand to benefit our patients greatly. I’d suggest looking at the table below and picking one new agent to become familiar with so that you can add that agent to your toolbox. 
Dr. Skolnik, professor, Department of Family Medicine, Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, Pennsylvania, and associate director, Department of Family Medicine, Abington Jefferson Health, Abington, Pennsylvania, disclosed ties with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, Bayer, and Teva.
A version of this article appeared on Medscape.com.
Does Headache Surgery Really Work? Neurologists Remain Unconvinced
Jeffrey E. Janis, MD, is on a mission. The professor of plastic surgery, surgery, neurosurgery, and neurology at The Ohio State University Wexner Medical Center, Columbus, Ohio, wants to convince neurologists of the safety and efficacy of nerve decompression surgery for treatment-resistant headache. However, many neurologists remain unconvinced.
There’s 24 years of evidence behind this surgical technique across hundreds of different studies with different study designs,” Dr. Janis said.
Yet this treatment approach — surgery on peripheral nerves rather than the brain or spinal cord — hasn’t garnered much support from neurologists. A scan of the agenda of topics at the recently held 2024 annual meeting of the American Headache Society showed few if any studies or presentations on this topic. And neurologists this news organization spoke to said they believe the surgery is experimental and unproven.
Experts do agree drugs don’t work for all patients with migraines. Up to 30% of patients don’t respond to the “laundry list of medications” available to treat the condition, said Dr. Janis.
Many patients have also tried, and failed, alternative treatment approaches such as massage, acupuncture, craniosacral therapy, transdermal patches, electrical stimulation, cryoablation, neurostimulation, and radiofrequency ablation.
If nothing else works, is surgery for headaches the answer?
Long-Held Theory
The idea that pinched, irritated, or compressed peripheral nerves can trigger migraine attacks has been around for nearly 25 years. Studies suggest that in addition to migraine, nerve compression can lead to other headache conditions, including occipital neuralgia, supraorbital neuralgia , and post-traumatic headaches.
This has led to the development of surgical techniques to deactivate various compression trigger sites — what Dr. Janis calls “pinch points” — which could involve muscles, bone, fascia, blood vessels, or scar tissue from prior trauma or surgery.
The procedure is predominantly performed by plastic surgeons, but to a lesser degree by neurosurgeons and ear, nose, and throat specialists.
Target nerves in surgical interventions include those in the frontal region of the head above the eye, temporal region, neck region, and nasal region. Affected areas are usually identified either through patient self-reports or by using a nerve block agent such as lidocaine or Botox at specific points, Dr. Janis noted. If pain subsides after an injection, that location is marked as a target.
One of the barriers to referring complicated patients for surgery is that neurologists evaluating migraine treatments “speak a different language” than surgeons performing the procedure, said Dr. Janis.
Neurologists tend to focus on reduction in monthly migraine days (MMD), while surgeons typically use the Migraine Headache Index that incorporates the frequency, intensity, and duration of migraine attacks.
“Rather than try to convince somebody to speak a different language, we thought, why don’t we just learn their language so we can build bridges and take down barriers,” said Dr. Janis, coauthor of a systematic review and meta-analysis published online recently in Plastic and Reconstructive Surgery.
Investigators examined 19 studies in the review, including five randomized controlled trials (RCTs), published from January 2020 to September 2023, with a total of 1603 participants who were mostly female and ranged in age from 9 to 72 years. Study follow-ups extended from 6 to 38 months. All but three studies were carried out in the United States, and six different compression sites were addressed during surgery.
Investigators found that across studies and by a number of measures, migraine frequency and severity improved after surgery.
Monthly migraine days decreased by 36%-92% and the number of overall migraine attacks per month dropped 25%-87.5%. Patients also reported decreases in attack duration of 41%-75% and intensity of 28%-82% across studies.
“Even using the neurologist-standard language of monthly migraine days, this surgery works,” said Dr. Janis. “Now this is documented both in the surgical literature and the nonsurgical literature.”
The most common complications were ecchymosis, hair loss or thinning, itching, dryness, and rhinorrhea, all of which Dr. Janis described as “fairly minor.” Major complications such as intraoperative bleeding and wound dehiscence were rare, occurring in 1% or less of participants.
‘One And Done?’
These surgeries are usually done on an outpatient basis and generally offer long-term results, Dr. Janis said.
“The idea is one and done,” he said. “The literature around this type of surgery says that whatever type of effect you get at 1 year is likely to be permanent.”
The American Society of Plastic Surgeons agrees. A 2018 position paper developed by experts and commissioned by the society reports that the intervention is safe and effective for appropriate patients, based on a comprehensive literature search and review of a large body of peer-reviewed scientific evidence.
“There is substantial, extensively replicated clinical data that demonstrates a significant reduction in [migraine headache] symptoms and frequency (even complete elimination of headache pain) following trigger site surgery,” the authors noted.
Pamela Blake, MD, a neurologist, board-certified headache specialist, and medical director at the Headache Center of River Oaks, Houston, is a proponent of what she said can be “lifesaving” headache surgery.
“If a doctor told you that you can either treat this problem with medications that you’ll need to take for the rest of your life or you can have a surgical procedure as an outpatient that has extremely low risk and has, in my experience, a 75% chance of reducing or eliminating your pain, you probably would be interested in surgery,” she said.
Continued Skepticism
However, other neurologists and clinicians appear doubtful about this intervention, including Hans-Christoph Diener, MD, PhD, professor of neurology and director, Essen Headache Centre, University of Duisburg-Essen in Germany.
During a debate on the topic a decade ago at the International Headache Congress, Dr. Diener argued that, as migraine is a complex multigene-related disorder of the brain, it doesn’t make sense that surgery would affect the epigenetics of 22 different genes.
Recently, he said that his views have not changed.
The topic remains controversial, and some neurologists are uncomfortable even openly discussing the procedure. Two clinicians who previously commented on this article later asked not to be included.
One neurologist, who asked to remain anonymous, said that Dr. Janis’s review article is “merely a review collecting 19 studies over the previous 10-plus years.”
Other limitations cited by this neurologist are the lack of consistency in procedures among the various studies and the inclusion of only four RCTs, the most recent of which was published 8 years ago, suggesting “the study was probably done closer to 9 or 10 years ago,” the neurologist said.
Dr. Blake suggested some neurologists’ reluctance could be due to limited background on the procedure, which she said isn’t widely discussed at headache meetings and is covered mostly in plastic surgery journals, not neurology literature. Access to surgery is further limited by a lack of specialists who perform the procedure and inconsistent insurance coverage.
A closer collaboration between neurologists and surgeons who perform the procedure could benefit patients, Dr. Blake noted.
“The headache doctor’s role is to identify who’s a candidate for surgery, who meets the criteria for nerve compression, and then follow that patient postoperatively, managing their medications, although usually we get them off their medications,” she added.
From Dr. Janis’s perspective, things are starting to change.
“I’m definitely seeing a greater comfort level among neurologists who are understanding where this sits in the algorithm for treatment, especially for complicated patients,” he said.
Dr. Janis receives royalties from Thieme and Springer Publishing. Dr. Blake reported no relevant conflicts. Dr. Diener received research support from the German Research Council; serves on the editorial boards of Cephalalgia, Lancet Neurology, and Drugs; and has received honoraria for participation in clinical trials, contribution to advisory boards, or oral presentations from AbbVie, Lilly, Lundbeck, Novartis, Pfizer, Teva, Weber & Weber, and WebMD.
A version of this article appeared on Medscape.com.
Jeffrey E. Janis, MD, is on a mission. The professor of plastic surgery, surgery, neurosurgery, and neurology at The Ohio State University Wexner Medical Center, Columbus, Ohio, wants to convince neurologists of the safety and efficacy of nerve decompression surgery for treatment-resistant headache. However, many neurologists remain unconvinced.
There’s 24 years of evidence behind this surgical technique across hundreds of different studies with different study designs,” Dr. Janis said.
Yet this treatment approach — surgery on peripheral nerves rather than the brain or spinal cord — hasn’t garnered much support from neurologists. A scan of the agenda of topics at the recently held 2024 annual meeting of the American Headache Society showed few if any studies or presentations on this topic. And neurologists this news organization spoke to said they believe the surgery is experimental and unproven.
Experts do agree drugs don’t work for all patients with migraines. Up to 30% of patients don’t respond to the “laundry list of medications” available to treat the condition, said Dr. Janis.
Many patients have also tried, and failed, alternative treatment approaches such as massage, acupuncture, craniosacral therapy, transdermal patches, electrical stimulation, cryoablation, neurostimulation, and radiofrequency ablation.
If nothing else works, is surgery for headaches the answer?
Long-Held Theory
The idea that pinched, irritated, or compressed peripheral nerves can trigger migraine attacks has been around for nearly 25 years. Studies suggest that in addition to migraine, nerve compression can lead to other headache conditions, including occipital neuralgia, supraorbital neuralgia , and post-traumatic headaches.
This has led to the development of surgical techniques to deactivate various compression trigger sites — what Dr. Janis calls “pinch points” — which could involve muscles, bone, fascia, blood vessels, or scar tissue from prior trauma or surgery.
The procedure is predominantly performed by plastic surgeons, but to a lesser degree by neurosurgeons and ear, nose, and throat specialists.
Target nerves in surgical interventions include those in the frontal region of the head above the eye, temporal region, neck region, and nasal region. Affected areas are usually identified either through patient self-reports or by using a nerve block agent such as lidocaine or Botox at specific points, Dr. Janis noted. If pain subsides after an injection, that location is marked as a target.
One of the barriers to referring complicated patients for surgery is that neurologists evaluating migraine treatments “speak a different language” than surgeons performing the procedure, said Dr. Janis.
Neurologists tend to focus on reduction in monthly migraine days (MMD), while surgeons typically use the Migraine Headache Index that incorporates the frequency, intensity, and duration of migraine attacks.
“Rather than try to convince somebody to speak a different language, we thought, why don’t we just learn their language so we can build bridges and take down barriers,” said Dr. Janis, coauthor of a systematic review and meta-analysis published online recently in Plastic and Reconstructive Surgery.
Investigators examined 19 studies in the review, including five randomized controlled trials (RCTs), published from January 2020 to September 2023, with a total of 1603 participants who were mostly female and ranged in age from 9 to 72 years. Study follow-ups extended from 6 to 38 months. All but three studies were carried out in the United States, and six different compression sites were addressed during surgery.
Investigators found that across studies and by a number of measures, migraine frequency and severity improved after surgery.
Monthly migraine days decreased by 36%-92% and the number of overall migraine attacks per month dropped 25%-87.5%. Patients also reported decreases in attack duration of 41%-75% and intensity of 28%-82% across studies.
“Even using the neurologist-standard language of monthly migraine days, this surgery works,” said Dr. Janis. “Now this is documented both in the surgical literature and the nonsurgical literature.”
The most common complications were ecchymosis, hair loss or thinning, itching, dryness, and rhinorrhea, all of which Dr. Janis described as “fairly minor.” Major complications such as intraoperative bleeding and wound dehiscence were rare, occurring in 1% or less of participants.
‘One And Done?’
These surgeries are usually done on an outpatient basis and generally offer long-term results, Dr. Janis said.
“The idea is one and done,” he said. “The literature around this type of surgery says that whatever type of effect you get at 1 year is likely to be permanent.”
The American Society of Plastic Surgeons agrees. A 2018 position paper developed by experts and commissioned by the society reports that the intervention is safe and effective for appropriate patients, based on a comprehensive literature search and review of a large body of peer-reviewed scientific evidence.
“There is substantial, extensively replicated clinical data that demonstrates a significant reduction in [migraine headache] symptoms and frequency (even complete elimination of headache pain) following trigger site surgery,” the authors noted.
Pamela Blake, MD, a neurologist, board-certified headache specialist, and medical director at the Headache Center of River Oaks, Houston, is a proponent of what she said can be “lifesaving” headache surgery.
“If a doctor told you that you can either treat this problem with medications that you’ll need to take for the rest of your life or you can have a surgical procedure as an outpatient that has extremely low risk and has, in my experience, a 75% chance of reducing or eliminating your pain, you probably would be interested in surgery,” she said.
Continued Skepticism
However, other neurologists and clinicians appear doubtful about this intervention, including Hans-Christoph Diener, MD, PhD, professor of neurology and director, Essen Headache Centre, University of Duisburg-Essen in Germany.
During a debate on the topic a decade ago at the International Headache Congress, Dr. Diener argued that, as migraine is a complex multigene-related disorder of the brain, it doesn’t make sense that surgery would affect the epigenetics of 22 different genes.
Recently, he said that his views have not changed.
The topic remains controversial, and some neurologists are uncomfortable even openly discussing the procedure. Two clinicians who previously commented on this article later asked not to be included.
One neurologist, who asked to remain anonymous, said that Dr. Janis’s review article is “merely a review collecting 19 studies over the previous 10-plus years.”
Other limitations cited by this neurologist are the lack of consistency in procedures among the various studies and the inclusion of only four RCTs, the most recent of which was published 8 years ago, suggesting “the study was probably done closer to 9 or 10 years ago,” the neurologist said.
Dr. Blake suggested some neurologists’ reluctance could be due to limited background on the procedure, which she said isn’t widely discussed at headache meetings and is covered mostly in plastic surgery journals, not neurology literature. Access to surgery is further limited by a lack of specialists who perform the procedure and inconsistent insurance coverage.
A closer collaboration between neurologists and surgeons who perform the procedure could benefit patients, Dr. Blake noted.
“The headache doctor’s role is to identify who’s a candidate for surgery, who meets the criteria for nerve compression, and then follow that patient postoperatively, managing their medications, although usually we get them off their medications,” she added.
From Dr. Janis’s perspective, things are starting to change.
“I’m definitely seeing a greater comfort level among neurologists who are understanding where this sits in the algorithm for treatment, especially for complicated patients,” he said.
Dr. Janis receives royalties from Thieme and Springer Publishing. Dr. Blake reported no relevant conflicts. Dr. Diener received research support from the German Research Council; serves on the editorial boards of Cephalalgia, Lancet Neurology, and Drugs; and has received honoraria for participation in clinical trials, contribution to advisory boards, or oral presentations from AbbVie, Lilly, Lundbeck, Novartis, Pfizer, Teva, Weber & Weber, and WebMD.
A version of this article appeared on Medscape.com.
Jeffrey E. Janis, MD, is on a mission. The professor of plastic surgery, surgery, neurosurgery, and neurology at The Ohio State University Wexner Medical Center, Columbus, Ohio, wants to convince neurologists of the safety and efficacy of nerve decompression surgery for treatment-resistant headache. However, many neurologists remain unconvinced.
There’s 24 years of evidence behind this surgical technique across hundreds of different studies with different study designs,” Dr. Janis said.
Yet this treatment approach — surgery on peripheral nerves rather than the brain or spinal cord — hasn’t garnered much support from neurologists. A scan of the agenda of topics at the recently held 2024 annual meeting of the American Headache Society showed few if any studies or presentations on this topic. And neurologists this news organization spoke to said they believe the surgery is experimental and unproven.
Experts do agree drugs don’t work for all patients with migraines. Up to 30% of patients don’t respond to the “laundry list of medications” available to treat the condition, said Dr. Janis.
Many patients have also tried, and failed, alternative treatment approaches such as massage, acupuncture, craniosacral therapy, transdermal patches, electrical stimulation, cryoablation, neurostimulation, and radiofrequency ablation.
If nothing else works, is surgery for headaches the answer?
Long-Held Theory
The idea that pinched, irritated, or compressed peripheral nerves can trigger migraine attacks has been around for nearly 25 years. Studies suggest that in addition to migraine, nerve compression can lead to other headache conditions, including occipital neuralgia, supraorbital neuralgia , and post-traumatic headaches.
This has led to the development of surgical techniques to deactivate various compression trigger sites — what Dr. Janis calls “pinch points” — which could involve muscles, bone, fascia, blood vessels, or scar tissue from prior trauma or surgery.
The procedure is predominantly performed by plastic surgeons, but to a lesser degree by neurosurgeons and ear, nose, and throat specialists.
Target nerves in surgical interventions include those in the frontal region of the head above the eye, temporal region, neck region, and nasal region. Affected areas are usually identified either through patient self-reports or by using a nerve block agent such as lidocaine or Botox at specific points, Dr. Janis noted. If pain subsides after an injection, that location is marked as a target.
One of the barriers to referring complicated patients for surgery is that neurologists evaluating migraine treatments “speak a different language” than surgeons performing the procedure, said Dr. Janis.
Neurologists tend to focus on reduction in monthly migraine days (MMD), while surgeons typically use the Migraine Headache Index that incorporates the frequency, intensity, and duration of migraine attacks.
“Rather than try to convince somebody to speak a different language, we thought, why don’t we just learn their language so we can build bridges and take down barriers,” said Dr. Janis, coauthor of a systematic review and meta-analysis published online recently in Plastic and Reconstructive Surgery.
Investigators examined 19 studies in the review, including five randomized controlled trials (RCTs), published from January 2020 to September 2023, with a total of 1603 participants who were mostly female and ranged in age from 9 to 72 years. Study follow-ups extended from 6 to 38 months. All but three studies were carried out in the United States, and six different compression sites were addressed during surgery.
Investigators found that across studies and by a number of measures, migraine frequency and severity improved after surgery.
Monthly migraine days decreased by 36%-92% and the number of overall migraine attacks per month dropped 25%-87.5%. Patients also reported decreases in attack duration of 41%-75% and intensity of 28%-82% across studies.
“Even using the neurologist-standard language of monthly migraine days, this surgery works,” said Dr. Janis. “Now this is documented both in the surgical literature and the nonsurgical literature.”
The most common complications were ecchymosis, hair loss or thinning, itching, dryness, and rhinorrhea, all of which Dr. Janis described as “fairly minor.” Major complications such as intraoperative bleeding and wound dehiscence were rare, occurring in 1% or less of participants.
‘One And Done?’
These surgeries are usually done on an outpatient basis and generally offer long-term results, Dr. Janis said.
“The idea is one and done,” he said. “The literature around this type of surgery says that whatever type of effect you get at 1 year is likely to be permanent.”
The American Society of Plastic Surgeons agrees. A 2018 position paper developed by experts and commissioned by the society reports that the intervention is safe and effective for appropriate patients, based on a comprehensive literature search and review of a large body of peer-reviewed scientific evidence.
“There is substantial, extensively replicated clinical data that demonstrates a significant reduction in [migraine headache] symptoms and frequency (even complete elimination of headache pain) following trigger site surgery,” the authors noted.
Pamela Blake, MD, a neurologist, board-certified headache specialist, and medical director at the Headache Center of River Oaks, Houston, is a proponent of what she said can be “lifesaving” headache surgery.
“If a doctor told you that you can either treat this problem with medications that you’ll need to take for the rest of your life or you can have a surgical procedure as an outpatient that has extremely low risk and has, in my experience, a 75% chance of reducing or eliminating your pain, you probably would be interested in surgery,” she said.
Continued Skepticism
However, other neurologists and clinicians appear doubtful about this intervention, including Hans-Christoph Diener, MD, PhD, professor of neurology and director, Essen Headache Centre, University of Duisburg-Essen in Germany.
During a debate on the topic a decade ago at the International Headache Congress, Dr. Diener argued that, as migraine is a complex multigene-related disorder of the brain, it doesn’t make sense that surgery would affect the epigenetics of 22 different genes.
Recently, he said that his views have not changed.
The topic remains controversial, and some neurologists are uncomfortable even openly discussing the procedure. Two clinicians who previously commented on this article later asked not to be included.
One neurologist, who asked to remain anonymous, said that Dr. Janis’s review article is “merely a review collecting 19 studies over the previous 10-plus years.”
Other limitations cited by this neurologist are the lack of consistency in procedures among the various studies and the inclusion of only four RCTs, the most recent of which was published 8 years ago, suggesting “the study was probably done closer to 9 or 10 years ago,” the neurologist said.
Dr. Blake suggested some neurologists’ reluctance could be due to limited background on the procedure, which she said isn’t widely discussed at headache meetings and is covered mostly in plastic surgery journals, not neurology literature. Access to surgery is further limited by a lack of specialists who perform the procedure and inconsistent insurance coverage.
A closer collaboration between neurologists and surgeons who perform the procedure could benefit patients, Dr. Blake noted.
“The headache doctor’s role is to identify who’s a candidate for surgery, who meets the criteria for nerve compression, and then follow that patient postoperatively, managing their medications, although usually we get them off their medications,” she added.
From Dr. Janis’s perspective, things are starting to change.
“I’m definitely seeing a greater comfort level among neurologists who are understanding where this sits in the algorithm for treatment, especially for complicated patients,” he said.
Dr. Janis receives royalties from Thieme and Springer Publishing. Dr. Blake reported no relevant conflicts. Dr. Diener received research support from the German Research Council; serves on the editorial boards of Cephalalgia, Lancet Neurology, and Drugs; and has received honoraria for participation in clinical trials, contribution to advisory boards, or oral presentations from AbbVie, Lilly, Lundbeck, Novartis, Pfizer, Teva, Weber & Weber, and WebMD.
A version of this article appeared on Medscape.com.
Long COVID & Chronic Fatigue: The Similarities are Uncanny
An estimated two million people in England and Scotland were experiencing symptoms of long COVID as of March 2024, according to the Office for National Statistics. Of these, 1.5 million said the condition was adversely affecting their day-to-day activities.
As more research emerges about long COVID, some experts are noticing that its trigger factors, symptoms, and causative mechanisms overlap with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS).
ME/CFS is characterized by severe fatigue that does not improve with rest, in addition to pain and cognitive problems. One in four patients are bed- or house-bound with severe forms of the condition, sometimes experiencing atypical seizures, and speech and swallowing difficulties.
Despite affecting around 250,000 people in the UK and around 2 million people in the European Union (EU), it is a relatively poorly funded disease research area. Increased research into long COVID is thus providing a much-needed boost to ME/CFS research.
“What we already know about the possible causation of ME/CFS is helping research into the causes of long COVID. At the same time, research into long COVID is opening up new avenues of research that may also be relevant to ME/CFS. It is becoming a two-way process,” Dr. Charles Shepherd, honorary medical adviser to the UK-based ME Association, told this news organization.
While funding remains an issue, promising research is currently underway in the UK to improve diagnosis, treatment, and understanding of the pathology of ME/CFS.
Viral Reactivation
Dr. David Newton is research director at ME Research UK. “Viral infection is commonly reported as a trigger for [ME/CFS, meaning that the disease] may be caused by reactivation of latent viruses, including human herpes viruses and enteroviruses,” he said.
Herpes viruses can lie dormant in their host’s immune system for long periods of time. They can be reactivated by factors including infections, stress, and a weakened immune system, and may cause temporary symptoms or persistent disease.
A 2021 pilot study found that people with ME/CFS have a higher concentration of human herpesvirus 6B (HHV-6B) DNA in their saliva, and that concentration correlates with symptom severity. HHV-6B is a common virus typically contracted during infancy and childhood.
A continuation of this research is now underway at Brunel University to improve understanding of HHV-6B’s role in the onset and progression of ME/CFS, and to support the development of diagnostic and prognostic markers, as well as therapeutics such as antiviral therapies.
Mitochondrial Dysfunction
Dr. Shepherd explained that there is now sound evidence demonstrating that biochemical abnormalities in ME/CFS affect how mitochondria produce energy after physical exertion. Research is thus underway to see if treating mitochondrial dysfunction improves ME/CFS symptoms.
A phase 2a placebo-controlled clinical trial from 2023 found that AXA1125, a drug that works by modulating energy metabolism, significantly improved symptoms of fatigue in patients with fatigue-dominant long COVID, although it did not improve mitochondrial respiration.
“[The findings suggest] that improving mitochondrial health may be one way to restore normal functioning among people with long COVID, and by extension CFS,” study author Betty Raman, associate professor of cardiovascular medicine at the University of Oxford, told this news organization. She noted, however, that plans for a phase III trial have stalled due to insufficient funding.
Meanwhile, researchers from the Quadram Institute in Norwich and the University of East Anglia are conducting a pilot study to see if red light therapy can relieve symptoms of ME/CFS. Red light can be absorbed by mitochondria and is used to boost energy production. The trial will monitor patients remotely from their homes and will assess cognitive function and physical activity levels.
Gut Dysbiosis
Many studies have found that people with ME/CFS have altered gut microbiota, which suggests that changes in gut bacteria may contribute to the condition. Researchers at the Quadram Institute will thus conduct a clinical trial called RESTORE-ME to see whether fecal microbiota transplants (FMT) can treat the condition.
Rik Haagmans is a research scientist and PhD candidate at the Quadram Institute. He told this news organization: “Our FMT studies, if effective, could provide a longer lasting or even permanent relief of ME/CFS, as restoring the gut microbial composition wouldn’t require continuous medication,” he said.
Biobank and Biomarkers
Europe’s first ME/CFS-specific biobank is in the UK and is called UKMEB. It now has more than 30,000 blood samples from patients with ME/CFS, multiple sclerosis, and healthy controls. Uniquely, it includes samples from people with ME/CFS who are house- and bed-bound. Caroline Kingdon, RN, MSc, a research fellow and biobank lead at the London School of Hygiene and Tropical Medicine, told this news organization that samples and data from the UKMEB have been provided to research groups all over the world and have contributed to widely cited literature.
One group making use of these samples is led by Fatima Labeed, PhD, senior lecturer in human biology at the University of Surrey. Dr. Labeed and her team are developing a diagnostic test for ME/CFS based on electrical properties in white blood cells.
“To date, studies of ME/CFS have focused on the biochemical behavior of cells: the amount and type of proteins that cells use. We have taken a different approach, studying the electrical properties,” she explained to this news organization.
Her research builds on initial observations from 2019 that found differences in the electrical impedance of white blood cells between people with ME/CFS and controls. While the biological implications remain unknown, the findings may represent a biomarker for the condition.
Using blood samples from the UKMEB, the researchers are now investigating this potential biomarker with improved techniques and a larger patient cohort, including those with mild/moderate and severe forms of ME/CFS. So far, they have received more than 100 blood samples and have analyzed the electrical properties of 42.
“Based on the results we have so far, we are very close to having a biomarker for diagnosis. Our results so far show a high degree of accuracy and are able to distinguish between ME/CFS and other diseases,” said Dr. Labeed.
Genetic Test
Another promising avenue for diagnostics comes from a research team at the University of Edinburgh led by Professor Chris Ponting at the university’s Institute of Genetics and Cancer. They are currently working on DecodeMe, a large genetic study of ME using data from more than 26,000 people.
“We are studying blood-based biomarkers that distinguish people with ME from population controls. We’ve found a large number — including some found previously in other studies — and are writing these results up for publication,” said Ponting. The results should be published in early 2025.
The Future
While research into ME/CFS has picked up pace in recent years, funding remains a key bottleneck.
“Over the last 10 years, only £8.05m has been spent on ME research,” Sonya Chowdhury, chief executive of UK charity Action for ME told this news organization. She believes this amount is not equitably comparable to research funding allocated to other diseases.
In 2022, the UK government announced its intention to develop a cross-government interim delivery plan on ME/CFS for England, however publication of the final plan has been delayed numerous times.
Dr. Shepherd agreed that increased funding is crucial for progress to be made. He said the biggest help to ME/CFS research would be to end the disparity in government research funding for the disease, and match what is given for many other disabling long-term conditions.
“It’s not fair to continue to rely on the charity sector to fund almost all of the biomedical research into ME/CFS here in the UK,” he said.
A version of this article appeared on Medscape.com.
An estimated two million people in England and Scotland were experiencing symptoms of long COVID as of March 2024, according to the Office for National Statistics. Of these, 1.5 million said the condition was adversely affecting their day-to-day activities.
As more research emerges about long COVID, some experts are noticing that its trigger factors, symptoms, and causative mechanisms overlap with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS).
ME/CFS is characterized by severe fatigue that does not improve with rest, in addition to pain and cognitive problems. One in four patients are bed- or house-bound with severe forms of the condition, sometimes experiencing atypical seizures, and speech and swallowing difficulties.
Despite affecting around 250,000 people in the UK and around 2 million people in the European Union (EU), it is a relatively poorly funded disease research area. Increased research into long COVID is thus providing a much-needed boost to ME/CFS research.
“What we already know about the possible causation of ME/CFS is helping research into the causes of long COVID. At the same time, research into long COVID is opening up new avenues of research that may also be relevant to ME/CFS. It is becoming a two-way process,” Dr. Charles Shepherd, honorary medical adviser to the UK-based ME Association, told this news organization.
While funding remains an issue, promising research is currently underway in the UK to improve diagnosis, treatment, and understanding of the pathology of ME/CFS.
Viral Reactivation
Dr. David Newton is research director at ME Research UK. “Viral infection is commonly reported as a trigger for [ME/CFS, meaning that the disease] may be caused by reactivation of latent viruses, including human herpes viruses and enteroviruses,” he said.
Herpes viruses can lie dormant in their host’s immune system for long periods of time. They can be reactivated by factors including infections, stress, and a weakened immune system, and may cause temporary symptoms or persistent disease.
A 2021 pilot study found that people with ME/CFS have a higher concentration of human herpesvirus 6B (HHV-6B) DNA in their saliva, and that concentration correlates with symptom severity. HHV-6B is a common virus typically contracted during infancy and childhood.
A continuation of this research is now underway at Brunel University to improve understanding of HHV-6B’s role in the onset and progression of ME/CFS, and to support the development of diagnostic and prognostic markers, as well as therapeutics such as antiviral therapies.
Mitochondrial Dysfunction
Dr. Shepherd explained that there is now sound evidence demonstrating that biochemical abnormalities in ME/CFS affect how mitochondria produce energy after physical exertion. Research is thus underway to see if treating mitochondrial dysfunction improves ME/CFS symptoms.
A phase 2a placebo-controlled clinical trial from 2023 found that AXA1125, a drug that works by modulating energy metabolism, significantly improved symptoms of fatigue in patients with fatigue-dominant long COVID, although it did not improve mitochondrial respiration.
“[The findings suggest] that improving mitochondrial health may be one way to restore normal functioning among people with long COVID, and by extension CFS,” study author Betty Raman, associate professor of cardiovascular medicine at the University of Oxford, told this news organization. She noted, however, that plans for a phase III trial have stalled due to insufficient funding.
Meanwhile, researchers from the Quadram Institute in Norwich and the University of East Anglia are conducting a pilot study to see if red light therapy can relieve symptoms of ME/CFS. Red light can be absorbed by mitochondria and is used to boost energy production. The trial will monitor patients remotely from their homes and will assess cognitive function and physical activity levels.
Gut Dysbiosis
Many studies have found that people with ME/CFS have altered gut microbiota, which suggests that changes in gut bacteria may contribute to the condition. Researchers at the Quadram Institute will thus conduct a clinical trial called RESTORE-ME to see whether fecal microbiota transplants (FMT) can treat the condition.
Rik Haagmans is a research scientist and PhD candidate at the Quadram Institute. He told this news organization: “Our FMT studies, if effective, could provide a longer lasting or even permanent relief of ME/CFS, as restoring the gut microbial composition wouldn’t require continuous medication,” he said.
Biobank and Biomarkers
Europe’s first ME/CFS-specific biobank is in the UK and is called UKMEB. It now has more than 30,000 blood samples from patients with ME/CFS, multiple sclerosis, and healthy controls. Uniquely, it includes samples from people with ME/CFS who are house- and bed-bound. Caroline Kingdon, RN, MSc, a research fellow and biobank lead at the London School of Hygiene and Tropical Medicine, told this news organization that samples and data from the UKMEB have been provided to research groups all over the world and have contributed to widely cited literature.
One group making use of these samples is led by Fatima Labeed, PhD, senior lecturer in human biology at the University of Surrey. Dr. Labeed and her team are developing a diagnostic test for ME/CFS based on electrical properties in white blood cells.
“To date, studies of ME/CFS have focused on the biochemical behavior of cells: the amount and type of proteins that cells use. We have taken a different approach, studying the electrical properties,” she explained to this news organization.
Her research builds on initial observations from 2019 that found differences in the electrical impedance of white blood cells between people with ME/CFS and controls. While the biological implications remain unknown, the findings may represent a biomarker for the condition.
Using blood samples from the UKMEB, the researchers are now investigating this potential biomarker with improved techniques and a larger patient cohort, including those with mild/moderate and severe forms of ME/CFS. So far, they have received more than 100 blood samples and have analyzed the electrical properties of 42.
“Based on the results we have so far, we are very close to having a biomarker for diagnosis. Our results so far show a high degree of accuracy and are able to distinguish between ME/CFS and other diseases,” said Dr. Labeed.
Genetic Test
Another promising avenue for diagnostics comes from a research team at the University of Edinburgh led by Professor Chris Ponting at the university’s Institute of Genetics and Cancer. They are currently working on DecodeMe, a large genetic study of ME using data from more than 26,000 people.
“We are studying blood-based biomarkers that distinguish people with ME from population controls. We’ve found a large number — including some found previously in other studies — and are writing these results up for publication,” said Ponting. The results should be published in early 2025.
The Future
While research into ME/CFS has picked up pace in recent years, funding remains a key bottleneck.
“Over the last 10 years, only £8.05m has been spent on ME research,” Sonya Chowdhury, chief executive of UK charity Action for ME told this news organization. She believes this amount is not equitably comparable to research funding allocated to other diseases.
In 2022, the UK government announced its intention to develop a cross-government interim delivery plan on ME/CFS for England, however publication of the final plan has been delayed numerous times.
Dr. Shepherd agreed that increased funding is crucial for progress to be made. He said the biggest help to ME/CFS research would be to end the disparity in government research funding for the disease, and match what is given for many other disabling long-term conditions.
“It’s not fair to continue to rely on the charity sector to fund almost all of the biomedical research into ME/CFS here in the UK,” he said.
A version of this article appeared on Medscape.com.
An estimated two million people in England and Scotland were experiencing symptoms of long COVID as of March 2024, according to the Office for National Statistics. Of these, 1.5 million said the condition was adversely affecting their day-to-day activities.
As more research emerges about long COVID, some experts are noticing that its trigger factors, symptoms, and causative mechanisms overlap with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS).
ME/CFS is characterized by severe fatigue that does not improve with rest, in addition to pain and cognitive problems. One in four patients are bed- or house-bound with severe forms of the condition, sometimes experiencing atypical seizures, and speech and swallowing difficulties.
Despite affecting around 250,000 people in the UK and around 2 million people in the European Union (EU), it is a relatively poorly funded disease research area. Increased research into long COVID is thus providing a much-needed boost to ME/CFS research.
“What we already know about the possible causation of ME/CFS is helping research into the causes of long COVID. At the same time, research into long COVID is opening up new avenues of research that may also be relevant to ME/CFS. It is becoming a two-way process,” Dr. Charles Shepherd, honorary medical adviser to the UK-based ME Association, told this news organization.
While funding remains an issue, promising research is currently underway in the UK to improve diagnosis, treatment, and understanding of the pathology of ME/CFS.
Viral Reactivation
Dr. David Newton is research director at ME Research UK. “Viral infection is commonly reported as a trigger for [ME/CFS, meaning that the disease] may be caused by reactivation of latent viruses, including human herpes viruses and enteroviruses,” he said.
Herpes viruses can lie dormant in their host’s immune system for long periods of time. They can be reactivated by factors including infections, stress, and a weakened immune system, and may cause temporary symptoms or persistent disease.
A 2021 pilot study found that people with ME/CFS have a higher concentration of human herpesvirus 6B (HHV-6B) DNA in their saliva, and that concentration correlates with symptom severity. HHV-6B is a common virus typically contracted during infancy and childhood.
A continuation of this research is now underway at Brunel University to improve understanding of HHV-6B’s role in the onset and progression of ME/CFS, and to support the development of diagnostic and prognostic markers, as well as therapeutics such as antiviral therapies.
Mitochondrial Dysfunction
Dr. Shepherd explained that there is now sound evidence demonstrating that biochemical abnormalities in ME/CFS affect how mitochondria produce energy after physical exertion. Research is thus underway to see if treating mitochondrial dysfunction improves ME/CFS symptoms.
A phase 2a placebo-controlled clinical trial from 2023 found that AXA1125, a drug that works by modulating energy metabolism, significantly improved symptoms of fatigue in patients with fatigue-dominant long COVID, although it did not improve mitochondrial respiration.
“[The findings suggest] that improving mitochondrial health may be one way to restore normal functioning among people with long COVID, and by extension CFS,” study author Betty Raman, associate professor of cardiovascular medicine at the University of Oxford, told this news organization. She noted, however, that plans for a phase III trial have stalled due to insufficient funding.
Meanwhile, researchers from the Quadram Institute in Norwich and the University of East Anglia are conducting a pilot study to see if red light therapy can relieve symptoms of ME/CFS. Red light can be absorbed by mitochondria and is used to boost energy production. The trial will monitor patients remotely from their homes and will assess cognitive function and physical activity levels.
Gut Dysbiosis
Many studies have found that people with ME/CFS have altered gut microbiota, which suggests that changes in gut bacteria may contribute to the condition. Researchers at the Quadram Institute will thus conduct a clinical trial called RESTORE-ME to see whether fecal microbiota transplants (FMT) can treat the condition.
Rik Haagmans is a research scientist and PhD candidate at the Quadram Institute. He told this news organization: “Our FMT studies, if effective, could provide a longer lasting or even permanent relief of ME/CFS, as restoring the gut microbial composition wouldn’t require continuous medication,” he said.
Biobank and Biomarkers
Europe’s first ME/CFS-specific biobank is in the UK and is called UKMEB. It now has more than 30,000 blood samples from patients with ME/CFS, multiple sclerosis, and healthy controls. Uniquely, it includes samples from people with ME/CFS who are house- and bed-bound. Caroline Kingdon, RN, MSc, a research fellow and biobank lead at the London School of Hygiene and Tropical Medicine, told this news organization that samples and data from the UKMEB have been provided to research groups all over the world and have contributed to widely cited literature.
One group making use of these samples is led by Fatima Labeed, PhD, senior lecturer in human biology at the University of Surrey. Dr. Labeed and her team are developing a diagnostic test for ME/CFS based on electrical properties in white blood cells.
“To date, studies of ME/CFS have focused on the biochemical behavior of cells: the amount and type of proteins that cells use. We have taken a different approach, studying the electrical properties,” she explained to this news organization.
Her research builds on initial observations from 2019 that found differences in the electrical impedance of white blood cells between people with ME/CFS and controls. While the biological implications remain unknown, the findings may represent a biomarker for the condition.
Using blood samples from the UKMEB, the researchers are now investigating this potential biomarker with improved techniques and a larger patient cohort, including those with mild/moderate and severe forms of ME/CFS. So far, they have received more than 100 blood samples and have analyzed the electrical properties of 42.
“Based on the results we have so far, we are very close to having a biomarker for diagnosis. Our results so far show a high degree of accuracy and are able to distinguish between ME/CFS and other diseases,” said Dr. Labeed.
Genetic Test
Another promising avenue for diagnostics comes from a research team at the University of Edinburgh led by Professor Chris Ponting at the university’s Institute of Genetics and Cancer. They are currently working on DecodeMe, a large genetic study of ME using data from more than 26,000 people.
“We are studying blood-based biomarkers that distinguish people with ME from population controls. We’ve found a large number — including some found previously in other studies — and are writing these results up for publication,” said Ponting. The results should be published in early 2025.
The Future
While research into ME/CFS has picked up pace in recent years, funding remains a key bottleneck.
“Over the last 10 years, only £8.05m has been spent on ME research,” Sonya Chowdhury, chief executive of UK charity Action for ME told this news organization. She believes this amount is not equitably comparable to research funding allocated to other diseases.
In 2022, the UK government announced its intention to develop a cross-government interim delivery plan on ME/CFS for England, however publication of the final plan has been delayed numerous times.
Dr. Shepherd agreed that increased funding is crucial for progress to be made. He said the biggest help to ME/CFS research would be to end the disparity in government research funding for the disease, and match what is given for many other disabling long-term conditions.
“It’s not fair to continue to rely on the charity sector to fund almost all of the biomedical research into ME/CFS here in the UK,” he said.
A version of this article appeared on Medscape.com.
Almost 10% of Infected Pregnant People Develop Long COVID
Almost 1 in 10 pregnant people infected with COVID-19 end up developing long COVID, according to a study published in Obstetrics & Gynecology.
Researchers at University of Utah Health looked at the medical records of more than 1500 people who got COVID-19 while pregnant and checked their self-reported symptoms at least 6 months after infection, according to a news release from the school.
The scientists discovered that 9.3% of those people reported long COVID symptoms, such as fatigue and issues in their gut.
To make sure those long COVID symptoms were not actually symptoms of pregnancy, the research team did a second analysis of people who reported symptoms more than 12 weeks after giving birth. The risk of long COVID was about the same as in the first analysis.
“It was surprising to me that the prevalence was that high,” Torri D. Metz, MD, vice chair for research of obstetrics and gynecology at the school and co-leader of the study, said in the release. “This is something that does continue to affect otherwise reasonably healthy and young populations.”
The school said this is the first study to look at long COVID risks in pregnant people. Previous research found other dangers for pregnant people who get COVID, such as a higher chance of hospitalization or death, or complications such as preterm birth.
In the general population, research shows that 10%-20% of people who get COVID develop long COVID.
Dr. Metz said healthcare providers need to remain alert about long COVID, including in pregnant people.
“We need to have this on our radar as we’re seeing patients. It’s something we really don’t want to miss. And we want to get people referred to appropriate specialists who treat long COVID,” she said.
A version of this article first appeared on WebMD.com.
Almost 1 in 10 pregnant people infected with COVID-19 end up developing long COVID, according to a study published in Obstetrics & Gynecology.
Researchers at University of Utah Health looked at the medical records of more than 1500 people who got COVID-19 while pregnant and checked their self-reported symptoms at least 6 months after infection, according to a news release from the school.
The scientists discovered that 9.3% of those people reported long COVID symptoms, such as fatigue and issues in their gut.
To make sure those long COVID symptoms were not actually symptoms of pregnancy, the research team did a second analysis of people who reported symptoms more than 12 weeks after giving birth. The risk of long COVID was about the same as in the first analysis.
“It was surprising to me that the prevalence was that high,” Torri D. Metz, MD, vice chair for research of obstetrics and gynecology at the school and co-leader of the study, said in the release. “This is something that does continue to affect otherwise reasonably healthy and young populations.”
The school said this is the first study to look at long COVID risks in pregnant people. Previous research found other dangers for pregnant people who get COVID, such as a higher chance of hospitalization or death, or complications such as preterm birth.
In the general population, research shows that 10%-20% of people who get COVID develop long COVID.
Dr. Metz said healthcare providers need to remain alert about long COVID, including in pregnant people.
“We need to have this on our radar as we’re seeing patients. It’s something we really don’t want to miss. And we want to get people referred to appropriate specialists who treat long COVID,” she said.
A version of this article first appeared on WebMD.com.
Almost 1 in 10 pregnant people infected with COVID-19 end up developing long COVID, according to a study published in Obstetrics & Gynecology.
Researchers at University of Utah Health looked at the medical records of more than 1500 people who got COVID-19 while pregnant and checked their self-reported symptoms at least 6 months after infection, according to a news release from the school.
The scientists discovered that 9.3% of those people reported long COVID symptoms, such as fatigue and issues in their gut.
To make sure those long COVID symptoms were not actually symptoms of pregnancy, the research team did a second analysis of people who reported symptoms more than 12 weeks after giving birth. The risk of long COVID was about the same as in the first analysis.
“It was surprising to me that the prevalence was that high,” Torri D. Metz, MD, vice chair for research of obstetrics and gynecology at the school and co-leader of the study, said in the release. “This is something that does continue to affect otherwise reasonably healthy and young populations.”
The school said this is the first study to look at long COVID risks in pregnant people. Previous research found other dangers for pregnant people who get COVID, such as a higher chance of hospitalization or death, or complications such as preterm birth.
In the general population, research shows that 10%-20% of people who get COVID develop long COVID.
Dr. Metz said healthcare providers need to remain alert about long COVID, including in pregnant people.
“We need to have this on our radar as we’re seeing patients. It’s something we really don’t want to miss. And we want to get people referred to appropriate specialists who treat long COVID,” she said.
A version of this article first appeared on WebMD.com.
FROM OBSTETRICS & GYNECOLOGY
MDs’ One-Word Summary of Long COVID Progress: ‘Frustration’
Stuart Malcolm, MD, a primary care physician who practices in Oregon and northern California, started seeing patients with long COVID early in the pandemic. Back then, he was frustrated by the obstacles and lack of standard diagnostic tests and treatments. Four years later, well, he still is.
“Something I learned the last few years is the logistics to get people care is really, really hard,” he said. “There’s a lot of frustration. It’s mostly frustration.”
For long COVID doctors and patients, there has been little to no progress addressing the challenges, leaving many discouraged. Researchers and clinicians now have a greater understanding of what health agencies formally call post-COVID condition, but the wide spectrum of symptoms, slow progress in launching pharmacologic clinical trials, and the research toward understanding the underlying causes mean standardized diagnostic tests and definitive treatments remain elusive.
“The frustration is that we aren’t able to help everyone with our current knowledge base. And I think the frustration lies not just with us physicians but also with patients because they’re at the point where if they tried everything, literally everything and haven’t gotten better,” said Zijian Chen, MD, director of the Mount Sinai Center for Post-COVID Care in New York City.
Wanted: More Funding, More Doctors, More Clinics
Between 10% and 20% of the estimated hundreds of millions of people infected worldwide with SARS-CoV-2 in the first 2 years went on to develop long-term symptoms. While many recover over time, doctors who have treated long COVID since 2020 said they see some patients still wrestling with the condition after 4 years.
The latest Centers for Disease Control and Prevention Household Pulse Survey, taken between March 5 and April 1, 2024, estimated that nearly 7% of the adult population — more than 18 million people — currently have long COVID. Data from other countries also suggest that millions have been living with long COVID for years now, and hundreds of thousands have seen their day-to-day activities significantly affected.
There is an urgent need for more funding, long COVID clinicians, multidisciplinary clinics, and education for non–long COVID physicians and specialists, doctors said. Instead, funding remains limited, clinics are closing, wait times are “horrendously long,” patients are left in limbo, and physicians are burning out.
“What’s changed in some ways is that there’s even less access to COVID rehab, which sounds crazy because there was very little to begin with,” said Alexandra Rendely, MD, a physical medicine and rehabilitation physician with the interdisciplinary Toronto Rehab, a part of the University Health Network of teaching hospitals in Toronto, Ontario, Canada.
“Patients are still being diagnosed every day, yet the resources available are becoming less and less.”
COVID-19 money earmarked during the pandemic was mostly limited to temporary emergency measures. As those funds dwindled, governments and institutions have decreased financial support. The Long COVID Moonshot campaign, organized by patients with long COVID, is pushing Congress to support $1 billion in annual research funding to close the financial chasm.
The Clinical Trial Conundrum
While long COVID clinics have come a long way in helping patients, gaps remain. Doctors may be unwilling to prescribe off-label treatments without proper clinical trials due to the potential risks and liabilities involved or due to the controversial or unconventional nature of the therapies, said Dr. Malcolm, who left his primary care practice more than 2 years ago to focus on long COVID.
In the absence of standard treatments, Dr. Malcolm and other doctors said they must take a trial-and-error approach in treating patients with long COVID that centers on addressing symptoms and not the underlying condition.
“There are actually a lot of treatments and a lot of them are not curative, but they can help people,” he said.
Dr. Malcolm, who is a medical director at Real Time Health Monitoring, a private clinic in the San Francisco Bay Area that specializes in long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), said it was important for him to be with a clinical team that understood and was supportive of his treatment decisions and was able to offer clinical support for those treatments if needed.
For physicians looking for clinical data before prescribing certain medications, the wait may be long. More than $1.5 billion in US federal funding has been earmarked to study long COVID, but the National Institutes of Health (NIH) has faced criticism from patients and scientists alike for its slow progress and emphasis on observational studies instead of research that could unravel the biological roots of long COVID. Among the clinical trials announced by the NIH’s RECOVER initiative, only a handful involve studying pharmaceutical treatments.
A 2023 editorial published in The Lancet called out the “dismal state of clinical research relative to the substantial burden of [long COVID]” and said, “we are clearly lacking tested pharmacological interventions that treat the underlying pathophysiology.” At the time of publication, it noted that of the 386 long COVID trials listed on ClinicalTrials.gov, only 12 were actually testing pharmacologic interventions.
There are also diagnostic and insurance barriers. The specialized tests that can detect long COVID anomalies are neither commonly known by primary care practitioners nor easily requested at the local lab, can be expensive, and are typically not covered by insurance, Dr. Malcolm explained.
Patients with long COVID also have the added barrier of being unable to advocate as easily because of their energy limitations, doctors said. Patients may appear outwardly fine, but fatigue and brain fog are among the many problems that cannot be measured in appearances. The condition has upended lives, some losing jobs, even homes, and the mental toll is why there is a “not insignificant” suicide rate.
One Patient’s 4-Year Journey
Charlie McCone, 34, used to be a tennis player and an active musician. But he’s spent the past 4 years mostly housebound, grappling with the aftermath of a SARS-CoV-2 infection he contracted in March 2020. He went from biking daily to work 10 miles and back to having at most 2 hours of energy per day.
In the first year alone, Mr. McCone saw more than two dozen doctors and specialists. The conditions now associated with long COVID, like ME/CFS, mast cell activation syndrome (a condition in which a patient experiences episodes of allergic symptoms such as hives, swelling, low blood pressure, and difficulty breathing), or dysautonomia (conditions that affect the autonomic nervous system, which controls automatic processes in the body) were not on physicians’ radars.
Then in 2021, he became bedbound for more than half a year after a Delta variant reinfection. He developed neurologic symptoms, including incapacitating fatigue, post-exertional malaise (where symptoms worsened after minimal physical or mental activity), left-sided weakness, and cognitive impairment. He stopped working altogether. But the worst was the shortness of breath he felt 24/7, even at rest. A battery of lab tests revealed nothing abnormal. He tried numerous drugs and the classic respiratory treatments.
Mr. McCone eventually connected with Dr. Malcolm over X and developed what he describes as an effective patient-doctor collaboration. When studies came out suggesting microclots were a common issue with patients with long COVID and positive outcomes were reported from anticoagulant therapy, they knew it could be one of the answers.
“After 3 weeks on [the antiplatelet drug], I was like, oh my god, my lungs are finally opening up,” said Mr. McCone. He has taken the medication for more than a year and a half, and some days he doesn’t even think about his respiratory symptoms.
“That trial-and-error process is just really long and hard and costly,” said Dr. Malcolm.
Today, fatigue and cognitive stamina are Mr. McCone’s main challenges, and he is far from recovered.
“[I had a] very fulfilling, happy life and now, it’s hard to think about. I’ve come a long way with my mental health and all this, but I’ve lost 4 years,” Mr. McCone said. “The prospect of me being here when I’m 40 seems very real ... so it’s pretty devastating.”
Lessons Learned, Hope Amid Ongoing Research
Despite the daunting obstacle, doctors said the science has come a long way for a new disease. We now know long COVID is likely caused by a combination of triggers, including viral reservoir in the tissue, inflammation, autoimmunity, and microclots; severity of infection is not necessarily an accurate risk factor predictor — long COVID can strike even those who had a mild infection; upward of 200 symptoms have been identified; and we know more about potential biomarkers that could lead to better diagnostic tools.
Unlike many other diseases and conditions with standard treatment protocols, long COVID treatments are typically aimed at addressing individual symptoms.
“It is very detailed and individualized to the patient’s specific symptoms and to the patient’s specific needs,” Dr. Rendely said. Symptoms can also fluctuate, relapse, or wax and wane, for example, so what ails a patient at their first doctor’s appointment could be completely different at the next appointment 2 months later.
Doctors are still hopeful the RECOVER research, which includes trials that look at autonomic and cognitive dysfunctions, will pave the way for more effective long COVID therapies. In Canada, Dr. Rendely is also eying the RECLAIM trial that is currently testing the effectiveness of pentoxifylline, which helps blood flow, and ibudilast, an anti-inflammatory drug.
Doctors are also hopeful when they see patients who have made “tremendous gains” or even full recoveries through their clinics. “It’s a new diagnosis, so I always tell my patients to think of this as a journey because I’m learning along with you,” said Jai Marathe, MD, an infectious disease physician at Boston Medical Center and an assistant professor of infectious diseases at Boston University Chobanian & Avedisian School of Medicine.
“Now we have 4 years of experience, but at the same time, no two long COVID patients are alike.”
Long COVID has also changed the way physicians view healthcare and how they practice medicine.
“I am a completely different person than I used to be because of this illness, and I don’t even have it. That is how profoundly it has affected how I view the universe,” said Dr. Malcolm. “I’ve been doing this for 4 years, and I’m very hopeful. But I don’t think about this in terms of months anymore. I think about this in terms of years.”
A version of this article first appeared on Medscape.com.
Stuart Malcolm, MD, a primary care physician who practices in Oregon and northern California, started seeing patients with long COVID early in the pandemic. Back then, he was frustrated by the obstacles and lack of standard diagnostic tests and treatments. Four years later, well, he still is.
“Something I learned the last few years is the logistics to get people care is really, really hard,” he said. “There’s a lot of frustration. It’s mostly frustration.”
For long COVID doctors and patients, there has been little to no progress addressing the challenges, leaving many discouraged. Researchers and clinicians now have a greater understanding of what health agencies formally call post-COVID condition, but the wide spectrum of symptoms, slow progress in launching pharmacologic clinical trials, and the research toward understanding the underlying causes mean standardized diagnostic tests and definitive treatments remain elusive.
“The frustration is that we aren’t able to help everyone with our current knowledge base. And I think the frustration lies not just with us physicians but also with patients because they’re at the point where if they tried everything, literally everything and haven’t gotten better,” said Zijian Chen, MD, director of the Mount Sinai Center for Post-COVID Care in New York City.
Wanted: More Funding, More Doctors, More Clinics
Between 10% and 20% of the estimated hundreds of millions of people infected worldwide with SARS-CoV-2 in the first 2 years went on to develop long-term symptoms. While many recover over time, doctors who have treated long COVID since 2020 said they see some patients still wrestling with the condition after 4 years.
The latest Centers for Disease Control and Prevention Household Pulse Survey, taken between March 5 and April 1, 2024, estimated that nearly 7% of the adult population — more than 18 million people — currently have long COVID. Data from other countries also suggest that millions have been living with long COVID for years now, and hundreds of thousands have seen their day-to-day activities significantly affected.
There is an urgent need for more funding, long COVID clinicians, multidisciplinary clinics, and education for non–long COVID physicians and specialists, doctors said. Instead, funding remains limited, clinics are closing, wait times are “horrendously long,” patients are left in limbo, and physicians are burning out.
“What’s changed in some ways is that there’s even less access to COVID rehab, which sounds crazy because there was very little to begin with,” said Alexandra Rendely, MD, a physical medicine and rehabilitation physician with the interdisciplinary Toronto Rehab, a part of the University Health Network of teaching hospitals in Toronto, Ontario, Canada.
“Patients are still being diagnosed every day, yet the resources available are becoming less and less.”
COVID-19 money earmarked during the pandemic was mostly limited to temporary emergency measures. As those funds dwindled, governments and institutions have decreased financial support. The Long COVID Moonshot campaign, organized by patients with long COVID, is pushing Congress to support $1 billion in annual research funding to close the financial chasm.
The Clinical Trial Conundrum
While long COVID clinics have come a long way in helping patients, gaps remain. Doctors may be unwilling to prescribe off-label treatments without proper clinical trials due to the potential risks and liabilities involved or due to the controversial or unconventional nature of the therapies, said Dr. Malcolm, who left his primary care practice more than 2 years ago to focus on long COVID.
In the absence of standard treatments, Dr. Malcolm and other doctors said they must take a trial-and-error approach in treating patients with long COVID that centers on addressing symptoms and not the underlying condition.
“There are actually a lot of treatments and a lot of them are not curative, but they can help people,” he said.
Dr. Malcolm, who is a medical director at Real Time Health Monitoring, a private clinic in the San Francisco Bay Area that specializes in long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), said it was important for him to be with a clinical team that understood and was supportive of his treatment decisions and was able to offer clinical support for those treatments if needed.
For physicians looking for clinical data before prescribing certain medications, the wait may be long. More than $1.5 billion in US federal funding has been earmarked to study long COVID, but the National Institutes of Health (NIH) has faced criticism from patients and scientists alike for its slow progress and emphasis on observational studies instead of research that could unravel the biological roots of long COVID. Among the clinical trials announced by the NIH’s RECOVER initiative, only a handful involve studying pharmaceutical treatments.
A 2023 editorial published in The Lancet called out the “dismal state of clinical research relative to the substantial burden of [long COVID]” and said, “we are clearly lacking tested pharmacological interventions that treat the underlying pathophysiology.” At the time of publication, it noted that of the 386 long COVID trials listed on ClinicalTrials.gov, only 12 were actually testing pharmacologic interventions.
There are also diagnostic and insurance barriers. The specialized tests that can detect long COVID anomalies are neither commonly known by primary care practitioners nor easily requested at the local lab, can be expensive, and are typically not covered by insurance, Dr. Malcolm explained.
Patients with long COVID also have the added barrier of being unable to advocate as easily because of their energy limitations, doctors said. Patients may appear outwardly fine, but fatigue and brain fog are among the many problems that cannot be measured in appearances. The condition has upended lives, some losing jobs, even homes, and the mental toll is why there is a “not insignificant” suicide rate.
One Patient’s 4-Year Journey
Charlie McCone, 34, used to be a tennis player and an active musician. But he’s spent the past 4 years mostly housebound, grappling with the aftermath of a SARS-CoV-2 infection he contracted in March 2020. He went from biking daily to work 10 miles and back to having at most 2 hours of energy per day.
In the first year alone, Mr. McCone saw more than two dozen doctors and specialists. The conditions now associated with long COVID, like ME/CFS, mast cell activation syndrome (a condition in which a patient experiences episodes of allergic symptoms such as hives, swelling, low blood pressure, and difficulty breathing), or dysautonomia (conditions that affect the autonomic nervous system, which controls automatic processes in the body) were not on physicians’ radars.
Then in 2021, he became bedbound for more than half a year after a Delta variant reinfection. He developed neurologic symptoms, including incapacitating fatigue, post-exertional malaise (where symptoms worsened after minimal physical or mental activity), left-sided weakness, and cognitive impairment. He stopped working altogether. But the worst was the shortness of breath he felt 24/7, even at rest. A battery of lab tests revealed nothing abnormal. He tried numerous drugs and the classic respiratory treatments.
Mr. McCone eventually connected with Dr. Malcolm over X and developed what he describes as an effective patient-doctor collaboration. When studies came out suggesting microclots were a common issue with patients with long COVID and positive outcomes were reported from anticoagulant therapy, they knew it could be one of the answers.
“After 3 weeks on [the antiplatelet drug], I was like, oh my god, my lungs are finally opening up,” said Mr. McCone. He has taken the medication for more than a year and a half, and some days he doesn’t even think about his respiratory symptoms.
“That trial-and-error process is just really long and hard and costly,” said Dr. Malcolm.
Today, fatigue and cognitive stamina are Mr. McCone’s main challenges, and he is far from recovered.
“[I had a] very fulfilling, happy life and now, it’s hard to think about. I’ve come a long way with my mental health and all this, but I’ve lost 4 years,” Mr. McCone said. “The prospect of me being here when I’m 40 seems very real ... so it’s pretty devastating.”
Lessons Learned, Hope Amid Ongoing Research
Despite the daunting obstacle, doctors said the science has come a long way for a new disease. We now know long COVID is likely caused by a combination of triggers, including viral reservoir in the tissue, inflammation, autoimmunity, and microclots; severity of infection is not necessarily an accurate risk factor predictor — long COVID can strike even those who had a mild infection; upward of 200 symptoms have been identified; and we know more about potential biomarkers that could lead to better diagnostic tools.
Unlike many other diseases and conditions with standard treatment protocols, long COVID treatments are typically aimed at addressing individual symptoms.
“It is very detailed and individualized to the patient’s specific symptoms and to the patient’s specific needs,” Dr. Rendely said. Symptoms can also fluctuate, relapse, or wax and wane, for example, so what ails a patient at their first doctor’s appointment could be completely different at the next appointment 2 months later.
Doctors are still hopeful the RECOVER research, which includes trials that look at autonomic and cognitive dysfunctions, will pave the way for more effective long COVID therapies. In Canada, Dr. Rendely is also eying the RECLAIM trial that is currently testing the effectiveness of pentoxifylline, which helps blood flow, and ibudilast, an anti-inflammatory drug.
Doctors are also hopeful when they see patients who have made “tremendous gains” or even full recoveries through their clinics. “It’s a new diagnosis, so I always tell my patients to think of this as a journey because I’m learning along with you,” said Jai Marathe, MD, an infectious disease physician at Boston Medical Center and an assistant professor of infectious diseases at Boston University Chobanian & Avedisian School of Medicine.
“Now we have 4 years of experience, but at the same time, no two long COVID patients are alike.”
Long COVID has also changed the way physicians view healthcare and how they practice medicine.
“I am a completely different person than I used to be because of this illness, and I don’t even have it. That is how profoundly it has affected how I view the universe,” said Dr. Malcolm. “I’ve been doing this for 4 years, and I’m very hopeful. But I don’t think about this in terms of months anymore. I think about this in terms of years.”
A version of this article first appeared on Medscape.com.
Stuart Malcolm, MD, a primary care physician who practices in Oregon and northern California, started seeing patients with long COVID early in the pandemic. Back then, he was frustrated by the obstacles and lack of standard diagnostic tests and treatments. Four years later, well, he still is.
“Something I learned the last few years is the logistics to get people care is really, really hard,” he said. “There’s a lot of frustration. It’s mostly frustration.”
For long COVID doctors and patients, there has been little to no progress addressing the challenges, leaving many discouraged. Researchers and clinicians now have a greater understanding of what health agencies formally call post-COVID condition, but the wide spectrum of symptoms, slow progress in launching pharmacologic clinical trials, and the research toward understanding the underlying causes mean standardized diagnostic tests and definitive treatments remain elusive.
“The frustration is that we aren’t able to help everyone with our current knowledge base. And I think the frustration lies not just with us physicians but also with patients because they’re at the point where if they tried everything, literally everything and haven’t gotten better,” said Zijian Chen, MD, director of the Mount Sinai Center for Post-COVID Care in New York City.
Wanted: More Funding, More Doctors, More Clinics
Between 10% and 20% of the estimated hundreds of millions of people infected worldwide with SARS-CoV-2 in the first 2 years went on to develop long-term symptoms. While many recover over time, doctors who have treated long COVID since 2020 said they see some patients still wrestling with the condition after 4 years.
The latest Centers for Disease Control and Prevention Household Pulse Survey, taken between March 5 and April 1, 2024, estimated that nearly 7% of the adult population — more than 18 million people — currently have long COVID. Data from other countries also suggest that millions have been living with long COVID for years now, and hundreds of thousands have seen their day-to-day activities significantly affected.
There is an urgent need for more funding, long COVID clinicians, multidisciplinary clinics, and education for non–long COVID physicians and specialists, doctors said. Instead, funding remains limited, clinics are closing, wait times are “horrendously long,” patients are left in limbo, and physicians are burning out.
“What’s changed in some ways is that there’s even less access to COVID rehab, which sounds crazy because there was very little to begin with,” said Alexandra Rendely, MD, a physical medicine and rehabilitation physician with the interdisciplinary Toronto Rehab, a part of the University Health Network of teaching hospitals in Toronto, Ontario, Canada.
“Patients are still being diagnosed every day, yet the resources available are becoming less and less.”
COVID-19 money earmarked during the pandemic was mostly limited to temporary emergency measures. As those funds dwindled, governments and institutions have decreased financial support. The Long COVID Moonshot campaign, organized by patients with long COVID, is pushing Congress to support $1 billion in annual research funding to close the financial chasm.
The Clinical Trial Conundrum
While long COVID clinics have come a long way in helping patients, gaps remain. Doctors may be unwilling to prescribe off-label treatments without proper clinical trials due to the potential risks and liabilities involved or due to the controversial or unconventional nature of the therapies, said Dr. Malcolm, who left his primary care practice more than 2 years ago to focus on long COVID.
In the absence of standard treatments, Dr. Malcolm and other doctors said they must take a trial-and-error approach in treating patients with long COVID that centers on addressing symptoms and not the underlying condition.
“There are actually a lot of treatments and a lot of them are not curative, but they can help people,” he said.
Dr. Malcolm, who is a medical director at Real Time Health Monitoring, a private clinic in the San Francisco Bay Area that specializes in long COVID and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), said it was important for him to be with a clinical team that understood and was supportive of his treatment decisions and was able to offer clinical support for those treatments if needed.
For physicians looking for clinical data before prescribing certain medications, the wait may be long. More than $1.5 billion in US federal funding has been earmarked to study long COVID, but the National Institutes of Health (NIH) has faced criticism from patients and scientists alike for its slow progress and emphasis on observational studies instead of research that could unravel the biological roots of long COVID. Among the clinical trials announced by the NIH’s RECOVER initiative, only a handful involve studying pharmaceutical treatments.
A 2023 editorial published in The Lancet called out the “dismal state of clinical research relative to the substantial burden of [long COVID]” and said, “we are clearly lacking tested pharmacological interventions that treat the underlying pathophysiology.” At the time of publication, it noted that of the 386 long COVID trials listed on ClinicalTrials.gov, only 12 were actually testing pharmacologic interventions.
There are also diagnostic and insurance barriers. The specialized tests that can detect long COVID anomalies are neither commonly known by primary care practitioners nor easily requested at the local lab, can be expensive, and are typically not covered by insurance, Dr. Malcolm explained.
Patients with long COVID also have the added barrier of being unable to advocate as easily because of their energy limitations, doctors said. Patients may appear outwardly fine, but fatigue and brain fog are among the many problems that cannot be measured in appearances. The condition has upended lives, some losing jobs, even homes, and the mental toll is why there is a “not insignificant” suicide rate.
One Patient’s 4-Year Journey
Charlie McCone, 34, used to be a tennis player and an active musician. But he’s spent the past 4 years mostly housebound, grappling with the aftermath of a SARS-CoV-2 infection he contracted in March 2020. He went from biking daily to work 10 miles and back to having at most 2 hours of energy per day.
In the first year alone, Mr. McCone saw more than two dozen doctors and specialists. The conditions now associated with long COVID, like ME/CFS, mast cell activation syndrome (a condition in which a patient experiences episodes of allergic symptoms such as hives, swelling, low blood pressure, and difficulty breathing), or dysautonomia (conditions that affect the autonomic nervous system, which controls automatic processes in the body) were not on physicians’ radars.
Then in 2021, he became bedbound for more than half a year after a Delta variant reinfection. He developed neurologic symptoms, including incapacitating fatigue, post-exertional malaise (where symptoms worsened after minimal physical or mental activity), left-sided weakness, and cognitive impairment. He stopped working altogether. But the worst was the shortness of breath he felt 24/7, even at rest. A battery of lab tests revealed nothing abnormal. He tried numerous drugs and the classic respiratory treatments.
Mr. McCone eventually connected with Dr. Malcolm over X and developed what he describes as an effective patient-doctor collaboration. When studies came out suggesting microclots were a common issue with patients with long COVID and positive outcomes were reported from anticoagulant therapy, they knew it could be one of the answers.
“After 3 weeks on [the antiplatelet drug], I was like, oh my god, my lungs are finally opening up,” said Mr. McCone. He has taken the medication for more than a year and a half, and some days he doesn’t even think about his respiratory symptoms.
“That trial-and-error process is just really long and hard and costly,” said Dr. Malcolm.
Today, fatigue and cognitive stamina are Mr. McCone’s main challenges, and he is far from recovered.
“[I had a] very fulfilling, happy life and now, it’s hard to think about. I’ve come a long way with my mental health and all this, but I’ve lost 4 years,” Mr. McCone said. “The prospect of me being here when I’m 40 seems very real ... so it’s pretty devastating.”
Lessons Learned, Hope Amid Ongoing Research
Despite the daunting obstacle, doctors said the science has come a long way for a new disease. We now know long COVID is likely caused by a combination of triggers, including viral reservoir in the tissue, inflammation, autoimmunity, and microclots; severity of infection is not necessarily an accurate risk factor predictor — long COVID can strike even those who had a mild infection; upward of 200 symptoms have been identified; and we know more about potential biomarkers that could lead to better diagnostic tools.
Unlike many other diseases and conditions with standard treatment protocols, long COVID treatments are typically aimed at addressing individual symptoms.
“It is very detailed and individualized to the patient’s specific symptoms and to the patient’s specific needs,” Dr. Rendely said. Symptoms can also fluctuate, relapse, or wax and wane, for example, so what ails a patient at their first doctor’s appointment could be completely different at the next appointment 2 months later.
Doctors are still hopeful the RECOVER research, which includes trials that look at autonomic and cognitive dysfunctions, will pave the way for more effective long COVID therapies. In Canada, Dr. Rendely is also eying the RECLAIM trial that is currently testing the effectiveness of pentoxifylline, which helps blood flow, and ibudilast, an anti-inflammatory drug.
Doctors are also hopeful when they see patients who have made “tremendous gains” or even full recoveries through their clinics. “It’s a new diagnosis, so I always tell my patients to think of this as a journey because I’m learning along with you,” said Jai Marathe, MD, an infectious disease physician at Boston Medical Center and an assistant professor of infectious diseases at Boston University Chobanian & Avedisian School of Medicine.
“Now we have 4 years of experience, but at the same time, no two long COVID patients are alike.”
Long COVID has also changed the way physicians view healthcare and how they practice medicine.
“I am a completely different person than I used to be because of this illness, and I don’t even have it. That is how profoundly it has affected how I view the universe,” said Dr. Malcolm. “I’ve been doing this for 4 years, and I’m very hopeful. But I don’t think about this in terms of months anymore. I think about this in terms of years.”
A version of this article first appeared on Medscape.com.
The Push to Get More People Into Long COVID Studies
When Ezra Spier was diagnosed with long COVID in late 2022, his main symptom, postexertional malaise, caused fatigue so severe that it forced him to quit his job as a technology entrepreneur. Since then, it’s been a tough road for Spier, 37, who said he wouldn’t wish his hellish condition on anyone.
Last spring, he enrolled in a clinical trial of a new long COVID therapy at Stanford University, and he’s about to start another at the University of California, San Francisco.
For Spier, who lives in Oakland, California, being part of the clinical trials connected him with people dealing with similar health issues while also moving the needle toward better treatments for everyone. Yet many potential participants are unaware that these clinical trials exist. Clinical trial researchers also express frustration over the challenge of enrolling participants.
That’s why Spier created a new website to help match long COVID patients with clinical trials that can help.
“I wanted a way to make long COVID clinical trials more accessible to the general public,” he said. Spier’s website, aptly named Long Covid Studies, launched in March. The site already includes details from about 550 trials globally and, in the future, will include many more.
It’s Not the Number of Studies, It’s Navigating Them
In all, nearly 9300 long COVID trials are listed on ClinicalTrials.gov. But many patients find the site difficult to navigate, said David F. Putrino, PhD, who runs the long COVID clinic at Mount Sinai Health System in New York City. He said Spier’s website helps make trials easier for patients to manage in ways that remove the enrollment challenges.
“Ezra’s platform pulls data from ClinicalTrials.gov and puts it into a space that’s much easier for patients to manage,” said Dr. Putrino. The site only includes the most relevant information, such as the study location, eligibility, and purpose and how to sign up.
Another of Spier’s goals is to make the process easier for patients who are already marginalized and often excluded from the healthcare system. Long COVID disproportionately impacts people in minority ethnic groups and women, as well as those who are impoverished or live in rural areas.
According to the National Institutes of Health (NIH), 1 in 4 patients with severe long COVID-19 are Black or Hispanic whereas only 1 in 7 are White. Yet participation by White persons in clinical trials is much higher overall: 77% of participants are White, compared with only 14% for Black persons and 15% for Hispanic persons. Without more balanced representation, research becomes skewed and less accurate, said Grace McComsey, MD, who leads one of the 15 nationwide long COVID centers funded by the federal RECOVER (Researching COVID to Enhance Recovery) Initiative in Cleveland.
Websites that are easier for the layperson to access would allow for wider participation, said McComsey.
Too Many Barriers to Entry
A study published in the Journal of Applied Gerontology found that transportation plays an outsized role in influencing study participation, which may also lead to less diverse participation.
Decentralized trials — in which participants receive therapy at home — also make enrolling in clinical trials easier for marginalized patients and those too sick to make it to a research center, said Dr. Putrino. Research published recently in The American Journal of Medicine demonstrated that for many patients, remote studies are the future of COVID research. The study, focusing on the efficacy of Paxlovid, recruited patients living in the 48 contiguous US states. Participation was entirely remote.
“We need to have more consideration for bedbound and housebound patients in our research,” said Dr. Putrino. “Some people don’t have the ability to show up to a prestigious university to take part in an academic trial.”
Dr. Putrino and colleagues at Yale School of Medicine’s Yale COVID Recovery Study plan to release a paper in the near future on the methodology for running decentralized or remote studies that could provide guidance for researchers elsewhere.
Decentralized studies serve a larger audience, but they’re also more expensive and cost has plagued long COVID research from the start, said Michael Peluso, MD, an assistant research professor of infectious medicine at UCSF School of Medicine, University of California, San Francisco.
“You need to have a staff in place that’s trained to do home visits in order to conduct remote trials,” Dr. Peluso said, adding that his biggest challenge has been connecting patients to appropriate clinical trials.
Individual eligibility has been an ongoing issue. For example, Dr. Peluso’s current trials are testing monoclonal antibodies — antibodies produced by cloning unique white blood cells to target viral persistence, which is thought to be a cause of long COVID. Only patients who were infected with certain variants of acute COVID are eligible because of the antibodies needed to target SARS-CoV-2 spike proteins.
“This can lead to a lot of frustration among patients who might think they can participate, but aren’t eligible,” said Dr. Peluso.
Long Fight for Better Long COVID Research
For Spier, one of the hardest parts of his health issues and lack of energy is that they have sharply curtailed his social interactions with friends and colleagues.
He has channeled his energies into researching new treatments that could potentially improve his symptoms. That research is partly what drove him to create the Long Covid Studies website.
His goal is still to help others with long COVID find trials that can improve their symptoms as well. The more people who participate, the closer scientists will come to providing effective treatments for everyone, he said.
“For all my frustrations, we’re still at the forefront of science globally,” he said. “And if we have the level of funding the NIH is equipped to provide, we can show the world what’s possible with long COVID research.”
A version of this article first appeared on Medscape.com.
When Ezra Spier was diagnosed with long COVID in late 2022, his main symptom, postexertional malaise, caused fatigue so severe that it forced him to quit his job as a technology entrepreneur. Since then, it’s been a tough road for Spier, 37, who said he wouldn’t wish his hellish condition on anyone.
Last spring, he enrolled in a clinical trial of a new long COVID therapy at Stanford University, and he’s about to start another at the University of California, San Francisco.
For Spier, who lives in Oakland, California, being part of the clinical trials connected him with people dealing with similar health issues while also moving the needle toward better treatments for everyone. Yet many potential participants are unaware that these clinical trials exist. Clinical trial researchers also express frustration over the challenge of enrolling participants.
That’s why Spier created a new website to help match long COVID patients with clinical trials that can help.
“I wanted a way to make long COVID clinical trials more accessible to the general public,” he said. Spier’s website, aptly named Long Covid Studies, launched in March. The site already includes details from about 550 trials globally and, in the future, will include many more.
It’s Not the Number of Studies, It’s Navigating Them
In all, nearly 9300 long COVID trials are listed on ClinicalTrials.gov. But many patients find the site difficult to navigate, said David F. Putrino, PhD, who runs the long COVID clinic at Mount Sinai Health System in New York City. He said Spier’s website helps make trials easier for patients to manage in ways that remove the enrollment challenges.
“Ezra’s platform pulls data from ClinicalTrials.gov and puts it into a space that’s much easier for patients to manage,” said Dr. Putrino. The site only includes the most relevant information, such as the study location, eligibility, and purpose and how to sign up.
Another of Spier’s goals is to make the process easier for patients who are already marginalized and often excluded from the healthcare system. Long COVID disproportionately impacts people in minority ethnic groups and women, as well as those who are impoverished or live in rural areas.
According to the National Institutes of Health (NIH), 1 in 4 patients with severe long COVID-19 are Black or Hispanic whereas only 1 in 7 are White. Yet participation by White persons in clinical trials is much higher overall: 77% of participants are White, compared with only 14% for Black persons and 15% for Hispanic persons. Without more balanced representation, research becomes skewed and less accurate, said Grace McComsey, MD, who leads one of the 15 nationwide long COVID centers funded by the federal RECOVER (Researching COVID to Enhance Recovery) Initiative in Cleveland.
Websites that are easier for the layperson to access would allow for wider participation, said McComsey.
Too Many Barriers to Entry
A study published in the Journal of Applied Gerontology found that transportation plays an outsized role in influencing study participation, which may also lead to less diverse participation.
Decentralized trials — in which participants receive therapy at home — also make enrolling in clinical trials easier for marginalized patients and those too sick to make it to a research center, said Dr. Putrino. Research published recently in The American Journal of Medicine demonstrated that for many patients, remote studies are the future of COVID research. The study, focusing on the efficacy of Paxlovid, recruited patients living in the 48 contiguous US states. Participation was entirely remote.
“We need to have more consideration for bedbound and housebound patients in our research,” said Dr. Putrino. “Some people don’t have the ability to show up to a prestigious university to take part in an academic trial.”
Dr. Putrino and colleagues at Yale School of Medicine’s Yale COVID Recovery Study plan to release a paper in the near future on the methodology for running decentralized or remote studies that could provide guidance for researchers elsewhere.
Decentralized studies serve a larger audience, but they’re also more expensive and cost has plagued long COVID research from the start, said Michael Peluso, MD, an assistant research professor of infectious medicine at UCSF School of Medicine, University of California, San Francisco.
“You need to have a staff in place that’s trained to do home visits in order to conduct remote trials,” Dr. Peluso said, adding that his biggest challenge has been connecting patients to appropriate clinical trials.
Individual eligibility has been an ongoing issue. For example, Dr. Peluso’s current trials are testing monoclonal antibodies — antibodies produced by cloning unique white blood cells to target viral persistence, which is thought to be a cause of long COVID. Only patients who were infected with certain variants of acute COVID are eligible because of the antibodies needed to target SARS-CoV-2 spike proteins.
“This can lead to a lot of frustration among patients who might think they can participate, but aren’t eligible,” said Dr. Peluso.
Long Fight for Better Long COVID Research
For Spier, one of the hardest parts of his health issues and lack of energy is that they have sharply curtailed his social interactions with friends and colleagues.
He has channeled his energies into researching new treatments that could potentially improve his symptoms. That research is partly what drove him to create the Long Covid Studies website.
His goal is still to help others with long COVID find trials that can improve their symptoms as well. The more people who participate, the closer scientists will come to providing effective treatments for everyone, he said.
“For all my frustrations, we’re still at the forefront of science globally,” he said. “And if we have the level of funding the NIH is equipped to provide, we can show the world what’s possible with long COVID research.”
A version of this article first appeared on Medscape.com.
When Ezra Spier was diagnosed with long COVID in late 2022, his main symptom, postexertional malaise, caused fatigue so severe that it forced him to quit his job as a technology entrepreneur. Since then, it’s been a tough road for Spier, 37, who said he wouldn’t wish his hellish condition on anyone.
Last spring, he enrolled in a clinical trial of a new long COVID therapy at Stanford University, and he’s about to start another at the University of California, San Francisco.
For Spier, who lives in Oakland, California, being part of the clinical trials connected him with people dealing with similar health issues while also moving the needle toward better treatments for everyone. Yet many potential participants are unaware that these clinical trials exist. Clinical trial researchers also express frustration over the challenge of enrolling participants.
That’s why Spier created a new website to help match long COVID patients with clinical trials that can help.
“I wanted a way to make long COVID clinical trials more accessible to the general public,” he said. Spier’s website, aptly named Long Covid Studies, launched in March. The site already includes details from about 550 trials globally and, in the future, will include many more.
It’s Not the Number of Studies, It’s Navigating Them
In all, nearly 9300 long COVID trials are listed on ClinicalTrials.gov. But many patients find the site difficult to navigate, said David F. Putrino, PhD, who runs the long COVID clinic at Mount Sinai Health System in New York City. He said Spier’s website helps make trials easier for patients to manage in ways that remove the enrollment challenges.
“Ezra’s platform pulls data from ClinicalTrials.gov and puts it into a space that’s much easier for patients to manage,” said Dr. Putrino. The site only includes the most relevant information, such as the study location, eligibility, and purpose and how to sign up.
Another of Spier’s goals is to make the process easier for patients who are already marginalized and often excluded from the healthcare system. Long COVID disproportionately impacts people in minority ethnic groups and women, as well as those who are impoverished or live in rural areas.
According to the National Institutes of Health (NIH), 1 in 4 patients with severe long COVID-19 are Black or Hispanic whereas only 1 in 7 are White. Yet participation by White persons in clinical trials is much higher overall: 77% of participants are White, compared with only 14% for Black persons and 15% for Hispanic persons. Without more balanced representation, research becomes skewed and less accurate, said Grace McComsey, MD, who leads one of the 15 nationwide long COVID centers funded by the federal RECOVER (Researching COVID to Enhance Recovery) Initiative in Cleveland.
Websites that are easier for the layperson to access would allow for wider participation, said McComsey.
Too Many Barriers to Entry
A study published in the Journal of Applied Gerontology found that transportation plays an outsized role in influencing study participation, which may also lead to less diverse participation.
Decentralized trials — in which participants receive therapy at home — also make enrolling in clinical trials easier for marginalized patients and those too sick to make it to a research center, said Dr. Putrino. Research published recently in The American Journal of Medicine demonstrated that for many patients, remote studies are the future of COVID research. The study, focusing on the efficacy of Paxlovid, recruited patients living in the 48 contiguous US states. Participation was entirely remote.
“We need to have more consideration for bedbound and housebound patients in our research,” said Dr. Putrino. “Some people don’t have the ability to show up to a prestigious university to take part in an academic trial.”
Dr. Putrino and colleagues at Yale School of Medicine’s Yale COVID Recovery Study plan to release a paper in the near future on the methodology for running decentralized or remote studies that could provide guidance for researchers elsewhere.
Decentralized studies serve a larger audience, but they’re also more expensive and cost has plagued long COVID research from the start, said Michael Peluso, MD, an assistant research professor of infectious medicine at UCSF School of Medicine, University of California, San Francisco.
“You need to have a staff in place that’s trained to do home visits in order to conduct remote trials,” Dr. Peluso said, adding that his biggest challenge has been connecting patients to appropriate clinical trials.
Individual eligibility has been an ongoing issue. For example, Dr. Peluso’s current trials are testing monoclonal antibodies — antibodies produced by cloning unique white blood cells to target viral persistence, which is thought to be a cause of long COVID. Only patients who were infected with certain variants of acute COVID are eligible because of the antibodies needed to target SARS-CoV-2 spike proteins.
“This can lead to a lot of frustration among patients who might think they can participate, but aren’t eligible,” said Dr. Peluso.
Long Fight for Better Long COVID Research
For Spier, one of the hardest parts of his health issues and lack of energy is that they have sharply curtailed his social interactions with friends and colleagues.
He has channeled his energies into researching new treatments that could potentially improve his symptoms. That research is partly what drove him to create the Long Covid Studies website.
His goal is still to help others with long COVID find trials that can improve their symptoms as well. The more people who participate, the closer scientists will come to providing effective treatments for everyone, he said.
“For all my frustrations, we’re still at the forefront of science globally,” he said. “And if we have the level of funding the NIH is equipped to provide, we can show the world what’s possible with long COVID research.”
A version of this article first appeared on Medscape.com.
COVID Vaccines and New-Onset Seizures: New Data
There is no association between the SARS-CoV-2 vaccine and the risk for new-onset seizure, data from a new meta-analysis of six randomized, placebo-controlled clinical trials (RCTs) showed.
Results of the pooled analysis that included 63,500 individuals vaccinated with SARS-CoV-2 and 55,000 who received a placebo vaccine showed there was no significant difference between the two groups with respect to new-onset seizures at 28- or 43-day follow-up.
Regarding new-onset seizures in the general population, there was no statistically significant difference in risk for seizure incidence among vaccinated individuals vs placebo recipients, according to our meta-analysis, wrote the investigators, led by Ali Rafati, MD, MPH, Iran University of Medical Sciences in Tehran.
The findings were published online in JAMA Neurology.
Mixed Results
Results from previous research have been mixed regarding the link between the SARS-CoV-2 vaccination and new-onset seizures, with some showing an association.
To learn more about the possible association between the vaccines and new-onset seizures, the researchers conducted a literature review and identified six RCTs that measured adverse events following SARS-CoV-2 vaccinations (including messenger RNA, viral vector, and inactivated virus) vs placebo or other vaccines.
While five of the studies defined new-onset seizures according to the Medical Dictionary for Regulatory Activities, trial investigators in the sixth RCT assessed and determined new-onset seizures in participants.
Participants received two vaccinations 28 days apart in five RCTs and only one vaccine in the sixth trial.
The research team searched the data for new-onset seizure in the 28 days following one or both COVID vaccinations.
No Link Found
After comparing the incidence of new-onset seizure between the 63,500 vaccine (nine new-onset seizures, 0.014%) and 55,000 placebo recipients (one new-onset seizure, 0.002%), investigators found no significant difference between the two groups (odds ratio [OR], 2.70; 95% CI, 0.76-9.57; P = .12)
Investigators also sliced the data several ways to see if it would yield different results. When they analyzed data by vaccine platform (viral vector) and age group (children), they didn’t observe significant differences in new-onset data.
The researchers also searched for data beyond the month following the injection to encompass the entire blinded phase, so they analyzed the results of three RCTs that reported adverse events up to 162 days after the vaccine.
After pooling the results from the three studies, investigators found no statistical difference between the vaccine and placebo groups in terms of the new-onset seizure (OR, 2.31; 95% CI, 0.86%-3.23; P > .99)
Study limitations included the missing information on vaccine doses or risk factors for the development of seizures. Also, the RCTs included in the meta-analysis were conducted at different times, so the SARS-CoV-2 vaccines may have differed in their composition and efficacy.
“The global vaccination drive against SARS-CoV-2 has been a monumental effort in combating the pandemic. SARS-CoV-2 vaccinations that are now available appear safe and appropriate,” the authors wrote.
There were no study funding sources or disclosures reported.
A version of this article appeared on Medscape.com.
There is no association between the SARS-CoV-2 vaccine and the risk for new-onset seizure, data from a new meta-analysis of six randomized, placebo-controlled clinical trials (RCTs) showed.
Results of the pooled analysis that included 63,500 individuals vaccinated with SARS-CoV-2 and 55,000 who received a placebo vaccine showed there was no significant difference between the two groups with respect to new-onset seizures at 28- or 43-day follow-up.
Regarding new-onset seizures in the general population, there was no statistically significant difference in risk for seizure incidence among vaccinated individuals vs placebo recipients, according to our meta-analysis, wrote the investigators, led by Ali Rafati, MD, MPH, Iran University of Medical Sciences in Tehran.
The findings were published online in JAMA Neurology.
Mixed Results
Results from previous research have been mixed regarding the link between the SARS-CoV-2 vaccination and new-onset seizures, with some showing an association.
To learn more about the possible association between the vaccines and new-onset seizures, the researchers conducted a literature review and identified six RCTs that measured adverse events following SARS-CoV-2 vaccinations (including messenger RNA, viral vector, and inactivated virus) vs placebo or other vaccines.
While five of the studies defined new-onset seizures according to the Medical Dictionary for Regulatory Activities, trial investigators in the sixth RCT assessed and determined new-onset seizures in participants.
Participants received two vaccinations 28 days apart in five RCTs and only one vaccine in the sixth trial.
The research team searched the data for new-onset seizure in the 28 days following one or both COVID vaccinations.
No Link Found
After comparing the incidence of new-onset seizure between the 63,500 vaccine (nine new-onset seizures, 0.014%) and 55,000 placebo recipients (one new-onset seizure, 0.002%), investigators found no significant difference between the two groups (odds ratio [OR], 2.70; 95% CI, 0.76-9.57; P = .12)
Investigators also sliced the data several ways to see if it would yield different results. When they analyzed data by vaccine platform (viral vector) and age group (children), they didn’t observe significant differences in new-onset data.
The researchers also searched for data beyond the month following the injection to encompass the entire blinded phase, so they analyzed the results of three RCTs that reported adverse events up to 162 days after the vaccine.
After pooling the results from the three studies, investigators found no statistical difference between the vaccine and placebo groups in terms of the new-onset seizure (OR, 2.31; 95% CI, 0.86%-3.23; P > .99)
Study limitations included the missing information on vaccine doses or risk factors for the development of seizures. Also, the RCTs included in the meta-analysis were conducted at different times, so the SARS-CoV-2 vaccines may have differed in their composition and efficacy.
“The global vaccination drive against SARS-CoV-2 has been a monumental effort in combating the pandemic. SARS-CoV-2 vaccinations that are now available appear safe and appropriate,” the authors wrote.
There were no study funding sources or disclosures reported.
A version of this article appeared on Medscape.com.
There is no association between the SARS-CoV-2 vaccine and the risk for new-onset seizure, data from a new meta-analysis of six randomized, placebo-controlled clinical trials (RCTs) showed.
Results of the pooled analysis that included 63,500 individuals vaccinated with SARS-CoV-2 and 55,000 who received a placebo vaccine showed there was no significant difference between the two groups with respect to new-onset seizures at 28- or 43-day follow-up.
Regarding new-onset seizures in the general population, there was no statistically significant difference in risk for seizure incidence among vaccinated individuals vs placebo recipients, according to our meta-analysis, wrote the investigators, led by Ali Rafati, MD, MPH, Iran University of Medical Sciences in Tehran.
The findings were published online in JAMA Neurology.
Mixed Results
Results from previous research have been mixed regarding the link between the SARS-CoV-2 vaccination and new-onset seizures, with some showing an association.
To learn more about the possible association between the vaccines and new-onset seizures, the researchers conducted a literature review and identified six RCTs that measured adverse events following SARS-CoV-2 vaccinations (including messenger RNA, viral vector, and inactivated virus) vs placebo or other vaccines.
While five of the studies defined new-onset seizures according to the Medical Dictionary for Regulatory Activities, trial investigators in the sixth RCT assessed and determined new-onset seizures in participants.
Participants received two vaccinations 28 days apart in five RCTs and only one vaccine in the sixth trial.
The research team searched the data for new-onset seizure in the 28 days following one or both COVID vaccinations.
No Link Found
After comparing the incidence of new-onset seizure between the 63,500 vaccine (nine new-onset seizures, 0.014%) and 55,000 placebo recipients (one new-onset seizure, 0.002%), investigators found no significant difference between the two groups (odds ratio [OR], 2.70; 95% CI, 0.76-9.57; P = .12)
Investigators also sliced the data several ways to see if it would yield different results. When they analyzed data by vaccine platform (viral vector) and age group (children), they didn’t observe significant differences in new-onset data.
The researchers also searched for data beyond the month following the injection to encompass the entire blinded phase, so they analyzed the results of three RCTs that reported adverse events up to 162 days after the vaccine.
After pooling the results from the three studies, investigators found no statistical difference between the vaccine and placebo groups in terms of the new-onset seizure (OR, 2.31; 95% CI, 0.86%-3.23; P > .99)
Study limitations included the missing information on vaccine doses or risk factors for the development of seizures. Also, the RCTs included in the meta-analysis were conducted at different times, so the SARS-CoV-2 vaccines may have differed in their composition and efficacy.
“The global vaccination drive against SARS-CoV-2 has been a monumental effort in combating the pandemic. SARS-CoV-2 vaccinations that are now available appear safe and appropriate,” the authors wrote.
There were no study funding sources or disclosures reported.
A version of this article appeared on Medscape.com.
Federal Trade Commission Bans Noncompete Agreements, Urges More Protections for Healthcare Workers
But business groups have vowed to challenge the decision in court.
The proposed final rule passed on a 3-2 vote, with the dissenting commissioners disputing the FTC’s authority to broadly ban noncompetes.
Tensions around noncompetes have been building for years. In 2021, President Biden issued an executive order supporting measures to improve economic competition, in which he urged the FTC to consider its rulemaking authority to address noncompete clauses that unfairly limit workers’ mobility. In January 2023, per that directive, the agency proposed ending the restrictive covenants.
While the FTC estimates that the final rule will reduce healthcare costs by up to $194 billion over the next decade and increase worker earnings by $300 million annually, the ruling faces legal hurdles.
US Chamber of Commerce president and CEO Suzanne P. Clark said in a statement that the move is a “blatant power grab” that will undermine competitive business practices, adding that the Chamber will sue to block the measure.
The FTC received more than 26,000 comments on noncompetes during the public feedback period, with about 25,000 supporting the measure, said Benjamin Cady, JD, an FTC attorney.
Mr. Cady called the feedback “compelling,” citing instances of workers who were forced to commute long distances, uproot their families, or risk expensive litigation for wanting to pursue job opportunities.
For example, a comment from a physician working in Appalachia highlights the potential real-life implications of the agreements. “With hospital systems merging, providers with aggressive noncompetes must abandon the community that they serve if they [choose] to leave their employer. Healthcare providers feel trapped in their current employment situation, leading to significant burnout that can shorten their [career] longevity.”
Commissioner Alvaro Bedoya said physicians have had their lives upended by cumbersome noncompetes, often having to move out of state to practice. “A pandemic killed a million people in this country, and there are doctors who cannot work because of a noncompete,” he said.
It’s unclear whether physicians and others who work for nonprofit healthcare groups or hospitals will be covered by the new ban. FTC Commissioner Rebecca Slaughter acknowledged that the agency’s jurisdictional limitations mean that employees of “certain nonprofit organizations” may not benefit from the rule.
“We want to be transparent about the limitation and recognize there are workers, especially healthcare workers, who are bound by anticompetitive and unfair noncompete clauses, that our rule will struggle to reach,” she said. To cover nonprofit healthcare employees, Ms. Slaughter urged Congress to pass legislation banning noncompetes, such as the Workforce Mobility Act of 2021 and the Freedom to Compete Act of 2023.
The FTC final rule will take effect 120 days after it is published in the federal register, and new noncompete agreements will be banned as of this date. However, existing contracts for senior executives will remain in effect because these individuals are less likely to experience “acute harm” due to their ability to negotiate accordingly, said Mr. Cady.
States, AMA Take Aim at Noncompetes
Before the federal ban, several states had already passed legislation limiting the reach of noncompetes. According to a recent article in the Journal of the American College of Cardiology, 12 states prohibit noncompete clauses for physicians: Alabama, California, Colorado, Delaware, Massachusetts, Montana, New Hampshire, New Mexico, North Dakota, Oklahoma, Rhode Island, and South Dakota.
The remaining states allow noncompetes in some form, often excluding them for employees earning below a certain threshold. For example, in Oregon, noncompete agreements may apply to employees earning more than $113,241. Most states have provisions to adjust the threshold annually. The District of Columbia permits 2-year noncompetes for “medical specialists” earning over $250,000 annually.
Indiana employers can no longer enter into noncompete agreements with primary care providers. Other specialties may be subject to the clauses, except when the physician terminates the contract for cause or when an employer terminates the contract without cause.
Rachel Marcus, MD, a cardiologist in Washington, DC, found out how limiting her employment contract’s noncompete clause was when she wanted to leave a former position. Due to the restrictions, she told this news organization that she couldn’t work locally for a competitor for 2 years. The closest location she could seek employment without violating the agreement was Baltimore, approximately 40 miles away.
Dr. Marcus ultimately moved to another position within the same organization because of the company’s reputation for being “aggressive” in their enforcement actions.
Although the American Medical Association (AMA) does not support a total ban, its House of Delegates adopted policies last year to support the prohibition of noncompete contracts for physicians employed by for-profit or nonprofit hospitals, hospital systems, or staffing companies.
Challenges Await
The American Hospital Association, which opposed the proposed rule, called it “bad policy.” The decision “will likely be short-lived, with courts almost certain to stop it before it can do damage to hospitals’ ability to care for their patients and communities,” the association said in a statement.
To ease the transition to the new rule, the FTC also released a model language for employers to use when discussing the changes with their employees. “All employers need to do to comply with the rule is to stop enforcing existing noncompetes with workers other than senior executives and provide notice to such workers,” he said.
Dr. Marcus hopes the ban improves doctors’ lives. “Your employer is going to have to treat you better because they know that you can easily go across town to a place that has a higher salary, and your patient can go with you.”
A version of this article appeared on Medscape.com.
But business groups have vowed to challenge the decision in court.
The proposed final rule passed on a 3-2 vote, with the dissenting commissioners disputing the FTC’s authority to broadly ban noncompetes.
Tensions around noncompetes have been building for years. In 2021, President Biden issued an executive order supporting measures to improve economic competition, in which he urged the FTC to consider its rulemaking authority to address noncompete clauses that unfairly limit workers’ mobility. In January 2023, per that directive, the agency proposed ending the restrictive covenants.
While the FTC estimates that the final rule will reduce healthcare costs by up to $194 billion over the next decade and increase worker earnings by $300 million annually, the ruling faces legal hurdles.
US Chamber of Commerce president and CEO Suzanne P. Clark said in a statement that the move is a “blatant power grab” that will undermine competitive business practices, adding that the Chamber will sue to block the measure.
The FTC received more than 26,000 comments on noncompetes during the public feedback period, with about 25,000 supporting the measure, said Benjamin Cady, JD, an FTC attorney.
Mr. Cady called the feedback “compelling,” citing instances of workers who were forced to commute long distances, uproot their families, or risk expensive litigation for wanting to pursue job opportunities.
For example, a comment from a physician working in Appalachia highlights the potential real-life implications of the agreements. “With hospital systems merging, providers with aggressive noncompetes must abandon the community that they serve if they [choose] to leave their employer. Healthcare providers feel trapped in their current employment situation, leading to significant burnout that can shorten their [career] longevity.”
Commissioner Alvaro Bedoya said physicians have had their lives upended by cumbersome noncompetes, often having to move out of state to practice. “A pandemic killed a million people in this country, and there are doctors who cannot work because of a noncompete,” he said.
It’s unclear whether physicians and others who work for nonprofit healthcare groups or hospitals will be covered by the new ban. FTC Commissioner Rebecca Slaughter acknowledged that the agency’s jurisdictional limitations mean that employees of “certain nonprofit organizations” may not benefit from the rule.
“We want to be transparent about the limitation and recognize there are workers, especially healthcare workers, who are bound by anticompetitive and unfair noncompete clauses, that our rule will struggle to reach,” she said. To cover nonprofit healthcare employees, Ms. Slaughter urged Congress to pass legislation banning noncompetes, such as the Workforce Mobility Act of 2021 and the Freedom to Compete Act of 2023.
The FTC final rule will take effect 120 days after it is published in the federal register, and new noncompete agreements will be banned as of this date. However, existing contracts for senior executives will remain in effect because these individuals are less likely to experience “acute harm” due to their ability to negotiate accordingly, said Mr. Cady.
States, AMA Take Aim at Noncompetes
Before the federal ban, several states had already passed legislation limiting the reach of noncompetes. According to a recent article in the Journal of the American College of Cardiology, 12 states prohibit noncompete clauses for physicians: Alabama, California, Colorado, Delaware, Massachusetts, Montana, New Hampshire, New Mexico, North Dakota, Oklahoma, Rhode Island, and South Dakota.
The remaining states allow noncompetes in some form, often excluding them for employees earning below a certain threshold. For example, in Oregon, noncompete agreements may apply to employees earning more than $113,241. Most states have provisions to adjust the threshold annually. The District of Columbia permits 2-year noncompetes for “medical specialists” earning over $250,000 annually.
Indiana employers can no longer enter into noncompete agreements with primary care providers. Other specialties may be subject to the clauses, except when the physician terminates the contract for cause or when an employer terminates the contract without cause.
Rachel Marcus, MD, a cardiologist in Washington, DC, found out how limiting her employment contract’s noncompete clause was when she wanted to leave a former position. Due to the restrictions, she told this news organization that she couldn’t work locally for a competitor for 2 years. The closest location she could seek employment without violating the agreement was Baltimore, approximately 40 miles away.
Dr. Marcus ultimately moved to another position within the same organization because of the company’s reputation for being “aggressive” in their enforcement actions.
Although the American Medical Association (AMA) does not support a total ban, its House of Delegates adopted policies last year to support the prohibition of noncompete contracts for physicians employed by for-profit or nonprofit hospitals, hospital systems, or staffing companies.
Challenges Await
The American Hospital Association, which opposed the proposed rule, called it “bad policy.” The decision “will likely be short-lived, with courts almost certain to stop it before it can do damage to hospitals’ ability to care for their patients and communities,” the association said in a statement.
To ease the transition to the new rule, the FTC also released a model language for employers to use when discussing the changes with their employees. “All employers need to do to comply with the rule is to stop enforcing existing noncompetes with workers other than senior executives and provide notice to such workers,” he said.
Dr. Marcus hopes the ban improves doctors’ lives. “Your employer is going to have to treat you better because they know that you can easily go across town to a place that has a higher salary, and your patient can go with you.”
A version of this article appeared on Medscape.com.
But business groups have vowed to challenge the decision in court.
The proposed final rule passed on a 3-2 vote, with the dissenting commissioners disputing the FTC’s authority to broadly ban noncompetes.
Tensions around noncompetes have been building for years. In 2021, President Biden issued an executive order supporting measures to improve economic competition, in which he urged the FTC to consider its rulemaking authority to address noncompete clauses that unfairly limit workers’ mobility. In January 2023, per that directive, the agency proposed ending the restrictive covenants.
While the FTC estimates that the final rule will reduce healthcare costs by up to $194 billion over the next decade and increase worker earnings by $300 million annually, the ruling faces legal hurdles.
US Chamber of Commerce president and CEO Suzanne P. Clark said in a statement that the move is a “blatant power grab” that will undermine competitive business practices, adding that the Chamber will sue to block the measure.
The FTC received more than 26,000 comments on noncompetes during the public feedback period, with about 25,000 supporting the measure, said Benjamin Cady, JD, an FTC attorney.
Mr. Cady called the feedback “compelling,” citing instances of workers who were forced to commute long distances, uproot their families, or risk expensive litigation for wanting to pursue job opportunities.
For example, a comment from a physician working in Appalachia highlights the potential real-life implications of the agreements. “With hospital systems merging, providers with aggressive noncompetes must abandon the community that they serve if they [choose] to leave their employer. Healthcare providers feel trapped in their current employment situation, leading to significant burnout that can shorten their [career] longevity.”
Commissioner Alvaro Bedoya said physicians have had their lives upended by cumbersome noncompetes, often having to move out of state to practice. “A pandemic killed a million people in this country, and there are doctors who cannot work because of a noncompete,” he said.
It’s unclear whether physicians and others who work for nonprofit healthcare groups or hospitals will be covered by the new ban. FTC Commissioner Rebecca Slaughter acknowledged that the agency’s jurisdictional limitations mean that employees of “certain nonprofit organizations” may not benefit from the rule.
“We want to be transparent about the limitation and recognize there are workers, especially healthcare workers, who are bound by anticompetitive and unfair noncompete clauses, that our rule will struggle to reach,” she said. To cover nonprofit healthcare employees, Ms. Slaughter urged Congress to pass legislation banning noncompetes, such as the Workforce Mobility Act of 2021 and the Freedom to Compete Act of 2023.
The FTC final rule will take effect 120 days after it is published in the federal register, and new noncompete agreements will be banned as of this date. However, existing contracts for senior executives will remain in effect because these individuals are less likely to experience “acute harm” due to their ability to negotiate accordingly, said Mr. Cady.
States, AMA Take Aim at Noncompetes
Before the federal ban, several states had already passed legislation limiting the reach of noncompetes. According to a recent article in the Journal of the American College of Cardiology, 12 states prohibit noncompete clauses for physicians: Alabama, California, Colorado, Delaware, Massachusetts, Montana, New Hampshire, New Mexico, North Dakota, Oklahoma, Rhode Island, and South Dakota.
The remaining states allow noncompetes in some form, often excluding them for employees earning below a certain threshold. For example, in Oregon, noncompete agreements may apply to employees earning more than $113,241. Most states have provisions to adjust the threshold annually. The District of Columbia permits 2-year noncompetes for “medical specialists” earning over $250,000 annually.
Indiana employers can no longer enter into noncompete agreements with primary care providers. Other specialties may be subject to the clauses, except when the physician terminates the contract for cause or when an employer terminates the contract without cause.
Rachel Marcus, MD, a cardiologist in Washington, DC, found out how limiting her employment contract’s noncompete clause was when she wanted to leave a former position. Due to the restrictions, she told this news organization that she couldn’t work locally for a competitor for 2 years. The closest location she could seek employment without violating the agreement was Baltimore, approximately 40 miles away.
Dr. Marcus ultimately moved to another position within the same organization because of the company’s reputation for being “aggressive” in their enforcement actions.
Although the American Medical Association (AMA) does not support a total ban, its House of Delegates adopted policies last year to support the prohibition of noncompete contracts for physicians employed by for-profit or nonprofit hospitals, hospital systems, or staffing companies.
Challenges Await
The American Hospital Association, which opposed the proposed rule, called it “bad policy.” The decision “will likely be short-lived, with courts almost certain to stop it before it can do damage to hospitals’ ability to care for their patients and communities,” the association said in a statement.
To ease the transition to the new rule, the FTC also released a model language for employers to use when discussing the changes with their employees. “All employers need to do to comply with the rule is to stop enforcing existing noncompetes with workers other than senior executives and provide notice to such workers,” he said.
Dr. Marcus hopes the ban improves doctors’ lives. “Your employer is going to have to treat you better because they know that you can easily go across town to a place that has a higher salary, and your patient can go with you.”
A version of this article appeared on Medscape.com.
COVID Vaccinations Less Prevalent in Marginalized Patients
Primary care physicians who served marginalized communities had the highest proportion of patients who were unvaccinated against COVID-19, Canadian data suggested.
A study of more than 9000 family physicians in Ontario also found that the physicians with the largest proportion of unvaccinated patients were more likely to be male, to have trained outside Canada, to be older, and to work in an enhanced fee-for-service model than their counterparts who had lower proportions of unvaccinated patients.
“The family physicians with the most unvaccinated patients were also more likely to be solo practitioners and less likely to practice in team-based models, meaning they may have fewer support staff in their clinics,” lead author Jennifer Shuldiner, PhD, a scientist at Women’s College Hospital in Toronto, Ontario, Canada, told this news organization.
The findings were published in CMAJ.
Need vs Resources
Dr. Shuldiner and her team had been working on a project to provide additional support to family physicians with large numbers of patients who had not received their COVID-19 vaccinations. Their goal was to encourage family physicians to support these patients in getting vaccinated.
“As we were designing this project, we wondered how these physicians and their patients might differ. What characteristics might they have that would enable us to design and implement an intervention with high uptake and impact?” she said.
The researchers conducted a cross-sectional, population-based cohort study using linked administrative datasets in Ontario. They calculated the percentage of patients unvaccinated against SARS-CoV-2 who were enrolled with each comprehensive care family physician, ranked physicians according to the proportion of unvaccinated patients, and identified 906 physicians in the top 10% of unvaccinated patients. These physicians were compared with the remaining 90% of family physicians.
The physicians with the highest proportion of unvaccinated patients cared for 259,130 unvaccinated patients as of November 1, 2021. The proportion of patients who received two or more doses of the SARS-CoV-2 vaccine in this group was 74.2%. In comparison, the proportion of patients who received two or more doses of the vaccine was 87.0% in the remaining 90% of physicians.
Physicians with the largest proportion of unvaccinated patients were more likely to be male (64.6% vs 48.1%), to have trained outside Canada (46.9% vs 29.3%), to be older (mean age, 56 years vs 49 years), and to work in an enhanced fee-for-service model (49% vs 28%).
The study also found that patients enrolled with physicians in the most unvaccinated group tended to live in places with more ethnic diversity, higher material deprivation, and lower incomes. The proportion of recent immigrants was higher in this group.
“Clinics or practices with a large number of unvaccinated patients could be viable targets for efforts to coordinate public health and primary care,” said Dr. Shuldiner.
The findings indicate “the ongoing inverse relationship between the need for care and its accessibility and utilization. In other words, the practices with the highest need receive the fewest resources,” she noted.
“We know that relationships with trusted family physicians can positively influence patients’ decisions. Our study highlights the need to create equitable systems and processes that create opportunities for primary care teams to play a crucial role in influencing general and COVID-19-specific vaccine-related decision-making.”
Helping Primary Care Physicians
Commenting on the study for this news organization, Sabrina Wong, RN, PhD, professor of nursing at the University of British Columbia in Vancouver, British Columbia, Canada, said, “They did quite a nice analysis to show this using administrative data, and I think the information they’ve uncovered will be helpful in trying to fill the gaps and provide these practitioners with more support.”
Dr. Wong did not participate in the study. “The information they provide will be useful in helping us to move forward working with underserved, underresourced communities and also hopefully provide the clinicians, family physicians, and nurse practitioners working in these areas with more resources,” she said.
“The authors also point out that there needs to be more collaboration between public health and primary care to support these communities in their efforts to get the vaccines to the people in these communities who need them.”
The study was supported by a Canadian Institutes of Health Research grant. Dr. Shuldiner and Dr. Wong reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Primary care physicians who served marginalized communities had the highest proportion of patients who were unvaccinated against COVID-19, Canadian data suggested.
A study of more than 9000 family physicians in Ontario also found that the physicians with the largest proportion of unvaccinated patients were more likely to be male, to have trained outside Canada, to be older, and to work in an enhanced fee-for-service model than their counterparts who had lower proportions of unvaccinated patients.
“The family physicians with the most unvaccinated patients were also more likely to be solo practitioners and less likely to practice in team-based models, meaning they may have fewer support staff in their clinics,” lead author Jennifer Shuldiner, PhD, a scientist at Women’s College Hospital in Toronto, Ontario, Canada, told this news organization.
The findings were published in CMAJ.
Need vs Resources
Dr. Shuldiner and her team had been working on a project to provide additional support to family physicians with large numbers of patients who had not received their COVID-19 vaccinations. Their goal was to encourage family physicians to support these patients in getting vaccinated.
“As we were designing this project, we wondered how these physicians and their patients might differ. What characteristics might they have that would enable us to design and implement an intervention with high uptake and impact?” she said.
The researchers conducted a cross-sectional, population-based cohort study using linked administrative datasets in Ontario. They calculated the percentage of patients unvaccinated against SARS-CoV-2 who were enrolled with each comprehensive care family physician, ranked physicians according to the proportion of unvaccinated patients, and identified 906 physicians in the top 10% of unvaccinated patients. These physicians were compared with the remaining 90% of family physicians.
The physicians with the highest proportion of unvaccinated patients cared for 259,130 unvaccinated patients as of November 1, 2021. The proportion of patients who received two or more doses of the SARS-CoV-2 vaccine in this group was 74.2%. In comparison, the proportion of patients who received two or more doses of the vaccine was 87.0% in the remaining 90% of physicians.
Physicians with the largest proportion of unvaccinated patients were more likely to be male (64.6% vs 48.1%), to have trained outside Canada (46.9% vs 29.3%), to be older (mean age, 56 years vs 49 years), and to work in an enhanced fee-for-service model (49% vs 28%).
The study also found that patients enrolled with physicians in the most unvaccinated group tended to live in places with more ethnic diversity, higher material deprivation, and lower incomes. The proportion of recent immigrants was higher in this group.
“Clinics or practices with a large number of unvaccinated patients could be viable targets for efforts to coordinate public health and primary care,” said Dr. Shuldiner.
The findings indicate “the ongoing inverse relationship between the need for care and its accessibility and utilization. In other words, the practices with the highest need receive the fewest resources,” she noted.
“We know that relationships with trusted family physicians can positively influence patients’ decisions. Our study highlights the need to create equitable systems and processes that create opportunities for primary care teams to play a crucial role in influencing general and COVID-19-specific vaccine-related decision-making.”
Helping Primary Care Physicians
Commenting on the study for this news organization, Sabrina Wong, RN, PhD, professor of nursing at the University of British Columbia in Vancouver, British Columbia, Canada, said, “They did quite a nice analysis to show this using administrative data, and I think the information they’ve uncovered will be helpful in trying to fill the gaps and provide these practitioners with more support.”
Dr. Wong did not participate in the study. “The information they provide will be useful in helping us to move forward working with underserved, underresourced communities and also hopefully provide the clinicians, family physicians, and nurse practitioners working in these areas with more resources,” she said.
“The authors also point out that there needs to be more collaboration between public health and primary care to support these communities in their efforts to get the vaccines to the people in these communities who need them.”
The study was supported by a Canadian Institutes of Health Research grant. Dr. Shuldiner and Dr. Wong reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Primary care physicians who served marginalized communities had the highest proportion of patients who were unvaccinated against COVID-19, Canadian data suggested.
A study of more than 9000 family physicians in Ontario also found that the physicians with the largest proportion of unvaccinated patients were more likely to be male, to have trained outside Canada, to be older, and to work in an enhanced fee-for-service model than their counterparts who had lower proportions of unvaccinated patients.
“The family physicians with the most unvaccinated patients were also more likely to be solo practitioners and less likely to practice in team-based models, meaning they may have fewer support staff in their clinics,” lead author Jennifer Shuldiner, PhD, a scientist at Women’s College Hospital in Toronto, Ontario, Canada, told this news organization.
The findings were published in CMAJ.
Need vs Resources
Dr. Shuldiner and her team had been working on a project to provide additional support to family physicians with large numbers of patients who had not received their COVID-19 vaccinations. Their goal was to encourage family physicians to support these patients in getting vaccinated.
“As we were designing this project, we wondered how these physicians and their patients might differ. What characteristics might they have that would enable us to design and implement an intervention with high uptake and impact?” she said.
The researchers conducted a cross-sectional, population-based cohort study using linked administrative datasets in Ontario. They calculated the percentage of patients unvaccinated against SARS-CoV-2 who were enrolled with each comprehensive care family physician, ranked physicians according to the proportion of unvaccinated patients, and identified 906 physicians in the top 10% of unvaccinated patients. These physicians were compared with the remaining 90% of family physicians.
The physicians with the highest proportion of unvaccinated patients cared for 259,130 unvaccinated patients as of November 1, 2021. The proportion of patients who received two or more doses of the SARS-CoV-2 vaccine in this group was 74.2%. In comparison, the proportion of patients who received two or more doses of the vaccine was 87.0% in the remaining 90% of physicians.
Physicians with the largest proportion of unvaccinated patients were more likely to be male (64.6% vs 48.1%), to have trained outside Canada (46.9% vs 29.3%), to be older (mean age, 56 years vs 49 years), and to work in an enhanced fee-for-service model (49% vs 28%).
The study also found that patients enrolled with physicians in the most unvaccinated group tended to live in places with more ethnic diversity, higher material deprivation, and lower incomes. The proportion of recent immigrants was higher in this group.
“Clinics or practices with a large number of unvaccinated patients could be viable targets for efforts to coordinate public health and primary care,” said Dr. Shuldiner.
The findings indicate “the ongoing inverse relationship between the need for care and its accessibility and utilization. In other words, the practices with the highest need receive the fewest resources,” she noted.
“We know that relationships with trusted family physicians can positively influence patients’ decisions. Our study highlights the need to create equitable systems and processes that create opportunities for primary care teams to play a crucial role in influencing general and COVID-19-specific vaccine-related decision-making.”
Helping Primary Care Physicians
Commenting on the study for this news organization, Sabrina Wong, RN, PhD, professor of nursing at the University of British Columbia in Vancouver, British Columbia, Canada, said, “They did quite a nice analysis to show this using administrative data, and I think the information they’ve uncovered will be helpful in trying to fill the gaps and provide these practitioners with more support.”
Dr. Wong did not participate in the study. “The information they provide will be useful in helping us to move forward working with underserved, underresourced communities and also hopefully provide the clinicians, family physicians, and nurse practitioners working in these areas with more resources,” she said.
“The authors also point out that there needs to be more collaboration between public health and primary care to support these communities in their efforts to get the vaccines to the people in these communities who need them.”
The study was supported by a Canadian Institutes of Health Research grant. Dr. Shuldiner and Dr. Wong reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CMAJ