User login
Hair disorder treatments are evolving
“No matter who the patient is, whether a child, adolescent, or adult, the key to figuring out hair disease is getting a good history,” Maria Hordinsky, MD, professor and chair of the department of dermatology at the University of Minnesota, Minneapolis, said at the Medscape Live Women’s and Pediatric Dermatology Seminar.
. She also urged physicians and other health care providers to use the electronic medical record and to be thorough in documenting information – noting nutrition, hair care habits, supplement use, and other details.
Lab tests should be selected based on that history, she said. For instance, low iron stores can be associated with hair shedding; and thyroid function studies might be needed.
Other highlights of her presentation included comments on different types of alopecia, and some new treatment approaches:
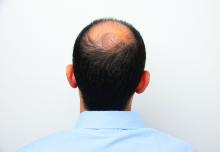
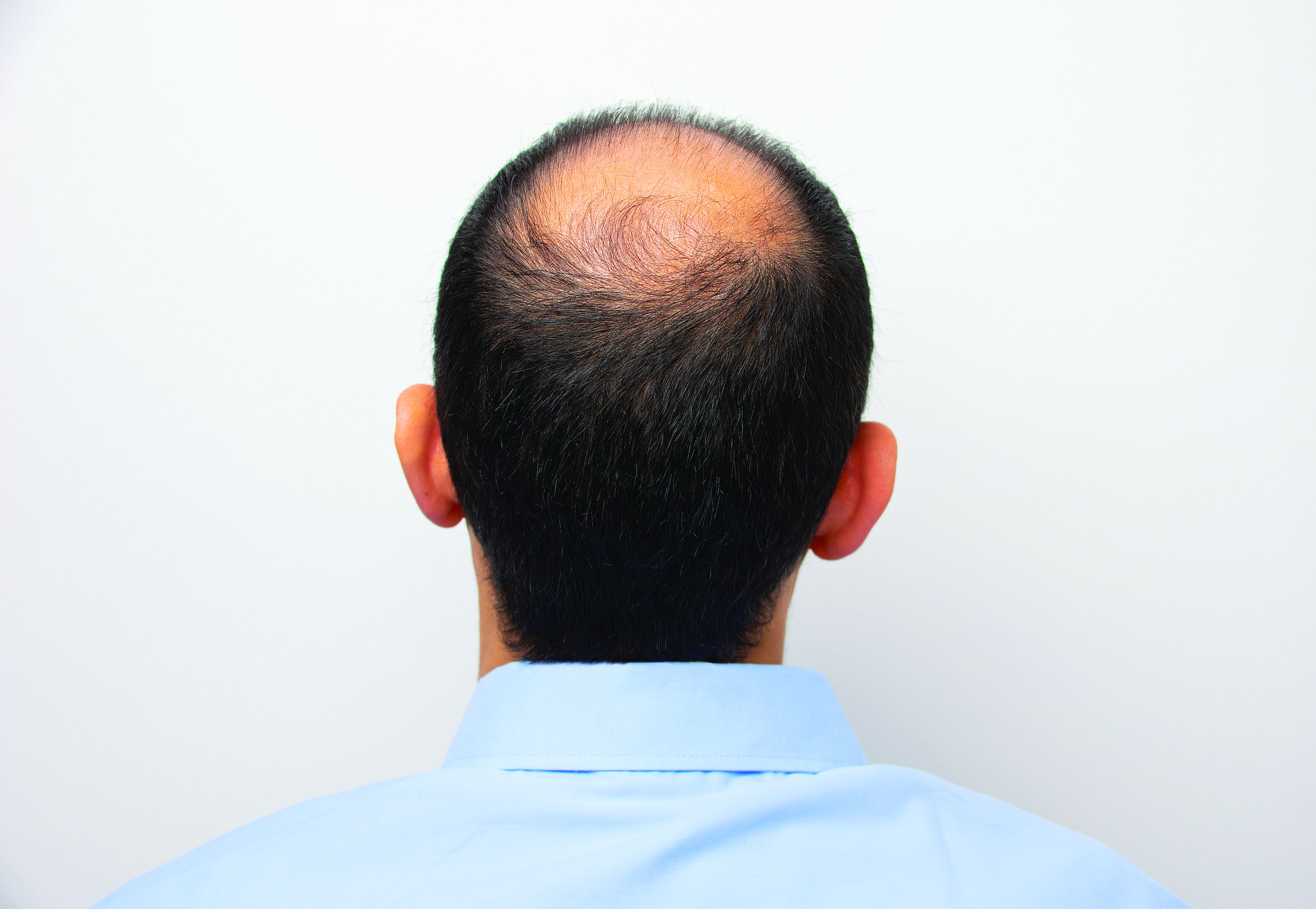
Androgenetic alopecia. In a meta-analysis and systematic review published in 2017, all treatments tested (2% and 5% minoxidil in men, 1 mg finasteride in men, 2% minoxidil in women, and low-level laser light therapy in men) were superior to placebo. Several photobiomodulation (PBM) devices (also known as low-level laser light) for home use have been cleared for androgenetic alopecia by the Food and Drug Administration; a clinician’s guide, published in 2018, provides information on these devices.
Hair and hormones. Combination therapy for female-pattern hair loss – low-dose minoxidil and spironolactone – is important to know about, she said, adding there are data from an observational pilot study supporting this treatment. Women should not become pregnant while on this treatment, Dr. Hordinsky cautioned.
PRP (platelet rich plasma). This treatment for hair loss can be costly, she cautioned, as it’s viewed as a cosmetic technique, “but it actually can work rather well.”
Hair regrowth measures. Traditionally, measures center on global assessment, the patient’s self-assessment, investigator assessment, and an independent photo review. Enter the dermatoscope. “We can now get pictures as a baseline. Patients can see, and also see the health of their scalp,” and if treatments make it look better or worse, she noted.
Alopecia areata (AA). Patients and families need to be made aware that this is an autoimmune disease that can recur, and if it does recur, the extent of hair loss is not predictable. According to Dr. Hordinsky, the most widely used tool to halt disease activity has been treatment with a corticosteroid (topical, intralesional, oral, or even intravenous corticosteroids).
Clinical trials and publications from 2018 to 2020 have triggered interest in off-label use and further studies of JAK inhibitors for treating AA, which include baricitinib, ruxolitinib, and tofacitinib. At the American Academy of Dermatology meeting in March 2022, results of the ALLEGRO phase 2b/3 trial found that the JAK inhibitor ritlecitinib (50 mg or 20 mg daily, with or without a 200-mg loading dose), was efficacious in adults and adolescents with AA, compared with placebo, with no safety concerns noted. “This looks to be very, very promising,” she said, “and also very safe.” Two phase 3 trials of baricitinib also presented at the same meeting found it was superior to placebo for hair regrowth in adults with severe AA at 36 weeks. (On June 13, shortly after Dr. Hordinsky spoke at the meeting, the FDA approved baricitinib for treating AA in adults, making this the first systemic treatment to be approved for AA).
Research on topical JAK inhibitors for AA has been disappointing, Dr. Hordinsky said.
Alopecia areata and atopic dermatitis. For patients with both AA and AD, dupilumab may provide relief, she said. She referred to a recently published phase 2a trial in patients with AA (including some with both AA and AD), which found that Severity of Alopecia Tool (SALT) scores improved after 48 weeks of treatment, with higher response rates among those with baseline IgE levels of 200 IU/mL or higher. “If your patient has both, and their immunoglobulin-E level is greater than 200, then they may be a good candidate for dupilumab and both diseases may respond,” she said.
Scalp symptoms. It can be challenging when patients complain of itch, pain, or burning on the scalp, but have no obvious skin disease, Dr. Hordinsky said. Her tips: Some of these patients may be experiencing scalp symptoms secondary to a neuropathy; others may have mast cell degranulation, but for others, the basis of the symptoms may be unclear. Special nerve studies may be needed. For relief, a trial of antihistamines or topical or oral gabapentin may be needed, she said.
Frontal fibrosing alopecia (FFA). This condition, first described in postmenopausal women, is now reported in men and in younger women. While sunscreen has been suspected, there are no good data that have proven that link, she said. Cosmetics are also considered a possible culprit. For treatment, “the first thing we try to do is treat the inflammation,” Dr. Hordinsky said. Treatment options include topical high-potency corticosteroids, intralesional steroids, and topical nonsteroid anti-inflammatory creams (tier 1); hydroxychloroquine, low-dose antibiotics, and acitretin (tier 2); and cyclosporin and mycophenolate mofetil (tier 3).
In an observational study of mostly women with FFA, she noted, treatment with dutasteride was more effective than commonly used systemic treatments.
“Don’t forget to address the psychosocial needs of the hair loss patient,” Dr. Hordinsky advised. “Hair loss patients are very distressed, and you have to learn how to be fast and nimble and address those needs.” Working with a behavioral health specialist or therapist can help, she said.
She also recommended directing patients to appropriate organizations such as the National Alopecia Areata Foundation and the Scarring Alopecia Foundation, as well as conferences, such as the upcoming NAAF conference in Washington. “These organizations do give good information that should complement what you are doing.”
Medscape Live and this news organization are owned by the same parent company. Dr. Hordinsky reported no disclosures.
“No matter who the patient is, whether a child, adolescent, or adult, the key to figuring out hair disease is getting a good history,” Maria Hordinsky, MD, professor and chair of the department of dermatology at the University of Minnesota, Minneapolis, said at the Medscape Live Women’s and Pediatric Dermatology Seminar.
. She also urged physicians and other health care providers to use the electronic medical record and to be thorough in documenting information – noting nutrition, hair care habits, supplement use, and other details.
Lab tests should be selected based on that history, she said. For instance, low iron stores can be associated with hair shedding; and thyroid function studies might be needed.
Other highlights of her presentation included comments on different types of alopecia, and some new treatment approaches:
Androgenetic alopecia. In a meta-analysis and systematic review published in 2017, all treatments tested (2% and 5% minoxidil in men, 1 mg finasteride in men, 2% minoxidil in women, and low-level laser light therapy in men) were superior to placebo. Several photobiomodulation (PBM) devices (also known as low-level laser light) for home use have been cleared for androgenetic alopecia by the Food and Drug Administration; a clinician’s guide, published in 2018, provides information on these devices.
Hair and hormones. Combination therapy for female-pattern hair loss – low-dose minoxidil and spironolactone – is important to know about, she said, adding there are data from an observational pilot study supporting this treatment. Women should not become pregnant while on this treatment, Dr. Hordinsky cautioned.
PRP (platelet rich plasma). This treatment for hair loss can be costly, she cautioned, as it’s viewed as a cosmetic technique, “but it actually can work rather well.”
Hair regrowth measures. Traditionally, measures center on global assessment, the patient’s self-assessment, investigator assessment, and an independent photo review. Enter the dermatoscope. “We can now get pictures as a baseline. Patients can see, and also see the health of their scalp,” and if treatments make it look better or worse, she noted.
Alopecia areata (AA). Patients and families need to be made aware that this is an autoimmune disease that can recur, and if it does recur, the extent of hair loss is not predictable. According to Dr. Hordinsky, the most widely used tool to halt disease activity has been treatment with a corticosteroid (topical, intralesional, oral, or even intravenous corticosteroids).
Clinical trials and publications from 2018 to 2020 have triggered interest in off-label use and further studies of JAK inhibitors for treating AA, which include baricitinib, ruxolitinib, and tofacitinib. At the American Academy of Dermatology meeting in March 2022, results of the ALLEGRO phase 2b/3 trial found that the JAK inhibitor ritlecitinib (50 mg or 20 mg daily, with or without a 200-mg loading dose), was efficacious in adults and adolescents with AA, compared with placebo, with no safety concerns noted. “This looks to be very, very promising,” she said, “and also very safe.” Two phase 3 trials of baricitinib also presented at the same meeting found it was superior to placebo for hair regrowth in adults with severe AA at 36 weeks. (On June 13, shortly after Dr. Hordinsky spoke at the meeting, the FDA approved baricitinib for treating AA in adults, making this the first systemic treatment to be approved for AA).
Research on topical JAK inhibitors for AA has been disappointing, Dr. Hordinsky said.
Alopecia areata and atopic dermatitis. For patients with both AA and AD, dupilumab may provide relief, she said. She referred to a recently published phase 2a trial in patients with AA (including some with both AA and AD), which found that Severity of Alopecia Tool (SALT) scores improved after 48 weeks of treatment, with higher response rates among those with baseline IgE levels of 200 IU/mL or higher. “If your patient has both, and their immunoglobulin-E level is greater than 200, then they may be a good candidate for dupilumab and both diseases may respond,” she said.
Scalp symptoms. It can be challenging when patients complain of itch, pain, or burning on the scalp, but have no obvious skin disease, Dr. Hordinsky said. Her tips: Some of these patients may be experiencing scalp symptoms secondary to a neuropathy; others may have mast cell degranulation, but for others, the basis of the symptoms may be unclear. Special nerve studies may be needed. For relief, a trial of antihistamines or topical or oral gabapentin may be needed, she said.
Frontal fibrosing alopecia (FFA). This condition, first described in postmenopausal women, is now reported in men and in younger women. While sunscreen has been suspected, there are no good data that have proven that link, she said. Cosmetics are also considered a possible culprit. For treatment, “the first thing we try to do is treat the inflammation,” Dr. Hordinsky said. Treatment options include topical high-potency corticosteroids, intralesional steroids, and topical nonsteroid anti-inflammatory creams (tier 1); hydroxychloroquine, low-dose antibiotics, and acitretin (tier 2); and cyclosporin and mycophenolate mofetil (tier 3).
In an observational study of mostly women with FFA, she noted, treatment with dutasteride was more effective than commonly used systemic treatments.
“Don’t forget to address the psychosocial needs of the hair loss patient,” Dr. Hordinsky advised. “Hair loss patients are very distressed, and you have to learn how to be fast and nimble and address those needs.” Working with a behavioral health specialist or therapist can help, she said.
She also recommended directing patients to appropriate organizations such as the National Alopecia Areata Foundation and the Scarring Alopecia Foundation, as well as conferences, such as the upcoming NAAF conference in Washington. “These organizations do give good information that should complement what you are doing.”
Medscape Live and this news organization are owned by the same parent company. Dr. Hordinsky reported no disclosures.
“No matter who the patient is, whether a child, adolescent, or adult, the key to figuring out hair disease is getting a good history,” Maria Hordinsky, MD, professor and chair of the department of dermatology at the University of Minnesota, Minneapolis, said at the Medscape Live Women’s and Pediatric Dermatology Seminar.
. She also urged physicians and other health care providers to use the electronic medical record and to be thorough in documenting information – noting nutrition, hair care habits, supplement use, and other details.
Lab tests should be selected based on that history, she said. For instance, low iron stores can be associated with hair shedding; and thyroid function studies might be needed.
Other highlights of her presentation included comments on different types of alopecia, and some new treatment approaches:
Androgenetic alopecia. In a meta-analysis and systematic review published in 2017, all treatments tested (2% and 5% minoxidil in men, 1 mg finasteride in men, 2% minoxidil in women, and low-level laser light therapy in men) were superior to placebo. Several photobiomodulation (PBM) devices (also known as low-level laser light) for home use have been cleared for androgenetic alopecia by the Food and Drug Administration; a clinician’s guide, published in 2018, provides information on these devices.
Hair and hormones. Combination therapy for female-pattern hair loss – low-dose minoxidil and spironolactone – is important to know about, she said, adding there are data from an observational pilot study supporting this treatment. Women should not become pregnant while on this treatment, Dr. Hordinsky cautioned.
PRP (platelet rich plasma). This treatment for hair loss can be costly, she cautioned, as it’s viewed as a cosmetic technique, “but it actually can work rather well.”
Hair regrowth measures. Traditionally, measures center on global assessment, the patient’s self-assessment, investigator assessment, and an independent photo review. Enter the dermatoscope. “We can now get pictures as a baseline. Patients can see, and also see the health of their scalp,” and if treatments make it look better or worse, she noted.
Alopecia areata (AA). Patients and families need to be made aware that this is an autoimmune disease that can recur, and if it does recur, the extent of hair loss is not predictable. According to Dr. Hordinsky, the most widely used tool to halt disease activity has been treatment with a corticosteroid (topical, intralesional, oral, or even intravenous corticosteroids).
Clinical trials and publications from 2018 to 2020 have triggered interest in off-label use and further studies of JAK inhibitors for treating AA, which include baricitinib, ruxolitinib, and tofacitinib. At the American Academy of Dermatology meeting in March 2022, results of the ALLEGRO phase 2b/3 trial found that the JAK inhibitor ritlecitinib (50 mg or 20 mg daily, with or without a 200-mg loading dose), was efficacious in adults and adolescents with AA, compared with placebo, with no safety concerns noted. “This looks to be very, very promising,” she said, “and also very safe.” Two phase 3 trials of baricitinib also presented at the same meeting found it was superior to placebo for hair regrowth in adults with severe AA at 36 weeks. (On June 13, shortly after Dr. Hordinsky spoke at the meeting, the FDA approved baricitinib for treating AA in adults, making this the first systemic treatment to be approved for AA).
Research on topical JAK inhibitors for AA has been disappointing, Dr. Hordinsky said.
Alopecia areata and atopic dermatitis. For patients with both AA and AD, dupilumab may provide relief, she said. She referred to a recently published phase 2a trial in patients with AA (including some with both AA and AD), which found that Severity of Alopecia Tool (SALT) scores improved after 48 weeks of treatment, with higher response rates among those with baseline IgE levels of 200 IU/mL or higher. “If your patient has both, and their immunoglobulin-E level is greater than 200, then they may be a good candidate for dupilumab and both diseases may respond,” she said.
Scalp symptoms. It can be challenging when patients complain of itch, pain, or burning on the scalp, but have no obvious skin disease, Dr. Hordinsky said. Her tips: Some of these patients may be experiencing scalp symptoms secondary to a neuropathy; others may have mast cell degranulation, but for others, the basis of the symptoms may be unclear. Special nerve studies may be needed. For relief, a trial of antihistamines or topical or oral gabapentin may be needed, she said.
Frontal fibrosing alopecia (FFA). This condition, first described in postmenopausal women, is now reported in men and in younger women. While sunscreen has been suspected, there are no good data that have proven that link, she said. Cosmetics are also considered a possible culprit. For treatment, “the first thing we try to do is treat the inflammation,” Dr. Hordinsky said. Treatment options include topical high-potency corticosteroids, intralesional steroids, and topical nonsteroid anti-inflammatory creams (tier 1); hydroxychloroquine, low-dose antibiotics, and acitretin (tier 2); and cyclosporin and mycophenolate mofetil (tier 3).
In an observational study of mostly women with FFA, she noted, treatment with dutasteride was more effective than commonly used systemic treatments.
“Don’t forget to address the psychosocial needs of the hair loss patient,” Dr. Hordinsky advised. “Hair loss patients are very distressed, and you have to learn how to be fast and nimble and address those needs.” Working with a behavioral health specialist or therapist can help, she said.
She also recommended directing patients to appropriate organizations such as the National Alopecia Areata Foundation and the Scarring Alopecia Foundation, as well as conferences, such as the upcoming NAAF conference in Washington. “These organizations do give good information that should complement what you are doing.”
Medscape Live and this news organization are owned by the same parent company. Dr. Hordinsky reported no disclosures.
FROM MEDSCAPELIVE WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
Atopic dermatitis: Options abound, and more are coming
, said at MedscapeLive’s Women’s & Pediatric Dermatology Seminar.
More and more treatment options are available and even more are in the pipeline, said Dr. Eichenfield, professor of dermatology and pediatrics and vice chair of dermatology at the University of California, San Diego and Rady Children’s Hospital. As he put it: “We got pills, injections, things to smear on the skin.”
Those options are welcome and needed, as AD affects up to 20% of children and up to 10% of adults. The course is variable, as is severity, and quality of life is impacted.
Besides new treatment options, there is a new understanding about comorbidities, environmental effects, and triggers, Dr. Eichenfield said. Among the potential comorbidities health care providers should be aware of are allergies, such as food allergies; asthma; rhinitis; mental health issues (depression, anxiety, ADHD, learning disabilities, or in adults, substance abuse); bone health; skin infections; immune disorders such as alopecia areata or urticaria; and cardiovascular issues that could affect adults.
Environmental effects can play a role in aggravating AD, as providers learned after visits for AD increased after Northern California wildfires and also in other areas with high air pollution, Dr. Eichenfield said. “I actually discuss this with my families,” when making them aware of factors that may affect AD, he noted.
Dr. Eichenfield provided an overview of available treatment options, and what treatments may be coming next. Among the highlights:
Topical ruxolitinib: A JAK1,2 inhibitor in a cream formulation, it is now approved for patients with mild to moderate AD aged 12 years and older in the United States. Of the two strengths studied, the higher strength, 1.5%, was approved, Dr. Eichenfield said. How well did it work? In two phase 3 studies in patients aged 12 and older, of those on 1.5%, 53% were clear or almost clear at 8 weeks, versus 11% in the control group given the vehicle; 52% had at least a 4-point reduction in itch from baseline, versus 15.4% on vehicle. Quality of life improved in up to 73.2% of those given the medication versus 19.7% of those on the vehicle. There was a marked and quick improvement in itch, as early as 12 hours, and safety measures also look good, he said.
Topical tapinarof: Approved in May 2022, for adults with plaque psoriasis, phase 3 trials began in September, 2021, for adults and children with AD, according to the manufacturer. Activation of the aryl hydrocarbon receptor mediates its anti-inflammatory properties.
Topical roflumilast: A potent PDE-4 inhibitor, phase 3 AD studies are underway. It appears to be well tolerated, Dr. Eichenfield said.
Dupilumab: An IL-4/13 blocker, this biologic produced an itch reduction of 50% and EASI of 80%, improved quality of life, and reduced anxiety and depression. The drug “led the revolution in systemic therapy for atopic dermatitis,” he said. First approved for treating AD in patients aged 18 years and up in 2017, approval for patients 12 years and up followed in March 2019, then for age 6 years and up May 2020.
At the meeting on June 3, Dr. Eichenfield said that approval in children 5 years and under was imminent, and on June 7, the FDA approved dupilumab for use in children aged 6 months to 5 years. In a phase 3, 16-week trial, 28% of children treated with dupilumab added on to low-potency topical corticosteroids met the endpoint of clear or nearly clear skin, compared with 4% of those on the corticosteroids alone (P < .0001).
Tralokinumab: There is no approved indication yet for adolescents, but the injected biologic, an interleukin-13 antagonist, is approved for adults with moderate to severe AD who are not well-controlled with topicals, or who cannot use topicals.
Oral JAK inhibitors: These include abrocitinib and upadacitinib, both approved by the FDA in January 2022 for treating moderate to severe AD, and baricitinib (the latter not in the United States). “For AD, you probably won’t see it in the U.S.,” Dr. Eichenfield said, referring to baricitinib. However, it might get approved for alopecia areata, he noted.
Upadacitinib is approved for adolescents 12 and older with AD. Abrocitinib is approved for adults 18 and older with AD.
Regarding safety and tolerance concerns with oral JAK inhibitors, Dr. Eichenfield cites headache, acne, nausea, and upper respiratory tract infections as relatively common, while herpes zoster, venous thromboembolism, and lab anomalies (neutropenia, elevated CPK) are uncommon.
As the options for AD treatments increase, and expectations by families and clinicians change, Dr. Eichenfield said he often focuses on “bucket duty” – whether a specific patient should be in the topical bucket or the systemic one. It’s a decision that will continue to be crucial, he said.
When presented with treatment options, patients – and parents – often worry about side effects, said Vivian Shi, MD, associate professor of dermatology at the University of Arkansas Medical Center, Little Rock, who also spoke at the meeting. She gently tells them: “The worst side effect you can have is probably not treating the disease itself.”
Medscape Live and this news organization are owned by the same parent company. Dr. Eichenfield is a consultant or investigator for numerous companies that manufacture treatments for AD, but based his discussion on evidence-based recommendations and public presentations or publications.
, said at MedscapeLive’s Women’s & Pediatric Dermatology Seminar.
More and more treatment options are available and even more are in the pipeline, said Dr. Eichenfield, professor of dermatology and pediatrics and vice chair of dermatology at the University of California, San Diego and Rady Children’s Hospital. As he put it: “We got pills, injections, things to smear on the skin.”
Those options are welcome and needed, as AD affects up to 20% of children and up to 10% of adults. The course is variable, as is severity, and quality of life is impacted.
Besides new treatment options, there is a new understanding about comorbidities, environmental effects, and triggers, Dr. Eichenfield said. Among the potential comorbidities health care providers should be aware of are allergies, such as food allergies; asthma; rhinitis; mental health issues (depression, anxiety, ADHD, learning disabilities, or in adults, substance abuse); bone health; skin infections; immune disorders such as alopecia areata or urticaria; and cardiovascular issues that could affect adults.
Environmental effects can play a role in aggravating AD, as providers learned after visits for AD increased after Northern California wildfires and also in other areas with high air pollution, Dr. Eichenfield said. “I actually discuss this with my families,” when making them aware of factors that may affect AD, he noted.
Dr. Eichenfield provided an overview of available treatment options, and what treatments may be coming next. Among the highlights:
Topical ruxolitinib: A JAK1,2 inhibitor in a cream formulation, it is now approved for patients with mild to moderate AD aged 12 years and older in the United States. Of the two strengths studied, the higher strength, 1.5%, was approved, Dr. Eichenfield said. How well did it work? In two phase 3 studies in patients aged 12 and older, of those on 1.5%, 53% were clear or almost clear at 8 weeks, versus 11% in the control group given the vehicle; 52% had at least a 4-point reduction in itch from baseline, versus 15.4% on vehicle. Quality of life improved in up to 73.2% of those given the medication versus 19.7% of those on the vehicle. There was a marked and quick improvement in itch, as early as 12 hours, and safety measures also look good, he said.
Topical tapinarof: Approved in May 2022, for adults with plaque psoriasis, phase 3 trials began in September, 2021, for adults and children with AD, according to the manufacturer. Activation of the aryl hydrocarbon receptor mediates its anti-inflammatory properties.
Topical roflumilast: A potent PDE-4 inhibitor, phase 3 AD studies are underway. It appears to be well tolerated, Dr. Eichenfield said.
Dupilumab: An IL-4/13 blocker, this biologic produced an itch reduction of 50% and EASI of 80%, improved quality of life, and reduced anxiety and depression. The drug “led the revolution in systemic therapy for atopic dermatitis,” he said. First approved for treating AD in patients aged 18 years and up in 2017, approval for patients 12 years and up followed in March 2019, then for age 6 years and up May 2020.
At the meeting on June 3, Dr. Eichenfield said that approval in children 5 years and under was imminent, and on June 7, the FDA approved dupilumab for use in children aged 6 months to 5 years. In a phase 3, 16-week trial, 28% of children treated with dupilumab added on to low-potency topical corticosteroids met the endpoint of clear or nearly clear skin, compared with 4% of those on the corticosteroids alone (P < .0001).
Tralokinumab: There is no approved indication yet for adolescents, but the injected biologic, an interleukin-13 antagonist, is approved for adults with moderate to severe AD who are not well-controlled with topicals, or who cannot use topicals.
Oral JAK inhibitors: These include abrocitinib and upadacitinib, both approved by the FDA in January 2022 for treating moderate to severe AD, and baricitinib (the latter not in the United States). “For AD, you probably won’t see it in the U.S.,” Dr. Eichenfield said, referring to baricitinib. However, it might get approved for alopecia areata, he noted.
Upadacitinib is approved for adolescents 12 and older with AD. Abrocitinib is approved for adults 18 and older with AD.
Regarding safety and tolerance concerns with oral JAK inhibitors, Dr. Eichenfield cites headache, acne, nausea, and upper respiratory tract infections as relatively common, while herpes zoster, venous thromboembolism, and lab anomalies (neutropenia, elevated CPK) are uncommon.
As the options for AD treatments increase, and expectations by families and clinicians change, Dr. Eichenfield said he often focuses on “bucket duty” – whether a specific patient should be in the topical bucket or the systemic one. It’s a decision that will continue to be crucial, he said.
When presented with treatment options, patients – and parents – often worry about side effects, said Vivian Shi, MD, associate professor of dermatology at the University of Arkansas Medical Center, Little Rock, who also spoke at the meeting. She gently tells them: “The worst side effect you can have is probably not treating the disease itself.”
Medscape Live and this news organization are owned by the same parent company. Dr. Eichenfield is a consultant or investigator for numerous companies that manufacture treatments for AD, but based his discussion on evidence-based recommendations and public presentations or publications.
, said at MedscapeLive’s Women’s & Pediatric Dermatology Seminar.
More and more treatment options are available and even more are in the pipeline, said Dr. Eichenfield, professor of dermatology and pediatrics and vice chair of dermatology at the University of California, San Diego and Rady Children’s Hospital. As he put it: “We got pills, injections, things to smear on the skin.”
Those options are welcome and needed, as AD affects up to 20% of children and up to 10% of adults. The course is variable, as is severity, and quality of life is impacted.
Besides new treatment options, there is a new understanding about comorbidities, environmental effects, and triggers, Dr. Eichenfield said. Among the potential comorbidities health care providers should be aware of are allergies, such as food allergies; asthma; rhinitis; mental health issues (depression, anxiety, ADHD, learning disabilities, or in adults, substance abuse); bone health; skin infections; immune disorders such as alopecia areata or urticaria; and cardiovascular issues that could affect adults.
Environmental effects can play a role in aggravating AD, as providers learned after visits for AD increased after Northern California wildfires and also in other areas with high air pollution, Dr. Eichenfield said. “I actually discuss this with my families,” when making them aware of factors that may affect AD, he noted.
Dr. Eichenfield provided an overview of available treatment options, and what treatments may be coming next. Among the highlights:
Topical ruxolitinib: A JAK1,2 inhibitor in a cream formulation, it is now approved for patients with mild to moderate AD aged 12 years and older in the United States. Of the two strengths studied, the higher strength, 1.5%, was approved, Dr. Eichenfield said. How well did it work? In two phase 3 studies in patients aged 12 and older, of those on 1.5%, 53% were clear or almost clear at 8 weeks, versus 11% in the control group given the vehicle; 52% had at least a 4-point reduction in itch from baseline, versus 15.4% on vehicle. Quality of life improved in up to 73.2% of those given the medication versus 19.7% of those on the vehicle. There was a marked and quick improvement in itch, as early as 12 hours, and safety measures also look good, he said.
Topical tapinarof: Approved in May 2022, for adults with plaque psoriasis, phase 3 trials began in September, 2021, for adults and children with AD, according to the manufacturer. Activation of the aryl hydrocarbon receptor mediates its anti-inflammatory properties.
Topical roflumilast: A potent PDE-4 inhibitor, phase 3 AD studies are underway. It appears to be well tolerated, Dr. Eichenfield said.
Dupilumab: An IL-4/13 blocker, this biologic produced an itch reduction of 50% and EASI of 80%, improved quality of life, and reduced anxiety and depression. The drug “led the revolution in systemic therapy for atopic dermatitis,” he said. First approved for treating AD in patients aged 18 years and up in 2017, approval for patients 12 years and up followed in March 2019, then for age 6 years and up May 2020.
At the meeting on June 3, Dr. Eichenfield said that approval in children 5 years and under was imminent, and on June 7, the FDA approved dupilumab for use in children aged 6 months to 5 years. In a phase 3, 16-week trial, 28% of children treated with dupilumab added on to low-potency topical corticosteroids met the endpoint of clear or nearly clear skin, compared with 4% of those on the corticosteroids alone (P < .0001).
Tralokinumab: There is no approved indication yet for adolescents, but the injected biologic, an interleukin-13 antagonist, is approved for adults with moderate to severe AD who are not well-controlled with topicals, or who cannot use topicals.
Oral JAK inhibitors: These include abrocitinib and upadacitinib, both approved by the FDA in January 2022 for treating moderate to severe AD, and baricitinib (the latter not in the United States). “For AD, you probably won’t see it in the U.S.,” Dr. Eichenfield said, referring to baricitinib. However, it might get approved for alopecia areata, he noted.
Upadacitinib is approved for adolescents 12 and older with AD. Abrocitinib is approved for adults 18 and older with AD.
Regarding safety and tolerance concerns with oral JAK inhibitors, Dr. Eichenfield cites headache, acne, nausea, and upper respiratory tract infections as relatively common, while herpes zoster, venous thromboembolism, and lab anomalies (neutropenia, elevated CPK) are uncommon.
As the options for AD treatments increase, and expectations by families and clinicians change, Dr. Eichenfield said he often focuses on “bucket duty” – whether a specific patient should be in the topical bucket or the systemic one. It’s a decision that will continue to be crucial, he said.
When presented with treatment options, patients – and parents – often worry about side effects, said Vivian Shi, MD, associate professor of dermatology at the University of Arkansas Medical Center, Little Rock, who also spoke at the meeting. She gently tells them: “The worst side effect you can have is probably not treating the disease itself.”
Medscape Live and this news organization are owned by the same parent company. Dr. Eichenfield is a consultant or investigator for numerous companies that manufacture treatments for AD, but based his discussion on evidence-based recommendations and public presentations or publications.
FROM MEDSCAPELIVE WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
Biologics, Women, and Pregnancy: What’s Known?
As the and the child’s development.
“I get asked a lot about fertility,” Vivian Shi, MD, associate professor of dermatology at the University of Arkansas, Little Rock, said at MedscapeLive’s Women’s and Pediatric Dermatology Seminar. Patients want to know, she said, if they go on a specific drug, whether it will affect their chances of conceiving and what else they need to know about safety.
She told the audience what she tells her patients: The answers are not complete but are evolving at a steady pace.
“Putting this talk together was kind of like a scavenger hunt,” said Dr. Shi, who gathered data from pregnancy exposure registries, published research, the Food and Drug Administration, and other sources on biologics. As more studies emerge each year, she said, recommendations will become stronger for considering treatment by certain biological drugs, taking into account effects on fertility, pregnancy, lactation, and the infant.
Among the biologics commonly used in dermatology are:
- Tumor necrosis factor (TNF) inhibitors (etanercept, adalimumab, infliximab, certolizumab).
- Interleukin (IL)–12 and -23 antagonist (ustekinumab).
- IL-17 antagonists (ixekizumab, secukinumab, brodalumab).
- IL-23 antagonists (risankizumab, tildrakizumab, guselkumab).
- IL-4, -13 antagonist (dupilumab) and IL-13 antagonist (tralokinumab).
- CD20-directed cytolytic antibody (rituximab).
To help with decision-making, Dr. Shi discussed the relatively new FDA labeling regulations as well as pregnancy exposure registries, research studies, and recommendations.
FDA pregnancy risk summaries
Under the previous system of classification of drugs in pregnancy, the FDA rated drugs as A, B, C, D, X. These categories ranged from showing no risks to the fetus to clear risk, but were oversimplistic and confusing, Dr. Shi said. Category C was especially confusing, as a drug with no animal or human data was put in the same category as a drug with adverse fetal effects on animals, she noted.
However, effective June 30, 2015, the FDA replaced pregnancy categories with risk summaries by medication. As of June, 2020, all prescription drugs were to remove pregnancy letter labeling. The risk summaries note human data when they are available and also note when no data are available. This information, Dr. Shi said, originates from many sources, including studies published in the medical literature, postmarketing studies conducted by companies, and pregnancy exposure registries, conducted by some companies and others. The FDA does not endorse any specific registries, but does post a list of such registries. Another helpful resource, she said, is Mother to Baby, a service of the nonprofit Organization of Teratology Information Specialists (OTIS).
Known, not known
Citing published literature, Dr. Shi said that TNF inhibitors have the most robust safety data from preconception to after birth. Less is known, she said, about the reproductive safety effects of other biologics used for dermatologic conditions, as they are newer than the anti-TNF medicines.
She reviewed a variety of research studies evaluating the safety of biologics during pregnancy and beyond. Highlights include results from a large registry, the Psoriasis Longitudinal Assessment and Registry (PSOLAR), of 298 pregnancies in about 220 women from 2007 to 2019, looking at 13 different biologics. The overall and live-birth outcomes in the women on biologics for psoriasis were similar to those for the general population and the rate of congenital anomalies was 0.8%, researchers reported in 2021, lower than the generally cited annual figure of U.S. births.
Studies evaluating biologics for nondermatologic conditions suggest safety. A prospective cohort study of women who took adalimumab in pregnancy (for rheumatoid arthritis or Crohn’s disease) found no increased risk for birth defects. In another study looking at women who were breastfeeding, researchers found no increased risk of infections or delay in developmental milestones in the children of women taking biologics for inflammatory bowel disease, compared with those not on the medications.
A report using data from the World Health Organization concludes that dupilumab appears to be safe during pregnancy, based on an evaluation of 36 pregnancy-related reports among more than 37,000 unique adverse event reports related to dupilumab in a global database.
Recommendations about biologic use from different organizations don’t always mesh, Dr. Shi said, noting that European guidelines tend to be stricter, as some reviews show.
If a mother is exposed to any biologic therapy other than certolizumab during the third trimester, after 27 weeks, Dr. Shi said, “you want to consider avoiding a live vaccine for the first 6 months of the baby’s life.” It turns out, she said, the only recommended live vaccine during that period is the rotavirus vaccine, and she suggests doctors recommend postponing that one until the babies are older if women have been on biologics other than certolizumab.
Her other take-home messages: TNF inhibitors have the most robust safety data from before conception through lactation. Under current guidelines, certolizumab is viewed as the safest to use throughout pregnancy. Dr. Shi’s message to her colleagues fielding the same questions she gets from patients: “There is more data coming out every year. Ultimately, we will have better information to inform our patients.”
At the conference, Lawrence F. Eichenfield, MD, a course director and professor of dermatology and pediatrics at the University of California, San Diego and Rady Children’s Hospital San Diego, encouraged Dr. Shi to write up her presentation as a resource for other dermatologists – which she said is in progress.
Medscape Live and this news organization are owned by the same parent company. Dr. Shi disclosed consulting and investigative and research funding from several pharmaceutical firms, but not directly related to the content of her presentation.
As the and the child’s development.
“I get asked a lot about fertility,” Vivian Shi, MD, associate professor of dermatology at the University of Arkansas, Little Rock, said at MedscapeLive’s Women’s and Pediatric Dermatology Seminar. Patients want to know, she said, if they go on a specific drug, whether it will affect their chances of conceiving and what else they need to know about safety.
She told the audience what she tells her patients: The answers are not complete but are evolving at a steady pace.
“Putting this talk together was kind of like a scavenger hunt,” said Dr. Shi, who gathered data from pregnancy exposure registries, published research, the Food and Drug Administration, and other sources on biologics. As more studies emerge each year, she said, recommendations will become stronger for considering treatment by certain biological drugs, taking into account effects on fertility, pregnancy, lactation, and the infant.
Among the biologics commonly used in dermatology are:
- Tumor necrosis factor (TNF) inhibitors (etanercept, adalimumab, infliximab, certolizumab).
- Interleukin (IL)–12 and -23 antagonist (ustekinumab).
- IL-17 antagonists (ixekizumab, secukinumab, brodalumab).
- IL-23 antagonists (risankizumab, tildrakizumab, guselkumab).
- IL-4, -13 antagonist (dupilumab) and IL-13 antagonist (tralokinumab).
- CD20-directed cytolytic antibody (rituximab).
To help with decision-making, Dr. Shi discussed the relatively new FDA labeling regulations as well as pregnancy exposure registries, research studies, and recommendations.
FDA pregnancy risk summaries
Under the previous system of classification of drugs in pregnancy, the FDA rated drugs as A, B, C, D, X. These categories ranged from showing no risks to the fetus to clear risk, but were oversimplistic and confusing, Dr. Shi said. Category C was especially confusing, as a drug with no animal or human data was put in the same category as a drug with adverse fetal effects on animals, she noted.
However, effective June 30, 2015, the FDA replaced pregnancy categories with risk summaries by medication. As of June, 2020, all prescription drugs were to remove pregnancy letter labeling. The risk summaries note human data when they are available and also note when no data are available. This information, Dr. Shi said, originates from many sources, including studies published in the medical literature, postmarketing studies conducted by companies, and pregnancy exposure registries, conducted by some companies and others. The FDA does not endorse any specific registries, but does post a list of such registries. Another helpful resource, she said, is Mother to Baby, a service of the nonprofit Organization of Teratology Information Specialists (OTIS).
Known, not known
Citing published literature, Dr. Shi said that TNF inhibitors have the most robust safety data from preconception to after birth. Less is known, she said, about the reproductive safety effects of other biologics used for dermatologic conditions, as they are newer than the anti-TNF medicines.
She reviewed a variety of research studies evaluating the safety of biologics during pregnancy and beyond. Highlights include results from a large registry, the Psoriasis Longitudinal Assessment and Registry (PSOLAR), of 298 pregnancies in about 220 women from 2007 to 2019, looking at 13 different biologics. The overall and live-birth outcomes in the women on biologics for psoriasis were similar to those for the general population and the rate of congenital anomalies was 0.8%, researchers reported in 2021, lower than the generally cited annual figure of U.S. births.
Studies evaluating biologics for nondermatologic conditions suggest safety. A prospective cohort study of women who took adalimumab in pregnancy (for rheumatoid arthritis or Crohn’s disease) found no increased risk for birth defects. In another study looking at women who were breastfeeding, researchers found no increased risk of infections or delay in developmental milestones in the children of women taking biologics for inflammatory bowel disease, compared with those not on the medications.
A report using data from the World Health Organization concludes that dupilumab appears to be safe during pregnancy, based on an evaluation of 36 pregnancy-related reports among more than 37,000 unique adverse event reports related to dupilumab in a global database.
Recommendations about biologic use from different organizations don’t always mesh, Dr. Shi said, noting that European guidelines tend to be stricter, as some reviews show.
If a mother is exposed to any biologic therapy other than certolizumab during the third trimester, after 27 weeks, Dr. Shi said, “you want to consider avoiding a live vaccine for the first 6 months of the baby’s life.” It turns out, she said, the only recommended live vaccine during that period is the rotavirus vaccine, and she suggests doctors recommend postponing that one until the babies are older if women have been on biologics other than certolizumab.
Her other take-home messages: TNF inhibitors have the most robust safety data from before conception through lactation. Under current guidelines, certolizumab is viewed as the safest to use throughout pregnancy. Dr. Shi’s message to her colleagues fielding the same questions she gets from patients: “There is more data coming out every year. Ultimately, we will have better information to inform our patients.”
At the conference, Lawrence F. Eichenfield, MD, a course director and professor of dermatology and pediatrics at the University of California, San Diego and Rady Children’s Hospital San Diego, encouraged Dr. Shi to write up her presentation as a resource for other dermatologists – which she said is in progress.
Medscape Live and this news organization are owned by the same parent company. Dr. Shi disclosed consulting and investigative and research funding from several pharmaceutical firms, but not directly related to the content of her presentation.
As the and the child’s development.
“I get asked a lot about fertility,” Vivian Shi, MD, associate professor of dermatology at the University of Arkansas, Little Rock, said at MedscapeLive’s Women’s and Pediatric Dermatology Seminar. Patients want to know, she said, if they go on a specific drug, whether it will affect their chances of conceiving and what else they need to know about safety.
She told the audience what she tells her patients: The answers are not complete but are evolving at a steady pace.
“Putting this talk together was kind of like a scavenger hunt,” said Dr. Shi, who gathered data from pregnancy exposure registries, published research, the Food and Drug Administration, and other sources on biologics. As more studies emerge each year, she said, recommendations will become stronger for considering treatment by certain biological drugs, taking into account effects on fertility, pregnancy, lactation, and the infant.
Among the biologics commonly used in dermatology are:
- Tumor necrosis factor (TNF) inhibitors (etanercept, adalimumab, infliximab, certolizumab).
- Interleukin (IL)–12 and -23 antagonist (ustekinumab).
- IL-17 antagonists (ixekizumab, secukinumab, brodalumab).
- IL-23 antagonists (risankizumab, tildrakizumab, guselkumab).
- IL-4, -13 antagonist (dupilumab) and IL-13 antagonist (tralokinumab).
- CD20-directed cytolytic antibody (rituximab).
To help with decision-making, Dr. Shi discussed the relatively new FDA labeling regulations as well as pregnancy exposure registries, research studies, and recommendations.
FDA pregnancy risk summaries
Under the previous system of classification of drugs in pregnancy, the FDA rated drugs as A, B, C, D, X. These categories ranged from showing no risks to the fetus to clear risk, but were oversimplistic and confusing, Dr. Shi said. Category C was especially confusing, as a drug with no animal or human data was put in the same category as a drug with adverse fetal effects on animals, she noted.
However, effective June 30, 2015, the FDA replaced pregnancy categories with risk summaries by medication. As of June, 2020, all prescription drugs were to remove pregnancy letter labeling. The risk summaries note human data when they are available and also note when no data are available. This information, Dr. Shi said, originates from many sources, including studies published in the medical literature, postmarketing studies conducted by companies, and pregnancy exposure registries, conducted by some companies and others. The FDA does not endorse any specific registries, but does post a list of such registries. Another helpful resource, she said, is Mother to Baby, a service of the nonprofit Organization of Teratology Information Specialists (OTIS).
Known, not known
Citing published literature, Dr. Shi said that TNF inhibitors have the most robust safety data from preconception to after birth. Less is known, she said, about the reproductive safety effects of other biologics used for dermatologic conditions, as they are newer than the anti-TNF medicines.
She reviewed a variety of research studies evaluating the safety of biologics during pregnancy and beyond. Highlights include results from a large registry, the Psoriasis Longitudinal Assessment and Registry (PSOLAR), of 298 pregnancies in about 220 women from 2007 to 2019, looking at 13 different biologics. The overall and live-birth outcomes in the women on biologics for psoriasis were similar to those for the general population and the rate of congenital anomalies was 0.8%, researchers reported in 2021, lower than the generally cited annual figure of U.S. births.
Studies evaluating biologics for nondermatologic conditions suggest safety. A prospective cohort study of women who took adalimumab in pregnancy (for rheumatoid arthritis or Crohn’s disease) found no increased risk for birth defects. In another study looking at women who were breastfeeding, researchers found no increased risk of infections or delay in developmental milestones in the children of women taking biologics for inflammatory bowel disease, compared with those not on the medications.
A report using data from the World Health Organization concludes that dupilumab appears to be safe during pregnancy, based on an evaluation of 36 pregnancy-related reports among more than 37,000 unique adverse event reports related to dupilumab in a global database.
Recommendations about biologic use from different organizations don’t always mesh, Dr. Shi said, noting that European guidelines tend to be stricter, as some reviews show.
If a mother is exposed to any biologic therapy other than certolizumab during the third trimester, after 27 weeks, Dr. Shi said, “you want to consider avoiding a live vaccine for the first 6 months of the baby’s life.” It turns out, she said, the only recommended live vaccine during that period is the rotavirus vaccine, and she suggests doctors recommend postponing that one until the babies are older if women have been on biologics other than certolizumab.
Her other take-home messages: TNF inhibitors have the most robust safety data from before conception through lactation. Under current guidelines, certolizumab is viewed as the safest to use throughout pregnancy. Dr. Shi’s message to her colleagues fielding the same questions she gets from patients: “There is more data coming out every year. Ultimately, we will have better information to inform our patients.”
At the conference, Lawrence F. Eichenfield, MD, a course director and professor of dermatology and pediatrics at the University of California, San Diego and Rady Children’s Hospital San Diego, encouraged Dr. Shi to write up her presentation as a resource for other dermatologists – which she said is in progress.
Medscape Live and this news organization are owned by the same parent company. Dr. Shi disclosed consulting and investigative and research funding from several pharmaceutical firms, but not directly related to the content of her presentation.
FROM MEDSCAPELIVE WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
CDC says about 20% get long COVID. New models try to define it
As the number of people reporting persistent, and sometimes debilitating, symptoms from COVID-19 increases, researchers have struggled to pinpoint exactly how common so-called “long COVID” is, as well as how to clearly define exactly who has it or who is likely to get it.
Now, Centers for Disease Control and Prevention researchers have concluded that one in five adults aged 18 and older have at least one health condition that might be related to their previous COVID-19 illness; that number goes up to one in four among those 65 and older. Their data was published in the CDC’s Morbidity and Mortality Weekly Report.
The conditions associated with what’s been officially termed postacute sequelae of COVID-19, or PASC, include kidney failure, blood clots, other vascular issues, respiratory issues, heart problems, mental health or neurologic problems, and musculoskeletal conditions. But none of those conditions is unique to long COVID.
Another new study, published in The Lancet Digital Health, is trying to help better characterize what long COVID is, and what it isn’t.
that could help identify those likely to develop it.
CDC data
The CDC team came to its conclusions by evaluating the EHRs of more than 353,000 adults who were diagnosed with COVID-19 or got a positive test result, then comparing those records with 1.6 million patients who had a medical visit in the same month without a positive test result or a COVID-19 diagnosis.
They looked at data from March 2020 to November 2021, tagging 26 conditions often linked to post-COVID issues.
Overall, more than 38% of the COVID patients and 16% of those without COVID had at least one of these 26 conditions. They assessed the absolute risk difference between the patients and the non-COVID patients who developed one of the conditions, finding a 20.8–percentage point difference for those 18-64, yielding the one in five figure, and a 26.9–percentage point difference for those 65 and above, translating to about one in four.
“These findings suggest the need for increased awareness for post-COVID conditions so that improved post-COVID care and management of patients who survived COVID-19 can be developed and implemented,” said study author Lara Bull-Otterson, PhD, MPH, colead of data analytics at the Healthcare Data Advisory Unit of the CDC.
Pinpointing long COVID characteristics
Long COVID is difficult to identify, because many of its symptoms are similar to those of other conditions, so researchers are looking for better ways to characterize it to help improve both diagnosis and treatment.
Researchers on the Lancet study evaluated data from the National COVID Cohort Collaborative, N3C, a national NIH database that includes information from more than 8 million people. The team looked at the health records of 98,000 adult COVID patients and used that information, along with data from about nearly 600 long-COVID patients treated at three long-COVID clinics, to create three machine learning models for identifying long-COVID patients.
The models aimed to identify long-COVID patients in three groups: all patients, those hospitalized with COVID, and those with COVID but not hospitalized. The models were judged by the researchers to be accurate because those identified at risk for long COVID from the database were similar to those actually treated for long COVID at the clinics.
“Our algorithm is not intended to diagnose long COVID,” said lead author Emily Pfaff, PhD, research assistant professor of medicine at the University of North Carolina at Chapel Hill. “Rather, it is intended to identify patients in EHR data who ‘look like’ patients seen by physicians for long COVID.’’
Next, the researchers say, they will incorporate the new patterns they found with a diagnosis code for COVID and include it in the models to further test their accuracy. The models could also be used to help recruit patients for clinical trials, the researchers say.
Perspective and caveats
The figures of one in five and one in four found by the CDC researchers don’t surprise David Putrino, PT, PhD, director of rehabilitation innovation for Mount Sinai Health System in New York and director of its Abilities Research Center, which cares for long-COVID patients.
“Those numbers are high and it’s alarming,” he said. “But we’ve been sounding the alarm for quite some time, and we’ve been assuming that about one in five end up with long COVID.”
He does see a limitation to the CDC research – that some symptoms could have emerged later, and some in the control group could have had an undiagnosed COVID infection and gone on to develop long COVID.
As for machine learning, “this is something we need to approach with caution,” Dr. Putrino said. “There are a lot of variables we don’t understand about long COVID,’’ and that could result in spurious conclusions.
“Although I am supportive of this work going on, I am saying, ‘Scrutinize the tools with a grain of salt.’ Electronic records, Dr. Putrino points out, include information that the doctors enter, not what the patient says.
Dr. Pfaff responds: “It is entirely appropriate to approach both machine learning and EHR data with relevant caveats in mind. There are many clinical factors that are not recorded in the EHR, and the EHR is not representative of all persons with long COVID.” Those data can only reflect those who seek care for a condition, a natural limitation.
When it comes to algorithms, they are limited by data they have access to, such as the electronic health records in this research. However, the immense size and diversity in the data used “does allow us to make some assertations with much more confidence than if we were using data from a single or small number of health care systems,” she said.
A version of this article first appeared on Medscape.com.
As the number of people reporting persistent, and sometimes debilitating, symptoms from COVID-19 increases, researchers have struggled to pinpoint exactly how common so-called “long COVID” is, as well as how to clearly define exactly who has it or who is likely to get it.
Now, Centers for Disease Control and Prevention researchers have concluded that one in five adults aged 18 and older have at least one health condition that might be related to their previous COVID-19 illness; that number goes up to one in four among those 65 and older. Their data was published in the CDC’s Morbidity and Mortality Weekly Report.
The conditions associated with what’s been officially termed postacute sequelae of COVID-19, or PASC, include kidney failure, blood clots, other vascular issues, respiratory issues, heart problems, mental health or neurologic problems, and musculoskeletal conditions. But none of those conditions is unique to long COVID.
Another new study, published in The Lancet Digital Health, is trying to help better characterize what long COVID is, and what it isn’t.
that could help identify those likely to develop it.
CDC data
The CDC team came to its conclusions by evaluating the EHRs of more than 353,000 adults who were diagnosed with COVID-19 or got a positive test result, then comparing those records with 1.6 million patients who had a medical visit in the same month without a positive test result or a COVID-19 diagnosis.
They looked at data from March 2020 to November 2021, tagging 26 conditions often linked to post-COVID issues.
Overall, more than 38% of the COVID patients and 16% of those without COVID had at least one of these 26 conditions. They assessed the absolute risk difference between the patients and the non-COVID patients who developed one of the conditions, finding a 20.8–percentage point difference for those 18-64, yielding the one in five figure, and a 26.9–percentage point difference for those 65 and above, translating to about one in four.
“These findings suggest the need for increased awareness for post-COVID conditions so that improved post-COVID care and management of patients who survived COVID-19 can be developed and implemented,” said study author Lara Bull-Otterson, PhD, MPH, colead of data analytics at the Healthcare Data Advisory Unit of the CDC.
Pinpointing long COVID characteristics
Long COVID is difficult to identify, because many of its symptoms are similar to those of other conditions, so researchers are looking for better ways to characterize it to help improve both diagnosis and treatment.
Researchers on the Lancet study evaluated data from the National COVID Cohort Collaborative, N3C, a national NIH database that includes information from more than 8 million people. The team looked at the health records of 98,000 adult COVID patients and used that information, along with data from about nearly 600 long-COVID patients treated at three long-COVID clinics, to create three machine learning models for identifying long-COVID patients.
The models aimed to identify long-COVID patients in three groups: all patients, those hospitalized with COVID, and those with COVID but not hospitalized. The models were judged by the researchers to be accurate because those identified at risk for long COVID from the database were similar to those actually treated for long COVID at the clinics.
“Our algorithm is not intended to diagnose long COVID,” said lead author Emily Pfaff, PhD, research assistant professor of medicine at the University of North Carolina at Chapel Hill. “Rather, it is intended to identify patients in EHR data who ‘look like’ patients seen by physicians for long COVID.’’
Next, the researchers say, they will incorporate the new patterns they found with a diagnosis code for COVID and include it in the models to further test their accuracy. The models could also be used to help recruit patients for clinical trials, the researchers say.
Perspective and caveats
The figures of one in five and one in four found by the CDC researchers don’t surprise David Putrino, PT, PhD, director of rehabilitation innovation for Mount Sinai Health System in New York and director of its Abilities Research Center, which cares for long-COVID patients.
“Those numbers are high and it’s alarming,” he said. “But we’ve been sounding the alarm for quite some time, and we’ve been assuming that about one in five end up with long COVID.”
He does see a limitation to the CDC research – that some symptoms could have emerged later, and some in the control group could have had an undiagnosed COVID infection and gone on to develop long COVID.
As for machine learning, “this is something we need to approach with caution,” Dr. Putrino said. “There are a lot of variables we don’t understand about long COVID,’’ and that could result in spurious conclusions.
“Although I am supportive of this work going on, I am saying, ‘Scrutinize the tools with a grain of salt.’ Electronic records, Dr. Putrino points out, include information that the doctors enter, not what the patient says.
Dr. Pfaff responds: “It is entirely appropriate to approach both machine learning and EHR data with relevant caveats in mind. There are many clinical factors that are not recorded in the EHR, and the EHR is not representative of all persons with long COVID.” Those data can only reflect those who seek care for a condition, a natural limitation.
When it comes to algorithms, they are limited by data they have access to, such as the electronic health records in this research. However, the immense size and diversity in the data used “does allow us to make some assertations with much more confidence than if we were using data from a single or small number of health care systems,” she said.
A version of this article first appeared on Medscape.com.
As the number of people reporting persistent, and sometimes debilitating, symptoms from COVID-19 increases, researchers have struggled to pinpoint exactly how common so-called “long COVID” is, as well as how to clearly define exactly who has it or who is likely to get it.
Now, Centers for Disease Control and Prevention researchers have concluded that one in five adults aged 18 and older have at least one health condition that might be related to their previous COVID-19 illness; that number goes up to one in four among those 65 and older. Their data was published in the CDC’s Morbidity and Mortality Weekly Report.
The conditions associated with what’s been officially termed postacute sequelae of COVID-19, or PASC, include kidney failure, blood clots, other vascular issues, respiratory issues, heart problems, mental health or neurologic problems, and musculoskeletal conditions. But none of those conditions is unique to long COVID.
Another new study, published in The Lancet Digital Health, is trying to help better characterize what long COVID is, and what it isn’t.
that could help identify those likely to develop it.
CDC data
The CDC team came to its conclusions by evaluating the EHRs of more than 353,000 adults who were diagnosed with COVID-19 or got a positive test result, then comparing those records with 1.6 million patients who had a medical visit in the same month without a positive test result or a COVID-19 diagnosis.
They looked at data from March 2020 to November 2021, tagging 26 conditions often linked to post-COVID issues.
Overall, more than 38% of the COVID patients and 16% of those without COVID had at least one of these 26 conditions. They assessed the absolute risk difference between the patients and the non-COVID patients who developed one of the conditions, finding a 20.8–percentage point difference for those 18-64, yielding the one in five figure, and a 26.9–percentage point difference for those 65 and above, translating to about one in four.
“These findings suggest the need for increased awareness for post-COVID conditions so that improved post-COVID care and management of patients who survived COVID-19 can be developed and implemented,” said study author Lara Bull-Otterson, PhD, MPH, colead of data analytics at the Healthcare Data Advisory Unit of the CDC.
Pinpointing long COVID characteristics
Long COVID is difficult to identify, because many of its symptoms are similar to those of other conditions, so researchers are looking for better ways to characterize it to help improve both diagnosis and treatment.
Researchers on the Lancet study evaluated data from the National COVID Cohort Collaborative, N3C, a national NIH database that includes information from more than 8 million people. The team looked at the health records of 98,000 adult COVID patients and used that information, along with data from about nearly 600 long-COVID patients treated at three long-COVID clinics, to create three machine learning models for identifying long-COVID patients.
The models aimed to identify long-COVID patients in three groups: all patients, those hospitalized with COVID, and those with COVID but not hospitalized. The models were judged by the researchers to be accurate because those identified at risk for long COVID from the database were similar to those actually treated for long COVID at the clinics.
“Our algorithm is not intended to diagnose long COVID,” said lead author Emily Pfaff, PhD, research assistant professor of medicine at the University of North Carolina at Chapel Hill. “Rather, it is intended to identify patients in EHR data who ‘look like’ patients seen by physicians for long COVID.’’
Next, the researchers say, they will incorporate the new patterns they found with a diagnosis code for COVID and include it in the models to further test their accuracy. The models could also be used to help recruit patients for clinical trials, the researchers say.
Perspective and caveats
The figures of one in five and one in four found by the CDC researchers don’t surprise David Putrino, PT, PhD, director of rehabilitation innovation for Mount Sinai Health System in New York and director of its Abilities Research Center, which cares for long-COVID patients.
“Those numbers are high and it’s alarming,” he said. “But we’ve been sounding the alarm for quite some time, and we’ve been assuming that about one in five end up with long COVID.”
He does see a limitation to the CDC research – that some symptoms could have emerged later, and some in the control group could have had an undiagnosed COVID infection and gone on to develop long COVID.
As for machine learning, “this is something we need to approach with caution,” Dr. Putrino said. “There are a lot of variables we don’t understand about long COVID,’’ and that could result in spurious conclusions.
“Although I am supportive of this work going on, I am saying, ‘Scrutinize the tools with a grain of salt.’ Electronic records, Dr. Putrino points out, include information that the doctors enter, not what the patient says.
Dr. Pfaff responds: “It is entirely appropriate to approach both machine learning and EHR data with relevant caveats in mind. There are many clinical factors that are not recorded in the EHR, and the EHR is not representative of all persons with long COVID.” Those data can only reflect those who seek care for a condition, a natural limitation.
When it comes to algorithms, they are limited by data they have access to, such as the electronic health records in this research. However, the immense size and diversity in the data used “does allow us to make some assertations with much more confidence than if we were using data from a single or small number of health care systems,” she said.
A version of this article first appeared on Medscape.com.
Childhood survivors of gun violence: What’s the long-term outlook?
As the parents of the 19 children shot dead Tuesday in Uvalde, Tex., by a teen gunman grapple with unspeakable grief and funeral preparations, the survivors and their families are dealing with their own angst and likely much more.
While the parents understandably feel lucky that their children made it out, what about the long-term effect on their children of witnessing that carnage, of seeing classmates, friends, and teachers die violently as they stood by helpless and fearful?
The outcome over the next few days, months, and years depends on many factors, but how parents address the trauma both immediately and long-term can make a huge difference, experts say.
Posttraumatic growth
Best long-term case scenario? Survivors can experience what experts call posttraumatic growth – reaching out to give back to society, to make the world a better place, and changing who they are and their view of the world.
A prime example of posttraumatic growth: A month after a teen gunman killed 17 students at Marjory Stoneman Douglas High School in Parkland, Fla., on Valentine’s Day 2018, an army of survivors from that day’s bloodbath headed to Washington, D.C., for the now-famous March for Our Lives. The student-led demonstration, with hundreds of thousands of supporters marching, called for gun control legislation and an end to gun violence. It remains a vibrant, nonprofit organization still advocating for universal background checks and increased support of mental health services.
No sign of future violence
While most children and teens who witness school violence won’t become high-profile activists, as survivors of Parkland and the numerous other school shootings have, neither will they become the next active shooter, mental health experts say. They can’t point to a study that follows the gun violence victims that shows who does OK and who doesn’t, but they know immediate support and therapy can go a long way to recovery.
“I can’t tell you how any particular child will do,” says Robin Gurwitch, PhD, psychologist and professor at Duke University Medical Center, Durham, N.C. “I can tell you the majority of kids will be OK.”
However, that doesn’t mean a surviving child won’t have behavior and other issues, she says. Research does suggest the next few days, weeks, or months will be rough.
What parents and other caretakers do in the days after the violence will help predict the long-term outcome. Dr. Gurwitch and other experts say it’s important to first focus on what they call “psychological first aid,” then phase in therapy such as trauma-focused cognitive behavioral therapy, if and when it’s needed.
First, ‘psychological first aid’
“Psychological first aid is designed to minimize the impact down the road,” Dr. Gurwitch says. “Validate that they are feeling scared or worried.”
Some may be angry, another understandable emotion. In the first few days of witnessing violence – or even just hearing about it – parents should expect clinginess, sleep problems, behavior meltdowns, and irritability, she says.
“Those kinds of changes are likely to last a few weeks,” she says.
If day-to-day functioning is very difficult, “don’t wait for those to pass,” Dr. Gurwitch says. “Reach out for help. Resources will be available. Check with your pediatrician or family physician.”
At home, parents can address specific problems related to the experience, Dr. Gurwitch says. If it’s sleep, she says, parents and kids can work together to figure out how to ease sleep, such as listening to their favorite music before bedtime.
While parents may be inclined to baby the kids after the violence, Dr. Gurwitch says it’s important to maintain routines. So it’s not cruel to insist they do their chores.
Expect change
Things won’t be the same.
“Anytime we go through a particular traumatic event, we are changed,” Dr. Gurwitch says. ‘’The question is, what do we do about it? How do we incorporate that change into who we are and have become?”
Also important is figuring out how to make meaning out of what happened.
“I am so impressed by the families at Sandy Hook (the Connecticut elementary school where a gunman killed 26 in 2012),” she says.
They set up foundations and did other advocacy work.
“These types of events are life-changing events,” agrees David Schonfeld, MD, a pediatrician and director of the National Center for Schools Crisis and Bereavement at Children’s Hospital Los Angeles, California. “They will change who children are as people, but it doesn’t mean they are damaged for life. They will remember it as long as they live, and it will also change who they are as a person.”
While people tend to stress the potential negative effects – and there certainly are some – ‘’some individuals actually emerge from these events with a renewed sense of purpose.’’
He tells parents: “Yes, your child has changed, and you can’t go back. But it doesn’t mean they are destined to never be able to cope [with trauma].”
Research
The effects of gun violence on children can be serious and dramatic, research shows.
- Exposure to neighborhood gun violence is linked with an increase in children’s mental health issues, have found. Children living within two or three blocks of gun violence had nearly twice the risk of going to the emergency department with a mental health complaint in the 14 days following the shooting.
- Exposure to gun violence should be classified, along with maltreatment, household dysfunction, and other issues known to impact children negatively, as an adverse childhood experience, other experts
- Direct gun violence exposure, witnessing it, and hearing gunshots are all associated with children being victimized in other ways, another found. And that poly-victimization, as it is called, was strongly associated with having posttraumatic symptoms.
Adverse Childhood Events, as these sorts of experiences are known, can have long-lasting effects on physical and mental health, as well as on even the economic future of a person, says Hansa Bhargava, MD, a pediatrician and chief medical officer of Medscape, WebMD’s sister site for medical professionals.
“Kids who have suffered through violent events can have brain development affected, as well as their immune systems,” she says. “They are more likely to have chronic disease, substance use disorder, sexually transmitted diseases, teen pregnancy, and lifelong depression. A high risk of [posttraumatic stress disorder] is likely for them and their families.”
The impact of family support
The gun violence and deaths are likely to remind children of other losses they have experienced, Dr. Schonfeld says, and that can make coping more difficult.
If the trauma from the Tuesday shootings is ‘’layered” on top of trauma from COVID-19 deaths or other trauma such as domestic violence, those children may have a more difficult time, says Allan Chrisman, MD, professor emeritus of psychiatry and behavioral sciences at Duke University Health System. However, protective factors such as the family response and the community response can build resilience in survivors, he says.
“The way in which parents handle it for themselves will have a huge impact on the kids,” Dr. Chrisman says. “The worst outcomes are linked with [parents saying], ‘We don’t want to talk about it.’ ”
The parents are understandably upset, Dr. Gurwitch says. It’s OK to show sadness, anger, and other emotions, but she tells parents: “It’s not OK to completely decompose.” It’s important for the children to see that parents can pull themselves together.
Longer-term effects
As time goes on, ‘’a very large percentage will have posttraumatic reactions,” Dr. Schonfeld says. “Those reactions tend to improve over time.”
While people talk about PTSD directly after an incident such as a school shooting, it isn’t officially diagnosed as PTSD until the symptoms describing PTSD have persisted for a month, Dr. Schonfeld says. However, ‘’that doesn’t mean you don’t have a problem” that needs attention from a mental health professional.
“As a country we are already struggling with a mental health crisis,” Dr. Bhargava says. “Events such as this serve to exacerbate even more crisis in a group of innocent children whose only crime was to attend school. We must address the ‘epidemic’ of gun violence and school shootings head on. For the sake of our children and their health. For all of us.”
Therapy that works
Cognitive behavioral therapy (CBT) approaches are effective in reducing the trauma, Dr. Gurwitch says.
She often recommends one type of CBT, called trauma-focused cognitive behavioral therapy. This approach involves children and parents and focuses on safety, coping skills, and gradual exposure. It’s a structured and short-term treatment of about eight to 25 sessions.
A version of this article first appeared on Medscape.com.
As the parents of the 19 children shot dead Tuesday in Uvalde, Tex., by a teen gunman grapple with unspeakable grief and funeral preparations, the survivors and their families are dealing with their own angst and likely much more.
While the parents understandably feel lucky that their children made it out, what about the long-term effect on their children of witnessing that carnage, of seeing classmates, friends, and teachers die violently as they stood by helpless and fearful?
The outcome over the next few days, months, and years depends on many factors, but how parents address the trauma both immediately and long-term can make a huge difference, experts say.
Posttraumatic growth
Best long-term case scenario? Survivors can experience what experts call posttraumatic growth – reaching out to give back to society, to make the world a better place, and changing who they are and their view of the world.
A prime example of posttraumatic growth: A month after a teen gunman killed 17 students at Marjory Stoneman Douglas High School in Parkland, Fla., on Valentine’s Day 2018, an army of survivors from that day’s bloodbath headed to Washington, D.C., for the now-famous March for Our Lives. The student-led demonstration, with hundreds of thousands of supporters marching, called for gun control legislation and an end to gun violence. It remains a vibrant, nonprofit organization still advocating for universal background checks and increased support of mental health services.
No sign of future violence
While most children and teens who witness school violence won’t become high-profile activists, as survivors of Parkland and the numerous other school shootings have, neither will they become the next active shooter, mental health experts say. They can’t point to a study that follows the gun violence victims that shows who does OK and who doesn’t, but they know immediate support and therapy can go a long way to recovery.
“I can’t tell you how any particular child will do,” says Robin Gurwitch, PhD, psychologist and professor at Duke University Medical Center, Durham, N.C. “I can tell you the majority of kids will be OK.”
However, that doesn’t mean a surviving child won’t have behavior and other issues, she says. Research does suggest the next few days, weeks, or months will be rough.
What parents and other caretakers do in the days after the violence will help predict the long-term outcome. Dr. Gurwitch and other experts say it’s important to first focus on what they call “psychological first aid,” then phase in therapy such as trauma-focused cognitive behavioral therapy, if and when it’s needed.
First, ‘psychological first aid’
“Psychological first aid is designed to minimize the impact down the road,” Dr. Gurwitch says. “Validate that they are feeling scared or worried.”
Some may be angry, another understandable emotion. In the first few days of witnessing violence – or even just hearing about it – parents should expect clinginess, sleep problems, behavior meltdowns, and irritability, she says.
“Those kinds of changes are likely to last a few weeks,” she says.
If day-to-day functioning is very difficult, “don’t wait for those to pass,” Dr. Gurwitch says. “Reach out for help. Resources will be available. Check with your pediatrician or family physician.”
At home, parents can address specific problems related to the experience, Dr. Gurwitch says. If it’s sleep, she says, parents and kids can work together to figure out how to ease sleep, such as listening to their favorite music before bedtime.
While parents may be inclined to baby the kids after the violence, Dr. Gurwitch says it’s important to maintain routines. So it’s not cruel to insist they do their chores.
Expect change
Things won’t be the same.
“Anytime we go through a particular traumatic event, we are changed,” Dr. Gurwitch says. ‘’The question is, what do we do about it? How do we incorporate that change into who we are and have become?”
Also important is figuring out how to make meaning out of what happened.
“I am so impressed by the families at Sandy Hook (the Connecticut elementary school where a gunman killed 26 in 2012),” she says.
They set up foundations and did other advocacy work.
“These types of events are life-changing events,” agrees David Schonfeld, MD, a pediatrician and director of the National Center for Schools Crisis and Bereavement at Children’s Hospital Los Angeles, California. “They will change who children are as people, but it doesn’t mean they are damaged for life. They will remember it as long as they live, and it will also change who they are as a person.”
While people tend to stress the potential negative effects – and there certainly are some – ‘’some individuals actually emerge from these events with a renewed sense of purpose.’’
He tells parents: “Yes, your child has changed, and you can’t go back. But it doesn’t mean they are destined to never be able to cope [with trauma].”
Research
The effects of gun violence on children can be serious and dramatic, research shows.
- Exposure to neighborhood gun violence is linked with an increase in children’s mental health issues, have found. Children living within two or three blocks of gun violence had nearly twice the risk of going to the emergency department with a mental health complaint in the 14 days following the shooting.
- Exposure to gun violence should be classified, along with maltreatment, household dysfunction, and other issues known to impact children negatively, as an adverse childhood experience, other experts
- Direct gun violence exposure, witnessing it, and hearing gunshots are all associated with children being victimized in other ways, another found. And that poly-victimization, as it is called, was strongly associated with having posttraumatic symptoms.
Adverse Childhood Events, as these sorts of experiences are known, can have long-lasting effects on physical and mental health, as well as on even the economic future of a person, says Hansa Bhargava, MD, a pediatrician and chief medical officer of Medscape, WebMD’s sister site for medical professionals.
“Kids who have suffered through violent events can have brain development affected, as well as their immune systems,” she says. “They are more likely to have chronic disease, substance use disorder, sexually transmitted diseases, teen pregnancy, and lifelong depression. A high risk of [posttraumatic stress disorder] is likely for them and their families.”
The impact of family support
The gun violence and deaths are likely to remind children of other losses they have experienced, Dr. Schonfeld says, and that can make coping more difficult.
If the trauma from the Tuesday shootings is ‘’layered” on top of trauma from COVID-19 deaths or other trauma such as domestic violence, those children may have a more difficult time, says Allan Chrisman, MD, professor emeritus of psychiatry and behavioral sciences at Duke University Health System. However, protective factors such as the family response and the community response can build resilience in survivors, he says.
“The way in which parents handle it for themselves will have a huge impact on the kids,” Dr. Chrisman says. “The worst outcomes are linked with [parents saying], ‘We don’t want to talk about it.’ ”
The parents are understandably upset, Dr. Gurwitch says. It’s OK to show sadness, anger, and other emotions, but she tells parents: “It’s not OK to completely decompose.” It’s important for the children to see that parents can pull themselves together.
Longer-term effects
As time goes on, ‘’a very large percentage will have posttraumatic reactions,” Dr. Schonfeld says. “Those reactions tend to improve over time.”
While people talk about PTSD directly after an incident such as a school shooting, it isn’t officially diagnosed as PTSD until the symptoms describing PTSD have persisted for a month, Dr. Schonfeld says. However, ‘’that doesn’t mean you don’t have a problem” that needs attention from a mental health professional.
“As a country we are already struggling with a mental health crisis,” Dr. Bhargava says. “Events such as this serve to exacerbate even more crisis in a group of innocent children whose only crime was to attend school. We must address the ‘epidemic’ of gun violence and school shootings head on. For the sake of our children and their health. For all of us.”
Therapy that works
Cognitive behavioral therapy (CBT) approaches are effective in reducing the trauma, Dr. Gurwitch says.
She often recommends one type of CBT, called trauma-focused cognitive behavioral therapy. This approach involves children and parents and focuses on safety, coping skills, and gradual exposure. It’s a structured and short-term treatment of about eight to 25 sessions.
A version of this article first appeared on Medscape.com.
As the parents of the 19 children shot dead Tuesday in Uvalde, Tex., by a teen gunman grapple with unspeakable grief and funeral preparations, the survivors and their families are dealing with their own angst and likely much more.
While the parents understandably feel lucky that their children made it out, what about the long-term effect on their children of witnessing that carnage, of seeing classmates, friends, and teachers die violently as they stood by helpless and fearful?
The outcome over the next few days, months, and years depends on many factors, but how parents address the trauma both immediately and long-term can make a huge difference, experts say.
Posttraumatic growth
Best long-term case scenario? Survivors can experience what experts call posttraumatic growth – reaching out to give back to society, to make the world a better place, and changing who they are and their view of the world.
A prime example of posttraumatic growth: A month after a teen gunman killed 17 students at Marjory Stoneman Douglas High School in Parkland, Fla., on Valentine’s Day 2018, an army of survivors from that day’s bloodbath headed to Washington, D.C., for the now-famous March for Our Lives. The student-led demonstration, with hundreds of thousands of supporters marching, called for gun control legislation and an end to gun violence. It remains a vibrant, nonprofit organization still advocating for universal background checks and increased support of mental health services.
No sign of future violence
While most children and teens who witness school violence won’t become high-profile activists, as survivors of Parkland and the numerous other school shootings have, neither will they become the next active shooter, mental health experts say. They can’t point to a study that follows the gun violence victims that shows who does OK and who doesn’t, but they know immediate support and therapy can go a long way to recovery.
“I can’t tell you how any particular child will do,” says Robin Gurwitch, PhD, psychologist and professor at Duke University Medical Center, Durham, N.C. “I can tell you the majority of kids will be OK.”
However, that doesn’t mean a surviving child won’t have behavior and other issues, she says. Research does suggest the next few days, weeks, or months will be rough.
What parents and other caretakers do in the days after the violence will help predict the long-term outcome. Dr. Gurwitch and other experts say it’s important to first focus on what they call “psychological first aid,” then phase in therapy such as trauma-focused cognitive behavioral therapy, if and when it’s needed.
First, ‘psychological first aid’
“Psychological first aid is designed to minimize the impact down the road,” Dr. Gurwitch says. “Validate that they are feeling scared or worried.”
Some may be angry, another understandable emotion. In the first few days of witnessing violence – or even just hearing about it – parents should expect clinginess, sleep problems, behavior meltdowns, and irritability, she says.
“Those kinds of changes are likely to last a few weeks,” she says.
If day-to-day functioning is very difficult, “don’t wait for those to pass,” Dr. Gurwitch says. “Reach out for help. Resources will be available. Check with your pediatrician or family physician.”
At home, parents can address specific problems related to the experience, Dr. Gurwitch says. If it’s sleep, she says, parents and kids can work together to figure out how to ease sleep, such as listening to their favorite music before bedtime.
While parents may be inclined to baby the kids after the violence, Dr. Gurwitch says it’s important to maintain routines. So it’s not cruel to insist they do their chores.
Expect change
Things won’t be the same.
“Anytime we go through a particular traumatic event, we are changed,” Dr. Gurwitch says. ‘’The question is, what do we do about it? How do we incorporate that change into who we are and have become?”
Also important is figuring out how to make meaning out of what happened.
“I am so impressed by the families at Sandy Hook (the Connecticut elementary school where a gunman killed 26 in 2012),” she says.
They set up foundations and did other advocacy work.
“These types of events are life-changing events,” agrees David Schonfeld, MD, a pediatrician and director of the National Center for Schools Crisis and Bereavement at Children’s Hospital Los Angeles, California. “They will change who children are as people, but it doesn’t mean they are damaged for life. They will remember it as long as they live, and it will also change who they are as a person.”
While people tend to stress the potential negative effects – and there certainly are some – ‘’some individuals actually emerge from these events with a renewed sense of purpose.’’
He tells parents: “Yes, your child has changed, and you can’t go back. But it doesn’t mean they are destined to never be able to cope [with trauma].”
Research
The effects of gun violence on children can be serious and dramatic, research shows.
- Exposure to neighborhood gun violence is linked with an increase in children’s mental health issues, have found. Children living within two or three blocks of gun violence had nearly twice the risk of going to the emergency department with a mental health complaint in the 14 days following the shooting.
- Exposure to gun violence should be classified, along with maltreatment, household dysfunction, and other issues known to impact children negatively, as an adverse childhood experience, other experts
- Direct gun violence exposure, witnessing it, and hearing gunshots are all associated with children being victimized in other ways, another found. And that poly-victimization, as it is called, was strongly associated with having posttraumatic symptoms.
Adverse Childhood Events, as these sorts of experiences are known, can have long-lasting effects on physical and mental health, as well as on even the economic future of a person, says Hansa Bhargava, MD, a pediatrician and chief medical officer of Medscape, WebMD’s sister site for medical professionals.
“Kids who have suffered through violent events can have brain development affected, as well as their immune systems,” she says. “They are more likely to have chronic disease, substance use disorder, sexually transmitted diseases, teen pregnancy, and lifelong depression. A high risk of [posttraumatic stress disorder] is likely for them and their families.”
The impact of family support
The gun violence and deaths are likely to remind children of other losses they have experienced, Dr. Schonfeld says, and that can make coping more difficult.
If the trauma from the Tuesday shootings is ‘’layered” on top of trauma from COVID-19 deaths or other trauma such as domestic violence, those children may have a more difficult time, says Allan Chrisman, MD, professor emeritus of psychiatry and behavioral sciences at Duke University Health System. However, protective factors such as the family response and the community response can build resilience in survivors, he says.
“The way in which parents handle it for themselves will have a huge impact on the kids,” Dr. Chrisman says. “The worst outcomes are linked with [parents saying], ‘We don’t want to talk about it.’ ”
The parents are understandably upset, Dr. Gurwitch says. It’s OK to show sadness, anger, and other emotions, but she tells parents: “It’s not OK to completely decompose.” It’s important for the children to see that parents can pull themselves together.
Longer-term effects
As time goes on, ‘’a very large percentage will have posttraumatic reactions,” Dr. Schonfeld says. “Those reactions tend to improve over time.”
While people talk about PTSD directly after an incident such as a school shooting, it isn’t officially diagnosed as PTSD until the symptoms describing PTSD have persisted for a month, Dr. Schonfeld says. However, ‘’that doesn’t mean you don’t have a problem” that needs attention from a mental health professional.
“As a country we are already struggling with a mental health crisis,” Dr. Bhargava says. “Events such as this serve to exacerbate even more crisis in a group of innocent children whose only crime was to attend school. We must address the ‘epidemic’ of gun violence and school shootings head on. For the sake of our children and their health. For all of us.”
Therapy that works
Cognitive behavioral therapy (CBT) approaches are effective in reducing the trauma, Dr. Gurwitch says.
She often recommends one type of CBT, called trauma-focused cognitive behavioral therapy. This approach involves children and parents and focuses on safety, coping skills, and gradual exposure. It’s a structured and short-term treatment of about eight to 25 sessions.
A version of this article first appeared on Medscape.com.
Pick your sunscreen carefully: 75% don’t pass muster
Just in time for Memorial Day outings, a new report on sunscreens is out.
The news isn’t all sunny. , a nonprofit research and advocacy group that just issued its 16th annual Guide to Sunscreens.
In response, dermatologists, including the president of the American Academy of Dermatology, say that although some concerns have been raised about the safety of some sunscreen ingredients, sunscreens themselves remain an important tool in the fight against skin cancer. According to the Skin Cancer Foundation, 1 in 5 Americans will get skin cancer by age 70. Melanoma, the most deadly, has a 5-year survival rate of 99% if caught early.
2022 report
Overall, the Environmental Working Group found that about 1 in 4 sunscreens, or about 500 products, met their standards for providing adequate sun protection and avoiding ingredients linked to known health harms. Products meant for babies and children did slightly better, with about 1 in 3 meeting the standards. The group evaluated mineral sunscreens, also called physical sunscreens, and non-mineral sunscreens, also called chemical sunscreens. Mineral sunscreens contain zinc oxide or titanium dioxide and sit on the skin to deflect the sun’s rays. Chemical sunscreens, with ingredients such as oxybenzone or avobenzone, are partially absorbed into the skin.
Among the group’s concerns:
- The use of oxybenzone in the non-mineral sunscreens. About 30% of the non-mineral sunscreens have it, says Carla Burns, senior director for cosmetic science for the Environmental Working Group. Oxybenzone is a potential hormone disrupter and a skin sensitizer that may harm children and adults, she says. Some progress has been made, as the group found oxybenzone in 66% of the non-mineral sunscreens it reviewed in 2019. (The FDA is seeking more information on oxybenzone and many other sunscreen ingredients.)
- Contamination of sunscreens with benzene, which has been linked to leukemia and other blood disorders, according to the National Cancer Institute. But industry experts stress that that chemical is found in trace amounts in personal care products and does not pose a safety concern. “Benzene is a chemical that is ubiquitous in the environment and not an intentionally added ingredient in personal care products. People worldwide are exposed daily to benzene from indoor and outdoor sources, including air, drinking water, and food and beverages,” the Personal Care Products Council, an industry group, said in a statement.
- Protection from ultraviolet A (UVA) rays is often inadequate, according to research published last year by the Environmental Working Group.
Products on the ‘best’ list
The Environmental Working Group found that 282 recreational sunscreens met its criteria. Among them:
- Coral Safe Sunscreen Lotion, SPF 30
- Neutrogena Sheer Zinc Mineral Sunscreen Lotion, SPF 30
- Mad Hippie Facial Sunscreen Lotion, SPF 30+
The group chose 86 non-mineral sunscreens as better options, including:
- Alba Botanica Hawaiian Sunscreen Lotion, Aloe Vera, SPF 30
- Banana Boat Sport Ultra Sunscreen Stick, SPF 50+
- Black Girl Sunscreen Melanin Boosting Moisturizing Sunscreen Lotion, SPF 30
And 70 sunscreens made the kids’ best list, including:
- True Baby Everyday Play Sunscreen Lotion, SPF 30+
- Sun Biologic Kids’ Sunscreen Stick, SPF 30+
- Kiss My Face Organic Kids’ Defense Sunscreen Lotion, SPF 30
Industry response, FDA actions
In a statement, Alexandra Kowcz, chief scientist at the Personal Care Products Council, pointed out that “as part of a daily safe-sun regimen, sunscreen products help prevent sunburn and reduce skin cancer risk. It is unfortunate that as Americans spend more time outdoors, the Environmental Working Group’s (EWG) 2022 Guide to Sunscreens resorts to fear-mongering with misleading information that could keep consumers from using sunscreens altogether.”
The FDA has asked for more information about certain ingredients to further evaluate products, she says, and industry is working with the agency. The FDA says it is attempting to improve the quality, safety and effectiveness of over-the-counter sunscreen products. In September, 2021, the FDA issued a proposal for regulating OTC sunscreen products, as required under the CARES (Coronavirus Aid, Relief and Economic Security) Act. The effective date for the final order can’t be earlier than September 2022, the CARES Act says.
Dermatologists weigh in
“Every time something like this gets published, my patients come in hysterical,” says Michele Green, MD, a New York City dermatologist who reviewed the report for WebMD. She acknowledges that more research is needed on some sunscreen ingredients. “We really do not know the long-term consequence of oxybenzone,” she says.
Her advice: If her patients have melasma (a skin condition with brown patches on the face), she advises them to use both a chemical and a mineral sunscreen. “I don’t tell my patients in general not to use the chemical [sunscreens].”
For children, she says, the mineral sunscreens may be preferred. On her own children, who are teens, she uses the mineral sunscreens, due to possible concern about hormone disruption.
In a statement, Mark D. Kaufmann, MD, president of the American Academy of Dermatology, says that “sunscreen is an important part of a comprehensive sun protection strategy.”
Besides a broad-spectrum, water-resistant sunscreen with an SPF of 30 or higher for exposed skin, the academy recommends seeking shade and wearing sun-protective clothing to reduce skin cancer risk.
A version of this article first appeared on WebMD.com.
Just in time for Memorial Day outings, a new report on sunscreens is out.
The news isn’t all sunny. , a nonprofit research and advocacy group that just issued its 16th annual Guide to Sunscreens.
In response, dermatologists, including the president of the American Academy of Dermatology, say that although some concerns have been raised about the safety of some sunscreen ingredients, sunscreens themselves remain an important tool in the fight against skin cancer. According to the Skin Cancer Foundation, 1 in 5 Americans will get skin cancer by age 70. Melanoma, the most deadly, has a 5-year survival rate of 99% if caught early.
2022 report
Overall, the Environmental Working Group found that about 1 in 4 sunscreens, or about 500 products, met their standards for providing adequate sun protection and avoiding ingredients linked to known health harms. Products meant for babies and children did slightly better, with about 1 in 3 meeting the standards. The group evaluated mineral sunscreens, also called physical sunscreens, and non-mineral sunscreens, also called chemical sunscreens. Mineral sunscreens contain zinc oxide or titanium dioxide and sit on the skin to deflect the sun’s rays. Chemical sunscreens, with ingredients such as oxybenzone or avobenzone, are partially absorbed into the skin.
Among the group’s concerns:
- The use of oxybenzone in the non-mineral sunscreens. About 30% of the non-mineral sunscreens have it, says Carla Burns, senior director for cosmetic science for the Environmental Working Group. Oxybenzone is a potential hormone disrupter and a skin sensitizer that may harm children and adults, she says. Some progress has been made, as the group found oxybenzone in 66% of the non-mineral sunscreens it reviewed in 2019. (The FDA is seeking more information on oxybenzone and many other sunscreen ingredients.)
- Contamination of sunscreens with benzene, which has been linked to leukemia and other blood disorders, according to the National Cancer Institute. But industry experts stress that that chemical is found in trace amounts in personal care products and does not pose a safety concern. “Benzene is a chemical that is ubiquitous in the environment and not an intentionally added ingredient in personal care products. People worldwide are exposed daily to benzene from indoor and outdoor sources, including air, drinking water, and food and beverages,” the Personal Care Products Council, an industry group, said in a statement.
- Protection from ultraviolet A (UVA) rays is often inadequate, according to research published last year by the Environmental Working Group.
Products on the ‘best’ list
The Environmental Working Group found that 282 recreational sunscreens met its criteria. Among them:
- Coral Safe Sunscreen Lotion, SPF 30
- Neutrogena Sheer Zinc Mineral Sunscreen Lotion, SPF 30
- Mad Hippie Facial Sunscreen Lotion, SPF 30+
The group chose 86 non-mineral sunscreens as better options, including:
- Alba Botanica Hawaiian Sunscreen Lotion, Aloe Vera, SPF 30
- Banana Boat Sport Ultra Sunscreen Stick, SPF 50+
- Black Girl Sunscreen Melanin Boosting Moisturizing Sunscreen Lotion, SPF 30
And 70 sunscreens made the kids’ best list, including:
- True Baby Everyday Play Sunscreen Lotion, SPF 30+
- Sun Biologic Kids’ Sunscreen Stick, SPF 30+
- Kiss My Face Organic Kids’ Defense Sunscreen Lotion, SPF 30
Industry response, FDA actions
In a statement, Alexandra Kowcz, chief scientist at the Personal Care Products Council, pointed out that “as part of a daily safe-sun regimen, sunscreen products help prevent sunburn and reduce skin cancer risk. It is unfortunate that as Americans spend more time outdoors, the Environmental Working Group’s (EWG) 2022 Guide to Sunscreens resorts to fear-mongering with misleading information that could keep consumers from using sunscreens altogether.”
The FDA has asked for more information about certain ingredients to further evaluate products, she says, and industry is working with the agency. The FDA says it is attempting to improve the quality, safety and effectiveness of over-the-counter sunscreen products. In September, 2021, the FDA issued a proposal for regulating OTC sunscreen products, as required under the CARES (Coronavirus Aid, Relief and Economic Security) Act. The effective date for the final order can’t be earlier than September 2022, the CARES Act says.
Dermatologists weigh in
“Every time something like this gets published, my patients come in hysterical,” says Michele Green, MD, a New York City dermatologist who reviewed the report for WebMD. She acknowledges that more research is needed on some sunscreen ingredients. “We really do not know the long-term consequence of oxybenzone,” she says.
Her advice: If her patients have melasma (a skin condition with brown patches on the face), she advises them to use both a chemical and a mineral sunscreen. “I don’t tell my patients in general not to use the chemical [sunscreens].”
For children, she says, the mineral sunscreens may be preferred. On her own children, who are teens, she uses the mineral sunscreens, due to possible concern about hormone disruption.
In a statement, Mark D. Kaufmann, MD, president of the American Academy of Dermatology, says that “sunscreen is an important part of a comprehensive sun protection strategy.”
Besides a broad-spectrum, water-resistant sunscreen with an SPF of 30 or higher for exposed skin, the academy recommends seeking shade and wearing sun-protective clothing to reduce skin cancer risk.
A version of this article first appeared on WebMD.com.
Just in time for Memorial Day outings, a new report on sunscreens is out.
The news isn’t all sunny. , a nonprofit research and advocacy group that just issued its 16th annual Guide to Sunscreens.
In response, dermatologists, including the president of the American Academy of Dermatology, say that although some concerns have been raised about the safety of some sunscreen ingredients, sunscreens themselves remain an important tool in the fight against skin cancer. According to the Skin Cancer Foundation, 1 in 5 Americans will get skin cancer by age 70. Melanoma, the most deadly, has a 5-year survival rate of 99% if caught early.
2022 report
Overall, the Environmental Working Group found that about 1 in 4 sunscreens, or about 500 products, met their standards for providing adequate sun protection and avoiding ingredients linked to known health harms. Products meant for babies and children did slightly better, with about 1 in 3 meeting the standards. The group evaluated mineral sunscreens, also called physical sunscreens, and non-mineral sunscreens, also called chemical sunscreens. Mineral sunscreens contain zinc oxide or titanium dioxide and sit on the skin to deflect the sun’s rays. Chemical sunscreens, with ingredients such as oxybenzone or avobenzone, are partially absorbed into the skin.
Among the group’s concerns:
- The use of oxybenzone in the non-mineral sunscreens. About 30% of the non-mineral sunscreens have it, says Carla Burns, senior director for cosmetic science for the Environmental Working Group. Oxybenzone is a potential hormone disrupter and a skin sensitizer that may harm children and adults, she says. Some progress has been made, as the group found oxybenzone in 66% of the non-mineral sunscreens it reviewed in 2019. (The FDA is seeking more information on oxybenzone and many other sunscreen ingredients.)
- Contamination of sunscreens with benzene, which has been linked to leukemia and other blood disorders, according to the National Cancer Institute. But industry experts stress that that chemical is found in trace amounts in personal care products and does not pose a safety concern. “Benzene is a chemical that is ubiquitous in the environment and not an intentionally added ingredient in personal care products. People worldwide are exposed daily to benzene from indoor and outdoor sources, including air, drinking water, and food and beverages,” the Personal Care Products Council, an industry group, said in a statement.
- Protection from ultraviolet A (UVA) rays is often inadequate, according to research published last year by the Environmental Working Group.
Products on the ‘best’ list
The Environmental Working Group found that 282 recreational sunscreens met its criteria. Among them:
- Coral Safe Sunscreen Lotion, SPF 30
- Neutrogena Sheer Zinc Mineral Sunscreen Lotion, SPF 30
- Mad Hippie Facial Sunscreen Lotion, SPF 30+
The group chose 86 non-mineral sunscreens as better options, including:
- Alba Botanica Hawaiian Sunscreen Lotion, Aloe Vera, SPF 30
- Banana Boat Sport Ultra Sunscreen Stick, SPF 50+
- Black Girl Sunscreen Melanin Boosting Moisturizing Sunscreen Lotion, SPF 30
And 70 sunscreens made the kids’ best list, including:
- True Baby Everyday Play Sunscreen Lotion, SPF 30+
- Sun Biologic Kids’ Sunscreen Stick, SPF 30+
- Kiss My Face Organic Kids’ Defense Sunscreen Lotion, SPF 30
Industry response, FDA actions
In a statement, Alexandra Kowcz, chief scientist at the Personal Care Products Council, pointed out that “as part of a daily safe-sun regimen, sunscreen products help prevent sunburn and reduce skin cancer risk. It is unfortunate that as Americans spend more time outdoors, the Environmental Working Group’s (EWG) 2022 Guide to Sunscreens resorts to fear-mongering with misleading information that could keep consumers from using sunscreens altogether.”
The FDA has asked for more information about certain ingredients to further evaluate products, she says, and industry is working with the agency. The FDA says it is attempting to improve the quality, safety and effectiveness of over-the-counter sunscreen products. In September, 2021, the FDA issued a proposal for regulating OTC sunscreen products, as required under the CARES (Coronavirus Aid, Relief and Economic Security) Act. The effective date for the final order can’t be earlier than September 2022, the CARES Act says.
Dermatologists weigh in
“Every time something like this gets published, my patients come in hysterical,” says Michele Green, MD, a New York City dermatologist who reviewed the report for WebMD. She acknowledges that more research is needed on some sunscreen ingredients. “We really do not know the long-term consequence of oxybenzone,” she says.
Her advice: If her patients have melasma (a skin condition with brown patches on the face), she advises them to use both a chemical and a mineral sunscreen. “I don’t tell my patients in general not to use the chemical [sunscreens].”
For children, she says, the mineral sunscreens may be preferred. On her own children, who are teens, she uses the mineral sunscreens, due to possible concern about hormone disruption.
In a statement, Mark D. Kaufmann, MD, president of the American Academy of Dermatology, says that “sunscreen is an important part of a comprehensive sun protection strategy.”
Besides a broad-spectrum, water-resistant sunscreen with an SPF of 30 or higher for exposed skin, the academy recommends seeking shade and wearing sun-protective clothing to reduce skin cancer risk.
A version of this article first appeared on WebMD.com.
FDA warns companies selling OTC skin lighteners
The as the active ingredient, and don’t meet the requirements to be sold legally over the counter. The letters were dated April 13.
The 12 products with hydroquinone are “unapproved drugs and are not generally recognized as safe and effective” (abbreviated as GRASE), the FDA said.
Among the side effects associated with hydroquinone products reported to the FDA are skin rashes, facial swelling, and skin discoloration or ochronosis. The discoloration can be permanent, the FDA said. The lighteners are marketed for use on age or dark spots on the skin associated with melasma.
Tri-Luma, a prescription product for the treatment of moderate to severe melasma of the face, is the only FDA-approved drug containing hydroquinone, according to the FDA. It contains 4% hydroquinone and two other ingredients. It is meant to be used under the supervision of a health care professional. Tri-Luma is indicated for up to 8 weeks of treatment for moderate to severe melasma of the face. The OTC products contain up to 2%. (Generic versions of 4% hydroquinone are available by prescription, dermatologists said.)
“Hydroquinone is a very effective medication, and that’s exactly what it is, a medication,” said Lily Talakoub, MD, a dermatologist in McLean, Va., who supports the FDA action. “It’s very effective and very safe to use in the right hands, but when it is overused or used in the wrong situation, it can cause problems.” Those problems often occur, she said, when there is no health care professional overseeing the use of the OTC products, and when people use them over the long term.
The FDA action to ban the OTC products is “very appropriate,” said dermatologist Pooja Sodha, MD, assistant professor and director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington. “We know patients pick this up [an OTC product] and use it without physician oversight.” When patients use the products longer than is appropriate, which is also common, it can worsen the initial skin issue, she said.
The action follows reforms finalized under the CARES Act (Coronavirus Aid, Relief and Economic Security Act), which included not only COVID-19 response efforts but also updated the method in which certain OTC drugs are regulated. Manufacturers of the skin lightening products that don’t have FDA approval had been told to remove the products from the market by September 2020.
The recent letters were sent to a dozen companies still marketing their products without an FDA new drug approval. The agency asked the companies to take prompt action and respond with 15 days, stating what they have done to correct the violations.
The 12 companies are AMBI Enterprises, Clinical Formula, Elements Brands Inc., Genomma Lab USA, Intilight/Dr Thomas Balshi, M&M Beauty and Wellness, Neoteric Cosmetics/Scott’s Liquid Gold, Skin Authority, Skin Pro, Skin PS Brands, True Earth Health Products, and Ultimark Products.
Health care professionals and consumers can report adverse reactions associated with these products to the FDA’s MedWatch Adverse Event Reporting program.
A version of this article first appeared on Medscape.com.
The as the active ingredient, and don’t meet the requirements to be sold legally over the counter. The letters were dated April 13.
The 12 products with hydroquinone are “unapproved drugs and are not generally recognized as safe and effective” (abbreviated as GRASE), the FDA said.
Among the side effects associated with hydroquinone products reported to the FDA are skin rashes, facial swelling, and skin discoloration or ochronosis. The discoloration can be permanent, the FDA said. The lighteners are marketed for use on age or dark spots on the skin associated with melasma.
Tri-Luma, a prescription product for the treatment of moderate to severe melasma of the face, is the only FDA-approved drug containing hydroquinone, according to the FDA. It contains 4% hydroquinone and two other ingredients. It is meant to be used under the supervision of a health care professional. Tri-Luma is indicated for up to 8 weeks of treatment for moderate to severe melasma of the face. The OTC products contain up to 2%. (Generic versions of 4% hydroquinone are available by prescription, dermatologists said.)
“Hydroquinone is a very effective medication, and that’s exactly what it is, a medication,” said Lily Talakoub, MD, a dermatologist in McLean, Va., who supports the FDA action. “It’s very effective and very safe to use in the right hands, but when it is overused or used in the wrong situation, it can cause problems.” Those problems often occur, she said, when there is no health care professional overseeing the use of the OTC products, and when people use them over the long term.
The FDA action to ban the OTC products is “very appropriate,” said dermatologist Pooja Sodha, MD, assistant professor and director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington. “We know patients pick this up [an OTC product] and use it without physician oversight.” When patients use the products longer than is appropriate, which is also common, it can worsen the initial skin issue, she said.
The action follows reforms finalized under the CARES Act (Coronavirus Aid, Relief and Economic Security Act), which included not only COVID-19 response efforts but also updated the method in which certain OTC drugs are regulated. Manufacturers of the skin lightening products that don’t have FDA approval had been told to remove the products from the market by September 2020.
The recent letters were sent to a dozen companies still marketing their products without an FDA new drug approval. The agency asked the companies to take prompt action and respond with 15 days, stating what they have done to correct the violations.
The 12 companies are AMBI Enterprises, Clinical Formula, Elements Brands Inc., Genomma Lab USA, Intilight/Dr Thomas Balshi, M&M Beauty and Wellness, Neoteric Cosmetics/Scott’s Liquid Gold, Skin Authority, Skin Pro, Skin PS Brands, True Earth Health Products, and Ultimark Products.
Health care professionals and consumers can report adverse reactions associated with these products to the FDA’s MedWatch Adverse Event Reporting program.
A version of this article first appeared on Medscape.com.
The as the active ingredient, and don’t meet the requirements to be sold legally over the counter. The letters were dated April 13.
The 12 products with hydroquinone are “unapproved drugs and are not generally recognized as safe and effective” (abbreviated as GRASE), the FDA said.
Among the side effects associated with hydroquinone products reported to the FDA are skin rashes, facial swelling, and skin discoloration or ochronosis. The discoloration can be permanent, the FDA said. The lighteners are marketed for use on age or dark spots on the skin associated with melasma.
Tri-Luma, a prescription product for the treatment of moderate to severe melasma of the face, is the only FDA-approved drug containing hydroquinone, according to the FDA. It contains 4% hydroquinone and two other ingredients. It is meant to be used under the supervision of a health care professional. Tri-Luma is indicated for up to 8 weeks of treatment for moderate to severe melasma of the face. The OTC products contain up to 2%. (Generic versions of 4% hydroquinone are available by prescription, dermatologists said.)
“Hydroquinone is a very effective medication, and that’s exactly what it is, a medication,” said Lily Talakoub, MD, a dermatologist in McLean, Va., who supports the FDA action. “It’s very effective and very safe to use in the right hands, but when it is overused or used in the wrong situation, it can cause problems.” Those problems often occur, she said, when there is no health care professional overseeing the use of the OTC products, and when people use them over the long term.
The FDA action to ban the OTC products is “very appropriate,” said dermatologist Pooja Sodha, MD, assistant professor and director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington. “We know patients pick this up [an OTC product] and use it without physician oversight.” When patients use the products longer than is appropriate, which is also common, it can worsen the initial skin issue, she said.
The action follows reforms finalized under the CARES Act (Coronavirus Aid, Relief and Economic Security Act), which included not only COVID-19 response efforts but also updated the method in which certain OTC drugs are regulated. Manufacturers of the skin lightening products that don’t have FDA approval had been told to remove the products from the market by September 2020.
The recent letters were sent to a dozen companies still marketing their products without an FDA new drug approval. The agency asked the companies to take prompt action and respond with 15 days, stating what they have done to correct the violations.
The 12 companies are AMBI Enterprises, Clinical Formula, Elements Brands Inc., Genomma Lab USA, Intilight/Dr Thomas Balshi, M&M Beauty and Wellness, Neoteric Cosmetics/Scott’s Liquid Gold, Skin Authority, Skin Pro, Skin PS Brands, True Earth Health Products, and Ultimark Products.
Health care professionals and consumers can report adverse reactions associated with these products to the FDA’s MedWatch Adverse Event Reporting program.
A version of this article first appeared on Medscape.com.
Strawberries, spinach, kale: high on the ‘Dirty Dozen’ list
Once again, of the foods.
The yearly report comes from the Environmental Working Group, a nonprofit organization dedicated to improving human health and the environment, and also includes a “Clean 15” list of produce.
An industry group for growers of organic and nonorganic produce, along with some dietitians, make strong objections to the report, saying it raises unnecessary alarm and could discourage people from eating enough fruits and vegetables.
The report gives people valuable information, says the Environmental Working Group’s Alexis Temkin, PhD, a toxicologist, so they can make informed choices about the fruits and vegetables they buy.
Environmental Working Group researchers get data from the U.S. Department of Agriculture’s samplings of pesticide residue on produce done yearly or every 2 years, and from the Food and Drug Administration for honeydew melon, which the USDA doesn’t test for.
2022 results: Dirty Dozen
More than 70% of the conventionally grown produce had detectable pesticide residue, the Environmental Working Group found. These fruits and vegetables were found to have the most pesticide residues this year:
- 1. Strawberries
- 2. Spinach
- 3. Kale and collard and mustard greens
- 4. Nectarines
- 5. Apples
- 6. Grapes
- 7. Bell and hot peppers
- 8. Cherries
- 9. Peaches
- 10. Pears
- 11. Celery
- 12. Tomatoes
2022 results: Clean 15
Almost 70% of the Clean Fifteen fruit and vegetable samples had no detectable residues of pesticides, the Environmental Working Group found. Avocados and sweet corn were the cleanest, with less than 2% of samples showing any detectable pesticides.
- 1. Avocados
- 2. Sweet corn
- 3. Pineapple
- 4. Onions
- 5. Papaya
- 6. Sweet peas (frozen)
- 7. Asparagus
- 8. Honeydew melon
- 9. Kiwi
- 10. Cabbage
- 11. Mushrooms
- 12. Cantaloupe
- 13. Mangoes
- 14. Watermelon
- 15. Sweet potatoes
More on methods
To produce the report, the Environmental Working Group analyzed more than 44,000 samples taken by the FDA and USDA, which tests a subset of produce each year.
Before testing, USDA scientists prepare each fruit or vegetable the way people tend to do themselves, such as peeling those with inedible peels and rinsing produce with edible peels.
The Environmental Working Group takes six measures of pesticide contamination into account:
- Percent of samples tested with detectable pesticides
- Percent with two or more detectable pesticides
- Average number of pesticides in a single sample
- Average amount of pesticides, expressed in parts per million
- Maximum number of pesticides on a single sample
- Total number of pesticides found
Next, the Environmental Working Group researchers ranked the 46 fruits and vegetables analyzed, calculated a total score, and drew up the lists.
Industry criticism
The Alliance for Food and Farming, an industry group that represents organic and nonorganic farmers, growers, and shippers, takes strong issue with the annual report, noting that pesticide residues on conventional produce are low, if present at all.
“Ignore or discount the list,” says Teresa Thorne, executive director of the alliance. Like others, she fears that if an organic fruit or vegetable costs more, as they often do, consumers will bypass produce altogether, especially low-income consumers. “Pick what’s best for you and your family,” she says.
Temkin of the Environmental Working Group acknowledges that all the residues found were within legal limits set by the Environmental Protection Agency. “Although the levels are legal, that doesn’t necessarily mean they are safe,” she says.
The point of the rankings, she says, is to give people information so they can choose whether to buy organic or nonorganic produce. “Our recommendation is to buy the ones on the ‘Dirty Dozen’ list organic when available, or focus on the ‘Clean 15’ list.”
The Environmental Working Group depends on a broad base of support overall, according to information on its website, including companies that produce organic products such as Stonyfield Farms, Earthbound Farms, and Organic Valley.
But according to Iris Myers, an Environmental Working Group spokesperson, the Shopper’s Guide with the clean and dirty produce rankings “isn’t funded by any companies – only grants and individual donors. We don’t allow companies to sponsor any of our research reports.”
In the report, the Environmental Working Group also notes that the EPA has taken action to prohibit the pesticide chlorpyrifos in food, after the group and others spent years asking for the ban.
Dietitians weigh in
The report uses “fear-branded messages to steer people away from eating conventionally grown fruits and veggies,” says Christine Rosenbloom, PhD, a retired Georgia State University professor and an Atlanta nutrition consultant.
She reminds people that “both organic and conventional agriculture use pesticides to protect the crop. Organic famers use different pesticides that are described as ‘natural,’ but natural doesn’t mean safer, better, or chemical-free,” she says.
She refers people to the Pesticide Residue Calculator from toxicologists at the University of California, Riverside, posted on the consumer site the Alliance for Food and Farming.
The calculator helps reassure people that trace amounts of chemicals on conventionally grown produce are not a hazard to your health, Dr. Rosenbloom says. “Using myself as an example, I could eat 850 apples or 13,225 servings of blueberries in one day without any effect, even in the worst-case scenario of the fruit having the highest pesticide residue recorded by the USDA.”
“It’s one more example of putting good and bad food labels on foods when it isn’t deserved,” says Connie Diekman, a food and nutrition consultant in St. Louis and a former president of the Academy of Nutrition and Dietetics. “The amounts they are measuring are so much below the tolerance level set by the EPA.”
The report shouldn’t scare people, including parents worried about serving their children conventional produce, she says.
As for how much produce to eat, “the best advice is to have half your plate be fruits and vegetables,” Ms. Diekman says. Under current Dietary Guidelines for Americans, an intake of 2½ “cups equivalent” of vegetables and 2 “cups equivalent” of fruits is recommended daily for adults.
Ms. Diekman is on the Bayer LEAD Network, Leaders Engaged in Advancing Dialogue. Dr. Rosenbloom reports an honorarium from a bean industry group for developing a webinar on healthy aging.
A version of this article first appeared on WebMD.com.
Once again, of the foods.
The yearly report comes from the Environmental Working Group, a nonprofit organization dedicated to improving human health and the environment, and also includes a “Clean 15” list of produce.
An industry group for growers of organic and nonorganic produce, along with some dietitians, make strong objections to the report, saying it raises unnecessary alarm and could discourage people from eating enough fruits and vegetables.
The report gives people valuable information, says the Environmental Working Group’s Alexis Temkin, PhD, a toxicologist, so they can make informed choices about the fruits and vegetables they buy.
Environmental Working Group researchers get data from the U.S. Department of Agriculture’s samplings of pesticide residue on produce done yearly or every 2 years, and from the Food and Drug Administration for honeydew melon, which the USDA doesn’t test for.
2022 results: Dirty Dozen
More than 70% of the conventionally grown produce had detectable pesticide residue, the Environmental Working Group found. These fruits and vegetables were found to have the most pesticide residues this year:
- 1. Strawberries
- 2. Spinach
- 3. Kale and collard and mustard greens
- 4. Nectarines
- 5. Apples
- 6. Grapes
- 7. Bell and hot peppers
- 8. Cherries
- 9. Peaches
- 10. Pears
- 11. Celery
- 12. Tomatoes
2022 results: Clean 15
Almost 70% of the Clean Fifteen fruit and vegetable samples had no detectable residues of pesticides, the Environmental Working Group found. Avocados and sweet corn were the cleanest, with less than 2% of samples showing any detectable pesticides.
- 1. Avocados
- 2. Sweet corn
- 3. Pineapple
- 4. Onions
- 5. Papaya
- 6. Sweet peas (frozen)
- 7. Asparagus
- 8. Honeydew melon
- 9. Kiwi
- 10. Cabbage
- 11. Mushrooms
- 12. Cantaloupe
- 13. Mangoes
- 14. Watermelon
- 15. Sweet potatoes
More on methods
To produce the report, the Environmental Working Group analyzed more than 44,000 samples taken by the FDA and USDA, which tests a subset of produce each year.
Before testing, USDA scientists prepare each fruit or vegetable the way people tend to do themselves, such as peeling those with inedible peels and rinsing produce with edible peels.
The Environmental Working Group takes six measures of pesticide contamination into account:
- Percent of samples tested with detectable pesticides
- Percent with two or more detectable pesticides
- Average number of pesticides in a single sample
- Average amount of pesticides, expressed in parts per million
- Maximum number of pesticides on a single sample
- Total number of pesticides found
Next, the Environmental Working Group researchers ranked the 46 fruits and vegetables analyzed, calculated a total score, and drew up the lists.
Industry criticism
The Alliance for Food and Farming, an industry group that represents organic and nonorganic farmers, growers, and shippers, takes strong issue with the annual report, noting that pesticide residues on conventional produce are low, if present at all.
“Ignore or discount the list,” says Teresa Thorne, executive director of the alliance. Like others, she fears that if an organic fruit or vegetable costs more, as they often do, consumers will bypass produce altogether, especially low-income consumers. “Pick what’s best for you and your family,” she says.
Temkin of the Environmental Working Group acknowledges that all the residues found were within legal limits set by the Environmental Protection Agency. “Although the levels are legal, that doesn’t necessarily mean they are safe,” she says.
The point of the rankings, she says, is to give people information so they can choose whether to buy organic or nonorganic produce. “Our recommendation is to buy the ones on the ‘Dirty Dozen’ list organic when available, or focus on the ‘Clean 15’ list.”
The Environmental Working Group depends on a broad base of support overall, according to information on its website, including companies that produce organic products such as Stonyfield Farms, Earthbound Farms, and Organic Valley.
But according to Iris Myers, an Environmental Working Group spokesperson, the Shopper’s Guide with the clean and dirty produce rankings “isn’t funded by any companies – only grants and individual donors. We don’t allow companies to sponsor any of our research reports.”
In the report, the Environmental Working Group also notes that the EPA has taken action to prohibit the pesticide chlorpyrifos in food, after the group and others spent years asking for the ban.
Dietitians weigh in
The report uses “fear-branded messages to steer people away from eating conventionally grown fruits and veggies,” says Christine Rosenbloom, PhD, a retired Georgia State University professor and an Atlanta nutrition consultant.
She reminds people that “both organic and conventional agriculture use pesticides to protect the crop. Organic famers use different pesticides that are described as ‘natural,’ but natural doesn’t mean safer, better, or chemical-free,” she says.
She refers people to the Pesticide Residue Calculator from toxicologists at the University of California, Riverside, posted on the consumer site the Alliance for Food and Farming.
The calculator helps reassure people that trace amounts of chemicals on conventionally grown produce are not a hazard to your health, Dr. Rosenbloom says. “Using myself as an example, I could eat 850 apples or 13,225 servings of blueberries in one day without any effect, even in the worst-case scenario of the fruit having the highest pesticide residue recorded by the USDA.”
“It’s one more example of putting good and bad food labels on foods when it isn’t deserved,” says Connie Diekman, a food and nutrition consultant in St. Louis and a former president of the Academy of Nutrition and Dietetics. “The amounts they are measuring are so much below the tolerance level set by the EPA.”
The report shouldn’t scare people, including parents worried about serving their children conventional produce, she says.
As for how much produce to eat, “the best advice is to have half your plate be fruits and vegetables,” Ms. Diekman says. Under current Dietary Guidelines for Americans, an intake of 2½ “cups equivalent” of vegetables and 2 “cups equivalent” of fruits is recommended daily for adults.
Ms. Diekman is on the Bayer LEAD Network, Leaders Engaged in Advancing Dialogue. Dr. Rosenbloom reports an honorarium from a bean industry group for developing a webinar on healthy aging.
A version of this article first appeared on WebMD.com.
Once again, of the foods.
The yearly report comes from the Environmental Working Group, a nonprofit organization dedicated to improving human health and the environment, and also includes a “Clean 15” list of produce.
An industry group for growers of organic and nonorganic produce, along with some dietitians, make strong objections to the report, saying it raises unnecessary alarm and could discourage people from eating enough fruits and vegetables.
The report gives people valuable information, says the Environmental Working Group’s Alexis Temkin, PhD, a toxicologist, so they can make informed choices about the fruits and vegetables they buy.
Environmental Working Group researchers get data from the U.S. Department of Agriculture’s samplings of pesticide residue on produce done yearly or every 2 years, and from the Food and Drug Administration for honeydew melon, which the USDA doesn’t test for.
2022 results: Dirty Dozen
More than 70% of the conventionally grown produce had detectable pesticide residue, the Environmental Working Group found. These fruits and vegetables were found to have the most pesticide residues this year:
- 1. Strawberries
- 2. Spinach
- 3. Kale and collard and mustard greens
- 4. Nectarines
- 5. Apples
- 6. Grapes
- 7. Bell and hot peppers
- 8. Cherries
- 9. Peaches
- 10. Pears
- 11. Celery
- 12. Tomatoes
2022 results: Clean 15
Almost 70% of the Clean Fifteen fruit and vegetable samples had no detectable residues of pesticides, the Environmental Working Group found. Avocados and sweet corn were the cleanest, with less than 2% of samples showing any detectable pesticides.
- 1. Avocados
- 2. Sweet corn
- 3. Pineapple
- 4. Onions
- 5. Papaya
- 6. Sweet peas (frozen)
- 7. Asparagus
- 8. Honeydew melon
- 9. Kiwi
- 10. Cabbage
- 11. Mushrooms
- 12. Cantaloupe
- 13. Mangoes
- 14. Watermelon
- 15. Sweet potatoes
More on methods
To produce the report, the Environmental Working Group analyzed more than 44,000 samples taken by the FDA and USDA, which tests a subset of produce each year.
Before testing, USDA scientists prepare each fruit or vegetable the way people tend to do themselves, such as peeling those with inedible peels and rinsing produce with edible peels.
The Environmental Working Group takes six measures of pesticide contamination into account:
- Percent of samples tested with detectable pesticides
- Percent with two or more detectable pesticides
- Average number of pesticides in a single sample
- Average amount of pesticides, expressed in parts per million
- Maximum number of pesticides on a single sample
- Total number of pesticides found
Next, the Environmental Working Group researchers ranked the 46 fruits and vegetables analyzed, calculated a total score, and drew up the lists.
Industry criticism
The Alliance for Food and Farming, an industry group that represents organic and nonorganic farmers, growers, and shippers, takes strong issue with the annual report, noting that pesticide residues on conventional produce are low, if present at all.
“Ignore or discount the list,” says Teresa Thorne, executive director of the alliance. Like others, she fears that if an organic fruit or vegetable costs more, as they often do, consumers will bypass produce altogether, especially low-income consumers. “Pick what’s best for you and your family,” she says.
Temkin of the Environmental Working Group acknowledges that all the residues found were within legal limits set by the Environmental Protection Agency. “Although the levels are legal, that doesn’t necessarily mean they are safe,” she says.
The point of the rankings, she says, is to give people information so they can choose whether to buy organic or nonorganic produce. “Our recommendation is to buy the ones on the ‘Dirty Dozen’ list organic when available, or focus on the ‘Clean 15’ list.”
The Environmental Working Group depends on a broad base of support overall, according to information on its website, including companies that produce organic products such as Stonyfield Farms, Earthbound Farms, and Organic Valley.
But according to Iris Myers, an Environmental Working Group spokesperson, the Shopper’s Guide with the clean and dirty produce rankings “isn’t funded by any companies – only grants and individual donors. We don’t allow companies to sponsor any of our research reports.”
In the report, the Environmental Working Group also notes that the EPA has taken action to prohibit the pesticide chlorpyrifos in food, after the group and others spent years asking for the ban.
Dietitians weigh in
The report uses “fear-branded messages to steer people away from eating conventionally grown fruits and veggies,” says Christine Rosenbloom, PhD, a retired Georgia State University professor and an Atlanta nutrition consultant.
She reminds people that “both organic and conventional agriculture use pesticides to protect the crop. Organic famers use different pesticides that are described as ‘natural,’ but natural doesn’t mean safer, better, or chemical-free,” she says.
She refers people to the Pesticide Residue Calculator from toxicologists at the University of California, Riverside, posted on the consumer site the Alliance for Food and Farming.
The calculator helps reassure people that trace amounts of chemicals on conventionally grown produce are not a hazard to your health, Dr. Rosenbloom says. “Using myself as an example, I could eat 850 apples or 13,225 servings of blueberries in one day without any effect, even in the worst-case scenario of the fruit having the highest pesticide residue recorded by the USDA.”
“It’s one more example of putting good and bad food labels on foods when it isn’t deserved,” says Connie Diekman, a food and nutrition consultant in St. Louis and a former president of the Academy of Nutrition and Dietetics. “The amounts they are measuring are so much below the tolerance level set by the EPA.”
The report shouldn’t scare people, including parents worried about serving their children conventional produce, she says.
As for how much produce to eat, “the best advice is to have half your plate be fruits and vegetables,” Ms. Diekman says. Under current Dietary Guidelines for Americans, an intake of 2½ “cups equivalent” of vegetables and 2 “cups equivalent” of fruits is recommended daily for adults.
Ms. Diekman is on the Bayer LEAD Network, Leaders Engaged in Advancing Dialogue. Dr. Rosenbloom reports an honorarium from a bean industry group for developing a webinar on healthy aging.
A version of this article first appeared on WebMD.com.
Oral tofacitinib produces hair regrowth in children with alopecia areata
and published in Pediatric Dermatology.
The 11 pediatric patients, ages 8-18 years, all with a diagnosis of AA, were treated with tofacitinib. Eight patients, or nearly 73%, experienced hair regrowth, while the other three (27.3%) did not, as the investigators reported in the retrospective chart review.
“A success rate of 73% is very good,” said lead author Cory A. Dunnick, MD, professor of dermatology and director of clinical trials at the University of Colorado at Denver, Aurora. No serious adverse events occurred, and adverse events of any kind were limited, the researchers found.
“It is important to get information into the literature about potential treatments for severe alopecia areata because there is no [Food and Drug Administration]–approved therapy at the present time,” Dr. Dunnick told this news organization. Patients’ insurance plans often deny non–FDA-approved therapies unless there are data to support their use.
The researchers found no correlation between the dose, duration of treatment, or the presence of comorbidities and clinical response.
Oral tofacitinib has been shown to be effective and well tolerated for AA in adults, the researchers said. They referred to recent studies that have used JAK inhibitors, including tofacitinib, “in an effort to inhibit T-cell activation and halt disease progression in adult and pediatric patients” with AA.
Study details
Of the 11 patients evaluated, 6 had alopecia universalis, 1 had alopecia totalis, and 4 had patchy AA. Concomitant medical conditions known to be associated with AA affected four patients. These included atopic dermatitis, autoimmune hypothyroidism, and asthma. One patient reported having two brothers with AA.
The median disease duration was 6 years. “In my experience, JAK inhibitors are less effective for patients with longstanding – more than 10 years – alopecia totalis or alopecia universalis,” Dr. Dunnick said.
Previously, patients had been given methotrexate, oral prednisone, intralesional triamcinolone, topical corticosteroids, and topical diphenylcyclopropenone. During treatment with tofacitinib, 5 of the 11 patients also received topical steroid treatment.
The study was a retrospective chart review, so dosing was not standardized, the researchers said. Most took 5-10 mg twice daily. Median treatment time was 32 months, with a range of 5-39 months.
Patients with a complete or near complete clinical response were categorized as responders; subjectively, these were the patients who had persistent hair regrowth over more than 50% of affected areas. Five patients had complete regrowth of hair on the scalp, eyebrows, and body during treatment. Others had incomplete responses. For instance, one patient had improved growth of eyelashes and eyebrows but not on the scalp. Once the medication was increased to 15 mg daily, the patient had complete regrowth of body hair, eyelashes, and eyebrows but slow regrowth on the scalp after 1 year of treatment.
“Patients are very happy with regrowth of their hair,” Dr. Dunnick said, noting that severe AA affects self-esteem and quality of life.
Other research
In a retrospective study that looked at the effects of oral tofacitinib given to 14 preadolescent patients with AA, 9 experienced “clinically significant improvement” in their Severity of Alopecia Tool score. Three had complete remission, and seven (63.6%) had more than a 50% improvement in the score.
Mechanisms, concerns
The researchers of the current study explained that interferon signaling activity through the JAK pathways is a key mediator of the inflammation and cytotoxic T-cell response in AA. That modulation of the signaling may decrease disease progression, as the results of the current chart review suggest.
A main concern, the researchers wrote, is the potential for significant adverse events. Although this chart review did not find any, the researchers did see some transient lab abnormalities. One study found lab abnormalities in such measures as triglycerides and cholesterol.
Asked to comment on the study results, Brett King, MD, PHD, associate professor of dermatology at Yale University, New Haven, Conn., said that the study “is an important addition to a series of articles dating back to 2017 showing efficacy of tofacitinib in the pediatric age group.” The results are similar to those of previous studies, “showing that severe AA can be treated effectively with tofacitinib. Cumulatively, there is significant data to support treatment of this age group with JAK inhibitors,” he said.
At the 2021 European Academy of Dermatology and Venereology meeting, Dr. King presented the results of two phase 3 studies, which found that treatment with the oral JAK inhibitor baricitinib resulted in substantial hair growth in adults with AA. He and colleagues have also reported positive results of tofacitinib in treating AA in four children ages 8-10, with alopecia totalis and alopecia universalis, and in adolescents with AA.
Currently, three large, randomized, phase 3 clinical trials of other JAK inhibitors for AA are underway – ritlecitinib, baricitinib, and ruxolitinib – and the ritlecitinib trial includes adolescents (ages 12 years and older). “It is the results of these trials that we eagerly await, because FDA approval will bring greater access to these treatments,” Dr. King said.
Dr. Dunnick has disclosed no relevant financial relationships. Dr. King has served on advisory boards and/or is a consultant and/or a clinical trial investigator for AbbVie, Bristol-Myers Squibb, Concert Pharmaceuticals, Eli Lilly, Incyte, Pfizer, and others. He is on speaker bureaus for AbbVie, Incyte, Pfizer, and others.
A version of this article first appeared on Medscape.com.
and published in Pediatric Dermatology.
The 11 pediatric patients, ages 8-18 years, all with a diagnosis of AA, were treated with tofacitinib. Eight patients, or nearly 73%, experienced hair regrowth, while the other three (27.3%) did not, as the investigators reported in the retrospective chart review.
“A success rate of 73% is very good,” said lead author Cory A. Dunnick, MD, professor of dermatology and director of clinical trials at the University of Colorado at Denver, Aurora. No serious adverse events occurred, and adverse events of any kind were limited, the researchers found.
“It is important to get information into the literature about potential treatments for severe alopecia areata because there is no [Food and Drug Administration]–approved therapy at the present time,” Dr. Dunnick told this news organization. Patients’ insurance plans often deny non–FDA-approved therapies unless there are data to support their use.
The researchers found no correlation between the dose, duration of treatment, or the presence of comorbidities and clinical response.
Oral tofacitinib has been shown to be effective and well tolerated for AA in adults, the researchers said. They referred to recent studies that have used JAK inhibitors, including tofacitinib, “in an effort to inhibit T-cell activation and halt disease progression in adult and pediatric patients” with AA.
Study details
Of the 11 patients evaluated, 6 had alopecia universalis, 1 had alopecia totalis, and 4 had patchy AA. Concomitant medical conditions known to be associated with AA affected four patients. These included atopic dermatitis, autoimmune hypothyroidism, and asthma. One patient reported having two brothers with AA.
The median disease duration was 6 years. “In my experience, JAK inhibitors are less effective for patients with longstanding – more than 10 years – alopecia totalis or alopecia universalis,” Dr. Dunnick said.
Previously, patients had been given methotrexate, oral prednisone, intralesional triamcinolone, topical corticosteroids, and topical diphenylcyclopropenone. During treatment with tofacitinib, 5 of the 11 patients also received topical steroid treatment.
The study was a retrospective chart review, so dosing was not standardized, the researchers said. Most took 5-10 mg twice daily. Median treatment time was 32 months, with a range of 5-39 months.
Patients with a complete or near complete clinical response were categorized as responders; subjectively, these were the patients who had persistent hair regrowth over more than 50% of affected areas. Five patients had complete regrowth of hair on the scalp, eyebrows, and body during treatment. Others had incomplete responses. For instance, one patient had improved growth of eyelashes and eyebrows but not on the scalp. Once the medication was increased to 15 mg daily, the patient had complete regrowth of body hair, eyelashes, and eyebrows but slow regrowth on the scalp after 1 year of treatment.
“Patients are very happy with regrowth of their hair,” Dr. Dunnick said, noting that severe AA affects self-esteem and quality of life.
Other research
In a retrospective study that looked at the effects of oral tofacitinib given to 14 preadolescent patients with AA, 9 experienced “clinically significant improvement” in their Severity of Alopecia Tool score. Three had complete remission, and seven (63.6%) had more than a 50% improvement in the score.
Mechanisms, concerns
The researchers of the current study explained that interferon signaling activity through the JAK pathways is a key mediator of the inflammation and cytotoxic T-cell response in AA. That modulation of the signaling may decrease disease progression, as the results of the current chart review suggest.
A main concern, the researchers wrote, is the potential for significant adverse events. Although this chart review did not find any, the researchers did see some transient lab abnormalities. One study found lab abnormalities in such measures as triglycerides and cholesterol.
Asked to comment on the study results, Brett King, MD, PHD, associate professor of dermatology at Yale University, New Haven, Conn., said that the study “is an important addition to a series of articles dating back to 2017 showing efficacy of tofacitinib in the pediatric age group.” The results are similar to those of previous studies, “showing that severe AA can be treated effectively with tofacitinib. Cumulatively, there is significant data to support treatment of this age group with JAK inhibitors,” he said.
At the 2021 European Academy of Dermatology and Venereology meeting, Dr. King presented the results of two phase 3 studies, which found that treatment with the oral JAK inhibitor baricitinib resulted in substantial hair growth in adults with AA. He and colleagues have also reported positive results of tofacitinib in treating AA in four children ages 8-10, with alopecia totalis and alopecia universalis, and in adolescents with AA.
Currently, three large, randomized, phase 3 clinical trials of other JAK inhibitors for AA are underway – ritlecitinib, baricitinib, and ruxolitinib – and the ritlecitinib trial includes adolescents (ages 12 years and older). “It is the results of these trials that we eagerly await, because FDA approval will bring greater access to these treatments,” Dr. King said.
Dr. Dunnick has disclosed no relevant financial relationships. Dr. King has served on advisory boards and/or is a consultant and/or a clinical trial investigator for AbbVie, Bristol-Myers Squibb, Concert Pharmaceuticals, Eli Lilly, Incyte, Pfizer, and others. He is on speaker bureaus for AbbVie, Incyte, Pfizer, and others.
A version of this article first appeared on Medscape.com.
and published in Pediatric Dermatology.
The 11 pediatric patients, ages 8-18 years, all with a diagnosis of AA, were treated with tofacitinib. Eight patients, or nearly 73%, experienced hair regrowth, while the other three (27.3%) did not, as the investigators reported in the retrospective chart review.
“A success rate of 73% is very good,” said lead author Cory A. Dunnick, MD, professor of dermatology and director of clinical trials at the University of Colorado at Denver, Aurora. No serious adverse events occurred, and adverse events of any kind were limited, the researchers found.
“It is important to get information into the literature about potential treatments for severe alopecia areata because there is no [Food and Drug Administration]–approved therapy at the present time,” Dr. Dunnick told this news organization. Patients’ insurance plans often deny non–FDA-approved therapies unless there are data to support their use.
The researchers found no correlation between the dose, duration of treatment, or the presence of comorbidities and clinical response.
Oral tofacitinib has been shown to be effective and well tolerated for AA in adults, the researchers said. They referred to recent studies that have used JAK inhibitors, including tofacitinib, “in an effort to inhibit T-cell activation and halt disease progression in adult and pediatric patients” with AA.
Study details
Of the 11 patients evaluated, 6 had alopecia universalis, 1 had alopecia totalis, and 4 had patchy AA. Concomitant medical conditions known to be associated with AA affected four patients. These included atopic dermatitis, autoimmune hypothyroidism, and asthma. One patient reported having two brothers with AA.
The median disease duration was 6 years. “In my experience, JAK inhibitors are less effective for patients with longstanding – more than 10 years – alopecia totalis or alopecia universalis,” Dr. Dunnick said.
Previously, patients had been given methotrexate, oral prednisone, intralesional triamcinolone, topical corticosteroids, and topical diphenylcyclopropenone. During treatment with tofacitinib, 5 of the 11 patients also received topical steroid treatment.
The study was a retrospective chart review, so dosing was not standardized, the researchers said. Most took 5-10 mg twice daily. Median treatment time was 32 months, with a range of 5-39 months.
Patients with a complete or near complete clinical response were categorized as responders; subjectively, these were the patients who had persistent hair regrowth over more than 50% of affected areas. Five patients had complete regrowth of hair on the scalp, eyebrows, and body during treatment. Others had incomplete responses. For instance, one patient had improved growth of eyelashes and eyebrows but not on the scalp. Once the medication was increased to 15 mg daily, the patient had complete regrowth of body hair, eyelashes, and eyebrows but slow regrowth on the scalp after 1 year of treatment.
“Patients are very happy with regrowth of their hair,” Dr. Dunnick said, noting that severe AA affects self-esteem and quality of life.
Other research
In a retrospective study that looked at the effects of oral tofacitinib given to 14 preadolescent patients with AA, 9 experienced “clinically significant improvement” in their Severity of Alopecia Tool score. Three had complete remission, and seven (63.6%) had more than a 50% improvement in the score.
Mechanisms, concerns
The researchers of the current study explained that interferon signaling activity through the JAK pathways is a key mediator of the inflammation and cytotoxic T-cell response in AA. That modulation of the signaling may decrease disease progression, as the results of the current chart review suggest.
A main concern, the researchers wrote, is the potential for significant adverse events. Although this chart review did not find any, the researchers did see some transient lab abnormalities. One study found lab abnormalities in such measures as triglycerides and cholesterol.
Asked to comment on the study results, Brett King, MD, PHD, associate professor of dermatology at Yale University, New Haven, Conn., said that the study “is an important addition to a series of articles dating back to 2017 showing efficacy of tofacitinib in the pediatric age group.” The results are similar to those of previous studies, “showing that severe AA can be treated effectively with tofacitinib. Cumulatively, there is significant data to support treatment of this age group with JAK inhibitors,” he said.
At the 2021 European Academy of Dermatology and Venereology meeting, Dr. King presented the results of two phase 3 studies, which found that treatment with the oral JAK inhibitor baricitinib resulted in substantial hair growth in adults with AA. He and colleagues have also reported positive results of tofacitinib in treating AA in four children ages 8-10, with alopecia totalis and alopecia universalis, and in adolescents with AA.
Currently, three large, randomized, phase 3 clinical trials of other JAK inhibitors for AA are underway – ritlecitinib, baricitinib, and ruxolitinib – and the ritlecitinib trial includes adolescents (ages 12 years and older). “It is the results of these trials that we eagerly await, because FDA approval will bring greater access to these treatments,” Dr. King said.
Dr. Dunnick has disclosed no relevant financial relationships. Dr. King has served on advisory boards and/or is a consultant and/or a clinical trial investigator for AbbVie, Bristol-Myers Squibb, Concert Pharmaceuticals, Eli Lilly, Incyte, Pfizer, and others. He is on speaker bureaus for AbbVie, Incyte, Pfizer, and others.
A version of this article first appeared on Medscape.com.
FROM PEDIATRIC DERMATOLOGY
Third transplant patient cured of HIV marks important firsts
that has plagued the world for decades.
But while this case is certainly cause for celebration, experts involved in the effort say we are still a long way from a universal cure.
Researcher Yvonne Bryson, MD, chief of pediatric infectious diseases at the University of California, Los Angeles, told those attending the Conference on Retroviruses and Opportunistic Infections that this case is special. The patient was a woman living with HIV who is multiracial. The previous two patients were men: one white, one Latinx.
The woman in this case was given transplants of stem cells and umbilical cord blood to treat leukemia. The treatment not only sent her cancer into remission, but her HIV as well.
The success of this case suggests that cord stem cell transplants should be considered to produce remission and cure for those with HIV who also have cancers and other diseases, the researchers said.
While the news was met with excitement in the scientific community, the approach will not be available universally, since the transplants were all done to treat cancers in the three HIV-infected patients. Overall, Dr. Bryson estimates that about 50 people per year may benefit from this procedure.
Even so, other experts say the approach could provide insight into other ways to find cures. And Dr. Bryson says it opens up options for more diverse populations.
“A bone marrow transplant is not a viable large-scale strategy for curing HIV, but it does present a proof of concept that HIV can be cured,” said Sharon Lewin, MD, president-elect of the International AIDS Society. “It also further strengthens using gene therapy as a viable strategy for an HIV cure.”
The woman needed a stem cell transplant after being diagnosed with leukemia. The stem cell transplant technique used was also novel, Dr. Bryson said. The medical team used a combination of adult stem cells from a relative’s blood and umbilical cord blood from a cord-blood bank that had a rare mutation that makes the immune system resistant to HIV.
In the previous two cases of HIV cures after transplants, both patients were treated with stem cell transplants, with the same mutation, but from bone marrow transplants, a more difficult procedure. And no cord blood was used for those.
The combination of adult cells and cord-blood cells proved to be the ticket to success. Using the adult cells provides a kind of bridge that helps until the cord blood takes over, the researchers said. By day 100 after the transplant, Dr. Bryson said, the woman basically had a new immune system.
HIV remained undetectable in T cells and in bone marrow. And 37 months after the transplant, the woman stopped taking the antiretroviral treatment commonly given to treat HIV infection.
‘’She is currently clinically well,” Dr. Bryson said. Her cancer is in remission.
Case histories: Three patients
The woman, who is middle-aged, has requested privacy, asking that neither her age nor other details be released. But the researchers did provide some background on her medical history and her route back to health. She was diagnosed with HIV in 2013 and began treatment with antiretroviral therapy (ART). Four years after her HIV diagnosis, she developed high-risk acute myelogenous leukemia. The transplant was done to treat that.
Her recovery was much less bumpy than that of the previous two patients, the researchers said. She left the hospital 17 days after the transplant. She didn’t have serious complications like the first two, who developed a condition that occurs when donor bone marrow or stem cells attack the recipient.
“This case also suggests that it’s the transplant of HIV-resistant cells that was key to achieving a cure here,” said Dr. Lewin. The first patient who had HIV remission after a stem cell transplant, a White man, stayed in remission for 12 years and was termed cured. But he died of leukemia in September 2020. The other, a Latinx man, has been in remission for more than 30 months.
HIV statistics, ethnic/racial burdens
In the United States, about 1.2 million people have HIV, according to HIV.gov. Thirteen percent of those who have it do not know they have it. In 2019, 34,800 new infections were diagnosed.
Certain ethnic and racial groups are more affected by HIV than others, given their proportions in the U.S. population, federal statistics suggest. In 2019, for instance, African Americans were 13% of the U.S. population but 40% of those with HIV. Hispanics/Latinx represented 18.5% of the total population but 25% of those diagnosed with HIV.
Disparities also affect women unequally, with Black women disproportionately affected, compared to women of other ethnic and racial groups. Annual HIV infections remained stable overall among Black women from 2015 to 2019, but the rate of new HIV infections among Black women is 11 times that of White women and 4 times that of Latinx, according to federal statistics.
Expert perspective, reactions
Vincent Marconi, MD, professor of infectious diseases at Emory University, Atlanta, whose research focuses on disparities in HIV treatment responses, called the news “an exciting development for the cure agenda. This is the first woman to have been cured for at least 14 months, and they used cord blood, which could allow for potentially less toxic regimens and fewer adverse effects.”
Although the approach, meant to be used to treat the cancers, will not be widely available, he said that ‘’it does provide insight into somewhat related alternative models of cure involving gene therapy.”
Meanwhile, Dr. Marconi and other researchers are focusing on the concept of long-term HIV remission if a cure is not possible. Among the strategies under study are gene editing and immune-based treatments. HIV remission is generally defined as having an HIV viral load that is not detectable after stopping treatment.
A version of this article first appeared on WebMD.com.
that has plagued the world for decades.
But while this case is certainly cause for celebration, experts involved in the effort say we are still a long way from a universal cure.
Researcher Yvonne Bryson, MD, chief of pediatric infectious diseases at the University of California, Los Angeles, told those attending the Conference on Retroviruses and Opportunistic Infections that this case is special. The patient was a woman living with HIV who is multiracial. The previous two patients were men: one white, one Latinx.
The woman in this case was given transplants of stem cells and umbilical cord blood to treat leukemia. The treatment not only sent her cancer into remission, but her HIV as well.
The success of this case suggests that cord stem cell transplants should be considered to produce remission and cure for those with HIV who also have cancers and other diseases, the researchers said.
While the news was met with excitement in the scientific community, the approach will not be available universally, since the transplants were all done to treat cancers in the three HIV-infected patients. Overall, Dr. Bryson estimates that about 50 people per year may benefit from this procedure.
Even so, other experts say the approach could provide insight into other ways to find cures. And Dr. Bryson says it opens up options for more diverse populations.
“A bone marrow transplant is not a viable large-scale strategy for curing HIV, but it does present a proof of concept that HIV can be cured,” said Sharon Lewin, MD, president-elect of the International AIDS Society. “It also further strengthens using gene therapy as a viable strategy for an HIV cure.”
The woman needed a stem cell transplant after being diagnosed with leukemia. The stem cell transplant technique used was also novel, Dr. Bryson said. The medical team used a combination of adult stem cells from a relative’s blood and umbilical cord blood from a cord-blood bank that had a rare mutation that makes the immune system resistant to HIV.
In the previous two cases of HIV cures after transplants, both patients were treated with stem cell transplants, with the same mutation, but from bone marrow transplants, a more difficult procedure. And no cord blood was used for those.
The combination of adult cells and cord-blood cells proved to be the ticket to success. Using the adult cells provides a kind of bridge that helps until the cord blood takes over, the researchers said. By day 100 after the transplant, Dr. Bryson said, the woman basically had a new immune system.
HIV remained undetectable in T cells and in bone marrow. And 37 months after the transplant, the woman stopped taking the antiretroviral treatment commonly given to treat HIV infection.
‘’She is currently clinically well,” Dr. Bryson said. Her cancer is in remission.
Case histories: Three patients
The woman, who is middle-aged, has requested privacy, asking that neither her age nor other details be released. But the researchers did provide some background on her medical history and her route back to health. She was diagnosed with HIV in 2013 and began treatment with antiretroviral therapy (ART). Four years after her HIV diagnosis, she developed high-risk acute myelogenous leukemia. The transplant was done to treat that.
Her recovery was much less bumpy than that of the previous two patients, the researchers said. She left the hospital 17 days after the transplant. She didn’t have serious complications like the first two, who developed a condition that occurs when donor bone marrow or stem cells attack the recipient.
“This case also suggests that it’s the transplant of HIV-resistant cells that was key to achieving a cure here,” said Dr. Lewin. The first patient who had HIV remission after a stem cell transplant, a White man, stayed in remission for 12 years and was termed cured. But he died of leukemia in September 2020. The other, a Latinx man, has been in remission for more than 30 months.
HIV statistics, ethnic/racial burdens
In the United States, about 1.2 million people have HIV, according to HIV.gov. Thirteen percent of those who have it do not know they have it. In 2019, 34,800 new infections were diagnosed.
Certain ethnic and racial groups are more affected by HIV than others, given their proportions in the U.S. population, federal statistics suggest. In 2019, for instance, African Americans were 13% of the U.S. population but 40% of those with HIV. Hispanics/Latinx represented 18.5% of the total population but 25% of those diagnosed with HIV.
Disparities also affect women unequally, with Black women disproportionately affected, compared to women of other ethnic and racial groups. Annual HIV infections remained stable overall among Black women from 2015 to 2019, but the rate of new HIV infections among Black women is 11 times that of White women and 4 times that of Latinx, according to federal statistics.
Expert perspective, reactions
Vincent Marconi, MD, professor of infectious diseases at Emory University, Atlanta, whose research focuses on disparities in HIV treatment responses, called the news “an exciting development for the cure agenda. This is the first woman to have been cured for at least 14 months, and they used cord blood, which could allow for potentially less toxic regimens and fewer adverse effects.”
Although the approach, meant to be used to treat the cancers, will not be widely available, he said that ‘’it does provide insight into somewhat related alternative models of cure involving gene therapy.”
Meanwhile, Dr. Marconi and other researchers are focusing on the concept of long-term HIV remission if a cure is not possible. Among the strategies under study are gene editing and immune-based treatments. HIV remission is generally defined as having an HIV viral load that is not detectable after stopping treatment.
A version of this article first appeared on WebMD.com.
that has plagued the world for decades.
But while this case is certainly cause for celebration, experts involved in the effort say we are still a long way from a universal cure.
Researcher Yvonne Bryson, MD, chief of pediatric infectious diseases at the University of California, Los Angeles, told those attending the Conference on Retroviruses and Opportunistic Infections that this case is special. The patient was a woman living with HIV who is multiracial. The previous two patients were men: one white, one Latinx.
The woman in this case was given transplants of stem cells and umbilical cord blood to treat leukemia. The treatment not only sent her cancer into remission, but her HIV as well.
The success of this case suggests that cord stem cell transplants should be considered to produce remission and cure for those with HIV who also have cancers and other diseases, the researchers said.
While the news was met with excitement in the scientific community, the approach will not be available universally, since the transplants were all done to treat cancers in the three HIV-infected patients. Overall, Dr. Bryson estimates that about 50 people per year may benefit from this procedure.
Even so, other experts say the approach could provide insight into other ways to find cures. And Dr. Bryson says it opens up options for more diverse populations.
“A bone marrow transplant is not a viable large-scale strategy for curing HIV, but it does present a proof of concept that HIV can be cured,” said Sharon Lewin, MD, president-elect of the International AIDS Society. “It also further strengthens using gene therapy as a viable strategy for an HIV cure.”
The woman needed a stem cell transplant after being diagnosed with leukemia. The stem cell transplant technique used was also novel, Dr. Bryson said. The medical team used a combination of adult stem cells from a relative’s blood and umbilical cord blood from a cord-blood bank that had a rare mutation that makes the immune system resistant to HIV.
In the previous two cases of HIV cures after transplants, both patients were treated with stem cell transplants, with the same mutation, but from bone marrow transplants, a more difficult procedure. And no cord blood was used for those.
The combination of adult cells and cord-blood cells proved to be the ticket to success. Using the adult cells provides a kind of bridge that helps until the cord blood takes over, the researchers said. By day 100 after the transplant, Dr. Bryson said, the woman basically had a new immune system.
HIV remained undetectable in T cells and in bone marrow. And 37 months after the transplant, the woman stopped taking the antiretroviral treatment commonly given to treat HIV infection.
‘’She is currently clinically well,” Dr. Bryson said. Her cancer is in remission.
Case histories: Three patients
The woman, who is middle-aged, has requested privacy, asking that neither her age nor other details be released. But the researchers did provide some background on her medical history and her route back to health. She was diagnosed with HIV in 2013 and began treatment with antiretroviral therapy (ART). Four years after her HIV diagnosis, she developed high-risk acute myelogenous leukemia. The transplant was done to treat that.
Her recovery was much less bumpy than that of the previous two patients, the researchers said. She left the hospital 17 days after the transplant. She didn’t have serious complications like the first two, who developed a condition that occurs when donor bone marrow or stem cells attack the recipient.
“This case also suggests that it’s the transplant of HIV-resistant cells that was key to achieving a cure here,” said Dr. Lewin. The first patient who had HIV remission after a stem cell transplant, a White man, stayed in remission for 12 years and was termed cured. But he died of leukemia in September 2020. The other, a Latinx man, has been in remission for more than 30 months.
HIV statistics, ethnic/racial burdens
In the United States, about 1.2 million people have HIV, according to HIV.gov. Thirteen percent of those who have it do not know they have it. In 2019, 34,800 new infections were diagnosed.
Certain ethnic and racial groups are more affected by HIV than others, given their proportions in the U.S. population, federal statistics suggest. In 2019, for instance, African Americans were 13% of the U.S. population but 40% of those with HIV. Hispanics/Latinx represented 18.5% of the total population but 25% of those diagnosed with HIV.
Disparities also affect women unequally, with Black women disproportionately affected, compared to women of other ethnic and racial groups. Annual HIV infections remained stable overall among Black women from 2015 to 2019, but the rate of new HIV infections among Black women is 11 times that of White women and 4 times that of Latinx, according to federal statistics.
Expert perspective, reactions
Vincent Marconi, MD, professor of infectious diseases at Emory University, Atlanta, whose research focuses on disparities in HIV treatment responses, called the news “an exciting development for the cure agenda. This is the first woman to have been cured for at least 14 months, and they used cord blood, which could allow for potentially less toxic regimens and fewer adverse effects.”
Although the approach, meant to be used to treat the cancers, will not be widely available, he said that ‘’it does provide insight into somewhat related alternative models of cure involving gene therapy.”
Meanwhile, Dr. Marconi and other researchers are focusing on the concept of long-term HIV remission if a cure is not possible. Among the strategies under study are gene editing and immune-based treatments. HIV remission is generally defined as having an HIV viral load that is not detectable after stopping treatment.
A version of this article first appeared on WebMD.com.
FROM CROI 22