User login
<p>For MD-IQ and ClinicalEdge use only</p>
AGA Clinical Practice Guideline: Coagulation in cirrhosis
A clinical update from the American Gastroenterological Association focuses on bleeding and thrombosis-related questions in patients with cirrhosis. It provides guidance on test strategies for bleeding risk, preprocedure management of bleeding risk, venous thromboembolism (VTE) prophylaxis, screening for portal vein thrombosis (PVT), and anticoagulation therapies. It is aimed at primary care providers, gastroenterologists, and hepatologists, among other health care providers.
In cirrhosis, there are often changes to platelet (PLT) counts and prothrombin time/international normalized ratio (PT/INR), among other parameters, and historically these changes led to concerns that patients were at greater risk of bleeding or thrombosis. More recent evidence has led to a nuanced view. Neither factor necessarily suggests increased bleeding risk, and the severity of coagulopathy predicted by them does not predict the risk of bleeding complications.
Patients with cirrhosis are at greater risk of thrombosis, but clinicians may be hesitant to prescribe anticoagulants because of uncertain risk profiles, and test strategies employing PT/INR to estimate bleeding risk and track treatment endpoints in patients receiving vitamin K antagonists may not work in cirrhosis patients with alterations in procoagulant and anticoagulant measures. Recent efforts to address this led to testing of fibrin clot formation and lysis to better gauge the variety of abnormalities in cirrhosis patients.
The guideline, published in Gastroenterology, was informed by a technical review that focused on both bleeding-related and thrombosis-related questions. Bleeding-related questions included testing strategies and preprocedure prophylaxis to reduce bleeding risk. Thrombosis-related questions included whether VTE prophylaxis may be useful in hospitalized patients with cirrhosis, whether patients should be screened for PVT, potential therapies for nontumoral PVT, and whether or not anticoagulation is safe and effective when atrial fibrillation is present alongside cirrhosis.
Because of a lack of evidence, the guideline provides no recommendations on visco-elastic testing for bleeding risk in advance of common gastrointestinal procedures for patients with stable cirrhosis. It recommends against use of extensive preprocedural testing, such as repeated PT/INR or PLT count testing.
The guideline also looked at whether preprocedural efforts to correct coagulation parameters could reduce bleeding risk in patients with cirrhosis. It recommends against giving blood products ahead of the procedure for patients with stable cirrhosis without severe thrombocytopenia or severe coagulopathy. Such interventions can be considered for patients in the latter categories who are undergoing procedures with high bleeding risk after consideration of risks and benefits, and consultation with a hematologist.
Thrombopoietin receptor agonists (TPO-RAs) are also not recommended in patients with thrombocytopenia and stable cirrhosis undergoing common procedures, but they can be considered for patients who are more concerned about reduction of bleeding events and less concerned about the risk of PVT.
Patients who are hospitalized and meet the requirements should receive VTE prophylaxis. Although there is little available evidence about the effects of thromboprophylaxis in patients with cirrhosis, there is strong evidence of benefit in acutely ill hospitalized patients, and patients with cirrhosis are believed to be at a similar risk of VTE. There is evidence of increased bleed risk, but this is of very low certainty.
PVT should not be routinely tested for, but such testing can be offered to patients with a high level of concern over PVT and are not as worried about potential harms of treatment. This recommendation does not apply to patients waiting for a liver transplant.
Patients with non-umoral PVT should receive anticoagulation therapy, but patients who have high levels of concern about bleeding risk from anticoagulation and put a lower value on possible benefits of anticoagulation may choose not to receive it.
The guideline recommends anticoagulation for patients with atrial fibrillation and cirrhosis who are indicated for it. Patients with more concern about the bleeding risk of anticoagulation and place lower value on the reduction in stroke risk may choose to not receive anticoagulation. This is particularly true for those with more advanced cirrhosis (Child-Turcotte-Pugh Class C) and/or low CHA2DS2-VASC scores.
Nearly all of the recommendations in the guideline are conditional, reflecting a lack of data and a range of knowledge gaps that need filling. The authors call for additional research to identify specific patients who are at high risk for bleeding or thrombosis “to appropriately provide prophylaxis using blood product transfusion or TPO-RAs in patients at risk for clinically significant bleeding, to screen for and treat PVT, and to prevent clinically significant thromboembolic events.”
The development of the guideline was funded fully by the AGA. Members of the panel submitted conflict of interest information, and these statements are maintained at AGA headquarters.
A clinical update from the American Gastroenterological Association focuses on bleeding and thrombosis-related questions in patients with cirrhosis. It provides guidance on test strategies for bleeding risk, preprocedure management of bleeding risk, venous thromboembolism (VTE) prophylaxis, screening for portal vein thrombosis (PVT), and anticoagulation therapies. It is aimed at primary care providers, gastroenterologists, and hepatologists, among other health care providers.
In cirrhosis, there are often changes to platelet (PLT) counts and prothrombin time/international normalized ratio (PT/INR), among other parameters, and historically these changes led to concerns that patients were at greater risk of bleeding or thrombosis. More recent evidence has led to a nuanced view. Neither factor necessarily suggests increased bleeding risk, and the severity of coagulopathy predicted by them does not predict the risk of bleeding complications.
Patients with cirrhosis are at greater risk of thrombosis, but clinicians may be hesitant to prescribe anticoagulants because of uncertain risk profiles, and test strategies employing PT/INR to estimate bleeding risk and track treatment endpoints in patients receiving vitamin K antagonists may not work in cirrhosis patients with alterations in procoagulant and anticoagulant measures. Recent efforts to address this led to testing of fibrin clot formation and lysis to better gauge the variety of abnormalities in cirrhosis patients.
The guideline, published in Gastroenterology, was informed by a technical review that focused on both bleeding-related and thrombosis-related questions. Bleeding-related questions included testing strategies and preprocedure prophylaxis to reduce bleeding risk. Thrombosis-related questions included whether VTE prophylaxis may be useful in hospitalized patients with cirrhosis, whether patients should be screened for PVT, potential therapies for nontumoral PVT, and whether or not anticoagulation is safe and effective when atrial fibrillation is present alongside cirrhosis.
Because of a lack of evidence, the guideline provides no recommendations on visco-elastic testing for bleeding risk in advance of common gastrointestinal procedures for patients with stable cirrhosis. It recommends against use of extensive preprocedural testing, such as repeated PT/INR or PLT count testing.
The guideline also looked at whether preprocedural efforts to correct coagulation parameters could reduce bleeding risk in patients with cirrhosis. It recommends against giving blood products ahead of the procedure for patients with stable cirrhosis without severe thrombocytopenia or severe coagulopathy. Such interventions can be considered for patients in the latter categories who are undergoing procedures with high bleeding risk after consideration of risks and benefits, and consultation with a hematologist.
Thrombopoietin receptor agonists (TPO-RAs) are also not recommended in patients with thrombocytopenia and stable cirrhosis undergoing common procedures, but they can be considered for patients who are more concerned about reduction of bleeding events and less concerned about the risk of PVT.
Patients who are hospitalized and meet the requirements should receive VTE prophylaxis. Although there is little available evidence about the effects of thromboprophylaxis in patients with cirrhosis, there is strong evidence of benefit in acutely ill hospitalized patients, and patients with cirrhosis are believed to be at a similar risk of VTE. There is evidence of increased bleed risk, but this is of very low certainty.
PVT should not be routinely tested for, but such testing can be offered to patients with a high level of concern over PVT and are not as worried about potential harms of treatment. This recommendation does not apply to patients waiting for a liver transplant.
Patients with non-umoral PVT should receive anticoagulation therapy, but patients who have high levels of concern about bleeding risk from anticoagulation and put a lower value on possible benefits of anticoagulation may choose not to receive it.
The guideline recommends anticoagulation for patients with atrial fibrillation and cirrhosis who are indicated for it. Patients with more concern about the bleeding risk of anticoagulation and place lower value on the reduction in stroke risk may choose to not receive anticoagulation. This is particularly true for those with more advanced cirrhosis (Child-Turcotte-Pugh Class C) and/or low CHA2DS2-VASC scores.
Nearly all of the recommendations in the guideline are conditional, reflecting a lack of data and a range of knowledge gaps that need filling. The authors call for additional research to identify specific patients who are at high risk for bleeding or thrombosis “to appropriately provide prophylaxis using blood product transfusion or TPO-RAs in patients at risk for clinically significant bleeding, to screen for and treat PVT, and to prevent clinically significant thromboembolic events.”
The development of the guideline was funded fully by the AGA. Members of the panel submitted conflict of interest information, and these statements are maintained at AGA headquarters.
A clinical update from the American Gastroenterological Association focuses on bleeding and thrombosis-related questions in patients with cirrhosis. It provides guidance on test strategies for bleeding risk, preprocedure management of bleeding risk, venous thromboembolism (VTE) prophylaxis, screening for portal vein thrombosis (PVT), and anticoagulation therapies. It is aimed at primary care providers, gastroenterologists, and hepatologists, among other health care providers.
In cirrhosis, there are often changes to platelet (PLT) counts and prothrombin time/international normalized ratio (PT/INR), among other parameters, and historically these changes led to concerns that patients were at greater risk of bleeding or thrombosis. More recent evidence has led to a nuanced view. Neither factor necessarily suggests increased bleeding risk, and the severity of coagulopathy predicted by them does not predict the risk of bleeding complications.
Patients with cirrhosis are at greater risk of thrombosis, but clinicians may be hesitant to prescribe anticoagulants because of uncertain risk profiles, and test strategies employing PT/INR to estimate bleeding risk and track treatment endpoints in patients receiving vitamin K antagonists may not work in cirrhosis patients with alterations in procoagulant and anticoagulant measures. Recent efforts to address this led to testing of fibrin clot formation and lysis to better gauge the variety of abnormalities in cirrhosis patients.
The guideline, published in Gastroenterology, was informed by a technical review that focused on both bleeding-related and thrombosis-related questions. Bleeding-related questions included testing strategies and preprocedure prophylaxis to reduce bleeding risk. Thrombosis-related questions included whether VTE prophylaxis may be useful in hospitalized patients with cirrhosis, whether patients should be screened for PVT, potential therapies for nontumoral PVT, and whether or not anticoagulation is safe and effective when atrial fibrillation is present alongside cirrhosis.
Because of a lack of evidence, the guideline provides no recommendations on visco-elastic testing for bleeding risk in advance of common gastrointestinal procedures for patients with stable cirrhosis. It recommends against use of extensive preprocedural testing, such as repeated PT/INR or PLT count testing.
The guideline also looked at whether preprocedural efforts to correct coagulation parameters could reduce bleeding risk in patients with cirrhosis. It recommends against giving blood products ahead of the procedure for patients with stable cirrhosis without severe thrombocytopenia or severe coagulopathy. Such interventions can be considered for patients in the latter categories who are undergoing procedures with high bleeding risk after consideration of risks and benefits, and consultation with a hematologist.
Thrombopoietin receptor agonists (TPO-RAs) are also not recommended in patients with thrombocytopenia and stable cirrhosis undergoing common procedures, but they can be considered for patients who are more concerned about reduction of bleeding events and less concerned about the risk of PVT.
Patients who are hospitalized and meet the requirements should receive VTE prophylaxis. Although there is little available evidence about the effects of thromboprophylaxis in patients with cirrhosis, there is strong evidence of benefit in acutely ill hospitalized patients, and patients with cirrhosis are believed to be at a similar risk of VTE. There is evidence of increased bleed risk, but this is of very low certainty.
PVT should not be routinely tested for, but such testing can be offered to patients with a high level of concern over PVT and are not as worried about potential harms of treatment. This recommendation does not apply to patients waiting for a liver transplant.
Patients with non-umoral PVT should receive anticoagulation therapy, but patients who have high levels of concern about bleeding risk from anticoagulation and put a lower value on possible benefits of anticoagulation may choose not to receive it.
The guideline recommends anticoagulation for patients with atrial fibrillation and cirrhosis who are indicated for it. Patients with more concern about the bleeding risk of anticoagulation and place lower value on the reduction in stroke risk may choose to not receive anticoagulation. This is particularly true for those with more advanced cirrhosis (Child-Turcotte-Pugh Class C) and/or low CHA2DS2-VASC scores.
Nearly all of the recommendations in the guideline are conditional, reflecting a lack of data and a range of knowledge gaps that need filling. The authors call for additional research to identify specific patients who are at high risk for bleeding or thrombosis “to appropriately provide prophylaxis using blood product transfusion or TPO-RAs in patients at risk for clinically significant bleeding, to screen for and treat PVT, and to prevent clinically significant thromboembolic events.”
The development of the guideline was funded fully by the AGA. Members of the panel submitted conflict of interest information, and these statements are maintained at AGA headquarters.
FROM GASTROENTEROLOGY
Lipoprotein(a) Elevation: A New Diagnostic Code with Relevance to Service Members and Veterans (FULL)
Cardiovascular disease (CVD) remains the leading cause of global mortality. In 2015, 41.5% of the US population had at least 1 form of CVD and CVD accounted for nearly 18 million deaths worldwide.1,2 The major disease categories represented include myocardial infarction (MI), sudden death, strokes, calcific aortic valve stenosis (CAVS), and peripheral vascular disease.1,2 In terms of health care costs, quality of life, and caregiver burden, the overall impact of disease prevalence continues to rise.1,3-6 There is an urgent need for more precise and earlier CVD risk assessment to guide lifestyle and therapeutic interventions for prevention of disease progression as well as potential reversal of preclinical disease. Even at a young age, visible coronary atherosclerosis has been found in up to 11% of “healthy” active individuals during autopsies for trauma fatalities.7,8
The impact of CVD on the US and global populations is profound. In 2011, CVD prevalence was predicted to reach 40% by 2030.9 That estimate was exceeded in 2015, and it is now predicted that by 2035, 45% of the US population will suffer from some form of clinical or preclinical CVD. In 2015, the decadeslong decline in CVD mortality was reversed for the first time since 1969, showing a 1% increase in deaths from CVD.1 Nearly 300,000 of those using US Department of Veterans Affairs (VA) services were hospitalized for CVD between 2010 and 2014.10 The annual direct and indirect costs related to CVD in the US are estimated at $329.7 billion, and these costs are predicted to top $1 trillion by 2035.1 Heart attack, coronary atherosclerosis, and stroke accounted for 3 of the 10 most expensive conditions treated in US hospitals in 2013.11 Globally, the estimate for CVD-related direct and indirect costs was $863 billion in 2010 and may exceed $1 trillion by 2030.12
The nature of military service adds additional risk factors, such as posttraumatic stress disorder, depression, sleep disorders and physical trauma which increase CVD morbidity/ mortality in service members, veterans, and their families.13-16 In addition, living in lowerincome areas (countries or neighborhoods) can increase the risk of both CVD incidence and fatalities, particularly in younger individuals.17-20 The Military Health System (MHS) and VA are responsible for the care of those individuals who have voluntarily taken on these additional risks through their time in service. This responsibility calls for rapid translation to practice tools and resources that can support interventions to minimize as many modifiable risk factors as possible and improve longterm health. This strategy aligns with the World Health Organization’s (WHO) focus on prevention of disease progression through interventions targeting modifiable risk.3-6,21-23 The driving force behind the launch of the US Department of Health and Human Services (HHS) Million Hearts program was the goal of preventing 1 million heart attacks and strokes by 2017 with risk reduction through aspirin, blood pressure control, cholesterol management, smoking cessation, sodium reduction, and physical activity.24,25 While some reductions in CVD events have been documented, the outcomes fell short of the goals set, highlighting both the need and value of continued and expanded efforts for CVD risk reduction.26
More precise assessment of risk factors during preventative care, as well as after a diagnosis of CVD, may improve the timeliness and precision of earlier interventions (both lifestyle and therapeutic) that reduce CVD morbidity and mortality.27 Personalized or precision medicine approaches take into account differences in socioeconomic, environmental, and lifestyle factors that are potentially reversible, as well as gender, race, and ethnicity.28-31 Current methods of predicting CVD risk have considerable room for improvement.27 About 40% of patients with newly diagnosed CVD have normal traditional cholesterol profiles, including those whose first cardiac event proves fatal.29-33 Currently available risk scores (hundreds have been described in the literature) mischaracterize risk in minority populations and women, and have shown deficiencies in identifying preclinical atherosclerosis.34,35 The failure to recognize preclinical CVD in military personnel during their active duty life cycle results in missed opportunities for improved health and readiness sustainment.
Most CVD risk prediction models incorporate some form of blood lipids. Total cholesterol (TC) is most commonly used in clinical practice, along with high-density lipoprotein (HDLC), low-density lipoprotein (LDLC), and triglycerides (TG).23,27,36 High LDLC and/or TC are well established as lipid-related CVD risk factors and are incorporated into many CVD risk scoring systems/models described in the literature.27 LDLC reduction is commonly recommended as CVD prevention, but even with optimal statin treatment, there is still considerable residual risk for new and recurrent CVD events.28,32,34,35,37-42
Incorporating novel biomarkers and alternative lipid measurements may improve risk prediction and aid targeted treatment, ultimately reducing CVD events.27 Apolipoprotein B (ApoB) is a major atherogenic component embedded in LDL and VLDL correlating to non-HDLC and may be useful in the setting of triglycerides ≥ 200 mg/d as levels > 130 mg/ dL appear to be risk-enhancing, but measurements may be unreliable.43 According to the 2018 Cholesterol Guidelines, lipoprotein(a) [Lp(a)] elevation also is recognized as a risk-enhancing factor that is particularly implicated when there is a strong family history of premature atherosclerotic CVD or personal history of CVD not explained by major risk factors.43
Lp(a) elevation is a largely underrecognized category of lipid disorder that impacts up to 20% to 30% of the population globally and within the US, although there is considerable variability by geographic location and ethnicity.44 Globally, Lp(a) elevation places > 1 billion people at moderate to high risk for CVD.44 Lp(a) has a strong genetic component and is recognized as a distinct and independent risk factor for MI, sudden death, strokes and CAVS. Lp(a) has an extensive body of evidence to support its distinct role both as a causal factor in CVD and as an augmentation to traditional risk factors.44-48
Lipoproteni(a) Elevation Use For Diagnosis
The importance of Lp(a) elevation as a clinical diagnosis rather than a laboratory abnormality alone was brought forward by the Lipoprotein(a) Foundation. Its founder, Sandra Tremulis, is a survivor of an acute coronary event that occurred when she was 39-years old, despite running marathons and having none of the traditional CVD lifestyle risk factors.49 This experience inspired her to create the Lipoprotein(a) Foundation to give a voice to families living with or at risk for CVD due to Lp(a) elevation.
As often happens in the progress of medicine, patients and their families drive change based on their personal experiences with the gaps in standard clinical practice. It was this foundation—not a member of the medical establishment—that submitted the formal request for the addition of new ICD-10-CM diagnostic and family history codes for Lp(a) elevation during the Centers for Disease Control and Prevention (CDC) September 2017 ICD-10-CM Coordination and Maintenance Committee meeting.50 In June 2018, the final ICD-10-CM code addenda for 2019 was released and included the new codes E78.41 (Elevated Lp[a]) and Z83.430 (Family history of elevated Lp[a]).52 After the new codes were approved, both the American Heart Association and the National Lipid Association added recommendations regarding Lp(a) testing to their clinical practice guidelines.43,52
Practically, these codes standardize billing and payment for legitimate clinical work and laboratory testing. Prior to the addition of Lp(a) elevation as a clinical diagnosis, testing and treatment of Lp(a) elevation was considered experimental and not medically necessary until after a cardiovascular event had already occurred. Services for Lp(a) elevation were therefore not reimbursed by many healthcare organizations and insurance companies. The new ICD-10-CM codes encourage the assessment of Lp(a) both in individuals with early onset major CVD events and in presumably fit, healthy individuals, particularly when there is a family history of Lp(a) elevation. Given that Lp(a) levels do not change significantly over time, the current understanding is that only a single measurement is needed to define the individual risk over a lifetime.41,42,44,45 As therapies targeting Lp(a) levels evolve, repeated measurements may be indicated to monitor response and direct changes in management. “Elevated Lipoprotein(a)” is the first laboratory testing abnormality that has achieved the status of a clinical diagnosis.
Lp(a) Measurements
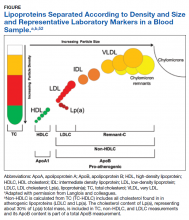
There is considerable complexity to the measurement of lipoproteins in blood samples due to heterogeneity in both density and size of particles as illustrated in the Figure.53
For traditional lipids measured in clinical practice, the size and density ranges from small high-density lipoprotein (HDL) through LDLC and intermediate- density lipoprotein (IDL) to the largest least dense particles in the very low-density lipoprotein (VLDL) and chylomicron remnant fractions. Standard lipid profiles consist of mass concentration measurements (mg/dL) of TC, TG, HDLC, and LDLC.53 Non-HDLC (calculated as: TC−HDLC) consists of all cholesterol found in atherogenic lipoproteins, including remnant-C and Lp(a). Until recently, the cholesterol content of Lp(a), corresponding to about 30% of Lp(a) total mass, was included in the TC, non-HDLC and LDLC measurements with no separate reporting by the majority of clinical laboratories.
After > 50 years of research on the structure and biochemistry of Lp(a), the physiology and biological functions of these complex and polymorphic lipoprotein particles are not fully understood. Lp(a) is composed of a lipoprotein particle similar in composition to LDL (protein and lipid), containing 1 molecule of ApoB wrapped around a core of cholesteryl ester and triglyceride with phospholipids and unesterified cholesterol at its surface.48 The presence of a unique hydrophilic, highly glycosylated protein referred to as apolopoprotienA (apo[a]), covalently attached to ApoB-100 by a single disulfide bridge, differentiates Lp(a) from LDL.48 Cholesterol rich ApoB is an important component within many lipoproteins pathogenic for atherosclerosis and CVD.45,47,53
The apo(a) contributes to the increased density of Lp(a) compared to LDLC with associated reduced binding affinity to the LDL receptor. This reduced receptor binding affinity is a presumed mechanism for the lack of Lp(a) plasma level response to statin therapies, which increase hepatic LDL receptor activity.47 Apo(a) evolved from the plasminogen gene through duplication and remodeling and demonstrates extensive heterogeneity in protein size, with > 40 different apo(a) isoforms resulting in > 40 different Lp(a) particle sizes. Size of the apo(a) particle is determined by the number of pleated structures known as kringles. Most people (> 80%) carry 2 different-sized apo(a) isoforms. Plasma Lp(a) level is determined by the net production of apo(a) in each isoform, and the smaller apo(a) isoforms are associated with higher plasma levels of Lp(a).45
Given the heterogeneity in Lp(a) molecular weight, which can vary even within individuals, recommendations have been made for reporting results as particle numbers or concentrations (nmol/L or mmol/L) rather than as mass concentration (mg/dL).55 However, the majority of the large CVD morbidity and mortality outcomes studies used Lp(a) mass concentration levels in mg/ dL to characterize risk levels.56,57 There is no standardized method to convert Lp(a) measurements from mg/dL to nmol/L.55 Current assays using WHO standardized reagents and controls are reliable for categorizing risk levels.58
The European Atherosclerosis Society consensus panel recommended that desirable Lp(a) levels should be below the 80th percentile (< 50 mg/dL or < 125 nmol/L) in patients with intermediate or high CVD risk.59 Subsequent epidemiological and Mendelian randomization studies have been performed in general populations with no history of CVD and demonstrated that increased CVD risk can be detected with Lp(a) levels as low as 25 to 30 mg/dL.56,60-63 In secondary prevention populations with prior CVD and optimal treatment (statins, antiplatelet drugs), recurrent event risk was also increased with elevated Lp(a).63-66
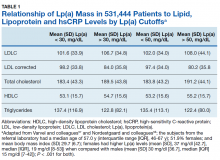
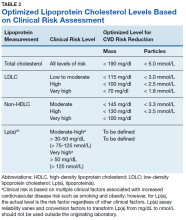
Using immunoturbidometric assays, Varvel and colleagues reported the prevalence of elevated Lp(a) mass concentration levels (mg/dL) in > 500,000 US patients undergoing clinical evaluations based on data from a referral laboratory of patients.58 The mean Lp(a) levels were 34.0 mg/dL with median (interquartile range [IQR]) levels at 17 (7-47) mg/dL and overall range of 0 to 907 mg/dL.58 Females had higher Lp(a) levels compared to males but no ethnic or racial breakdown was provided. Lp(a) levels > 30 mg/dL and > 50 mg/dL were present in 35% and 24% of subjects, respectively. Table 1 displays the relationship between various Lp(a) level cut-offs to mean levels of LDLC, estimated LDLC corrected for Lp(a), TC, HDLC, and TG.58 The data demonstrate that Lp(a) elevation cannot be inferred from LDLC levels nor from any of the other traditional lipoprotein measures. Patients with high risk Lp(a) levels may have normal LDLC. While Lp(a) thresholds have been identified for stratification of CVD risk, the target levels for risk reduction have not been specifically defined, particularly since therapies are not widely available for reduction of Lp(a). Table 2 provides an overview of clinical lipoprotein measurements that may be reasonable targets for therapeutic interventions and reduction of CVD risk.44,53,55 In general, existing studies suggest that radical reduction (> 80%) is required to impact long-term outcomes, particularly in individuals with severe disease.68,69
LDLC reduction alone leaves a residual CVD risk that is greater than the risk reduced.40 In addition, the autoimmune inflammation and lipid specific autoantibodies play an important role in increased CVD morbidity and mortality risk.70,71 The presence of autoantibodies such as antiphospholipid antibodies (without a specific autoimmune disease diagnosis) increases the risk of subclinical atherosclerosis.72,73 Certain autoimmune diseases such as systemic lupus erythematosus are recognized as independent risk factors for CVD.74,75 Autoantibodies appear to mediate CVD events and mortality risk, independent of traditional therapies for risk reduction.73 Further research is needed to clarify the role of autoantibodies as markers of increased or decreased CVD risk and their mechanism of action.
Autoantibodies directed at new antigens in lipoproteins within atherosclerotic lesions can modulate the impact of atherosclerosis via activation of the innate and adaptive immune system.76 The lipid-associated neopeptides are recognized as damage-associated or danger- associated molecular patterns (DAMPs), also known as alarmins, which signal molecules that can trigger and perpetuate noninfectious inflammatory responses.77-79 Plasma autoantibodies (immunoglobulin M and G [IgM, IgG]) modify proinflammatory oxidation-specific epitopes on oxidized phospholipids (oxPL) within lipoproteins and are linked with markers of inflammation and CVD events.80-82 Modified LDLC and ApoB-100 immune complexes with specific autoantibodies in the IgG class are associated with increased CVD.76 These and other risk-modulating autoantibodies may explain some of the variability in CVD outcomes by ethnicity and between individuals.
Some antibodies to oxidized LDL (ox-LDL) may have a protective role in the development of atherosclerosis.83,84 In a cohort of > 500 women, the number of carotid atherosclerotic plaques and total carotid plaque area were inversely correlated with a specific IgM autoantibody (MDA-p210).84 High concentrations of Lp(a)- containing circulating immune complexes and Lp(a)-specific IgM and IgG have been described in patients with coronary heart disease (CHD).85 Like ox-LDL, oxidized Lp(a) [ox-Lp(a)] is more potent than native Lp(a) in increasing atherosclerosis risk and is increased in patients with CHD compared to healthy controls.86-88 Ox-Lp(a) levels may represent an even stronger risk marker for CVD than ox-LDL.85
Possible Mechanisms of Pathogenesis
While the precise quantification of Lp(a) in human plasma (or serum) has been challenging, current clinical laboratories use standardized international reference reagents and controls in their assays. Most current Lp(a) assays are based on immunological methods (eg, immunonephelometry, immunoturbidimetry, or enzyme linked immunosorbent assay [ELISA]) using antibodies against apo(a).89 Apo(a) contains 10 subtypes of kringle IV and 1 copy of kringle V. Some assays use antibodies against kringle-IV type 2; however, it has been recommended that newer methods should use antibodies against the specific bridging kringle-IV Type 9 domain, which has a more stable bond and is present as a single copy.48,89 Other approaches to Lp(a) measurement include ultraperformance liquid chromatography/mass spectrometry that can determine both the concentration and particle size of apo(a).48,90 For routine clinical care, currently available assays reporting in mg/dL can be considered fairly accurate for separating low-risk from moderate-to-high-risk patients.45
The physiologic role of Lp(a) in humans remains to be fully defined and individuals with extremely low plasma Lp(a) levels present no disease or deficiency syndromes.91 Lp(a) accumulates in endothelial injuries and binds to components of the vessel wall and subendothelial matrix, presumably due to the strong lysine binding site in apo(a).46 Mediated by apo(a), the binding stimulates chemotactic activation of monocytes/macrophages and thereby modulating angiogenesis and inflammation.89 Lp(a) may contribute to CVD and CAVS via its LDL-like component, with proinflammatory effects of oxidized phospholipids (OxPL) on both ApoB and apo(a) and antifibrinolytic/prothrombotic effects of apo(a).92 In Vitro studies have demonstrated that apo(a) modifies cellular function of cultured vascular endothelial cells (promoting stress fiber formation, endothelial contraction and vascular permeability), smooth muscles, and monocytes/ macrophages (promoting differentiation of proinflammatory M1-1 type macrophages) via complex mechanisms of cell signaling and cytokine production.89 Lp(a) is the only monogenetic risk factor for aortic valve calcification and stenosis93 and is strongly linked specifically with the single nucleotide polymorphism (SNP) rs10455872 in the gene LPA encoding for apo(a).94
CVD Risk Predictive Value
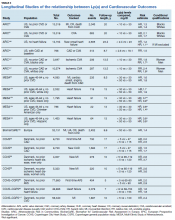
There are a large number of studies demonstrating that Lp(a) elevations are an independent predictor of adverse cardiovascular outcomes including MI, sudden death, strokes, calcific aortic valve stenosis and peripheral vascular disease (Table 3). The Copenhagen City Heart Study and Copenhagen General Population Study are well known prospective population- based cohort studies that track outcomes through national patient registries.95 These studies demonstrate increased risk for MI, CHD, CAVS, and heart failure when subjects with very high Lp(a) levels (50-115 mg/dL) are compared with subjects with very low Lp(a) levels (< 5 mg/dL).96-100 Subjects with less extreme Lp(a) elevations (> 30 mg/dL) also show increased risk of CVD when they have comorbid LDLC elevations.101 However, the Copenhagen studies are composed exclusively of white subjects and the effects of Lp(a) are known to vary with race or ethnicity.
The Multi-Ethnic Study of Atherosclerosis (MESA) recruited an ethnically diverse sample of > 6,000 Americans, aged 45 to 84 years, without CVD, into an ongoing prospective cohort study. Research using subjects from this study has found consistently increased risk of CHD, heart failure, subclinical aortic valve calcification, and more severe CAVS in white subjects with elevated Lp(a).60,102,103 Black subjects with elevated Lp(a) had increased risk of CHD and more severe CAVS and Hispanic subjects with Lp(a) elevation were at higher risk for CHD.60,102 So far, no studies of MESA subjects have identified a relationship between Lp(a) elevation and CVD events for Asian-Americans subjects (predominantly of Chinese descent). There is a need for ongoing research to more precisely define relevant cut-off levels by race, ethnicity and sex.
The Atherosclerosis Risk in Communities (ARIC) Study was a prospective multiethnic cohort study including > 15,000 US adults, aged 45 to 64 years.103 Lp(a) elevations in this cohort were associated with greater risks for first CVD events, heart failure, and recurrent CVD events.61,64,105 The risk of stroke for subjects with elevated Lp(a) was greater for black and white women, and for black men.61,106 However, a meta-analysis of case-control studies showed increased ischemic stroke risk in both men and women with elevated Lp(a).57
A recent European meta-analysis collected blood samples and outcome data from > 50,000 subjects in 7 prospective cohort studies. Using a central laboratory to standardize Lp(a) measurements, researchers found increased risk of major coronary events and new CVD in subjects with Lp(a) > 50 mg/dL compared to those below that threshold.107
Although many of these studies show modest increases in risk of CVD events with Lp(a) elevation, it should be noted that other studies do not demonstrate such consistent associations. This is particularly true in studies of women and nonwhite ethnic groups.103,108-112 The variability of study results may be due to other confounding factors such as autoantibodies that either upregulate or downregulate atherogenicity of LDLC and potentially other lipoproteins. This is particularly relevant to women who have an increased risk for autoimmune disease.
Lp(a) has significant genetic heritability—75% in Europeans and 85% in African Americans.113 In whites, the LPA gene on chromosome 6p26- 27 with the polymorphism genetic variants rs10455872 and rs3798220 is consistently associated with elevated Lp(a) levels.63,100,113 However, the degree of Lp(a) elevation associated with these specific genetic variants varies by ethnicity.78,113,115
Lifestyle and Cardiovascular Health
It is noteworthy that the Lp(a) genetic risks can also be modified by lifestyle risk reduction even in the absence of significant blood level reductions. For example, Khera and colleagues constructed a genetic risk profile for CVD that included genes related to Lp(a).116 Subjects with high genetic risk were more likely to experience CVD events compared with subjects with low genetic risk. However, risks for CVD were attenuated by 4 healthy lifestyle factors: current nonsmoker, body mass index < 30, at least weekly physical activity, and a healthy diet. Subjects with high genetic risk and an unhealthy lifestyle (0 or 1 of the 4 healthy lifestyle factors) were the most likely to develop CVD (Hazard ratio [HR], 3.5), but that risk was lower for subjects with healthy (3 or 4 of the 4 healthy lifestyle factors) and intermediate lifestyles (2 of the 4 healthy lifestyle factors) (HR, 1.9 and 2.2, respectively), despite despite high genetic risk for CVD.
While the independent CVD risk associated with elevated Lp(a) does not appear to be responsive to lifestyle risk reduction alone, certainly elevated LDLC and traditional risk factors can increase the overall CVD risk and are worthy of preventive interventions. In particular, inflammation from any source exacerbates CVD risk. Proatherogenic diet, insufficient sleep, lack of exercise, and maladaptive stress responses are other targets for personalized CVD risk reduction. 28,117 Studies of dietary modifications and other lifestyle factors have shown reduced risk of CVD events, despite lack of reduction in Lp(a) levels.119,120 It is noteworthy that statin therapy (with or without ezetimibe) fails to impact CAVS progression, likely because statins either raise or have no effect on Lp(a) levels.92,119
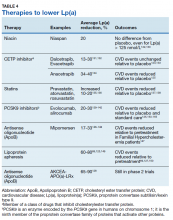
Until recently, there has been no evidence supporting any therapeutic intervention causing clinically meaningful reductions in Lp(a). Table 4 lists major drug classes and their effects on Lp(a) and CVD outcomes; however, a detailed discussion of each of these therapies is beyond the scope of this review. Drugs that reduce Lp(a) by 20-30% have varying effects on CVD outcomes, from no effect122,123 to a 10% to 20% decrease in CVD events when compared with a placebo.124,125 Because these drugs also produce substantial reductions in LDLC, it is not possible to determine how much of the beneficial effects are due to reductions in Lp(a).
Lipoprotein apheresis produces profound reductions in Lp(a) of 60 to 80% in very highrisk populations.69,126 Within-subjects comparisons show up to 80% reductions in CVD events, relative to event rates prior to treatment initiation.69,127 Early trials of antisense oligonucleotide against apo(a) therapies show potential to produce similar outcomes.128,129 These treatments may be particularly effective in patients with isolated Lp(a) elevations.
Summary
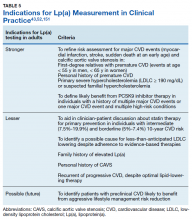
Lp(a) elevation is a major contributor to cardiovascular disease risk and has been recognized as an ICD-10-CM coded clinical diagnosis, the first laboratory abnormality to be defined a clinical disease in the asymptomatic healthy young individuals. This change addresses currently under- diagnosed CVD risk independent of LDLC reduction strategies. A brief overview of recent guidelines for the clinical use of Lp(a) testing from the American Heart Association43,151 and the National Lipid Association52 can be found in Table 5. Although drug therapies for lowering Lp(a) levels remain limited, new treatment options are actively being developed.
Many Americans with high Lp(a) have not yet been identified. Expanded one-time screening can inform these patients of their cardiovascular risk and increase their access to early, aggressive lifestyle modification and optimal lipid-lowering therapy. Given the further increased CVD risk factors for military service members and veterans, a case can be made for broader screening and enhanced surveillance of elevated Lp(a) in these presumably healthy and fit individuals as well as management focused on modifiable risk factors.
Acknowledgements
This program initiative was conducted by the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. as part of the Integrative Cardiac Health Project at Walter Reed National Military Medical Center (WRNMMC), and is made possible by a cooperative agreement that was awarded and administered by the US Army Medical Research & Materiel Command (USAMRMC), at Fort Detrick under Contract Number: W81XWH-16-2-0007. It reflects literature review preparatory work for a research protocol but does not involve an actual research project. The work in this manuscript was supported by the staff of the Integrative Cardiac Health Project (ICHP) with special thanks to Claire Fuller, Elaine Walizer, Dr. Mariam Kashani and the entire health coaching team.
1. American Heart Association. Cardiovascular disease: a costly burden for America, projections through 2035. http://www.heart.org/idc/groups /heart-public/@wcm/@adv/documents/downloadable /ucm_491543.pdf. Accessed October 10, 2019.
2. Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67-e492.
3. Roth GA, Johnson C, Abajobir A, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70(1):1-25.
4. Thrift AG, Cadilhac DA, Thayabaranathan T, et al. Global stroke statistics. Int J Stroke. 2014;9(1):6-18.
5. Murray CJ, Barber RM, Foreman KJ, et al; GBD 2013 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990-2013: quantifying the epidemiological transition. Lancet. 2015;386(10009):2145-2191.
6. Mukherjee D, Patil CG. Epidemiology and the global burden of stroke. World Neurosurg. 2011;76(6 suppl):S85-S90.
7. Joseph A, Ackerman D, Talley JD, Johnstone J, Kupersmith J. Manifestations of coronary atherosclerosis in young trauma victims—an autopsy study. J Am Coll Cardiol. 1993;22(2):459-467.
8. Webber BJ, Seguin PG, Burnett DG, Clark LL, Otto JL. Prevalence of and risk factors for autopsy-determined atherosclerosis among US service members, 2001-2011. JAMA. 2012;308(24):2577-2583.
9. Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123(8):933-944.
10. Krishnamurthi N, Francis J, Fihn SD, Meyer CS, Whooley MA. Leading causes of cardiovascular hospitalization in 8.45 million US veterans. PLoS One. 2018;13(3):e0193996.
11. Torio CM, Moore BJ. National inpatient hospital costs: the most expensive conditions by payer. Agency for Healthcare Research and Quality Statistical Brief No. 204. http:// www.hcup-us.ahrq.gov/reports/statbriefs/sb204-Most -Expensive-Hospital-Conditions.pdf. Published May 2016. Accessed October 10, 2019.
12. Bloom DE, Cafiero ET, Jané-Llopis E, et al. The global economic burden of noncommunicable diseases. https:// www.weforum.org/reports/global-economic-burden-non -communicable-diseases. Published September 18, 2011. Accessed October 10, 2019.
13. Crum-Cianflone NF, Bagnell ME, Schaller E, et al. Impact of combat deployment and posttraumatic stress disorder on newly reported coronary heart disease among US active duty and reserve forces. Circulation. 2014;129(18):1813-1820.
14. Fryar CD, Herrick K, Afful J, Ogden CL. Cardiovascular disease risk factors among male veterans, U.S., 2009- 2012. Am J Prev Med. 2016;50(1):101-105.
15. Ulmer CS, Bosworth HB, Germain A, et al; VA Mid-Atlantic Mental Illness Research Education and Clinical Center Registry Workgroup. Associations between sleep difficulties and risk factors for cardiovascular disease in veterans and active duty military personnel of the Iraq and Afghanistan conflicts. J Behav Med. 2015;38(3):544-555.
16. Lutwak N, Dill C. Military sexual trauma increases risk of post-traumatic stress disorder and depression thereby amplifying the possibility of suicidal ideation and cardiovascular disease. Mil Med. 2013;178(4):359-361.
17. Bowry ADK, Lewey J, Dugani SB, Choudhry NK. The burden of cardiovascular disease in low- and middle-income countries: epidemiology and management. Can J Cardiol. 2015;31(9):1151-1159.
18. Reinier K, Stecker EC, Vickers C, Gunson K, Jui J, Chugh SS. Incidence of sudden cardiac arrest is higher in areas of low socioeconomic status: a prospective two year study in a large United States community. Resuscitation. 2006;70(2):186-192.
19. Reinier K, Thomas E, Andrusiek DL, et al; Resuscitation Outcomes Consortium Investigators. Socioeconomic status and incidence of sudden cardiac arrest. CMAJ. 2011;183(15):1705-1712.
20. Yusuf S, Rangarajan S, Teo K, et al; PURE Investigators. Cardiovascular risk and events in 17 low-, middle-, and high-income countries. N Engl J Med. 2014;371(9):818-827.
21. World Health Organization. Health topics: cardiovascular disease. http://www.who.int/cardiovascular_diseases/en/. Updated 2019. Accessed October 10, 2019.
22. Berkowitz AL. Stroke and the noncommunicable diseases: A global burden in need of global advocacy. Neurology. 2015;84(21):2183-2184.
23. Holt T. Predicting cardiovascular disease. BMJ. 2016;353:i2621.
24. Centers for Disease Control and Prevention. Million hearts: strategies to reduce the prevalence of leading cardiovascular disease risk factors—United States, 2011. MMWR Morb Mortal Wkly Rep. 2011;60(36):1248-1251.
25. Fryar CD, Chen TC, Li X. Prevalence of uncontrolled risk factors for cardiovascular disease: United States, 1999- 2010. NCHS Data Brief. 2012;103:1-8.
26. Ritchey MD, Loustalot F, Wall HK, et al. Million Hearts: description of the national surveillance and modeling methodology used to monitor the number of cardiovascular events prevented during 2012-2016. J Am Heart Assoc. 2017;6(5):pii:e00602.
27. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/ AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2935-2959.
28. Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): casecontrol study. Lancet. 2004;364(9438):937-952.
29. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2960-2984.
30. Bansilal S, Castellano JM, Fuster V. Global burden of CVD: focus on secondary prevention of cardiovascular disease. Int J Cardiol. 2015;201(suppl 1):S1-S7.
31. Havranek EP, Mujahid MS, Barr DA, et al. Social determinants of risk and outcomes for cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2015;132(9):873-898.
32. Miedema MD, Garberich RF, Schnaidt LJ, et al. Statin eligibility and outpatient care prior to ST-segment elevation myocardial infarction. J Am Heart Assoc. 2017;6(4): pii: e005333.
33. Noheria A, Teodorescu C, Uy-Evanado A, et al. Distinctive profile of sudden cardiac arrest in middle-aged vs. older adults: a community-based study. Int J Cardiol. 2013;168(4):3495-3499.
34. Lieb W, Enserro DM, Larson MG, Vasan RS. Residual cardiovascular risk in individuals on lipid-lowering treatment: quantifying absolute and relative risk in the community. Open Heart. 2018;5(1):e000722.
35. Sachdeva A, Cannon CP, Deedwania PC, et al. Lipid levels in patients hospitalized with coronary artery disease: an analysis of 136,905 hospitalizations in Get With The Guidelines. Am Heart J. 2009;157(1):111-117.e2.
36. Damen JA, Hooft L, Schuit E, et al. Prediction models for cardiovascular disease risk in the general population: systematic review. BMJ. 2016;353:i2416.
37. Fulcher J, O’Connell R, Voysey M, et al; Cholesterol Treatment Trialists (CTT) Collaboration. Efficacy and safety of LDL-lowering therapy among men and women: metaanalysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385(9976):1397-1405.
38. Perrone V, Sangiorgi D, Buda S, Degli Esposti L. Residual cardiovascular risk in patients who received lipid-lowering treatment in a real-life setting: retrospective study. Clinicoecon Outcomes Res. 2016;8:649-655.
39. Sirimarco G, Labreuche J, Bruckert E, et al; PERFORM and SPARCL Investigators. Atherogenic dyslipidemia and residual cardiovascular risk in statin-treated patients. Stroke. 2014;45(5):1429-1436.
40. Kones R. Molecular sources of residual cardiovascular risk, clinical signals, and innovative solutions: relationship with subclinical disease, undertreatment, and poor adherence: implications of new evidence upon optimizing cardiovascular patient outcomes. Vasc Health Risk Manag. 2013;9:617-670.
41. Hayashi M, Shimizu W, Albert CM. The spectrum of epidemiology underlying sudden cardiac death. Circ Res. 2015;116(12):1887-1906.
42. Downs JR, O’Malley PG. Management of dyslipidemia for cardiovascular disease risk reduction: synopsis of the 2014 U.S. Department of Veterans Affairs and U.S. Department of Defense clinical practice guideline. Ann Intern Med. 2015;163(4):291-297.
43. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/ AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/ NLA/PCNA Guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):3168-3209.
44. Tsimikas S, Fazio S, Ferdinand KC, et al. NHLBI Working Group recommendations to reduce lipoprotein(a)-mediated risk of cardiovascular disease and aortic stenosis. J Am Coll Cardiol. 2018;71(2):177-192.
45. Tsimikas S. A test in context: Lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol. 2017;69(6):692-711.
46. Ellis KL, Boffa MB, Sahebkar A, Koschinsky ML, Watts GF. The renaissance of lipoprotein(a): brave new world for preventive cardiology? Prog Lipid Res. 2017;68:57-82.
47. Thompson GR, Seed M. Lipoprotein(a): the underestimated cardiovascular risk factor. Heart. 2014;100(7):534-535.
48. Marcovina SM, Albers JJ. Lipoprotein (a) measurements for clinical application. J Lipid Res. 2016;57(4):526-537.
49. Tremulis SR. Founder’s Story: Lipoprotein(a) Foundation. https://www.lipoproteinafoundation.org/page /Sandrastory. Accessed October 10, 2019.
50. Centers for Disease Control and Prevention. ICD-10 Coordination and Maintenance Committee meeting, September 12-13, 2017 diagnosis agenda. https://www.cdc. gov/nchs/data/icd/Topic_Packet_Sept_2017.pdf. Accessed October 10, 2019.
51. Centers for Medicare & Medicaid Services. 2019 ICD- 10-CM codes descriptions in tabular order. https://www. cms.gov/Medicare/Coding/ICD10/2019-ICD-10-CM.html. Accessed October 10, 2019.
52. Wilson DP, Jacobson TA, Jones PH, et al. Use of Lipoprotein(a) in clinical practice: a biomarker whose time has come. A scientific statement from the National Lipid Association. J Clin Lipidol. 2019;13(3):374-392.
53. Langlois MR, Chapman MJ, Cobbaert C, et al. Quantifying atherogenic lipoproteins: current and future challenges in the era of personalized medicine and very low concentrations of ldl cholesterol. A consensus statement from EAS and EFLM. Clin Chem. 2018;64(7):1006-1033.
54. Shapiro MD, Fazio S. Apolipoprotein B-containing lipoproteins and atherosclerotic cardiovascular disease. F1000Res. 2017;6:134.
55. Tsimikas S, Fazio S, Viney NJ, Xia S, Witztum JL, Marcovina SM. Relationship of lipoprotein(a) molar concentrations and mass according to lipoprotein(a) thresholds and apolipoprotein(a) isoform size. J Clin Lipidol. 2018;12(5):1313-1323.
56. Erqou S, Kaptoge S, Perry PL, et al; Emerging Risk Factors Collaboration. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302(4):412-423.
57. Nave AH, Lange KS, Leonards CO, et al. Lipoprotein (a) as a risk factor for ischemic stroke: a meta-analysis. Atherosclerosis. 2015;242(2):496-503.
58. Varvel S, McConnell JP, Tsimikas S. Prevalence of elevated Lp(a) mass levels and patient thresholds in 532,359 patients in the United States. Arterioscler Thromb Vasc Biol. 2016;36(11):2239-2245.
59. Nordestgaard BG, Chapman MJ, Ray K, et al; European Atherosclerosis Society Consensus Panel. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31(23):2844-2853.
60. Guan W, Cao J, Steffen BT, et al. Race is a key variable in assigning lipoprotein(a) cutoff values for coronary heart disease risk assessment: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35(4):996-1001.
61. Virani SS, Brautbar A, Davis BC, et al. Associations between lipoprotein(a) levels and cardiovascular outcomes in black and white subjects: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2012;125(2):241-249.
62. Tsimikas S, Mallat Z, Talmud PJ, et al. Oxidation-specific biomarkers, lipoprotein(a), and risk of fatal and nonfatal coronary events. J Am Coll Cardiol. 2010;56(12):946-955.
63. Clarke R, Peden JF, Hopewell JC, et al; PROCARDIS Consortium. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. New Eng J Med. 2009;361(26):2518-2528.
64. Wattanakit K, Folsom AR, Chambless LE, Nieto FJ. Risk factors for cardiovascular event recurrence in the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2005;149(4):606-612.
65. Ruotolo G, Lincoff MA, Menon V, et al. Lipoprotein(a) is a determinant of residual cardiovascular risk in the setting of optimal LDL-C in statin-treated patients with atherosclerotic cardiovascular disease [Abstract 17400]. Circulation. 2018;136(suppl 1):A17400.
66. Suwa S, Ogita M, Miyauchi K, et al. Impact of lipoprotein (a) on long-term outcomes in patients with coronary artery disease treated with statin after a first percutaneous coronary intervention. J Atheroscler Thromb. 2017;24(11):1125-1131.
67. Nestel PJ, Barnes EH, Tonkin AM, et al. Plasma lipoprotein(a) concentration predicts future coronary and cardiovascular events in patients with stable coronary heart disease. Arterioscler Thromb Vasc Biol. 2013;33(12):2902-2908.
68. Burgess S, Ference BA, et al. Association of LPA variants with risk of coronary disease and the implications for lipoprotein(a)-lowering therapies: a Mendelian randomization analysis. JAMA Cardiol. 2018;3(7):619-627.
69. Roeseler E, Julius U, Heigl F, et al; Pro(a)LiFe-Study Group. Lipoprotein apheresis for lipoprotein(a)-associated cardiovascular disease: prospective 5 years of followup and apolipoprotein(a) characterization. Arterioscler Thromb Vasc Biol. 2016;36(9):2019-2027.
70. Matsuura E, Atzeni F, Sarzi-Puttini P, Turiel M, Lopez LR, Nurmohamed MT. Is atherosclerosis an autoimmune disease? BMC Med. 2014;12:47.
71. Ahearn J, Shields KJ, Liu CC, Manzi S. Cardiovascular disease biomarkers across autoimmune diseases. Clin Immunol. 2015;161(1):59-63.
72. Di Minno MND, Emmi G, Ambrosino P, et al. Subclinical atherosclerosis in asymptomatic carriers of persistent antiphospholipid antibodies positivity: a cross-sectional study. Int J Cardiol. 2019;274:1-6.
72. Di Minno MND, Emmi G, Ambrosino P, et al. Subclinical atherosclerosis in asymptomatic carriers of persistent antiphospholipid antibodies positivity: a cross-sectional study. Int J Cardiol. 2019;274:1-6.
73. Iseme RA, McEvoy M, Kelly B, et al. A role for autoantibodies in atherogenesis. Cardiovasc Res. 2017;113(10):1102-1112.
74. Sinicato NA, da Silva Cardoso PA, Appenzeller S. Risk factors in cardiovascular disease in systemic lupus erythematosus. Curr Cardiol Rev. 2013;9(1):15-19.
75. Sciatti E, Cavazzana I, Vizzardi E, et al. Systemic lupus erythematosus and endothelial dysfunction: a close relationship. Curr Rheumatol Rev. 2018;15(3):177-188.
76. Prasad A, Clopton P, Ayers C, et al. Relationship of autoantibodies to MDA-LDL and ApoB-Immune complexes to sex, ethnicity, subclinical atherosclerosis, and cardiovascular events. Arterioscler Thromb Vasc Biol. 2017;37(6):1213-1221.
77. Miller YI, Choi SH, Wiesner P, et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res. 2011;108(2):235-248.
78. Libby P, Lichtman AH, Hansson GK. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity. 2013;38(6):1092-1104.
79. Binder CJ, Papac-Milicevic N, Witztum JL. Innate sensing of oxidation-specific epitopes in health and disease. Nat Rev Immunol. 2016;16(8):485-497.
80. Freigang S. The regulation of inflammation by oxidized phospholipids. Eur J Immunol. 2016;46(8):1818-1825.
81. Ravandi A, Boekholdt SM, Mallat Z, et al. Relationship of oxidized LDL with markers of oxidation and inflammation and cardiovascular events: results from the EPIC-Norfolk Study. J Lipid Res. 2011;52(10):1829-1836.
82. Tsimikas S, Willeit P, Willeit J, et al. Oxidation-specific biomarkers, prospective 15-year cardiovascular and stroke outcomes, and net reclassification of cardiovascular events. J Am Coll Cardiol. 2012;60(21):2218-2229.
83. Cinoku I, Mavragani CP, Tellis CC, Nezos A, Tselepis AD, Moutsopoulos HM. Autoantibodies to ox-LDL in Sjogren’s syndrome: are they atheroprotective? Clin Exp Rheumatol. 2018;36 Suppl 112(3):61-67.
84. Fagerberg B, Prahl Gullberg U, Alm R, Nilsson J, Fredrikson GN. Circulating autoantibodies against the apolipoprotein B-100 peptides p45 and p210 in relation to the occurrence of carotid plaques in 64-year-old women. PLoS One. 2015;10(3):e0120744.
85. Klesareva EA, Afanas’eva OI, Donskikh VV, Adamova IY, Pokrovskii SN. Characteristics of lipoprotein(a)-containing circulating immune complexes as markers of coronary heart disease. Bull Exp Biol Med. 2016;162(2):231-236.
86. Morishita R, Ishii J, Kusumi Y, et al. Association of serum oxidized lipoprotein(a) concentration with coronary artery disease: potential role of oxidized lipoprotein(a) in the vasucular wall. J Atheroscler Thromb. 2009;16(4):410-418.
87. Wang J, Zhang C, Gong J, et al. Development of new enzyme-linked immunosorbent assay for oxidized lipoprotein(a) by using purified human oxidized lipoprotein(a) autoantibodies as capture antibody. Clin Chim Acta. 2007;385(1-2):73-78.
88. Wang JJ, Han AZ, Meng Y, et al. Measurement of oxidized lipoprotein (a) in patients with acute coronary syndromes and stable coronary artery disease by 2 ELISAs: using different capture antibody against oxidized lipoprotein (a) or oxidized LDL. Clin Biochem. 2010;43(6):571-575.
89. Orso E, Schmitz G. Lipoprotein(a) and its role in inflammation, atherosclerosis and malignancies. Clin Res Cardiol Suppl. 2017;12(Suppl 1):31-37.
90. Lassman ME, McLaughlin TM, Zhou H, et al. Simultaneous quantitation and size characterization of apolipoprotein(a) by ultra-performance liquid chromatography/ mass spectrometry. Rapid Commun Mass Spectrom. 2014;28(10):1101-1106.
91. Lippi G, Guidi G. Lipoprotein(a): from ancestral benefit to modern pathogen? QJM. 2000;93(2):75-84.
92. van der Valk FM, Bekkering S, Kroon J, et al. Oxidized phospholipids on lipoprotein(a) elicit arterial wall inflammation and an inflammatory monocyte response in humans. Circulation. 2016;134(8):611-624.
93. Yeang C, Wilkinson MJ, Tsimikas S. Lipoprotein(a) and oxidized phospholipids in calcific aortic valve stenosis. Curr Opin Cardiol. 2016;31(4):440-450.
94. Thanassoulis G, Campbell CY, Owens DS, et al; CHARGE Extracoronary Calcium Working Group. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368(6):503-512.
95. Aguib Y, Al Suwaidi J. The Copenhagen City Heart Study (Osterbroundersogelsen). Glob Cardiol Sci Pract. 2015;2015(3):33.
96. Kamstrup PR, Benn M, Tybjaerg-Hansen A, Nordestgaard BG. Extreme lipoprotein(a) levels and risk of myocardial infarction in the general population: the Copenhagen City Heart Study. Circulation. 2008;117(2):176-184.
97. Kamstrup PR, Tybjærg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301(22):2331-2339.
98. Kamstrup PR, Tybjaerg-Hansen A, Nordestgaard BG. Extreme lipoprotein(a) levels and improved cardiovascular risk prediction. J Am Coll Cardiol.2013;61(11):1146-1156.
99. Kamstrup PR, Tybjaerg-Hansen A, Nordestgaard BG. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J Am Coll Cardiol. 2014;63(5):470-477.
100. Kamstrup PR, Nordestgaard BG. Elevated lipoprotein(a) levels, LPA risk genotypes, and increased risk of heart failure in the general population. JACC Heart Fail.2016;4(1):78-87.
101. Verbeek R, Hoogeveen RM, Langsted A, et al. Cardiovascular disease risk associated with elevated lipoprotein(a) attenuates at low low-density lipoprotein cholesterol levels in a primary prevention setting. Eur Heart J. 2018;39(27):2589-2596.
102. Cao J, Steffen BT, Budoff M, et al. Lipoprotein(a) levels are associated with subclinical calcific aortic valve disease in white and black individuals: the multi-ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol. 2016;36(5):1003-1009.
103. Steffen BT, Duprez D, Bertoni AG, Guan W, Tsai M. Lp(a) [lipoprotein(a)]-related risk of heart failure is evident in whites but not in other racial/ethnic groups.Arterioscler Thromb Vasc Biol. 2018;38(10):2498-2504.
104. ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129(4):687-702.
105. Agarwala A, Pokharel Y, Saeed A, et al. The association of lipoprotein(a) with incident heart failure hospitalization: Atherosclerosis Risk in Communities study. Atherosclerosis. 2017;262:131-137.
106. Ohira T, Schreiner PJ, Morrisett JD, Chambless LE, Rosamond WD, Folsom AR. Lipoprotein(a) and incident ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2006;37(6):1407-1412.
107. Waldeyer C, Makarova N, Zeller T, et al. Lipoprotein(a) and the risk of cardiovascular disease in the European population: results from the BiomarCaRE consortium. Eur Heart J. 2017;38(32):2490-2498.
108. Cook NR, Mora S, Ridker PM. Lipoprotein(a) and cardiovascular risk prediction among women. J Am Coll Cardiol. 2018;72(3):287-296.
109. Suk Danik J, Rifai N, Buring JE, Ridker PM. Lipoprotein(a), measured with an assay independent of apolipoprotein(a) isoform size, and risk of future cardiovascular events among initially healthy women. JAMA. 2006;296(11):1363-1370.
110. Suk Danik J, Rifai N, Buring JE, Ridker PM. Lipoprotein(a), hormone replacement therapy, and risk of future cardiovascular events. J Am Coll Cardiol. 2008;52(2):124-131.
111. Chien KL, Hsu HC, Su TC, Sung FC, Chen MF, Lee YT. Lipoprotein(a) and cardiovascular disease in ethnic Chinese: the Chin-Shan Community Cardiovascular Cohort Study. Clin Chem. 2008;54(2):285-291.
112. Lee SR, Prasad A, Choi YS, et al. LPA gene, ethnicity, and cardiovascular events. Circulation.2017;135(3):251-263.
113. Zekavat SM, Ruotsalainen S, Handsaker RE, et al. Deep coverage whole genome sequences and plasma lipoprotein(a) in individuals of European and African ancestries. Nat Commun.2018;9(1):2606.
114. Zewinger S, Kleber ME, Tragante V, et al. Relations between lipoprotein(a) concentrations, LPA genetic variants, and the risk of mortality in patients with established coronary heart disease: a molecular and genetic association study. Lancet Diabetes Endocrinol. 2017;5(7):534-543.
115. Li J, Lange LA, Sabourin J, et al. Genome- and exomewide association study of serum lipoprotein (a) in the Jackson Heart Study. J Hum Genet. 2015;60(12):755-761.
116. Khera AV, Emdin CA, Drake I, et al, Kathiresan S. Genetic risk, adherence to a healthy lifestyle, and coronary disease. N Engl J Med.2016;375(24):2349-2358.
117. Nordestgaard BG, Langsted A. Lipoprotein(a) as a cause of cardiovascular disease: insights from epidemiology, genetics, and biology. J Lipid Res.2016;57(11):1953-1975.
118. Sofi F, Cesari F, Casini A, Macchi C, Abbate R, Gensini GF. Insomnia and risk of cardiovascular disease: a metaanalysis. Eur J Prev Cardiol.2014;21(1):57-64.
119. Estruch R, Ros E, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with a mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med.2018;378(25):e34.
120. Perrot N, Verbeek R, Sandhu M, et al. Ideal cardiovascular health influences cardiovascular disease risk associated with high lipoprotein(a) levels and genotype: The EPICNorfolk prospective population study. Atherosclerosis. 2017;256:47-52.
121. Teo KK, Corsi DJ, Tam JW, Dumesnil JG, Chan KL. Lipid lowering on progression of mild to moderate aortic stenosis: meta-analysis of the randomized placebocontrolled clinical trials on 2344 patients. Can J Cardiol. 2011;27(6):800-808.
122. Albers JJ, Slee A, O’Brien KD, et al. Relationship of apolipoproteins A-1 and B, and lipoprotein(a) to cardiovascular outcomes: the AIM-HIGH trial (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglyceride and Impact on Global Health Outcomes). J Am Coll Cardiol. 2013;62(17):1575-1579.
123. Lincoff AM, Nicholls SJ, Riesmeyer JS, et al; ACCELERATE Investigators. Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med. 2017;376(20):1933-1942.
124. Schmidt AF, Pearce LS, Wilkins JT, Overington JP, Hingorani AD, Casas JP. PCSK9 monoclonal antibodies for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev.2017;4:CD011748.
125. Bowman L, Hopewell JC, Chen F, et al; PHS3/TIM155-REVEAL Collaborative Group. Effects of anacetrapib in patients with atherosclerotic vascular disease.
126. Leebmann J, Roeseler E, Julius U, et al; Pro(a)LiFe Study Group. Lipoprotein apheresis in patients with maximally tolerated lipid-lowering therapy, lipoprotein(a)-hyperlipoproteinemia, and progressive cardiovascular disease: prospective observational multicenter study. Circulation. 2013;128(24):2567-2576.
127. Heigl F, Hettich R, Lotz N, et al. Efficacy, safety, and tolerability of long-term lipoprotein apheresis in patients with LDL- or Lp(a) hyperlipoproteinemia: Findings gathered from more than 36,000 treatments at one center in Germany. Atheroscler Suppl. 2015;18:154-162.
128. Viney NJ, van Capelleveen JC, Geary RS, et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet. 2016;388(10057):2239-2253.
129. Graham MJ, Viney N, Crooke RM, Tsimikas S. Antisense inhibition of apolipoprotein (a) to lower plasma lipoprotein (a) levels in humans. J Lipid Res. 2016;57(3):340-351.
130. Keene D, Price C, Shun-Shin MJ, Francis DP. Effect on cardiovascular risk of high density lipoprotein targeted drug treatments niacin, fibrates, and CETP inhibitors: meta-analysis of randomised controlled trials including 117,411 patients. BMJ. 2014;349:g4379.
131. Nicholls SJ, Ruotolo G, Brewer HB, et al. Evacetrapib alone or in combination with statins lowers lipoprotein(a) and total and small LDL particle concentrations in mildly hypercholesterolemic patients. J Clin Lipidol. 2016;10(3):519-527.e4.
132. Schwartz GG, Ballantyne CM, Barter PJ, et al. Association of lipoprotein(a) with risk of recurrent ischemic events following acute coronary syndrome: analysis of the dal-outcomes randomized clinical trial. JAMA Cardiol.2018;3(2):164-168.
133. Schwartz GG, Olsson AG, Abt M, et al; dal-OUTCOMES Investigators. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med.2012;367(22):2089-2099.
134. Thomas T, Zhou H, Karmally W, et al. CETP (Cholesteryl Ester Transfer Protein) inhibition with anacetrapib decreases production of lipoprotein(a) in mildly hypercholesterolemic subjects. Arterioscler Thromb Vasc Biol.2017;37(9):1770-1775.
135. Khera AV, Everett BM, Caulfield MP, et al. Lipoprotein(a) concentrations, rosuvastatin therapy, and residual vascular risk: an analysis from the JUPITER Trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin). Circulation. 2014;129(6):635-642.
136. Yeang C, Hung MY, Byun YS, et al. Effect of therapeutic interventions on oxidized phospholipids on apolipoprotein B100 and lipoprotein(a). J Clin Lipidol. 2016;10(3):594-603.
137. Zhou Z, Rahme E, Pilote L. Are statins created equal? Evidence from randomized trials of pravastatin, simvastatin, and atorvastatin for cardiovascular disease prevention.Am Heart J. 2006;151(2):273-281.
138. Ridker PM, MacFadyen JG, Fonseca FA, et al; JUPITER Study Group. Number needed to treat with rosuvastatin to prevent first cardiovascular events and death among men and women with low low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein: justification for the use of statins in prevention: an intervention trial evaluating rosuvastatin (JUPITER). Circ Cardiovasc Qual Outcomes. 2009;2(6):616-623.
139. Raal FJ, Giugliano RP, Sabatine MS, et al. Reduction in lipoprotein(a) with PCSK9 monoclonal antibody evolocumab (AMG 145): a pooled analysis of more than 1,300 patients in 4 phase II trials. J Am Coll Cardiol.2014;63(13):1278-1288.
140. Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1500-1509.
141. Koren MJ, Sabatine MS, Giugliano RP, et al. Long-term low-density lipoprotein cholesterol-lowering efficacy, persistence, and safety of evolocumab in treatment of hypercholesterolemia: results up to 4 years from the open-label OSLER-1 extension study. JAMA Cardiol.2017;2(6):598-607.
142. Desai NR, Kohli P, Giugliano RP, et al. AMG145, a monoclonal antibody against proprotein convertase subtilisin kexin type 9, significantly reduces lipoprotein(a) in hypercholesterolemic patients receiving statin therapy: an analysis from the LDL-C Assessment with Proprotein Convertase Subtilisin Kexin Type 9 Monoclonal Antibody Inhibition Combined with Statin Therapy (LAPLACE)-Thrombolysis in Myocardial Infarction (TIMI) 57 trial. Circulation.2013;128(9):962-969.
143. Schwartz GG, Steg PG, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and cardiovascular outcomes after acute coronary syndrome.N Engl J Med. 2018;379(22):2097-2107.
144. Sabatine MS, Giugliano RP, Keech AC, et al; FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular Disease.N Engl J Med. 2017;376(18):1713-1722.
145. Karatasakis A, Danek BA, Karacsonyi J, et al. Effect of PCSK9 inhibitors on clinical outcomes in patients with hypercholesterolemia: A meta-analysis of 35 randomized controlled trials. J Am Heart Assoc. 2017;6(12):e006910.
146. Santos RD, Duell PB, East C, et al. Long-term efficacy and safety of mipomersen in patients with familial hypercholesterolaemia: 2-year interim results of an open-label extension.Eur Heart J. 2015;36(9):566-575.
147. Duell PB, Santos RD, Kirwan BA, Witztum JL, Tsimikas S, Kastelein JJP. Long-term mipomersen treatment is associated with a reduction in cardiovascular events in patients with familial hypercholesterolemia. J Clin Lipidol. 2016;10(4):1011-1021.
148. McGowan MP, Tardif JC, Ceska R, et al. Randomized, placebo-controlled trial of mipomersen in patients with severe hypercholesterolemia receiving maximally tolerated lipid-lowering therapy. PLoS One.2012;7(11):e49006.
149. Jaeger BR, Richter Y, Nagel D, et al. Longitudinal cohort study on the effectiveness of lipid apheresis treatment to reduce high lipoprotein(a) levels and prevent major adverse coronary events. Nat Clin Pract Cardiovasc Med.2009;6(3):229-239.
150. Rosada A, Kassner U, Vogt A, Willhauck M, Parhofer K, Steinhagen-Thiessen E. Does regular lipid apheresis in Does regular lipid apheresis in patients with isolated elevated lipoprotein(a) levels reduce the incidence of cardiovascular events? Artif Organs. 2014;38(2):135-141.
151. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596-e646.
Cardiovascular disease (CVD) remains the leading cause of global mortality. In 2015, 41.5% of the US population had at least 1 form of CVD and CVD accounted for nearly 18 million deaths worldwide.1,2 The major disease categories represented include myocardial infarction (MI), sudden death, strokes, calcific aortic valve stenosis (CAVS), and peripheral vascular disease.1,2 In terms of health care costs, quality of life, and caregiver burden, the overall impact of disease prevalence continues to rise.1,3-6 There is an urgent need for more precise and earlier CVD risk assessment to guide lifestyle and therapeutic interventions for prevention of disease progression as well as potential reversal of preclinical disease. Even at a young age, visible coronary atherosclerosis has been found in up to 11% of “healthy” active individuals during autopsies for trauma fatalities.7,8
The impact of CVD on the US and global populations is profound. In 2011, CVD prevalence was predicted to reach 40% by 2030.9 That estimate was exceeded in 2015, and it is now predicted that by 2035, 45% of the US population will suffer from some form of clinical or preclinical CVD. In 2015, the decadeslong decline in CVD mortality was reversed for the first time since 1969, showing a 1% increase in deaths from CVD.1 Nearly 300,000 of those using US Department of Veterans Affairs (VA) services were hospitalized for CVD between 2010 and 2014.10 The annual direct and indirect costs related to CVD in the US are estimated at $329.7 billion, and these costs are predicted to top $1 trillion by 2035.1 Heart attack, coronary atherosclerosis, and stroke accounted for 3 of the 10 most expensive conditions treated in US hospitals in 2013.11 Globally, the estimate for CVD-related direct and indirect costs was $863 billion in 2010 and may exceed $1 trillion by 2030.12
The nature of military service adds additional risk factors, such as posttraumatic stress disorder, depression, sleep disorders and physical trauma which increase CVD morbidity/ mortality in service members, veterans, and their families.13-16 In addition, living in lowerincome areas (countries or neighborhoods) can increase the risk of both CVD incidence and fatalities, particularly in younger individuals.17-20 The Military Health System (MHS) and VA are responsible for the care of those individuals who have voluntarily taken on these additional risks through their time in service. This responsibility calls for rapid translation to practice tools and resources that can support interventions to minimize as many modifiable risk factors as possible and improve longterm health. This strategy aligns with the World Health Organization’s (WHO) focus on prevention of disease progression through interventions targeting modifiable risk.3-6,21-23 The driving force behind the launch of the US Department of Health and Human Services (HHS) Million Hearts program was the goal of preventing 1 million heart attacks and strokes by 2017 with risk reduction through aspirin, blood pressure control, cholesterol management, smoking cessation, sodium reduction, and physical activity.24,25 While some reductions in CVD events have been documented, the outcomes fell short of the goals set, highlighting both the need and value of continued and expanded efforts for CVD risk reduction.26
More precise assessment of risk factors during preventative care, as well as after a diagnosis of CVD, may improve the timeliness and precision of earlier interventions (both lifestyle and therapeutic) that reduce CVD morbidity and mortality.27 Personalized or precision medicine approaches take into account differences in socioeconomic, environmental, and lifestyle factors that are potentially reversible, as well as gender, race, and ethnicity.28-31 Current methods of predicting CVD risk have considerable room for improvement.27 About 40% of patients with newly diagnosed CVD have normal traditional cholesterol profiles, including those whose first cardiac event proves fatal.29-33 Currently available risk scores (hundreds have been described in the literature) mischaracterize risk in minority populations and women, and have shown deficiencies in identifying preclinical atherosclerosis.34,35 The failure to recognize preclinical CVD in military personnel during their active duty life cycle results in missed opportunities for improved health and readiness sustainment.
Most CVD risk prediction models incorporate some form of blood lipids. Total cholesterol (TC) is most commonly used in clinical practice, along with high-density lipoprotein (HDLC), low-density lipoprotein (LDLC), and triglycerides (TG).23,27,36 High LDLC and/or TC are well established as lipid-related CVD risk factors and are incorporated into many CVD risk scoring systems/models described in the literature.27 LDLC reduction is commonly recommended as CVD prevention, but even with optimal statin treatment, there is still considerable residual risk for new and recurrent CVD events.28,32,34,35,37-42
Incorporating novel biomarkers and alternative lipid measurements may improve risk prediction and aid targeted treatment, ultimately reducing CVD events.27 Apolipoprotein B (ApoB) is a major atherogenic component embedded in LDL and VLDL correlating to non-HDLC and may be useful in the setting of triglycerides ≥ 200 mg/d as levels > 130 mg/ dL appear to be risk-enhancing, but measurements may be unreliable.43 According to the 2018 Cholesterol Guidelines, lipoprotein(a) [Lp(a)] elevation also is recognized as a risk-enhancing factor that is particularly implicated when there is a strong family history of premature atherosclerotic CVD or personal history of CVD not explained by major risk factors.43
Lp(a) elevation is a largely underrecognized category of lipid disorder that impacts up to 20% to 30% of the population globally and within the US, although there is considerable variability by geographic location and ethnicity.44 Globally, Lp(a) elevation places > 1 billion people at moderate to high risk for CVD.44 Lp(a) has a strong genetic component and is recognized as a distinct and independent risk factor for MI, sudden death, strokes and CAVS. Lp(a) has an extensive body of evidence to support its distinct role both as a causal factor in CVD and as an augmentation to traditional risk factors.44-48
Lipoproteni(a) Elevation Use For Diagnosis
The importance of Lp(a) elevation as a clinical diagnosis rather than a laboratory abnormality alone was brought forward by the Lipoprotein(a) Foundation. Its founder, Sandra Tremulis, is a survivor of an acute coronary event that occurred when she was 39-years old, despite running marathons and having none of the traditional CVD lifestyle risk factors.49 This experience inspired her to create the Lipoprotein(a) Foundation to give a voice to families living with or at risk for CVD due to Lp(a) elevation.
As often happens in the progress of medicine, patients and their families drive change based on their personal experiences with the gaps in standard clinical practice. It was this foundation—not a member of the medical establishment—that submitted the formal request for the addition of new ICD-10-CM diagnostic and family history codes for Lp(a) elevation during the Centers for Disease Control and Prevention (CDC) September 2017 ICD-10-CM Coordination and Maintenance Committee meeting.50 In June 2018, the final ICD-10-CM code addenda for 2019 was released and included the new codes E78.41 (Elevated Lp[a]) and Z83.430 (Family history of elevated Lp[a]).52 After the new codes were approved, both the American Heart Association and the National Lipid Association added recommendations regarding Lp(a) testing to their clinical practice guidelines.43,52
Practically, these codes standardize billing and payment for legitimate clinical work and laboratory testing. Prior to the addition of Lp(a) elevation as a clinical diagnosis, testing and treatment of Lp(a) elevation was considered experimental and not medically necessary until after a cardiovascular event had already occurred. Services for Lp(a) elevation were therefore not reimbursed by many healthcare organizations and insurance companies. The new ICD-10-CM codes encourage the assessment of Lp(a) both in individuals with early onset major CVD events and in presumably fit, healthy individuals, particularly when there is a family history of Lp(a) elevation. Given that Lp(a) levels do not change significantly over time, the current understanding is that only a single measurement is needed to define the individual risk over a lifetime.41,42,44,45 As therapies targeting Lp(a) levels evolve, repeated measurements may be indicated to monitor response and direct changes in management. “Elevated Lipoprotein(a)” is the first laboratory testing abnormality that has achieved the status of a clinical diagnosis.
Lp(a) Measurements
There is considerable complexity to the measurement of lipoproteins in blood samples due to heterogeneity in both density and size of particles as illustrated in the Figure.53
For traditional lipids measured in clinical practice, the size and density ranges from small high-density lipoprotein (HDL) through LDLC and intermediate- density lipoprotein (IDL) to the largest least dense particles in the very low-density lipoprotein (VLDL) and chylomicron remnant fractions. Standard lipid profiles consist of mass concentration measurements (mg/dL) of TC, TG, HDLC, and LDLC.53 Non-HDLC (calculated as: TC−HDLC) consists of all cholesterol found in atherogenic lipoproteins, including remnant-C and Lp(a). Until recently, the cholesterol content of Lp(a), corresponding to about 30% of Lp(a) total mass, was included in the TC, non-HDLC and LDLC measurements with no separate reporting by the majority of clinical laboratories.
After > 50 years of research on the structure and biochemistry of Lp(a), the physiology and biological functions of these complex and polymorphic lipoprotein particles are not fully understood. Lp(a) is composed of a lipoprotein particle similar in composition to LDL (protein and lipid), containing 1 molecule of ApoB wrapped around a core of cholesteryl ester and triglyceride with phospholipids and unesterified cholesterol at its surface.48 The presence of a unique hydrophilic, highly glycosylated protein referred to as apolopoprotienA (apo[a]), covalently attached to ApoB-100 by a single disulfide bridge, differentiates Lp(a) from LDL.48 Cholesterol rich ApoB is an important component within many lipoproteins pathogenic for atherosclerosis and CVD.45,47,53
The apo(a) contributes to the increased density of Lp(a) compared to LDLC with associated reduced binding affinity to the LDL receptor. This reduced receptor binding affinity is a presumed mechanism for the lack of Lp(a) plasma level response to statin therapies, which increase hepatic LDL receptor activity.47 Apo(a) evolved from the plasminogen gene through duplication and remodeling and demonstrates extensive heterogeneity in protein size, with > 40 different apo(a) isoforms resulting in > 40 different Lp(a) particle sizes. Size of the apo(a) particle is determined by the number of pleated structures known as kringles. Most people (> 80%) carry 2 different-sized apo(a) isoforms. Plasma Lp(a) level is determined by the net production of apo(a) in each isoform, and the smaller apo(a) isoforms are associated with higher plasma levels of Lp(a).45
Given the heterogeneity in Lp(a) molecular weight, which can vary even within individuals, recommendations have been made for reporting results as particle numbers or concentrations (nmol/L or mmol/L) rather than as mass concentration (mg/dL).55 However, the majority of the large CVD morbidity and mortality outcomes studies used Lp(a) mass concentration levels in mg/ dL to characterize risk levels.56,57 There is no standardized method to convert Lp(a) measurements from mg/dL to nmol/L.55 Current assays using WHO standardized reagents and controls are reliable for categorizing risk levels.58
The European Atherosclerosis Society consensus panel recommended that desirable Lp(a) levels should be below the 80th percentile (< 50 mg/dL or < 125 nmol/L) in patients with intermediate or high CVD risk.59 Subsequent epidemiological and Mendelian randomization studies have been performed in general populations with no history of CVD and demonstrated that increased CVD risk can be detected with Lp(a) levels as low as 25 to 30 mg/dL.56,60-63 In secondary prevention populations with prior CVD and optimal treatment (statins, antiplatelet drugs), recurrent event risk was also increased with elevated Lp(a).63-66
Using immunoturbidometric assays, Varvel and colleagues reported the prevalence of elevated Lp(a) mass concentration levels (mg/dL) in > 500,000 US patients undergoing clinical evaluations based on data from a referral laboratory of patients.58 The mean Lp(a) levels were 34.0 mg/dL with median (interquartile range [IQR]) levels at 17 (7-47) mg/dL and overall range of 0 to 907 mg/dL.58 Females had higher Lp(a) levels compared to males but no ethnic or racial breakdown was provided. Lp(a) levels > 30 mg/dL and > 50 mg/dL were present in 35% and 24% of subjects, respectively. Table 1 displays the relationship between various Lp(a) level cut-offs to mean levels of LDLC, estimated LDLC corrected for Lp(a), TC, HDLC, and TG.58 The data demonstrate that Lp(a) elevation cannot be inferred from LDLC levels nor from any of the other traditional lipoprotein measures. Patients with high risk Lp(a) levels may have normal LDLC. While Lp(a) thresholds have been identified for stratification of CVD risk, the target levels for risk reduction have not been specifically defined, particularly since therapies are not widely available for reduction of Lp(a). Table 2 provides an overview of clinical lipoprotein measurements that may be reasonable targets for therapeutic interventions and reduction of CVD risk.44,53,55 In general, existing studies suggest that radical reduction (> 80%) is required to impact long-term outcomes, particularly in individuals with severe disease.68,69
LDLC reduction alone leaves a residual CVD risk that is greater than the risk reduced.40 In addition, the autoimmune inflammation and lipid specific autoantibodies play an important role in increased CVD morbidity and mortality risk.70,71 The presence of autoantibodies such as antiphospholipid antibodies (without a specific autoimmune disease diagnosis) increases the risk of subclinical atherosclerosis.72,73 Certain autoimmune diseases such as systemic lupus erythematosus are recognized as independent risk factors for CVD.74,75 Autoantibodies appear to mediate CVD events and mortality risk, independent of traditional therapies for risk reduction.73 Further research is needed to clarify the role of autoantibodies as markers of increased or decreased CVD risk and their mechanism of action.
Autoantibodies directed at new antigens in lipoproteins within atherosclerotic lesions can modulate the impact of atherosclerosis via activation of the innate and adaptive immune system.76 The lipid-associated neopeptides are recognized as damage-associated or danger- associated molecular patterns (DAMPs), also known as alarmins, which signal molecules that can trigger and perpetuate noninfectious inflammatory responses.77-79 Plasma autoantibodies (immunoglobulin M and G [IgM, IgG]) modify proinflammatory oxidation-specific epitopes on oxidized phospholipids (oxPL) within lipoproteins and are linked with markers of inflammation and CVD events.80-82 Modified LDLC and ApoB-100 immune complexes with specific autoantibodies in the IgG class are associated with increased CVD.76 These and other risk-modulating autoantibodies may explain some of the variability in CVD outcomes by ethnicity and between individuals.
Some antibodies to oxidized LDL (ox-LDL) may have a protective role in the development of atherosclerosis.83,84 In a cohort of > 500 women, the number of carotid atherosclerotic plaques and total carotid plaque area were inversely correlated with a specific IgM autoantibody (MDA-p210).84 High concentrations of Lp(a)- containing circulating immune complexes and Lp(a)-specific IgM and IgG have been described in patients with coronary heart disease (CHD).85 Like ox-LDL, oxidized Lp(a) [ox-Lp(a)] is more potent than native Lp(a) in increasing atherosclerosis risk and is increased in patients with CHD compared to healthy controls.86-88 Ox-Lp(a) levels may represent an even stronger risk marker for CVD than ox-LDL.85
Possible Mechanisms of Pathogenesis
While the precise quantification of Lp(a) in human plasma (or serum) has been challenging, current clinical laboratories use standardized international reference reagents and controls in their assays. Most current Lp(a) assays are based on immunological methods (eg, immunonephelometry, immunoturbidimetry, or enzyme linked immunosorbent assay [ELISA]) using antibodies against apo(a).89 Apo(a) contains 10 subtypes of kringle IV and 1 copy of kringle V. Some assays use antibodies against kringle-IV type 2; however, it has been recommended that newer methods should use antibodies against the specific bridging kringle-IV Type 9 domain, which has a more stable bond and is present as a single copy.48,89 Other approaches to Lp(a) measurement include ultraperformance liquid chromatography/mass spectrometry that can determine both the concentration and particle size of apo(a).48,90 For routine clinical care, currently available assays reporting in mg/dL can be considered fairly accurate for separating low-risk from moderate-to-high-risk patients.45
The physiologic role of Lp(a) in humans remains to be fully defined and individuals with extremely low plasma Lp(a) levels present no disease or deficiency syndromes.91 Lp(a) accumulates in endothelial injuries and binds to components of the vessel wall and subendothelial matrix, presumably due to the strong lysine binding site in apo(a).46 Mediated by apo(a), the binding stimulates chemotactic activation of monocytes/macrophages and thereby modulating angiogenesis and inflammation.89 Lp(a) may contribute to CVD and CAVS via its LDL-like component, with proinflammatory effects of oxidized phospholipids (OxPL) on both ApoB and apo(a) and antifibrinolytic/prothrombotic effects of apo(a).92 In Vitro studies have demonstrated that apo(a) modifies cellular function of cultured vascular endothelial cells (promoting stress fiber formation, endothelial contraction and vascular permeability), smooth muscles, and monocytes/ macrophages (promoting differentiation of proinflammatory M1-1 type macrophages) via complex mechanisms of cell signaling and cytokine production.89 Lp(a) is the only monogenetic risk factor for aortic valve calcification and stenosis93 and is strongly linked specifically with the single nucleotide polymorphism (SNP) rs10455872 in the gene LPA encoding for apo(a).94
CVD Risk Predictive Value
There are a large number of studies demonstrating that Lp(a) elevations are an independent predictor of adverse cardiovascular outcomes including MI, sudden death, strokes, calcific aortic valve stenosis and peripheral vascular disease (Table 3). The Copenhagen City Heart Study and Copenhagen General Population Study are well known prospective population- based cohort studies that track outcomes through national patient registries.95 These studies demonstrate increased risk for MI, CHD, CAVS, and heart failure when subjects with very high Lp(a) levels (50-115 mg/dL) are compared with subjects with very low Lp(a) levels (< 5 mg/dL).96-100 Subjects with less extreme Lp(a) elevations (> 30 mg/dL) also show increased risk of CVD when they have comorbid LDLC elevations.101 However, the Copenhagen studies are composed exclusively of white subjects and the effects of Lp(a) are known to vary with race or ethnicity.
The Multi-Ethnic Study of Atherosclerosis (MESA) recruited an ethnically diverse sample of > 6,000 Americans, aged 45 to 84 years, without CVD, into an ongoing prospective cohort study. Research using subjects from this study has found consistently increased risk of CHD, heart failure, subclinical aortic valve calcification, and more severe CAVS in white subjects with elevated Lp(a).60,102,103 Black subjects with elevated Lp(a) had increased risk of CHD and more severe CAVS and Hispanic subjects with Lp(a) elevation were at higher risk for CHD.60,102 So far, no studies of MESA subjects have identified a relationship between Lp(a) elevation and CVD events for Asian-Americans subjects (predominantly of Chinese descent). There is a need for ongoing research to more precisely define relevant cut-off levels by race, ethnicity and sex.
The Atherosclerosis Risk in Communities (ARIC) Study was a prospective multiethnic cohort study including > 15,000 US adults, aged 45 to 64 years.103 Lp(a) elevations in this cohort were associated with greater risks for first CVD events, heart failure, and recurrent CVD events.61,64,105 The risk of stroke for subjects with elevated Lp(a) was greater for black and white women, and for black men.61,106 However, a meta-analysis of case-control studies showed increased ischemic stroke risk in both men and women with elevated Lp(a).57
A recent European meta-analysis collected blood samples and outcome data from > 50,000 subjects in 7 prospective cohort studies. Using a central laboratory to standardize Lp(a) measurements, researchers found increased risk of major coronary events and new CVD in subjects with Lp(a) > 50 mg/dL compared to those below that threshold.107
Although many of these studies show modest increases in risk of CVD events with Lp(a) elevation, it should be noted that other studies do not demonstrate such consistent associations. This is particularly true in studies of women and nonwhite ethnic groups.103,108-112 The variability of study results may be due to other confounding factors such as autoantibodies that either upregulate or downregulate atherogenicity of LDLC and potentially other lipoproteins. This is particularly relevant to women who have an increased risk for autoimmune disease.
Lp(a) has significant genetic heritability—75% in Europeans and 85% in African Americans.113 In whites, the LPA gene on chromosome 6p26- 27 with the polymorphism genetic variants rs10455872 and rs3798220 is consistently associated with elevated Lp(a) levels.63,100,113 However, the degree of Lp(a) elevation associated with these specific genetic variants varies by ethnicity.78,113,115
Lifestyle and Cardiovascular Health
It is noteworthy that the Lp(a) genetic risks can also be modified by lifestyle risk reduction even in the absence of significant blood level reductions. For example, Khera and colleagues constructed a genetic risk profile for CVD that included genes related to Lp(a).116 Subjects with high genetic risk were more likely to experience CVD events compared with subjects with low genetic risk. However, risks for CVD were attenuated by 4 healthy lifestyle factors: current nonsmoker, body mass index < 30, at least weekly physical activity, and a healthy diet. Subjects with high genetic risk and an unhealthy lifestyle (0 or 1 of the 4 healthy lifestyle factors) were the most likely to develop CVD (Hazard ratio [HR], 3.5), but that risk was lower for subjects with healthy (3 or 4 of the 4 healthy lifestyle factors) and intermediate lifestyles (2 of the 4 healthy lifestyle factors) (HR, 1.9 and 2.2, respectively), despite despite high genetic risk for CVD.
While the independent CVD risk associated with elevated Lp(a) does not appear to be responsive to lifestyle risk reduction alone, certainly elevated LDLC and traditional risk factors can increase the overall CVD risk and are worthy of preventive interventions. In particular, inflammation from any source exacerbates CVD risk. Proatherogenic diet, insufficient sleep, lack of exercise, and maladaptive stress responses are other targets for personalized CVD risk reduction. 28,117 Studies of dietary modifications and other lifestyle factors have shown reduced risk of CVD events, despite lack of reduction in Lp(a) levels.119,120 It is noteworthy that statin therapy (with or without ezetimibe) fails to impact CAVS progression, likely because statins either raise or have no effect on Lp(a) levels.92,119
Until recently, there has been no evidence supporting any therapeutic intervention causing clinically meaningful reductions in Lp(a). Table 4 lists major drug classes and their effects on Lp(a) and CVD outcomes; however, a detailed discussion of each of these therapies is beyond the scope of this review. Drugs that reduce Lp(a) by 20-30% have varying effects on CVD outcomes, from no effect122,123 to a 10% to 20% decrease in CVD events when compared with a placebo.124,125 Because these drugs also produce substantial reductions in LDLC, it is not possible to determine how much of the beneficial effects are due to reductions in Lp(a).
Lipoprotein apheresis produces profound reductions in Lp(a) of 60 to 80% in very highrisk populations.69,126 Within-subjects comparisons show up to 80% reductions in CVD events, relative to event rates prior to treatment initiation.69,127 Early trials of antisense oligonucleotide against apo(a) therapies show potential to produce similar outcomes.128,129 These treatments may be particularly effective in patients with isolated Lp(a) elevations.
Summary
Lp(a) elevation is a major contributor to cardiovascular disease risk and has been recognized as an ICD-10-CM coded clinical diagnosis, the first laboratory abnormality to be defined a clinical disease in the asymptomatic healthy young individuals. This change addresses currently under- diagnosed CVD risk independent of LDLC reduction strategies. A brief overview of recent guidelines for the clinical use of Lp(a) testing from the American Heart Association43,151 and the National Lipid Association52 can be found in Table 5. Although drug therapies for lowering Lp(a) levels remain limited, new treatment options are actively being developed.
Many Americans with high Lp(a) have not yet been identified. Expanded one-time screening can inform these patients of their cardiovascular risk and increase their access to early, aggressive lifestyle modification and optimal lipid-lowering therapy. Given the further increased CVD risk factors for military service members and veterans, a case can be made for broader screening and enhanced surveillance of elevated Lp(a) in these presumably healthy and fit individuals as well as management focused on modifiable risk factors.
Acknowledgements
This program initiative was conducted by the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. as part of the Integrative Cardiac Health Project at Walter Reed National Military Medical Center (WRNMMC), and is made possible by a cooperative agreement that was awarded and administered by the US Army Medical Research & Materiel Command (USAMRMC), at Fort Detrick under Contract Number: W81XWH-16-2-0007. It reflects literature review preparatory work for a research protocol but does not involve an actual research project. The work in this manuscript was supported by the staff of the Integrative Cardiac Health Project (ICHP) with special thanks to Claire Fuller, Elaine Walizer, Dr. Mariam Kashani and the entire health coaching team.
Cardiovascular disease (CVD) remains the leading cause of global mortality. In 2015, 41.5% of the US population had at least 1 form of CVD and CVD accounted for nearly 18 million deaths worldwide.1,2 The major disease categories represented include myocardial infarction (MI), sudden death, strokes, calcific aortic valve stenosis (CAVS), and peripheral vascular disease.1,2 In terms of health care costs, quality of life, and caregiver burden, the overall impact of disease prevalence continues to rise.1,3-6 There is an urgent need for more precise and earlier CVD risk assessment to guide lifestyle and therapeutic interventions for prevention of disease progression as well as potential reversal of preclinical disease. Even at a young age, visible coronary atherosclerosis has been found in up to 11% of “healthy” active individuals during autopsies for trauma fatalities.7,8
The impact of CVD on the US and global populations is profound. In 2011, CVD prevalence was predicted to reach 40% by 2030.9 That estimate was exceeded in 2015, and it is now predicted that by 2035, 45% of the US population will suffer from some form of clinical or preclinical CVD. In 2015, the decadeslong decline in CVD mortality was reversed for the first time since 1969, showing a 1% increase in deaths from CVD.1 Nearly 300,000 of those using US Department of Veterans Affairs (VA) services were hospitalized for CVD between 2010 and 2014.10 The annual direct and indirect costs related to CVD in the US are estimated at $329.7 billion, and these costs are predicted to top $1 trillion by 2035.1 Heart attack, coronary atherosclerosis, and stroke accounted for 3 of the 10 most expensive conditions treated in US hospitals in 2013.11 Globally, the estimate for CVD-related direct and indirect costs was $863 billion in 2010 and may exceed $1 trillion by 2030.12
The nature of military service adds additional risk factors, such as posttraumatic stress disorder, depression, sleep disorders and physical trauma which increase CVD morbidity/ mortality in service members, veterans, and their families.13-16 In addition, living in lowerincome areas (countries or neighborhoods) can increase the risk of both CVD incidence and fatalities, particularly in younger individuals.17-20 The Military Health System (MHS) and VA are responsible for the care of those individuals who have voluntarily taken on these additional risks through their time in service. This responsibility calls for rapid translation to practice tools and resources that can support interventions to minimize as many modifiable risk factors as possible and improve longterm health. This strategy aligns with the World Health Organization’s (WHO) focus on prevention of disease progression through interventions targeting modifiable risk.3-6,21-23 The driving force behind the launch of the US Department of Health and Human Services (HHS) Million Hearts program was the goal of preventing 1 million heart attacks and strokes by 2017 with risk reduction through aspirin, blood pressure control, cholesterol management, smoking cessation, sodium reduction, and physical activity.24,25 While some reductions in CVD events have been documented, the outcomes fell short of the goals set, highlighting both the need and value of continued and expanded efforts for CVD risk reduction.26
More precise assessment of risk factors during preventative care, as well as after a diagnosis of CVD, may improve the timeliness and precision of earlier interventions (both lifestyle and therapeutic) that reduce CVD morbidity and mortality.27 Personalized or precision medicine approaches take into account differences in socioeconomic, environmental, and lifestyle factors that are potentially reversible, as well as gender, race, and ethnicity.28-31 Current methods of predicting CVD risk have considerable room for improvement.27 About 40% of patients with newly diagnosed CVD have normal traditional cholesterol profiles, including those whose first cardiac event proves fatal.29-33 Currently available risk scores (hundreds have been described in the literature) mischaracterize risk in minority populations and women, and have shown deficiencies in identifying preclinical atherosclerosis.34,35 The failure to recognize preclinical CVD in military personnel during their active duty life cycle results in missed opportunities for improved health and readiness sustainment.
Most CVD risk prediction models incorporate some form of blood lipids. Total cholesterol (TC) is most commonly used in clinical practice, along with high-density lipoprotein (HDLC), low-density lipoprotein (LDLC), and triglycerides (TG).23,27,36 High LDLC and/or TC are well established as lipid-related CVD risk factors and are incorporated into many CVD risk scoring systems/models described in the literature.27 LDLC reduction is commonly recommended as CVD prevention, but even with optimal statin treatment, there is still considerable residual risk for new and recurrent CVD events.28,32,34,35,37-42
Incorporating novel biomarkers and alternative lipid measurements may improve risk prediction and aid targeted treatment, ultimately reducing CVD events.27 Apolipoprotein B (ApoB) is a major atherogenic component embedded in LDL and VLDL correlating to non-HDLC and may be useful in the setting of triglycerides ≥ 200 mg/d as levels > 130 mg/ dL appear to be risk-enhancing, but measurements may be unreliable.43 According to the 2018 Cholesterol Guidelines, lipoprotein(a) [Lp(a)] elevation also is recognized as a risk-enhancing factor that is particularly implicated when there is a strong family history of premature atherosclerotic CVD or personal history of CVD not explained by major risk factors.43
Lp(a) elevation is a largely underrecognized category of lipid disorder that impacts up to 20% to 30% of the population globally and within the US, although there is considerable variability by geographic location and ethnicity.44 Globally, Lp(a) elevation places > 1 billion people at moderate to high risk for CVD.44 Lp(a) has a strong genetic component and is recognized as a distinct and independent risk factor for MI, sudden death, strokes and CAVS. Lp(a) has an extensive body of evidence to support its distinct role both as a causal factor in CVD and as an augmentation to traditional risk factors.44-48
Lipoproteni(a) Elevation Use For Diagnosis
The importance of Lp(a) elevation as a clinical diagnosis rather than a laboratory abnormality alone was brought forward by the Lipoprotein(a) Foundation. Its founder, Sandra Tremulis, is a survivor of an acute coronary event that occurred when she was 39-years old, despite running marathons and having none of the traditional CVD lifestyle risk factors.49 This experience inspired her to create the Lipoprotein(a) Foundation to give a voice to families living with or at risk for CVD due to Lp(a) elevation.
As often happens in the progress of medicine, patients and their families drive change based on their personal experiences with the gaps in standard clinical practice. It was this foundation—not a member of the medical establishment—that submitted the formal request for the addition of new ICD-10-CM diagnostic and family history codes for Lp(a) elevation during the Centers for Disease Control and Prevention (CDC) September 2017 ICD-10-CM Coordination and Maintenance Committee meeting.50 In June 2018, the final ICD-10-CM code addenda for 2019 was released and included the new codes E78.41 (Elevated Lp[a]) and Z83.430 (Family history of elevated Lp[a]).52 After the new codes were approved, both the American Heart Association and the National Lipid Association added recommendations regarding Lp(a) testing to their clinical practice guidelines.43,52
Practically, these codes standardize billing and payment for legitimate clinical work and laboratory testing. Prior to the addition of Lp(a) elevation as a clinical diagnosis, testing and treatment of Lp(a) elevation was considered experimental and not medically necessary until after a cardiovascular event had already occurred. Services for Lp(a) elevation were therefore not reimbursed by many healthcare organizations and insurance companies. The new ICD-10-CM codes encourage the assessment of Lp(a) both in individuals with early onset major CVD events and in presumably fit, healthy individuals, particularly when there is a family history of Lp(a) elevation. Given that Lp(a) levels do not change significantly over time, the current understanding is that only a single measurement is needed to define the individual risk over a lifetime.41,42,44,45 As therapies targeting Lp(a) levels evolve, repeated measurements may be indicated to monitor response and direct changes in management. “Elevated Lipoprotein(a)” is the first laboratory testing abnormality that has achieved the status of a clinical diagnosis.
Lp(a) Measurements
There is considerable complexity to the measurement of lipoproteins in blood samples due to heterogeneity in both density and size of particles as illustrated in the Figure.53
For traditional lipids measured in clinical practice, the size and density ranges from small high-density lipoprotein (HDL) through LDLC and intermediate- density lipoprotein (IDL) to the largest least dense particles in the very low-density lipoprotein (VLDL) and chylomicron remnant fractions. Standard lipid profiles consist of mass concentration measurements (mg/dL) of TC, TG, HDLC, and LDLC.53 Non-HDLC (calculated as: TC−HDLC) consists of all cholesterol found in atherogenic lipoproteins, including remnant-C and Lp(a). Until recently, the cholesterol content of Lp(a), corresponding to about 30% of Lp(a) total mass, was included in the TC, non-HDLC and LDLC measurements with no separate reporting by the majority of clinical laboratories.
After > 50 years of research on the structure and biochemistry of Lp(a), the physiology and biological functions of these complex and polymorphic lipoprotein particles are not fully understood. Lp(a) is composed of a lipoprotein particle similar in composition to LDL (protein and lipid), containing 1 molecule of ApoB wrapped around a core of cholesteryl ester and triglyceride with phospholipids and unesterified cholesterol at its surface.48 The presence of a unique hydrophilic, highly glycosylated protein referred to as apolopoprotienA (apo[a]), covalently attached to ApoB-100 by a single disulfide bridge, differentiates Lp(a) from LDL.48 Cholesterol rich ApoB is an important component within many lipoproteins pathogenic for atherosclerosis and CVD.45,47,53
The apo(a) contributes to the increased density of Lp(a) compared to LDLC with associated reduced binding affinity to the LDL receptor. This reduced receptor binding affinity is a presumed mechanism for the lack of Lp(a) plasma level response to statin therapies, which increase hepatic LDL receptor activity.47 Apo(a) evolved from the plasminogen gene through duplication and remodeling and demonstrates extensive heterogeneity in protein size, with > 40 different apo(a) isoforms resulting in > 40 different Lp(a) particle sizes. Size of the apo(a) particle is determined by the number of pleated structures known as kringles. Most people (> 80%) carry 2 different-sized apo(a) isoforms. Plasma Lp(a) level is determined by the net production of apo(a) in each isoform, and the smaller apo(a) isoforms are associated with higher plasma levels of Lp(a).45
Given the heterogeneity in Lp(a) molecular weight, which can vary even within individuals, recommendations have been made for reporting results as particle numbers or concentrations (nmol/L or mmol/L) rather than as mass concentration (mg/dL).55 However, the majority of the large CVD morbidity and mortality outcomes studies used Lp(a) mass concentration levels in mg/ dL to characterize risk levels.56,57 There is no standardized method to convert Lp(a) measurements from mg/dL to nmol/L.55 Current assays using WHO standardized reagents and controls are reliable for categorizing risk levels.58
The European Atherosclerosis Society consensus panel recommended that desirable Lp(a) levels should be below the 80th percentile (< 50 mg/dL or < 125 nmol/L) in patients with intermediate or high CVD risk.59 Subsequent epidemiological and Mendelian randomization studies have been performed in general populations with no history of CVD and demonstrated that increased CVD risk can be detected with Lp(a) levels as low as 25 to 30 mg/dL.56,60-63 In secondary prevention populations with prior CVD and optimal treatment (statins, antiplatelet drugs), recurrent event risk was also increased with elevated Lp(a).63-66
Using immunoturbidometric assays, Varvel and colleagues reported the prevalence of elevated Lp(a) mass concentration levels (mg/dL) in > 500,000 US patients undergoing clinical evaluations based on data from a referral laboratory of patients.58 The mean Lp(a) levels were 34.0 mg/dL with median (interquartile range [IQR]) levels at 17 (7-47) mg/dL and overall range of 0 to 907 mg/dL.58 Females had higher Lp(a) levels compared to males but no ethnic or racial breakdown was provided. Lp(a) levels > 30 mg/dL and > 50 mg/dL were present in 35% and 24% of subjects, respectively. Table 1 displays the relationship between various Lp(a) level cut-offs to mean levels of LDLC, estimated LDLC corrected for Lp(a), TC, HDLC, and TG.58 The data demonstrate that Lp(a) elevation cannot be inferred from LDLC levels nor from any of the other traditional lipoprotein measures. Patients with high risk Lp(a) levels may have normal LDLC. While Lp(a) thresholds have been identified for stratification of CVD risk, the target levels for risk reduction have not been specifically defined, particularly since therapies are not widely available for reduction of Lp(a). Table 2 provides an overview of clinical lipoprotein measurements that may be reasonable targets for therapeutic interventions and reduction of CVD risk.44,53,55 In general, existing studies suggest that radical reduction (> 80%) is required to impact long-term outcomes, particularly in individuals with severe disease.68,69
LDLC reduction alone leaves a residual CVD risk that is greater than the risk reduced.40 In addition, the autoimmune inflammation and lipid specific autoantibodies play an important role in increased CVD morbidity and mortality risk.70,71 The presence of autoantibodies such as antiphospholipid antibodies (without a specific autoimmune disease diagnosis) increases the risk of subclinical atherosclerosis.72,73 Certain autoimmune diseases such as systemic lupus erythematosus are recognized as independent risk factors for CVD.74,75 Autoantibodies appear to mediate CVD events and mortality risk, independent of traditional therapies for risk reduction.73 Further research is needed to clarify the role of autoantibodies as markers of increased or decreased CVD risk and their mechanism of action.
Autoantibodies directed at new antigens in lipoproteins within atherosclerotic lesions can modulate the impact of atherosclerosis via activation of the innate and adaptive immune system.76 The lipid-associated neopeptides are recognized as damage-associated or danger- associated molecular patterns (DAMPs), also known as alarmins, which signal molecules that can trigger and perpetuate noninfectious inflammatory responses.77-79 Plasma autoantibodies (immunoglobulin M and G [IgM, IgG]) modify proinflammatory oxidation-specific epitopes on oxidized phospholipids (oxPL) within lipoproteins and are linked with markers of inflammation and CVD events.80-82 Modified LDLC and ApoB-100 immune complexes with specific autoantibodies in the IgG class are associated with increased CVD.76 These and other risk-modulating autoantibodies may explain some of the variability in CVD outcomes by ethnicity and between individuals.
Some antibodies to oxidized LDL (ox-LDL) may have a protective role in the development of atherosclerosis.83,84 In a cohort of > 500 women, the number of carotid atherosclerotic plaques and total carotid plaque area were inversely correlated with a specific IgM autoantibody (MDA-p210).84 High concentrations of Lp(a)- containing circulating immune complexes and Lp(a)-specific IgM and IgG have been described in patients with coronary heart disease (CHD).85 Like ox-LDL, oxidized Lp(a) [ox-Lp(a)] is more potent than native Lp(a) in increasing atherosclerosis risk and is increased in patients with CHD compared to healthy controls.86-88 Ox-Lp(a) levels may represent an even stronger risk marker for CVD than ox-LDL.85
Possible Mechanisms of Pathogenesis
While the precise quantification of Lp(a) in human plasma (or serum) has been challenging, current clinical laboratories use standardized international reference reagents and controls in their assays. Most current Lp(a) assays are based on immunological methods (eg, immunonephelometry, immunoturbidimetry, or enzyme linked immunosorbent assay [ELISA]) using antibodies against apo(a).89 Apo(a) contains 10 subtypes of kringle IV and 1 copy of kringle V. Some assays use antibodies against kringle-IV type 2; however, it has been recommended that newer methods should use antibodies against the specific bridging kringle-IV Type 9 domain, which has a more stable bond and is present as a single copy.48,89 Other approaches to Lp(a) measurement include ultraperformance liquid chromatography/mass spectrometry that can determine both the concentration and particle size of apo(a).48,90 For routine clinical care, currently available assays reporting in mg/dL can be considered fairly accurate for separating low-risk from moderate-to-high-risk patients.45
The physiologic role of Lp(a) in humans remains to be fully defined and individuals with extremely low plasma Lp(a) levels present no disease or deficiency syndromes.91 Lp(a) accumulates in endothelial injuries and binds to components of the vessel wall and subendothelial matrix, presumably due to the strong lysine binding site in apo(a).46 Mediated by apo(a), the binding stimulates chemotactic activation of monocytes/macrophages and thereby modulating angiogenesis and inflammation.89 Lp(a) may contribute to CVD and CAVS via its LDL-like component, with proinflammatory effects of oxidized phospholipids (OxPL) on both ApoB and apo(a) and antifibrinolytic/prothrombotic effects of apo(a).92 In Vitro studies have demonstrated that apo(a) modifies cellular function of cultured vascular endothelial cells (promoting stress fiber formation, endothelial contraction and vascular permeability), smooth muscles, and monocytes/ macrophages (promoting differentiation of proinflammatory M1-1 type macrophages) via complex mechanisms of cell signaling and cytokine production.89 Lp(a) is the only monogenetic risk factor for aortic valve calcification and stenosis93 and is strongly linked specifically with the single nucleotide polymorphism (SNP) rs10455872 in the gene LPA encoding for apo(a).94
CVD Risk Predictive Value
There are a large number of studies demonstrating that Lp(a) elevations are an independent predictor of adverse cardiovascular outcomes including MI, sudden death, strokes, calcific aortic valve stenosis and peripheral vascular disease (Table 3). The Copenhagen City Heart Study and Copenhagen General Population Study are well known prospective population- based cohort studies that track outcomes through national patient registries.95 These studies demonstrate increased risk for MI, CHD, CAVS, and heart failure when subjects with very high Lp(a) levels (50-115 mg/dL) are compared with subjects with very low Lp(a) levels (< 5 mg/dL).96-100 Subjects with less extreme Lp(a) elevations (> 30 mg/dL) also show increased risk of CVD when they have comorbid LDLC elevations.101 However, the Copenhagen studies are composed exclusively of white subjects and the effects of Lp(a) are known to vary with race or ethnicity.
The Multi-Ethnic Study of Atherosclerosis (MESA) recruited an ethnically diverse sample of > 6,000 Americans, aged 45 to 84 years, without CVD, into an ongoing prospective cohort study. Research using subjects from this study has found consistently increased risk of CHD, heart failure, subclinical aortic valve calcification, and more severe CAVS in white subjects with elevated Lp(a).60,102,103 Black subjects with elevated Lp(a) had increased risk of CHD and more severe CAVS and Hispanic subjects with Lp(a) elevation were at higher risk for CHD.60,102 So far, no studies of MESA subjects have identified a relationship between Lp(a) elevation and CVD events for Asian-Americans subjects (predominantly of Chinese descent). There is a need for ongoing research to more precisely define relevant cut-off levels by race, ethnicity and sex.
The Atherosclerosis Risk in Communities (ARIC) Study was a prospective multiethnic cohort study including > 15,000 US adults, aged 45 to 64 years.103 Lp(a) elevations in this cohort were associated with greater risks for first CVD events, heart failure, and recurrent CVD events.61,64,105 The risk of stroke for subjects with elevated Lp(a) was greater for black and white women, and for black men.61,106 However, a meta-analysis of case-control studies showed increased ischemic stroke risk in both men and women with elevated Lp(a).57
A recent European meta-analysis collected blood samples and outcome data from > 50,000 subjects in 7 prospective cohort studies. Using a central laboratory to standardize Lp(a) measurements, researchers found increased risk of major coronary events and new CVD in subjects with Lp(a) > 50 mg/dL compared to those below that threshold.107
Although many of these studies show modest increases in risk of CVD events with Lp(a) elevation, it should be noted that other studies do not demonstrate such consistent associations. This is particularly true in studies of women and nonwhite ethnic groups.103,108-112 The variability of study results may be due to other confounding factors such as autoantibodies that either upregulate or downregulate atherogenicity of LDLC and potentially other lipoproteins. This is particularly relevant to women who have an increased risk for autoimmune disease.
Lp(a) has significant genetic heritability—75% in Europeans and 85% in African Americans.113 In whites, the LPA gene on chromosome 6p26- 27 with the polymorphism genetic variants rs10455872 and rs3798220 is consistently associated with elevated Lp(a) levels.63,100,113 However, the degree of Lp(a) elevation associated with these specific genetic variants varies by ethnicity.78,113,115
Lifestyle and Cardiovascular Health
It is noteworthy that the Lp(a) genetic risks can also be modified by lifestyle risk reduction even in the absence of significant blood level reductions. For example, Khera and colleagues constructed a genetic risk profile for CVD that included genes related to Lp(a).116 Subjects with high genetic risk were more likely to experience CVD events compared with subjects with low genetic risk. However, risks for CVD were attenuated by 4 healthy lifestyle factors: current nonsmoker, body mass index < 30, at least weekly physical activity, and a healthy diet. Subjects with high genetic risk and an unhealthy lifestyle (0 or 1 of the 4 healthy lifestyle factors) were the most likely to develop CVD (Hazard ratio [HR], 3.5), but that risk was lower for subjects with healthy (3 or 4 of the 4 healthy lifestyle factors) and intermediate lifestyles (2 of the 4 healthy lifestyle factors) (HR, 1.9 and 2.2, respectively), despite despite high genetic risk for CVD.
While the independent CVD risk associated with elevated Lp(a) does not appear to be responsive to lifestyle risk reduction alone, certainly elevated LDLC and traditional risk factors can increase the overall CVD risk and are worthy of preventive interventions. In particular, inflammation from any source exacerbates CVD risk. Proatherogenic diet, insufficient sleep, lack of exercise, and maladaptive stress responses are other targets for personalized CVD risk reduction. 28,117 Studies of dietary modifications and other lifestyle factors have shown reduced risk of CVD events, despite lack of reduction in Lp(a) levels.119,120 It is noteworthy that statin therapy (with or without ezetimibe) fails to impact CAVS progression, likely because statins either raise or have no effect on Lp(a) levels.92,119
Until recently, there has been no evidence supporting any therapeutic intervention causing clinically meaningful reductions in Lp(a). Table 4 lists major drug classes and their effects on Lp(a) and CVD outcomes; however, a detailed discussion of each of these therapies is beyond the scope of this review. Drugs that reduce Lp(a) by 20-30% have varying effects on CVD outcomes, from no effect122,123 to a 10% to 20% decrease in CVD events when compared with a placebo.124,125 Because these drugs also produce substantial reductions in LDLC, it is not possible to determine how much of the beneficial effects are due to reductions in Lp(a).
Lipoprotein apheresis produces profound reductions in Lp(a) of 60 to 80% in very highrisk populations.69,126 Within-subjects comparisons show up to 80% reductions in CVD events, relative to event rates prior to treatment initiation.69,127 Early trials of antisense oligonucleotide against apo(a) therapies show potential to produce similar outcomes.128,129 These treatments may be particularly effective in patients with isolated Lp(a) elevations.
Summary
Lp(a) elevation is a major contributor to cardiovascular disease risk and has been recognized as an ICD-10-CM coded clinical diagnosis, the first laboratory abnormality to be defined a clinical disease in the asymptomatic healthy young individuals. This change addresses currently under- diagnosed CVD risk independent of LDLC reduction strategies. A brief overview of recent guidelines for the clinical use of Lp(a) testing from the American Heart Association43,151 and the National Lipid Association52 can be found in Table 5. Although drug therapies for lowering Lp(a) levels remain limited, new treatment options are actively being developed.
Many Americans with high Lp(a) have not yet been identified. Expanded one-time screening can inform these patients of their cardiovascular risk and increase their access to early, aggressive lifestyle modification and optimal lipid-lowering therapy. Given the further increased CVD risk factors for military service members and veterans, a case can be made for broader screening and enhanced surveillance of elevated Lp(a) in these presumably healthy and fit individuals as well as management focused on modifiable risk factors.
Acknowledgements
This program initiative was conducted by the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. as part of the Integrative Cardiac Health Project at Walter Reed National Military Medical Center (WRNMMC), and is made possible by a cooperative agreement that was awarded and administered by the US Army Medical Research & Materiel Command (USAMRMC), at Fort Detrick under Contract Number: W81XWH-16-2-0007. It reflects literature review preparatory work for a research protocol but does not involve an actual research project. The work in this manuscript was supported by the staff of the Integrative Cardiac Health Project (ICHP) with special thanks to Claire Fuller, Elaine Walizer, Dr. Mariam Kashani and the entire health coaching team.
1. American Heart Association. Cardiovascular disease: a costly burden for America, projections through 2035. http://www.heart.org/idc/groups /heart-public/@wcm/@adv/documents/downloadable /ucm_491543.pdf. Accessed October 10, 2019.
2. Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67-e492.
3. Roth GA, Johnson C, Abajobir A, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70(1):1-25.
4. Thrift AG, Cadilhac DA, Thayabaranathan T, et al. Global stroke statistics. Int J Stroke. 2014;9(1):6-18.
5. Murray CJ, Barber RM, Foreman KJ, et al; GBD 2013 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990-2013: quantifying the epidemiological transition. Lancet. 2015;386(10009):2145-2191.
6. Mukherjee D, Patil CG. Epidemiology and the global burden of stroke. World Neurosurg. 2011;76(6 suppl):S85-S90.
7. Joseph A, Ackerman D, Talley JD, Johnstone J, Kupersmith J. Manifestations of coronary atherosclerosis in young trauma victims—an autopsy study. J Am Coll Cardiol. 1993;22(2):459-467.
8. Webber BJ, Seguin PG, Burnett DG, Clark LL, Otto JL. Prevalence of and risk factors for autopsy-determined atherosclerosis among US service members, 2001-2011. JAMA. 2012;308(24):2577-2583.
9. Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123(8):933-944.
10. Krishnamurthi N, Francis J, Fihn SD, Meyer CS, Whooley MA. Leading causes of cardiovascular hospitalization in 8.45 million US veterans. PLoS One. 2018;13(3):e0193996.
11. Torio CM, Moore BJ. National inpatient hospital costs: the most expensive conditions by payer. Agency for Healthcare Research and Quality Statistical Brief No. 204. http:// www.hcup-us.ahrq.gov/reports/statbriefs/sb204-Most -Expensive-Hospital-Conditions.pdf. Published May 2016. Accessed October 10, 2019.
12. Bloom DE, Cafiero ET, Jané-Llopis E, et al. The global economic burden of noncommunicable diseases. https:// www.weforum.org/reports/global-economic-burden-non -communicable-diseases. Published September 18, 2011. Accessed October 10, 2019.
13. Crum-Cianflone NF, Bagnell ME, Schaller E, et al. Impact of combat deployment and posttraumatic stress disorder on newly reported coronary heart disease among US active duty and reserve forces. Circulation. 2014;129(18):1813-1820.
14. Fryar CD, Herrick K, Afful J, Ogden CL. Cardiovascular disease risk factors among male veterans, U.S., 2009- 2012. Am J Prev Med. 2016;50(1):101-105.
15. Ulmer CS, Bosworth HB, Germain A, et al; VA Mid-Atlantic Mental Illness Research Education and Clinical Center Registry Workgroup. Associations between sleep difficulties and risk factors for cardiovascular disease in veterans and active duty military personnel of the Iraq and Afghanistan conflicts. J Behav Med. 2015;38(3):544-555.
16. Lutwak N, Dill C. Military sexual trauma increases risk of post-traumatic stress disorder and depression thereby amplifying the possibility of suicidal ideation and cardiovascular disease. Mil Med. 2013;178(4):359-361.
17. Bowry ADK, Lewey J, Dugani SB, Choudhry NK. The burden of cardiovascular disease in low- and middle-income countries: epidemiology and management. Can J Cardiol. 2015;31(9):1151-1159.
18. Reinier K, Stecker EC, Vickers C, Gunson K, Jui J, Chugh SS. Incidence of sudden cardiac arrest is higher in areas of low socioeconomic status: a prospective two year study in a large United States community. Resuscitation. 2006;70(2):186-192.
19. Reinier K, Thomas E, Andrusiek DL, et al; Resuscitation Outcomes Consortium Investigators. Socioeconomic status and incidence of sudden cardiac arrest. CMAJ. 2011;183(15):1705-1712.
20. Yusuf S, Rangarajan S, Teo K, et al; PURE Investigators. Cardiovascular risk and events in 17 low-, middle-, and high-income countries. N Engl J Med. 2014;371(9):818-827.
21. World Health Organization. Health topics: cardiovascular disease. http://www.who.int/cardiovascular_diseases/en/. Updated 2019. Accessed October 10, 2019.
22. Berkowitz AL. Stroke and the noncommunicable diseases: A global burden in need of global advocacy. Neurology. 2015;84(21):2183-2184.
23. Holt T. Predicting cardiovascular disease. BMJ. 2016;353:i2621.
24. Centers for Disease Control and Prevention. Million hearts: strategies to reduce the prevalence of leading cardiovascular disease risk factors—United States, 2011. MMWR Morb Mortal Wkly Rep. 2011;60(36):1248-1251.
25. Fryar CD, Chen TC, Li X. Prevalence of uncontrolled risk factors for cardiovascular disease: United States, 1999- 2010. NCHS Data Brief. 2012;103:1-8.
26. Ritchey MD, Loustalot F, Wall HK, et al. Million Hearts: description of the national surveillance and modeling methodology used to monitor the number of cardiovascular events prevented during 2012-2016. J Am Heart Assoc. 2017;6(5):pii:e00602.
27. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/ AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2935-2959.
28. Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): casecontrol study. Lancet. 2004;364(9438):937-952.
29. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2960-2984.
30. Bansilal S, Castellano JM, Fuster V. Global burden of CVD: focus on secondary prevention of cardiovascular disease. Int J Cardiol. 2015;201(suppl 1):S1-S7.
31. Havranek EP, Mujahid MS, Barr DA, et al. Social determinants of risk and outcomes for cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2015;132(9):873-898.
32. Miedema MD, Garberich RF, Schnaidt LJ, et al. Statin eligibility and outpatient care prior to ST-segment elevation myocardial infarction. J Am Heart Assoc. 2017;6(4): pii: e005333.
33. Noheria A, Teodorescu C, Uy-Evanado A, et al. Distinctive profile of sudden cardiac arrest in middle-aged vs. older adults: a community-based study. Int J Cardiol. 2013;168(4):3495-3499.
34. Lieb W, Enserro DM, Larson MG, Vasan RS. Residual cardiovascular risk in individuals on lipid-lowering treatment: quantifying absolute and relative risk in the community. Open Heart. 2018;5(1):e000722.
35. Sachdeva A, Cannon CP, Deedwania PC, et al. Lipid levels in patients hospitalized with coronary artery disease: an analysis of 136,905 hospitalizations in Get With The Guidelines. Am Heart J. 2009;157(1):111-117.e2.
36. Damen JA, Hooft L, Schuit E, et al. Prediction models for cardiovascular disease risk in the general population: systematic review. BMJ. 2016;353:i2416.
37. Fulcher J, O’Connell R, Voysey M, et al; Cholesterol Treatment Trialists (CTT) Collaboration. Efficacy and safety of LDL-lowering therapy among men and women: metaanalysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385(9976):1397-1405.
38. Perrone V, Sangiorgi D, Buda S, Degli Esposti L. Residual cardiovascular risk in patients who received lipid-lowering treatment in a real-life setting: retrospective study. Clinicoecon Outcomes Res. 2016;8:649-655.
39. Sirimarco G, Labreuche J, Bruckert E, et al; PERFORM and SPARCL Investigators. Atherogenic dyslipidemia and residual cardiovascular risk in statin-treated patients. Stroke. 2014;45(5):1429-1436.
40. Kones R. Molecular sources of residual cardiovascular risk, clinical signals, and innovative solutions: relationship with subclinical disease, undertreatment, and poor adherence: implications of new evidence upon optimizing cardiovascular patient outcomes. Vasc Health Risk Manag. 2013;9:617-670.
41. Hayashi M, Shimizu W, Albert CM. The spectrum of epidemiology underlying sudden cardiac death. Circ Res. 2015;116(12):1887-1906.
42. Downs JR, O’Malley PG. Management of dyslipidemia for cardiovascular disease risk reduction: synopsis of the 2014 U.S. Department of Veterans Affairs and U.S. Department of Defense clinical practice guideline. Ann Intern Med. 2015;163(4):291-297.
43. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/ AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/ NLA/PCNA Guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):3168-3209.
44. Tsimikas S, Fazio S, Ferdinand KC, et al. NHLBI Working Group recommendations to reduce lipoprotein(a)-mediated risk of cardiovascular disease and aortic stenosis. J Am Coll Cardiol. 2018;71(2):177-192.
45. Tsimikas S. A test in context: Lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol. 2017;69(6):692-711.
46. Ellis KL, Boffa MB, Sahebkar A, Koschinsky ML, Watts GF. The renaissance of lipoprotein(a): brave new world for preventive cardiology? Prog Lipid Res. 2017;68:57-82.
47. Thompson GR, Seed M. Lipoprotein(a): the underestimated cardiovascular risk factor. Heart. 2014;100(7):534-535.
48. Marcovina SM, Albers JJ. Lipoprotein (a) measurements for clinical application. J Lipid Res. 2016;57(4):526-537.
49. Tremulis SR. Founder’s Story: Lipoprotein(a) Foundation. https://www.lipoproteinafoundation.org/page /Sandrastory. Accessed October 10, 2019.
50. Centers for Disease Control and Prevention. ICD-10 Coordination and Maintenance Committee meeting, September 12-13, 2017 diagnosis agenda. https://www.cdc. gov/nchs/data/icd/Topic_Packet_Sept_2017.pdf. Accessed October 10, 2019.
51. Centers for Medicare & Medicaid Services. 2019 ICD- 10-CM codes descriptions in tabular order. https://www. cms.gov/Medicare/Coding/ICD10/2019-ICD-10-CM.html. Accessed October 10, 2019.
52. Wilson DP, Jacobson TA, Jones PH, et al. Use of Lipoprotein(a) in clinical practice: a biomarker whose time has come. A scientific statement from the National Lipid Association. J Clin Lipidol. 2019;13(3):374-392.
53. Langlois MR, Chapman MJ, Cobbaert C, et al. Quantifying atherogenic lipoproteins: current and future challenges in the era of personalized medicine and very low concentrations of ldl cholesterol. A consensus statement from EAS and EFLM. Clin Chem. 2018;64(7):1006-1033.
54. Shapiro MD, Fazio S. Apolipoprotein B-containing lipoproteins and atherosclerotic cardiovascular disease. F1000Res. 2017;6:134.
55. Tsimikas S, Fazio S, Viney NJ, Xia S, Witztum JL, Marcovina SM. Relationship of lipoprotein(a) molar concentrations and mass according to lipoprotein(a) thresholds and apolipoprotein(a) isoform size. J Clin Lipidol. 2018;12(5):1313-1323.
56. Erqou S, Kaptoge S, Perry PL, et al; Emerging Risk Factors Collaboration. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302(4):412-423.
57. Nave AH, Lange KS, Leonards CO, et al. Lipoprotein (a) as a risk factor for ischemic stroke: a meta-analysis. Atherosclerosis. 2015;242(2):496-503.
58. Varvel S, McConnell JP, Tsimikas S. Prevalence of elevated Lp(a) mass levels and patient thresholds in 532,359 patients in the United States. Arterioscler Thromb Vasc Biol. 2016;36(11):2239-2245.
59. Nordestgaard BG, Chapman MJ, Ray K, et al; European Atherosclerosis Society Consensus Panel. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31(23):2844-2853.
60. Guan W, Cao J, Steffen BT, et al. Race is a key variable in assigning lipoprotein(a) cutoff values for coronary heart disease risk assessment: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35(4):996-1001.
61. Virani SS, Brautbar A, Davis BC, et al. Associations between lipoprotein(a) levels and cardiovascular outcomes in black and white subjects: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2012;125(2):241-249.
62. Tsimikas S, Mallat Z, Talmud PJ, et al. Oxidation-specific biomarkers, lipoprotein(a), and risk of fatal and nonfatal coronary events. J Am Coll Cardiol. 2010;56(12):946-955.
63. Clarke R, Peden JF, Hopewell JC, et al; PROCARDIS Consortium. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. New Eng J Med. 2009;361(26):2518-2528.
64. Wattanakit K, Folsom AR, Chambless LE, Nieto FJ. Risk factors for cardiovascular event recurrence in the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2005;149(4):606-612.
65. Ruotolo G, Lincoff MA, Menon V, et al. Lipoprotein(a) is a determinant of residual cardiovascular risk in the setting of optimal LDL-C in statin-treated patients with atherosclerotic cardiovascular disease [Abstract 17400]. Circulation. 2018;136(suppl 1):A17400.
66. Suwa S, Ogita M, Miyauchi K, et al. Impact of lipoprotein (a) on long-term outcomes in patients with coronary artery disease treated with statin after a first percutaneous coronary intervention. J Atheroscler Thromb. 2017;24(11):1125-1131.
67. Nestel PJ, Barnes EH, Tonkin AM, et al. Plasma lipoprotein(a) concentration predicts future coronary and cardiovascular events in patients with stable coronary heart disease. Arterioscler Thromb Vasc Biol. 2013;33(12):2902-2908.
68. Burgess S, Ference BA, et al. Association of LPA variants with risk of coronary disease and the implications for lipoprotein(a)-lowering therapies: a Mendelian randomization analysis. JAMA Cardiol. 2018;3(7):619-627.
69. Roeseler E, Julius U, Heigl F, et al; Pro(a)LiFe-Study Group. Lipoprotein apheresis for lipoprotein(a)-associated cardiovascular disease: prospective 5 years of followup and apolipoprotein(a) characterization. Arterioscler Thromb Vasc Biol. 2016;36(9):2019-2027.
70. Matsuura E, Atzeni F, Sarzi-Puttini P, Turiel M, Lopez LR, Nurmohamed MT. Is atherosclerosis an autoimmune disease? BMC Med. 2014;12:47.
71. Ahearn J, Shields KJ, Liu CC, Manzi S. Cardiovascular disease biomarkers across autoimmune diseases. Clin Immunol. 2015;161(1):59-63.
72. Di Minno MND, Emmi G, Ambrosino P, et al. Subclinical atherosclerosis in asymptomatic carriers of persistent antiphospholipid antibodies positivity: a cross-sectional study. Int J Cardiol. 2019;274:1-6.
72. Di Minno MND, Emmi G, Ambrosino P, et al. Subclinical atherosclerosis in asymptomatic carriers of persistent antiphospholipid antibodies positivity: a cross-sectional study. Int J Cardiol. 2019;274:1-6.
73. Iseme RA, McEvoy M, Kelly B, et al. A role for autoantibodies in atherogenesis. Cardiovasc Res. 2017;113(10):1102-1112.
74. Sinicato NA, da Silva Cardoso PA, Appenzeller S. Risk factors in cardiovascular disease in systemic lupus erythematosus. Curr Cardiol Rev. 2013;9(1):15-19.
75. Sciatti E, Cavazzana I, Vizzardi E, et al. Systemic lupus erythematosus and endothelial dysfunction: a close relationship. Curr Rheumatol Rev. 2018;15(3):177-188.
76. Prasad A, Clopton P, Ayers C, et al. Relationship of autoantibodies to MDA-LDL and ApoB-Immune complexes to sex, ethnicity, subclinical atherosclerosis, and cardiovascular events. Arterioscler Thromb Vasc Biol. 2017;37(6):1213-1221.
77. Miller YI, Choi SH, Wiesner P, et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res. 2011;108(2):235-248.
78. Libby P, Lichtman AH, Hansson GK. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity. 2013;38(6):1092-1104.
79. Binder CJ, Papac-Milicevic N, Witztum JL. Innate sensing of oxidation-specific epitopes in health and disease. Nat Rev Immunol. 2016;16(8):485-497.
80. Freigang S. The regulation of inflammation by oxidized phospholipids. Eur J Immunol. 2016;46(8):1818-1825.
81. Ravandi A, Boekholdt SM, Mallat Z, et al. Relationship of oxidized LDL with markers of oxidation and inflammation and cardiovascular events: results from the EPIC-Norfolk Study. J Lipid Res. 2011;52(10):1829-1836.
82. Tsimikas S, Willeit P, Willeit J, et al. Oxidation-specific biomarkers, prospective 15-year cardiovascular and stroke outcomes, and net reclassification of cardiovascular events. J Am Coll Cardiol. 2012;60(21):2218-2229.
83. Cinoku I, Mavragani CP, Tellis CC, Nezos A, Tselepis AD, Moutsopoulos HM. Autoantibodies to ox-LDL in Sjogren’s syndrome: are they atheroprotective? Clin Exp Rheumatol. 2018;36 Suppl 112(3):61-67.
84. Fagerberg B, Prahl Gullberg U, Alm R, Nilsson J, Fredrikson GN. Circulating autoantibodies against the apolipoprotein B-100 peptides p45 and p210 in relation to the occurrence of carotid plaques in 64-year-old women. PLoS One. 2015;10(3):e0120744.
85. Klesareva EA, Afanas’eva OI, Donskikh VV, Adamova IY, Pokrovskii SN. Characteristics of lipoprotein(a)-containing circulating immune complexes as markers of coronary heart disease. Bull Exp Biol Med. 2016;162(2):231-236.
86. Morishita R, Ishii J, Kusumi Y, et al. Association of serum oxidized lipoprotein(a) concentration with coronary artery disease: potential role of oxidized lipoprotein(a) in the vasucular wall. J Atheroscler Thromb. 2009;16(4):410-418.
87. Wang J, Zhang C, Gong J, et al. Development of new enzyme-linked immunosorbent assay for oxidized lipoprotein(a) by using purified human oxidized lipoprotein(a) autoantibodies as capture antibody. Clin Chim Acta. 2007;385(1-2):73-78.
88. Wang JJ, Han AZ, Meng Y, et al. Measurement of oxidized lipoprotein (a) in patients with acute coronary syndromes and stable coronary artery disease by 2 ELISAs: using different capture antibody against oxidized lipoprotein (a) or oxidized LDL. Clin Biochem. 2010;43(6):571-575.
89. Orso E, Schmitz G. Lipoprotein(a) and its role in inflammation, atherosclerosis and malignancies. Clin Res Cardiol Suppl. 2017;12(Suppl 1):31-37.
90. Lassman ME, McLaughlin TM, Zhou H, et al. Simultaneous quantitation and size characterization of apolipoprotein(a) by ultra-performance liquid chromatography/ mass spectrometry. Rapid Commun Mass Spectrom. 2014;28(10):1101-1106.
91. Lippi G, Guidi G. Lipoprotein(a): from ancestral benefit to modern pathogen? QJM. 2000;93(2):75-84.
92. van der Valk FM, Bekkering S, Kroon J, et al. Oxidized phospholipids on lipoprotein(a) elicit arterial wall inflammation and an inflammatory monocyte response in humans. Circulation. 2016;134(8):611-624.
93. Yeang C, Wilkinson MJ, Tsimikas S. Lipoprotein(a) and oxidized phospholipids in calcific aortic valve stenosis. Curr Opin Cardiol. 2016;31(4):440-450.
94. Thanassoulis G, Campbell CY, Owens DS, et al; CHARGE Extracoronary Calcium Working Group. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368(6):503-512.
95. Aguib Y, Al Suwaidi J. The Copenhagen City Heart Study (Osterbroundersogelsen). Glob Cardiol Sci Pract. 2015;2015(3):33.
96. Kamstrup PR, Benn M, Tybjaerg-Hansen A, Nordestgaard BG. Extreme lipoprotein(a) levels and risk of myocardial infarction in the general population: the Copenhagen City Heart Study. Circulation. 2008;117(2):176-184.
97. Kamstrup PR, Tybjærg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301(22):2331-2339.
98. Kamstrup PR, Tybjaerg-Hansen A, Nordestgaard BG. Extreme lipoprotein(a) levels and improved cardiovascular risk prediction. J Am Coll Cardiol.2013;61(11):1146-1156.
99. Kamstrup PR, Tybjaerg-Hansen A, Nordestgaard BG. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J Am Coll Cardiol. 2014;63(5):470-477.
100. Kamstrup PR, Nordestgaard BG. Elevated lipoprotein(a) levels, LPA risk genotypes, and increased risk of heart failure in the general population. JACC Heart Fail.2016;4(1):78-87.
101. Verbeek R, Hoogeveen RM, Langsted A, et al. Cardiovascular disease risk associated with elevated lipoprotein(a) attenuates at low low-density lipoprotein cholesterol levels in a primary prevention setting. Eur Heart J. 2018;39(27):2589-2596.
102. Cao J, Steffen BT, Budoff M, et al. Lipoprotein(a) levels are associated with subclinical calcific aortic valve disease in white and black individuals: the multi-ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol. 2016;36(5):1003-1009.
103. Steffen BT, Duprez D, Bertoni AG, Guan W, Tsai M. Lp(a) [lipoprotein(a)]-related risk of heart failure is evident in whites but not in other racial/ethnic groups.Arterioscler Thromb Vasc Biol. 2018;38(10):2498-2504.
104. ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129(4):687-702.
105. Agarwala A, Pokharel Y, Saeed A, et al. The association of lipoprotein(a) with incident heart failure hospitalization: Atherosclerosis Risk in Communities study. Atherosclerosis. 2017;262:131-137.
106. Ohira T, Schreiner PJ, Morrisett JD, Chambless LE, Rosamond WD, Folsom AR. Lipoprotein(a) and incident ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2006;37(6):1407-1412.
107. Waldeyer C, Makarova N, Zeller T, et al. Lipoprotein(a) and the risk of cardiovascular disease in the European population: results from the BiomarCaRE consortium. Eur Heart J. 2017;38(32):2490-2498.
108. Cook NR, Mora S, Ridker PM. Lipoprotein(a) and cardiovascular risk prediction among women. J Am Coll Cardiol. 2018;72(3):287-296.
109. Suk Danik J, Rifai N, Buring JE, Ridker PM. Lipoprotein(a), measured with an assay independent of apolipoprotein(a) isoform size, and risk of future cardiovascular events among initially healthy women. JAMA. 2006;296(11):1363-1370.
110. Suk Danik J, Rifai N, Buring JE, Ridker PM. Lipoprotein(a), hormone replacement therapy, and risk of future cardiovascular events. J Am Coll Cardiol. 2008;52(2):124-131.
111. Chien KL, Hsu HC, Su TC, Sung FC, Chen MF, Lee YT. Lipoprotein(a) and cardiovascular disease in ethnic Chinese: the Chin-Shan Community Cardiovascular Cohort Study. Clin Chem. 2008;54(2):285-291.
112. Lee SR, Prasad A, Choi YS, et al. LPA gene, ethnicity, and cardiovascular events. Circulation.2017;135(3):251-263.
113. Zekavat SM, Ruotsalainen S, Handsaker RE, et al. Deep coverage whole genome sequences and plasma lipoprotein(a) in individuals of European and African ancestries. Nat Commun.2018;9(1):2606.
114. Zewinger S, Kleber ME, Tragante V, et al. Relations between lipoprotein(a) concentrations, LPA genetic variants, and the risk of mortality in patients with established coronary heart disease: a molecular and genetic association study. Lancet Diabetes Endocrinol. 2017;5(7):534-543.
115. Li J, Lange LA, Sabourin J, et al. Genome- and exomewide association study of serum lipoprotein (a) in the Jackson Heart Study. J Hum Genet. 2015;60(12):755-761.
116. Khera AV, Emdin CA, Drake I, et al, Kathiresan S. Genetic risk, adherence to a healthy lifestyle, and coronary disease. N Engl J Med.2016;375(24):2349-2358.
117. Nordestgaard BG, Langsted A. Lipoprotein(a) as a cause of cardiovascular disease: insights from epidemiology, genetics, and biology. J Lipid Res.2016;57(11):1953-1975.
118. Sofi F, Cesari F, Casini A, Macchi C, Abbate R, Gensini GF. Insomnia and risk of cardiovascular disease: a metaanalysis. Eur J Prev Cardiol.2014;21(1):57-64.
119. Estruch R, Ros E, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with a mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med.2018;378(25):e34.
120. Perrot N, Verbeek R, Sandhu M, et al. Ideal cardiovascular health influences cardiovascular disease risk associated with high lipoprotein(a) levels and genotype: The EPICNorfolk prospective population study. Atherosclerosis. 2017;256:47-52.
121. Teo KK, Corsi DJ, Tam JW, Dumesnil JG, Chan KL. Lipid lowering on progression of mild to moderate aortic stenosis: meta-analysis of the randomized placebocontrolled clinical trials on 2344 patients. Can J Cardiol. 2011;27(6):800-808.
122. Albers JJ, Slee A, O’Brien KD, et al. Relationship of apolipoproteins A-1 and B, and lipoprotein(a) to cardiovascular outcomes: the AIM-HIGH trial (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglyceride and Impact on Global Health Outcomes). J Am Coll Cardiol. 2013;62(17):1575-1579.
123. Lincoff AM, Nicholls SJ, Riesmeyer JS, et al; ACCELERATE Investigators. Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med. 2017;376(20):1933-1942.
124. Schmidt AF, Pearce LS, Wilkins JT, Overington JP, Hingorani AD, Casas JP. PCSK9 monoclonal antibodies for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev.2017;4:CD011748.
125. Bowman L, Hopewell JC, Chen F, et al; PHS3/TIM155-REVEAL Collaborative Group. Effects of anacetrapib in patients with atherosclerotic vascular disease.
126. Leebmann J, Roeseler E, Julius U, et al; Pro(a)LiFe Study Group. Lipoprotein apheresis in patients with maximally tolerated lipid-lowering therapy, lipoprotein(a)-hyperlipoproteinemia, and progressive cardiovascular disease: prospective observational multicenter study. Circulation. 2013;128(24):2567-2576.
127. Heigl F, Hettich R, Lotz N, et al. Efficacy, safety, and tolerability of long-term lipoprotein apheresis in patients with LDL- or Lp(a) hyperlipoproteinemia: Findings gathered from more than 36,000 treatments at one center in Germany. Atheroscler Suppl. 2015;18:154-162.
128. Viney NJ, van Capelleveen JC, Geary RS, et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet. 2016;388(10057):2239-2253.
129. Graham MJ, Viney N, Crooke RM, Tsimikas S. Antisense inhibition of apolipoprotein (a) to lower plasma lipoprotein (a) levels in humans. J Lipid Res. 2016;57(3):340-351.
130. Keene D, Price C, Shun-Shin MJ, Francis DP. Effect on cardiovascular risk of high density lipoprotein targeted drug treatments niacin, fibrates, and CETP inhibitors: meta-analysis of randomised controlled trials including 117,411 patients. BMJ. 2014;349:g4379.
131. Nicholls SJ, Ruotolo G, Brewer HB, et al. Evacetrapib alone or in combination with statins lowers lipoprotein(a) and total and small LDL particle concentrations in mildly hypercholesterolemic patients. J Clin Lipidol. 2016;10(3):519-527.e4.
132. Schwartz GG, Ballantyne CM, Barter PJ, et al. Association of lipoprotein(a) with risk of recurrent ischemic events following acute coronary syndrome: analysis of the dal-outcomes randomized clinical trial. JAMA Cardiol.2018;3(2):164-168.
133. Schwartz GG, Olsson AG, Abt M, et al; dal-OUTCOMES Investigators. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med.2012;367(22):2089-2099.
134. Thomas T, Zhou H, Karmally W, et al. CETP (Cholesteryl Ester Transfer Protein) inhibition with anacetrapib decreases production of lipoprotein(a) in mildly hypercholesterolemic subjects. Arterioscler Thromb Vasc Biol.2017;37(9):1770-1775.
135. Khera AV, Everett BM, Caulfield MP, et al. Lipoprotein(a) concentrations, rosuvastatin therapy, and residual vascular risk: an analysis from the JUPITER Trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin). Circulation. 2014;129(6):635-642.
136. Yeang C, Hung MY, Byun YS, et al. Effect of therapeutic interventions on oxidized phospholipids on apolipoprotein B100 and lipoprotein(a). J Clin Lipidol. 2016;10(3):594-603.
137. Zhou Z, Rahme E, Pilote L. Are statins created equal? Evidence from randomized trials of pravastatin, simvastatin, and atorvastatin for cardiovascular disease prevention.Am Heart J. 2006;151(2):273-281.
138. Ridker PM, MacFadyen JG, Fonseca FA, et al; JUPITER Study Group. Number needed to treat with rosuvastatin to prevent first cardiovascular events and death among men and women with low low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein: justification for the use of statins in prevention: an intervention trial evaluating rosuvastatin (JUPITER). Circ Cardiovasc Qual Outcomes. 2009;2(6):616-623.
139. Raal FJ, Giugliano RP, Sabatine MS, et al. Reduction in lipoprotein(a) with PCSK9 monoclonal antibody evolocumab (AMG 145): a pooled analysis of more than 1,300 patients in 4 phase II trials. J Am Coll Cardiol.2014;63(13):1278-1288.
140. Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1500-1509.
141. Koren MJ, Sabatine MS, Giugliano RP, et al. Long-term low-density lipoprotein cholesterol-lowering efficacy, persistence, and safety of evolocumab in treatment of hypercholesterolemia: results up to 4 years from the open-label OSLER-1 extension study. JAMA Cardiol.2017;2(6):598-607.
142. Desai NR, Kohli P, Giugliano RP, et al. AMG145, a monoclonal antibody against proprotein convertase subtilisin kexin type 9, significantly reduces lipoprotein(a) in hypercholesterolemic patients receiving statin therapy: an analysis from the LDL-C Assessment with Proprotein Convertase Subtilisin Kexin Type 9 Monoclonal Antibody Inhibition Combined with Statin Therapy (LAPLACE)-Thrombolysis in Myocardial Infarction (TIMI) 57 trial. Circulation.2013;128(9):962-969.
143. Schwartz GG, Steg PG, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and cardiovascular outcomes after acute coronary syndrome.N Engl J Med. 2018;379(22):2097-2107.
144. Sabatine MS, Giugliano RP, Keech AC, et al; FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular Disease.N Engl J Med. 2017;376(18):1713-1722.
145. Karatasakis A, Danek BA, Karacsonyi J, et al. Effect of PCSK9 inhibitors on clinical outcomes in patients with hypercholesterolemia: A meta-analysis of 35 randomized controlled trials. J Am Heart Assoc. 2017;6(12):e006910.
146. Santos RD, Duell PB, East C, et al. Long-term efficacy and safety of mipomersen in patients with familial hypercholesterolaemia: 2-year interim results of an open-label extension.Eur Heart J. 2015;36(9):566-575.
147. Duell PB, Santos RD, Kirwan BA, Witztum JL, Tsimikas S, Kastelein JJP. Long-term mipomersen treatment is associated with a reduction in cardiovascular events in patients with familial hypercholesterolemia. J Clin Lipidol. 2016;10(4):1011-1021.
148. McGowan MP, Tardif JC, Ceska R, et al. Randomized, placebo-controlled trial of mipomersen in patients with severe hypercholesterolemia receiving maximally tolerated lipid-lowering therapy. PLoS One.2012;7(11):e49006.
149. Jaeger BR, Richter Y, Nagel D, et al. Longitudinal cohort study on the effectiveness of lipid apheresis treatment to reduce high lipoprotein(a) levels and prevent major adverse coronary events. Nat Clin Pract Cardiovasc Med.2009;6(3):229-239.
150. Rosada A, Kassner U, Vogt A, Willhauck M, Parhofer K, Steinhagen-Thiessen E. Does regular lipid apheresis in Does regular lipid apheresis in patients with isolated elevated lipoprotein(a) levels reduce the incidence of cardiovascular events? Artif Organs. 2014;38(2):135-141.
151. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596-e646.
1. American Heart Association. Cardiovascular disease: a costly burden for America, projections through 2035. http://www.heart.org/idc/groups /heart-public/@wcm/@adv/documents/downloadable /ucm_491543.pdf. Accessed October 10, 2019.
2. Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67-e492.
3. Roth GA, Johnson C, Abajobir A, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70(1):1-25.
4. Thrift AG, Cadilhac DA, Thayabaranathan T, et al. Global stroke statistics. Int J Stroke. 2014;9(1):6-18.
5. Murray CJ, Barber RM, Foreman KJ, et al; GBD 2013 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990-2013: quantifying the epidemiological transition. Lancet. 2015;386(10009):2145-2191.
6. Mukherjee D, Patil CG. Epidemiology and the global burden of stroke. World Neurosurg. 2011;76(6 suppl):S85-S90.
7. Joseph A, Ackerman D, Talley JD, Johnstone J, Kupersmith J. Manifestations of coronary atherosclerosis in young trauma victims—an autopsy study. J Am Coll Cardiol. 1993;22(2):459-467.
8. Webber BJ, Seguin PG, Burnett DG, Clark LL, Otto JL. Prevalence of and risk factors for autopsy-determined atherosclerosis among US service members, 2001-2011. JAMA. 2012;308(24):2577-2583.
9. Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123(8):933-944.
10. Krishnamurthi N, Francis J, Fihn SD, Meyer CS, Whooley MA. Leading causes of cardiovascular hospitalization in 8.45 million US veterans. PLoS One. 2018;13(3):e0193996.
11. Torio CM, Moore BJ. National inpatient hospital costs: the most expensive conditions by payer. Agency for Healthcare Research and Quality Statistical Brief No. 204. http:// www.hcup-us.ahrq.gov/reports/statbriefs/sb204-Most -Expensive-Hospital-Conditions.pdf. Published May 2016. Accessed October 10, 2019.
12. Bloom DE, Cafiero ET, Jané-Llopis E, et al. The global economic burden of noncommunicable diseases. https:// www.weforum.org/reports/global-economic-burden-non -communicable-diseases. Published September 18, 2011. Accessed October 10, 2019.
13. Crum-Cianflone NF, Bagnell ME, Schaller E, et al. Impact of combat deployment and posttraumatic stress disorder on newly reported coronary heart disease among US active duty and reserve forces. Circulation. 2014;129(18):1813-1820.
14. Fryar CD, Herrick K, Afful J, Ogden CL. Cardiovascular disease risk factors among male veterans, U.S., 2009- 2012. Am J Prev Med. 2016;50(1):101-105.
15. Ulmer CS, Bosworth HB, Germain A, et al; VA Mid-Atlantic Mental Illness Research Education and Clinical Center Registry Workgroup. Associations between sleep difficulties and risk factors for cardiovascular disease in veterans and active duty military personnel of the Iraq and Afghanistan conflicts. J Behav Med. 2015;38(3):544-555.
16. Lutwak N, Dill C. Military sexual trauma increases risk of post-traumatic stress disorder and depression thereby amplifying the possibility of suicidal ideation and cardiovascular disease. Mil Med. 2013;178(4):359-361.
17. Bowry ADK, Lewey J, Dugani SB, Choudhry NK. The burden of cardiovascular disease in low- and middle-income countries: epidemiology and management. Can J Cardiol. 2015;31(9):1151-1159.
18. Reinier K, Stecker EC, Vickers C, Gunson K, Jui J, Chugh SS. Incidence of sudden cardiac arrest is higher in areas of low socioeconomic status: a prospective two year study in a large United States community. Resuscitation. 2006;70(2):186-192.
19. Reinier K, Thomas E, Andrusiek DL, et al; Resuscitation Outcomes Consortium Investigators. Socioeconomic status and incidence of sudden cardiac arrest. CMAJ. 2011;183(15):1705-1712.
20. Yusuf S, Rangarajan S, Teo K, et al; PURE Investigators. Cardiovascular risk and events in 17 low-, middle-, and high-income countries. N Engl J Med. 2014;371(9):818-827.
21. World Health Organization. Health topics: cardiovascular disease. http://www.who.int/cardiovascular_diseases/en/. Updated 2019. Accessed October 10, 2019.
22. Berkowitz AL. Stroke and the noncommunicable diseases: A global burden in need of global advocacy. Neurology. 2015;84(21):2183-2184.
23. Holt T. Predicting cardiovascular disease. BMJ. 2016;353:i2621.
24. Centers for Disease Control and Prevention. Million hearts: strategies to reduce the prevalence of leading cardiovascular disease risk factors—United States, 2011. MMWR Morb Mortal Wkly Rep. 2011;60(36):1248-1251.
25. Fryar CD, Chen TC, Li X. Prevalence of uncontrolled risk factors for cardiovascular disease: United States, 1999- 2010. NCHS Data Brief. 2012;103:1-8.
26. Ritchey MD, Loustalot F, Wall HK, et al. Million Hearts: description of the national surveillance and modeling methodology used to monitor the number of cardiovascular events prevented during 2012-2016. J Am Heart Assoc. 2017;6(5):pii:e00602.
27. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/ AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2935-2959.
28. Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): casecontrol study. Lancet. 2004;364(9438):937-952.
29. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2960-2984.
30. Bansilal S, Castellano JM, Fuster V. Global burden of CVD: focus on secondary prevention of cardiovascular disease. Int J Cardiol. 2015;201(suppl 1):S1-S7.
31. Havranek EP, Mujahid MS, Barr DA, et al. Social determinants of risk and outcomes for cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2015;132(9):873-898.
32. Miedema MD, Garberich RF, Schnaidt LJ, et al. Statin eligibility and outpatient care prior to ST-segment elevation myocardial infarction. J Am Heart Assoc. 2017;6(4): pii: e005333.
33. Noheria A, Teodorescu C, Uy-Evanado A, et al. Distinctive profile of sudden cardiac arrest in middle-aged vs. older adults: a community-based study. Int J Cardiol. 2013;168(4):3495-3499.
34. Lieb W, Enserro DM, Larson MG, Vasan RS. Residual cardiovascular risk in individuals on lipid-lowering treatment: quantifying absolute and relative risk in the community. Open Heart. 2018;5(1):e000722.
35. Sachdeva A, Cannon CP, Deedwania PC, et al. Lipid levels in patients hospitalized with coronary artery disease: an analysis of 136,905 hospitalizations in Get With The Guidelines. Am Heart J. 2009;157(1):111-117.e2.
36. Damen JA, Hooft L, Schuit E, et al. Prediction models for cardiovascular disease risk in the general population: systematic review. BMJ. 2016;353:i2416.
37. Fulcher J, O’Connell R, Voysey M, et al; Cholesterol Treatment Trialists (CTT) Collaboration. Efficacy and safety of LDL-lowering therapy among men and women: metaanalysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385(9976):1397-1405.
38. Perrone V, Sangiorgi D, Buda S, Degli Esposti L. Residual cardiovascular risk in patients who received lipid-lowering treatment in a real-life setting: retrospective study. Clinicoecon Outcomes Res. 2016;8:649-655.
39. Sirimarco G, Labreuche J, Bruckert E, et al; PERFORM and SPARCL Investigators. Atherogenic dyslipidemia and residual cardiovascular risk in statin-treated patients. Stroke. 2014;45(5):1429-1436.
40. Kones R. Molecular sources of residual cardiovascular risk, clinical signals, and innovative solutions: relationship with subclinical disease, undertreatment, and poor adherence: implications of new evidence upon optimizing cardiovascular patient outcomes. Vasc Health Risk Manag. 2013;9:617-670.
41. Hayashi M, Shimizu W, Albert CM. The spectrum of epidemiology underlying sudden cardiac death. Circ Res. 2015;116(12):1887-1906.
42. Downs JR, O’Malley PG. Management of dyslipidemia for cardiovascular disease risk reduction: synopsis of the 2014 U.S. Department of Veterans Affairs and U.S. Department of Defense clinical practice guideline. Ann Intern Med. 2015;163(4):291-297.
43. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/ AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/ NLA/PCNA Guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):3168-3209.
44. Tsimikas S, Fazio S, Ferdinand KC, et al. NHLBI Working Group recommendations to reduce lipoprotein(a)-mediated risk of cardiovascular disease and aortic stenosis. J Am Coll Cardiol. 2018;71(2):177-192.
45. Tsimikas S. A test in context: Lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol. 2017;69(6):692-711.
46. Ellis KL, Boffa MB, Sahebkar A, Koschinsky ML, Watts GF. The renaissance of lipoprotein(a): brave new world for preventive cardiology? Prog Lipid Res. 2017;68:57-82.
47. Thompson GR, Seed M. Lipoprotein(a): the underestimated cardiovascular risk factor. Heart. 2014;100(7):534-535.
48. Marcovina SM, Albers JJ. Lipoprotein (a) measurements for clinical application. J Lipid Res. 2016;57(4):526-537.
49. Tremulis SR. Founder’s Story: Lipoprotein(a) Foundation. https://www.lipoproteinafoundation.org/page /Sandrastory. Accessed October 10, 2019.
50. Centers for Disease Control and Prevention. ICD-10 Coordination and Maintenance Committee meeting, September 12-13, 2017 diagnosis agenda. https://www.cdc. gov/nchs/data/icd/Topic_Packet_Sept_2017.pdf. Accessed October 10, 2019.
51. Centers for Medicare & Medicaid Services. 2019 ICD- 10-CM codes descriptions in tabular order. https://www. cms.gov/Medicare/Coding/ICD10/2019-ICD-10-CM.html. Accessed October 10, 2019.
52. Wilson DP, Jacobson TA, Jones PH, et al. Use of Lipoprotein(a) in clinical practice: a biomarker whose time has come. A scientific statement from the National Lipid Association. J Clin Lipidol. 2019;13(3):374-392.
53. Langlois MR, Chapman MJ, Cobbaert C, et al. Quantifying atherogenic lipoproteins: current and future challenges in the era of personalized medicine and very low concentrations of ldl cholesterol. A consensus statement from EAS and EFLM. Clin Chem. 2018;64(7):1006-1033.
54. Shapiro MD, Fazio S. Apolipoprotein B-containing lipoproteins and atherosclerotic cardiovascular disease. F1000Res. 2017;6:134.
55. Tsimikas S, Fazio S, Viney NJ, Xia S, Witztum JL, Marcovina SM. Relationship of lipoprotein(a) molar concentrations and mass according to lipoprotein(a) thresholds and apolipoprotein(a) isoform size. J Clin Lipidol. 2018;12(5):1313-1323.
56. Erqou S, Kaptoge S, Perry PL, et al; Emerging Risk Factors Collaboration. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302(4):412-423.
57. Nave AH, Lange KS, Leonards CO, et al. Lipoprotein (a) as a risk factor for ischemic stroke: a meta-analysis. Atherosclerosis. 2015;242(2):496-503.
58. Varvel S, McConnell JP, Tsimikas S. Prevalence of elevated Lp(a) mass levels and patient thresholds in 532,359 patients in the United States. Arterioscler Thromb Vasc Biol. 2016;36(11):2239-2245.
59. Nordestgaard BG, Chapman MJ, Ray K, et al; European Atherosclerosis Society Consensus Panel. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31(23):2844-2853.
60. Guan W, Cao J, Steffen BT, et al. Race is a key variable in assigning lipoprotein(a) cutoff values for coronary heart disease risk assessment: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35(4):996-1001.
61. Virani SS, Brautbar A, Davis BC, et al. Associations between lipoprotein(a) levels and cardiovascular outcomes in black and white subjects: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2012;125(2):241-249.
62. Tsimikas S, Mallat Z, Talmud PJ, et al. Oxidation-specific biomarkers, lipoprotein(a), and risk of fatal and nonfatal coronary events. J Am Coll Cardiol. 2010;56(12):946-955.
63. Clarke R, Peden JF, Hopewell JC, et al; PROCARDIS Consortium. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. New Eng J Med. 2009;361(26):2518-2528.
64. Wattanakit K, Folsom AR, Chambless LE, Nieto FJ. Risk factors for cardiovascular event recurrence in the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2005;149(4):606-612.
65. Ruotolo G, Lincoff MA, Menon V, et al. Lipoprotein(a) is a determinant of residual cardiovascular risk in the setting of optimal LDL-C in statin-treated patients with atherosclerotic cardiovascular disease [Abstract 17400]. Circulation. 2018;136(suppl 1):A17400.
66. Suwa S, Ogita M, Miyauchi K, et al. Impact of lipoprotein (a) on long-term outcomes in patients with coronary artery disease treated with statin after a first percutaneous coronary intervention. J Atheroscler Thromb. 2017;24(11):1125-1131.
67. Nestel PJ, Barnes EH, Tonkin AM, et al. Plasma lipoprotein(a) concentration predicts future coronary and cardiovascular events in patients with stable coronary heart disease. Arterioscler Thromb Vasc Biol. 2013;33(12):2902-2908.
68. Burgess S, Ference BA, et al. Association of LPA variants with risk of coronary disease and the implications for lipoprotein(a)-lowering therapies: a Mendelian randomization analysis. JAMA Cardiol. 2018;3(7):619-627.
69. Roeseler E, Julius U, Heigl F, et al; Pro(a)LiFe-Study Group. Lipoprotein apheresis for lipoprotein(a)-associated cardiovascular disease: prospective 5 years of followup and apolipoprotein(a) characterization. Arterioscler Thromb Vasc Biol. 2016;36(9):2019-2027.
70. Matsuura E, Atzeni F, Sarzi-Puttini P, Turiel M, Lopez LR, Nurmohamed MT. Is atherosclerosis an autoimmune disease? BMC Med. 2014;12:47.
71. Ahearn J, Shields KJ, Liu CC, Manzi S. Cardiovascular disease biomarkers across autoimmune diseases. Clin Immunol. 2015;161(1):59-63.
72. Di Minno MND, Emmi G, Ambrosino P, et al. Subclinical atherosclerosis in asymptomatic carriers of persistent antiphospholipid antibodies positivity: a cross-sectional study. Int J Cardiol. 2019;274:1-6.
72. Di Minno MND, Emmi G, Ambrosino P, et al. Subclinical atherosclerosis in asymptomatic carriers of persistent antiphospholipid antibodies positivity: a cross-sectional study. Int J Cardiol. 2019;274:1-6.
73. Iseme RA, McEvoy M, Kelly B, et al. A role for autoantibodies in atherogenesis. Cardiovasc Res. 2017;113(10):1102-1112.
74. Sinicato NA, da Silva Cardoso PA, Appenzeller S. Risk factors in cardiovascular disease in systemic lupus erythematosus. Curr Cardiol Rev. 2013;9(1):15-19.
75. Sciatti E, Cavazzana I, Vizzardi E, et al. Systemic lupus erythematosus and endothelial dysfunction: a close relationship. Curr Rheumatol Rev. 2018;15(3):177-188.
76. Prasad A, Clopton P, Ayers C, et al. Relationship of autoantibodies to MDA-LDL and ApoB-Immune complexes to sex, ethnicity, subclinical atherosclerosis, and cardiovascular events. Arterioscler Thromb Vasc Biol. 2017;37(6):1213-1221.
77. Miller YI, Choi SH, Wiesner P, et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res. 2011;108(2):235-248.
78. Libby P, Lichtman AH, Hansson GK. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity. 2013;38(6):1092-1104.
79. Binder CJ, Papac-Milicevic N, Witztum JL. Innate sensing of oxidation-specific epitopes in health and disease. Nat Rev Immunol. 2016;16(8):485-497.
80. Freigang S. The regulation of inflammation by oxidized phospholipids. Eur J Immunol. 2016;46(8):1818-1825.
81. Ravandi A, Boekholdt SM, Mallat Z, et al. Relationship of oxidized LDL with markers of oxidation and inflammation and cardiovascular events: results from the EPIC-Norfolk Study. J Lipid Res. 2011;52(10):1829-1836.
82. Tsimikas S, Willeit P, Willeit J, et al. Oxidation-specific biomarkers, prospective 15-year cardiovascular and stroke outcomes, and net reclassification of cardiovascular events. J Am Coll Cardiol. 2012;60(21):2218-2229.
83. Cinoku I, Mavragani CP, Tellis CC, Nezos A, Tselepis AD, Moutsopoulos HM. Autoantibodies to ox-LDL in Sjogren’s syndrome: are they atheroprotective? Clin Exp Rheumatol. 2018;36 Suppl 112(3):61-67.
84. Fagerberg B, Prahl Gullberg U, Alm R, Nilsson J, Fredrikson GN. Circulating autoantibodies against the apolipoprotein B-100 peptides p45 and p210 in relation to the occurrence of carotid plaques in 64-year-old women. PLoS One. 2015;10(3):e0120744.
85. Klesareva EA, Afanas’eva OI, Donskikh VV, Adamova IY, Pokrovskii SN. Characteristics of lipoprotein(a)-containing circulating immune complexes as markers of coronary heart disease. Bull Exp Biol Med. 2016;162(2):231-236.
86. Morishita R, Ishii J, Kusumi Y, et al. Association of serum oxidized lipoprotein(a) concentration with coronary artery disease: potential role of oxidized lipoprotein(a) in the vasucular wall. J Atheroscler Thromb. 2009;16(4):410-418.
87. Wang J, Zhang C, Gong J, et al. Development of new enzyme-linked immunosorbent assay for oxidized lipoprotein(a) by using purified human oxidized lipoprotein(a) autoantibodies as capture antibody. Clin Chim Acta. 2007;385(1-2):73-78.
88. Wang JJ, Han AZ, Meng Y, et al. Measurement of oxidized lipoprotein (a) in patients with acute coronary syndromes and stable coronary artery disease by 2 ELISAs: using different capture antibody against oxidized lipoprotein (a) or oxidized LDL. Clin Biochem. 2010;43(6):571-575.
89. Orso E, Schmitz G. Lipoprotein(a) and its role in inflammation, atherosclerosis and malignancies. Clin Res Cardiol Suppl. 2017;12(Suppl 1):31-37.
90. Lassman ME, McLaughlin TM, Zhou H, et al. Simultaneous quantitation and size characterization of apolipoprotein(a) by ultra-performance liquid chromatography/ mass spectrometry. Rapid Commun Mass Spectrom. 2014;28(10):1101-1106.
91. Lippi G, Guidi G. Lipoprotein(a): from ancestral benefit to modern pathogen? QJM. 2000;93(2):75-84.
92. van der Valk FM, Bekkering S, Kroon J, et al. Oxidized phospholipids on lipoprotein(a) elicit arterial wall inflammation and an inflammatory monocyte response in humans. Circulation. 2016;134(8):611-624.
93. Yeang C, Wilkinson MJ, Tsimikas S. Lipoprotein(a) and oxidized phospholipids in calcific aortic valve stenosis. Curr Opin Cardiol. 2016;31(4):440-450.
94. Thanassoulis G, Campbell CY, Owens DS, et al; CHARGE Extracoronary Calcium Working Group. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368(6):503-512.
95. Aguib Y, Al Suwaidi J. The Copenhagen City Heart Study (Osterbroundersogelsen). Glob Cardiol Sci Pract. 2015;2015(3):33.
96. Kamstrup PR, Benn M, Tybjaerg-Hansen A, Nordestgaard BG. Extreme lipoprotein(a) levels and risk of myocardial infarction in the general population: the Copenhagen City Heart Study. Circulation. 2008;117(2):176-184.
97. Kamstrup PR, Tybjærg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301(22):2331-2339.
98. Kamstrup PR, Tybjaerg-Hansen A, Nordestgaard BG. Extreme lipoprotein(a) levels and improved cardiovascular risk prediction. J Am Coll Cardiol.2013;61(11):1146-1156.
99. Kamstrup PR, Tybjaerg-Hansen A, Nordestgaard BG. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J Am Coll Cardiol. 2014;63(5):470-477.
100. Kamstrup PR, Nordestgaard BG. Elevated lipoprotein(a) levels, LPA risk genotypes, and increased risk of heart failure in the general population. JACC Heart Fail.2016;4(1):78-87.
101. Verbeek R, Hoogeveen RM, Langsted A, et al. Cardiovascular disease risk associated with elevated lipoprotein(a) attenuates at low low-density lipoprotein cholesterol levels in a primary prevention setting. Eur Heart J. 2018;39(27):2589-2596.
102. Cao J, Steffen BT, Budoff M, et al. Lipoprotein(a) levels are associated with subclinical calcific aortic valve disease in white and black individuals: the multi-ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol. 2016;36(5):1003-1009.
103. Steffen BT, Duprez D, Bertoni AG, Guan W, Tsai M. Lp(a) [lipoprotein(a)]-related risk of heart failure is evident in whites but not in other racial/ethnic groups.Arterioscler Thromb Vasc Biol. 2018;38(10):2498-2504.
104. ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129(4):687-702.
105. Agarwala A, Pokharel Y, Saeed A, et al. The association of lipoprotein(a) with incident heart failure hospitalization: Atherosclerosis Risk in Communities study. Atherosclerosis. 2017;262:131-137.
106. Ohira T, Schreiner PJ, Morrisett JD, Chambless LE, Rosamond WD, Folsom AR. Lipoprotein(a) and incident ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2006;37(6):1407-1412.
107. Waldeyer C, Makarova N, Zeller T, et al. Lipoprotein(a) and the risk of cardiovascular disease in the European population: results from the BiomarCaRE consortium. Eur Heart J. 2017;38(32):2490-2498.
108. Cook NR, Mora S, Ridker PM. Lipoprotein(a) and cardiovascular risk prediction among women. J Am Coll Cardiol. 2018;72(3):287-296.
109. Suk Danik J, Rifai N, Buring JE, Ridker PM. Lipoprotein(a), measured with an assay independent of apolipoprotein(a) isoform size, and risk of future cardiovascular events among initially healthy women. JAMA. 2006;296(11):1363-1370.
110. Suk Danik J, Rifai N, Buring JE, Ridker PM. Lipoprotein(a), hormone replacement therapy, and risk of future cardiovascular events. J Am Coll Cardiol. 2008;52(2):124-131.
111. Chien KL, Hsu HC, Su TC, Sung FC, Chen MF, Lee YT. Lipoprotein(a) and cardiovascular disease in ethnic Chinese: the Chin-Shan Community Cardiovascular Cohort Study. Clin Chem. 2008;54(2):285-291.
112. Lee SR, Prasad A, Choi YS, et al. LPA gene, ethnicity, and cardiovascular events. Circulation.2017;135(3):251-263.
113. Zekavat SM, Ruotsalainen S, Handsaker RE, et al. Deep coverage whole genome sequences and plasma lipoprotein(a) in individuals of European and African ancestries. Nat Commun.2018;9(1):2606.
114. Zewinger S, Kleber ME, Tragante V, et al. Relations between lipoprotein(a) concentrations, LPA genetic variants, and the risk of mortality in patients with established coronary heart disease: a molecular and genetic association study. Lancet Diabetes Endocrinol. 2017;5(7):534-543.
115. Li J, Lange LA, Sabourin J, et al. Genome- and exomewide association study of serum lipoprotein (a) in the Jackson Heart Study. J Hum Genet. 2015;60(12):755-761.
116. Khera AV, Emdin CA, Drake I, et al, Kathiresan S. Genetic risk, adherence to a healthy lifestyle, and coronary disease. N Engl J Med.2016;375(24):2349-2358.
117. Nordestgaard BG, Langsted A. Lipoprotein(a) as a cause of cardiovascular disease: insights from epidemiology, genetics, and biology. J Lipid Res.2016;57(11):1953-1975.
118. Sofi F, Cesari F, Casini A, Macchi C, Abbate R, Gensini GF. Insomnia and risk of cardiovascular disease: a metaanalysis. Eur J Prev Cardiol.2014;21(1):57-64.
119. Estruch R, Ros E, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with a mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med.2018;378(25):e34.
120. Perrot N, Verbeek R, Sandhu M, et al. Ideal cardiovascular health influences cardiovascular disease risk associated with high lipoprotein(a) levels and genotype: The EPICNorfolk prospective population study. Atherosclerosis. 2017;256:47-52.
121. Teo KK, Corsi DJ, Tam JW, Dumesnil JG, Chan KL. Lipid lowering on progression of mild to moderate aortic stenosis: meta-analysis of the randomized placebocontrolled clinical trials on 2344 patients. Can J Cardiol. 2011;27(6):800-808.
122. Albers JJ, Slee A, O’Brien KD, et al. Relationship of apolipoproteins A-1 and B, and lipoprotein(a) to cardiovascular outcomes: the AIM-HIGH trial (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglyceride and Impact on Global Health Outcomes). J Am Coll Cardiol. 2013;62(17):1575-1579.
123. Lincoff AM, Nicholls SJ, Riesmeyer JS, et al; ACCELERATE Investigators. Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med. 2017;376(20):1933-1942.
124. Schmidt AF, Pearce LS, Wilkins JT, Overington JP, Hingorani AD, Casas JP. PCSK9 monoclonal antibodies for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev.2017;4:CD011748.
125. Bowman L, Hopewell JC, Chen F, et al; PHS3/TIM155-REVEAL Collaborative Group. Effects of anacetrapib in patients with atherosclerotic vascular disease.
126. Leebmann J, Roeseler E, Julius U, et al; Pro(a)LiFe Study Group. Lipoprotein apheresis in patients with maximally tolerated lipid-lowering therapy, lipoprotein(a)-hyperlipoproteinemia, and progressive cardiovascular disease: prospective observational multicenter study. Circulation. 2013;128(24):2567-2576.
127. Heigl F, Hettich R, Lotz N, et al. Efficacy, safety, and tolerability of long-term lipoprotein apheresis in patients with LDL- or Lp(a) hyperlipoproteinemia: Findings gathered from more than 36,000 treatments at one center in Germany. Atheroscler Suppl. 2015;18:154-162.
128. Viney NJ, van Capelleveen JC, Geary RS, et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet. 2016;388(10057):2239-2253.
129. Graham MJ, Viney N, Crooke RM, Tsimikas S. Antisense inhibition of apolipoprotein (a) to lower plasma lipoprotein (a) levels in humans. J Lipid Res. 2016;57(3):340-351.
130. Keene D, Price C, Shun-Shin MJ, Francis DP. Effect on cardiovascular risk of high density lipoprotein targeted drug treatments niacin, fibrates, and CETP inhibitors: meta-analysis of randomised controlled trials including 117,411 patients. BMJ. 2014;349:g4379.
131. Nicholls SJ, Ruotolo G, Brewer HB, et al. Evacetrapib alone or in combination with statins lowers lipoprotein(a) and total and small LDL particle concentrations in mildly hypercholesterolemic patients. J Clin Lipidol. 2016;10(3):519-527.e4.
132. Schwartz GG, Ballantyne CM, Barter PJ, et al. Association of lipoprotein(a) with risk of recurrent ischemic events following acute coronary syndrome: analysis of the dal-outcomes randomized clinical trial. JAMA Cardiol.2018;3(2):164-168.
133. Schwartz GG, Olsson AG, Abt M, et al; dal-OUTCOMES Investigators. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med.2012;367(22):2089-2099.
134. Thomas T, Zhou H, Karmally W, et al. CETP (Cholesteryl Ester Transfer Protein) inhibition with anacetrapib decreases production of lipoprotein(a) in mildly hypercholesterolemic subjects. Arterioscler Thromb Vasc Biol.2017;37(9):1770-1775.
135. Khera AV, Everett BM, Caulfield MP, et al. Lipoprotein(a) concentrations, rosuvastatin therapy, and residual vascular risk: an analysis from the JUPITER Trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin). Circulation. 2014;129(6):635-642.
136. Yeang C, Hung MY, Byun YS, et al. Effect of therapeutic interventions on oxidized phospholipids on apolipoprotein B100 and lipoprotein(a). J Clin Lipidol. 2016;10(3):594-603.
137. Zhou Z, Rahme E, Pilote L. Are statins created equal? Evidence from randomized trials of pravastatin, simvastatin, and atorvastatin for cardiovascular disease prevention.Am Heart J. 2006;151(2):273-281.
138. Ridker PM, MacFadyen JG, Fonseca FA, et al; JUPITER Study Group. Number needed to treat with rosuvastatin to prevent first cardiovascular events and death among men and women with low low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein: justification for the use of statins in prevention: an intervention trial evaluating rosuvastatin (JUPITER). Circ Cardiovasc Qual Outcomes. 2009;2(6):616-623.
139. Raal FJ, Giugliano RP, Sabatine MS, et al. Reduction in lipoprotein(a) with PCSK9 monoclonal antibody evolocumab (AMG 145): a pooled analysis of more than 1,300 patients in 4 phase II trials. J Am Coll Cardiol.2014;63(13):1278-1288.
140. Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1500-1509.
141. Koren MJ, Sabatine MS, Giugliano RP, et al. Long-term low-density lipoprotein cholesterol-lowering efficacy, persistence, and safety of evolocumab in treatment of hypercholesterolemia: results up to 4 years from the open-label OSLER-1 extension study. JAMA Cardiol.2017;2(6):598-607.
142. Desai NR, Kohli P, Giugliano RP, et al. AMG145, a monoclonal antibody against proprotein convertase subtilisin kexin type 9, significantly reduces lipoprotein(a) in hypercholesterolemic patients receiving statin therapy: an analysis from the LDL-C Assessment with Proprotein Convertase Subtilisin Kexin Type 9 Monoclonal Antibody Inhibition Combined with Statin Therapy (LAPLACE)-Thrombolysis in Myocardial Infarction (TIMI) 57 trial. Circulation.2013;128(9):962-969.
143. Schwartz GG, Steg PG, Szarek M, et al; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and cardiovascular outcomes after acute coronary syndrome.N Engl J Med. 2018;379(22):2097-2107.
144. Sabatine MS, Giugliano RP, Keech AC, et al; FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular Disease.N Engl J Med. 2017;376(18):1713-1722.
145. Karatasakis A, Danek BA, Karacsonyi J, et al. Effect of PCSK9 inhibitors on clinical outcomes in patients with hypercholesterolemia: A meta-analysis of 35 randomized controlled trials. J Am Heart Assoc. 2017;6(12):e006910.
146. Santos RD, Duell PB, East C, et al. Long-term efficacy and safety of mipomersen in patients with familial hypercholesterolaemia: 2-year interim results of an open-label extension.Eur Heart J. 2015;36(9):566-575.
147. Duell PB, Santos RD, Kirwan BA, Witztum JL, Tsimikas S, Kastelein JJP. Long-term mipomersen treatment is associated with a reduction in cardiovascular events in patients with familial hypercholesterolemia. J Clin Lipidol. 2016;10(4):1011-1021.
148. McGowan MP, Tardif JC, Ceska R, et al. Randomized, placebo-controlled trial of mipomersen in patients with severe hypercholesterolemia receiving maximally tolerated lipid-lowering therapy. PLoS One.2012;7(11):e49006.
149. Jaeger BR, Richter Y, Nagel D, et al. Longitudinal cohort study on the effectiveness of lipid apheresis treatment to reduce high lipoprotein(a) levels and prevent major adverse coronary events. Nat Clin Pract Cardiovasc Med.2009;6(3):229-239.
150. Rosada A, Kassner U, Vogt A, Willhauck M, Parhofer K, Steinhagen-Thiessen E. Does regular lipid apheresis in Does regular lipid apheresis in patients with isolated elevated lipoprotein(a) levels reduce the incidence of cardiovascular events? Artif Organs. 2014;38(2):135-141.
151. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596-e646.
Evaluating a Veterans Affairs Home-Based Primary Care Population for Patients at High Risk of Osteoporosis
Osteoporosis is a disease characterized by the loss of bone density.1 Bone is normally porous and is in a state of flux due to changes in regeneration caused by osteoclast or osteoblast activity. However, age and other factors can accelerate loss in bone density and lead to decreased bone strength and an increased risk of fracture. In men, bone mineral density (BMD) can begin to decline as early as age 30 to 40 years. By age 80 years, 25% of total bone mass may be lost.2
Of the 44 million Americans with low BMD or osteoporosis, 20% are men.1 This group accounts for up to 40% of all osteoporotic fractures. About 1 in 4 men aged ≥ 50 years may experience a lifetime fracture. Fractures may lead to chronic pain, disability, increased dependence, and potentially death. These complications cause expenditures upward of $4.1 billion annually in North America alone.3,4 About 80,000 US men will experience a hip fracture each year, one-third of whom will die within that year. This constitutes a mortality rate 2 to 3 times higher than that of women. Osteoporosis often goes undiagnosed and untreated due to a lack of symptoms until a fracture occurs, underlining the potential benefit of preemptive screening.
In 2007, Shekell and colleagues outlined how the US Department of Veterans Affairs (VA) screened men for osteoporosis.5 At the time, 95% of the VA population was male, though it has since dropped to 91%.6 Shekell and colleagues estimated that about 200,0000 to 400,0000 male veterans had osteoporosis.5 Osteoporotic risk factors deemed specific to veterans were excessive alcohol use, spinal cord injury and lack of weight-bearing exercise, prolonged corticosteroid use, and androgen deprivation therapy in prostate cancer. Different screening techniques were assessed, and the VA recommended the Osteoporosis Self-Assessment Tool (OST).5 Many organizations have developed clinical guidance, including who should be screened; however, screening for men remains a controversial area due to a lack of any strong recommendations (Table 1).
Endocrine Society screening guidelines for men are the most specific: testing BMD in men aged ≥ 70 years, or if aged 50 to 69 years with an additional risk factor (eg, low body weight, smoking, chronic obstructive pulmonary disease, chronic steroid use).1 The Fracture Risk Assessment tool (FRAX) score is often cited as a common screening tool. It is a free online questionnaire that provides a 10-year probability risk of hip or major osteoporotic fracture.11 However, this tool is limited by age, weight, and the assumption that all questions are answered accurately. Some of the information required includes the presence of a number of risk factors, such as alcohol use, glucocorticoids, and medical history of rheumatoid arthritis, among others (Table 2). The OST score, on the other hand, is a calculation that does not take into account other risk factors (Figure 1). This tool categorizes the patient into low, moderate, or high risk for osteoporosis.8
In a study of 4,000 men aged ≥ 70 years,
A 2017 VA Office of Rural Health study examined the utility of OST to screen referred patients aged > 50 years to receive DEXA scans in patient aligned care team (PACT) clinics at 3 different VA locations.13 The study excluded patients who had been screened previously or treated for osteoporosis, were receiving hospice care; 1 site excluded patients aged > 88 years. Two of the sites also reviewed the patient’s medications to screen for agents that may contribute to increased fracture risk. Veterans identified as high risk were referred for education and offered a DEXA scan and treatment. In total, 867 veterans were screened; 19% (168) were deemed high risk, and 6% (53) underwent DEXA scans. The study noted that only 15 patients had reportable DEXA scans and 10 were positive for bone disease.
As there has been documented success in the PACT setting in implementing standardized protocols for screening and treating veterans, it is reasonable to extend the concept into other VA services. The home-based primary care (HBPC) population is especially vulnerable due to the age of patients, limited weight-bearing exercise to improve bone strength, and limited access to DEXA scans due to difficulty traveling outside of the home. Despite these issues, a goal of the HBPC service is to provide continual care for veterans and improve their health so they may return to the community setting. As a result, patients are followed frequently, providing many opportunities for interventions. This study aims to determine the proportion of HBPC patients who are at high risk for osteoporosis and can receive a DEXA scan for evaluation.
Methods
This study was a retrospective chart analysis using descriptive statistics. It was reviewed and approved by the institutional review board at Captain James A. Lovell Federal Health Care Center (FHCC). Patients were included in the study if they were enrolled in the HBPC program at FHCC. Patients were excluded if they were receiving hospice or palliative care, had a limited life expectancy per the HBPC provider, or had a diagnosis of osteoporosis that was being managed by a VA endocrinologist, rheumatologist, or non-VA provider.
The study was conducted from February 1, 2018, through November 30, 2018. All chart reviews were done through the FHCC electronic health record. A minimum of 80 and maximum of 150 charts were reviewed as this was the typical patient volume in the HBPC program. Basic demographic information was collected and analyzed by calculating FRAX and OST scores. With the results, patients were classified as low or high risk of developing osteoporosis, and whether a DEXA scan should be recommended.
Results
After chart review, 83 patients were enrolled in the FHCC HBPC program during the study period. Out of these, 5 patients were excluded due to hospice or palliative care status, limited life expectancy, or had their osteoporosis managed by another non-HBPC provider. As a result, 78 patients were analyzed to determine their risk of osteoporosis (Figure 2). Most of the patients were white males with a median age of 82 years. A majority of the patients did not have any current or previous treatment with bisphosphonates, 77% had normal vitamin D levels, and only 13% (10) were current smokers; of the male patients only 21% (15) had a previous DEXA scan (Table 3).
The FRAX and OST scores for each male patient were calculated (Table 4). Half the patients were low risk for osteoporosis. Just 20% (14) of the patients were at high risk for osteoporosis, and only 6 of those had DEXA scans. However, if expanding the criteria to OST scores of < 2, then only 24% (10) received DEXA scans. When calculating FRAX scores, 30% (21) had ≥ 9.3% for major osteoporotic fracture risk, and only 19% (4) had received a DEXA scan.
Discussion
Based on the collected data, many of the male HBPC patients have not had an evaluation for osteoporosis despite being in a high-risk population and meeting some of the screening guidelines by various organizations.1 Based on Diem and colleagues and the 2007 VA report, utilizing OST scores could help capture a subset of patients that would be referred for DEXA scans.5,12 Of the 60% (42) of patients that met OST scores of < 2, 76% (32) of them could have been referred for DEXA scans for osteoporosis evaluation. However, at the time of publication of this article, 50% (16) of the patients have been discharged from the service without interventions. Of the remaining 16 patients, only 2 were referred for a DEXA scan, and 1 patient had confirmed osteoporosis. Currently, these results have been reviewed by the HBPC provider, and plans are in place for DEXA scan referrals for the remaining patients. In addition, for new patients admitted to the program and during annual reviews, the plan is to use OST scores to help screen for osteoporosis.
Limitations
The HBPC population is often in flux due to discharges as patients pass away, become eligible for long-term care, advance to hospice or palliative care status, or see an improvement in their condition to transition back into the community. Along with patients who are bed-bound, have poor prognosis, and barriers to access (eg, transportation issues), interventions for DEXA scan referrals are often not clinically indicated. During calculations of the FRAX score, documentation is often missing from a patient’s medical chart, making it difficult to answer all questions on the questionnaire. This does increase the utility of the OST score as the calculation is much easier and does not rely on other osteoporotic factors. Despite these restrictions for offering DEXA scans, the HBPC service has a high standard of excellence in preventing falls, a major contributor to fractures. Physical therapy services are readily available, nursing visits are frequent and as clinically indicated, vitamin D levels are maintained within normal limits via supplementation, and medication management is performed at least quarterly among other interventions.
Conclusions
The retrospective chart review of patients in the HBPC program suggests that there may be a lack of standardized screening for osteoporosis in the male patient population. As seen within the data, there is great potential for interventions as many of the patients would be candidates for screening based on the OST score. The tool is easy to use and readily accessible to all health care providers and staff. By increasing screening of eligible patients, it also increases the identification of those who would benefit from osteoporosis treatment. While the HBPC population has access limitations (eg, homebound, limited life expectancy), the implementation of a protocol and extension of concepts from this study can be extrapolated into other PACT clinics at VA facilities. Osteoporosis in the male population is often overlooked, but screening procedures can help reduce health care expenditures.
1. Watts NB, Adler RA, Bilezikian JP, et al; Endocrine Society. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(6):1802-1822.
2. Holt G, Smith R, Duncan K, Hutchison JD, Gregori A. Gender differences in epidemiology and outcome after hip fracture: evidence from the Scottish Hip Fracture Audit. J Bone Joint Surg Br. 2008;90(4):480-483.
3. Ackman JM, Lata PF, Schuna AA, Elliott ME. Bone health evaluation in a veteran population: a need for the Fracture Risk Assessment tool (FRAX). Ann Pharmacother. 2014;48(10):1288-1293.
4. International Osteoporosis Foundation. Osteoporosis in men: why change needs to happen. http://share.iofbone-health.org/WOD/2014/thematic-report/WOD14-Report.pdf. Published 2014. Accessed September 16, 2019.
5. Shekell P, Munjas B, Liu H, et al. Screening Men for Osteoporosis: Who & How. Evidence-based Synthesis Program. Washington, DC: Department of Veterans Affairs; 2007.
6. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Veteran population. https://www.va.gov/vetdata/Veteran_Population.asp. Accessed September 16, 2019.
7. Rao SS, Budhwar N, Ashfaque A. Osteoporosis in men. Am Fam Physician. 2010;82(5):503-508.
8. US Preventive Services Task Force, Curry SJ, Krist AH, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA. 2018;319(24):2521-2531.
9. Viswanathan M, Reddy S, Berkman N, et al. Screening to prevent osteoporotic fractures updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319(24):2532-2551.
10. Cosman F, de Beur SJ, LeBoff MS, et al; National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2381.
11. Centre for Metabolic Bone Diseases, University of Sheffield, UK. FRAX Fracture Risk Assessment Tool. http://www.sheffield.ac.uk/FRAX/tool.aspx?country=9. Accessed September 16, 2019.
12. Diem SJ, Peters KW, Gourlay ML, et al; Osteoporotic Fractures in Men Research Group. Screening for osteoporosis in older men: operating characteristics of proposed strategies for selecting men for BMD testing. J Gen Intern Med. 2017;32(11):1235-1241.
13. US Department of Veterans Affairs, Office of Rural Health. Osteoporosis risk assessment using Osteoporosis Self-Assessment Tool (OST) and other interventions at rural facilities. https://www.ruralhealth.va.gov/docs/promise/2017_02_01_OST_Issue%20Brief_v2.pdf. Published February 7, 2019. Accessed September 16, 2019.
Osteoporosis is a disease characterized by the loss of bone density.1 Bone is normally porous and is in a state of flux due to changes in regeneration caused by osteoclast or osteoblast activity. However, age and other factors can accelerate loss in bone density and lead to decreased bone strength and an increased risk of fracture. In men, bone mineral density (BMD) can begin to decline as early as age 30 to 40 years. By age 80 years, 25% of total bone mass may be lost.2
Of the 44 million Americans with low BMD or osteoporosis, 20% are men.1 This group accounts for up to 40% of all osteoporotic fractures. About 1 in 4 men aged ≥ 50 years may experience a lifetime fracture. Fractures may lead to chronic pain, disability, increased dependence, and potentially death. These complications cause expenditures upward of $4.1 billion annually in North America alone.3,4 About 80,000 US men will experience a hip fracture each year, one-third of whom will die within that year. This constitutes a mortality rate 2 to 3 times higher than that of women. Osteoporosis often goes undiagnosed and untreated due to a lack of symptoms until a fracture occurs, underlining the potential benefit of preemptive screening.
In 2007, Shekell and colleagues outlined how the US Department of Veterans Affairs (VA) screened men for osteoporosis.5 At the time, 95% of the VA population was male, though it has since dropped to 91%.6 Shekell and colleagues estimated that about 200,0000 to 400,0000 male veterans had osteoporosis.5 Osteoporotic risk factors deemed specific to veterans were excessive alcohol use, spinal cord injury and lack of weight-bearing exercise, prolonged corticosteroid use, and androgen deprivation therapy in prostate cancer. Different screening techniques were assessed, and the VA recommended the Osteoporosis Self-Assessment Tool (OST).5 Many organizations have developed clinical guidance, including who should be screened; however, screening for men remains a controversial area due to a lack of any strong recommendations (Table 1).
Endocrine Society screening guidelines for men are the most specific: testing BMD in men aged ≥ 70 years, or if aged 50 to 69 years with an additional risk factor (eg, low body weight, smoking, chronic obstructive pulmonary disease, chronic steroid use).1 The Fracture Risk Assessment tool (FRAX) score is often cited as a common screening tool. It is a free online questionnaire that provides a 10-year probability risk of hip or major osteoporotic fracture.11 However, this tool is limited by age, weight, and the assumption that all questions are answered accurately. Some of the information required includes the presence of a number of risk factors, such as alcohol use, glucocorticoids, and medical history of rheumatoid arthritis, among others (Table 2). The OST score, on the other hand, is a calculation that does not take into account other risk factors (Figure 1). This tool categorizes the patient into low, moderate, or high risk for osteoporosis.8
In a study of 4,000 men aged ≥ 70 years,
A 2017 VA Office of Rural Health study examined the utility of OST to screen referred patients aged > 50 years to receive DEXA scans in patient aligned care team (PACT) clinics at 3 different VA locations.13 The study excluded patients who had been screened previously or treated for osteoporosis, were receiving hospice care; 1 site excluded patients aged > 88 years. Two of the sites also reviewed the patient’s medications to screen for agents that may contribute to increased fracture risk. Veterans identified as high risk were referred for education and offered a DEXA scan and treatment. In total, 867 veterans were screened; 19% (168) were deemed high risk, and 6% (53) underwent DEXA scans. The study noted that only 15 patients had reportable DEXA scans and 10 were positive for bone disease.
As there has been documented success in the PACT setting in implementing standardized protocols for screening and treating veterans, it is reasonable to extend the concept into other VA services. The home-based primary care (HBPC) population is especially vulnerable due to the age of patients, limited weight-bearing exercise to improve bone strength, and limited access to DEXA scans due to difficulty traveling outside of the home. Despite these issues, a goal of the HBPC service is to provide continual care for veterans and improve their health so they may return to the community setting. As a result, patients are followed frequently, providing many opportunities for interventions. This study aims to determine the proportion of HBPC patients who are at high risk for osteoporosis and can receive a DEXA scan for evaluation.
Methods
This study was a retrospective chart analysis using descriptive statistics. It was reviewed and approved by the institutional review board at Captain James A. Lovell Federal Health Care Center (FHCC). Patients were included in the study if they were enrolled in the HBPC program at FHCC. Patients were excluded if they were receiving hospice or palliative care, had a limited life expectancy per the HBPC provider, or had a diagnosis of osteoporosis that was being managed by a VA endocrinologist, rheumatologist, or non-VA provider.
The study was conducted from February 1, 2018, through November 30, 2018. All chart reviews were done through the FHCC electronic health record. A minimum of 80 and maximum of 150 charts were reviewed as this was the typical patient volume in the HBPC program. Basic demographic information was collected and analyzed by calculating FRAX and OST scores. With the results, patients were classified as low or high risk of developing osteoporosis, and whether a DEXA scan should be recommended.
Results
After chart review, 83 patients were enrolled in the FHCC HBPC program during the study period. Out of these, 5 patients were excluded due to hospice or palliative care status, limited life expectancy, or had their osteoporosis managed by another non-HBPC provider. As a result, 78 patients were analyzed to determine their risk of osteoporosis (Figure 2). Most of the patients were white males with a median age of 82 years. A majority of the patients did not have any current or previous treatment with bisphosphonates, 77% had normal vitamin D levels, and only 13% (10) were current smokers; of the male patients only 21% (15) had a previous DEXA scan (Table 3).
The FRAX and OST scores for each male patient were calculated (Table 4). Half the patients were low risk for osteoporosis. Just 20% (14) of the patients were at high risk for osteoporosis, and only 6 of those had DEXA scans. However, if expanding the criteria to OST scores of < 2, then only 24% (10) received DEXA scans. When calculating FRAX scores, 30% (21) had ≥ 9.3% for major osteoporotic fracture risk, and only 19% (4) had received a DEXA scan.
Discussion
Based on the collected data, many of the male HBPC patients have not had an evaluation for osteoporosis despite being in a high-risk population and meeting some of the screening guidelines by various organizations.1 Based on Diem and colleagues and the 2007 VA report, utilizing OST scores could help capture a subset of patients that would be referred for DEXA scans.5,12 Of the 60% (42) of patients that met OST scores of < 2, 76% (32) of them could have been referred for DEXA scans for osteoporosis evaluation. However, at the time of publication of this article, 50% (16) of the patients have been discharged from the service without interventions. Of the remaining 16 patients, only 2 were referred for a DEXA scan, and 1 patient had confirmed osteoporosis. Currently, these results have been reviewed by the HBPC provider, and plans are in place for DEXA scan referrals for the remaining patients. In addition, for new patients admitted to the program and during annual reviews, the plan is to use OST scores to help screen for osteoporosis.
Limitations
The HBPC population is often in flux due to discharges as patients pass away, become eligible for long-term care, advance to hospice or palliative care status, or see an improvement in their condition to transition back into the community. Along with patients who are bed-bound, have poor prognosis, and barriers to access (eg, transportation issues), interventions for DEXA scan referrals are often not clinically indicated. During calculations of the FRAX score, documentation is often missing from a patient’s medical chart, making it difficult to answer all questions on the questionnaire. This does increase the utility of the OST score as the calculation is much easier and does not rely on other osteoporotic factors. Despite these restrictions for offering DEXA scans, the HBPC service has a high standard of excellence in preventing falls, a major contributor to fractures. Physical therapy services are readily available, nursing visits are frequent and as clinically indicated, vitamin D levels are maintained within normal limits via supplementation, and medication management is performed at least quarterly among other interventions.
Conclusions
The retrospective chart review of patients in the HBPC program suggests that there may be a lack of standardized screening for osteoporosis in the male patient population. As seen within the data, there is great potential for interventions as many of the patients would be candidates for screening based on the OST score. The tool is easy to use and readily accessible to all health care providers and staff. By increasing screening of eligible patients, it also increases the identification of those who would benefit from osteoporosis treatment. While the HBPC population has access limitations (eg, homebound, limited life expectancy), the implementation of a protocol and extension of concepts from this study can be extrapolated into other PACT clinics at VA facilities. Osteoporosis in the male population is often overlooked, but screening procedures can help reduce health care expenditures.
Osteoporosis is a disease characterized by the loss of bone density.1 Bone is normally porous and is in a state of flux due to changes in regeneration caused by osteoclast or osteoblast activity. However, age and other factors can accelerate loss in bone density and lead to decreased bone strength and an increased risk of fracture. In men, bone mineral density (BMD) can begin to decline as early as age 30 to 40 years. By age 80 years, 25% of total bone mass may be lost.2
Of the 44 million Americans with low BMD or osteoporosis, 20% are men.1 This group accounts for up to 40% of all osteoporotic fractures. About 1 in 4 men aged ≥ 50 years may experience a lifetime fracture. Fractures may lead to chronic pain, disability, increased dependence, and potentially death. These complications cause expenditures upward of $4.1 billion annually in North America alone.3,4 About 80,000 US men will experience a hip fracture each year, one-third of whom will die within that year. This constitutes a mortality rate 2 to 3 times higher than that of women. Osteoporosis often goes undiagnosed and untreated due to a lack of symptoms until a fracture occurs, underlining the potential benefit of preemptive screening.
In 2007, Shekell and colleagues outlined how the US Department of Veterans Affairs (VA) screened men for osteoporosis.5 At the time, 95% of the VA population was male, though it has since dropped to 91%.6 Shekell and colleagues estimated that about 200,0000 to 400,0000 male veterans had osteoporosis.5 Osteoporotic risk factors deemed specific to veterans were excessive alcohol use, spinal cord injury and lack of weight-bearing exercise, prolonged corticosteroid use, and androgen deprivation therapy in prostate cancer. Different screening techniques were assessed, and the VA recommended the Osteoporosis Self-Assessment Tool (OST).5 Many organizations have developed clinical guidance, including who should be screened; however, screening for men remains a controversial area due to a lack of any strong recommendations (Table 1).
Endocrine Society screening guidelines for men are the most specific: testing BMD in men aged ≥ 70 years, or if aged 50 to 69 years with an additional risk factor (eg, low body weight, smoking, chronic obstructive pulmonary disease, chronic steroid use).1 The Fracture Risk Assessment tool (FRAX) score is often cited as a common screening tool. It is a free online questionnaire that provides a 10-year probability risk of hip or major osteoporotic fracture.11 However, this tool is limited by age, weight, and the assumption that all questions are answered accurately. Some of the information required includes the presence of a number of risk factors, such as alcohol use, glucocorticoids, and medical history of rheumatoid arthritis, among others (Table 2). The OST score, on the other hand, is a calculation that does not take into account other risk factors (Figure 1). This tool categorizes the patient into low, moderate, or high risk for osteoporosis.8
In a study of 4,000 men aged ≥ 70 years,
A 2017 VA Office of Rural Health study examined the utility of OST to screen referred patients aged > 50 years to receive DEXA scans in patient aligned care team (PACT) clinics at 3 different VA locations.13 The study excluded patients who had been screened previously or treated for osteoporosis, were receiving hospice care; 1 site excluded patients aged > 88 years. Two of the sites also reviewed the patient’s medications to screen for agents that may contribute to increased fracture risk. Veterans identified as high risk were referred for education and offered a DEXA scan and treatment. In total, 867 veterans were screened; 19% (168) were deemed high risk, and 6% (53) underwent DEXA scans. The study noted that only 15 patients had reportable DEXA scans and 10 were positive for bone disease.
As there has been documented success in the PACT setting in implementing standardized protocols for screening and treating veterans, it is reasonable to extend the concept into other VA services. The home-based primary care (HBPC) population is especially vulnerable due to the age of patients, limited weight-bearing exercise to improve bone strength, and limited access to DEXA scans due to difficulty traveling outside of the home. Despite these issues, a goal of the HBPC service is to provide continual care for veterans and improve their health so they may return to the community setting. As a result, patients are followed frequently, providing many opportunities for interventions. This study aims to determine the proportion of HBPC patients who are at high risk for osteoporosis and can receive a DEXA scan for evaluation.
Methods
This study was a retrospective chart analysis using descriptive statistics. It was reviewed and approved by the institutional review board at Captain James A. Lovell Federal Health Care Center (FHCC). Patients were included in the study if they were enrolled in the HBPC program at FHCC. Patients were excluded if they were receiving hospice or palliative care, had a limited life expectancy per the HBPC provider, or had a diagnosis of osteoporosis that was being managed by a VA endocrinologist, rheumatologist, or non-VA provider.
The study was conducted from February 1, 2018, through November 30, 2018. All chart reviews were done through the FHCC electronic health record. A minimum of 80 and maximum of 150 charts were reviewed as this was the typical patient volume in the HBPC program. Basic demographic information was collected and analyzed by calculating FRAX and OST scores. With the results, patients were classified as low or high risk of developing osteoporosis, and whether a DEXA scan should be recommended.
Results
After chart review, 83 patients were enrolled in the FHCC HBPC program during the study period. Out of these, 5 patients were excluded due to hospice or palliative care status, limited life expectancy, or had their osteoporosis managed by another non-HBPC provider. As a result, 78 patients were analyzed to determine their risk of osteoporosis (Figure 2). Most of the patients were white males with a median age of 82 years. A majority of the patients did not have any current or previous treatment with bisphosphonates, 77% had normal vitamin D levels, and only 13% (10) were current smokers; of the male patients only 21% (15) had a previous DEXA scan (Table 3).
The FRAX and OST scores for each male patient were calculated (Table 4). Half the patients were low risk for osteoporosis. Just 20% (14) of the patients were at high risk for osteoporosis, and only 6 of those had DEXA scans. However, if expanding the criteria to OST scores of < 2, then only 24% (10) received DEXA scans. When calculating FRAX scores, 30% (21) had ≥ 9.3% for major osteoporotic fracture risk, and only 19% (4) had received a DEXA scan.
Discussion
Based on the collected data, many of the male HBPC patients have not had an evaluation for osteoporosis despite being in a high-risk population and meeting some of the screening guidelines by various organizations.1 Based on Diem and colleagues and the 2007 VA report, utilizing OST scores could help capture a subset of patients that would be referred for DEXA scans.5,12 Of the 60% (42) of patients that met OST scores of < 2, 76% (32) of them could have been referred for DEXA scans for osteoporosis evaluation. However, at the time of publication of this article, 50% (16) of the patients have been discharged from the service without interventions. Of the remaining 16 patients, only 2 were referred for a DEXA scan, and 1 patient had confirmed osteoporosis. Currently, these results have been reviewed by the HBPC provider, and plans are in place for DEXA scan referrals for the remaining patients. In addition, for new patients admitted to the program and during annual reviews, the plan is to use OST scores to help screen for osteoporosis.
Limitations
The HBPC population is often in flux due to discharges as patients pass away, become eligible for long-term care, advance to hospice or palliative care status, or see an improvement in their condition to transition back into the community. Along with patients who are bed-bound, have poor prognosis, and barriers to access (eg, transportation issues), interventions for DEXA scan referrals are often not clinically indicated. During calculations of the FRAX score, documentation is often missing from a patient’s medical chart, making it difficult to answer all questions on the questionnaire. This does increase the utility of the OST score as the calculation is much easier and does not rely on other osteoporotic factors. Despite these restrictions for offering DEXA scans, the HBPC service has a high standard of excellence in preventing falls, a major contributor to fractures. Physical therapy services are readily available, nursing visits are frequent and as clinically indicated, vitamin D levels are maintained within normal limits via supplementation, and medication management is performed at least quarterly among other interventions.
Conclusions
The retrospective chart review of patients in the HBPC program suggests that there may be a lack of standardized screening for osteoporosis in the male patient population. As seen within the data, there is great potential for interventions as many of the patients would be candidates for screening based on the OST score. The tool is easy to use and readily accessible to all health care providers and staff. By increasing screening of eligible patients, it also increases the identification of those who would benefit from osteoporosis treatment. While the HBPC population has access limitations (eg, homebound, limited life expectancy), the implementation of a protocol and extension of concepts from this study can be extrapolated into other PACT clinics at VA facilities. Osteoporosis in the male population is often overlooked, but screening procedures can help reduce health care expenditures.
1. Watts NB, Adler RA, Bilezikian JP, et al; Endocrine Society. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(6):1802-1822.
2. Holt G, Smith R, Duncan K, Hutchison JD, Gregori A. Gender differences in epidemiology and outcome after hip fracture: evidence from the Scottish Hip Fracture Audit. J Bone Joint Surg Br. 2008;90(4):480-483.
3. Ackman JM, Lata PF, Schuna AA, Elliott ME. Bone health evaluation in a veteran population: a need for the Fracture Risk Assessment tool (FRAX). Ann Pharmacother. 2014;48(10):1288-1293.
4. International Osteoporosis Foundation. Osteoporosis in men: why change needs to happen. http://share.iofbone-health.org/WOD/2014/thematic-report/WOD14-Report.pdf. Published 2014. Accessed September 16, 2019.
5. Shekell P, Munjas B, Liu H, et al. Screening Men for Osteoporosis: Who & How. Evidence-based Synthesis Program. Washington, DC: Department of Veterans Affairs; 2007.
6. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Veteran population. https://www.va.gov/vetdata/Veteran_Population.asp. Accessed September 16, 2019.
7. Rao SS, Budhwar N, Ashfaque A. Osteoporosis in men. Am Fam Physician. 2010;82(5):503-508.
8. US Preventive Services Task Force, Curry SJ, Krist AH, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA. 2018;319(24):2521-2531.
9. Viswanathan M, Reddy S, Berkman N, et al. Screening to prevent osteoporotic fractures updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319(24):2532-2551.
10. Cosman F, de Beur SJ, LeBoff MS, et al; National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2381.
11. Centre for Metabolic Bone Diseases, University of Sheffield, UK. FRAX Fracture Risk Assessment Tool. http://www.sheffield.ac.uk/FRAX/tool.aspx?country=9. Accessed September 16, 2019.
12. Diem SJ, Peters KW, Gourlay ML, et al; Osteoporotic Fractures in Men Research Group. Screening for osteoporosis in older men: operating characteristics of proposed strategies for selecting men for BMD testing. J Gen Intern Med. 2017;32(11):1235-1241.
13. US Department of Veterans Affairs, Office of Rural Health. Osteoporosis risk assessment using Osteoporosis Self-Assessment Tool (OST) and other interventions at rural facilities. https://www.ruralhealth.va.gov/docs/promise/2017_02_01_OST_Issue%20Brief_v2.pdf. Published February 7, 2019. Accessed September 16, 2019.
1. Watts NB, Adler RA, Bilezikian JP, et al; Endocrine Society. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(6):1802-1822.
2. Holt G, Smith R, Duncan K, Hutchison JD, Gregori A. Gender differences in epidemiology and outcome after hip fracture: evidence from the Scottish Hip Fracture Audit. J Bone Joint Surg Br. 2008;90(4):480-483.
3. Ackman JM, Lata PF, Schuna AA, Elliott ME. Bone health evaluation in a veteran population: a need for the Fracture Risk Assessment tool (FRAX). Ann Pharmacother. 2014;48(10):1288-1293.
4. International Osteoporosis Foundation. Osteoporosis in men: why change needs to happen. http://share.iofbone-health.org/WOD/2014/thematic-report/WOD14-Report.pdf. Published 2014. Accessed September 16, 2019.
5. Shekell P, Munjas B, Liu H, et al. Screening Men for Osteoporosis: Who & How. Evidence-based Synthesis Program. Washington, DC: Department of Veterans Affairs; 2007.
6. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Veteran population. https://www.va.gov/vetdata/Veteran_Population.asp. Accessed September 16, 2019.
7. Rao SS, Budhwar N, Ashfaque A. Osteoporosis in men. Am Fam Physician. 2010;82(5):503-508.
8. US Preventive Services Task Force, Curry SJ, Krist AH, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA. 2018;319(24):2521-2531.
9. Viswanathan M, Reddy S, Berkman N, et al. Screening to prevent osteoporotic fractures updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319(24):2532-2551.
10. Cosman F, de Beur SJ, LeBoff MS, et al; National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2381.
11. Centre for Metabolic Bone Diseases, University of Sheffield, UK. FRAX Fracture Risk Assessment Tool. http://www.sheffield.ac.uk/FRAX/tool.aspx?country=9. Accessed September 16, 2019.
12. Diem SJ, Peters KW, Gourlay ML, et al; Osteoporotic Fractures in Men Research Group. Screening for osteoporosis in older men: operating characteristics of proposed strategies for selecting men for BMD testing. J Gen Intern Med. 2017;32(11):1235-1241.
13. US Department of Veterans Affairs, Office of Rural Health. Osteoporosis risk assessment using Osteoporosis Self-Assessment Tool (OST) and other interventions at rural facilities. https://www.ruralhealth.va.gov/docs/promise/2017_02_01_OST_Issue%20Brief_v2.pdf. Published February 7, 2019. Accessed September 16, 2019.
Conflicts of interest common among authors of ASCO guidelines
A significant number of physicians who author practice guidelines are not reporting financial conflicts of interest, a study finds.
Lead author Ramy R. Saleh, MD, of the University of Toronto, and colleagues searched The American Society of Clinical Oncology (ASCO) website to identify all clinical practice guidelines (CPGs) for systemic therapy published between August 2013 and June 2018. Investigators analyzed self-reported author financial conflicts of interest and funding sources and also reviewed The Open Payments database to identify compensation to guideline authors. Researchers categorized conflicts of interest into two groups: research funding (which could include departmental and/or hospital funding) and nonresearch payments (including travel expenses, honoraria, employment, and stock ownership to the individual author).
The initial search identified 121 CPGs published by ASCO between August 2013 and August 2018 of which 26 guidelines were selected because of their focus on systemic treatment. Findings showed that 239 guideline authors who were not exempt from reporting received industry payments, but only 184 (77%) disclosed these payments, according to the study in Cancer. The mean total of all undisclosed payments from 2013 to 2017 received by CPG authors was $187,503 and the median was $30,500. Of the 55 authors with undisclosed conflicts of interest, 34 authors (62%) received more than $1,000 of nonresearch funding, and 19 authors (35%) received more than $5,000 per calendar year.
The majority of the authors with undisclosed conflicts were medical oncologists, the investigators found. Radiation oncologists and surgeons had similar proportions of undisclosed financial conflicts.
The researchers concluded that financial conflicts of interest among authors of ASCO guidelines are common and are not disclosed in a substantial number of cases. The findings indicate that current self-disclosure practices are not adequate for accurately reporting conflicts, they noted.
“Improved transparency of [financial conflicts of interest should become standard practice among CPG authors,” the investigators wrote. “Professional societies and journal editors need to create a mechanism to verify self-reported [financial conflicts of interest].”
Source: Saleh et. al. 2019 July 29 doi: 10.1002/cncr.32408.
The study by Saleh et al. illustrates the need for a better disclosure system that is more consistent and allows for potential conflicts of interest to be more easily identified and managed, says Clifford A. Hudis, MD, of The American Society of Clinical Oncology.
In an editorial accompanying Dr. Saleh’s study in the July 29 issue of Cancer, Dr. Hudis and coauthor Robert W. Carlson, MD, of the National Comprehensive Cancer Network, write that while disclosure compliance is important, they do not believe the lack of disclosures reported in the analysis “represent malintent or malfeasance on the part of authors or a lack of diligence by the involved institutions.
“Instead, this represents one more in a potentially endless number of illustrative specific examples of all that is wrong — and must be fixed—with disclosure as currently practiced in the United States,” the authors wrote.
Dr. Hudis and Dr. Carlson outlined several possible solutions for a better disclosure system, including making the definitions of research funding, consultancy, honoraria, and travel support standardized and applied consistently. In addition, one source of universal disclosure should be developed within the house of medicine that provides a simple, easy-to-use, easily vetted, shared, and accessible resource that allows for the easy documentation, confirmation, and sharing of potential conflicts, according to the authors. Finally, companies that are subject to sunshine reporting should be required to notify covered individuals, in nearly real time, “when and what they are reporting so that there is no disconnect or time lag,” the doctors wrote.
Clifford A. Hudis is CEO for the American Society of Clinical Oncology and Robert W. Carlson is CEO for the National Comprehensive Cancer Network. Dr. Carlson reports being issued US patent D848,448S for Evidence Blocks (part of National Comprehensive Cancer Network guidelines).
The study by Saleh et al. illustrates the need for a better disclosure system that is more consistent and allows for potential conflicts of interest to be more easily identified and managed, says Clifford A. Hudis, MD, of The American Society of Clinical Oncology.
In an editorial accompanying Dr. Saleh’s study in the July 29 issue of Cancer, Dr. Hudis and coauthor Robert W. Carlson, MD, of the National Comprehensive Cancer Network, write that while disclosure compliance is important, they do not believe the lack of disclosures reported in the analysis “represent malintent or malfeasance on the part of authors or a lack of diligence by the involved institutions.
“Instead, this represents one more in a potentially endless number of illustrative specific examples of all that is wrong — and must be fixed—with disclosure as currently practiced in the United States,” the authors wrote.
Dr. Hudis and Dr. Carlson outlined several possible solutions for a better disclosure system, including making the definitions of research funding, consultancy, honoraria, and travel support standardized and applied consistently. In addition, one source of universal disclosure should be developed within the house of medicine that provides a simple, easy-to-use, easily vetted, shared, and accessible resource that allows for the easy documentation, confirmation, and sharing of potential conflicts, according to the authors. Finally, companies that are subject to sunshine reporting should be required to notify covered individuals, in nearly real time, “when and what they are reporting so that there is no disconnect or time lag,” the doctors wrote.
Clifford A. Hudis is CEO for the American Society of Clinical Oncology and Robert W. Carlson is CEO for the National Comprehensive Cancer Network. Dr. Carlson reports being issued US patent D848,448S for Evidence Blocks (part of National Comprehensive Cancer Network guidelines).
The study by Saleh et al. illustrates the need for a better disclosure system that is more consistent and allows for potential conflicts of interest to be more easily identified and managed, says Clifford A. Hudis, MD, of The American Society of Clinical Oncology.
In an editorial accompanying Dr. Saleh’s study in the July 29 issue of Cancer, Dr. Hudis and coauthor Robert W. Carlson, MD, of the National Comprehensive Cancer Network, write that while disclosure compliance is important, they do not believe the lack of disclosures reported in the analysis “represent malintent or malfeasance on the part of authors or a lack of diligence by the involved institutions.
“Instead, this represents one more in a potentially endless number of illustrative specific examples of all that is wrong — and must be fixed—with disclosure as currently practiced in the United States,” the authors wrote.
Dr. Hudis and Dr. Carlson outlined several possible solutions for a better disclosure system, including making the definitions of research funding, consultancy, honoraria, and travel support standardized and applied consistently. In addition, one source of universal disclosure should be developed within the house of medicine that provides a simple, easy-to-use, easily vetted, shared, and accessible resource that allows for the easy documentation, confirmation, and sharing of potential conflicts, according to the authors. Finally, companies that are subject to sunshine reporting should be required to notify covered individuals, in nearly real time, “when and what they are reporting so that there is no disconnect or time lag,” the doctors wrote.
Clifford A. Hudis is CEO for the American Society of Clinical Oncology and Robert W. Carlson is CEO for the National Comprehensive Cancer Network. Dr. Carlson reports being issued US patent D848,448S for Evidence Blocks (part of National Comprehensive Cancer Network guidelines).
A significant number of physicians who author practice guidelines are not reporting financial conflicts of interest, a study finds.
Lead author Ramy R. Saleh, MD, of the University of Toronto, and colleagues searched The American Society of Clinical Oncology (ASCO) website to identify all clinical practice guidelines (CPGs) for systemic therapy published between August 2013 and June 2018. Investigators analyzed self-reported author financial conflicts of interest and funding sources and also reviewed The Open Payments database to identify compensation to guideline authors. Researchers categorized conflicts of interest into two groups: research funding (which could include departmental and/or hospital funding) and nonresearch payments (including travel expenses, honoraria, employment, and stock ownership to the individual author).
The initial search identified 121 CPGs published by ASCO between August 2013 and August 2018 of which 26 guidelines were selected because of their focus on systemic treatment. Findings showed that 239 guideline authors who were not exempt from reporting received industry payments, but only 184 (77%) disclosed these payments, according to the study in Cancer. The mean total of all undisclosed payments from 2013 to 2017 received by CPG authors was $187,503 and the median was $30,500. Of the 55 authors with undisclosed conflicts of interest, 34 authors (62%) received more than $1,000 of nonresearch funding, and 19 authors (35%) received more than $5,000 per calendar year.
The majority of the authors with undisclosed conflicts were medical oncologists, the investigators found. Radiation oncologists and surgeons had similar proportions of undisclosed financial conflicts.
The researchers concluded that financial conflicts of interest among authors of ASCO guidelines are common and are not disclosed in a substantial number of cases. The findings indicate that current self-disclosure practices are not adequate for accurately reporting conflicts, they noted.
“Improved transparency of [financial conflicts of interest should become standard practice among CPG authors,” the investigators wrote. “Professional societies and journal editors need to create a mechanism to verify self-reported [financial conflicts of interest].”
Source: Saleh et. al. 2019 July 29 doi: 10.1002/cncr.32408.
A significant number of physicians who author practice guidelines are not reporting financial conflicts of interest, a study finds.
Lead author Ramy R. Saleh, MD, of the University of Toronto, and colleagues searched The American Society of Clinical Oncology (ASCO) website to identify all clinical practice guidelines (CPGs) for systemic therapy published between August 2013 and June 2018. Investigators analyzed self-reported author financial conflicts of interest and funding sources and also reviewed The Open Payments database to identify compensation to guideline authors. Researchers categorized conflicts of interest into two groups: research funding (which could include departmental and/or hospital funding) and nonresearch payments (including travel expenses, honoraria, employment, and stock ownership to the individual author).
The initial search identified 121 CPGs published by ASCO between August 2013 and August 2018 of which 26 guidelines were selected because of their focus on systemic treatment. Findings showed that 239 guideline authors who were not exempt from reporting received industry payments, but only 184 (77%) disclosed these payments, according to the study in Cancer. The mean total of all undisclosed payments from 2013 to 2017 received by CPG authors was $187,503 and the median was $30,500. Of the 55 authors with undisclosed conflicts of interest, 34 authors (62%) received more than $1,000 of nonresearch funding, and 19 authors (35%) received more than $5,000 per calendar year.
The majority of the authors with undisclosed conflicts were medical oncologists, the investigators found. Radiation oncologists and surgeons had similar proportions of undisclosed financial conflicts.
The researchers concluded that financial conflicts of interest among authors of ASCO guidelines are common and are not disclosed in a substantial number of cases. The findings indicate that current self-disclosure practices are not adequate for accurately reporting conflicts, they noted.
“Improved transparency of [financial conflicts of interest should become standard practice among CPG authors,” the investigators wrote. “Professional societies and journal editors need to create a mechanism to verify self-reported [financial conflicts of interest].”
Source: Saleh et. al. 2019 July 29 doi: 10.1002/cncr.32408.
Effects of Process Improvement on Guideline-Concordant Cardiac Enzyme Testing
In recent years, driven by accelerating health care costs and desire for improved health care value, major specialty group guidelines have incorporated resource utilization and value calculations into their recommendations. High-value care has the characteristics of enhancing outcomes, safety, and patient satisfaction at a reasonable cost. As one example, the American College of Cardiology (ACC) recently published a consensus statement on its clinical practice guidelines with a specific focus on cost and value.1 This guideline acknowledges the difficulty in incorporating value into clinical decision making but stresses a need for increased transparency and consistency to boost value in everyday practice.
Chest pain and related symptoms were listed as the second leading principle reasons for emergency department visits in the US in 2011 with 14% of patients undergoing cardiac enzyme testing.2 The ACC guidelines advocate use of troponin as the preferred laboratory test for the initial evaluation of acute coronary syndrome (ACS). Fractionated creatine kinase (CK-MB) is an acceptable alternative only when a cardiac troponin test is not available.3 Furthermore, troponins should be obtained no more than 3 times for the initial evaluation of a single event, and further trending provides no additional benefit or prognostic information.
A recent study from an academic hospital showed that process improvement interventions focused on eliminating unnecessary cardiac enzyme testing led to a 1-year cost savings of $1.25 million while increasing the rate of ACS diagnosis.4 Common clinical practice at Naval Medical Center Portsmouth (NMCP) in Virginia still routinely includes both troponin as well as a CK panel comprised of CK, CK-MB, and a calculated CK-MB/CK index. Our study focuses on the implementation of quality improvement efforts described by Larochelle and colleagues at NMCP.4 The study aimed to determine the impact of implementing interventions designed to improve the ordering practices and reduce the cost of cardiac enzyme testing.
Methods
The primary focus of the intervention was on ordering practices of the emergency medicine department (EMD), internal medicine (IM) inpatient services, and cardiology inpatient services. Specific interventions were: (1) removal of the CK panel from the chest pain order set in the EMD electronic health record (EHR); (2) removal of the CK panel from the inpatient cardiology order set; (3) education of staff on the changes in CK panel utility via direct communication during IM academic seminars; (4) education of nursing staff ordering laboratory results on behalf of physicians on the cardiology service at the morning and evening huddles; and (5) addition of “max of 3 tests indicated” comment to the inpatient EHR ordering page of the troponin test
Data Source
The process improvement interventions were considered exempt from institutional review board (IRB) approval; however, we obtained expedited IRB approval with waiver of consent for the research aspect of the project. We obtained clinical administrative data from the Military Health System Data Repository (MDR). We identified all adult patients aged ≥ 18 years who had a troponin test, CK-MB, or both drawn at NMCP on the following services: the EMD, IM, and cardiology. A troponin or CK-MB test was defined using Current Procedural Terminology (CPT) codes and unique Logical Observation Identifiers Names and Codes (LOINC).
Measures
The study was divided into 3 periods: the preintervention period from August 1, 2013 to July 31, 2014; the intervention period from August 1, 2014 to January 31, 2015; and the postintervention period February 1, 2015 to January 31, 2016.
The primary outcomes measured were the frequency of guideline concordance and total costs for tests ordered per month using the Centers for Medicare and Medicaid Services (CMS) clinical laboratory fee schedule of $13.40 for troponin and $16.17 for CK-MB.5Concordance was defined as ≤ 3 troponin tests and no CK-MB tests ordered during 1 encounter for a patient without an ACS diagnosis in the preceding 7 days. Due to faster cellular release kinetics of CK-MB compared with that of troponin, this test has utility in evaluating new or worsening chest pain in the setting of a recent myocardial infarction (MI). Therefore, we excluded any patient who had a MI within the preceding 7 days of an order for either CK-MB or troponin tests. Additionally, the number of tests, both CK-MB and troponin, ordered per patient encounter (hereafter referred to as an episode) were measured. Finally, we measured the monthly prevalence of ACS diagnosis and percentage of visits having that diagnosis.
Data Analysis
Descriptive statistics were used to calculate population demographics of age group, sex, beneficiary category, sponsor service, and clinical setting. Monthly data were grouped into the preintervention and postintervention periods. The analysis was performed using t tests to compare mean values and CIs before and after the intervention. Simple linear regression with attention to correlation was used to create best fit lines with confidence bands before and after the intervention. Interrupted time series (ITS) regression was used to describe all data points throughout the study. Consistency between these various methods was verified. Mean values and CIs were reported from the t tests. Statistical significance was reported when appropriate. Equations and confidence predictions on the simple linear regressions were produced and reported. These were used to identify values at the start, midpoint, and end of the pre- and postintervention periods.
Results
There were a total of 6,281 patients in the study population. More patients were seen during the postintervention period than in the preintervention period. The mean age of patients was slightly higher during the preintervention period (Table 1).
Guideline Concordance
To determine whether ordering practices for cardiac enzyme testing improved, we assessed the changes in the frequency of guideline concordance during the pre- and postintervention period. On average during the preintervention year, the percentage of tests ordered that met guideline concordance was 10.1% (95% CI, 7.4%-12.9%), increasing by 0.80% (95% CI, 0.17%-1.42%) each month.
Costs
We assessed changes in total dollars spent on cardiac enzyme testing during the pre- and postintervention periods. During the preintervention year, $9,400 (95% CI, $8,700-$10,100) was spent on average each month, which did not change significantly throughout the period. During the postintervention year, the cost was stable at $5,000 (95% CI, $4,600-$5,300) on average each month, a reduction of $4,400 (95% CI, $3,700-$5,100) (Figure 2).
CK-MB and Troponin Tests per Patient
To further assess ordering practices for cardiac enzyme testing, we compared the changes in the monthly number of tests and the average number of CK-MB and troponin tests ordered per episode pre- and postintervention. On average during the preintervention year, 297 tests (95% CI, 278-315) were run per month, with an average of 1.21 CK tests (95% CI, 1.15-1.27) per episode (Table 2, Figure 3).
The changes in troponin testing were not as dramatic. The counts of tests each month remained similar, with a preintervention year average of 341 (95% CI, 306-377) and postintervention year average of 310 (95% CI, 287-332), which were not statistically different. However, there was a statistically significant decrease in the number of tests per episode. During the preintervention year, 1.38 troponin tests (95% CI, 1.31-1.45) were ordered per patient on average. This dropped by 0.17 (95% CI, 0.09-0.24) to the postintervention average of 1.21 (95% CI, 1.17-1.25) (Table 2, Figure 4).
ACS Prevalence
To determine whether there was an impact on ACS diagnoses, we looked at the numbers of ACS diagnoses and their prevalence among visits before and after the intervention. During the preintervention year, the average monthly number of diagnoses was 29.7 (95% CI, 26.1-33.2), and prevalence of ACS was 0.56% (95% CI, 0.48%-0.63%) of all episodes. Although the monthly rate was statistically decreasing by 0.022% (95% CI, 0.003-0.41), this has little meaning since the level of correlation (r2 = 0.2522, not displayed) was poor due to the essentially nonexistent correlation in number of visits each month (r2 = 0.0112, not displayed). During the postintervention year, the average number of diagnoses was 32.2 (95% CI, 27.9-36.6), and the prevalence of ACS was 0.62% (95% CI, 0.54-0.65). Neither of these values changed significantly between the pre- and postintervention period. All ICD-9 and ICD-10 diagnosis codes used for the analysis are available upon request from the authors.
Discussion
Our data demonstrate the ability of simple process improvement interventions to decrease unnecessary testing in the workup of ACS, increasing the rate of guideline concordant testing by > 70% at a single military treatment facility (MTF). In particular, with the now widespread use of EHR, the order set presents a high-yield target for process improvement in an easily implemented, durable fashion. We had expected to see some decrease in the efficacy of the intervention at a time of staff turnover in the summer of 2015 because ongoing dedicated teaching sessions were not performed. Despite that, the intervention remained effective without further dedicated teaching sessions. This outcome was certainly attributable to the hardwired interventions made (mainly via order sets), but possibly indicates an institutional memory that can take hold after an initial concerted effort is made.
We reduced the estimated preintervention annual cost of $113,000 by $53,000 (95% CI, $42,000-$64,000). Although on a much smaller scale than the study by Larochelle, our study represents a nearly 50% reduction in the total cost of initial testing for possible ACS and a > 80% reduction in unnecessary CK-MB testing.4 This result was achieved with no statistical change in the prevalence of ACS. The cost reduction does not account for the labor costs to clinically follow-up and address additional unnecessary lab results. The estimated cost of intervention was limited to the time required to educate residents, interns, and nursing staff as well as the implementation of the automated, reflexive laboratory results ordering process.
Unique to our study, we also demonstrated an intervention that satisfied all the major stakeholders in the ordering of these laboratory results. By instituting the reflexive ordering of CK-MB tests for positive troponins, we obtained the support of the facility’s interventional cardiology department, which finds value in that data. Appreciating the time-sensitive nature of an ACS diagnosis, the reflexive ordering minimized the delay in receiving these data while still greatly reducing the number of tests performed. That being said, if the current trend away from CK-MB in favor of exclusively testing troponin continues, removing the reflexive ordering for positive laboratory results protocol would be an easy follow-on intervention.
Limitations
Our study presented several limitations. First, reporting errors due to improper or insufficient medical coding as well as data entry errors may exist within the MDR; therefore, the results of this analysis may be over- or underestimated. Specifically, CPT codes for troponin and CK-MB were available only in 1 of the 2 data sets used for this study, which primarily contains outpatient patient encounters. For this reason, most of the laboratory testing comes from the EMD rather than from inpatient services. However, because we excluded all patients who eventually had an ACS diagnosis (patients who likely had more inpatient time and better indication for repeat troponin), we feel that our intervention was still thoroughly investigated. Second, the number of tests drawn per patient was significantly < 2, the expected minimum number of tests to rule out ACS in patients with appropriate symptoms.
This study was not designed to answer the source of variation from guidelines. Many patients had only 1 test, which we feel represents an opportunity for future study to identify other ways cardiac enzyme testing is being used clinically. These tests might be used for patients without convincing symptoms and signs of coronary syndromes or for patients with other primary problems. Third, by using the ITS analysis, we assumed that the outcome during each intervention period follows a linear pattern. However, changes may follow a nonlinear pattern over a long period. Finally, our intervention was limited to only a single MTF, which may limit generalizability to other facilities across military medicine. However, we feel this study should serve as a guide for other MTFs as well as US Department of Veterans Affairs facilities that could institute similar process improvements.
Conclusion
We made easily implemented and durable process improvement interventions that changed institution-wide ordering practices. These changes dramatically increased the rate of guideline-concordant testing, decreasing cost and furthering the goal of high-value medical care.
1. Anderson JL, Heidenreich PA, Barnett PG, et al; ACC/AHA Task Force on Performance Measures; ACC/AHA Task Force on Practice Guidelines. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. Circulation. 2014;129(22):2329-2345.
2. Centers for Disease Control and Prevention, National Center for Health Statistics. National hospital ambulatory medical care survey: 2010 emergency department summary tables. https://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2010_ed_web_tables.pdf. Accessed March 15, 2019.
3. Morrow DA, Cannon CP, Jesse RL, et al; National Academy of Clinical Biochemistry. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: Clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation. 2007;115(13):e356-e375.
4. Larochelle MR, Knight AM, Pantle H, Riedel S, Trost JC. Reducing excess cardiac biomarker testing at an academic medical center. J Gen Intern Med. 2014;29(11):1468-1474.
5. Centers for Medicare and Medicaid Services. 2016 clinical laboratory fee schedule. https://www.cms.gov/Medicare/Medicare-Fee -for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files-Items/16CLAB.html?DLPage=1&DLEntries=10&DLSort=2&DLSortDir=descending. Accessed March 15, 2019.
In recent years, driven by accelerating health care costs and desire for improved health care value, major specialty group guidelines have incorporated resource utilization and value calculations into their recommendations. High-value care has the characteristics of enhancing outcomes, safety, and patient satisfaction at a reasonable cost. As one example, the American College of Cardiology (ACC) recently published a consensus statement on its clinical practice guidelines with a specific focus on cost and value.1 This guideline acknowledges the difficulty in incorporating value into clinical decision making but stresses a need for increased transparency and consistency to boost value in everyday practice.
Chest pain and related symptoms were listed as the second leading principle reasons for emergency department visits in the US in 2011 with 14% of patients undergoing cardiac enzyme testing.2 The ACC guidelines advocate use of troponin as the preferred laboratory test for the initial evaluation of acute coronary syndrome (ACS). Fractionated creatine kinase (CK-MB) is an acceptable alternative only when a cardiac troponin test is not available.3 Furthermore, troponins should be obtained no more than 3 times for the initial evaluation of a single event, and further trending provides no additional benefit or prognostic information.
A recent study from an academic hospital showed that process improvement interventions focused on eliminating unnecessary cardiac enzyme testing led to a 1-year cost savings of $1.25 million while increasing the rate of ACS diagnosis.4 Common clinical practice at Naval Medical Center Portsmouth (NMCP) in Virginia still routinely includes both troponin as well as a CK panel comprised of CK, CK-MB, and a calculated CK-MB/CK index. Our study focuses on the implementation of quality improvement efforts described by Larochelle and colleagues at NMCP.4 The study aimed to determine the impact of implementing interventions designed to improve the ordering practices and reduce the cost of cardiac enzyme testing.
Methods
The primary focus of the intervention was on ordering practices of the emergency medicine department (EMD), internal medicine (IM) inpatient services, and cardiology inpatient services. Specific interventions were: (1) removal of the CK panel from the chest pain order set in the EMD electronic health record (EHR); (2) removal of the CK panel from the inpatient cardiology order set; (3) education of staff on the changes in CK panel utility via direct communication during IM academic seminars; (4) education of nursing staff ordering laboratory results on behalf of physicians on the cardiology service at the morning and evening huddles; and (5) addition of “max of 3 tests indicated” comment to the inpatient EHR ordering page of the troponin test
Data Source
The process improvement interventions were considered exempt from institutional review board (IRB) approval; however, we obtained expedited IRB approval with waiver of consent for the research aspect of the project. We obtained clinical administrative data from the Military Health System Data Repository (MDR). We identified all adult patients aged ≥ 18 years who had a troponin test, CK-MB, or both drawn at NMCP on the following services: the EMD, IM, and cardiology. A troponin or CK-MB test was defined using Current Procedural Terminology (CPT) codes and unique Logical Observation Identifiers Names and Codes (LOINC).
Measures
The study was divided into 3 periods: the preintervention period from August 1, 2013 to July 31, 2014; the intervention period from August 1, 2014 to January 31, 2015; and the postintervention period February 1, 2015 to January 31, 2016.
The primary outcomes measured were the frequency of guideline concordance and total costs for tests ordered per month using the Centers for Medicare and Medicaid Services (CMS) clinical laboratory fee schedule of $13.40 for troponin and $16.17 for CK-MB.5Concordance was defined as ≤ 3 troponin tests and no CK-MB tests ordered during 1 encounter for a patient without an ACS diagnosis in the preceding 7 days. Due to faster cellular release kinetics of CK-MB compared with that of troponin, this test has utility in evaluating new or worsening chest pain in the setting of a recent myocardial infarction (MI). Therefore, we excluded any patient who had a MI within the preceding 7 days of an order for either CK-MB or troponin tests. Additionally, the number of tests, both CK-MB and troponin, ordered per patient encounter (hereafter referred to as an episode) were measured. Finally, we measured the monthly prevalence of ACS diagnosis and percentage of visits having that diagnosis.
Data Analysis
Descriptive statistics were used to calculate population demographics of age group, sex, beneficiary category, sponsor service, and clinical setting. Monthly data were grouped into the preintervention and postintervention periods. The analysis was performed using t tests to compare mean values and CIs before and after the intervention. Simple linear regression with attention to correlation was used to create best fit lines with confidence bands before and after the intervention. Interrupted time series (ITS) regression was used to describe all data points throughout the study. Consistency between these various methods was verified. Mean values and CIs were reported from the t tests. Statistical significance was reported when appropriate. Equations and confidence predictions on the simple linear regressions were produced and reported. These were used to identify values at the start, midpoint, and end of the pre- and postintervention periods.
Results
There were a total of 6,281 patients in the study population. More patients were seen during the postintervention period than in the preintervention period. The mean age of patients was slightly higher during the preintervention period (Table 1).
Guideline Concordance
To determine whether ordering practices for cardiac enzyme testing improved, we assessed the changes in the frequency of guideline concordance during the pre- and postintervention period. On average during the preintervention year, the percentage of tests ordered that met guideline concordance was 10.1% (95% CI, 7.4%-12.9%), increasing by 0.80% (95% CI, 0.17%-1.42%) each month.
Costs
We assessed changes in total dollars spent on cardiac enzyme testing during the pre- and postintervention periods. During the preintervention year, $9,400 (95% CI, $8,700-$10,100) was spent on average each month, which did not change significantly throughout the period. During the postintervention year, the cost was stable at $5,000 (95% CI, $4,600-$5,300) on average each month, a reduction of $4,400 (95% CI, $3,700-$5,100) (Figure 2).
CK-MB and Troponin Tests per Patient
To further assess ordering practices for cardiac enzyme testing, we compared the changes in the monthly number of tests and the average number of CK-MB and troponin tests ordered per episode pre- and postintervention. On average during the preintervention year, 297 tests (95% CI, 278-315) were run per month, with an average of 1.21 CK tests (95% CI, 1.15-1.27) per episode (Table 2, Figure 3).
The changes in troponin testing were not as dramatic. The counts of tests each month remained similar, with a preintervention year average of 341 (95% CI, 306-377) and postintervention year average of 310 (95% CI, 287-332), which were not statistically different. However, there was a statistically significant decrease in the number of tests per episode. During the preintervention year, 1.38 troponin tests (95% CI, 1.31-1.45) were ordered per patient on average. This dropped by 0.17 (95% CI, 0.09-0.24) to the postintervention average of 1.21 (95% CI, 1.17-1.25) (Table 2, Figure 4).
ACS Prevalence
To determine whether there was an impact on ACS diagnoses, we looked at the numbers of ACS diagnoses and their prevalence among visits before and after the intervention. During the preintervention year, the average monthly number of diagnoses was 29.7 (95% CI, 26.1-33.2), and prevalence of ACS was 0.56% (95% CI, 0.48%-0.63%) of all episodes. Although the monthly rate was statistically decreasing by 0.022% (95% CI, 0.003-0.41), this has little meaning since the level of correlation (r2 = 0.2522, not displayed) was poor due to the essentially nonexistent correlation in number of visits each month (r2 = 0.0112, not displayed). During the postintervention year, the average number of diagnoses was 32.2 (95% CI, 27.9-36.6), and the prevalence of ACS was 0.62% (95% CI, 0.54-0.65). Neither of these values changed significantly between the pre- and postintervention period. All ICD-9 and ICD-10 diagnosis codes used for the analysis are available upon request from the authors.
Discussion
Our data demonstrate the ability of simple process improvement interventions to decrease unnecessary testing in the workup of ACS, increasing the rate of guideline concordant testing by > 70% at a single military treatment facility (MTF). In particular, with the now widespread use of EHR, the order set presents a high-yield target for process improvement in an easily implemented, durable fashion. We had expected to see some decrease in the efficacy of the intervention at a time of staff turnover in the summer of 2015 because ongoing dedicated teaching sessions were not performed. Despite that, the intervention remained effective without further dedicated teaching sessions. This outcome was certainly attributable to the hardwired interventions made (mainly via order sets), but possibly indicates an institutional memory that can take hold after an initial concerted effort is made.
We reduced the estimated preintervention annual cost of $113,000 by $53,000 (95% CI, $42,000-$64,000). Although on a much smaller scale than the study by Larochelle, our study represents a nearly 50% reduction in the total cost of initial testing for possible ACS and a > 80% reduction in unnecessary CK-MB testing.4 This result was achieved with no statistical change in the prevalence of ACS. The cost reduction does not account for the labor costs to clinically follow-up and address additional unnecessary lab results. The estimated cost of intervention was limited to the time required to educate residents, interns, and nursing staff as well as the implementation of the automated, reflexive laboratory results ordering process.
Unique to our study, we also demonstrated an intervention that satisfied all the major stakeholders in the ordering of these laboratory results. By instituting the reflexive ordering of CK-MB tests for positive troponins, we obtained the support of the facility’s interventional cardiology department, which finds value in that data. Appreciating the time-sensitive nature of an ACS diagnosis, the reflexive ordering minimized the delay in receiving these data while still greatly reducing the number of tests performed. That being said, if the current trend away from CK-MB in favor of exclusively testing troponin continues, removing the reflexive ordering for positive laboratory results protocol would be an easy follow-on intervention.
Limitations
Our study presented several limitations. First, reporting errors due to improper or insufficient medical coding as well as data entry errors may exist within the MDR; therefore, the results of this analysis may be over- or underestimated. Specifically, CPT codes for troponin and CK-MB were available only in 1 of the 2 data sets used for this study, which primarily contains outpatient patient encounters. For this reason, most of the laboratory testing comes from the EMD rather than from inpatient services. However, because we excluded all patients who eventually had an ACS diagnosis (patients who likely had more inpatient time and better indication for repeat troponin), we feel that our intervention was still thoroughly investigated. Second, the number of tests drawn per patient was significantly < 2, the expected minimum number of tests to rule out ACS in patients with appropriate symptoms.
This study was not designed to answer the source of variation from guidelines. Many patients had only 1 test, which we feel represents an opportunity for future study to identify other ways cardiac enzyme testing is being used clinically. These tests might be used for patients without convincing symptoms and signs of coronary syndromes or for patients with other primary problems. Third, by using the ITS analysis, we assumed that the outcome during each intervention period follows a linear pattern. However, changes may follow a nonlinear pattern over a long period. Finally, our intervention was limited to only a single MTF, which may limit generalizability to other facilities across military medicine. However, we feel this study should serve as a guide for other MTFs as well as US Department of Veterans Affairs facilities that could institute similar process improvements.
Conclusion
We made easily implemented and durable process improvement interventions that changed institution-wide ordering practices. These changes dramatically increased the rate of guideline-concordant testing, decreasing cost and furthering the goal of high-value medical care.
In recent years, driven by accelerating health care costs and desire for improved health care value, major specialty group guidelines have incorporated resource utilization and value calculations into their recommendations. High-value care has the characteristics of enhancing outcomes, safety, and patient satisfaction at a reasonable cost. As one example, the American College of Cardiology (ACC) recently published a consensus statement on its clinical practice guidelines with a specific focus on cost and value.1 This guideline acknowledges the difficulty in incorporating value into clinical decision making but stresses a need for increased transparency and consistency to boost value in everyday practice.
Chest pain and related symptoms were listed as the second leading principle reasons for emergency department visits in the US in 2011 with 14% of patients undergoing cardiac enzyme testing.2 The ACC guidelines advocate use of troponin as the preferred laboratory test for the initial evaluation of acute coronary syndrome (ACS). Fractionated creatine kinase (CK-MB) is an acceptable alternative only when a cardiac troponin test is not available.3 Furthermore, troponins should be obtained no more than 3 times for the initial evaluation of a single event, and further trending provides no additional benefit or prognostic information.
A recent study from an academic hospital showed that process improvement interventions focused on eliminating unnecessary cardiac enzyme testing led to a 1-year cost savings of $1.25 million while increasing the rate of ACS diagnosis.4 Common clinical practice at Naval Medical Center Portsmouth (NMCP) in Virginia still routinely includes both troponin as well as a CK panel comprised of CK, CK-MB, and a calculated CK-MB/CK index. Our study focuses on the implementation of quality improvement efforts described by Larochelle and colleagues at NMCP.4 The study aimed to determine the impact of implementing interventions designed to improve the ordering practices and reduce the cost of cardiac enzyme testing.
Methods
The primary focus of the intervention was on ordering practices of the emergency medicine department (EMD), internal medicine (IM) inpatient services, and cardiology inpatient services. Specific interventions were: (1) removal of the CK panel from the chest pain order set in the EMD electronic health record (EHR); (2) removal of the CK panel from the inpatient cardiology order set; (3) education of staff on the changes in CK panel utility via direct communication during IM academic seminars; (4) education of nursing staff ordering laboratory results on behalf of physicians on the cardiology service at the morning and evening huddles; and (5) addition of “max of 3 tests indicated” comment to the inpatient EHR ordering page of the troponin test
Data Source
The process improvement interventions were considered exempt from institutional review board (IRB) approval; however, we obtained expedited IRB approval with waiver of consent for the research aspect of the project. We obtained clinical administrative data from the Military Health System Data Repository (MDR). We identified all adult patients aged ≥ 18 years who had a troponin test, CK-MB, or both drawn at NMCP on the following services: the EMD, IM, and cardiology. A troponin or CK-MB test was defined using Current Procedural Terminology (CPT) codes and unique Logical Observation Identifiers Names and Codes (LOINC).
Measures
The study was divided into 3 periods: the preintervention period from August 1, 2013 to July 31, 2014; the intervention period from August 1, 2014 to January 31, 2015; and the postintervention period February 1, 2015 to January 31, 2016.
The primary outcomes measured were the frequency of guideline concordance and total costs for tests ordered per month using the Centers for Medicare and Medicaid Services (CMS) clinical laboratory fee schedule of $13.40 for troponin and $16.17 for CK-MB.5Concordance was defined as ≤ 3 troponin tests and no CK-MB tests ordered during 1 encounter for a patient without an ACS diagnosis in the preceding 7 days. Due to faster cellular release kinetics of CK-MB compared with that of troponin, this test has utility in evaluating new or worsening chest pain in the setting of a recent myocardial infarction (MI). Therefore, we excluded any patient who had a MI within the preceding 7 days of an order for either CK-MB or troponin tests. Additionally, the number of tests, both CK-MB and troponin, ordered per patient encounter (hereafter referred to as an episode) were measured. Finally, we measured the monthly prevalence of ACS diagnosis and percentage of visits having that diagnosis.
Data Analysis
Descriptive statistics were used to calculate population demographics of age group, sex, beneficiary category, sponsor service, and clinical setting. Monthly data were grouped into the preintervention and postintervention periods. The analysis was performed using t tests to compare mean values and CIs before and after the intervention. Simple linear regression with attention to correlation was used to create best fit lines with confidence bands before and after the intervention. Interrupted time series (ITS) regression was used to describe all data points throughout the study. Consistency between these various methods was verified. Mean values and CIs were reported from the t tests. Statistical significance was reported when appropriate. Equations and confidence predictions on the simple linear regressions were produced and reported. These were used to identify values at the start, midpoint, and end of the pre- and postintervention periods.
Results
There were a total of 6,281 patients in the study population. More patients were seen during the postintervention period than in the preintervention period. The mean age of patients was slightly higher during the preintervention period (Table 1).
Guideline Concordance
To determine whether ordering practices for cardiac enzyme testing improved, we assessed the changes in the frequency of guideline concordance during the pre- and postintervention period. On average during the preintervention year, the percentage of tests ordered that met guideline concordance was 10.1% (95% CI, 7.4%-12.9%), increasing by 0.80% (95% CI, 0.17%-1.42%) each month.
Costs
We assessed changes in total dollars spent on cardiac enzyme testing during the pre- and postintervention periods. During the preintervention year, $9,400 (95% CI, $8,700-$10,100) was spent on average each month, which did not change significantly throughout the period. During the postintervention year, the cost was stable at $5,000 (95% CI, $4,600-$5,300) on average each month, a reduction of $4,400 (95% CI, $3,700-$5,100) (Figure 2).
CK-MB and Troponin Tests per Patient
To further assess ordering practices for cardiac enzyme testing, we compared the changes in the monthly number of tests and the average number of CK-MB and troponin tests ordered per episode pre- and postintervention. On average during the preintervention year, 297 tests (95% CI, 278-315) were run per month, with an average of 1.21 CK tests (95% CI, 1.15-1.27) per episode (Table 2, Figure 3).
The changes in troponin testing were not as dramatic. The counts of tests each month remained similar, with a preintervention year average of 341 (95% CI, 306-377) and postintervention year average of 310 (95% CI, 287-332), which were not statistically different. However, there was a statistically significant decrease in the number of tests per episode. During the preintervention year, 1.38 troponin tests (95% CI, 1.31-1.45) were ordered per patient on average. This dropped by 0.17 (95% CI, 0.09-0.24) to the postintervention average of 1.21 (95% CI, 1.17-1.25) (Table 2, Figure 4).
ACS Prevalence
To determine whether there was an impact on ACS diagnoses, we looked at the numbers of ACS diagnoses and their prevalence among visits before and after the intervention. During the preintervention year, the average monthly number of diagnoses was 29.7 (95% CI, 26.1-33.2), and prevalence of ACS was 0.56% (95% CI, 0.48%-0.63%) of all episodes. Although the monthly rate was statistically decreasing by 0.022% (95% CI, 0.003-0.41), this has little meaning since the level of correlation (r2 = 0.2522, not displayed) was poor due to the essentially nonexistent correlation in number of visits each month (r2 = 0.0112, not displayed). During the postintervention year, the average number of diagnoses was 32.2 (95% CI, 27.9-36.6), and the prevalence of ACS was 0.62% (95% CI, 0.54-0.65). Neither of these values changed significantly between the pre- and postintervention period. All ICD-9 and ICD-10 diagnosis codes used for the analysis are available upon request from the authors.
Discussion
Our data demonstrate the ability of simple process improvement interventions to decrease unnecessary testing in the workup of ACS, increasing the rate of guideline concordant testing by > 70% at a single military treatment facility (MTF). In particular, with the now widespread use of EHR, the order set presents a high-yield target for process improvement in an easily implemented, durable fashion. We had expected to see some decrease in the efficacy of the intervention at a time of staff turnover in the summer of 2015 because ongoing dedicated teaching sessions were not performed. Despite that, the intervention remained effective without further dedicated teaching sessions. This outcome was certainly attributable to the hardwired interventions made (mainly via order sets), but possibly indicates an institutional memory that can take hold after an initial concerted effort is made.
We reduced the estimated preintervention annual cost of $113,000 by $53,000 (95% CI, $42,000-$64,000). Although on a much smaller scale than the study by Larochelle, our study represents a nearly 50% reduction in the total cost of initial testing for possible ACS and a > 80% reduction in unnecessary CK-MB testing.4 This result was achieved with no statistical change in the prevalence of ACS. The cost reduction does not account for the labor costs to clinically follow-up and address additional unnecessary lab results. The estimated cost of intervention was limited to the time required to educate residents, interns, and nursing staff as well as the implementation of the automated, reflexive laboratory results ordering process.
Unique to our study, we also demonstrated an intervention that satisfied all the major stakeholders in the ordering of these laboratory results. By instituting the reflexive ordering of CK-MB tests for positive troponins, we obtained the support of the facility’s interventional cardiology department, which finds value in that data. Appreciating the time-sensitive nature of an ACS diagnosis, the reflexive ordering minimized the delay in receiving these data while still greatly reducing the number of tests performed. That being said, if the current trend away from CK-MB in favor of exclusively testing troponin continues, removing the reflexive ordering for positive laboratory results protocol would be an easy follow-on intervention.
Limitations
Our study presented several limitations. First, reporting errors due to improper or insufficient medical coding as well as data entry errors may exist within the MDR; therefore, the results of this analysis may be over- or underestimated. Specifically, CPT codes for troponin and CK-MB were available only in 1 of the 2 data sets used for this study, which primarily contains outpatient patient encounters. For this reason, most of the laboratory testing comes from the EMD rather than from inpatient services. However, because we excluded all patients who eventually had an ACS diagnosis (patients who likely had more inpatient time and better indication for repeat troponin), we feel that our intervention was still thoroughly investigated. Second, the number of tests drawn per patient was significantly < 2, the expected minimum number of tests to rule out ACS in patients with appropriate symptoms.
This study was not designed to answer the source of variation from guidelines. Many patients had only 1 test, which we feel represents an opportunity for future study to identify other ways cardiac enzyme testing is being used clinically. These tests might be used for patients without convincing symptoms and signs of coronary syndromes or for patients with other primary problems. Third, by using the ITS analysis, we assumed that the outcome during each intervention period follows a linear pattern. However, changes may follow a nonlinear pattern over a long period. Finally, our intervention was limited to only a single MTF, which may limit generalizability to other facilities across military medicine. However, we feel this study should serve as a guide for other MTFs as well as US Department of Veterans Affairs facilities that could institute similar process improvements.
Conclusion
We made easily implemented and durable process improvement interventions that changed institution-wide ordering practices. These changes dramatically increased the rate of guideline-concordant testing, decreasing cost and furthering the goal of high-value medical care.
1. Anderson JL, Heidenreich PA, Barnett PG, et al; ACC/AHA Task Force on Performance Measures; ACC/AHA Task Force on Practice Guidelines. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. Circulation. 2014;129(22):2329-2345.
2. Centers for Disease Control and Prevention, National Center for Health Statistics. National hospital ambulatory medical care survey: 2010 emergency department summary tables. https://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2010_ed_web_tables.pdf. Accessed March 15, 2019.
3. Morrow DA, Cannon CP, Jesse RL, et al; National Academy of Clinical Biochemistry. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: Clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation. 2007;115(13):e356-e375.
4. Larochelle MR, Knight AM, Pantle H, Riedel S, Trost JC. Reducing excess cardiac biomarker testing at an academic medical center. J Gen Intern Med. 2014;29(11):1468-1474.
5. Centers for Medicare and Medicaid Services. 2016 clinical laboratory fee schedule. https://www.cms.gov/Medicare/Medicare-Fee -for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files-Items/16CLAB.html?DLPage=1&DLEntries=10&DLSort=2&DLSortDir=descending. Accessed March 15, 2019.
1. Anderson JL, Heidenreich PA, Barnett PG, et al; ACC/AHA Task Force on Performance Measures; ACC/AHA Task Force on Practice Guidelines. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. Circulation. 2014;129(22):2329-2345.
2. Centers for Disease Control and Prevention, National Center for Health Statistics. National hospital ambulatory medical care survey: 2010 emergency department summary tables. https://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2010_ed_web_tables.pdf. Accessed March 15, 2019.
3. Morrow DA, Cannon CP, Jesse RL, et al; National Academy of Clinical Biochemistry. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: Clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation. 2007;115(13):e356-e375.
4. Larochelle MR, Knight AM, Pantle H, Riedel S, Trost JC. Reducing excess cardiac biomarker testing at an academic medical center. J Gen Intern Med. 2014;29(11):1468-1474.
5. Centers for Medicare and Medicaid Services. 2016 clinical laboratory fee schedule. https://www.cms.gov/Medicare/Medicare-Fee -for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files-Items/16CLAB.html?DLPage=1&DLEntries=10&DLSort=2&DLSortDir=descending. Accessed March 15, 2019.
Use of GBCA in MRIs for High-Risk Patients
To the Editor:
We read with interest the case report of nephrogenic systemic fibrosis (NSF) by Chuang, Kaneshiro, and Betancourt in the June 2018 issue of Federal Practitioner.1 It was reported that a 61-year-old Hispanic male patient with a history of IV heroin abuse with end-stage renal disease (ESRD) secondary to membranous glomerulonephritis on hemodialysis and chronic hepatitis C infection received 15 mL gadoversetamide, a linear gadolinium-based contrast agent (GBCA) during magnetic resonance imaging (MRI) of the brain. Hemodialysis was performed 18 hours after the contrast administration.
Eight weeks after his initial presentation, the patient developed pyoderma gangrenosum on his right forearm, which was treated with high-dose steroids. He then developed thickening and induration of his bilateral forearm skin with peau d’orange appearance. NSF was confirmed by a skin biopsy. The patient developed contractures of his upper and lower extremities and was finally wheelchair bound.
This case is very concerning since no NSF cases in patients receiving GBCA have been published since 2009. Unfortunately, the authors give no information on the occurrence of this particular case. Thus, it is unclear whether this case was observed before or after the switch to macrocyclic agents in patients with reduced renal function. The reported patient with ESRD was on hemodialysis and received 15 mL gadoversetamide during MRI of the brain. In 2007 the ESUR (European Society of Urogenital Radiology) published guidelines indicating linear GBCA (gadodiamide, gadoversetamide, gadopentetate dimeglumine) as high-risk agents that may not be used in patients with eGFR < 30 mL/min/1.73 m2.2,3
Consequently in 2007, the European Medicines Agency contraindicated these linear GBCA in patients with chronic kidney disease grades 4 and 5. Also in 2007 the US Food and Drug Administration (FDA) requested a revision of the prescribing information for all 5 GBCA approved in the US.4 In response to accumulating more informative data, in 2010 the FDA again used this class labeling approach to more explicitly describe differences in NSF risks among the agents.4 FDA regulation and contraindication of the use of low-stability GBCA in patients with advanced renal impairment and robust local policies on the safe use of these agents have resulted in marked reduction in the prevalence of NSF in the US. This case report needs to clarify why a high-risk linear agent was administered to a patient with ESRD.
In 2006 Grobner and Marckmann and colleagues reported their observations of a previously unrecognized link between exposure to gadodiamide and the development of NSF.5,6 It soon became clear that NSF is a delayed adverse contrast reaction that may cause severe disability and even death. Advanced renal disease and high-risk linear GBCA are the main factors in the pathogenesis of NSF. Additionally, the dose of the agent may play a role. NSF can occur from hours to years after exposure to GBCA. Not all patients with severe kidney disease exposed to high-risk agents developed NSF. Thus, additional factors were proposed to play a role in the pathogenesis of NSF. Among those factors were erythropoietin, metabolic acidosis, anion gap, iron, increased phosphate, zinc loss, proinflammatory conditions/inflammation and angiotensin-converting enzyme (ACE) inhibitors.7 Although there is little proof with these assumptions, special care must be taken as shown by this reported patient with multiple inflammatory disorders.
- Gertraud Heinz, MD, MBA; Aart van der Molen, MD; and Giles Roditi, MD; on behalf of the ESUR Contrast Media Safety Committee
Author affiliations: Gertraud Heinz is former President ESUR and Head of the Department of Radiology, Diagnostics and Intervention University Hospital St. Pölten Karl Landsteiner University of Health Sciences.
Correspondence: Gertraud Heinz (gertraud.heinz@stpoelten .lknoe.at)
Disclosures: The authors report no conflict of interest with regard to this article.
References
1. Chuang K, Kaneshiro C, Betancourt J. Nephrogenic systemic fibrosis in a patient with multiple inflammatory disorders. Fed Pract. 2018;35(6):40-43.
2. Thomsen HS; European Society of Urogenital Radiology (ESUR). ESUR guideline: gadolinium based contrast media and nephrogenic systemic fibrosis. Eur Radiol. 2007;17(10):2692-2696.
3. Thomsen HS, Morcos SK, Almén T, et al; ESUR Contrast Medium Safety Committee. Nephrogenic systemic fibrosis and gadolinium-based contrast media: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol. 2013;23(2):307-318
4. Yang L, Krefting I, Gorovets A, et al. Nephrogenic systemic fibrosis and class labeling of gadolinium-based agents by the Food and Drug Administration. Radiology. 2012;265(1):248-253.
5. Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21(4):1104-1108.
6. Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17(9):2359-2362.
7. Thomsen HS, Bennett CL. Six years after. Acta Radiol. 2012;53(8):827-829.
To the Editor:
With great interest, I read the case report by Chuang, Kaneshiro, and Betancourt.1 Patients with nephrogenic systemic fibrosis (NSF) are of special interest because the disease is still unclear as mentioned by the authors. Although new cases may occur,2 this case raises some concerns that I would like to address.
First, it would be of great interest to know the date when the patient received the high-risk gadolinium-based contrast agent (GBCA) gadoversetamide. Unfortunately, the authors did not mention the date of the injection of the GBCA that probably caused NSF. Due to the obvious association between the applications of special GBCAs in 2006, the US Food and Drug Administration (FDA) warned physicians not to inject these contrast agents in patients with compromised kidney function.3 Moreover, in 2007 the American College of Radiology (ACR) published guidelines for the safe use of GBCAs in patients with renal failure.4 Also, the European Medicines Agency (EMA) demanded that companies provide warning in product inserts about the acquisition of NSF in patients with severe kidney injury.5
Second, the clinical illustration of the case is inadequate. In the manuscript, we read that the patient acquired NSF-characteristic lesions like peau d’orange skin lesions and contractures of his extremities, but unfortunately, Chuang, Kaneshiro, and Betancourt did not provide figures that show them. On the other hand, Figure 1 shows an uncharacteristic dermal induration around inflammatory and ulcerated skin lesion (pyoderma gangrenosum).1 Such clinical signs are well known and occur perilesional of different conditions independently of NSF.6-8
Third, the histological features described as presence of fibrotic tissue in the deep dermis in Figure 2, and dermal fibrosis with thick collagen deposition in Figure 31 do not confirm the existence of NSF.
Taken together, the case presented by Chuang, Kaneshiro, and Betancourt contains some unclear aspects; therefore, it is questionable whether the published case describes a patient with NSF or not. In the current presentation, the diagnosis NSF seems to be an overestimation.
NSF still is a poorly understood disorder. Therefore, exactly documented new cases could be of clinical value when providing interesting information. Even single cases could shed some light in the darkness of the pathological mechanisms of this entity. On the other hand, we should not mix the existing cohort of published NSF cases with other scleroderma-like diseases, because this will lead to a confusion. Moreover, such a practice could inhibit the discovery of the pathophysiology of NSF.
- Ingrid Böhm, MD
Author affiliations: Ingrid Böhm is a Physician in the Department of Diagnostics, Interventional and Pediatric Radiology at the University Hospital of Bern, Inselspital, University of Bern in Bern, Switzerland.
Correspondence: Ingrid Böhm (ingrid.boehm@insel.ch)
Disclosures: The author reports no conflict of interest with regard to this article.
References
1. Chuang K, Kaneshiro C, Betancourt J. Nephrogenic systemic fibrosis in a patient with multiple inflammatory disorders. Fed Pract . 2018;35(6):40-43.
2. Larson KN, Gagnon AL, Darling MD, Patterson JW, Cropley TG. Nephrogenic systemic fibrosis manifesting a decade after exposure to gadolinium. JAMA Dermatol. 2015;151(10):1117-1120.
3. US Food and Drug Administration. A Public Health Advisory. Gadolinium-containing contrast agents for magnetic resonance imaging (MRI). http://wayback.archive-it.org/7993/20170112033022/http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformation forPatientsandProviders/ucm053112.htm. Published June 8, 2006. Accessed March 15, 2019.
4. Kanal E, Barkovich AJ, Bell C, et al; ACR Blue Ribbon Panel on MR Safety. ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol. 2007;188(6):1447-1474.
5. European Medicines Agency. Public statement: Vasovist and nephrogenic systemic fibrosis (NSF). https://www.ema.europa.eu/en/news/public-statement-vasovist-nephrogenic-systemic-fibrosis-nsf. Published February 7, 2007. Accessed March 15, 2019.
6. Luke JC. The etiology and modern treatment of varicose ulcer. Can Med Assoc J. 1940;43(3):217-221.
7. Paulsen E, Bygum A. Keratin gel as an adjuvant in the treatment of recalcitrant pyoderma gangrenosum ulcers: a case report. Acta Derm Venereol. 2019;99(2):234-235.
8. Boehm I, Bauer R. Low-dose methotrexate controls a severe form of polyarteritis nodosa. Arch Dermatol. 2000; 136(2):167-169.
Response:
We thank Drs. Heinz, van der Molen, and Roditi for their valuable response. The following is the opinion of the authors and is not representative of the views or policies of our institution. The patient in this case received a gadolinium-based contrast agent (GBCA) in 2015 and was diagnosed with nephrogenic systemic fibrosis (NSF) 8 weeks later. We agree with the correspondents that linear GBCAs should not be used in patients with eGFR < 30 mL/min/1.73 m2. To date, a few cases of patients who received GBCA and developed NSF since 2009 have unfortunately continued to be reported in the literature.1-3 Our intention in publishing this case was to provide ongoing education to the medical community regarding this serious condition to ensure prevention of future cases.
We thank Dr. Böhm for her important inquiry. The patient received a histopathologic diagnosis of NSF. The report from the patient’s left dorsal forearm skin punch biopsy was read by our pathologist as “fibrosis and inflammation consistent with nephrogenic systemic fibrosis,” a diagnosis agreed upon by our colleagues in the dermatology and rheumatology departments based on the rapidity of his symptom onset and progression. While we acknowledge that this patient had other inflammatory disorders of the skin that may have coexisted with the diagnosis, after weighing the preponderance of clinical evidence in support of the biopsy results, we believe that this represents a case of NSF, which is associated with high morbidity and mortality. Thankfully, the patient in this case engaged extensively in physical and occupational therapy and is still alive nearly 4 years later. We would like to thank all the letter writers for their correspondence.
Author Affiliations: Kelley Chuang and Casey Kaneshiro are Hospitalists and Jaime Betancourt is a Pulmonologist, all in the Department of Medicine at the VA Greater Los Angeles Healthcare System in California.
Correspondence: Kelley Chuang (kelleychuang@mednet.ucla.edu)
Disclosures: The authors report no conflict of interest with regard to this article.
References
1. Aggarwal A, Froehlich AA, Essah P, Brinster N, High WA, Downs RW. Complications of nephrogenic systemic fibrosis following repeated exposure to gadolinium in a man with hypothyroidism: a case report. J Med Case Rep. 2011;5:566.
2. Fuah KW, Lim CT. Erythema nodosum masking nephrogenic systemic fibrosis as initial skin manifestation. BMC Nephrol. 2017;18(1):249.
3. Koratala A, Bhatti V. Nephrogenic systemic fibrosis. Clin Case Rep. 2017;5(7):1184-1185.
To the Editor:
We read with interest the case report of nephrogenic systemic fibrosis (NSF) by Chuang, Kaneshiro, and Betancourt in the June 2018 issue of Federal Practitioner.1 It was reported that a 61-year-old Hispanic male patient with a history of IV heroin abuse with end-stage renal disease (ESRD) secondary to membranous glomerulonephritis on hemodialysis and chronic hepatitis C infection received 15 mL gadoversetamide, a linear gadolinium-based contrast agent (GBCA) during magnetic resonance imaging (MRI) of the brain. Hemodialysis was performed 18 hours after the contrast administration.
Eight weeks after his initial presentation, the patient developed pyoderma gangrenosum on his right forearm, which was treated with high-dose steroids. He then developed thickening and induration of his bilateral forearm skin with peau d’orange appearance. NSF was confirmed by a skin biopsy. The patient developed contractures of his upper and lower extremities and was finally wheelchair bound.
This case is very concerning since no NSF cases in patients receiving GBCA have been published since 2009. Unfortunately, the authors give no information on the occurrence of this particular case. Thus, it is unclear whether this case was observed before or after the switch to macrocyclic agents in patients with reduced renal function. The reported patient with ESRD was on hemodialysis and received 15 mL gadoversetamide during MRI of the brain. In 2007 the ESUR (European Society of Urogenital Radiology) published guidelines indicating linear GBCA (gadodiamide, gadoversetamide, gadopentetate dimeglumine) as high-risk agents that may not be used in patients with eGFR < 30 mL/min/1.73 m2.2,3
Consequently in 2007, the European Medicines Agency contraindicated these linear GBCA in patients with chronic kidney disease grades 4 and 5. Also in 2007 the US Food and Drug Administration (FDA) requested a revision of the prescribing information for all 5 GBCA approved in the US.4 In response to accumulating more informative data, in 2010 the FDA again used this class labeling approach to more explicitly describe differences in NSF risks among the agents.4 FDA regulation and contraindication of the use of low-stability GBCA in patients with advanced renal impairment and robust local policies on the safe use of these agents have resulted in marked reduction in the prevalence of NSF in the US. This case report needs to clarify why a high-risk linear agent was administered to a patient with ESRD.
In 2006 Grobner and Marckmann and colleagues reported their observations of a previously unrecognized link between exposure to gadodiamide and the development of NSF.5,6 It soon became clear that NSF is a delayed adverse contrast reaction that may cause severe disability and even death. Advanced renal disease and high-risk linear GBCA are the main factors in the pathogenesis of NSF. Additionally, the dose of the agent may play a role. NSF can occur from hours to years after exposure to GBCA. Not all patients with severe kidney disease exposed to high-risk agents developed NSF. Thus, additional factors were proposed to play a role in the pathogenesis of NSF. Among those factors were erythropoietin, metabolic acidosis, anion gap, iron, increased phosphate, zinc loss, proinflammatory conditions/inflammation and angiotensin-converting enzyme (ACE) inhibitors.7 Although there is little proof with these assumptions, special care must be taken as shown by this reported patient with multiple inflammatory disorders.
- Gertraud Heinz, MD, MBA; Aart van der Molen, MD; and Giles Roditi, MD; on behalf of the ESUR Contrast Media Safety Committee
Author affiliations: Gertraud Heinz is former President ESUR and Head of the Department of Radiology, Diagnostics and Intervention University Hospital St. Pölten Karl Landsteiner University of Health Sciences.
Correspondence: Gertraud Heinz (gertraud.heinz@stpoelten .lknoe.at)
Disclosures: The authors report no conflict of interest with regard to this article.
References
1. Chuang K, Kaneshiro C, Betancourt J. Nephrogenic systemic fibrosis in a patient with multiple inflammatory disorders. Fed Pract. 2018;35(6):40-43.
2. Thomsen HS; European Society of Urogenital Radiology (ESUR). ESUR guideline: gadolinium based contrast media and nephrogenic systemic fibrosis. Eur Radiol. 2007;17(10):2692-2696.
3. Thomsen HS, Morcos SK, Almén T, et al; ESUR Contrast Medium Safety Committee. Nephrogenic systemic fibrosis and gadolinium-based contrast media: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol. 2013;23(2):307-318
4. Yang L, Krefting I, Gorovets A, et al. Nephrogenic systemic fibrosis and class labeling of gadolinium-based agents by the Food and Drug Administration. Radiology. 2012;265(1):248-253.
5. Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21(4):1104-1108.
6. Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17(9):2359-2362.
7. Thomsen HS, Bennett CL. Six years after. Acta Radiol. 2012;53(8):827-829.
To the Editor:
With great interest, I read the case report by Chuang, Kaneshiro, and Betancourt.1 Patients with nephrogenic systemic fibrosis (NSF) are of special interest because the disease is still unclear as mentioned by the authors. Although new cases may occur,2 this case raises some concerns that I would like to address.
First, it would be of great interest to know the date when the patient received the high-risk gadolinium-based contrast agent (GBCA) gadoversetamide. Unfortunately, the authors did not mention the date of the injection of the GBCA that probably caused NSF. Due to the obvious association between the applications of special GBCAs in 2006, the US Food and Drug Administration (FDA) warned physicians not to inject these contrast agents in patients with compromised kidney function.3 Moreover, in 2007 the American College of Radiology (ACR) published guidelines for the safe use of GBCAs in patients with renal failure.4 Also, the European Medicines Agency (EMA) demanded that companies provide warning in product inserts about the acquisition of NSF in patients with severe kidney injury.5
Second, the clinical illustration of the case is inadequate. In the manuscript, we read that the patient acquired NSF-characteristic lesions like peau d’orange skin lesions and contractures of his extremities, but unfortunately, Chuang, Kaneshiro, and Betancourt did not provide figures that show them. On the other hand, Figure 1 shows an uncharacteristic dermal induration around inflammatory and ulcerated skin lesion (pyoderma gangrenosum).1 Such clinical signs are well known and occur perilesional of different conditions independently of NSF.6-8
Third, the histological features described as presence of fibrotic tissue in the deep dermis in Figure 2, and dermal fibrosis with thick collagen deposition in Figure 31 do not confirm the existence of NSF.
Taken together, the case presented by Chuang, Kaneshiro, and Betancourt contains some unclear aspects; therefore, it is questionable whether the published case describes a patient with NSF or not. In the current presentation, the diagnosis NSF seems to be an overestimation.
NSF still is a poorly understood disorder. Therefore, exactly documented new cases could be of clinical value when providing interesting information. Even single cases could shed some light in the darkness of the pathological mechanisms of this entity. On the other hand, we should not mix the existing cohort of published NSF cases with other scleroderma-like diseases, because this will lead to a confusion. Moreover, such a practice could inhibit the discovery of the pathophysiology of NSF.
- Ingrid Böhm, MD
Author affiliations: Ingrid Böhm is a Physician in the Department of Diagnostics, Interventional and Pediatric Radiology at the University Hospital of Bern, Inselspital, University of Bern in Bern, Switzerland.
Correspondence: Ingrid Böhm (ingrid.boehm@insel.ch)
Disclosures: The author reports no conflict of interest with regard to this article.
References
1. Chuang K, Kaneshiro C, Betancourt J. Nephrogenic systemic fibrosis in a patient with multiple inflammatory disorders. Fed Pract . 2018;35(6):40-43.
2. Larson KN, Gagnon AL, Darling MD, Patterson JW, Cropley TG. Nephrogenic systemic fibrosis manifesting a decade after exposure to gadolinium. JAMA Dermatol. 2015;151(10):1117-1120.
3. US Food and Drug Administration. A Public Health Advisory. Gadolinium-containing contrast agents for magnetic resonance imaging (MRI). http://wayback.archive-it.org/7993/20170112033022/http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformation forPatientsandProviders/ucm053112.htm. Published June 8, 2006. Accessed March 15, 2019.
4. Kanal E, Barkovich AJ, Bell C, et al; ACR Blue Ribbon Panel on MR Safety. ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol. 2007;188(6):1447-1474.
5. European Medicines Agency. Public statement: Vasovist and nephrogenic systemic fibrosis (NSF). https://www.ema.europa.eu/en/news/public-statement-vasovist-nephrogenic-systemic-fibrosis-nsf. Published February 7, 2007. Accessed March 15, 2019.
6. Luke JC. The etiology and modern treatment of varicose ulcer. Can Med Assoc J. 1940;43(3):217-221.
7. Paulsen E, Bygum A. Keratin gel as an adjuvant in the treatment of recalcitrant pyoderma gangrenosum ulcers: a case report. Acta Derm Venereol. 2019;99(2):234-235.
8. Boehm I, Bauer R. Low-dose methotrexate controls a severe form of polyarteritis nodosa. Arch Dermatol. 2000; 136(2):167-169.
Response:
We thank Drs. Heinz, van der Molen, and Roditi for their valuable response. The following is the opinion of the authors and is not representative of the views or policies of our institution. The patient in this case received a gadolinium-based contrast agent (GBCA) in 2015 and was diagnosed with nephrogenic systemic fibrosis (NSF) 8 weeks later. We agree with the correspondents that linear GBCAs should not be used in patients with eGFR < 30 mL/min/1.73 m2. To date, a few cases of patients who received GBCA and developed NSF since 2009 have unfortunately continued to be reported in the literature.1-3 Our intention in publishing this case was to provide ongoing education to the medical community regarding this serious condition to ensure prevention of future cases.
We thank Dr. Böhm for her important inquiry. The patient received a histopathologic diagnosis of NSF. The report from the patient’s left dorsal forearm skin punch biopsy was read by our pathologist as “fibrosis and inflammation consistent with nephrogenic systemic fibrosis,” a diagnosis agreed upon by our colleagues in the dermatology and rheumatology departments based on the rapidity of his symptom onset and progression. While we acknowledge that this patient had other inflammatory disorders of the skin that may have coexisted with the diagnosis, after weighing the preponderance of clinical evidence in support of the biopsy results, we believe that this represents a case of NSF, which is associated with high morbidity and mortality. Thankfully, the patient in this case engaged extensively in physical and occupational therapy and is still alive nearly 4 years later. We would like to thank all the letter writers for their correspondence.
Author Affiliations: Kelley Chuang and Casey Kaneshiro are Hospitalists and Jaime Betancourt is a Pulmonologist, all in the Department of Medicine at the VA Greater Los Angeles Healthcare System in California.
Correspondence: Kelley Chuang (kelleychuang@mednet.ucla.edu)
Disclosures: The authors report no conflict of interest with regard to this article.
References
1. Aggarwal A, Froehlich AA, Essah P, Brinster N, High WA, Downs RW. Complications of nephrogenic systemic fibrosis following repeated exposure to gadolinium in a man with hypothyroidism: a case report. J Med Case Rep. 2011;5:566.
2. Fuah KW, Lim CT. Erythema nodosum masking nephrogenic systemic fibrosis as initial skin manifestation. BMC Nephrol. 2017;18(1):249.
3. Koratala A, Bhatti V. Nephrogenic systemic fibrosis. Clin Case Rep. 2017;5(7):1184-1185.
To the Editor:
We read with interest the case report of nephrogenic systemic fibrosis (NSF) by Chuang, Kaneshiro, and Betancourt in the June 2018 issue of Federal Practitioner.1 It was reported that a 61-year-old Hispanic male patient with a history of IV heroin abuse with end-stage renal disease (ESRD) secondary to membranous glomerulonephritis on hemodialysis and chronic hepatitis C infection received 15 mL gadoversetamide, a linear gadolinium-based contrast agent (GBCA) during magnetic resonance imaging (MRI) of the brain. Hemodialysis was performed 18 hours after the contrast administration.
Eight weeks after his initial presentation, the patient developed pyoderma gangrenosum on his right forearm, which was treated with high-dose steroids. He then developed thickening and induration of his bilateral forearm skin with peau d’orange appearance. NSF was confirmed by a skin biopsy. The patient developed contractures of his upper and lower extremities and was finally wheelchair bound.
This case is very concerning since no NSF cases in patients receiving GBCA have been published since 2009. Unfortunately, the authors give no information on the occurrence of this particular case. Thus, it is unclear whether this case was observed before or after the switch to macrocyclic agents in patients with reduced renal function. The reported patient with ESRD was on hemodialysis and received 15 mL gadoversetamide during MRI of the brain. In 2007 the ESUR (European Society of Urogenital Radiology) published guidelines indicating linear GBCA (gadodiamide, gadoversetamide, gadopentetate dimeglumine) as high-risk agents that may not be used in patients with eGFR < 30 mL/min/1.73 m2.2,3
Consequently in 2007, the European Medicines Agency contraindicated these linear GBCA in patients with chronic kidney disease grades 4 and 5. Also in 2007 the US Food and Drug Administration (FDA) requested a revision of the prescribing information for all 5 GBCA approved in the US.4 In response to accumulating more informative data, in 2010 the FDA again used this class labeling approach to more explicitly describe differences in NSF risks among the agents.4 FDA regulation and contraindication of the use of low-stability GBCA in patients with advanced renal impairment and robust local policies on the safe use of these agents have resulted in marked reduction in the prevalence of NSF in the US. This case report needs to clarify why a high-risk linear agent was administered to a patient with ESRD.
In 2006 Grobner and Marckmann and colleagues reported their observations of a previously unrecognized link between exposure to gadodiamide and the development of NSF.5,6 It soon became clear that NSF is a delayed adverse contrast reaction that may cause severe disability and even death. Advanced renal disease and high-risk linear GBCA are the main factors in the pathogenesis of NSF. Additionally, the dose of the agent may play a role. NSF can occur from hours to years after exposure to GBCA. Not all patients with severe kidney disease exposed to high-risk agents developed NSF. Thus, additional factors were proposed to play a role in the pathogenesis of NSF. Among those factors were erythropoietin, metabolic acidosis, anion gap, iron, increased phosphate, zinc loss, proinflammatory conditions/inflammation and angiotensin-converting enzyme (ACE) inhibitors.7 Although there is little proof with these assumptions, special care must be taken as shown by this reported patient with multiple inflammatory disorders.
- Gertraud Heinz, MD, MBA; Aart van der Molen, MD; and Giles Roditi, MD; on behalf of the ESUR Contrast Media Safety Committee
Author affiliations: Gertraud Heinz is former President ESUR and Head of the Department of Radiology, Diagnostics and Intervention University Hospital St. Pölten Karl Landsteiner University of Health Sciences.
Correspondence: Gertraud Heinz (gertraud.heinz@stpoelten .lknoe.at)
Disclosures: The authors report no conflict of interest with regard to this article.
References
1. Chuang K, Kaneshiro C, Betancourt J. Nephrogenic systemic fibrosis in a patient with multiple inflammatory disorders. Fed Pract. 2018;35(6):40-43.
2. Thomsen HS; European Society of Urogenital Radiology (ESUR). ESUR guideline: gadolinium based contrast media and nephrogenic systemic fibrosis. Eur Radiol. 2007;17(10):2692-2696.
3. Thomsen HS, Morcos SK, Almén T, et al; ESUR Contrast Medium Safety Committee. Nephrogenic systemic fibrosis and gadolinium-based contrast media: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol. 2013;23(2):307-318
4. Yang L, Krefting I, Gorovets A, et al. Nephrogenic systemic fibrosis and class labeling of gadolinium-based agents by the Food and Drug Administration. Radiology. 2012;265(1):248-253.
5. Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21(4):1104-1108.
6. Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17(9):2359-2362.
7. Thomsen HS, Bennett CL. Six years after. Acta Radiol. 2012;53(8):827-829.
To the Editor:
With great interest, I read the case report by Chuang, Kaneshiro, and Betancourt.1 Patients with nephrogenic systemic fibrosis (NSF) are of special interest because the disease is still unclear as mentioned by the authors. Although new cases may occur,2 this case raises some concerns that I would like to address.
First, it would be of great interest to know the date when the patient received the high-risk gadolinium-based contrast agent (GBCA) gadoversetamide. Unfortunately, the authors did not mention the date of the injection of the GBCA that probably caused NSF. Due to the obvious association between the applications of special GBCAs in 2006, the US Food and Drug Administration (FDA) warned physicians not to inject these contrast agents in patients with compromised kidney function.3 Moreover, in 2007 the American College of Radiology (ACR) published guidelines for the safe use of GBCAs in patients with renal failure.4 Also, the European Medicines Agency (EMA) demanded that companies provide warning in product inserts about the acquisition of NSF in patients with severe kidney injury.5
Second, the clinical illustration of the case is inadequate. In the manuscript, we read that the patient acquired NSF-characteristic lesions like peau d’orange skin lesions and contractures of his extremities, but unfortunately, Chuang, Kaneshiro, and Betancourt did not provide figures that show them. On the other hand, Figure 1 shows an uncharacteristic dermal induration around inflammatory and ulcerated skin lesion (pyoderma gangrenosum).1 Such clinical signs are well known and occur perilesional of different conditions independently of NSF.6-8
Third, the histological features described as presence of fibrotic tissue in the deep dermis in Figure 2, and dermal fibrosis with thick collagen deposition in Figure 31 do not confirm the existence of NSF.
Taken together, the case presented by Chuang, Kaneshiro, and Betancourt contains some unclear aspects; therefore, it is questionable whether the published case describes a patient with NSF or not. In the current presentation, the diagnosis NSF seems to be an overestimation.
NSF still is a poorly understood disorder. Therefore, exactly documented new cases could be of clinical value when providing interesting information. Even single cases could shed some light in the darkness of the pathological mechanisms of this entity. On the other hand, we should not mix the existing cohort of published NSF cases with other scleroderma-like diseases, because this will lead to a confusion. Moreover, such a practice could inhibit the discovery of the pathophysiology of NSF.
- Ingrid Böhm, MD
Author affiliations: Ingrid Böhm is a Physician in the Department of Diagnostics, Interventional and Pediatric Radiology at the University Hospital of Bern, Inselspital, University of Bern in Bern, Switzerland.
Correspondence: Ingrid Böhm (ingrid.boehm@insel.ch)
Disclosures: The author reports no conflict of interest with regard to this article.
References
1. Chuang K, Kaneshiro C, Betancourt J. Nephrogenic systemic fibrosis in a patient with multiple inflammatory disorders. Fed Pract . 2018;35(6):40-43.
2. Larson KN, Gagnon AL, Darling MD, Patterson JW, Cropley TG. Nephrogenic systemic fibrosis manifesting a decade after exposure to gadolinium. JAMA Dermatol. 2015;151(10):1117-1120.
3. US Food and Drug Administration. A Public Health Advisory. Gadolinium-containing contrast agents for magnetic resonance imaging (MRI). http://wayback.archive-it.org/7993/20170112033022/http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformation forPatientsandProviders/ucm053112.htm. Published June 8, 2006. Accessed March 15, 2019.
4. Kanal E, Barkovich AJ, Bell C, et al; ACR Blue Ribbon Panel on MR Safety. ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol. 2007;188(6):1447-1474.
5. European Medicines Agency. Public statement: Vasovist and nephrogenic systemic fibrosis (NSF). https://www.ema.europa.eu/en/news/public-statement-vasovist-nephrogenic-systemic-fibrosis-nsf. Published February 7, 2007. Accessed March 15, 2019.
6. Luke JC. The etiology and modern treatment of varicose ulcer. Can Med Assoc J. 1940;43(3):217-221.
7. Paulsen E, Bygum A. Keratin gel as an adjuvant in the treatment of recalcitrant pyoderma gangrenosum ulcers: a case report. Acta Derm Venereol. 2019;99(2):234-235.
8. Boehm I, Bauer R. Low-dose methotrexate controls a severe form of polyarteritis nodosa. Arch Dermatol. 2000; 136(2):167-169.
Response:
We thank Drs. Heinz, van der Molen, and Roditi for their valuable response. The following is the opinion of the authors and is not representative of the views or policies of our institution. The patient in this case received a gadolinium-based contrast agent (GBCA) in 2015 and was diagnosed with nephrogenic systemic fibrosis (NSF) 8 weeks later. We agree with the correspondents that linear GBCAs should not be used in patients with eGFR < 30 mL/min/1.73 m2. To date, a few cases of patients who received GBCA and developed NSF since 2009 have unfortunately continued to be reported in the literature.1-3 Our intention in publishing this case was to provide ongoing education to the medical community regarding this serious condition to ensure prevention of future cases.
We thank Dr. Böhm for her important inquiry. The patient received a histopathologic diagnosis of NSF. The report from the patient’s left dorsal forearm skin punch biopsy was read by our pathologist as “fibrosis and inflammation consistent with nephrogenic systemic fibrosis,” a diagnosis agreed upon by our colleagues in the dermatology and rheumatology departments based on the rapidity of his symptom onset and progression. While we acknowledge that this patient had other inflammatory disorders of the skin that may have coexisted with the diagnosis, after weighing the preponderance of clinical evidence in support of the biopsy results, we believe that this represents a case of NSF, which is associated with high morbidity and mortality. Thankfully, the patient in this case engaged extensively in physical and occupational therapy and is still alive nearly 4 years later. We would like to thank all the letter writers for their correspondence.
Author Affiliations: Kelley Chuang and Casey Kaneshiro are Hospitalists and Jaime Betancourt is a Pulmonologist, all in the Department of Medicine at the VA Greater Los Angeles Healthcare System in California.
Correspondence: Kelley Chuang (kelleychuang@mednet.ucla.edu)
Disclosures: The authors report no conflict of interest with regard to this article.
References
1. Aggarwal A, Froehlich AA, Essah P, Brinster N, High WA, Downs RW. Complications of nephrogenic systemic fibrosis following repeated exposure to gadolinium in a man with hypothyroidism: a case report. J Med Case Rep. 2011;5:566.
2. Fuah KW, Lim CT. Erythema nodosum masking nephrogenic systemic fibrosis as initial skin manifestation. BMC Nephrol. 2017;18(1):249.
3. Koratala A, Bhatti V. Nephrogenic systemic fibrosis. Clin Case Rep. 2017;5(7):1184-1185.
Pharmacogenomics testing: What the FDA says
Mr. R, age 30, is referred to you by his primary care physician, who diagnosed him with depression approximately 2 years ago. When he was first diagnosed, Mr. R was prescribed
Mr. R says that based on his primary care physician’s recommendation, he had undergone pharmacogenomics testing to help guide therapy. He presents the results to you, and you notice that he has the cytochrome P450 (CYP) 2C19 *2/*3 genotype and a CYP2D6*4/*5 genotype. Both are associated with a poor metabolism phenotype. Should you use these findings to determine which medication Mr. R should be treated with next?
While the field of pharmacogenomics is not new, within the last few years this science has begun to transition into clinical practice. A recent meta-analysis found support for using pharmacogenomics testing results in clinical practice.1 This study included more than 1,700 patients who took part in 5 controlled trials that randomized participants to either pharmacogenetics-guided or unguided (ie, standard) treatment. Each participant was assessed using the Hamilton Depression Rating Scale-17 (HDRS-17) a minimum of 3 times over a minimum of 8 weeks.1 While the exact inclusion and exclusion criteria for each trial differed, they all defined remission of depression as achieving an HDRS-17 score ≤7. Overall, the authors concluded that based on the random-effects pooled risk ratio, there was a significant association between pharmacogenetics-guided prescribing and remission (relative risk = 1.71, 95% confidence interval [CI], 1.17 to 2.48; P = .005). The results of this meta-analysis are controversial, however, because all 5 studies were industry-funded, and interpretation of the testing results was based on proprietary algorithms.
Experts in the field and professional societies, such as the International Society of Psychiatric Genetics (ISPG), have issued policy statements on genetic testing within psychiatry.2,3 While the ISPG did not necessarily endorse use of pharmacogenomics in practice, they recommended that clinicians follow good medical practice and stay current on changes to drug labeling and adverse event reports.3 The ISPG also noted that useful but not exhaustive lists of pharmacogenetic tests are maintained by the Clinical Pharmacogenetics Implementation Consortium (CPIC) and the US FDA.3
Laboratory developed vs direct-to-consumer tests
In a previous Savvy Psychopharmacology article,4 we had discussed the role of CPIC, but not the role of the FDA. This issue is key because there is a lack of clarity regarding pharmacogenomics tests and whether they are considered Class II devices by the FDA, which would require their review and approval. Until recently, the FDA was fairly quiet regarding pharmacogenomics tests because most of these tests were considered laboratory developed tests, which were regulated under the Clinic Laboratory Improvements Amendments program. The critical distinction of a laboratory developed test is that it is developed and performed in a single laboratory and is offered to patients only when prescribed by a clinician. Due to this distinction, laboratory developed pharmacogenomics tests did not need FDA 510(k) clearance, which is a premarket submission common for medical devices.
Direct-to-consumer pharmacogenomics tests are different in that the FDA has classified these platforms as medical devices; however, they are reviewed by the FDA only if they are being used for moderate- to high-risk medical purposes, or if the results of the testing may have a higher impact on medical care. As part of its review, the FDA examines test accuracy and reliably measures to determine if the measurement is predictive of a certain state of health and supported by what the company claims about the test and how well it works. Additionally, the FDA examines the company-provided descriptive information to ensure that consumers can easily understand it without the help of a clinician.5
Conflicting FDA statements
Recently the FDA issued 2 statements—one a policy statement and the other a safety communication—about laboratory developed tests and direct-to-consumer tests. The statements appear to contradict themselves, despite focusing on using pharmacogenomics testing in practice.
Continue to: The FDA's first statement
The FDA’s first statement. On October 31, 2018, the FDA released a policy statement that they had “permitted marketing, with special controls,” of the Personal Genome Service Pharmacogenetic Reports test through 23andMe (a direct-to-consumer genetic testing company) for 33 different variants within specific pharmacogenomic genes (CYP2C19, CYP2C9, CYP3A5, UGT1A1, DPYD, TPMT, SLC01B1, and CYP2D6) that may impact drug metabolism or response.6 As part of its review of this Personal Genome Service Pharmacogenetic Reports test, the FDA found that the company-provided data showed that the test is accurate and can correctly identify the 33 specific genetic variants. The FDA review also showed that the testing results were reproducible, and the test instructions and reports could be understood by consumers.
While the specific reports related to this testing are not yet available within 23andMe, this approval allows for greater oversight by the FDA with regard to the pharmacogenomics information provided through this company’s Personal Genome Service Pharmacogenetic Reports test. The FDA noted that this approval was only for adults age >185 and that consumers “should not use the test results to stop or change any medication.”6 Further, the FDA stated that the results of the direct-to-consumer test should be confirmed with independent pharmacogenomics testing before making any medical decision. Unfortunately, the FDA did not offer guidance on what would be an appropriate independent pharmacogenomics test, but it did provide a link to a list of FDA-approved nucleic acid–based tests, on which 23andMe’s Personal Genome Service Pharmacogenetic Reports test is included.7
The FDA’s second statement. On November 1, 2018, the FDA issued a separate safety communication that cautioned clinicians and patients that most of the current commercially available testing platforms for pharmacogenomics have not been FDA-reviewed, meaning that they may lack clinical evidence supporting their use.8 Further, the FDA safety communication stated, “Changing drug treatment based on the results from such a genetic test could lead to inappropriate treatment decisions and potentially serious health consequences for the patient.”8
Taken together, these FDA statements appear to support pharmacogenomics testing with approval of the 23andMe’s Personal Genome Service Pharmacogenetic Reports test but warn that the testing results should not be used to make treatment decisions, and that they should be verified. However, the FDA does not offer any guidance on what an appropriate testing platform would be
What the FDA advises
The FDA has provided some guidance to clinicians and patients regarding next steps for patients who are interested in having pharmacogenomics testing or who have already undergone testing. The FDA’s first point is that both clinicians and patients need to be aware that pharmacogenomics testing is not FDA-reviewed, that patients should discuss the results of their testing with their clinicians, and that they should not stop their medication based on the results of the testing. Additionally, the FDA recommends that clinicians and patients should be aware that any claims made by the testing companies regarding the specific effect of a medication may not be supported by evidence. Furthermore, the FDA strongly recommends that clinicians consult the FDA-approved drug label, or the label of the FDA-cleared or FDA-approved genetic test, for information regarding how genetic information should be used in making treatment decisions. The FDA recommends reviewing the Warning section, as well as the Indications and Usage, Dosage and Administration, or Use in Specific Populations sections of the FDA-approved drug labeling.
Continue to: Unfortunately, this information...
Unfortunately, this information might be difficult to locate due to the lack of consistency regarding where it is placed in the FDA-approved drug labeling. The Pharmacogenomics Knowledgebase (https://www.pharmgkb.org/) can help clinicians quickly identify information regarding medications, their metabolic pathways, CPIC dosing guidelines, and the FDA-approved drug labeling information.9 By searching for specific medications within the Pharmacogenomic Knowledge Base, information regarding the FDA-approved drug labeling can be easily found, which is important because currently >120 medications contain pharmacogenomics information in their FDA-approved drug labeling.10
CASE CONTINUED
Overall Mr. R’s pharmacogenomics testing results indicate that he has 2 genotypes that are associated with poor metabolism phenotypes and could result in reduced metabolism of medications that are metabolized by these CYP enzymes, leading to higher blood levels and an increased risk of adverse effects. The Table11 lists pharmacogenomics information from the FDA-approved drug labeling and from the Pharmacogenomics Knowledgebase for both the medications Mr. R has previously been prescribed and for several potential medications to consider.
It would be prudent to first discuss with Mr. R the FDA’s recent policy statement and safety communication. While you could recommend that he pursue additional pharmacogenomics testing, it is unclear which specific laboratory is available to conduct this confirmatory analysis.
Because Mr. R has had unsuccessful trials of several medications that primarily fall in the selective serotonin reuptake inhibitors class, it might be time to consider a medication from a different class. A quick review of the FDA-approved drug labeling for
Related Resources
- Gammal RS, Gardner KN, Burghardt KJ. Where to find guidance on using pharmacogenomics in psychiatric practice. Current Psychiatry. 2016;15(9):93-94.
- Clinical Pharmacogenomics Implementation Consortium. What is CPIC? https://www.pharmgkb.org/page/cpic.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Citalopram • Celexa
Paroxetine • Paxil
Sertraline • Zoloft
Venlafaxine • Effexor
1. Bousman CA, Arandjelovic K, Mancuso SG, et al. Pharmacogenetic tests and depressive symptom remission: a meta-analysis of randomized controlled trials. Pharmacogenomics. 2019;20(1):37-47.
2. Zubenko GS, Sommer BR, Cohen BM. Pharmacogenetics in psychiatry: a companion, rather than competitor, to protocol-based care-reply. JAMA Psychiatry. 2018;75(10):1090-1091.
3. International Society for Psychiatric Genetics. Genetic testing statement: genetic testing and psychiatric disorders: a statement from the International Society of Psychiatric Genetics. https://ispg.net/genetic-testing-statement/. Revised January 26, 2017. Accessed January 1, 2019.
4. Ellingrod VL, Ward KM. Using pharmacogenetics guidelines when prescribing: what’s available. Current Psychiatry. 2018;17(1):43-46.
5. U.S. Food and Drug Administration. Medical devices: direct-to-consumer tests. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm624726.htm. Published November 1, 2018. Accessed January 1, 2019.
6. U.S. Food and Drug Administration. FDA news releases: FDA authorizes first direct-to consumer test for detecting variants that may be associated with medication metabolism. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm624753.htm. Published October 31, 2018. Accessed January 1, 2019.
7. U.S. Food and Drug Administration. Medical devices: nucleic acid based tests. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm330711.htm. Published February 5, 2019. Accessed March 1, 2019.
8. U.S. Food and Drug Administration. Medical devices. The FDA warns against the use of many genetic tests with unapproved claims to predict patient response to specific medications: FDA Safety Communications. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm624725.htm. Published November 1, 2018. Accessed January 1, 2019.
9. Whirl-Carrillo EM, McDonagh JM, Hebert L, et al. Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther. 2012;92(4):414-417.
10. U.S. Food and Drug Administration. Drugs. Table of pharmacogenomic biomarkers in drug labeling. https://www.fda.gov/Drugs/ScienceResearch/ucm572698.htm. Published August 3, 2018. Accessed January 1, 2019.
11. U.S. Food and Drug Administration. Drugs@FDA: FDA approved drug products. https://www.accessdata.fda.gov/scripts/cder/daf. Accessed March 4, 2019.
Mr. R, age 30, is referred to you by his primary care physician, who diagnosed him with depression approximately 2 years ago. When he was first diagnosed, Mr. R was prescribed
Mr. R says that based on his primary care physician’s recommendation, he had undergone pharmacogenomics testing to help guide therapy. He presents the results to you, and you notice that he has the cytochrome P450 (CYP) 2C19 *2/*3 genotype and a CYP2D6*4/*5 genotype. Both are associated with a poor metabolism phenotype. Should you use these findings to determine which medication Mr. R should be treated with next?
While the field of pharmacogenomics is not new, within the last few years this science has begun to transition into clinical practice. A recent meta-analysis found support for using pharmacogenomics testing results in clinical practice.1 This study included more than 1,700 patients who took part in 5 controlled trials that randomized participants to either pharmacogenetics-guided or unguided (ie, standard) treatment. Each participant was assessed using the Hamilton Depression Rating Scale-17 (HDRS-17) a minimum of 3 times over a minimum of 8 weeks.1 While the exact inclusion and exclusion criteria for each trial differed, they all defined remission of depression as achieving an HDRS-17 score ≤7. Overall, the authors concluded that based on the random-effects pooled risk ratio, there was a significant association between pharmacogenetics-guided prescribing and remission (relative risk = 1.71, 95% confidence interval [CI], 1.17 to 2.48; P = .005). The results of this meta-analysis are controversial, however, because all 5 studies were industry-funded, and interpretation of the testing results was based on proprietary algorithms.
Experts in the field and professional societies, such as the International Society of Psychiatric Genetics (ISPG), have issued policy statements on genetic testing within psychiatry.2,3 While the ISPG did not necessarily endorse use of pharmacogenomics in practice, they recommended that clinicians follow good medical practice and stay current on changes to drug labeling and adverse event reports.3 The ISPG also noted that useful but not exhaustive lists of pharmacogenetic tests are maintained by the Clinical Pharmacogenetics Implementation Consortium (CPIC) and the US FDA.3
Laboratory developed vs direct-to-consumer tests
In a previous Savvy Psychopharmacology article,4 we had discussed the role of CPIC, but not the role of the FDA. This issue is key because there is a lack of clarity regarding pharmacogenomics tests and whether they are considered Class II devices by the FDA, which would require their review and approval. Until recently, the FDA was fairly quiet regarding pharmacogenomics tests because most of these tests were considered laboratory developed tests, which were regulated under the Clinic Laboratory Improvements Amendments program. The critical distinction of a laboratory developed test is that it is developed and performed in a single laboratory and is offered to patients only when prescribed by a clinician. Due to this distinction, laboratory developed pharmacogenomics tests did not need FDA 510(k) clearance, which is a premarket submission common for medical devices.
Direct-to-consumer pharmacogenomics tests are different in that the FDA has classified these platforms as medical devices; however, they are reviewed by the FDA only if they are being used for moderate- to high-risk medical purposes, or if the results of the testing may have a higher impact on medical care. As part of its review, the FDA examines test accuracy and reliably measures to determine if the measurement is predictive of a certain state of health and supported by what the company claims about the test and how well it works. Additionally, the FDA examines the company-provided descriptive information to ensure that consumers can easily understand it without the help of a clinician.5
Conflicting FDA statements
Recently the FDA issued 2 statements—one a policy statement and the other a safety communication—about laboratory developed tests and direct-to-consumer tests. The statements appear to contradict themselves, despite focusing on using pharmacogenomics testing in practice.
Continue to: The FDA's first statement
The FDA’s first statement. On October 31, 2018, the FDA released a policy statement that they had “permitted marketing, with special controls,” of the Personal Genome Service Pharmacogenetic Reports test through 23andMe (a direct-to-consumer genetic testing company) for 33 different variants within specific pharmacogenomic genes (CYP2C19, CYP2C9, CYP3A5, UGT1A1, DPYD, TPMT, SLC01B1, and CYP2D6) that may impact drug metabolism or response.6 As part of its review of this Personal Genome Service Pharmacogenetic Reports test, the FDA found that the company-provided data showed that the test is accurate and can correctly identify the 33 specific genetic variants. The FDA review also showed that the testing results were reproducible, and the test instructions and reports could be understood by consumers.
While the specific reports related to this testing are not yet available within 23andMe, this approval allows for greater oversight by the FDA with regard to the pharmacogenomics information provided through this company’s Personal Genome Service Pharmacogenetic Reports test. The FDA noted that this approval was only for adults age >185 and that consumers “should not use the test results to stop or change any medication.”6 Further, the FDA stated that the results of the direct-to-consumer test should be confirmed with independent pharmacogenomics testing before making any medical decision. Unfortunately, the FDA did not offer guidance on what would be an appropriate independent pharmacogenomics test, but it did provide a link to a list of FDA-approved nucleic acid–based tests, on which 23andMe’s Personal Genome Service Pharmacogenetic Reports test is included.7
The FDA’s second statement. On November 1, 2018, the FDA issued a separate safety communication that cautioned clinicians and patients that most of the current commercially available testing platforms for pharmacogenomics have not been FDA-reviewed, meaning that they may lack clinical evidence supporting their use.8 Further, the FDA safety communication stated, “Changing drug treatment based on the results from such a genetic test could lead to inappropriate treatment decisions and potentially serious health consequences for the patient.”8
Taken together, these FDA statements appear to support pharmacogenomics testing with approval of the 23andMe’s Personal Genome Service Pharmacogenetic Reports test but warn that the testing results should not be used to make treatment decisions, and that they should be verified. However, the FDA does not offer any guidance on what an appropriate testing platform would be
What the FDA advises
The FDA has provided some guidance to clinicians and patients regarding next steps for patients who are interested in having pharmacogenomics testing or who have already undergone testing. The FDA’s first point is that both clinicians and patients need to be aware that pharmacogenomics testing is not FDA-reviewed, that patients should discuss the results of their testing with their clinicians, and that they should not stop their medication based on the results of the testing. Additionally, the FDA recommends that clinicians and patients should be aware that any claims made by the testing companies regarding the specific effect of a medication may not be supported by evidence. Furthermore, the FDA strongly recommends that clinicians consult the FDA-approved drug label, or the label of the FDA-cleared or FDA-approved genetic test, for information regarding how genetic information should be used in making treatment decisions. The FDA recommends reviewing the Warning section, as well as the Indications and Usage, Dosage and Administration, or Use in Specific Populations sections of the FDA-approved drug labeling.
Continue to: Unfortunately, this information...
Unfortunately, this information might be difficult to locate due to the lack of consistency regarding where it is placed in the FDA-approved drug labeling. The Pharmacogenomics Knowledgebase (https://www.pharmgkb.org/) can help clinicians quickly identify information regarding medications, their metabolic pathways, CPIC dosing guidelines, and the FDA-approved drug labeling information.9 By searching for specific medications within the Pharmacogenomic Knowledge Base, information regarding the FDA-approved drug labeling can be easily found, which is important because currently >120 medications contain pharmacogenomics information in their FDA-approved drug labeling.10
CASE CONTINUED
Overall Mr. R’s pharmacogenomics testing results indicate that he has 2 genotypes that are associated with poor metabolism phenotypes and could result in reduced metabolism of medications that are metabolized by these CYP enzymes, leading to higher blood levels and an increased risk of adverse effects. The Table11 lists pharmacogenomics information from the FDA-approved drug labeling and from the Pharmacogenomics Knowledgebase for both the medications Mr. R has previously been prescribed and for several potential medications to consider.
It would be prudent to first discuss with Mr. R the FDA’s recent policy statement and safety communication. While you could recommend that he pursue additional pharmacogenomics testing, it is unclear which specific laboratory is available to conduct this confirmatory analysis.
Because Mr. R has had unsuccessful trials of several medications that primarily fall in the selective serotonin reuptake inhibitors class, it might be time to consider a medication from a different class. A quick review of the FDA-approved drug labeling for
Related Resources
- Gammal RS, Gardner KN, Burghardt KJ. Where to find guidance on using pharmacogenomics in psychiatric practice. Current Psychiatry. 2016;15(9):93-94.
- Clinical Pharmacogenomics Implementation Consortium. What is CPIC? https://www.pharmgkb.org/page/cpic.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Citalopram • Celexa
Paroxetine • Paxil
Sertraline • Zoloft
Venlafaxine • Effexor
Mr. R, age 30, is referred to you by his primary care physician, who diagnosed him with depression approximately 2 years ago. When he was first diagnosed, Mr. R was prescribed
Mr. R says that based on his primary care physician’s recommendation, he had undergone pharmacogenomics testing to help guide therapy. He presents the results to you, and you notice that he has the cytochrome P450 (CYP) 2C19 *2/*3 genotype and a CYP2D6*4/*5 genotype. Both are associated with a poor metabolism phenotype. Should you use these findings to determine which medication Mr. R should be treated with next?
While the field of pharmacogenomics is not new, within the last few years this science has begun to transition into clinical practice. A recent meta-analysis found support for using pharmacogenomics testing results in clinical practice.1 This study included more than 1,700 patients who took part in 5 controlled trials that randomized participants to either pharmacogenetics-guided or unguided (ie, standard) treatment. Each participant was assessed using the Hamilton Depression Rating Scale-17 (HDRS-17) a minimum of 3 times over a minimum of 8 weeks.1 While the exact inclusion and exclusion criteria for each trial differed, they all defined remission of depression as achieving an HDRS-17 score ≤7. Overall, the authors concluded that based on the random-effects pooled risk ratio, there was a significant association between pharmacogenetics-guided prescribing and remission (relative risk = 1.71, 95% confidence interval [CI], 1.17 to 2.48; P = .005). The results of this meta-analysis are controversial, however, because all 5 studies were industry-funded, and interpretation of the testing results was based on proprietary algorithms.
Experts in the field and professional societies, such as the International Society of Psychiatric Genetics (ISPG), have issued policy statements on genetic testing within psychiatry.2,3 While the ISPG did not necessarily endorse use of pharmacogenomics in practice, they recommended that clinicians follow good medical practice and stay current on changes to drug labeling and adverse event reports.3 The ISPG also noted that useful but not exhaustive lists of pharmacogenetic tests are maintained by the Clinical Pharmacogenetics Implementation Consortium (CPIC) and the US FDA.3
Laboratory developed vs direct-to-consumer tests
In a previous Savvy Psychopharmacology article,4 we had discussed the role of CPIC, but not the role of the FDA. This issue is key because there is a lack of clarity regarding pharmacogenomics tests and whether they are considered Class II devices by the FDA, which would require their review and approval. Until recently, the FDA was fairly quiet regarding pharmacogenomics tests because most of these tests were considered laboratory developed tests, which were regulated under the Clinic Laboratory Improvements Amendments program. The critical distinction of a laboratory developed test is that it is developed and performed in a single laboratory and is offered to patients only when prescribed by a clinician. Due to this distinction, laboratory developed pharmacogenomics tests did not need FDA 510(k) clearance, which is a premarket submission common for medical devices.
Direct-to-consumer pharmacogenomics tests are different in that the FDA has classified these platforms as medical devices; however, they are reviewed by the FDA only if they are being used for moderate- to high-risk medical purposes, or if the results of the testing may have a higher impact on medical care. As part of its review, the FDA examines test accuracy and reliably measures to determine if the measurement is predictive of a certain state of health and supported by what the company claims about the test and how well it works. Additionally, the FDA examines the company-provided descriptive information to ensure that consumers can easily understand it without the help of a clinician.5
Conflicting FDA statements
Recently the FDA issued 2 statements—one a policy statement and the other a safety communication—about laboratory developed tests and direct-to-consumer tests. The statements appear to contradict themselves, despite focusing on using pharmacogenomics testing in practice.
Continue to: The FDA's first statement
The FDA’s first statement. On October 31, 2018, the FDA released a policy statement that they had “permitted marketing, with special controls,” of the Personal Genome Service Pharmacogenetic Reports test through 23andMe (a direct-to-consumer genetic testing company) for 33 different variants within specific pharmacogenomic genes (CYP2C19, CYP2C9, CYP3A5, UGT1A1, DPYD, TPMT, SLC01B1, and CYP2D6) that may impact drug metabolism or response.6 As part of its review of this Personal Genome Service Pharmacogenetic Reports test, the FDA found that the company-provided data showed that the test is accurate and can correctly identify the 33 specific genetic variants. The FDA review also showed that the testing results were reproducible, and the test instructions and reports could be understood by consumers.
While the specific reports related to this testing are not yet available within 23andMe, this approval allows for greater oversight by the FDA with regard to the pharmacogenomics information provided through this company’s Personal Genome Service Pharmacogenetic Reports test. The FDA noted that this approval was only for adults age >185 and that consumers “should not use the test results to stop or change any medication.”6 Further, the FDA stated that the results of the direct-to-consumer test should be confirmed with independent pharmacogenomics testing before making any medical decision. Unfortunately, the FDA did not offer guidance on what would be an appropriate independent pharmacogenomics test, but it did provide a link to a list of FDA-approved nucleic acid–based tests, on which 23andMe’s Personal Genome Service Pharmacogenetic Reports test is included.7
The FDA’s second statement. On November 1, 2018, the FDA issued a separate safety communication that cautioned clinicians and patients that most of the current commercially available testing platforms for pharmacogenomics have not been FDA-reviewed, meaning that they may lack clinical evidence supporting their use.8 Further, the FDA safety communication stated, “Changing drug treatment based on the results from such a genetic test could lead to inappropriate treatment decisions and potentially serious health consequences for the patient.”8
Taken together, these FDA statements appear to support pharmacogenomics testing with approval of the 23andMe’s Personal Genome Service Pharmacogenetic Reports test but warn that the testing results should not be used to make treatment decisions, and that they should be verified. However, the FDA does not offer any guidance on what an appropriate testing platform would be
What the FDA advises
The FDA has provided some guidance to clinicians and patients regarding next steps for patients who are interested in having pharmacogenomics testing or who have already undergone testing. The FDA’s first point is that both clinicians and patients need to be aware that pharmacogenomics testing is not FDA-reviewed, that patients should discuss the results of their testing with their clinicians, and that they should not stop their medication based on the results of the testing. Additionally, the FDA recommends that clinicians and patients should be aware that any claims made by the testing companies regarding the specific effect of a medication may not be supported by evidence. Furthermore, the FDA strongly recommends that clinicians consult the FDA-approved drug label, or the label of the FDA-cleared or FDA-approved genetic test, for information regarding how genetic information should be used in making treatment decisions. The FDA recommends reviewing the Warning section, as well as the Indications and Usage, Dosage and Administration, or Use in Specific Populations sections of the FDA-approved drug labeling.
Continue to: Unfortunately, this information...
Unfortunately, this information might be difficult to locate due to the lack of consistency regarding where it is placed in the FDA-approved drug labeling. The Pharmacogenomics Knowledgebase (https://www.pharmgkb.org/) can help clinicians quickly identify information regarding medications, their metabolic pathways, CPIC dosing guidelines, and the FDA-approved drug labeling information.9 By searching for specific medications within the Pharmacogenomic Knowledge Base, information regarding the FDA-approved drug labeling can be easily found, which is important because currently >120 medications contain pharmacogenomics information in their FDA-approved drug labeling.10
CASE CONTINUED
Overall Mr. R’s pharmacogenomics testing results indicate that he has 2 genotypes that are associated with poor metabolism phenotypes and could result in reduced metabolism of medications that are metabolized by these CYP enzymes, leading to higher blood levels and an increased risk of adverse effects. The Table11 lists pharmacogenomics information from the FDA-approved drug labeling and from the Pharmacogenomics Knowledgebase for both the medications Mr. R has previously been prescribed and for several potential medications to consider.
It would be prudent to first discuss with Mr. R the FDA’s recent policy statement and safety communication. While you could recommend that he pursue additional pharmacogenomics testing, it is unclear which specific laboratory is available to conduct this confirmatory analysis.
Because Mr. R has had unsuccessful trials of several medications that primarily fall in the selective serotonin reuptake inhibitors class, it might be time to consider a medication from a different class. A quick review of the FDA-approved drug labeling for
Related Resources
- Gammal RS, Gardner KN, Burghardt KJ. Where to find guidance on using pharmacogenomics in psychiatric practice. Current Psychiatry. 2016;15(9):93-94.
- Clinical Pharmacogenomics Implementation Consortium. What is CPIC? https://www.pharmgkb.org/page/cpic.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Citalopram • Celexa
Paroxetine • Paxil
Sertraline • Zoloft
Venlafaxine • Effexor
1. Bousman CA, Arandjelovic K, Mancuso SG, et al. Pharmacogenetic tests and depressive symptom remission: a meta-analysis of randomized controlled trials. Pharmacogenomics. 2019;20(1):37-47.
2. Zubenko GS, Sommer BR, Cohen BM. Pharmacogenetics in psychiatry: a companion, rather than competitor, to protocol-based care-reply. JAMA Psychiatry. 2018;75(10):1090-1091.
3. International Society for Psychiatric Genetics. Genetic testing statement: genetic testing and psychiatric disorders: a statement from the International Society of Psychiatric Genetics. https://ispg.net/genetic-testing-statement/. Revised January 26, 2017. Accessed January 1, 2019.
4. Ellingrod VL, Ward KM. Using pharmacogenetics guidelines when prescribing: what’s available. Current Psychiatry. 2018;17(1):43-46.
5. U.S. Food and Drug Administration. Medical devices: direct-to-consumer tests. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm624726.htm. Published November 1, 2018. Accessed January 1, 2019.
6. U.S. Food and Drug Administration. FDA news releases: FDA authorizes first direct-to consumer test for detecting variants that may be associated with medication metabolism. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm624753.htm. Published October 31, 2018. Accessed January 1, 2019.
7. U.S. Food and Drug Administration. Medical devices: nucleic acid based tests. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm330711.htm. Published February 5, 2019. Accessed March 1, 2019.
8. U.S. Food and Drug Administration. Medical devices. The FDA warns against the use of many genetic tests with unapproved claims to predict patient response to specific medications: FDA Safety Communications. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm624725.htm. Published November 1, 2018. Accessed January 1, 2019.
9. Whirl-Carrillo EM, McDonagh JM, Hebert L, et al. Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther. 2012;92(4):414-417.
10. U.S. Food and Drug Administration. Drugs. Table of pharmacogenomic biomarkers in drug labeling. https://www.fda.gov/Drugs/ScienceResearch/ucm572698.htm. Published August 3, 2018. Accessed January 1, 2019.
11. U.S. Food and Drug Administration. Drugs@FDA: FDA approved drug products. https://www.accessdata.fda.gov/scripts/cder/daf. Accessed March 4, 2019.
1. Bousman CA, Arandjelovic K, Mancuso SG, et al. Pharmacogenetic tests and depressive symptom remission: a meta-analysis of randomized controlled trials. Pharmacogenomics. 2019;20(1):37-47.
2. Zubenko GS, Sommer BR, Cohen BM. Pharmacogenetics in psychiatry: a companion, rather than competitor, to protocol-based care-reply. JAMA Psychiatry. 2018;75(10):1090-1091.
3. International Society for Psychiatric Genetics. Genetic testing statement: genetic testing and psychiatric disorders: a statement from the International Society of Psychiatric Genetics. https://ispg.net/genetic-testing-statement/. Revised January 26, 2017. Accessed January 1, 2019.
4. Ellingrod VL, Ward KM. Using pharmacogenetics guidelines when prescribing: what’s available. Current Psychiatry. 2018;17(1):43-46.
5. U.S. Food and Drug Administration. Medical devices: direct-to-consumer tests. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm624726.htm. Published November 1, 2018. Accessed January 1, 2019.
6. U.S. Food and Drug Administration. FDA news releases: FDA authorizes first direct-to consumer test for detecting variants that may be associated with medication metabolism. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm624753.htm. Published October 31, 2018. Accessed January 1, 2019.
7. U.S. Food and Drug Administration. Medical devices: nucleic acid based tests. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm330711.htm. Published February 5, 2019. Accessed March 1, 2019.
8. U.S. Food and Drug Administration. Medical devices. The FDA warns against the use of many genetic tests with unapproved claims to predict patient response to specific medications: FDA Safety Communications. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm624725.htm. Published November 1, 2018. Accessed January 1, 2019.
9. Whirl-Carrillo EM, McDonagh JM, Hebert L, et al. Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther. 2012;92(4):414-417.
10. U.S. Food and Drug Administration. Drugs. Table of pharmacogenomic biomarkers in drug labeling. https://www.fda.gov/Drugs/ScienceResearch/ucm572698.htm. Published August 3, 2018. Accessed January 1, 2019.
11. U.S. Food and Drug Administration. Drugs@FDA: FDA approved drug products. https://www.accessdata.fda.gov/scripts/cder/daf. Accessed March 4, 2019.
Pharmacologic performance enhancement: What to consider before prescribing
Performance enhancement in sports (“doping”) dates back to Ancient Greece. This was an era when Olympic athletes would attempt to improve their physical performance by consuming magic potions, herbal medications, and even exotic meats such as sheep testicles—a delicacy high in testosterone. Advances in medical and pharmaceutical technologies have increased both the range of enhancement agents available and their efficacy, leading to the development of anti-doping agencies and routine screening for doping in athletics. This has led to the renouncement of titles, medals, and financial sponsorship of athletes found to have been using prohibited substances during competition.
While doping in elite athletes often forms the nidus of media attention, the pressure to compete and perform at, or even beyond, one’s potential extends into many facets of today’s achievementfocused society. In the face of these pressures, individuals are increasingly seeking medications to enhance their performance across numerous domains, including cognitive, athletic, and artistic endeavors. Medication classes used to enhance performance include stimulants, which increase attention, executive function, and energy; cholinesterase inhibitors, which may ameliorate age-related memory decline; and beta-blockers, which decrease physiologic symptoms of anxiety and have been demonstrated to be beneficial for musical performance.1 Fifty-three percent of college athletes report using prescription medications to enhance athletic performance,2 and most college students who take stimulants without a prescription use them to study (84%) or stay awake (51%).3
Pharmacologic performance enhancement is the use of medications by healthy individuals to improve function in the absence of mental illness. Psychiatrists are increasingly finding themselves in the controversial position of “gatekeeper” of these medications for enhancement purposes. In this article we:
- outline arguments that support the use of psychopharmacology for performance enhancement, as well as some serious concerns with this practice
- discuss special considerations for pediatric populations and the risk of malpractice when prescribing for performance enhancement
- offer practice guidelines for approaching requests for psychopharmacologic performance enhancement.
Performance enhancement: The wave of the future?
The ethical principle that supports providing medication for performance enhancement is beneficence, the promotion of the patient’s well-being. In other words, it is a physician’s duty to help his or her patient in need. Individuals seeking performance enhancement typically present with suffering, and the principle of beneficence would call upon the psychiatrist to help ameliorate that suffering. Furthermore, patients who seek performance enhancement may present with impairing “subsyndromal” psychiatric symptoms (for example, low-grade attentional difficulty that occurs only in one setting), which, even if they do not rise to the threshold of a DSM diagnosis, may improve with psychiatric medications.
Using medical knowledge and skills beyond the traditional physician duty to diagnose and treat medical conditions is not unprecedented (eg, when surgeons perform cosmetic enhancement). Might elective enhancement of cognition and psychological performance through the judicious use of medication be part of the future of psychiatry? If cognitive and emotional enhancement becomes a more widely accepted standard of care, might this increase both individual and societal innovation and productivity?
Dilemma: Cautions against performance enhancement
One of the major cautions against prescribing psychotropics for the purpose of performance enhancement is the lack of clearly supported efficacy. Psychiatric medications generally are studied in individuals who meet criteria for mental illness, and they are FDA-approved for use in ill persons. It may be erroneous to extrapolate that a medication that improves symptoms in a patient with an illness would achieve the same target effect in a healthy individual. For example, data on whether stimulants provide neurocognitive enhancement in healthy individuals without attention-deficit/hyperactivity disorder is mixed, and these agents may even promote risky behavior in healthy controls.4 Furthermore, dopamine agonism may compress cognitive performance in both directions,5 as it has been observed that methylphenidate improves executive function in healthy controls, but is less beneficial for those with strong executive function at baseline.6
In the face of unclear benefit, it is particularly important to consider the risk of medications used for performance enhancement. Pharmacologic performance enhancement in individuals without psychopathology can be considered an “elective” intervention, for which individuals typically tolerate less risk. Physical risks, including medication-related adverse effects, must be considered, particularly in settings where there may be temptation to use more than prescribed, or to divert medication to others who may use it without medical monitoring. In addition to physical harm, there may be psychological harm associated with prescribing performance enhancers, such as pathologizing variants of “normal,” diminishing one’s sense of self-efficacy, or decreasing one’s ability to bear failure.
Continue to: Finally, there are ethical quandaries
Finally, there are ethical quandaries regarding using medications for performance enhancement. Widespread adoption of pharmacologic performance enhancement may lead to implicit coercion for all individuals to enhance their abilities. As a greater proportion of society receives pharmacologic enhancement, society as a whole faces stronger pressures to seek pharmacologic enhancement, ultimately constricting an individual’s freedom of choice to enhance.6 In this setting, distributive justice would become a consideration, because insurance companies are unlikely to reimburse for medications used for enhancement,7 which would give an advantage to individuals with higher socioeconomic status. Research shows that children from higher socioeconomic communities and from states with higher academic standards are more likely to use stimulants.8
Areas of controversy
Pediatric populations. There are special considerations when prescribing performance-enhancing medications for children and adolescents. First, such prescribing may inhibit normal child development, shifting the focus away from the normative tasks of social and emotional development that occur through leisure and creativity, experimentation, and play to an emphasis on performance and outcomes-based achievement.9 Second, during childhood and adolescence, one develops a sense of his or her identity, morals, and values. Taking a medication during childhood to enhance performance may inhibit the process of learning to tolerate failure, become aware of one’s weaknesses, and value effort in addition to outcome.
Malpractice risk. Practicing medicine beyond the scope of one’s expertise is unethical and unlawful. In the past 30 years, medical malpractice has become one of the most difficult health care issues in the U.S.10 In addition to billions of dollars in legal fees and court costs, medical malpractice premiums in the U.S. total more than $5 billion annually,11 and “defensive medicine”— procedures performed to protect against litigation—is estimated to cost more than $14 billion a year.12
When considering performance-enhancing treatment, it is the physician’s duty to conduct a diagnostic assessment, including noting target symptoms that are interfering with the patient’s function, and to tailor such treatment toward measurable goals and outcomes. Aside from medication, this could include a therapeutic approach to improving performance that might include cognitive-behavioral therapy and promotion of a healthy diet and exercise.
Treatment rises to the level of malpractice when there is a dereliction of duty that directly leads to damages.13 Part of a physician’s duty is to educate patients about the pros and cons of different treatment options. For performance-enhancing medications, the risks of addiction and dependence are adverse effects that require discussion. And for a pediatric patient, this would require the guardian’s engagement and understanding.
Continue to: What to do if you decide to prescribe
What to do if you decide to prescribe
Inevitably, the decision to prescribe psychotropic medications for performance enhancement is a physician-specific one. Certainly, psychiatrists should not feel obligated to prescribe performance enhancers. Given our current state of pharmacology, it is unclear whether medications would be helpful in the absence of psychopathology. When deciding whether to prescribe for performance enhancement in the absence of psychopathology, we suggest first carefully considering how to maintain the ethical value of nonmaleficence by weighing both the potential physical and psychologic harms of prescribing as well as the legal risks and rules of applicable sport governing bodies.
For a psychiatrist who chooses to prescribe for performance enhancement, we recommend conducting a thorough psychiatric assessment to determine whether the patient has a treatable mental illness. If so, then effective treatment of that illness should take priority. Before prescribing, the psychiatrist and patient should discuss the patient’s specific performance goals and how to measure them.
Any prescription for a performance-enhancing medication should be given in conjunction with nonpharmacologic approaches, including optimizing diet, exercise, and sleep. Therapy to address problem-solving techniques and skills to cope with stress may also be appropriate. The patient and psychiatrist should engage in regular follow-up to assess the efficacy of the medication, as well as its safety and tolerability. Finally, if a medication is not efficacious as a performance enhancer, then both the patient and psychiatrist should be open to re-evaluating the treatment plan, and when appropriate, stopping the medication.
1. Brantigan CO, Brantigan TA, Joseph N. Effect of beta blockade and beta stimulation on stage fright. Am J Med. 1982;72(1):88-94.
2. Hoyte CO, Albert D, Heard KJ. The use of energy drinks, dietary supplements, and prescription medications by United States college students to enhance athletic performance. J Community Health. 2013;38(3):575-850.
3. Advokat CD, Guidry D, Martino L. Licit and illicit use of medications for attention-deficit hyperactivity disorder in undergraduate college students. J Am Coll Health. 2008;56(6):601-606.
4. Advokat C, Scheithauer M. Attention-deficit hyperactivity disorder (ADHD) stimulant medications as cognitive enhancers. Front Neurosci. 2013;7:82.
5. Kimberg DY, D’Esposito M, Farah MJ. Effects of bromocriptine on human subjects depend on working memory capacity. Neuroreport. 1997;8(16):3581-3585.
6. Farah MJ, Illes J, Cook-Deegan R, et al. Neurocognitive enhancement: what can we do and what should we do? Nat Rev Neurosci. 2004;5(5):421-425.
7. Larriviere D, Williams MA, Rizzo M, et al; AAN Ethics, Law and Humanities Committee. Responding to requests from adult patients for neuroenhancements: guidance of the Ethics, Law and Humanities Committee. Neurology. 2009;73(17):1406-1412.
8. Colaneri N, Sheldon M, Adesman A. Pharmacological cognitive enhancement in pediatrics. Curr Opin Pediatr. 2018;30(3):430-437.
9. Gaucher N, Payot A, Racine E. Cognitive enhancement in children and adolescents: Is it in their best interests? Acta Paediatr. 2013;102(12):1118-1124.
10. Moore PJ, Adler, NE, Robertson, PA. Medical malpractice; the effect of doctor-patient relations on medical patient perceptions and malpractice intentions. West J Med. 2000;173(4):244-250.
11. Hiatt H. Medical malpractice. Bull N Y Acad Med. 1992;68(2):254-260.
12. Rubin RJ, Mendelson DN. How much does defensive medicine cost? J Am Health Policy. 1994;4(4):7-15.
13. Kloss D. The duty of care: medical negligence. Br Med J (Clin Res Ed). 1984;289(6436):66-68.
Performance enhancement in sports (“doping”) dates back to Ancient Greece. This was an era when Olympic athletes would attempt to improve their physical performance by consuming magic potions, herbal medications, and even exotic meats such as sheep testicles—a delicacy high in testosterone. Advances in medical and pharmaceutical technologies have increased both the range of enhancement agents available and their efficacy, leading to the development of anti-doping agencies and routine screening for doping in athletics. This has led to the renouncement of titles, medals, and financial sponsorship of athletes found to have been using prohibited substances during competition.
While doping in elite athletes often forms the nidus of media attention, the pressure to compete and perform at, or even beyond, one’s potential extends into many facets of today’s achievementfocused society. In the face of these pressures, individuals are increasingly seeking medications to enhance their performance across numerous domains, including cognitive, athletic, and artistic endeavors. Medication classes used to enhance performance include stimulants, which increase attention, executive function, and energy; cholinesterase inhibitors, which may ameliorate age-related memory decline; and beta-blockers, which decrease physiologic symptoms of anxiety and have been demonstrated to be beneficial for musical performance.1 Fifty-three percent of college athletes report using prescription medications to enhance athletic performance,2 and most college students who take stimulants without a prescription use them to study (84%) or stay awake (51%).3
Pharmacologic performance enhancement is the use of medications by healthy individuals to improve function in the absence of mental illness. Psychiatrists are increasingly finding themselves in the controversial position of “gatekeeper” of these medications for enhancement purposes. In this article we:
- outline arguments that support the use of psychopharmacology for performance enhancement, as well as some serious concerns with this practice
- discuss special considerations for pediatric populations and the risk of malpractice when prescribing for performance enhancement
- offer practice guidelines for approaching requests for psychopharmacologic performance enhancement.
Performance enhancement: The wave of the future?
The ethical principle that supports providing medication for performance enhancement is beneficence, the promotion of the patient’s well-being. In other words, it is a physician’s duty to help his or her patient in need. Individuals seeking performance enhancement typically present with suffering, and the principle of beneficence would call upon the psychiatrist to help ameliorate that suffering. Furthermore, patients who seek performance enhancement may present with impairing “subsyndromal” psychiatric symptoms (for example, low-grade attentional difficulty that occurs only in one setting), which, even if they do not rise to the threshold of a DSM diagnosis, may improve with psychiatric medications.
Using medical knowledge and skills beyond the traditional physician duty to diagnose and treat medical conditions is not unprecedented (eg, when surgeons perform cosmetic enhancement). Might elective enhancement of cognition and psychological performance through the judicious use of medication be part of the future of psychiatry? If cognitive and emotional enhancement becomes a more widely accepted standard of care, might this increase both individual and societal innovation and productivity?
Dilemma: Cautions against performance enhancement
One of the major cautions against prescribing psychotropics for the purpose of performance enhancement is the lack of clearly supported efficacy. Psychiatric medications generally are studied in individuals who meet criteria for mental illness, and they are FDA-approved for use in ill persons. It may be erroneous to extrapolate that a medication that improves symptoms in a patient with an illness would achieve the same target effect in a healthy individual. For example, data on whether stimulants provide neurocognitive enhancement in healthy individuals without attention-deficit/hyperactivity disorder is mixed, and these agents may even promote risky behavior in healthy controls.4 Furthermore, dopamine agonism may compress cognitive performance in both directions,5 as it has been observed that methylphenidate improves executive function in healthy controls, but is less beneficial for those with strong executive function at baseline.6
In the face of unclear benefit, it is particularly important to consider the risk of medications used for performance enhancement. Pharmacologic performance enhancement in individuals without psychopathology can be considered an “elective” intervention, for which individuals typically tolerate less risk. Physical risks, including medication-related adverse effects, must be considered, particularly in settings where there may be temptation to use more than prescribed, or to divert medication to others who may use it without medical monitoring. In addition to physical harm, there may be psychological harm associated with prescribing performance enhancers, such as pathologizing variants of “normal,” diminishing one’s sense of self-efficacy, or decreasing one’s ability to bear failure.
Continue to: Finally, there are ethical quandaries
Finally, there are ethical quandaries regarding using medications for performance enhancement. Widespread adoption of pharmacologic performance enhancement may lead to implicit coercion for all individuals to enhance their abilities. As a greater proportion of society receives pharmacologic enhancement, society as a whole faces stronger pressures to seek pharmacologic enhancement, ultimately constricting an individual’s freedom of choice to enhance.6 In this setting, distributive justice would become a consideration, because insurance companies are unlikely to reimburse for medications used for enhancement,7 which would give an advantage to individuals with higher socioeconomic status. Research shows that children from higher socioeconomic communities and from states with higher academic standards are more likely to use stimulants.8
Areas of controversy
Pediatric populations. There are special considerations when prescribing performance-enhancing medications for children and adolescents. First, such prescribing may inhibit normal child development, shifting the focus away from the normative tasks of social and emotional development that occur through leisure and creativity, experimentation, and play to an emphasis on performance and outcomes-based achievement.9 Second, during childhood and adolescence, one develops a sense of his or her identity, morals, and values. Taking a medication during childhood to enhance performance may inhibit the process of learning to tolerate failure, become aware of one’s weaknesses, and value effort in addition to outcome.
Malpractice risk. Practicing medicine beyond the scope of one’s expertise is unethical and unlawful. In the past 30 years, medical malpractice has become one of the most difficult health care issues in the U.S.10 In addition to billions of dollars in legal fees and court costs, medical malpractice premiums in the U.S. total more than $5 billion annually,11 and “defensive medicine”— procedures performed to protect against litigation—is estimated to cost more than $14 billion a year.12
When considering performance-enhancing treatment, it is the physician’s duty to conduct a diagnostic assessment, including noting target symptoms that are interfering with the patient’s function, and to tailor such treatment toward measurable goals and outcomes. Aside from medication, this could include a therapeutic approach to improving performance that might include cognitive-behavioral therapy and promotion of a healthy diet and exercise.
Treatment rises to the level of malpractice when there is a dereliction of duty that directly leads to damages.13 Part of a physician’s duty is to educate patients about the pros and cons of different treatment options. For performance-enhancing medications, the risks of addiction and dependence are adverse effects that require discussion. And for a pediatric patient, this would require the guardian’s engagement and understanding.
Continue to: What to do if you decide to prescribe
What to do if you decide to prescribe
Inevitably, the decision to prescribe psychotropic medications for performance enhancement is a physician-specific one. Certainly, psychiatrists should not feel obligated to prescribe performance enhancers. Given our current state of pharmacology, it is unclear whether medications would be helpful in the absence of psychopathology. When deciding whether to prescribe for performance enhancement in the absence of psychopathology, we suggest first carefully considering how to maintain the ethical value of nonmaleficence by weighing both the potential physical and psychologic harms of prescribing as well as the legal risks and rules of applicable sport governing bodies.
For a psychiatrist who chooses to prescribe for performance enhancement, we recommend conducting a thorough psychiatric assessment to determine whether the patient has a treatable mental illness. If so, then effective treatment of that illness should take priority. Before prescribing, the psychiatrist and patient should discuss the patient’s specific performance goals and how to measure them.
Any prescription for a performance-enhancing medication should be given in conjunction with nonpharmacologic approaches, including optimizing diet, exercise, and sleep. Therapy to address problem-solving techniques and skills to cope with stress may also be appropriate. The patient and psychiatrist should engage in regular follow-up to assess the efficacy of the medication, as well as its safety and tolerability. Finally, if a medication is not efficacious as a performance enhancer, then both the patient and psychiatrist should be open to re-evaluating the treatment plan, and when appropriate, stopping the medication.
Performance enhancement in sports (“doping”) dates back to Ancient Greece. This was an era when Olympic athletes would attempt to improve their physical performance by consuming magic potions, herbal medications, and even exotic meats such as sheep testicles—a delicacy high in testosterone. Advances in medical and pharmaceutical technologies have increased both the range of enhancement agents available and their efficacy, leading to the development of anti-doping agencies and routine screening for doping in athletics. This has led to the renouncement of titles, medals, and financial sponsorship of athletes found to have been using prohibited substances during competition.
While doping in elite athletes often forms the nidus of media attention, the pressure to compete and perform at, or even beyond, one’s potential extends into many facets of today’s achievementfocused society. In the face of these pressures, individuals are increasingly seeking medications to enhance their performance across numerous domains, including cognitive, athletic, and artistic endeavors. Medication classes used to enhance performance include stimulants, which increase attention, executive function, and energy; cholinesterase inhibitors, which may ameliorate age-related memory decline; and beta-blockers, which decrease physiologic symptoms of anxiety and have been demonstrated to be beneficial for musical performance.1 Fifty-three percent of college athletes report using prescription medications to enhance athletic performance,2 and most college students who take stimulants without a prescription use them to study (84%) or stay awake (51%).3
Pharmacologic performance enhancement is the use of medications by healthy individuals to improve function in the absence of mental illness. Psychiatrists are increasingly finding themselves in the controversial position of “gatekeeper” of these medications for enhancement purposes. In this article we:
- outline arguments that support the use of psychopharmacology for performance enhancement, as well as some serious concerns with this practice
- discuss special considerations for pediatric populations and the risk of malpractice when prescribing for performance enhancement
- offer practice guidelines for approaching requests for psychopharmacologic performance enhancement.
Performance enhancement: The wave of the future?
The ethical principle that supports providing medication for performance enhancement is beneficence, the promotion of the patient’s well-being. In other words, it is a physician’s duty to help his or her patient in need. Individuals seeking performance enhancement typically present with suffering, and the principle of beneficence would call upon the psychiatrist to help ameliorate that suffering. Furthermore, patients who seek performance enhancement may present with impairing “subsyndromal” psychiatric symptoms (for example, low-grade attentional difficulty that occurs only in one setting), which, even if they do not rise to the threshold of a DSM diagnosis, may improve with psychiatric medications.
Using medical knowledge and skills beyond the traditional physician duty to diagnose and treat medical conditions is not unprecedented (eg, when surgeons perform cosmetic enhancement). Might elective enhancement of cognition and psychological performance through the judicious use of medication be part of the future of psychiatry? If cognitive and emotional enhancement becomes a more widely accepted standard of care, might this increase both individual and societal innovation and productivity?
Dilemma: Cautions against performance enhancement
One of the major cautions against prescribing psychotropics for the purpose of performance enhancement is the lack of clearly supported efficacy. Psychiatric medications generally are studied in individuals who meet criteria for mental illness, and they are FDA-approved for use in ill persons. It may be erroneous to extrapolate that a medication that improves symptoms in a patient with an illness would achieve the same target effect in a healthy individual. For example, data on whether stimulants provide neurocognitive enhancement in healthy individuals without attention-deficit/hyperactivity disorder is mixed, and these agents may even promote risky behavior in healthy controls.4 Furthermore, dopamine agonism may compress cognitive performance in both directions,5 as it has been observed that methylphenidate improves executive function in healthy controls, but is less beneficial for those with strong executive function at baseline.6
In the face of unclear benefit, it is particularly important to consider the risk of medications used for performance enhancement. Pharmacologic performance enhancement in individuals without psychopathology can be considered an “elective” intervention, for which individuals typically tolerate less risk. Physical risks, including medication-related adverse effects, must be considered, particularly in settings where there may be temptation to use more than prescribed, or to divert medication to others who may use it without medical monitoring. In addition to physical harm, there may be psychological harm associated with prescribing performance enhancers, such as pathologizing variants of “normal,” diminishing one’s sense of self-efficacy, or decreasing one’s ability to bear failure.
Continue to: Finally, there are ethical quandaries
Finally, there are ethical quandaries regarding using medications for performance enhancement. Widespread adoption of pharmacologic performance enhancement may lead to implicit coercion for all individuals to enhance their abilities. As a greater proportion of society receives pharmacologic enhancement, society as a whole faces stronger pressures to seek pharmacologic enhancement, ultimately constricting an individual’s freedom of choice to enhance.6 In this setting, distributive justice would become a consideration, because insurance companies are unlikely to reimburse for medications used for enhancement,7 which would give an advantage to individuals with higher socioeconomic status. Research shows that children from higher socioeconomic communities and from states with higher academic standards are more likely to use stimulants.8
Areas of controversy
Pediatric populations. There are special considerations when prescribing performance-enhancing medications for children and adolescents. First, such prescribing may inhibit normal child development, shifting the focus away from the normative tasks of social and emotional development that occur through leisure and creativity, experimentation, and play to an emphasis on performance and outcomes-based achievement.9 Second, during childhood and adolescence, one develops a sense of his or her identity, morals, and values. Taking a medication during childhood to enhance performance may inhibit the process of learning to tolerate failure, become aware of one’s weaknesses, and value effort in addition to outcome.
Malpractice risk. Practicing medicine beyond the scope of one’s expertise is unethical and unlawful. In the past 30 years, medical malpractice has become one of the most difficult health care issues in the U.S.10 In addition to billions of dollars in legal fees and court costs, medical malpractice premiums in the U.S. total more than $5 billion annually,11 and “defensive medicine”— procedures performed to protect against litigation—is estimated to cost more than $14 billion a year.12
When considering performance-enhancing treatment, it is the physician’s duty to conduct a diagnostic assessment, including noting target symptoms that are interfering with the patient’s function, and to tailor such treatment toward measurable goals and outcomes. Aside from medication, this could include a therapeutic approach to improving performance that might include cognitive-behavioral therapy and promotion of a healthy diet and exercise.
Treatment rises to the level of malpractice when there is a dereliction of duty that directly leads to damages.13 Part of a physician’s duty is to educate patients about the pros and cons of different treatment options. For performance-enhancing medications, the risks of addiction and dependence are adverse effects that require discussion. And for a pediatric patient, this would require the guardian’s engagement and understanding.
Continue to: What to do if you decide to prescribe
What to do if you decide to prescribe
Inevitably, the decision to prescribe psychotropic medications for performance enhancement is a physician-specific one. Certainly, psychiatrists should not feel obligated to prescribe performance enhancers. Given our current state of pharmacology, it is unclear whether medications would be helpful in the absence of psychopathology. When deciding whether to prescribe for performance enhancement in the absence of psychopathology, we suggest first carefully considering how to maintain the ethical value of nonmaleficence by weighing both the potential physical and psychologic harms of prescribing as well as the legal risks and rules of applicable sport governing bodies.
For a psychiatrist who chooses to prescribe for performance enhancement, we recommend conducting a thorough psychiatric assessment to determine whether the patient has a treatable mental illness. If so, then effective treatment of that illness should take priority. Before prescribing, the psychiatrist and patient should discuss the patient’s specific performance goals and how to measure them.
Any prescription for a performance-enhancing medication should be given in conjunction with nonpharmacologic approaches, including optimizing diet, exercise, and sleep. Therapy to address problem-solving techniques and skills to cope with stress may also be appropriate. The patient and psychiatrist should engage in regular follow-up to assess the efficacy of the medication, as well as its safety and tolerability. Finally, if a medication is not efficacious as a performance enhancer, then both the patient and psychiatrist should be open to re-evaluating the treatment plan, and when appropriate, stopping the medication.
1. Brantigan CO, Brantigan TA, Joseph N. Effect of beta blockade and beta stimulation on stage fright. Am J Med. 1982;72(1):88-94.
2. Hoyte CO, Albert D, Heard KJ. The use of energy drinks, dietary supplements, and prescription medications by United States college students to enhance athletic performance. J Community Health. 2013;38(3):575-850.
3. Advokat CD, Guidry D, Martino L. Licit and illicit use of medications for attention-deficit hyperactivity disorder in undergraduate college students. J Am Coll Health. 2008;56(6):601-606.
4. Advokat C, Scheithauer M. Attention-deficit hyperactivity disorder (ADHD) stimulant medications as cognitive enhancers. Front Neurosci. 2013;7:82.
5. Kimberg DY, D’Esposito M, Farah MJ. Effects of bromocriptine on human subjects depend on working memory capacity. Neuroreport. 1997;8(16):3581-3585.
6. Farah MJ, Illes J, Cook-Deegan R, et al. Neurocognitive enhancement: what can we do and what should we do? Nat Rev Neurosci. 2004;5(5):421-425.
7. Larriviere D, Williams MA, Rizzo M, et al; AAN Ethics, Law and Humanities Committee. Responding to requests from adult patients for neuroenhancements: guidance of the Ethics, Law and Humanities Committee. Neurology. 2009;73(17):1406-1412.
8. Colaneri N, Sheldon M, Adesman A. Pharmacological cognitive enhancement in pediatrics. Curr Opin Pediatr. 2018;30(3):430-437.
9. Gaucher N, Payot A, Racine E. Cognitive enhancement in children and adolescents: Is it in their best interests? Acta Paediatr. 2013;102(12):1118-1124.
10. Moore PJ, Adler, NE, Robertson, PA. Medical malpractice; the effect of doctor-patient relations on medical patient perceptions and malpractice intentions. West J Med. 2000;173(4):244-250.
11. Hiatt H. Medical malpractice. Bull N Y Acad Med. 1992;68(2):254-260.
12. Rubin RJ, Mendelson DN. How much does defensive medicine cost? J Am Health Policy. 1994;4(4):7-15.
13. Kloss D. The duty of care: medical negligence. Br Med J (Clin Res Ed). 1984;289(6436):66-68.
1. Brantigan CO, Brantigan TA, Joseph N. Effect of beta blockade and beta stimulation on stage fright. Am J Med. 1982;72(1):88-94.
2. Hoyte CO, Albert D, Heard KJ. The use of energy drinks, dietary supplements, and prescription medications by United States college students to enhance athletic performance. J Community Health. 2013;38(3):575-850.
3. Advokat CD, Guidry D, Martino L. Licit and illicit use of medications for attention-deficit hyperactivity disorder in undergraduate college students. J Am Coll Health. 2008;56(6):601-606.
4. Advokat C, Scheithauer M. Attention-deficit hyperactivity disorder (ADHD) stimulant medications as cognitive enhancers. Front Neurosci. 2013;7:82.
5. Kimberg DY, D’Esposito M, Farah MJ. Effects of bromocriptine on human subjects depend on working memory capacity. Neuroreport. 1997;8(16):3581-3585.
6. Farah MJ, Illes J, Cook-Deegan R, et al. Neurocognitive enhancement: what can we do and what should we do? Nat Rev Neurosci. 2004;5(5):421-425.
7. Larriviere D, Williams MA, Rizzo M, et al; AAN Ethics, Law and Humanities Committee. Responding to requests from adult patients for neuroenhancements: guidance of the Ethics, Law and Humanities Committee. Neurology. 2009;73(17):1406-1412.
8. Colaneri N, Sheldon M, Adesman A. Pharmacological cognitive enhancement in pediatrics. Curr Opin Pediatr. 2018;30(3):430-437.
9. Gaucher N, Payot A, Racine E. Cognitive enhancement in children and adolescents: Is it in their best interests? Acta Paediatr. 2013;102(12):1118-1124.
10. Moore PJ, Adler, NE, Robertson, PA. Medical malpractice; the effect of doctor-patient relations on medical patient perceptions and malpractice intentions. West J Med. 2000;173(4):244-250.
11. Hiatt H. Medical malpractice. Bull N Y Acad Med. 1992;68(2):254-260.
12. Rubin RJ, Mendelson DN. How much does defensive medicine cost? J Am Health Policy. 1994;4(4):7-15.
13. Kloss D. The duty of care: medical negligence. Br Med J (Clin Res Ed). 1984;289(6436):66-68.
Distress Screen Implementation and Quality Improvement
Background: To best address the psychosocial concerns experienced by patients with cancer, the 2007 report of the IOM, Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs, described the importance of distress screening and identifying psychosocial needs to optimize the quality of the cancer care. This may be especially critical when treating the Veteran population, where psychosocial needs as a whole may be elevated compared to non-VA institutions. The NCCN distress thermometer screening tool is a commonly used, validated, and easily administered screen of distress (eg, Hoffman et al, 2004). However, challenges can arise in successful implementation, adherence, and responsiveness to the information gleaned from this screen (eg, Zebrack et al, 2015).
As an institution accredited by the commission on cancer, it is important to not only meet the distress screening standard (ie, assess and identify psychosocial needs) but to understand barriers to identifying psychosocial needs and to appropriately triage when psychosocial concerns are identified. Goals of this project were to understand challenges with distress screening, address barriers to distress screening, and improve quality of assessment and referrals following positive screens.
Results: At Hines VAMC, we rolled out distress screening in 2015 and 2016, with rates of screening administration increasing over the course of the first year. However, without continued monitoring and re-education, successful adherence decreased overtime. Additionally, of the 862 screens administered to date, 37% were found to be considered “positive.” We will discuss the various barriers and challenges associated with managing referrals to nonmedical providers.
Our team has identified several essential aspects of successful screening and follow-up including staff/nursing education, continued tracking and re-education over time, and establishing and maintaining relationships with psychosocial clinicians to best address these aspects of care and to optimize quality of cancer care overall. We will discuss the impact of the above interventions on adherence and responsiveness.
Background: To best address the psychosocial concerns experienced by patients with cancer, the 2007 report of the IOM, Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs, described the importance of distress screening and identifying psychosocial needs to optimize the quality of the cancer care. This may be especially critical when treating the Veteran population, where psychosocial needs as a whole may be elevated compared to non-VA institutions. The NCCN distress thermometer screening tool is a commonly used, validated, and easily administered screen of distress (eg, Hoffman et al, 2004). However, challenges can arise in successful implementation, adherence, and responsiveness to the information gleaned from this screen (eg, Zebrack et al, 2015).
As an institution accredited by the commission on cancer, it is important to not only meet the distress screening standard (ie, assess and identify psychosocial needs) but to understand barriers to identifying psychosocial needs and to appropriately triage when psychosocial concerns are identified. Goals of this project were to understand challenges with distress screening, address barriers to distress screening, and improve quality of assessment and referrals following positive screens.
Results: At Hines VAMC, we rolled out distress screening in 2015 and 2016, with rates of screening administration increasing over the course of the first year. However, without continued monitoring and re-education, successful adherence decreased overtime. Additionally, of the 862 screens administered to date, 37% were found to be considered “positive.” We will discuss the various barriers and challenges associated with managing referrals to nonmedical providers.
Our team has identified several essential aspects of successful screening and follow-up including staff/nursing education, continued tracking and re-education over time, and establishing and maintaining relationships with psychosocial clinicians to best address these aspects of care and to optimize quality of cancer care overall. We will discuss the impact of the above interventions on adherence and responsiveness.
Background: To best address the psychosocial concerns experienced by patients with cancer, the 2007 report of the IOM, Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs, described the importance of distress screening and identifying psychosocial needs to optimize the quality of the cancer care. This may be especially critical when treating the Veteran population, where psychosocial needs as a whole may be elevated compared to non-VA institutions. The NCCN distress thermometer screening tool is a commonly used, validated, and easily administered screen of distress (eg, Hoffman et al, 2004). However, challenges can arise in successful implementation, adherence, and responsiveness to the information gleaned from this screen (eg, Zebrack et al, 2015).
As an institution accredited by the commission on cancer, it is important to not only meet the distress screening standard (ie, assess and identify psychosocial needs) but to understand barriers to identifying psychosocial needs and to appropriately triage when psychosocial concerns are identified. Goals of this project were to understand challenges with distress screening, address barriers to distress screening, and improve quality of assessment and referrals following positive screens.
Results: At Hines VAMC, we rolled out distress screening in 2015 and 2016, with rates of screening administration increasing over the course of the first year. However, without continued monitoring and re-education, successful adherence decreased overtime. Additionally, of the 862 screens administered to date, 37% were found to be considered “positive.” We will discuss the various barriers and challenges associated with managing referrals to nonmedical providers.
Our team has identified several essential aspects of successful screening and follow-up including staff/nursing education, continued tracking and re-education over time, and establishing and maintaining relationships with psychosocial clinicians to best address these aspects of care and to optimize quality of cancer care overall. We will discuss the impact of the above interventions on adherence and responsiveness.
Improve Patient Access, Turnaround Times and Customized Results Notification While Improving Mammography Program’s Ability to Detect and Follow High-Risk Patients
Purpose: Improve patient access, turnaround times and customized results notification while improving mammography program’s ability to detect and follow high-risk patients.
Background: Barriers to care is of high concern when outsourcing services into the community. Therefore, having a tracking system to ensure clinicians and patients are aware of results is of vital importance. A committee was formed to review mammogram barriers and processes. The desire was to achieve a faster turnaround time for patients from consult placement to appointment time and the ability to follow abnormal results along with high-risk detection.
Methods: A mammogram committee was formed to review general work processes and identify barriers which existed. Implementation of high-risk patient assessment and turnaround time from consult to appointment was also reviewed. Initial data showed that from consult placement to completion could range up to 220 days with the average of 158 days. There were multiple steps involved from placement of the consult until the patient was scheduled. High-risk patient screening was not utilized and it was recognized as a significant weakness in the work process.
Results: The review of the current process revealed many steps involved in obtaining an appointment and test results. An algorithm was developed to decrease the steps necessary from consult to appointment and a process was started where all mammogram orders/results were associated with one VA provider and fax number. Consult turnaround time was decreased from an average of 158 days to 35 days. Implementation of a women’s health navigator position enabled the process of detecting high-risk patients for breast cancer through a phone interview with new enrollees.
Implications: New women Veteran enrollees are receiving personalized phone appointments to assess them for risk factors in many areas of women’s health, including breast cancer screening. This has improved our ability to provide earlier detection through genetic testing, screening procedures, and prophylactic treatments. Decreasing average turnaround time by 167 days has improved patient satisfaction and decreased time in treatment for abnormalities that are found in screening. Centralizing all mammogram ordering and results received have enabled process streamlining and now allow customized patient result notification.
Purpose: Improve patient access, turnaround times and customized results notification while improving mammography program’s ability to detect and follow high-risk patients.
Background: Barriers to care is of high concern when outsourcing services into the community. Therefore, having a tracking system to ensure clinicians and patients are aware of results is of vital importance. A committee was formed to review mammogram barriers and processes. The desire was to achieve a faster turnaround time for patients from consult placement to appointment time and the ability to follow abnormal results along with high-risk detection.
Methods: A mammogram committee was formed to review general work processes and identify barriers which existed. Implementation of high-risk patient assessment and turnaround time from consult to appointment was also reviewed. Initial data showed that from consult placement to completion could range up to 220 days with the average of 158 days. There were multiple steps involved from placement of the consult until the patient was scheduled. High-risk patient screening was not utilized and it was recognized as a significant weakness in the work process.
Results: The review of the current process revealed many steps involved in obtaining an appointment and test results. An algorithm was developed to decrease the steps necessary from consult to appointment and a process was started where all mammogram orders/results were associated with one VA provider and fax number. Consult turnaround time was decreased from an average of 158 days to 35 days. Implementation of a women’s health navigator position enabled the process of detecting high-risk patients for breast cancer through a phone interview with new enrollees.
Implications: New women Veteran enrollees are receiving personalized phone appointments to assess them for risk factors in many areas of women’s health, including breast cancer screening. This has improved our ability to provide earlier detection through genetic testing, screening procedures, and prophylactic treatments. Decreasing average turnaround time by 167 days has improved patient satisfaction and decreased time in treatment for abnormalities that are found in screening. Centralizing all mammogram ordering and results received have enabled process streamlining and now allow customized patient result notification.
Purpose: Improve patient access, turnaround times and customized results notification while improving mammography program’s ability to detect and follow high-risk patients.
Background: Barriers to care is of high concern when outsourcing services into the community. Therefore, having a tracking system to ensure clinicians and patients are aware of results is of vital importance. A committee was formed to review mammogram barriers and processes. The desire was to achieve a faster turnaround time for patients from consult placement to appointment time and the ability to follow abnormal results along with high-risk detection.
Methods: A mammogram committee was formed to review general work processes and identify barriers which existed. Implementation of high-risk patient assessment and turnaround time from consult to appointment was also reviewed. Initial data showed that from consult placement to completion could range up to 220 days with the average of 158 days. There were multiple steps involved from placement of the consult until the patient was scheduled. High-risk patient screening was not utilized and it was recognized as a significant weakness in the work process.
Results: The review of the current process revealed many steps involved in obtaining an appointment and test results. An algorithm was developed to decrease the steps necessary from consult to appointment and a process was started where all mammogram orders/results were associated with one VA provider and fax number. Consult turnaround time was decreased from an average of 158 days to 35 days. Implementation of a women’s health navigator position enabled the process of detecting high-risk patients for breast cancer through a phone interview with new enrollees.
Implications: New women Veteran enrollees are receiving personalized phone appointments to assess them for risk factors in many areas of women’s health, including breast cancer screening. This has improved our ability to provide earlier detection through genetic testing, screening procedures, and prophylactic treatments. Decreasing average turnaround time by 167 days has improved patient satisfaction and decreased time in treatment for abnormalities that are found in screening. Centralizing all mammogram ordering and results received have enabled process streamlining and now allow customized patient result notification.