User login
A Fixed Drug Eruption to Medroxyprogesterone Acetate Injectable Suspension
To the Editor:
A fixed drug eruption (FDE) is a well-documented form of cutaneous hypersensitivity that typically manifests as a sharply demarcated, dusky, round to oval, edematous, red-violaceous macule or patch on the skin and mucous membranes. The lesion often resolves with residual postinflammatory hyperpigmentation, most commonly as a reaction to ingested drugs or drug components.1 Lesions generally occur at the same anatomic site with repeated exposure to the offending drug. Typically, a single site is affected, but additional sites with more generalized involvement have been reported to occur with subsequent exposure to the offending medication. The diagnosis usually is clinical, but histopathologic findings can help confirm the diagnosis in unusual presentations. We present a novel case of a patient with an FDE from medroxyprogesterone acetate, a contraceptive injection that contains the hormone progestin.
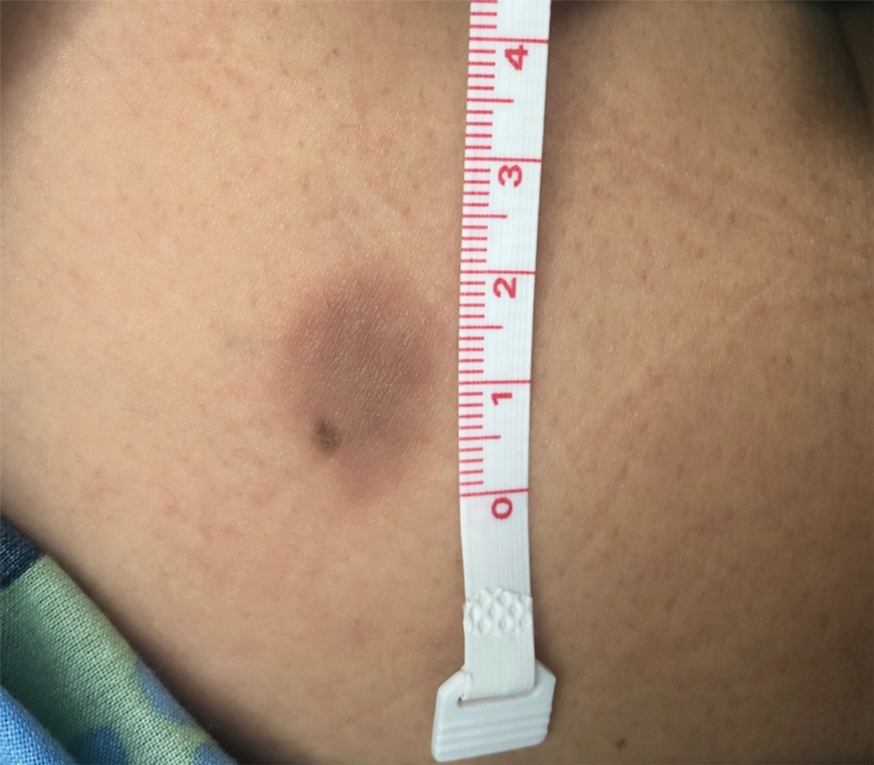
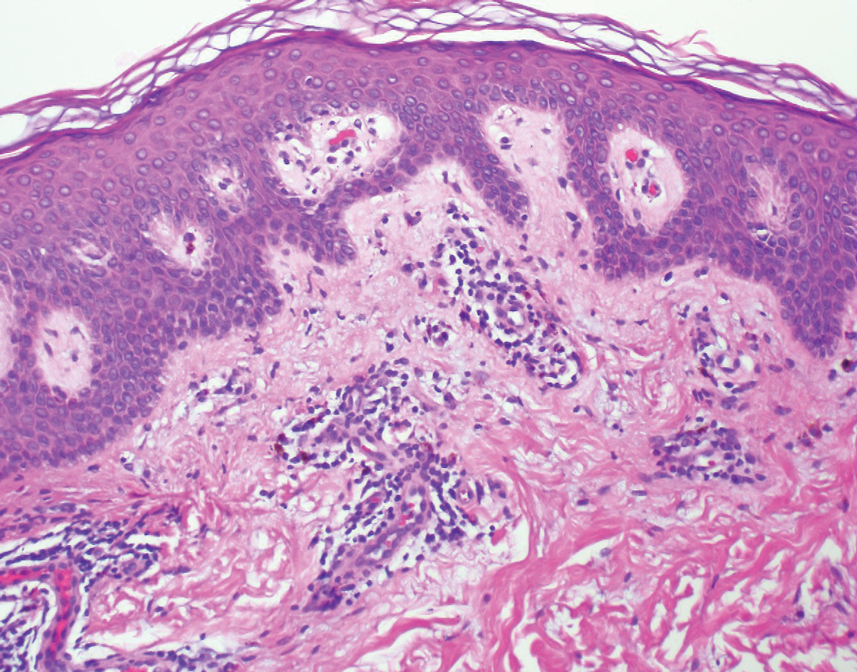
A 35-year-old woman presented to the dermatology clinic for evaluation of a lesion on the left lower buttock of 1 year’s duration. She reported periodic swelling and associated pruritus of the lesion. She denied any growth in size, and no other similar lesions were present. The patient reported a medication history of medroxyprogesterone acetate for birth control, but she denied any other prescription or over-the-counter medication, oral supplements, or recreational drug use. Upon further inquiry, she reported that the recurrence of symptoms appeared to coincide with each administration of medroxyprogesterone acetate, which occurred approximately every 3 months. The eruption cleared between injections and recurred in the same location following subsequent injections. The lesion appeared approximately 2 weeks after the first injection (approximately 1 year prior to presentation to dermatology) and within 2 to 3 days after each subsequent injection. Physical examination revealed a 2×2-cm, circular, slightly violaceous patch on the left buttock (Figure 1). A biopsy was recommended to aid in diagnosis, and the patient was offered a topical steroid for symptomatic relief. A punch biopsy revealed subtle interface dermatitis with superficial perivascular lymphoid infiltrate and marked pigmentary incontinence consistent with an FDE (Figure 2).
An FDE was first reported in 1889 by Bourns,2 and over time more implicated agents and varying clinical presentations have been linked to the disease. The FDE can be accompanied by symptoms of pruritus or paresthesia. Most cases are devoid of systemic symptoms. An FDE can be located anywhere on the body, but it most frequently manifests on the lips, face, hands, feet, and genitalia. Although the eruption often heals with residual postinflammatory hyperpigmentation, a nonpigmenting FDE due to pseudoephedrine has been reported.3
Common culprits include antibiotics (eg, sulfonamides, trimethoprim, fluoroquinolones, tetracyclines), nonsteroidal anti-inflammatory medications (eg, naproxen sodium, ibuprofen, celecoxib), barbiturates, antimalarials, and anticonvulsants. Rare cases of FDE induced by foods and food additives also have been reported.4 Oral fluconazole, levocetirizine dihydrochloride, loperamide, and multivitamin-mineral preparations are other rare inducers of FDE.5-8 In 2004, Ritter and Meffert9 described an FDE to the green dye used in inactive oral contraceptive pills. A similar case was reported by Rea et al10 that described an FDE from the inactive sugar pills in ethinyl estradiol and levonorgestrel, which is another combined oral contraceptive.
The time between ingestion of the offending agent and the manifestation of the disease usually is 1 to 2 weeks; however, upon subsequent exposure, the disease has been reported to manifest within hours.1 CD8+ memory T cells have been shown to be major players in the development of FDE and can be found along the dermoepidermal junction as part of a delayed type IV hypersensitivity reaction.11 Histopathology reveals superficial and deep interstitial and perivascular infiltrates consisting of lymphocytes with admixed eosinophils and possibly neutrophils in the dermis. In the epidermis, necrotic keratinocytes can be present. In rare cases, FDE may have atypical features, such as in generalized bullous FDE and nonpigmenting FDE, the latter of which more commonly is associated with pseudoephedrine.1
The differential diagnosis for FDE includes erythema multiforme, Stevens-Johnson syndrome/toxic epidermal necrolysis, autoimmune progesterone dermatitis, and large plaque parapsoriasis. The number and morphology of lesions in erythema multiforme help differentiate it from FDE, as erythema multiforme presents with multiple targetoid lesions. The lesions of generalized bullous FDE can be similar to those of Stevens-Johnson syndrome/toxic epidermal necrolysis, and the pigmented patches of FDE can resemble large plaque parapsoriasis.
It is important to consider any medication ingested in the 1- to 2-week period before FDE onset, including over-the-counter medications, health food supplements, and prescription medications. Discontinuation of the implicated medication or any medication potentially cross-reacting with another medication is the most important step in management. Wound care may be needed for any bullous or eroded lesions. Lesions typically resolve within a few days to weeks of stopping the offending agent. Importantly, patients should be counseled on the secondary pigment alterations that may be persistent for several months. Other treatment for FDEs is aimed at symptomatic relief and may include topical corticosteroids and oral antihistamines.1
Medroxyprogesterone acetate is a highly effective contraceptive drug with low rates of failure.12 It is a weak androgenic progestin that is administered as a single 150-mg intramuscular injection every 3 months and inhibits gonadotropins. Common side effects include local injection-site reactions, unscheduled bleeding, amenorrhea, weight gain, headache, and mood changes. However, FDE has not been reported as an adverse effect to medroxyprogesterone acetate, both in official US Food and Drug Administration information and in the current literature.12
Autoimmune progesterone dermatitis (also known as progestin hypersensitivity) is a well-characterized cyclic hypersensitivity reaction to the hormone progesterone that occurs during the luteal phase of the menstrual cycle. It is known to have a variable clinical presentation including urticaria, erythema multiforme, eczema, and angioedema.13 Autoimmune progesterone dermatitis also has been reported to present as an FDE.14-16 The onset of the cutaneous manifestation often starts a few days before the onset of menses, with spontaneous resolution occurring after the onset of menstruation. The mechanism by which endogenous progesterone or other secretory products become antigenic is unknown. It has been suggested that there is an alteration in the properties of the hormone that would predispose it to be antigenic as it would not be considered self. In 2001, Warin17 proposed the following diagnostic criteria for autoimmune progesterone dermatitis: (1) skin lesions associated with menstrual cycle (premenstrual flare); (2) a positive response to the progesterone intradermal or intramuscular test; and (3) symptomatic improvement after inhibiting progesterone secretion by suppressing ovulation.17 The treatment includes antiallergy medications, progesterone desensitization, omalizumab injection, and leuprolide acetate injection.
Our case represents FDE from medroxyprogesterone acetate. Although we did not formally investigate the antigenicity of the exogenous progesterone, we postulate that the pathophysiology likely is similar to an FDE associated with endogenous progesterone. This reasoning is supported by the time course of the patient’s lesion as well as the worsening of symptoms in the days following the administration of the medication. Additionally, the patient had no history of skin lesions prior to the initiation of medroxyprogesterone acetate or similar lesions associated with her menstrual cycles.
A careful and detailed review of medication history is necessary to evaluate FDEs. Our case emphasizes that not only endogenous but also exogenous forms of progesterone may cause hypersensitivity, leading to an FDE. With more than 2 million prescriptions of medroxyprogesterone acetate written every year, dermatologists should be aware of the rare but potential risk for an FDE in patients using this medication.18
- Bolognia J, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. Mosby; 2008.
- Bourns DCG. Unusual effects of antipyrine. Br Med J. 1889;2:818-820.
- Shelley WB, Shelley ED. Nonpigmenting fixed drug eruption as a distinctive reaction pattern: examples caused by sensitivity to pseudoephedrine hydrochloride and tetrahydrozoline. J Am Acad Dermatol. 1987;17:403-407.
- Sohn KH, Kim BK, Kim JY, et al. Fixed food eruption caused by Actinidia arguta (hardy kiwi): a case report and literature review. Allergy Asthma Immunol Res. 2017;9:182-184.
- Nakai N, Katoh N. Fixed drug eruption caused by fluconazole: a case report and mini-review of the literature. Allergol Int. 2013;6:139-141.
- An I, Demir V, Ibiloglu I, et al. Fixed drug eruption induced by levocetirizine. Indian Dermatol Online J. 2017;8:276-278.
- Matarredona J, Borrás Blasco J, Navarro-Ruiz A, et al. Fixed drug eruption associated to loperamide [in Spanish]. Med Clin (Barc). 2005;124:198-199.
- Gohel D. Fixed drug eruption due to multi-vitamin multi-mineral preparation. J Assoc Physicians India. 2000;48:268.
- Ritter SE, Meffert J. A refractory fixed drug reaction to a dye used in an oral contraceptive. Cutis. 2004;74:243-244.
- Rea S, McMeniman E, Darch K, et al. A fixed drug eruption to the sugar pills of a combined oral contraceptive. Poster presented at: The Australasian College of Dermatologists 51st Annual Scientific Meeting; May 22, 2018; Queensland, Australia.
- Shiohara T, Mizukawa Y. Fixed drug eruption: a disease mediated by self-inflicted responses of intraepidermal T cells. Eur J Dermatol. 2007;17:201-208.
- Depo-Provera CI. Prescribing information. Pfizer; 2020. Accessed March 10, 2022. https://labeling.pfizer.com/ShowLabeling.aspx?format=PDF&id=522
- George R, Badawy SZ. Autoimmune progesterone dermatitis: a case report. Case Rep Obstet Gynecol. 2012;2012:757854.
- Mokhtari R, Sepaskhah M, Aslani FS, et al. Autoimmune progesterone dermatitis presenting as fixed drug eruption: a case report. Dermatol Online J. 2017;23:13030/qt685685p4.
- Asai J, Katoh N, Nakano M, et al. Case of autoimmune progesterone dermatitis presenting as fixed drug eruption. J Dermatol. 2009;36:643-645.
- Bhardwaj N, Jindal R, Chauhan P. Autoimmune progesterone dermatitis presenting as fixed drug eruption. BMJ Case Rep. 2019;12:E231873.
- Warin AP. Case 2. diagnosis: erythema multiforme as a presentation of autoimmune progesterone dermatitis. Clin Exp Dermatol. 2001;26:107-108.
- Medroxyprogesterone Drug Usage Statistics, United States, 2013-2019. ClinCalc website. Updated September 15, 2021. Accessed March 17, 2022. https://clincalc.com/DrugStats/Drugs/Medroxyprogesterone
To the Editor:
A fixed drug eruption (FDE) is a well-documented form of cutaneous hypersensitivity that typically manifests as a sharply demarcated, dusky, round to oval, edematous, red-violaceous macule or patch on the skin and mucous membranes. The lesion often resolves with residual postinflammatory hyperpigmentation, most commonly as a reaction to ingested drugs or drug components.1 Lesions generally occur at the same anatomic site with repeated exposure to the offending drug. Typically, a single site is affected, but additional sites with more generalized involvement have been reported to occur with subsequent exposure to the offending medication. The diagnosis usually is clinical, but histopathologic findings can help confirm the diagnosis in unusual presentations. We present a novel case of a patient with an FDE from medroxyprogesterone acetate, a contraceptive injection that contains the hormone progestin.
A 35-year-old woman presented to the dermatology clinic for evaluation of a lesion on the left lower buttock of 1 year’s duration. She reported periodic swelling and associated pruritus of the lesion. She denied any growth in size, and no other similar lesions were present. The patient reported a medication history of medroxyprogesterone acetate for birth control, but she denied any other prescription or over-the-counter medication, oral supplements, or recreational drug use. Upon further inquiry, she reported that the recurrence of symptoms appeared to coincide with each administration of medroxyprogesterone acetate, which occurred approximately every 3 months. The eruption cleared between injections and recurred in the same location following subsequent injections. The lesion appeared approximately 2 weeks after the first injection (approximately 1 year prior to presentation to dermatology) and within 2 to 3 days after each subsequent injection. Physical examination revealed a 2×2-cm, circular, slightly violaceous patch on the left buttock (Figure 1). A biopsy was recommended to aid in diagnosis, and the patient was offered a topical steroid for symptomatic relief. A punch biopsy revealed subtle interface dermatitis with superficial perivascular lymphoid infiltrate and marked pigmentary incontinence consistent with an FDE (Figure 2).
An FDE was first reported in 1889 by Bourns,2 and over time more implicated agents and varying clinical presentations have been linked to the disease. The FDE can be accompanied by symptoms of pruritus or paresthesia. Most cases are devoid of systemic symptoms. An FDE can be located anywhere on the body, but it most frequently manifests on the lips, face, hands, feet, and genitalia. Although the eruption often heals with residual postinflammatory hyperpigmentation, a nonpigmenting FDE due to pseudoephedrine has been reported.3
Common culprits include antibiotics (eg, sulfonamides, trimethoprim, fluoroquinolones, tetracyclines), nonsteroidal anti-inflammatory medications (eg, naproxen sodium, ibuprofen, celecoxib), barbiturates, antimalarials, and anticonvulsants. Rare cases of FDE induced by foods and food additives also have been reported.4 Oral fluconazole, levocetirizine dihydrochloride, loperamide, and multivitamin-mineral preparations are other rare inducers of FDE.5-8 In 2004, Ritter and Meffert9 described an FDE to the green dye used in inactive oral contraceptive pills. A similar case was reported by Rea et al10 that described an FDE from the inactive sugar pills in ethinyl estradiol and levonorgestrel, which is another combined oral contraceptive.
The time between ingestion of the offending agent and the manifestation of the disease usually is 1 to 2 weeks; however, upon subsequent exposure, the disease has been reported to manifest within hours.1 CD8+ memory T cells have been shown to be major players in the development of FDE and can be found along the dermoepidermal junction as part of a delayed type IV hypersensitivity reaction.11 Histopathology reveals superficial and deep interstitial and perivascular infiltrates consisting of lymphocytes with admixed eosinophils and possibly neutrophils in the dermis. In the epidermis, necrotic keratinocytes can be present. In rare cases, FDE may have atypical features, such as in generalized bullous FDE and nonpigmenting FDE, the latter of which more commonly is associated with pseudoephedrine.1
The differential diagnosis for FDE includes erythema multiforme, Stevens-Johnson syndrome/toxic epidermal necrolysis, autoimmune progesterone dermatitis, and large plaque parapsoriasis. The number and morphology of lesions in erythema multiforme help differentiate it from FDE, as erythema multiforme presents with multiple targetoid lesions. The lesions of generalized bullous FDE can be similar to those of Stevens-Johnson syndrome/toxic epidermal necrolysis, and the pigmented patches of FDE can resemble large plaque parapsoriasis.
It is important to consider any medication ingested in the 1- to 2-week period before FDE onset, including over-the-counter medications, health food supplements, and prescription medications. Discontinuation of the implicated medication or any medication potentially cross-reacting with another medication is the most important step in management. Wound care may be needed for any bullous or eroded lesions. Lesions typically resolve within a few days to weeks of stopping the offending agent. Importantly, patients should be counseled on the secondary pigment alterations that may be persistent for several months. Other treatment for FDEs is aimed at symptomatic relief and may include topical corticosteroids and oral antihistamines.1
Medroxyprogesterone acetate is a highly effective contraceptive drug with low rates of failure.12 It is a weak androgenic progestin that is administered as a single 150-mg intramuscular injection every 3 months and inhibits gonadotropins. Common side effects include local injection-site reactions, unscheduled bleeding, amenorrhea, weight gain, headache, and mood changes. However, FDE has not been reported as an adverse effect to medroxyprogesterone acetate, both in official US Food and Drug Administration information and in the current literature.12
Autoimmune progesterone dermatitis (also known as progestin hypersensitivity) is a well-characterized cyclic hypersensitivity reaction to the hormone progesterone that occurs during the luteal phase of the menstrual cycle. It is known to have a variable clinical presentation including urticaria, erythema multiforme, eczema, and angioedema.13 Autoimmune progesterone dermatitis also has been reported to present as an FDE.14-16 The onset of the cutaneous manifestation often starts a few days before the onset of menses, with spontaneous resolution occurring after the onset of menstruation. The mechanism by which endogenous progesterone or other secretory products become antigenic is unknown. It has been suggested that there is an alteration in the properties of the hormone that would predispose it to be antigenic as it would not be considered self. In 2001, Warin17 proposed the following diagnostic criteria for autoimmune progesterone dermatitis: (1) skin lesions associated with menstrual cycle (premenstrual flare); (2) a positive response to the progesterone intradermal or intramuscular test; and (3) symptomatic improvement after inhibiting progesterone secretion by suppressing ovulation.17 The treatment includes antiallergy medications, progesterone desensitization, omalizumab injection, and leuprolide acetate injection.
Our case represents FDE from medroxyprogesterone acetate. Although we did not formally investigate the antigenicity of the exogenous progesterone, we postulate that the pathophysiology likely is similar to an FDE associated with endogenous progesterone. This reasoning is supported by the time course of the patient’s lesion as well as the worsening of symptoms in the days following the administration of the medication. Additionally, the patient had no history of skin lesions prior to the initiation of medroxyprogesterone acetate or similar lesions associated with her menstrual cycles.
A careful and detailed review of medication history is necessary to evaluate FDEs. Our case emphasizes that not only endogenous but also exogenous forms of progesterone may cause hypersensitivity, leading to an FDE. With more than 2 million prescriptions of medroxyprogesterone acetate written every year, dermatologists should be aware of the rare but potential risk for an FDE in patients using this medication.18
To the Editor:
A fixed drug eruption (FDE) is a well-documented form of cutaneous hypersensitivity that typically manifests as a sharply demarcated, dusky, round to oval, edematous, red-violaceous macule or patch on the skin and mucous membranes. The lesion often resolves with residual postinflammatory hyperpigmentation, most commonly as a reaction to ingested drugs or drug components.1 Lesions generally occur at the same anatomic site with repeated exposure to the offending drug. Typically, a single site is affected, but additional sites with more generalized involvement have been reported to occur with subsequent exposure to the offending medication. The diagnosis usually is clinical, but histopathologic findings can help confirm the diagnosis in unusual presentations. We present a novel case of a patient with an FDE from medroxyprogesterone acetate, a contraceptive injection that contains the hormone progestin.
A 35-year-old woman presented to the dermatology clinic for evaluation of a lesion on the left lower buttock of 1 year’s duration. She reported periodic swelling and associated pruritus of the lesion. She denied any growth in size, and no other similar lesions were present. The patient reported a medication history of medroxyprogesterone acetate for birth control, but she denied any other prescription or over-the-counter medication, oral supplements, or recreational drug use. Upon further inquiry, she reported that the recurrence of symptoms appeared to coincide with each administration of medroxyprogesterone acetate, which occurred approximately every 3 months. The eruption cleared between injections and recurred in the same location following subsequent injections. The lesion appeared approximately 2 weeks after the first injection (approximately 1 year prior to presentation to dermatology) and within 2 to 3 days after each subsequent injection. Physical examination revealed a 2×2-cm, circular, slightly violaceous patch on the left buttock (Figure 1). A biopsy was recommended to aid in diagnosis, and the patient was offered a topical steroid for symptomatic relief. A punch biopsy revealed subtle interface dermatitis with superficial perivascular lymphoid infiltrate and marked pigmentary incontinence consistent with an FDE (Figure 2).
An FDE was first reported in 1889 by Bourns,2 and over time more implicated agents and varying clinical presentations have been linked to the disease. The FDE can be accompanied by symptoms of pruritus or paresthesia. Most cases are devoid of systemic symptoms. An FDE can be located anywhere on the body, but it most frequently manifests on the lips, face, hands, feet, and genitalia. Although the eruption often heals with residual postinflammatory hyperpigmentation, a nonpigmenting FDE due to pseudoephedrine has been reported.3
Common culprits include antibiotics (eg, sulfonamides, trimethoprim, fluoroquinolones, tetracyclines), nonsteroidal anti-inflammatory medications (eg, naproxen sodium, ibuprofen, celecoxib), barbiturates, antimalarials, and anticonvulsants. Rare cases of FDE induced by foods and food additives also have been reported.4 Oral fluconazole, levocetirizine dihydrochloride, loperamide, and multivitamin-mineral preparations are other rare inducers of FDE.5-8 In 2004, Ritter and Meffert9 described an FDE to the green dye used in inactive oral contraceptive pills. A similar case was reported by Rea et al10 that described an FDE from the inactive sugar pills in ethinyl estradiol and levonorgestrel, which is another combined oral contraceptive.
The time between ingestion of the offending agent and the manifestation of the disease usually is 1 to 2 weeks; however, upon subsequent exposure, the disease has been reported to manifest within hours.1 CD8+ memory T cells have been shown to be major players in the development of FDE and can be found along the dermoepidermal junction as part of a delayed type IV hypersensitivity reaction.11 Histopathology reveals superficial and deep interstitial and perivascular infiltrates consisting of lymphocytes with admixed eosinophils and possibly neutrophils in the dermis. In the epidermis, necrotic keratinocytes can be present. In rare cases, FDE may have atypical features, such as in generalized bullous FDE and nonpigmenting FDE, the latter of which more commonly is associated with pseudoephedrine.1
The differential diagnosis for FDE includes erythema multiforme, Stevens-Johnson syndrome/toxic epidermal necrolysis, autoimmune progesterone dermatitis, and large plaque parapsoriasis. The number and morphology of lesions in erythema multiforme help differentiate it from FDE, as erythema multiforme presents with multiple targetoid lesions. The lesions of generalized bullous FDE can be similar to those of Stevens-Johnson syndrome/toxic epidermal necrolysis, and the pigmented patches of FDE can resemble large plaque parapsoriasis.
It is important to consider any medication ingested in the 1- to 2-week period before FDE onset, including over-the-counter medications, health food supplements, and prescription medications. Discontinuation of the implicated medication or any medication potentially cross-reacting with another medication is the most important step in management. Wound care may be needed for any bullous or eroded lesions. Lesions typically resolve within a few days to weeks of stopping the offending agent. Importantly, patients should be counseled on the secondary pigment alterations that may be persistent for several months. Other treatment for FDEs is aimed at symptomatic relief and may include topical corticosteroids and oral antihistamines.1
Medroxyprogesterone acetate is a highly effective contraceptive drug with low rates of failure.12 It is a weak androgenic progestin that is administered as a single 150-mg intramuscular injection every 3 months and inhibits gonadotropins. Common side effects include local injection-site reactions, unscheduled bleeding, amenorrhea, weight gain, headache, and mood changes. However, FDE has not been reported as an adverse effect to medroxyprogesterone acetate, both in official US Food and Drug Administration information and in the current literature.12
Autoimmune progesterone dermatitis (also known as progestin hypersensitivity) is a well-characterized cyclic hypersensitivity reaction to the hormone progesterone that occurs during the luteal phase of the menstrual cycle. It is known to have a variable clinical presentation including urticaria, erythema multiforme, eczema, and angioedema.13 Autoimmune progesterone dermatitis also has been reported to present as an FDE.14-16 The onset of the cutaneous manifestation often starts a few days before the onset of menses, with spontaneous resolution occurring after the onset of menstruation. The mechanism by which endogenous progesterone or other secretory products become antigenic is unknown. It has been suggested that there is an alteration in the properties of the hormone that would predispose it to be antigenic as it would not be considered self. In 2001, Warin17 proposed the following diagnostic criteria for autoimmune progesterone dermatitis: (1) skin lesions associated with menstrual cycle (premenstrual flare); (2) a positive response to the progesterone intradermal or intramuscular test; and (3) symptomatic improvement after inhibiting progesterone secretion by suppressing ovulation.17 The treatment includes antiallergy medications, progesterone desensitization, omalizumab injection, and leuprolide acetate injection.
Our case represents FDE from medroxyprogesterone acetate. Although we did not formally investigate the antigenicity of the exogenous progesterone, we postulate that the pathophysiology likely is similar to an FDE associated with endogenous progesterone. This reasoning is supported by the time course of the patient’s lesion as well as the worsening of symptoms in the days following the administration of the medication. Additionally, the patient had no history of skin lesions prior to the initiation of medroxyprogesterone acetate or similar lesions associated with her menstrual cycles.
A careful and detailed review of medication history is necessary to evaluate FDEs. Our case emphasizes that not only endogenous but also exogenous forms of progesterone may cause hypersensitivity, leading to an FDE. With more than 2 million prescriptions of medroxyprogesterone acetate written every year, dermatologists should be aware of the rare but potential risk for an FDE in patients using this medication.18
- Bolognia J, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. Mosby; 2008.
- Bourns DCG. Unusual effects of antipyrine. Br Med J. 1889;2:818-820.
- Shelley WB, Shelley ED. Nonpigmenting fixed drug eruption as a distinctive reaction pattern: examples caused by sensitivity to pseudoephedrine hydrochloride and tetrahydrozoline. J Am Acad Dermatol. 1987;17:403-407.
- Sohn KH, Kim BK, Kim JY, et al. Fixed food eruption caused by Actinidia arguta (hardy kiwi): a case report and literature review. Allergy Asthma Immunol Res. 2017;9:182-184.
- Nakai N, Katoh N. Fixed drug eruption caused by fluconazole: a case report and mini-review of the literature. Allergol Int. 2013;6:139-141.
- An I, Demir V, Ibiloglu I, et al. Fixed drug eruption induced by levocetirizine. Indian Dermatol Online J. 2017;8:276-278.
- Matarredona J, Borrás Blasco J, Navarro-Ruiz A, et al. Fixed drug eruption associated to loperamide [in Spanish]. Med Clin (Barc). 2005;124:198-199.
- Gohel D. Fixed drug eruption due to multi-vitamin multi-mineral preparation. J Assoc Physicians India. 2000;48:268.
- Ritter SE, Meffert J. A refractory fixed drug reaction to a dye used in an oral contraceptive. Cutis. 2004;74:243-244.
- Rea S, McMeniman E, Darch K, et al. A fixed drug eruption to the sugar pills of a combined oral contraceptive. Poster presented at: The Australasian College of Dermatologists 51st Annual Scientific Meeting; May 22, 2018; Queensland, Australia.
- Shiohara T, Mizukawa Y. Fixed drug eruption: a disease mediated by self-inflicted responses of intraepidermal T cells. Eur J Dermatol. 2007;17:201-208.
- Depo-Provera CI. Prescribing information. Pfizer; 2020. Accessed March 10, 2022. https://labeling.pfizer.com/ShowLabeling.aspx?format=PDF&id=522
- George R, Badawy SZ. Autoimmune progesterone dermatitis: a case report. Case Rep Obstet Gynecol. 2012;2012:757854.
- Mokhtari R, Sepaskhah M, Aslani FS, et al. Autoimmune progesterone dermatitis presenting as fixed drug eruption: a case report. Dermatol Online J. 2017;23:13030/qt685685p4.
- Asai J, Katoh N, Nakano M, et al. Case of autoimmune progesterone dermatitis presenting as fixed drug eruption. J Dermatol. 2009;36:643-645.
- Bhardwaj N, Jindal R, Chauhan P. Autoimmune progesterone dermatitis presenting as fixed drug eruption. BMJ Case Rep. 2019;12:E231873.
- Warin AP. Case 2. diagnosis: erythema multiforme as a presentation of autoimmune progesterone dermatitis. Clin Exp Dermatol. 2001;26:107-108.
- Medroxyprogesterone Drug Usage Statistics, United States, 2013-2019. ClinCalc website. Updated September 15, 2021. Accessed March 17, 2022. https://clincalc.com/DrugStats/Drugs/Medroxyprogesterone
- Bolognia J, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. Mosby; 2008.
- Bourns DCG. Unusual effects of antipyrine. Br Med J. 1889;2:818-820.
- Shelley WB, Shelley ED. Nonpigmenting fixed drug eruption as a distinctive reaction pattern: examples caused by sensitivity to pseudoephedrine hydrochloride and tetrahydrozoline. J Am Acad Dermatol. 1987;17:403-407.
- Sohn KH, Kim BK, Kim JY, et al. Fixed food eruption caused by Actinidia arguta (hardy kiwi): a case report and literature review. Allergy Asthma Immunol Res. 2017;9:182-184.
- Nakai N, Katoh N. Fixed drug eruption caused by fluconazole: a case report and mini-review of the literature. Allergol Int. 2013;6:139-141.
- An I, Demir V, Ibiloglu I, et al. Fixed drug eruption induced by levocetirizine. Indian Dermatol Online J. 2017;8:276-278.
- Matarredona J, Borrás Blasco J, Navarro-Ruiz A, et al. Fixed drug eruption associated to loperamide [in Spanish]. Med Clin (Barc). 2005;124:198-199.
- Gohel D. Fixed drug eruption due to multi-vitamin multi-mineral preparation. J Assoc Physicians India. 2000;48:268.
- Ritter SE, Meffert J. A refractory fixed drug reaction to a dye used in an oral contraceptive. Cutis. 2004;74:243-244.
- Rea S, McMeniman E, Darch K, et al. A fixed drug eruption to the sugar pills of a combined oral contraceptive. Poster presented at: The Australasian College of Dermatologists 51st Annual Scientific Meeting; May 22, 2018; Queensland, Australia.
- Shiohara T, Mizukawa Y. Fixed drug eruption: a disease mediated by self-inflicted responses of intraepidermal T cells. Eur J Dermatol. 2007;17:201-208.
- Depo-Provera CI. Prescribing information. Pfizer; 2020. Accessed March 10, 2022. https://labeling.pfizer.com/ShowLabeling.aspx?format=PDF&id=522
- George R, Badawy SZ. Autoimmune progesterone dermatitis: a case report. Case Rep Obstet Gynecol. 2012;2012:757854.
- Mokhtari R, Sepaskhah M, Aslani FS, et al. Autoimmune progesterone dermatitis presenting as fixed drug eruption: a case report. Dermatol Online J. 2017;23:13030/qt685685p4.
- Asai J, Katoh N, Nakano M, et al. Case of autoimmune progesterone dermatitis presenting as fixed drug eruption. J Dermatol. 2009;36:643-645.
- Bhardwaj N, Jindal R, Chauhan P. Autoimmune progesterone dermatitis presenting as fixed drug eruption. BMJ Case Rep. 2019;12:E231873.
- Warin AP. Case 2. diagnosis: erythema multiforme as a presentation of autoimmune progesterone dermatitis. Clin Exp Dermatol. 2001;26:107-108.
- Medroxyprogesterone Drug Usage Statistics, United States, 2013-2019. ClinCalc website. Updated September 15, 2021. Accessed March 17, 2022. https://clincalc.com/DrugStats/Drugs/Medroxyprogesterone
Practice Points
- Exogenous progesterone from the administration of the contraceptive injectable medroxyprogesterone acetate has the potential to cause a cutaneous hypersensitivity reaction in the form of a fixed drug eruption (FDE).
- Dermatologists should perform a careful and detailed review of medication history to evaluate drug eruptions.
Pediatric-Onset Refractory Lupus Erythematosus Panniculitis Treated With Rituximab
To the Editor:
Lupus erythematosus panniculitis (LEP) is rare in the pediatric population. It can be difficult to manage, as patients may not respond to conventional treatments including hydroxychloroquine and prednisone. We report the use of rituximab in the treatment of a 20-year-old woman with LEP of the face, legs, and arms that was refractory to standard treatments. She also had a history of hemophagocytic lymphohistiocytosis (HLH). Further studies are warranted to determine the role of rituximab in the treatment of pediatric patients with LEP.
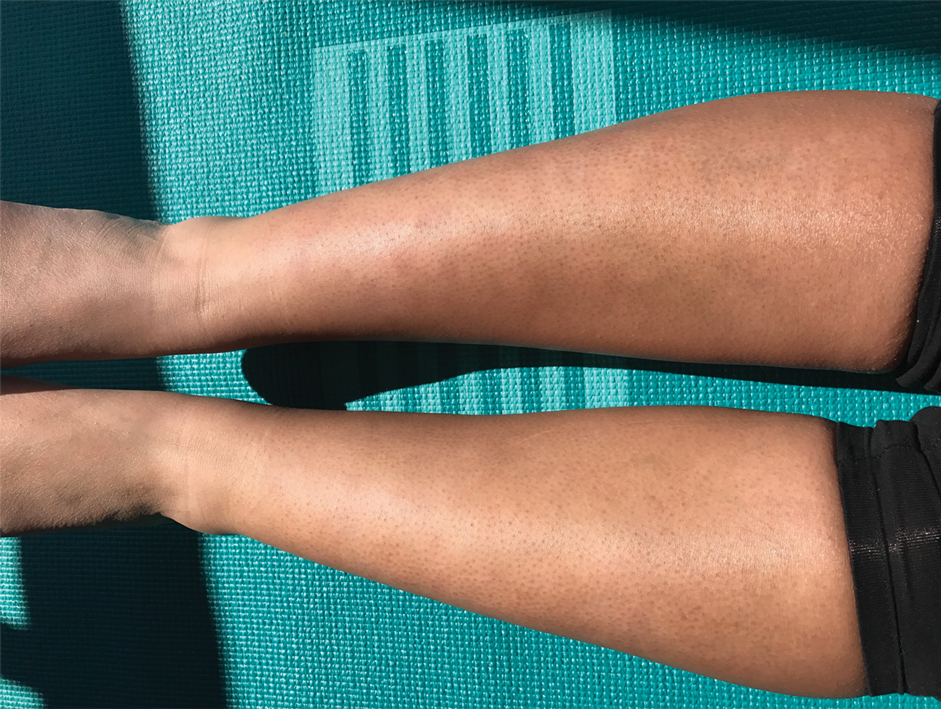
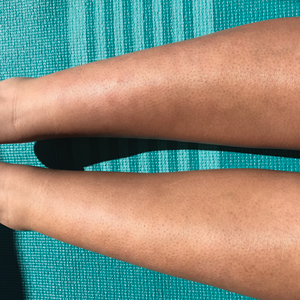
A 20-year-old woman with history of LEP and HLH initially presented with migratory violaceous nodules on the face 16 years prior to the current presentation. A skin biopsy 3 years after that initial presentation suggested a diagnosis of cutaneous lupus erythematosus. Six years later, numerous asymptomatic lesions appeared on the legs, predominantly on the calves; she was successfully treated with hydroxychloroquine and high-dose prednisone. Four years prior to the current presentation, a febrile illness prompted discontinuation of hydroxychloroquine and hospitalization, where she was first was diagnosed with HLH; she achieved remission with cyclosporine. At the current presentation, she continued to have persistent violaceous lesions on the face, lower arms, and legs with underlying nodularity (Figure 1). Skin biopsies revealed LEP and were less suggestive of HLH. She was restarted on hydroxychloroquine, which did not adequately control the disease. Rheumatologic workup was only notable for an antinuclear antibody titer of 1:80 (reference range, <1:80) in a speckled pattern.
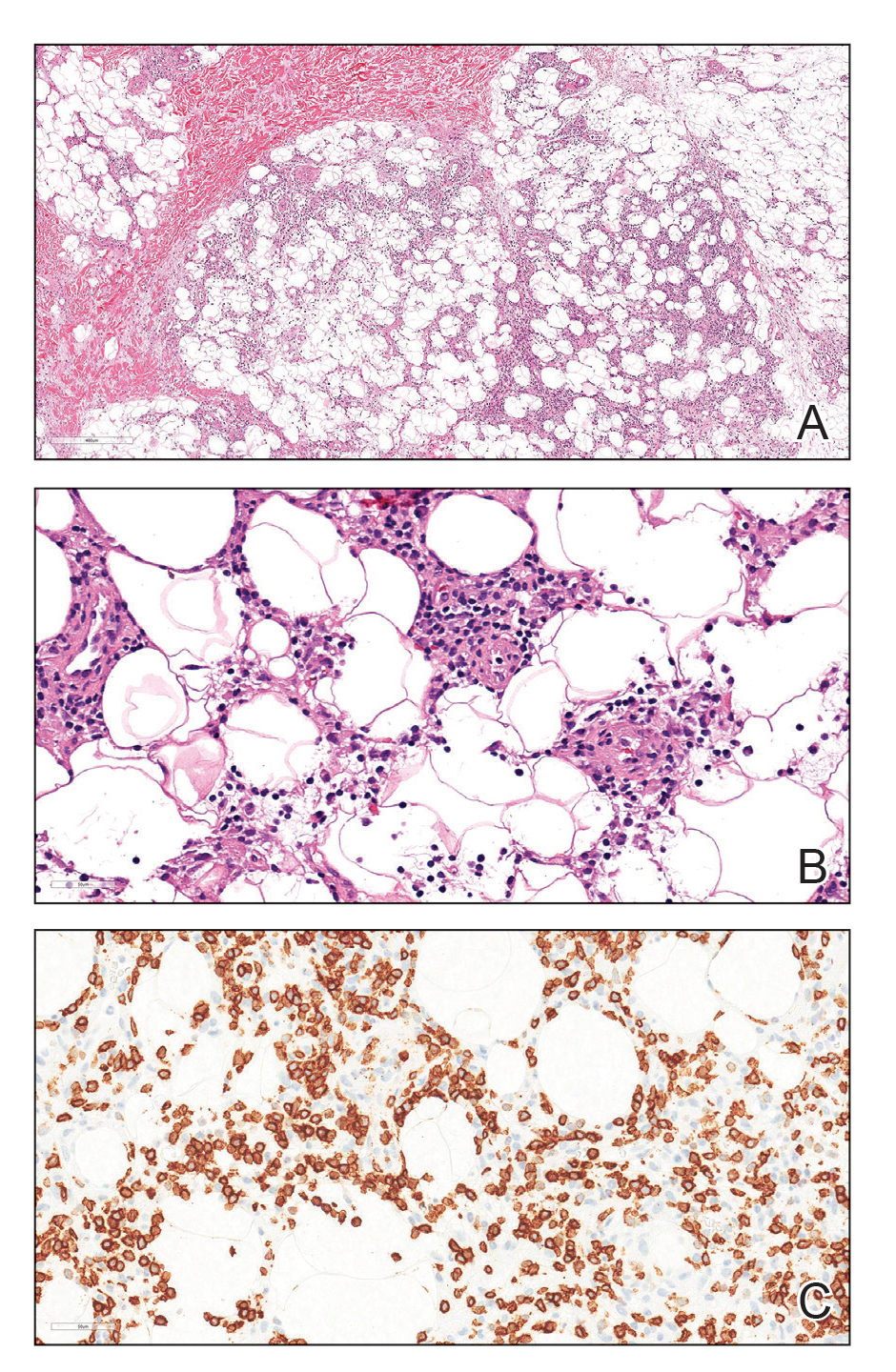
Due to the refractory nature of her condition, continued lesion development despite standard treatment, and concerns of possible scarring, we considered a trial of rituximab. Because HLH and LEP can mimic subcutaneous T-cell lymphoma, another skin biopsy was performed, which revealed a deep dermal and subcutaneous lymphohistiocytic infiltrate composed of predominantly CD3+ T cells with a mixed population of CD4+ and CD8+ cells (Figure 2). There was no evidence of transformation into lymphoma. Pathologic findings were most compatible with LEP rather than an HLH-associated panniculitis due to the lack of definitive phagocytosis. She received rituximab using body surface area–based dosing at 375 mg/m2. CD19 levels decreased to undetectable levels after the first dose. Rituximab was dosed based on clinical response; she tolerated treatment well and experienced considerable improvement in the number of lesions following completion of 4 doses at weeks 0, 1, 5, and 7 (Figure 3). She developed a flare at 7 months and improved again after another dose of rituximab.
Lupus erythematosus panniculitis is a rare variant of lupus erythematosus with an average age of presentation between 30 and 60 years.1 In children, LEP presents as recurrent subcutaneous nodules and plaques, commonly involving the face and upper arms.1,2 Long-term sequelae include local swelling and skin atrophy.3 Conventional treatment options for pediatric patients include hydroxychloroquine and corticosteroids.1 Management can be challenging due to the lack of response to conventional treatments as well as the chronic progressive nature of LEP.2 In refractory cases, cyclosporine, azathioprine, sulfones, thalidomide, mycophenolate mofetil, and cyclophosphamide are alternative treatment options.1-4
Rituximab, a chimeric monoclonal antibody targeting B-cell surface marker CD20, results in depletion of mature B cells. Use of rituximab for LEP has been described in multiple case reports involving an 8-year-old boy, 22-year-old girl, and 2 middle-aged women.2-4 In addition, a recently published case series of 4 patients with childhood-onset refractory LEP described improvement of disease activity with rituximab.5 It is important to rule out subcutaneous T-cell lymphoma before treatment with rituximab, as its histopathology can closely resemble that seen in LEP and HLH-associated cytophagic histiocytic panniculitis.1,6
Rituximab may be an effective treatment option in pediatric patients with refractory LEP. Larger studies on the use of rituximab in the pediatric population are necessary.
- Weingartner JS, Zedek DC, Burkhart CN, et al. Lupus erythematosus panniculitis in children: report of three cases and review of previously reported cases. Pediatr Dermatol. 2011;29:169-176.
- Moreno-Suárez F, Pulpillo-Ruiz Á. Rituximab for the treatment of lupus erythematosus panniculitis. Dermatol Ther. 2013;26:415-418.
- Guissa VR, Trudes G, Jesus AA, et al. Lupus erythematosus panniculitis in children and adolescents. Acta Reumatol Port. 2012;37:82-85.
- Mcardle A, Baker JF. A case of “refractory” lupus erythematosus profundus responsive to rituximab. Clin Rheumatol. 2009;28:745-746.
- Correll CK, Miller DD, Maguiness SM. Treatment of childhood-onset lupus erythematosus panniculitis with rituximab. JAMA Dermatol. 2020;156:566-569.
- Aronson IK, Worobec SM. Cytophagic histiocytic panniculitis and hemophagocytic lymphohistiocytosis: an overview. Dermatol Ther. 2010;23:389-402.
To the Editor:
Lupus erythematosus panniculitis (LEP) is rare in the pediatric population. It can be difficult to manage, as patients may not respond to conventional treatments including hydroxychloroquine and prednisone. We report the use of rituximab in the treatment of a 20-year-old woman with LEP of the face, legs, and arms that was refractory to standard treatments. She also had a history of hemophagocytic lymphohistiocytosis (HLH). Further studies are warranted to determine the role of rituximab in the treatment of pediatric patients with LEP.
A 20-year-old woman with history of LEP and HLH initially presented with migratory violaceous nodules on the face 16 years prior to the current presentation. A skin biopsy 3 years after that initial presentation suggested a diagnosis of cutaneous lupus erythematosus. Six years later, numerous asymptomatic lesions appeared on the legs, predominantly on the calves; she was successfully treated with hydroxychloroquine and high-dose prednisone. Four years prior to the current presentation, a febrile illness prompted discontinuation of hydroxychloroquine and hospitalization, where she was first was diagnosed with HLH; she achieved remission with cyclosporine. At the current presentation, she continued to have persistent violaceous lesions on the face, lower arms, and legs with underlying nodularity (Figure 1). Skin biopsies revealed LEP and were less suggestive of HLH. She was restarted on hydroxychloroquine, which did not adequately control the disease. Rheumatologic workup was only notable for an antinuclear antibody titer of 1:80 (reference range, <1:80) in a speckled pattern.
Due to the refractory nature of her condition, continued lesion development despite standard treatment, and concerns of possible scarring, we considered a trial of rituximab. Because HLH and LEP can mimic subcutaneous T-cell lymphoma, another skin biopsy was performed, which revealed a deep dermal and subcutaneous lymphohistiocytic infiltrate composed of predominantly CD3+ T cells with a mixed population of CD4+ and CD8+ cells (Figure 2). There was no evidence of transformation into lymphoma. Pathologic findings were most compatible with LEP rather than an HLH-associated panniculitis due to the lack of definitive phagocytosis. She received rituximab using body surface area–based dosing at 375 mg/m2. CD19 levels decreased to undetectable levels after the first dose. Rituximab was dosed based on clinical response; she tolerated treatment well and experienced considerable improvement in the number of lesions following completion of 4 doses at weeks 0, 1, 5, and 7 (Figure 3). She developed a flare at 7 months and improved again after another dose of rituximab.
Lupus erythematosus panniculitis is a rare variant of lupus erythematosus with an average age of presentation between 30 and 60 years.1 In children, LEP presents as recurrent subcutaneous nodules and plaques, commonly involving the face and upper arms.1,2 Long-term sequelae include local swelling and skin atrophy.3 Conventional treatment options for pediatric patients include hydroxychloroquine and corticosteroids.1 Management can be challenging due to the lack of response to conventional treatments as well as the chronic progressive nature of LEP.2 In refractory cases, cyclosporine, azathioprine, sulfones, thalidomide, mycophenolate mofetil, and cyclophosphamide are alternative treatment options.1-4
Rituximab, a chimeric monoclonal antibody targeting B-cell surface marker CD20, results in depletion of mature B cells. Use of rituximab for LEP has been described in multiple case reports involving an 8-year-old boy, 22-year-old girl, and 2 middle-aged women.2-4 In addition, a recently published case series of 4 patients with childhood-onset refractory LEP described improvement of disease activity with rituximab.5 It is important to rule out subcutaneous T-cell lymphoma before treatment with rituximab, as its histopathology can closely resemble that seen in LEP and HLH-associated cytophagic histiocytic panniculitis.1,6
Rituximab may be an effective treatment option in pediatric patients with refractory LEP. Larger studies on the use of rituximab in the pediatric population are necessary.
To the Editor:
Lupus erythematosus panniculitis (LEP) is rare in the pediatric population. It can be difficult to manage, as patients may not respond to conventional treatments including hydroxychloroquine and prednisone. We report the use of rituximab in the treatment of a 20-year-old woman with LEP of the face, legs, and arms that was refractory to standard treatments. She also had a history of hemophagocytic lymphohistiocytosis (HLH). Further studies are warranted to determine the role of rituximab in the treatment of pediatric patients with LEP.
A 20-year-old woman with history of LEP and HLH initially presented with migratory violaceous nodules on the face 16 years prior to the current presentation. A skin biopsy 3 years after that initial presentation suggested a diagnosis of cutaneous lupus erythematosus. Six years later, numerous asymptomatic lesions appeared on the legs, predominantly on the calves; she was successfully treated with hydroxychloroquine and high-dose prednisone. Four years prior to the current presentation, a febrile illness prompted discontinuation of hydroxychloroquine and hospitalization, where she was first was diagnosed with HLH; she achieved remission with cyclosporine. At the current presentation, she continued to have persistent violaceous lesions on the face, lower arms, and legs with underlying nodularity (Figure 1). Skin biopsies revealed LEP and were less suggestive of HLH. She was restarted on hydroxychloroquine, which did not adequately control the disease. Rheumatologic workup was only notable for an antinuclear antibody titer of 1:80 (reference range, <1:80) in a speckled pattern.
Due to the refractory nature of her condition, continued lesion development despite standard treatment, and concerns of possible scarring, we considered a trial of rituximab. Because HLH and LEP can mimic subcutaneous T-cell lymphoma, another skin biopsy was performed, which revealed a deep dermal and subcutaneous lymphohistiocytic infiltrate composed of predominantly CD3+ T cells with a mixed population of CD4+ and CD8+ cells (Figure 2). There was no evidence of transformation into lymphoma. Pathologic findings were most compatible with LEP rather than an HLH-associated panniculitis due to the lack of definitive phagocytosis. She received rituximab using body surface area–based dosing at 375 mg/m2. CD19 levels decreased to undetectable levels after the first dose. Rituximab was dosed based on clinical response; she tolerated treatment well and experienced considerable improvement in the number of lesions following completion of 4 doses at weeks 0, 1, 5, and 7 (Figure 3). She developed a flare at 7 months and improved again after another dose of rituximab.
Lupus erythematosus panniculitis is a rare variant of lupus erythematosus with an average age of presentation between 30 and 60 years.1 In children, LEP presents as recurrent subcutaneous nodules and plaques, commonly involving the face and upper arms.1,2 Long-term sequelae include local swelling and skin atrophy.3 Conventional treatment options for pediatric patients include hydroxychloroquine and corticosteroids.1 Management can be challenging due to the lack of response to conventional treatments as well as the chronic progressive nature of LEP.2 In refractory cases, cyclosporine, azathioprine, sulfones, thalidomide, mycophenolate mofetil, and cyclophosphamide are alternative treatment options.1-4
Rituximab, a chimeric monoclonal antibody targeting B-cell surface marker CD20, results in depletion of mature B cells. Use of rituximab for LEP has been described in multiple case reports involving an 8-year-old boy, 22-year-old girl, and 2 middle-aged women.2-4 In addition, a recently published case series of 4 patients with childhood-onset refractory LEP described improvement of disease activity with rituximab.5 It is important to rule out subcutaneous T-cell lymphoma before treatment with rituximab, as its histopathology can closely resemble that seen in LEP and HLH-associated cytophagic histiocytic panniculitis.1,6
Rituximab may be an effective treatment option in pediatric patients with refractory LEP. Larger studies on the use of rituximab in the pediatric population are necessary.
- Weingartner JS, Zedek DC, Burkhart CN, et al. Lupus erythematosus panniculitis in children: report of three cases and review of previously reported cases. Pediatr Dermatol. 2011;29:169-176.
- Moreno-Suárez F, Pulpillo-Ruiz Á. Rituximab for the treatment of lupus erythematosus panniculitis. Dermatol Ther. 2013;26:415-418.
- Guissa VR, Trudes G, Jesus AA, et al. Lupus erythematosus panniculitis in children and adolescents. Acta Reumatol Port. 2012;37:82-85.
- Mcardle A, Baker JF. A case of “refractory” lupus erythematosus profundus responsive to rituximab. Clin Rheumatol. 2009;28:745-746.
- Correll CK, Miller DD, Maguiness SM. Treatment of childhood-onset lupus erythematosus panniculitis with rituximab. JAMA Dermatol. 2020;156:566-569.
- Aronson IK, Worobec SM. Cytophagic histiocytic panniculitis and hemophagocytic lymphohistiocytosis: an overview. Dermatol Ther. 2010;23:389-402.
- Weingartner JS, Zedek DC, Burkhart CN, et al. Lupus erythematosus panniculitis in children: report of three cases and review of previously reported cases. Pediatr Dermatol. 2011;29:169-176.
- Moreno-Suárez F, Pulpillo-Ruiz Á. Rituximab for the treatment of lupus erythematosus panniculitis. Dermatol Ther. 2013;26:415-418.
- Guissa VR, Trudes G, Jesus AA, et al. Lupus erythematosus panniculitis in children and adolescents. Acta Reumatol Port. 2012;37:82-85.
- Mcardle A, Baker JF. A case of “refractory” lupus erythematosus profundus responsive to rituximab. Clin Rheumatol. 2009;28:745-746.
- Correll CK, Miller DD, Maguiness SM. Treatment of childhood-onset lupus erythematosus panniculitis with rituximab. JAMA Dermatol. 2020;156:566-569.
- Aronson IK, Worobec SM. Cytophagic histiocytic panniculitis and hemophagocytic lymphohistiocytosis: an overview. Dermatol Ther. 2010;23:389-402.
Practice Points
- Lupus erythematosus panniculitis (LEP) is rare in the pediatric population and often is difficult to treat.
- Rituximab can be an effective treatment option for refractory LEP.
- Before the initiation of rituximab, a biopsy is warranted to rule out subcutaneous T-cell lymphoma, which can mimic LEP and hemophagocytic lymphohistiocytosis–associated panniculitis.
Active Comparator Trial Designs Used to Promote Development of Innovative New Medications
Spending on medications is expected to grow from $344 billion in 2018 to $420 billion in 2023, largely driven by the introduction of new branded drugs.1 These costs place substantial financial burden on patients, with nearly 30% of patients not taking their prescriptions as directed because of costs. Although many new medications have transformed how we care for patients, others may not offer meaningful benefit over existing less-costly alternatives that are supported by declining effect sizes of conventional placebo-controlled trials.2 Most medications are approved based on placebo-controlled trial data that does not include an arm comparing the new drug to standard of care, leaving clinicians and patients unable to make meaningful comparisons when deciding on the most appropriate or cost-effective treatment. We consider ways in which clinicians, patients, payers, and regulators could compel more meaningful trials from industry.
Although we often look to the US Food and Drug Administration (FDA) to ensure rigorous and appropriate testing of new medications, the primary mission of the FDA is to ensure efficacy and safety. As a result, pharmaceutical companies seeking approval in the United States have little incentive to go beyond providing the minimal level of evidence required: placebo-controlled randomized trials. Although these trials provide important data on whether a treatment works and its associated risks, they do not provide data on comparative effectiveness. When relevant inexpensive medications are already on the market for the same indication, these placebo-controlled trials provide inadequate evidence to guide clinical decision-making. This issue is particularly relevant in dermatology given how easily topical medications can be combined or reformulated to pursue additional market exclusivity. The addition of an active comparator arm represents an important opportunity to improve the value of these studies.
In the pivotal trials of clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel for the treatment of acne, the experimental group was not only compared to vehicle but also the active comparator arms of clindamycin alone and benzoyl peroxide alone. The mean percentage change in total lesions was 47.9% with clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel, 41.6% with the active comparator arm of benzoyl peroxide alone, 40.4% with the active comparator arm of clindamycin alone, and 26.2% for vehicle.3 With these data in mind, clinicians and patients can decide whether the additional benefit of this new product over benzoyl peroxide alone is worth the increased cost.
In contrast, the trials of dapsone gel 7.5% for the treatment of acne did not include an active comparator. The mean percentage change in total lesions was 48.9% for dapsone gel and 43.2% for vehicle.4 Given these data, it is possible that dapsone gel may be no more effective, or possibly less effective, than alternatives such as benzoyl peroxide or other topical antibiotics. Nevertheless, dapsone annual sales were more than $200 million in 2016,5 suggesting that effectively marketed new products can achieve high sales even without convincing evidence of their value compared to standard of care. Although dapsone may be a useful treatment, we cannot effectively make patient-centered clinical decisions given the lack of an active comparator trial design.
This issue is not limited to acne. Phase 3 trials of halobetasol propionate foam 0.05% for psoriasis and crisaborole for atopic dermatitis also did not include an active comparator arm.6,7 Given that topical steroids—and calcineurin inhibitors for atopic dermatitis—are mainstays of treatment for each condition, it is difficult to determine whether these new treatments offer meaningful advantages over existing options and how to incorporate them into our management strategies.
Unfortunately, expensive new medications that are adopted without convincing evidence of their benefit above standard of care can put patients at risk for financial toxicity, either directly through higher out-of-pocket costs or indirectly through higher premiums. Given the impact of rising medication costs on clinicians, patients, and payers, we propose several approaches these stakeholders could adopt to encourage the use of active comparator trial designs.
Clinicians and patients can encourage these trials by remaining skeptical of new treatments that were only compared to vehicle or placebo. Because new medications often are more expensive, clinicians and patients could avoid using these treatments without evidence of either increased efficacy or improved safety and tolerability. In addition, health care institutions should consider reducing pharmaceutical representatives’ access to clinicians to encourage treatment decisions based on the published literature and comparative effectiveness data rather than marketing.
Payers, such as Medicare, also could play a role by requiring active comparator trials for coverage of new medications, particularly when there are already other effective treatments available or other medications in the same class. Payers also could give preferred coverage tier or step therapy status to medications that demonstrate value over existing options.
Although regulatory approaches to increase use of active comparator designs may be more politically challenging to introduce, these options would be more administratively robust. The FDA or a novel regulatory body could require that new treatments demonstrate value in addition to safety and efficacy. This approach would be similar to the role of The National Institute for Health and Care Excellence in the United Kingdom or the recommendations of the European Medicines Agency. Such a group also could provide independent adjudication to ensure appropriate selection of a relevant active comparator. Another approach would be to give extended market exclusivity to medications that are approved based on trials including an additional active comparator arm, an approach used by the European Medicines Agency.
Any approach that encourages increased use of active comparator trials is not without potential downsides. It will be important to avoid unintended consequences of reduced research for rare diseases with smaller markets that may not be able to support the increased cost of these trials. As a result, it would be reasonable to forgo active comparator designs for mediations indicated for rare and orphan diseases or for medications with novel mechanisms of action.
Another argument against including an active comparator arm is that it may stifle innovation by driving up the cost of conducting trials; however, if a product is so marginally innovative that it cannot demonstrate superior safety or efficacy to an existing product, such a new treatment may not be worth the increased cost. In addition, patients provide a notable contribution by participating in these trials, and it is important to ensure that their efforts result in the highest-quality data possible. Furthermore, given the adverse physical and psychosocial impact of a wide variety of dermatologic diseases, the inclusion of an active comparator arm reduces the likelihood that patients will receive placebo, which will make these trials more ethical when effective treatments are available.8 By raising the bar, we can encourage pharmaceutical companies to pursue novel approaches that are more likely to have a revolutionary impact rather than minor modifications or formulations that offer little to no benefit at substantially increased cost.
Although some recent clinical trials in dermatology have included active comparators, many new medications continue to be introduced without any evidence of how they compare to existing standards of care. Until clinicians, patients, payers, and regulators demand that pharmaceutical companies conduct the necessary trials to not only demonstrate whether a treatment is effective and safe but also how it provides value, there will be continued introduction of marginal innovations rather than revolutionary treatments that improve patients’ lives. The next time a new medication is approved, as clinicians, patients, and payers, we must ask ourselves, is this treatment worth it?
- Aitken M, Kleinrock M. Medicine Use and Spending in the U.S.: A Review of 2018 and Outlook to 2023. IQVIA Institute for Human Data Science. https://www.iqvia.com/insights/the-iqvia-institute/reports/medicine-use-and-spending-in-the-us-a-review-of-2018-and-outlook-to-2023. Published May 9, 2019. Accessed August 15, 2020.
- Olfson M, Marcus SC. Decline in placebo-controlled trial results suggests new directions for comparative effectiveness research. Health Aff Proj Hope. 2019;32:1116-1125.
- Thiboutot D, Zaenglein A, Weiss J, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol. 2008;59:792-800.
- Eichenfield LF, Lain T, Frankel EH, et al. Efficacy and safety of once-daily dapsone gel, 7.5% for treatment of adolescents and adults with acne vulgaris: second of two identically designed, large, multicenter, randomized, vehicle-controlled trials. J Drugs Dermatol. 2016;15:962-969.
- Allergan. 2017 Form 10-K. https://www.abbvie.com/content/dam/abbvie-dotcom/uploads/PDFs/allergan/allergan-annual-report-form-10K-123117.pdf. Accessed August 19, 2020.
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75:494-503.e6.
- Bhatia N, Stein Gold L, Kircik LH, et al. Two multicenter, randomized, double-blind, parallel group comparison studies of a novel foam formulation of halobetasol propionate, 0.05% vs its vehicle in adult subjects with plaque psoriasis. J Drugs Dermatol. 2019;18:790-796.
- Temple R, Ellenberg SS. Placebo-controlled trials and active-control trials in the evaluation of new treatments. part 1: ethical and scientific issues. Ann Intern Med. 2000;133:455-463.
Spending on medications is expected to grow from $344 billion in 2018 to $420 billion in 2023, largely driven by the introduction of new branded drugs.1 These costs place substantial financial burden on patients, with nearly 30% of patients not taking their prescriptions as directed because of costs. Although many new medications have transformed how we care for patients, others may not offer meaningful benefit over existing less-costly alternatives that are supported by declining effect sizes of conventional placebo-controlled trials.2 Most medications are approved based on placebo-controlled trial data that does not include an arm comparing the new drug to standard of care, leaving clinicians and patients unable to make meaningful comparisons when deciding on the most appropriate or cost-effective treatment. We consider ways in which clinicians, patients, payers, and regulators could compel more meaningful trials from industry.
Although we often look to the US Food and Drug Administration (FDA) to ensure rigorous and appropriate testing of new medications, the primary mission of the FDA is to ensure efficacy and safety. As a result, pharmaceutical companies seeking approval in the United States have little incentive to go beyond providing the minimal level of evidence required: placebo-controlled randomized trials. Although these trials provide important data on whether a treatment works and its associated risks, they do not provide data on comparative effectiveness. When relevant inexpensive medications are already on the market for the same indication, these placebo-controlled trials provide inadequate evidence to guide clinical decision-making. This issue is particularly relevant in dermatology given how easily topical medications can be combined or reformulated to pursue additional market exclusivity. The addition of an active comparator arm represents an important opportunity to improve the value of these studies.
In the pivotal trials of clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel for the treatment of acne, the experimental group was not only compared to vehicle but also the active comparator arms of clindamycin alone and benzoyl peroxide alone. The mean percentage change in total lesions was 47.9% with clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel, 41.6% with the active comparator arm of benzoyl peroxide alone, 40.4% with the active comparator arm of clindamycin alone, and 26.2% for vehicle.3 With these data in mind, clinicians and patients can decide whether the additional benefit of this new product over benzoyl peroxide alone is worth the increased cost.
In contrast, the trials of dapsone gel 7.5% for the treatment of acne did not include an active comparator. The mean percentage change in total lesions was 48.9% for dapsone gel and 43.2% for vehicle.4 Given these data, it is possible that dapsone gel may be no more effective, or possibly less effective, than alternatives such as benzoyl peroxide or other topical antibiotics. Nevertheless, dapsone annual sales were more than $200 million in 2016,5 suggesting that effectively marketed new products can achieve high sales even without convincing evidence of their value compared to standard of care. Although dapsone may be a useful treatment, we cannot effectively make patient-centered clinical decisions given the lack of an active comparator trial design.
This issue is not limited to acne. Phase 3 trials of halobetasol propionate foam 0.05% for psoriasis and crisaborole for atopic dermatitis also did not include an active comparator arm.6,7 Given that topical steroids—and calcineurin inhibitors for atopic dermatitis—are mainstays of treatment for each condition, it is difficult to determine whether these new treatments offer meaningful advantages over existing options and how to incorporate them into our management strategies.
Unfortunately, expensive new medications that are adopted without convincing evidence of their benefit above standard of care can put patients at risk for financial toxicity, either directly through higher out-of-pocket costs or indirectly through higher premiums. Given the impact of rising medication costs on clinicians, patients, and payers, we propose several approaches these stakeholders could adopt to encourage the use of active comparator trial designs.
Clinicians and patients can encourage these trials by remaining skeptical of new treatments that were only compared to vehicle or placebo. Because new medications often are more expensive, clinicians and patients could avoid using these treatments without evidence of either increased efficacy or improved safety and tolerability. In addition, health care institutions should consider reducing pharmaceutical representatives’ access to clinicians to encourage treatment decisions based on the published literature and comparative effectiveness data rather than marketing.
Payers, such as Medicare, also could play a role by requiring active comparator trials for coverage of new medications, particularly when there are already other effective treatments available or other medications in the same class. Payers also could give preferred coverage tier or step therapy status to medications that demonstrate value over existing options.
Although regulatory approaches to increase use of active comparator designs may be more politically challenging to introduce, these options would be more administratively robust. The FDA or a novel regulatory body could require that new treatments demonstrate value in addition to safety and efficacy. This approach would be similar to the role of The National Institute for Health and Care Excellence in the United Kingdom or the recommendations of the European Medicines Agency. Such a group also could provide independent adjudication to ensure appropriate selection of a relevant active comparator. Another approach would be to give extended market exclusivity to medications that are approved based on trials including an additional active comparator arm, an approach used by the European Medicines Agency.
Any approach that encourages increased use of active comparator trials is not without potential downsides. It will be important to avoid unintended consequences of reduced research for rare diseases with smaller markets that may not be able to support the increased cost of these trials. As a result, it would be reasonable to forgo active comparator designs for mediations indicated for rare and orphan diseases or for medications with novel mechanisms of action.
Another argument against including an active comparator arm is that it may stifle innovation by driving up the cost of conducting trials; however, if a product is so marginally innovative that it cannot demonstrate superior safety or efficacy to an existing product, such a new treatment may not be worth the increased cost. In addition, patients provide a notable contribution by participating in these trials, and it is important to ensure that their efforts result in the highest-quality data possible. Furthermore, given the adverse physical and psychosocial impact of a wide variety of dermatologic diseases, the inclusion of an active comparator arm reduces the likelihood that patients will receive placebo, which will make these trials more ethical when effective treatments are available.8 By raising the bar, we can encourage pharmaceutical companies to pursue novel approaches that are more likely to have a revolutionary impact rather than minor modifications or formulations that offer little to no benefit at substantially increased cost.
Although some recent clinical trials in dermatology have included active comparators, many new medications continue to be introduced without any evidence of how they compare to existing standards of care. Until clinicians, patients, payers, and regulators demand that pharmaceutical companies conduct the necessary trials to not only demonstrate whether a treatment is effective and safe but also how it provides value, there will be continued introduction of marginal innovations rather than revolutionary treatments that improve patients’ lives. The next time a new medication is approved, as clinicians, patients, and payers, we must ask ourselves, is this treatment worth it?
Spending on medications is expected to grow from $344 billion in 2018 to $420 billion in 2023, largely driven by the introduction of new branded drugs.1 These costs place substantial financial burden on patients, with nearly 30% of patients not taking their prescriptions as directed because of costs. Although many new medications have transformed how we care for patients, others may not offer meaningful benefit over existing less-costly alternatives that are supported by declining effect sizes of conventional placebo-controlled trials.2 Most medications are approved based on placebo-controlled trial data that does not include an arm comparing the new drug to standard of care, leaving clinicians and patients unable to make meaningful comparisons when deciding on the most appropriate or cost-effective treatment. We consider ways in which clinicians, patients, payers, and regulators could compel more meaningful trials from industry.
Although we often look to the US Food and Drug Administration (FDA) to ensure rigorous and appropriate testing of new medications, the primary mission of the FDA is to ensure efficacy and safety. As a result, pharmaceutical companies seeking approval in the United States have little incentive to go beyond providing the minimal level of evidence required: placebo-controlled randomized trials. Although these trials provide important data on whether a treatment works and its associated risks, they do not provide data on comparative effectiveness. When relevant inexpensive medications are already on the market for the same indication, these placebo-controlled trials provide inadequate evidence to guide clinical decision-making. This issue is particularly relevant in dermatology given how easily topical medications can be combined or reformulated to pursue additional market exclusivity. The addition of an active comparator arm represents an important opportunity to improve the value of these studies.
In the pivotal trials of clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel for the treatment of acne, the experimental group was not only compared to vehicle but also the active comparator arms of clindamycin alone and benzoyl peroxide alone. The mean percentage change in total lesions was 47.9% with clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel, 41.6% with the active comparator arm of benzoyl peroxide alone, 40.4% with the active comparator arm of clindamycin alone, and 26.2% for vehicle.3 With these data in mind, clinicians and patients can decide whether the additional benefit of this new product over benzoyl peroxide alone is worth the increased cost.
In contrast, the trials of dapsone gel 7.5% for the treatment of acne did not include an active comparator. The mean percentage change in total lesions was 48.9% for dapsone gel and 43.2% for vehicle.4 Given these data, it is possible that dapsone gel may be no more effective, or possibly less effective, than alternatives such as benzoyl peroxide or other topical antibiotics. Nevertheless, dapsone annual sales were more than $200 million in 2016,5 suggesting that effectively marketed new products can achieve high sales even without convincing evidence of their value compared to standard of care. Although dapsone may be a useful treatment, we cannot effectively make patient-centered clinical decisions given the lack of an active comparator trial design.
This issue is not limited to acne. Phase 3 trials of halobetasol propionate foam 0.05% for psoriasis and crisaborole for atopic dermatitis also did not include an active comparator arm.6,7 Given that topical steroids—and calcineurin inhibitors for atopic dermatitis—are mainstays of treatment for each condition, it is difficult to determine whether these new treatments offer meaningful advantages over existing options and how to incorporate them into our management strategies.
Unfortunately, expensive new medications that are adopted without convincing evidence of their benefit above standard of care can put patients at risk for financial toxicity, either directly through higher out-of-pocket costs or indirectly through higher premiums. Given the impact of rising medication costs on clinicians, patients, and payers, we propose several approaches these stakeholders could adopt to encourage the use of active comparator trial designs.
Clinicians and patients can encourage these trials by remaining skeptical of new treatments that were only compared to vehicle or placebo. Because new medications often are more expensive, clinicians and patients could avoid using these treatments without evidence of either increased efficacy or improved safety and tolerability. In addition, health care institutions should consider reducing pharmaceutical representatives’ access to clinicians to encourage treatment decisions based on the published literature and comparative effectiveness data rather than marketing.
Payers, such as Medicare, also could play a role by requiring active comparator trials for coverage of new medications, particularly when there are already other effective treatments available or other medications in the same class. Payers also could give preferred coverage tier or step therapy status to medications that demonstrate value over existing options.
Although regulatory approaches to increase use of active comparator designs may be more politically challenging to introduce, these options would be more administratively robust. The FDA or a novel regulatory body could require that new treatments demonstrate value in addition to safety and efficacy. This approach would be similar to the role of The National Institute for Health and Care Excellence in the United Kingdom or the recommendations of the European Medicines Agency. Such a group also could provide independent adjudication to ensure appropriate selection of a relevant active comparator. Another approach would be to give extended market exclusivity to medications that are approved based on trials including an additional active comparator arm, an approach used by the European Medicines Agency.
Any approach that encourages increased use of active comparator trials is not without potential downsides. It will be important to avoid unintended consequences of reduced research for rare diseases with smaller markets that may not be able to support the increased cost of these trials. As a result, it would be reasonable to forgo active comparator designs for mediations indicated for rare and orphan diseases or for medications with novel mechanisms of action.
Another argument against including an active comparator arm is that it may stifle innovation by driving up the cost of conducting trials; however, if a product is so marginally innovative that it cannot demonstrate superior safety or efficacy to an existing product, such a new treatment may not be worth the increased cost. In addition, patients provide a notable contribution by participating in these trials, and it is important to ensure that their efforts result in the highest-quality data possible. Furthermore, given the adverse physical and psychosocial impact of a wide variety of dermatologic diseases, the inclusion of an active comparator arm reduces the likelihood that patients will receive placebo, which will make these trials more ethical when effective treatments are available.8 By raising the bar, we can encourage pharmaceutical companies to pursue novel approaches that are more likely to have a revolutionary impact rather than minor modifications or formulations that offer little to no benefit at substantially increased cost.
Although some recent clinical trials in dermatology have included active comparators, many new medications continue to be introduced without any evidence of how they compare to existing standards of care. Until clinicians, patients, payers, and regulators demand that pharmaceutical companies conduct the necessary trials to not only demonstrate whether a treatment is effective and safe but also how it provides value, there will be continued introduction of marginal innovations rather than revolutionary treatments that improve patients’ lives. The next time a new medication is approved, as clinicians, patients, and payers, we must ask ourselves, is this treatment worth it?
- Aitken M, Kleinrock M. Medicine Use and Spending in the U.S.: A Review of 2018 and Outlook to 2023. IQVIA Institute for Human Data Science. https://www.iqvia.com/insights/the-iqvia-institute/reports/medicine-use-and-spending-in-the-us-a-review-of-2018-and-outlook-to-2023. Published May 9, 2019. Accessed August 15, 2020.
- Olfson M, Marcus SC. Decline in placebo-controlled trial results suggests new directions for comparative effectiveness research. Health Aff Proj Hope. 2019;32:1116-1125.
- Thiboutot D, Zaenglein A, Weiss J, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol. 2008;59:792-800.
- Eichenfield LF, Lain T, Frankel EH, et al. Efficacy and safety of once-daily dapsone gel, 7.5% for treatment of adolescents and adults with acne vulgaris: second of two identically designed, large, multicenter, randomized, vehicle-controlled trials. J Drugs Dermatol. 2016;15:962-969.
- Allergan. 2017 Form 10-K. https://www.abbvie.com/content/dam/abbvie-dotcom/uploads/PDFs/allergan/allergan-annual-report-form-10K-123117.pdf. Accessed August 19, 2020.
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75:494-503.e6.
- Bhatia N, Stein Gold L, Kircik LH, et al. Two multicenter, randomized, double-blind, parallel group comparison studies of a novel foam formulation of halobetasol propionate, 0.05% vs its vehicle in adult subjects with plaque psoriasis. J Drugs Dermatol. 2019;18:790-796.
- Temple R, Ellenberg SS. Placebo-controlled trials and active-control trials in the evaluation of new treatments. part 1: ethical and scientific issues. Ann Intern Med. 2000;133:455-463.
- Aitken M, Kleinrock M. Medicine Use and Spending in the U.S.: A Review of 2018 and Outlook to 2023. IQVIA Institute for Human Data Science. https://www.iqvia.com/insights/the-iqvia-institute/reports/medicine-use-and-spending-in-the-us-a-review-of-2018-and-outlook-to-2023. Published May 9, 2019. Accessed August 15, 2020.
- Olfson M, Marcus SC. Decline in placebo-controlled trial results suggests new directions for comparative effectiveness research. Health Aff Proj Hope. 2019;32:1116-1125.
- Thiboutot D, Zaenglein A, Weiss J, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol. 2008;59:792-800.
- Eichenfield LF, Lain T, Frankel EH, et al. Efficacy and safety of once-daily dapsone gel, 7.5% for treatment of adolescents and adults with acne vulgaris: second of two identically designed, large, multicenter, randomized, vehicle-controlled trials. J Drugs Dermatol. 2016;15:962-969.
- Allergan. 2017 Form 10-K. https://www.abbvie.com/content/dam/abbvie-dotcom/uploads/PDFs/allergan/allergan-annual-report-form-10K-123117.pdf. Accessed August 19, 2020.
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75:494-503.e6.
- Bhatia N, Stein Gold L, Kircik LH, et al. Two multicenter, randomized, double-blind, parallel group comparison studies of a novel foam formulation of halobetasol propionate, 0.05% vs its vehicle in adult subjects with plaque psoriasis. J Drugs Dermatol. 2019;18:790-796.
- Temple R, Ellenberg SS. Placebo-controlled trials and active-control trials in the evaluation of new treatments. part 1: ethical and scientific issues. Ann Intern Med. 2000;133:455-463.
Practice Points
- When evaluating a new treatment, it is important to consider not only whether it is effective but also whether it provides additional value compared to existing treatment options.
- Encouraging active comparator trials will provide clinicians and patients with important data to guide decision-making regarding the most appropriate treatment options.
Concordance Between Dermatologist Self-reported and Industry-Reported Interactions at a National Dermatology Conference
Interactions between industry and physicians, including dermatologists, are widely prevalent.1-3 Proper reporting of industry relationships is essential for transparency, objectivity, and management of potential biases and conflicts of interest. There has been increasing public scrutiny regarding these interactions.
The Physician Payments Sunshine Act established Open Payments (OP), a publicly available database that collects and displays industry-reported physician-industry interactions.4,5 For the medical community and public, the OP database may be used to assess transparency by comparing the data with physician self-disclosures. There is a paucity of studies in the literature examining the concordance of industry-reported disclosures and physician self-reported data, with even fewer studies utilizing OP as a source of industry disclosures, and none exists for dermatology.6-12 It also is not clear to what extent the OP database captures all possible dermatologist-industry interactions, as the Sunshine Act only mandates reporting by applicable US-based manufacturers and group purchasing organizations that produce or purchase drugs or devices that require a prescription and are reimbursable by a government-run health care program.5 As a result, certain companies, such as cosmeceuticals, may not be represented.
In this study we aimed to evaluate the concordance of dermatologist self-disclosure of industry relationships and those reported on OP. Specifically, we focused on interactions disclosed by presenters at the American Academy of Dermatology (AAD) 73rd Annual Meeting in San Francisco, California (March 20–24, 2015), and those by industry in the 2014 OP database.
Methods
In this retrospective cohort study, we compared publicly available data from the OP database to presenter disclosures found in the publicly available AAD 73rd Annual Meeting program (AADMP). The AAD required speakers to disclose financial relationships with industry within the 12 months preceding the presentation, as outlined in the Accreditation Council for Continuing Medical Education guidelines.13 All AAD presenters who were dermatologists practicing in the United States were included in the analysis, whereas residents, fellows, nonphysicians, nondermatologist physicians, and international dermatologists were excluded.
We examined general, research, and associated research payments to specific dermatologists using the 2014 OP data, which contained industry payments made between January 1 and December 31, 2014. Open Payments defined research payments as direct payment to the physician for different types of research activities and associated research payments as indirect payments made to a research institution or entity where the physician was named the principal investigator.5 We chose the 2014 database because it most closely matched the period of required disclosures defined by the AAD for the 2015 meeting. Our review of the OP data occurred after the June 2016 update and thus included the most accurate and up-to-date financial interactions.
We conducted our analysis in 2 major steps. First, we determined whether each industry interaction reported in the OP database was present in the AADMP, which provided an assessment of interaction-level concordance. Second, we determined whether all the industry interactions for any given dermatologist listed in the OP also were present in AADMP, which provided an assessment of dermatologist-level concordance.
First, to establish interaction-level concordance for each industry interaction, the company name and the type of interaction (eg, consultant, speaker, investigator) listed in the AADMP were compared with the data in OP to verify a match. Each interaction was assigned into one of the categories of concordant disclosure (a match of both the company name and type of interaction details in OP and the AADMP), overdisclosure (the presence of an AADMP interaction not found in OP, such as an additional type of interaction or company), or underdisclosure (a company name or type of interaction found in OP but not reported in the AADMP). For underdisclosure, we further classified into company present or company absent based on whether the dermatologist disclosed any relationship with a particular company in the AADMP. We considered the type of interaction to be matching if they were identical or similar in nature (eg, consulting in OP and advisory board in the AADMP), as the types of interactions are reported differently in OP and the AADMP. Otherwise, if they were not similar enough (eg, education in OP and stockholder in the AADMP), it was classified as underdisclosure. Some types of interactions reported in OP were not available on the AAD disclosure form. For example, food and beverage as well as travel and lodging were types of interactions in OP that did not exist in the AADMP. These 2 types of interactions comprised a large majority of OP payment entries but only accounted for a small percentage of the payment amount. Analysis was performed both including and excluding interactions for food, beverage, travel, and lodging (f/b/t/l) to best account for differences in interaction categories between OP and the AADMP.
Second, each dermatologist was assigned to an overall disclosure category of dermatologist-level concordance based on the status for all his/her interactions. Categories included no disclosure (no industry interactions in OP and the AADMP), concordant (all industry interactions reported in OP and the AADMP match), overdisclosure only (no industry interactions on OP but self-reported interactions present in the AADMP), and discordant (not all OP interactions were disclosed in the AADMP). The discordant category was further divided into with overdisclosure and without overdisclosure, depending on the presence or absence of industry relationships listed in the AADMP but not in OP, respectively.
To ensure uniformity, one individual (A.F.S.) reviewed and collected the data from OP and the AADMP. Information on gender and academic affiliation of study participants was obtained from information listed in the AADMP and Google searches. Data management was performed with Microsoft Excel software (Microsoft Excel 2010, Version 14.0, Microsoft Corporation). The New York University School of Medicine’s (New York, New York) institutional review board exempted this study.
Results
Of the 938 presenters listed in the AADMP, 768 individuals met the inclusion criteria. The most commonly cited type of relationship with industry listed in the AADMP was serving as an investigator, consultant, or advisory board member, comprising 34%, 26%, and 18%, respectively (Table 1). The forms of payment most frequently reported in the AADMP were honoraria and grants/research funding, comprising 49% and 25%, respectively (Table 2).
In 2014, there were a total of 20,761 industry payments totaling $35,627,365 for general, research, and associated research payments in the OP database related to the dermatologists who met inclusion criteria. There were 8678 payments totaling $466,622 for food and beverage and 3238 payments totaling $1,357,770 for travel and lodging. After excluding payments for f/b/t/l, there were 8845 payments totaling $33,802,973, with highest percentages of payment amounts for associated research (67.1%), consulting fees (11.5%), research (7.9%), and speaker fees (7.2%)(Table 3). For presenters with industry payments, the range of disbursements excluding f/b/t/l was $6.52 to $1,933,705, with a mean (standard deviation) of $107,997 ($249,941), a median of $18,247, and an interquartile range of $3422 to $97,375 (data not shown).
In assessing interaction-level concordance, 63% of all payment amounts in OP were classified as concordant disclosures. Regarding the number of OP payments, 27% were concordant disclosures, 34% were underdisclosures due to f/b/t/l payments, and 39% were underdisclosures due to non–f/b/t/l payments. When f/b/t/l payment entries in OP were excluded, the status of concordant disclosure for the amount and number of OP payments increased to 66% ($22,242,638) and 63% (5549), respectively. The percentage of payment entries with concordant disclosure status ranged from 43% to 71% depending on the payment amount. Payment entries at both ends of the spectrum had the lowest concordant disclosure rates, with 43% for payment entries between $0.01 and $100 and 58% for entries greater than $100,000 (Table 4). The concordance status also differed by the type of interactions. None of the OP payments for gift and royalty or license were disclosed in the AADMP, as there were no suitable corresponding categories. The proportion of payments with concordant disclosure for honoraria (45%), education (48%), and associated research (61%) was lower than the proportion of payments with concordant disclosure for research (90%), speaker fees (75%–79%), and consulting fees (74%)(Table 5).
In assessing dermatologist-level concordance including all OP entries, the number of dermatologists with no disclosure, overdisclosure only, concordant disclosure, discordant with overdisclosure, and discordant without overdisclosure statuses were 234 (30%), 70 (9%), 9 (1%), 251 (33%), and 204 (27%), respectively. When f/b/t/l entries were excluded, those figures changed to 347 (45%), 108 (14%), 79 (10%), 157 (20%), and 77 (10%), respectively. The characteristics of these dermatologists and their associated industry interactions by disclosure status are shown in the eTable. Dermatologists in the discordant with overdisclosure group had the highest median number and amount of OP payments, followed by those in the concordant disclosure and discordant without overdisclosure groups. Additionally, discordant with overdisclosure dermatologists also had the highest median and mean number of unique industry interactions not on OP, followed by those in the overdisclosure only and no disclosure groups. Academic and private practice settings did not impact dermatologists’ disclosure status. The percentage of female and male dermatologists in the discordant group was 25% and 36%, respectively.
Dermatologists reported a total of 1756 unique industry relationships in the AADMP that were not found on OP. Of these, 1440 (82%) relationships were from 236 dermatologists who had industry payments on OP. The remaining 316 relationships were from 108 dermatologists who had no payments on OP. Although 114 companies reported payments to dermatologists on OP, dermatologists in the AADMP reported interactions with an additional 430 companies.
Comment
In this study, we demonstrated discordance between dermatologist self-reported financial interactions in the AADMP compared with those reported by industry via OP. After excluding f/b/t/l entries, approximately two-thirds of the total amount and number of payments in OP were disclosed, while 31% of dermatologists had discordant disclosure status.
Prior investigations in other medical fields showed high discrepancy rates between industry-reported and physician-reported relationships ranging from 23% to 62%, with studies utilizing various methodologies.6-9,11,12,14,15 Only a few studies have utilized the OP database.8,12,15 Thompson et al12 compared OP payment data with physician financial disclosure at an annual gynecology scientific meeting and found although 209 of 335 (62%) physicians had interactions listed in the OP database, only 24 (7%) listed at least 1 company in the meeting financial disclosure section. Of these 24 physicians, only 5 (21%) accurately disclosed financial relationships with all of the companies listed in OP. The investigators found 129 (38.5%) physicians and 33.7% of the $1.99 million OP payments had concordant disclosure status. When they excluded physicians who received less than $100, 53% of individuals had concordant disclosure.12 Hannon et al8 reported on inconsistencies between disclosures in the OP database and the American Academy of Orthopedic Surgeons Annual Meeting and found 259 (23%) of 1113 physicians meeting inclusion criteria had financial interactions listed in the OP database that were not reported in the meeting disclosures. Yee et al15 also utilized the OP database and compared it with author disclosures in 3 major ophthalmology journals.Of 670 authors, 367 (54.8%) had complete concordance, with 68 (10.1%) more reporting additional overdisclosures, leading to a discordant with underdisclosure rate of 35.1%. Additionally, $1.46 million (44.6%) of the $3.27 million OP payments had concordant disclosure status.15 Other studies compared individual companies’ online reports of physician payments with physician self-disclosures in annual meeting programs, clinical guidelines, and peer-reviewed publications.6,7,9,11,14
Our study demonstrated variation in disclosure status. Compared with other groups, dermatologists in the discordant with overdisclosure group on average had more interactions with and received higher payments from industry, which is consistent with studies in the orthopedic surgery literature.8,9 Male dermatologists had 11% more discordant disclosures than their female counterparts, which may be influenced by men having more industry interactions than women.3 Although small industry payments possessed the lowest concordant rate in our study, which has been observed,12 payments greater than $100,000 had the second-lowest concordance rate at 58%, which may be skewed by the small sample size. Rates of concordant disclosure differed among types of interactions, such as between research and associated research payments. This particular difference may be attributed to the incorrect listing of dermatologists as principal investigators or reduced awareness of payments, as associated research payments were made to the institution and not the individual.
Reasons for discrepancies between industry-reported and dermatologist-reported disclosures may include reporting time differences, lack of physician awareness of OP, industry reporting inaccuracies, dearth of contextual information associated with individual payment entries, and misunderstandings. Prior research demonstrated that the most common reasons for physician nondisclosure included misunderstanding disclosure requirements, unintentional omission of payment, and a lack of relationship between the industry payment and presentation topic.9,12 These factors likely contributed to the disclosure inconsistencies in our study. Similarly high rates of inconsistencies across different specialties suggest systemic concerns.
We found a substantial number of dermatologist-industry interactions listed in the AADMP that were not captured by OP, with 108 dermatologists (35%) having overdisclosures even when excluding f/b/t/l entries. The number of companies in these overdisclosures approximated 4 times the number of companies on OP. Other studies have also observed physician-industry interactions not displayed on OP.8,12,15 Because the Sunshine Act requires reporting only by certain companies, interactions surrounding products such as over-the-counter merchandise, cosmetics, lasers, novel devices, and new medications are generally not included. Further, OP may not capture nonmonetary industry relationships.
There were several limitations to this study. The most notable limitation was the differences in the categorizations of industry relationships by OP and the AADMP. These differences can overemphasize some types of interactions at the expense of other types, such as f/b/t/l. As such, analyses were repeated after excluding f/b/t/l. Another limitation was the inexact overlap of time frames for OP and the AADMP, which may have led to discrepancies. However, we used the best available data and expect the vast majority of interactions to have occurred by the AAD disclosure deadline. It is possible that the presenters may have had a more updated conflict-of-interest disclosure slide at the time of the meeting presentation. The most important limitation was that we were unable to determine whether discrepancies resulted from underreporting by dermatologists or inaccurate reporting by industry. It was unlikely that OP or the AADMP alone completely represented all dermatologist-industry financial relationships.
Conclusion
With a growing emphasis on physician-industry transparency, we identified rates of differences in dermatology consistent with those in other medical fields when comparing the publicly available OP database with disclosures at national conferences. Although the differences in the categorization and requirements for disclosure between the OP database and the AADMP may account for some of the discordance, dermatologists should be aware of potentially negative public perceptions regarding the transparency and prevalence of physician-industry interactions.
Acknowledgment
The first two authors contributed equally to this research/article
- Campbell EG, Gruen RL, Mountford J, et al. A national survey of physician-industry relationships. N Engl J Med. 2007;356:1742-1750.
- Marshall DC, Jackson ME, Hattangadi-Gluth JA. Disclosure of industry payments to physicians: an epidemiologic analysis of early data from the open payments program. Mayo Clin Proc. 2016;91:84-96.
- Feng H, Wu P, Leger M. Exploring the industry-dermatologist financial relationship: insight from the open payment data. JAMA Dermatol. 2016;152:1307-1313.
- Kirschner NM, Sulmasy LS, Kesselheim AS. Health policy basics: the physician payment Sunshine Act and the open payments program. Ann Intern Med. 2014;161:519-521.
- Search Open Payment. Centers for Medicare & Medicaid Services. https://openpaymentsdata.cms.gov. Accessed October 21, 2019.
- Buerba RA, Fu MC, Grauer JN. Discrepancies in spine surgeon conflict of interest disclosures between a national meeting and physician payment listings on device manufacturer web sites. Spine J. 2013;13:1780-1788.
- Chimonas S, Frosch Z, Rothman DJ. From disclosure to transparency: the use of company payment data. Arch Intern Med. 2011;171:81-86.
- Hannon CP, Chalmers PN, Carpiniello MF, et al. Inconsistencies between physician-reported disclosures at the AAOS annual meeting and industry-reported financial disclosures in the open payments database. J Bone Joint Surg. 2016;98:E90.
- Okike K, Kocher MS, Wei EX, et al. Accuracy of conflict-of-interest disclosures reported by physicians. N Engl J Med. 2009;361:1466-1474.
- Ramm O, Brubaker L. Conflicts-of-interest disclosures at the 2010 AUGS Scientific Meeting. Female Pelvic Med Reconstr Surg. 2012;18:79-81.
- Tanzer D, Smith K, Tanzer M. American Academy of Orthopaedic Surgeons disclosure policy fails to accurately inform its members of potential conflicts of interest. Am J Orthop (Belle Mead NJ). 2015;44:E207-E210.
- Thompson JC, Volpe KA, Bridgewater LK, et al. Sunshine Act: shedding light on inaccurate disclosures at a gynecologic annual meeting. Am J Obstet Gynecol. 2016;215:661.
- Disclosure of Potential Conflicts of Interest. American Academy of Dermatology and AAD Association Web site. https://aad.org/Forms/Policies/Uploads/AR/
AR%20Disclosure%20of%20Potential%20Conflicts%
20of%20Interest-2.pdf. Accessed October 21, 2019. - Hockenberry JM, Weigel P, Auerbach A, et al. Financial payments by orthopedic device makers to orthopedic surgeons. Arch Intern Med. 2011;171:1759-1765.
- Yee C, Greenberg PB, Margo CE, et al. Financial disclosures in academic publications and the Sunshine Act: a concordance dtudy. Br J Med Med Res. 2015;10:1-6.
Interactions between industry and physicians, including dermatologists, are widely prevalent.1-3 Proper reporting of industry relationships is essential for transparency, objectivity, and management of potential biases and conflicts of interest. There has been increasing public scrutiny regarding these interactions.
The Physician Payments Sunshine Act established Open Payments (OP), a publicly available database that collects and displays industry-reported physician-industry interactions.4,5 For the medical community and public, the OP database may be used to assess transparency by comparing the data with physician self-disclosures. There is a paucity of studies in the literature examining the concordance of industry-reported disclosures and physician self-reported data, with even fewer studies utilizing OP as a source of industry disclosures, and none exists for dermatology.6-12 It also is not clear to what extent the OP database captures all possible dermatologist-industry interactions, as the Sunshine Act only mandates reporting by applicable US-based manufacturers and group purchasing organizations that produce or purchase drugs or devices that require a prescription and are reimbursable by a government-run health care program.5 As a result, certain companies, such as cosmeceuticals, may not be represented.
In this study we aimed to evaluate the concordance of dermatologist self-disclosure of industry relationships and those reported on OP. Specifically, we focused on interactions disclosed by presenters at the American Academy of Dermatology (AAD) 73rd Annual Meeting in San Francisco, California (March 20–24, 2015), and those by industry in the 2014 OP database.
Methods
In this retrospective cohort study, we compared publicly available data from the OP database to presenter disclosures found in the publicly available AAD 73rd Annual Meeting program (AADMP). The AAD required speakers to disclose financial relationships with industry within the 12 months preceding the presentation, as outlined in the Accreditation Council for Continuing Medical Education guidelines.13 All AAD presenters who were dermatologists practicing in the United States were included in the analysis, whereas residents, fellows, nonphysicians, nondermatologist physicians, and international dermatologists were excluded.
We examined general, research, and associated research payments to specific dermatologists using the 2014 OP data, which contained industry payments made between January 1 and December 31, 2014. Open Payments defined research payments as direct payment to the physician for different types of research activities and associated research payments as indirect payments made to a research institution or entity where the physician was named the principal investigator.5 We chose the 2014 database because it most closely matched the period of required disclosures defined by the AAD for the 2015 meeting. Our review of the OP data occurred after the June 2016 update and thus included the most accurate and up-to-date financial interactions.
We conducted our analysis in 2 major steps. First, we determined whether each industry interaction reported in the OP database was present in the AADMP, which provided an assessment of interaction-level concordance. Second, we determined whether all the industry interactions for any given dermatologist listed in the OP also were present in AADMP, which provided an assessment of dermatologist-level concordance.
First, to establish interaction-level concordance for each industry interaction, the company name and the type of interaction (eg, consultant, speaker, investigator) listed in the AADMP were compared with the data in OP to verify a match. Each interaction was assigned into one of the categories of concordant disclosure (a match of both the company name and type of interaction details in OP and the AADMP), overdisclosure (the presence of an AADMP interaction not found in OP, such as an additional type of interaction or company), or underdisclosure (a company name or type of interaction found in OP but not reported in the AADMP). For underdisclosure, we further classified into company present or company absent based on whether the dermatologist disclosed any relationship with a particular company in the AADMP. We considered the type of interaction to be matching if they were identical or similar in nature (eg, consulting in OP and advisory board in the AADMP), as the types of interactions are reported differently in OP and the AADMP. Otherwise, if they were not similar enough (eg, education in OP and stockholder in the AADMP), it was classified as underdisclosure. Some types of interactions reported in OP were not available on the AAD disclosure form. For example, food and beverage as well as travel and lodging were types of interactions in OP that did not exist in the AADMP. These 2 types of interactions comprised a large majority of OP payment entries but only accounted for a small percentage of the payment amount. Analysis was performed both including and excluding interactions for food, beverage, travel, and lodging (f/b/t/l) to best account for differences in interaction categories between OP and the AADMP.
Second, each dermatologist was assigned to an overall disclosure category of dermatologist-level concordance based on the status for all his/her interactions. Categories included no disclosure (no industry interactions in OP and the AADMP), concordant (all industry interactions reported in OP and the AADMP match), overdisclosure only (no industry interactions on OP but self-reported interactions present in the AADMP), and discordant (not all OP interactions were disclosed in the AADMP). The discordant category was further divided into with overdisclosure and without overdisclosure, depending on the presence or absence of industry relationships listed in the AADMP but not in OP, respectively.
To ensure uniformity, one individual (A.F.S.) reviewed and collected the data from OP and the AADMP. Information on gender and academic affiliation of study participants was obtained from information listed in the AADMP and Google searches. Data management was performed with Microsoft Excel software (Microsoft Excel 2010, Version 14.0, Microsoft Corporation). The New York University School of Medicine’s (New York, New York) institutional review board exempted this study.
Results
Of the 938 presenters listed in the AADMP, 768 individuals met the inclusion criteria. The most commonly cited type of relationship with industry listed in the AADMP was serving as an investigator, consultant, or advisory board member, comprising 34%, 26%, and 18%, respectively (Table 1). The forms of payment most frequently reported in the AADMP were honoraria and grants/research funding, comprising 49% and 25%, respectively (Table 2).
In 2014, there were a total of 20,761 industry payments totaling $35,627,365 for general, research, and associated research payments in the OP database related to the dermatologists who met inclusion criteria. There were 8678 payments totaling $466,622 for food and beverage and 3238 payments totaling $1,357,770 for travel and lodging. After excluding payments for f/b/t/l, there were 8845 payments totaling $33,802,973, with highest percentages of payment amounts for associated research (67.1%), consulting fees (11.5%), research (7.9%), and speaker fees (7.2%)(Table 3). For presenters with industry payments, the range of disbursements excluding f/b/t/l was $6.52 to $1,933,705, with a mean (standard deviation) of $107,997 ($249,941), a median of $18,247, and an interquartile range of $3422 to $97,375 (data not shown).
In assessing interaction-level concordance, 63% of all payment amounts in OP were classified as concordant disclosures. Regarding the number of OP payments, 27% were concordant disclosures, 34% were underdisclosures due to f/b/t/l payments, and 39% were underdisclosures due to non–f/b/t/l payments. When f/b/t/l payment entries in OP were excluded, the status of concordant disclosure for the amount and number of OP payments increased to 66% ($22,242,638) and 63% (5549), respectively. The percentage of payment entries with concordant disclosure status ranged from 43% to 71% depending on the payment amount. Payment entries at both ends of the spectrum had the lowest concordant disclosure rates, with 43% for payment entries between $0.01 and $100 and 58% for entries greater than $100,000 (Table 4). The concordance status also differed by the type of interactions. None of the OP payments for gift and royalty or license were disclosed in the AADMP, as there were no suitable corresponding categories. The proportion of payments with concordant disclosure for honoraria (45%), education (48%), and associated research (61%) was lower than the proportion of payments with concordant disclosure for research (90%), speaker fees (75%–79%), and consulting fees (74%)(Table 5).
In assessing dermatologist-level concordance including all OP entries, the number of dermatologists with no disclosure, overdisclosure only, concordant disclosure, discordant with overdisclosure, and discordant without overdisclosure statuses were 234 (30%), 70 (9%), 9 (1%), 251 (33%), and 204 (27%), respectively. When f/b/t/l entries were excluded, those figures changed to 347 (45%), 108 (14%), 79 (10%), 157 (20%), and 77 (10%), respectively. The characteristics of these dermatologists and their associated industry interactions by disclosure status are shown in the eTable. Dermatologists in the discordant with overdisclosure group had the highest median number and amount of OP payments, followed by those in the concordant disclosure and discordant without overdisclosure groups. Additionally, discordant with overdisclosure dermatologists also had the highest median and mean number of unique industry interactions not on OP, followed by those in the overdisclosure only and no disclosure groups. Academic and private practice settings did not impact dermatologists’ disclosure status. The percentage of female and male dermatologists in the discordant group was 25% and 36%, respectively.
Dermatologists reported a total of 1756 unique industry relationships in the AADMP that were not found on OP. Of these, 1440 (82%) relationships were from 236 dermatologists who had industry payments on OP. The remaining 316 relationships were from 108 dermatologists who had no payments on OP. Although 114 companies reported payments to dermatologists on OP, dermatologists in the AADMP reported interactions with an additional 430 companies.
Comment
In this study, we demonstrated discordance between dermatologist self-reported financial interactions in the AADMP compared with those reported by industry via OP. After excluding f/b/t/l entries, approximately two-thirds of the total amount and number of payments in OP were disclosed, while 31% of dermatologists had discordant disclosure status.
Prior investigations in other medical fields showed high discrepancy rates between industry-reported and physician-reported relationships ranging from 23% to 62%, with studies utilizing various methodologies.6-9,11,12,14,15 Only a few studies have utilized the OP database.8,12,15 Thompson et al12 compared OP payment data with physician financial disclosure at an annual gynecology scientific meeting and found although 209 of 335 (62%) physicians had interactions listed in the OP database, only 24 (7%) listed at least 1 company in the meeting financial disclosure section. Of these 24 physicians, only 5 (21%) accurately disclosed financial relationships with all of the companies listed in OP. The investigators found 129 (38.5%) physicians and 33.7% of the $1.99 million OP payments had concordant disclosure status. When they excluded physicians who received less than $100, 53% of individuals had concordant disclosure.12 Hannon et al8 reported on inconsistencies between disclosures in the OP database and the American Academy of Orthopedic Surgeons Annual Meeting and found 259 (23%) of 1113 physicians meeting inclusion criteria had financial interactions listed in the OP database that were not reported in the meeting disclosures. Yee et al15 also utilized the OP database and compared it with author disclosures in 3 major ophthalmology journals.Of 670 authors, 367 (54.8%) had complete concordance, with 68 (10.1%) more reporting additional overdisclosures, leading to a discordant with underdisclosure rate of 35.1%. Additionally, $1.46 million (44.6%) of the $3.27 million OP payments had concordant disclosure status.15 Other studies compared individual companies’ online reports of physician payments with physician self-disclosures in annual meeting programs, clinical guidelines, and peer-reviewed publications.6,7,9,11,14
Our study demonstrated variation in disclosure status. Compared with other groups, dermatologists in the discordant with overdisclosure group on average had more interactions with and received higher payments from industry, which is consistent with studies in the orthopedic surgery literature.8,9 Male dermatologists had 11% more discordant disclosures than their female counterparts, which may be influenced by men having more industry interactions than women.3 Although small industry payments possessed the lowest concordant rate in our study, which has been observed,12 payments greater than $100,000 had the second-lowest concordance rate at 58%, which may be skewed by the small sample size. Rates of concordant disclosure differed among types of interactions, such as between research and associated research payments. This particular difference may be attributed to the incorrect listing of dermatologists as principal investigators or reduced awareness of payments, as associated research payments were made to the institution and not the individual.
Reasons for discrepancies between industry-reported and dermatologist-reported disclosures may include reporting time differences, lack of physician awareness of OP, industry reporting inaccuracies, dearth of contextual information associated with individual payment entries, and misunderstandings. Prior research demonstrated that the most common reasons for physician nondisclosure included misunderstanding disclosure requirements, unintentional omission of payment, and a lack of relationship between the industry payment and presentation topic.9,12 These factors likely contributed to the disclosure inconsistencies in our study. Similarly high rates of inconsistencies across different specialties suggest systemic concerns.
We found a substantial number of dermatologist-industry interactions listed in the AADMP that were not captured by OP, with 108 dermatologists (35%) having overdisclosures even when excluding f/b/t/l entries. The number of companies in these overdisclosures approximated 4 times the number of companies on OP. Other studies have also observed physician-industry interactions not displayed on OP.8,12,15 Because the Sunshine Act requires reporting only by certain companies, interactions surrounding products such as over-the-counter merchandise, cosmetics, lasers, novel devices, and new medications are generally not included. Further, OP may not capture nonmonetary industry relationships.
There were several limitations to this study. The most notable limitation was the differences in the categorizations of industry relationships by OP and the AADMP. These differences can overemphasize some types of interactions at the expense of other types, such as f/b/t/l. As such, analyses were repeated after excluding f/b/t/l. Another limitation was the inexact overlap of time frames for OP and the AADMP, which may have led to discrepancies. However, we used the best available data and expect the vast majority of interactions to have occurred by the AAD disclosure deadline. It is possible that the presenters may have had a more updated conflict-of-interest disclosure slide at the time of the meeting presentation. The most important limitation was that we were unable to determine whether discrepancies resulted from underreporting by dermatologists or inaccurate reporting by industry. It was unlikely that OP or the AADMP alone completely represented all dermatologist-industry financial relationships.
Conclusion
With a growing emphasis on physician-industry transparency, we identified rates of differences in dermatology consistent with those in other medical fields when comparing the publicly available OP database with disclosures at national conferences. Although the differences in the categorization and requirements for disclosure between the OP database and the AADMP may account for some of the discordance, dermatologists should be aware of potentially negative public perceptions regarding the transparency and prevalence of physician-industry interactions.
Acknowledgment
The first two authors contributed equally to this research/article
Interactions between industry and physicians, including dermatologists, are widely prevalent.1-3 Proper reporting of industry relationships is essential for transparency, objectivity, and management of potential biases and conflicts of interest. There has been increasing public scrutiny regarding these interactions.
The Physician Payments Sunshine Act established Open Payments (OP), a publicly available database that collects and displays industry-reported physician-industry interactions.4,5 For the medical community and public, the OP database may be used to assess transparency by comparing the data with physician self-disclosures. There is a paucity of studies in the literature examining the concordance of industry-reported disclosures and physician self-reported data, with even fewer studies utilizing OP as a source of industry disclosures, and none exists for dermatology.6-12 It also is not clear to what extent the OP database captures all possible dermatologist-industry interactions, as the Sunshine Act only mandates reporting by applicable US-based manufacturers and group purchasing organizations that produce or purchase drugs or devices that require a prescription and are reimbursable by a government-run health care program.5 As a result, certain companies, such as cosmeceuticals, may not be represented.
In this study we aimed to evaluate the concordance of dermatologist self-disclosure of industry relationships and those reported on OP. Specifically, we focused on interactions disclosed by presenters at the American Academy of Dermatology (AAD) 73rd Annual Meeting in San Francisco, California (March 20–24, 2015), and those by industry in the 2014 OP database.
Methods
In this retrospective cohort study, we compared publicly available data from the OP database to presenter disclosures found in the publicly available AAD 73rd Annual Meeting program (AADMP). The AAD required speakers to disclose financial relationships with industry within the 12 months preceding the presentation, as outlined in the Accreditation Council for Continuing Medical Education guidelines.13 All AAD presenters who were dermatologists practicing in the United States were included in the analysis, whereas residents, fellows, nonphysicians, nondermatologist physicians, and international dermatologists were excluded.
We examined general, research, and associated research payments to specific dermatologists using the 2014 OP data, which contained industry payments made between January 1 and December 31, 2014. Open Payments defined research payments as direct payment to the physician for different types of research activities and associated research payments as indirect payments made to a research institution or entity where the physician was named the principal investigator.5 We chose the 2014 database because it most closely matched the period of required disclosures defined by the AAD for the 2015 meeting. Our review of the OP data occurred after the June 2016 update and thus included the most accurate and up-to-date financial interactions.
We conducted our analysis in 2 major steps. First, we determined whether each industry interaction reported in the OP database was present in the AADMP, which provided an assessment of interaction-level concordance. Second, we determined whether all the industry interactions for any given dermatologist listed in the OP also were present in AADMP, which provided an assessment of dermatologist-level concordance.
First, to establish interaction-level concordance for each industry interaction, the company name and the type of interaction (eg, consultant, speaker, investigator) listed in the AADMP were compared with the data in OP to verify a match. Each interaction was assigned into one of the categories of concordant disclosure (a match of both the company name and type of interaction details in OP and the AADMP), overdisclosure (the presence of an AADMP interaction not found in OP, such as an additional type of interaction or company), or underdisclosure (a company name or type of interaction found in OP but not reported in the AADMP). For underdisclosure, we further classified into company present or company absent based on whether the dermatologist disclosed any relationship with a particular company in the AADMP. We considered the type of interaction to be matching if they were identical or similar in nature (eg, consulting in OP and advisory board in the AADMP), as the types of interactions are reported differently in OP and the AADMP. Otherwise, if they were not similar enough (eg, education in OP and stockholder in the AADMP), it was classified as underdisclosure. Some types of interactions reported in OP were not available on the AAD disclosure form. For example, food and beverage as well as travel and lodging were types of interactions in OP that did not exist in the AADMP. These 2 types of interactions comprised a large majority of OP payment entries but only accounted for a small percentage of the payment amount. Analysis was performed both including and excluding interactions for food, beverage, travel, and lodging (f/b/t/l) to best account for differences in interaction categories between OP and the AADMP.
Second, each dermatologist was assigned to an overall disclosure category of dermatologist-level concordance based on the status for all his/her interactions. Categories included no disclosure (no industry interactions in OP and the AADMP), concordant (all industry interactions reported in OP and the AADMP match), overdisclosure only (no industry interactions on OP but self-reported interactions present in the AADMP), and discordant (not all OP interactions were disclosed in the AADMP). The discordant category was further divided into with overdisclosure and without overdisclosure, depending on the presence or absence of industry relationships listed in the AADMP but not in OP, respectively.
To ensure uniformity, one individual (A.F.S.) reviewed and collected the data from OP and the AADMP. Information on gender and academic affiliation of study participants was obtained from information listed in the AADMP and Google searches. Data management was performed with Microsoft Excel software (Microsoft Excel 2010, Version 14.0, Microsoft Corporation). The New York University School of Medicine’s (New York, New York) institutional review board exempted this study.
Results
Of the 938 presenters listed in the AADMP, 768 individuals met the inclusion criteria. The most commonly cited type of relationship with industry listed in the AADMP was serving as an investigator, consultant, or advisory board member, comprising 34%, 26%, and 18%, respectively (Table 1). The forms of payment most frequently reported in the AADMP were honoraria and grants/research funding, comprising 49% and 25%, respectively (Table 2).
In 2014, there were a total of 20,761 industry payments totaling $35,627,365 for general, research, and associated research payments in the OP database related to the dermatologists who met inclusion criteria. There were 8678 payments totaling $466,622 for food and beverage and 3238 payments totaling $1,357,770 for travel and lodging. After excluding payments for f/b/t/l, there were 8845 payments totaling $33,802,973, with highest percentages of payment amounts for associated research (67.1%), consulting fees (11.5%), research (7.9%), and speaker fees (7.2%)(Table 3). For presenters with industry payments, the range of disbursements excluding f/b/t/l was $6.52 to $1,933,705, with a mean (standard deviation) of $107,997 ($249,941), a median of $18,247, and an interquartile range of $3422 to $97,375 (data not shown).
In assessing interaction-level concordance, 63% of all payment amounts in OP were classified as concordant disclosures. Regarding the number of OP payments, 27% were concordant disclosures, 34% were underdisclosures due to f/b/t/l payments, and 39% were underdisclosures due to non–f/b/t/l payments. When f/b/t/l payment entries in OP were excluded, the status of concordant disclosure for the amount and number of OP payments increased to 66% ($22,242,638) and 63% (5549), respectively. The percentage of payment entries with concordant disclosure status ranged from 43% to 71% depending on the payment amount. Payment entries at both ends of the spectrum had the lowest concordant disclosure rates, with 43% for payment entries between $0.01 and $100 and 58% for entries greater than $100,000 (Table 4). The concordance status also differed by the type of interactions. None of the OP payments for gift and royalty or license were disclosed in the AADMP, as there were no suitable corresponding categories. The proportion of payments with concordant disclosure for honoraria (45%), education (48%), and associated research (61%) was lower than the proportion of payments with concordant disclosure for research (90%), speaker fees (75%–79%), and consulting fees (74%)(Table 5).
In assessing dermatologist-level concordance including all OP entries, the number of dermatologists with no disclosure, overdisclosure only, concordant disclosure, discordant with overdisclosure, and discordant without overdisclosure statuses were 234 (30%), 70 (9%), 9 (1%), 251 (33%), and 204 (27%), respectively. When f/b/t/l entries were excluded, those figures changed to 347 (45%), 108 (14%), 79 (10%), 157 (20%), and 77 (10%), respectively. The characteristics of these dermatologists and their associated industry interactions by disclosure status are shown in the eTable. Dermatologists in the discordant with overdisclosure group had the highest median number and amount of OP payments, followed by those in the concordant disclosure and discordant without overdisclosure groups. Additionally, discordant with overdisclosure dermatologists also had the highest median and mean number of unique industry interactions not on OP, followed by those in the overdisclosure only and no disclosure groups. Academic and private practice settings did not impact dermatologists’ disclosure status. The percentage of female and male dermatologists in the discordant group was 25% and 36%, respectively.
Dermatologists reported a total of 1756 unique industry relationships in the AADMP that were not found on OP. Of these, 1440 (82%) relationships were from 236 dermatologists who had industry payments on OP. The remaining 316 relationships were from 108 dermatologists who had no payments on OP. Although 114 companies reported payments to dermatologists on OP, dermatologists in the AADMP reported interactions with an additional 430 companies.
Comment
In this study, we demonstrated discordance between dermatologist self-reported financial interactions in the AADMP compared with those reported by industry via OP. After excluding f/b/t/l entries, approximately two-thirds of the total amount and number of payments in OP were disclosed, while 31% of dermatologists had discordant disclosure status.
Prior investigations in other medical fields showed high discrepancy rates between industry-reported and physician-reported relationships ranging from 23% to 62%, with studies utilizing various methodologies.6-9,11,12,14,15 Only a few studies have utilized the OP database.8,12,15 Thompson et al12 compared OP payment data with physician financial disclosure at an annual gynecology scientific meeting and found although 209 of 335 (62%) physicians had interactions listed in the OP database, only 24 (7%) listed at least 1 company in the meeting financial disclosure section. Of these 24 physicians, only 5 (21%) accurately disclosed financial relationships with all of the companies listed in OP. The investigators found 129 (38.5%) physicians and 33.7% of the $1.99 million OP payments had concordant disclosure status. When they excluded physicians who received less than $100, 53% of individuals had concordant disclosure.12 Hannon et al8 reported on inconsistencies between disclosures in the OP database and the American Academy of Orthopedic Surgeons Annual Meeting and found 259 (23%) of 1113 physicians meeting inclusion criteria had financial interactions listed in the OP database that were not reported in the meeting disclosures. Yee et al15 also utilized the OP database and compared it with author disclosures in 3 major ophthalmology journals.Of 670 authors, 367 (54.8%) had complete concordance, with 68 (10.1%) more reporting additional overdisclosures, leading to a discordant with underdisclosure rate of 35.1%. Additionally, $1.46 million (44.6%) of the $3.27 million OP payments had concordant disclosure status.15 Other studies compared individual companies’ online reports of physician payments with physician self-disclosures in annual meeting programs, clinical guidelines, and peer-reviewed publications.6,7,9,11,14
Our study demonstrated variation in disclosure status. Compared with other groups, dermatologists in the discordant with overdisclosure group on average had more interactions with and received higher payments from industry, which is consistent with studies in the orthopedic surgery literature.8,9 Male dermatologists had 11% more discordant disclosures than their female counterparts, which may be influenced by men having more industry interactions than women.3 Although small industry payments possessed the lowest concordant rate in our study, which has been observed,12 payments greater than $100,000 had the second-lowest concordance rate at 58%, which may be skewed by the small sample size. Rates of concordant disclosure differed among types of interactions, such as between research and associated research payments. This particular difference may be attributed to the incorrect listing of dermatologists as principal investigators or reduced awareness of payments, as associated research payments were made to the institution and not the individual.
Reasons for discrepancies between industry-reported and dermatologist-reported disclosures may include reporting time differences, lack of physician awareness of OP, industry reporting inaccuracies, dearth of contextual information associated with individual payment entries, and misunderstandings. Prior research demonstrated that the most common reasons for physician nondisclosure included misunderstanding disclosure requirements, unintentional omission of payment, and a lack of relationship between the industry payment and presentation topic.9,12 These factors likely contributed to the disclosure inconsistencies in our study. Similarly high rates of inconsistencies across different specialties suggest systemic concerns.
We found a substantial number of dermatologist-industry interactions listed in the AADMP that were not captured by OP, with 108 dermatologists (35%) having overdisclosures even when excluding f/b/t/l entries. The number of companies in these overdisclosures approximated 4 times the number of companies on OP. Other studies have also observed physician-industry interactions not displayed on OP.8,12,15 Because the Sunshine Act requires reporting only by certain companies, interactions surrounding products such as over-the-counter merchandise, cosmetics, lasers, novel devices, and new medications are generally not included. Further, OP may not capture nonmonetary industry relationships.
There were several limitations to this study. The most notable limitation was the differences in the categorizations of industry relationships by OP and the AADMP. These differences can overemphasize some types of interactions at the expense of other types, such as f/b/t/l. As such, analyses were repeated after excluding f/b/t/l. Another limitation was the inexact overlap of time frames for OP and the AADMP, which may have led to discrepancies. However, we used the best available data and expect the vast majority of interactions to have occurred by the AAD disclosure deadline. It is possible that the presenters may have had a more updated conflict-of-interest disclosure slide at the time of the meeting presentation. The most important limitation was that we were unable to determine whether discrepancies resulted from underreporting by dermatologists or inaccurate reporting by industry. It was unlikely that OP or the AADMP alone completely represented all dermatologist-industry financial relationships.
Conclusion
With a growing emphasis on physician-industry transparency, we identified rates of differences in dermatology consistent with those in other medical fields when comparing the publicly available OP database with disclosures at national conferences. Although the differences in the categorization and requirements for disclosure between the OP database and the AADMP may account for some of the discordance, dermatologists should be aware of potentially negative public perceptions regarding the transparency and prevalence of physician-industry interactions.
Acknowledgment
The first two authors contributed equally to this research/article
- Campbell EG, Gruen RL, Mountford J, et al. A national survey of physician-industry relationships. N Engl J Med. 2007;356:1742-1750.
- Marshall DC, Jackson ME, Hattangadi-Gluth JA. Disclosure of industry payments to physicians: an epidemiologic analysis of early data from the open payments program. Mayo Clin Proc. 2016;91:84-96.
- Feng H, Wu P, Leger M. Exploring the industry-dermatologist financial relationship: insight from the open payment data. JAMA Dermatol. 2016;152:1307-1313.
- Kirschner NM, Sulmasy LS, Kesselheim AS. Health policy basics: the physician payment Sunshine Act and the open payments program. Ann Intern Med. 2014;161:519-521.
- Search Open Payment. Centers for Medicare & Medicaid Services. https://openpaymentsdata.cms.gov. Accessed October 21, 2019.
- Buerba RA, Fu MC, Grauer JN. Discrepancies in spine surgeon conflict of interest disclosures between a national meeting and physician payment listings on device manufacturer web sites. Spine J. 2013;13:1780-1788.
- Chimonas S, Frosch Z, Rothman DJ. From disclosure to transparency: the use of company payment data. Arch Intern Med. 2011;171:81-86.
- Hannon CP, Chalmers PN, Carpiniello MF, et al. Inconsistencies between physician-reported disclosures at the AAOS annual meeting and industry-reported financial disclosures in the open payments database. J Bone Joint Surg. 2016;98:E90.
- Okike K, Kocher MS, Wei EX, et al. Accuracy of conflict-of-interest disclosures reported by physicians. N Engl J Med. 2009;361:1466-1474.
- Ramm O, Brubaker L. Conflicts-of-interest disclosures at the 2010 AUGS Scientific Meeting. Female Pelvic Med Reconstr Surg. 2012;18:79-81.
- Tanzer D, Smith K, Tanzer M. American Academy of Orthopaedic Surgeons disclosure policy fails to accurately inform its members of potential conflicts of interest. Am J Orthop (Belle Mead NJ). 2015;44:E207-E210.
- Thompson JC, Volpe KA, Bridgewater LK, et al. Sunshine Act: shedding light on inaccurate disclosures at a gynecologic annual meeting. Am J Obstet Gynecol. 2016;215:661.
- Disclosure of Potential Conflicts of Interest. American Academy of Dermatology and AAD Association Web site. https://aad.org/Forms/Policies/Uploads/AR/
AR%20Disclosure%20of%20Potential%20Conflicts%
20of%20Interest-2.pdf. Accessed October 21, 2019. - Hockenberry JM, Weigel P, Auerbach A, et al. Financial payments by orthopedic device makers to orthopedic surgeons. Arch Intern Med. 2011;171:1759-1765.
- Yee C, Greenberg PB, Margo CE, et al. Financial disclosures in academic publications and the Sunshine Act: a concordance dtudy. Br J Med Med Res. 2015;10:1-6.
- Campbell EG, Gruen RL, Mountford J, et al. A national survey of physician-industry relationships. N Engl J Med. 2007;356:1742-1750.
- Marshall DC, Jackson ME, Hattangadi-Gluth JA. Disclosure of industry payments to physicians: an epidemiologic analysis of early data from the open payments program. Mayo Clin Proc. 2016;91:84-96.
- Feng H, Wu P, Leger M. Exploring the industry-dermatologist financial relationship: insight from the open payment data. JAMA Dermatol. 2016;152:1307-1313.
- Kirschner NM, Sulmasy LS, Kesselheim AS. Health policy basics: the physician payment Sunshine Act and the open payments program. Ann Intern Med. 2014;161:519-521.
- Search Open Payment. Centers for Medicare & Medicaid Services. https://openpaymentsdata.cms.gov. Accessed October 21, 2019.
- Buerba RA, Fu MC, Grauer JN. Discrepancies in spine surgeon conflict of interest disclosures between a national meeting and physician payment listings on device manufacturer web sites. Spine J. 2013;13:1780-1788.
- Chimonas S, Frosch Z, Rothman DJ. From disclosure to transparency: the use of company payment data. Arch Intern Med. 2011;171:81-86.
- Hannon CP, Chalmers PN, Carpiniello MF, et al. Inconsistencies between physician-reported disclosures at the AAOS annual meeting and industry-reported financial disclosures in the open payments database. J Bone Joint Surg. 2016;98:E90.
- Okike K, Kocher MS, Wei EX, et al. Accuracy of conflict-of-interest disclosures reported by physicians. N Engl J Med. 2009;361:1466-1474.
- Ramm O, Brubaker L. Conflicts-of-interest disclosures at the 2010 AUGS Scientific Meeting. Female Pelvic Med Reconstr Surg. 2012;18:79-81.
- Tanzer D, Smith K, Tanzer M. American Academy of Orthopaedic Surgeons disclosure policy fails to accurately inform its members of potential conflicts of interest. Am J Orthop (Belle Mead NJ). 2015;44:E207-E210.
- Thompson JC, Volpe KA, Bridgewater LK, et al. Sunshine Act: shedding light on inaccurate disclosures at a gynecologic annual meeting. Am J Obstet Gynecol. 2016;215:661.
- Disclosure of Potential Conflicts of Interest. American Academy of Dermatology and AAD Association Web site. https://aad.org/Forms/Policies/Uploads/AR/
AR%20Disclosure%20of%20Potential%20Conflicts%
20of%20Interest-2.pdf. Accessed October 21, 2019. - Hockenberry JM, Weigel P, Auerbach A, et al. Financial payments by orthopedic device makers to orthopedic surgeons. Arch Intern Med. 2011;171:1759-1765.
- Yee C, Greenberg PB, Margo CE, et al. Financial disclosures in academic publications and the Sunshine Act: a concordance dtudy. Br J Med Med Res. 2015;10:1-6.
Practice Points
- There is heightening public attention to conflicts of interest since the start of the government-mandated reporting of physician-industry interactions.
- When compared with an industry-reported physician-interaction database, approximately two-thirds of dermatologists who presented at a national dermatology conference self-disclosed all interactions.
- This rate of discordance is consistent with other specialties, but it may reflect differences in the database reporting methods.