User login
From Breakouts to Bargains: Strategies for Patient-Centered, Cost-effective Acne Care
In the United States, acne affects 85% of adolescents and can persist into adulthood at a prevalence of 30% to 50% in adult women. 1,2 The pathogenesis of acne is multifactorial and involves hyperkeratinization of the follicle, bacterial colonization with Cutibacterium acnes , and increased androgen-induced sebum production, which together lead to inflammation. 3,4 A wide range of treatment guideline–recommended options are available, including benzoyl peroxide (BPO), topical retinoids, topical and oral antibiotics, antiandrogens, and isotretinoin. 5 However, these options vary widely in their clinical uses, effectiveness, and costs.
Why Cost-effective Acne Care Matters
Out-of-pocket spending by patients on acne treatments can be substantial, with surveys finding that acne patients often spend hundreds to thousands of dollars per year.6,7 In a poll conducted in 2019 by the Kaiser Family Foundation, 3 in 10 patients said they had not taken their medicine as prescribed because of costs.8 A mixed methods study by Ryskina et al9 found that 65% (17/26) of participants who reported primary nonadherence—intended to fill prescriptions but were unable to do so—cited cost or coverage-related barriers as the reason. With the continued rise of dermatologic drug prices and increased prevalence of high-deductible health plans, cost-effective treatment continues to grow in importance. Failure to consider cost-effective, patient-centered care may lead to increased financial toxicity, reduced adherence, and ultimately worse outcomes and patient satisfaction. We aim to review the cost-effectiveness of current prescription therapies for acne management and highlight the most cost-effective approaches to patients with mild to moderate acne as well as moderate to severe acne.
In this review, we will take a value-oriented framework.10 Value can be defined as the cost per outcome of interest. Therefore, a treatment does not necessarily need to be inexpensive to provide high value if it delivers outstanding clinical outcomes. In addition, we will focus on incremental cost-effectiveness relative to common alternatives (eg, a retinoid could deliver high value relative to a vehicle but still provide limited value compared to other available retinoids if it is more expensive but not more efficacious). When possible, we present data from cost-effectiveness studies.11,12 We also use recent available price data obtained from GoodRx on August 11, 2023, to guide this discussion.13 However, as comparative-effectiveness and cost-effectiveness studies rarely are performed for acne medications, much of this discussion will be based on expert opinion.
Treatment Categories
Topical Retinoids—There currently are 4 topical retinoids that are approved by the US Food and Drug Administration (FDA) for the treatment of acne: tretinoin, tazarotene, trifarotene, and adapalene. These drugs are vitamin A derivatives that bind retinoic acid receptors and function as comedolytic and anti-inflammatory agents.5 In general, generic tretinoin and adapalene products have the lowest cost (Table).

In network meta-analyses, tretinoin and adapalene often are highly ranked topical treatment options with respect to efficacy.14 Combined with their low cost, generic tretinoin and adapalene likely are excellent initial options for topical therapy from the standpoint of cost-effectiveness.15 Adapalene may be preferred in many situations because of its better photostability and compatibility with BPO.
Due to the importance of the vehicle in determining retinoid tolerability, efforts have been made to use encapsulation and polymeric emulsion technology to improve tolerability. Recently, polymeric lotion formulations of tretinoin and tazarotene have become available. In a phase 2 study, tazarotene lotion 0.045% was found to have equivalent efficacy and superior tolerability to tazarotene cream 0.1%.16 Although head-to-head data are not available, it is likely that tretinoin lotion may offer similar tolerability improvements.17 Although these formulations currently are more costly, this improved tolerability may be critical for some patients to be able to use topical retinoids, and the additional cost may be worthwhile. In addition, as these products lose market exclusivity, they may become more affordable and similarly priced to other topical retinoids. It is important to keep in mind that in clinical trials of tretinoin and adapalene, rates of dropout due to adverse events typically were 1% to 2%; therefore, because many patients can tolerate generic tretinoin and adapalene, at current prices the lotion formulations of retinoids may not be cost-effective relative to these generics.14
Trifarotene cream 0.005%, a fourth-generation topical retinoid that is highly sensitive for retinoic acid receptor γ, recently was FDA approved for the treatment of acne. Although trifarotene is efficacious for both facial and truncal acne, there is a lack of active comparator data compared to other topical retinoids.18 In a 2023 network meta-analysis, trifarotene was found to be both less efficacious and less tolerable compared to other topical retinoids.19 Thus, it is unclear if trifarotene offers any improved efficacy compared to other options, and it comes at a much higher cost (Table). In a tolerability study, trifarotene was found to be significantly more irritating than tazarotene lotion 0.045% and adapalene gel 0.3% (P<.05).20 Therefore, trifarotene cream 0.005% is unlikely to be a cost-effective option; in fact, it may be overall inferior to other topical retinoids, given its potentially lower tolerability.
Topical Antibiotics—There are 4 commonly prescribed topical antibiotics that are approved by the FDA for the treatment of acne: clindamycin, erythromycin, dapsone, and minocycline. The American Academy of Dermatology guidelines for the treatment of acne recommend concomitant use of BPO to prevent antibiotic resistance.5 Clindamycin is favored over erythromycin because of increasing antibiotic resistance to erythromycin.21 Inexpensive generic options in multiple vehicles (eg, solution, foam, gel) make clindamycin a highly cost-effective option when antibiotic therapy is desired as part of a topical regimen (Table).
The cost-effectiveness of dapsone gel and minocycline foam relative to clindamycin are less certain. Rates of resistance to minocycline are lower than clindamycin, and minocycline foam may be a reasonable alternative in patients who have not had success with other topical antibiotics, such as clindamycin.22 However, given the absence of comparative effectiveness data to suggest minocycline is more effective than clindamycin, it is difficult to justify the substantially higher cost for the typical patient. Although dapsone gel has been suggested as an option for adult women with acne, there are no data to support that it is any more effective than other topical antibiotics in this patient population.23 As generic dapsone prices decrease, it may become a reasonable alternative to clindamycin. In addition, the antineutrophil properties of dapsone may be useful in other acneform and inflammatory eruptions, such as scalp folliculitis and folliculitis decalvans.24
Combination Topicals—Current combination topical products include antibiotic and BPO, antibiotic and retinoid, and retinoid and BPO. Use of combination agents is recommended to reduce the risk for resistance and to enhance effectiveness. Combination products offer improved convenience, which is associated with better adherence and outcomes.25 Generic fixed-dose adapalene-BPO can be a highly cost-effective option that can sometimes be less expensive than the individual component products (Table). Similarly, fixed-dose clindamycin-BPO also is likely to be highly cost-effective. A network meta-analysis found fixed-dose adapalene-BPO to be the most efficacious topical treatment, though it also was found to be the most irritating—more so than fixed-dose clindamycin-BPO, which may have similar efficacy.14,26,27 Generic fixed-dose tretinoin-clindamycin offers improved convenience and adherence compared to the individual components, but it is more expensive, and its cost-effectiveness may be influenced by the importance of convenience for the patient.25 An encapsulated, fixed-dose tretinoin 0.1%–BPO 3% cream is FDA approved for acne, but the cost is high and there is a lack of comparative effectiveness data demonstrating advantages over generic fixed-dose adapalene-BPO products.
Topical Antiandrogen—Clascoterone was introduced in 2020 as the first FDA-approved topical medication to target the hormonal pathogenesis of acne, inhibiting the androgen receptors in the sebaceous gland.28 Because it is rapidly metabolized to cortexolone and does not have systemic antiandrogen effects, clascoterone can be used in both men and women with acne. In clinical trials, it had minimal side effects, including no evidence of irritability, which is an advantage over topical retinoids and BPO.29 In addition, a phase 2 study found that clascoterone may have similar to superior efficacy to tretinoin cream 0.05%.30 Although clascoterone has several strengths, including its efficacy, tolerability, and unique mechanism of action, its cost-effectiveness is limited due to its high cost (Table) and the need for twice-daily application, which reduces convenience. Clascoterone likely is best reserved for patients with a strong hormonal pathogenesis of their acne or difficulty tolerating other topicals, or as an additional therapy to complement other topicals.
Oral Antibiotics—Oral antibiotics are the most commonly prescribed systemic treatments for acne, particularly tetracyclines such as doxycycline, minocycline, and sarecycline.31-34 Doxycycline and minocycline are considered first-line oral antibiotic therapy in the United States and are inexpensive and easily accessible.5 Doxycycline generally is recommended over minocycline given lack of evidence of superior efficacy of minocycline and concerns about severe adverse cutaneous reactions and drug-induced lupus with minocycline.35
In recent years, there has been growing concern of the development of antibiotic resistance.5 Sarecycline is a narrow-spectrum tetracycline that was FDA approved for acne in 2018. In vitro studies demonstrate sarecycline maintains high efficacy against C acnes with less activity against other bacteria, particularly gram-negative enterobes.36 The selectivity of sarecycline may lessen alterations of the gut microbiome seen with other oral antibiotics and reduce gastrointestinal tract side effects. Although comparative effectiveness studies are lacking, sarecycline was efficacious in phase 3 trials with few side effects compared with placebo.37 However, at this time, given the absence of comparative effectiveness data and its high cost (Table), sarecycline likely is best reserved for patients with comorbidities (eg, gastrointestinal disease), those requiring long-term antibiotic therapy, or those with acne that has failed to respond to other oral antibiotics.
Hormonal Treatments—Hormonal treatments such as combined oral contraceptives (COCs) and spironolactone often are considered second-line options, though they may represent cost-effective and safe alternatives to oral antibiotics for women with moderate to severe acne.38-41 There currently are 4 COCs approved by the FDA for the treatment of moderate acne in postmenarcheal females: drospirenone-ethinyl estradiol (Yaz [Bayer HealthCare Pharmaceuticals, Inc]), ethinyl estradiol-norgestimate (Ortho Tri-Cyclen [Ortho-McNeil Pharmaceuticals, Inc]), drospirenone-ethinyl estradiol-levomefolate (Beyaz [Bayer HealthCare Pharmaceuticals, Inc]), and ethinyl estradiol-norethindrone acetate-ferrous fumarate (Estrostep Fe [Allergan USA, Inc]).5 Treatment with COCs has been shown to cause substantial reductions in lesion counts across all lesion types compared to placebo, and a meta-analysis of 24 randomized trials conducted by Arowojolu et al42 demonstrated no consistent differences in acne reduction among different COCs.43,44 Although oral antibiotics are associated with faster improvement than COCs, there is some evidence that they have similar efficacy at 6 months of therapy.45 Combined oral contraceptives are inexpensive and likely reflect a highly cost-effective option (Table).
Spironolactone is an aldosterone inhibitor and androgen receptor blocker that is used off label to treat acne. It is one of the least expensive systemic medications for acne (Table). Although randomized controlled trials are lacking, several large case series support the effectiveness of spironolactone for women with acne.38,46 In addition, observational data suggest spironolactone may have similar effectiveness to oral antibiotics.41 Spironolactone generally is well tolerated, with the most common adverse effects being menstrual irregularities, breast tenderness, and diuresis.47,48 Many of these adverse effects are dose dependent and less likely with the dosing used in acne care. Additionally, menstrual irregularities can be reduced by concomitant use of a COC.48
Although frequent potassium monitoring remains common among patients being treated with spironolactone, there is growing evidence to suggest that potassium monitoring is of low value in young healthy women with acne.49-51 Reducing this laboratory monitoring likely represents an opportunity to provide higher-value care to patients being treated with spironolactone. However, laboratory monitoring should be considered if risk factors for hyperkalemia are present (eg, older age, comorbidities, medications).51
Isotretinoin—Isotretinoin is the most efficacious treatment available for acne and has the unique property of being able to induce a remission of acne activity for many patients.5 Although it remains modestly expensive (Table), it may be less costly overall relative to other treatments that may need continued use over many years because it can induce a remission of acne activity. As with spironolactone, frequent laboratory monitoring remains common among patients being treated with isotretinoin. There is no evidence to support checking complete blood cell counts.52 Several observational studies and a Delphi consensus support reduced monitoring, such as checking lipids and alanine aminotransferase at baseline and peak dose in otherwise young healthy patients.53,54 A recent critically appraised topic published in the British Journal of Dermatology has proposed eliminating laboratory monitoring entirely.55 Reducing laboratory monitoring for patients being treated with isotretinoin has been estimated to potentially save $100 million to $200 million per year in the United States.52-54
Other Strategies to Reduce Patient Costs
Although choosing a cost-effective treatment approach is critical to preventing financial toxicity given poor coverage for acne care and the growth of high-deductible insurance plans, some patients may still experience high treatment costs.56 Because pharmacy costs often are inflated, potentially related to practices of pharmacy benefit managers, it often is possible to find better prices than the presented list price, either by using platforms such as GoodRx or through direct-to-patient mail-order pharmacies such as Cost Plus Drug.57 For branded medications, some patients may be eligible for patient-assistance programs, though they typically are not available for those with public insurance such as Medicare or Medicaid. Compounding pharmacies offer another approach to reduce cost and improve convenience for patients, but because the vehicle can influence the efficacy and tolerability of some topical medications, it is possible that these compounded formulations may not perform similarly to the original FDA-approved products.
Conclusion
For mild to moderate acne, multimodal topical therapy often is required. Fixed-dose combination adapalene-BPO and clindamycin-BPO are highly cost-effective options for most patients. Lotion formulations of topical retinoids may be useful in patients with difficulty tolerating other formulations. Clascoterone is a novel topical antiandrogen that is more expensive than other topical therapies but can complement other topical therapies and is well tolerated.
For moderate to severe acne, doxycycline or hormonal therapy (ie, COCs, spironolactone) are highly cost-effective options. Isotretinoin is recommended for severe or scarring acne. Reduced laboratory monitoring for spironolactone and isotretinoin is an opportunity to provide higher-value care.
- Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474-485. doi:10.1111/bjd.12149
- Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59. doi:10.1016/j.jaad.2007.06.045
- Webster GF. The pathophysiology of acne. Cutis. 2005;76(2 suppl):4-7.
- Degitz K, Placzek M, Borelli C, et al. Pathophysiology of acne. J Dtsch Dermatol Ges. 2007;5:316-323. doi:10.1111/j.1610-0387.2007.06274.x
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33. doi:10.1016/j.jaad.2015.12.037
- Felmingham C, Kerr A, Veysey E. Costs incurred by patients with acne prior to dermatological consultation and their relation to patient income. Australas J Dermatol. 2020;61:384-386. doi:10.1111/ajd.13324
- Perche P, Singh R, Feldman S. Patient preferences for acne vulgaris treatment and barriers to care: a survey study. J Drugs Dermatol. 2022;21:1191-1195. doi:10.36849/JDD.6940
- KFF Health Tracking Poll—February 2019. Accessed August 9, 2023. https://files.kff.org/attachment/Topline-KFF-Health-Tracking-Poll-February-2019
- Ryskina KL, Goldberg E, Lott B, et al. The role of the physician in patient perceptions of barriers to primary adherence with acne medications. JAMA Dermatol. 2018;154:456-459. doi:10.1001/jamadermatol.2017.6144
- Porter ME. What is value in health care? N Engl J Med. 2010;363:2477-2481. doi:10.1056/NEJMp1011024
- Barbieri JS, Tan JKL, Adamson AS. Active comparator trial designs used to promote development of innovative new medications. Cutis. 2020;106:E4-E6. doi:10.12788/cutis.0067
- Miller J, Ly S, Mostaghimi A, et al. Use of active comparator trials for topical medications in dermatology. JAMA Dermatol. 2021;157:597-599. doi:10.1001/jamadermatol.2021.0356
- GoodRx. Accessed August 11, 2023. https://www.goodrx.com
- Stuart B, Maund E, Wilcox C, et al. Topical preparations for the treatment of mild‐to‐moderate acne vulgaris: systematic review and network meta‐analysis. Br J Dermatol. 2021;185:512-525. doi:10.1111/bjd.20080
- Mavranezouli I, Welton NJ, Daly CH, et al. Cost-effectiveness of topical pharmacological, oral pharmacological, physical and combined treatments for acne vulgaris. Clin Exp Dermatol. 2022;47:2176-2187. doi:10.1111/ced.15356
- Tanghetti E, Werschler W, Lain T, et al. Tazarotene 0.045% lotion for once-daily treatment of moderate-to-severe acne vulgaris: results from two phase 3 trials. J Drugs Dermatol. 2020;19:70-77. doi:10.36849/JDD.2020.3977
- Tyring SK, Kircik LH, Pariser DM, et al. Novel tretinoin 0.05% lotion for the once-daily treatment of moderate-to-severe acne vulgaris: assessment of efficacy and safety in patients aged 9 years and older. J Drugs Dermatol. 2018;17:1084-1091.
- Tan J, Thiboutot D, Popp G, et al. Randomized phase 3 evaluation of trifarotene 50 μg/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol. 2019;80:1691-1699. doi:10.1016/j.jaad.2019.02.044
- Huang CY, Chang IJ, Bolick N, et al. Comparative efficacy of pharmacological treatments for acne vulgaris: a network meta-analysis of 221 randomized controlled trials. Ann Fam Med. 2023;21:358-369. doi:10.1370/afm.2995
- Draelos ZD. Low irritation potential of tazarotene 0.045% lotion: head-to-head comparison to adapalene 0.3% gel and trifarotene 0.005% cream in two studies. J Dermatolog Treat. 2023;34:2166346. doi:10.1080/09546634.2023.2166346
- Dessinioti C, Katsambas A. Antibiotics and antimicrobial resistance in acne: epidemiological trends and clinical practice considerations. Yale J Biol Med. 2022;95:429-443.
- Gold LS, Dhawan S, Weiss J, et al. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: results of 2 randomized, double-blind, phase 3 studies. J Am Acad Dermatol. 2019;80:168-177. doi:10.1016/j.jaad.2018.08.020
- Wang X, Wang Z, Sun L, et al. Efficacy and safety of dapsone gel for acne: a systematic review and meta-analysis. Ann Palliat Med. 2022;11:611-620. doi:10.21037/apm-21-3935
- Melián-Olivera A, Burgos-Blasco P, Selda-Enríquez G, et al. Topical dapsone for folliculitis decalvans: a retrospective cohort study. J Am Acad Dermatol. 2022;87:150-151. doi:10.1016/j.jaad.2021.07.004
- Yentzer BA, Ade RA, Fountain JM, et al. Simplifying regimens promotes greater adherence and outcomes with topical acne medications: a randomized controlled trial. Cutis. 2010;86:103-108.
- Ting W. Randomized, observer-blind, split-face study to compare the irritation potential of 2 topical acne formulations over a 14-day treatment period. Cutis. 2012;90:91-96.
- Aschoff R, Möller S, Haase R, et al. Tolerability and efficacy ofclindamycin/tretinoin versus adapalene/benzoyl peroxide in the treatment of acne vulgaris. J Drugs Dermatol. 2021;20:295-301. doi:10.36849/JDD.2021.5641
- Rosette C, Agan FJ, Mazzetti A, et al. Cortexolone 17α-propionate (clascoterone) is a novel androgen receptor antagonist that inhibits production of lipids and inflammatory cytokines from sebocytes in vitro. J Drugs Dermatol. 2019;18:412-418.
- Hebert A, Thiboutot D, Stein Gold L, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:621-630. doi:10.1001/jamadermatol.2020.0465
- Trifu V, Tiplica GS, Naumescu E, et al. Cortexolone 17α-propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. a pilot randomized, double-blind comparative study vs. placebo and tretinoin 0·05% cream. Br J Dermatol. 2011;165:177-183. doi:10.1111/j.1365-2133.2011.10332.x
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Guzman AK, Barbieri JS. Comparative analysis of prescribing patterns of tetracycline class antibiotics and spironolactone between advanced practice providers and physicians in the treatment of acne vulgaris. J Am Acad Dermatol. 2021;84:1119-1121. doi:10.1016/j.jaad.2020.06.044
- Barbieri JS, James WD, Margolis DJ. Trends in prescribing behavior of systemic agents used in the treatment of acne among dermatologists and nondermatologists: a retrospective analysis, 2004-2013. J Am Acad Dermatol. 2017;77:456-463.e4. doi:10.1016/j.jaad.2017.04.016
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016. JAMA Dermatol. 2019;155:290-297. doi:10.1001/jamadermatol.2018.4944
- Garner SE, Eady A, Bennett C, et al. Minocycline for acne vulgaris: efficacy and safety. Cochrane Database Syst Rev. 2012;2012:CD002086. doi:10.1002/14651858.CD002086.pub2
- Zhanel G, Critchley I, Lin LY, et al. Microbiological profile of sarecycline, a novel targeted spectrum tetracycline for the treatment of acne vulgaris. Antimicrob Agents Chemother. 2018;63:e01297-18. doi:10.1128/AAC.01297-18
- Moore A, Green LJ, Bruce S, et al. Once-daily oral sarecycline 1.5 mg/kg/day is effective for moderate to severe acne vulgaris: results from two identically designed, phase 3, randomized, double-blind clinical trials. J Drugs Dermatol. 2018;17:987-996.
- Garg V, Choi JK, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355. doi:10.1016/j.jaad.2020.12.071
- Barbieri JS, Choi JK, James WD, et al. Real-world drug usage survival of spironolactone versus oral antibiotics for the management of female patients with acne. J Am Acad Dermatol. 2019;81:848-851. doi:10.1016/j.jaad.2019.03.036
- Barbieri JS, Spaccarelli N, Margolis DJ, et al. Approaches to limit systemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments. J Am Acad Dermatol. 2019;80:538-549. doi:10.1016/j.jaad.2018.09.055
- Barbieri JS, Choi JK, Mitra N, et al. Frequency of treatment switching for spironolactone compared to oral tetracycline-class antibiotics for women with acne: a retrospective cohort study 2010-2016. J Drugs Dermatol. 2018;17:632-638.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;7:CD004425. doi:10.1002/14651858.CD004425.pub6
- Maloney JM, Dietze P, Watson D, et al. Treatment of acne using a 3-milligram drospirenone/20-microgram ethinyl estradiol oral contraceptive administered in a 24/4 regimen. Obstet Gynecol. 2008;112:773-781. doi:10.1097/AOG.0b013e318187e1c5
- Lucky AW, Koltun W, Thiboutot D, et al. A combined oral contraceptive containing 3-mg drospirenone/20-microg ethinyl estradiol in the treatment of acne vulgaris: a randomized, double-blind, placebo-controlled study evaluating lesion counts and participant self-assessment. Cutis. 2008;82:143-150.
- Koo EB, Petersen TD, Kimball AB. Meta-analysis comparing efficacy of antibiotics versus oral contraceptives in acne vulgaris. J Am Acad Dermatol. 2014;71:450-459. doi:10.1016/j.jaad.2014.03.051
- Roberts EE, Nowsheen S, Davis DMR, et al. Use of spironolactone to treat acne in adolescent females. Pediatr Dermatol. 2021;38:72-76. doi:10.1111/pde.14391
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502. doi:10.1067/mjd.2000.105557
- Layton AM, Eady EA, Whitehouse H, et al. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol. 2017;18:169-191. doi:10.1007/s40257-016-0245-x
- Barbieri JS, Margolis DJ, Mostaghimi A. Temporal trends and clinician variability in potassium monitoring of healthy young women treated for acne with spironolactone. JAMA Dermatol. 2021;157:296-300. doi:10.1001/jamadermatol.2020.5468
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944. doi:10.1001/jamadermatol.2015.34
- Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157. doi:10.1016/j.ijwd.2019.04.024
- Barbieri JS, Shin DB, Wang S, et al. The clinical utility of laboratory monitoring during isotretinoin therapy for acne and changes to monitoring practices over time. J Am Acad Dermatol. 2020;82:72-79. doi:10.1016/j.jaad.2019.06.025
- Lee YH, Scharnitz TP, Muscat J, et al. Laboratory monitoring during isotretinoin therapy for acne: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:35-44. doi:10.1001/jamadermatol.2015.3091
- Xia E, Han J, Faletsky A, et al. Isotretinoin laboratory monitoring in acne treatment: a Delphi consensus study. JAMA Dermatol. 2022;158:942-948. doi:10.1001/jamadermatol.2022.2044
- Affleck A, Jackson D, Williams HC, et al. Is routine laboratory testing in healthy young patients taking isotretinoin necessary: a critically appraised topic. Br J Dermatol. 2022;187:857-865. doi:10.1111/bjd.21840
- Barbieri JS, LaChance A, Albrecht J. Double standards and inconsistencies in access to care-what constitutes a cosmetic treatment? JAMA Dermatol. 2023;159:245-246. doi:10.1001/jamadermatol.2022.6322
- Trish E, Van Nuys K, Popovian R. US consumers overpay for generic drugs. Schaeffer Center White Paper Series. May 31, 2022. doi:10.25549/m589-2268
In the United States, acne affects 85% of adolescents and can persist into adulthood at a prevalence of 30% to 50% in adult women. 1,2 The pathogenesis of acne is multifactorial and involves hyperkeratinization of the follicle, bacterial colonization with Cutibacterium acnes , and increased androgen-induced sebum production, which together lead to inflammation. 3,4 A wide range of treatment guideline–recommended options are available, including benzoyl peroxide (BPO), topical retinoids, topical and oral antibiotics, antiandrogens, and isotretinoin. 5 However, these options vary widely in their clinical uses, effectiveness, and costs.
Why Cost-effective Acne Care Matters
Out-of-pocket spending by patients on acne treatments can be substantial, with surveys finding that acne patients often spend hundreds to thousands of dollars per year.6,7 In a poll conducted in 2019 by the Kaiser Family Foundation, 3 in 10 patients said they had not taken their medicine as prescribed because of costs.8 A mixed methods study by Ryskina et al9 found that 65% (17/26) of participants who reported primary nonadherence—intended to fill prescriptions but were unable to do so—cited cost or coverage-related barriers as the reason. With the continued rise of dermatologic drug prices and increased prevalence of high-deductible health plans, cost-effective treatment continues to grow in importance. Failure to consider cost-effective, patient-centered care may lead to increased financial toxicity, reduced adherence, and ultimately worse outcomes and patient satisfaction. We aim to review the cost-effectiveness of current prescription therapies for acne management and highlight the most cost-effective approaches to patients with mild to moderate acne as well as moderate to severe acne.
In this review, we will take a value-oriented framework.10 Value can be defined as the cost per outcome of interest. Therefore, a treatment does not necessarily need to be inexpensive to provide high value if it delivers outstanding clinical outcomes. In addition, we will focus on incremental cost-effectiveness relative to common alternatives (eg, a retinoid could deliver high value relative to a vehicle but still provide limited value compared to other available retinoids if it is more expensive but not more efficacious). When possible, we present data from cost-effectiveness studies.11,12 We also use recent available price data obtained from GoodRx on August 11, 2023, to guide this discussion.13 However, as comparative-effectiveness and cost-effectiveness studies rarely are performed for acne medications, much of this discussion will be based on expert opinion.
Treatment Categories
Topical Retinoids—There currently are 4 topical retinoids that are approved by the US Food and Drug Administration (FDA) for the treatment of acne: tretinoin, tazarotene, trifarotene, and adapalene. These drugs are vitamin A derivatives that bind retinoic acid receptors and function as comedolytic and anti-inflammatory agents.5 In general, generic tretinoin and adapalene products have the lowest cost (Table).

In network meta-analyses, tretinoin and adapalene often are highly ranked topical treatment options with respect to efficacy.14 Combined with their low cost, generic tretinoin and adapalene likely are excellent initial options for topical therapy from the standpoint of cost-effectiveness.15 Adapalene may be preferred in many situations because of its better photostability and compatibility with BPO.
Due to the importance of the vehicle in determining retinoid tolerability, efforts have been made to use encapsulation and polymeric emulsion technology to improve tolerability. Recently, polymeric lotion formulations of tretinoin and tazarotene have become available. In a phase 2 study, tazarotene lotion 0.045% was found to have equivalent efficacy and superior tolerability to tazarotene cream 0.1%.16 Although head-to-head data are not available, it is likely that tretinoin lotion may offer similar tolerability improvements.17 Although these formulations currently are more costly, this improved tolerability may be critical for some patients to be able to use topical retinoids, and the additional cost may be worthwhile. In addition, as these products lose market exclusivity, they may become more affordable and similarly priced to other topical retinoids. It is important to keep in mind that in clinical trials of tretinoin and adapalene, rates of dropout due to adverse events typically were 1% to 2%; therefore, because many patients can tolerate generic tretinoin and adapalene, at current prices the lotion formulations of retinoids may not be cost-effective relative to these generics.14
Trifarotene cream 0.005%, a fourth-generation topical retinoid that is highly sensitive for retinoic acid receptor γ, recently was FDA approved for the treatment of acne. Although trifarotene is efficacious for both facial and truncal acne, there is a lack of active comparator data compared to other topical retinoids.18 In a 2023 network meta-analysis, trifarotene was found to be both less efficacious and less tolerable compared to other topical retinoids.19 Thus, it is unclear if trifarotene offers any improved efficacy compared to other options, and it comes at a much higher cost (Table). In a tolerability study, trifarotene was found to be significantly more irritating than tazarotene lotion 0.045% and adapalene gel 0.3% (P<.05).20 Therefore, trifarotene cream 0.005% is unlikely to be a cost-effective option; in fact, it may be overall inferior to other topical retinoids, given its potentially lower tolerability.
Topical Antibiotics—There are 4 commonly prescribed topical antibiotics that are approved by the FDA for the treatment of acne: clindamycin, erythromycin, dapsone, and minocycline. The American Academy of Dermatology guidelines for the treatment of acne recommend concomitant use of BPO to prevent antibiotic resistance.5 Clindamycin is favored over erythromycin because of increasing antibiotic resistance to erythromycin.21 Inexpensive generic options in multiple vehicles (eg, solution, foam, gel) make clindamycin a highly cost-effective option when antibiotic therapy is desired as part of a topical regimen (Table).
The cost-effectiveness of dapsone gel and minocycline foam relative to clindamycin are less certain. Rates of resistance to minocycline are lower than clindamycin, and minocycline foam may be a reasonable alternative in patients who have not had success with other topical antibiotics, such as clindamycin.22 However, given the absence of comparative effectiveness data to suggest minocycline is more effective than clindamycin, it is difficult to justify the substantially higher cost for the typical patient. Although dapsone gel has been suggested as an option for adult women with acne, there are no data to support that it is any more effective than other topical antibiotics in this patient population.23 As generic dapsone prices decrease, it may become a reasonable alternative to clindamycin. In addition, the antineutrophil properties of dapsone may be useful in other acneform and inflammatory eruptions, such as scalp folliculitis and folliculitis decalvans.24
Combination Topicals—Current combination topical products include antibiotic and BPO, antibiotic and retinoid, and retinoid and BPO. Use of combination agents is recommended to reduce the risk for resistance and to enhance effectiveness. Combination products offer improved convenience, which is associated with better adherence and outcomes.25 Generic fixed-dose adapalene-BPO can be a highly cost-effective option that can sometimes be less expensive than the individual component products (Table). Similarly, fixed-dose clindamycin-BPO also is likely to be highly cost-effective. A network meta-analysis found fixed-dose adapalene-BPO to be the most efficacious topical treatment, though it also was found to be the most irritating—more so than fixed-dose clindamycin-BPO, which may have similar efficacy.14,26,27 Generic fixed-dose tretinoin-clindamycin offers improved convenience and adherence compared to the individual components, but it is more expensive, and its cost-effectiveness may be influenced by the importance of convenience for the patient.25 An encapsulated, fixed-dose tretinoin 0.1%–BPO 3% cream is FDA approved for acne, but the cost is high and there is a lack of comparative effectiveness data demonstrating advantages over generic fixed-dose adapalene-BPO products.
Topical Antiandrogen—Clascoterone was introduced in 2020 as the first FDA-approved topical medication to target the hormonal pathogenesis of acne, inhibiting the androgen receptors in the sebaceous gland.28 Because it is rapidly metabolized to cortexolone and does not have systemic antiandrogen effects, clascoterone can be used in both men and women with acne. In clinical trials, it had minimal side effects, including no evidence of irritability, which is an advantage over topical retinoids and BPO.29 In addition, a phase 2 study found that clascoterone may have similar to superior efficacy to tretinoin cream 0.05%.30 Although clascoterone has several strengths, including its efficacy, tolerability, and unique mechanism of action, its cost-effectiveness is limited due to its high cost (Table) and the need for twice-daily application, which reduces convenience. Clascoterone likely is best reserved for patients with a strong hormonal pathogenesis of their acne or difficulty tolerating other topicals, or as an additional therapy to complement other topicals.
Oral Antibiotics—Oral antibiotics are the most commonly prescribed systemic treatments for acne, particularly tetracyclines such as doxycycline, minocycline, and sarecycline.31-34 Doxycycline and minocycline are considered first-line oral antibiotic therapy in the United States and are inexpensive and easily accessible.5 Doxycycline generally is recommended over minocycline given lack of evidence of superior efficacy of minocycline and concerns about severe adverse cutaneous reactions and drug-induced lupus with minocycline.35
In recent years, there has been growing concern of the development of antibiotic resistance.5 Sarecycline is a narrow-spectrum tetracycline that was FDA approved for acne in 2018. In vitro studies demonstrate sarecycline maintains high efficacy against C acnes with less activity against other bacteria, particularly gram-negative enterobes.36 The selectivity of sarecycline may lessen alterations of the gut microbiome seen with other oral antibiotics and reduce gastrointestinal tract side effects. Although comparative effectiveness studies are lacking, sarecycline was efficacious in phase 3 trials with few side effects compared with placebo.37 However, at this time, given the absence of comparative effectiveness data and its high cost (Table), sarecycline likely is best reserved for patients with comorbidities (eg, gastrointestinal disease), those requiring long-term antibiotic therapy, or those with acne that has failed to respond to other oral antibiotics.
Hormonal Treatments—Hormonal treatments such as combined oral contraceptives (COCs) and spironolactone often are considered second-line options, though they may represent cost-effective and safe alternatives to oral antibiotics for women with moderate to severe acne.38-41 There currently are 4 COCs approved by the FDA for the treatment of moderate acne in postmenarcheal females: drospirenone-ethinyl estradiol (Yaz [Bayer HealthCare Pharmaceuticals, Inc]), ethinyl estradiol-norgestimate (Ortho Tri-Cyclen [Ortho-McNeil Pharmaceuticals, Inc]), drospirenone-ethinyl estradiol-levomefolate (Beyaz [Bayer HealthCare Pharmaceuticals, Inc]), and ethinyl estradiol-norethindrone acetate-ferrous fumarate (Estrostep Fe [Allergan USA, Inc]).5 Treatment with COCs has been shown to cause substantial reductions in lesion counts across all lesion types compared to placebo, and a meta-analysis of 24 randomized trials conducted by Arowojolu et al42 demonstrated no consistent differences in acne reduction among different COCs.43,44 Although oral antibiotics are associated with faster improvement than COCs, there is some evidence that they have similar efficacy at 6 months of therapy.45 Combined oral contraceptives are inexpensive and likely reflect a highly cost-effective option (Table).
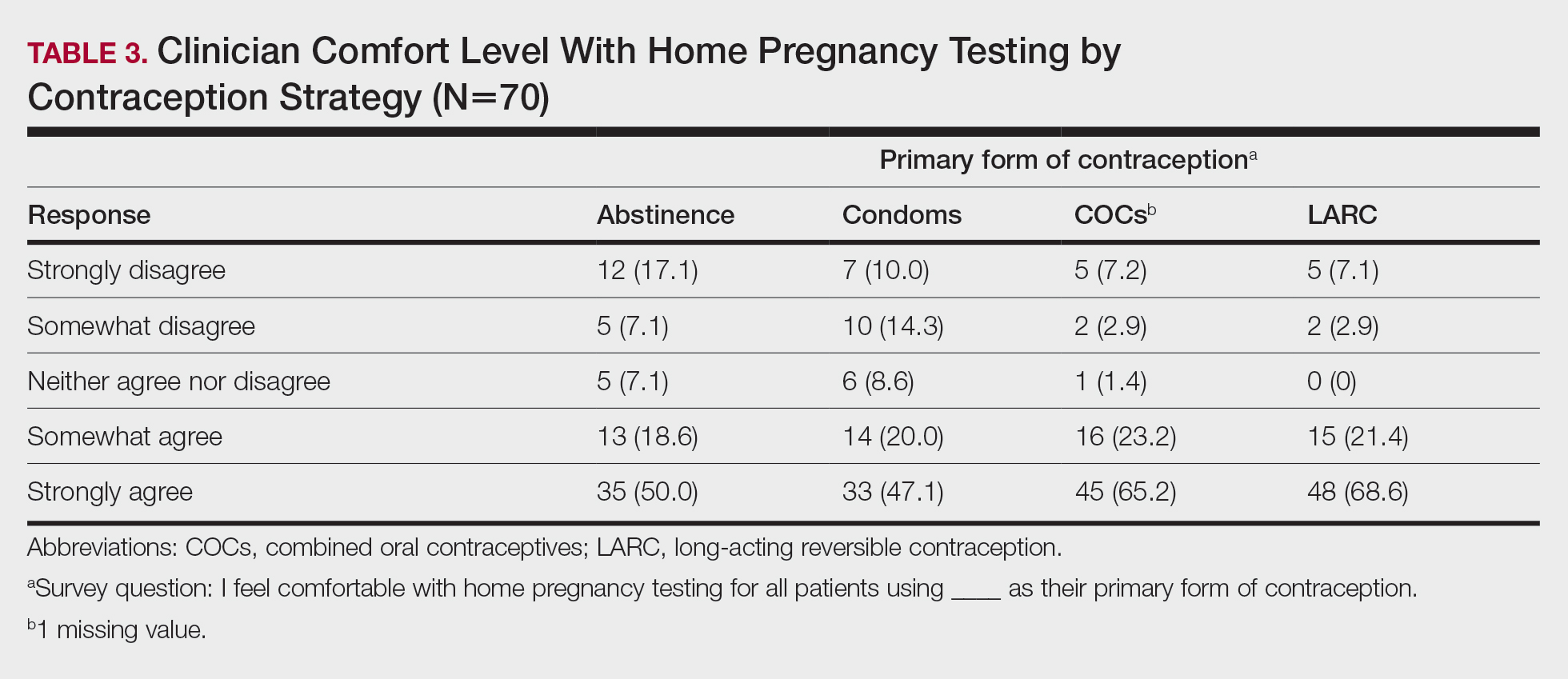
Spironolactone is an aldosterone inhibitor and androgen receptor blocker that is used off label to treat acne. It is one of the least expensive systemic medications for acne (Table). Although randomized controlled trials are lacking, several large case series support the effectiveness of spironolactone for women with acne.38,46 In addition, observational data suggest spironolactone may have similar effectiveness to oral antibiotics.41 Spironolactone generally is well tolerated, with the most common adverse effects being menstrual irregularities, breast tenderness, and diuresis.47,48 Many of these adverse effects are dose dependent and less likely with the dosing used in acne care. Additionally, menstrual irregularities can be reduced by concomitant use of a COC.48
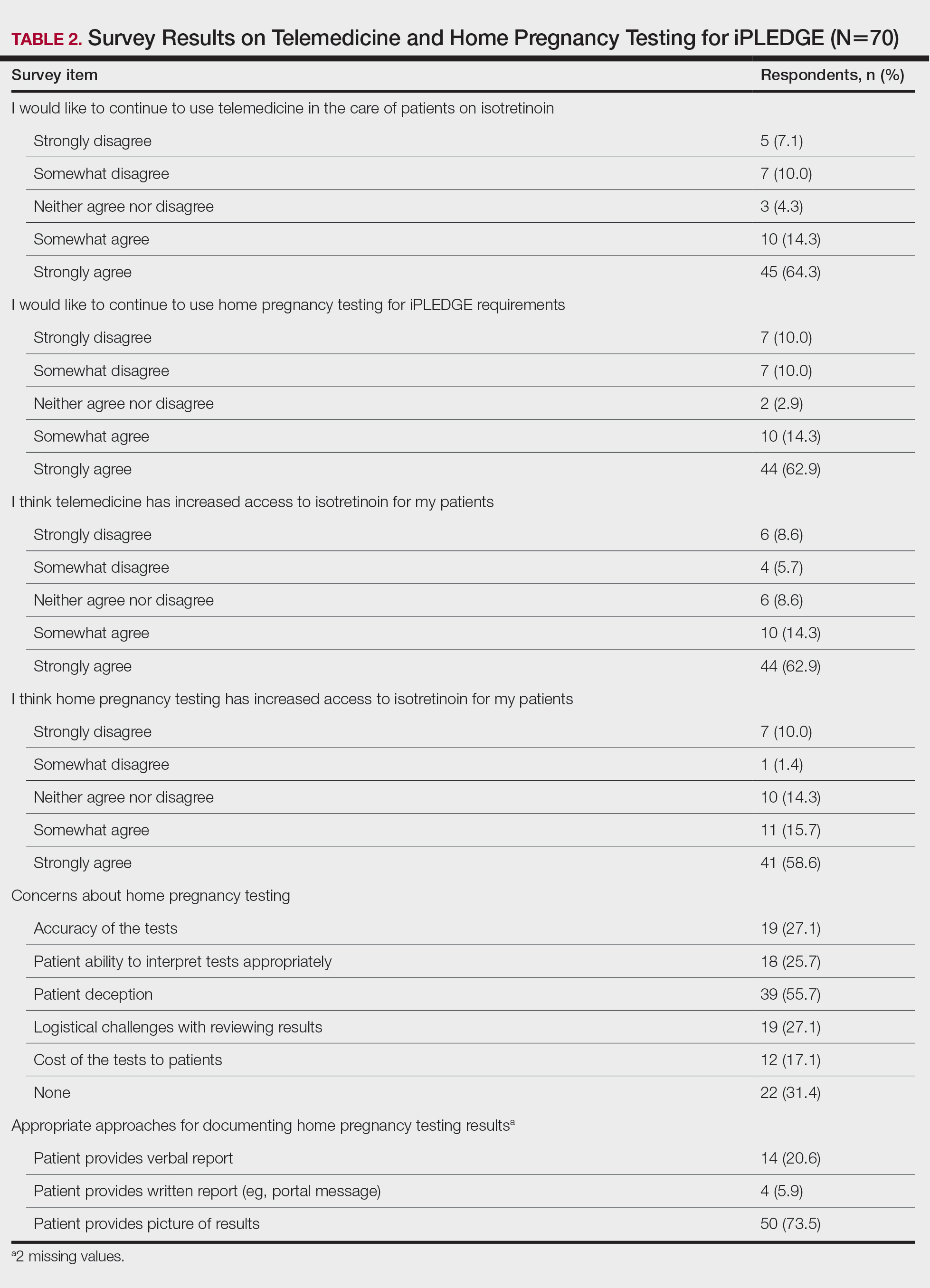
Although frequent potassium monitoring remains common among patients being treated with spironolactone, there is growing evidence to suggest that potassium monitoring is of low value in young healthy women with acne.49-51 Reducing this laboratory monitoring likely represents an opportunity to provide higher-value care to patients being treated with spironolactone. However, laboratory monitoring should be considered if risk factors for hyperkalemia are present (eg, older age, comorbidities, medications).51
Isotretinoin—Isotretinoin is the most efficacious treatment available for acne and has the unique property of being able to induce a remission of acne activity for many patients.5 Although it remains modestly expensive (Table), it may be less costly overall relative to other treatments that may need continued use over many years because it can induce a remission of acne activity. As with spironolactone, frequent laboratory monitoring remains common among patients being treated with isotretinoin. There is no evidence to support checking complete blood cell counts.52 Several observational studies and a Delphi consensus support reduced monitoring, such as checking lipids and alanine aminotransferase at baseline and peak dose in otherwise young healthy patients.53,54 A recent critically appraised topic published in the British Journal of Dermatology has proposed eliminating laboratory monitoring entirely.55 Reducing laboratory monitoring for patients being treated with isotretinoin has been estimated to potentially save $100 million to $200 million per year in the United States.52-54
Other Strategies to Reduce Patient Costs
Although choosing a cost-effective treatment approach is critical to preventing financial toxicity given poor coverage for acne care and the growth of high-deductible insurance plans, some patients may still experience high treatment costs.56 Because pharmacy costs often are inflated, potentially related to practices of pharmacy benefit managers, it often is possible to find better prices than the presented list price, either by using platforms such as GoodRx or through direct-to-patient mail-order pharmacies such as Cost Plus Drug.57 For branded medications, some patients may be eligible for patient-assistance programs, though they typically are not available for those with public insurance such as Medicare or Medicaid. Compounding pharmacies offer another approach to reduce cost and improve convenience for patients, but because the vehicle can influence the efficacy and tolerability of some topical medications, it is possible that these compounded formulations may not perform similarly to the original FDA-approved products.
Conclusion
For mild to moderate acne, multimodal topical therapy often is required. Fixed-dose combination adapalene-BPO and clindamycin-BPO are highly cost-effective options for most patients. Lotion formulations of topical retinoids may be useful in patients with difficulty tolerating other formulations. Clascoterone is a novel topical antiandrogen that is more expensive than other topical therapies but can complement other topical therapies and is well tolerated.
For moderate to severe acne, doxycycline or hormonal therapy (ie, COCs, spironolactone) are highly cost-effective options. Isotretinoin is recommended for severe or scarring acne. Reduced laboratory monitoring for spironolactone and isotretinoin is an opportunity to provide higher-value care.
In the United States, acne affects 85% of adolescents and can persist into adulthood at a prevalence of 30% to 50% in adult women. 1,2 The pathogenesis of acne is multifactorial and involves hyperkeratinization of the follicle, bacterial colonization with Cutibacterium acnes , and increased androgen-induced sebum production, which together lead to inflammation. 3,4 A wide range of treatment guideline–recommended options are available, including benzoyl peroxide (BPO), topical retinoids, topical and oral antibiotics, antiandrogens, and isotretinoin. 5 However, these options vary widely in their clinical uses, effectiveness, and costs.
Why Cost-effective Acne Care Matters
Out-of-pocket spending by patients on acne treatments can be substantial, with surveys finding that acne patients often spend hundreds to thousands of dollars per year.6,7 In a poll conducted in 2019 by the Kaiser Family Foundation, 3 in 10 patients said they had not taken their medicine as prescribed because of costs.8 A mixed methods study by Ryskina et al9 found that 65% (17/26) of participants who reported primary nonadherence—intended to fill prescriptions but were unable to do so—cited cost or coverage-related barriers as the reason. With the continued rise of dermatologic drug prices and increased prevalence of high-deductible health plans, cost-effective treatment continues to grow in importance. Failure to consider cost-effective, patient-centered care may lead to increased financial toxicity, reduced adherence, and ultimately worse outcomes and patient satisfaction. We aim to review the cost-effectiveness of current prescription therapies for acne management and highlight the most cost-effective approaches to patients with mild to moderate acne as well as moderate to severe acne.
In this review, we will take a value-oriented framework.10 Value can be defined as the cost per outcome of interest. Therefore, a treatment does not necessarily need to be inexpensive to provide high value if it delivers outstanding clinical outcomes. In addition, we will focus on incremental cost-effectiveness relative to common alternatives (eg, a retinoid could deliver high value relative to a vehicle but still provide limited value compared to other available retinoids if it is more expensive but not more efficacious). When possible, we present data from cost-effectiveness studies.11,12 We also use recent available price data obtained from GoodRx on August 11, 2023, to guide this discussion.13 However, as comparative-effectiveness and cost-effectiveness studies rarely are performed for acne medications, much of this discussion will be based on expert opinion.
Treatment Categories
Topical Retinoids—There currently are 4 topical retinoids that are approved by the US Food and Drug Administration (FDA) for the treatment of acne: tretinoin, tazarotene, trifarotene, and adapalene. These drugs are vitamin A derivatives that bind retinoic acid receptors and function as comedolytic and anti-inflammatory agents.5 In general, generic tretinoin and adapalene products have the lowest cost (Table).

In network meta-analyses, tretinoin and adapalene often are highly ranked topical treatment options with respect to efficacy.14 Combined with their low cost, generic tretinoin and adapalene likely are excellent initial options for topical therapy from the standpoint of cost-effectiveness.15 Adapalene may be preferred in many situations because of its better photostability and compatibility with BPO.
Due to the importance of the vehicle in determining retinoid tolerability, efforts have been made to use encapsulation and polymeric emulsion technology to improve tolerability. Recently, polymeric lotion formulations of tretinoin and tazarotene have become available. In a phase 2 study, tazarotene lotion 0.045% was found to have equivalent efficacy and superior tolerability to tazarotene cream 0.1%.16 Although head-to-head data are not available, it is likely that tretinoin lotion may offer similar tolerability improvements.17 Although these formulations currently are more costly, this improved tolerability may be critical for some patients to be able to use topical retinoids, and the additional cost may be worthwhile. In addition, as these products lose market exclusivity, they may become more affordable and similarly priced to other topical retinoids. It is important to keep in mind that in clinical trials of tretinoin and adapalene, rates of dropout due to adverse events typically were 1% to 2%; therefore, because many patients can tolerate generic tretinoin and adapalene, at current prices the lotion formulations of retinoids may not be cost-effective relative to these generics.14
Trifarotene cream 0.005%, a fourth-generation topical retinoid that is highly sensitive for retinoic acid receptor γ, recently was FDA approved for the treatment of acne. Although trifarotene is efficacious for both facial and truncal acne, there is a lack of active comparator data compared to other topical retinoids.18 In a 2023 network meta-analysis, trifarotene was found to be both less efficacious and less tolerable compared to other topical retinoids.19 Thus, it is unclear if trifarotene offers any improved efficacy compared to other options, and it comes at a much higher cost (Table). In a tolerability study, trifarotene was found to be significantly more irritating than tazarotene lotion 0.045% and adapalene gel 0.3% (P<.05).20 Therefore, trifarotene cream 0.005% is unlikely to be a cost-effective option; in fact, it may be overall inferior to other topical retinoids, given its potentially lower tolerability.
Topical Antibiotics—There are 4 commonly prescribed topical antibiotics that are approved by the FDA for the treatment of acne: clindamycin, erythromycin, dapsone, and minocycline. The American Academy of Dermatology guidelines for the treatment of acne recommend concomitant use of BPO to prevent antibiotic resistance.5 Clindamycin is favored over erythromycin because of increasing antibiotic resistance to erythromycin.21 Inexpensive generic options in multiple vehicles (eg, solution, foam, gel) make clindamycin a highly cost-effective option when antibiotic therapy is desired as part of a topical regimen (Table).
The cost-effectiveness of dapsone gel and minocycline foam relative to clindamycin are less certain. Rates of resistance to minocycline are lower than clindamycin, and minocycline foam may be a reasonable alternative in patients who have not had success with other topical antibiotics, such as clindamycin.22 However, given the absence of comparative effectiveness data to suggest minocycline is more effective than clindamycin, it is difficult to justify the substantially higher cost for the typical patient. Although dapsone gel has been suggested as an option for adult women with acne, there are no data to support that it is any more effective than other topical antibiotics in this patient population.23 As generic dapsone prices decrease, it may become a reasonable alternative to clindamycin. In addition, the antineutrophil properties of dapsone may be useful in other acneform and inflammatory eruptions, such as scalp folliculitis and folliculitis decalvans.24
Combination Topicals—Current combination topical products include antibiotic and BPO, antibiotic and retinoid, and retinoid and BPO. Use of combination agents is recommended to reduce the risk for resistance and to enhance effectiveness. Combination products offer improved convenience, which is associated with better adherence and outcomes.25 Generic fixed-dose adapalene-BPO can be a highly cost-effective option that can sometimes be less expensive than the individual component products (Table). Similarly, fixed-dose clindamycin-BPO also is likely to be highly cost-effective. A network meta-analysis found fixed-dose adapalene-BPO to be the most efficacious topical treatment, though it also was found to be the most irritating—more so than fixed-dose clindamycin-BPO, which may have similar efficacy.14,26,27 Generic fixed-dose tretinoin-clindamycin offers improved convenience and adherence compared to the individual components, but it is more expensive, and its cost-effectiveness may be influenced by the importance of convenience for the patient.25 An encapsulated, fixed-dose tretinoin 0.1%–BPO 3% cream is FDA approved for acne, but the cost is high and there is a lack of comparative effectiveness data demonstrating advantages over generic fixed-dose adapalene-BPO products.
Topical Antiandrogen—Clascoterone was introduced in 2020 as the first FDA-approved topical medication to target the hormonal pathogenesis of acne, inhibiting the androgen receptors in the sebaceous gland.28 Because it is rapidly metabolized to cortexolone and does not have systemic antiandrogen effects, clascoterone can be used in both men and women with acne. In clinical trials, it had minimal side effects, including no evidence of irritability, which is an advantage over topical retinoids and BPO.29 In addition, a phase 2 study found that clascoterone may have similar to superior efficacy to tretinoin cream 0.05%.30 Although clascoterone has several strengths, including its efficacy, tolerability, and unique mechanism of action, its cost-effectiveness is limited due to its high cost (Table) and the need for twice-daily application, which reduces convenience. Clascoterone likely is best reserved for patients with a strong hormonal pathogenesis of their acne or difficulty tolerating other topicals, or as an additional therapy to complement other topicals.
Oral Antibiotics—Oral antibiotics are the most commonly prescribed systemic treatments for acne, particularly tetracyclines such as doxycycline, minocycline, and sarecycline.31-34 Doxycycline and minocycline are considered first-line oral antibiotic therapy in the United States and are inexpensive and easily accessible.5 Doxycycline generally is recommended over minocycline given lack of evidence of superior efficacy of minocycline and concerns about severe adverse cutaneous reactions and drug-induced lupus with minocycline.35
In recent years, there has been growing concern of the development of antibiotic resistance.5 Sarecycline is a narrow-spectrum tetracycline that was FDA approved for acne in 2018. In vitro studies demonstrate sarecycline maintains high efficacy against C acnes with less activity against other bacteria, particularly gram-negative enterobes.36 The selectivity of sarecycline may lessen alterations of the gut microbiome seen with other oral antibiotics and reduce gastrointestinal tract side effects. Although comparative effectiveness studies are lacking, sarecycline was efficacious in phase 3 trials with few side effects compared with placebo.37 However, at this time, given the absence of comparative effectiveness data and its high cost (Table), sarecycline likely is best reserved for patients with comorbidities (eg, gastrointestinal disease), those requiring long-term antibiotic therapy, or those with acne that has failed to respond to other oral antibiotics.
Hormonal Treatments—Hormonal treatments such as combined oral contraceptives (COCs) and spironolactone often are considered second-line options, though they may represent cost-effective and safe alternatives to oral antibiotics for women with moderate to severe acne.38-41 There currently are 4 COCs approved by the FDA for the treatment of moderate acne in postmenarcheal females: drospirenone-ethinyl estradiol (Yaz [Bayer HealthCare Pharmaceuticals, Inc]), ethinyl estradiol-norgestimate (Ortho Tri-Cyclen [Ortho-McNeil Pharmaceuticals, Inc]), drospirenone-ethinyl estradiol-levomefolate (Beyaz [Bayer HealthCare Pharmaceuticals, Inc]), and ethinyl estradiol-norethindrone acetate-ferrous fumarate (Estrostep Fe [Allergan USA, Inc]).5 Treatment with COCs has been shown to cause substantial reductions in lesion counts across all lesion types compared to placebo, and a meta-analysis of 24 randomized trials conducted by Arowojolu et al42 demonstrated no consistent differences in acne reduction among different COCs.43,44 Although oral antibiotics are associated with faster improvement than COCs, there is some evidence that they have similar efficacy at 6 months of therapy.45 Combined oral contraceptives are inexpensive and likely reflect a highly cost-effective option (Table).
Spironolactone is an aldosterone inhibitor and androgen receptor blocker that is used off label to treat acne. It is one of the least expensive systemic medications for acne (Table). Although randomized controlled trials are lacking, several large case series support the effectiveness of spironolactone for women with acne.38,46 In addition, observational data suggest spironolactone may have similar effectiveness to oral antibiotics.41 Spironolactone generally is well tolerated, with the most common adverse effects being menstrual irregularities, breast tenderness, and diuresis.47,48 Many of these adverse effects are dose dependent and less likely with the dosing used in acne care. Additionally, menstrual irregularities can be reduced by concomitant use of a COC.48
Although frequent potassium monitoring remains common among patients being treated with spironolactone, there is growing evidence to suggest that potassium monitoring is of low value in young healthy women with acne.49-51 Reducing this laboratory monitoring likely represents an opportunity to provide higher-value care to patients being treated with spironolactone. However, laboratory monitoring should be considered if risk factors for hyperkalemia are present (eg, older age, comorbidities, medications).51
Isotretinoin—Isotretinoin is the most efficacious treatment available for acne and has the unique property of being able to induce a remission of acne activity for many patients.5 Although it remains modestly expensive (Table), it may be less costly overall relative to other treatments that may need continued use over many years because it can induce a remission of acne activity. As with spironolactone, frequent laboratory monitoring remains common among patients being treated with isotretinoin. There is no evidence to support checking complete blood cell counts.52 Several observational studies and a Delphi consensus support reduced monitoring, such as checking lipids and alanine aminotransferase at baseline and peak dose in otherwise young healthy patients.53,54 A recent critically appraised topic published in the British Journal of Dermatology has proposed eliminating laboratory monitoring entirely.55 Reducing laboratory monitoring for patients being treated with isotretinoin has been estimated to potentially save $100 million to $200 million per year in the United States.52-54
Other Strategies to Reduce Patient Costs
Although choosing a cost-effective treatment approach is critical to preventing financial toxicity given poor coverage for acne care and the growth of high-deductible insurance plans, some patients may still experience high treatment costs.56 Because pharmacy costs often are inflated, potentially related to practices of pharmacy benefit managers, it often is possible to find better prices than the presented list price, either by using platforms such as GoodRx or through direct-to-patient mail-order pharmacies such as Cost Plus Drug.57 For branded medications, some patients may be eligible for patient-assistance programs, though they typically are not available for those with public insurance such as Medicare or Medicaid. Compounding pharmacies offer another approach to reduce cost and improve convenience for patients, but because the vehicle can influence the efficacy and tolerability of some topical medications, it is possible that these compounded formulations may not perform similarly to the original FDA-approved products.
Conclusion
For mild to moderate acne, multimodal topical therapy often is required. Fixed-dose combination adapalene-BPO and clindamycin-BPO are highly cost-effective options for most patients. Lotion formulations of topical retinoids may be useful in patients with difficulty tolerating other formulations. Clascoterone is a novel topical antiandrogen that is more expensive than other topical therapies but can complement other topical therapies and is well tolerated.
For moderate to severe acne, doxycycline or hormonal therapy (ie, COCs, spironolactone) are highly cost-effective options. Isotretinoin is recommended for severe or scarring acne. Reduced laboratory monitoring for spironolactone and isotretinoin is an opportunity to provide higher-value care.
- Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474-485. doi:10.1111/bjd.12149
- Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59. doi:10.1016/j.jaad.2007.06.045
- Webster GF. The pathophysiology of acne. Cutis. 2005;76(2 suppl):4-7.
- Degitz K, Placzek M, Borelli C, et al. Pathophysiology of acne. J Dtsch Dermatol Ges. 2007;5:316-323. doi:10.1111/j.1610-0387.2007.06274.x
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33. doi:10.1016/j.jaad.2015.12.037
- Felmingham C, Kerr A, Veysey E. Costs incurred by patients with acne prior to dermatological consultation and their relation to patient income. Australas J Dermatol. 2020;61:384-386. doi:10.1111/ajd.13324
- Perche P, Singh R, Feldman S. Patient preferences for acne vulgaris treatment and barriers to care: a survey study. J Drugs Dermatol. 2022;21:1191-1195. doi:10.36849/JDD.6940
- KFF Health Tracking Poll—February 2019. Accessed August 9, 2023. https://files.kff.org/attachment/Topline-KFF-Health-Tracking-Poll-February-2019
- Ryskina KL, Goldberg E, Lott B, et al. The role of the physician in patient perceptions of barriers to primary adherence with acne medications. JAMA Dermatol. 2018;154:456-459. doi:10.1001/jamadermatol.2017.6144
- Porter ME. What is value in health care? N Engl J Med. 2010;363:2477-2481. doi:10.1056/NEJMp1011024
- Barbieri JS, Tan JKL, Adamson AS. Active comparator trial designs used to promote development of innovative new medications. Cutis. 2020;106:E4-E6. doi:10.12788/cutis.0067
- Miller J, Ly S, Mostaghimi A, et al. Use of active comparator trials for topical medications in dermatology. JAMA Dermatol. 2021;157:597-599. doi:10.1001/jamadermatol.2021.0356
- GoodRx. Accessed August 11, 2023. https://www.goodrx.com
- Stuart B, Maund E, Wilcox C, et al. Topical preparations for the treatment of mild‐to‐moderate acne vulgaris: systematic review and network meta‐analysis. Br J Dermatol. 2021;185:512-525. doi:10.1111/bjd.20080
- Mavranezouli I, Welton NJ, Daly CH, et al. Cost-effectiveness of topical pharmacological, oral pharmacological, physical and combined treatments for acne vulgaris. Clin Exp Dermatol. 2022;47:2176-2187. doi:10.1111/ced.15356
- Tanghetti E, Werschler W, Lain T, et al. Tazarotene 0.045% lotion for once-daily treatment of moderate-to-severe acne vulgaris: results from two phase 3 trials. J Drugs Dermatol. 2020;19:70-77. doi:10.36849/JDD.2020.3977
- Tyring SK, Kircik LH, Pariser DM, et al. Novel tretinoin 0.05% lotion for the once-daily treatment of moderate-to-severe acne vulgaris: assessment of efficacy and safety in patients aged 9 years and older. J Drugs Dermatol. 2018;17:1084-1091.
- Tan J, Thiboutot D, Popp G, et al. Randomized phase 3 evaluation of trifarotene 50 μg/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol. 2019;80:1691-1699. doi:10.1016/j.jaad.2019.02.044
- Huang CY, Chang IJ, Bolick N, et al. Comparative efficacy of pharmacological treatments for acne vulgaris: a network meta-analysis of 221 randomized controlled trials. Ann Fam Med. 2023;21:358-369. doi:10.1370/afm.2995
- Draelos ZD. Low irritation potential of tazarotene 0.045% lotion: head-to-head comparison to adapalene 0.3% gel and trifarotene 0.005% cream in two studies. J Dermatolog Treat. 2023;34:2166346. doi:10.1080/09546634.2023.2166346
- Dessinioti C, Katsambas A. Antibiotics and antimicrobial resistance in acne: epidemiological trends and clinical practice considerations. Yale J Biol Med. 2022;95:429-443.
- Gold LS, Dhawan S, Weiss J, et al. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: results of 2 randomized, double-blind, phase 3 studies. J Am Acad Dermatol. 2019;80:168-177. doi:10.1016/j.jaad.2018.08.020
- Wang X, Wang Z, Sun L, et al. Efficacy and safety of dapsone gel for acne: a systematic review and meta-analysis. Ann Palliat Med. 2022;11:611-620. doi:10.21037/apm-21-3935
- Melián-Olivera A, Burgos-Blasco P, Selda-Enríquez G, et al. Topical dapsone for folliculitis decalvans: a retrospective cohort study. J Am Acad Dermatol. 2022;87:150-151. doi:10.1016/j.jaad.2021.07.004
- Yentzer BA, Ade RA, Fountain JM, et al. Simplifying regimens promotes greater adherence and outcomes with topical acne medications: a randomized controlled trial. Cutis. 2010;86:103-108.
- Ting W. Randomized, observer-blind, split-face study to compare the irritation potential of 2 topical acne formulations over a 14-day treatment period. Cutis. 2012;90:91-96.
- Aschoff R, Möller S, Haase R, et al. Tolerability and efficacy ofclindamycin/tretinoin versus adapalene/benzoyl peroxide in the treatment of acne vulgaris. J Drugs Dermatol. 2021;20:295-301. doi:10.36849/JDD.2021.5641
- Rosette C, Agan FJ, Mazzetti A, et al. Cortexolone 17α-propionate (clascoterone) is a novel androgen receptor antagonist that inhibits production of lipids and inflammatory cytokines from sebocytes in vitro. J Drugs Dermatol. 2019;18:412-418.
- Hebert A, Thiboutot D, Stein Gold L, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:621-630. doi:10.1001/jamadermatol.2020.0465
- Trifu V, Tiplica GS, Naumescu E, et al. Cortexolone 17α-propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. a pilot randomized, double-blind comparative study vs. placebo and tretinoin 0·05% cream. Br J Dermatol. 2011;165:177-183. doi:10.1111/j.1365-2133.2011.10332.x
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Guzman AK, Barbieri JS. Comparative analysis of prescribing patterns of tetracycline class antibiotics and spironolactone between advanced practice providers and physicians in the treatment of acne vulgaris. J Am Acad Dermatol. 2021;84:1119-1121. doi:10.1016/j.jaad.2020.06.044
- Barbieri JS, James WD, Margolis DJ. Trends in prescribing behavior of systemic agents used in the treatment of acne among dermatologists and nondermatologists: a retrospective analysis, 2004-2013. J Am Acad Dermatol. 2017;77:456-463.e4. doi:10.1016/j.jaad.2017.04.016
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016. JAMA Dermatol. 2019;155:290-297. doi:10.1001/jamadermatol.2018.4944
- Garner SE, Eady A, Bennett C, et al. Minocycline for acne vulgaris: efficacy and safety. Cochrane Database Syst Rev. 2012;2012:CD002086. doi:10.1002/14651858.CD002086.pub2
- Zhanel G, Critchley I, Lin LY, et al. Microbiological profile of sarecycline, a novel targeted spectrum tetracycline for the treatment of acne vulgaris. Antimicrob Agents Chemother. 2018;63:e01297-18. doi:10.1128/AAC.01297-18
- Moore A, Green LJ, Bruce S, et al. Once-daily oral sarecycline 1.5 mg/kg/day is effective for moderate to severe acne vulgaris: results from two identically designed, phase 3, randomized, double-blind clinical trials. J Drugs Dermatol. 2018;17:987-996.
- Garg V, Choi JK, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355. doi:10.1016/j.jaad.2020.12.071
- Barbieri JS, Choi JK, James WD, et al. Real-world drug usage survival of spironolactone versus oral antibiotics for the management of female patients with acne. J Am Acad Dermatol. 2019;81:848-851. doi:10.1016/j.jaad.2019.03.036
- Barbieri JS, Spaccarelli N, Margolis DJ, et al. Approaches to limit systemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments. J Am Acad Dermatol. 2019;80:538-549. doi:10.1016/j.jaad.2018.09.055
- Barbieri JS, Choi JK, Mitra N, et al. Frequency of treatment switching for spironolactone compared to oral tetracycline-class antibiotics for women with acne: a retrospective cohort study 2010-2016. J Drugs Dermatol. 2018;17:632-638.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;7:CD004425. doi:10.1002/14651858.CD004425.pub6
- Maloney JM, Dietze P, Watson D, et al. Treatment of acne using a 3-milligram drospirenone/20-microgram ethinyl estradiol oral contraceptive administered in a 24/4 regimen. Obstet Gynecol. 2008;112:773-781. doi:10.1097/AOG.0b013e318187e1c5
- Lucky AW, Koltun W, Thiboutot D, et al. A combined oral contraceptive containing 3-mg drospirenone/20-microg ethinyl estradiol in the treatment of acne vulgaris: a randomized, double-blind, placebo-controlled study evaluating lesion counts and participant self-assessment. Cutis. 2008;82:143-150.
- Koo EB, Petersen TD, Kimball AB. Meta-analysis comparing efficacy of antibiotics versus oral contraceptives in acne vulgaris. J Am Acad Dermatol. 2014;71:450-459. doi:10.1016/j.jaad.2014.03.051
- Roberts EE, Nowsheen S, Davis DMR, et al. Use of spironolactone to treat acne in adolescent females. Pediatr Dermatol. 2021;38:72-76. doi:10.1111/pde.14391
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502. doi:10.1067/mjd.2000.105557
- Layton AM, Eady EA, Whitehouse H, et al. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol. 2017;18:169-191. doi:10.1007/s40257-016-0245-x
- Barbieri JS, Margolis DJ, Mostaghimi A. Temporal trends and clinician variability in potassium monitoring of healthy young women treated for acne with spironolactone. JAMA Dermatol. 2021;157:296-300. doi:10.1001/jamadermatol.2020.5468
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944. doi:10.1001/jamadermatol.2015.34
- Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157. doi:10.1016/j.ijwd.2019.04.024
- Barbieri JS, Shin DB, Wang S, et al. The clinical utility of laboratory monitoring during isotretinoin therapy for acne and changes to monitoring practices over time. J Am Acad Dermatol. 2020;82:72-79. doi:10.1016/j.jaad.2019.06.025
- Lee YH, Scharnitz TP, Muscat J, et al. Laboratory monitoring during isotretinoin therapy for acne: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:35-44. doi:10.1001/jamadermatol.2015.3091
- Xia E, Han J, Faletsky A, et al. Isotretinoin laboratory monitoring in acne treatment: a Delphi consensus study. JAMA Dermatol. 2022;158:942-948. doi:10.1001/jamadermatol.2022.2044
- Affleck A, Jackson D, Williams HC, et al. Is routine laboratory testing in healthy young patients taking isotretinoin necessary: a critically appraised topic. Br J Dermatol. 2022;187:857-865. doi:10.1111/bjd.21840
- Barbieri JS, LaChance A, Albrecht J. Double standards and inconsistencies in access to care-what constitutes a cosmetic treatment? JAMA Dermatol. 2023;159:245-246. doi:10.1001/jamadermatol.2022.6322
- Trish E, Van Nuys K, Popovian R. US consumers overpay for generic drugs. Schaeffer Center White Paper Series. May 31, 2022. doi:10.25549/m589-2268
- Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474-485. doi:10.1111/bjd.12149
- Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59. doi:10.1016/j.jaad.2007.06.045
- Webster GF. The pathophysiology of acne. Cutis. 2005;76(2 suppl):4-7.
- Degitz K, Placzek M, Borelli C, et al. Pathophysiology of acne. J Dtsch Dermatol Ges. 2007;5:316-323. doi:10.1111/j.1610-0387.2007.06274.x
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33. doi:10.1016/j.jaad.2015.12.037
- Felmingham C, Kerr A, Veysey E. Costs incurred by patients with acne prior to dermatological consultation and their relation to patient income. Australas J Dermatol. 2020;61:384-386. doi:10.1111/ajd.13324
- Perche P, Singh R, Feldman S. Patient preferences for acne vulgaris treatment and barriers to care: a survey study. J Drugs Dermatol. 2022;21:1191-1195. doi:10.36849/JDD.6940
- KFF Health Tracking Poll—February 2019. Accessed August 9, 2023. https://files.kff.org/attachment/Topline-KFF-Health-Tracking-Poll-February-2019
- Ryskina KL, Goldberg E, Lott B, et al. The role of the physician in patient perceptions of barriers to primary adherence with acne medications. JAMA Dermatol. 2018;154:456-459. doi:10.1001/jamadermatol.2017.6144
- Porter ME. What is value in health care? N Engl J Med. 2010;363:2477-2481. doi:10.1056/NEJMp1011024
- Barbieri JS, Tan JKL, Adamson AS. Active comparator trial designs used to promote development of innovative new medications. Cutis. 2020;106:E4-E6. doi:10.12788/cutis.0067
- Miller J, Ly S, Mostaghimi A, et al. Use of active comparator trials for topical medications in dermatology. JAMA Dermatol. 2021;157:597-599. doi:10.1001/jamadermatol.2021.0356
- GoodRx. Accessed August 11, 2023. https://www.goodrx.com
- Stuart B, Maund E, Wilcox C, et al. Topical preparations for the treatment of mild‐to‐moderate acne vulgaris: systematic review and network meta‐analysis. Br J Dermatol. 2021;185:512-525. doi:10.1111/bjd.20080
- Mavranezouli I, Welton NJ, Daly CH, et al. Cost-effectiveness of topical pharmacological, oral pharmacological, physical and combined treatments for acne vulgaris. Clin Exp Dermatol. 2022;47:2176-2187. doi:10.1111/ced.15356
- Tanghetti E, Werschler W, Lain T, et al. Tazarotene 0.045% lotion for once-daily treatment of moderate-to-severe acne vulgaris: results from two phase 3 trials. J Drugs Dermatol. 2020;19:70-77. doi:10.36849/JDD.2020.3977
- Tyring SK, Kircik LH, Pariser DM, et al. Novel tretinoin 0.05% lotion for the once-daily treatment of moderate-to-severe acne vulgaris: assessment of efficacy and safety in patients aged 9 years and older. J Drugs Dermatol. 2018;17:1084-1091.
- Tan J, Thiboutot D, Popp G, et al. Randomized phase 3 evaluation of trifarotene 50 μg/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol. 2019;80:1691-1699. doi:10.1016/j.jaad.2019.02.044
- Huang CY, Chang IJ, Bolick N, et al. Comparative efficacy of pharmacological treatments for acne vulgaris: a network meta-analysis of 221 randomized controlled trials. Ann Fam Med. 2023;21:358-369. doi:10.1370/afm.2995
- Draelos ZD. Low irritation potential of tazarotene 0.045% lotion: head-to-head comparison to adapalene 0.3% gel and trifarotene 0.005% cream in two studies. J Dermatolog Treat. 2023;34:2166346. doi:10.1080/09546634.2023.2166346
- Dessinioti C, Katsambas A. Antibiotics and antimicrobial resistance in acne: epidemiological trends and clinical practice considerations. Yale J Biol Med. 2022;95:429-443.
- Gold LS, Dhawan S, Weiss J, et al. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: results of 2 randomized, double-blind, phase 3 studies. J Am Acad Dermatol. 2019;80:168-177. doi:10.1016/j.jaad.2018.08.020
- Wang X, Wang Z, Sun L, et al. Efficacy and safety of dapsone gel for acne: a systematic review and meta-analysis. Ann Palliat Med. 2022;11:611-620. doi:10.21037/apm-21-3935
- Melián-Olivera A, Burgos-Blasco P, Selda-Enríquez G, et al. Topical dapsone for folliculitis decalvans: a retrospective cohort study. J Am Acad Dermatol. 2022;87:150-151. doi:10.1016/j.jaad.2021.07.004
- Yentzer BA, Ade RA, Fountain JM, et al. Simplifying regimens promotes greater adherence and outcomes with topical acne medications: a randomized controlled trial. Cutis. 2010;86:103-108.
- Ting W. Randomized, observer-blind, split-face study to compare the irritation potential of 2 topical acne formulations over a 14-day treatment period. Cutis. 2012;90:91-96.
- Aschoff R, Möller S, Haase R, et al. Tolerability and efficacy ofclindamycin/tretinoin versus adapalene/benzoyl peroxide in the treatment of acne vulgaris. J Drugs Dermatol. 2021;20:295-301. doi:10.36849/JDD.2021.5641
- Rosette C, Agan FJ, Mazzetti A, et al. Cortexolone 17α-propionate (clascoterone) is a novel androgen receptor antagonist that inhibits production of lipids and inflammatory cytokines from sebocytes in vitro. J Drugs Dermatol. 2019;18:412-418.
- Hebert A, Thiboutot D, Stein Gold L, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:621-630. doi:10.1001/jamadermatol.2020.0465
- Trifu V, Tiplica GS, Naumescu E, et al. Cortexolone 17α-propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. a pilot randomized, double-blind comparative study vs. placebo and tretinoin 0·05% cream. Br J Dermatol. 2011;165:177-183. doi:10.1111/j.1365-2133.2011.10332.x
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Guzman AK, Barbieri JS. Comparative analysis of prescribing patterns of tetracycline class antibiotics and spironolactone between advanced practice providers and physicians in the treatment of acne vulgaris. J Am Acad Dermatol. 2021;84:1119-1121. doi:10.1016/j.jaad.2020.06.044
- Barbieri JS, James WD, Margolis DJ. Trends in prescribing behavior of systemic agents used in the treatment of acne among dermatologists and nondermatologists: a retrospective analysis, 2004-2013. J Am Acad Dermatol. 2017;77:456-463.e4. doi:10.1016/j.jaad.2017.04.016
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016. JAMA Dermatol. 2019;155:290-297. doi:10.1001/jamadermatol.2018.4944
- Garner SE, Eady A, Bennett C, et al. Minocycline for acne vulgaris: efficacy and safety. Cochrane Database Syst Rev. 2012;2012:CD002086. doi:10.1002/14651858.CD002086.pub2
- Zhanel G, Critchley I, Lin LY, et al. Microbiological profile of sarecycline, a novel targeted spectrum tetracycline for the treatment of acne vulgaris. Antimicrob Agents Chemother. 2018;63:e01297-18. doi:10.1128/AAC.01297-18
- Moore A, Green LJ, Bruce S, et al. Once-daily oral sarecycline 1.5 mg/kg/day is effective for moderate to severe acne vulgaris: results from two identically designed, phase 3, randomized, double-blind clinical trials. J Drugs Dermatol. 2018;17:987-996.
- Garg V, Choi JK, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355. doi:10.1016/j.jaad.2020.12.071
- Barbieri JS, Choi JK, James WD, et al. Real-world drug usage survival of spironolactone versus oral antibiotics for the management of female patients with acne. J Am Acad Dermatol. 2019;81:848-851. doi:10.1016/j.jaad.2019.03.036
- Barbieri JS, Spaccarelli N, Margolis DJ, et al. Approaches to limit systemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments. J Am Acad Dermatol. 2019;80:538-549. doi:10.1016/j.jaad.2018.09.055
- Barbieri JS, Choi JK, Mitra N, et al. Frequency of treatment switching for spironolactone compared to oral tetracycline-class antibiotics for women with acne: a retrospective cohort study 2010-2016. J Drugs Dermatol. 2018;17:632-638.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;7:CD004425. doi:10.1002/14651858.CD004425.pub6
- Maloney JM, Dietze P, Watson D, et al. Treatment of acne using a 3-milligram drospirenone/20-microgram ethinyl estradiol oral contraceptive administered in a 24/4 regimen. Obstet Gynecol. 2008;112:773-781. doi:10.1097/AOG.0b013e318187e1c5
- Lucky AW, Koltun W, Thiboutot D, et al. A combined oral contraceptive containing 3-mg drospirenone/20-microg ethinyl estradiol in the treatment of acne vulgaris: a randomized, double-blind, placebo-controlled study evaluating lesion counts and participant self-assessment. Cutis. 2008;82:143-150.
- Koo EB, Petersen TD, Kimball AB. Meta-analysis comparing efficacy of antibiotics versus oral contraceptives in acne vulgaris. J Am Acad Dermatol. 2014;71:450-459. doi:10.1016/j.jaad.2014.03.051
- Roberts EE, Nowsheen S, Davis DMR, et al. Use of spironolactone to treat acne in adolescent females. Pediatr Dermatol. 2021;38:72-76. doi:10.1111/pde.14391
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502. doi:10.1067/mjd.2000.105557
- Layton AM, Eady EA, Whitehouse H, et al. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol. 2017;18:169-191. doi:10.1007/s40257-016-0245-x
- Barbieri JS, Margolis DJ, Mostaghimi A. Temporal trends and clinician variability in potassium monitoring of healthy young women treated for acne with spironolactone. JAMA Dermatol. 2021;157:296-300. doi:10.1001/jamadermatol.2020.5468
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944. doi:10.1001/jamadermatol.2015.34
- Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157. doi:10.1016/j.ijwd.2019.04.024
- Barbieri JS, Shin DB, Wang S, et al. The clinical utility of laboratory monitoring during isotretinoin therapy for acne and changes to monitoring practices over time. J Am Acad Dermatol. 2020;82:72-79. doi:10.1016/j.jaad.2019.06.025
- Lee YH, Scharnitz TP, Muscat J, et al. Laboratory monitoring during isotretinoin therapy for acne: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:35-44. doi:10.1001/jamadermatol.2015.3091
- Xia E, Han J, Faletsky A, et al. Isotretinoin laboratory monitoring in acne treatment: a Delphi consensus study. JAMA Dermatol. 2022;158:942-948. doi:10.1001/jamadermatol.2022.2044
- Affleck A, Jackson D, Williams HC, et al. Is routine laboratory testing in healthy young patients taking isotretinoin necessary: a critically appraised topic. Br J Dermatol. 2022;187:857-865. doi:10.1111/bjd.21840
- Barbieri JS, LaChance A, Albrecht J. Double standards and inconsistencies in access to care-what constitutes a cosmetic treatment? JAMA Dermatol. 2023;159:245-246. doi:10.1001/jamadermatol.2022.6322
- Trish E, Van Nuys K, Popovian R. US consumers overpay for generic drugs. Schaeffer Center White Paper Series. May 31, 2022. doi:10.25549/m589-2268
Practice Points
- For mild to moderate acne, fixed-dose combination adapalene–benzoyl peroxide and clindamycin–benzoyl peroxide are highly cost-effective options for most patients.
- For moderate to severe acne, doxycycline or hormonal therapy (ie, combined oral contraceptives, spironolactone) are highly cost-effective options.
- Reduction of laboratory monitoring for spironolactone and isotretinoin is an opportunity to provide higher-value care.
Telemedicine and Home Pregnancy Testing for iPLEDGE: A Survey of Clinician Perspectives
To the Editor:
In response to the challenges of the COVID-19 pandemic, iPLEDGE announced that they would accept results from home pregnancy tests and explicitly permit telemedicine.1 Given the financial and logistical burdens associated with iPLEDGE, these changes have the potential to increase access.2 However, it is unclear whether these modifications will be allowed to continue. We sought to evaluate clinician perspectives on the role of telemedicine and home pregnancy testing for iPLEDGE.
After piloting among several clinicians, a 13-question survey was distributed using the Qualtrics platform to members of the American Acne & Rosacea Society between April 14, 2021, and June 14, 2021. This survey consisted of items addressing provider practices and perspectives on telemedicine and home pregnancy testing for patients taking isotretinoin (eTable). Respondents were asked whether they think telemedicine and home pregnancy testing have improved access to care and whether they would like to continue these practices going forward. In addition, participants were asked about their concerns with home pregnancy testing and how comfortable they feel with home pregnancy testing for various contraceptive strategies (abstinence, condoms, combined oral contraceptives, and long-acting reversible contraception). This study was deemed exempt (category 2) by the University of Pennsylvania (Philadelphia, Pennsylvania) institutional review board (Protocol #844549).
Among 70 clinicians who completed the survey (response rate, 6.4%), 33 (47.1%) practiced in an academic setting. At the peak of the COVID-19 pandemic, clinicians reported using telemedicine for a median of 90% (IQR=50%–100%) of their patients on isotretinoin, and 57 respondents (81.4%) reported having patients use a home pregnancy test for iPLEDGE (Table 1). More than 75% (55/70) agreed that they would like to continue to use telemedicine for patients on isotretinoin, and more than 75% (54/70) agreed that they would like to continue using home pregnancy testing for patients outside the setting of the COVID-19 pandemic. More than 75% (54/70) agreed that telemedicine has increased access for their patients, and more than 70% (52/70) agreed that home pregnancy testing has increased access (Table 2). Clinicians agreed that they would be comfortable using home pregnancy testing for patients choosing long-acting reversible contraception (63/70 [90.0%]), combined oral contraceptives (61/69 [88.4%]), condoms (47/70 [67.1%]), or abstinence (48/70 [68.6%])(Table 3).
The most common concerns about home pregnancy testing were patient deception (39/70 [55.7%]), logistical challenges with reviewing results (19/70 [27.1%]), accuracy of the tests (19/70 [27.1%]), and patient ability to interpret tests appropriately (18/70 [25.7%]). To document testing results, 50 respondents (73.5%) would require a picture of results, 4 (5.9%) would accept a written report from the patient, and 14 (20.6%) would accept a verbal report from the patient (Table 2).
In this survey, clinicians expressed interest in continuing to use telemedicine and home pregnancy testing to care for patients with acne treated with isotretinoin. More than 75% agreed that these changes have increased access, which is notable, as several studies have identified that female and minority patients may face iPLEDGE-associated access barriers.3,4 Continuing to allow home pregnancy testing and explicitly permitting telemedicine can enable clinicians to provide patient-centered care.2
Although clinicians felt comfortable with a variety of contraceptive strategies, particularly those with high reported effectiveness,5 there were concerns about deception and interpretation of test results. Future studies are needed to identify optimal workflows for home pregnancy testing and whether patients should be required to provide a photograph of the results.
This survey study is limited by the possibility of sampling and response bias due to the low response rate. Although the use of national listservs was employed to maximize the generalizability of the results, given the response rate, future studies are needed to evaluate whether these findings generalize to other settings. In addition, given iPLEDGE-associated access barriers, further research is needed to examine how changes such as telemedicine and home pregnancy testing influence both access to isotretinoin and pregnancy prevention.
Acknowledgments—We would like to thank Stacey Moore (Montclair, New Jersey) and the American Acne & Rosacea Society for their help distributing the survey.
- Kane S, Admani S. COVID-19 pandemic leading to the accelerated development of a virtual health model for isotretinoin. J Dermatol Nurses Assoc. 2021;13:54-57.
- Barbieri JS, Frieden IJ, Nagler AR. Isotretinoin, patient safety, and patient-centered care-time to reform iPLEDGE. JAMA Dermatol. 2020;156:21-22.
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319.
- Charrow A, Xia FD, Lu J, et al. Differences in isotretinoin start, interruption, and early termination across race and sex in the iPLEDGE era. PloS One. 2019;14:E0210445.
- Barbieri JS, Roe AH, Mostaghimi A. Simplifying contraception requirements for iPLEDGE: a decision analysis. J Am Acad Dermatol. 2020;83:104-108.
To the Editor:
In response to the challenges of the COVID-19 pandemic, iPLEDGE announced that they would accept results from home pregnancy tests and explicitly permit telemedicine.1 Given the financial and logistical burdens associated with iPLEDGE, these changes have the potential to increase access.2 However, it is unclear whether these modifications will be allowed to continue. We sought to evaluate clinician perspectives on the role of telemedicine and home pregnancy testing for iPLEDGE.
After piloting among several clinicians, a 13-question survey was distributed using the Qualtrics platform to members of the American Acne & Rosacea Society between April 14, 2021, and June 14, 2021. This survey consisted of items addressing provider practices and perspectives on telemedicine and home pregnancy testing for patients taking isotretinoin (eTable). Respondents were asked whether they think telemedicine and home pregnancy testing have improved access to care and whether they would like to continue these practices going forward. In addition, participants were asked about their concerns with home pregnancy testing and how comfortable they feel with home pregnancy testing for various contraceptive strategies (abstinence, condoms, combined oral contraceptives, and long-acting reversible contraception). This study was deemed exempt (category 2) by the University of Pennsylvania (Philadelphia, Pennsylvania) institutional review board (Protocol #844549).
Among 70 clinicians who completed the survey (response rate, 6.4%), 33 (47.1%) practiced in an academic setting. At the peak of the COVID-19 pandemic, clinicians reported using telemedicine for a median of 90% (IQR=50%–100%) of their patients on isotretinoin, and 57 respondents (81.4%) reported having patients use a home pregnancy test for iPLEDGE (Table 1). More than 75% (55/70) agreed that they would like to continue to use telemedicine for patients on isotretinoin, and more than 75% (54/70) agreed that they would like to continue using home pregnancy testing for patients outside the setting of the COVID-19 pandemic. More than 75% (54/70) agreed that telemedicine has increased access for their patients, and more than 70% (52/70) agreed that home pregnancy testing has increased access (Table 2). Clinicians agreed that they would be comfortable using home pregnancy testing for patients choosing long-acting reversible contraception (63/70 [90.0%]), combined oral contraceptives (61/69 [88.4%]), condoms (47/70 [67.1%]), or abstinence (48/70 [68.6%])(Table 3).
The most common concerns about home pregnancy testing were patient deception (39/70 [55.7%]), logistical challenges with reviewing results (19/70 [27.1%]), accuracy of the tests (19/70 [27.1%]), and patient ability to interpret tests appropriately (18/70 [25.7%]). To document testing results, 50 respondents (73.5%) would require a picture of results, 4 (5.9%) would accept a written report from the patient, and 14 (20.6%) would accept a verbal report from the patient (Table 2).
In this survey, clinicians expressed interest in continuing to use telemedicine and home pregnancy testing to care for patients with acne treated with isotretinoin. More than 75% agreed that these changes have increased access, which is notable, as several studies have identified that female and minority patients may face iPLEDGE-associated access barriers.3,4 Continuing to allow home pregnancy testing and explicitly permitting telemedicine can enable clinicians to provide patient-centered care.2
Although clinicians felt comfortable with a variety of contraceptive strategies, particularly those with high reported effectiveness,5 there were concerns about deception and interpretation of test results. Future studies are needed to identify optimal workflows for home pregnancy testing and whether patients should be required to provide a photograph of the results.
This survey study is limited by the possibility of sampling and response bias due to the low response rate. Although the use of national listservs was employed to maximize the generalizability of the results, given the response rate, future studies are needed to evaluate whether these findings generalize to other settings. In addition, given iPLEDGE-associated access barriers, further research is needed to examine how changes such as telemedicine and home pregnancy testing influence both access to isotretinoin and pregnancy prevention.
Acknowledgments—We would like to thank Stacey Moore (Montclair, New Jersey) and the American Acne & Rosacea Society for their help distributing the survey.
To the Editor:
In response to the challenges of the COVID-19 pandemic, iPLEDGE announced that they would accept results from home pregnancy tests and explicitly permit telemedicine.1 Given the financial and logistical burdens associated with iPLEDGE, these changes have the potential to increase access.2 However, it is unclear whether these modifications will be allowed to continue. We sought to evaluate clinician perspectives on the role of telemedicine and home pregnancy testing for iPLEDGE.
After piloting among several clinicians, a 13-question survey was distributed using the Qualtrics platform to members of the American Acne & Rosacea Society between April 14, 2021, and June 14, 2021. This survey consisted of items addressing provider practices and perspectives on telemedicine and home pregnancy testing for patients taking isotretinoin (eTable). Respondents were asked whether they think telemedicine and home pregnancy testing have improved access to care and whether they would like to continue these practices going forward. In addition, participants were asked about their concerns with home pregnancy testing and how comfortable they feel with home pregnancy testing for various contraceptive strategies (abstinence, condoms, combined oral contraceptives, and long-acting reversible contraception). This study was deemed exempt (category 2) by the University of Pennsylvania (Philadelphia, Pennsylvania) institutional review board (Protocol #844549).
Among 70 clinicians who completed the survey (response rate, 6.4%), 33 (47.1%) practiced in an academic setting. At the peak of the COVID-19 pandemic, clinicians reported using telemedicine for a median of 90% (IQR=50%–100%) of their patients on isotretinoin, and 57 respondents (81.4%) reported having patients use a home pregnancy test for iPLEDGE (Table 1). More than 75% (55/70) agreed that they would like to continue to use telemedicine for patients on isotretinoin, and more than 75% (54/70) agreed that they would like to continue using home pregnancy testing for patients outside the setting of the COVID-19 pandemic. More than 75% (54/70) agreed that telemedicine has increased access for their patients, and more than 70% (52/70) agreed that home pregnancy testing has increased access (Table 2). Clinicians agreed that they would be comfortable using home pregnancy testing for patients choosing long-acting reversible contraception (63/70 [90.0%]), combined oral contraceptives (61/69 [88.4%]), condoms (47/70 [67.1%]), or abstinence (48/70 [68.6%])(Table 3).
The most common concerns about home pregnancy testing were patient deception (39/70 [55.7%]), logistical challenges with reviewing results (19/70 [27.1%]), accuracy of the tests (19/70 [27.1%]), and patient ability to interpret tests appropriately (18/70 [25.7%]). To document testing results, 50 respondents (73.5%) would require a picture of results, 4 (5.9%) would accept a written report from the patient, and 14 (20.6%) would accept a verbal report from the patient (Table 2).
In this survey, clinicians expressed interest in continuing to use telemedicine and home pregnancy testing to care for patients with acne treated with isotretinoin. More than 75% agreed that these changes have increased access, which is notable, as several studies have identified that female and minority patients may face iPLEDGE-associated access barriers.3,4 Continuing to allow home pregnancy testing and explicitly permitting telemedicine can enable clinicians to provide patient-centered care.2
Although clinicians felt comfortable with a variety of contraceptive strategies, particularly those with high reported effectiveness,5 there were concerns about deception and interpretation of test results. Future studies are needed to identify optimal workflows for home pregnancy testing and whether patients should be required to provide a photograph of the results.
This survey study is limited by the possibility of sampling and response bias due to the low response rate. Although the use of national listservs was employed to maximize the generalizability of the results, given the response rate, future studies are needed to evaluate whether these findings generalize to other settings. In addition, given iPLEDGE-associated access barriers, further research is needed to examine how changes such as telemedicine and home pregnancy testing influence both access to isotretinoin and pregnancy prevention.
Acknowledgments—We would like to thank Stacey Moore (Montclair, New Jersey) and the American Acne & Rosacea Society for their help distributing the survey.
- Kane S, Admani S. COVID-19 pandemic leading to the accelerated development of a virtual health model for isotretinoin. J Dermatol Nurses Assoc. 2021;13:54-57.
- Barbieri JS, Frieden IJ, Nagler AR. Isotretinoin, patient safety, and patient-centered care-time to reform iPLEDGE. JAMA Dermatol. 2020;156:21-22.
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319.
- Charrow A, Xia FD, Lu J, et al. Differences in isotretinoin start, interruption, and early termination across race and sex in the iPLEDGE era. PloS One. 2019;14:E0210445.
- Barbieri JS, Roe AH, Mostaghimi A. Simplifying contraception requirements for iPLEDGE: a decision analysis. J Am Acad Dermatol. 2020;83:104-108.
- Kane S, Admani S. COVID-19 pandemic leading to the accelerated development of a virtual health model for isotretinoin. J Dermatol Nurses Assoc. 2021;13:54-57.
- Barbieri JS, Frieden IJ, Nagler AR. Isotretinoin, patient safety, and patient-centered care-time to reform iPLEDGE. JAMA Dermatol. 2020;156:21-22.
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319.
- Charrow A, Xia FD, Lu J, et al. Differences in isotretinoin start, interruption, and early termination across race and sex in the iPLEDGE era. PloS One. 2019;14:E0210445.
- Barbieri JS, Roe AH, Mostaghimi A. Simplifying contraception requirements for iPLEDGE: a decision analysis. J Am Acad Dermatol. 2020;83:104-108.
PRACTICE POINTS
- The majority of clinicians report that the use of telemedicine and home pregnancy testing for iPLEDGE has improved access to care and that they would like to continue these practices.
- Continuing to allow home pregnancy testing and explicitly permitting telemedicine can enable clinicians to provide patient-centered care for patients treated with isotretinoin.
Comparison of Shave and Punch Biopsy Utilization Among Dermatology Practices
In 2019, the 2 Current Procedural Terminology (CPT) codes for skin biopsies (11100 and 11101) were replaced with 6 new CPT codes that specify biopsy technique and associated procedural complexity. 1,2 Prior to the coding changes, all biopsies were reimbursed at the same payment level, but a punch biopsy (11104; national nonfacility Medicare payment, $133.29) is now reimbursed more than a shave biopsy (11102; national nonfacility Medicare payment, $106.42). 3 We sought to evaluate whether the decrease in reimbursement for shave biopsies and concurrent increase in reimbursement for punch biopsies led to a shift from shave to punch biopsy utilization.
Methods
We examined shave and punch biopsies submitted for pathologic examination at Brigham and Women’s Hospital, Massachusetts General Hospital, and Massachusetts General Physician’s Organization (all in Boston, Massachusetts), and Penn Medicine, University of Pennsylvania Health System (Philadelphia, Pennsylvania), in May 2018 vs May 2019 (four months after new codes were implemented). This study was approved by Partners HealthCare (Boston, Massachusetts) and the University of Pennsylvania institutional review boards.
We included shave and punch biopsies of skin performed by physician dermatologists and mid-level providers (ie, physician assistants, nurse practitioners) at dermatology practices. All biopsies performed by a technique other than shave or punch, unspecified biopsy type, consultation cases, nonskin biopsies (eg, mucosa), and biopsies performed at nondermatology practices were excluded. We also excluded biopsies by providers who were not present during both study periods to account for provider mix.
Statistical Analysis
To evaluate for changes in the ratio of shave to punch biopsy utilization over time, we performed χ2 tests. Because care practices may differ between private and academic settings as well as between physicians and mid-level providers, we performed subgroup analyses by practice setting and provider type.4
Results
We identified 11,785 biopsies (12.11% of which were punch) submitted for pathologic examination in May 2018 compared to 11,291 biopsies (12.08% of which were punch) in May 2019 (Table). The overall use of punch biopsies relative to shave biopsies did not change between the years. There was a relative decrease in punch biopsy use among academic practices (−1.88%; P=.032) and a relative increase in punch biopsy use among private practices (+0.90%; P=.043). Provider type was not associated with differing utilization of biopsy type.
Comment
Overall, there was not a considerable shift in utilization behavior from shave to punch biopsies after the introduction of new coding changes. However, our study does demonstrate a small yet significant increase in punch biopsy utilization among private practices, and a decrease among academic practices. Although the change in biopsy utilization behavior is small in magnitude, it may have a substantial impact when extrapolated to behavior across the entire United States.
We were unable to assess additional factors, such as clinical diagnosis, body site, and cosmetic concerns, that may impact biopsy type selection in this study. Although we included multiple study sites to improve generalizability, our findings may not be representative of all biopsies performed in the dermatology setting. The baseline difference in relative punch biopsy use in academic vs private practices may reflect differences in patient populations and chief concerns, but assuming these features are stable over a 1-year time period, our findings should remain valid. Future studies should focus on qualitative evaluations of physician decision-making and evaluate whether similar trends persist over time.
Conclusion
Skin biopsy utilization trends among differing practice and provider types should continue to be monitored to assess for longitudinal trends in utilization within the context of updated billing codes and associated reimbursements.
- Grider D. 2019 CPT® coding for skin biopsies. ICD10 monitor website. September 17, 2018. Updated January 7, 2019. Accessed February 17, 2021. https://www.icd10monitor.com/2019-cpt-coding-for-skin-biopsies 2.
- Tongdee E, Siegel DM, Markowitz O. New diagnostic procedure codes and reimbursement. Cutis. 2019;103:208-211.
- Search the physician fee schedule. Centers for Medicare & Medicaid Services website. Updated January 20, 2021. Accessed February 17, 2021. https://www.cms.gov/medicare/physician-fee-schedule/search
- Zhang M, Zippin J, Kaffenberger B. Trends and scope of dermatology procedures billed by advanced practice professionals from 2012 through 2015. JAMA Dermatol. 2018;154:1040-1044.
In 2019, the 2 Current Procedural Terminology (CPT) codes for skin biopsies (11100 and 11101) were replaced with 6 new CPT codes that specify biopsy technique and associated procedural complexity. 1,2 Prior to the coding changes, all biopsies were reimbursed at the same payment level, but a punch biopsy (11104; national nonfacility Medicare payment, $133.29) is now reimbursed more than a shave biopsy (11102; national nonfacility Medicare payment, $106.42). 3 We sought to evaluate whether the decrease in reimbursement for shave biopsies and concurrent increase in reimbursement for punch biopsies led to a shift from shave to punch biopsy utilization.
Methods
We examined shave and punch biopsies submitted for pathologic examination at Brigham and Women’s Hospital, Massachusetts General Hospital, and Massachusetts General Physician’s Organization (all in Boston, Massachusetts), and Penn Medicine, University of Pennsylvania Health System (Philadelphia, Pennsylvania), in May 2018 vs May 2019 (four months after new codes were implemented). This study was approved by Partners HealthCare (Boston, Massachusetts) and the University of Pennsylvania institutional review boards.
We included shave and punch biopsies of skin performed by physician dermatologists and mid-level providers (ie, physician assistants, nurse practitioners) at dermatology practices. All biopsies performed by a technique other than shave or punch, unspecified biopsy type, consultation cases, nonskin biopsies (eg, mucosa), and biopsies performed at nondermatology practices were excluded. We also excluded biopsies by providers who were not present during both study periods to account for provider mix.
Statistical Analysis
To evaluate for changes in the ratio of shave to punch biopsy utilization over time, we performed χ2 tests. Because care practices may differ between private and academic settings as well as between physicians and mid-level providers, we performed subgroup analyses by practice setting and provider type.4
Results
We identified 11,785 biopsies (12.11% of which were punch) submitted for pathologic examination in May 2018 compared to 11,291 biopsies (12.08% of which were punch) in May 2019 (Table). The overall use of punch biopsies relative to shave biopsies did not change between the years. There was a relative decrease in punch biopsy use among academic practices (−1.88%; P=.032) and a relative increase in punch biopsy use among private practices (+0.90%; P=.043). Provider type was not associated with differing utilization of biopsy type.
Comment
Overall, there was not a considerable shift in utilization behavior from shave to punch biopsies after the introduction of new coding changes. However, our study does demonstrate a small yet significant increase in punch biopsy utilization among private practices, and a decrease among academic practices. Although the change in biopsy utilization behavior is small in magnitude, it may have a substantial impact when extrapolated to behavior across the entire United States.
We were unable to assess additional factors, such as clinical diagnosis, body site, and cosmetic concerns, that may impact biopsy type selection in this study. Although we included multiple study sites to improve generalizability, our findings may not be representative of all biopsies performed in the dermatology setting. The baseline difference in relative punch biopsy use in academic vs private practices may reflect differences in patient populations and chief concerns, but assuming these features are stable over a 1-year time period, our findings should remain valid. Future studies should focus on qualitative evaluations of physician decision-making and evaluate whether similar trends persist over time.
Conclusion
Skin biopsy utilization trends among differing practice and provider types should continue to be monitored to assess for longitudinal trends in utilization within the context of updated billing codes and associated reimbursements.
In 2019, the 2 Current Procedural Terminology (CPT) codes for skin biopsies (11100 and 11101) were replaced with 6 new CPT codes that specify biopsy technique and associated procedural complexity. 1,2 Prior to the coding changes, all biopsies were reimbursed at the same payment level, but a punch biopsy (11104; national nonfacility Medicare payment, $133.29) is now reimbursed more than a shave biopsy (11102; national nonfacility Medicare payment, $106.42). 3 We sought to evaluate whether the decrease in reimbursement for shave biopsies and concurrent increase in reimbursement for punch biopsies led to a shift from shave to punch biopsy utilization.
Methods
We examined shave and punch biopsies submitted for pathologic examination at Brigham and Women’s Hospital, Massachusetts General Hospital, and Massachusetts General Physician’s Organization (all in Boston, Massachusetts), and Penn Medicine, University of Pennsylvania Health System (Philadelphia, Pennsylvania), in May 2018 vs May 2019 (four months after new codes were implemented). This study was approved by Partners HealthCare (Boston, Massachusetts) and the University of Pennsylvania institutional review boards.
We included shave and punch biopsies of skin performed by physician dermatologists and mid-level providers (ie, physician assistants, nurse practitioners) at dermatology practices. All biopsies performed by a technique other than shave or punch, unspecified biopsy type, consultation cases, nonskin biopsies (eg, mucosa), and biopsies performed at nondermatology practices were excluded. We also excluded biopsies by providers who were not present during both study periods to account for provider mix.
Statistical Analysis
To evaluate for changes in the ratio of shave to punch biopsy utilization over time, we performed χ2 tests. Because care practices may differ between private and academic settings as well as between physicians and mid-level providers, we performed subgroup analyses by practice setting and provider type.4
Results
We identified 11,785 biopsies (12.11% of which were punch) submitted for pathologic examination in May 2018 compared to 11,291 biopsies (12.08% of which were punch) in May 2019 (Table). The overall use of punch biopsies relative to shave biopsies did not change between the years. There was a relative decrease in punch biopsy use among academic practices (−1.88%; P=.032) and a relative increase in punch biopsy use among private practices (+0.90%; P=.043). Provider type was not associated with differing utilization of biopsy type.
Comment
Overall, there was not a considerable shift in utilization behavior from shave to punch biopsies after the introduction of new coding changes. However, our study does demonstrate a small yet significant increase in punch biopsy utilization among private practices, and a decrease among academic practices. Although the change in biopsy utilization behavior is small in magnitude, it may have a substantial impact when extrapolated to behavior across the entire United States.
We were unable to assess additional factors, such as clinical diagnosis, body site, and cosmetic concerns, that may impact biopsy type selection in this study. Although we included multiple study sites to improve generalizability, our findings may not be representative of all biopsies performed in the dermatology setting. The baseline difference in relative punch biopsy use in academic vs private practices may reflect differences in patient populations and chief concerns, but assuming these features are stable over a 1-year time period, our findings should remain valid. Future studies should focus on qualitative evaluations of physician decision-making and evaluate whether similar trends persist over time.
Conclusion
Skin biopsy utilization trends among differing practice and provider types should continue to be monitored to assess for longitudinal trends in utilization within the context of updated billing codes and associated reimbursements.
- Grider D. 2019 CPT® coding for skin biopsies. ICD10 monitor website. September 17, 2018. Updated January 7, 2019. Accessed February 17, 2021. https://www.icd10monitor.com/2019-cpt-coding-for-skin-biopsies 2.
- Tongdee E, Siegel DM, Markowitz O. New diagnostic procedure codes and reimbursement. Cutis. 2019;103:208-211.
- Search the physician fee schedule. Centers for Medicare & Medicaid Services website. Updated January 20, 2021. Accessed February 17, 2021. https://www.cms.gov/medicare/physician-fee-schedule/search
- Zhang M, Zippin J, Kaffenberger B. Trends and scope of dermatology procedures billed by advanced practice professionals from 2012 through 2015. JAMA Dermatol. 2018;154:1040-1044.
- Grider D. 2019 CPT® coding for skin biopsies. ICD10 monitor website. September 17, 2018. Updated January 7, 2019. Accessed February 17, 2021. https://www.icd10monitor.com/2019-cpt-coding-for-skin-biopsies 2.
- Tongdee E, Siegel DM, Markowitz O. New diagnostic procedure codes and reimbursement. Cutis. 2019;103:208-211.
- Search the physician fee schedule. Centers for Medicare & Medicaid Services website. Updated January 20, 2021. Accessed February 17, 2021. https://www.cms.gov/medicare/physician-fee-schedule/search
- Zhang M, Zippin J, Kaffenberger B. Trends and scope of dermatology procedures billed by advanced practice professionals from 2012 through 2015. JAMA Dermatol. 2018;154:1040-1044.
Practice Points
- Dermatologists should be aware that skin biopsy billing codes and reimbursements were changed in 2019 to reflect their level of complexity, which may impact how often each type of biopsy is used.
- Even small shifts in biopsy utilization behavior among dermatologists in the context of reimbursement changes can have a large impact on net reimbursements.
Active Comparator Trial Designs Used to Promote Development of Innovative New Medications
Spending on medications is expected to grow from $344 billion in 2018 to $420 billion in 2023, largely driven by the introduction of new branded drugs.1 These costs place substantial financial burden on patients, with nearly 30% of patients not taking their prescriptions as directed because of costs. Although many new medications have transformed how we care for patients, others may not offer meaningful benefit over existing less-costly alternatives that are supported by declining effect sizes of conventional placebo-controlled trials.2 Most medications are approved based on placebo-controlled trial data that does not include an arm comparing the new drug to standard of care, leaving clinicians and patients unable to make meaningful comparisons when deciding on the most appropriate or cost-effective treatment. We consider ways in which clinicians, patients, payers, and regulators could compel more meaningful trials from industry.
Although we often look to the US Food and Drug Administration (FDA) to ensure rigorous and appropriate testing of new medications, the primary mission of the FDA is to ensure efficacy and safety. As a result, pharmaceutical companies seeking approval in the United States have little incentive to go beyond providing the minimal level of evidence required: placebo-controlled randomized trials. Although these trials provide important data on whether a treatment works and its associated risks, they do not provide data on comparative effectiveness. When relevant inexpensive medications are already on the market for the same indication, these placebo-controlled trials provide inadequate evidence to guide clinical decision-making. This issue is particularly relevant in dermatology given how easily topical medications can be combined or reformulated to pursue additional market exclusivity. The addition of an active comparator arm represents an important opportunity to improve the value of these studies.
In the pivotal trials of clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel for the treatment of acne, the experimental group was not only compared to vehicle but also the active comparator arms of clindamycin alone and benzoyl peroxide alone. The mean percentage change in total lesions was 47.9% with clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel, 41.6% with the active comparator arm of benzoyl peroxide alone, 40.4% with the active comparator arm of clindamycin alone, and 26.2% for vehicle.3 With these data in mind, clinicians and patients can decide whether the additional benefit of this new product over benzoyl peroxide alone is worth the increased cost.
In contrast, the trials of dapsone gel 7.5% for the treatment of acne did not include an active comparator. The mean percentage change in total lesions was 48.9% for dapsone gel and 43.2% for vehicle.4 Given these data, it is possible that dapsone gel may be no more effective, or possibly less effective, than alternatives such as benzoyl peroxide or other topical antibiotics. Nevertheless, dapsone annual sales were more than $200 million in 2016,5 suggesting that effectively marketed new products can achieve high sales even without convincing evidence of their value compared to standard of care. Although dapsone may be a useful treatment, we cannot effectively make patient-centered clinical decisions given the lack of an active comparator trial design.
This issue is not limited to acne. Phase 3 trials of halobetasol propionate foam 0.05% for psoriasis and crisaborole for atopic dermatitis also did not include an active comparator arm.6,7 Given that topical steroids—and calcineurin inhibitors for atopic dermatitis—are mainstays of treatment for each condition, it is difficult to determine whether these new treatments offer meaningful advantages over existing options and how to incorporate them into our management strategies.
Unfortunately, expensive new medications that are adopted without convincing evidence of their benefit above standard of care can put patients at risk for financial toxicity, either directly through higher out-of-pocket costs or indirectly through higher premiums. Given the impact of rising medication costs on clinicians, patients, and payers, we propose several approaches these stakeholders could adopt to encourage the use of active comparator trial designs.
Clinicians and patients can encourage these trials by remaining skeptical of new treatments that were only compared to vehicle or placebo. Because new medications often are more expensive, clinicians and patients could avoid using these treatments without evidence of either increased efficacy or improved safety and tolerability. In addition, health care institutions should consider reducing pharmaceutical representatives’ access to clinicians to encourage treatment decisions based on the published literature and comparative effectiveness data rather than marketing.
Payers, such as Medicare, also could play a role by requiring active comparator trials for coverage of new medications, particularly when there are already other effective treatments available or other medications in the same class. Payers also could give preferred coverage tier or step therapy status to medications that demonstrate value over existing options.
Although regulatory approaches to increase use of active comparator designs may be more politically challenging to introduce, these options would be more administratively robust. The FDA or a novel regulatory body could require that new treatments demonstrate value in addition to safety and efficacy. This approach would be similar to the role of The National Institute for Health and Care Excellence in the United Kingdom or the recommendations of the European Medicines Agency. Such a group also could provide independent adjudication to ensure appropriate selection of a relevant active comparator. Another approach would be to give extended market exclusivity to medications that are approved based on trials including an additional active comparator arm, an approach used by the European Medicines Agency.
Any approach that encourages increased use of active comparator trials is not without potential downsides. It will be important to avoid unintended consequences of reduced research for rare diseases with smaller markets that may not be able to support the increased cost of these trials. As a result, it would be reasonable to forgo active comparator designs for mediations indicated for rare and orphan diseases or for medications with novel mechanisms of action.
Another argument against including an active comparator arm is that it may stifle innovation by driving up the cost of conducting trials; however, if a product is so marginally innovative that it cannot demonstrate superior safety or efficacy to an existing product, such a new treatment may not be worth the increased cost. In addition, patients provide a notable contribution by participating in these trials, and it is important to ensure that their efforts result in the highest-quality data possible. Furthermore, given the adverse physical and psychosocial impact of a wide variety of dermatologic diseases, the inclusion of an active comparator arm reduces the likelihood that patients will receive placebo, which will make these trials more ethical when effective treatments are available.8 By raising the bar, we can encourage pharmaceutical companies to pursue novel approaches that are more likely to have a revolutionary impact rather than minor modifications or formulations that offer little to no benefit at substantially increased cost.
Although some recent clinical trials in dermatology have included active comparators, many new medications continue to be introduced without any evidence of how they compare to existing standards of care. Until clinicians, patients, payers, and regulators demand that pharmaceutical companies conduct the necessary trials to not only demonstrate whether a treatment is effective and safe but also how it provides value, there will be continued introduction of marginal innovations rather than revolutionary treatments that improve patients’ lives. The next time a new medication is approved, as clinicians, patients, and payers, we must ask ourselves, is this treatment worth it?
- Aitken M, Kleinrock M. Medicine Use and Spending in the U.S.: A Review of 2018 and Outlook to 2023. IQVIA Institute for Human Data Science. https://www.iqvia.com/insights/the-iqvia-institute/reports/medicine-use-and-spending-in-the-us-a-review-of-2018-and-outlook-to-2023. Published May 9, 2019. Accessed August 15, 2020.
- Olfson M, Marcus SC. Decline in placebo-controlled trial results suggests new directions for comparative effectiveness research. Health Aff Proj Hope. 2019;32:1116-1125.
- Thiboutot D, Zaenglein A, Weiss J, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol. 2008;59:792-800.
- Eichenfield LF, Lain T, Frankel EH, et al. Efficacy and safety of once-daily dapsone gel, 7.5% for treatment of adolescents and adults with acne vulgaris: second of two identically designed, large, multicenter, randomized, vehicle-controlled trials. J Drugs Dermatol. 2016;15:962-969.
- Allergan. 2017 Form 10-K. https://www.abbvie.com/content/dam/abbvie-dotcom/uploads/PDFs/allergan/allergan-annual-report-form-10K-123117.pdf. Accessed August 19, 2020.
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75:494-503.e6.
- Bhatia N, Stein Gold L, Kircik LH, et al. Two multicenter, randomized, double-blind, parallel group comparison studies of a novel foam formulation of halobetasol propionate, 0.05% vs its vehicle in adult subjects with plaque psoriasis. J Drugs Dermatol. 2019;18:790-796.
- Temple R, Ellenberg SS. Placebo-controlled trials and active-control trials in the evaluation of new treatments. part 1: ethical and scientific issues. Ann Intern Med. 2000;133:455-463.
Spending on medications is expected to grow from $344 billion in 2018 to $420 billion in 2023, largely driven by the introduction of new branded drugs.1 These costs place substantial financial burden on patients, with nearly 30% of patients not taking their prescriptions as directed because of costs. Although many new medications have transformed how we care for patients, others may not offer meaningful benefit over existing less-costly alternatives that are supported by declining effect sizes of conventional placebo-controlled trials.2 Most medications are approved based on placebo-controlled trial data that does not include an arm comparing the new drug to standard of care, leaving clinicians and patients unable to make meaningful comparisons when deciding on the most appropriate or cost-effective treatment. We consider ways in which clinicians, patients, payers, and regulators could compel more meaningful trials from industry.
Although we often look to the US Food and Drug Administration (FDA) to ensure rigorous and appropriate testing of new medications, the primary mission of the FDA is to ensure efficacy and safety. As a result, pharmaceutical companies seeking approval in the United States have little incentive to go beyond providing the minimal level of evidence required: placebo-controlled randomized trials. Although these trials provide important data on whether a treatment works and its associated risks, they do not provide data on comparative effectiveness. When relevant inexpensive medications are already on the market for the same indication, these placebo-controlled trials provide inadequate evidence to guide clinical decision-making. This issue is particularly relevant in dermatology given how easily topical medications can be combined or reformulated to pursue additional market exclusivity. The addition of an active comparator arm represents an important opportunity to improve the value of these studies.
In the pivotal trials of clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel for the treatment of acne, the experimental group was not only compared to vehicle but also the active comparator arms of clindamycin alone and benzoyl peroxide alone. The mean percentage change in total lesions was 47.9% with clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel, 41.6% with the active comparator arm of benzoyl peroxide alone, 40.4% with the active comparator arm of clindamycin alone, and 26.2% for vehicle.3 With these data in mind, clinicians and patients can decide whether the additional benefit of this new product over benzoyl peroxide alone is worth the increased cost.
In contrast, the trials of dapsone gel 7.5% for the treatment of acne did not include an active comparator. The mean percentage change in total lesions was 48.9% for dapsone gel and 43.2% for vehicle.4 Given these data, it is possible that dapsone gel may be no more effective, or possibly less effective, than alternatives such as benzoyl peroxide or other topical antibiotics. Nevertheless, dapsone annual sales were more than $200 million in 2016,5 suggesting that effectively marketed new products can achieve high sales even without convincing evidence of their value compared to standard of care. Although dapsone may be a useful treatment, we cannot effectively make patient-centered clinical decisions given the lack of an active comparator trial design.
This issue is not limited to acne. Phase 3 trials of halobetasol propionate foam 0.05% for psoriasis and crisaborole for atopic dermatitis also did not include an active comparator arm.6,7 Given that topical steroids—and calcineurin inhibitors for atopic dermatitis—are mainstays of treatment for each condition, it is difficult to determine whether these new treatments offer meaningful advantages over existing options and how to incorporate them into our management strategies.
Unfortunately, expensive new medications that are adopted without convincing evidence of their benefit above standard of care can put patients at risk for financial toxicity, either directly through higher out-of-pocket costs or indirectly through higher premiums. Given the impact of rising medication costs on clinicians, patients, and payers, we propose several approaches these stakeholders could adopt to encourage the use of active comparator trial designs.
Clinicians and patients can encourage these trials by remaining skeptical of new treatments that were only compared to vehicle or placebo. Because new medications often are more expensive, clinicians and patients could avoid using these treatments without evidence of either increased efficacy or improved safety and tolerability. In addition, health care institutions should consider reducing pharmaceutical representatives’ access to clinicians to encourage treatment decisions based on the published literature and comparative effectiveness data rather than marketing.
Payers, such as Medicare, also could play a role by requiring active comparator trials for coverage of new medications, particularly when there are already other effective treatments available or other medications in the same class. Payers also could give preferred coverage tier or step therapy status to medications that demonstrate value over existing options.
Although regulatory approaches to increase use of active comparator designs may be more politically challenging to introduce, these options would be more administratively robust. The FDA or a novel regulatory body could require that new treatments demonstrate value in addition to safety and efficacy. This approach would be similar to the role of The National Institute for Health and Care Excellence in the United Kingdom or the recommendations of the European Medicines Agency. Such a group also could provide independent adjudication to ensure appropriate selection of a relevant active comparator. Another approach would be to give extended market exclusivity to medications that are approved based on trials including an additional active comparator arm, an approach used by the European Medicines Agency.
Any approach that encourages increased use of active comparator trials is not without potential downsides. It will be important to avoid unintended consequences of reduced research for rare diseases with smaller markets that may not be able to support the increased cost of these trials. As a result, it would be reasonable to forgo active comparator designs for mediations indicated for rare and orphan diseases or for medications with novel mechanisms of action.
Another argument against including an active comparator arm is that it may stifle innovation by driving up the cost of conducting trials; however, if a product is so marginally innovative that it cannot demonstrate superior safety or efficacy to an existing product, such a new treatment may not be worth the increased cost. In addition, patients provide a notable contribution by participating in these trials, and it is important to ensure that their efforts result in the highest-quality data possible. Furthermore, given the adverse physical and psychosocial impact of a wide variety of dermatologic diseases, the inclusion of an active comparator arm reduces the likelihood that patients will receive placebo, which will make these trials more ethical when effective treatments are available.8 By raising the bar, we can encourage pharmaceutical companies to pursue novel approaches that are more likely to have a revolutionary impact rather than minor modifications or formulations that offer little to no benefit at substantially increased cost.
Although some recent clinical trials in dermatology have included active comparators, many new medications continue to be introduced without any evidence of how they compare to existing standards of care. Until clinicians, patients, payers, and regulators demand that pharmaceutical companies conduct the necessary trials to not only demonstrate whether a treatment is effective and safe but also how it provides value, there will be continued introduction of marginal innovations rather than revolutionary treatments that improve patients’ lives. The next time a new medication is approved, as clinicians, patients, and payers, we must ask ourselves, is this treatment worth it?
Spending on medications is expected to grow from $344 billion in 2018 to $420 billion in 2023, largely driven by the introduction of new branded drugs.1 These costs place substantial financial burden on patients, with nearly 30% of patients not taking their prescriptions as directed because of costs. Although many new medications have transformed how we care for patients, others may not offer meaningful benefit over existing less-costly alternatives that are supported by declining effect sizes of conventional placebo-controlled trials.2 Most medications are approved based on placebo-controlled trial data that does not include an arm comparing the new drug to standard of care, leaving clinicians and patients unable to make meaningful comparisons when deciding on the most appropriate or cost-effective treatment. We consider ways in which clinicians, patients, payers, and regulators could compel more meaningful trials from industry.
Although we often look to the US Food and Drug Administration (FDA) to ensure rigorous and appropriate testing of new medications, the primary mission of the FDA is to ensure efficacy and safety. As a result, pharmaceutical companies seeking approval in the United States have little incentive to go beyond providing the minimal level of evidence required: placebo-controlled randomized trials. Although these trials provide important data on whether a treatment works and its associated risks, they do not provide data on comparative effectiveness. When relevant inexpensive medications are already on the market for the same indication, these placebo-controlled trials provide inadequate evidence to guide clinical decision-making. This issue is particularly relevant in dermatology given how easily topical medications can be combined or reformulated to pursue additional market exclusivity. The addition of an active comparator arm represents an important opportunity to improve the value of these studies.
In the pivotal trials of clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel for the treatment of acne, the experimental group was not only compared to vehicle but also the active comparator arms of clindamycin alone and benzoyl peroxide alone. The mean percentage change in total lesions was 47.9% with clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel, 41.6% with the active comparator arm of benzoyl peroxide alone, 40.4% with the active comparator arm of clindamycin alone, and 26.2% for vehicle.3 With these data in mind, clinicians and patients can decide whether the additional benefit of this new product over benzoyl peroxide alone is worth the increased cost.
In contrast, the trials of dapsone gel 7.5% for the treatment of acne did not include an active comparator. The mean percentage change in total lesions was 48.9% for dapsone gel and 43.2% for vehicle.4 Given these data, it is possible that dapsone gel may be no more effective, or possibly less effective, than alternatives such as benzoyl peroxide or other topical antibiotics. Nevertheless, dapsone annual sales were more than $200 million in 2016,5 suggesting that effectively marketed new products can achieve high sales even without convincing evidence of their value compared to standard of care. Although dapsone may be a useful treatment, we cannot effectively make patient-centered clinical decisions given the lack of an active comparator trial design.
This issue is not limited to acne. Phase 3 trials of halobetasol propionate foam 0.05% for psoriasis and crisaborole for atopic dermatitis also did not include an active comparator arm.6,7 Given that topical steroids—and calcineurin inhibitors for atopic dermatitis—are mainstays of treatment for each condition, it is difficult to determine whether these new treatments offer meaningful advantages over existing options and how to incorporate them into our management strategies.
Unfortunately, expensive new medications that are adopted without convincing evidence of their benefit above standard of care can put patients at risk for financial toxicity, either directly through higher out-of-pocket costs or indirectly through higher premiums. Given the impact of rising medication costs on clinicians, patients, and payers, we propose several approaches these stakeholders could adopt to encourage the use of active comparator trial designs.
Clinicians and patients can encourage these trials by remaining skeptical of new treatments that were only compared to vehicle or placebo. Because new medications often are more expensive, clinicians and patients could avoid using these treatments without evidence of either increased efficacy or improved safety and tolerability. In addition, health care institutions should consider reducing pharmaceutical representatives’ access to clinicians to encourage treatment decisions based on the published literature and comparative effectiveness data rather than marketing.
Payers, such as Medicare, also could play a role by requiring active comparator trials for coverage of new medications, particularly when there are already other effective treatments available or other medications in the same class. Payers also could give preferred coverage tier or step therapy status to medications that demonstrate value over existing options.
Although regulatory approaches to increase use of active comparator designs may be more politically challenging to introduce, these options would be more administratively robust. The FDA or a novel regulatory body could require that new treatments demonstrate value in addition to safety and efficacy. This approach would be similar to the role of The National Institute for Health and Care Excellence in the United Kingdom or the recommendations of the European Medicines Agency. Such a group also could provide independent adjudication to ensure appropriate selection of a relevant active comparator. Another approach would be to give extended market exclusivity to medications that are approved based on trials including an additional active comparator arm, an approach used by the European Medicines Agency.
Any approach that encourages increased use of active comparator trials is not without potential downsides. It will be important to avoid unintended consequences of reduced research for rare diseases with smaller markets that may not be able to support the increased cost of these trials. As a result, it would be reasonable to forgo active comparator designs for mediations indicated for rare and orphan diseases or for medications with novel mechanisms of action.
Another argument against including an active comparator arm is that it may stifle innovation by driving up the cost of conducting trials; however, if a product is so marginally innovative that it cannot demonstrate superior safety or efficacy to an existing product, such a new treatment may not be worth the increased cost. In addition, patients provide a notable contribution by participating in these trials, and it is important to ensure that their efforts result in the highest-quality data possible. Furthermore, given the adverse physical and psychosocial impact of a wide variety of dermatologic diseases, the inclusion of an active comparator arm reduces the likelihood that patients will receive placebo, which will make these trials more ethical when effective treatments are available.8 By raising the bar, we can encourage pharmaceutical companies to pursue novel approaches that are more likely to have a revolutionary impact rather than minor modifications or formulations that offer little to no benefit at substantially increased cost.
Although some recent clinical trials in dermatology have included active comparators, many new medications continue to be introduced without any evidence of how they compare to existing standards of care. Until clinicians, patients, payers, and regulators demand that pharmaceutical companies conduct the necessary trials to not only demonstrate whether a treatment is effective and safe but also how it provides value, there will be continued introduction of marginal innovations rather than revolutionary treatments that improve patients’ lives. The next time a new medication is approved, as clinicians, patients, and payers, we must ask ourselves, is this treatment worth it?
- Aitken M, Kleinrock M. Medicine Use and Spending in the U.S.: A Review of 2018 and Outlook to 2023. IQVIA Institute for Human Data Science. https://www.iqvia.com/insights/the-iqvia-institute/reports/medicine-use-and-spending-in-the-us-a-review-of-2018-and-outlook-to-2023. Published May 9, 2019. Accessed August 15, 2020.
- Olfson M, Marcus SC. Decline in placebo-controlled trial results suggests new directions for comparative effectiveness research. Health Aff Proj Hope. 2019;32:1116-1125.
- Thiboutot D, Zaenglein A, Weiss J, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol. 2008;59:792-800.
- Eichenfield LF, Lain T, Frankel EH, et al. Efficacy and safety of once-daily dapsone gel, 7.5% for treatment of adolescents and adults with acne vulgaris: second of two identically designed, large, multicenter, randomized, vehicle-controlled trials. J Drugs Dermatol. 2016;15:962-969.
- Allergan. 2017 Form 10-K. https://www.abbvie.com/content/dam/abbvie-dotcom/uploads/PDFs/allergan/allergan-annual-report-form-10K-123117.pdf. Accessed August 19, 2020.
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75:494-503.e6.
- Bhatia N, Stein Gold L, Kircik LH, et al. Two multicenter, randomized, double-blind, parallel group comparison studies of a novel foam formulation of halobetasol propionate, 0.05% vs its vehicle in adult subjects with plaque psoriasis. J Drugs Dermatol. 2019;18:790-796.
- Temple R, Ellenberg SS. Placebo-controlled trials and active-control trials in the evaluation of new treatments. part 1: ethical and scientific issues. Ann Intern Med. 2000;133:455-463.
- Aitken M, Kleinrock M. Medicine Use and Spending in the U.S.: A Review of 2018 and Outlook to 2023. IQVIA Institute for Human Data Science. https://www.iqvia.com/insights/the-iqvia-institute/reports/medicine-use-and-spending-in-the-us-a-review-of-2018-and-outlook-to-2023. Published May 9, 2019. Accessed August 15, 2020.
- Olfson M, Marcus SC. Decline in placebo-controlled trial results suggests new directions for comparative effectiveness research. Health Aff Proj Hope. 2019;32:1116-1125.
- Thiboutot D, Zaenglein A, Weiss J, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol. 2008;59:792-800.
- Eichenfield LF, Lain T, Frankel EH, et al. Efficacy and safety of once-daily dapsone gel, 7.5% for treatment of adolescents and adults with acne vulgaris: second of two identically designed, large, multicenter, randomized, vehicle-controlled trials. J Drugs Dermatol. 2016;15:962-969.
- Allergan. 2017 Form 10-K. https://www.abbvie.com/content/dam/abbvie-dotcom/uploads/PDFs/allergan/allergan-annual-report-form-10K-123117.pdf. Accessed August 19, 2020.
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75:494-503.e6.
- Bhatia N, Stein Gold L, Kircik LH, et al. Two multicenter, randomized, double-blind, parallel group comparison studies of a novel foam formulation of halobetasol propionate, 0.05% vs its vehicle in adult subjects with plaque psoriasis. J Drugs Dermatol. 2019;18:790-796.
- Temple R, Ellenberg SS. Placebo-controlled trials and active-control trials in the evaluation of new treatments. part 1: ethical and scientific issues. Ann Intern Med. 2000;133:455-463.
Practice Points
- When evaluating a new treatment, it is important to consider not only whether it is effective but also whether it provides additional value compared to existing treatment options.
- Encouraging active comparator trials will provide clinicians and patients with important data to guide decision-making regarding the most appropriate treatment options.
Amylase Testing for Acute Pancreatitis
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A 37‐year‐old man presents to the emergency department complaining of acute onset abdominal pain associated with nausea and vomiting. The pain is constant and achy in nature. It is located in the upper abdomen and radiates to the back. The patient reports binge alcohol consumption the day prior to the onset of his pain.
His physical examination is remarkable for fever, with a temperature of 100.6F and epigastric tenderness to palpation without rebound or guarding. He is not hypotensive, and there is no evidence of the Cullen sign or Grey‐Turner sign.
In this patient presenting with acute abdominal pain, is ordering amylase alone, lipase alone, or amylase and lipase together the most high‐value method to evaluate him for acute pancreatitis?
WHY YOU MIGHT THINK AMYLASE TESTING IS HELPFUL
Amylase was one of the earliest, easily measurable laboratory tests that provided a relatively high degree of sensitivity and specificity for identifying patients with acute pancreatitis among those presenting with acute abdominal pain.[1] Since the introduction of amylase, additional tests, including lipase, have been introduced into clinical practice, which offer superior sensitivity and specificity compared to amylase for the diagnosis of acute pancreatitis.[2] However, amylase testing is routinely ordered at many healthcare institutions, with co‐ordering of these tests occurring greater than 90% of the time in some cases.[3, 4] Amylase testing may remain in clinical practice for several reasons including: (1) greater experience with amylase given its earlier introduction into clinical practice, (2) the belief that co‐ordering amylase and lipase provides greater accuracy than either test alone, (3) the notion that pancreatic enzymes provide prognostic information or allow monitoring of clinical progress, and (4) coupling of amylase and lipase in electronic order sets or including the tests together as part of routine lab panels used for the evaluation of abdominal pain.
WHY AMYLASE TESTING OFFERS NO ADDITIONAL VALUE TO LIPASE TESTING
Is Amylase More Accurate Than Lipase in the Diagnosis of Acute Pancreatitis?
In a number of studies, lipase has generally been found to be both more sensitive and more specific than amylase for the diagnosis of acute pancreatitis.[5, 6, 7, 8, 9] A large study by Smith and colleagues, which included 8937 patients who were initially evaluated in the emergency department, found that lipase had a superior area under the receiver operating curve when compared to amylase (0.948 vs 0.906). At a diagnostic threshold of 208 U/L for lipase and 114 U/L for amylase, the authors found that lipase compared to amylase had a superior sensitivity (90.3% vs 78.7%), specificity (93.0% vs 92.6%), positive likelihood ratio (14.1 vs 10.6), and a similar negative likelihood ratio (0.1 vs 0.1).[5]
The observed superiority of lipase over amylase may be related to a number of underlying factors. For instance, amylase measurements often include ‐amylase from the salivary glands and various macroamylase molecules that may not be related to pancreatic injury, whereas lipase measurements are more specific to the pancreas itself.[5] As a result, amylase can be elevated in a number of conditions that are unrelated to acute pancreatitis including parotitis, macroamylasemia, and some cancers.[10] In addition, because lipase remains elevated longer than amylase, it may be more accurate in the setting of delayed presentations of acute pancreatitis.
Does Amylase Co‐Ordered With Lipase Increase Diagnostic Accuracy?
Multiple studies have explored whether amylase provides additional diagnostic information when co‐ordered with lipase. A study by Chase et al. found that amylase and lipase were closely correlated, making them likely redundant measures.[7] Viel and colleagues developed a logistic regression model exploring the value of various parameters in the diagnosis of acute pancreatitis. Although they found that lipase and amylase were both accurate in univariate analyses, a multivariate analysis found that the addition of amylase did not improve the model when compared to lipase alone.[9] In a more recent study by Treacy et al., the investigators explored the accuracy of amylase and lipase in the diagnosis of acute pancreatitis, either alone or in combination, at days 1, 2, and 3 following presentation. In addition to showing that lipase was more accurate than amylase, their results also demonstrated that amylase in addition to lipase did not provide additional diagnostic accuracy compared to lipase alone, as assessed by partial area under the receiver operating curve (0.125 vs 0.128 at day 1, 0.050 vs 0.054 at day 3).[6] Finally, although some early reports suggested that the lipase to amylase ratio could be helpful to distinguish alcoholic pancreatitis from nonalcoholic pancreatitis, later studies have not confirmed these results.[11, 12]
Does Either Amylase or Lipase Add Prognostic Information?
Although both amylase and lipase are useful in the diagnosis of pancreatitis, neither correlates well with severity of illness or clinical resolution of pancreatitis.[10] As a result, the level of elevation of pancreatic enzymes is not included in the major tools used to assess severity of illness, including Ranson's criteria,[13] APACHE II (Acute Physiology and Chronic Health Evaluation II), or the computed tomography (CT) severity index.[14] Newer scoring systems, including the Bedside Index of Severity in Acute Pancreatitis[15] and the Harmless Acute Pancreatitis Score[16] also do not include pancreatic enzymes in their algorithms.
What Do Guidelines and Thought Leaders Say About Using Amylase?
The 2006 Practice Guidelines in Acute Pancreatitis state that it is not necessary to order amylase and lipase together under normal circumstances, and also note that serum lipase is the preferred diagnostic study. In addition, these guidelines state that daily measurement of pancreatic enzymes after the initial diagnosis has limited value in assessing the clinical progress of the illness or ultimate prognosis.[10] The 2013 American College of Gastroenterology guidelines provide stronger support for ordering lipase alone, stating serum amylase alone cannot be used reliably and that serum lipase is preferred.[2]
International guidelines also support the use of lipase alone for the diagnosis of acute pancreatitis. The UK guidelines for the management of acute pancreatitis offer their strongest recommendation supporting the use of lipase alone rather than amylase, unless lipase testing is not available.[17] The Japanese guidelines state the lipase level is the best pancreatic enzyme parameter and additionally do not support the co‐ordering of amylase and lipase together.[18]
Finally, a group of experts in pathology and laboratory medicine at major academic medical centers have identified serum amylase as one of the top 10 antiquated tests within the clinical pathology laboratory.[19]
WHAT YOU SHOULD DO INSTEAD: ORDER LIPASE ALONE
In all cases where a patient presents with abdominal pain concerning for acute pancreatitis, we recommend ordering lipase alone rather than either amylase alone or co‐ordering amylase and lipase. In addition, we suggest that healthcare providers do not perform daily measurement of pancreatic enzymes.
When lipase testing is available, amylase testing provides no clinical value in assisting with the diagnosis or management of acute pancreatitis, but comes at a significant cost to patients. At an average charge of $35 per test, amylase testing represents at least $19 million in annual charges to Medicare alone, according to 2013 payment data.[20] Given that the average charge for a lipase test is $41, it is also difficult to argue that amylase testing is significantly less costly than lipase testing. In addition to the direct costs, amylase tests in these settings can result in diagnostic delays or misdiagnosis, imposing additional costs on patients and the health system. For instance, a patient who presents with symptoms of pancreatitis, a positive lipase, and a negative amylase could receive an unnecessary CT scan to further assess for acute pancreatitis, despite already meeting diagnostic criteria based on the symptoms and lipase test alone.
At institutions where amylase and lipase are listed together in common order sets, removing amylase from these order sets may be a simple, durable intervention to reduce amylase testing.[3] Educational interventions aimed at alleviating some of the cognitive factors associated with amylase and lipase co‐ordering described above should also be considered.
RECOMMENDATIONS
- In patients suspected of having acute pancreatitis, lipase should be ordered alone rather than ordering either amylase alone or amylase and lipase together.
- Pancreatic enzymes should not be repeated after making the diagnosis of acute pancreatitis, as this practice does not provide additional information that is of clinical utility.
CONCLUSION
In the evaluation of acute pancreatitis, the majority of the evidence suggests that amylase, when compared to lipase, has inferior sensitivity and specificity, adds no additional diagnostic information when co‐ordered, and does not provide additional prognostic information (Table 1). In this setting, many guidelines and thought leaders recommend ordering lipase alone rather than either amylase alone or co‐ordering amylase and lipase. In addition, daily monitoring of pancreatic enzymes is not recommended because it does not help assess clinical progress or severity of illness. As a result, we believe that amylase testing should no longer be ordered for patients with suspected acute pancreatitis. Ordering amylase for the evaluation of abdominal pain is a thing we do for no reason.
| Test Characteristic | Amylase | Lipase |
|---|---|---|
| Sensitivity3 | 78.7% | 90.3% |
| Specificity3 | 92.6% | 93.0% |
| Useful when presentation of pancreatitis is delayed | Sometimes | Almost always |
| Useful to assess severity | No | No |
| Useful to assess clinical resolution | No | No |
| Peak | 1272 hours | 24 hours |
| Return to normal | 35 days | 814 days |
Disclosure: Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing TWDFNR@hospitalmedicine.org.
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A 37‐year‐old man presents to the emergency department complaining of acute onset abdominal pain associated with nausea and vomiting. The pain is constant and achy in nature. It is located in the upper abdomen and radiates to the back. The patient reports binge alcohol consumption the day prior to the onset of his pain.
His physical examination is remarkable for fever, with a temperature of 100.6F and epigastric tenderness to palpation without rebound or guarding. He is not hypotensive, and there is no evidence of the Cullen sign or Grey‐Turner sign.
In this patient presenting with acute abdominal pain, is ordering amylase alone, lipase alone, or amylase and lipase together the most high‐value method to evaluate him for acute pancreatitis?
WHY YOU MIGHT THINK AMYLASE TESTING IS HELPFUL
Amylase was one of the earliest, easily measurable laboratory tests that provided a relatively high degree of sensitivity and specificity for identifying patients with acute pancreatitis among those presenting with acute abdominal pain.[1] Since the introduction of amylase, additional tests, including lipase, have been introduced into clinical practice, which offer superior sensitivity and specificity compared to amylase for the diagnosis of acute pancreatitis.[2] However, amylase testing is routinely ordered at many healthcare institutions, with co‐ordering of these tests occurring greater than 90% of the time in some cases.[3, 4] Amylase testing may remain in clinical practice for several reasons including: (1) greater experience with amylase given its earlier introduction into clinical practice, (2) the belief that co‐ordering amylase and lipase provides greater accuracy than either test alone, (3) the notion that pancreatic enzymes provide prognostic information or allow monitoring of clinical progress, and (4) coupling of amylase and lipase in electronic order sets or including the tests together as part of routine lab panels used for the evaluation of abdominal pain.
WHY AMYLASE TESTING OFFERS NO ADDITIONAL VALUE TO LIPASE TESTING
Is Amylase More Accurate Than Lipase in the Diagnosis of Acute Pancreatitis?
In a number of studies, lipase has generally been found to be both more sensitive and more specific than amylase for the diagnosis of acute pancreatitis.[5, 6, 7, 8, 9] A large study by Smith and colleagues, which included 8937 patients who were initially evaluated in the emergency department, found that lipase had a superior area under the receiver operating curve when compared to amylase (0.948 vs 0.906). At a diagnostic threshold of 208 U/L for lipase and 114 U/L for amylase, the authors found that lipase compared to amylase had a superior sensitivity (90.3% vs 78.7%), specificity (93.0% vs 92.6%), positive likelihood ratio (14.1 vs 10.6), and a similar negative likelihood ratio (0.1 vs 0.1).[5]
The observed superiority of lipase over amylase may be related to a number of underlying factors. For instance, amylase measurements often include ‐amylase from the salivary glands and various macroamylase molecules that may not be related to pancreatic injury, whereas lipase measurements are more specific to the pancreas itself.[5] As a result, amylase can be elevated in a number of conditions that are unrelated to acute pancreatitis including parotitis, macroamylasemia, and some cancers.[10] In addition, because lipase remains elevated longer than amylase, it may be more accurate in the setting of delayed presentations of acute pancreatitis.
Does Amylase Co‐Ordered With Lipase Increase Diagnostic Accuracy?
Multiple studies have explored whether amylase provides additional diagnostic information when co‐ordered with lipase. A study by Chase et al. found that amylase and lipase were closely correlated, making them likely redundant measures.[7] Viel and colleagues developed a logistic regression model exploring the value of various parameters in the diagnosis of acute pancreatitis. Although they found that lipase and amylase were both accurate in univariate analyses, a multivariate analysis found that the addition of amylase did not improve the model when compared to lipase alone.[9] In a more recent study by Treacy et al., the investigators explored the accuracy of amylase and lipase in the diagnosis of acute pancreatitis, either alone or in combination, at days 1, 2, and 3 following presentation. In addition to showing that lipase was more accurate than amylase, their results also demonstrated that amylase in addition to lipase did not provide additional diagnostic accuracy compared to lipase alone, as assessed by partial area under the receiver operating curve (0.125 vs 0.128 at day 1, 0.050 vs 0.054 at day 3).[6] Finally, although some early reports suggested that the lipase to amylase ratio could be helpful to distinguish alcoholic pancreatitis from nonalcoholic pancreatitis, later studies have not confirmed these results.[11, 12]
Does Either Amylase or Lipase Add Prognostic Information?
Although both amylase and lipase are useful in the diagnosis of pancreatitis, neither correlates well with severity of illness or clinical resolution of pancreatitis.[10] As a result, the level of elevation of pancreatic enzymes is not included in the major tools used to assess severity of illness, including Ranson's criteria,[13] APACHE II (Acute Physiology and Chronic Health Evaluation II), or the computed tomography (CT) severity index.[14] Newer scoring systems, including the Bedside Index of Severity in Acute Pancreatitis[15] and the Harmless Acute Pancreatitis Score[16] also do not include pancreatic enzymes in their algorithms.
What Do Guidelines and Thought Leaders Say About Using Amylase?
The 2006 Practice Guidelines in Acute Pancreatitis state that it is not necessary to order amylase and lipase together under normal circumstances, and also note that serum lipase is the preferred diagnostic study. In addition, these guidelines state that daily measurement of pancreatic enzymes after the initial diagnosis has limited value in assessing the clinical progress of the illness or ultimate prognosis.[10] The 2013 American College of Gastroenterology guidelines provide stronger support for ordering lipase alone, stating serum amylase alone cannot be used reliably and that serum lipase is preferred.[2]
International guidelines also support the use of lipase alone for the diagnosis of acute pancreatitis. The UK guidelines for the management of acute pancreatitis offer their strongest recommendation supporting the use of lipase alone rather than amylase, unless lipase testing is not available.[17] The Japanese guidelines state the lipase level is the best pancreatic enzyme parameter and additionally do not support the co‐ordering of amylase and lipase together.[18]
Finally, a group of experts in pathology and laboratory medicine at major academic medical centers have identified serum amylase as one of the top 10 antiquated tests within the clinical pathology laboratory.[19]
WHAT YOU SHOULD DO INSTEAD: ORDER LIPASE ALONE
In all cases where a patient presents with abdominal pain concerning for acute pancreatitis, we recommend ordering lipase alone rather than either amylase alone or co‐ordering amylase and lipase. In addition, we suggest that healthcare providers do not perform daily measurement of pancreatic enzymes.
When lipase testing is available, amylase testing provides no clinical value in assisting with the diagnosis or management of acute pancreatitis, but comes at a significant cost to patients. At an average charge of $35 per test, amylase testing represents at least $19 million in annual charges to Medicare alone, according to 2013 payment data.[20] Given that the average charge for a lipase test is $41, it is also difficult to argue that amylase testing is significantly less costly than lipase testing. In addition to the direct costs, amylase tests in these settings can result in diagnostic delays or misdiagnosis, imposing additional costs on patients and the health system. For instance, a patient who presents with symptoms of pancreatitis, a positive lipase, and a negative amylase could receive an unnecessary CT scan to further assess for acute pancreatitis, despite already meeting diagnostic criteria based on the symptoms and lipase test alone.
At institutions where amylase and lipase are listed together in common order sets, removing amylase from these order sets may be a simple, durable intervention to reduce amylase testing.[3] Educational interventions aimed at alleviating some of the cognitive factors associated with amylase and lipase co‐ordering described above should also be considered.
RECOMMENDATIONS
- In patients suspected of having acute pancreatitis, lipase should be ordered alone rather than ordering either amylase alone or amylase and lipase together.
- Pancreatic enzymes should not be repeated after making the diagnosis of acute pancreatitis, as this practice does not provide additional information that is of clinical utility.
CONCLUSION
In the evaluation of acute pancreatitis, the majority of the evidence suggests that amylase, when compared to lipase, has inferior sensitivity and specificity, adds no additional diagnostic information when co‐ordered, and does not provide additional prognostic information (Table 1). In this setting, many guidelines and thought leaders recommend ordering lipase alone rather than either amylase alone or co‐ordering amylase and lipase. In addition, daily monitoring of pancreatic enzymes is not recommended because it does not help assess clinical progress or severity of illness. As a result, we believe that amylase testing should no longer be ordered for patients with suspected acute pancreatitis. Ordering amylase for the evaluation of abdominal pain is a thing we do for no reason.
| Test Characteristic | Amylase | Lipase |
|---|---|---|
| Sensitivity3 | 78.7% | 90.3% |
| Specificity3 | 92.6% | 93.0% |
| Useful when presentation of pancreatitis is delayed | Sometimes | Almost always |
| Useful to assess severity | No | No |
| Useful to assess clinical resolution | No | No |
| Peak | 1272 hours | 24 hours |
| Return to normal | 35 days | 814 days |
Disclosure: Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing TWDFNR@hospitalmedicine.org.
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A 37‐year‐old man presents to the emergency department complaining of acute onset abdominal pain associated with nausea and vomiting. The pain is constant and achy in nature. It is located in the upper abdomen and radiates to the back. The patient reports binge alcohol consumption the day prior to the onset of his pain.
His physical examination is remarkable for fever, with a temperature of 100.6F and epigastric tenderness to palpation without rebound or guarding. He is not hypotensive, and there is no evidence of the Cullen sign or Grey‐Turner sign.
In this patient presenting with acute abdominal pain, is ordering amylase alone, lipase alone, or amylase and lipase together the most high‐value method to evaluate him for acute pancreatitis?
WHY YOU MIGHT THINK AMYLASE TESTING IS HELPFUL
Amylase was one of the earliest, easily measurable laboratory tests that provided a relatively high degree of sensitivity and specificity for identifying patients with acute pancreatitis among those presenting with acute abdominal pain.[1] Since the introduction of amylase, additional tests, including lipase, have been introduced into clinical practice, which offer superior sensitivity and specificity compared to amylase for the diagnosis of acute pancreatitis.[2] However, amylase testing is routinely ordered at many healthcare institutions, with co‐ordering of these tests occurring greater than 90% of the time in some cases.[3, 4] Amylase testing may remain in clinical practice for several reasons including: (1) greater experience with amylase given its earlier introduction into clinical practice, (2) the belief that co‐ordering amylase and lipase provides greater accuracy than either test alone, (3) the notion that pancreatic enzymes provide prognostic information or allow monitoring of clinical progress, and (4) coupling of amylase and lipase in electronic order sets or including the tests together as part of routine lab panels used for the evaluation of abdominal pain.
WHY AMYLASE TESTING OFFERS NO ADDITIONAL VALUE TO LIPASE TESTING
Is Amylase More Accurate Than Lipase in the Diagnosis of Acute Pancreatitis?
In a number of studies, lipase has generally been found to be both more sensitive and more specific than amylase for the diagnosis of acute pancreatitis.[5, 6, 7, 8, 9] A large study by Smith and colleagues, which included 8937 patients who were initially evaluated in the emergency department, found that lipase had a superior area under the receiver operating curve when compared to amylase (0.948 vs 0.906). At a diagnostic threshold of 208 U/L for lipase and 114 U/L for amylase, the authors found that lipase compared to amylase had a superior sensitivity (90.3% vs 78.7%), specificity (93.0% vs 92.6%), positive likelihood ratio (14.1 vs 10.6), and a similar negative likelihood ratio (0.1 vs 0.1).[5]
The observed superiority of lipase over amylase may be related to a number of underlying factors. For instance, amylase measurements often include ‐amylase from the salivary glands and various macroamylase molecules that may not be related to pancreatic injury, whereas lipase measurements are more specific to the pancreas itself.[5] As a result, amylase can be elevated in a number of conditions that are unrelated to acute pancreatitis including parotitis, macroamylasemia, and some cancers.[10] In addition, because lipase remains elevated longer than amylase, it may be more accurate in the setting of delayed presentations of acute pancreatitis.
Does Amylase Co‐Ordered With Lipase Increase Diagnostic Accuracy?
Multiple studies have explored whether amylase provides additional diagnostic information when co‐ordered with lipase. A study by Chase et al. found that amylase and lipase were closely correlated, making them likely redundant measures.[7] Viel and colleagues developed a logistic regression model exploring the value of various parameters in the diagnosis of acute pancreatitis. Although they found that lipase and amylase were both accurate in univariate analyses, a multivariate analysis found that the addition of amylase did not improve the model when compared to lipase alone.[9] In a more recent study by Treacy et al., the investigators explored the accuracy of amylase and lipase in the diagnosis of acute pancreatitis, either alone or in combination, at days 1, 2, and 3 following presentation. In addition to showing that lipase was more accurate than amylase, their results also demonstrated that amylase in addition to lipase did not provide additional diagnostic accuracy compared to lipase alone, as assessed by partial area under the receiver operating curve (0.125 vs 0.128 at day 1, 0.050 vs 0.054 at day 3).[6] Finally, although some early reports suggested that the lipase to amylase ratio could be helpful to distinguish alcoholic pancreatitis from nonalcoholic pancreatitis, later studies have not confirmed these results.[11, 12]
Does Either Amylase or Lipase Add Prognostic Information?
Although both amylase and lipase are useful in the diagnosis of pancreatitis, neither correlates well with severity of illness or clinical resolution of pancreatitis.[10] As a result, the level of elevation of pancreatic enzymes is not included in the major tools used to assess severity of illness, including Ranson's criteria,[13] APACHE II (Acute Physiology and Chronic Health Evaluation II), or the computed tomography (CT) severity index.[14] Newer scoring systems, including the Bedside Index of Severity in Acute Pancreatitis[15] and the Harmless Acute Pancreatitis Score[16] also do not include pancreatic enzymes in their algorithms.
What Do Guidelines and Thought Leaders Say About Using Amylase?
The 2006 Practice Guidelines in Acute Pancreatitis state that it is not necessary to order amylase and lipase together under normal circumstances, and also note that serum lipase is the preferred diagnostic study. In addition, these guidelines state that daily measurement of pancreatic enzymes after the initial diagnosis has limited value in assessing the clinical progress of the illness or ultimate prognosis.[10] The 2013 American College of Gastroenterology guidelines provide stronger support for ordering lipase alone, stating serum amylase alone cannot be used reliably and that serum lipase is preferred.[2]
International guidelines also support the use of lipase alone for the diagnosis of acute pancreatitis. The UK guidelines for the management of acute pancreatitis offer their strongest recommendation supporting the use of lipase alone rather than amylase, unless lipase testing is not available.[17] The Japanese guidelines state the lipase level is the best pancreatic enzyme parameter and additionally do not support the co‐ordering of amylase and lipase together.[18]
Finally, a group of experts in pathology and laboratory medicine at major academic medical centers have identified serum amylase as one of the top 10 antiquated tests within the clinical pathology laboratory.[19]
WHAT YOU SHOULD DO INSTEAD: ORDER LIPASE ALONE
In all cases where a patient presents with abdominal pain concerning for acute pancreatitis, we recommend ordering lipase alone rather than either amylase alone or co‐ordering amylase and lipase. In addition, we suggest that healthcare providers do not perform daily measurement of pancreatic enzymes.
When lipase testing is available, amylase testing provides no clinical value in assisting with the diagnosis or management of acute pancreatitis, but comes at a significant cost to patients. At an average charge of $35 per test, amylase testing represents at least $19 million in annual charges to Medicare alone, according to 2013 payment data.[20] Given that the average charge for a lipase test is $41, it is also difficult to argue that amylase testing is significantly less costly than lipase testing. In addition to the direct costs, amylase tests in these settings can result in diagnostic delays or misdiagnosis, imposing additional costs on patients and the health system. For instance, a patient who presents with symptoms of pancreatitis, a positive lipase, and a negative amylase could receive an unnecessary CT scan to further assess for acute pancreatitis, despite already meeting diagnostic criteria based on the symptoms and lipase test alone.
At institutions where amylase and lipase are listed together in common order sets, removing amylase from these order sets may be a simple, durable intervention to reduce amylase testing.[3] Educational interventions aimed at alleviating some of the cognitive factors associated with amylase and lipase co‐ordering described above should also be considered.
RECOMMENDATIONS
- In patients suspected of having acute pancreatitis, lipase should be ordered alone rather than ordering either amylase alone or amylase and lipase together.
- Pancreatic enzymes should not be repeated after making the diagnosis of acute pancreatitis, as this practice does not provide additional information that is of clinical utility.
CONCLUSION
In the evaluation of acute pancreatitis, the majority of the evidence suggests that amylase, when compared to lipase, has inferior sensitivity and specificity, adds no additional diagnostic information when co‐ordered, and does not provide additional prognostic information (Table 1). In this setting, many guidelines and thought leaders recommend ordering lipase alone rather than either amylase alone or co‐ordering amylase and lipase. In addition, daily monitoring of pancreatic enzymes is not recommended because it does not help assess clinical progress or severity of illness. As a result, we believe that amylase testing should no longer be ordered for patients with suspected acute pancreatitis. Ordering amylase for the evaluation of abdominal pain is a thing we do for no reason.
| Test Characteristic | Amylase | Lipase |
|---|---|---|
| Sensitivity3 | 78.7% | 90.3% |
| Specificity3 | 92.6% | 93.0% |
| Useful when presentation of pancreatitis is delayed | Sometimes | Almost always |
| Useful to assess severity | No | No |
| Useful to assess clinical resolution | No | No |
| Peak | 1272 hours | 24 hours |
| Return to normal | 35 days | 814 days |
Disclosure: Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing TWDFNR@hospitalmedicine.org.
© 2016 Society of Hospital Medicine