User login
A focus on women with diabetes and their offspring
In 2021, diabetes and related complications was the 8th leading cause of death in the United States.1 As of 2022, more than 11% of the U.S. population had diabetes and 38% of the adult U.S. population had prediabetes.2 Diabetes is the most expensive chronic condition in the United States, where $1 of every $4 in health care costs is spent on care.3
Where this is most concerning is diabetes in pregnancy. While childbirth rates in the United States have decreased since the 2007 high of 4.32 million births4 to 3.66 million in 2021,5 the incidence of diabetes in pregnancy – both pregestational and gestational – has increased. The rate of pregestational diabetes in 2021 was 10.9 per 1,000 births, a 27% increase from 2016 (8.6 per 1,000).6 The percentage of those giving birth who also were diagnosed with gestational diabetes mellitus (GDM) was 8.3% in 2021, up from 6.0% in 2016.7
Adverse outcomes for an infant born to a mother with diabetes include a higher risk of obesity and diabetes as adults, potentially leading to a forward-feeding cycle.
We and our colleagues established the Diabetes in Pregnancy Study Group of North America in 1997 because we had witnessed too frequently the devastating diabetes-induced pregnancy complications in our patients. The mission we set forth was to provide a forum for dialogue among maternal-fetal medicine subspecialists. The three main goals we set forth to support this mission were to provide a catalyst for research, contribute to the creation and refinement of medical policies, and influence professional practices in diabetes in pregnancy.8
In the last quarter century, DPSG-NA, through its annual and biennial meetings, has brought together several hundred practitioners that include physicians, nurses, statisticians, researchers, nutritionists, and allied health professionals, among others. As a group, it has improved the detection and management of diabetes in pregnant women and their offspring through knowledge sharing and influencing policies on GDM screening, diagnosis, management, and treatment. Our members have shown that preconceptional counseling for women with diabetes can significantly reduce congenital malformation and perinatal mortality compared with those women with pregestational diabetes who receive no counseling.9,10
We have addressed a wide variety of topics including the paucity of data in determining the timing of delivery for women with diabetes and the Institute of Medicine/National Academy of Medicine recommendations of gestational weight gain and risks of not adhering to them. We have learned about new scientific discoveries that reveal underlying mechanisms to diabetes-related birth defects and potential therapeutic targets; and we have discussed the health literacy requirements, ethics, and opportunities for lifestyle intervention.11-16
But we need to do more.
Two risk factors are at play: Women continue to choose to have babies at later ages and their pregnancies continue to be complicated by the rising incidence of obesity (see Figure 1 and Figure 2). 
The global obesity epidemic has become a significant concern for all aspects of health and particularly for diabetes in pregnancy. 
In 1990, 24.9% of women in the United States were obese; in 2010, 35.8%; and now more than 41%. Some experts project that by 2030 more than 80% of women in the United States will be overweight or obese.21
If we are to stop this cycle of diabetes begets more diabetes, now more than ever we need to come together and accelerate the research and education around the diabetes in pregnancy. Join us at this year’s DPSG-NA meeting Oct. 26-28 to take part in the knowledge sharing, discussions, and planning. More information can be found online at https://events.dpsg-na.com/home.
Dr. Miodovnik is adjunct professor of obstetrics, gynecology, and reproductive sciences at University of Maryland School of Medicine. Dr. Reece is professor of obstetrics, gynecology, and reproductive sciences and senior scientist at the Center for Birth Defects Research at University of Maryland School of Medicine.
References
1. Xu J et al. Mortality in the United States, 2021. NCHS Data Brief. 2022 Dec;(456):1-8. PMID: 36598387.
2. Centers for Disease Control and Prevention, diabetes data and statistics.
3. American Diabetes Association. The Cost of Diabetes.
4. Martin JA et al. Births: Final data for 2007. Natl Vital Stat Rep. 2010 Aug 9;58(24):1-85. PMID: 21254725.
5. Osterman MJK et al. Births: Final data for 2021. Natl Vital Stat Rep. 2023 Jan;72(1):1-53. PMID: 36723449.
6. Gregory ECW and Ely DM. Trends and characteristics in prepregnancy diabetes: United States, 2016-2021. Natl Vital Stat Rep. 2023 May;72(6):1-13. PMID: 37256333.
7. QuickStats: Percentage of mothers with gestational diabetes, by maternal age – National Vital Statistics System, United States, 2016 and 2021. MMWR Morb Mortal Wkly Rep. 2023 Jan 6;72(1):16. doi: 10.15585/mmwr.mm7201a4.
8. Langer O et al. The Diabetes in Pregnancy Study Group of North America – Introduction and summary statement. Prenat Neonat Med. 1998;3(6):514-6.
9. Willhoite MB et al. The impact of preconception counseling on pregnancy outcomes. The experience of the Maine Diabetes in Pregnancy Program. Diabetes Care. 1993 Feb;16(2):450-5. doi: 10.2337/diacare.16.2.450.
10. McElvy SS et al. A focused preconceptional and early pregnancy program in women with type 1 diabetes reduces perinatal mortality and malformation rates to general population levels. J Matern Fetal Med. 2000 Jan-Feb;9(1):14-20. doi: 10.1002/(SICI)1520-6661(200001/02)9:1<14::AID-MFM5>3.0.CO;2-K.
11. Rosen JA et al. The history and contributions of the Diabetes in Pregnancy Study Group of North America (1997-2015). Am J Perinatol. 2016 Nov;33(13):1223-6. doi: 10.1055/s-0036-1585082.
12. Driggers RW and Baschat A. The 12th meeting of the Diabetes in Pregnancy Study Group of North America (DPSG-NA): Introduction and overview. J Matern Fetal Neonatal Med. 2012 Jan;25(1):3-4. doi: 10.3109/14767058.2012.626917.
13. Langer O et al. The proceedings of the Diabetes in Pregnancy Study Group of North America 2009 conference. J Matern Fetal Neonatal Med. 2010 Mar;23(3):196-8. doi: 10.3109/14767050903550634.
14. Reece EA et al. A consensus report of the Diabetes in Pregnancy Study Group of North America Conference, Little Rock, Ark., May 2002. J Matern Fetal Neonatal Med. 2002 Dec;12(6):362-4. doi: 10.1080/jmf.12.6.362.364.
15. Reece EA and Maulik D. A consensus conference of the Diabetes in Pregnancy Study Group of North America. J Matern Fetal Neonatal Med. 2002 Dec;12(6):361. doi: 10.1080/jmf.12.6.361.361.
16. Gabbe SG. Summation of the second meeting of the Diabetes in Pregnancy Study Group of North America (DPSG-NA). J Matern Fetal Med. 2000 Jan-Feb;9(1):3-9.
17. Vital Statistics of the United States 1990: Volume I – Natality.
18. Martin JA et al. Births: final data for 2000. Natl Vital Stat Rep. 2002 Feb 12;50(5):1-101. PMID: 11876093.
19. Martin JA et al. Births: final data for 2010. Natl Vital Stat Rep. 2012 Aug 28;61(1):1-72. PMID: 24974589.
20. CDC Website. Normal weight, overweight, and obesity among adults aged 20 and over, by selected characteristics: United States.
21. Wang Y et al. Has the prevalence of overweight, obesity, and central obesity levelled off in the United States? Trends, patterns, disparities, and future projections for the obesity epidemic. Int J Epidemiol. 2020 Jun 1;49(3):810-23. doi: 10.1093/ije/dyz273.
In 2021, diabetes and related complications was the 8th leading cause of death in the United States.1 As of 2022, more than 11% of the U.S. population had diabetes and 38% of the adult U.S. population had prediabetes.2 Diabetes is the most expensive chronic condition in the United States, where $1 of every $4 in health care costs is spent on care.3
Where this is most concerning is diabetes in pregnancy. While childbirth rates in the United States have decreased since the 2007 high of 4.32 million births4 to 3.66 million in 2021,5 the incidence of diabetes in pregnancy – both pregestational and gestational – has increased. The rate of pregestational diabetes in 2021 was 10.9 per 1,000 births, a 27% increase from 2016 (8.6 per 1,000).6 The percentage of those giving birth who also were diagnosed with gestational diabetes mellitus (GDM) was 8.3% in 2021, up from 6.0% in 2016.7
Adverse outcomes for an infant born to a mother with diabetes include a higher risk of obesity and diabetes as adults, potentially leading to a forward-feeding cycle.
We and our colleagues established the Diabetes in Pregnancy Study Group of North America in 1997 because we had witnessed too frequently the devastating diabetes-induced pregnancy complications in our patients. The mission we set forth was to provide a forum for dialogue among maternal-fetal medicine subspecialists. The three main goals we set forth to support this mission were to provide a catalyst for research, contribute to the creation and refinement of medical policies, and influence professional practices in diabetes in pregnancy.8
In the last quarter century, DPSG-NA, through its annual and biennial meetings, has brought together several hundred practitioners that include physicians, nurses, statisticians, researchers, nutritionists, and allied health professionals, among others. As a group, it has improved the detection and management of diabetes in pregnant women and their offspring through knowledge sharing and influencing policies on GDM screening, diagnosis, management, and treatment. Our members have shown that preconceptional counseling for women with diabetes can significantly reduce congenital malformation and perinatal mortality compared with those women with pregestational diabetes who receive no counseling.9,10
We have addressed a wide variety of topics including the paucity of data in determining the timing of delivery for women with diabetes and the Institute of Medicine/National Academy of Medicine recommendations of gestational weight gain and risks of not adhering to them. We have learned about new scientific discoveries that reveal underlying mechanisms to diabetes-related birth defects and potential therapeutic targets; and we have discussed the health literacy requirements, ethics, and opportunities for lifestyle intervention.11-16
But we need to do more.
Two risk factors are at play: Women continue to choose to have babies at later ages and their pregnancies continue to be complicated by the rising incidence of obesity (see Figure 1 and Figure 2). 
The global obesity epidemic has become a significant concern for all aspects of health and particularly for diabetes in pregnancy. 
In 1990, 24.9% of women in the United States were obese; in 2010, 35.8%; and now more than 41%. Some experts project that by 2030 more than 80% of women in the United States will be overweight or obese.21
If we are to stop this cycle of diabetes begets more diabetes, now more than ever we need to come together and accelerate the research and education around the diabetes in pregnancy. Join us at this year’s DPSG-NA meeting Oct. 26-28 to take part in the knowledge sharing, discussions, and planning. More information can be found online at https://events.dpsg-na.com/home.
Dr. Miodovnik is adjunct professor of obstetrics, gynecology, and reproductive sciences at University of Maryland School of Medicine. Dr. Reece is professor of obstetrics, gynecology, and reproductive sciences and senior scientist at the Center for Birth Defects Research at University of Maryland School of Medicine.
References
1. Xu J et al. Mortality in the United States, 2021. NCHS Data Brief. 2022 Dec;(456):1-8. PMID: 36598387.
2. Centers for Disease Control and Prevention, diabetes data and statistics.
3. American Diabetes Association. The Cost of Diabetes.
4. Martin JA et al. Births: Final data for 2007. Natl Vital Stat Rep. 2010 Aug 9;58(24):1-85. PMID: 21254725.
5. Osterman MJK et al. Births: Final data for 2021. Natl Vital Stat Rep. 2023 Jan;72(1):1-53. PMID: 36723449.
6. Gregory ECW and Ely DM. Trends and characteristics in prepregnancy diabetes: United States, 2016-2021. Natl Vital Stat Rep. 2023 May;72(6):1-13. PMID: 37256333.
7. QuickStats: Percentage of mothers with gestational diabetes, by maternal age – National Vital Statistics System, United States, 2016 and 2021. MMWR Morb Mortal Wkly Rep. 2023 Jan 6;72(1):16. doi: 10.15585/mmwr.mm7201a4.
8. Langer O et al. The Diabetes in Pregnancy Study Group of North America – Introduction and summary statement. Prenat Neonat Med. 1998;3(6):514-6.
9. Willhoite MB et al. The impact of preconception counseling on pregnancy outcomes. The experience of the Maine Diabetes in Pregnancy Program. Diabetes Care. 1993 Feb;16(2):450-5. doi: 10.2337/diacare.16.2.450.
10. McElvy SS et al. A focused preconceptional and early pregnancy program in women with type 1 diabetes reduces perinatal mortality and malformation rates to general population levels. J Matern Fetal Med. 2000 Jan-Feb;9(1):14-20. doi: 10.1002/(SICI)1520-6661(200001/02)9:1<14::AID-MFM5>3.0.CO;2-K.
11. Rosen JA et al. The history and contributions of the Diabetes in Pregnancy Study Group of North America (1997-2015). Am J Perinatol. 2016 Nov;33(13):1223-6. doi: 10.1055/s-0036-1585082.
12. Driggers RW and Baschat A. The 12th meeting of the Diabetes in Pregnancy Study Group of North America (DPSG-NA): Introduction and overview. J Matern Fetal Neonatal Med. 2012 Jan;25(1):3-4. doi: 10.3109/14767058.2012.626917.
13. Langer O et al. The proceedings of the Diabetes in Pregnancy Study Group of North America 2009 conference. J Matern Fetal Neonatal Med. 2010 Mar;23(3):196-8. doi: 10.3109/14767050903550634.
14. Reece EA et al. A consensus report of the Diabetes in Pregnancy Study Group of North America Conference, Little Rock, Ark., May 2002. J Matern Fetal Neonatal Med. 2002 Dec;12(6):362-4. doi: 10.1080/jmf.12.6.362.364.
15. Reece EA and Maulik D. A consensus conference of the Diabetes in Pregnancy Study Group of North America. J Matern Fetal Neonatal Med. 2002 Dec;12(6):361. doi: 10.1080/jmf.12.6.361.361.
16. Gabbe SG. Summation of the second meeting of the Diabetes in Pregnancy Study Group of North America (DPSG-NA). J Matern Fetal Med. 2000 Jan-Feb;9(1):3-9.
17. Vital Statistics of the United States 1990: Volume I – Natality.
18. Martin JA et al. Births: final data for 2000. Natl Vital Stat Rep. 2002 Feb 12;50(5):1-101. PMID: 11876093.
19. Martin JA et al. Births: final data for 2010. Natl Vital Stat Rep. 2012 Aug 28;61(1):1-72. PMID: 24974589.
20. CDC Website. Normal weight, overweight, and obesity among adults aged 20 and over, by selected characteristics: United States.
21. Wang Y et al. Has the prevalence of overweight, obesity, and central obesity levelled off in the United States? Trends, patterns, disparities, and future projections for the obesity epidemic. Int J Epidemiol. 2020 Jun 1;49(3):810-23. doi: 10.1093/ije/dyz273.
In 2021, diabetes and related complications was the 8th leading cause of death in the United States.1 As of 2022, more than 11% of the U.S. population had diabetes and 38% of the adult U.S. population had prediabetes.2 Diabetes is the most expensive chronic condition in the United States, where $1 of every $4 in health care costs is spent on care.3
Where this is most concerning is diabetes in pregnancy. While childbirth rates in the United States have decreased since the 2007 high of 4.32 million births4 to 3.66 million in 2021,5 the incidence of diabetes in pregnancy – both pregestational and gestational – has increased. The rate of pregestational diabetes in 2021 was 10.9 per 1,000 births, a 27% increase from 2016 (8.6 per 1,000).6 The percentage of those giving birth who also were diagnosed with gestational diabetes mellitus (GDM) was 8.3% in 2021, up from 6.0% in 2016.7
Adverse outcomes for an infant born to a mother with diabetes include a higher risk of obesity and diabetes as adults, potentially leading to a forward-feeding cycle.
We and our colleagues established the Diabetes in Pregnancy Study Group of North America in 1997 because we had witnessed too frequently the devastating diabetes-induced pregnancy complications in our patients. The mission we set forth was to provide a forum for dialogue among maternal-fetal medicine subspecialists. The three main goals we set forth to support this mission were to provide a catalyst for research, contribute to the creation and refinement of medical policies, and influence professional practices in diabetes in pregnancy.8
In the last quarter century, DPSG-NA, through its annual and biennial meetings, has brought together several hundred practitioners that include physicians, nurses, statisticians, researchers, nutritionists, and allied health professionals, among others. As a group, it has improved the detection and management of diabetes in pregnant women and their offspring through knowledge sharing and influencing policies on GDM screening, diagnosis, management, and treatment. Our members have shown that preconceptional counseling for women with diabetes can significantly reduce congenital malformation and perinatal mortality compared with those women with pregestational diabetes who receive no counseling.9,10
We have addressed a wide variety of topics including the paucity of data in determining the timing of delivery for women with diabetes and the Institute of Medicine/National Academy of Medicine recommendations of gestational weight gain and risks of not adhering to them. We have learned about new scientific discoveries that reveal underlying mechanisms to diabetes-related birth defects and potential therapeutic targets; and we have discussed the health literacy requirements, ethics, and opportunities for lifestyle intervention.11-16
But we need to do more.
Two risk factors are at play: Women continue to choose to have babies at later ages and their pregnancies continue to be complicated by the rising incidence of obesity (see Figure 1 and Figure 2). 
The global obesity epidemic has become a significant concern for all aspects of health and particularly for diabetes in pregnancy. 
In 1990, 24.9% of women in the United States were obese; in 2010, 35.8%; and now more than 41%. Some experts project that by 2030 more than 80% of women in the United States will be overweight or obese.21
If we are to stop this cycle of diabetes begets more diabetes, now more than ever we need to come together and accelerate the research and education around the diabetes in pregnancy. Join us at this year’s DPSG-NA meeting Oct. 26-28 to take part in the knowledge sharing, discussions, and planning. More information can be found online at https://events.dpsg-na.com/home.
Dr. Miodovnik is adjunct professor of obstetrics, gynecology, and reproductive sciences at University of Maryland School of Medicine. Dr. Reece is professor of obstetrics, gynecology, and reproductive sciences and senior scientist at the Center for Birth Defects Research at University of Maryland School of Medicine.
References
1. Xu J et al. Mortality in the United States, 2021. NCHS Data Brief. 2022 Dec;(456):1-8. PMID: 36598387.
2. Centers for Disease Control and Prevention, diabetes data and statistics.
3. American Diabetes Association. The Cost of Diabetes.
4. Martin JA et al. Births: Final data for 2007. Natl Vital Stat Rep. 2010 Aug 9;58(24):1-85. PMID: 21254725.
5. Osterman MJK et al. Births: Final data for 2021. Natl Vital Stat Rep. 2023 Jan;72(1):1-53. PMID: 36723449.
6. Gregory ECW and Ely DM. Trends and characteristics in prepregnancy diabetes: United States, 2016-2021. Natl Vital Stat Rep. 2023 May;72(6):1-13. PMID: 37256333.
7. QuickStats: Percentage of mothers with gestational diabetes, by maternal age – National Vital Statistics System, United States, 2016 and 2021. MMWR Morb Mortal Wkly Rep. 2023 Jan 6;72(1):16. doi: 10.15585/mmwr.mm7201a4.
8. Langer O et al. The Diabetes in Pregnancy Study Group of North America – Introduction and summary statement. Prenat Neonat Med. 1998;3(6):514-6.
9. Willhoite MB et al. The impact of preconception counseling on pregnancy outcomes. The experience of the Maine Diabetes in Pregnancy Program. Diabetes Care. 1993 Feb;16(2):450-5. doi: 10.2337/diacare.16.2.450.
10. McElvy SS et al. A focused preconceptional and early pregnancy program in women with type 1 diabetes reduces perinatal mortality and malformation rates to general population levels. J Matern Fetal Med. 2000 Jan-Feb;9(1):14-20. doi: 10.1002/(SICI)1520-6661(200001/02)9:1<14::AID-MFM5>3.0.CO;2-K.
11. Rosen JA et al. The history and contributions of the Diabetes in Pregnancy Study Group of North America (1997-2015). Am J Perinatol. 2016 Nov;33(13):1223-6. doi: 10.1055/s-0036-1585082.
12. Driggers RW and Baschat A. The 12th meeting of the Diabetes in Pregnancy Study Group of North America (DPSG-NA): Introduction and overview. J Matern Fetal Neonatal Med. 2012 Jan;25(1):3-4. doi: 10.3109/14767058.2012.626917.
13. Langer O et al. The proceedings of the Diabetes in Pregnancy Study Group of North America 2009 conference. J Matern Fetal Neonatal Med. 2010 Mar;23(3):196-8. doi: 10.3109/14767050903550634.
14. Reece EA et al. A consensus report of the Diabetes in Pregnancy Study Group of North America Conference, Little Rock, Ark., May 2002. J Matern Fetal Neonatal Med. 2002 Dec;12(6):362-4. doi: 10.1080/jmf.12.6.362.364.
15. Reece EA and Maulik D. A consensus conference of the Diabetes in Pregnancy Study Group of North America. J Matern Fetal Neonatal Med. 2002 Dec;12(6):361. doi: 10.1080/jmf.12.6.361.361.
16. Gabbe SG. Summation of the second meeting of the Diabetes in Pregnancy Study Group of North America (DPSG-NA). J Matern Fetal Med. 2000 Jan-Feb;9(1):3-9.
17. Vital Statistics of the United States 1990: Volume I – Natality.
18. Martin JA et al. Births: final data for 2000. Natl Vital Stat Rep. 2002 Feb 12;50(5):1-101. PMID: 11876093.
19. Martin JA et al. Births: final data for 2010. Natl Vital Stat Rep. 2012 Aug 28;61(1):1-72. PMID: 24974589.
20. CDC Website. Normal weight, overweight, and obesity among adults aged 20 and over, by selected characteristics: United States.
21. Wang Y et al. Has the prevalence of overweight, obesity, and central obesity levelled off in the United States? Trends, patterns, disparities, and future projections for the obesity epidemic. Int J Epidemiol. 2020 Jun 1;49(3):810-23. doi: 10.1093/ije/dyz273.
Decoding mechanisms of diabetic embryopathy suggests therapeutic targets
Before the introduction of insulin, there were few reported cases of pregnancy complicated by diabetes because women with the disease too often did not live to childbearing age, and when they did, they were often counseled to terminate their pregnancies. Perinatal and maternal mortality in the limited number of reported pregnancies were 70% and 40%, respectively,1 making the risks of continuing the pregnancy quite high.
After insulin became available, maternal mortality dropped dramatically, down to a few percent. Perinatal mortality also declined, but it took several decades to achieve a similar magnitude of reduction.2 Today, with insulin therapy and tight glucose control as well as improved perinatal care, almost all women with diabetes can contemplate pregnancy with greater hope for normal outcomes.
Problems persist, however. Maternal diabetes continues to cause a variety of adverse outcomes, including infants large for gestational age, prematurity, and structural birth defects. Birth defects and prematurity, in fact, are the top causes of the unacceptably high infant mortality rate in the United States – a rate that is about 70% higher than the average in comparable developed countries.3
Infant mortality is considered an indicator of population health and of the development of a country; to reduce its rate, we must address these two areas.
Women with type 1 and type 2 diabetes are five times more likely to have a child with birth defects than are nondiabetic women.4 Up to 10% of women with preexisting diabetes will have fetuses with a major congenital malformation.5
Over the years we have been striving in our Center for Birth Defects Research to understand the pathomechanisms and the molecular and epigenetic alterations behind the high rates of birth defects in the offspring of women with preexisting diabetes. We have focused on heart defects and neural tube defects (particularly the latter), which together cause significant mortality, morbidity, disability, and human suffering.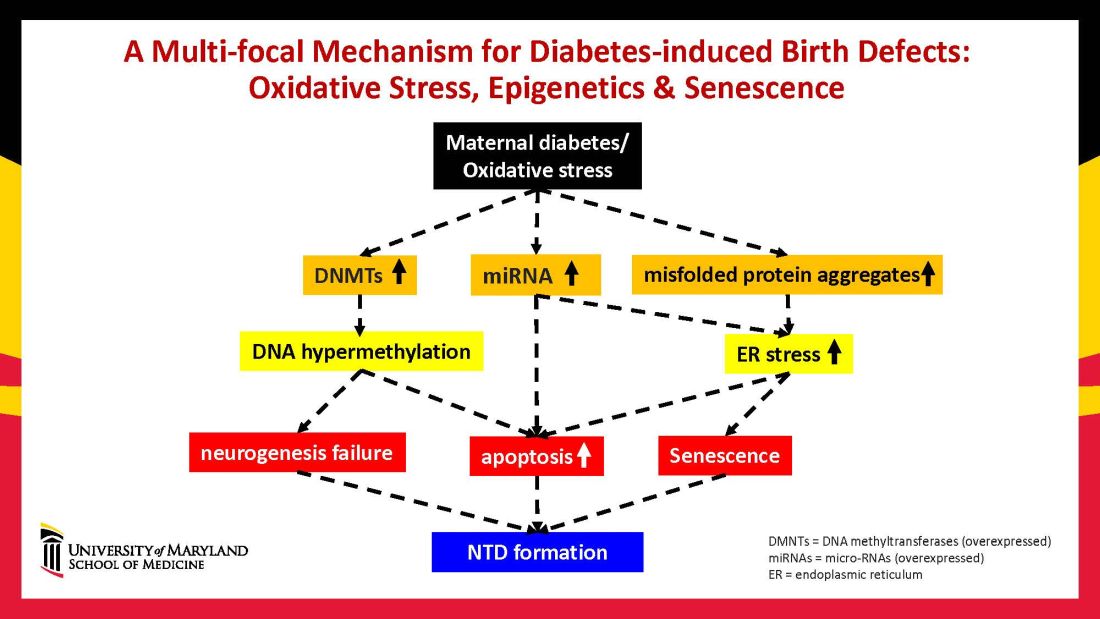
Using animal models that mimic human diabetic pregnancy, we have made significant strides in our understanding of the mechanisms, uncovering molecular pathways involving oxidative stress, senescence/premature cellular aging, and epigenetic modifications (Figure 1). Understanding these pathways is providing us, in turn, with potential therapeutic targets and approaches that may be used in the future to prevent birth defects in women who enter pregnancy with type 1 or type 2 diabetes.
Unraveling the role of oxidative stress
Our mouse models accurately reflect the human conditions of diabetes in pregnancy and diabetic embryopathy. Offspring of mice with type 1 and type 2 diabetes have a similarly higher rate of neural tube defects and congenital heart disease, compared to mice without diabetes. We observe a similar incidence of anencephaly and spina bifida, and of cardiac septation defects in the mouse embryo hearts, for instance.
A primary mechanism and causal event of diabetic embryopathy is hyperglycemia-induced apoptosis in embryonic cells. Excessive cell death in the neural epithelium or in the developing heart leads to abnormal organogenesis and dysfunctional developmental events that cause birth defects. We have identified pathways leading to apoptosis, and have found that many of these pathways crosstalk with each other.
Hyperglycemia induces oxidative stress – one of these pathways – by causing sustained generation of reactive oxygen species. The cells’ mitochondrial function is significantly impaired by the hyperglycemia response, and this diabetes-induced mitochondrial dysfunction further increases the production of reactive oxygen species and a weakening of the endogenous cellular antioxidant systems, both of which then exacerbate oxidative stress.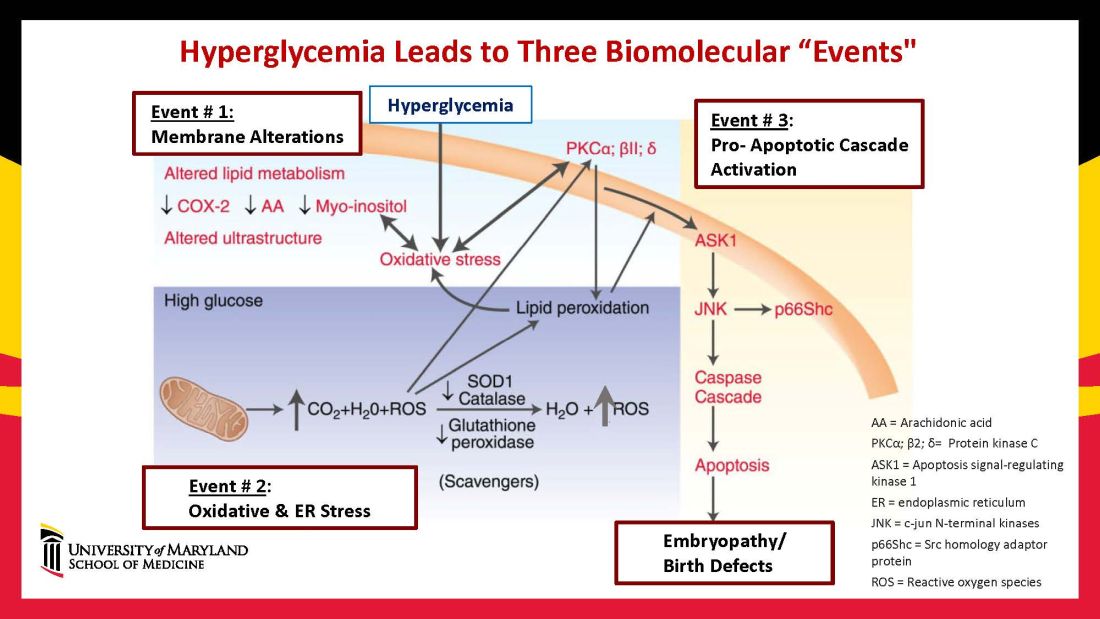
Our research has detailed what happens downstream. We’ve learned that oxidative stress in embryos exposed to maternal diabetes activates a cascade of proapoptotic kinase signaling molecules – for example, protein kinase C isoforms such as PKCalpha; apoptosis signal-regulating kinase 1; and c-Jun-N-terminal kinases – that ultimately lead to abnormal cell death in the neuroepithelium before neural tube closure (Figure 2).5
Hyperglycemia also alters membrane biochemistry in the developing embryo, suppressing lipids including arachidonic acid and myoinositol, and induces the elevation of other molecules that cause newly synthesized proteins to be misfolded. A build-up of misfolded/unfolded proteins triggers or exacerbates endoplasmic reticulum stress, which, like oxidative stress, plays a role in the activation of proapoptotic kinase signaling and apoptosis.6
When we’ve deleted genes for some of the proapoptotic kinase–signaling intermediates, or otherwise inhibited oxidative and endoplasmic reticulum stresses, we’ve been able to ameliorate neural cell apoptosis and the formation of neural tube defects. Studying the processes both forward and backward gives us confidence that the pathways are real and important, and that altering the pathways can alter the outcomes.
Reduced autophagy and induction of cellular senescence
Just as mitochondria are negatively affected by hyperglycemic conditions, so are autophagosomes – organelles that play a key role in removing abnormal or damaged stem cells and cellular components (including unfolded protein aggregates) and in maintaining cellular homeostasis. A high level of autophagy is essential for neural tube closure as well as cardiac morphogenesis.
In our models, maternal diabetes significantly suppressed the process of autophagy in neuroepithelial cells. We have identified responsible molecular intermediates and a key regulating gene for autophagy impairment and have found that deletion of the gene restores autophagy and reduces the development of neural tube defects.4 Administration of a naturally occurring compound, trehalose, which reactivates autophagy, had a similar effect.7Exposure to hyperglycemia not only causes cell death and suppresses autophagy, it also impairs other aspects of cellular function. More recently, we have shown that cells in the neuroepithelium become quiescent and cease proliferating. The quiescent cells, those cells with premature aging markers, also produce cytokines that influence the functioning and development of neighboring cells, causing additional cell death.
All told, premature senescence in the neuroepithelium adversely affects the neurulation process, leading to neural tube defects. In our mouse model, the senomorphic agent rapamycin suppressed cellular senescence, reduced the number of apoptotic neuroepithelial cells, and reduced the formation of neural tube defects.8
The role of epigenetics, future interventions
Epigenetics – the process by which gene expression and function can be modified by environmental conditions without modification of the DNA sequence – has become an additional area of focus in diabetic embryopathy. Our lab has studied the overexpression of both DNA methyltransferases (DNMTs) that cause DNA hypermethylation, and of microRNAs (miRNAs) that can suppress gene expression at the posttranscriptional level. Both are considered to be primary epigenetic mechanisms involved in human diseases and it appears that they are influential in the incidence of birth defects in diabetic mothers.
In our mouse models, maternal diabetes induces DNA hypermethylation via the increase of DNMTs, leading to the silencing of genes essential for neural tube closure and formation of the developing heart. MiRNAs also play a role; in addition to finding altered DNMT activity in the neural epithelium and other tissues of diabetes-exposed embryos, we also found altered miRNA expression. By deleting miRNA genes or by inhibiting DNMT activity through treatment with antioxidants, we saw significant reductions in birth defects.
In one study of the green tea polyphenol epigallocatechin gallate (EGCG), we demonstrated inhibition of diabetes-elevated DNMT expression and activity and suppression of DNA hypermethylation. The expression of genes essential for neural tube closure was restored, with a subsequent reduction in neural tube defects from 29.5% to 2% in embryos treated with EGCG.9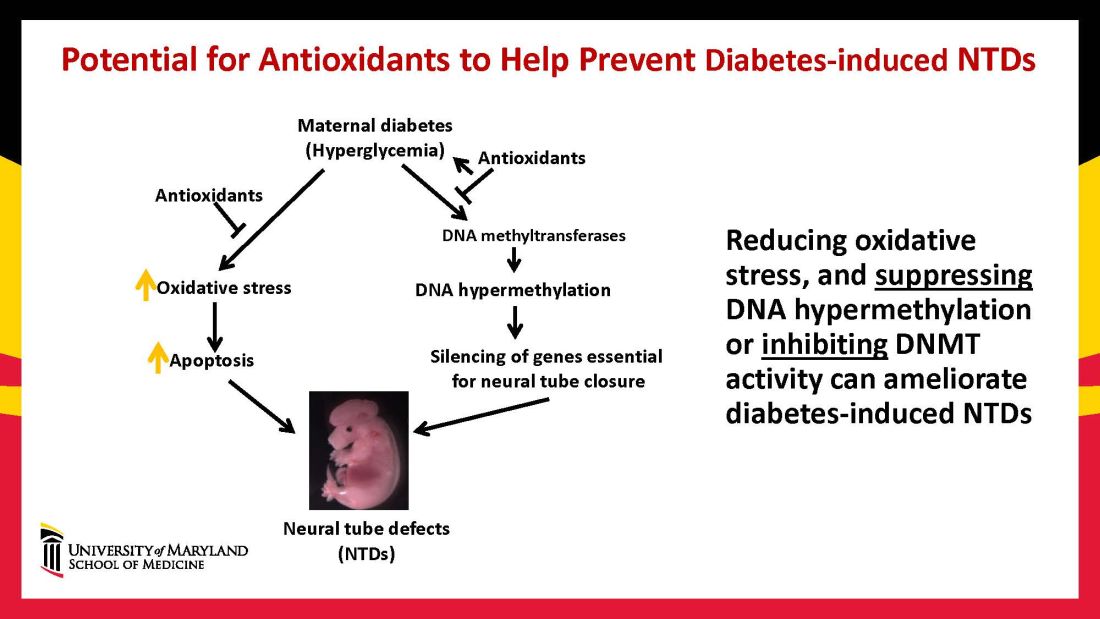
Our interventions to reverse or alter the mechanisms and pathways leading to birth defects have not only helped prove causation, but have given us hope for the future. Antioxidants are among the compounds that could be used as dietary supplements during pregnancy to prevent structural birth defects (Figure 3). Other compounds could activate the process of autophagy (for example, trehalose) and antisenescence compounds similar to rapamycin could be used to reduce numbers of senescent cells in the neuroepithelium or the developing heart.
Dr. Reece and Dr. Yang reported no relevant disclosures.
Dr. Reece, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president, endowed professor and director of CARTI, and codirector of the Center for Birth Defects.
*This story was updated on Nov. 3, 2022
References
1. Z Zhiyong and Reece EA. Clin Lab Med. 2013;33(2)207-33.
2. Reece EA and Coustan DR. Diabetes and obesity in women. Wolters Kluwer: 2019. 4th ed. (https://www.amazon.com/Diabetes-Obesity-Women-Albert-Reece/dp/1496390547).
3. The Peterson-KFF Health System Tracker. www.healthsystemtracker.org.
4. Wang F et al. Nat. Commun. 2017;8:15182.
5. Yang P et al. Am J Obstet Gynecol. 2015;212(5):569-79.
6. Li X et al. Diabetes. 2013 Feb;62(2):599-608.
7. Xu C et al. Am J Physiol Endocrinol Metab. 2013 Sep 1;305(5):E667-78.
8. Xu C et al. Sci Adv. 2021;7(27):eabf5089.
9. Zhong J et al. Am J Obstet Gynecol. 2016 Sep;215(3):368.e1-10.
Before the introduction of insulin, there were few reported cases of pregnancy complicated by diabetes because women with the disease too often did not live to childbearing age, and when they did, they were often counseled to terminate their pregnancies. Perinatal and maternal mortality in the limited number of reported pregnancies were 70% and 40%, respectively,1 making the risks of continuing the pregnancy quite high.
After insulin became available, maternal mortality dropped dramatically, down to a few percent. Perinatal mortality also declined, but it took several decades to achieve a similar magnitude of reduction.2 Today, with insulin therapy and tight glucose control as well as improved perinatal care, almost all women with diabetes can contemplate pregnancy with greater hope for normal outcomes.
Problems persist, however. Maternal diabetes continues to cause a variety of adverse outcomes, including infants large for gestational age, prematurity, and structural birth defects. Birth defects and prematurity, in fact, are the top causes of the unacceptably high infant mortality rate in the United States – a rate that is about 70% higher than the average in comparable developed countries.3
Infant mortality is considered an indicator of population health and of the development of a country; to reduce its rate, we must address these two areas.
Women with type 1 and type 2 diabetes are five times more likely to have a child with birth defects than are nondiabetic women.4 Up to 10% of women with preexisting diabetes will have fetuses with a major congenital malformation.5
Over the years we have been striving in our Center for Birth Defects Research to understand the pathomechanisms and the molecular and epigenetic alterations behind the high rates of birth defects in the offspring of women with preexisting diabetes. We have focused on heart defects and neural tube defects (particularly the latter), which together cause significant mortality, morbidity, disability, and human suffering.
Using animal models that mimic human diabetic pregnancy, we have made significant strides in our understanding of the mechanisms, uncovering molecular pathways involving oxidative stress, senescence/premature cellular aging, and epigenetic modifications (Figure 1). Understanding these pathways is providing us, in turn, with potential therapeutic targets and approaches that may be used in the future to prevent birth defects in women who enter pregnancy with type 1 or type 2 diabetes.
Unraveling the role of oxidative stress
Our mouse models accurately reflect the human conditions of diabetes in pregnancy and diabetic embryopathy. Offspring of mice with type 1 and type 2 diabetes have a similarly higher rate of neural tube defects and congenital heart disease, compared to mice without diabetes. We observe a similar incidence of anencephaly and spina bifida, and of cardiac septation defects in the mouse embryo hearts, for instance.
A primary mechanism and causal event of diabetic embryopathy is hyperglycemia-induced apoptosis in embryonic cells. Excessive cell death in the neural epithelium or in the developing heart leads to abnormal organogenesis and dysfunctional developmental events that cause birth defects. We have identified pathways leading to apoptosis, and have found that many of these pathways crosstalk with each other.
Hyperglycemia induces oxidative stress – one of these pathways – by causing sustained generation of reactive oxygen species. The cells’ mitochondrial function is significantly impaired by the hyperglycemia response, and this diabetes-induced mitochondrial dysfunction further increases the production of reactive oxygen species and a weakening of the endogenous cellular antioxidant systems, both of which then exacerbate oxidative stress.
Our research has detailed what happens downstream. We’ve learned that oxidative stress in embryos exposed to maternal diabetes activates a cascade of proapoptotic kinase signaling molecules – for example, protein kinase C isoforms such as PKCalpha; apoptosis signal-regulating kinase 1; and c-Jun-N-terminal kinases – that ultimately lead to abnormal cell death in the neuroepithelium before neural tube closure (Figure 2).5
Hyperglycemia also alters membrane biochemistry in the developing embryo, suppressing lipids including arachidonic acid and myoinositol, and induces the elevation of other molecules that cause newly synthesized proteins to be misfolded. A build-up of misfolded/unfolded proteins triggers or exacerbates endoplasmic reticulum stress, which, like oxidative stress, plays a role in the activation of proapoptotic kinase signaling and apoptosis.6
When we’ve deleted genes for some of the proapoptotic kinase–signaling intermediates, or otherwise inhibited oxidative and endoplasmic reticulum stresses, we’ve been able to ameliorate neural cell apoptosis and the formation of neural tube defects. Studying the processes both forward and backward gives us confidence that the pathways are real and important, and that altering the pathways can alter the outcomes.
Reduced autophagy and induction of cellular senescence
Just as mitochondria are negatively affected by hyperglycemic conditions, so are autophagosomes – organelles that play a key role in removing abnormal or damaged stem cells and cellular components (including unfolded protein aggregates) and in maintaining cellular homeostasis. A high level of autophagy is essential for neural tube closure as well as cardiac morphogenesis.
In our models, maternal diabetes significantly suppressed the process of autophagy in neuroepithelial cells. We have identified responsible molecular intermediates and a key regulating gene for autophagy impairment and have found that deletion of the gene restores autophagy and reduces the development of neural tube defects.4 Administration of a naturally occurring compound, trehalose, which reactivates autophagy, had a similar effect.7Exposure to hyperglycemia not only causes cell death and suppresses autophagy, it also impairs other aspects of cellular function. More recently, we have shown that cells in the neuroepithelium become quiescent and cease proliferating. The quiescent cells, those cells with premature aging markers, also produce cytokines that influence the functioning and development of neighboring cells, causing additional cell death.
All told, premature senescence in the neuroepithelium adversely affects the neurulation process, leading to neural tube defects. In our mouse model, the senomorphic agent rapamycin suppressed cellular senescence, reduced the number of apoptotic neuroepithelial cells, and reduced the formation of neural tube defects.8
The role of epigenetics, future interventions
Epigenetics – the process by which gene expression and function can be modified by environmental conditions without modification of the DNA sequence – has become an additional area of focus in diabetic embryopathy. Our lab has studied the overexpression of both DNA methyltransferases (DNMTs) that cause DNA hypermethylation, and of microRNAs (miRNAs) that can suppress gene expression at the posttranscriptional level. Both are considered to be primary epigenetic mechanisms involved in human diseases and it appears that they are influential in the incidence of birth defects in diabetic mothers.
In our mouse models, maternal diabetes induces DNA hypermethylation via the increase of DNMTs, leading to the silencing of genes essential for neural tube closure and formation of the developing heart. MiRNAs also play a role; in addition to finding altered DNMT activity in the neural epithelium and other tissues of diabetes-exposed embryos, we also found altered miRNA expression. By deleting miRNA genes or by inhibiting DNMT activity through treatment with antioxidants, we saw significant reductions in birth defects.
In one study of the green tea polyphenol epigallocatechin gallate (EGCG), we demonstrated inhibition of diabetes-elevated DNMT expression and activity and suppression of DNA hypermethylation. The expression of genes essential for neural tube closure was restored, with a subsequent reduction in neural tube defects from 29.5% to 2% in embryos treated with EGCG.9
Our interventions to reverse or alter the mechanisms and pathways leading to birth defects have not only helped prove causation, but have given us hope for the future. Antioxidants are among the compounds that could be used as dietary supplements during pregnancy to prevent structural birth defects (Figure 3). Other compounds could activate the process of autophagy (for example, trehalose) and antisenescence compounds similar to rapamycin could be used to reduce numbers of senescent cells in the neuroepithelium or the developing heart.
Dr. Reece and Dr. Yang reported no relevant disclosures.
Dr. Reece, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president, endowed professor and director of CARTI, and codirector of the Center for Birth Defects.
*This story was updated on Nov. 3, 2022
References
1. Z Zhiyong and Reece EA. Clin Lab Med. 2013;33(2)207-33.
2. Reece EA and Coustan DR. Diabetes and obesity in women. Wolters Kluwer: 2019. 4th ed. (https://www.amazon.com/Diabetes-Obesity-Women-Albert-Reece/dp/1496390547).
3. The Peterson-KFF Health System Tracker. www.healthsystemtracker.org.
4. Wang F et al. Nat. Commun. 2017;8:15182.
5. Yang P et al. Am J Obstet Gynecol. 2015;212(5):569-79.
6. Li X et al. Diabetes. 2013 Feb;62(2):599-608.
7. Xu C et al. Am J Physiol Endocrinol Metab. 2013 Sep 1;305(5):E667-78.
8. Xu C et al. Sci Adv. 2021;7(27):eabf5089.
9. Zhong J et al. Am J Obstet Gynecol. 2016 Sep;215(3):368.e1-10.
Before the introduction of insulin, there were few reported cases of pregnancy complicated by diabetes because women with the disease too often did not live to childbearing age, and when they did, they were often counseled to terminate their pregnancies. Perinatal and maternal mortality in the limited number of reported pregnancies were 70% and 40%, respectively,1 making the risks of continuing the pregnancy quite high.
After insulin became available, maternal mortality dropped dramatically, down to a few percent. Perinatal mortality also declined, but it took several decades to achieve a similar magnitude of reduction.2 Today, with insulin therapy and tight glucose control as well as improved perinatal care, almost all women with diabetes can contemplate pregnancy with greater hope for normal outcomes.
Problems persist, however. Maternal diabetes continues to cause a variety of adverse outcomes, including infants large for gestational age, prematurity, and structural birth defects. Birth defects and prematurity, in fact, are the top causes of the unacceptably high infant mortality rate in the United States – a rate that is about 70% higher than the average in comparable developed countries.3
Infant mortality is considered an indicator of population health and of the development of a country; to reduce its rate, we must address these two areas.
Women with type 1 and type 2 diabetes are five times more likely to have a child with birth defects than are nondiabetic women.4 Up to 10% of women with preexisting diabetes will have fetuses with a major congenital malformation.5
Over the years we have been striving in our Center for Birth Defects Research to understand the pathomechanisms and the molecular and epigenetic alterations behind the high rates of birth defects in the offspring of women with preexisting diabetes. We have focused on heart defects and neural tube defects (particularly the latter), which together cause significant mortality, morbidity, disability, and human suffering.
Using animal models that mimic human diabetic pregnancy, we have made significant strides in our understanding of the mechanisms, uncovering molecular pathways involving oxidative stress, senescence/premature cellular aging, and epigenetic modifications (Figure 1). Understanding these pathways is providing us, in turn, with potential therapeutic targets and approaches that may be used in the future to prevent birth defects in women who enter pregnancy with type 1 or type 2 diabetes.
Unraveling the role of oxidative stress
Our mouse models accurately reflect the human conditions of diabetes in pregnancy and diabetic embryopathy. Offspring of mice with type 1 and type 2 diabetes have a similarly higher rate of neural tube defects and congenital heart disease, compared to mice without diabetes. We observe a similar incidence of anencephaly and spina bifida, and of cardiac septation defects in the mouse embryo hearts, for instance.
A primary mechanism and causal event of diabetic embryopathy is hyperglycemia-induced apoptosis in embryonic cells. Excessive cell death in the neural epithelium or in the developing heart leads to abnormal organogenesis and dysfunctional developmental events that cause birth defects. We have identified pathways leading to apoptosis, and have found that many of these pathways crosstalk with each other.
Hyperglycemia induces oxidative stress – one of these pathways – by causing sustained generation of reactive oxygen species. The cells’ mitochondrial function is significantly impaired by the hyperglycemia response, and this diabetes-induced mitochondrial dysfunction further increases the production of reactive oxygen species and a weakening of the endogenous cellular antioxidant systems, both of which then exacerbate oxidative stress.
Our research has detailed what happens downstream. We’ve learned that oxidative stress in embryos exposed to maternal diabetes activates a cascade of proapoptotic kinase signaling molecules – for example, protein kinase C isoforms such as PKCalpha; apoptosis signal-regulating kinase 1; and c-Jun-N-terminal kinases – that ultimately lead to abnormal cell death in the neuroepithelium before neural tube closure (Figure 2).5
Hyperglycemia also alters membrane biochemistry in the developing embryo, suppressing lipids including arachidonic acid and myoinositol, and induces the elevation of other molecules that cause newly synthesized proteins to be misfolded. A build-up of misfolded/unfolded proteins triggers or exacerbates endoplasmic reticulum stress, which, like oxidative stress, plays a role in the activation of proapoptotic kinase signaling and apoptosis.6
When we’ve deleted genes for some of the proapoptotic kinase–signaling intermediates, or otherwise inhibited oxidative and endoplasmic reticulum stresses, we’ve been able to ameliorate neural cell apoptosis and the formation of neural tube defects. Studying the processes both forward and backward gives us confidence that the pathways are real and important, and that altering the pathways can alter the outcomes.
Reduced autophagy and induction of cellular senescence
Just as mitochondria are negatively affected by hyperglycemic conditions, so are autophagosomes – organelles that play a key role in removing abnormal or damaged stem cells and cellular components (including unfolded protein aggregates) and in maintaining cellular homeostasis. A high level of autophagy is essential for neural tube closure as well as cardiac morphogenesis.
In our models, maternal diabetes significantly suppressed the process of autophagy in neuroepithelial cells. We have identified responsible molecular intermediates and a key regulating gene for autophagy impairment and have found that deletion of the gene restores autophagy and reduces the development of neural tube defects.4 Administration of a naturally occurring compound, trehalose, which reactivates autophagy, had a similar effect.7Exposure to hyperglycemia not only causes cell death and suppresses autophagy, it also impairs other aspects of cellular function. More recently, we have shown that cells in the neuroepithelium become quiescent and cease proliferating. The quiescent cells, those cells with premature aging markers, also produce cytokines that influence the functioning and development of neighboring cells, causing additional cell death.
All told, premature senescence in the neuroepithelium adversely affects the neurulation process, leading to neural tube defects. In our mouse model, the senomorphic agent rapamycin suppressed cellular senescence, reduced the number of apoptotic neuroepithelial cells, and reduced the formation of neural tube defects.8
The role of epigenetics, future interventions
Epigenetics – the process by which gene expression and function can be modified by environmental conditions without modification of the DNA sequence – has become an additional area of focus in diabetic embryopathy. Our lab has studied the overexpression of both DNA methyltransferases (DNMTs) that cause DNA hypermethylation, and of microRNAs (miRNAs) that can suppress gene expression at the posttranscriptional level. Both are considered to be primary epigenetic mechanisms involved in human diseases and it appears that they are influential in the incidence of birth defects in diabetic mothers.
In our mouse models, maternal diabetes induces DNA hypermethylation via the increase of DNMTs, leading to the silencing of genes essential for neural tube closure and formation of the developing heart. MiRNAs also play a role; in addition to finding altered DNMT activity in the neural epithelium and other tissues of diabetes-exposed embryos, we also found altered miRNA expression. By deleting miRNA genes or by inhibiting DNMT activity through treatment with antioxidants, we saw significant reductions in birth defects.
In one study of the green tea polyphenol epigallocatechin gallate (EGCG), we demonstrated inhibition of diabetes-elevated DNMT expression and activity and suppression of DNA hypermethylation. The expression of genes essential for neural tube closure was restored, with a subsequent reduction in neural tube defects from 29.5% to 2% in embryos treated with EGCG.9
Our interventions to reverse or alter the mechanisms and pathways leading to birth defects have not only helped prove causation, but have given us hope for the future. Antioxidants are among the compounds that could be used as dietary supplements during pregnancy to prevent structural birth defects (Figure 3). Other compounds could activate the process of autophagy (for example, trehalose) and antisenescence compounds similar to rapamycin could be used to reduce numbers of senescent cells in the neuroepithelium or the developing heart.
Dr. Reece and Dr. Yang reported no relevant disclosures.
Dr. Reece, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president, endowed professor and director of CARTI, and codirector of the Center for Birth Defects.
*This story was updated on Nov. 3, 2022
References
1. Z Zhiyong and Reece EA. Clin Lab Med. 2013;33(2)207-33.
2. Reece EA and Coustan DR. Diabetes and obesity in women. Wolters Kluwer: 2019. 4th ed. (https://www.amazon.com/Diabetes-Obesity-Women-Albert-Reece/dp/1496390547).
3. The Peterson-KFF Health System Tracker. www.healthsystemtracker.org.
4. Wang F et al. Nat. Commun. 2017;8:15182.
5. Yang P et al. Am J Obstet Gynecol. 2015;212(5):569-79.
6. Li X et al. Diabetes. 2013 Feb;62(2):599-608.
7. Xu C et al. Am J Physiol Endocrinol Metab. 2013 Sep 1;305(5):E667-78.
8. Xu C et al. Sci Adv. 2021;7(27):eabf5089.
9. Zhong J et al. Am J Obstet Gynecol. 2016 Sep;215(3):368.e1-10.
Managing maternal mortality with multifetal pregnancy reduction
For over 2 years, the world has reeled from the COVID-19 pandemic. Life has changed dramatically, priorities have been re-examined, and the collective approach to health care has shifted tremendously. While concerns regarding coronavirus and its variants are warranted, another “pandemic” is ravaging the world and has yet to be fully addressed: pregnancy-related maternal mortality.
The rate of pregnancy-related deaths in the United States is unconscionable. Compared with other developed nations – such as Germany, the United Kingdom, and Canada – we lag far behind. Data published in 2020 showed that the rate of maternal deaths per 100,000 live births in the United States was 17.4, more than double that of France (8.7 deaths per 100,000 live births),1 the country with the next-highest rate. Americans like being first – first to invent the light bulb, first to perform a successful solid organ xenotransplantation, first to go to the moon – but holding “first place” in maternal mortality is not something we should wish to maintain.
Ob.gyns. have long raised the alarm regarding the exceedingly high rates of pregnancy-related deaths in the United States. While there have been many advances in antenatal care to reduce these severe adverse events – improvements in surveillance and data reporting, maternal-focused telemedicine services, multidisciplinary care team models, and numerous research initiatives by federal and nonprofit organizations2 – the recent wave of legislation restricting reproductive choice may also have the unintended consequence of further increasing the rate of pregnancy-related maternal morbidity and mortality.3
While we have an obligation to provide our maternal and fetal patients with the best possible care, under some circumstances, that care may require prioritizing the mother’s health above all else.
To discuss the judicious use of multifetal pregnancy reduction, we have invited Dr. Joanne Stone, The Ellen and Howard C. Katz Chairman’s Chair, and Dr. Chelsea DeBolt, clinical fellow in maternal-fetal medicine, both in the Raquel and Jaime Gilinski Department of Obstetrics, Gynecology, and Reproductive Science at the Icahn School of Medicine at Mount Sinai.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
References
1. Tikkanen R et al. The Commonwealth Fund. Nov 2020. doi: 10.26099/411v-9255
2. Ahn R et al. Ann Intern Med. 2020;173(11 Suppl):S3-10. doi: 10.7326/M19-3258.
3. Pabayo R et al. Int J Environ Res Public Health. 2020;17(11):3773. doi: 10.3390/ijerph17113773.
For over 2 years, the world has reeled from the COVID-19 pandemic. Life has changed dramatically, priorities have been re-examined, and the collective approach to health care has shifted tremendously. While concerns regarding coronavirus and its variants are warranted, another “pandemic” is ravaging the world and has yet to be fully addressed: pregnancy-related maternal mortality.
The rate of pregnancy-related deaths in the United States is unconscionable. Compared with other developed nations – such as Germany, the United Kingdom, and Canada – we lag far behind. Data published in 2020 showed that the rate of maternal deaths per 100,000 live births in the United States was 17.4, more than double that of France (8.7 deaths per 100,000 live births),1 the country with the next-highest rate. Americans like being first – first to invent the light bulb, first to perform a successful solid organ xenotransplantation, first to go to the moon – but holding “first place” in maternal mortality is not something we should wish to maintain.
Ob.gyns. have long raised the alarm regarding the exceedingly high rates of pregnancy-related deaths in the United States. While there have been many advances in antenatal care to reduce these severe adverse events – improvements in surveillance and data reporting, maternal-focused telemedicine services, multidisciplinary care team models, and numerous research initiatives by federal and nonprofit organizations2 – the recent wave of legislation restricting reproductive choice may also have the unintended consequence of further increasing the rate of pregnancy-related maternal morbidity and mortality.3
While we have an obligation to provide our maternal and fetal patients with the best possible care, under some circumstances, that care may require prioritizing the mother’s health above all else.
To discuss the judicious use of multifetal pregnancy reduction, we have invited Dr. Joanne Stone, The Ellen and Howard C. Katz Chairman’s Chair, and Dr. Chelsea DeBolt, clinical fellow in maternal-fetal medicine, both in the Raquel and Jaime Gilinski Department of Obstetrics, Gynecology, and Reproductive Science at the Icahn School of Medicine at Mount Sinai.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
References
1. Tikkanen R et al. The Commonwealth Fund. Nov 2020. doi: 10.26099/411v-9255
2. Ahn R et al. Ann Intern Med. 2020;173(11 Suppl):S3-10. doi: 10.7326/M19-3258.
3. Pabayo R et al. Int J Environ Res Public Health. 2020;17(11):3773. doi: 10.3390/ijerph17113773.
For over 2 years, the world has reeled from the COVID-19 pandemic. Life has changed dramatically, priorities have been re-examined, and the collective approach to health care has shifted tremendously. While concerns regarding coronavirus and its variants are warranted, another “pandemic” is ravaging the world and has yet to be fully addressed: pregnancy-related maternal mortality.
The rate of pregnancy-related deaths in the United States is unconscionable. Compared with other developed nations – such as Germany, the United Kingdom, and Canada – we lag far behind. Data published in 2020 showed that the rate of maternal deaths per 100,000 live births in the United States was 17.4, more than double that of France (8.7 deaths per 100,000 live births),1 the country with the next-highest rate. Americans like being first – first to invent the light bulb, first to perform a successful solid organ xenotransplantation, first to go to the moon – but holding “first place” in maternal mortality is not something we should wish to maintain.
Ob.gyns. have long raised the alarm regarding the exceedingly high rates of pregnancy-related deaths in the United States. While there have been many advances in antenatal care to reduce these severe adverse events – improvements in surveillance and data reporting, maternal-focused telemedicine services, multidisciplinary care team models, and numerous research initiatives by federal and nonprofit organizations2 – the recent wave of legislation restricting reproductive choice may also have the unintended consequence of further increasing the rate of pregnancy-related maternal morbidity and mortality.3
While we have an obligation to provide our maternal and fetal patients with the best possible care, under some circumstances, that care may require prioritizing the mother’s health above all else.
To discuss the judicious use of multifetal pregnancy reduction, we have invited Dr. Joanne Stone, The Ellen and Howard C. Katz Chairman’s Chair, and Dr. Chelsea DeBolt, clinical fellow in maternal-fetal medicine, both in the Raquel and Jaime Gilinski Department of Obstetrics, Gynecology, and Reproductive Science at the Icahn School of Medicine at Mount Sinai.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
References
1. Tikkanen R et al. The Commonwealth Fund. Nov 2020. doi: 10.26099/411v-9255
2. Ahn R et al. Ann Intern Med. 2020;173(11 Suppl):S3-10. doi: 10.7326/M19-3258.
3. Pabayo R et al. Int J Environ Res Public Health. 2020;17(11):3773. doi: 10.3390/ijerph17113773.
Insulin in pregnancy: A look back at history for Diabetes Awareness Month
Each November, Diabetes Awareness Month, we commemorate the myriad advances that have made living with diabetes possible. This year is especially auspicious as it marks the 100th anniversary of the discovery of insulin by Frederick Banting, MD, and Charles Best, MD. The miracle of insulin cannot be overstated. In the preinsulin era, life expectancy after a diabetes diagnosis was 4-7 years for a 30-year-old patient. Within 3 years after the introduction of insulin, life expectancy after diagnosis jumped to about 17 years, a 167% increase.1
For ob.gyns. and their patients, insulin was a godsend. In the early 1920s, patients with pre-existing diabetes and pregnancy (recall that gestational diabetes mellitus would not be recognized as a unique condition until the 1960s)2 were advised to terminate the pregnancy; those who did not do so faced almost certain death for the fetus and, sometimes, themselves.3 By 1935, approximately 10 years after the introduction of insulin into practice, perinatal mortality dropped by 25%. By 1955, it had dropped by nearly 63%.4
The advent of technologies such as continuous glucose monitors, mobile phone–based health applications, and the artificial pancreas, have further transformed diabetes care.5 In addition, studies using animal models of diabetic pregnancy have revealed the molecular mechanisms responsible for hyperglycemia-induced birth defects – including alterations in lipid metabolism, excess generation of free radicals, and aberrant cell death – and uncovered potential strategies for prevention.6
To reflect on the herculean accomplishments in ob.gyn. since the discovery of insulin, we have invited two pillars of the diabetes in pregnancy research and clinical care communities: Steven G. Gabbe, MD, current professor of ob.gyn. at The Ohio State University (OSU) College of Medicine, former chair of ob.gyn. at OSU and University of Washington Medical Center, former senior vice president for health sciences and CEO of the OSU Medical Center, and former dean of Vanderbilt University School of Medicine; and Mark B. Landon, MD, the Richard L. Meiling professor and chair of ob.gyn. at OSU.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He has no relevant financial disclosures. Contact him at obnews@mdedge.com.
References
1. Brostoff JM et al. Diabetologia. 2007;50(6):1351-3.
2. Panaitescu AM and Peltecu G. Acta Endocrinol (Buchar). 2016;12(3):331-4.
3. Joslin EP. Boston Med Surg J 1915;173:841-9.
4. Gabbe SG and Graves CR. Obstet Gynecol. 2003;102(4):857-68.
5. Crimmins SD et al. Clin Diabetes. 2020;38(5):486-94.
6. Gabbay-Benziv R et al. World J Diabetes. 2015;6(3):481-8.
Each November, Diabetes Awareness Month, we commemorate the myriad advances that have made living with diabetes possible. This year is especially auspicious as it marks the 100th anniversary of the discovery of insulin by Frederick Banting, MD, and Charles Best, MD. The miracle of insulin cannot be overstated. In the preinsulin era, life expectancy after a diabetes diagnosis was 4-7 years for a 30-year-old patient. Within 3 years after the introduction of insulin, life expectancy after diagnosis jumped to about 17 years, a 167% increase.1
For ob.gyns. and their patients, insulin was a godsend. In the early 1920s, patients with pre-existing diabetes and pregnancy (recall that gestational diabetes mellitus would not be recognized as a unique condition until the 1960s)2 were advised to terminate the pregnancy; those who did not do so faced almost certain death for the fetus and, sometimes, themselves.3 By 1935, approximately 10 years after the introduction of insulin into practice, perinatal mortality dropped by 25%. By 1955, it had dropped by nearly 63%.4
The advent of technologies such as continuous glucose monitors, mobile phone–based health applications, and the artificial pancreas, have further transformed diabetes care.5 In addition, studies using animal models of diabetic pregnancy have revealed the molecular mechanisms responsible for hyperglycemia-induced birth defects – including alterations in lipid metabolism, excess generation of free radicals, and aberrant cell death – and uncovered potential strategies for prevention.6
To reflect on the herculean accomplishments in ob.gyn. since the discovery of insulin, we have invited two pillars of the diabetes in pregnancy research and clinical care communities: Steven G. Gabbe, MD, current professor of ob.gyn. at The Ohio State University (OSU) College of Medicine, former chair of ob.gyn. at OSU and University of Washington Medical Center, former senior vice president for health sciences and CEO of the OSU Medical Center, and former dean of Vanderbilt University School of Medicine; and Mark B. Landon, MD, the Richard L. Meiling professor and chair of ob.gyn. at OSU.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He has no relevant financial disclosures. Contact him at obnews@mdedge.com.
References
1. Brostoff JM et al. Diabetologia. 2007;50(6):1351-3.
2. Panaitescu AM and Peltecu G. Acta Endocrinol (Buchar). 2016;12(3):331-4.
3. Joslin EP. Boston Med Surg J 1915;173:841-9.
4. Gabbe SG and Graves CR. Obstet Gynecol. 2003;102(4):857-68.
5. Crimmins SD et al. Clin Diabetes. 2020;38(5):486-94.
6. Gabbay-Benziv R et al. World J Diabetes. 2015;6(3):481-8.
Each November, Diabetes Awareness Month, we commemorate the myriad advances that have made living with diabetes possible. This year is especially auspicious as it marks the 100th anniversary of the discovery of insulin by Frederick Banting, MD, and Charles Best, MD. The miracle of insulin cannot be overstated. In the preinsulin era, life expectancy after a diabetes diagnosis was 4-7 years for a 30-year-old patient. Within 3 years after the introduction of insulin, life expectancy after diagnosis jumped to about 17 years, a 167% increase.1
For ob.gyns. and their patients, insulin was a godsend. In the early 1920s, patients with pre-existing diabetes and pregnancy (recall that gestational diabetes mellitus would not be recognized as a unique condition until the 1960s)2 were advised to terminate the pregnancy; those who did not do so faced almost certain death for the fetus and, sometimes, themselves.3 By 1935, approximately 10 years after the introduction of insulin into practice, perinatal mortality dropped by 25%. By 1955, it had dropped by nearly 63%.4
The advent of technologies such as continuous glucose monitors, mobile phone–based health applications, and the artificial pancreas, have further transformed diabetes care.5 In addition, studies using animal models of diabetic pregnancy have revealed the molecular mechanisms responsible for hyperglycemia-induced birth defects – including alterations in lipid metabolism, excess generation of free radicals, and aberrant cell death – and uncovered potential strategies for prevention.6
To reflect on the herculean accomplishments in ob.gyn. since the discovery of insulin, we have invited two pillars of the diabetes in pregnancy research and clinical care communities: Steven G. Gabbe, MD, current professor of ob.gyn. at The Ohio State University (OSU) College of Medicine, former chair of ob.gyn. at OSU and University of Washington Medical Center, former senior vice president for health sciences and CEO of the OSU Medical Center, and former dean of Vanderbilt University School of Medicine; and Mark B. Landon, MD, the Richard L. Meiling professor and chair of ob.gyn. at OSU.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He has no relevant financial disclosures. Contact him at obnews@mdedge.com.
References
1. Brostoff JM et al. Diabetologia. 2007;50(6):1351-3.
2. Panaitescu AM and Peltecu G. Acta Endocrinol (Buchar). 2016;12(3):331-4.
3. Joslin EP. Boston Med Surg J 1915;173:841-9.
4. Gabbe SG and Graves CR. Obstet Gynecol. 2003;102(4):857-68.
5. Crimmins SD et al. Clin Diabetes. 2020;38(5):486-94.
6. Gabbay-Benziv R et al. World J Diabetes. 2015;6(3):481-8.
How advances in genomics have informed obstetrics practice
The publication of the draft sequence for the human genome changed the research and clinical medicine landscape forever. This genetic map created the possibility to develop more personalized health care and targeted therapeutics. It opened the door to the age of “big data” sets in biomedical research, fusing science, computer technology, and mathematics – the “s,” “t,” and “m” of “STEM.”
In the 20 years that followed the publication of the human genome, many advances in biomedicine occurred. Improvements in DNA sequencing technologies, built upon the original sequencing project, made the noninvasive prenatal screening test (NIPT) possible. The ease, speed, and cost effectiveness of sequencing has made diagnosing fetal structural anomalies using whole-exome sequencing a reality.
However, uncovering humanity’s genetic code introduced new quandaries and reopened old wounds: How would a person’s genetic data be used? Could a person’s risk for disease, identified through sequencing, lead to overdiagnosis? Would knowing the human genome reinforce age-old ideas that genes make one group superior or inferior? Could we now create “designer babies”?
This last question has become even more pressing with the advent of human gene editing technology, also known by its acronym “CRISPR.” , but it also has the potential for bringing us to the precipice of a Wellsian reality. The alarming claim that scientists had used CRISPR to edit the genes of human babies (Nature. 2020;577[7789]:154-5; doi:10.1038/d41586-020-00001-y) has rippled through the biomedical community and spurred numerous debates on the ethics of using such a powerful tool (Human Genome Editing: Science, Ethics, and Governance; doi: 10.17226/24623).
The passage of the Genetic Information Non-discrimination Act (GINA; https://www.eeoc.gov/statutes/genetic-information-nondiscrimination-act-2008) in 2008 ensured that health insurance companies and employers could not use a person’s genome against them, creating a balance between the forces of “can we?” and “should we?” Yet, many ethical questions remain.
We have invited two experts from the University of Maryland (Baltimore) School of Medicine’s department of obstetrics, gynecology & reproductive sciences, Christopher Harman, MD, professor and chair, and Amanda Higgs, MGC, CGC, senior genetic counselor, to address how advances in genomics affect patient care and counseling.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
The publication of the draft sequence for the human genome changed the research and clinical medicine landscape forever. This genetic map created the possibility to develop more personalized health care and targeted therapeutics. It opened the door to the age of “big data” sets in biomedical research, fusing science, computer technology, and mathematics – the “s,” “t,” and “m” of “STEM.”
In the 20 years that followed the publication of the human genome, many advances in biomedicine occurred. Improvements in DNA sequencing technologies, built upon the original sequencing project, made the noninvasive prenatal screening test (NIPT) possible. The ease, speed, and cost effectiveness of sequencing has made diagnosing fetal structural anomalies using whole-exome sequencing a reality.
However, uncovering humanity’s genetic code introduced new quandaries and reopened old wounds: How would a person’s genetic data be used? Could a person’s risk for disease, identified through sequencing, lead to overdiagnosis? Would knowing the human genome reinforce age-old ideas that genes make one group superior or inferior? Could we now create “designer babies”?
This last question has become even more pressing with the advent of human gene editing technology, also known by its acronym “CRISPR.” , but it also has the potential for bringing us to the precipice of a Wellsian reality. The alarming claim that scientists had used CRISPR to edit the genes of human babies (Nature. 2020;577[7789]:154-5; doi:10.1038/d41586-020-00001-y) has rippled through the biomedical community and spurred numerous debates on the ethics of using such a powerful tool (Human Genome Editing: Science, Ethics, and Governance; doi: 10.17226/24623).
The passage of the Genetic Information Non-discrimination Act (GINA; https://www.eeoc.gov/statutes/genetic-information-nondiscrimination-act-2008) in 2008 ensured that health insurance companies and employers could not use a person’s genome against them, creating a balance between the forces of “can we?” and “should we?” Yet, many ethical questions remain.
We have invited two experts from the University of Maryland (Baltimore) School of Medicine’s department of obstetrics, gynecology & reproductive sciences, Christopher Harman, MD, professor and chair, and Amanda Higgs, MGC, CGC, senior genetic counselor, to address how advances in genomics affect patient care and counseling.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
The publication of the draft sequence for the human genome changed the research and clinical medicine landscape forever. This genetic map created the possibility to develop more personalized health care and targeted therapeutics. It opened the door to the age of “big data” sets in biomedical research, fusing science, computer technology, and mathematics – the “s,” “t,” and “m” of “STEM.”
In the 20 years that followed the publication of the human genome, many advances in biomedicine occurred. Improvements in DNA sequencing technologies, built upon the original sequencing project, made the noninvasive prenatal screening test (NIPT) possible. The ease, speed, and cost effectiveness of sequencing has made diagnosing fetal structural anomalies using whole-exome sequencing a reality.
However, uncovering humanity’s genetic code introduced new quandaries and reopened old wounds: How would a person’s genetic data be used? Could a person’s risk for disease, identified through sequencing, lead to overdiagnosis? Would knowing the human genome reinforce age-old ideas that genes make one group superior or inferior? Could we now create “designer babies”?
This last question has become even more pressing with the advent of human gene editing technology, also known by its acronym “CRISPR.” , but it also has the potential for bringing us to the precipice of a Wellsian reality. The alarming claim that scientists had used CRISPR to edit the genes of human babies (Nature. 2020;577[7789]:154-5; doi:10.1038/d41586-020-00001-y) has rippled through the biomedical community and spurred numerous debates on the ethics of using such a powerful tool (Human Genome Editing: Science, Ethics, and Governance; doi: 10.17226/24623).
The passage of the Genetic Information Non-discrimination Act (GINA; https://www.eeoc.gov/statutes/genetic-information-nondiscrimination-act-2008) in 2008 ensured that health insurance companies and employers could not use a person’s genome against them, creating a balance between the forces of “can we?” and “should we?” Yet, many ethical questions remain.
We have invited two experts from the University of Maryland (Baltimore) School of Medicine’s department of obstetrics, gynecology & reproductive sciences, Christopher Harman, MD, professor and chair, and Amanda Higgs, MGC, CGC, senior genetic counselor, to address how advances in genomics affect patient care and counseling.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
The fourth trimester
As we approach the end of this year, one of the most surreal times in human history, we will look back on the many things we taught ourselves, the many things we took for granted, the many things we were grateful for, the many things we missed, and the many things we plan to do once we can do things again. Among the many things 2020 taught us to appreciate was the very real manifestation of the old adage, “prevention is the best medicine.” To prevent transmission of SARS-CoV-2, we wore masks, we sanitized everything, we avoided crowds, we traded in-person meetings for virtual meetings, we learned how to homeschool our children, and we delayed seeing relatives and friends.
Ob.gyns. in small and large practices around the world had the tremendous challenge of balancing necessary in-person prenatal care services with keeping their patients and babies safe. Labor and delivery units had even greater demands to keep women and neonates free of SARS-CoV-2 infection. Practices quickly put into place new treatment protocols and new management strategies to maintain the health of their staff while ensuring a high quality of care.
While we have focused much of our attention on greater precautions during pregnancy and childbirth, an important component of care is the immediate postpartum period – colloquially referred to as the “fourth trimester” – which remains critical to maintaining physical and mental health and well-being.
Despite concerns regarding COVID-19 safety, we should continue monitoring our patients during these crucial first weeks after childbirth. This year of social isolation, financial strain, and incredible uncertainty has created additional stress in many women’s lives. The usual support that some women would receive from family members, friends, and other mothers in the early days post partum may not be available. The pandemic also has further highlighted inequities in access to health care for vulnerable groups. In addition, restrictions have increased the incidence of intimate partner violence as many women and children have needed to shelter with their abusers. Perhaps now more than any time previously, ob.gyns. must be attuned to their patients’ needs and be ready to provide compassionate and sensitive care.
In this final month of the year, we have invited George A. Macones, MD, professor and chair of the department of women’s health at the University of Texas, Austin, to address the importance of care in the final “trimester” of pregnancy – the first 3 months post partum.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
*This version has been updated to correct an erroneous byline, photo, and bio.
As we approach the end of this year, one of the most surreal times in human history, we will look back on the many things we taught ourselves, the many things we took for granted, the many things we were grateful for, the many things we missed, and the many things we plan to do once we can do things again. Among the many things 2020 taught us to appreciate was the very real manifestation of the old adage, “prevention is the best medicine.” To prevent transmission of SARS-CoV-2, we wore masks, we sanitized everything, we avoided crowds, we traded in-person meetings for virtual meetings, we learned how to homeschool our children, and we delayed seeing relatives and friends.
Ob.gyns. in small and large practices around the world had the tremendous challenge of balancing necessary in-person prenatal care services with keeping their patients and babies safe. Labor and delivery units had even greater demands to keep women and neonates free of SARS-CoV-2 infection. Practices quickly put into place new treatment protocols and new management strategies to maintain the health of their staff while ensuring a high quality of care.
While we have focused much of our attention on greater precautions during pregnancy and childbirth, an important component of care is the immediate postpartum period – colloquially referred to as the “fourth trimester” – which remains critical to maintaining physical and mental health and well-being.
Despite concerns regarding COVID-19 safety, we should continue monitoring our patients during these crucial first weeks after childbirth. This year of social isolation, financial strain, and incredible uncertainty has created additional stress in many women’s lives. The usual support that some women would receive from family members, friends, and other mothers in the early days post partum may not be available. The pandemic also has further highlighted inequities in access to health care for vulnerable groups. In addition, restrictions have increased the incidence of intimate partner violence as many women and children have needed to shelter with their abusers. Perhaps now more than any time previously, ob.gyns. must be attuned to their patients’ needs and be ready to provide compassionate and sensitive care.
In this final month of the year, we have invited George A. Macones, MD, professor and chair of the department of women’s health at the University of Texas, Austin, to address the importance of care in the final “trimester” of pregnancy – the first 3 months post partum.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
*This version has been updated to correct an erroneous byline, photo, and bio.
As we approach the end of this year, one of the most surreal times in human history, we will look back on the many things we taught ourselves, the many things we took for granted, the many things we were grateful for, the many things we missed, and the many things we plan to do once we can do things again. Among the many things 2020 taught us to appreciate was the very real manifestation of the old adage, “prevention is the best medicine.” To prevent transmission of SARS-CoV-2, we wore masks, we sanitized everything, we avoided crowds, we traded in-person meetings for virtual meetings, we learned how to homeschool our children, and we delayed seeing relatives and friends.
Ob.gyns. in small and large practices around the world had the tremendous challenge of balancing necessary in-person prenatal care services with keeping their patients and babies safe. Labor and delivery units had even greater demands to keep women and neonates free of SARS-CoV-2 infection. Practices quickly put into place new treatment protocols and new management strategies to maintain the health of their staff while ensuring a high quality of care.
While we have focused much of our attention on greater precautions during pregnancy and childbirth, an important component of care is the immediate postpartum period – colloquially referred to as the “fourth trimester” – which remains critical to maintaining physical and mental health and well-being.
Despite concerns regarding COVID-19 safety, we should continue monitoring our patients during these crucial first weeks after childbirth. This year of social isolation, financial strain, and incredible uncertainty has created additional stress in many women’s lives. The usual support that some women would receive from family members, friends, and other mothers in the early days post partum may not be available. The pandemic also has further highlighted inequities in access to health care for vulnerable groups. In addition, restrictions have increased the incidence of intimate partner violence as many women and children have needed to shelter with their abusers. Perhaps now more than any time previously, ob.gyns. must be attuned to their patients’ needs and be ready to provide compassionate and sensitive care.
In this final month of the year, we have invited George A. Macones, MD, professor and chair of the department of women’s health at the University of Texas, Austin, to address the importance of care in the final “trimester” of pregnancy – the first 3 months post partum.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
*This version has been updated to correct an erroneous byline, photo, and bio.
Breast cancer screening complexities
Breast cancer in women remains one of the most common types of cancer in the United States, affecting about one in eight women1 over the course of their lifetime. Despite its pervasiveness, the 5-year survival rate for women with breast cancer remains high, estimated at around 90%2 based on data from 2010-2016, in large part because of early detection and treatment through screening. However, many organizations disagree on when to start and how often to screen women at average risk.
Important to discussions about breast cancer screening is the trend that many women delay childbirth until their 30s and 40s. In 2018 the birth rate increased for women ages 35-44, and the mean age of first birth increased from the prior year across all racial and ethnic groups.3 Therefore, ob.gyns. may need to consider that their patients not only may have increased risk of developing breast cancer based on age alone – women aged 35-44 have four times greater risk of disease than women aged 20-342 – but that the pregnancy itself may further exacerbate risk in older women. A 2019 pooled analysis found that women who were older at first birth had a greater chance of developing breast cancer compared with women with no children.4
In addition, ob.gyns. should consider that their patients may have received a breast cancer diagnosis prior to initiation or completion of their family plans or that their patients are cancer survivors – in 2013-2017, breast cancer was the most common form of cancer in adolescents and young adults.5 Thus, practitioners should be prepared to discuss not only options for fertility preservation but the evidence regarding cancer recurrence after pregnancy.
We have invited Dr. Katherine Tkaczuk, professor of medicine at the University of Maryland School of Medicine* and director of the breast evaluation and treatment program at the Marlene and Stewart Greenebaum Comprehensive Cancer Center, to discuss the vital role of screening in the shared decision-making process of breast cancer prevention.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore,* as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
Correction, 1/8/21: *An earlier version of this article misstated the university affiliations for Dr. Tkaczuk and Dr. Reece.
References
1. U.S. Breast Cancer Statistics. breastcancer.org.
2. “Cancer Stat Facts: Female Breast Cancer,” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
3. Martin JA et al. “Births: Final Data for 2018.” National Vital Statistics Reports. 2019 Nov 27;68(13):1-46.
4. Nichols HB et al. Ann Intern Med. 2019 Jan;170(1):22-30.
5. “Cancer Stat Facts: Cancer Among Adolescents and Young Adults (AYAs) (Ages 15-39),” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
Breast cancer in women remains one of the most common types of cancer in the United States, affecting about one in eight women1 over the course of their lifetime. Despite its pervasiveness, the 5-year survival rate for women with breast cancer remains high, estimated at around 90%2 based on data from 2010-2016, in large part because of early detection and treatment through screening. However, many organizations disagree on when to start and how often to screen women at average risk.
Important to discussions about breast cancer screening is the trend that many women delay childbirth until their 30s and 40s. In 2018 the birth rate increased for women ages 35-44, and the mean age of first birth increased from the prior year across all racial and ethnic groups.3 Therefore, ob.gyns. may need to consider that their patients not only may have increased risk of developing breast cancer based on age alone – women aged 35-44 have four times greater risk of disease than women aged 20-342 – but that the pregnancy itself may further exacerbate risk in older women. A 2019 pooled analysis found that women who were older at first birth had a greater chance of developing breast cancer compared with women with no children.4
In addition, ob.gyns. should consider that their patients may have received a breast cancer diagnosis prior to initiation or completion of their family plans or that their patients are cancer survivors – in 2013-2017, breast cancer was the most common form of cancer in adolescents and young adults.5 Thus, practitioners should be prepared to discuss not only options for fertility preservation but the evidence regarding cancer recurrence after pregnancy.
We have invited Dr. Katherine Tkaczuk, professor of medicine at the University of Maryland School of Medicine* and director of the breast evaluation and treatment program at the Marlene and Stewart Greenebaum Comprehensive Cancer Center, to discuss the vital role of screening in the shared decision-making process of breast cancer prevention.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore,* as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
Correction, 1/8/21: *An earlier version of this article misstated the university affiliations for Dr. Tkaczuk and Dr. Reece.
References
1. U.S. Breast Cancer Statistics. breastcancer.org.
2. “Cancer Stat Facts: Female Breast Cancer,” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
3. Martin JA et al. “Births: Final Data for 2018.” National Vital Statistics Reports. 2019 Nov 27;68(13):1-46.
4. Nichols HB et al. Ann Intern Med. 2019 Jan;170(1):22-30.
5. “Cancer Stat Facts: Cancer Among Adolescents and Young Adults (AYAs) (Ages 15-39),” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
Breast cancer in women remains one of the most common types of cancer in the United States, affecting about one in eight women1 over the course of their lifetime. Despite its pervasiveness, the 5-year survival rate for women with breast cancer remains high, estimated at around 90%2 based on data from 2010-2016, in large part because of early detection and treatment through screening. However, many organizations disagree on when to start and how often to screen women at average risk.
Important to discussions about breast cancer screening is the trend that many women delay childbirth until their 30s and 40s. In 2018 the birth rate increased for women ages 35-44, and the mean age of first birth increased from the prior year across all racial and ethnic groups.3 Therefore, ob.gyns. may need to consider that their patients not only may have increased risk of developing breast cancer based on age alone – women aged 35-44 have four times greater risk of disease than women aged 20-342 – but that the pregnancy itself may further exacerbate risk in older women. A 2019 pooled analysis found that women who were older at first birth had a greater chance of developing breast cancer compared with women with no children.4
In addition, ob.gyns. should consider that their patients may have received a breast cancer diagnosis prior to initiation or completion of their family plans or that their patients are cancer survivors – in 2013-2017, breast cancer was the most common form of cancer in adolescents and young adults.5 Thus, practitioners should be prepared to discuss not only options for fertility preservation but the evidence regarding cancer recurrence after pregnancy.
We have invited Dr. Katherine Tkaczuk, professor of medicine at the University of Maryland School of Medicine* and director of the breast evaluation and treatment program at the Marlene and Stewart Greenebaum Comprehensive Cancer Center, to discuss the vital role of screening in the shared decision-making process of breast cancer prevention.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore,* as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
Correction, 1/8/21: *An earlier version of this article misstated the university affiliations for Dr. Tkaczuk and Dr. Reece.
References
1. U.S. Breast Cancer Statistics. breastcancer.org.
2. “Cancer Stat Facts: Female Breast Cancer,” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
3. Martin JA et al. “Births: Final Data for 2018.” National Vital Statistics Reports. 2019 Nov 27;68(13):1-46.
4. Nichols HB et al. Ann Intern Med. 2019 Jan;170(1):22-30.
5. “Cancer Stat Facts: Cancer Among Adolescents and Young Adults (AYAs) (Ages 15-39),” Surveillance, Epidemiology, and End Results Program. National Cancer Institute.
Vaccines for maternal and fetal health
Biomedical science is ever changing, and what may be believed in one era – for instance, bloodletting can cure disease or lobotomies can treat psychiatric disorders – may not be accepted in the next. However, one medical advance stands out in terms of maintaining and sustaining our health: vaccines. The data comparing morbidity and mortality before and after widespread vaccination are staggering. Before the smallpox vaccine, nearly 49,000 people were infected and more than 1,500 died annually from smallpox; by 1977, the vaccine eradicated the disease in the United States.1 Polio caused paralytic disease in more than 16,000 people per year in the United States, including, perhaps most famously, President Franklin Roosevelt. After development of the polio vaccine, cases and deaths dropped to zero.2
Despite the evidence indicating the effectiveness of vaccines to reduce disease and death, rates of vaccination in the United States remain low among adults, ranging from about 23% for pneumococcal disease to 45% for seasonal influenza.3 Childhood immunization in 2017 hovered around 70% for those receiving all the recommended vaccines.4 Clearly there is room for improvement.
A woman’s ob.gyn. may be the only medical professional she sees regularly, and her annual well visit may be the only time she receives information regarding her weight and blood pressure, or reviews her current medications. For women who are planning pregnancy, pregnant, or post partum, ob.gyn. consultations present unique opportunities to increase patient engagement in healthy behaviors, such as diet, exercise, and regular sleep, because women are highly motivated to do what is best for their babies.
Immunization during pregnancy not only reduces the mother’s risk of severe disease, which can lead to complications, defects, and fetal or perinatal death, but also has been shown to improve the neonate’s ability to fight infection and may reduce vertical transmission of certain diseases. In this era of COVID-19 where we have no vaccine but we have evidence that pregnant women may be at greater risk for severe disease,5 routine immunizations are vital to maternal and fetal health.
We have invited Laura E. Riley, MD, chair of obstetrics and gynecology at Weill Cornell Medicine, New York, to address the importance of vaccination and the role of the ob.gyn. in advocating for this life-saving preventive health measure. Dr. Riley disclosed she is an author for Up to Date and was a consultant to GlaxoSmithKline about a cytomegalovirus vaccine.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore County, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
References
1. MMWR Morb Mortal Wkly Rep. 1999 Apr 2;48(12);243-8.
2. JAMA. 2007 Nov 14;298(18):2155-63.
3. MMWR Morb Mortal Wkly Rep. 2017 May 5;66(11);1-28.
4. CDC National Center for Health Statistics FastStats on Immunization.
5. MMWR Morb Mortal Wkly Rep. 2020 Jun 26;69(25);769-75.
Biomedical science is ever changing, and what may be believed in one era – for instance, bloodletting can cure disease or lobotomies can treat psychiatric disorders – may not be accepted in the next. However, one medical advance stands out in terms of maintaining and sustaining our health: vaccines. The data comparing morbidity and mortality before and after widespread vaccination are staggering. Before the smallpox vaccine, nearly 49,000 people were infected and more than 1,500 died annually from smallpox; by 1977, the vaccine eradicated the disease in the United States.1 Polio caused paralytic disease in more than 16,000 people per year in the United States, including, perhaps most famously, President Franklin Roosevelt. After development of the polio vaccine, cases and deaths dropped to zero.2
Despite the evidence indicating the effectiveness of vaccines to reduce disease and death, rates of vaccination in the United States remain low among adults, ranging from about 23% for pneumococcal disease to 45% for seasonal influenza.3 Childhood immunization in 2017 hovered around 70% for those receiving all the recommended vaccines.4 Clearly there is room for improvement.
A woman’s ob.gyn. may be the only medical professional she sees regularly, and her annual well visit may be the only time she receives information regarding her weight and blood pressure, or reviews her current medications. For women who are planning pregnancy, pregnant, or post partum, ob.gyn. consultations present unique opportunities to increase patient engagement in healthy behaviors, such as diet, exercise, and regular sleep, because women are highly motivated to do what is best for their babies.
Immunization during pregnancy not only reduces the mother’s risk of severe disease, which can lead to complications, defects, and fetal or perinatal death, but also has been shown to improve the neonate’s ability to fight infection and may reduce vertical transmission of certain diseases. In this era of COVID-19 where we have no vaccine but we have evidence that pregnant women may be at greater risk for severe disease,5 routine immunizations are vital to maternal and fetal health.
We have invited Laura E. Riley, MD, chair of obstetrics and gynecology at Weill Cornell Medicine, New York, to address the importance of vaccination and the role of the ob.gyn. in advocating for this life-saving preventive health measure. Dr. Riley disclosed she is an author for Up to Date and was a consultant to GlaxoSmithKline about a cytomegalovirus vaccine.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore County, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
References
1. MMWR Morb Mortal Wkly Rep. 1999 Apr 2;48(12);243-8.
2. JAMA. 2007 Nov 14;298(18):2155-63.
3. MMWR Morb Mortal Wkly Rep. 2017 May 5;66(11);1-28.
4. CDC National Center for Health Statistics FastStats on Immunization.
5. MMWR Morb Mortal Wkly Rep. 2020 Jun 26;69(25);769-75.
Biomedical science is ever changing, and what may be believed in one era – for instance, bloodletting can cure disease or lobotomies can treat psychiatric disorders – may not be accepted in the next. However, one medical advance stands out in terms of maintaining and sustaining our health: vaccines. The data comparing morbidity and mortality before and after widespread vaccination are staggering. Before the smallpox vaccine, nearly 49,000 people were infected and more than 1,500 died annually from smallpox; by 1977, the vaccine eradicated the disease in the United States.1 Polio caused paralytic disease in more than 16,000 people per year in the United States, including, perhaps most famously, President Franklin Roosevelt. After development of the polio vaccine, cases and deaths dropped to zero.2
Despite the evidence indicating the effectiveness of vaccines to reduce disease and death, rates of vaccination in the United States remain low among adults, ranging from about 23% for pneumococcal disease to 45% for seasonal influenza.3 Childhood immunization in 2017 hovered around 70% for those receiving all the recommended vaccines.4 Clearly there is room for improvement.
A woman’s ob.gyn. may be the only medical professional she sees regularly, and her annual well visit may be the only time she receives information regarding her weight and blood pressure, or reviews her current medications. For women who are planning pregnancy, pregnant, or post partum, ob.gyn. consultations present unique opportunities to increase patient engagement in healthy behaviors, such as diet, exercise, and regular sleep, because women are highly motivated to do what is best for their babies.
Immunization during pregnancy not only reduces the mother’s risk of severe disease, which can lead to complications, defects, and fetal or perinatal death, but also has been shown to improve the neonate’s ability to fight infection and may reduce vertical transmission of certain diseases. In this era of COVID-19 where we have no vaccine but we have evidence that pregnant women may be at greater risk for severe disease,5 routine immunizations are vital to maternal and fetal health.
We have invited Laura E. Riley, MD, chair of obstetrics and gynecology at Weill Cornell Medicine, New York, to address the importance of vaccination and the role of the ob.gyn. in advocating for this life-saving preventive health measure. Dr. Riley disclosed she is an author for Up to Date and was a consultant to GlaxoSmithKline about a cytomegalovirus vaccine.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore County, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
References
1. MMWR Morb Mortal Wkly Rep. 1999 Apr 2;48(12);243-8.
2. JAMA. 2007 Nov 14;298(18):2155-63.
3. MMWR Morb Mortal Wkly Rep. 2017 May 5;66(11);1-28.
4. CDC National Center for Health Statistics FastStats on Immunization.
5. MMWR Morb Mortal Wkly Rep. 2020 Jun 26;69(25);769-75.
Obstetrics during the COVID-19 pandemic
The identification of the SARS coronavirus (SARS-CoV-2) and emergence of the associated infectious respiratory disease, COVID-19, in late 2019 catapulted the citizens of the world, especially those in the health care professions, into an era of considerable uncertainty. At this moment in human history, calm reassurance – founded in fact and evidence – seems its greatest need. Much of the focus within the biomedical community has been on containment, prevention, and treatment of this highly contagious and, for some, extremely virulent disease.
However, for ob.gyns on the front lines of the COVID-19 fight, there is the additional challenge of caring for at least two patients simultaneously: the mother and her unborn baby. Studies in mother-baby dyads, while being published at an incredible pace, are still quite scarce. In addition, published reports are limited by the small sample size of the patient population (many are single-case reports), lack of uniformity in the timing and types of clinical samples collected, testing delays, and varying isolation protocols in cases where the mother has confirmed SARS-CoV-2.
Five months into a pandemic that has swept the world, we still know very little about COVID-19 infection in the general population, let alone the obstetric one. We do not know if having and resolving COVID-19 infection provides any long-term protection against future disease. We do not know if vertical transmission of SARS-CoV-2 occurs. We do not know if maternal infection confers any immunologic benefit to the neonate. The list goes on.
What we do know is that taking extra precautions works. Use of personal protective equipment saves health care practitioner and patient lives. Prohibiting or restricting visitors to only one person in hospitals reduces risk of transmission to vulnerable patients.
Additionally, we know that leading with compassion is vital to easing patient – and practitioner – anxiety and stress. Most importantly, we know that people are extraordinarily resilient, especially when it comes to safeguarding the health of their families.
To address some of the major concerns that many ob.gyns. have regarding their risk of coronavirus exposure when caring for patients, we have invited Ray Bahado-Singh, MD, professor and chair of obstetrics and gynecology at Oakland University, Rochester, Mich., and health system chair for obstetrics and gynecology at Beaumont Health System, who works in a suburb of Detroit, one of our nation’s COVID-19 hot spots.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
The identification of the SARS coronavirus (SARS-CoV-2) and emergence of the associated infectious respiratory disease, COVID-19, in late 2019 catapulted the citizens of the world, especially those in the health care professions, into an era of considerable uncertainty. At this moment in human history, calm reassurance – founded in fact and evidence – seems its greatest need. Much of the focus within the biomedical community has been on containment, prevention, and treatment of this highly contagious and, for some, extremely virulent disease.
However, for ob.gyns on the front lines of the COVID-19 fight, there is the additional challenge of caring for at least two patients simultaneously: the mother and her unborn baby. Studies in mother-baby dyads, while being published at an incredible pace, are still quite scarce. In addition, published reports are limited by the small sample size of the patient population (many are single-case reports), lack of uniformity in the timing and types of clinical samples collected, testing delays, and varying isolation protocols in cases where the mother has confirmed SARS-CoV-2.
Five months into a pandemic that has swept the world, we still know very little about COVID-19 infection in the general population, let alone the obstetric one. We do not know if having and resolving COVID-19 infection provides any long-term protection against future disease. We do not know if vertical transmission of SARS-CoV-2 occurs. We do not know if maternal infection confers any immunologic benefit to the neonate. The list goes on.
What we do know is that taking extra precautions works. Use of personal protective equipment saves health care practitioner and patient lives. Prohibiting or restricting visitors to only one person in hospitals reduces risk of transmission to vulnerable patients.
Additionally, we know that leading with compassion is vital to easing patient – and practitioner – anxiety and stress. Most importantly, we know that people are extraordinarily resilient, especially when it comes to safeguarding the health of their families.
To address some of the major concerns that many ob.gyns. have regarding their risk of coronavirus exposure when caring for patients, we have invited Ray Bahado-Singh, MD, professor and chair of obstetrics and gynecology at Oakland University, Rochester, Mich., and health system chair for obstetrics and gynecology at Beaumont Health System, who works in a suburb of Detroit, one of our nation’s COVID-19 hot spots.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
The identification of the SARS coronavirus (SARS-CoV-2) and emergence of the associated infectious respiratory disease, COVID-19, in late 2019 catapulted the citizens of the world, especially those in the health care professions, into an era of considerable uncertainty. At this moment in human history, calm reassurance – founded in fact and evidence – seems its greatest need. Much of the focus within the biomedical community has been on containment, prevention, and treatment of this highly contagious and, for some, extremely virulent disease.
However, for ob.gyns on the front lines of the COVID-19 fight, there is the additional challenge of caring for at least two patients simultaneously: the mother and her unborn baby. Studies in mother-baby dyads, while being published at an incredible pace, are still quite scarce. In addition, published reports are limited by the small sample size of the patient population (many are single-case reports), lack of uniformity in the timing and types of clinical samples collected, testing delays, and varying isolation protocols in cases where the mother has confirmed SARS-CoV-2.
Five months into a pandemic that has swept the world, we still know very little about COVID-19 infection in the general population, let alone the obstetric one. We do not know if having and resolving COVID-19 infection provides any long-term protection against future disease. We do not know if vertical transmission of SARS-CoV-2 occurs. We do not know if maternal infection confers any immunologic benefit to the neonate. The list goes on.
What we do know is that taking extra precautions works. Use of personal protective equipment saves health care practitioner and patient lives. Prohibiting or restricting visitors to only one person in hospitals reduces risk of transmission to vulnerable patients.
Additionally, we know that leading with compassion is vital to easing patient – and practitioner – anxiety and stress. Most importantly, we know that people are extraordinarily resilient, especially when it comes to safeguarding the health of their families.
To address some of the major concerns that many ob.gyns. have regarding their risk of coronavirus exposure when caring for patients, we have invited Ray Bahado-Singh, MD, professor and chair of obstetrics and gynecology at Oakland University, Rochester, Mich., and health system chair for obstetrics and gynecology at Beaumont Health System, who works in a suburb of Detroit, one of our nation’s COVID-19 hot spots.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
CVH in pregnant women: Ample room for improvement
Cardiovascular disease is both common and chronic, and it remains the leading cause of death in women. Because it is a life-long condition, cardiovascular disease must be managed over the entire lifespan. In recognition of the important role of obstetricians and gynecologists in monitoring women’s health, the American Heart Association/American College of Obstetricians and Gynecologists 2018 guidelines1 promoted the use of “Life’s Simple 7”2 for assessing cardiovascular health (CVH) in women.
These seven metrics include diet, physical activity, smoking status, body mass index (BMI), blood pressure, total cholesterol, and fasting blood glucose levels. They have been shown to predict positive health outcomes in nonpregnant adults. However, until now, CVH had not been assessed in pregnant women.
Perak et al. recently performed the first cross-sectional study of the prevalence of CVH metrics in pregnant women using the AHA definition.3 Using data from the National Health and Nutrition Examination Surveys (NHANES), they used the Life’s Simple 7 metrics to assess CVH in 1,117 pregnant and 8,200 nonpregnant women in the United States aged 20-44 years. Each of the Life’s Simple 7 metrics was scored 0, 1, or 2 points, corresponding to a rating of poor, intermediate, or ideal, respectively. Thus, the total CVH score ranged from 0-14 points, with total scores of 0-7 indicating low CVH, 8-11 indicating moderate CVH, and 12-14 indicating high CVH.
which was even worse than in nonpregnant women, of whom only 13% were scored as having ideal CVH. Ideal scores were observed for 0.1% of pregnant women for diet, 27% for physical activity, 39% for cholesterol levels, 51% for BMI, 78% for smoking, 90% for blood pressure, and 92% for fasting blood glucose. Physical activity and cholesterol levels appeared to be the major drivers of the lower CVH scores in pregnant women.
Although further studies are warranted to determine the relevance of CVH during pregnancy to outcomes for both mother and offspring, the study by Perak et al. is an important step toward the development of pregnancy-specific guidelines and definitions for CVH metrics. These are stated goals of the AHA/ACOG that will help promote CVH in women across their lifespans, but which have not been possible due to scant data.
Emerging data suggest that cumulative lifetime exposure is a significant factor in cardiovascular disease outcomes; therefore, earlier intervention would have a more significant impact. Just as gestational diabetes is a predictor of future type 2 diabetes, CVH earlier in a woman’s life predicts cardiovascular disease later in life.4-7 The best data in this regard come from genetic and other studies of hyperlipidemia, which suggest that lowering lipid levels before symptoms develop may prevent cardiovascular disease. In contrast, treatment of patients with clinically manifest disease neither offers a cure nor prevents the occurrence of most cardiovascular events.
It is a particularly salient point in this regard that there currently are no guidelines on treatment of hypercholesterolemia during pregnancy. Notably, the study by Perak et al. suggested that cholesterol levels may have a significant impact on CVH in pregnant women. There also is emerging data supporting the importance of controlling blood pressure across the lifespan,7,8 including during pregnancy.9
For many women, their ob.gyn. is their primary care physician, and pregnancy is often the first time that a woman will have a substantial interaction with the health care system. The AHA/ACOG advisory panel described pregnancy as a “physiological stress test” for women that offers the opportunity to identify those at increased risk of cardiovascular disease.1
As pregnancy is a time when women particularly are motivated to improve their health,10 it also presents a valuable opportunity for physicians, including ob.gyns., to make a lifelong impact on the CVH of their patients through early identification, education, and intervention.
Dr. Charles Hong is the Melvin Sharoky, MD, Professor of Medicine and director of cardiovascular research in the department of medicine at the University of Maryland School of Medicine. Dr. E. Albert Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Neither physician had any relevant financial disclosures. Contact him at obnews@mdedge.com.
References
1. Circulation. 2018;137:e843–e852.
2. Circulation. 2010 Jan 20;121(4):586–613.
3. J Am Heart Assoc. 2020 Feb 17;9:e015123.
4. J Am Coll Cardiol. 2018 Sep 4;72(10):1141-56.
5. N Engl J Med. 2016 Dec 1;375:2144-53.
6. Nat Rev Cardiol. 2011 Nov 1;8(12):721-5.
7. J Am Coll Cardiol. 2019 Jul 23;74(3):330-41.
8. Circulation. 2020 Mar 2:141:725-7.
9. Circulation. 2013 Feb 12;127(6):681-90.
10. Nutrients. 2018 Aug 8. doi: 10.3390/nu10081032.
Cardiovascular disease is both common and chronic, and it remains the leading cause of death in women. Because it is a life-long condition, cardiovascular disease must be managed over the entire lifespan. In recognition of the important role of obstetricians and gynecologists in monitoring women’s health, the American Heart Association/American College of Obstetricians and Gynecologists 2018 guidelines1 promoted the use of “Life’s Simple 7”2 for assessing cardiovascular health (CVH) in women.
These seven metrics include diet, physical activity, smoking status, body mass index (BMI), blood pressure, total cholesterol, and fasting blood glucose levels. They have been shown to predict positive health outcomes in nonpregnant adults. However, until now, CVH had not been assessed in pregnant women.
Perak et al. recently performed the first cross-sectional study of the prevalence of CVH metrics in pregnant women using the AHA definition.3 Using data from the National Health and Nutrition Examination Surveys (NHANES), they used the Life’s Simple 7 metrics to assess CVH in 1,117 pregnant and 8,200 nonpregnant women in the United States aged 20-44 years. Each of the Life’s Simple 7 metrics was scored 0, 1, or 2 points, corresponding to a rating of poor, intermediate, or ideal, respectively. Thus, the total CVH score ranged from 0-14 points, with total scores of 0-7 indicating low CVH, 8-11 indicating moderate CVH, and 12-14 indicating high CVH.
which was even worse than in nonpregnant women, of whom only 13% were scored as having ideal CVH. Ideal scores were observed for 0.1% of pregnant women for diet, 27% for physical activity, 39% for cholesterol levels, 51% for BMI, 78% for smoking, 90% for blood pressure, and 92% for fasting blood glucose. Physical activity and cholesterol levels appeared to be the major drivers of the lower CVH scores in pregnant women.
Although further studies are warranted to determine the relevance of CVH during pregnancy to outcomes for both mother and offspring, the study by Perak et al. is an important step toward the development of pregnancy-specific guidelines and definitions for CVH metrics. These are stated goals of the AHA/ACOG that will help promote CVH in women across their lifespans, but which have not been possible due to scant data.
Emerging data suggest that cumulative lifetime exposure is a significant factor in cardiovascular disease outcomes; therefore, earlier intervention would have a more significant impact. Just as gestational diabetes is a predictor of future type 2 diabetes, CVH earlier in a woman’s life predicts cardiovascular disease later in life.4-7 The best data in this regard come from genetic and other studies of hyperlipidemia, which suggest that lowering lipid levels before symptoms develop may prevent cardiovascular disease. In contrast, treatment of patients with clinically manifest disease neither offers a cure nor prevents the occurrence of most cardiovascular events.
It is a particularly salient point in this regard that there currently are no guidelines on treatment of hypercholesterolemia during pregnancy. Notably, the study by Perak et al. suggested that cholesterol levels may have a significant impact on CVH in pregnant women. There also is emerging data supporting the importance of controlling blood pressure across the lifespan,7,8 including during pregnancy.9
For many women, their ob.gyn. is their primary care physician, and pregnancy is often the first time that a woman will have a substantial interaction with the health care system. The AHA/ACOG advisory panel described pregnancy as a “physiological stress test” for women that offers the opportunity to identify those at increased risk of cardiovascular disease.1
As pregnancy is a time when women particularly are motivated to improve their health,10 it also presents a valuable opportunity for physicians, including ob.gyns., to make a lifelong impact on the CVH of their patients through early identification, education, and intervention.
Dr. Charles Hong is the Melvin Sharoky, MD, Professor of Medicine and director of cardiovascular research in the department of medicine at the University of Maryland School of Medicine. Dr. E. Albert Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Neither physician had any relevant financial disclosures. Contact him at obnews@mdedge.com.
References
1. Circulation. 2018;137:e843–e852.
2. Circulation. 2010 Jan 20;121(4):586–613.
3. J Am Heart Assoc. 2020 Feb 17;9:e015123.
4. J Am Coll Cardiol. 2018 Sep 4;72(10):1141-56.
5. N Engl J Med. 2016 Dec 1;375:2144-53.
6. Nat Rev Cardiol. 2011 Nov 1;8(12):721-5.
7. J Am Coll Cardiol. 2019 Jul 23;74(3):330-41.
8. Circulation. 2020 Mar 2:141:725-7.
9. Circulation. 2013 Feb 12;127(6):681-90.
10. Nutrients. 2018 Aug 8. doi: 10.3390/nu10081032.
Cardiovascular disease is both common and chronic, and it remains the leading cause of death in women. Because it is a life-long condition, cardiovascular disease must be managed over the entire lifespan. In recognition of the important role of obstetricians and gynecologists in monitoring women’s health, the American Heart Association/American College of Obstetricians and Gynecologists 2018 guidelines1 promoted the use of “Life’s Simple 7”2 for assessing cardiovascular health (CVH) in women.
These seven metrics include diet, physical activity, smoking status, body mass index (BMI), blood pressure, total cholesterol, and fasting blood glucose levels. They have been shown to predict positive health outcomes in nonpregnant adults. However, until now, CVH had not been assessed in pregnant women.
Perak et al. recently performed the first cross-sectional study of the prevalence of CVH metrics in pregnant women using the AHA definition.3 Using data from the National Health and Nutrition Examination Surveys (NHANES), they used the Life’s Simple 7 metrics to assess CVH in 1,117 pregnant and 8,200 nonpregnant women in the United States aged 20-44 years. Each of the Life’s Simple 7 metrics was scored 0, 1, or 2 points, corresponding to a rating of poor, intermediate, or ideal, respectively. Thus, the total CVH score ranged from 0-14 points, with total scores of 0-7 indicating low CVH, 8-11 indicating moderate CVH, and 12-14 indicating high CVH.
which was even worse than in nonpregnant women, of whom only 13% were scored as having ideal CVH. Ideal scores were observed for 0.1% of pregnant women for diet, 27% for physical activity, 39% for cholesterol levels, 51% for BMI, 78% for smoking, 90% for blood pressure, and 92% for fasting blood glucose. Physical activity and cholesterol levels appeared to be the major drivers of the lower CVH scores in pregnant women.
Although further studies are warranted to determine the relevance of CVH during pregnancy to outcomes for both mother and offspring, the study by Perak et al. is an important step toward the development of pregnancy-specific guidelines and definitions for CVH metrics. These are stated goals of the AHA/ACOG that will help promote CVH in women across their lifespans, but which have not been possible due to scant data.
Emerging data suggest that cumulative lifetime exposure is a significant factor in cardiovascular disease outcomes; therefore, earlier intervention would have a more significant impact. Just as gestational diabetes is a predictor of future type 2 diabetes, CVH earlier in a woman’s life predicts cardiovascular disease later in life.4-7 The best data in this regard come from genetic and other studies of hyperlipidemia, which suggest that lowering lipid levels before symptoms develop may prevent cardiovascular disease. In contrast, treatment of patients with clinically manifest disease neither offers a cure nor prevents the occurrence of most cardiovascular events.
It is a particularly salient point in this regard that there currently are no guidelines on treatment of hypercholesterolemia during pregnancy. Notably, the study by Perak et al. suggested that cholesterol levels may have a significant impact on CVH in pregnant women. There also is emerging data supporting the importance of controlling blood pressure across the lifespan,7,8 including during pregnancy.9
For many women, their ob.gyn. is their primary care physician, and pregnancy is often the first time that a woman will have a substantial interaction with the health care system. The AHA/ACOG advisory panel described pregnancy as a “physiological stress test” for women that offers the opportunity to identify those at increased risk of cardiovascular disease.1
As pregnancy is a time when women particularly are motivated to improve their health,10 it also presents a valuable opportunity for physicians, including ob.gyns., to make a lifelong impact on the CVH of their patients through early identification, education, and intervention.
Dr. Charles Hong is the Melvin Sharoky, MD, Professor of Medicine and director of cardiovascular research in the department of medicine at the University of Maryland School of Medicine. Dr. E. Albert Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Neither physician had any relevant financial disclosures. Contact him at obnews@mdedge.com.
References
1. Circulation. 2018;137:e843–e852.
2. Circulation. 2010 Jan 20;121(4):586–613.
3. J Am Heart Assoc. 2020 Feb 17;9:e015123.
4. J Am Coll Cardiol. 2018 Sep 4;72(10):1141-56.
5. N Engl J Med. 2016 Dec 1;375:2144-53.
6. Nat Rev Cardiol. 2011 Nov 1;8(12):721-5.
7. J Am Coll Cardiol. 2019 Jul 23;74(3):330-41.
8. Circulation. 2020 Mar 2:141:725-7.
9. Circulation. 2013 Feb 12;127(6):681-90.
10. Nutrients. 2018 Aug 8. doi: 10.3390/nu10081032.





