User login
Transcervical ablation of symptomatic uterine fibroids under US guidance
On Aug. 29, 2019, the first commercial case utilizing the Sonata system to transcervically ablate symptomatic uterine fibroids under ultrasound guidance was performed at Stamford (Conn.) Hospital. This truly minimally invasive new treatment expands our options in the surgical management of uterine fibroids.
Uterine fibroids are the most common benign tumors of the reproductive tract. It has been estimated that nearly half of the 70%-80% of women who develop fibroids during their reproductive years are symptomatic. Given that some patients present with fertility concerns, it also has been estimated that at least one in three women with fibroids have symptoms such as heavy bleeding (menorrhagia) and bulk symptoms, pain (dyspareunia, dysmenorrhea, noncyclic pain), and increased urinary frequency.
Fibroids are the most common cause of hysterectomy in the United States, with 240,000 (40% of 600,000) performed annually, yet research shows that many women are interested in minimally invasive options and in uterine conservation. In a 2013 national survey published in the American Journal of Obstetrics and Gynecology, 79% of women expressed an interest in minimally invasive approaches for fibroid treatment, and over 50% reported a desire for uterine conservation.1
Both myomectomy and uterine artery embolization are uterine-sparing procedures. However, uterine artery embolization should not be performed in a woman interested in pregnancy. Moreover, there are reports of ovarian reserve issues when the procedure is performed in women in their later reproductive years.
Depending on the technique performed, women undergoing hysteroscopic myomectomy are at risk of fluid overload, hyponatremia, gas-related embolism, and postoperative adhesions. The suture requirements of a laparoscopic myomectomy make this approach an often-difficult one to master, even with robotic assistance. It also requires intubation and potentially places the patient at risk for bleeding and infection. Furthermore, long-term risks include adhesions and the need for C-section with pregnancy.
The impact of uterine fibroids on patients’ lives and their desire for uterine conservation has spurred growing interest in the use of radiofrequency (RF) energy to ablate uterine fibroids. In a 2018 systematic review of nonresective treatments for uterine fibroids published in the International Journal of Hyperthermia, investigators found that the pooled fibroid volume reductions at 6 months after RF ablation and uterine artery embolization were 70% and 54%, respectively.2
The first commercially available system utilizing RF frequency to shrink fibrosis – Acessa – involves laparoscopy, and thus requires abdominal incisions. In August 2018, the Sonata system (Gynesonics: Redwood, Calif.) received Food and Drug Administration clearance after having received European CE-Mark approval in 2010 (for the original device, the VizAblate) and in 2014 (for the next-generation device, the Sonata).
The technology
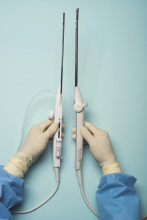
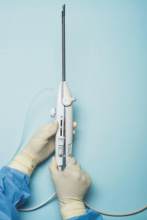
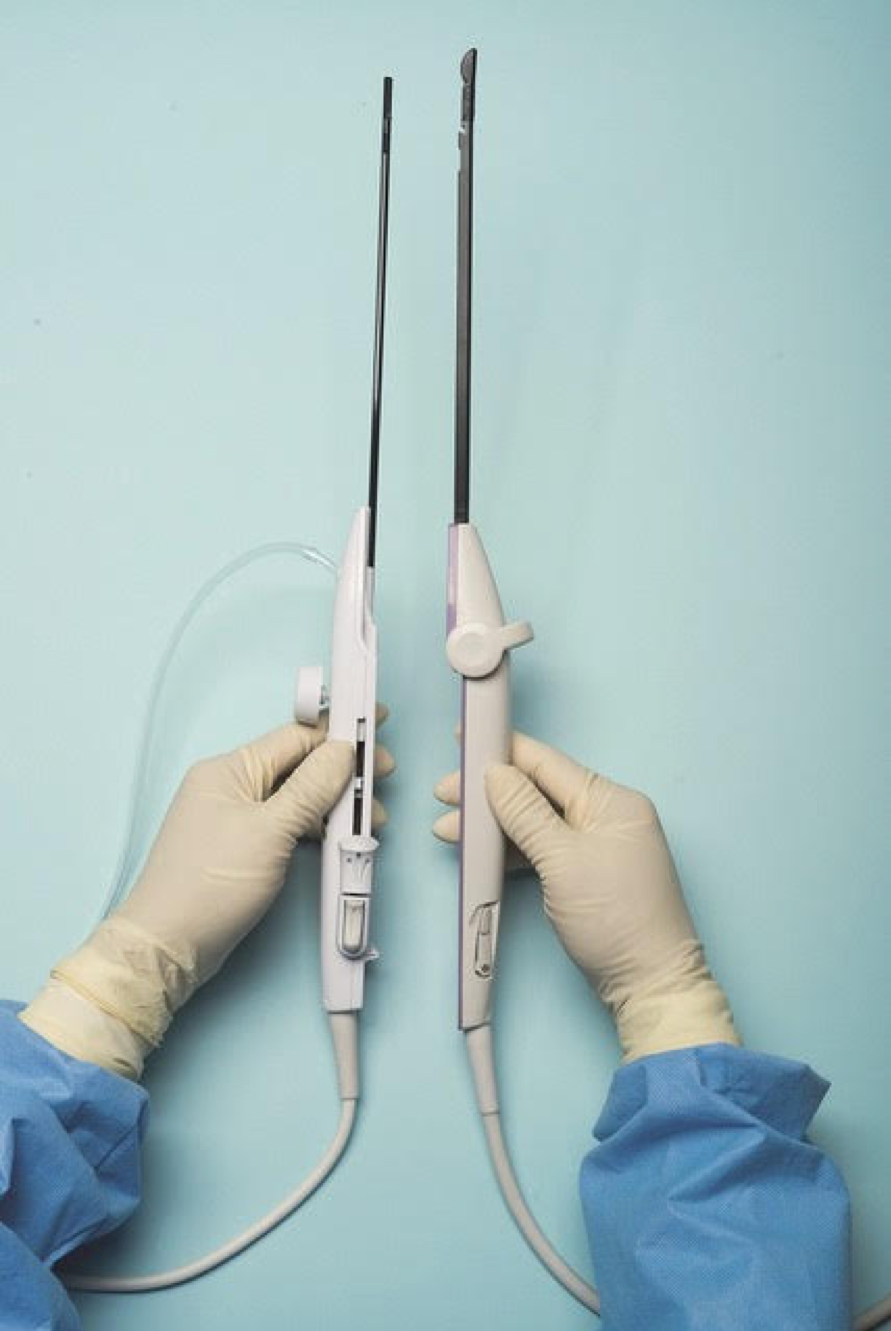
For a complete description of transcervical, intrauterine sonography–guided radiofrequency ablation of uterine fibroids, one can refer to the excellent outline by David Toub, MD, in Current Obstetrics and Gynecology Reports.3 Basically, the Sonata system allows for real-time, image-guided treatment through the use of a reusable intrauterine ultrasound (IUUS) probe, a single-use RF ablation (RFA) handpiece, and graphical guidance software for diagnosis and targeting.
Initially, the IUUS probe enables identification of fibroids from within the uterine cavity, then guides deployment of an introducer and needle electrode into the targeted fibroid(s). The probe image is curvilinear, penetrates more than 9 cm, and provides a 90-degree field of view.
The RFA handpiece contains the introducer and needle electrode array. It snaps together with the IUUS probe to form and integrate into a single treatment device that contains all controls needed to place and size the ablation. Mechanical stops and lockouts within the RFA handpiece further enhance proper localization and sizing of the ablation.
The system’s graphical guidance software, also known as the SMART Guide, is a real-time graphical overlay on the ultrasound display, which enables one to visually select deployment length, width, and position of the ablation guides. In so doing, the mechanical stops for the introducer and needle electrodes are determined prior to their insertion into the targeted fibroid(s). This was validated in more than 4,000 ablations in bovine muscle and human-extirpated uteri, as well as in vivo at time of laparotomy.
By displaying the ellipsoidal region where the ablation will take place (ablation zone) along with a surrounding ellipsoid (thermal safety border) where tissue temperature will be elevated, the SMART Guide provides a safer and more accurate understanding of the ablation than if it showed only the ablation zone.
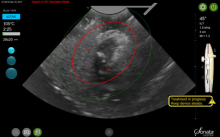
As with transabdominal or transvaginal sonography, the serosa will appear hyperechoic at the time of intrauterine ultrasound. By using the SMART Guide, the ablation is sized and positioned to encompass as much of the fibroid as possible while maintaining thermal energy within the uterine serosal margin. Once the desired ablation size has been selected, and safe placement of the needle electrodes is confirmed by rotating the IUUS probe in multiple planes, therapeutic RF energy is delivered to the fibroid; the fixed treatment cycle is dependent on ablation size.
The system will modulate power (up to 150W) to keep temperature at the tips of the needle electrode at 105° C. Moreover, the time of energy delivery at the temperature of 105° – 2-7 minutes – is automatically set based on ablation size, which is a continuum up to 4 cm wide and up to 5 cm long. Multiple ablations may be utilized in a particularly large fibroid.
Unlike hysteroscopic myomectomy, only a small amount of hypotonic solution is instilled within the uterine cavity to enhance acoustic coupling. Furthermore, the treatment device (RFA handpiece and IUUS probe) is only 8.3 mm in diameter. This requires Hegar dilatation of the cervix to 9.
The procedure
After administering anesthesia (regional or sedation), dispersive electrode pads are placed on the anterior thighs. After the cervix is dilated to Hegar dilatation of 9, the treatment device is inserted transcervically into the uterine cavity and the fibroid(s) are identified with the ultrasound probe. The physician plans and optimizes the ablation by sizing and aligning the graphical overlay targeting guide (the SMART Guide) over the live image. Once the size and location of the ablation are set, the trocar-tipped introducer is advanced into the fibroid. After ensuring the guide is within the serosal boundary, the needle electrodes are deployed.
A second visual safety check is completed, and the delivery of RF energy is initiated using a footswitch control. The time of energy delivery is determined based on the size of the desired ablation, up to 7 minutes for the largest ablation size (5 cm x 4 cm). The targeting and treatment steps are repeated as required to treat additional fibroids. Once the treatment is completed, the needle electrodes and introducer are retracted, and the treatment device removed.
Study results and the future
The 12-month safety and effectiveness data for ultrasound-guided transcervical ablation of uterine fibroids were reported in January 2019 in Obstetrics & Gynecology.4 Women enrolled in the prospective, multicenter, single-arm, interventional trial had 1-10 fibroids – the International Federation of Gynecology and Obstetrics (FIGO) types 1, 2, 3, 4, and 2-5 (pedunculated fibroids excluded) – with diameters of 1-5 centimeters. Patients also were required to have at least one fibroid indenting or impinging on the endometrial cavity (FIGO type 1, 2, 3, or 2-5).
Upon study entry, the pictorial assessment blood loss was required to be 150-500 cc. The study included 147 patients. Both coprimary endpoints were satisfied at 12 months; that is, 65% of patients experienced a 50% or greater reduction in menstrual bleeding, and 99% were free from surgical intervention at 1 year.
The mean pictorial blood loss decreased by 39%, 48%, and 51% at 3, 6, and 12 months respectively. Moreover, 95% of the study population experienced some reduction in menstrual bleeding at 12 months. There also were mean improvements in symptom severity and health-related quality-of-life parameters. Mean maximal fibroid volume reduction per patient was 62%.
More than half of the patients returned to normal activity within 1 day, 96% of patients reported symptom improvement at 12 months, and 97% expressed satisfaction with the procedure and results at 12 months. There were no device-related adverse events.
I am the lead author for the 2-year follow-up study utilizing transcervical RFA of symptomatic uterine fibroids, which currently is in press. Suffice it to say, the quality-of-life data, symptom improvement, and lower rate of surgical reintervention all are significant and compelling. Ultimately, I believe Sonata will not only be a treatment of choice in the appropriate patient presenting with heavy menstrual flow or bulk symptoms secondary to uterine fibroids, but will prove to be beneficial in women with impinging or deep submucosal fibroids and implantation failure.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. Dr. Miller disclosed that he is a consultant for Gynesonics and holds a stock option agreement with the company.
References
1. Am J Obstet Gynecol. 2013 Oct;209(4):319.e1-319.e20.
2. Int J Hyperthermia. 2019;36(1):295-301.
3. Curr Obstet Gynecol Rep. 2017; 6(1): 67-73.
4. Obstet Gynecol. 2019 Jan;133(1):13-22.
On Aug. 29, 2019, the first commercial case utilizing the Sonata system to transcervically ablate symptomatic uterine fibroids under ultrasound guidance was performed at Stamford (Conn.) Hospital. This truly minimally invasive new treatment expands our options in the surgical management of uterine fibroids.
Uterine fibroids are the most common benign tumors of the reproductive tract. It has been estimated that nearly half of the 70%-80% of women who develop fibroids during their reproductive years are symptomatic. Given that some patients present with fertility concerns, it also has been estimated that at least one in three women with fibroids have symptoms such as heavy bleeding (menorrhagia) and bulk symptoms, pain (dyspareunia, dysmenorrhea, noncyclic pain), and increased urinary frequency.
Fibroids are the most common cause of hysterectomy in the United States, with 240,000 (40% of 600,000) performed annually, yet research shows that many women are interested in minimally invasive options and in uterine conservation. In a 2013 national survey published in the American Journal of Obstetrics and Gynecology, 79% of women expressed an interest in minimally invasive approaches for fibroid treatment, and over 50% reported a desire for uterine conservation.1
Both myomectomy and uterine artery embolization are uterine-sparing procedures. However, uterine artery embolization should not be performed in a woman interested in pregnancy. Moreover, there are reports of ovarian reserve issues when the procedure is performed in women in their later reproductive years.
Depending on the technique performed, women undergoing hysteroscopic myomectomy are at risk of fluid overload, hyponatremia, gas-related embolism, and postoperative adhesions. The suture requirements of a laparoscopic myomectomy make this approach an often-difficult one to master, even with robotic assistance. It also requires intubation and potentially places the patient at risk for bleeding and infection. Furthermore, long-term risks include adhesions and the need for C-section with pregnancy.
The impact of uterine fibroids on patients’ lives and their desire for uterine conservation has spurred growing interest in the use of radiofrequency (RF) energy to ablate uterine fibroids. In a 2018 systematic review of nonresective treatments for uterine fibroids published in the International Journal of Hyperthermia, investigators found that the pooled fibroid volume reductions at 6 months after RF ablation and uterine artery embolization were 70% and 54%, respectively.2
The first commercially available system utilizing RF frequency to shrink fibrosis – Acessa – involves laparoscopy, and thus requires abdominal incisions. In August 2018, the Sonata system (Gynesonics: Redwood, Calif.) received Food and Drug Administration clearance after having received European CE-Mark approval in 2010 (for the original device, the VizAblate) and in 2014 (for the next-generation device, the Sonata).
The technology
For a complete description of transcervical, intrauterine sonography–guided radiofrequency ablation of uterine fibroids, one can refer to the excellent outline by David Toub, MD, in Current Obstetrics and Gynecology Reports.3 Basically, the Sonata system allows for real-time, image-guided treatment through the use of a reusable intrauterine ultrasound (IUUS) probe, a single-use RF ablation (RFA) handpiece, and graphical guidance software for diagnosis and targeting.
Initially, the IUUS probe enables identification of fibroids from within the uterine cavity, then guides deployment of an introducer and needle electrode into the targeted fibroid(s). The probe image is curvilinear, penetrates more than 9 cm, and provides a 90-degree field of view.
The RFA handpiece contains the introducer and needle electrode array. It snaps together with the IUUS probe to form and integrate into a single treatment device that contains all controls needed to place and size the ablation. Mechanical stops and lockouts within the RFA handpiece further enhance proper localization and sizing of the ablation.
The system’s graphical guidance software, also known as the SMART Guide, is a real-time graphical overlay on the ultrasound display, which enables one to visually select deployment length, width, and position of the ablation guides. In so doing, the mechanical stops for the introducer and needle electrodes are determined prior to their insertion into the targeted fibroid(s). This was validated in more than 4,000 ablations in bovine muscle and human-extirpated uteri, as well as in vivo at time of laparotomy.
By displaying the ellipsoidal region where the ablation will take place (ablation zone) along with a surrounding ellipsoid (thermal safety border) where tissue temperature will be elevated, the SMART Guide provides a safer and more accurate understanding of the ablation than if it showed only the ablation zone.
As with transabdominal or transvaginal sonography, the serosa will appear hyperechoic at the time of intrauterine ultrasound. By using the SMART Guide, the ablation is sized and positioned to encompass as much of the fibroid as possible while maintaining thermal energy within the uterine serosal margin. Once the desired ablation size has been selected, and safe placement of the needle electrodes is confirmed by rotating the IUUS probe in multiple planes, therapeutic RF energy is delivered to the fibroid; the fixed treatment cycle is dependent on ablation size.
The system will modulate power (up to 150W) to keep temperature at the tips of the needle electrode at 105° C. Moreover, the time of energy delivery at the temperature of 105° – 2-7 minutes – is automatically set based on ablation size, which is a continuum up to 4 cm wide and up to 5 cm long. Multiple ablations may be utilized in a particularly large fibroid.
Unlike hysteroscopic myomectomy, only a small amount of hypotonic solution is instilled within the uterine cavity to enhance acoustic coupling. Furthermore, the treatment device (RFA handpiece and IUUS probe) is only 8.3 mm in diameter. This requires Hegar dilatation of the cervix to 9.
The procedure
After administering anesthesia (regional or sedation), dispersive electrode pads are placed on the anterior thighs. After the cervix is dilated to Hegar dilatation of 9, the treatment device is inserted transcervically into the uterine cavity and the fibroid(s) are identified with the ultrasound probe. The physician plans and optimizes the ablation by sizing and aligning the graphical overlay targeting guide (the SMART Guide) over the live image. Once the size and location of the ablation are set, the trocar-tipped introducer is advanced into the fibroid. After ensuring the guide is within the serosal boundary, the needle electrodes are deployed.
A second visual safety check is completed, and the delivery of RF energy is initiated using a footswitch control. The time of energy delivery is determined based on the size of the desired ablation, up to 7 minutes for the largest ablation size (5 cm x 4 cm). The targeting and treatment steps are repeated as required to treat additional fibroids. Once the treatment is completed, the needle electrodes and introducer are retracted, and the treatment device removed.
Study results and the future
The 12-month safety and effectiveness data for ultrasound-guided transcervical ablation of uterine fibroids were reported in January 2019 in Obstetrics & Gynecology.4 Women enrolled in the prospective, multicenter, single-arm, interventional trial had 1-10 fibroids – the International Federation of Gynecology and Obstetrics (FIGO) types 1, 2, 3, 4, and 2-5 (pedunculated fibroids excluded) – with diameters of 1-5 centimeters. Patients also were required to have at least one fibroid indenting or impinging on the endometrial cavity (FIGO type 1, 2, 3, or 2-5).
Upon study entry, the pictorial assessment blood loss was required to be 150-500 cc. The study included 147 patients. Both coprimary endpoints were satisfied at 12 months; that is, 65% of patients experienced a 50% or greater reduction in menstrual bleeding, and 99% were free from surgical intervention at 1 year.
The mean pictorial blood loss decreased by 39%, 48%, and 51% at 3, 6, and 12 months respectively. Moreover, 95% of the study population experienced some reduction in menstrual bleeding at 12 months. There also were mean improvements in symptom severity and health-related quality-of-life parameters. Mean maximal fibroid volume reduction per patient was 62%.
More than half of the patients returned to normal activity within 1 day, 96% of patients reported symptom improvement at 12 months, and 97% expressed satisfaction with the procedure and results at 12 months. There were no device-related adverse events.
I am the lead author for the 2-year follow-up study utilizing transcervical RFA of symptomatic uterine fibroids, which currently is in press. Suffice it to say, the quality-of-life data, symptom improvement, and lower rate of surgical reintervention all are significant and compelling. Ultimately, I believe Sonata will not only be a treatment of choice in the appropriate patient presenting with heavy menstrual flow or bulk symptoms secondary to uterine fibroids, but will prove to be beneficial in women with impinging or deep submucosal fibroids and implantation failure.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. Dr. Miller disclosed that he is a consultant for Gynesonics and holds a stock option agreement with the company.
References
1. Am J Obstet Gynecol. 2013 Oct;209(4):319.e1-319.e20.
2. Int J Hyperthermia. 2019;36(1):295-301.
3. Curr Obstet Gynecol Rep. 2017; 6(1): 67-73.
4. Obstet Gynecol. 2019 Jan;133(1):13-22.
On Aug. 29, 2019, the first commercial case utilizing the Sonata system to transcervically ablate symptomatic uterine fibroids under ultrasound guidance was performed at Stamford (Conn.) Hospital. This truly minimally invasive new treatment expands our options in the surgical management of uterine fibroids.
Uterine fibroids are the most common benign tumors of the reproductive tract. It has been estimated that nearly half of the 70%-80% of women who develop fibroids during their reproductive years are symptomatic. Given that some patients present with fertility concerns, it also has been estimated that at least one in three women with fibroids have symptoms such as heavy bleeding (menorrhagia) and bulk symptoms, pain (dyspareunia, dysmenorrhea, noncyclic pain), and increased urinary frequency.
Fibroids are the most common cause of hysterectomy in the United States, with 240,000 (40% of 600,000) performed annually, yet research shows that many women are interested in minimally invasive options and in uterine conservation. In a 2013 national survey published in the American Journal of Obstetrics and Gynecology, 79% of women expressed an interest in minimally invasive approaches for fibroid treatment, and over 50% reported a desire for uterine conservation.1
Both myomectomy and uterine artery embolization are uterine-sparing procedures. However, uterine artery embolization should not be performed in a woman interested in pregnancy. Moreover, there are reports of ovarian reserve issues when the procedure is performed in women in their later reproductive years.
Depending on the technique performed, women undergoing hysteroscopic myomectomy are at risk of fluid overload, hyponatremia, gas-related embolism, and postoperative adhesions. The suture requirements of a laparoscopic myomectomy make this approach an often-difficult one to master, even with robotic assistance. It also requires intubation and potentially places the patient at risk for bleeding and infection. Furthermore, long-term risks include adhesions and the need for C-section with pregnancy.
The impact of uterine fibroids on patients’ lives and their desire for uterine conservation has spurred growing interest in the use of radiofrequency (RF) energy to ablate uterine fibroids. In a 2018 systematic review of nonresective treatments for uterine fibroids published in the International Journal of Hyperthermia, investigators found that the pooled fibroid volume reductions at 6 months after RF ablation and uterine artery embolization were 70% and 54%, respectively.2
The first commercially available system utilizing RF frequency to shrink fibrosis – Acessa – involves laparoscopy, and thus requires abdominal incisions. In August 2018, the Sonata system (Gynesonics: Redwood, Calif.) received Food and Drug Administration clearance after having received European CE-Mark approval in 2010 (for the original device, the VizAblate) and in 2014 (for the next-generation device, the Sonata).
The technology
For a complete description of transcervical, intrauterine sonography–guided radiofrequency ablation of uterine fibroids, one can refer to the excellent outline by David Toub, MD, in Current Obstetrics and Gynecology Reports.3 Basically, the Sonata system allows for real-time, image-guided treatment through the use of a reusable intrauterine ultrasound (IUUS) probe, a single-use RF ablation (RFA) handpiece, and graphical guidance software for diagnosis and targeting.
Initially, the IUUS probe enables identification of fibroids from within the uterine cavity, then guides deployment of an introducer and needle electrode into the targeted fibroid(s). The probe image is curvilinear, penetrates more than 9 cm, and provides a 90-degree field of view.
The RFA handpiece contains the introducer and needle electrode array. It snaps together with the IUUS probe to form and integrate into a single treatment device that contains all controls needed to place and size the ablation. Mechanical stops and lockouts within the RFA handpiece further enhance proper localization and sizing of the ablation.
The system’s graphical guidance software, also known as the SMART Guide, is a real-time graphical overlay on the ultrasound display, which enables one to visually select deployment length, width, and position of the ablation guides. In so doing, the mechanical stops for the introducer and needle electrodes are determined prior to their insertion into the targeted fibroid(s). This was validated in more than 4,000 ablations in bovine muscle and human-extirpated uteri, as well as in vivo at time of laparotomy.
By displaying the ellipsoidal region where the ablation will take place (ablation zone) along with a surrounding ellipsoid (thermal safety border) where tissue temperature will be elevated, the SMART Guide provides a safer and more accurate understanding of the ablation than if it showed only the ablation zone.
As with transabdominal or transvaginal sonography, the serosa will appear hyperechoic at the time of intrauterine ultrasound. By using the SMART Guide, the ablation is sized and positioned to encompass as much of the fibroid as possible while maintaining thermal energy within the uterine serosal margin. Once the desired ablation size has been selected, and safe placement of the needle electrodes is confirmed by rotating the IUUS probe in multiple planes, therapeutic RF energy is delivered to the fibroid; the fixed treatment cycle is dependent on ablation size.
The system will modulate power (up to 150W) to keep temperature at the tips of the needle electrode at 105° C. Moreover, the time of energy delivery at the temperature of 105° – 2-7 minutes – is automatically set based on ablation size, which is a continuum up to 4 cm wide and up to 5 cm long. Multiple ablations may be utilized in a particularly large fibroid.
Unlike hysteroscopic myomectomy, only a small amount of hypotonic solution is instilled within the uterine cavity to enhance acoustic coupling. Furthermore, the treatment device (RFA handpiece and IUUS probe) is only 8.3 mm in diameter. This requires Hegar dilatation of the cervix to 9.
The procedure
After administering anesthesia (regional or sedation), dispersive electrode pads are placed on the anterior thighs. After the cervix is dilated to Hegar dilatation of 9, the treatment device is inserted transcervically into the uterine cavity and the fibroid(s) are identified with the ultrasound probe. The physician plans and optimizes the ablation by sizing and aligning the graphical overlay targeting guide (the SMART Guide) over the live image. Once the size and location of the ablation are set, the trocar-tipped introducer is advanced into the fibroid. After ensuring the guide is within the serosal boundary, the needle electrodes are deployed.
A second visual safety check is completed, and the delivery of RF energy is initiated using a footswitch control. The time of energy delivery is determined based on the size of the desired ablation, up to 7 minutes for the largest ablation size (5 cm x 4 cm). The targeting and treatment steps are repeated as required to treat additional fibroids. Once the treatment is completed, the needle electrodes and introducer are retracted, and the treatment device removed.
Study results and the future
The 12-month safety and effectiveness data for ultrasound-guided transcervical ablation of uterine fibroids were reported in January 2019 in Obstetrics & Gynecology.4 Women enrolled in the prospective, multicenter, single-arm, interventional trial had 1-10 fibroids – the International Federation of Gynecology and Obstetrics (FIGO) types 1, 2, 3, 4, and 2-5 (pedunculated fibroids excluded) – with diameters of 1-5 centimeters. Patients also were required to have at least one fibroid indenting or impinging on the endometrial cavity (FIGO type 1, 2, 3, or 2-5).
Upon study entry, the pictorial assessment blood loss was required to be 150-500 cc. The study included 147 patients. Both coprimary endpoints were satisfied at 12 months; that is, 65% of patients experienced a 50% or greater reduction in menstrual bleeding, and 99% were free from surgical intervention at 1 year.
The mean pictorial blood loss decreased by 39%, 48%, and 51% at 3, 6, and 12 months respectively. Moreover, 95% of the study population experienced some reduction in menstrual bleeding at 12 months. There also were mean improvements in symptom severity and health-related quality-of-life parameters. Mean maximal fibroid volume reduction per patient was 62%.
More than half of the patients returned to normal activity within 1 day, 96% of patients reported symptom improvement at 12 months, and 97% expressed satisfaction with the procedure and results at 12 months. There were no device-related adverse events.
I am the lead author for the 2-year follow-up study utilizing transcervical RFA of symptomatic uterine fibroids, which currently is in press. Suffice it to say, the quality-of-life data, symptom improvement, and lower rate of surgical reintervention all are significant and compelling. Ultimately, I believe Sonata will not only be a treatment of choice in the appropriate patient presenting with heavy menstrual flow or bulk symptoms secondary to uterine fibroids, but will prove to be beneficial in women with impinging or deep submucosal fibroids and implantation failure.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. Dr. Miller disclosed that he is a consultant for Gynesonics and holds a stock option agreement with the company.
References
1. Am J Obstet Gynecol. 2013 Oct;209(4):319.e1-319.e20.
2. Int J Hyperthermia. 2019;36(1):295-301.
3. Curr Obstet Gynecol Rep. 2017; 6(1): 67-73.
4. Obstet Gynecol. 2019 Jan;133(1):13-22.
Treating uterine fibroids
Uterine fibroids are the most common benign tumor in women originating from the smooth muscles of the myometrium. While some women are asymptomatic, others experience pelvic pain, pressure, and abnormal uterine bleeding. Uterine fibroids also are associated with gastrointestinal disturbances; urinary problems; infertility; and obstetrical complications including miscarriages, preterm delivery, and cesarean sections.
The first successful abdominal myomectomy was described in 1845 but the procedure quickly fell out of favor because of unacceptably high mortality rates. Myomectomies require special skills and, at times, are associated with bleeding resulting in massive transfusions or sometimes unwanted hysterectomies. In 1922, Victor Bonney developed a uterine artery clamp which significantly decreased bleeding associated with morbidity and mortality.1
The latter part of the 20th century belonged to the minimally invasive surgery (MIS) evolution. Currently, video- or robotic-assisted laparoscopic myomectomies are increasingly employed in fertility-sparing surgery. In 2014, electromechanical morcellators came under scrutiny with concerns about iatrogenic dissemination of both benign and malignant tissues. A media storm ensued, resulting in the 2014 Food and Drug Administration black-box warning, and electromechanical morcellators were pulled from shelves. Data are being collected to quantify and understand these risks more clearly.
While exposing patients to even a small risk of dissemination of an occult uterine malignancy is unwise, MIS should not be abandoned altogether given its advantages to patients.2 Most recently, the American College of Obstetricians and Gynecologists concluded that, although abdominal hysterectomy or myomectomy may reduce the chance of spreading undiagnosed leiomyosarcoma cells, it is associated with increased morbidity, compared with noninvasive approaches, and ob.gyns. should engage in open decision-making processes and explain nonsurgical options with patients.3
The author of this Master Class, Dr. Charles Miller, a world-renowned MIS surgeon, will enlighten readers on the latest development in noninvasive treatment of symptomatic patients. The Sonata system, a promising transcervical (and thus incisionless) treatment modality utilizing intrauterine sonography–guided radiofrequency ablation for uterine fibroids which does not require general anesthesia or hospitalization. He believes that Sonata “will not only be a treatment of choice in the appropriate patient presenting with heavy menstrual flow or bulk symptoms secondary to uterine fibroids, but will prove to be beneficial in women with impinging or deep submucosal fibroids and implantation failure.”
Dr. Miller is on the editorial advisory boards of numerous academic journals and serves as the editor of the award-winning Master Class in Gynecologic Surgery column. For this installment, he has stepped into the role of guest author. Dr. Miller has received numerous awards for his educational contributions and was recently granted the distinct honor of taking the lead in the March 28, 2020 Worldwide EndoMarch–Chicago. It is my pleasure to take part in this introduction.
Dr. Nezhat is director of minimally invasive surgery and robotics as well as the medical director of training and education at Northside Hospital, both in Atlanta. He is fellowship director at Atlanta Center for Special Minimally Invasive Surgery & Reproductive Medicine. Dr. Nezhat also is an adjunct professor of gynecology and obstetrics at Emory University, Atlanta, and is past president of the Society of Reproductive Surgeons and the AAGL. He reported that he has no disclosures relevant to this Master Class. Email him at obnews@mdedge.com.
References
1. BJOG. 2018 Apr;125(5):586.
2. JAMA Oncol. 2015;1(1):78-9.
3. Obstet Gynecol. 2019 Mar;133(3):e238-48.
Uterine fibroids are the most common benign tumor in women originating from the smooth muscles of the myometrium. While some women are asymptomatic, others experience pelvic pain, pressure, and abnormal uterine bleeding. Uterine fibroids also are associated with gastrointestinal disturbances; urinary problems; infertility; and obstetrical complications including miscarriages, preterm delivery, and cesarean sections.
The first successful abdominal myomectomy was described in 1845 but the procedure quickly fell out of favor because of unacceptably high mortality rates. Myomectomies require special skills and, at times, are associated with bleeding resulting in massive transfusions or sometimes unwanted hysterectomies. In 1922, Victor Bonney developed a uterine artery clamp which significantly decreased bleeding associated with morbidity and mortality.1
The latter part of the 20th century belonged to the minimally invasive surgery (MIS) evolution. Currently, video- or robotic-assisted laparoscopic myomectomies are increasingly employed in fertility-sparing surgery. In 2014, electromechanical morcellators came under scrutiny with concerns about iatrogenic dissemination of both benign and malignant tissues. A media storm ensued, resulting in the 2014 Food and Drug Administration black-box warning, and electromechanical morcellators were pulled from shelves. Data are being collected to quantify and understand these risks more clearly.
While exposing patients to even a small risk of dissemination of an occult uterine malignancy is unwise, MIS should not be abandoned altogether given its advantages to patients.2 Most recently, the American College of Obstetricians and Gynecologists concluded that, although abdominal hysterectomy or myomectomy may reduce the chance of spreading undiagnosed leiomyosarcoma cells, it is associated with increased morbidity, compared with noninvasive approaches, and ob.gyns. should engage in open decision-making processes and explain nonsurgical options with patients.3
The author of this Master Class, Dr. Charles Miller, a world-renowned MIS surgeon, will enlighten readers on the latest development in noninvasive treatment of symptomatic patients. The Sonata system, a promising transcervical (and thus incisionless) treatment modality utilizing intrauterine sonography–guided radiofrequency ablation for uterine fibroids which does not require general anesthesia or hospitalization. He believes that Sonata “will not only be a treatment of choice in the appropriate patient presenting with heavy menstrual flow or bulk symptoms secondary to uterine fibroids, but will prove to be beneficial in women with impinging or deep submucosal fibroids and implantation failure.”
Dr. Miller is on the editorial advisory boards of numerous academic journals and serves as the editor of the award-winning Master Class in Gynecologic Surgery column. For this installment, he has stepped into the role of guest author. Dr. Miller has received numerous awards for his educational contributions and was recently granted the distinct honor of taking the lead in the March 28, 2020 Worldwide EndoMarch–Chicago. It is my pleasure to take part in this introduction.
Dr. Nezhat is director of minimally invasive surgery and robotics as well as the medical director of training and education at Northside Hospital, both in Atlanta. He is fellowship director at Atlanta Center for Special Minimally Invasive Surgery & Reproductive Medicine. Dr. Nezhat also is an adjunct professor of gynecology and obstetrics at Emory University, Atlanta, and is past president of the Society of Reproductive Surgeons and the AAGL. He reported that he has no disclosures relevant to this Master Class. Email him at obnews@mdedge.com.
References
1. BJOG. 2018 Apr;125(5):586.
2. JAMA Oncol. 2015;1(1):78-9.
3. Obstet Gynecol. 2019 Mar;133(3):e238-48.
Uterine fibroids are the most common benign tumor in women originating from the smooth muscles of the myometrium. While some women are asymptomatic, others experience pelvic pain, pressure, and abnormal uterine bleeding. Uterine fibroids also are associated with gastrointestinal disturbances; urinary problems; infertility; and obstetrical complications including miscarriages, preterm delivery, and cesarean sections.
The first successful abdominal myomectomy was described in 1845 but the procedure quickly fell out of favor because of unacceptably high mortality rates. Myomectomies require special skills and, at times, are associated with bleeding resulting in massive transfusions or sometimes unwanted hysterectomies. In 1922, Victor Bonney developed a uterine artery clamp which significantly decreased bleeding associated with morbidity and mortality.1
The latter part of the 20th century belonged to the minimally invasive surgery (MIS) evolution. Currently, video- or robotic-assisted laparoscopic myomectomies are increasingly employed in fertility-sparing surgery. In 2014, electromechanical morcellators came under scrutiny with concerns about iatrogenic dissemination of both benign and malignant tissues. A media storm ensued, resulting in the 2014 Food and Drug Administration black-box warning, and electromechanical morcellators were pulled from shelves. Data are being collected to quantify and understand these risks more clearly.
While exposing patients to even a small risk of dissemination of an occult uterine malignancy is unwise, MIS should not be abandoned altogether given its advantages to patients.2 Most recently, the American College of Obstetricians and Gynecologists concluded that, although abdominal hysterectomy or myomectomy may reduce the chance of spreading undiagnosed leiomyosarcoma cells, it is associated with increased morbidity, compared with noninvasive approaches, and ob.gyns. should engage in open decision-making processes and explain nonsurgical options with patients.3
The author of this Master Class, Dr. Charles Miller, a world-renowned MIS surgeon, will enlighten readers on the latest development in noninvasive treatment of symptomatic patients. The Sonata system, a promising transcervical (and thus incisionless) treatment modality utilizing intrauterine sonography–guided radiofrequency ablation for uterine fibroids which does not require general anesthesia or hospitalization. He believes that Sonata “will not only be a treatment of choice in the appropriate patient presenting with heavy menstrual flow or bulk symptoms secondary to uterine fibroids, but will prove to be beneficial in women with impinging or deep submucosal fibroids and implantation failure.”
Dr. Miller is on the editorial advisory boards of numerous academic journals and serves as the editor of the award-winning Master Class in Gynecologic Surgery column. For this installment, he has stepped into the role of guest author. Dr. Miller has received numerous awards for his educational contributions and was recently granted the distinct honor of taking the lead in the March 28, 2020 Worldwide EndoMarch–Chicago. It is my pleasure to take part in this introduction.
Dr. Nezhat is director of minimally invasive surgery and robotics as well as the medical director of training and education at Northside Hospital, both in Atlanta. He is fellowship director at Atlanta Center for Special Minimally Invasive Surgery & Reproductive Medicine. Dr. Nezhat also is an adjunct professor of gynecology and obstetrics at Emory University, Atlanta, and is past president of the Society of Reproductive Surgeons and the AAGL. He reported that he has no disclosures relevant to this Master Class. Email him at obnews@mdedge.com.
References
1. BJOG. 2018 Apr;125(5):586.
2. JAMA Oncol. 2015;1(1):78-9.
3. Obstet Gynecol. 2019 Mar;133(3):e238-48.
Use of genetic testing for congenital heart defect management
The average student in America learns that genes form the building blocks of what makes us human by the time they receive their high school diploma. Indeed, the completion of the Human Genome Project in 2003 paved the way for our genetic makeup, much like our medical history, to become a routine part of our health care. For example, our faculty at the University of Maryland School of Medicine discovered an important gene – CYP2C19 – which is involved in the metabolism of the antiplatelet medicine clopidogrel (Plavix). Although most people have this gene, some don’t. Therefore, when we manage a patient with coronary disease, we use a genetic screen to determine whether that patient has CYP2C19 and then modify therapy based on these results.
Our genes also have become commodities – from companies willing to analyze our genes to determine our racial and ethnic ancestry or propensity for certain diseases to those that can sequence the family dog’s genes.
Advances in genomics similarly have impacted ob.gyn. practice. Because of rapidly evolving gene analysis tools, we can now, for example, noninvasively test a developing fetus’s risk for chromosomal abnormalities and determine a baby’s sex by merely examining fetal DNA in a pregnant woman’s bloodstream. Although not diagnostic, these gene-based prenatal screening tests have reduced the need for unnecessary, costly, and highly invasive procedures for many of our patients.
Importantly, our recognition that certain genes can confer a higher risk of disease has meant that performing a prenatal genetic evaluation can greatly inform the mother and her care team about potential problems her baby may have that may require additional management. For babies who have congenital heart defects, a genetic evaluation performed in addition to sonographic examination can provide ob.gyns. with crucial details to enhance pregnancy management and postnatal care decisions.
The importance of genetic testing and analysis in the detection, treatment, and prevention of congenital heart defects is the topic of part two of this two-part Master Class series authored by Shifa Turan, MD, associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine and director of the Fetal Heart Program at the University of Maryland Medical Center. By using a combination of three- and four-dimensional ultrasound with gene assays, Dr. Turan and her colleagues can greatly enhance and personalize the care they deliver to their patients.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
The average student in America learns that genes form the building blocks of what makes us human by the time they receive their high school diploma. Indeed, the completion of the Human Genome Project in 2003 paved the way for our genetic makeup, much like our medical history, to become a routine part of our health care. For example, our faculty at the University of Maryland School of Medicine discovered an important gene – CYP2C19 – which is involved in the metabolism of the antiplatelet medicine clopidogrel (Plavix). Although most people have this gene, some don’t. Therefore, when we manage a patient with coronary disease, we use a genetic screen to determine whether that patient has CYP2C19 and then modify therapy based on these results.
Our genes also have become commodities – from companies willing to analyze our genes to determine our racial and ethnic ancestry or propensity for certain diseases to those that can sequence the family dog’s genes.
Advances in genomics similarly have impacted ob.gyn. practice. Because of rapidly evolving gene analysis tools, we can now, for example, noninvasively test a developing fetus’s risk for chromosomal abnormalities and determine a baby’s sex by merely examining fetal DNA in a pregnant woman’s bloodstream. Although not diagnostic, these gene-based prenatal screening tests have reduced the need for unnecessary, costly, and highly invasive procedures for many of our patients.
Importantly, our recognition that certain genes can confer a higher risk of disease has meant that performing a prenatal genetic evaluation can greatly inform the mother and her care team about potential problems her baby may have that may require additional management. For babies who have congenital heart defects, a genetic evaluation performed in addition to sonographic examination can provide ob.gyns. with crucial details to enhance pregnancy management and postnatal care decisions.
The importance of genetic testing and analysis in the detection, treatment, and prevention of congenital heart defects is the topic of part two of this two-part Master Class series authored by Shifa Turan, MD, associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine and director of the Fetal Heart Program at the University of Maryland Medical Center. By using a combination of three- and four-dimensional ultrasound with gene assays, Dr. Turan and her colleagues can greatly enhance and personalize the care they deliver to their patients.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
The average student in America learns that genes form the building blocks of what makes us human by the time they receive their high school diploma. Indeed, the completion of the Human Genome Project in 2003 paved the way for our genetic makeup, much like our medical history, to become a routine part of our health care. For example, our faculty at the University of Maryland School of Medicine discovered an important gene – CYP2C19 – which is involved in the metabolism of the antiplatelet medicine clopidogrel (Plavix). Although most people have this gene, some don’t. Therefore, when we manage a patient with coronary disease, we use a genetic screen to determine whether that patient has CYP2C19 and then modify therapy based on these results.
Our genes also have become commodities – from companies willing to analyze our genes to determine our racial and ethnic ancestry or propensity for certain diseases to those that can sequence the family dog’s genes.
Advances in genomics similarly have impacted ob.gyn. practice. Because of rapidly evolving gene analysis tools, we can now, for example, noninvasively test a developing fetus’s risk for chromosomal abnormalities and determine a baby’s sex by merely examining fetal DNA in a pregnant woman’s bloodstream. Although not diagnostic, these gene-based prenatal screening tests have reduced the need for unnecessary, costly, and highly invasive procedures for many of our patients.
Importantly, our recognition that certain genes can confer a higher risk of disease has meant that performing a prenatal genetic evaluation can greatly inform the mother and her care team about potential problems her baby may have that may require additional management. For babies who have congenital heart defects, a genetic evaluation performed in addition to sonographic examination can provide ob.gyns. with crucial details to enhance pregnancy management and postnatal care decisions.
The importance of genetic testing and analysis in the detection, treatment, and prevention of congenital heart defects is the topic of part two of this two-part Master Class series authored by Shifa Turan, MD, associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine and director of the Fetal Heart Program at the University of Maryland Medical Center. By using a combination of three- and four-dimensional ultrasound with gene assays, Dr. Turan and her colleagues can greatly enhance and personalize the care they deliver to their patients.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
Genetic assessment for CHD: Case-specific, stepwise
Congenital heart defects (CHDs) are etiologically heterogeneous, but in recent years it has become clear that genetics plays a larger role in the development of CHDs than was previously thought. Research has been shifting from a focus on risk – estimating the magnitude of increased risk, for instance, based on maternal or familial risk factors – to a focus on the etiology of cardiac defects.
In practice, advances in genetic testing technologies have made the underlying causes of CHDs increasingly detectable. Chromosomal microarray analysis (CMA) – technology that detects significantly more and smaller changes in the amount of chromosomal material than traditional karyotype – has been proven to increase the diagnostic yield in cases of isolated CHDs and CHDs with extracardiac anomalies. Targeted next-generation sequencing also is now available as an additional approach in selective cases, and a clinically viable option for whole-exome sequencing is fast approaching.
For researchers, genetic evaluation carries the potential to unravel remaining mysteries about underlying causes of CHDs – to provide pathological insights and identify potential therapeutic targets. Currently, about 6 % of the total pie of presumed genetic determinants of CHDs is attributed to chromosomal anomalies, 10% to copy number variants, and 12% to single-gene defects. The remaining 72% of etiology, approximately, is undetermined.
As Helen Taussig, MD, (known as the founder of pediatric cardiology) once said, common cardiac malformations occurring in otherwise “normal” individuals “must be genetic in origin.”1 Greater use of genetic testing – and in particular, of whole-exome sequencing – will drive down this “undetermined” piece of the genetics pie.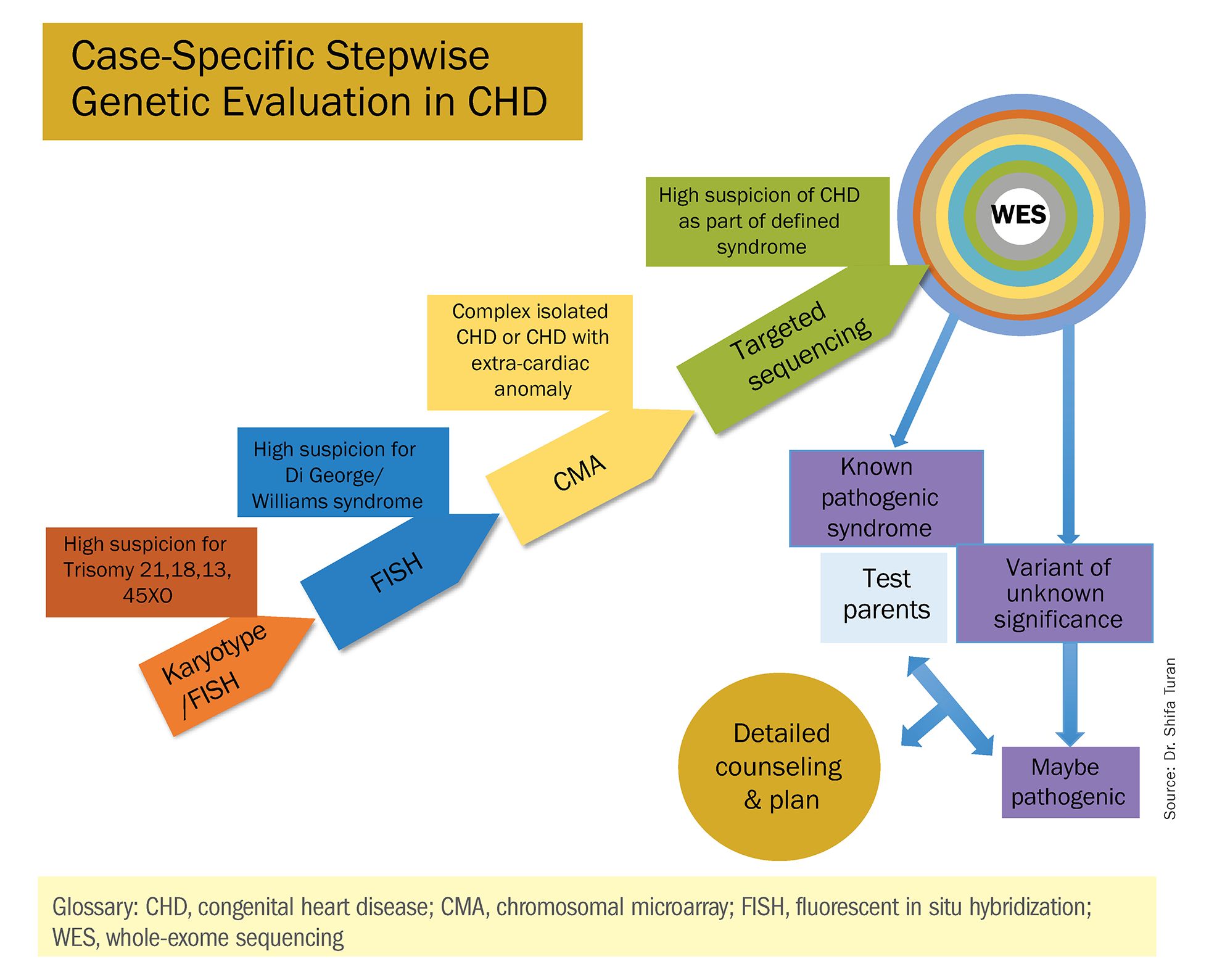
For clinicians and patients, prenatal genetic evaluation can inform clinical management, guiding decisions on the mode, timing, and location of delivery. Genetic assessments help guide the neonatal health care team in taking optimal care of the infant, and the surgeon in preparing for neonatal surgeries and postsurgical complications.
In a recent analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database, prenatal diagnosis was associated with a lower overall prevalence of major preoperative risk factors for cardiac surgery.2 Surgical outcomes themselves also have been shown to be better after the prenatal diagnosis of complex CHDs, mainly because of improvements in perioperative care.3
When genetic etiology is elucidated, the cardiologist also is better able to counsel patients about anticipated challenges – such as the propensity, with certain genetic variants of CHD, to develop neurodevelopmental delays or other cardiac complications – and to target patient follow-up. Patients also can make informed decisions about termination or continuation of a current pregnancy and about family planning in the future.
Fortunately, advances in genetics technology have paralleled technological advancements in ultrasound. As I discussed in part one of this two-part Master Class series, it is now possible to detect many major CHDs well before 16 weeks’ gestation. Checking the structure of the fetal heart at the first-trimester screening and sonography (11-14 weeks of gestation) offers the opportunity for early genetic assessment, counseling, and planning when anomalies are detected.
A personalized approach
There has been growing interest in recent years in CMA for the prenatal genetic workup of CHDs. Microarray targets chromosomal regions at a much higher resolution than traditional karyotype. Traditional karyotype assesses both changes in chromosome number as well as more subtle structural changes such as chromosomal deletions and duplications. CMA finds what traditional karyotype identifies, but in addition, it identifies much smaller, clinically relevant chromosomal deletions and duplications that are not detected by karyotype performed with or without fluorescence in-situ hybridization (FISH). FISH uses DNA probes that carry fluorescent tags to detect chromosomal DNA.
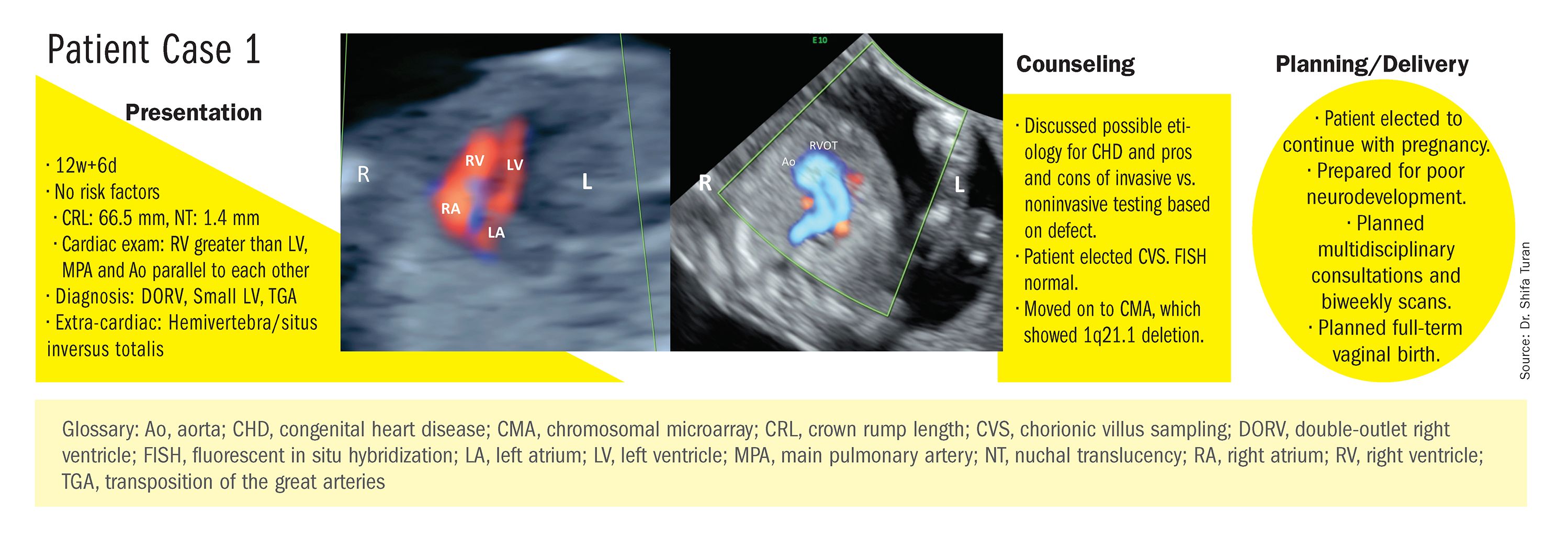
At our center, we studied the prenatal genetic test results of 145 fetuses diagnosed with CHDs. Each case involved FISH for aneuploidy/karyotype, followed by CMA in cases of a negative karyotype result. CMA increased the diagnostic yield in cases of CHD by 19.8% overall – 17.4% in cases of isolated CHD and 24.5% in cases of CHD plus extracardiac anomalies.4
Indeed, although a microarray costs more and takes an additional 2 weeks to run, CMA should be strongly considered as first-line testing for the prenatal genetic evaluation of fetuses with major structural cardiac abnormalities detected by ultrasound. However, there still are cases in which a karyotype might be sufficient. For instance, if I see that a fetus has an atrial-ventricular septal defect on a prenatal ultrasound, and there are markers for trisomy 21, 13, or 18, or Turner’s syndrome (45 XO), I usually recommend a karyotype or FISH rather than an initial CMA. If the karyotype is abnormal – which is likely in such a scenario – there isn’t a need for more extensive testing.
Similarly, when there is high suspicion for DiGeorge syndrome (the 22q11.2 deletion, which often includes cleft palate and aortic arch abnormalities), usually it is most appropriate to perform a FISH test.
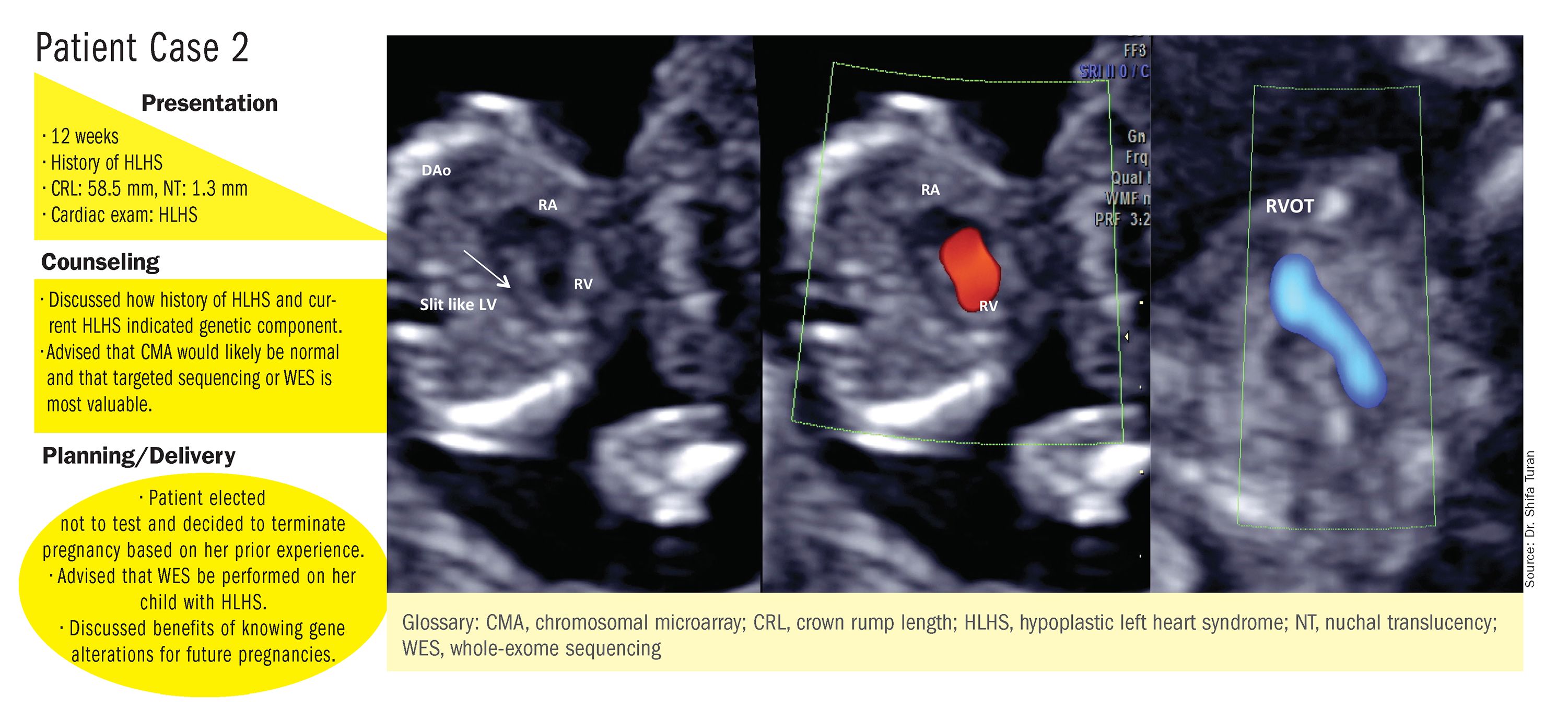
CMA is the preferred first modality, however, when prenatal imaging suggests severe CHD – for instance, when there are signs of hypoplastic left heart syndrome or tetralogy of Fallot (a conotruncal defect) – or complex CHD with extracardiac anomalies. In these cases, there is a high likelihood of detecting a small deletion or duplication that would be missed with karyotype.
In the past decade, karyotype and CMA have become the major methods used in our practice. However, targeted next‐generation sequencing and whole‐exome sequencing may become more widely used because these technologies enable rapid analysis of a large number of gene sequences and facilitate discovery of novel causative genes in many genetic diseases that cause CHDs.
Currently, targeted next-generation sequencing has mainly been used in the postnatal setting, and there are limited data available on its prenatal use. Compared with whole-exome sequencing, which sequences all of the protein-coding regions of the genome, targeted next-generation sequencing panels select regions of genes that are known to be associated with diseases of interest.
For CHDs, some perinatal centers have begun using a customized gene panel that targets 77 CHD-associated genes. This particular panel has been shown to be useful in addition to current methods and is an effective tool for prenatal genetic diagnosis.5
Whole-exome sequencing is currently expensive and time consuming. While sometimes it is used in the postnatal context, it is not yet part of routine practice as a prenatal diagnostic tool. As technology advances this will change – early in the next decade, I believe. For now, whole-exome sequencing may be an option for some patients who want to know more when severe CHD is evident on ultrasound and there are negative results from CMA or targeted sequencing. We have diagnosed some rare genetic syndromes using whole-exome sequencing; these diagnoses helped us to better manage the pregnancies.
These choices are part of the case-specific, stepwise approach to genetic evaluation that we take in our fetal heart program. Our goal is to pursue information that will be accurate and valuable for the patient and clinicians, in the most cost-effective and timely manner.
Limitations of noninvasive screening
In our fetal heart program we see increasing numbers of referred patients who have chosen noninvasive cell-free fetal DNA screening (cfDNA) after a cardiac anomaly is detected on ultrasound examination, and who believe that their “low risk” results demonstrate very little or no risk of CHD. Many of these patients express a belief that noninvasive testing is highly sensitive and accurate for fetal anomalies, including CHDs, and are not easily convinced of the value of other genetic tests.
We recently conducted a retrospective chart analysis (unpublished) in which we found that 41% of cases of CHD with abnormal genetics results were not detectable by cfDNA screening.
In the case of atrial-ventricular septal defects and conotruncal abnormalities that often are more associated with common aneuploidies (trisomy 21, 18, 13, and 45 XO), a “high-risk” result from cfDNA screening may offer the family and cardiology/neonatal team some guidance, but a “low-risk” result does not eliminate the risk of a microarray abnormality and thus may provide false reassurance.
Other research has shown that noninvasive screening will miss up to 7.3% of karyotype abnormalities in pregnancies at high risk for common aneuploidies.6
While invasive testing poses a very small risk of miscarriage, it is hard without such testing to elucidate the potential genetic etiologies of CHDs and truly understand the problems. We must take time to thoughtfully counsel patients who decline invasive testing about the limitations of cfDNA screening for CHDs and other anomalies.
Dr. Turan is an associate professor of obstetrics, gynecology, and reproductive sciences, and director of the fetal heart program at the University of Maryland School of Medicine and director of the Fetal Heart Program at the University of Maryland Medical Center. Dr. Turan reported that she has no disclosures relevant to this Master Class. Email her at obnews@mdedge.com.
References
1. J Am Coll Cardiol. 1988 Oct;12(4):1079-86.
2. Pediatr Cardiol. 2019 Mar;40(3):489-96.
3. Ann Pediatr Cardiol. 2017 May-Aug;10(2):126-30.
4. Eur J Obstet Gynecol Reprod Biol 2018;221:172-76.
5. Ultrasound Obstet Gynecol. 2018 Aug;52(2):205-11.
6. PLoS One. 2016 Jan 15;11(1):e0146794.
Congenital heart defects (CHDs) are etiologically heterogeneous, but in recent years it has become clear that genetics plays a larger role in the development of CHDs than was previously thought. Research has been shifting from a focus on risk – estimating the magnitude of increased risk, for instance, based on maternal or familial risk factors – to a focus on the etiology of cardiac defects.
In practice, advances in genetic testing technologies have made the underlying causes of CHDs increasingly detectable. Chromosomal microarray analysis (CMA) – technology that detects significantly more and smaller changes in the amount of chromosomal material than traditional karyotype – has been proven to increase the diagnostic yield in cases of isolated CHDs and CHDs with extracardiac anomalies. Targeted next-generation sequencing also is now available as an additional approach in selective cases, and a clinically viable option for whole-exome sequencing is fast approaching.
For researchers, genetic evaluation carries the potential to unravel remaining mysteries about underlying causes of CHDs – to provide pathological insights and identify potential therapeutic targets. Currently, about 6 % of the total pie of presumed genetic determinants of CHDs is attributed to chromosomal anomalies, 10% to copy number variants, and 12% to single-gene defects. The remaining 72% of etiology, approximately, is undetermined.
As Helen Taussig, MD, (known as the founder of pediatric cardiology) once said, common cardiac malformations occurring in otherwise “normal” individuals “must be genetic in origin.”1 Greater use of genetic testing – and in particular, of whole-exome sequencing – will drive down this “undetermined” piece of the genetics pie.
For clinicians and patients, prenatal genetic evaluation can inform clinical management, guiding decisions on the mode, timing, and location of delivery. Genetic assessments help guide the neonatal health care team in taking optimal care of the infant, and the surgeon in preparing for neonatal surgeries and postsurgical complications.
In a recent analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database, prenatal diagnosis was associated with a lower overall prevalence of major preoperative risk factors for cardiac surgery.2 Surgical outcomes themselves also have been shown to be better after the prenatal diagnosis of complex CHDs, mainly because of improvements in perioperative care.3
When genetic etiology is elucidated, the cardiologist also is better able to counsel patients about anticipated challenges – such as the propensity, with certain genetic variants of CHD, to develop neurodevelopmental delays or other cardiac complications – and to target patient follow-up. Patients also can make informed decisions about termination or continuation of a current pregnancy and about family planning in the future.
Fortunately, advances in genetics technology have paralleled technological advancements in ultrasound. As I discussed in part one of this two-part Master Class series, it is now possible to detect many major CHDs well before 16 weeks’ gestation. Checking the structure of the fetal heart at the first-trimester screening and sonography (11-14 weeks of gestation) offers the opportunity for early genetic assessment, counseling, and planning when anomalies are detected.
A personalized approach
There has been growing interest in recent years in CMA for the prenatal genetic workup of CHDs. Microarray targets chromosomal regions at a much higher resolution than traditional karyotype. Traditional karyotype assesses both changes in chromosome number as well as more subtle structural changes such as chromosomal deletions and duplications. CMA finds what traditional karyotype identifies, but in addition, it identifies much smaller, clinically relevant chromosomal deletions and duplications that are not detected by karyotype performed with or without fluorescence in-situ hybridization (FISH). FISH uses DNA probes that carry fluorescent tags to detect chromosomal DNA.

At our center, we studied the prenatal genetic test results of 145 fetuses diagnosed with CHDs. Each case involved FISH for aneuploidy/karyotype, followed by CMA in cases of a negative karyotype result. CMA increased the diagnostic yield in cases of CHD by 19.8% overall – 17.4% in cases of isolated CHD and 24.5% in cases of CHD plus extracardiac anomalies.4
Indeed, although a microarray costs more and takes an additional 2 weeks to run, CMA should be strongly considered as first-line testing for the prenatal genetic evaluation of fetuses with major structural cardiac abnormalities detected by ultrasound. However, there still are cases in which a karyotype might be sufficient. For instance, if I see that a fetus has an atrial-ventricular septal defect on a prenatal ultrasound, and there are markers for trisomy 21, 13, or 18, or Turner’s syndrome (45 XO), I usually recommend a karyotype or FISH rather than an initial CMA. If the karyotype is abnormal – which is likely in such a scenario – there isn’t a need for more extensive testing.
Similarly, when there is high suspicion for DiGeorge syndrome (the 22q11.2 deletion, which often includes cleft palate and aortic arch abnormalities), usually it is most appropriate to perform a FISH test.
CMA is the preferred first modality, however, when prenatal imaging suggests severe CHD – for instance, when there are signs of hypoplastic left heart syndrome or tetralogy of Fallot (a conotruncal defect) – or complex CHD with extracardiac anomalies. In these cases, there is a high likelihood of detecting a small deletion or duplication that would be missed with karyotype.
In the past decade, karyotype and CMA have become the major methods used in our practice. However, targeted next‐generation sequencing and whole‐exome sequencing may become more widely used because these technologies enable rapid analysis of a large number of gene sequences and facilitate discovery of novel causative genes in many genetic diseases that cause CHDs.
Currently, targeted next-generation sequencing has mainly been used in the postnatal setting, and there are limited data available on its prenatal use. Compared with whole-exome sequencing, which sequences all of the protein-coding regions of the genome, targeted next-generation sequencing panels select regions of genes that are known to be associated with diseases of interest.
For CHDs, some perinatal centers have begun using a customized gene panel that targets 77 CHD-associated genes. This particular panel has been shown to be useful in addition to current methods and is an effective tool for prenatal genetic diagnosis.5
Whole-exome sequencing is currently expensive and time consuming. While sometimes it is used in the postnatal context, it is not yet part of routine practice as a prenatal diagnostic tool. As technology advances this will change – early in the next decade, I believe. For now, whole-exome sequencing may be an option for some patients who want to know more when severe CHD is evident on ultrasound and there are negative results from CMA or targeted sequencing. We have diagnosed some rare genetic syndromes using whole-exome sequencing; these diagnoses helped us to better manage the pregnancies.

These choices are part of the case-specific, stepwise approach to genetic evaluation that we take in our fetal heart program. Our goal is to pursue information that will be accurate and valuable for the patient and clinicians, in the most cost-effective and timely manner.
Limitations of noninvasive screening
In our fetal heart program we see increasing numbers of referred patients who have chosen noninvasive cell-free fetal DNA screening (cfDNA) after a cardiac anomaly is detected on ultrasound examination, and who believe that their “low risk” results demonstrate very little or no risk of CHD. Many of these patients express a belief that noninvasive testing is highly sensitive and accurate for fetal anomalies, including CHDs, and are not easily convinced of the value of other genetic tests.
We recently conducted a retrospective chart analysis (unpublished) in which we found that 41% of cases of CHD with abnormal genetics results were not detectable by cfDNA screening.
In the case of atrial-ventricular septal defects and conotruncal abnormalities that often are more associated with common aneuploidies (trisomy 21, 18, 13, and 45 XO), a “high-risk” result from cfDNA screening may offer the family and cardiology/neonatal team some guidance, but a “low-risk” result does not eliminate the risk of a microarray abnormality and thus may provide false reassurance.
Other research has shown that noninvasive screening will miss up to 7.3% of karyotype abnormalities in pregnancies at high risk for common aneuploidies.6
While invasive testing poses a very small risk of miscarriage, it is hard without such testing to elucidate the potential genetic etiologies of CHDs and truly understand the problems. We must take time to thoughtfully counsel patients who decline invasive testing about the limitations of cfDNA screening for CHDs and other anomalies.
Dr. Turan is an associate professor of obstetrics, gynecology, and reproductive sciences, and director of the fetal heart program at the University of Maryland School of Medicine and director of the Fetal Heart Program at the University of Maryland Medical Center. Dr. Turan reported that she has no disclosures relevant to this Master Class. Email her at obnews@mdedge.com.
References
1. J Am Coll Cardiol. 1988 Oct;12(4):1079-86.
2. Pediatr Cardiol. 2019 Mar;40(3):489-96.
3. Ann Pediatr Cardiol. 2017 May-Aug;10(2):126-30.
4. Eur J Obstet Gynecol Reprod Biol 2018;221:172-76.
5. Ultrasound Obstet Gynecol. 2018 Aug;52(2):205-11.
6. PLoS One. 2016 Jan 15;11(1):e0146794.
Congenital heart defects (CHDs) are etiologically heterogeneous, but in recent years it has become clear that genetics plays a larger role in the development of CHDs than was previously thought. Research has been shifting from a focus on risk – estimating the magnitude of increased risk, for instance, based on maternal or familial risk factors – to a focus on the etiology of cardiac defects.
In practice, advances in genetic testing technologies have made the underlying causes of CHDs increasingly detectable. Chromosomal microarray analysis (CMA) – technology that detects significantly more and smaller changes in the amount of chromosomal material than traditional karyotype – has been proven to increase the diagnostic yield in cases of isolated CHDs and CHDs with extracardiac anomalies. Targeted next-generation sequencing also is now available as an additional approach in selective cases, and a clinically viable option for whole-exome sequencing is fast approaching.
For researchers, genetic evaluation carries the potential to unravel remaining mysteries about underlying causes of CHDs – to provide pathological insights and identify potential therapeutic targets. Currently, about 6 % of the total pie of presumed genetic determinants of CHDs is attributed to chromosomal anomalies, 10% to copy number variants, and 12% to single-gene defects. The remaining 72% of etiology, approximately, is undetermined.
As Helen Taussig, MD, (known as the founder of pediatric cardiology) once said, common cardiac malformations occurring in otherwise “normal” individuals “must be genetic in origin.”1 Greater use of genetic testing – and in particular, of whole-exome sequencing – will drive down this “undetermined” piece of the genetics pie.
For clinicians and patients, prenatal genetic evaluation can inform clinical management, guiding decisions on the mode, timing, and location of delivery. Genetic assessments help guide the neonatal health care team in taking optimal care of the infant, and the surgeon in preparing for neonatal surgeries and postsurgical complications.
In a recent analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database, prenatal diagnosis was associated with a lower overall prevalence of major preoperative risk factors for cardiac surgery.2 Surgical outcomes themselves also have been shown to be better after the prenatal diagnosis of complex CHDs, mainly because of improvements in perioperative care.3
When genetic etiology is elucidated, the cardiologist also is better able to counsel patients about anticipated challenges – such as the propensity, with certain genetic variants of CHD, to develop neurodevelopmental delays or other cardiac complications – and to target patient follow-up. Patients also can make informed decisions about termination or continuation of a current pregnancy and about family planning in the future.
Fortunately, advances in genetics technology have paralleled technological advancements in ultrasound. As I discussed in part one of this two-part Master Class series, it is now possible to detect many major CHDs well before 16 weeks’ gestation. Checking the structure of the fetal heart at the first-trimester screening and sonography (11-14 weeks of gestation) offers the opportunity for early genetic assessment, counseling, and planning when anomalies are detected.
A personalized approach
There has been growing interest in recent years in CMA for the prenatal genetic workup of CHDs. Microarray targets chromosomal regions at a much higher resolution than traditional karyotype. Traditional karyotype assesses both changes in chromosome number as well as more subtle structural changes such as chromosomal deletions and duplications. CMA finds what traditional karyotype identifies, but in addition, it identifies much smaller, clinically relevant chromosomal deletions and duplications that are not detected by karyotype performed with or without fluorescence in-situ hybridization (FISH). FISH uses DNA probes that carry fluorescent tags to detect chromosomal DNA.

At our center, we studied the prenatal genetic test results of 145 fetuses diagnosed with CHDs. Each case involved FISH for aneuploidy/karyotype, followed by CMA in cases of a negative karyotype result. CMA increased the diagnostic yield in cases of CHD by 19.8% overall – 17.4% in cases of isolated CHD and 24.5% in cases of CHD plus extracardiac anomalies.4
Indeed, although a microarray costs more and takes an additional 2 weeks to run, CMA should be strongly considered as first-line testing for the prenatal genetic evaluation of fetuses with major structural cardiac abnormalities detected by ultrasound. However, there still are cases in which a karyotype might be sufficient. For instance, if I see that a fetus has an atrial-ventricular septal defect on a prenatal ultrasound, and there are markers for trisomy 21, 13, or 18, or Turner’s syndrome (45 XO), I usually recommend a karyotype or FISH rather than an initial CMA. If the karyotype is abnormal – which is likely in such a scenario – there isn’t a need for more extensive testing.
Similarly, when there is high suspicion for DiGeorge syndrome (the 22q11.2 deletion, which often includes cleft palate and aortic arch abnormalities), usually it is most appropriate to perform a FISH test.
CMA is the preferred first modality, however, when prenatal imaging suggests severe CHD – for instance, when there are signs of hypoplastic left heart syndrome or tetralogy of Fallot (a conotruncal defect) – or complex CHD with extracardiac anomalies. In these cases, there is a high likelihood of detecting a small deletion or duplication that would be missed with karyotype.
In the past decade, karyotype and CMA have become the major methods used in our practice. However, targeted next‐generation sequencing and whole‐exome sequencing may become more widely used because these technologies enable rapid analysis of a large number of gene sequences and facilitate discovery of novel causative genes in many genetic diseases that cause CHDs.
Currently, targeted next-generation sequencing has mainly been used in the postnatal setting, and there are limited data available on its prenatal use. Compared with whole-exome sequencing, which sequences all of the protein-coding regions of the genome, targeted next-generation sequencing panels select regions of genes that are known to be associated with diseases of interest.
For CHDs, some perinatal centers have begun using a customized gene panel that targets 77 CHD-associated genes. This particular panel has been shown to be useful in addition to current methods and is an effective tool for prenatal genetic diagnosis.5
Whole-exome sequencing is currently expensive and time consuming. While sometimes it is used in the postnatal context, it is not yet part of routine practice as a prenatal diagnostic tool. As technology advances this will change – early in the next decade, I believe. For now, whole-exome sequencing may be an option for some patients who want to know more when severe CHD is evident on ultrasound and there are negative results from CMA or targeted sequencing. We have diagnosed some rare genetic syndromes using whole-exome sequencing; these diagnoses helped us to better manage the pregnancies.

These choices are part of the case-specific, stepwise approach to genetic evaluation that we take in our fetal heart program. Our goal is to pursue information that will be accurate and valuable for the patient and clinicians, in the most cost-effective and timely manner.
Limitations of noninvasive screening
In our fetal heart program we see increasing numbers of referred patients who have chosen noninvasive cell-free fetal DNA screening (cfDNA) after a cardiac anomaly is detected on ultrasound examination, and who believe that their “low risk” results demonstrate very little or no risk of CHD. Many of these patients express a belief that noninvasive testing is highly sensitive and accurate for fetal anomalies, including CHDs, and are not easily convinced of the value of other genetic tests.
We recently conducted a retrospective chart analysis (unpublished) in which we found that 41% of cases of CHD with abnormal genetics results were not detectable by cfDNA screening.
In the case of atrial-ventricular septal defects and conotruncal abnormalities that often are more associated with common aneuploidies (trisomy 21, 18, 13, and 45 XO), a “high-risk” result from cfDNA screening may offer the family and cardiology/neonatal team some guidance, but a “low-risk” result does not eliminate the risk of a microarray abnormality and thus may provide false reassurance.
Other research has shown that noninvasive screening will miss up to 7.3% of karyotype abnormalities in pregnancies at high risk for common aneuploidies.6
While invasive testing poses a very small risk of miscarriage, it is hard without such testing to elucidate the potential genetic etiologies of CHDs and truly understand the problems. We must take time to thoughtfully counsel patients who decline invasive testing about the limitations of cfDNA screening for CHDs and other anomalies.
Dr. Turan is an associate professor of obstetrics, gynecology, and reproductive sciences, and director of the fetal heart program at the University of Maryland School of Medicine and director of the Fetal Heart Program at the University of Maryland Medical Center. Dr. Turan reported that she has no disclosures relevant to this Master Class. Email her at obnews@mdedge.com.
References
1. J Am Coll Cardiol. 1988 Oct;12(4):1079-86.
2. Pediatr Cardiol. 2019 Mar;40(3):489-96.
3. Ann Pediatr Cardiol. 2017 May-Aug;10(2):126-30.
4. Eur J Obstet Gynecol Reprod Biol 2018;221:172-76.
5. Ultrasound Obstet Gynecol. 2018 Aug;52(2):205-11.
6. PLoS One. 2016 Jan 15;11(1):e0146794.
Diagnosis, treatment, and prevention of ovarian remnant syndrome
Ovarian remnant syndrome (ORS) is an uncommon problem, but one that seems to be increasing in incidence and one that is important to diagnose and treat properly, as well as prevent. Retrospective cohort studies published in the past 15 years or so have improved our understanding of its presentation and the outcomes of surgical management – and recent literature has demonstrated that a minimally invasive surgical approach with either conventional laparoscopy or robot-assisted laparoscopy yields improved outcomes in a skilled surgeon’s hands.
Diagnosis is based on clinical history and should be further supported with imaging and laboratory evaluation. A definitive diagnosis of the disease comes through surgical intervention and pathological findings.
Surgery therefore is technically challenging, usually requiring complete ureterolysis, careful adhesiolysis (often enterolysis), and excision of much of the pelvic sidewall peritoneum with extirpation of the remnant and endometriosis. High ligation of the ovarian vasculature also often is required.
This complexity and the consequent risk of intraoperative injury to the bowel, bladder, and ureters requires careful preoperative preparation. When an ovarian remnant is suspected, it may be important to have other surgeons – such as gynecologic oncologists, urologists, colorectal surgeons, or general surgeons – either present or on standby during the surgical intervention. In expert hands, surgical intervention has been shown to resolve or improve pain in the majority of patients, with no recurrence of the syndrome.
Diagnosis of ORS

Courtesy Dr. Charles E. Miller and Dr. Kirsten J. Sasaki
Patients with ORS have had previous oophorectomies with incomplete removal of ovarian tissue. Pelvic pain, either cyclical or most commonly chronic, is a common symptom. Other symptoms can include dyspareunia, dysuria and other urinary symptoms, and bowel symptoms. Ovarian remnants may have an expanding cystic structure – oftentimes secondary to endometriosis – that causes mass-like effects leading to pain and inflammation and to symptoms such as low back pain, constipation, and even urinary retention.
It also is important to discuss the patient’s history of menopausal symptoms, because the absence of these symptoms after oophorectomy may be a sign that ovarian tissue has been left behind. Menopausal symptoms do not exclude the diagnosis, however. Endometriosis, extensive surgical history, and other diseases that lead to significant adhesion formation – and a higher risk of incomplete removal of ovarian tissue, theoretically – also should be explored during history-taking.
Laboratory assessment of serum follicle-stimulating hormone (FSH) and estradiol can be helpful. Values that are indicative of ovarian function – FSH less than 30 mIU/mL and estradiol greater than 35 pg/mL – point towards ORS, but the absence of such premenopausal values should not rule out the possibility of an ovarian remnant.
The literature shows that FSH and estradiol levels are variable in women with ORS. A retrospective review published in 2005 by Paul M. Magtibay, MD, and colleagues at the Mayo Clinic, Scottsdale, Ariz., and Rochester, Minn., involved 186 patients treated surgically from 1985 to 2003 with a mean follow-up, via questionnaire, of 1.2 years. This is the largest series published thus far of patients with pathologically confirmed ORS. It reported premenopausal levels of FSH and estradiol in 69% and 63% of patients, respectively, who had preoperative hormonal evaluations.1
In another retrospective cohort study published in 2011 of 30 women – also with pathologically confirmed ovarian remnants – Deborah Arden, MD, and Ted Lee, MD, of the University of Pittsburgh Medical Center reported premenopausal levels of FSH and estradiol in 59% and 71%, respectively, of women whose concentrations were measured.2
ORS often involves a pelvic mass, and preoperative imaging is important in this regard. In Dr. Magtibay’s series, a pelvic mass was identified in 93%, 92%, and 78% of those who were imaged presurgically with ultrasonography, computed tomography, and magnetic resonance imaging, respectively.1 As with laboratory testing, however, a negative result does not rule out the presence of an ovarian remnant.
Some authors have advocated the use of clomiphene citrate stimulation before preoperative imaging – or before repeat imaging – to identify remnant ovarian tissue. Typically, clomiphene citrate 100 mg is administered for 10 days prior to imaging to potentially induce ovulation in patients with suspected ORS. Alternatively, at the Advanced Gynecologic Surgery Institute in Naperville and Park Ridge, Ill., ovarian stimulation is performed using FSH 300 IUs for 5 days. A finding of cystic structures consistent with ovarian follicles will help narrow the diagnosis.
Use of gonadotropins is superior in that an intact pituitary-ovarian axis is not required. Moreover, monitoring can be in real time; increasing estradiol levels and increasing mass size on ultrasound can be monitored as gonadotropin treatment is rendered. Again, however, negative findings should not necessarily rule out ORS. Unfortunately, there have been no clinical studies looking at the use of controlled ovarian stimulation as a definitive test.
The differential diagnosis includes supernumerary ovary (a rare gynecologic congenital anomaly) and residual ovary syndrome (a condition in which an ovary is intentionally or unintentionally left in place during a hysterectomy, as well as often an intended bilateral oophorectomy, and later causes pain). The latter occurs when surgical anatomy is poor and the surgery is consequently very difficult.
Surgical principles and approach
Previously, laparotomy was believed to be the best approach for minimizing intraoperative complications and achieving the extensive dissections necessary for effective treatment of ORS. In recent years, conventional laparoscopy and robot-assisted laparoscopy have been shown in retrospective reviews such as that by Arden et al.2 and a 2007 review by Rosanne M. Kho, MD,3 to be just as safe and effective provided that the same surgical principles – extensive retroperitoneal dissections and ureterolysis – are applied.
Good outcomes can be achieved with less blood loss, shorter operating room time, and less time in the hospital. The better visualization with greater magnification afforded by a minimally invasive approach offers a distinct advantage for such complex dissections.
A remnant of ovarian tissue can be located anywhere along the pelvic sidewall, which makes the surgical protocol largely individualized and based on the suspected location of the remnant.
Still, there are certain standard components of any surgical approach to ORS: The retroperitoneum should be entered at the level of the pelvic brim and the ureter must be clearly identified; usually, a partial or complete ureterolysis is necessary. Then, a window into the broad ligament inferior to the infundibulopelvic (IP) ligament is created, or the peritoneum of the broad ligament is removed, in order to completely isolate both the IP ligament and the ureter.
Once the ovarian remnant is isolated, a wide excision at least 2 cm from all ovarian tissue is performed. This wide surgical clearance is critical to prevent recurrence.
These standard components form the crux of the most basic and straightforward surgery for ORS. In some cases, more extensive dissections such as a cystectomy or even a bowel resection might be necessary. Ligation of the IP ligament as high because its connection to the aortic bifurcation also may be necessary – depending, again, on the location of the ovarian remnant.
The risk of intraoperative injury to the bowel, bladder, and ureters is not insignificant, but with careful planning and the involvement of other surgeons in the most complex cases, these risks can be minimized.
For patients who have a significant surgical history and do not want more surgery, pharmacologic therapy, such as leuprolide (Lupron) or danazol, is an option for ORS. It’s important to note, however, that no studies have been done to demonstrate that medical therapy is a curative option. In addition, one must consider the small risk that remnants may harbor or develop malignancy.
Malignancy has been reported in ovarian remnant tissue. While the risk is believed to be very small, 2 of the 20 patients in Dr. Kho’s cohort had malignancy in remnant tissue,3 and it is generally recommended that surgeons send frozen sections of suspected ovarian tissue to pathology. Frozen-section diagnosis of ovarian tissue is about 95% accurate.
Preventing ovarian remnants
Oophorectomy is a common procedure performed by gynecologic surgeons. While routine, it is imperative that it be performed correctly to prevent ovarian remnants from occurring. When performing a laparoscopic or robot-assisted laparoscopic oophorectomy, it is important to optimize visualization of the ovary and the IP ligament, and to account for the significant magnification provided by laparoscopic cameras.
Surgeons must make sure all adhesions are completely cleared in order to optimally transect the IP ligament. Furthermore, wide excision around ovarian tissue is critical. Accessory ovarian tissue has been found up to 1.4 cm away from the ovary itself, which is why we recommend that surgeons excise at least 2-3 cm away from the IP in order to safely ensure complete removal of ovarian tissue.
Dr. Kooperman completed the American Association of Gynecologic Laparoscopists (AAGL) Fellowship Program in Minimally Invasive Gynecologic Surgery at Advocate Lutheran General Hospital, Park Ridge, Ill., and will be starting practice at the Highland Park (Ill.) North Shore Hospital System in August 2019. He reported no relevant disclosures.
References
1. Am J Obstet Gynecol. 2005;193(6):2062-6.
2. J Minim Invasive Gynecol. 2011;18(2):194-9.
3. Fertil Steril. 2007;87(5):1005-9.
Ovarian remnant syndrome (ORS) is an uncommon problem, but one that seems to be increasing in incidence and one that is important to diagnose and treat properly, as well as prevent. Retrospective cohort studies published in the past 15 years or so have improved our understanding of its presentation and the outcomes of surgical management – and recent literature has demonstrated that a minimally invasive surgical approach with either conventional laparoscopy or robot-assisted laparoscopy yields improved outcomes in a skilled surgeon’s hands.
Diagnosis is based on clinical history and should be further supported with imaging and laboratory evaluation. A definitive diagnosis of the disease comes through surgical intervention and pathological findings.
Surgery therefore is technically challenging, usually requiring complete ureterolysis, careful adhesiolysis (often enterolysis), and excision of much of the pelvic sidewall peritoneum with extirpation of the remnant and endometriosis. High ligation of the ovarian vasculature also often is required.
This complexity and the consequent risk of intraoperative injury to the bowel, bladder, and ureters requires careful preoperative preparation. When an ovarian remnant is suspected, it may be important to have other surgeons – such as gynecologic oncologists, urologists, colorectal surgeons, or general surgeons – either present or on standby during the surgical intervention. In expert hands, surgical intervention has been shown to resolve or improve pain in the majority of patients, with no recurrence of the syndrome.
Diagnosis of ORS

Courtesy Dr. Charles E. Miller and Dr. Kirsten J. Sasaki
Patients with ORS have had previous oophorectomies with incomplete removal of ovarian tissue. Pelvic pain, either cyclical or most commonly chronic, is a common symptom. Other symptoms can include dyspareunia, dysuria and other urinary symptoms, and bowel symptoms. Ovarian remnants may have an expanding cystic structure – oftentimes secondary to endometriosis – that causes mass-like effects leading to pain and inflammation and to symptoms such as low back pain, constipation, and even urinary retention.
It also is important to discuss the patient’s history of menopausal symptoms, because the absence of these symptoms after oophorectomy may be a sign that ovarian tissue has been left behind. Menopausal symptoms do not exclude the diagnosis, however. Endometriosis, extensive surgical history, and other diseases that lead to significant adhesion formation – and a higher risk of incomplete removal of ovarian tissue, theoretically – also should be explored during history-taking.
Laboratory assessment of serum follicle-stimulating hormone (FSH) and estradiol can be helpful. Values that are indicative of ovarian function – FSH less than 30 mIU/mL and estradiol greater than 35 pg/mL – point towards ORS, but the absence of such premenopausal values should not rule out the possibility of an ovarian remnant.
The literature shows that FSH and estradiol levels are variable in women with ORS. A retrospective review published in 2005 by Paul M. Magtibay, MD, and colleagues at the Mayo Clinic, Scottsdale, Ariz., and Rochester, Minn., involved 186 patients treated surgically from 1985 to 2003 with a mean follow-up, via questionnaire, of 1.2 years. This is the largest series published thus far of patients with pathologically confirmed ORS. It reported premenopausal levels of FSH and estradiol in 69% and 63% of patients, respectively, who had preoperative hormonal evaluations.1
In another retrospective cohort study published in 2011 of 30 women – also with pathologically confirmed ovarian remnants – Deborah Arden, MD, and Ted Lee, MD, of the University of Pittsburgh Medical Center reported premenopausal levels of FSH and estradiol in 59% and 71%, respectively, of women whose concentrations were measured.2
ORS often involves a pelvic mass, and preoperative imaging is important in this regard. In Dr. Magtibay’s series, a pelvic mass was identified in 93%, 92%, and 78% of those who were imaged presurgically with ultrasonography, computed tomography, and magnetic resonance imaging, respectively.1 As with laboratory testing, however, a negative result does not rule out the presence of an ovarian remnant.
Some authors have advocated the use of clomiphene citrate stimulation before preoperative imaging – or before repeat imaging – to identify remnant ovarian tissue. Typically, clomiphene citrate 100 mg is administered for 10 days prior to imaging to potentially induce ovulation in patients with suspected ORS. Alternatively, at the Advanced Gynecologic Surgery Institute in Naperville and Park Ridge, Ill., ovarian stimulation is performed using FSH 300 IUs for 5 days. A finding of cystic structures consistent with ovarian follicles will help narrow the diagnosis.
Use of gonadotropins is superior in that an intact pituitary-ovarian axis is not required. Moreover, monitoring can be in real time; increasing estradiol levels and increasing mass size on ultrasound can be monitored as gonadotropin treatment is rendered. Again, however, negative findings should not necessarily rule out ORS. Unfortunately, there have been no clinical studies looking at the use of controlled ovarian stimulation as a definitive test.
The differential diagnosis includes supernumerary ovary (a rare gynecologic congenital anomaly) and residual ovary syndrome (a condition in which an ovary is intentionally or unintentionally left in place during a hysterectomy, as well as often an intended bilateral oophorectomy, and later causes pain). The latter occurs when surgical anatomy is poor and the surgery is consequently very difficult.
Surgical principles and approach
Previously, laparotomy was believed to be the best approach for minimizing intraoperative complications and achieving the extensive dissections necessary for effective treatment of ORS. In recent years, conventional laparoscopy and robot-assisted laparoscopy have been shown in retrospective reviews such as that by Arden et al.2 and a 2007 review by Rosanne M. Kho, MD,3 to be just as safe and effective provided that the same surgical principles – extensive retroperitoneal dissections and ureterolysis – are applied.
Good outcomes can be achieved with less blood loss, shorter operating room time, and less time in the hospital. The better visualization with greater magnification afforded by a minimally invasive approach offers a distinct advantage for such complex dissections.
A remnant of ovarian tissue can be located anywhere along the pelvic sidewall, which makes the surgical protocol largely individualized and based on the suspected location of the remnant.
Still, there are certain standard components of any surgical approach to ORS: The retroperitoneum should be entered at the level of the pelvic brim and the ureter must be clearly identified; usually, a partial or complete ureterolysis is necessary. Then, a window into the broad ligament inferior to the infundibulopelvic (IP) ligament is created, or the peritoneum of the broad ligament is removed, in order to completely isolate both the IP ligament and the ureter.
Once the ovarian remnant is isolated, a wide excision at least 2 cm from all ovarian tissue is performed. This wide surgical clearance is critical to prevent recurrence.
These standard components form the crux of the most basic and straightforward surgery for ORS. In some cases, more extensive dissections such as a cystectomy or even a bowel resection might be necessary. Ligation of the IP ligament as high because its connection to the aortic bifurcation also may be necessary – depending, again, on the location of the ovarian remnant.
The risk of intraoperative injury to the bowel, bladder, and ureters is not insignificant, but with careful planning and the involvement of other surgeons in the most complex cases, these risks can be minimized.
For patients who have a significant surgical history and do not want more surgery, pharmacologic therapy, such as leuprolide (Lupron) or danazol, is an option for ORS. It’s important to note, however, that no studies have been done to demonstrate that medical therapy is a curative option. In addition, one must consider the small risk that remnants may harbor or develop malignancy.
Malignancy has been reported in ovarian remnant tissue. While the risk is believed to be very small, 2 of the 20 patients in Dr. Kho’s cohort had malignancy in remnant tissue,3 and it is generally recommended that surgeons send frozen sections of suspected ovarian tissue to pathology. Frozen-section diagnosis of ovarian tissue is about 95% accurate.
Preventing ovarian remnants
Oophorectomy is a common procedure performed by gynecologic surgeons. While routine, it is imperative that it be performed correctly to prevent ovarian remnants from occurring. When performing a laparoscopic or robot-assisted laparoscopic oophorectomy, it is important to optimize visualization of the ovary and the IP ligament, and to account for the significant magnification provided by laparoscopic cameras.
Surgeons must make sure all adhesions are completely cleared in order to optimally transect the IP ligament. Furthermore, wide excision around ovarian tissue is critical. Accessory ovarian tissue has been found up to 1.4 cm away from the ovary itself, which is why we recommend that surgeons excise at least 2-3 cm away from the IP in order to safely ensure complete removal of ovarian tissue.
Dr. Kooperman completed the American Association of Gynecologic Laparoscopists (AAGL) Fellowship Program in Minimally Invasive Gynecologic Surgery at Advocate Lutheran General Hospital, Park Ridge, Ill., and will be starting practice at the Highland Park (Ill.) North Shore Hospital System in August 2019. He reported no relevant disclosures.
References
1. Am J Obstet Gynecol. 2005;193(6):2062-6.
2. J Minim Invasive Gynecol. 2011;18(2):194-9.
3. Fertil Steril. 2007;87(5):1005-9.
Ovarian remnant syndrome (ORS) is an uncommon problem, but one that seems to be increasing in incidence and one that is important to diagnose and treat properly, as well as prevent. Retrospective cohort studies published in the past 15 years or so have improved our understanding of its presentation and the outcomes of surgical management – and recent literature has demonstrated that a minimally invasive surgical approach with either conventional laparoscopy or robot-assisted laparoscopy yields improved outcomes in a skilled surgeon’s hands.
Diagnosis is based on clinical history and should be further supported with imaging and laboratory evaluation. A definitive diagnosis of the disease comes through surgical intervention and pathological findings.
Surgery therefore is technically challenging, usually requiring complete ureterolysis, careful adhesiolysis (often enterolysis), and excision of much of the pelvic sidewall peritoneum with extirpation of the remnant and endometriosis. High ligation of the ovarian vasculature also often is required.
This complexity and the consequent risk of intraoperative injury to the bowel, bladder, and ureters requires careful preoperative preparation. When an ovarian remnant is suspected, it may be important to have other surgeons – such as gynecologic oncologists, urologists, colorectal surgeons, or general surgeons – either present or on standby during the surgical intervention. In expert hands, surgical intervention has been shown to resolve or improve pain in the majority of patients, with no recurrence of the syndrome.
Diagnosis of ORS

Courtesy Dr. Charles E. Miller and Dr. Kirsten J. Sasaki
Patients with ORS have had previous oophorectomies with incomplete removal of ovarian tissue. Pelvic pain, either cyclical or most commonly chronic, is a common symptom. Other symptoms can include dyspareunia, dysuria and other urinary symptoms, and bowel symptoms. Ovarian remnants may have an expanding cystic structure – oftentimes secondary to endometriosis – that causes mass-like effects leading to pain and inflammation and to symptoms such as low back pain, constipation, and even urinary retention.
It also is important to discuss the patient’s history of menopausal symptoms, because the absence of these symptoms after oophorectomy may be a sign that ovarian tissue has been left behind. Menopausal symptoms do not exclude the diagnosis, however. Endometriosis, extensive surgical history, and other diseases that lead to significant adhesion formation – and a higher risk of incomplete removal of ovarian tissue, theoretically – also should be explored during history-taking.
Laboratory assessment of serum follicle-stimulating hormone (FSH) and estradiol can be helpful. Values that are indicative of ovarian function – FSH less than 30 mIU/mL and estradiol greater than 35 pg/mL – point towards ORS, but the absence of such premenopausal values should not rule out the possibility of an ovarian remnant.
The literature shows that FSH and estradiol levels are variable in women with ORS. A retrospective review published in 2005 by Paul M. Magtibay, MD, and colleagues at the Mayo Clinic, Scottsdale, Ariz., and Rochester, Minn., involved 186 patients treated surgically from 1985 to 2003 with a mean follow-up, via questionnaire, of 1.2 years. This is the largest series published thus far of patients with pathologically confirmed ORS. It reported premenopausal levels of FSH and estradiol in 69% and 63% of patients, respectively, who had preoperative hormonal evaluations.1
In another retrospective cohort study published in 2011 of 30 women – also with pathologically confirmed ovarian remnants – Deborah Arden, MD, and Ted Lee, MD, of the University of Pittsburgh Medical Center reported premenopausal levels of FSH and estradiol in 59% and 71%, respectively, of women whose concentrations were measured.2
ORS often involves a pelvic mass, and preoperative imaging is important in this regard. In Dr. Magtibay’s series, a pelvic mass was identified in 93%, 92%, and 78% of those who were imaged presurgically with ultrasonography, computed tomography, and magnetic resonance imaging, respectively.1 As with laboratory testing, however, a negative result does not rule out the presence of an ovarian remnant.
Some authors have advocated the use of clomiphene citrate stimulation before preoperative imaging – or before repeat imaging – to identify remnant ovarian tissue. Typically, clomiphene citrate 100 mg is administered for 10 days prior to imaging to potentially induce ovulation in patients with suspected ORS. Alternatively, at the Advanced Gynecologic Surgery Institute in Naperville and Park Ridge, Ill., ovarian stimulation is performed using FSH 300 IUs for 5 days. A finding of cystic structures consistent with ovarian follicles will help narrow the diagnosis.
Use of gonadotropins is superior in that an intact pituitary-ovarian axis is not required. Moreover, monitoring can be in real time; increasing estradiol levels and increasing mass size on ultrasound can be monitored as gonadotropin treatment is rendered. Again, however, negative findings should not necessarily rule out ORS. Unfortunately, there have been no clinical studies looking at the use of controlled ovarian stimulation as a definitive test.
The differential diagnosis includes supernumerary ovary (a rare gynecologic congenital anomaly) and residual ovary syndrome (a condition in which an ovary is intentionally or unintentionally left in place during a hysterectomy, as well as often an intended bilateral oophorectomy, and later causes pain). The latter occurs when surgical anatomy is poor and the surgery is consequently very difficult.
Surgical principles and approach
Previously, laparotomy was believed to be the best approach for minimizing intraoperative complications and achieving the extensive dissections necessary for effective treatment of ORS. In recent years, conventional laparoscopy and robot-assisted laparoscopy have been shown in retrospective reviews such as that by Arden et al.2 and a 2007 review by Rosanne M. Kho, MD,3 to be just as safe and effective provided that the same surgical principles – extensive retroperitoneal dissections and ureterolysis – are applied.
Good outcomes can be achieved with less blood loss, shorter operating room time, and less time in the hospital. The better visualization with greater magnification afforded by a minimally invasive approach offers a distinct advantage for such complex dissections.
A remnant of ovarian tissue can be located anywhere along the pelvic sidewall, which makes the surgical protocol largely individualized and based on the suspected location of the remnant.
Still, there are certain standard components of any surgical approach to ORS: The retroperitoneum should be entered at the level of the pelvic brim and the ureter must be clearly identified; usually, a partial or complete ureterolysis is necessary. Then, a window into the broad ligament inferior to the infundibulopelvic (IP) ligament is created, or the peritoneum of the broad ligament is removed, in order to completely isolate both the IP ligament and the ureter.
Once the ovarian remnant is isolated, a wide excision at least 2 cm from all ovarian tissue is performed. This wide surgical clearance is critical to prevent recurrence.
These standard components form the crux of the most basic and straightforward surgery for ORS. In some cases, more extensive dissections such as a cystectomy or even a bowel resection might be necessary. Ligation of the IP ligament as high because its connection to the aortic bifurcation also may be necessary – depending, again, on the location of the ovarian remnant.
The risk of intraoperative injury to the bowel, bladder, and ureters is not insignificant, but with careful planning and the involvement of other surgeons in the most complex cases, these risks can be minimized.
For patients who have a significant surgical history and do not want more surgery, pharmacologic therapy, such as leuprolide (Lupron) or danazol, is an option for ORS. It’s important to note, however, that no studies have been done to demonstrate that medical therapy is a curative option. In addition, one must consider the small risk that remnants may harbor or develop malignancy.
Malignancy has been reported in ovarian remnant tissue. While the risk is believed to be very small, 2 of the 20 patients in Dr. Kho’s cohort had malignancy in remnant tissue,3 and it is generally recommended that surgeons send frozen sections of suspected ovarian tissue to pathology. Frozen-section diagnosis of ovarian tissue is about 95% accurate.
Preventing ovarian remnants
Oophorectomy is a common procedure performed by gynecologic surgeons. While routine, it is imperative that it be performed correctly to prevent ovarian remnants from occurring. When performing a laparoscopic or robot-assisted laparoscopic oophorectomy, it is important to optimize visualization of the ovary and the IP ligament, and to account for the significant magnification provided by laparoscopic cameras.
Surgeons must make sure all adhesions are completely cleared in order to optimally transect the IP ligament. Furthermore, wide excision around ovarian tissue is critical. Accessory ovarian tissue has been found up to 1.4 cm away from the ovary itself, which is why we recommend that surgeons excise at least 2-3 cm away from the IP in order to safely ensure complete removal of ovarian tissue.
Dr. Kooperman completed the American Association of Gynecologic Laparoscopists (AAGL) Fellowship Program in Minimally Invasive Gynecologic Surgery at Advocate Lutheran General Hospital, Park Ridge, Ill., and will be starting practice at the Highland Park (Ill.) North Shore Hospital System in August 2019. He reported no relevant disclosures.
References
1. Am J Obstet Gynecol. 2005;193(6):2062-6.
2. J Minim Invasive Gynecol. 2011;18(2):194-9.
3. Fertil Steril. 2007;87(5):1005-9.
The ovarian remnant syndrome
A 45-year old woman was referred by her physician to my clinic for continued pain after total hysterectomy and bilateral salpingo-oophorectomy. The patient initially had undergone a robot-assisted total laparoscopic hysterectomy, bilateral salpingectomy, and excision of stage 1 endometriosis secondary to pelvic pain. Because of continued pain and new onset of persistent ovarian cysts, she once again underwent robotic-assisted laparoscopic surgery, this time to remove both ovaries. Interestingly, severe periadnexal adhesions were noted in the second surgical report. A hemorrhagic cyst and a corpus luteal cyst were noted. Unfortunately, the patient continued to have left lower abdominal pain; thus, the referral to my clinic.
Given the history of pelvic pain, especially in light of severe periadnexal adhesions at the second surgery, I voiced my concern about possible ovarian remnant syndrome. At the patient’s initial visit, an estradiol (E2), progesterone (P4) and follicle-stimulating hormone (FSH) test were ordered. Interestingly, while the E2 and P4 were quite low, the FSH was 10.9 IU/mL. Certainly, this was not consistent with menopause but could point to ovarian remnant syndrome.
A follow-up examination and ultrasound revealed a 15-mm exquisitely tender left adnexal mass, again consistent with ovarian remnant syndrome. My plan now is to proceed with surgery with the presumptive diagnosis of ovarian remnant syndrome.
Ovarian remnant syndrome (ORS), first described by Shemwell and Weed in 1970, is defined as a pelvic mass with residual ovarian tissue postoophorectomy.1-3 ORS may be associated with endometriosis or ovarian cancer. Remnant ovarian tissue also may stimulate endometriosis and cyclic pelvic pain, similar to symptoms of the remnant itself.4
Pelvic adhesions may be secondary to previous surgery, intraoperative bleeding, previous appendectomy, inflammatory bowel disease, pelvic inflammatory disease, or endometriosis, the latter of which is the most common cause of initial oophorectomy. Moreover, surgical technique may be causal. This includes inability to achieve adequate exposure, inability to restore normal anatomy, and imprecise site of surgical incision.5-7
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Ryan S. Kooperman, DO, who recently completed his 2-year American Association of Gynecologic Laparoscopists (AAGL) Fellowship in Minimally Invasive Gynecologic Surgery at Advocate Lutheran General Hospital in Park Ridge, Ill., where I am currently the program director.
In 2016, Dr. Kooperman was the recipient of the National Outstanding Resident of the Year in Obstetrics and Gynecology (American Osteopathic Foundation/Medical Education Foundation of the American College of Osteopathic Obstetricians and Gynecologists). Dr. Kooperman is a very skilled surgeon and adroit clinician. He will be starting practice at Highland Park (Ill.) North Shore Hospital System in August 2019. It is a pleasure to welcome Dr. Kooperman to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital. He has no disclosures relevant to this Master Class.
References
1. Obstet Gynecol. 1970 Aug;36(2):299-303.
2. Aust N Z J Obstet Gynaecol. 1989 Nov;29(4):433-5.
3. Curr Opin Obstet Gynecol. 2012 Aug;24(4):210-4.
4. Int J Gynaecol Obstet. 1988 Feb;26(1):93-103.
5. Oncol Lett. 2014 Jul;8(1):3-6.
6. J Minim Invasive Gynecol. 2011 Mar-Apr;18(2):194-9.
7. Fertil Steril. 2007 May;87(5):1005-9.
A 45-year old woman was referred by her physician to my clinic for continued pain after total hysterectomy and bilateral salpingo-oophorectomy. The patient initially had undergone a robot-assisted total laparoscopic hysterectomy, bilateral salpingectomy, and excision of stage 1 endometriosis secondary to pelvic pain. Because of continued pain and new onset of persistent ovarian cysts, she once again underwent robotic-assisted laparoscopic surgery, this time to remove both ovaries. Interestingly, severe periadnexal adhesions were noted in the second surgical report. A hemorrhagic cyst and a corpus luteal cyst were noted. Unfortunately, the patient continued to have left lower abdominal pain; thus, the referral to my clinic.
Given the history of pelvic pain, especially in light of severe periadnexal adhesions at the second surgery, I voiced my concern about possible ovarian remnant syndrome. At the patient’s initial visit, an estradiol (E2), progesterone (P4) and follicle-stimulating hormone (FSH) test were ordered. Interestingly, while the E2 and P4 were quite low, the FSH was 10.9 IU/mL. Certainly, this was not consistent with menopause but could point to ovarian remnant syndrome.
A follow-up examination and ultrasound revealed a 15-mm exquisitely tender left adnexal mass, again consistent with ovarian remnant syndrome. My plan now is to proceed with surgery with the presumptive diagnosis of ovarian remnant syndrome.
Ovarian remnant syndrome (ORS), first described by Shemwell and Weed in 1970, is defined as a pelvic mass with residual ovarian tissue postoophorectomy.1-3 ORS may be associated with endometriosis or ovarian cancer. Remnant ovarian tissue also may stimulate endometriosis and cyclic pelvic pain, similar to symptoms of the remnant itself.4
Pelvic adhesions may be secondary to previous surgery, intraoperative bleeding, previous appendectomy, inflammatory bowel disease, pelvic inflammatory disease, or endometriosis, the latter of which is the most common cause of initial oophorectomy. Moreover, surgical technique may be causal. This includes inability to achieve adequate exposure, inability to restore normal anatomy, and imprecise site of surgical incision.5-7
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Ryan S. Kooperman, DO, who recently completed his 2-year American Association of Gynecologic Laparoscopists (AAGL) Fellowship in Minimally Invasive Gynecologic Surgery at Advocate Lutheran General Hospital in Park Ridge, Ill., where I am currently the program director.
In 2016, Dr. Kooperman was the recipient of the National Outstanding Resident of the Year in Obstetrics and Gynecology (American Osteopathic Foundation/Medical Education Foundation of the American College of Osteopathic Obstetricians and Gynecologists). Dr. Kooperman is a very skilled surgeon and adroit clinician. He will be starting practice at Highland Park (Ill.) North Shore Hospital System in August 2019. It is a pleasure to welcome Dr. Kooperman to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital. He has no disclosures relevant to this Master Class.
References
1. Obstet Gynecol. 1970 Aug;36(2):299-303.
2. Aust N Z J Obstet Gynaecol. 1989 Nov;29(4):433-5.
3. Curr Opin Obstet Gynecol. 2012 Aug;24(4):210-4.
4. Int J Gynaecol Obstet. 1988 Feb;26(1):93-103.
5. Oncol Lett. 2014 Jul;8(1):3-6.
6. J Minim Invasive Gynecol. 2011 Mar-Apr;18(2):194-9.
7. Fertil Steril. 2007 May;87(5):1005-9.
A 45-year old woman was referred by her physician to my clinic for continued pain after total hysterectomy and bilateral salpingo-oophorectomy. The patient initially had undergone a robot-assisted total laparoscopic hysterectomy, bilateral salpingectomy, and excision of stage 1 endometriosis secondary to pelvic pain. Because of continued pain and new onset of persistent ovarian cysts, she once again underwent robotic-assisted laparoscopic surgery, this time to remove both ovaries. Interestingly, severe periadnexal adhesions were noted in the second surgical report. A hemorrhagic cyst and a corpus luteal cyst were noted. Unfortunately, the patient continued to have left lower abdominal pain; thus, the referral to my clinic.
Given the history of pelvic pain, especially in light of severe periadnexal adhesions at the second surgery, I voiced my concern about possible ovarian remnant syndrome. At the patient’s initial visit, an estradiol (E2), progesterone (P4) and follicle-stimulating hormone (FSH) test were ordered. Interestingly, while the E2 and P4 were quite low, the FSH was 10.9 IU/mL. Certainly, this was not consistent with menopause but could point to ovarian remnant syndrome.
A follow-up examination and ultrasound revealed a 15-mm exquisitely tender left adnexal mass, again consistent with ovarian remnant syndrome. My plan now is to proceed with surgery with the presumptive diagnosis of ovarian remnant syndrome.
Ovarian remnant syndrome (ORS), first described by Shemwell and Weed in 1970, is defined as a pelvic mass with residual ovarian tissue postoophorectomy.1-3 ORS may be associated with endometriosis or ovarian cancer. Remnant ovarian tissue also may stimulate endometriosis and cyclic pelvic pain, similar to symptoms of the remnant itself.4
Pelvic adhesions may be secondary to previous surgery, intraoperative bleeding, previous appendectomy, inflammatory bowel disease, pelvic inflammatory disease, or endometriosis, the latter of which is the most common cause of initial oophorectomy. Moreover, surgical technique may be causal. This includes inability to achieve adequate exposure, inability to restore normal anatomy, and imprecise site of surgical incision.5-7
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Ryan S. Kooperman, DO, who recently completed his 2-year American Association of Gynecologic Laparoscopists (AAGL) Fellowship in Minimally Invasive Gynecologic Surgery at Advocate Lutheran General Hospital in Park Ridge, Ill., where I am currently the program director.
In 2016, Dr. Kooperman was the recipient of the National Outstanding Resident of the Year in Obstetrics and Gynecology (American Osteopathic Foundation/Medical Education Foundation of the American College of Osteopathic Obstetricians and Gynecologists). Dr. Kooperman is a very skilled surgeon and adroit clinician. He will be starting practice at Highland Park (Ill.) North Shore Hospital System in August 2019. It is a pleasure to welcome Dr. Kooperman to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital. He has no disclosures relevant to this Master Class.
References
1. Obstet Gynecol. 1970 Aug;36(2):299-303.
2. Aust N Z J Obstet Gynaecol. 1989 Nov;29(4):433-5.
3. Curr Opin Obstet Gynecol. 2012 Aug;24(4):210-4.
4. Int J Gynaecol Obstet. 1988 Feb;26(1):93-103.
5. Oncol Lett. 2014 Jul;8(1):3-6.
6. J Minim Invasive Gynecol. 2011 Mar-Apr;18(2):194-9.
7. Fertil Steril. 2007 May;87(5):1005-9.
The benefits of first-trimester fetal heart evaluation
The fetal heart typically is examined during the routine 18-20 week obstetric ultrasound screening, and pregnancies with abnormalities on this routine scan are referred for detailed fetal echocardiography. Per multiple practice guidelines, patients deemed to be at high risk of congenital heart defects (CHDs) are referred for fetal echocardiography as well between 18 and 24 weeks’ gestation.
However, with technological advancements in ultrasound, it is possible for obstetricians to detect many major CHDs well before 16 weeks’ gestation. First-trimester fetal heart assessment – and early detection of CHDs – has numerous advantages: It enables early genetic testing, early decision making about continuation or termination of pregnancy, and earlier planning for appropriate management during and after pregnancy. Perioperative outcomes are improved.
At least 75% of CHDs occur in pregnancies with no identifiable maternal, familial, or fetal risk factors. It only seems fitting, therefore, that we check the structure of the fetal heart in all women at the time of their first-trimester screening and sonography at 11-14 weeks. In addition to a determination of fetal viability and gestational age, nuchal translucency measurement, and a check of basic anatomy, .
The value of early detection
Women who have diabetes, congenital defects, in vitro fertilization pregnancies, twin and multiple pregnancies, and certain medication and drug exposures are at high risk for their fetus having a CHD and should undergo fetal echocardiography. Lupus, Sjögren’s, and other medical disorders also are risk factors, as are abnormal biochemical test results.
During the last 10 years, the first-trimester fetal heart evaluation has been performed for all patients who come for a first-trimester screening scan at the University of Maryland’s fetal heart program, part of the Center for Advanced Fetal Care. Approximately 45% of indications for detailed first-trimester fetal heart evaluation have been driven by maternal history, and almost 40% by abnormal basic first-trimester ultrasound findings such as increased nuchal translucency, tricuspid regurgitation, abnormal ductus venosus blood flow, and other structural anomalies.
An estimated 50%-60% of serious cardiac malformations can be detected with a four-chamber heart view during routine first-trimester ultrasound. When the outflow tract relationship and three-vessel views also are examined in the first trimester – as is now recommended in guidelines for second-trimester protocols – an estimated 85%-95% of major CHDs can be detected. One should see the great arteries originating from the left and right sides and crisscrossing each other by a transabdominal scan, or by a transvaginal scan if the transabdominal approach fails to show these features of the fetal heart.
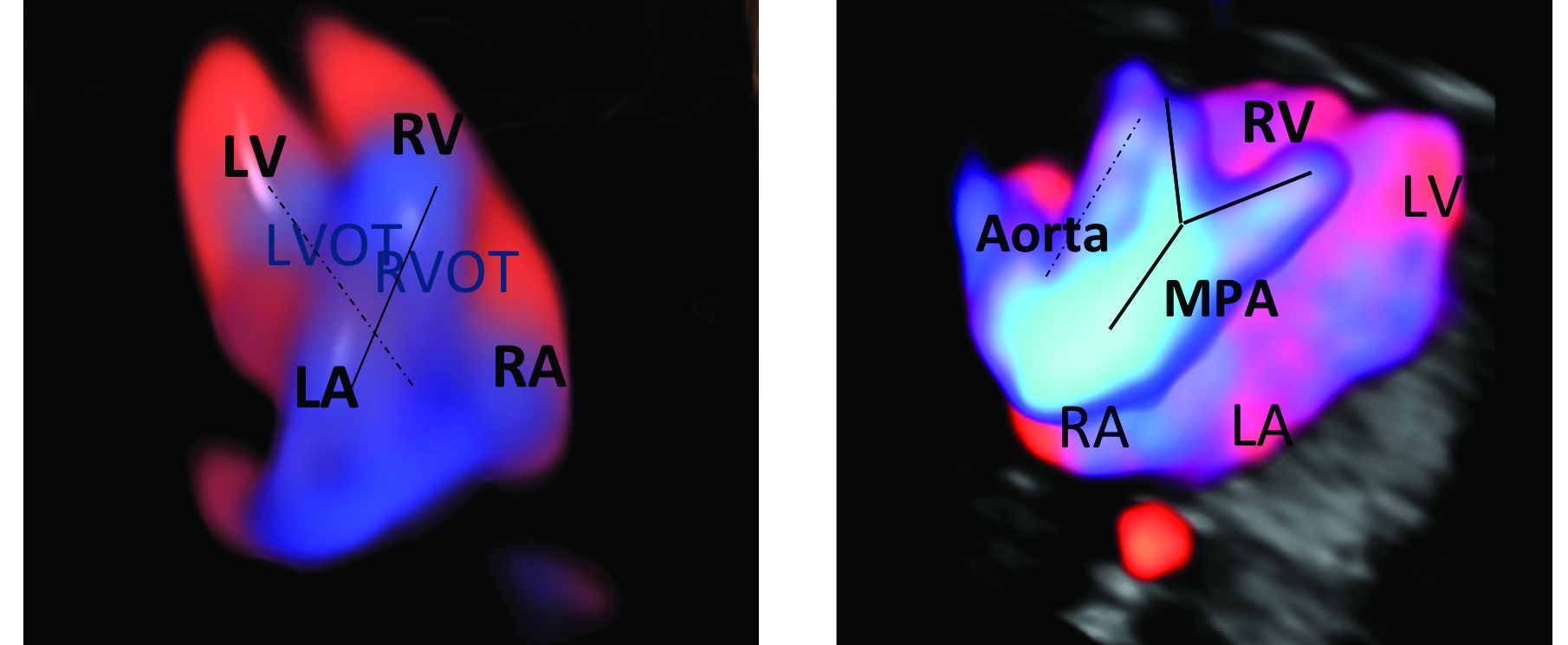
Early sonography not only has been shown to have a high sensitivity but also a specificity of greater than 95% in identifying CHDs. Multiple studies also have demonstrated high negative predictive values in cases with normal findings.1
When defects seen or suspected on routine obstetric ultrasound are then confirmed and diagnosed with detailed fetal echocardiography, women are counseled about outcomes, management options, and mortality – and some patients will choose to terminate their pregnancies.
Psychologically, for the mother, earlier termination is less traumatic. A cross-sectional study of 254 women conducted 2-7 years after pregnancy termination for fetal anomalies found that advanced gestational age at termination was associated with higher levels of grief and posttraumatic stress symptoms, and that long-term psychological morbidity was rare when termination occurred before 14 weeks’ gestation.2 Others studies have shown similar results, with grief and posttraumatic stress time shorter with earlier termination.
First-trimester termination also involves significantly less maternal morbidity and risk, as shown in a retrospective study of 844 patients who underwent a termination of pregnancy after a positive amniocentesis or chorionic villus sampling. Hemorrhages, transfusions, infections, and other complications were significantly higher in second-trimester terminations than in earlier terminations.3
Early fetal heart evaluation can reassure high-risk patients – and low-risk patients as well – when a normal four-chamber heart and great arteries are seen. And when defects are spotted, early evaluation allows appropriate time to test for associated chromosomal abnormalities and genetic syndromes, which in turn improves management. It also gives patients and providers more time to plan and prepare for delivery, surgery, and other specific needs at delivery and after birth.
In our fetal heart program, patients are cared for by a multidisciplinary team of perinatologists with special expertise in the fetal heart, geneticists, cardiologists, cardiac surgeons, and neonatologists. Perioperative outcomes are improved when CHDs are diagnosed prenatally. One meta-analysis showed that prenatal diagnosis reduced the risk of death prior to planned cardiac surgery by about one-fourth relative to patients with a comparable postnatal diagnosis.4
Prenatal diagnosis appears to have generally been improving, although rates remain too low overall. According to the National Institute for Cardiovascular Outcomes Research, which collects data from centers across the United Kingdom and Republic of Ireland, prenatal detection rates of CHDs requiring a procedure in the first year of life moved from about 25% in 2004-2005 to just over 50% between 2010 and 2016.5 More complex lesions, such as hypoplastic left heart syndrome, were more likely to be detected prenatally (80%).
Trends in the United States appear to be similar. A study utilizing the Society of Thoracic Surgeons Congenital Heart Surgery Database found that prenatal detection increased from 26% in 2006 to 42% in 2012.6
A first-trimester evaluation cannot replace the second-trimester echocardiography that currently is performed for high-risk patients, because a small percentage of CHDs – aortic coarctation, valve stenosis, mild tetralogy of Fallot, and hypoplastic left heart, for instance – have the potential to evolve past the first trimester. High-risk patients whose first-trimester evaluations are normal still should undergo another evaluation at 18-20 weeks. The fetal heart completes its embryologic development over the first 8 weeks of gestation, and the majority of CHDs are present at the time of the first-trimester screening (11-14 weeks).
Early evaluation of the fetal heart does not appear to be impacted by obesity. We compared the early evaluation of fetal heart landmarks using two-dimensional sonography with color/power Doppler in obese and nonobese women and found that there were no significant differences in experienced sonographers’ ability to evaluate the four-chamber view, outflow tract relationship, and transverse arches views.
In about 6% of obese women, the evaluation at 11-14 weeks’ gestation required additional imaging with transvaginal sonography. The chances of needing transvaginal ultrasound rose as body mass index rose.1 The median scan time was only 5 minutes longer in the obese group, however, so there is no reason that obesity should be a contraindication to look at the fetal heart.
In fact, it is extremely important that we do early fetal heart evaluations in women who are obese, because the risk of having a fetus with CHD is increasingly being found to be higher in obese women, and because fetal heart assessment with transvaginal ultrasound is an option only in early gestation, when the fetal heart is within the depth of penetration of the vaginal probe. With advancing gestational age, a combined abdominal/transvaginal approach becomes increasingly difficult. Our study also demonstrated a dose-response relationship between maternal obesity and CHD risk.
Preexisting diabetes mellitus, which can occur in conjunction with obesity, has been found to increase the risk for all types of CHDs, especially conotruncal abnormalities. While the pathophysiology is not completely understood, elevated oxidative stress is believed to be the primary trigger.7
First-trimester echocardiography benefits
Patients referred to our fetal heart program for detailed first-trimester fetal heart evaluation – again, a significant number of whom have been found on standard 2-D ultrasound to have increased nuchal translucency thickness or other abnormalities – undergo a four-dimensional fetal echocardiographic technique that utilizes spatiotemporal image correlation and tomographic ultrasound imaging display (STIC-TUI echo) along with color Doppler. The heart is swept from top to bottom in about 10 seconds, and tomographic ultrasound imaging is used offline, after the patient leaves, to develop volume datasets that simultaneously display multiple cross-sectional images.
This method has been implemented into our routine scan at the first trimester as well, and all of our staff have been trained to perform it. Obtaining STIC-TUI by color Doppler allows us to assess all of the important landmarks of the cardiac anatomy in one picture.
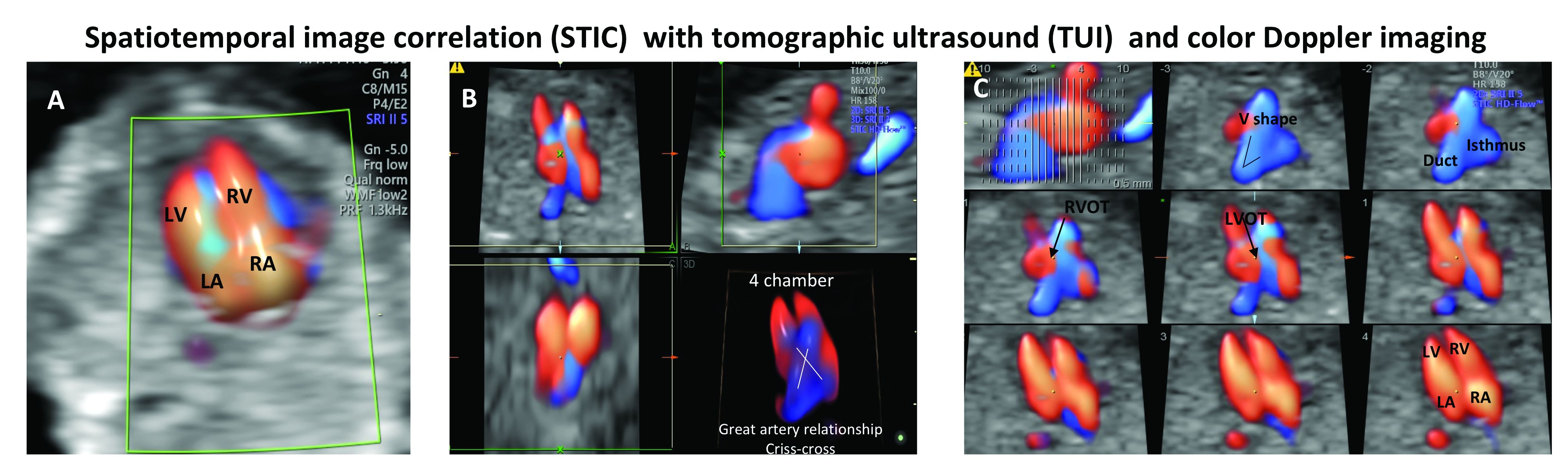
In a prospective study of 164 fetuses from 152 patients, we found that first-trimester STIC-TUI echo had 91% sensitivity and 100% specificity for the detection of CHD. Most anomalies were evident in the four-chamber view plane of the TUI display, and the rest were diagnosed in the outflow tract planes. Two cases of CHD missed by this first-trimester evaluation were diagnosed on second-trimester echo and neither involved a major CHD.8
Dr. Turan is associate professor of obstetrics, gynecology, and reproductive sciences, and director of the fetal heart program at the University of Maryland, Baltimore.
References
1. J Ultrasound Med. 2019 May;38(5):1269-77.
2. Prenat Diagn. 2005 Mar;25(3):253-60.
3. J Perinat Med. 2018 May 24;46(4):373-8.
4. Ultrasound Obstet Gynecol. 2015 Jun;45(6):631-8.
5. National Congenital Heart Disease Audit Report 2013-2016.
6. Pediatrics. 2015. doi: 10.1542/peds.2014-3783.
7. Echocardiography. 2018 Feb;35(2):244-57.
8. Ultrasound Obstet Gynecol. 2014 Nov;44(5):562-7.
The fetal heart typically is examined during the routine 18-20 week obstetric ultrasound screening, and pregnancies with abnormalities on this routine scan are referred for detailed fetal echocardiography. Per multiple practice guidelines, patients deemed to be at high risk of congenital heart defects (CHDs) are referred for fetal echocardiography as well between 18 and 24 weeks’ gestation.
However, with technological advancements in ultrasound, it is possible for obstetricians to detect many major CHDs well before 16 weeks’ gestation. First-trimester fetal heart assessment – and early detection of CHDs – has numerous advantages: It enables early genetic testing, early decision making about continuation or termination of pregnancy, and earlier planning for appropriate management during and after pregnancy. Perioperative outcomes are improved.
At least 75% of CHDs occur in pregnancies with no identifiable maternal, familial, or fetal risk factors. It only seems fitting, therefore, that we check the structure of the fetal heart in all women at the time of their first-trimester screening and sonography at 11-14 weeks. In addition to a determination of fetal viability and gestational age, nuchal translucency measurement, and a check of basic anatomy, .
The value of early detection
Women who have diabetes, congenital defects, in vitro fertilization pregnancies, twin and multiple pregnancies, and certain medication and drug exposures are at high risk for their fetus having a CHD and should undergo fetal echocardiography. Lupus, Sjögren’s, and other medical disorders also are risk factors, as are abnormal biochemical test results.
During the last 10 years, the first-trimester fetal heart evaluation has been performed for all patients who come for a first-trimester screening scan at the University of Maryland’s fetal heart program, part of the Center for Advanced Fetal Care. Approximately 45% of indications for detailed first-trimester fetal heart evaluation have been driven by maternal history, and almost 40% by abnormal basic first-trimester ultrasound findings such as increased nuchal translucency, tricuspid regurgitation, abnormal ductus venosus blood flow, and other structural anomalies.
An estimated 50%-60% of serious cardiac malformations can be detected with a four-chamber heart view during routine first-trimester ultrasound. When the outflow tract relationship and three-vessel views also are examined in the first trimester – as is now recommended in guidelines for second-trimester protocols – an estimated 85%-95% of major CHDs can be detected. One should see the great arteries originating from the left and right sides and crisscrossing each other by a transabdominal scan, or by a transvaginal scan if the transabdominal approach fails to show these features of the fetal heart.

Early sonography not only has been shown to have a high sensitivity but also a specificity of greater than 95% in identifying CHDs. Multiple studies also have demonstrated high negative predictive values in cases with normal findings.1
When defects seen or suspected on routine obstetric ultrasound are then confirmed and diagnosed with detailed fetal echocardiography, women are counseled about outcomes, management options, and mortality – and some patients will choose to terminate their pregnancies.
Psychologically, for the mother, earlier termination is less traumatic. A cross-sectional study of 254 women conducted 2-7 years after pregnancy termination for fetal anomalies found that advanced gestational age at termination was associated with higher levels of grief and posttraumatic stress symptoms, and that long-term psychological morbidity was rare when termination occurred before 14 weeks’ gestation.2 Others studies have shown similar results, with grief and posttraumatic stress time shorter with earlier termination.
First-trimester termination also involves significantly less maternal morbidity and risk, as shown in a retrospective study of 844 patients who underwent a termination of pregnancy after a positive amniocentesis or chorionic villus sampling. Hemorrhages, transfusions, infections, and other complications were significantly higher in second-trimester terminations than in earlier terminations.3
Early fetal heart evaluation can reassure high-risk patients – and low-risk patients as well – when a normal four-chamber heart and great arteries are seen. And when defects are spotted, early evaluation allows appropriate time to test for associated chromosomal abnormalities and genetic syndromes, which in turn improves management. It also gives patients and providers more time to plan and prepare for delivery, surgery, and other specific needs at delivery and after birth.
In our fetal heart program, patients are cared for by a multidisciplinary team of perinatologists with special expertise in the fetal heart, geneticists, cardiologists, cardiac surgeons, and neonatologists. Perioperative outcomes are improved when CHDs are diagnosed prenatally. One meta-analysis showed that prenatal diagnosis reduced the risk of death prior to planned cardiac surgery by about one-fourth relative to patients with a comparable postnatal diagnosis.4
Prenatal diagnosis appears to have generally been improving, although rates remain too low overall. According to the National Institute for Cardiovascular Outcomes Research, which collects data from centers across the United Kingdom and Republic of Ireland, prenatal detection rates of CHDs requiring a procedure in the first year of life moved from about 25% in 2004-2005 to just over 50% between 2010 and 2016.5 More complex lesions, such as hypoplastic left heart syndrome, were more likely to be detected prenatally (80%).
Trends in the United States appear to be similar. A study utilizing the Society of Thoracic Surgeons Congenital Heart Surgery Database found that prenatal detection increased from 26% in 2006 to 42% in 2012.6
A first-trimester evaluation cannot replace the second-trimester echocardiography that currently is performed for high-risk patients, because a small percentage of CHDs – aortic coarctation, valve stenosis, mild tetralogy of Fallot, and hypoplastic left heart, for instance – have the potential to evolve past the first trimester. High-risk patients whose first-trimester evaluations are normal still should undergo another evaluation at 18-20 weeks. The fetal heart completes its embryologic development over the first 8 weeks of gestation, and the majority of CHDs are present at the time of the first-trimester screening (11-14 weeks).
Early evaluation of the fetal heart does not appear to be impacted by obesity. We compared the early evaluation of fetal heart landmarks using two-dimensional sonography with color/power Doppler in obese and nonobese women and found that there were no significant differences in experienced sonographers’ ability to evaluate the four-chamber view, outflow tract relationship, and transverse arches views.
In about 6% of obese women, the evaluation at 11-14 weeks’ gestation required additional imaging with transvaginal sonography. The chances of needing transvaginal ultrasound rose as body mass index rose.1 The median scan time was only 5 minutes longer in the obese group, however, so there is no reason that obesity should be a contraindication to look at the fetal heart.
In fact, it is extremely important that we do early fetal heart evaluations in women who are obese, because the risk of having a fetus with CHD is increasingly being found to be higher in obese women, and because fetal heart assessment with transvaginal ultrasound is an option only in early gestation, when the fetal heart is within the depth of penetration of the vaginal probe. With advancing gestational age, a combined abdominal/transvaginal approach becomes increasingly difficult. Our study also demonstrated a dose-response relationship between maternal obesity and CHD risk.
Preexisting diabetes mellitus, which can occur in conjunction with obesity, has been found to increase the risk for all types of CHDs, especially conotruncal abnormalities. While the pathophysiology is not completely understood, elevated oxidative stress is believed to be the primary trigger.7
First-trimester echocardiography benefits
Patients referred to our fetal heart program for detailed first-trimester fetal heart evaluation – again, a significant number of whom have been found on standard 2-D ultrasound to have increased nuchal translucency thickness or other abnormalities – undergo a four-dimensional fetal echocardiographic technique that utilizes spatiotemporal image correlation and tomographic ultrasound imaging display (STIC-TUI echo) along with color Doppler. The heart is swept from top to bottom in about 10 seconds, and tomographic ultrasound imaging is used offline, after the patient leaves, to develop volume datasets that simultaneously display multiple cross-sectional images.
This method has been implemented into our routine scan at the first trimester as well, and all of our staff have been trained to perform it. Obtaining STIC-TUI by color Doppler allows us to assess all of the important landmarks of the cardiac anatomy in one picture.

In a prospective study of 164 fetuses from 152 patients, we found that first-trimester STIC-TUI echo had 91% sensitivity and 100% specificity for the detection of CHD. Most anomalies were evident in the four-chamber view plane of the TUI display, and the rest were diagnosed in the outflow tract planes. Two cases of CHD missed by this first-trimester evaluation were diagnosed on second-trimester echo and neither involved a major CHD.8
Dr. Turan is associate professor of obstetrics, gynecology, and reproductive sciences, and director of the fetal heart program at the University of Maryland, Baltimore.
References
1. J Ultrasound Med. 2019 May;38(5):1269-77.
2. Prenat Diagn. 2005 Mar;25(3):253-60.
3. J Perinat Med. 2018 May 24;46(4):373-8.
4. Ultrasound Obstet Gynecol. 2015 Jun;45(6):631-8.
5. National Congenital Heart Disease Audit Report 2013-2016.
6. Pediatrics. 2015. doi: 10.1542/peds.2014-3783.
7. Echocardiography. 2018 Feb;35(2):244-57.
8. Ultrasound Obstet Gynecol. 2014 Nov;44(5):562-7.
The fetal heart typically is examined during the routine 18-20 week obstetric ultrasound screening, and pregnancies with abnormalities on this routine scan are referred for detailed fetal echocardiography. Per multiple practice guidelines, patients deemed to be at high risk of congenital heart defects (CHDs) are referred for fetal echocardiography as well between 18 and 24 weeks’ gestation.
However, with technological advancements in ultrasound, it is possible for obstetricians to detect many major CHDs well before 16 weeks’ gestation. First-trimester fetal heart assessment – and early detection of CHDs – has numerous advantages: It enables early genetic testing, early decision making about continuation or termination of pregnancy, and earlier planning for appropriate management during and after pregnancy. Perioperative outcomes are improved.
At least 75% of CHDs occur in pregnancies with no identifiable maternal, familial, or fetal risk factors. It only seems fitting, therefore, that we check the structure of the fetal heart in all women at the time of their first-trimester screening and sonography at 11-14 weeks. In addition to a determination of fetal viability and gestational age, nuchal translucency measurement, and a check of basic anatomy, .
The value of early detection
Women who have diabetes, congenital defects, in vitro fertilization pregnancies, twin and multiple pregnancies, and certain medication and drug exposures are at high risk for their fetus having a CHD and should undergo fetal echocardiography. Lupus, Sjögren’s, and other medical disorders also are risk factors, as are abnormal biochemical test results.
During the last 10 years, the first-trimester fetal heart evaluation has been performed for all patients who come for a first-trimester screening scan at the University of Maryland’s fetal heart program, part of the Center for Advanced Fetal Care. Approximately 45% of indications for detailed first-trimester fetal heart evaluation have been driven by maternal history, and almost 40% by abnormal basic first-trimester ultrasound findings such as increased nuchal translucency, tricuspid regurgitation, abnormal ductus venosus blood flow, and other structural anomalies.
An estimated 50%-60% of serious cardiac malformations can be detected with a four-chamber heart view during routine first-trimester ultrasound. When the outflow tract relationship and three-vessel views also are examined in the first trimester – as is now recommended in guidelines for second-trimester protocols – an estimated 85%-95% of major CHDs can be detected. One should see the great arteries originating from the left and right sides and crisscrossing each other by a transabdominal scan, or by a transvaginal scan if the transabdominal approach fails to show these features of the fetal heart.

Early sonography not only has been shown to have a high sensitivity but also a specificity of greater than 95% in identifying CHDs. Multiple studies also have demonstrated high negative predictive values in cases with normal findings.1
When defects seen or suspected on routine obstetric ultrasound are then confirmed and diagnosed with detailed fetal echocardiography, women are counseled about outcomes, management options, and mortality – and some patients will choose to terminate their pregnancies.
Psychologically, for the mother, earlier termination is less traumatic. A cross-sectional study of 254 women conducted 2-7 years after pregnancy termination for fetal anomalies found that advanced gestational age at termination was associated with higher levels of grief and posttraumatic stress symptoms, and that long-term psychological morbidity was rare when termination occurred before 14 weeks’ gestation.2 Others studies have shown similar results, with grief and posttraumatic stress time shorter with earlier termination.
First-trimester termination also involves significantly less maternal morbidity and risk, as shown in a retrospective study of 844 patients who underwent a termination of pregnancy after a positive amniocentesis or chorionic villus sampling. Hemorrhages, transfusions, infections, and other complications were significantly higher in second-trimester terminations than in earlier terminations.3
Early fetal heart evaluation can reassure high-risk patients – and low-risk patients as well – when a normal four-chamber heart and great arteries are seen. And when defects are spotted, early evaluation allows appropriate time to test for associated chromosomal abnormalities and genetic syndromes, which in turn improves management. It also gives patients and providers more time to plan and prepare for delivery, surgery, and other specific needs at delivery and after birth.
In our fetal heart program, patients are cared for by a multidisciplinary team of perinatologists with special expertise in the fetal heart, geneticists, cardiologists, cardiac surgeons, and neonatologists. Perioperative outcomes are improved when CHDs are diagnosed prenatally. One meta-analysis showed that prenatal diagnosis reduced the risk of death prior to planned cardiac surgery by about one-fourth relative to patients with a comparable postnatal diagnosis.4
Prenatal diagnosis appears to have generally been improving, although rates remain too low overall. According to the National Institute for Cardiovascular Outcomes Research, which collects data from centers across the United Kingdom and Republic of Ireland, prenatal detection rates of CHDs requiring a procedure in the first year of life moved from about 25% in 2004-2005 to just over 50% between 2010 and 2016.5 More complex lesions, such as hypoplastic left heart syndrome, were more likely to be detected prenatally (80%).
Trends in the United States appear to be similar. A study utilizing the Society of Thoracic Surgeons Congenital Heart Surgery Database found that prenatal detection increased from 26% in 2006 to 42% in 2012.6
A first-trimester evaluation cannot replace the second-trimester echocardiography that currently is performed for high-risk patients, because a small percentage of CHDs – aortic coarctation, valve stenosis, mild tetralogy of Fallot, and hypoplastic left heart, for instance – have the potential to evolve past the first trimester. High-risk patients whose first-trimester evaluations are normal still should undergo another evaluation at 18-20 weeks. The fetal heart completes its embryologic development over the first 8 weeks of gestation, and the majority of CHDs are present at the time of the first-trimester screening (11-14 weeks).
Early evaluation of the fetal heart does not appear to be impacted by obesity. We compared the early evaluation of fetal heart landmarks using two-dimensional sonography with color/power Doppler in obese and nonobese women and found that there were no significant differences in experienced sonographers’ ability to evaluate the four-chamber view, outflow tract relationship, and transverse arches views.
In about 6% of obese women, the evaluation at 11-14 weeks’ gestation required additional imaging with transvaginal sonography. The chances of needing transvaginal ultrasound rose as body mass index rose.1 The median scan time was only 5 minutes longer in the obese group, however, so there is no reason that obesity should be a contraindication to look at the fetal heart.
In fact, it is extremely important that we do early fetal heart evaluations in women who are obese, because the risk of having a fetus with CHD is increasingly being found to be higher in obese women, and because fetal heart assessment with transvaginal ultrasound is an option only in early gestation, when the fetal heart is within the depth of penetration of the vaginal probe. With advancing gestational age, a combined abdominal/transvaginal approach becomes increasingly difficult. Our study also demonstrated a dose-response relationship between maternal obesity and CHD risk.
Preexisting diabetes mellitus, which can occur in conjunction with obesity, has been found to increase the risk for all types of CHDs, especially conotruncal abnormalities. While the pathophysiology is not completely understood, elevated oxidative stress is believed to be the primary trigger.7
First-trimester echocardiography benefits
Patients referred to our fetal heart program for detailed first-trimester fetal heart evaluation – again, a significant number of whom have been found on standard 2-D ultrasound to have increased nuchal translucency thickness or other abnormalities – undergo a four-dimensional fetal echocardiographic technique that utilizes spatiotemporal image correlation and tomographic ultrasound imaging display (STIC-TUI echo) along with color Doppler. The heart is swept from top to bottom in about 10 seconds, and tomographic ultrasound imaging is used offline, after the patient leaves, to develop volume datasets that simultaneously display multiple cross-sectional images.
This method has been implemented into our routine scan at the first trimester as well, and all of our staff have been trained to perform it. Obtaining STIC-TUI by color Doppler allows us to assess all of the important landmarks of the cardiac anatomy in one picture.

In a prospective study of 164 fetuses from 152 patients, we found that first-trimester STIC-TUI echo had 91% sensitivity and 100% specificity for the detection of CHD. Most anomalies were evident in the four-chamber view plane of the TUI display, and the rest were diagnosed in the outflow tract planes. Two cases of CHD missed by this first-trimester evaluation were diagnosed on second-trimester echo and neither involved a major CHD.8
Dr. Turan is associate professor of obstetrics, gynecology, and reproductive sciences, and director of the fetal heart program at the University of Maryland, Baltimore.
References
1. J Ultrasound Med. 2019 May;38(5):1269-77.
2. Prenat Diagn. 2005 Mar;25(3):253-60.
3. J Perinat Med. 2018 May 24;46(4):373-8.
4. Ultrasound Obstet Gynecol. 2015 Jun;45(6):631-8.
5. National Congenital Heart Disease Audit Report 2013-2016.
6. Pediatrics. 2015. doi: 10.1542/peds.2014-3783.
7. Echocardiography. 2018 Feb;35(2):244-57.
8. Ultrasound Obstet Gynecol. 2014 Nov;44(5):562-7.
Considering congenital heart defects early
Regardless of political or ideological views, detecting the embryonic heartbeat in the first trimester is a major milestone for a patient. Measured via ultrasound, normal beating of 90-110 bpm around 6 weeks’ gestation indicates a high probability of a successful pregnancy. Once the embryo becomes a fetus, around gestational weeks 8-9, a strong fetal heartbeat of 140-170 bpm should be detected. Finding a heartbeat is a reassuring sign. However, simply seeing and/or hearing the heart is not enough to ensure that the fetus will develop without problems.
Congenital heart defects (CHDs) are the most common birth defects worldwide and, although many CHDs can be mild forms, approximately 25% are severe forms requiring early detection and intervention.1 In addition, CHDs in the fetus can cause miscarriage, stillbirth, and infant deaths.
A 2014 analysis of data from the Wisconsin Stillbirth Service Program revealed that 2 An analysis of the Active Malformations Surveillance Program at Brigham and Women’s Hospital also revealed CHDs as a major cause of stillbirths.3 In addition, a retrospective study of the Metropolitan Atlanta Congenital Defects program showed that, although 1-year survival of infants with severe CHDs has improved over the last 4 decades, mortality remains high.1
Because advances in medicine and surgical procedures have significantly reduced deaths attributable to CHDs, more women with a preexisting heart condition are becoming pregnant. Women who have a CHD, even if corrected, can experience pregnancy complications such as arrhythmias, thrombosis, and cardiac dysfunction. In addition, babies of women with CHDs have a higher risk of developing cardiac defects as well.
Therefore, it is critical that we closely monitor our patients – both the mother and her baby – to ensure that the fetal heart is present, functional, and developing normally. We have invited Dr. Shifa Turan, associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland and director of the Fetal Heart Program at the University of Maryland Medical Center, both in Baltimore, to discuss the fetal heart. In this first section of a two-part series, Dr. Turan addresses how we can and should monitor fetal heart development.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
References
1. Pediatrics. 2013 May. doi: 10.1542/peds.2012-3435).
2. Am J Med Genet A. 2014 Mar. doi: 10.1002/ajmg.a.36366.
3. Birth Defects Res. 2018 Jan. 29. doi: 10.1002/bdr2.1097.
Regardless of political or ideological views, detecting the embryonic heartbeat in the first trimester is a major milestone for a patient. Measured via ultrasound, normal beating of 90-110 bpm around 6 weeks’ gestation indicates a high probability of a successful pregnancy. Once the embryo becomes a fetus, around gestational weeks 8-9, a strong fetal heartbeat of 140-170 bpm should be detected. Finding a heartbeat is a reassuring sign. However, simply seeing and/or hearing the heart is not enough to ensure that the fetus will develop without problems.
Congenital heart defects (CHDs) are the most common birth defects worldwide and, although many CHDs can be mild forms, approximately 25% are severe forms requiring early detection and intervention.1 In addition, CHDs in the fetus can cause miscarriage, stillbirth, and infant deaths.
A 2014 analysis of data from the Wisconsin Stillbirth Service Program revealed that 2 An analysis of the Active Malformations Surveillance Program at Brigham and Women’s Hospital also revealed CHDs as a major cause of stillbirths.3 In addition, a retrospective study of the Metropolitan Atlanta Congenital Defects program showed that, although 1-year survival of infants with severe CHDs has improved over the last 4 decades, mortality remains high.1
Because advances in medicine and surgical procedures have significantly reduced deaths attributable to CHDs, more women with a preexisting heart condition are becoming pregnant. Women who have a CHD, even if corrected, can experience pregnancy complications such as arrhythmias, thrombosis, and cardiac dysfunction. In addition, babies of women with CHDs have a higher risk of developing cardiac defects as well.
Therefore, it is critical that we closely monitor our patients – both the mother and her baby – to ensure that the fetal heart is present, functional, and developing normally. We have invited Dr. Shifa Turan, associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland and director of the Fetal Heart Program at the University of Maryland Medical Center, both in Baltimore, to discuss the fetal heart. In this first section of a two-part series, Dr. Turan addresses how we can and should monitor fetal heart development.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
References
1. Pediatrics. 2013 May. doi: 10.1542/peds.2012-3435).
2. Am J Med Genet A. 2014 Mar. doi: 10.1002/ajmg.a.36366.
3. Birth Defects Res. 2018 Jan. 29. doi: 10.1002/bdr2.1097.
Regardless of political or ideological views, detecting the embryonic heartbeat in the first trimester is a major milestone for a patient. Measured via ultrasound, normal beating of 90-110 bpm around 6 weeks’ gestation indicates a high probability of a successful pregnancy. Once the embryo becomes a fetus, around gestational weeks 8-9, a strong fetal heartbeat of 140-170 bpm should be detected. Finding a heartbeat is a reassuring sign. However, simply seeing and/or hearing the heart is not enough to ensure that the fetus will develop without problems.
Congenital heart defects (CHDs) are the most common birth defects worldwide and, although many CHDs can be mild forms, approximately 25% are severe forms requiring early detection and intervention.1 In addition, CHDs in the fetus can cause miscarriage, stillbirth, and infant deaths.
A 2014 analysis of data from the Wisconsin Stillbirth Service Program revealed that 2 An analysis of the Active Malformations Surveillance Program at Brigham and Women’s Hospital also revealed CHDs as a major cause of stillbirths.3 In addition, a retrospective study of the Metropolitan Atlanta Congenital Defects program showed that, although 1-year survival of infants with severe CHDs has improved over the last 4 decades, mortality remains high.1
Because advances in medicine and surgical procedures have significantly reduced deaths attributable to CHDs, more women with a preexisting heart condition are becoming pregnant. Women who have a CHD, even if corrected, can experience pregnancy complications such as arrhythmias, thrombosis, and cardiac dysfunction. In addition, babies of women with CHDs have a higher risk of developing cardiac defects as well.
Therefore, it is critical that we closely monitor our patients – both the mother and her baby – to ensure that the fetal heart is present, functional, and developing normally. We have invited Dr. Shifa Turan, associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland and director of the Fetal Heart Program at the University of Maryland Medical Center, both in Baltimore, to discuss the fetal heart. In this first section of a two-part series, Dr. Turan addresses how we can and should monitor fetal heart development.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at obnews@mdedge.com.
References
1. Pediatrics. 2013 May. doi: 10.1542/peds.2012-3435).
2. Am J Med Genet A. 2014 Mar. doi: 10.1002/ajmg.a.36366.
3. Birth Defects Res. 2018 Jan. 29. doi: 10.1002/bdr2.1097.
The genesis of vaginal anomalies
According to our guest author Marc R. Laufer, MD, the “development of the female genital tract is a complex process that is dependent upon a series of events involving cellular differentiation, migration, fusion, and canalization. Failure of any one of these processes results in a congenital anomaly.”1
In 1933, A.K. Koff coined the terms sinovaginal bulb and vaginal plate. He proposed that the upper 80% of the vagina is derived from Müllerian epithelium and the lower 20% derived from urogenital sinus epithelium.2 In 1957, D. Bulmer proposed that vaginal epithelium derives solely from urogenital sinus epithelium.3 And in 2017, Robboy et al. supported Bulmer’s proposal that human vaginal epithelium derives solely from urogenital sinus epithelium and differs from mouse vaginal development.4
Beginning at 3 weeks of embryogenesis and continuing into the second trimester of pregnancy, development of the female genital tract takes place. The sinovaginal bulbs originate in the urogenital sinus at the distal aspect of the Müllerian tubercle. At approximately 13 weeks, these two solid evaginations grow out of the pelvic part of the urogenital sinus and proliferate into the caudal end of the uterovaginal canal to become a solid vaginal plate. Degeneration of the central cells of this vaginal plate, which occur in a cephalad direction, enables creation of the lower vagina. Canalization is generally completed by 20 weeks’ gestation.
Agenesis or absence of the lower vagina is usually associated with normal development of the upper vagina, cervix, uterus, and ovaries. It is the result of abnormal development of the sinovaginal bulbs and vaginal plate.
The hymenal membrane separates the vaginal lumen from the urogenital sinus. Secondary to degeneration of the central epithelial cells, the hymen typically ruptures, leaving a thin fold of mucous membrane around the vaginal introitus. Hymenal anatomic variants include microperforate, septate, or cribriform. They occur secondary to incomplete degeneration of the central portion of the hymen.
Dr. Laufer is chief of the division of gynecology, codirector of the Center for Young Women’s Health, and director of the Boston Center for Endometriosis, all at Boston Children’s Hospital. He also is professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston. Dr. Laufer is an acclaimed physician, surgeon, clinical researcher, author, and teacher, and it is truly my pleasure to welcome him to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He reported no disclosures relevant to this Master Class. Email him at pdnews@mdedge.com.
References
1. Laufer M. Congenital anomalies of the hymen and vagina. Uptodate (accessed April 2019).
2. Contrib Embryol. 1933 Sep;24(140):59-91.
3. J Anat. 1957 Oct;91(4):490-509.
4. Differentiation. 2017 Sep-Oct;97:9-22.
According to our guest author Marc R. Laufer, MD, the “development of the female genital tract is a complex process that is dependent upon a series of events involving cellular differentiation, migration, fusion, and canalization. Failure of any one of these processes results in a congenital anomaly.”1
In 1933, A.K. Koff coined the terms sinovaginal bulb and vaginal plate. He proposed that the upper 80% of the vagina is derived from Müllerian epithelium and the lower 20% derived from urogenital sinus epithelium.2 In 1957, D. Bulmer proposed that vaginal epithelium derives solely from urogenital sinus epithelium.3 And in 2017, Robboy et al. supported Bulmer’s proposal that human vaginal epithelium derives solely from urogenital sinus epithelium and differs from mouse vaginal development.4
Beginning at 3 weeks of embryogenesis and continuing into the second trimester of pregnancy, development of the female genital tract takes place. The sinovaginal bulbs originate in the urogenital sinus at the distal aspect of the Müllerian tubercle. At approximately 13 weeks, these two solid evaginations grow out of the pelvic part of the urogenital sinus and proliferate into the caudal end of the uterovaginal canal to become a solid vaginal plate. Degeneration of the central cells of this vaginal plate, which occur in a cephalad direction, enables creation of the lower vagina. Canalization is generally completed by 20 weeks’ gestation.
Agenesis or absence of the lower vagina is usually associated with normal development of the upper vagina, cervix, uterus, and ovaries. It is the result of abnormal development of the sinovaginal bulbs and vaginal plate.
The hymenal membrane separates the vaginal lumen from the urogenital sinus. Secondary to degeneration of the central epithelial cells, the hymen typically ruptures, leaving a thin fold of mucous membrane around the vaginal introitus. Hymenal anatomic variants include microperforate, septate, or cribriform. They occur secondary to incomplete degeneration of the central portion of the hymen.
Dr. Laufer is chief of the division of gynecology, codirector of the Center for Young Women’s Health, and director of the Boston Center for Endometriosis, all at Boston Children’s Hospital. He also is professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston. Dr. Laufer is an acclaimed physician, surgeon, clinical researcher, author, and teacher, and it is truly my pleasure to welcome him to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He reported no disclosures relevant to this Master Class. Email him at pdnews@mdedge.com.
References
1. Laufer M. Congenital anomalies of the hymen and vagina. Uptodate (accessed April 2019).
2. Contrib Embryol. 1933 Sep;24(140):59-91.
3. J Anat. 1957 Oct;91(4):490-509.
4. Differentiation. 2017 Sep-Oct;97:9-22.
According to our guest author Marc R. Laufer, MD, the “development of the female genital tract is a complex process that is dependent upon a series of events involving cellular differentiation, migration, fusion, and canalization. Failure of any one of these processes results in a congenital anomaly.”1
In 1933, A.K. Koff coined the terms sinovaginal bulb and vaginal plate. He proposed that the upper 80% of the vagina is derived from Müllerian epithelium and the lower 20% derived from urogenital sinus epithelium.2 In 1957, D. Bulmer proposed that vaginal epithelium derives solely from urogenital sinus epithelium.3 And in 2017, Robboy et al. supported Bulmer’s proposal that human vaginal epithelium derives solely from urogenital sinus epithelium and differs from mouse vaginal development.4
Beginning at 3 weeks of embryogenesis and continuing into the second trimester of pregnancy, development of the female genital tract takes place. The sinovaginal bulbs originate in the urogenital sinus at the distal aspect of the Müllerian tubercle. At approximately 13 weeks, these two solid evaginations grow out of the pelvic part of the urogenital sinus and proliferate into the caudal end of the uterovaginal canal to become a solid vaginal plate. Degeneration of the central cells of this vaginal plate, which occur in a cephalad direction, enables creation of the lower vagina. Canalization is generally completed by 20 weeks’ gestation.
Agenesis or absence of the lower vagina is usually associated with normal development of the upper vagina, cervix, uterus, and ovaries. It is the result of abnormal development of the sinovaginal bulbs and vaginal plate.
The hymenal membrane separates the vaginal lumen from the urogenital sinus. Secondary to degeneration of the central epithelial cells, the hymen typically ruptures, leaving a thin fold of mucous membrane around the vaginal introitus. Hymenal anatomic variants include microperforate, septate, or cribriform. They occur secondary to incomplete degeneration of the central portion of the hymen.
Dr. Laufer is chief of the division of gynecology, codirector of the Center for Young Women’s Health, and director of the Boston Center for Endometriosis, all at Boston Children’s Hospital. He also is professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston. Dr. Laufer is an acclaimed physician, surgeon, clinical researcher, author, and teacher, and it is truly my pleasure to welcome him to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He reported no disclosures relevant to this Master Class. Email him at pdnews@mdedge.com.
References
1. Laufer M. Congenital anomalies of the hymen and vagina. Uptodate (accessed April 2019).
2. Contrib Embryol. 1933 Sep;24(140):59-91.
3. J Anat. 1957 Oct;91(4):490-509.
4. Differentiation. 2017 Sep-Oct;97:9-22.
Vaginal anomalies and their surgical correction
Congenital obstructive anomalies of the vagina are unusual and can be challenging to diagnose and manage. Two of the most challenging are obstructive hemivagina with ipsilateral renal agenesis (Figure 1a) and agenesis of the lower vagina (Figure 1b), the latter of which must be differentiated most commonly from imperforate hymen (Figure 1c). Evaluation and treatment of these anomalies is dependent upon the age of the patient, as well as the symptoms, and the timing of treatment should be individualized.
Agenesis of the lower vagina
Agenesis of the lower vagina and imperforate hymen may present either in the newborn period as a bulging introitus caused by mucocolpos from vaginal secretions stimulated by maternal estradiol or during adolescence at the time of menarche. In neonates, it often is best not to intervene when obstructive anomalies are suspected as long as there is no fever; pain; or compromise of respiration, urinary and bowel function, and other functionality. It will be easier to differentiate agenesis of the lower vagina and imperforate hymen – the latter of which is one of the most common obstructive lesions of the female genital tract – later on. And if the hymen remains imperforate, the mucus will be reabsorbed and the patient usually will remain asymptomatic until menarche.
In the adolescent time period, both anomalies often are identified when the patient presents with pelvic pain – usually cyclic pelvic pain with primary amenorrhea. Because the onset of menses typically occurs 2-3 years after the onset of estrogenization and breast development, evaluating breast development can help us to determine the timing of expected menarche. An obstructive anomaly should be suspected in an adolescent who presents with pain during this time period, after evaluation for an acute abdomen (Figure 2a).
When a vaginal orifice is visualized upon evaluation of the external genitalia and separation of the labia, a higher anomaly such as a transverse vaginal septum should be suspected. When an introitus cannot be visualized, evaluation for an imperforate hymen or agenesis of the lower vagina is necessary (Figure 1b and 1c).
The simplest way to differentiate imperforate hymen from agenesis of the lower vagina is with visualization of the obstructing tissue on exam and usage of transperitoneal ultrasound. With the transducer placed on the vulva, we can evaluate the distance from the normal location of an introitus to the level of the obstruction. If the distance is in millimeters, then typically there is an imperforate hymen. If the distance is larger – more than several millimeters – then the differential diagnosis typically is agenesis of the lower vagina, an anomaly that results from abnormal development of the sinovaginal bulbs and vaginal plate.
The distance as measured by transperitoneal ultrasound also will indicate whether or not pull-through vaginoplasty (Figure 2b) – our standard treatment for lower vaginal agenesis – is possible using native vaginal mucosa from the upper vagina. Most commonly, the distance is less than 5 cm and we are able to make a transverse incision where the hymenal ring should be located, carry the dissection to the upper vagina, drain the obstruction, and mobilize the upper vaginal mucosa, suturing it to the newly created introitus to formulate a patent vaginal tract.
A rectoabdominal examination similarly can be helpful in making the diagnosis of lower vaginal agenesis and in determining whether there is enough tissue available for a pull-through procedure (Figures 2a and 2b). Because patients with this anomaly generally have normal cyclic pituitary-ovarian-endometrial function at menarche, the upper vagina will distend with blood products and secretions that can be palpated on the rectoabdominal exam. If the obstructed vaginal tissue is not felt with the rectal finger at midline, the obstructed agenesis of the vagina probably is too high for a straightforward pull-through procedure. Alternatively, the patient may have a unicornuate system with agenesis of the lower vagina; in this case, the obstructed upper vaginal tissue will not be in the midline but off to one side. MRI also may be helpful for defining the pelvic anatomy.
The optimal timing for a pull-through vaginoplasty (Figure 2b) is when a large hematocolpos (Figure 2a) is present, as the blood acts as a natural expander of the native vaginal tissue, increasing the amount of tissue available for a primary reanastomosis. This emphasizes the importance of an accurate initial diagnosis. Too often, obstructions that are actually lower vaginal agenesis are presumed to be imperforate hymen, and the hematocolpos is subsequently evacuated after a transverse incision and dissection of excess tissue, causing the upper vagina to retract and shrink. This mistake can result in the formation of a fistulous tract from the previously obstructed upper vagina to the level of the introitus.
The vaginoplasty is carried out with the patient in the dorsal lithotomy position. A Foley catheter is placed into the bladder to avoid an inadvertent anterior entry into the posterior wall of the bladder, and the labia are grasped and pulled down and out.
The hymenal tissue should be visible. A transverse incision is made, with electrocautery, where the introitus should be located, and a dissection is carried out to reach the obstructed upper vaginal tissue. Care is needed to keep the dissection in the midline and avoid the bladder above and the rectum below. In cases in which it is difficult to identify the area of obstruction, intraoperative ultrasound can be helpful. A spinal needle with a 10-cc syringe also can be used to identify a track through which to access the fluid.
The linear incision then is made with electrocautery and the obstructed hemivagina is entered. Allis clamps are used to grasp the vaginal mucosa from the previously obstructed upper vagina to help identify the tissue that needs to be mobilized. The tissue then is further dissected to free the upper vagina, and the edges are pulled down to the level of the introitus with Allis clamps. “Relaxing” incisions are made at 1, 5,7, and 11 o’clock to avoid a circumferential scar. The upper vaginal mucosa is sewn to the newly created introitus with a 2-0 vicryl suture on a UR6 (a smaller curved urology needle).
When the distance from normal introitus location to obstruction is greater than 5 cm, we sometimes use vaginal dilators to lessen the distance and reach the obstruction for a pull-through procedure. Alternatively, the upper vagina may be mobilized from above either robotically or laparoscopically so that the upper vaginal mucosa may be pulled down without entering the bladder. Occasionally, with greater distances over 5 cm, the vaginoplasty may require utilization of a buccal mucosal graft or a bowel segment.
Intraoperative ultrasound can be especially helpful for locating the obstructed vagina in women with a unicornuate system because the upper vagina will not be in the midline and ultrasound can help determine the appropriate angle for dissection.
Prophylactic antibiotics initiated postoperatively are important with pull-through vaginoplasty, because the uterus and fallopian tubes may contain blood (an excellent growth media) and because there is a risk of bacteria ascending into what becomes an open system.
Postoperatively, we guide patients on the use of flexible Milex dilators (CooperSurgical) to ensure that the vagina heals without restenosis. The length of postoperative dilation therapy can vary from 2-12 months, depending on healing. The dilator is worn 24 hours a day, 7 days a week, and is removed only for urination, defecation, and cleaning. With adequate postoperative dilation, patients will have normal sexual and reproductive function, and vaginal delivery should be possible.
Obstructed hemivagina
An obstructed hemivagina, an uncommon Müllerian duct anomaly, occurs most often with ipsilateral renal agenesis and is commonly referred to as OHVIRA. Because the formation of the reproductive system is closely associated with the development of the urinary system, it is not unusual for renal anomalies to occur alongside Müllerian anomalies and vaginal anomalies. There should be a high index of suspicion for a reproductive tract anomaly in any patient known to have a horseshoe kidney, duplex collecting system, unilateral renal agenesis, or other renal anomaly.
Patients with obstructed hemivagina typically present in adolescence with pelvic pain or dysmenorrhea, and commonly are misdiagnosed as having endometriomas or vaginal cysts. On vaginal examination, the obstructed hemivagina may be visualized as a bulge coming from the lateral vaginal sidewall. While only one cervix is appreciated on a vaginal exam, an ultrasound examination often will show two uteri and two cervices. MRI also is helpful for diagnosis.
Obstructed hemivagina requires surgical correction to open the obstruction, excise the excess vaginal tissue, and create one vagina with access to the second cervix. Great care must be taken to avoid not only the bladder and rectum but the cervices. It is not unusual for the two cervices to be at different levels, with one cervix sharing medial aspects of the vaginal wall of the second vagina (Figure 1a). The tissue between the two cervices should be left in place to avoid compromising their blood supply.
We manage this anomaly primarily through a single-stage vaginoplasty. For the nonobstructed side to be visualized, a longitudinal incision into the obstructed hemivagina should be made at the point at which it is most easily palpated. As with agenesis of the lower vagina, the fluid to be drained tends to be old menstrual blood that is thick and dark brown. It is useful to set up two suction units at the time of surgery because tubing can become clogged.
The use of vaginal side wall retractors helps with visualization. Alternatively, I tend to use malleable abdominal wall retractors vaginally, as they can be bent to conform to the angle needed and come in different widths. When it is difficult to identify the area of obstruction, a spinal needle with a 10-cc syringe again can be used to identify a track for accessing the fluid. The linear incision then is made with electrocautery, and the obstructed hemivagina is entered.
Allis clamps are used to grasp the vaginal mucosa from the previously obstructed hemivagina to help identify the tissue that needs to be excised. Once the fluid is evacuated, a finger also can be placed into the obstructed vagina is help identify excess tissue. This three-dimensional elliptical area is similar to a septum but becomes the obstructed hemivagina as it attaches to the vaginal wall (Figure 1a).
Retrograde menses and endometriosis occur commonly with obstructive anomalies like obstructed hemivagina and agenesis of the lower vagina, but laparoscopy with the goal of treating endometriosis is not indicated. We discourage its use at the time of repair because there is evidence that almost all endometriosis will completely resorb on its own once the anomalies are corrected.1,2
As with repair of lower vagina agenesis, antibiotics to prevent an ascending infection should be taken after surgical correction of obstructed hemivagina. Patients with obstructed hemivagina can have a vaginal delivery if there are no other contraindications. Women with obstructed hemivagina and ipsilateral renal anomaly have essentially two unicornuate systems and thus are at risk of breech presentation and preterm delivery.
Dr. Laufer is chief of the division of gynecology, codirector of the Center for Young Women’s Health, and director of the Boston Center for Endometriosis, all at Boston Children’s Hospital. He also is professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston.
References
1. Am J Obstet Gynecol. 1986;154:39.
2. J Pediatr Adolesc Gynecol. 2010;23(2):e89.
Congenital obstructive anomalies of the vagina are unusual and can be challenging to diagnose and manage. Two of the most challenging are obstructive hemivagina with ipsilateral renal agenesis (Figure 1a) and agenesis of the lower vagina (Figure 1b), the latter of which must be differentiated most commonly from imperforate hymen (Figure 1c). Evaluation and treatment of these anomalies is dependent upon the age of the patient, as well as the symptoms, and the timing of treatment should be individualized.
Agenesis of the lower vagina
Agenesis of the lower vagina and imperforate hymen may present either in the newborn period as a bulging introitus caused by mucocolpos from vaginal secretions stimulated by maternal estradiol or during adolescence at the time of menarche. In neonates, it often is best not to intervene when obstructive anomalies are suspected as long as there is no fever; pain; or compromise of respiration, urinary and bowel function, and other functionality. It will be easier to differentiate agenesis of the lower vagina and imperforate hymen – the latter of which is one of the most common obstructive lesions of the female genital tract – later on. And if the hymen remains imperforate, the mucus will be reabsorbed and the patient usually will remain asymptomatic until menarche.
In the adolescent time period, both anomalies often are identified when the patient presents with pelvic pain – usually cyclic pelvic pain with primary amenorrhea. Because the onset of menses typically occurs 2-3 years after the onset of estrogenization and breast development, evaluating breast development can help us to determine the timing of expected menarche. An obstructive anomaly should be suspected in an adolescent who presents with pain during this time period, after evaluation for an acute abdomen (Figure 2a).
When a vaginal orifice is visualized upon evaluation of the external genitalia and separation of the labia, a higher anomaly such as a transverse vaginal septum should be suspected. When an introitus cannot be visualized, evaluation for an imperforate hymen or agenesis of the lower vagina is necessary (Figure 1b and 1c).
The simplest way to differentiate imperforate hymen from agenesis of the lower vagina is with visualization of the obstructing tissue on exam and usage of transperitoneal ultrasound. With the transducer placed on the vulva, we can evaluate the distance from the normal location of an introitus to the level of the obstruction. If the distance is in millimeters, then typically there is an imperforate hymen. If the distance is larger – more than several millimeters – then the differential diagnosis typically is agenesis of the lower vagina, an anomaly that results from abnormal development of the sinovaginal bulbs and vaginal plate.
The distance as measured by transperitoneal ultrasound also will indicate whether or not pull-through vaginoplasty (Figure 2b) – our standard treatment for lower vaginal agenesis – is possible using native vaginal mucosa from the upper vagina. Most commonly, the distance is less than 5 cm and we are able to make a transverse incision where the hymenal ring should be located, carry the dissection to the upper vagina, drain the obstruction, and mobilize the upper vaginal mucosa, suturing it to the newly created introitus to formulate a patent vaginal tract.
A rectoabdominal examination similarly can be helpful in making the diagnosis of lower vaginal agenesis and in determining whether there is enough tissue available for a pull-through procedure (Figures 2a and 2b). Because patients with this anomaly generally have normal cyclic pituitary-ovarian-endometrial function at menarche, the upper vagina will distend with blood products and secretions that can be palpated on the rectoabdominal exam. If the obstructed vaginal tissue is not felt with the rectal finger at midline, the obstructed agenesis of the vagina probably is too high for a straightforward pull-through procedure. Alternatively, the patient may have a unicornuate system with agenesis of the lower vagina; in this case, the obstructed upper vaginal tissue will not be in the midline but off to one side. MRI also may be helpful for defining the pelvic anatomy.
The optimal timing for a pull-through vaginoplasty (Figure 2b) is when a large hematocolpos (Figure 2a) is present, as the blood acts as a natural expander of the native vaginal tissue, increasing the amount of tissue available for a primary reanastomosis. This emphasizes the importance of an accurate initial diagnosis. Too often, obstructions that are actually lower vaginal agenesis are presumed to be imperforate hymen, and the hematocolpos is subsequently evacuated after a transverse incision and dissection of excess tissue, causing the upper vagina to retract and shrink. This mistake can result in the formation of a fistulous tract from the previously obstructed upper vagina to the level of the introitus.
The vaginoplasty is carried out with the patient in the dorsal lithotomy position. A Foley catheter is placed into the bladder to avoid an inadvertent anterior entry into the posterior wall of the bladder, and the labia are grasped and pulled down and out.
The hymenal tissue should be visible. A transverse incision is made, with electrocautery, where the introitus should be located, and a dissection is carried out to reach the obstructed upper vaginal tissue. Care is needed to keep the dissection in the midline and avoid the bladder above and the rectum below. In cases in which it is difficult to identify the area of obstruction, intraoperative ultrasound can be helpful. A spinal needle with a 10-cc syringe also can be used to identify a track through which to access the fluid.
The linear incision then is made with electrocautery and the obstructed hemivagina is entered. Allis clamps are used to grasp the vaginal mucosa from the previously obstructed upper vagina to help identify the tissue that needs to be mobilized. The tissue then is further dissected to free the upper vagina, and the edges are pulled down to the level of the introitus with Allis clamps. “Relaxing” incisions are made at 1, 5,7, and 11 o’clock to avoid a circumferential scar. The upper vaginal mucosa is sewn to the newly created introitus with a 2-0 vicryl suture on a UR6 (a smaller curved urology needle).
When the distance from normal introitus location to obstruction is greater than 5 cm, we sometimes use vaginal dilators to lessen the distance and reach the obstruction for a pull-through procedure. Alternatively, the upper vagina may be mobilized from above either robotically or laparoscopically so that the upper vaginal mucosa may be pulled down without entering the bladder. Occasionally, with greater distances over 5 cm, the vaginoplasty may require utilization of a buccal mucosal graft or a bowel segment.
Intraoperative ultrasound can be especially helpful for locating the obstructed vagina in women with a unicornuate system because the upper vagina will not be in the midline and ultrasound can help determine the appropriate angle for dissection.
Prophylactic antibiotics initiated postoperatively are important with pull-through vaginoplasty, because the uterus and fallopian tubes may contain blood (an excellent growth media) and because there is a risk of bacteria ascending into what becomes an open system.
Postoperatively, we guide patients on the use of flexible Milex dilators (CooperSurgical) to ensure that the vagina heals without restenosis. The length of postoperative dilation therapy can vary from 2-12 months, depending on healing. The dilator is worn 24 hours a day, 7 days a week, and is removed only for urination, defecation, and cleaning. With adequate postoperative dilation, patients will have normal sexual and reproductive function, and vaginal delivery should be possible.
Obstructed hemivagina
An obstructed hemivagina, an uncommon Müllerian duct anomaly, occurs most often with ipsilateral renal agenesis and is commonly referred to as OHVIRA. Because the formation of the reproductive system is closely associated with the development of the urinary system, it is not unusual for renal anomalies to occur alongside Müllerian anomalies and vaginal anomalies. There should be a high index of suspicion for a reproductive tract anomaly in any patient known to have a horseshoe kidney, duplex collecting system, unilateral renal agenesis, or other renal anomaly.
Patients with obstructed hemivagina typically present in adolescence with pelvic pain or dysmenorrhea, and commonly are misdiagnosed as having endometriomas or vaginal cysts. On vaginal examination, the obstructed hemivagina may be visualized as a bulge coming from the lateral vaginal sidewall. While only one cervix is appreciated on a vaginal exam, an ultrasound examination often will show two uteri and two cervices. MRI also is helpful for diagnosis.
Obstructed hemivagina requires surgical correction to open the obstruction, excise the excess vaginal tissue, and create one vagina with access to the second cervix. Great care must be taken to avoid not only the bladder and rectum but the cervices. It is not unusual for the two cervices to be at different levels, with one cervix sharing medial aspects of the vaginal wall of the second vagina (Figure 1a). The tissue between the two cervices should be left in place to avoid compromising their blood supply.
We manage this anomaly primarily through a single-stage vaginoplasty. For the nonobstructed side to be visualized, a longitudinal incision into the obstructed hemivagina should be made at the point at which it is most easily palpated. As with agenesis of the lower vagina, the fluid to be drained tends to be old menstrual blood that is thick and dark brown. It is useful to set up two suction units at the time of surgery because tubing can become clogged.
The use of vaginal side wall retractors helps with visualization. Alternatively, I tend to use malleable abdominal wall retractors vaginally, as they can be bent to conform to the angle needed and come in different widths. When it is difficult to identify the area of obstruction, a spinal needle with a 10-cc syringe again can be used to identify a track for accessing the fluid. The linear incision then is made with electrocautery, and the obstructed hemivagina is entered.
Allis clamps are used to grasp the vaginal mucosa from the previously obstructed hemivagina to help identify the tissue that needs to be excised. Once the fluid is evacuated, a finger also can be placed into the obstructed vagina is help identify excess tissue. This three-dimensional elliptical area is similar to a septum but becomes the obstructed hemivagina as it attaches to the vaginal wall (Figure 1a).
Retrograde menses and endometriosis occur commonly with obstructive anomalies like obstructed hemivagina and agenesis of the lower vagina, but laparoscopy with the goal of treating endometriosis is not indicated. We discourage its use at the time of repair because there is evidence that almost all endometriosis will completely resorb on its own once the anomalies are corrected.1,2
As with repair of lower vagina agenesis, antibiotics to prevent an ascending infection should be taken after surgical correction of obstructed hemivagina. Patients with obstructed hemivagina can have a vaginal delivery if there are no other contraindications. Women with obstructed hemivagina and ipsilateral renal anomaly have essentially two unicornuate systems and thus are at risk of breech presentation and preterm delivery.
Dr. Laufer is chief of the division of gynecology, codirector of the Center for Young Women’s Health, and director of the Boston Center for Endometriosis, all at Boston Children’s Hospital. He also is professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston.
References
1. Am J Obstet Gynecol. 1986;154:39.
2. J Pediatr Adolesc Gynecol. 2010;23(2):e89.
Congenital obstructive anomalies of the vagina are unusual and can be challenging to diagnose and manage. Two of the most challenging are obstructive hemivagina with ipsilateral renal agenesis (Figure 1a) and agenesis of the lower vagina (Figure 1b), the latter of which must be differentiated most commonly from imperforate hymen (Figure 1c). Evaluation and treatment of these anomalies is dependent upon the age of the patient, as well as the symptoms, and the timing of treatment should be individualized.
Agenesis of the lower vagina
Agenesis of the lower vagina and imperforate hymen may present either in the newborn period as a bulging introitus caused by mucocolpos from vaginal secretions stimulated by maternal estradiol or during adolescence at the time of menarche. In neonates, it often is best not to intervene when obstructive anomalies are suspected as long as there is no fever; pain; or compromise of respiration, urinary and bowel function, and other functionality. It will be easier to differentiate agenesis of the lower vagina and imperforate hymen – the latter of which is one of the most common obstructive lesions of the female genital tract – later on. And if the hymen remains imperforate, the mucus will be reabsorbed and the patient usually will remain asymptomatic until menarche.
In the adolescent time period, both anomalies often are identified when the patient presents with pelvic pain – usually cyclic pelvic pain with primary amenorrhea. Because the onset of menses typically occurs 2-3 years after the onset of estrogenization and breast development, evaluating breast development can help us to determine the timing of expected menarche. An obstructive anomaly should be suspected in an adolescent who presents with pain during this time period, after evaluation for an acute abdomen (Figure 2a).
When a vaginal orifice is visualized upon evaluation of the external genitalia and separation of the labia, a higher anomaly such as a transverse vaginal septum should be suspected. When an introitus cannot be visualized, evaluation for an imperforate hymen or agenesis of the lower vagina is necessary (Figure 1b and 1c).
The simplest way to differentiate imperforate hymen from agenesis of the lower vagina is with visualization of the obstructing tissue on exam and usage of transperitoneal ultrasound. With the transducer placed on the vulva, we can evaluate the distance from the normal location of an introitus to the level of the obstruction. If the distance is in millimeters, then typically there is an imperforate hymen. If the distance is larger – more than several millimeters – then the differential diagnosis typically is agenesis of the lower vagina, an anomaly that results from abnormal development of the sinovaginal bulbs and vaginal plate.
The distance as measured by transperitoneal ultrasound also will indicate whether or not pull-through vaginoplasty (Figure 2b) – our standard treatment for lower vaginal agenesis – is possible using native vaginal mucosa from the upper vagina. Most commonly, the distance is less than 5 cm and we are able to make a transverse incision where the hymenal ring should be located, carry the dissection to the upper vagina, drain the obstruction, and mobilize the upper vaginal mucosa, suturing it to the newly created introitus to formulate a patent vaginal tract.
A rectoabdominal examination similarly can be helpful in making the diagnosis of lower vaginal agenesis and in determining whether there is enough tissue available for a pull-through procedure (Figures 2a and 2b). Because patients with this anomaly generally have normal cyclic pituitary-ovarian-endometrial function at menarche, the upper vagina will distend with blood products and secretions that can be palpated on the rectoabdominal exam. If the obstructed vaginal tissue is not felt with the rectal finger at midline, the obstructed agenesis of the vagina probably is too high for a straightforward pull-through procedure. Alternatively, the patient may have a unicornuate system with agenesis of the lower vagina; in this case, the obstructed upper vaginal tissue will not be in the midline but off to one side. MRI also may be helpful for defining the pelvic anatomy.
The optimal timing for a pull-through vaginoplasty (Figure 2b) is when a large hematocolpos (Figure 2a) is present, as the blood acts as a natural expander of the native vaginal tissue, increasing the amount of tissue available for a primary reanastomosis. This emphasizes the importance of an accurate initial diagnosis. Too often, obstructions that are actually lower vaginal agenesis are presumed to be imperforate hymen, and the hematocolpos is subsequently evacuated after a transverse incision and dissection of excess tissue, causing the upper vagina to retract and shrink. This mistake can result in the formation of a fistulous tract from the previously obstructed upper vagina to the level of the introitus.
The vaginoplasty is carried out with the patient in the dorsal lithotomy position. A Foley catheter is placed into the bladder to avoid an inadvertent anterior entry into the posterior wall of the bladder, and the labia are grasped and pulled down and out.
The hymenal tissue should be visible. A transverse incision is made, with electrocautery, where the introitus should be located, and a dissection is carried out to reach the obstructed upper vaginal tissue. Care is needed to keep the dissection in the midline and avoid the bladder above and the rectum below. In cases in which it is difficult to identify the area of obstruction, intraoperative ultrasound can be helpful. A spinal needle with a 10-cc syringe also can be used to identify a track through which to access the fluid.
The linear incision then is made with electrocautery and the obstructed hemivagina is entered. Allis clamps are used to grasp the vaginal mucosa from the previously obstructed upper vagina to help identify the tissue that needs to be mobilized. The tissue then is further dissected to free the upper vagina, and the edges are pulled down to the level of the introitus with Allis clamps. “Relaxing” incisions are made at 1, 5,7, and 11 o’clock to avoid a circumferential scar. The upper vaginal mucosa is sewn to the newly created introitus with a 2-0 vicryl suture on a UR6 (a smaller curved urology needle).
When the distance from normal introitus location to obstruction is greater than 5 cm, we sometimes use vaginal dilators to lessen the distance and reach the obstruction for a pull-through procedure. Alternatively, the upper vagina may be mobilized from above either robotically or laparoscopically so that the upper vaginal mucosa may be pulled down without entering the bladder. Occasionally, with greater distances over 5 cm, the vaginoplasty may require utilization of a buccal mucosal graft or a bowel segment.
Intraoperative ultrasound can be especially helpful for locating the obstructed vagina in women with a unicornuate system because the upper vagina will not be in the midline and ultrasound can help determine the appropriate angle for dissection.
Prophylactic antibiotics initiated postoperatively are important with pull-through vaginoplasty, because the uterus and fallopian tubes may contain blood (an excellent growth media) and because there is a risk of bacteria ascending into what becomes an open system.
Postoperatively, we guide patients on the use of flexible Milex dilators (CooperSurgical) to ensure that the vagina heals without restenosis. The length of postoperative dilation therapy can vary from 2-12 months, depending on healing. The dilator is worn 24 hours a day, 7 days a week, and is removed only for urination, defecation, and cleaning. With adequate postoperative dilation, patients will have normal sexual and reproductive function, and vaginal delivery should be possible.
Obstructed hemivagina
An obstructed hemivagina, an uncommon Müllerian duct anomaly, occurs most often with ipsilateral renal agenesis and is commonly referred to as OHVIRA. Because the formation of the reproductive system is closely associated with the development of the urinary system, it is not unusual for renal anomalies to occur alongside Müllerian anomalies and vaginal anomalies. There should be a high index of suspicion for a reproductive tract anomaly in any patient known to have a horseshoe kidney, duplex collecting system, unilateral renal agenesis, or other renal anomaly.
Patients with obstructed hemivagina typically present in adolescence with pelvic pain or dysmenorrhea, and commonly are misdiagnosed as having endometriomas or vaginal cysts. On vaginal examination, the obstructed hemivagina may be visualized as a bulge coming from the lateral vaginal sidewall. While only one cervix is appreciated on a vaginal exam, an ultrasound examination often will show two uteri and two cervices. MRI also is helpful for diagnosis.
Obstructed hemivagina requires surgical correction to open the obstruction, excise the excess vaginal tissue, and create one vagina with access to the second cervix. Great care must be taken to avoid not only the bladder and rectum but the cervices. It is not unusual for the two cervices to be at different levels, with one cervix sharing medial aspects of the vaginal wall of the second vagina (Figure 1a). The tissue between the two cervices should be left in place to avoid compromising their blood supply.
We manage this anomaly primarily through a single-stage vaginoplasty. For the nonobstructed side to be visualized, a longitudinal incision into the obstructed hemivagina should be made at the point at which it is most easily palpated. As with agenesis of the lower vagina, the fluid to be drained tends to be old menstrual blood that is thick and dark brown. It is useful to set up two suction units at the time of surgery because tubing can become clogged.
The use of vaginal side wall retractors helps with visualization. Alternatively, I tend to use malleable abdominal wall retractors vaginally, as they can be bent to conform to the angle needed and come in different widths. When it is difficult to identify the area of obstruction, a spinal needle with a 10-cc syringe again can be used to identify a track for accessing the fluid. The linear incision then is made with electrocautery, and the obstructed hemivagina is entered.
Allis clamps are used to grasp the vaginal mucosa from the previously obstructed hemivagina to help identify the tissue that needs to be excised. Once the fluid is evacuated, a finger also can be placed into the obstructed vagina is help identify excess tissue. This three-dimensional elliptical area is similar to a septum but becomes the obstructed hemivagina as it attaches to the vaginal wall (Figure 1a).
Retrograde menses and endometriosis occur commonly with obstructive anomalies like obstructed hemivagina and agenesis of the lower vagina, but laparoscopy with the goal of treating endometriosis is not indicated. We discourage its use at the time of repair because there is evidence that almost all endometriosis will completely resorb on its own once the anomalies are corrected.1,2
As with repair of lower vagina agenesis, antibiotics to prevent an ascending infection should be taken after surgical correction of obstructed hemivagina. Patients with obstructed hemivagina can have a vaginal delivery if there are no other contraindications. Women with obstructed hemivagina and ipsilateral renal anomaly have essentially two unicornuate systems and thus are at risk of breech presentation and preterm delivery.
Dr. Laufer is chief of the division of gynecology, codirector of the Center for Young Women’s Health, and director of the Boston Center for Endometriosis, all at Boston Children’s Hospital. He also is professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston.
References
1. Am J Obstet Gynecol. 1986;154:39.
2. J Pediatr Adolesc Gynecol. 2010;23(2):e89.