User login
Maternal immunization is a priority
Maternal immunization remains a priority for ob.gyns. – an opportunity to provide protection against serious infectious diseases for both the mother and the baby. With influenza vaccination rates in pregnant women still hovering around 50% and the emerging public health problem of vaccine hesitancy, we must fully embrace our responsibility to recommend immunizations and to effectively communicate what is known about their efficacy and safety. Ideally, we should offer them as well.
One reason for the low rates of influenza vaccination – one of the two vaccinations routinely recommended for all pregnant women in the United States – is that pregnant women do not always know the importance of the vaccine. This is actionable: Data clearly show that the physician’s recommendation makes a difference and that a clinician’s offer to administer the vaccination has an even greater impact.
A 2017 Centers for Disease Control and Prevention analysis of data from Internet panel surveys1 shows that women who reported receiving both a clinician recommendation and offer of vaccination had higher coverage during the 2015-2016 and 2016-2017 influenza seasons (63.7% and 70.5%) than did women who reported receiving a clinician recommendation but no offer (37.5% and 43.7%) and women who reported receiving no recommendation for vaccination (12.8% and 14.8%).
The analysis suggests there are consistently missed opportunities: Fewer than 70% (67.3%) of pregnant women in the 2016-2017 flu season reported receiving a clinician recommendation for and offer of vaccination. This is similar to the prior three flu seasons, according to the CDC.
This year, with the COVID-19 pandemic ensuing, the prevention of severe influenza illness – and other vaccine-preventable illnesses – takes on even greater importance. It is not known what the impact of two potentially devastating respiratory infections could be for pregnant individuals. Therefore, maximal protection against at least influenza will be critical.
Influenza and Tdap
Poor outcomes and disproportionately high death rates for pregnant women were observed in both the influenza pandemic of 1918-1919 and the 1957 “Asian flu” pandemic. Maternal immunization for influenza has been recommended in the United States since 2004 (part of the recommendation that everyone over the age of 6 months receive an annual flu vaccine), but it was the H1N1 influenza pandemic of 2009 that reinforced its value and led our field to more fully embrace influenza vaccination as a priority for prenatal care.
Surprisingly, most of the pregnant women who became severely ill from the H1N1 virus were young and healthy and did not have a coexisting condition known to increase risk, such as asthma or diabetes. In an analysis of California epidemiologic data, 2 only one-third of 94 pregnant women who were hospitalized with 2009 H1N1 influenza had established risk factors for complications from influenza, compared with almost two-thirds of nonpregnant women of reproductive age.
Nationally, 75 deaths of pregnant women were confirmed as because of H1N1 and 34 were possibly related to H1N1, most of which (64.3%) occurred in the third trimester.3 Records of the 1957 pandemic similarly show that pregnant women in the second and third trimesters were particularly affected.
That healthy pregnant women became so ill during the H1N1 pandemic raised several flags. For one, it became clearer that pregnancy is its own significant risk factor for severe illness from the influenza virus. Physiological changes believed to make a pregnant woman more susceptible to becoming ill include decreased lung capacity, increased nasal congestion, reduced colloid oncotic pressure, and changes in the immune system. The morbidity and mortality from H1N1 influenza also increased our drive as a specialty to convince women that vaccination is an important strategy in each influenza season.
The flu vaccine can be administered at any point during pregnancy. There is no evidence that the safety profile is any different during one trimester than another.
Patients should be reassured that vaccines recommended in pregnancy have undergone rigorous testing and that the influenza vaccine has been given to millions of pregnant women over decades. They also should understand that contracting influenza has risks for the fetus; research has demonstrated that pregnant women who contract influenza are at greater risk of spontaneous abortion as well as preterm birth and low birth weight.4
In addition, the issue of flu vaccine efficacy needs to be properly teased apart. Women read every year that the vaccine is not effective, so we need to discuss with patients what efficacy means. Does the vaccine prevent illness altogether, or does it prevent severe illness? For the most part, whereas influenza vaccines often do not offer an exact match for the year’s circulating strains – and therefore may not prevent all illness – data show that the vaccine can prevent severe illness.5 That is a worthy outcome.
Also worthy is the impact of influenza vaccination on the newborn. That maternal immunization also protects the baby – it can reduce the risk for influenza in infants under 6 months of age – is underappreciated and should be part of patient counseling. There is clear evidence that maternal immunization boosts the concentration of maternal antibodies that can cross the placenta and that infants benefit from this passive antibody protection.6
The Tdap vaccine (tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis), the second vaccine routinely recommended during each pregnancy, is administered as early as possible during the third trimester precisely for this reason – to boost maternal immune response and maximize the passive transfer of antibodies to the newborn. The target is the prevention of pertussis and associated hospitalizations and death during the first 2 months of life in an era when sporadic and unpredictable outbreaks of the infection are occurring.
Data from the CDC of morbidity and mortality from pertussis in children (2001-2011) prior to routine maternal vaccination show that the highest rates of pediatric hospitalizations and deaths occurred in newborns. Research has demonstrated that the Tdap vaccine is highly effective in preventing infections and hospitalizations in newborns: Case-control and cohort studies in the United Kingdom7,8 have shown vaccine effectiveness of 91%-93%, and similar research9 done in the U.S. has demonstrated effectiveness of 78%-85%.
The Tdap vaccine is recommended for pregnant women at 27-36 weeks of gestation – in each pregnancy. The reason for revaccination with each pregnancy is that antibody levels do not remain high for too long; at 8 months post immunization, research has shown, maternal antibody levels have begun to wane.
The vaccine also is recommended for all individuals who will be in close contact with infants younger than 12 months (for example, parents, grandparents, and child-care providers) and who have not previously received it. However, “cocooning” the newborn is effective only when the mother also is immunized – a point that ob.gyns. need to better explain to their patients so that they understand the purpose of this strategy.
Other vaccines in pregnancy and post partum
As described in the American College of Obstetricians and Gynecologists’ committee opinion on maternal immunization, 4 it is the responsibility of the ob.gyn. or obstetric care provider to routinely assess the immunization status of every pregnant patient and recommend additional vaccines for those patients who have conditions or social/behavioral practices that put them at higher risk of acquiring vaccine-preventable diseases.
Patients who have asthma or diabetes, who smoke, or who have never been vaccinated for the prevention of pneumococcal disease should receive the PPV23 pneumococcal vaccine, for instance. For pregnant women with immune deficiencies such as HIV, the PCV13 vaccine followed by PPV23 is recommended. There are approximately 500,000 cases of invasive pneumococcal disease in the United States each year, resulting in 40,000 deaths, and many multidrug-resistant strains of Streptococcus pneumoniae.
Hepatitis A and B vaccines – both recombinant vaccines with no safety concerns – also can be given during pregnancy and are officially recommended for women who have high-risk exposures. In the case of hepatitis A, high risk entails traveling to countries where the disease is endemic. High-risk behavior for hepatitis B includes sex work or being the household contact or sexual partner of a person positive for hepatitis B surface antigen.
Other travel-related vaccines, such as Japanese encephalitis, yellow fever, smallpox, and inactivated polio vaccine, can be considered in pregnancy, but decisions should be driven by more in-depth conversations about potential risks and benefits. Unlike for other vaccinations, there are limited data on the safety of travel-related immunizations in pregnancy. Sometimes, the question of whether travel is advisable in the middle of pregnancy – whether potential risks are worth taking – is a valid question to pose in conversations with patients.
Standard obstetric practice includes assessment of rubella susceptibility at the beginning of pregnancy. In some locations such as New York, measles susceptibility is also routinely evaluated. After delivery, seronegative women should be vaccinated with MMR (measles, mumps, and rubella) vaccine prior to discharge. In recent years, with the growing problem of vaccine refusal and an increasingly mobile and global society, we’re seeing sporadic outbreaks of measles and rubella – diseases that were once eradicated.
Measles in particular is highly contagious and requires a herd immunity threshold of 92%-94% to prevent sustained spread of the disease. Postpartum immunization has important maternal and pediatric implications for subsequent pregnancies, before which vaccination is often missed.
Both the MMR vaccine and the varicella vaccine (another vaccine that can be initiated post partum) are live vaccines and therefore contraindicated during pregnancy but should be administered post partum, including to people who are breastfeeding.
Other immunizations that hold some promise to protect either the mother or fetus/neonate or both are in various stages of development or testing. These include vaccines for cytomegalovirus, malaria, respiratory syncytial virus, and group B streptococcus.
A word about COVID-19
In mid-July there were more than 120 vaccine candidates for COVID-19 in various phases of study and a host of questions. Will a vaccine be efficacious? Will it prevent severe illness, or illness altogether? And will it be safe for pregnant women?
Vaccines work by manipulating the immune system, and it is important to appreciate the possibility that there may be unique pregnancy-related issues to consider with future COVID-19 vaccines – issues that could influence the effectiveness, safety, and timing of vaccination – and to understand that with any new immunization, there will likely be reluctance on the part of pregnant women who routinely prioritize fetal safety over their own health.
Pregnant women have been excluded from COVID-19 vaccine trials, but there may come a time when experts decide that a vaccine against COVID-19 is beneficial in pregnancy. Thus far, we know that the disease is clearly different from influenza. A growing knowledge of the impact of COVID-19 on the health of pregnant women, particularly the risk of developing severe illness, will be important for the future of COVID-19 immunization, as many women will not want to accept any potential risk of a vaccine unless they believe there is a significant benefit.
References
1. MMWR Morb Mortal Wkly Rep. 2017 Sep 29;66(38):1016-22.
2. N Engl J Med. 2010 Jan 7;362(1):27-35.
3. Obstet Gynecol. 2015 Sep;126(3):486-90.
4. Obstet Gynecol. 2018 Jun;131(6):e214-e217.
5. MMWR Morb Mortal Wkly Rep. 2019 Feb 15;68(6):135-9.
6. Obstet Gynecol. 2019 Apr;133(4):739-53.
7. Lancet. 2014 Oct 25;384(9953):1521-8.
8. Clin Infect Dis. 2015 Feb 1;60(3):333-7.
9. Clin Infect Dis. 2017 Jan 1;64(1):9-14.
Maternal immunization remains a priority for ob.gyns. – an opportunity to provide protection against serious infectious diseases for both the mother and the baby. With influenza vaccination rates in pregnant women still hovering around 50% and the emerging public health problem of vaccine hesitancy, we must fully embrace our responsibility to recommend immunizations and to effectively communicate what is known about their efficacy and safety. Ideally, we should offer them as well.
One reason for the low rates of influenza vaccination – one of the two vaccinations routinely recommended for all pregnant women in the United States – is that pregnant women do not always know the importance of the vaccine. This is actionable: Data clearly show that the physician’s recommendation makes a difference and that a clinician’s offer to administer the vaccination has an even greater impact.
A 2017 Centers for Disease Control and Prevention analysis of data from Internet panel surveys1 shows that women who reported receiving both a clinician recommendation and offer of vaccination had higher coverage during the 2015-2016 and 2016-2017 influenza seasons (63.7% and 70.5%) than did women who reported receiving a clinician recommendation but no offer (37.5% and 43.7%) and women who reported receiving no recommendation for vaccination (12.8% and 14.8%).
The analysis suggests there are consistently missed opportunities: Fewer than 70% (67.3%) of pregnant women in the 2016-2017 flu season reported receiving a clinician recommendation for and offer of vaccination. This is similar to the prior three flu seasons, according to the CDC.
This year, with the COVID-19 pandemic ensuing, the prevention of severe influenza illness – and other vaccine-preventable illnesses – takes on even greater importance. It is not known what the impact of two potentially devastating respiratory infections could be for pregnant individuals. Therefore, maximal protection against at least influenza will be critical.
Influenza and Tdap
Poor outcomes and disproportionately high death rates for pregnant women were observed in both the influenza pandemic of 1918-1919 and the 1957 “Asian flu” pandemic. Maternal immunization for influenza has been recommended in the United States since 2004 (part of the recommendation that everyone over the age of 6 months receive an annual flu vaccine), but it was the H1N1 influenza pandemic of 2009 that reinforced its value and led our field to more fully embrace influenza vaccination as a priority for prenatal care.
Surprisingly, most of the pregnant women who became severely ill from the H1N1 virus were young and healthy and did not have a coexisting condition known to increase risk, such as asthma or diabetes. In an analysis of California epidemiologic data, 2 only one-third of 94 pregnant women who were hospitalized with 2009 H1N1 influenza had established risk factors for complications from influenza, compared with almost two-thirds of nonpregnant women of reproductive age.
Nationally, 75 deaths of pregnant women were confirmed as because of H1N1 and 34 were possibly related to H1N1, most of which (64.3%) occurred in the third trimester.3 Records of the 1957 pandemic similarly show that pregnant women in the second and third trimesters were particularly affected.
That healthy pregnant women became so ill during the H1N1 pandemic raised several flags. For one, it became clearer that pregnancy is its own significant risk factor for severe illness from the influenza virus. Physiological changes believed to make a pregnant woman more susceptible to becoming ill include decreased lung capacity, increased nasal congestion, reduced colloid oncotic pressure, and changes in the immune system. The morbidity and mortality from H1N1 influenza also increased our drive as a specialty to convince women that vaccination is an important strategy in each influenza season.
The flu vaccine can be administered at any point during pregnancy. There is no evidence that the safety profile is any different during one trimester than another.
Patients should be reassured that vaccines recommended in pregnancy have undergone rigorous testing and that the influenza vaccine has been given to millions of pregnant women over decades. They also should understand that contracting influenza has risks for the fetus; research has demonstrated that pregnant women who contract influenza are at greater risk of spontaneous abortion as well as preterm birth and low birth weight.4
In addition, the issue of flu vaccine efficacy needs to be properly teased apart. Women read every year that the vaccine is not effective, so we need to discuss with patients what efficacy means. Does the vaccine prevent illness altogether, or does it prevent severe illness? For the most part, whereas influenza vaccines often do not offer an exact match for the year’s circulating strains – and therefore may not prevent all illness – data show that the vaccine can prevent severe illness.5 That is a worthy outcome.
Also worthy is the impact of influenza vaccination on the newborn. That maternal immunization also protects the baby – it can reduce the risk for influenza in infants under 6 months of age – is underappreciated and should be part of patient counseling. There is clear evidence that maternal immunization boosts the concentration of maternal antibodies that can cross the placenta and that infants benefit from this passive antibody protection.6
The Tdap vaccine (tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis), the second vaccine routinely recommended during each pregnancy, is administered as early as possible during the third trimester precisely for this reason – to boost maternal immune response and maximize the passive transfer of antibodies to the newborn. The target is the prevention of pertussis and associated hospitalizations and death during the first 2 months of life in an era when sporadic and unpredictable outbreaks of the infection are occurring.
Data from the CDC of morbidity and mortality from pertussis in children (2001-2011) prior to routine maternal vaccination show that the highest rates of pediatric hospitalizations and deaths occurred in newborns. Research has demonstrated that the Tdap vaccine is highly effective in preventing infections and hospitalizations in newborns: Case-control and cohort studies in the United Kingdom7,8 have shown vaccine effectiveness of 91%-93%, and similar research9 done in the U.S. has demonstrated effectiveness of 78%-85%.
The Tdap vaccine is recommended for pregnant women at 27-36 weeks of gestation – in each pregnancy. The reason for revaccination with each pregnancy is that antibody levels do not remain high for too long; at 8 months post immunization, research has shown, maternal antibody levels have begun to wane.
The vaccine also is recommended for all individuals who will be in close contact with infants younger than 12 months (for example, parents, grandparents, and child-care providers) and who have not previously received it. However, “cocooning” the newborn is effective only when the mother also is immunized – a point that ob.gyns. need to better explain to their patients so that they understand the purpose of this strategy.
Other vaccines in pregnancy and post partum
As described in the American College of Obstetricians and Gynecologists’ committee opinion on maternal immunization, 4 it is the responsibility of the ob.gyn. or obstetric care provider to routinely assess the immunization status of every pregnant patient and recommend additional vaccines for those patients who have conditions or social/behavioral practices that put them at higher risk of acquiring vaccine-preventable diseases.
Patients who have asthma or diabetes, who smoke, or who have never been vaccinated for the prevention of pneumococcal disease should receive the PPV23 pneumococcal vaccine, for instance. For pregnant women with immune deficiencies such as HIV, the PCV13 vaccine followed by PPV23 is recommended. There are approximately 500,000 cases of invasive pneumococcal disease in the United States each year, resulting in 40,000 deaths, and many multidrug-resistant strains of Streptococcus pneumoniae.
Hepatitis A and B vaccines – both recombinant vaccines with no safety concerns – also can be given during pregnancy and are officially recommended for women who have high-risk exposures. In the case of hepatitis A, high risk entails traveling to countries where the disease is endemic. High-risk behavior for hepatitis B includes sex work or being the household contact or sexual partner of a person positive for hepatitis B surface antigen.
Other travel-related vaccines, such as Japanese encephalitis, yellow fever, smallpox, and inactivated polio vaccine, can be considered in pregnancy, but decisions should be driven by more in-depth conversations about potential risks and benefits. Unlike for other vaccinations, there are limited data on the safety of travel-related immunizations in pregnancy. Sometimes, the question of whether travel is advisable in the middle of pregnancy – whether potential risks are worth taking – is a valid question to pose in conversations with patients.
Standard obstetric practice includes assessment of rubella susceptibility at the beginning of pregnancy. In some locations such as New York, measles susceptibility is also routinely evaluated. After delivery, seronegative women should be vaccinated with MMR (measles, mumps, and rubella) vaccine prior to discharge. In recent years, with the growing problem of vaccine refusal and an increasingly mobile and global society, we’re seeing sporadic outbreaks of measles and rubella – diseases that were once eradicated.
Measles in particular is highly contagious and requires a herd immunity threshold of 92%-94% to prevent sustained spread of the disease. Postpartum immunization has important maternal and pediatric implications for subsequent pregnancies, before which vaccination is often missed.
Both the MMR vaccine and the varicella vaccine (another vaccine that can be initiated post partum) are live vaccines and therefore contraindicated during pregnancy but should be administered post partum, including to people who are breastfeeding.
Other immunizations that hold some promise to protect either the mother or fetus/neonate or both are in various stages of development or testing. These include vaccines for cytomegalovirus, malaria, respiratory syncytial virus, and group B streptococcus.
A word about COVID-19
In mid-July there were more than 120 vaccine candidates for COVID-19 in various phases of study and a host of questions. Will a vaccine be efficacious? Will it prevent severe illness, or illness altogether? And will it be safe for pregnant women?
Vaccines work by manipulating the immune system, and it is important to appreciate the possibility that there may be unique pregnancy-related issues to consider with future COVID-19 vaccines – issues that could influence the effectiveness, safety, and timing of vaccination – and to understand that with any new immunization, there will likely be reluctance on the part of pregnant women who routinely prioritize fetal safety over their own health.
Pregnant women have been excluded from COVID-19 vaccine trials, but there may come a time when experts decide that a vaccine against COVID-19 is beneficial in pregnancy. Thus far, we know that the disease is clearly different from influenza. A growing knowledge of the impact of COVID-19 on the health of pregnant women, particularly the risk of developing severe illness, will be important for the future of COVID-19 immunization, as many women will not want to accept any potential risk of a vaccine unless they believe there is a significant benefit.
References
1. MMWR Morb Mortal Wkly Rep. 2017 Sep 29;66(38):1016-22.
2. N Engl J Med. 2010 Jan 7;362(1):27-35.
3. Obstet Gynecol. 2015 Sep;126(3):486-90.
4. Obstet Gynecol. 2018 Jun;131(6):e214-e217.
5. MMWR Morb Mortal Wkly Rep. 2019 Feb 15;68(6):135-9.
6. Obstet Gynecol. 2019 Apr;133(4):739-53.
7. Lancet. 2014 Oct 25;384(9953):1521-8.
8. Clin Infect Dis. 2015 Feb 1;60(3):333-7.
9. Clin Infect Dis. 2017 Jan 1;64(1):9-14.
Maternal immunization remains a priority for ob.gyns. – an opportunity to provide protection against serious infectious diseases for both the mother and the baby. With influenza vaccination rates in pregnant women still hovering around 50% and the emerging public health problem of vaccine hesitancy, we must fully embrace our responsibility to recommend immunizations and to effectively communicate what is known about their efficacy and safety. Ideally, we should offer them as well.
One reason for the low rates of influenza vaccination – one of the two vaccinations routinely recommended for all pregnant women in the United States – is that pregnant women do not always know the importance of the vaccine. This is actionable: Data clearly show that the physician’s recommendation makes a difference and that a clinician’s offer to administer the vaccination has an even greater impact.
A 2017 Centers for Disease Control and Prevention analysis of data from Internet panel surveys1 shows that women who reported receiving both a clinician recommendation and offer of vaccination had higher coverage during the 2015-2016 and 2016-2017 influenza seasons (63.7% and 70.5%) than did women who reported receiving a clinician recommendation but no offer (37.5% and 43.7%) and women who reported receiving no recommendation for vaccination (12.8% and 14.8%).
The analysis suggests there are consistently missed opportunities: Fewer than 70% (67.3%) of pregnant women in the 2016-2017 flu season reported receiving a clinician recommendation for and offer of vaccination. This is similar to the prior three flu seasons, according to the CDC.
This year, with the COVID-19 pandemic ensuing, the prevention of severe influenza illness – and other vaccine-preventable illnesses – takes on even greater importance. It is not known what the impact of two potentially devastating respiratory infections could be for pregnant individuals. Therefore, maximal protection against at least influenza will be critical.
Influenza and Tdap
Poor outcomes and disproportionately high death rates for pregnant women were observed in both the influenza pandemic of 1918-1919 and the 1957 “Asian flu” pandemic. Maternal immunization for influenza has been recommended in the United States since 2004 (part of the recommendation that everyone over the age of 6 months receive an annual flu vaccine), but it was the H1N1 influenza pandemic of 2009 that reinforced its value and led our field to more fully embrace influenza vaccination as a priority for prenatal care.
Surprisingly, most of the pregnant women who became severely ill from the H1N1 virus were young and healthy and did not have a coexisting condition known to increase risk, such as asthma or diabetes. In an analysis of California epidemiologic data, 2 only one-third of 94 pregnant women who were hospitalized with 2009 H1N1 influenza had established risk factors for complications from influenza, compared with almost two-thirds of nonpregnant women of reproductive age.
Nationally, 75 deaths of pregnant women were confirmed as because of H1N1 and 34 were possibly related to H1N1, most of which (64.3%) occurred in the third trimester.3 Records of the 1957 pandemic similarly show that pregnant women in the second and third trimesters were particularly affected.
That healthy pregnant women became so ill during the H1N1 pandemic raised several flags. For one, it became clearer that pregnancy is its own significant risk factor for severe illness from the influenza virus. Physiological changes believed to make a pregnant woman more susceptible to becoming ill include decreased lung capacity, increased nasal congestion, reduced colloid oncotic pressure, and changes in the immune system. The morbidity and mortality from H1N1 influenza also increased our drive as a specialty to convince women that vaccination is an important strategy in each influenza season.
The flu vaccine can be administered at any point during pregnancy. There is no evidence that the safety profile is any different during one trimester than another.
Patients should be reassured that vaccines recommended in pregnancy have undergone rigorous testing and that the influenza vaccine has been given to millions of pregnant women over decades. They also should understand that contracting influenza has risks for the fetus; research has demonstrated that pregnant women who contract influenza are at greater risk of spontaneous abortion as well as preterm birth and low birth weight.4
In addition, the issue of flu vaccine efficacy needs to be properly teased apart. Women read every year that the vaccine is not effective, so we need to discuss with patients what efficacy means. Does the vaccine prevent illness altogether, or does it prevent severe illness? For the most part, whereas influenza vaccines often do not offer an exact match for the year’s circulating strains – and therefore may not prevent all illness – data show that the vaccine can prevent severe illness.5 That is a worthy outcome.
Also worthy is the impact of influenza vaccination on the newborn. That maternal immunization also protects the baby – it can reduce the risk for influenza in infants under 6 months of age – is underappreciated and should be part of patient counseling. There is clear evidence that maternal immunization boosts the concentration of maternal antibodies that can cross the placenta and that infants benefit from this passive antibody protection.6
The Tdap vaccine (tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis), the second vaccine routinely recommended during each pregnancy, is administered as early as possible during the third trimester precisely for this reason – to boost maternal immune response and maximize the passive transfer of antibodies to the newborn. The target is the prevention of pertussis and associated hospitalizations and death during the first 2 months of life in an era when sporadic and unpredictable outbreaks of the infection are occurring.
Data from the CDC of morbidity and mortality from pertussis in children (2001-2011) prior to routine maternal vaccination show that the highest rates of pediatric hospitalizations and deaths occurred in newborns. Research has demonstrated that the Tdap vaccine is highly effective in preventing infections and hospitalizations in newborns: Case-control and cohort studies in the United Kingdom7,8 have shown vaccine effectiveness of 91%-93%, and similar research9 done in the U.S. has demonstrated effectiveness of 78%-85%.
The Tdap vaccine is recommended for pregnant women at 27-36 weeks of gestation – in each pregnancy. The reason for revaccination with each pregnancy is that antibody levels do not remain high for too long; at 8 months post immunization, research has shown, maternal antibody levels have begun to wane.
The vaccine also is recommended for all individuals who will be in close contact with infants younger than 12 months (for example, parents, grandparents, and child-care providers) and who have not previously received it. However, “cocooning” the newborn is effective only when the mother also is immunized – a point that ob.gyns. need to better explain to their patients so that they understand the purpose of this strategy.
Other vaccines in pregnancy and post partum
As described in the American College of Obstetricians and Gynecologists’ committee opinion on maternal immunization, 4 it is the responsibility of the ob.gyn. or obstetric care provider to routinely assess the immunization status of every pregnant patient and recommend additional vaccines for those patients who have conditions or social/behavioral practices that put them at higher risk of acquiring vaccine-preventable diseases.
Patients who have asthma or diabetes, who smoke, or who have never been vaccinated for the prevention of pneumococcal disease should receive the PPV23 pneumococcal vaccine, for instance. For pregnant women with immune deficiencies such as HIV, the PCV13 vaccine followed by PPV23 is recommended. There are approximately 500,000 cases of invasive pneumococcal disease in the United States each year, resulting in 40,000 deaths, and many multidrug-resistant strains of Streptococcus pneumoniae.
Hepatitis A and B vaccines – both recombinant vaccines with no safety concerns – also can be given during pregnancy and are officially recommended for women who have high-risk exposures. In the case of hepatitis A, high risk entails traveling to countries where the disease is endemic. High-risk behavior for hepatitis B includes sex work or being the household contact or sexual partner of a person positive for hepatitis B surface antigen.
Other travel-related vaccines, such as Japanese encephalitis, yellow fever, smallpox, and inactivated polio vaccine, can be considered in pregnancy, but decisions should be driven by more in-depth conversations about potential risks and benefits. Unlike for other vaccinations, there are limited data on the safety of travel-related immunizations in pregnancy. Sometimes, the question of whether travel is advisable in the middle of pregnancy – whether potential risks are worth taking – is a valid question to pose in conversations with patients.
Standard obstetric practice includes assessment of rubella susceptibility at the beginning of pregnancy. In some locations such as New York, measles susceptibility is also routinely evaluated. After delivery, seronegative women should be vaccinated with MMR (measles, mumps, and rubella) vaccine prior to discharge. In recent years, with the growing problem of vaccine refusal and an increasingly mobile and global society, we’re seeing sporadic outbreaks of measles and rubella – diseases that were once eradicated.
Measles in particular is highly contagious and requires a herd immunity threshold of 92%-94% to prevent sustained spread of the disease. Postpartum immunization has important maternal and pediatric implications for subsequent pregnancies, before which vaccination is often missed.
Both the MMR vaccine and the varicella vaccine (another vaccine that can be initiated post partum) are live vaccines and therefore contraindicated during pregnancy but should be administered post partum, including to people who are breastfeeding.
Other immunizations that hold some promise to protect either the mother or fetus/neonate or both are in various stages of development or testing. These include vaccines for cytomegalovirus, malaria, respiratory syncytial virus, and group B streptococcus.
A word about COVID-19
In mid-July there were more than 120 vaccine candidates for COVID-19 in various phases of study and a host of questions. Will a vaccine be efficacious? Will it prevent severe illness, or illness altogether? And will it be safe for pregnant women?
Vaccines work by manipulating the immune system, and it is important to appreciate the possibility that there may be unique pregnancy-related issues to consider with future COVID-19 vaccines – issues that could influence the effectiveness, safety, and timing of vaccination – and to understand that with any new immunization, there will likely be reluctance on the part of pregnant women who routinely prioritize fetal safety over their own health.
Pregnant women have been excluded from COVID-19 vaccine trials, but there may come a time when experts decide that a vaccine against COVID-19 is beneficial in pregnancy. Thus far, we know that the disease is clearly different from influenza. A growing knowledge of the impact of COVID-19 on the health of pregnant women, particularly the risk of developing severe illness, will be important for the future of COVID-19 immunization, as many women will not want to accept any potential risk of a vaccine unless they believe there is a significant benefit.
References
1. MMWR Morb Mortal Wkly Rep. 2017 Sep 29;66(38):1016-22.
2. N Engl J Med. 2010 Jan 7;362(1):27-35.
3. Obstet Gynecol. 2015 Sep;126(3):486-90.
4. Obstet Gynecol. 2018 Jun;131(6):e214-e217.
5. MMWR Morb Mortal Wkly Rep. 2019 Feb 15;68(6):135-9.
6. Obstet Gynecol. 2019 Apr;133(4):739-53.
7. Lancet. 2014 Oct 25;384(9953):1521-8.
8. Clin Infect Dis. 2015 Feb 1;60(3):333-7.
9. Clin Infect Dis. 2017 Jan 1;64(1):9-14.
Triage, L&D, postpartum care during the COVID-19 pandemic
The meteoric rise in the number of test-positive and clinical cases of COVID-19 because of infection with the SARS coronavirus (SARS-CoV-2) in states and cities across the United States has added urgency to the efforts to develop protocols for hospital triage, admission, labor and delivery management, and other aspects of obstetrical care.
Emerging data suggest that, while SARS-CoV-2 is less lethal overall than the severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) proved to be, it is significantly more contagious. Although a severe disease, the limited worldwide data so far available (as of early May) do not indicate that pregnant women are at greater risk of severe disease, compared with the general population. However, there remains a critical need for data on maternal and perinatal outcomes in women infected with SARS-CoV-2.
Multiple physiological changes in pregnancy, from reduced cell-based immune competence to changes in respiratory tract and pulmonary function – e.g., edema of the respiratory tract, increases in secretions and oxygen consumption, elevation of the diaphragm, and decrease in functional residual capacity – have historically contributed to worse obstetric outcomes in pregnant women who have had viral pneumonias. Furthermore, limited published experience with COVID-19 in China suggests worse perinatal outcomes in some affected pregnancies, including prematurity and perinatal death.
With evolution of the pandemic and accumulation of experience, it is expected that data-driven guidelines on assessment and management of infected pregnant women will contribute to improved maternal and perinatal outcomes. What is clear now, however, is that,
Here are my recommendations, based on a currently limited body of literature on COVID-19 and other communicable viral respiratory disorders, as well my experience in the greater Detroit area, a COVID-19 hot spot.
Preparing for hospital evaluation and admission
The obstetric triage or labor and delivery (L&D) unit should be notified prior to the arrival of a patient suspected of or known to be infected with the virus. This will minimize staff exposure and allow sufficient time to prepare appropriate accommodations, equipment, and supplies for the patient’s care. Hospital infection control should be promptly notified by L&D of the expected arrival of such a patient. Placement ideally should be in a negative-pressure room, which allows outside air to flow into the room but prevents contaminated air from escaping. In the absence of a negative-pressure room, an infection isolation area should be utilized.
The patient and one accompanying support individual should wear either medical-grade masks brought from home or supplied upon entry to the hospital or homemade masks or bandanas. This will reduce the risk of viral transmission to hospital workers and other individuals encountered in the hospital prior to arriving in L&D. An ideal setup is to have separate entry areas, access corridors, and elevators for patients known or suspected to have COVID-19 infection. The patient and visitor should be expeditiously escorted to the prepared area for evaluation. Patients who are not known or suspected to be infected ideally should be tested.
Screening of patients & support individuals
Proper screening of patients and support individuals is critical to protecting both patients and staff in the L&D unit. This should include an expanded questionnaire that asks about disturbances of smell and taste and GI symptoms like loss of appetite – not only the more commonly queried symptoms of fever, shortness of breath, coughing, and exposure to someone who may have been ill.
Recent studies regarding presenting symptoms cast significant doubt, in fact, on the validity of patients with “asymptomatic COVID-19.” Over 15% of patients with confirmed infection in one published case series had solely GI symptoms and almost all had some digestive symptoms, for example, and almost 90% in another study had absent or reduced sense of smell and/or taste.1,2 In fact, the use of the term “paucisymptomatic” rather than “asymptomatic” may be most appropriate.
Support individuals also should undergo temperature screening, ideally with laser noncontact thermometers on entry to the hospital or triage.
Visitor policy
The number of visitors/support individuals should be kept to a minimum to reduce transmission risk. The actual number will be determined by hospital or state policy, but up to one visitor in the labor room appears reasonable. Very strong individual justification should be required to exceed this threshold! The visitor should not only be screened for an expanded list of symptoms, but they also should be queried for underlying illnesses (e.g., diabetes, cardiovascular disease, significant lung disease, undergoing cancer therapy) as well as for age over 65 years, each of which increase the chances of severe COVID-19 disease should infection occur. The visitor should be informed of such risks and, especially when accompanying a patient with known or suspected COVID-19, provided the option of voluntarily revoking their visitor status. A visitor with known or suspected COVID-19 infection based on testing or screening should not be allowed into the L&D unit.
In addition, institutions may be considered to have obligations to the visitor/support person beyond screening. These include instructions in proper mask usage, hand washing, and limiting the touching of surfaces to lower infection risk.
“Visitor relays” where one visitor replaces another should be strongly discouraged. Visitors should similarly not be allowed to wander around the hospital (to use phones, for instance); transiting back and forth to obtain food and coffee should be kept to a strict minimum. For visitors accompanying COVID-19–-infected women, “visitor’s plates” provided by the hospital at reasonable cost is a much-preferred arrangement for obtaining meals during the course of the hospital stay. In addition, visitors should be sent out of the room during the performance of aerosolizing procedures.
Labor and delivery management
The successful management of patients with COVID-19 requires a rigorous infection control protocol informed by guidelines from national entities, such as the Centers for Disease Control and Prevention, the Society for Maternal-Fetal Medicine, and the American College of Obstetricians and Gynecologists, and by state health departments when available.
Strict limits on the number of obstetricians and other health care workers (HCWs) entering the patient’s room should be enforced and documented to minimize risk to the HCWs attending to patients who have a positive diagnosis or who are under investigation. Only in cases of demonstrable clinical benefit should repeat visits by the same or additional HCWs be permitted. Conventional and electronic tablets present an excellent opportunity for patient follow-up visits without room entry. In our institution, this has been successfully piloted in nonpregnant patients. Obstetricians and others caring for obstetrical patients – especially those who are infected or under investigation for infection – should always wear a properly fitted N95 mask.
Because patients with COVID-19 may have or go on to develop a constellation of organ abnormalities (e.g., cardiovascular, renal, pulmonary), it is vital that a standardized panel of baseline laboratory studies be developed for pregnant patients. This will minimize the need for repeated blood draws and other testing which may increase HCW exposure.
A negative screen based on nonreport of symptoms, lack of temperature elevation, and reported nonexposure to individuals with COVID-19 symptoms still has limitations in terms of disease detection. A recent report from a tertiary care hospital in New York City found that close to one-third of pregnant patients with confirmed COVID-19 admitted over a 2-week period had no viral symptoms or instructive history on initial admission.3 This is consistent with our clinical experience. Most importantly, therefore, routine quantitative reverse transcription polymerase chain reaction testing should be performed on all patients admitted to the L&D unit.
Given the reported variability in the accuracy of polymerase chain reaction testing induced by variable effectiveness of sampling techniques, stage of infection, and inherent test accuracy issues, symptomatic patients with a negative test should first obtain clearance from infectious disease specialists before isolation precautions are discontinued. Repeat testing in 24 hours, including testing of multiple sites, may subsequently yield a positive result in persistently symptomatic patients.
Intrapartum management
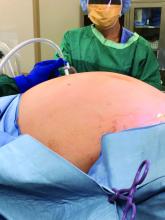
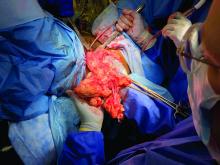
As much as possible, standard obstetric indications should guide the timing and route of delivery. In the case of a COVID-19–positive patient or a patient under investigation, nonobstetric factors may bear heavily on decision making, and management flexibility is of great value. For example, in cases of severe or critical disease status, evidence suggests that early delivery regardless of gestational age can improve maternal oxygenation; this supports the liberal use of C-sections in these circumstances. In addition, shortening labor length as well as duration of hospitalization may be expected to reduce the risk of transmission to HCWs, other staff, and other patients.
High rates of cesarean delivery unsurprisingly have been reported thus far: One review of 108 case reports and series of test-positive COVID-19 pregnancies found a 92% C-section rate, and another review and meta-analysis of studies of SARS, MERS, and COVID-19 during pregnancy similarly found that the majority of patients – 84% across all coronavirus infections and 91% in COVID-19 pregnancies – were delivered by C-section.4,5 Given these high rates of cesarean deliveries, the early placement of neuraxial anesthesia while the patient is stable appears to be prudent and obviates the need for intubation, the latter of which is associated with increased aerosol generation and increased virus transmission risk.
Strict protocols for the optimal protection of staff should be observed, including proper personal protective equipment (PPE) protection. Protocols have been detailed in various guidelines and publications; they include the wearing of shoe covers, gowns, N95 masks, goggles, face shields, and two layers of gloves.
For institutions that currently do not offer routine COVID-19 testing to pregnant patients – especially those in areas of outbreaks – N95 masks and eye protection should still be provided to all HCWs involved in the intrapartum management of untested asymptomatic patients, particularly those in the active phase of labor. This protection is justified given the limitations of symptom- and history-based screening and the not-uncommon experience of the patient with a negative screen who subsequently develops the clinical syndrome.
Obstetric management of labor requires close patient contact that potentially elevates the risk of contamination and infection. During the active stage of labor, patient shouting, rapid mouth breathing, and other behaviors inherent to labor all increase the risk of aerosolization of oronasal secretions. In addition, nasal-prong oxygen administration is believed to independently increase the risk of aerosolization of secretions. The casual practice of nasal oxygen application should thus be discontinued and, where felt to be absolutely necessary, a mask should be worn on top of the prongs.
Regarding operative delivery, each participating obstetric surgeon should observe guidelines and recommendations of governing national organizations and professional groups – including the American College of Surgeons – regarding the safe conduct of operations on patients with COVID-19. Written guidelines should be tailored as needed to the performance of C-sections and readily available in L&D. Drills and simulations are generally valuable, and expertise and support should always be available in the labor room to assist with donning and doffing of PPE.
Postpartum care
Expeditious separation of the COVID-19–positive mother from her infant is recommended, including avoidance of delayed cord clamping because of insufficient evidence of benefit to the infant. Insufficient evidence exists to support vertical transmission, but the possibility of maternal-infant transmission is clinically accepted based on small case reports of infection in a neonate at 30 hours of life and in infants of mothers with suspected or confirmed COVID-19.6,7 Accordingly, it is recommended that the benefit of early infant separation should be discussed with the mother. If approved, the infant should be kept in a separate isolation area and observed.
There is no evidence of breast milk transmission of the virus. For those electing to breastfeed, the patient should be provided with a breast pump to express and store the milk for subsequent bottle feeding. For mothers who elect to room in with the infant, a separation distance of 6 feet is recommended with an intervening barrier curtain. For COVID-19–positive mothers who elect breastfeeding, meticulous hand and face washing, continuous wearing of a mask, and cleansing of the breast prior to feeding needs to be maintained.
Restrictive visiting policies of no more than one visitor should be maintained. For severely or critically ill patients with COVID-19, it has been suggested that no visitors be allowed. As with other hospitalizations of COVID-19 patients, the HCW contact should be kept at a justifiable minimum to reduce the risk of transmission.
Protecting the obstetrician and other HCWs
Protecting the health of obstetricians and other HCWs is central to any successful strategy to fight the COVID-19 epidemic. For the individual obstetrician, careful attention to national and local hospital guidelines is required as these are rapidly evolving.
Physicians and their leadership must maintain an ongoing dialogue with hospital leadership to continually upgrade and optimize infection prevention and control measures, and to uphold best practices. The experience in Wuhan, China, illustrates the effectiveness of the proper use of PPE along with population control measures to reduce infections in HCWs. Prior to understanding the mechanism of virus transmission and using protective equipment, infection rates of 3%-29% were reported among HCWs. With the meticulous utilization of mitigation strategies and population control measures – including consistent use of PPE – the rate of infection of HCWs reportedly fell to zero.
In outpatient offices, all staff and HCWs should wear masks at all times and engage in social distancing and in frequent hand sanitization. Patients should be strongly encouraged to wear masks during office visits and on all other occasions when they will be in physical proximity to other individuals outside of the home.
Reports from epidemic areas describe transmission from household sources as a significant cause of HCW infection. The information emphasizes the need for ongoing vigilance and attention to sanitization measures even when at home with one’s family. An additional benefit is reduced risk of transmission from HCWs to family members.
Dr. Bahado-Singh is professor and chair of obstetrics and gynecology at Oakland University, Rochester, Mich., and health system chair for obstetrics and gynecology at Beaumont Health System.
References
1. Luo S et al. Clin Gastroenterol Hepatol. 2020 Mar 20. doi: 10.1016/j.cgh.2020.03.043.
2. Lechien JR et al. Eur Arch Otorhinolaryngol. 2020 Apr 6. doi: 10.1007/s00405-020-05965-1.
3. Breslin N et al. Am J Obstet Gynecol MFM. 2020 Apr 9. doi: 10.1016/j.ajogmf.2020.100118.
4. Zaigham M, Andersson O. Acta Obstet Gynecol Scand. 2020 Apr 7. doi: 10.1111/aogs.13867.
5. Di Mascio D et al. Am J Obstet Gynecol MFM. 2020 Mar 25. doi: 10.1016/j.ajogmf.2020.100107.
6. Ital J. Pediatr 2020;46(1) doi: 10.1186/s13052-020-0820-x.
7. Int J Gynaecol Obstet. 2020;149(2):130-6.
*This article was updated 5/6/2020.
The meteoric rise in the number of test-positive and clinical cases of COVID-19 because of infection with the SARS coronavirus (SARS-CoV-2) in states and cities across the United States has added urgency to the efforts to develop protocols for hospital triage, admission, labor and delivery management, and other aspects of obstetrical care.
Emerging data suggest that, while SARS-CoV-2 is less lethal overall than the severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) proved to be, it is significantly more contagious. Although a severe disease, the limited worldwide data so far available (as of early May) do not indicate that pregnant women are at greater risk of severe disease, compared with the general population. However, there remains a critical need for data on maternal and perinatal outcomes in women infected with SARS-CoV-2.
Multiple physiological changes in pregnancy, from reduced cell-based immune competence to changes in respiratory tract and pulmonary function – e.g., edema of the respiratory tract, increases in secretions and oxygen consumption, elevation of the diaphragm, and decrease in functional residual capacity – have historically contributed to worse obstetric outcomes in pregnant women who have had viral pneumonias. Furthermore, limited published experience with COVID-19 in China suggests worse perinatal outcomes in some affected pregnancies, including prematurity and perinatal death.
With evolution of the pandemic and accumulation of experience, it is expected that data-driven guidelines on assessment and management of infected pregnant women will contribute to improved maternal and perinatal outcomes. What is clear now, however, is that,
Here are my recommendations, based on a currently limited body of literature on COVID-19 and other communicable viral respiratory disorders, as well my experience in the greater Detroit area, a COVID-19 hot spot.
Preparing for hospital evaluation and admission
The obstetric triage or labor and delivery (L&D) unit should be notified prior to the arrival of a patient suspected of or known to be infected with the virus. This will minimize staff exposure and allow sufficient time to prepare appropriate accommodations, equipment, and supplies for the patient’s care. Hospital infection control should be promptly notified by L&D of the expected arrival of such a patient. Placement ideally should be in a negative-pressure room, which allows outside air to flow into the room but prevents contaminated air from escaping. In the absence of a negative-pressure room, an infection isolation area should be utilized.
The patient and one accompanying support individual should wear either medical-grade masks brought from home or supplied upon entry to the hospital or homemade masks or bandanas. This will reduce the risk of viral transmission to hospital workers and other individuals encountered in the hospital prior to arriving in L&D. An ideal setup is to have separate entry areas, access corridors, and elevators for patients known or suspected to have COVID-19 infection. The patient and visitor should be expeditiously escorted to the prepared area for evaluation. Patients who are not known or suspected to be infected ideally should be tested.
Screening of patients & support individuals
Proper screening of patients and support individuals is critical to protecting both patients and staff in the L&D unit. This should include an expanded questionnaire that asks about disturbances of smell and taste and GI symptoms like loss of appetite – not only the more commonly queried symptoms of fever, shortness of breath, coughing, and exposure to someone who may have been ill.
Recent studies regarding presenting symptoms cast significant doubt, in fact, on the validity of patients with “asymptomatic COVID-19.” Over 15% of patients with confirmed infection in one published case series had solely GI symptoms and almost all had some digestive symptoms, for example, and almost 90% in another study had absent or reduced sense of smell and/or taste.1,2 In fact, the use of the term “paucisymptomatic” rather than “asymptomatic” may be most appropriate.
Support individuals also should undergo temperature screening, ideally with laser noncontact thermometers on entry to the hospital or triage.
Visitor policy
The number of visitors/support individuals should be kept to a minimum to reduce transmission risk. The actual number will be determined by hospital or state policy, but up to one visitor in the labor room appears reasonable. Very strong individual justification should be required to exceed this threshold! The visitor should not only be screened for an expanded list of symptoms, but they also should be queried for underlying illnesses (e.g., diabetes, cardiovascular disease, significant lung disease, undergoing cancer therapy) as well as for age over 65 years, each of which increase the chances of severe COVID-19 disease should infection occur. The visitor should be informed of such risks and, especially when accompanying a patient with known or suspected COVID-19, provided the option of voluntarily revoking their visitor status. A visitor with known or suspected COVID-19 infection based on testing or screening should not be allowed into the L&D unit.
In addition, institutions may be considered to have obligations to the visitor/support person beyond screening. These include instructions in proper mask usage, hand washing, and limiting the touching of surfaces to lower infection risk.
“Visitor relays” where one visitor replaces another should be strongly discouraged. Visitors should similarly not be allowed to wander around the hospital (to use phones, for instance); transiting back and forth to obtain food and coffee should be kept to a strict minimum. For visitors accompanying COVID-19–-infected women, “visitor’s plates” provided by the hospital at reasonable cost is a much-preferred arrangement for obtaining meals during the course of the hospital stay. In addition, visitors should be sent out of the room during the performance of aerosolizing procedures.
Labor and delivery management
The successful management of patients with COVID-19 requires a rigorous infection control protocol informed by guidelines from national entities, such as the Centers for Disease Control and Prevention, the Society for Maternal-Fetal Medicine, and the American College of Obstetricians and Gynecologists, and by state health departments when available.
Strict limits on the number of obstetricians and other health care workers (HCWs) entering the patient’s room should be enforced and documented to minimize risk to the HCWs attending to patients who have a positive diagnosis or who are under investigation. Only in cases of demonstrable clinical benefit should repeat visits by the same or additional HCWs be permitted. Conventional and electronic tablets present an excellent opportunity for patient follow-up visits without room entry. In our institution, this has been successfully piloted in nonpregnant patients. Obstetricians and others caring for obstetrical patients – especially those who are infected or under investigation for infection – should always wear a properly fitted N95 mask.
Because patients with COVID-19 may have or go on to develop a constellation of organ abnormalities (e.g., cardiovascular, renal, pulmonary), it is vital that a standardized panel of baseline laboratory studies be developed for pregnant patients. This will minimize the need for repeated blood draws and other testing which may increase HCW exposure.
A negative screen based on nonreport of symptoms, lack of temperature elevation, and reported nonexposure to individuals with COVID-19 symptoms still has limitations in terms of disease detection. A recent report from a tertiary care hospital in New York City found that close to one-third of pregnant patients with confirmed COVID-19 admitted over a 2-week period had no viral symptoms or instructive history on initial admission.3 This is consistent with our clinical experience. Most importantly, therefore, routine quantitative reverse transcription polymerase chain reaction testing should be performed on all patients admitted to the L&D unit.
Given the reported variability in the accuracy of polymerase chain reaction testing induced by variable effectiveness of sampling techniques, stage of infection, and inherent test accuracy issues, symptomatic patients with a negative test should first obtain clearance from infectious disease specialists before isolation precautions are discontinued. Repeat testing in 24 hours, including testing of multiple sites, may subsequently yield a positive result in persistently symptomatic patients.
Intrapartum management
As much as possible, standard obstetric indications should guide the timing and route of delivery. In the case of a COVID-19–positive patient or a patient under investigation, nonobstetric factors may bear heavily on decision making, and management flexibility is of great value. For example, in cases of severe or critical disease status, evidence suggests that early delivery regardless of gestational age can improve maternal oxygenation; this supports the liberal use of C-sections in these circumstances. In addition, shortening labor length as well as duration of hospitalization may be expected to reduce the risk of transmission to HCWs, other staff, and other patients.
High rates of cesarean delivery unsurprisingly have been reported thus far: One review of 108 case reports and series of test-positive COVID-19 pregnancies found a 92% C-section rate, and another review and meta-analysis of studies of SARS, MERS, and COVID-19 during pregnancy similarly found that the majority of patients – 84% across all coronavirus infections and 91% in COVID-19 pregnancies – were delivered by C-section.4,5 Given these high rates of cesarean deliveries, the early placement of neuraxial anesthesia while the patient is stable appears to be prudent and obviates the need for intubation, the latter of which is associated with increased aerosol generation and increased virus transmission risk.
Strict protocols for the optimal protection of staff should be observed, including proper personal protective equipment (PPE) protection. Protocols have been detailed in various guidelines and publications; they include the wearing of shoe covers, gowns, N95 masks, goggles, face shields, and two layers of gloves.
For institutions that currently do not offer routine COVID-19 testing to pregnant patients – especially those in areas of outbreaks – N95 masks and eye protection should still be provided to all HCWs involved in the intrapartum management of untested asymptomatic patients, particularly those in the active phase of labor. This protection is justified given the limitations of symptom- and history-based screening and the not-uncommon experience of the patient with a negative screen who subsequently develops the clinical syndrome.
Obstetric management of labor requires close patient contact that potentially elevates the risk of contamination and infection. During the active stage of labor, patient shouting, rapid mouth breathing, and other behaviors inherent to labor all increase the risk of aerosolization of oronasal secretions. In addition, nasal-prong oxygen administration is believed to independently increase the risk of aerosolization of secretions. The casual practice of nasal oxygen application should thus be discontinued and, where felt to be absolutely necessary, a mask should be worn on top of the prongs.
Regarding operative delivery, each participating obstetric surgeon should observe guidelines and recommendations of governing national organizations and professional groups – including the American College of Surgeons – regarding the safe conduct of operations on patients with COVID-19. Written guidelines should be tailored as needed to the performance of C-sections and readily available in L&D. Drills and simulations are generally valuable, and expertise and support should always be available in the labor room to assist with donning and doffing of PPE.
Postpartum care
Expeditious separation of the COVID-19–positive mother from her infant is recommended, including avoidance of delayed cord clamping because of insufficient evidence of benefit to the infant. Insufficient evidence exists to support vertical transmission, but the possibility of maternal-infant transmission is clinically accepted based on small case reports of infection in a neonate at 30 hours of life and in infants of mothers with suspected or confirmed COVID-19.6,7 Accordingly, it is recommended that the benefit of early infant separation should be discussed with the mother. If approved, the infant should be kept in a separate isolation area and observed.
There is no evidence of breast milk transmission of the virus. For those electing to breastfeed, the patient should be provided with a breast pump to express and store the milk for subsequent bottle feeding. For mothers who elect to room in with the infant, a separation distance of 6 feet is recommended with an intervening barrier curtain. For COVID-19–positive mothers who elect breastfeeding, meticulous hand and face washing, continuous wearing of a mask, and cleansing of the breast prior to feeding needs to be maintained.
Restrictive visiting policies of no more than one visitor should be maintained. For severely or critically ill patients with COVID-19, it has been suggested that no visitors be allowed. As with other hospitalizations of COVID-19 patients, the HCW contact should be kept at a justifiable minimum to reduce the risk of transmission.
Protecting the obstetrician and other HCWs
Protecting the health of obstetricians and other HCWs is central to any successful strategy to fight the COVID-19 epidemic. For the individual obstetrician, careful attention to national and local hospital guidelines is required as these are rapidly evolving.
Physicians and their leadership must maintain an ongoing dialogue with hospital leadership to continually upgrade and optimize infection prevention and control measures, and to uphold best practices. The experience in Wuhan, China, illustrates the effectiveness of the proper use of PPE along with population control measures to reduce infections in HCWs. Prior to understanding the mechanism of virus transmission and using protective equipment, infection rates of 3%-29% were reported among HCWs. With the meticulous utilization of mitigation strategies and population control measures – including consistent use of PPE – the rate of infection of HCWs reportedly fell to zero.
In outpatient offices, all staff and HCWs should wear masks at all times and engage in social distancing and in frequent hand sanitization. Patients should be strongly encouraged to wear masks during office visits and on all other occasions when they will be in physical proximity to other individuals outside of the home.
Reports from epidemic areas describe transmission from household sources as a significant cause of HCW infection. The information emphasizes the need for ongoing vigilance and attention to sanitization measures even when at home with one’s family. An additional benefit is reduced risk of transmission from HCWs to family members.
Dr. Bahado-Singh is professor and chair of obstetrics and gynecology at Oakland University, Rochester, Mich., and health system chair for obstetrics and gynecology at Beaumont Health System.
References
1. Luo S et al. Clin Gastroenterol Hepatol. 2020 Mar 20. doi: 10.1016/j.cgh.2020.03.043.
2. Lechien JR et al. Eur Arch Otorhinolaryngol. 2020 Apr 6. doi: 10.1007/s00405-020-05965-1.
3. Breslin N et al. Am J Obstet Gynecol MFM. 2020 Apr 9. doi: 10.1016/j.ajogmf.2020.100118.
4. Zaigham M, Andersson O. Acta Obstet Gynecol Scand. 2020 Apr 7. doi: 10.1111/aogs.13867.
5. Di Mascio D et al. Am J Obstet Gynecol MFM. 2020 Mar 25. doi: 10.1016/j.ajogmf.2020.100107.
6. Ital J. Pediatr 2020;46(1) doi: 10.1186/s13052-020-0820-x.
7. Int J Gynaecol Obstet. 2020;149(2):130-6.
*This article was updated 5/6/2020.
The meteoric rise in the number of test-positive and clinical cases of COVID-19 because of infection with the SARS coronavirus (SARS-CoV-2) in states and cities across the United States has added urgency to the efforts to develop protocols for hospital triage, admission, labor and delivery management, and other aspects of obstetrical care.
Emerging data suggest that, while SARS-CoV-2 is less lethal overall than the severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) proved to be, it is significantly more contagious. Although a severe disease, the limited worldwide data so far available (as of early May) do not indicate that pregnant women are at greater risk of severe disease, compared with the general population. However, there remains a critical need for data on maternal and perinatal outcomes in women infected with SARS-CoV-2.
Multiple physiological changes in pregnancy, from reduced cell-based immune competence to changes in respiratory tract and pulmonary function – e.g., edema of the respiratory tract, increases in secretions and oxygen consumption, elevation of the diaphragm, and decrease in functional residual capacity – have historically contributed to worse obstetric outcomes in pregnant women who have had viral pneumonias. Furthermore, limited published experience with COVID-19 in China suggests worse perinatal outcomes in some affected pregnancies, including prematurity and perinatal death.
With evolution of the pandemic and accumulation of experience, it is expected that data-driven guidelines on assessment and management of infected pregnant women will contribute to improved maternal and perinatal outcomes. What is clear now, however, is that,
Here are my recommendations, based on a currently limited body of literature on COVID-19 and other communicable viral respiratory disorders, as well my experience in the greater Detroit area, a COVID-19 hot spot.
Preparing for hospital evaluation and admission
The obstetric triage or labor and delivery (L&D) unit should be notified prior to the arrival of a patient suspected of or known to be infected with the virus. This will minimize staff exposure and allow sufficient time to prepare appropriate accommodations, equipment, and supplies for the patient’s care. Hospital infection control should be promptly notified by L&D of the expected arrival of such a patient. Placement ideally should be in a negative-pressure room, which allows outside air to flow into the room but prevents contaminated air from escaping. In the absence of a negative-pressure room, an infection isolation area should be utilized.
The patient and one accompanying support individual should wear either medical-grade masks brought from home or supplied upon entry to the hospital or homemade masks or bandanas. This will reduce the risk of viral transmission to hospital workers and other individuals encountered in the hospital prior to arriving in L&D. An ideal setup is to have separate entry areas, access corridors, and elevators for patients known or suspected to have COVID-19 infection. The patient and visitor should be expeditiously escorted to the prepared area for evaluation. Patients who are not known or suspected to be infected ideally should be tested.
Screening of patients & support individuals
Proper screening of patients and support individuals is critical to protecting both patients and staff in the L&D unit. This should include an expanded questionnaire that asks about disturbances of smell and taste and GI symptoms like loss of appetite – not only the more commonly queried symptoms of fever, shortness of breath, coughing, and exposure to someone who may have been ill.
Recent studies regarding presenting symptoms cast significant doubt, in fact, on the validity of patients with “asymptomatic COVID-19.” Over 15% of patients with confirmed infection in one published case series had solely GI symptoms and almost all had some digestive symptoms, for example, and almost 90% in another study had absent or reduced sense of smell and/or taste.1,2 In fact, the use of the term “paucisymptomatic” rather than “asymptomatic” may be most appropriate.
Support individuals also should undergo temperature screening, ideally with laser noncontact thermometers on entry to the hospital or triage.
Visitor policy
The number of visitors/support individuals should be kept to a minimum to reduce transmission risk. The actual number will be determined by hospital or state policy, but up to one visitor in the labor room appears reasonable. Very strong individual justification should be required to exceed this threshold! The visitor should not only be screened for an expanded list of symptoms, but they also should be queried for underlying illnesses (e.g., diabetes, cardiovascular disease, significant lung disease, undergoing cancer therapy) as well as for age over 65 years, each of which increase the chances of severe COVID-19 disease should infection occur. The visitor should be informed of such risks and, especially when accompanying a patient with known or suspected COVID-19, provided the option of voluntarily revoking their visitor status. A visitor with known or suspected COVID-19 infection based on testing or screening should not be allowed into the L&D unit.
In addition, institutions may be considered to have obligations to the visitor/support person beyond screening. These include instructions in proper mask usage, hand washing, and limiting the touching of surfaces to lower infection risk.
“Visitor relays” where one visitor replaces another should be strongly discouraged. Visitors should similarly not be allowed to wander around the hospital (to use phones, for instance); transiting back and forth to obtain food and coffee should be kept to a strict minimum. For visitors accompanying COVID-19–-infected women, “visitor’s plates” provided by the hospital at reasonable cost is a much-preferred arrangement for obtaining meals during the course of the hospital stay. In addition, visitors should be sent out of the room during the performance of aerosolizing procedures.
Labor and delivery management
The successful management of patients with COVID-19 requires a rigorous infection control protocol informed by guidelines from national entities, such as the Centers for Disease Control and Prevention, the Society for Maternal-Fetal Medicine, and the American College of Obstetricians and Gynecologists, and by state health departments when available.
Strict limits on the number of obstetricians and other health care workers (HCWs) entering the patient’s room should be enforced and documented to minimize risk to the HCWs attending to patients who have a positive diagnosis or who are under investigation. Only in cases of demonstrable clinical benefit should repeat visits by the same or additional HCWs be permitted. Conventional and electronic tablets present an excellent opportunity for patient follow-up visits without room entry. In our institution, this has been successfully piloted in nonpregnant patients. Obstetricians and others caring for obstetrical patients – especially those who are infected or under investigation for infection – should always wear a properly fitted N95 mask.
Because patients with COVID-19 may have or go on to develop a constellation of organ abnormalities (e.g., cardiovascular, renal, pulmonary), it is vital that a standardized panel of baseline laboratory studies be developed for pregnant patients. This will minimize the need for repeated blood draws and other testing which may increase HCW exposure.
A negative screen based on nonreport of symptoms, lack of temperature elevation, and reported nonexposure to individuals with COVID-19 symptoms still has limitations in terms of disease detection. A recent report from a tertiary care hospital in New York City found that close to one-third of pregnant patients with confirmed COVID-19 admitted over a 2-week period had no viral symptoms or instructive history on initial admission.3 This is consistent with our clinical experience. Most importantly, therefore, routine quantitative reverse transcription polymerase chain reaction testing should be performed on all patients admitted to the L&D unit.
Given the reported variability in the accuracy of polymerase chain reaction testing induced by variable effectiveness of sampling techniques, stage of infection, and inherent test accuracy issues, symptomatic patients with a negative test should first obtain clearance from infectious disease specialists before isolation precautions are discontinued. Repeat testing in 24 hours, including testing of multiple sites, may subsequently yield a positive result in persistently symptomatic patients.
Intrapartum management
As much as possible, standard obstetric indications should guide the timing and route of delivery. In the case of a COVID-19–positive patient or a patient under investigation, nonobstetric factors may bear heavily on decision making, and management flexibility is of great value. For example, in cases of severe or critical disease status, evidence suggests that early delivery regardless of gestational age can improve maternal oxygenation; this supports the liberal use of C-sections in these circumstances. In addition, shortening labor length as well as duration of hospitalization may be expected to reduce the risk of transmission to HCWs, other staff, and other patients.
High rates of cesarean delivery unsurprisingly have been reported thus far: One review of 108 case reports and series of test-positive COVID-19 pregnancies found a 92% C-section rate, and another review and meta-analysis of studies of SARS, MERS, and COVID-19 during pregnancy similarly found that the majority of patients – 84% across all coronavirus infections and 91% in COVID-19 pregnancies – were delivered by C-section.4,5 Given these high rates of cesarean deliveries, the early placement of neuraxial anesthesia while the patient is stable appears to be prudent and obviates the need for intubation, the latter of which is associated with increased aerosol generation and increased virus transmission risk.
Strict protocols for the optimal protection of staff should be observed, including proper personal protective equipment (PPE) protection. Protocols have been detailed in various guidelines and publications; they include the wearing of shoe covers, gowns, N95 masks, goggles, face shields, and two layers of gloves.
For institutions that currently do not offer routine COVID-19 testing to pregnant patients – especially those in areas of outbreaks – N95 masks and eye protection should still be provided to all HCWs involved in the intrapartum management of untested asymptomatic patients, particularly those in the active phase of labor. This protection is justified given the limitations of symptom- and history-based screening and the not-uncommon experience of the patient with a negative screen who subsequently develops the clinical syndrome.
Obstetric management of labor requires close patient contact that potentially elevates the risk of contamination and infection. During the active stage of labor, patient shouting, rapid mouth breathing, and other behaviors inherent to labor all increase the risk of aerosolization of oronasal secretions. In addition, nasal-prong oxygen administration is believed to independently increase the risk of aerosolization of secretions. The casual practice of nasal oxygen application should thus be discontinued and, where felt to be absolutely necessary, a mask should be worn on top of the prongs.
Regarding operative delivery, each participating obstetric surgeon should observe guidelines and recommendations of governing national organizations and professional groups – including the American College of Surgeons – regarding the safe conduct of operations on patients with COVID-19. Written guidelines should be tailored as needed to the performance of C-sections and readily available in L&D. Drills and simulations are generally valuable, and expertise and support should always be available in the labor room to assist with donning and doffing of PPE.
Postpartum care
Expeditious separation of the COVID-19–positive mother from her infant is recommended, including avoidance of delayed cord clamping because of insufficient evidence of benefit to the infant. Insufficient evidence exists to support vertical transmission, but the possibility of maternal-infant transmission is clinically accepted based on small case reports of infection in a neonate at 30 hours of life and in infants of mothers with suspected or confirmed COVID-19.6,7 Accordingly, it is recommended that the benefit of early infant separation should be discussed with the mother. If approved, the infant should be kept in a separate isolation area and observed.
There is no evidence of breast milk transmission of the virus. For those electing to breastfeed, the patient should be provided with a breast pump to express and store the milk for subsequent bottle feeding. For mothers who elect to room in with the infant, a separation distance of 6 feet is recommended with an intervening barrier curtain. For COVID-19–positive mothers who elect breastfeeding, meticulous hand and face washing, continuous wearing of a mask, and cleansing of the breast prior to feeding needs to be maintained.
Restrictive visiting policies of no more than one visitor should be maintained. For severely or critically ill patients with COVID-19, it has been suggested that no visitors be allowed. As with other hospitalizations of COVID-19 patients, the HCW contact should be kept at a justifiable minimum to reduce the risk of transmission.
Protecting the obstetrician and other HCWs
Protecting the health of obstetricians and other HCWs is central to any successful strategy to fight the COVID-19 epidemic. For the individual obstetrician, careful attention to national and local hospital guidelines is required as these are rapidly evolving.
Physicians and their leadership must maintain an ongoing dialogue with hospital leadership to continually upgrade and optimize infection prevention and control measures, and to uphold best practices. The experience in Wuhan, China, illustrates the effectiveness of the proper use of PPE along with population control measures to reduce infections in HCWs. Prior to understanding the mechanism of virus transmission and using protective equipment, infection rates of 3%-29% were reported among HCWs. With the meticulous utilization of mitigation strategies and population control measures – including consistent use of PPE – the rate of infection of HCWs reportedly fell to zero.
In outpatient offices, all staff and HCWs should wear masks at all times and engage in social distancing and in frequent hand sanitization. Patients should be strongly encouraged to wear masks during office visits and on all other occasions when they will be in physical proximity to other individuals outside of the home.
Reports from epidemic areas describe transmission from household sources as a significant cause of HCW infection. The information emphasizes the need for ongoing vigilance and attention to sanitization measures even when at home with one’s family. An additional benefit is reduced risk of transmission from HCWs to family members.
Dr. Bahado-Singh is professor and chair of obstetrics and gynecology at Oakland University, Rochester, Mich., and health system chair for obstetrics and gynecology at Beaumont Health System.
References
1. Luo S et al. Clin Gastroenterol Hepatol. 2020 Mar 20. doi: 10.1016/j.cgh.2020.03.043.
2. Lechien JR et al. Eur Arch Otorhinolaryngol. 2020 Apr 6. doi: 10.1007/s00405-020-05965-1.
3. Breslin N et al. Am J Obstet Gynecol MFM. 2020 Apr 9. doi: 10.1016/j.ajogmf.2020.100118.
4. Zaigham M, Andersson O. Acta Obstet Gynecol Scand. 2020 Apr 7. doi: 10.1111/aogs.13867.
5. Di Mascio D et al. Am J Obstet Gynecol MFM. 2020 Mar 25. doi: 10.1016/j.ajogmf.2020.100107.
6. Ital J. Pediatr 2020;46(1) doi: 10.1186/s13052-020-0820-x.
7. Int J Gynaecol Obstet. 2020;149(2):130-6.
*This article was updated 5/6/2020.
Obstetrics during the COVID-19 pandemic
The identification of the SARS coronavirus (SARS-CoV-2) and emergence of the associated infectious respiratory disease, COVID-19, in late 2019 catapulted the citizens of the world, especially those in the health care professions, into an era of considerable uncertainty. At this moment in human history, calm reassurance – founded in fact and evidence – seems its greatest need. Much of the focus within the biomedical community has been on containment, prevention, and treatment of this highly contagious and, for some, extremely virulent disease.
However, for ob.gyns on the front lines of the COVID-19 fight, there is the additional challenge of caring for at least two patients simultaneously: the mother and her unborn baby. Studies in mother-baby dyads, while being published at an incredible pace, are still quite scarce. In addition, published reports are limited by the small sample size of the patient population (many are single-case reports), lack of uniformity in the timing and types of clinical samples collected, testing delays, and varying isolation protocols in cases where the mother has confirmed SARS-CoV-2.
Five months into a pandemic that has swept the world, we still know very little about COVID-19 infection in the general population, let alone the obstetric one. We do not know if having and resolving COVID-19 infection provides any long-term protection against future disease. We do not know if vertical transmission of SARS-CoV-2 occurs. We do not know if maternal infection confers any immunologic benefit to the neonate. The list goes on.
What we do know is that taking extra precautions works. Use of personal protective equipment saves health care practitioner and patient lives. Prohibiting or restricting visitors to only one person in hospitals reduces risk of transmission to vulnerable patients.
Additionally, we know that leading with compassion is vital to easing patient – and practitioner – anxiety and stress. Most importantly, we know that people are extraordinarily resilient, especially when it comes to safeguarding the health of their families.
To address some of the major concerns that many ob.gyns. have regarding their risk of coronavirus exposure when caring for patients, we have invited Ray Bahado-Singh, MD, professor and chair of obstetrics and gynecology at Oakland University, Rochester, Mich., and health system chair for obstetrics and gynecology at Beaumont Health System, who works in a suburb of Detroit, one of our nation’s COVID-19 hot spots.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
The identification of the SARS coronavirus (SARS-CoV-2) and emergence of the associated infectious respiratory disease, COVID-19, in late 2019 catapulted the citizens of the world, especially those in the health care professions, into an era of considerable uncertainty. At this moment in human history, calm reassurance – founded in fact and evidence – seems its greatest need. Much of the focus within the biomedical community has been on containment, prevention, and treatment of this highly contagious and, for some, extremely virulent disease.
However, for ob.gyns on the front lines of the COVID-19 fight, there is the additional challenge of caring for at least two patients simultaneously: the mother and her unborn baby. Studies in mother-baby dyads, while being published at an incredible pace, are still quite scarce. In addition, published reports are limited by the small sample size of the patient population (many are single-case reports), lack of uniformity in the timing and types of clinical samples collected, testing delays, and varying isolation protocols in cases where the mother has confirmed SARS-CoV-2.
Five months into a pandemic that has swept the world, we still know very little about COVID-19 infection in the general population, let alone the obstetric one. We do not know if having and resolving COVID-19 infection provides any long-term protection against future disease. We do not know if vertical transmission of SARS-CoV-2 occurs. We do not know if maternal infection confers any immunologic benefit to the neonate. The list goes on.
What we do know is that taking extra precautions works. Use of personal protective equipment saves health care practitioner and patient lives. Prohibiting or restricting visitors to only one person in hospitals reduces risk of transmission to vulnerable patients.
Additionally, we know that leading with compassion is vital to easing patient – and practitioner – anxiety and stress. Most importantly, we know that people are extraordinarily resilient, especially when it comes to safeguarding the health of their families.
To address some of the major concerns that many ob.gyns. have regarding their risk of coronavirus exposure when caring for patients, we have invited Ray Bahado-Singh, MD, professor and chair of obstetrics and gynecology at Oakland University, Rochester, Mich., and health system chair for obstetrics and gynecology at Beaumont Health System, who works in a suburb of Detroit, one of our nation’s COVID-19 hot spots.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
The identification of the SARS coronavirus (SARS-CoV-2) and emergence of the associated infectious respiratory disease, COVID-19, in late 2019 catapulted the citizens of the world, especially those in the health care professions, into an era of considerable uncertainty. At this moment in human history, calm reassurance – founded in fact and evidence – seems its greatest need. Much of the focus within the biomedical community has been on containment, prevention, and treatment of this highly contagious and, for some, extremely virulent disease.
However, for ob.gyns on the front lines of the COVID-19 fight, there is the additional challenge of caring for at least two patients simultaneously: the mother and her unborn baby. Studies in mother-baby dyads, while being published at an incredible pace, are still quite scarce. In addition, published reports are limited by the small sample size of the patient population (many are single-case reports), lack of uniformity in the timing and types of clinical samples collected, testing delays, and varying isolation protocols in cases where the mother has confirmed SARS-CoV-2.
Five months into a pandemic that has swept the world, we still know very little about COVID-19 infection in the general population, let alone the obstetric one. We do not know if having and resolving COVID-19 infection provides any long-term protection against future disease. We do not know if vertical transmission of SARS-CoV-2 occurs. We do not know if maternal infection confers any immunologic benefit to the neonate. The list goes on.
What we do know is that taking extra precautions works. Use of personal protective equipment saves health care practitioner and patient lives. Prohibiting or restricting visitors to only one person in hospitals reduces risk of transmission to vulnerable patients.
Additionally, we know that leading with compassion is vital to easing patient – and practitioner – anxiety and stress. Most importantly, we know that people are extraordinarily resilient, especially when it comes to safeguarding the health of their families.
To address some of the major concerns that many ob.gyns. have regarding their risk of coronavirus exposure when caring for patients, we have invited Ray Bahado-Singh, MD, professor and chair of obstetrics and gynecology at Oakland University, Rochester, Mich., and health system chair for obstetrics and gynecology at Beaumont Health System, who works in a suburb of Detroit, one of our nation’s COVID-19 hot spots.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
Understanding the cervicovaginal microbiome and how it affects preterm birth
Prematurity remains the leading cause of neonatal morbidity and mortality, accounting for $26 billion a year in immediate costs, despite the implementation in obstetrics of a host of risk stratification algorithms and strategies for risk reduction, including the use of some medications.
It now is questionable whether injectable 17-alpha hydroxyprogesterone caproate (Makena) truly is efficacious in women who’ve had a prior spontaneous preterm birth (sPTB) – a Food and Drug Administration advisory committee last year recommended withdrawing it from the market based on results of an FDA confirmatory study. Even if the drug were efficacious, only a small percentage of the women who have an sPTB have had a prior one. The majority of sPTB occurs among women without such a history.
Vaginal progesterone appears to confer some protection in women found to have a short cervix during the second trimester, but this approach also has limited reach: Only 9% of women with sPTB had an antecedent short cervix in a 2017 study.1 Like a history of sPTB, screening for short cervical length is a potentially helpful strategy for risk reduction, but it is not a strategy that will significantly impact the overall rate of prematurity.
We’ve fallen short in our goals to significantly reduce the public health impact of prematurity partly because we still do not understand the exact pathways and mechanisms by which sPTB occurs. The main working paradigm for myself and many other researchers over the past 2 decades has centered on infection in the uterus triggering inflammation, followed by cervical remodeling and ripening. Research in animal models, as well as human clinical trials targeting various infections and inflammation, have led to some insights and discoveries, but no successful interventions.
In the past decade, however, our research framework for understanding sPTB incorporates new questions about immunologic, microbiological, and molecular/cellular events that happen in the cervicovaginal space. We’ve learned more about the cervicovaginal microbiota, and most recently, our research at the University of Pennsylvania has elucidated the role that nonoptimal bacteria play in disrupting the cervical endothelial barrier and initiating the process of cervical remodeling that likely precedes sPTB.
We also know that this association is stronger in black women and may help explain some of the observed racial disparities in sPTB. Although more research is needed to determine specific therapeutic strategies, new doors are open.
Host immune-microbial interactions
This new research paradigm has involved stepping back and asking basic questions, such as, what do we really know about the cervicovaginal space? In actuality, we know very little. We know little about the immune function of the vaginal and cervical epithelial cells in pregnancy, for instance, and there is a large gap in knowledge regarding the biomechanics of the cervix – a remarkable organ that can change shape and function in a matter of minutes. Studies on the biomechanics of the cervix during pregnancy and in labor are still in their infancy.
However, lessons can be drawn from research on inflammatory bowel disease and other disorders involving the gut. In the gastrointestinal tract, epithelial cells have been found to act as sentinels, forming a mucosal barrier against bacterial pathogens and secreting various immune factors. Research in this field also has shown that microbes living in the gut produce metabolites; that these microbial metabolites may be the key messengers from the microbial communities to the epithelial barrier; and that the microbes, microbial metabolites, and immune responses are responsible for triggering inflammatory processes in the tissues underneath.
In 2011, Jacques Ravel, PhD, who was part of the National Institutes of Health’s Human Microbiome Project, characterized the vaginal microbiome of reproductive-age women for the first time.2 His paper classified the vaginal microbial communities of approximately 400 asymptomatic women of various ethnicities into five “community state types” (CSTs) based on the predominant bacteria found in the cervicovaginal space.3
On the heels of his research, Dr. Ravel and I launched an NIH-funded study involving a prospective cohort of 2,000 women with singleton pregnancies – the Motherhood & Microbiome cohort – to look at the cervicovaginal microbiota, the local immune response, and the risk of sPTB.4 Cervicovaginal samples were collected at 16-20 weeks’ gestation and during two subsequent clinical visits. From this cohort, which was composed mostly of African American women (74.5%), we conducted a nested case-controlled study of 103 cases of sPTB and 432 women who delivered at term, matched for race.
We carefully adjudicated the deliveries in our 2,000-person cohort so that we homed in on sPTB as opposed to preterm births that are medically indicated for reasons such as fetal distress or preeclampsia. (Several prior studies looking at the associations between the cervicovaginal microbiome had a heterogeneous phenotyping of PTB that made it hard to draw definitive conclusions.)
Our focus in assessing the microbiome and immunologic profiles was on the samples collected at the earliest time points in pregnancy because we hoped to detect a “signature” that could predict an outcome months later. Indeed, we found that the nonoptimal microbiota, known in microbiological terms as CST IV, was associated with about a 150% increased risk of sPTB. This community comprises a dominant array of anaerobic bacteria and a paucity of Lactobacillus species.
We also found that a larger proportion of African American women, compared with non–African American women, had this nonoptimal microbiota early in pregnancy (40% vs. 15%), which is consistent with previous studies in pregnancy and nonpregnancy showing lower levels of Lactobacillus species in the cervicovaginal microbiome of African American women.
Even more interesting was the finding that, although the rate of sPTB was higher in African American women and the effect of CST IV on sPTB was stronger in these women, the risk of sPTB couldn’t be explained solely by the presence of CST IV. Some women with this nonoptimal microbiome delivered at term, whereas others with more optimal microbiome types had sPTBs. This suggests that other factors contribute to African American women having a nonoptimal microbiota and being especially predisposed to sPTB.
Through the study’s immunologic profiling, we found a significant difference in the cervicovaginal levels of an immune factor, beta-defensin 2, between African American women who delivered at term and those who had a sPTB. Women who had a sPTB, even those who had higher levels of Lactobacillus species, had lower levels of beta-defensin 2. This association was not found in non–African American women.
Beta-defensin 2 is a host-derived antimicrobial peptide that, like other antimicrobial peptides, works at epithelial-mucosal barriers to combat bacteria; we have knowledge of its action from research on the gut, as well as some studies of the vaginal space in nonpregnant women that have focused on sexually transmitted infections.
Most exciting for us was the finding that higher levels of beta-defensin 2 appeared to lower the risk of sPTB in women who had a nonoptimal cervicovaginal microbiota. There’s an interplay between the host and the microbiota, in other words, and it’s one that could be essential to manipulate as we seek to reduce sPTB.
The cervical epithelial barrier
In the laboratory, meanwhile, we are learning how certain microbes are mechanistically involved in the pathogenesis of sPTB. Research over the last decade has suggested that disruption or breakdown of the cervical epithelial barrier drives cervical remodeling processes that precede sPTB. The question now is, do cervicovaginal bacteria associated with sPTB, or a nonoptimal cervicovaginal microbiota, cause disruption of the vaginal and cervical epithelial barrier – and how?
Using an in vitro model system, we found that Mobiluncus curtisii/mulieris, the bacterial taxa with the strongest association with sPTB in our Motherhood & Microbiome cohort and one that has long been associated with bacterial vaginosis, had a plethora of effects. It increased cell permeability and the expression of inflammatory mediators associated with cervical epithelial breakdown, and it altered expression of microRNAs that have been associated with sPTB in human studies.
Our study on Mobiluncus has served as proof of concept to us that, not only is the bacteria associated with sPTB, but that there are multiple mechanisms by which it can disrupt the cervicovaginal barrier and lead to cervical remodeling.5
The findings echo previous in vitro research on Gardnerella vaginalis, another anaerobic bacterium that has been associated with bacterial vaginosis and adverse obstetric outcomes, including sPTB.6 Using similar models, we found that G. vaginalis disrupts the cervical epithelial barrier through diverse mechanisms including the cleavage of certain proteins, the up-regulation of proinflammatory immune mediators, and altered gene expression.
Lactobacillus crispatus, on the other hand, conferred protection to the cervical epithelial barrier in this study by mitigating various G. vaginalis–induced effects.
Learning more about host-microbe interactions and the role of microbial metabolites in these interactions, as well as the role of altered gene expression in cervical function, will help us to more fully understand the biological mechanisms regulating cervicovaginal epithelial cells. At this point, we know that, as in the gut, bacteria commonly found in the cervicovaginal space play a significant role in regulating the function of epithelial cells (in both optimal and nonoptimal microbiota), and that various bacteria associated with sPTB contribute to poor outcomes by breaking down the cervical epithelium.
Therapeutic implications
Our growing knowledge of the cervicovaginal microbiota does not yet support screening or any particular interventions. We don’t know, for instance, that administering probiotics or prebiotics orally or vaginally will have any effect on rates of sPTB.
Ongoing research at all levels holds promise, however, for the development of diagnostics to identify women at risk for sPTB, and for the development of therapeutic strategies that aim to modify the microbiome and/or modify the immune response. We know from other areas of medicine that there are realistic ways to modulate the immune response and/or microbiota in a system to alter risk.
We need to more thoroughly understand the risk of particular microbiota and immune response factors – and how they vary by race and ethnicity – and we need to study the cervicovaginal microbiota of women before and during pregnancy to learn whether there is something about pregnancy or even about intercourse that can change one’s microbiome to a less favorable state.
It may well be possible in the near future to identify high-risk states of nonoptimal microbiota before conception – microbiota that, in and of themselves, may not be pathogenic but that become detrimental during pregnancy – and it should be possible to screen women early in pregnancy for microbial or immune signatures or both.
The question often arises in medicine of the validity of screening without having achieved certainty about treatments. However, in obstetrics, where we have different levels of care and the ability to personalize monitoring and care, identifying those at greatest risk still has value. Ultimately, with enough investment in all levels of research (basic, translational, and clinical), we can develop interventions and therapeutics that address a biologically plausible mechanism of sPTB and, as a result, achieve significant reductions in the rate of prematurity.
Dr. Elovitz is the Hilarie L. Morgan and Mitchell L. Morgan President’s Distinguished Professor in Women’s Health, vice chair of translational research, and director of the Maternal and Child Health Research Center, department of obstetrics and gynecology, at the University of Pennsylvania, Philadelphia. She disclosed holding a patent on a method to determine risk of preterm birth that relates to the microbiome. Email her at obnews@mdedge.com.
References
1. JAMA. 2017 Mar 14;317(10):1047-56.
2. NIH Human Microbiome Project. https://hmpdacc.org/.
3. PNAS. 2011 Mar 15;108 (Supplement 1):4680-7.
4. Nat Commun. 2019 Mar 21. doi: 10.1038/s41467-019-09285-9.
5. Anaerobe. 2019 Nov 21. doi: 10.1016/j.anaerobe.2019.102127.
6. Front Microbiol. 2018 Oct 8. doi: 10.3389/fmicb.2018.02181.
Prematurity remains the leading cause of neonatal morbidity and mortality, accounting for $26 billion a year in immediate costs, despite the implementation in obstetrics of a host of risk stratification algorithms and strategies for risk reduction, including the use of some medications.
It now is questionable whether injectable 17-alpha hydroxyprogesterone caproate (Makena) truly is efficacious in women who’ve had a prior spontaneous preterm birth (sPTB) – a Food and Drug Administration advisory committee last year recommended withdrawing it from the market based on results of an FDA confirmatory study. Even if the drug were efficacious, only a small percentage of the women who have an sPTB have had a prior one. The majority of sPTB occurs among women without such a history.
Vaginal progesterone appears to confer some protection in women found to have a short cervix during the second trimester, but this approach also has limited reach: Only 9% of women with sPTB had an antecedent short cervix in a 2017 study.1 Like a history of sPTB, screening for short cervical length is a potentially helpful strategy for risk reduction, but it is not a strategy that will significantly impact the overall rate of prematurity.
We’ve fallen short in our goals to significantly reduce the public health impact of prematurity partly because we still do not understand the exact pathways and mechanisms by which sPTB occurs. The main working paradigm for myself and many other researchers over the past 2 decades has centered on infection in the uterus triggering inflammation, followed by cervical remodeling and ripening. Research in animal models, as well as human clinical trials targeting various infections and inflammation, have led to some insights and discoveries, but no successful interventions.
In the past decade, however, our research framework for understanding sPTB incorporates new questions about immunologic, microbiological, and molecular/cellular events that happen in the cervicovaginal space. We’ve learned more about the cervicovaginal microbiota, and most recently, our research at the University of Pennsylvania has elucidated the role that nonoptimal bacteria play in disrupting the cervical endothelial barrier and initiating the process of cervical remodeling that likely precedes sPTB.
We also know that this association is stronger in black women and may help explain some of the observed racial disparities in sPTB. Although more research is needed to determine specific therapeutic strategies, new doors are open.
Host immune-microbial interactions
This new research paradigm has involved stepping back and asking basic questions, such as, what do we really know about the cervicovaginal space? In actuality, we know very little. We know little about the immune function of the vaginal and cervical epithelial cells in pregnancy, for instance, and there is a large gap in knowledge regarding the biomechanics of the cervix – a remarkable organ that can change shape and function in a matter of minutes. Studies on the biomechanics of the cervix during pregnancy and in labor are still in their infancy.
However, lessons can be drawn from research on inflammatory bowel disease and other disorders involving the gut. In the gastrointestinal tract, epithelial cells have been found to act as sentinels, forming a mucosal barrier against bacterial pathogens and secreting various immune factors. Research in this field also has shown that microbes living in the gut produce metabolites; that these microbial metabolites may be the key messengers from the microbial communities to the epithelial barrier; and that the microbes, microbial metabolites, and immune responses are responsible for triggering inflammatory processes in the tissues underneath.
In 2011, Jacques Ravel, PhD, who was part of the National Institutes of Health’s Human Microbiome Project, characterized the vaginal microbiome of reproductive-age women for the first time.2 His paper classified the vaginal microbial communities of approximately 400 asymptomatic women of various ethnicities into five “community state types” (CSTs) based on the predominant bacteria found in the cervicovaginal space.3
On the heels of his research, Dr. Ravel and I launched an NIH-funded study involving a prospective cohort of 2,000 women with singleton pregnancies – the Motherhood & Microbiome cohort – to look at the cervicovaginal microbiota, the local immune response, and the risk of sPTB.4 Cervicovaginal samples were collected at 16-20 weeks’ gestation and during two subsequent clinical visits. From this cohort, which was composed mostly of African American women (74.5%), we conducted a nested case-controlled study of 103 cases of sPTB and 432 women who delivered at term, matched for race.
We carefully adjudicated the deliveries in our 2,000-person cohort so that we homed in on sPTB as opposed to preterm births that are medically indicated for reasons such as fetal distress or preeclampsia. (Several prior studies looking at the associations between the cervicovaginal microbiome had a heterogeneous phenotyping of PTB that made it hard to draw definitive conclusions.)
Our focus in assessing the microbiome and immunologic profiles was on the samples collected at the earliest time points in pregnancy because we hoped to detect a “signature” that could predict an outcome months later. Indeed, we found that the nonoptimal microbiota, known in microbiological terms as CST IV, was associated with about a 150% increased risk of sPTB. This community comprises a dominant array of anaerobic bacteria and a paucity of Lactobacillus species.
We also found that a larger proportion of African American women, compared with non–African American women, had this nonoptimal microbiota early in pregnancy (40% vs. 15%), which is consistent with previous studies in pregnancy and nonpregnancy showing lower levels of Lactobacillus species in the cervicovaginal microbiome of African American women.
Even more interesting was the finding that, although the rate of sPTB was higher in African American women and the effect of CST IV on sPTB was stronger in these women, the risk of sPTB couldn’t be explained solely by the presence of CST IV. Some women with this nonoptimal microbiome delivered at term, whereas others with more optimal microbiome types had sPTBs. This suggests that other factors contribute to African American women having a nonoptimal microbiota and being especially predisposed to sPTB.
Through the study’s immunologic profiling, we found a significant difference in the cervicovaginal levels of an immune factor, beta-defensin 2, between African American women who delivered at term and those who had a sPTB. Women who had a sPTB, even those who had higher levels of Lactobacillus species, had lower levels of beta-defensin 2. This association was not found in non–African American women.
Beta-defensin 2 is a host-derived antimicrobial peptide that, like other antimicrobial peptides, works at epithelial-mucosal barriers to combat bacteria; we have knowledge of its action from research on the gut, as well as some studies of the vaginal space in nonpregnant women that have focused on sexually transmitted infections.
Most exciting for us was the finding that higher levels of beta-defensin 2 appeared to lower the risk of sPTB in women who had a nonoptimal cervicovaginal microbiota. There’s an interplay between the host and the microbiota, in other words, and it’s one that could be essential to manipulate as we seek to reduce sPTB.
The cervical epithelial barrier
In the laboratory, meanwhile, we are learning how certain microbes are mechanistically involved in the pathogenesis of sPTB. Research over the last decade has suggested that disruption or breakdown of the cervical epithelial barrier drives cervical remodeling processes that precede sPTB. The question now is, do cervicovaginal bacteria associated with sPTB, or a nonoptimal cervicovaginal microbiota, cause disruption of the vaginal and cervical epithelial barrier – and how?
Using an in vitro model system, we found that Mobiluncus curtisii/mulieris, the bacterial taxa with the strongest association with sPTB in our Motherhood & Microbiome cohort and one that has long been associated with bacterial vaginosis, had a plethora of effects. It increased cell permeability and the expression of inflammatory mediators associated with cervical epithelial breakdown, and it altered expression of microRNAs that have been associated with sPTB in human studies.
Our study on Mobiluncus has served as proof of concept to us that, not only is the bacteria associated with sPTB, but that there are multiple mechanisms by which it can disrupt the cervicovaginal barrier and lead to cervical remodeling.5
The findings echo previous in vitro research on Gardnerella vaginalis, another anaerobic bacterium that has been associated with bacterial vaginosis and adverse obstetric outcomes, including sPTB.6 Using similar models, we found that G. vaginalis disrupts the cervical epithelial barrier through diverse mechanisms including the cleavage of certain proteins, the up-regulation of proinflammatory immune mediators, and altered gene expression.
Lactobacillus crispatus, on the other hand, conferred protection to the cervical epithelial barrier in this study by mitigating various G. vaginalis–induced effects.
Learning more about host-microbe interactions and the role of microbial metabolites in these interactions, as well as the role of altered gene expression in cervical function, will help us to more fully understand the biological mechanisms regulating cervicovaginal epithelial cells. At this point, we know that, as in the gut, bacteria commonly found in the cervicovaginal space play a significant role in regulating the function of epithelial cells (in both optimal and nonoptimal microbiota), and that various bacteria associated with sPTB contribute to poor outcomes by breaking down the cervical epithelium.
Therapeutic implications
Our growing knowledge of the cervicovaginal microbiota does not yet support screening or any particular interventions. We don’t know, for instance, that administering probiotics or prebiotics orally or vaginally will have any effect on rates of sPTB.
Ongoing research at all levels holds promise, however, for the development of diagnostics to identify women at risk for sPTB, and for the development of therapeutic strategies that aim to modify the microbiome and/or modify the immune response. We know from other areas of medicine that there are realistic ways to modulate the immune response and/or microbiota in a system to alter risk.
We need to more thoroughly understand the risk of particular microbiota and immune response factors – and how they vary by race and ethnicity – and we need to study the cervicovaginal microbiota of women before and during pregnancy to learn whether there is something about pregnancy or even about intercourse that can change one’s microbiome to a less favorable state.
It may well be possible in the near future to identify high-risk states of nonoptimal microbiota before conception – microbiota that, in and of themselves, may not be pathogenic but that become detrimental during pregnancy – and it should be possible to screen women early in pregnancy for microbial or immune signatures or both.
The question often arises in medicine of the validity of screening without having achieved certainty about treatments. However, in obstetrics, where we have different levels of care and the ability to personalize monitoring and care, identifying those at greatest risk still has value. Ultimately, with enough investment in all levels of research (basic, translational, and clinical), we can develop interventions and therapeutics that address a biologically plausible mechanism of sPTB and, as a result, achieve significant reductions in the rate of prematurity.
Dr. Elovitz is the Hilarie L. Morgan and Mitchell L. Morgan President’s Distinguished Professor in Women’s Health, vice chair of translational research, and director of the Maternal and Child Health Research Center, department of obstetrics and gynecology, at the University of Pennsylvania, Philadelphia. She disclosed holding a patent on a method to determine risk of preterm birth that relates to the microbiome. Email her at obnews@mdedge.com.
References
1. JAMA. 2017 Mar 14;317(10):1047-56.
2. NIH Human Microbiome Project. https://hmpdacc.org/.
3. PNAS. 2011 Mar 15;108 (Supplement 1):4680-7.
4. Nat Commun. 2019 Mar 21. doi: 10.1038/s41467-019-09285-9.
5. Anaerobe. 2019 Nov 21. doi: 10.1016/j.anaerobe.2019.102127.
6. Front Microbiol. 2018 Oct 8. doi: 10.3389/fmicb.2018.02181.
Prematurity remains the leading cause of neonatal morbidity and mortality, accounting for $26 billion a year in immediate costs, despite the implementation in obstetrics of a host of risk stratification algorithms and strategies for risk reduction, including the use of some medications.
It now is questionable whether injectable 17-alpha hydroxyprogesterone caproate (Makena) truly is efficacious in women who’ve had a prior spontaneous preterm birth (sPTB) – a Food and Drug Administration advisory committee last year recommended withdrawing it from the market based on results of an FDA confirmatory study. Even if the drug were efficacious, only a small percentage of the women who have an sPTB have had a prior one. The majority of sPTB occurs among women without such a history.
Vaginal progesterone appears to confer some protection in women found to have a short cervix during the second trimester, but this approach also has limited reach: Only 9% of women with sPTB had an antecedent short cervix in a 2017 study.1 Like a history of sPTB, screening for short cervical length is a potentially helpful strategy for risk reduction, but it is not a strategy that will significantly impact the overall rate of prematurity.
We’ve fallen short in our goals to significantly reduce the public health impact of prematurity partly because we still do not understand the exact pathways and mechanisms by which sPTB occurs. The main working paradigm for myself and many other researchers over the past 2 decades has centered on infection in the uterus triggering inflammation, followed by cervical remodeling and ripening. Research in animal models, as well as human clinical trials targeting various infections and inflammation, have led to some insights and discoveries, but no successful interventions.
In the past decade, however, our research framework for understanding sPTB incorporates new questions about immunologic, microbiological, and molecular/cellular events that happen in the cervicovaginal space. We’ve learned more about the cervicovaginal microbiota, and most recently, our research at the University of Pennsylvania has elucidated the role that nonoptimal bacteria play in disrupting the cervical endothelial barrier and initiating the process of cervical remodeling that likely precedes sPTB.
We also know that this association is stronger in black women and may help explain some of the observed racial disparities in sPTB. Although more research is needed to determine specific therapeutic strategies, new doors are open.
Host immune-microbial interactions
This new research paradigm has involved stepping back and asking basic questions, such as, what do we really know about the cervicovaginal space? In actuality, we know very little. We know little about the immune function of the vaginal and cervical epithelial cells in pregnancy, for instance, and there is a large gap in knowledge regarding the biomechanics of the cervix – a remarkable organ that can change shape and function in a matter of minutes. Studies on the biomechanics of the cervix during pregnancy and in labor are still in their infancy.
However, lessons can be drawn from research on inflammatory bowel disease and other disorders involving the gut. In the gastrointestinal tract, epithelial cells have been found to act as sentinels, forming a mucosal barrier against bacterial pathogens and secreting various immune factors. Research in this field also has shown that microbes living in the gut produce metabolites; that these microbial metabolites may be the key messengers from the microbial communities to the epithelial barrier; and that the microbes, microbial metabolites, and immune responses are responsible for triggering inflammatory processes in the tissues underneath.
In 2011, Jacques Ravel, PhD, who was part of the National Institutes of Health’s Human Microbiome Project, characterized the vaginal microbiome of reproductive-age women for the first time.2 His paper classified the vaginal microbial communities of approximately 400 asymptomatic women of various ethnicities into five “community state types” (CSTs) based on the predominant bacteria found in the cervicovaginal space.3
On the heels of his research, Dr. Ravel and I launched an NIH-funded study involving a prospective cohort of 2,000 women with singleton pregnancies – the Motherhood & Microbiome cohort – to look at the cervicovaginal microbiota, the local immune response, and the risk of sPTB.4 Cervicovaginal samples were collected at 16-20 weeks’ gestation and during two subsequent clinical visits. From this cohort, which was composed mostly of African American women (74.5%), we conducted a nested case-controlled study of 103 cases of sPTB and 432 women who delivered at term, matched for race.
We carefully adjudicated the deliveries in our 2,000-person cohort so that we homed in on sPTB as opposed to preterm births that are medically indicated for reasons such as fetal distress or preeclampsia. (Several prior studies looking at the associations between the cervicovaginal microbiome had a heterogeneous phenotyping of PTB that made it hard to draw definitive conclusions.)
Our focus in assessing the microbiome and immunologic profiles was on the samples collected at the earliest time points in pregnancy because we hoped to detect a “signature” that could predict an outcome months later. Indeed, we found that the nonoptimal microbiota, known in microbiological terms as CST IV, was associated with about a 150% increased risk of sPTB. This community comprises a dominant array of anaerobic bacteria and a paucity of Lactobacillus species.
We also found that a larger proportion of African American women, compared with non–African American women, had this nonoptimal microbiota early in pregnancy (40% vs. 15%), which is consistent with previous studies in pregnancy and nonpregnancy showing lower levels of Lactobacillus species in the cervicovaginal microbiome of African American women.
Even more interesting was the finding that, although the rate of sPTB was higher in African American women and the effect of CST IV on sPTB was stronger in these women, the risk of sPTB couldn’t be explained solely by the presence of CST IV. Some women with this nonoptimal microbiome delivered at term, whereas others with more optimal microbiome types had sPTBs. This suggests that other factors contribute to African American women having a nonoptimal microbiota and being especially predisposed to sPTB.
Through the study’s immunologic profiling, we found a significant difference in the cervicovaginal levels of an immune factor, beta-defensin 2, between African American women who delivered at term and those who had a sPTB. Women who had a sPTB, even those who had higher levels of Lactobacillus species, had lower levels of beta-defensin 2. This association was not found in non–African American women.
Beta-defensin 2 is a host-derived antimicrobial peptide that, like other antimicrobial peptides, works at epithelial-mucosal barriers to combat bacteria; we have knowledge of its action from research on the gut, as well as some studies of the vaginal space in nonpregnant women that have focused on sexually transmitted infections.
Most exciting for us was the finding that higher levels of beta-defensin 2 appeared to lower the risk of sPTB in women who had a nonoptimal cervicovaginal microbiota. There’s an interplay between the host and the microbiota, in other words, and it’s one that could be essential to manipulate as we seek to reduce sPTB.
The cervical epithelial barrier
In the laboratory, meanwhile, we are learning how certain microbes are mechanistically involved in the pathogenesis of sPTB. Research over the last decade has suggested that disruption or breakdown of the cervical epithelial barrier drives cervical remodeling processes that precede sPTB. The question now is, do cervicovaginal bacteria associated with sPTB, or a nonoptimal cervicovaginal microbiota, cause disruption of the vaginal and cervical epithelial barrier – and how?
Using an in vitro model system, we found that Mobiluncus curtisii/mulieris, the bacterial taxa with the strongest association with sPTB in our Motherhood & Microbiome cohort and one that has long been associated with bacterial vaginosis, had a plethora of effects. It increased cell permeability and the expression of inflammatory mediators associated with cervical epithelial breakdown, and it altered expression of microRNAs that have been associated with sPTB in human studies.
Our study on Mobiluncus has served as proof of concept to us that, not only is the bacteria associated with sPTB, but that there are multiple mechanisms by which it can disrupt the cervicovaginal barrier and lead to cervical remodeling.5
The findings echo previous in vitro research on Gardnerella vaginalis, another anaerobic bacterium that has been associated with bacterial vaginosis and adverse obstetric outcomes, including sPTB.6 Using similar models, we found that G. vaginalis disrupts the cervical epithelial barrier through diverse mechanisms including the cleavage of certain proteins, the up-regulation of proinflammatory immune mediators, and altered gene expression.
Lactobacillus crispatus, on the other hand, conferred protection to the cervical epithelial barrier in this study by mitigating various G. vaginalis–induced effects.
Learning more about host-microbe interactions and the role of microbial metabolites in these interactions, as well as the role of altered gene expression in cervical function, will help us to more fully understand the biological mechanisms regulating cervicovaginal epithelial cells. At this point, we know that, as in the gut, bacteria commonly found in the cervicovaginal space play a significant role in regulating the function of epithelial cells (in both optimal and nonoptimal microbiota), and that various bacteria associated with sPTB contribute to poor outcomes by breaking down the cervical epithelium.
Therapeutic implications
Our growing knowledge of the cervicovaginal microbiota does not yet support screening or any particular interventions. We don’t know, for instance, that administering probiotics or prebiotics orally or vaginally will have any effect on rates of sPTB.
Ongoing research at all levels holds promise, however, for the development of diagnostics to identify women at risk for sPTB, and for the development of therapeutic strategies that aim to modify the microbiome and/or modify the immune response. We know from other areas of medicine that there are realistic ways to modulate the immune response and/or microbiota in a system to alter risk.
We need to more thoroughly understand the risk of particular microbiota and immune response factors – and how they vary by race and ethnicity – and we need to study the cervicovaginal microbiota of women before and during pregnancy to learn whether there is something about pregnancy or even about intercourse that can change one’s microbiome to a less favorable state.
It may well be possible in the near future to identify high-risk states of nonoptimal microbiota before conception – microbiota that, in and of themselves, may not be pathogenic but that become detrimental during pregnancy – and it should be possible to screen women early in pregnancy for microbial or immune signatures or both.
The question often arises in medicine of the validity of screening without having achieved certainty about treatments. However, in obstetrics, where we have different levels of care and the ability to personalize monitoring and care, identifying those at greatest risk still has value. Ultimately, with enough investment in all levels of research (basic, translational, and clinical), we can develop interventions and therapeutics that address a biologically plausible mechanism of sPTB and, as a result, achieve significant reductions in the rate of prematurity.
Dr. Elovitz is the Hilarie L. Morgan and Mitchell L. Morgan President’s Distinguished Professor in Women’s Health, vice chair of translational research, and director of the Maternal and Child Health Research Center, department of obstetrics and gynecology, at the University of Pennsylvania, Philadelphia. She disclosed holding a patent on a method to determine risk of preterm birth that relates to the microbiome. Email her at obnews@mdedge.com.
References
1. JAMA. 2017 Mar 14;317(10):1047-56.
2. NIH Human Microbiome Project. https://hmpdacc.org/.
3. PNAS. 2011 Mar 15;108 (Supplement 1):4680-7.
4. Nat Commun. 2019 Mar 21. doi: 10.1038/s41467-019-09285-9.
5. Anaerobe. 2019 Nov 21. doi: 10.1016/j.anaerobe.2019.102127.
6. Front Microbiol. 2018 Oct 8. doi: 10.3389/fmicb.2018.02181.
Preterm birth: Under the microscope
Preventing infant mortality remains a significant challenge for ob.gyns. Despite the availability of a multitude of preventive and treatment options and some of the best possible medical care offered in the world, the United States lags behind many other developed and developing countries in its rate of infant deaths, which was an estimated 5.8 deaths per 1,000 live births in 2017. We can, and must, do better.
One of the major contributing factors to infant mortality is preterm birth. Defined as birth occurring prior to 37 weeks’ gestation, preterm birth is associated with a myriad of severe neonatal sequelae: low birth weight, bacterial sepsis, neonatal hemorrhage, and respiratory distress syndrome, among others. Therefore, many within the clinical and biomedical research spheres recognize that preventing preterm birth means reducing infant deaths.
However, therein lies the conundrum. We know very little about what causes preterm birth, which renders the current therapeutic strategies – such as use of progesterone supplements or cerclage placement – good for some but not all patients. It is thus vital to continue research to unravel the underlying mechanisms of preterm birth.
A promising area of investigation is the field of microbiome research, which has made great strides in advancing our awareness of the critical role of the millions of organisms living on and within us in maintaining health and fighting disease. For example, we now realize that eradicating all the commensals in our gastrointestinal tract has unintended and very negative consequences and, for patients whose good bacteria have been eliminated, fecal transplant is a therapeutic option. Therefore, it stands to reason that the microbes found in the vagina contribute significantly to women’s overall reproductive health.
The publication of the groundbreaking study characterizing the vaginal microbiome species in reproductive-age women opened new avenues of research into how these organisms contribute to women’s health. Importantly, this work, led initially by Jacques Ravel, PhD, a professor in the department of microbiology & immunology and associate director of the Institute for Genome Sciences at the University of Maryland School of Medicine, has spawned additional investigations into the potential role of the vaginal microbiome in preterm birth.
To provide some insight into the research around how the microorganisms in the vagina may induce or prevent preterm birth is our guest author, Michal A. Elovitz, MD, the Hilarie L. Morgan and Mitchell L. Morgan President’s Distinguished Professor in Women’s Health, vice chair of translational research, and director of the Maternal and Child Health Research Center, department of obstetrics and gynecology, at the University of Pennsylvania, Philadelphia.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
Preventing infant mortality remains a significant challenge for ob.gyns. Despite the availability of a multitude of preventive and treatment options and some of the best possible medical care offered in the world, the United States lags behind many other developed and developing countries in its rate of infant deaths, which was an estimated 5.8 deaths per 1,000 live births in 2017. We can, and must, do better.
One of the major contributing factors to infant mortality is preterm birth. Defined as birth occurring prior to 37 weeks’ gestation, preterm birth is associated with a myriad of severe neonatal sequelae: low birth weight, bacterial sepsis, neonatal hemorrhage, and respiratory distress syndrome, among others. Therefore, many within the clinical and biomedical research spheres recognize that preventing preterm birth means reducing infant deaths.
However, therein lies the conundrum. We know very little about what causes preterm birth, which renders the current therapeutic strategies – such as use of progesterone supplements or cerclage placement – good for some but not all patients. It is thus vital to continue research to unravel the underlying mechanisms of preterm birth.
A promising area of investigation is the field of microbiome research, which has made great strides in advancing our awareness of the critical role of the millions of organisms living on and within us in maintaining health and fighting disease. For example, we now realize that eradicating all the commensals in our gastrointestinal tract has unintended and very negative consequences and, for patients whose good bacteria have been eliminated, fecal transplant is a therapeutic option. Therefore, it stands to reason that the microbes found in the vagina contribute significantly to women’s overall reproductive health.
The publication of the groundbreaking study characterizing the vaginal microbiome species in reproductive-age women opened new avenues of research into how these organisms contribute to women’s health. Importantly, this work, led initially by Jacques Ravel, PhD, a professor in the department of microbiology & immunology and associate director of the Institute for Genome Sciences at the University of Maryland School of Medicine, has spawned additional investigations into the potential role of the vaginal microbiome in preterm birth.
To provide some insight into the research around how the microorganisms in the vagina may induce or prevent preterm birth is our guest author, Michal A. Elovitz, MD, the Hilarie L. Morgan and Mitchell L. Morgan President’s Distinguished Professor in Women’s Health, vice chair of translational research, and director of the Maternal and Child Health Research Center, department of obstetrics and gynecology, at the University of Pennsylvania, Philadelphia.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
Preventing infant mortality remains a significant challenge for ob.gyns. Despite the availability of a multitude of preventive and treatment options and some of the best possible medical care offered in the world, the United States lags behind many other developed and developing countries in its rate of infant deaths, which was an estimated 5.8 deaths per 1,000 live births in 2017. We can, and must, do better.
One of the major contributing factors to infant mortality is preterm birth. Defined as birth occurring prior to 37 weeks’ gestation, preterm birth is associated with a myriad of severe neonatal sequelae: low birth weight, bacterial sepsis, neonatal hemorrhage, and respiratory distress syndrome, among others. Therefore, many within the clinical and biomedical research spheres recognize that preventing preterm birth means reducing infant deaths.
However, therein lies the conundrum. We know very little about what causes preterm birth, which renders the current therapeutic strategies – such as use of progesterone supplements or cerclage placement – good for some but not all patients. It is thus vital to continue research to unravel the underlying mechanisms of preterm birth.
A promising area of investigation is the field of microbiome research, which has made great strides in advancing our awareness of the critical role of the millions of organisms living on and within us in maintaining health and fighting disease. For example, we now realize that eradicating all the commensals in our gastrointestinal tract has unintended and very negative consequences and, for patients whose good bacteria have been eliminated, fecal transplant is a therapeutic option. Therefore, it stands to reason that the microbes found in the vagina contribute significantly to women’s overall reproductive health.
The publication of the groundbreaking study characterizing the vaginal microbiome species in reproductive-age women opened new avenues of research into how these organisms contribute to women’s health. Importantly, this work, led initially by Jacques Ravel, PhD, a professor in the department of microbiology & immunology and associate director of the Institute for Genome Sciences at the University of Maryland School of Medicine, has spawned additional investigations into the potential role of the vaginal microbiome in preterm birth.
To provide some insight into the research around how the microorganisms in the vagina may induce or prevent preterm birth is our guest author, Michal A. Elovitz, MD, the Hilarie L. Morgan and Mitchell L. Morgan President’s Distinguished Professor in Women’s Health, vice chair of translational research, and director of the Maternal and Child Health Research Center, department of obstetrics and gynecology, at the University of Pennsylvania, Philadelphia.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
The removal of the multiple-kilogram uterus using MIGSs
It has now been 30 years since the first total laparoscopic hysterectomy was performed. The benefits of minimally invasive gynecologic surgery (MIGS) – and of minimally invasive hysterectomy specifically – are now well documented. Since this milestone procedure, both instrumentation and technique have improved significantly.
This includes traditional laparoscopy, as well as the robotically assisted laparoscopic approach. However, certain patient characteristics also may influence the choice. A uterus that is undescended, combined with a narrow introitus, for instance, can be a contributory factor in choosing to perform laparoscopic hysterectomy. Additionally, so can an extremely large uterus and an extremely high body mass index (BMI).
These latter two factors – a very large uterus (which we define as more than 15-16 weeks’ gestational size) and a BMI over 60 kg/m2 – historically were considered to be contraindications to laparoscopic hysterectomy. But as the proficiency, comfort, and skill of a new generation of laparoscopic surgeons increases, the tide is shifting with respect to both morbid obesity and the very large uterus.
With growing experience and improved instrumentation, the majority of gynecologists who are fellowship-trained in MIGS are able to routinely and safely perform laparoscopic hysterectomy for uteri weighing 1-2 kg and in patients who have extreme morbid obesity. The literature, moreover, increasingly features case reports of laparoscopic removal of very large uteri and reviews/discussions of total laparoscopic hysterectomy being feasible.
In our own experience, total laparoscopic hysterectomy (TLH) of the very large uterus can be safely and advantageously performed using key instruments and refinements in technique, as well as thorough patient counseling regarding the risk of unexpected sarcomas. Recently, we safely performed total laparoscopic hysterectomy for a patient with a uterus that – somewhat unexpectedly – weighed 7.4 kg.
Surgical pearls
Performing safe and effective total laparoscopic hysterectomy for large uteri – and for morbidly obese patients – hinges largely on modifications in entry and port placement, patient positioning, and choice of instrumentation. With these modifications, we can achieve adequate visualization of critical anatomy and can minimize bleeding. Otherwise, the surgery itself is largely the same. Here are the principles we find most helpful.
Entry and port placement
Traditionally, for TLHs, a camera port is placed at the umbilicus to provide a full view of the pelvis. For the larger uterus – and in women who are extremely obese – we aim to introduce the laparoscope higher. A reliable landmark is the Palmer’s point in the left upper quadrant. From here, we can identify areas for the placement of additional trocars.
In general, we place ancillary 5-mm ports more cephalad and lateral to the uterus than we otherwise would. Such placement facilitates effective visualization while accommodating manipulation of the uterus and allows us to avoid bleeding around the vascular upper pedicles. Overall, we have much better control through all parts of the surgery when we operate lateral to the uterus.
Patient positioning
In addition to the Trendelenburg position, we have adopted an “airplaning” technique for patients with a very large uterus in which the bed is tilted from side to side so that the left and right sides of the body are rotated upward as needed. This allows for gravitational-assisted retraction when it otherwise is not possible.
Instrumentation
For morbidly obese patients, we use Kii Fios advanced fixation trocars. These come in 5- and 10-mm sizes and are equipped with an intraperitoneal balloon that can be inflated to prevent sliding of the trocar out of the abdominal wall.
By far the most valuable instrument for the morbidly obese and the very large uterus is a 30-degree laparoscope. With our higher port placement as described, the 30-degree scope provides visualization of critical structures that wouldn’t be possible with a 0-degree scope.
The Rumi uterine manipulator comes with cups that come in different sizes and can fit around the cervix and help delineate the cervicouterine junction. We use this manipulator for all laparoscopic hysterectomies, but it is a must for the very large uterus.
Extensive desiccation of the utero-ovarian pedicles and uterine arteries is critical, and for this we advise using the rotating bipolar RoBi instrument. Use of the conventional bipolar instrument allows us to use targeted and anatomically guided application of energy. This ensures certainty that vessels whose limits exceed the diameter for advanced bipolar devices (typically 7 mm) are completely sealed. In-depth knowledge of pelvic anatomy and advanced laparoscopic dissection is paramount during these steps to ensure that vital structures are not damaged by the wider thermal spread of the traditional bipolar device. For cutting, the use of ultrasonic energy is important to prevent energy from spreading laterally.
Lastly, we recommend a good suction irrigator because, if bleeding occurs, it tends to be heavy because of the enlarged nature of feeding vasculature. When placed through an umbilical or suprapubic port, the suction irrigator also may be used to help with the rotational vectors and traction for further uterine manipulation.
Technique
We usually operate from top to bottom, transecting the upper pedicles such as the infundibulopelvic (IP) ligaments or utero-ovarian ligaments first, rather than the round ligaments. This helps us achieve additional mobility of the uterus. Some surgeons believe that retroperitoneal dissection and ligation of the uterine arteries at their origin is essential, but we find that, with good uterine manipulation and the use of a 30-degree scope, we achieve adequate visualization for identifying the ureter and uterine artery on the sidewall and consequently do not need to dissect retroperitoneally.
When using the uterine manipulator with the colpotomy cup, the uterus is pushed upward, increasing the distance between the vaginal fornix and the ureters. Uterine arteries can easily be identified and desiccated using conventional bipolar energy. When the colpotomy cup is pushed cephalad, the application of the bipolar energy within the limits of the cup is safe. The thermal spread does not pose a threat to the ureters, which are displaced 1.5-2 cm laterally. Large fibroids often contribute to distorted anatomical planes, and a colpotomy cup provides a firm palpable surface between the cervix and vagina during dissection.
When dealing with large uteri, one must sometimes think outside the box and deviate from standard technique. For instance, in patients with distorted anatomy because of large fibroids, it helps to first control the pedicles that are most easily accessible. Sometimes it is acceptable to perform oophorectomy if the IP ligament is more accessible and the utero-ovarian pedicle is distorted by dilated veins and adherent to the uterus. After transection of each pedicle, we gain more mobility of the uterus and better visualization for the next step.
Inserting the camera through ancillary ports – a technique known as “port hopping” – helps to visualize and take down adhesions much better and more safely than using the camera from the umbilical port only. Port hopping with a 30-degree laparoscope helps to obtain a 360-degree view of adhesions and anatomy, which is exceedingly helpful in cases in which crucial anatomical structures are within close proximity of one another.
In general it is more challenging to perform TLH on a patient with a broad uterus or a patient with low posterior fibroids that are occupying the pelvis than on a patient with fibroids in the upper abdomen. The main challenge for the surgeon is to safely secure the uterine arteries and control the blood supply to the uterus.
Access to the pelvic sidewall is obtained with the combination of 30-degree scope, uterine manipulator, and the suction irrigator introduced through the midline port; the cervix and uterus are deviated upward. Instead of the suction irrigator or blunt dissector used for internal uterine manipulation, some surgeons use myoma screws or a 5-mm single-tooth tenaculum to manipulate a large uterus. Both of those instruments are valuable and work well, but often a large uterus requires extensive manipulation. Repositioning of any sharp instruments that pierce the serosa can often lead to additional blood loss. It is preferable to avoid this blood loss on a large uterus at all costs because it can be brisk and stains the surgical pedicles, making the remainder of the procedure unnecessarily difficult.
Once the uterine arteries are desiccated, if fibroids are obscuring the view, the corpus of the uterus can be detached from the cervix as in supracervical hysterectomy fashion. From there, the uterus can be placed in the upper abdomen while colpotomy can be performed.
In patients with multiple fibroids, we do not recommend performing myomectomy first, unless the fibroid is pedunculated and on a very small stalk. Improved uterine manipulation and retroperitoneal dissection are preferred over myomectomy to safely complete hysterectomy for the broad uterus. In our opinion, any attempt at myomectomy would lead to unnecessary blood loss and additional operative time with minimal benefit.
In patients with fibroids that grow into the broad ligament and pelvic sidewall, the natural course of the ureter becomes displaced laterally. This is contrary to the popular misconception that the ureter is more medially located in the setting of broad-ligament fibroids. To ensure safe access to the uterine arteries, the vesicouterine peritoneum can be incised and extended cephalad along the broad ligament and, then, using the above-mentioned technique, by pushing the uterus and the fibroid to the contralateral side via the suction irrigator, the uterine arteries can be easily accessed.
Another useful technique is to use diluted vasopressin injected into the lower pole of the uterus to cause vasoconstriction and minimize the bleeding. The concentration is 1 cc of 20 units of vasopressin in 100-400 cc of saline. This technique is very useful for myomectomies, and some surgeons find it also helpful for hysterectomy. The plasma half-life of vasopressin is 10-20 minutes, and a large quantity is needed to help with vasoconstriction in a big uterus. The safe upper limits of vasopressin dosing are not firmly established. A fibroid uterus with aberrant vasculature may require a greater-than-acceptable dose to control bleeding.
It is important to ensure that patients have an optimized hemoglobin level preoperatively. We use a hemoglobin level of 8 g/dL as a lowest cutoff value for performing TLH without preoperative transfusion. Regarding bowel preparation, neither the literature nor our own experience support its value, so we typically do not use it.
Morcellation and patient counseling
Uteri up to 12 weeks’ gestational size usually can be extracted transvaginally, and most uteri regardless of size can be morcellated and extracted through the vagina, providing that the vaginal fornix is accessible from below. In some cases, such as when the apex is too high, a minilaparotomic incision is needed to extract the uterus, or when available, power morcellation can be performed.
A major challenge, given our growing ability to laparoscopically remove very larger uteri, is that uteri heavier than about 2.5 kg in weight cannot be morcellated inside a morcellation bag. The risk of upstaging a known or suspected uterine malignancy, or of spreading an unknown malignant sarcoma (presumed benign myoma), should be incorporated in each patient’s decision making.
Thorough counseling about surgical options and on the risks of morcellating a very large uterus without containment in a bag is essential. Each patient must understand the risks and decide whether the benefits of minimally invasive surgery outweigh these risks. While MRI can sometimes provide increased suspicion of a leiomyosarcoma, malignancy can never be completed excluded preoperatively.
Removal of a 7.4-kg uterus
Our patient was a 44-year-old with a markedly enlarged fibroid uterus. Having been told by other providers that she was not a candidate for minimally invasive hysterectomy, she had delayed surgical management for a number of years, allowing for such a generous uterine size to develop.
The patient was knowledgeable about her condition and, given her comorbid obesity, she requested a minimally invasive approach. Preoperative imaging included an ultrasound, which had to be completed abdominally because of the size of her uterus, and an additional MRI was needed to further characterize the extent and nature of her uterus. A very detailed discussion regarding risk of leiomyosarcoma, operative complications, and conversion to laparotomy ensued.
Intraoperatively, we placed the first 5 mm port in the left upper quadrant initially to survey the anatomy for feasibility of laparoscopic hysterectomy. The left utero-ovarian pedicle was easily viewed by airplaning the bed alone. While the right utero-ovarian pedicle was much more skewed and enlarged, the right IP was easily accessible and the ureter well visualized.
The decision was made to place additional ports and proceed with laparoscopic hysterectomy. The 5-mm assistant ports were placed lateral and directly above the upper vascular pedicles. Operative time was 4 hours and 12 minutes, and blood loss was only 700 cc. Her preoperative hemoglobin was optimized at 13.3 g/dL and dropped to 11.3 g/dL postoperatively. The patient was discharged home the next morning and had a normal recovery with no complications.
Dr. Pasic is professor of obstetrics, gynecology & women’s health; director of the section of advanced gynecologic endoscopy; and codirector of the AAGL fellowship in minimally invasive gynecologic surgery at the University of Louisville (Ky.). Dr. Pasic is the current president of the International Society of Gynecologic Endoscopy. He is also a past president of the AAGL (2009). Dr. Cesta is Dr. Pasic’s current fellow in minimally invasive gynecologic surgery as well as an instructor in obstetrics and gynecology at the University of Louisville. Dr. Pasic disclosed he is a consultant for Ethicon Endo, Medtronic, and Olympus and is a speaker for Cooper Surgical, which manufactures some of the instruments mentioned in this article. Dr. Cesta had no relevant financial disclosures.
It has now been 30 years since the first total laparoscopic hysterectomy was performed. The benefits of minimally invasive gynecologic surgery (MIGS) – and of minimally invasive hysterectomy specifically – are now well documented. Since this milestone procedure, both instrumentation and technique have improved significantly.
This includes traditional laparoscopy, as well as the robotically assisted laparoscopic approach. However, certain patient characteristics also may influence the choice. A uterus that is undescended, combined with a narrow introitus, for instance, can be a contributory factor in choosing to perform laparoscopic hysterectomy. Additionally, so can an extremely large uterus and an extremely high body mass index (BMI).
These latter two factors – a very large uterus (which we define as more than 15-16 weeks’ gestational size) and a BMI over 60 kg/m2 – historically were considered to be contraindications to laparoscopic hysterectomy. But as the proficiency, comfort, and skill of a new generation of laparoscopic surgeons increases, the tide is shifting with respect to both morbid obesity and the very large uterus.
With growing experience and improved instrumentation, the majority of gynecologists who are fellowship-trained in MIGS are able to routinely and safely perform laparoscopic hysterectomy for uteri weighing 1-2 kg and in patients who have extreme morbid obesity. The literature, moreover, increasingly features case reports of laparoscopic removal of very large uteri and reviews/discussions of total laparoscopic hysterectomy being feasible.
In our own experience, total laparoscopic hysterectomy (TLH) of the very large uterus can be safely and advantageously performed using key instruments and refinements in technique, as well as thorough patient counseling regarding the risk of unexpected sarcomas. Recently, we safely performed total laparoscopic hysterectomy for a patient with a uterus that – somewhat unexpectedly – weighed 7.4 kg.
Surgical pearls
Performing safe and effective total laparoscopic hysterectomy for large uteri – and for morbidly obese patients – hinges largely on modifications in entry and port placement, patient positioning, and choice of instrumentation. With these modifications, we can achieve adequate visualization of critical anatomy and can minimize bleeding. Otherwise, the surgery itself is largely the same. Here are the principles we find most helpful.
Entry and port placement
Traditionally, for TLHs, a camera port is placed at the umbilicus to provide a full view of the pelvis. For the larger uterus – and in women who are extremely obese – we aim to introduce the laparoscope higher. A reliable landmark is the Palmer’s point in the left upper quadrant. From here, we can identify areas for the placement of additional trocars.
In general, we place ancillary 5-mm ports more cephalad and lateral to the uterus than we otherwise would. Such placement facilitates effective visualization while accommodating manipulation of the uterus and allows us to avoid bleeding around the vascular upper pedicles. Overall, we have much better control through all parts of the surgery when we operate lateral to the uterus.
Patient positioning
In addition to the Trendelenburg position, we have adopted an “airplaning” technique for patients with a very large uterus in which the bed is tilted from side to side so that the left and right sides of the body are rotated upward as needed. This allows for gravitational-assisted retraction when it otherwise is not possible.
Instrumentation
For morbidly obese patients, we use Kii Fios advanced fixation trocars. These come in 5- and 10-mm sizes and are equipped with an intraperitoneal balloon that can be inflated to prevent sliding of the trocar out of the abdominal wall.
By far the most valuable instrument for the morbidly obese and the very large uterus is a 30-degree laparoscope. With our higher port placement as described, the 30-degree scope provides visualization of critical structures that wouldn’t be possible with a 0-degree scope.
The Rumi uterine manipulator comes with cups that come in different sizes and can fit around the cervix and help delineate the cervicouterine junction. We use this manipulator for all laparoscopic hysterectomies, but it is a must for the very large uterus.
Extensive desiccation of the utero-ovarian pedicles and uterine arteries is critical, and for this we advise using the rotating bipolar RoBi instrument. Use of the conventional bipolar instrument allows us to use targeted and anatomically guided application of energy. This ensures certainty that vessels whose limits exceed the diameter for advanced bipolar devices (typically 7 mm) are completely sealed. In-depth knowledge of pelvic anatomy and advanced laparoscopic dissection is paramount during these steps to ensure that vital structures are not damaged by the wider thermal spread of the traditional bipolar device. For cutting, the use of ultrasonic energy is important to prevent energy from spreading laterally.
Lastly, we recommend a good suction irrigator because, if bleeding occurs, it tends to be heavy because of the enlarged nature of feeding vasculature. When placed through an umbilical or suprapubic port, the suction irrigator also may be used to help with the rotational vectors and traction for further uterine manipulation.
Technique
We usually operate from top to bottom, transecting the upper pedicles such as the infundibulopelvic (IP) ligaments or utero-ovarian ligaments first, rather than the round ligaments. This helps us achieve additional mobility of the uterus. Some surgeons believe that retroperitoneal dissection and ligation of the uterine arteries at their origin is essential, but we find that, with good uterine manipulation and the use of a 30-degree scope, we achieve adequate visualization for identifying the ureter and uterine artery on the sidewall and consequently do not need to dissect retroperitoneally.
When using the uterine manipulator with the colpotomy cup, the uterus is pushed upward, increasing the distance between the vaginal fornix and the ureters. Uterine arteries can easily be identified and desiccated using conventional bipolar energy. When the colpotomy cup is pushed cephalad, the application of the bipolar energy within the limits of the cup is safe. The thermal spread does not pose a threat to the ureters, which are displaced 1.5-2 cm laterally. Large fibroids often contribute to distorted anatomical planes, and a colpotomy cup provides a firm palpable surface between the cervix and vagina during dissection.
When dealing with large uteri, one must sometimes think outside the box and deviate from standard technique. For instance, in patients with distorted anatomy because of large fibroids, it helps to first control the pedicles that are most easily accessible. Sometimes it is acceptable to perform oophorectomy if the IP ligament is more accessible and the utero-ovarian pedicle is distorted by dilated veins and adherent to the uterus. After transection of each pedicle, we gain more mobility of the uterus and better visualization for the next step.
Inserting the camera through ancillary ports – a technique known as “port hopping” – helps to visualize and take down adhesions much better and more safely than using the camera from the umbilical port only. Port hopping with a 30-degree laparoscope helps to obtain a 360-degree view of adhesions and anatomy, which is exceedingly helpful in cases in which crucial anatomical structures are within close proximity of one another.
In general it is more challenging to perform TLH on a patient with a broad uterus or a patient with low posterior fibroids that are occupying the pelvis than on a patient with fibroids in the upper abdomen. The main challenge for the surgeon is to safely secure the uterine arteries and control the blood supply to the uterus.
Access to the pelvic sidewall is obtained with the combination of 30-degree scope, uterine manipulator, and the suction irrigator introduced through the midline port; the cervix and uterus are deviated upward. Instead of the suction irrigator or blunt dissector used for internal uterine manipulation, some surgeons use myoma screws or a 5-mm single-tooth tenaculum to manipulate a large uterus. Both of those instruments are valuable and work well, but often a large uterus requires extensive manipulation. Repositioning of any sharp instruments that pierce the serosa can often lead to additional blood loss. It is preferable to avoid this blood loss on a large uterus at all costs because it can be brisk and stains the surgical pedicles, making the remainder of the procedure unnecessarily difficult.
Once the uterine arteries are desiccated, if fibroids are obscuring the view, the corpus of the uterus can be detached from the cervix as in supracervical hysterectomy fashion. From there, the uterus can be placed in the upper abdomen while colpotomy can be performed.
In patients with multiple fibroids, we do not recommend performing myomectomy first, unless the fibroid is pedunculated and on a very small stalk. Improved uterine manipulation and retroperitoneal dissection are preferred over myomectomy to safely complete hysterectomy for the broad uterus. In our opinion, any attempt at myomectomy would lead to unnecessary blood loss and additional operative time with minimal benefit.
In patients with fibroids that grow into the broad ligament and pelvic sidewall, the natural course of the ureter becomes displaced laterally. This is contrary to the popular misconception that the ureter is more medially located in the setting of broad-ligament fibroids. To ensure safe access to the uterine arteries, the vesicouterine peritoneum can be incised and extended cephalad along the broad ligament and, then, using the above-mentioned technique, by pushing the uterus and the fibroid to the contralateral side via the suction irrigator, the uterine arteries can be easily accessed.
Another useful technique is to use diluted vasopressin injected into the lower pole of the uterus to cause vasoconstriction and minimize the bleeding. The concentration is 1 cc of 20 units of vasopressin in 100-400 cc of saline. This technique is very useful for myomectomies, and some surgeons find it also helpful for hysterectomy. The plasma half-life of vasopressin is 10-20 minutes, and a large quantity is needed to help with vasoconstriction in a big uterus. The safe upper limits of vasopressin dosing are not firmly established. A fibroid uterus with aberrant vasculature may require a greater-than-acceptable dose to control bleeding.
It is important to ensure that patients have an optimized hemoglobin level preoperatively. We use a hemoglobin level of 8 g/dL as a lowest cutoff value for performing TLH without preoperative transfusion. Regarding bowel preparation, neither the literature nor our own experience support its value, so we typically do not use it.
Morcellation and patient counseling
Uteri up to 12 weeks’ gestational size usually can be extracted transvaginally, and most uteri regardless of size can be morcellated and extracted through the vagina, providing that the vaginal fornix is accessible from below. In some cases, such as when the apex is too high, a minilaparotomic incision is needed to extract the uterus, or when available, power morcellation can be performed.
A major challenge, given our growing ability to laparoscopically remove very larger uteri, is that uteri heavier than about 2.5 kg in weight cannot be morcellated inside a morcellation bag. The risk of upstaging a known or suspected uterine malignancy, or of spreading an unknown malignant sarcoma (presumed benign myoma), should be incorporated in each patient’s decision making.
Thorough counseling about surgical options and on the risks of morcellating a very large uterus without containment in a bag is essential. Each patient must understand the risks and decide whether the benefits of minimally invasive surgery outweigh these risks. While MRI can sometimes provide increased suspicion of a leiomyosarcoma, malignancy can never be completed excluded preoperatively.
Removal of a 7.4-kg uterus
Our patient was a 44-year-old with a markedly enlarged fibroid uterus. Having been told by other providers that she was not a candidate for minimally invasive hysterectomy, she had delayed surgical management for a number of years, allowing for such a generous uterine size to develop.
The patient was knowledgeable about her condition and, given her comorbid obesity, she requested a minimally invasive approach. Preoperative imaging included an ultrasound, which had to be completed abdominally because of the size of her uterus, and an additional MRI was needed to further characterize the extent and nature of her uterus. A very detailed discussion regarding risk of leiomyosarcoma, operative complications, and conversion to laparotomy ensued.
Intraoperatively, we placed the first 5 mm port in the left upper quadrant initially to survey the anatomy for feasibility of laparoscopic hysterectomy. The left utero-ovarian pedicle was easily viewed by airplaning the bed alone. While the right utero-ovarian pedicle was much more skewed and enlarged, the right IP was easily accessible and the ureter well visualized.
The decision was made to place additional ports and proceed with laparoscopic hysterectomy. The 5-mm assistant ports were placed lateral and directly above the upper vascular pedicles. Operative time was 4 hours and 12 minutes, and blood loss was only 700 cc. Her preoperative hemoglobin was optimized at 13.3 g/dL and dropped to 11.3 g/dL postoperatively. The patient was discharged home the next morning and had a normal recovery with no complications.
Dr. Pasic is professor of obstetrics, gynecology & women’s health; director of the section of advanced gynecologic endoscopy; and codirector of the AAGL fellowship in minimally invasive gynecologic surgery at the University of Louisville (Ky.). Dr. Pasic is the current president of the International Society of Gynecologic Endoscopy. He is also a past president of the AAGL (2009). Dr. Cesta is Dr. Pasic’s current fellow in minimally invasive gynecologic surgery as well as an instructor in obstetrics and gynecology at the University of Louisville. Dr. Pasic disclosed he is a consultant for Ethicon Endo, Medtronic, and Olympus and is a speaker for Cooper Surgical, which manufactures some of the instruments mentioned in this article. Dr. Cesta had no relevant financial disclosures.
It has now been 30 years since the first total laparoscopic hysterectomy was performed. The benefits of minimally invasive gynecologic surgery (MIGS) – and of minimally invasive hysterectomy specifically – are now well documented. Since this milestone procedure, both instrumentation and technique have improved significantly.
This includes traditional laparoscopy, as well as the robotically assisted laparoscopic approach. However, certain patient characteristics also may influence the choice. A uterus that is undescended, combined with a narrow introitus, for instance, can be a contributory factor in choosing to perform laparoscopic hysterectomy. Additionally, so can an extremely large uterus and an extremely high body mass index (BMI).
These latter two factors – a very large uterus (which we define as more than 15-16 weeks’ gestational size) and a BMI over 60 kg/m2 – historically were considered to be contraindications to laparoscopic hysterectomy. But as the proficiency, comfort, and skill of a new generation of laparoscopic surgeons increases, the tide is shifting with respect to both morbid obesity and the very large uterus.
With growing experience and improved instrumentation, the majority of gynecologists who are fellowship-trained in MIGS are able to routinely and safely perform laparoscopic hysterectomy for uteri weighing 1-2 kg and in patients who have extreme morbid obesity. The literature, moreover, increasingly features case reports of laparoscopic removal of very large uteri and reviews/discussions of total laparoscopic hysterectomy being feasible.
In our own experience, total laparoscopic hysterectomy (TLH) of the very large uterus can be safely and advantageously performed using key instruments and refinements in technique, as well as thorough patient counseling regarding the risk of unexpected sarcomas. Recently, we safely performed total laparoscopic hysterectomy for a patient with a uterus that – somewhat unexpectedly – weighed 7.4 kg.
Surgical pearls
Performing safe and effective total laparoscopic hysterectomy for large uteri – and for morbidly obese patients – hinges largely on modifications in entry and port placement, patient positioning, and choice of instrumentation. With these modifications, we can achieve adequate visualization of critical anatomy and can minimize bleeding. Otherwise, the surgery itself is largely the same. Here are the principles we find most helpful.
Entry and port placement
Traditionally, for TLHs, a camera port is placed at the umbilicus to provide a full view of the pelvis. For the larger uterus – and in women who are extremely obese – we aim to introduce the laparoscope higher. A reliable landmark is the Palmer’s point in the left upper quadrant. From here, we can identify areas for the placement of additional trocars.
In general, we place ancillary 5-mm ports more cephalad and lateral to the uterus than we otherwise would. Such placement facilitates effective visualization while accommodating manipulation of the uterus and allows us to avoid bleeding around the vascular upper pedicles. Overall, we have much better control through all parts of the surgery when we operate lateral to the uterus.
Patient positioning
In addition to the Trendelenburg position, we have adopted an “airplaning” technique for patients with a very large uterus in which the bed is tilted from side to side so that the left and right sides of the body are rotated upward as needed. This allows for gravitational-assisted retraction when it otherwise is not possible.
Instrumentation
For morbidly obese patients, we use Kii Fios advanced fixation trocars. These come in 5- and 10-mm sizes and are equipped with an intraperitoneal balloon that can be inflated to prevent sliding of the trocar out of the abdominal wall.
By far the most valuable instrument for the morbidly obese and the very large uterus is a 30-degree laparoscope. With our higher port placement as described, the 30-degree scope provides visualization of critical structures that wouldn’t be possible with a 0-degree scope.
The Rumi uterine manipulator comes with cups that come in different sizes and can fit around the cervix and help delineate the cervicouterine junction. We use this manipulator for all laparoscopic hysterectomies, but it is a must for the very large uterus.
Extensive desiccation of the utero-ovarian pedicles and uterine arteries is critical, and for this we advise using the rotating bipolar RoBi instrument. Use of the conventional bipolar instrument allows us to use targeted and anatomically guided application of energy. This ensures certainty that vessels whose limits exceed the diameter for advanced bipolar devices (typically 7 mm) are completely sealed. In-depth knowledge of pelvic anatomy and advanced laparoscopic dissection is paramount during these steps to ensure that vital structures are not damaged by the wider thermal spread of the traditional bipolar device. For cutting, the use of ultrasonic energy is important to prevent energy from spreading laterally.
Lastly, we recommend a good suction irrigator because, if bleeding occurs, it tends to be heavy because of the enlarged nature of feeding vasculature. When placed through an umbilical or suprapubic port, the suction irrigator also may be used to help with the rotational vectors and traction for further uterine manipulation.
Technique
We usually operate from top to bottom, transecting the upper pedicles such as the infundibulopelvic (IP) ligaments or utero-ovarian ligaments first, rather than the round ligaments. This helps us achieve additional mobility of the uterus. Some surgeons believe that retroperitoneal dissection and ligation of the uterine arteries at their origin is essential, but we find that, with good uterine manipulation and the use of a 30-degree scope, we achieve adequate visualization for identifying the ureter and uterine artery on the sidewall and consequently do not need to dissect retroperitoneally.
When using the uterine manipulator with the colpotomy cup, the uterus is pushed upward, increasing the distance between the vaginal fornix and the ureters. Uterine arteries can easily be identified and desiccated using conventional bipolar energy. When the colpotomy cup is pushed cephalad, the application of the bipolar energy within the limits of the cup is safe. The thermal spread does not pose a threat to the ureters, which are displaced 1.5-2 cm laterally. Large fibroids often contribute to distorted anatomical planes, and a colpotomy cup provides a firm palpable surface between the cervix and vagina during dissection.
When dealing with large uteri, one must sometimes think outside the box and deviate from standard technique. For instance, in patients with distorted anatomy because of large fibroids, it helps to first control the pedicles that are most easily accessible. Sometimes it is acceptable to perform oophorectomy if the IP ligament is more accessible and the utero-ovarian pedicle is distorted by dilated veins and adherent to the uterus. After transection of each pedicle, we gain more mobility of the uterus and better visualization for the next step.
Inserting the camera through ancillary ports – a technique known as “port hopping” – helps to visualize and take down adhesions much better and more safely than using the camera from the umbilical port only. Port hopping with a 30-degree laparoscope helps to obtain a 360-degree view of adhesions and anatomy, which is exceedingly helpful in cases in which crucial anatomical structures are within close proximity of one another.
In general it is more challenging to perform TLH on a patient with a broad uterus or a patient with low posterior fibroids that are occupying the pelvis than on a patient with fibroids in the upper abdomen. The main challenge for the surgeon is to safely secure the uterine arteries and control the blood supply to the uterus.
Access to the pelvic sidewall is obtained with the combination of 30-degree scope, uterine manipulator, and the suction irrigator introduced through the midline port; the cervix and uterus are deviated upward. Instead of the suction irrigator or blunt dissector used for internal uterine manipulation, some surgeons use myoma screws or a 5-mm single-tooth tenaculum to manipulate a large uterus. Both of those instruments are valuable and work well, but often a large uterus requires extensive manipulation. Repositioning of any sharp instruments that pierce the serosa can often lead to additional blood loss. It is preferable to avoid this blood loss on a large uterus at all costs because it can be brisk and stains the surgical pedicles, making the remainder of the procedure unnecessarily difficult.
Once the uterine arteries are desiccated, if fibroids are obscuring the view, the corpus of the uterus can be detached from the cervix as in supracervical hysterectomy fashion. From there, the uterus can be placed in the upper abdomen while colpotomy can be performed.
In patients with multiple fibroids, we do not recommend performing myomectomy first, unless the fibroid is pedunculated and on a very small stalk. Improved uterine manipulation and retroperitoneal dissection are preferred over myomectomy to safely complete hysterectomy for the broad uterus. In our opinion, any attempt at myomectomy would lead to unnecessary blood loss and additional operative time with minimal benefit.
In patients with fibroids that grow into the broad ligament and pelvic sidewall, the natural course of the ureter becomes displaced laterally. This is contrary to the popular misconception that the ureter is more medially located in the setting of broad-ligament fibroids. To ensure safe access to the uterine arteries, the vesicouterine peritoneum can be incised and extended cephalad along the broad ligament and, then, using the above-mentioned technique, by pushing the uterus and the fibroid to the contralateral side via the suction irrigator, the uterine arteries can be easily accessed.
Another useful technique is to use diluted vasopressin injected into the lower pole of the uterus to cause vasoconstriction and minimize the bleeding. The concentration is 1 cc of 20 units of vasopressin in 100-400 cc of saline. This technique is very useful for myomectomies, and some surgeons find it also helpful for hysterectomy. The plasma half-life of vasopressin is 10-20 minutes, and a large quantity is needed to help with vasoconstriction in a big uterus. The safe upper limits of vasopressin dosing are not firmly established. A fibroid uterus with aberrant vasculature may require a greater-than-acceptable dose to control bleeding.
It is important to ensure that patients have an optimized hemoglobin level preoperatively. We use a hemoglobin level of 8 g/dL as a lowest cutoff value for performing TLH without preoperative transfusion. Regarding bowel preparation, neither the literature nor our own experience support its value, so we typically do not use it.
Morcellation and patient counseling
Uteri up to 12 weeks’ gestational size usually can be extracted transvaginally, and most uteri regardless of size can be morcellated and extracted through the vagina, providing that the vaginal fornix is accessible from below. In some cases, such as when the apex is too high, a minilaparotomic incision is needed to extract the uterus, or when available, power morcellation can be performed.
A major challenge, given our growing ability to laparoscopically remove very larger uteri, is that uteri heavier than about 2.5 kg in weight cannot be morcellated inside a morcellation bag. The risk of upstaging a known or suspected uterine malignancy, or of spreading an unknown malignant sarcoma (presumed benign myoma), should be incorporated in each patient’s decision making.
Thorough counseling about surgical options and on the risks of morcellating a very large uterus without containment in a bag is essential. Each patient must understand the risks and decide whether the benefits of minimally invasive surgery outweigh these risks. While MRI can sometimes provide increased suspicion of a leiomyosarcoma, malignancy can never be completed excluded preoperatively.
Removal of a 7.4-kg uterus
Our patient was a 44-year-old with a markedly enlarged fibroid uterus. Having been told by other providers that she was not a candidate for minimally invasive hysterectomy, she had delayed surgical management for a number of years, allowing for such a generous uterine size to develop.
The patient was knowledgeable about her condition and, given her comorbid obesity, she requested a minimally invasive approach. Preoperative imaging included an ultrasound, which had to be completed abdominally because of the size of her uterus, and an additional MRI was needed to further characterize the extent and nature of her uterus. A very detailed discussion regarding risk of leiomyosarcoma, operative complications, and conversion to laparotomy ensued.
Intraoperatively, we placed the first 5 mm port in the left upper quadrant initially to survey the anatomy for feasibility of laparoscopic hysterectomy. The left utero-ovarian pedicle was easily viewed by airplaning the bed alone. While the right utero-ovarian pedicle was much more skewed and enlarged, the right IP was easily accessible and the ureter well visualized.
The decision was made to place additional ports and proceed with laparoscopic hysterectomy. The 5-mm assistant ports were placed lateral and directly above the upper vascular pedicles. Operative time was 4 hours and 12 minutes, and blood loss was only 700 cc. Her preoperative hemoglobin was optimized at 13.3 g/dL and dropped to 11.3 g/dL postoperatively. The patient was discharged home the next morning and had a normal recovery with no complications.
Dr. Pasic is professor of obstetrics, gynecology & women’s health; director of the section of advanced gynecologic endoscopy; and codirector of the AAGL fellowship in minimally invasive gynecologic surgery at the University of Louisville (Ky.). Dr. Pasic is the current president of the International Society of Gynecologic Endoscopy. He is also a past president of the AAGL (2009). Dr. Cesta is Dr. Pasic’s current fellow in minimally invasive gynecologic surgery as well as an instructor in obstetrics and gynecology at the University of Louisville. Dr. Pasic disclosed he is a consultant for Ethicon Endo, Medtronic, and Olympus and is a speaker for Cooper Surgical, which manufactures some of the instruments mentioned in this article. Dr. Cesta had no relevant financial disclosures.
Safely pushing the limits of MIGS surgery
In his excellent treatise on the history of hysterectomy, Chris Sutton, MBBch, noted that, while Themison of Athens in 50 bc and Soranus of Ephesus in 120 ad were reported to have performed vaginal hysterectomy, these cases essentially were emergency amputation of severely prolapsed uteri, which usually involved cutting both ureters and the bladder (J Minim Invas Gynecol. 2010 Jul;17[4]:421–35). It was not until 1801 that the first planned elective vaginal hysterectomy was performed, and it was not until the mid 19th century, in 1853, that Walter Burnham, MD, in Lowell, Mass., performed the first abdominal hysterectomy resulting in patient survival.
Seemingly incredible, it was only 125 years later, in autumn of 1988 at William Nesbitt Memorial Hospital in Kingston, Pa., that Harry Reich, MD, performed the first total laparoscopically assisted hysterectomy.
Since Dr. Reich’s groundbreaking procedure, the performance of laparoscopic hysterectomy has advanced at a feverish pace. In my own practice, I have not performed an abdominal hysterectomy since 1998. My two partners, who are both fellowship-trained in minimally invasive gynecologic surgery (MIGS), Aarathi Cholkeri-Singh, MD, who joined my practice in 2007, and Kristen Sasaki, MD, who joined my practice in 2014, have never performed an open hysterectomy since starting practice. Despite these advances, One of the most difficult situations is the truly large uterus – greater than 2,500 grams.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Paya Pasic, MD, and Megan Cesta, MD, to discuss the next frontier: the removal of the multiple kilogram uterus.
Dr. Pasic is an internationally recognized leader in laparoscopic MIGS. He is professor of obstetrics, gynecology & women’s health; director, section of advanced gynecologic endoscopy, and codirector of the AAGL fellowship in MIGS at the University of Louisville (Ky.). Dr. Pasic is the current president of the International Society of Gynecologic Endoscopy. He is also a past president of the AAGL (2009). Dr. Pasic is published in the field of MIGS, having authored many publications, book chapters, monographs, and textbooks.
Dr. Cesta is Dr. Pasic’s current fellow in MIGS and an instructor in obstetrics and gynecology at the university.
It is truly a pleasure to welcome Dr. Pasic and Dr. Cesta to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class. Email him at obnews@mdedge.com.
In his excellent treatise on the history of hysterectomy, Chris Sutton, MBBch, noted that, while Themison of Athens in 50 bc and Soranus of Ephesus in 120 ad were reported to have performed vaginal hysterectomy, these cases essentially were emergency amputation of severely prolapsed uteri, which usually involved cutting both ureters and the bladder (J Minim Invas Gynecol. 2010 Jul;17[4]:421–35). It was not until 1801 that the first planned elective vaginal hysterectomy was performed, and it was not until the mid 19th century, in 1853, that Walter Burnham, MD, in Lowell, Mass., performed the first abdominal hysterectomy resulting in patient survival.
Seemingly incredible, it was only 125 years later, in autumn of 1988 at William Nesbitt Memorial Hospital in Kingston, Pa., that Harry Reich, MD, performed the first total laparoscopically assisted hysterectomy.
Since Dr. Reich’s groundbreaking procedure, the performance of laparoscopic hysterectomy has advanced at a feverish pace. In my own practice, I have not performed an abdominal hysterectomy since 1998. My two partners, who are both fellowship-trained in minimally invasive gynecologic surgery (MIGS), Aarathi Cholkeri-Singh, MD, who joined my practice in 2007, and Kristen Sasaki, MD, who joined my practice in 2014, have never performed an open hysterectomy since starting practice. Despite these advances, One of the most difficult situations is the truly large uterus – greater than 2,500 grams.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Paya Pasic, MD, and Megan Cesta, MD, to discuss the next frontier: the removal of the multiple kilogram uterus.
Dr. Pasic is an internationally recognized leader in laparoscopic MIGS. He is professor of obstetrics, gynecology & women’s health; director, section of advanced gynecologic endoscopy, and codirector of the AAGL fellowship in MIGS at the University of Louisville (Ky.). Dr. Pasic is the current president of the International Society of Gynecologic Endoscopy. He is also a past president of the AAGL (2009). Dr. Pasic is published in the field of MIGS, having authored many publications, book chapters, monographs, and textbooks.
Dr. Cesta is Dr. Pasic’s current fellow in MIGS and an instructor in obstetrics and gynecology at the university.
It is truly a pleasure to welcome Dr. Pasic and Dr. Cesta to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class. Email him at obnews@mdedge.com.
In his excellent treatise on the history of hysterectomy, Chris Sutton, MBBch, noted that, while Themison of Athens in 50 bc and Soranus of Ephesus in 120 ad were reported to have performed vaginal hysterectomy, these cases essentially were emergency amputation of severely prolapsed uteri, which usually involved cutting both ureters and the bladder (J Minim Invas Gynecol. 2010 Jul;17[4]:421–35). It was not until 1801 that the first planned elective vaginal hysterectomy was performed, and it was not until the mid 19th century, in 1853, that Walter Burnham, MD, in Lowell, Mass., performed the first abdominal hysterectomy resulting in patient survival.
Seemingly incredible, it was only 125 years later, in autumn of 1988 at William Nesbitt Memorial Hospital in Kingston, Pa., that Harry Reich, MD, performed the first total laparoscopically assisted hysterectomy.
Since Dr. Reich’s groundbreaking procedure, the performance of laparoscopic hysterectomy has advanced at a feverish pace. In my own practice, I have not performed an abdominal hysterectomy since 1998. My two partners, who are both fellowship-trained in minimally invasive gynecologic surgery (MIGS), Aarathi Cholkeri-Singh, MD, who joined my practice in 2007, and Kristen Sasaki, MD, who joined my practice in 2014, have never performed an open hysterectomy since starting practice. Despite these advances, One of the most difficult situations is the truly large uterus – greater than 2,500 grams.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Paya Pasic, MD, and Megan Cesta, MD, to discuss the next frontier: the removal of the multiple kilogram uterus.
Dr. Pasic is an internationally recognized leader in laparoscopic MIGS. He is professor of obstetrics, gynecology & women’s health; director, section of advanced gynecologic endoscopy, and codirector of the AAGL fellowship in MIGS at the University of Louisville (Ky.). Dr. Pasic is the current president of the International Society of Gynecologic Endoscopy. He is also a past president of the AAGL (2009). Dr. Pasic is published in the field of MIGS, having authored many publications, book chapters, monographs, and textbooks.
Dr. Cesta is Dr. Pasic’s current fellow in MIGS and an instructor in obstetrics and gynecology at the university.
It is truly a pleasure to welcome Dr. Pasic and Dr. Cesta to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class. Email him at obnews@mdedge.com.
Ask about vaping and e-cigarette use
When we studied the knowledge and practice of e-cigarette use among pregnant women in one of our outpatient practices, we found that 43% of more than 300 survey participants believed e-cigarettes are less harmful to a fetus than traditional cigarettes. Just over half – 57% – believed that e-cigarettes contain nicotine.
This study from 5 years ago demonstrated the need for more patient education.1 Today, we have even more clarity that, while there may be health benefits of switching to noncombustible forms of nicotine consumption outside of pregnancy, these potential benefits do not extend to pregnancy. Both human and animal studies have demonstrated that nicotine itself is harmful to the developing fetus; the Centers for Disease Control and Prevention warns against the use of e-cigarettes in pregnancy for this reason.
A 2018 literature review on the use of e-cigarettes in pregnancy and the effects on perinatal/neonatal outcomes reported that the amount of nicotine consumed by e-cigarette users is similar to that of cigarette smokers and that most animal studies suggest a potential danger to the fetus, primarily because of the nicotine.2 Effects on the immune system, neural development, lung function, and cardiac function were all noted in the review. Other research has shown that e-cigarette fluid can contain formaldehyde and other harmful substances.
A new analysis of data from the 2014-2017 National Health Interview Survey shows a significantly lower prevalence of conventional cigarette use among pregnant women than in nonpregnant women, and an almost identical prevalence of e-cigarette use among pregnant and nonpregnant women of reproductive age.3 This discrepancy again suggests that women may not be aware of the potential harms of e-cigarettes in pregnancy, which is not surprising considering that prenatal care clinicians often are not appropriately screening or counseling regarding e-cigarette use.4
and counsel women that the use of e-cigarettes is not a safer alternative to cigarette smoking. I urge patients who have switched to e-cigarettes as a means of smoking cessation or as a choice they perceive to be safer to work together with me to find another way to reduce potential harm to their baby.
References
1. J Addict Med. 2015 Jul-Aug;9(4):266-72.
2. Obstet Gynecol Surv. 2018 Sep;73(9):544-9.
3. JAMA Pediatr. 2019 Jun 1;173(6):600-2.
4. Am J Obstet Gynecol. 2014 Dec;211(6):695.e1-7.
When we studied the knowledge and practice of e-cigarette use among pregnant women in one of our outpatient practices, we found that 43% of more than 300 survey participants believed e-cigarettes are less harmful to a fetus than traditional cigarettes. Just over half – 57% – believed that e-cigarettes contain nicotine.
This study from 5 years ago demonstrated the need for more patient education.1 Today, we have even more clarity that, while there may be health benefits of switching to noncombustible forms of nicotine consumption outside of pregnancy, these potential benefits do not extend to pregnancy. Both human and animal studies have demonstrated that nicotine itself is harmful to the developing fetus; the Centers for Disease Control and Prevention warns against the use of e-cigarettes in pregnancy for this reason.
A 2018 literature review on the use of e-cigarettes in pregnancy and the effects on perinatal/neonatal outcomes reported that the amount of nicotine consumed by e-cigarette users is similar to that of cigarette smokers and that most animal studies suggest a potential danger to the fetus, primarily because of the nicotine.2 Effects on the immune system, neural development, lung function, and cardiac function were all noted in the review. Other research has shown that e-cigarette fluid can contain formaldehyde and other harmful substances.
A new analysis of data from the 2014-2017 National Health Interview Survey shows a significantly lower prevalence of conventional cigarette use among pregnant women than in nonpregnant women, and an almost identical prevalence of e-cigarette use among pregnant and nonpregnant women of reproductive age.3 This discrepancy again suggests that women may not be aware of the potential harms of e-cigarettes in pregnancy, which is not surprising considering that prenatal care clinicians often are not appropriately screening or counseling regarding e-cigarette use.4
and counsel women that the use of e-cigarettes is not a safer alternative to cigarette smoking. I urge patients who have switched to e-cigarettes as a means of smoking cessation or as a choice they perceive to be safer to work together with me to find another way to reduce potential harm to their baby.
References
1. J Addict Med. 2015 Jul-Aug;9(4):266-72.
2. Obstet Gynecol Surv. 2018 Sep;73(9):544-9.
3. JAMA Pediatr. 2019 Jun 1;173(6):600-2.
4. Am J Obstet Gynecol. 2014 Dec;211(6):695.e1-7.
When we studied the knowledge and practice of e-cigarette use among pregnant women in one of our outpatient practices, we found that 43% of more than 300 survey participants believed e-cigarettes are less harmful to a fetus than traditional cigarettes. Just over half – 57% – believed that e-cigarettes contain nicotine.
This study from 5 years ago demonstrated the need for more patient education.1 Today, we have even more clarity that, while there may be health benefits of switching to noncombustible forms of nicotine consumption outside of pregnancy, these potential benefits do not extend to pregnancy. Both human and animal studies have demonstrated that nicotine itself is harmful to the developing fetus; the Centers for Disease Control and Prevention warns against the use of e-cigarettes in pregnancy for this reason.
A 2018 literature review on the use of e-cigarettes in pregnancy and the effects on perinatal/neonatal outcomes reported that the amount of nicotine consumed by e-cigarette users is similar to that of cigarette smokers and that most animal studies suggest a potential danger to the fetus, primarily because of the nicotine.2 Effects on the immune system, neural development, lung function, and cardiac function were all noted in the review. Other research has shown that e-cigarette fluid can contain formaldehyde and other harmful substances.
A new analysis of data from the 2014-2017 National Health Interview Survey shows a significantly lower prevalence of conventional cigarette use among pregnant women than in nonpregnant women, and an almost identical prevalence of e-cigarette use among pregnant and nonpregnant women of reproductive age.3 This discrepancy again suggests that women may not be aware of the potential harms of e-cigarettes in pregnancy, which is not surprising considering that prenatal care clinicians often are not appropriately screening or counseling regarding e-cigarette use.4
and counsel women that the use of e-cigarettes is not a safer alternative to cigarette smoking. I urge patients who have switched to e-cigarettes as a means of smoking cessation or as a choice they perceive to be safer to work together with me to find another way to reduce potential harm to their baby.
References
1. J Addict Med. 2015 Jul-Aug;9(4):266-72.
2. Obstet Gynecol Surv. 2018 Sep;73(9):544-9.
3. JAMA Pediatr. 2019 Jun 1;173(6):600-2.
4. Am J Obstet Gynecol. 2014 Dec;211(6):695.e1-7.
Cannabis and prenatal care
We know that the environment significantly impacts our health. People who live in areas prone to industrial waste, poor air or water quality, and crime have higher risks for cardiovascular disease, severe asthma, and stress-induced illnesses. Children who grow up under these conditions can experience a failure to thrive.
As ob.gyns., we also recognize that the intrauterine environment plays a key role in influencing embryonic and fetal development. For this reason, we counsel our pregnant patients to eat well-balanced diets, drink healthy amounts of water, get plenty of rest, and incorporate physical activity into their daily routines. Indeed, the seminal work by Sir David Barker demonstrated that the roots of chronic diseases – including hypertension, stroke, and type 2 diabetes – begin in utero. We truly are where we live – from before birth up through adulthood.
Because the womb environment, where we spend the first critical 9 months of life, dramatically affects our lifelong health, we advise against the use of certain medications and other substances during pregnancy. Some of these recommendations seem clear-cut: Don’t smoke and significantly reduce or abstain from alcohol consumption; illicit drugs – such as cocaine or heroin – should never be used. However, gray areas exist. For example, although anticonvulsants carry higher risks for congenital malformations, patients who experience seizures may need to continue taking antiepileptic drugs during pregnancy, especially those with long safety records.
One of the newer challenges the medical community in general must face is the broadened use and wider societal acceptance of cannabis. Currently legal in 33 U.S. states and Washington, D.C., medical marijuana now is viewed as another legitimate tool in the health care arsenal, rather than the off-limits, off-label substance it was less than a generation ago.
Although proponents may tout the health benefits of cannabis and related products like cannabidiol, it remains unclear what the long-term effects of routine use may have on development, especially fetal development. However, how we as ob.gyns. navigate conversations with our patients around substance use remains crucial to our delivery of the best possible prenatal care.
We have invited Katrina S. Mark, MD, associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, to examine use of cannabis in pregnancy and the need for maintaining trust in the patient-practitioner relationship when discussing substance use during prenatal counseling.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
We know that the environment significantly impacts our health. People who live in areas prone to industrial waste, poor air or water quality, and crime have higher risks for cardiovascular disease, severe asthma, and stress-induced illnesses. Children who grow up under these conditions can experience a failure to thrive.
As ob.gyns., we also recognize that the intrauterine environment plays a key role in influencing embryonic and fetal development. For this reason, we counsel our pregnant patients to eat well-balanced diets, drink healthy amounts of water, get plenty of rest, and incorporate physical activity into their daily routines. Indeed, the seminal work by Sir David Barker demonstrated that the roots of chronic diseases – including hypertension, stroke, and type 2 diabetes – begin in utero. We truly are where we live – from before birth up through adulthood.
Because the womb environment, where we spend the first critical 9 months of life, dramatically affects our lifelong health, we advise against the use of certain medications and other substances during pregnancy. Some of these recommendations seem clear-cut: Don’t smoke and significantly reduce or abstain from alcohol consumption; illicit drugs – such as cocaine or heroin – should never be used. However, gray areas exist. For example, although anticonvulsants carry higher risks for congenital malformations, patients who experience seizures may need to continue taking antiepileptic drugs during pregnancy, especially those with long safety records.
One of the newer challenges the medical community in general must face is the broadened use and wider societal acceptance of cannabis. Currently legal in 33 U.S. states and Washington, D.C., medical marijuana now is viewed as another legitimate tool in the health care arsenal, rather than the off-limits, off-label substance it was less than a generation ago.
Although proponents may tout the health benefits of cannabis and related products like cannabidiol, it remains unclear what the long-term effects of routine use may have on development, especially fetal development. However, how we as ob.gyns. navigate conversations with our patients around substance use remains crucial to our delivery of the best possible prenatal care.
We have invited Katrina S. Mark, MD, associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, to examine use of cannabis in pregnancy and the need for maintaining trust in the patient-practitioner relationship when discussing substance use during prenatal counseling.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
We know that the environment significantly impacts our health. People who live in areas prone to industrial waste, poor air or water quality, and crime have higher risks for cardiovascular disease, severe asthma, and stress-induced illnesses. Children who grow up under these conditions can experience a failure to thrive.
As ob.gyns., we also recognize that the intrauterine environment plays a key role in influencing embryonic and fetal development. For this reason, we counsel our pregnant patients to eat well-balanced diets, drink healthy amounts of water, get plenty of rest, and incorporate physical activity into their daily routines. Indeed, the seminal work by Sir David Barker demonstrated that the roots of chronic diseases – including hypertension, stroke, and type 2 diabetes – begin in utero. We truly are where we live – from before birth up through adulthood.
Because the womb environment, where we spend the first critical 9 months of life, dramatically affects our lifelong health, we advise against the use of certain medications and other substances during pregnancy. Some of these recommendations seem clear-cut: Don’t smoke and significantly reduce or abstain from alcohol consumption; illicit drugs – such as cocaine or heroin – should never be used. However, gray areas exist. For example, although anticonvulsants carry higher risks for congenital malformations, patients who experience seizures may need to continue taking antiepileptic drugs during pregnancy, especially those with long safety records.
One of the newer challenges the medical community in general must face is the broadened use and wider societal acceptance of cannabis. Currently legal in 33 U.S. states and Washington, D.C., medical marijuana now is viewed as another legitimate tool in the health care arsenal, rather than the off-limits, off-label substance it was less than a generation ago.
Although proponents may tout the health benefits of cannabis and related products like cannabidiol, it remains unclear what the long-term effects of routine use may have on development, especially fetal development. However, how we as ob.gyns. navigate conversations with our patients around substance use remains crucial to our delivery of the best possible prenatal care.
We have invited Katrina S. Mark, MD, associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, to examine use of cannabis in pregnancy and the need for maintaining trust in the patient-practitioner relationship when discussing substance use during prenatal counseling.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
Counseling on cannabis use in pregnancy
A flurry of research papers published this year has simultaneously documented a rise in the use of cannabis during pregnancy and offered more data about its potential harms. This confluence of findings is concerning and highlights the importance of screening our patients for cannabis use and engaging with them in a way in which we can maintain their trust and their commitment to prenatal care.
A retrospective cohort study involving 661,617 women in Ontario found a significant association between self-reported cannabis use in pregnancy and an increased risk of preterm birth (relative risk, 1.41), as well as a greater likelihood of small-for-gestational-age babies (RR, 1.53), placental abruption (RR, 1.72), and transfer to neonatal intensive care (RR, 1.40).1 The study, reported in JAMA in July 2019, carefully matched users with nonusers who had the same characteristics – for example, tobacco use or not.
This new information builds upon other meta-analyses that have demonstrated a decrease in birth weight and greater admittance to the neonatal ICU associated with cannabis use in pregnancy – and it supplements what some research suggests about long-term neurologic development and a potentially increased risk of attention and behavioral problems. Other outcomes that have been noted in long-term neurologic studies of children who were exposed to cannabis in utero include impaired visual acuity, verbal reasoning and comprehension, and short-term memory.2
Increases in use were recently documented in two studies. One, an analysis of data from the National Survey on Drug Use and Health (NSDUH) published in JAMA in June 2019, showed that, between 2002-2003 and 2016-2017, the use of cannabis “in the past month” increased from 3.4% to 7.0% among pregnant women overall, and from 6% to 12% during the first trimester.3
The use of cannabis on a daily or near-daily basis, moreover, increased from 0.9% to 3% among pregnant women overall and from 2% to 5% during the first trimester. The data were collected during face-to-face interviews and were adjusted for age, race/ethnicity, and family income.
In the second study – a cross-sectional study of 367,403 pregnancies among women who filled out a questionnaire on cannabis use during standard prenatal care at Kaiser Permanente Northern California – the adjusted prevalence of use in the year before pregnancy increased from 7% in 2009 to 13% in 2017, and the adjusted prevalence during pregnancy increased from 2% to 3%.4
As in the NSDUH analysis, daily use increased most rapidly (compared with weekly or monthly) such that, by 2017, 25% of those who reported using cannabis in the year before pregnancy – and 21% of those who used cannabis during pregnancy – were daily users. It is notable that Kaiser’s population is diverse in all respects, and that the annual relative rates of increase in cannabis use before and during pregnancy (at each level of frequency) were consistent across racial/ethnic and household income groups.
It’s also worth noting that, in earlier research covering a similar time period (2009-2016), the investigators found significant increases in use via urine toxicology testing that occurs at the first prenatal visit at Kaiser. The increase found through questionnaires, therefore, reflects more than a greater willingness to self-report.
Choosing a screening tool
Universal prenatal substance use screening is recommended by the American College of Obstetricians and Gynecologists and the Centers for Disease Control and Prevention, but we don’t have any specific recommendations on what this means. Who should be screening, and what should that screening look like? Should we use a biologic screen, a standardized screening tool, or simply ask patients whether they use illicit substances?
Screening tools seem advantageous in that they are low cost, noninvasive, potentially comprehensive, and not subject to false-positive results as biologic screens can be – but which tool or tools are best? There are several validated screening tools that can be used outside of pregnancy to determine an individual’s use of illicit substances and whether or not that use is problematic, but previous studies have not used biologic markers to validate substance use screeners in pregnancy. Nor have studies compared screeners in pregnancy.
In our prenatal population in Baltimore, we have not been getting the answers we want using various nonvalidated screening tools. Approximately 30% of patients are positive for cannabis by urine screen, but only half tell us about their use.
Through research in our two prenatal care practices (one serving mostly privately insured and the other serving primarily Medicaid-eligible patients), we assessed both the accuracy and the acceptability of three substance use screening tools that are brief and that have been validated (for the general population) by the World Health Organization for screening of multiple substances: the 4P’s Plus (Parents, Partner, Past, and Pregnancy), the National Institute on Drug Abuse Quick Screen–ASSIST (Modified Alcohol, Smoking and Substance Involvement Screening Test), and the SURP-P (Substance Use Risk Profile–Pregnancy) scale.
In one study, published in May 2019 in Obstetrics & Gynecology, we recruited 500 pregnant women and administered these three tests to each of them.5 We then compared results with those of urine and hair drug testing, and checked the test-retest reliability of each test by readministering them (albeit by telephone) a week later. Although hair testing is not an indicator of current substance use, we used it to validate the screening tools on less-recent use.
The tests with the highest sensitivity and negative predictive values – the qualities we most want for screening – were the SURP-P and the 4P’s Plus (sensitivity of 92.4% and 90.2%, respectively). Overall they were highly sensitive screening tools across all trimesters, races, and age groups, making them more ideal screening tests than the NIDA Quick Screen–ASSIST.
Of the two tests, the 4P’s Plus screening tool was the one preferred by staff from both practices. In a companion qualitative study, we conducted focus-group discussions with 40 practice staff who were responsible for administering or overseeing patient screening.6 The staff, who were unaware of the sensitivity findings, were asked what they thought about the acceptability to patients of each of the three tools and their usability in practice.
Most of the participating staff preferred the 4P’s Plus screening tool for several reasons: It is easy to understand, is brief and to the point, and it has nonjudgmental language and tone. The screener first asks the patient about her parents’ and her partner’s use of alcohol and drugs, and then asks the patient about her own use of alcohol and tobacco. Affirmative responses to these questions lead to additional questions.
The premise is that one’s genetics, history, and current exposures – as well as one’s own use of tobacco and alcohol – are significantly associated with the use of illicit substances. If the patient reports no parental history or partner usage, and has never drank or smoked before, it’s extremely unlikely that she is using other drugs. The progression of questions does indeed seem less judgmental than immediately asking: “Do you use drugs?”
For us, the insight from this staff perception study combined with the findings on accuracy mean that the 4P’s Plus may be the most useful and acceptable screening tool for routine use in prenatal care.
Talking with our patients
The increase in the use of cannabis before and after pregnancy parallels the movement toward state legalization and decriminalization. Historically, clinicians often have relied on illegality as their main focus of counseling when giving recommendations for cessation and abstinence in pregnancy.2 This approach not only leads to punitive counseling, which can fracture the doctor-patient relationship, but increasingly it is no longer valid. In our changing legal climate, we need to provide medically based counseling and be very clear with our patients that legalization does not equate to safety.
It is important that we neither minimize nor overstate the risks. The evidence base for adverse birth outcomes of cannabis use in pregnancy is quite robust, but the associations can be subtle and are moderated by other behaviors and environmental factors that continue to challenge researchers.
As with alcohol, there likely are dose-or trimester-dependent differences in perinatal outcomes, and it’s quite possible that different cannabis products and routes of consumption have different effects. At this point, however, we don’t know the full story, nor do we know the extent to which the literature is biased toward positive correlations – the reporting of adverse effects – compared with negative findings. It is our job as medical care providers to be comfortable in that gray area and to still counsel patients on what we do know, providing the best-possible medical advice based on the information available to us.
In talking with patients, I explain that cannabis may cause a spectrum of problems and that there certainly are risks. I also tell them that we’re uncertain about the conditions and magnitude of that risk and that some babies who are exposed to cannabis in utero may have no perceivable consequences. Such honesty is important for maintaining trust, especially as some patients may see friends and relatives who also are cannabis users have normal pregnancy outcomes.
Much of my concern about cannabis in pregnancy centers on its effect on the developing brain and on long-term neurologic development. I share this with patients – I tell them that cannabis crosses the placenta and may well affect their baby’s brain as it is developing. I explain that I do not know whether this effect would be big or small, but that it’s not a chance I’m willing to take for their baby.
It is also important to educate patients that cannabis products are untested and unregulated and that they may be contaminated with heavy metals, pesticides, and other toxins that may be harmful to themselves and their babies. Patients also should know that the potency of cannabis has been dramatically increasing; research shows that the tetrahydrocannabinol – the psychoactive component – concentration has tripled over the past 2 decades.7
Research tells us that women who use illicit drugs and alcohol categorically engage in some form of harm reduction once they learn they are pregnant, and the same is true for cannabis. This is seen in dramatically different rates of first- and third-trimester use in the new analysis of NSDUH data; third-trimester use is approximately halved.
Some women will not be able to discontinue use, however, or they may try to quit and fail in their attempts. As we should with substance use more broadly, we must meet patients where they are, view cannabis use as a chronic medical problem, offer our assistance in helping them reduce harms of their use, and understand that quitting is a process.
Screening for mental health disorders and trauma is, of course, especially important in patients who use cannabis and other substances recreationally. In cases of medical marijuana usage, I recommend, as ACOG and other have done, that we discuss the risks and benefits of continuing cannabis versus shifting to alternative medications if options exist.
All patients should be welcomed, congratulated on their pregnancy and on coming for prenatal care, and engaged in the overall process of optimizing their health and the health of their baby. Like any other health issue during pregnancy, cannabis use needs to be screened for and treated in an evidence-based manner, but it does not define the trajectory or success of a woman’s pregnancy or her ability to be a successful parent.
Dr. Mark is associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine.
References
1. JAMA. 2019 Jul 9;322(2):145-52.
2. Preventive Medicine 2017 May 18;104:46-9.
3. JAMA. 2019 Jul 9;322(2):167-9.
4. JAMA Netw Open. 2019 Jul 3;2(7):e196471.
5. Obstet Gynecol. 2019 May;133(5):952-61.
6. J. Addict Med. 2019 May 10. doi: 10.1097/ADM.0000000000000543.
7. Biol Psychiatry. 2016 Apr 1;79(7):613-9.
A flurry of research papers published this year has simultaneously documented a rise in the use of cannabis during pregnancy and offered more data about its potential harms. This confluence of findings is concerning and highlights the importance of screening our patients for cannabis use and engaging with them in a way in which we can maintain their trust and their commitment to prenatal care.
A retrospective cohort study involving 661,617 women in Ontario found a significant association between self-reported cannabis use in pregnancy and an increased risk of preterm birth (relative risk, 1.41), as well as a greater likelihood of small-for-gestational-age babies (RR, 1.53), placental abruption (RR, 1.72), and transfer to neonatal intensive care (RR, 1.40).1 The study, reported in JAMA in July 2019, carefully matched users with nonusers who had the same characteristics – for example, tobacco use or not.
This new information builds upon other meta-analyses that have demonstrated a decrease in birth weight and greater admittance to the neonatal ICU associated with cannabis use in pregnancy – and it supplements what some research suggests about long-term neurologic development and a potentially increased risk of attention and behavioral problems. Other outcomes that have been noted in long-term neurologic studies of children who were exposed to cannabis in utero include impaired visual acuity, verbal reasoning and comprehension, and short-term memory.2
Increases in use were recently documented in two studies. One, an analysis of data from the National Survey on Drug Use and Health (NSDUH) published in JAMA in June 2019, showed that, between 2002-2003 and 2016-2017, the use of cannabis “in the past month” increased from 3.4% to 7.0% among pregnant women overall, and from 6% to 12% during the first trimester.3
The use of cannabis on a daily or near-daily basis, moreover, increased from 0.9% to 3% among pregnant women overall and from 2% to 5% during the first trimester. The data were collected during face-to-face interviews and were adjusted for age, race/ethnicity, and family income.
In the second study – a cross-sectional study of 367,403 pregnancies among women who filled out a questionnaire on cannabis use during standard prenatal care at Kaiser Permanente Northern California – the adjusted prevalence of use in the year before pregnancy increased from 7% in 2009 to 13% in 2017, and the adjusted prevalence during pregnancy increased from 2% to 3%.4
As in the NSDUH analysis, daily use increased most rapidly (compared with weekly or monthly) such that, by 2017, 25% of those who reported using cannabis in the year before pregnancy – and 21% of those who used cannabis during pregnancy – were daily users. It is notable that Kaiser’s population is diverse in all respects, and that the annual relative rates of increase in cannabis use before and during pregnancy (at each level of frequency) were consistent across racial/ethnic and household income groups.
It’s also worth noting that, in earlier research covering a similar time period (2009-2016), the investigators found significant increases in use via urine toxicology testing that occurs at the first prenatal visit at Kaiser. The increase found through questionnaires, therefore, reflects more than a greater willingness to self-report.
Choosing a screening tool
Universal prenatal substance use screening is recommended by the American College of Obstetricians and Gynecologists and the Centers for Disease Control and Prevention, but we don’t have any specific recommendations on what this means. Who should be screening, and what should that screening look like? Should we use a biologic screen, a standardized screening tool, or simply ask patients whether they use illicit substances?
Screening tools seem advantageous in that they are low cost, noninvasive, potentially comprehensive, and not subject to false-positive results as biologic screens can be – but which tool or tools are best? There are several validated screening tools that can be used outside of pregnancy to determine an individual’s use of illicit substances and whether or not that use is problematic, but previous studies have not used biologic markers to validate substance use screeners in pregnancy. Nor have studies compared screeners in pregnancy.
In our prenatal population in Baltimore, we have not been getting the answers we want using various nonvalidated screening tools. Approximately 30% of patients are positive for cannabis by urine screen, but only half tell us about their use.
Through research in our two prenatal care practices (one serving mostly privately insured and the other serving primarily Medicaid-eligible patients), we assessed both the accuracy and the acceptability of three substance use screening tools that are brief and that have been validated (for the general population) by the World Health Organization for screening of multiple substances: the 4P’s Plus (Parents, Partner, Past, and Pregnancy), the National Institute on Drug Abuse Quick Screen–ASSIST (Modified Alcohol, Smoking and Substance Involvement Screening Test), and the SURP-P (Substance Use Risk Profile–Pregnancy) scale.
In one study, published in May 2019 in Obstetrics & Gynecology, we recruited 500 pregnant women and administered these three tests to each of them.5 We then compared results with those of urine and hair drug testing, and checked the test-retest reliability of each test by readministering them (albeit by telephone) a week later. Although hair testing is not an indicator of current substance use, we used it to validate the screening tools on less-recent use.
The tests with the highest sensitivity and negative predictive values – the qualities we most want for screening – were the SURP-P and the 4P’s Plus (sensitivity of 92.4% and 90.2%, respectively). Overall they were highly sensitive screening tools across all trimesters, races, and age groups, making them more ideal screening tests than the NIDA Quick Screen–ASSIST.
Of the two tests, the 4P’s Plus screening tool was the one preferred by staff from both practices. In a companion qualitative study, we conducted focus-group discussions with 40 practice staff who were responsible for administering or overseeing patient screening.6 The staff, who were unaware of the sensitivity findings, were asked what they thought about the acceptability to patients of each of the three tools and their usability in practice.
Most of the participating staff preferred the 4P’s Plus screening tool for several reasons: It is easy to understand, is brief and to the point, and it has nonjudgmental language and tone. The screener first asks the patient about her parents’ and her partner’s use of alcohol and drugs, and then asks the patient about her own use of alcohol and tobacco. Affirmative responses to these questions lead to additional questions.
The premise is that one’s genetics, history, and current exposures – as well as one’s own use of tobacco and alcohol – are significantly associated with the use of illicit substances. If the patient reports no parental history or partner usage, and has never drank or smoked before, it’s extremely unlikely that she is using other drugs. The progression of questions does indeed seem less judgmental than immediately asking: “Do you use drugs?”
For us, the insight from this staff perception study combined with the findings on accuracy mean that the 4P’s Plus may be the most useful and acceptable screening tool for routine use in prenatal care.
Talking with our patients
The increase in the use of cannabis before and after pregnancy parallels the movement toward state legalization and decriminalization. Historically, clinicians often have relied on illegality as their main focus of counseling when giving recommendations for cessation and abstinence in pregnancy.2 This approach not only leads to punitive counseling, which can fracture the doctor-patient relationship, but increasingly it is no longer valid. In our changing legal climate, we need to provide medically based counseling and be very clear with our patients that legalization does not equate to safety.
It is important that we neither minimize nor overstate the risks. The evidence base for adverse birth outcomes of cannabis use in pregnancy is quite robust, but the associations can be subtle and are moderated by other behaviors and environmental factors that continue to challenge researchers.
As with alcohol, there likely are dose-or trimester-dependent differences in perinatal outcomes, and it’s quite possible that different cannabis products and routes of consumption have different effects. At this point, however, we don’t know the full story, nor do we know the extent to which the literature is biased toward positive correlations – the reporting of adverse effects – compared with negative findings. It is our job as medical care providers to be comfortable in that gray area and to still counsel patients on what we do know, providing the best-possible medical advice based on the information available to us.
In talking with patients, I explain that cannabis may cause a spectrum of problems and that there certainly are risks. I also tell them that we’re uncertain about the conditions and magnitude of that risk and that some babies who are exposed to cannabis in utero may have no perceivable consequences. Such honesty is important for maintaining trust, especially as some patients may see friends and relatives who also are cannabis users have normal pregnancy outcomes.
Much of my concern about cannabis in pregnancy centers on its effect on the developing brain and on long-term neurologic development. I share this with patients – I tell them that cannabis crosses the placenta and may well affect their baby’s brain as it is developing. I explain that I do not know whether this effect would be big or small, but that it’s not a chance I’m willing to take for their baby.
It is also important to educate patients that cannabis products are untested and unregulated and that they may be contaminated with heavy metals, pesticides, and other toxins that may be harmful to themselves and their babies. Patients also should know that the potency of cannabis has been dramatically increasing; research shows that the tetrahydrocannabinol – the psychoactive component – concentration has tripled over the past 2 decades.7
Research tells us that women who use illicit drugs and alcohol categorically engage in some form of harm reduction once they learn they are pregnant, and the same is true for cannabis. This is seen in dramatically different rates of first- and third-trimester use in the new analysis of NSDUH data; third-trimester use is approximately halved.
Some women will not be able to discontinue use, however, or they may try to quit and fail in their attempts. As we should with substance use more broadly, we must meet patients where they are, view cannabis use as a chronic medical problem, offer our assistance in helping them reduce harms of their use, and understand that quitting is a process.
Screening for mental health disorders and trauma is, of course, especially important in patients who use cannabis and other substances recreationally. In cases of medical marijuana usage, I recommend, as ACOG and other have done, that we discuss the risks and benefits of continuing cannabis versus shifting to alternative medications if options exist.
All patients should be welcomed, congratulated on their pregnancy and on coming for prenatal care, and engaged in the overall process of optimizing their health and the health of their baby. Like any other health issue during pregnancy, cannabis use needs to be screened for and treated in an evidence-based manner, but it does not define the trajectory or success of a woman’s pregnancy or her ability to be a successful parent.
Dr. Mark is associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine.
References
1. JAMA. 2019 Jul 9;322(2):145-52.
2. Preventive Medicine 2017 May 18;104:46-9.
3. JAMA. 2019 Jul 9;322(2):167-9.
4. JAMA Netw Open. 2019 Jul 3;2(7):e196471.
5. Obstet Gynecol. 2019 May;133(5):952-61.
6. J. Addict Med. 2019 May 10. doi: 10.1097/ADM.0000000000000543.
7. Biol Psychiatry. 2016 Apr 1;79(7):613-9.
A flurry of research papers published this year has simultaneously documented a rise in the use of cannabis during pregnancy and offered more data about its potential harms. This confluence of findings is concerning and highlights the importance of screening our patients for cannabis use and engaging with them in a way in which we can maintain their trust and their commitment to prenatal care.
A retrospective cohort study involving 661,617 women in Ontario found a significant association between self-reported cannabis use in pregnancy and an increased risk of preterm birth (relative risk, 1.41), as well as a greater likelihood of small-for-gestational-age babies (RR, 1.53), placental abruption (RR, 1.72), and transfer to neonatal intensive care (RR, 1.40).1 The study, reported in JAMA in July 2019, carefully matched users with nonusers who had the same characteristics – for example, tobacco use or not.
This new information builds upon other meta-analyses that have demonstrated a decrease in birth weight and greater admittance to the neonatal ICU associated with cannabis use in pregnancy – and it supplements what some research suggests about long-term neurologic development and a potentially increased risk of attention and behavioral problems. Other outcomes that have been noted in long-term neurologic studies of children who were exposed to cannabis in utero include impaired visual acuity, verbal reasoning and comprehension, and short-term memory.2
Increases in use were recently documented in two studies. One, an analysis of data from the National Survey on Drug Use and Health (NSDUH) published in JAMA in June 2019, showed that, between 2002-2003 and 2016-2017, the use of cannabis “in the past month” increased from 3.4% to 7.0% among pregnant women overall, and from 6% to 12% during the first trimester.3
The use of cannabis on a daily or near-daily basis, moreover, increased from 0.9% to 3% among pregnant women overall and from 2% to 5% during the first trimester. The data were collected during face-to-face interviews and were adjusted for age, race/ethnicity, and family income.
In the second study – a cross-sectional study of 367,403 pregnancies among women who filled out a questionnaire on cannabis use during standard prenatal care at Kaiser Permanente Northern California – the adjusted prevalence of use in the year before pregnancy increased from 7% in 2009 to 13% in 2017, and the adjusted prevalence during pregnancy increased from 2% to 3%.4
As in the NSDUH analysis, daily use increased most rapidly (compared with weekly or monthly) such that, by 2017, 25% of those who reported using cannabis in the year before pregnancy – and 21% of those who used cannabis during pregnancy – were daily users. It is notable that Kaiser’s population is diverse in all respects, and that the annual relative rates of increase in cannabis use before and during pregnancy (at each level of frequency) were consistent across racial/ethnic and household income groups.
It’s also worth noting that, in earlier research covering a similar time period (2009-2016), the investigators found significant increases in use via urine toxicology testing that occurs at the first prenatal visit at Kaiser. The increase found through questionnaires, therefore, reflects more than a greater willingness to self-report.
Choosing a screening tool
Universal prenatal substance use screening is recommended by the American College of Obstetricians and Gynecologists and the Centers for Disease Control and Prevention, but we don’t have any specific recommendations on what this means. Who should be screening, and what should that screening look like? Should we use a biologic screen, a standardized screening tool, or simply ask patients whether they use illicit substances?
Screening tools seem advantageous in that they are low cost, noninvasive, potentially comprehensive, and not subject to false-positive results as biologic screens can be – but which tool or tools are best? There are several validated screening tools that can be used outside of pregnancy to determine an individual’s use of illicit substances and whether or not that use is problematic, but previous studies have not used biologic markers to validate substance use screeners in pregnancy. Nor have studies compared screeners in pregnancy.
In our prenatal population in Baltimore, we have not been getting the answers we want using various nonvalidated screening tools. Approximately 30% of patients are positive for cannabis by urine screen, but only half tell us about their use.
Through research in our two prenatal care practices (one serving mostly privately insured and the other serving primarily Medicaid-eligible patients), we assessed both the accuracy and the acceptability of three substance use screening tools that are brief and that have been validated (for the general population) by the World Health Organization for screening of multiple substances: the 4P’s Plus (Parents, Partner, Past, and Pregnancy), the National Institute on Drug Abuse Quick Screen–ASSIST (Modified Alcohol, Smoking and Substance Involvement Screening Test), and the SURP-P (Substance Use Risk Profile–Pregnancy) scale.
In one study, published in May 2019 in Obstetrics & Gynecology, we recruited 500 pregnant women and administered these three tests to each of them.5 We then compared results with those of urine and hair drug testing, and checked the test-retest reliability of each test by readministering them (albeit by telephone) a week later. Although hair testing is not an indicator of current substance use, we used it to validate the screening tools on less-recent use.
The tests with the highest sensitivity and negative predictive values – the qualities we most want for screening – were the SURP-P and the 4P’s Plus (sensitivity of 92.4% and 90.2%, respectively). Overall they were highly sensitive screening tools across all trimesters, races, and age groups, making them more ideal screening tests than the NIDA Quick Screen–ASSIST.
Of the two tests, the 4P’s Plus screening tool was the one preferred by staff from both practices. In a companion qualitative study, we conducted focus-group discussions with 40 practice staff who were responsible for administering or overseeing patient screening.6 The staff, who were unaware of the sensitivity findings, were asked what they thought about the acceptability to patients of each of the three tools and their usability in practice.
Most of the participating staff preferred the 4P’s Plus screening tool for several reasons: It is easy to understand, is brief and to the point, and it has nonjudgmental language and tone. The screener first asks the patient about her parents’ and her partner’s use of alcohol and drugs, and then asks the patient about her own use of alcohol and tobacco. Affirmative responses to these questions lead to additional questions.
The premise is that one’s genetics, history, and current exposures – as well as one’s own use of tobacco and alcohol – are significantly associated with the use of illicit substances. If the patient reports no parental history or partner usage, and has never drank or smoked before, it’s extremely unlikely that she is using other drugs. The progression of questions does indeed seem less judgmental than immediately asking: “Do you use drugs?”
For us, the insight from this staff perception study combined with the findings on accuracy mean that the 4P’s Plus may be the most useful and acceptable screening tool for routine use in prenatal care.
Talking with our patients
The increase in the use of cannabis before and after pregnancy parallels the movement toward state legalization and decriminalization. Historically, clinicians often have relied on illegality as their main focus of counseling when giving recommendations for cessation and abstinence in pregnancy.2 This approach not only leads to punitive counseling, which can fracture the doctor-patient relationship, but increasingly it is no longer valid. In our changing legal climate, we need to provide medically based counseling and be very clear with our patients that legalization does not equate to safety.
It is important that we neither minimize nor overstate the risks. The evidence base for adverse birth outcomes of cannabis use in pregnancy is quite robust, but the associations can be subtle and are moderated by other behaviors and environmental factors that continue to challenge researchers.
As with alcohol, there likely are dose-or trimester-dependent differences in perinatal outcomes, and it’s quite possible that different cannabis products and routes of consumption have different effects. At this point, however, we don’t know the full story, nor do we know the extent to which the literature is biased toward positive correlations – the reporting of adverse effects – compared with negative findings. It is our job as medical care providers to be comfortable in that gray area and to still counsel patients on what we do know, providing the best-possible medical advice based on the information available to us.
In talking with patients, I explain that cannabis may cause a spectrum of problems and that there certainly are risks. I also tell them that we’re uncertain about the conditions and magnitude of that risk and that some babies who are exposed to cannabis in utero may have no perceivable consequences. Such honesty is important for maintaining trust, especially as some patients may see friends and relatives who also are cannabis users have normal pregnancy outcomes.
Much of my concern about cannabis in pregnancy centers on its effect on the developing brain and on long-term neurologic development. I share this with patients – I tell them that cannabis crosses the placenta and may well affect their baby’s brain as it is developing. I explain that I do not know whether this effect would be big or small, but that it’s not a chance I’m willing to take for their baby.
It is also important to educate patients that cannabis products are untested and unregulated and that they may be contaminated with heavy metals, pesticides, and other toxins that may be harmful to themselves and their babies. Patients also should know that the potency of cannabis has been dramatically increasing; research shows that the tetrahydrocannabinol – the psychoactive component – concentration has tripled over the past 2 decades.7
Research tells us that women who use illicit drugs and alcohol categorically engage in some form of harm reduction once they learn they are pregnant, and the same is true for cannabis. This is seen in dramatically different rates of first- and third-trimester use in the new analysis of NSDUH data; third-trimester use is approximately halved.
Some women will not be able to discontinue use, however, or they may try to quit and fail in their attempts. As we should with substance use more broadly, we must meet patients where they are, view cannabis use as a chronic medical problem, offer our assistance in helping them reduce harms of their use, and understand that quitting is a process.
Screening for mental health disorders and trauma is, of course, especially important in patients who use cannabis and other substances recreationally. In cases of medical marijuana usage, I recommend, as ACOG and other have done, that we discuss the risks and benefits of continuing cannabis versus shifting to alternative medications if options exist.
All patients should be welcomed, congratulated on their pregnancy and on coming for prenatal care, and engaged in the overall process of optimizing their health and the health of their baby. Like any other health issue during pregnancy, cannabis use needs to be screened for and treated in an evidence-based manner, but it does not define the trajectory or success of a woman’s pregnancy or her ability to be a successful parent.
Dr. Mark is associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine.
References
1. JAMA. 2019 Jul 9;322(2):145-52.
2. Preventive Medicine 2017 May 18;104:46-9.
3. JAMA. 2019 Jul 9;322(2):167-9.
4. JAMA Netw Open. 2019 Jul 3;2(7):e196471.
5. Obstet Gynecol. 2019 May;133(5):952-61.
6. J. Addict Med. 2019 May 10. doi: 10.1097/ADM.0000000000000543.
7. Biol Psychiatry. 2016 Apr 1;79(7):613-9.