User login
Colorectal Cancer: New Primary Care Method Predicts Onset Within Next 2 Years
TOPLINE:
Up to 16% of primary care patients are non-compliant with FIT, which is the gold standard for predicting CRC.
METHODOLOGY:
- This study was retrospective cohort of 50,387 UK Biobank participants reporting a CRC symptom in primary care at age ≥ 40 years.
- The novel method, called an integrated risk model, used a combination of a polygenic risk score from genetic testing, symptoms, and patient characteristics to identify patients likely to develop CRC in the next 2 years.
- The primary outcome was the risk model’s performance in classifying a CRC case according to a statistical metric, the receiver operating characteristic area under the curve. Values range from 0 to 1, where 1 indicates perfect discriminative power and 0.5 indicates no discriminative power.
TAKEAWAY:
- The cohort of 50,387 participants was found to have 438 cases of CRC and 49,949 controls without CRC within 2 years of symptom reporting. CRC cases were diagnosed by hospital records, cancer registries, or death records.
- Increased risk of a CRC diagnosis was identified by a combination of six variables: older age at index date of symptom, higher polygenic risk score, which included 201 variants, male sex, previous smoking, rectal bleeding, and change in bowel habit.
- The polygenic risk score alone had good ability to distinguish cases from controls because 1.45% of participants in the highest quintile and 0.42% in the lowest quintile were later diagnosed with CRC.
- The variables were used to calculate an integrated risk model, which estimated the cross-sectional risk (in 80% of the final cohort) of a subsequent CRC diagnosis within 2 years. The highest scoring integrated risk model in this study was found to have a receiver operating characteristic area under the curve value of 0.76 with a 95% CI of 0.71-0.81. (A value of this magnitude indicates moderate discriminative ability to distinguish cases from controls because it falls between 0.7 and 0.8. A higher value [above 0.8] is considered strong and a lower value [< 0.7] is considered weak.)
IN PRACTICE:
The authors concluded, “The [integrated risk model] developed in this study predicts, with good accuracy, which patients presenting with CRC symptoms in a primary care setting are likely to be diagnosed with CRC within the next 2 years.”
The integrated risk model has “potential to be immediately actionable in the clinical setting … [by] inform[ing] patient triage, improving early diagnostic rates and health outcomes and reducing pressure on diagnostic secondary care services.”
SOURCE:
The corresponding author is Harry D. Green of the University of Exeter, England. The study (2024 Aug 1. doi: 10.1038/s41431-024-01654-3) appeared in the European Journal of Human Genetics.
LIMITATIONS:
Limitations included an observational design and the inability of the integrated risk model to outperform FIT, which has a receiver operating characteristic area under the curve of 0.95.
DISCLOSURES:
None of the authors reported competing interests. The funding sources included the National Institute for Health and Care Research and others.
A version of this article first appeared on Medscape.com.
TOPLINE:
Up to 16% of primary care patients are non-compliant with FIT, which is the gold standard for predicting CRC.
METHODOLOGY:
- This study was retrospective cohort of 50,387 UK Biobank participants reporting a CRC symptom in primary care at age ≥ 40 years.
- The novel method, called an integrated risk model, used a combination of a polygenic risk score from genetic testing, symptoms, and patient characteristics to identify patients likely to develop CRC in the next 2 years.
- The primary outcome was the risk model’s performance in classifying a CRC case according to a statistical metric, the receiver operating characteristic area under the curve. Values range from 0 to 1, where 1 indicates perfect discriminative power and 0.5 indicates no discriminative power.
TAKEAWAY:
- The cohort of 50,387 participants was found to have 438 cases of CRC and 49,949 controls without CRC within 2 years of symptom reporting. CRC cases were diagnosed by hospital records, cancer registries, or death records.
- Increased risk of a CRC diagnosis was identified by a combination of six variables: older age at index date of symptom, higher polygenic risk score, which included 201 variants, male sex, previous smoking, rectal bleeding, and change in bowel habit.
- The polygenic risk score alone had good ability to distinguish cases from controls because 1.45% of participants in the highest quintile and 0.42% in the lowest quintile were later diagnosed with CRC.
- The variables were used to calculate an integrated risk model, which estimated the cross-sectional risk (in 80% of the final cohort) of a subsequent CRC diagnosis within 2 years. The highest scoring integrated risk model in this study was found to have a receiver operating characteristic area under the curve value of 0.76 with a 95% CI of 0.71-0.81. (A value of this magnitude indicates moderate discriminative ability to distinguish cases from controls because it falls between 0.7 and 0.8. A higher value [above 0.8] is considered strong and a lower value [< 0.7] is considered weak.)
IN PRACTICE:
The authors concluded, “The [integrated risk model] developed in this study predicts, with good accuracy, which patients presenting with CRC symptoms in a primary care setting are likely to be diagnosed with CRC within the next 2 years.”
The integrated risk model has “potential to be immediately actionable in the clinical setting … [by] inform[ing] patient triage, improving early diagnostic rates and health outcomes and reducing pressure on diagnostic secondary care services.”
SOURCE:
The corresponding author is Harry D. Green of the University of Exeter, England. The study (2024 Aug 1. doi: 10.1038/s41431-024-01654-3) appeared in the European Journal of Human Genetics.
LIMITATIONS:
Limitations included an observational design and the inability of the integrated risk model to outperform FIT, which has a receiver operating characteristic area under the curve of 0.95.
DISCLOSURES:
None of the authors reported competing interests. The funding sources included the National Institute for Health and Care Research and others.
A version of this article first appeared on Medscape.com.
TOPLINE:
Up to 16% of primary care patients are non-compliant with FIT, which is the gold standard for predicting CRC.
METHODOLOGY:
- This study was retrospective cohort of 50,387 UK Biobank participants reporting a CRC symptom in primary care at age ≥ 40 years.
- The novel method, called an integrated risk model, used a combination of a polygenic risk score from genetic testing, symptoms, and patient characteristics to identify patients likely to develop CRC in the next 2 years.
- The primary outcome was the risk model’s performance in classifying a CRC case according to a statistical metric, the receiver operating characteristic area under the curve. Values range from 0 to 1, where 1 indicates perfect discriminative power and 0.5 indicates no discriminative power.
TAKEAWAY:
- The cohort of 50,387 participants was found to have 438 cases of CRC and 49,949 controls without CRC within 2 years of symptom reporting. CRC cases were diagnosed by hospital records, cancer registries, or death records.
- Increased risk of a CRC diagnosis was identified by a combination of six variables: older age at index date of symptom, higher polygenic risk score, which included 201 variants, male sex, previous smoking, rectal bleeding, and change in bowel habit.
- The polygenic risk score alone had good ability to distinguish cases from controls because 1.45% of participants in the highest quintile and 0.42% in the lowest quintile were later diagnosed with CRC.
- The variables were used to calculate an integrated risk model, which estimated the cross-sectional risk (in 80% of the final cohort) of a subsequent CRC diagnosis within 2 years. The highest scoring integrated risk model in this study was found to have a receiver operating characteristic area under the curve value of 0.76 with a 95% CI of 0.71-0.81. (A value of this magnitude indicates moderate discriminative ability to distinguish cases from controls because it falls between 0.7 and 0.8. A higher value [above 0.8] is considered strong and a lower value [< 0.7] is considered weak.)
IN PRACTICE:
The authors concluded, “The [integrated risk model] developed in this study predicts, with good accuracy, which patients presenting with CRC symptoms in a primary care setting are likely to be diagnosed with CRC within the next 2 years.”
The integrated risk model has “potential to be immediately actionable in the clinical setting … [by] inform[ing] patient triage, improving early diagnostic rates and health outcomes and reducing pressure on diagnostic secondary care services.”
SOURCE:
The corresponding author is Harry D. Green of the University of Exeter, England. The study (2024 Aug 1. doi: 10.1038/s41431-024-01654-3) appeared in the European Journal of Human Genetics.
LIMITATIONS:
Limitations included an observational design and the inability of the integrated risk model to outperform FIT, which has a receiver operating characteristic area under the curve of 0.95.
DISCLOSURES:
None of the authors reported competing interests. The funding sources included the National Institute for Health and Care Research and others.
A version of this article first appeared on Medscape.com.
Is Buprenorphine/Naloxone Safer Than Buprenorphine Alone During Pregnancy?
TOPLINE:
Buprenorphine combined with naloxone during pregnancy is associated with lower risks for neonatal abstinence syndrome and neonatal intensive care unit admission than buprenorphine alone. The study also found no significant differences in major congenital malformations between the two treatments.
METHODOLOGY:
- Researchers conducted a population-based cohort study using healthcare utilization data of people who were insured by Medicaid between 2000 and 2018.
- A total of 8695 pregnant individuals were included, with 3369 exposed to buprenorphine/naloxone and 5326 exposed to buprenorphine alone during the first trimester.
- Outcome measures included major congenital malformations, low birth weight, neonatal abstinence syndrome, neonatal intensive care unit admission, preterm birth, respiratory symptoms, small for gestational age, cesarean delivery, and maternal morbidity.
- The study excluded pregnancies with chromosomal anomalies, first-trimester exposure to known teratogens, or methadone use during baseline or the first trimester.
TAKEAWAY:
- According to the authors, buprenorphine/naloxone exposure during pregnancy was associated with a lower risk for neonatal abstinence syndrome (weighted risk ratio [RR], 0.77; 95% CI, 0.70-0.84) than buprenorphine alone.
- The researchers found a modestly lower risk for neonatal intensive care unit admission (weighted RR, 0.91; 95% CI, 0.85-0.98) and small risk for gestational age (weighted RR, 0.86; 95% CI, 0.75-0.98) in the buprenorphine/naloxone group.
- No significant differences were observed between the two groups in major congenital malformations, low birth weight, preterm birth, respiratory symptoms, or cesarean delivery.
IN PRACTICE:
“For the outcomes assessed, compared with buprenorphine alone, buprenorphine combined with naloxone during pregnancy appears to be a safe treatment option. This supports the view that both formulations are reasonable options for treatment of OUD in pregnancy, affirming flexibility in collaborative treatment decision-making,” the study authors wrote.
SOURCE:
The study was led by Loreen Straub, MD, MS, of the Division of Pharmacoepidemiology and Pharmacoeconomics at Brigham and Women’s Hospital and Harvard Medical School in Boston. It was published online in JAMA.
LIMITATIONS:
Some potential confounders, such as alcohol use and cigarette smoking, may not have been recorded in claims data. The findings for many of the neonatal and maternal outcomes suggest that confounding by unmeasured factors is an unlikely explanation for the associations observed. Individuals identified as exposed based on filled prescriptions might not have used the medication. The study used outcome algorithms with relatively high positive predictive values to minimize outcome misclassification. The cohort was restricted to live births to enable linkage to infants and to assess neonatal outcomes.
DISCLOSURES:
Various authors reported receiving grants and personal fees from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute on Drug Abuse, Roche, Moderna, Takeda, and Janssen Global, among others.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Buprenorphine combined with naloxone during pregnancy is associated with lower risks for neonatal abstinence syndrome and neonatal intensive care unit admission than buprenorphine alone. The study also found no significant differences in major congenital malformations between the two treatments.
METHODOLOGY:
- Researchers conducted a population-based cohort study using healthcare utilization data of people who were insured by Medicaid between 2000 and 2018.
- A total of 8695 pregnant individuals were included, with 3369 exposed to buprenorphine/naloxone and 5326 exposed to buprenorphine alone during the first trimester.
- Outcome measures included major congenital malformations, low birth weight, neonatal abstinence syndrome, neonatal intensive care unit admission, preterm birth, respiratory symptoms, small for gestational age, cesarean delivery, and maternal morbidity.
- The study excluded pregnancies with chromosomal anomalies, first-trimester exposure to known teratogens, or methadone use during baseline or the first trimester.
TAKEAWAY:
- According to the authors, buprenorphine/naloxone exposure during pregnancy was associated with a lower risk for neonatal abstinence syndrome (weighted risk ratio [RR], 0.77; 95% CI, 0.70-0.84) than buprenorphine alone.
- The researchers found a modestly lower risk for neonatal intensive care unit admission (weighted RR, 0.91; 95% CI, 0.85-0.98) and small risk for gestational age (weighted RR, 0.86; 95% CI, 0.75-0.98) in the buprenorphine/naloxone group.
- No significant differences were observed between the two groups in major congenital malformations, low birth weight, preterm birth, respiratory symptoms, or cesarean delivery.
IN PRACTICE:
“For the outcomes assessed, compared with buprenorphine alone, buprenorphine combined with naloxone during pregnancy appears to be a safe treatment option. This supports the view that both formulations are reasonable options for treatment of OUD in pregnancy, affirming flexibility in collaborative treatment decision-making,” the study authors wrote.
SOURCE:
The study was led by Loreen Straub, MD, MS, of the Division of Pharmacoepidemiology and Pharmacoeconomics at Brigham and Women’s Hospital and Harvard Medical School in Boston. It was published online in JAMA.
LIMITATIONS:
Some potential confounders, such as alcohol use and cigarette smoking, may not have been recorded in claims data. The findings for many of the neonatal and maternal outcomes suggest that confounding by unmeasured factors is an unlikely explanation for the associations observed. Individuals identified as exposed based on filled prescriptions might not have used the medication. The study used outcome algorithms with relatively high positive predictive values to minimize outcome misclassification. The cohort was restricted to live births to enable linkage to infants and to assess neonatal outcomes.
DISCLOSURES:
Various authors reported receiving grants and personal fees from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute on Drug Abuse, Roche, Moderna, Takeda, and Janssen Global, among others.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Buprenorphine combined with naloxone during pregnancy is associated with lower risks for neonatal abstinence syndrome and neonatal intensive care unit admission than buprenorphine alone. The study also found no significant differences in major congenital malformations between the two treatments.
METHODOLOGY:
- Researchers conducted a population-based cohort study using healthcare utilization data of people who were insured by Medicaid between 2000 and 2018.
- A total of 8695 pregnant individuals were included, with 3369 exposed to buprenorphine/naloxone and 5326 exposed to buprenorphine alone during the first trimester.
- Outcome measures included major congenital malformations, low birth weight, neonatal abstinence syndrome, neonatal intensive care unit admission, preterm birth, respiratory symptoms, small for gestational age, cesarean delivery, and maternal morbidity.
- The study excluded pregnancies with chromosomal anomalies, first-trimester exposure to known teratogens, or methadone use during baseline or the first trimester.
TAKEAWAY:
- According to the authors, buprenorphine/naloxone exposure during pregnancy was associated with a lower risk for neonatal abstinence syndrome (weighted risk ratio [RR], 0.77; 95% CI, 0.70-0.84) than buprenorphine alone.
- The researchers found a modestly lower risk for neonatal intensive care unit admission (weighted RR, 0.91; 95% CI, 0.85-0.98) and small risk for gestational age (weighted RR, 0.86; 95% CI, 0.75-0.98) in the buprenorphine/naloxone group.
- No significant differences were observed between the two groups in major congenital malformations, low birth weight, preterm birth, respiratory symptoms, or cesarean delivery.
IN PRACTICE:
“For the outcomes assessed, compared with buprenorphine alone, buprenorphine combined with naloxone during pregnancy appears to be a safe treatment option. This supports the view that both formulations are reasonable options for treatment of OUD in pregnancy, affirming flexibility in collaborative treatment decision-making,” the study authors wrote.
SOURCE:
The study was led by Loreen Straub, MD, MS, of the Division of Pharmacoepidemiology and Pharmacoeconomics at Brigham and Women’s Hospital and Harvard Medical School in Boston. It was published online in JAMA.
LIMITATIONS:
Some potential confounders, such as alcohol use and cigarette smoking, may not have been recorded in claims data. The findings for many of the neonatal and maternal outcomes suggest that confounding by unmeasured factors is an unlikely explanation for the associations observed. Individuals identified as exposed based on filled prescriptions might not have used the medication. The study used outcome algorithms with relatively high positive predictive values to minimize outcome misclassification. The cohort was restricted to live births to enable linkage to infants and to assess neonatal outcomes.
DISCLOSURES:
Various authors reported receiving grants and personal fees from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute on Drug Abuse, Roche, Moderna, Takeda, and Janssen Global, among others.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Viral Season 2024-2025: Try for An Ounce of Prevention
We are quickly approaching the typical cold and flu season. But can we call anything typical since 2020? Since 2020, there have been different recommendations for prevention, testing, return to work, and treatment since our world was rocked by the pandemic. Now that we are in the “post-pandemic” era, family physicians and other primary care professionals are the front line for discussions on prevention, evaluation, and treatment of the typical upper-respiratory infections, influenza, and COVID-19.
Let’s start with prevention. We have all heard the old adage, an ounce of prevention is worth a pound of cure. In primary care, we need to focus on prevention. Vaccination is often one of our best tools against the myriad of infections we are hoping to help patients prevent during cold and flu season. Most recently, we have fall vaccinations aimed to prevent COVID-19, influenza, and respiratory syncytial virus (RSV).
The number and timing of each of these vaccinations has different recommendations based on a variety of factors including age, pregnancy status, and whether or not the patient is immunocompromised. For the 2024-2025 season, the Centers for Disease Control and Prevention has recommended updated vaccines for both influenza and COVID-19.1 They have also updated the RSV vaccine recommendations to “People 75 or older, or between 60-74 with certain chronic health conditions or living in a nursing home should get one dose of the RSV vaccine to provide an extra layer of protection.”2
In addition to vaccines as prevention, there is also hygiene, staying home when sick and away from others who are sick, following guidelines for where and when to wear a face mask, and the general tools of eating well, and getting sufficient sleep and exercise to help maintain the healthiest immune system.
Despite the best of intentions, there will still be many who experience viral infections in this upcoming season. The CDC is currently recommending persons to stay away from others for at least 24 hours after their symptoms improve and they are fever-free without antipyretics. In addition to isolation while sick, general symptom management is something that we can recommend for all of these illnesses.
There is more to consider, though, as our patients face these illnesses. The first question is how to determine the diagnosis — and if that diagnosis is even necessary. Unfortunately, many of these viral illnesses can look the same. They can all cause fevers, chills, and other upper respiratory symptoms. They are all fairly contagious. All of these viruses can cause serious illness associated with additional complications. It is not truly possible to determine which virus someone has by symptoms alone, our patients can have multiple viruses at the same time and diagnosis of one does not preclude having another.3
Instead, we truly do need a test for diagnosis. In-office testing is available for RSV, influenza, and COVID-19. Additionally, despite not being as freely available as they were during the pandemic, patients are able to do home COVID tests and then call in with their results. At the time of writing this, at-home rapid influenza tests have also been approved by the FDA but are not yet readily available to the public. These tests are important for determining if the patient is eligible for treatment. Both influenza and COVID-19 have antiviral treatments available to help decrease the severity of the illness and potentially the length of illness and time contagious. According to the CDC, both treatments are underutilized.
This could be because of a lack of testing and diagnosis. It may also be because of a lack of familiarity with the available treatments.4,5Influenza treatment is recommended as soon as possible for those with suspected or confirmed diagnosis, immediately for anyone hospitalized, anyone with severe, complicated, or progressing illness, and for those at high risk of severe illness including but not limited to those under 2 years old, those over 65, those who are pregnant, and those with many chronic conditions.
Treatment can also be used for those who are not high risk when diagnosed within 48 hours. In the United States, four antivirals are recommended to treat influenza: oseltamivir phosphate, zanamivir, peramivir, and baloxavir marboxil. For COVID-19, treatments are also available for mild or moderate disease in those at risk for severe disease. Both remdesivir and nimatrelvir with ritonavir are treatment options that can be used for COVID-19 infection. Unfortunately, no specific antiviral is available for the other viral illnesses we see often during this season.
In primary care, we have some important roles to play. We need to continue to discuss all methods of prevention. Not only do vaccine recommendations change at least annually, our patients’ situations change and we have to reassess them. Additionally, people often need to hear things more than once before committing — so it never hurts to continue having those conversations. Combining the conversation about vaccines with other prevention measures is also important so that it does not seem like we are only recommending one thing. We should also start talking about treatment options before our patients are sick. We can communicate what is available as long as they let us know they are sick early. We can also be there to help our patients determine when they are at risk for severe illness and when they should consider a higher level of care.
The availability of home testing gives us the opportunity to provide these treatments via telehealth and even potentially in times when these illnesses are everywhere — with standing orders with our clinical teams. Although it is a busy time for us in the clinic, “cold and flu” season is definitely one of those times when our primary care relationship can truly help our patients.
References
1. CDC Recommends Updated 2024-2025 COVID-19 and Flu Vaccines for Fall/Winter Virus Season. https://www.cdc.gov/media/releases/2024/s-t0627-vaccine-recommendations.html. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention.
2. CDC Updates RSV Vaccination Recommendation for Adults. https://www.cdc.gov/media/releases/2024/s-0626-vaccination-adults.html. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention.
3. Similarities and Differences between Flu and COVID-19. https://www.cdc.gov/flu/symptoms/flu-vs-covid19.htm. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases.
4. Respiratory Virus Guidance. https://www.cdc.gov/respiratory-viruses/guidance/index.html. Accessed August 9, 2024. Source: National Center for Immunization and Respiratory Diseases.
5. Provider Toolkit: Preparing Patients for the Fall and Winter Virus Season. https://www.cdc.gov/respiratory-viruses/hcp/tools-resources/index.html. Accessed August 9, 2024. Source: Centers for Disease Control and Prevention.
We are quickly approaching the typical cold and flu season. But can we call anything typical since 2020? Since 2020, there have been different recommendations for prevention, testing, return to work, and treatment since our world was rocked by the pandemic. Now that we are in the “post-pandemic” era, family physicians and other primary care professionals are the front line for discussions on prevention, evaluation, and treatment of the typical upper-respiratory infections, influenza, and COVID-19.
Let’s start with prevention. We have all heard the old adage, an ounce of prevention is worth a pound of cure. In primary care, we need to focus on prevention. Vaccination is often one of our best tools against the myriad of infections we are hoping to help patients prevent during cold and flu season. Most recently, we have fall vaccinations aimed to prevent COVID-19, influenza, and respiratory syncytial virus (RSV).
The number and timing of each of these vaccinations has different recommendations based on a variety of factors including age, pregnancy status, and whether or not the patient is immunocompromised. For the 2024-2025 season, the Centers for Disease Control and Prevention has recommended updated vaccines for both influenza and COVID-19.1 They have also updated the RSV vaccine recommendations to “People 75 or older, or between 60-74 with certain chronic health conditions or living in a nursing home should get one dose of the RSV vaccine to provide an extra layer of protection.”2
In addition to vaccines as prevention, there is also hygiene, staying home when sick and away from others who are sick, following guidelines for where and when to wear a face mask, and the general tools of eating well, and getting sufficient sleep and exercise to help maintain the healthiest immune system.
Despite the best of intentions, there will still be many who experience viral infections in this upcoming season. The CDC is currently recommending persons to stay away from others for at least 24 hours after their symptoms improve and they are fever-free without antipyretics. In addition to isolation while sick, general symptom management is something that we can recommend for all of these illnesses.
There is more to consider, though, as our patients face these illnesses. The first question is how to determine the diagnosis — and if that diagnosis is even necessary. Unfortunately, many of these viral illnesses can look the same. They can all cause fevers, chills, and other upper respiratory symptoms. They are all fairly contagious. All of these viruses can cause serious illness associated with additional complications. It is not truly possible to determine which virus someone has by symptoms alone, our patients can have multiple viruses at the same time and diagnosis of one does not preclude having another.3
Instead, we truly do need a test for diagnosis. In-office testing is available for RSV, influenza, and COVID-19. Additionally, despite not being as freely available as they were during the pandemic, patients are able to do home COVID tests and then call in with their results. At the time of writing this, at-home rapid influenza tests have also been approved by the FDA but are not yet readily available to the public. These tests are important for determining if the patient is eligible for treatment. Both influenza and COVID-19 have antiviral treatments available to help decrease the severity of the illness and potentially the length of illness and time contagious. According to the CDC, both treatments are underutilized.
This could be because of a lack of testing and diagnosis. It may also be because of a lack of familiarity with the available treatments.4,5Influenza treatment is recommended as soon as possible for those with suspected or confirmed diagnosis, immediately for anyone hospitalized, anyone with severe, complicated, or progressing illness, and for those at high risk of severe illness including but not limited to those under 2 years old, those over 65, those who are pregnant, and those with many chronic conditions.
Treatment can also be used for those who are not high risk when diagnosed within 48 hours. In the United States, four antivirals are recommended to treat influenza: oseltamivir phosphate, zanamivir, peramivir, and baloxavir marboxil. For COVID-19, treatments are also available for mild or moderate disease in those at risk for severe disease. Both remdesivir and nimatrelvir with ritonavir are treatment options that can be used for COVID-19 infection. Unfortunately, no specific antiviral is available for the other viral illnesses we see often during this season.
In primary care, we have some important roles to play. We need to continue to discuss all methods of prevention. Not only do vaccine recommendations change at least annually, our patients’ situations change and we have to reassess them. Additionally, people often need to hear things more than once before committing — so it never hurts to continue having those conversations. Combining the conversation about vaccines with other prevention measures is also important so that it does not seem like we are only recommending one thing. We should also start talking about treatment options before our patients are sick. We can communicate what is available as long as they let us know they are sick early. We can also be there to help our patients determine when they are at risk for severe illness and when they should consider a higher level of care.
The availability of home testing gives us the opportunity to provide these treatments via telehealth and even potentially in times when these illnesses are everywhere — with standing orders with our clinical teams. Although it is a busy time for us in the clinic, “cold and flu” season is definitely one of those times when our primary care relationship can truly help our patients.
References
1. CDC Recommends Updated 2024-2025 COVID-19 and Flu Vaccines for Fall/Winter Virus Season. https://www.cdc.gov/media/releases/2024/s-t0627-vaccine-recommendations.html. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention.
2. CDC Updates RSV Vaccination Recommendation for Adults. https://www.cdc.gov/media/releases/2024/s-0626-vaccination-adults.html. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention.
3. Similarities and Differences between Flu and COVID-19. https://www.cdc.gov/flu/symptoms/flu-vs-covid19.htm. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases.
4. Respiratory Virus Guidance. https://www.cdc.gov/respiratory-viruses/guidance/index.html. Accessed August 9, 2024. Source: National Center for Immunization and Respiratory Diseases.
5. Provider Toolkit: Preparing Patients for the Fall and Winter Virus Season. https://www.cdc.gov/respiratory-viruses/hcp/tools-resources/index.html. Accessed August 9, 2024. Source: Centers for Disease Control and Prevention.
We are quickly approaching the typical cold and flu season. But can we call anything typical since 2020? Since 2020, there have been different recommendations for prevention, testing, return to work, and treatment since our world was rocked by the pandemic. Now that we are in the “post-pandemic” era, family physicians and other primary care professionals are the front line for discussions on prevention, evaluation, and treatment of the typical upper-respiratory infections, influenza, and COVID-19.
Let’s start with prevention. We have all heard the old adage, an ounce of prevention is worth a pound of cure. In primary care, we need to focus on prevention. Vaccination is often one of our best tools against the myriad of infections we are hoping to help patients prevent during cold and flu season. Most recently, we have fall vaccinations aimed to prevent COVID-19, influenza, and respiratory syncytial virus (RSV).
The number and timing of each of these vaccinations has different recommendations based on a variety of factors including age, pregnancy status, and whether or not the patient is immunocompromised. For the 2024-2025 season, the Centers for Disease Control and Prevention has recommended updated vaccines for both influenza and COVID-19.1 They have also updated the RSV vaccine recommendations to “People 75 or older, or between 60-74 with certain chronic health conditions or living in a nursing home should get one dose of the RSV vaccine to provide an extra layer of protection.”2
In addition to vaccines as prevention, there is also hygiene, staying home when sick and away from others who are sick, following guidelines for where and when to wear a face mask, and the general tools of eating well, and getting sufficient sleep and exercise to help maintain the healthiest immune system.
Despite the best of intentions, there will still be many who experience viral infections in this upcoming season. The CDC is currently recommending persons to stay away from others for at least 24 hours after their symptoms improve and they are fever-free without antipyretics. In addition to isolation while sick, general symptom management is something that we can recommend for all of these illnesses.
There is more to consider, though, as our patients face these illnesses. The first question is how to determine the diagnosis — and if that diagnosis is even necessary. Unfortunately, many of these viral illnesses can look the same. They can all cause fevers, chills, and other upper respiratory symptoms. They are all fairly contagious. All of these viruses can cause serious illness associated with additional complications. It is not truly possible to determine which virus someone has by symptoms alone, our patients can have multiple viruses at the same time and diagnosis of one does not preclude having another.3
Instead, we truly do need a test for diagnosis. In-office testing is available for RSV, influenza, and COVID-19. Additionally, despite not being as freely available as they were during the pandemic, patients are able to do home COVID tests and then call in with their results. At the time of writing this, at-home rapid influenza tests have also been approved by the FDA but are not yet readily available to the public. These tests are important for determining if the patient is eligible for treatment. Both influenza and COVID-19 have antiviral treatments available to help decrease the severity of the illness and potentially the length of illness and time contagious. According to the CDC, both treatments are underutilized.
This could be because of a lack of testing and diagnosis. It may also be because of a lack of familiarity with the available treatments.4,5Influenza treatment is recommended as soon as possible for those with suspected or confirmed diagnosis, immediately for anyone hospitalized, anyone with severe, complicated, or progressing illness, and for those at high risk of severe illness including but not limited to those under 2 years old, those over 65, those who are pregnant, and those with many chronic conditions.
Treatment can also be used for those who are not high risk when diagnosed within 48 hours. In the United States, four antivirals are recommended to treat influenza: oseltamivir phosphate, zanamivir, peramivir, and baloxavir marboxil. For COVID-19, treatments are also available for mild or moderate disease in those at risk for severe disease. Both remdesivir and nimatrelvir with ritonavir are treatment options that can be used for COVID-19 infection. Unfortunately, no specific antiviral is available for the other viral illnesses we see often during this season.
In primary care, we have some important roles to play. We need to continue to discuss all methods of prevention. Not only do vaccine recommendations change at least annually, our patients’ situations change and we have to reassess them. Additionally, people often need to hear things more than once before committing — so it never hurts to continue having those conversations. Combining the conversation about vaccines with other prevention measures is also important so that it does not seem like we are only recommending one thing. We should also start talking about treatment options before our patients are sick. We can communicate what is available as long as they let us know they are sick early. We can also be there to help our patients determine when they are at risk for severe illness and when they should consider a higher level of care.
The availability of home testing gives us the opportunity to provide these treatments via telehealth and even potentially in times when these illnesses are everywhere — with standing orders with our clinical teams. Although it is a busy time for us in the clinic, “cold and flu” season is definitely one of those times when our primary care relationship can truly help our patients.
References
1. CDC Recommends Updated 2024-2025 COVID-19 and Flu Vaccines for Fall/Winter Virus Season. https://www.cdc.gov/media/releases/2024/s-t0627-vaccine-recommendations.html. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention.
2. CDC Updates RSV Vaccination Recommendation for Adults. https://www.cdc.gov/media/releases/2024/s-0626-vaccination-adults.html. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention.
3. Similarities and Differences between Flu and COVID-19. https://www.cdc.gov/flu/symptoms/flu-vs-covid19.htm. Accessed August 8, 2024. Source: Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases.
4. Respiratory Virus Guidance. https://www.cdc.gov/respiratory-viruses/guidance/index.html. Accessed August 9, 2024. Source: National Center for Immunization and Respiratory Diseases.
5. Provider Toolkit: Preparing Patients for the Fall and Winter Virus Season. https://www.cdc.gov/respiratory-viruses/hcp/tools-resources/index.html. Accessed August 9, 2024. Source: Centers for Disease Control and Prevention.
Remission or Not, Biologics May Mitigate Cardiovascular Risks of RA
TOPLINE:
, suggesting that biologics may reduce cardiovascular risk in RA even if remission is not achieved.
METHODOLOGY:
- Studies reported reduced cardiovascular risk in patients with RA who respond to tumor necrosis factor inhibitors but not in nonresponders, highlighting the importance of controlling inflammation for cardiovascular protection.
- Researchers assessed whether bDMARDs modify the impact of disease activity and systemic inflammation on cardiovascular risk in 4370 patients (mean age, 55 years) with RA without cardiovascular disease from a 10-country observational cohort.
- The severity of RA disease activity was assessed using C-reactive protein (CRP) levels and 28-joint Disease Activity Score based on CRP (DAS28-CRP).
- Endpoints were time to first MACE — a composite of cardiovascular death, myocardial infarction, and stroke — and time to first ischemic cardiovascular event (iCVE) — a composite of MACE plus revascularization, angina, transient ischemic attack, and peripheral arterial disease.
TAKEAWAY:
- The interaction between use of bDMARD and DAS28-CRP (P = .017) or CRP (P = .011) was significant for MACE.
- Each unit increase in DAS28-CRP increased the risk for MACE in bDMARD nonusers (hazard ratio [HR], 1.21; P = .002) but not in users.
- The per log unit increase in CRP was associated with a risk for MACE in bDMARD nonusers (HR, 1.16; P = .009) but not in users.
- No interaction was observed between bDMARD use and DAS28-CRP or CRP for the iCVE risk.
IN PRACTICE:
“This may indicate additional bDMARD-specific benefits directly on arterial wall inflammation and atherosclerotic plaque anatomy, stability, and biology, independently of systemic inflammation,” the authors wrote.
SOURCE:
The study, led by George Athanasios Karpouzas, MD, The Lundquist Institute, Torrance, California, was published online in RMD Open.
LIMITATIONS:
Patients with a particular interest in RA-associated cardiovascular disease were included, which may have introduced referral bias and affected the generalizability of the findings. Standard definitions were used for selected outcomes; however, differences in the reporting of outcomes may be plausible. Some patients were evaluated prospectively, while others were evaluated retrospectively, leading to differences in surveillance.
DISCLOSURES:
The study was supported by Pfizer. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
, suggesting that biologics may reduce cardiovascular risk in RA even if remission is not achieved.
METHODOLOGY:
- Studies reported reduced cardiovascular risk in patients with RA who respond to tumor necrosis factor inhibitors but not in nonresponders, highlighting the importance of controlling inflammation for cardiovascular protection.
- Researchers assessed whether bDMARDs modify the impact of disease activity and systemic inflammation on cardiovascular risk in 4370 patients (mean age, 55 years) with RA without cardiovascular disease from a 10-country observational cohort.
- The severity of RA disease activity was assessed using C-reactive protein (CRP) levels and 28-joint Disease Activity Score based on CRP (DAS28-CRP).
- Endpoints were time to first MACE — a composite of cardiovascular death, myocardial infarction, and stroke — and time to first ischemic cardiovascular event (iCVE) — a composite of MACE plus revascularization, angina, transient ischemic attack, and peripheral arterial disease.
TAKEAWAY:
- The interaction between use of bDMARD and DAS28-CRP (P = .017) or CRP (P = .011) was significant for MACE.
- Each unit increase in DAS28-CRP increased the risk for MACE in bDMARD nonusers (hazard ratio [HR], 1.21; P = .002) but not in users.
- The per log unit increase in CRP was associated with a risk for MACE in bDMARD nonusers (HR, 1.16; P = .009) but not in users.
- No interaction was observed between bDMARD use and DAS28-CRP or CRP for the iCVE risk.
IN PRACTICE:
“This may indicate additional bDMARD-specific benefits directly on arterial wall inflammation and atherosclerotic plaque anatomy, stability, and biology, independently of systemic inflammation,” the authors wrote.
SOURCE:
The study, led by George Athanasios Karpouzas, MD, The Lundquist Institute, Torrance, California, was published online in RMD Open.
LIMITATIONS:
Patients with a particular interest in RA-associated cardiovascular disease were included, which may have introduced referral bias and affected the generalizability of the findings. Standard definitions were used for selected outcomes; however, differences in the reporting of outcomes may be plausible. Some patients were evaluated prospectively, while others were evaluated retrospectively, leading to differences in surveillance.
DISCLOSURES:
The study was supported by Pfizer. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
, suggesting that biologics may reduce cardiovascular risk in RA even if remission is not achieved.
METHODOLOGY:
- Studies reported reduced cardiovascular risk in patients with RA who respond to tumor necrosis factor inhibitors but not in nonresponders, highlighting the importance of controlling inflammation for cardiovascular protection.
- Researchers assessed whether bDMARDs modify the impact of disease activity and systemic inflammation on cardiovascular risk in 4370 patients (mean age, 55 years) with RA without cardiovascular disease from a 10-country observational cohort.
- The severity of RA disease activity was assessed using C-reactive protein (CRP) levels and 28-joint Disease Activity Score based on CRP (DAS28-CRP).
- Endpoints were time to first MACE — a composite of cardiovascular death, myocardial infarction, and stroke — and time to first ischemic cardiovascular event (iCVE) — a composite of MACE plus revascularization, angina, transient ischemic attack, and peripheral arterial disease.
TAKEAWAY:
- The interaction between use of bDMARD and DAS28-CRP (P = .017) or CRP (P = .011) was significant for MACE.
- Each unit increase in DAS28-CRP increased the risk for MACE in bDMARD nonusers (hazard ratio [HR], 1.21; P = .002) but not in users.
- The per log unit increase in CRP was associated with a risk for MACE in bDMARD nonusers (HR, 1.16; P = .009) but not in users.
- No interaction was observed between bDMARD use and DAS28-CRP or CRP for the iCVE risk.
IN PRACTICE:
“This may indicate additional bDMARD-specific benefits directly on arterial wall inflammation and atherosclerotic plaque anatomy, stability, and biology, independently of systemic inflammation,” the authors wrote.
SOURCE:
The study, led by George Athanasios Karpouzas, MD, The Lundquist Institute, Torrance, California, was published online in RMD Open.
LIMITATIONS:
Patients with a particular interest in RA-associated cardiovascular disease were included, which may have introduced referral bias and affected the generalizability of the findings. Standard definitions were used for selected outcomes; however, differences in the reporting of outcomes may be plausible. Some patients were evaluated prospectively, while others were evaluated retrospectively, leading to differences in surveillance.
DISCLOSURES:
The study was supported by Pfizer. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
These Four Factors Account for 18 Years of Life Expectancy
This transcript has been edited for clarity.
Two individuals in the United States are celebrating their 30th birthdays. It’s a good day. They are entering the prime of their lives. One is a married White woman with a university degree. The other is a never-married White man with a high school diploma.
How many more years of life can these two individuals look forward to?
There’s a fairly dramatic difference. The man can expect 37.1 more years of life on average, living to be about 67. The woman can expect to live to age 85. That’s a life-expectancy discrepancy of 18 years based solely on gender, education, and marital status.
I’m using these cases to illustrate the extremes of life expectancy across four key social determinants of health: sex, race, marital status, and education. We all have some sense of how these factors play out in terms of health, but a new study suggests that it’s actually quite a bit more complicated than we thought.
Let me start by acknowledging my own bias here. As a clinical researcher, I sometimes find it hard to appreciate the value of actuarial-type studies that look at life expectancy (or any metric, really) between groups defined by marital status, for example. I’m never quite sure what to do with the conclusion. Married people live longer, the headline says. Okay, but as a doctor, what am I supposed to do about that? Encourage my patients to settle down and commit? Studies showing that women live longer than men or that White people live longer than Black people are also hard for me to incorporate into my practice. These are not easily changeable states.
But studies examining these groups are a reasonable starting point to ask more relevant questions. Why do women live longer than men? Is it behavioral (men take more risks and are less likely to see doctors)? Or is it hormonal (estrogen has a lot of protective effects that testosterone does not)? Or is it something else?
Integrating these social determinants of health into a cohesive story is a bit harder than it might seem, as this study, appearing in BMJ Open, illustrates.
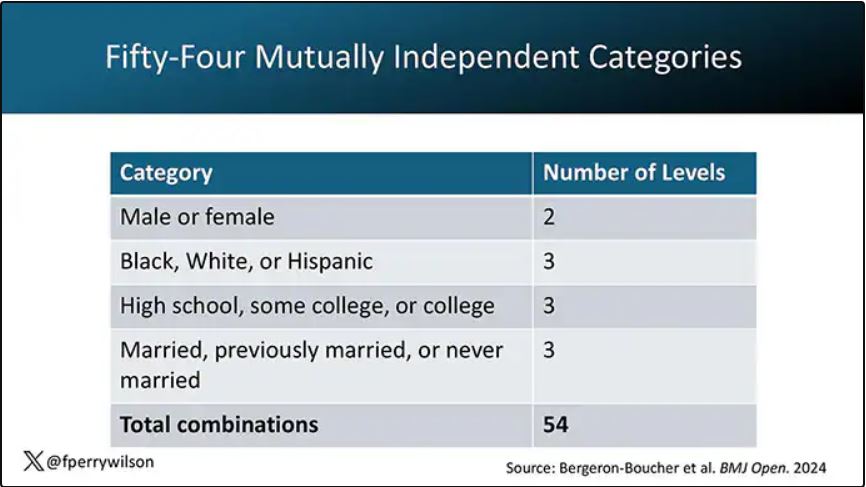
In the context of this study, every person in America can be placed into one of 54 mutually exclusive groups. You can be male or female. You can be Black, White, or Hispanic. You can have a high school diploma or less, an associate degree, or a college degree; and you can be married, previously married, or never married.
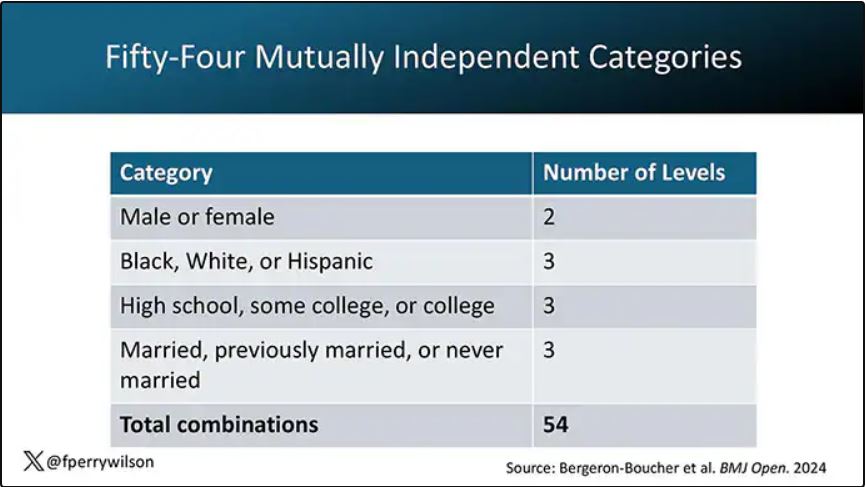
Of course, this does not capture the beautiful tapestry that is American life, but let’s give them a pass. They are working with data from the American Community Survey, which contains 8634 people — the statistics would run into trouble with more granular divisions. This survey can be population weighted, so you can scale up the results to reasonably represent the population of the United States.
The survey collected data on the four broad categories of sex, race, education, and marital status and linked those survey results to the Multiple Cause of Death dataset from the CDC. From there, it’s a pretty simple task to rank the 54 categories in order from longest to shortest life expectancy, as you can see here.
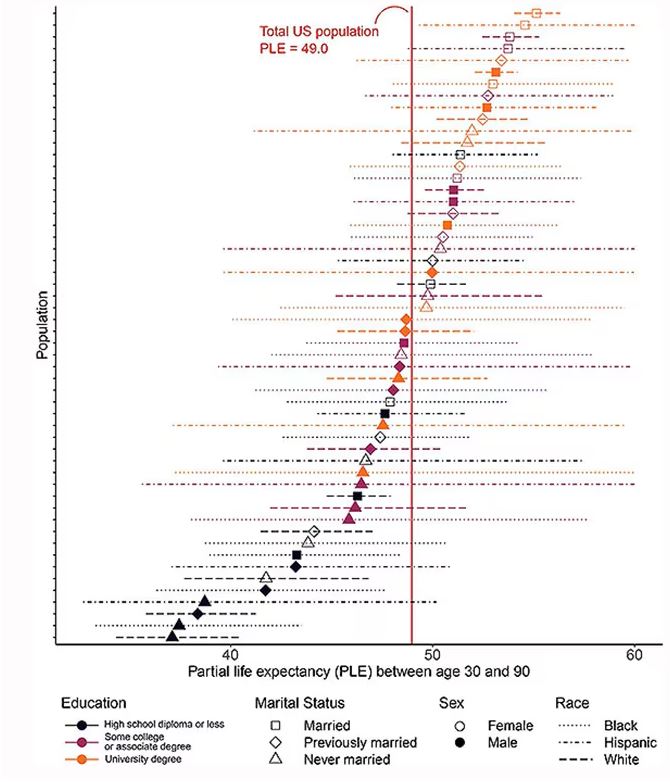
But that’s not really the interesting part of this study. Sure, there is a lot of variation; it’s interesting that these four factors explain about 18 years’ difference in life expectancy in this country. What strikes me here, actually, is the lack of an entirely consistent message across this spectrum.
Let me walk you through the second figure in this paper, because this nicely illustrates the surprising heterogeneity that exists here.
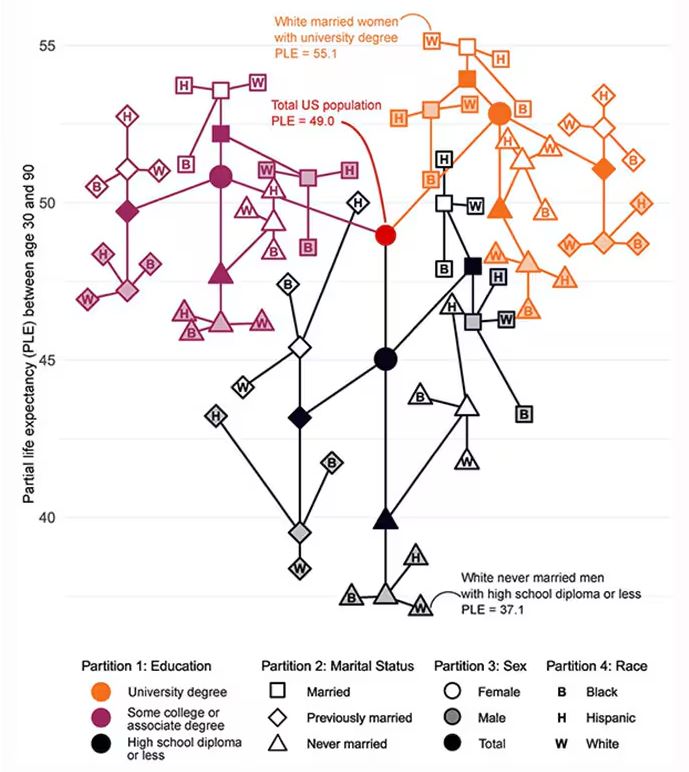
This may seem overwhelming, but basically, shapes that are higher up on the Y-axis represent the groups with longer life expectancy.
You can tell, for example, that shapes that are black in color (groups with high school educations or less) are generally lower. But not universally so. This box represents married, Hispanic females who do quite well in terms of life expectancy, even at that lower educational level.
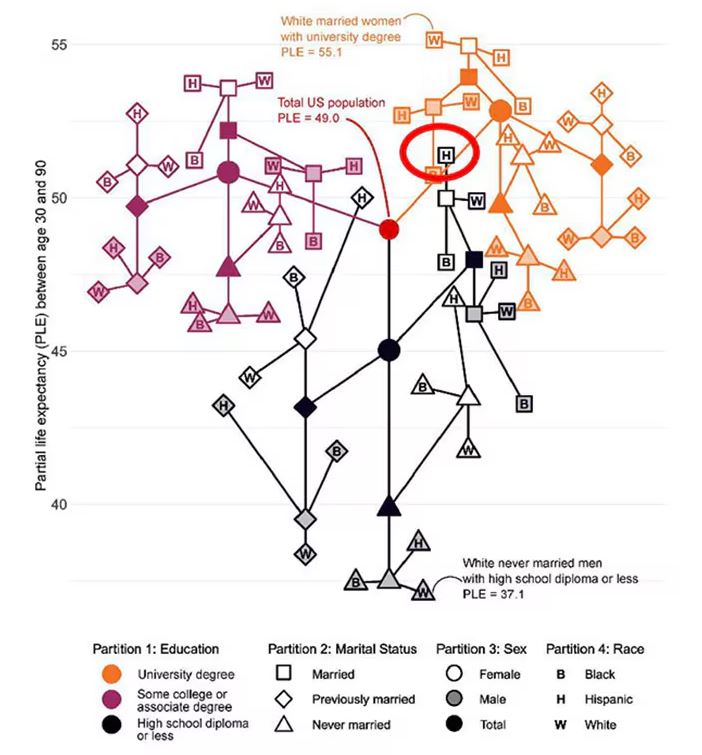
The authors quantify this phenomenon by creating a mortality risk score that integrates these findings. It looks something like this, with 0 being average morality for the United States.
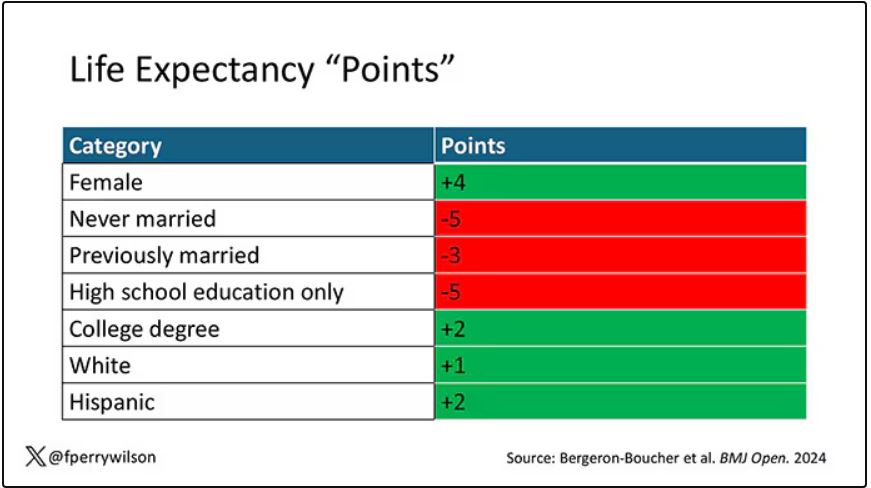
As you can see, you get a bunch of points for being female, but you lose a bunch for not being married. Education plays a large role, with a big hit for those who have a high school diploma or less, and a bonus for those with a college degree. Race plays a relatively more minor role.
This is all very interesting, but as I said at the beginning, this isn’t terribly useful to me as a physician. More important is figuring out why these differences exist. And there are some clues in the study data, particularly when we examine causes of death. This figure ranks those 54 groups again, from the married, White, college-educated females down to the never-married, White, high school–educated males. The boxes show how much more or less likely this group is to die of a given condition than the general population.
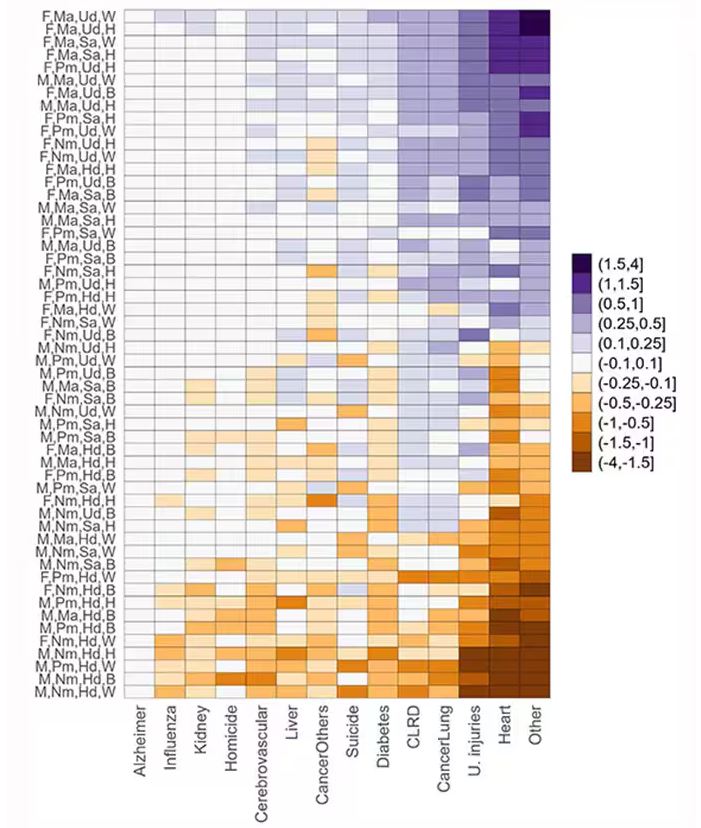
Looking at the bottom groups, you can see a dramatically increased risk for death from unintentional injuries, heart disease, and lung cancer. You see an increased risk for suicide as well. In the upper tiers, the only place where risk seems higher than expected is for the category of “other cancers,” reminding us that many types of cancer do not respect definitions of socioeconomic status.
You can even update the risk-scoring system to reflect the risk for different causes of death. You can see here how White people, for example, are at higher risk for death from unintentional injuries relative to other populations, despite having a lower mortality overall.
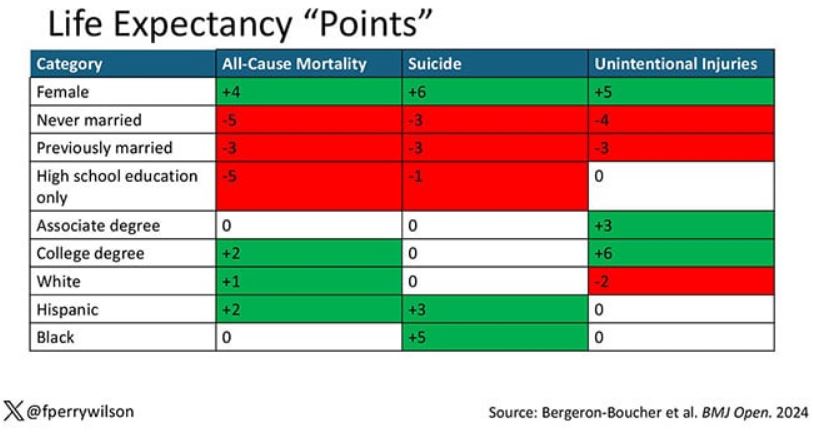
So maybe, through cause of death, we get a little closer to the answer of why. But this paper is really just a start. Its primary effect should be to surprise us — that in a country as wealthy as the United States, such dramatic variation exists based on factors that, with the exception of sex, I suppose, are not really biological. Which means that to find the why, we may need to turn from physiology to sociology.
Dr. Wilson is associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Two individuals in the United States are celebrating their 30th birthdays. It’s a good day. They are entering the prime of their lives. One is a married White woman with a university degree. The other is a never-married White man with a high school diploma.
How many more years of life can these two individuals look forward to?
There’s a fairly dramatic difference. The man can expect 37.1 more years of life on average, living to be about 67. The woman can expect to live to age 85. That’s a life-expectancy discrepancy of 18 years based solely on gender, education, and marital status.
I’m using these cases to illustrate the extremes of life expectancy across four key social determinants of health: sex, race, marital status, and education. We all have some sense of how these factors play out in terms of health, but a new study suggests that it’s actually quite a bit more complicated than we thought.
Let me start by acknowledging my own bias here. As a clinical researcher, I sometimes find it hard to appreciate the value of actuarial-type studies that look at life expectancy (or any metric, really) between groups defined by marital status, for example. I’m never quite sure what to do with the conclusion. Married people live longer, the headline says. Okay, but as a doctor, what am I supposed to do about that? Encourage my patients to settle down and commit? Studies showing that women live longer than men or that White people live longer than Black people are also hard for me to incorporate into my practice. These are not easily changeable states.
But studies examining these groups are a reasonable starting point to ask more relevant questions. Why do women live longer than men? Is it behavioral (men take more risks and are less likely to see doctors)? Or is it hormonal (estrogen has a lot of protective effects that testosterone does not)? Or is it something else?
Integrating these social determinants of health into a cohesive story is a bit harder than it might seem, as this study, appearing in BMJ Open, illustrates.
In the context of this study, every person in America can be placed into one of 54 mutually exclusive groups. You can be male or female. You can be Black, White, or Hispanic. You can have a high school diploma or less, an associate degree, or a college degree; and you can be married, previously married, or never married.

Of course, this does not capture the beautiful tapestry that is American life, but let’s give them a pass. They are working with data from the American Community Survey, which contains 8634 people — the statistics would run into trouble with more granular divisions. This survey can be population weighted, so you can scale up the results to reasonably represent the population of the United States.
The survey collected data on the four broad categories of sex, race, education, and marital status and linked those survey results to the Multiple Cause of Death dataset from the CDC. From there, it’s a pretty simple task to rank the 54 categories in order from longest to shortest life expectancy, as you can see here.

But that’s not really the interesting part of this study. Sure, there is a lot of variation; it’s interesting that these four factors explain about 18 years’ difference in life expectancy in this country. What strikes me here, actually, is the lack of an entirely consistent message across this spectrum.
Let me walk you through the second figure in this paper, because this nicely illustrates the surprising heterogeneity that exists here.

This may seem overwhelming, but basically, shapes that are higher up on the Y-axis represent the groups with longer life expectancy.
You can tell, for example, that shapes that are black in color (groups with high school educations or less) are generally lower. But not universally so. This box represents married, Hispanic females who do quite well in terms of life expectancy, even at that lower educational level.

The authors quantify this phenomenon by creating a mortality risk score that integrates these findings. It looks something like this, with 0 being average morality for the United States.

As you can see, you get a bunch of points for being female, but you lose a bunch for not being married. Education plays a large role, with a big hit for those who have a high school diploma or less, and a bonus for those with a college degree. Race plays a relatively more minor role.
This is all very interesting, but as I said at the beginning, this isn’t terribly useful to me as a physician. More important is figuring out why these differences exist. And there are some clues in the study data, particularly when we examine causes of death. This figure ranks those 54 groups again, from the married, White, college-educated females down to the never-married, White, high school–educated males. The boxes show how much more or less likely this group is to die of a given condition than the general population.

Looking at the bottom groups, you can see a dramatically increased risk for death from unintentional injuries, heart disease, and lung cancer. You see an increased risk for suicide as well. In the upper tiers, the only place where risk seems higher than expected is for the category of “other cancers,” reminding us that many types of cancer do not respect definitions of socioeconomic status.
You can even update the risk-scoring system to reflect the risk for different causes of death. You can see here how White people, for example, are at higher risk for death from unintentional injuries relative to other populations, despite having a lower mortality overall.

So maybe, through cause of death, we get a little closer to the answer of why. But this paper is really just a start. Its primary effect should be to surprise us — that in a country as wealthy as the United States, such dramatic variation exists based on factors that, with the exception of sex, I suppose, are not really biological. Which means that to find the why, we may need to turn from physiology to sociology.
Dr. Wilson is associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Two individuals in the United States are celebrating their 30th birthdays. It’s a good day. They are entering the prime of their lives. One is a married White woman with a university degree. The other is a never-married White man with a high school diploma.
How many more years of life can these two individuals look forward to?
There’s a fairly dramatic difference. The man can expect 37.1 more years of life on average, living to be about 67. The woman can expect to live to age 85. That’s a life-expectancy discrepancy of 18 years based solely on gender, education, and marital status.
I’m using these cases to illustrate the extremes of life expectancy across four key social determinants of health: sex, race, marital status, and education. We all have some sense of how these factors play out in terms of health, but a new study suggests that it’s actually quite a bit more complicated than we thought.
Let me start by acknowledging my own bias here. As a clinical researcher, I sometimes find it hard to appreciate the value of actuarial-type studies that look at life expectancy (or any metric, really) between groups defined by marital status, for example. I’m never quite sure what to do with the conclusion. Married people live longer, the headline says. Okay, but as a doctor, what am I supposed to do about that? Encourage my patients to settle down and commit? Studies showing that women live longer than men or that White people live longer than Black people are also hard for me to incorporate into my practice. These are not easily changeable states.
But studies examining these groups are a reasonable starting point to ask more relevant questions. Why do women live longer than men? Is it behavioral (men take more risks and are less likely to see doctors)? Or is it hormonal (estrogen has a lot of protective effects that testosterone does not)? Or is it something else?
Integrating these social determinants of health into a cohesive story is a bit harder than it might seem, as this study, appearing in BMJ Open, illustrates.
In the context of this study, every person in America can be placed into one of 54 mutually exclusive groups. You can be male or female. You can be Black, White, or Hispanic. You can have a high school diploma or less, an associate degree, or a college degree; and you can be married, previously married, or never married.

Of course, this does not capture the beautiful tapestry that is American life, but let’s give them a pass. They are working with data from the American Community Survey, which contains 8634 people — the statistics would run into trouble with more granular divisions. This survey can be population weighted, so you can scale up the results to reasonably represent the population of the United States.
The survey collected data on the four broad categories of sex, race, education, and marital status and linked those survey results to the Multiple Cause of Death dataset from the CDC. From there, it’s a pretty simple task to rank the 54 categories in order from longest to shortest life expectancy, as you can see here.

But that’s not really the interesting part of this study. Sure, there is a lot of variation; it’s interesting that these four factors explain about 18 years’ difference in life expectancy in this country. What strikes me here, actually, is the lack of an entirely consistent message across this spectrum.
Let me walk you through the second figure in this paper, because this nicely illustrates the surprising heterogeneity that exists here.

This may seem overwhelming, but basically, shapes that are higher up on the Y-axis represent the groups with longer life expectancy.
You can tell, for example, that shapes that are black in color (groups with high school educations or less) are generally lower. But not universally so. This box represents married, Hispanic females who do quite well in terms of life expectancy, even at that lower educational level.

The authors quantify this phenomenon by creating a mortality risk score that integrates these findings. It looks something like this, with 0 being average morality for the United States.

As you can see, you get a bunch of points for being female, but you lose a bunch for not being married. Education plays a large role, with a big hit for those who have a high school diploma or less, and a bonus for those with a college degree. Race plays a relatively more minor role.
This is all very interesting, but as I said at the beginning, this isn’t terribly useful to me as a physician. More important is figuring out why these differences exist. And there are some clues in the study data, particularly when we examine causes of death. This figure ranks those 54 groups again, from the married, White, college-educated females down to the never-married, White, high school–educated males. The boxes show how much more or less likely this group is to die of a given condition than the general population.

Looking at the bottom groups, you can see a dramatically increased risk for death from unintentional injuries, heart disease, and lung cancer. You see an increased risk for suicide as well. In the upper tiers, the only place where risk seems higher than expected is for the category of “other cancers,” reminding us that many types of cancer do not respect definitions of socioeconomic status.
You can even update the risk-scoring system to reflect the risk for different causes of death. You can see here how White people, for example, are at higher risk for death from unintentional injuries relative to other populations, despite having a lower mortality overall.

So maybe, through cause of death, we get a little closer to the answer of why. But this paper is really just a start. Its primary effect should be to surprise us — that in a country as wealthy as the United States, such dramatic variation exists based on factors that, with the exception of sex, I suppose, are not really biological. Which means that to find the why, we may need to turn from physiology to sociology.
Dr. Wilson is associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Are Your Patients Using Any of These Six Potentially Hepatotoxic Botanicals?
TOPLINE:
The estimated number of US adults who consumed at least one of the six most frequently reported hepatotoxic botanicals in the last 30 days is similar to the number of patients prescribed potentially hepatotoxic drugs, including nonsteroidal anti-inflammatory drugs (NSAIDs) and simvastatin.
METHODOLOGY:
- Herbal and dietary supplements (HDS) are an increasingly common source of drug hepatotoxicity cases, but their prevalence and the reasons for their use among the general population are uncertain.
- This survey study evaluated nationally representative data from 9685 adults (mean age, 47.5 years; 51.8% women) enrolled in the National Health and Nutrition Examination Survey (NHANES) between January 2017 and March 2020.
- Participants reported their use of HDS and prescription drugs through personal interviews for a 30-day period prior to the survey date.
- Researchers compared the clinical features and baseline demographic characteristics of users of six potentially hepatotoxic botanicals (ie, turmeric, green tea, Garcinia cambogia, black cohosh, red yeast rice, and ashwagandha) with those of nonusers.
- The prevalence of use of these at-risk botanicals was compared with that of widely prescribed potentially hepatotoxic medications, including NSAIDs, simvastatin, and sertraline.
TAKEAWAY:
- In the cohort of 9685 participants, 4.7% of individuals reported consumption of at least one of the six potentially hepatotoxic botanicals in the past 30 days, with turmeric being the most common, followed by green tea.
- Extrapolating the survey data, researchers estimated that 15.6 million US adults use at least one of these six botanicals, which is comparable to the number of those prescribed potentially hepatotoxic drugs, including NSAIDs (14.8 million) and simvastatin (14.0 million). Sertraline use was lower (7.7 million).
- Most individuals used these botanicals without the recommendation of their healthcare provider.
- Those using botanicals were more likely to be older (adjusted odds ratio [aOR], 2.36; P = .04 for 40-59 years; aOR, 3.96; P = .001 for ≥ 60 years), to have some college education (aOR, 4.78; P < .001), and to have arthritis (aOR, 2.27; P < .001) than nonusers.
- The most common reasons for using any of these six potential hepatotoxic botanicals were to improve or maintain health or to prevent health problems or boost immunity.
IN PRACTICE:
“In light of the lack of regulatory oversight on the manufacturing and testing of botanical products, it is recommended that clinicians obtain a full medication and HDS use history when evaluating patients with unexplained symptoms or liver test abnormalities,” the authors wrote.
SOURCE:
The study, led by Alisa Likhitsup, MD, MPH, Division of Gastroenterology and Hepatology, University of Michigan, Ann Arbor, Michigan, was published online in JAMA Network Open.
LIMITATIONS:
The survey response rate was low at 43.9% for adults aged ≥ 20 years. As NHANES is a cross-sectional study, the causal relationship between consumption of the six botanicals of interest and the development of liver injury could not be determined. The use of HDS products and medications was self-reported in NHANES and not independently verified using source documents.
DISCLOSURES:
This study did not report any source of funding. Two authors declared receiving grants from pharmaceutical companies outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
The estimated number of US adults who consumed at least one of the six most frequently reported hepatotoxic botanicals in the last 30 days is similar to the number of patients prescribed potentially hepatotoxic drugs, including nonsteroidal anti-inflammatory drugs (NSAIDs) and simvastatin.
METHODOLOGY:
- Herbal and dietary supplements (HDS) are an increasingly common source of drug hepatotoxicity cases, but their prevalence and the reasons for their use among the general population are uncertain.
- This survey study evaluated nationally representative data from 9685 adults (mean age, 47.5 years; 51.8% women) enrolled in the National Health and Nutrition Examination Survey (NHANES) between January 2017 and March 2020.
- Participants reported their use of HDS and prescription drugs through personal interviews for a 30-day period prior to the survey date.
- Researchers compared the clinical features and baseline demographic characteristics of users of six potentially hepatotoxic botanicals (ie, turmeric, green tea, Garcinia cambogia, black cohosh, red yeast rice, and ashwagandha) with those of nonusers.
- The prevalence of use of these at-risk botanicals was compared with that of widely prescribed potentially hepatotoxic medications, including NSAIDs, simvastatin, and sertraline.
TAKEAWAY:
- In the cohort of 9685 participants, 4.7% of individuals reported consumption of at least one of the six potentially hepatotoxic botanicals in the past 30 days, with turmeric being the most common, followed by green tea.
- Extrapolating the survey data, researchers estimated that 15.6 million US adults use at least one of these six botanicals, which is comparable to the number of those prescribed potentially hepatotoxic drugs, including NSAIDs (14.8 million) and simvastatin (14.0 million). Sertraline use was lower (7.7 million).
- Most individuals used these botanicals without the recommendation of their healthcare provider.
- Those using botanicals were more likely to be older (adjusted odds ratio [aOR], 2.36; P = .04 for 40-59 years; aOR, 3.96; P = .001 for ≥ 60 years), to have some college education (aOR, 4.78; P < .001), and to have arthritis (aOR, 2.27; P < .001) than nonusers.
- The most common reasons for using any of these six potential hepatotoxic botanicals were to improve or maintain health or to prevent health problems or boost immunity.
IN PRACTICE:
“In light of the lack of regulatory oversight on the manufacturing and testing of botanical products, it is recommended that clinicians obtain a full medication and HDS use history when evaluating patients with unexplained symptoms or liver test abnormalities,” the authors wrote.
SOURCE:
The study, led by Alisa Likhitsup, MD, MPH, Division of Gastroenterology and Hepatology, University of Michigan, Ann Arbor, Michigan, was published online in JAMA Network Open.
LIMITATIONS:
The survey response rate was low at 43.9% for adults aged ≥ 20 years. As NHANES is a cross-sectional study, the causal relationship between consumption of the six botanicals of interest and the development of liver injury could not be determined. The use of HDS products and medications was self-reported in NHANES and not independently verified using source documents.
DISCLOSURES:
This study did not report any source of funding. Two authors declared receiving grants from pharmaceutical companies outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
The estimated number of US adults who consumed at least one of the six most frequently reported hepatotoxic botanicals in the last 30 days is similar to the number of patients prescribed potentially hepatotoxic drugs, including nonsteroidal anti-inflammatory drugs (NSAIDs) and simvastatin.
METHODOLOGY:
- Herbal and dietary supplements (HDS) are an increasingly common source of drug hepatotoxicity cases, but their prevalence and the reasons for their use among the general population are uncertain.
- This survey study evaluated nationally representative data from 9685 adults (mean age, 47.5 years; 51.8% women) enrolled in the National Health and Nutrition Examination Survey (NHANES) between January 2017 and March 2020.
- Participants reported their use of HDS and prescription drugs through personal interviews for a 30-day period prior to the survey date.
- Researchers compared the clinical features and baseline demographic characteristics of users of six potentially hepatotoxic botanicals (ie, turmeric, green tea, Garcinia cambogia, black cohosh, red yeast rice, and ashwagandha) with those of nonusers.
- The prevalence of use of these at-risk botanicals was compared with that of widely prescribed potentially hepatotoxic medications, including NSAIDs, simvastatin, and sertraline.
TAKEAWAY:
- In the cohort of 9685 participants, 4.7% of individuals reported consumption of at least one of the six potentially hepatotoxic botanicals in the past 30 days, with turmeric being the most common, followed by green tea.
- Extrapolating the survey data, researchers estimated that 15.6 million US adults use at least one of these six botanicals, which is comparable to the number of those prescribed potentially hepatotoxic drugs, including NSAIDs (14.8 million) and simvastatin (14.0 million). Sertraline use was lower (7.7 million).
- Most individuals used these botanicals without the recommendation of their healthcare provider.
- Those using botanicals were more likely to be older (adjusted odds ratio [aOR], 2.36; P = .04 for 40-59 years; aOR, 3.96; P = .001 for ≥ 60 years), to have some college education (aOR, 4.78; P < .001), and to have arthritis (aOR, 2.27; P < .001) than nonusers.
- The most common reasons for using any of these six potential hepatotoxic botanicals were to improve or maintain health or to prevent health problems or boost immunity.
IN PRACTICE:
“In light of the lack of regulatory oversight on the manufacturing and testing of botanical products, it is recommended that clinicians obtain a full medication and HDS use history when evaluating patients with unexplained symptoms or liver test abnormalities,” the authors wrote.
SOURCE:
The study, led by Alisa Likhitsup, MD, MPH, Division of Gastroenterology and Hepatology, University of Michigan, Ann Arbor, Michigan, was published online in JAMA Network Open.
LIMITATIONS:
The survey response rate was low at 43.9% for adults aged ≥ 20 years. As NHANES is a cross-sectional study, the causal relationship between consumption of the six botanicals of interest and the development of liver injury could not be determined. The use of HDS products and medications was self-reported in NHANES and not independently verified using source documents.
DISCLOSURES:
This study did not report any source of funding. Two authors declared receiving grants from pharmaceutical companies outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
New Study Links Sweetener to Heart Risk: What to Know
Is going sugar free really good advice for patients with cardiometabolic risk factors?
That’s the question raised by new Cleveland Clinic research, which suggests that consuming erythritol, a sweetener widely found in sugar-free and keto food products, could spur a prothrombotic response.
In the study, published in Arteriosclerosis, Thrombosis, and Vascular Biology, 10 healthy participants ate 30 grams of erythritol. Thirty minutes later, their blood showed enhanced platelet aggregation and increased markers of platelet responsiveness and activation.
Specifically, the researchers saw enhanced stimulus-dependent release of serotonin (a marker of platelet dense granules) and CXCL4 (a platelet alpha-granule marker).
“ With every single person, you see a prothrombotic effect with every single test that we did,” said study author Stanley Hazen, MD, PhD, chair of the Department of Cardiovascular & Metabolic Sciences at Cleveland Clinic in Ohio. By contrast, participants who ate 30 grams of glucose saw no such effect.
The erythritol itself does not activate the platelets, Dr. Hazen said, rather it lowers the threshold for triggering a response. This could make someone more prone to clotting, raising heart attack and stroke risk over time.
Though the mechanism is unknown, Dr. Hazen has an idea.
“There appears to be a receptor on platelets that is recognizing and sensing these sugar alcohols,” Dr. Hazen said, “much in the same way your taste bud for sweet is a receptor for recognizing a glucose or sugar molecule.”
“We’re very interested in trying to figure out what the receptor is,” Dr. Hazen said, “because I think that then becomes a very interesting potential target for further investigation and study into how this is linked to causing heart disease.”
The Past and Future of Erythritol Research
In 2001, the Food and Drug Administration classified erythritol as a “generally recognized as safe” food additive. A sugar alcohol that occurs naturally in foods like melon and grapes, erythritol is also manufactured by fermenting sugars. It’s about 70% as sweet as table sugar. Humans also produce small amounts of erythritol naturally: Our blood cells make it from glucose via the pentose phosphate pathway.
Previous research from Dr. Hazen’s group linked erythritol to a risk for major adverse cardiovascular events and clotting.
“Based on their previous study, I think this was a really important study to do in healthy individuals,” said Martha Field, PhD, assistant professor in the Division of Nutritional Sciences at Cornell University, Ithaca, New York, who was not involved in the study.
The earlier paper analyzed blood samples from participants with unknown erythritol intake, including some taken before the sweetener, and it was as widespread as it is today. That made disentangling the effects of eating erythritol vs naturally producing it more difficult.
By showing that eating erythritol raises markers associated with thrombosis, the new paper reinforces the importance of thinking about and developing a deeper understanding of what we put into our bodies.
“This paper was conducted in healthy individuals — might this be particularly dangerous for individuals who are at increased risk of clotting?” asked Dr. Field. “There are lots of genetic polymorphisms that increase your risk for clotting disorders or your propensity to form thrombosis.”
Field would like to see similar analyses of xylitol and sorbitol, other sugar alcohols found in sugar-free foods. And she called for more studies on erythritol that look at lower erythritol consumption over longer time periods.
Registered dietitian nutritionist Valisa E. Hedrick, PhD, agreed: Much more work is needed in this area, particularly in higher-risk groups, such as those with prediabetes and diabetes, said Dr. Hedrick, an associate professor in the Department of Human Nutrition, Foods, and Exercise at Virginia Tech, Blacksburg, who was not involved in the study.
“Because this study was conducted in healthy individuals, the impact of a small dose of glucose was negligible, as their body can effectively regulate blood glucose levels,” she said. “Because high blood glucose concentrations have also been shown to increase platelet reactivity, and consequently increase thrombosis potential, individuals who are not able to regulate their blood glucose levels, such as those with prediabetes and diabetes, could potentially see a similar effect on the body as erythritol when consuming large amounts of sugar.”
At the same time, “individuals with diabetes or prediabetes may be more inclined to consume erythritol as an alternative to sugar,” Dr. Hedrick added. “It will be important to design studies that include these individuals to determine if erythritol has an additive adverse effect on cardiac event risk.”
Criticism and Impact
Critics have suggested the 30-gram dose of erythritol ingested by study participants is unrealistic. Dr. Hazen said that it’s not.
Erythritol is often recommended as a one-to-one sugar replacement. And you could top 30 grams with a few servings of erythritol-sweetened ice cream or soda, Dr. Hazen said.
“The dose that we used, it’s on the high end, but it’s well within a physiologically relevant level,” he said.
Still others say the results are only relevant for people with preexisting heart trouble. But Dr. Hazen said they matter for the masses.
“I think there’s a significant health concern at a population level that this work is underscoring,” he said.
After all, heart disease risk factors like obesity, hypertension, diabetes, and smoking are common and quickly add up.
“If you look at middle-aged America, most people who experience a heart attack or stroke do not know that they have coronary artery disease, and the first recognition of it is that event,” Dr. Hazen said.
For now, Dr. Hazen recommends eating real sugar in moderation. He hopes future research will reveal a nonnutritive sweetener that doesn’t activate platelets.
The Bigger Picture
The new research adds yet another piece to the puzzle of whether nonnutritive sweeteners are better than sugar.
“I think these results are concerning,” said JoAnn E. Manson, MD, chief of the Division of Preventive Medicine at Brigham and Women’s Hospital and a professor of medicine at Harvard Medical School, both in Boston, Massachusetts. They “ may help explain the surprising results in some observational studies that artificial sweeteners are linked to an increased risk of cardiovascular disease.”
Dr. Manson, who was not involved in the new study, has conducted other research linking artificial sweetener use with stroke risk.
In an upcoming randomized clinical study, her team is comparing head-to-head sugar-sweetened beverages, drinks sweetened with calorie-free substitutes, and water to determine which is best for a range of cardiometabolic outcomes.
“We need more research on this question,” she said, “because these artificial sweeteners are commonly used, and many people are assuming that their health outcomes will be better with the artificial sweeteners than with sugar-sweetened products.”
A version of this article first appeared on Medscape.com.
Is going sugar free really good advice for patients with cardiometabolic risk factors?
That’s the question raised by new Cleveland Clinic research, which suggests that consuming erythritol, a sweetener widely found in sugar-free and keto food products, could spur a prothrombotic response.
In the study, published in Arteriosclerosis, Thrombosis, and Vascular Biology, 10 healthy participants ate 30 grams of erythritol. Thirty minutes later, their blood showed enhanced platelet aggregation and increased markers of platelet responsiveness and activation.
Specifically, the researchers saw enhanced stimulus-dependent release of serotonin (a marker of platelet dense granules) and CXCL4 (a platelet alpha-granule marker).
“ With every single person, you see a prothrombotic effect with every single test that we did,” said study author Stanley Hazen, MD, PhD, chair of the Department of Cardiovascular & Metabolic Sciences at Cleveland Clinic in Ohio. By contrast, participants who ate 30 grams of glucose saw no such effect.
The erythritol itself does not activate the platelets, Dr. Hazen said, rather it lowers the threshold for triggering a response. This could make someone more prone to clotting, raising heart attack and stroke risk over time.
Though the mechanism is unknown, Dr. Hazen has an idea.
“There appears to be a receptor on platelets that is recognizing and sensing these sugar alcohols,” Dr. Hazen said, “much in the same way your taste bud for sweet is a receptor for recognizing a glucose or sugar molecule.”
“We’re very interested in trying to figure out what the receptor is,” Dr. Hazen said, “because I think that then becomes a very interesting potential target for further investigation and study into how this is linked to causing heart disease.”
The Past and Future of Erythritol Research
In 2001, the Food and Drug Administration classified erythritol as a “generally recognized as safe” food additive. A sugar alcohol that occurs naturally in foods like melon and grapes, erythritol is also manufactured by fermenting sugars. It’s about 70% as sweet as table sugar. Humans also produce small amounts of erythritol naturally: Our blood cells make it from glucose via the pentose phosphate pathway.
Previous research from Dr. Hazen’s group linked erythritol to a risk for major adverse cardiovascular events and clotting.
“Based on their previous study, I think this was a really important study to do in healthy individuals,” said Martha Field, PhD, assistant professor in the Division of Nutritional Sciences at Cornell University, Ithaca, New York, who was not involved in the study.
The earlier paper analyzed blood samples from participants with unknown erythritol intake, including some taken before the sweetener, and it was as widespread as it is today. That made disentangling the effects of eating erythritol vs naturally producing it more difficult.
By showing that eating erythritol raises markers associated with thrombosis, the new paper reinforces the importance of thinking about and developing a deeper understanding of what we put into our bodies.
“This paper was conducted in healthy individuals — might this be particularly dangerous for individuals who are at increased risk of clotting?” asked Dr. Field. “There are lots of genetic polymorphisms that increase your risk for clotting disorders or your propensity to form thrombosis.”
Field would like to see similar analyses of xylitol and sorbitol, other sugar alcohols found in sugar-free foods. And she called for more studies on erythritol that look at lower erythritol consumption over longer time periods.
Registered dietitian nutritionist Valisa E. Hedrick, PhD, agreed: Much more work is needed in this area, particularly in higher-risk groups, such as those with prediabetes and diabetes, said Dr. Hedrick, an associate professor in the Department of Human Nutrition, Foods, and Exercise at Virginia Tech, Blacksburg, who was not involved in the study.
“Because this study was conducted in healthy individuals, the impact of a small dose of glucose was negligible, as their body can effectively regulate blood glucose levels,” she said. “Because high blood glucose concentrations have also been shown to increase platelet reactivity, and consequently increase thrombosis potential, individuals who are not able to regulate their blood glucose levels, such as those with prediabetes and diabetes, could potentially see a similar effect on the body as erythritol when consuming large amounts of sugar.”
At the same time, “individuals with diabetes or prediabetes may be more inclined to consume erythritol as an alternative to sugar,” Dr. Hedrick added. “It will be important to design studies that include these individuals to determine if erythritol has an additive adverse effect on cardiac event risk.”
Criticism and Impact
Critics have suggested the 30-gram dose of erythritol ingested by study participants is unrealistic. Dr. Hazen said that it’s not.
Erythritol is often recommended as a one-to-one sugar replacement. And you could top 30 grams with a few servings of erythritol-sweetened ice cream or soda, Dr. Hazen said.
“The dose that we used, it’s on the high end, but it’s well within a physiologically relevant level,” he said.
Still others say the results are only relevant for people with preexisting heart trouble. But Dr. Hazen said they matter for the masses.
“I think there’s a significant health concern at a population level that this work is underscoring,” he said.
After all, heart disease risk factors like obesity, hypertension, diabetes, and smoking are common and quickly add up.
“If you look at middle-aged America, most people who experience a heart attack or stroke do not know that they have coronary artery disease, and the first recognition of it is that event,” Dr. Hazen said.
For now, Dr. Hazen recommends eating real sugar in moderation. He hopes future research will reveal a nonnutritive sweetener that doesn’t activate platelets.
The Bigger Picture
The new research adds yet another piece to the puzzle of whether nonnutritive sweeteners are better than sugar.
“I think these results are concerning,” said JoAnn E. Manson, MD, chief of the Division of Preventive Medicine at Brigham and Women’s Hospital and a professor of medicine at Harvard Medical School, both in Boston, Massachusetts. They “ may help explain the surprising results in some observational studies that artificial sweeteners are linked to an increased risk of cardiovascular disease.”
Dr. Manson, who was not involved in the new study, has conducted other research linking artificial sweetener use with stroke risk.
In an upcoming randomized clinical study, her team is comparing head-to-head sugar-sweetened beverages, drinks sweetened with calorie-free substitutes, and water to determine which is best for a range of cardiometabolic outcomes.
“We need more research on this question,” she said, “because these artificial sweeteners are commonly used, and many people are assuming that their health outcomes will be better with the artificial sweeteners than with sugar-sweetened products.”
A version of this article first appeared on Medscape.com.
Is going sugar free really good advice for patients with cardiometabolic risk factors?
That’s the question raised by new Cleveland Clinic research, which suggests that consuming erythritol, a sweetener widely found in sugar-free and keto food products, could spur a prothrombotic response.
In the study, published in Arteriosclerosis, Thrombosis, and Vascular Biology, 10 healthy participants ate 30 grams of erythritol. Thirty minutes later, their blood showed enhanced platelet aggregation and increased markers of platelet responsiveness and activation.
Specifically, the researchers saw enhanced stimulus-dependent release of serotonin (a marker of platelet dense granules) and CXCL4 (a platelet alpha-granule marker).
“ With every single person, you see a prothrombotic effect with every single test that we did,” said study author Stanley Hazen, MD, PhD, chair of the Department of Cardiovascular & Metabolic Sciences at Cleveland Clinic in Ohio. By contrast, participants who ate 30 grams of glucose saw no such effect.
The erythritol itself does not activate the platelets, Dr. Hazen said, rather it lowers the threshold for triggering a response. This could make someone more prone to clotting, raising heart attack and stroke risk over time.
Though the mechanism is unknown, Dr. Hazen has an idea.
“There appears to be a receptor on platelets that is recognizing and sensing these sugar alcohols,” Dr. Hazen said, “much in the same way your taste bud for sweet is a receptor for recognizing a glucose or sugar molecule.”
“We’re very interested in trying to figure out what the receptor is,” Dr. Hazen said, “because I think that then becomes a very interesting potential target for further investigation and study into how this is linked to causing heart disease.”
The Past and Future of Erythritol Research
In 2001, the Food and Drug Administration classified erythritol as a “generally recognized as safe” food additive. A sugar alcohol that occurs naturally in foods like melon and grapes, erythritol is also manufactured by fermenting sugars. It’s about 70% as sweet as table sugar. Humans also produce small amounts of erythritol naturally: Our blood cells make it from glucose via the pentose phosphate pathway.
Previous research from Dr. Hazen’s group linked erythritol to a risk for major adverse cardiovascular events and clotting.
“Based on their previous study, I think this was a really important study to do in healthy individuals,” said Martha Field, PhD, assistant professor in the Division of Nutritional Sciences at Cornell University, Ithaca, New York, who was not involved in the study.
The earlier paper analyzed blood samples from participants with unknown erythritol intake, including some taken before the sweetener, and it was as widespread as it is today. That made disentangling the effects of eating erythritol vs naturally producing it more difficult.
By showing that eating erythritol raises markers associated with thrombosis, the new paper reinforces the importance of thinking about and developing a deeper understanding of what we put into our bodies.
“This paper was conducted in healthy individuals — might this be particularly dangerous for individuals who are at increased risk of clotting?” asked Dr. Field. “There are lots of genetic polymorphisms that increase your risk for clotting disorders or your propensity to form thrombosis.”
Field would like to see similar analyses of xylitol and sorbitol, other sugar alcohols found in sugar-free foods. And she called for more studies on erythritol that look at lower erythritol consumption over longer time periods.
Registered dietitian nutritionist Valisa E. Hedrick, PhD, agreed: Much more work is needed in this area, particularly in higher-risk groups, such as those with prediabetes and diabetes, said Dr. Hedrick, an associate professor in the Department of Human Nutrition, Foods, and Exercise at Virginia Tech, Blacksburg, who was not involved in the study.
“Because this study was conducted in healthy individuals, the impact of a small dose of glucose was negligible, as their body can effectively regulate blood glucose levels,” she said. “Because high blood glucose concentrations have also been shown to increase platelet reactivity, and consequently increase thrombosis potential, individuals who are not able to regulate their blood glucose levels, such as those with prediabetes and diabetes, could potentially see a similar effect on the body as erythritol when consuming large amounts of sugar.”
At the same time, “individuals with diabetes or prediabetes may be more inclined to consume erythritol as an alternative to sugar,” Dr. Hedrick added. “It will be important to design studies that include these individuals to determine if erythritol has an additive adverse effect on cardiac event risk.”
Criticism and Impact
Critics have suggested the 30-gram dose of erythritol ingested by study participants is unrealistic. Dr. Hazen said that it’s not.
Erythritol is often recommended as a one-to-one sugar replacement. And you could top 30 grams with a few servings of erythritol-sweetened ice cream or soda, Dr. Hazen said.
“The dose that we used, it’s on the high end, but it’s well within a physiologically relevant level,” he said.
Still others say the results are only relevant for people with preexisting heart trouble. But Dr. Hazen said they matter for the masses.
“I think there’s a significant health concern at a population level that this work is underscoring,” he said.
After all, heart disease risk factors like obesity, hypertension, diabetes, and smoking are common and quickly add up.
“If you look at middle-aged America, most people who experience a heart attack or stroke do not know that they have coronary artery disease, and the first recognition of it is that event,” Dr. Hazen said.
For now, Dr. Hazen recommends eating real sugar in moderation. He hopes future research will reveal a nonnutritive sweetener that doesn’t activate platelets.
The Bigger Picture
The new research adds yet another piece to the puzzle of whether nonnutritive sweeteners are better than sugar.
“I think these results are concerning,” said JoAnn E. Manson, MD, chief of the Division of Preventive Medicine at Brigham and Women’s Hospital and a professor of medicine at Harvard Medical School, both in Boston, Massachusetts. They “ may help explain the surprising results in some observational studies that artificial sweeteners are linked to an increased risk of cardiovascular disease.”
Dr. Manson, who was not involved in the new study, has conducted other research linking artificial sweetener use with stroke risk.
In an upcoming randomized clinical study, her team is comparing head-to-head sugar-sweetened beverages, drinks sweetened with calorie-free substitutes, and water to determine which is best for a range of cardiometabolic outcomes.
“We need more research on this question,” she said, “because these artificial sweeteners are commonly used, and many people are assuming that their health outcomes will be better with the artificial sweeteners than with sugar-sweetened products.”
A version of this article first appeared on Medscape.com.
FROM ARTERIOSCLEROSIS, THROMBOSIS, AND VASCULAR BIOLOGY
Did Statin Decision-Making Just Get Harder?
The new American Heart Association Predicting Risk of cardiovascular disease EVENTs (PREVENT) equation outperforms the standard pooled cohort equation (PCE). But there is a problem. A big one, actually.
The new score incorporates kidney function and social situation, and it eliminates race from the estimate. It was derived from larger, more modern datasets and can be applied to younger adults.
Two luminaries in preventive cardiology recently called the PREVENT calculator a “substantial improvement over the PCE in terms of accuracy and precision of risk estimates over the entire population and within demographic subgroups.”
Now to the Problem of PREVENT vs PCE
A recent study comparing PREVENT and PCE found that the PREVENT equation would assign lower 10-year risks to millions of US adults.
The authors estimated that the more accurate calculator would result in an estimated 14 million adults no longer reaching the statin eligibility risk threshold of 7.5% over 10 years. Nearly 3 million adults would also not reach the threshold for blood pressure therapy.
Because statins and blood pressure drugs reduce cardiac events, the authors further estimated that more than 100,000 excess myocardial infarctions (MIs) would occur if the PREVENT equation was used along with the current risk thresholds for statin eligibility.
The change in eligibility induced by PREVENT would affect more men than women and a greater proportion of Black adults than White adults.
The Tension of Arbitrary Thresholds
Modern cardiac therapeutics are amazing, but it’s still better to prevent an event than to treat it.
Statin drugs reduce cardiac risk by about 20%-25% at all absolute risks. American experts chose a 10-year risk of 7.5% as the threshold where statin benefit exceed risk. The USPSTF chose 10%. But the thresholds are arbitrary and derived only by opinion.
If your frame is population health, the more patients who take statins, the fewer cardiac events there will be. Anything that reduces statin use increases cardiac events.
The tension occurs because a more accurate equation decreases the number of people who meet eligibility for primary prevention therapy and therefore increases the number of cardiac events.
I write from the perspective of both a clinician and a possible patient. As a clinician, patients often ask me whether they should take a statin. (Sadly, most have not had a risk-based discussion with their clinician. But that is another column.)
The incidence of MI or stroke in a population has no effect on either of these scenarios. I see three broad categories of patients: minimizers, maximizers, and those in between.
I am a minimizer. I don’t worry much about heart disease. First, I won’t ignore symptoms, and I know that we have great treatments. Second, my wife, Staci, practiced hospice and palliative care medicine, and this taught me that worrying about one specific disease is folly. In the next decade, I, like anyone my age, could have many other bad things happen: cancer, trauma, infection, etc. Given these competing risks for serious disease, a PREVENT-calculated risk of 4% or a PCE-calculated risk of 8% makes no difference. I don’t like pills, and, with risks in this range, I decline statin drugs.
Then there are the maximizers. This person wants to avoid heart disease. Maybe they have family or friends who had terrible cardiac events. This person will maximize everything to avoid heart disease. The calculated 10-year risk makes little difference to a maximizer. Whether it is 4% or 8% matters not. They will take a statin or blood pressure drugs to reduce risk to as low as possible.
There are people between minimizers and maximizers. I am not sure that there are that many truly undecided people, but I challenge you to translate a difference of a few percent over a decade to them. I feel comfortable with numbers but struggle to sort out these small absolute differences over such a long time frame.
Other Issues With Risk-Based Decisions
Venk Murthy, MD, PhD, from the University of Michigan, wrote on X about two other issues with a risk-based decision. One is that it does not consider life-years lost. If a 50-year-old person has a fatal MI, that counts as one event. But in life-years lost, that one event is much worse than a fatal MI in a 79-year-old. Cardiac prevention, therefore, may have a greater effect in lower-risk younger people.
Another point Dr. Murthy made is that risk and benefit are driven by many different preferences and rare events. Minimizers and maximizers come to the decision with widely disparate preferences. Risk-based decisions treat patients as if they were automatons who make decisions based simply on calculated probabilities. Clinicians know how untrue that is.
Conclusion
If you carry forward the logic of being disturbed by the estimate of more MIs using the PREVENT score, then you could justify putting statins in the water — because that would reduce population estimates of MIs.
I am not disturbed by the PREVENT score. Clinicians treat individuals, not populations. Individuals want a more accurate score. They don’t need expert-based thresholds. Clinician and patient can discuss the evidence and come up with an agreeable decision, one that is concordant with a person’s goals. The next patient may have a different decision despite seeing the same evidence.
The tension created by this comparative study exposes the gap between population health and basic clinical care. I don’t think clinicians need to worry about populations.
Dr. Mandrola, a clinical electrophysiologist at Baptist Medical Associates, Louisville, Kentucky, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
The new American Heart Association Predicting Risk of cardiovascular disease EVENTs (PREVENT) equation outperforms the standard pooled cohort equation (PCE). But there is a problem. A big one, actually.
The new score incorporates kidney function and social situation, and it eliminates race from the estimate. It was derived from larger, more modern datasets and can be applied to younger adults.
Two luminaries in preventive cardiology recently called the PREVENT calculator a “substantial improvement over the PCE in terms of accuracy and precision of risk estimates over the entire population and within demographic subgroups.”
Now to the Problem of PREVENT vs PCE
A recent study comparing PREVENT and PCE found that the PREVENT equation would assign lower 10-year risks to millions of US adults.
The authors estimated that the more accurate calculator would result in an estimated 14 million adults no longer reaching the statin eligibility risk threshold of 7.5% over 10 years. Nearly 3 million adults would also not reach the threshold for blood pressure therapy.
Because statins and blood pressure drugs reduce cardiac events, the authors further estimated that more than 100,000 excess myocardial infarctions (MIs) would occur if the PREVENT equation was used along with the current risk thresholds for statin eligibility.
The change in eligibility induced by PREVENT would affect more men than women and a greater proportion of Black adults than White adults.
The Tension of Arbitrary Thresholds
Modern cardiac therapeutics are amazing, but it’s still better to prevent an event than to treat it.
Statin drugs reduce cardiac risk by about 20%-25% at all absolute risks. American experts chose a 10-year risk of 7.5% as the threshold where statin benefit exceed risk. The USPSTF chose 10%. But the thresholds are arbitrary and derived only by opinion.
If your frame is population health, the more patients who take statins, the fewer cardiac events there will be. Anything that reduces statin use increases cardiac events.
The tension occurs because a more accurate equation decreases the number of people who meet eligibility for primary prevention therapy and therefore increases the number of cardiac events.
I write from the perspective of both a clinician and a possible patient. As a clinician, patients often ask me whether they should take a statin. (Sadly, most have not had a risk-based discussion with their clinician. But that is another column.)
The incidence of MI or stroke in a population has no effect on either of these scenarios. I see three broad categories of patients: minimizers, maximizers, and those in between.
I am a minimizer. I don’t worry much about heart disease. First, I won’t ignore symptoms, and I know that we have great treatments. Second, my wife, Staci, practiced hospice and palliative care medicine, and this taught me that worrying about one specific disease is folly. In the next decade, I, like anyone my age, could have many other bad things happen: cancer, trauma, infection, etc. Given these competing risks for serious disease, a PREVENT-calculated risk of 4% or a PCE-calculated risk of 8% makes no difference. I don’t like pills, and, with risks in this range, I decline statin drugs.
Then there are the maximizers. This person wants to avoid heart disease. Maybe they have family or friends who had terrible cardiac events. This person will maximize everything to avoid heart disease. The calculated 10-year risk makes little difference to a maximizer. Whether it is 4% or 8% matters not. They will take a statin or blood pressure drugs to reduce risk to as low as possible.
There are people between minimizers and maximizers. I am not sure that there are that many truly undecided people, but I challenge you to translate a difference of a few percent over a decade to them. I feel comfortable with numbers but struggle to sort out these small absolute differences over such a long time frame.
Other Issues With Risk-Based Decisions
Venk Murthy, MD, PhD, from the University of Michigan, wrote on X about two other issues with a risk-based decision. One is that it does not consider life-years lost. If a 50-year-old person has a fatal MI, that counts as one event. But in life-years lost, that one event is much worse than a fatal MI in a 79-year-old. Cardiac prevention, therefore, may have a greater effect in lower-risk younger people.
Another point Dr. Murthy made is that risk and benefit are driven by many different preferences and rare events. Minimizers and maximizers come to the decision with widely disparate preferences. Risk-based decisions treat patients as if they were automatons who make decisions based simply on calculated probabilities. Clinicians know how untrue that is.
Conclusion
If you carry forward the logic of being disturbed by the estimate of more MIs using the PREVENT score, then you could justify putting statins in the water — because that would reduce population estimates of MIs.
I am not disturbed by the PREVENT score. Clinicians treat individuals, not populations. Individuals want a more accurate score. They don’t need expert-based thresholds. Clinician and patient can discuss the evidence and come up with an agreeable decision, one that is concordant with a person’s goals. The next patient may have a different decision despite seeing the same evidence.
The tension created by this comparative study exposes the gap between population health and basic clinical care. I don’t think clinicians need to worry about populations.
Dr. Mandrola, a clinical electrophysiologist at Baptist Medical Associates, Louisville, Kentucky, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
The new American Heart Association Predicting Risk of cardiovascular disease EVENTs (PREVENT) equation outperforms the standard pooled cohort equation (PCE). But there is a problem. A big one, actually.
The new score incorporates kidney function and social situation, and it eliminates race from the estimate. It was derived from larger, more modern datasets and can be applied to younger adults.
Two luminaries in preventive cardiology recently called the PREVENT calculator a “substantial improvement over the PCE in terms of accuracy and precision of risk estimates over the entire population and within demographic subgroups.”
Now to the Problem of PREVENT vs PCE
A recent study comparing PREVENT and PCE found that the PREVENT equation would assign lower 10-year risks to millions of US adults.
The authors estimated that the more accurate calculator would result in an estimated 14 million adults no longer reaching the statin eligibility risk threshold of 7.5% over 10 years. Nearly 3 million adults would also not reach the threshold for blood pressure therapy.
Because statins and blood pressure drugs reduce cardiac events, the authors further estimated that more than 100,000 excess myocardial infarctions (MIs) would occur if the PREVENT equation was used along with the current risk thresholds for statin eligibility.
The change in eligibility induced by PREVENT would affect more men than women and a greater proportion of Black adults than White adults.
The Tension of Arbitrary Thresholds
Modern cardiac therapeutics are amazing, but it’s still better to prevent an event than to treat it.
Statin drugs reduce cardiac risk by about 20%-25% at all absolute risks. American experts chose a 10-year risk of 7.5% as the threshold where statin benefit exceed risk. The USPSTF chose 10%. But the thresholds are arbitrary and derived only by opinion.
If your frame is population health, the more patients who take statins, the fewer cardiac events there will be. Anything that reduces statin use increases cardiac events.
The tension occurs because a more accurate equation decreases the number of people who meet eligibility for primary prevention therapy and therefore increases the number of cardiac events.
I write from the perspective of both a clinician and a possible patient. As a clinician, patients often ask me whether they should take a statin. (Sadly, most have not had a risk-based discussion with their clinician. But that is another column.)
The incidence of MI or stroke in a population has no effect on either of these scenarios. I see three broad categories of patients: minimizers, maximizers, and those in between.
I am a minimizer. I don’t worry much about heart disease. First, I won’t ignore symptoms, and I know that we have great treatments. Second, my wife, Staci, practiced hospice and palliative care medicine, and this taught me that worrying about one specific disease is folly. In the next decade, I, like anyone my age, could have many other bad things happen: cancer, trauma, infection, etc. Given these competing risks for serious disease, a PREVENT-calculated risk of 4% or a PCE-calculated risk of 8% makes no difference. I don’t like pills, and, with risks in this range, I decline statin drugs.
Then there are the maximizers. This person wants to avoid heart disease. Maybe they have family or friends who had terrible cardiac events. This person will maximize everything to avoid heart disease. The calculated 10-year risk makes little difference to a maximizer. Whether it is 4% or 8% matters not. They will take a statin or blood pressure drugs to reduce risk to as low as possible.
There are people between minimizers and maximizers. I am not sure that there are that many truly undecided people, but I challenge you to translate a difference of a few percent over a decade to them. I feel comfortable with numbers but struggle to sort out these small absolute differences over such a long time frame.
Other Issues With Risk-Based Decisions
Venk Murthy, MD, PhD, from the University of Michigan, wrote on X about two other issues with a risk-based decision. One is that it does not consider life-years lost. If a 50-year-old person has a fatal MI, that counts as one event. But in life-years lost, that one event is much worse than a fatal MI in a 79-year-old. Cardiac prevention, therefore, may have a greater effect in lower-risk younger people.
Another point Dr. Murthy made is that risk and benefit are driven by many different preferences and rare events. Minimizers and maximizers come to the decision with widely disparate preferences. Risk-based decisions treat patients as if they were automatons who make decisions based simply on calculated probabilities. Clinicians know how untrue that is.
Conclusion
If you carry forward the logic of being disturbed by the estimate of more MIs using the PREVENT score, then you could justify putting statins in the water — because that would reduce population estimates of MIs.
I am not disturbed by the PREVENT score. Clinicians treat individuals, not populations. Individuals want a more accurate score. They don’t need expert-based thresholds. Clinician and patient can discuss the evidence and come up with an agreeable decision, one that is concordant with a person’s goals. The next patient may have a different decision despite seeing the same evidence.
The tension created by this comparative study exposes the gap between population health and basic clinical care. I don’t think clinicians need to worry about populations.
Dr. Mandrola, a clinical electrophysiologist at Baptist Medical Associates, Louisville, Kentucky, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
On Second Thought: The Truth About Beta-Blockers
This transcript has been edited for clarity.
Giving patients a beta-blocker after a myocardial infarction is standard of care. It’s in the guidelines. It’s one of the performance measures used by the American College of Cardiology (ACC) and the American Heart Association (AHA). If you aren’t putting your post–acute coronary syndrome (ACS) patients on a beta-blocker, the ACC and the AHA both think you suck.
They are very disappointed in you, just like your mother was when you told her you didn’t want to become a surgeon because you don’t like waking up early, your hands shake when you get nervous, it’s not your fault, there’s nothing you can do about it, so just leave me alone!
The data on beta-blockers are decades old. In the time before stents, statins, angiotensin-converting enzyme inhibitors, and dual antiplatelet therapy, when patients either died or got better on their own, beta-blockers showed major benefits. Studies like the Norwegian Multicenter Study Group, the BHAT trial, and the ISIS-1 trial proved the benefits of beta blockade. These studies date back to the 1980s, when you could call a study ISIS without controversy.
It was a simpler time, when all you had to worry about was the Cold War, apartheid, and the global AIDS pandemic. It was a time when doctors smoked in their offices, and patients had bigger infarcts that caused large scars and systolic dysfunction. That world is no longer our world, except for the war, the global pandemic, and the out-of-control gas prices.
The reality is that, before troponins, we probably missed most small heart attacks. Now, most infarcts are small, and most patients walk away from their heart attacks with essentially normal hearts. Do beta-blockers still matter? If you’re a fan of Cochrane reviews, the answer is yes.
In 2021, Cochrane published a review of beta-blockers in patients without heart failure after myocardial infarction (MI). The authors of that analysis concluded, after the usual caveats about heterogeneity, potential bias, and the whims of a random universe, that, yes, beta-blockers do reduce mortality. The risk ratio for max all-cause mortality was 0.81.
What does that mean practically? The absolute risk was reduced from 10.9% to 8.7%, a 2.2–percentage point absolute decrease and about a 20% relative drop. A little math gives us a third number: 46. That’s the number needed to treat. If you think about how many patients you admit during a typical week of critical care unit with an MI, a number needed to treat of 46 is a pretty good trade-off for a fairly inexpensive medication with fairly minimal side effects.
Of course, these are the same people who claim that masks don’t stop the spread of COVID-19. Sure, were they the only people who thought that handwashing was the best way to stop a respiratory virus? No. We all believed that fantasy for far longer than we should have. Not everybody can bat a thousand, if by batting a thousand, you mean reflecting on how your words will impact on a broader population primed to believe misinformation because of the increasingly toxic social media environment and worsening politicization and radicalization of our politics.
By the way, if any of you want to come to Canada, you can stay with me. Things are incrementally better here. In this day and age, incrementally better is the best we can hope for.
Here’s the wrinkle with the Cochrane beta-blocker review: Many of the studies took place before early revascularization became the norm and before our current armamentarium of drugs became standard of care.
Back in the day, bed rest and the power of positive thinking were the mainstays of cardiac treatment. Also, many of these studies mixed together ST-segment MI (STEMI) and non-STEMI patients, so you’re obviously going to see more benefits in STEMI patients who are at higher risk. Some of them used intravenous (IV) beta-blockers right away, whereas some were looking only at oral beta-blockers started days after the infarct.
We don’t use IV beta-blockers that much anymore because of the risk for shock.
Also, some studies had short-term follow-up where the benefits were less pronounced, and some studies used doses and types of beta-blockers rarely used today. Some of the studies had a mix of coronary and heart failure patients, which muddies the water because the heart failure patients would clearly benefit from being on a beta-blocker.
Basically, the data are not definitive because they are old and don’t reflect our current standard of care. The data contain a heterogeneous mix of patients that aren’t really relevant to the question that we’re asking. The question we’re asking is, should you put all your post-MI patients on a beta-blocker routinely, even if they don’t have heart failure?
The REDUCE-AMI trial is the first of a few trials testing, or to be more accurate, retesting, whether beta-blockers are useful after an MI. BETAMI, REBOOT, DANBLOCK— you’ll be hearing these names in the next few years, either because the studies get published or because they’re the Twitter handles of people harassing you online. Either/or. (By the way, I’ll be cold in my grave before I call it X.)
For now, REDUCE-AMI is the first across the finish line, and at least in cardiology, finishing first is a good thing. This study enrolled patients with ACS, both STEMI and non-STEMI, with a post-MI ejection fraction ≥ 50%, and the result was nothing. The risk ratio for all-cause mortality was 0.94 and was not statistically significant.
In absolute terms, that’s a reduction from 4.1% to 3.9%, or a 0.2–percentage point decrease; this translates into a number needed to treat of 500, which is 10 times higher than what the Cochrane review found. That’s if you assume that there is, in fact, a small benefit amidst all the statistical noise, which there probably isn’t.
Now, studies like this can never rule out small effects, either positive or negative, so maybe there is a small benefit from using beta-blockers. If it’s there, it’s really small. Do beta-blockers work? Well, yes, obviously, for heart failure and atrial fibrillation — which, let’s face it, are not exactly rare and often coexist in patients with heart disease. They probably aren’t that great as blood pressure pills, but that’s a story for another day and another video.
Yes, beta-blockers are useful pills, and they are standard of care, just maybe not for post-MI patients with normal ejection fractions because they probably don’t really need them. They worked in the pre-stent, pre-aspirin, pre-anything era.
That’s not our world anymore. Things change. It’s not the 1980s. That’s why I don’t have a mullet, and that’s why you need to update your kitchen.
Dr. Labos, a cardiologist at Kirkland Medical Center, Montreal, Quebec, Canada, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Giving patients a beta-blocker after a myocardial infarction is standard of care. It’s in the guidelines. It’s one of the performance measures used by the American College of Cardiology (ACC) and the American Heart Association (AHA). If you aren’t putting your post–acute coronary syndrome (ACS) patients on a beta-blocker, the ACC and the AHA both think you suck.
They are very disappointed in you, just like your mother was when you told her you didn’t want to become a surgeon because you don’t like waking up early, your hands shake when you get nervous, it’s not your fault, there’s nothing you can do about it, so just leave me alone!
The data on beta-blockers are decades old. In the time before stents, statins, angiotensin-converting enzyme inhibitors, and dual antiplatelet therapy, when patients either died or got better on their own, beta-blockers showed major benefits. Studies like the Norwegian Multicenter Study Group, the BHAT trial, and the ISIS-1 trial proved the benefits of beta blockade. These studies date back to the 1980s, when you could call a study ISIS without controversy.
It was a simpler time, when all you had to worry about was the Cold War, apartheid, and the global AIDS pandemic. It was a time when doctors smoked in their offices, and patients had bigger infarcts that caused large scars and systolic dysfunction. That world is no longer our world, except for the war, the global pandemic, and the out-of-control gas prices.
The reality is that, before troponins, we probably missed most small heart attacks. Now, most infarcts are small, and most patients walk away from their heart attacks with essentially normal hearts. Do beta-blockers still matter? If you’re a fan of Cochrane reviews, the answer is yes.
In 2021, Cochrane published a review of beta-blockers in patients without heart failure after myocardial infarction (MI). The authors of that analysis concluded, after the usual caveats about heterogeneity, potential bias, and the whims of a random universe, that, yes, beta-blockers do reduce mortality. The risk ratio for max all-cause mortality was 0.81.
What does that mean practically? The absolute risk was reduced from 10.9% to 8.7%, a 2.2–percentage point absolute decrease and about a 20% relative drop. A little math gives us a third number: 46. That’s the number needed to treat. If you think about how many patients you admit during a typical week of critical care unit with an MI, a number needed to treat of 46 is a pretty good trade-off for a fairly inexpensive medication with fairly minimal side effects.
Of course, these are the same people who claim that masks don’t stop the spread of COVID-19. Sure, were they the only people who thought that handwashing was the best way to stop a respiratory virus? No. We all believed that fantasy for far longer than we should have. Not everybody can bat a thousand, if by batting a thousand, you mean reflecting on how your words will impact on a broader population primed to believe misinformation because of the increasingly toxic social media environment and worsening politicization and radicalization of our politics.
By the way, if any of you want to come to Canada, you can stay with me. Things are incrementally better here. In this day and age, incrementally better is the best we can hope for.
Here’s the wrinkle with the Cochrane beta-blocker review: Many of the studies took place before early revascularization became the norm and before our current armamentarium of drugs became standard of care.
Back in the day, bed rest and the power of positive thinking were the mainstays of cardiac treatment. Also, many of these studies mixed together ST-segment MI (STEMI) and non-STEMI patients, so you’re obviously going to see more benefits in STEMI patients who are at higher risk. Some of them used intravenous (IV) beta-blockers right away, whereas some were looking only at oral beta-blockers started days after the infarct.
We don’t use IV beta-blockers that much anymore because of the risk for shock.
Also, some studies had short-term follow-up where the benefits were less pronounced, and some studies used doses and types of beta-blockers rarely used today. Some of the studies had a mix of coronary and heart failure patients, which muddies the water because the heart failure patients would clearly benefit from being on a beta-blocker.
Basically, the data are not definitive because they are old and don’t reflect our current standard of care. The data contain a heterogeneous mix of patients that aren’t really relevant to the question that we’re asking. The question we’re asking is, should you put all your post-MI patients on a beta-blocker routinely, even if they don’t have heart failure?
The REDUCE-AMI trial is the first of a few trials testing, or to be more accurate, retesting, whether beta-blockers are useful after an MI. BETAMI, REBOOT, DANBLOCK— you’ll be hearing these names in the next few years, either because the studies get published or because they’re the Twitter handles of people harassing you online. Either/or. (By the way, I’ll be cold in my grave before I call it X.)
For now, REDUCE-AMI is the first across the finish line, and at least in cardiology, finishing first is a good thing. This study enrolled patients with ACS, both STEMI and non-STEMI, with a post-MI ejection fraction ≥ 50%, and the result was nothing. The risk ratio for all-cause mortality was 0.94 and was not statistically significant.
In absolute terms, that’s a reduction from 4.1% to 3.9%, or a 0.2–percentage point decrease; this translates into a number needed to treat of 500, which is 10 times higher than what the Cochrane review found. That’s if you assume that there is, in fact, a small benefit amidst all the statistical noise, which there probably isn’t.
Now, studies like this can never rule out small effects, either positive or negative, so maybe there is a small benefit from using beta-blockers. If it’s there, it’s really small. Do beta-blockers work? Well, yes, obviously, for heart failure and atrial fibrillation — which, let’s face it, are not exactly rare and often coexist in patients with heart disease. They probably aren’t that great as blood pressure pills, but that’s a story for another day and another video.
Yes, beta-blockers are useful pills, and they are standard of care, just maybe not for post-MI patients with normal ejection fractions because they probably don’t really need them. They worked in the pre-stent, pre-aspirin, pre-anything era.
That’s not our world anymore. Things change. It’s not the 1980s. That’s why I don’t have a mullet, and that’s why you need to update your kitchen.
Dr. Labos, a cardiologist at Kirkland Medical Center, Montreal, Quebec, Canada, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Giving patients a beta-blocker after a myocardial infarction is standard of care. It’s in the guidelines. It’s one of the performance measures used by the American College of Cardiology (ACC) and the American Heart Association (AHA). If you aren’t putting your post–acute coronary syndrome (ACS) patients on a beta-blocker, the ACC and the AHA both think you suck.
They are very disappointed in you, just like your mother was when you told her you didn’t want to become a surgeon because you don’t like waking up early, your hands shake when you get nervous, it’s not your fault, there’s nothing you can do about it, so just leave me alone!
The data on beta-blockers are decades old. In the time before stents, statins, angiotensin-converting enzyme inhibitors, and dual antiplatelet therapy, when patients either died or got better on their own, beta-blockers showed major benefits. Studies like the Norwegian Multicenter Study Group, the BHAT trial, and the ISIS-1 trial proved the benefits of beta blockade. These studies date back to the 1980s, when you could call a study ISIS without controversy.
It was a simpler time, when all you had to worry about was the Cold War, apartheid, and the global AIDS pandemic. It was a time when doctors smoked in their offices, and patients had bigger infarcts that caused large scars and systolic dysfunction. That world is no longer our world, except for the war, the global pandemic, and the out-of-control gas prices.
The reality is that, before troponins, we probably missed most small heart attacks. Now, most infarcts are small, and most patients walk away from their heart attacks with essentially normal hearts. Do beta-blockers still matter? If you’re a fan of Cochrane reviews, the answer is yes.
In 2021, Cochrane published a review of beta-blockers in patients without heart failure after myocardial infarction (MI). The authors of that analysis concluded, after the usual caveats about heterogeneity, potential bias, and the whims of a random universe, that, yes, beta-blockers do reduce mortality. The risk ratio for max all-cause mortality was 0.81.
What does that mean practically? The absolute risk was reduced from 10.9% to 8.7%, a 2.2–percentage point absolute decrease and about a 20% relative drop. A little math gives us a third number: 46. That’s the number needed to treat. If you think about how many patients you admit during a typical week of critical care unit with an MI, a number needed to treat of 46 is a pretty good trade-off for a fairly inexpensive medication with fairly minimal side effects.
Of course, these are the same people who claim that masks don’t stop the spread of COVID-19. Sure, were they the only people who thought that handwashing was the best way to stop a respiratory virus? No. We all believed that fantasy for far longer than we should have. Not everybody can bat a thousand, if by batting a thousand, you mean reflecting on how your words will impact on a broader population primed to believe misinformation because of the increasingly toxic social media environment and worsening politicization and radicalization of our politics.
By the way, if any of you want to come to Canada, you can stay with me. Things are incrementally better here. In this day and age, incrementally better is the best we can hope for.
Here’s the wrinkle with the Cochrane beta-blocker review: Many of the studies took place before early revascularization became the norm and before our current armamentarium of drugs became standard of care.
Back in the day, bed rest and the power of positive thinking were the mainstays of cardiac treatment. Also, many of these studies mixed together ST-segment MI (STEMI) and non-STEMI patients, so you’re obviously going to see more benefits in STEMI patients who are at higher risk. Some of them used intravenous (IV) beta-blockers right away, whereas some were looking only at oral beta-blockers started days after the infarct.
We don’t use IV beta-blockers that much anymore because of the risk for shock.
Also, some studies had short-term follow-up where the benefits were less pronounced, and some studies used doses and types of beta-blockers rarely used today. Some of the studies had a mix of coronary and heart failure patients, which muddies the water because the heart failure patients would clearly benefit from being on a beta-blocker.
Basically, the data are not definitive because they are old and don’t reflect our current standard of care. The data contain a heterogeneous mix of patients that aren’t really relevant to the question that we’re asking. The question we’re asking is, should you put all your post-MI patients on a beta-blocker routinely, even if they don’t have heart failure?
The REDUCE-AMI trial is the first of a few trials testing, or to be more accurate, retesting, whether beta-blockers are useful after an MI. BETAMI, REBOOT, DANBLOCK— you’ll be hearing these names in the next few years, either because the studies get published or because they’re the Twitter handles of people harassing you online. Either/or. (By the way, I’ll be cold in my grave before I call it X.)
For now, REDUCE-AMI is the first across the finish line, and at least in cardiology, finishing first is a good thing. This study enrolled patients with ACS, both STEMI and non-STEMI, with a post-MI ejection fraction ≥ 50%, and the result was nothing. The risk ratio for all-cause mortality was 0.94 and was not statistically significant.
In absolute terms, that’s a reduction from 4.1% to 3.9%, or a 0.2–percentage point decrease; this translates into a number needed to treat of 500, which is 10 times higher than what the Cochrane review found. That’s if you assume that there is, in fact, a small benefit amidst all the statistical noise, which there probably isn’t.
Now, studies like this can never rule out small effects, either positive or negative, so maybe there is a small benefit from using beta-blockers. If it’s there, it’s really small. Do beta-blockers work? Well, yes, obviously, for heart failure and atrial fibrillation — which, let’s face it, are not exactly rare and often coexist in patients with heart disease. They probably aren’t that great as blood pressure pills, but that’s a story for another day and another video.
Yes, beta-blockers are useful pills, and they are standard of care, just maybe not for post-MI patients with normal ejection fractions because they probably don’t really need them. They worked in the pre-stent, pre-aspirin, pre-anything era.
That’s not our world anymore. Things change. It’s not the 1980s. That’s why I don’t have a mullet, and that’s why you need to update your kitchen.
Dr. Labos, a cardiologist at Kirkland Medical Center, Montreal, Quebec, Canada, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
New First-Line Therapies for Migraine Prevention
This transcript has been edited for clarity.
Today I am going to talk about the position statement from the American Headache Society (AHS) “Calcitonin gene-related peptide [CGRP]–targeting therapies are a first-line option for the prevention of migraine”. This update is of critical importance because about three fourths of people with migraine get their care from a primary care clinician, not from a neurologist or a headache specialist. CGRP-targeting therapies have transformed migraine care at the specialty level, but many in primary care are not yet familiar with this class of medicines. Until this new statement was released, CGRPs were not viewed as first-line agents for migraine. That has now changed.
Two main types of therapy for people with migraine headache are: (1) acute or abortive therapy (when a headache develops, it is treated), and (2) preventive therapy. Preventive therapy is typically used when the patient has headaches on 4 or more days per month. Preventive therapy is aimed at reducing the frequency and severity of headaches. About 40% of patients with migraine qualify for preventive therapy, but only a minority are receiving it.
The armamentarium for preventive therapy of migraines had not changed in a long time — until now. First-line preventive therapy has traditionally consisted of three classes of agents: beta-blockers, tricyclic antidepressants, and topiramate. These medicines were developed for different therapeutic purposes, yet they work for migraines. These drugs may have off-target effects that can make them difficult to tolerate.
Based on new evidence, candesartan — an angiotensin receptor blocker (ARB) — is now also a first-line drug for migraine. This is good news, because ARBs are a drug class that we have a lot of experience with, are easy to use, and could be an excellent choice for people with concomitant hypertension or chronic kidney disease. The serotonin-norepinephrine reuptake inhibitors (venlafaxine and duloxetine) are also considered first-line agents for migraine treatment.
In the AHS’s new position statement, the two main drug classes are small-molecule CGRP receptor antagonists and monoclonal antibodies.
The role of the neuropeptide CGRP in migraine was originally discovered after finding that blood levels of CGRP were elevated during migraine attacks. This led to the discovery of agents that blocked CGRP, initially for acute treatment of migraine, and then for preventive therapy. Multiple clinical studies show the CGRP targeting therapies to be as or even more effective than traditional first-line agents at decreasing the number of migraine days per month.
The efficacy and safety of these agents have been demonstrated in both randomized trials and in real-world studies. Other important positive endpoints include fewer days of migraine, reduced acute medication use, and improvements in many quality-of-life outcomes. Studies also have shown that CGRP-targeting therapies are well tolerated and safe, with very few serious adverse events.
Furthermore, studies have shown the CGRP targeting therapies are effective in individuals who have failed multiple other first-line therapies. They fit now both as first-line agents and as agents that can be used in difficult-to-treat patients as well as in patients who struggle with acute medication overuse, which is often very challenging.
To quote from the AHS statement,
Side effects are uncommon and can include hypertension, constipation, and Raynaud phenomenon.
The position statement is strong and is based on a lot of evidence and clinical experience. CGRP-targeting therapies are now first-line agents for the prevention of migraine headache. We should learn more about and begin to feel comfortable using this class of agents because they stand to benefit our patients greatly. I’d suggest looking at the table below and picking one new agent to become familiar with so that you can add that agent to your toolbox. 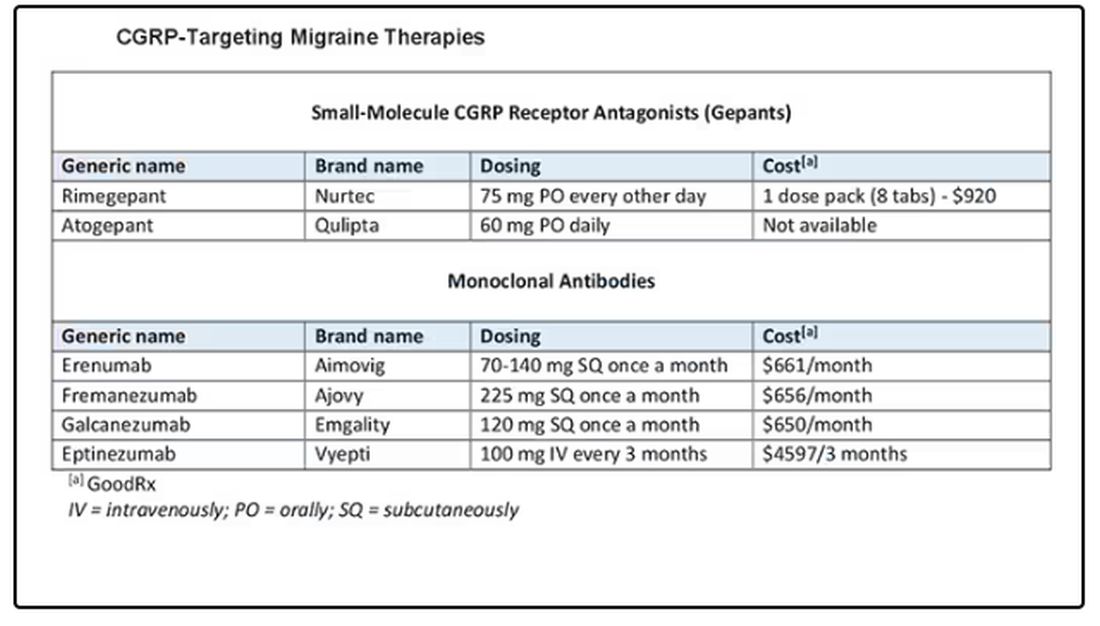
Dr. Skolnik, professor, Department of Family Medicine, Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, Pennsylvania, and associate director, Department of Family Medicine, Abington Jefferson Health, Abington, Pennsylvania, disclosed ties with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, Bayer, and Teva.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Today I am going to talk about the position statement from the American Headache Society (AHS) “Calcitonin gene-related peptide [CGRP]–targeting therapies are a first-line option for the prevention of migraine”. This update is of critical importance because about three fourths of people with migraine get their care from a primary care clinician, not from a neurologist or a headache specialist. CGRP-targeting therapies have transformed migraine care at the specialty level, but many in primary care are not yet familiar with this class of medicines. Until this new statement was released, CGRPs were not viewed as first-line agents for migraine. That has now changed.
Two main types of therapy for people with migraine headache are: (1) acute or abortive therapy (when a headache develops, it is treated), and (2) preventive therapy. Preventive therapy is typically used when the patient has headaches on 4 or more days per month. Preventive therapy is aimed at reducing the frequency and severity of headaches. About 40% of patients with migraine qualify for preventive therapy, but only a minority are receiving it.
The armamentarium for preventive therapy of migraines had not changed in a long time — until now. First-line preventive therapy has traditionally consisted of three classes of agents: beta-blockers, tricyclic antidepressants, and topiramate. These medicines were developed for different therapeutic purposes, yet they work for migraines. These drugs may have off-target effects that can make them difficult to tolerate.
Based on new evidence, candesartan — an angiotensin receptor blocker (ARB) — is now also a first-line drug for migraine. This is good news, because ARBs are a drug class that we have a lot of experience with, are easy to use, and could be an excellent choice for people with concomitant hypertension or chronic kidney disease. The serotonin-norepinephrine reuptake inhibitors (venlafaxine and duloxetine) are also considered first-line agents for migraine treatment.
In the AHS’s new position statement, the two main drug classes are small-molecule CGRP receptor antagonists and monoclonal antibodies.
The role of the neuropeptide CGRP in migraine was originally discovered after finding that blood levels of CGRP were elevated during migraine attacks. This led to the discovery of agents that blocked CGRP, initially for acute treatment of migraine, and then for preventive therapy. Multiple clinical studies show the CGRP targeting therapies to be as or even more effective than traditional first-line agents at decreasing the number of migraine days per month.
The efficacy and safety of these agents have been demonstrated in both randomized trials and in real-world studies. Other important positive endpoints include fewer days of migraine, reduced acute medication use, and improvements in many quality-of-life outcomes. Studies also have shown that CGRP-targeting therapies are well tolerated and safe, with very few serious adverse events.
Furthermore, studies have shown the CGRP targeting therapies are effective in individuals who have failed multiple other first-line therapies. They fit now both as first-line agents and as agents that can be used in difficult-to-treat patients as well as in patients who struggle with acute medication overuse, which is often very challenging.
To quote from the AHS statement,
Side effects are uncommon and can include hypertension, constipation, and Raynaud phenomenon.
The position statement is strong and is based on a lot of evidence and clinical experience. CGRP-targeting therapies are now first-line agents for the prevention of migraine headache. We should learn more about and begin to feel comfortable using this class of agents because they stand to benefit our patients greatly. I’d suggest looking at the table below and picking one new agent to become familiar with so that you can add that agent to your toolbox. 
Dr. Skolnik, professor, Department of Family Medicine, Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, Pennsylvania, and associate director, Department of Family Medicine, Abington Jefferson Health, Abington, Pennsylvania, disclosed ties with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, Bayer, and Teva.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Today I am going to talk about the position statement from the American Headache Society (AHS) “Calcitonin gene-related peptide [CGRP]–targeting therapies are a first-line option for the prevention of migraine”. This update is of critical importance because about three fourths of people with migraine get their care from a primary care clinician, not from a neurologist or a headache specialist. CGRP-targeting therapies have transformed migraine care at the specialty level, but many in primary care are not yet familiar with this class of medicines. Until this new statement was released, CGRPs were not viewed as first-line agents for migraine. That has now changed.
Two main types of therapy for people with migraine headache are: (1) acute or abortive therapy (when a headache develops, it is treated), and (2) preventive therapy. Preventive therapy is typically used when the patient has headaches on 4 or more days per month. Preventive therapy is aimed at reducing the frequency and severity of headaches. About 40% of patients with migraine qualify for preventive therapy, but only a minority are receiving it.
The armamentarium for preventive therapy of migraines had not changed in a long time — until now. First-line preventive therapy has traditionally consisted of three classes of agents: beta-blockers, tricyclic antidepressants, and topiramate. These medicines were developed for different therapeutic purposes, yet they work for migraines. These drugs may have off-target effects that can make them difficult to tolerate.
Based on new evidence, candesartan — an angiotensin receptor blocker (ARB) — is now also a first-line drug for migraine. This is good news, because ARBs are a drug class that we have a lot of experience with, are easy to use, and could be an excellent choice for people with concomitant hypertension or chronic kidney disease. The serotonin-norepinephrine reuptake inhibitors (venlafaxine and duloxetine) are also considered first-line agents for migraine treatment.
In the AHS’s new position statement, the two main drug classes are small-molecule CGRP receptor antagonists and monoclonal antibodies.
The role of the neuropeptide CGRP in migraine was originally discovered after finding that blood levels of CGRP were elevated during migraine attacks. This led to the discovery of agents that blocked CGRP, initially for acute treatment of migraine, and then for preventive therapy. Multiple clinical studies show the CGRP targeting therapies to be as or even more effective than traditional first-line agents at decreasing the number of migraine days per month.
The efficacy and safety of these agents have been demonstrated in both randomized trials and in real-world studies. Other important positive endpoints include fewer days of migraine, reduced acute medication use, and improvements in many quality-of-life outcomes. Studies also have shown that CGRP-targeting therapies are well tolerated and safe, with very few serious adverse events.
Furthermore, studies have shown the CGRP targeting therapies are effective in individuals who have failed multiple other first-line therapies. They fit now both as first-line agents and as agents that can be used in difficult-to-treat patients as well as in patients who struggle with acute medication overuse, which is often very challenging.
To quote from the AHS statement,
Side effects are uncommon and can include hypertension, constipation, and Raynaud phenomenon.
The position statement is strong and is based on a lot of evidence and clinical experience. CGRP-targeting therapies are now first-line agents for the prevention of migraine headache. We should learn more about and begin to feel comfortable using this class of agents because they stand to benefit our patients greatly. I’d suggest looking at the table below and picking one new agent to become familiar with so that you can add that agent to your toolbox. 
Dr. Skolnik, professor, Department of Family Medicine, Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, Pennsylvania, and associate director, Department of Family Medicine, Abington Jefferson Health, Abington, Pennsylvania, disclosed ties with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, Bayer, and Teva.
A version of this article appeared on Medscape.com.