User login
How docs in firearm-friendly states talk gun safety
Samuel Mathis, MD, tries to cover a lot of ground during a wellness exam for his patients. Nutrition, immunizations, dental hygiene, and staying safe at school are a few of the topics on his list. And the Texas pediatrician asks one more question of children and their parents: “Are there any firearms in the house?”
If the answer is “yes,” Dr. Mathis discusses safety courses and other ideas with the families. “Rather than ask a bunch of questions, often I will say it’s recommended to keep them locked up and don’t forget toddlers can climb heights that you never would have envisioned,” said Dr. Mathis, an assistant professor at the University of Texas Medical Branch, Galveston.
Dr. Mathis said some of his physician colleagues are wary of bringing up the topic of guns in a state that leads the nation with more than 1 million registered firearms. “My discussion is more on firearm responsibility and just making sure they are taking extra steps to keep themselves and everyone around them safe. That works much better in these discussions.”
Gun safety: Public health concern, not politics
The statistics tell why:
- Unintentional shooting deaths by children rose by nearly one third in a 3-month period in 2020, compared with the same period in 2019.
- Of every 10 gun deaths in the United States, 6 are by suicide.
- As of July 28, 372 mass shootings have occured.
- Firearms now represent the leading cause of death among the nation’s youth.
In 2018, the editors of Annals of Internal Medicine urged physicians in the United States to sign a pledge to talk with their patients about guns in the home. To date, at least 3,664 have done so.
In 2019, the American Academy of Family Medicine, with other leading physician and public health organizations, issued a “call to action,” recommending ways to reduce firearm-related injury and death in the United States. Physicians can and should address the issue, it said, by counseling patients about firearm safety.
“This is just another part of healthcare,” said Sarah C. Nosal, MD, a member of the board of directors of the AAFP, who practices at the Urban Horizons Family Health Center, New York.
Dr. Nosal said she asks about firearms during every well-child visit. She also focuses on patients with a history of depression or suicide attempts and those who have experienced domestic violence.
Are physicians counseling patients about gun safety?
A 2018 survey of physicians found that 73% of the 71 who responded agreed to discuss gun safety with at-risk patients. But just 5% said they always talk to those at-risk patients, according to Melanie G. Hagen, MD, professor of internal medicine at the University of Florida, Gainesville, who led the study. While the overwhelming majority agreed that gun safety is a public health issue, only 55% said they felt comfortable initiating conversations about firearms with their patients.
Have things changed since then? “Probably not,” Dr. Hagen said in an interview. She cited some reasons, at least in her state.
One obstacle is that many people, including physicians, believe that Florida’s physician gag law, which prohibited physicians from asking about a patient’s firearm ownership, was still in effect. The law, passed in 2011, was overturned in 2017. In her survey, 76% said they were aware it had been overturned. But that awareness appears not to be universal, she said.
In a 2020 report about physician involvement in promoting gun safety, researchers noted four main challenges: lingering fears about the overturned law and potential liability from violating it, feeling unprepared, worry that patients don’t want to discuss the topic, and lack of time to talk about it during a rushed office visit.
But recent research suggests that patients are often open to talking about gun safety, and another study found that if physicians are given educational materials on firearm safety, more will counsel patients about gun safety.
Are patients and parents receptive?
Parents welcome discussion from health care providers about gun safety, according to a study from the University of Pennsylvania, Philadelphia.
Researchers asked roughly 100 parents to watch a short video about a firearm safety program designed to prevent accidents and suicides from guns. The program, still under study, involves a discussion between a parent and a pediatrician, with information given on secure storage of guns and the offering of a free cable lock.
The parents, about equally divided between gun owners and non–gun owners, said they were open to discussion about firearm safety, especially when the conversation involves their child’s pediatrician. Among the gun owners, only one in three said all their firearms were locked, unloaded, and stored properly. But after getting the safety information, 64% said they would change the way they stored their firearms.
A different program that offered pediatricians educational materials on firearm safety, as well as free firearm locks for distribution, increased the likelihood that the physicians would counsel patients on gun safety, other researchers reported.
Getting the conversation started
Some patients “bristle” when they’re asked about guns, Dr. Hagen said. Focusing on the “why” of the question can soften their response. One of her patients, a man in his 80s, had worked as a prison guard. After he was diagnosed with clinical depression, she asked him if he ever thought about ending his life. He said yes.
“And in Florida, I know a lot of people have guns,” she said. The state ranks second in the nation, with more than a half million registered weapons.
When Dr. Hagen asked him if he had firearms at home, he balked. Why did she need to know? “People do get defensive,” she said. “Luckily, I had a good relationship with this man, and he was willing to listen to me. If it’s someone I have a good relationship with, and I have this initial bristling, if I say: ‘I’m worried about you, I’m worried about your safety,’ that changes the entire conversation.”
She talked through the best plan for this patient, and he agreed to give his weapons to his son to keep.
Likewise, she talks with family members of dementia patients, urging them to be sure the weapons are stored and locked to prevent tragic accidents.
Dr. Nosal said reading the room is key. “Often, we are having the conversation with a parent with a child present,” she said. “Perhaps that is not the conversation the parent or guardian wanted to have with the child present.” In such a situation, she suggests asking the parent if they would talk about it solo.
“It can be a challenge to know the appropriate way to start the conversation,” Dr. Mathis said. The topic is not taught in medical school, although many experts think it should be. Dr. Hagen recently delivered a lecture to medical students about how to broach the topic with patients. She said she hopes it will become a regular event.
“It really comes down to being willing to be open and just ask that first question in a nonjudgmental way,” Dr. Mathis said. It helps, too, he said, for physicians to remember what he always tries to keep in mind: “My job isn’t politics, my job is health.”
Among the points Dr. Hagen makes in her lecture about talking to patients about guns are the following:
- Every day, more than 110 Americans are killed with guns.
- Gun violence accounts for just 1%-2% of those deaths, but mass shootings serve to shine a light on the issue of gun safety.
- 110,000 firearm injuries a year require medical or legal attention. Each year, more than 1,200 children in this country die from gun-related injuries.
- More than 33,000 people, on average, die in the United States each year from gun violence, including more than 21,000 from suicide.
- About 31% of all U.S. households have firearms; 22% of U.S. adults own one or more.
- Guns are 70% less likely to be stored locked and unloaded in homes where suicides or unintentional gun injuries occur.
- Action points: Identify risk, counsel patients at risk, act when someone is in imminent danger (such as unsafe practices or suicide threats).
- Focus on identifying adults who have a risk of inflicting violence on self or others.
- Focus on health and well-being with all; be conversational and educational.
- Clinicians should ask five crucial questions, all with an “L,” if firearms are in the home: Is it Loaded? Locked? Are Little children present? Is the owner feeling Low? Are they Learned [educated] in gun safety?
A version of this article first appeared on Medscape.com.
Samuel Mathis, MD, tries to cover a lot of ground during a wellness exam for his patients. Nutrition, immunizations, dental hygiene, and staying safe at school are a few of the topics on his list. And the Texas pediatrician asks one more question of children and their parents: “Are there any firearms in the house?”
If the answer is “yes,” Dr. Mathis discusses safety courses and other ideas with the families. “Rather than ask a bunch of questions, often I will say it’s recommended to keep them locked up and don’t forget toddlers can climb heights that you never would have envisioned,” said Dr. Mathis, an assistant professor at the University of Texas Medical Branch, Galveston.
Dr. Mathis said some of his physician colleagues are wary of bringing up the topic of guns in a state that leads the nation with more than 1 million registered firearms. “My discussion is more on firearm responsibility and just making sure they are taking extra steps to keep themselves and everyone around them safe. That works much better in these discussions.”
Gun safety: Public health concern, not politics
The statistics tell why:
- Unintentional shooting deaths by children rose by nearly one third in a 3-month period in 2020, compared with the same period in 2019.
- Of every 10 gun deaths in the United States, 6 are by suicide.
- As of July 28, 372 mass shootings have occured.
- Firearms now represent the leading cause of death among the nation’s youth.
In 2018, the editors of Annals of Internal Medicine urged physicians in the United States to sign a pledge to talk with their patients about guns in the home. To date, at least 3,664 have done so.
In 2019, the American Academy of Family Medicine, with other leading physician and public health organizations, issued a “call to action,” recommending ways to reduce firearm-related injury and death in the United States. Physicians can and should address the issue, it said, by counseling patients about firearm safety.
“This is just another part of healthcare,” said Sarah C. Nosal, MD, a member of the board of directors of the AAFP, who practices at the Urban Horizons Family Health Center, New York.
Dr. Nosal said she asks about firearms during every well-child visit. She also focuses on patients with a history of depression or suicide attempts and those who have experienced domestic violence.
Are physicians counseling patients about gun safety?
A 2018 survey of physicians found that 73% of the 71 who responded agreed to discuss gun safety with at-risk patients. But just 5% said they always talk to those at-risk patients, according to Melanie G. Hagen, MD, professor of internal medicine at the University of Florida, Gainesville, who led the study. While the overwhelming majority agreed that gun safety is a public health issue, only 55% said they felt comfortable initiating conversations about firearms with their patients.
Have things changed since then? “Probably not,” Dr. Hagen said in an interview. She cited some reasons, at least in her state.
One obstacle is that many people, including physicians, believe that Florida’s physician gag law, which prohibited physicians from asking about a patient’s firearm ownership, was still in effect. The law, passed in 2011, was overturned in 2017. In her survey, 76% said they were aware it had been overturned. But that awareness appears not to be universal, she said.
In a 2020 report about physician involvement in promoting gun safety, researchers noted four main challenges: lingering fears about the overturned law and potential liability from violating it, feeling unprepared, worry that patients don’t want to discuss the topic, and lack of time to talk about it during a rushed office visit.
But recent research suggests that patients are often open to talking about gun safety, and another study found that if physicians are given educational materials on firearm safety, more will counsel patients about gun safety.
Are patients and parents receptive?
Parents welcome discussion from health care providers about gun safety, according to a study from the University of Pennsylvania, Philadelphia.
Researchers asked roughly 100 parents to watch a short video about a firearm safety program designed to prevent accidents and suicides from guns. The program, still under study, involves a discussion between a parent and a pediatrician, with information given on secure storage of guns and the offering of a free cable lock.
The parents, about equally divided between gun owners and non–gun owners, said they were open to discussion about firearm safety, especially when the conversation involves their child’s pediatrician. Among the gun owners, only one in three said all their firearms were locked, unloaded, and stored properly. But after getting the safety information, 64% said they would change the way they stored their firearms.
A different program that offered pediatricians educational materials on firearm safety, as well as free firearm locks for distribution, increased the likelihood that the physicians would counsel patients on gun safety, other researchers reported.
Getting the conversation started
Some patients “bristle” when they’re asked about guns, Dr. Hagen said. Focusing on the “why” of the question can soften their response. One of her patients, a man in his 80s, had worked as a prison guard. After he was diagnosed with clinical depression, she asked him if he ever thought about ending his life. He said yes.
“And in Florida, I know a lot of people have guns,” she said. The state ranks second in the nation, with more than a half million registered weapons.
When Dr. Hagen asked him if he had firearms at home, he balked. Why did she need to know? “People do get defensive,” she said. “Luckily, I had a good relationship with this man, and he was willing to listen to me. If it’s someone I have a good relationship with, and I have this initial bristling, if I say: ‘I’m worried about you, I’m worried about your safety,’ that changes the entire conversation.”
She talked through the best plan for this patient, and he agreed to give his weapons to his son to keep.
Likewise, she talks with family members of dementia patients, urging them to be sure the weapons are stored and locked to prevent tragic accidents.
Dr. Nosal said reading the room is key. “Often, we are having the conversation with a parent with a child present,” she said. “Perhaps that is not the conversation the parent or guardian wanted to have with the child present.” In such a situation, she suggests asking the parent if they would talk about it solo.
“It can be a challenge to know the appropriate way to start the conversation,” Dr. Mathis said. The topic is not taught in medical school, although many experts think it should be. Dr. Hagen recently delivered a lecture to medical students about how to broach the topic with patients. She said she hopes it will become a regular event.
“It really comes down to being willing to be open and just ask that first question in a nonjudgmental way,” Dr. Mathis said. It helps, too, he said, for physicians to remember what he always tries to keep in mind: “My job isn’t politics, my job is health.”
Among the points Dr. Hagen makes in her lecture about talking to patients about guns are the following:
- Every day, more than 110 Americans are killed with guns.
- Gun violence accounts for just 1%-2% of those deaths, but mass shootings serve to shine a light on the issue of gun safety.
- 110,000 firearm injuries a year require medical or legal attention. Each year, more than 1,200 children in this country die from gun-related injuries.
- More than 33,000 people, on average, die in the United States each year from gun violence, including more than 21,000 from suicide.
- About 31% of all U.S. households have firearms; 22% of U.S. adults own one or more.
- Guns are 70% less likely to be stored locked and unloaded in homes where suicides or unintentional gun injuries occur.
- Action points: Identify risk, counsel patients at risk, act when someone is in imminent danger (such as unsafe practices or suicide threats).
- Focus on identifying adults who have a risk of inflicting violence on self or others.
- Focus on health and well-being with all; be conversational and educational.
- Clinicians should ask five crucial questions, all with an “L,” if firearms are in the home: Is it Loaded? Locked? Are Little children present? Is the owner feeling Low? Are they Learned [educated] in gun safety?
A version of this article first appeared on Medscape.com.
Samuel Mathis, MD, tries to cover a lot of ground during a wellness exam for his patients. Nutrition, immunizations, dental hygiene, and staying safe at school are a few of the topics on his list. And the Texas pediatrician asks one more question of children and their parents: “Are there any firearms in the house?”
If the answer is “yes,” Dr. Mathis discusses safety courses and other ideas with the families. “Rather than ask a bunch of questions, often I will say it’s recommended to keep them locked up and don’t forget toddlers can climb heights that you never would have envisioned,” said Dr. Mathis, an assistant professor at the University of Texas Medical Branch, Galveston.
Dr. Mathis said some of his physician colleagues are wary of bringing up the topic of guns in a state that leads the nation with more than 1 million registered firearms. “My discussion is more on firearm responsibility and just making sure they are taking extra steps to keep themselves and everyone around them safe. That works much better in these discussions.”
Gun safety: Public health concern, not politics
The statistics tell why:
- Unintentional shooting deaths by children rose by nearly one third in a 3-month period in 2020, compared with the same period in 2019.
- Of every 10 gun deaths in the United States, 6 are by suicide.
- As of July 28, 372 mass shootings have occured.
- Firearms now represent the leading cause of death among the nation’s youth.
In 2018, the editors of Annals of Internal Medicine urged physicians in the United States to sign a pledge to talk with their patients about guns in the home. To date, at least 3,664 have done so.
In 2019, the American Academy of Family Medicine, with other leading physician and public health organizations, issued a “call to action,” recommending ways to reduce firearm-related injury and death in the United States. Physicians can and should address the issue, it said, by counseling patients about firearm safety.
“This is just another part of healthcare,” said Sarah C. Nosal, MD, a member of the board of directors of the AAFP, who practices at the Urban Horizons Family Health Center, New York.
Dr. Nosal said she asks about firearms during every well-child visit. She also focuses on patients with a history of depression or suicide attempts and those who have experienced domestic violence.
Are physicians counseling patients about gun safety?
A 2018 survey of physicians found that 73% of the 71 who responded agreed to discuss gun safety with at-risk patients. But just 5% said they always talk to those at-risk patients, according to Melanie G. Hagen, MD, professor of internal medicine at the University of Florida, Gainesville, who led the study. While the overwhelming majority agreed that gun safety is a public health issue, only 55% said they felt comfortable initiating conversations about firearms with their patients.
Have things changed since then? “Probably not,” Dr. Hagen said in an interview. She cited some reasons, at least in her state.
One obstacle is that many people, including physicians, believe that Florida’s physician gag law, which prohibited physicians from asking about a patient’s firearm ownership, was still in effect. The law, passed in 2011, was overturned in 2017. In her survey, 76% said they were aware it had been overturned. But that awareness appears not to be universal, she said.
In a 2020 report about physician involvement in promoting gun safety, researchers noted four main challenges: lingering fears about the overturned law and potential liability from violating it, feeling unprepared, worry that patients don’t want to discuss the topic, and lack of time to talk about it during a rushed office visit.
But recent research suggests that patients are often open to talking about gun safety, and another study found that if physicians are given educational materials on firearm safety, more will counsel patients about gun safety.
Are patients and parents receptive?
Parents welcome discussion from health care providers about gun safety, according to a study from the University of Pennsylvania, Philadelphia.
Researchers asked roughly 100 parents to watch a short video about a firearm safety program designed to prevent accidents and suicides from guns. The program, still under study, involves a discussion between a parent and a pediatrician, with information given on secure storage of guns and the offering of a free cable lock.
The parents, about equally divided between gun owners and non–gun owners, said they were open to discussion about firearm safety, especially when the conversation involves their child’s pediatrician. Among the gun owners, only one in three said all their firearms were locked, unloaded, and stored properly. But after getting the safety information, 64% said they would change the way they stored their firearms.
A different program that offered pediatricians educational materials on firearm safety, as well as free firearm locks for distribution, increased the likelihood that the physicians would counsel patients on gun safety, other researchers reported.
Getting the conversation started
Some patients “bristle” when they’re asked about guns, Dr. Hagen said. Focusing on the “why” of the question can soften their response. One of her patients, a man in his 80s, had worked as a prison guard. After he was diagnosed with clinical depression, she asked him if he ever thought about ending his life. He said yes.
“And in Florida, I know a lot of people have guns,” she said. The state ranks second in the nation, with more than a half million registered weapons.
When Dr. Hagen asked him if he had firearms at home, he balked. Why did she need to know? “People do get defensive,” she said. “Luckily, I had a good relationship with this man, and he was willing to listen to me. If it’s someone I have a good relationship with, and I have this initial bristling, if I say: ‘I’m worried about you, I’m worried about your safety,’ that changes the entire conversation.”
She talked through the best plan for this patient, and he agreed to give his weapons to his son to keep.
Likewise, she talks with family members of dementia patients, urging them to be sure the weapons are stored and locked to prevent tragic accidents.
Dr. Nosal said reading the room is key. “Often, we are having the conversation with a parent with a child present,” she said. “Perhaps that is not the conversation the parent or guardian wanted to have with the child present.” In such a situation, she suggests asking the parent if they would talk about it solo.
“It can be a challenge to know the appropriate way to start the conversation,” Dr. Mathis said. The topic is not taught in medical school, although many experts think it should be. Dr. Hagen recently delivered a lecture to medical students about how to broach the topic with patients. She said she hopes it will become a regular event.
“It really comes down to being willing to be open and just ask that first question in a nonjudgmental way,” Dr. Mathis said. It helps, too, he said, for physicians to remember what he always tries to keep in mind: “My job isn’t politics, my job is health.”
Among the points Dr. Hagen makes in her lecture about talking to patients about guns are the following:
- Every day, more than 110 Americans are killed with guns.
- Gun violence accounts for just 1%-2% of those deaths, but mass shootings serve to shine a light on the issue of gun safety.
- 110,000 firearm injuries a year require medical or legal attention. Each year, more than 1,200 children in this country die from gun-related injuries.
- More than 33,000 people, on average, die in the United States each year from gun violence, including more than 21,000 from suicide.
- About 31% of all U.S. households have firearms; 22% of U.S. adults own one or more.
- Guns are 70% less likely to be stored locked and unloaded in homes where suicides or unintentional gun injuries occur.
- Action points: Identify risk, counsel patients at risk, act when someone is in imminent danger (such as unsafe practices or suicide threats).
- Focus on identifying adults who have a risk of inflicting violence on self or others.
- Focus on health and well-being with all; be conversational and educational.
- Clinicians should ask five crucial questions, all with an “L,” if firearms are in the home: Is it Loaded? Locked? Are Little children present? Is the owner feeling Low? Are they Learned [educated] in gun safety?
A version of this article first appeared on Medscape.com.
Experts: EPA should assess risk of sunscreens’ UV filters
The , an expert panel of the National Academies of Sciences, Engineering, and Medicine (NAS) said on Aug. 9.
The assessment is urgently needed, the experts said, and the results should be shared with the Food and Drug Administration, which oversees sunscreens.
In its 400-page report, titled the Review of Fate, Exposure, and Effects of Sunscreens in Aquatic Environments and Implications for Sunscreen Usage and Human Health, the panel does not make recommendations but suggests that such an EPA risk assessment should highlight gaps in knowledge.
“We are teeing up the critical information that will be used to take on the challenge of risk assessment,” Charles A. Menzie, PhD, chair of the committee that wrote the report, said at a media briefing Aug. 9 when the report was released. Dr. Menzie is a principal at Exponent, Inc., an engineering and scientific consulting firm. He is former executive director of the Society of Environmental Toxicology and Chemistry.
The EPA sponsored the study, which was conducted by a committee of the National Academy of Sciences, a nonprofit, nongovernmental organization authorized by Congress that studies issues related to science, technology, and medicine.
Balancing aquatic, human health concerns
Such an EPA assessment, Dr. Menzie said in a statement, will help inform efforts to understand the environmental effects of UV filters as well as clarify a path forward for managing sunscreens. For years, concerns have been raised about the potential toxicity of sunscreens regarding many marine and freshwater aquatic organisms, especially coral. That concern, however, must be balanced against the benefits of sunscreens, which are known to protect against skin cancer. A low percentage of people use sunscreen regularly, Dr. Menzie and other panel members said.
“Only about a third of the U.S. population regularly uses sunscreen,” Mark Cullen, MD, vice chair of the NAS committee and former director of the Center for Population Health Sciences, Stanford (Calif.) University, said at the briefing. About 70% or 80% of people use it at the beach or outdoors, he said.
Report background, details
UV filters are the active ingredients in physical as well as chemical sunscreen products. They decrease the amount of UV radiation that reaches the skin. They have been found in water, sediments, and marine organisms, both saltwater and freshwater.
Currently, 17 UV filters are used in U.S. sunscreens; 15 of those are organic, such as oxybenzone and avobenzone, and are used in chemical sunscreens. They work by absorbing the rays before they damage the skin. In addition, two inorganic filters, which are used in physical sunscreens, sit on the skin and as a shield to block the rays.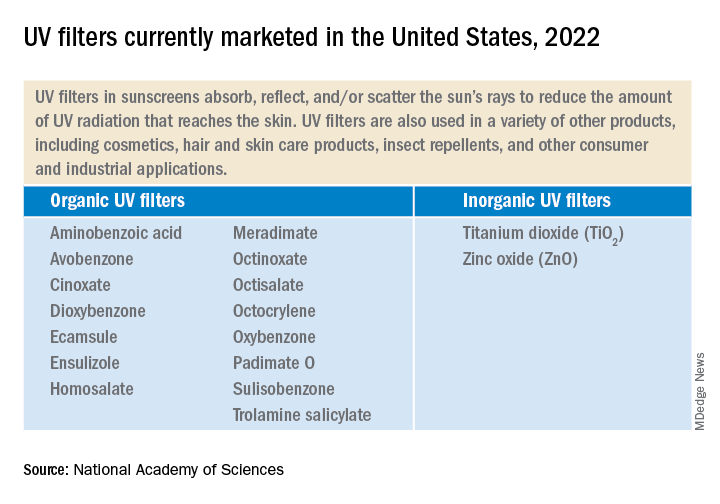
UV filters enter bodies of water by direct release, as when sunscreens rinse off people while swimming or while engaging in other water activities. They also enter bodies of water in storm water runoff and wastewater.
Lab toxicity tests, which are the most widely used, provide effects data for ecologic risk assessment. The tests are more often used in the study of short-term, not long-term exposure. Test results have shown that in high enough concentrations, some UV filters can be toxic to algal, invertebrate, and fish species.
But much information is lacking, the experts said. Toxicity data for many species, for instance, are limited. There are few studies on the longer-term environmental effects of UV filter exposure. Not enough is known about the rate at which the filters degrade in the environment. The filters accumulate in higher amounts in different areas. Recreational water areas have higher concentrations.
The recommendations
The panel is urging the EPA to complete a formal risk assessment of the UV filters “with some urgency,” Dr. Cullen said. That will enable decisions to be made about the use of the products. The risks to aquatic life must be balanced against the need for sun protection to reduce skin cancer risk.
The experts made two recommendations:
- The EPA should conduct ecologic risk assessments for all the UV filters now marketed and for all new ones. The assessment should evaluate the filters individually as well as the risk from co-occurring filters. The assessments should take into account the different exposure scenarios.
- The EPA, along with partner agencies, and sunscreen and UV filter manufacturers should fund, support, and conduct research and share data. Research should include study of human health outcomes if usage and availability of sunscreens change.
Dermatologists should “continue to emphasize the importance of protection from UV radiation in every way that can be done,” Dr. Cullen said, including the use of sunscreen as well as other protective practices, such as wearing long sleeves and hats, seeking shade, and avoiding the sun during peak hours.
A dermatologist’s perspective
“I applaud their scientific curiosity to know one way or the other whether this is an issue,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, DC. “I welcome this investigation.”
The multitude of studies, Dr. Friedman said, don’t always agree about whether the filters pose dangers. He noted that the concentration of UV filters detected in water is often lower than the concentrations found to be harmful in a lab setting to marine life, specifically coral.
However, he said, “these studies are snapshots.” For that reason, calling for more assessment of risk is desirable, Dr. Friedman said, but “I want to be sure the call to do more research is not an admission of guilt. It’s very easy to vilify sunscreens – but the facts we know are that UV light causes skin cancer and aging, and sunscreen protects us against this.”
Dr. Friedman has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The , an expert panel of the National Academies of Sciences, Engineering, and Medicine (NAS) said on Aug. 9.
The assessment is urgently needed, the experts said, and the results should be shared with the Food and Drug Administration, which oversees sunscreens.
In its 400-page report, titled the Review of Fate, Exposure, and Effects of Sunscreens in Aquatic Environments and Implications for Sunscreen Usage and Human Health, the panel does not make recommendations but suggests that such an EPA risk assessment should highlight gaps in knowledge.
“We are teeing up the critical information that will be used to take on the challenge of risk assessment,” Charles A. Menzie, PhD, chair of the committee that wrote the report, said at a media briefing Aug. 9 when the report was released. Dr. Menzie is a principal at Exponent, Inc., an engineering and scientific consulting firm. He is former executive director of the Society of Environmental Toxicology and Chemistry.
The EPA sponsored the study, which was conducted by a committee of the National Academy of Sciences, a nonprofit, nongovernmental organization authorized by Congress that studies issues related to science, technology, and medicine.
Balancing aquatic, human health concerns
Such an EPA assessment, Dr. Menzie said in a statement, will help inform efforts to understand the environmental effects of UV filters as well as clarify a path forward for managing sunscreens. For years, concerns have been raised about the potential toxicity of sunscreens regarding many marine and freshwater aquatic organisms, especially coral. That concern, however, must be balanced against the benefits of sunscreens, which are known to protect against skin cancer. A low percentage of people use sunscreen regularly, Dr. Menzie and other panel members said.
“Only about a third of the U.S. population regularly uses sunscreen,” Mark Cullen, MD, vice chair of the NAS committee and former director of the Center for Population Health Sciences, Stanford (Calif.) University, said at the briefing. About 70% or 80% of people use it at the beach or outdoors, he said.
Report background, details
UV filters are the active ingredients in physical as well as chemical sunscreen products. They decrease the amount of UV radiation that reaches the skin. They have been found in water, sediments, and marine organisms, both saltwater and freshwater.
Currently, 17 UV filters are used in U.S. sunscreens; 15 of those are organic, such as oxybenzone and avobenzone, and are used in chemical sunscreens. They work by absorbing the rays before they damage the skin. In addition, two inorganic filters, which are used in physical sunscreens, sit on the skin and as a shield to block the rays.
UV filters enter bodies of water by direct release, as when sunscreens rinse off people while swimming or while engaging in other water activities. They also enter bodies of water in storm water runoff and wastewater.
Lab toxicity tests, which are the most widely used, provide effects data for ecologic risk assessment. The tests are more often used in the study of short-term, not long-term exposure. Test results have shown that in high enough concentrations, some UV filters can be toxic to algal, invertebrate, and fish species.
But much information is lacking, the experts said. Toxicity data for many species, for instance, are limited. There are few studies on the longer-term environmental effects of UV filter exposure. Not enough is known about the rate at which the filters degrade in the environment. The filters accumulate in higher amounts in different areas. Recreational water areas have higher concentrations.
The recommendations
The panel is urging the EPA to complete a formal risk assessment of the UV filters “with some urgency,” Dr. Cullen said. That will enable decisions to be made about the use of the products. The risks to aquatic life must be balanced against the need for sun protection to reduce skin cancer risk.
The experts made two recommendations:
- The EPA should conduct ecologic risk assessments for all the UV filters now marketed and for all new ones. The assessment should evaluate the filters individually as well as the risk from co-occurring filters. The assessments should take into account the different exposure scenarios.
- The EPA, along with partner agencies, and sunscreen and UV filter manufacturers should fund, support, and conduct research and share data. Research should include study of human health outcomes if usage and availability of sunscreens change.
Dermatologists should “continue to emphasize the importance of protection from UV radiation in every way that can be done,” Dr. Cullen said, including the use of sunscreen as well as other protective practices, such as wearing long sleeves and hats, seeking shade, and avoiding the sun during peak hours.
A dermatologist’s perspective
“I applaud their scientific curiosity to know one way or the other whether this is an issue,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, DC. “I welcome this investigation.”
The multitude of studies, Dr. Friedman said, don’t always agree about whether the filters pose dangers. He noted that the concentration of UV filters detected in water is often lower than the concentrations found to be harmful in a lab setting to marine life, specifically coral.
However, he said, “these studies are snapshots.” For that reason, calling for more assessment of risk is desirable, Dr. Friedman said, but “I want to be sure the call to do more research is not an admission of guilt. It’s very easy to vilify sunscreens – but the facts we know are that UV light causes skin cancer and aging, and sunscreen protects us against this.”
Dr. Friedman has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The , an expert panel of the National Academies of Sciences, Engineering, and Medicine (NAS) said on Aug. 9.
The assessment is urgently needed, the experts said, and the results should be shared with the Food and Drug Administration, which oversees sunscreens.
In its 400-page report, titled the Review of Fate, Exposure, and Effects of Sunscreens in Aquatic Environments and Implications for Sunscreen Usage and Human Health, the panel does not make recommendations but suggests that such an EPA risk assessment should highlight gaps in knowledge.
“We are teeing up the critical information that will be used to take on the challenge of risk assessment,” Charles A. Menzie, PhD, chair of the committee that wrote the report, said at a media briefing Aug. 9 when the report was released. Dr. Menzie is a principal at Exponent, Inc., an engineering and scientific consulting firm. He is former executive director of the Society of Environmental Toxicology and Chemistry.
The EPA sponsored the study, which was conducted by a committee of the National Academy of Sciences, a nonprofit, nongovernmental organization authorized by Congress that studies issues related to science, technology, and medicine.
Balancing aquatic, human health concerns
Such an EPA assessment, Dr. Menzie said in a statement, will help inform efforts to understand the environmental effects of UV filters as well as clarify a path forward for managing sunscreens. For years, concerns have been raised about the potential toxicity of sunscreens regarding many marine and freshwater aquatic organisms, especially coral. That concern, however, must be balanced against the benefits of sunscreens, which are known to protect against skin cancer. A low percentage of people use sunscreen regularly, Dr. Menzie and other panel members said.
“Only about a third of the U.S. population regularly uses sunscreen,” Mark Cullen, MD, vice chair of the NAS committee and former director of the Center for Population Health Sciences, Stanford (Calif.) University, said at the briefing. About 70% or 80% of people use it at the beach or outdoors, he said.
Report background, details
UV filters are the active ingredients in physical as well as chemical sunscreen products. They decrease the amount of UV radiation that reaches the skin. They have been found in water, sediments, and marine organisms, both saltwater and freshwater.
Currently, 17 UV filters are used in U.S. sunscreens; 15 of those are organic, such as oxybenzone and avobenzone, and are used in chemical sunscreens. They work by absorbing the rays before they damage the skin. In addition, two inorganic filters, which are used in physical sunscreens, sit on the skin and as a shield to block the rays.
UV filters enter bodies of water by direct release, as when sunscreens rinse off people while swimming or while engaging in other water activities. They also enter bodies of water in storm water runoff and wastewater.
Lab toxicity tests, which are the most widely used, provide effects data for ecologic risk assessment. The tests are more often used in the study of short-term, not long-term exposure. Test results have shown that in high enough concentrations, some UV filters can be toxic to algal, invertebrate, and fish species.
But much information is lacking, the experts said. Toxicity data for many species, for instance, are limited. There are few studies on the longer-term environmental effects of UV filter exposure. Not enough is known about the rate at which the filters degrade in the environment. The filters accumulate in higher amounts in different areas. Recreational water areas have higher concentrations.
The recommendations
The panel is urging the EPA to complete a formal risk assessment of the UV filters “with some urgency,” Dr. Cullen said. That will enable decisions to be made about the use of the products. The risks to aquatic life must be balanced against the need for sun protection to reduce skin cancer risk.
The experts made two recommendations:
- The EPA should conduct ecologic risk assessments for all the UV filters now marketed and for all new ones. The assessment should evaluate the filters individually as well as the risk from co-occurring filters. The assessments should take into account the different exposure scenarios.
- The EPA, along with partner agencies, and sunscreen and UV filter manufacturers should fund, support, and conduct research and share data. Research should include study of human health outcomes if usage and availability of sunscreens change.
Dermatologists should “continue to emphasize the importance of protection from UV radiation in every way that can be done,” Dr. Cullen said, including the use of sunscreen as well as other protective practices, such as wearing long sleeves and hats, seeking shade, and avoiding the sun during peak hours.
A dermatologist’s perspective
“I applaud their scientific curiosity to know one way or the other whether this is an issue,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, DC. “I welcome this investigation.”
The multitude of studies, Dr. Friedman said, don’t always agree about whether the filters pose dangers. He noted that the concentration of UV filters detected in water is often lower than the concentrations found to be harmful in a lab setting to marine life, specifically coral.
However, he said, “these studies are snapshots.” For that reason, calling for more assessment of risk is desirable, Dr. Friedman said, but “I want to be sure the call to do more research is not an admission of guilt. It’s very easy to vilify sunscreens – but the facts we know are that UV light causes skin cancer and aging, and sunscreen protects us against this.”
Dr. Friedman has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Summer flu, RSV in July, ‘super colds?’
Richard Martinello, MD, a professor of medicine and pediatric infectious diseases at Yale University, New haven, Conn., doesn’t expect to see a child hospitalized with respiratory syncytial virus (RSV) in the middle of summer. The illness, which can strike infants and older adults especially hard, is known as a “winter virus.”
But not this year. Over the last several weeks, he says, admissions for children with RSV have increased at the Yale New Haven Children’s Hospital. While the numbers aren’t large, they are out of the ordinary, he says, “because usually, at this time of year, we see zero. For lack of a better term, it’s weird.”
Likewise, William Schaffner, MD, a professor of infectious diseases at Vanderbilt University in Nashville, says RSV is on the rise there. Tennessee is one of 10 states taking part in a Centers for Disease Control and Prevention surveillance system that tracks influenza, RSV, and COVID-19.
He says RSV cases have risen by at least a third during the past week, including all age ranges. At this time of year, he says, “We aren’t supposed to have any RSV.”
RSV isn’t the only virus thriving out of season or otherwise acting strangely. Since the pandemic began, flu seasons have been out of whack – sometimes nearly nonexistent and other times extending well beyond “normal” seasons. Some experts say one influenza “B” strain may now be extinct, while others say it will be back.
Severe colds – what some call “super colds” – also seem to be on the rise in recent warm-weather months, although that evidence is mostly based on personal experience, not science.
Trying to explain these out-of-season variations has sparked much discussion among epidemiologists and virologists, Dr. Schaffner says, with debates ongoing about whether human behavior and habits or the seasons play a bigger role in the transmission of viral illness.
On top of that, scientists are also looking at the interactions between the SARS-CoV-2 virus that causes COVID-19 and other viruses. When people get hit with COVID-19 and other viruses at the same time, does that make COVID-19 more severe, or less?
Research is conflicting.
Summer of 2022: A repeat of 2021?
RSV. Most children contract the virus by age 2, and while it’s generally mild, about 58,000 children under age 5 years are hospitalized each year. During the pandemic, RSV cases decreased from January to April 2020, the CDC reported, and then remained at “historically low levels”: less than 1% positive RSV results a week, for the next year.
But cases began rising in April 2021.
“Last year, we did have an unusual summer,” Dr. Schaffner says. After lockdown ended, to everyone’s surprise, RSV infections rose.
That increase triggered a CDC health advisory in June 2021, telling doctors and caregivers about the increase in “interseasonal” RSV cases across parts of the Southern United States, recommending broader testing for RSV in patients who had a respiratory illness but tested negative for COVID.
Because of the reduced circulation of RSV during the winter of 2020 to 2021, the CDC warned, older infants and toddlers might have a higher risk of RSV since they weren’t exposed to typical levels of RSV for the previous 15 months.
What about 2022? “At the moment,” Dr. Schaffner says, “it looks like we are having a repeat [of 2021].”
On Twitter, other pediatricians, including those from Maine and Texas, have reported an increase in RSV cases this summer.
Influenza. From October 2020 until May 2021, flu activity was lower than during any previous flu season since at least 1997, according to the CDC.
In late 2021, researchers suggested that one line of influenza known as B/Yamagata may have become extinct.
The 2021-2022 flu season has been mild, the CDC says, but it has come in two waves, with the second wave lingering longer than previous ones. While flu activity is decreasing, last week the CDC said doctors should be alert to flu infections throughout the summer.
Colds. In reports on colds that aren’t based on science, several doctors say they are seeing more colds than usual in the summer, and they’re more severe than usual. According to the CDC, common coronaviruses and respiratory adenoviruses have been increasing since early 2021, and rhinoviruses since June 2020.
Behavior vs. seasons
In explaining the spread of viral respiratory diseases, infectious disease doctors consider two things. “One is that temperature and humidity in the winter favors longer survival of some viruses, leading to longer periods of possible transmission,” says Dean Blumberg, MD, a professor of pediatrics and chief of pediatric infectious disease at University of California Davis Health.
“The other is differences in human behavior, with people spending more time outside in the summer, which results in more distancing and [less] virus concentration due to very large air volume,” he says, and vice versa in winter.
What about the “super colds?” COVID-19 lockdowns and social distancing greatly reduced people’s exposure to common viruses like those that cause colds, says Neil A. Mabbott, PhD, a professor of immunopathology at the University of Edinburgh (Scotland).
“Immunity to these common cold viruses gained through natural infection is considered to last around 8 or 9 months or so,” he says. “Each winter, when we are exposed to the new circulating variants of these viruses, our immunity receives a natural boost.”
That explains why most people get a cold that’s relatively mild. But with all the pandemic lockdowns and the use of hand sanitizers, most people had limited exposure to other viruses, including the common cold. When people emerged from lockdown, the common cold viruses were beginning to circulate again.
“Our immune systems were less able to clear the infection than previously,” Dr. Mabbott says. “As a consequence, some may have experienced increased symptoms, giving the impression of being infected with a ‘super cold.’ ”
“The colds themselves are probably not different to those we got prepandemic,” says Ian Mackay, PhD, a virologist at the University of Queensland, Brisbane, Australia. “But there might be more of them. So I doubt they are ‘super colds’ as much as they are ‘super-perfect circumstances.’ ”
The colds themselves are probably not different to those we got prepandemic. But there might be more of them.
Those super-perfect circumstances, he says, include people gathering after lockdown; a lack of immunity in new babies; viruses that have remained, even if at low levels, but continue to mutate; and our waning immunity to the range of viruses we’d normally encounter.
While lack of exposure may partly explain why some viruses become rampant out of season, it’s likely not the only reason. For example, the reduced circulation of RSV in the population as a whole also may have reduced the transfer of immunity from mothers to infants, some researchers say, making those infants more vulnerable than usual.
Interactions of viruses
Another thing that may be driving the different behavior of viruses is that the SARS-CoV-2 virus could somehow be interacting with other respiratory viruses, Dr. Schaffner says. “And if so, what sort of interactions?”
Many researchers are looking into that, and how coinfections with other respiratory diseases, including the common cold and flu, may affect the course of COVID-19. Some studies have found that the T cells – a source of deeper, cellular immunity in people – generated after a common cold “may also provide cross-protection in some people against COVID-19.”
But another study found immunity against common cold–causing coronaviruses might make COVID-19 more severe.
When researchers in the United Kingdom studied nearly 7,000 patients infected with COVID-19, including 583 also infected with RSV, flu, or adenoviruses (causing flulike or coldlike illness), those with flu or adenovirus, compared with the others, were at higher risk of death.
To be continued …
Exactly how COVID-19 will be changing what we know of other viruses is yet to be determined, too.
Even before the pandemic, Dr. Martinello says, there were already some shifts in RSV. Florida, for instance, has an RSV season longer than the rest of the country, mimicking the pattern in the tropics.
Will the atypical patterns continue? “My guess is that this will settle out,” he says, with some sort of pattern developing. At this point, there are many unknowns. “We still can’t answer whether there will be some seasonality to COVID.”
A version of this article first appeared on WebMD.com.
Richard Martinello, MD, a professor of medicine and pediatric infectious diseases at Yale University, New haven, Conn., doesn’t expect to see a child hospitalized with respiratory syncytial virus (RSV) in the middle of summer. The illness, which can strike infants and older adults especially hard, is known as a “winter virus.”
But not this year. Over the last several weeks, he says, admissions for children with RSV have increased at the Yale New Haven Children’s Hospital. While the numbers aren’t large, they are out of the ordinary, he says, “because usually, at this time of year, we see zero. For lack of a better term, it’s weird.”
Likewise, William Schaffner, MD, a professor of infectious diseases at Vanderbilt University in Nashville, says RSV is on the rise there. Tennessee is one of 10 states taking part in a Centers for Disease Control and Prevention surveillance system that tracks influenza, RSV, and COVID-19.
He says RSV cases have risen by at least a third during the past week, including all age ranges. At this time of year, he says, “We aren’t supposed to have any RSV.”
RSV isn’t the only virus thriving out of season or otherwise acting strangely. Since the pandemic began, flu seasons have been out of whack – sometimes nearly nonexistent and other times extending well beyond “normal” seasons. Some experts say one influenza “B” strain may now be extinct, while others say it will be back.
Severe colds – what some call “super colds” – also seem to be on the rise in recent warm-weather months, although that evidence is mostly based on personal experience, not science.
Trying to explain these out-of-season variations has sparked much discussion among epidemiologists and virologists, Dr. Schaffner says, with debates ongoing about whether human behavior and habits or the seasons play a bigger role in the transmission of viral illness.
On top of that, scientists are also looking at the interactions between the SARS-CoV-2 virus that causes COVID-19 and other viruses. When people get hit with COVID-19 and other viruses at the same time, does that make COVID-19 more severe, or less?
Research is conflicting.
Summer of 2022: A repeat of 2021?
RSV. Most children contract the virus by age 2, and while it’s generally mild, about 58,000 children under age 5 years are hospitalized each year. During the pandemic, RSV cases decreased from January to April 2020, the CDC reported, and then remained at “historically low levels”: less than 1% positive RSV results a week, for the next year.
But cases began rising in April 2021.
“Last year, we did have an unusual summer,” Dr. Schaffner says. After lockdown ended, to everyone’s surprise, RSV infections rose.
That increase triggered a CDC health advisory in June 2021, telling doctors and caregivers about the increase in “interseasonal” RSV cases across parts of the Southern United States, recommending broader testing for RSV in patients who had a respiratory illness but tested negative for COVID.
Because of the reduced circulation of RSV during the winter of 2020 to 2021, the CDC warned, older infants and toddlers might have a higher risk of RSV since they weren’t exposed to typical levels of RSV for the previous 15 months.
What about 2022? “At the moment,” Dr. Schaffner says, “it looks like we are having a repeat [of 2021].”
On Twitter, other pediatricians, including those from Maine and Texas, have reported an increase in RSV cases this summer.
Influenza. From October 2020 until May 2021, flu activity was lower than during any previous flu season since at least 1997, according to the CDC.
In late 2021, researchers suggested that one line of influenza known as B/Yamagata may have become extinct.
The 2021-2022 flu season has been mild, the CDC says, but it has come in two waves, with the second wave lingering longer than previous ones. While flu activity is decreasing, last week the CDC said doctors should be alert to flu infections throughout the summer.
Colds. In reports on colds that aren’t based on science, several doctors say they are seeing more colds than usual in the summer, and they’re more severe than usual. According to the CDC, common coronaviruses and respiratory adenoviruses have been increasing since early 2021, and rhinoviruses since June 2020.
Behavior vs. seasons
In explaining the spread of viral respiratory diseases, infectious disease doctors consider two things. “One is that temperature and humidity in the winter favors longer survival of some viruses, leading to longer periods of possible transmission,” says Dean Blumberg, MD, a professor of pediatrics and chief of pediatric infectious disease at University of California Davis Health.
“The other is differences in human behavior, with people spending more time outside in the summer, which results in more distancing and [less] virus concentration due to very large air volume,” he says, and vice versa in winter.
What about the “super colds?” COVID-19 lockdowns and social distancing greatly reduced people’s exposure to common viruses like those that cause colds, says Neil A. Mabbott, PhD, a professor of immunopathology at the University of Edinburgh (Scotland).
“Immunity to these common cold viruses gained through natural infection is considered to last around 8 or 9 months or so,” he says. “Each winter, when we are exposed to the new circulating variants of these viruses, our immunity receives a natural boost.”
That explains why most people get a cold that’s relatively mild. But with all the pandemic lockdowns and the use of hand sanitizers, most people had limited exposure to other viruses, including the common cold. When people emerged from lockdown, the common cold viruses were beginning to circulate again.
“Our immune systems were less able to clear the infection than previously,” Dr. Mabbott says. “As a consequence, some may have experienced increased symptoms, giving the impression of being infected with a ‘super cold.’ ”
“The colds themselves are probably not different to those we got prepandemic,” says Ian Mackay, PhD, a virologist at the University of Queensland, Brisbane, Australia. “But there might be more of them. So I doubt they are ‘super colds’ as much as they are ‘super-perfect circumstances.’ ”
The colds themselves are probably not different to those we got prepandemic. But there might be more of them.
Those super-perfect circumstances, he says, include people gathering after lockdown; a lack of immunity in new babies; viruses that have remained, even if at low levels, but continue to mutate; and our waning immunity to the range of viruses we’d normally encounter.
While lack of exposure may partly explain why some viruses become rampant out of season, it’s likely not the only reason. For example, the reduced circulation of RSV in the population as a whole also may have reduced the transfer of immunity from mothers to infants, some researchers say, making those infants more vulnerable than usual.
Interactions of viruses
Another thing that may be driving the different behavior of viruses is that the SARS-CoV-2 virus could somehow be interacting with other respiratory viruses, Dr. Schaffner says. “And if so, what sort of interactions?”
Many researchers are looking into that, and how coinfections with other respiratory diseases, including the common cold and flu, may affect the course of COVID-19. Some studies have found that the T cells – a source of deeper, cellular immunity in people – generated after a common cold “may also provide cross-protection in some people against COVID-19.”
But another study found immunity against common cold–causing coronaviruses might make COVID-19 more severe.
When researchers in the United Kingdom studied nearly 7,000 patients infected with COVID-19, including 583 also infected with RSV, flu, or adenoviruses (causing flulike or coldlike illness), those with flu or adenovirus, compared with the others, were at higher risk of death.
To be continued …
Exactly how COVID-19 will be changing what we know of other viruses is yet to be determined, too.
Even before the pandemic, Dr. Martinello says, there were already some shifts in RSV. Florida, for instance, has an RSV season longer than the rest of the country, mimicking the pattern in the tropics.
Will the atypical patterns continue? “My guess is that this will settle out,” he says, with some sort of pattern developing. At this point, there are many unknowns. “We still can’t answer whether there will be some seasonality to COVID.”
A version of this article first appeared on WebMD.com.
Richard Martinello, MD, a professor of medicine and pediatric infectious diseases at Yale University, New haven, Conn., doesn’t expect to see a child hospitalized with respiratory syncytial virus (RSV) in the middle of summer. The illness, which can strike infants and older adults especially hard, is known as a “winter virus.”
But not this year. Over the last several weeks, he says, admissions for children with RSV have increased at the Yale New Haven Children’s Hospital. While the numbers aren’t large, they are out of the ordinary, he says, “because usually, at this time of year, we see zero. For lack of a better term, it’s weird.”
Likewise, William Schaffner, MD, a professor of infectious diseases at Vanderbilt University in Nashville, says RSV is on the rise there. Tennessee is one of 10 states taking part in a Centers for Disease Control and Prevention surveillance system that tracks influenza, RSV, and COVID-19.
He says RSV cases have risen by at least a third during the past week, including all age ranges. At this time of year, he says, “We aren’t supposed to have any RSV.”
RSV isn’t the only virus thriving out of season or otherwise acting strangely. Since the pandemic began, flu seasons have been out of whack – sometimes nearly nonexistent and other times extending well beyond “normal” seasons. Some experts say one influenza “B” strain may now be extinct, while others say it will be back.
Severe colds – what some call “super colds” – also seem to be on the rise in recent warm-weather months, although that evidence is mostly based on personal experience, not science.
Trying to explain these out-of-season variations has sparked much discussion among epidemiologists and virologists, Dr. Schaffner says, with debates ongoing about whether human behavior and habits or the seasons play a bigger role in the transmission of viral illness.
On top of that, scientists are also looking at the interactions between the SARS-CoV-2 virus that causes COVID-19 and other viruses. When people get hit with COVID-19 and other viruses at the same time, does that make COVID-19 more severe, or less?
Research is conflicting.
Summer of 2022: A repeat of 2021?
RSV. Most children contract the virus by age 2, and while it’s generally mild, about 58,000 children under age 5 years are hospitalized each year. During the pandemic, RSV cases decreased from January to April 2020, the CDC reported, and then remained at “historically low levels”: less than 1% positive RSV results a week, for the next year.
But cases began rising in April 2021.
“Last year, we did have an unusual summer,” Dr. Schaffner says. After lockdown ended, to everyone’s surprise, RSV infections rose.
That increase triggered a CDC health advisory in June 2021, telling doctors and caregivers about the increase in “interseasonal” RSV cases across parts of the Southern United States, recommending broader testing for RSV in patients who had a respiratory illness but tested negative for COVID.
Because of the reduced circulation of RSV during the winter of 2020 to 2021, the CDC warned, older infants and toddlers might have a higher risk of RSV since they weren’t exposed to typical levels of RSV for the previous 15 months.
What about 2022? “At the moment,” Dr. Schaffner says, “it looks like we are having a repeat [of 2021].”
On Twitter, other pediatricians, including those from Maine and Texas, have reported an increase in RSV cases this summer.
Influenza. From October 2020 until May 2021, flu activity was lower than during any previous flu season since at least 1997, according to the CDC.
In late 2021, researchers suggested that one line of influenza known as B/Yamagata may have become extinct.
The 2021-2022 flu season has been mild, the CDC says, but it has come in two waves, with the second wave lingering longer than previous ones. While flu activity is decreasing, last week the CDC said doctors should be alert to flu infections throughout the summer.
Colds. In reports on colds that aren’t based on science, several doctors say they are seeing more colds than usual in the summer, and they’re more severe than usual. According to the CDC, common coronaviruses and respiratory adenoviruses have been increasing since early 2021, and rhinoviruses since June 2020.
Behavior vs. seasons
In explaining the spread of viral respiratory diseases, infectious disease doctors consider two things. “One is that temperature and humidity in the winter favors longer survival of some viruses, leading to longer periods of possible transmission,” says Dean Blumberg, MD, a professor of pediatrics and chief of pediatric infectious disease at University of California Davis Health.
“The other is differences in human behavior, with people spending more time outside in the summer, which results in more distancing and [less] virus concentration due to very large air volume,” he says, and vice versa in winter.
What about the “super colds?” COVID-19 lockdowns and social distancing greatly reduced people’s exposure to common viruses like those that cause colds, says Neil A. Mabbott, PhD, a professor of immunopathology at the University of Edinburgh (Scotland).
“Immunity to these common cold viruses gained through natural infection is considered to last around 8 or 9 months or so,” he says. “Each winter, when we are exposed to the new circulating variants of these viruses, our immunity receives a natural boost.”
That explains why most people get a cold that’s relatively mild. But with all the pandemic lockdowns and the use of hand sanitizers, most people had limited exposure to other viruses, including the common cold. When people emerged from lockdown, the common cold viruses were beginning to circulate again.
“Our immune systems were less able to clear the infection than previously,” Dr. Mabbott says. “As a consequence, some may have experienced increased symptoms, giving the impression of being infected with a ‘super cold.’ ”
“The colds themselves are probably not different to those we got prepandemic,” says Ian Mackay, PhD, a virologist at the University of Queensland, Brisbane, Australia. “But there might be more of them. So I doubt they are ‘super colds’ as much as they are ‘super-perfect circumstances.’ ”
The colds themselves are probably not different to those we got prepandemic. But there might be more of them.
Those super-perfect circumstances, he says, include people gathering after lockdown; a lack of immunity in new babies; viruses that have remained, even if at low levels, but continue to mutate; and our waning immunity to the range of viruses we’d normally encounter.
While lack of exposure may partly explain why some viruses become rampant out of season, it’s likely not the only reason. For example, the reduced circulation of RSV in the population as a whole also may have reduced the transfer of immunity from mothers to infants, some researchers say, making those infants more vulnerable than usual.
Interactions of viruses
Another thing that may be driving the different behavior of viruses is that the SARS-CoV-2 virus could somehow be interacting with other respiratory viruses, Dr. Schaffner says. “And if so, what sort of interactions?”
Many researchers are looking into that, and how coinfections with other respiratory diseases, including the common cold and flu, may affect the course of COVID-19. Some studies have found that the T cells – a source of deeper, cellular immunity in people – generated after a common cold “may also provide cross-protection in some people against COVID-19.”
But another study found immunity against common cold–causing coronaviruses might make COVID-19 more severe.
When researchers in the United Kingdom studied nearly 7,000 patients infected with COVID-19, including 583 also infected with RSV, flu, or adenoviruses (causing flulike or coldlike illness), those with flu or adenovirus, compared with the others, were at higher risk of death.
To be continued …
Exactly how COVID-19 will be changing what we know of other viruses is yet to be determined, too.
Even before the pandemic, Dr. Martinello says, there were already some shifts in RSV. Florida, for instance, has an RSV season longer than the rest of the country, mimicking the pattern in the tropics.
Will the atypical patterns continue? “My guess is that this will settle out,” he says, with some sort of pattern developing. At this point, there are many unknowns. “We still can’t answer whether there will be some seasonality to COVID.”
A version of this article first appeared on WebMD.com.
Evusheld for COVID-19: Lifesaving and free, but still few takers
Evusheld (AstraZeneca), a medication used to prevent SARS-CoV-2 infection in patients at high risk, has problems: Namely, that supplies of the potentially lifesaving drug outweigh demand.
At least 7 million people who are immunocompromised could benefit from it, as could many others who are undergoing cancer treatment, have received a transplant, or who are allergic to the COVID-19 vaccines. The medication has laboratory-produced antibodies against SARS-CoV-2 and helps the body protect itself. It can slash the chances of becoming infected by 77%, according to the U.S. Food and Drug Administration.
And it’s free to eligible patients (although there may be an out-of-pocket administrative fee in some cases).
To meet demand, the Biden administration secured 1.7 million doses of the medicine, which was granted emergency use authorization by the FDA in December 2021. As of July 25, however, 793,348 doses have been ordered by the administration sites, and only 398,181 doses have been reported as used, a spokesperson for the Department of Health & Human Services tells this news organization.
Each week, a certain amount of doses from the 1.7 million dose stockpile is made available to state and territorial health departments. States have not been asking for their full allotment, the spokesperson said July 28.
Now, HHS and AstraZeneca have taken a number of steps to increase awareness of the medication and access to it.
- On July 27, HHS announced that individual providers and smaller sites of care that don’t currently receive Evusheld through the federal distribution process via the HHS Health Partner Order Portal can now order up to three patient courses of the medicine. These can be
- Health care providers can use the HHS’s COVID-19 Therapeutics Locator to find Evusheld in their area.
- AstraZeneca has launched a new website with educational materials and says it is working closely with patient and professional groups to inform patients and health care providers.
- A direct-to-consumer ad launched on June 22 and will run in the United States online and on TV (Yahoo, Fox, CBS Sports, MSN, ESPN) and be amplified on social and digital channels through year’s end, an AstraZeneca spokesperson said in an interview.
- AstraZeneca set up a toll-free number for providers: 1-833-EVUSHLD.
Evusheld includes two monoclonal antibodies, tixagevimab and cilgavimab. The medication is given as two consecutive intramuscular injections during a single visit to a doctor’s office, infusion center, or other health care facility. The antibodies bind to the SARS-CoV-2 spike protein and prevent the virus from getting into human cells and infecting them. It’s authorized for use in children and adults aged 12 years and older who weigh at least 88 pounds.
Studies have found that the medication decreases the risk of getting COVID-19 for up to 6 months after it is given. The FDA recommends repeat dosing every 6 months with the doses of 300 mg of each monoclonal antibody. In clinical trials, Evusheld reduced the incidence of COVID-19 symptomatic illness by 77%, compared with placebo.
Physicians monitor patients for an hour after administering Evusheld for allergic reactions. Other possible side effects include cardiac events, but they are not common.
Doctors and patients weigh in
Physicians – and patients – from the United States to the United Kingdom and beyond are questioning why the medication is underused while lauding the recent efforts to expand access and increase awareness.
The U.S. federal government may have underestimated the amount of communication needed to increase awareness of the medication and its applications, said infectious disease specialist William Schaffner, MD, professor of preventive medicine at Vanderbilt University School of Medicine, Nashville, Tenn.
“HHS hasn’t made a major educational effort to promote it,” he said in an interview.
Many physicians who need to know about it, such as transplant doctors and rheumatologists, are outside the typical public health communications loop, he said.
Eric Topol, MD, director of the Scripps Research Transational Institute and editor-in-chief of Medscape, has taken to social media to bemoan the lack of awareness.
Another infectious disease expert agrees. “In my experience, the awareness of Evusheld is low amongst many patients as well as many providers,” said Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security, Baltimore.
“Initially, there were scarce supplies of the drug, and certain hospital systems tiered eligibility based on degrees of immunosuppression, and only the most immunosuppressed were proactively approached for treatment.”
“Also, many community hospitals never initially ordered Evusheld – they may have been crowded out by academic centers who treat many more immunosuppressed patients and may not currently see it as a priority,” Dr. Adalja said in an interview. “As such, many immunosuppressed patients would have to seek treatment at academic medical centers, where the drug is more likely to be available.”
A version of this article first appeared on Medscape.com.
Evusheld (AstraZeneca), a medication used to prevent SARS-CoV-2 infection in patients at high risk, has problems: Namely, that supplies of the potentially lifesaving drug outweigh demand.
At least 7 million people who are immunocompromised could benefit from it, as could many others who are undergoing cancer treatment, have received a transplant, or who are allergic to the COVID-19 vaccines. The medication has laboratory-produced antibodies against SARS-CoV-2 and helps the body protect itself. It can slash the chances of becoming infected by 77%, according to the U.S. Food and Drug Administration.
And it’s free to eligible patients (although there may be an out-of-pocket administrative fee in some cases).
To meet demand, the Biden administration secured 1.7 million doses of the medicine, which was granted emergency use authorization by the FDA in December 2021. As of July 25, however, 793,348 doses have been ordered by the administration sites, and only 398,181 doses have been reported as used, a spokesperson for the Department of Health & Human Services tells this news organization.
Each week, a certain amount of doses from the 1.7 million dose stockpile is made available to state and territorial health departments. States have not been asking for their full allotment, the spokesperson said July 28.
Now, HHS and AstraZeneca have taken a number of steps to increase awareness of the medication and access to it.
- On July 27, HHS announced that individual providers and smaller sites of care that don’t currently receive Evusheld through the federal distribution process via the HHS Health Partner Order Portal can now order up to three patient courses of the medicine. These can be
- Health care providers can use the HHS’s COVID-19 Therapeutics Locator to find Evusheld in their area.
- AstraZeneca has launched a new website with educational materials and says it is working closely with patient and professional groups to inform patients and health care providers.
- A direct-to-consumer ad launched on June 22 and will run in the United States online and on TV (Yahoo, Fox, CBS Sports, MSN, ESPN) and be amplified on social and digital channels through year’s end, an AstraZeneca spokesperson said in an interview.
- AstraZeneca set up a toll-free number for providers: 1-833-EVUSHLD.
Evusheld includes two monoclonal antibodies, tixagevimab and cilgavimab. The medication is given as two consecutive intramuscular injections during a single visit to a doctor’s office, infusion center, or other health care facility. The antibodies bind to the SARS-CoV-2 spike protein and prevent the virus from getting into human cells and infecting them. It’s authorized for use in children and adults aged 12 years and older who weigh at least 88 pounds.
Studies have found that the medication decreases the risk of getting COVID-19 for up to 6 months after it is given. The FDA recommends repeat dosing every 6 months with the doses of 300 mg of each monoclonal antibody. In clinical trials, Evusheld reduced the incidence of COVID-19 symptomatic illness by 77%, compared with placebo.
Physicians monitor patients for an hour after administering Evusheld for allergic reactions. Other possible side effects include cardiac events, but they are not common.
Doctors and patients weigh in
Physicians – and patients – from the United States to the United Kingdom and beyond are questioning why the medication is underused while lauding the recent efforts to expand access and increase awareness.
The U.S. federal government may have underestimated the amount of communication needed to increase awareness of the medication and its applications, said infectious disease specialist William Schaffner, MD, professor of preventive medicine at Vanderbilt University School of Medicine, Nashville, Tenn.
“HHS hasn’t made a major educational effort to promote it,” he said in an interview.
Many physicians who need to know about it, such as transplant doctors and rheumatologists, are outside the typical public health communications loop, he said.
Eric Topol, MD, director of the Scripps Research Transational Institute and editor-in-chief of Medscape, has taken to social media to bemoan the lack of awareness.
Another infectious disease expert agrees. “In my experience, the awareness of Evusheld is low amongst many patients as well as many providers,” said Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security, Baltimore.
“Initially, there were scarce supplies of the drug, and certain hospital systems tiered eligibility based on degrees of immunosuppression, and only the most immunosuppressed were proactively approached for treatment.”
“Also, many community hospitals never initially ordered Evusheld – they may have been crowded out by academic centers who treat many more immunosuppressed patients and may not currently see it as a priority,” Dr. Adalja said in an interview. “As such, many immunosuppressed patients would have to seek treatment at academic medical centers, where the drug is more likely to be available.”
A version of this article first appeared on Medscape.com.
Evusheld (AstraZeneca), a medication used to prevent SARS-CoV-2 infection in patients at high risk, has problems: Namely, that supplies of the potentially lifesaving drug outweigh demand.
At least 7 million people who are immunocompromised could benefit from it, as could many others who are undergoing cancer treatment, have received a transplant, or who are allergic to the COVID-19 vaccines. The medication has laboratory-produced antibodies against SARS-CoV-2 and helps the body protect itself. It can slash the chances of becoming infected by 77%, according to the U.S. Food and Drug Administration.
And it’s free to eligible patients (although there may be an out-of-pocket administrative fee in some cases).
To meet demand, the Biden administration secured 1.7 million doses of the medicine, which was granted emergency use authorization by the FDA in December 2021. As of July 25, however, 793,348 doses have been ordered by the administration sites, and only 398,181 doses have been reported as used, a spokesperson for the Department of Health & Human Services tells this news organization.
Each week, a certain amount of doses from the 1.7 million dose stockpile is made available to state and territorial health departments. States have not been asking for their full allotment, the spokesperson said July 28.
Now, HHS and AstraZeneca have taken a number of steps to increase awareness of the medication and access to it.
- On July 27, HHS announced that individual providers and smaller sites of care that don’t currently receive Evusheld through the federal distribution process via the HHS Health Partner Order Portal can now order up to three patient courses of the medicine. These can be
- Health care providers can use the HHS’s COVID-19 Therapeutics Locator to find Evusheld in their area.
- AstraZeneca has launched a new website with educational materials and says it is working closely with patient and professional groups to inform patients and health care providers.
- A direct-to-consumer ad launched on June 22 and will run in the United States online and on TV (Yahoo, Fox, CBS Sports, MSN, ESPN) and be amplified on social and digital channels through year’s end, an AstraZeneca spokesperson said in an interview.
- AstraZeneca set up a toll-free number for providers: 1-833-EVUSHLD.
Evusheld includes two monoclonal antibodies, tixagevimab and cilgavimab. The medication is given as two consecutive intramuscular injections during a single visit to a doctor’s office, infusion center, or other health care facility. The antibodies bind to the SARS-CoV-2 spike protein and prevent the virus from getting into human cells and infecting them. It’s authorized for use in children and adults aged 12 years and older who weigh at least 88 pounds.
Studies have found that the medication decreases the risk of getting COVID-19 for up to 6 months after it is given. The FDA recommends repeat dosing every 6 months with the doses of 300 mg of each monoclonal antibody. In clinical trials, Evusheld reduced the incidence of COVID-19 symptomatic illness by 77%, compared with placebo.
Physicians monitor patients for an hour after administering Evusheld for allergic reactions. Other possible side effects include cardiac events, but they are not common.
Doctors and patients weigh in
Physicians – and patients – from the United States to the United Kingdom and beyond are questioning why the medication is underused while lauding the recent efforts to expand access and increase awareness.
The U.S. federal government may have underestimated the amount of communication needed to increase awareness of the medication and its applications, said infectious disease specialist William Schaffner, MD, professor of preventive medicine at Vanderbilt University School of Medicine, Nashville, Tenn.
“HHS hasn’t made a major educational effort to promote it,” he said in an interview.
Many physicians who need to know about it, such as transplant doctors and rheumatologists, are outside the typical public health communications loop, he said.
Eric Topol, MD, director of the Scripps Research Transational Institute and editor-in-chief of Medscape, has taken to social media to bemoan the lack of awareness.
Another infectious disease expert agrees. “In my experience, the awareness of Evusheld is low amongst many patients as well as many providers,” said Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security, Baltimore.
“Initially, there were scarce supplies of the drug, and certain hospital systems tiered eligibility based on degrees of immunosuppression, and only the most immunosuppressed were proactively approached for treatment.”
“Also, many community hospitals never initially ordered Evusheld – they may have been crowded out by academic centers who treat many more immunosuppressed patients and may not currently see it as a priority,” Dr. Adalja said in an interview. “As such, many immunosuppressed patients would have to seek treatment at academic medical centers, where the drug is more likely to be available.”
A version of this article first appeared on Medscape.com.
Aesthetics abound for the aging face
At the MedscapeLive’s Women’s and Pediatric Dermatology Seminar, Jacqueline Watchmaker, MD, a dermatologist in Scottsdale, Ariz., provided an overview of current options, along with advice on how to keep patients’ expectations realistic and how to properly choose the best candidates for the best procedures.
“One of the most common concerns patients come to me with are wrinkles on the upper face,” but this is far from their only concern, Dr. Watchmaker said. Wrinkles and sagging of the lower face, areas under the eyes, nasolabial folds, marionette lines, and the neck also draw concern. Uneven coloration is another common concern, she said.
“So, what can we do for all of this?” she asked. The options are plentiful. Wrinkles of the upper face are easy to address with neuromodulators, she said, and soft-tissue fillers help the jawline and cheek areas.
“For the lower face, skin tightening devices really shine,” she added. And lasers can help correct uneven coloration. Surgery, of course, can also produce good results, but many patients want to stick with noninvasive or minimally invasive procedures.
Case: 83-year-old woman
Dr. Watchmaker discussed an 83-year old patient, who had malar mounds and accentuation of the infraorbital hollowness resulting from changes in subcutaneous fat and ligament laxity. She also had uneven coloration from photo damage, wrinkles on the upper face, linear appearance of zygoma related to underlying bony changes and fat compartment descent, and nasolabial folds and jowls related to decreased bony compartments, ligament laxity, and shifting of fat. She was naive to any cosmetic procedure.
Despite her age, this patient had no wrinkling on the upper forehead. Dr. Watchmaker did not inject neuromodulator in the upper forehead, as this patient also had a slightly heavy eyelid. “If you inject too much, it can cause some drooping of the eyelid and eyebrow,” she said.
For filler, she used a combination of high G (firmness, support) hyaluronic acid filler, a medium G acid filler, and a low G filler. The result: The woman’s face became more balanced, the mid-face volumization lifted the lower face, and the glabellar and periocular lines were softer, although still present. “It’s important to counsel patients that neuromodulators won’t make the lines go away the first time, but they will be softened.”
Practice tips
It’s important to titrate neuromodulators to fit the patient, Dr. Watchmaker said. Ask: What are their goals: Reversal of static lines? Softening wrinkles? Maintaining current status? “There’s not one dosing regimen,” and both dosing and frequency of neuromodulators can be titrated to fit each patient’s aesthetic goals, she said. For older patients who want to soften or maintain appearance, she suggested treatment every 4-6 months. And some patients just want to maintain the status quo, she noted.
Ideal candidates
For neuromodulators and fillers, who is an ideal candidate? “I think it’s anyone who has realistic expectations,” she said. Patients need to know how many treatments are needed and how much it will cost. For patients with extensive wrinkling and sagging, she said, she does extensive counseling about what results to expect “because I don’t want them to feel like they wasted their time or their money.”
She also suggests a surgical consult, as some may opt for that route after learning about the options and expected results.
Skin tightening
Both radiofrequency and microfocused ultrasound are noninvasive and additional options. Radiofrequency uses radio waves, with electromagnetic energy to stimulate heat. Ultrasound uses ultrasound waves to stimulate heat. Both approaches cause collagen contraction, neocollagenesis, and skin tightening.
These procedures do well for the lower face, Dr. Watchmaker said, but “I am relatively unimpressed for how well they do for the upper face.” Ideal candidates have mild to moderate skin laxity and want to avoid surgery. She also tells patients that collagen isn’t made overnight. “You won’t see much for 3-6 months after.” The good news? Usually the treatments need to be repeated only every 1.5-2 years, she said.
Lasers
“There are so many lasers out there,” said Dr. Watchmaker, who groups them into three categories: those used for wrinkles, dyschromia, and erythema. Her picks: ablative lasers (CO2 and erbium) and erbium-doped YAG 1550 nm laser for rhytids. Thulium 1927 and QS and picosecond lasers are her picks for dyschromia, and for erythema, pulsed dye and KTP lasers.
Some laser treatments are not a “walk in the park,” as she warns patients. For example, after treatment with ablative lasers, there is pain, post-procedure redness, and crusting.
Take-home points
A combination of noninvasive and minimally invasive procedures can produce appearance-improving results. That’s more likely if dermatologists choose ideal candidates, personalize the treatment, and set realistic expectations. “We have a finite number of tools,” she said, but they can be used in a variety of ways.
At the interactive panel discussion following her presentation, Dr. Watchmaker was asked what she tells patients about sun protection. “I talk a lot about sunscreens,’’ she said, always urging patients to use them. While the options for rejuvenation are numerous, taking care of the skin is still crucial.
Dr. Watchmaker had no disclosures. MedscapeLive and this news organization are owned by the same parent company.
At the MedscapeLive’s Women’s and Pediatric Dermatology Seminar, Jacqueline Watchmaker, MD, a dermatologist in Scottsdale, Ariz., provided an overview of current options, along with advice on how to keep patients’ expectations realistic and how to properly choose the best candidates for the best procedures.
“One of the most common concerns patients come to me with are wrinkles on the upper face,” but this is far from their only concern, Dr. Watchmaker said. Wrinkles and sagging of the lower face, areas under the eyes, nasolabial folds, marionette lines, and the neck also draw concern. Uneven coloration is another common concern, she said.
“So, what can we do for all of this?” she asked. The options are plentiful. Wrinkles of the upper face are easy to address with neuromodulators, she said, and soft-tissue fillers help the jawline and cheek areas.
“For the lower face, skin tightening devices really shine,” she added. And lasers can help correct uneven coloration. Surgery, of course, can also produce good results, but many patients want to stick with noninvasive or minimally invasive procedures.
Case: 83-year-old woman
Dr. Watchmaker discussed an 83-year old patient, who had malar mounds and accentuation of the infraorbital hollowness resulting from changes in subcutaneous fat and ligament laxity. She also had uneven coloration from photo damage, wrinkles on the upper face, linear appearance of zygoma related to underlying bony changes and fat compartment descent, and nasolabial folds and jowls related to decreased bony compartments, ligament laxity, and shifting of fat. She was naive to any cosmetic procedure.
Despite her age, this patient had no wrinkling on the upper forehead. Dr. Watchmaker did not inject neuromodulator in the upper forehead, as this patient also had a slightly heavy eyelid. “If you inject too much, it can cause some drooping of the eyelid and eyebrow,” she said.
For filler, she used a combination of high G (firmness, support) hyaluronic acid filler, a medium G acid filler, and a low G filler. The result: The woman’s face became more balanced, the mid-face volumization lifted the lower face, and the glabellar and periocular lines were softer, although still present. “It’s important to counsel patients that neuromodulators won’t make the lines go away the first time, but they will be softened.”
Practice tips
It’s important to titrate neuromodulators to fit the patient, Dr. Watchmaker said. Ask: What are their goals: Reversal of static lines? Softening wrinkles? Maintaining current status? “There’s not one dosing regimen,” and both dosing and frequency of neuromodulators can be titrated to fit each patient’s aesthetic goals, she said. For older patients who want to soften or maintain appearance, she suggested treatment every 4-6 months. And some patients just want to maintain the status quo, she noted.
Ideal candidates
For neuromodulators and fillers, who is an ideal candidate? “I think it’s anyone who has realistic expectations,” she said. Patients need to know how many treatments are needed and how much it will cost. For patients with extensive wrinkling and sagging, she said, she does extensive counseling about what results to expect “because I don’t want them to feel like they wasted their time or their money.”
She also suggests a surgical consult, as some may opt for that route after learning about the options and expected results.
Skin tightening
Both radiofrequency and microfocused ultrasound are noninvasive and additional options. Radiofrequency uses radio waves, with electromagnetic energy to stimulate heat. Ultrasound uses ultrasound waves to stimulate heat. Both approaches cause collagen contraction, neocollagenesis, and skin tightening.
These procedures do well for the lower face, Dr. Watchmaker said, but “I am relatively unimpressed for how well they do for the upper face.” Ideal candidates have mild to moderate skin laxity and want to avoid surgery. She also tells patients that collagen isn’t made overnight. “You won’t see much for 3-6 months after.” The good news? Usually the treatments need to be repeated only every 1.5-2 years, she said.
Lasers
“There are so many lasers out there,” said Dr. Watchmaker, who groups them into three categories: those used for wrinkles, dyschromia, and erythema. Her picks: ablative lasers (CO2 and erbium) and erbium-doped YAG 1550 nm laser for rhytids. Thulium 1927 and QS and picosecond lasers are her picks for dyschromia, and for erythema, pulsed dye and KTP lasers.
Some laser treatments are not a “walk in the park,” as she warns patients. For example, after treatment with ablative lasers, there is pain, post-procedure redness, and crusting.
Take-home points
A combination of noninvasive and minimally invasive procedures can produce appearance-improving results. That’s more likely if dermatologists choose ideal candidates, personalize the treatment, and set realistic expectations. “We have a finite number of tools,” she said, but they can be used in a variety of ways.
At the interactive panel discussion following her presentation, Dr. Watchmaker was asked what she tells patients about sun protection. “I talk a lot about sunscreens,’’ she said, always urging patients to use them. While the options for rejuvenation are numerous, taking care of the skin is still crucial.
Dr. Watchmaker had no disclosures. MedscapeLive and this news organization are owned by the same parent company.
At the MedscapeLive’s Women’s and Pediatric Dermatology Seminar, Jacqueline Watchmaker, MD, a dermatologist in Scottsdale, Ariz., provided an overview of current options, along with advice on how to keep patients’ expectations realistic and how to properly choose the best candidates for the best procedures.
“One of the most common concerns patients come to me with are wrinkles on the upper face,” but this is far from their only concern, Dr. Watchmaker said. Wrinkles and sagging of the lower face, areas under the eyes, nasolabial folds, marionette lines, and the neck also draw concern. Uneven coloration is another common concern, she said.
“So, what can we do for all of this?” she asked. The options are plentiful. Wrinkles of the upper face are easy to address with neuromodulators, she said, and soft-tissue fillers help the jawline and cheek areas.
“For the lower face, skin tightening devices really shine,” she added. And lasers can help correct uneven coloration. Surgery, of course, can also produce good results, but many patients want to stick with noninvasive or minimally invasive procedures.
Case: 83-year-old woman
Dr. Watchmaker discussed an 83-year old patient, who had malar mounds and accentuation of the infraorbital hollowness resulting from changes in subcutaneous fat and ligament laxity. She also had uneven coloration from photo damage, wrinkles on the upper face, linear appearance of zygoma related to underlying bony changes and fat compartment descent, and nasolabial folds and jowls related to decreased bony compartments, ligament laxity, and shifting of fat. She was naive to any cosmetic procedure.
Despite her age, this patient had no wrinkling on the upper forehead. Dr. Watchmaker did not inject neuromodulator in the upper forehead, as this patient also had a slightly heavy eyelid. “If you inject too much, it can cause some drooping of the eyelid and eyebrow,” she said.
For filler, she used a combination of high G (firmness, support) hyaluronic acid filler, a medium G acid filler, and a low G filler. The result: The woman’s face became more balanced, the mid-face volumization lifted the lower face, and the glabellar and periocular lines were softer, although still present. “It’s important to counsel patients that neuromodulators won’t make the lines go away the first time, but they will be softened.”
Practice tips
It’s important to titrate neuromodulators to fit the patient, Dr. Watchmaker said. Ask: What are their goals: Reversal of static lines? Softening wrinkles? Maintaining current status? “There’s not one dosing regimen,” and both dosing and frequency of neuromodulators can be titrated to fit each patient’s aesthetic goals, she said. For older patients who want to soften or maintain appearance, she suggested treatment every 4-6 months. And some patients just want to maintain the status quo, she noted.
Ideal candidates
For neuromodulators and fillers, who is an ideal candidate? “I think it’s anyone who has realistic expectations,” she said. Patients need to know how many treatments are needed and how much it will cost. For patients with extensive wrinkling and sagging, she said, she does extensive counseling about what results to expect “because I don’t want them to feel like they wasted their time or their money.”
She also suggests a surgical consult, as some may opt for that route after learning about the options and expected results.
Skin tightening
Both radiofrequency and microfocused ultrasound are noninvasive and additional options. Radiofrequency uses radio waves, with electromagnetic energy to stimulate heat. Ultrasound uses ultrasound waves to stimulate heat. Both approaches cause collagen contraction, neocollagenesis, and skin tightening.
These procedures do well for the lower face, Dr. Watchmaker said, but “I am relatively unimpressed for how well they do for the upper face.” Ideal candidates have mild to moderate skin laxity and want to avoid surgery. She also tells patients that collagen isn’t made overnight. “You won’t see much for 3-6 months after.” The good news? Usually the treatments need to be repeated only every 1.5-2 years, she said.
Lasers
“There are so many lasers out there,” said Dr. Watchmaker, who groups them into three categories: those used for wrinkles, dyschromia, and erythema. Her picks: ablative lasers (CO2 and erbium) and erbium-doped YAG 1550 nm laser for rhytids. Thulium 1927 and QS and picosecond lasers are her picks for dyschromia, and for erythema, pulsed dye and KTP lasers.
Some laser treatments are not a “walk in the park,” as she warns patients. For example, after treatment with ablative lasers, there is pain, post-procedure redness, and crusting.
Take-home points
A combination of noninvasive and minimally invasive procedures can produce appearance-improving results. That’s more likely if dermatologists choose ideal candidates, personalize the treatment, and set realistic expectations. “We have a finite number of tools,” she said, but they can be used in a variety of ways.
At the interactive panel discussion following her presentation, Dr. Watchmaker was asked what she tells patients about sun protection. “I talk a lot about sunscreens,’’ she said, always urging patients to use them. While the options for rejuvenation are numerous, taking care of the skin is still crucial.
Dr. Watchmaker had no disclosures. MedscapeLive and this news organization are owned by the same parent company.
FROM MEDSCAPELIVE WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
Fertility doctors, IVF families, post Roe: ‘We’re anxious’
Married for nearly 5 years, Jessica King, 34, and her wife, Sarah, agreed on some things right from the start. “We always knew kids were in the equation,” Jessica says.
Now, Jessica is nearly 20 weeks pregnant, thanks to in vitro fertilization, or IVF. They did “reciprocal” IVF, with Sarah’s egg mixed with donor sperm and the embryo transferred into Jessica. “We’re excited – and terrified,” Jessica says.
But that terror goes beyond the typical concerns of excess weight gain and long labors. They live in Missouri, one of 13 states with so-called trigger laws that went into effect after the Supreme Court overturned Roe v. Wade and the constitutional right to abortion, giving states the power to regulate it. States with trigger laws either banned abortion immediately or within a specified time frame after the ruling. In all, 26 states are expected to have abortion restrictions.
Missouri now allows abortion only for medical emergencies. If her upcoming ultrasound shows serious issues, Jessica says they could easily travel to another state and pay for an abortion. She realizes not everyone can.
However, the concern about trigger laws goes well beyond abortion. Many experts worry about the “spill-over” effects the abortion laws – both the existing ones and future proposals – may have on fertility care and treatments.
‘Personhood’ laws drive the concern
“The current trigger laws on the books are not impacting people’s access to IVF,” says Barbara Collura, president and CEO of RESOLVE, an advocacy group for those with fertility issues. “What we are concerned about is they will come back and make them stronger.”
The chief concern for reproductive rights advocates is so-called “personhood” legislation. According to the Guttmacher Institute, at least six bills about personhood have been introduced in five states, including Iowa, Oklahoma, South Carolina, Vermont, and West Virginia. One of the two Oklahoma bills has gone the farthest, passed by one chamber.
Since the Guttmacher report, Ohio introduced its own personhood legislation July 11, recognizing the personhood of an unborn person from conception.
Personhood legislation defines a fertilized egg or embryo as a legal human entity, says Sean Tipton, chief policy and advocacy officer for the Washington-based American Society of Reproductive Medicine, a nonprofit advocacy group.
“If the legal status of fertilized eggs or early embryos is codified, in vitro fertilization procedures may become legally risky for patients, physicians and staff,” Mr. Tipton wrote in late June in Contemporary OB/GYN Journal. The American Society for Reproductive Medicine has posted a report on state abortion trigger laws and their potential implications for reproductive medicine. Of the 13 with trigger laws in effect, the report found concern about the potential effect on IVF only with Utah’s.
‘Safe’ states?
Even in states without trigger laws or personhood bills, IVF patients say they are anxious about how the Supreme Court ruling may ultimately affect care. Thanks to IVF, Shelly Battista and her husband Robert are expecting twins in December, little sisters to their daughter Emilia, who is 2½.
They live in Illinois, where abortion is legal. “Even though we are safe now, I think the overturning of Roe has made it clear to all of us that none of our freedoms are safe, especially reproductive rights,” Ms. Shelly says.
About one in eight U.S. couples are infertile, according to RESOLVE, In 2019, 2% of all babies born in the United States, or about 78,000 infants, were conceived with the use of assisted reproductive technologies, according to the Centers for Disease Control and Prevention. The most common assisted reproductive technology is IVF, in which the sperm fertilizes the egg outside the body and an embryo is then transferred. The standard of practice is to transfer a single embryo, freezing others for future use.
Trigger state doctors weigh in
Some fertility doctors in those “trigger” states are keeping a close eye on proposed legislation and talking to legislators for interpretation of current and proposed laws.
Eli Reshef, MD, a reproductive endocrinologist and fertility specialist at Bennett Fertility Institute in Oklahoma City, notes that his state has “the strictest abortion law in the land.” The law prohibits all abortions with few exceptions, such as the removal of an ectopic pregnancy (when a fertilized egg implants outside the uterus, such as in the fallopian tubes).
While IVF will not be affected for now, he worries that the Oklahoma law allows a private citizen to sue a health care provider that they feel is performing abortion. The Oklahoma law leaves interpretation of abortion up to the general public, who may be unfamiliar with the language of the law, House Bill 4327.
Dean Moutos, MD, a reproductive endocrinologist and medical director of Arkansas Fertility and Gynecology in Little Rock, says his state’s current trigger law should not affect IVF. “When you read the bill, it says abortion means to terminate the pregnancy of a woman.” Still, he says, “we are concerned about what might happen in the future” and the possibility that some legislators may interpret that differently.
A minority approach
John David Gordon, MD, a reproductive endocrinologist and medical director of Southeastern Fertility Center for Fertility and Reproductive Surgery in Knoxville, Tenn., is also in a trigger state. However, it’s not likely any personhood laws would affect his practice.
That’s because his center, which he acknowledges is clearly in the minority, only performs natural-cycle IVF, which usually results in a single egg, or “mini-stim IVF,” which usually results in three to eight eggs in order to limit the number that may be potentially fertilized. Often, he says, patients choose to freeze unfertilized eggs (alone) to avoid creating an excessive number of embryos. He has a “no discard” program, with any viable embryos frozen or transferred. Abandoned embryos are donated to others.
“This may work for young women,” says Marcelle Cedars, MD, director of reproductive endocrinology at the University of California, San Francisco, and president of the American Society of Reproductive Medicine. However, she says, it will be very inefficient for older patients, since they have a higher percentage of abnormal eggs.
Overall, that approach will also drive up costs, especially for older women, Dr. Cedars says. An average cycle of IVF costs $12,400, and most Americans’ insurance plans don’t cover IVF, according to Mr. Tipton.
Top concerns for IVF
“Personhood” legislation has the potential to upend many common IVF practices, experts say.
Of greatest concern to fertility practices are potential restrictions on the freezing or discarding of embryos, Dr. Cedars says. “This could have a critical impact on practicing the safest, most evidence-based medicine,” she says.
Most children born in the United States as a result of IVF procedures are born from frozen embryos, according to the Society for Assisted Reproductive Technology, an organization for reproductive specialists.
“The practice of IVF really requires that we generate more embryos than will be used in a given [IVF] cycle,” agrees Kara Goldman, MD, associate professor of obstetrics and gynecology and medical director of the fertility preservation program at Northwestern University, Chicago. She performed the embryo transfer for the Battistas.
In nature, she says, it’s known that only a small number of eggs will be competent to generate a baby. “We see the same thing in IVF.” In a single cycle, 20 eggs may be retrieved, but many fewer typically reach successful fertilization and are able to be implanted.
When patients have completed their family, unused embryos are donated to research, donated for adoption, or destroyed. If embryo destruction is outlawed, Dr. Goldman says, it will have serious ramifications for the practice of IVF.
And if personhood legislation prohibits destroying any embryos, others wonder: Would a lab technician who accidentally dropped and destroyed an embryo be subject to charges? If laws prohibit destruction of embryos, others wonder if will families be forced to pay the embryo storage fees, generally $500-$1,000 a year, in perpetuity.
If an embryo is declared a person, it could also affect a practice called preimplantation genetic testing, or PGT. In PGT, cells are retrieved from an embryo and checked for genetic disorders such as sickle cell anemia and cystic fibrosis, with some parents choosing to discard embryos that are found to be affected.
Some potential parents choose this testing because they know they are carriers for genetic diseases that are serious and even incompatible with life, says Art Caplan, PhD, head of the division of medical ethics at New York University. They may choose to discard embryos that show evidence of the diseases.
Also under fire could be “selective reduction,” reducing multiple fetuses to a single or twin, to reduce risks to babies and mother.
Dr. Caplan predicts if states have many restrictions, some providers will adopt the attitude that “if no one reports, it did not happen.” And those prospective parents with the means, he says, will go to court and fight restrictions. “When they do it, they are saying, ‘You say you are pro-life; I’m trying to have a child. What are you doing getting in my way?’”
IVF families: Tough decisions, emotional times
The Battistas, of Illinois, have had an especially rough road. Shelly was diagnosed with a fast-growing breast cancer in 2020, when Emilia was just an infant. Warned that the chemotherapy she needed would suppress her ovaries, Shelly underwent egg retrieval before starting the cancer treatment.
She opted to have a double mastectomy and her ovaries removed after learning she carried the BRCA1 genetic mutation, boosting the risk of both breast and ovarian cancer.
Once she was cancer-free, she was cleared to start IVF. The first two embryo transfers failed. The third transfer, of a single embryo, was successful. But it split, a rare occurrence, producing two embryos. “It was a big shock, but in the best way,” she says about learning they were having twins. “Now we are over the moon.”
Five frozen embryos remain. At the start, the Battistas decided to discard unused embryos. She and Robert are discussing what to do next. If they decide they are done building their family after the twins’ birth, she wonders, “do we need to discard our [other] embryos before that becomes something that isn’t eligible [possible] for us any longer?” She doesn’t want to be rushed into that decision, however, especially with her medical history.
Jessica King and Sarah have 20 more embryos.
The couple had decided to donate unused embryos for research, when the time comes, and for different reasons. Her wife’s decision is based on her belief in science, while Jessica cites her faith. “As a Jew, it is part of our faith, that we should be doing everything we can to advance humanity,” she says.
In the midst of all the uncertainty, Jessica says, only half-jokingly, that she is tempted to claim the frozen embryos as dependents. “If you are truly going to claim these are precious human lives, you should be giving me all the benefits from having children,” she says.
Shelly knows that having one daughter, with two more on the way, affects her thinking about the court’s ruling. “My overall wish would be that Roe v. Wade is reinstated, and my daughters have the same rights and options that I have … or I did have until my current 36 years of life.”
A version of this article first appeared on WebMD.com.
Married for nearly 5 years, Jessica King, 34, and her wife, Sarah, agreed on some things right from the start. “We always knew kids were in the equation,” Jessica says.
Now, Jessica is nearly 20 weeks pregnant, thanks to in vitro fertilization, or IVF. They did “reciprocal” IVF, with Sarah’s egg mixed with donor sperm and the embryo transferred into Jessica. “We’re excited – and terrified,” Jessica says.
But that terror goes beyond the typical concerns of excess weight gain and long labors. They live in Missouri, one of 13 states with so-called trigger laws that went into effect after the Supreme Court overturned Roe v. Wade and the constitutional right to abortion, giving states the power to regulate it. States with trigger laws either banned abortion immediately or within a specified time frame after the ruling. In all, 26 states are expected to have abortion restrictions.
Missouri now allows abortion only for medical emergencies. If her upcoming ultrasound shows serious issues, Jessica says they could easily travel to another state and pay for an abortion. She realizes not everyone can.
However, the concern about trigger laws goes well beyond abortion. Many experts worry about the “spill-over” effects the abortion laws – both the existing ones and future proposals – may have on fertility care and treatments.
‘Personhood’ laws drive the concern
“The current trigger laws on the books are not impacting people’s access to IVF,” says Barbara Collura, president and CEO of RESOLVE, an advocacy group for those with fertility issues. “What we are concerned about is they will come back and make them stronger.”
The chief concern for reproductive rights advocates is so-called “personhood” legislation. According to the Guttmacher Institute, at least six bills about personhood have been introduced in five states, including Iowa, Oklahoma, South Carolina, Vermont, and West Virginia. One of the two Oklahoma bills has gone the farthest, passed by one chamber.
Since the Guttmacher report, Ohio introduced its own personhood legislation July 11, recognizing the personhood of an unborn person from conception.
Personhood legislation defines a fertilized egg or embryo as a legal human entity, says Sean Tipton, chief policy and advocacy officer for the Washington-based American Society of Reproductive Medicine, a nonprofit advocacy group.
“If the legal status of fertilized eggs or early embryos is codified, in vitro fertilization procedures may become legally risky for patients, physicians and staff,” Mr. Tipton wrote in late June in Contemporary OB/GYN Journal. The American Society for Reproductive Medicine has posted a report on state abortion trigger laws and their potential implications for reproductive medicine. Of the 13 with trigger laws in effect, the report found concern about the potential effect on IVF only with Utah’s.
‘Safe’ states?
Even in states without trigger laws or personhood bills, IVF patients say they are anxious about how the Supreme Court ruling may ultimately affect care. Thanks to IVF, Shelly Battista and her husband Robert are expecting twins in December, little sisters to their daughter Emilia, who is 2½.
They live in Illinois, where abortion is legal. “Even though we are safe now, I think the overturning of Roe has made it clear to all of us that none of our freedoms are safe, especially reproductive rights,” Ms. Shelly says.
About one in eight U.S. couples are infertile, according to RESOLVE, In 2019, 2% of all babies born in the United States, or about 78,000 infants, were conceived with the use of assisted reproductive technologies, according to the Centers for Disease Control and Prevention. The most common assisted reproductive technology is IVF, in which the sperm fertilizes the egg outside the body and an embryo is then transferred. The standard of practice is to transfer a single embryo, freezing others for future use.
Trigger state doctors weigh in
Some fertility doctors in those “trigger” states are keeping a close eye on proposed legislation and talking to legislators for interpretation of current and proposed laws.
Eli Reshef, MD, a reproductive endocrinologist and fertility specialist at Bennett Fertility Institute in Oklahoma City, notes that his state has “the strictest abortion law in the land.” The law prohibits all abortions with few exceptions, such as the removal of an ectopic pregnancy (when a fertilized egg implants outside the uterus, such as in the fallopian tubes).
While IVF will not be affected for now, he worries that the Oklahoma law allows a private citizen to sue a health care provider that they feel is performing abortion. The Oklahoma law leaves interpretation of abortion up to the general public, who may be unfamiliar with the language of the law, House Bill 4327.
Dean Moutos, MD, a reproductive endocrinologist and medical director of Arkansas Fertility and Gynecology in Little Rock, says his state’s current trigger law should not affect IVF. “When you read the bill, it says abortion means to terminate the pregnancy of a woman.” Still, he says, “we are concerned about what might happen in the future” and the possibility that some legislators may interpret that differently.
A minority approach
John David Gordon, MD, a reproductive endocrinologist and medical director of Southeastern Fertility Center for Fertility and Reproductive Surgery in Knoxville, Tenn., is also in a trigger state. However, it’s not likely any personhood laws would affect his practice.
That’s because his center, which he acknowledges is clearly in the minority, only performs natural-cycle IVF, which usually results in a single egg, or “mini-stim IVF,” which usually results in three to eight eggs in order to limit the number that may be potentially fertilized. Often, he says, patients choose to freeze unfertilized eggs (alone) to avoid creating an excessive number of embryos. He has a “no discard” program, with any viable embryos frozen or transferred. Abandoned embryos are donated to others.
“This may work for young women,” says Marcelle Cedars, MD, director of reproductive endocrinology at the University of California, San Francisco, and president of the American Society of Reproductive Medicine. However, she says, it will be very inefficient for older patients, since they have a higher percentage of abnormal eggs.
Overall, that approach will also drive up costs, especially for older women, Dr. Cedars says. An average cycle of IVF costs $12,400, and most Americans’ insurance plans don’t cover IVF, according to Mr. Tipton.
Top concerns for IVF
“Personhood” legislation has the potential to upend many common IVF practices, experts say.
Of greatest concern to fertility practices are potential restrictions on the freezing or discarding of embryos, Dr. Cedars says. “This could have a critical impact on practicing the safest, most evidence-based medicine,” she says.
Most children born in the United States as a result of IVF procedures are born from frozen embryos, according to the Society for Assisted Reproductive Technology, an organization for reproductive specialists.
“The practice of IVF really requires that we generate more embryos than will be used in a given [IVF] cycle,” agrees Kara Goldman, MD, associate professor of obstetrics and gynecology and medical director of the fertility preservation program at Northwestern University, Chicago. She performed the embryo transfer for the Battistas.
In nature, she says, it’s known that only a small number of eggs will be competent to generate a baby. “We see the same thing in IVF.” In a single cycle, 20 eggs may be retrieved, but many fewer typically reach successful fertilization and are able to be implanted.
When patients have completed their family, unused embryos are donated to research, donated for adoption, or destroyed. If embryo destruction is outlawed, Dr. Goldman says, it will have serious ramifications for the practice of IVF.
And if personhood legislation prohibits destroying any embryos, others wonder: Would a lab technician who accidentally dropped and destroyed an embryo be subject to charges? If laws prohibit destruction of embryos, others wonder if will families be forced to pay the embryo storage fees, generally $500-$1,000 a year, in perpetuity.
If an embryo is declared a person, it could also affect a practice called preimplantation genetic testing, or PGT. In PGT, cells are retrieved from an embryo and checked for genetic disorders such as sickle cell anemia and cystic fibrosis, with some parents choosing to discard embryos that are found to be affected.
Some potential parents choose this testing because they know they are carriers for genetic diseases that are serious and even incompatible with life, says Art Caplan, PhD, head of the division of medical ethics at New York University. They may choose to discard embryos that show evidence of the diseases.
Also under fire could be “selective reduction,” reducing multiple fetuses to a single or twin, to reduce risks to babies and mother.
Dr. Caplan predicts if states have many restrictions, some providers will adopt the attitude that “if no one reports, it did not happen.” And those prospective parents with the means, he says, will go to court and fight restrictions. “When they do it, they are saying, ‘You say you are pro-life; I’m trying to have a child. What are you doing getting in my way?’”
IVF families: Tough decisions, emotional times
The Battistas, of Illinois, have had an especially rough road. Shelly was diagnosed with a fast-growing breast cancer in 2020, when Emilia was just an infant. Warned that the chemotherapy she needed would suppress her ovaries, Shelly underwent egg retrieval before starting the cancer treatment.
She opted to have a double mastectomy and her ovaries removed after learning she carried the BRCA1 genetic mutation, boosting the risk of both breast and ovarian cancer.
Once she was cancer-free, she was cleared to start IVF. The first two embryo transfers failed. The third transfer, of a single embryo, was successful. But it split, a rare occurrence, producing two embryos. “It was a big shock, but in the best way,” she says about learning they were having twins. “Now we are over the moon.”
Five frozen embryos remain. At the start, the Battistas decided to discard unused embryos. She and Robert are discussing what to do next. If they decide they are done building their family after the twins’ birth, she wonders, “do we need to discard our [other] embryos before that becomes something that isn’t eligible [possible] for us any longer?” She doesn’t want to be rushed into that decision, however, especially with her medical history.
Jessica King and Sarah have 20 more embryos.
The couple had decided to donate unused embryos for research, when the time comes, and for different reasons. Her wife’s decision is based on her belief in science, while Jessica cites her faith. “As a Jew, it is part of our faith, that we should be doing everything we can to advance humanity,” she says.
In the midst of all the uncertainty, Jessica says, only half-jokingly, that she is tempted to claim the frozen embryos as dependents. “If you are truly going to claim these are precious human lives, you should be giving me all the benefits from having children,” she says.
Shelly knows that having one daughter, with two more on the way, affects her thinking about the court’s ruling. “My overall wish would be that Roe v. Wade is reinstated, and my daughters have the same rights and options that I have … or I did have until my current 36 years of life.”
A version of this article first appeared on WebMD.com.
Married for nearly 5 years, Jessica King, 34, and her wife, Sarah, agreed on some things right from the start. “We always knew kids were in the equation,” Jessica says.
Now, Jessica is nearly 20 weeks pregnant, thanks to in vitro fertilization, or IVF. They did “reciprocal” IVF, with Sarah’s egg mixed with donor sperm and the embryo transferred into Jessica. “We’re excited – and terrified,” Jessica says.
But that terror goes beyond the typical concerns of excess weight gain and long labors. They live in Missouri, one of 13 states with so-called trigger laws that went into effect after the Supreme Court overturned Roe v. Wade and the constitutional right to abortion, giving states the power to regulate it. States with trigger laws either banned abortion immediately or within a specified time frame after the ruling. In all, 26 states are expected to have abortion restrictions.
Missouri now allows abortion only for medical emergencies. If her upcoming ultrasound shows serious issues, Jessica says they could easily travel to another state and pay for an abortion. She realizes not everyone can.
However, the concern about trigger laws goes well beyond abortion. Many experts worry about the “spill-over” effects the abortion laws – both the existing ones and future proposals – may have on fertility care and treatments.
‘Personhood’ laws drive the concern
“The current trigger laws on the books are not impacting people’s access to IVF,” says Barbara Collura, president and CEO of RESOLVE, an advocacy group for those with fertility issues. “What we are concerned about is they will come back and make them stronger.”
The chief concern for reproductive rights advocates is so-called “personhood” legislation. According to the Guttmacher Institute, at least six bills about personhood have been introduced in five states, including Iowa, Oklahoma, South Carolina, Vermont, and West Virginia. One of the two Oklahoma bills has gone the farthest, passed by one chamber.
Since the Guttmacher report, Ohio introduced its own personhood legislation July 11, recognizing the personhood of an unborn person from conception.
Personhood legislation defines a fertilized egg or embryo as a legal human entity, says Sean Tipton, chief policy and advocacy officer for the Washington-based American Society of Reproductive Medicine, a nonprofit advocacy group.
“If the legal status of fertilized eggs or early embryos is codified, in vitro fertilization procedures may become legally risky for patients, physicians and staff,” Mr. Tipton wrote in late June in Contemporary OB/GYN Journal. The American Society for Reproductive Medicine has posted a report on state abortion trigger laws and their potential implications for reproductive medicine. Of the 13 with trigger laws in effect, the report found concern about the potential effect on IVF only with Utah’s.
‘Safe’ states?
Even in states without trigger laws or personhood bills, IVF patients say they are anxious about how the Supreme Court ruling may ultimately affect care. Thanks to IVF, Shelly Battista and her husband Robert are expecting twins in December, little sisters to their daughter Emilia, who is 2½.
They live in Illinois, where abortion is legal. “Even though we are safe now, I think the overturning of Roe has made it clear to all of us that none of our freedoms are safe, especially reproductive rights,” Ms. Shelly says.
About one in eight U.S. couples are infertile, according to RESOLVE, In 2019, 2% of all babies born in the United States, or about 78,000 infants, were conceived with the use of assisted reproductive technologies, according to the Centers for Disease Control and Prevention. The most common assisted reproductive technology is IVF, in which the sperm fertilizes the egg outside the body and an embryo is then transferred. The standard of practice is to transfer a single embryo, freezing others for future use.
Trigger state doctors weigh in
Some fertility doctors in those “trigger” states are keeping a close eye on proposed legislation and talking to legislators for interpretation of current and proposed laws.
Eli Reshef, MD, a reproductive endocrinologist and fertility specialist at Bennett Fertility Institute in Oklahoma City, notes that his state has “the strictest abortion law in the land.” The law prohibits all abortions with few exceptions, such as the removal of an ectopic pregnancy (when a fertilized egg implants outside the uterus, such as in the fallopian tubes).
While IVF will not be affected for now, he worries that the Oklahoma law allows a private citizen to sue a health care provider that they feel is performing abortion. The Oklahoma law leaves interpretation of abortion up to the general public, who may be unfamiliar with the language of the law, House Bill 4327.
Dean Moutos, MD, a reproductive endocrinologist and medical director of Arkansas Fertility and Gynecology in Little Rock, says his state’s current trigger law should not affect IVF. “When you read the bill, it says abortion means to terminate the pregnancy of a woman.” Still, he says, “we are concerned about what might happen in the future” and the possibility that some legislators may interpret that differently.
A minority approach
John David Gordon, MD, a reproductive endocrinologist and medical director of Southeastern Fertility Center for Fertility and Reproductive Surgery in Knoxville, Tenn., is also in a trigger state. However, it’s not likely any personhood laws would affect his practice.
That’s because his center, which he acknowledges is clearly in the minority, only performs natural-cycle IVF, which usually results in a single egg, or “mini-stim IVF,” which usually results in three to eight eggs in order to limit the number that may be potentially fertilized. Often, he says, patients choose to freeze unfertilized eggs (alone) to avoid creating an excessive number of embryos. He has a “no discard” program, with any viable embryos frozen or transferred. Abandoned embryos are donated to others.
“This may work for young women,” says Marcelle Cedars, MD, director of reproductive endocrinology at the University of California, San Francisco, and president of the American Society of Reproductive Medicine. However, she says, it will be very inefficient for older patients, since they have a higher percentage of abnormal eggs.
Overall, that approach will also drive up costs, especially for older women, Dr. Cedars says. An average cycle of IVF costs $12,400, and most Americans’ insurance plans don’t cover IVF, according to Mr. Tipton.
Top concerns for IVF
“Personhood” legislation has the potential to upend many common IVF practices, experts say.
Of greatest concern to fertility practices are potential restrictions on the freezing or discarding of embryos, Dr. Cedars says. “This could have a critical impact on practicing the safest, most evidence-based medicine,” she says.
Most children born in the United States as a result of IVF procedures are born from frozen embryos, according to the Society for Assisted Reproductive Technology, an organization for reproductive specialists.
“The practice of IVF really requires that we generate more embryos than will be used in a given [IVF] cycle,” agrees Kara Goldman, MD, associate professor of obstetrics and gynecology and medical director of the fertility preservation program at Northwestern University, Chicago. She performed the embryo transfer for the Battistas.
In nature, she says, it’s known that only a small number of eggs will be competent to generate a baby. “We see the same thing in IVF.” In a single cycle, 20 eggs may be retrieved, but many fewer typically reach successful fertilization and are able to be implanted.
When patients have completed their family, unused embryos are donated to research, donated for adoption, or destroyed. If embryo destruction is outlawed, Dr. Goldman says, it will have serious ramifications for the practice of IVF.
And if personhood legislation prohibits destroying any embryos, others wonder: Would a lab technician who accidentally dropped and destroyed an embryo be subject to charges? If laws prohibit destruction of embryos, others wonder if will families be forced to pay the embryo storage fees, generally $500-$1,000 a year, in perpetuity.
If an embryo is declared a person, it could also affect a practice called preimplantation genetic testing, or PGT. In PGT, cells are retrieved from an embryo and checked for genetic disorders such as sickle cell anemia and cystic fibrosis, with some parents choosing to discard embryos that are found to be affected.
Some potential parents choose this testing because they know they are carriers for genetic diseases that are serious and even incompatible with life, says Art Caplan, PhD, head of the division of medical ethics at New York University. They may choose to discard embryos that show evidence of the diseases.
Also under fire could be “selective reduction,” reducing multiple fetuses to a single or twin, to reduce risks to babies and mother.
Dr. Caplan predicts if states have many restrictions, some providers will adopt the attitude that “if no one reports, it did not happen.” And those prospective parents with the means, he says, will go to court and fight restrictions. “When they do it, they are saying, ‘You say you are pro-life; I’m trying to have a child. What are you doing getting in my way?’”
IVF families: Tough decisions, emotional times
The Battistas, of Illinois, have had an especially rough road. Shelly was diagnosed with a fast-growing breast cancer in 2020, when Emilia was just an infant. Warned that the chemotherapy she needed would suppress her ovaries, Shelly underwent egg retrieval before starting the cancer treatment.
She opted to have a double mastectomy and her ovaries removed after learning she carried the BRCA1 genetic mutation, boosting the risk of both breast and ovarian cancer.
Once she was cancer-free, she was cleared to start IVF. The first two embryo transfers failed. The third transfer, of a single embryo, was successful. But it split, a rare occurrence, producing two embryos. “It was a big shock, but in the best way,” she says about learning they were having twins. “Now we are over the moon.”
Five frozen embryos remain. At the start, the Battistas decided to discard unused embryos. She and Robert are discussing what to do next. If they decide they are done building their family after the twins’ birth, she wonders, “do we need to discard our [other] embryos before that becomes something that isn’t eligible [possible] for us any longer?” She doesn’t want to be rushed into that decision, however, especially with her medical history.
Jessica King and Sarah have 20 more embryos.
The couple had decided to donate unused embryos for research, when the time comes, and for different reasons. Her wife’s decision is based on her belief in science, while Jessica cites her faith. “As a Jew, it is part of our faith, that we should be doing everything we can to advance humanity,” she says.
In the midst of all the uncertainty, Jessica says, only half-jokingly, that she is tempted to claim the frozen embryos as dependents. “If you are truly going to claim these are precious human lives, you should be giving me all the benefits from having children,” she says.
Shelly knows that having one daughter, with two more on the way, affects her thinking about the court’s ruling. “My overall wish would be that Roe v. Wade is reinstated, and my daughters have the same rights and options that I have … or I did have until my current 36 years of life.”
A version of this article first appeared on WebMD.com.
Coming soon: More breathable, more comfortable face masks
Sitting at his desk in Sea Girt, N.J., John Schwind is eager to demonstrate his ReadiMask 365. He holds up what looks like a white sheet of memo paper, peels off a protective liner, and sticks the mask first to his nose. He glides his fingers down his face, over his cheeks, and to his chin, sealing the mask and then demonstrating how easy it is to talk with it in place.
The mask’s medical adhesive sticks directly to the face, without causing breakouts, he said. It doesn’t let air leak and won’t fog his glasses. It’s strapless, so it won’t hurt his ears or make them stick out.
This fall, Mr. Schwind, the CEO of Global Safety First, is hoping to take home $150,000 as one of the two top winners of the federal Mask Innovation Challenge. He has made it to the top 10 but realizes he still has a ton of competition.
After the challenge launched in late 2021, nearly 1,500 submissions were received, says Kumiko Lippold, PhD, a health scientist and manager of the Mask Innovation Challenge. The challenge is run by Dr. Lippold and others at the Division of Research, Innovation, and Ventures (DRIVe), which is part of the Biomedical Advanced Research and Development Authority (BARDA) at the U.S. Department of Health & Human Services.
Like the rest of us, Dr. Lippold knows that masks desperately need a makeover. The aim is not only to get us through this pandemic but also future pandemics and other public health emergencies. “We are focused on building masks for the next pandemic, the next wildfires,” she says.
The project is a partnership among BARDA’s DRIVe, the National Institute for Occupational Safety and Health (NIOSH), and the National Institute of Standards and Technology (NIST).
While NIOSH is a partner in the challenge, giving feedback to mask developers, “the mask challenge is entirely separate from the NIOSH approval process,” Dr. Lippold says. Companies can then pursue NIOSH approval on their own, later, if they wish. The agency certifies only masks and respirators.
Preview of masks to come
“We’ve seen some really amazing things,” Dr. Lippold said of the new designs. She didn’t want to play favorites, so she gave an overview of innovations. Some designs have transparent materials, or partially see-through materials, so facial expressions can be read. “We’ve also seen really unique bio-based materials that are derived from natural products. We’ve seen sensors in some.”
One mask model has origami folds, which increase overall surface and breathing area. Some 3D-printed masks promise a custom fit and take into account whether a person’s nose bridge is low or high.
And the finalists are ...
ReadiMask 365: “I can wear this all day long,” Mr. Schwind said of his new design. It has a nano fiber filter and is flexible. Besides the one in the BARDA challenge, the company has other ReadiMasks on the market. “The most important thing is comfort,” he says. “Second is protection. If they don’t feel they have a good seal, users don’t have confidence in the mask.”
He offers various sizes of ReadiMasks, from small sizes designed for women with smaller faces to extra-large, “for NFL linemen.”
ClearMask: “We are the original clear mask,” says Aaron Hsu, CEO and co-founder of ClearMask in Baltimore. The company began in 2017, and the clear design was inspired by a company co-founder who is deaf. She was scheduled to have surgery, and her sign language interpreter did not show up, leaving her to try to communicate in the operating room with masked health care providers. There were no transparent masks available then, Mr. Hsu says.
“Being able to work with BARDA and getting their wisdom is invaluable,” he says.
The makers of ClearMask think masks are here to stay, at least for some. “I think a certain percentage of the population will continue to wear them, regardless,” said Mr. Hsu. He predicts health care settings will become stricter about wearing masks.
“Even now, when you even walk in to a hospital, you might be required to wear a mask,” he says, even as a visitor. His company’s masks are easy to adjust and are secured around the head, so your ears don’t get sore, he says.
4C Air: The BreSafe transparent mask is semi-transparent and is made of a nanomaterial that provides high levels of filtration and breathability with some transparency.
Air99: Based on origami principles, the Airgami mask is meant to improve fit, breathability, and aesthetics over existing masks. “Airgami fits better, works better and looks better,” says Min Xiao, a company spokesperson. “It won’t fall off the nose or collapse onto the mouth, and eyeglasses fog less, she says. Voices are less muffled.” It’s also reusable, rinseable and can be heat disinfected, she says. It went on the market in November 2020.
Air Flo Labs: Flo Mask Pro, like the company’s other designs, conducted over 100 3D facial scans across many ethnicities to produce a better fit, says Kevin Ngo, its creator. For the adult masks, two nose bridge sizes are offered. And users can choose a Pro Filter, with 99% filtration, or an Everyday, which is meant to be much more breathable than other masks. “Our silicone gasket is incredibly soft and gentle on the skin,” Mr. Ngo says. “In addition,we offer indents for glasses, which prevent any fogging.” The company began shipping in May; several thousand masks are in use now, Mr. Ngo said.
Georgetown University: This team’s smart mask is made of metallic foams that can be cleaned and reused.
Levi Strauss: The form of the mask can be made by any basic garment factory. It aims to activate the apparel supply chain as another source of low-cost, high-performance masks.
Matregenix: This mask, made of a transparent nanofiber, allows for easier communication while having high filtration.
SEAL Lab: The SINEW mask stands for Smart, Individualized, Near-Face, Extended Wear. The mask used technology to overcome flaws of traditional respirators, with the same degree of protection. It doesn’t make contact with the skin of the wearer’s face.
StaySafeNow: A team from Harvard University developed Crystal Guard, a reusable, cost-effective clear mask. Its developers say it’s meant to be especially useful for essential workers, teachers, and others who have to communicate to do their work.
Bye-bye N95?
“From our perspective, our goal with the mask challenge was not to replace the N95 respirator,” Dr. Lippold says. N95 masks, which NIOSH certifies, are valuable and protect people in high-risk settings. “With the mask challenge, our goal was really to provide the public with a comparable alternative that really meets their specific level of risk.” Working in a health care setting carries a different risk, she says, than going to the grocery store.
“A common complaint with the N95 is that they are very uncomfortable.” It’s a major barrier to compliance, “and we wanted to address that gap. We didn’t directly compare [the entries] to an N95,” she says, although their testing was similar to NIOSH’s. A number of finalists say they will pursue NIOSH approval, she says.
Meanwhile, some of the finalists’ masks are for sale. Air Flo Labs, for instance, has its Flo Mask Pro for sale online, noting that BARDA allowed it to release the test results from NIOSH and NIST.
Getting from 1,500 to 10
In the first phase of the challenge, Dr. Lippold says, “the goal was to engage as wide an audience as possible.” With the second phase, the bar was set a bit higher. Instead of just submitting ideas on paper, companies had to submit prototypes for lab testing. “We got about 80 submissions,” she says.
Those 80 were whittled down to 10 finalists. Teams had sent prototypes, and experts, including those from NIOSH and NIST, rated them, sometimes looking at multiple copies of the masks. Experts looked at how well the masks filtered the air, how breathable they were, and other data. Once the feedback was given to the mask companies, they entered a redesign period. “Scientists can take this data and basically make these prototypes better,” Dr. Lippold says.
The final round of testing will be in September, and the winners will be announced in the fall. The opportunity allowed companies to have their products go through testing they might not otherwise have been able to get, she says.
A version of this article first appeared on WebMD.com.
Sitting at his desk in Sea Girt, N.J., John Schwind is eager to demonstrate his ReadiMask 365. He holds up what looks like a white sheet of memo paper, peels off a protective liner, and sticks the mask first to his nose. He glides his fingers down his face, over his cheeks, and to his chin, sealing the mask and then demonstrating how easy it is to talk with it in place.
The mask’s medical adhesive sticks directly to the face, without causing breakouts, he said. It doesn’t let air leak and won’t fog his glasses. It’s strapless, so it won’t hurt his ears or make them stick out.
This fall, Mr. Schwind, the CEO of Global Safety First, is hoping to take home $150,000 as one of the two top winners of the federal Mask Innovation Challenge. He has made it to the top 10 but realizes he still has a ton of competition.
After the challenge launched in late 2021, nearly 1,500 submissions were received, says Kumiko Lippold, PhD, a health scientist and manager of the Mask Innovation Challenge. The challenge is run by Dr. Lippold and others at the Division of Research, Innovation, and Ventures (DRIVe), which is part of the Biomedical Advanced Research and Development Authority (BARDA) at the U.S. Department of Health & Human Services.
Like the rest of us, Dr. Lippold knows that masks desperately need a makeover. The aim is not only to get us through this pandemic but also future pandemics and other public health emergencies. “We are focused on building masks for the next pandemic, the next wildfires,” she says.
The project is a partnership among BARDA’s DRIVe, the National Institute for Occupational Safety and Health (NIOSH), and the National Institute of Standards and Technology (NIST).
While NIOSH is a partner in the challenge, giving feedback to mask developers, “the mask challenge is entirely separate from the NIOSH approval process,” Dr. Lippold says. Companies can then pursue NIOSH approval on their own, later, if they wish. The agency certifies only masks and respirators.
Preview of masks to come
“We’ve seen some really amazing things,” Dr. Lippold said of the new designs. She didn’t want to play favorites, so she gave an overview of innovations. Some designs have transparent materials, or partially see-through materials, so facial expressions can be read. “We’ve also seen really unique bio-based materials that are derived from natural products. We’ve seen sensors in some.”
One mask model has origami folds, which increase overall surface and breathing area. Some 3D-printed masks promise a custom fit and take into account whether a person’s nose bridge is low or high.
And the finalists are ...
ReadiMask 365: “I can wear this all day long,” Mr. Schwind said of his new design. It has a nano fiber filter and is flexible. Besides the one in the BARDA challenge, the company has other ReadiMasks on the market. “The most important thing is comfort,” he says. “Second is protection. If they don’t feel they have a good seal, users don’t have confidence in the mask.”
He offers various sizes of ReadiMasks, from small sizes designed for women with smaller faces to extra-large, “for NFL linemen.”
ClearMask: “We are the original clear mask,” says Aaron Hsu, CEO and co-founder of ClearMask in Baltimore. The company began in 2017, and the clear design was inspired by a company co-founder who is deaf. She was scheduled to have surgery, and her sign language interpreter did not show up, leaving her to try to communicate in the operating room with masked health care providers. There were no transparent masks available then, Mr. Hsu says.
“Being able to work with BARDA and getting their wisdom is invaluable,” he says.
The makers of ClearMask think masks are here to stay, at least for some. “I think a certain percentage of the population will continue to wear them, regardless,” said Mr. Hsu. He predicts health care settings will become stricter about wearing masks.
“Even now, when you even walk in to a hospital, you might be required to wear a mask,” he says, even as a visitor. His company’s masks are easy to adjust and are secured around the head, so your ears don’t get sore, he says.
4C Air: The BreSafe transparent mask is semi-transparent and is made of a nanomaterial that provides high levels of filtration and breathability with some transparency.
Air99: Based on origami principles, the Airgami mask is meant to improve fit, breathability, and aesthetics over existing masks. “Airgami fits better, works better and looks better,” says Min Xiao, a company spokesperson. “It won’t fall off the nose or collapse onto the mouth, and eyeglasses fog less, she says. Voices are less muffled.” It’s also reusable, rinseable and can be heat disinfected, she says. It went on the market in November 2020.
Air Flo Labs: Flo Mask Pro, like the company’s other designs, conducted over 100 3D facial scans across many ethnicities to produce a better fit, says Kevin Ngo, its creator. For the adult masks, two nose bridge sizes are offered. And users can choose a Pro Filter, with 99% filtration, or an Everyday, which is meant to be much more breathable than other masks. “Our silicone gasket is incredibly soft and gentle on the skin,” Mr. Ngo says. “In addition,we offer indents for glasses, which prevent any fogging.” The company began shipping in May; several thousand masks are in use now, Mr. Ngo said.
Georgetown University: This team’s smart mask is made of metallic foams that can be cleaned and reused.
Levi Strauss: The form of the mask can be made by any basic garment factory. It aims to activate the apparel supply chain as another source of low-cost, high-performance masks.
Matregenix: This mask, made of a transparent nanofiber, allows for easier communication while having high filtration.
SEAL Lab: The SINEW mask stands for Smart, Individualized, Near-Face, Extended Wear. The mask used technology to overcome flaws of traditional respirators, with the same degree of protection. It doesn’t make contact with the skin of the wearer’s face.
StaySafeNow: A team from Harvard University developed Crystal Guard, a reusable, cost-effective clear mask. Its developers say it’s meant to be especially useful for essential workers, teachers, and others who have to communicate to do their work.
Bye-bye N95?
“From our perspective, our goal with the mask challenge was not to replace the N95 respirator,” Dr. Lippold says. N95 masks, which NIOSH certifies, are valuable and protect people in high-risk settings. “With the mask challenge, our goal was really to provide the public with a comparable alternative that really meets their specific level of risk.” Working in a health care setting carries a different risk, she says, than going to the grocery store.
“A common complaint with the N95 is that they are very uncomfortable.” It’s a major barrier to compliance, “and we wanted to address that gap. We didn’t directly compare [the entries] to an N95,” she says, although their testing was similar to NIOSH’s. A number of finalists say they will pursue NIOSH approval, she says.
Meanwhile, some of the finalists’ masks are for sale. Air Flo Labs, for instance, has its Flo Mask Pro for sale online, noting that BARDA allowed it to release the test results from NIOSH and NIST.
Getting from 1,500 to 10
In the first phase of the challenge, Dr. Lippold says, “the goal was to engage as wide an audience as possible.” With the second phase, the bar was set a bit higher. Instead of just submitting ideas on paper, companies had to submit prototypes for lab testing. “We got about 80 submissions,” she says.
Those 80 were whittled down to 10 finalists. Teams had sent prototypes, and experts, including those from NIOSH and NIST, rated them, sometimes looking at multiple copies of the masks. Experts looked at how well the masks filtered the air, how breathable they were, and other data. Once the feedback was given to the mask companies, they entered a redesign period. “Scientists can take this data and basically make these prototypes better,” Dr. Lippold says.
The final round of testing will be in September, and the winners will be announced in the fall. The opportunity allowed companies to have their products go through testing they might not otherwise have been able to get, she says.
A version of this article first appeared on WebMD.com.
Sitting at his desk in Sea Girt, N.J., John Schwind is eager to demonstrate his ReadiMask 365. He holds up what looks like a white sheet of memo paper, peels off a protective liner, and sticks the mask first to his nose. He glides his fingers down his face, over his cheeks, and to his chin, sealing the mask and then demonstrating how easy it is to talk with it in place.
The mask’s medical adhesive sticks directly to the face, without causing breakouts, he said. It doesn’t let air leak and won’t fog his glasses. It’s strapless, so it won’t hurt his ears or make them stick out.
This fall, Mr. Schwind, the CEO of Global Safety First, is hoping to take home $150,000 as one of the two top winners of the federal Mask Innovation Challenge. He has made it to the top 10 but realizes he still has a ton of competition.
After the challenge launched in late 2021, nearly 1,500 submissions were received, says Kumiko Lippold, PhD, a health scientist and manager of the Mask Innovation Challenge. The challenge is run by Dr. Lippold and others at the Division of Research, Innovation, and Ventures (DRIVe), which is part of the Biomedical Advanced Research and Development Authority (BARDA) at the U.S. Department of Health & Human Services.
Like the rest of us, Dr. Lippold knows that masks desperately need a makeover. The aim is not only to get us through this pandemic but also future pandemics and other public health emergencies. “We are focused on building masks for the next pandemic, the next wildfires,” she says.
The project is a partnership among BARDA’s DRIVe, the National Institute for Occupational Safety and Health (NIOSH), and the National Institute of Standards and Technology (NIST).
While NIOSH is a partner in the challenge, giving feedback to mask developers, “the mask challenge is entirely separate from the NIOSH approval process,” Dr. Lippold says. Companies can then pursue NIOSH approval on their own, later, if they wish. The agency certifies only masks and respirators.
Preview of masks to come
“We’ve seen some really amazing things,” Dr. Lippold said of the new designs. She didn’t want to play favorites, so she gave an overview of innovations. Some designs have transparent materials, or partially see-through materials, so facial expressions can be read. “We’ve also seen really unique bio-based materials that are derived from natural products. We’ve seen sensors in some.”
One mask model has origami folds, which increase overall surface and breathing area. Some 3D-printed masks promise a custom fit and take into account whether a person’s nose bridge is low or high.
And the finalists are ...
ReadiMask 365: “I can wear this all day long,” Mr. Schwind said of his new design. It has a nano fiber filter and is flexible. Besides the one in the BARDA challenge, the company has other ReadiMasks on the market. “The most important thing is comfort,” he says. “Second is protection. If they don’t feel they have a good seal, users don’t have confidence in the mask.”
He offers various sizes of ReadiMasks, from small sizes designed for women with smaller faces to extra-large, “for NFL linemen.”
ClearMask: “We are the original clear mask,” says Aaron Hsu, CEO and co-founder of ClearMask in Baltimore. The company began in 2017, and the clear design was inspired by a company co-founder who is deaf. She was scheduled to have surgery, and her sign language interpreter did not show up, leaving her to try to communicate in the operating room with masked health care providers. There were no transparent masks available then, Mr. Hsu says.
“Being able to work with BARDA and getting their wisdom is invaluable,” he says.
The makers of ClearMask think masks are here to stay, at least for some. “I think a certain percentage of the population will continue to wear them, regardless,” said Mr. Hsu. He predicts health care settings will become stricter about wearing masks.
“Even now, when you even walk in to a hospital, you might be required to wear a mask,” he says, even as a visitor. His company’s masks are easy to adjust and are secured around the head, so your ears don’t get sore, he says.
4C Air: The BreSafe transparent mask is semi-transparent and is made of a nanomaterial that provides high levels of filtration and breathability with some transparency.
Air99: Based on origami principles, the Airgami mask is meant to improve fit, breathability, and aesthetics over existing masks. “Airgami fits better, works better and looks better,” says Min Xiao, a company spokesperson. “It won’t fall off the nose or collapse onto the mouth, and eyeglasses fog less, she says. Voices are less muffled.” It’s also reusable, rinseable and can be heat disinfected, she says. It went on the market in November 2020.
Air Flo Labs: Flo Mask Pro, like the company’s other designs, conducted over 100 3D facial scans across many ethnicities to produce a better fit, says Kevin Ngo, its creator. For the adult masks, two nose bridge sizes are offered. And users can choose a Pro Filter, with 99% filtration, or an Everyday, which is meant to be much more breathable than other masks. “Our silicone gasket is incredibly soft and gentle on the skin,” Mr. Ngo says. “In addition,we offer indents for glasses, which prevent any fogging.” The company began shipping in May; several thousand masks are in use now, Mr. Ngo said.
Georgetown University: This team’s smart mask is made of metallic foams that can be cleaned and reused.
Levi Strauss: The form of the mask can be made by any basic garment factory. It aims to activate the apparel supply chain as another source of low-cost, high-performance masks.
Matregenix: This mask, made of a transparent nanofiber, allows for easier communication while having high filtration.
SEAL Lab: The SINEW mask stands for Smart, Individualized, Near-Face, Extended Wear. The mask used technology to overcome flaws of traditional respirators, with the same degree of protection. It doesn’t make contact with the skin of the wearer’s face.
StaySafeNow: A team from Harvard University developed Crystal Guard, a reusable, cost-effective clear mask. Its developers say it’s meant to be especially useful for essential workers, teachers, and others who have to communicate to do their work.
Bye-bye N95?
“From our perspective, our goal with the mask challenge was not to replace the N95 respirator,” Dr. Lippold says. N95 masks, which NIOSH certifies, are valuable and protect people in high-risk settings. “With the mask challenge, our goal was really to provide the public with a comparable alternative that really meets their specific level of risk.” Working in a health care setting carries a different risk, she says, than going to the grocery store.
“A common complaint with the N95 is that they are very uncomfortable.” It’s a major barrier to compliance, “and we wanted to address that gap. We didn’t directly compare [the entries] to an N95,” she says, although their testing was similar to NIOSH’s. A number of finalists say they will pursue NIOSH approval, she says.
Meanwhile, some of the finalists’ masks are for sale. Air Flo Labs, for instance, has its Flo Mask Pro for sale online, noting that BARDA allowed it to release the test results from NIOSH and NIST.
Getting from 1,500 to 10
In the first phase of the challenge, Dr. Lippold says, “the goal was to engage as wide an audience as possible.” With the second phase, the bar was set a bit higher. Instead of just submitting ideas on paper, companies had to submit prototypes for lab testing. “We got about 80 submissions,” she says.
Those 80 were whittled down to 10 finalists. Teams had sent prototypes, and experts, including those from NIOSH and NIST, rated them, sometimes looking at multiple copies of the masks. Experts looked at how well the masks filtered the air, how breathable they were, and other data. Once the feedback was given to the mask companies, they entered a redesign period. “Scientists can take this data and basically make these prototypes better,” Dr. Lippold says.
The final round of testing will be in September, and the winners will be announced in the fall. The opportunity allowed companies to have their products go through testing they might not otherwise have been able to get, she says.
A version of this article first appeared on WebMD.com.
Can dietary tweaks improve some skin diseases?
Since 1950, the terms “diet and skin” in the medical literature have markedly increased, said Vivian Shi, MD associate professor of dermatology at the University of Arkansas for Medical Sciences, Little Rock, who talked about nutritional approaches for select skin diseases at MedscapeLive’s Women’s and Pediatric Dermatology Seminar.
Myths abound, but some associations of diet with skin diseases hold water, and she said.
Acne
What’s known, Dr. Shi said, is that the prevalence of acne is substantially lower in non-Westernized countries, and that diets in those countries generally have a low glycemic load, which decreases IGF-1 insulinlike growth factor 1 (IGF-1) concentrations, an accepted risk factor for acne. The Western diet also includes the hormonal effects of cow’s milk products.
Whey protein, which is popular as a supplement, isn’t good for acne, Dr. Shi said. It takes a couple of hours to digest, while casein protein digests more slowly, over 5-7 hours. If casein protein isn’t acceptable, good alternatives to whey protein are hemp seed, plant protein blends (peas, seeds, berries), egg white, brown rice isolate, and soy isolate protein.
Dairy products increase IGF-1 levels, hormonal mediators that can make acne worse. In addition, industrial cow’s milk can contain anabolic steroids and growth factor, leading to sebogenesis, Dr. Shi said. As for the type of milk, skim milk tends to be the most acnegenic and associated with the highest blood levels of IGF-1.
Supplementing with omega-3 fatty acids and gamma-linolenic acid improved mild to moderate acne in a double-blind, controlled study. Researchers randomized 45 patients with mild to moderate acne to an omega-3 fatty acid group (2,000 mg of eicosapentaenoic acid and docosahexaenoic acid), a gamma-linolenic acid group (borage oil with 400 mg gamma-linolenic acid) or a control group. After 10 weeks in both treatment groups, there was a significant reduction in inflammatory and noninflammatory lesions.
Those with acne are more likely to be deficient in Vitamin D, research suggests. Researchers also found that among those who had vitamin D deficiency, supplementing with 1,000 IU daily for 2 months reduced inflammatory lesions by 35% after 8 weeks, compared with a 6% reduction in the control group.
Other research has found that those with a low serum zinc level had more severe acne and that 30-200 mg of zinc orally for 2-4 months reduced inflammatory acne. However, Dr. Shi cautioned that those taking zinc for more than 2 months also need a copper supplement, as zinc reduces the amount of copper absorbed by the body.
Dr. Shi’s “do’s” diet list for acne patients is a follows: Paleolithic and Mediterranean diets, omega-3 fatty acids, gamma-linolenic acids, Vitamin D, zinc, tubers, legumes, vegetables, fruits, and fish.
Unknowns, she said, include chocolate, caffeine, green tea, and high salt.
Hidradenitis suppurativa
Patents with HS who follow a Mediterranean diet most closely have less severe disease, research has found. In this study, those patients with HS with the lowest adherence had a Sartorius HS score of 59.38, while those who followed it the most closely had a score of 39 (of 80).
In another study, patients with HS reported the following foods as exacerbating HS: sweets, bread/pasta/rice, dairy, and high-fat foods. Alleviating foods included vegetables, fruit, chicken, and fish.
Dr. Shi’s dietary recommendations for patients with HS: Follow a Mediterranean diet, avoid high fat foods and highly processed foods, and focus on eating more vegetables, fresh fruit, corn-based cereal, white meat, and fish.
A retrospective study of patients with Hurley stage 1 and 2 found that oral zinc gluconate, 90 mg a day, combined with 2% topical triclosan twice a day, resulted in significantly decreased HS scores and nodules and improved quality of life after 3 months. Expect vitamin D deficiency, she added.
Lastly, Dr. Shi recommended, if necessary, “weight loss to reduce the inflammatory burden.”
Rosacea
Dietary triggers for rosacea are thought to include high-fat foods, dairy foods, spicy foods, hot drinks, cinnamon, and vanilla.
A population-based case-control study in China, which evaluated 1,347 rosacea patients and 1,290 healthy controls, found that a high intake of fatty foods positively correlated with erythematotelangiectatic rosacea (ETR) and phymatous rosacea. High-frequency dairy intake negatively correlated with ETR and papulopustular rosacea, which was a surprise, she said. And in this study, no significant correlations were found between sweets, coffee, and spicy foods. That goes against the traditional thinking, she said, but this was a Chinese cohort and their diet is probably vastly different than those in the United States.
Other rosacea triggers, Dr. Shi said, are niacin-containing foods such as turkey, chicken breast, crustaceans, dried Shiitake mushrooms, peanuts, tuna, and liver, as well as cold drinks, and formalin-containing foods (fish, squid, tofu, wet noodles).
As the field of nutrigenics – how genes affect how the body responds to food – evolves, more answers about the impact of diet on these diseases will be forthcoming, Dr. Shi said.
In an interactive panel discussion, she was asked if she talks about diet with all her patients with acne, rosacea, and HS, or just those not responding to traditional therapy.
“I think it’s an important conversation to have,” Dr. Shi responded. “When I’m done with the medication [instructions], I say: ‘There is something else you can do to augment what I just told you.’ ” That’s when she explains the dietary information. She also has a handout on diet and routinely refers patients for dietary counseling.
MedscapeLive and this news organization are owned by the same parent company. Dr. Shi disclosed consulting, investigative and research funding from several sources, but not directly related to the content of her talk.
Since 1950, the terms “diet and skin” in the medical literature have markedly increased, said Vivian Shi, MD associate professor of dermatology at the University of Arkansas for Medical Sciences, Little Rock, who talked about nutritional approaches for select skin diseases at MedscapeLive’s Women’s and Pediatric Dermatology Seminar.
Myths abound, but some associations of diet with skin diseases hold water, and she said.
Acne
What’s known, Dr. Shi said, is that the prevalence of acne is substantially lower in non-Westernized countries, and that diets in those countries generally have a low glycemic load, which decreases IGF-1 insulinlike growth factor 1 (IGF-1) concentrations, an accepted risk factor for acne. The Western diet also includes the hormonal effects of cow’s milk products.
Whey protein, which is popular as a supplement, isn’t good for acne, Dr. Shi said. It takes a couple of hours to digest, while casein protein digests more slowly, over 5-7 hours. If casein protein isn’t acceptable, good alternatives to whey protein are hemp seed, plant protein blends (peas, seeds, berries), egg white, brown rice isolate, and soy isolate protein.
Dairy products increase IGF-1 levels, hormonal mediators that can make acne worse. In addition, industrial cow’s milk can contain anabolic steroids and growth factor, leading to sebogenesis, Dr. Shi said. As for the type of milk, skim milk tends to be the most acnegenic and associated with the highest blood levels of IGF-1.
Supplementing with omega-3 fatty acids and gamma-linolenic acid improved mild to moderate acne in a double-blind, controlled study. Researchers randomized 45 patients with mild to moderate acne to an omega-3 fatty acid group (2,000 mg of eicosapentaenoic acid and docosahexaenoic acid), a gamma-linolenic acid group (borage oil with 400 mg gamma-linolenic acid) or a control group. After 10 weeks in both treatment groups, there was a significant reduction in inflammatory and noninflammatory lesions.
Those with acne are more likely to be deficient in Vitamin D, research suggests. Researchers also found that among those who had vitamin D deficiency, supplementing with 1,000 IU daily for 2 months reduced inflammatory lesions by 35% after 8 weeks, compared with a 6% reduction in the control group.
Other research has found that those with a low serum zinc level had more severe acne and that 30-200 mg of zinc orally for 2-4 months reduced inflammatory acne. However, Dr. Shi cautioned that those taking zinc for more than 2 months also need a copper supplement, as zinc reduces the amount of copper absorbed by the body.
Dr. Shi’s “do’s” diet list for acne patients is a follows: Paleolithic and Mediterranean diets, omega-3 fatty acids, gamma-linolenic acids, Vitamin D, zinc, tubers, legumes, vegetables, fruits, and fish.
Unknowns, she said, include chocolate, caffeine, green tea, and high salt.
Hidradenitis suppurativa
Patents with HS who follow a Mediterranean diet most closely have less severe disease, research has found. In this study, those patients with HS with the lowest adherence had a Sartorius HS score of 59.38, while those who followed it the most closely had a score of 39 (of 80).
In another study, patients with HS reported the following foods as exacerbating HS: sweets, bread/pasta/rice, dairy, and high-fat foods. Alleviating foods included vegetables, fruit, chicken, and fish.
Dr. Shi’s dietary recommendations for patients with HS: Follow a Mediterranean diet, avoid high fat foods and highly processed foods, and focus on eating more vegetables, fresh fruit, corn-based cereal, white meat, and fish.
A retrospective study of patients with Hurley stage 1 and 2 found that oral zinc gluconate, 90 mg a day, combined with 2% topical triclosan twice a day, resulted in significantly decreased HS scores and nodules and improved quality of life after 3 months. Expect vitamin D deficiency, she added.
Lastly, Dr. Shi recommended, if necessary, “weight loss to reduce the inflammatory burden.”
Rosacea
Dietary triggers for rosacea are thought to include high-fat foods, dairy foods, spicy foods, hot drinks, cinnamon, and vanilla.
A population-based case-control study in China, which evaluated 1,347 rosacea patients and 1,290 healthy controls, found that a high intake of fatty foods positively correlated with erythematotelangiectatic rosacea (ETR) and phymatous rosacea. High-frequency dairy intake negatively correlated with ETR and papulopustular rosacea, which was a surprise, she said. And in this study, no significant correlations were found between sweets, coffee, and spicy foods. That goes against the traditional thinking, she said, but this was a Chinese cohort and their diet is probably vastly different than those in the United States.
Other rosacea triggers, Dr. Shi said, are niacin-containing foods such as turkey, chicken breast, crustaceans, dried Shiitake mushrooms, peanuts, tuna, and liver, as well as cold drinks, and formalin-containing foods (fish, squid, tofu, wet noodles).
As the field of nutrigenics – how genes affect how the body responds to food – evolves, more answers about the impact of diet on these diseases will be forthcoming, Dr. Shi said.
In an interactive panel discussion, she was asked if she talks about diet with all her patients with acne, rosacea, and HS, or just those not responding to traditional therapy.
“I think it’s an important conversation to have,” Dr. Shi responded. “When I’m done with the medication [instructions], I say: ‘There is something else you can do to augment what I just told you.’ ” That’s when she explains the dietary information. She also has a handout on diet and routinely refers patients for dietary counseling.
MedscapeLive and this news organization are owned by the same parent company. Dr. Shi disclosed consulting, investigative and research funding from several sources, but not directly related to the content of her talk.
Since 1950, the terms “diet and skin” in the medical literature have markedly increased, said Vivian Shi, MD associate professor of dermatology at the University of Arkansas for Medical Sciences, Little Rock, who talked about nutritional approaches for select skin diseases at MedscapeLive’s Women’s and Pediatric Dermatology Seminar.
Myths abound, but some associations of diet with skin diseases hold water, and she said.
Acne
What’s known, Dr. Shi said, is that the prevalence of acne is substantially lower in non-Westernized countries, and that diets in those countries generally have a low glycemic load, which decreases IGF-1 insulinlike growth factor 1 (IGF-1) concentrations, an accepted risk factor for acne. The Western diet also includes the hormonal effects of cow’s milk products.
Whey protein, which is popular as a supplement, isn’t good for acne, Dr. Shi said. It takes a couple of hours to digest, while casein protein digests more slowly, over 5-7 hours. If casein protein isn’t acceptable, good alternatives to whey protein are hemp seed, plant protein blends (peas, seeds, berries), egg white, brown rice isolate, and soy isolate protein.
Dairy products increase IGF-1 levels, hormonal mediators that can make acne worse. In addition, industrial cow’s milk can contain anabolic steroids and growth factor, leading to sebogenesis, Dr. Shi said. As for the type of milk, skim milk tends to be the most acnegenic and associated with the highest blood levels of IGF-1.
Supplementing with omega-3 fatty acids and gamma-linolenic acid improved mild to moderate acne in a double-blind, controlled study. Researchers randomized 45 patients with mild to moderate acne to an omega-3 fatty acid group (2,000 mg of eicosapentaenoic acid and docosahexaenoic acid), a gamma-linolenic acid group (borage oil with 400 mg gamma-linolenic acid) or a control group. After 10 weeks in both treatment groups, there was a significant reduction in inflammatory and noninflammatory lesions.
Those with acne are more likely to be deficient in Vitamin D, research suggests. Researchers also found that among those who had vitamin D deficiency, supplementing with 1,000 IU daily for 2 months reduced inflammatory lesions by 35% after 8 weeks, compared with a 6% reduction in the control group.
Other research has found that those with a low serum zinc level had more severe acne and that 30-200 mg of zinc orally for 2-4 months reduced inflammatory acne. However, Dr. Shi cautioned that those taking zinc for more than 2 months also need a copper supplement, as zinc reduces the amount of copper absorbed by the body.
Dr. Shi’s “do’s” diet list for acne patients is a follows: Paleolithic and Mediterranean diets, omega-3 fatty acids, gamma-linolenic acids, Vitamin D, zinc, tubers, legumes, vegetables, fruits, and fish.
Unknowns, she said, include chocolate, caffeine, green tea, and high salt.
Hidradenitis suppurativa
Patents with HS who follow a Mediterranean diet most closely have less severe disease, research has found. In this study, those patients with HS with the lowest adherence had a Sartorius HS score of 59.38, while those who followed it the most closely had a score of 39 (of 80).
In another study, patients with HS reported the following foods as exacerbating HS: sweets, bread/pasta/rice, dairy, and high-fat foods. Alleviating foods included vegetables, fruit, chicken, and fish.
Dr. Shi’s dietary recommendations for patients with HS: Follow a Mediterranean diet, avoid high fat foods and highly processed foods, and focus on eating more vegetables, fresh fruit, corn-based cereal, white meat, and fish.
A retrospective study of patients with Hurley stage 1 and 2 found that oral zinc gluconate, 90 mg a day, combined with 2% topical triclosan twice a day, resulted in significantly decreased HS scores and nodules and improved quality of life after 3 months. Expect vitamin D deficiency, she added.
Lastly, Dr. Shi recommended, if necessary, “weight loss to reduce the inflammatory burden.”
Rosacea
Dietary triggers for rosacea are thought to include high-fat foods, dairy foods, spicy foods, hot drinks, cinnamon, and vanilla.
A population-based case-control study in China, which evaluated 1,347 rosacea patients and 1,290 healthy controls, found that a high intake of fatty foods positively correlated with erythematotelangiectatic rosacea (ETR) and phymatous rosacea. High-frequency dairy intake negatively correlated with ETR and papulopustular rosacea, which was a surprise, she said. And in this study, no significant correlations were found between sweets, coffee, and spicy foods. That goes against the traditional thinking, she said, but this was a Chinese cohort and their diet is probably vastly different than those in the United States.
Other rosacea triggers, Dr. Shi said, are niacin-containing foods such as turkey, chicken breast, crustaceans, dried Shiitake mushrooms, peanuts, tuna, and liver, as well as cold drinks, and formalin-containing foods (fish, squid, tofu, wet noodles).
As the field of nutrigenics – how genes affect how the body responds to food – evolves, more answers about the impact of diet on these diseases will be forthcoming, Dr. Shi said.
In an interactive panel discussion, she was asked if she talks about diet with all her patients with acne, rosacea, and HS, or just those not responding to traditional therapy.
“I think it’s an important conversation to have,” Dr. Shi responded. “When I’m done with the medication [instructions], I say: ‘There is something else you can do to augment what I just told you.’ ” That’s when she explains the dietary information. She also has a handout on diet and routinely refers patients for dietary counseling.
MedscapeLive and this news organization are owned by the same parent company. Dr. Shi disclosed consulting, investigative and research funding from several sources, but not directly related to the content of her talk.
FROM MEDSCAPELIVE WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
Post–Roe v. Wade: What’s next?
The U.S. Supreme Court’s decision to overturn Roe v. Wade, the landmark ruling in 1973 establishing a constitutional right to abortion, has spurred abortion rights supporters and opponents into action, speeding up their efforts to protect or remove access to abortion.
For now, the fight moves to the states, where so-called trigger laws have already banned nearly all abortions in a handful of states. More will likely take effect soon.
“Half of [the states] are going to have quite restrictive abortion laws, and about half will pretty much maintain the status quo,” said Ron Allen, JD, a constitutional law expert and professor of law at Northwestern University, Chicago. “My guess is, the largest population will be in those states that maintain the status quo, [though] that’s not terribly consoling to somebody in Arkansas, [which has a trigger law.]”
Federal and state officials spoke out quickly about what protections are still in place for access to abortion, and some governors have taken new actions to expand that protection.
While abortion rights advocates called on Congress to pass legislation legalizing abortion access nationwide, others, including former Vice President Mike Pence, said a national ban on abortions should be the next step.
Federal, state protections
President Joe Biden quickly addressed the issue of women needing to travel out of state to access abortion. In his statement on June 24, he said: “So if a woman lives in a state that restricts abortion, the Supreme Court’s decision does not prevent her from traveling from her home state to the state that allows it. It does not prevent a doctor in that state from treating her.”
In a statement also issued June 24, Attorney General Merrick Garland expressed strong disagreement with the court’s decision and also pointed out it does not mean that states can’t keep abortion legal within their borders. Nor can states ban reproductive services provided to their residents outside their own borders.
Women living in states banning access to abortion, “must be free to seek care in states where it is legal.” Others are free to inform and counsel each other about reproductive care available in other states, he said, citing the First Amendment.
Doctors who provide abortion services in states where the services remain legal, as well as patients who receive the services, will be protected under the Freedom of Access to Clinic Entrances Act, Mr. Garland said in a statement from the Department of Justice.
States reiterated protection for health care providers. For instance, California Gov. Gavin Newsom signed a law June 24 protecting California abortion providers from civil liability when they provide care for women traveling from states where abortion is banned or access to it is narrowed.
Officials from other states with abortion access began publicizing their status as “safe havens.” New York Attorney General Letitia James tweeted: “While other states strip away the fundamental right to choose, New York will always be a safe haven for anyone seeking an abortion.”
Gov. Newsom, too, among other state officials, has promised his state would be a sanctuary for women in need.
After the ruling, New York Gov. Kathy Hochul and the New York State Department of Health launched a new website and campaign, Abortion Access Always, providing a single destination for information about rights, providers, support, and other details.
Abortion pill
Mr. Garland and President Biden strongly warned states not to try to interfere with access to the so-called abortion pill. Approved 20 years ago by the FDA to safely end early pregnancies, the medication, mifepristone (formerly called RU-486) is taken along with misoprostol, a drug also used to prevent stomach ulcers. Medication abortion now accounts for more than half of all abortions, according to the Guttmacher Institute.
In his statement, Mr. Garland noted that the “FDA has approved the use of the medication mifepristone. States may not ban mifepristone based on disagreement with the FDA’s expert judgment about its safety and efficacy.”
Plan C, an information campaign for abortion services, has a state-by-state directory of ways to find the pills, even in states restricting access to abortion, said Elisa Wells, Plan C’s cofounder and codirector.
Calls for national access
On June 24, President Biden called on Congress to restore the protections of Roe v. Wade as federal law. “No executive action from the president can do that,” he said. If Congress lacks the vote to do that now, voters need to make their voices heard, he said.
“The Supreme Court is but one of many government bodies that can protect the right to abortion,” Nancy Northup, JD, president and CEO of the Center for Reproductive Rights, New York, said June 24. “We will be looking to the Congress to pass the Women’s Health Protection Act. Congress can solve this as a national problem. We’ll be looking to the Biden administration to use the extent of its powers.”
The Women’s Health Protection Act would prohibit government restrictions on access to abortion services.
Sen. Bernie Sanders (I-Vt.) tweeted: “Democrats must now end the filibuster in the Senate, codify Roe v. Wade, and once again make abortion legal and safe.”
“The federal government can do a lot of things,” said Mr. Allen. “It’s interesting that we focus on the administrative agencies. The fight over Roe is a fight in large measure over who should be deciding and whether these are issues that should be decided by agencies or a court or legislators.”
Anger, he said, “should be directed at legislators, and that’s who should be acting here, and that means people have to get out and vote.”
Calls for a national ban
Former Vice President Pence told far-right publication Breitbart News that the court’s decision should lead to a national ban on abortion.
He also took to Twitter. Among other posts, he said: “Having been given this second chance for Life, we must not rest and must not relent until the sanctity of life is restored to the center of American law in every state in the land!”
Organizations’ actions
Organizations on both sides of the issue have mobilization and expansion plans.
NRLC: The National Right to Life Committee will now focus on state legislatures, said Laura Echevarria, the group’s communications director.
“We will continue to work on these [antiabortion] laws in the states we can get these passed,” she said. There’s no one size fits all. “New York is not going to pass a law that Alabama is going to pass. Every state is going to be doing something different.”
“The next big thing is to build that safety net” for women who decide to avoid abortion, she said. More than 2,700 “pregnancy help” centers operate in the United States. “We don’t run them, they are independent.” But the NRLC supports them. The centers provide pregnancy support and financial help, “two big reasons why women get abortions.”
She added: “The prolife movement often gets a bad rap, like we don’t care about women, and we do.” In an open letter issued May 12 to state lawmakers, the NRLC said: “We state unequivocally that we do not support any measure seeking to criminalize or punish women and we stand firmly opposed to include such penalties in legislation.”
ACLU: Anthony D. Romero, JD, executive director of the American Civil Liberties Union, issued a statement on Jun 24 that read in part: “Second-class status for women has once again become the law because of today’s decisions.”
As the fight plays out in the court, the ACLU urges voters to head to the polls, noting that state constitutional amendments to preserve reproductive freedom are on the ballot in Kansas in August and in Vermont and Kentucky in November.
Planned Parenthood
“A majority of justices ruled to throw away nearly 50 years of precedent and take away the right to control our bodies and personal health care decisions,” the Planned Parenthood site posted.
On June 25, the Planned Parenthood Association of Utah filed suit in Utah state court, planning to request a temporary restraining order against the state’s ban on abortion at any point in pregnancy. The law took effect June 24.
Abortion rights offers of help
As legislators and public officials focused on what the next steps should be, social media lit up over the weekend with offers of help for women in states without access to abortion.
One meme posted on social media focused on “camping.” Reportedly created by a woman who needed abortions before the 1973 Roe v. Wade decision, it reads: “If you are a person who suddenly finds yourself with a need to go camping in another state friendly towards camping, just know that I will happily drive you, support you, and not talk about the camping trip to anyone ever.”
While the camping code word quickly picked up steam, one Twitter user who favored the court’s decision called the trend of using camping as a code word to help people access abortions “horrible.”
TikTok users also offered their homes and help to women from other states who might need either. And one Airbnb host posted this invitation on Facebook: “My Airbnb is free for any American woman coming to Los Angeles for an abortion. Hugs and cute kittens, too.”
A version of this article first appeared on Medscape.com.
The U.S. Supreme Court’s decision to overturn Roe v. Wade, the landmark ruling in 1973 establishing a constitutional right to abortion, has spurred abortion rights supporters and opponents into action, speeding up their efforts to protect or remove access to abortion.
For now, the fight moves to the states, where so-called trigger laws have already banned nearly all abortions in a handful of states. More will likely take effect soon.
“Half of [the states] are going to have quite restrictive abortion laws, and about half will pretty much maintain the status quo,” said Ron Allen, JD, a constitutional law expert and professor of law at Northwestern University, Chicago. “My guess is, the largest population will be in those states that maintain the status quo, [though] that’s not terribly consoling to somebody in Arkansas, [which has a trigger law.]”
Federal and state officials spoke out quickly about what protections are still in place for access to abortion, and some governors have taken new actions to expand that protection.
While abortion rights advocates called on Congress to pass legislation legalizing abortion access nationwide, others, including former Vice President Mike Pence, said a national ban on abortions should be the next step.
Federal, state protections
President Joe Biden quickly addressed the issue of women needing to travel out of state to access abortion. In his statement on June 24, he said: “So if a woman lives in a state that restricts abortion, the Supreme Court’s decision does not prevent her from traveling from her home state to the state that allows it. It does not prevent a doctor in that state from treating her.”
In a statement also issued June 24, Attorney General Merrick Garland expressed strong disagreement with the court’s decision and also pointed out it does not mean that states can’t keep abortion legal within their borders. Nor can states ban reproductive services provided to their residents outside their own borders.
Women living in states banning access to abortion, “must be free to seek care in states where it is legal.” Others are free to inform and counsel each other about reproductive care available in other states, he said, citing the First Amendment.
Doctors who provide abortion services in states where the services remain legal, as well as patients who receive the services, will be protected under the Freedom of Access to Clinic Entrances Act, Mr. Garland said in a statement from the Department of Justice.
States reiterated protection for health care providers. For instance, California Gov. Gavin Newsom signed a law June 24 protecting California abortion providers from civil liability when they provide care for women traveling from states where abortion is banned or access to it is narrowed.
Officials from other states with abortion access began publicizing their status as “safe havens.” New York Attorney General Letitia James tweeted: “While other states strip away the fundamental right to choose, New York will always be a safe haven for anyone seeking an abortion.”
Gov. Newsom, too, among other state officials, has promised his state would be a sanctuary for women in need.
After the ruling, New York Gov. Kathy Hochul and the New York State Department of Health launched a new website and campaign, Abortion Access Always, providing a single destination for information about rights, providers, support, and other details.
Abortion pill
Mr. Garland and President Biden strongly warned states not to try to interfere with access to the so-called abortion pill. Approved 20 years ago by the FDA to safely end early pregnancies, the medication, mifepristone (formerly called RU-486) is taken along with misoprostol, a drug also used to prevent stomach ulcers. Medication abortion now accounts for more than half of all abortions, according to the Guttmacher Institute.
In his statement, Mr. Garland noted that the “FDA has approved the use of the medication mifepristone. States may not ban mifepristone based on disagreement with the FDA’s expert judgment about its safety and efficacy.”
Plan C, an information campaign for abortion services, has a state-by-state directory of ways to find the pills, even in states restricting access to abortion, said Elisa Wells, Plan C’s cofounder and codirector.
Calls for national access
On June 24, President Biden called on Congress to restore the protections of Roe v. Wade as federal law. “No executive action from the president can do that,” he said. If Congress lacks the vote to do that now, voters need to make their voices heard, he said.
“The Supreme Court is but one of many government bodies that can protect the right to abortion,” Nancy Northup, JD, president and CEO of the Center for Reproductive Rights, New York, said June 24. “We will be looking to the Congress to pass the Women’s Health Protection Act. Congress can solve this as a national problem. We’ll be looking to the Biden administration to use the extent of its powers.”
The Women’s Health Protection Act would prohibit government restrictions on access to abortion services.
Sen. Bernie Sanders (I-Vt.) tweeted: “Democrats must now end the filibuster in the Senate, codify Roe v. Wade, and once again make abortion legal and safe.”
“The federal government can do a lot of things,” said Mr. Allen. “It’s interesting that we focus on the administrative agencies. The fight over Roe is a fight in large measure over who should be deciding and whether these are issues that should be decided by agencies or a court or legislators.”
Anger, he said, “should be directed at legislators, and that’s who should be acting here, and that means people have to get out and vote.”
Calls for a national ban
Former Vice President Pence told far-right publication Breitbart News that the court’s decision should lead to a national ban on abortion.
He also took to Twitter. Among other posts, he said: “Having been given this second chance for Life, we must not rest and must not relent until the sanctity of life is restored to the center of American law in every state in the land!”
Organizations’ actions
Organizations on both sides of the issue have mobilization and expansion plans.
NRLC: The National Right to Life Committee will now focus on state legislatures, said Laura Echevarria, the group’s communications director.
“We will continue to work on these [antiabortion] laws in the states we can get these passed,” she said. There’s no one size fits all. “New York is not going to pass a law that Alabama is going to pass. Every state is going to be doing something different.”
“The next big thing is to build that safety net” for women who decide to avoid abortion, she said. More than 2,700 “pregnancy help” centers operate in the United States. “We don’t run them, they are independent.” But the NRLC supports them. The centers provide pregnancy support and financial help, “two big reasons why women get abortions.”
She added: “The prolife movement often gets a bad rap, like we don’t care about women, and we do.” In an open letter issued May 12 to state lawmakers, the NRLC said: “We state unequivocally that we do not support any measure seeking to criminalize or punish women and we stand firmly opposed to include such penalties in legislation.”
ACLU: Anthony D. Romero, JD, executive director of the American Civil Liberties Union, issued a statement on Jun 24 that read in part: “Second-class status for women has once again become the law because of today’s decisions.”
As the fight plays out in the court, the ACLU urges voters to head to the polls, noting that state constitutional amendments to preserve reproductive freedom are on the ballot in Kansas in August and in Vermont and Kentucky in November.
Planned Parenthood
“A majority of justices ruled to throw away nearly 50 years of precedent and take away the right to control our bodies and personal health care decisions,” the Planned Parenthood site posted.
On June 25, the Planned Parenthood Association of Utah filed suit in Utah state court, planning to request a temporary restraining order against the state’s ban on abortion at any point in pregnancy. The law took effect June 24.
Abortion rights offers of help
As legislators and public officials focused on what the next steps should be, social media lit up over the weekend with offers of help for women in states without access to abortion.
One meme posted on social media focused on “camping.” Reportedly created by a woman who needed abortions before the 1973 Roe v. Wade decision, it reads: “If you are a person who suddenly finds yourself with a need to go camping in another state friendly towards camping, just know that I will happily drive you, support you, and not talk about the camping trip to anyone ever.”
While the camping code word quickly picked up steam, one Twitter user who favored the court’s decision called the trend of using camping as a code word to help people access abortions “horrible.”
TikTok users also offered their homes and help to women from other states who might need either. And one Airbnb host posted this invitation on Facebook: “My Airbnb is free for any American woman coming to Los Angeles for an abortion. Hugs and cute kittens, too.”
A version of this article first appeared on Medscape.com.
The U.S. Supreme Court’s decision to overturn Roe v. Wade, the landmark ruling in 1973 establishing a constitutional right to abortion, has spurred abortion rights supporters and opponents into action, speeding up their efforts to protect or remove access to abortion.
For now, the fight moves to the states, where so-called trigger laws have already banned nearly all abortions in a handful of states. More will likely take effect soon.
“Half of [the states] are going to have quite restrictive abortion laws, and about half will pretty much maintain the status quo,” said Ron Allen, JD, a constitutional law expert and professor of law at Northwestern University, Chicago. “My guess is, the largest population will be in those states that maintain the status quo, [though] that’s not terribly consoling to somebody in Arkansas, [which has a trigger law.]”
Federal and state officials spoke out quickly about what protections are still in place for access to abortion, and some governors have taken new actions to expand that protection.
While abortion rights advocates called on Congress to pass legislation legalizing abortion access nationwide, others, including former Vice President Mike Pence, said a national ban on abortions should be the next step.
Federal, state protections
President Joe Biden quickly addressed the issue of women needing to travel out of state to access abortion. In his statement on June 24, he said: “So if a woman lives in a state that restricts abortion, the Supreme Court’s decision does not prevent her from traveling from her home state to the state that allows it. It does not prevent a doctor in that state from treating her.”
In a statement also issued June 24, Attorney General Merrick Garland expressed strong disagreement with the court’s decision and also pointed out it does not mean that states can’t keep abortion legal within their borders. Nor can states ban reproductive services provided to their residents outside their own borders.
Women living in states banning access to abortion, “must be free to seek care in states where it is legal.” Others are free to inform and counsel each other about reproductive care available in other states, he said, citing the First Amendment.
Doctors who provide abortion services in states where the services remain legal, as well as patients who receive the services, will be protected under the Freedom of Access to Clinic Entrances Act, Mr. Garland said in a statement from the Department of Justice.
States reiterated protection for health care providers. For instance, California Gov. Gavin Newsom signed a law June 24 protecting California abortion providers from civil liability when they provide care for women traveling from states where abortion is banned or access to it is narrowed.
Officials from other states with abortion access began publicizing their status as “safe havens.” New York Attorney General Letitia James tweeted: “While other states strip away the fundamental right to choose, New York will always be a safe haven for anyone seeking an abortion.”
Gov. Newsom, too, among other state officials, has promised his state would be a sanctuary for women in need.
After the ruling, New York Gov. Kathy Hochul and the New York State Department of Health launched a new website and campaign, Abortion Access Always, providing a single destination for information about rights, providers, support, and other details.
Abortion pill
Mr. Garland and President Biden strongly warned states not to try to interfere with access to the so-called abortion pill. Approved 20 years ago by the FDA to safely end early pregnancies, the medication, mifepristone (formerly called RU-486) is taken along with misoprostol, a drug also used to prevent stomach ulcers. Medication abortion now accounts for more than half of all abortions, according to the Guttmacher Institute.
In his statement, Mr. Garland noted that the “FDA has approved the use of the medication mifepristone. States may not ban mifepristone based on disagreement with the FDA’s expert judgment about its safety and efficacy.”
Plan C, an information campaign for abortion services, has a state-by-state directory of ways to find the pills, even in states restricting access to abortion, said Elisa Wells, Plan C’s cofounder and codirector.
Calls for national access
On June 24, President Biden called on Congress to restore the protections of Roe v. Wade as federal law. “No executive action from the president can do that,” he said. If Congress lacks the vote to do that now, voters need to make their voices heard, he said.
“The Supreme Court is but one of many government bodies that can protect the right to abortion,” Nancy Northup, JD, president and CEO of the Center for Reproductive Rights, New York, said June 24. “We will be looking to the Congress to pass the Women’s Health Protection Act. Congress can solve this as a national problem. We’ll be looking to the Biden administration to use the extent of its powers.”
The Women’s Health Protection Act would prohibit government restrictions on access to abortion services.
Sen. Bernie Sanders (I-Vt.) tweeted: “Democrats must now end the filibuster in the Senate, codify Roe v. Wade, and once again make abortion legal and safe.”
“The federal government can do a lot of things,” said Mr. Allen. “It’s interesting that we focus on the administrative agencies. The fight over Roe is a fight in large measure over who should be deciding and whether these are issues that should be decided by agencies or a court or legislators.”
Anger, he said, “should be directed at legislators, and that’s who should be acting here, and that means people have to get out and vote.”
Calls for a national ban
Former Vice President Pence told far-right publication Breitbart News that the court’s decision should lead to a national ban on abortion.
He also took to Twitter. Among other posts, he said: “Having been given this second chance for Life, we must not rest and must not relent until the sanctity of life is restored to the center of American law in every state in the land!”
Organizations’ actions
Organizations on both sides of the issue have mobilization and expansion plans.
NRLC: The National Right to Life Committee will now focus on state legislatures, said Laura Echevarria, the group’s communications director.
“We will continue to work on these [antiabortion] laws in the states we can get these passed,” she said. There’s no one size fits all. “New York is not going to pass a law that Alabama is going to pass. Every state is going to be doing something different.”
“The next big thing is to build that safety net” for women who decide to avoid abortion, she said. More than 2,700 “pregnancy help” centers operate in the United States. “We don’t run them, they are independent.” But the NRLC supports them. The centers provide pregnancy support and financial help, “two big reasons why women get abortions.”
She added: “The prolife movement often gets a bad rap, like we don’t care about women, and we do.” In an open letter issued May 12 to state lawmakers, the NRLC said: “We state unequivocally that we do not support any measure seeking to criminalize or punish women and we stand firmly opposed to include such penalties in legislation.”
ACLU: Anthony D. Romero, JD, executive director of the American Civil Liberties Union, issued a statement on Jun 24 that read in part: “Second-class status for women has once again become the law because of today’s decisions.”
As the fight plays out in the court, the ACLU urges voters to head to the polls, noting that state constitutional amendments to preserve reproductive freedom are on the ballot in Kansas in August and in Vermont and Kentucky in November.
Planned Parenthood
“A majority of justices ruled to throw away nearly 50 years of precedent and take away the right to control our bodies and personal health care decisions,” the Planned Parenthood site posted.
On June 25, the Planned Parenthood Association of Utah filed suit in Utah state court, planning to request a temporary restraining order against the state’s ban on abortion at any point in pregnancy. The law took effect June 24.
Abortion rights offers of help
As legislators and public officials focused on what the next steps should be, social media lit up over the weekend with offers of help for women in states without access to abortion.
One meme posted on social media focused on “camping.” Reportedly created by a woman who needed abortions before the 1973 Roe v. Wade decision, it reads: “If you are a person who suddenly finds yourself with a need to go camping in another state friendly towards camping, just know that I will happily drive you, support you, and not talk about the camping trip to anyone ever.”
While the camping code word quickly picked up steam, one Twitter user who favored the court’s decision called the trend of using camping as a code word to help people access abortions “horrible.”
TikTok users also offered their homes and help to women from other states who might need either. And one Airbnb host posted this invitation on Facebook: “My Airbnb is free for any American woman coming to Los Angeles for an abortion. Hugs and cute kittens, too.”
A version of this article first appeared on Medscape.com.
Biden moves to limit nicotine levels in cigarettes
The Department of Health and Human Services posted a notice that details plans for a new rule to create a maximum allowed amount of nicotine in certain tobacco products. The Food and Drug Administration would take the action, the notice said, “to reduce addictiveness to certain tobacco products, thus giving addicted users a greater ability to quit.” The product standard would also help keep nonsmokers interested in trying tobacco, mainly youth, from starting to smoke and become regulars.
“Lowering nicotine levels to minimally addictive or non-addictive levels would decrease the likelihood that future generations of young people become addicted to cigarettes and help more currently addicted smokers to quit,” FDA Commissioner Robert Califf, MD, said in a statement.
The FDA, in charge of regulating cigarettes, issues a proposed rule when changes are discussed. That would be followed by a period for public comments before a final rule could be issued.
The proposed rule was first reported by The Washington Post.
The FDA in 2018 published a study in the New England Journal of Medicine that estimated that a potential limit on nicotine in cigarettes could, by the year 2100, prevent more than 33 million people from becoming regular smokers, and prevent the deaths of more than 8 million people from tobacco-related illnesses.
The action to reduce nicotine levels would fit in with President Joe Biden’s goal of reducing cancer death rates by half over 25 years. Each year, according to the American Cancer Society, about 480,000 deaths (about 1 in 5) are related to smoking. Currently, about 34 million American adults still smoke cigarettes.
Matthew Myers, president of the Campaign for Tobacco-Free Kids, called the proposed rule a “truly game-changing proposal.”
“There is no other single action our country can take that would prevent more young people from becoming addicted to tobacco or have a greater impact on reducing deaths from cancer, cardiovascular disease and respiratory disease,” Mr. Myers said in a statement.
However, he said, “these gains will only be realized if the administration and the FDA demonstrate a full-throated commitment to finalizing and implementing this proposal.”
The FDA proposed the nicotine reduction strategy in talks with the White House and the Department of Health and Human Services early in 2021, according to the Post.
Earlier this year, the FDA issued a proposed rule to ban menthol flavoring in cigarettes. The agency is accepting public comments though July 5.
The action of reducing nicotine levels would likely take years to complete, Mitch Zeller, JD, recently retired director of the FDA Center for Tobacco Products, told the Post.
In 2018, the FDA issued a proposed ruling to set a standard for maximum nicotine levels in cigarettes.
Advocates say the action of slashing nicotine, the active – and addictive – ingredient in cigarettes, would save millions of lives for generations to come. Opponents liken it to the prohibition of alcohol in the 1920s and predict the action will fail.
Others say that if limits are put on nicotine levels, adults should have greater access to noncombustible alternatives.
A version of this article first appeared on WebMD.com.
The Department of Health and Human Services posted a notice that details plans for a new rule to create a maximum allowed amount of nicotine in certain tobacco products. The Food and Drug Administration would take the action, the notice said, “to reduce addictiveness to certain tobacco products, thus giving addicted users a greater ability to quit.” The product standard would also help keep nonsmokers interested in trying tobacco, mainly youth, from starting to smoke and become regulars.
“Lowering nicotine levels to minimally addictive or non-addictive levels would decrease the likelihood that future generations of young people become addicted to cigarettes and help more currently addicted smokers to quit,” FDA Commissioner Robert Califf, MD, said in a statement.
The FDA, in charge of regulating cigarettes, issues a proposed rule when changes are discussed. That would be followed by a period for public comments before a final rule could be issued.
The proposed rule was first reported by The Washington Post.
The FDA in 2018 published a study in the New England Journal of Medicine that estimated that a potential limit on nicotine in cigarettes could, by the year 2100, prevent more than 33 million people from becoming regular smokers, and prevent the deaths of more than 8 million people from tobacco-related illnesses.
The action to reduce nicotine levels would fit in with President Joe Biden’s goal of reducing cancer death rates by half over 25 years. Each year, according to the American Cancer Society, about 480,000 deaths (about 1 in 5) are related to smoking. Currently, about 34 million American adults still smoke cigarettes.
Matthew Myers, president of the Campaign for Tobacco-Free Kids, called the proposed rule a “truly game-changing proposal.”
“There is no other single action our country can take that would prevent more young people from becoming addicted to tobacco or have a greater impact on reducing deaths from cancer, cardiovascular disease and respiratory disease,” Mr. Myers said in a statement.
However, he said, “these gains will only be realized if the administration and the FDA demonstrate a full-throated commitment to finalizing and implementing this proposal.”
The FDA proposed the nicotine reduction strategy in talks with the White House and the Department of Health and Human Services early in 2021, according to the Post.
Earlier this year, the FDA issued a proposed rule to ban menthol flavoring in cigarettes. The agency is accepting public comments though July 5.
The action of reducing nicotine levels would likely take years to complete, Mitch Zeller, JD, recently retired director of the FDA Center for Tobacco Products, told the Post.
In 2018, the FDA issued a proposed ruling to set a standard for maximum nicotine levels in cigarettes.
Advocates say the action of slashing nicotine, the active – and addictive – ingredient in cigarettes, would save millions of lives for generations to come. Opponents liken it to the prohibition of alcohol in the 1920s and predict the action will fail.
Others say that if limits are put on nicotine levels, adults should have greater access to noncombustible alternatives.
A version of this article first appeared on WebMD.com.
The Department of Health and Human Services posted a notice that details plans for a new rule to create a maximum allowed amount of nicotine in certain tobacco products. The Food and Drug Administration would take the action, the notice said, “to reduce addictiveness to certain tobacco products, thus giving addicted users a greater ability to quit.” The product standard would also help keep nonsmokers interested in trying tobacco, mainly youth, from starting to smoke and become regulars.
“Lowering nicotine levels to minimally addictive or non-addictive levels would decrease the likelihood that future generations of young people become addicted to cigarettes and help more currently addicted smokers to quit,” FDA Commissioner Robert Califf, MD, said in a statement.
The FDA, in charge of regulating cigarettes, issues a proposed rule when changes are discussed. That would be followed by a period for public comments before a final rule could be issued.
The proposed rule was first reported by The Washington Post.
The FDA in 2018 published a study in the New England Journal of Medicine that estimated that a potential limit on nicotine in cigarettes could, by the year 2100, prevent more than 33 million people from becoming regular smokers, and prevent the deaths of more than 8 million people from tobacco-related illnesses.
The action to reduce nicotine levels would fit in with President Joe Biden’s goal of reducing cancer death rates by half over 25 years. Each year, according to the American Cancer Society, about 480,000 deaths (about 1 in 5) are related to smoking. Currently, about 34 million American adults still smoke cigarettes.
Matthew Myers, president of the Campaign for Tobacco-Free Kids, called the proposed rule a “truly game-changing proposal.”
“There is no other single action our country can take that would prevent more young people from becoming addicted to tobacco or have a greater impact on reducing deaths from cancer, cardiovascular disease and respiratory disease,” Mr. Myers said in a statement.
However, he said, “these gains will only be realized if the administration and the FDA demonstrate a full-throated commitment to finalizing and implementing this proposal.”
The FDA proposed the nicotine reduction strategy in talks with the White House and the Department of Health and Human Services early in 2021, according to the Post.
Earlier this year, the FDA issued a proposed rule to ban menthol flavoring in cigarettes. The agency is accepting public comments though July 5.
The action of reducing nicotine levels would likely take years to complete, Mitch Zeller, JD, recently retired director of the FDA Center for Tobacco Products, told the Post.
In 2018, the FDA issued a proposed ruling to set a standard for maximum nicotine levels in cigarettes.
Advocates say the action of slashing nicotine, the active – and addictive – ingredient in cigarettes, would save millions of lives for generations to come. Opponents liken it to the prohibition of alcohol in the 1920s and predict the action will fail.
Others say that if limits are put on nicotine levels, adults should have greater access to noncombustible alternatives.
A version of this article first appeared on WebMD.com.




