User login
Infectious Diseases Society of America (IDSA)/ Society for Healthcare Epidemiology of America (SHEA)/ HIV Medicine Association (HIVMA)/ Pediatric Infectious Diseases Society (PIDS): IDWeek 2015
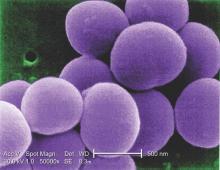
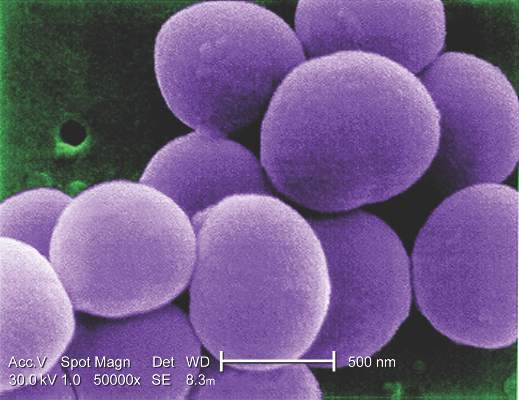
IDWEEK: Cefazolin beats ceftriaxone for MSSA bacteremia treatment
SAN DIEGO – Veteran patients treated with ceftriaxone for methicillin-susceptible Staphylococcus aureus bacteremia were more likely to have treatment failure, compared with those who received cefazolin, a retrospective observational study found.
“To date, no study has specifically evaluated cefazolin versus ceftriaxone for MSSA bacteremia,” Dustin Carr, Pharm.D., said at an annual scientific meeting on infectious diseases. “The main design of this study was to inform our current site and give us additional antimicrobial stewardship opportunities to ensure appropriate selection, dosing, route, and duration.”
In a study conducted during Dr. Carr’s residency training at Louis Stokes Cleveland VA Medical Center, he and his colleagues retrospectively evaluated veteran patients with MSSA bacteremia from January 2009 to August 2014 who received at least 14 days of parenteral cefazolin or ceftriaxone. The use of concomitant anti-staphylococcal agents were excluded, as were patients with polymicrobial infections and those who received empiric antibiotics greater than 72 hours after cultures were finalized. Dr. Carr noted that infectious diseases clinicians are consulted for all cases of MSSA bacteremia at the Louis Stokes Cleveland VA Medical Center, which accounts for about 25% of all outpatient parenteral antimicrobial therapy cases.
Treatment failure was defined as unplanned extension of parenteral antimicrobial therapy, failure to complete a course of parenteral therapy, relapse or recurrence of infection within 90 days, addition of suppressive oral antimicrobial therapy, readmission or unanticipated surgical intervention within 90 days, and being lost to follow-up. Secondary outcomes were relapse and recurrence within 90 days of treatment, overall 90-day mortality, rate of Clostridium difficile infection within 90 days, adverse drug reactions, and cost of IV antimicrobials. The researchers used logistic regression to assess variables of treatment failure.
Dr. Carr, who is currently an infectious diseases specialty resident at Wake Forest Baptist Health, Winston-Salem, N.C., reported results from 71 patients: 38 who received cefazolin (the cefazolin group) and 33 who received ceftriaxone (the ceftriaxone group). Patients in the cefazolin group were more likely to be on dialysis, compared with those in the ceftriaxone group (24% vs. 0%, respectively; P = .003) and they were more likely to have a prosthesis (53% vs. 24%; P = .015).
The researchers observed no significant differences in the comorbidities between the two groups, but there was more IV drug use in the cefazolin group (13% vs. 0%). The primary source of infection did not differ between the groups, but SSTIs were more likely to be treated with ceftriaxone, compared with cefazolin (33% vs. 8%; P = .086).
Dr. Carr reported that 28.9% of patients in the cefazolin group experienced a treatment failure, compared with 54.5% of those in the ceftriaxone group, a difference that was statistically significant (P = .029). There were no significant differences in the rate of most secondary outcomes between the two groups, with the exception of cost and loss to follow-up. The mean cost of IV therapy per patient was $746.51 in the cefazolin group, compared with $60.30 in the ceftriaxone group, while 5.3% of patients in the cefazolin group were lost to follow-up, compared with 12.1% in the ceftriaxone group.
By setting of outpatient parenteral antibiotic therapy, treatment failure occurred most often in the community skilled nursing facility setting (71%), followed by home settings (41%) and the VA long-term care facility attached to Louis Stokes Cleveland VA Medical Center (17%).
On logistic regression, the only significant predictors of treatment failure among all patients were duration of IV therapy (OR 1.05; P = .015), having heart failure (OR 7.93; P less than .001), as well as being treated in an outside community skilled nursing facility, compared with being treated at the long-term care setting attached to Louis Stokes Cleveland VA Medical Center (P = .008).
Dr. Carr acknowledged certain limitations of the study, including its retrospective design and the fact that it lacked data on antimicrobial susceptibilities and minimum inhibitory concentrations. Other limitations, he said, were that patients were allowed to receive cefazolin prior to ceftriaxone use and that there were low frequencies of secondary outcomes.
IDWeek marks the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. The researchers reported having no financial disclosures.
SAN DIEGO – Veteran patients treated with ceftriaxone for methicillin-susceptible Staphylococcus aureus bacteremia were more likely to have treatment failure, compared with those who received cefazolin, a retrospective observational study found.
“To date, no study has specifically evaluated cefazolin versus ceftriaxone for MSSA bacteremia,” Dustin Carr, Pharm.D., said at an annual scientific meeting on infectious diseases. “The main design of this study was to inform our current site and give us additional antimicrobial stewardship opportunities to ensure appropriate selection, dosing, route, and duration.”
In a study conducted during Dr. Carr’s residency training at Louis Stokes Cleveland VA Medical Center, he and his colleagues retrospectively evaluated veteran patients with MSSA bacteremia from January 2009 to August 2014 who received at least 14 days of parenteral cefazolin or ceftriaxone. The use of concomitant anti-staphylococcal agents were excluded, as were patients with polymicrobial infections and those who received empiric antibiotics greater than 72 hours after cultures were finalized. Dr. Carr noted that infectious diseases clinicians are consulted for all cases of MSSA bacteremia at the Louis Stokes Cleveland VA Medical Center, which accounts for about 25% of all outpatient parenteral antimicrobial therapy cases.
Treatment failure was defined as unplanned extension of parenteral antimicrobial therapy, failure to complete a course of parenteral therapy, relapse or recurrence of infection within 90 days, addition of suppressive oral antimicrobial therapy, readmission or unanticipated surgical intervention within 90 days, and being lost to follow-up. Secondary outcomes were relapse and recurrence within 90 days of treatment, overall 90-day mortality, rate of Clostridium difficile infection within 90 days, adverse drug reactions, and cost of IV antimicrobials. The researchers used logistic regression to assess variables of treatment failure.
Dr. Carr, who is currently an infectious diseases specialty resident at Wake Forest Baptist Health, Winston-Salem, N.C., reported results from 71 patients: 38 who received cefazolin (the cefazolin group) and 33 who received ceftriaxone (the ceftriaxone group). Patients in the cefazolin group were more likely to be on dialysis, compared with those in the ceftriaxone group (24% vs. 0%, respectively; P = .003) and they were more likely to have a prosthesis (53% vs. 24%; P = .015).
The researchers observed no significant differences in the comorbidities between the two groups, but there was more IV drug use in the cefazolin group (13% vs. 0%). The primary source of infection did not differ between the groups, but SSTIs were more likely to be treated with ceftriaxone, compared with cefazolin (33% vs. 8%; P = .086).
Dr. Carr reported that 28.9% of patients in the cefazolin group experienced a treatment failure, compared with 54.5% of those in the ceftriaxone group, a difference that was statistically significant (P = .029). There were no significant differences in the rate of most secondary outcomes between the two groups, with the exception of cost and loss to follow-up. The mean cost of IV therapy per patient was $746.51 in the cefazolin group, compared with $60.30 in the ceftriaxone group, while 5.3% of patients in the cefazolin group were lost to follow-up, compared with 12.1% in the ceftriaxone group.
By setting of outpatient parenteral antibiotic therapy, treatment failure occurred most often in the community skilled nursing facility setting (71%), followed by home settings (41%) and the VA long-term care facility attached to Louis Stokes Cleveland VA Medical Center (17%).
On logistic regression, the only significant predictors of treatment failure among all patients were duration of IV therapy (OR 1.05; P = .015), having heart failure (OR 7.93; P less than .001), as well as being treated in an outside community skilled nursing facility, compared with being treated at the long-term care setting attached to Louis Stokes Cleveland VA Medical Center (P = .008).
Dr. Carr acknowledged certain limitations of the study, including its retrospective design and the fact that it lacked data on antimicrobial susceptibilities and minimum inhibitory concentrations. Other limitations, he said, were that patients were allowed to receive cefazolin prior to ceftriaxone use and that there were low frequencies of secondary outcomes.
IDWeek marks the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. The researchers reported having no financial disclosures.
SAN DIEGO – Veteran patients treated with ceftriaxone for methicillin-susceptible Staphylococcus aureus bacteremia were more likely to have treatment failure, compared with those who received cefazolin, a retrospective observational study found.
“To date, no study has specifically evaluated cefazolin versus ceftriaxone for MSSA bacteremia,” Dustin Carr, Pharm.D., said at an annual scientific meeting on infectious diseases. “The main design of this study was to inform our current site and give us additional antimicrobial stewardship opportunities to ensure appropriate selection, dosing, route, and duration.”
In a study conducted during Dr. Carr’s residency training at Louis Stokes Cleveland VA Medical Center, he and his colleagues retrospectively evaluated veteran patients with MSSA bacteremia from January 2009 to August 2014 who received at least 14 days of parenteral cefazolin or ceftriaxone. The use of concomitant anti-staphylococcal agents were excluded, as were patients with polymicrobial infections and those who received empiric antibiotics greater than 72 hours after cultures were finalized. Dr. Carr noted that infectious diseases clinicians are consulted for all cases of MSSA bacteremia at the Louis Stokes Cleveland VA Medical Center, which accounts for about 25% of all outpatient parenteral antimicrobial therapy cases.
Treatment failure was defined as unplanned extension of parenteral antimicrobial therapy, failure to complete a course of parenteral therapy, relapse or recurrence of infection within 90 days, addition of suppressive oral antimicrobial therapy, readmission or unanticipated surgical intervention within 90 days, and being lost to follow-up. Secondary outcomes were relapse and recurrence within 90 days of treatment, overall 90-day mortality, rate of Clostridium difficile infection within 90 days, adverse drug reactions, and cost of IV antimicrobials. The researchers used logistic regression to assess variables of treatment failure.
Dr. Carr, who is currently an infectious diseases specialty resident at Wake Forest Baptist Health, Winston-Salem, N.C., reported results from 71 patients: 38 who received cefazolin (the cefazolin group) and 33 who received ceftriaxone (the ceftriaxone group). Patients in the cefazolin group were more likely to be on dialysis, compared with those in the ceftriaxone group (24% vs. 0%, respectively; P = .003) and they were more likely to have a prosthesis (53% vs. 24%; P = .015).
The researchers observed no significant differences in the comorbidities between the two groups, but there was more IV drug use in the cefazolin group (13% vs. 0%). The primary source of infection did not differ between the groups, but SSTIs were more likely to be treated with ceftriaxone, compared with cefazolin (33% vs. 8%; P = .086).
Dr. Carr reported that 28.9% of patients in the cefazolin group experienced a treatment failure, compared with 54.5% of those in the ceftriaxone group, a difference that was statistically significant (P = .029). There were no significant differences in the rate of most secondary outcomes between the two groups, with the exception of cost and loss to follow-up. The mean cost of IV therapy per patient was $746.51 in the cefazolin group, compared with $60.30 in the ceftriaxone group, while 5.3% of patients in the cefazolin group were lost to follow-up, compared with 12.1% in the ceftriaxone group.
By setting of outpatient parenteral antibiotic therapy, treatment failure occurred most often in the community skilled nursing facility setting (71%), followed by home settings (41%) and the VA long-term care facility attached to Louis Stokes Cleveland VA Medical Center (17%).
On logistic regression, the only significant predictors of treatment failure among all patients were duration of IV therapy (OR 1.05; P = .015), having heart failure (OR 7.93; P less than .001), as well as being treated in an outside community skilled nursing facility, compared with being treated at the long-term care setting attached to Louis Stokes Cleveland VA Medical Center (P = .008).
Dr. Carr acknowledged certain limitations of the study, including its retrospective design and the fact that it lacked data on antimicrobial susceptibilities and minimum inhibitory concentrations. Other limitations, he said, were that patients were allowed to receive cefazolin prior to ceftriaxone use and that there were low frequencies of secondary outcomes.
IDWeek marks the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. The researchers reported having no financial disclosures.
AT IDWEEK 2015
Key clinical point: In a veteran population, ceftriaxone had a higher rate of treatment failure than did cefazolin for parenteral treatment of MSSA infection.
Major finding: More than one-fourth of patients in the cefazolin group (28.9%) experienced a treatment failure, compared with 54.5% of those in the ceftriaxone group (P = .029).
Data source: A retrospective, observational analysis of 71patients with MSSA bacteremia who received at least 14 days of parenteral cefazolin or ceftriaxone.
Disclosures: The researchers reported having no financial disclosures.
Invasive candidiasis hospitalizations down overall
SAN DIEGO – The incidence of hospitalizations associated with invasive candidiasis decreased between 2007 and 2012, but both elderly and black patients remain at greatest risk for the infection, according to an analysis of national data.
“It’s been noted previously that the incidence of neonatal candidiasis seems to be going down, but we wanted to focus on older populations,” Sara Strollo, M.P.H., a trainee in the division of intramural research at the National Institute of Allergy and Infectious Diseases, Rockville., Md., said in an interview at an annual scientific meeting on infectious diseases.
For the study, which is the first of its kind, Ms. Strollo and her associates analyzed data from the State Inpatient Database from the Agency for Healthcare Research and Quality, which represents 97% of all community hospital discharges. They excluded neonatal cases.
The age-adjusted annual incidence of hospitalizations associated with invasive candidiasis ranged from 4.3 to 6.0 per 100,000 persons between 2002 and 2012. The incidence increased from 2002-2005, was stable through 2007, and decreased significantly between 2007 and 2012 -- by 6.7% among men and by 7.4% among women.
The highest incidence of hospitalization for invasive candidiasis occurred among the oldest age groups and among men. For example, compared with persons aged 50-64, the average annual incidence among those over age 80 years old was 2.6-fold higher among women (7.6 vs. 19.7 per 100,000 persons) and 3.9-fold higher among men (7.6 vs. 30 per 100,000 persons).
The researchers also found that among persons older than 50 years of age, black men and women had more than a two-fold higher incidence, compared with white men and women (23.7 vs. 11.7 per 100,000 persons and 22 vs. 10.4 per 100,000 persons, respectively).
During the overall study period, Ms. Strollo and her associates observed a nearly three-fold variation in the average annual incidence of hospital discharges for candidiasis per 100,000 persons, from 2.7 in Oregon to 7.2 in Florida. States with the highest incidence were Florida, Maryland, Missouri, Michigan, California, and Texas, but temporal trends were similar across states and no clear regional patterns among states were observed.
The investigators limited their analysis to 24 states with continuous reporting from 2002 through 2012, which represents 65% of the United States population. The researchers extracted records for discharges where ICD-9 codes for invasive candidiasis were listed in the primary or secondary discharge fields, including disseminated candidiasis (112.5), candidal endocarditis (112.81), and candidal meningitis (112.83). Age, gender, hospitalization year, and state data were extracted, and U.S. Census Bureau data were used as the denominator for state hospitalization incidence and trends. Poisson regression was used to assess significance of trends.
IDWeek marks the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. The study was supported by a training grant from the National Institute of Child Health and Human Development. The researchers reported having no financial disclosures.
SAN DIEGO – The incidence of hospitalizations associated with invasive candidiasis decreased between 2007 and 2012, but both elderly and black patients remain at greatest risk for the infection, according to an analysis of national data.
“It’s been noted previously that the incidence of neonatal candidiasis seems to be going down, but we wanted to focus on older populations,” Sara Strollo, M.P.H., a trainee in the division of intramural research at the National Institute of Allergy and Infectious Diseases, Rockville., Md., said in an interview at an annual scientific meeting on infectious diseases.
For the study, which is the first of its kind, Ms. Strollo and her associates analyzed data from the State Inpatient Database from the Agency for Healthcare Research and Quality, which represents 97% of all community hospital discharges. They excluded neonatal cases.
The age-adjusted annual incidence of hospitalizations associated with invasive candidiasis ranged from 4.3 to 6.0 per 100,000 persons between 2002 and 2012. The incidence increased from 2002-2005, was stable through 2007, and decreased significantly between 2007 and 2012 -- by 6.7% among men and by 7.4% among women.
The highest incidence of hospitalization for invasive candidiasis occurred among the oldest age groups and among men. For example, compared with persons aged 50-64, the average annual incidence among those over age 80 years old was 2.6-fold higher among women (7.6 vs. 19.7 per 100,000 persons) and 3.9-fold higher among men (7.6 vs. 30 per 100,000 persons).
The researchers also found that among persons older than 50 years of age, black men and women had more than a two-fold higher incidence, compared with white men and women (23.7 vs. 11.7 per 100,000 persons and 22 vs. 10.4 per 100,000 persons, respectively).
During the overall study period, Ms. Strollo and her associates observed a nearly three-fold variation in the average annual incidence of hospital discharges for candidiasis per 100,000 persons, from 2.7 in Oregon to 7.2 in Florida. States with the highest incidence were Florida, Maryland, Missouri, Michigan, California, and Texas, but temporal trends were similar across states and no clear regional patterns among states were observed.
The investigators limited their analysis to 24 states with continuous reporting from 2002 through 2012, which represents 65% of the United States population. The researchers extracted records for discharges where ICD-9 codes for invasive candidiasis were listed in the primary or secondary discharge fields, including disseminated candidiasis (112.5), candidal endocarditis (112.81), and candidal meningitis (112.83). Age, gender, hospitalization year, and state data were extracted, and U.S. Census Bureau data were used as the denominator for state hospitalization incidence and trends. Poisson regression was used to assess significance of trends.
IDWeek marks the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. The study was supported by a training grant from the National Institute of Child Health and Human Development. The researchers reported having no financial disclosures.
SAN DIEGO – The incidence of hospitalizations associated with invasive candidiasis decreased between 2007 and 2012, but both elderly and black patients remain at greatest risk for the infection, according to an analysis of national data.
“It’s been noted previously that the incidence of neonatal candidiasis seems to be going down, but we wanted to focus on older populations,” Sara Strollo, M.P.H., a trainee in the division of intramural research at the National Institute of Allergy and Infectious Diseases, Rockville., Md., said in an interview at an annual scientific meeting on infectious diseases.
For the study, which is the first of its kind, Ms. Strollo and her associates analyzed data from the State Inpatient Database from the Agency for Healthcare Research and Quality, which represents 97% of all community hospital discharges. They excluded neonatal cases.
The age-adjusted annual incidence of hospitalizations associated with invasive candidiasis ranged from 4.3 to 6.0 per 100,000 persons between 2002 and 2012. The incidence increased from 2002-2005, was stable through 2007, and decreased significantly between 2007 and 2012 -- by 6.7% among men and by 7.4% among women.
The highest incidence of hospitalization for invasive candidiasis occurred among the oldest age groups and among men. For example, compared with persons aged 50-64, the average annual incidence among those over age 80 years old was 2.6-fold higher among women (7.6 vs. 19.7 per 100,000 persons) and 3.9-fold higher among men (7.6 vs. 30 per 100,000 persons).
The researchers also found that among persons older than 50 years of age, black men and women had more than a two-fold higher incidence, compared with white men and women (23.7 vs. 11.7 per 100,000 persons and 22 vs. 10.4 per 100,000 persons, respectively).
During the overall study period, Ms. Strollo and her associates observed a nearly three-fold variation in the average annual incidence of hospital discharges for candidiasis per 100,000 persons, from 2.7 in Oregon to 7.2 in Florida. States with the highest incidence were Florida, Maryland, Missouri, Michigan, California, and Texas, but temporal trends were similar across states and no clear regional patterns among states were observed.
The investigators limited their analysis to 24 states with continuous reporting from 2002 through 2012, which represents 65% of the United States population. The researchers extracted records for discharges where ICD-9 codes for invasive candidiasis were listed in the primary or secondary discharge fields, including disseminated candidiasis (112.5), candidal endocarditis (112.81), and candidal meningitis (112.83). Age, gender, hospitalization year, and state data were extracted, and U.S. Census Bureau data were used as the denominator for state hospitalization incidence and trends. Poisson regression was used to assess significance of trends.
IDWeek marks the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. The study was supported by a training grant from the National Institute of Child Health and Human Development. The researchers reported having no financial disclosures.
AT IDWEEK 2015
Key clinical point: As of 2007, the incidence of hospital-associated invasive candidiasis appears to be decreasing.
Major finding: Between 2007 and 2012, the age-adjusted annual incidence of hospitalizations associated with invasive candidiasis in the United States decreased by 6.7% among men and by 7.4% among women.
Data source: A long-term analysis of data from the State Inpatient Database from the Agency for Healthcare Research and Quality.
Disclosures: The researchers reported having no financial disclosures.
Staphylococcus aureus vaccine tolerable, immunogenic in preliminary study
SAN DIEGO – A vaccine designed to lower the risk of postoperative Staphylococcus aureus infections is being tested in a phase IIb study of adults who are preparing to undergo elective spinal fusion surgery.
In phase I-II trial results, reported at an annual scientific meeting on infectious diseases, the vaccine proved to be well tolerated, and it induced durable functional antibody responses.
The phase IIb trial aims to enroll 2,600 patients undergoing elective spinal fusion surgery, and is scheduled to end in 2017, said Dr. Buddy Creech of Vanderbilt University in Nashville, Tenn.
“We’re at a point where the disease frequency of Staphylococcus aureus is high enough that we could make the case for universal vaccination,” said Dr. Creech. “If we can prove it in high-risk hosts, I think that’s a win.”
Invasive staphylococcal disease causes more deaths in the United States than AIDS, viral hepatitis, and tuberculosis combined, but the complex virulence factors of S. aureus have eluded vaccine researchers for years. Other investigational vaccines failed in previous large phase III trials. The tetravalent vaccine now being tested targets type 5 and 8 capsular polysaccharides (CP5 and CP8), which are expressed by 95% of hospital-associated S. aureus strains; an adhesion molecule; and an essential recombinant manganese transport protein C.
The vaccine was well tolerated in a phase I study of healthy adults earlier this year. Single doses of vaccine or placebo were administered to 285 healthy adults aged 65-84 years in a randomized, multicenter double-blind trial. Patients averaged 71 years in age, and half were women, Dr. Creech said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Similar rates of mild to moderate adverse events occurred in both groups, and no participants died or had serious vaccine-related side effects, Dr. Creech said.
At 1 month after vaccination, all tetravalent vaccine recipients and 5% of placebo recipients had achieved a predetermined threshold for CP5 opsonophagocytic activity. Likewise, about 92% of vaccinated recipients and 19% of placebo recipients achieved the CP8 opsonophagocytic activity threshold. Further, 84% of vaccinated recipients and none of the placebo recipients had at least a fourfold rise in antibody titers against the adhesion molecule.
In vaccine recipients, an immunoassay found substantial rises in antibodies against all four antigens by day 8; levels peaked around day 15, Dr. Creech said. “There was a decline of antibody titers over the course of the next year, as one would expect with a single-dose vaccine, but they remained over prespecified thresholds,” he added.
“Durability of response is especially important for patients who are scheduling surgeries and others who have a definitive risk period” for infection, he said.
Pfizer is funding the research, and Dr. Creech is a grant investigator for the company. One coauthor is also a grant investigator for Pfizer, nine coauthors are Pfizer employees and shareholders, and three declared no relevant conflicts of interest.
SAN DIEGO – A vaccine designed to lower the risk of postoperative Staphylococcus aureus infections is being tested in a phase IIb study of adults who are preparing to undergo elective spinal fusion surgery.
In phase I-II trial results, reported at an annual scientific meeting on infectious diseases, the vaccine proved to be well tolerated, and it induced durable functional antibody responses.
The phase IIb trial aims to enroll 2,600 patients undergoing elective spinal fusion surgery, and is scheduled to end in 2017, said Dr. Buddy Creech of Vanderbilt University in Nashville, Tenn.
“We’re at a point where the disease frequency of Staphylococcus aureus is high enough that we could make the case for universal vaccination,” said Dr. Creech. “If we can prove it in high-risk hosts, I think that’s a win.”
Invasive staphylococcal disease causes more deaths in the United States than AIDS, viral hepatitis, and tuberculosis combined, but the complex virulence factors of S. aureus have eluded vaccine researchers for years. Other investigational vaccines failed in previous large phase III trials. The tetravalent vaccine now being tested targets type 5 and 8 capsular polysaccharides (CP5 and CP8), which are expressed by 95% of hospital-associated S. aureus strains; an adhesion molecule; and an essential recombinant manganese transport protein C.
The vaccine was well tolerated in a phase I study of healthy adults earlier this year. Single doses of vaccine or placebo were administered to 285 healthy adults aged 65-84 years in a randomized, multicenter double-blind trial. Patients averaged 71 years in age, and half were women, Dr. Creech said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Similar rates of mild to moderate adverse events occurred in both groups, and no participants died or had serious vaccine-related side effects, Dr. Creech said.
At 1 month after vaccination, all tetravalent vaccine recipients and 5% of placebo recipients had achieved a predetermined threshold for CP5 opsonophagocytic activity. Likewise, about 92% of vaccinated recipients and 19% of placebo recipients achieved the CP8 opsonophagocytic activity threshold. Further, 84% of vaccinated recipients and none of the placebo recipients had at least a fourfold rise in antibody titers against the adhesion molecule.
In vaccine recipients, an immunoassay found substantial rises in antibodies against all four antigens by day 8; levels peaked around day 15, Dr. Creech said. “There was a decline of antibody titers over the course of the next year, as one would expect with a single-dose vaccine, but they remained over prespecified thresholds,” he added.
“Durability of response is especially important for patients who are scheduling surgeries and others who have a definitive risk period” for infection, he said.
Pfizer is funding the research, and Dr. Creech is a grant investigator for the company. One coauthor is also a grant investigator for Pfizer, nine coauthors are Pfizer employees and shareholders, and three declared no relevant conflicts of interest.
SAN DIEGO – A vaccine designed to lower the risk of postoperative Staphylococcus aureus infections is being tested in a phase IIb study of adults who are preparing to undergo elective spinal fusion surgery.
In phase I-II trial results, reported at an annual scientific meeting on infectious diseases, the vaccine proved to be well tolerated, and it induced durable functional antibody responses.
The phase IIb trial aims to enroll 2,600 patients undergoing elective spinal fusion surgery, and is scheduled to end in 2017, said Dr. Buddy Creech of Vanderbilt University in Nashville, Tenn.
“We’re at a point where the disease frequency of Staphylococcus aureus is high enough that we could make the case for universal vaccination,” said Dr. Creech. “If we can prove it in high-risk hosts, I think that’s a win.”
Invasive staphylococcal disease causes more deaths in the United States than AIDS, viral hepatitis, and tuberculosis combined, but the complex virulence factors of S. aureus have eluded vaccine researchers for years. Other investigational vaccines failed in previous large phase III trials. The tetravalent vaccine now being tested targets type 5 and 8 capsular polysaccharides (CP5 and CP8), which are expressed by 95% of hospital-associated S. aureus strains; an adhesion molecule; and an essential recombinant manganese transport protein C.
The vaccine was well tolerated in a phase I study of healthy adults earlier this year. Single doses of vaccine or placebo were administered to 285 healthy adults aged 65-84 years in a randomized, multicenter double-blind trial. Patients averaged 71 years in age, and half were women, Dr. Creech said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Similar rates of mild to moderate adverse events occurred in both groups, and no participants died or had serious vaccine-related side effects, Dr. Creech said.
At 1 month after vaccination, all tetravalent vaccine recipients and 5% of placebo recipients had achieved a predetermined threshold for CP5 opsonophagocytic activity. Likewise, about 92% of vaccinated recipients and 19% of placebo recipients achieved the CP8 opsonophagocytic activity threshold. Further, 84% of vaccinated recipients and none of the placebo recipients had at least a fourfold rise in antibody titers against the adhesion molecule.
In vaccine recipients, an immunoassay found substantial rises in antibodies against all four antigens by day 8; levels peaked around day 15, Dr. Creech said. “There was a decline of antibody titers over the course of the next year, as one would expect with a single-dose vaccine, but they remained over prespecified thresholds,” he added.
“Durability of response is especially important for patients who are scheduling surgeries and others who have a definitive risk period” for infection, he said.
Pfizer is funding the research, and Dr. Creech is a grant investigator for the company. One coauthor is also a grant investigator for Pfizer, nine coauthors are Pfizer employees and shareholders, and three declared no relevant conflicts of interest.
AT IDWEEK 2015
Key clinical point: A four-antigen vaccine against Staphylococcus aureus yielded durable functional antibody responses and no major safety signals in a phase I-II trial.
Major finding: There were no vaccine-related serious adverse effects and no signs of dose-related reactogenicity, and researchers observed sustained bacterial killing at month 12.
Data source: A multicenter, randomized, double-blind placebo-controlled trial of 285 healthy adults aged 65-84 years.
Disclosures: Pfizer is funding the research. Dr. Creech is a grant investigator for the company. One coauthor is also a grant investigator for Pfizer, nine coauthors are Pfizer employees and shareholders, and three declared no relevant conflicts of interest.
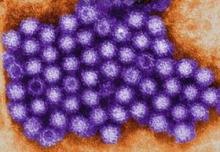
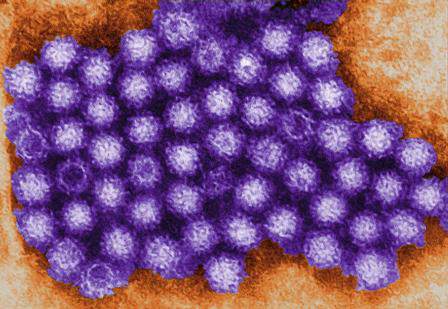
Norovirus sends 1.6 million to doctors every year
SAN DIEGO – Norovirus infections send about 1.6 million people to the doctor every year in the United States, according to the first active surveillance study to cover all age groups here.
“This provides a baseline burden of norovirus disease to assess the potential impact of future vaccines,” said Dr. Aron Hall of the Centers for Disease Control and Prevention in Atlanta. Estimated infection rates generally resembled those from past studies, but were more robust and granular, Dr. Hall said at an annual scientific meeting on infectious diseases.
About 19-21 million Americans suffer acute gastroenteritis because of norovirus infection every year, Dr. Hall noted. About 400,000 of these patients seek urgent care, at least 56,000 are hospitalized, and 570-800 ultimately die from associated complications. However, several factors have impeded large epidemiologic studies of norovirus disease, he added. Most infected patients do not seek care, those who do often do not undergo stool testing, there is no national norovirus case reporting system, turnaround times and the sensitivity of clinical assays remain suboptimal, and there are no specific ICD codes for norovirus gastroenteritis, he said.
To overcome these obstacles, Dr. Hall and his associates spent a year studying 480,000 Kaiser Permanente Northwest enrollees in the Portland, Ore. area. Enrollees seek care almost entirely in-network and demographically resemble other residents in the region, Dr. Hall said. The researchers used automated software to identify about 19,000 patients with ICD-9 codes for acute gastroenteritis. They called about half of these patients to request stool samples within 3 days of their appointments, while they were likely to still be shedding pathogens. They aimed to test all patients who were younger than 5 years or older than 65 years, and called a random sample of 35% of the other patients.
In all, the researchers tested stool specimens for 84% (1,467) of patients whom they reached within the 3-day window, said Dr. Hall. About 13% of these patients tested positive for norovirus, of which 85% were genotype II, while the rest were genotype I. The overall incidence of medically attended acute gastroenteritis due to norovirus was 5 cases per 100,00 person-years, but rates were four to five times higher among infants and children under the age of 2 years (22.7 and 29.1 cases per 100,000 person-years, respectively), and about 50% higher among adults aged 85 years and older (7.4 cases per 100,000 person-years).
“Extrapolation to the U.S. population would yield 1.6 million norovirus-associated MAAGE [medically attended acute gastroenteritis] encounters per year,” Dr. Hall concluded. The research team is now sequencing norovirus genotypes, testing stool samples for other enteric pathogens, and studying high-risk subgroups, clinical severity, and cases of potential household transmission, he added.
Dr. Hall spoke at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. He had no disclosures. One of his coauthors reported receiving research support from GlaxoSmithKline.
SAN DIEGO – Norovirus infections send about 1.6 million people to the doctor every year in the United States, according to the first active surveillance study to cover all age groups here.
“This provides a baseline burden of norovirus disease to assess the potential impact of future vaccines,” said Dr. Aron Hall of the Centers for Disease Control and Prevention in Atlanta. Estimated infection rates generally resembled those from past studies, but were more robust and granular, Dr. Hall said at an annual scientific meeting on infectious diseases.
About 19-21 million Americans suffer acute gastroenteritis because of norovirus infection every year, Dr. Hall noted. About 400,000 of these patients seek urgent care, at least 56,000 are hospitalized, and 570-800 ultimately die from associated complications. However, several factors have impeded large epidemiologic studies of norovirus disease, he added. Most infected patients do not seek care, those who do often do not undergo stool testing, there is no national norovirus case reporting system, turnaround times and the sensitivity of clinical assays remain suboptimal, and there are no specific ICD codes for norovirus gastroenteritis, he said.
To overcome these obstacles, Dr. Hall and his associates spent a year studying 480,000 Kaiser Permanente Northwest enrollees in the Portland, Ore. area. Enrollees seek care almost entirely in-network and demographically resemble other residents in the region, Dr. Hall said. The researchers used automated software to identify about 19,000 patients with ICD-9 codes for acute gastroenteritis. They called about half of these patients to request stool samples within 3 days of their appointments, while they were likely to still be shedding pathogens. They aimed to test all patients who were younger than 5 years or older than 65 years, and called a random sample of 35% of the other patients.
In all, the researchers tested stool specimens for 84% (1,467) of patients whom they reached within the 3-day window, said Dr. Hall. About 13% of these patients tested positive for norovirus, of which 85% were genotype II, while the rest were genotype I. The overall incidence of medically attended acute gastroenteritis due to norovirus was 5 cases per 100,00 person-years, but rates were four to five times higher among infants and children under the age of 2 years (22.7 and 29.1 cases per 100,000 person-years, respectively), and about 50% higher among adults aged 85 years and older (7.4 cases per 100,000 person-years).
“Extrapolation to the U.S. population would yield 1.6 million norovirus-associated MAAGE [medically attended acute gastroenteritis] encounters per year,” Dr. Hall concluded. The research team is now sequencing norovirus genotypes, testing stool samples for other enteric pathogens, and studying high-risk subgroups, clinical severity, and cases of potential household transmission, he added.
Dr. Hall spoke at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. He had no disclosures. One of his coauthors reported receiving research support from GlaxoSmithKline.
SAN DIEGO – Norovirus infections send about 1.6 million people to the doctor every year in the United States, according to the first active surveillance study to cover all age groups here.
“This provides a baseline burden of norovirus disease to assess the potential impact of future vaccines,” said Dr. Aron Hall of the Centers for Disease Control and Prevention in Atlanta. Estimated infection rates generally resembled those from past studies, but were more robust and granular, Dr. Hall said at an annual scientific meeting on infectious diseases.
About 19-21 million Americans suffer acute gastroenteritis because of norovirus infection every year, Dr. Hall noted. About 400,000 of these patients seek urgent care, at least 56,000 are hospitalized, and 570-800 ultimately die from associated complications. However, several factors have impeded large epidemiologic studies of norovirus disease, he added. Most infected patients do not seek care, those who do often do not undergo stool testing, there is no national norovirus case reporting system, turnaround times and the sensitivity of clinical assays remain suboptimal, and there are no specific ICD codes for norovirus gastroenteritis, he said.
To overcome these obstacles, Dr. Hall and his associates spent a year studying 480,000 Kaiser Permanente Northwest enrollees in the Portland, Ore. area. Enrollees seek care almost entirely in-network and demographically resemble other residents in the region, Dr. Hall said. The researchers used automated software to identify about 19,000 patients with ICD-9 codes for acute gastroenteritis. They called about half of these patients to request stool samples within 3 days of their appointments, while they were likely to still be shedding pathogens. They aimed to test all patients who were younger than 5 years or older than 65 years, and called a random sample of 35% of the other patients.
In all, the researchers tested stool specimens for 84% (1,467) of patients whom they reached within the 3-day window, said Dr. Hall. About 13% of these patients tested positive for norovirus, of which 85% were genotype II, while the rest were genotype I. The overall incidence of medically attended acute gastroenteritis due to norovirus was 5 cases per 100,00 person-years, but rates were four to five times higher among infants and children under the age of 2 years (22.7 and 29.1 cases per 100,000 person-years, respectively), and about 50% higher among adults aged 85 years and older (7.4 cases per 100,000 person-years).
“Extrapolation to the U.S. population would yield 1.6 million norovirus-associated MAAGE [medically attended acute gastroenteritis] encounters per year,” Dr. Hall concluded. The research team is now sequencing norovirus genotypes, testing stool samples for other enteric pathogens, and studying high-risk subgroups, clinical severity, and cases of potential household transmission, he added.
Dr. Hall spoke at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. He had no disclosures. One of his coauthors reported receiving research support from GlaxoSmithKline.
AT IDWEEK 2015
Key clinical point: Norovirus infection is a common cause of medically attended acute gastroenteritis.
Major finding: Every year, about 1.6 million patients in the United States seek medical care for norovirus-associated MAAGE.
Data source: Active surveillance of 480,000 health maintenance organization enrollees in the urban Pacific Northwest between March 2014 and April 2015.
Disclosures: Dr. Hall had no disclosures. A coauthor reported receiving research support from GlaxoSmithKline.
Duodenoscopes often cultured high-concern organisms
High-concern organisms were cultured from 92% of duodenoscopes immediately after endoscopic retrograde cholangiopancreatography (ERCP), and from 17% of scopes after reprocessing, based on a small single-center study reported at an annual scientific meeting on infectious diseases.
“We think the high percentage of high-concern organisms is key to highlight, because it shows the importance of vigilant reprocessing techniques,” said Dr. Michaela Gazdik, who led the study at Intermountain Healthcare in Salt Lake City. A 17% prevalence of clinically significant residual contamination after reprocessing “aligns with reports from the literature, indicating that reprocessing protocols are likely effective,” she added.
But “no growth” on a quantitative plate does not necessarily mean there are no microbes on a duodenoscope, Dr. Gazdik emphasized. Swabbing the duodenoscope, and particularly the notoriously hard to clean elevator mechanism, could improve sampling, she said.
In 2013 and 2014, the FDA received reports of about 135 multidrug resistant infections acquired after ERCP. “After investigation, many of these outbreaks were attributed to the duodenoscope’s elevator mechanism,” Dr. Gazdik noted. The elevator mechanism contains microscopic crevices that can remain contaminated even after cleaning with a manual brush as recommended by manufacturers. To study the efficacy of their reprocessing system, Dr. Gazdik and her associates cultured 12 scopes immediately after ERCP and again after reprocessing. They also took conducted surveillance cultures of 11 scopes stored at other Intermountain Healthcare facilities.
Immediately after ERCP, 91.7% (11 of 12) scopes cultured out high-concern organisms, including Pseudomonas aeruginosa, Enterococcus species, Klebsiella pneumoniae, Enterobacter asburiae, and E. cloacae, said Dr. Gazdik. Three-quarters of the scopes also yielded gram-negative organisms. No organisms were resistant to carbapenem or vancomycin, based on testing with selective media.
After reprocessing, 17% of the scopes still yielded high-concern organisms, and 9% had more quantitative growth than desired, Dr. Gazdik reported. “We did tell our reprocessing departments that their protocols seem to be as effective as others out there,” she said. “But two scopes showed growth in broth that matched the pre-reprocessing growth, indicating it was left over after reprocessing. We thought that was an interesting result, because the interim CDC guidance does not include a swab portion.”
To culture the scopes, investigators flushed 5 mL of sterile saline through the elevator channel, brushed the cantilevered elevator mechanism in both the up and down positions, and rapidly swirled the brush in the flush saline. They performed quantitative colony counts by serially diluting the collected saline. For the swab-broth culture, they passed a sterile swab over and under the distal elevator mechanism and inoculating the swab into 5 mL tryptic soy broth. They also subcultured growth from the broth to identify bacteria.
Surveillance cultures of the 11 stored scopes identified no organisms from quantitative plate counts, but swab-inoculated broth cultures grew Micrococcus from three of 11 scopes, and non-CRE [carbapenem-resistant Enterobacteriaceae] K. pneumonia from one scope, Dr. Gazdik also reported. “Routine cultures play a role, mainly to continue heightened vigilance during reprocessing,” she said. “The sampling method we are going to be using will be simplified from the CDC procedure so that we can train endoscopy technicians to do it and then send samples to us.”
Dr. Gazdik and her coauthors reported no funding sources and had no conflicts of interest.
High-concern organisms were cultured from 92% of duodenoscopes immediately after endoscopic retrograde cholangiopancreatography (ERCP), and from 17% of scopes after reprocessing, based on a small single-center study reported at an annual scientific meeting on infectious diseases.
“We think the high percentage of high-concern organisms is key to highlight, because it shows the importance of vigilant reprocessing techniques,” said Dr. Michaela Gazdik, who led the study at Intermountain Healthcare in Salt Lake City. A 17% prevalence of clinically significant residual contamination after reprocessing “aligns with reports from the literature, indicating that reprocessing protocols are likely effective,” she added.
But “no growth” on a quantitative plate does not necessarily mean there are no microbes on a duodenoscope, Dr. Gazdik emphasized. Swabbing the duodenoscope, and particularly the notoriously hard to clean elevator mechanism, could improve sampling, she said.
In 2013 and 2014, the FDA received reports of about 135 multidrug resistant infections acquired after ERCP. “After investigation, many of these outbreaks were attributed to the duodenoscope’s elevator mechanism,” Dr. Gazdik noted. The elevator mechanism contains microscopic crevices that can remain contaminated even after cleaning with a manual brush as recommended by manufacturers. To study the efficacy of their reprocessing system, Dr. Gazdik and her associates cultured 12 scopes immediately after ERCP and again after reprocessing. They also took conducted surveillance cultures of 11 scopes stored at other Intermountain Healthcare facilities.
Immediately after ERCP, 91.7% (11 of 12) scopes cultured out high-concern organisms, including Pseudomonas aeruginosa, Enterococcus species, Klebsiella pneumoniae, Enterobacter asburiae, and E. cloacae, said Dr. Gazdik. Three-quarters of the scopes also yielded gram-negative organisms. No organisms were resistant to carbapenem or vancomycin, based on testing with selective media.
After reprocessing, 17% of the scopes still yielded high-concern organisms, and 9% had more quantitative growth than desired, Dr. Gazdik reported. “We did tell our reprocessing departments that their protocols seem to be as effective as others out there,” she said. “But two scopes showed growth in broth that matched the pre-reprocessing growth, indicating it was left over after reprocessing. We thought that was an interesting result, because the interim CDC guidance does not include a swab portion.”
To culture the scopes, investigators flushed 5 mL of sterile saline through the elevator channel, brushed the cantilevered elevator mechanism in both the up and down positions, and rapidly swirled the brush in the flush saline. They performed quantitative colony counts by serially diluting the collected saline. For the swab-broth culture, they passed a sterile swab over and under the distal elevator mechanism and inoculating the swab into 5 mL tryptic soy broth. They also subcultured growth from the broth to identify bacteria.
Surveillance cultures of the 11 stored scopes identified no organisms from quantitative plate counts, but swab-inoculated broth cultures grew Micrococcus from three of 11 scopes, and non-CRE [carbapenem-resistant Enterobacteriaceae] K. pneumonia from one scope, Dr. Gazdik also reported. “Routine cultures play a role, mainly to continue heightened vigilance during reprocessing,” she said. “The sampling method we are going to be using will be simplified from the CDC procedure so that we can train endoscopy technicians to do it and then send samples to us.”
Dr. Gazdik and her coauthors reported no funding sources and had no conflicts of interest.
High-concern organisms were cultured from 92% of duodenoscopes immediately after endoscopic retrograde cholangiopancreatography (ERCP), and from 17% of scopes after reprocessing, based on a small single-center study reported at an annual scientific meeting on infectious diseases.
“We think the high percentage of high-concern organisms is key to highlight, because it shows the importance of vigilant reprocessing techniques,” said Dr. Michaela Gazdik, who led the study at Intermountain Healthcare in Salt Lake City. A 17% prevalence of clinically significant residual contamination after reprocessing “aligns with reports from the literature, indicating that reprocessing protocols are likely effective,” she added.
But “no growth” on a quantitative plate does not necessarily mean there are no microbes on a duodenoscope, Dr. Gazdik emphasized. Swabbing the duodenoscope, and particularly the notoriously hard to clean elevator mechanism, could improve sampling, she said.
In 2013 and 2014, the FDA received reports of about 135 multidrug resistant infections acquired after ERCP. “After investigation, many of these outbreaks were attributed to the duodenoscope’s elevator mechanism,” Dr. Gazdik noted. The elevator mechanism contains microscopic crevices that can remain contaminated even after cleaning with a manual brush as recommended by manufacturers. To study the efficacy of their reprocessing system, Dr. Gazdik and her associates cultured 12 scopes immediately after ERCP and again after reprocessing. They also took conducted surveillance cultures of 11 scopes stored at other Intermountain Healthcare facilities.
Immediately after ERCP, 91.7% (11 of 12) scopes cultured out high-concern organisms, including Pseudomonas aeruginosa, Enterococcus species, Klebsiella pneumoniae, Enterobacter asburiae, and E. cloacae, said Dr. Gazdik. Three-quarters of the scopes also yielded gram-negative organisms. No organisms were resistant to carbapenem or vancomycin, based on testing with selective media.
After reprocessing, 17% of the scopes still yielded high-concern organisms, and 9% had more quantitative growth than desired, Dr. Gazdik reported. “We did tell our reprocessing departments that their protocols seem to be as effective as others out there,” she said. “But two scopes showed growth in broth that matched the pre-reprocessing growth, indicating it was left over after reprocessing. We thought that was an interesting result, because the interim CDC guidance does not include a swab portion.”
To culture the scopes, investigators flushed 5 mL of sterile saline through the elevator channel, brushed the cantilevered elevator mechanism in both the up and down positions, and rapidly swirled the brush in the flush saline. They performed quantitative colony counts by serially diluting the collected saline. For the swab-broth culture, they passed a sterile swab over and under the distal elevator mechanism and inoculating the swab into 5 mL tryptic soy broth. They also subcultured growth from the broth to identify bacteria.
Surveillance cultures of the 11 stored scopes identified no organisms from quantitative plate counts, but swab-inoculated broth cultures grew Micrococcus from three of 11 scopes, and non-CRE [carbapenem-resistant Enterobacteriaceae] K. pneumonia from one scope, Dr. Gazdik also reported. “Routine cultures play a role, mainly to continue heightened vigilance during reprocessing,” she said. “The sampling method we are going to be using will be simplified from the CDC procedure so that we can train endoscopy technicians to do it and then send samples to us.”
Dr. Gazdik and her coauthors reported no funding sources and had no conflicts of interest.
AT IDWEEK 2015
Key clinical point: High-concern organisms often contaminated duodenoscopes after ERCP.
Major finding: Investigators cultured these bacteria from 92% of duodenoscopes immediately after ERCP, and from 17% of scopes after reprocessing.
Data source: Cultures of 12 duodenoscopes immediately after ERCP and again after reprocessing, and surveillance cultures of 11 stored duodenoscopes.
Disclosures: Dr. Gazdik and her coauthors reported no funding sources and had no conflicts of interest.
Impact of health care–associated meningitis or ventriculitis spotlighted
SAN DIEGO – Health care–associated meningitis or ventriculitis continues to occur despite well established preventive methods and is associated with significant morbidity and mortality, a long-term analysis at two hospitals showed.
“Health care–associated meningitis or ventriculitis remains challenging for providers in terms of diagnosis, treatment, and prevention,” Dr. Chanunya Srihawan said at an annual scientific meeting on infectious diseases. “Even though there are a lot of well described methods to minimize the risk of infection, we still see patients with health care–associated meningitis or ventriculitis in the hospital, and most of them have a poor outcome.”
Dr. Srihawan, of the division of infectious diseases in the department of internal medicine at the University of Texas, Houston, and her associates set out to describe the clinical characteristics of patients with health care–associated meningitis or ventriculitis, and to identify risk factors associated with clinical outcomes. They examined data from adult and pediatric patients with a diagnosis of health care–associated meningitis or ventriculitis based on the 2015 Centers for Disease Control and Prevention/National Healthcare Safety Network surveillance definition who were treated at two large tertiary care hospitals in Houston from July 2003 to November 2014.
Patients were prospectively identified by infection control clinicians and by screening of all cerebrospinal fluid samples sent to a central laboratory. The researchers collected patient information on demographics, clinical presentations, laboratory results, imaging studies, treatments, and clinical outcomes. They used Pearson chi-square and Fischer’s exact test for bivariate analysis between baseline variables and outcomes, followed by logistic regression analysis with bootstrap.
Dr. Srihawan reported results from 166 adult and 49 pediatric patients. The median age of patients was 45 years, 45% were white, 26% were Hispanic, 20% were African American, and the remainder were from other ethnic groups. The two most common indications for neurosurgical intervention were hemorrhage (49%) and hydrocephalus (48%), followed by trauma (18%), and brain tumor (11%). The top three neurological signs and symptoms reported were headache (48%), changes in mental status (41%), and nausea/vomiting (39%), followed by focal neurological deficits (33%), neck stiffness (19%), seizures (10%), and photophobia (6%). Nearly three quarters of patients (71%) were admitted to the ICU and 43% received mechanical ventilation for a median of 9 days.
A positive cerebrospinal fluid culture was observed in 106 patients (49%), with the majority of the etiologies being Staphylococcus and Gram-negative rods. An adverse clinical outcome occurred in 167 patients (78%) and was defined as death in 20 patients (9%), persistent vegetative state in 31 patients (14%), severe disability in 77 patients (36%), and moderate disability in 39 patients (18%).
Baseline variables associated with adverse clinical outcomes included age 45 years or older (odds ratio, 11.39), CNS bleeding (OR, 4.37), abnormal neurological exam (OR, 6.51), ICU admission (OR, 5.81), and use of mechanical ventilation (OR, 12.59; P less than .001 for all comparisons). Use of a ventriculoperitoneal shunt was found to be a protective variable (OR, 0.17), which, Dr. Srihawan said, could be explained by the fact most patients who received a ventriculoperitoneal shunt were children who had fewer comorbidities “and tended to be less sick.”
After logistic regression, only three variables remained significantly associated with adverse clinical outcomes: age 45 years or older (OR, 6.47), abnormal neurological exam (OR, 3.04), and use of mechanical ventilation (OR, 5.34).
IDWeek marks the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. The researchers reported having no financial disclosures.
SAN DIEGO – Health care–associated meningitis or ventriculitis continues to occur despite well established preventive methods and is associated with significant morbidity and mortality, a long-term analysis at two hospitals showed.
“Health care–associated meningitis or ventriculitis remains challenging for providers in terms of diagnosis, treatment, and prevention,” Dr. Chanunya Srihawan said at an annual scientific meeting on infectious diseases. “Even though there are a lot of well described methods to minimize the risk of infection, we still see patients with health care–associated meningitis or ventriculitis in the hospital, and most of them have a poor outcome.”
Dr. Srihawan, of the division of infectious diseases in the department of internal medicine at the University of Texas, Houston, and her associates set out to describe the clinical characteristics of patients with health care–associated meningitis or ventriculitis, and to identify risk factors associated with clinical outcomes. They examined data from adult and pediatric patients with a diagnosis of health care–associated meningitis or ventriculitis based on the 2015 Centers for Disease Control and Prevention/National Healthcare Safety Network surveillance definition who were treated at two large tertiary care hospitals in Houston from July 2003 to November 2014.
Patients were prospectively identified by infection control clinicians and by screening of all cerebrospinal fluid samples sent to a central laboratory. The researchers collected patient information on demographics, clinical presentations, laboratory results, imaging studies, treatments, and clinical outcomes. They used Pearson chi-square and Fischer’s exact test for bivariate analysis between baseline variables and outcomes, followed by logistic regression analysis with bootstrap.
Dr. Srihawan reported results from 166 adult and 49 pediatric patients. The median age of patients was 45 years, 45% were white, 26% were Hispanic, 20% were African American, and the remainder were from other ethnic groups. The two most common indications for neurosurgical intervention were hemorrhage (49%) and hydrocephalus (48%), followed by trauma (18%), and brain tumor (11%). The top three neurological signs and symptoms reported were headache (48%), changes in mental status (41%), and nausea/vomiting (39%), followed by focal neurological deficits (33%), neck stiffness (19%), seizures (10%), and photophobia (6%). Nearly three quarters of patients (71%) were admitted to the ICU and 43% received mechanical ventilation for a median of 9 days.
A positive cerebrospinal fluid culture was observed in 106 patients (49%), with the majority of the etiologies being Staphylococcus and Gram-negative rods. An adverse clinical outcome occurred in 167 patients (78%) and was defined as death in 20 patients (9%), persistent vegetative state in 31 patients (14%), severe disability in 77 patients (36%), and moderate disability in 39 patients (18%).
Baseline variables associated with adverse clinical outcomes included age 45 years or older (odds ratio, 11.39), CNS bleeding (OR, 4.37), abnormal neurological exam (OR, 6.51), ICU admission (OR, 5.81), and use of mechanical ventilation (OR, 12.59; P less than .001 for all comparisons). Use of a ventriculoperitoneal shunt was found to be a protective variable (OR, 0.17), which, Dr. Srihawan said, could be explained by the fact most patients who received a ventriculoperitoneal shunt were children who had fewer comorbidities “and tended to be less sick.”
After logistic regression, only three variables remained significantly associated with adverse clinical outcomes: age 45 years or older (OR, 6.47), abnormal neurological exam (OR, 3.04), and use of mechanical ventilation (OR, 5.34).
IDWeek marks the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. The researchers reported having no financial disclosures.
SAN DIEGO – Health care–associated meningitis or ventriculitis continues to occur despite well established preventive methods and is associated with significant morbidity and mortality, a long-term analysis at two hospitals showed.
“Health care–associated meningitis or ventriculitis remains challenging for providers in terms of diagnosis, treatment, and prevention,” Dr. Chanunya Srihawan said at an annual scientific meeting on infectious diseases. “Even though there are a lot of well described methods to minimize the risk of infection, we still see patients with health care–associated meningitis or ventriculitis in the hospital, and most of them have a poor outcome.”
Dr. Srihawan, of the division of infectious diseases in the department of internal medicine at the University of Texas, Houston, and her associates set out to describe the clinical characteristics of patients with health care–associated meningitis or ventriculitis, and to identify risk factors associated with clinical outcomes. They examined data from adult and pediatric patients with a diagnosis of health care–associated meningitis or ventriculitis based on the 2015 Centers for Disease Control and Prevention/National Healthcare Safety Network surveillance definition who were treated at two large tertiary care hospitals in Houston from July 2003 to November 2014.
Patients were prospectively identified by infection control clinicians and by screening of all cerebrospinal fluid samples sent to a central laboratory. The researchers collected patient information on demographics, clinical presentations, laboratory results, imaging studies, treatments, and clinical outcomes. They used Pearson chi-square and Fischer’s exact test for bivariate analysis between baseline variables and outcomes, followed by logistic regression analysis with bootstrap.
Dr. Srihawan reported results from 166 adult and 49 pediatric patients. The median age of patients was 45 years, 45% were white, 26% were Hispanic, 20% were African American, and the remainder were from other ethnic groups. The two most common indications for neurosurgical intervention were hemorrhage (49%) and hydrocephalus (48%), followed by trauma (18%), and brain tumor (11%). The top three neurological signs and symptoms reported were headache (48%), changes in mental status (41%), and nausea/vomiting (39%), followed by focal neurological deficits (33%), neck stiffness (19%), seizures (10%), and photophobia (6%). Nearly three quarters of patients (71%) were admitted to the ICU and 43% received mechanical ventilation for a median of 9 days.
A positive cerebrospinal fluid culture was observed in 106 patients (49%), with the majority of the etiologies being Staphylococcus and Gram-negative rods. An adverse clinical outcome occurred in 167 patients (78%) and was defined as death in 20 patients (9%), persistent vegetative state in 31 patients (14%), severe disability in 77 patients (36%), and moderate disability in 39 patients (18%).
Baseline variables associated with adverse clinical outcomes included age 45 years or older (odds ratio, 11.39), CNS bleeding (OR, 4.37), abnormal neurological exam (OR, 6.51), ICU admission (OR, 5.81), and use of mechanical ventilation (OR, 12.59; P less than .001 for all comparisons). Use of a ventriculoperitoneal shunt was found to be a protective variable (OR, 0.17), which, Dr. Srihawan said, could be explained by the fact most patients who received a ventriculoperitoneal shunt were children who had fewer comorbidities “and tended to be less sick.”
After logistic regression, only three variables remained significantly associated with adverse clinical outcomes: age 45 years or older (OR, 6.47), abnormal neurological exam (OR, 3.04), and use of mechanical ventilation (OR, 5.34).
IDWeek marks the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. The researchers reported having no financial disclosures.
AT IDWEEK 2015
Key clinical point: The majority of patients treated for health care–associated meningitis or ventriculitis had an adverse outcome.
Major finding: An adverse clinical outcome occurred in 78% of patients treated for health care–associated meningitis or ventriculitis.
Data source: An analysis of 215 adult and pediatric patients with a diagnosis of health care–associated meningitis or ventriculitis who were treated at two large tertiary care hospitals in Houston from July 2003 to November 2014.
Disclosures: The researchers reported having no financial disclosures.
Experts debate fecal transplants as first-line therapy for CDI
SAN DIEGO – Fecal microbiota transplantation (FMT) is now first-line therapy for Clostridium difficile infection (CDI) in much of Scandinavia. At an annual conference on infectious diseases, two specialists debated whether that should be the case in the United States, too.
The epidemiology of CDI has changed greatly in the past decade, as other researchers have noted (Infect Drug Resist. 2014;7:63-72). Incidence, severity, and case-fatality rates have risen substantially, and individuals who lack the usual risk factors for CDI are now acquiring it in community settings. Moreover, CDI adds about 5-6 days to a patient’s average hospital stay, and almost one in three affected patients is rehospitalized (Am J Infect Control. 2015 Apr 1;43[4]:314-7.) – most often within a week of discharge, said Dr. Thomas Moore, who is at the University of Kansas, Wichita.
“Should FMT be used as first-line therapy? Not just yes, but hell, yes! It has superior efficacy,” Dr. Moore said.
He pointed to the recent landmark study (N Engl J Med. 2013;368:407-15) of CDI in which duodenal infusions of donor feces more than tripled the rates of relapse-free cure, compared with vancomycin monotherapy or vancomycin with bowel lavage (P less than .001 for both comparisons). Moreover, diarrhea resolved for 81% of patients after the first fecal infusion, and the observed superiority over the vancomycin regimens was so marked that investigators stopped the study after the interim analysis.
Evidence suggests FMT is safe as well as effective, said Dr. Moore. Centers in Norway, Sweden, Denmark, Finland, and Holland have treated at least 900 patients with no reported adverse effects and with cure rates of about 90%, he noted. Of more than 1,000 published FMT studies worldwide, there has been one only report of peritonitis after colonoscopy, one case of irritable bowel syndrome, three reports of mild enteritis, one case of upper gastrointestinal bleeding, one death from sepsis from a dislodged gastrostomy tube, and one case of new-onset obesity, which occurred after a patient received fecal microbiota donated by an obese relative, he added (Open Forum Infect Dis. 2015 Feb 1. doi: 10.1093/ofid/ofv004). Patients now receive fecal microbiota donations from normal-weight individuals, he noted (http://www.openbiome.org/stool-donation/).
“Fecal microbiota transplantation could save lives,” Dr. Moore concluded. Donor material is “cheap and unlimited, the procedure is cost-effective, easy to perform, can even be done at home, and patient satisfaction is very high.”
But Dr. Johan S. Bakken, an infectious disease specialist at St. Luke’s Hospital in Duluth, Minn., argued that FMT is not ready for first-line use for CDI in the United States. “There are no published FMT practice guidelines for initial therapy, even in Scandinavia, and no randomized controlled trials of FMT conducted anywhere,” said Dr. Bakken. He noted that because the Food and Drug Administration has not approved FMT for first-line use in CDI, utilization could require an approved Investigational New Drug application, leading to “unavoidable” treatment delays.
Clinicians also should not gloss over concerns about adverse effects with FMT, Dr. Bakken said. In addition to the case of new-onset obesity, there are risks of aspirating fecal material or perforating hollow viscera. Furthermore, the potential long-term adverse consequences of FMT are unknown, he said.
In contrast, the rate of resolution of initial CDI with per oral vancomycin or fidaxomicin is more than 80%, Dr. Bakken said. Moreover, liquid vancomycin at an appropriate dose and frequency for CDI costs about $4.25 per day in Duluth, he added. “No comparative outcomes data are available for FMT, but it is more costly than vancomycin, may not be locally available, and requires several days or weeks of planning,” he emphasized.
Cost-reimbursement issues with third-party payers are also likely with FMT, according to Dr. Bakken. “There also are potential or perceived medicolegal issues with FMT,” he said. “Keep in mind that about 80% of the world’s lawyers work and practice in the U.S.A.”
Dr. Moore had no disclosures. Dr. Bakken reported being an advisory board member of Rebiotix, which is developing a biologic drug to treat recurrent CDI.
 |
| Dr. Christina Surawicz |
Fecal microbiota transplant is the best treatment for multiple recurrences of C. difficile infection that have not responded to standard therapy including a good pulse and taper course of vancomycin. There is such enthusiasm for FMT because of its simplicity and the ready availability of stool, which is a natural product. Why use an antibiotic to treat an illness that is usually a result of antibiotics? Is FMT therefore the best treatment for a primary infection of C. difficile infection? While treatment for recurrent C. difficile infection is supported by several randomized, controlled trials, including comparison with vancomycin, gut lavage, and sham colonoscopy, there are no randomized, controlled trials for FMT as a treatment of first episodes of CDI. Moreover, 80% of people with their first infection respond to standard antibiotic therapy. In addition, we are learning how important the microbiome is but we do not know the long-term consequences of FMT on an individual. I must agree with Dr. Bakken, it is not wise at this time to use FMT for first episodes of uncomplicated CDI.
Dr. Christina Surawicz is a professor of medicine at University of Washington, Seattle. She has no conflicts of interest.
 |
| Dr. Christina Surawicz |
Fecal microbiota transplant is the best treatment for multiple recurrences of C. difficile infection that have not responded to standard therapy including a good pulse and taper course of vancomycin. There is such enthusiasm for FMT because of its simplicity and the ready availability of stool, which is a natural product. Why use an antibiotic to treat an illness that is usually a result of antibiotics? Is FMT therefore the best treatment for a primary infection of C. difficile infection? While treatment for recurrent C. difficile infection is supported by several randomized, controlled trials, including comparison with vancomycin, gut lavage, and sham colonoscopy, there are no randomized, controlled trials for FMT as a treatment of first episodes of CDI. Moreover, 80% of people with their first infection respond to standard antibiotic therapy. In addition, we are learning how important the microbiome is but we do not know the long-term consequences of FMT on an individual. I must agree with Dr. Bakken, it is not wise at this time to use FMT for first episodes of uncomplicated CDI.
Dr. Christina Surawicz is a professor of medicine at University of Washington, Seattle. She has no conflicts of interest.
 |
| Dr. Christina Surawicz |
Fecal microbiota transplant is the best treatment for multiple recurrences of C. difficile infection that have not responded to standard therapy including a good pulse and taper course of vancomycin. There is such enthusiasm for FMT because of its simplicity and the ready availability of stool, which is a natural product. Why use an antibiotic to treat an illness that is usually a result of antibiotics? Is FMT therefore the best treatment for a primary infection of C. difficile infection? While treatment for recurrent C. difficile infection is supported by several randomized, controlled trials, including comparison with vancomycin, gut lavage, and sham colonoscopy, there are no randomized, controlled trials for FMT as a treatment of first episodes of CDI. Moreover, 80% of people with their first infection respond to standard antibiotic therapy. In addition, we are learning how important the microbiome is but we do not know the long-term consequences of FMT on an individual. I must agree with Dr. Bakken, it is not wise at this time to use FMT for first episodes of uncomplicated CDI.
Dr. Christina Surawicz is a professor of medicine at University of Washington, Seattle. She has no conflicts of interest.
SAN DIEGO – Fecal microbiota transplantation (FMT) is now first-line therapy for Clostridium difficile infection (CDI) in much of Scandinavia. At an annual conference on infectious diseases, two specialists debated whether that should be the case in the United States, too.
The epidemiology of CDI has changed greatly in the past decade, as other researchers have noted (Infect Drug Resist. 2014;7:63-72). Incidence, severity, and case-fatality rates have risen substantially, and individuals who lack the usual risk factors for CDI are now acquiring it in community settings. Moreover, CDI adds about 5-6 days to a patient’s average hospital stay, and almost one in three affected patients is rehospitalized (Am J Infect Control. 2015 Apr 1;43[4]:314-7.) – most often within a week of discharge, said Dr. Thomas Moore, who is at the University of Kansas, Wichita.
“Should FMT be used as first-line therapy? Not just yes, but hell, yes! It has superior efficacy,” Dr. Moore said.
He pointed to the recent landmark study (N Engl J Med. 2013;368:407-15) of CDI in which duodenal infusions of donor feces more than tripled the rates of relapse-free cure, compared with vancomycin monotherapy or vancomycin with bowel lavage (P less than .001 for both comparisons). Moreover, diarrhea resolved for 81% of patients after the first fecal infusion, and the observed superiority over the vancomycin regimens was so marked that investigators stopped the study after the interim analysis.
Evidence suggests FMT is safe as well as effective, said Dr. Moore. Centers in Norway, Sweden, Denmark, Finland, and Holland have treated at least 900 patients with no reported adverse effects and with cure rates of about 90%, he noted. Of more than 1,000 published FMT studies worldwide, there has been one only report of peritonitis after colonoscopy, one case of irritable bowel syndrome, three reports of mild enteritis, one case of upper gastrointestinal bleeding, one death from sepsis from a dislodged gastrostomy tube, and one case of new-onset obesity, which occurred after a patient received fecal microbiota donated by an obese relative, he added (Open Forum Infect Dis. 2015 Feb 1. doi: 10.1093/ofid/ofv004). Patients now receive fecal microbiota donations from normal-weight individuals, he noted (http://www.openbiome.org/stool-donation/).
“Fecal microbiota transplantation could save lives,” Dr. Moore concluded. Donor material is “cheap and unlimited, the procedure is cost-effective, easy to perform, can even be done at home, and patient satisfaction is very high.”
But Dr. Johan S. Bakken, an infectious disease specialist at St. Luke’s Hospital in Duluth, Minn., argued that FMT is not ready for first-line use for CDI in the United States. “There are no published FMT practice guidelines for initial therapy, even in Scandinavia, and no randomized controlled trials of FMT conducted anywhere,” said Dr. Bakken. He noted that because the Food and Drug Administration has not approved FMT for first-line use in CDI, utilization could require an approved Investigational New Drug application, leading to “unavoidable” treatment delays.
Clinicians also should not gloss over concerns about adverse effects with FMT, Dr. Bakken said. In addition to the case of new-onset obesity, there are risks of aspirating fecal material or perforating hollow viscera. Furthermore, the potential long-term adverse consequences of FMT are unknown, he said.
In contrast, the rate of resolution of initial CDI with per oral vancomycin or fidaxomicin is more than 80%, Dr. Bakken said. Moreover, liquid vancomycin at an appropriate dose and frequency for CDI costs about $4.25 per day in Duluth, he added. “No comparative outcomes data are available for FMT, but it is more costly than vancomycin, may not be locally available, and requires several days or weeks of planning,” he emphasized.
Cost-reimbursement issues with third-party payers are also likely with FMT, according to Dr. Bakken. “There also are potential or perceived medicolegal issues with FMT,” he said. “Keep in mind that about 80% of the world’s lawyers work and practice in the U.S.A.”
Dr. Moore had no disclosures. Dr. Bakken reported being an advisory board member of Rebiotix, which is developing a biologic drug to treat recurrent CDI.
SAN DIEGO – Fecal microbiota transplantation (FMT) is now first-line therapy for Clostridium difficile infection (CDI) in much of Scandinavia. At an annual conference on infectious diseases, two specialists debated whether that should be the case in the United States, too.
The epidemiology of CDI has changed greatly in the past decade, as other researchers have noted (Infect Drug Resist. 2014;7:63-72). Incidence, severity, and case-fatality rates have risen substantially, and individuals who lack the usual risk factors for CDI are now acquiring it in community settings. Moreover, CDI adds about 5-6 days to a patient’s average hospital stay, and almost one in three affected patients is rehospitalized (Am J Infect Control. 2015 Apr 1;43[4]:314-7.) – most often within a week of discharge, said Dr. Thomas Moore, who is at the University of Kansas, Wichita.
“Should FMT be used as first-line therapy? Not just yes, but hell, yes! It has superior efficacy,” Dr. Moore said.
He pointed to the recent landmark study (N Engl J Med. 2013;368:407-15) of CDI in which duodenal infusions of donor feces more than tripled the rates of relapse-free cure, compared with vancomycin monotherapy or vancomycin with bowel lavage (P less than .001 for both comparisons). Moreover, diarrhea resolved for 81% of patients after the first fecal infusion, and the observed superiority over the vancomycin regimens was so marked that investigators stopped the study after the interim analysis.
Evidence suggests FMT is safe as well as effective, said Dr. Moore. Centers in Norway, Sweden, Denmark, Finland, and Holland have treated at least 900 patients with no reported adverse effects and with cure rates of about 90%, he noted. Of more than 1,000 published FMT studies worldwide, there has been one only report of peritonitis after colonoscopy, one case of irritable bowel syndrome, three reports of mild enteritis, one case of upper gastrointestinal bleeding, one death from sepsis from a dislodged gastrostomy tube, and one case of new-onset obesity, which occurred after a patient received fecal microbiota donated by an obese relative, he added (Open Forum Infect Dis. 2015 Feb 1. doi: 10.1093/ofid/ofv004). Patients now receive fecal microbiota donations from normal-weight individuals, he noted (http://www.openbiome.org/stool-donation/).
“Fecal microbiota transplantation could save lives,” Dr. Moore concluded. Donor material is “cheap and unlimited, the procedure is cost-effective, easy to perform, can even be done at home, and patient satisfaction is very high.”
But Dr. Johan S. Bakken, an infectious disease specialist at St. Luke’s Hospital in Duluth, Minn., argued that FMT is not ready for first-line use for CDI in the United States. “There are no published FMT practice guidelines for initial therapy, even in Scandinavia, and no randomized controlled trials of FMT conducted anywhere,” said Dr. Bakken. He noted that because the Food and Drug Administration has not approved FMT for first-line use in CDI, utilization could require an approved Investigational New Drug application, leading to “unavoidable” treatment delays.
Clinicians also should not gloss over concerns about adverse effects with FMT, Dr. Bakken said. In addition to the case of new-onset obesity, there are risks of aspirating fecal material or perforating hollow viscera. Furthermore, the potential long-term adverse consequences of FMT are unknown, he said.
In contrast, the rate of resolution of initial CDI with per oral vancomycin or fidaxomicin is more than 80%, Dr. Bakken said. Moreover, liquid vancomycin at an appropriate dose and frequency for CDI costs about $4.25 per day in Duluth, he added. “No comparative outcomes data are available for FMT, but it is more costly than vancomycin, may not be locally available, and requires several days or weeks of planning,” he emphasized.
Cost-reimbursement issues with third-party payers are also likely with FMT, according to Dr. Bakken. “There also are potential or perceived medicolegal issues with FMT,” he said. “Keep in mind that about 80% of the world’s lawyers work and practice in the U.S.A.”
Dr. Moore had no disclosures. Dr. Bakken reported being an advisory board member of Rebiotix, which is developing a biologic drug to treat recurrent CDI.
EXPERT ANALYSIS AT IDWEEK 2015
IDWeek: EV-D68 found more virulent but not more deadly in children
SAN DIEGO – Enterovirus D68 appeared more virulent – but not more lethal – than rhinovirus and other strains of enterovirus among children, said the authors of a single-center study.
The pulmonary pathogen was linked to higher rates of respiratory distress, hospital admission, and magnesium sulfate therapy, but patients were no more likely to die or require critical care unit admission than were those infected with other EV genotypes or rhinovirus, said Dr. Dominik Mertz of the division of infectious diseases at McMaster University, in Hamilton, Ont.
The study also uncovered no evidence of EV-68 transmission at the hospital, Dr. Mertz and his associates said at an annual scientific meeting on infectious diseases.
In 2014, an outbreak of EV-D68 in the United States included more than 1,000 confirmed cases, almost all among children, and many of whom had comorbid asthma or a history of wheezing. Fourteen patients died, and the Centers for Disease Control and Prevention noted that millions more individuals probably had milder EV-D68 infections for which they were never tested.
Dr. Mertz and his associates studied children who presented consecutively to the hospital during the 3 months between Aug. 1 and Oct. 31, 2014. During that time, nasopharyngeal swabs that were positive for EV or rhinovirus were automatically tested for EV-D68. The researchers matched EV-D68–positive patients with children who were positive for rhinovirus or other EVs on the basis of sex, age, and date of presentation to the hospital.
Almost a third (93 of 297; 31%) of rhinovirus or EV samples were positive for EV-D68. Among 87 matched pairs, EV-D68 infection was associated with a threefold greater odds of respiratory distress (95% confidence interval, 1.47-6.14), and a more than twofold rise in the odds of needing magnesium sulfate therapy (odds ratio, 2.62; 95% CI, 1.06-6.47). There was a trend toward greater risk of hospital admission with EV-D68, although it was not statistically significant (OR, 2.29; 95% CI, 0.96-5.46; P = .06).
Notably, EV-68 did not increase the likelihood of death or CCU admission, while, influenza causes dozens of deaths among children in the United States every year, Dr. Mertz and his coinvestigator Dr. Jeffrey Pernica noted in an interview (MMWR. 2014 Jun 6:63[22];483-90). Studies have yet to compare the morbidity and mortality burdens of influenza, respiratory syncytial virus, and EV-D68, and they would like to do so, they said.
“There was a lot of fuss made about EV-D68,” said Dr. Mertz. “But we have flu every year, and many more kids get the flu and die of flu than EV-D68.”
Patients with EV-D68 infection were more likely than others to have a family history of atopy (OR, 2.25) and a personal history of asthma or wheezing (OR, 1.77), hay fever (1.22), and eosinophilia (4.5), although none of these associations reached statistical significance, the investigators reported. “It seems reasonable to hypothesize that EV-D68 is a more virulent pulmonary pathogen to those with preexisting atopic disease than other rhinoviruses and enteroviruses,” Dr. Mertz and his associates wrote in an associated article (CMAJ. 2015 Oct. 13. doi: 10.1503/cmaj.150619). “We can hypothesize that these children are more likely to have respiratory distress because of the combination of allergy and EV-D68, but why the same doesn’t hold true for rhinovirus or other enterovirus strains, we don’t know,” Dr. Mertz said.
The investigators received no funding for the study and reported no conflicts of interest.
SAN DIEGO – Enterovirus D68 appeared more virulent – but not more lethal – than rhinovirus and other strains of enterovirus among children, said the authors of a single-center study.
The pulmonary pathogen was linked to higher rates of respiratory distress, hospital admission, and magnesium sulfate therapy, but patients were no more likely to die or require critical care unit admission than were those infected with other EV genotypes or rhinovirus, said Dr. Dominik Mertz of the division of infectious diseases at McMaster University, in Hamilton, Ont.
The study also uncovered no evidence of EV-68 transmission at the hospital, Dr. Mertz and his associates said at an annual scientific meeting on infectious diseases.
In 2014, an outbreak of EV-D68 in the United States included more than 1,000 confirmed cases, almost all among children, and many of whom had comorbid asthma or a history of wheezing. Fourteen patients died, and the Centers for Disease Control and Prevention noted that millions more individuals probably had milder EV-D68 infections for which they were never tested.
Dr. Mertz and his associates studied children who presented consecutively to the hospital during the 3 months between Aug. 1 and Oct. 31, 2014. During that time, nasopharyngeal swabs that were positive for EV or rhinovirus were automatically tested for EV-D68. The researchers matched EV-D68–positive patients with children who were positive for rhinovirus or other EVs on the basis of sex, age, and date of presentation to the hospital.
Almost a third (93 of 297; 31%) of rhinovirus or EV samples were positive for EV-D68. Among 87 matched pairs, EV-D68 infection was associated with a threefold greater odds of respiratory distress (95% confidence interval, 1.47-6.14), and a more than twofold rise in the odds of needing magnesium sulfate therapy (odds ratio, 2.62; 95% CI, 1.06-6.47). There was a trend toward greater risk of hospital admission with EV-D68, although it was not statistically significant (OR, 2.29; 95% CI, 0.96-5.46; P = .06).
Notably, EV-68 did not increase the likelihood of death or CCU admission, while, influenza causes dozens of deaths among children in the United States every year, Dr. Mertz and his coinvestigator Dr. Jeffrey Pernica noted in an interview (MMWR. 2014 Jun 6:63[22];483-90). Studies have yet to compare the morbidity and mortality burdens of influenza, respiratory syncytial virus, and EV-D68, and they would like to do so, they said.
“There was a lot of fuss made about EV-D68,” said Dr. Mertz. “But we have flu every year, and many more kids get the flu and die of flu than EV-D68.”
Patients with EV-D68 infection were more likely than others to have a family history of atopy (OR, 2.25) and a personal history of asthma or wheezing (OR, 1.77), hay fever (1.22), and eosinophilia (4.5), although none of these associations reached statistical significance, the investigators reported. “It seems reasonable to hypothesize that EV-D68 is a more virulent pulmonary pathogen to those with preexisting atopic disease than other rhinoviruses and enteroviruses,” Dr. Mertz and his associates wrote in an associated article (CMAJ. 2015 Oct. 13. doi: 10.1503/cmaj.150619). “We can hypothesize that these children are more likely to have respiratory distress because of the combination of allergy and EV-D68, but why the same doesn’t hold true for rhinovirus or other enterovirus strains, we don’t know,” Dr. Mertz said.
The investigators received no funding for the study and reported no conflicts of interest.
SAN DIEGO – Enterovirus D68 appeared more virulent – but not more lethal – than rhinovirus and other strains of enterovirus among children, said the authors of a single-center study.
The pulmonary pathogen was linked to higher rates of respiratory distress, hospital admission, and magnesium sulfate therapy, but patients were no more likely to die or require critical care unit admission than were those infected with other EV genotypes or rhinovirus, said Dr. Dominik Mertz of the division of infectious diseases at McMaster University, in Hamilton, Ont.
The study also uncovered no evidence of EV-68 transmission at the hospital, Dr. Mertz and his associates said at an annual scientific meeting on infectious diseases.
In 2014, an outbreak of EV-D68 in the United States included more than 1,000 confirmed cases, almost all among children, and many of whom had comorbid asthma or a history of wheezing. Fourteen patients died, and the Centers for Disease Control and Prevention noted that millions more individuals probably had milder EV-D68 infections for which they were never tested.
Dr. Mertz and his associates studied children who presented consecutively to the hospital during the 3 months between Aug. 1 and Oct. 31, 2014. During that time, nasopharyngeal swabs that were positive for EV or rhinovirus were automatically tested for EV-D68. The researchers matched EV-D68–positive patients with children who were positive for rhinovirus or other EVs on the basis of sex, age, and date of presentation to the hospital.
Almost a third (93 of 297; 31%) of rhinovirus or EV samples were positive for EV-D68. Among 87 matched pairs, EV-D68 infection was associated with a threefold greater odds of respiratory distress (95% confidence interval, 1.47-6.14), and a more than twofold rise in the odds of needing magnesium sulfate therapy (odds ratio, 2.62; 95% CI, 1.06-6.47). There was a trend toward greater risk of hospital admission with EV-D68, although it was not statistically significant (OR, 2.29; 95% CI, 0.96-5.46; P = .06).
Notably, EV-68 did not increase the likelihood of death or CCU admission, while, influenza causes dozens of deaths among children in the United States every year, Dr. Mertz and his coinvestigator Dr. Jeffrey Pernica noted in an interview (MMWR. 2014 Jun 6:63[22];483-90). Studies have yet to compare the morbidity and mortality burdens of influenza, respiratory syncytial virus, and EV-D68, and they would like to do so, they said.
“There was a lot of fuss made about EV-D68,” said Dr. Mertz. “But we have flu every year, and many more kids get the flu and die of flu than EV-D68.”
Patients with EV-D68 infection were more likely than others to have a family history of atopy (OR, 2.25) and a personal history of asthma or wheezing (OR, 1.77), hay fever (1.22), and eosinophilia (4.5), although none of these associations reached statistical significance, the investigators reported. “It seems reasonable to hypothesize that EV-D68 is a more virulent pulmonary pathogen to those with preexisting atopic disease than other rhinoviruses and enteroviruses,” Dr. Mertz and his associates wrote in an associated article (CMAJ. 2015 Oct. 13. doi: 10.1503/cmaj.150619). “We can hypothesize that these children are more likely to have respiratory distress because of the combination of allergy and EV-D68, but why the same doesn’t hold true for rhinovirus or other enterovirus strains, we don’t know,” Dr. Mertz said.
The investigators received no funding for the study and reported no conflicts of interest.
AT IDWEEK 2015
Key clinical point: Enterovirus D68 was more virulent – but not more lethal – than other strains of EV and rhinovirus.
Major finding: Among 87 matched pairs, EV-D68 infection was associated with a threefold greater odds of respiratory distress (95% CI, 1.47-6.14), and a more than twofold rise in the odds of needing magnesium sulfate therapy (OR, 2.62; 95% CI, 1.06-6.47).
Data source: Matched cohort study of 87 pairs of children seen at one hospital during August-October 2014.
Disclosures: The study received no funding. The investigators reported having no conflicts of interest.
Anesthesia-related medical malpractice claims falling in the U.S.
SAN DIEGO – Between 2005 and 2013, the number of anesthesia-related medical malpractice payments decreased by 41%, and the reduction in payments was more significant in inpatient settings, compared with outpatient settings.
Those are key findings from an analysis of national data presented by lead author Dr. Richard J. Kelly at an annual meeting of the American Society of Anesthesiologists.
“It was gratifying to see that overall, the trends of anesthesia-related claims have gone down over the years,” said Dr. Kelly of the department of anesthesiology and perioperative care at the University of California, Irvine. “We did see that because the number of inpatient payments has fallen faster than outpatient payments, the proportion of outpatient payments has increased relative to inpatient claims.”
For the study, the researchers used the U.S. National Practitioner Data Bank to compare inpatient and outpatient anesthesia-related medical malpractice claims made against physicians during 2005-2013. They looked at the number and size of inpatient and outpatient payments over time and compared patient age, patient sex, clinical outcome, and type of medical error for each type of these payments.
Over the 9-year period the frequency of anesthesia-related payments decreased 41.4% (4.6%/year). Payments for inpatient claims decreased a total of 45.5% (5.1%/year) while outpatient payments decreased 24.3% (2.7%/year). The most common patient age group was 40-49 years (40.1% of anesthesia-related claims) and slightly more than half of claims involved females (54.4%). Court judgments made up only 2.7% of claims, while the remainder were settlements.
Cumulative payments for malpractice claims decreased by 47.8% during the study period, from $174.4 million in 2005 to $91.1 million in 2013, and the decrease was greater for inpatient claims (51.4%, compared with 25.9% for outpatient claims). The median payment for all claims was $245,000, and inpatient payments were significantly more expensive than were outpatient claims ($261,742 vs. $189,349; P less than .001).
Death was the most common clinical outcome for all of the paid claims (38.4%) and made up a larger proportion of inpatient than outpatient payments (39.8% vs. 33.9%, respectively). Major injury represented 29.8% of all payments, with no significant difference observed between treatment settings. Compared with inpatient payments, outpatient payments were more likely to involve minor injuries (31.2% vs. 18.2%) and were less likely to involve debilitating injuries (6.4% vs. 9.9%). The study findings “tell us that there are a lot more surgeries being done in an outpatient arena now,” Dr. Kelly said. “And, because of that, surgeons and anesthesia providers should be careful to make sure that patients are appropriately selected for an outpatient setting.”
Dr. Kelly reported having no financial disclosures.
SAN DIEGO – Between 2005 and 2013, the number of anesthesia-related medical malpractice payments decreased by 41%, and the reduction in payments was more significant in inpatient settings, compared with outpatient settings.
Those are key findings from an analysis of national data presented by lead author Dr. Richard J. Kelly at an annual meeting of the American Society of Anesthesiologists.
“It was gratifying to see that overall, the trends of anesthesia-related claims have gone down over the years,” said Dr. Kelly of the department of anesthesiology and perioperative care at the University of California, Irvine. “We did see that because the number of inpatient payments has fallen faster than outpatient payments, the proportion of outpatient payments has increased relative to inpatient claims.”
For the study, the researchers used the U.S. National Practitioner Data Bank to compare inpatient and outpatient anesthesia-related medical malpractice claims made against physicians during 2005-2013. They looked at the number and size of inpatient and outpatient payments over time and compared patient age, patient sex, clinical outcome, and type of medical error for each type of these payments.
Over the 9-year period the frequency of anesthesia-related payments decreased 41.4% (4.6%/year). Payments for inpatient claims decreased a total of 45.5% (5.1%/year) while outpatient payments decreased 24.3% (2.7%/year). The most common patient age group was 40-49 years (40.1% of anesthesia-related claims) and slightly more than half of claims involved females (54.4%). Court judgments made up only 2.7% of claims, while the remainder were settlements.
Cumulative payments for malpractice claims decreased by 47.8% during the study period, from $174.4 million in 2005 to $91.1 million in 2013, and the decrease was greater for inpatient claims (51.4%, compared with 25.9% for outpatient claims). The median payment for all claims was $245,000, and inpatient payments were significantly more expensive than were outpatient claims ($261,742 vs. $189,349; P less than .001).
Death was the most common clinical outcome for all of the paid claims (38.4%) and made up a larger proportion of inpatient than outpatient payments (39.8% vs. 33.9%, respectively). Major injury represented 29.8% of all payments, with no significant difference observed between treatment settings. Compared with inpatient payments, outpatient payments were more likely to involve minor injuries (31.2% vs. 18.2%) and were less likely to involve debilitating injuries (6.4% vs. 9.9%). The study findings “tell us that there are a lot more surgeries being done in an outpatient arena now,” Dr. Kelly said. “And, because of that, surgeons and anesthesia providers should be careful to make sure that patients are appropriately selected for an outpatient setting.”
Dr. Kelly reported having no financial disclosures.
SAN DIEGO – Between 2005 and 2013, the number of anesthesia-related medical malpractice payments decreased by 41%, and the reduction in payments was more significant in inpatient settings, compared with outpatient settings.
Those are key findings from an analysis of national data presented by lead author Dr. Richard J. Kelly at an annual meeting of the American Society of Anesthesiologists.
“It was gratifying to see that overall, the trends of anesthesia-related claims have gone down over the years,” said Dr. Kelly of the department of anesthesiology and perioperative care at the University of California, Irvine. “We did see that because the number of inpatient payments has fallen faster than outpatient payments, the proportion of outpatient payments has increased relative to inpatient claims.”
For the study, the researchers used the U.S. National Practitioner Data Bank to compare inpatient and outpatient anesthesia-related medical malpractice claims made against physicians during 2005-2013. They looked at the number and size of inpatient and outpatient payments over time and compared patient age, patient sex, clinical outcome, and type of medical error for each type of these payments.
Over the 9-year period the frequency of anesthesia-related payments decreased 41.4% (4.6%/year). Payments for inpatient claims decreased a total of 45.5% (5.1%/year) while outpatient payments decreased 24.3% (2.7%/year). The most common patient age group was 40-49 years (40.1% of anesthesia-related claims) and slightly more than half of claims involved females (54.4%). Court judgments made up only 2.7% of claims, while the remainder were settlements.
Cumulative payments for malpractice claims decreased by 47.8% during the study period, from $174.4 million in 2005 to $91.1 million in 2013, and the decrease was greater for inpatient claims (51.4%, compared with 25.9% for outpatient claims). The median payment for all claims was $245,000, and inpatient payments were significantly more expensive than were outpatient claims ($261,742 vs. $189,349; P less than .001).
Death was the most common clinical outcome for all of the paid claims (38.4%) and made up a larger proportion of inpatient than outpatient payments (39.8% vs. 33.9%, respectively). Major injury represented 29.8% of all payments, with no significant difference observed between treatment settings. Compared with inpatient payments, outpatient payments were more likely to involve minor injuries (31.2% vs. 18.2%) and were less likely to involve debilitating injuries (6.4% vs. 9.9%). The study findings “tell us that there are a lot more surgeries being done in an outpatient arena now,” Dr. Kelly said. “And, because of that, surgeons and anesthesia providers should be careful to make sure that patients are appropriately selected for an outpatient setting.”
Dr. Kelly reported having no financial disclosures.
AT THE ASA ANNUAL MEETING
Experts debate infection control merits of ‘bare beneath the elbows’
SAN DIEGO – Going tieless and “bare beneath the elbows” has been touted for infection control. But while some clinicians endorse the practice, others call it inconvenient, unprofessional, and distracting. At an annual conference on infectious diseases, two specialists in the field debated going “BBE” and its evidence base.
Widespread practice of BBE dates to at least 2008, when the National Health Service in the United Kingdom mandated it as part of a set of measures to decrease nosocomial transmission of methicillin-resistant Staphylococcus aureus (MRSA) and Clostridium difficile. Clinicians at NHS were directed to leave jewelry, neckties, and wrist watches at home, hang up their lab coats, and wear short sleeves. The policy aims not only to reduce points of physical contact between providers and patients, but also to improve hand and wrist washing, said Dr. Michael Edmond, who is at the University of Iowa Hospitals and Clinics in Iowa City.
Some evidence supports going BBE, said Dr. Edmond. Pathogenic gram-negative rods have been cultured from neckties, scrubs, uniforms, and white coats in multiple studies, he added. Inadequate laundering is part of the problem – clinical faculty in one study reported washing their coats about once every 2 weeks, even less often than medical students did.
“So when is biological plausibility enough to support a change in practice?” Dr. Edmond asked. “There is a potential for benefit in going BBE. There is no risk for harm. And there is minimal cost. On the basis of the same evidence and assumptions, we are willing to wrap ourselves in plastic and confine patients to their hospital rooms – that is, to use contact precautions. And yet, we are not willing to eliminate white coats and ties.”
Patient perception is not at issue, Dr. Edmond argued. Only about half of patients at one British hospital said they wanted physicians to wear traditional white coats, and that proportion dropped to 22% after patients received educational materials on clothing contamination, he noted. In another study, patients ranked their physician’s appearance behind knowledge, compassion, and politeness when asked which characteristics they valued most.
“Without strong evidence for benefit, we should recommend – not mandate – this new practice,” Dr. Edmond concluded.
But Dr. Neil O. Fishman disagreed, calling BBE “an evidence-free zone.” Dr. Fishman, who is at the University of Pennsylvania in Philadelphia, noted a total lack of randomized, controlled trials or well-performed observational studies supporting BBE. “No clinical studies have demonstrated cross-transmission of health care–associated pathogens from a health care provider to a patient,” he said.
Moreover, BBE does not prevent contamination, Dr. Fishman said. Bacterial cultures of the hands of BBE clinicians and controls revealed no differences in total bacteria counts or numbers of clinically significant pathogens, he said. Cultures of white coats and the undersides of wrists also were similar in terms of total bacteria and MRSA counts, he added.
Despite the lack of evidence, BBE has been implemented at NHS “mainly as a political gesture and has had unintended consequences,” Dr. Fishman said. Informal attire has promoted a less-robust view of infection control, junior doctors have adopted scruffy attire and “slovenly” personal hygiene, and all the focus on clothing has distracted from hand washing, he added.
Furthermore, less than 12% of clinicians have complied with BBE, according to Dr. Fishman. Abstainers report feeling cold and not knowing what time it is. Women, in particular, say they have no pockets to carry work essentials. “This is a gender equity issue,” Dr. Fishman said. “Can we afford to promote practices based on limited evidence, a theoretical rationale, or individual opinions? This is a lack of focus on what really matters.”
Dr. Edmond and Dr. Fishman reported no disclosures.
SAN DIEGO – Going tieless and “bare beneath the elbows” has been touted for infection control. But while some clinicians endorse the practice, others call it inconvenient, unprofessional, and distracting. At an annual conference on infectious diseases, two specialists in the field debated going “BBE” and its evidence base.
Widespread practice of BBE dates to at least 2008, when the National Health Service in the United Kingdom mandated it as part of a set of measures to decrease nosocomial transmission of methicillin-resistant Staphylococcus aureus (MRSA) and Clostridium difficile. Clinicians at NHS were directed to leave jewelry, neckties, and wrist watches at home, hang up their lab coats, and wear short sleeves. The policy aims not only to reduce points of physical contact between providers and patients, but also to improve hand and wrist washing, said Dr. Michael Edmond, who is at the University of Iowa Hospitals and Clinics in Iowa City.
Some evidence supports going BBE, said Dr. Edmond. Pathogenic gram-negative rods have been cultured from neckties, scrubs, uniforms, and white coats in multiple studies, he added. Inadequate laundering is part of the problem – clinical faculty in one study reported washing their coats about once every 2 weeks, even less often than medical students did.
“So when is biological plausibility enough to support a change in practice?” Dr. Edmond asked. “There is a potential for benefit in going BBE. There is no risk for harm. And there is minimal cost. On the basis of the same evidence and assumptions, we are willing to wrap ourselves in plastic and confine patients to their hospital rooms – that is, to use contact precautions. And yet, we are not willing to eliminate white coats and ties.”
Patient perception is not at issue, Dr. Edmond argued. Only about half of patients at one British hospital said they wanted physicians to wear traditional white coats, and that proportion dropped to 22% after patients received educational materials on clothing contamination, he noted. In another study, patients ranked their physician’s appearance behind knowledge, compassion, and politeness when asked which characteristics they valued most.
“Without strong evidence for benefit, we should recommend – not mandate – this new practice,” Dr. Edmond concluded.
But Dr. Neil O. Fishman disagreed, calling BBE “an evidence-free zone.” Dr. Fishman, who is at the University of Pennsylvania in Philadelphia, noted a total lack of randomized, controlled trials or well-performed observational studies supporting BBE. “No clinical studies have demonstrated cross-transmission of health care–associated pathogens from a health care provider to a patient,” he said.
Moreover, BBE does not prevent contamination, Dr. Fishman said. Bacterial cultures of the hands of BBE clinicians and controls revealed no differences in total bacteria counts or numbers of clinically significant pathogens, he said. Cultures of white coats and the undersides of wrists also were similar in terms of total bacteria and MRSA counts, he added.
Despite the lack of evidence, BBE has been implemented at NHS “mainly as a political gesture and has had unintended consequences,” Dr. Fishman said. Informal attire has promoted a less-robust view of infection control, junior doctors have adopted scruffy attire and “slovenly” personal hygiene, and all the focus on clothing has distracted from hand washing, he added.
Furthermore, less than 12% of clinicians have complied with BBE, according to Dr. Fishman. Abstainers report feeling cold and not knowing what time it is. Women, in particular, say they have no pockets to carry work essentials. “This is a gender equity issue,” Dr. Fishman said. “Can we afford to promote practices based on limited evidence, a theoretical rationale, or individual opinions? This is a lack of focus on what really matters.”
Dr. Edmond and Dr. Fishman reported no disclosures.
SAN DIEGO – Going tieless and “bare beneath the elbows” has been touted for infection control. But while some clinicians endorse the practice, others call it inconvenient, unprofessional, and distracting. At an annual conference on infectious diseases, two specialists in the field debated going “BBE” and its evidence base.
Widespread practice of BBE dates to at least 2008, when the National Health Service in the United Kingdom mandated it as part of a set of measures to decrease nosocomial transmission of methicillin-resistant Staphylococcus aureus (MRSA) and Clostridium difficile. Clinicians at NHS were directed to leave jewelry, neckties, and wrist watches at home, hang up their lab coats, and wear short sleeves. The policy aims not only to reduce points of physical contact between providers and patients, but also to improve hand and wrist washing, said Dr. Michael Edmond, who is at the University of Iowa Hospitals and Clinics in Iowa City.
Some evidence supports going BBE, said Dr. Edmond. Pathogenic gram-negative rods have been cultured from neckties, scrubs, uniforms, and white coats in multiple studies, he added. Inadequate laundering is part of the problem – clinical faculty in one study reported washing their coats about once every 2 weeks, even less often than medical students did.
“So when is biological plausibility enough to support a change in practice?” Dr. Edmond asked. “There is a potential for benefit in going BBE. There is no risk for harm. And there is minimal cost. On the basis of the same evidence and assumptions, we are willing to wrap ourselves in plastic and confine patients to their hospital rooms – that is, to use contact precautions. And yet, we are not willing to eliminate white coats and ties.”
Patient perception is not at issue, Dr. Edmond argued. Only about half of patients at one British hospital said they wanted physicians to wear traditional white coats, and that proportion dropped to 22% after patients received educational materials on clothing contamination, he noted. In another study, patients ranked their physician’s appearance behind knowledge, compassion, and politeness when asked which characteristics they valued most.
“Without strong evidence for benefit, we should recommend – not mandate – this new practice,” Dr. Edmond concluded.
But Dr. Neil O. Fishman disagreed, calling BBE “an evidence-free zone.” Dr. Fishman, who is at the University of Pennsylvania in Philadelphia, noted a total lack of randomized, controlled trials or well-performed observational studies supporting BBE. “No clinical studies have demonstrated cross-transmission of health care–associated pathogens from a health care provider to a patient,” he said.
Moreover, BBE does not prevent contamination, Dr. Fishman said. Bacterial cultures of the hands of BBE clinicians and controls revealed no differences in total bacteria counts or numbers of clinically significant pathogens, he said. Cultures of white coats and the undersides of wrists also were similar in terms of total bacteria and MRSA counts, he added.
Despite the lack of evidence, BBE has been implemented at NHS “mainly as a political gesture and has had unintended consequences,” Dr. Fishman said. Informal attire has promoted a less-robust view of infection control, junior doctors have adopted scruffy attire and “slovenly” personal hygiene, and all the focus on clothing has distracted from hand washing, he added.
Furthermore, less than 12% of clinicians have complied with BBE, according to Dr. Fishman. Abstainers report feeling cold and not knowing what time it is. Women, in particular, say they have no pockets to carry work essentials. “This is a gender equity issue,” Dr. Fishman said. “Can we afford to promote practices based on limited evidence, a theoretical rationale, or individual opinions? This is a lack of focus on what really matters.”
Dr. Edmond and Dr. Fishman reported no disclosures.
EXPERT ANALYSIS AT IDWEEK 2015