User login
Risk for Appendicitis, Cholecystitis, or Diverticulitis in Patients With Psoriasis
Psoriasis is a chronic skin condition affecting approximately 2% to 3% of the population.1,2 Beyond cutaneous manifestations, psoriasis is a systemic inflammatory state that is associated with an increased risk for cardiovascular disease, including obesity,3,4 type 2 diabetes mellitus,5,6 hypertension,5 dyslipidemia,3,7 metabolic syndrome,7 atherosclerosis,8 peripheral vascular disease,9 coronary artery calcification,10 myocardial infarction,11-13 stroke,9,14 and cardiac death.15,16
Psoriasis also has been associated with inflammatory bowel disease (IBD), possibly because of similar autoimmune mechanisms in the pathogenesis of both diseases.17,18 However, there is no literature regarding the risk for acute gastrointestinal pathologies such as appendicitis, cholecystitis, or diverticulitis in patients with psoriasis.
The primary objective of this study was to examine if patients with psoriasis are at increased risk for appendicitis, cholecystitis, or diverticulitis compared to the general population. The secondary objective was to determine if patients with severe psoriasis (ie, patients treated with phototherapy or systemic therapy) are at a higher risk for these conditions compared to patients with mild psoriasis.
Methods
Patients and Tools
A descriptive, population-based cohort study design with controls from a matched cohort was used to ascertain the effect of psoriasis status on patients’ risk for appendicitis, cholecystitis, or diverticulitis. Our cohort was selected using administrative data from Kaiser Permanente Southern California (KPSC) during the study period (January 1, 2004, through December 31, 2016).
Kaiser Permanente Southern California is a large integrated health maintenance organization that includes approximately 4 million patients as of December 31, 2016, and includes roughly 20% of the region’s population. The geographic area served extends from Bakersfield in the lower California Central Valley to San Diego on the border with Mexico. Membership demographics, socioeconomic status, and ethnicity composition are representative of California.
Patients were included if they had a diagnosis of psoriasis (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 696.1; International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] codes L40.0, L40.4, L40.8, or L40.9) for at least 3 visits between January 1, 2004, and December 31, 2016. Patients were not excluded if they also had a diagnosis of psoriatic arthritis (ICD-9-CM code 696.0; ICD-10-CM code L40.5x). Patients also must have been continuously enrolled for at least 1 year before and 1 year after the index date, which was defined as the date of the third psoriasis diagnosis.
Each patient with psoriasis was assigned to 1 of 2 cohorts: (1) severe psoriasis: patients who received UVB phototherapy, psoralen plus UVA phototherapy, methotrexate, acitretin, cyclosporine, apremilast, etanercept, adalimumab, infliximab, ustekinumab, efalizumab, alefacept, secukinumab, or ixekizumab during the study period; and (2) mild psoriasis: patients who had a diagnosis of psoriasis who did not receive one of these therapies during the study period.
Patients were excluded if they had a history of appendicitis, cholecystitis, or diverticulitis at any time before the index date. Only patients older than 18 years were included.
Patients with psoriasis were frequency matched (1:5) with healthy patients, also from the KPSC network. Individuals were matched by age, sex, and ethnicity.
Statistical Analysis
Baseline characteristics were described with means and SD for continuous variables as well as percentages for categorical variables. Chi-square tests for categorical variables and the Mann-Whitney U Test for continuous variables were used to compare the patients’ characteristics by psoriasis status. Cox proportional hazards regression models were used to examine the risk for appendicitis, cholecystitis, or diverticulitis among patients with and without psoriasis and among patients with mild and severe psoriasis. Proportionality assumption was validated using Pearson product moment correlation between the scaled Schoenfeld residuals and log transformed time for each covariate.
Results were presented as crude (unadjusted) hazard ratios (HRs) and adjusted HRs, where confounding factors (ie, age, sex, ethnicity, body mass index [BMI], alcohol use, smoking status, income, education, and membership length) were adjusted. All tests were performed with SAS EG 5.1 and R software. P<.05 was considered statistically significant. Results are reported with the 95% confidence interval (CI), when appropriate.
Results
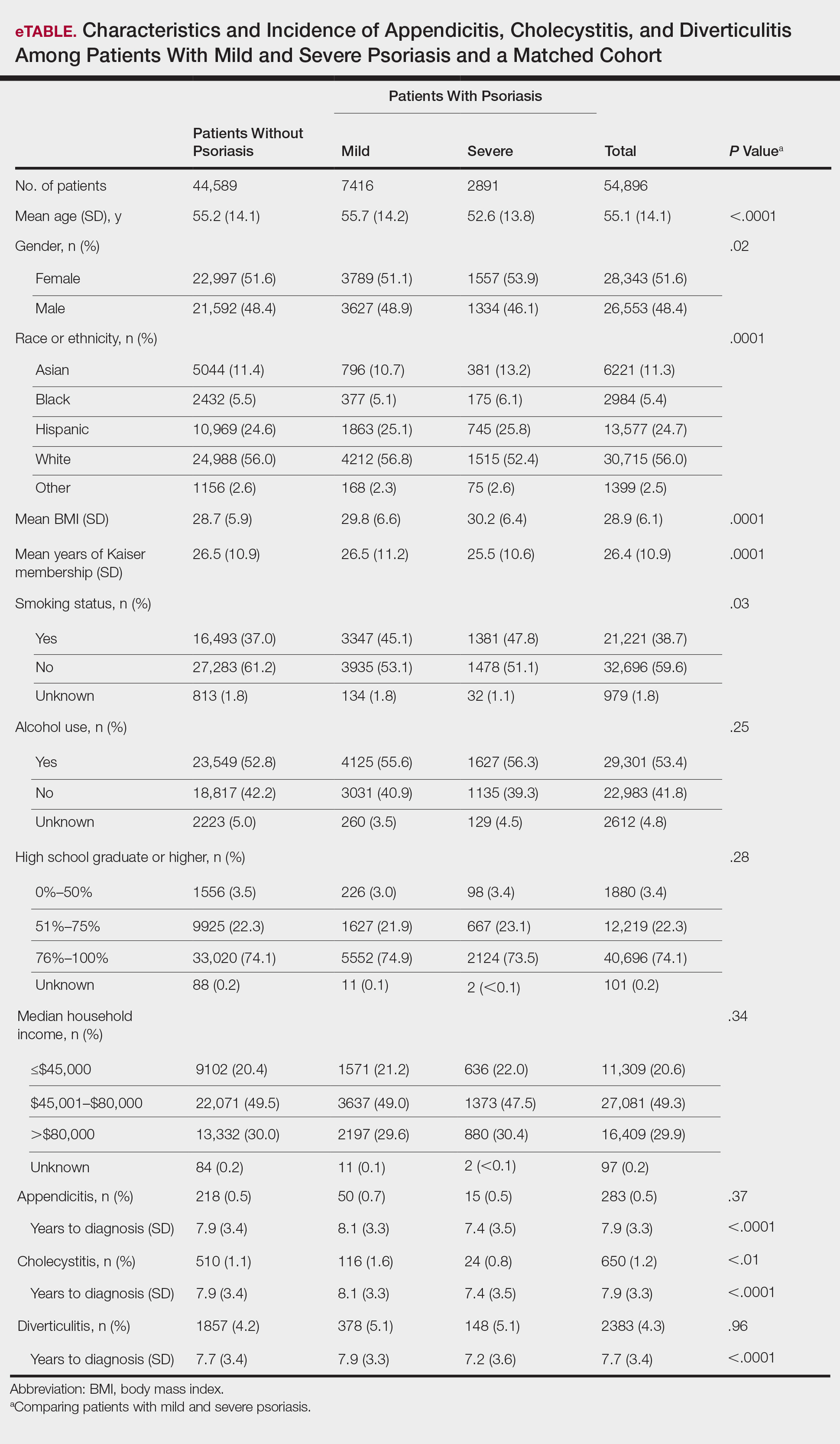
A total of 1,690,214 KPSC patients were eligible for the study; 10,307 (0.6%) met diagnostic and inclusion criteria for the psoriasis cohort. Patients with psoriasis had a significantly higher mean BMI (29.9 vs 28.7; P<.0001) as well as higher mean rates of alcohol use (56% vs 53%; P<.0001) and smoking (47% vs 38%; P<.01) compared to controls. Psoriasis patients had a shorter average duration of membership within the Kaiser network (P=.0001) compared to controls.
A total of 7416 patients met criteria for mild psoriasis and 2891 patients met criteria for severe psoriasis (eTable). Patients with severe psoriasis were significantly younger and had significantly higher mean BMI compared to patients with mild psoriasis (P<.0001 and P=.0001, respectively). No significant difference in rates of alcohol or tobacco use was detected among patients with mild and severe psoriasis.
Appendicitis
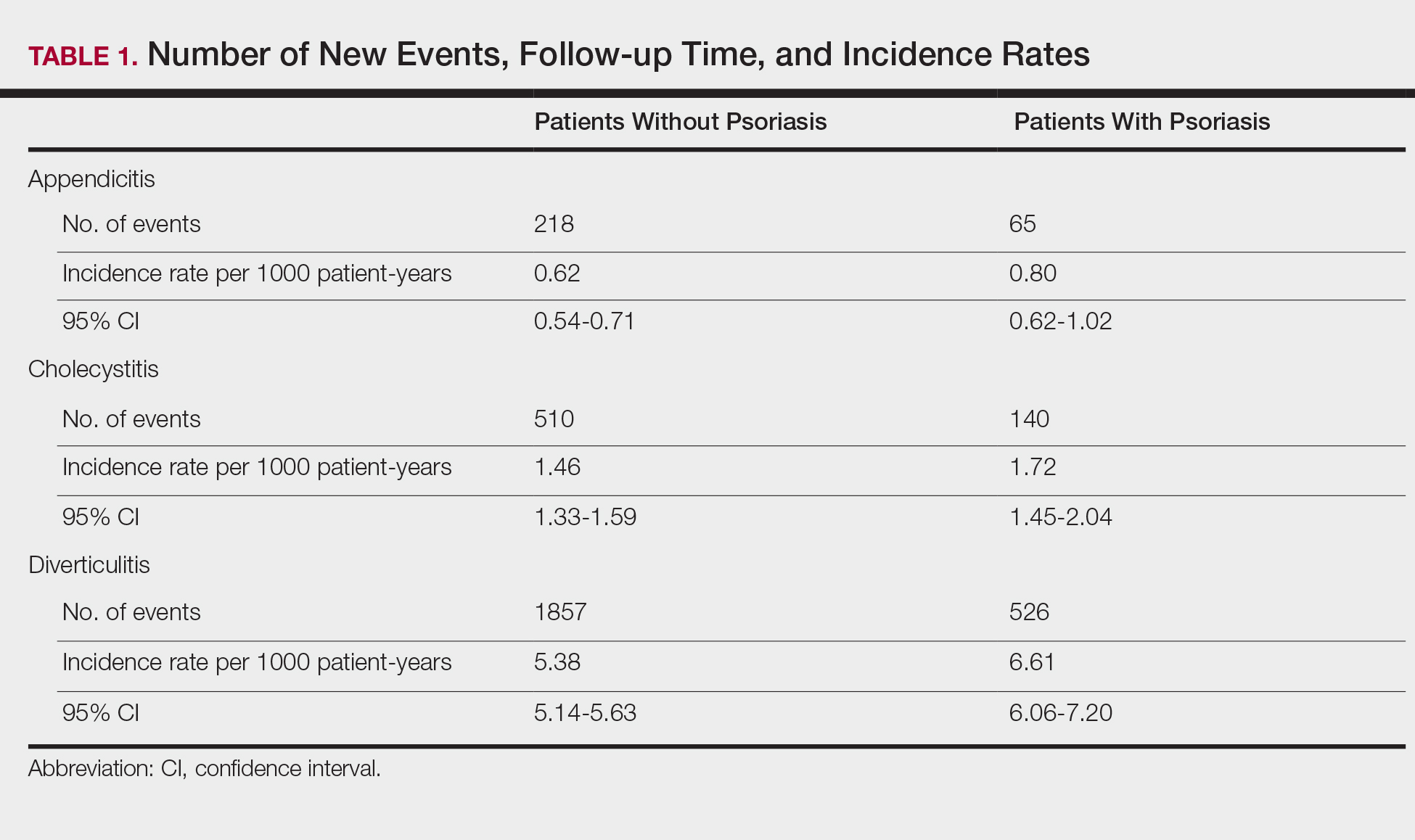
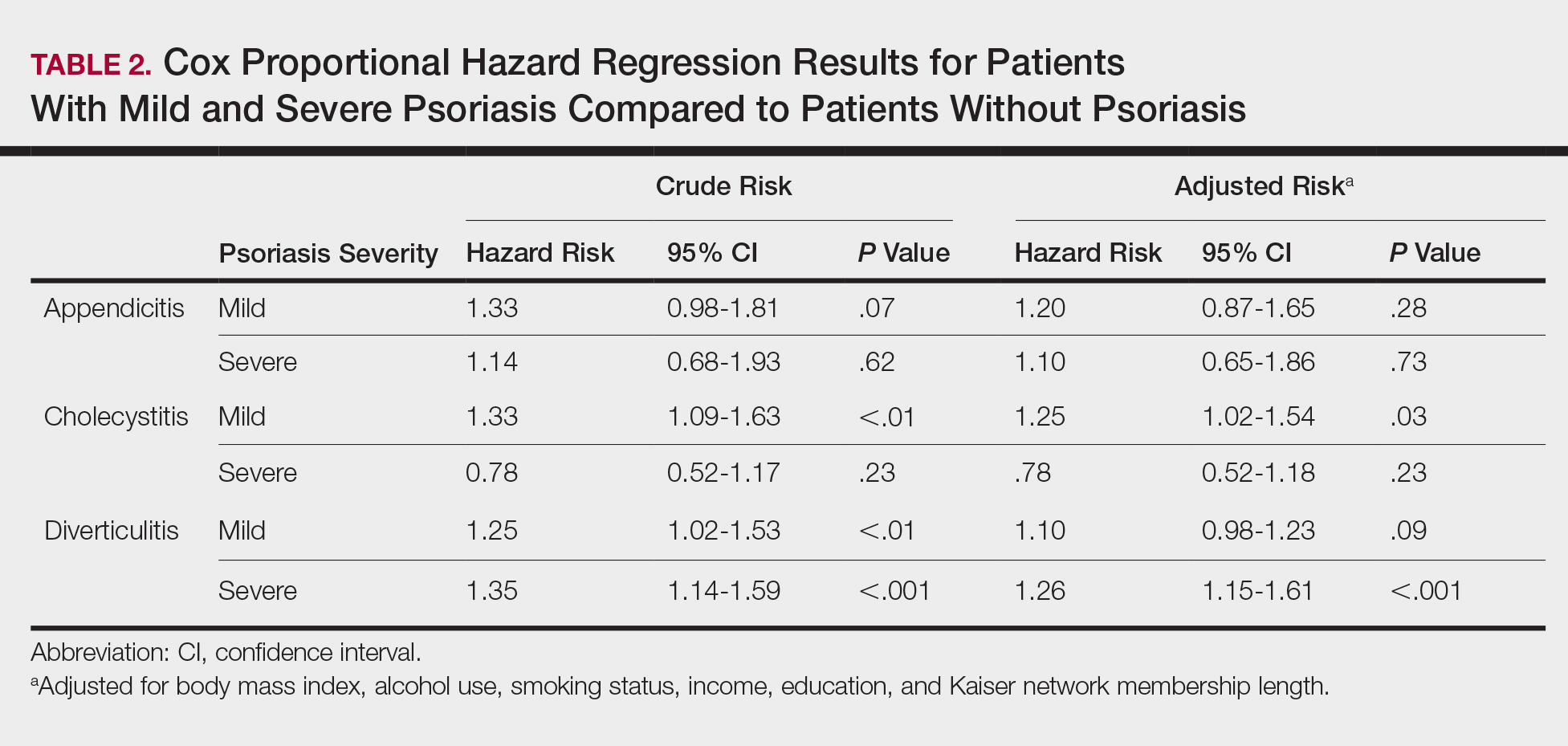
The prevalence of appendicitis was not significantly different between patients with and without psoriasis or between patients with mild and severe psoriasis, though the incidence rate was slightly higher among patients with psoriasis (0.80 per 1000 patient-years compared to 0.62 per 1000 patient-years among patients without psoriasis)(Table 1). However, there was not a significant difference in risk for appendicitis between healthy patients, patients with severe psoriasis, and patients with mild psoriasis after adjusting for potential confounding factors (Table 2). Interestingly, patients with severe psoriasis who had a diagnosis of appendicitis had a significantly shorter time to diagnosis of appendicitis compared to patients with mild psoriasis (7.4 years vs 8.1 years; P<.0001).
Cholecystitis
Psoriasis patients also did not have an increased prevalence of cholecystitis compared to healthy patients. However, patients with severe psoriasis had a significantly higher prevalence of cholecystitis compared to patients with mild psoriasis (P=.0038). Overall, patients with psoriasis had a slightly higher incidence rate (1.72 per 1000 patient-years) compared to healthy patients (1.46 per 1000 patient-years). Moreover, the time to diagnosis of cholecystitis was significantly shorter for patients with severe psoriasis than for patients with mild psoriasis (7.4 years vs 8.1 years; P<.0001). Mild psoriasis was associated with a significantly increased risk (HR, 1.33; 95% CI, 1.09-1.63; P<.01) for cholecystitis compared to individuals without psoriasis in both the crude and adjusted models (Table 2). There was no difference between mild psoriasis patients and severe psoriasis patients in risk for cholecystitis.
Diverticulitis
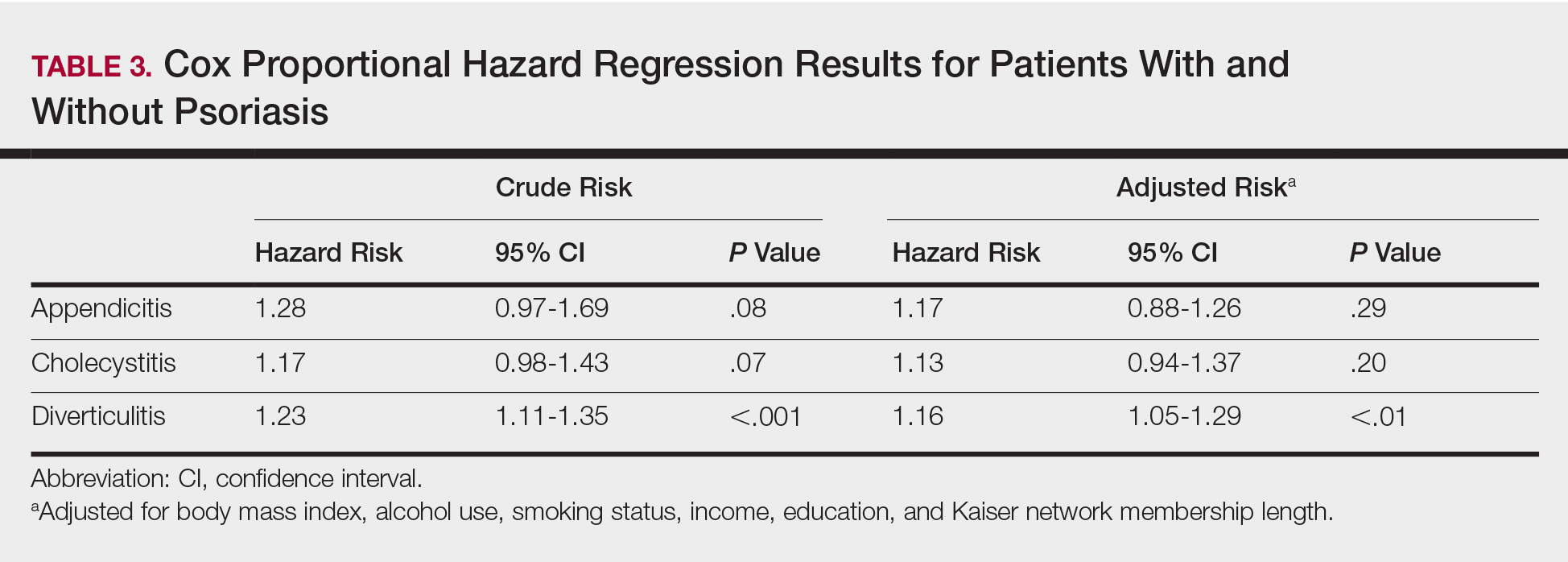
Patients with psoriasis had a significantly greater prevalence of diverticulitis compared to the control cohort (5.1% vs 4.2%; P<.0001). There was no difference in prevalence between the severe psoriasis group and the mild psoriasis group (P=.96), but the time to diagnosis of diverticulitis was shorter in the severe psoriasis group than in the mild psoriasis group (7.2 years vs 7.9 years; P<.0001). Psoriasis patients had an incidence rate of diverticulitis of 6.61 per 1000 patient-years compared to 5.38 per 1000 patient-years in the control group. Psoriasis conferred a higher risk for diverticulitis in both the crude and adjusted models (HR, 1.23; 95% CI, 1.11-1.35 [P<.001] and HR, 1.16; 95% CI, 1.05-1.29; [P<.01], respectively)(Table 3); however, when stratified by disease severity, only patients with severe psoriasis were found to be at higher risk (HR, 1.26; 95% CI, 1.15-1.61; P<.001 for the adjusted model).
Comment
The objective of this study was to examine the background risks for specific gastrointestinal pathologies in a large cohort of patients with psoriasis compared to the general population. After adjusting for measured confounders, patients with severe psoriasis had a significantly higher risk of diverticulitis compared to the general population. Although more patients with severe psoriasis developed appendicitis or cholecystitis, the difference was not significant.
The pathogenesis of diverticulosis and diverticulitis has been thought to be related to increased intracolonic pressure and decreased dietary fiber intake, leading to formation of diverticula in the colon.19 Our study did not correct for differences in diet between the 2 groups, making it a possible confounding variable. Studies evaluating dietary habits of psoriatic patients have found that adult males with psoriasis might consume less fiber compared to healthy patients,20 and psoriasis patients also might consume less whole-grain fiber.21 Furthermore, fiber deficiency also might affect gut flora, causing low-grade chronic inflammation,18 which also has been supported by response to anti-inflammatory medications such as mesalazine.22 Given the autoimmune association between psoriasis and IBD, it is possible that psoriasis also might create an environment of chronic inflammation in the gut, predisposing patients with psoriasis to diverticulitis. However, further research is needed to better evaluate this possibility.
Our study also does not address any potential effects on outcomes of specific treatments for psoriasis. Brandl et al23 found that patients on immunosuppressive therapy for autoimmune diseases had longer hospital and intensive care unit stays, higher rates of emergency operations, and higher mortality while hospitalized. Because our results suggest that patients with severe psoriasis, who are therefore more likely to require treatment with an immunomodulator, are at higher risk for diverticulitis, these patients also might be at risk for poorer outcomes.
There is no literature evaluating the relationship between psoriasis and appendicitis. Our study found a slightly lower incidence rate compared to the national trend (9.38 per 10,000 patient-years in the United States in 2008) in both healthy patients and psoriasis patients.24 Of note, this statistic includes children, whereas our study did not, which might in part account for the lower rate. However, Cheluvappa et al25 hypothesized a relationship between appendicitis and subsequent appendectomy at a young age and protection against IBD. They also found that the mechanism for protection involves downregulation of the helper T cell (TH17) pathway,25 which also has been found to play a role in psoriasis pathogenesis.26,27 Although our results suggest that the risk for appendicitis is not increased for patients with psoriasis, further research might be able to determine if appendicitis and subsequent appendectomy also can offer protection against development of psoriasis.
We found that patients with severe psoriasis had a higher incidence rate of cholecystitis compared to patients with mild psoriasis. Egeberg et al28 found an increased risk for cholelithiasis among patients with psoriasis, which may contribute to a higher rate of cholecystitis. Although both acute and chronic cholecystitis were incorporated in this study, a Russian study found that chronic cholecystitis may be a predictor of progression of psoriasis.29 Moreover, patients with severe psoriasis had a shorter duration to diagnosis of cholecystitis than patients with mild psoriasis. It is possible that patients with severe psoriasis are in a state of greater chronic inflammation than those with mild psoriasis, and therefore, when combined with other risk factors for cholecystitis, may progress to disease more quickly. Alternatively, this finding could be treatment related, as there have been reported cases of cholecystitis related to etanercept use in patients treated for psoriasis and juvenile polyarticular rheumatoid arthritis.30,31 The relationship is not yet well defined, however, and further research is necessary to evaluate this association.
Study Strengths
Key strengths of this study include the large sample size and diversity of the patient population. Kaiser Permanente Southern California membership generally is representative of the broader community, making our results fairly generalizable to populations with health insurance. Use of a matched control cohort allows the results to be more specific to the disease of interest, and the population-based design minimizes bias.
Study Limitations
This study has several limitations. Although the cohorts were categorized based on type of treatment received, exact therapies were not specified. As a retrospective study, it is difficult to control for potential confounding variables that are not included in the electronic medical record. The results of this study also demonstrated significantly shorter durations to diagnosis of all 3 conditions, indicating that surveillance bias may be present.
Conclusion
Patients with psoriasis may be at an increased risk for diverticulitis compared to patients without psoriasis, which could be due to the chronic inflammatory state induced by psoriasis. Therefore, it may be beneficial for clinicians to evaluate psoriasis patients for other risk factors for diverticulitis and subsequently provide counseling to these patients to minimize their risk for diverticulitis. Psoriasis patients do not appear to be at an increased risk for appendicitis or cholecystitis compared to controls; however, further research is needed for confirmation.
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Channual J, Wu JJ, Dann FJ. Effects of tumor necrosis factor-α blockade on metabolic syndrome in psoriasis and psoriatic arthritis and additional lessons learned from rheumatoid arthritis. Dermatol Ther. 2009;22:61-73.
- Koebnick C, Black MH, Smith N, et al. The association of psoriasis and elevated blood lipids in overweight and obese children. J Pediatr. 2011;159:577-583.
- Herron MD, Hinckley M, Hoffman MS, et al. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol. 2005;141:1527-1534.
- Qureshi AA, Choi HK, Setty AR, et al. Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch Dermatol. 2009;145:379-382.
- Shapiro J, Cohen AD, David M, et al. The association between psoriasis, diabetes mellitus, and atherosclerosis in Israel: a case-control study. J Am Acad Dermatol. 2007;56:629-634.
- Love TJ, Qureshi AA, Karlson EW, et al. Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, 2003-2006. Arch Dermatol. 2011;147:419-424.
- El-Mongy S, Fathy H, Abdelaziz A, et al. Subclinical atherosclerosis in patients with chronic psoriasis: a potential association. J Eur Acad Dermatol Venereol. 2010;24:661-666.
- Prodanovich S, Kirsner RS, Kravetz JD, et al. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol. 2009;145:700-703.
- Ludwig RJ, Herzog C, Rostock A, et al. Psoriasis: a possible risk factor for development of coronary artery calcification. Br J Dermatol. 2007;156:271-276.
- Kaye JA, Li L, Jick SS. Incidence of risk factors for myocardial infarction and other vascular diseases in patients with psoriasis. Br J Dermatol. 2008;159:895-902.
- Kimball AB, Robinson D Jr, Wu Y, et al. Cardiovascular disease and risk factors among psoriasis patients in two US healthcare databases, 2001-2002. Dermatology. 2008;217:27-37.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Mehta NN, Azfar RS, Shin DB, et al. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31:1000-1006.
- Abuabara K, Azfar RS, Shin DB, et al. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the United Kingdom. Br J Dermatol. 2010;163:586-592.
- Christophers E. Comorbidities in psoriasis. Clin Dermatol. 2007;25:529-534.
- Wu JJ, Nguyen TU, Poon KY, et al. The association of psoriasis with autoimmune diseases. J Am Acad Dermatol. 2012;67:924-930.
- Floch MH, Bina I. The natural history of diverticulitis: fact and theory. Clin Gastroenterol. 2004;38(5, suppl 1):S2-S7.
- Barrea L, Macchia PE, Tarantino G, et al. Nutrition: a key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J Transl Med. 2015;13:303.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. National Survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Matrana MR, Margolin DA. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg. 2009;22:141-146.
- Brandl A, Kratzer T, Kafka-Ritsch R, et al. Diverticulitis in immunosuppressed patients: a fatal outcome requiring a new approach? Can J Surg. 2016;59:254-261.
- Buckius MT, McGrath B, Monk J, et al. Changing epidemiology of acute appendicitis in the United States: study period 1993-2008. J Surg Res. 2012;175:185-190.
- Cheluvappa R, Luo AS, Grimm MC. T helper type 17 pathway suppression by appendicitis and appendectomy protects against colitis. Clin Exp Immunol. 2014;175:316-322.
- Lynde CW, Poulin Y, Vender R, et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141-150.
- Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-α, IFN-γ, IL6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005:2005;273-279.
- Egeberg A, Anderson YMF, Gislason GH, et al. Gallstone risk in adult patients with atopic dermatitis and psoriasis: possible effect of overweight and obesity. Acta Derm Venereol. 2017;97:627-631.
- Smirnova SV, Barilo AA, Smolnikova MV. Hepatobiliary system diseases as the predictors of psoriasis progression [in Russian]. Vestn Ross Akad Med Nauk. 2016:102-108.
- Bagel J, Lynde C, Tyring S, et al. Moderate to severe plaque psoriasis with scalp involvement: a randomized, double-blind, placebo-controlled study of etanercept. J Am Acad Dermatol. 2012;67:86-92.
- Foeldvari I, Krüger E, Schneider T. Acute, non-obstructive, sterile cholecystitis associated with etanercept and infliximab for the treatment of juvenile polyarticular rheumatoid arthritis. Ann Rheum Dis. 2003;62:908-909.
Psoriasis is a chronic skin condition affecting approximately 2% to 3% of the population.1,2 Beyond cutaneous manifestations, psoriasis is a systemic inflammatory state that is associated with an increased risk for cardiovascular disease, including obesity,3,4 type 2 diabetes mellitus,5,6 hypertension,5 dyslipidemia,3,7 metabolic syndrome,7 atherosclerosis,8 peripheral vascular disease,9 coronary artery calcification,10 myocardial infarction,11-13 stroke,9,14 and cardiac death.15,16
Psoriasis also has been associated with inflammatory bowel disease (IBD), possibly because of similar autoimmune mechanisms in the pathogenesis of both diseases.17,18 However, there is no literature regarding the risk for acute gastrointestinal pathologies such as appendicitis, cholecystitis, or diverticulitis in patients with psoriasis.
The primary objective of this study was to examine if patients with psoriasis are at increased risk for appendicitis, cholecystitis, or diverticulitis compared to the general population. The secondary objective was to determine if patients with severe psoriasis (ie, patients treated with phototherapy or systemic therapy) are at a higher risk for these conditions compared to patients with mild psoriasis.
Methods
Patients and Tools
A descriptive, population-based cohort study design with controls from a matched cohort was used to ascertain the effect of psoriasis status on patients’ risk for appendicitis, cholecystitis, or diverticulitis. Our cohort was selected using administrative data from Kaiser Permanente Southern California (KPSC) during the study period (January 1, 2004, through December 31, 2016).
Kaiser Permanente Southern California is a large integrated health maintenance organization that includes approximately 4 million patients as of December 31, 2016, and includes roughly 20% of the region’s population. The geographic area served extends from Bakersfield in the lower California Central Valley to San Diego on the border with Mexico. Membership demographics, socioeconomic status, and ethnicity composition are representative of California.
Patients were included if they had a diagnosis of psoriasis (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 696.1; International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] codes L40.0, L40.4, L40.8, or L40.9) for at least 3 visits between January 1, 2004, and December 31, 2016. Patients were not excluded if they also had a diagnosis of psoriatic arthritis (ICD-9-CM code 696.0; ICD-10-CM code L40.5x). Patients also must have been continuously enrolled for at least 1 year before and 1 year after the index date, which was defined as the date of the third psoriasis diagnosis.
Each patient with psoriasis was assigned to 1 of 2 cohorts: (1) severe psoriasis: patients who received UVB phototherapy, psoralen plus UVA phototherapy, methotrexate, acitretin, cyclosporine, apremilast, etanercept, adalimumab, infliximab, ustekinumab, efalizumab, alefacept, secukinumab, or ixekizumab during the study period; and (2) mild psoriasis: patients who had a diagnosis of psoriasis who did not receive one of these therapies during the study period.
Patients were excluded if they had a history of appendicitis, cholecystitis, or diverticulitis at any time before the index date. Only patients older than 18 years were included.
Patients with psoriasis were frequency matched (1:5) with healthy patients, also from the KPSC network. Individuals were matched by age, sex, and ethnicity.
Statistical Analysis
Baseline characteristics were described with means and SD for continuous variables as well as percentages for categorical variables. Chi-square tests for categorical variables and the Mann-Whitney U Test for continuous variables were used to compare the patients’ characteristics by psoriasis status. Cox proportional hazards regression models were used to examine the risk for appendicitis, cholecystitis, or diverticulitis among patients with and without psoriasis and among patients with mild and severe psoriasis. Proportionality assumption was validated using Pearson product moment correlation between the scaled Schoenfeld residuals and log transformed time for each covariate.
Results were presented as crude (unadjusted) hazard ratios (HRs) and adjusted HRs, where confounding factors (ie, age, sex, ethnicity, body mass index [BMI], alcohol use, smoking status, income, education, and membership length) were adjusted. All tests were performed with SAS EG 5.1 and R software. P<.05 was considered statistically significant. Results are reported with the 95% confidence interval (CI), when appropriate.
Results
A total of 1,690,214 KPSC patients were eligible for the study; 10,307 (0.6%) met diagnostic and inclusion criteria for the psoriasis cohort. Patients with psoriasis had a significantly higher mean BMI (29.9 vs 28.7; P<.0001) as well as higher mean rates of alcohol use (56% vs 53%; P<.0001) and smoking (47% vs 38%; P<.01) compared to controls. Psoriasis patients had a shorter average duration of membership within the Kaiser network (P=.0001) compared to controls.
A total of 7416 patients met criteria for mild psoriasis and 2891 patients met criteria for severe psoriasis (eTable). Patients with severe psoriasis were significantly younger and had significantly higher mean BMI compared to patients with mild psoriasis (P<.0001 and P=.0001, respectively). No significant difference in rates of alcohol or tobacco use was detected among patients with mild and severe psoriasis.
Appendicitis
The prevalence of appendicitis was not significantly different between patients with and without psoriasis or between patients with mild and severe psoriasis, though the incidence rate was slightly higher among patients with psoriasis (0.80 per 1000 patient-years compared to 0.62 per 1000 patient-years among patients without psoriasis)(Table 1). However, there was not a significant difference in risk for appendicitis between healthy patients, patients with severe psoriasis, and patients with mild psoriasis after adjusting for potential confounding factors (Table 2). Interestingly, patients with severe psoriasis who had a diagnosis of appendicitis had a significantly shorter time to diagnosis of appendicitis compared to patients with mild psoriasis (7.4 years vs 8.1 years; P<.0001).
Cholecystitis
Psoriasis patients also did not have an increased prevalence of cholecystitis compared to healthy patients. However, patients with severe psoriasis had a significantly higher prevalence of cholecystitis compared to patients with mild psoriasis (P=.0038). Overall, patients with psoriasis had a slightly higher incidence rate (1.72 per 1000 patient-years) compared to healthy patients (1.46 per 1000 patient-years). Moreover, the time to diagnosis of cholecystitis was significantly shorter for patients with severe psoriasis than for patients with mild psoriasis (7.4 years vs 8.1 years; P<.0001). Mild psoriasis was associated with a significantly increased risk (HR, 1.33; 95% CI, 1.09-1.63; P<.01) for cholecystitis compared to individuals without psoriasis in both the crude and adjusted models (Table 2). There was no difference between mild psoriasis patients and severe psoriasis patients in risk for cholecystitis.
Diverticulitis
Patients with psoriasis had a significantly greater prevalence of diverticulitis compared to the control cohort (5.1% vs 4.2%; P<.0001). There was no difference in prevalence between the severe psoriasis group and the mild psoriasis group (P=.96), but the time to diagnosis of diverticulitis was shorter in the severe psoriasis group than in the mild psoriasis group (7.2 years vs 7.9 years; P<.0001). Psoriasis patients had an incidence rate of diverticulitis of 6.61 per 1000 patient-years compared to 5.38 per 1000 patient-years in the control group. Psoriasis conferred a higher risk for diverticulitis in both the crude and adjusted models (HR, 1.23; 95% CI, 1.11-1.35 [P<.001] and HR, 1.16; 95% CI, 1.05-1.29; [P<.01], respectively)(Table 3); however, when stratified by disease severity, only patients with severe psoriasis were found to be at higher risk (HR, 1.26; 95% CI, 1.15-1.61; P<.001 for the adjusted model).
Comment
The objective of this study was to examine the background risks for specific gastrointestinal pathologies in a large cohort of patients with psoriasis compared to the general population. After adjusting for measured confounders, patients with severe psoriasis had a significantly higher risk of diverticulitis compared to the general population. Although more patients with severe psoriasis developed appendicitis or cholecystitis, the difference was not significant.
The pathogenesis of diverticulosis and diverticulitis has been thought to be related to increased intracolonic pressure and decreased dietary fiber intake, leading to formation of diverticula in the colon.19 Our study did not correct for differences in diet between the 2 groups, making it a possible confounding variable. Studies evaluating dietary habits of psoriatic patients have found that adult males with psoriasis might consume less fiber compared to healthy patients,20 and psoriasis patients also might consume less whole-grain fiber.21 Furthermore, fiber deficiency also might affect gut flora, causing low-grade chronic inflammation,18 which also has been supported by response to anti-inflammatory medications such as mesalazine.22 Given the autoimmune association between psoriasis and IBD, it is possible that psoriasis also might create an environment of chronic inflammation in the gut, predisposing patients with psoriasis to diverticulitis. However, further research is needed to better evaluate this possibility.
Our study also does not address any potential effects on outcomes of specific treatments for psoriasis. Brandl et al23 found that patients on immunosuppressive therapy for autoimmune diseases had longer hospital and intensive care unit stays, higher rates of emergency operations, and higher mortality while hospitalized. Because our results suggest that patients with severe psoriasis, who are therefore more likely to require treatment with an immunomodulator, are at higher risk for diverticulitis, these patients also might be at risk for poorer outcomes.
There is no literature evaluating the relationship between psoriasis and appendicitis. Our study found a slightly lower incidence rate compared to the national trend (9.38 per 10,000 patient-years in the United States in 2008) in both healthy patients and psoriasis patients.24 Of note, this statistic includes children, whereas our study did not, which might in part account for the lower rate. However, Cheluvappa et al25 hypothesized a relationship between appendicitis and subsequent appendectomy at a young age and protection against IBD. They also found that the mechanism for protection involves downregulation of the helper T cell (TH17) pathway,25 which also has been found to play a role in psoriasis pathogenesis.26,27 Although our results suggest that the risk for appendicitis is not increased for patients with psoriasis, further research might be able to determine if appendicitis and subsequent appendectomy also can offer protection against development of psoriasis.
We found that patients with severe psoriasis had a higher incidence rate of cholecystitis compared to patients with mild psoriasis. Egeberg et al28 found an increased risk for cholelithiasis among patients with psoriasis, which may contribute to a higher rate of cholecystitis. Although both acute and chronic cholecystitis were incorporated in this study, a Russian study found that chronic cholecystitis may be a predictor of progression of psoriasis.29 Moreover, patients with severe psoriasis had a shorter duration to diagnosis of cholecystitis than patients with mild psoriasis. It is possible that patients with severe psoriasis are in a state of greater chronic inflammation than those with mild psoriasis, and therefore, when combined with other risk factors for cholecystitis, may progress to disease more quickly. Alternatively, this finding could be treatment related, as there have been reported cases of cholecystitis related to etanercept use in patients treated for psoriasis and juvenile polyarticular rheumatoid arthritis.30,31 The relationship is not yet well defined, however, and further research is necessary to evaluate this association.
Study Strengths
Key strengths of this study include the large sample size and diversity of the patient population. Kaiser Permanente Southern California membership generally is representative of the broader community, making our results fairly generalizable to populations with health insurance. Use of a matched control cohort allows the results to be more specific to the disease of interest, and the population-based design minimizes bias.
Study Limitations
This study has several limitations. Although the cohorts were categorized based on type of treatment received, exact therapies were not specified. As a retrospective study, it is difficult to control for potential confounding variables that are not included in the electronic medical record. The results of this study also demonstrated significantly shorter durations to diagnosis of all 3 conditions, indicating that surveillance bias may be present.
Conclusion
Patients with psoriasis may be at an increased risk for diverticulitis compared to patients without psoriasis, which could be due to the chronic inflammatory state induced by psoriasis. Therefore, it may be beneficial for clinicians to evaluate psoriasis patients for other risk factors for diverticulitis and subsequently provide counseling to these patients to minimize their risk for diverticulitis. Psoriasis patients do not appear to be at an increased risk for appendicitis or cholecystitis compared to controls; however, further research is needed for confirmation.
Psoriasis is a chronic skin condition affecting approximately 2% to 3% of the population.1,2 Beyond cutaneous manifestations, psoriasis is a systemic inflammatory state that is associated with an increased risk for cardiovascular disease, including obesity,3,4 type 2 diabetes mellitus,5,6 hypertension,5 dyslipidemia,3,7 metabolic syndrome,7 atherosclerosis,8 peripheral vascular disease,9 coronary artery calcification,10 myocardial infarction,11-13 stroke,9,14 and cardiac death.15,16
Psoriasis also has been associated with inflammatory bowel disease (IBD), possibly because of similar autoimmune mechanisms in the pathogenesis of both diseases.17,18 However, there is no literature regarding the risk for acute gastrointestinal pathologies such as appendicitis, cholecystitis, or diverticulitis in patients with psoriasis.
The primary objective of this study was to examine if patients with psoriasis are at increased risk for appendicitis, cholecystitis, or diverticulitis compared to the general population. The secondary objective was to determine if patients with severe psoriasis (ie, patients treated with phototherapy or systemic therapy) are at a higher risk for these conditions compared to patients with mild psoriasis.
Methods
Patients and Tools
A descriptive, population-based cohort study design with controls from a matched cohort was used to ascertain the effect of psoriasis status on patients’ risk for appendicitis, cholecystitis, or diverticulitis. Our cohort was selected using administrative data from Kaiser Permanente Southern California (KPSC) during the study period (January 1, 2004, through December 31, 2016).
Kaiser Permanente Southern California is a large integrated health maintenance organization that includes approximately 4 million patients as of December 31, 2016, and includes roughly 20% of the region’s population. The geographic area served extends from Bakersfield in the lower California Central Valley to San Diego on the border with Mexico. Membership demographics, socioeconomic status, and ethnicity composition are representative of California.
Patients were included if they had a diagnosis of psoriasis (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 696.1; International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] codes L40.0, L40.4, L40.8, or L40.9) for at least 3 visits between January 1, 2004, and December 31, 2016. Patients were not excluded if they also had a diagnosis of psoriatic arthritis (ICD-9-CM code 696.0; ICD-10-CM code L40.5x). Patients also must have been continuously enrolled for at least 1 year before and 1 year after the index date, which was defined as the date of the third psoriasis diagnosis.
Each patient with psoriasis was assigned to 1 of 2 cohorts: (1) severe psoriasis: patients who received UVB phototherapy, psoralen plus UVA phototherapy, methotrexate, acitretin, cyclosporine, apremilast, etanercept, adalimumab, infliximab, ustekinumab, efalizumab, alefacept, secukinumab, or ixekizumab during the study period; and (2) mild psoriasis: patients who had a diagnosis of psoriasis who did not receive one of these therapies during the study period.
Patients were excluded if they had a history of appendicitis, cholecystitis, or diverticulitis at any time before the index date. Only patients older than 18 years were included.
Patients with psoriasis were frequency matched (1:5) with healthy patients, also from the KPSC network. Individuals were matched by age, sex, and ethnicity.
Statistical Analysis
Baseline characteristics were described with means and SD for continuous variables as well as percentages for categorical variables. Chi-square tests for categorical variables and the Mann-Whitney U Test for continuous variables were used to compare the patients’ characteristics by psoriasis status. Cox proportional hazards regression models were used to examine the risk for appendicitis, cholecystitis, or diverticulitis among patients with and without psoriasis and among patients with mild and severe psoriasis. Proportionality assumption was validated using Pearson product moment correlation between the scaled Schoenfeld residuals and log transformed time for each covariate.
Results were presented as crude (unadjusted) hazard ratios (HRs) and adjusted HRs, where confounding factors (ie, age, sex, ethnicity, body mass index [BMI], alcohol use, smoking status, income, education, and membership length) were adjusted. All tests were performed with SAS EG 5.1 and R software. P<.05 was considered statistically significant. Results are reported with the 95% confidence interval (CI), when appropriate.
Results
A total of 1,690,214 KPSC patients were eligible for the study; 10,307 (0.6%) met diagnostic and inclusion criteria for the psoriasis cohort. Patients with psoriasis had a significantly higher mean BMI (29.9 vs 28.7; P<.0001) as well as higher mean rates of alcohol use (56% vs 53%; P<.0001) and smoking (47% vs 38%; P<.01) compared to controls. Psoriasis patients had a shorter average duration of membership within the Kaiser network (P=.0001) compared to controls.
A total of 7416 patients met criteria for mild psoriasis and 2891 patients met criteria for severe psoriasis (eTable). Patients with severe psoriasis were significantly younger and had significantly higher mean BMI compared to patients with mild psoriasis (P<.0001 and P=.0001, respectively). No significant difference in rates of alcohol or tobacco use was detected among patients with mild and severe psoriasis.
Appendicitis
The prevalence of appendicitis was not significantly different between patients with and without psoriasis or between patients with mild and severe psoriasis, though the incidence rate was slightly higher among patients with psoriasis (0.80 per 1000 patient-years compared to 0.62 per 1000 patient-years among patients without psoriasis)(Table 1). However, there was not a significant difference in risk for appendicitis between healthy patients, patients with severe psoriasis, and patients with mild psoriasis after adjusting for potential confounding factors (Table 2). Interestingly, patients with severe psoriasis who had a diagnosis of appendicitis had a significantly shorter time to diagnosis of appendicitis compared to patients with mild psoriasis (7.4 years vs 8.1 years; P<.0001).
Cholecystitis
Psoriasis patients also did not have an increased prevalence of cholecystitis compared to healthy patients. However, patients with severe psoriasis had a significantly higher prevalence of cholecystitis compared to patients with mild psoriasis (P=.0038). Overall, patients with psoriasis had a slightly higher incidence rate (1.72 per 1000 patient-years) compared to healthy patients (1.46 per 1000 patient-years). Moreover, the time to diagnosis of cholecystitis was significantly shorter for patients with severe psoriasis than for patients with mild psoriasis (7.4 years vs 8.1 years; P<.0001). Mild psoriasis was associated with a significantly increased risk (HR, 1.33; 95% CI, 1.09-1.63; P<.01) for cholecystitis compared to individuals without psoriasis in both the crude and adjusted models (Table 2). There was no difference between mild psoriasis patients and severe psoriasis patients in risk for cholecystitis.
Diverticulitis
Patients with psoriasis had a significantly greater prevalence of diverticulitis compared to the control cohort (5.1% vs 4.2%; P<.0001). There was no difference in prevalence between the severe psoriasis group and the mild psoriasis group (P=.96), but the time to diagnosis of diverticulitis was shorter in the severe psoriasis group than in the mild psoriasis group (7.2 years vs 7.9 years; P<.0001). Psoriasis patients had an incidence rate of diverticulitis of 6.61 per 1000 patient-years compared to 5.38 per 1000 patient-years in the control group. Psoriasis conferred a higher risk for diverticulitis in both the crude and adjusted models (HR, 1.23; 95% CI, 1.11-1.35 [P<.001] and HR, 1.16; 95% CI, 1.05-1.29; [P<.01], respectively)(Table 3); however, when stratified by disease severity, only patients with severe psoriasis were found to be at higher risk (HR, 1.26; 95% CI, 1.15-1.61; P<.001 for the adjusted model).
Comment
The objective of this study was to examine the background risks for specific gastrointestinal pathologies in a large cohort of patients with psoriasis compared to the general population. After adjusting for measured confounders, patients with severe psoriasis had a significantly higher risk of diverticulitis compared to the general population. Although more patients with severe psoriasis developed appendicitis or cholecystitis, the difference was not significant.
The pathogenesis of diverticulosis and diverticulitis has been thought to be related to increased intracolonic pressure and decreased dietary fiber intake, leading to formation of diverticula in the colon.19 Our study did not correct for differences in diet between the 2 groups, making it a possible confounding variable. Studies evaluating dietary habits of psoriatic patients have found that adult males with psoriasis might consume less fiber compared to healthy patients,20 and psoriasis patients also might consume less whole-grain fiber.21 Furthermore, fiber deficiency also might affect gut flora, causing low-grade chronic inflammation,18 which also has been supported by response to anti-inflammatory medications such as mesalazine.22 Given the autoimmune association between psoriasis and IBD, it is possible that psoriasis also might create an environment of chronic inflammation in the gut, predisposing patients with psoriasis to diverticulitis. However, further research is needed to better evaluate this possibility.
Our study also does not address any potential effects on outcomes of specific treatments for psoriasis. Brandl et al23 found that patients on immunosuppressive therapy for autoimmune diseases had longer hospital and intensive care unit stays, higher rates of emergency operations, and higher mortality while hospitalized. Because our results suggest that patients with severe psoriasis, who are therefore more likely to require treatment with an immunomodulator, are at higher risk for diverticulitis, these patients also might be at risk for poorer outcomes.
There is no literature evaluating the relationship between psoriasis and appendicitis. Our study found a slightly lower incidence rate compared to the national trend (9.38 per 10,000 patient-years in the United States in 2008) in both healthy patients and psoriasis patients.24 Of note, this statistic includes children, whereas our study did not, which might in part account for the lower rate. However, Cheluvappa et al25 hypothesized a relationship between appendicitis and subsequent appendectomy at a young age and protection against IBD. They also found that the mechanism for protection involves downregulation of the helper T cell (TH17) pathway,25 which also has been found to play a role in psoriasis pathogenesis.26,27 Although our results suggest that the risk for appendicitis is not increased for patients with psoriasis, further research might be able to determine if appendicitis and subsequent appendectomy also can offer protection against development of psoriasis.
We found that patients with severe psoriasis had a higher incidence rate of cholecystitis compared to patients with mild psoriasis. Egeberg et al28 found an increased risk for cholelithiasis among patients with psoriasis, which may contribute to a higher rate of cholecystitis. Although both acute and chronic cholecystitis were incorporated in this study, a Russian study found that chronic cholecystitis may be a predictor of progression of psoriasis.29 Moreover, patients with severe psoriasis had a shorter duration to diagnosis of cholecystitis than patients with mild psoriasis. It is possible that patients with severe psoriasis are in a state of greater chronic inflammation than those with mild psoriasis, and therefore, when combined with other risk factors for cholecystitis, may progress to disease more quickly. Alternatively, this finding could be treatment related, as there have been reported cases of cholecystitis related to etanercept use in patients treated for psoriasis and juvenile polyarticular rheumatoid arthritis.30,31 The relationship is not yet well defined, however, and further research is necessary to evaluate this association.
Study Strengths
Key strengths of this study include the large sample size and diversity of the patient population. Kaiser Permanente Southern California membership generally is representative of the broader community, making our results fairly generalizable to populations with health insurance. Use of a matched control cohort allows the results to be more specific to the disease of interest, and the population-based design minimizes bias.
Study Limitations
This study has several limitations. Although the cohorts were categorized based on type of treatment received, exact therapies were not specified. As a retrospective study, it is difficult to control for potential confounding variables that are not included in the electronic medical record. The results of this study also demonstrated significantly shorter durations to diagnosis of all 3 conditions, indicating that surveillance bias may be present.
Conclusion
Patients with psoriasis may be at an increased risk for diverticulitis compared to patients without psoriasis, which could be due to the chronic inflammatory state induced by psoriasis. Therefore, it may be beneficial for clinicians to evaluate psoriasis patients for other risk factors for diverticulitis and subsequently provide counseling to these patients to minimize their risk for diverticulitis. Psoriasis patients do not appear to be at an increased risk for appendicitis or cholecystitis compared to controls; however, further research is needed for confirmation.
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Channual J, Wu JJ, Dann FJ. Effects of tumor necrosis factor-α blockade on metabolic syndrome in psoriasis and psoriatic arthritis and additional lessons learned from rheumatoid arthritis. Dermatol Ther. 2009;22:61-73.
- Koebnick C, Black MH, Smith N, et al. The association of psoriasis and elevated blood lipids in overweight and obese children. J Pediatr. 2011;159:577-583.
- Herron MD, Hinckley M, Hoffman MS, et al. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol. 2005;141:1527-1534.
- Qureshi AA, Choi HK, Setty AR, et al. Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch Dermatol. 2009;145:379-382.
- Shapiro J, Cohen AD, David M, et al. The association between psoriasis, diabetes mellitus, and atherosclerosis in Israel: a case-control study. J Am Acad Dermatol. 2007;56:629-634.
- Love TJ, Qureshi AA, Karlson EW, et al. Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, 2003-2006. Arch Dermatol. 2011;147:419-424.
- El-Mongy S, Fathy H, Abdelaziz A, et al. Subclinical atherosclerosis in patients with chronic psoriasis: a potential association. J Eur Acad Dermatol Venereol. 2010;24:661-666.
- Prodanovich S, Kirsner RS, Kravetz JD, et al. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol. 2009;145:700-703.
- Ludwig RJ, Herzog C, Rostock A, et al. Psoriasis: a possible risk factor for development of coronary artery calcification. Br J Dermatol. 2007;156:271-276.
- Kaye JA, Li L, Jick SS. Incidence of risk factors for myocardial infarction and other vascular diseases in patients with psoriasis. Br J Dermatol. 2008;159:895-902.
- Kimball AB, Robinson D Jr, Wu Y, et al. Cardiovascular disease and risk factors among psoriasis patients in two US healthcare databases, 2001-2002. Dermatology. 2008;217:27-37.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Mehta NN, Azfar RS, Shin DB, et al. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31:1000-1006.
- Abuabara K, Azfar RS, Shin DB, et al. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the United Kingdom. Br J Dermatol. 2010;163:586-592.
- Christophers E. Comorbidities in psoriasis. Clin Dermatol. 2007;25:529-534.
- Wu JJ, Nguyen TU, Poon KY, et al. The association of psoriasis with autoimmune diseases. J Am Acad Dermatol. 2012;67:924-930.
- Floch MH, Bina I. The natural history of diverticulitis: fact and theory. Clin Gastroenterol. 2004;38(5, suppl 1):S2-S7.
- Barrea L, Macchia PE, Tarantino G, et al. Nutrition: a key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J Transl Med. 2015;13:303.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. National Survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Matrana MR, Margolin DA. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg. 2009;22:141-146.
- Brandl A, Kratzer T, Kafka-Ritsch R, et al. Diverticulitis in immunosuppressed patients: a fatal outcome requiring a new approach? Can J Surg. 2016;59:254-261.
- Buckius MT, McGrath B, Monk J, et al. Changing epidemiology of acute appendicitis in the United States: study period 1993-2008. J Surg Res. 2012;175:185-190.
- Cheluvappa R, Luo AS, Grimm MC. T helper type 17 pathway suppression by appendicitis and appendectomy protects against colitis. Clin Exp Immunol. 2014;175:316-322.
- Lynde CW, Poulin Y, Vender R, et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141-150.
- Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-α, IFN-γ, IL6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005:2005;273-279.
- Egeberg A, Anderson YMF, Gislason GH, et al. Gallstone risk in adult patients with atopic dermatitis and psoriasis: possible effect of overweight and obesity. Acta Derm Venereol. 2017;97:627-631.
- Smirnova SV, Barilo AA, Smolnikova MV. Hepatobiliary system diseases as the predictors of psoriasis progression [in Russian]. Vestn Ross Akad Med Nauk. 2016:102-108.
- Bagel J, Lynde C, Tyring S, et al. Moderate to severe plaque psoriasis with scalp involvement: a randomized, double-blind, placebo-controlled study of etanercept. J Am Acad Dermatol. 2012;67:86-92.
- Foeldvari I, Krüger E, Schneider T. Acute, non-obstructive, sterile cholecystitis associated with etanercept and infliximab for the treatment of juvenile polyarticular rheumatoid arthritis. Ann Rheum Dis. 2003;62:908-909.
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Channual J, Wu JJ, Dann FJ. Effects of tumor necrosis factor-α blockade on metabolic syndrome in psoriasis and psoriatic arthritis and additional lessons learned from rheumatoid arthritis. Dermatol Ther. 2009;22:61-73.
- Koebnick C, Black MH, Smith N, et al. The association of psoriasis and elevated blood lipids in overweight and obese children. J Pediatr. 2011;159:577-583.
- Herron MD, Hinckley M, Hoffman MS, et al. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol. 2005;141:1527-1534.
- Qureshi AA, Choi HK, Setty AR, et al. Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch Dermatol. 2009;145:379-382.
- Shapiro J, Cohen AD, David M, et al. The association between psoriasis, diabetes mellitus, and atherosclerosis in Israel: a case-control study. J Am Acad Dermatol. 2007;56:629-634.
- Love TJ, Qureshi AA, Karlson EW, et al. Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, 2003-2006. Arch Dermatol. 2011;147:419-424.
- El-Mongy S, Fathy H, Abdelaziz A, et al. Subclinical atherosclerosis in patients with chronic psoriasis: a potential association. J Eur Acad Dermatol Venereol. 2010;24:661-666.
- Prodanovich S, Kirsner RS, Kravetz JD, et al. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol. 2009;145:700-703.
- Ludwig RJ, Herzog C, Rostock A, et al. Psoriasis: a possible risk factor for development of coronary artery calcification. Br J Dermatol. 2007;156:271-276.
- Kaye JA, Li L, Jick SS. Incidence of risk factors for myocardial infarction and other vascular diseases in patients with psoriasis. Br J Dermatol. 2008;159:895-902.
- Kimball AB, Robinson D Jr, Wu Y, et al. Cardiovascular disease and risk factors among psoriasis patients in two US healthcare databases, 2001-2002. Dermatology. 2008;217:27-37.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Mehta NN, Azfar RS, Shin DB, et al. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31:1000-1006.
- Abuabara K, Azfar RS, Shin DB, et al. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the United Kingdom. Br J Dermatol. 2010;163:586-592.
- Christophers E. Comorbidities in psoriasis. Clin Dermatol. 2007;25:529-534.
- Wu JJ, Nguyen TU, Poon KY, et al. The association of psoriasis with autoimmune diseases. J Am Acad Dermatol. 2012;67:924-930.
- Floch MH, Bina I. The natural history of diverticulitis: fact and theory. Clin Gastroenterol. 2004;38(5, suppl 1):S2-S7.
- Barrea L, Macchia PE, Tarantino G, et al. Nutrition: a key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J Transl Med. 2015;13:303.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. National Survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Matrana MR, Margolin DA. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg. 2009;22:141-146.
- Brandl A, Kratzer T, Kafka-Ritsch R, et al. Diverticulitis in immunosuppressed patients: a fatal outcome requiring a new approach? Can J Surg. 2016;59:254-261.
- Buckius MT, McGrath B, Monk J, et al. Changing epidemiology of acute appendicitis in the United States: study period 1993-2008. J Surg Res. 2012;175:185-190.
- Cheluvappa R, Luo AS, Grimm MC. T helper type 17 pathway suppression by appendicitis and appendectomy protects against colitis. Clin Exp Immunol. 2014;175:316-322.
- Lynde CW, Poulin Y, Vender R, et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141-150.
- Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-α, IFN-γ, IL6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005:2005;273-279.
- Egeberg A, Anderson YMF, Gislason GH, et al. Gallstone risk in adult patients with atopic dermatitis and psoriasis: possible effect of overweight and obesity. Acta Derm Venereol. 2017;97:627-631.
- Smirnova SV, Barilo AA, Smolnikova MV. Hepatobiliary system diseases as the predictors of psoriasis progression [in Russian]. Vestn Ross Akad Med Nauk. 2016:102-108.
- Bagel J, Lynde C, Tyring S, et al. Moderate to severe plaque psoriasis with scalp involvement: a randomized, double-blind, placebo-controlled study of etanercept. J Am Acad Dermatol. 2012;67:86-92.
- Foeldvari I, Krüger E, Schneider T. Acute, non-obstructive, sterile cholecystitis associated with etanercept and infliximab for the treatment of juvenile polyarticular rheumatoid arthritis. Ann Rheum Dis. 2003;62:908-909.
Practice Points
- Patients with psoriasis may have elevated risk of diverticulitis compared to healthy patients. However, psoriasis patients do not appear to have increased risk of appendicitis or cholecystitis.
- Clinicians treating psoriasis patients should consider assessing for other risk factors of diverticulitis at regular intervals.
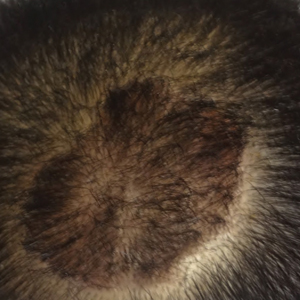
Clearance of Psoriasis After Ischemic Stroke
The etiology of psoriasis is multifactorial, and it is attributed to both genetic and environmental components.1 One of the lesser-studied aspects of psoriasis pathogenesis is the involvement of the nervous system. It is thought that the pathogenesis involves inflammation of the cutaneous nerves,2 and cutaneous denervation has been shown to improve acanthosis and IL-23 expression in mice with psoriasiform skin.3 There also have been reports of psoriasis remission following peripheral and central nervous system injury from surgical nerve resection4 as well as cerebrovascular accident.5 We present a case of total psoriasis clearance following ischemic stroke.
Case Report
A 52-year-old man with psoriasis presented to the dermatology clinic for follow-up. The patient had been using topical clobetasol and apremilast with limited success but had not previously tried biologics. On physical examination he was noted to have erythematous, scaly, indurated papules and plaques on the chest, abdomen, back, arms, and legs, consistent with psoriasis. Affected body surface area was approximately 10%. Ustekinumab was prescribed, but the patient did not pick it up from the pharmacy.
Approximately 1 month later, the patient presented to the emergency department with left-sided weakness and numbness. He was hospitalized for treatment of stroke. During hospitalization, the patient was started on lisinopril, aspirin, and atorvastatin. He also was given subcutaneous enoxaparin with plans to initiate warfarin as an outpatient. His psoriasis was not treated with topical or systemic medications during the course of his admission. He was discharged to a skilled nursing facility after 3 days.
Three months following discharge, the patient returned to the dermatology clinic for follow-up. After his stroke, he reported that his psoriasis had cleared and had not returned. On physical examination his skin was clear of psoriatic lesions.
Comment
The nervous system is thought to play an important role in the pathophysiology of psoriasis. Evidence for this involvement includes the exacerbation of psoriasis with stress and the often symmetric distribution of psoriatic lesions.6
Moreover, numerous neuropeptides have been identified in the pathophysiology of psoriasis. Farber et al7 first proposed that release of substance P (SP) from cutaneous sensory nerve fibers causes a local neurogenic response that triggers psoriasis in predisposed individuals. The role of SP in psoriasis is unclear, as there have been reports of both higher8 and lower9 levels in involved and noninvolved skin of psoriatic patients compared to skin in healthy individuals. It has been suggested that numerous other neuropeptides, including nerve growth factor (NGF), calcitonin gene-related peptide, and vasoactive intestinal peptide, play a part in psoriasis.2,10 Specifically, NGF prevents apoptosis of keratinocytes11 and is found in higher levels in psoriatic skin compared to controls.12 Calcitonin gene-related peptide has been shown to stimulate keratinocyte proliferation13 and has been found at increased levels in psoriatic skin.14 Vasoactive intestinal peptide-positive nerve fibers in the epidermis and dermis are found in higher quantities in psoriatic plaques compared to nonlesional and normal skin.8
Neuropeptides also might play a role in the itching and Köbner phenomenon that accompany psoriasis. Increased levels of NGF in nonlesional skin of patients with psoriasis is thought to contribute to the development of psoriatic plaques following trauma by inducing an inflammatory response that upregulates other neuropeptides, such as SP and calcitonin gene-related peptide. These neuropeptides induce keratinocyte proliferation, which further increases NGF expression, thus creating a cycle of inflammation and formation of psoriatic lesions.6 Moreover, there is a notable correlation between pruritus severity and density of NGF-immunoreactive keratinocytes, high-affinity NGF receptors, protein gene product 9.5–immunoreactive intraepidermal fibers, and immunoreactive vessels for E-selectin.15
Spontaneous remission of psoriasis after cerebrovascular accident was first reported in 1998.5 Moreover, there have been cases of protective effects from psoriasis and psoriatic arthritis in limbs affected by poliomyelitis.16,17 In cases in which patients regained neurologic function, Zhu et al10 found that recurrence of skin lesions in areas corresponding to nervous system injury also occurred. However, in cases of permanent nerve damage, psoriasis did not return,10 confirming the role of peripheral nerves in the pathogenesis of psoriasis. It is thought that peripheral nerve damage results in decreased secretion of neuropeptides3 and that central nervous system injury also can cause similar downstream effects.10
Other reasons for the patient’s remission also were considered. Although it is possible that the sudden change in the patient’s usual environment could have induced remission of psoriasis, it seems more likely that the stress of the situation would have worsened his symptoms. Medications used during the patient’s hospitalization also were considered as reasons for symptom improvement. One study using a case-control and case-crossover design found psoriasis to be associated with nonsteroidal anti-inflammatory drugs and angiotensin-converting enzyme inhibitors (odds ratio, 4.0 and 2.1, respectively).18 Atorvastatin has been investigated as a potential treatment of psoriasis, though no therapeutic benefit has been proven.19,20 Heparin has been shown in case reports to improve psoriasis symptoms but was used in addition to standard psoriasis therapies and not as monotherapy.21
A more thorough understanding of which neuropeptides are directly implicated in the neurologic-mediated clearance of psoriasis might contribute to better targeted therapies. For example, infusion of peptide T, a vasoactive intestinal peptide analogue, was shown to have some effect in clearing the skin in 14 psoriasis patients.22 Although this finding has not been replicated, it demonstrates the potential utility of therapies targeted toward the neurologic aspects of psoriasis. More research is needed to evaluate the potential of targeting other neuropeptides for treatment of psoriatic plaques.
- Boehncke WH. Etiology and pathogenesis of psoriasis. Rheum Dis Clin North Am. 2015;41:665-675.
- Saraceno R, Kleyn CE, Terenghi G, et al. The role of neuropeptides in psoriasis. Br J Dermatol. 2006;155:876-882.
- Ostrowski SM, Belkai A, Loyd CM, et al. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530-1538.
- Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Arch Dermatol. 1971;104:220-221.
- Stratigos AJ, Katoulis AK, Stavrianeas NG. Spontaneous clearing of psoriasis after stroke. J Am Acad Dermatol. 1998;38(5, pt 1):768-770.
- Raychaudhuri SP, Farber EM. Neuroimmunologic aspects of psoriasis. Cutis. 2000;66:357-362.
- Farber EM, Nickoloff BJ, Recht B, et al. Stress, symmetry, and psoriasis: possible role of neuropeptides. J Am Acad Dermatol. 1986;14(2, pt 1):305-311.
- Al’Abadie MS, Senior HJ, Bleehen SS, et al. Neuropeptides and general neuronal marker in psoriasis—an immunohistochemical study. Clin Exp Dermatol. 1995;20:384-389.
- Pincelli C, Fantini F, Romualdi P, et al. Substance P is diminished and vasoactive intestinal peptide is augmented in psoriatic lesions and these peptides exert disparate effects on the proliferation of cultured human keratinocytes. J Invest Dermatol. 1992;98:421-427.
- Zhu TH, Nakamura M, Farahnik B, et al. The role of the nervous system in the pathophysiology of psoriasis: a review of cases of psoriasis remission or improvement following denervation injury. Am J Clin Dermatol. 2016;17:257-263.
- Pincelli C. Nerve growth factor and keratinocytes: a role in psoriasis. Eur J Dermatol. 2000;10:85-90.
- Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol. 1998;78:84-86.
- He Y, Ding G, Wang X, et al. Calcitonin gene‐related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J (Engl). 2000;113:747-751.
- Chu DQ, Choy M, Foster P, et al. A comparative study of the ability of calcitonin gene‐related peptide and adrenomedullin13–52 to modulate microvascular but not thermal hyperalgesia responses. Br J Pharmacol. 2000;130:1589-1596.
- Nakamura M, Toyoda M, Morohashi M. Pruritogenic mediators in psoriasis vulgaris: comparative evaluation of itch-associated cutaneous factors. Br J Dermatol. 2003;149:718-730.
- Wang TS, Tsai TF. Psoriasis sparing the lower limb with postpoliomeylitis residual paralysis. Br J Dermatol. 2014;171:429-431.
- Weiner SR, Bassett LW, Reichman RP. Protective effect of poliomyelitis on psoriatic arthritis. Arthritis Rheum. 1985;28:703-706.
- Cohen AD, Bonneh DY, Reuveni H, et al. Drug exposure and psoriasis vulgaris: case control and case-crossover studies. Acta Derm Venereol. 2005;85:299-303.
- Faghihi T, Radfar M, Mehrabian Z, et al. Atorvastatin for the treatment of plaque-type psoriasis. Pharmacotherapy. 2011;31:1045-1050.
- Chua SHH, Tioleco GMS, Dayrit CAF, et al. Atorvastatin as adjunctive therapy for chronic plaque type psoriasis versus betamethasone valerate alone: a randomized, double-blind, placebo-controlled trial. Indian J Dermatol Venereol Leprol. 2017;83:441-447.
- Jekel LG. Use of heparin in treatment of psoriasis. AMA Arch Derm Syphilol. 1953;68:80-82.
- Farber EM, Cohen EN, Trozak DJ, et al. Peptide T improves psoriasis when infused into lesions in nanogram amounts. J Am Acad Dermatol. 1991;25:658-664.
The etiology of psoriasis is multifactorial, and it is attributed to both genetic and environmental components.1 One of the lesser-studied aspects of psoriasis pathogenesis is the involvement of the nervous system. It is thought that the pathogenesis involves inflammation of the cutaneous nerves,2 and cutaneous denervation has been shown to improve acanthosis and IL-23 expression in mice with psoriasiform skin.3 There also have been reports of psoriasis remission following peripheral and central nervous system injury from surgical nerve resection4 as well as cerebrovascular accident.5 We present a case of total psoriasis clearance following ischemic stroke.
Case Report
A 52-year-old man with psoriasis presented to the dermatology clinic for follow-up. The patient had been using topical clobetasol and apremilast with limited success but had not previously tried biologics. On physical examination he was noted to have erythematous, scaly, indurated papules and plaques on the chest, abdomen, back, arms, and legs, consistent with psoriasis. Affected body surface area was approximately 10%. Ustekinumab was prescribed, but the patient did not pick it up from the pharmacy.
Approximately 1 month later, the patient presented to the emergency department with left-sided weakness and numbness. He was hospitalized for treatment of stroke. During hospitalization, the patient was started on lisinopril, aspirin, and atorvastatin. He also was given subcutaneous enoxaparin with plans to initiate warfarin as an outpatient. His psoriasis was not treated with topical or systemic medications during the course of his admission. He was discharged to a skilled nursing facility after 3 days.
Three months following discharge, the patient returned to the dermatology clinic for follow-up. After his stroke, he reported that his psoriasis had cleared and had not returned. On physical examination his skin was clear of psoriatic lesions.
Comment
The nervous system is thought to play an important role in the pathophysiology of psoriasis. Evidence for this involvement includes the exacerbation of psoriasis with stress and the often symmetric distribution of psoriatic lesions.6
Moreover, numerous neuropeptides have been identified in the pathophysiology of psoriasis. Farber et al7 first proposed that release of substance P (SP) from cutaneous sensory nerve fibers causes a local neurogenic response that triggers psoriasis in predisposed individuals. The role of SP in psoriasis is unclear, as there have been reports of both higher8 and lower9 levels in involved and noninvolved skin of psoriatic patients compared to skin in healthy individuals. It has been suggested that numerous other neuropeptides, including nerve growth factor (NGF), calcitonin gene-related peptide, and vasoactive intestinal peptide, play a part in psoriasis.2,10 Specifically, NGF prevents apoptosis of keratinocytes11 and is found in higher levels in psoriatic skin compared to controls.12 Calcitonin gene-related peptide has been shown to stimulate keratinocyte proliferation13 and has been found at increased levels in psoriatic skin.14 Vasoactive intestinal peptide-positive nerve fibers in the epidermis and dermis are found in higher quantities in psoriatic plaques compared to nonlesional and normal skin.8
Neuropeptides also might play a role in the itching and Köbner phenomenon that accompany psoriasis. Increased levels of NGF in nonlesional skin of patients with psoriasis is thought to contribute to the development of psoriatic plaques following trauma by inducing an inflammatory response that upregulates other neuropeptides, such as SP and calcitonin gene-related peptide. These neuropeptides induce keratinocyte proliferation, which further increases NGF expression, thus creating a cycle of inflammation and formation of psoriatic lesions.6 Moreover, there is a notable correlation between pruritus severity and density of NGF-immunoreactive keratinocytes, high-affinity NGF receptors, protein gene product 9.5–immunoreactive intraepidermal fibers, and immunoreactive vessels for E-selectin.15
Spontaneous remission of psoriasis after cerebrovascular accident was first reported in 1998.5 Moreover, there have been cases of protective effects from psoriasis and psoriatic arthritis in limbs affected by poliomyelitis.16,17 In cases in which patients regained neurologic function, Zhu et al10 found that recurrence of skin lesions in areas corresponding to nervous system injury also occurred. However, in cases of permanent nerve damage, psoriasis did not return,10 confirming the role of peripheral nerves in the pathogenesis of psoriasis. It is thought that peripheral nerve damage results in decreased secretion of neuropeptides3 and that central nervous system injury also can cause similar downstream effects.10
Other reasons for the patient’s remission also were considered. Although it is possible that the sudden change in the patient’s usual environment could have induced remission of psoriasis, it seems more likely that the stress of the situation would have worsened his symptoms. Medications used during the patient’s hospitalization also were considered as reasons for symptom improvement. One study using a case-control and case-crossover design found psoriasis to be associated with nonsteroidal anti-inflammatory drugs and angiotensin-converting enzyme inhibitors (odds ratio, 4.0 and 2.1, respectively).18 Atorvastatin has been investigated as a potential treatment of psoriasis, though no therapeutic benefit has been proven.19,20 Heparin has been shown in case reports to improve psoriasis symptoms but was used in addition to standard psoriasis therapies and not as monotherapy.21
A more thorough understanding of which neuropeptides are directly implicated in the neurologic-mediated clearance of psoriasis might contribute to better targeted therapies. For example, infusion of peptide T, a vasoactive intestinal peptide analogue, was shown to have some effect in clearing the skin in 14 psoriasis patients.22 Although this finding has not been replicated, it demonstrates the potential utility of therapies targeted toward the neurologic aspects of psoriasis. More research is needed to evaluate the potential of targeting other neuropeptides for treatment of psoriatic plaques.
The etiology of psoriasis is multifactorial, and it is attributed to both genetic and environmental components.1 One of the lesser-studied aspects of psoriasis pathogenesis is the involvement of the nervous system. It is thought that the pathogenesis involves inflammation of the cutaneous nerves,2 and cutaneous denervation has been shown to improve acanthosis and IL-23 expression in mice with psoriasiform skin.3 There also have been reports of psoriasis remission following peripheral and central nervous system injury from surgical nerve resection4 as well as cerebrovascular accident.5 We present a case of total psoriasis clearance following ischemic stroke.
Case Report
A 52-year-old man with psoriasis presented to the dermatology clinic for follow-up. The patient had been using topical clobetasol and apremilast with limited success but had not previously tried biologics. On physical examination he was noted to have erythematous, scaly, indurated papules and plaques on the chest, abdomen, back, arms, and legs, consistent with psoriasis. Affected body surface area was approximately 10%. Ustekinumab was prescribed, but the patient did not pick it up from the pharmacy.
Approximately 1 month later, the patient presented to the emergency department with left-sided weakness and numbness. He was hospitalized for treatment of stroke. During hospitalization, the patient was started on lisinopril, aspirin, and atorvastatin. He also was given subcutaneous enoxaparin with plans to initiate warfarin as an outpatient. His psoriasis was not treated with topical or systemic medications during the course of his admission. He was discharged to a skilled nursing facility after 3 days.
Three months following discharge, the patient returned to the dermatology clinic for follow-up. After his stroke, he reported that his psoriasis had cleared and had not returned. On physical examination his skin was clear of psoriatic lesions.
Comment
The nervous system is thought to play an important role in the pathophysiology of psoriasis. Evidence for this involvement includes the exacerbation of psoriasis with stress and the often symmetric distribution of psoriatic lesions.6
Moreover, numerous neuropeptides have been identified in the pathophysiology of psoriasis. Farber et al7 first proposed that release of substance P (SP) from cutaneous sensory nerve fibers causes a local neurogenic response that triggers psoriasis in predisposed individuals. The role of SP in psoriasis is unclear, as there have been reports of both higher8 and lower9 levels in involved and noninvolved skin of psoriatic patients compared to skin in healthy individuals. It has been suggested that numerous other neuropeptides, including nerve growth factor (NGF), calcitonin gene-related peptide, and vasoactive intestinal peptide, play a part in psoriasis.2,10 Specifically, NGF prevents apoptosis of keratinocytes11 and is found in higher levels in psoriatic skin compared to controls.12 Calcitonin gene-related peptide has been shown to stimulate keratinocyte proliferation13 and has been found at increased levels in psoriatic skin.14 Vasoactive intestinal peptide-positive nerve fibers in the epidermis and dermis are found in higher quantities in psoriatic plaques compared to nonlesional and normal skin.8
Neuropeptides also might play a role in the itching and Köbner phenomenon that accompany psoriasis. Increased levels of NGF in nonlesional skin of patients with psoriasis is thought to contribute to the development of psoriatic plaques following trauma by inducing an inflammatory response that upregulates other neuropeptides, such as SP and calcitonin gene-related peptide. These neuropeptides induce keratinocyte proliferation, which further increases NGF expression, thus creating a cycle of inflammation and formation of psoriatic lesions.6 Moreover, there is a notable correlation between pruritus severity and density of NGF-immunoreactive keratinocytes, high-affinity NGF receptors, protein gene product 9.5–immunoreactive intraepidermal fibers, and immunoreactive vessels for E-selectin.15
Spontaneous remission of psoriasis after cerebrovascular accident was first reported in 1998.5 Moreover, there have been cases of protective effects from psoriasis and psoriatic arthritis in limbs affected by poliomyelitis.16,17 In cases in which patients regained neurologic function, Zhu et al10 found that recurrence of skin lesions in areas corresponding to nervous system injury also occurred. However, in cases of permanent nerve damage, psoriasis did not return,10 confirming the role of peripheral nerves in the pathogenesis of psoriasis. It is thought that peripheral nerve damage results in decreased secretion of neuropeptides3 and that central nervous system injury also can cause similar downstream effects.10
Other reasons for the patient’s remission also were considered. Although it is possible that the sudden change in the patient’s usual environment could have induced remission of psoriasis, it seems more likely that the stress of the situation would have worsened his symptoms. Medications used during the patient’s hospitalization also were considered as reasons for symptom improvement. One study using a case-control and case-crossover design found psoriasis to be associated with nonsteroidal anti-inflammatory drugs and angiotensin-converting enzyme inhibitors (odds ratio, 4.0 and 2.1, respectively).18 Atorvastatin has been investigated as a potential treatment of psoriasis, though no therapeutic benefit has been proven.19,20 Heparin has been shown in case reports to improve psoriasis symptoms but was used in addition to standard psoriasis therapies and not as monotherapy.21
A more thorough understanding of which neuropeptides are directly implicated in the neurologic-mediated clearance of psoriasis might contribute to better targeted therapies. For example, infusion of peptide T, a vasoactive intestinal peptide analogue, was shown to have some effect in clearing the skin in 14 psoriasis patients.22 Although this finding has not been replicated, it demonstrates the potential utility of therapies targeted toward the neurologic aspects of psoriasis. More research is needed to evaluate the potential of targeting other neuropeptides for treatment of psoriatic plaques.
- Boehncke WH. Etiology and pathogenesis of psoriasis. Rheum Dis Clin North Am. 2015;41:665-675.
- Saraceno R, Kleyn CE, Terenghi G, et al. The role of neuropeptides in psoriasis. Br J Dermatol. 2006;155:876-882.
- Ostrowski SM, Belkai A, Loyd CM, et al. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530-1538.
- Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Arch Dermatol. 1971;104:220-221.
- Stratigos AJ, Katoulis AK, Stavrianeas NG. Spontaneous clearing of psoriasis after stroke. J Am Acad Dermatol. 1998;38(5, pt 1):768-770.
- Raychaudhuri SP, Farber EM. Neuroimmunologic aspects of psoriasis. Cutis. 2000;66:357-362.
- Farber EM, Nickoloff BJ, Recht B, et al. Stress, symmetry, and psoriasis: possible role of neuropeptides. J Am Acad Dermatol. 1986;14(2, pt 1):305-311.
- Al’Abadie MS, Senior HJ, Bleehen SS, et al. Neuropeptides and general neuronal marker in psoriasis—an immunohistochemical study. Clin Exp Dermatol. 1995;20:384-389.
- Pincelli C, Fantini F, Romualdi P, et al. Substance P is diminished and vasoactive intestinal peptide is augmented in psoriatic lesions and these peptides exert disparate effects on the proliferation of cultured human keratinocytes. J Invest Dermatol. 1992;98:421-427.
- Zhu TH, Nakamura M, Farahnik B, et al. The role of the nervous system in the pathophysiology of psoriasis: a review of cases of psoriasis remission or improvement following denervation injury. Am J Clin Dermatol. 2016;17:257-263.
- Pincelli C. Nerve growth factor and keratinocytes: a role in psoriasis. Eur J Dermatol. 2000;10:85-90.
- Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol. 1998;78:84-86.
- He Y, Ding G, Wang X, et al. Calcitonin gene‐related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J (Engl). 2000;113:747-751.
- Chu DQ, Choy M, Foster P, et al. A comparative study of the ability of calcitonin gene‐related peptide and adrenomedullin13–52 to modulate microvascular but not thermal hyperalgesia responses. Br J Pharmacol. 2000;130:1589-1596.
- Nakamura M, Toyoda M, Morohashi M. Pruritogenic mediators in psoriasis vulgaris: comparative evaluation of itch-associated cutaneous factors. Br J Dermatol. 2003;149:718-730.
- Wang TS, Tsai TF. Psoriasis sparing the lower limb with postpoliomeylitis residual paralysis. Br J Dermatol. 2014;171:429-431.
- Weiner SR, Bassett LW, Reichman RP. Protective effect of poliomyelitis on psoriatic arthritis. Arthritis Rheum. 1985;28:703-706.
- Cohen AD, Bonneh DY, Reuveni H, et al. Drug exposure and psoriasis vulgaris: case control and case-crossover studies. Acta Derm Venereol. 2005;85:299-303.
- Faghihi T, Radfar M, Mehrabian Z, et al. Atorvastatin for the treatment of plaque-type psoriasis. Pharmacotherapy. 2011;31:1045-1050.
- Chua SHH, Tioleco GMS, Dayrit CAF, et al. Atorvastatin as adjunctive therapy for chronic plaque type psoriasis versus betamethasone valerate alone: a randomized, double-blind, placebo-controlled trial. Indian J Dermatol Venereol Leprol. 2017;83:441-447.
- Jekel LG. Use of heparin in treatment of psoriasis. AMA Arch Derm Syphilol. 1953;68:80-82.
- Farber EM, Cohen EN, Trozak DJ, et al. Peptide T improves psoriasis when infused into lesions in nanogram amounts. J Am Acad Dermatol. 1991;25:658-664.
- Boehncke WH. Etiology and pathogenesis of psoriasis. Rheum Dis Clin North Am. 2015;41:665-675.
- Saraceno R, Kleyn CE, Terenghi G, et al. The role of neuropeptides in psoriasis. Br J Dermatol. 2006;155:876-882.
- Ostrowski SM, Belkai A, Loyd CM, et al. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530-1538.
- Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Arch Dermatol. 1971;104:220-221.
- Stratigos AJ, Katoulis AK, Stavrianeas NG. Spontaneous clearing of psoriasis after stroke. J Am Acad Dermatol. 1998;38(5, pt 1):768-770.
- Raychaudhuri SP, Farber EM. Neuroimmunologic aspects of psoriasis. Cutis. 2000;66:357-362.
- Farber EM, Nickoloff BJ, Recht B, et al. Stress, symmetry, and psoriasis: possible role of neuropeptides. J Am Acad Dermatol. 1986;14(2, pt 1):305-311.
- Al’Abadie MS, Senior HJ, Bleehen SS, et al. Neuropeptides and general neuronal marker in psoriasis—an immunohistochemical study. Clin Exp Dermatol. 1995;20:384-389.
- Pincelli C, Fantini F, Romualdi P, et al. Substance P is diminished and vasoactive intestinal peptide is augmented in psoriatic lesions and these peptides exert disparate effects on the proliferation of cultured human keratinocytes. J Invest Dermatol. 1992;98:421-427.
- Zhu TH, Nakamura M, Farahnik B, et al. The role of the nervous system in the pathophysiology of psoriasis: a review of cases of psoriasis remission or improvement following denervation injury. Am J Clin Dermatol. 2016;17:257-263.
- Pincelli C. Nerve growth factor and keratinocytes: a role in psoriasis. Eur J Dermatol. 2000;10:85-90.
- Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol. 1998;78:84-86.
- He Y, Ding G, Wang X, et al. Calcitonin gene‐related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J (Engl). 2000;113:747-751.
- Chu DQ, Choy M, Foster P, et al. A comparative study of the ability of calcitonin gene‐related peptide and adrenomedullin13–52 to modulate microvascular but not thermal hyperalgesia responses. Br J Pharmacol. 2000;130:1589-1596.
- Nakamura M, Toyoda M, Morohashi M. Pruritogenic mediators in psoriasis vulgaris: comparative evaluation of itch-associated cutaneous factors. Br J Dermatol. 2003;149:718-730.
- Wang TS, Tsai TF. Psoriasis sparing the lower limb with postpoliomeylitis residual paralysis. Br J Dermatol. 2014;171:429-431.
- Weiner SR, Bassett LW, Reichman RP. Protective effect of poliomyelitis on psoriatic arthritis. Arthritis Rheum. 1985;28:703-706.
- Cohen AD, Bonneh DY, Reuveni H, et al. Drug exposure and psoriasis vulgaris: case control and case-crossover studies. Acta Derm Venereol. 2005;85:299-303.
- Faghihi T, Radfar M, Mehrabian Z, et al. Atorvastatin for the treatment of plaque-type psoriasis. Pharmacotherapy. 2011;31:1045-1050.
- Chua SHH, Tioleco GMS, Dayrit CAF, et al. Atorvastatin as adjunctive therapy for chronic plaque type psoriasis versus betamethasone valerate alone: a randomized, double-blind, placebo-controlled trial. Indian J Dermatol Venereol Leprol. 2017;83:441-447.
- Jekel LG. Use of heparin in treatment of psoriasis. AMA Arch Derm Syphilol. 1953;68:80-82.
- Farber EM, Cohen EN, Trozak DJ, et al. Peptide T improves psoriasis when infused into lesions in nanogram amounts. J Am Acad Dermatol. 1991;25:658-664.
Practice Points
- Psoriasis is exacerbated in the presence of stress, and psoriatic lesions often have a symmetric distribution, which is evidence that the nervous system is involved in the pathophysiology of the condition.
- Various neuropeptides are involved in the pathophysiology of psoriasis, including substance P, nerve growth factor, calcitonin gene-related peptide, and vasoactive intestinal peptide.
- Peripheral nerve damage results in decreased secretion of neuropeptides, which can lead to remission of psoriasis.
Psoriasis Risk Factors and Triggers
Psoriasis is a chronic autoimmune skin disease affecting approximately 6.7 million adults in the United States.1 Although its pathogenesis is not yet clear, risk factors and triggers provide insight into potential pathways by which psoriasis can occur. There is notable overlap between risk factors and triggers of psoriasis; perceived risk factors might, in fact, be triggers causing manifestation of disease in predisposed persons. In this review, we summarize the key factors contributing to onset and exacerbation of psoriasis. When learning to manage this chronic disease, it also may be helpful to educate patients about how these elements may affect the course of psoriasis.
Genetics
The pathogenesis of psoriasis has a strong genetic component, with approximately 70% and 20% concordance rates in monozygotic and dizygotic twins, respectively.2 Moreover, studies have shown a positive family history in approximately 35% of patients.3,4 Family-based studies have found a 50% risk of developing psoriasis in patients with 2 affected parents.5 However, the genetics of psoriasis are complex and are attributed to many different genes. Thus far, genes involving antigen presentation, T-cell receptor development and polarization, and the nuclear factor κβ (NF-κβ) pathway have been identified.6
HLA-Cw6
The most well-studied gene implicated in psoriasis is HLA-Cw6, which encodes a major histocompatibility complex class I allele supporting psoriasis as a T cell–mediated reaction to an autoantigen.6 Two potential antigens for HLA-Cw6 recently have been identified: LL-37, a cathelicidin-related antimicrobial peptide, and the A disintegrin and metalloproteinase with thrombospondin motifs-like protein 5 (ADAMTSL5), found on melanocytes and keratinocytes.7 The percentage of psoriasis patients with HLA-Cw6 ranges from 10.5% to 77.2%, with higher frequency in white individuals than in Asians.7
HLA-Cw6 manifests as specific features in psoriasis, including onset of disease before 21 years of age.8 It also is more strongly associated with guttate-type psoriasis, greater body surface area involvement, and higher incidence of Köbner phenomenon. Patients with positive HLA-Cw6 also reported worsening of psoriasis during and after throat infection.9
Caspase Recruitment Domain Family Member 14
Another gene mutation implicated in psoriasis pathogenesis is caspase recruitment domain family member 14, CARD14 (formerly PSORS2), a gene encoding a scaffolding protein important in the activation of NF-κβ.10,11 Missense CARD14 mutations cause upregulation of NF-κβ through formation of a complex with adapter protein B-cell lymphoma 10 (BCL10) and mucosa-associated lymphoid tissue lymphoma translocation protein 1 (MALT1),12 which, in turn, causes increased transcription of cytokines IL-8, C-C motif chemokine ligand 20 (CCL-20), and IL-36 gamma in the keratinocyte.13 Mutations in CARD14 alone lead to psoriasiform skin in mice through amplified activation of the IL-23/IL-17 axis.14,15 Patients with a mutation in a CARD14 variant (p.Arg820Trp) have demonstrated better response to tumor necrosis factor (TNF) inhibitors.16
Further characterization of the genetic pathogenesis of psoriasis might lead to better targeted therapies, including the possibility of MALT1 inhibitors as a treatment option.12
Infection
Streptococcus
The association between streptococcal infection and psoriasis was first documented more than 100 years ago, specifically the onset of acute guttate psoriasis.17,18 Although classically described following throat infection, psoriasis also occurs following streptococcal vulvovaginitis and perianal streptococcal infection.19,20
This type of psoriasis is typically self-limited but can recur with subsequent streptococcal infections or initiate a more chronic plaque psoriasis. Patients have a 1 in 3 risk of developing chronic psoriasis within 10 years of a single episode of acute guttate psoriasis.21 Moreover, in many patients with existing plaque psoriasis, throat infection exacerbates psoriatic symptoms.22 The mechanism of exacerbation is likely due to cross-reactivity between streptococcal M surface antigen and human keratinocytes and might also be influenced by inherited abnormalities in immune response.23-26 Therefore, tonsillectomy has been studied as a possible treatment of psoriasis but is likely helpful only in patients with exacerbations of disease that are closely associated with recurrent tonsillitis.27
Human Immunodeficiency Virus
The prevalence of psoriasis in human immunodeficiency virus (HIV) patients is similar to or greater than the general population.28 Human immunodeficiency virus infection causes new onset of psoriasis and exacerbation of existing psoriasis; severity often is correlated with worsening immune function.28,29
The clinical subtypes of psoriasis that occur most frequently with HIV include guttate, inverse, and erythrodermic, though patients may present with any subtype.28 The mechanism is puzzling because HIV is primarily mediated by helper T cell 2 (TH2) cytokines, whereas psoriasis is mainly driven by helper T cell 1 (TH1) cytokines.30 Furthermore, despite increased severity with lower CD4+ counts, treatments further lowering T-cell counts paradoxically improve symptoms.31 Current literature suggests that expansion of CD8+ memory T cells might be the primary mechanism in the exacerbation of psoriasis in HIV-mediated immunosuppression.30
Treatment of HIV-associated psoriasis presents challenges because many therapeutics cause further immunosuppression. The National Psoriasis Foundation recommends topical preparations as first-line agents for mild to moderate psoriasis.32 For moderate to severe psoriasis, retroviral agents may be effective as first-line monotherapy or when supplemented by phototherapy with UVB or psoralen plus UVA. Retinoids can be used as second-line agents.32 For cases of severe refractory psoriasis, cyclosporine, methotrexate, TNF inhibitors, or hydroxyurea can be considered. There also is evidence that apremilast is effective without risk for worsening immune function.33
Other Infections
Other bacteria associated with triggering or exacerbating psoriasis include Staphylococcus aureus and Helicobacter pylori.34,35 Fungi, such as species of the genera Malassezia and Candida, and other viruses, including papillomaviruses and retroviruses, also have been implicated.34
Medications
Numerous medications can trigger psoriasis, including lithium, nonsteroidal anti-inflammatory drugs, antimalarials, beta-blockers, and angiotensin-converting enzyme inhibitors.34 More recent literature suggests that TNF inhibitors also can paradoxically induce psoriasis in rare cases.35
Lithium
Psoriasis is the most common cutaneous adverse effect of lithium.34 It is more likely to exacerbate existing disease but also can induce onset of psoriasis; it also can cause disease that is more refractory to treatment.34,36 Current literature hypothesizes that lithium triggers psoriasis by interference of intracellular calcium channels through reduction of inositol, thereby affecting keratinocyte proliferation and differentiation.34 Lithium also inhibits glycogen synthase kinase-3 (GSK-3), a serine threonine kinase, which, in turn, induces human keratinocyte proliferation.37 However, it is unlikely lithium alone can induce psoriasis; genetic predisposition is necessary.
TNF Inhibitors
Tumor necrosis factor inhibitors such as adalimumab, etanercept, certolizumab pegol, golimumab, and infliximab are used in various inflammatory diseases, including psoriasis. Interestingly, there have been more than 200 reported cases of suspected TNF inhibitor–induced or –exacerbated psoriasis.38 This phenomenon appears to occur more frequently with infliximab and is most likely to occur in the first year of treatment of Crohn disease and rheumatoid arthritis.38 Plaque psoriasis is the most common form, but 15% to 26% of cases presented with 2 or more morphologies.38,39
Treatment options include discontinuing therapy, though many patients experience resolution while continuing treatment or switching to another TNF inhibitor.38-40 Traditional topical therapies also have been used with success.40 The pathogenesis of this phenomenon is still unclear but is thought to involve both the IL-23/helper T cell 17 (TH17) axis and dysregulation of IFN-α in the setting of TNF suppression.38
Lifestyle
Obesity is a chronic low-grade inflammatory state that can contribute to the onset of psoriasis or exacerbation of exist
The relationship between psoriasis and alcohol consumption is less clear than it is between psoriasis and obesity or smoking; greater consumption is found in psoriasis patients, but evidence is insufficient to deem alcohol a risk factor.44
Conclusion
Various factors, including genetics, infection, pharmacotherapeutic, and lifestyle, can all contribute to the induction or exacerbation of psoriasis. These factors can provide clues to the pathogenesis of psoriasis as well as help clinicians better counsel patients about their disease.
- Helmick CG, Lee-Han H, Hirsch SC, et al. Prevalence of psoriasis among adults in the U.S.: 2003-2006 and 2009-2010 National Health and Nutrition Examination Surveys. Am J Prev Med. 2014;47:37-45.
- Bowcock AM. The genetics of psoriasis and autoimmunity. Annu Rev Genomics Hum Genet. 2005;6:93-122.
- Swanbeck G, Inerot A, Martinsson T, et al. A population genetic study of psoriasis. Br J Dermatol. 1994;131:32-39.
- Kimberling W, Dobson RL. The inheritance of psoriasis. J Invest Dermatol. 1973;60:538-540.
- Gupta R, Debbaneh MG, Liao W. Genetic epidemiology of psoriasis. Curr Dermatol Rep. 2014;3:61-78.
- Harden JL, Krueger JG, Bowcock AM. The immunogenetics of psoriasis: a comprehensive review. J Autoimmun. 2015;64:66-73.
- Chen L, Tsai TF. HLA-Cw6 and psoriasis. Br J Dermatol. 2018;178:854-862.
- Enerbäck C, Martinsson T, Ineraot A, et al. Evidence that HLA-Cw6 determines early onset of psoriasis, obtained using sequence-specific primers (PCR-SSP). Acta Derm Venereol. 1997;77:273-276.
- Gudjónsson JE, Kárason A, Antonsdóttir EH, et al. HLA-Cw6-positive and HLA-Cw6-negative patients with psoriasis vulgaris have distinct clinical features. J Invest Dermatol. 2002;118:362-365.
- Tomfohrde J, Silverman A, Barnes R, et al. Gene for familial psoriasis susceptibility mapped to distal end of human chromosome 17q. Science. 1994;264:1141-1145.
- Blonska M, Lin X. NF-κB signaling pathways regulated by CARMA family of scaffold proteins. Cell Res. 2011;21:55-70.
- Van Nuffel E, Schmitt A, Afonina IS, et al. CARD14-mediated activation of paracaspase MALT1 in keratinocytes: implications for psoriasis. J Invest Dermatol. 2017;137:569-575.
- Jordan CT, Cao L, Roberson ED, et al. PSORS2 is due to mutations in CARD14. Am J Hum Genet. 2012;90:784-795.
- Wang M, Zhang S, Zheng G, et al. Gain-of-function mutation of Card14 leads to spontaneous psoriasis-like skin inflammation through enhanced keratinocyte response to IL-17A. Immunity. 2018;49:66-79.
- Mellet M, Meier B, Mohanan D, et al. CARD14 gain-of-function mutation alone is sufficient to drive IL-23/IL-17-mediated psoriasiform skin inflammation in vivo. J Invest Dermatol. 2018;138:2010-2023.
- Coto-Segura P, González-Fernández D, Batalla A, et al. Common and rare CARD14 gene variants affect the antitumour necrosis factor response among patients with psoriasis. Br J Dermatol. 2016;175:134-141.
- Winfield JM. Psoriasis as a sequel to acute inflammations of the tonsils: a clinical note. J Cutan Dis. 1916;34:441-443.
- Telfer NR, Chalmers RJG, Whale K, et al. The role of streptococcal infection in the initiation of guttate psoriasis. Arch Dermatol. 1992;128:39-42.
- Hernandez M, Simms-Cendan J, Zendell K. Guttate psoriasis following streptococcal vulvovaginitis in a five-year-old girl. J Pediatr Adolesc Gynecol. 2015;28:e127-e129.
- Herbst RA, Hoch O, Kapp A, et al. Guttate psoriasis triggered by perianal streptococcal dermatitis in a four-year-old boy. J Am Acad Dermatol. 2000;42(5, pt 2):885-887.
- Martin BA, Chalmers RJ, Telfer NR. How great is the risk of further psoriasis following a single episode of acute guttate psoriasis? Arch Dermatol. 1996;132:717-718.
- Thorleifsdottir RH, Eysteinsdóttir, Olafsson JH, et al. Throat infections are associated with exacerbation in a substantial proportion of patients with chronic plaque psoriasis. Acta Derm Venereol. 2016;96:788-791.
- McFadden J, Valdimarsson H, Fry L. Cross-reactivity between streptococcal M surface antigen and human skin. Br J Dermatol. 1991;125:443-447.
- Validmarsson H, Thorleifsdottir RH, Sigurdardottir SL, et al. Psoriasis—as an autoimmune disease caused by molecular mimicry. Trends Immunol. 2009;30:494-501.
- Muto M, Fujikara Y, Hamamoto Y, et al. Immune response to Streptococcus pyogenes and the susceptibility to psoriasis. Australas J Dermatol. 1996;37(suppl 1):S54-S55.
- Weisenseel P, Laumbacher B, Besgen P, et al. Streptococcal infection distinguishes different types of psoriasis. J Med Genet. 2002;39:767-768.
- Rachakonda TD, Dhillon JS, Florek AG, et al. Effect of tonsillectomy on psoriasis: a systematic review. J Am Acad Dermatol. 2015;72:261-275.
- Mallon E, Bunker CB. HIV-associated psoriasis. AIDS Patient Care STDS. 2000;14:239-246.
- Duvic M, Johnson TM, Rapini RP, et al. Acquired immunodeficiency syndrome-associated psoriasis and Reiter’s syndrome. Arch Dermatol. 1987;123:1622-1632.
- Fife DJ, Waller JM, Jeffes EW, et al. Unraveling the paradoxes of HIV-associated psoriasis: a review of T-cell subsets and cytokine profiles. Dermatol Online J. 2007;13:4.
- Ortonne JP, Lebwohl M, Em Griffiths C; Alefacept Clinical Study Group. Alefacept-induced decreases in circulating blood lymphocyte counts correlate with clinical response in patients with chronic plaque psoriasis. Eur J Dermatol. 2003;13:117-123.
- Menon K, Van Voorhees AS, Bebo BF Jr, et al; National Psoriasis Foundation. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299.
- Reddy SP, Shah VV, Wu JJ. Apremilast for a psoriasis patient with HIV and hepatitis C. J Eur Acad Dermatol Venereol. 2017;31:e481-e482.
- Fry L, Baker BS. Triggering psoriasis: the role of infections and medications. Clin Dermatol. 2007;25:606-615.
- Sfikakis PP, Iliopoulos A, Elezoglou A, et al. Psoriasis induced by anti-tumor necrosis factor therapy: a paradoxical adverse reaction. Arthritis Rheum. 2005;52:2513-2518.
- Yeung CK, Chan HH. Cutaneous adverse effects of lithium: epidemiology and management. Am J Clin Dermatol. 2004;5:3-8.
- Hampton PJ, Jans R, Flockhart RJ, et al. Lithium regulates keratinocyte proliferation via glycogen synthase kinase 3 and NFAT 2 (nuclear factor of activated T cells 2). J Cell Physiol. 2012;227:1529-1537.
- Brown G, Wang E, Leon A, et al. Tumor necrosis factor-α inhibitor-induced psoriasis: systematic review of clinical features, histopathological findings, and management experience. J Am Acad Dermatol. 2017;76:334-341.
- Collamer AN, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: clinical features and possible immunopathogenesis. Semin Arthritis Rheum. 2010;40:233-240.
- Collamer AN, Guerrero KT, Henning JS, et al. Psoriatic skin lesions induced by tumor antagonist therapy: a literature review and potential mechanisms of action. Arthritis Rheum. 2008;59:996-1001.
- Jensen P, Skov L. Psoriasis and obesity. Dermatology. 2016;232:633-639.
- Barrea L, Nappi F, Di Somma C, et al. Environmental risk factors in psoriasis: the point of view of the nutritionist. Int J Environ Res Public Health. 2016;13:743.
- Lee EJ, Han KD, Han JH, et al. Smoking and risk of psoriasis: a nationwide cohort study. J Am Acad Dermatol. 2017;77:573-575.
- Brenaut E, Horreau C, Pouplard C, et al. Alcohol consumption and psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2013;27(suppl 3):30-35.
Psoriasis is a chronic autoimmune skin disease affecting approximately 6.7 million adults in the United States.1 Although its pathogenesis is not yet clear, risk factors and triggers provide insight into potential pathways by which psoriasis can occur. There is notable overlap between risk factors and triggers of psoriasis; perceived risk factors might, in fact, be triggers causing manifestation of disease in predisposed persons. In this review, we summarize the key factors contributing to onset and exacerbation of psoriasis. When learning to manage this chronic disease, it also may be helpful to educate patients about how these elements may affect the course of psoriasis.
Genetics
The pathogenesis of psoriasis has a strong genetic component, with approximately 70% and 20% concordance rates in monozygotic and dizygotic twins, respectively.2 Moreover, studies have shown a positive family history in approximately 35% of patients.3,4 Family-based studies have found a 50% risk of developing psoriasis in patients with 2 affected parents.5 However, the genetics of psoriasis are complex and are attributed to many different genes. Thus far, genes involving antigen presentation, T-cell receptor development and polarization, and the nuclear factor κβ (NF-κβ) pathway have been identified.6
HLA-Cw6
The most well-studied gene implicated in psoriasis is HLA-Cw6, which encodes a major histocompatibility complex class I allele supporting psoriasis as a T cell–mediated reaction to an autoantigen.6 Two potential antigens for HLA-Cw6 recently have been identified: LL-37, a cathelicidin-related antimicrobial peptide, and the A disintegrin and metalloproteinase with thrombospondin motifs-like protein 5 (ADAMTSL5), found on melanocytes and keratinocytes.7 The percentage of psoriasis patients with HLA-Cw6 ranges from 10.5% to 77.2%, with higher frequency in white individuals than in Asians.7
HLA-Cw6 manifests as specific features in psoriasis, including onset of disease before 21 years of age.8 It also is more strongly associated with guttate-type psoriasis, greater body surface area involvement, and higher incidence of Köbner phenomenon. Patients with positive HLA-Cw6 also reported worsening of psoriasis during and after throat infection.9
Caspase Recruitment Domain Family Member 14
Another gene mutation implicated in psoriasis pathogenesis is caspase recruitment domain family member 14, CARD14 (formerly PSORS2), a gene encoding a scaffolding protein important in the activation of NF-κβ.10,11 Missense CARD14 mutations cause upregulation of NF-κβ through formation of a complex with adapter protein B-cell lymphoma 10 (BCL10) and mucosa-associated lymphoid tissue lymphoma translocation protein 1 (MALT1),12 which, in turn, causes increased transcription of cytokines IL-8, C-C motif chemokine ligand 20 (CCL-20), and IL-36 gamma in the keratinocyte.13 Mutations in CARD14 alone lead to psoriasiform skin in mice through amplified activation of the IL-23/IL-17 axis.14,15 Patients with a mutation in a CARD14 variant (p.Arg820Trp) have demonstrated better response to tumor necrosis factor (TNF) inhibitors.16
Further characterization of the genetic pathogenesis of psoriasis might lead to better targeted therapies, including the possibility of MALT1 inhibitors as a treatment option.12
Infection
Streptococcus
The association between streptococcal infection and psoriasis was first documented more than 100 years ago, specifically the onset of acute guttate psoriasis.17,18 Although classically described following throat infection, psoriasis also occurs following streptococcal vulvovaginitis and perianal streptococcal infection.19,20
This type of psoriasis is typically self-limited but can recur with subsequent streptococcal infections or initiate a more chronic plaque psoriasis. Patients have a 1 in 3 risk of developing chronic psoriasis within 10 years of a single episode of acute guttate psoriasis.21 Moreover, in many patients with existing plaque psoriasis, throat infection exacerbates psoriatic symptoms.22 The mechanism of exacerbation is likely due to cross-reactivity between streptococcal M surface antigen and human keratinocytes and might also be influenced by inherited abnormalities in immune response.23-26 Therefore, tonsillectomy has been studied as a possible treatment of psoriasis but is likely helpful only in patients with exacerbations of disease that are closely associated with recurrent tonsillitis.27
Human Immunodeficiency Virus
The prevalence of psoriasis in human immunodeficiency virus (HIV) patients is similar to or greater than the general population.28 Human immunodeficiency virus infection causes new onset of psoriasis and exacerbation of existing psoriasis; severity often is correlated with worsening immune function.28,29
The clinical subtypes of psoriasis that occur most frequently with HIV include guttate, inverse, and erythrodermic, though patients may present with any subtype.28 The mechanism is puzzling because HIV is primarily mediated by helper T cell 2 (TH2) cytokines, whereas psoriasis is mainly driven by helper T cell 1 (TH1) cytokines.30 Furthermore, despite increased severity with lower CD4+ counts, treatments further lowering T-cell counts paradoxically improve symptoms.31 Current literature suggests that expansion of CD8+ memory T cells might be the primary mechanism in the exacerbation of psoriasis in HIV-mediated immunosuppression.30
Treatment of HIV-associated psoriasis presents challenges because many therapeutics cause further immunosuppression. The National Psoriasis Foundation recommends topical preparations as first-line agents for mild to moderate psoriasis.32 For moderate to severe psoriasis, retroviral agents may be effective as first-line monotherapy or when supplemented by phototherapy with UVB or psoralen plus UVA. Retinoids can be used as second-line agents.32 For cases of severe refractory psoriasis, cyclosporine, methotrexate, TNF inhibitors, or hydroxyurea can be considered. There also is evidence that apremilast is effective without risk for worsening immune function.33
Other Infections
Other bacteria associated with triggering or exacerbating psoriasis include Staphylococcus aureus and Helicobacter pylori.34,35 Fungi, such as species of the genera Malassezia and Candida, and other viruses, including papillomaviruses and retroviruses, also have been implicated.34
Medications
Numerous medications can trigger psoriasis, including lithium, nonsteroidal anti-inflammatory drugs, antimalarials, beta-blockers, and angiotensin-converting enzyme inhibitors.34 More recent literature suggests that TNF inhibitors also can paradoxically induce psoriasis in rare cases.35
Lithium
Psoriasis is the most common cutaneous adverse effect of lithium.34 It is more likely to exacerbate existing disease but also can induce onset of psoriasis; it also can cause disease that is more refractory to treatment.34,36 Current literature hypothesizes that lithium triggers psoriasis by interference of intracellular calcium channels through reduction of inositol, thereby affecting keratinocyte proliferation and differentiation.34 Lithium also inhibits glycogen synthase kinase-3 (GSK-3), a serine threonine kinase, which, in turn, induces human keratinocyte proliferation.37 However, it is unlikely lithium alone can induce psoriasis; genetic predisposition is necessary.
TNF Inhibitors
Tumor necrosis factor inhibitors such as adalimumab, etanercept, certolizumab pegol, golimumab, and infliximab are used in various inflammatory diseases, including psoriasis. Interestingly, there have been more than 200 reported cases of suspected TNF inhibitor–induced or –exacerbated psoriasis.38 This phenomenon appears to occur more frequently with infliximab and is most likely to occur in the first year of treatment of Crohn disease and rheumatoid arthritis.38 Plaque psoriasis is the most common form, but 15% to 26% of cases presented with 2 or more morphologies.38,39
Treatment options include discontinuing therapy, though many patients experience resolution while continuing treatment or switching to another TNF inhibitor.38-40 Traditional topical therapies also have been used with success.40 The pathogenesis of this phenomenon is still unclear but is thought to involve both the IL-23/helper T cell 17 (TH17) axis and dysregulation of IFN-α in the setting of TNF suppression.38
Lifestyle
Obesity is a chronic low-grade inflammatory state that can contribute to the onset of psoriasis or exacerbation of exist
The relationship between psoriasis and alcohol consumption is less clear than it is between psoriasis and obesity or smoking; greater consumption is found in psoriasis patients, but evidence is insufficient to deem alcohol a risk factor.44
Conclusion
Various factors, including genetics, infection, pharmacotherapeutic, and lifestyle, can all contribute to the induction or exacerbation of psoriasis. These factors can provide clues to the pathogenesis of psoriasis as well as help clinicians better counsel patients about their disease.
Psoriasis is a chronic autoimmune skin disease affecting approximately 6.7 million adults in the United States.1 Although its pathogenesis is not yet clear, risk factors and triggers provide insight into potential pathways by which psoriasis can occur. There is notable overlap between risk factors and triggers of psoriasis; perceived risk factors might, in fact, be triggers causing manifestation of disease in predisposed persons. In this review, we summarize the key factors contributing to onset and exacerbation of psoriasis. When learning to manage this chronic disease, it also may be helpful to educate patients about how these elements may affect the course of psoriasis.
Genetics
The pathogenesis of psoriasis has a strong genetic component, with approximately 70% and 20% concordance rates in monozygotic and dizygotic twins, respectively.2 Moreover, studies have shown a positive family history in approximately 35% of patients.3,4 Family-based studies have found a 50% risk of developing psoriasis in patients with 2 affected parents.5 However, the genetics of psoriasis are complex and are attributed to many different genes. Thus far, genes involving antigen presentation, T-cell receptor development and polarization, and the nuclear factor κβ (NF-κβ) pathway have been identified.6
HLA-Cw6
The most well-studied gene implicated in psoriasis is HLA-Cw6, which encodes a major histocompatibility complex class I allele supporting psoriasis as a T cell–mediated reaction to an autoantigen.6 Two potential antigens for HLA-Cw6 recently have been identified: LL-37, a cathelicidin-related antimicrobial peptide, and the A disintegrin and metalloproteinase with thrombospondin motifs-like protein 5 (ADAMTSL5), found on melanocytes and keratinocytes.7 The percentage of psoriasis patients with HLA-Cw6 ranges from 10.5% to 77.2%, with higher frequency in white individuals than in Asians.7
HLA-Cw6 manifests as specific features in psoriasis, including onset of disease before 21 years of age.8 It also is more strongly associated with guttate-type psoriasis, greater body surface area involvement, and higher incidence of Köbner phenomenon. Patients with positive HLA-Cw6 also reported worsening of psoriasis during and after throat infection.9
Caspase Recruitment Domain Family Member 14
Another gene mutation implicated in psoriasis pathogenesis is caspase recruitment domain family member 14, CARD14 (formerly PSORS2), a gene encoding a scaffolding protein important in the activation of NF-κβ.10,11 Missense CARD14 mutations cause upregulation of NF-κβ through formation of a complex with adapter protein B-cell lymphoma 10 (BCL10) and mucosa-associated lymphoid tissue lymphoma translocation protein 1 (MALT1),12 which, in turn, causes increased transcription of cytokines IL-8, C-C motif chemokine ligand 20 (CCL-20), and IL-36 gamma in the keratinocyte.13 Mutations in CARD14 alone lead to psoriasiform skin in mice through amplified activation of the IL-23/IL-17 axis.14,15 Patients with a mutation in a CARD14 variant (p.Arg820Trp) have demonstrated better response to tumor necrosis factor (TNF) inhibitors.16
Further characterization of the genetic pathogenesis of psoriasis might lead to better targeted therapies, including the possibility of MALT1 inhibitors as a treatment option.12
Infection
Streptococcus
The association between streptococcal infection and psoriasis was first documented more than 100 years ago, specifically the onset of acute guttate psoriasis.17,18 Although classically described following throat infection, psoriasis also occurs following streptococcal vulvovaginitis and perianal streptococcal infection.19,20
This type of psoriasis is typically self-limited but can recur with subsequent streptococcal infections or initiate a more chronic plaque psoriasis. Patients have a 1 in 3 risk of developing chronic psoriasis within 10 years of a single episode of acute guttate psoriasis.21 Moreover, in many patients with existing plaque psoriasis, throat infection exacerbates psoriatic symptoms.22 The mechanism of exacerbation is likely due to cross-reactivity between streptococcal M surface antigen and human keratinocytes and might also be influenced by inherited abnormalities in immune response.23-26 Therefore, tonsillectomy has been studied as a possible treatment of psoriasis but is likely helpful only in patients with exacerbations of disease that are closely associated with recurrent tonsillitis.27
Human Immunodeficiency Virus
The prevalence of psoriasis in human immunodeficiency virus (HIV) patients is similar to or greater than the general population.28 Human immunodeficiency virus infection causes new onset of psoriasis and exacerbation of existing psoriasis; severity often is correlated with worsening immune function.28,29
The clinical subtypes of psoriasis that occur most frequently with HIV include guttate, inverse, and erythrodermic, though patients may present with any subtype.28 The mechanism is puzzling because HIV is primarily mediated by helper T cell 2 (TH2) cytokines, whereas psoriasis is mainly driven by helper T cell 1 (TH1) cytokines.30 Furthermore, despite increased severity with lower CD4+ counts, treatments further lowering T-cell counts paradoxically improve symptoms.31 Current literature suggests that expansion of CD8+ memory T cells might be the primary mechanism in the exacerbation of psoriasis in HIV-mediated immunosuppression.30
Treatment of HIV-associated psoriasis presents challenges because many therapeutics cause further immunosuppression. The National Psoriasis Foundation recommends topical preparations as first-line agents for mild to moderate psoriasis.32 For moderate to severe psoriasis, retroviral agents may be effective as first-line monotherapy or when supplemented by phototherapy with UVB or psoralen plus UVA. Retinoids can be used as second-line agents.32 For cases of severe refractory psoriasis, cyclosporine, methotrexate, TNF inhibitors, or hydroxyurea can be considered. There also is evidence that apremilast is effective without risk for worsening immune function.33
Other Infections
Other bacteria associated with triggering or exacerbating psoriasis include Staphylococcus aureus and Helicobacter pylori.34,35 Fungi, such as species of the genera Malassezia and Candida, and other viruses, including papillomaviruses and retroviruses, also have been implicated.34
Medications
Numerous medications can trigger psoriasis, including lithium, nonsteroidal anti-inflammatory drugs, antimalarials, beta-blockers, and angiotensin-converting enzyme inhibitors.34 More recent literature suggests that TNF inhibitors also can paradoxically induce psoriasis in rare cases.35
Lithium
Psoriasis is the most common cutaneous adverse effect of lithium.34 It is more likely to exacerbate existing disease but also can induce onset of psoriasis; it also can cause disease that is more refractory to treatment.34,36 Current literature hypothesizes that lithium triggers psoriasis by interference of intracellular calcium channels through reduction of inositol, thereby affecting keratinocyte proliferation and differentiation.34 Lithium also inhibits glycogen synthase kinase-3 (GSK-3), a serine threonine kinase, which, in turn, induces human keratinocyte proliferation.37 However, it is unlikely lithium alone can induce psoriasis; genetic predisposition is necessary.
TNF Inhibitors
Tumor necrosis factor inhibitors such as adalimumab, etanercept, certolizumab pegol, golimumab, and infliximab are used in various inflammatory diseases, including psoriasis. Interestingly, there have been more than 200 reported cases of suspected TNF inhibitor–induced or –exacerbated psoriasis.38 This phenomenon appears to occur more frequently with infliximab and is most likely to occur in the first year of treatment of Crohn disease and rheumatoid arthritis.38 Plaque psoriasis is the most common form, but 15% to 26% of cases presented with 2 or more morphologies.38,39
Treatment options include discontinuing therapy, though many patients experience resolution while continuing treatment or switching to another TNF inhibitor.38-40 Traditional topical therapies also have been used with success.40 The pathogenesis of this phenomenon is still unclear but is thought to involve both the IL-23/helper T cell 17 (TH17) axis and dysregulation of IFN-α in the setting of TNF suppression.38
Lifestyle
Obesity is a chronic low-grade inflammatory state that can contribute to the onset of psoriasis or exacerbation of exist
The relationship between psoriasis and alcohol consumption is less clear than it is between psoriasis and obesity or smoking; greater consumption is found in psoriasis patients, but evidence is insufficient to deem alcohol a risk factor.44
Conclusion
Various factors, including genetics, infection, pharmacotherapeutic, and lifestyle, can all contribute to the induction or exacerbation of psoriasis. These factors can provide clues to the pathogenesis of psoriasis as well as help clinicians better counsel patients about their disease.
- Helmick CG, Lee-Han H, Hirsch SC, et al. Prevalence of psoriasis among adults in the U.S.: 2003-2006 and 2009-2010 National Health and Nutrition Examination Surveys. Am J Prev Med. 2014;47:37-45.
- Bowcock AM. The genetics of psoriasis and autoimmunity. Annu Rev Genomics Hum Genet. 2005;6:93-122.
- Swanbeck G, Inerot A, Martinsson T, et al. A population genetic study of psoriasis. Br J Dermatol. 1994;131:32-39.
- Kimberling W, Dobson RL. The inheritance of psoriasis. J Invest Dermatol. 1973;60:538-540.
- Gupta R, Debbaneh MG, Liao W. Genetic epidemiology of psoriasis. Curr Dermatol Rep. 2014;3:61-78.
- Harden JL, Krueger JG, Bowcock AM. The immunogenetics of psoriasis: a comprehensive review. J Autoimmun. 2015;64:66-73.
- Chen L, Tsai TF. HLA-Cw6 and psoriasis. Br J Dermatol. 2018;178:854-862.
- Enerbäck C, Martinsson T, Ineraot A, et al. Evidence that HLA-Cw6 determines early onset of psoriasis, obtained using sequence-specific primers (PCR-SSP). Acta Derm Venereol. 1997;77:273-276.
- Gudjónsson JE, Kárason A, Antonsdóttir EH, et al. HLA-Cw6-positive and HLA-Cw6-negative patients with psoriasis vulgaris have distinct clinical features. J Invest Dermatol. 2002;118:362-365.
- Tomfohrde J, Silverman A, Barnes R, et al. Gene for familial psoriasis susceptibility mapped to distal end of human chromosome 17q. Science. 1994;264:1141-1145.
- Blonska M, Lin X. NF-κB signaling pathways regulated by CARMA family of scaffold proteins. Cell Res. 2011;21:55-70.
- Van Nuffel E, Schmitt A, Afonina IS, et al. CARD14-mediated activation of paracaspase MALT1 in keratinocytes: implications for psoriasis. J Invest Dermatol. 2017;137:569-575.
- Jordan CT, Cao L, Roberson ED, et al. PSORS2 is due to mutations in CARD14. Am J Hum Genet. 2012;90:784-795.
- Wang M, Zhang S, Zheng G, et al. Gain-of-function mutation of Card14 leads to spontaneous psoriasis-like skin inflammation through enhanced keratinocyte response to IL-17A. Immunity. 2018;49:66-79.
- Mellet M, Meier B, Mohanan D, et al. CARD14 gain-of-function mutation alone is sufficient to drive IL-23/IL-17-mediated psoriasiform skin inflammation in vivo. J Invest Dermatol. 2018;138:2010-2023.
- Coto-Segura P, González-Fernández D, Batalla A, et al. Common and rare CARD14 gene variants affect the antitumour necrosis factor response among patients with psoriasis. Br J Dermatol. 2016;175:134-141.
- Winfield JM. Psoriasis as a sequel to acute inflammations of the tonsils: a clinical note. J Cutan Dis. 1916;34:441-443.
- Telfer NR, Chalmers RJG, Whale K, et al. The role of streptococcal infection in the initiation of guttate psoriasis. Arch Dermatol. 1992;128:39-42.
- Hernandez M, Simms-Cendan J, Zendell K. Guttate psoriasis following streptococcal vulvovaginitis in a five-year-old girl. J Pediatr Adolesc Gynecol. 2015;28:e127-e129.
- Herbst RA, Hoch O, Kapp A, et al. Guttate psoriasis triggered by perianal streptococcal dermatitis in a four-year-old boy. J Am Acad Dermatol. 2000;42(5, pt 2):885-887.
- Martin BA, Chalmers RJ, Telfer NR. How great is the risk of further psoriasis following a single episode of acute guttate psoriasis? Arch Dermatol. 1996;132:717-718.
- Thorleifsdottir RH, Eysteinsdóttir, Olafsson JH, et al. Throat infections are associated with exacerbation in a substantial proportion of patients with chronic plaque psoriasis. Acta Derm Venereol. 2016;96:788-791.
- McFadden J, Valdimarsson H, Fry L. Cross-reactivity between streptococcal M surface antigen and human skin. Br J Dermatol. 1991;125:443-447.
- Validmarsson H, Thorleifsdottir RH, Sigurdardottir SL, et al. Psoriasis—as an autoimmune disease caused by molecular mimicry. Trends Immunol. 2009;30:494-501.
- Muto M, Fujikara Y, Hamamoto Y, et al. Immune response to Streptococcus pyogenes and the susceptibility to psoriasis. Australas J Dermatol. 1996;37(suppl 1):S54-S55.
- Weisenseel P, Laumbacher B, Besgen P, et al. Streptococcal infection distinguishes different types of psoriasis. J Med Genet. 2002;39:767-768.
- Rachakonda TD, Dhillon JS, Florek AG, et al. Effect of tonsillectomy on psoriasis: a systematic review. J Am Acad Dermatol. 2015;72:261-275.
- Mallon E, Bunker CB. HIV-associated psoriasis. AIDS Patient Care STDS. 2000;14:239-246.
- Duvic M, Johnson TM, Rapini RP, et al. Acquired immunodeficiency syndrome-associated psoriasis and Reiter’s syndrome. Arch Dermatol. 1987;123:1622-1632.
- Fife DJ, Waller JM, Jeffes EW, et al. Unraveling the paradoxes of HIV-associated psoriasis: a review of T-cell subsets and cytokine profiles. Dermatol Online J. 2007;13:4.
- Ortonne JP, Lebwohl M, Em Griffiths C; Alefacept Clinical Study Group. Alefacept-induced decreases in circulating blood lymphocyte counts correlate with clinical response in patients with chronic plaque psoriasis. Eur J Dermatol. 2003;13:117-123.
- Menon K, Van Voorhees AS, Bebo BF Jr, et al; National Psoriasis Foundation. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299.
- Reddy SP, Shah VV, Wu JJ. Apremilast for a psoriasis patient with HIV and hepatitis C. J Eur Acad Dermatol Venereol. 2017;31:e481-e482.
- Fry L, Baker BS. Triggering psoriasis: the role of infections and medications. Clin Dermatol. 2007;25:606-615.
- Sfikakis PP, Iliopoulos A, Elezoglou A, et al. Psoriasis induced by anti-tumor necrosis factor therapy: a paradoxical adverse reaction. Arthritis Rheum. 2005;52:2513-2518.
- Yeung CK, Chan HH. Cutaneous adverse effects of lithium: epidemiology and management. Am J Clin Dermatol. 2004;5:3-8.
- Hampton PJ, Jans R, Flockhart RJ, et al. Lithium regulates keratinocyte proliferation via glycogen synthase kinase 3 and NFAT 2 (nuclear factor of activated T cells 2). J Cell Physiol. 2012;227:1529-1537.
- Brown G, Wang E, Leon A, et al. Tumor necrosis factor-α inhibitor-induced psoriasis: systematic review of clinical features, histopathological findings, and management experience. J Am Acad Dermatol. 2017;76:334-341.
- Collamer AN, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: clinical features and possible immunopathogenesis. Semin Arthritis Rheum. 2010;40:233-240.
- Collamer AN, Guerrero KT, Henning JS, et al. Psoriatic skin lesions induced by tumor antagonist therapy: a literature review and potential mechanisms of action. Arthritis Rheum. 2008;59:996-1001.
- Jensen P, Skov L. Psoriasis and obesity. Dermatology. 2016;232:633-639.
- Barrea L, Nappi F, Di Somma C, et al. Environmental risk factors in psoriasis: the point of view of the nutritionist. Int J Environ Res Public Health. 2016;13:743.
- Lee EJ, Han KD, Han JH, et al. Smoking and risk of psoriasis: a nationwide cohort study. J Am Acad Dermatol. 2017;77:573-575.
- Brenaut E, Horreau C, Pouplard C, et al. Alcohol consumption and psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2013;27(suppl 3):30-35.
- Helmick CG, Lee-Han H, Hirsch SC, et al. Prevalence of psoriasis among adults in the U.S.: 2003-2006 and 2009-2010 National Health and Nutrition Examination Surveys. Am J Prev Med. 2014;47:37-45.
- Bowcock AM. The genetics of psoriasis and autoimmunity. Annu Rev Genomics Hum Genet. 2005;6:93-122.
- Swanbeck G, Inerot A, Martinsson T, et al. A population genetic study of psoriasis. Br J Dermatol. 1994;131:32-39.
- Kimberling W, Dobson RL. The inheritance of psoriasis. J Invest Dermatol. 1973;60:538-540.
- Gupta R, Debbaneh MG, Liao W. Genetic epidemiology of psoriasis. Curr Dermatol Rep. 2014;3:61-78.
- Harden JL, Krueger JG, Bowcock AM. The immunogenetics of psoriasis: a comprehensive review. J Autoimmun. 2015;64:66-73.
- Chen L, Tsai TF. HLA-Cw6 and psoriasis. Br J Dermatol. 2018;178:854-862.
- Enerbäck C, Martinsson T, Ineraot A, et al. Evidence that HLA-Cw6 determines early onset of psoriasis, obtained using sequence-specific primers (PCR-SSP). Acta Derm Venereol. 1997;77:273-276.
- Gudjónsson JE, Kárason A, Antonsdóttir EH, et al. HLA-Cw6-positive and HLA-Cw6-negative patients with psoriasis vulgaris have distinct clinical features. J Invest Dermatol. 2002;118:362-365.
- Tomfohrde J, Silverman A, Barnes R, et al. Gene for familial psoriasis susceptibility mapped to distal end of human chromosome 17q. Science. 1994;264:1141-1145.
- Blonska M, Lin X. NF-κB signaling pathways regulated by CARMA family of scaffold proteins. Cell Res. 2011;21:55-70.
- Van Nuffel E, Schmitt A, Afonina IS, et al. CARD14-mediated activation of paracaspase MALT1 in keratinocytes: implications for psoriasis. J Invest Dermatol. 2017;137:569-575.
- Jordan CT, Cao L, Roberson ED, et al. PSORS2 is due to mutations in CARD14. Am J Hum Genet. 2012;90:784-795.
- Wang M, Zhang S, Zheng G, et al. Gain-of-function mutation of Card14 leads to spontaneous psoriasis-like skin inflammation through enhanced keratinocyte response to IL-17A. Immunity. 2018;49:66-79.
- Mellet M, Meier B, Mohanan D, et al. CARD14 gain-of-function mutation alone is sufficient to drive IL-23/IL-17-mediated psoriasiform skin inflammation in vivo. J Invest Dermatol. 2018;138:2010-2023.
- Coto-Segura P, González-Fernández D, Batalla A, et al. Common and rare CARD14 gene variants affect the antitumour necrosis factor response among patients with psoriasis. Br J Dermatol. 2016;175:134-141.
- Winfield JM. Psoriasis as a sequel to acute inflammations of the tonsils: a clinical note. J Cutan Dis. 1916;34:441-443.
- Telfer NR, Chalmers RJG, Whale K, et al. The role of streptococcal infection in the initiation of guttate psoriasis. Arch Dermatol. 1992;128:39-42.
- Hernandez M, Simms-Cendan J, Zendell K. Guttate psoriasis following streptococcal vulvovaginitis in a five-year-old girl. J Pediatr Adolesc Gynecol. 2015;28:e127-e129.
- Herbst RA, Hoch O, Kapp A, et al. Guttate psoriasis triggered by perianal streptococcal dermatitis in a four-year-old boy. J Am Acad Dermatol. 2000;42(5, pt 2):885-887.
- Martin BA, Chalmers RJ, Telfer NR. How great is the risk of further psoriasis following a single episode of acute guttate psoriasis? Arch Dermatol. 1996;132:717-718.
- Thorleifsdottir RH, Eysteinsdóttir, Olafsson JH, et al. Throat infections are associated with exacerbation in a substantial proportion of patients with chronic plaque psoriasis. Acta Derm Venereol. 2016;96:788-791.
- McFadden J, Valdimarsson H, Fry L. Cross-reactivity between streptococcal M surface antigen and human skin. Br J Dermatol. 1991;125:443-447.
- Validmarsson H, Thorleifsdottir RH, Sigurdardottir SL, et al. Psoriasis—as an autoimmune disease caused by molecular mimicry. Trends Immunol. 2009;30:494-501.
- Muto M, Fujikara Y, Hamamoto Y, et al. Immune response to Streptococcus pyogenes and the susceptibility to psoriasis. Australas J Dermatol. 1996;37(suppl 1):S54-S55.
- Weisenseel P, Laumbacher B, Besgen P, et al. Streptococcal infection distinguishes different types of psoriasis. J Med Genet. 2002;39:767-768.
- Rachakonda TD, Dhillon JS, Florek AG, et al. Effect of tonsillectomy on psoriasis: a systematic review. J Am Acad Dermatol. 2015;72:261-275.
- Mallon E, Bunker CB. HIV-associated psoriasis. AIDS Patient Care STDS. 2000;14:239-246.
- Duvic M, Johnson TM, Rapini RP, et al. Acquired immunodeficiency syndrome-associated psoriasis and Reiter’s syndrome. Arch Dermatol. 1987;123:1622-1632.
- Fife DJ, Waller JM, Jeffes EW, et al. Unraveling the paradoxes of HIV-associated psoriasis: a review of T-cell subsets and cytokine profiles. Dermatol Online J. 2007;13:4.
- Ortonne JP, Lebwohl M, Em Griffiths C; Alefacept Clinical Study Group. Alefacept-induced decreases in circulating blood lymphocyte counts correlate with clinical response in patients with chronic plaque psoriasis. Eur J Dermatol. 2003;13:117-123.
- Menon K, Van Voorhees AS, Bebo BF Jr, et al; National Psoriasis Foundation. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299.
- Reddy SP, Shah VV, Wu JJ. Apremilast for a psoriasis patient with HIV and hepatitis C. J Eur Acad Dermatol Venereol. 2017;31:e481-e482.
- Fry L, Baker BS. Triggering psoriasis: the role of infections and medications. Clin Dermatol. 2007;25:606-615.
- Sfikakis PP, Iliopoulos A, Elezoglou A, et al. Psoriasis induced by anti-tumor necrosis factor therapy: a paradoxical adverse reaction. Arthritis Rheum. 2005;52:2513-2518.
- Yeung CK, Chan HH. Cutaneous adverse effects of lithium: epidemiology and management. Am J Clin Dermatol. 2004;5:3-8.
- Hampton PJ, Jans R, Flockhart RJ, et al. Lithium regulates keratinocyte proliferation via glycogen synthase kinase 3 and NFAT 2 (nuclear factor of activated T cells 2). J Cell Physiol. 2012;227:1529-1537.
- Brown G, Wang E, Leon A, et al. Tumor necrosis factor-α inhibitor-induced psoriasis: systematic review of clinical features, histopathological findings, and management experience. J Am Acad Dermatol. 2017;76:334-341.
- Collamer AN, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: clinical features and possible immunopathogenesis. Semin Arthritis Rheum. 2010;40:233-240.
- Collamer AN, Guerrero KT, Henning JS, et al. Psoriatic skin lesions induced by tumor antagonist therapy: a literature review and potential mechanisms of action. Arthritis Rheum. 2008;59:996-1001.
- Jensen P, Skov L. Psoriasis and obesity. Dermatology. 2016;232:633-639.
- Barrea L, Nappi F, Di Somma C, et al. Environmental risk factors in psoriasis: the point of view of the nutritionist. Int J Environ Res Public Health. 2016;13:743.
- Lee EJ, Han KD, Han JH, et al. Smoking and risk of psoriasis: a nationwide cohort study. J Am Acad Dermatol. 2017;77:573-575.
- Brenaut E, Horreau C, Pouplard C, et al. Alcohol consumption and psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2013;27(suppl 3):30-35.
Practice Points
- HLA-Cw6 and CARD14 are genetic factors associated with psoriasis.
- Psoriasis in the setting of human immunodeficiency virus infection may be treated with topical steroids, phototherapy, systemic retinoids, or apremilast.
- Psoriasis is a potential adverse effect in patients taking lithium or tumor necrosis factor inhibitors.
- Patients should be counseled about the role of obesity and smoking on psoriasis.
Scalp Psoriasis With Increased Hair Density
Case Report
A 19-year-old man first presented to our outpatient dermatology clinic for evaluation of a rash on the elbows and knees of 2 to 3 months’ duration. The lesions were asymptomatic. A review of symptoms including joint pain was largely negative. His medical history was remarkable for terminal ileitis, Crohn disease, anal fissure, rhabdomyolysis, and viral gastroenteritis. Physical examination revealed a well-nourished man with red, scaly, indurated papules and plaques involving approximately 0.5% of the body surface area. A diagnosis of plaque psoriasis was made, and he was treated with topical corticosteroids for 2 weeks and as needed thereafter.
The patient remained stable for 5 years before presenting again to the dermatology clinic for psoriasis that had now spread to the scalp. Clinical examination revealed a very thin, faintly erythematous, scaly patch associated with increased hair density of the right frontal and parietal scalp (Figure). The patient denied any trauma or injury to the area or application of hair dye. We prescribed clobetasol solution 0.05% twice daily to the affected area of the scalp for 2 weeks, which resulted in minimal resolution of the psoriatic scalp lesion.

Comment
The scalp is a site of predilection in psoriasis, as approximately 80% of psoriasis patients report involvement of the scalp.1 Scalp involvement can dramatically affect a patient’s quality of life and often poses considerable therapeutic challenges for dermatologists.1 Alopecia in the setting of scalp psoriasis is common but is not well understood.2 First described by Shuster3 in 1972, psoriatic alopecia is associated with diminished hair density, follicular miniaturization, sebaceous gland atrophy, and an increased number of dystrophic bulbs in psoriatic plaques.4 It clinically presents as pink scaly plaques consistent with psoriasis with overlying alopecia. There are few instances of psoriatic alopecia reported as cicatricial hair loss and generalized telogen effluvium.2 It is known that a higher proportion of telogen and catagen hairs exist in patients with psoriatic alopecia.5 Additionally, psoriasis patients have more dystrophic hairs in affected and unaffected skin despite no differences in skin when compared to unaffected patients. Many patients achieve hair regrowth following treatment of psoriasis.2
We described a patient with scalp psoriasis who had increased and preserved hair density. Our case suggests that while most patients with scalp psoriasis experience psoriatic alopecia of the lesional skin, some may unconventionally experience increased hair density, which is contradictory to propositions that the friction associated with the application of topical treatments results in breakage of telogen hairs.2 Additionally, the presence of increased hair density in scalp psoriasis can further complicate antipsoriatic treatment by making the scalp inaccessible and topical therapies even more difficult to apply.
- Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280-284.
- George SM, Taylor MR, Farrant PB. Psoriatic alopecia. Clin Exp Dermatol. 2015;40:717-721.
- Shuster S. Psoriatic alopecia. Br J Dermatol. 1972;87:73-77.
- Wyatt E, Bottoms E, Comaish S. Abnormal hair shafts in psoriasis on scanning electron microscopy. Br J Dermatol. 1972;87:368-373.
- Schoorl WJ, van Baar HJ, van de Kerkhof PC. The hair root pattern in psoriasis of the scalp. Acta Derm Venereol. 1992;72:141-142.
Case Report
A 19-year-old man first presented to our outpatient dermatology clinic for evaluation of a rash on the elbows and knees of 2 to 3 months’ duration. The lesions were asymptomatic. A review of symptoms including joint pain was largely negative. His medical history was remarkable for terminal ileitis, Crohn disease, anal fissure, rhabdomyolysis, and viral gastroenteritis. Physical examination revealed a well-nourished man with red, scaly, indurated papules and plaques involving approximately 0.5% of the body surface area. A diagnosis of plaque psoriasis was made, and he was treated with topical corticosteroids for 2 weeks and as needed thereafter.
The patient remained stable for 5 years before presenting again to the dermatology clinic for psoriasis that had now spread to the scalp. Clinical examination revealed a very thin, faintly erythematous, scaly patch associated with increased hair density of the right frontal and parietal scalp (Figure). The patient denied any trauma or injury to the area or application of hair dye. We prescribed clobetasol solution 0.05% twice daily to the affected area of the scalp for 2 weeks, which resulted in minimal resolution of the psoriatic scalp lesion.

Comment
The scalp is a site of predilection in psoriasis, as approximately 80% of psoriasis patients report involvement of the scalp.1 Scalp involvement can dramatically affect a patient’s quality of life and often poses considerable therapeutic challenges for dermatologists.1 Alopecia in the setting of scalp psoriasis is common but is not well understood.2 First described by Shuster3 in 1972, psoriatic alopecia is associated with diminished hair density, follicular miniaturization, sebaceous gland atrophy, and an increased number of dystrophic bulbs in psoriatic plaques.4 It clinically presents as pink scaly plaques consistent with psoriasis with overlying alopecia. There are few instances of psoriatic alopecia reported as cicatricial hair loss and generalized telogen effluvium.2 It is known that a higher proportion of telogen and catagen hairs exist in patients with psoriatic alopecia.5 Additionally, psoriasis patients have more dystrophic hairs in affected and unaffected skin despite no differences in skin when compared to unaffected patients. Many patients achieve hair regrowth following treatment of psoriasis.2
We described a patient with scalp psoriasis who had increased and preserved hair density. Our case suggests that while most patients with scalp psoriasis experience psoriatic alopecia of the lesional skin, some may unconventionally experience increased hair density, which is contradictory to propositions that the friction associated with the application of topical treatments results in breakage of telogen hairs.2 Additionally, the presence of increased hair density in scalp psoriasis can further complicate antipsoriatic treatment by making the scalp inaccessible and topical therapies even more difficult to apply.
Case Report
A 19-year-old man first presented to our outpatient dermatology clinic for evaluation of a rash on the elbows and knees of 2 to 3 months’ duration. The lesions were asymptomatic. A review of symptoms including joint pain was largely negative. His medical history was remarkable for terminal ileitis, Crohn disease, anal fissure, rhabdomyolysis, and viral gastroenteritis. Physical examination revealed a well-nourished man with red, scaly, indurated papules and plaques involving approximately 0.5% of the body surface area. A diagnosis of plaque psoriasis was made, and he was treated with topical corticosteroids for 2 weeks and as needed thereafter.
The patient remained stable for 5 years before presenting again to the dermatology clinic for psoriasis that had now spread to the scalp. Clinical examination revealed a very thin, faintly erythematous, scaly patch associated with increased hair density of the right frontal and parietal scalp (Figure). The patient denied any trauma or injury to the area or application of hair dye. We prescribed clobetasol solution 0.05% twice daily to the affected area of the scalp for 2 weeks, which resulted in minimal resolution of the psoriatic scalp lesion.

Comment
The scalp is a site of predilection in psoriasis, as approximately 80% of psoriasis patients report involvement of the scalp.1 Scalp involvement can dramatically affect a patient’s quality of life and often poses considerable therapeutic challenges for dermatologists.1 Alopecia in the setting of scalp psoriasis is common but is not well understood.2 First described by Shuster3 in 1972, psoriatic alopecia is associated with diminished hair density, follicular miniaturization, sebaceous gland atrophy, and an increased number of dystrophic bulbs in psoriatic plaques.4 It clinically presents as pink scaly plaques consistent with psoriasis with overlying alopecia. There are few instances of psoriatic alopecia reported as cicatricial hair loss and generalized telogen effluvium.2 It is known that a higher proportion of telogen and catagen hairs exist in patients with psoriatic alopecia.5 Additionally, psoriasis patients have more dystrophic hairs in affected and unaffected skin despite no differences in skin when compared to unaffected patients. Many patients achieve hair regrowth following treatment of psoriasis.2
We described a patient with scalp psoriasis who had increased and preserved hair density. Our case suggests that while most patients with scalp psoriasis experience psoriatic alopecia of the lesional skin, some may unconventionally experience increased hair density, which is contradictory to propositions that the friction associated with the application of topical treatments results in breakage of telogen hairs.2 Additionally, the presence of increased hair density in scalp psoriasis can further complicate antipsoriatic treatment by making the scalp inaccessible and topical therapies even more difficult to apply.
- Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280-284.
- George SM, Taylor MR, Farrant PB. Psoriatic alopecia. Clin Exp Dermatol. 2015;40:717-721.
- Shuster S. Psoriatic alopecia. Br J Dermatol. 1972;87:73-77.
- Wyatt E, Bottoms E, Comaish S. Abnormal hair shafts in psoriasis on scanning electron microscopy. Br J Dermatol. 1972;87:368-373.
- Schoorl WJ, van Baar HJ, van de Kerkhof PC. The hair root pattern in psoriasis of the scalp. Acta Derm Venereol. 1992;72:141-142.
- Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280-284.
- George SM, Taylor MR, Farrant PB. Psoriatic alopecia. Clin Exp Dermatol. 2015;40:717-721.
- Shuster S. Psoriatic alopecia. Br J Dermatol. 1972;87:73-77.
- Wyatt E, Bottoms E, Comaish S. Abnormal hair shafts in psoriasis on scanning electron microscopy. Br J Dermatol. 1972;87:368-373.
- Schoorl WJ, van Baar HJ, van de Kerkhof PC. The hair root pattern in psoriasis of the scalp. Acta Derm Venereol. 1992;72:141-142.
Practice Points
- Scalp psoriasis may present with hair loss or increased hair density.
- Psoriasis with increased hair density may make topical medications more difficult to apply.
Emerging Therapies In Psoriasis: A Systematic Review
Psoriasis is a chronic, autoimmune-mediated disease estimated to affect 2.8% of the US population.1 The pathogenesis of psoriasis is thought to involve a complex process triggered by a combination of genetic and environmental factors that induce tumor necrosis factor (TNF) α secretion by keratinocytes, which in turn activates dendritic cells. Activated dendritic cells produce IL-23, leading to helper T cell (TH17) differentiation.2,3 TH17 cells secrete IL-17A, which has been shown to promote psoriatic skin changes.4 Therefore, TNF-α, IL-23, and IL-17A have been recognized as key targets for psoriasis therapy.
The newest biologic agents targeting IL-17–mediated pathways include ixekizumab, brodalumab, and bimekizumab. Secukinumab, the first US Food and Drug Administration (FDA)–approved IL-17 inhibitor, has been available since 2015 and therefore is not included in this review. IL-23 inhibitors that are FDA approved or being evaluated in clinical trials include guselkumab, tildrakizumab, and risankizumab. In addition, certolizumab pegol, a TNF-α inhibitor, is being studied for use in psoriasis.
METHODS
We reviewed the published results of phase 3 clinical trials for ixekizumab, brodalumab, bimekizumab, guselkumab, tildrakizumab, risankizumab, and certolizumab pegol. We performed an English-language literature search (January 1, 2012 to October 15, 2017) of articles indexed for PubMed/MEDLINE using the following combinations of keywords: IL-23 and psoriasis; IL-17 and psoriasis; tumor necrosis factor and psoriasis; [drug name] and psoriasis. If data from phase 3 clinical trials were not yet available, data from phase 2 clinical trials were incorporated in our analysis. We also reviewed citations within articles to identify relevant sources.
RESULTS
Phase 3 clinical trial design, efficacy, and adverse events (AEs) for ixekizumab and brodalumab are reported in eTable 15-10 and for guselkumab and tildrakizumab in eTable 2.11-14 Phase 2 clinical trial design, efficacy, and AEs are presented for risankizumab in eTable 315-18 and for certolizumab pegol in eTable 4.17,19 No published clinical trial data were found for bimekizumab.
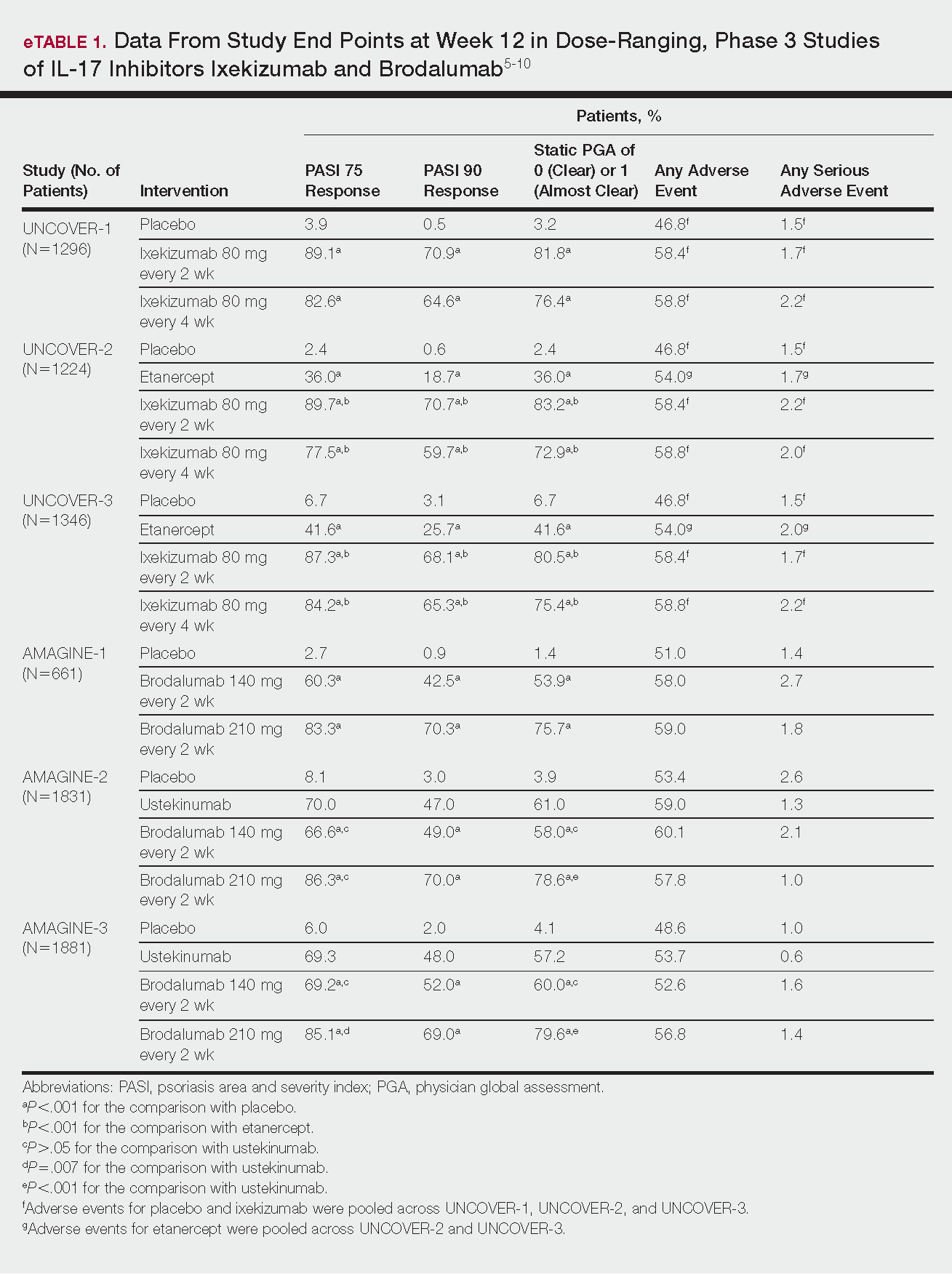
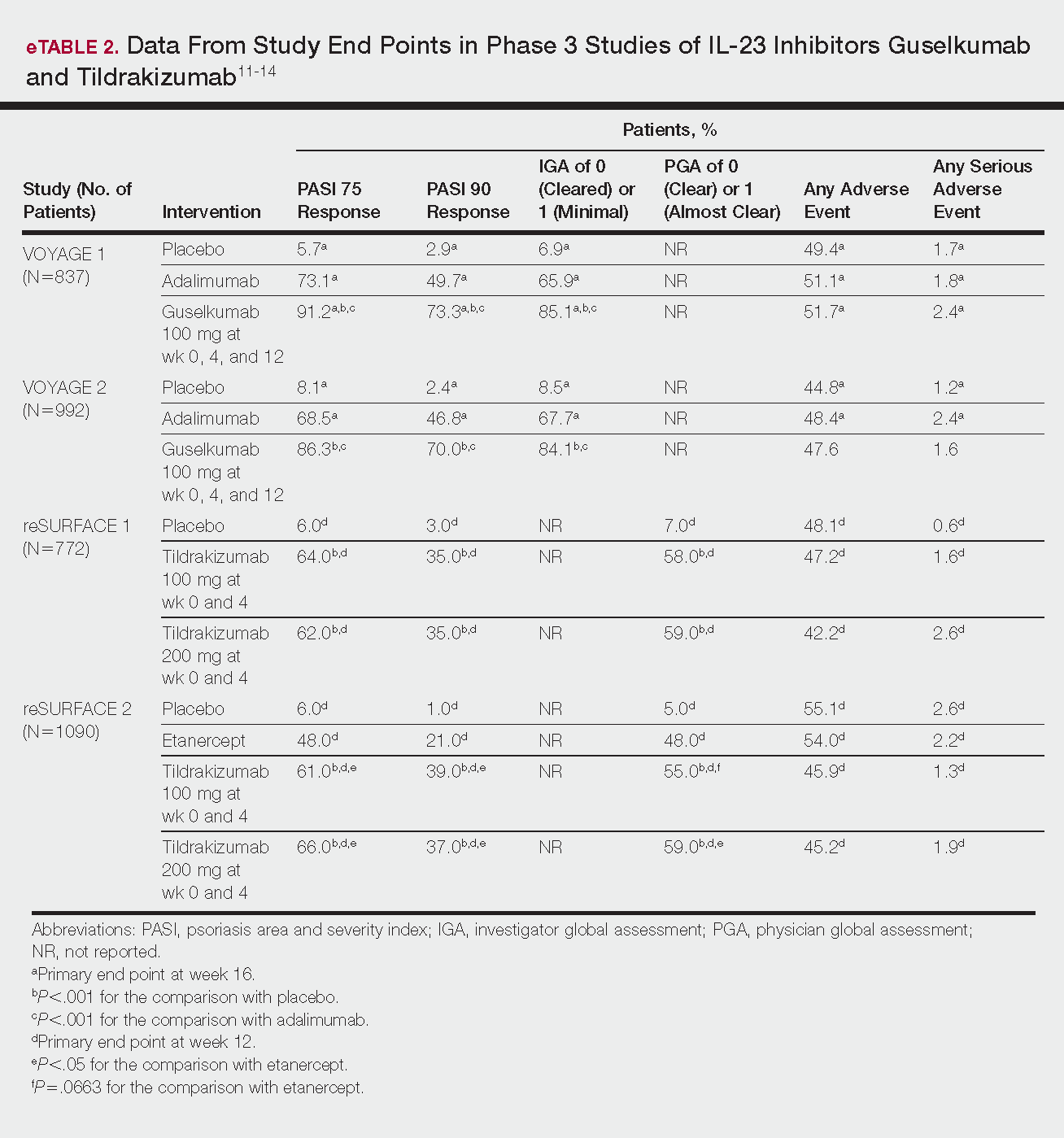
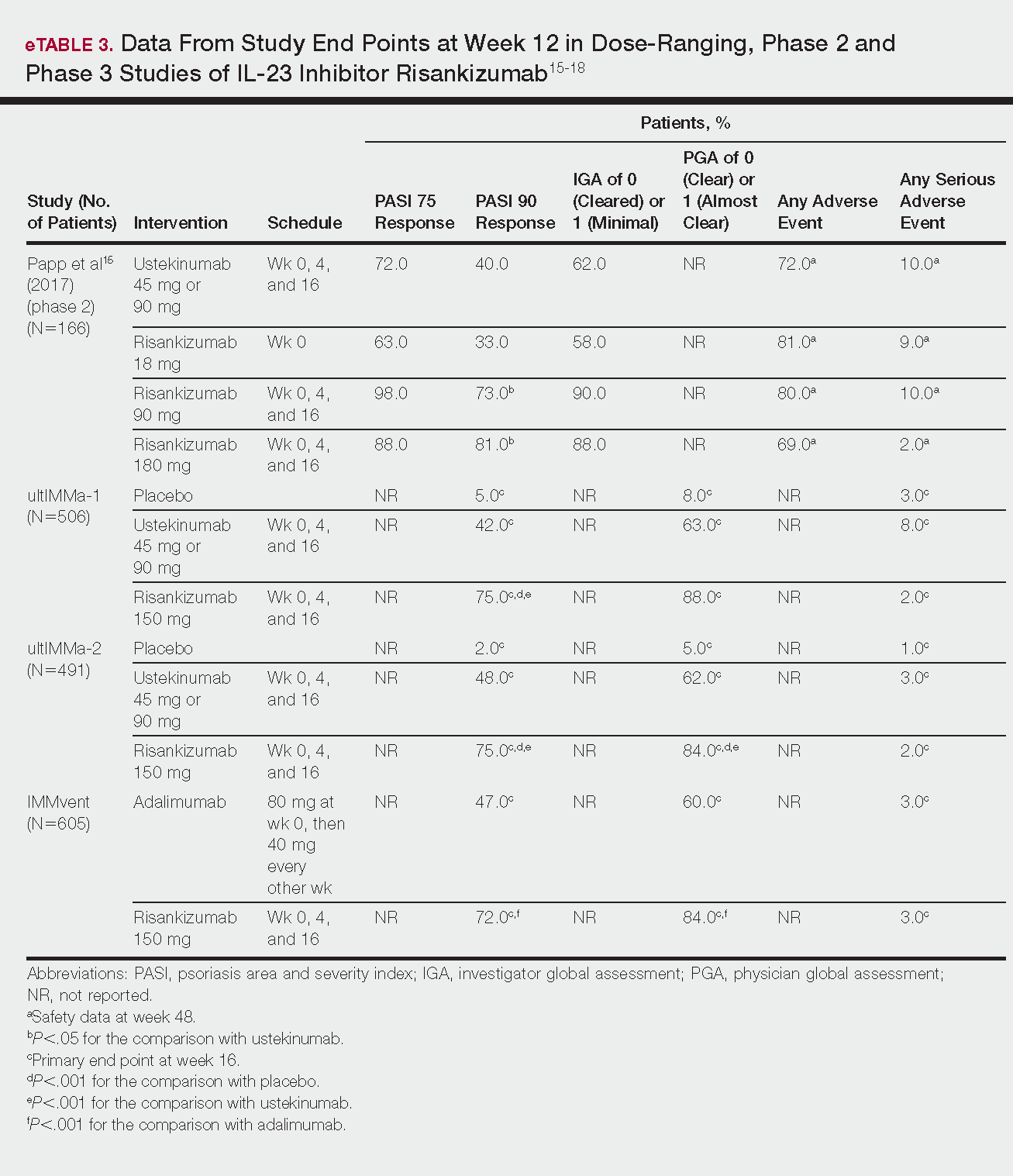
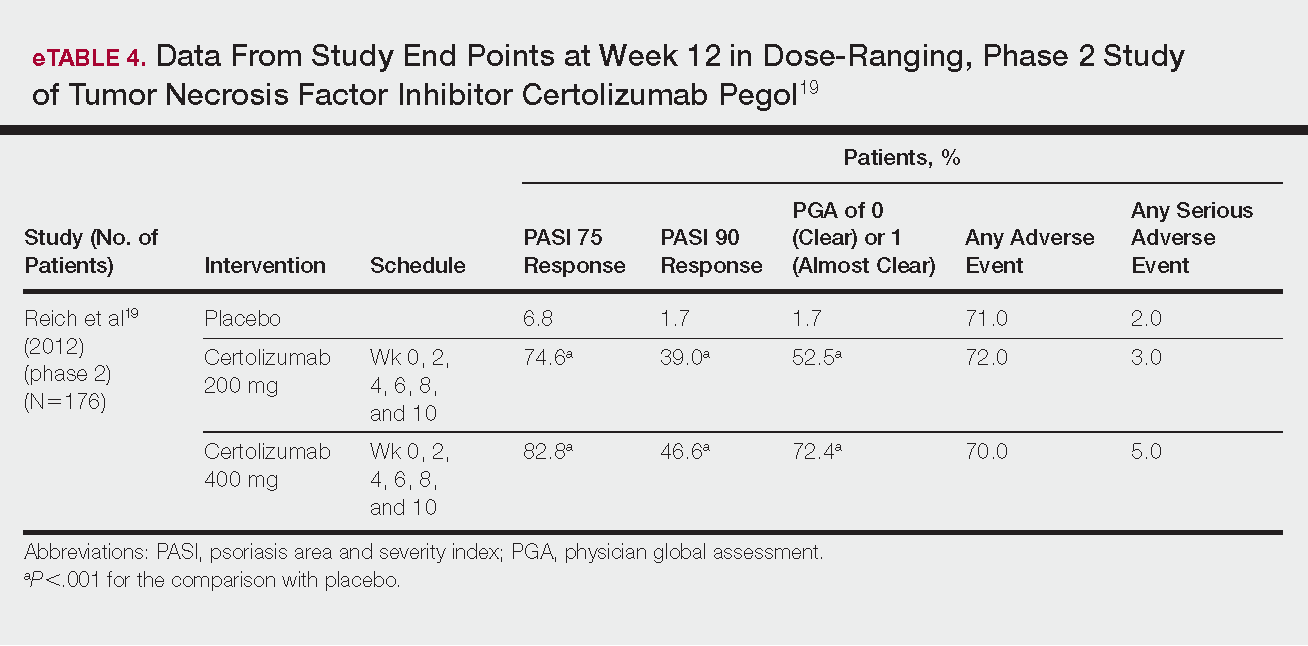
IL-17 Inhibitors
Ixekizumab
This recombinant, high-affinity IgG4κ antibody selectively binds and neutralizes IL-17A.5,6 Three phase 3 clinical trials—UNCOVER-1, UNCOVER-2, and UNCOVER-3—evaluated ixekizumab for moderate to severe plaque psoriasis.7
The 3 UNCOVER trials were randomized, double-blind, phase 3 trials of 1296, 1224, and 1346 patients, respectively, assigned to a placebo group; a group treated with ixekizumab 80 mg every 2 weeks; and a group treated with ixekizumab 80 mg every 4 weeks. Both ixekizumab groups received a loading dose of 160 mg at week 0.5,6 UNCOVER-2 and UNCOVER-3 also included a comparator group of patients on etanercept 50 mg.5 Co-primary end points included the percentage of patients reaching a psoriasis area and severity index (PASI) of 75 and with a static physician global assessment (PGA) score of clear (0) or almost clear (1) at week 12.5,6
Ixekizumab achieved greater efficacy than placebo: 89.1%, 89.7%, and 87.3% of patients achieved PASI 75 in the every 2-week dosing group, and 82.6%, 77.5% and 84.2% achieved PASI 75 in the every 4-week dosing group in UNCOVER-1, UNCOVER-2, and UNCOVER-3, respectively (P<.001 for both treatment arms compared to placebo in all trials). The percentage of patients achieving a static PGA score of 0 or 1 also was higher in the ixekizumab groups in the 2-week and 4-week dosing groups in all UNCOVER trials—81.8% and 76.4% in UNCOVER-1, 83.2% and 72.9% in UNCOVER-2, and 80.5% and 75.4% in UNCOVER-3—compared to 3.2%, 2.4%, and 6.7% in the placebo groups of the 3 trials (P<.001 for both ixekizumab groups compared to placebo in all trials).5,6 Ixekizumab also was found to be more effective than etanercept for both co-primary end points in both UNCOVER-2 and UNCOVER-3 (eTable 1).5
Safety data for all UNCOVER trials were pooled and reported.6 At week 12 the rate of at least 1 AE was 58.4% in patients on ixekizumab every 2 weeks and 58.8% in patients on ixekizumab every 4 weeks compared to 54.0% in the etanercept group in UNCOVER-2 and UNCOVER-3 and 46.8% in the placebo group. At week 12, 72 nonfatal serious AEs were reported: 12 in the placebo group, 14 in the etanercept group, 20 in the ixekizumab every 2 weeks group, and 26 in the ixekizumab every 4 weeks group.6
The most common AE across all groups was nasopharyngitis. Overall, infections were more frequent in patients treated with ixekizumab than in patients treated with placebo or etanercept. Specifically, oral candidiasis occurred more frequently in the ixekizumab groups, with a higher rate in the 2-week dosing group than in the 4-week dosing group.6 Two myocardial infarctions (MIs) occurred: 1 in the etanercept group and 1 in the placebo group.5
Brodalumab
This human monoclonal antibody binds to IL-17ra.8,9 Three double-blind, placebo-controlled, phase 3 trials—AMAGINE-1, AMAGINE-2, and AMAGINE-3—evaluated its use for plaque psoriasis.10
In AMAGINE-1 (N=661), patients were randomized to receive brodalumab 140 mg or 210 mg (every 2 weeks for 12 weeks), or placebo.8 In AMAGINE-2 (N=1831) and AMAGINE-3 (N=1881), patients were randomized to receive brodalumab 140 mg or 210 mg (every 2 weeks for 12 weeks), ustekinumab 45 mg or 90 mg by weight (at weeks 0 and 4, then every 12 weeks thereafter), or placebo. In all trials, patients on brodalumab received a dose at week 0 and week 1. Co-primary end points were PASI 75 and a static PGA score of 0 or 1 at 12 weeks compared to placebo and to ustekinumab (in AMAGINE-2 and AMAGINE-3 only).8
At week 12, 83.3%, 86.3%, and 85.1% of patients on brodalumab 210 mg, and 60.3%, 66.6%, and 69.2% of patients on brodalumab 140 mg, achieved PASI 75 in AMAGINE-1, AMAGINE-2, and AMAGINE-3, respectively, compared to 2.7%, 8.1%, and 6.0% in the placebo groups (P<.001 between both brodalumab groups and placebo in all trials).8 Both brodalumab groups were noninferior but not significantly superior to ustekinumab, which achieved a PASI 75 of 70.0% in AMAGINE-2 and 69.3% in AMAGINE-3. The PASI 90 rate was higher, however, in both brodalumab groups compared to ustekinumab but significance was not reported (eTable 1).9 For both brodalumab groups, significantly more patients achieved a static PGA value of 0 or 1 compared to placebo (P<.001 across all trials). However, only the brodalumab 210-mg group achieved a significantly higher rate of static PGA 0 or 1 compared to ustekinumab in AMAGINE-2 and AMAGINE-3 (P<.001).9
After 12 weeks, the percentage of patients reporting at least 1 AE was 59.0%, 57.8%, and 56.8% in the brodalumab 210-mg group in AMAGINE-1, AMAGINE-2, and AMAGINE-3, respectively; 58.0%, 60.1%, and 52.6% in the brodalumab 140-mg group; and 51.0%, 53.4%, and 48.6% in the placebo group. Patients taking ustekinumab had an AE rate of 59.0% in AMAGINE-2 and 53.7% in AMAGINE-3. The most common AE was nasopharyngitis, followed by upper respiratory infection (URI) and headache across all trials.8,9 Serious AEs were rare: 10 in AMAGINE-1, 31 in AMAGINE-2, and 24 in AMAGINE-3 across all groups. One death occurred from stroke in the brodalumab 210-mg group in AMAGINE-2.9
IL-23 Inhibitors
Guselkumab
This drug is a human IgG1κ antibody that binds to the p19 subunit of IL-23, thereby inhibiting IL-23 signaling.11,12 Guselkumab was approved by the FDA in July 2017 for moderate to severe plaque psoriasis.13
VOYAGE 1 and VOYAGE 2 were phase 3, double-blind, placebo- and active comparator–controlled trials of 837 and 992 patients, respectively, randomized to receive adalimumab (80 mg at week 0 and 40 mg at week 1, then at 40 mg every 2 weeks thereafter), guselkumab 100 mg at weeks 0, 4, and 12, or placebo.11 Co-primary end points for both trials were the percentage of patients reaching PASI 90 and an investigator global assessment (IGA) score of cleared (0) or minimal (1) at week 16.11
By week 16 of both trials, PASI 90 values were statistically superior for guselkumab (VOYAGE 1, 73.3%; VOYAGE 2, 70.0%) compared to adalimumab (VOYAGE 1, 49.7%; VOYAGE 2, 46.8%) and placebo (VOYAGE 1, 2.9%; VOYAGE 2, 2.4%)(P<.001). Moreover, patients on guselkumab achieved a higher rate of IGA values of 0 and 1 at week 12 (85.1% in VOYAGE 1 and 84.1% in VOYAGE 2) than patients on adalimumab (65.9% in VOYAGE 1 and 67.7% in VOYAGE 2) and placebo (6.9% in VOYAGE 1 and 8.5% in VOYAGE 2)(P<.001).11,12
The frequency of AEs was comparable across all groups in both trials.11,12 During the 16-week treatment period, 51.7% and 47.6% of the guselkumab groups in VOYAGE 1 and VOYAGE 2, respectively; 51.1% and 48.4% of the adalimumab groups; and 49.4% and 44.8% of the placebo groups reported at least 1 AE. The most common AEs in all groups were nasopharyngitis, headache, and URI.11,12
Serious AEs also occurred at similar rates: 2.4% and 1.6% in the guselkumab group in VOYAGE 1 and VOYAGE 2, respectively; 2.4% and 1.8% in the adalimumab group; and 1.7% and 1.2% in the placebo group.11,12 One case of malignancy occurred in the VOYAGE 1 trial: basal cell carcinoma in the guselkumab group.11 Three major cardiovascular events occurred across both trials: 1 MI in the guselkumab group in each trial and 1 MI in the adalimumab group in VOYAGE 1.11,12
Tildrakizumab
A high-affinity, humanized IgG1κ antibody, tildrakizumab targets the p19 subunit of IL-23. As of February 2018, 2 double-blind, randomized phase 3 trials have studied tildrakizumab with published results: reSURFACE 1 and reSURFACE 2.14
reSURFACE 1 (N=772) and reSURFACE 2 (N=1090) randomized patients to receive tildrakizumab 100 or 200 mg (at weeks 0 and 4), etanercept 50 mg (twice weekly) for 12 weeks (reSURFACE 2 only), or placebo. Co-primary end points were the percentage of patients achieving PASI 75 and the percentage of patients achieving a PGA score of 0 or 1 at week 12.14
In reSURFACE 1, significantly more patients receiving tildrakizumab attained PASI 75 at week 12 compared to placebo: 200 mg, 62.0%; 100 mg, 64.0%; and placebo, 6.0% (P<.001 for tildrakizumab groups compared to placebo). Moreover, significantly proportionally more patients received a PGA score of 0 or 1 compared to placebo: 100 mg, 59%; 200 mg, 58.0%; placebo, 7.0% (P<.001 for both tildrakizumab groups compared to placebo).14
In reSURFACE 2, significantly more patients receiving tildrakizumab achieved PASI 75 compared to etanercept and placebo at week 12: 200 mg, 66.0%; 100mg, 61.0%; etanercept, 48.0%; placebo, 6.0% (P<.001 for both tildrakizumab groups compared to placebo; P<.05 for both tildrakizumab groups compared to etanercept). Additionally, significantly more patients in the tildrakizumab groups experienced a PGA score of 0 or 1 at week 12 compared to placebo: 200 mg, 59%; 100 mg, 55.0%; placebo, 5% (P<.001 for both tildrakizumab groups compared to placebo).14
Adverse events were reported at a similar rate across all groups. For reSURFACE 1 and reSURFACE 2, at least 1 AE by week 12 was reported by 42.2% and 45.2% of patients in the 200-mg group; 47.2% and 45.9% in the 100-mg group; and 48.1% and 55.1% in the placebo groups.14The most common AEs were nasopharyngitis, URI (reSURFACE 1), and erythema at the injection site (reSURFACE 2). One case of serious infection was reported in each of the tildrakizumab groups: 1 case of drug-related hypersensitivity reaction in the 200-mg group, and 1 major cardiovascular event in the 100-mg group of reSURFACE 1. There was 1 serious AE in reSURFACE 2 that led to death in which the cause was undetermined.14
Risankizumab
This humanized IgG1 antibody binds the p19 unit of IL-23.15,16 The drug is undergoing 3 phase 3 trials—ultIMMa-1, ultIMMa-2, and IMMvent—for which only preliminary data have been published and are reported here.16,17 There is 1 phase 2 randomized, dose-ranging trial with published data.15
ultIMMa-1 and ultIMMa-2 comprised 506 and 491 patients, respectively, randomized to receive risankizumab (150 mg at weeks 0, 4, and 16), ustekinumab (45 mg or 90 mg, by weight, at weeks 0, 4, and 16), or placebo. Co-primary end points were PASI 90 and a PGA score of 0 or 1 at week 16.17
In ultIMMa-1 and ultIMMa-2, 75.0% and 75.0% of patients on risankizumab 150 mg achieved PASI 90 compared to 42.0% and 48.0% on ustekinumab and 5.0% and 2.0% on placebo at 16 weeks (P<.001 between both placebo and ustekinumab in both trials).17 In both trials, patients receiving risankizumab achieved higher rates of a static PGA score of 0 or 1 (88.0% and 84.0%) compared to ustekinumab (63.0% and 62.0%) and placebo (8.0% and 5.0%) at 16 weeks (P<.001 for both trials).18
At week 16, 2.0% of patients on risankizumab reported a serious AE in both trials, compared to 8.0% and 3.0% of patients on ustekinumab and 3.0% and 1.0% on placebo. No new safety concerns were noted.17
In the phase 3 IMMvent trial, 605 patients were randomized to receive risankizumab (150 mg at weeks 0, 4, and 16) or adalimumab (80 mg at week 0, 40 mg at week 1, then 40 mg every 2 weeks). Co-primary end points were PASI 90 and a static PGA score of 0 or 1 at week 16.17
In IMMvent, risankizumab was significantly more effective than adalimumab for PASI 75 (risankizumab, 72.0%; adalimumab, 47.0%) and a static PGA score of 0 or 1 (risankizumab 84.0%; adalimumab, 60.0%) (P<.001 risankizumab compared to adalimumab for both end points).17
At week 16, serious AEs were reported in 3.0% of patients on risankizumab and 3.0% of patients on adalimumab. One patient receiving risankizumab died of an acute MI during the treatment phase.17
TNF Inhibitor
Certolizumab Pegol
Certolizumab pegol is a human PEGylated anti-TNF agent. In vitro studies have shown that certolizumab binds to soluble and membrane-bound TNF.19 Unlike other TNF inhibitors, certolizumab pegol is a Fab‘ portion of anti-TNF conjugated to a molecule of polyethylene glycol.19 The drug is approved in the United States for treating psoriatic arthritis, Crohn disease, and rheumatoid arthritis; its potential for treating psoriasis has been confirmed. Results of 1 phase 2 trial have been published19; data from 3 phase 3 trials are forthcoming.
This randomized, placebo-controlled, double-blind phase 2 study comprised 176 patients who received certolizumab 200 mg, certolizumab 400 mg, or placebo. The dosing schedule was 400 mg at week 0, followed by either 200 or 400 mg every other week until week 10. Co-primary end points were PASI 75 and a PGA score of 0 or 1 at week 12.19
Certolizumab was significantly more effective than placebo at week 12: 74.6% of the 200-mg group and 82.8% of the 400-mg group achieved PASI 75 compared to 6.8% of the placebo group (P<.001). Certolizumab also performed better for the PGA score: 52.5% and 72.4% of patients attained a score of 0 or 1 in the 200-mg and 400-mg groups compared to 1.7% in the placebo group.19
Adverse events were reported equally across all groups: 72% of patients in the 200-mg group, 70% in the 400-mg group, and 71% in the placebo group reported at least 1 AE, most commonly nasopharyngitis, headache, and pruritis.19
COMMENT
With the development of new insights into the pathogenesis of psoriasis, therapies that are targeted toward key cytokines may contribute to improved management of the disease. The results of these clinical trials demonstrate numerous promising options for psoriatic patients.
IL-17 Inhibitors Ixekizumab and Brodalumab
When comparing these 2 biologics, it is important to consider that these studies were not performed head to head, thereby inhibiting direct comparisons. Moreover, dosage ranges of the investigative drugs were not identical, which also makes comparisons challenging. However, when looking at the highest dosages of ixekizumab and brodalumab, results indicate that ixekizumab may be slightly more effective than brodalumab based on the percentage of patients who achieved a PASI 75 and a static PGA score of 0 or 1 (eTable 1).
Phase 3 trials have shown ixekizumab to maintain efficacy over 60 weeks of treatment.6 Ixekizumab also has been shown to alleviate other symptoms of psoriasis, such as itching, pain, and nail involvement.20,21 Furthermore, ixekizumab appears to be equally effective in patients with or without prior exposure to biologics22; therefore, ixekizumab may benefit patients who have not experienced success with other biologics.
Across the UNCOVER trials, 11 cases of inflammatory bowel disease were reported in patients receiving ixekizumab (ulcerative colitis in 7; Crohn disease in 4)6; it appears that at least 3 of these cases were new diagnoses. In light of a study suggesting that IL-17A might have a protective function in the intestine,23 these findings may have important clinical implications and require follow-up studies.
Brodalumab also has been shown to maintain efficacy and acceptable safety for as long as 120 weeks.24 In the extension period of the AMAGINE-1 trial, patients who experienced a return of disease during a withdrawal period recaptured static PGA success with re-treatment for 12 weeks (re-treatment was successful in 97% of those given a dosage of 210 mg and in 84% of those given 140 mg).8
Furthermore, phase 2 trials also have shown that brodalumab is effective in patients with a history of biologic use.25 Across all AMAGINE trials, only 1 case of Crohn disease was reported in a patient taking brodalumab.9 There are concerns about depression, despite data from AMAGINE-1 stating patients on brodalumab actually had greater improvements in Hospital Anxiety and Depression Scale scores after 12 weeks of treatment (P<.001) for both brodalumab 140 mg and 210 mg compared to placebo.8 Regardless, brodalumab has a black-box warning for suicidal ideation and behavior, and availability is restricted through a Risk Evaluation and Mitigation Strategy (REMS) program.26
Bimekizumab
Although no phase 2 or phase 3 clinical trial data have been published for bimekizumab (phase 2 trials are underway), it has been shown in a phase 1 trial to be effective for psoriasis. Bimekizumab also is unique; it is the first dual inhibitor of IL-17A and IL-17F.18
IL-23 Inhibitors Guselkumab, Tildrakizumab, and Risankizumab
Making comparisons among the IL-23 inhibitors also is difficult; studies were not head-to-head comparison trials, and the VOYAGE and reSURFACE studies used different time points for primary end points. Furthermore, only phase 2 trial data are available for risankizumab. Despite these limitations, results of these trials suggest that guselkumab and risankizumab may be slightly more efficacious than tildrakizumab. However, future studies, including head-to-head studies, would ultimately provide further information on how these agents compare.
Guselkumab was shown to remain efficacious at 48 weeks, though patients on maintenance dosing had better results than those who were re-treated.12 Moreover, guselkumab was found to be effective in hard-to-treat areas, such as the scalp,11 and in patients who did not respond to adalimumab. Guselkumab may therefore benefit patients who have experienced limited clinical improvement on other biologics.12
Tildrakizumab was shown to improve PASI 75 and PGA scores through week 28 of treatment. Moreover, a higher percentage of patients taking tildrakizumab scored 0 or 1 on the dermatology life quality index, suggesting that the drug improves quality of life.14 No specific safety concerns arose in either reSURFACE trial; however, long-term studies are needed for further evaluation.
Risankizumab appears to be a promising new therapy based on phase 2 trial results. Improvements also were seen in dermatology life quality index scores, scalp and fingernail symptoms, and palmoplantar psoriasis.15 Of note, neutralizing antidrug antibodies were found in 3 patients during this study,15 which may present potential problems for long-term efficacy. However, preliminary data from 3 phase 3 trials—ultIMMa-1, ultIMMa-2, and IMMvent—are promising.17
CONCLUSION
Advances in the understanding of psoriasis have led to new targeted therapies. Ongoing clinical trials have shown encouraging results for treating physical and psychological symptoms of psoriasis. The findings of these trials support the idea that therapies targeting IL-23, specifically its p19 subunit, are effective against psoriasis while sparing IL-12. Long-term data from open-label extension studies would help guide clinical recommendations regarding the safety profiles of these agents and determine their long-term utility.
- Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(suppl 2):ii18-ii23; discussion, ii24, ii25.
- Lynde CW, Poulin Y, Vender R, et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141-150.
- Amin M, Darji K, No DJ, et al. Review of phase III trial data on IL-23 inhibitors tildrakizumab and guselkumab for psoriasis. J Eur Acad Dermatol Venereol. 2017;31:1627-1632.
- Arican O, Aral M, Sasmaz S, et al. Levels of TNF-alpha, IFN-gamma, IL6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005:273-279.
- Griffiths CE, Reich K, Lebwohl M, et al; UNCOVER-2 and UNCOVER-3 investigators. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541-551.
- Gordon KB, Blauvelt A, Papp KA, et al; UNCOVER-1 study group, UNCOVER-2 study group, UNCOVER-3 study group. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- FDA approves new psoriasis drug Taltz [news release]. Silver Spring, MD: US Food and Drug Administration; March 22, 2016. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm491872.htm. Accessed January 29, 2018.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273-286.
- Lebwohl M, Strober B, Mentor A, et al. Phase 3 studies comparing brodalumab with ustekinumab for psoriasis. N Engl J Med. 2015;373:1318-1328.
- FDA approves new psoriasis drug [news release]. Silver Spring, MD: US Food and Drug Administration; February 15, 2017. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm541981.htm. Accessed January 29, 2018.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate-to-severe plaque psoriasis: results from the phase III, double-blinded placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405-417.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Janssen announces U.S. FDA approval of Tremfya™ (guselkumab) for the treatment of moderate to severe plaque psoriasis [news release]. Horsham, PA: Johnson & Johnson; July 13, 2017. https://www.jnj.com/media-center/press-releases/janssen-announces-us-fda-approval-of-tremfya-guselkumab-for-the-treatment-of-moderate-to-severe-plaque-psoriasis. Accessed January 29, 2018.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE1 and reSURFACE 2): results from two randomized controlled, phase 3 trials. Lancet. 2017;390:276-288.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376:1551-1560.
- Risankizumab. AbbVie Inc website. https://www.abbvie.com/our-science/pipeline/risankizumab.html. Accessed January 29, 2018.
- Risankizumab meets all co-primary and ranked secondary endpoints, achieving significantly greater efficacy versus standard biologic therapies in three pivotal phase 3 psoriasis studies [news release]. North Chicago, IL: AbbVie Inc; October 26, 2017. https://news.abbvie.com/news/risankizumab-meets-all-co-primary-and-ranked-secondary-endpoints-achieving-significantly-greater-efficacy-versus-standard-biologic-therapies-in-three-pivotal-phase-3-psoriasis-studies.htm. Accessed January 29, 2018.
- Glatt S, Helmer E, Haier B, et al. First-in-human randomized study of bimekizumab, a humanized monoclonal antibody and selective dual inhibitor of IL-17A and IL-17F, in mild psoriasis. Br J Clin Pharmacol. 2017;83:991-1001.
- Reich K, Ortonne JP, Gottlieb AB, et al. Successful treatment of moderate to severe plaque psoriasis with the PEGylated Fab‘ certolizumab pegol: results of a phase II randomized, placebo-controlled trial with a re-treatment extension. Br J Dermatol. 2012;167:180-190.
- Kimball AB, Luger T, Gottlieb A, et al. Impact of ixekizumab on psoriasis itch severity and other psoriasis symptoms: results from 3 phase III psoriasis clinical trials. J Am Acad Dermatol. 2016;75:1156-1161.
- Dennehy EB, Zhang L, Amato D, et al. Ixekizumab is effective in subjects with moderate to severe plaque psoriasis with significant nail involvement: results from UNCOVER 3. J Drugs Dermatol. 2016;15:958-961.
- Gottlieb AB, Lacour JP, Korman N, et al. Treatment outcomes with ixekizumab in patients with moderate-to-severe psoriasis who have not received prior biological therapies: an integrated analysis of two phase III randomized studies. J Eur Acad Dermatol Venereol. 2017;31:679-685.
- Hueber W, Sands BE, Lewitsky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693-1700.
- Papp K, Leonardi C, Menter A, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71:1183-1190.
- Papp K, Menter A, Strober B, et al. Efficacy and safety of brodalumab in subpopulations of patients with difficult-to-treat moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2015;72:436-439.
- SILIQ [package insert]. Thousand Oaks, CA: Amgen, Inc; 2017.
Psoriasis is a chronic, autoimmune-mediated disease estimated to affect 2.8% of the US population.1 The pathogenesis of psoriasis is thought to involve a complex process triggered by a combination of genetic and environmental factors that induce tumor necrosis factor (TNF) α secretion by keratinocytes, which in turn activates dendritic cells. Activated dendritic cells produce IL-23, leading to helper T cell (TH17) differentiation.2,3 TH17 cells secrete IL-17A, which has been shown to promote psoriatic skin changes.4 Therefore, TNF-α, IL-23, and IL-17A have been recognized as key targets for psoriasis therapy.
The newest biologic agents targeting IL-17–mediated pathways include ixekizumab, brodalumab, and bimekizumab. Secukinumab, the first US Food and Drug Administration (FDA)–approved IL-17 inhibitor, has been available since 2015 and therefore is not included in this review. IL-23 inhibitors that are FDA approved or being evaluated in clinical trials include guselkumab, tildrakizumab, and risankizumab. In addition, certolizumab pegol, a TNF-α inhibitor, is being studied for use in psoriasis.
METHODS
We reviewed the published results of phase 3 clinical trials for ixekizumab, brodalumab, bimekizumab, guselkumab, tildrakizumab, risankizumab, and certolizumab pegol. We performed an English-language literature search (January 1, 2012 to October 15, 2017) of articles indexed for PubMed/MEDLINE using the following combinations of keywords: IL-23 and psoriasis; IL-17 and psoriasis; tumor necrosis factor and psoriasis; [drug name] and psoriasis. If data from phase 3 clinical trials were not yet available, data from phase 2 clinical trials were incorporated in our analysis. We also reviewed citations within articles to identify relevant sources.
RESULTS
Phase 3 clinical trial design, efficacy, and adverse events (AEs) for ixekizumab and brodalumab are reported in eTable 15-10 and for guselkumab and tildrakizumab in eTable 2.11-14 Phase 2 clinical trial design, efficacy, and AEs are presented for risankizumab in eTable 315-18 and for certolizumab pegol in eTable 4.17,19 No published clinical trial data were found for bimekizumab.




IL-17 Inhibitors
Ixekizumab
This recombinant, high-affinity IgG4κ antibody selectively binds and neutralizes IL-17A.5,6 Three phase 3 clinical trials—UNCOVER-1, UNCOVER-2, and UNCOVER-3—evaluated ixekizumab for moderate to severe plaque psoriasis.7
The 3 UNCOVER trials were randomized, double-blind, phase 3 trials of 1296, 1224, and 1346 patients, respectively, assigned to a placebo group; a group treated with ixekizumab 80 mg every 2 weeks; and a group treated with ixekizumab 80 mg every 4 weeks. Both ixekizumab groups received a loading dose of 160 mg at week 0.5,6 UNCOVER-2 and UNCOVER-3 also included a comparator group of patients on etanercept 50 mg.5 Co-primary end points included the percentage of patients reaching a psoriasis area and severity index (PASI) of 75 and with a static physician global assessment (PGA) score of clear (0) or almost clear (1) at week 12.5,6
Ixekizumab achieved greater efficacy than placebo: 89.1%, 89.7%, and 87.3% of patients achieved PASI 75 in the every 2-week dosing group, and 82.6%, 77.5% and 84.2% achieved PASI 75 in the every 4-week dosing group in UNCOVER-1, UNCOVER-2, and UNCOVER-3, respectively (P<.001 for both treatment arms compared to placebo in all trials). The percentage of patients achieving a static PGA score of 0 or 1 also was higher in the ixekizumab groups in the 2-week and 4-week dosing groups in all UNCOVER trials—81.8% and 76.4% in UNCOVER-1, 83.2% and 72.9% in UNCOVER-2, and 80.5% and 75.4% in UNCOVER-3—compared to 3.2%, 2.4%, and 6.7% in the placebo groups of the 3 trials (P<.001 for both ixekizumab groups compared to placebo in all trials).5,6 Ixekizumab also was found to be more effective than etanercept for both co-primary end points in both UNCOVER-2 and UNCOVER-3 (eTable 1).5
Safety data for all UNCOVER trials were pooled and reported.6 At week 12 the rate of at least 1 AE was 58.4% in patients on ixekizumab every 2 weeks and 58.8% in patients on ixekizumab every 4 weeks compared to 54.0% in the etanercept group in UNCOVER-2 and UNCOVER-3 and 46.8% in the placebo group. At week 12, 72 nonfatal serious AEs were reported: 12 in the placebo group, 14 in the etanercept group, 20 in the ixekizumab every 2 weeks group, and 26 in the ixekizumab every 4 weeks group.6
The most common AE across all groups was nasopharyngitis. Overall, infections were more frequent in patients treated with ixekizumab than in patients treated with placebo or etanercept. Specifically, oral candidiasis occurred more frequently in the ixekizumab groups, with a higher rate in the 2-week dosing group than in the 4-week dosing group.6 Two myocardial infarctions (MIs) occurred: 1 in the etanercept group and 1 in the placebo group.5
Brodalumab
This human monoclonal antibody binds to IL-17ra.8,9 Three double-blind, placebo-controlled, phase 3 trials—AMAGINE-1, AMAGINE-2, and AMAGINE-3—evaluated its use for plaque psoriasis.10
In AMAGINE-1 (N=661), patients were randomized to receive brodalumab 140 mg or 210 mg (every 2 weeks for 12 weeks), or placebo.8 In AMAGINE-2 (N=1831) and AMAGINE-3 (N=1881), patients were randomized to receive brodalumab 140 mg or 210 mg (every 2 weeks for 12 weeks), ustekinumab 45 mg or 90 mg by weight (at weeks 0 and 4, then every 12 weeks thereafter), or placebo. In all trials, patients on brodalumab received a dose at week 0 and week 1. Co-primary end points were PASI 75 and a static PGA score of 0 or 1 at 12 weeks compared to placebo and to ustekinumab (in AMAGINE-2 and AMAGINE-3 only).8
At week 12, 83.3%, 86.3%, and 85.1% of patients on brodalumab 210 mg, and 60.3%, 66.6%, and 69.2% of patients on brodalumab 140 mg, achieved PASI 75 in AMAGINE-1, AMAGINE-2, and AMAGINE-3, respectively, compared to 2.7%, 8.1%, and 6.0% in the placebo groups (P<.001 between both brodalumab groups and placebo in all trials).8 Both brodalumab groups were noninferior but not significantly superior to ustekinumab, which achieved a PASI 75 of 70.0% in AMAGINE-2 and 69.3% in AMAGINE-3. The PASI 90 rate was higher, however, in both brodalumab groups compared to ustekinumab but significance was not reported (eTable 1).9 For both brodalumab groups, significantly more patients achieved a static PGA value of 0 or 1 compared to placebo (P<.001 across all trials). However, only the brodalumab 210-mg group achieved a significantly higher rate of static PGA 0 or 1 compared to ustekinumab in AMAGINE-2 and AMAGINE-3 (P<.001).9
After 12 weeks, the percentage of patients reporting at least 1 AE was 59.0%, 57.8%, and 56.8% in the brodalumab 210-mg group in AMAGINE-1, AMAGINE-2, and AMAGINE-3, respectively; 58.0%, 60.1%, and 52.6% in the brodalumab 140-mg group; and 51.0%, 53.4%, and 48.6% in the placebo group. Patients taking ustekinumab had an AE rate of 59.0% in AMAGINE-2 and 53.7% in AMAGINE-3. The most common AE was nasopharyngitis, followed by upper respiratory infection (URI) and headache across all trials.8,9 Serious AEs were rare: 10 in AMAGINE-1, 31 in AMAGINE-2, and 24 in AMAGINE-3 across all groups. One death occurred from stroke in the brodalumab 210-mg group in AMAGINE-2.9
IL-23 Inhibitors
Guselkumab
This drug is a human IgG1κ antibody that binds to the p19 subunit of IL-23, thereby inhibiting IL-23 signaling.11,12 Guselkumab was approved by the FDA in July 2017 for moderate to severe plaque psoriasis.13
VOYAGE 1 and VOYAGE 2 were phase 3, double-blind, placebo- and active comparator–controlled trials of 837 and 992 patients, respectively, randomized to receive adalimumab (80 mg at week 0 and 40 mg at week 1, then at 40 mg every 2 weeks thereafter), guselkumab 100 mg at weeks 0, 4, and 12, or placebo.11 Co-primary end points for both trials were the percentage of patients reaching PASI 90 and an investigator global assessment (IGA) score of cleared (0) or minimal (1) at week 16.11
By week 16 of both trials, PASI 90 values were statistically superior for guselkumab (VOYAGE 1, 73.3%; VOYAGE 2, 70.0%) compared to adalimumab (VOYAGE 1, 49.7%; VOYAGE 2, 46.8%) and placebo (VOYAGE 1, 2.9%; VOYAGE 2, 2.4%)(P<.001). Moreover, patients on guselkumab achieved a higher rate of IGA values of 0 and 1 at week 12 (85.1% in VOYAGE 1 and 84.1% in VOYAGE 2) than patients on adalimumab (65.9% in VOYAGE 1 and 67.7% in VOYAGE 2) and placebo (6.9% in VOYAGE 1 and 8.5% in VOYAGE 2)(P<.001).11,12
The frequency of AEs was comparable across all groups in both trials.11,12 During the 16-week treatment period, 51.7% and 47.6% of the guselkumab groups in VOYAGE 1 and VOYAGE 2, respectively; 51.1% and 48.4% of the adalimumab groups; and 49.4% and 44.8% of the placebo groups reported at least 1 AE. The most common AEs in all groups were nasopharyngitis, headache, and URI.11,12
Serious AEs also occurred at similar rates: 2.4% and 1.6% in the guselkumab group in VOYAGE 1 and VOYAGE 2, respectively; 2.4% and 1.8% in the adalimumab group; and 1.7% and 1.2% in the placebo group.11,12 One case of malignancy occurred in the VOYAGE 1 trial: basal cell carcinoma in the guselkumab group.11 Three major cardiovascular events occurred across both trials: 1 MI in the guselkumab group in each trial and 1 MI in the adalimumab group in VOYAGE 1.11,12
Tildrakizumab
A high-affinity, humanized IgG1κ antibody, tildrakizumab targets the p19 subunit of IL-23. As of February 2018, 2 double-blind, randomized phase 3 trials have studied tildrakizumab with published results: reSURFACE 1 and reSURFACE 2.14
reSURFACE 1 (N=772) and reSURFACE 2 (N=1090) randomized patients to receive tildrakizumab 100 or 200 mg (at weeks 0 and 4), etanercept 50 mg (twice weekly) for 12 weeks (reSURFACE 2 only), or placebo. Co-primary end points were the percentage of patients achieving PASI 75 and the percentage of patients achieving a PGA score of 0 or 1 at week 12.14
In reSURFACE 1, significantly more patients receiving tildrakizumab attained PASI 75 at week 12 compared to placebo: 200 mg, 62.0%; 100 mg, 64.0%; and placebo, 6.0% (P<.001 for tildrakizumab groups compared to placebo). Moreover, significantly proportionally more patients received a PGA score of 0 or 1 compared to placebo: 100 mg, 59%; 200 mg, 58.0%; placebo, 7.0% (P<.001 for both tildrakizumab groups compared to placebo).14
In reSURFACE 2, significantly more patients receiving tildrakizumab achieved PASI 75 compared to etanercept and placebo at week 12: 200 mg, 66.0%; 100mg, 61.0%; etanercept, 48.0%; placebo, 6.0% (P<.001 for both tildrakizumab groups compared to placebo; P<.05 for both tildrakizumab groups compared to etanercept). Additionally, significantly more patients in the tildrakizumab groups experienced a PGA score of 0 or 1 at week 12 compared to placebo: 200 mg, 59%; 100 mg, 55.0%; placebo, 5% (P<.001 for both tildrakizumab groups compared to placebo).14
Adverse events were reported at a similar rate across all groups. For reSURFACE 1 and reSURFACE 2, at least 1 AE by week 12 was reported by 42.2% and 45.2% of patients in the 200-mg group; 47.2% and 45.9% in the 100-mg group; and 48.1% and 55.1% in the placebo groups.14The most common AEs were nasopharyngitis, URI (reSURFACE 1), and erythema at the injection site (reSURFACE 2). One case of serious infection was reported in each of the tildrakizumab groups: 1 case of drug-related hypersensitivity reaction in the 200-mg group, and 1 major cardiovascular event in the 100-mg group of reSURFACE 1. There was 1 serious AE in reSURFACE 2 that led to death in which the cause was undetermined.14
Risankizumab
This humanized IgG1 antibody binds the p19 unit of IL-23.15,16 The drug is undergoing 3 phase 3 trials—ultIMMa-1, ultIMMa-2, and IMMvent—for which only preliminary data have been published and are reported here.16,17 There is 1 phase 2 randomized, dose-ranging trial with published data.15
ultIMMa-1 and ultIMMa-2 comprised 506 and 491 patients, respectively, randomized to receive risankizumab (150 mg at weeks 0, 4, and 16), ustekinumab (45 mg or 90 mg, by weight, at weeks 0, 4, and 16), or placebo. Co-primary end points were PASI 90 and a PGA score of 0 or 1 at week 16.17
In ultIMMa-1 and ultIMMa-2, 75.0% and 75.0% of patients on risankizumab 150 mg achieved PASI 90 compared to 42.0% and 48.0% on ustekinumab and 5.0% and 2.0% on placebo at 16 weeks (P<.001 between both placebo and ustekinumab in both trials).17 In both trials, patients receiving risankizumab achieved higher rates of a static PGA score of 0 or 1 (88.0% and 84.0%) compared to ustekinumab (63.0% and 62.0%) and placebo (8.0% and 5.0%) at 16 weeks (P<.001 for both trials).18
At week 16, 2.0% of patients on risankizumab reported a serious AE in both trials, compared to 8.0% and 3.0% of patients on ustekinumab and 3.0% and 1.0% on placebo. No new safety concerns were noted.17
In the phase 3 IMMvent trial, 605 patients were randomized to receive risankizumab (150 mg at weeks 0, 4, and 16) or adalimumab (80 mg at week 0, 40 mg at week 1, then 40 mg every 2 weeks). Co-primary end points were PASI 90 and a static PGA score of 0 or 1 at week 16.17
In IMMvent, risankizumab was significantly more effective than adalimumab for PASI 75 (risankizumab, 72.0%; adalimumab, 47.0%) and a static PGA score of 0 or 1 (risankizumab 84.0%; adalimumab, 60.0%) (P<.001 risankizumab compared to adalimumab for both end points).17
At week 16, serious AEs were reported in 3.0% of patients on risankizumab and 3.0% of patients on adalimumab. One patient receiving risankizumab died of an acute MI during the treatment phase.17
TNF Inhibitor
Certolizumab Pegol
Certolizumab pegol is a human PEGylated anti-TNF agent. In vitro studies have shown that certolizumab binds to soluble and membrane-bound TNF.19 Unlike other TNF inhibitors, certolizumab pegol is a Fab‘ portion of anti-TNF conjugated to a molecule of polyethylene glycol.19 The drug is approved in the United States for treating psoriatic arthritis, Crohn disease, and rheumatoid arthritis; its potential for treating psoriasis has been confirmed. Results of 1 phase 2 trial have been published19; data from 3 phase 3 trials are forthcoming.
This randomized, placebo-controlled, double-blind phase 2 study comprised 176 patients who received certolizumab 200 mg, certolizumab 400 mg, or placebo. The dosing schedule was 400 mg at week 0, followed by either 200 or 400 mg every other week until week 10. Co-primary end points were PASI 75 and a PGA score of 0 or 1 at week 12.19
Certolizumab was significantly more effective than placebo at week 12: 74.6% of the 200-mg group and 82.8% of the 400-mg group achieved PASI 75 compared to 6.8% of the placebo group (P<.001). Certolizumab also performed better for the PGA score: 52.5% and 72.4% of patients attained a score of 0 or 1 in the 200-mg and 400-mg groups compared to 1.7% in the placebo group.19
Adverse events were reported equally across all groups: 72% of patients in the 200-mg group, 70% in the 400-mg group, and 71% in the placebo group reported at least 1 AE, most commonly nasopharyngitis, headache, and pruritis.19
COMMENT
With the development of new insights into the pathogenesis of psoriasis, therapies that are targeted toward key cytokines may contribute to improved management of the disease. The results of these clinical trials demonstrate numerous promising options for psoriatic patients.
IL-17 Inhibitors Ixekizumab and Brodalumab
When comparing these 2 biologics, it is important to consider that these studies were not performed head to head, thereby inhibiting direct comparisons. Moreover, dosage ranges of the investigative drugs were not identical, which also makes comparisons challenging. However, when looking at the highest dosages of ixekizumab and brodalumab, results indicate that ixekizumab may be slightly more effective than brodalumab based on the percentage of patients who achieved a PASI 75 and a static PGA score of 0 or 1 (eTable 1).
Phase 3 trials have shown ixekizumab to maintain efficacy over 60 weeks of treatment.6 Ixekizumab also has been shown to alleviate other symptoms of psoriasis, such as itching, pain, and nail involvement.20,21 Furthermore, ixekizumab appears to be equally effective in patients with or without prior exposure to biologics22; therefore, ixekizumab may benefit patients who have not experienced success with other biologics.
Across the UNCOVER trials, 11 cases of inflammatory bowel disease were reported in patients receiving ixekizumab (ulcerative colitis in 7; Crohn disease in 4)6; it appears that at least 3 of these cases were new diagnoses. In light of a study suggesting that IL-17A might have a protective function in the intestine,23 these findings may have important clinical implications and require follow-up studies.
Brodalumab also has been shown to maintain efficacy and acceptable safety for as long as 120 weeks.24 In the extension period of the AMAGINE-1 trial, patients who experienced a return of disease during a withdrawal period recaptured static PGA success with re-treatment for 12 weeks (re-treatment was successful in 97% of those given a dosage of 210 mg and in 84% of those given 140 mg).8
Furthermore, phase 2 trials also have shown that brodalumab is effective in patients with a history of biologic use.25 Across all AMAGINE trials, only 1 case of Crohn disease was reported in a patient taking brodalumab.9 There are concerns about depression, despite data from AMAGINE-1 stating patients on brodalumab actually had greater improvements in Hospital Anxiety and Depression Scale scores after 12 weeks of treatment (P<.001) for both brodalumab 140 mg and 210 mg compared to placebo.8 Regardless, brodalumab has a black-box warning for suicidal ideation and behavior, and availability is restricted through a Risk Evaluation and Mitigation Strategy (REMS) program.26
Bimekizumab
Although no phase 2 or phase 3 clinical trial data have been published for bimekizumab (phase 2 trials are underway), it has been shown in a phase 1 trial to be effective for psoriasis. Bimekizumab also is unique; it is the first dual inhibitor of IL-17A and IL-17F.18
IL-23 Inhibitors Guselkumab, Tildrakizumab, and Risankizumab
Making comparisons among the IL-23 inhibitors also is difficult; studies were not head-to-head comparison trials, and the VOYAGE and reSURFACE studies used different time points for primary end points. Furthermore, only phase 2 trial data are available for risankizumab. Despite these limitations, results of these trials suggest that guselkumab and risankizumab may be slightly more efficacious than tildrakizumab. However, future studies, including head-to-head studies, would ultimately provide further information on how these agents compare.
Guselkumab was shown to remain efficacious at 48 weeks, though patients on maintenance dosing had better results than those who were re-treated.12 Moreover, guselkumab was found to be effective in hard-to-treat areas, such as the scalp,11 and in patients who did not respond to adalimumab. Guselkumab may therefore benefit patients who have experienced limited clinical improvement on other biologics.12
Tildrakizumab was shown to improve PASI 75 and PGA scores through week 28 of treatment. Moreover, a higher percentage of patients taking tildrakizumab scored 0 or 1 on the dermatology life quality index, suggesting that the drug improves quality of life.14 No specific safety concerns arose in either reSURFACE trial; however, long-term studies are needed for further evaluation.
Risankizumab appears to be a promising new therapy based on phase 2 trial results. Improvements also were seen in dermatology life quality index scores, scalp and fingernail symptoms, and palmoplantar psoriasis.15 Of note, neutralizing antidrug antibodies were found in 3 patients during this study,15 which may present potential problems for long-term efficacy. However, preliminary data from 3 phase 3 trials—ultIMMa-1, ultIMMa-2, and IMMvent—are promising.17
CONCLUSION
Advances in the understanding of psoriasis have led to new targeted therapies. Ongoing clinical trials have shown encouraging results for treating physical and psychological symptoms of psoriasis. The findings of these trials support the idea that therapies targeting IL-23, specifically its p19 subunit, are effective against psoriasis while sparing IL-12. Long-term data from open-label extension studies would help guide clinical recommendations regarding the safety profiles of these agents and determine their long-term utility.
Psoriasis is a chronic, autoimmune-mediated disease estimated to affect 2.8% of the US population.1 The pathogenesis of psoriasis is thought to involve a complex process triggered by a combination of genetic and environmental factors that induce tumor necrosis factor (TNF) α secretion by keratinocytes, which in turn activates dendritic cells. Activated dendritic cells produce IL-23, leading to helper T cell (TH17) differentiation.2,3 TH17 cells secrete IL-17A, which has been shown to promote psoriatic skin changes.4 Therefore, TNF-α, IL-23, and IL-17A have been recognized as key targets for psoriasis therapy.
The newest biologic agents targeting IL-17–mediated pathways include ixekizumab, brodalumab, and bimekizumab. Secukinumab, the first US Food and Drug Administration (FDA)–approved IL-17 inhibitor, has been available since 2015 and therefore is not included in this review. IL-23 inhibitors that are FDA approved or being evaluated in clinical trials include guselkumab, tildrakizumab, and risankizumab. In addition, certolizumab pegol, a TNF-α inhibitor, is being studied for use in psoriasis.
METHODS
We reviewed the published results of phase 3 clinical trials for ixekizumab, brodalumab, bimekizumab, guselkumab, tildrakizumab, risankizumab, and certolizumab pegol. We performed an English-language literature search (January 1, 2012 to October 15, 2017) of articles indexed for PubMed/MEDLINE using the following combinations of keywords: IL-23 and psoriasis; IL-17 and psoriasis; tumor necrosis factor and psoriasis; [drug name] and psoriasis. If data from phase 3 clinical trials were not yet available, data from phase 2 clinical trials were incorporated in our analysis. We also reviewed citations within articles to identify relevant sources.
RESULTS
Phase 3 clinical trial design, efficacy, and adverse events (AEs) for ixekizumab and brodalumab are reported in eTable 15-10 and for guselkumab and tildrakizumab in eTable 2.11-14 Phase 2 clinical trial design, efficacy, and AEs are presented for risankizumab in eTable 315-18 and for certolizumab pegol in eTable 4.17,19 No published clinical trial data were found for bimekizumab.




IL-17 Inhibitors
Ixekizumab
This recombinant, high-affinity IgG4κ antibody selectively binds and neutralizes IL-17A.5,6 Three phase 3 clinical trials—UNCOVER-1, UNCOVER-2, and UNCOVER-3—evaluated ixekizumab for moderate to severe plaque psoriasis.7
The 3 UNCOVER trials were randomized, double-blind, phase 3 trials of 1296, 1224, and 1346 patients, respectively, assigned to a placebo group; a group treated with ixekizumab 80 mg every 2 weeks; and a group treated with ixekizumab 80 mg every 4 weeks. Both ixekizumab groups received a loading dose of 160 mg at week 0.5,6 UNCOVER-2 and UNCOVER-3 also included a comparator group of patients on etanercept 50 mg.5 Co-primary end points included the percentage of patients reaching a psoriasis area and severity index (PASI) of 75 and with a static physician global assessment (PGA) score of clear (0) or almost clear (1) at week 12.5,6
Ixekizumab achieved greater efficacy than placebo: 89.1%, 89.7%, and 87.3% of patients achieved PASI 75 in the every 2-week dosing group, and 82.6%, 77.5% and 84.2% achieved PASI 75 in the every 4-week dosing group in UNCOVER-1, UNCOVER-2, and UNCOVER-3, respectively (P<.001 for both treatment arms compared to placebo in all trials). The percentage of patients achieving a static PGA score of 0 or 1 also was higher in the ixekizumab groups in the 2-week and 4-week dosing groups in all UNCOVER trials—81.8% and 76.4% in UNCOVER-1, 83.2% and 72.9% in UNCOVER-2, and 80.5% and 75.4% in UNCOVER-3—compared to 3.2%, 2.4%, and 6.7% in the placebo groups of the 3 trials (P<.001 for both ixekizumab groups compared to placebo in all trials).5,6 Ixekizumab also was found to be more effective than etanercept for both co-primary end points in both UNCOVER-2 and UNCOVER-3 (eTable 1).5
Safety data for all UNCOVER trials were pooled and reported.6 At week 12 the rate of at least 1 AE was 58.4% in patients on ixekizumab every 2 weeks and 58.8% in patients on ixekizumab every 4 weeks compared to 54.0% in the etanercept group in UNCOVER-2 and UNCOVER-3 and 46.8% in the placebo group. At week 12, 72 nonfatal serious AEs were reported: 12 in the placebo group, 14 in the etanercept group, 20 in the ixekizumab every 2 weeks group, and 26 in the ixekizumab every 4 weeks group.6
The most common AE across all groups was nasopharyngitis. Overall, infections were more frequent in patients treated with ixekizumab than in patients treated with placebo or etanercept. Specifically, oral candidiasis occurred more frequently in the ixekizumab groups, with a higher rate in the 2-week dosing group than in the 4-week dosing group.6 Two myocardial infarctions (MIs) occurred: 1 in the etanercept group and 1 in the placebo group.5
Brodalumab
This human monoclonal antibody binds to IL-17ra.8,9 Three double-blind, placebo-controlled, phase 3 trials—AMAGINE-1, AMAGINE-2, and AMAGINE-3—evaluated its use for plaque psoriasis.10
In AMAGINE-1 (N=661), patients were randomized to receive brodalumab 140 mg or 210 mg (every 2 weeks for 12 weeks), or placebo.8 In AMAGINE-2 (N=1831) and AMAGINE-3 (N=1881), patients were randomized to receive brodalumab 140 mg or 210 mg (every 2 weeks for 12 weeks), ustekinumab 45 mg or 90 mg by weight (at weeks 0 and 4, then every 12 weeks thereafter), or placebo. In all trials, patients on brodalumab received a dose at week 0 and week 1. Co-primary end points were PASI 75 and a static PGA score of 0 or 1 at 12 weeks compared to placebo and to ustekinumab (in AMAGINE-2 and AMAGINE-3 only).8
At week 12, 83.3%, 86.3%, and 85.1% of patients on brodalumab 210 mg, and 60.3%, 66.6%, and 69.2% of patients on brodalumab 140 mg, achieved PASI 75 in AMAGINE-1, AMAGINE-2, and AMAGINE-3, respectively, compared to 2.7%, 8.1%, and 6.0% in the placebo groups (P<.001 between both brodalumab groups and placebo in all trials).8 Both brodalumab groups were noninferior but not significantly superior to ustekinumab, which achieved a PASI 75 of 70.0% in AMAGINE-2 and 69.3% in AMAGINE-3. The PASI 90 rate was higher, however, in both brodalumab groups compared to ustekinumab but significance was not reported (eTable 1).9 For both brodalumab groups, significantly more patients achieved a static PGA value of 0 or 1 compared to placebo (P<.001 across all trials). However, only the brodalumab 210-mg group achieved a significantly higher rate of static PGA 0 or 1 compared to ustekinumab in AMAGINE-2 and AMAGINE-3 (P<.001).9
After 12 weeks, the percentage of patients reporting at least 1 AE was 59.0%, 57.8%, and 56.8% in the brodalumab 210-mg group in AMAGINE-1, AMAGINE-2, and AMAGINE-3, respectively; 58.0%, 60.1%, and 52.6% in the brodalumab 140-mg group; and 51.0%, 53.4%, and 48.6% in the placebo group. Patients taking ustekinumab had an AE rate of 59.0% in AMAGINE-2 and 53.7% in AMAGINE-3. The most common AE was nasopharyngitis, followed by upper respiratory infection (URI) and headache across all trials.8,9 Serious AEs were rare: 10 in AMAGINE-1, 31 in AMAGINE-2, and 24 in AMAGINE-3 across all groups. One death occurred from stroke in the brodalumab 210-mg group in AMAGINE-2.9
IL-23 Inhibitors
Guselkumab
This drug is a human IgG1κ antibody that binds to the p19 subunit of IL-23, thereby inhibiting IL-23 signaling.11,12 Guselkumab was approved by the FDA in July 2017 for moderate to severe plaque psoriasis.13
VOYAGE 1 and VOYAGE 2 were phase 3, double-blind, placebo- and active comparator–controlled trials of 837 and 992 patients, respectively, randomized to receive adalimumab (80 mg at week 0 and 40 mg at week 1, then at 40 mg every 2 weeks thereafter), guselkumab 100 mg at weeks 0, 4, and 12, or placebo.11 Co-primary end points for both trials were the percentage of patients reaching PASI 90 and an investigator global assessment (IGA) score of cleared (0) or minimal (1) at week 16.11
By week 16 of both trials, PASI 90 values were statistically superior for guselkumab (VOYAGE 1, 73.3%; VOYAGE 2, 70.0%) compared to adalimumab (VOYAGE 1, 49.7%; VOYAGE 2, 46.8%) and placebo (VOYAGE 1, 2.9%; VOYAGE 2, 2.4%)(P<.001). Moreover, patients on guselkumab achieved a higher rate of IGA values of 0 and 1 at week 12 (85.1% in VOYAGE 1 and 84.1% in VOYAGE 2) than patients on adalimumab (65.9% in VOYAGE 1 and 67.7% in VOYAGE 2) and placebo (6.9% in VOYAGE 1 and 8.5% in VOYAGE 2)(P<.001).11,12
The frequency of AEs was comparable across all groups in both trials.11,12 During the 16-week treatment period, 51.7% and 47.6% of the guselkumab groups in VOYAGE 1 and VOYAGE 2, respectively; 51.1% and 48.4% of the adalimumab groups; and 49.4% and 44.8% of the placebo groups reported at least 1 AE. The most common AEs in all groups were nasopharyngitis, headache, and URI.11,12
Serious AEs also occurred at similar rates: 2.4% and 1.6% in the guselkumab group in VOYAGE 1 and VOYAGE 2, respectively; 2.4% and 1.8% in the adalimumab group; and 1.7% and 1.2% in the placebo group.11,12 One case of malignancy occurred in the VOYAGE 1 trial: basal cell carcinoma in the guselkumab group.11 Three major cardiovascular events occurred across both trials: 1 MI in the guselkumab group in each trial and 1 MI in the adalimumab group in VOYAGE 1.11,12
Tildrakizumab
A high-affinity, humanized IgG1κ antibody, tildrakizumab targets the p19 subunit of IL-23. As of February 2018, 2 double-blind, randomized phase 3 trials have studied tildrakizumab with published results: reSURFACE 1 and reSURFACE 2.14
reSURFACE 1 (N=772) and reSURFACE 2 (N=1090) randomized patients to receive tildrakizumab 100 or 200 mg (at weeks 0 and 4), etanercept 50 mg (twice weekly) for 12 weeks (reSURFACE 2 only), or placebo. Co-primary end points were the percentage of patients achieving PASI 75 and the percentage of patients achieving a PGA score of 0 or 1 at week 12.14
In reSURFACE 1, significantly more patients receiving tildrakizumab attained PASI 75 at week 12 compared to placebo: 200 mg, 62.0%; 100 mg, 64.0%; and placebo, 6.0% (P<.001 for tildrakizumab groups compared to placebo). Moreover, significantly proportionally more patients received a PGA score of 0 or 1 compared to placebo: 100 mg, 59%; 200 mg, 58.0%; placebo, 7.0% (P<.001 for both tildrakizumab groups compared to placebo).14
In reSURFACE 2, significantly more patients receiving tildrakizumab achieved PASI 75 compared to etanercept and placebo at week 12: 200 mg, 66.0%; 100mg, 61.0%; etanercept, 48.0%; placebo, 6.0% (P<.001 for both tildrakizumab groups compared to placebo; P<.05 for both tildrakizumab groups compared to etanercept). Additionally, significantly more patients in the tildrakizumab groups experienced a PGA score of 0 or 1 at week 12 compared to placebo: 200 mg, 59%; 100 mg, 55.0%; placebo, 5% (P<.001 for both tildrakizumab groups compared to placebo).14
Adverse events were reported at a similar rate across all groups. For reSURFACE 1 and reSURFACE 2, at least 1 AE by week 12 was reported by 42.2% and 45.2% of patients in the 200-mg group; 47.2% and 45.9% in the 100-mg group; and 48.1% and 55.1% in the placebo groups.14The most common AEs were nasopharyngitis, URI (reSURFACE 1), and erythema at the injection site (reSURFACE 2). One case of serious infection was reported in each of the tildrakizumab groups: 1 case of drug-related hypersensitivity reaction in the 200-mg group, and 1 major cardiovascular event in the 100-mg group of reSURFACE 1. There was 1 serious AE in reSURFACE 2 that led to death in which the cause was undetermined.14
Risankizumab
This humanized IgG1 antibody binds the p19 unit of IL-23.15,16 The drug is undergoing 3 phase 3 trials—ultIMMa-1, ultIMMa-2, and IMMvent—for which only preliminary data have been published and are reported here.16,17 There is 1 phase 2 randomized, dose-ranging trial with published data.15
ultIMMa-1 and ultIMMa-2 comprised 506 and 491 patients, respectively, randomized to receive risankizumab (150 mg at weeks 0, 4, and 16), ustekinumab (45 mg or 90 mg, by weight, at weeks 0, 4, and 16), or placebo. Co-primary end points were PASI 90 and a PGA score of 0 or 1 at week 16.17
In ultIMMa-1 and ultIMMa-2, 75.0% and 75.0% of patients on risankizumab 150 mg achieved PASI 90 compared to 42.0% and 48.0% on ustekinumab and 5.0% and 2.0% on placebo at 16 weeks (P<.001 between both placebo and ustekinumab in both trials).17 In both trials, patients receiving risankizumab achieved higher rates of a static PGA score of 0 or 1 (88.0% and 84.0%) compared to ustekinumab (63.0% and 62.0%) and placebo (8.0% and 5.0%) at 16 weeks (P<.001 for both trials).18
At week 16, 2.0% of patients on risankizumab reported a serious AE in both trials, compared to 8.0% and 3.0% of patients on ustekinumab and 3.0% and 1.0% on placebo. No new safety concerns were noted.17
In the phase 3 IMMvent trial, 605 patients were randomized to receive risankizumab (150 mg at weeks 0, 4, and 16) or adalimumab (80 mg at week 0, 40 mg at week 1, then 40 mg every 2 weeks). Co-primary end points were PASI 90 and a static PGA score of 0 or 1 at week 16.17
In IMMvent, risankizumab was significantly more effective than adalimumab for PASI 75 (risankizumab, 72.0%; adalimumab, 47.0%) and a static PGA score of 0 or 1 (risankizumab 84.0%; adalimumab, 60.0%) (P<.001 risankizumab compared to adalimumab for both end points).17
At week 16, serious AEs were reported in 3.0% of patients on risankizumab and 3.0% of patients on adalimumab. One patient receiving risankizumab died of an acute MI during the treatment phase.17
TNF Inhibitor
Certolizumab Pegol
Certolizumab pegol is a human PEGylated anti-TNF agent. In vitro studies have shown that certolizumab binds to soluble and membrane-bound TNF.19 Unlike other TNF inhibitors, certolizumab pegol is a Fab‘ portion of anti-TNF conjugated to a molecule of polyethylene glycol.19 The drug is approved in the United States for treating psoriatic arthritis, Crohn disease, and rheumatoid arthritis; its potential for treating psoriasis has been confirmed. Results of 1 phase 2 trial have been published19; data from 3 phase 3 trials are forthcoming.
This randomized, placebo-controlled, double-blind phase 2 study comprised 176 patients who received certolizumab 200 mg, certolizumab 400 mg, or placebo. The dosing schedule was 400 mg at week 0, followed by either 200 or 400 mg every other week until week 10. Co-primary end points were PASI 75 and a PGA score of 0 or 1 at week 12.19
Certolizumab was significantly more effective than placebo at week 12: 74.6% of the 200-mg group and 82.8% of the 400-mg group achieved PASI 75 compared to 6.8% of the placebo group (P<.001). Certolizumab also performed better for the PGA score: 52.5% and 72.4% of patients attained a score of 0 or 1 in the 200-mg and 400-mg groups compared to 1.7% in the placebo group.19
Adverse events were reported equally across all groups: 72% of patients in the 200-mg group, 70% in the 400-mg group, and 71% in the placebo group reported at least 1 AE, most commonly nasopharyngitis, headache, and pruritis.19
COMMENT
With the development of new insights into the pathogenesis of psoriasis, therapies that are targeted toward key cytokines may contribute to improved management of the disease. The results of these clinical trials demonstrate numerous promising options for psoriatic patients.
IL-17 Inhibitors Ixekizumab and Brodalumab
When comparing these 2 biologics, it is important to consider that these studies were not performed head to head, thereby inhibiting direct comparisons. Moreover, dosage ranges of the investigative drugs were not identical, which also makes comparisons challenging. However, when looking at the highest dosages of ixekizumab and brodalumab, results indicate that ixekizumab may be slightly more effective than brodalumab based on the percentage of patients who achieved a PASI 75 and a static PGA score of 0 or 1 (eTable 1).
Phase 3 trials have shown ixekizumab to maintain efficacy over 60 weeks of treatment.6 Ixekizumab also has been shown to alleviate other symptoms of psoriasis, such as itching, pain, and nail involvement.20,21 Furthermore, ixekizumab appears to be equally effective in patients with or without prior exposure to biologics22; therefore, ixekizumab may benefit patients who have not experienced success with other biologics.
Across the UNCOVER trials, 11 cases of inflammatory bowel disease were reported in patients receiving ixekizumab (ulcerative colitis in 7; Crohn disease in 4)6; it appears that at least 3 of these cases were new diagnoses. In light of a study suggesting that IL-17A might have a protective function in the intestine,23 these findings may have important clinical implications and require follow-up studies.
Brodalumab also has been shown to maintain efficacy and acceptable safety for as long as 120 weeks.24 In the extension period of the AMAGINE-1 trial, patients who experienced a return of disease during a withdrawal period recaptured static PGA success with re-treatment for 12 weeks (re-treatment was successful in 97% of those given a dosage of 210 mg and in 84% of those given 140 mg).8
Furthermore, phase 2 trials also have shown that brodalumab is effective in patients with a history of biologic use.25 Across all AMAGINE trials, only 1 case of Crohn disease was reported in a patient taking brodalumab.9 There are concerns about depression, despite data from AMAGINE-1 stating patients on brodalumab actually had greater improvements in Hospital Anxiety and Depression Scale scores after 12 weeks of treatment (P<.001) for both brodalumab 140 mg and 210 mg compared to placebo.8 Regardless, brodalumab has a black-box warning for suicidal ideation and behavior, and availability is restricted through a Risk Evaluation and Mitigation Strategy (REMS) program.26
Bimekizumab
Although no phase 2 or phase 3 clinical trial data have been published for bimekizumab (phase 2 trials are underway), it has been shown in a phase 1 trial to be effective for psoriasis. Bimekizumab also is unique; it is the first dual inhibitor of IL-17A and IL-17F.18
IL-23 Inhibitors Guselkumab, Tildrakizumab, and Risankizumab
Making comparisons among the IL-23 inhibitors also is difficult; studies were not head-to-head comparison trials, and the VOYAGE and reSURFACE studies used different time points for primary end points. Furthermore, only phase 2 trial data are available for risankizumab. Despite these limitations, results of these trials suggest that guselkumab and risankizumab may be slightly more efficacious than tildrakizumab. However, future studies, including head-to-head studies, would ultimately provide further information on how these agents compare.
Guselkumab was shown to remain efficacious at 48 weeks, though patients on maintenance dosing had better results than those who were re-treated.12 Moreover, guselkumab was found to be effective in hard-to-treat areas, such as the scalp,11 and in patients who did not respond to adalimumab. Guselkumab may therefore benefit patients who have experienced limited clinical improvement on other biologics.12
Tildrakizumab was shown to improve PASI 75 and PGA scores through week 28 of treatment. Moreover, a higher percentage of patients taking tildrakizumab scored 0 or 1 on the dermatology life quality index, suggesting that the drug improves quality of life.14 No specific safety concerns arose in either reSURFACE trial; however, long-term studies are needed for further evaluation.
Risankizumab appears to be a promising new therapy based on phase 2 trial results. Improvements also were seen in dermatology life quality index scores, scalp and fingernail symptoms, and palmoplantar psoriasis.15 Of note, neutralizing antidrug antibodies were found in 3 patients during this study,15 which may present potential problems for long-term efficacy. However, preliminary data from 3 phase 3 trials—ultIMMa-1, ultIMMa-2, and IMMvent—are promising.17
CONCLUSION
Advances in the understanding of psoriasis have led to new targeted therapies. Ongoing clinical trials have shown encouraging results for treating physical and psychological symptoms of psoriasis. The findings of these trials support the idea that therapies targeting IL-23, specifically its p19 subunit, are effective against psoriasis while sparing IL-12. Long-term data from open-label extension studies would help guide clinical recommendations regarding the safety profiles of these agents and determine their long-term utility.
- Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(suppl 2):ii18-ii23; discussion, ii24, ii25.
- Lynde CW, Poulin Y, Vender R, et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141-150.
- Amin M, Darji K, No DJ, et al. Review of phase III trial data on IL-23 inhibitors tildrakizumab and guselkumab for psoriasis. J Eur Acad Dermatol Venereol. 2017;31:1627-1632.
- Arican O, Aral M, Sasmaz S, et al. Levels of TNF-alpha, IFN-gamma, IL6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005:273-279.
- Griffiths CE, Reich K, Lebwohl M, et al; UNCOVER-2 and UNCOVER-3 investigators. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541-551.
- Gordon KB, Blauvelt A, Papp KA, et al; UNCOVER-1 study group, UNCOVER-2 study group, UNCOVER-3 study group. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- FDA approves new psoriasis drug Taltz [news release]. Silver Spring, MD: US Food and Drug Administration; March 22, 2016. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm491872.htm. Accessed January 29, 2018.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273-286.
- Lebwohl M, Strober B, Mentor A, et al. Phase 3 studies comparing brodalumab with ustekinumab for psoriasis. N Engl J Med. 2015;373:1318-1328.
- FDA approves new psoriasis drug [news release]. Silver Spring, MD: US Food and Drug Administration; February 15, 2017. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm541981.htm. Accessed January 29, 2018.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate-to-severe plaque psoriasis: results from the phase III, double-blinded placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405-417.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Janssen announces U.S. FDA approval of Tremfya™ (guselkumab) for the treatment of moderate to severe plaque psoriasis [news release]. Horsham, PA: Johnson & Johnson; July 13, 2017. https://www.jnj.com/media-center/press-releases/janssen-announces-us-fda-approval-of-tremfya-guselkumab-for-the-treatment-of-moderate-to-severe-plaque-psoriasis. Accessed January 29, 2018.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE1 and reSURFACE 2): results from two randomized controlled, phase 3 trials. Lancet. 2017;390:276-288.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376:1551-1560.
- Risankizumab. AbbVie Inc website. https://www.abbvie.com/our-science/pipeline/risankizumab.html. Accessed January 29, 2018.
- Risankizumab meets all co-primary and ranked secondary endpoints, achieving significantly greater efficacy versus standard biologic therapies in three pivotal phase 3 psoriasis studies [news release]. North Chicago, IL: AbbVie Inc; October 26, 2017. https://news.abbvie.com/news/risankizumab-meets-all-co-primary-and-ranked-secondary-endpoints-achieving-significantly-greater-efficacy-versus-standard-biologic-therapies-in-three-pivotal-phase-3-psoriasis-studies.htm. Accessed January 29, 2018.
- Glatt S, Helmer E, Haier B, et al. First-in-human randomized study of bimekizumab, a humanized monoclonal antibody and selective dual inhibitor of IL-17A and IL-17F, in mild psoriasis. Br J Clin Pharmacol. 2017;83:991-1001.
- Reich K, Ortonne JP, Gottlieb AB, et al. Successful treatment of moderate to severe plaque psoriasis with the PEGylated Fab‘ certolizumab pegol: results of a phase II randomized, placebo-controlled trial with a re-treatment extension. Br J Dermatol. 2012;167:180-190.
- Kimball AB, Luger T, Gottlieb A, et al. Impact of ixekizumab on psoriasis itch severity and other psoriasis symptoms: results from 3 phase III psoriasis clinical trials. J Am Acad Dermatol. 2016;75:1156-1161.
- Dennehy EB, Zhang L, Amato D, et al. Ixekizumab is effective in subjects with moderate to severe plaque psoriasis with significant nail involvement: results from UNCOVER 3. J Drugs Dermatol. 2016;15:958-961.
- Gottlieb AB, Lacour JP, Korman N, et al. Treatment outcomes with ixekizumab in patients with moderate-to-severe psoriasis who have not received prior biological therapies: an integrated analysis of two phase III randomized studies. J Eur Acad Dermatol Venereol. 2017;31:679-685.
- Hueber W, Sands BE, Lewitsky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693-1700.
- Papp K, Leonardi C, Menter A, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71:1183-1190.
- Papp K, Menter A, Strober B, et al. Efficacy and safety of brodalumab in subpopulations of patients with difficult-to-treat moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2015;72:436-439.
- SILIQ [package insert]. Thousand Oaks, CA: Amgen, Inc; 2017.
- Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(suppl 2):ii18-ii23; discussion, ii24, ii25.
- Lynde CW, Poulin Y, Vender R, et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141-150.
- Amin M, Darji K, No DJ, et al. Review of phase III trial data on IL-23 inhibitors tildrakizumab and guselkumab for psoriasis. J Eur Acad Dermatol Venereol. 2017;31:1627-1632.
- Arican O, Aral M, Sasmaz S, et al. Levels of TNF-alpha, IFN-gamma, IL6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005:273-279.
- Griffiths CE, Reich K, Lebwohl M, et al; UNCOVER-2 and UNCOVER-3 investigators. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541-551.
- Gordon KB, Blauvelt A, Papp KA, et al; UNCOVER-1 study group, UNCOVER-2 study group, UNCOVER-3 study group. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- FDA approves new psoriasis drug Taltz [news release]. Silver Spring, MD: US Food and Drug Administration; March 22, 2016. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm491872.htm. Accessed January 29, 2018.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273-286.
- Lebwohl M, Strober B, Mentor A, et al. Phase 3 studies comparing brodalumab with ustekinumab for psoriasis. N Engl J Med. 2015;373:1318-1328.
- FDA approves new psoriasis drug [news release]. Silver Spring, MD: US Food and Drug Administration; February 15, 2017. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm541981.htm. Accessed January 29, 2018.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate-to-severe plaque psoriasis: results from the phase III, double-blinded placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405-417.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Janssen announces U.S. FDA approval of Tremfya™ (guselkumab) for the treatment of moderate to severe plaque psoriasis [news release]. Horsham, PA: Johnson & Johnson; July 13, 2017. https://www.jnj.com/media-center/press-releases/janssen-announces-us-fda-approval-of-tremfya-guselkumab-for-the-treatment-of-moderate-to-severe-plaque-psoriasis. Accessed January 29, 2018.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE1 and reSURFACE 2): results from two randomized controlled, phase 3 trials. Lancet. 2017;390:276-288.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376:1551-1560.
- Risankizumab. AbbVie Inc website. https://www.abbvie.com/our-science/pipeline/risankizumab.html. Accessed January 29, 2018.
- Risankizumab meets all co-primary and ranked secondary endpoints, achieving significantly greater efficacy versus standard biologic therapies in three pivotal phase 3 psoriasis studies [news release]. North Chicago, IL: AbbVie Inc; October 26, 2017. https://news.abbvie.com/news/risankizumab-meets-all-co-primary-and-ranked-secondary-endpoints-achieving-significantly-greater-efficacy-versus-standard-biologic-therapies-in-three-pivotal-phase-3-psoriasis-studies.htm. Accessed January 29, 2018.
- Glatt S, Helmer E, Haier B, et al. First-in-human randomized study of bimekizumab, a humanized monoclonal antibody and selective dual inhibitor of IL-17A and IL-17F, in mild psoriasis. Br J Clin Pharmacol. 2017;83:991-1001.
- Reich K, Ortonne JP, Gottlieb AB, et al. Successful treatment of moderate to severe plaque psoriasis with the PEGylated Fab‘ certolizumab pegol: results of a phase II randomized, placebo-controlled trial with a re-treatment extension. Br J Dermatol. 2012;167:180-190.
- Kimball AB, Luger T, Gottlieb A, et al. Impact of ixekizumab on psoriasis itch severity and other psoriasis symptoms: results from 3 phase III psoriasis clinical trials. J Am Acad Dermatol. 2016;75:1156-1161.
- Dennehy EB, Zhang L, Amato D, et al. Ixekizumab is effective in subjects with moderate to severe plaque psoriasis with significant nail involvement: results from UNCOVER 3. J Drugs Dermatol. 2016;15:958-961.
- Gottlieb AB, Lacour JP, Korman N, et al. Treatment outcomes with ixekizumab in patients with moderate-to-severe psoriasis who have not received prior biological therapies: an integrated analysis of two phase III randomized studies. J Eur Acad Dermatol Venereol. 2017;31:679-685.
- Hueber W, Sands BE, Lewitsky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693-1700.
- Papp K, Leonardi C, Menter A, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71:1183-1190.
- Papp K, Menter A, Strober B, et al. Efficacy and safety of brodalumab in subpopulations of patients with difficult-to-treat moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2015;72:436-439.
- SILIQ [package insert]. Thousand Oaks, CA: Amgen, Inc; 2017.
Practice Points
- Tumor necrosis factor α, IL-23, and IL-17A are key targets for psoriasis therapy based on an understanding of the key role that these cytokines play in the pathophysiology of disease.
- The biologic agents secukinumab and ixekizumab are approved for use in the management of psoriasis. Other biologics—brodalumab, bimekizumab, guselkumab, tildrakizumab, risankizumab, and certolizumab pegol—have been (and some continue to be) the focus of phase 2 and phase 3 clinical trials.
- Findings of several of those trials support the idea that therapies targeting IL-23, specifically its p19 subunit, but that spare IL-12 are effective against psoriasis.
- Longer-term studies are needed to determine whether the agents reviewed here, including those approved for clinical use, are suitable for prolonged administration.
Do Psoriasis Patients Engage In Vigorous Physical Activity?
Psoriasis is a chronic inflammatory disease that affects approximately 2% to 3% of the US population.1 Patients with psoriasis are more likely to have cardiovascular risk factors (eg, obesity, metabolic syndrome) than individuals without psoriasis.2 In fact, recent evidence has suggested that a diagnosis of psoriasis is an independent risk factor for cardiometabolic diseases including diabetes, major adverse cardiovascular events, and obesity.3 Given the well-recognized health benefits of physical activity and the associated reduction in coronary heart disease risk,4 patients with psoriasis specifically may benefit from regular participation in physical activity. Thus, an enhanced understanding of the relationship between psoriasis and vigorous physical activity would help determine the role of initiating and recommending interventions that implement physical activity for patients with psoriasis. A review was conducted to determine the relationship between psoriasis and vigorous physical activity.
Methods
An English-language literature search of PubMed articles indexed for MEDLINE (January 1, 1946–October 15, 2017) as well as articles in the Embase database (January 1, 1947–October 15, 2017) and Cochrane Library (January 1, 1992–October 15, 2017) using the terms psoriasis and physical activity was performed. The search strategy was established based on a prior review of vigorous physical activity in eczema.5 The article titles and/or abstracts were reviewed, and the studies were excluded if they did not evaluate physical activity in patients with psoriasis. Studies without a control group also were excluded. Articles on patients with psoriatic arthritis and studies that involved modification of dietary intake also were excluded.
Two reviewers (M.A. and E.B.L.) independently extracted data from the studies and compiled the results. The following factors were included in the data extracted: study year, location, and design; method of diagnosis of psoriasis; total number of patients included in the study; and age, gender, and level of physical activity of the study patients. Level of physical activity was the exposure, and diagnosis of psoriasis was the dependent variable. Physical activity was defined differently across the studies that were evaluated. To determine study quality, we implemented the Newcastle–Ottawa Scale (NOS), a 9-star scoring system that includes items such as selection criteria, comparability, and study outcome.6 Studies with an NOS score of 7 or higher were included in the meta-analysis.
Results
The literature search generated 353 nonduplicate articles. A thorough review of the articles yielded 4 studies that were incorporated in the final analysis.7-10 We aimed to perform a meta-analysis; however, only 1 of the studies included in the final analysis had an NOS score of 7 or higher along with adequate data to be incorporated into our study.10 As a result, the meta-analysis was converted to a regular review.
The cross-sectional study we reviewed, which had an NOS score of 7, included males and females in the United States aged 20 to 59 years.10 Data were collected using the population-based National Health and Nutrition Examination Survey from 2003 to 2006. The survey measured the likelihood of participation in leisure-time moderate to vigorous physical activity (MVPA) and metabolic equivalent task (MET) minutes of MVPA in the past 30 days. Of 6549 participants, 385 were excluded from the analysis due to missing values for 1 or more of the study variables. Of the remaining 6164 participants, 84 (1.4%) reported having a diagnosis of psoriasis with few or no psoriasis patches at the time of the survey, and 71 (1.2%) reported having a diagnosis of psoriasis with few to extensive patches at the time of the survey.10
Participants with psoriasis were less likely to participate in MVPA in the previous 30 days compared to participants without psoriasis, but the association was not statistically significant.10 The study demonstrated that, on average, participants with psoriasis spent 31% (95% confidence interval [CI], −0.57 to −0.05) fewer MET minutes on leisure-time MVPA versus participants without psoriasis; however, this association was not statistically significant. It is important to note that the diagnosis of psoriasis was self-reported, and measures of disease duration or areas of involvement were not incorporated.
Comment
Our review revealed that vigorous physical activity may be reduced in patients with psoriasis compared to those without psoriasis. Initially, we aimed to perform a systematic review of the literature; however, only 1 study met the criteria for the systematic review, highlighting the need for more robust studies evaluating this subject.
Do et al10 demonstrated that psoriasis patients were less likely to participate in MVPA, but the findings were not statistically significant. Of those who participated in MVPA, MET minutes were fewer among patients with few to extensive skin lesions compared to those without psoriasis. The investigators suggested that psoriasis patients with more severe disease tend to exercise less and ultimately would benefit from regular vigorous physical activity.
Frankel et al7 performed a prospective cohort study in US women to evaluate the role of physical activity in preventing psoriasis. The investigators reported that the most physically active quintile had a lower multivariate relative risk of psoriasis (0.72; 95% CI, 0.59–0.89; P<.001 for trend) compared to the least active quintile.7 Additionally, vigorous physical activity, which was defined as 6 or more MET minutes, was associated with a significantly lower risk of incident psoriasis (0.66; 95% CI, 0.54–0.81; P<.001 for trend), which maintained significance after adjusting for body mass index (BMI). The investigators suggested that, by decreasing chronic inflammation and lowering levels of proinflammatory cytokines, vigorous physical activity may reduce the risk of psoriasis development in women.7 It is plausible that vigorous physical activity modifies the state of chronic inflammation, which could subsequently reduce the risk of developing psoriasis; however, further long-term, randomized, prospective studies are needed to verify the relationship between physical activity and development of psoriasis.
Torres et al8 performed a cross-sectional questionnaire study to assess physical activity in patients with severe psoriasis (defined as >10% body surface area involvement and/or disease requiring systemic therapy or phototherapy) versus healthy controls. Physical activity level was measured using the International Physical Activity Questionnaire. The odds ratio of low-level physical activity compared to non–low-level physical activity among psoriasis patients versus controls was 3.42 (95% CI, 1.47–7.91; P=.002). Additionally, the average total MET minutes of psoriasis patients were significantly reduced compared to those of the healthy controls (P=.001). Thus, the investigators suggested that vigorous physical activity is less likely in psoriasis patients, which may contribute to the increased risk of cardiovascular disease in this population.8 Vigorous physical activity would benefit patients with psoriasis to help lower the chronic state of inflammation and cardiometabolic comorbidities.
Demirel et al9 performed a study to compare aerobic exercise capacity and daily physical activity level in psoriasis patients (n=30) compared to controls (n=30). Daily physical activity, measured with an accelerometer, was significantly higher in male patients with psoriasis compared to controls (P=.021). No significant difference was reported in maximal aerobic capacity in both male and female psoriasis patients versus controls. The investigators suggested that the level of daily physical activity is not limited in psoriasis patients, yet the small sample size may limit the generalizability of the study.
The ability to dissipate heat during exercise seems to be diminished in patients with psoriasis. Specifically, it has been suggested that psoriasis lesions interfere with normal perspiration.11 Moreover, joint involvement in patients with psoriatic arthritis may lead to physical functional disabilities that can interfere with the ability of these patients to participate in regular physical activity.12-14 For this reason, our review excluded articles that evaluated patients with psoriatic arthritis. Despite this exclusion, it is important to consider that comorbid psoriatic arthritis in clinical practice may impede patients with psoriasis from participating in physical activity. Additionally, various social aspects also may limit physical activity in psoriasis patients; for instance, psoriasis patients often avoid activities that involve increased exposure of the skin (eg, communal showers, wearing sports attire).15
Furthermore, obese psoriasis patients are less likely to exercise compared to obese individuals without psoriasis.16 In patients with higher BMI, the risk of psoriasis is increased.17 A systematic review suggested that weight loss may improve psoriasis severity.18 Bariatric surgery also may improve psoriasis.19 Moreover, obesity may interfere with response to biologic therapies for psoriasis. Specifically, higher BMI is linked with lower response to fixed-dose biologic therapies compared to weight-based biologic options (eg, infliximab).20,21
Conclusion
Given the increased risk of myocardial infarction in patients with psoriasis, it is important to recognize the barriers to physical activity that psoriasis patients face.22 Due to the considerable health benefits associated with regular physical activity, physicians should encourage patients with psoriasis to participate in physical activity as tolerated. Of note, the studies included in this review varied in their definitions of psoriasis disease severity and measures of physical activity level. Long-term, randomized, prospective studies are needed to clarify the relationship between psoriasis and physical activity. Evidence from these studies would help guide clinical recommendations regarding the role of physical activity for patients with psoriasis.
- Takeshita J, Gelfand JM, Li P, et al. Psoriasis in the US Medicare population: prevalence, treatment, and factors associated with biologic use. J Invest Dermatol. 2015;135:2955-2963.
- Prey S, Paul C, Bronsard V, et al. Cardiovascular risk factors in patients with plaque psoriasis: a systematic review of epidemiological studies. J Eur Acad Dermatol Venereol. 2010;24(suppl 2):23-30.
- Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76:377-390.
- Leon AS. Biological mechanisms for the cardioprotective effects of aerobic exercise. Am J Lifestyle Med. 2009;3:32S-34S.
- Kim A, Silverberg JI. A systematic review of vigorous physical activity in eczema. Br J Dermatol. 2016;174:660-662.
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. The Ottawa Hospital Research Institute website. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed February 23, 2018.
- Frankel HC, Han J, Li T, et al. The association between physical activity and the risk of incident psoriasis. Arch Dermatol. 2012;148:918-924.
- Torres T, Alexandre JM, Mendonça D, et al. Levels of physical activity in patients with severe psoriasis: a cross-sectional questionnaire study. Am J Clin Dermatol. 2014;15:129-135.
- Demirel R, Genc A, Ucok K, et al. Do patients with mild to moderate psoriasis really have a sedentary lifestyle? Int J Dermatol. 2013;52:1129-1134.
- Do YK, Lakhani N, Malhotra R, et al. Association between psoriasis and leisure‐time physical activity: findings from the National Health and Nutrition Examination Survey. J Dermatol. 2015;42:148-153.
- Leibowitz E, Seidman DS, Laor A, et al. Are psoriatic patients at risk of heat intolerance? Br J Dermatol. 1991;124:439-442.
- Husted JA, Tom BD, Farewell VT, et al. Description and prediction of physical functional disability in psoriatic arthritis: a longitudinal analysis using a Markov model approach. Arthritis Rheum. 2005;53:404-409.
- Wilson FC, Icen M, Crowson CS, et al. Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: a population‐based study. Arthritis Rheum. 2009;61:233-239.
- Shih M, Hootman JM, Kruger J, et al. Physical activity in men and women with arthritis: National Health Interview Survey, 2002. Am J Prev Med. 2006;30:385-393.
- Ramsay B, O’Reagan M. A survey of the social and psychological effects of psoriasis. Br J Dermatol. 1988;118:195-201.
- Herron MD, Hinckley M, Hoffman MS, et al. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol. 2005;141:1527-1534.
- Kumar S, Han J, Li T, et al. Obesity, waist circumference, weight change and the risk of psoriasis in US women. J Eur Acad Dermatol Venereol. 2013;27:1293-1298.
- Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes (Lond). 2015;39:1197-1202.
- Sako EY, Famenini S, Wu JJ. Bariatric surgery and psoriasis. J Am Acad Dermatol. 2014;70:774-779.
- Clark L, Lebwohl M. The effect of weight on the efficacy of biologic therapy in patients with psoriasis. J Am Acad Dermatol. 2008;58:443-446.
- Puig L. Obesity and psoriasis: body weight and body mass index influence the response to biological treatment. J Eur Acad Dermatol Venereol. 2011;25:1007-1011.
- Wu JJ, Choi YM, Bebchuk JD. Risk of myocardial infarction in psoriasis patients: a retrospective cohort study. J Dermatolog Treat. 2015;26:230-234.
Psoriasis is a chronic inflammatory disease that affects approximately 2% to 3% of the US population.1 Patients with psoriasis are more likely to have cardiovascular risk factors (eg, obesity, metabolic syndrome) than individuals without psoriasis.2 In fact, recent evidence has suggested that a diagnosis of psoriasis is an independent risk factor for cardiometabolic diseases including diabetes, major adverse cardiovascular events, and obesity.3 Given the well-recognized health benefits of physical activity and the associated reduction in coronary heart disease risk,4 patients with psoriasis specifically may benefit from regular participation in physical activity. Thus, an enhanced understanding of the relationship between psoriasis and vigorous physical activity would help determine the role of initiating and recommending interventions that implement physical activity for patients with psoriasis. A review was conducted to determine the relationship between psoriasis and vigorous physical activity.
Methods
An English-language literature search of PubMed articles indexed for MEDLINE (January 1, 1946–October 15, 2017) as well as articles in the Embase database (January 1, 1947–October 15, 2017) and Cochrane Library (January 1, 1992–October 15, 2017) using the terms psoriasis and physical activity was performed. The search strategy was established based on a prior review of vigorous physical activity in eczema.5 The article titles and/or abstracts were reviewed, and the studies were excluded if they did not evaluate physical activity in patients with psoriasis. Studies without a control group also were excluded. Articles on patients with psoriatic arthritis and studies that involved modification of dietary intake also were excluded.
Two reviewers (M.A. and E.B.L.) independently extracted data from the studies and compiled the results. The following factors were included in the data extracted: study year, location, and design; method of diagnosis of psoriasis; total number of patients included in the study; and age, gender, and level of physical activity of the study patients. Level of physical activity was the exposure, and diagnosis of psoriasis was the dependent variable. Physical activity was defined differently across the studies that were evaluated. To determine study quality, we implemented the Newcastle–Ottawa Scale (NOS), a 9-star scoring system that includes items such as selection criteria, comparability, and study outcome.6 Studies with an NOS score of 7 or higher were included in the meta-analysis.
Results
The literature search generated 353 nonduplicate articles. A thorough review of the articles yielded 4 studies that were incorporated in the final analysis.7-10 We aimed to perform a meta-analysis; however, only 1 of the studies included in the final analysis had an NOS score of 7 or higher along with adequate data to be incorporated into our study.10 As a result, the meta-analysis was converted to a regular review.
The cross-sectional study we reviewed, which had an NOS score of 7, included males and females in the United States aged 20 to 59 years.10 Data were collected using the population-based National Health and Nutrition Examination Survey from 2003 to 2006. The survey measured the likelihood of participation in leisure-time moderate to vigorous physical activity (MVPA) and metabolic equivalent task (MET) minutes of MVPA in the past 30 days. Of 6549 participants, 385 were excluded from the analysis due to missing values for 1 or more of the study variables. Of the remaining 6164 participants, 84 (1.4%) reported having a diagnosis of psoriasis with few or no psoriasis patches at the time of the survey, and 71 (1.2%) reported having a diagnosis of psoriasis with few to extensive patches at the time of the survey.10
Participants with psoriasis were less likely to participate in MVPA in the previous 30 days compared to participants without psoriasis, but the association was not statistically significant.10 The study demonstrated that, on average, participants with psoriasis spent 31% (95% confidence interval [CI], −0.57 to −0.05) fewer MET minutes on leisure-time MVPA versus participants without psoriasis; however, this association was not statistically significant. It is important to note that the diagnosis of psoriasis was self-reported, and measures of disease duration or areas of involvement were not incorporated.
Comment
Our review revealed that vigorous physical activity may be reduced in patients with psoriasis compared to those without psoriasis. Initially, we aimed to perform a systematic review of the literature; however, only 1 study met the criteria for the systematic review, highlighting the need for more robust studies evaluating this subject.
Do et al10 demonstrated that psoriasis patients were less likely to participate in MVPA, but the findings were not statistically significant. Of those who participated in MVPA, MET minutes were fewer among patients with few to extensive skin lesions compared to those without psoriasis. The investigators suggested that psoriasis patients with more severe disease tend to exercise less and ultimately would benefit from regular vigorous physical activity.
Frankel et al7 performed a prospective cohort study in US women to evaluate the role of physical activity in preventing psoriasis. The investigators reported that the most physically active quintile had a lower multivariate relative risk of psoriasis (0.72; 95% CI, 0.59–0.89; P<.001 for trend) compared to the least active quintile.7 Additionally, vigorous physical activity, which was defined as 6 or more MET minutes, was associated with a significantly lower risk of incident psoriasis (0.66; 95% CI, 0.54–0.81; P<.001 for trend), which maintained significance after adjusting for body mass index (BMI). The investigators suggested that, by decreasing chronic inflammation and lowering levels of proinflammatory cytokines, vigorous physical activity may reduce the risk of psoriasis development in women.7 It is plausible that vigorous physical activity modifies the state of chronic inflammation, which could subsequently reduce the risk of developing psoriasis; however, further long-term, randomized, prospective studies are needed to verify the relationship between physical activity and development of psoriasis.
Torres et al8 performed a cross-sectional questionnaire study to assess physical activity in patients with severe psoriasis (defined as >10% body surface area involvement and/or disease requiring systemic therapy or phototherapy) versus healthy controls. Physical activity level was measured using the International Physical Activity Questionnaire. The odds ratio of low-level physical activity compared to non–low-level physical activity among psoriasis patients versus controls was 3.42 (95% CI, 1.47–7.91; P=.002). Additionally, the average total MET minutes of psoriasis patients were significantly reduced compared to those of the healthy controls (P=.001). Thus, the investigators suggested that vigorous physical activity is less likely in psoriasis patients, which may contribute to the increased risk of cardiovascular disease in this population.8 Vigorous physical activity would benefit patients with psoriasis to help lower the chronic state of inflammation and cardiometabolic comorbidities.
Demirel et al9 performed a study to compare aerobic exercise capacity and daily physical activity level in psoriasis patients (n=30) compared to controls (n=30). Daily physical activity, measured with an accelerometer, was significantly higher in male patients with psoriasis compared to controls (P=.021). No significant difference was reported in maximal aerobic capacity in both male and female psoriasis patients versus controls. The investigators suggested that the level of daily physical activity is not limited in psoriasis patients, yet the small sample size may limit the generalizability of the study.
The ability to dissipate heat during exercise seems to be diminished in patients with psoriasis. Specifically, it has been suggested that psoriasis lesions interfere with normal perspiration.11 Moreover, joint involvement in patients with psoriatic arthritis may lead to physical functional disabilities that can interfere with the ability of these patients to participate in regular physical activity.12-14 For this reason, our review excluded articles that evaluated patients with psoriatic arthritis. Despite this exclusion, it is important to consider that comorbid psoriatic arthritis in clinical practice may impede patients with psoriasis from participating in physical activity. Additionally, various social aspects also may limit physical activity in psoriasis patients; for instance, psoriasis patients often avoid activities that involve increased exposure of the skin (eg, communal showers, wearing sports attire).15
Furthermore, obese psoriasis patients are less likely to exercise compared to obese individuals without psoriasis.16 In patients with higher BMI, the risk of psoriasis is increased.17 A systematic review suggested that weight loss may improve psoriasis severity.18 Bariatric surgery also may improve psoriasis.19 Moreover, obesity may interfere with response to biologic therapies for psoriasis. Specifically, higher BMI is linked with lower response to fixed-dose biologic therapies compared to weight-based biologic options (eg, infliximab).20,21
Conclusion
Given the increased risk of myocardial infarction in patients with psoriasis, it is important to recognize the barriers to physical activity that psoriasis patients face.22 Due to the considerable health benefits associated with regular physical activity, physicians should encourage patients with psoriasis to participate in physical activity as tolerated. Of note, the studies included in this review varied in their definitions of psoriasis disease severity and measures of physical activity level. Long-term, randomized, prospective studies are needed to clarify the relationship between psoriasis and physical activity. Evidence from these studies would help guide clinical recommendations regarding the role of physical activity for patients with psoriasis.
Psoriasis is a chronic inflammatory disease that affects approximately 2% to 3% of the US population.1 Patients with psoriasis are more likely to have cardiovascular risk factors (eg, obesity, metabolic syndrome) than individuals without psoriasis.2 In fact, recent evidence has suggested that a diagnosis of psoriasis is an independent risk factor for cardiometabolic diseases including diabetes, major adverse cardiovascular events, and obesity.3 Given the well-recognized health benefits of physical activity and the associated reduction in coronary heart disease risk,4 patients with psoriasis specifically may benefit from regular participation in physical activity. Thus, an enhanced understanding of the relationship between psoriasis and vigorous physical activity would help determine the role of initiating and recommending interventions that implement physical activity for patients with psoriasis. A review was conducted to determine the relationship between psoriasis and vigorous physical activity.
Methods
An English-language literature search of PubMed articles indexed for MEDLINE (January 1, 1946–October 15, 2017) as well as articles in the Embase database (January 1, 1947–October 15, 2017) and Cochrane Library (January 1, 1992–October 15, 2017) using the terms psoriasis and physical activity was performed. The search strategy was established based on a prior review of vigorous physical activity in eczema.5 The article titles and/or abstracts were reviewed, and the studies were excluded if they did not evaluate physical activity in patients with psoriasis. Studies without a control group also were excluded. Articles on patients with psoriatic arthritis and studies that involved modification of dietary intake also were excluded.
Two reviewers (M.A. and E.B.L.) independently extracted data from the studies and compiled the results. The following factors were included in the data extracted: study year, location, and design; method of diagnosis of psoriasis; total number of patients included in the study; and age, gender, and level of physical activity of the study patients. Level of physical activity was the exposure, and diagnosis of psoriasis was the dependent variable. Physical activity was defined differently across the studies that were evaluated. To determine study quality, we implemented the Newcastle–Ottawa Scale (NOS), a 9-star scoring system that includes items such as selection criteria, comparability, and study outcome.6 Studies with an NOS score of 7 or higher were included in the meta-analysis.
Results
The literature search generated 353 nonduplicate articles. A thorough review of the articles yielded 4 studies that were incorporated in the final analysis.7-10 We aimed to perform a meta-analysis; however, only 1 of the studies included in the final analysis had an NOS score of 7 or higher along with adequate data to be incorporated into our study.10 As a result, the meta-analysis was converted to a regular review.
The cross-sectional study we reviewed, which had an NOS score of 7, included males and females in the United States aged 20 to 59 years.10 Data were collected using the population-based National Health and Nutrition Examination Survey from 2003 to 2006. The survey measured the likelihood of participation in leisure-time moderate to vigorous physical activity (MVPA) and metabolic equivalent task (MET) minutes of MVPA in the past 30 days. Of 6549 participants, 385 were excluded from the analysis due to missing values for 1 or more of the study variables. Of the remaining 6164 participants, 84 (1.4%) reported having a diagnosis of psoriasis with few or no psoriasis patches at the time of the survey, and 71 (1.2%) reported having a diagnosis of psoriasis with few to extensive patches at the time of the survey.10
Participants with psoriasis were less likely to participate in MVPA in the previous 30 days compared to participants without psoriasis, but the association was not statistically significant.10 The study demonstrated that, on average, participants with psoriasis spent 31% (95% confidence interval [CI], −0.57 to −0.05) fewer MET minutes on leisure-time MVPA versus participants without psoriasis; however, this association was not statistically significant. It is important to note that the diagnosis of psoriasis was self-reported, and measures of disease duration or areas of involvement were not incorporated.
Comment
Our review revealed that vigorous physical activity may be reduced in patients with psoriasis compared to those without psoriasis. Initially, we aimed to perform a systematic review of the literature; however, only 1 study met the criteria for the systematic review, highlighting the need for more robust studies evaluating this subject.
Do et al10 demonstrated that psoriasis patients were less likely to participate in MVPA, but the findings were not statistically significant. Of those who participated in MVPA, MET minutes were fewer among patients with few to extensive skin lesions compared to those without psoriasis. The investigators suggested that psoriasis patients with more severe disease tend to exercise less and ultimately would benefit from regular vigorous physical activity.
Frankel et al7 performed a prospective cohort study in US women to evaluate the role of physical activity in preventing psoriasis. The investigators reported that the most physically active quintile had a lower multivariate relative risk of psoriasis (0.72; 95% CI, 0.59–0.89; P<.001 for trend) compared to the least active quintile.7 Additionally, vigorous physical activity, which was defined as 6 or more MET minutes, was associated with a significantly lower risk of incident psoriasis (0.66; 95% CI, 0.54–0.81; P<.001 for trend), which maintained significance after adjusting for body mass index (BMI). The investigators suggested that, by decreasing chronic inflammation and lowering levels of proinflammatory cytokines, vigorous physical activity may reduce the risk of psoriasis development in women.7 It is plausible that vigorous physical activity modifies the state of chronic inflammation, which could subsequently reduce the risk of developing psoriasis; however, further long-term, randomized, prospective studies are needed to verify the relationship between physical activity and development of psoriasis.
Torres et al8 performed a cross-sectional questionnaire study to assess physical activity in patients with severe psoriasis (defined as >10% body surface area involvement and/or disease requiring systemic therapy or phototherapy) versus healthy controls. Physical activity level was measured using the International Physical Activity Questionnaire. The odds ratio of low-level physical activity compared to non–low-level physical activity among psoriasis patients versus controls was 3.42 (95% CI, 1.47–7.91; P=.002). Additionally, the average total MET minutes of psoriasis patients were significantly reduced compared to those of the healthy controls (P=.001). Thus, the investigators suggested that vigorous physical activity is less likely in psoriasis patients, which may contribute to the increased risk of cardiovascular disease in this population.8 Vigorous physical activity would benefit patients with psoriasis to help lower the chronic state of inflammation and cardiometabolic comorbidities.
Demirel et al9 performed a study to compare aerobic exercise capacity and daily physical activity level in psoriasis patients (n=30) compared to controls (n=30). Daily physical activity, measured with an accelerometer, was significantly higher in male patients with psoriasis compared to controls (P=.021). No significant difference was reported in maximal aerobic capacity in both male and female psoriasis patients versus controls. The investigators suggested that the level of daily physical activity is not limited in psoriasis patients, yet the small sample size may limit the generalizability of the study.
The ability to dissipate heat during exercise seems to be diminished in patients with psoriasis. Specifically, it has been suggested that psoriasis lesions interfere with normal perspiration.11 Moreover, joint involvement in patients with psoriatic arthritis may lead to physical functional disabilities that can interfere with the ability of these patients to participate in regular physical activity.12-14 For this reason, our review excluded articles that evaluated patients with psoriatic arthritis. Despite this exclusion, it is important to consider that comorbid psoriatic arthritis in clinical practice may impede patients with psoriasis from participating in physical activity. Additionally, various social aspects also may limit physical activity in psoriasis patients; for instance, psoriasis patients often avoid activities that involve increased exposure of the skin (eg, communal showers, wearing sports attire).15
Furthermore, obese psoriasis patients are less likely to exercise compared to obese individuals without psoriasis.16 In patients with higher BMI, the risk of psoriasis is increased.17 A systematic review suggested that weight loss may improve psoriasis severity.18 Bariatric surgery also may improve psoriasis.19 Moreover, obesity may interfere with response to biologic therapies for psoriasis. Specifically, higher BMI is linked with lower response to fixed-dose biologic therapies compared to weight-based biologic options (eg, infliximab).20,21
Conclusion
Given the increased risk of myocardial infarction in patients with psoriasis, it is important to recognize the barriers to physical activity that psoriasis patients face.22 Due to the considerable health benefits associated with regular physical activity, physicians should encourage patients with psoriasis to participate in physical activity as tolerated. Of note, the studies included in this review varied in their definitions of psoriasis disease severity and measures of physical activity level. Long-term, randomized, prospective studies are needed to clarify the relationship between psoriasis and physical activity. Evidence from these studies would help guide clinical recommendations regarding the role of physical activity for patients with psoriasis.
- Takeshita J, Gelfand JM, Li P, et al. Psoriasis in the US Medicare population: prevalence, treatment, and factors associated with biologic use. J Invest Dermatol. 2015;135:2955-2963.
- Prey S, Paul C, Bronsard V, et al. Cardiovascular risk factors in patients with plaque psoriasis: a systematic review of epidemiological studies. J Eur Acad Dermatol Venereol. 2010;24(suppl 2):23-30.
- Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76:377-390.
- Leon AS. Biological mechanisms for the cardioprotective effects of aerobic exercise. Am J Lifestyle Med. 2009;3:32S-34S.
- Kim A, Silverberg JI. A systematic review of vigorous physical activity in eczema. Br J Dermatol. 2016;174:660-662.
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. The Ottawa Hospital Research Institute website. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed February 23, 2018.
- Frankel HC, Han J, Li T, et al. The association between physical activity and the risk of incident psoriasis. Arch Dermatol. 2012;148:918-924.
- Torres T, Alexandre JM, Mendonça D, et al. Levels of physical activity in patients with severe psoriasis: a cross-sectional questionnaire study. Am J Clin Dermatol. 2014;15:129-135.
- Demirel R, Genc A, Ucok K, et al. Do patients with mild to moderate psoriasis really have a sedentary lifestyle? Int J Dermatol. 2013;52:1129-1134.
- Do YK, Lakhani N, Malhotra R, et al. Association between psoriasis and leisure‐time physical activity: findings from the National Health and Nutrition Examination Survey. J Dermatol. 2015;42:148-153.
- Leibowitz E, Seidman DS, Laor A, et al. Are psoriatic patients at risk of heat intolerance? Br J Dermatol. 1991;124:439-442.
- Husted JA, Tom BD, Farewell VT, et al. Description and prediction of physical functional disability in psoriatic arthritis: a longitudinal analysis using a Markov model approach. Arthritis Rheum. 2005;53:404-409.
- Wilson FC, Icen M, Crowson CS, et al. Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: a population‐based study. Arthritis Rheum. 2009;61:233-239.
- Shih M, Hootman JM, Kruger J, et al. Physical activity in men and women with arthritis: National Health Interview Survey, 2002. Am J Prev Med. 2006;30:385-393.
- Ramsay B, O’Reagan M. A survey of the social and psychological effects of psoriasis. Br J Dermatol. 1988;118:195-201.
- Herron MD, Hinckley M, Hoffman MS, et al. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol. 2005;141:1527-1534.
- Kumar S, Han J, Li T, et al. Obesity, waist circumference, weight change and the risk of psoriasis in US women. J Eur Acad Dermatol Venereol. 2013;27:1293-1298.
- Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes (Lond). 2015;39:1197-1202.
- Sako EY, Famenini S, Wu JJ. Bariatric surgery and psoriasis. J Am Acad Dermatol. 2014;70:774-779.
- Clark L, Lebwohl M. The effect of weight on the efficacy of biologic therapy in patients with psoriasis. J Am Acad Dermatol. 2008;58:443-446.
- Puig L. Obesity and psoriasis: body weight and body mass index influence the response to biological treatment. J Eur Acad Dermatol Venereol. 2011;25:1007-1011.
- Wu JJ, Choi YM, Bebchuk JD. Risk of myocardial infarction in psoriasis patients: a retrospective cohort study. J Dermatolog Treat. 2015;26:230-234.
- Takeshita J, Gelfand JM, Li P, et al. Psoriasis in the US Medicare population: prevalence, treatment, and factors associated with biologic use. J Invest Dermatol. 2015;135:2955-2963.
- Prey S, Paul C, Bronsard V, et al. Cardiovascular risk factors in patients with plaque psoriasis: a systematic review of epidemiological studies. J Eur Acad Dermatol Venereol. 2010;24(suppl 2):23-30.
- Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76:377-390.
- Leon AS. Biological mechanisms for the cardioprotective effects of aerobic exercise. Am J Lifestyle Med. 2009;3:32S-34S.
- Kim A, Silverberg JI. A systematic review of vigorous physical activity in eczema. Br J Dermatol. 2016;174:660-662.
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. The Ottawa Hospital Research Institute website. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed February 23, 2018.
- Frankel HC, Han J, Li T, et al. The association between physical activity and the risk of incident psoriasis. Arch Dermatol. 2012;148:918-924.
- Torres T, Alexandre JM, Mendonça D, et al. Levels of physical activity in patients with severe psoriasis: a cross-sectional questionnaire study. Am J Clin Dermatol. 2014;15:129-135.
- Demirel R, Genc A, Ucok K, et al. Do patients with mild to moderate psoriasis really have a sedentary lifestyle? Int J Dermatol. 2013;52:1129-1134.
- Do YK, Lakhani N, Malhotra R, et al. Association between psoriasis and leisure‐time physical activity: findings from the National Health and Nutrition Examination Survey. J Dermatol. 2015;42:148-153.
- Leibowitz E, Seidman DS, Laor A, et al. Are psoriatic patients at risk of heat intolerance? Br J Dermatol. 1991;124:439-442.
- Husted JA, Tom BD, Farewell VT, et al. Description and prediction of physical functional disability in psoriatic arthritis: a longitudinal analysis using a Markov model approach. Arthritis Rheum. 2005;53:404-409.
- Wilson FC, Icen M, Crowson CS, et al. Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: a population‐based study. Arthritis Rheum. 2009;61:233-239.
- Shih M, Hootman JM, Kruger J, et al. Physical activity in men and women with arthritis: National Health Interview Survey, 2002. Am J Prev Med. 2006;30:385-393.
- Ramsay B, O’Reagan M. A survey of the social and psychological effects of psoriasis. Br J Dermatol. 1988;118:195-201.
- Herron MD, Hinckley M, Hoffman MS, et al. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol. 2005;141:1527-1534.
- Kumar S, Han J, Li T, et al. Obesity, waist circumference, weight change and the risk of psoriasis in US women. J Eur Acad Dermatol Venereol. 2013;27:1293-1298.
- Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes (Lond). 2015;39:1197-1202.
- Sako EY, Famenini S, Wu JJ. Bariatric surgery and psoriasis. J Am Acad Dermatol. 2014;70:774-779.
- Clark L, Lebwohl M. The effect of weight on the efficacy of biologic therapy in patients with psoriasis. J Am Acad Dermatol. 2008;58:443-446.
- Puig L. Obesity and psoriasis: body weight and body mass index influence the response to biological treatment. J Eur Acad Dermatol Venereol. 2011;25:1007-1011.
- Wu JJ, Choi YM, Bebchuk JD. Risk of myocardial infarction in psoriasis patients: a retrospective cohort study. J Dermatolog Treat. 2015;26:230-234.
Practice Points
- Psoriasis is associated with comorbid disease conditions, including cardiovascular disease.
- Regular physical activity is known to decrease the risk of developing cardiovascular disease.
- Patients with psoriasis would likely benefit from regular participation in vigorous physical activity to help reduce the risk of developing cardiovascular disease.