User login
Risk for Appendicitis, Cholecystitis, or Diverticulitis in Patients With Psoriasis
Psoriasis is a chronic skin condition affecting approximately 2% to 3% of the population.1,2 Beyond cutaneous manifestations, psoriasis is a systemic inflammatory state that is associated with an increased risk for cardiovascular disease, including obesity,3,4 type 2 diabetes mellitus,5,6 hypertension,5 dyslipidemia,3,7 metabolic syndrome,7 atherosclerosis,8 peripheral vascular disease,9 coronary artery calcification,10 myocardial infarction,11-13 stroke,9,14 and cardiac death.15,16
Psoriasis also has been associated with inflammatory bowel disease (IBD), possibly because of similar autoimmune mechanisms in the pathogenesis of both diseases.17,18 However, there is no literature regarding the risk for acute gastrointestinal pathologies such as appendicitis, cholecystitis, or diverticulitis in patients with psoriasis.
The primary objective of this study was to examine if patients with psoriasis are at increased risk for appendicitis, cholecystitis, or diverticulitis compared to the general population. The secondary objective was to determine if patients with severe psoriasis (ie, patients treated with phototherapy or systemic therapy) are at a higher risk for these conditions compared to patients with mild psoriasis.
Methods
Patients and Tools
A descriptive, population-based cohort study design with controls from a matched cohort was used to ascertain the effect of psoriasis status on patients’ risk for appendicitis, cholecystitis, or diverticulitis. Our cohort was selected using administrative data from Kaiser Permanente Southern California (KPSC) during the study period (January 1, 2004, through December 31, 2016).
Kaiser Permanente Southern California is a large integrated health maintenance organization that includes approximately 4 million patients as of December 31, 2016, and includes roughly 20% of the region’s population. The geographic area served extends from Bakersfield in the lower California Central Valley to San Diego on the border with Mexico. Membership demographics, socioeconomic status, and ethnicity composition are representative of California.
Patients were included if they had a diagnosis of psoriasis (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 696.1; International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] codes L40.0, L40.4, L40.8, or L40.9) for at least 3 visits between January 1, 2004, and December 31, 2016. Patients were not excluded if they also had a diagnosis of psoriatic arthritis (ICD-9-CM code 696.0; ICD-10-CM code L40.5x). Patients also must have been continuously enrolled for at least 1 year before and 1 year after the index date, which was defined as the date of the third psoriasis diagnosis.
Each patient with psoriasis was assigned to 1 of 2 cohorts: (1) severe psoriasis: patients who received UVB phototherapy, psoralen plus UVA phototherapy, methotrexate, acitretin, cyclosporine, apremilast, etanercept, adalimumab, infliximab, ustekinumab, efalizumab, alefacept, secukinumab, or ixekizumab during the study period; and (2) mild psoriasis: patients who had a diagnosis of psoriasis who did not receive one of these therapies during the study period.
Patients were excluded if they had a history of appendicitis, cholecystitis, or diverticulitis at any time before the index date. Only patients older than 18 years were included.
Patients with psoriasis were frequency matched (1:5) with healthy patients, also from the KPSC network. Individuals were matched by age, sex, and ethnicity.
Statistical Analysis
Baseline characteristics were described with means and SD for continuous variables as well as percentages for categorical variables. Chi-square tests for categorical variables and the Mann-Whitney U Test for continuous variables were used to compare the patients’ characteristics by psoriasis status. Cox proportional hazards regression models were used to examine the risk for appendicitis, cholecystitis, or diverticulitis among patients with and without psoriasis and among patients with mild and severe psoriasis. Proportionality assumption was validated using Pearson product moment correlation between the scaled Schoenfeld residuals and log transformed time for each covariate.
Results were presented as crude (unadjusted) hazard ratios (HRs) and adjusted HRs, where confounding factors (ie, age, sex, ethnicity, body mass index [BMI], alcohol use, smoking status, income, education, and membership length) were adjusted. All tests were performed with SAS EG 5.1 and R software. P<.05 was considered statistically significant. Results are reported with the 95% confidence interval (CI), when appropriate.
Results
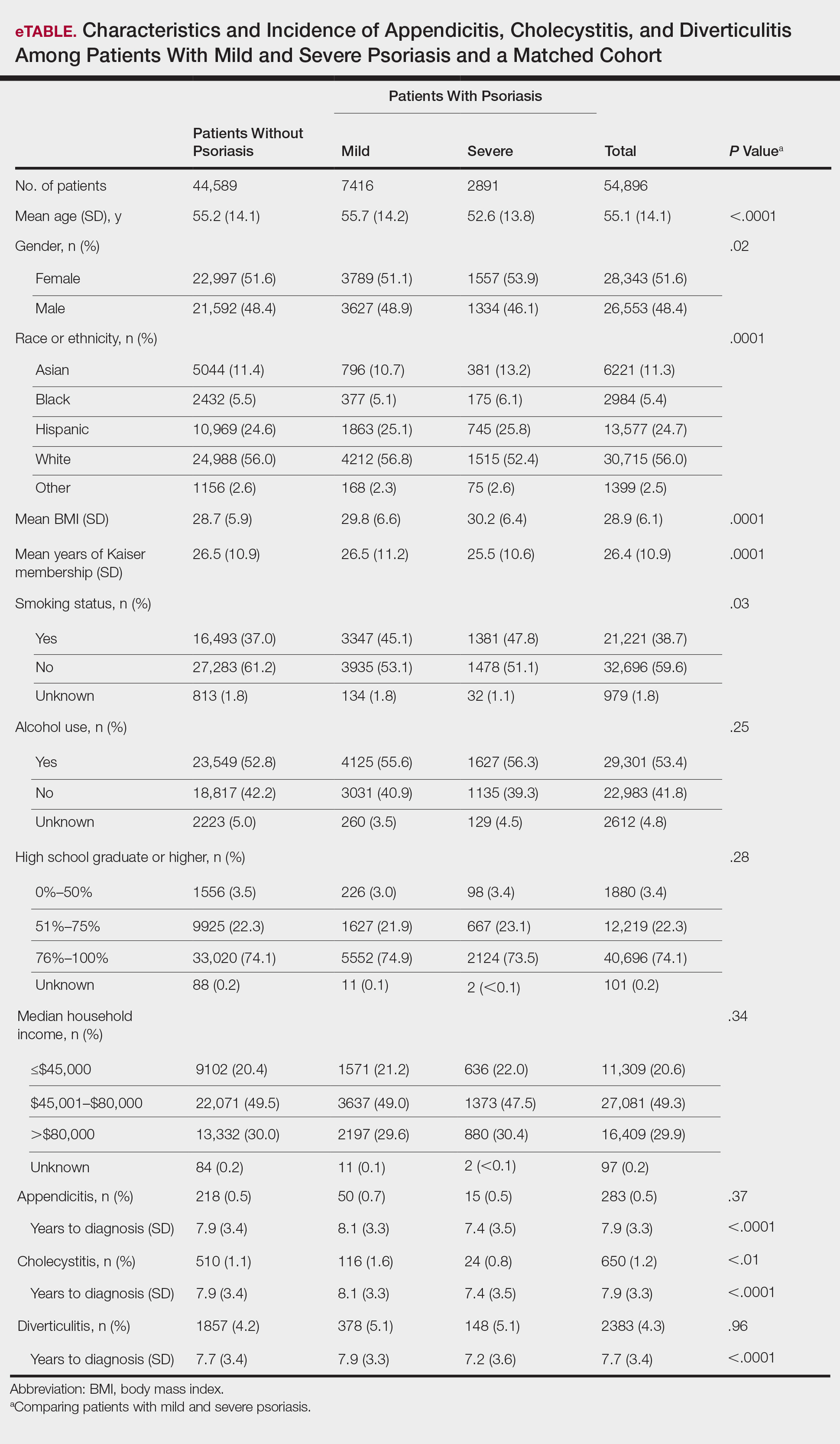
A total of 1,690,214 KPSC patients were eligible for the study; 10,307 (0.6%) met diagnostic and inclusion criteria for the psoriasis cohort. Patients with psoriasis had a significantly higher mean BMI (29.9 vs 28.7; P<.0001) as well as higher mean rates of alcohol use (56% vs 53%; P<.0001) and smoking (47% vs 38%; P<.01) compared to controls. Psoriasis patients had a shorter average duration of membership within the Kaiser network (P=.0001) compared to controls.
A total of 7416 patients met criteria for mild psoriasis and 2891 patients met criteria for severe psoriasis (eTable). Patients with severe psoriasis were significantly younger and had significantly higher mean BMI compared to patients with mild psoriasis (P<.0001 and P=.0001, respectively). No significant difference in rates of alcohol or tobacco use was detected among patients with mild and severe psoriasis.
Appendicitis
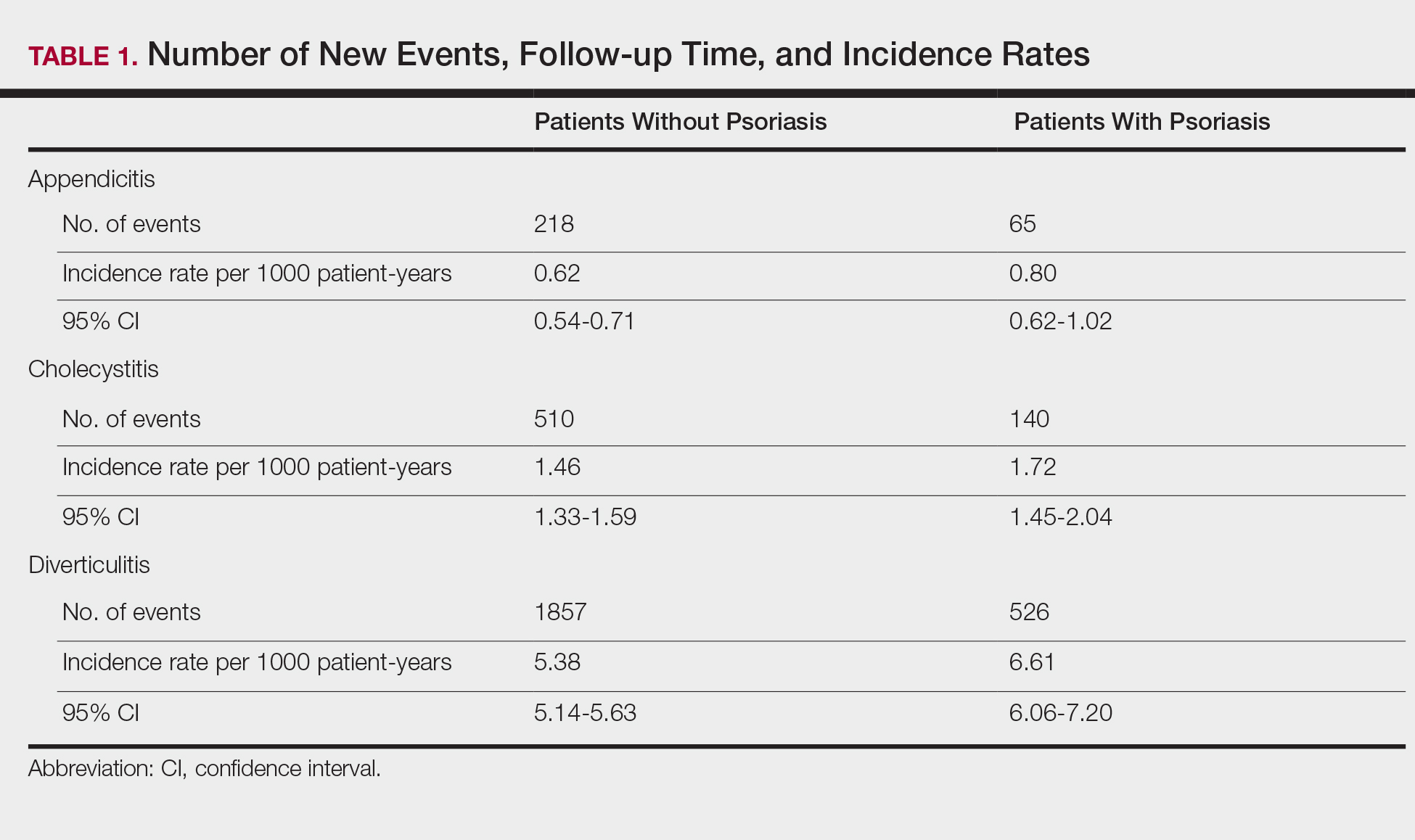
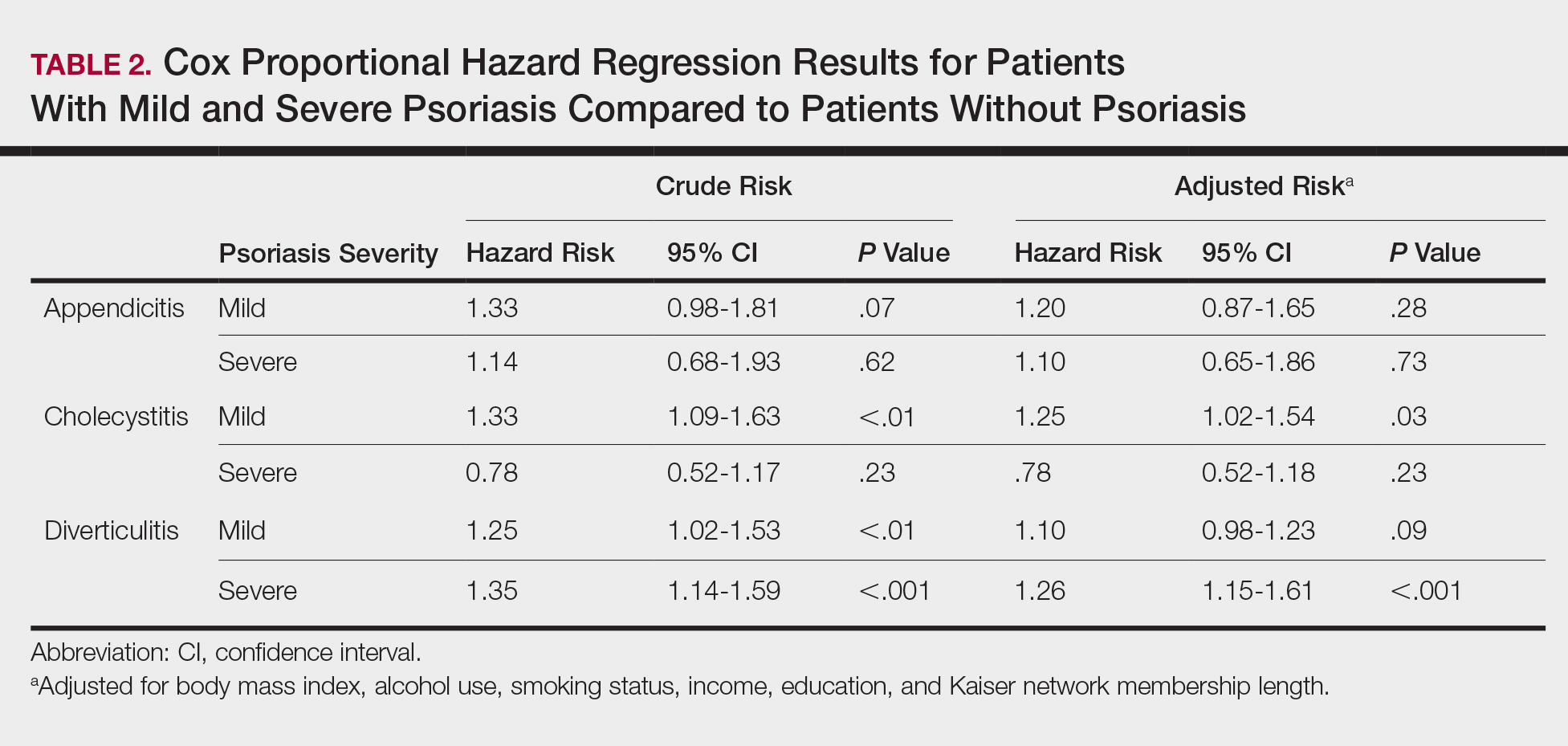
The prevalence of appendicitis was not significantly different between patients with and without psoriasis or between patients with mild and severe psoriasis, though the incidence rate was slightly higher among patients with psoriasis (0.80 per 1000 patient-years compared to 0.62 per 1000 patient-years among patients without psoriasis)(Table 1). However, there was not a significant difference in risk for appendicitis between healthy patients, patients with severe psoriasis, and patients with mild psoriasis after adjusting for potential confounding factors (Table 2). Interestingly, patients with severe psoriasis who had a diagnosis of appendicitis had a significantly shorter time to diagnosis of appendicitis compared to patients with mild psoriasis (7.4 years vs 8.1 years; P<.0001).
Cholecystitis
Psoriasis patients also did not have an increased prevalence of cholecystitis compared to healthy patients. However, patients with severe psoriasis had a significantly higher prevalence of cholecystitis compared to patients with mild psoriasis (P=.0038). Overall, patients with psoriasis had a slightly higher incidence rate (1.72 per 1000 patient-years) compared to healthy patients (1.46 per 1000 patient-years). Moreover, the time to diagnosis of cholecystitis was significantly shorter for patients with severe psoriasis than for patients with mild psoriasis (7.4 years vs 8.1 years; P<.0001). Mild psoriasis was associated with a significantly increased risk (HR, 1.33; 95% CI, 1.09-1.63; P<.01) for cholecystitis compared to individuals without psoriasis in both the crude and adjusted models (Table 2). There was no difference between mild psoriasis patients and severe psoriasis patients in risk for cholecystitis.
Diverticulitis
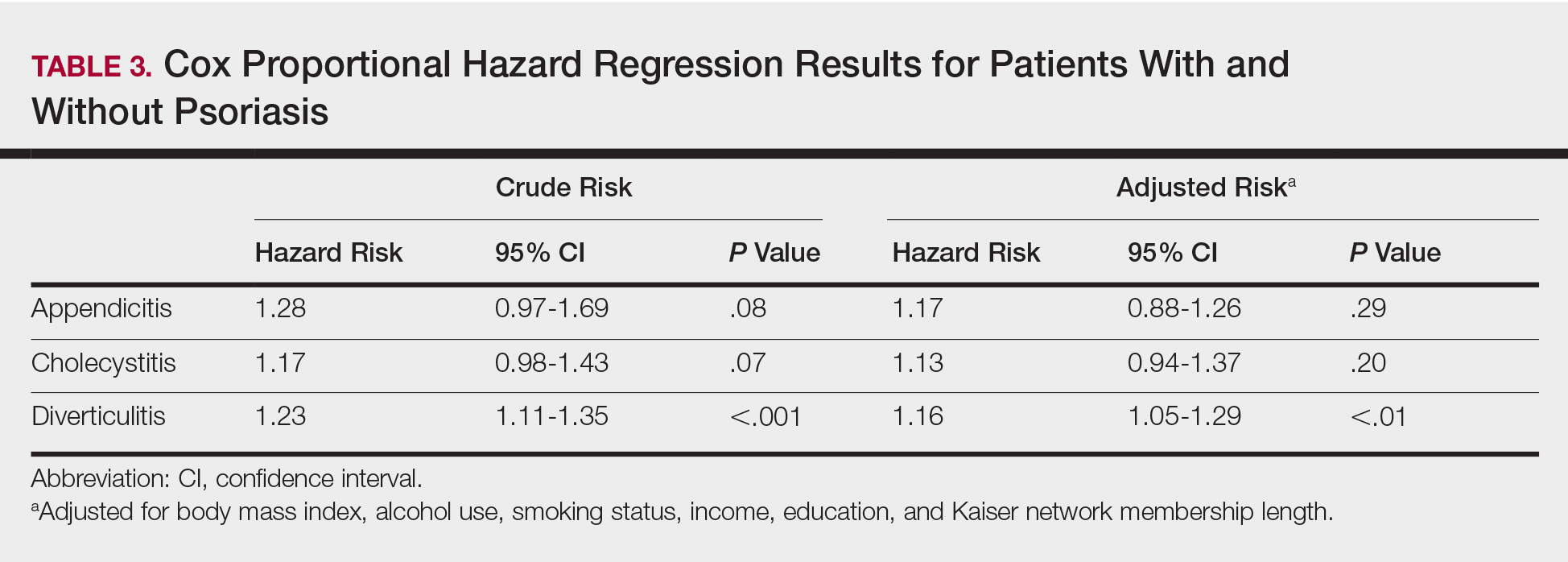
Patients with psoriasis had a significantly greater prevalence of diverticulitis compared to the control cohort (5.1% vs 4.2%; P<.0001). There was no difference in prevalence between the severe psoriasis group and the mild psoriasis group (P=.96), but the time to diagnosis of diverticulitis was shorter in the severe psoriasis group than in the mild psoriasis group (7.2 years vs 7.9 years; P<.0001). Psoriasis patients had an incidence rate of diverticulitis of 6.61 per 1000 patient-years compared to 5.38 per 1000 patient-years in the control group. Psoriasis conferred a higher risk for diverticulitis in both the crude and adjusted models (HR, 1.23; 95% CI, 1.11-1.35 [P<.001] and HR, 1.16; 95% CI, 1.05-1.29; [P<.01], respectively)(Table 3); however, when stratified by disease severity, only patients with severe psoriasis were found to be at higher risk (HR, 1.26; 95% CI, 1.15-1.61; P<.001 for the adjusted model).
Comment
The objective of this study was to examine the background risks for specific gastrointestinal pathologies in a large cohort of patients with psoriasis compared to the general population. After adjusting for measured confounders, patients with severe psoriasis had a significantly higher risk of diverticulitis compared to the general population. Although more patients with severe psoriasis developed appendicitis or cholecystitis, the difference was not significant.
The pathogenesis of diverticulosis and diverticulitis has been thought to be related to increased intracolonic pressure and decreased dietary fiber intake, leading to formation of diverticula in the colon.19 Our study did not correct for differences in diet between the 2 groups, making it a possible confounding variable. Studies evaluating dietary habits of psoriatic patients have found that adult males with psoriasis might consume less fiber compared to healthy patients,20 and psoriasis patients also might consume less whole-grain fiber.21 Furthermore, fiber deficiency also might affect gut flora, causing low-grade chronic inflammation,18 which also has been supported by response to anti-inflammatory medications such as mesalazine.22 Given the autoimmune association between psoriasis and IBD, it is possible that psoriasis also might create an environment of chronic inflammation in the gut, predisposing patients with psoriasis to diverticulitis. However, further research is needed to better evaluate this possibility.
Our study also does not address any potential effects on outcomes of specific treatments for psoriasis. Brandl et al23 found that patients on immunosuppressive therapy for autoimmune diseases had longer hospital and intensive care unit stays, higher rates of emergency operations, and higher mortality while hospitalized. Because our results suggest that patients with severe psoriasis, who are therefore more likely to require treatment with an immunomodulator, are at higher risk for diverticulitis, these patients also might be at risk for poorer outcomes.
There is no literature evaluating the relationship between psoriasis and appendicitis. Our study found a slightly lower incidence rate compared to the national trend (9.38 per 10,000 patient-years in the United States in 2008) in both healthy patients and psoriasis patients.24 Of note, this statistic includes children, whereas our study did not, which might in part account for the lower rate. However, Cheluvappa et al25 hypothesized a relationship between appendicitis and subsequent appendectomy at a young age and protection against IBD. They also found that the mechanism for protection involves downregulation of the helper T cell (TH17) pathway,25 which also has been found to play a role in psoriasis pathogenesis.26,27 Although our results suggest that the risk for appendicitis is not increased for patients with psoriasis, further research might be able to determine if appendicitis and subsequent appendectomy also can offer protection against development of psoriasis.
We found that patients with severe psoriasis had a higher incidence rate of cholecystitis compared to patients with mild psoriasis. Egeberg et al28 found an increased risk for cholelithiasis among patients with psoriasis, which may contribute to a higher rate of cholecystitis. Although both acute and chronic cholecystitis were incorporated in this study, a Russian study found that chronic cholecystitis may be a predictor of progression of psoriasis.29 Moreover, patients with severe psoriasis had a shorter duration to diagnosis of cholecystitis than patients with mild psoriasis. It is possible that patients with severe psoriasis are in a state of greater chronic inflammation than those with mild psoriasis, and therefore, when combined with other risk factors for cholecystitis, may progress to disease more quickly. Alternatively, this finding could be treatment related, as there have been reported cases of cholecystitis related to etanercept use in patients treated for psoriasis and juvenile polyarticular rheumatoid arthritis.30,31 The relationship is not yet well defined, however, and further research is necessary to evaluate this association.
Study Strengths
Key strengths of this study include the large sample size and diversity of the patient population. Kaiser Permanente Southern California membership generally is representative of the broader community, making our results fairly generalizable to populations with health insurance. Use of a matched control cohort allows the results to be more specific to the disease of interest, and the population-based design minimizes bias.
Study Limitations
This study has several limitations. Although the cohorts were categorized based on type of treatment received, exact therapies were not specified. As a retrospective study, it is difficult to control for potential confounding variables that are not included in the electronic medical record. The results of this study also demonstrated significantly shorter durations to diagnosis of all 3 conditions, indicating that surveillance bias may be present.
Conclusion
Patients with psoriasis may be at an increased risk for diverticulitis compared to patients without psoriasis, which could be due to the chronic inflammatory state induced by psoriasis. Therefore, it may be beneficial for clinicians to evaluate psoriasis patients for other risk factors for diverticulitis and subsequently provide counseling to these patients to minimize their risk for diverticulitis. Psoriasis patients do not appear to be at an increased risk for appendicitis or cholecystitis compared to controls; however, further research is needed for confirmation.
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Channual J, Wu JJ, Dann FJ. Effects of tumor necrosis factor-α blockade on metabolic syndrome in psoriasis and psoriatic arthritis and additional lessons learned from rheumatoid arthritis. Dermatol Ther. 2009;22:61-73.
- Koebnick C, Black MH, Smith N, et al. The association of psoriasis and elevated blood lipids in overweight and obese children. J Pediatr. 2011;159:577-583.
- Herron MD, Hinckley M, Hoffman MS, et al. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol. 2005;141:1527-1534.
- Qureshi AA, Choi HK, Setty AR, et al. Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch Dermatol. 2009;145:379-382.
- Shapiro J, Cohen AD, David M, et al. The association between psoriasis, diabetes mellitus, and atherosclerosis in Israel: a case-control study. J Am Acad Dermatol. 2007;56:629-634.
- Love TJ, Qureshi AA, Karlson EW, et al. Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, 2003-2006. Arch Dermatol. 2011;147:419-424.
- El-Mongy S, Fathy H, Abdelaziz A, et al. Subclinical atherosclerosis in patients with chronic psoriasis: a potential association. J Eur Acad Dermatol Venereol. 2010;24:661-666.
- Prodanovich S, Kirsner RS, Kravetz JD, et al. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol. 2009;145:700-703.
- Ludwig RJ, Herzog C, Rostock A, et al. Psoriasis: a possible risk factor for development of coronary artery calcification. Br J Dermatol. 2007;156:271-276.
- Kaye JA, Li L, Jick SS. Incidence of risk factors for myocardial infarction and other vascular diseases in patients with psoriasis. Br J Dermatol. 2008;159:895-902.
- Kimball AB, Robinson D Jr, Wu Y, et al. Cardiovascular disease and risk factors among psoriasis patients in two US healthcare databases, 2001-2002. Dermatology. 2008;217:27-37.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Mehta NN, Azfar RS, Shin DB, et al. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31:1000-1006.
- Abuabara K, Azfar RS, Shin DB, et al. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the United Kingdom. Br J Dermatol. 2010;163:586-592.
- Christophers E. Comorbidities in psoriasis. Clin Dermatol. 2007;25:529-534.
- Wu JJ, Nguyen TU, Poon KY, et al. The association of psoriasis with autoimmune diseases. J Am Acad Dermatol. 2012;67:924-930.
- Floch MH, Bina I. The natural history of diverticulitis: fact and theory. Clin Gastroenterol. 2004;38(5, suppl 1):S2-S7.
- Barrea L, Macchia PE, Tarantino G, et al. Nutrition: a key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J Transl Med. 2015;13:303.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. National Survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Matrana MR, Margolin DA. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg. 2009;22:141-146.
- Brandl A, Kratzer T, Kafka-Ritsch R, et al. Diverticulitis in immunosuppressed patients: a fatal outcome requiring a new approach? Can J Surg. 2016;59:254-261.
- Buckius MT, McGrath B, Monk J, et al. Changing epidemiology of acute appendicitis in the United States: study period 1993-2008. J Surg Res. 2012;175:185-190.
- Cheluvappa R, Luo AS, Grimm MC. T helper type 17 pathway suppression by appendicitis and appendectomy protects against colitis. Clin Exp Immunol. 2014;175:316-322.
- Lynde CW, Poulin Y, Vender R, et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141-150.
- Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-α, IFN-γ, IL6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005:2005;273-279.
- Egeberg A, Anderson YMF, Gislason GH, et al. Gallstone risk in adult patients with atopic dermatitis and psoriasis: possible effect of overweight and obesity. Acta Derm Venereol. 2017;97:627-631.
- Smirnova SV, Barilo AA, Smolnikova MV. Hepatobiliary system diseases as the predictors of psoriasis progression [in Russian]. Vestn Ross Akad Med Nauk. 2016:102-108.
- Bagel J, Lynde C, Tyring S, et al. Moderate to severe plaque psoriasis with scalp involvement: a randomized, double-blind, placebo-controlled study of etanercept. J Am Acad Dermatol. 2012;67:86-92.
- Foeldvari I, Krüger E, Schneider T. Acute, non-obstructive, sterile cholecystitis associated with etanercept and infliximab for the treatment of juvenile polyarticular rheumatoid arthritis. Ann Rheum Dis. 2003;62:908-909.
Psoriasis is a chronic skin condition affecting approximately 2% to 3% of the population.1,2 Beyond cutaneous manifestations, psoriasis is a systemic inflammatory state that is associated with an increased risk for cardiovascular disease, including obesity,3,4 type 2 diabetes mellitus,5,6 hypertension,5 dyslipidemia,3,7 metabolic syndrome,7 atherosclerosis,8 peripheral vascular disease,9 coronary artery calcification,10 myocardial infarction,11-13 stroke,9,14 and cardiac death.15,16
Psoriasis also has been associated with inflammatory bowel disease (IBD), possibly because of similar autoimmune mechanisms in the pathogenesis of both diseases.17,18 However, there is no literature regarding the risk for acute gastrointestinal pathologies such as appendicitis, cholecystitis, or diverticulitis in patients with psoriasis.
The primary objective of this study was to examine if patients with psoriasis are at increased risk for appendicitis, cholecystitis, or diverticulitis compared to the general population. The secondary objective was to determine if patients with severe psoriasis (ie, patients treated with phototherapy or systemic therapy) are at a higher risk for these conditions compared to patients with mild psoriasis.
Methods
Patients and Tools
A descriptive, population-based cohort study design with controls from a matched cohort was used to ascertain the effect of psoriasis status on patients’ risk for appendicitis, cholecystitis, or diverticulitis. Our cohort was selected using administrative data from Kaiser Permanente Southern California (KPSC) during the study period (January 1, 2004, through December 31, 2016).
Kaiser Permanente Southern California is a large integrated health maintenance organization that includes approximately 4 million patients as of December 31, 2016, and includes roughly 20% of the region’s population. The geographic area served extends from Bakersfield in the lower California Central Valley to San Diego on the border with Mexico. Membership demographics, socioeconomic status, and ethnicity composition are representative of California.
Patients were included if they had a diagnosis of psoriasis (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 696.1; International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] codes L40.0, L40.4, L40.8, or L40.9) for at least 3 visits between January 1, 2004, and December 31, 2016. Patients were not excluded if they also had a diagnosis of psoriatic arthritis (ICD-9-CM code 696.0; ICD-10-CM code L40.5x). Patients also must have been continuously enrolled for at least 1 year before and 1 year after the index date, which was defined as the date of the third psoriasis diagnosis.
Each patient with psoriasis was assigned to 1 of 2 cohorts: (1) severe psoriasis: patients who received UVB phototherapy, psoralen plus UVA phototherapy, methotrexate, acitretin, cyclosporine, apremilast, etanercept, adalimumab, infliximab, ustekinumab, efalizumab, alefacept, secukinumab, or ixekizumab during the study period; and (2) mild psoriasis: patients who had a diagnosis of psoriasis who did not receive one of these therapies during the study period.
Patients were excluded if they had a history of appendicitis, cholecystitis, or diverticulitis at any time before the index date. Only patients older than 18 years were included.
Patients with psoriasis were frequency matched (1:5) with healthy patients, also from the KPSC network. Individuals were matched by age, sex, and ethnicity.
Statistical Analysis
Baseline characteristics were described with means and SD for continuous variables as well as percentages for categorical variables. Chi-square tests for categorical variables and the Mann-Whitney U Test for continuous variables were used to compare the patients’ characteristics by psoriasis status. Cox proportional hazards regression models were used to examine the risk for appendicitis, cholecystitis, or diverticulitis among patients with and without psoriasis and among patients with mild and severe psoriasis. Proportionality assumption was validated using Pearson product moment correlation between the scaled Schoenfeld residuals and log transformed time for each covariate.
Results were presented as crude (unadjusted) hazard ratios (HRs) and adjusted HRs, where confounding factors (ie, age, sex, ethnicity, body mass index [BMI], alcohol use, smoking status, income, education, and membership length) were adjusted. All tests were performed with SAS EG 5.1 and R software. P<.05 was considered statistically significant. Results are reported with the 95% confidence interval (CI), when appropriate.
Results
A total of 1,690,214 KPSC patients were eligible for the study; 10,307 (0.6%) met diagnostic and inclusion criteria for the psoriasis cohort. Patients with psoriasis had a significantly higher mean BMI (29.9 vs 28.7; P<.0001) as well as higher mean rates of alcohol use (56% vs 53%; P<.0001) and smoking (47% vs 38%; P<.01) compared to controls. Psoriasis patients had a shorter average duration of membership within the Kaiser network (P=.0001) compared to controls.
A total of 7416 patients met criteria for mild psoriasis and 2891 patients met criteria for severe psoriasis (eTable). Patients with severe psoriasis were significantly younger and had significantly higher mean BMI compared to patients with mild psoriasis (P<.0001 and P=.0001, respectively). No significant difference in rates of alcohol or tobacco use was detected among patients with mild and severe psoriasis.
Appendicitis
The prevalence of appendicitis was not significantly different between patients with and without psoriasis or between patients with mild and severe psoriasis, though the incidence rate was slightly higher among patients with psoriasis (0.80 per 1000 patient-years compared to 0.62 per 1000 patient-years among patients without psoriasis)(Table 1). However, there was not a significant difference in risk for appendicitis between healthy patients, patients with severe psoriasis, and patients with mild psoriasis after adjusting for potential confounding factors (Table 2). Interestingly, patients with severe psoriasis who had a diagnosis of appendicitis had a significantly shorter time to diagnosis of appendicitis compared to patients with mild psoriasis (7.4 years vs 8.1 years; P<.0001).
Cholecystitis
Psoriasis patients also did not have an increased prevalence of cholecystitis compared to healthy patients. However, patients with severe psoriasis had a significantly higher prevalence of cholecystitis compared to patients with mild psoriasis (P=.0038). Overall, patients with psoriasis had a slightly higher incidence rate (1.72 per 1000 patient-years) compared to healthy patients (1.46 per 1000 patient-years). Moreover, the time to diagnosis of cholecystitis was significantly shorter for patients with severe psoriasis than for patients with mild psoriasis (7.4 years vs 8.1 years; P<.0001). Mild psoriasis was associated with a significantly increased risk (HR, 1.33; 95% CI, 1.09-1.63; P<.01) for cholecystitis compared to individuals without psoriasis in both the crude and adjusted models (Table 2). There was no difference between mild psoriasis patients and severe psoriasis patients in risk for cholecystitis.
Diverticulitis
Patients with psoriasis had a significantly greater prevalence of diverticulitis compared to the control cohort (5.1% vs 4.2%; P<.0001). There was no difference in prevalence between the severe psoriasis group and the mild psoriasis group (P=.96), but the time to diagnosis of diverticulitis was shorter in the severe psoriasis group than in the mild psoriasis group (7.2 years vs 7.9 years; P<.0001). Psoriasis patients had an incidence rate of diverticulitis of 6.61 per 1000 patient-years compared to 5.38 per 1000 patient-years in the control group. Psoriasis conferred a higher risk for diverticulitis in both the crude and adjusted models (HR, 1.23; 95% CI, 1.11-1.35 [P<.001] and HR, 1.16; 95% CI, 1.05-1.29; [P<.01], respectively)(Table 3); however, when stratified by disease severity, only patients with severe psoriasis were found to be at higher risk (HR, 1.26; 95% CI, 1.15-1.61; P<.001 for the adjusted model).
Comment
The objective of this study was to examine the background risks for specific gastrointestinal pathologies in a large cohort of patients with psoriasis compared to the general population. After adjusting for measured confounders, patients with severe psoriasis had a significantly higher risk of diverticulitis compared to the general population. Although more patients with severe psoriasis developed appendicitis or cholecystitis, the difference was not significant.
The pathogenesis of diverticulosis and diverticulitis has been thought to be related to increased intracolonic pressure and decreased dietary fiber intake, leading to formation of diverticula in the colon.19 Our study did not correct for differences in diet between the 2 groups, making it a possible confounding variable. Studies evaluating dietary habits of psoriatic patients have found that adult males with psoriasis might consume less fiber compared to healthy patients,20 and psoriasis patients also might consume less whole-grain fiber.21 Furthermore, fiber deficiency also might affect gut flora, causing low-grade chronic inflammation,18 which also has been supported by response to anti-inflammatory medications such as mesalazine.22 Given the autoimmune association between psoriasis and IBD, it is possible that psoriasis also might create an environment of chronic inflammation in the gut, predisposing patients with psoriasis to diverticulitis. However, further research is needed to better evaluate this possibility.
Our study also does not address any potential effects on outcomes of specific treatments for psoriasis. Brandl et al23 found that patients on immunosuppressive therapy for autoimmune diseases had longer hospital and intensive care unit stays, higher rates of emergency operations, and higher mortality while hospitalized. Because our results suggest that patients with severe psoriasis, who are therefore more likely to require treatment with an immunomodulator, are at higher risk for diverticulitis, these patients also might be at risk for poorer outcomes.
There is no literature evaluating the relationship between psoriasis and appendicitis. Our study found a slightly lower incidence rate compared to the national trend (9.38 per 10,000 patient-years in the United States in 2008) in both healthy patients and psoriasis patients.24 Of note, this statistic includes children, whereas our study did not, which might in part account for the lower rate. However, Cheluvappa et al25 hypothesized a relationship between appendicitis and subsequent appendectomy at a young age and protection against IBD. They also found that the mechanism for protection involves downregulation of the helper T cell (TH17) pathway,25 which also has been found to play a role in psoriasis pathogenesis.26,27 Although our results suggest that the risk for appendicitis is not increased for patients with psoriasis, further research might be able to determine if appendicitis and subsequent appendectomy also can offer protection against development of psoriasis.
We found that patients with severe psoriasis had a higher incidence rate of cholecystitis compared to patients with mild psoriasis. Egeberg et al28 found an increased risk for cholelithiasis among patients with psoriasis, which may contribute to a higher rate of cholecystitis. Although both acute and chronic cholecystitis were incorporated in this study, a Russian study found that chronic cholecystitis may be a predictor of progression of psoriasis.29 Moreover, patients with severe psoriasis had a shorter duration to diagnosis of cholecystitis than patients with mild psoriasis. It is possible that patients with severe psoriasis are in a state of greater chronic inflammation than those with mild psoriasis, and therefore, when combined with other risk factors for cholecystitis, may progress to disease more quickly. Alternatively, this finding could be treatment related, as there have been reported cases of cholecystitis related to etanercept use in patients treated for psoriasis and juvenile polyarticular rheumatoid arthritis.30,31 The relationship is not yet well defined, however, and further research is necessary to evaluate this association.
Study Strengths
Key strengths of this study include the large sample size and diversity of the patient population. Kaiser Permanente Southern California membership generally is representative of the broader community, making our results fairly generalizable to populations with health insurance. Use of a matched control cohort allows the results to be more specific to the disease of interest, and the population-based design minimizes bias.
Study Limitations
This study has several limitations. Although the cohorts were categorized based on type of treatment received, exact therapies were not specified. As a retrospective study, it is difficult to control for potential confounding variables that are not included in the electronic medical record. The results of this study also demonstrated significantly shorter durations to diagnosis of all 3 conditions, indicating that surveillance bias may be present.
Conclusion
Patients with psoriasis may be at an increased risk for diverticulitis compared to patients without psoriasis, which could be due to the chronic inflammatory state induced by psoriasis. Therefore, it may be beneficial for clinicians to evaluate psoriasis patients for other risk factors for diverticulitis and subsequently provide counseling to these patients to minimize their risk for diverticulitis. Psoriasis patients do not appear to be at an increased risk for appendicitis or cholecystitis compared to controls; however, further research is needed for confirmation.
Psoriasis is a chronic skin condition affecting approximately 2% to 3% of the population.1,2 Beyond cutaneous manifestations, psoriasis is a systemic inflammatory state that is associated with an increased risk for cardiovascular disease, including obesity,3,4 type 2 diabetes mellitus,5,6 hypertension,5 dyslipidemia,3,7 metabolic syndrome,7 atherosclerosis,8 peripheral vascular disease,9 coronary artery calcification,10 myocardial infarction,11-13 stroke,9,14 and cardiac death.15,16
Psoriasis also has been associated with inflammatory bowel disease (IBD), possibly because of similar autoimmune mechanisms in the pathogenesis of both diseases.17,18 However, there is no literature regarding the risk for acute gastrointestinal pathologies such as appendicitis, cholecystitis, or diverticulitis in patients with psoriasis.
The primary objective of this study was to examine if patients with psoriasis are at increased risk for appendicitis, cholecystitis, or diverticulitis compared to the general population. The secondary objective was to determine if patients with severe psoriasis (ie, patients treated with phototherapy or systemic therapy) are at a higher risk for these conditions compared to patients with mild psoriasis.
Methods
Patients and Tools
A descriptive, population-based cohort study design with controls from a matched cohort was used to ascertain the effect of psoriasis status on patients’ risk for appendicitis, cholecystitis, or diverticulitis. Our cohort was selected using administrative data from Kaiser Permanente Southern California (KPSC) during the study period (January 1, 2004, through December 31, 2016).
Kaiser Permanente Southern California is a large integrated health maintenance organization that includes approximately 4 million patients as of December 31, 2016, and includes roughly 20% of the region’s population. The geographic area served extends from Bakersfield in the lower California Central Valley to San Diego on the border with Mexico. Membership demographics, socioeconomic status, and ethnicity composition are representative of California.
Patients were included if they had a diagnosis of psoriasis (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 696.1; International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] codes L40.0, L40.4, L40.8, or L40.9) for at least 3 visits between January 1, 2004, and December 31, 2016. Patients were not excluded if they also had a diagnosis of psoriatic arthritis (ICD-9-CM code 696.0; ICD-10-CM code L40.5x). Patients also must have been continuously enrolled for at least 1 year before and 1 year after the index date, which was defined as the date of the third psoriasis diagnosis.
Each patient with psoriasis was assigned to 1 of 2 cohorts: (1) severe psoriasis: patients who received UVB phototherapy, psoralen plus UVA phototherapy, methotrexate, acitretin, cyclosporine, apremilast, etanercept, adalimumab, infliximab, ustekinumab, efalizumab, alefacept, secukinumab, or ixekizumab during the study period; and (2) mild psoriasis: patients who had a diagnosis of psoriasis who did not receive one of these therapies during the study period.
Patients were excluded if they had a history of appendicitis, cholecystitis, or diverticulitis at any time before the index date. Only patients older than 18 years were included.
Patients with psoriasis were frequency matched (1:5) with healthy patients, also from the KPSC network. Individuals were matched by age, sex, and ethnicity.
Statistical Analysis
Baseline characteristics were described with means and SD for continuous variables as well as percentages for categorical variables. Chi-square tests for categorical variables and the Mann-Whitney U Test for continuous variables were used to compare the patients’ characteristics by psoriasis status. Cox proportional hazards regression models were used to examine the risk for appendicitis, cholecystitis, or diverticulitis among patients with and without psoriasis and among patients with mild and severe psoriasis. Proportionality assumption was validated using Pearson product moment correlation between the scaled Schoenfeld residuals and log transformed time for each covariate.
Results were presented as crude (unadjusted) hazard ratios (HRs) and adjusted HRs, where confounding factors (ie, age, sex, ethnicity, body mass index [BMI], alcohol use, smoking status, income, education, and membership length) were adjusted. All tests were performed with SAS EG 5.1 and R software. P<.05 was considered statistically significant. Results are reported with the 95% confidence interval (CI), when appropriate.
Results
A total of 1,690,214 KPSC patients were eligible for the study; 10,307 (0.6%) met diagnostic and inclusion criteria for the psoriasis cohort. Patients with psoriasis had a significantly higher mean BMI (29.9 vs 28.7; P<.0001) as well as higher mean rates of alcohol use (56% vs 53%; P<.0001) and smoking (47% vs 38%; P<.01) compared to controls. Psoriasis patients had a shorter average duration of membership within the Kaiser network (P=.0001) compared to controls.
A total of 7416 patients met criteria for mild psoriasis and 2891 patients met criteria for severe psoriasis (eTable). Patients with severe psoriasis were significantly younger and had significantly higher mean BMI compared to patients with mild psoriasis (P<.0001 and P=.0001, respectively). No significant difference in rates of alcohol or tobacco use was detected among patients with mild and severe psoriasis.
Appendicitis
The prevalence of appendicitis was not significantly different between patients with and without psoriasis or between patients with mild and severe psoriasis, though the incidence rate was slightly higher among patients with psoriasis (0.80 per 1000 patient-years compared to 0.62 per 1000 patient-years among patients without psoriasis)(Table 1). However, there was not a significant difference in risk for appendicitis between healthy patients, patients with severe psoriasis, and patients with mild psoriasis after adjusting for potential confounding factors (Table 2). Interestingly, patients with severe psoriasis who had a diagnosis of appendicitis had a significantly shorter time to diagnosis of appendicitis compared to patients with mild psoriasis (7.4 years vs 8.1 years; P<.0001).
Cholecystitis
Psoriasis patients also did not have an increased prevalence of cholecystitis compared to healthy patients. However, patients with severe psoriasis had a significantly higher prevalence of cholecystitis compared to patients with mild psoriasis (P=.0038). Overall, patients with psoriasis had a slightly higher incidence rate (1.72 per 1000 patient-years) compared to healthy patients (1.46 per 1000 patient-years). Moreover, the time to diagnosis of cholecystitis was significantly shorter for patients with severe psoriasis than for patients with mild psoriasis (7.4 years vs 8.1 years; P<.0001). Mild psoriasis was associated with a significantly increased risk (HR, 1.33; 95% CI, 1.09-1.63; P<.01) for cholecystitis compared to individuals without psoriasis in both the crude and adjusted models (Table 2). There was no difference between mild psoriasis patients and severe psoriasis patients in risk for cholecystitis.
Diverticulitis
Patients with psoriasis had a significantly greater prevalence of diverticulitis compared to the control cohort (5.1% vs 4.2%; P<.0001). There was no difference in prevalence between the severe psoriasis group and the mild psoriasis group (P=.96), but the time to diagnosis of diverticulitis was shorter in the severe psoriasis group than in the mild psoriasis group (7.2 years vs 7.9 years; P<.0001). Psoriasis patients had an incidence rate of diverticulitis of 6.61 per 1000 patient-years compared to 5.38 per 1000 patient-years in the control group. Psoriasis conferred a higher risk for diverticulitis in both the crude and adjusted models (HR, 1.23; 95% CI, 1.11-1.35 [P<.001] and HR, 1.16; 95% CI, 1.05-1.29; [P<.01], respectively)(Table 3); however, when stratified by disease severity, only patients with severe psoriasis were found to be at higher risk (HR, 1.26; 95% CI, 1.15-1.61; P<.001 for the adjusted model).
Comment
The objective of this study was to examine the background risks for specific gastrointestinal pathologies in a large cohort of patients with psoriasis compared to the general population. After adjusting for measured confounders, patients with severe psoriasis had a significantly higher risk of diverticulitis compared to the general population. Although more patients with severe psoriasis developed appendicitis or cholecystitis, the difference was not significant.
The pathogenesis of diverticulosis and diverticulitis has been thought to be related to increased intracolonic pressure and decreased dietary fiber intake, leading to formation of diverticula in the colon.19 Our study did not correct for differences in diet between the 2 groups, making it a possible confounding variable. Studies evaluating dietary habits of psoriatic patients have found that adult males with psoriasis might consume less fiber compared to healthy patients,20 and psoriasis patients also might consume less whole-grain fiber.21 Furthermore, fiber deficiency also might affect gut flora, causing low-grade chronic inflammation,18 which also has been supported by response to anti-inflammatory medications such as mesalazine.22 Given the autoimmune association between psoriasis and IBD, it is possible that psoriasis also might create an environment of chronic inflammation in the gut, predisposing patients with psoriasis to diverticulitis. However, further research is needed to better evaluate this possibility.
Our study also does not address any potential effects on outcomes of specific treatments for psoriasis. Brandl et al23 found that patients on immunosuppressive therapy for autoimmune diseases had longer hospital and intensive care unit stays, higher rates of emergency operations, and higher mortality while hospitalized. Because our results suggest that patients with severe psoriasis, who are therefore more likely to require treatment with an immunomodulator, are at higher risk for diverticulitis, these patients also might be at risk for poorer outcomes.
There is no literature evaluating the relationship between psoriasis and appendicitis. Our study found a slightly lower incidence rate compared to the national trend (9.38 per 10,000 patient-years in the United States in 2008) in both healthy patients and psoriasis patients.24 Of note, this statistic includes children, whereas our study did not, which might in part account for the lower rate. However, Cheluvappa et al25 hypothesized a relationship between appendicitis and subsequent appendectomy at a young age and protection against IBD. They also found that the mechanism for protection involves downregulation of the helper T cell (TH17) pathway,25 which also has been found to play a role in psoriasis pathogenesis.26,27 Although our results suggest that the risk for appendicitis is not increased for patients with psoriasis, further research might be able to determine if appendicitis and subsequent appendectomy also can offer protection against development of psoriasis.
We found that patients with severe psoriasis had a higher incidence rate of cholecystitis compared to patients with mild psoriasis. Egeberg et al28 found an increased risk for cholelithiasis among patients with psoriasis, which may contribute to a higher rate of cholecystitis. Although both acute and chronic cholecystitis were incorporated in this study, a Russian study found that chronic cholecystitis may be a predictor of progression of psoriasis.29 Moreover, patients with severe psoriasis had a shorter duration to diagnosis of cholecystitis than patients with mild psoriasis. It is possible that patients with severe psoriasis are in a state of greater chronic inflammation than those with mild psoriasis, and therefore, when combined with other risk factors for cholecystitis, may progress to disease more quickly. Alternatively, this finding could be treatment related, as there have been reported cases of cholecystitis related to etanercept use in patients treated for psoriasis and juvenile polyarticular rheumatoid arthritis.30,31 The relationship is not yet well defined, however, and further research is necessary to evaluate this association.
Study Strengths
Key strengths of this study include the large sample size and diversity of the patient population. Kaiser Permanente Southern California membership generally is representative of the broader community, making our results fairly generalizable to populations with health insurance. Use of a matched control cohort allows the results to be more specific to the disease of interest, and the population-based design minimizes bias.
Study Limitations
This study has several limitations. Although the cohorts were categorized based on type of treatment received, exact therapies were not specified. As a retrospective study, it is difficult to control for potential confounding variables that are not included in the electronic medical record. The results of this study also demonstrated significantly shorter durations to diagnosis of all 3 conditions, indicating that surveillance bias may be present.
Conclusion
Patients with psoriasis may be at an increased risk for diverticulitis compared to patients without psoriasis, which could be due to the chronic inflammatory state induced by psoriasis. Therefore, it may be beneficial for clinicians to evaluate psoriasis patients for other risk factors for diverticulitis and subsequently provide counseling to these patients to minimize their risk for diverticulitis. Psoriasis patients do not appear to be at an increased risk for appendicitis or cholecystitis compared to controls; however, further research is needed for confirmation.
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Channual J, Wu JJ, Dann FJ. Effects of tumor necrosis factor-α blockade on metabolic syndrome in psoriasis and psoriatic arthritis and additional lessons learned from rheumatoid arthritis. Dermatol Ther. 2009;22:61-73.
- Koebnick C, Black MH, Smith N, et al. The association of psoriasis and elevated blood lipids in overweight and obese children. J Pediatr. 2011;159:577-583.
- Herron MD, Hinckley M, Hoffman MS, et al. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol. 2005;141:1527-1534.
- Qureshi AA, Choi HK, Setty AR, et al. Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch Dermatol. 2009;145:379-382.
- Shapiro J, Cohen AD, David M, et al. The association between psoriasis, diabetes mellitus, and atherosclerosis in Israel: a case-control study. J Am Acad Dermatol. 2007;56:629-634.
- Love TJ, Qureshi AA, Karlson EW, et al. Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, 2003-2006. Arch Dermatol. 2011;147:419-424.
- El-Mongy S, Fathy H, Abdelaziz A, et al. Subclinical atherosclerosis in patients with chronic psoriasis: a potential association. J Eur Acad Dermatol Venereol. 2010;24:661-666.
- Prodanovich S, Kirsner RS, Kravetz JD, et al. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol. 2009;145:700-703.
- Ludwig RJ, Herzog C, Rostock A, et al. Psoriasis: a possible risk factor for development of coronary artery calcification. Br J Dermatol. 2007;156:271-276.
- Kaye JA, Li L, Jick SS. Incidence of risk factors for myocardial infarction and other vascular diseases in patients with psoriasis. Br J Dermatol. 2008;159:895-902.
- Kimball AB, Robinson D Jr, Wu Y, et al. Cardiovascular disease and risk factors among psoriasis patients in two US healthcare databases, 2001-2002. Dermatology. 2008;217:27-37.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Mehta NN, Azfar RS, Shin DB, et al. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31:1000-1006.
- Abuabara K, Azfar RS, Shin DB, et al. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the United Kingdom. Br J Dermatol. 2010;163:586-592.
- Christophers E. Comorbidities in psoriasis. Clin Dermatol. 2007;25:529-534.
- Wu JJ, Nguyen TU, Poon KY, et al. The association of psoriasis with autoimmune diseases. J Am Acad Dermatol. 2012;67:924-930.
- Floch MH, Bina I. The natural history of diverticulitis: fact and theory. Clin Gastroenterol. 2004;38(5, suppl 1):S2-S7.
- Barrea L, Macchia PE, Tarantino G, et al. Nutrition: a key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J Transl Med. 2015;13:303.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. National Survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Matrana MR, Margolin DA. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg. 2009;22:141-146.
- Brandl A, Kratzer T, Kafka-Ritsch R, et al. Diverticulitis in immunosuppressed patients: a fatal outcome requiring a new approach? Can J Surg. 2016;59:254-261.
- Buckius MT, McGrath B, Monk J, et al. Changing epidemiology of acute appendicitis in the United States: study period 1993-2008. J Surg Res. 2012;175:185-190.
- Cheluvappa R, Luo AS, Grimm MC. T helper type 17 pathway suppression by appendicitis and appendectomy protects against colitis. Clin Exp Immunol. 2014;175:316-322.
- Lynde CW, Poulin Y, Vender R, et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141-150.
- Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-α, IFN-γ, IL6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005:2005;273-279.
- Egeberg A, Anderson YMF, Gislason GH, et al. Gallstone risk in adult patients with atopic dermatitis and psoriasis: possible effect of overweight and obesity. Acta Derm Venereol. 2017;97:627-631.
- Smirnova SV, Barilo AA, Smolnikova MV. Hepatobiliary system diseases as the predictors of psoriasis progression [in Russian]. Vestn Ross Akad Med Nauk. 2016:102-108.
- Bagel J, Lynde C, Tyring S, et al. Moderate to severe plaque psoriasis with scalp involvement: a randomized, double-blind, placebo-controlled study of etanercept. J Am Acad Dermatol. 2012;67:86-92.
- Foeldvari I, Krüger E, Schneider T. Acute, non-obstructive, sterile cholecystitis associated with etanercept and infliximab for the treatment of juvenile polyarticular rheumatoid arthritis. Ann Rheum Dis. 2003;62:908-909.
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Channual J, Wu JJ, Dann FJ. Effects of tumor necrosis factor-α blockade on metabolic syndrome in psoriasis and psoriatic arthritis and additional lessons learned from rheumatoid arthritis. Dermatol Ther. 2009;22:61-73.
- Koebnick C, Black MH, Smith N, et al. The association of psoriasis and elevated blood lipids in overweight and obese children. J Pediatr. 2011;159:577-583.
- Herron MD, Hinckley M, Hoffman MS, et al. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol. 2005;141:1527-1534.
- Qureshi AA, Choi HK, Setty AR, et al. Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch Dermatol. 2009;145:379-382.
- Shapiro J, Cohen AD, David M, et al. The association between psoriasis, diabetes mellitus, and atherosclerosis in Israel: a case-control study. J Am Acad Dermatol. 2007;56:629-634.
- Love TJ, Qureshi AA, Karlson EW, et al. Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, 2003-2006. Arch Dermatol. 2011;147:419-424.
- El-Mongy S, Fathy H, Abdelaziz A, et al. Subclinical atherosclerosis in patients with chronic psoriasis: a potential association. J Eur Acad Dermatol Venereol. 2010;24:661-666.
- Prodanovich S, Kirsner RS, Kravetz JD, et al. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol. 2009;145:700-703.
- Ludwig RJ, Herzog C, Rostock A, et al. Psoriasis: a possible risk factor for development of coronary artery calcification. Br J Dermatol. 2007;156:271-276.
- Kaye JA, Li L, Jick SS. Incidence of risk factors for myocardial infarction and other vascular diseases in patients with psoriasis. Br J Dermatol. 2008;159:895-902.
- Kimball AB, Robinson D Jr, Wu Y, et al. Cardiovascular disease and risk factors among psoriasis patients in two US healthcare databases, 2001-2002. Dermatology. 2008;217:27-37.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Mehta NN, Azfar RS, Shin DB, et al. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31:1000-1006.
- Abuabara K, Azfar RS, Shin DB, et al. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the United Kingdom. Br J Dermatol. 2010;163:586-592.
- Christophers E. Comorbidities in psoriasis. Clin Dermatol. 2007;25:529-534.
- Wu JJ, Nguyen TU, Poon KY, et al. The association of psoriasis with autoimmune diseases. J Am Acad Dermatol. 2012;67:924-930.
- Floch MH, Bina I. The natural history of diverticulitis: fact and theory. Clin Gastroenterol. 2004;38(5, suppl 1):S2-S7.
- Barrea L, Macchia PE, Tarantino G, et al. Nutrition: a key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J Transl Med. 2015;13:303.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. National Survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Matrana MR, Margolin DA. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg. 2009;22:141-146.
- Brandl A, Kratzer T, Kafka-Ritsch R, et al. Diverticulitis in immunosuppressed patients: a fatal outcome requiring a new approach? Can J Surg. 2016;59:254-261.
- Buckius MT, McGrath B, Monk J, et al. Changing epidemiology of acute appendicitis in the United States: study period 1993-2008. J Surg Res. 2012;175:185-190.
- Cheluvappa R, Luo AS, Grimm MC. T helper type 17 pathway suppression by appendicitis and appendectomy protects against colitis. Clin Exp Immunol. 2014;175:316-322.
- Lynde CW, Poulin Y, Vender R, et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141-150.
- Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-α, IFN-γ, IL6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005:2005;273-279.
- Egeberg A, Anderson YMF, Gislason GH, et al. Gallstone risk in adult patients with atopic dermatitis and psoriasis: possible effect of overweight and obesity. Acta Derm Venereol. 2017;97:627-631.
- Smirnova SV, Barilo AA, Smolnikova MV. Hepatobiliary system diseases as the predictors of psoriasis progression [in Russian]. Vestn Ross Akad Med Nauk. 2016:102-108.
- Bagel J, Lynde C, Tyring S, et al. Moderate to severe plaque psoriasis with scalp involvement: a randomized, double-blind, placebo-controlled study of etanercept. J Am Acad Dermatol. 2012;67:86-92.
- Foeldvari I, Krüger E, Schneider T. Acute, non-obstructive, sterile cholecystitis associated with etanercept and infliximab for the treatment of juvenile polyarticular rheumatoid arthritis. Ann Rheum Dis. 2003;62:908-909.
Practice Points
- Patients with psoriasis may have elevated risk of diverticulitis compared to healthy patients. However, psoriasis patients do not appear to have increased risk of appendicitis or cholecystitis.
- Clinicians treating psoriasis patients should consider assessing for other risk factors of diverticulitis at regular intervals.
The Role of Biologic Therapy for Psoriasis in Cardiovascular Risk Reduction
The cardiovascular comorbidities associated with psoriasis have been well documented; however, the mechanism by which psoriasis increases the risk for cardiovascular disease (CVD) remains unclear. Elevated systemic inflammatory cytokines and mediators may play a key role in their association, which prompts the questions: Do systemic medications have a protective effect? Do patients on systemic antipsoriatic treatment have a decreased risk for major adverse cardiovascular events (MACEs) compared with untreated patients?
We believe the shared inflammatory processes involved in psoriasis and atherosclerosis formation are potential targets for therapy in reducing the incidence of CVD and its associated complications. A growing amount of evidence suggests cardioprotective effects associated with antipsoriatic treatments such as tumor necrosis factor (TNF) inhibitors and methotrexate. Gkalpakiotis et al1 demonstrated a reduction in serum E-selectin (mean [standard deviation], 53.04 [23.54] ng/mL vs 35.32 [8.70] ng/mL; P<.001) and IL-22 (25.11 [19.9] pg/mL vs 12.83 [8.42] pg/mL; P<.001) after 3 months of adalimumab administration in patients with moderate to severe psoriasis. Both E-selectin and IL-22 are associated with the development of atherosclerosis, endothelial dysfunction, and an increased incidence of CVD. Similarly, Wu et al2 demonstrated a statistically significant reduction (–5.04 mg/dL [95% confidence interval [CI], –8.24 to –2.12; P<.01) in C-reactive protein in patients with psoriasis, psoriatic arthritis, and rheumatoid arthritis after concurrent use of methotrexate and TNF inhibitors.
Solomon et al3 compared the rate of newly diagnosed diabetes mellitus among psoriasis and rheumatoid arthritis patients treated with TNF inhibitors, methotrexate, hydroxychloroquine, and other nonbiologic disease-modifying antirheumatic drugs. The authors’ findings suggest that those who take a TNF inhibitor (hazard ratio [HR], 0.62; 95% CI, 0.42-0.91) and hydroxychloroquine (HR, 0.54; 95% CI, 0.36-0.80) are at lower risk for diabetes mellitus compared to those treated with nonbiologic disease-modifying antirheumatic drugs. Conversely, the methotrexate (HR, 0.77; 95% CI, 0.53-1.13) cohort did not show a statistically significant reduction in diabetes risk.3
Pina et al4 revealed improvement in endothelial function after 6 months of adalimumab use in patients with moderate to severe psoriasis. To evaluate the presence of subclinical endothelial dysfunction, the authors assessed brachial artery reactivity by measuring flow-mediated dilation and carotid artery stiffness by pulse wave velocity. Patients showed an increase in flow-mediated dilation (mean [SD], 6.19% [2.44%] vs 7.46% [2.43%]; P=.008) and reduction in pulse wave velocity (6.28 [1.04] m/s vs 5.69 [1.31] m/s; P=.03) compared to baseline measurements, indicating an improvement of endothelial function.4
Ahlehoff et al5 observed for improvements in subclinical left ventricular dysfunction in psoriasis patients after treatment with biologics. Using echocardiography, they assessed for changes in diastolic function and left ventricular systolic deformation (defined by global longitudinal strain). Of patients who received 3 months of biologic therapy (TNF inhibitor orIL-12/23 inhibitor) and maintained at minimum a psoriasis area and severity index 50 response, all demonstrated an improvement in diastolic function (mean [SD], 8.1 [2.1] vs 6.7 [1.9]; P<.001) and global longitudinal strain (mean [SD], –16.8% [2.1%] vs –18.3% [2.3%]; P<.001). Of note, patients who did not achieve a psoriasis area and severity index 50 response at follow-up did not exhibit an improvement in subclinical myocardial function.5
Moreover, a Danish nationwide study with up to 5-year follow-up evaluated the risk for MACE (ie, cardiovascular death, myocardial infarction, stroke) in patients with severe psoriasis receiving systemic anti-inflammatory medications and nonsystemic therapies including topical treatments, phototherapy, and climate therapy.6 Compared to nonsystemic therapies, methotrexate use (HR, 0.53; 95% CI, 0.34-0.83) was associated with a decreased risk for cardiovascular events. However, a protective decreased risk was not found among patients who used systemic cyclosporine (HR, 1.06; 95% CI, 0.26-4.27) or retinoids (HR, 1.80; 95% CI, 1.03-2.96). Any biological drug use had a comparable but nonsignificant reduction of cardiovascular events (HR, 0.58; 95% CI, 0.30-1.10). After multivariable adjustment, TNF inhibitors were associated with a statistically significant decreased risk for cardiovascular events (HR, 0.46; 95% CI, 0.22-0.98; P=.04) compared to nonsystemic therapies. The IL-12/23 inhibitor did not demonstrate this relationship (HR, 1.52; 95% CI, 0.47-4.94).6
Lastly, Wu et al7 compared the risk for MACE (ie, myocardial infarction, stroke, unstable angina, transient ischemic attack) between patients with psoriasis who received TNF inhibitors or methotrexate. The TNF inhibitor and methotrexate cohorts were observed for a median of 12 months and 9 months, respectively. After adjusting for potential confounding factors, they found a 45% reduction (HR, 0.55; 95% CI, 0.45-0.67) in cardiovascular event risk in the TNF inhibitor cohort compared with the methotrexate cohort. Notably, analyses also showed comparatively fewer cardiovascular events in the TNF inhibitor cohort throughout all time points—6, 12, 18, 24, 60 months—in the observation period. Regression analysis revealed an 11% reduction in cardiovascular events (HR, 0.89; 95% CI, 0.80-0.98) with each additional 6 months of cumulative TNF inhibitor exposure.
The current sum of evidence suggests cardioprotective effects of TNF inhibitor and methotrexate use. However, given the cumulative systemic toxicity and inferior cutaneous efficacy of methotrexate, TNF inhibitors will likely play a more significant role going forward. The role of methotrexate may be for its simultaneous use with biologic therapies to limit immunogenicity. Newer biologic agents such as IL-12/23 and IL-17 inhibitors have not yet been as extensively studied for their effects on cardiovascular risk as their TNF inhibitor counterparts. However, because of their shared ability to target specific immunological pathways, it is plausible that IL-12/23 and IL-17 agents may exhibit cardioprotective effects.8
Patients with psoriasis should be counseled and educated about the increased risk for CVD and its associated morbidity and mortality risk. Screening for modifiable risk factors and recommending therapeutic lifestyle changes also is appropriate. Future studies should help define the role of specific systemic drugs in reducing the risk for CVD in patients with psoriasis.
- Gkalpakiotis S, Arenbergerova M, Gkalpakioti P, et al. Impact of adalimumab treatment on cardiovascular risk biomarkers in psoriasis: results of a pilot study [published online October 24, 2016]. J Dermatol. doi:10.1111/1346-8138.13661.
- Wu JJ, Rowan CG, Bebchuk JD, et al. Association between tumor necrosis factor inhibitor (TNFi) therapy and changes in C-reactive protein (CRP), blood pressure, and alanine aminotransferase (ALT) among patients with psoriasis, psoriatic arthritis, or rheumatoid arthritis [published online March 5, 2015]. J Am Acad Dermatol. 2015;72:917-919.
- Solomon DH, Massarotti E, Garg R, et al. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA. 2011;305:2525-2531.
- Pina T, Corrales A, Lopez-Mejias R, et al. Anti-tumor necrosis factor-alpha therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: a 6-month prospective study. J Dermatol. 2016;43:1267-1272.
- Ahlehoff O, Hansen PR, Gislason GH, et al. Myocardial function and effects of biologic therapy in patients with severe psoriasis: a prospective echocardiographic study [published online April 6, 2015]. J Eur Acad Dermatol Venereol. 2016;30:819-823.
- Ahlehoff O, Skov L, Gislason G, et al. Cardiovascular outcomes and systemic anti-inflammatory drugs in patients with severe psoriasis: 5-year follow-up of a Danish nationwide cohort [published online October 10, 2014]. J Eur Acad Dermatol Venereol. 2015;29:1128-1134.
- Wu JJ, Guérin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-α inhibitors versus methotrexate [published online October 26, 2016]. J Am Acad Dermatol. 2017;76:81-90.
- Egeberg A, Skov L. Management of cardiovascular disease in patients with psoriasis. Expert Opin Pharmacother. 2016;17:1509-1516.
The cardiovascular comorbidities associated with psoriasis have been well documented; however, the mechanism by which psoriasis increases the risk for cardiovascular disease (CVD) remains unclear. Elevated systemic inflammatory cytokines and mediators may play a key role in their association, which prompts the questions: Do systemic medications have a protective effect? Do patients on systemic antipsoriatic treatment have a decreased risk for major adverse cardiovascular events (MACEs) compared with untreated patients?
We believe the shared inflammatory processes involved in psoriasis and atherosclerosis formation are potential targets for therapy in reducing the incidence of CVD and its associated complications. A growing amount of evidence suggests cardioprotective effects associated with antipsoriatic treatments such as tumor necrosis factor (TNF) inhibitors and methotrexate. Gkalpakiotis et al1 demonstrated a reduction in serum E-selectin (mean [standard deviation], 53.04 [23.54] ng/mL vs 35.32 [8.70] ng/mL; P<.001) and IL-22 (25.11 [19.9] pg/mL vs 12.83 [8.42] pg/mL; P<.001) after 3 months of adalimumab administration in patients with moderate to severe psoriasis. Both E-selectin and IL-22 are associated with the development of atherosclerosis, endothelial dysfunction, and an increased incidence of CVD. Similarly, Wu et al2 demonstrated a statistically significant reduction (–5.04 mg/dL [95% confidence interval [CI], –8.24 to –2.12; P<.01) in C-reactive protein in patients with psoriasis, psoriatic arthritis, and rheumatoid arthritis after concurrent use of methotrexate and TNF inhibitors.
Solomon et al3 compared the rate of newly diagnosed diabetes mellitus among psoriasis and rheumatoid arthritis patients treated with TNF inhibitors, methotrexate, hydroxychloroquine, and other nonbiologic disease-modifying antirheumatic drugs. The authors’ findings suggest that those who take a TNF inhibitor (hazard ratio [HR], 0.62; 95% CI, 0.42-0.91) and hydroxychloroquine (HR, 0.54; 95% CI, 0.36-0.80) are at lower risk for diabetes mellitus compared to those treated with nonbiologic disease-modifying antirheumatic drugs. Conversely, the methotrexate (HR, 0.77; 95% CI, 0.53-1.13) cohort did not show a statistically significant reduction in diabetes risk.3
Pina et al4 revealed improvement in endothelial function after 6 months of adalimumab use in patients with moderate to severe psoriasis. To evaluate the presence of subclinical endothelial dysfunction, the authors assessed brachial artery reactivity by measuring flow-mediated dilation and carotid artery stiffness by pulse wave velocity. Patients showed an increase in flow-mediated dilation (mean [SD], 6.19% [2.44%] vs 7.46% [2.43%]; P=.008) and reduction in pulse wave velocity (6.28 [1.04] m/s vs 5.69 [1.31] m/s; P=.03) compared to baseline measurements, indicating an improvement of endothelial function.4
Ahlehoff et al5 observed for improvements in subclinical left ventricular dysfunction in psoriasis patients after treatment with biologics. Using echocardiography, they assessed for changes in diastolic function and left ventricular systolic deformation (defined by global longitudinal strain). Of patients who received 3 months of biologic therapy (TNF inhibitor orIL-12/23 inhibitor) and maintained at minimum a psoriasis area and severity index 50 response, all demonstrated an improvement in diastolic function (mean [SD], 8.1 [2.1] vs 6.7 [1.9]; P<.001) and global longitudinal strain (mean [SD], –16.8% [2.1%] vs –18.3% [2.3%]; P<.001). Of note, patients who did not achieve a psoriasis area and severity index 50 response at follow-up did not exhibit an improvement in subclinical myocardial function.5
Moreover, a Danish nationwide study with up to 5-year follow-up evaluated the risk for MACE (ie, cardiovascular death, myocardial infarction, stroke) in patients with severe psoriasis receiving systemic anti-inflammatory medications and nonsystemic therapies including topical treatments, phototherapy, and climate therapy.6 Compared to nonsystemic therapies, methotrexate use (HR, 0.53; 95% CI, 0.34-0.83) was associated with a decreased risk for cardiovascular events. However, a protective decreased risk was not found among patients who used systemic cyclosporine (HR, 1.06; 95% CI, 0.26-4.27) or retinoids (HR, 1.80; 95% CI, 1.03-2.96). Any biological drug use had a comparable but nonsignificant reduction of cardiovascular events (HR, 0.58; 95% CI, 0.30-1.10). After multivariable adjustment, TNF inhibitors were associated with a statistically significant decreased risk for cardiovascular events (HR, 0.46; 95% CI, 0.22-0.98; P=.04) compared to nonsystemic therapies. The IL-12/23 inhibitor did not demonstrate this relationship (HR, 1.52; 95% CI, 0.47-4.94).6
Lastly, Wu et al7 compared the risk for MACE (ie, myocardial infarction, stroke, unstable angina, transient ischemic attack) between patients with psoriasis who received TNF inhibitors or methotrexate. The TNF inhibitor and methotrexate cohorts were observed for a median of 12 months and 9 months, respectively. After adjusting for potential confounding factors, they found a 45% reduction (HR, 0.55; 95% CI, 0.45-0.67) in cardiovascular event risk in the TNF inhibitor cohort compared with the methotrexate cohort. Notably, analyses also showed comparatively fewer cardiovascular events in the TNF inhibitor cohort throughout all time points—6, 12, 18, 24, 60 months—in the observation period. Regression analysis revealed an 11% reduction in cardiovascular events (HR, 0.89; 95% CI, 0.80-0.98) with each additional 6 months of cumulative TNF inhibitor exposure.
The current sum of evidence suggests cardioprotective effects of TNF inhibitor and methotrexate use. However, given the cumulative systemic toxicity and inferior cutaneous efficacy of methotrexate, TNF inhibitors will likely play a more significant role going forward. The role of methotrexate may be for its simultaneous use with biologic therapies to limit immunogenicity. Newer biologic agents such as IL-12/23 and IL-17 inhibitors have not yet been as extensively studied for their effects on cardiovascular risk as their TNF inhibitor counterparts. However, because of their shared ability to target specific immunological pathways, it is plausible that IL-12/23 and IL-17 agents may exhibit cardioprotective effects.8
Patients with psoriasis should be counseled and educated about the increased risk for CVD and its associated morbidity and mortality risk. Screening for modifiable risk factors and recommending therapeutic lifestyle changes also is appropriate. Future studies should help define the role of specific systemic drugs in reducing the risk for CVD in patients with psoriasis.
The cardiovascular comorbidities associated with psoriasis have been well documented; however, the mechanism by which psoriasis increases the risk for cardiovascular disease (CVD) remains unclear. Elevated systemic inflammatory cytokines and mediators may play a key role in their association, which prompts the questions: Do systemic medications have a protective effect? Do patients on systemic antipsoriatic treatment have a decreased risk for major adverse cardiovascular events (MACEs) compared with untreated patients?
We believe the shared inflammatory processes involved in psoriasis and atherosclerosis formation are potential targets for therapy in reducing the incidence of CVD and its associated complications. A growing amount of evidence suggests cardioprotective effects associated with antipsoriatic treatments such as tumor necrosis factor (TNF) inhibitors and methotrexate. Gkalpakiotis et al1 demonstrated a reduction in serum E-selectin (mean [standard deviation], 53.04 [23.54] ng/mL vs 35.32 [8.70] ng/mL; P<.001) and IL-22 (25.11 [19.9] pg/mL vs 12.83 [8.42] pg/mL; P<.001) after 3 months of adalimumab administration in patients with moderate to severe psoriasis. Both E-selectin and IL-22 are associated with the development of atherosclerosis, endothelial dysfunction, and an increased incidence of CVD. Similarly, Wu et al2 demonstrated a statistically significant reduction (–5.04 mg/dL [95% confidence interval [CI], –8.24 to –2.12; P<.01) in C-reactive protein in patients with psoriasis, psoriatic arthritis, and rheumatoid arthritis after concurrent use of methotrexate and TNF inhibitors.
Solomon et al3 compared the rate of newly diagnosed diabetes mellitus among psoriasis and rheumatoid arthritis patients treated with TNF inhibitors, methotrexate, hydroxychloroquine, and other nonbiologic disease-modifying antirheumatic drugs. The authors’ findings suggest that those who take a TNF inhibitor (hazard ratio [HR], 0.62; 95% CI, 0.42-0.91) and hydroxychloroquine (HR, 0.54; 95% CI, 0.36-0.80) are at lower risk for diabetes mellitus compared to those treated with nonbiologic disease-modifying antirheumatic drugs. Conversely, the methotrexate (HR, 0.77; 95% CI, 0.53-1.13) cohort did not show a statistically significant reduction in diabetes risk.3
Pina et al4 revealed improvement in endothelial function after 6 months of adalimumab use in patients with moderate to severe psoriasis. To evaluate the presence of subclinical endothelial dysfunction, the authors assessed brachial artery reactivity by measuring flow-mediated dilation and carotid artery stiffness by pulse wave velocity. Patients showed an increase in flow-mediated dilation (mean [SD], 6.19% [2.44%] vs 7.46% [2.43%]; P=.008) and reduction in pulse wave velocity (6.28 [1.04] m/s vs 5.69 [1.31] m/s; P=.03) compared to baseline measurements, indicating an improvement of endothelial function.4
Ahlehoff et al5 observed for improvements in subclinical left ventricular dysfunction in psoriasis patients after treatment with biologics. Using echocardiography, they assessed for changes in diastolic function and left ventricular systolic deformation (defined by global longitudinal strain). Of patients who received 3 months of biologic therapy (TNF inhibitor orIL-12/23 inhibitor) and maintained at minimum a psoriasis area and severity index 50 response, all demonstrated an improvement in diastolic function (mean [SD], 8.1 [2.1] vs 6.7 [1.9]; P<.001) and global longitudinal strain (mean [SD], –16.8% [2.1%] vs –18.3% [2.3%]; P<.001). Of note, patients who did not achieve a psoriasis area and severity index 50 response at follow-up did not exhibit an improvement in subclinical myocardial function.5
Moreover, a Danish nationwide study with up to 5-year follow-up evaluated the risk for MACE (ie, cardiovascular death, myocardial infarction, stroke) in patients with severe psoriasis receiving systemic anti-inflammatory medications and nonsystemic therapies including topical treatments, phototherapy, and climate therapy.6 Compared to nonsystemic therapies, methotrexate use (HR, 0.53; 95% CI, 0.34-0.83) was associated with a decreased risk for cardiovascular events. However, a protective decreased risk was not found among patients who used systemic cyclosporine (HR, 1.06; 95% CI, 0.26-4.27) or retinoids (HR, 1.80; 95% CI, 1.03-2.96). Any biological drug use had a comparable but nonsignificant reduction of cardiovascular events (HR, 0.58; 95% CI, 0.30-1.10). After multivariable adjustment, TNF inhibitors were associated with a statistically significant decreased risk for cardiovascular events (HR, 0.46; 95% CI, 0.22-0.98; P=.04) compared to nonsystemic therapies. The IL-12/23 inhibitor did not demonstrate this relationship (HR, 1.52; 95% CI, 0.47-4.94).6
Lastly, Wu et al7 compared the risk for MACE (ie, myocardial infarction, stroke, unstable angina, transient ischemic attack) between patients with psoriasis who received TNF inhibitors or methotrexate. The TNF inhibitor and methotrexate cohorts were observed for a median of 12 months and 9 months, respectively. After adjusting for potential confounding factors, they found a 45% reduction (HR, 0.55; 95% CI, 0.45-0.67) in cardiovascular event risk in the TNF inhibitor cohort compared with the methotrexate cohort. Notably, analyses also showed comparatively fewer cardiovascular events in the TNF inhibitor cohort throughout all time points—6, 12, 18, 24, 60 months—in the observation period. Regression analysis revealed an 11% reduction in cardiovascular events (HR, 0.89; 95% CI, 0.80-0.98) with each additional 6 months of cumulative TNF inhibitor exposure.
The current sum of evidence suggests cardioprotective effects of TNF inhibitor and methotrexate use. However, given the cumulative systemic toxicity and inferior cutaneous efficacy of methotrexate, TNF inhibitors will likely play a more significant role going forward. The role of methotrexate may be for its simultaneous use with biologic therapies to limit immunogenicity. Newer biologic agents such as IL-12/23 and IL-17 inhibitors have not yet been as extensively studied for their effects on cardiovascular risk as their TNF inhibitor counterparts. However, because of their shared ability to target specific immunological pathways, it is plausible that IL-12/23 and IL-17 agents may exhibit cardioprotective effects.8
Patients with psoriasis should be counseled and educated about the increased risk for CVD and its associated morbidity and mortality risk. Screening for modifiable risk factors and recommending therapeutic lifestyle changes also is appropriate. Future studies should help define the role of specific systemic drugs in reducing the risk for CVD in patients with psoriasis.
- Gkalpakiotis S, Arenbergerova M, Gkalpakioti P, et al. Impact of adalimumab treatment on cardiovascular risk biomarkers in psoriasis: results of a pilot study [published online October 24, 2016]. J Dermatol. doi:10.1111/1346-8138.13661.
- Wu JJ, Rowan CG, Bebchuk JD, et al. Association between tumor necrosis factor inhibitor (TNFi) therapy and changes in C-reactive protein (CRP), blood pressure, and alanine aminotransferase (ALT) among patients with psoriasis, psoriatic arthritis, or rheumatoid arthritis [published online March 5, 2015]. J Am Acad Dermatol. 2015;72:917-919.
- Solomon DH, Massarotti E, Garg R, et al. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA. 2011;305:2525-2531.
- Pina T, Corrales A, Lopez-Mejias R, et al. Anti-tumor necrosis factor-alpha therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: a 6-month prospective study. J Dermatol. 2016;43:1267-1272.
- Ahlehoff O, Hansen PR, Gislason GH, et al. Myocardial function and effects of biologic therapy in patients with severe psoriasis: a prospective echocardiographic study [published online April 6, 2015]. J Eur Acad Dermatol Venereol. 2016;30:819-823.
- Ahlehoff O, Skov L, Gislason G, et al. Cardiovascular outcomes and systemic anti-inflammatory drugs in patients with severe psoriasis: 5-year follow-up of a Danish nationwide cohort [published online October 10, 2014]. J Eur Acad Dermatol Venereol. 2015;29:1128-1134.
- Wu JJ, Guérin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-α inhibitors versus methotrexate [published online October 26, 2016]. J Am Acad Dermatol. 2017;76:81-90.
- Egeberg A, Skov L. Management of cardiovascular disease in patients with psoriasis. Expert Opin Pharmacother. 2016;17:1509-1516.
- Gkalpakiotis S, Arenbergerova M, Gkalpakioti P, et al. Impact of adalimumab treatment on cardiovascular risk biomarkers in psoriasis: results of a pilot study [published online October 24, 2016]. J Dermatol. doi:10.1111/1346-8138.13661.
- Wu JJ, Rowan CG, Bebchuk JD, et al. Association between tumor necrosis factor inhibitor (TNFi) therapy and changes in C-reactive protein (CRP), blood pressure, and alanine aminotransferase (ALT) among patients with psoriasis, psoriatic arthritis, or rheumatoid arthritis [published online March 5, 2015]. J Am Acad Dermatol. 2015;72:917-919.
- Solomon DH, Massarotti E, Garg R, et al. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA. 2011;305:2525-2531.
- Pina T, Corrales A, Lopez-Mejias R, et al. Anti-tumor necrosis factor-alpha therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: a 6-month prospective study. J Dermatol. 2016;43:1267-1272.
- Ahlehoff O, Hansen PR, Gislason GH, et al. Myocardial function and effects of biologic therapy in patients with severe psoriasis: a prospective echocardiographic study [published online April 6, 2015]. J Eur Acad Dermatol Venereol. 2016;30:819-823.
- Ahlehoff O, Skov L, Gislason G, et al. Cardiovascular outcomes and systemic anti-inflammatory drugs in patients with severe psoriasis: 5-year follow-up of a Danish nationwide cohort [published online October 10, 2014]. J Eur Acad Dermatol Venereol. 2015;29:1128-1134.
- Wu JJ, Guérin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-α inhibitors versus methotrexate [published online October 26, 2016]. J Am Acad Dermatol. 2017;76:81-90.
- Egeberg A, Skov L. Management of cardiovascular disease in patients with psoriasis. Expert Opin Pharmacother. 2016;17:1509-1516.