User login
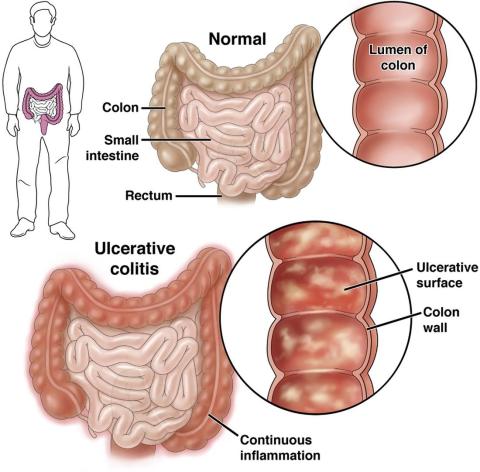
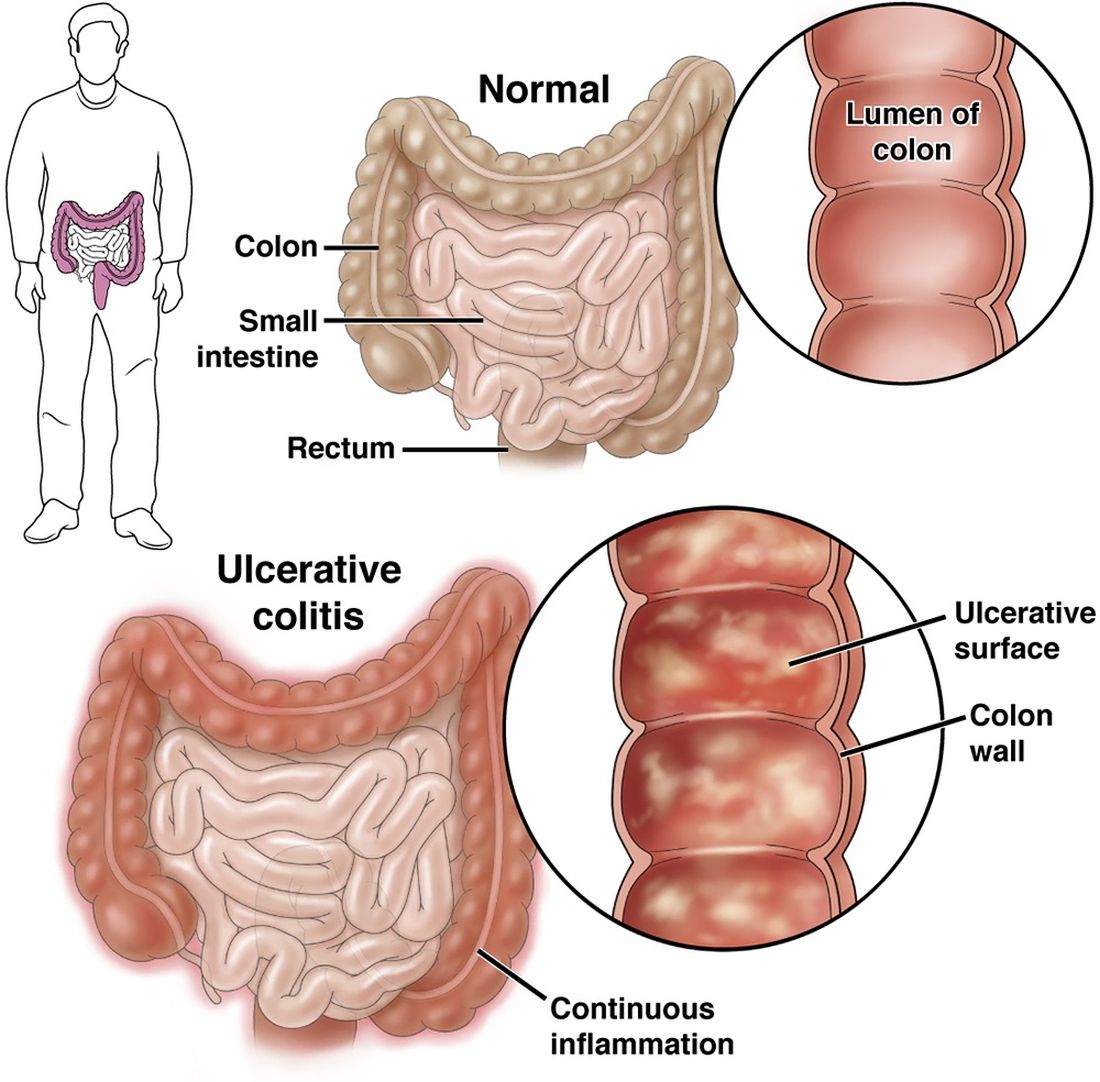
Environment More Than Genes Impacts Age at Inflammatory Bowel Disease Diagnosis
a large study of IBD patients reported.
Published in Clinical Gastroenterology and Hepatology , the study found that environment influences the onset of both ulcerative colitis (UC) and Crohn’s disease (CD), and exposures typical in Western society lower the age of diagnosis. These factors include birth in a developed nation, delivery by C-section, and more bathrooms in the home, according to Oriana M. Damas, MD, MSCTI, an associate professor of clinical medicine at the University of Miami Miller School of Medicine in Florida and colleagues.
Environmental factors explained 21% of the variance in age of CD diagnosis and 39% of the variance in age of UC diagnosis. In models incorporating both genetic and environmental risk scores, the environment was the only significant factor associated with younger age of IBD diagnosis in all groups.
Several epidemiologic studies have examined environmental culprits in IBD, and others have examined genetic risk factors, Dr. Damas said in an interview. “But we had not seen any studies that examined the influence of both [of] these on age of IBD development.” Her group’s working hypothesis that environment would have a greater effect than genetics was borne out.
“Additionally, very few studies have examined the contribution of genetics or environmental factors in Hispanic individuals, and our study examined the contribution of these factors in this understudied population,” she added.
According to Dr. Damas, the findings’ most immediate clinical relevance is for counseling people with a family history of IBD. “I think it’s important for concerned patients to know that IBD is not solely genetic and that several environmental factors can shape disease risk to a greater extent than genetic predisposition,” she said
Westernization is increasingly considered a contributor to the global increase in IBD, which has been diagnosed in an estimated 2.39 million Americans . In genetically predisposed individuals, environmental culprits in developed countries are thought to negatively shape the intestinal microbiome’s composition into a less tolerant and more proinflammatory state, the authors noted.
According to the “hygiene hypothesis,” the oversanitization of life in the developed world is partly to blame. “A cleaner environment at home, part of the hygiene hypothesis, has been postulated as a theory to help explain the rise of autoimmune diseases in the 21st century and may play an important part in explaining our study findings,” the authors wrote.
Population-based studies have also pointed to antibiotics, nonsteroidal anti-inflammatory drugs, smoking, cesarean delivery, lack of breastfeeding, and nonexposure to farm animals as other risk factors for IBD.
Study Details
To compare the effect of environmental vs genetic risk factors, the questionnaire-based study surveyed 2952 IBD patients from a tertiary care referral center — 58.9% with CD, 45.83% of Hispanic background, and 53.18% of non-Hispanic White (NHW) ethnicity. There were too few available Black and Asian patients to be included in the cohort. Data were collected from 2017 to 2022.
The mean age of patients was 39.71 years, and 34.14% were defined as born outside of the US mainland. Foreign-born patients were further characterized as from developed nations vs developing nations; 81.3% in this subgroup came from the latter. A detailed questionnaire probed 13 potential environmental factors from type of birth to domestic living conditions, medications, and smoking across several different age groups. Blood was drawn to genotype participants and to create a genetic risk score.
Early plastic water bottle use — which has been linked to inflammatory microplastics in the intestines — and residing in homes with more than one bathroom (and presumably less exposure to infections) were also associated with younger age at diagnosis. Susceptibility to environmental exposures was similar in Hispanic and NHW patients.
“It was interesting to find an association between reported plastic water bottle use and younger age of IBD diagnosis,” said Dr. Damas. “Because this is a self-reported intake, we need more studies to confirm this. However, this finding falls in line with other recent studies showing a potential association between microplastics and disease states, including IBD. The next step is to measure for traces of environmental contaminants in human samples of patients with IBD.”
Unlike previous studies, this analysis did not find parasitic infections, pets, and antibiotics to be associated with age of IBD diagnosis.
“This is an interesting and important study,” commented Ashwin Ananthakrishnan, MBBS, MPH, AGAF, director of the Crohn’s and Colitis Center at Massachusetts General Hospital in Boston, who was not involved in the study. “There are few environmental risk factor studies looking at non-White populations and to that end, this is a very large and well-done analysis looking at environmental factors among Hispanic patients with IBD.”
He added that, while most studies have just compared factors between cases and controls, “this is an interesting examination of the impact of such factors on age of onset.”
Dr. Ananthakrishnan stressed, however, that further work is needed to expand on these findings.” The addition of a control group would help determine how these factors actually modify disease risk. It is also intriguing that environmental factors more strongly predict age of onset than genetic risk. That only highlights the fact that IBD is in large part an environmentally influenced disease, suggesting there is exciting opportunity for environmental modification to address disease onset.”
Offering another outsider’s perspective, Manasi Agrawal, MD, MS, an assistant professor of medicine at Icahn School of Medicine at Mount Sinai in New York City and not a participant in the study, agreed that the findings highlight the contribution of early life and childhood environmental factors to IBD risk relative to genetic variants. “The relative importance of the environment compared to genetic risk toward IBD, timing of exposure, and impact on age at IBD diagnosis is a novel and important finding. These data will help contextualize how we communicate disease risk and potential prevention approaches.”
She added that future research should measure various exposures, such as pollutants in preclinical biological samples. “Mechanistic data on their downstream effects are needed to understand IBD pathogenesis and develop prevention efforts.”
According to the authors, theirs is the first study of its kind to examine the contribution of cumulative environmental factors, age-dependent exposures, and genetic predisposition to age of IBD diagnosis in a diverse IBD cohort.
The authors listed no specific funding for this study and had no conflicts of interest to declare. Dr. Ananthakrishnan and Dr. Agrawal had no relevant competing interests.
A version of this article appeared on Medscape.com.
a large study of IBD patients reported.
Published in Clinical Gastroenterology and Hepatology , the study found that environment influences the onset of both ulcerative colitis (UC) and Crohn’s disease (CD), and exposures typical in Western society lower the age of diagnosis. These factors include birth in a developed nation, delivery by C-section, and more bathrooms in the home, according to Oriana M. Damas, MD, MSCTI, an associate professor of clinical medicine at the University of Miami Miller School of Medicine in Florida and colleagues.
Environmental factors explained 21% of the variance in age of CD diagnosis and 39% of the variance in age of UC diagnosis. In models incorporating both genetic and environmental risk scores, the environment was the only significant factor associated with younger age of IBD diagnosis in all groups.
Several epidemiologic studies have examined environmental culprits in IBD, and others have examined genetic risk factors, Dr. Damas said in an interview. “But we had not seen any studies that examined the influence of both [of] these on age of IBD development.” Her group’s working hypothesis that environment would have a greater effect than genetics was borne out.
“Additionally, very few studies have examined the contribution of genetics or environmental factors in Hispanic individuals, and our study examined the contribution of these factors in this understudied population,” she added.
According to Dr. Damas, the findings’ most immediate clinical relevance is for counseling people with a family history of IBD. “I think it’s important for concerned patients to know that IBD is not solely genetic and that several environmental factors can shape disease risk to a greater extent than genetic predisposition,” she said
Westernization is increasingly considered a contributor to the global increase in IBD, which has been diagnosed in an estimated 2.39 million Americans . In genetically predisposed individuals, environmental culprits in developed countries are thought to negatively shape the intestinal microbiome’s composition into a less tolerant and more proinflammatory state, the authors noted.
According to the “hygiene hypothesis,” the oversanitization of life in the developed world is partly to blame. “A cleaner environment at home, part of the hygiene hypothesis, has been postulated as a theory to help explain the rise of autoimmune diseases in the 21st century and may play an important part in explaining our study findings,” the authors wrote.
Population-based studies have also pointed to antibiotics, nonsteroidal anti-inflammatory drugs, smoking, cesarean delivery, lack of breastfeeding, and nonexposure to farm animals as other risk factors for IBD.
Study Details
To compare the effect of environmental vs genetic risk factors, the questionnaire-based study surveyed 2952 IBD patients from a tertiary care referral center — 58.9% with CD, 45.83% of Hispanic background, and 53.18% of non-Hispanic White (NHW) ethnicity. There were too few available Black and Asian patients to be included in the cohort. Data were collected from 2017 to 2022.
The mean age of patients was 39.71 years, and 34.14% were defined as born outside of the US mainland. Foreign-born patients were further characterized as from developed nations vs developing nations; 81.3% in this subgroup came from the latter. A detailed questionnaire probed 13 potential environmental factors from type of birth to domestic living conditions, medications, and smoking across several different age groups. Blood was drawn to genotype participants and to create a genetic risk score.
Early plastic water bottle use — which has been linked to inflammatory microplastics in the intestines — and residing in homes with more than one bathroom (and presumably less exposure to infections) were also associated with younger age at diagnosis. Susceptibility to environmental exposures was similar in Hispanic and NHW patients.
“It was interesting to find an association between reported plastic water bottle use and younger age of IBD diagnosis,” said Dr. Damas. “Because this is a self-reported intake, we need more studies to confirm this. However, this finding falls in line with other recent studies showing a potential association between microplastics and disease states, including IBD. The next step is to measure for traces of environmental contaminants in human samples of patients with IBD.”
Unlike previous studies, this analysis did not find parasitic infections, pets, and antibiotics to be associated with age of IBD diagnosis.
“This is an interesting and important study,” commented Ashwin Ananthakrishnan, MBBS, MPH, AGAF, director of the Crohn’s and Colitis Center at Massachusetts General Hospital in Boston, who was not involved in the study. “There are few environmental risk factor studies looking at non-White populations and to that end, this is a very large and well-done analysis looking at environmental factors among Hispanic patients with IBD.”
He added that, while most studies have just compared factors between cases and controls, “this is an interesting examination of the impact of such factors on age of onset.”
Dr. Ananthakrishnan stressed, however, that further work is needed to expand on these findings.” The addition of a control group would help determine how these factors actually modify disease risk. It is also intriguing that environmental factors more strongly predict age of onset than genetic risk. That only highlights the fact that IBD is in large part an environmentally influenced disease, suggesting there is exciting opportunity for environmental modification to address disease onset.”
Offering another outsider’s perspective, Manasi Agrawal, MD, MS, an assistant professor of medicine at Icahn School of Medicine at Mount Sinai in New York City and not a participant in the study, agreed that the findings highlight the contribution of early life and childhood environmental factors to IBD risk relative to genetic variants. “The relative importance of the environment compared to genetic risk toward IBD, timing of exposure, and impact on age at IBD diagnosis is a novel and important finding. These data will help contextualize how we communicate disease risk and potential prevention approaches.”
She added that future research should measure various exposures, such as pollutants in preclinical biological samples. “Mechanistic data on their downstream effects are needed to understand IBD pathogenesis and develop prevention efforts.”
According to the authors, theirs is the first study of its kind to examine the contribution of cumulative environmental factors, age-dependent exposures, and genetic predisposition to age of IBD diagnosis in a diverse IBD cohort.
The authors listed no specific funding for this study and had no conflicts of interest to declare. Dr. Ananthakrishnan and Dr. Agrawal had no relevant competing interests.
A version of this article appeared on Medscape.com.
a large study of IBD patients reported.
Published in Clinical Gastroenterology and Hepatology , the study found that environment influences the onset of both ulcerative colitis (UC) and Crohn’s disease (CD), and exposures typical in Western society lower the age of diagnosis. These factors include birth in a developed nation, delivery by C-section, and more bathrooms in the home, according to Oriana M. Damas, MD, MSCTI, an associate professor of clinical medicine at the University of Miami Miller School of Medicine in Florida and colleagues.
Environmental factors explained 21% of the variance in age of CD diagnosis and 39% of the variance in age of UC diagnosis. In models incorporating both genetic and environmental risk scores, the environment was the only significant factor associated with younger age of IBD diagnosis in all groups.
Several epidemiologic studies have examined environmental culprits in IBD, and others have examined genetic risk factors, Dr. Damas said in an interview. “But we had not seen any studies that examined the influence of both [of] these on age of IBD development.” Her group’s working hypothesis that environment would have a greater effect than genetics was borne out.
“Additionally, very few studies have examined the contribution of genetics or environmental factors in Hispanic individuals, and our study examined the contribution of these factors in this understudied population,” she added.
According to Dr. Damas, the findings’ most immediate clinical relevance is for counseling people with a family history of IBD. “I think it’s important for concerned patients to know that IBD is not solely genetic and that several environmental factors can shape disease risk to a greater extent than genetic predisposition,” she said
Westernization is increasingly considered a contributor to the global increase in IBD, which has been diagnosed in an estimated 2.39 million Americans . In genetically predisposed individuals, environmental culprits in developed countries are thought to negatively shape the intestinal microbiome’s composition into a less tolerant and more proinflammatory state, the authors noted.
According to the “hygiene hypothesis,” the oversanitization of life in the developed world is partly to blame. “A cleaner environment at home, part of the hygiene hypothesis, has been postulated as a theory to help explain the rise of autoimmune diseases in the 21st century and may play an important part in explaining our study findings,” the authors wrote.
Population-based studies have also pointed to antibiotics, nonsteroidal anti-inflammatory drugs, smoking, cesarean delivery, lack of breastfeeding, and nonexposure to farm animals as other risk factors for IBD.
Study Details
To compare the effect of environmental vs genetic risk factors, the questionnaire-based study surveyed 2952 IBD patients from a tertiary care referral center — 58.9% with CD, 45.83% of Hispanic background, and 53.18% of non-Hispanic White (NHW) ethnicity. There were too few available Black and Asian patients to be included in the cohort. Data were collected from 2017 to 2022.
The mean age of patients was 39.71 years, and 34.14% were defined as born outside of the US mainland. Foreign-born patients were further characterized as from developed nations vs developing nations; 81.3% in this subgroup came from the latter. A detailed questionnaire probed 13 potential environmental factors from type of birth to domestic living conditions, medications, and smoking across several different age groups. Blood was drawn to genotype participants and to create a genetic risk score.
Early plastic water bottle use — which has been linked to inflammatory microplastics in the intestines — and residing in homes with more than one bathroom (and presumably less exposure to infections) were also associated with younger age at diagnosis. Susceptibility to environmental exposures was similar in Hispanic and NHW patients.
“It was interesting to find an association between reported plastic water bottle use and younger age of IBD diagnosis,” said Dr. Damas. “Because this is a self-reported intake, we need more studies to confirm this. However, this finding falls in line with other recent studies showing a potential association between microplastics and disease states, including IBD. The next step is to measure for traces of environmental contaminants in human samples of patients with IBD.”
Unlike previous studies, this analysis did not find parasitic infections, pets, and antibiotics to be associated with age of IBD diagnosis.
“This is an interesting and important study,” commented Ashwin Ananthakrishnan, MBBS, MPH, AGAF, director of the Crohn’s and Colitis Center at Massachusetts General Hospital in Boston, who was not involved in the study. “There are few environmental risk factor studies looking at non-White populations and to that end, this is a very large and well-done analysis looking at environmental factors among Hispanic patients with IBD.”
He added that, while most studies have just compared factors between cases and controls, “this is an interesting examination of the impact of such factors on age of onset.”
Dr. Ananthakrishnan stressed, however, that further work is needed to expand on these findings.” The addition of a control group would help determine how these factors actually modify disease risk. It is also intriguing that environmental factors more strongly predict age of onset than genetic risk. That only highlights the fact that IBD is in large part an environmentally influenced disease, suggesting there is exciting opportunity for environmental modification to address disease onset.”
Offering another outsider’s perspective, Manasi Agrawal, MD, MS, an assistant professor of medicine at Icahn School of Medicine at Mount Sinai in New York City and not a participant in the study, agreed that the findings highlight the contribution of early life and childhood environmental factors to IBD risk relative to genetic variants. “The relative importance of the environment compared to genetic risk toward IBD, timing of exposure, and impact on age at IBD diagnosis is a novel and important finding. These data will help contextualize how we communicate disease risk and potential prevention approaches.”
She added that future research should measure various exposures, such as pollutants in preclinical biological samples. “Mechanistic data on their downstream effects are needed to understand IBD pathogenesis and develop prevention efforts.”
According to the authors, theirs is the first study of its kind to examine the contribution of cumulative environmental factors, age-dependent exposures, and genetic predisposition to age of IBD diagnosis in a diverse IBD cohort.
The authors listed no specific funding for this study and had no conflicts of interest to declare. Dr. Ananthakrishnan and Dr. Agrawal had no relevant competing interests.
A version of this article appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Family Size, Dog Ownership Linked With Reduced Risk of Crohn’s
, according to investigators.
Those who live with a pet bird may be more likely to develop CD, although few participants in the study lived with birds, requiring a cautious interpretation of this latter finding, lead author Mingyue Xue, PhD, of Mount Sinai Hospital, Toronto, Ontario, Canada, and colleagues reported.
“Environmental factors, such as smoking, large families, urban environments, and exposure to pets, have been shown to be associated with the risk of CD development,” the investigators wrote in Clinical Gastroenterology and Hepatology. “However, most of these studies were based on a retrospective study design, which makes it challenging to understand when and how environmental factors trigger the biological changes that lead to disease.”
The present study prospectively followed 4289 asymptomatic first-degree relatives (FDRs) of patients with CD. Environmental factors were identified via regression models that also considered biological factors, including gut inflammation via fecal calprotectin (FCP) levels, altered intestinal permeability measured by urinary fractional excretion of lactulose to mannitol ratio (LMR), and fecal microbiome composition through 16S rRNA sequencing.
After a median follow-up period of 5.62 years, 86 FDRs (1.9%) developed CD.
Living in a household of at least three people in the first year of life was associated with a 57% reduced risk of CD development (hazard ratio [HR], 0.43; P = .019). Similarly, living with a pet dog between the ages of 5 and 15 also demonstrated a protective effect, dropping risk of CD by 39% (HR, 0.61; P = .025).
“Our analysis revealed a protective trend of living with dogs that transcends the age of exposure, suggesting that dog ownership could confer health benefits in reducing the risk of CD,” the investigators wrote. “Our study also found that living in a large family during the first year of life is significantly associated with the future onset of CD, aligning with prior research that indicates that a larger family size in the first year of life can reduce the risk of developing IBD.”
In contrast, the study identified bird ownership at time of recruitment as a risk factor for CD, increasing risk almost three-fold (HR, 2.84; P = .005). The investigators urged a careful interpretation of this latter finding, however, as relatively few FDRs lived with birds.
“[A]lthough our sample size can be considered large, some environmental variables were uncommon, such as the participants having birds as pets, and would greatly benefit from replication of our findings in other cohorts,” Dr. Xue and colleagues noted.
They suggested several possible ways in which the above environmental factors may impact CD risk, including effects on subclinical inflammation, microbiome composition, and gut permeability.
“Understanding the relationship between CD-related environmental factors and these predisease biomarkers may shed light on the underlying mechanisms by which environmental factors impact host health and ultimately lead to CD onset,” the investigators concluded.
The study was supported by Crohn’s and Colitis Canada, Canadian Institutes of Health Research, the Helmsley Charitable Trust, and others. The investigators disclosed no conflicts of interest.
, according to investigators.
Those who live with a pet bird may be more likely to develop CD, although few participants in the study lived with birds, requiring a cautious interpretation of this latter finding, lead author Mingyue Xue, PhD, of Mount Sinai Hospital, Toronto, Ontario, Canada, and colleagues reported.
“Environmental factors, such as smoking, large families, urban environments, and exposure to pets, have been shown to be associated with the risk of CD development,” the investigators wrote in Clinical Gastroenterology and Hepatology. “However, most of these studies were based on a retrospective study design, which makes it challenging to understand when and how environmental factors trigger the biological changes that lead to disease.”
The present study prospectively followed 4289 asymptomatic first-degree relatives (FDRs) of patients with CD. Environmental factors were identified via regression models that also considered biological factors, including gut inflammation via fecal calprotectin (FCP) levels, altered intestinal permeability measured by urinary fractional excretion of lactulose to mannitol ratio (LMR), and fecal microbiome composition through 16S rRNA sequencing.
After a median follow-up period of 5.62 years, 86 FDRs (1.9%) developed CD.
Living in a household of at least three people in the first year of life was associated with a 57% reduced risk of CD development (hazard ratio [HR], 0.43; P = .019). Similarly, living with a pet dog between the ages of 5 and 15 also demonstrated a protective effect, dropping risk of CD by 39% (HR, 0.61; P = .025).
“Our analysis revealed a protective trend of living with dogs that transcends the age of exposure, suggesting that dog ownership could confer health benefits in reducing the risk of CD,” the investigators wrote. “Our study also found that living in a large family during the first year of life is significantly associated with the future onset of CD, aligning with prior research that indicates that a larger family size in the first year of life can reduce the risk of developing IBD.”
In contrast, the study identified bird ownership at time of recruitment as a risk factor for CD, increasing risk almost three-fold (HR, 2.84; P = .005). The investigators urged a careful interpretation of this latter finding, however, as relatively few FDRs lived with birds.
“[A]lthough our sample size can be considered large, some environmental variables were uncommon, such as the participants having birds as pets, and would greatly benefit from replication of our findings in other cohorts,” Dr. Xue and colleagues noted.
They suggested several possible ways in which the above environmental factors may impact CD risk, including effects on subclinical inflammation, microbiome composition, and gut permeability.
“Understanding the relationship between CD-related environmental factors and these predisease biomarkers may shed light on the underlying mechanisms by which environmental factors impact host health and ultimately lead to CD onset,” the investigators concluded.
The study was supported by Crohn’s and Colitis Canada, Canadian Institutes of Health Research, the Helmsley Charitable Trust, and others. The investigators disclosed no conflicts of interest.
, according to investigators.
Those who live with a pet bird may be more likely to develop CD, although few participants in the study lived with birds, requiring a cautious interpretation of this latter finding, lead author Mingyue Xue, PhD, of Mount Sinai Hospital, Toronto, Ontario, Canada, and colleagues reported.
“Environmental factors, such as smoking, large families, urban environments, and exposure to pets, have been shown to be associated with the risk of CD development,” the investigators wrote in Clinical Gastroenterology and Hepatology. “However, most of these studies were based on a retrospective study design, which makes it challenging to understand when and how environmental factors trigger the biological changes that lead to disease.”
The present study prospectively followed 4289 asymptomatic first-degree relatives (FDRs) of patients with CD. Environmental factors were identified via regression models that also considered biological factors, including gut inflammation via fecal calprotectin (FCP) levels, altered intestinal permeability measured by urinary fractional excretion of lactulose to mannitol ratio (LMR), and fecal microbiome composition through 16S rRNA sequencing.
After a median follow-up period of 5.62 years, 86 FDRs (1.9%) developed CD.
Living in a household of at least three people in the first year of life was associated with a 57% reduced risk of CD development (hazard ratio [HR], 0.43; P = .019). Similarly, living with a pet dog between the ages of 5 and 15 also demonstrated a protective effect, dropping risk of CD by 39% (HR, 0.61; P = .025).
“Our analysis revealed a protective trend of living with dogs that transcends the age of exposure, suggesting that dog ownership could confer health benefits in reducing the risk of CD,” the investigators wrote. “Our study also found that living in a large family during the first year of life is significantly associated with the future onset of CD, aligning with prior research that indicates that a larger family size in the first year of life can reduce the risk of developing IBD.”
In contrast, the study identified bird ownership at time of recruitment as a risk factor for CD, increasing risk almost three-fold (HR, 2.84; P = .005). The investigators urged a careful interpretation of this latter finding, however, as relatively few FDRs lived with birds.
“[A]lthough our sample size can be considered large, some environmental variables were uncommon, such as the participants having birds as pets, and would greatly benefit from replication of our findings in other cohorts,” Dr. Xue and colleagues noted.
They suggested several possible ways in which the above environmental factors may impact CD risk, including effects on subclinical inflammation, microbiome composition, and gut permeability.
“Understanding the relationship between CD-related environmental factors and these predisease biomarkers may shed light on the underlying mechanisms by which environmental factors impact host health and ultimately lead to CD onset,” the investigators concluded.
The study was supported by Crohn’s and Colitis Canada, Canadian Institutes of Health Research, the Helmsley Charitable Trust, and others. The investigators disclosed no conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Check Out Our New Irritable Bowel Syndrome Clinician Toolkit
AGA’s new irritable bowel syndrome toolkit gathers all our clinical guidance, continuing education materials, patient education resources, and FAQs in one convenient place. Be sure to check it out and bookmark it for easy access!
Curious about our other toolkits? Visit gastro.org/clinical-guidance to explore our toolkits on ulcerative colitis and Crohn’s disease. Keep an eye out for more coming soon!
The toolkit includes clinical guidance on:
- Pharmacological management of irritable bowel syndrome with diarrhea (IBS-D)
- Pharmacological management of irritable bowel syndrome with constipation (IBS-C)
For more resources for ulcerative colitis patients, visit the Patient Center on the AGA website. The AGA Patient Center has a variety of information that can be shared with your patients, including tips on diet, vaccine recommendations, and information on biosimilars.
AGA’s new irritable bowel syndrome toolkit gathers all our clinical guidance, continuing education materials, patient education resources, and FAQs in one convenient place. Be sure to check it out and bookmark it for easy access!
Curious about our other toolkits? Visit gastro.org/clinical-guidance to explore our toolkits on ulcerative colitis and Crohn’s disease. Keep an eye out for more coming soon!
The toolkit includes clinical guidance on:
- Pharmacological management of irritable bowel syndrome with diarrhea (IBS-D)
- Pharmacological management of irritable bowel syndrome with constipation (IBS-C)
For more resources for ulcerative colitis patients, visit the Patient Center on the AGA website. The AGA Patient Center has a variety of information that can be shared with your patients, including tips on diet, vaccine recommendations, and information on biosimilars.
AGA’s new irritable bowel syndrome toolkit gathers all our clinical guidance, continuing education materials, patient education resources, and FAQs in one convenient place. Be sure to check it out and bookmark it for easy access!
Curious about our other toolkits? Visit gastro.org/clinical-guidance to explore our toolkits on ulcerative colitis and Crohn’s disease. Keep an eye out for more coming soon!
The toolkit includes clinical guidance on:
- Pharmacological management of irritable bowel syndrome with diarrhea (IBS-D)
- Pharmacological management of irritable bowel syndrome with constipation (IBS-C)
For more resources for ulcerative colitis patients, visit the Patient Center on the AGA website. The AGA Patient Center has a variety of information that can be shared with your patients, including tips on diet, vaccine recommendations, and information on biosimilars.
August 2024 – ICYMI
Gastroenterology
April 2024
Shah I, et al. Disparities in Colorectal Cancer Screening Among Asian American Populations and Strategies to Address These Disparities. Gastroenterology. 2024 Apr;166(4):549-552. doi: 10.1053/j.gastro.2024.02.009. PMID: 38521575.
Shiha MG, et al. Accuracy of the No-Biopsy Approach for the Diagnosis of Celiac Disease in Adults: A Systematic Review and Meta-Analysis. Gastroenterology. 2024 Apr;166(4):620-630. doi: 10.1053/j.gastro.2023.12.023. Epub 2024 Jan 2. PMID: 38176661.
Goltstein LCMJ, et al. Standard of Care Versus Octreotide in Angiodysplasia-Related Bleeding (the OCEAN Study): A Multicenter Randomized Controlled Trial. Gastroenterology. 2024 Apr;166(4):690-703. doi: 10.1053/j.gastro.2023.12.020. Epub 2023 Dec 28. PMID: 38158089.
May 2024
Robertson DJ, et al. Colonoscopy vs the Fecal Immunochemical Test: Which is Best? Gastroenterology. 2024 May;166(5):758-771. doi: 10.1053/j.gastro.2023.12.027. Epub 2024 Feb 9. PMID: 38342196.
Mårild K, et al. Histologic Remission in Inflammatory Bowel Disease and Female Fertility: A Nationwide Study. Gastroenterology. 2024 May;166(5):802-814.e18. doi: 10.1053/j.gastro.2024.01.018. Epub 2024 Feb 6. PMID: 38331202.
June 2024
Trivedi PJ, et al. Immunopathogenesis of Primary Biliary Cholangitis, Primary Sclerosing Cholangitis and Autoimmune Hepatitis: Themes and Concepts. Gastroenterology. 2024 Jun;166(6):995-1019. doi: 10.1053/j.gastro.2024.01.049. Epub 2024 Feb 10. PMID: 38342195.
Rubenstein JH, et al. AGA Clinical Practice Guideline on Endoscopic Eradication Therapy of Barrett’s Esophagus and Related Neoplasia. Gastroenterology. 2024 Jun;166(6):1020-1055. doi: 10.1053/j.gastro.2024.03.019. PMID: 38763697.
Ridtitid W, et al. Endoscopic Gallbladder Stenting to Prevent Recurrent Cholecystitis in Deferred Cholecystectomy: A Randomized Trial. Gastroenterology. 2024 Jun;166(6):1145-1155. doi: 10.1053/j.gastro.2024.02.007. Epub 2024 Feb 14. PMID: 38360274.
Clinical Gastroenterology and Hepatology
April 2024
Berwald G, et al. The Diagnostic Performance of Fecal Immunochemical Tests for Detecting Advanced Neoplasia at Surveillance Colonoscopy. Clin Gastroenterol Hepatol. 2024 Apr;22(4):878-885.e2. doi: 10.1016/j.cgh.2023.09.016. Epub 2023 Sep 22. PMID: 37743036.
Hashash JG, et al. AGA Rapid Clinical Practice Update on the Management of Patients Taking GLP-1 Receptor Agonists Prior to Endoscopy: Communication. Clin Gastroenterol Hepatol. 2024 Apr;22(4):705-707. doi: 10.1016/j.cgh.2023.11.002. Epub 2023 Nov 7. PMID: 37944573.
Sharma R, et al. Statins Are Associated With a Decreased Risk of Severe Liver Disease in Individuals With Noncirrhotic Chronic Liver Disease. Clin Gastroenterol Hepatol. 2024 Apr;22(4):749-759.e19. doi: 10.1016/j.cgh.2023.04.017. Epub 2023 Apr 28. PMID: 37121528.
May 2024
Overbeek KA, et al; PrescrAIP Study Group. Type 1 Autoimmune Pancreatitis in Europe: Clinical Profile and Response to Treatment. Clin Gastroenterol Hepatol. 2024 May;22(5):994-1004.e10. doi: 10.1016/j.cgh.2023.12.010. Epub 2024 Jan 5. Erratum in: Clin Gastroenterol Hepatol. 2024 Jun 1:S1542-3565(24)00446-4. doi: 10.1016/j.cgh.2024.05.005. PMID: 38184096.
Jairath V, et al. ENTERPRET: A Randomized Controlled Trial of Vedolizumab Dose Optimization in Patients With Ulcerative Colitis Who Have Early Nonresponse. Clin Gastroenterol Hepatol. 2024 May;22(5):1077-1086.e13. doi: 10.1016/j.cgh.2023.10.029. Epub 2023 Nov 10. PMID: 37951560.
Gunby SA, et al. Smoking and Alcohol Consumption and Risk of Incident Diverticulitis in Women. Clin Gastroenterol Hepatol. 2024 May;22(5):1108-1116. doi: 10.1016/j.cgh.2023.11.036. Epub 2023 Dec 19. PMID: 38122959; PMCID: PMC11045313.
June 2024
Krause AJ, et al. Validated Clinical Score to Predict Gastroesophageal Reflux in Patients With Chronic Laryngeal Symptoms: COuGH RefluX. Clin Gastroenterol Hepatol. 2024 Jun;22(6):1200-1209.e1. doi: 10.1016/j.cgh.2024.01.021. Epub 2024 Feb 2. PMID: 38309491; PMCID: PMC11128352.
Peng X, et al. Efficacy and Safety of Vonoprazan-Amoxicillin Dual Regimen With Varying Dose and Duration for Helicobacter pylori Eradication: A Multicenter, Prospective, Randomized Study. Clin Gastroenterol Hepatol. 2024 Jun;22(6):1210-1216. doi: 10.1016/j.cgh.2024.01.022. Epub 2024 Feb 1. PMID: 38309492.
Kedia S, et al. Coconut Water Induces Clinical Remission in Mild to Moderate Ulcerative Colitis: Double-blind Placebo-controlled Trial. Clin Gastroenterol Hepatol. 2024 Jun;22(6):1295-1306.e7. doi: 10.1016/j.cgh.2024.01.013. Epub 2024 Jan 24. PMID: 38278200.
Techniques and Innovations in Gastrointestinal Endoscopy
Ogura T, et al. Step-Up Strategy for Endoscopic Hemostasis Using PuraStat After Endoscopic Sphincterotomy Bleeding (STOP Trial). Tech Innov Gastrointest Endosc. 2024 March 16. doi: 10.1016/j.tige.2024.03.005.
Nakai Y, et al. Cyst Detection Rate: A Quality Indicator in the Era of Pancreatic Screening Endoscopic Ultrasonography. Tech Innov Gastrointest Endosc. 2024 May. doi: 10.1016/j.tige.2024.04.001.
Gastro Hep Advances
Kimura Y, et al. Early Sonographic Improvement Predicts Clinical Remission and Mucosal Healing With Molecular-Targeted Drugs in Ulcerative Colitis. Gastro Hep Adv. 2024 April 22. doi: 10.1016/j.gastha.2024.04.007.
Hunaut T, et al. Long-Term Neoplastic Risk Associated With Colorectal Strictures in Crohn’s Disease: A Multicenter Study. Gastro Hep Adv. 2024 May 15. doi: 10.1016/j.gastha.2024.05.003.
Gastroenterology
April 2024
Shah I, et al. Disparities in Colorectal Cancer Screening Among Asian American Populations and Strategies to Address These Disparities. Gastroenterology. 2024 Apr;166(4):549-552. doi: 10.1053/j.gastro.2024.02.009. PMID: 38521575.
Shiha MG, et al. Accuracy of the No-Biopsy Approach for the Diagnosis of Celiac Disease in Adults: A Systematic Review and Meta-Analysis. Gastroenterology. 2024 Apr;166(4):620-630. doi: 10.1053/j.gastro.2023.12.023. Epub 2024 Jan 2. PMID: 38176661.
Goltstein LCMJ, et al. Standard of Care Versus Octreotide in Angiodysplasia-Related Bleeding (the OCEAN Study): A Multicenter Randomized Controlled Trial. Gastroenterology. 2024 Apr;166(4):690-703. doi: 10.1053/j.gastro.2023.12.020. Epub 2023 Dec 28. PMID: 38158089.
May 2024
Robertson DJ, et al. Colonoscopy vs the Fecal Immunochemical Test: Which is Best? Gastroenterology. 2024 May;166(5):758-771. doi: 10.1053/j.gastro.2023.12.027. Epub 2024 Feb 9. PMID: 38342196.
Mårild K, et al. Histologic Remission in Inflammatory Bowel Disease and Female Fertility: A Nationwide Study. Gastroenterology. 2024 May;166(5):802-814.e18. doi: 10.1053/j.gastro.2024.01.018. Epub 2024 Feb 6. PMID: 38331202.
June 2024
Trivedi PJ, et al. Immunopathogenesis of Primary Biliary Cholangitis, Primary Sclerosing Cholangitis and Autoimmune Hepatitis: Themes and Concepts. Gastroenterology. 2024 Jun;166(6):995-1019. doi: 10.1053/j.gastro.2024.01.049. Epub 2024 Feb 10. PMID: 38342195.
Rubenstein JH, et al. AGA Clinical Practice Guideline on Endoscopic Eradication Therapy of Barrett’s Esophagus and Related Neoplasia. Gastroenterology. 2024 Jun;166(6):1020-1055. doi: 10.1053/j.gastro.2024.03.019. PMID: 38763697.
Ridtitid W, et al. Endoscopic Gallbladder Stenting to Prevent Recurrent Cholecystitis in Deferred Cholecystectomy: A Randomized Trial. Gastroenterology. 2024 Jun;166(6):1145-1155. doi: 10.1053/j.gastro.2024.02.007. Epub 2024 Feb 14. PMID: 38360274.
Clinical Gastroenterology and Hepatology
April 2024
Berwald G, et al. The Diagnostic Performance of Fecal Immunochemical Tests for Detecting Advanced Neoplasia at Surveillance Colonoscopy. Clin Gastroenterol Hepatol. 2024 Apr;22(4):878-885.e2. doi: 10.1016/j.cgh.2023.09.016. Epub 2023 Sep 22. PMID: 37743036.
Hashash JG, et al. AGA Rapid Clinical Practice Update on the Management of Patients Taking GLP-1 Receptor Agonists Prior to Endoscopy: Communication. Clin Gastroenterol Hepatol. 2024 Apr;22(4):705-707. doi: 10.1016/j.cgh.2023.11.002. Epub 2023 Nov 7. PMID: 37944573.
Sharma R, et al. Statins Are Associated With a Decreased Risk of Severe Liver Disease in Individuals With Noncirrhotic Chronic Liver Disease. Clin Gastroenterol Hepatol. 2024 Apr;22(4):749-759.e19. doi: 10.1016/j.cgh.2023.04.017. Epub 2023 Apr 28. PMID: 37121528.
May 2024
Overbeek KA, et al; PrescrAIP Study Group. Type 1 Autoimmune Pancreatitis in Europe: Clinical Profile and Response to Treatment. Clin Gastroenterol Hepatol. 2024 May;22(5):994-1004.e10. doi: 10.1016/j.cgh.2023.12.010. Epub 2024 Jan 5. Erratum in: Clin Gastroenterol Hepatol. 2024 Jun 1:S1542-3565(24)00446-4. doi: 10.1016/j.cgh.2024.05.005. PMID: 38184096.
Jairath V, et al. ENTERPRET: A Randomized Controlled Trial of Vedolizumab Dose Optimization in Patients With Ulcerative Colitis Who Have Early Nonresponse. Clin Gastroenterol Hepatol. 2024 May;22(5):1077-1086.e13. doi: 10.1016/j.cgh.2023.10.029. Epub 2023 Nov 10. PMID: 37951560.
Gunby SA, et al. Smoking and Alcohol Consumption and Risk of Incident Diverticulitis in Women. Clin Gastroenterol Hepatol. 2024 May;22(5):1108-1116. doi: 10.1016/j.cgh.2023.11.036. Epub 2023 Dec 19. PMID: 38122959; PMCID: PMC11045313.
June 2024
Krause AJ, et al. Validated Clinical Score to Predict Gastroesophageal Reflux in Patients With Chronic Laryngeal Symptoms: COuGH RefluX. Clin Gastroenterol Hepatol. 2024 Jun;22(6):1200-1209.e1. doi: 10.1016/j.cgh.2024.01.021. Epub 2024 Feb 2. PMID: 38309491; PMCID: PMC11128352.
Peng X, et al. Efficacy and Safety of Vonoprazan-Amoxicillin Dual Regimen With Varying Dose and Duration for Helicobacter pylori Eradication: A Multicenter, Prospective, Randomized Study. Clin Gastroenterol Hepatol. 2024 Jun;22(6):1210-1216. doi: 10.1016/j.cgh.2024.01.022. Epub 2024 Feb 1. PMID: 38309492.
Kedia S, et al. Coconut Water Induces Clinical Remission in Mild to Moderate Ulcerative Colitis: Double-blind Placebo-controlled Trial. Clin Gastroenterol Hepatol. 2024 Jun;22(6):1295-1306.e7. doi: 10.1016/j.cgh.2024.01.013. Epub 2024 Jan 24. PMID: 38278200.
Techniques and Innovations in Gastrointestinal Endoscopy
Ogura T, et al. Step-Up Strategy for Endoscopic Hemostasis Using PuraStat After Endoscopic Sphincterotomy Bleeding (STOP Trial). Tech Innov Gastrointest Endosc. 2024 March 16. doi: 10.1016/j.tige.2024.03.005.
Nakai Y, et al. Cyst Detection Rate: A Quality Indicator in the Era of Pancreatic Screening Endoscopic Ultrasonography. Tech Innov Gastrointest Endosc. 2024 May. doi: 10.1016/j.tige.2024.04.001.
Gastro Hep Advances
Kimura Y, et al. Early Sonographic Improvement Predicts Clinical Remission and Mucosal Healing With Molecular-Targeted Drugs in Ulcerative Colitis. Gastro Hep Adv. 2024 April 22. doi: 10.1016/j.gastha.2024.04.007.
Hunaut T, et al. Long-Term Neoplastic Risk Associated With Colorectal Strictures in Crohn’s Disease: A Multicenter Study. Gastro Hep Adv. 2024 May 15. doi: 10.1016/j.gastha.2024.05.003.
Gastroenterology
April 2024
Shah I, et al. Disparities in Colorectal Cancer Screening Among Asian American Populations and Strategies to Address These Disparities. Gastroenterology. 2024 Apr;166(4):549-552. doi: 10.1053/j.gastro.2024.02.009. PMID: 38521575.
Shiha MG, et al. Accuracy of the No-Biopsy Approach for the Diagnosis of Celiac Disease in Adults: A Systematic Review and Meta-Analysis. Gastroenterology. 2024 Apr;166(4):620-630. doi: 10.1053/j.gastro.2023.12.023. Epub 2024 Jan 2. PMID: 38176661.
Goltstein LCMJ, et al. Standard of Care Versus Octreotide in Angiodysplasia-Related Bleeding (the OCEAN Study): A Multicenter Randomized Controlled Trial. Gastroenterology. 2024 Apr;166(4):690-703. doi: 10.1053/j.gastro.2023.12.020. Epub 2023 Dec 28. PMID: 38158089.
May 2024
Robertson DJ, et al. Colonoscopy vs the Fecal Immunochemical Test: Which is Best? Gastroenterology. 2024 May;166(5):758-771. doi: 10.1053/j.gastro.2023.12.027. Epub 2024 Feb 9. PMID: 38342196.
Mårild K, et al. Histologic Remission in Inflammatory Bowel Disease and Female Fertility: A Nationwide Study. Gastroenterology. 2024 May;166(5):802-814.e18. doi: 10.1053/j.gastro.2024.01.018. Epub 2024 Feb 6. PMID: 38331202.
June 2024
Trivedi PJ, et al. Immunopathogenesis of Primary Biliary Cholangitis, Primary Sclerosing Cholangitis and Autoimmune Hepatitis: Themes and Concepts. Gastroenterology. 2024 Jun;166(6):995-1019. doi: 10.1053/j.gastro.2024.01.049. Epub 2024 Feb 10. PMID: 38342195.
Rubenstein JH, et al. AGA Clinical Practice Guideline on Endoscopic Eradication Therapy of Barrett’s Esophagus and Related Neoplasia. Gastroenterology. 2024 Jun;166(6):1020-1055. doi: 10.1053/j.gastro.2024.03.019. PMID: 38763697.
Ridtitid W, et al. Endoscopic Gallbladder Stenting to Prevent Recurrent Cholecystitis in Deferred Cholecystectomy: A Randomized Trial. Gastroenterology. 2024 Jun;166(6):1145-1155. doi: 10.1053/j.gastro.2024.02.007. Epub 2024 Feb 14. PMID: 38360274.
Clinical Gastroenterology and Hepatology
April 2024
Berwald G, et al. The Diagnostic Performance of Fecal Immunochemical Tests for Detecting Advanced Neoplasia at Surveillance Colonoscopy. Clin Gastroenterol Hepatol. 2024 Apr;22(4):878-885.e2. doi: 10.1016/j.cgh.2023.09.016. Epub 2023 Sep 22. PMID: 37743036.
Hashash JG, et al. AGA Rapid Clinical Practice Update on the Management of Patients Taking GLP-1 Receptor Agonists Prior to Endoscopy: Communication. Clin Gastroenterol Hepatol. 2024 Apr;22(4):705-707. doi: 10.1016/j.cgh.2023.11.002. Epub 2023 Nov 7. PMID: 37944573.
Sharma R, et al. Statins Are Associated With a Decreased Risk of Severe Liver Disease in Individuals With Noncirrhotic Chronic Liver Disease. Clin Gastroenterol Hepatol. 2024 Apr;22(4):749-759.e19. doi: 10.1016/j.cgh.2023.04.017. Epub 2023 Apr 28. PMID: 37121528.
May 2024
Overbeek KA, et al; PrescrAIP Study Group. Type 1 Autoimmune Pancreatitis in Europe: Clinical Profile and Response to Treatment. Clin Gastroenterol Hepatol. 2024 May;22(5):994-1004.e10. doi: 10.1016/j.cgh.2023.12.010. Epub 2024 Jan 5. Erratum in: Clin Gastroenterol Hepatol. 2024 Jun 1:S1542-3565(24)00446-4. doi: 10.1016/j.cgh.2024.05.005. PMID: 38184096.
Jairath V, et al. ENTERPRET: A Randomized Controlled Trial of Vedolizumab Dose Optimization in Patients With Ulcerative Colitis Who Have Early Nonresponse. Clin Gastroenterol Hepatol. 2024 May;22(5):1077-1086.e13. doi: 10.1016/j.cgh.2023.10.029. Epub 2023 Nov 10. PMID: 37951560.
Gunby SA, et al. Smoking and Alcohol Consumption and Risk of Incident Diverticulitis in Women. Clin Gastroenterol Hepatol. 2024 May;22(5):1108-1116. doi: 10.1016/j.cgh.2023.11.036. Epub 2023 Dec 19. PMID: 38122959; PMCID: PMC11045313.
June 2024
Krause AJ, et al. Validated Clinical Score to Predict Gastroesophageal Reflux in Patients With Chronic Laryngeal Symptoms: COuGH RefluX. Clin Gastroenterol Hepatol. 2024 Jun;22(6):1200-1209.e1. doi: 10.1016/j.cgh.2024.01.021. Epub 2024 Feb 2. PMID: 38309491; PMCID: PMC11128352.
Peng X, et al. Efficacy and Safety of Vonoprazan-Amoxicillin Dual Regimen With Varying Dose and Duration for Helicobacter pylori Eradication: A Multicenter, Prospective, Randomized Study. Clin Gastroenterol Hepatol. 2024 Jun;22(6):1210-1216. doi: 10.1016/j.cgh.2024.01.022. Epub 2024 Feb 1. PMID: 38309492.
Kedia S, et al. Coconut Water Induces Clinical Remission in Mild to Moderate Ulcerative Colitis: Double-blind Placebo-controlled Trial. Clin Gastroenterol Hepatol. 2024 Jun;22(6):1295-1306.e7. doi: 10.1016/j.cgh.2024.01.013. Epub 2024 Jan 24. PMID: 38278200.
Techniques and Innovations in Gastrointestinal Endoscopy
Ogura T, et al. Step-Up Strategy for Endoscopic Hemostasis Using PuraStat After Endoscopic Sphincterotomy Bleeding (STOP Trial). Tech Innov Gastrointest Endosc. 2024 March 16. doi: 10.1016/j.tige.2024.03.005.
Nakai Y, et al. Cyst Detection Rate: A Quality Indicator in the Era of Pancreatic Screening Endoscopic Ultrasonography. Tech Innov Gastrointest Endosc. 2024 May. doi: 10.1016/j.tige.2024.04.001.
Gastro Hep Advances
Kimura Y, et al. Early Sonographic Improvement Predicts Clinical Remission and Mucosal Healing With Molecular-Targeted Drugs in Ulcerative Colitis. Gastro Hep Adv. 2024 April 22. doi: 10.1016/j.gastha.2024.04.007.
Hunaut T, et al. Long-Term Neoplastic Risk Associated With Colorectal Strictures in Crohn’s Disease: A Multicenter Study. Gastro Hep Adv. 2024 May 15. doi: 10.1016/j.gastha.2024.05.003.
Check Out Our New Ulcerative Colitis Clinician Toolkit
Check out and bookmark AGA’s new ulcerative colitis toolkit, which compiles all our ulcerative colitis clinical guidance, continuing education resources, patient education, and FAQs into one convenient toolkit.
Curious about our other toolkits? Check out our toolkit on Crohn’s disease.
The new UC toolkit includes clinical guidance on:
- Role of biomarkers for the management of ulcerative colitis
- Medical management of moderate to severe ulcerative colitis
- Management of pouchitis and inflammatory pouch disorders
For more resources for ulcerative colitis patients, visit the Patient Center on the AGA website.
The AGA Patient Center has a variety of information that can be shared with your patients, including tips on diet, vaccine recommendations, and information on biosimilars.
Check out and bookmark AGA’s new ulcerative colitis toolkit, which compiles all our ulcerative colitis clinical guidance, continuing education resources, patient education, and FAQs into one convenient toolkit.
Curious about our other toolkits? Check out our toolkit on Crohn’s disease.
The new UC toolkit includes clinical guidance on:
- Role of biomarkers for the management of ulcerative colitis
- Medical management of moderate to severe ulcerative colitis
- Management of pouchitis and inflammatory pouch disorders
For more resources for ulcerative colitis patients, visit the Patient Center on the AGA website.
The AGA Patient Center has a variety of information that can be shared with your patients, including tips on diet, vaccine recommendations, and information on biosimilars.
Check out and bookmark AGA’s new ulcerative colitis toolkit, which compiles all our ulcerative colitis clinical guidance, continuing education resources, patient education, and FAQs into one convenient toolkit.
Curious about our other toolkits? Check out our toolkit on Crohn’s disease.
The new UC toolkit includes clinical guidance on:
- Role of biomarkers for the management of ulcerative colitis
- Medical management of moderate to severe ulcerative colitis
- Management of pouchitis and inflammatory pouch disorders
For more resources for ulcerative colitis patients, visit the Patient Center on the AGA website.
The AGA Patient Center has a variety of information that can be shared with your patients, including tips on diet, vaccine recommendations, and information on biosimilars.
A Fitbit for the Gut May Aid in Detection of GI Disorders
, new research revealed.
Traditional methods for locating, measuring, and monitoring gasses associated with such disorders as irritable bowel syndrome, inflammatory bowel disease, food intolerances, and gastric cancers are often invasive and typically require hospital-based procedures.
This experimental system, developed by a team at the University of Southern California’s Viterbi School of Engineering, Los Angeles, represents “a significant step forward in ingestible technology,” according to principal investigator Yasser Khan, PhD, and colleagues.
The novel ingestible could someday serve as a “Fitbit for the gut” and aid in early disease detection, Dr. Khan said.
The team’s work was published online in Cell Reports Physical Science.
Real-Time Tracking
While wearables with sensors are a promising way to monitor body functions, the ability to track ingestible devices once they are inside the body has been limited.
To solve this problem, the researchers developed a system that includes a wearable coil (placed on a T-shirt for this study) and an ingestible pill with a 3D-printed shell made from a biocompatible resin.
The pill is equipped with a gas-permeable membrane, an optical gas-sensing membrane, an optical filter, and a printed circuit board that houses its electronic components. The gas sensor can detect oxygen in the 0%-20% range and ammonia in the 0-100 ppm concentration range.
The researchers developed various algorithms and conducted experiments to test the system’s ability to decode the pill’s location in a human gut model and in an ex vivo animal intestine. To simulate the in vivo environment, they tested the system in an agar phantom solution, which enabled them to track the pill’s movement.
So, how does it work?
Simply put, once the patient ingests the pill, a phone application connects to the pill over Bluetooth and sends a command to initiate the target gas and magnetic field measurements.
Next, the wearable coil generates a magnetic field, which is captured by a magnetic sensor on the pill, enabling the pill’s location to be decoded in real time.
Then, using optical absorption spectroscopy with a light-emitting diode, a photodiode, and the pill’s gas-sensing membrane, gasses such as oxygen and ammonia can be measured and mapped in 3D while the pill is in the gut.
Notably, elevated levels of ammonia, which is produced by Helicobacter pylori, could serve as a signal for peptic ulcers, gastric cancer, or irritable bowel syndrome, Dr. Khan said.
“The ingestible system with the wearable coil is both compact and practical, offering a clear path for application in human health,” he said. The work also could “empower patients to conveniently assess their GI gas profiles from home and manage their digestive health.”
The next step is to test the wearable in animal models to assess, among other factors, whether the gas-sensing system “will operate properly in biological tissue and whether clogging or coating with GI liquids and food particles causes sensor fouling and affects the measurement accuracy,” Dr. Khan and colleagues noted.
Dr. Khan acknowledges support from USC Viterbi School of Engineering. A provisional patent application has been filed based on the technology described in this work. During the preparation of this work, the authors used ChatGPT to check for grammatical errors in the writing. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
A version of this article first appeared on Medscape.com.
, new research revealed.
Traditional methods for locating, measuring, and monitoring gasses associated with such disorders as irritable bowel syndrome, inflammatory bowel disease, food intolerances, and gastric cancers are often invasive and typically require hospital-based procedures.
This experimental system, developed by a team at the University of Southern California’s Viterbi School of Engineering, Los Angeles, represents “a significant step forward in ingestible technology,” according to principal investigator Yasser Khan, PhD, and colleagues.
The novel ingestible could someday serve as a “Fitbit for the gut” and aid in early disease detection, Dr. Khan said.
The team’s work was published online in Cell Reports Physical Science.
Real-Time Tracking
While wearables with sensors are a promising way to monitor body functions, the ability to track ingestible devices once they are inside the body has been limited.
To solve this problem, the researchers developed a system that includes a wearable coil (placed on a T-shirt for this study) and an ingestible pill with a 3D-printed shell made from a biocompatible resin.
The pill is equipped with a gas-permeable membrane, an optical gas-sensing membrane, an optical filter, and a printed circuit board that houses its electronic components. The gas sensor can detect oxygen in the 0%-20% range and ammonia in the 0-100 ppm concentration range.
The researchers developed various algorithms and conducted experiments to test the system’s ability to decode the pill’s location in a human gut model and in an ex vivo animal intestine. To simulate the in vivo environment, they tested the system in an agar phantom solution, which enabled them to track the pill’s movement.
So, how does it work?
Simply put, once the patient ingests the pill, a phone application connects to the pill over Bluetooth and sends a command to initiate the target gas and magnetic field measurements.
Next, the wearable coil generates a magnetic field, which is captured by a magnetic sensor on the pill, enabling the pill’s location to be decoded in real time.
Then, using optical absorption spectroscopy with a light-emitting diode, a photodiode, and the pill’s gas-sensing membrane, gasses such as oxygen and ammonia can be measured and mapped in 3D while the pill is in the gut.
Notably, elevated levels of ammonia, which is produced by Helicobacter pylori, could serve as a signal for peptic ulcers, gastric cancer, or irritable bowel syndrome, Dr. Khan said.
“The ingestible system with the wearable coil is both compact and practical, offering a clear path for application in human health,” he said. The work also could “empower patients to conveniently assess their GI gas profiles from home and manage their digestive health.”
The next step is to test the wearable in animal models to assess, among other factors, whether the gas-sensing system “will operate properly in biological tissue and whether clogging or coating with GI liquids and food particles causes sensor fouling and affects the measurement accuracy,” Dr. Khan and colleagues noted.
Dr. Khan acknowledges support from USC Viterbi School of Engineering. A provisional patent application has been filed based on the technology described in this work. During the preparation of this work, the authors used ChatGPT to check for grammatical errors in the writing. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
A version of this article first appeared on Medscape.com.
, new research revealed.
Traditional methods for locating, measuring, and monitoring gasses associated with such disorders as irritable bowel syndrome, inflammatory bowel disease, food intolerances, and gastric cancers are often invasive and typically require hospital-based procedures.
This experimental system, developed by a team at the University of Southern California’s Viterbi School of Engineering, Los Angeles, represents “a significant step forward in ingestible technology,” according to principal investigator Yasser Khan, PhD, and colleagues.
The novel ingestible could someday serve as a “Fitbit for the gut” and aid in early disease detection, Dr. Khan said.
The team’s work was published online in Cell Reports Physical Science.
Real-Time Tracking
While wearables with sensors are a promising way to monitor body functions, the ability to track ingestible devices once they are inside the body has been limited.
To solve this problem, the researchers developed a system that includes a wearable coil (placed on a T-shirt for this study) and an ingestible pill with a 3D-printed shell made from a biocompatible resin.
The pill is equipped with a gas-permeable membrane, an optical gas-sensing membrane, an optical filter, and a printed circuit board that houses its electronic components. The gas sensor can detect oxygen in the 0%-20% range and ammonia in the 0-100 ppm concentration range.
The researchers developed various algorithms and conducted experiments to test the system’s ability to decode the pill’s location in a human gut model and in an ex vivo animal intestine. To simulate the in vivo environment, they tested the system in an agar phantom solution, which enabled them to track the pill’s movement.
So, how does it work?
Simply put, once the patient ingests the pill, a phone application connects to the pill over Bluetooth and sends a command to initiate the target gas and magnetic field measurements.
Next, the wearable coil generates a magnetic field, which is captured by a magnetic sensor on the pill, enabling the pill’s location to be decoded in real time.
Then, using optical absorption spectroscopy with a light-emitting diode, a photodiode, and the pill’s gas-sensing membrane, gasses such as oxygen and ammonia can be measured and mapped in 3D while the pill is in the gut.
Notably, elevated levels of ammonia, which is produced by Helicobacter pylori, could serve as a signal for peptic ulcers, gastric cancer, or irritable bowel syndrome, Dr. Khan said.
“The ingestible system with the wearable coil is both compact and practical, offering a clear path for application in human health,” he said. The work also could “empower patients to conveniently assess their GI gas profiles from home and manage their digestive health.”
The next step is to test the wearable in animal models to assess, among other factors, whether the gas-sensing system “will operate properly in biological tissue and whether clogging or coating with GI liquids and food particles causes sensor fouling and affects the measurement accuracy,” Dr. Khan and colleagues noted.
Dr. Khan acknowledges support from USC Viterbi School of Engineering. A provisional patent application has been filed based on the technology described in this work. During the preparation of this work, the authors used ChatGPT to check for grammatical errors in the writing. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
A version of this article first appeared on Medscape.com.
FROM CELL REPORTS PHYSICAL SCIENCE
FDA Approves Skyrizi for Ulcerative Colitis
This approval makes it the first specific anti-interleukin 23 monoclonal antibody indicated for both ulcerative colitis and moderate to severe Crohn’s disease.
The drug is also approved in the United States for the treatment of adults with active psoriatic arthritis and moderate to severe plaque psoriasis.
The safety and efficacy of Skyrizi for ulcerative colitis is supported by data from two phase 3 clinical trials: a 12-week induction study (INSPIRE) and a 52-week maintenance study (COMMAND).
The data showed that clinical remission, the primary endpoint in both the induction and maintenance studies, was achieved along with endoscopic improvement, which was a key secondary endpoint.
“When treating patients with ulcerative colitis, it’s important to prioritize both early and sustained clinical remission as well as endoscopic improvement,” Edward V. Loftus Jr., MD, AGAF, gastroenterologist at Mayo Clinic in Rochester, Minnesota, said in a news release. “This approval for Skyrizi is an important step toward addressing these treatment goals.”
For the treatment of ulcerative colitis, dosing includes a 12-week induction period with three 1200-mg doses delivered every 4 weeks followed by maintenance therapy of either 180 mg or 360 mg delivered every 8 weeks.
After the induction period, Skyrizi treatment can be maintained at home using an on-body injector (OBI). “The OBI is a hands-free device designed with patients in mind that adheres to the body and takes about 5 minutes to deliver the medication following preparation steps,” according to the news release.
Full prescribing information is available online.
A version of this article appeared on Medscape.com.
This approval makes it the first specific anti-interleukin 23 monoclonal antibody indicated for both ulcerative colitis and moderate to severe Crohn’s disease.
The drug is also approved in the United States for the treatment of adults with active psoriatic arthritis and moderate to severe plaque psoriasis.
The safety and efficacy of Skyrizi for ulcerative colitis is supported by data from two phase 3 clinical trials: a 12-week induction study (INSPIRE) and a 52-week maintenance study (COMMAND).
The data showed that clinical remission, the primary endpoint in both the induction and maintenance studies, was achieved along with endoscopic improvement, which was a key secondary endpoint.
“When treating patients with ulcerative colitis, it’s important to prioritize both early and sustained clinical remission as well as endoscopic improvement,” Edward V. Loftus Jr., MD, AGAF, gastroenterologist at Mayo Clinic in Rochester, Minnesota, said in a news release. “This approval for Skyrizi is an important step toward addressing these treatment goals.”
For the treatment of ulcerative colitis, dosing includes a 12-week induction period with three 1200-mg doses delivered every 4 weeks followed by maintenance therapy of either 180 mg or 360 mg delivered every 8 weeks.
After the induction period, Skyrizi treatment can be maintained at home using an on-body injector (OBI). “The OBI is a hands-free device designed with patients in mind that adheres to the body and takes about 5 minutes to deliver the medication following preparation steps,” according to the news release.
Full prescribing information is available online.
A version of this article appeared on Medscape.com.
This approval makes it the first specific anti-interleukin 23 monoclonal antibody indicated for both ulcerative colitis and moderate to severe Crohn’s disease.
The drug is also approved in the United States for the treatment of adults with active psoriatic arthritis and moderate to severe plaque psoriasis.
The safety and efficacy of Skyrizi for ulcerative colitis is supported by data from two phase 3 clinical trials: a 12-week induction study (INSPIRE) and a 52-week maintenance study (COMMAND).
The data showed that clinical remission, the primary endpoint in both the induction and maintenance studies, was achieved along with endoscopic improvement, which was a key secondary endpoint.
“When treating patients with ulcerative colitis, it’s important to prioritize both early and sustained clinical remission as well as endoscopic improvement,” Edward V. Loftus Jr., MD, AGAF, gastroenterologist at Mayo Clinic in Rochester, Minnesota, said in a news release. “This approval for Skyrizi is an important step toward addressing these treatment goals.”
For the treatment of ulcerative colitis, dosing includes a 12-week induction period with three 1200-mg doses delivered every 4 weeks followed by maintenance therapy of either 180 mg or 360 mg delivered every 8 weeks.
After the induction period, Skyrizi treatment can be maintained at home using an on-body injector (OBI). “The OBI is a hands-free device designed with patients in mind that adheres to the body and takes about 5 minutes to deliver the medication following preparation steps,” according to the news release.
Full prescribing information is available online.
A version of this article appeared on Medscape.com.
Autonomous AI Outperforms Humans in Optical Diagnosis of Colorectal Polyps
, while providing greater alignment with pathology-based surveillance intervals, based on a randomized controlled trial.
These findings suggest that autonomous AI may one day replace histologic assessment of diminutive polyps, reported lead author Roupen Djinbachian, MD, of the Montreal University Hospital Research Center, Montreal, Quebec, Canada, and colleagues.Optical diagnosis of diminutive colorectal polyps has been proposed as a cost-effective alternative to histologic diagnosis, but its implementation in general clinical practice has been hindered by endoscopists’ concerns about incorrect diagnoses, the investigators wrote in Gastroenterology.“AI-based systems (CADx) have been proposed as a solution to these barriers to implementation, with studies showing high adherence to Preservation and Incorporation of Valuable Endoscopic Innovations (PIVI) thresholds when using AI-H,” they wrote. “However, the efficacy and safety of autonomous AI-based diagnostic platforms have not yet been evaluated.”
To address this knowledge gap, Dr. Djinbachian and colleagues conducted a randomized controlled noninferiority trial involving 467 patients, all of whom underwent elective colonoscopies at a single academic institution.
Participants were randomly assigned to one of two groups. The first group received an optical diagnosis of diminutive (1-5 mm) colorectal polyps using an autonomous AI-based CADx system without any human input. The second group had diagnoses performed by endoscopists who used AI-H to make their optical diagnoses.
The primary outcome was the accuracy of optical diagnosis compared with the gold standard of histologic evaluation. Secondarily, the investigators explored associations between pathology-based surveillance intervals and various measures of accuracy, including sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV).
The results showed that the accuracy of optical diagnosis for diminutive polyps was similar between the two groups, supporting noninferiority. Autonomous AI achieved an accuracy rate of 77.2%, while the AI-H group had an accuracy of 72.1%, which was not statistically significant (P = .86).
But when it came to pathology-based surveillance intervals, autonomous AI showed a clear advantage; the autonomous AI system achieved a 91.5% agreement rate, compared with 82.1% for the AI-H group (P = .016).
“These findings indicate that autonomous AI not only matches but also surpasses AI-H in accuracy for determining surveillance intervals,” the investigators wrote, noting that this finding highlights the “complexities of human interaction with AI modules where human intervention could lead to worse outcomes.”
Further analysis revealed that the sensitivity of autonomous AI for identifying adenomas was 84.8%, slightly higher than the 83.6% sensitivity of the AI-H group. Specificity was 64.4% for autonomous AI vs 63.8% for AI-H. While PPV was higher in the autonomous AI group (85.6%), compared with the AI-H group (78.6%), NPV was lower for autonomous AI than AI-H (63.0% vs 71.0%).
Dr. Djinbachian and colleagues suggested that future research should focus on larger, multicenter trials to validate these findings and further explore the integration of autonomous AI systems in clinical practice. They also noted that improving AI algorithms to accurately diagnose sessile serrated lesions could enhance the overall effectiveness of AI-based optical diagnosis.
“The performance of autonomous AI in accurately diagnosing diminutive polyps and determining appropriate surveillance intervals suggests that it could play a crucial role in streamlining colorectal cancer screening processes, reducing the burden on pathologists, and potentially lowering healthcare costs,” the investigators concluded.The study was supported by Fujifilm, which had no role in the study design or data analysis. Dr. von Renteln reported additional research funding from Vantage and Fujifilm.
In the era of computer vision for endoscopy and colonoscopy, current paradigms rely on AI as a co-pilot or second observer, with the physician serving as the final arbiter in procedure-related decision-making. This study by Djinbachian and Haumesser et al brings up the interesting wrinkle of autonomous AI as a potentially superior (or noninferior) option in narrow, task-specific use cases.
In this study, human input from the endoscopist after CADx diagnosis led to lower agreement between the AI-predicted diagnosis and corresponding surveillance intervals; human oversight more often incorrectly changed the resultant diagnosis and led to shorter than recommended surveillance intervals.
This study offers a small but very important update to the growing body of literature on CADx in colonoscopy. So far, prospective validation of CADx compared with the human eye for in-situ diagnosis of polyps has provided mixed results. This study is one of the first to examine the potential role of “automatic” CADx without additional human input and sheds light on the importance of the AI-human hybrid in medical care. How do the ways in which humans interact with the user interface and output of AI lead to changes in outcome? How can we optimize the AI-human interaction in order to provide optimal results?
Jeremy R. Glissen Brown is an assistant professor in the Department of Internal Medicine and Division of Gastroenterology at Duke University Medical Center, Durham, North Carolina. He has served as a consultant for Medtronic and Olympus, and on the advisory board for Odin Vision.
In the era of computer vision for endoscopy and colonoscopy, current paradigms rely on AI as a co-pilot or second observer, with the physician serving as the final arbiter in procedure-related decision-making. This study by Djinbachian and Haumesser et al brings up the interesting wrinkle of autonomous AI as a potentially superior (or noninferior) option in narrow, task-specific use cases.
In this study, human input from the endoscopist after CADx diagnosis led to lower agreement between the AI-predicted diagnosis and corresponding surveillance intervals; human oversight more often incorrectly changed the resultant diagnosis and led to shorter than recommended surveillance intervals.
This study offers a small but very important update to the growing body of literature on CADx in colonoscopy. So far, prospective validation of CADx compared with the human eye for in-situ diagnosis of polyps has provided mixed results. This study is one of the first to examine the potential role of “automatic” CADx without additional human input and sheds light on the importance of the AI-human hybrid in medical care. How do the ways in which humans interact with the user interface and output of AI lead to changes in outcome? How can we optimize the AI-human interaction in order to provide optimal results?
Jeremy R. Glissen Brown is an assistant professor in the Department of Internal Medicine and Division of Gastroenterology at Duke University Medical Center, Durham, North Carolina. He has served as a consultant for Medtronic and Olympus, and on the advisory board for Odin Vision.
In the era of computer vision for endoscopy and colonoscopy, current paradigms rely on AI as a co-pilot or second observer, with the physician serving as the final arbiter in procedure-related decision-making. This study by Djinbachian and Haumesser et al brings up the interesting wrinkle of autonomous AI as a potentially superior (or noninferior) option in narrow, task-specific use cases.
In this study, human input from the endoscopist after CADx diagnosis led to lower agreement between the AI-predicted diagnosis and corresponding surveillance intervals; human oversight more often incorrectly changed the resultant diagnosis and led to shorter than recommended surveillance intervals.
This study offers a small but very important update to the growing body of literature on CADx in colonoscopy. So far, prospective validation of CADx compared with the human eye for in-situ diagnosis of polyps has provided mixed results. This study is one of the first to examine the potential role of “automatic” CADx without additional human input and sheds light on the importance of the AI-human hybrid in medical care. How do the ways in which humans interact with the user interface and output of AI lead to changes in outcome? How can we optimize the AI-human interaction in order to provide optimal results?
Jeremy R. Glissen Brown is an assistant professor in the Department of Internal Medicine and Division of Gastroenterology at Duke University Medical Center, Durham, North Carolina. He has served as a consultant for Medtronic and Olympus, and on the advisory board for Odin Vision.
, while providing greater alignment with pathology-based surveillance intervals, based on a randomized controlled trial.
These findings suggest that autonomous AI may one day replace histologic assessment of diminutive polyps, reported lead author Roupen Djinbachian, MD, of the Montreal University Hospital Research Center, Montreal, Quebec, Canada, and colleagues.Optical diagnosis of diminutive colorectal polyps has been proposed as a cost-effective alternative to histologic diagnosis, but its implementation in general clinical practice has been hindered by endoscopists’ concerns about incorrect diagnoses, the investigators wrote in Gastroenterology.“AI-based systems (CADx) have been proposed as a solution to these barriers to implementation, with studies showing high adherence to Preservation and Incorporation of Valuable Endoscopic Innovations (PIVI) thresholds when using AI-H,” they wrote. “However, the efficacy and safety of autonomous AI-based diagnostic platforms have not yet been evaluated.”
To address this knowledge gap, Dr. Djinbachian and colleagues conducted a randomized controlled noninferiority trial involving 467 patients, all of whom underwent elective colonoscopies at a single academic institution.
Participants were randomly assigned to one of two groups. The first group received an optical diagnosis of diminutive (1-5 mm) colorectal polyps using an autonomous AI-based CADx system without any human input. The second group had diagnoses performed by endoscopists who used AI-H to make their optical diagnoses.
The primary outcome was the accuracy of optical diagnosis compared with the gold standard of histologic evaluation. Secondarily, the investigators explored associations between pathology-based surveillance intervals and various measures of accuracy, including sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV).
The results showed that the accuracy of optical diagnosis for diminutive polyps was similar between the two groups, supporting noninferiority. Autonomous AI achieved an accuracy rate of 77.2%, while the AI-H group had an accuracy of 72.1%, which was not statistically significant (P = .86).
But when it came to pathology-based surveillance intervals, autonomous AI showed a clear advantage; the autonomous AI system achieved a 91.5% agreement rate, compared with 82.1% for the AI-H group (P = .016).
“These findings indicate that autonomous AI not only matches but also surpasses AI-H in accuracy for determining surveillance intervals,” the investigators wrote, noting that this finding highlights the “complexities of human interaction with AI modules where human intervention could lead to worse outcomes.”
Further analysis revealed that the sensitivity of autonomous AI for identifying adenomas was 84.8%, slightly higher than the 83.6% sensitivity of the AI-H group. Specificity was 64.4% for autonomous AI vs 63.8% for AI-H. While PPV was higher in the autonomous AI group (85.6%), compared with the AI-H group (78.6%), NPV was lower for autonomous AI than AI-H (63.0% vs 71.0%).
Dr. Djinbachian and colleagues suggested that future research should focus on larger, multicenter trials to validate these findings and further explore the integration of autonomous AI systems in clinical practice. They also noted that improving AI algorithms to accurately diagnose sessile serrated lesions could enhance the overall effectiveness of AI-based optical diagnosis.
“The performance of autonomous AI in accurately diagnosing diminutive polyps and determining appropriate surveillance intervals suggests that it could play a crucial role in streamlining colorectal cancer screening processes, reducing the burden on pathologists, and potentially lowering healthcare costs,” the investigators concluded.The study was supported by Fujifilm, which had no role in the study design or data analysis. Dr. von Renteln reported additional research funding from Vantage and Fujifilm.
, while providing greater alignment with pathology-based surveillance intervals, based on a randomized controlled trial.
These findings suggest that autonomous AI may one day replace histologic assessment of diminutive polyps, reported lead author Roupen Djinbachian, MD, of the Montreal University Hospital Research Center, Montreal, Quebec, Canada, and colleagues.Optical diagnosis of diminutive colorectal polyps has been proposed as a cost-effective alternative to histologic diagnosis, but its implementation in general clinical practice has been hindered by endoscopists’ concerns about incorrect diagnoses, the investigators wrote in Gastroenterology.“AI-based systems (CADx) have been proposed as a solution to these barriers to implementation, with studies showing high adherence to Preservation and Incorporation of Valuable Endoscopic Innovations (PIVI) thresholds when using AI-H,” they wrote. “However, the efficacy and safety of autonomous AI-based diagnostic platforms have not yet been evaluated.”
To address this knowledge gap, Dr. Djinbachian and colleagues conducted a randomized controlled noninferiority trial involving 467 patients, all of whom underwent elective colonoscopies at a single academic institution.
Participants were randomly assigned to one of two groups. The first group received an optical diagnosis of diminutive (1-5 mm) colorectal polyps using an autonomous AI-based CADx system without any human input. The second group had diagnoses performed by endoscopists who used AI-H to make their optical diagnoses.
The primary outcome was the accuracy of optical diagnosis compared with the gold standard of histologic evaluation. Secondarily, the investigators explored associations between pathology-based surveillance intervals and various measures of accuracy, including sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV).
The results showed that the accuracy of optical diagnosis for diminutive polyps was similar between the two groups, supporting noninferiority. Autonomous AI achieved an accuracy rate of 77.2%, while the AI-H group had an accuracy of 72.1%, which was not statistically significant (P = .86).
But when it came to pathology-based surveillance intervals, autonomous AI showed a clear advantage; the autonomous AI system achieved a 91.5% agreement rate, compared with 82.1% for the AI-H group (P = .016).
“These findings indicate that autonomous AI not only matches but also surpasses AI-H in accuracy for determining surveillance intervals,” the investigators wrote, noting that this finding highlights the “complexities of human interaction with AI modules where human intervention could lead to worse outcomes.”
Further analysis revealed that the sensitivity of autonomous AI for identifying adenomas was 84.8%, slightly higher than the 83.6% sensitivity of the AI-H group. Specificity was 64.4% for autonomous AI vs 63.8% for AI-H. While PPV was higher in the autonomous AI group (85.6%), compared with the AI-H group (78.6%), NPV was lower for autonomous AI than AI-H (63.0% vs 71.0%).
Dr. Djinbachian and colleagues suggested that future research should focus on larger, multicenter trials to validate these findings and further explore the integration of autonomous AI systems in clinical practice. They also noted that improving AI algorithms to accurately diagnose sessile serrated lesions could enhance the overall effectiveness of AI-based optical diagnosis.
“The performance of autonomous AI in accurately diagnosing diminutive polyps and determining appropriate surveillance intervals suggests that it could play a crucial role in streamlining colorectal cancer screening processes, reducing the burden on pathologists, and potentially lowering healthcare costs,” the investigators concluded.The study was supported by Fujifilm, which had no role in the study design or data analysis. Dr. von Renteln reported additional research funding from Vantage and Fujifilm.
FROM GASTROENTEROLOGY
Targeting Enteroendocrine Cells Could Hold Promise for IBD
, according to investigators.
These findings suggest that restoring EEC function could alleviate some of the more general abdominal symptoms associated with IBD, reported lead author Zachariah Raouf, MD, of Johns Hopkins University School of Medicine, Baltimore, and colleagues.
“The symptoms experienced by patients with IBD, especially ulcerative colitis, may include those that are colonic in nature, such as bloody stools, abdominal pain, and weight loss, as well as those that are more general in nature, such as severe nausea and abdominal bloating,” the investigators wrote in Cellular and Molecular Gastroenterology and Hepatology . “Although the first set of symptoms may be attributable to the effects of colonic inflammation itself, those that are more vague seem to overlap with the symptoms that patients with small intestinal dysmotility experience, such as occur in response to medications, or diabetes.”
Supporting this notion, several previous studies have reported the onset of intestinal dysmotility in experimental models of colitis, which is believed to be caused by impaired enteric nervous system function. But the precise mechanisms behind the impaired intestinal motility observed in colitis patients remain unclear.
To learn more, Dr. Raouf and colleagues conducted experiments involving three groups of mice: wild-type mice, mice genetically engineered to overexpress EECs, and mice lacking EECs.
To induce colitis, the mice were administered dextran sulfate sodium (DSS) in drinking water at concentrations of 2.5% or 5% for 7 days. Small intestinal motility was evaluated by measuring the transit of fluorescein isothiocyanate (FITC)-dextran. Immunohistochemical analyses were conducted to assess EEC number and differentiation, while quantitative reverse-transcriptase polymerase chain reaction was used to examine the expression of genes related to serotonin synthesis and transport.
The researchers examined colon length and signs of colonic inflammation, monitored weight loss, and measured the expression of proinflammatory cytokines. Histological analyses of colon and small intestine tissues were performed to further understand the effects of colitis. The presence and number of EEC cells was evaluated using chromogranin A (ChgA) staining, while apoptosis in EECs was measured via TUNEL staining. The expression of serotonin-related genes was also assessed.
These experiments revealed that DSS-induced colitis led to significant small-bowel hypomotility and a reduction in EEC density. Of note, genetic overexpression of EECs or treatment with prucalopride, a 5-hydroxytryptamine receptor 4 agonist, improved small intestinal motility.
“It is noteworthy that there were no significant changes in the density of other intestinal epithelial cells, or in other cell types that are linked to motility, such as enteric glia and neurons, suggesting the specificity of the effect,” the investigators wrote. “Importantly, treatment with a serotonin agonist ameliorated the colitis-induced, small-bowel hypomotility and attenuated the severity of colitis, providing potential clinical relevance of the current findings. Taken together, these results identify mechanisms to explain the intestinal hypomotility observed in the setting of colitis.”Dr. Raouf and colleagues called for human clinical trials to their findings. Specifically, they suggested exploring therapies targeting enteroendocrine cells or serotonin pathways and examining the role of different EEC types in gut motility during inflammation. The study was supported by the National Institutes of Health. The investigators disclosed no conflicts of interest.
Inflammatory bowel disease (IBD) typically manifests with colonic symptoms but is also associated with intestinal inflammation and dysmotility of the small intestine. Clinical research debates whether IBD causes small intestine hypermotility or hypomotility, but these motility dysfunctions are often attributed to alterations of the gut’s intrinsic nervous system.
Dr. Raouf and colleagues focus on the role of enteroendocrine cells, an epithelial cell subtype with neuron-like features that secrete serotonin, one of the most important regulators of intestinal motility. Their population is reduced in colitis, and the subsequent alteration of serotonin signaling induces small intestine dysmotility. The observed loss of enteroendocrine cells in the small bowel may result from low-grade local inflammation increasing enteroendocrine cell apoptosis, or impaired gene expression in their differentiation pathways. However, more research is required to elucidate the underlying mechanisms of this loss.
This study enhances our understanding of the small intestine dysfunction associated with colitis and raises the exciting possibility of enteroendocrine cell-based therapeutic approaches in IBD.
Jacques A. Gonzales, PhD, is a postdoctoral fellow in the Gulbransen laboratory at Michigan State University, East Lansing. He has no conflicts of interest.
Inflammatory bowel disease (IBD) typically manifests with colonic symptoms but is also associated with intestinal inflammation and dysmotility of the small intestine. Clinical research debates whether IBD causes small intestine hypermotility or hypomotility, but these motility dysfunctions are often attributed to alterations of the gut’s intrinsic nervous system.
Dr. Raouf and colleagues focus on the role of enteroendocrine cells, an epithelial cell subtype with neuron-like features that secrete serotonin, one of the most important regulators of intestinal motility. Their population is reduced in colitis, and the subsequent alteration of serotonin signaling induces small intestine dysmotility. The observed loss of enteroendocrine cells in the small bowel may result from low-grade local inflammation increasing enteroendocrine cell apoptosis, or impaired gene expression in their differentiation pathways. However, more research is required to elucidate the underlying mechanisms of this loss.
This study enhances our understanding of the small intestine dysfunction associated with colitis and raises the exciting possibility of enteroendocrine cell-based therapeutic approaches in IBD.
Jacques A. Gonzales, PhD, is a postdoctoral fellow in the Gulbransen laboratory at Michigan State University, East Lansing. He has no conflicts of interest.
Inflammatory bowel disease (IBD) typically manifests with colonic symptoms but is also associated with intestinal inflammation and dysmotility of the small intestine. Clinical research debates whether IBD causes small intestine hypermotility or hypomotility, but these motility dysfunctions are often attributed to alterations of the gut’s intrinsic nervous system.
Dr. Raouf and colleagues focus on the role of enteroendocrine cells, an epithelial cell subtype with neuron-like features that secrete serotonin, one of the most important regulators of intestinal motility. Their population is reduced in colitis, and the subsequent alteration of serotonin signaling induces small intestine dysmotility. The observed loss of enteroendocrine cells in the small bowel may result from low-grade local inflammation increasing enteroendocrine cell apoptosis, or impaired gene expression in their differentiation pathways. However, more research is required to elucidate the underlying mechanisms of this loss.
This study enhances our understanding of the small intestine dysfunction associated with colitis and raises the exciting possibility of enteroendocrine cell-based therapeutic approaches in IBD.
Jacques A. Gonzales, PhD, is a postdoctoral fellow in the Gulbransen laboratory at Michigan State University, East Lansing. He has no conflicts of interest.
, according to investigators.
These findings suggest that restoring EEC function could alleviate some of the more general abdominal symptoms associated with IBD, reported lead author Zachariah Raouf, MD, of Johns Hopkins University School of Medicine, Baltimore, and colleagues.
“The symptoms experienced by patients with IBD, especially ulcerative colitis, may include those that are colonic in nature, such as bloody stools, abdominal pain, and weight loss, as well as those that are more general in nature, such as severe nausea and abdominal bloating,” the investigators wrote in Cellular and Molecular Gastroenterology and Hepatology . “Although the first set of symptoms may be attributable to the effects of colonic inflammation itself, those that are more vague seem to overlap with the symptoms that patients with small intestinal dysmotility experience, such as occur in response to medications, or diabetes.”
Supporting this notion, several previous studies have reported the onset of intestinal dysmotility in experimental models of colitis, which is believed to be caused by impaired enteric nervous system function. But the precise mechanisms behind the impaired intestinal motility observed in colitis patients remain unclear.
To learn more, Dr. Raouf and colleagues conducted experiments involving three groups of mice: wild-type mice, mice genetically engineered to overexpress EECs, and mice lacking EECs.
To induce colitis, the mice were administered dextran sulfate sodium (DSS) in drinking water at concentrations of 2.5% or 5% for 7 days. Small intestinal motility was evaluated by measuring the transit of fluorescein isothiocyanate (FITC)-dextran. Immunohistochemical analyses were conducted to assess EEC number and differentiation, while quantitative reverse-transcriptase polymerase chain reaction was used to examine the expression of genes related to serotonin synthesis and transport.
The researchers examined colon length and signs of colonic inflammation, monitored weight loss, and measured the expression of proinflammatory cytokines. Histological analyses of colon and small intestine tissues were performed to further understand the effects of colitis. The presence and number of EEC cells was evaluated using chromogranin A (ChgA) staining, while apoptosis in EECs was measured via TUNEL staining. The expression of serotonin-related genes was also assessed.
These experiments revealed that DSS-induced colitis led to significant small-bowel hypomotility and a reduction in EEC density. Of note, genetic overexpression of EECs or treatment with prucalopride, a 5-hydroxytryptamine receptor 4 agonist, improved small intestinal motility.
“It is noteworthy that there were no significant changes in the density of other intestinal epithelial cells, or in other cell types that are linked to motility, such as enteric glia and neurons, suggesting the specificity of the effect,” the investigators wrote. “Importantly, treatment with a serotonin agonist ameliorated the colitis-induced, small-bowel hypomotility and attenuated the severity of colitis, providing potential clinical relevance of the current findings. Taken together, these results identify mechanisms to explain the intestinal hypomotility observed in the setting of colitis.”Dr. Raouf and colleagues called for human clinical trials to their findings. Specifically, they suggested exploring therapies targeting enteroendocrine cells or serotonin pathways and examining the role of different EEC types in gut motility during inflammation. The study was supported by the National Institutes of Health. The investigators disclosed no conflicts of interest.
, according to investigators.
These findings suggest that restoring EEC function could alleviate some of the more general abdominal symptoms associated with IBD, reported lead author Zachariah Raouf, MD, of Johns Hopkins University School of Medicine, Baltimore, and colleagues.
“The symptoms experienced by patients with IBD, especially ulcerative colitis, may include those that are colonic in nature, such as bloody stools, abdominal pain, and weight loss, as well as those that are more general in nature, such as severe nausea and abdominal bloating,” the investigators wrote in Cellular and Molecular Gastroenterology and Hepatology . “Although the first set of symptoms may be attributable to the effects of colonic inflammation itself, those that are more vague seem to overlap with the symptoms that patients with small intestinal dysmotility experience, such as occur in response to medications, or diabetes.”
Supporting this notion, several previous studies have reported the onset of intestinal dysmotility in experimental models of colitis, which is believed to be caused by impaired enteric nervous system function. But the precise mechanisms behind the impaired intestinal motility observed in colitis patients remain unclear.
To learn more, Dr. Raouf and colleagues conducted experiments involving three groups of mice: wild-type mice, mice genetically engineered to overexpress EECs, and mice lacking EECs.
To induce colitis, the mice were administered dextran sulfate sodium (DSS) in drinking water at concentrations of 2.5% or 5% for 7 days. Small intestinal motility was evaluated by measuring the transit of fluorescein isothiocyanate (FITC)-dextran. Immunohistochemical analyses were conducted to assess EEC number and differentiation, while quantitative reverse-transcriptase polymerase chain reaction was used to examine the expression of genes related to serotonin synthesis and transport.
The researchers examined colon length and signs of colonic inflammation, monitored weight loss, and measured the expression of proinflammatory cytokines. Histological analyses of colon and small intestine tissues were performed to further understand the effects of colitis. The presence and number of EEC cells was evaluated using chromogranin A (ChgA) staining, while apoptosis in EECs was measured via TUNEL staining. The expression of serotonin-related genes was also assessed.
These experiments revealed that DSS-induced colitis led to significant small-bowel hypomotility and a reduction in EEC density. Of note, genetic overexpression of EECs or treatment with prucalopride, a 5-hydroxytryptamine receptor 4 agonist, improved small intestinal motility.
“It is noteworthy that there were no significant changes in the density of other intestinal epithelial cells, or in other cell types that are linked to motility, such as enteric glia and neurons, suggesting the specificity of the effect,” the investigators wrote. “Importantly, treatment with a serotonin agonist ameliorated the colitis-induced, small-bowel hypomotility and attenuated the severity of colitis, providing potential clinical relevance of the current findings. Taken together, these results identify mechanisms to explain the intestinal hypomotility observed in the setting of colitis.”Dr. Raouf and colleagues called for human clinical trials to their findings. Specifically, they suggested exploring therapies targeting enteroendocrine cells or serotonin pathways and examining the role of different EEC types in gut motility during inflammation. The study was supported by the National Institutes of Health. The investigators disclosed no conflicts of interest.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Gastroenterology Data Trends 2024
In this issue:
- Eosinophilic Gastrointestinal Diseases: Beyond EoE
Nirmala Gonsalves, MD, AGAF, FACG - The Changing Face of IBD: Beyond the Western World
Gilaad G. Kaplan, MD, MPH, AGAF; Paulo Kotze, MD, MS, PhD; Siew C. Ng, MBBS, PhD, AGAF - Role of Non-invasive Biomarkers in the Evaluation and Management of MASLD
Julia J. Wattacheril, MD, MPH - The Emerging Role of Liquid Biopsy in the Diagnosis and Management of CRC
David Lieberman, MD, AGAF - Cannabinoids and Digestive Disorders
Jami A. Kinnucan, MD, AGAF, FACG - AI and Machine Learning in IBD: Promising Applications and Remaining Challenges
Shirley Cohen-Mekelburg, MD, MS - Simulation-Based Training in Endoscopy: Benefits and Challenges
Richa Shukla, MD - Fluid Management in Acute Pancreatitis
Jorge D. Machicado, MD, MPH
In this issue:
- Eosinophilic Gastrointestinal Diseases: Beyond EoE
Nirmala Gonsalves, MD, AGAF, FACG - The Changing Face of IBD: Beyond the Western World
Gilaad G. Kaplan, MD, MPH, AGAF; Paulo Kotze, MD, MS, PhD; Siew C. Ng, MBBS, PhD, AGAF - Role of Non-invasive Biomarkers in the Evaluation and Management of MASLD
Julia J. Wattacheril, MD, MPH - The Emerging Role of Liquid Biopsy in the Diagnosis and Management of CRC
David Lieberman, MD, AGAF - Cannabinoids and Digestive Disorders
Jami A. Kinnucan, MD, AGAF, FACG - AI and Machine Learning in IBD: Promising Applications and Remaining Challenges
Shirley Cohen-Mekelburg, MD, MS - Simulation-Based Training in Endoscopy: Benefits and Challenges
Richa Shukla, MD - Fluid Management in Acute Pancreatitis
Jorge D. Machicado, MD, MPH
In this issue:
- Eosinophilic Gastrointestinal Diseases: Beyond EoE
Nirmala Gonsalves, MD, AGAF, FACG - The Changing Face of IBD: Beyond the Western World
Gilaad G. Kaplan, MD, MPH, AGAF; Paulo Kotze, MD, MS, PhD; Siew C. Ng, MBBS, PhD, AGAF - Role of Non-invasive Biomarkers in the Evaluation and Management of MASLD
Julia J. Wattacheril, MD, MPH - The Emerging Role of Liquid Biopsy in the Diagnosis and Management of CRC
David Lieberman, MD, AGAF - Cannabinoids and Digestive Disorders
Jami A. Kinnucan, MD, AGAF, FACG - AI and Machine Learning in IBD: Promising Applications and Remaining Challenges
Shirley Cohen-Mekelburg, MD, MS - Simulation-Based Training in Endoscopy: Benefits and Challenges
Richa Shukla, MD - Fluid Management in Acute Pancreatitis
Jorge D. Machicado, MD, MPH