User login
Can California solve its ob.gyn. shortage?
Three patients were waiting in a queue for their telemedicine visit. Four others were in exam rooms, waiting for their appointments. Another patient was on the phone, requesting a prescription renewal.
On a sunny Wednesday afternoon in February, David Ahdoot, MD, FACOG, an ob.gyn. in Burbank, Calif., about 10 miles north of downtown Los Angeles, knows he’ll be working late.
“Normally, we would be closed on Wednesday afternoon,” he said. That time would ordinarily be used to schedule surgeries, make dictation, and perform other tasks. But those were the old days, before the COVID-19 pandemic, before the ob.gyn. shortage got even worse, and before many of the other obstacles that make his practice more burdensome worsened.
Those Wednesday afternoon tasks must be done another time. “There are too many patients to see in the office,” said Dr. Ahdoot, who’s also an assistant clinical professor at UCLA. Because of the shortage of primary care physicians, he has taken on new patients, although he said he would like to focus on his existing ones.
Many of those existing patients have been coming to Dr. Ahdoot for years. “I love my job,” he said, and it shows.
His patient reviews online include the usual grumblings about waiting time and being rushed, but many, especially those from new parents, praise him as caring, compassionate, exceptional – the kind of doctor women trust to deliver their first baby and their next ones, then guide them through menopause and other issues.
The shortage of ob.gyns. in California, as elsewhere, is real, as Dr. Ahdoot’s day-to-day attests. The implications are in evidence well beyond his higher patient loads. Lately, Dr. Ahdoot said, the calls from headhunters seeking to fill positions for locum tenens have increased from twice a month to three times a day. Despite his love for his practice, he admits he thinks about stepping away. He is 56, 8 years short of the average retirement age for ob.gyns. nationally, according to a 2018 report.
Projected shortages
The shortage of primary care doctors, including ob.gyns., is nationwide. Dr. Ahdoot is one of many faces behind the statistics. According to a 2021 update from the U.S. Department of Health & Human Services, the number of ob.gyns. nationwide is expected to decrease 7% between 2018 and 2030, from 50,850 to 47,490. Meanwhile, demand is headed in the other direction – it is projected to rise 4%, from 50,850 to 52,660 ob.gyns. needed. The need for nurse-midwives, nurse-practitioners, and physician assistants who provide women’s health care is also expected to exceed the supply in coming years.
Some areas are harder hit. The Northeast is expected to have enough maternal health care providers to meet the current average level of care nationally but the West, Midwest, and South will not, according to HHS.
California will likely need an additional 4,700 primary care clinicians by 2025, according to projections by the HealthForce Center at the University of California, San Francisco.
Solutions in sight?
Efforts are increasing to make it easier or more appealing for ob.gyns. to practice, or remain in practice, in California. Some existing programs have received funding, while new initiatives to improve the situation are launching.
Some of these efforts and programs will be viewed as a model by some other states, said Janet Coffman, PhD, associate professor at UC San Francisco and a health policy expert who is familiar with new programs and established ones.
“I would say that California offers an example of a multifaceted approach to addressing the shortage of reproductive health providers in general and abortion providers in particular.”
The state has not sat idly in the face of dire predictions of shortfalls in the number of ob.gyns. Over the past decade, Dr. Coffman said, the legislature has “substantially” boosted funding for grants to support ob.gyn. residency programs through CalMedForce and the Song-Brown Healthcare Workforce Training Program. The result: an 18% increase in the number of residents entering the field over the past decade.
“These programs have also substantially increased funding for family medicine residency programs, which are important because family physicians are trained to provide preventive reproductive health services and manage low-risk deliveries,” she added. “Funding for midwifery, nurse midwifery, and nurse practitioner education has been more modest, which I find disappointing because they are qualified to provide many reproductive health services and are more likely to care for underserved populations.”
Other new programs and legislation are focused on expanding the scope of practice for nonphysician health care providers who care for women. Many of these measures are meant to ensure continued access to abortion services not just for California residents, who are guaranteed that right in the state constitution, but for the influx of women expected from states that limited or prohibited abortion after the overturn of Roe v. Wade.
Gavin Newsom, the state’s Democratic governor, has promoted California as a safe haven for women seeking abortions. In September, Gov. Newsom’s reelection campaign rented billboards in six states that have restrictive abortion laws with messages directing women to a website informing them “abortion is legal and protected in California.” The website includes a search function for women looking for providers – representing a further potential strain on the already stressed pool of clinicians. Each year, an estimated 8,000 to 16,100 more people are expected to travel to California for abortions, according to projections made in 2022 by the UCLA Center on Reproductive Health, Law, and Policy.
The questions are, will the efforts be enough to stall or reverse the shortage, and will the efforts to expand other health care providers’ scope of practice be met with cooperation or resistance by MDs?
Just launched: California reproductive health service corps
Brand new, as of January 2023, is the California Reproductive Health Service Corps, created by a bill Gov. Newsom signed into law last September. The program operates within the Department of Health Care Access and Information. Rajeena Victoria Bisla, a spokesperson for assemblywoman Cottie Petrie-Norris (D-Irvine), who authored the bill, said: “The Corps will be responsible for recruiting, training, and retaining a diverse workforce of health care professionals who will be part of reproductive health care teams assigned to work in underserved areas.”
The teams will include MDs as well as licensed midwives, nurses, physician’s assistants, doulas, and medical assistants. They will provide abortion care, contraception, perinatal care, gynecology services, and gender-affirming care, among other needs, Ms. Bisla said.
The California Medical Association’s philanthropic arm, Physicians for a Healthy California (PHC), has two programs that aim to grow and diversify the physician workforce and invest in the state’s underserved areas, according to Lupe Alonzo-Diaz, CEO and president of PHC.
CalMedForce gives annual grants to fund new residency positions at graduate medical education (GME) programs throughout the state. The goal, Ms. Alonzo-Diaz said, is to expand the physician training pool. Funds were generated by Proposition 56, which was passed in 2016. The legislation generates tax on tobacco products. To date, GME programs have received more than $112 million to retain and expand primary care GME programs.
A second program, CalHealthCares, also funded by Proposition 56, offers a loan repayment program of up to $300,000 for physicians who meet certain criteria. “We are incentivizing young physicians and dentists to practice in Medi-Cal communities,” Ms. Alonzo-Diaz said, referring to the state’s Medicaid program. Clinicians must have graduated within the past 5 years (since Jan. 1, 2018) or will be graduating from a residency or fellowship program no later than June 30, 2023. Dentists applying for the practice support grant must have graduated from dental school or residency program within the past 15 years (since Jan. 1, 2008).
In exchange for the loan repayment, the health care providers are asked to commit to 5 years of service in the underserved community. So far, about 800 providers are part of the program, she said. According to Ms. Alonzo-Diaz, the average educational debt for health care providers in California is $315,000 to $350,000. That is as much as $100,000 above the national average.
What else is needed? Shannan Velayas, a spokesperson for the California Medical Association, said the state should invest in the Medi-Cal system to improve “meaningful access” to health care services and to expand loan repayment and residency programs like CalHealthCares and CalMedForce.
“Workforce shortages are not a reason to sacrifice quality of care or compromise patient safety but do warrant additional investment to increase access to medical providers working within their scope of practice,” Ms. Velayas said.
Widening scopes
Efforts are also underway to expand the scope of practice for nurse-practitioners, certified nurse-midwives, and physician assistants. Triggering these efforts has been the fallout and expected consequences of the overturning of Roe v. Wade, removing the federal right to abortion care.
Effective January 2023, trained and qualified nurse-practitioners and certified nurse-midwives in California can perform first-trimester abortions without a doctor’s supervision. Toni Atkins (D-San Diego), now president pro tempore of the California State Senate, authored the bill, SB1375. The measure builds on a 2013 law she spearheaded that allowed certain advanced-practice providers to perform first-trimester abortions with physician supervision.
On Feb. 13, Ms. Atkins introduced SB385, which gives physician assistants the same ability to become qualified in abortion care.
Ms. Atkins expressed confidence that teamwork would prevail in the efforts to have enough providers in the state. “One of the biggest lessons I learned working at a women’s health clinic [prior to her assuming her legislative positions] is that providers put their patients above all else, whether they are doctors, registered nurses, nurse practitioners, certified nurse-midwives, or physician assistants,” she said. “Everyone is on the same team when it comes to breaking down barriers and ensuring all Californians get the care they need without delay.”
Will other states follow suit? “This is pure speculation, but I believe states in which the political leadership supports abortion rights may see the California Reproductive Service Corps and the changes to scope-of-practice laws that allow specially trained CNMs, NPs, and PAs to provide abortions as a model for preserving access to abortion in their states,” Dr. Coffman said.
However, she said, “other states are less likely to view CalMedForce and CalHealthCares as models, because other states have had similar programs for many years, and some have historically invested larger shares of state budget resources into these programs, especially some rural states.”
Reports from the trenches
Laurie Love, DNP, RN, is a family nurse practitioner in Valencia and a clinical instructor and lecturer at the UCLA School of Nursing. When a patient becomes pregnant, she refers her to one of four local ob.gyns.
The working relationships she has with them, she said, “are extremely collaborative. There is no animosity or lack of respect because I don’t have an MD behind my name.”
One of those doctors is Dr. Ahdoot, who said he welcomes the expansion of scope of practice for non-MD health care providers. Some of his colleagues, he said, have tried to fight it, but many have come to the point of welcoming the help. “The consensus is you can’t practice without a nurse practitioner anymore,” Dr. Ahdoot told this news organization.
Expanding the scope of practice for other clinicians helps everyone, including patients, he said. He thinks about how the shortage affects them. “For patients, there is frustration,” he said. He said he often hears women saying they can’t schedule a pap smear for 3 months, or they can’t get a return call from their doctor.
Nalo Hamilton, PhD, an ob.gyn. nurse practitioner and associate professor at UCLA, said the physicians she interacts with support the expanded scope of practice. “Many are confused about details, about what it means and how it will impact them,” she said. “Those who understand it, yes, they agree with it. Doctors will simply have more health care providers who are able to do independent practice.” And she makes another point clear: “We won’t replace ob.gyns.”
None of the persons quoted in this story have disclosed any relevant financial relationships.
A version of this article first appeared on Medscape.com.
Three patients were waiting in a queue for their telemedicine visit. Four others were in exam rooms, waiting for their appointments. Another patient was on the phone, requesting a prescription renewal.
On a sunny Wednesday afternoon in February, David Ahdoot, MD, FACOG, an ob.gyn. in Burbank, Calif., about 10 miles north of downtown Los Angeles, knows he’ll be working late.
“Normally, we would be closed on Wednesday afternoon,” he said. That time would ordinarily be used to schedule surgeries, make dictation, and perform other tasks. But those were the old days, before the COVID-19 pandemic, before the ob.gyn. shortage got even worse, and before many of the other obstacles that make his practice more burdensome worsened.
Those Wednesday afternoon tasks must be done another time. “There are too many patients to see in the office,” said Dr. Ahdoot, who’s also an assistant clinical professor at UCLA. Because of the shortage of primary care physicians, he has taken on new patients, although he said he would like to focus on his existing ones.
Many of those existing patients have been coming to Dr. Ahdoot for years. “I love my job,” he said, and it shows.
His patient reviews online include the usual grumblings about waiting time and being rushed, but many, especially those from new parents, praise him as caring, compassionate, exceptional – the kind of doctor women trust to deliver their first baby and their next ones, then guide them through menopause and other issues.
The shortage of ob.gyns. in California, as elsewhere, is real, as Dr. Ahdoot’s day-to-day attests. The implications are in evidence well beyond his higher patient loads. Lately, Dr. Ahdoot said, the calls from headhunters seeking to fill positions for locum tenens have increased from twice a month to three times a day. Despite his love for his practice, he admits he thinks about stepping away. He is 56, 8 years short of the average retirement age for ob.gyns. nationally, according to a 2018 report.
Projected shortages
The shortage of primary care doctors, including ob.gyns., is nationwide. Dr. Ahdoot is one of many faces behind the statistics. According to a 2021 update from the U.S. Department of Health & Human Services, the number of ob.gyns. nationwide is expected to decrease 7% between 2018 and 2030, from 50,850 to 47,490. Meanwhile, demand is headed in the other direction – it is projected to rise 4%, from 50,850 to 52,660 ob.gyns. needed. The need for nurse-midwives, nurse-practitioners, and physician assistants who provide women’s health care is also expected to exceed the supply in coming years.
Some areas are harder hit. The Northeast is expected to have enough maternal health care providers to meet the current average level of care nationally but the West, Midwest, and South will not, according to HHS.
California will likely need an additional 4,700 primary care clinicians by 2025, according to projections by the HealthForce Center at the University of California, San Francisco.
Solutions in sight?
Efforts are increasing to make it easier or more appealing for ob.gyns. to practice, or remain in practice, in California. Some existing programs have received funding, while new initiatives to improve the situation are launching.
Some of these efforts and programs will be viewed as a model by some other states, said Janet Coffman, PhD, associate professor at UC San Francisco and a health policy expert who is familiar with new programs and established ones.
“I would say that California offers an example of a multifaceted approach to addressing the shortage of reproductive health providers in general and abortion providers in particular.”
The state has not sat idly in the face of dire predictions of shortfalls in the number of ob.gyns. Over the past decade, Dr. Coffman said, the legislature has “substantially” boosted funding for grants to support ob.gyn. residency programs through CalMedForce and the Song-Brown Healthcare Workforce Training Program. The result: an 18% increase in the number of residents entering the field over the past decade.
“These programs have also substantially increased funding for family medicine residency programs, which are important because family physicians are trained to provide preventive reproductive health services and manage low-risk deliveries,” she added. “Funding for midwifery, nurse midwifery, and nurse practitioner education has been more modest, which I find disappointing because they are qualified to provide many reproductive health services and are more likely to care for underserved populations.”
Other new programs and legislation are focused on expanding the scope of practice for nonphysician health care providers who care for women. Many of these measures are meant to ensure continued access to abortion services not just for California residents, who are guaranteed that right in the state constitution, but for the influx of women expected from states that limited or prohibited abortion after the overturn of Roe v. Wade.
Gavin Newsom, the state’s Democratic governor, has promoted California as a safe haven for women seeking abortions. In September, Gov. Newsom’s reelection campaign rented billboards in six states that have restrictive abortion laws with messages directing women to a website informing them “abortion is legal and protected in California.” The website includes a search function for women looking for providers – representing a further potential strain on the already stressed pool of clinicians. Each year, an estimated 8,000 to 16,100 more people are expected to travel to California for abortions, according to projections made in 2022 by the UCLA Center on Reproductive Health, Law, and Policy.
The questions are, will the efforts be enough to stall or reverse the shortage, and will the efforts to expand other health care providers’ scope of practice be met with cooperation or resistance by MDs?
Just launched: California reproductive health service corps
Brand new, as of January 2023, is the California Reproductive Health Service Corps, created by a bill Gov. Newsom signed into law last September. The program operates within the Department of Health Care Access and Information. Rajeena Victoria Bisla, a spokesperson for assemblywoman Cottie Petrie-Norris (D-Irvine), who authored the bill, said: “The Corps will be responsible for recruiting, training, and retaining a diverse workforce of health care professionals who will be part of reproductive health care teams assigned to work in underserved areas.”
The teams will include MDs as well as licensed midwives, nurses, physician’s assistants, doulas, and medical assistants. They will provide abortion care, contraception, perinatal care, gynecology services, and gender-affirming care, among other needs, Ms. Bisla said.
The California Medical Association’s philanthropic arm, Physicians for a Healthy California (PHC), has two programs that aim to grow and diversify the physician workforce and invest in the state’s underserved areas, according to Lupe Alonzo-Diaz, CEO and president of PHC.
CalMedForce gives annual grants to fund new residency positions at graduate medical education (GME) programs throughout the state. The goal, Ms. Alonzo-Diaz said, is to expand the physician training pool. Funds were generated by Proposition 56, which was passed in 2016. The legislation generates tax on tobacco products. To date, GME programs have received more than $112 million to retain and expand primary care GME programs.
A second program, CalHealthCares, also funded by Proposition 56, offers a loan repayment program of up to $300,000 for physicians who meet certain criteria. “We are incentivizing young physicians and dentists to practice in Medi-Cal communities,” Ms. Alonzo-Diaz said, referring to the state’s Medicaid program. Clinicians must have graduated within the past 5 years (since Jan. 1, 2018) or will be graduating from a residency or fellowship program no later than June 30, 2023. Dentists applying for the practice support grant must have graduated from dental school or residency program within the past 15 years (since Jan. 1, 2008).
In exchange for the loan repayment, the health care providers are asked to commit to 5 years of service in the underserved community. So far, about 800 providers are part of the program, she said. According to Ms. Alonzo-Diaz, the average educational debt for health care providers in California is $315,000 to $350,000. That is as much as $100,000 above the national average.
What else is needed? Shannan Velayas, a spokesperson for the California Medical Association, said the state should invest in the Medi-Cal system to improve “meaningful access” to health care services and to expand loan repayment and residency programs like CalHealthCares and CalMedForce.
“Workforce shortages are not a reason to sacrifice quality of care or compromise patient safety but do warrant additional investment to increase access to medical providers working within their scope of practice,” Ms. Velayas said.
Widening scopes
Efforts are also underway to expand the scope of practice for nurse-practitioners, certified nurse-midwives, and physician assistants. Triggering these efforts has been the fallout and expected consequences of the overturning of Roe v. Wade, removing the federal right to abortion care.
Effective January 2023, trained and qualified nurse-practitioners and certified nurse-midwives in California can perform first-trimester abortions without a doctor’s supervision. Toni Atkins (D-San Diego), now president pro tempore of the California State Senate, authored the bill, SB1375. The measure builds on a 2013 law she spearheaded that allowed certain advanced-practice providers to perform first-trimester abortions with physician supervision.
On Feb. 13, Ms. Atkins introduced SB385, which gives physician assistants the same ability to become qualified in abortion care.
Ms. Atkins expressed confidence that teamwork would prevail in the efforts to have enough providers in the state. “One of the biggest lessons I learned working at a women’s health clinic [prior to her assuming her legislative positions] is that providers put their patients above all else, whether they are doctors, registered nurses, nurse practitioners, certified nurse-midwives, or physician assistants,” she said. “Everyone is on the same team when it comes to breaking down barriers and ensuring all Californians get the care they need without delay.”
Will other states follow suit? “This is pure speculation, but I believe states in which the political leadership supports abortion rights may see the California Reproductive Service Corps and the changes to scope-of-practice laws that allow specially trained CNMs, NPs, and PAs to provide abortions as a model for preserving access to abortion in their states,” Dr. Coffman said.
However, she said, “other states are less likely to view CalMedForce and CalHealthCares as models, because other states have had similar programs for many years, and some have historically invested larger shares of state budget resources into these programs, especially some rural states.”
Reports from the trenches
Laurie Love, DNP, RN, is a family nurse practitioner in Valencia and a clinical instructor and lecturer at the UCLA School of Nursing. When a patient becomes pregnant, she refers her to one of four local ob.gyns.
The working relationships she has with them, she said, “are extremely collaborative. There is no animosity or lack of respect because I don’t have an MD behind my name.”
One of those doctors is Dr. Ahdoot, who said he welcomes the expansion of scope of practice for non-MD health care providers. Some of his colleagues, he said, have tried to fight it, but many have come to the point of welcoming the help. “The consensus is you can’t practice without a nurse practitioner anymore,” Dr. Ahdoot told this news organization.
Expanding the scope of practice for other clinicians helps everyone, including patients, he said. He thinks about how the shortage affects them. “For patients, there is frustration,” he said. He said he often hears women saying they can’t schedule a pap smear for 3 months, or they can’t get a return call from their doctor.
Nalo Hamilton, PhD, an ob.gyn. nurse practitioner and associate professor at UCLA, said the physicians she interacts with support the expanded scope of practice. “Many are confused about details, about what it means and how it will impact them,” she said. “Those who understand it, yes, they agree with it. Doctors will simply have more health care providers who are able to do independent practice.” And she makes another point clear: “We won’t replace ob.gyns.”
None of the persons quoted in this story have disclosed any relevant financial relationships.
A version of this article first appeared on Medscape.com.
Three patients were waiting in a queue for their telemedicine visit. Four others were in exam rooms, waiting for their appointments. Another patient was on the phone, requesting a prescription renewal.
On a sunny Wednesday afternoon in February, David Ahdoot, MD, FACOG, an ob.gyn. in Burbank, Calif., about 10 miles north of downtown Los Angeles, knows he’ll be working late.
“Normally, we would be closed on Wednesday afternoon,” he said. That time would ordinarily be used to schedule surgeries, make dictation, and perform other tasks. But those were the old days, before the COVID-19 pandemic, before the ob.gyn. shortage got even worse, and before many of the other obstacles that make his practice more burdensome worsened.
Those Wednesday afternoon tasks must be done another time. “There are too many patients to see in the office,” said Dr. Ahdoot, who’s also an assistant clinical professor at UCLA. Because of the shortage of primary care physicians, he has taken on new patients, although he said he would like to focus on his existing ones.
Many of those existing patients have been coming to Dr. Ahdoot for years. “I love my job,” he said, and it shows.
His patient reviews online include the usual grumblings about waiting time and being rushed, but many, especially those from new parents, praise him as caring, compassionate, exceptional – the kind of doctor women trust to deliver their first baby and their next ones, then guide them through menopause and other issues.
The shortage of ob.gyns. in California, as elsewhere, is real, as Dr. Ahdoot’s day-to-day attests. The implications are in evidence well beyond his higher patient loads. Lately, Dr. Ahdoot said, the calls from headhunters seeking to fill positions for locum tenens have increased from twice a month to three times a day. Despite his love for his practice, he admits he thinks about stepping away. He is 56, 8 years short of the average retirement age for ob.gyns. nationally, according to a 2018 report.
Projected shortages
The shortage of primary care doctors, including ob.gyns., is nationwide. Dr. Ahdoot is one of many faces behind the statistics. According to a 2021 update from the U.S. Department of Health & Human Services, the number of ob.gyns. nationwide is expected to decrease 7% between 2018 and 2030, from 50,850 to 47,490. Meanwhile, demand is headed in the other direction – it is projected to rise 4%, from 50,850 to 52,660 ob.gyns. needed. The need for nurse-midwives, nurse-practitioners, and physician assistants who provide women’s health care is also expected to exceed the supply in coming years.
Some areas are harder hit. The Northeast is expected to have enough maternal health care providers to meet the current average level of care nationally but the West, Midwest, and South will not, according to HHS.
California will likely need an additional 4,700 primary care clinicians by 2025, according to projections by the HealthForce Center at the University of California, San Francisco.
Solutions in sight?
Efforts are increasing to make it easier or more appealing for ob.gyns. to practice, or remain in practice, in California. Some existing programs have received funding, while new initiatives to improve the situation are launching.
Some of these efforts and programs will be viewed as a model by some other states, said Janet Coffman, PhD, associate professor at UC San Francisco and a health policy expert who is familiar with new programs and established ones.
“I would say that California offers an example of a multifaceted approach to addressing the shortage of reproductive health providers in general and abortion providers in particular.”
The state has not sat idly in the face of dire predictions of shortfalls in the number of ob.gyns. Over the past decade, Dr. Coffman said, the legislature has “substantially” boosted funding for grants to support ob.gyn. residency programs through CalMedForce and the Song-Brown Healthcare Workforce Training Program. The result: an 18% increase in the number of residents entering the field over the past decade.
“These programs have also substantially increased funding for family medicine residency programs, which are important because family physicians are trained to provide preventive reproductive health services and manage low-risk deliveries,” she added. “Funding for midwifery, nurse midwifery, and nurse practitioner education has been more modest, which I find disappointing because they are qualified to provide many reproductive health services and are more likely to care for underserved populations.”
Other new programs and legislation are focused on expanding the scope of practice for nonphysician health care providers who care for women. Many of these measures are meant to ensure continued access to abortion services not just for California residents, who are guaranteed that right in the state constitution, but for the influx of women expected from states that limited or prohibited abortion after the overturn of Roe v. Wade.
Gavin Newsom, the state’s Democratic governor, has promoted California as a safe haven for women seeking abortions. In September, Gov. Newsom’s reelection campaign rented billboards in six states that have restrictive abortion laws with messages directing women to a website informing them “abortion is legal and protected in California.” The website includes a search function for women looking for providers – representing a further potential strain on the already stressed pool of clinicians. Each year, an estimated 8,000 to 16,100 more people are expected to travel to California for abortions, according to projections made in 2022 by the UCLA Center on Reproductive Health, Law, and Policy.
The questions are, will the efforts be enough to stall or reverse the shortage, and will the efforts to expand other health care providers’ scope of practice be met with cooperation or resistance by MDs?
Just launched: California reproductive health service corps
Brand new, as of January 2023, is the California Reproductive Health Service Corps, created by a bill Gov. Newsom signed into law last September. The program operates within the Department of Health Care Access and Information. Rajeena Victoria Bisla, a spokesperson for assemblywoman Cottie Petrie-Norris (D-Irvine), who authored the bill, said: “The Corps will be responsible for recruiting, training, and retaining a diverse workforce of health care professionals who will be part of reproductive health care teams assigned to work in underserved areas.”
The teams will include MDs as well as licensed midwives, nurses, physician’s assistants, doulas, and medical assistants. They will provide abortion care, contraception, perinatal care, gynecology services, and gender-affirming care, among other needs, Ms. Bisla said.
The California Medical Association’s philanthropic arm, Physicians for a Healthy California (PHC), has two programs that aim to grow and diversify the physician workforce and invest in the state’s underserved areas, according to Lupe Alonzo-Diaz, CEO and president of PHC.
CalMedForce gives annual grants to fund new residency positions at graduate medical education (GME) programs throughout the state. The goal, Ms. Alonzo-Diaz said, is to expand the physician training pool. Funds were generated by Proposition 56, which was passed in 2016. The legislation generates tax on tobacco products. To date, GME programs have received more than $112 million to retain and expand primary care GME programs.
A second program, CalHealthCares, also funded by Proposition 56, offers a loan repayment program of up to $300,000 for physicians who meet certain criteria. “We are incentivizing young physicians and dentists to practice in Medi-Cal communities,” Ms. Alonzo-Diaz said, referring to the state’s Medicaid program. Clinicians must have graduated within the past 5 years (since Jan. 1, 2018) or will be graduating from a residency or fellowship program no later than June 30, 2023. Dentists applying for the practice support grant must have graduated from dental school or residency program within the past 15 years (since Jan. 1, 2008).
In exchange for the loan repayment, the health care providers are asked to commit to 5 years of service in the underserved community. So far, about 800 providers are part of the program, she said. According to Ms. Alonzo-Diaz, the average educational debt for health care providers in California is $315,000 to $350,000. That is as much as $100,000 above the national average.
What else is needed? Shannan Velayas, a spokesperson for the California Medical Association, said the state should invest in the Medi-Cal system to improve “meaningful access” to health care services and to expand loan repayment and residency programs like CalHealthCares and CalMedForce.
“Workforce shortages are not a reason to sacrifice quality of care or compromise patient safety but do warrant additional investment to increase access to medical providers working within their scope of practice,” Ms. Velayas said.
Widening scopes
Efforts are also underway to expand the scope of practice for nurse-practitioners, certified nurse-midwives, and physician assistants. Triggering these efforts has been the fallout and expected consequences of the overturning of Roe v. Wade, removing the federal right to abortion care.
Effective January 2023, trained and qualified nurse-practitioners and certified nurse-midwives in California can perform first-trimester abortions without a doctor’s supervision. Toni Atkins (D-San Diego), now president pro tempore of the California State Senate, authored the bill, SB1375. The measure builds on a 2013 law she spearheaded that allowed certain advanced-practice providers to perform first-trimester abortions with physician supervision.
On Feb. 13, Ms. Atkins introduced SB385, which gives physician assistants the same ability to become qualified in abortion care.
Ms. Atkins expressed confidence that teamwork would prevail in the efforts to have enough providers in the state. “One of the biggest lessons I learned working at a women’s health clinic [prior to her assuming her legislative positions] is that providers put their patients above all else, whether they are doctors, registered nurses, nurse practitioners, certified nurse-midwives, or physician assistants,” she said. “Everyone is on the same team when it comes to breaking down barriers and ensuring all Californians get the care they need without delay.”
Will other states follow suit? “This is pure speculation, but I believe states in which the political leadership supports abortion rights may see the California Reproductive Service Corps and the changes to scope-of-practice laws that allow specially trained CNMs, NPs, and PAs to provide abortions as a model for preserving access to abortion in their states,” Dr. Coffman said.
However, she said, “other states are less likely to view CalMedForce and CalHealthCares as models, because other states have had similar programs for many years, and some have historically invested larger shares of state budget resources into these programs, especially some rural states.”
Reports from the trenches
Laurie Love, DNP, RN, is a family nurse practitioner in Valencia and a clinical instructor and lecturer at the UCLA School of Nursing. When a patient becomes pregnant, she refers her to one of four local ob.gyns.
The working relationships she has with them, she said, “are extremely collaborative. There is no animosity or lack of respect because I don’t have an MD behind my name.”
One of those doctors is Dr. Ahdoot, who said he welcomes the expansion of scope of practice for non-MD health care providers. Some of his colleagues, he said, have tried to fight it, but many have come to the point of welcoming the help. “The consensus is you can’t practice without a nurse practitioner anymore,” Dr. Ahdoot told this news organization.
Expanding the scope of practice for other clinicians helps everyone, including patients, he said. He thinks about how the shortage affects them. “For patients, there is frustration,” he said. He said he often hears women saying they can’t schedule a pap smear for 3 months, or they can’t get a return call from their doctor.
Nalo Hamilton, PhD, an ob.gyn. nurse practitioner and associate professor at UCLA, said the physicians she interacts with support the expanded scope of practice. “Many are confused about details, about what it means and how it will impact them,” she said. “Those who understand it, yes, they agree with it. Doctors will simply have more health care providers who are able to do independent practice.” And she makes another point clear: “We won’t replace ob.gyns.”
None of the persons quoted in this story have disclosed any relevant financial relationships.
A version of this article first appeared on Medscape.com.
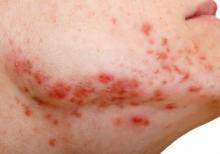
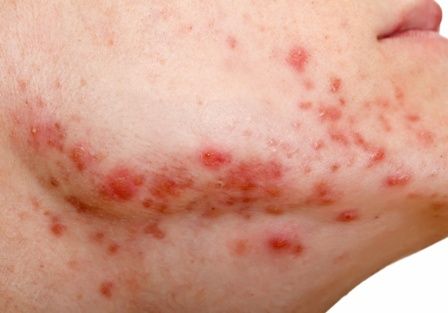
Embattled iPLEDGE program: Changes ahead?
In December 2021, major changes took effect in the iPLEDGE program, the Food and Drug Administration–required safety program for managing the risks of isotretinoin’s teratogenicity and preventing exposure during pregnancy. Now, more modifications may be coming to the acne drug’s safety program.
The risk evaluation and mitigation strategy (REMS) requirements. The aim, according to the FDA meeting announcement, is “to minimize burden on patients, pharmacies, and prescribers while maintaining safe use of isotretinoin oral capsules for patients.”
Isotretinoin is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane. Its former brand name was Accutane.
Problems began to surface days after a new, gender-neutral approach to the risk mitigation program was launched on Dec. 13, 2021. That program had been approved earlier by the FDA.
However, the problems that were encountered were a result of glitches in changes in the platform that had been planned, and were not related to the gender-neutral changes. The iPLEDGE program had transitioned to the new platform, and the rollout was far from smooth. Dermatologists, pharmacists, patients, parents of patients, and others were frustrated and angry that they could not access the new platform and obtain the medication promptly. Reaching the help line to sort out problems was another exercise in frustration. Wait times while on hold were unbearably long, or problems were not resolved over the phone.
(The new gender-neutral approach, which advocates said was needed to preserve inclusiveness of their patients, including transgender patients, places potential patients into two categories: those who can become pregnant, and those who cannot. Previously, there were three categories into which patients were classified: females who have reproductive potential, females who do not have reproductive potential, and males.)
Before pharmacists can fill a prescription for isotretinoin, a medical provider must confirm a patient’s negative pregnancy test and inform a patient with reproductive potential of the risks of the medication.
In January 2022, to deal with the chaotic launch and subsequent problems, the FDA said it would continue to meet with the Isotretinoin Products Manufacturers Group (IPMG) to resolve the problems reported by clinicians, pharmacists, and patients.
The American Academy of Dermatology Association formed an iPLEDGE work group to address the issues and suggest solutions. It has made several requests of and suggestions for the IPMG, which manages the program, according to Andrea L. Zaenglein, MD, professor of dermatology and pediatrics at Penn State Hershey (Pa.) Medical Center, and a member of the work group.
“We are asking them to eliminate the monthly attestation for patients who can’t get pregnant and to review and modify restrictive and punitive waiting and lockout periods for all patients,” she told this news organization.
As of February 2023, most of the platform glitches had been smoothed out, Dr. Zaenglein said. Still, “improvements to the design of the website could improve the user interface,” she added.
The FDA has established a docket for the public to submit comments before the meeting. The docket number is FDA-2022-N-3071. The electronic filing system will accept comments until 11:59 p.m. Eastern time on March 27. Background material and a link to the live webcast of the panel meeting will be available to the public no later than 2 days before the meeting and will be posted on the FDA web page or at the time of the meeting.
A version of this article first appeared on Medscape.com.
In December 2021, major changes took effect in the iPLEDGE program, the Food and Drug Administration–required safety program for managing the risks of isotretinoin’s teratogenicity and preventing exposure during pregnancy. Now, more modifications may be coming to the acne drug’s safety program.
The risk evaluation and mitigation strategy (REMS) requirements. The aim, according to the FDA meeting announcement, is “to minimize burden on patients, pharmacies, and prescribers while maintaining safe use of isotretinoin oral capsules for patients.”
Isotretinoin is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane. Its former brand name was Accutane.
Problems began to surface days after a new, gender-neutral approach to the risk mitigation program was launched on Dec. 13, 2021. That program had been approved earlier by the FDA.
However, the problems that were encountered were a result of glitches in changes in the platform that had been planned, and were not related to the gender-neutral changes. The iPLEDGE program had transitioned to the new platform, and the rollout was far from smooth. Dermatologists, pharmacists, patients, parents of patients, and others were frustrated and angry that they could not access the new platform and obtain the medication promptly. Reaching the help line to sort out problems was another exercise in frustration. Wait times while on hold were unbearably long, or problems were not resolved over the phone.
(The new gender-neutral approach, which advocates said was needed to preserve inclusiveness of their patients, including transgender patients, places potential patients into two categories: those who can become pregnant, and those who cannot. Previously, there were three categories into which patients were classified: females who have reproductive potential, females who do not have reproductive potential, and males.)
Before pharmacists can fill a prescription for isotretinoin, a medical provider must confirm a patient’s negative pregnancy test and inform a patient with reproductive potential of the risks of the medication.
In January 2022, to deal with the chaotic launch and subsequent problems, the FDA said it would continue to meet with the Isotretinoin Products Manufacturers Group (IPMG) to resolve the problems reported by clinicians, pharmacists, and patients.
The American Academy of Dermatology Association formed an iPLEDGE work group to address the issues and suggest solutions. It has made several requests of and suggestions for the IPMG, which manages the program, according to Andrea L. Zaenglein, MD, professor of dermatology and pediatrics at Penn State Hershey (Pa.) Medical Center, and a member of the work group.
“We are asking them to eliminate the monthly attestation for patients who can’t get pregnant and to review and modify restrictive and punitive waiting and lockout periods for all patients,” she told this news organization.
As of February 2023, most of the platform glitches had been smoothed out, Dr. Zaenglein said. Still, “improvements to the design of the website could improve the user interface,” she added.
The FDA has established a docket for the public to submit comments before the meeting. The docket number is FDA-2022-N-3071. The electronic filing system will accept comments until 11:59 p.m. Eastern time on March 27. Background material and a link to the live webcast of the panel meeting will be available to the public no later than 2 days before the meeting and will be posted on the FDA web page or at the time of the meeting.
A version of this article first appeared on Medscape.com.
In December 2021, major changes took effect in the iPLEDGE program, the Food and Drug Administration–required safety program for managing the risks of isotretinoin’s teratogenicity and preventing exposure during pregnancy. Now, more modifications may be coming to the acne drug’s safety program.
The risk evaluation and mitigation strategy (REMS) requirements. The aim, according to the FDA meeting announcement, is “to minimize burden on patients, pharmacies, and prescribers while maintaining safe use of isotretinoin oral capsules for patients.”
Isotretinoin is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane. Its former brand name was Accutane.
Problems began to surface days after a new, gender-neutral approach to the risk mitigation program was launched on Dec. 13, 2021. That program had been approved earlier by the FDA.
However, the problems that were encountered were a result of glitches in changes in the platform that had been planned, and were not related to the gender-neutral changes. The iPLEDGE program had transitioned to the new platform, and the rollout was far from smooth. Dermatologists, pharmacists, patients, parents of patients, and others were frustrated and angry that they could not access the new platform and obtain the medication promptly. Reaching the help line to sort out problems was another exercise in frustration. Wait times while on hold were unbearably long, or problems were not resolved over the phone.
(The new gender-neutral approach, which advocates said was needed to preserve inclusiveness of their patients, including transgender patients, places potential patients into two categories: those who can become pregnant, and those who cannot. Previously, there were three categories into which patients were classified: females who have reproductive potential, females who do not have reproductive potential, and males.)
Before pharmacists can fill a prescription for isotretinoin, a medical provider must confirm a patient’s negative pregnancy test and inform a patient with reproductive potential of the risks of the medication.
In January 2022, to deal with the chaotic launch and subsequent problems, the FDA said it would continue to meet with the Isotretinoin Products Manufacturers Group (IPMG) to resolve the problems reported by clinicians, pharmacists, and patients.
The American Academy of Dermatology Association formed an iPLEDGE work group to address the issues and suggest solutions. It has made several requests of and suggestions for the IPMG, which manages the program, according to Andrea L. Zaenglein, MD, professor of dermatology and pediatrics at Penn State Hershey (Pa.) Medical Center, and a member of the work group.
“We are asking them to eliminate the monthly attestation for patients who can’t get pregnant and to review and modify restrictive and punitive waiting and lockout periods for all patients,” she told this news organization.
As of February 2023, most of the platform glitches had been smoothed out, Dr. Zaenglein said. Still, “improvements to the design of the website could improve the user interface,” she added.
The FDA has established a docket for the public to submit comments before the meeting. The docket number is FDA-2022-N-3071. The electronic filing system will accept comments until 11:59 p.m. Eastern time on March 27. Background material and a link to the live webcast of the panel meeting will be available to the public no later than 2 days before the meeting and will be posted on the FDA web page or at the time of the meeting.
A version of this article first appeared on Medscape.com.
Is it time for yet another COVID booster? It’s complicated
For some people who have received a two-dose primary series and all the recommended boosters, that could mean a sixth shot since COVID-19 vaccines became available. But is even that enough (or too much)?
At this point, no one knows for sure, but new guidance may be on the docket.
On Jan. 26, the FDA’s Vaccines and Related Biological Products Advisory Committee is meeting. On the agenda is discussion about plans for future vaccinations for COVID-19.The committee, made up of external advisers, evaluates data on vaccines and other products for the agency.
According to the FDA announcement, after the meeting, “the FDA will consider whether to recommend adjustments to the current authorizations and approvals, and the FDA will consider the most efficient and transparent process to use for selection of strains for inclusion in the primary and booster vaccines.”
From there, the CDC will take up the issue and decide on recommendations.
The issue is important, as more than 550 Americans a day are still dying from COVID-19, as of the week ending Jan. 13, the CDC reported. That’s up from 346 a day for the week ending Dec. 28.
Yet, uptake of the newest vaccine, the bivalent booster, has been slow. As of Jan. 11, just 15.9% of the population 5 years and up has gotten it; for those most vulnerable to COVID19 – those 65 and up – the number is just 39%.
COVID vaccines, 2023 and beyond
Meanwhile, infectious disease experts have widely differing views on what the vaccination landscape of 2023 and beyond should look like. Among the areas of disagreement are how effective the bivalent vaccine is, which people most need another shot, and what type of vaccine is best.
“I think we probably will need another booster,” says Peter Hotez, MD, PhD, dean of the National School of Tropical Medicine at Baylor College of Medicine, and codirector of the Center for Vaccine Development at Texas Children’s Hospital in Houston. “The question is, what is it going to be? Is it going to be the same bivalent that we just got, or will it be a new bivalent or even a trivalent?”
The trivalent booster, he suggested, might include something more protective against XBB.1.5.
The bivalent booster gives “broadened immunity” that is improved from the original booster shots, says Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape, WebMD’s sister site for health professionals.
In his publication Ground Truths, Dr. Topol on Jan. 11 explained how new data caused him to reverse his previously skeptical view of how the FDA authorized the bivalent vaccine in September without data on how it affected humans at the time.
Paul Offit, MD, director of the Vaccine Education Center and a professor of pediatrics at the Children’s Hospital of Philadelphia, is a member of the FDA advisory committee for vaccines. He still takes a dimmer view of more bivalent booster vaccines, at least as a blanket recommendation.
While he acknowledges that boosters can help some groups – such as older adults, people with multiple health conditions, and those with compromised immune systems – he opposes a recommendation that’s population-wide.
“People who fall into those three groups do benefit,” he says, “but the recommendation is everyone over 6 months get the bivalent, and what I’m asking is, ‘Where is the data that a healthy 12-year-old boy needs a booster to stay out of the hospital?’ ”
Evolving research
“We are trying to understand how to stay one step ahead rather than several steps behind [the virus],“ says Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota.
Among the key questions: How well can a vaccine work against a single subvariant, when no one can say for sure what the next predominant subvariant will be?
Much more research has become available recently about the bivalent vaccine and its effectiveness, Dr. Osterholm says. “The bivalent vaccine is working as well as we could have expected,” he says, especially in high-risk people and in those over age 65. “The challenge we have is, what does that mean going forward?”
In his review, Dr. Topol concludes: “There is now more than ample, highly consistent evidence via lab studies and clinical outcomes to support the bivalent’s benefit over the original booster.”
Among other evidence, he looked at eight studies, including four that used a live virus as part of the research. Six of the eight studies showed the bivalent booster is more effective against the BA.5 variant, compared with the original booster shots. Two others showed no real difference.
“The four live virus studies offer consistent evidence of broadened immunity for the BA.5 vaccine that is improved over the original booster shots,” Dr. Topol wrote. The evidence also found the bivalent antibody response superior against XBB, he wrote.
Dr. Topol also cited CDC data that supports the benefits of the bivalent shot on hospitalization in older adults. During November, hospitalization of adults 65 and above was 2.5 times higher for those vaccinated who did not get the booster, compared to those who got the updated bivalent booster.
Boosters do matter, Dr. Offit says. “But not for all.” In a perspective published Jan. 11 in the New England Journal of Medicine – the same issue that published the two studies finding few differences between the original and bivalent – Dr. Offit wrote that boosting is best reserved for vulnerable groups.
Chasing the variants with a bivalent vaccine, he says, “has not panned out. There remains no evidence that a bivalent vaccine is any better than what we had. Please, show me the data that one is better than the other.”
Dr. Offit believes the goal should not be to prevent all symptomatic infections in healthy, young people by boosting them “with vaccines containing mRNA from strains that might disappear a few months later.”
The CDC needs to parse the data by subgroups, Dr. Offit says. “The critical question is, ‘Who gets hospitalized and who is dying? Who are they?’ ”
That data should take into account age, ethnicity, vaccine history, and other factors, Dr. Offit says, because right now, there is no great data to say, “OK, everyone gets a boost.”
Future vaccine costs
Another debate – for not only current boosters but future ones, too – centers on cost. Without congressional action to fund more vaccines, vaccine makers have suggested their prices may reach $130 a dose, compared with the average $20-per-dose cost the federal government pays now, according to a Kaiser Family Foundation report.
The government has spent more than $30 billion on COVID-19 vaccines, including the bivalent, to provide them free of charge.
The suggested price increase infuriated many. On Jan. 10, Sen. Bernie Sanders (I-Vt.), incoming chair of the Senate Committee on Health, Education, Labor and Pensions, sent a letter to Moderna CEO Stéphane Bancel, urging him to reconsider and refrain from any price increase.
“The huge increase in price that you have proposed will have a significantly negative impact on the budgets of Medicaid, Medicare and other government programs that will continue covering the vaccine without cost-sharing for patients.”
He pointed out, too, the $19 billion in profits Moderna has made over the past 2 years.
While most people with health insurance would likely still get the vaccines and booster for free, according to the Kaiser analysis, will a higher price discourage people from keeping up with recommended vaccinations, including a possible new booster?
“I think so, yes,” Dr. Hotez says, noting that vaccine reluctance is high as it is, even with free vaccinations and easy access.
“The government is balking at paying for the boosters,” he says. “I think it’s very tone deaf from the pharmaceutical companies [to increase the price]. Given all the help they’ve gotten from the American people, I think they should not be gouging at this point.”
He noted that the federal government provided not just money to the companies for the vaccines, but a “glide path” through the FDA for the vaccine approvals.
Are new, variant-specific boosters coming?
Are Moderna, Pfizer-BioNTech, and others developing more variant-specific vaccines, boosters, or other advances?
Novavax, approved in July 2022 as a primary series and in some cases as a booster, is “also developing an Omicron-containing bivalent vaccine at the direction of public health agencies,” says spokesperson Alison Chartan.
Pfizer responded: “When and if we have something to share we will let you know.”
Moderna did not respond.
A version of this article first appeared on WebMD.com.
For some people who have received a two-dose primary series and all the recommended boosters, that could mean a sixth shot since COVID-19 vaccines became available. But is even that enough (or too much)?
At this point, no one knows for sure, but new guidance may be on the docket.
On Jan. 26, the FDA’s Vaccines and Related Biological Products Advisory Committee is meeting. On the agenda is discussion about plans for future vaccinations for COVID-19.The committee, made up of external advisers, evaluates data on vaccines and other products for the agency.
According to the FDA announcement, after the meeting, “the FDA will consider whether to recommend adjustments to the current authorizations and approvals, and the FDA will consider the most efficient and transparent process to use for selection of strains for inclusion in the primary and booster vaccines.”
From there, the CDC will take up the issue and decide on recommendations.
The issue is important, as more than 550 Americans a day are still dying from COVID-19, as of the week ending Jan. 13, the CDC reported. That’s up from 346 a day for the week ending Dec. 28.
Yet, uptake of the newest vaccine, the bivalent booster, has been slow. As of Jan. 11, just 15.9% of the population 5 years and up has gotten it; for those most vulnerable to COVID19 – those 65 and up – the number is just 39%.
COVID vaccines, 2023 and beyond
Meanwhile, infectious disease experts have widely differing views on what the vaccination landscape of 2023 and beyond should look like. Among the areas of disagreement are how effective the bivalent vaccine is, which people most need another shot, and what type of vaccine is best.
“I think we probably will need another booster,” says Peter Hotez, MD, PhD, dean of the National School of Tropical Medicine at Baylor College of Medicine, and codirector of the Center for Vaccine Development at Texas Children’s Hospital in Houston. “The question is, what is it going to be? Is it going to be the same bivalent that we just got, or will it be a new bivalent or even a trivalent?”
The trivalent booster, he suggested, might include something more protective against XBB.1.5.
The bivalent booster gives “broadened immunity” that is improved from the original booster shots, says Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape, WebMD’s sister site for health professionals.
In his publication Ground Truths, Dr. Topol on Jan. 11 explained how new data caused him to reverse his previously skeptical view of how the FDA authorized the bivalent vaccine in September without data on how it affected humans at the time.
Paul Offit, MD, director of the Vaccine Education Center and a professor of pediatrics at the Children’s Hospital of Philadelphia, is a member of the FDA advisory committee for vaccines. He still takes a dimmer view of more bivalent booster vaccines, at least as a blanket recommendation.
While he acknowledges that boosters can help some groups – such as older adults, people with multiple health conditions, and those with compromised immune systems – he opposes a recommendation that’s population-wide.
“People who fall into those three groups do benefit,” he says, “but the recommendation is everyone over 6 months get the bivalent, and what I’m asking is, ‘Where is the data that a healthy 12-year-old boy needs a booster to stay out of the hospital?’ ”
Evolving research
“We are trying to understand how to stay one step ahead rather than several steps behind [the virus],“ says Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota.
Among the key questions: How well can a vaccine work against a single subvariant, when no one can say for sure what the next predominant subvariant will be?
Much more research has become available recently about the bivalent vaccine and its effectiveness, Dr. Osterholm says. “The bivalent vaccine is working as well as we could have expected,” he says, especially in high-risk people and in those over age 65. “The challenge we have is, what does that mean going forward?”
In his review, Dr. Topol concludes: “There is now more than ample, highly consistent evidence via lab studies and clinical outcomes to support the bivalent’s benefit over the original booster.”
Among other evidence, he looked at eight studies, including four that used a live virus as part of the research. Six of the eight studies showed the bivalent booster is more effective against the BA.5 variant, compared with the original booster shots. Two others showed no real difference.
“The four live virus studies offer consistent evidence of broadened immunity for the BA.5 vaccine that is improved over the original booster shots,” Dr. Topol wrote. The evidence also found the bivalent antibody response superior against XBB, he wrote.
Dr. Topol also cited CDC data that supports the benefits of the bivalent shot on hospitalization in older adults. During November, hospitalization of adults 65 and above was 2.5 times higher for those vaccinated who did not get the booster, compared to those who got the updated bivalent booster.
Boosters do matter, Dr. Offit says. “But not for all.” In a perspective published Jan. 11 in the New England Journal of Medicine – the same issue that published the two studies finding few differences between the original and bivalent – Dr. Offit wrote that boosting is best reserved for vulnerable groups.
Chasing the variants with a bivalent vaccine, he says, “has not panned out. There remains no evidence that a bivalent vaccine is any better than what we had. Please, show me the data that one is better than the other.”
Dr. Offit believes the goal should not be to prevent all symptomatic infections in healthy, young people by boosting them “with vaccines containing mRNA from strains that might disappear a few months later.”
The CDC needs to parse the data by subgroups, Dr. Offit says. “The critical question is, ‘Who gets hospitalized and who is dying? Who are they?’ ”
That data should take into account age, ethnicity, vaccine history, and other factors, Dr. Offit says, because right now, there is no great data to say, “OK, everyone gets a boost.”
Future vaccine costs
Another debate – for not only current boosters but future ones, too – centers on cost. Without congressional action to fund more vaccines, vaccine makers have suggested their prices may reach $130 a dose, compared with the average $20-per-dose cost the federal government pays now, according to a Kaiser Family Foundation report.
The government has spent more than $30 billion on COVID-19 vaccines, including the bivalent, to provide them free of charge.
The suggested price increase infuriated many. On Jan. 10, Sen. Bernie Sanders (I-Vt.), incoming chair of the Senate Committee on Health, Education, Labor and Pensions, sent a letter to Moderna CEO Stéphane Bancel, urging him to reconsider and refrain from any price increase.
“The huge increase in price that you have proposed will have a significantly negative impact on the budgets of Medicaid, Medicare and other government programs that will continue covering the vaccine without cost-sharing for patients.”
He pointed out, too, the $19 billion in profits Moderna has made over the past 2 years.
While most people with health insurance would likely still get the vaccines and booster for free, according to the Kaiser analysis, will a higher price discourage people from keeping up with recommended vaccinations, including a possible new booster?
“I think so, yes,” Dr. Hotez says, noting that vaccine reluctance is high as it is, even with free vaccinations and easy access.
“The government is balking at paying for the boosters,” he says. “I think it’s very tone deaf from the pharmaceutical companies [to increase the price]. Given all the help they’ve gotten from the American people, I think they should not be gouging at this point.”
He noted that the federal government provided not just money to the companies for the vaccines, but a “glide path” through the FDA for the vaccine approvals.
Are new, variant-specific boosters coming?
Are Moderna, Pfizer-BioNTech, and others developing more variant-specific vaccines, boosters, or other advances?
Novavax, approved in July 2022 as a primary series and in some cases as a booster, is “also developing an Omicron-containing bivalent vaccine at the direction of public health agencies,” says spokesperson Alison Chartan.
Pfizer responded: “When and if we have something to share we will let you know.”
Moderna did not respond.
A version of this article first appeared on WebMD.com.
For some people who have received a two-dose primary series and all the recommended boosters, that could mean a sixth shot since COVID-19 vaccines became available. But is even that enough (or too much)?
At this point, no one knows for sure, but new guidance may be on the docket.
On Jan. 26, the FDA’s Vaccines and Related Biological Products Advisory Committee is meeting. On the agenda is discussion about plans for future vaccinations for COVID-19.The committee, made up of external advisers, evaluates data on vaccines and other products for the agency.
According to the FDA announcement, after the meeting, “the FDA will consider whether to recommend adjustments to the current authorizations and approvals, and the FDA will consider the most efficient and transparent process to use for selection of strains for inclusion in the primary and booster vaccines.”
From there, the CDC will take up the issue and decide on recommendations.
The issue is important, as more than 550 Americans a day are still dying from COVID-19, as of the week ending Jan. 13, the CDC reported. That’s up from 346 a day for the week ending Dec. 28.
Yet, uptake of the newest vaccine, the bivalent booster, has been slow. As of Jan. 11, just 15.9% of the population 5 years and up has gotten it; for those most vulnerable to COVID19 – those 65 and up – the number is just 39%.
COVID vaccines, 2023 and beyond
Meanwhile, infectious disease experts have widely differing views on what the vaccination landscape of 2023 and beyond should look like. Among the areas of disagreement are how effective the bivalent vaccine is, which people most need another shot, and what type of vaccine is best.
“I think we probably will need another booster,” says Peter Hotez, MD, PhD, dean of the National School of Tropical Medicine at Baylor College of Medicine, and codirector of the Center for Vaccine Development at Texas Children’s Hospital in Houston. “The question is, what is it going to be? Is it going to be the same bivalent that we just got, or will it be a new bivalent or even a trivalent?”
The trivalent booster, he suggested, might include something more protective against XBB.1.5.
The bivalent booster gives “broadened immunity” that is improved from the original booster shots, says Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape, WebMD’s sister site for health professionals.
In his publication Ground Truths, Dr. Topol on Jan. 11 explained how new data caused him to reverse his previously skeptical view of how the FDA authorized the bivalent vaccine in September without data on how it affected humans at the time.
Paul Offit, MD, director of the Vaccine Education Center and a professor of pediatrics at the Children’s Hospital of Philadelphia, is a member of the FDA advisory committee for vaccines. He still takes a dimmer view of more bivalent booster vaccines, at least as a blanket recommendation.
While he acknowledges that boosters can help some groups – such as older adults, people with multiple health conditions, and those with compromised immune systems – he opposes a recommendation that’s population-wide.
“People who fall into those three groups do benefit,” he says, “but the recommendation is everyone over 6 months get the bivalent, and what I’m asking is, ‘Where is the data that a healthy 12-year-old boy needs a booster to stay out of the hospital?’ ”
Evolving research
“We are trying to understand how to stay one step ahead rather than several steps behind [the virus],“ says Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota.
Among the key questions: How well can a vaccine work against a single subvariant, when no one can say for sure what the next predominant subvariant will be?
Much more research has become available recently about the bivalent vaccine and its effectiveness, Dr. Osterholm says. “The bivalent vaccine is working as well as we could have expected,” he says, especially in high-risk people and in those over age 65. “The challenge we have is, what does that mean going forward?”
In his review, Dr. Topol concludes: “There is now more than ample, highly consistent evidence via lab studies and clinical outcomes to support the bivalent’s benefit over the original booster.”
Among other evidence, he looked at eight studies, including four that used a live virus as part of the research. Six of the eight studies showed the bivalent booster is more effective against the BA.5 variant, compared with the original booster shots. Two others showed no real difference.
“The four live virus studies offer consistent evidence of broadened immunity for the BA.5 vaccine that is improved over the original booster shots,” Dr. Topol wrote. The evidence also found the bivalent antibody response superior against XBB, he wrote.
Dr. Topol also cited CDC data that supports the benefits of the bivalent shot on hospitalization in older adults. During November, hospitalization of adults 65 and above was 2.5 times higher for those vaccinated who did not get the booster, compared to those who got the updated bivalent booster.
Boosters do matter, Dr. Offit says. “But not for all.” In a perspective published Jan. 11 in the New England Journal of Medicine – the same issue that published the two studies finding few differences between the original and bivalent – Dr. Offit wrote that boosting is best reserved for vulnerable groups.
Chasing the variants with a bivalent vaccine, he says, “has not panned out. There remains no evidence that a bivalent vaccine is any better than what we had. Please, show me the data that one is better than the other.”
Dr. Offit believes the goal should not be to prevent all symptomatic infections in healthy, young people by boosting them “with vaccines containing mRNA from strains that might disappear a few months later.”
The CDC needs to parse the data by subgroups, Dr. Offit says. “The critical question is, ‘Who gets hospitalized and who is dying? Who are they?’ ”
That data should take into account age, ethnicity, vaccine history, and other factors, Dr. Offit says, because right now, there is no great data to say, “OK, everyone gets a boost.”
Future vaccine costs
Another debate – for not only current boosters but future ones, too – centers on cost. Without congressional action to fund more vaccines, vaccine makers have suggested their prices may reach $130 a dose, compared with the average $20-per-dose cost the federal government pays now, according to a Kaiser Family Foundation report.
The government has spent more than $30 billion on COVID-19 vaccines, including the bivalent, to provide them free of charge.
The suggested price increase infuriated many. On Jan. 10, Sen. Bernie Sanders (I-Vt.), incoming chair of the Senate Committee on Health, Education, Labor and Pensions, sent a letter to Moderna CEO Stéphane Bancel, urging him to reconsider and refrain from any price increase.
“The huge increase in price that you have proposed will have a significantly negative impact on the budgets of Medicaid, Medicare and other government programs that will continue covering the vaccine without cost-sharing for patients.”
He pointed out, too, the $19 billion in profits Moderna has made over the past 2 years.
While most people with health insurance would likely still get the vaccines and booster for free, according to the Kaiser analysis, will a higher price discourage people from keeping up with recommended vaccinations, including a possible new booster?
“I think so, yes,” Dr. Hotez says, noting that vaccine reluctance is high as it is, even with free vaccinations and easy access.
“The government is balking at paying for the boosters,” he says. “I think it’s very tone deaf from the pharmaceutical companies [to increase the price]. Given all the help they’ve gotten from the American people, I think they should not be gouging at this point.”
He noted that the federal government provided not just money to the companies for the vaccines, but a “glide path” through the FDA for the vaccine approvals.
Are new, variant-specific boosters coming?
Are Moderna, Pfizer-BioNTech, and others developing more variant-specific vaccines, boosters, or other advances?
Novavax, approved in July 2022 as a primary series and in some cases as a booster, is “also developing an Omicron-containing bivalent vaccine at the direction of public health agencies,” says spokesperson Alison Chartan.
Pfizer responded: “When and if we have something to share we will let you know.”
Moderna did not respond.
A version of this article first appeared on WebMD.com.
What’s next for COVID? Here’s what to know
As holiday celebrations wind down in the United States, COVID is on the rise.
Will there be another winter surge? If so, can we minimize it? How big a role might the boosters play in that? Are more mandates coming, along with a return to closed offices and businesses? Read on for a look at the latest info.
Cases, hospitalizations, deaths
As of Dec. 27, the latest statistics, the Centers for Disease Control and Prevention reports more than 487,000 weekly cases, compared to about 265,000 for the week ending Oct. 12. On average, 4,938 people were admitted to the hospital daily from Dec. 19 to 25, down about 6% from the 5,257 admitted daily the week before.
Deaths totaled 2,952 weekly as of Dec. 21, up from 2,699 on Dec. 14.
“What’s sobering overall is still seeing about 400 deaths a day in the U.S.,” said Peter Chin-Hong, MD, professor of medicine and infectious disease specialist at the University of California, San Francisco. “It’s still very high.”
As of Dec. 17, the variants predominating are BQ.1, BQ.1.1, and XBB. Experts said they are paying close attention to XBB, which is increasing quickly in the Northeast.
Predicting a winter surge
Experts tracking the pandemic agree there will be a surge.
“We are in the midst of it now,” said Eric Topol, MD, founder and director of the Scripps Research Translational Institute, La Jolla, Calif., and editor-in-chief of Medscape (MDedge’s sister site). “It’s not nearly like what we’ve had in Omicron or other waves; it’s not as severe. But it’s being particularly felt by seniors.”
One bit of good news: “Outside of that group it doesn’t look like – so far – it is going to be as bad a wave [as in the past],” Dr. Topol said.
Predicting the extent of the post-holiday surge “is the billion-dollar question right now,” said Katelyn Jetelina, PhD, a San Diego epidemiologist and author of the newsletter Your Local Epidemiologist.
“Much of these waves are not being driven by subvariants of concern but rather behavior,” she said.
People are opening up their social networks to gather for celebrations and family time. That’s unique to this winter, she said.
“I think our numbers will continue to go up, but certainly not like 2021 or 2020,” Dr. Chin-Hong said.
Others point out that the surge doesn’t involve just COVID.
“We are expecting a Christmas surge and we are concerned it might be a triple surge,” said William Schaffner, MD, professor of infectious diseases at Vanderbilt University, Nashville, Tenn., referring to the rising cases of flu and RSV (respiratory syncytial virus).
Dr. Jetelina shares that concern, worrying that those illnesses may be what overwhelms hospital capacity.
Another wild card is the situation in China. With the easing of China’s “zero COVID” policies, cases there are rising dramatically. Some models are predicting up to 1 million COVID deaths could occur in China in 2023. (The United States is now requiring travelers from China to show a negative COVID test before entering. Italy and Japan have taken similar measures.)
“The suffering that is going to occur in China is not good news at all,” Dr. Topol said. “We are going to be seeing that for many weeks if not months ahead.”
Theoretically, uncontained spread such as what is expected there could generate a whole new family of variants, he said. But “the main hit is going to be in China,” he predicted. “But it’s hard to project with accuracy.”
“China is 20% of the global population, so we can’t ignore it,” Dr. Jetelina said. “The question is, what’s the probability of a subvariant of concern coming from China? I think the probability is pretty low, but the possibility is there.”
What happens with cases in China may “throw a wrench” in the transition from pandemic to endemic, Dr. Chin-Hong said. But even if the rising cases in China do result in a new variant, “there’s so much T cell and B cell immunity [here], your average person is still not going to get seriously ill, even if the variant looks really scary.”
Minimizing the damage
Experts echo the same advice on stemming the surge, especially for adults who are 65 or older: Get the bivalent booster, and get it now.
“The same with the influenza vaccine,” Dr. Schaffner said.
Both the booster vaccine and the flu vaccine have been underused this year, he said. “It’s part of the general vaccine fatigue.”
The low uptake of the booster vaccine is concerning, Dr. Topol said, especially among adults aged 65 and older, the age group most vulnerable to severe disease. Just 35.7% of U.S. adults 65 and older have gotten the booster, according to the CDC. Dr. Topol calls that a tragedy.
Younger people have not taken to the booster, either. Overall, only 14.1% of people aged 5 and up have gotten an updated booster dose, according to the CDC.
Recent studies find value in the boosters. One study looked only at adults age 65 or older, finding that the bivalent booster reduced the risk of hospitalization by 84% compared to someone not vaccinated, and 73% compared to someone who had received only the monovalent vaccine. Another study of adults found those who had gotten the bivalent were less likely to need COVID-related emergency room care or urgent care.
In a Dec. 21 report in the New England Journal of Medicine, researchers took plasma samples from people who had gotten either one or two monovalent boosters or the bivalent to determine how well they worked against the circulating Omicron subvariants BA.1, BA.5, BA.2.75.2, BQ.1.1, and XBB. The bivalent worked better than the monovalent against all the Omicron subvariants, but especially against BA.2.75.2, BQ.1.1, and XBB.
Rapid testing can help minimize transmission. On Dec. 15, the Biden administration announced its Winter Preparedness Plan, urging Americans to test before and after travel as well as indoor visiting with vulnerable individuals, providing another round of free at-home tests, continuing to make community testing available and continuing to provide vaccines.
Besides the general precautions, Dr. Schaffner suggested: “Look at yourself. Who are you? If you are older than 65, or have underlying illness or are immunocompromised, or are pregnant, please put your mask back on. And think about social distancing. It might be time to worship at home and stream a movie,” instead of going to the theaters, he said.
Back to mandates?
On Dec. 9, the New York City Commissioner of Health and Mental Hygiene urged a return to masking indoors, saying people “should” mask up, including in schools, stores, offices, and when in crowded outdoor settings.
On the same date, the County of Los Angeles Public Health urged a return to masking for everyone aged 2 and older when indoors, including at schools, in transit, or in work sites when around others.
While the CDC order requiring masks on public transportation is no longer in effect, the agency continues to recommend that those using public transportation do so.
But some are taking that further. In Philadelphia, for example, School Superintendent Tony B. Watlington Sr., EdD, announced before the winter break that indoor masking would be required for all students and staff for the first 2 weeks of school return, through Jan. 13, citing guidance from the Philadelphia Department of Public Health.
Universal masking in schools does reduce COVID transmission, as a study published in late November suggests. After Massachusetts dropped the statewide universal masking policy in public schools in February 2022, researchers compared the incidence of COVID in 70 school districts there that dropped the mandate with two school districts that kept it. In the 15 weeks after the policy was rescinded, the lifting of the mandate was linked with an additional 44.9 cases of COVID per 1,000 students and staff. That corresponded to an estimated 11,901 cases and to nearly 30% of the cases in all districts during that time.
That said, experts see mandates as the exception rather than the rule, at least for now, citing public backlash against mandates to mask or follow other restrictions.
“Mandating, we know, it shuts people off,” Dr. Topol said. “It’s unenforceable. If you have a very strong recommendation, that’s probably as good as you’re going to be able to do right now.”
There may be communities where mandates go over better than others, Dr. Schaffner said, such as communities where people have confidence in their public health authorities.
Glimmers of hope
Despite uncertainties, experts offered some not-so-dismal perspectives as well.
“I think our numbers will continue to go up, but certainly not like 2021 or 2020,” Dr. Chin-Hong said.
A version of this article first appeared on WebMD.com.
As holiday celebrations wind down in the United States, COVID is on the rise.
Will there be another winter surge? If so, can we minimize it? How big a role might the boosters play in that? Are more mandates coming, along with a return to closed offices and businesses? Read on for a look at the latest info.
Cases, hospitalizations, deaths
As of Dec. 27, the latest statistics, the Centers for Disease Control and Prevention reports more than 487,000 weekly cases, compared to about 265,000 for the week ending Oct. 12. On average, 4,938 people were admitted to the hospital daily from Dec. 19 to 25, down about 6% from the 5,257 admitted daily the week before.
Deaths totaled 2,952 weekly as of Dec. 21, up from 2,699 on Dec. 14.
“What’s sobering overall is still seeing about 400 deaths a day in the U.S.,” said Peter Chin-Hong, MD, professor of medicine and infectious disease specialist at the University of California, San Francisco. “It’s still very high.”
As of Dec. 17, the variants predominating are BQ.1, BQ.1.1, and XBB. Experts said they are paying close attention to XBB, which is increasing quickly in the Northeast.
Predicting a winter surge
Experts tracking the pandemic agree there will be a surge.
“We are in the midst of it now,” said Eric Topol, MD, founder and director of the Scripps Research Translational Institute, La Jolla, Calif., and editor-in-chief of Medscape (MDedge’s sister site). “It’s not nearly like what we’ve had in Omicron or other waves; it’s not as severe. But it’s being particularly felt by seniors.”
One bit of good news: “Outside of that group it doesn’t look like – so far – it is going to be as bad a wave [as in the past],” Dr. Topol said.
Predicting the extent of the post-holiday surge “is the billion-dollar question right now,” said Katelyn Jetelina, PhD, a San Diego epidemiologist and author of the newsletter Your Local Epidemiologist.
“Much of these waves are not being driven by subvariants of concern but rather behavior,” she said.
People are opening up their social networks to gather for celebrations and family time. That’s unique to this winter, she said.
“I think our numbers will continue to go up, but certainly not like 2021 or 2020,” Dr. Chin-Hong said.
Others point out that the surge doesn’t involve just COVID.
“We are expecting a Christmas surge and we are concerned it might be a triple surge,” said William Schaffner, MD, professor of infectious diseases at Vanderbilt University, Nashville, Tenn., referring to the rising cases of flu and RSV (respiratory syncytial virus).
Dr. Jetelina shares that concern, worrying that those illnesses may be what overwhelms hospital capacity.
Another wild card is the situation in China. With the easing of China’s “zero COVID” policies, cases there are rising dramatically. Some models are predicting up to 1 million COVID deaths could occur in China in 2023. (The United States is now requiring travelers from China to show a negative COVID test before entering. Italy and Japan have taken similar measures.)
“The suffering that is going to occur in China is not good news at all,” Dr. Topol said. “We are going to be seeing that for many weeks if not months ahead.”
Theoretically, uncontained spread such as what is expected there could generate a whole new family of variants, he said. But “the main hit is going to be in China,” he predicted. “But it’s hard to project with accuracy.”
“China is 20% of the global population, so we can’t ignore it,” Dr. Jetelina said. “The question is, what’s the probability of a subvariant of concern coming from China? I think the probability is pretty low, but the possibility is there.”
What happens with cases in China may “throw a wrench” in the transition from pandemic to endemic, Dr. Chin-Hong said. But even if the rising cases in China do result in a new variant, “there’s so much T cell and B cell immunity [here], your average person is still not going to get seriously ill, even if the variant looks really scary.”
Minimizing the damage
Experts echo the same advice on stemming the surge, especially for adults who are 65 or older: Get the bivalent booster, and get it now.
“The same with the influenza vaccine,” Dr. Schaffner said.
Both the booster vaccine and the flu vaccine have been underused this year, he said. “It’s part of the general vaccine fatigue.”
The low uptake of the booster vaccine is concerning, Dr. Topol said, especially among adults aged 65 and older, the age group most vulnerable to severe disease. Just 35.7% of U.S. adults 65 and older have gotten the booster, according to the CDC. Dr. Topol calls that a tragedy.
Younger people have not taken to the booster, either. Overall, only 14.1% of people aged 5 and up have gotten an updated booster dose, according to the CDC.
Recent studies find value in the boosters. One study looked only at adults age 65 or older, finding that the bivalent booster reduced the risk of hospitalization by 84% compared to someone not vaccinated, and 73% compared to someone who had received only the monovalent vaccine. Another study of adults found those who had gotten the bivalent were less likely to need COVID-related emergency room care or urgent care.
In a Dec. 21 report in the New England Journal of Medicine, researchers took plasma samples from people who had gotten either one or two monovalent boosters or the bivalent to determine how well they worked against the circulating Omicron subvariants BA.1, BA.5, BA.2.75.2, BQ.1.1, and XBB. The bivalent worked better than the monovalent against all the Omicron subvariants, but especially against BA.2.75.2, BQ.1.1, and XBB.
Rapid testing can help minimize transmission. On Dec. 15, the Biden administration announced its Winter Preparedness Plan, urging Americans to test before and after travel as well as indoor visiting with vulnerable individuals, providing another round of free at-home tests, continuing to make community testing available and continuing to provide vaccines.
Besides the general precautions, Dr. Schaffner suggested: “Look at yourself. Who are you? If you are older than 65, or have underlying illness or are immunocompromised, or are pregnant, please put your mask back on. And think about social distancing. It might be time to worship at home and stream a movie,” instead of going to the theaters, he said.
Back to mandates?
On Dec. 9, the New York City Commissioner of Health and Mental Hygiene urged a return to masking indoors, saying people “should” mask up, including in schools, stores, offices, and when in crowded outdoor settings.
On the same date, the County of Los Angeles Public Health urged a return to masking for everyone aged 2 and older when indoors, including at schools, in transit, or in work sites when around others.
While the CDC order requiring masks on public transportation is no longer in effect, the agency continues to recommend that those using public transportation do so.
But some are taking that further. In Philadelphia, for example, School Superintendent Tony B. Watlington Sr., EdD, announced before the winter break that indoor masking would be required for all students and staff for the first 2 weeks of school return, through Jan. 13, citing guidance from the Philadelphia Department of Public Health.
Universal masking in schools does reduce COVID transmission, as a study published in late November suggests. After Massachusetts dropped the statewide universal masking policy in public schools in February 2022, researchers compared the incidence of COVID in 70 school districts there that dropped the mandate with two school districts that kept it. In the 15 weeks after the policy was rescinded, the lifting of the mandate was linked with an additional 44.9 cases of COVID per 1,000 students and staff. That corresponded to an estimated 11,901 cases and to nearly 30% of the cases in all districts during that time.
That said, experts see mandates as the exception rather than the rule, at least for now, citing public backlash against mandates to mask or follow other restrictions.
“Mandating, we know, it shuts people off,” Dr. Topol said. “It’s unenforceable. If you have a very strong recommendation, that’s probably as good as you’re going to be able to do right now.”
There may be communities where mandates go over better than others, Dr. Schaffner said, such as communities where people have confidence in their public health authorities.
Glimmers of hope
Despite uncertainties, experts offered some not-so-dismal perspectives as well.
“I think our numbers will continue to go up, but certainly not like 2021 or 2020,” Dr. Chin-Hong said.
A version of this article first appeared on WebMD.com.
As holiday celebrations wind down in the United States, COVID is on the rise.
Will there be another winter surge? If so, can we minimize it? How big a role might the boosters play in that? Are more mandates coming, along with a return to closed offices and businesses? Read on for a look at the latest info.
Cases, hospitalizations, deaths
As of Dec. 27, the latest statistics, the Centers for Disease Control and Prevention reports more than 487,000 weekly cases, compared to about 265,000 for the week ending Oct. 12. On average, 4,938 people were admitted to the hospital daily from Dec. 19 to 25, down about 6% from the 5,257 admitted daily the week before.
Deaths totaled 2,952 weekly as of Dec. 21, up from 2,699 on Dec. 14.
“What’s sobering overall is still seeing about 400 deaths a day in the U.S.,” said Peter Chin-Hong, MD, professor of medicine and infectious disease specialist at the University of California, San Francisco. “It’s still very high.”
As of Dec. 17, the variants predominating are BQ.1, BQ.1.1, and XBB. Experts said they are paying close attention to XBB, which is increasing quickly in the Northeast.
Predicting a winter surge
Experts tracking the pandemic agree there will be a surge.
“We are in the midst of it now,” said Eric Topol, MD, founder and director of the Scripps Research Translational Institute, La Jolla, Calif., and editor-in-chief of Medscape (MDedge’s sister site). “It’s not nearly like what we’ve had in Omicron or other waves; it’s not as severe. But it’s being particularly felt by seniors.”
One bit of good news: “Outside of that group it doesn’t look like – so far – it is going to be as bad a wave [as in the past],” Dr. Topol said.
Predicting the extent of the post-holiday surge “is the billion-dollar question right now,” said Katelyn Jetelina, PhD, a San Diego epidemiologist and author of the newsletter Your Local Epidemiologist.
“Much of these waves are not being driven by subvariants of concern but rather behavior,” she said.
People are opening up their social networks to gather for celebrations and family time. That’s unique to this winter, she said.
“I think our numbers will continue to go up, but certainly not like 2021 or 2020,” Dr. Chin-Hong said.
Others point out that the surge doesn’t involve just COVID.
“We are expecting a Christmas surge and we are concerned it might be a triple surge,” said William Schaffner, MD, professor of infectious diseases at Vanderbilt University, Nashville, Tenn., referring to the rising cases of flu and RSV (respiratory syncytial virus).
Dr. Jetelina shares that concern, worrying that those illnesses may be what overwhelms hospital capacity.
Another wild card is the situation in China. With the easing of China’s “zero COVID” policies, cases there are rising dramatically. Some models are predicting up to 1 million COVID deaths could occur in China in 2023. (The United States is now requiring travelers from China to show a negative COVID test before entering. Italy and Japan have taken similar measures.)
“The suffering that is going to occur in China is not good news at all,” Dr. Topol said. “We are going to be seeing that for many weeks if not months ahead.”
Theoretically, uncontained spread such as what is expected there could generate a whole new family of variants, he said. But “the main hit is going to be in China,” he predicted. “But it’s hard to project with accuracy.”
“China is 20% of the global population, so we can’t ignore it,” Dr. Jetelina said. “The question is, what’s the probability of a subvariant of concern coming from China? I think the probability is pretty low, but the possibility is there.”
What happens with cases in China may “throw a wrench” in the transition from pandemic to endemic, Dr. Chin-Hong said. But even if the rising cases in China do result in a new variant, “there’s so much T cell and B cell immunity [here], your average person is still not going to get seriously ill, even if the variant looks really scary.”
Minimizing the damage
Experts echo the same advice on stemming the surge, especially for adults who are 65 or older: Get the bivalent booster, and get it now.
“The same with the influenza vaccine,” Dr. Schaffner said.
Both the booster vaccine and the flu vaccine have been underused this year, he said. “It’s part of the general vaccine fatigue.”
The low uptake of the booster vaccine is concerning, Dr. Topol said, especially among adults aged 65 and older, the age group most vulnerable to severe disease. Just 35.7% of U.S. adults 65 and older have gotten the booster, according to the CDC. Dr. Topol calls that a tragedy.
Younger people have not taken to the booster, either. Overall, only 14.1% of people aged 5 and up have gotten an updated booster dose, according to the CDC.
Recent studies find value in the boosters. One study looked only at adults age 65 or older, finding that the bivalent booster reduced the risk of hospitalization by 84% compared to someone not vaccinated, and 73% compared to someone who had received only the monovalent vaccine. Another study of adults found those who had gotten the bivalent were less likely to need COVID-related emergency room care or urgent care.
In a Dec. 21 report in the New England Journal of Medicine, researchers took plasma samples from people who had gotten either one or two monovalent boosters or the bivalent to determine how well they worked against the circulating Omicron subvariants BA.1, BA.5, BA.2.75.2, BQ.1.1, and XBB. The bivalent worked better than the monovalent against all the Omicron subvariants, but especially against BA.2.75.2, BQ.1.1, and XBB.
Rapid testing can help minimize transmission. On Dec. 15, the Biden administration announced its Winter Preparedness Plan, urging Americans to test before and after travel as well as indoor visiting with vulnerable individuals, providing another round of free at-home tests, continuing to make community testing available and continuing to provide vaccines.
Besides the general precautions, Dr. Schaffner suggested: “Look at yourself. Who are you? If you are older than 65, or have underlying illness or are immunocompromised, or are pregnant, please put your mask back on. And think about social distancing. It might be time to worship at home and stream a movie,” instead of going to the theaters, he said.
Back to mandates?
On Dec. 9, the New York City Commissioner of Health and Mental Hygiene urged a return to masking indoors, saying people “should” mask up, including in schools, stores, offices, and when in crowded outdoor settings.
On the same date, the County of Los Angeles Public Health urged a return to masking for everyone aged 2 and older when indoors, including at schools, in transit, or in work sites when around others.
While the CDC order requiring masks on public transportation is no longer in effect, the agency continues to recommend that those using public transportation do so.
But some are taking that further. In Philadelphia, for example, School Superintendent Tony B. Watlington Sr., EdD, announced before the winter break that indoor masking would be required for all students and staff for the first 2 weeks of school return, through Jan. 13, citing guidance from the Philadelphia Department of Public Health.
Universal masking in schools does reduce COVID transmission, as a study published in late November suggests. After Massachusetts dropped the statewide universal masking policy in public schools in February 2022, researchers compared the incidence of COVID in 70 school districts there that dropped the mandate with two school districts that kept it. In the 15 weeks after the policy was rescinded, the lifting of the mandate was linked with an additional 44.9 cases of COVID per 1,000 students and staff. That corresponded to an estimated 11,901 cases and to nearly 30% of the cases in all districts during that time.
That said, experts see mandates as the exception rather than the rule, at least for now, citing public backlash against mandates to mask or follow other restrictions.
“Mandating, we know, it shuts people off,” Dr. Topol said. “It’s unenforceable. If you have a very strong recommendation, that’s probably as good as you’re going to be able to do right now.”
There may be communities where mandates go over better than others, Dr. Schaffner said, such as communities where people have confidence in their public health authorities.
Glimmers of hope
Despite uncertainties, experts offered some not-so-dismal perspectives as well.
“I think our numbers will continue to go up, but certainly not like 2021 or 2020,” Dr. Chin-Hong said.
A version of this article first appeared on WebMD.com.
Best diets in 2023: Mediterranean diet wins again
After all, weight loss usually lands one of the top spots on New Year’s resolution surveys.
And just in time, there’s guidance to pick the best plan, as U.S. News & World Report’s annual rankings of the best diet plans were released on Jan. 3.
Once again, the Mediterranean diet, which emphasizes fruits, vegetables, olive oil, and fish, got the top spot, as best diet overall. It’s the sixth consecutive year for that win. But many other diets got top marks as well.
In 2023, U.S. News, with the help of more than 30 nutritionists, doctors, and epidemiologists, ranked 24 diets in several categories to help people find a plan that meets their goals, whether it’s finding the best weight loss diet, easiest one to follow, or plans for other goals, such as managing diabetes or heart disease. Two new categories were added: Best Diets for Bone & Joint Health and Best Family-Friendly Diets.
In previous years, the publication ranked 40 diets. Even if a diet is no longer ranked, its profile with detailed information remains on the site.
“Each year we ask ourselves what we can do better or differently next time,” said Gretel Schueller, managing editor of health for U.S. News. When the publication got feedback from their experts this year, they had requests to consider sustainability of diets and whether they meet a busy family’s needs, in addition to considering many other factors.
This year’s report ranks plans in 11 categories.
The winners and the categories:
Best diets overall
After the Mediterranean diet, two others tied for second place:
- DASH (Dietary Approaches to Stop Hypertension) diet, which fights high blood pressure and emphasizes fruits, vegetables, whole grains, lean protein, and low-fat dairy.
- Flexitarian diet, which focuses on fruits, vegetables, and other healthy foods but also allows occasional meat.
Best weight-loss diets
WW, formerly known as Weight Watchers, got first place. The plan emphasizes not only weight loss but healthier eating and regular activity. The Points program, which assigns specific points to foods, with a daily Points budget, is more personalized than in the past.
- DASH got second place.
- Mayo Clinic Diet and TLC diet tied for third place. The Mayo Clinic Diet focuses on fruits, vegetables, and whole grains. It helps people improve their eating habits. The TLC diet (Therapeutic Lifestyle Changes) focuses on vegetables, fruit, lean protein, and reducing cholesterol levels.
Best fast weight-loss diets
The keto diet got first place. It’s a high-fat, low-carb diet that aims to achieve weight loss through fat burning. Four others tied for second place:
- Atkins, a diet created by the cardiologist Robert Atkins, which begins with very few carbs and then recommends progressively eating more until the weight loss goal is achieved
- Nutrisystem, a commercial program that includes prepackaged meals and focuses on high-protein, lower-glycemic foods to stabilize blood sugar levels
- Optavia, a plan focused on low-carb, low-calorie foods and including fortified meal replacements
- SlimFast Diet, a plan of shakes, smoothies, and meal bars to replace two of three meals a day
Best diets for healthy eating
- Mediterranean
- DASH
- Flexitarian
Best heart-healthy diets
- DASH
- Mediterranean
- Flexitarian and Ornish tied for third. The Ornish Diet focuses on plant-based and whole foods and limiting animal products. It recommends daily exercise and stress reduction.
Best diets for diabetes
- DASH
- Mediterranean
- Flexitarian
Best diets for bone and joint health
DASH and Mediterranean are in a first-place tie, followed by the flexitarian diet.
Best family-friendly diets
This category has a three-way tie: the flexitarian, Mediterranean, and TLC diets.
Best plant-based diets
Mediterranean was first, then flexitarian and the MIND diet. The MIND diet combines the DASH and Mediterranean diets and focuses on “brain-healthy” foods.
Easiest diets to follow
Flexitarian and TLC tied for first, followed by a tie between DASH and Mediterranean.
Best diet programs (formerly called commercial plans)
- WW
- There was a tie for second place between Jenny Craig and Noom, the latter of which focuses on low-calorie foods, with personalized calorie ranges and coaching to help meet goals.
Methodology
A variety of factors were considered, such as whether a diet includes all food groups, how easy it is to follow, whether it can be customized to meet cultural and personal preferences, and if it has a realistic timeline for weight loss.
Response from diet plans
Representatives from two plans that received mixed reviews in the rankings responded.
Jenny Craig was ranked second for best diet program but much lower for family friendly, landing at 22nd place of 24.
“Our program is designed to address the needs of the individual through personalized experiences,” Jenny Craig CEO Mandy Dowson said. “We have many families that participate in our program together but are still evaluated separately to determine appropriate individual goals.”
Its high ranking for best diet program reflects feedback from satisfied members, she said. Among advances will be the new Jenny Fresh program, a line of entrées prepared fresh and delivered to customers’ doors.
Atkins got second place for best fast weight loss but ranked near the bottom for best overall, best weight loss, diabetes, healthy eating, and heart health. In response, Colette Heimowitz, vice president of nutrition and education for Simply Good Foods, which makes Atkins’s food products, said that low-carb eating approaches are a viable option for anyone today.
“There are more than 130 independent, peer-reviewed published studies that show the efficacy and safety of low-carb eating,” she said. “The studies have been conducted for several decades and counting.”
Expert perspective
Samantha Cassetty, a registered dietitian, nutritionist, and wellness expert in New York and author of Sugar Shock, reviewed the report for this news organization. She was not involved in the rankings.
“I think what this shows you is, the best diet overall is also the best for various conditions,” she said. For instance, the Mediterranean, the No. 1 overall, also got high ranking for diabetes, heart health, and bone and joint health.
For consumers trying to lose weight: “If you see fast weight loss, that should be a red flag. A healthy diet for weight loss is one you can sustain,” she said.
She’s not a fan of the programs with prepackaged foods. “It takes the guesswork out, but the portion sizes tend to be unsatisfying. They don’t teach you how to deal with some of the challenges [such as realizing an ‘ideal’ portion size].”
How to use the report
Ms. Schueller’s advice: “Recognize that no diet fits everyone.” When considering which plan to choose, she suggests thinking long-term.
“Whatever we choose has to work in the long run,” she said.
Consumers should consider expenses, meal prep time, and whether the diet fits their lifestyle.
Ideally, she said, the best diet “teaches you smart food preparation and how to make healthy choices, allows the flexibility to be social and eat with groups, whether family or friends.”
Before choosing a diet to follow, consult a medical professional for input on the decision, U.S. News cautioned.
A version of this article first appeared on Medscape.com.
After all, weight loss usually lands one of the top spots on New Year’s resolution surveys.
And just in time, there’s guidance to pick the best plan, as U.S. News & World Report’s annual rankings of the best diet plans were released on Jan. 3.
Once again, the Mediterranean diet, which emphasizes fruits, vegetables, olive oil, and fish, got the top spot, as best diet overall. It’s the sixth consecutive year for that win. But many other diets got top marks as well.
In 2023, U.S. News, with the help of more than 30 nutritionists, doctors, and epidemiologists, ranked 24 diets in several categories to help people find a plan that meets their goals, whether it’s finding the best weight loss diet, easiest one to follow, or plans for other goals, such as managing diabetes or heart disease. Two new categories were added: Best Diets for Bone & Joint Health and Best Family-Friendly Diets.
In previous years, the publication ranked 40 diets. Even if a diet is no longer ranked, its profile with detailed information remains on the site.
“Each year we ask ourselves what we can do better or differently next time,” said Gretel Schueller, managing editor of health for U.S. News. When the publication got feedback from their experts this year, they had requests to consider sustainability of diets and whether they meet a busy family’s needs, in addition to considering many other factors.
This year’s report ranks plans in 11 categories.
The winners and the categories:
Best diets overall
After the Mediterranean diet, two others tied for second place:
- DASH (Dietary Approaches to Stop Hypertension) diet, which fights high blood pressure and emphasizes fruits, vegetables, whole grains, lean protein, and low-fat dairy.
- Flexitarian diet, which focuses on fruits, vegetables, and other healthy foods but also allows occasional meat.
Best weight-loss diets
WW, formerly known as Weight Watchers, got first place. The plan emphasizes not only weight loss but healthier eating and regular activity. The Points program, which assigns specific points to foods, with a daily Points budget, is more personalized than in the past.
- DASH got second place.
- Mayo Clinic Diet and TLC diet tied for third place. The Mayo Clinic Diet focuses on fruits, vegetables, and whole grains. It helps people improve their eating habits. The TLC diet (Therapeutic Lifestyle Changes) focuses on vegetables, fruit, lean protein, and reducing cholesterol levels.
Best fast weight-loss diets
The keto diet got first place. It’s a high-fat, low-carb diet that aims to achieve weight loss through fat burning. Four others tied for second place:
- Atkins, a diet created by the cardiologist Robert Atkins, which begins with very few carbs and then recommends progressively eating more until the weight loss goal is achieved
- Nutrisystem, a commercial program that includes prepackaged meals and focuses on high-protein, lower-glycemic foods to stabilize blood sugar levels
- Optavia, a plan focused on low-carb, low-calorie foods and including fortified meal replacements
- SlimFast Diet, a plan of shakes, smoothies, and meal bars to replace two of three meals a day
Best diets for healthy eating
- Mediterranean
- DASH
- Flexitarian
Best heart-healthy diets
- DASH
- Mediterranean
- Flexitarian and Ornish tied for third. The Ornish Diet focuses on plant-based and whole foods and limiting animal products. It recommends daily exercise and stress reduction.
Best diets for diabetes
- DASH
- Mediterranean
- Flexitarian
Best diets for bone and joint health
DASH and Mediterranean are in a first-place tie, followed by the flexitarian diet.
Best family-friendly diets
This category has a three-way tie: the flexitarian, Mediterranean, and TLC diets.
Best plant-based diets
Mediterranean was first, then flexitarian and the MIND diet. The MIND diet combines the DASH and Mediterranean diets and focuses on “brain-healthy” foods.
Easiest diets to follow
Flexitarian and TLC tied for first, followed by a tie between DASH and Mediterranean.
Best diet programs (formerly called commercial plans)
- WW
- There was a tie for second place between Jenny Craig and Noom, the latter of which focuses on low-calorie foods, with personalized calorie ranges and coaching to help meet goals.
Methodology
A variety of factors were considered, such as whether a diet includes all food groups, how easy it is to follow, whether it can be customized to meet cultural and personal preferences, and if it has a realistic timeline for weight loss.
Response from diet plans
Representatives from two plans that received mixed reviews in the rankings responded.
Jenny Craig was ranked second for best diet program but much lower for family friendly, landing at 22nd place of 24.
“Our program is designed to address the needs of the individual through personalized experiences,” Jenny Craig CEO Mandy Dowson said. “We have many families that participate in our program together but are still evaluated separately to determine appropriate individual goals.”
Its high ranking for best diet program reflects feedback from satisfied members, she said. Among advances will be the new Jenny Fresh program, a line of entrées prepared fresh and delivered to customers’ doors.
Atkins got second place for best fast weight loss but ranked near the bottom for best overall, best weight loss, diabetes, healthy eating, and heart health. In response, Colette Heimowitz, vice president of nutrition and education for Simply Good Foods, which makes Atkins’s food products, said that low-carb eating approaches are a viable option for anyone today.
“There are more than 130 independent, peer-reviewed published studies that show the efficacy and safety of low-carb eating,” she said. “The studies have been conducted for several decades and counting.”
Expert perspective
Samantha Cassetty, a registered dietitian, nutritionist, and wellness expert in New York and author of Sugar Shock, reviewed the report for this news organization. She was not involved in the rankings.
“I think what this shows you is, the best diet overall is also the best for various conditions,” she said. For instance, the Mediterranean, the No. 1 overall, also got high ranking for diabetes, heart health, and bone and joint health.
For consumers trying to lose weight: “If you see fast weight loss, that should be a red flag. A healthy diet for weight loss is one you can sustain,” she said.
She’s not a fan of the programs with prepackaged foods. “It takes the guesswork out, but the portion sizes tend to be unsatisfying. They don’t teach you how to deal with some of the challenges [such as realizing an ‘ideal’ portion size].”
How to use the report
Ms. Schueller’s advice: “Recognize that no diet fits everyone.” When considering which plan to choose, she suggests thinking long-term.
“Whatever we choose has to work in the long run,” she said.
Consumers should consider expenses, meal prep time, and whether the diet fits their lifestyle.
Ideally, she said, the best diet “teaches you smart food preparation and how to make healthy choices, allows the flexibility to be social and eat with groups, whether family or friends.”
Before choosing a diet to follow, consult a medical professional for input on the decision, U.S. News cautioned.
A version of this article first appeared on Medscape.com.
After all, weight loss usually lands one of the top spots on New Year’s resolution surveys.
And just in time, there’s guidance to pick the best plan, as U.S. News & World Report’s annual rankings of the best diet plans were released on Jan. 3.
Once again, the Mediterranean diet, which emphasizes fruits, vegetables, olive oil, and fish, got the top spot, as best diet overall. It’s the sixth consecutive year for that win. But many other diets got top marks as well.
In 2023, U.S. News, with the help of more than 30 nutritionists, doctors, and epidemiologists, ranked 24 diets in several categories to help people find a plan that meets their goals, whether it’s finding the best weight loss diet, easiest one to follow, or plans for other goals, such as managing diabetes or heart disease. Two new categories were added: Best Diets for Bone & Joint Health and Best Family-Friendly Diets.
In previous years, the publication ranked 40 diets. Even if a diet is no longer ranked, its profile with detailed information remains on the site.
“Each year we ask ourselves what we can do better or differently next time,” said Gretel Schueller, managing editor of health for U.S. News. When the publication got feedback from their experts this year, they had requests to consider sustainability of diets and whether they meet a busy family’s needs, in addition to considering many other factors.
This year’s report ranks plans in 11 categories.
The winners and the categories:
Best diets overall
After the Mediterranean diet, two others tied for second place:
- DASH (Dietary Approaches to Stop Hypertension) diet, which fights high blood pressure and emphasizes fruits, vegetables, whole grains, lean protein, and low-fat dairy.
- Flexitarian diet, which focuses on fruits, vegetables, and other healthy foods but also allows occasional meat.
Best weight-loss diets
WW, formerly known as Weight Watchers, got first place. The plan emphasizes not only weight loss but healthier eating and regular activity. The Points program, which assigns specific points to foods, with a daily Points budget, is more personalized than in the past.
- DASH got second place.
- Mayo Clinic Diet and TLC diet tied for third place. The Mayo Clinic Diet focuses on fruits, vegetables, and whole grains. It helps people improve their eating habits. The TLC diet (Therapeutic Lifestyle Changes) focuses on vegetables, fruit, lean protein, and reducing cholesterol levels.
Best fast weight-loss diets
The keto diet got first place. It’s a high-fat, low-carb diet that aims to achieve weight loss through fat burning. Four others tied for second place:
- Atkins, a diet created by the cardiologist Robert Atkins, which begins with very few carbs and then recommends progressively eating more until the weight loss goal is achieved
- Nutrisystem, a commercial program that includes prepackaged meals and focuses on high-protein, lower-glycemic foods to stabilize blood sugar levels
- Optavia, a plan focused on low-carb, low-calorie foods and including fortified meal replacements
- SlimFast Diet, a plan of shakes, smoothies, and meal bars to replace two of three meals a day
Best diets for healthy eating
- Mediterranean
- DASH
- Flexitarian
Best heart-healthy diets
- DASH
- Mediterranean
- Flexitarian and Ornish tied for third. The Ornish Diet focuses on plant-based and whole foods and limiting animal products. It recommends daily exercise and stress reduction.
Best diets for diabetes
- DASH
- Mediterranean
- Flexitarian
Best diets for bone and joint health
DASH and Mediterranean are in a first-place tie, followed by the flexitarian diet.
Best family-friendly diets
This category has a three-way tie: the flexitarian, Mediterranean, and TLC diets.
Best plant-based diets
Mediterranean was first, then flexitarian and the MIND diet. The MIND diet combines the DASH and Mediterranean diets and focuses on “brain-healthy” foods.
Easiest diets to follow
Flexitarian and TLC tied for first, followed by a tie between DASH and Mediterranean.
Best diet programs (formerly called commercial plans)
- WW
- There was a tie for second place between Jenny Craig and Noom, the latter of which focuses on low-calorie foods, with personalized calorie ranges and coaching to help meet goals.
Methodology
A variety of factors were considered, such as whether a diet includes all food groups, how easy it is to follow, whether it can be customized to meet cultural and personal preferences, and if it has a realistic timeline for weight loss.
Response from diet plans
Representatives from two plans that received mixed reviews in the rankings responded.
Jenny Craig was ranked second for best diet program but much lower for family friendly, landing at 22nd place of 24.
“Our program is designed to address the needs of the individual through personalized experiences,” Jenny Craig CEO Mandy Dowson said. “We have many families that participate in our program together but are still evaluated separately to determine appropriate individual goals.”
Its high ranking for best diet program reflects feedback from satisfied members, she said. Among advances will be the new Jenny Fresh program, a line of entrées prepared fresh and delivered to customers’ doors.
Atkins got second place for best fast weight loss but ranked near the bottom for best overall, best weight loss, diabetes, healthy eating, and heart health. In response, Colette Heimowitz, vice president of nutrition and education for Simply Good Foods, which makes Atkins’s food products, said that low-carb eating approaches are a viable option for anyone today.
“There are more than 130 independent, peer-reviewed published studies that show the efficacy and safety of low-carb eating,” she said. “The studies have been conducted for several decades and counting.”
Expert perspective
Samantha Cassetty, a registered dietitian, nutritionist, and wellness expert in New York and author of Sugar Shock, reviewed the report for this news organization. She was not involved in the rankings.
“I think what this shows you is, the best diet overall is also the best for various conditions,” she said. For instance, the Mediterranean, the No. 1 overall, also got high ranking for diabetes, heart health, and bone and joint health.
For consumers trying to lose weight: “If you see fast weight loss, that should be a red flag. A healthy diet for weight loss is one you can sustain,” she said.
She’s not a fan of the programs with prepackaged foods. “It takes the guesswork out, but the portion sizes tend to be unsatisfying. They don’t teach you how to deal with some of the challenges [such as realizing an ‘ideal’ portion size].”
How to use the report
Ms. Schueller’s advice: “Recognize that no diet fits everyone.” When considering which plan to choose, she suggests thinking long-term.
“Whatever we choose has to work in the long run,” she said.
Consumers should consider expenses, meal prep time, and whether the diet fits their lifestyle.
Ideally, she said, the best diet “teaches you smart food preparation and how to make healthy choices, allows the flexibility to be social and eat with groups, whether family or friends.”
Before choosing a diet to follow, consult a medical professional for input on the decision, U.S. News cautioned.
A version of this article first appeared on Medscape.com.
As COVID treatments dwindle, are new ones waiting in the wings?
It was the last monoclonal antibody treatment standing. But less than 10 months after the U.S. Food and Drug Administration gave bebtelovimab its emergency use authorization (EUA) to fight COVID-19, it earlier this month de-authorized it, just as it had for other monoclonal antibody treatments, and for the same reason:
Bebtelovimab couldn’t neutralize the Omicron subvariants BQ.1 and BQ.1.1, the cause of nearly 60% of COVID cases nationally as of November 30.
Next on the chopping block, some predict, will be Evusheld, the combination of tixagevimab and cilgavimab given as a preventive monoclonal antibody to people who are immunocompromised and at high risk of contracting COVID and to those who can’t take the vaccine. In October, the FDA warned that Evusheld was not neutralizing circulating COVID variants.
As the options for treating and preventing COVID decline, will companies rally quickly to develop new ones, or cut their losses in developing treatments that may work for only a few months, given the speed of viral mutations?
But although monoclonal antibody treatments are off the table, at least for now, antiviral drugs – including Paxlovid – are still very much available, and some say underused.
Others suggest it’s time to resurrect interest in convalescent plasma, a treatment used early in the pandemic before drugs or vaccines were here and still authorized for use in those who are immunosuppressed or receiving immunosuppressive treatment.
And on the prevention front, staying up to date with booster vaccines, masking, and taking other precautions should be stressed more, others say, regardless of the number of treatment options, and especially now, as cases rise and people gather for the winter holidays.
‘A major setback’
The bebtelovimab de-authorization was “a major setback,” but an understandable one, said Arturo Casadevall, MD, PhD, professor and chair of molecular microbiology and immunology at the Johns Hopkins Bloomberg School of Public Health in Baltimore. “Monoclonal antibodies are great drugs. We are in an unfortunate situation in that they are vulnerable to changes in the virus” and can’t offer long-lasting protection.
Supplies of bebtelovimab will be retained, according to the FDA, in case variants susceptible to it return.
“What happened to bebtelovimab is no surprise,” agreed Amesh Adalja, MD, senior scholar at Johns Hopkins Center for Health Security. “This is what is going to happen when you are targeting a virus that mutates a lot.”
Monoclonal antibodies work by binding to the spike protein on the virus surface to prevent it from entering cells.
However, Dr. Adalja doesn’t view the disappearance of monoclonal antibody treatments as a major setback. Monoclonal antibodies were not the primary way COVID was treated, he said.
While he does believe it’s important that more monoclonal antibody treatments be developed, “I think it’s important to remember we still have Paxlovid while everyone is lamenting the loss of bebtelovimab.’’
Antivirals: What’s here, what’s coming
Compared with monoclonal antibodies, “Paxlovid remains a much easier drug to give,” Dr. Adalja told this news organization, because it is taken orally, not intravenously.
And it’s effective. In a recent study, researchers found that adults diagnosed with COVID given Paxlovid within 5 days of diagnosis had a 51% lower hospitalization rate within the next 30 days than those not given it. Another study shows it could also reduce a person’s risk of developing long COVID by 26%.
Paxlovid is underused, Dr. Adalja said, partly because the rebound potential got more press than the effectiveness. When a celebrity got rebound from Paxlovid, he said, that would make the news, overshadowing the research on its effectiveness.
Besides Paxlovid, the antivirals remdesivir (Veklury), given intravenously for 3 days, and molnupiravir (Lagevrio), taken orally, are also still available. Antivirals work by targeting specific parts of the virus to prevent it from multiplying.
In the lab, remdesivir, molnupiravir, and another antiviral, nirmatrelvir, all appear to be effective against both BQ.1.1 (a BA.5 subvariant) and XBB (a BA.2 subvariant), both rapidly rising in the United States, according to a report last week in the New England Journal of Medicine.
The researchers also tested several monoclonal antibodies and found they did not neutralize either of the subvariants BQ.1.1 and XBB.
A new oral antiviral, Xocova (ensitrelvir fumaric acid), from Japanese manufacturer Shionogi, received emergency approval in Japan on November 22. It’s taken once a day for 5 days. The goal is to expand access to it globally, according to the company.
Pardes Biosciences launched a phase 2 trial in September for its oral antiviral drug (PBI-0451), under study as a treatment and preventive for COVID. It expects data by the first quarter of 2023.
Pfizer, which makes Paxlovid, has partnered with Clear Creek Bio to develop another oral antiviral COVID drug.
Other approaches
A receptor protein known as ACE2 (angiotensin-converting enzyme 2) is the main “doorway” that SARS-CoV-2 uses to enter and infect cells.
Dana-Farber Cancer Institute scientists are developing a “decoy” drug that works by mimicking the ACE2 receptor on the surface of cells; when the virus tries to bind to it, the spike protein is destroyed. Human trials have not yet started.
Other researchers are investigating whether an already-approved drug used to treat a liver disease, Actigall (UDCA/ursodeoxycholic acid), could protect against COVID infection by reducing ACE2.
So far, the researchers have found in early research that people taking UDCA for liver conditions were less likely than those not taking the drug to have severe COVID. They also found that UDCA reduced SARS-CoV-2 infection in human lungs maintained outside the body.
Monoclonal antibody treatments?
After the FDA decision to withdraw the bebtelovimab EUA, which Eli Lilly said it agreed with, the company issued a statement, promising it wasn’t giving up on monoclonal antibody treatments.
“Lilly will continue to search and evaluate monoclonal antibodies to identify potential candidates for clinical development against new variants,” it read in part.
AstraZeneca, which makes Evusheld, is also continuing to work on monoclonal antibody development. According to a spokesperson, “We are also developing a new long-acting antibody combination – AZD5156 – which has been shown in the lab to neutralize emerging new variants and all known variants to date. We are working to accelerate the development of AZD5156 to make it available at the end of 2023.”
The AstraZeneca spokesperson said he could share no more information about what the combination would include.
A convalescent plasma comeback?
Although Paxlovid can help, there are many contraindications to it, such as drug-drug interactions, Dr. Casadevall told this news organization. And now that the monoclonal antibody treatments have been paused, convalescent plasma “is the only antibody-based therapy that is reliably available. Convalescent plasma includes thousands of different antibodies.”
With his colleagues, Dr. Casadevall evaluated plasma samples from 740 patients. Some had received booster vaccines and been infected with Omicron, others had received boosters and not been infected, and still others had not been vaccinated and became infected.
In a report (not yet peer-reviewed), they found the plasma from those who had been infected or boosted within the past 6 months neutralized the new Omicron variants BQ.1.1, XBB.1, and BF.7.
A push for boosters, masks
To get through the coming months, taking precautions like masking and distancing and staying up to date on booster vaccinations, especially for older adults, can make a difference, other experts say.
In a Twitter thread in early December, Peter Hotez, MD, PhD, professor of pediatrics and molecular virology and microbiology at Baylor College of Medicine, Houston, urged people to take COVID seriously as holiday parties and gatherings occur.
“The single most impactful thing you can do is get your bivalent booster,” he tweeted, as well as give your kids the booster, citing preliminary research that the bivalent mRNA booster broadens immunity against the Omicron subvariants.
For seniors, he said, ‘‘if you get breakthrough COVID, [it’s] really important to get Paxlovid.” Masks will help not only for COVID but also influenza, respiratory syncytial virus (RSV), and other conditions.
Mitigation measures have largely been abandoned, according to Eric Topol, MD, director of the Scripps Research Translational Institute, La Jolla, Calif., and editor-in-chief of Medscape. In an op-ed in the Los Angeles Times, and on his Twitter feed, he reminds people about masking and urges people to get the bivalent booster.
According to the Centers for Disease Control and Prevention, as of Dec. 8, only 13.5% of people aged 5 and older have gotten an updated booster, despite research that shows an increase in antibodies to BQ.1.1. Recent research has found that the bivalent booster increases antibodies to BQ.1.1 by up to 10-fold, Dr. Topol said.
Dr. Adalja is on advisory boards for Shionogi, GSK, and Pardes. Dr. Casadevall reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It was the last monoclonal antibody treatment standing. But less than 10 months after the U.S. Food and Drug Administration gave bebtelovimab its emergency use authorization (EUA) to fight COVID-19, it earlier this month de-authorized it, just as it had for other monoclonal antibody treatments, and for the same reason:
Bebtelovimab couldn’t neutralize the Omicron subvariants BQ.1 and BQ.1.1, the cause of nearly 60% of COVID cases nationally as of November 30.
Next on the chopping block, some predict, will be Evusheld, the combination of tixagevimab and cilgavimab given as a preventive monoclonal antibody to people who are immunocompromised and at high risk of contracting COVID and to those who can’t take the vaccine. In October, the FDA warned that Evusheld was not neutralizing circulating COVID variants.
As the options for treating and preventing COVID decline, will companies rally quickly to develop new ones, or cut their losses in developing treatments that may work for only a few months, given the speed of viral mutations?
But although monoclonal antibody treatments are off the table, at least for now, antiviral drugs – including Paxlovid – are still very much available, and some say underused.
Others suggest it’s time to resurrect interest in convalescent plasma, a treatment used early in the pandemic before drugs or vaccines were here and still authorized for use in those who are immunosuppressed or receiving immunosuppressive treatment.
And on the prevention front, staying up to date with booster vaccines, masking, and taking other precautions should be stressed more, others say, regardless of the number of treatment options, and especially now, as cases rise and people gather for the winter holidays.
‘A major setback’
The bebtelovimab de-authorization was “a major setback,” but an understandable one, said Arturo Casadevall, MD, PhD, professor and chair of molecular microbiology and immunology at the Johns Hopkins Bloomberg School of Public Health in Baltimore. “Monoclonal antibodies are great drugs. We are in an unfortunate situation in that they are vulnerable to changes in the virus” and can’t offer long-lasting protection.
Supplies of bebtelovimab will be retained, according to the FDA, in case variants susceptible to it return.
“What happened to bebtelovimab is no surprise,” agreed Amesh Adalja, MD, senior scholar at Johns Hopkins Center for Health Security. “This is what is going to happen when you are targeting a virus that mutates a lot.”
Monoclonal antibodies work by binding to the spike protein on the virus surface to prevent it from entering cells.
However, Dr. Adalja doesn’t view the disappearance of monoclonal antibody treatments as a major setback. Monoclonal antibodies were not the primary way COVID was treated, he said.
While he does believe it’s important that more monoclonal antibody treatments be developed, “I think it’s important to remember we still have Paxlovid while everyone is lamenting the loss of bebtelovimab.’’
Antivirals: What’s here, what’s coming
Compared with monoclonal antibodies, “Paxlovid remains a much easier drug to give,” Dr. Adalja told this news organization, because it is taken orally, not intravenously.
And it’s effective. In a recent study, researchers found that adults diagnosed with COVID given Paxlovid within 5 days of diagnosis had a 51% lower hospitalization rate within the next 30 days than those not given it. Another study shows it could also reduce a person’s risk of developing long COVID by 26%.
Paxlovid is underused, Dr. Adalja said, partly because the rebound potential got more press than the effectiveness. When a celebrity got rebound from Paxlovid, he said, that would make the news, overshadowing the research on its effectiveness.
Besides Paxlovid, the antivirals remdesivir (Veklury), given intravenously for 3 days, and molnupiravir (Lagevrio), taken orally, are also still available. Antivirals work by targeting specific parts of the virus to prevent it from multiplying.
In the lab, remdesivir, molnupiravir, and another antiviral, nirmatrelvir, all appear to be effective against both BQ.1.1 (a BA.5 subvariant) and XBB (a BA.2 subvariant), both rapidly rising in the United States, according to a report last week in the New England Journal of Medicine.
The researchers also tested several monoclonal antibodies and found they did not neutralize either of the subvariants BQ.1.1 and XBB.
A new oral antiviral, Xocova (ensitrelvir fumaric acid), from Japanese manufacturer Shionogi, received emergency approval in Japan on November 22. It’s taken once a day for 5 days. The goal is to expand access to it globally, according to the company.
Pardes Biosciences launched a phase 2 trial in September for its oral antiviral drug (PBI-0451), under study as a treatment and preventive for COVID. It expects data by the first quarter of 2023.
Pfizer, which makes Paxlovid, has partnered with Clear Creek Bio to develop another oral antiviral COVID drug.
Other approaches
A receptor protein known as ACE2 (angiotensin-converting enzyme 2) is the main “doorway” that SARS-CoV-2 uses to enter and infect cells.
Dana-Farber Cancer Institute scientists are developing a “decoy” drug that works by mimicking the ACE2 receptor on the surface of cells; when the virus tries to bind to it, the spike protein is destroyed. Human trials have not yet started.
Other researchers are investigating whether an already-approved drug used to treat a liver disease, Actigall (UDCA/ursodeoxycholic acid), could protect against COVID infection by reducing ACE2.
So far, the researchers have found in early research that people taking UDCA for liver conditions were less likely than those not taking the drug to have severe COVID. They also found that UDCA reduced SARS-CoV-2 infection in human lungs maintained outside the body.
Monoclonal antibody treatments?
After the FDA decision to withdraw the bebtelovimab EUA, which Eli Lilly said it agreed with, the company issued a statement, promising it wasn’t giving up on monoclonal antibody treatments.
“Lilly will continue to search and evaluate monoclonal antibodies to identify potential candidates for clinical development against new variants,” it read in part.
AstraZeneca, which makes Evusheld, is also continuing to work on monoclonal antibody development. According to a spokesperson, “We are also developing a new long-acting antibody combination – AZD5156 – which has been shown in the lab to neutralize emerging new variants and all known variants to date. We are working to accelerate the development of AZD5156 to make it available at the end of 2023.”
The AstraZeneca spokesperson said he could share no more information about what the combination would include.
A convalescent plasma comeback?
Although Paxlovid can help, there are many contraindications to it, such as drug-drug interactions, Dr. Casadevall told this news organization. And now that the monoclonal antibody treatments have been paused, convalescent plasma “is the only antibody-based therapy that is reliably available. Convalescent plasma includes thousands of different antibodies.”
With his colleagues, Dr. Casadevall evaluated plasma samples from 740 patients. Some had received booster vaccines and been infected with Omicron, others had received boosters and not been infected, and still others had not been vaccinated and became infected.
In a report (not yet peer-reviewed), they found the plasma from those who had been infected or boosted within the past 6 months neutralized the new Omicron variants BQ.1.1, XBB.1, and BF.7.
A push for boosters, masks
To get through the coming months, taking precautions like masking and distancing and staying up to date on booster vaccinations, especially for older adults, can make a difference, other experts say.
In a Twitter thread in early December, Peter Hotez, MD, PhD, professor of pediatrics and molecular virology and microbiology at Baylor College of Medicine, Houston, urged people to take COVID seriously as holiday parties and gatherings occur.
“The single most impactful thing you can do is get your bivalent booster,” he tweeted, as well as give your kids the booster, citing preliminary research that the bivalent mRNA booster broadens immunity against the Omicron subvariants.
For seniors, he said, ‘‘if you get breakthrough COVID, [it’s] really important to get Paxlovid.” Masks will help not only for COVID but also influenza, respiratory syncytial virus (RSV), and other conditions.
Mitigation measures have largely been abandoned, according to Eric Topol, MD, director of the Scripps Research Translational Institute, La Jolla, Calif., and editor-in-chief of Medscape. In an op-ed in the Los Angeles Times, and on his Twitter feed, he reminds people about masking and urges people to get the bivalent booster.
According to the Centers for Disease Control and Prevention, as of Dec. 8, only 13.5% of people aged 5 and older have gotten an updated booster, despite research that shows an increase in antibodies to BQ.1.1. Recent research has found that the bivalent booster increases antibodies to BQ.1.1 by up to 10-fold, Dr. Topol said.
Dr. Adalja is on advisory boards for Shionogi, GSK, and Pardes. Dr. Casadevall reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It was the last monoclonal antibody treatment standing. But less than 10 months after the U.S. Food and Drug Administration gave bebtelovimab its emergency use authorization (EUA) to fight COVID-19, it earlier this month de-authorized it, just as it had for other monoclonal antibody treatments, and for the same reason:
Bebtelovimab couldn’t neutralize the Omicron subvariants BQ.1 and BQ.1.1, the cause of nearly 60% of COVID cases nationally as of November 30.
Next on the chopping block, some predict, will be Evusheld, the combination of tixagevimab and cilgavimab given as a preventive monoclonal antibody to people who are immunocompromised and at high risk of contracting COVID and to those who can’t take the vaccine. In October, the FDA warned that Evusheld was not neutralizing circulating COVID variants.
As the options for treating and preventing COVID decline, will companies rally quickly to develop new ones, or cut their losses in developing treatments that may work for only a few months, given the speed of viral mutations?
But although monoclonal antibody treatments are off the table, at least for now, antiviral drugs – including Paxlovid – are still very much available, and some say underused.
Others suggest it’s time to resurrect interest in convalescent plasma, a treatment used early in the pandemic before drugs or vaccines were here and still authorized for use in those who are immunosuppressed or receiving immunosuppressive treatment.
And on the prevention front, staying up to date with booster vaccines, masking, and taking other precautions should be stressed more, others say, regardless of the number of treatment options, and especially now, as cases rise and people gather for the winter holidays.
‘A major setback’
The bebtelovimab de-authorization was “a major setback,” but an understandable one, said Arturo Casadevall, MD, PhD, professor and chair of molecular microbiology and immunology at the Johns Hopkins Bloomberg School of Public Health in Baltimore. “Monoclonal antibodies are great drugs. We are in an unfortunate situation in that they are vulnerable to changes in the virus” and can’t offer long-lasting protection.
Supplies of bebtelovimab will be retained, according to the FDA, in case variants susceptible to it return.
“What happened to bebtelovimab is no surprise,” agreed Amesh Adalja, MD, senior scholar at Johns Hopkins Center for Health Security. “This is what is going to happen when you are targeting a virus that mutates a lot.”
Monoclonal antibodies work by binding to the spike protein on the virus surface to prevent it from entering cells.
However, Dr. Adalja doesn’t view the disappearance of monoclonal antibody treatments as a major setback. Monoclonal antibodies were not the primary way COVID was treated, he said.
While he does believe it’s important that more monoclonal antibody treatments be developed, “I think it’s important to remember we still have Paxlovid while everyone is lamenting the loss of bebtelovimab.’’
Antivirals: What’s here, what’s coming
Compared with monoclonal antibodies, “Paxlovid remains a much easier drug to give,” Dr. Adalja told this news organization, because it is taken orally, not intravenously.
And it’s effective. In a recent study, researchers found that adults diagnosed with COVID given Paxlovid within 5 days of diagnosis had a 51% lower hospitalization rate within the next 30 days than those not given it. Another study shows it could also reduce a person’s risk of developing long COVID by 26%.
Paxlovid is underused, Dr. Adalja said, partly because the rebound potential got more press than the effectiveness. When a celebrity got rebound from Paxlovid, he said, that would make the news, overshadowing the research on its effectiveness.
Besides Paxlovid, the antivirals remdesivir (Veklury), given intravenously for 3 days, and molnupiravir (Lagevrio), taken orally, are also still available. Antivirals work by targeting specific parts of the virus to prevent it from multiplying.
In the lab, remdesivir, molnupiravir, and another antiviral, nirmatrelvir, all appear to be effective against both BQ.1.1 (a BA.5 subvariant) and XBB (a BA.2 subvariant), both rapidly rising in the United States, according to a report last week in the New England Journal of Medicine.
The researchers also tested several monoclonal antibodies and found they did not neutralize either of the subvariants BQ.1.1 and XBB.
A new oral antiviral, Xocova (ensitrelvir fumaric acid), from Japanese manufacturer Shionogi, received emergency approval in Japan on November 22. It’s taken once a day for 5 days. The goal is to expand access to it globally, according to the company.
Pardes Biosciences launched a phase 2 trial in September for its oral antiviral drug (PBI-0451), under study as a treatment and preventive for COVID. It expects data by the first quarter of 2023.
Pfizer, which makes Paxlovid, has partnered with Clear Creek Bio to develop another oral antiviral COVID drug.
Other approaches
A receptor protein known as ACE2 (angiotensin-converting enzyme 2) is the main “doorway” that SARS-CoV-2 uses to enter and infect cells.
Dana-Farber Cancer Institute scientists are developing a “decoy” drug that works by mimicking the ACE2 receptor on the surface of cells; when the virus tries to bind to it, the spike protein is destroyed. Human trials have not yet started.
Other researchers are investigating whether an already-approved drug used to treat a liver disease, Actigall (UDCA/ursodeoxycholic acid), could protect against COVID infection by reducing ACE2.
So far, the researchers have found in early research that people taking UDCA for liver conditions were less likely than those not taking the drug to have severe COVID. They also found that UDCA reduced SARS-CoV-2 infection in human lungs maintained outside the body.
Monoclonal antibody treatments?
After the FDA decision to withdraw the bebtelovimab EUA, which Eli Lilly said it agreed with, the company issued a statement, promising it wasn’t giving up on monoclonal antibody treatments.
“Lilly will continue to search and evaluate monoclonal antibodies to identify potential candidates for clinical development against new variants,” it read in part.
AstraZeneca, which makes Evusheld, is also continuing to work on monoclonal antibody development. According to a spokesperson, “We are also developing a new long-acting antibody combination – AZD5156 – which has been shown in the lab to neutralize emerging new variants and all known variants to date. We are working to accelerate the development of AZD5156 to make it available at the end of 2023.”
The AstraZeneca spokesperson said he could share no more information about what the combination would include.
A convalescent plasma comeback?
Although Paxlovid can help, there are many contraindications to it, such as drug-drug interactions, Dr. Casadevall told this news organization. And now that the monoclonal antibody treatments have been paused, convalescent plasma “is the only antibody-based therapy that is reliably available. Convalescent plasma includes thousands of different antibodies.”
With his colleagues, Dr. Casadevall evaluated plasma samples from 740 patients. Some had received booster vaccines and been infected with Omicron, others had received boosters and not been infected, and still others had not been vaccinated and became infected.
In a report (not yet peer-reviewed), they found the plasma from those who had been infected or boosted within the past 6 months neutralized the new Omicron variants BQ.1.1, XBB.1, and BF.7.
A push for boosters, masks
To get through the coming months, taking precautions like masking and distancing and staying up to date on booster vaccinations, especially for older adults, can make a difference, other experts say.
In a Twitter thread in early December, Peter Hotez, MD, PhD, professor of pediatrics and molecular virology and microbiology at Baylor College of Medicine, Houston, urged people to take COVID seriously as holiday parties and gatherings occur.
“The single most impactful thing you can do is get your bivalent booster,” he tweeted, as well as give your kids the booster, citing preliminary research that the bivalent mRNA booster broadens immunity against the Omicron subvariants.
For seniors, he said, ‘‘if you get breakthrough COVID, [it’s] really important to get Paxlovid.” Masks will help not only for COVID but also influenza, respiratory syncytial virus (RSV), and other conditions.
Mitigation measures have largely been abandoned, according to Eric Topol, MD, director of the Scripps Research Translational Institute, La Jolla, Calif., and editor-in-chief of Medscape. In an op-ed in the Los Angeles Times, and on his Twitter feed, he reminds people about masking and urges people to get the bivalent booster.
According to the Centers for Disease Control and Prevention, as of Dec. 8, only 13.5% of people aged 5 and older have gotten an updated booster, despite research that shows an increase in antibodies to BQ.1.1. Recent research has found that the bivalent booster increases antibodies to BQ.1.1 by up to 10-fold, Dr. Topol said.
Dr. Adalja is on advisory boards for Shionogi, GSK, and Pardes. Dr. Casadevall reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Ask knee OA patients about stair climbing difficulty
Asking knee osteoarthritis patients a simple question – do you have difficulty climbing stairs? – may predict the risk of future functional limitation, according to research presented at the annual meeting of the American College of Rheumatology. Finding out that the patient has difficulty also opens avenues for further evaluation and intervention, said Jason Jakiela, a PhD candidate at the University of Delaware, Newark, who led the study. “We like to view it as a kind of yellow flag,” Mr. Jakiela said in an interview.
Another expert agreed. “I think this is useful for clinical rheumatologists,” said C. Kent Kwoh, MD, professor of medicine and medical imaging at the University of Arizona, Tucson, and director of the University of Arizona Arthritis Center. He commented on the study findings but was not involved in the study. Another common question asked of OA patients, about pain, may not be as useful as asking about difficulty climbing stairs, he said. “Their pain level can go up and down and can be quite varied.”
Osteoarthritis affects more than 32.5 million adults, according to the CDC, and the knee is a common site.
Study details, results
Mr. Jakiela and his team, including Daniel White, PT, ScD, MSC, associate professor of physical therapy at the University of Delaware, Newark, used data from the Osteoarthritis Initiative (OAI). They assessed stair climbing difficulty at baseline with the question: Does your health now limit you in climbing several flights of stairs? Respondents could answer that they were limited a lot, a little, or not at all.
The researchers evaluated functional limitation using two measures: Walking speed and Western Ontario and McMaster Universities Osteoarthritis Index physical function (WOMAC-PF) scores. A walking speed of < 1.22 m/s over 20 meters, the speed needed to safely cross a timed intersection, represented poor function. A WOMAC-PF score of 28/68 or more was also used to define low functioning.
The analyses included only people free of functional limitations at baseline. Each measure was conducted at the start and then at 12, 24, 36, 48, 72, and 96 months’ follow-up visits.
While 2,952 participants (mean age 60.1, 54% female, mean body mass index 27.9) were in the walking speed sample, 3,983 participants (mean age 61.2, 57% female, mean BMI 28.2) were in the WOMAC-PF sample.
When compared with people who had no limitations, those limited a little had a 47% greater risk of gait speed functional limitation and those limited a lot had a 61% greater risk at follow-up. There was a 70% greater risk for functional limitation defined by WOMAC-PF score at follow-up among people who were limited a little in stair climbing when compared with those not limited at all, and people with a lot of limitations had 161% greater risk. Slow gait speed has been linked with mortality.
Over the 8-year follow-up, 973 in the walking speed sample and 578 in the WOMAC-PF sample developed functional limitation.
Starting the conversation
The question about stair climbing difficulty is a good “jumping-off point,” Mr. Jakiela said. “It opens up a line of questioning.” With knee OA, stair climbing difficulty is often the first reported limitation. That difficulty could capture a variety of issues, he said. Patients could be struggling with strength issues, cardiovascular problems, or balance deficits, for instance.
It signals there may be a trajectory of slow decline coming in this patient, Mr. Jakiela said.
“It’s a signal that something is not right,” Dr. White said in an interview. “We don’t know what is wrong.” While questions about stairs have routinely been asked of OA patients, the study findings suggest the answer to the question about having difficulty could help predict a patient’s future course, he said.
After patients reported a little or a lot of difficulty with stair climbing, the average time to reach functional limitation status was about 3 years, Mr. Jakiela said. That gives health care providers time to ask more questions about the patient’s condition and potentially intervene, depending on the details of the difficulty. If it’s a balance issue, physical therapy might help, for example.
While gait speed is a tried-and-true indication, collecting answers about stair climbing difficulty is easier and quicker for clinicians than assessing gait speed, which requires more time as well as office space, Mr. Jakiela said. It’s also intuitive for the patients to recall, the researchers said.
More practical takeaways
Finding out whether functional limitation is likely, based on the stair question, can help health care providers consider nonpharmacologic interventions, Dr. Kwoh agreed, such as physical therapy or braces. “It doesn’t have to be drugs. We have limited drugs for OA at the moment. We don’t have a so-called DMARD drug [for OA].”
NSAIDs have side effects, and people are very familiar with the issues of opioids, he said. It’s important, he added, for the health care provider, if referring to a physical therapist, to find the right one. To help those dealing with knee OA, a PT in sports medicine might be a good choice, he said.
Mr. Jakiela has no disclosures. Dr. Kwoh and Dr. White have no relevant disclosures.
Asking knee osteoarthritis patients a simple question – do you have difficulty climbing stairs? – may predict the risk of future functional limitation, according to research presented at the annual meeting of the American College of Rheumatology. Finding out that the patient has difficulty also opens avenues for further evaluation and intervention, said Jason Jakiela, a PhD candidate at the University of Delaware, Newark, who led the study. “We like to view it as a kind of yellow flag,” Mr. Jakiela said in an interview.
Another expert agreed. “I think this is useful for clinical rheumatologists,” said C. Kent Kwoh, MD, professor of medicine and medical imaging at the University of Arizona, Tucson, and director of the University of Arizona Arthritis Center. He commented on the study findings but was not involved in the study. Another common question asked of OA patients, about pain, may not be as useful as asking about difficulty climbing stairs, he said. “Their pain level can go up and down and can be quite varied.”
Osteoarthritis affects more than 32.5 million adults, according to the CDC, and the knee is a common site.
Study details, results
Mr. Jakiela and his team, including Daniel White, PT, ScD, MSC, associate professor of physical therapy at the University of Delaware, Newark, used data from the Osteoarthritis Initiative (OAI). They assessed stair climbing difficulty at baseline with the question: Does your health now limit you in climbing several flights of stairs? Respondents could answer that they were limited a lot, a little, or not at all.
The researchers evaluated functional limitation using two measures: Walking speed and Western Ontario and McMaster Universities Osteoarthritis Index physical function (WOMAC-PF) scores. A walking speed of < 1.22 m/s over 20 meters, the speed needed to safely cross a timed intersection, represented poor function. A WOMAC-PF score of 28/68 or more was also used to define low functioning.
The analyses included only people free of functional limitations at baseline. Each measure was conducted at the start and then at 12, 24, 36, 48, 72, and 96 months’ follow-up visits.
While 2,952 participants (mean age 60.1, 54% female, mean body mass index 27.9) were in the walking speed sample, 3,983 participants (mean age 61.2, 57% female, mean BMI 28.2) were in the WOMAC-PF sample.
When compared with people who had no limitations, those limited a little had a 47% greater risk of gait speed functional limitation and those limited a lot had a 61% greater risk at follow-up. There was a 70% greater risk for functional limitation defined by WOMAC-PF score at follow-up among people who were limited a little in stair climbing when compared with those not limited at all, and people with a lot of limitations had 161% greater risk. Slow gait speed has been linked with mortality.
Over the 8-year follow-up, 973 in the walking speed sample and 578 in the WOMAC-PF sample developed functional limitation.
Starting the conversation
The question about stair climbing difficulty is a good “jumping-off point,” Mr. Jakiela said. “It opens up a line of questioning.” With knee OA, stair climbing difficulty is often the first reported limitation. That difficulty could capture a variety of issues, he said. Patients could be struggling with strength issues, cardiovascular problems, or balance deficits, for instance.
It signals there may be a trajectory of slow decline coming in this patient, Mr. Jakiela said.
“It’s a signal that something is not right,” Dr. White said in an interview. “We don’t know what is wrong.” While questions about stairs have routinely been asked of OA patients, the study findings suggest the answer to the question about having difficulty could help predict a patient’s future course, he said.
After patients reported a little or a lot of difficulty with stair climbing, the average time to reach functional limitation status was about 3 years, Mr. Jakiela said. That gives health care providers time to ask more questions about the patient’s condition and potentially intervene, depending on the details of the difficulty. If it’s a balance issue, physical therapy might help, for example.
While gait speed is a tried-and-true indication, collecting answers about stair climbing difficulty is easier and quicker for clinicians than assessing gait speed, which requires more time as well as office space, Mr. Jakiela said. It’s also intuitive for the patients to recall, the researchers said.
More practical takeaways
Finding out whether functional limitation is likely, based on the stair question, can help health care providers consider nonpharmacologic interventions, Dr. Kwoh agreed, such as physical therapy or braces. “It doesn’t have to be drugs. We have limited drugs for OA at the moment. We don’t have a so-called DMARD drug [for OA].”
NSAIDs have side effects, and people are very familiar with the issues of opioids, he said. It’s important, he added, for the health care provider, if referring to a physical therapist, to find the right one. To help those dealing with knee OA, a PT in sports medicine might be a good choice, he said.
Mr. Jakiela has no disclosures. Dr. Kwoh and Dr. White have no relevant disclosures.
Asking knee osteoarthritis patients a simple question – do you have difficulty climbing stairs? – may predict the risk of future functional limitation, according to research presented at the annual meeting of the American College of Rheumatology. Finding out that the patient has difficulty also opens avenues for further evaluation and intervention, said Jason Jakiela, a PhD candidate at the University of Delaware, Newark, who led the study. “We like to view it as a kind of yellow flag,” Mr. Jakiela said in an interview.
Another expert agreed. “I think this is useful for clinical rheumatologists,” said C. Kent Kwoh, MD, professor of medicine and medical imaging at the University of Arizona, Tucson, and director of the University of Arizona Arthritis Center. He commented on the study findings but was not involved in the study. Another common question asked of OA patients, about pain, may not be as useful as asking about difficulty climbing stairs, he said. “Their pain level can go up and down and can be quite varied.”
Osteoarthritis affects more than 32.5 million adults, according to the CDC, and the knee is a common site.
Study details, results
Mr. Jakiela and his team, including Daniel White, PT, ScD, MSC, associate professor of physical therapy at the University of Delaware, Newark, used data from the Osteoarthritis Initiative (OAI). They assessed stair climbing difficulty at baseline with the question: Does your health now limit you in climbing several flights of stairs? Respondents could answer that they were limited a lot, a little, or not at all.
The researchers evaluated functional limitation using two measures: Walking speed and Western Ontario and McMaster Universities Osteoarthritis Index physical function (WOMAC-PF) scores. A walking speed of < 1.22 m/s over 20 meters, the speed needed to safely cross a timed intersection, represented poor function. A WOMAC-PF score of 28/68 or more was also used to define low functioning.
The analyses included only people free of functional limitations at baseline. Each measure was conducted at the start and then at 12, 24, 36, 48, 72, and 96 months’ follow-up visits.
While 2,952 participants (mean age 60.1, 54% female, mean body mass index 27.9) were in the walking speed sample, 3,983 participants (mean age 61.2, 57% female, mean BMI 28.2) were in the WOMAC-PF sample.
When compared with people who had no limitations, those limited a little had a 47% greater risk of gait speed functional limitation and those limited a lot had a 61% greater risk at follow-up. There was a 70% greater risk for functional limitation defined by WOMAC-PF score at follow-up among people who were limited a little in stair climbing when compared with those not limited at all, and people with a lot of limitations had 161% greater risk. Slow gait speed has been linked with mortality.
Over the 8-year follow-up, 973 in the walking speed sample and 578 in the WOMAC-PF sample developed functional limitation.
Starting the conversation
The question about stair climbing difficulty is a good “jumping-off point,” Mr. Jakiela said. “It opens up a line of questioning.” With knee OA, stair climbing difficulty is often the first reported limitation. That difficulty could capture a variety of issues, he said. Patients could be struggling with strength issues, cardiovascular problems, or balance deficits, for instance.
It signals there may be a trajectory of slow decline coming in this patient, Mr. Jakiela said.
“It’s a signal that something is not right,” Dr. White said in an interview. “We don’t know what is wrong.” While questions about stairs have routinely been asked of OA patients, the study findings suggest the answer to the question about having difficulty could help predict a patient’s future course, he said.
After patients reported a little or a lot of difficulty with stair climbing, the average time to reach functional limitation status was about 3 years, Mr. Jakiela said. That gives health care providers time to ask more questions about the patient’s condition and potentially intervene, depending on the details of the difficulty. If it’s a balance issue, physical therapy might help, for example.
While gait speed is a tried-and-true indication, collecting answers about stair climbing difficulty is easier and quicker for clinicians than assessing gait speed, which requires more time as well as office space, Mr. Jakiela said. It’s also intuitive for the patients to recall, the researchers said.
More practical takeaways
Finding out whether functional limitation is likely, based on the stair question, can help health care providers consider nonpharmacologic interventions, Dr. Kwoh agreed, such as physical therapy or braces. “It doesn’t have to be drugs. We have limited drugs for OA at the moment. We don’t have a so-called DMARD drug [for OA].”
NSAIDs have side effects, and people are very familiar with the issues of opioids, he said. It’s important, he added, for the health care provider, if referring to a physical therapist, to find the right one. To help those dealing with knee OA, a PT in sports medicine might be a good choice, he said.
Mr. Jakiela has no disclosures. Dr. Kwoh and Dr. White have no relevant disclosures.
FROM ACR 2022
Is it flu, RSV, or COVID? Experts fear the ‘tripledemic’
Just when we thought this holiday season, finally, would be the back-to-normal one, some infectious disease experts are warning that a so-called “tripledemic” – influenza, COVID-19, and RSV – may be in the forecast.
The warning isn’t without basis.
The flu season has gotten an early start. As of Oct. 21, early increases in seasonal flu activity have been reported in most of the country, the Centers for Disease Control and Prevention said, with the southeast and south-central areas having the highest activity levels.
Children’s hospitals and EDs are seeing a surge in children with RSV.
COVID-19 cases are trending down, according to the CDC, but epidemiologists – scientists who study disease outbreaks – always have their eyes on emerging variants.
said Justin Lessler, PhD, a professor of epidemiology at the University of North Carolina at Chapel Hill. Dr. Lessler is on the coordinating team for the COVID-19 Scenario Modeling Hub, which aims to predict the course COVID-19, and the Flu Scenario Modeling Hub, which does the same for influenza.
For COVID-19, some models are predicting some spikes before Christmas, he said, and others see a new wave in 2023. For the flu, the model is predicting an earlier-than-usual start, as the CDC has reported.
While flu activity is relatively low, the CDC said, the season is off to an early start. For the week ending Oct. 21, 1,674 patients were hospitalized for flu, higher than in the summer months but fewer than the 2,675 hospitalizations for the week of May 15, 2022.
As of Oct. 20, COVID-19 cases have declined 12% over the last 2 weeks, nationwide. But hospitalizations are up 10% in much of the Northeast, The New York Times reports, and the improvement in cases and deaths has been slowing down.
As of Oct. 15, 15% of RSV tests reported nationwide were positive, compared with about 11% at that time in 2021, the CDC said. The surveillance collects information from 75 counties in 12 states.
Experts point out that the viruses – all three are respiratory viruses – are simply playing catchup.
“They spread the same way and along with lots of other viruses, and you tend to see an increase in them during the cold months,” said Timothy Brewer, MD, professor of medicine and epidemiology at UCLA.
The increase in all three viruses “is almost predictable at this point in the pandemic,” said Dean Blumberg, MD, a professor and chief of pediatric infectious diseases at the University of California Davis Health. “All the respiratory viruses are out of whack.”
Last year, RSV cases were up, too, and began to appear very early, he said, in the summer instead of in the cooler months. Flu also appeared early in 2021, as it has in 2022.
That contrasts with the flu season of 2020-2021, when COVID precautions were nearly universal, and cases were down. At UC Davis, “we didn’t have one pediatric admission due to influenza in the 2020-2021 [flu] season,” Dr. Blumberg said.
The number of pediatric flu deaths usually range from 37 to 199 per year, according to CDC records. But in the 2020-2021 season, the CDC recorded one pediatric flu death in the U.S.
Both children and adults have had less contact with others the past two seasons, Dr. Blumberg said, “and they don’t get the immunity they got with those infections [previously]. That’s why we are seeing out-of-season, early season [viruses].”
Eventually, he said, the cases of flu and RSV will return to previous levels. “It could be as soon as next year,” Dr. Blumberg said. And COVID-19, hopefully, will become like influenza, he said.
“RSV has always come around in the fall and winter,” said Elizabeth Murray, DO, a pediatric emergency medicine doctor at the University of Rochester (N.Y.) Medical Center and a spokesperson for the American Academy of Pediatrics. In 2022, children are back in school and for the most part not masking. “It’s a perfect storm for all the germs to spread now. They’ve just been waiting for their opportunity to come back.”
Self-care vs. not
RSV can pose a risk for anyone, but most at risk are children under age 5, especially infants under age 1, and adults over age 65. There is no vaccine for it. Symptoms include a runny nose, decreased appetite, coughing, sneezing, fever, and wheezing. But in young infants, there may only be decreased activity, crankiness, and breathing issues, the CDC said.
Keep an eye on the breathing if RSV is suspected, Dr. Murray tells parents. If your child can’t breathe easily, is unable to lie down comfortably, can’t speak clearly, or is sucking in the chest muscles to breathe, get medical help. Most kids with RSV can stay home and recover, she said, but often will need to be checked by a medical professional.
She advises against getting an oximeter to measure oxygen levels for home use. “They are often not accurate,” she said. If in doubt about how serious your child’s symptoms are, “don’t wait it out,” and don’t hesitate to call 911.
Symptoms of flu, COVID, and RSV can overlap. But each can involve breathing problems, which can be an emergency.
“It’s important to seek medical attention for any concerning symptoms, but especially severe shortness of breath or difficulty breathing, as these could signal the need for supplemental oxygen or other emergency interventions,” said Mandy De Vries, a respiratory therapist and director of education at the American Association for Respiratory Care. Inhalation treatment or mechanical ventilation may be needed for severe respiratory issues.
Precautions
To avoid the tripledemic – or any single infection – Timothy Brewer, MD, a professor of medicine and epidemiology at the University of California, Los Angeles, suggests some familiar measures: “Stay home if you’re feeling sick. Make sure you are up to date on your vaccinations. Wear a mask indoors.”
A version of this article first appeared on Medscape.com.
Just when we thought this holiday season, finally, would be the back-to-normal one, some infectious disease experts are warning that a so-called “tripledemic” – influenza, COVID-19, and RSV – may be in the forecast.
The warning isn’t without basis.
The flu season has gotten an early start. As of Oct. 21, early increases in seasonal flu activity have been reported in most of the country, the Centers for Disease Control and Prevention said, with the southeast and south-central areas having the highest activity levels.
Children’s hospitals and EDs are seeing a surge in children with RSV.
COVID-19 cases are trending down, according to the CDC, but epidemiologists – scientists who study disease outbreaks – always have their eyes on emerging variants.
said Justin Lessler, PhD, a professor of epidemiology at the University of North Carolina at Chapel Hill. Dr. Lessler is on the coordinating team for the COVID-19 Scenario Modeling Hub, which aims to predict the course COVID-19, and the Flu Scenario Modeling Hub, which does the same for influenza.
For COVID-19, some models are predicting some spikes before Christmas, he said, and others see a new wave in 2023. For the flu, the model is predicting an earlier-than-usual start, as the CDC has reported.
While flu activity is relatively low, the CDC said, the season is off to an early start. For the week ending Oct. 21, 1,674 patients were hospitalized for flu, higher than in the summer months but fewer than the 2,675 hospitalizations for the week of May 15, 2022.
As of Oct. 20, COVID-19 cases have declined 12% over the last 2 weeks, nationwide. But hospitalizations are up 10% in much of the Northeast, The New York Times reports, and the improvement in cases and deaths has been slowing down.
As of Oct. 15, 15% of RSV tests reported nationwide were positive, compared with about 11% at that time in 2021, the CDC said. The surveillance collects information from 75 counties in 12 states.
Experts point out that the viruses – all three are respiratory viruses – are simply playing catchup.
“They spread the same way and along with lots of other viruses, and you tend to see an increase in them during the cold months,” said Timothy Brewer, MD, professor of medicine and epidemiology at UCLA.
The increase in all three viruses “is almost predictable at this point in the pandemic,” said Dean Blumberg, MD, a professor and chief of pediatric infectious diseases at the University of California Davis Health. “All the respiratory viruses are out of whack.”
Last year, RSV cases were up, too, and began to appear very early, he said, in the summer instead of in the cooler months. Flu also appeared early in 2021, as it has in 2022.
That contrasts with the flu season of 2020-2021, when COVID precautions were nearly universal, and cases were down. At UC Davis, “we didn’t have one pediatric admission due to influenza in the 2020-2021 [flu] season,” Dr. Blumberg said.
The number of pediatric flu deaths usually range from 37 to 199 per year, according to CDC records. But in the 2020-2021 season, the CDC recorded one pediatric flu death in the U.S.
Both children and adults have had less contact with others the past two seasons, Dr. Blumberg said, “and they don’t get the immunity they got with those infections [previously]. That’s why we are seeing out-of-season, early season [viruses].”
Eventually, he said, the cases of flu and RSV will return to previous levels. “It could be as soon as next year,” Dr. Blumberg said. And COVID-19, hopefully, will become like influenza, he said.
“RSV has always come around in the fall and winter,” said Elizabeth Murray, DO, a pediatric emergency medicine doctor at the University of Rochester (N.Y.) Medical Center and a spokesperson for the American Academy of Pediatrics. In 2022, children are back in school and for the most part not masking. “It’s a perfect storm for all the germs to spread now. They’ve just been waiting for their opportunity to come back.”
Self-care vs. not
RSV can pose a risk for anyone, but most at risk are children under age 5, especially infants under age 1, and adults over age 65. There is no vaccine for it. Symptoms include a runny nose, decreased appetite, coughing, sneezing, fever, and wheezing. But in young infants, there may only be decreased activity, crankiness, and breathing issues, the CDC said.
Keep an eye on the breathing if RSV is suspected, Dr. Murray tells parents. If your child can’t breathe easily, is unable to lie down comfortably, can’t speak clearly, or is sucking in the chest muscles to breathe, get medical help. Most kids with RSV can stay home and recover, she said, but often will need to be checked by a medical professional.
She advises against getting an oximeter to measure oxygen levels for home use. “They are often not accurate,” she said. If in doubt about how serious your child’s symptoms are, “don’t wait it out,” and don’t hesitate to call 911.
Symptoms of flu, COVID, and RSV can overlap. But each can involve breathing problems, which can be an emergency.
“It’s important to seek medical attention for any concerning symptoms, but especially severe shortness of breath or difficulty breathing, as these could signal the need for supplemental oxygen or other emergency interventions,” said Mandy De Vries, a respiratory therapist and director of education at the American Association for Respiratory Care. Inhalation treatment or mechanical ventilation may be needed for severe respiratory issues.
Precautions
To avoid the tripledemic – or any single infection – Timothy Brewer, MD, a professor of medicine and epidemiology at the University of California, Los Angeles, suggests some familiar measures: “Stay home if you’re feeling sick. Make sure you are up to date on your vaccinations. Wear a mask indoors.”
A version of this article first appeared on Medscape.com.
Just when we thought this holiday season, finally, would be the back-to-normal one, some infectious disease experts are warning that a so-called “tripledemic” – influenza, COVID-19, and RSV – may be in the forecast.
The warning isn’t without basis.
The flu season has gotten an early start. As of Oct. 21, early increases in seasonal flu activity have been reported in most of the country, the Centers for Disease Control and Prevention said, with the southeast and south-central areas having the highest activity levels.
Children’s hospitals and EDs are seeing a surge in children with RSV.
COVID-19 cases are trending down, according to the CDC, but epidemiologists – scientists who study disease outbreaks – always have their eyes on emerging variants.
said Justin Lessler, PhD, a professor of epidemiology at the University of North Carolina at Chapel Hill. Dr. Lessler is on the coordinating team for the COVID-19 Scenario Modeling Hub, which aims to predict the course COVID-19, and the Flu Scenario Modeling Hub, which does the same for influenza.
For COVID-19, some models are predicting some spikes before Christmas, he said, and others see a new wave in 2023. For the flu, the model is predicting an earlier-than-usual start, as the CDC has reported.
While flu activity is relatively low, the CDC said, the season is off to an early start. For the week ending Oct. 21, 1,674 patients were hospitalized for flu, higher than in the summer months but fewer than the 2,675 hospitalizations for the week of May 15, 2022.
As of Oct. 20, COVID-19 cases have declined 12% over the last 2 weeks, nationwide. But hospitalizations are up 10% in much of the Northeast, The New York Times reports, and the improvement in cases and deaths has been slowing down.
As of Oct. 15, 15% of RSV tests reported nationwide were positive, compared with about 11% at that time in 2021, the CDC said. The surveillance collects information from 75 counties in 12 states.
Experts point out that the viruses – all three are respiratory viruses – are simply playing catchup.
“They spread the same way and along with lots of other viruses, and you tend to see an increase in them during the cold months,” said Timothy Brewer, MD, professor of medicine and epidemiology at UCLA.
The increase in all three viruses “is almost predictable at this point in the pandemic,” said Dean Blumberg, MD, a professor and chief of pediatric infectious diseases at the University of California Davis Health. “All the respiratory viruses are out of whack.”
Last year, RSV cases were up, too, and began to appear very early, he said, in the summer instead of in the cooler months. Flu also appeared early in 2021, as it has in 2022.
That contrasts with the flu season of 2020-2021, when COVID precautions were nearly universal, and cases were down. At UC Davis, “we didn’t have one pediatric admission due to influenza in the 2020-2021 [flu] season,” Dr. Blumberg said.
The number of pediatric flu deaths usually range from 37 to 199 per year, according to CDC records. But in the 2020-2021 season, the CDC recorded one pediatric flu death in the U.S.
Both children and adults have had less contact with others the past two seasons, Dr. Blumberg said, “and they don’t get the immunity they got with those infections [previously]. That’s why we are seeing out-of-season, early season [viruses].”
Eventually, he said, the cases of flu and RSV will return to previous levels. “It could be as soon as next year,” Dr. Blumberg said. And COVID-19, hopefully, will become like influenza, he said.
“RSV has always come around in the fall and winter,” said Elizabeth Murray, DO, a pediatric emergency medicine doctor at the University of Rochester (N.Y.) Medical Center and a spokesperson for the American Academy of Pediatrics. In 2022, children are back in school and for the most part not masking. “It’s a perfect storm for all the germs to spread now. They’ve just been waiting for their opportunity to come back.”
Self-care vs. not
RSV can pose a risk for anyone, but most at risk are children under age 5, especially infants under age 1, and adults over age 65. There is no vaccine for it. Symptoms include a runny nose, decreased appetite, coughing, sneezing, fever, and wheezing. But in young infants, there may only be decreased activity, crankiness, and breathing issues, the CDC said.
Keep an eye on the breathing if RSV is suspected, Dr. Murray tells parents. If your child can’t breathe easily, is unable to lie down comfortably, can’t speak clearly, or is sucking in the chest muscles to breathe, get medical help. Most kids with RSV can stay home and recover, she said, but often will need to be checked by a medical professional.
She advises against getting an oximeter to measure oxygen levels for home use. “They are often not accurate,” she said. If in doubt about how serious your child’s symptoms are, “don’t wait it out,” and don’t hesitate to call 911.
Symptoms of flu, COVID, and RSV can overlap. But each can involve breathing problems, which can be an emergency.
“It’s important to seek medical attention for any concerning symptoms, but especially severe shortness of breath or difficulty breathing, as these could signal the need for supplemental oxygen or other emergency interventions,” said Mandy De Vries, a respiratory therapist and director of education at the American Association for Respiratory Care. Inhalation treatment or mechanical ventilation may be needed for severe respiratory issues.
Precautions
To avoid the tripledemic – or any single infection – Timothy Brewer, MD, a professor of medicine and epidemiology at the University of California, Los Angeles, suggests some familiar measures: “Stay home if you’re feeling sick. Make sure you are up to date on your vaccinations. Wear a mask indoors.”
A version of this article first appeared on Medscape.com.
Sexual health care for disabled youth: Tough and getting tougher
The developmentally disabled girl was just 10 years old when Margaret Thew, DNP, medical director of adolescent medicine at Children’s Wisconsin, Milwaukee, helped care for her. Providing that care was not emotionally easy. “Her brother’s friend sexually assaulted her and impregnated her,” Dr. Thew said.
The girl was able to obtain an abortion, a decision her parents supported. The alternative could have been deadly. “She was a tiny little person and would not have been able to carry a fetus,” Dr. Thew, a nurse practitioner, said.
Dr. Thew said she’s thankful that tragic case occurred before 2022. After the United States Supreme Court overturned Roe v. Wade in June, Wisconsin reverted to an 1849 law banning abortion. Although the law is currently being challenged, Dr. Thew wonders how the situation would have played out now. (Weeks after the Supreme Court’s decision, a similar case occurred in Ohio. In that case, a 10-year-old girl had to travel out of the state to obtain an abortion after having been raped.)
Talking to adolescents and young adults about reproductive health, whether regarding an unexpected pregnancy, the need for contraception, or to provide information about sexual activity, can be a challenge even for experienced health care providers.
The talks, decisions, and care are particularly complex when patients have developmental and intellectual disabilities. Among the many factors, Dr. Thew said, are dealing with menstruation, finding the right contraceptives, and counseling parents who might not want to acknowledge their children’s emerging sexuality.
Statistics: How many?
Because the definitions of disabilities vary and they represent a spectrum, estimates for how many youth have intellectual or developmental disabilities range widely.
In 2019, the National Survey of Children’s Health found that 1 in 4 children and adolescents aged 12-17 years have special health care needs because of disability. The American Community Survey estimates more than 1.3 million people aged 16-20 have a disability.
Intellectual disabilities can occur when a person’s IQ is below 70, significantly impeding the ability to perform activities of daily living, such as eating, dressing, and communicating. Developmental disabilities are impairments in physical, learning, language, and behavior, according to the United States Centers for Disease Control and Prevention. Among the conditions are attention-deficit/hyperactivity disorder, autism spectrum disorders, fragile X syndrome, learning and language problems, spina bifida, and other conditions.
Addressing common issues, concerns
April Kayser is a health educator for the Multnomah County Health Department, Portland, Ore. In 2016, Ms. Kayser and other experts conducted interviews with 11 youth with developmental and intellectual disabilities and 34 support people, either parents or professionals who provide services. The survey was part of the SHEIDD Project – short for Sexual Health Equity for Individuals with Intellectual/Developmental Disabilities – at Oregon Health and Science University (OHSU).
From their findings, the researchers compiled guidelines. They provided scenarios that health care providers need to be aware of and that they need to be ready to address:
- A boy, 14, who is unclear about what to do when he feels sexually excited and wants to masturbate but isn’t at home. He has been told that masturbation is appropriate in private.
- A 20-year-old woman who lives in a group home is pregnant. She confesses to her parents during a visit that another resident is her boyfriend and that he is the father of the child she is expecting.
- A 17-year-old boy wants to ask out another student, who is 15.
Some developmentally and intellectually disabled youth can’t turn to their parents for help. One person in the survey said his father told him, “You don’t need to worry about any of that stuff. You’re too young.” Another said the job of a health care provider was to offer reproductive and sex education “to make sure you don’t screw up in some bad way.”
One finding stood out: Health care providers were at the top of the list of those whom young people trusted for information about reproductive and sexual health, Ms. Kayser said. Yet in her experience, she said, health care professionals are hesitant to bring up the issues with all youth, “especially those with intellectual and developmental disabilities.”
Health care providers often talk both to the patient and to the parents. Those conversations can be critical when a child is developmentally or intellectually disabled.
Women with disabilities have been shown to have a higher risk for adverse outcomes of pregnancy, said Willi Horner-Johnson, PhD, associate professor at OHSU–Portland State University School of Public Health.
In a recent study, she and her colleagues analyzed data from the CDC’s National Survey of Family Growth that included self-reported disability status. They found that the number of women with disabilities who give birth is far higher than was previously thought.
The researchers found that 19.5% of respondents who gave birth reported at least one sensory, cognitive, or mobility-related disability, a rate that is much greater than the less than 1%-6.6% estimates that are based on hospital discharge data.
Her group reported other troubling findings: Women with disabilities are twice as likely to have smoked during their pregnancy (19% vs. 8.9%) and are more likely to have preterm and low-birthweight babies.
Clinicians play an important role
Dr. Horner-Johnson agreed with the finding from the Multnomah County survey that health care providers play an important role in providing those with intellectual and developmental disabilities reproductive health care that meets their needs. “Clinicians need to be asking people with disabilities about their reproductive plans,” she said.
In the Multnomah County report, the researchers advised health care providers to recognize that people with disabilities are social and sexual beings; to learn about their goals, including those regarding sex and reproductive health; and to help youth build skills for healthy relationships and sexual activity.
Dr. Horner-Johnson pointed out that the American College of Obstetricians and Gynecologists “recommends that clinicians discuss reproductive plans at every visit, for example, by asking one key question – ‘Would you like to become pregnant in the next year?’ – of every woman of reproductive age.”
Some women will not be able to answer that question, and health care providers at times must rely on a caregiver for input. But many women, even those with disabilities, could answer if given a chance. She estimated that only about 5% of disabled people are unable to communicate. “Clinicians defer to the caregiver more than they need to,” she said.
Clinicians are becoming better at providing care to those with disabilities, Dr. Horner-Johnson said, yet they have a way to go. Clinician biases may prevent some from asking all women, including those with disabilities, about their reproductive plans. “Women with disabilities have described clinicians treating them as nonsexual, assuming or implying that they would not or should not get pregnant,” she writes in her report.
Such biases, she said, could be reduced by increased education of providers. A 2018 study in Health Equity found that only 19.3% of ob.gyns. said they felt equipped to manage the pregnancy of a woman with disabilities.
Managing sexuality and sexual health for youth with disabilities can be highly complex, according to Margaret Thew, DNP, medical director of adolescent medicine at Children’s Wisconsin, Milwaukee. Challenges include the following:
- Parents often can’t deal with the reality that their teen or young adult is sexually active or may become so. Parents she helps often prefer to use the term “hormones,” not contraceptives, when talking about pregnancy prevention.
- Menstruation is a frequent concern, especially for youth with severe disabilities. Some react strongly to seeing a sanitary pad with blood, for example, by throwing it. Parents worry that caregivers will balk at changing pads regularly. As a result, some parents want complete menstrual suppression, Dr. Thew said. The American Academy of Pediatrics outlines how to approach menstrual suppression through methods such as the use of estrogen-progestin, progesterone, a ring, or a patch. In late August, the American College of Obstetricians and Gynecologists released its clinical consensus on medical management of menstrual suppression.
- Some parents want to know how to obtain a complete hysterectomy for the patient – an option Dr. Thew and the AAP discourage. “We will tell them that’s not the best and safest approach, as you want to have the estrogen for bone health,” she said.
- After a discussion of all the options, an intrauterine device proves best for many. “That gives 7-8 years of protection,” she said, which is the approved effective duration for such devices. “They are less apt to have heavy monthly menstrual bleeding.”
- Parents of boys with disabilities, especially those with Down syndrome, often ask for sex education and guidance when sexual desires develop.
- Many parents want effective birth control for their children because of fear that their teen or young adult will be assaulted, a fear that isn’t groundless. Such cases are common, and caregivers frequently are the perpetrators.
Ms. Kayser, Dr. Horner-Johnson, and Dr. Thew have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The developmentally disabled girl was just 10 years old when Margaret Thew, DNP, medical director of adolescent medicine at Children’s Wisconsin, Milwaukee, helped care for her. Providing that care was not emotionally easy. “Her brother’s friend sexually assaulted her and impregnated her,” Dr. Thew said.
The girl was able to obtain an abortion, a decision her parents supported. The alternative could have been deadly. “She was a tiny little person and would not have been able to carry a fetus,” Dr. Thew, a nurse practitioner, said.
Dr. Thew said she’s thankful that tragic case occurred before 2022. After the United States Supreme Court overturned Roe v. Wade in June, Wisconsin reverted to an 1849 law banning abortion. Although the law is currently being challenged, Dr. Thew wonders how the situation would have played out now. (Weeks after the Supreme Court’s decision, a similar case occurred in Ohio. In that case, a 10-year-old girl had to travel out of the state to obtain an abortion after having been raped.)
Talking to adolescents and young adults about reproductive health, whether regarding an unexpected pregnancy, the need for contraception, or to provide information about sexual activity, can be a challenge even for experienced health care providers.
The talks, decisions, and care are particularly complex when patients have developmental and intellectual disabilities. Among the many factors, Dr. Thew said, are dealing with menstruation, finding the right contraceptives, and counseling parents who might not want to acknowledge their children’s emerging sexuality.
Statistics: How many?
Because the definitions of disabilities vary and they represent a spectrum, estimates for how many youth have intellectual or developmental disabilities range widely.
In 2019, the National Survey of Children’s Health found that 1 in 4 children and adolescents aged 12-17 years have special health care needs because of disability. The American Community Survey estimates more than 1.3 million people aged 16-20 have a disability.
Intellectual disabilities can occur when a person’s IQ is below 70, significantly impeding the ability to perform activities of daily living, such as eating, dressing, and communicating. Developmental disabilities are impairments in physical, learning, language, and behavior, according to the United States Centers for Disease Control and Prevention. Among the conditions are attention-deficit/hyperactivity disorder, autism spectrum disorders, fragile X syndrome, learning and language problems, spina bifida, and other conditions.
Addressing common issues, concerns
April Kayser is a health educator for the Multnomah County Health Department, Portland, Ore. In 2016, Ms. Kayser and other experts conducted interviews with 11 youth with developmental and intellectual disabilities and 34 support people, either parents or professionals who provide services. The survey was part of the SHEIDD Project – short for Sexual Health Equity for Individuals with Intellectual/Developmental Disabilities – at Oregon Health and Science University (OHSU).
From their findings, the researchers compiled guidelines. They provided scenarios that health care providers need to be aware of and that they need to be ready to address:
- A boy, 14, who is unclear about what to do when he feels sexually excited and wants to masturbate but isn’t at home. He has been told that masturbation is appropriate in private.
- A 20-year-old woman who lives in a group home is pregnant. She confesses to her parents during a visit that another resident is her boyfriend and that he is the father of the child she is expecting.
- A 17-year-old boy wants to ask out another student, who is 15.
Some developmentally and intellectually disabled youth can’t turn to their parents for help. One person in the survey said his father told him, “You don’t need to worry about any of that stuff. You’re too young.” Another said the job of a health care provider was to offer reproductive and sex education “to make sure you don’t screw up in some bad way.”
One finding stood out: Health care providers were at the top of the list of those whom young people trusted for information about reproductive and sexual health, Ms. Kayser said. Yet in her experience, she said, health care professionals are hesitant to bring up the issues with all youth, “especially those with intellectual and developmental disabilities.”
Health care providers often talk both to the patient and to the parents. Those conversations can be critical when a child is developmentally or intellectually disabled.
Women with disabilities have been shown to have a higher risk for adverse outcomes of pregnancy, said Willi Horner-Johnson, PhD, associate professor at OHSU–Portland State University School of Public Health.
In a recent study, she and her colleagues analyzed data from the CDC’s National Survey of Family Growth that included self-reported disability status. They found that the number of women with disabilities who give birth is far higher than was previously thought.
The researchers found that 19.5% of respondents who gave birth reported at least one sensory, cognitive, or mobility-related disability, a rate that is much greater than the less than 1%-6.6% estimates that are based on hospital discharge data.
Her group reported other troubling findings: Women with disabilities are twice as likely to have smoked during their pregnancy (19% vs. 8.9%) and are more likely to have preterm and low-birthweight babies.
Clinicians play an important role
Dr. Horner-Johnson agreed with the finding from the Multnomah County survey that health care providers play an important role in providing those with intellectual and developmental disabilities reproductive health care that meets their needs. “Clinicians need to be asking people with disabilities about their reproductive plans,” she said.
In the Multnomah County report, the researchers advised health care providers to recognize that people with disabilities are social and sexual beings; to learn about their goals, including those regarding sex and reproductive health; and to help youth build skills for healthy relationships and sexual activity.
Dr. Horner-Johnson pointed out that the American College of Obstetricians and Gynecologists “recommends that clinicians discuss reproductive plans at every visit, for example, by asking one key question – ‘Would you like to become pregnant in the next year?’ – of every woman of reproductive age.”
Some women will not be able to answer that question, and health care providers at times must rely on a caregiver for input. But many women, even those with disabilities, could answer if given a chance. She estimated that only about 5% of disabled people are unable to communicate. “Clinicians defer to the caregiver more than they need to,” she said.
Clinicians are becoming better at providing care to those with disabilities, Dr. Horner-Johnson said, yet they have a way to go. Clinician biases may prevent some from asking all women, including those with disabilities, about their reproductive plans. “Women with disabilities have described clinicians treating them as nonsexual, assuming or implying that they would not or should not get pregnant,” she writes in her report.
Such biases, she said, could be reduced by increased education of providers. A 2018 study in Health Equity found that only 19.3% of ob.gyns. said they felt equipped to manage the pregnancy of a woman with disabilities.
Managing sexuality and sexual health for youth with disabilities can be highly complex, according to Margaret Thew, DNP, medical director of adolescent medicine at Children’s Wisconsin, Milwaukee. Challenges include the following:
- Parents often can’t deal with the reality that their teen or young adult is sexually active or may become so. Parents she helps often prefer to use the term “hormones,” not contraceptives, when talking about pregnancy prevention.
- Menstruation is a frequent concern, especially for youth with severe disabilities. Some react strongly to seeing a sanitary pad with blood, for example, by throwing it. Parents worry that caregivers will balk at changing pads regularly. As a result, some parents want complete menstrual suppression, Dr. Thew said. The American Academy of Pediatrics outlines how to approach menstrual suppression through methods such as the use of estrogen-progestin, progesterone, a ring, or a patch. In late August, the American College of Obstetricians and Gynecologists released its clinical consensus on medical management of menstrual suppression.
- Some parents want to know how to obtain a complete hysterectomy for the patient – an option Dr. Thew and the AAP discourage. “We will tell them that’s not the best and safest approach, as you want to have the estrogen for bone health,” she said.
- After a discussion of all the options, an intrauterine device proves best for many. “That gives 7-8 years of protection,” she said, which is the approved effective duration for such devices. “They are less apt to have heavy monthly menstrual bleeding.”
- Parents of boys with disabilities, especially those with Down syndrome, often ask for sex education and guidance when sexual desires develop.
- Many parents want effective birth control for their children because of fear that their teen or young adult will be assaulted, a fear that isn’t groundless. Such cases are common, and caregivers frequently are the perpetrators.
Ms. Kayser, Dr. Horner-Johnson, and Dr. Thew have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The developmentally disabled girl was just 10 years old when Margaret Thew, DNP, medical director of adolescent medicine at Children’s Wisconsin, Milwaukee, helped care for her. Providing that care was not emotionally easy. “Her brother’s friend sexually assaulted her and impregnated her,” Dr. Thew said.
The girl was able to obtain an abortion, a decision her parents supported. The alternative could have been deadly. “She was a tiny little person and would not have been able to carry a fetus,” Dr. Thew, a nurse practitioner, said.
Dr. Thew said she’s thankful that tragic case occurred before 2022. After the United States Supreme Court overturned Roe v. Wade in June, Wisconsin reverted to an 1849 law banning abortion. Although the law is currently being challenged, Dr. Thew wonders how the situation would have played out now. (Weeks after the Supreme Court’s decision, a similar case occurred in Ohio. In that case, a 10-year-old girl had to travel out of the state to obtain an abortion after having been raped.)
Talking to adolescents and young adults about reproductive health, whether regarding an unexpected pregnancy, the need for contraception, or to provide information about sexual activity, can be a challenge even for experienced health care providers.
The talks, decisions, and care are particularly complex when patients have developmental and intellectual disabilities. Among the many factors, Dr. Thew said, are dealing with menstruation, finding the right contraceptives, and counseling parents who might not want to acknowledge their children’s emerging sexuality.
Statistics: How many?
Because the definitions of disabilities vary and they represent a spectrum, estimates for how many youth have intellectual or developmental disabilities range widely.
In 2019, the National Survey of Children’s Health found that 1 in 4 children and adolescents aged 12-17 years have special health care needs because of disability. The American Community Survey estimates more than 1.3 million people aged 16-20 have a disability.
Intellectual disabilities can occur when a person’s IQ is below 70, significantly impeding the ability to perform activities of daily living, such as eating, dressing, and communicating. Developmental disabilities are impairments in physical, learning, language, and behavior, according to the United States Centers for Disease Control and Prevention. Among the conditions are attention-deficit/hyperactivity disorder, autism spectrum disorders, fragile X syndrome, learning and language problems, spina bifida, and other conditions.
Addressing common issues, concerns
April Kayser is a health educator for the Multnomah County Health Department, Portland, Ore. In 2016, Ms. Kayser and other experts conducted interviews with 11 youth with developmental and intellectual disabilities and 34 support people, either parents or professionals who provide services. The survey was part of the SHEIDD Project – short for Sexual Health Equity for Individuals with Intellectual/Developmental Disabilities – at Oregon Health and Science University (OHSU).
From their findings, the researchers compiled guidelines. They provided scenarios that health care providers need to be aware of and that they need to be ready to address:
- A boy, 14, who is unclear about what to do when he feels sexually excited and wants to masturbate but isn’t at home. He has been told that masturbation is appropriate in private.
- A 20-year-old woman who lives in a group home is pregnant. She confesses to her parents during a visit that another resident is her boyfriend and that he is the father of the child she is expecting.
- A 17-year-old boy wants to ask out another student, who is 15.
Some developmentally and intellectually disabled youth can’t turn to their parents for help. One person in the survey said his father told him, “You don’t need to worry about any of that stuff. You’re too young.” Another said the job of a health care provider was to offer reproductive and sex education “to make sure you don’t screw up in some bad way.”
One finding stood out: Health care providers were at the top of the list of those whom young people trusted for information about reproductive and sexual health, Ms. Kayser said. Yet in her experience, she said, health care professionals are hesitant to bring up the issues with all youth, “especially those with intellectual and developmental disabilities.”
Health care providers often talk both to the patient and to the parents. Those conversations can be critical when a child is developmentally or intellectually disabled.
Women with disabilities have been shown to have a higher risk for adverse outcomes of pregnancy, said Willi Horner-Johnson, PhD, associate professor at OHSU–Portland State University School of Public Health.
In a recent study, she and her colleagues analyzed data from the CDC’s National Survey of Family Growth that included self-reported disability status. They found that the number of women with disabilities who give birth is far higher than was previously thought.
The researchers found that 19.5% of respondents who gave birth reported at least one sensory, cognitive, or mobility-related disability, a rate that is much greater than the less than 1%-6.6% estimates that are based on hospital discharge data.
Her group reported other troubling findings: Women with disabilities are twice as likely to have smoked during their pregnancy (19% vs. 8.9%) and are more likely to have preterm and low-birthweight babies.
Clinicians play an important role
Dr. Horner-Johnson agreed with the finding from the Multnomah County survey that health care providers play an important role in providing those with intellectual and developmental disabilities reproductive health care that meets their needs. “Clinicians need to be asking people with disabilities about their reproductive plans,” she said.
In the Multnomah County report, the researchers advised health care providers to recognize that people with disabilities are social and sexual beings; to learn about their goals, including those regarding sex and reproductive health; and to help youth build skills for healthy relationships and sexual activity.
Dr. Horner-Johnson pointed out that the American College of Obstetricians and Gynecologists “recommends that clinicians discuss reproductive plans at every visit, for example, by asking one key question – ‘Would you like to become pregnant in the next year?’ – of every woman of reproductive age.”
Some women will not be able to answer that question, and health care providers at times must rely on a caregiver for input. But many women, even those with disabilities, could answer if given a chance. She estimated that only about 5% of disabled people are unable to communicate. “Clinicians defer to the caregiver more than they need to,” she said.
Clinicians are becoming better at providing care to those with disabilities, Dr. Horner-Johnson said, yet they have a way to go. Clinician biases may prevent some from asking all women, including those with disabilities, about their reproductive plans. “Women with disabilities have described clinicians treating them as nonsexual, assuming or implying that they would not or should not get pregnant,” she writes in her report.
Such biases, she said, could be reduced by increased education of providers. A 2018 study in Health Equity found that only 19.3% of ob.gyns. said they felt equipped to manage the pregnancy of a woman with disabilities.
Managing sexuality and sexual health for youth with disabilities can be highly complex, according to Margaret Thew, DNP, medical director of adolescent medicine at Children’s Wisconsin, Milwaukee. Challenges include the following:
- Parents often can’t deal with the reality that their teen or young adult is sexually active or may become so. Parents she helps often prefer to use the term “hormones,” not contraceptives, when talking about pregnancy prevention.
- Menstruation is a frequent concern, especially for youth with severe disabilities. Some react strongly to seeing a sanitary pad with blood, for example, by throwing it. Parents worry that caregivers will balk at changing pads regularly. As a result, some parents want complete menstrual suppression, Dr. Thew said. The American Academy of Pediatrics outlines how to approach menstrual suppression through methods such as the use of estrogen-progestin, progesterone, a ring, or a patch. In late August, the American College of Obstetricians and Gynecologists released its clinical consensus on medical management of menstrual suppression.
- Some parents want to know how to obtain a complete hysterectomy for the patient – an option Dr. Thew and the AAP discourage. “We will tell them that’s not the best and safest approach, as you want to have the estrogen for bone health,” she said.
- After a discussion of all the options, an intrauterine device proves best for many. “That gives 7-8 years of protection,” she said, which is the approved effective duration for such devices. “They are less apt to have heavy monthly menstrual bleeding.”
- Parents of boys with disabilities, especially those with Down syndrome, often ask for sex education and guidance when sexual desires develop.
- Many parents want effective birth control for their children because of fear that their teen or young adult will be assaulted, a fear that isn’t groundless. Such cases are common, and caregivers frequently are the perpetrators.
Ms. Kayser, Dr. Horner-Johnson, and Dr. Thew have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Dermatologists fear effects of Dobbs decision for patients on isotretinoin, methotrexate
More than 3 months after the Dobbs decision by the U.S. Supreme Court overturned Roe v. Wade and revoked the constitutional right to an abortion, Some have beefed up their already stringent instructions and lengthy conversations about avoiding pregnancy while on the medication.
The major fear is that a patient who is taking contraceptive precautions, in accordance with the isotretinoin risk-management program, iPLEDGE, but still becomes pregnant while on isotretinoin may find out about the pregnancy too late to undergo an abortion in her own state and may not be able to travel to another state – or the patient may live in a state where abortions are entirely prohibited and is unable to travel to another state.
Isotretinoin is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane; its former brand name was Accutane.
As of Oct. 7, a total of 14 states have banned most abortions, while 4 others have bans at 6, 15, 18, or 20 weeks. Attempts to restrict abortion on several other states are underway.
“To date, we don’t know of any specific effects of the Dobbs decision on isotretinoin prescribing, but with abortion access banned in many states, we anticipate that this could be a very real issue for individuals who accidentally become pregnant while taking isotretinoin,” said Ilona Frieden, MD, professor of dermatology and pediatrics at the University of California, San Francisco, and chair of the American Academy of Dermatology Association’s iPLEDGE Workgroup.
The iPLEDGE REMS (Risk Evaluation and Mitigation Strategy) is the Food and Drug Administration–required safety program that is in place to manage the risk of isotretinoin teratogenicity and minimize fetal exposure. The work group meets with the FDA and isotretinoin manufacturers to keep the program safe and operating smoothly. The iPLEDGE workgroup has not yet issued any specific statements on the implications of the Dobbs decision on prescribing isotretinoin.
But work on the issue is ongoing by the American Academy of Dermatology. In a statement issued in September, Mark D. Kaufmann, MD, president of the AAD, said that the academy “is continuing to work with its Patient Guidance for State Regulations Regarding Reproductive Health Task Force to help dermatologists best navigate state laws about how care should be implemented for patients who are or might become pregnant, and have been exposed to teratogenic medications.”
The task force, working with the academy, is “in the process of developing resources to help members better assist patients and have a productive and caring dialogue with them,” according to the statement. No specific timeline was given for when those resources might be available.
Methotrexate prescriptions
Also of concern are prescriptions for methotrexate, which is prescribed for psoriasis, atopic dermatitis, and other skin diseases. Soon after the Dobbs decision was announced on June 24, pharmacies began to require pharmacists in states that banned abortions to verify that a prescription for methotrexate was not intended for an abortion, since methotrexate is used in combination with misoprostol for termination of an early pregnancy.
The action was taken, spokespersons for several major pharmacies said, to comply with state laws. According to Kara Page, a CVS spokesperson: “Pharmacists are caught in the middle on this issue.” Laws in some states, she told this news organization, “restrict the dispensing of medications for the purpose of inducing an abortion. These laws, some of which include criminal penalties, have forced us to require pharmacists in these states to validate that the intended indication is not to terminate a pregnancy before they can fill a prescription for methotrexate.”
“New laws in various states require additional steps for dispensing certain prescriptions and apply to all pharmacies, including Walgreens,” Fraser Engerman, a spokesperson for Walgreens, told this news organization. “In these states, our pharmacists work closely with prescribers as needed, to fill lawful, clinically appropriate prescriptions. We provide ongoing training and information to help our pharmacists understand the latest requirements in their area, and with these supports, the expectation is they are empowered to fill these prescriptions.”
The iPLEDGE program has numerous requirements before a patient can begin isotretinoin treatment. Patients capable of becoming pregnant must agree to use two effective forms of birth control during the entire treatment period, which typically lasts 4 or 5 months, as well as 1 month before and 1 month after treatment, or commit to total abstinence during that time.
Perspective: A Georgia dermatologist
Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, who sees patients regularly, practices in Georgia, where abortion is now banned at about 6 weeks of pregnancy. Dr. Yeung worries that some dermatologists in Georgia and elsewhere may not even want to take the risk of prescribing isotretinoin, although the results in treating resistant acne are well documented.
That isn’t his only concern. “Some may not want to prescribe it to a patient who reports they are abstinent and instead require them to go on two forms [of contraception].” Or some women who are not sexually active with anyone who can get them pregnant may also be asked to go on contraception, he said. Abstinence is an alternative option in iPLEDGE.
In the past, he said, well before the Dobbs decision, some doctors have argued that iPLEDGE should not include abstinence as an option. That 2020 report was challenged by others who pointed out that removing the abstinence option would pose ethical issues and may disproportionately affect minorities and others.
Before the Dobbs decision, Dr. Yeung noted, dermatologists prescribing isotretinoin focused on pregnancy prevention but knew that if pregnancy accidentally occurred, abortion was available as an option. “The reality after the decision is, it may or may not be available to all our patients.”
Of the 14 states banning most abortions, 10 are clustered within the South and Southeast. A woman living in Arkansas, which bans most abortions, for example, is surrounded by 6 other states that do the same.
Perspective: An Arizona dermatologist
Christina Kranc, MD, is a general dermatologist in Phoenix and Scottsdale. Arizona now bans most abortions. However, this has not changed her practice much when prescribing isotretinoin, she told this news organization, because when selecting appropriate candidates for the medication, she is strict on the contraceptive requirement, and only very rarely agrees to a patient relying on abstinence.
And if a patient capable of becoming pregnant was only having sex with another patient capable of becoming pregnant? Dr. Kranc said she would still require contraception unless it was impossible for pregnancy to occur.
Among the many scenarios a dermatologist might have to consider are a lesbian cisgender woman who is having, or has only had, sexual activity with another cisgender women.
Perspective: A Connecticut dermatologist
The concern is not only about isotretinoin but all teratogenic drugs, according to Jane M. Grant-Kels, MD, vice chair of dermatology and professor of dermatology, pathology, and pediatrics at the University of Connecticut, Farmington. She often prescribes methotrexate, which is also teratogenic.
Her advice for colleagues: “Whether you believe in abortion or not is irrelevant; it’s something you discuss with your patients.” She, too, fears that doctors in states banning abortions will stop prescribing these medications, “and that is very sad.”
For those practicing in states limiting or banning abortions, Dr. Grant-Kels said, “They need to have an even longer discussion with their patients about how serious this is.” Those doctors need to talk about not only two or three types of birth control, but also discuss with the patient about the potential need for travel, should pregnancy occur and abortion be the chosen option.
Although the newer biologics are an option for psoriasis, they are expensive. And, she said, many insurers require a step-therapy approach, and “want you to start with cheaper medications,” such as methotrexate. As a result, “in some states you won’t have access to the targeted therapies unless a patient fails something like methotrexate.”
Dr. Grant-Kels worries in particular about low-income women who may not have the means to travel to get an abortion.
Need for EC education
In a recent survey of 57 pediatric dermatologists who prescribe isotretinoin, only a third said they felt confident in their understanding of emergency contraception.
The authors of the study noted that the most common reasons for pregnancies during isotretinoin therapy reported to the FDA from 2011 to 2017 “included ineffective or inconsistent use” of contraceptives and “unsuccessful abstinence,” and recommended that physicians who prescribe isotretinoin update and increase their understanding of emergency contraception.
Dr. Yeung, Dr. Kranc, Dr. Grant-Kels, and Dr. Frieden reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
More than 3 months after the Dobbs decision by the U.S. Supreme Court overturned Roe v. Wade and revoked the constitutional right to an abortion, Some have beefed up their already stringent instructions and lengthy conversations about avoiding pregnancy while on the medication.
The major fear is that a patient who is taking contraceptive precautions, in accordance with the isotretinoin risk-management program, iPLEDGE, but still becomes pregnant while on isotretinoin may find out about the pregnancy too late to undergo an abortion in her own state and may not be able to travel to another state – or the patient may live in a state where abortions are entirely prohibited and is unable to travel to another state.
Isotretinoin is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane; its former brand name was Accutane.
As of Oct. 7, a total of 14 states have banned most abortions, while 4 others have bans at 6, 15, 18, or 20 weeks. Attempts to restrict abortion on several other states are underway.
“To date, we don’t know of any specific effects of the Dobbs decision on isotretinoin prescribing, but with abortion access banned in many states, we anticipate that this could be a very real issue for individuals who accidentally become pregnant while taking isotretinoin,” said Ilona Frieden, MD, professor of dermatology and pediatrics at the University of California, San Francisco, and chair of the American Academy of Dermatology Association’s iPLEDGE Workgroup.
The iPLEDGE REMS (Risk Evaluation and Mitigation Strategy) is the Food and Drug Administration–required safety program that is in place to manage the risk of isotretinoin teratogenicity and minimize fetal exposure. The work group meets with the FDA and isotretinoin manufacturers to keep the program safe and operating smoothly. The iPLEDGE workgroup has not yet issued any specific statements on the implications of the Dobbs decision on prescribing isotretinoin.
But work on the issue is ongoing by the American Academy of Dermatology. In a statement issued in September, Mark D. Kaufmann, MD, president of the AAD, said that the academy “is continuing to work with its Patient Guidance for State Regulations Regarding Reproductive Health Task Force to help dermatologists best navigate state laws about how care should be implemented for patients who are or might become pregnant, and have been exposed to teratogenic medications.”
The task force, working with the academy, is “in the process of developing resources to help members better assist patients and have a productive and caring dialogue with them,” according to the statement. No specific timeline was given for when those resources might be available.
Methotrexate prescriptions
Also of concern are prescriptions for methotrexate, which is prescribed for psoriasis, atopic dermatitis, and other skin diseases. Soon after the Dobbs decision was announced on June 24, pharmacies began to require pharmacists in states that banned abortions to verify that a prescription for methotrexate was not intended for an abortion, since methotrexate is used in combination with misoprostol for termination of an early pregnancy.
The action was taken, spokespersons for several major pharmacies said, to comply with state laws. According to Kara Page, a CVS spokesperson: “Pharmacists are caught in the middle on this issue.” Laws in some states, she told this news organization, “restrict the dispensing of medications for the purpose of inducing an abortion. These laws, some of which include criminal penalties, have forced us to require pharmacists in these states to validate that the intended indication is not to terminate a pregnancy before they can fill a prescription for methotrexate.”
“New laws in various states require additional steps for dispensing certain prescriptions and apply to all pharmacies, including Walgreens,” Fraser Engerman, a spokesperson for Walgreens, told this news organization. “In these states, our pharmacists work closely with prescribers as needed, to fill lawful, clinically appropriate prescriptions. We provide ongoing training and information to help our pharmacists understand the latest requirements in their area, and with these supports, the expectation is they are empowered to fill these prescriptions.”
The iPLEDGE program has numerous requirements before a patient can begin isotretinoin treatment. Patients capable of becoming pregnant must agree to use two effective forms of birth control during the entire treatment period, which typically lasts 4 or 5 months, as well as 1 month before and 1 month after treatment, or commit to total abstinence during that time.
Perspective: A Georgia dermatologist
Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, who sees patients regularly, practices in Georgia, where abortion is now banned at about 6 weeks of pregnancy. Dr. Yeung worries that some dermatologists in Georgia and elsewhere may not even want to take the risk of prescribing isotretinoin, although the results in treating resistant acne are well documented.
That isn’t his only concern. “Some may not want to prescribe it to a patient who reports they are abstinent and instead require them to go on two forms [of contraception].” Or some women who are not sexually active with anyone who can get them pregnant may also be asked to go on contraception, he said. Abstinence is an alternative option in iPLEDGE.
In the past, he said, well before the Dobbs decision, some doctors have argued that iPLEDGE should not include abstinence as an option. That 2020 report was challenged by others who pointed out that removing the abstinence option would pose ethical issues and may disproportionately affect minorities and others.
Before the Dobbs decision, Dr. Yeung noted, dermatologists prescribing isotretinoin focused on pregnancy prevention but knew that if pregnancy accidentally occurred, abortion was available as an option. “The reality after the decision is, it may or may not be available to all our patients.”
Of the 14 states banning most abortions, 10 are clustered within the South and Southeast. A woman living in Arkansas, which bans most abortions, for example, is surrounded by 6 other states that do the same.
Perspective: An Arizona dermatologist
Christina Kranc, MD, is a general dermatologist in Phoenix and Scottsdale. Arizona now bans most abortions. However, this has not changed her practice much when prescribing isotretinoin, she told this news organization, because when selecting appropriate candidates for the medication, she is strict on the contraceptive requirement, and only very rarely agrees to a patient relying on abstinence.
And if a patient capable of becoming pregnant was only having sex with another patient capable of becoming pregnant? Dr. Kranc said she would still require contraception unless it was impossible for pregnancy to occur.
Among the many scenarios a dermatologist might have to consider are a lesbian cisgender woman who is having, or has only had, sexual activity with another cisgender women.
Perspective: A Connecticut dermatologist
The concern is not only about isotretinoin but all teratogenic drugs, according to Jane M. Grant-Kels, MD, vice chair of dermatology and professor of dermatology, pathology, and pediatrics at the University of Connecticut, Farmington. She often prescribes methotrexate, which is also teratogenic.
Her advice for colleagues: “Whether you believe in abortion or not is irrelevant; it’s something you discuss with your patients.” She, too, fears that doctors in states banning abortions will stop prescribing these medications, “and that is very sad.”
For those practicing in states limiting or banning abortions, Dr. Grant-Kels said, “They need to have an even longer discussion with their patients about how serious this is.” Those doctors need to talk about not only two or three types of birth control, but also discuss with the patient about the potential need for travel, should pregnancy occur and abortion be the chosen option.
Although the newer biologics are an option for psoriasis, they are expensive. And, she said, many insurers require a step-therapy approach, and “want you to start with cheaper medications,” such as methotrexate. As a result, “in some states you won’t have access to the targeted therapies unless a patient fails something like methotrexate.”
Dr. Grant-Kels worries in particular about low-income women who may not have the means to travel to get an abortion.
Need for EC education
In a recent survey of 57 pediatric dermatologists who prescribe isotretinoin, only a third said they felt confident in their understanding of emergency contraception.
The authors of the study noted that the most common reasons for pregnancies during isotretinoin therapy reported to the FDA from 2011 to 2017 “included ineffective or inconsistent use” of contraceptives and “unsuccessful abstinence,” and recommended that physicians who prescribe isotretinoin update and increase their understanding of emergency contraception.
Dr. Yeung, Dr. Kranc, Dr. Grant-Kels, and Dr. Frieden reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
More than 3 months after the Dobbs decision by the U.S. Supreme Court overturned Roe v. Wade and revoked the constitutional right to an abortion, Some have beefed up their already stringent instructions and lengthy conversations about avoiding pregnancy while on the medication.
The major fear is that a patient who is taking contraceptive precautions, in accordance with the isotretinoin risk-management program, iPLEDGE, but still becomes pregnant while on isotretinoin may find out about the pregnancy too late to undergo an abortion in her own state and may not be able to travel to another state – or the patient may live in a state where abortions are entirely prohibited and is unable to travel to another state.
Isotretinoin is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane; its former brand name was Accutane.
As of Oct. 7, a total of 14 states have banned most abortions, while 4 others have bans at 6, 15, 18, or 20 weeks. Attempts to restrict abortion on several other states are underway.
“To date, we don’t know of any specific effects of the Dobbs decision on isotretinoin prescribing, but with abortion access banned in many states, we anticipate that this could be a very real issue for individuals who accidentally become pregnant while taking isotretinoin,” said Ilona Frieden, MD, professor of dermatology and pediatrics at the University of California, San Francisco, and chair of the American Academy of Dermatology Association’s iPLEDGE Workgroup.
The iPLEDGE REMS (Risk Evaluation and Mitigation Strategy) is the Food and Drug Administration–required safety program that is in place to manage the risk of isotretinoin teratogenicity and minimize fetal exposure. The work group meets with the FDA and isotretinoin manufacturers to keep the program safe and operating smoothly. The iPLEDGE workgroup has not yet issued any specific statements on the implications of the Dobbs decision on prescribing isotretinoin.
But work on the issue is ongoing by the American Academy of Dermatology. In a statement issued in September, Mark D. Kaufmann, MD, president of the AAD, said that the academy “is continuing to work with its Patient Guidance for State Regulations Regarding Reproductive Health Task Force to help dermatologists best navigate state laws about how care should be implemented for patients who are or might become pregnant, and have been exposed to teratogenic medications.”
The task force, working with the academy, is “in the process of developing resources to help members better assist patients and have a productive and caring dialogue with them,” according to the statement. No specific timeline was given for when those resources might be available.
Methotrexate prescriptions
Also of concern are prescriptions for methotrexate, which is prescribed for psoriasis, atopic dermatitis, and other skin diseases. Soon after the Dobbs decision was announced on June 24, pharmacies began to require pharmacists in states that banned abortions to verify that a prescription for methotrexate was not intended for an abortion, since methotrexate is used in combination with misoprostol for termination of an early pregnancy.
The action was taken, spokespersons for several major pharmacies said, to comply with state laws. According to Kara Page, a CVS spokesperson: “Pharmacists are caught in the middle on this issue.” Laws in some states, she told this news organization, “restrict the dispensing of medications for the purpose of inducing an abortion. These laws, some of which include criminal penalties, have forced us to require pharmacists in these states to validate that the intended indication is not to terminate a pregnancy before they can fill a prescription for methotrexate.”
“New laws in various states require additional steps for dispensing certain prescriptions and apply to all pharmacies, including Walgreens,” Fraser Engerman, a spokesperson for Walgreens, told this news organization. “In these states, our pharmacists work closely with prescribers as needed, to fill lawful, clinically appropriate prescriptions. We provide ongoing training and information to help our pharmacists understand the latest requirements in their area, and with these supports, the expectation is they are empowered to fill these prescriptions.”
The iPLEDGE program has numerous requirements before a patient can begin isotretinoin treatment. Patients capable of becoming pregnant must agree to use two effective forms of birth control during the entire treatment period, which typically lasts 4 or 5 months, as well as 1 month before and 1 month after treatment, or commit to total abstinence during that time.
Perspective: A Georgia dermatologist
Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, who sees patients regularly, practices in Georgia, where abortion is now banned at about 6 weeks of pregnancy. Dr. Yeung worries that some dermatologists in Georgia and elsewhere may not even want to take the risk of prescribing isotretinoin, although the results in treating resistant acne are well documented.
That isn’t his only concern. “Some may not want to prescribe it to a patient who reports they are abstinent and instead require them to go on two forms [of contraception].” Or some women who are not sexually active with anyone who can get them pregnant may also be asked to go on contraception, he said. Abstinence is an alternative option in iPLEDGE.
In the past, he said, well before the Dobbs decision, some doctors have argued that iPLEDGE should not include abstinence as an option. That 2020 report was challenged by others who pointed out that removing the abstinence option would pose ethical issues and may disproportionately affect minorities and others.
Before the Dobbs decision, Dr. Yeung noted, dermatologists prescribing isotretinoin focused on pregnancy prevention but knew that if pregnancy accidentally occurred, abortion was available as an option. “The reality after the decision is, it may or may not be available to all our patients.”
Of the 14 states banning most abortions, 10 are clustered within the South and Southeast. A woman living in Arkansas, which bans most abortions, for example, is surrounded by 6 other states that do the same.
Perspective: An Arizona dermatologist
Christina Kranc, MD, is a general dermatologist in Phoenix and Scottsdale. Arizona now bans most abortions. However, this has not changed her practice much when prescribing isotretinoin, she told this news organization, because when selecting appropriate candidates for the medication, she is strict on the contraceptive requirement, and only very rarely agrees to a patient relying on abstinence.
And if a patient capable of becoming pregnant was only having sex with another patient capable of becoming pregnant? Dr. Kranc said she would still require contraception unless it was impossible for pregnancy to occur.
Among the many scenarios a dermatologist might have to consider are a lesbian cisgender woman who is having, or has only had, sexual activity with another cisgender women.
Perspective: A Connecticut dermatologist
The concern is not only about isotretinoin but all teratogenic drugs, according to Jane M. Grant-Kels, MD, vice chair of dermatology and professor of dermatology, pathology, and pediatrics at the University of Connecticut, Farmington. She often prescribes methotrexate, which is also teratogenic.
Her advice for colleagues: “Whether you believe in abortion or not is irrelevant; it’s something you discuss with your patients.” She, too, fears that doctors in states banning abortions will stop prescribing these medications, “and that is very sad.”
For those practicing in states limiting or banning abortions, Dr. Grant-Kels said, “They need to have an even longer discussion with their patients about how serious this is.” Those doctors need to talk about not only two or three types of birth control, but also discuss with the patient about the potential need for travel, should pregnancy occur and abortion be the chosen option.
Although the newer biologics are an option for psoriasis, they are expensive. And, she said, many insurers require a step-therapy approach, and “want you to start with cheaper medications,” such as methotrexate. As a result, “in some states you won’t have access to the targeted therapies unless a patient fails something like methotrexate.”
Dr. Grant-Kels worries in particular about low-income women who may not have the means to travel to get an abortion.
Need for EC education
In a recent survey of 57 pediatric dermatologists who prescribe isotretinoin, only a third said they felt confident in their understanding of emergency contraception.
The authors of the study noted that the most common reasons for pregnancies during isotretinoin therapy reported to the FDA from 2011 to 2017 “included ineffective or inconsistent use” of contraceptives and “unsuccessful abstinence,” and recommended that physicians who prescribe isotretinoin update and increase their understanding of emergency contraception.
Dr. Yeung, Dr. Kranc, Dr. Grant-Kels, and Dr. Frieden reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.