User login
Before the COVID-19 surge hits your facility, take steps to boost capacity
, according to a physician leader and a health workforce expert.
Polly Pittman, PhD, is hearing a lot of concern among health care workers that it’s difficult to find definitive and accurate information about how best to protect themselves and their families, she said during a webinar by the Alliance for Health Policy titled Health System Capacity: Protecting Frontline Health Workers. “The knowledge base is evolving very quickly,” said Dr. Pittman, Fitzhugh Mullan Professor of Health Workforce Equity at the Milken Institute School of Public Health, George Washington University, Washington.
Stephen Parodi, MD, agreed that effective communication is job one in the health care workplace during the crisis. “I can’t stress enough ... that communications are paramount and you can’t overcommunicate,” said Dr. Parodi, executive vice president of external affairs, communications, and brand at the Permanente Federation and associate executive director of the Permanente Medical Group, Vallejo, Calif.
“We’re in a situation of confusion and improvisation right now,” regarding protection of health care workers, said Dr. Pittman. The potential exists for “a downward spiral where you have the lack of training, the shortages in terms of protective gear, weakening of guidelines, and confusion regarding guidelines at federal level, creating a potential cascade” that may result in “moral distress and fatigue. ... That’s not occurring now, but that’s the danger” unless the personal protective equipment (PPE) situation is adequately addressed very soon, she said.
Dr. Pittman also pointed out the concerns that many of the 18 million U.S. health care workers have for their families should they themselves fall ill or transmit coronavirus to family members. “The danger exists of a mass exodus. People don’t have to show up at work, and they won’t show up at work if they don’t feel supported and safe.”
Dr. Parodi said that the Permanente organization is on a better footing than many workplaces. “We actually had an early experience because of the work that we did to support the Diamond Princess cruise ship evacuees from Yokahama in February.” That ship was quarantined upon arrival in Yokahama on Feb. 3 because a passenger had a confirmed test for SARS-CoV-2 infection, and a quarter of the 428 Americans on board subsequently tested positive. Most of them were evacuated to California or Texas. “That actually gave us the experience for providing care within the hospital setting – and also for containment strategies,” he said.
“We quickly understood that we needed to move to a mitigation strategy,” said Dr. Parodi. Use of PPE has been “tailored for how the virus is spread.” In the absence of the risk of aerosol transmission from certain procedures, health care workers use gowns, gloves, surgical masks, and goggles.
Because of anticipated “supply chain shortfalls,” Dr. Parodi said that his organization implemented Centers for Disease Control and Prevention guidelines for reuse and extended use of N95 respirators early on. “Even if you’re not in a locale that’s been hit, you need to be on wartime footing for preserving PPE.”
Telehealth, said Dr. Parodi, has been implemented “in a huge way” throughout the Permanente system. “We have reduced primary care visits by 90% in the past week, and also subspecialty visits by 50%. … A large amount of the workforce can work from home. We turned off elective surgeries more than a week ago to reduce the number of patients who are requiring intensive care.” Making these changes means the organization is more prepared now for a surge they expect in the coming weeks.
Dr. Pittman voiced an opinion widely shared by those who are implementing large-scale telehealth efforts “We’re going to learn a lot. Many of the traditional doctor-patient visits can be done by telemedicine in the future.”
Knowledge about local trends in infection rates is key to preparedness. “We’ve ramped up testing, to understand what’s happening in the community,” said Dr. Parodi, noting that test turnaround time is currently running 8-24 hours. Tightening up this window can free up resources when an admitted patient’s test is negative.
Still, some national projections forecast a need for hospital beds at two to three times current capacity – or even more, said Dr. Parodi.
He noted that Permanente is “working hand in glove with state authorities throughout the country.” Efforts include establishing alternative sites for assessment and testing, as well as opening up closed hospitals and working with the National Guard and the Department of Defense to prepare mobile hospital units that can be deployed in areas with peak infection rates. “Having all of those options available to us is critically important,” he said.
To mitigate potential provider shortages, Dr. Pittman said, “All members of the care team could potentially do more” than their current licenses allow. Expanding the scope of practice for pharmacists, clinical laboratory staff, licensed practical nurses, and medical assistants can help with efficient care delivery.
Other measures include expedited licensing for near-graduates and nonpracticing foreign medical graduates, as well as relicensing for retired health care personnel and those who are not currently working directly with patients, she said.
Getting these things done “requires leadership on behalf of the licensing bodies,” as well as coordination with state regulatory authorities, Dr. Pittman pointed out.
Dr. Parodi called for state and federal governments to implement emergency declarations that suspend some existing health codes to achieve repurposing of staff. Getting these measures in place now will allow facilities “to be able to provide that in-time training now before the surge occurs. ... We are actively developing plans knowing that there’s going to be a need for more critical care.”
The game plan at Permanente, he said, is to repurpose critical care physicians to provide consultations to multiple hospitalists who are providing the bulk of frontline care. At the same time, they plan to repurpose other specialists to backfill the hospitalists, and to repurpose family medicine physicians to supplement staff in emergency departments and other frontline intake areas.
All the organizational measures being taken won’t be in vain if they increase preparedness for the long battle ahead, he said. “We need to double down on the work. ... We need to continue social distancing, and we’ve got to ramp up testing. Until we do that we have to hold the line on basic public health measures.”
Dr. Parodi is employed by Permanente. The panelists reported no disclosures relevant to the presentation, which was sponsored by the Alliance for Health Policy, the Commonwealth Fund, and the National Institute for Health Care Management Foundation.
, according to a physician leader and a health workforce expert.
Polly Pittman, PhD, is hearing a lot of concern among health care workers that it’s difficult to find definitive and accurate information about how best to protect themselves and their families, she said during a webinar by the Alliance for Health Policy titled Health System Capacity: Protecting Frontline Health Workers. “The knowledge base is evolving very quickly,” said Dr. Pittman, Fitzhugh Mullan Professor of Health Workforce Equity at the Milken Institute School of Public Health, George Washington University, Washington.
Stephen Parodi, MD, agreed that effective communication is job one in the health care workplace during the crisis. “I can’t stress enough ... that communications are paramount and you can’t overcommunicate,” said Dr. Parodi, executive vice president of external affairs, communications, and brand at the Permanente Federation and associate executive director of the Permanente Medical Group, Vallejo, Calif.
“We’re in a situation of confusion and improvisation right now,” regarding protection of health care workers, said Dr. Pittman. The potential exists for “a downward spiral where you have the lack of training, the shortages in terms of protective gear, weakening of guidelines, and confusion regarding guidelines at federal level, creating a potential cascade” that may result in “moral distress and fatigue. ... That’s not occurring now, but that’s the danger” unless the personal protective equipment (PPE) situation is adequately addressed very soon, she said.
Dr. Pittman also pointed out the concerns that many of the 18 million U.S. health care workers have for their families should they themselves fall ill or transmit coronavirus to family members. “The danger exists of a mass exodus. People don’t have to show up at work, and they won’t show up at work if they don’t feel supported and safe.”
Dr. Parodi said that the Permanente organization is on a better footing than many workplaces. “We actually had an early experience because of the work that we did to support the Diamond Princess cruise ship evacuees from Yokahama in February.” That ship was quarantined upon arrival in Yokahama on Feb. 3 because a passenger had a confirmed test for SARS-CoV-2 infection, and a quarter of the 428 Americans on board subsequently tested positive. Most of them were evacuated to California or Texas. “That actually gave us the experience for providing care within the hospital setting – and also for containment strategies,” he said.
“We quickly understood that we needed to move to a mitigation strategy,” said Dr. Parodi. Use of PPE has been “tailored for how the virus is spread.” In the absence of the risk of aerosol transmission from certain procedures, health care workers use gowns, gloves, surgical masks, and goggles.
Because of anticipated “supply chain shortfalls,” Dr. Parodi said that his organization implemented Centers for Disease Control and Prevention guidelines for reuse and extended use of N95 respirators early on. “Even if you’re not in a locale that’s been hit, you need to be on wartime footing for preserving PPE.”
Telehealth, said Dr. Parodi, has been implemented “in a huge way” throughout the Permanente system. “We have reduced primary care visits by 90% in the past week, and also subspecialty visits by 50%. … A large amount of the workforce can work from home. We turned off elective surgeries more than a week ago to reduce the number of patients who are requiring intensive care.” Making these changes means the organization is more prepared now for a surge they expect in the coming weeks.
Dr. Pittman voiced an opinion widely shared by those who are implementing large-scale telehealth efforts “We’re going to learn a lot. Many of the traditional doctor-patient visits can be done by telemedicine in the future.”
Knowledge about local trends in infection rates is key to preparedness. “We’ve ramped up testing, to understand what’s happening in the community,” said Dr. Parodi, noting that test turnaround time is currently running 8-24 hours. Tightening up this window can free up resources when an admitted patient’s test is negative.
Still, some national projections forecast a need for hospital beds at two to three times current capacity – or even more, said Dr. Parodi.
He noted that Permanente is “working hand in glove with state authorities throughout the country.” Efforts include establishing alternative sites for assessment and testing, as well as opening up closed hospitals and working with the National Guard and the Department of Defense to prepare mobile hospital units that can be deployed in areas with peak infection rates. “Having all of those options available to us is critically important,” he said.
To mitigate potential provider shortages, Dr. Pittman said, “All members of the care team could potentially do more” than their current licenses allow. Expanding the scope of practice for pharmacists, clinical laboratory staff, licensed practical nurses, and medical assistants can help with efficient care delivery.
Other measures include expedited licensing for near-graduates and nonpracticing foreign medical graduates, as well as relicensing for retired health care personnel and those who are not currently working directly with patients, she said.
Getting these things done “requires leadership on behalf of the licensing bodies,” as well as coordination with state regulatory authorities, Dr. Pittman pointed out.
Dr. Parodi called for state and federal governments to implement emergency declarations that suspend some existing health codes to achieve repurposing of staff. Getting these measures in place now will allow facilities “to be able to provide that in-time training now before the surge occurs. ... We are actively developing plans knowing that there’s going to be a need for more critical care.”
The game plan at Permanente, he said, is to repurpose critical care physicians to provide consultations to multiple hospitalists who are providing the bulk of frontline care. At the same time, they plan to repurpose other specialists to backfill the hospitalists, and to repurpose family medicine physicians to supplement staff in emergency departments and other frontline intake areas.
All the organizational measures being taken won’t be in vain if they increase preparedness for the long battle ahead, he said. “We need to double down on the work. ... We need to continue social distancing, and we’ve got to ramp up testing. Until we do that we have to hold the line on basic public health measures.”
Dr. Parodi is employed by Permanente. The panelists reported no disclosures relevant to the presentation, which was sponsored by the Alliance for Health Policy, the Commonwealth Fund, and the National Institute for Health Care Management Foundation.
, according to a physician leader and a health workforce expert.
Polly Pittman, PhD, is hearing a lot of concern among health care workers that it’s difficult to find definitive and accurate information about how best to protect themselves and their families, she said during a webinar by the Alliance for Health Policy titled Health System Capacity: Protecting Frontline Health Workers. “The knowledge base is evolving very quickly,” said Dr. Pittman, Fitzhugh Mullan Professor of Health Workforce Equity at the Milken Institute School of Public Health, George Washington University, Washington.
Stephen Parodi, MD, agreed that effective communication is job one in the health care workplace during the crisis. “I can’t stress enough ... that communications are paramount and you can’t overcommunicate,” said Dr. Parodi, executive vice president of external affairs, communications, and brand at the Permanente Federation and associate executive director of the Permanente Medical Group, Vallejo, Calif.
“We’re in a situation of confusion and improvisation right now,” regarding protection of health care workers, said Dr. Pittman. The potential exists for “a downward spiral where you have the lack of training, the shortages in terms of protective gear, weakening of guidelines, and confusion regarding guidelines at federal level, creating a potential cascade” that may result in “moral distress and fatigue. ... That’s not occurring now, but that’s the danger” unless the personal protective equipment (PPE) situation is adequately addressed very soon, she said.
Dr. Pittman also pointed out the concerns that many of the 18 million U.S. health care workers have for their families should they themselves fall ill or transmit coronavirus to family members. “The danger exists of a mass exodus. People don’t have to show up at work, and they won’t show up at work if they don’t feel supported and safe.”
Dr. Parodi said that the Permanente organization is on a better footing than many workplaces. “We actually had an early experience because of the work that we did to support the Diamond Princess cruise ship evacuees from Yokahama in February.” That ship was quarantined upon arrival in Yokahama on Feb. 3 because a passenger had a confirmed test for SARS-CoV-2 infection, and a quarter of the 428 Americans on board subsequently tested positive. Most of them were evacuated to California or Texas. “That actually gave us the experience for providing care within the hospital setting – and also for containment strategies,” he said.
“We quickly understood that we needed to move to a mitigation strategy,” said Dr. Parodi. Use of PPE has been “tailored for how the virus is spread.” In the absence of the risk of aerosol transmission from certain procedures, health care workers use gowns, gloves, surgical masks, and goggles.
Because of anticipated “supply chain shortfalls,” Dr. Parodi said that his organization implemented Centers for Disease Control and Prevention guidelines for reuse and extended use of N95 respirators early on. “Even if you’re not in a locale that’s been hit, you need to be on wartime footing for preserving PPE.”
Telehealth, said Dr. Parodi, has been implemented “in a huge way” throughout the Permanente system. “We have reduced primary care visits by 90% in the past week, and also subspecialty visits by 50%. … A large amount of the workforce can work from home. We turned off elective surgeries more than a week ago to reduce the number of patients who are requiring intensive care.” Making these changes means the organization is more prepared now for a surge they expect in the coming weeks.
Dr. Pittman voiced an opinion widely shared by those who are implementing large-scale telehealth efforts “We’re going to learn a lot. Many of the traditional doctor-patient visits can be done by telemedicine in the future.”
Knowledge about local trends in infection rates is key to preparedness. “We’ve ramped up testing, to understand what’s happening in the community,” said Dr. Parodi, noting that test turnaround time is currently running 8-24 hours. Tightening up this window can free up resources when an admitted patient’s test is negative.
Still, some national projections forecast a need for hospital beds at two to three times current capacity – or even more, said Dr. Parodi.
He noted that Permanente is “working hand in glove with state authorities throughout the country.” Efforts include establishing alternative sites for assessment and testing, as well as opening up closed hospitals and working with the National Guard and the Department of Defense to prepare mobile hospital units that can be deployed in areas with peak infection rates. “Having all of those options available to us is critically important,” he said.
To mitigate potential provider shortages, Dr. Pittman said, “All members of the care team could potentially do more” than their current licenses allow. Expanding the scope of practice for pharmacists, clinical laboratory staff, licensed practical nurses, and medical assistants can help with efficient care delivery.
Other measures include expedited licensing for near-graduates and nonpracticing foreign medical graduates, as well as relicensing for retired health care personnel and those who are not currently working directly with patients, she said.
Getting these things done “requires leadership on behalf of the licensing bodies,” as well as coordination with state regulatory authorities, Dr. Pittman pointed out.
Dr. Parodi called for state and federal governments to implement emergency declarations that suspend some existing health codes to achieve repurposing of staff. Getting these measures in place now will allow facilities “to be able to provide that in-time training now before the surge occurs. ... We are actively developing plans knowing that there’s going to be a need for more critical care.”
The game plan at Permanente, he said, is to repurpose critical care physicians to provide consultations to multiple hospitalists who are providing the bulk of frontline care. At the same time, they plan to repurpose other specialists to backfill the hospitalists, and to repurpose family medicine physicians to supplement staff in emergency departments and other frontline intake areas.
All the organizational measures being taken won’t be in vain if they increase preparedness for the long battle ahead, he said. “We need to double down on the work. ... We need to continue social distancing, and we’ve got to ramp up testing. Until we do that we have to hold the line on basic public health measures.”
Dr. Parodi is employed by Permanente. The panelists reported no disclosures relevant to the presentation, which was sponsored by the Alliance for Health Policy, the Commonwealth Fund, and the National Institute for Health Care Management Foundation.
REPORTING FROM AN ALLIANCE FOR HEALTH POLICY WEBINAR
In older HIV patients, B/F/TAF regimen was noninferior to others
The single-tablet antiretroviral regimen of bictegravir, emtricitabine, and tenofovir alafenamide (B/F/TAF) was assessed as being noninferior to two dolutegravir (DTG)-containing regimens among adults aged 50 and over living with HIV, according to a new study.
The study pooled data from two phase 3, randomized, double-blind B/F/TAF studies comparing that regimen with DTG-containing regimens for adults living with HIV who were treatment naive. Anthony Mills, MD, a physician in private practice in West Hollywood, Calif., and his associates reported results at the 144-week mark for the two studies in a poster that was presented as part of the Conference on Retroviruses & Opportunistic Infections, which was presented online this year. CROI organizers chose to hold a virtual meeting because of concerns about the spread of COVID-19.
In the two trials, the B/F/TAF regimen was compared with a DTG and abacavir/lamivudine (DTG + ABC/3TC) regimen, as well as with DTG plus emtricitabine/tenofovir alafenamide (DTG + F/TAF). A total of 629 patients were enrolled in the first study, and 645 in the second. Of these, a total of 196 patients were aged 50 years or older. Across all study arms, most participants (73%-92%) were male, and 20%-37% were black or of African descent. Participants identifying as Hispanic or being of Latino ancestry made up 11%-27% of participants.
About 80%-90% of patients had asymptomatic HIV infection at the time of enrollment, with median CD4 counts ranging from 405-534 cells/mcL across study arms. Patients had a median 4.27-4.53 log (base 10) copies/mL at baseline. In both studies, patients had to have at least 500 HIV-1 RNA copies per mL, and couldn’t have known resistance to any of the study drugs.
At week 144, there were no statistically significant differences in virologic outcomes across study arms in the younger or older age subgroups: 81% of the B/F/TAF patients older than 50 years had fewer than 50 copies/mL of HIV-1 RNA, compared with 83% and 88% of the younger DTG/ABC/3TC and DTG + F/TAF groups meeting this mark, respectively.
Results were similar for the patients aged younger than 50 years, with 82%, 84%, and 83% of the B/F/TAF, DTG/ABC/3TC, and DTG + F/TAF groups having fewer than 50 copies/mL of HIV-1 RNA, respectively.
No patients in either age group showed resistance to any components of the treatment regimens. No treatment-emergent resistance was seen in any study participants, and few adverse events occurred. Those that were seen didn’t occur more frequently in older patients, compared with younger patients, and no patients discontinued their treatment because of renal issues.
Bone mineral density (BMD) was only measured in the study that compared B/F/TAF with DTG/ABC/3TC. Hip BMD decreased slightly in both groups, but the changes were comparable between older and younger participants. For both age groups, differences weren’t significant between the B/F/TAF group and those taking DTG/ABC/3TC. Spine density actually increased slightly in older patients taking B/F/TAF, but the difference between this measure and the slight decrease in older patients taking DTG/ABC/3TC was not significant.
Weight increased over time for all groups, ranging from a gain of 3.4 kg at 144 weeks for older patients taking DTG + F/TAF to 5.3 kg for the same regimen in younger patients, but none of the between-group differences were significant.
All fasting lipids rose for each study arm in both older and younger patients. Some of the between-group differences were statistically significant, but “there were no clinically significant differences in median changes from baseline in fasting lipids” among those aged 50 years or older, noted Dr. Mills and associates.
In terms of safety, no study drug-related discontinuations for renal adverse events occurred in either the B/F/TAF or the DTG + F/TAF groups, and the investigators saw no proximal renal tubulopathy.
The estimated glomerular filtration rate (eGFR) was calculated using the Cockroft-Gault equation. All three antiviral regimens were associated with early drops in eGFR, “consistent with inhibition of tubular creatinine secretion via organic cation transporter 2,” noted Dr. Mills and associates.
Other adverse events were rare in all groups in both older and younger patients, without significant differences between therapies.
The study was supported by Gilead Sciences, which also provided support to Dr. Mills. One coauthor is a Gilead employee.
SOURCE: Mills A et al. CROI 2020, Abstract 2886.
The single-tablet antiretroviral regimen of bictegravir, emtricitabine, and tenofovir alafenamide (B/F/TAF) was assessed as being noninferior to two dolutegravir (DTG)-containing regimens among adults aged 50 and over living with HIV, according to a new study.
The study pooled data from two phase 3, randomized, double-blind B/F/TAF studies comparing that regimen with DTG-containing regimens for adults living with HIV who were treatment naive. Anthony Mills, MD, a physician in private practice in West Hollywood, Calif., and his associates reported results at the 144-week mark for the two studies in a poster that was presented as part of the Conference on Retroviruses & Opportunistic Infections, which was presented online this year. CROI organizers chose to hold a virtual meeting because of concerns about the spread of COVID-19.
In the two trials, the B/F/TAF regimen was compared with a DTG and abacavir/lamivudine (DTG + ABC/3TC) regimen, as well as with DTG plus emtricitabine/tenofovir alafenamide (DTG + F/TAF). A total of 629 patients were enrolled in the first study, and 645 in the second. Of these, a total of 196 patients were aged 50 years or older. Across all study arms, most participants (73%-92%) were male, and 20%-37% were black or of African descent. Participants identifying as Hispanic or being of Latino ancestry made up 11%-27% of participants.
About 80%-90% of patients had asymptomatic HIV infection at the time of enrollment, with median CD4 counts ranging from 405-534 cells/mcL across study arms. Patients had a median 4.27-4.53 log (base 10) copies/mL at baseline. In both studies, patients had to have at least 500 HIV-1 RNA copies per mL, and couldn’t have known resistance to any of the study drugs.
At week 144, there were no statistically significant differences in virologic outcomes across study arms in the younger or older age subgroups: 81% of the B/F/TAF patients older than 50 years had fewer than 50 copies/mL of HIV-1 RNA, compared with 83% and 88% of the younger DTG/ABC/3TC and DTG + F/TAF groups meeting this mark, respectively.
Results were similar for the patients aged younger than 50 years, with 82%, 84%, and 83% of the B/F/TAF, DTG/ABC/3TC, and DTG + F/TAF groups having fewer than 50 copies/mL of HIV-1 RNA, respectively.
No patients in either age group showed resistance to any components of the treatment regimens. No treatment-emergent resistance was seen in any study participants, and few adverse events occurred. Those that were seen didn’t occur more frequently in older patients, compared with younger patients, and no patients discontinued their treatment because of renal issues.
Bone mineral density (BMD) was only measured in the study that compared B/F/TAF with DTG/ABC/3TC. Hip BMD decreased slightly in both groups, but the changes were comparable between older and younger participants. For both age groups, differences weren’t significant between the B/F/TAF group and those taking DTG/ABC/3TC. Spine density actually increased slightly in older patients taking B/F/TAF, but the difference between this measure and the slight decrease in older patients taking DTG/ABC/3TC was not significant.
Weight increased over time for all groups, ranging from a gain of 3.4 kg at 144 weeks for older patients taking DTG + F/TAF to 5.3 kg for the same regimen in younger patients, but none of the between-group differences were significant.
All fasting lipids rose for each study arm in both older and younger patients. Some of the between-group differences were statistically significant, but “there were no clinically significant differences in median changes from baseline in fasting lipids” among those aged 50 years or older, noted Dr. Mills and associates.
In terms of safety, no study drug-related discontinuations for renal adverse events occurred in either the B/F/TAF or the DTG + F/TAF groups, and the investigators saw no proximal renal tubulopathy.
The estimated glomerular filtration rate (eGFR) was calculated using the Cockroft-Gault equation. All three antiviral regimens were associated with early drops in eGFR, “consistent with inhibition of tubular creatinine secretion via organic cation transporter 2,” noted Dr. Mills and associates.
Other adverse events were rare in all groups in both older and younger patients, without significant differences between therapies.
The study was supported by Gilead Sciences, which also provided support to Dr. Mills. One coauthor is a Gilead employee.
SOURCE: Mills A et al. CROI 2020, Abstract 2886.
The single-tablet antiretroviral regimen of bictegravir, emtricitabine, and tenofovir alafenamide (B/F/TAF) was assessed as being noninferior to two dolutegravir (DTG)-containing regimens among adults aged 50 and over living with HIV, according to a new study.
The study pooled data from two phase 3, randomized, double-blind B/F/TAF studies comparing that regimen with DTG-containing regimens for adults living with HIV who were treatment naive. Anthony Mills, MD, a physician in private practice in West Hollywood, Calif., and his associates reported results at the 144-week mark for the two studies in a poster that was presented as part of the Conference on Retroviruses & Opportunistic Infections, which was presented online this year. CROI organizers chose to hold a virtual meeting because of concerns about the spread of COVID-19.
In the two trials, the B/F/TAF regimen was compared with a DTG and abacavir/lamivudine (DTG + ABC/3TC) regimen, as well as with DTG plus emtricitabine/tenofovir alafenamide (DTG + F/TAF). A total of 629 patients were enrolled in the first study, and 645 in the second. Of these, a total of 196 patients were aged 50 years or older. Across all study arms, most participants (73%-92%) were male, and 20%-37% were black or of African descent. Participants identifying as Hispanic or being of Latino ancestry made up 11%-27% of participants.
About 80%-90% of patients had asymptomatic HIV infection at the time of enrollment, with median CD4 counts ranging from 405-534 cells/mcL across study arms. Patients had a median 4.27-4.53 log (base 10) copies/mL at baseline. In both studies, patients had to have at least 500 HIV-1 RNA copies per mL, and couldn’t have known resistance to any of the study drugs.
At week 144, there were no statistically significant differences in virologic outcomes across study arms in the younger or older age subgroups: 81% of the B/F/TAF patients older than 50 years had fewer than 50 copies/mL of HIV-1 RNA, compared with 83% and 88% of the younger DTG/ABC/3TC and DTG + F/TAF groups meeting this mark, respectively.
Results were similar for the patients aged younger than 50 years, with 82%, 84%, and 83% of the B/F/TAF, DTG/ABC/3TC, and DTG + F/TAF groups having fewer than 50 copies/mL of HIV-1 RNA, respectively.
No patients in either age group showed resistance to any components of the treatment regimens. No treatment-emergent resistance was seen in any study participants, and few adverse events occurred. Those that were seen didn’t occur more frequently in older patients, compared with younger patients, and no patients discontinued their treatment because of renal issues.
Bone mineral density (BMD) was only measured in the study that compared B/F/TAF with DTG/ABC/3TC. Hip BMD decreased slightly in both groups, but the changes were comparable between older and younger participants. For both age groups, differences weren’t significant between the B/F/TAF group and those taking DTG/ABC/3TC. Spine density actually increased slightly in older patients taking B/F/TAF, but the difference between this measure and the slight decrease in older patients taking DTG/ABC/3TC was not significant.
Weight increased over time for all groups, ranging from a gain of 3.4 kg at 144 weeks for older patients taking DTG + F/TAF to 5.3 kg for the same regimen in younger patients, but none of the between-group differences were significant.
All fasting lipids rose for each study arm in both older and younger patients. Some of the between-group differences were statistically significant, but “there were no clinically significant differences in median changes from baseline in fasting lipids” among those aged 50 years or older, noted Dr. Mills and associates.
In terms of safety, no study drug-related discontinuations for renal adverse events occurred in either the B/F/TAF or the DTG + F/TAF groups, and the investigators saw no proximal renal tubulopathy.
The estimated glomerular filtration rate (eGFR) was calculated using the Cockroft-Gault equation. All three antiviral regimens were associated with early drops in eGFR, “consistent with inhibition of tubular creatinine secretion via organic cation transporter 2,” noted Dr. Mills and associates.
Other adverse events were rare in all groups in both older and younger patients, without significant differences between therapies.
The study was supported by Gilead Sciences, which also provided support to Dr. Mills. One coauthor is a Gilead employee.
SOURCE: Mills A et al. CROI 2020, Abstract 2886.
FROM CROI 2020
At U.S. Ground Zero for coronavirus, a hospital is transformed
David Baker, MD, a hospitalist at EvergreenHealth in Kirkland, Wash., had just come off a 7-day stretch of work and was early into his usual 7 days off. He’d helped care for some patients from a nearby assisted living facility who had been admitted with puzzlingly severe viral pneumonia that wasn’t influenza.
Though COVID-19, the novel coronavirus that was sickening tens of thousands in the Chinese province of Hubei, was in the back of everyone’s mind in late February, he said he wasn’t really expecting the call notifying him that two of the patients with pneumonia had tested positive for COVID-19.
Michael Chu, MD, was coming onto EvergreenHealth’s hospitalist service at about the time Dr. Baker was rotating off. He recalled learning of the first two positive COVID-19 tests on the evening of Feb. 28 – a Friday. He and his colleagues took in this information, coming to the realization that they were seeing other patients from the same facility who had viral pneumonia and negative influenza tests. “The first cohort of coronavirus patients all came from Life Care,” the Kirkland assisted living facility that was the epicenter of the first identified U.S. outbreak of community-transmitted coronavirus, said Dr. Chu. “They all fit a clinical syndrome” and many of them were critically ill or failing fast, since they were aged and with multiple risk factors, he said during the interviews he and his colleagues participated in.
As he processed the news of the positive tests and his inadvertent exposure to COVID-19, Dr. Baker realized that his duty schedule worked in his favor, since he wasn’t expected back for several more days. When he did come back to work after remaining asymptomatic, he found a much-changed environment as the coronavirus cases poured in and continual adaptations were made to accommodate these patients – and to keep staff and other patients safe.
The hospital adapts to a new normal
The usual protocol in EvergreenHealth’s ICU is for the nocturnist hospitalists, such as Dr. Baker, to staff that unit, with intensivists readily available for phone consultation. However, as the numbers of critically ill, ventilated COVID-19 patients climbed, the facility switched to 24/7 staffing with intensivists to augment the hospitalist team, said Nancy Marshall, MD, the director of EvergreenHealth’s hospitalist service.
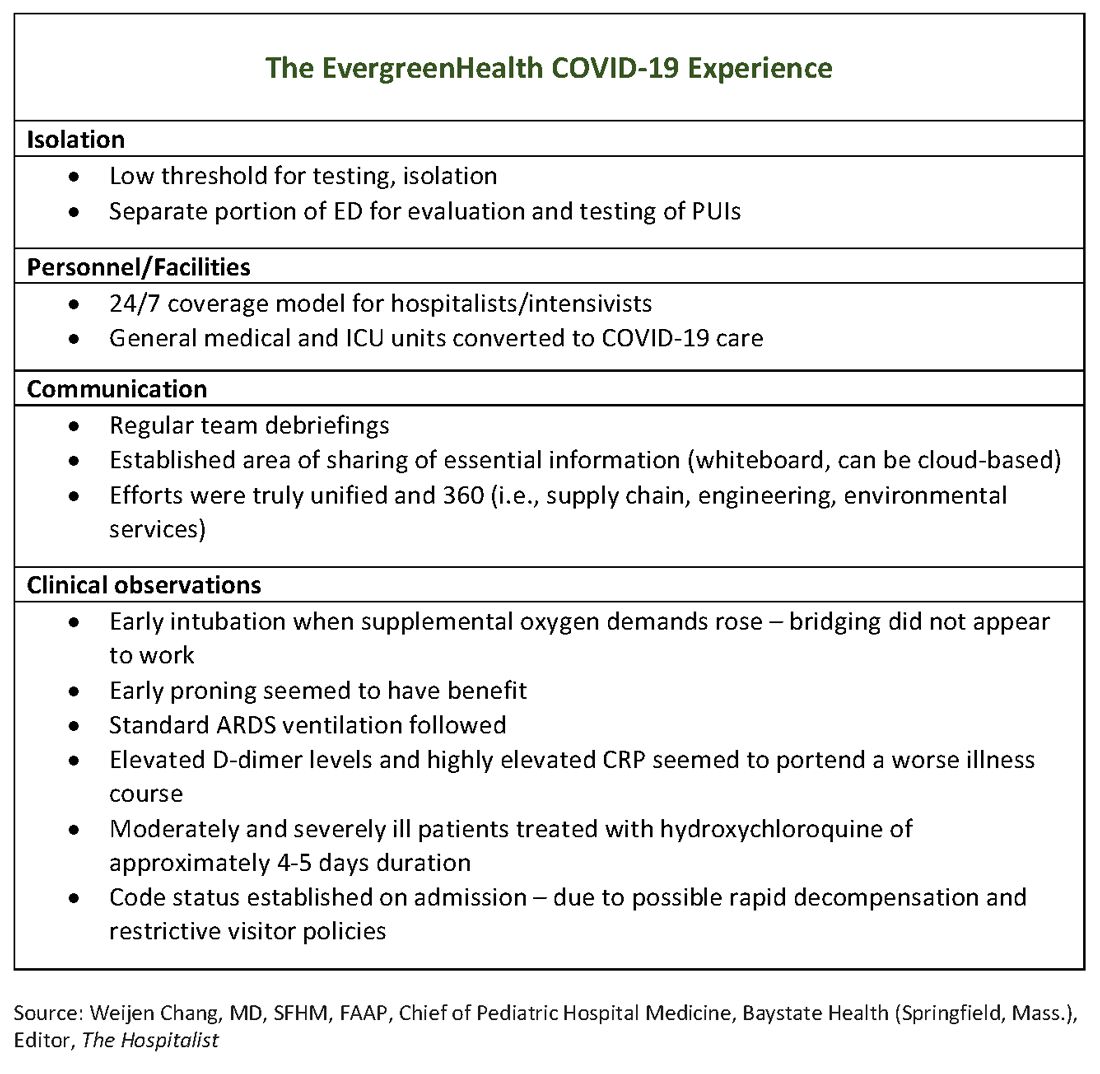
Dr. Marshall related how the entire hospital rallied to create appropriate – but flexible – staffing and environmental adaptations to the influx of coronavirus patients. “Early on, we established a separate portion of the emergency department to evaluate and test persons under investigation,” for COVID-19, she said. When they realized that they were seeing the nation’s first cluster of community coronavirus transmission, they used “appropriate isolation precautions” when indicated. Triggers for clinical suspicion included not just fever or cough, but also a new requirement for supplemental oxygen and new abnormal findings on chest radiographs.
Patients with confirmed or suspected coronavirus, once admitted, were placed in negative-pressure rooms, and droplet precautions were used with these patients. In the absence of aerosol-generating procedures, those caring for these patients used a standard surgical mask, goggles or face shield, an isolation gown, and gloves. For intubations, bronchoscopies, and other aerosol-generating procedures, N95 masks were used; the facility also has some powered and controlled air-purifying respirators.
In short order, once the size of the outbreak was appreciated, said Dr. Marshall, the entire ICU and half of another general medical floor in the hospital were converted to negative-pressure rooms.
Dr. Marshall said that having daily team debriefings has been essential. The hospitalist team room has a big whiteboard where essential information can be put up and shared. Frequent video conferencing has allowed physicians and advanced practice clinicians on the hospitalist team to ask questions, share concerns, and develop a shared knowledge base and vocabulary as they confronted this novel illness.
The rapid adaptations that EvergreenHealth successfully made depended on a responsive administration, good communication among physician services and with nursing staff, and the active participation of engineering and environmental services teams in adjusting to shifting patient needs, said Dr. Marshall.
“Preparedness is key,” Dr. Chu noted. “Managing this has required a unified effort” that addresses everything from the supply chain for personal protective equipment, to cleaning procedures, to engineering fixes that quickly added negative-pressure rooms.
“I can’t emphasize enough that this is a team sport,” said Dr. Marshall.
The unpredictable clinical course of COVID-19
The chimeric clinical course of COVID-19 means clinicians need to keep an open mind and be ready to act nimbly, said the EvergreenHealth hospitalists. Pattern recognition is a key to competent clinical management of hospitalized patients, but the course of coronavirus thus far defies any convenient application of heuristics.
Those first two patients had some characteristics in common, aside from their arrival from the same long-term care facility They each had unexplained acute respiratory distress syndrome and ground-glass opacities seen on chest CT, said Dr. Marshall. But all agreed it is still not clear who will fare well, and who will do poorly once they are admitted with coronavirus.
“We have noticed that these patients tend to have a rough course,” said Dr. Marshall. The “brisk inflammatory response” seen in some patients manifests in persistent fevers, big C-reactive protein (CRP) elevations, and likely is part of the picture of yet-unknown host factors that contribute to a worse disease course for some, she said. “These patients look toxic for a long time.”
Dr. Chu said that he’s seen even younger, healthier-looking patients admitted from the emergency department who are already quite dyspneic and may be headed for ventilation. These patients may have a low procalcitonin, and will often turn out to have an “impressive-looking” chest x-ray or CT that will show prominent bilateral infiltrates.
On the other hand, said Dr. Marshall, she and her colleagues have admitted frail-appearing nonagenarians who “just kind of sleep it off,” with little more than a cough and intermittent fevers.
Dr. Chu concurred: “So many of these patients had risk factors for severe disease and only had mild illness. Many were really quite stable.”
In terms of managing respiratory status, Dr. Baker said that the time to start planning for intubation is when the supplemental oxygen demands of COVID-19 patients start to go up. Unlike with patients who may be in some respiratory distress from other causes, once these patients have increased Fi02 needs, bridging “doesn’t work. ... They need to be intubated. Early intubation is important.” Clinicians’ level of concern should spike when they see increased work of breathing in a coronavirus patient, regardless of what the numbers are saying, he added.
For coronavirus patients with acute respiratory distress syndrome (ARDS), early proning also seems to provide some benefit, he said. At EvergreenHealth, standard ARDS ventilation protocols are being followed, including low tidal volume ventilation and positive end-expiratory pressure (PEEP) ladders. Coronavirus ventilation management has thus far been “pretty similar to standard practice with ARDS patients,” he said.
The hospitalist team was able to tap into the building knowledge base in China: Two of the EvergreenHealth hospitalists spoke fluent Mandarin, and one had contacts in China that allowed her to connect with Chinese physicians who had been treating COVID-19 patients since that outbreak had started. They established regular communication on WeChat, checking in frequently for updates on therapies and diagnostics being used in China as well.
One benefit of being in communication with colleagues in China, said Dr. Baker, was that they were able to get anecdotal evidence that elevated D-dimer levels and highly elevated CRP levels can portend a worse illness course. These findings seem to have held generally true for EvergreenHealth patients, he said. Dr. Marshall also spoke to the value of early communication with Chinese teams, who confirmed that the picture of a febrile illness with elevated CRP and leukopenia should raise the index of suspicion for coronavirus.
“Patients might improve over a few days, and then in the final 24 hours of their lives, we see changes in hemodynamics,” including reduced ejection fraction consistent with cardiogenic shock, as well as arrhythmias, said Dr. Baker. Some of the early patient deaths at EvergreenHealth followed this pattern, he said, noting that others have called for investigation into whether viral myocarditis is at play in some coronavirus deaths.
Moderately and severely ill coronavirus patients at EvergreenHealth currently receive a course of hydroxychloroquine of approximately 4-5 days’ duration. The hospital obtained remdesivir from Gilead through its compassionate-use program early on, and now is participating in a clinical trial for COVID-19 patients in the ICU.
By March 23, the facility had seen 162 confirmed COVID-19 cases, and 30 patients had died. Twenty-two inpatients had been discharged, and an additional 58 who were seen in the emergency department had been discharged home without admission.
Be suspicious – and prepared
When asked what he’d like his colleagues around the country to know as they diagnose and admit their first patients who are ill with coronavirus, Dr. Baker advised maintaining a high index of suspicion and a low threshold for testing. “I’ve given some thought to this,” he said. “From our reading and what information is out there, we are geared to pick up on the classic symptoms of coronavirus – cough, fever, some gastrointestinal symptoms.” However, many elderly patients “are not good historians. Some may have advanced dementia. ... When patients arrive with no history, we do our best to gather information,” but sometimes a case can still take clinicians by surprise, he said.
Dr. Baker told a cautionary tale of one of his patients, a woman who was admitted for a hip fracture after a fall at an assisted living facility. The patient was mildly hypoxic, but had an unremarkable physical exam, no fever, and a clear chest x-ray. She went to surgery and then to a postoperative floor with no isolation measures. When her respiratory status unexpectedly deteriorated, she was tested for COVID-19 – and was positive.
“When in doubt, isolate,” said Dr. Baker.
Dr. Chu concurred: “As soon as you suspect, move them, rather than testing first.”
Dr. Baker acknowledged, though, that when testing criteria and availability of personal protective equipment and test materials may vary by region, “it’s a challenge, especially with limited resources.”
Dr. Chu said that stringent isolation, though necessary, creates great hardship for patients and families. “It’s really important for us to check in with family members,” he said; patients are alone and afraid, and family members feel cut off – and also afraid on behalf of their ill loved ones. Workflow planning should acknowledge this and allocate extra time for patient connection and a little more time on the phone with families.
Dr. Chu offered a sobering final word. Make sure family members know their ill loved one’s wishes for care, he said: “There’s never been a better time to clarify code status on admission.”
Physicians at EvergreenHealth have created a document that contains consolidated information on what to anticipate and how to prepare for the arrival of COVID-19+ patients, recommendations on maximizing safety in the hospital environment, and key clinical management considerations. The document will be updated as new information arises.
Correction, 3/27/20: An earlier version of this article referenced white blood counts, presence of lymphopenia, and elevated hepatic enzymes for patients at EvergreenHealth when in fact that information pertained to patients in China. That paragraph has been deleted.
David Baker, MD, a hospitalist at EvergreenHealth in Kirkland, Wash., had just come off a 7-day stretch of work and was early into his usual 7 days off. He’d helped care for some patients from a nearby assisted living facility who had been admitted with puzzlingly severe viral pneumonia that wasn’t influenza.
Though COVID-19, the novel coronavirus that was sickening tens of thousands in the Chinese province of Hubei, was in the back of everyone’s mind in late February, he said he wasn’t really expecting the call notifying him that two of the patients with pneumonia had tested positive for COVID-19.
Michael Chu, MD, was coming onto EvergreenHealth’s hospitalist service at about the time Dr. Baker was rotating off. He recalled learning of the first two positive COVID-19 tests on the evening of Feb. 28 – a Friday. He and his colleagues took in this information, coming to the realization that they were seeing other patients from the same facility who had viral pneumonia and negative influenza tests. “The first cohort of coronavirus patients all came from Life Care,” the Kirkland assisted living facility that was the epicenter of the first identified U.S. outbreak of community-transmitted coronavirus, said Dr. Chu. “They all fit a clinical syndrome” and many of them were critically ill or failing fast, since they were aged and with multiple risk factors, he said during the interviews he and his colleagues participated in.
As he processed the news of the positive tests and his inadvertent exposure to COVID-19, Dr. Baker realized that his duty schedule worked in his favor, since he wasn’t expected back for several more days. When he did come back to work after remaining asymptomatic, he found a much-changed environment as the coronavirus cases poured in and continual adaptations were made to accommodate these patients – and to keep staff and other patients safe.

The hospital adapts to a new normal
The usual protocol in EvergreenHealth’s ICU is for the nocturnist hospitalists, such as Dr. Baker, to staff that unit, with intensivists readily available for phone consultation. However, as the numbers of critically ill, ventilated COVID-19 patients climbed, the facility switched to 24/7 staffing with intensivists to augment the hospitalist team, said Nancy Marshall, MD, the director of EvergreenHealth’s hospitalist service.
Dr. Marshall related how the entire hospital rallied to create appropriate – but flexible – staffing and environmental adaptations to the influx of coronavirus patients. “Early on, we established a separate portion of the emergency department to evaluate and test persons under investigation,” for COVID-19, she said. When they realized that they were seeing the nation’s first cluster of community coronavirus transmission, they used “appropriate isolation precautions” when indicated. Triggers for clinical suspicion included not just fever or cough, but also a new requirement for supplemental oxygen and new abnormal findings on chest radiographs.
Patients with confirmed or suspected coronavirus, once admitted, were placed in negative-pressure rooms, and droplet precautions were used with these patients. In the absence of aerosol-generating procedures, those caring for these patients used a standard surgical mask, goggles or face shield, an isolation gown, and gloves. For intubations, bronchoscopies, and other aerosol-generating procedures, N95 masks were used; the facility also has some powered and controlled air-purifying respirators.
In short order, once the size of the outbreak was appreciated, said Dr. Marshall, the entire ICU and half of another general medical floor in the hospital were converted to negative-pressure rooms.
Dr. Marshall said that having daily team debriefings has been essential. The hospitalist team room has a big whiteboard where essential information can be put up and shared. Frequent video conferencing has allowed physicians and advanced practice clinicians on the hospitalist team to ask questions, share concerns, and develop a shared knowledge base and vocabulary as they confronted this novel illness.
The rapid adaptations that EvergreenHealth successfully made depended on a responsive administration, good communication among physician services and with nursing staff, and the active participation of engineering and environmental services teams in adjusting to shifting patient needs, said Dr. Marshall.
“Preparedness is key,” Dr. Chu noted. “Managing this has required a unified effort” that addresses everything from the supply chain for personal protective equipment, to cleaning procedures, to engineering fixes that quickly added negative-pressure rooms.
“I can’t emphasize enough that this is a team sport,” said Dr. Marshall.
The unpredictable clinical course of COVID-19
The chimeric clinical course of COVID-19 means clinicians need to keep an open mind and be ready to act nimbly, said the EvergreenHealth hospitalists. Pattern recognition is a key to competent clinical management of hospitalized patients, but the course of coronavirus thus far defies any convenient application of heuristics.
Those first two patients had some characteristics in common, aside from their arrival from the same long-term care facility They each had unexplained acute respiratory distress syndrome and ground-glass opacities seen on chest CT, said Dr. Marshall. But all agreed it is still not clear who will fare well, and who will do poorly once they are admitted with coronavirus.
“We have noticed that these patients tend to have a rough course,” said Dr. Marshall. The “brisk inflammatory response” seen in some patients manifests in persistent fevers, big C-reactive protein (CRP) elevations, and likely is part of the picture of yet-unknown host factors that contribute to a worse disease course for some, she said. “These patients look toxic for a long time.”
Dr. Chu said that he’s seen even younger, healthier-looking patients admitted from the emergency department who are already quite dyspneic and may be headed for ventilation. These patients may have a low procalcitonin, and will often turn out to have an “impressive-looking” chest x-ray or CT that will show prominent bilateral infiltrates.
On the other hand, said Dr. Marshall, she and her colleagues have admitted frail-appearing nonagenarians who “just kind of sleep it off,” with little more than a cough and intermittent fevers.
Dr. Chu concurred: “So many of these patients had risk factors for severe disease and only had mild illness. Many were really quite stable.”
In terms of managing respiratory status, Dr. Baker said that the time to start planning for intubation is when the supplemental oxygen demands of COVID-19 patients start to go up. Unlike with patients who may be in some respiratory distress from other causes, once these patients have increased Fi02 needs, bridging “doesn’t work. ... They need to be intubated. Early intubation is important.” Clinicians’ level of concern should spike when they see increased work of breathing in a coronavirus patient, regardless of what the numbers are saying, he added.
For coronavirus patients with acute respiratory distress syndrome (ARDS), early proning also seems to provide some benefit, he said. At EvergreenHealth, standard ARDS ventilation protocols are being followed, including low tidal volume ventilation and positive end-expiratory pressure (PEEP) ladders. Coronavirus ventilation management has thus far been “pretty similar to standard practice with ARDS patients,” he said.
The hospitalist team was able to tap into the building knowledge base in China: Two of the EvergreenHealth hospitalists spoke fluent Mandarin, and one had contacts in China that allowed her to connect with Chinese physicians who had been treating COVID-19 patients since that outbreak had started. They established regular communication on WeChat, checking in frequently for updates on therapies and diagnostics being used in China as well.
One benefit of being in communication with colleagues in China, said Dr. Baker, was that they were able to get anecdotal evidence that elevated D-dimer levels and highly elevated CRP levels can portend a worse illness course. These findings seem to have held generally true for EvergreenHealth patients, he said. Dr. Marshall also spoke to the value of early communication with Chinese teams, who confirmed that the picture of a febrile illness with elevated CRP and leukopenia should raise the index of suspicion for coronavirus.
“Patients might improve over a few days, and then in the final 24 hours of their lives, we see changes in hemodynamics,” including reduced ejection fraction consistent with cardiogenic shock, as well as arrhythmias, said Dr. Baker. Some of the early patient deaths at EvergreenHealth followed this pattern, he said, noting that others have called for investigation into whether viral myocarditis is at play in some coronavirus deaths.
Moderately and severely ill coronavirus patients at EvergreenHealth currently receive a course of hydroxychloroquine of approximately 4-5 days’ duration. The hospital obtained remdesivir from Gilead through its compassionate-use program early on, and now is participating in a clinical trial for COVID-19 patients in the ICU.
By March 23, the facility had seen 162 confirmed COVID-19 cases, and 30 patients had died. Twenty-two inpatients had been discharged, and an additional 58 who were seen in the emergency department had been discharged home without admission.
Be suspicious – and prepared
When asked what he’d like his colleagues around the country to know as they diagnose and admit their first patients who are ill with coronavirus, Dr. Baker advised maintaining a high index of suspicion and a low threshold for testing. “I’ve given some thought to this,” he said. “From our reading and what information is out there, we are geared to pick up on the classic symptoms of coronavirus – cough, fever, some gastrointestinal symptoms.” However, many elderly patients “are not good historians. Some may have advanced dementia. ... When patients arrive with no history, we do our best to gather information,” but sometimes a case can still take clinicians by surprise, he said.
Dr. Baker told a cautionary tale of one of his patients, a woman who was admitted for a hip fracture after a fall at an assisted living facility. The patient was mildly hypoxic, but had an unremarkable physical exam, no fever, and a clear chest x-ray. She went to surgery and then to a postoperative floor with no isolation measures. When her respiratory status unexpectedly deteriorated, she was tested for COVID-19 – and was positive.
“When in doubt, isolate,” said Dr. Baker.
Dr. Chu concurred: “As soon as you suspect, move them, rather than testing first.”
Dr. Baker acknowledged, though, that when testing criteria and availability of personal protective equipment and test materials may vary by region, “it’s a challenge, especially with limited resources.”
Dr. Chu said that stringent isolation, though necessary, creates great hardship for patients and families. “It’s really important for us to check in with family members,” he said; patients are alone and afraid, and family members feel cut off – and also afraid on behalf of their ill loved ones. Workflow planning should acknowledge this and allocate extra time for patient connection and a little more time on the phone with families.
Dr. Chu offered a sobering final word. Make sure family members know their ill loved one’s wishes for care, he said: “There’s never been a better time to clarify code status on admission.”
Physicians at EvergreenHealth have created a document that contains consolidated information on what to anticipate and how to prepare for the arrival of COVID-19+ patients, recommendations on maximizing safety in the hospital environment, and key clinical management considerations. The document will be updated as new information arises.
Correction, 3/27/20: An earlier version of this article referenced white blood counts, presence of lymphopenia, and elevated hepatic enzymes for patients at EvergreenHealth when in fact that information pertained to patients in China. That paragraph has been deleted.
David Baker, MD, a hospitalist at EvergreenHealth in Kirkland, Wash., had just come off a 7-day stretch of work and was early into his usual 7 days off. He’d helped care for some patients from a nearby assisted living facility who had been admitted with puzzlingly severe viral pneumonia that wasn’t influenza.
Though COVID-19, the novel coronavirus that was sickening tens of thousands in the Chinese province of Hubei, was in the back of everyone’s mind in late February, he said he wasn’t really expecting the call notifying him that two of the patients with pneumonia had tested positive for COVID-19.
Michael Chu, MD, was coming onto EvergreenHealth’s hospitalist service at about the time Dr. Baker was rotating off. He recalled learning of the first two positive COVID-19 tests on the evening of Feb. 28 – a Friday. He and his colleagues took in this information, coming to the realization that they were seeing other patients from the same facility who had viral pneumonia and negative influenza tests. “The first cohort of coronavirus patients all came from Life Care,” the Kirkland assisted living facility that was the epicenter of the first identified U.S. outbreak of community-transmitted coronavirus, said Dr. Chu. “They all fit a clinical syndrome” and many of them were critically ill or failing fast, since they were aged and with multiple risk factors, he said during the interviews he and his colleagues participated in.
As he processed the news of the positive tests and his inadvertent exposure to COVID-19, Dr. Baker realized that his duty schedule worked in his favor, since he wasn’t expected back for several more days. When he did come back to work after remaining asymptomatic, he found a much-changed environment as the coronavirus cases poured in and continual adaptations were made to accommodate these patients – and to keep staff and other patients safe.

The hospital adapts to a new normal
The usual protocol in EvergreenHealth’s ICU is for the nocturnist hospitalists, such as Dr. Baker, to staff that unit, with intensivists readily available for phone consultation. However, as the numbers of critically ill, ventilated COVID-19 patients climbed, the facility switched to 24/7 staffing with intensivists to augment the hospitalist team, said Nancy Marshall, MD, the director of EvergreenHealth’s hospitalist service.
Dr. Marshall related how the entire hospital rallied to create appropriate – but flexible – staffing and environmental adaptations to the influx of coronavirus patients. “Early on, we established a separate portion of the emergency department to evaluate and test persons under investigation,” for COVID-19, she said. When they realized that they were seeing the nation’s first cluster of community coronavirus transmission, they used “appropriate isolation precautions” when indicated. Triggers for clinical suspicion included not just fever or cough, but also a new requirement for supplemental oxygen and new abnormal findings on chest radiographs.
Patients with confirmed or suspected coronavirus, once admitted, were placed in negative-pressure rooms, and droplet precautions were used with these patients. In the absence of aerosol-generating procedures, those caring for these patients used a standard surgical mask, goggles or face shield, an isolation gown, and gloves. For intubations, bronchoscopies, and other aerosol-generating procedures, N95 masks were used; the facility also has some powered and controlled air-purifying respirators.
In short order, once the size of the outbreak was appreciated, said Dr. Marshall, the entire ICU and half of another general medical floor in the hospital were converted to negative-pressure rooms.
Dr. Marshall said that having daily team debriefings has been essential. The hospitalist team room has a big whiteboard where essential information can be put up and shared. Frequent video conferencing has allowed physicians and advanced practice clinicians on the hospitalist team to ask questions, share concerns, and develop a shared knowledge base and vocabulary as they confronted this novel illness.
The rapid adaptations that EvergreenHealth successfully made depended on a responsive administration, good communication among physician services and with nursing staff, and the active participation of engineering and environmental services teams in adjusting to shifting patient needs, said Dr. Marshall.
“Preparedness is key,” Dr. Chu noted. “Managing this has required a unified effort” that addresses everything from the supply chain for personal protective equipment, to cleaning procedures, to engineering fixes that quickly added negative-pressure rooms.
“I can’t emphasize enough that this is a team sport,” said Dr. Marshall.
The unpredictable clinical course of COVID-19
The chimeric clinical course of COVID-19 means clinicians need to keep an open mind and be ready to act nimbly, said the EvergreenHealth hospitalists. Pattern recognition is a key to competent clinical management of hospitalized patients, but the course of coronavirus thus far defies any convenient application of heuristics.
Those first two patients had some characteristics in common, aside from their arrival from the same long-term care facility They each had unexplained acute respiratory distress syndrome and ground-glass opacities seen on chest CT, said Dr. Marshall. But all agreed it is still not clear who will fare well, and who will do poorly once they are admitted with coronavirus.
“We have noticed that these patients tend to have a rough course,” said Dr. Marshall. The “brisk inflammatory response” seen in some patients manifests in persistent fevers, big C-reactive protein (CRP) elevations, and likely is part of the picture of yet-unknown host factors that contribute to a worse disease course for some, she said. “These patients look toxic for a long time.”
Dr. Chu said that he’s seen even younger, healthier-looking patients admitted from the emergency department who are already quite dyspneic and may be headed for ventilation. These patients may have a low procalcitonin, and will often turn out to have an “impressive-looking” chest x-ray or CT that will show prominent bilateral infiltrates.
On the other hand, said Dr. Marshall, she and her colleagues have admitted frail-appearing nonagenarians who “just kind of sleep it off,” with little more than a cough and intermittent fevers.
Dr. Chu concurred: “So many of these patients had risk factors for severe disease and only had mild illness. Many were really quite stable.”
In terms of managing respiratory status, Dr. Baker said that the time to start planning for intubation is when the supplemental oxygen demands of COVID-19 patients start to go up. Unlike with patients who may be in some respiratory distress from other causes, once these patients have increased Fi02 needs, bridging “doesn’t work. ... They need to be intubated. Early intubation is important.” Clinicians’ level of concern should spike when they see increased work of breathing in a coronavirus patient, regardless of what the numbers are saying, he added.
For coronavirus patients with acute respiratory distress syndrome (ARDS), early proning also seems to provide some benefit, he said. At EvergreenHealth, standard ARDS ventilation protocols are being followed, including low tidal volume ventilation and positive end-expiratory pressure (PEEP) ladders. Coronavirus ventilation management has thus far been “pretty similar to standard practice with ARDS patients,” he said.
The hospitalist team was able to tap into the building knowledge base in China: Two of the EvergreenHealth hospitalists spoke fluent Mandarin, and one had contacts in China that allowed her to connect with Chinese physicians who had been treating COVID-19 patients since that outbreak had started. They established regular communication on WeChat, checking in frequently for updates on therapies and diagnostics being used in China as well.
One benefit of being in communication with colleagues in China, said Dr. Baker, was that they were able to get anecdotal evidence that elevated D-dimer levels and highly elevated CRP levels can portend a worse illness course. These findings seem to have held generally true for EvergreenHealth patients, he said. Dr. Marshall also spoke to the value of early communication with Chinese teams, who confirmed that the picture of a febrile illness with elevated CRP and leukopenia should raise the index of suspicion for coronavirus.
“Patients might improve over a few days, and then in the final 24 hours of their lives, we see changes in hemodynamics,” including reduced ejection fraction consistent with cardiogenic shock, as well as arrhythmias, said Dr. Baker. Some of the early patient deaths at EvergreenHealth followed this pattern, he said, noting that others have called for investigation into whether viral myocarditis is at play in some coronavirus deaths.
Moderately and severely ill coronavirus patients at EvergreenHealth currently receive a course of hydroxychloroquine of approximately 4-5 days’ duration. The hospital obtained remdesivir from Gilead through its compassionate-use program early on, and now is participating in a clinical trial for COVID-19 patients in the ICU.
By March 23, the facility had seen 162 confirmed COVID-19 cases, and 30 patients had died. Twenty-two inpatients had been discharged, and an additional 58 who were seen in the emergency department had been discharged home without admission.
Be suspicious – and prepared
When asked what he’d like his colleagues around the country to know as they diagnose and admit their first patients who are ill with coronavirus, Dr. Baker advised maintaining a high index of suspicion and a low threshold for testing. “I’ve given some thought to this,” he said. “From our reading and what information is out there, we are geared to pick up on the classic symptoms of coronavirus – cough, fever, some gastrointestinal symptoms.” However, many elderly patients “are not good historians. Some may have advanced dementia. ... When patients arrive with no history, we do our best to gather information,” but sometimes a case can still take clinicians by surprise, he said.
Dr. Baker told a cautionary tale of one of his patients, a woman who was admitted for a hip fracture after a fall at an assisted living facility. The patient was mildly hypoxic, but had an unremarkable physical exam, no fever, and a clear chest x-ray. She went to surgery and then to a postoperative floor with no isolation measures. When her respiratory status unexpectedly deteriorated, she was tested for COVID-19 – and was positive.
“When in doubt, isolate,” said Dr. Baker.
Dr. Chu concurred: “As soon as you suspect, move them, rather than testing first.”
Dr. Baker acknowledged, though, that when testing criteria and availability of personal protective equipment and test materials may vary by region, “it’s a challenge, especially with limited resources.”
Dr. Chu said that stringent isolation, though necessary, creates great hardship for patients and families. “It’s really important for us to check in with family members,” he said; patients are alone and afraid, and family members feel cut off – and also afraid on behalf of their ill loved ones. Workflow planning should acknowledge this and allocate extra time for patient connection and a little more time on the phone with families.
Dr. Chu offered a sobering final word. Make sure family members know their ill loved one’s wishes for care, he said: “There’s never been a better time to clarify code status on admission.”
Physicians at EvergreenHealth have created a document that contains consolidated information on what to anticipate and how to prepare for the arrival of COVID-19+ patients, recommendations on maximizing safety in the hospital environment, and key clinical management considerations. The document will be updated as new information arises.
Correction, 3/27/20: An earlier version of this article referenced white blood counts, presence of lymphopenia, and elevated hepatic enzymes for patients at EvergreenHealth when in fact that information pertained to patients in China. That paragraph has been deleted.
Coronavirus resources from AAD target safe office practices, new telemedicine guidance
The American Academy of Dermatology (
The guidance pages are publicly viewable. Additionally, AAD has made a collection of COVID-19 articles from the Journal of the American Academy of Dermatology freely available for the next 6 months.
George Hruza, MD, AAD president, detailed regulatory updates and other federal actions as well as guidance regarding telemedicine and clinical practice in a message to AAD members.
“While many questions still need answers, I have appointed an Ad Hoc Task Force to assess dermatology’s needs, share knowledge, and provide ongoing guidance and information throughout the crisis,” Dr. Hruza wrote.
“The situation is changing rapidly, and we are committed to keeping you updated with reliable and practical information to help you adapt to the circumstances,” he noted, referring dermatologists to the AAD’s information hub for the coronavirus outbreak. “We are keeping this page updated frequently, and it will serve as your primary source for what we know now,” he noted.
The Centers for Medicare & Medicaid Services has recently relaxed key regulations regarding technology to provide telemedicine so that physicians and patients can use existing platforms such as FaceTime and Skype for virtual visits. The usual fines for HIPAA noncompliance have been waived. Additionally, telemedicine visits can now be reimbursed at the same rate as in-person visits.
Private payers are beginning to follow suit, said Dr. Hruza, noting that the AAD Association is working to harmonize private coverage with public reimbursement. The AAD also is tracking which payers are coming in line with federal policies on its teledermatology page.
These changes in regulation around telemedicine apply to patient encounters for any purpose, not just coronavirus-related encounters, noted Dr. Hruza. “The good news is that the government has taken action to make it much easier for us to provide virtual consults to patients. Dermatology has always been a leader in telemedicine, and it will be an important way to offer care to patients who can’t or don’t need to come into the office or clinic,” he added.
Importantly, said Dr. Hruza, CMS is allowing practices to have discretion over whether copays are collected, or collected in full, so that these payments don’t present a barrier to patient care in the current crisis environment.
For dermatologists who are new to telemedicine, AAD has created an online resource that includes information about various telemedicine platforms, updated guidance regarding regulations, and best practices for accurate coding and documentation of telemedicine visits.
The Academy has also been developing dermatology-specific guidance, including how to address the concerns of patients who are receiving biologic therapies and how to conserve personal protective equipment while still protecting physicians, staff, and patients from COVID-19 infection.
For patients on biologic therapy who show no sign of coronavirus infection, the decision to continue or stop biologics should be made on a case-by-case basis. Factors to be considered include patient age, comorbidities, and the severity of the original indication for biologic use.
Initiation of biologics should only be done after a similar risk-benefit analysis, with a recommendation to consider deferring initiation for patients 60 and older and those with comorbidities that may portend a worse course in the event of coronavirus infection. Biologics should be discontinued for patients who test positive for COVID-19.
Dr. Hruza outlined some of the federal measures taken that may affect the business side of dermatology practices. These include a $20 million transfer to the Small Business Administration to offset administrative expenses associated with increased loan volumes related to the coronavirus outbreak. Eligible expenses for loans may include new devices and environmental adjustments to accommodate telehealth services.
Additionally, it is anticipated that as employers are required to provide paid sick and family leave, a payroll tax credit will be issued to employers. Some self-employed individuals will also be able to claim a tax credit for sick and family leave.
A recent news release from the AAD encourages the public to use high-emollient moisturizers after handwashing. The release also provides other tips, such as using petrolatum at bedtime for hands that are particularly dry and focusing on the fingertips when moisturizing, as these areas are prone to cracking. The release also reaffirms that the most effective way to clean hands is soap and water, and that moisturizing after handwashing does not negate the antiviral effects of cleansing, contrary to some social media reports.
koakes@mdedge.com
The American Academy of Dermatology (
The guidance pages are publicly viewable. Additionally, AAD has made a collection of COVID-19 articles from the Journal of the American Academy of Dermatology freely available for the next 6 months.
George Hruza, MD, AAD president, detailed regulatory updates and other federal actions as well as guidance regarding telemedicine and clinical practice in a message to AAD members.
“While many questions still need answers, I have appointed an Ad Hoc Task Force to assess dermatology’s needs, share knowledge, and provide ongoing guidance and information throughout the crisis,” Dr. Hruza wrote.
“The situation is changing rapidly, and we are committed to keeping you updated with reliable and practical information to help you adapt to the circumstances,” he noted, referring dermatologists to the AAD’s information hub for the coronavirus outbreak. “We are keeping this page updated frequently, and it will serve as your primary source for what we know now,” he noted.
The Centers for Medicare & Medicaid Services has recently relaxed key regulations regarding technology to provide telemedicine so that physicians and patients can use existing platforms such as FaceTime and Skype for virtual visits. The usual fines for HIPAA noncompliance have been waived. Additionally, telemedicine visits can now be reimbursed at the same rate as in-person visits.
Private payers are beginning to follow suit, said Dr. Hruza, noting that the AAD Association is working to harmonize private coverage with public reimbursement. The AAD also is tracking which payers are coming in line with federal policies on its teledermatology page.
These changes in regulation around telemedicine apply to patient encounters for any purpose, not just coronavirus-related encounters, noted Dr. Hruza. “The good news is that the government has taken action to make it much easier for us to provide virtual consults to patients. Dermatology has always been a leader in telemedicine, and it will be an important way to offer care to patients who can’t or don’t need to come into the office or clinic,” he added.
Importantly, said Dr. Hruza, CMS is allowing practices to have discretion over whether copays are collected, or collected in full, so that these payments don’t present a barrier to patient care in the current crisis environment.
For dermatologists who are new to telemedicine, AAD has created an online resource that includes information about various telemedicine platforms, updated guidance regarding regulations, and best practices for accurate coding and documentation of telemedicine visits.
The Academy has also been developing dermatology-specific guidance, including how to address the concerns of patients who are receiving biologic therapies and how to conserve personal protective equipment while still protecting physicians, staff, and patients from COVID-19 infection.
For patients on biologic therapy who show no sign of coronavirus infection, the decision to continue or stop biologics should be made on a case-by-case basis. Factors to be considered include patient age, comorbidities, and the severity of the original indication for biologic use.
Initiation of biologics should only be done after a similar risk-benefit analysis, with a recommendation to consider deferring initiation for patients 60 and older and those with comorbidities that may portend a worse course in the event of coronavirus infection. Biologics should be discontinued for patients who test positive for COVID-19.
Dr. Hruza outlined some of the federal measures taken that may affect the business side of dermatology practices. These include a $20 million transfer to the Small Business Administration to offset administrative expenses associated with increased loan volumes related to the coronavirus outbreak. Eligible expenses for loans may include new devices and environmental adjustments to accommodate telehealth services.
Additionally, it is anticipated that as employers are required to provide paid sick and family leave, a payroll tax credit will be issued to employers. Some self-employed individuals will also be able to claim a tax credit for sick and family leave.
A recent news release from the AAD encourages the public to use high-emollient moisturizers after handwashing. The release also provides other tips, such as using petrolatum at bedtime for hands that are particularly dry and focusing on the fingertips when moisturizing, as these areas are prone to cracking. The release also reaffirms that the most effective way to clean hands is soap and water, and that moisturizing after handwashing does not negate the antiviral effects of cleansing, contrary to some social media reports.
koakes@mdedge.com
The American Academy of Dermatology (
The guidance pages are publicly viewable. Additionally, AAD has made a collection of COVID-19 articles from the Journal of the American Academy of Dermatology freely available for the next 6 months.
George Hruza, MD, AAD president, detailed regulatory updates and other federal actions as well as guidance regarding telemedicine and clinical practice in a message to AAD members.
“While many questions still need answers, I have appointed an Ad Hoc Task Force to assess dermatology’s needs, share knowledge, and provide ongoing guidance and information throughout the crisis,” Dr. Hruza wrote.
“The situation is changing rapidly, and we are committed to keeping you updated with reliable and practical information to help you adapt to the circumstances,” he noted, referring dermatologists to the AAD’s information hub for the coronavirus outbreak. “We are keeping this page updated frequently, and it will serve as your primary source for what we know now,” he noted.
The Centers for Medicare & Medicaid Services has recently relaxed key regulations regarding technology to provide telemedicine so that physicians and patients can use existing platforms such as FaceTime and Skype for virtual visits. The usual fines for HIPAA noncompliance have been waived. Additionally, telemedicine visits can now be reimbursed at the same rate as in-person visits.
Private payers are beginning to follow suit, said Dr. Hruza, noting that the AAD Association is working to harmonize private coverage with public reimbursement. The AAD also is tracking which payers are coming in line with federal policies on its teledermatology page.
These changes in regulation around telemedicine apply to patient encounters for any purpose, not just coronavirus-related encounters, noted Dr. Hruza. “The good news is that the government has taken action to make it much easier for us to provide virtual consults to patients. Dermatology has always been a leader in telemedicine, and it will be an important way to offer care to patients who can’t or don’t need to come into the office or clinic,” he added.
Importantly, said Dr. Hruza, CMS is allowing practices to have discretion over whether copays are collected, or collected in full, so that these payments don’t present a barrier to patient care in the current crisis environment.
For dermatologists who are new to telemedicine, AAD has created an online resource that includes information about various telemedicine platforms, updated guidance regarding regulations, and best practices for accurate coding and documentation of telemedicine visits.
The Academy has also been developing dermatology-specific guidance, including how to address the concerns of patients who are receiving biologic therapies and how to conserve personal protective equipment while still protecting physicians, staff, and patients from COVID-19 infection.
For patients on biologic therapy who show no sign of coronavirus infection, the decision to continue or stop biologics should be made on a case-by-case basis. Factors to be considered include patient age, comorbidities, and the severity of the original indication for biologic use.
Initiation of biologics should only be done after a similar risk-benefit analysis, with a recommendation to consider deferring initiation for patients 60 and older and those with comorbidities that may portend a worse course in the event of coronavirus infection. Biologics should be discontinued for patients who test positive for COVID-19.
Dr. Hruza outlined some of the federal measures taken that may affect the business side of dermatology practices. These include a $20 million transfer to the Small Business Administration to offset administrative expenses associated with increased loan volumes related to the coronavirus outbreak. Eligible expenses for loans may include new devices and environmental adjustments to accommodate telehealth services.
Additionally, it is anticipated that as employers are required to provide paid sick and family leave, a payroll tax credit will be issued to employers. Some self-employed individuals will also be able to claim a tax credit for sick and family leave.
A recent news release from the AAD encourages the public to use high-emollient moisturizers after handwashing. The release also provides other tips, such as using petrolatum at bedtime for hands that are particularly dry and focusing on the fingertips when moisturizing, as these areas are prone to cracking. The release also reaffirms that the most effective way to clean hands is soap and water, and that moisturizing after handwashing does not negate the antiviral effects of cleansing, contrary to some social media reports.
koakes@mdedge.com
COVID-19 will test medical supply stocks
In a JAMA Live Stream interview, in the United States.
Dr. Fauci got into the details of what is known, what is unknown, what is being done in laboratories, and what clinical elements are still not understood about this disease.
The next several weeks, he said, are likely to tell the tale of whether our health care system is up to the challenge of care for the most ill among those who will be affected by COVID-19.
“It shouldn’t panic or frighten us, but we have to know we’re dealing with a very serious problem that we have to address, and we have to deal with it in a very bold way,” said Dr. Fauci, director of the National Institute of Allergy and Infectious Diseases at the National Institutes of Health.
Speaking in an interview with JAMA Editor in Chief Howard Bauchner, MD, Dr. Fauci said the situation favors action over fear. “Let’s apply that energy to doing the things that we know can mitigate this.”
He added that he heard the message loud and clear from health care leaders in Italy and France during a World Health Organization coronavirus call earlier in the day. Officials in those countries, he said, were “almost pleading with the rest of the world to please take this very seriously, because it happens all of a sudden – very abruptly. ... The best time to mitigate is before that happens, because if you wait until after it happens you’re playing catch-up.”
Dr. Bauchner, noting that strict social distancing has been underway in many parts of the United States for several days, posited that, by early April, “We’ll really have a sense if we can manage in terms of serious illness.” Seattle, New York, Boston, and the San Francisco Bay Area may experience demand that outstrips ICU capacity at that point, but the rest of the country, he said, “is doing relatively well.”
Stress test on the health care system
Dr. Fauci agreed with this statement and added: “We’re going to know – for better or worse – whether we have enough of what it takes to be able to practice the kind of medicine that we optimally would want to practice.
In the matter of a week or 2 ... I think we’ll get a feel for whether or not we really have enough of the supplies that it takes.”
The well-publicized regional shortages in personal protective equipment (PPE) are forcing tough choices in some areas. As expedited – and even drive-through – testing begins, some of the demand for testing-related PPE may abate, especially if protocols include self-administration of nasal swabs, he noted.
Dr. Fauci added that the strategic national stockpile of medical supplies and equipment has not yet been tapped, “but you need to backfill that as quickly as you can once you start drawing from the strategic national stockpile.”
Returning to work after COVID-19 infection
Regarding the thorny question of when health care workers should be permitted to return to work after coronavirus infection, “it’s an evolving story,” said Dr. Fauci. Current guidance advises that health care providers stay away from work until two negative tests after resolution of fever and improvement of respiratory symptoms, or 3 fever-free days.
“We are approaching a point where you’re going to get enough people who are getting infected that we aren’t going to be able to do that,” he said. Depending on the stress to the health care system in a given locality, he said that facilities are going to have to “decide with good judgment” when health care workers go back on the job after coronavirus infection.
Asked how soon an individual would reliably test positive for COVID-19 after exposure, Dr. Fauci said, “We don’t know the answer to that. ... We can surmise it ...” He noted that it’s a median of about 5 days with a range of 2 to 14 days, before an infected individual becomes symptomatic. “I can say it’s not going to happen immediately,” he added, noting that he wouldn’t expect to see a positive test until about 2 days after exposure at the earliest. “When you get to the point where you are symptomatic, you’re almost certainly going to be positive then. ... This is just an extrapolation,” rather than conclusions drawn from solid data, he emphasized.
Higher risk reported in cardiac patients
Dr. Bauchner, who was relaying questions sent in from physicians during the live-streamed interview, asked about a newly issued joint statement from the American Heart Association, American College of Cardiology, and the Heart Failure Society of America, which on March 17 affirmed that individuals on ACE inhibitors and angiotensin receptor blockers (ARBs) continue that therapy if they should become ill with COVID-19. The European Society of Cardiology issued a similar recommendation a few days prior.
Despite these societies’ statements, Dr. Fauci pointed to population-level data in Italy as suggesting that the case isn’t yet closed. “We really need to get data, and we need to get data fast. There’s a mechanistic rationale for the concern. It’s there, and it’s firm,” he said. The theoretical concern is that ACE inhibitors can upregulate expression of the ACE-2 protein on cell membranes, which is the entry point for SARS-Cov-2 to enter cells.
He added that he remains concerned about the number of coronavirus fatalities of patients in Italy who had hypertension as their only, or primary, underlying health problem.“That to me was a bit of a red flag,” he said. “Patients with hypertension almost certainly had a physician, and the physician almost certainly treated that person with medication. Why should someone who has hypertension that was well controlled have a much greater chance of dying?” he asked, noting that “I look at a person with well-controlled hypertension as a relatively healthy person. I don’t know what the answer is, but somebody has to look very carefully,” ideally by means of a natural history study that identifies medications used by those who died from coronavirus.
Potential therapies
Regarding potential therapies for COVID-19, Dr. Fauci acknowledged the social media buzz and flurry of medical letters and case reports about the use of hydroxychloroquine (Plaquenil) to treat active infection. He said that he and other researchers are “in active discussion” about how best to study the efficacy and safety of hydroxychloroquine, but he also acknowledged that many treating clinicians will use hydroxychloroquine empirically in the absence of other treatments with proven efficacy.
Clinical trials underway in China for antiviral medication are facing some enrollment challenges currently “because people want to get the drug,” said Dr. Fauci. “They don’t want to be in the trial; they just want to get the drug.” Though each of two trials has targeted approximately 500 participants as the number needed for sufficient statistical power, Dr. Fauci urged Chinese data safety monitoring boards to “take a close look” at the data already accrued for the several hundred patients who have already enrolled for the studies “to see if there’s any hint of efficacy.”
In a JAMA Live Stream interview, in the United States.
Dr. Fauci got into the details of what is known, what is unknown, what is being done in laboratories, and what clinical elements are still not understood about this disease.
The next several weeks, he said, are likely to tell the tale of whether our health care system is up to the challenge of care for the most ill among those who will be affected by COVID-19.
“It shouldn’t panic or frighten us, but we have to know we’re dealing with a very serious problem that we have to address, and we have to deal with it in a very bold way,” said Dr. Fauci, director of the National Institute of Allergy and Infectious Diseases at the National Institutes of Health.
Speaking in an interview with JAMA Editor in Chief Howard Bauchner, MD, Dr. Fauci said the situation favors action over fear. “Let’s apply that energy to doing the things that we know can mitigate this.”
He added that he heard the message loud and clear from health care leaders in Italy and France during a World Health Organization coronavirus call earlier in the day. Officials in those countries, he said, were “almost pleading with the rest of the world to please take this very seriously, because it happens all of a sudden – very abruptly. ... The best time to mitigate is before that happens, because if you wait until after it happens you’re playing catch-up.”
Dr. Bauchner, noting that strict social distancing has been underway in many parts of the United States for several days, posited that, by early April, “We’ll really have a sense if we can manage in terms of serious illness.” Seattle, New York, Boston, and the San Francisco Bay Area may experience demand that outstrips ICU capacity at that point, but the rest of the country, he said, “is doing relatively well.”
Stress test on the health care system
Dr. Fauci agreed with this statement and added: “We’re going to know – for better or worse – whether we have enough of what it takes to be able to practice the kind of medicine that we optimally would want to practice.
In the matter of a week or 2 ... I think we’ll get a feel for whether or not we really have enough of the supplies that it takes.”
The well-publicized regional shortages in personal protective equipment (PPE) are forcing tough choices in some areas. As expedited – and even drive-through – testing begins, some of the demand for testing-related PPE may abate, especially if protocols include self-administration of nasal swabs, he noted.
Dr. Fauci added that the strategic national stockpile of medical supplies and equipment has not yet been tapped, “but you need to backfill that as quickly as you can once you start drawing from the strategic national stockpile.”
Returning to work after COVID-19 infection
Regarding the thorny question of when health care workers should be permitted to return to work after coronavirus infection, “it’s an evolving story,” said Dr. Fauci. Current guidance advises that health care providers stay away from work until two negative tests after resolution of fever and improvement of respiratory symptoms, or 3 fever-free days.
“We are approaching a point where you’re going to get enough people who are getting infected that we aren’t going to be able to do that,” he said. Depending on the stress to the health care system in a given locality, he said that facilities are going to have to “decide with good judgment” when health care workers go back on the job after coronavirus infection.
Asked how soon an individual would reliably test positive for COVID-19 after exposure, Dr. Fauci said, “We don’t know the answer to that. ... We can surmise it ...” He noted that it’s a median of about 5 days with a range of 2 to 14 days, before an infected individual becomes symptomatic. “I can say it’s not going to happen immediately,” he added, noting that he wouldn’t expect to see a positive test until about 2 days after exposure at the earliest. “When you get to the point where you are symptomatic, you’re almost certainly going to be positive then. ... This is just an extrapolation,” rather than conclusions drawn from solid data, he emphasized.
Higher risk reported in cardiac patients
Dr. Bauchner, who was relaying questions sent in from physicians during the live-streamed interview, asked about a newly issued joint statement from the American Heart Association, American College of Cardiology, and the Heart Failure Society of America, which on March 17 affirmed that individuals on ACE inhibitors and angiotensin receptor blockers (ARBs) continue that therapy if they should become ill with COVID-19. The European Society of Cardiology issued a similar recommendation a few days prior.
Despite these societies’ statements, Dr. Fauci pointed to population-level data in Italy as suggesting that the case isn’t yet closed. “We really need to get data, and we need to get data fast. There’s a mechanistic rationale for the concern. It’s there, and it’s firm,” he said. The theoretical concern is that ACE inhibitors can upregulate expression of the ACE-2 protein on cell membranes, which is the entry point for SARS-Cov-2 to enter cells.
He added that he remains concerned about the number of coronavirus fatalities of patients in Italy who had hypertension as their only, or primary, underlying health problem.“That to me was a bit of a red flag,” he said. “Patients with hypertension almost certainly had a physician, and the physician almost certainly treated that person with medication. Why should someone who has hypertension that was well controlled have a much greater chance of dying?” he asked, noting that “I look at a person with well-controlled hypertension as a relatively healthy person. I don’t know what the answer is, but somebody has to look very carefully,” ideally by means of a natural history study that identifies medications used by those who died from coronavirus.
Potential therapies
Regarding potential therapies for COVID-19, Dr. Fauci acknowledged the social media buzz and flurry of medical letters and case reports about the use of hydroxychloroquine (Plaquenil) to treat active infection. He said that he and other researchers are “in active discussion” about how best to study the efficacy and safety of hydroxychloroquine, but he also acknowledged that many treating clinicians will use hydroxychloroquine empirically in the absence of other treatments with proven efficacy.
Clinical trials underway in China for antiviral medication are facing some enrollment challenges currently “because people want to get the drug,” said Dr. Fauci. “They don’t want to be in the trial; they just want to get the drug.” Though each of two trials has targeted approximately 500 participants as the number needed for sufficient statistical power, Dr. Fauci urged Chinese data safety monitoring boards to “take a close look” at the data already accrued for the several hundred patients who have already enrolled for the studies “to see if there’s any hint of efficacy.”
In a JAMA Live Stream interview, in the United States.
Dr. Fauci got into the details of what is known, what is unknown, what is being done in laboratories, and what clinical elements are still not understood about this disease.
The next several weeks, he said, are likely to tell the tale of whether our health care system is up to the challenge of care for the most ill among those who will be affected by COVID-19.
“It shouldn’t panic or frighten us, but we have to know we’re dealing with a very serious problem that we have to address, and we have to deal with it in a very bold way,” said Dr. Fauci, director of the National Institute of Allergy and Infectious Diseases at the National Institutes of Health.
Speaking in an interview with JAMA Editor in Chief Howard Bauchner, MD, Dr. Fauci said the situation favors action over fear. “Let’s apply that energy to doing the things that we know can mitigate this.”
He added that he heard the message loud and clear from health care leaders in Italy and France during a World Health Organization coronavirus call earlier in the day. Officials in those countries, he said, were “almost pleading with the rest of the world to please take this very seriously, because it happens all of a sudden – very abruptly. ... The best time to mitigate is before that happens, because if you wait until after it happens you’re playing catch-up.”
Dr. Bauchner, noting that strict social distancing has been underway in many parts of the United States for several days, posited that, by early April, “We’ll really have a sense if we can manage in terms of serious illness.” Seattle, New York, Boston, and the San Francisco Bay Area may experience demand that outstrips ICU capacity at that point, but the rest of the country, he said, “is doing relatively well.”
Stress test on the health care system
Dr. Fauci agreed with this statement and added: “We’re going to know – for better or worse – whether we have enough of what it takes to be able to practice the kind of medicine that we optimally would want to practice.
In the matter of a week or 2 ... I think we’ll get a feel for whether or not we really have enough of the supplies that it takes.”
The well-publicized regional shortages in personal protective equipment (PPE) are forcing tough choices in some areas. As expedited – and even drive-through – testing begins, some of the demand for testing-related PPE may abate, especially if protocols include self-administration of nasal swabs, he noted.
Dr. Fauci added that the strategic national stockpile of medical supplies and equipment has not yet been tapped, “but you need to backfill that as quickly as you can once you start drawing from the strategic national stockpile.”
Returning to work after COVID-19 infection
Regarding the thorny question of when health care workers should be permitted to return to work after coronavirus infection, “it’s an evolving story,” said Dr. Fauci. Current guidance advises that health care providers stay away from work until two negative tests after resolution of fever and improvement of respiratory symptoms, or 3 fever-free days.
“We are approaching a point where you’re going to get enough people who are getting infected that we aren’t going to be able to do that,” he said. Depending on the stress to the health care system in a given locality, he said that facilities are going to have to “decide with good judgment” when health care workers go back on the job after coronavirus infection.
Asked how soon an individual would reliably test positive for COVID-19 after exposure, Dr. Fauci said, “We don’t know the answer to that. ... We can surmise it ...” He noted that it’s a median of about 5 days with a range of 2 to 14 days, before an infected individual becomes symptomatic. “I can say it’s not going to happen immediately,” he added, noting that he wouldn’t expect to see a positive test until about 2 days after exposure at the earliest. “When you get to the point where you are symptomatic, you’re almost certainly going to be positive then. ... This is just an extrapolation,” rather than conclusions drawn from solid data, he emphasized.
Higher risk reported in cardiac patients
Dr. Bauchner, who was relaying questions sent in from physicians during the live-streamed interview, asked about a newly issued joint statement from the American Heart Association, American College of Cardiology, and the Heart Failure Society of America, which on March 17 affirmed that individuals on ACE inhibitors and angiotensin receptor blockers (ARBs) continue that therapy if they should become ill with COVID-19. The European Society of Cardiology issued a similar recommendation a few days prior.
Despite these societies’ statements, Dr. Fauci pointed to population-level data in Italy as suggesting that the case isn’t yet closed. “We really need to get data, and we need to get data fast. There’s a mechanistic rationale for the concern. It’s there, and it’s firm,” he said. The theoretical concern is that ACE inhibitors can upregulate expression of the ACE-2 protein on cell membranes, which is the entry point for SARS-Cov-2 to enter cells.
He added that he remains concerned about the number of coronavirus fatalities of patients in Italy who had hypertension as their only, or primary, underlying health problem.“That to me was a bit of a red flag,” he said. “Patients with hypertension almost certainly had a physician, and the physician almost certainly treated that person with medication. Why should someone who has hypertension that was well controlled have a much greater chance of dying?” he asked, noting that “I look at a person with well-controlled hypertension as a relatively healthy person. I don’t know what the answer is, but somebody has to look very carefully,” ideally by means of a natural history study that identifies medications used by those who died from coronavirus.
Potential therapies
Regarding potential therapies for COVID-19, Dr. Fauci acknowledged the social media buzz and flurry of medical letters and case reports about the use of hydroxychloroquine (Plaquenil) to treat active infection. He said that he and other researchers are “in active discussion” about how best to study the efficacy and safety of hydroxychloroquine, but he also acknowledged that many treating clinicians will use hydroxychloroquine empirically in the absence of other treatments with proven efficacy.
Clinical trials underway in China for antiviral medication are facing some enrollment challenges currently “because people want to get the drug,” said Dr. Fauci. “They don’t want to be in the trial; they just want to get the drug.” Though each of two trials has targeted approximately 500 participants as the number needed for sufficient statistical power, Dr. Fauci urged Chinese data safety monitoring boards to “take a close look” at the data already accrued for the several hundred patients who have already enrolled for the studies “to see if there’s any hint of efficacy.”
REPORTING FROM JAMA LIVE STREAM
Digestive Disease Week® 2020 is canceled because of coronavirus concerns
Digestive Disease Week (DDW) 2020, originally scheduled for May 2-5, 2020, in Chicago, has been canceled because of the coronavirus pandemic.
Organizers are exploring options for virtual presentation of some of the content material.
“While we are disappointed to miss the science, education, and networking that are hallmarks of DDW, we must focus on the health and safety of our community,” said DDW organizers in an email notification on March 18, 2020. “Thank you for your patience as we evaluated the status of DDW in light of the rapidly changing coronavirus pandemic.”
Citing the meeting’s long tradition of improving patient care and the understanding of digestive diseases, the organizers promised more information to come about opportunities for remote presentation of research and educational material.
All events associated with DDW are also canceled, said the email. A page of frequently asked questions is being maintained (https://digestivediseaseweek.freshdesk.com/support/solutions/43000366101), and questions may be asked by submitting a ticket to the DDW help desk (https://digestivediseaseweek.freshdesk.com/support/tickets/new).
Digestive Disease Week (DDW) 2020, originally scheduled for May 2-5, 2020, in Chicago, has been canceled because of the coronavirus pandemic.
Organizers are exploring options for virtual presentation of some of the content material.
“While we are disappointed to miss the science, education, and networking that are hallmarks of DDW, we must focus on the health and safety of our community,” said DDW organizers in an email notification on March 18, 2020. “Thank you for your patience as we evaluated the status of DDW in light of the rapidly changing coronavirus pandemic.”
Citing the meeting’s long tradition of improving patient care and the understanding of digestive diseases, the organizers promised more information to come about opportunities for remote presentation of research and educational material.
All events associated with DDW are also canceled, said the email. A page of frequently asked questions is being maintained (https://digestivediseaseweek.freshdesk.com/support/solutions/43000366101), and questions may be asked by submitting a ticket to the DDW help desk (https://digestivediseaseweek.freshdesk.com/support/tickets/new).
Digestive Disease Week (DDW) 2020, originally scheduled for May 2-5, 2020, in Chicago, has been canceled because of the coronavirus pandemic.
Organizers are exploring options for virtual presentation of some of the content material.
“While we are disappointed to miss the science, education, and networking that are hallmarks of DDW, we must focus on the health and safety of our community,” said DDW organizers in an email notification on March 18, 2020. “Thank you for your patience as we evaluated the status of DDW in light of the rapidly changing coronavirus pandemic.”
Citing the meeting’s long tradition of improving patient care and the understanding of digestive diseases, the organizers promised more information to come about opportunities for remote presentation of research and educational material.
All events associated with DDW are also canceled, said the email. A page of frequently asked questions is being maintained (https://digestivediseaseweek.freshdesk.com/support/solutions/43000366101), and questions may be asked by submitting a ticket to the DDW help desk (https://digestivediseaseweek.freshdesk.com/support/tickets/new).
More postpartum weight gain with dolutegravir-based ART
Women with HIV on dolutegravir-based antiretroviral therapy (ART) protocols had higher weights through 18 months of the postpartum period than women on efavirenz-based therapy, according to a recent study. However, women taking dolutegravir had similar postpartum weights to women who did not have HIV infection.
The results were shared by Jennifer Jao, MD, MPH, of Northwestern University, Chicago, in a video presentation of the research during the Conference on Retroviruses & Opportunistic Infections, which was presented online this year. CROI organizers chose to hold a virtual meeting because of concerns about the spread of COVID-19.
Dr. Jao, an internal medicine physician and pediatrician, and colleagues looked at the association between dolutegravir and postpartum weight for women with HIV, compared with women with HIV who were taking efavirenz-based ART and women who did not have HIV infection.
Though there was no significant difference among the three groups for body mass index at 4 weeks post partum (all were between 24 and 26 kg/m2), postpartum weight for the dolutegravir group was significantly higher.
Using a mixed models statistical approach that adjusted for potentially confounding variables, Dr. Jao and associates found that women on a dolutegravir-based regiment weighed an average of 5 kg more postpartum than women on an efavirenz-based regiment. (P less than .01).
Further adjustment that included CD4 count, viral load, and ART status at conception didn’t change the results from the original approach that included such variables as age, breastfeeding duration , gestational diabetes status, and second and third trimester weight gain (P = .04).
The study was a secondary analysis of the Tshilo Dikotla study conducted in Botswana. Dr. Jao said that the study addressed the known association of dolutegravir-based ART with higher weight gain than other ART regimens. Seeing how postpartum weight varies by regimen is important because “postpartum weight retention impacts cardiometabolic risk,” added Dr. Jao.
Of a total of 406 women, 170 were on dolutegravir-based therapy, 114 were on efavirenz-based therapy, and 122 weren’t HIV infected. Overall, the women on efavirenz-based therapy were older, with a median age of 33 years, compared with 28.5 and 25 years for the dolutegravir group and those without HIV, respectively. This and all other between-group differences were statistically significant at P less than .01.
Women without HIV had lower gravidity, with a median one pregnancy, compared with three in the other two groups. Other significant differences included a higher rate of weight gain in the second and third trimesters for the non–HIV-infected group, who gained at a rate of 0.3 kg/week, compared with 0.1 and 0.2 kg/week for the efavirenz and dolutegravir groups, respectively. Breastfeeding duration was longer in the non–HIV-infected group as well.
Finally, 86% of women on efavirenz-based therapy were on ART at the time of conception, compared with just 35.3% of women on dolutegravir-based treatment.
“Further studies to assess mechanisms of postpartum weight retention are needed,” said Dr. Jao.
The study was supported by the National Institutes of Health. Dr. Jao reported no relevant conflicts of interest.
SOURCE: Jao J et al. CROI 2020, Poster 00772.
Women with HIV on dolutegravir-based antiretroviral therapy (ART) protocols had higher weights through 18 months of the postpartum period than women on efavirenz-based therapy, according to a recent study. However, women taking dolutegravir had similar postpartum weights to women who did not have HIV infection.
The results were shared by Jennifer Jao, MD, MPH, of Northwestern University, Chicago, in a video presentation of the research during the Conference on Retroviruses & Opportunistic Infections, which was presented online this year. CROI organizers chose to hold a virtual meeting because of concerns about the spread of COVID-19.
Dr. Jao, an internal medicine physician and pediatrician, and colleagues looked at the association between dolutegravir and postpartum weight for women with HIV, compared with women with HIV who were taking efavirenz-based ART and women who did not have HIV infection.
Though there was no significant difference among the three groups for body mass index at 4 weeks post partum (all were between 24 and 26 kg/m2), postpartum weight for the dolutegravir group was significantly higher.
Using a mixed models statistical approach that adjusted for potentially confounding variables, Dr. Jao and associates found that women on a dolutegravir-based regiment weighed an average of 5 kg more postpartum than women on an efavirenz-based regiment. (P less than .01).
Further adjustment that included CD4 count, viral load, and ART status at conception didn’t change the results from the original approach that included such variables as age, breastfeeding duration , gestational diabetes status, and second and third trimester weight gain (P = .04).
The study was a secondary analysis of the Tshilo Dikotla study conducted in Botswana. Dr. Jao said that the study addressed the known association of dolutegravir-based ART with higher weight gain than other ART regimens. Seeing how postpartum weight varies by regimen is important because “postpartum weight retention impacts cardiometabolic risk,” added Dr. Jao.
Of a total of 406 women, 170 were on dolutegravir-based therapy, 114 were on efavirenz-based therapy, and 122 weren’t HIV infected. Overall, the women on efavirenz-based therapy were older, with a median age of 33 years, compared with 28.5 and 25 years for the dolutegravir group and those without HIV, respectively. This and all other between-group differences were statistically significant at P less than .01.
Women without HIV had lower gravidity, with a median one pregnancy, compared with three in the other two groups. Other significant differences included a higher rate of weight gain in the second and third trimesters for the non–HIV-infected group, who gained at a rate of 0.3 kg/week, compared with 0.1 and 0.2 kg/week for the efavirenz and dolutegravir groups, respectively. Breastfeeding duration was longer in the non–HIV-infected group as well.
Finally, 86% of women on efavirenz-based therapy were on ART at the time of conception, compared with just 35.3% of women on dolutegravir-based treatment.
“Further studies to assess mechanisms of postpartum weight retention are needed,” said Dr. Jao.
The study was supported by the National Institutes of Health. Dr. Jao reported no relevant conflicts of interest.
SOURCE: Jao J et al. CROI 2020, Poster 00772.
Women with HIV on dolutegravir-based antiretroviral therapy (ART) protocols had higher weights through 18 months of the postpartum period than women on efavirenz-based therapy, according to a recent study. However, women taking dolutegravir had similar postpartum weights to women who did not have HIV infection.
The results were shared by Jennifer Jao, MD, MPH, of Northwestern University, Chicago, in a video presentation of the research during the Conference on Retroviruses & Opportunistic Infections, which was presented online this year. CROI organizers chose to hold a virtual meeting because of concerns about the spread of COVID-19.
Dr. Jao, an internal medicine physician and pediatrician, and colleagues looked at the association between dolutegravir and postpartum weight for women with HIV, compared with women with HIV who were taking efavirenz-based ART and women who did not have HIV infection.
Though there was no significant difference among the three groups for body mass index at 4 weeks post partum (all were between 24 and 26 kg/m2), postpartum weight for the dolutegravir group was significantly higher.
Using a mixed models statistical approach that adjusted for potentially confounding variables, Dr. Jao and associates found that women on a dolutegravir-based regiment weighed an average of 5 kg more postpartum than women on an efavirenz-based regiment. (P less than .01).
Further adjustment that included CD4 count, viral load, and ART status at conception didn’t change the results from the original approach that included such variables as age, breastfeeding duration , gestational diabetes status, and second and third trimester weight gain (P = .04).
The study was a secondary analysis of the Tshilo Dikotla study conducted in Botswana. Dr. Jao said that the study addressed the known association of dolutegravir-based ART with higher weight gain than other ART regimens. Seeing how postpartum weight varies by regimen is important because “postpartum weight retention impacts cardiometabolic risk,” added Dr. Jao.
Of a total of 406 women, 170 were on dolutegravir-based therapy, 114 were on efavirenz-based therapy, and 122 weren’t HIV infected. Overall, the women on efavirenz-based therapy were older, with a median age of 33 years, compared with 28.5 and 25 years for the dolutegravir group and those without HIV, respectively. This and all other between-group differences were statistically significant at P less than .01.
Women without HIV had lower gravidity, with a median one pregnancy, compared with three in the other two groups. Other significant differences included a higher rate of weight gain in the second and third trimesters for the non–HIV-infected group, who gained at a rate of 0.3 kg/week, compared with 0.1 and 0.2 kg/week for the efavirenz and dolutegravir groups, respectively. Breastfeeding duration was longer in the non–HIV-infected group as well.
Finally, 86% of women on efavirenz-based therapy were on ART at the time of conception, compared with just 35.3% of women on dolutegravir-based treatment.
“Further studies to assess mechanisms of postpartum weight retention are needed,” said Dr. Jao.
The study was supported by the National Institutes of Health. Dr. Jao reported no relevant conflicts of interest.
SOURCE: Jao J et al. CROI 2020, Poster 00772.
FROM CROI 2020
HIV free 30 months after stem cell transplant, is the London patient cured?
A patient with HIV remission induced by stem cell transplantation continues to be disease free at the 30-month mark.
The individual, referred to as the London patient, received allogeneic hematopoietic stem cell transplantation (allo-HSCT) for stage IVB Hodgkin lymphoma. The transplant donor was homozygous for the CCR5 delta-32 mutation, which confers immunity to HIV because there’s no point of entry for the virus into immune cells.
After extensive sampling of various tissues, including gut, lymph node, blood, semen, and cerebrospinal fluid (CSF), Ravindra Kumar Gupta, MD, PhD, and colleagues found no detectable virus that was competent to replicate. However, they reported that the testing did detect some “fossilized” remnants of HIV DNA persisting in certain tissues.
The results were shared in a video presentation of the research during the Conference on Retroviruses & Opportunistic Infections, which was presented online this year. CROI organizers chose to hold a virtual meeting because of concerns about the spread of COVID-19.
The London patient’s HIV status had been reported the previous year at CROI 2019, but only blood samples were used in that analysis.
In a commentary accompanying the simultaneously published study in the Lancet, Jennifer Zerbato, PhD, and Sharon Lewin, FRACP, PHD, FAAHMS, asked: “A key question now for the area of HIV cure is how soon can one know if someone has been cured of HIV?
“We will need more than a handful of patients cured of HIV to really understand the duration of follow-up needed and the likelihood of an unexpected late rebound in virus replication,” continued Dr. Zerbato, of the University of Melbourne, and Dr. Lewin, of the Royal Melbourne Hospital and Monash University, also in Melbourne.
In their ongoing analysis of data from the London patient, Dr. Gupta, a virologist at the University of Cambridge (England), and associates constructed a mathematical model that maps the probability for lifetime remission or cure of HIV against several factors, including the degree of chimerism achieved with the stem cell transplant.
In this model, when chimerism reaches 80% in total HIV target cells, the probability of remission for life is 98%; when donor chimerism reaches 90%, the probability of lifetime remission is greater than 99%. Peripheral T-cell chimerism in the London patient has held steady at 99%.
Dr. Gupta and associates obtained some testing opportunistically: A PET-CT scan revealed an axillary lymph node that was biopsied after it was found to have avid radiotracer uptake. Similarly, the CSF sample was obtained in the course of a work-up for some neurologic symptoms that the London patient was having.
In contrast to the first patient who achieved ongoing HIV remission from a pair of stem cell transplants received over 13 years ago – the Berlin patient – the London patient did not receive whole-body radiation, but rather underwent a reduced-intensity conditioning regimen. The London patient experienced a bout of gut graft-versus-host disease (GVHD) about 2 months after his transplant, but has been free of GVHD in the interval. He hasn’t taken cytotoxic agents or any GVHD prophylaxis since 6 months post transplant.
Though there’s no sign of HIV that’s competent to replicate, “the London patient has shown somewhat slow CD4 reconstitution,” said Dr. Gupta and coauthors in discussing the results.
The patient had a reactivation of Epstein-Barr virus (EBV) about 21 months after analytic treatment interruption (ATI) of antiretroviral therapy that was managed without any specific treatment, but he hasn’t experienced any opportunistic infections. However, his CD4 count didn’t rebound to pretransplant levels until 28 months after ATI. At that point, his CD4 count was 430 cells per mcL, or 23.5% of total T cells. The CD4:CD8 ratio was 0.86; normal range is 1.5-2.5.
The researchers used quantitative real-time polymerase chain reaction (rt-PCR) to look for packaging site and envelope (env) DNA fragments, and droplet digital PCR to quantify HIV-1 DNA.
The patient’s HIV-1 plasma load measured at 30 months post ATI on an ultrasensitive assay was below the lower limit of detection (less than 1 copy per mL). Semen viremia measured at 21 months was also below the lower limit of detection, as was CSF measured at 25 months.
Samples were taken from the patient’s rectum, cecum, sigmoid colon, and terminal ileum during a colonoscopy conducted 22 months post ATI; all tested negative for HIV DNA via droplet digital PCR.
The lymph node had large numbers of EBV-positive cells and was positive for HIV-1 env and long-terminal repeat by double-drop PCR, but no integrase DNA was detected. Additionally, no intact proviral DNA was found on assay.
Dr. Gupta and associates speculated that “EBV reactivation could have triggered EBV-specific CD4 and CD8 T-cell responses and proliferation, potentially including CD4 T cells containing HIV-1 DNA.” Supporting this hypothesis, EBV-specific CD8 T-cell responses in peripheral blood were “robust,” and the researchers also saw some CD4 response.
“Similar to the Berlin patient, highly sensitive tests showed very low levels of so-called fossilized HIV-1 DNA in some tissue samples from the London patient. Residual HIV-1 DNA and axillary lymph node tissue could represent a defective clone that expanded during hyperplasia within the lymph note sampled,” noted Dr. Gupta and coauthors.
Responses of CD4 and CD8 T cells to HIV have also remained below the limit of detection, though cytomegalovirus-specific responses persist in the London patient.
As with the Berlin patient, standard enzyme-linked immunosorbent assay (ELISA) testing has remained positive in the London patient. “Standard ELISA testing, therefore, cannot be used as a marker for cure, although more work needs to be done to assess the role of detuned low-avidity antibody assays in defining cure,” noted Dr. Gupta and associates.
The ongoing follow-up plan for the London patient is to obtain viral load testing twice yearly up to 5 years post ATI, and then obtain yearly tests for a total of 10 years. Ongoing testing will confirm the investigators’ belief that “these findings probably represent the second recorded HIV-1 cure after CCR5 delta-32/delta-32 allo-HSCT, with evidence of residual low-level HIV-1 DNA.”
Dr. Zerbato and Dr. Lewin advised cautious optimism and ongoing surveillance: “In view of the many cells sampled in this case, and the absence of any intact virus, is the London patient truly cured? The additional data provided in this follow-up case report is certainly exciting and encouraging but, in the end, only time will tell.”
Dr. Gupta reported being a consultant for ViiV Healthcare and Gilead Sciences; several coauthors also reported financial relationships with pharmaceutical companies. The work was funded by amfAR, the American Foundation for AIDS Research, and the Wellcome Trust. Dr. Lewin reported grants from the National Health and Medical Research Council of Australia, the National Institutes of Health, the American Foundation for AIDS Research, Gilead Sciences, Merck, ViiV Healthcare, Leidos, the Wellcome Trust, the Australian Centre for HIV and Hepatitis Virology Research, and the Melbourne HIV Cure Consortium. Dr. Zerbato reported grants from the Melbourne HIV Cure Consortium,
SOURCE: Gupta R et al. Lancet. 2020 Mar 10. doi: 10.1016/ S2352-3018(20)30069-2.
A patient with HIV remission induced by stem cell transplantation continues to be disease free at the 30-month mark.
The individual, referred to as the London patient, received allogeneic hematopoietic stem cell transplantation (allo-HSCT) for stage IVB Hodgkin lymphoma. The transplant donor was homozygous for the CCR5 delta-32 mutation, which confers immunity to HIV because there’s no point of entry for the virus into immune cells.
After extensive sampling of various tissues, including gut, lymph node, blood, semen, and cerebrospinal fluid (CSF), Ravindra Kumar Gupta, MD, PhD, and colleagues found no detectable virus that was competent to replicate. However, they reported that the testing did detect some “fossilized” remnants of HIV DNA persisting in certain tissues.
The results were shared in a video presentation of the research during the Conference on Retroviruses & Opportunistic Infections, which was presented online this year. CROI organizers chose to hold a virtual meeting because of concerns about the spread of COVID-19.
The London patient’s HIV status had been reported the previous year at CROI 2019, but only blood samples were used in that analysis.
In a commentary accompanying the simultaneously published study in the Lancet, Jennifer Zerbato, PhD, and Sharon Lewin, FRACP, PHD, FAAHMS, asked: “A key question now for the area of HIV cure is how soon can one know if someone has been cured of HIV?
“We will need more than a handful of patients cured of HIV to really understand the duration of follow-up needed and the likelihood of an unexpected late rebound in virus replication,” continued Dr. Zerbato, of the University of Melbourne, and Dr. Lewin, of the Royal Melbourne Hospital and Monash University, also in Melbourne.
In their ongoing analysis of data from the London patient, Dr. Gupta, a virologist at the University of Cambridge (England), and associates constructed a mathematical model that maps the probability for lifetime remission or cure of HIV against several factors, including the degree of chimerism achieved with the stem cell transplant.
In this model, when chimerism reaches 80% in total HIV target cells, the probability of remission for life is 98%; when donor chimerism reaches 90%, the probability of lifetime remission is greater than 99%. Peripheral T-cell chimerism in the London patient has held steady at 99%.
Dr. Gupta and associates obtained some testing opportunistically: A PET-CT scan revealed an axillary lymph node that was biopsied after it was found to have avid radiotracer uptake. Similarly, the CSF sample was obtained in the course of a work-up for some neurologic symptoms that the London patient was having.
In contrast to the first patient who achieved ongoing HIV remission from a pair of stem cell transplants received over 13 years ago – the Berlin patient – the London patient did not receive whole-body radiation, but rather underwent a reduced-intensity conditioning regimen. The London patient experienced a bout of gut graft-versus-host disease (GVHD) about 2 months after his transplant, but has been free of GVHD in the interval. He hasn’t taken cytotoxic agents or any GVHD prophylaxis since 6 months post transplant.
Though there’s no sign of HIV that’s competent to replicate, “the London patient has shown somewhat slow CD4 reconstitution,” said Dr. Gupta and coauthors in discussing the results.
The patient had a reactivation of Epstein-Barr virus (EBV) about 21 months after analytic treatment interruption (ATI) of antiretroviral therapy that was managed without any specific treatment, but he hasn’t experienced any opportunistic infections. However, his CD4 count didn’t rebound to pretransplant levels until 28 months after ATI. At that point, his CD4 count was 430 cells per mcL, or 23.5% of total T cells. The CD4:CD8 ratio was 0.86; normal range is 1.5-2.5.
The researchers used quantitative real-time polymerase chain reaction (rt-PCR) to look for packaging site and envelope (env) DNA fragments, and droplet digital PCR to quantify HIV-1 DNA.
The patient’s HIV-1 plasma load measured at 30 months post ATI on an ultrasensitive assay was below the lower limit of detection (less than 1 copy per mL). Semen viremia measured at 21 months was also below the lower limit of detection, as was CSF measured at 25 months.
Samples were taken from the patient’s rectum, cecum, sigmoid colon, and terminal ileum during a colonoscopy conducted 22 months post ATI; all tested negative for HIV DNA via droplet digital PCR.
The lymph node had large numbers of EBV-positive cells and was positive for HIV-1 env and long-terminal repeat by double-drop PCR, but no integrase DNA was detected. Additionally, no intact proviral DNA was found on assay.
Dr. Gupta and associates speculated that “EBV reactivation could have triggered EBV-specific CD4 and CD8 T-cell responses and proliferation, potentially including CD4 T cells containing HIV-1 DNA.” Supporting this hypothesis, EBV-specific CD8 T-cell responses in peripheral blood were “robust,” and the researchers also saw some CD4 response.
“Similar to the Berlin patient, highly sensitive tests showed very low levels of so-called fossilized HIV-1 DNA in some tissue samples from the London patient. Residual HIV-1 DNA and axillary lymph node tissue could represent a defective clone that expanded during hyperplasia within the lymph note sampled,” noted Dr. Gupta and coauthors.
Responses of CD4 and CD8 T cells to HIV have also remained below the limit of detection, though cytomegalovirus-specific responses persist in the London patient.
As with the Berlin patient, standard enzyme-linked immunosorbent assay (ELISA) testing has remained positive in the London patient. “Standard ELISA testing, therefore, cannot be used as a marker for cure, although more work needs to be done to assess the role of detuned low-avidity antibody assays in defining cure,” noted Dr. Gupta and associates.
The ongoing follow-up plan for the London patient is to obtain viral load testing twice yearly up to 5 years post ATI, and then obtain yearly tests for a total of 10 years. Ongoing testing will confirm the investigators’ belief that “these findings probably represent the second recorded HIV-1 cure after CCR5 delta-32/delta-32 allo-HSCT, with evidence of residual low-level HIV-1 DNA.”
Dr. Zerbato and Dr. Lewin advised cautious optimism and ongoing surveillance: “In view of the many cells sampled in this case, and the absence of any intact virus, is the London patient truly cured? The additional data provided in this follow-up case report is certainly exciting and encouraging but, in the end, only time will tell.”
Dr. Gupta reported being a consultant for ViiV Healthcare and Gilead Sciences; several coauthors also reported financial relationships with pharmaceutical companies. The work was funded by amfAR, the American Foundation for AIDS Research, and the Wellcome Trust. Dr. Lewin reported grants from the National Health and Medical Research Council of Australia, the National Institutes of Health, the American Foundation for AIDS Research, Gilead Sciences, Merck, ViiV Healthcare, Leidos, the Wellcome Trust, the Australian Centre for HIV and Hepatitis Virology Research, and the Melbourne HIV Cure Consortium. Dr. Zerbato reported grants from the Melbourne HIV Cure Consortium,
SOURCE: Gupta R et al. Lancet. 2020 Mar 10. doi: 10.1016/ S2352-3018(20)30069-2.
A patient with HIV remission induced by stem cell transplantation continues to be disease free at the 30-month mark.
The individual, referred to as the London patient, received allogeneic hematopoietic stem cell transplantation (allo-HSCT) for stage IVB Hodgkin lymphoma. The transplant donor was homozygous for the CCR5 delta-32 mutation, which confers immunity to HIV because there’s no point of entry for the virus into immune cells.
After extensive sampling of various tissues, including gut, lymph node, blood, semen, and cerebrospinal fluid (CSF), Ravindra Kumar Gupta, MD, PhD, and colleagues found no detectable virus that was competent to replicate. However, they reported that the testing did detect some “fossilized” remnants of HIV DNA persisting in certain tissues.
The results were shared in a video presentation of the research during the Conference on Retroviruses & Opportunistic Infections, which was presented online this year. CROI organizers chose to hold a virtual meeting because of concerns about the spread of COVID-19.
The London patient’s HIV status had been reported the previous year at CROI 2019, but only blood samples were used in that analysis.
In a commentary accompanying the simultaneously published study in the Lancet, Jennifer Zerbato, PhD, and Sharon Lewin, FRACP, PHD, FAAHMS, asked: “A key question now for the area of HIV cure is how soon can one know if someone has been cured of HIV?
“We will need more than a handful of patients cured of HIV to really understand the duration of follow-up needed and the likelihood of an unexpected late rebound in virus replication,” continued Dr. Zerbato, of the University of Melbourne, and Dr. Lewin, of the Royal Melbourne Hospital and Monash University, also in Melbourne.
In their ongoing analysis of data from the London patient, Dr. Gupta, a virologist at the University of Cambridge (England), and associates constructed a mathematical model that maps the probability for lifetime remission or cure of HIV against several factors, including the degree of chimerism achieved with the stem cell transplant.
In this model, when chimerism reaches 80% in total HIV target cells, the probability of remission for life is 98%; when donor chimerism reaches 90%, the probability of lifetime remission is greater than 99%. Peripheral T-cell chimerism in the London patient has held steady at 99%.
Dr. Gupta and associates obtained some testing opportunistically: A PET-CT scan revealed an axillary lymph node that was biopsied after it was found to have avid radiotracer uptake. Similarly, the CSF sample was obtained in the course of a work-up for some neurologic symptoms that the London patient was having.
In contrast to the first patient who achieved ongoing HIV remission from a pair of stem cell transplants received over 13 years ago – the Berlin patient – the London patient did not receive whole-body radiation, but rather underwent a reduced-intensity conditioning regimen. The London patient experienced a bout of gut graft-versus-host disease (GVHD) about 2 months after his transplant, but has been free of GVHD in the interval. He hasn’t taken cytotoxic agents or any GVHD prophylaxis since 6 months post transplant.
Though there’s no sign of HIV that’s competent to replicate, “the London patient has shown somewhat slow CD4 reconstitution,” said Dr. Gupta and coauthors in discussing the results.
The patient had a reactivation of Epstein-Barr virus (EBV) about 21 months after analytic treatment interruption (ATI) of antiretroviral therapy that was managed without any specific treatment, but he hasn’t experienced any opportunistic infections. However, his CD4 count didn’t rebound to pretransplant levels until 28 months after ATI. At that point, his CD4 count was 430 cells per mcL, or 23.5% of total T cells. The CD4:CD8 ratio was 0.86; normal range is 1.5-2.5.
The researchers used quantitative real-time polymerase chain reaction (rt-PCR) to look for packaging site and envelope (env) DNA fragments, and droplet digital PCR to quantify HIV-1 DNA.
The patient’s HIV-1 plasma load measured at 30 months post ATI on an ultrasensitive assay was below the lower limit of detection (less than 1 copy per mL). Semen viremia measured at 21 months was also below the lower limit of detection, as was CSF measured at 25 months.
Samples were taken from the patient’s rectum, cecum, sigmoid colon, and terminal ileum during a colonoscopy conducted 22 months post ATI; all tested negative for HIV DNA via droplet digital PCR.
The lymph node had large numbers of EBV-positive cells and was positive for HIV-1 env and long-terminal repeat by double-drop PCR, but no integrase DNA was detected. Additionally, no intact proviral DNA was found on assay.
Dr. Gupta and associates speculated that “EBV reactivation could have triggered EBV-specific CD4 and CD8 T-cell responses and proliferation, potentially including CD4 T cells containing HIV-1 DNA.” Supporting this hypothesis, EBV-specific CD8 T-cell responses in peripheral blood were “robust,” and the researchers also saw some CD4 response.
“Similar to the Berlin patient, highly sensitive tests showed very low levels of so-called fossilized HIV-1 DNA in some tissue samples from the London patient. Residual HIV-1 DNA and axillary lymph node tissue could represent a defective clone that expanded during hyperplasia within the lymph note sampled,” noted Dr. Gupta and coauthors.
Responses of CD4 and CD8 T cells to HIV have also remained below the limit of detection, though cytomegalovirus-specific responses persist in the London patient.
As with the Berlin patient, standard enzyme-linked immunosorbent assay (ELISA) testing has remained positive in the London patient. “Standard ELISA testing, therefore, cannot be used as a marker for cure, although more work needs to be done to assess the role of detuned low-avidity antibody assays in defining cure,” noted Dr. Gupta and associates.
The ongoing follow-up plan for the London patient is to obtain viral load testing twice yearly up to 5 years post ATI, and then obtain yearly tests for a total of 10 years. Ongoing testing will confirm the investigators’ belief that “these findings probably represent the second recorded HIV-1 cure after CCR5 delta-32/delta-32 allo-HSCT, with evidence of residual low-level HIV-1 DNA.”
Dr. Zerbato and Dr. Lewin advised cautious optimism and ongoing surveillance: “In view of the many cells sampled in this case, and the absence of any intact virus, is the London patient truly cured? The additional data provided in this follow-up case report is certainly exciting and encouraging but, in the end, only time will tell.”
Dr. Gupta reported being a consultant for ViiV Healthcare and Gilead Sciences; several coauthors also reported financial relationships with pharmaceutical companies. The work was funded by amfAR, the American Foundation for AIDS Research, and the Wellcome Trust. Dr. Lewin reported grants from the National Health and Medical Research Council of Australia, the National Institutes of Health, the American Foundation for AIDS Research, Gilead Sciences, Merck, ViiV Healthcare, Leidos, the Wellcome Trust, the Australian Centre for HIV and Hepatitis Virology Research, and the Melbourne HIV Cure Consortium. Dr. Zerbato reported grants from the Melbourne HIV Cure Consortium,
SOURCE: Gupta R et al. Lancet. 2020 Mar 10. doi: 10.1016/ S2352-3018(20)30069-2.
FROM CROI 2020
FDA moves to expand coronavirus testing capacity; CDC clarifies testing criteria
The White House Coronavirus Task Force appeared at a press briefing March 2 to provide updates about testing strategies and public health coordination to address the current outbreak of the coronavirus COVID-19. Speaking at the briefing, led by Vice President Mike Pence, Centers for Disease Control and Prevention (CDC) director Robert Redfield, MD, said, “Working with our public health partners we continue to be able to identify new community cases and use our public health efforts to aggressively confirm, isolate, and do contact tracking.” Calling state, local, tribal, and territorial public health departments “the backbone of the public health system in our country,” Dr. Redfield noted that he expected many more confirmed COVID-19 cases to emerge.
At least some of the expected increase in confirmed cases of COVID-19 will occur because of expanded testing capacity, noted several of the task force members. On Feb. 29, the Food and Drug Administration issued a the virus that is causing the current outbreak of COVID-19.
Highly qualified laboratories, including both those run by public agencies and private labs, are now authorized to begin using their own validated test for the virus as long as they submit an Emergency Use Authorization (EUA) to the Food and Drug Administration within 15 days of notifying the agency of validation.
“To effectively respond to the COVID-19 outbreak, rapid detection of cases and contacts, appropriate clinical management and infection control, and implementation of community mitigation efforts are critical. This can best be achieved with wide availability of testing capabilities in health care settings, reference and commercial laboratories, and at the point of care,” the agency wrote in a press announcement of the expedited test expansion.
On Feb. 4, the Secretary of the Department of Health & Human Services declared a coronavirus public health emergency. The FDA was then authorized to allow individual laboratories with validated coronavirus tests to begin testing samples immediately. The goal is a more rapid and expanded testing capacity in the United States.
“The global emergence of COVID-19 is concerning, and we appreciate the efforts of the FDA to help bring more testing capability to the U.S.,” Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases (NCIRD), said in the press release.
The new guidance that permits the immediate use of clinical tests after individual development and validation, said the FDA, only applies to labs already certified to perform high complexity testing under Clinical Laboratory Improvement Amendments. Many governmental, academic, and private laboratories fall into this category, however.
“Under this policy, we expect certain laboratories who develop validated tests for coronavirus would begin using them right away prior to FDA review,” said Jeffrey Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health. “We believe this action will support laboratories across the country working on this urgent public health situation,” he added in the press release.
“By the end of this week, close to a million tests will be available,” FDA Commissioner Stephen M. Hahn, MD, said during the March 2 briefing.*
Updated criteria
The CDC is maintaining updated criteria for the virus testing on its website. Testing criteria are based both on clinical features and epidemiologic risk.
Individuals with less severe clinical features – those who have either fever or signs and symptoms of lower respiratory disease such as cough or shortness of breath, but who don’t require hospitalization – should be tested if they have high epidemiologic risk. “High risk” is defined by the CDC as any individual, including health care workers, who has had close contact with a person with confirmed COVID-19 within the past 2 weeks. For health care workers, testing can be considered even if they have relatively mild respiratory symptoms or have had contact with a person who is suspected, but not yet confirmed, to have coronavirus.
In its testing guidance, the CDC recognizes that defining close contact is difficult. General guidelines are that individuals are considered to have been in close contact with a person who has COVID-19 if they were within about six feet of the person for a prolonged period, or cared for or have spent a prolonged amount of time in the same room or house as a person with confirmed COVID-19.
Individuals who have both fever and signs or symptoms of lower respiratory illness who require hospitalization should be tested if they have a history of travel from any affected geographic area within 14 days of the onset of their symptoms. The CDC now defines “affected geographic area” as any country or region that has at least a CDC Level 2 Travel Health Notice for COVID-19, so that the testing criteria themselves don’t need to be updated when new geographic areas are included in these alerts. As of March 3, China, Iran, Italy, Japan, and South Korea all have Level 2 or 3 travel alerts.
The CDC now recommends that any patient who has severe acute lower respiratory illness that requires hospitalization and doesn’t have an alternative diagnosis should be tested, even without any identified source of exposure.
“Despite seeing these new cases, the risk to the American people is low,” said the CDC’s Dr. Redfield. In response to a question from the press about how fast the coronavirus will spread across the United States, Dr. Redfield said, “From the beginning we’ve anticipated seeing community cases pop up.” He added that as these cases arise, testing and public health strategies will focus on unearthing linkages and contacts to learn how the virus is spreading. “We’ll use the public health strategies that we can to limit that transmission,” he said.
*An earlier version of this article misattributed this quote.
The White House Coronavirus Task Force appeared at a press briefing March 2 to provide updates about testing strategies and public health coordination to address the current outbreak of the coronavirus COVID-19. Speaking at the briefing, led by Vice President Mike Pence, Centers for Disease Control and Prevention (CDC) director Robert Redfield, MD, said, “Working with our public health partners we continue to be able to identify new community cases and use our public health efforts to aggressively confirm, isolate, and do contact tracking.” Calling state, local, tribal, and territorial public health departments “the backbone of the public health system in our country,” Dr. Redfield noted that he expected many more confirmed COVID-19 cases to emerge.
At least some of the expected increase in confirmed cases of COVID-19 will occur because of expanded testing capacity, noted several of the task force members. On Feb. 29, the Food and Drug Administration issued a the virus that is causing the current outbreak of COVID-19.
Highly qualified laboratories, including both those run by public agencies and private labs, are now authorized to begin using their own validated test for the virus as long as they submit an Emergency Use Authorization (EUA) to the Food and Drug Administration within 15 days of notifying the agency of validation.
“To effectively respond to the COVID-19 outbreak, rapid detection of cases and contacts, appropriate clinical management and infection control, and implementation of community mitigation efforts are critical. This can best be achieved with wide availability of testing capabilities in health care settings, reference and commercial laboratories, and at the point of care,” the agency wrote in a press announcement of the expedited test expansion.
On Feb. 4, the Secretary of the Department of Health & Human Services declared a coronavirus public health emergency. The FDA was then authorized to allow individual laboratories with validated coronavirus tests to begin testing samples immediately. The goal is a more rapid and expanded testing capacity in the United States.
“The global emergence of COVID-19 is concerning, and we appreciate the efforts of the FDA to help bring more testing capability to the U.S.,” Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases (NCIRD), said in the press release.
The new guidance that permits the immediate use of clinical tests after individual development and validation, said the FDA, only applies to labs already certified to perform high complexity testing under Clinical Laboratory Improvement Amendments. Many governmental, academic, and private laboratories fall into this category, however.
“Under this policy, we expect certain laboratories who develop validated tests for coronavirus would begin using them right away prior to FDA review,” said Jeffrey Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health. “We believe this action will support laboratories across the country working on this urgent public health situation,” he added in the press release.
“By the end of this week, close to a million tests will be available,” FDA Commissioner Stephen M. Hahn, MD, said during the March 2 briefing.*
Updated criteria
The CDC is maintaining updated criteria for the virus testing on its website. Testing criteria are based both on clinical features and epidemiologic risk.
Individuals with less severe clinical features – those who have either fever or signs and symptoms of lower respiratory disease such as cough or shortness of breath, but who don’t require hospitalization – should be tested if they have high epidemiologic risk. “High risk” is defined by the CDC as any individual, including health care workers, who has had close contact with a person with confirmed COVID-19 within the past 2 weeks. For health care workers, testing can be considered even if they have relatively mild respiratory symptoms or have had contact with a person who is suspected, but not yet confirmed, to have coronavirus.
In its testing guidance, the CDC recognizes that defining close contact is difficult. General guidelines are that individuals are considered to have been in close contact with a person who has COVID-19 if they were within about six feet of the person for a prolonged period, or cared for or have spent a prolonged amount of time in the same room or house as a person with confirmed COVID-19.
Individuals who have both fever and signs or symptoms of lower respiratory illness who require hospitalization should be tested if they have a history of travel from any affected geographic area within 14 days of the onset of their symptoms. The CDC now defines “affected geographic area” as any country or region that has at least a CDC Level 2 Travel Health Notice for COVID-19, so that the testing criteria themselves don’t need to be updated when new geographic areas are included in these alerts. As of March 3, China, Iran, Italy, Japan, and South Korea all have Level 2 or 3 travel alerts.
The CDC now recommends that any patient who has severe acute lower respiratory illness that requires hospitalization and doesn’t have an alternative diagnosis should be tested, even without any identified source of exposure.
“Despite seeing these new cases, the risk to the American people is low,” said the CDC’s Dr. Redfield. In response to a question from the press about how fast the coronavirus will spread across the United States, Dr. Redfield said, “From the beginning we’ve anticipated seeing community cases pop up.” He added that as these cases arise, testing and public health strategies will focus on unearthing linkages and contacts to learn how the virus is spreading. “We’ll use the public health strategies that we can to limit that transmission,” he said.
*An earlier version of this article misattributed this quote.
The White House Coronavirus Task Force appeared at a press briefing March 2 to provide updates about testing strategies and public health coordination to address the current outbreak of the coronavirus COVID-19. Speaking at the briefing, led by Vice President Mike Pence, Centers for Disease Control and Prevention (CDC) director Robert Redfield, MD, said, “Working with our public health partners we continue to be able to identify new community cases and use our public health efforts to aggressively confirm, isolate, and do contact tracking.” Calling state, local, tribal, and territorial public health departments “the backbone of the public health system in our country,” Dr. Redfield noted that he expected many more confirmed COVID-19 cases to emerge.
At least some of the expected increase in confirmed cases of COVID-19 will occur because of expanded testing capacity, noted several of the task force members. On Feb. 29, the Food and Drug Administration issued a the virus that is causing the current outbreak of COVID-19.
Highly qualified laboratories, including both those run by public agencies and private labs, are now authorized to begin using their own validated test for the virus as long as they submit an Emergency Use Authorization (EUA) to the Food and Drug Administration within 15 days of notifying the agency of validation.
“To effectively respond to the COVID-19 outbreak, rapid detection of cases and contacts, appropriate clinical management and infection control, and implementation of community mitigation efforts are critical. This can best be achieved with wide availability of testing capabilities in health care settings, reference and commercial laboratories, and at the point of care,” the agency wrote in a press announcement of the expedited test expansion.
On Feb. 4, the Secretary of the Department of Health & Human Services declared a coronavirus public health emergency. The FDA was then authorized to allow individual laboratories with validated coronavirus tests to begin testing samples immediately. The goal is a more rapid and expanded testing capacity in the United States.
“The global emergence of COVID-19 is concerning, and we appreciate the efforts of the FDA to help bring more testing capability to the U.S.,” Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases (NCIRD), said in the press release.
The new guidance that permits the immediate use of clinical tests after individual development and validation, said the FDA, only applies to labs already certified to perform high complexity testing under Clinical Laboratory Improvement Amendments. Many governmental, academic, and private laboratories fall into this category, however.
“Under this policy, we expect certain laboratories who develop validated tests for coronavirus would begin using them right away prior to FDA review,” said Jeffrey Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health. “We believe this action will support laboratories across the country working on this urgent public health situation,” he added in the press release.
“By the end of this week, close to a million tests will be available,” FDA Commissioner Stephen M. Hahn, MD, said during the March 2 briefing.*
Updated criteria
The CDC is maintaining updated criteria for the virus testing on its website. Testing criteria are based both on clinical features and epidemiologic risk.
Individuals with less severe clinical features – those who have either fever or signs and symptoms of lower respiratory disease such as cough or shortness of breath, but who don’t require hospitalization – should be tested if they have high epidemiologic risk. “High risk” is defined by the CDC as any individual, including health care workers, who has had close contact with a person with confirmed COVID-19 within the past 2 weeks. For health care workers, testing can be considered even if they have relatively mild respiratory symptoms or have had contact with a person who is suspected, but not yet confirmed, to have coronavirus.
In its testing guidance, the CDC recognizes that defining close contact is difficult. General guidelines are that individuals are considered to have been in close contact with a person who has COVID-19 if they were within about six feet of the person for a prolonged period, or cared for or have spent a prolonged amount of time in the same room or house as a person with confirmed COVID-19.
Individuals who have both fever and signs or symptoms of lower respiratory illness who require hospitalization should be tested if they have a history of travel from any affected geographic area within 14 days of the onset of their symptoms. The CDC now defines “affected geographic area” as any country or region that has at least a CDC Level 2 Travel Health Notice for COVID-19, so that the testing criteria themselves don’t need to be updated when new geographic areas are included in these alerts. As of March 3, China, Iran, Italy, Japan, and South Korea all have Level 2 or 3 travel alerts.
The CDC now recommends that any patient who has severe acute lower respiratory illness that requires hospitalization and doesn’t have an alternative diagnosis should be tested, even without any identified source of exposure.
“Despite seeing these new cases, the risk to the American people is low,” said the CDC’s Dr. Redfield. In response to a question from the press about how fast the coronavirus will spread across the United States, Dr. Redfield said, “From the beginning we’ve anticipated seeing community cases pop up.” He added that as these cases arise, testing and public health strategies will focus on unearthing linkages and contacts to learn how the virus is spreading. “We’ll use the public health strategies that we can to limit that transmission,” he said.
*An earlier version of this article misattributed this quote.
FROM A PRESS BRIEFING BY THE WHITE HOUSE CORONAVIRUS TASK FORCE
Salpingectomy adds little time and no complications to cesarean delivery
GRAPEVINE, TEXAS – Performing a total salpingectomy at the time of cesarean delivery added just over 6 minutes of operative time, compared with cesarean delivery and conventional sterilization, according to a recent systematic review and meta-analysis.
Although surgery took a little longer with salpingectomy, there was no increase in surgical complications, Jared Roeckner, MD, said in an interview at the Pregnancy Meeting, sponsored by the Society for Maternal-Fetal Medicine. “Total salpingectomy could provide an effective means of contraception and reduce the risk of future ovarian cancer,” he said.
Dr. Roeckner, a maternal-fetal medicine fellow at the University of South Florida, Tampa, explained in an interview that the systematic review and meta-analysis comprised 11 studies and included 320,443 women who received salpingectomy or standard sterilization methods. Eight cohort studies and three randomized controlled trials were included in the analysis, which was presented in a poster session at the meeting and in a simultaneous publication in Obstetrics & Gynecology.
The review’s results, wrote Dr. Roeckner and colleagues, “suggest total salpingectomy should be offered to women interested in ovarian cancer risk-reduction interventions [who] plan to undergo sterilization at the time of cesarean delivery.”
The eight cohort studies included 7,303 women. In these studies, women who received total salpingectomy at the time of cesarean delivery had operative time – defined as the time from skin incision to skin closure – 6.3 minutes longer than women who received a standard sterilization method (95% confidence interval, 3.5-9.1). The difference in duration of procedure for the three randomized controlled trials was not statistically significant between the two procedures.
Dr. Roeckner and colleagues noted that two of the randomized controlled trials reported times for the sterilization procedures. One study found a duration of 5.6 minutes for salpingectomy with a bipolar device and 6.1 minutes for tubal interruption; the other study compared salpingectomy with suture ligation and tubal interruption, finding operative times of 18.5 and 6.9 minutes, respectively.
In addition to the primary outcome of operative time, Dr. Roeckner and colleagues looked at rates of a variety of complications. These included transfusion, estimated blood loss, change in hemoglobin, wound infection, internal organ damage, readmission, reoperation, and length of stay. Salpingectomy was not associated with higher rates of any of these complications.
“Our main finding was that salpingectomy at the time of cesarean delivery may be associated with a small increase in operative time, but it doesn’t appear to be associated with an increased rate of surgical complications,” the researchers wrote.
One concern that’s been raised about the strategy of salpingectomy is the possibility of reduction of ovarian reserve related to decreased blood supply to the ovaries. However, noted Dr. Roeckner and coinvestigators, other studies have not shown decreases in anti-Müllerian hormone levels or other real-world signals for reduced ovarian reserve.
It’s true, the investigators acknowledged, that there is no possibility for reanastomosis and future fertility with salpingectomy. However, they observed that if the possibility for future fertility exists, conventional tubal ligation should not be performed.
Despite the thoroughness of the review and the investigators’ emphasis on adhering to best practices for systematic reviews and meta-analysis, they acknowledged that there were few studies, which resulted in some difficulties with statistical power. Still, they said, “there does not appear to be a trend toward increased complications among the salpingectomy cohort.”
Dr. Roeckner reported no outside sources of funding and no conflicts of interest.
SOURCE: Roeckner J et al. Pregnancy Meeting, Abstract P180; Obstet Gynecol. 2020 Feb;135:3:550-7.
GRAPEVINE, TEXAS – Performing a total salpingectomy at the time of cesarean delivery added just over 6 minutes of operative time, compared with cesarean delivery and conventional sterilization, according to a recent systematic review and meta-analysis.
Although surgery took a little longer with salpingectomy, there was no increase in surgical complications, Jared Roeckner, MD, said in an interview at the Pregnancy Meeting, sponsored by the Society for Maternal-Fetal Medicine. “Total salpingectomy could provide an effective means of contraception and reduce the risk of future ovarian cancer,” he said.
Dr. Roeckner, a maternal-fetal medicine fellow at the University of South Florida, Tampa, explained in an interview that the systematic review and meta-analysis comprised 11 studies and included 320,443 women who received salpingectomy or standard sterilization methods. Eight cohort studies and three randomized controlled trials were included in the analysis, which was presented in a poster session at the meeting and in a simultaneous publication in Obstetrics & Gynecology.
The review’s results, wrote Dr. Roeckner and colleagues, “suggest total salpingectomy should be offered to women interested in ovarian cancer risk-reduction interventions [who] plan to undergo sterilization at the time of cesarean delivery.”
The eight cohort studies included 7,303 women. In these studies, women who received total salpingectomy at the time of cesarean delivery had operative time – defined as the time from skin incision to skin closure – 6.3 minutes longer than women who received a standard sterilization method (95% confidence interval, 3.5-9.1). The difference in duration of procedure for the three randomized controlled trials was not statistically significant between the two procedures.
Dr. Roeckner and colleagues noted that two of the randomized controlled trials reported times for the sterilization procedures. One study found a duration of 5.6 minutes for salpingectomy with a bipolar device and 6.1 minutes for tubal interruption; the other study compared salpingectomy with suture ligation and tubal interruption, finding operative times of 18.5 and 6.9 minutes, respectively.
In addition to the primary outcome of operative time, Dr. Roeckner and colleagues looked at rates of a variety of complications. These included transfusion, estimated blood loss, change in hemoglobin, wound infection, internal organ damage, readmission, reoperation, and length of stay. Salpingectomy was not associated with higher rates of any of these complications.
“Our main finding was that salpingectomy at the time of cesarean delivery may be associated with a small increase in operative time, but it doesn’t appear to be associated with an increased rate of surgical complications,” the researchers wrote.
One concern that’s been raised about the strategy of salpingectomy is the possibility of reduction of ovarian reserve related to decreased blood supply to the ovaries. However, noted Dr. Roeckner and coinvestigators, other studies have not shown decreases in anti-Müllerian hormone levels or other real-world signals for reduced ovarian reserve.
It’s true, the investigators acknowledged, that there is no possibility for reanastomosis and future fertility with salpingectomy. However, they observed that if the possibility for future fertility exists, conventional tubal ligation should not be performed.
Despite the thoroughness of the review and the investigators’ emphasis on adhering to best practices for systematic reviews and meta-analysis, they acknowledged that there were few studies, which resulted in some difficulties with statistical power. Still, they said, “there does not appear to be a trend toward increased complications among the salpingectomy cohort.”
Dr. Roeckner reported no outside sources of funding and no conflicts of interest.
SOURCE: Roeckner J et al. Pregnancy Meeting, Abstract P180; Obstet Gynecol. 2020 Feb;135:3:550-7.
GRAPEVINE, TEXAS – Performing a total salpingectomy at the time of cesarean delivery added just over 6 minutes of operative time, compared with cesarean delivery and conventional sterilization, according to a recent systematic review and meta-analysis.
Although surgery took a little longer with salpingectomy, there was no increase in surgical complications, Jared Roeckner, MD, said in an interview at the Pregnancy Meeting, sponsored by the Society for Maternal-Fetal Medicine. “Total salpingectomy could provide an effective means of contraception and reduce the risk of future ovarian cancer,” he said.
Dr. Roeckner, a maternal-fetal medicine fellow at the University of South Florida, Tampa, explained in an interview that the systematic review and meta-analysis comprised 11 studies and included 320,443 women who received salpingectomy or standard sterilization methods. Eight cohort studies and three randomized controlled trials were included in the analysis, which was presented in a poster session at the meeting and in a simultaneous publication in Obstetrics & Gynecology.
The review’s results, wrote Dr. Roeckner and colleagues, “suggest total salpingectomy should be offered to women interested in ovarian cancer risk-reduction interventions [who] plan to undergo sterilization at the time of cesarean delivery.”
The eight cohort studies included 7,303 women. In these studies, women who received total salpingectomy at the time of cesarean delivery had operative time – defined as the time from skin incision to skin closure – 6.3 minutes longer than women who received a standard sterilization method (95% confidence interval, 3.5-9.1). The difference in duration of procedure for the three randomized controlled trials was not statistically significant between the two procedures.
Dr. Roeckner and colleagues noted that two of the randomized controlled trials reported times for the sterilization procedures. One study found a duration of 5.6 minutes for salpingectomy with a bipolar device and 6.1 minutes for tubal interruption; the other study compared salpingectomy with suture ligation and tubal interruption, finding operative times of 18.5 and 6.9 minutes, respectively.
In addition to the primary outcome of operative time, Dr. Roeckner and colleagues looked at rates of a variety of complications. These included transfusion, estimated blood loss, change in hemoglobin, wound infection, internal organ damage, readmission, reoperation, and length of stay. Salpingectomy was not associated with higher rates of any of these complications.
“Our main finding was that salpingectomy at the time of cesarean delivery may be associated with a small increase in operative time, but it doesn’t appear to be associated with an increased rate of surgical complications,” the researchers wrote.
One concern that’s been raised about the strategy of salpingectomy is the possibility of reduction of ovarian reserve related to decreased blood supply to the ovaries. However, noted Dr. Roeckner and coinvestigators, other studies have not shown decreases in anti-Müllerian hormone levels or other real-world signals for reduced ovarian reserve.
It’s true, the investigators acknowledged, that there is no possibility for reanastomosis and future fertility with salpingectomy. However, they observed that if the possibility for future fertility exists, conventional tubal ligation should not be performed.
Despite the thoroughness of the review and the investigators’ emphasis on adhering to best practices for systematic reviews and meta-analysis, they acknowledged that there were few studies, which resulted in some difficulties with statistical power. Still, they said, “there does not appear to be a trend toward increased complications among the salpingectomy cohort.”
Dr. Roeckner reported no outside sources of funding and no conflicts of interest.
SOURCE: Roeckner J et al. Pregnancy Meeting, Abstract P180; Obstet Gynecol. 2020 Feb;135:3:550-7.
REPORTING FROM THE PREGNANCY MEETING