User login
Psoriasis-Associated Fatigue: Pathogenesis, Metrics, and Treatment
Fatigue is defined as “an overwhelming, sustained sense of exhaustion and decreased capacity for physical and mental work,”1 and it is experienced by most patients with chronic disease. There are 2 types of fatigue: acute and chronic.2 Acute fatigue typically is caused by an identified insult (ie, injury), is self-limited, and is relieved by rest. Chronic fatigue, which may have multiple unknown causes, may accompany chronic illness and lasts longer than 6 months.2 In chronic disease, fatigue can originate peripherally (neu romuscular dysfunction outside of the central nervous system) or centrally (neurotransmitter activity within the central nervous system). Generally, central fatigue is more relevant in patients with chronic disease; however, both central and peripheral fatigue frequently coexist.
Fatigue, even with its accepted definition, is a nonspecific symptom, making it difficult to measure. Because of its subjective nature and the lack of effective therapies, clinicians often ignore fatigue. Still, patients with chronic disease continue to cite fatigue as one of the most challenging aspects of their disease that frequently decreases their quality of life (QOL).2
Fatigue has been well recognized in a number of chronic inflammatory diseases such as rheumatoid arthritis,3,4 systemic lupus erythematosus,5 fibromyalgia,6 and primary Sjögren syndrome.7 Similarly, fatigue is a frequent concern among patients with psoriasis and psoriatic arthritis.8 Given the prevalence and significance of psoriasis-associated fatigue,9 new efforts are needed to understand its pathophysiology, to develop new metrics for its evaluation, and to investigate therapeutic strategies to target it clinically. The following discussion provides an overview of the association between fatigue and psoriatic disease as well as the commonly used metrics for evaluating fatigue. Possible therapeutic agents with which to manage fatigue in this patient population also are provided.
Pathogenesis of Psoriasis-Associated Fatigue
Immunologic/Molecular Basis for Psoriasis-Associated Fatigue
Several theories aim to explain the pathophysiology of fatigue in patients with psoriatic disease. Psoriasis is a chronic inflammatory disease characterized by sharply demarcated erythematous plaques with adherent scale (Figure 1). Many in vitro studies have demonstrated the complex cytokine network that underlies the histopathologic alterations we observe in psoriatic lesions.10,11 Until recently, psoriasis was considered a type I autoimmune disease with strong TH1 signaling, influenced by IFN-γ, IL-2, and IL-12.12 TH1-producing proinflammatory cytokines, tumor necrosis factor α (TNF-α), and IFN-γ are elevated in psoriatic lesions.13 Studies on the efficacy of ustekinumab, a monoclonal antibody targeting IL-12 and IL-23, demonstrate the integral role of the immune system in psoriasis pathogenesis as the production of IL-12 polarizes T cells into TH1 cells.14,15 However, in recent years, TH17 cells have been linked to autoimmune inflammation16 and have been localized to the dermis in psoriatic lesions.17


Among a milieu of inflammatory cytokines, IL-1 is crucial for the early differentiation of TH17 cells.18 The IL-1 family of cytokines serve as primary mediators of inflammation with members including the IL-1 agonists (IL-1α, IL-1β),19 IL-1 receptor antagonist (IL-1RA),20 and IL-1 receptor type II (IL-1RII).20 The latter two inhibit IL-1 agonists from binding to their receptor (IL-1RI).19,20 A study by Yoshinaga et al21 investigated the level of inflammatory cytokines within lesional and nonlesional psoriatic skin, finding elevated levels of IL-1β in lesional skin. Another study found that IL-1β expression was increased 357% within biopsied psoriasiform lesions from flaky-skin mice, a useful model to examine the hyperproliferative alterations in the skin. This same study revealed that in vivo IL-1β neutralization alleviated the psoriasiform features in these same mice, suggesting IL-1β is integral to psoriasis pathogenesis.22
Evidence indicates that the aforementioned inflammatory mediators may contribute to psoriasis-associated fatigue. When the peripheral immune system is continuously activated, such as in psoriasis, the peripherally produced proinflammatory cytokines and subsequent immune signaling are monitored by the brain via afferent nerves, cytokine transporters at the blood-brain barrier, and IL-1 receptors on macrophages and endothelial cells of brain venules.23 For example, subseptic doses of lipopolysaccharide injected into rats induced messenger RNA expression of IL-1β in the choroid plexus, circumventricular organs, and the meninges,24 sites where cytokines can enter the blood-brain barrier via diffusion or cytokine transporters.23 These results may suggest a pathway that relays the peripheral immune signals that underlie psoriatic disease to the brain, resulting in activation of brain circuitry that mediates various negative behavioral responses, including fatigue.23 Following a central IL-1β infusion in mice, investigators found a significant decrease in the running performance (P<.01)25; the same infusion increased lethargy, malaise, and fatigue in rats.26 Interestingly, administration of IL-1RA significantly increased run time to fatigue (P<.05), supporting the hypothesis that IL-1β plays an important role in fatigue.25 Other investigators found that administration of many cytokines (IL-1β, IL-6, TNF-α) into rats induced depressivelike behaviors27 and suppressed locomotor activity.28 Lastly, another investigation found that IL-1RI knockout mice were resistant to symptoms of sickness, such as social exploration, anorexia, immobility, and weight loss, following IL-1β injections.29 Although the translatability of these studies to humans is not entirely clear, one study found that the proinflammatory cytokines IL-1 and TNF-α were elevated in patients with chronic fatigue syndrome.30 Furthermore, a 2013 systematic review found that several serum inflammatory markers including IL-6 and TNF-α were elevated in patients with severe plaque psoriasis compared to healthy controls.31 Therefore, these shared inflammatory cytokines may contribute to and explain the pathogenesis of both fatigue and psoriasis.
Confounding Factors
Although fatigue may be partially explained by the joint effect of inflammatory mediators on both the skin and the brain, there is evidence to suggest that other confounding factors may modify this association and affect its clinical presentation. The pathophysiology of fatigue in psoriasis may not be strictly immunologic; the environmental, psychological, and physical effects of psoriasis may all contribute to and perpetuate fatigue.9,32,33 Interestingly, the pathophysiology of psoriasis involves many cytokines also known to contribute to features of the metabolic syndrome.34 For example, elevated levels of free fatty acids, TNF-α, and IL-6 act in concert to promote inflammation, alter glucose metabolism, and dysregulate endothelial cell function, contributing to dyslipidemia, insulin resistance, and cardiovascular disease.35 A systematic review found a high prevalence of metabolic syndrome in patients with psoriasis and have found that those with more severe disease have an even greater risk for developing metabolic syndrome.34
Numerous studies have documented that upward of 80% of patients consider psoriasis to have a major impact on their QOL.36-38 The National Psoriasis Foundation assessed patients’ perspectives on the social, physical, and psychological aspects of their disease, finding that health-related QOL is impaired in patients with psoriatic disease.36,39 Patients reported their disease interfered with overall emotional well-being and life enjoyment and cited feelings of anger, frustration, helplessness, embarrassment, and self-consciousness, all of which can influence fatigue.36,39 Pain and pruritus (Figure 2) can interrupt sleep and thus may also contribute to symptoms of fatigue.40 Patients with psoriatic disease have a higher incidence of both depression and anxiety compared with the general population. Another study found that patient-reported factors of disability, pain, and fatigue were associated with clinical depression and anxiety; however, these factors are commonly observed in this cohort of patients and thus it is unclear whether they are predictors of or the result of depression.38
Furthermore, psoriatic disease leads to considerable economic burdens; one study (N=5604) found that among respondents who were not employed, 92% reported they were unemployed solely due to their psoriatic disease.36 One study explored the relationship between fatigue, work disability, and psoriatic arthritis, finding that the association between fatigue and work productivity loss persisted after controlling for cutaneous/musculoskeletal activity.41 However, another investigation revealed contradicting results, finding that improvements in fatigue correlated with improvements in joint and skin pain.9
Therefore, we can conclude that the pathogenesis of psoriasis-associated fatigue is the result of a multifactorial immunologic, psychologic, and physiologic pathway that triggers symptoms of exhaustion and lethargy. Fatigue is a complex multidimensional symptom activated by psoriatic disease, directly by shared inflammatory cytokines and indirectly by factors of disease activity and psychiatric distress that inherently influence somatic manifestations of fatigue. Regardless of its pathogenesis, these data and observations highlight the importance of fatigue symptoms and the need for new therapeutics to target this debilitating disease.
Measurement of Fatigue in Psoriasis
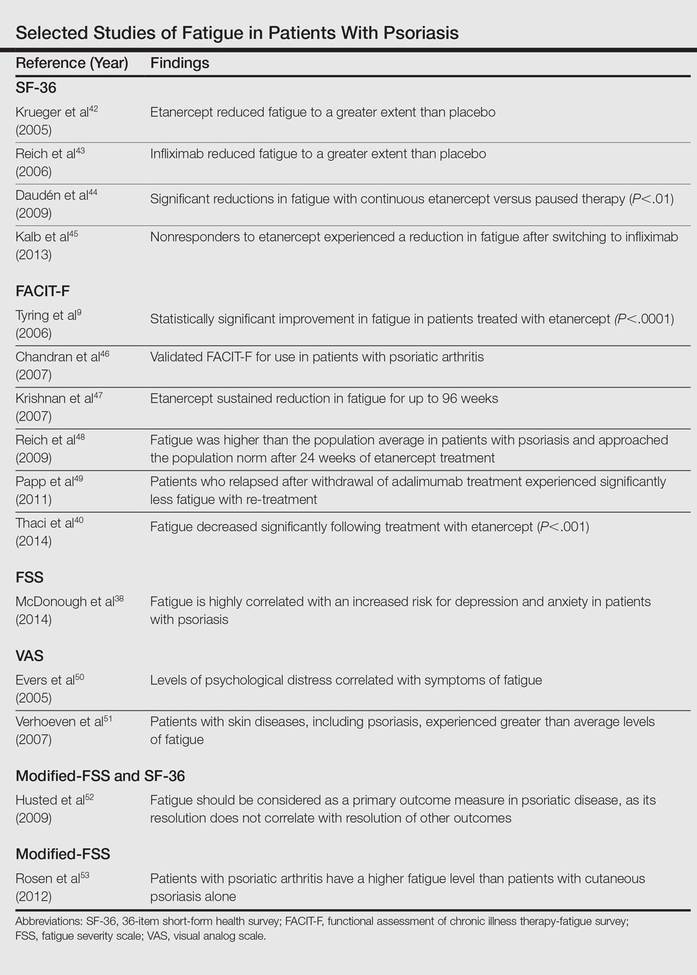
A patient’s level of fatigue is not objectively quantifiable. For this reason, clinicians and investigators have relied on self-report instruments to gauge fatigue (Table).9,38,40,42-53 These survey instruments each have distinct advantages and disadvantages, though all are subject to common difficulties. Many rely on the literacy of patients and their interpretation of each item, which can make completing the survey difficult and yield variability between subjects. Patients are inaccurate in self-reporting even measurable characteristics such as height and weight,54 which introduces an element of uncertainty in the reporting of subjective symptoms (ie, fatigue). Lastly, there are several biases implicit in self-reporting including recall bias, selective recall, and digit preference.55
When analyzing fatigue due to a chronic disease, several symptoms may be misconstrued or interfere with the interpretation of fatigue. For instance, patients with multiple sclerosis may confuse neuropathy-associated muscle weakness with fatigue. These interactions can be controlled for in self-report instruments and validated through careful study of many patients. Disease-specific questionnaires have been validated for use in several diseases,56-58 though none have been validated for cutaneous psoriasis in the absence of psoriatic arthritis. The need for validated instruments in psoriasis is great, as symptoms such as sleep disturbance and arthralgia may confound metrics of fatigue.
Thus far, 4 self-report instruments have been used to study fatigue in psoriasis: the medical outcomes 36-item short-form health survey (SF-36), the functional assessment of chronic illness therapy-fatigue, the fatigue severity scale (FSS), and the visual analog scale (VAS) for fatigue.
The SF-36 is a 36-item survey designed to measure 8 dimensions of health status in patients with chronic disease.59 Items are answered using a 3- to 6-point Likert scale, or in a yes/no format. Although the SF-36 is typically administered by a trained interviewer, it relies on a patient’s interpretation of language that must be used to describe their level of fatigue, which may not capture the full range of symptoms. Also, the length of the survey makes it impractical for use in clinical practice.
The functional assessment of chronic illness therapy-fatigue survey is validated for use in psoriatic arthritis. It is similar to the SF-36 in its use of a 5-point Likert scale to answer each of 13 items. It improves on the SF-36 model by including questions about associated symptoms (ie, pain, medication side effects) that may interfere with the measurement of fatigue. It also investigates the impact of fatigue on several areas of functioning. However, it is subject to the same pitfalls of interpretation and a rigid scale with which to answer questions.
The FSS is another Likert scale–based instrument that gauges both level of fatigue and its impact using 9 items and a 7-point scale. Investigators used the FSS to uncover an association between increasing fatigue scores and depression in patients with psoriatic disease.38
The VAS overcomes many of the language and interpretation issues inherent in Likert scale–based instruments. Patients are presented with a single item in which they are asked to plot their level of fatigue on a continuous line, with one end representing no fatigue and the other end the worst possible fatigue. Although VAS adds simplicity of response and removes some ambiguity from surveying, it provides no information about the functional impact of fatigue on patients. It also does not provide a method to control for other symptoms.
Treatment of Psoriasis-Associated Fatigue
Much of our understanding of psoriasis-associated fatigue arises from therapeutic clinical trials. Because increased concentrations of proinflammatory cytokines are associated with fatigue, it has been suggested that blocking these cytokines with biologic agents may relieve fatigue symptoms. For example, investigators found that patients treated with etanercept, a soluble TNF-α receptor fusion protein, had clinically meaningful improvement in fatigue compared to those receiving placebo, with sustained improvements at 96 weeks.9,47 We must note, however, that the decrease in fatigue correlated with improvements in cutaneous/arthritic pain. Nevertheless, another study found that treatment with the same drug decreased fatigue in patients with psoriasis, even after controlling for improvements in the psoriasis area severity index score.40 Adalimumab is another monoclonal antibody for TNF-α that has caused a notable decline in fatigue symptoms.49
These data suggest that biologic agents are useful in the treatment of fatigue. Biologic agents are frequently administered to patients with moderate to severe psoriasis in whom more conservative treatments previously failed. However, cutaneous/arthritic disease severity is not always correlated with fatigue, so these data may urge clinicians to lower their threshold for treatment with biologics in patients with substantial fatigue symptoms. Although further investigations are necessary, we may even consider using a biologic therapy for severe fatigue in those without severe psoriatic disease.
Conclusion
Fatigue is a multidimensional symptom, impacted both directly and indirectly by psoriasis pathophysiology. The prevalence of fatigue within this patient population suggests that clinicians need to recognize the symptom as a core domain in psoriasis evaluation. Although a host of metrics have been used to quantify/qualify fatigue, there remains a need for a validated instrument for assessing fatigue in patients with psoriatic disease.
Biologic agents have proven useful in the treatment of psoriasis-associated fatigue. The central role of proinflammatory cytokines to both fatigue and psoriasis pathogenesis provide insight into potential treatment targets. Understanding the overlapping pathophysiology of psoriasis and fatigue provides an avenue for developing innovative strategies to target molecules implicated in the activation of the immune system. In the future, it may be possible to predict the severity of fatigue by measuring the levels of serum inflammatory cytokines; in fact, a new study aims to identify a panel of soluble biomarkers that can predict joint damage in psoriatic arthritis.60 Taken together, the findings described suggest that further study is needed to characterize, measure, and treat psoriasis-associated fatigue.
- NANDA Nursing Diagnoses: Definitions and Classification, 1999-2000. Philadelphia, PA: NANDA International; 1999.
- Swain MG. Fatigue in chronic disease. Clin Sci (Lond). 2000;99:1-8.
- Wolfe F, Hawley DJ, Wilson K. The prevalence and meaning of fatigue in rheumatic disease. J Rheumatol. 1996;23:1407-1417.
- van Hoogmoed D, Fransen J, Bleijenberg G, et al. Physical and psychosocial correlates of severe fatigue in rheumatoid arthritis. Rheumatology (Oxford). 2010;49:1294-1302.
- Cleanthous S, Tyagi M, Isenberg DA, et al. What do we know about self-reported fatigue in systemic lupus erythematosus? Lupus. 2012;21:465-476.
- Ulus Y, Akyol Y, Tander B, et al. Sleep quality in fibromyalgia and rheumatoid arthritis: associations with pain, fatigue, depression, and disease activity. Clin Exp Rheumatol. 2011;29(6, suppl 69):S92-S96.
- Segal B, Thomas W, Rogers T, et al. Prevalence, severity, and predictors of fatigue in subjects with primary Sjögren’s syndrome. Arthritis Rheum. 2008;59:1780-1787.
- Gladman DD, Mease PJ, Strand V, et al. Consensus on a core set of domains for psoriatic arthritis. J Rheumatol. 2007;34:1167-1170.
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
- De Rosa G, Mignogna C. The histopathology of psoriasis. Reumatismo. 2007;59(suppl 1):46-48.
- Nickoloff BJ, Xin H, Nestle FO, et al. The cytokine and chemokine network in psoriasis. Clin Dermatol. 2007;25:568-573.
- Zaba LC, Fuentes-Duculan J, Eungdamrong NJ, et al. Psoriasis is characterized by accumulation of immunostimulatory and Th1/Th17 cell-polarizing myeloid dendritic cells. J Invest Dermatol. 2009;129:79-88.
- Austin LM, Ozawa M, Kikuchi T, et al. The majority of epidermal T cells in psoriasis vulgaris lesions can produce type 1 cytokines, interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J Invest Dermatol. 1999;113:752-759.
- Lebwohl M, Papp K, Han C, et al. Ustekinumab improves health-related quality of life in patients with moderate-to-severe psoriasis: results from the PHOENIX 1 trial. Br J Dermatol. 2010;162:137-146.
- Sabat R, Wolk K. Pathogenesis of psoriasis. In: Sterry W, Sabat R, Philipp S, eds. Psoriasis: Diagnosis and Management. Chichester, UK: John Wiley & Sons, Ltd; 2014:28-48.
- Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345-350.
- Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207-1211.
- Chung Y, Chang SH, Martinez GJ, et al. Critical regulation of early Th17 cell differentiation by IL-1 signaling. Immunity. 2009;30:576-587.
- Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720-3732.
- Jensen LE. Targeting the IL-1 family members in skin inflammation. Curr Opin Investig Drugs. 2010;11:1211-1220.
- Yoshinaga Y, Higaki M, Terajima S, et al. Detection of inflammatory cytokines in psoriatic skin. Arch Dermatol Res. 1995;287:158-164.
- Schon M, Behmenburg C, Denzer D, et al. Pathogenic function of IL-1 beta in psoriasiform skin lesions of flaky skin (fsn/fsn) mice. Clin Exp Immunol. 2001;123:505-510.
- Dantzer R, O’Connor JC, Freund GG, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46-56.
- Quan N, Stern EL, Whiteside MB, et al. Induction of pro-inflammatory cytokine mRNAs in the brain after peripheral injection of subseptic doses of lipopolysaccharide in the rat. J Neuroimmunol. 1999;93:72-80.
- Carmichael MD, Davis JM, Murphy EA, et al. Role of brain IL-1beta on fatigue after exercise-induced muscle damage. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1344-R1348.
- Swain MG, Beck P, Rioux K, et al. Augmented interleukin-1beta-induced depression of locomotor activity in cholestatic rats. Hepatology. 1998;28:1561-1565.
- Kent S, Bluthé RM, Kelley KW, et al. Sickness behavior as a new target for drug development. Trends Pharmacol Sci. 1992;13:24-28.
- Lacosta S, Merali Z, Anisman H. Influence of interleukin-1beta on exploratory behaviors, plasma ACTH, corticosterone, and central biogenic amines in mice. Psychopharmacology. 1998;137:351-361.
- Bluthé RM, Laye S, Michaud B, et al. Role of interleukin-1beta and tumour necrosis factor-alpha in lipopolysaccharide-induced sickness behaviour: a study with interleukin-1 type I receptor-deficient mice. Eur J Neurosci. 2000;12:4447-4456.
- Maes M, Twisk FN, Ringel K. Inflammatory and cell-mediated immune biomarkers in myalgic encephalomyelitis/chronic fatigue syndrome and depression: inflammatory markers are higher in myalgic encephalomyelitis/chronic fatigue syndrome than in depression. Psychother Psychosom. 2012;81:286-295.
- Dowlatshahi EA, van der Voort EAM, Arends LR, et al. Markers of systemic inflammation in psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2013;169:266-282.
- Jankovic S, Raznatovic M, Marinkovic J, et al. Health-related quality of life in patients with psoriasis.J Cutan Med Surg. 2011;15:29-36.
- Carneiro C, Chaves M, Verardino G, et al. Fatigue in psoriasis with arthritis. Skinmed. 2011;9:34-37.
- Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and metabolic syndrome: a systematic review and meta-analysis of observational studies. J Am Acad Dermatol. 2013;68:654-662.
- Sterry W, Strober BE, Menter A. Obesity in psoriasis: the metabolic, clinical and therapeutic implications. report of an interdisciplinary conference and review. Br J Dermatol. 2007;157:649-655.
- Armstrong AW, Schupp C, Wu J, et al. Quality of life and work productivity impairment among psoriasis patients: findings from the National Psoriasis Foundation survey data 2003-2011. PloS One. 2012;7:e52935.
- de Korte J, Sprangers MA, Mombers FM, et al. Quality of life in patients with psoriasis: a systematic literature review. J Invest Dermatol. 2004;9:140-147.
- McDonough E, Ayearst R, Eder L, et al. Depression and anxiety in psoriatic disease: prevalence and associated factors. J Rheumatol. 2014;41:887-896.
- Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280-284.
- Thaci D, Galimberti R, Amaya-Guerra M, et al. Improvement in aspects of sleep with etanercept and optional adjunctive topical therapy in patients with moderate-to-severe psoriasis: results from the PRISTINE trial. J Eur Acad Dermatol Venereol. 2014;28:900-906.
- Walsh JA, McFadden ML, Morgan MD, et al. Work productivity loss and fatigue in psoriatic arthritis. J Rheumatol. 2014;41:1670-1674.
- Krueger GG, Langley RG, Finlay AY, et al. Patient-reported outcomes of psoriasis improvement with etanercept therapy: results of a randomized phase III trial. Br J Dermatol. 2005;153:1192-1199.
- Reich K, Nestle FO, Papp K, et al. Improvement in quality of life with infliximab induction and maintenance therapy in patients with moderate-to-severe psoriasis: a randomized controlled trial. Br J Dermatol. 2006;154:1161-1168.
- Daudén E, Griffiths CE, Ortonne JP, et al. Improvements in patient-reported outcomes in moderate-to-severe psoriasis patients receiving continuous or paused etanercept treatment over 54 weeks: the CRYSTEL study. J Eur Acad Dermatol Venereol. 2009;23:1374-1382.
- Kalb RE, Blauvelt A, Sofen HL, et al. Effect of infliximab on health-related quality of life and disease activity by body region in patients with moderate-to-severe psoriasis and inadequate response to etanercept: results from the PSUNRISE trial. J Drugs Dermatol. 2013;12:874-880.
- Chandran V, Bhella S, Schentag C, et al. Functional assessment of chronic illness therapy-fatigue scale is valid in patients with psoriatic arthritis. Ann Rheumatic Dis. 2007;66:936-939.
- Krishnan R, Cella D, Leonardi C, et al. Effects of etanercept therapy on fatigue and symptoms of depression in subjects treated for moderate to severe plaque psoriasis for up to 96 weeks. Br J Dermatol. 2007;157:1275-1277.
- Reich K, Segaert S, Van de Kerkhof P, et al. Once-weekly administration of etanercept 50 mgimproves patient-reported outcomes in patients with moderate-to-severe plaque psoriasis. Dermatology. 2009;219:239-249.
- Papp K, Crowley J, Ortonne JP, et al. Adalimumab for moderate to severe chronic plaque psoriasis: efficacy and safety of retreatment and disease recurrence following withdrawal from therapy. Br J Dermatol. 2011;164:434-441.
- Evers AW, Lu Y, Duller P, et al. Common burden of chronic skin diseases? contributors to psychological distress in adults with psoriasis and atopic dermatitis. Br J Dermatol. 2005;152:1275-1281.
- Verhoeven EW, Kraaimaat FW, van de Kerkhof PC, et al. Prevalence of physical symptoms of itch, pain and fatigue in patients with skin diseases in general practice. Br J Dermatol. 2007;156:1346-1349.
- Husted JA, Tom BD, Schentag CT, et al. Occurrence and correlates of fatigue in psoriatic arthritis. Ann Rheum Dis. 2009;68:1553-1558.
- Rosen CF, Mussani F, Chandran V, et al. Patients with psoriatic arthritis have worse quality of life than those with psoriasis alone. Rheumatology. 2012;51:571-576.
- Gorber SC, Tremblay M, Moher D, et al. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: a systematic review. Obes Rev. 2007;8:307-326.
- Fadnes LT, Taube A, Tylleskär T. How to identify information bias due to self-reporting in epidemiological research. Int J Epidemiol. 2009;7:3.
- Brown RG, Dittner A, Findley L, et al. The Parkinson fatigue scale. Parkinsonism Relat Disord. 2005;11:49-55.
- Fisk JD, Ritvo PG, Ross L, et al. Measuring the functional impact of fatigue: initial validation of the fatigue impact scale. Clin Infect Dis. 1994;18(suppl 1):S79-S83.
- Bowman SJ, Booth DA, Platts RG. Measurement of fatigue and discomfort in primary Sjögren’s syndrome using a new questionnaire tool. Rheumatology. 2004;43:758-764.
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. conceptual framework and item selection. Med Care. 1992;30:473-483.
- FitzGerald O, Mease PJ. Biomarkers: project update from the GRAPPA 2012 annual meeting. J Rheumatol. 2013;40:1453-1454.
Fatigue is defined as “an overwhelming, sustained sense of exhaustion and decreased capacity for physical and mental work,”1 and it is experienced by most patients with chronic disease. There are 2 types of fatigue: acute and chronic.2 Acute fatigue typically is caused by an identified insult (ie, injury), is self-limited, and is relieved by rest. Chronic fatigue, which may have multiple unknown causes, may accompany chronic illness and lasts longer than 6 months.2 In chronic disease, fatigue can originate peripherally (neu romuscular dysfunction outside of the central nervous system) or centrally (neurotransmitter activity within the central nervous system). Generally, central fatigue is more relevant in patients with chronic disease; however, both central and peripheral fatigue frequently coexist.
Fatigue, even with its accepted definition, is a nonspecific symptom, making it difficult to measure. Because of its subjective nature and the lack of effective therapies, clinicians often ignore fatigue. Still, patients with chronic disease continue to cite fatigue as one of the most challenging aspects of their disease that frequently decreases their quality of life (QOL).2
Fatigue has been well recognized in a number of chronic inflammatory diseases such as rheumatoid arthritis,3,4 systemic lupus erythematosus,5 fibromyalgia,6 and primary Sjögren syndrome.7 Similarly, fatigue is a frequent concern among patients with psoriasis and psoriatic arthritis.8 Given the prevalence and significance of psoriasis-associated fatigue,9 new efforts are needed to understand its pathophysiology, to develop new metrics for its evaluation, and to investigate therapeutic strategies to target it clinically. The following discussion provides an overview of the association between fatigue and psoriatic disease as well as the commonly used metrics for evaluating fatigue. Possible therapeutic agents with which to manage fatigue in this patient population also are provided.
Pathogenesis of Psoriasis-Associated Fatigue
Immunologic/Molecular Basis for Psoriasis-Associated Fatigue
Several theories aim to explain the pathophysiology of fatigue in patients with psoriatic disease. Psoriasis is a chronic inflammatory disease characterized by sharply demarcated erythematous plaques with adherent scale (Figure 1). Many in vitro studies have demonstrated the complex cytokine network that underlies the histopathologic alterations we observe in psoriatic lesions.10,11 Until recently, psoriasis was considered a type I autoimmune disease with strong TH1 signaling, influenced by IFN-γ, IL-2, and IL-12.12 TH1-producing proinflammatory cytokines, tumor necrosis factor α (TNF-α), and IFN-γ are elevated in psoriatic lesions.13 Studies on the efficacy of ustekinumab, a monoclonal antibody targeting IL-12 and IL-23, demonstrate the integral role of the immune system in psoriasis pathogenesis as the production of IL-12 polarizes T cells into TH1 cells.14,15 However, in recent years, TH17 cells have been linked to autoimmune inflammation16 and have been localized to the dermis in psoriatic lesions.17


Among a milieu of inflammatory cytokines, IL-1 is crucial for the early differentiation of TH17 cells.18 The IL-1 family of cytokines serve as primary mediators of inflammation with members including the IL-1 agonists (IL-1α, IL-1β),19 IL-1 receptor antagonist (IL-1RA),20 and IL-1 receptor type II (IL-1RII).20 The latter two inhibit IL-1 agonists from binding to their receptor (IL-1RI).19,20 A study by Yoshinaga et al21 investigated the level of inflammatory cytokines within lesional and nonlesional psoriatic skin, finding elevated levels of IL-1β in lesional skin. Another study found that IL-1β expression was increased 357% within biopsied psoriasiform lesions from flaky-skin mice, a useful model to examine the hyperproliferative alterations in the skin. This same study revealed that in vivo IL-1β neutralization alleviated the psoriasiform features in these same mice, suggesting IL-1β is integral to psoriasis pathogenesis.22
Evidence indicates that the aforementioned inflammatory mediators may contribute to psoriasis-associated fatigue. When the peripheral immune system is continuously activated, such as in psoriasis, the peripherally produced proinflammatory cytokines and subsequent immune signaling are monitored by the brain via afferent nerves, cytokine transporters at the blood-brain barrier, and IL-1 receptors on macrophages and endothelial cells of brain venules.23 For example, subseptic doses of lipopolysaccharide injected into rats induced messenger RNA expression of IL-1β in the choroid plexus, circumventricular organs, and the meninges,24 sites where cytokines can enter the blood-brain barrier via diffusion or cytokine transporters.23 These results may suggest a pathway that relays the peripheral immune signals that underlie psoriatic disease to the brain, resulting in activation of brain circuitry that mediates various negative behavioral responses, including fatigue.23 Following a central IL-1β infusion in mice, investigators found a significant decrease in the running performance (P<.01)25; the same infusion increased lethargy, malaise, and fatigue in rats.26 Interestingly, administration of IL-1RA significantly increased run time to fatigue (P<.05), supporting the hypothesis that IL-1β plays an important role in fatigue.25 Other investigators found that administration of many cytokines (IL-1β, IL-6, TNF-α) into rats induced depressivelike behaviors27 and suppressed locomotor activity.28 Lastly, another investigation found that IL-1RI knockout mice were resistant to symptoms of sickness, such as social exploration, anorexia, immobility, and weight loss, following IL-1β injections.29 Although the translatability of these studies to humans is not entirely clear, one study found that the proinflammatory cytokines IL-1 and TNF-α were elevated in patients with chronic fatigue syndrome.30 Furthermore, a 2013 systematic review found that several serum inflammatory markers including IL-6 and TNF-α were elevated in patients with severe plaque psoriasis compared to healthy controls.31 Therefore, these shared inflammatory cytokines may contribute to and explain the pathogenesis of both fatigue and psoriasis.
Confounding Factors
Although fatigue may be partially explained by the joint effect of inflammatory mediators on both the skin and the brain, there is evidence to suggest that other confounding factors may modify this association and affect its clinical presentation. The pathophysiology of fatigue in psoriasis may not be strictly immunologic; the environmental, psychological, and physical effects of psoriasis may all contribute to and perpetuate fatigue.9,32,33 Interestingly, the pathophysiology of psoriasis involves many cytokines also known to contribute to features of the metabolic syndrome.34 For example, elevated levels of free fatty acids, TNF-α, and IL-6 act in concert to promote inflammation, alter glucose metabolism, and dysregulate endothelial cell function, contributing to dyslipidemia, insulin resistance, and cardiovascular disease.35 A systematic review found a high prevalence of metabolic syndrome in patients with psoriasis and have found that those with more severe disease have an even greater risk for developing metabolic syndrome.34
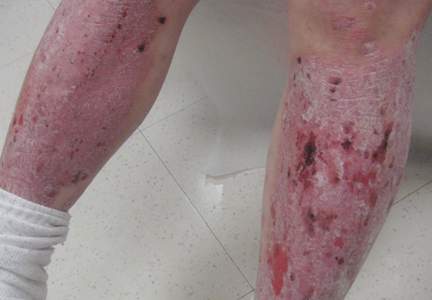
Numerous studies have documented that upward of 80% of patients consider psoriasis to have a major impact on their QOL.36-38 The National Psoriasis Foundation assessed patients’ perspectives on the social, physical, and psychological aspects of their disease, finding that health-related QOL is impaired in patients with psoriatic disease.36,39 Patients reported their disease interfered with overall emotional well-being and life enjoyment and cited feelings of anger, frustration, helplessness, embarrassment, and self-consciousness, all of which can influence fatigue.36,39 Pain and pruritus (Figure 2) can interrupt sleep and thus may also contribute to symptoms of fatigue.40 Patients with psoriatic disease have a higher incidence of both depression and anxiety compared with the general population. Another study found that patient-reported factors of disability, pain, and fatigue were associated with clinical depression and anxiety; however, these factors are commonly observed in this cohort of patients and thus it is unclear whether they are predictors of or the result of depression.38
Furthermore, psoriatic disease leads to considerable economic burdens; one study (N=5604) found that among respondents who were not employed, 92% reported they were unemployed solely due to their psoriatic disease.36 One study explored the relationship between fatigue, work disability, and psoriatic arthritis, finding that the association between fatigue and work productivity loss persisted after controlling for cutaneous/musculoskeletal activity.41 However, another investigation revealed contradicting results, finding that improvements in fatigue correlated with improvements in joint and skin pain.9
Therefore, we can conclude that the pathogenesis of psoriasis-associated fatigue is the result of a multifactorial immunologic, psychologic, and physiologic pathway that triggers symptoms of exhaustion and lethargy. Fatigue is a complex multidimensional symptom activated by psoriatic disease, directly by shared inflammatory cytokines and indirectly by factors of disease activity and psychiatric distress that inherently influence somatic manifestations of fatigue. Regardless of its pathogenesis, these data and observations highlight the importance of fatigue symptoms and the need for new therapeutics to target this debilitating disease.
Measurement of Fatigue in Psoriasis
A patient’s level of fatigue is not objectively quantifiable. For this reason, clinicians and investigators have relied on self-report instruments to gauge fatigue (Table).9,38,40,42-53 These survey instruments each have distinct advantages and disadvantages, though all are subject to common difficulties. Many rely on the literacy of patients and their interpretation of each item, which can make completing the survey difficult and yield variability between subjects. Patients are inaccurate in self-reporting even measurable characteristics such as height and weight,54 which introduces an element of uncertainty in the reporting of subjective symptoms (ie, fatigue). Lastly, there are several biases implicit in self-reporting including recall bias, selective recall, and digit preference.55
When analyzing fatigue due to a chronic disease, several symptoms may be misconstrued or interfere with the interpretation of fatigue. For instance, patients with multiple sclerosis may confuse neuropathy-associated muscle weakness with fatigue. These interactions can be controlled for in self-report instruments and validated through careful study of many patients. Disease-specific questionnaires have been validated for use in several diseases,56-58 though none have been validated for cutaneous psoriasis in the absence of psoriatic arthritis. The need for validated instruments in psoriasis is great, as symptoms such as sleep disturbance and arthralgia may confound metrics of fatigue.
Thus far, 4 self-report instruments have been used to study fatigue in psoriasis: the medical outcomes 36-item short-form health survey (SF-36), the functional assessment of chronic illness therapy-fatigue, the fatigue severity scale (FSS), and the visual analog scale (VAS) for fatigue.
The SF-36 is a 36-item survey designed to measure 8 dimensions of health status in patients with chronic disease.59 Items are answered using a 3- to 6-point Likert scale, or in a yes/no format. Although the SF-36 is typically administered by a trained interviewer, it relies on a patient’s interpretation of language that must be used to describe their level of fatigue, which may not capture the full range of symptoms. Also, the length of the survey makes it impractical for use in clinical practice.
The functional assessment of chronic illness therapy-fatigue survey is validated for use in psoriatic arthritis. It is similar to the SF-36 in its use of a 5-point Likert scale to answer each of 13 items. It improves on the SF-36 model by including questions about associated symptoms (ie, pain, medication side effects) that may interfere with the measurement of fatigue. It also investigates the impact of fatigue on several areas of functioning. However, it is subject to the same pitfalls of interpretation and a rigid scale with which to answer questions.
The FSS is another Likert scale–based instrument that gauges both level of fatigue and its impact using 9 items and a 7-point scale. Investigators used the FSS to uncover an association between increasing fatigue scores and depression in patients with psoriatic disease.38
The VAS overcomes many of the language and interpretation issues inherent in Likert scale–based instruments. Patients are presented with a single item in which they are asked to plot their level of fatigue on a continuous line, with one end representing no fatigue and the other end the worst possible fatigue. Although VAS adds simplicity of response and removes some ambiguity from surveying, it provides no information about the functional impact of fatigue on patients. It also does not provide a method to control for other symptoms.
Treatment of Psoriasis-Associated Fatigue
Much of our understanding of psoriasis-associated fatigue arises from therapeutic clinical trials. Because increased concentrations of proinflammatory cytokines are associated with fatigue, it has been suggested that blocking these cytokines with biologic agents may relieve fatigue symptoms. For example, investigators found that patients treated with etanercept, a soluble TNF-α receptor fusion protein, had clinically meaningful improvement in fatigue compared to those receiving placebo, with sustained improvements at 96 weeks.9,47 We must note, however, that the decrease in fatigue correlated with improvements in cutaneous/arthritic pain. Nevertheless, another study found that treatment with the same drug decreased fatigue in patients with psoriasis, even after controlling for improvements in the psoriasis area severity index score.40 Adalimumab is another monoclonal antibody for TNF-α that has caused a notable decline in fatigue symptoms.49
These data suggest that biologic agents are useful in the treatment of fatigue. Biologic agents are frequently administered to patients with moderate to severe psoriasis in whom more conservative treatments previously failed. However, cutaneous/arthritic disease severity is not always correlated with fatigue, so these data may urge clinicians to lower their threshold for treatment with biologics in patients with substantial fatigue symptoms. Although further investigations are necessary, we may even consider using a biologic therapy for severe fatigue in those without severe psoriatic disease.
Conclusion
Fatigue is a multidimensional symptom, impacted both directly and indirectly by psoriasis pathophysiology. The prevalence of fatigue within this patient population suggests that clinicians need to recognize the symptom as a core domain in psoriasis evaluation. Although a host of metrics have been used to quantify/qualify fatigue, there remains a need for a validated instrument for assessing fatigue in patients with psoriatic disease.
Biologic agents have proven useful in the treatment of psoriasis-associated fatigue. The central role of proinflammatory cytokines to both fatigue and psoriasis pathogenesis provide insight into potential treatment targets. Understanding the overlapping pathophysiology of psoriasis and fatigue provides an avenue for developing innovative strategies to target molecules implicated in the activation of the immune system. In the future, it may be possible to predict the severity of fatigue by measuring the levels of serum inflammatory cytokines; in fact, a new study aims to identify a panel of soluble biomarkers that can predict joint damage in psoriatic arthritis.60 Taken together, the findings described suggest that further study is needed to characterize, measure, and treat psoriasis-associated fatigue.
Fatigue is defined as “an overwhelming, sustained sense of exhaustion and decreased capacity for physical and mental work,”1 and it is experienced by most patients with chronic disease. There are 2 types of fatigue: acute and chronic.2 Acute fatigue typically is caused by an identified insult (ie, injury), is self-limited, and is relieved by rest. Chronic fatigue, which may have multiple unknown causes, may accompany chronic illness and lasts longer than 6 months.2 In chronic disease, fatigue can originate peripherally (neu romuscular dysfunction outside of the central nervous system) or centrally (neurotransmitter activity within the central nervous system). Generally, central fatigue is more relevant in patients with chronic disease; however, both central and peripheral fatigue frequently coexist.
Fatigue, even with its accepted definition, is a nonspecific symptom, making it difficult to measure. Because of its subjective nature and the lack of effective therapies, clinicians often ignore fatigue. Still, patients with chronic disease continue to cite fatigue as one of the most challenging aspects of their disease that frequently decreases their quality of life (QOL).2
Fatigue has been well recognized in a number of chronic inflammatory diseases such as rheumatoid arthritis,3,4 systemic lupus erythematosus,5 fibromyalgia,6 and primary Sjögren syndrome.7 Similarly, fatigue is a frequent concern among patients with psoriasis and psoriatic arthritis.8 Given the prevalence and significance of psoriasis-associated fatigue,9 new efforts are needed to understand its pathophysiology, to develop new metrics for its evaluation, and to investigate therapeutic strategies to target it clinically. The following discussion provides an overview of the association between fatigue and psoriatic disease as well as the commonly used metrics for evaluating fatigue. Possible therapeutic agents with which to manage fatigue in this patient population also are provided.
Pathogenesis of Psoriasis-Associated Fatigue
Immunologic/Molecular Basis for Psoriasis-Associated Fatigue
Several theories aim to explain the pathophysiology of fatigue in patients with psoriatic disease. Psoriasis is a chronic inflammatory disease characterized by sharply demarcated erythematous plaques with adherent scale (Figure 1). Many in vitro studies have demonstrated the complex cytokine network that underlies the histopathologic alterations we observe in psoriatic lesions.10,11 Until recently, psoriasis was considered a type I autoimmune disease with strong TH1 signaling, influenced by IFN-γ, IL-2, and IL-12.12 TH1-producing proinflammatory cytokines, tumor necrosis factor α (TNF-α), and IFN-γ are elevated in psoriatic lesions.13 Studies on the efficacy of ustekinumab, a monoclonal antibody targeting IL-12 and IL-23, demonstrate the integral role of the immune system in psoriasis pathogenesis as the production of IL-12 polarizes T cells into TH1 cells.14,15 However, in recent years, TH17 cells have been linked to autoimmune inflammation16 and have been localized to the dermis in psoriatic lesions.17


Among a milieu of inflammatory cytokines, IL-1 is crucial for the early differentiation of TH17 cells.18 The IL-1 family of cytokines serve as primary mediators of inflammation with members including the IL-1 agonists (IL-1α, IL-1β),19 IL-1 receptor antagonist (IL-1RA),20 and IL-1 receptor type II (IL-1RII).20 The latter two inhibit IL-1 agonists from binding to their receptor (IL-1RI).19,20 A study by Yoshinaga et al21 investigated the level of inflammatory cytokines within lesional and nonlesional psoriatic skin, finding elevated levels of IL-1β in lesional skin. Another study found that IL-1β expression was increased 357% within biopsied psoriasiform lesions from flaky-skin mice, a useful model to examine the hyperproliferative alterations in the skin. This same study revealed that in vivo IL-1β neutralization alleviated the psoriasiform features in these same mice, suggesting IL-1β is integral to psoriasis pathogenesis.22
Evidence indicates that the aforementioned inflammatory mediators may contribute to psoriasis-associated fatigue. When the peripheral immune system is continuously activated, such as in psoriasis, the peripherally produced proinflammatory cytokines and subsequent immune signaling are monitored by the brain via afferent nerves, cytokine transporters at the blood-brain barrier, and IL-1 receptors on macrophages and endothelial cells of brain venules.23 For example, subseptic doses of lipopolysaccharide injected into rats induced messenger RNA expression of IL-1β in the choroid plexus, circumventricular organs, and the meninges,24 sites where cytokines can enter the blood-brain barrier via diffusion or cytokine transporters.23 These results may suggest a pathway that relays the peripheral immune signals that underlie psoriatic disease to the brain, resulting in activation of brain circuitry that mediates various negative behavioral responses, including fatigue.23 Following a central IL-1β infusion in mice, investigators found a significant decrease in the running performance (P<.01)25; the same infusion increased lethargy, malaise, and fatigue in rats.26 Interestingly, administration of IL-1RA significantly increased run time to fatigue (P<.05), supporting the hypothesis that IL-1β plays an important role in fatigue.25 Other investigators found that administration of many cytokines (IL-1β, IL-6, TNF-α) into rats induced depressivelike behaviors27 and suppressed locomotor activity.28 Lastly, another investigation found that IL-1RI knockout mice were resistant to symptoms of sickness, such as social exploration, anorexia, immobility, and weight loss, following IL-1β injections.29 Although the translatability of these studies to humans is not entirely clear, one study found that the proinflammatory cytokines IL-1 and TNF-α were elevated in patients with chronic fatigue syndrome.30 Furthermore, a 2013 systematic review found that several serum inflammatory markers including IL-6 and TNF-α were elevated in patients with severe plaque psoriasis compared to healthy controls.31 Therefore, these shared inflammatory cytokines may contribute to and explain the pathogenesis of both fatigue and psoriasis.
Confounding Factors
Although fatigue may be partially explained by the joint effect of inflammatory mediators on both the skin and the brain, there is evidence to suggest that other confounding factors may modify this association and affect its clinical presentation. The pathophysiology of fatigue in psoriasis may not be strictly immunologic; the environmental, psychological, and physical effects of psoriasis may all contribute to and perpetuate fatigue.9,32,33 Interestingly, the pathophysiology of psoriasis involves many cytokines also known to contribute to features of the metabolic syndrome.34 For example, elevated levels of free fatty acids, TNF-α, and IL-6 act in concert to promote inflammation, alter glucose metabolism, and dysregulate endothelial cell function, contributing to dyslipidemia, insulin resistance, and cardiovascular disease.35 A systematic review found a high prevalence of metabolic syndrome in patients with psoriasis and have found that those with more severe disease have an even greater risk for developing metabolic syndrome.34
Numerous studies have documented that upward of 80% of patients consider psoriasis to have a major impact on their QOL.36-38 The National Psoriasis Foundation assessed patients’ perspectives on the social, physical, and psychological aspects of their disease, finding that health-related QOL is impaired in patients with psoriatic disease.36,39 Patients reported their disease interfered with overall emotional well-being and life enjoyment and cited feelings of anger, frustration, helplessness, embarrassment, and self-consciousness, all of which can influence fatigue.36,39 Pain and pruritus (Figure 2) can interrupt sleep and thus may also contribute to symptoms of fatigue.40 Patients with psoriatic disease have a higher incidence of both depression and anxiety compared with the general population. Another study found that patient-reported factors of disability, pain, and fatigue were associated with clinical depression and anxiety; however, these factors are commonly observed in this cohort of patients and thus it is unclear whether they are predictors of or the result of depression.38
Furthermore, psoriatic disease leads to considerable economic burdens; one study (N=5604) found that among respondents who were not employed, 92% reported they were unemployed solely due to their psoriatic disease.36 One study explored the relationship between fatigue, work disability, and psoriatic arthritis, finding that the association between fatigue and work productivity loss persisted after controlling for cutaneous/musculoskeletal activity.41 However, another investigation revealed contradicting results, finding that improvements in fatigue correlated with improvements in joint and skin pain.9
Therefore, we can conclude that the pathogenesis of psoriasis-associated fatigue is the result of a multifactorial immunologic, psychologic, and physiologic pathway that triggers symptoms of exhaustion and lethargy. Fatigue is a complex multidimensional symptom activated by psoriatic disease, directly by shared inflammatory cytokines and indirectly by factors of disease activity and psychiatric distress that inherently influence somatic manifestations of fatigue. Regardless of its pathogenesis, these data and observations highlight the importance of fatigue symptoms and the need for new therapeutics to target this debilitating disease.
Measurement of Fatigue in Psoriasis
A patient’s level of fatigue is not objectively quantifiable. For this reason, clinicians and investigators have relied on self-report instruments to gauge fatigue (Table).9,38,40,42-53 These survey instruments each have distinct advantages and disadvantages, though all are subject to common difficulties. Many rely on the literacy of patients and their interpretation of each item, which can make completing the survey difficult and yield variability between subjects. Patients are inaccurate in self-reporting even measurable characteristics such as height and weight,54 which introduces an element of uncertainty in the reporting of subjective symptoms (ie, fatigue). Lastly, there are several biases implicit in self-reporting including recall bias, selective recall, and digit preference.55
When analyzing fatigue due to a chronic disease, several symptoms may be misconstrued or interfere with the interpretation of fatigue. For instance, patients with multiple sclerosis may confuse neuropathy-associated muscle weakness with fatigue. These interactions can be controlled for in self-report instruments and validated through careful study of many patients. Disease-specific questionnaires have been validated for use in several diseases,56-58 though none have been validated for cutaneous psoriasis in the absence of psoriatic arthritis. The need for validated instruments in psoriasis is great, as symptoms such as sleep disturbance and arthralgia may confound metrics of fatigue.
Thus far, 4 self-report instruments have been used to study fatigue in psoriasis: the medical outcomes 36-item short-form health survey (SF-36), the functional assessment of chronic illness therapy-fatigue, the fatigue severity scale (FSS), and the visual analog scale (VAS) for fatigue.
The SF-36 is a 36-item survey designed to measure 8 dimensions of health status in patients with chronic disease.59 Items are answered using a 3- to 6-point Likert scale, or in a yes/no format. Although the SF-36 is typically administered by a trained interviewer, it relies on a patient’s interpretation of language that must be used to describe their level of fatigue, which may not capture the full range of symptoms. Also, the length of the survey makes it impractical for use in clinical practice.
The functional assessment of chronic illness therapy-fatigue survey is validated for use in psoriatic arthritis. It is similar to the SF-36 in its use of a 5-point Likert scale to answer each of 13 items. It improves on the SF-36 model by including questions about associated symptoms (ie, pain, medication side effects) that may interfere with the measurement of fatigue. It also investigates the impact of fatigue on several areas of functioning. However, it is subject to the same pitfalls of interpretation and a rigid scale with which to answer questions.
The FSS is another Likert scale–based instrument that gauges both level of fatigue and its impact using 9 items and a 7-point scale. Investigators used the FSS to uncover an association between increasing fatigue scores and depression in patients with psoriatic disease.38
The VAS overcomes many of the language and interpretation issues inherent in Likert scale–based instruments. Patients are presented with a single item in which they are asked to plot their level of fatigue on a continuous line, with one end representing no fatigue and the other end the worst possible fatigue. Although VAS adds simplicity of response and removes some ambiguity from surveying, it provides no information about the functional impact of fatigue on patients. It also does not provide a method to control for other symptoms.
Treatment of Psoriasis-Associated Fatigue
Much of our understanding of psoriasis-associated fatigue arises from therapeutic clinical trials. Because increased concentrations of proinflammatory cytokines are associated with fatigue, it has been suggested that blocking these cytokines with biologic agents may relieve fatigue symptoms. For example, investigators found that patients treated with etanercept, a soluble TNF-α receptor fusion protein, had clinically meaningful improvement in fatigue compared to those receiving placebo, with sustained improvements at 96 weeks.9,47 We must note, however, that the decrease in fatigue correlated with improvements in cutaneous/arthritic pain. Nevertheless, another study found that treatment with the same drug decreased fatigue in patients with psoriasis, even after controlling for improvements in the psoriasis area severity index score.40 Adalimumab is another monoclonal antibody for TNF-α that has caused a notable decline in fatigue symptoms.49
These data suggest that biologic agents are useful in the treatment of fatigue. Biologic agents are frequently administered to patients with moderate to severe psoriasis in whom more conservative treatments previously failed. However, cutaneous/arthritic disease severity is not always correlated with fatigue, so these data may urge clinicians to lower their threshold for treatment with biologics in patients with substantial fatigue symptoms. Although further investigations are necessary, we may even consider using a biologic therapy for severe fatigue in those without severe psoriatic disease.
Conclusion
Fatigue is a multidimensional symptom, impacted both directly and indirectly by psoriasis pathophysiology. The prevalence of fatigue within this patient population suggests that clinicians need to recognize the symptom as a core domain in psoriasis evaluation. Although a host of metrics have been used to quantify/qualify fatigue, there remains a need for a validated instrument for assessing fatigue in patients with psoriatic disease.
Biologic agents have proven useful in the treatment of psoriasis-associated fatigue. The central role of proinflammatory cytokines to both fatigue and psoriasis pathogenesis provide insight into potential treatment targets. Understanding the overlapping pathophysiology of psoriasis and fatigue provides an avenue for developing innovative strategies to target molecules implicated in the activation of the immune system. In the future, it may be possible to predict the severity of fatigue by measuring the levels of serum inflammatory cytokines; in fact, a new study aims to identify a panel of soluble biomarkers that can predict joint damage in psoriatic arthritis.60 Taken together, the findings described suggest that further study is needed to characterize, measure, and treat psoriasis-associated fatigue.
- NANDA Nursing Diagnoses: Definitions and Classification, 1999-2000. Philadelphia, PA: NANDA International; 1999.
- Swain MG. Fatigue in chronic disease. Clin Sci (Lond). 2000;99:1-8.
- Wolfe F, Hawley DJ, Wilson K. The prevalence and meaning of fatigue in rheumatic disease. J Rheumatol. 1996;23:1407-1417.
- van Hoogmoed D, Fransen J, Bleijenberg G, et al. Physical and psychosocial correlates of severe fatigue in rheumatoid arthritis. Rheumatology (Oxford). 2010;49:1294-1302.
- Cleanthous S, Tyagi M, Isenberg DA, et al. What do we know about self-reported fatigue in systemic lupus erythematosus? Lupus. 2012;21:465-476.
- Ulus Y, Akyol Y, Tander B, et al. Sleep quality in fibromyalgia and rheumatoid arthritis: associations with pain, fatigue, depression, and disease activity. Clin Exp Rheumatol. 2011;29(6, suppl 69):S92-S96.
- Segal B, Thomas W, Rogers T, et al. Prevalence, severity, and predictors of fatigue in subjects with primary Sjögren’s syndrome. Arthritis Rheum. 2008;59:1780-1787.
- Gladman DD, Mease PJ, Strand V, et al. Consensus on a core set of domains for psoriatic arthritis. J Rheumatol. 2007;34:1167-1170.
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
- De Rosa G, Mignogna C. The histopathology of psoriasis. Reumatismo. 2007;59(suppl 1):46-48.
- Nickoloff BJ, Xin H, Nestle FO, et al. The cytokine and chemokine network in psoriasis. Clin Dermatol. 2007;25:568-573.
- Zaba LC, Fuentes-Duculan J, Eungdamrong NJ, et al. Psoriasis is characterized by accumulation of immunostimulatory and Th1/Th17 cell-polarizing myeloid dendritic cells. J Invest Dermatol. 2009;129:79-88.
- Austin LM, Ozawa M, Kikuchi T, et al. The majority of epidermal T cells in psoriasis vulgaris lesions can produce type 1 cytokines, interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J Invest Dermatol. 1999;113:752-759.
- Lebwohl M, Papp K, Han C, et al. Ustekinumab improves health-related quality of life in patients with moderate-to-severe psoriasis: results from the PHOENIX 1 trial. Br J Dermatol. 2010;162:137-146.
- Sabat R, Wolk K. Pathogenesis of psoriasis. In: Sterry W, Sabat R, Philipp S, eds. Psoriasis: Diagnosis and Management. Chichester, UK: John Wiley & Sons, Ltd; 2014:28-48.
- Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345-350.
- Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207-1211.
- Chung Y, Chang SH, Martinez GJ, et al. Critical regulation of early Th17 cell differentiation by IL-1 signaling. Immunity. 2009;30:576-587.
- Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720-3732.
- Jensen LE. Targeting the IL-1 family members in skin inflammation. Curr Opin Investig Drugs. 2010;11:1211-1220.
- Yoshinaga Y, Higaki M, Terajima S, et al. Detection of inflammatory cytokines in psoriatic skin. Arch Dermatol Res. 1995;287:158-164.
- Schon M, Behmenburg C, Denzer D, et al. Pathogenic function of IL-1 beta in psoriasiform skin lesions of flaky skin (fsn/fsn) mice. Clin Exp Immunol. 2001;123:505-510.
- Dantzer R, O’Connor JC, Freund GG, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46-56.
- Quan N, Stern EL, Whiteside MB, et al. Induction of pro-inflammatory cytokine mRNAs in the brain after peripheral injection of subseptic doses of lipopolysaccharide in the rat. J Neuroimmunol. 1999;93:72-80.
- Carmichael MD, Davis JM, Murphy EA, et al. Role of brain IL-1beta on fatigue after exercise-induced muscle damage. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1344-R1348.
- Swain MG, Beck P, Rioux K, et al. Augmented interleukin-1beta-induced depression of locomotor activity in cholestatic rats. Hepatology. 1998;28:1561-1565.
- Kent S, Bluthé RM, Kelley KW, et al. Sickness behavior as a new target for drug development. Trends Pharmacol Sci. 1992;13:24-28.
- Lacosta S, Merali Z, Anisman H. Influence of interleukin-1beta on exploratory behaviors, plasma ACTH, corticosterone, and central biogenic amines in mice. Psychopharmacology. 1998;137:351-361.
- Bluthé RM, Laye S, Michaud B, et al. Role of interleukin-1beta and tumour necrosis factor-alpha in lipopolysaccharide-induced sickness behaviour: a study with interleukin-1 type I receptor-deficient mice. Eur J Neurosci. 2000;12:4447-4456.
- Maes M, Twisk FN, Ringel K. Inflammatory and cell-mediated immune biomarkers in myalgic encephalomyelitis/chronic fatigue syndrome and depression: inflammatory markers are higher in myalgic encephalomyelitis/chronic fatigue syndrome than in depression. Psychother Psychosom. 2012;81:286-295.
- Dowlatshahi EA, van der Voort EAM, Arends LR, et al. Markers of systemic inflammation in psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2013;169:266-282.
- Jankovic S, Raznatovic M, Marinkovic J, et al. Health-related quality of life in patients with psoriasis.J Cutan Med Surg. 2011;15:29-36.
- Carneiro C, Chaves M, Verardino G, et al. Fatigue in psoriasis with arthritis. Skinmed. 2011;9:34-37.
- Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and metabolic syndrome: a systematic review and meta-analysis of observational studies. J Am Acad Dermatol. 2013;68:654-662.
- Sterry W, Strober BE, Menter A. Obesity in psoriasis: the metabolic, clinical and therapeutic implications. report of an interdisciplinary conference and review. Br J Dermatol. 2007;157:649-655.
- Armstrong AW, Schupp C, Wu J, et al. Quality of life and work productivity impairment among psoriasis patients: findings from the National Psoriasis Foundation survey data 2003-2011. PloS One. 2012;7:e52935.
- de Korte J, Sprangers MA, Mombers FM, et al. Quality of life in patients with psoriasis: a systematic literature review. J Invest Dermatol. 2004;9:140-147.
- McDonough E, Ayearst R, Eder L, et al. Depression and anxiety in psoriatic disease: prevalence and associated factors. J Rheumatol. 2014;41:887-896.
- Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280-284.
- Thaci D, Galimberti R, Amaya-Guerra M, et al. Improvement in aspects of sleep with etanercept and optional adjunctive topical therapy in patients with moderate-to-severe psoriasis: results from the PRISTINE trial. J Eur Acad Dermatol Venereol. 2014;28:900-906.
- Walsh JA, McFadden ML, Morgan MD, et al. Work productivity loss and fatigue in psoriatic arthritis. J Rheumatol. 2014;41:1670-1674.
- Krueger GG, Langley RG, Finlay AY, et al. Patient-reported outcomes of psoriasis improvement with etanercept therapy: results of a randomized phase III trial. Br J Dermatol. 2005;153:1192-1199.
- Reich K, Nestle FO, Papp K, et al. Improvement in quality of life with infliximab induction and maintenance therapy in patients with moderate-to-severe psoriasis: a randomized controlled trial. Br J Dermatol. 2006;154:1161-1168.
- Daudén E, Griffiths CE, Ortonne JP, et al. Improvements in patient-reported outcomes in moderate-to-severe psoriasis patients receiving continuous or paused etanercept treatment over 54 weeks: the CRYSTEL study. J Eur Acad Dermatol Venereol. 2009;23:1374-1382.
- Kalb RE, Blauvelt A, Sofen HL, et al. Effect of infliximab on health-related quality of life and disease activity by body region in patients with moderate-to-severe psoriasis and inadequate response to etanercept: results from the PSUNRISE trial. J Drugs Dermatol. 2013;12:874-880.
- Chandran V, Bhella S, Schentag C, et al. Functional assessment of chronic illness therapy-fatigue scale is valid in patients with psoriatic arthritis. Ann Rheumatic Dis. 2007;66:936-939.
- Krishnan R, Cella D, Leonardi C, et al. Effects of etanercept therapy on fatigue and symptoms of depression in subjects treated for moderate to severe plaque psoriasis for up to 96 weeks. Br J Dermatol. 2007;157:1275-1277.
- Reich K, Segaert S, Van de Kerkhof P, et al. Once-weekly administration of etanercept 50 mgimproves patient-reported outcomes in patients with moderate-to-severe plaque psoriasis. Dermatology. 2009;219:239-249.
- Papp K, Crowley J, Ortonne JP, et al. Adalimumab for moderate to severe chronic plaque psoriasis: efficacy and safety of retreatment and disease recurrence following withdrawal from therapy. Br J Dermatol. 2011;164:434-441.
- Evers AW, Lu Y, Duller P, et al. Common burden of chronic skin diseases? contributors to psychological distress in adults with psoriasis and atopic dermatitis. Br J Dermatol. 2005;152:1275-1281.
- Verhoeven EW, Kraaimaat FW, van de Kerkhof PC, et al. Prevalence of physical symptoms of itch, pain and fatigue in patients with skin diseases in general practice. Br J Dermatol. 2007;156:1346-1349.
- Husted JA, Tom BD, Schentag CT, et al. Occurrence and correlates of fatigue in psoriatic arthritis. Ann Rheum Dis. 2009;68:1553-1558.
- Rosen CF, Mussani F, Chandran V, et al. Patients with psoriatic arthritis have worse quality of life than those with psoriasis alone. Rheumatology. 2012;51:571-576.
- Gorber SC, Tremblay M, Moher D, et al. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: a systematic review. Obes Rev. 2007;8:307-326.
- Fadnes LT, Taube A, Tylleskär T. How to identify information bias due to self-reporting in epidemiological research. Int J Epidemiol. 2009;7:3.
- Brown RG, Dittner A, Findley L, et al. The Parkinson fatigue scale. Parkinsonism Relat Disord. 2005;11:49-55.
- Fisk JD, Ritvo PG, Ross L, et al. Measuring the functional impact of fatigue: initial validation of the fatigue impact scale. Clin Infect Dis. 1994;18(suppl 1):S79-S83.
- Bowman SJ, Booth DA, Platts RG. Measurement of fatigue and discomfort in primary Sjögren’s syndrome using a new questionnaire tool. Rheumatology. 2004;43:758-764.
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. conceptual framework and item selection. Med Care. 1992;30:473-483.
- FitzGerald O, Mease PJ. Biomarkers: project update from the GRAPPA 2012 annual meeting. J Rheumatol. 2013;40:1453-1454.
- NANDA Nursing Diagnoses: Definitions and Classification, 1999-2000. Philadelphia, PA: NANDA International; 1999.
- Swain MG. Fatigue in chronic disease. Clin Sci (Lond). 2000;99:1-8.
- Wolfe F, Hawley DJ, Wilson K. The prevalence and meaning of fatigue in rheumatic disease. J Rheumatol. 1996;23:1407-1417.
- van Hoogmoed D, Fransen J, Bleijenberg G, et al. Physical and psychosocial correlates of severe fatigue in rheumatoid arthritis. Rheumatology (Oxford). 2010;49:1294-1302.
- Cleanthous S, Tyagi M, Isenberg DA, et al. What do we know about self-reported fatigue in systemic lupus erythematosus? Lupus. 2012;21:465-476.
- Ulus Y, Akyol Y, Tander B, et al. Sleep quality in fibromyalgia and rheumatoid arthritis: associations with pain, fatigue, depression, and disease activity. Clin Exp Rheumatol. 2011;29(6, suppl 69):S92-S96.
- Segal B, Thomas W, Rogers T, et al. Prevalence, severity, and predictors of fatigue in subjects with primary Sjögren’s syndrome. Arthritis Rheum. 2008;59:1780-1787.
- Gladman DD, Mease PJ, Strand V, et al. Consensus on a core set of domains for psoriatic arthritis. J Rheumatol. 2007;34:1167-1170.
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
- De Rosa G, Mignogna C. The histopathology of psoriasis. Reumatismo. 2007;59(suppl 1):46-48.
- Nickoloff BJ, Xin H, Nestle FO, et al. The cytokine and chemokine network in psoriasis. Clin Dermatol. 2007;25:568-573.
- Zaba LC, Fuentes-Duculan J, Eungdamrong NJ, et al. Psoriasis is characterized by accumulation of immunostimulatory and Th1/Th17 cell-polarizing myeloid dendritic cells. J Invest Dermatol. 2009;129:79-88.
- Austin LM, Ozawa M, Kikuchi T, et al. The majority of epidermal T cells in psoriasis vulgaris lesions can produce type 1 cytokines, interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J Invest Dermatol. 1999;113:752-759.
- Lebwohl M, Papp K, Han C, et al. Ustekinumab improves health-related quality of life in patients with moderate-to-severe psoriasis: results from the PHOENIX 1 trial. Br J Dermatol. 2010;162:137-146.
- Sabat R, Wolk K. Pathogenesis of psoriasis. In: Sterry W, Sabat R, Philipp S, eds. Psoriasis: Diagnosis and Management. Chichester, UK: John Wiley & Sons, Ltd; 2014:28-48.
- Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345-350.
- Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207-1211.
- Chung Y, Chang SH, Martinez GJ, et al. Critical regulation of early Th17 cell differentiation by IL-1 signaling. Immunity. 2009;30:576-587.
- Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720-3732.
- Jensen LE. Targeting the IL-1 family members in skin inflammation. Curr Opin Investig Drugs. 2010;11:1211-1220.
- Yoshinaga Y, Higaki M, Terajima S, et al. Detection of inflammatory cytokines in psoriatic skin. Arch Dermatol Res. 1995;287:158-164.
- Schon M, Behmenburg C, Denzer D, et al. Pathogenic function of IL-1 beta in psoriasiform skin lesions of flaky skin (fsn/fsn) mice. Clin Exp Immunol. 2001;123:505-510.
- Dantzer R, O’Connor JC, Freund GG, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46-56.
- Quan N, Stern EL, Whiteside MB, et al. Induction of pro-inflammatory cytokine mRNAs in the brain after peripheral injection of subseptic doses of lipopolysaccharide in the rat. J Neuroimmunol. 1999;93:72-80.
- Carmichael MD, Davis JM, Murphy EA, et al. Role of brain IL-1beta on fatigue after exercise-induced muscle damage. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1344-R1348.
- Swain MG, Beck P, Rioux K, et al. Augmented interleukin-1beta-induced depression of locomotor activity in cholestatic rats. Hepatology. 1998;28:1561-1565.
- Kent S, Bluthé RM, Kelley KW, et al. Sickness behavior as a new target for drug development. Trends Pharmacol Sci. 1992;13:24-28.
- Lacosta S, Merali Z, Anisman H. Influence of interleukin-1beta on exploratory behaviors, plasma ACTH, corticosterone, and central biogenic amines in mice. Psychopharmacology. 1998;137:351-361.
- Bluthé RM, Laye S, Michaud B, et al. Role of interleukin-1beta and tumour necrosis factor-alpha in lipopolysaccharide-induced sickness behaviour: a study with interleukin-1 type I receptor-deficient mice. Eur J Neurosci. 2000;12:4447-4456.
- Maes M, Twisk FN, Ringel K. Inflammatory and cell-mediated immune biomarkers in myalgic encephalomyelitis/chronic fatigue syndrome and depression: inflammatory markers are higher in myalgic encephalomyelitis/chronic fatigue syndrome than in depression. Psychother Psychosom. 2012;81:286-295.
- Dowlatshahi EA, van der Voort EAM, Arends LR, et al. Markers of systemic inflammation in psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2013;169:266-282.
- Jankovic S, Raznatovic M, Marinkovic J, et al. Health-related quality of life in patients with psoriasis.J Cutan Med Surg. 2011;15:29-36.
- Carneiro C, Chaves M, Verardino G, et al. Fatigue in psoriasis with arthritis. Skinmed. 2011;9:34-37.
- Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and metabolic syndrome: a systematic review and meta-analysis of observational studies. J Am Acad Dermatol. 2013;68:654-662.
- Sterry W, Strober BE, Menter A. Obesity in psoriasis: the metabolic, clinical and therapeutic implications. report of an interdisciplinary conference and review. Br J Dermatol. 2007;157:649-655.
- Armstrong AW, Schupp C, Wu J, et al. Quality of life and work productivity impairment among psoriasis patients: findings from the National Psoriasis Foundation survey data 2003-2011. PloS One. 2012;7:e52935.
- de Korte J, Sprangers MA, Mombers FM, et al. Quality of life in patients with psoriasis: a systematic literature review. J Invest Dermatol. 2004;9:140-147.
- McDonough E, Ayearst R, Eder L, et al. Depression and anxiety in psoriatic disease: prevalence and associated factors. J Rheumatol. 2014;41:887-896.
- Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280-284.
- Thaci D, Galimberti R, Amaya-Guerra M, et al. Improvement in aspects of sleep with etanercept and optional adjunctive topical therapy in patients with moderate-to-severe psoriasis: results from the PRISTINE trial. J Eur Acad Dermatol Venereol. 2014;28:900-906.
- Walsh JA, McFadden ML, Morgan MD, et al. Work productivity loss and fatigue in psoriatic arthritis. J Rheumatol. 2014;41:1670-1674.
- Krueger GG, Langley RG, Finlay AY, et al. Patient-reported outcomes of psoriasis improvement with etanercept therapy: results of a randomized phase III trial. Br J Dermatol. 2005;153:1192-1199.
- Reich K, Nestle FO, Papp K, et al. Improvement in quality of life with infliximab induction and maintenance therapy in patients with moderate-to-severe psoriasis: a randomized controlled trial. Br J Dermatol. 2006;154:1161-1168.
- Daudén E, Griffiths CE, Ortonne JP, et al. Improvements in patient-reported outcomes in moderate-to-severe psoriasis patients receiving continuous or paused etanercept treatment over 54 weeks: the CRYSTEL study. J Eur Acad Dermatol Venereol. 2009;23:1374-1382.
- Kalb RE, Blauvelt A, Sofen HL, et al. Effect of infliximab on health-related quality of life and disease activity by body region in patients with moderate-to-severe psoriasis and inadequate response to etanercept: results from the PSUNRISE trial. J Drugs Dermatol. 2013;12:874-880.
- Chandran V, Bhella S, Schentag C, et al. Functional assessment of chronic illness therapy-fatigue scale is valid in patients with psoriatic arthritis. Ann Rheumatic Dis. 2007;66:936-939.
- Krishnan R, Cella D, Leonardi C, et al. Effects of etanercept therapy on fatigue and symptoms of depression in subjects treated for moderate to severe plaque psoriasis for up to 96 weeks. Br J Dermatol. 2007;157:1275-1277.
- Reich K, Segaert S, Van de Kerkhof P, et al. Once-weekly administration of etanercept 50 mgimproves patient-reported outcomes in patients with moderate-to-severe plaque psoriasis. Dermatology. 2009;219:239-249.
- Papp K, Crowley J, Ortonne JP, et al. Adalimumab for moderate to severe chronic plaque psoriasis: efficacy and safety of retreatment and disease recurrence following withdrawal from therapy. Br J Dermatol. 2011;164:434-441.
- Evers AW, Lu Y, Duller P, et al. Common burden of chronic skin diseases? contributors to psychological distress in adults with psoriasis and atopic dermatitis. Br J Dermatol. 2005;152:1275-1281.
- Verhoeven EW, Kraaimaat FW, van de Kerkhof PC, et al. Prevalence of physical symptoms of itch, pain and fatigue in patients with skin diseases in general practice. Br J Dermatol. 2007;156:1346-1349.
- Husted JA, Tom BD, Schentag CT, et al. Occurrence and correlates of fatigue in psoriatic arthritis. Ann Rheum Dis. 2009;68:1553-1558.
- Rosen CF, Mussani F, Chandran V, et al. Patients with psoriatic arthritis have worse quality of life than those with psoriasis alone. Rheumatology. 2012;51:571-576.
- Gorber SC, Tremblay M, Moher D, et al. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: a systematic review. Obes Rev. 2007;8:307-326.
- Fadnes LT, Taube A, Tylleskär T. How to identify information bias due to self-reporting in epidemiological research. Int J Epidemiol. 2009;7:3.
- Brown RG, Dittner A, Findley L, et al. The Parkinson fatigue scale. Parkinsonism Relat Disord. 2005;11:49-55.
- Fisk JD, Ritvo PG, Ross L, et al. Measuring the functional impact of fatigue: initial validation of the fatigue impact scale. Clin Infect Dis. 1994;18(suppl 1):S79-S83.
- Bowman SJ, Booth DA, Platts RG. Measurement of fatigue and discomfort in primary Sjögren’s syndrome using a new questionnaire tool. Rheumatology. 2004;43:758-764.
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. conceptual framework and item selection. Med Care. 1992;30:473-483.
- FitzGerald O, Mease PJ. Biomarkers: project update from the GRAPPA 2012 annual meeting. J Rheumatol. 2013;40:1453-1454.
Practice Points
- Psoriasis-associated fatigue results from the impact of the inflammatory cascade on the central nervous system and from the negative influences of disease on patients.
- Although psoriasis-associated fatigue is common, there is a lack of validated systems to quantify its severity and guide therapy.
- Given the overlapping pathophysiology of psoriasis and fatigue, biologic agents may be beneficial for treating psoriasis-associated fatigue.
Overuse of Antibiotics for Acne Vulgaris: Too Much of a Good Thing
In recent years, resistance to antimicrobial drugs has become increasingly widespread, resulting in a health threat of epidemic proportions. The long list of drug-resistant bacteria continues to expand at an accelerated pace. What does this mean in the dermatology world? We are not the only problem but are certainly part of the problem, representing 5% of all antibiotic prescriptions annually even though we represent only 1% of all physicians in the United States. These prescriptions certainly do not just include skin and soft tissue functions, as a survey-based study by Chouake et al (J Drugs Dermatol. 2014;13:119-124.) showed that dermatologists are overusing antibiotics in the treatment of simple skin abscesses such as acne vulgaris, one of the most common inflammatory skin diseases.
Although the inappropriate utilization of antibiotics for acne has been a subject of great discourse for years, it recently reentered the limelight in a study by Nagler et al published online in October 2015 in the Journal of the American Academy of Dermatology. They showed that patients who ultimately were treated with isotretinoin had been receiving antibiotics for months without any sign of therapeutic life or course end in sight. This retrospective chart review evaluated the duration of systemic antibiotic use prior to starting isotretinoin in 137 patients with inflammatory/nodulocystic acne. Antibiotic use continued for a mean of 331.3 days (median, 238 days). Duration of antibiotic use was divided into categories: 3 months or less (15.3%), 6 months or more (64.2%), or 1 year or more (33.6%).
Let’s take a broad look at antimicrobial resistance. Bacterial drug resistance has numerous negative effects on medicine and society. Drug-resistant bacterial infections result in higher doses of drugs, the addition of treatments with higher toxicity, longer hospital stays, and increased mortality. In the United States, infections due to antibiotic-resistant bacteria add $20 billion to total health care costs plus $35 billion in costs to society.
Unfortunately, it is relatively easy for bacterium to develop drug resistance through 3 simple steps: acquisition by microbes of resistance genes, expression of those resistance genes, and selection for pathogens expressing those resistance genes. The selective pressure in favor of resistance occurs whenever microbes are exposed to a drug but not eradicated, either by the killing effects of the drug itself or by inhibitory effects of the drug followed by killing by the host’s immune system. In any setting that creates this selective pressure in favor of drug resistance, such as poor patient compliance (ie, infrequent dosing, taking an antibiotic for too long as we see with the use of antibiotics for the treatment of inflammatory skin diseases such as acne), the likelihood of that resistance actually developing is increased. In addition, drugs that inhibit but do not kill microbes are more likely to allow some microbial cells to live and therefore develop resistance when exposed to a drug, which accounts for the majority of antibiotics in our armament. Lastly, abuse of broad-spectrum antibiotics has further spurred the emergence of many antibiotic-resistant strains. For instance, Pseudomonas aeruginosa is one of many evolving multidrug-resistant microorganisms that have been collectively coined the “ESKAPE” pathogens (Enterococcus faecalis, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, P aeruginosa, Enterobacter species) to emphasize the fact that they “escape” the effects of many antibacterial agents.
All of the above does not take into account the environmental factors that play a role in this resistance. The close quarters, mass/public transportation, and stressful pace of life of urban living not only bring these organisms together to share resistance genes but also increase our susceptibility.
What’s the issue?
We can all do our part in the fight against microbial resistance and join the antimicrobial stewardship. Here are a couple tips for dermatologists:
- Stop using over-the-counter antibiotic ointment for every biopsy or minor procedure, which is one of the recommendations of the American Academy of Dermatology based on the ABIM Foundation’s Choosing Wisely campaign.
- Oral and topical antibiotics for inflammatory skin diseases such as acne, rosacea, and hidradenitis suppurativa should only be used temporarily or at subantimicrobial dosing. Always combine a benzoyl peroxide–containing wash with a topical or oral antibiotic to hit the bacteria with multiple mechanisms of antibacterial activity to limit resistance. Don’t use benzoyl peroxide stronger than 2.5% for the face; make sure to wash it off completely to avoid staining your towels, sheets, and clothing.
We can all play our part in the fight against antimicrobial resistance. How do you fight the resistance?
Suggested Readings
Boucher HW. Challenges in anti-infective development in the era of bad bugs, no drugs: a regulatory perspective using the example of bloodstream infection as an indication. Clin Infect Dis. 2010;50(suppl 1):S4-S9.
Spellberg B, Guidos R, Gilbert D, et al. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:155-164.
In recent years, resistance to antimicrobial drugs has become increasingly widespread, resulting in a health threat of epidemic proportions. The long list of drug-resistant bacteria continues to expand at an accelerated pace. What does this mean in the dermatology world? We are not the only problem but are certainly part of the problem, representing 5% of all antibiotic prescriptions annually even though we represent only 1% of all physicians in the United States. These prescriptions certainly do not just include skin and soft tissue functions, as a survey-based study by Chouake et al (J Drugs Dermatol. 2014;13:119-124.) showed that dermatologists are overusing antibiotics in the treatment of simple skin abscesses such as acne vulgaris, one of the most common inflammatory skin diseases.
Although the inappropriate utilization of antibiotics for acne has been a subject of great discourse for years, it recently reentered the limelight in a study by Nagler et al published online in October 2015 in the Journal of the American Academy of Dermatology. They showed that patients who ultimately were treated with isotretinoin had been receiving antibiotics for months without any sign of therapeutic life or course end in sight. This retrospective chart review evaluated the duration of systemic antibiotic use prior to starting isotretinoin in 137 patients with inflammatory/nodulocystic acne. Antibiotic use continued for a mean of 331.3 days (median, 238 days). Duration of antibiotic use was divided into categories: 3 months or less (15.3%), 6 months or more (64.2%), or 1 year or more (33.6%).
Let’s take a broad look at antimicrobial resistance. Bacterial drug resistance has numerous negative effects on medicine and society. Drug-resistant bacterial infections result in higher doses of drugs, the addition of treatments with higher toxicity, longer hospital stays, and increased mortality. In the United States, infections due to antibiotic-resistant bacteria add $20 billion to total health care costs plus $35 billion in costs to society.
Unfortunately, it is relatively easy for bacterium to develop drug resistance through 3 simple steps: acquisition by microbes of resistance genes, expression of those resistance genes, and selection for pathogens expressing those resistance genes. The selective pressure in favor of resistance occurs whenever microbes are exposed to a drug but not eradicated, either by the killing effects of the drug itself or by inhibitory effects of the drug followed by killing by the host’s immune system. In any setting that creates this selective pressure in favor of drug resistance, such as poor patient compliance (ie, infrequent dosing, taking an antibiotic for too long as we see with the use of antibiotics for the treatment of inflammatory skin diseases such as acne), the likelihood of that resistance actually developing is increased. In addition, drugs that inhibit but do not kill microbes are more likely to allow some microbial cells to live and therefore develop resistance when exposed to a drug, which accounts for the majority of antibiotics in our armament. Lastly, abuse of broad-spectrum antibiotics has further spurred the emergence of many antibiotic-resistant strains. For instance, Pseudomonas aeruginosa is one of many evolving multidrug-resistant microorganisms that have been collectively coined the “ESKAPE” pathogens (Enterococcus faecalis, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, P aeruginosa, Enterobacter species) to emphasize the fact that they “escape” the effects of many antibacterial agents.
All of the above does not take into account the environmental factors that play a role in this resistance. The close quarters, mass/public transportation, and stressful pace of life of urban living not only bring these organisms together to share resistance genes but also increase our susceptibility.
What’s the issue?
We can all do our part in the fight against microbial resistance and join the antimicrobial stewardship. Here are a couple tips for dermatologists:
- Stop using over-the-counter antibiotic ointment for every biopsy or minor procedure, which is one of the recommendations of the American Academy of Dermatology based on the ABIM Foundation’s Choosing Wisely campaign.
- Oral and topical antibiotics for inflammatory skin diseases such as acne, rosacea, and hidradenitis suppurativa should only be used temporarily or at subantimicrobial dosing. Always combine a benzoyl peroxide–containing wash with a topical or oral antibiotic to hit the bacteria with multiple mechanisms of antibacterial activity to limit resistance. Don’t use benzoyl peroxide stronger than 2.5% for the face; make sure to wash it off completely to avoid staining your towels, sheets, and clothing.
We can all play our part in the fight against antimicrobial resistance. How do you fight the resistance?
In recent years, resistance to antimicrobial drugs has become increasingly widespread, resulting in a health threat of epidemic proportions. The long list of drug-resistant bacteria continues to expand at an accelerated pace. What does this mean in the dermatology world? We are not the only problem but are certainly part of the problem, representing 5% of all antibiotic prescriptions annually even though we represent only 1% of all physicians in the United States. These prescriptions certainly do not just include skin and soft tissue functions, as a survey-based study by Chouake et al (J Drugs Dermatol. 2014;13:119-124.) showed that dermatologists are overusing antibiotics in the treatment of simple skin abscesses such as acne vulgaris, one of the most common inflammatory skin diseases.
Although the inappropriate utilization of antibiotics for acne has been a subject of great discourse for years, it recently reentered the limelight in a study by Nagler et al published online in October 2015 in the Journal of the American Academy of Dermatology. They showed that patients who ultimately were treated with isotretinoin had been receiving antibiotics for months without any sign of therapeutic life or course end in sight. This retrospective chart review evaluated the duration of systemic antibiotic use prior to starting isotretinoin in 137 patients with inflammatory/nodulocystic acne. Antibiotic use continued for a mean of 331.3 days (median, 238 days). Duration of antibiotic use was divided into categories: 3 months or less (15.3%), 6 months or more (64.2%), or 1 year or more (33.6%).
Let’s take a broad look at antimicrobial resistance. Bacterial drug resistance has numerous negative effects on medicine and society. Drug-resistant bacterial infections result in higher doses of drugs, the addition of treatments with higher toxicity, longer hospital stays, and increased mortality. In the United States, infections due to antibiotic-resistant bacteria add $20 billion to total health care costs plus $35 billion in costs to society.
Unfortunately, it is relatively easy for bacterium to develop drug resistance through 3 simple steps: acquisition by microbes of resistance genes, expression of those resistance genes, and selection for pathogens expressing those resistance genes. The selective pressure in favor of resistance occurs whenever microbes are exposed to a drug but not eradicated, either by the killing effects of the drug itself or by inhibitory effects of the drug followed by killing by the host’s immune system. In any setting that creates this selective pressure in favor of drug resistance, such as poor patient compliance (ie, infrequent dosing, taking an antibiotic for too long as we see with the use of antibiotics for the treatment of inflammatory skin diseases such as acne), the likelihood of that resistance actually developing is increased. In addition, drugs that inhibit but do not kill microbes are more likely to allow some microbial cells to live and therefore develop resistance when exposed to a drug, which accounts for the majority of antibiotics in our armament. Lastly, abuse of broad-spectrum antibiotics has further spurred the emergence of many antibiotic-resistant strains. For instance, Pseudomonas aeruginosa is one of many evolving multidrug-resistant microorganisms that have been collectively coined the “ESKAPE” pathogens (Enterococcus faecalis, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, P aeruginosa, Enterobacter species) to emphasize the fact that they “escape” the effects of many antibacterial agents.
All of the above does not take into account the environmental factors that play a role in this resistance. The close quarters, mass/public transportation, and stressful pace of life of urban living not only bring these organisms together to share resistance genes but also increase our susceptibility.
What’s the issue?
We can all do our part in the fight against microbial resistance and join the antimicrobial stewardship. Here are a couple tips for dermatologists:
- Stop using over-the-counter antibiotic ointment for every biopsy or minor procedure, which is one of the recommendations of the American Academy of Dermatology based on the ABIM Foundation’s Choosing Wisely campaign.
- Oral and topical antibiotics for inflammatory skin diseases such as acne, rosacea, and hidradenitis suppurativa should only be used temporarily or at subantimicrobial dosing. Always combine a benzoyl peroxide–containing wash with a topical or oral antibiotic to hit the bacteria with multiple mechanisms of antibacterial activity to limit resistance. Don’t use benzoyl peroxide stronger than 2.5% for the face; make sure to wash it off completely to avoid staining your towels, sheets, and clothing.
We can all play our part in the fight against antimicrobial resistance. How do you fight the resistance?
Suggested Readings
Boucher HW. Challenges in anti-infective development in the era of bad bugs, no drugs: a regulatory perspective using the example of bloodstream infection as an indication. Clin Infect Dis. 2010;50(suppl 1):S4-S9.
Spellberg B, Guidos R, Gilbert D, et al. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:155-164.
Suggested Readings
Boucher HW. Challenges in anti-infective development in the era of bad bugs, no drugs: a regulatory perspective using the example of bloodstream infection as an indication. Clin Infect Dis. 2010;50(suppl 1):S4-S9.
Spellberg B, Guidos R, Gilbert D, et al. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:155-164.
Acne as a Potential New Target for Soy Isoflavones
While the pathophysiology of acne vulgaris is, at a minimum, complex (and that’s putting it lightly), it is generally accepted that androgens such as dihydrotestosterone (DHT) can play a prominent role, especially in adult women with acne. Although it is not approved by the US Food and Drug Administration, the utilization of antiandrogens such as spironolactone (see my discussion of spironolactone use in adult females in the October issue of Cutis) has become standard practice for many US dermatologists who treat this patient population. Joined only by combined oral contraceptives, antihormonal therapies for acne are somewhat limited. Therefore, effective as well as safe additions are needed.
In a study published online on July 20 in Dermato-Endocrinology, Riyanto et al evaluated the potential of orally administered soy isoflavones for treatment of acne in adult women based on both lesion count over time and corresponding changes in DHT levels. Soy isoflavones such as genistein, daidzein, and glycitein have established effects on androgen metabolism through inhibition of 3β-hydroxysteroid dehydrogenase, 17β-hydroxysteroid dehydrogenase, and the 5α-reductases. The study was double-blinded and conducted over 12 weeks, and various confounders were accounted for, including body mass index and menstrual irregularities; however, the sample size was relatively small (N=40), with participants equally randomized to treatment with either a placebo or the soybean isoflavone (160 mg daily). The results were determined to be significant (P<.05) based on the statistical analysis, which found that the isoflavone group had a lower lesion count after 12 weeks as well as a drop in serum DHT levels. Baseline lesion counts and serum DHT levels were not statistically significant when compared to the placebo group.
What’s the Issue?
Am I saying you should recommend to all of your adult female acne patients that they should run out and buy soy isoflavone supplements? Probably not. Forgetting even the study limitations, we face a daily struggle with reproducibility when it comes to over-the-counter supplements given these products are not regulated with the same scrutiny as prescription products or devices. Unfortunately, the degree of variability between 1 manufacturer to another can be broad, with shelf life stability often being the greatest issue. Are all soy isoflavone supplements created equal? I don’t know, and I can assure you that most regulatory bodies don’t know either. Walking down the vitamin aisle with countless versions of the same product can be acne inducing in itself.
The data is certainly interesting and novel for this disease state. A larger study certainly is warranted, although as we increase the number of studies, I wonder if we will receive mixed data as witnessed with the breast cancer prevention studies with soy; some showed intake was advantageous, other did not (see suggested readings below if interested in learning more). To end on a positive note, the way I see it is that soy isoflavones could possibly become a cheaper addition to—not a replacement for—our vast yet active ingredient–lacking armament of acne treatments. Time will hopefully tell. How do you think these study results will impact the treatment of acne?
We want to know your views! Tell us what you think.
Suggested Readings
- Travis RC, Allen NE, Appleby PN, et al. A prospective study of vegetarianism and isoflavone intake in relation to breast cancer risk in British women. Int J Cancer. 2008;122:705-710.
- Key TJ, Sharp GB, Appleby PN, et al. Soya foods and breast cancer risk: a prospective study in Hiroshima and Nagasaki, Japan. Br J Cancer. 1999;81:1248-1256.
- Zaineddin AK, Buck K, Vrieling A, et al. The association between dietary lignans, phytoestrogen-rich foods, and fiber intake and postmenopausal breast cancer risk: a German case-control study. Nutr Cancer. 2012;64:652-665.
While the pathophysiology of acne vulgaris is, at a minimum, complex (and that’s putting it lightly), it is generally accepted that androgens such as dihydrotestosterone (DHT) can play a prominent role, especially in adult women with acne. Although it is not approved by the US Food and Drug Administration, the utilization of antiandrogens such as spironolactone (see my discussion of spironolactone use in adult females in the October issue of Cutis) has become standard practice for many US dermatologists who treat this patient population. Joined only by combined oral contraceptives, antihormonal therapies for acne are somewhat limited. Therefore, effective as well as safe additions are needed.
In a study published online on July 20 in Dermato-Endocrinology, Riyanto et al evaluated the potential of orally administered soy isoflavones for treatment of acne in adult women based on both lesion count over time and corresponding changes in DHT levels. Soy isoflavones such as genistein, daidzein, and glycitein have established effects on androgen metabolism through inhibition of 3β-hydroxysteroid dehydrogenase, 17β-hydroxysteroid dehydrogenase, and the 5α-reductases. The study was double-blinded and conducted over 12 weeks, and various confounders were accounted for, including body mass index and menstrual irregularities; however, the sample size was relatively small (N=40), with participants equally randomized to treatment with either a placebo or the soybean isoflavone (160 mg daily). The results were determined to be significant (P<.05) based on the statistical analysis, which found that the isoflavone group had a lower lesion count after 12 weeks as well as a drop in serum DHT levels. Baseline lesion counts and serum DHT levels were not statistically significant when compared to the placebo group.
What’s the Issue?
Am I saying you should recommend to all of your adult female acne patients that they should run out and buy soy isoflavone supplements? Probably not. Forgetting even the study limitations, we face a daily struggle with reproducibility when it comes to over-the-counter supplements given these products are not regulated with the same scrutiny as prescription products or devices. Unfortunately, the degree of variability between 1 manufacturer to another can be broad, with shelf life stability often being the greatest issue. Are all soy isoflavone supplements created equal? I don’t know, and I can assure you that most regulatory bodies don’t know either. Walking down the vitamin aisle with countless versions of the same product can be acne inducing in itself.
The data is certainly interesting and novel for this disease state. A larger study certainly is warranted, although as we increase the number of studies, I wonder if we will receive mixed data as witnessed with the breast cancer prevention studies with soy; some showed intake was advantageous, other did not (see suggested readings below if interested in learning more). To end on a positive note, the way I see it is that soy isoflavones could possibly become a cheaper addition to—not a replacement for—our vast yet active ingredient–lacking armament of acne treatments. Time will hopefully tell. How do you think these study results will impact the treatment of acne?
We want to know your views! Tell us what you think.
Suggested Readings
- Travis RC, Allen NE, Appleby PN, et al. A prospective study of vegetarianism and isoflavone intake in relation to breast cancer risk in British women. Int J Cancer. 2008;122:705-710.
- Key TJ, Sharp GB, Appleby PN, et al. Soya foods and breast cancer risk: a prospective study in Hiroshima and Nagasaki, Japan. Br J Cancer. 1999;81:1248-1256.
- Zaineddin AK, Buck K, Vrieling A, et al. The association between dietary lignans, phytoestrogen-rich foods, and fiber intake and postmenopausal breast cancer risk: a German case-control study. Nutr Cancer. 2012;64:652-665.
While the pathophysiology of acne vulgaris is, at a minimum, complex (and that’s putting it lightly), it is generally accepted that androgens such as dihydrotestosterone (DHT) can play a prominent role, especially in adult women with acne. Although it is not approved by the US Food and Drug Administration, the utilization of antiandrogens such as spironolactone (see my discussion of spironolactone use in adult females in the October issue of Cutis) has become standard practice for many US dermatologists who treat this patient population. Joined only by combined oral contraceptives, antihormonal therapies for acne are somewhat limited. Therefore, effective as well as safe additions are needed.
In a study published online on July 20 in Dermato-Endocrinology, Riyanto et al evaluated the potential of orally administered soy isoflavones for treatment of acne in adult women based on both lesion count over time and corresponding changes in DHT levels. Soy isoflavones such as genistein, daidzein, and glycitein have established effects on androgen metabolism through inhibition of 3β-hydroxysteroid dehydrogenase, 17β-hydroxysteroid dehydrogenase, and the 5α-reductases. The study was double-blinded and conducted over 12 weeks, and various confounders were accounted for, including body mass index and menstrual irregularities; however, the sample size was relatively small (N=40), with participants equally randomized to treatment with either a placebo or the soybean isoflavone (160 mg daily). The results were determined to be significant (P<.05) based on the statistical analysis, which found that the isoflavone group had a lower lesion count after 12 weeks as well as a drop in serum DHT levels. Baseline lesion counts and serum DHT levels were not statistically significant when compared to the placebo group.
What’s the Issue?
Am I saying you should recommend to all of your adult female acne patients that they should run out and buy soy isoflavone supplements? Probably not. Forgetting even the study limitations, we face a daily struggle with reproducibility when it comes to over-the-counter supplements given these products are not regulated with the same scrutiny as prescription products or devices. Unfortunately, the degree of variability between 1 manufacturer to another can be broad, with shelf life stability often being the greatest issue. Are all soy isoflavone supplements created equal? I don’t know, and I can assure you that most regulatory bodies don’t know either. Walking down the vitamin aisle with countless versions of the same product can be acne inducing in itself.
The data is certainly interesting and novel for this disease state. A larger study certainly is warranted, although as we increase the number of studies, I wonder if we will receive mixed data as witnessed with the breast cancer prevention studies with soy; some showed intake was advantageous, other did not (see suggested readings below if interested in learning more). To end on a positive note, the way I see it is that soy isoflavones could possibly become a cheaper addition to—not a replacement for—our vast yet active ingredient–lacking armament of acne treatments. Time will hopefully tell. How do you think these study results will impact the treatment of acne?
We want to know your views! Tell us what you think.
Suggested Readings
- Travis RC, Allen NE, Appleby PN, et al. A prospective study of vegetarianism and isoflavone intake in relation to breast cancer risk in British women. Int J Cancer. 2008;122:705-710.
- Key TJ, Sharp GB, Appleby PN, et al. Soya foods and breast cancer risk: a prospective study in Hiroshima and Nagasaki, Japan. Br J Cancer. 1999;81:1248-1256.
- Zaineddin AK, Buck K, Vrieling A, et al. The association between dietary lignans, phytoestrogen-rich foods, and fiber intake and postmenopausal breast cancer risk: a German case-control study. Nutr Cancer. 2012;64:652-665.
Spironolactone for Adult Female Acne
What should you do during the first visit for a patient you may start on spironolactone?
Some women will come in asking about spironolactone for acne, so it is important to identify potential candidates for antihormonal therapy:
- Women with acne flares that cycle with menstruation
- Women with adult-onset acne or persistent-recurrent acne past teenaged years, even in the absence of clinical or laboratory signs of hyperandrogenism
- Women on oral contraceptives (OCs) who exhibit moderate to severe acne, especially with a hormonal pattern clinically
- Women not responding to conventional therapy and not wanting to use oral isotretinoin or who are not candidates for oral isotretinoin
Evaluation of these women with acne for the possibility of hormonal imbalance may be necessary, with the 2 most common causes of hyperandrogenism being polycystic ovary syndrome and congenital adrenal hyperplasia. The presence of alopecia, hirsutism, acanthosis nigricans, or other signs of androgen excess, in combination with dysmenorrhea or amenorrhea, may be an indication that the patient has an underlying medical condition that needs to be addressed. Blood tests including testosterone, dehydroepiandrosterone, follicle-stimulating hormone, and luteinizing hormone would be appropriate screening tests and should be performed during the menstrual period or week prior; the patient should not be on an OC or have been on one within the last 6 weeks of testing.
Prior to initiating therapy with spironolactone, it is important to establish that there is no history of renal dysfunction; that the patient does not utilize salt substitutes, which may contain potassium in place of sodium; and that the patient is not taking potassium supplements, other potassium-sparing diuretics (ie, amiloride, triamterene), angiotensin-converting enzyme inhibitors, or angiotensin II receptor blockers.
Of note, the patient should not be currently or actively trying to become pregnant. Even though it has a category C rating, there is substantial theoretical risk for teratogenicity, especially in a male fetus (ie, feminization of a male fetus). However, there are no reports linking spironolactone with human congenital defects, and no well-controlled, prospective studies evaluating spironolactone exposure in pregnant women.
What does the patient need to know at the first visit?
Because patients have Dr. Internet on call within seconds on their smartphones and tablets, there are several points I review with patients as a semipreemptive strike.
Spironolactone is not approved by the US Food and Drug Administration for the treatment of acne; however, it has been used for decades for acne and even longer for the management of high blood pressure (since 1957!). Because it is a potassium-sparing diuretic, patients need to be careful not to get too much of a good thing (ie, potassium). I counsel patients on potassium intake, including sources such as diet (ie, fruit/fruit drinks), coconut water (very popular right now), and over-the-counter nutritional supplements.
Spironolactone is used in varying doses depending on the situation (25–200 mg daily), but it is important to start with a lower dose and escalate in a stepwise fashion, if needed, depending on how the patient is doing. I usually tell the patient it requires at least one boost in the dosage (around 50 mg twice daily) to appreciate notable results; however, patients will often have some improvement even at the lowest dose of 25 mg twice daily within 4 weeks of treatment initiation, which is when I have them return for reevaluation.
Spironolactone will help with acne on the face, back, and chest.
The majority of sides effects associated with spironolactone are dose dependent; low-dose therapy (25–50 mg daily) generally is well tolerated, and even 100 mg daily is not problematic in most cases. Dose-dependent side effects include frequent urination, menstrual irregularities, breast tenderness and/or enlargement, low blood pressure, hyperkalemia, and reduced libido. Of note, a recent study (Plovanich et al) found that the incidence of hyperkalemia in healthy young women taking spironolactone for acne is equivalent to the baseline rate of hyperkalemia in this specific population. Therefore, routine potassium monitoring is unnecessary for healthy women taking spironolactone for acne. I tend not to check potassium in these patients unless I head to higher doses due to poor response or I am treating female pattern alopecia, which often requires higher dosing.
Spironolactone has sufficient data to suggest that long-term use appears to be safe overall. There was one long-term study with patients who received spironolactone for up to 8 years for the treatment of acne vulgaris (Shaw and White).
Spironolactone can be used as monotherapy or in combination with OCs safely. In fact, by prescribing spironolactone in combination with OCs you can kill 3 birds with 1 stone from efficacy (the synergy between the two often allows for lower dosing of spironolactone without compromising impact), contraception prevention, and dysmenorrhea perspectives. I do offer OCs to eligible patients who are starting on spironolactone. In general, spironolactone can be used safely in combination with oral antibiotics, though oral antibiotic use should be short-term to limit rising rates of antimicrobial resistance. Of note, there may be risk for hyperkalemia when spironolactone is combined with trimethoprim-sulfamethoxazole, so its use should be avoided in this setting.
How do you keep patients compliant with treatment?
If androgens are playing a notable role in the patient’s acne, some response is usually noted by even the first return visit, which I always make for 4 weeks later, unlike with other acne treatment regimens, which I usually make for 7 to 8 weeks later. Even though most treatments require at least 8 weeks to show any sign of improvement, even spironolactone at times, close follow-up allows me to increase the dose, which is often needed, or change to another medication if the patient is not tolerating it. Given that I stress it will require taking the medication every day in a consistent fashion to allow me to effectively evaluate it, the short time frame between visits also enhances compliance, as it encourages the patient to actually take the medication and incorporate it into her routine.
What do you do if patients refuse treatment?
I always tell my patients they are the captains and I am helping them navigate through their disease. I will, however, discuss the chronicity of acne as well as the long-term sequelae of this inflammatory disease including scarring and postinflammatory pigment alteration for which there are no great treatments. I also tell them that if there is any issue with the medication, we simply stop, and the likelihood for severe adverse events is exceedingly low based on the evidence and anecdotal experience.
Suggested Readings
Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
Schmidt TH, Shinkai K. Evidence-based approach to cutaneous hyperandrogenism in women. J Am Acad Dermatol. 2015;73:672-690.
Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year followup study. J Cutan Med Surg. 2002;6:541-545.
What should you do during the first visit for a patient you may start on spironolactone?
Some women will come in asking about spironolactone for acne, so it is important to identify potential candidates for antihormonal therapy:
- Women with acne flares that cycle with menstruation
- Women with adult-onset acne or persistent-recurrent acne past teenaged years, even in the absence of clinical or laboratory signs of hyperandrogenism
- Women on oral contraceptives (OCs) who exhibit moderate to severe acne, especially with a hormonal pattern clinically
- Women not responding to conventional therapy and not wanting to use oral isotretinoin or who are not candidates for oral isotretinoin
Evaluation of these women with acne for the possibility of hormonal imbalance may be necessary, with the 2 most common causes of hyperandrogenism being polycystic ovary syndrome and congenital adrenal hyperplasia. The presence of alopecia, hirsutism, acanthosis nigricans, or other signs of androgen excess, in combination with dysmenorrhea or amenorrhea, may be an indication that the patient has an underlying medical condition that needs to be addressed. Blood tests including testosterone, dehydroepiandrosterone, follicle-stimulating hormone, and luteinizing hormone would be appropriate screening tests and should be performed during the menstrual period or week prior; the patient should not be on an OC or have been on one within the last 6 weeks of testing.
Prior to initiating therapy with spironolactone, it is important to establish that there is no history of renal dysfunction; that the patient does not utilize salt substitutes, which may contain potassium in place of sodium; and that the patient is not taking potassium supplements, other potassium-sparing diuretics (ie, amiloride, triamterene), angiotensin-converting enzyme inhibitors, or angiotensin II receptor blockers.
Of note, the patient should not be currently or actively trying to become pregnant. Even though it has a category C rating, there is substantial theoretical risk for teratogenicity, especially in a male fetus (ie, feminization of a male fetus). However, there are no reports linking spironolactone with human congenital defects, and no well-controlled, prospective studies evaluating spironolactone exposure in pregnant women.
What does the patient need to know at the first visit?
Because patients have Dr. Internet on call within seconds on their smartphones and tablets, there are several points I review with patients as a semipreemptive strike.
Spironolactone is not approved by the US Food and Drug Administration for the treatment of acne; however, it has been used for decades for acne and even longer for the management of high blood pressure (since 1957!). Because it is a potassium-sparing diuretic, patients need to be careful not to get too much of a good thing (ie, potassium). I counsel patients on potassium intake, including sources such as diet (ie, fruit/fruit drinks), coconut water (very popular right now), and over-the-counter nutritional supplements.
Spironolactone is used in varying doses depending on the situation (25–200 mg daily), but it is important to start with a lower dose and escalate in a stepwise fashion, if needed, depending on how the patient is doing. I usually tell the patient it requires at least one boost in the dosage (around 50 mg twice daily) to appreciate notable results; however, patients will often have some improvement even at the lowest dose of 25 mg twice daily within 4 weeks of treatment initiation, which is when I have them return for reevaluation.
Spironolactone will help with acne on the face, back, and chest.
The majority of sides effects associated with spironolactone are dose dependent; low-dose therapy (25–50 mg daily) generally is well tolerated, and even 100 mg daily is not problematic in most cases. Dose-dependent side effects include frequent urination, menstrual irregularities, breast tenderness and/or enlargement, low blood pressure, hyperkalemia, and reduced libido. Of note, a recent study (Plovanich et al) found that the incidence of hyperkalemia in healthy young women taking spironolactone for acne is equivalent to the baseline rate of hyperkalemia in this specific population. Therefore, routine potassium monitoring is unnecessary for healthy women taking spironolactone for acne. I tend not to check potassium in these patients unless I head to higher doses due to poor response or I am treating female pattern alopecia, which often requires higher dosing.
Spironolactone has sufficient data to suggest that long-term use appears to be safe overall. There was one long-term study with patients who received spironolactone for up to 8 years for the treatment of acne vulgaris (Shaw and White).
Spironolactone can be used as monotherapy or in combination with OCs safely. In fact, by prescribing spironolactone in combination with OCs you can kill 3 birds with 1 stone from efficacy (the synergy between the two often allows for lower dosing of spironolactone without compromising impact), contraception prevention, and dysmenorrhea perspectives. I do offer OCs to eligible patients who are starting on spironolactone. In general, spironolactone can be used safely in combination with oral antibiotics, though oral antibiotic use should be short-term to limit rising rates of antimicrobial resistance. Of note, there may be risk for hyperkalemia when spironolactone is combined with trimethoprim-sulfamethoxazole, so its use should be avoided in this setting.
How do you keep patients compliant with treatment?
If androgens are playing a notable role in the patient’s acne, some response is usually noted by even the first return visit, which I always make for 4 weeks later, unlike with other acne treatment regimens, which I usually make for 7 to 8 weeks later. Even though most treatments require at least 8 weeks to show any sign of improvement, even spironolactone at times, close follow-up allows me to increase the dose, which is often needed, or change to another medication if the patient is not tolerating it. Given that I stress it will require taking the medication every day in a consistent fashion to allow me to effectively evaluate it, the short time frame between visits also enhances compliance, as it encourages the patient to actually take the medication and incorporate it into her routine.
What do you do if patients refuse treatment?
I always tell my patients they are the captains and I am helping them navigate through their disease. I will, however, discuss the chronicity of acne as well as the long-term sequelae of this inflammatory disease including scarring and postinflammatory pigment alteration for which there are no great treatments. I also tell them that if there is any issue with the medication, we simply stop, and the likelihood for severe adverse events is exceedingly low based on the evidence and anecdotal experience.
What should you do during the first visit for a patient you may start on spironolactone?
Some women will come in asking about spironolactone for acne, so it is important to identify potential candidates for antihormonal therapy:
- Women with acne flares that cycle with menstruation
- Women with adult-onset acne or persistent-recurrent acne past teenaged years, even in the absence of clinical or laboratory signs of hyperandrogenism
- Women on oral contraceptives (OCs) who exhibit moderate to severe acne, especially with a hormonal pattern clinically
- Women not responding to conventional therapy and not wanting to use oral isotretinoin or who are not candidates for oral isotretinoin
Evaluation of these women with acne for the possibility of hormonal imbalance may be necessary, with the 2 most common causes of hyperandrogenism being polycystic ovary syndrome and congenital adrenal hyperplasia. The presence of alopecia, hirsutism, acanthosis nigricans, or other signs of androgen excess, in combination with dysmenorrhea or amenorrhea, may be an indication that the patient has an underlying medical condition that needs to be addressed. Blood tests including testosterone, dehydroepiandrosterone, follicle-stimulating hormone, and luteinizing hormone would be appropriate screening tests and should be performed during the menstrual period or week prior; the patient should not be on an OC or have been on one within the last 6 weeks of testing.
Prior to initiating therapy with spironolactone, it is important to establish that there is no history of renal dysfunction; that the patient does not utilize salt substitutes, which may contain potassium in place of sodium; and that the patient is not taking potassium supplements, other potassium-sparing diuretics (ie, amiloride, triamterene), angiotensin-converting enzyme inhibitors, or angiotensin II receptor blockers.
Of note, the patient should not be currently or actively trying to become pregnant. Even though it has a category C rating, there is substantial theoretical risk for teratogenicity, especially in a male fetus (ie, feminization of a male fetus). However, there are no reports linking spironolactone with human congenital defects, and no well-controlled, prospective studies evaluating spironolactone exposure in pregnant women.
What does the patient need to know at the first visit?
Because patients have Dr. Internet on call within seconds on their smartphones and tablets, there are several points I review with patients as a semipreemptive strike.
Spironolactone is not approved by the US Food and Drug Administration for the treatment of acne; however, it has been used for decades for acne and even longer for the management of high blood pressure (since 1957!). Because it is a potassium-sparing diuretic, patients need to be careful not to get too much of a good thing (ie, potassium). I counsel patients on potassium intake, including sources such as diet (ie, fruit/fruit drinks), coconut water (very popular right now), and over-the-counter nutritional supplements.
Spironolactone is used in varying doses depending on the situation (25–200 mg daily), but it is important to start with a lower dose and escalate in a stepwise fashion, if needed, depending on how the patient is doing. I usually tell the patient it requires at least one boost in the dosage (around 50 mg twice daily) to appreciate notable results; however, patients will often have some improvement even at the lowest dose of 25 mg twice daily within 4 weeks of treatment initiation, which is when I have them return for reevaluation.
Spironolactone will help with acne on the face, back, and chest.
The majority of sides effects associated with spironolactone are dose dependent; low-dose therapy (25–50 mg daily) generally is well tolerated, and even 100 mg daily is not problematic in most cases. Dose-dependent side effects include frequent urination, menstrual irregularities, breast tenderness and/or enlargement, low blood pressure, hyperkalemia, and reduced libido. Of note, a recent study (Plovanich et al) found that the incidence of hyperkalemia in healthy young women taking spironolactone for acne is equivalent to the baseline rate of hyperkalemia in this specific population. Therefore, routine potassium monitoring is unnecessary for healthy women taking spironolactone for acne. I tend not to check potassium in these patients unless I head to higher doses due to poor response or I am treating female pattern alopecia, which often requires higher dosing.
Spironolactone has sufficient data to suggest that long-term use appears to be safe overall. There was one long-term study with patients who received spironolactone for up to 8 years for the treatment of acne vulgaris (Shaw and White).
Spironolactone can be used as monotherapy or in combination with OCs safely. In fact, by prescribing spironolactone in combination with OCs you can kill 3 birds with 1 stone from efficacy (the synergy between the two often allows for lower dosing of spironolactone without compromising impact), contraception prevention, and dysmenorrhea perspectives. I do offer OCs to eligible patients who are starting on spironolactone. In general, spironolactone can be used safely in combination with oral antibiotics, though oral antibiotic use should be short-term to limit rising rates of antimicrobial resistance. Of note, there may be risk for hyperkalemia when spironolactone is combined with trimethoprim-sulfamethoxazole, so its use should be avoided in this setting.
How do you keep patients compliant with treatment?
If androgens are playing a notable role in the patient’s acne, some response is usually noted by even the first return visit, which I always make for 4 weeks later, unlike with other acne treatment regimens, which I usually make for 7 to 8 weeks later. Even though most treatments require at least 8 weeks to show any sign of improvement, even spironolactone at times, close follow-up allows me to increase the dose, which is often needed, or change to another medication if the patient is not tolerating it. Given that I stress it will require taking the medication every day in a consistent fashion to allow me to effectively evaluate it, the short time frame between visits also enhances compliance, as it encourages the patient to actually take the medication and incorporate it into her routine.
What do you do if patients refuse treatment?
I always tell my patients they are the captains and I am helping them navigate through their disease. I will, however, discuss the chronicity of acne as well as the long-term sequelae of this inflammatory disease including scarring and postinflammatory pigment alteration for which there are no great treatments. I also tell them that if there is any issue with the medication, we simply stop, and the likelihood for severe adverse events is exceedingly low based on the evidence and anecdotal experience.
Suggested Readings
Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
Schmidt TH, Shinkai K. Evidence-based approach to cutaneous hyperandrogenism in women. J Am Acad Dermatol. 2015;73:672-690.
Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year followup study. J Cutan Med Surg. 2002;6:541-545.
Suggested Readings
Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
Schmidt TH, Shinkai K. Evidence-based approach to cutaneous hyperandrogenism in women. J Am Acad Dermatol. 2015;73:672-690.
Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year followup study. J Cutan Med Surg. 2002;6:541-545.
Acne and Melanoma: What to Do With the Reported Connection?
Dermatologists have become accustomed to reading about the associations of dermatologic disease with extracutaneous comorbidities (psoriasis certainly takes the lead). One may see the headline “Study finds increased risk for melanoma in female acne patients” and say “Sure, why not?” However, before we all jump on the association bandwagon, let’s better appreciate this finding.
A study published online January 8 in Cancer by Zhang et al followed 99,128 female nurses in the Nurses’ Health Study II cohort for 20 years. This cohort has been utilized for numerous prospective studies over the year. Even after adjusting for known risk factors, investigators discovered that women with a history of severe cystic teenage acne had a hazard ratio of 1.44 for melanoma. The authors replicated the association with an independent melanoma case-control study of 930 cases and 1026 controls, finding an odds ratio of 1.27. They also found that individuals with teenage acne were more likely to have nevi (52.7% vs 50.1% in the cohort study; 55.2% vs 45.1% in the control study).
These data points ultimately led the team to conclude that acne may serve as an independent risk factor for melanoma, attributing androgens in female acne as a possible and plausible explanation due to their known effect on telomere elongation; melanocytes with longer telomere lengths have more opportunity to develop mutations, which could lead to malignant transformation, as the extended length ultimately delays initiation of cellular senescence. The longer these cells are “awake,” more moles can form, which means more room for trouble.
What’s the issue?
The size of this cohort certainly gives credibility to the data and statistics presented. Although the study is powered very well by the numbers, it is a unique cohort because all participants were nurses, narrowing down the demographics to some degree given general patterns, behaviors, and backgrounds when it comes to this group, an issue that has been previously raised with using this cohort. That said, more research is certainly warranted to elucidate the proposed mechanism and further clarify the association.
From a purely clinical standpoint, this paper is powerful ammo that can be used in our war against skin cancer. This very large cohort probably does not follow the American Academy of Dermatology guidelines for sun protection, skin cancer prevention, and surveillance. It could be a nice tidbit for patients at the end of your spiel on acne and then work in the photoprotection discussion, something we haven’t been the best at according to a recent study published in JAMA Dermatology (JAMA Dermatol. 2014;150:51-55)! Would it be such a bad thing if this paper helped us encourage all women with moderate to severe acne to undertake more effective sun-safe behaviors and to visit their dermatologist every year for total-body skin examinations?
Dermatologists have become accustomed to reading about the associations of dermatologic disease with extracutaneous comorbidities (psoriasis certainly takes the lead). One may see the headline “Study finds increased risk for melanoma in female acne patients” and say “Sure, why not?” However, before we all jump on the association bandwagon, let’s better appreciate this finding.
A study published online January 8 in Cancer by Zhang et al followed 99,128 female nurses in the Nurses’ Health Study II cohort for 20 years. This cohort has been utilized for numerous prospective studies over the year. Even after adjusting for known risk factors, investigators discovered that women with a history of severe cystic teenage acne had a hazard ratio of 1.44 for melanoma. The authors replicated the association with an independent melanoma case-control study of 930 cases and 1026 controls, finding an odds ratio of 1.27. They also found that individuals with teenage acne were more likely to have nevi (52.7% vs 50.1% in the cohort study; 55.2% vs 45.1% in the control study).
These data points ultimately led the team to conclude that acne may serve as an independent risk factor for melanoma, attributing androgens in female acne as a possible and plausible explanation due to their known effect on telomere elongation; melanocytes with longer telomere lengths have more opportunity to develop mutations, which could lead to malignant transformation, as the extended length ultimately delays initiation of cellular senescence. The longer these cells are “awake,” more moles can form, which means more room for trouble.
What’s the issue?
The size of this cohort certainly gives credibility to the data and statistics presented. Although the study is powered very well by the numbers, it is a unique cohort because all participants were nurses, narrowing down the demographics to some degree given general patterns, behaviors, and backgrounds when it comes to this group, an issue that has been previously raised with using this cohort. That said, more research is certainly warranted to elucidate the proposed mechanism and further clarify the association.
From a purely clinical standpoint, this paper is powerful ammo that can be used in our war against skin cancer. This very large cohort probably does not follow the American Academy of Dermatology guidelines for sun protection, skin cancer prevention, and surveillance. It could be a nice tidbit for patients at the end of your spiel on acne and then work in the photoprotection discussion, something we haven’t been the best at according to a recent study published in JAMA Dermatology (JAMA Dermatol. 2014;150:51-55)! Would it be such a bad thing if this paper helped us encourage all women with moderate to severe acne to undertake more effective sun-safe behaviors and to visit their dermatologist every year for total-body skin examinations?
Dermatologists have become accustomed to reading about the associations of dermatologic disease with extracutaneous comorbidities (psoriasis certainly takes the lead). One may see the headline “Study finds increased risk for melanoma in female acne patients” and say “Sure, why not?” However, before we all jump on the association bandwagon, let’s better appreciate this finding.
A study published online January 8 in Cancer by Zhang et al followed 99,128 female nurses in the Nurses’ Health Study II cohort for 20 years. This cohort has been utilized for numerous prospective studies over the year. Even after adjusting for known risk factors, investigators discovered that women with a history of severe cystic teenage acne had a hazard ratio of 1.44 for melanoma. The authors replicated the association with an independent melanoma case-control study of 930 cases and 1026 controls, finding an odds ratio of 1.27. They also found that individuals with teenage acne were more likely to have nevi (52.7% vs 50.1% in the cohort study; 55.2% vs 45.1% in the control study).
These data points ultimately led the team to conclude that acne may serve as an independent risk factor for melanoma, attributing androgens in female acne as a possible and plausible explanation due to their known effect on telomere elongation; melanocytes with longer telomere lengths have more opportunity to develop mutations, which could lead to malignant transformation, as the extended length ultimately delays initiation of cellular senescence. The longer these cells are “awake,” more moles can form, which means more room for trouble.
What’s the issue?
The size of this cohort certainly gives credibility to the data and statistics presented. Although the study is powered very well by the numbers, it is a unique cohort because all participants were nurses, narrowing down the demographics to some degree given general patterns, behaviors, and backgrounds when it comes to this group, an issue that has been previously raised with using this cohort. That said, more research is certainly warranted to elucidate the proposed mechanism and further clarify the association.
From a purely clinical standpoint, this paper is powerful ammo that can be used in our war against skin cancer. This very large cohort probably does not follow the American Academy of Dermatology guidelines for sun protection, skin cancer prevention, and surveillance. It could be a nice tidbit for patients at the end of your spiel on acne and then work in the photoprotection discussion, something we haven’t been the best at according to a recent study published in JAMA Dermatology (JAMA Dermatol. 2014;150:51-55)! Would it be such a bad thing if this paper helped us encourage all women with moderate to severe acne to undertake more effective sun-safe behaviors and to visit their dermatologist every year for total-body skin examinations?
Changing the Paradigm: New Thoughts on Pathophysiology and Drugable Targets in Acne
Who is tired of the same old stuff when it comes to acne? Innovation in therapy has been stagnant with a flurry of “me too” reformulated fixed combinations. The only true advance has been in drug delivery, with new vehicles allowing for the solubilization of established drugs such as dapsone or the combination of incompatible actives such as benzoyl peroxide and a retinoid. Before we can welcome new drugs with open arms, we must first expand the construct of acne pathophysiology to identify more appropriate targets for said new drugs. In a recent article published online in the Journal of the American Academy of Dermatology in June, Metiko et al highlight this sentiment. Generations of dermatologists were taught the 3- to 4-step process (depending on the teacher) through which an acne lesion forms: (1) follicular epidermal hyperproliferation, (2) Propionibacterium acnes colonization, and (3) inflammation. However, the molecular underpinnings of this theory have been challenged for more than a decade, with research highlighting the presence of preclinical inflammation, most recently found to be mediated by IL-1ß through a specific inflammasome pathway, NLRP3 (NOD-like receptor family, pyrin domain containing 3). Maybe we are missing a bridge between this stellar basic science and the clinical dermatologist who contends that the pesky microcomedone is the acne instigator. This short but sweet letter once again calls this antiquated prose into question in a highly visible clinical dermatology journal.
In thinking of new pathways and targets, Gupta et al published an article online in Archives of Dermatological Research on May 19 on the role of peroxisome proliferator-activated receptors (PPARs) and PPAR agonists in the treatment of multiple dermatologic diseases. For our purposes, I will highlight the section on acne and will start at the end: More research is needed. Peroxisome proliferator-activated receptors are nuclear hormone receptors that regulate gene expression, cell growth and differentiation, apoptosis, inflammatory responses, and tumorigenesis after binding with specific ligands. With respect to acne specifically, PPARs influence 2 of the pathophysiologic factors—sebum production and inflammation—due to their effect on lipid deposition in the sebocytes and inhibition of proinflammatory gene expression and downregulation of inflammatory cytokines. It appears that activation or inhibition of specific PPAR subtypes can either increase or decrease sebum production or be pro- or anti-inflammatory. The tough part is which receptors to activate and which to inhibit. This review related to an interesting clinical study that evaluated oral zileuton 600 mg administered 4 times daily for 3 months for acne. Zileuton inhibits leukotriene B4 production, which, as it turns out, is a natural ligand for PPARα. The idea here is that this blockade would be anti-inflammatory and indirectly inhibit the sebum production via PPARα suppression. The pilot study was reported as successful, with a decrease in the papulopustular acne severity index in a time-dependent manner in subjects evaluated.
What’s the issue?
So, what’s the point of this long-winded, double-paper review? We need to expand our acne horizons. We need new bench-to-bedside approaches. Which is your favorite target?
Who is tired of the same old stuff when it comes to acne? Innovation in therapy has been stagnant with a flurry of “me too” reformulated fixed combinations. The only true advance has been in drug delivery, with new vehicles allowing for the solubilization of established drugs such as dapsone or the combination of incompatible actives such as benzoyl peroxide and a retinoid. Before we can welcome new drugs with open arms, we must first expand the construct of acne pathophysiology to identify more appropriate targets for said new drugs. In a recent article published online in the Journal of the American Academy of Dermatology in June, Metiko et al highlight this sentiment. Generations of dermatologists were taught the 3- to 4-step process (depending on the teacher) through which an acne lesion forms: (1) follicular epidermal hyperproliferation, (2) Propionibacterium acnes colonization, and (3) inflammation. However, the molecular underpinnings of this theory have been challenged for more than a decade, with research highlighting the presence of preclinical inflammation, most recently found to be mediated by IL-1ß through a specific inflammasome pathway, NLRP3 (NOD-like receptor family, pyrin domain containing 3). Maybe we are missing a bridge between this stellar basic science and the clinical dermatologist who contends that the pesky microcomedone is the acne instigator. This short but sweet letter once again calls this antiquated prose into question in a highly visible clinical dermatology journal.
In thinking of new pathways and targets, Gupta et al published an article online in Archives of Dermatological Research on May 19 on the role of peroxisome proliferator-activated receptors (PPARs) and PPAR agonists in the treatment of multiple dermatologic diseases. For our purposes, I will highlight the section on acne and will start at the end: More research is needed. Peroxisome proliferator-activated receptors are nuclear hormone receptors that regulate gene expression, cell growth and differentiation, apoptosis, inflammatory responses, and tumorigenesis after binding with specific ligands. With respect to acne specifically, PPARs influence 2 of the pathophysiologic factors—sebum production and inflammation—due to their effect on lipid deposition in the sebocytes and inhibition of proinflammatory gene expression and downregulation of inflammatory cytokines. It appears that activation or inhibition of specific PPAR subtypes can either increase or decrease sebum production or be pro- or anti-inflammatory. The tough part is which receptors to activate and which to inhibit. This review related to an interesting clinical study that evaluated oral zileuton 600 mg administered 4 times daily for 3 months for acne. Zileuton inhibits leukotriene B4 production, which, as it turns out, is a natural ligand for PPARα. The idea here is that this blockade would be anti-inflammatory and indirectly inhibit the sebum production via PPARα suppression. The pilot study was reported as successful, with a decrease in the papulopustular acne severity index in a time-dependent manner in subjects evaluated.
What’s the issue?
So, what’s the point of this long-winded, double-paper review? We need to expand our acne horizons. We need new bench-to-bedside approaches. Which is your favorite target?
Who is tired of the same old stuff when it comes to acne? Innovation in therapy has been stagnant with a flurry of “me too” reformulated fixed combinations. The only true advance has been in drug delivery, with new vehicles allowing for the solubilization of established drugs such as dapsone or the combination of incompatible actives such as benzoyl peroxide and a retinoid. Before we can welcome new drugs with open arms, we must first expand the construct of acne pathophysiology to identify more appropriate targets for said new drugs. In a recent article published online in the Journal of the American Academy of Dermatology in June, Metiko et al highlight this sentiment. Generations of dermatologists were taught the 3- to 4-step process (depending on the teacher) through which an acne lesion forms: (1) follicular epidermal hyperproliferation, (2) Propionibacterium acnes colonization, and (3) inflammation. However, the molecular underpinnings of this theory have been challenged for more than a decade, with research highlighting the presence of preclinical inflammation, most recently found to be mediated by IL-1ß through a specific inflammasome pathway, NLRP3 (NOD-like receptor family, pyrin domain containing 3). Maybe we are missing a bridge between this stellar basic science and the clinical dermatologist who contends that the pesky microcomedone is the acne instigator. This short but sweet letter once again calls this antiquated prose into question in a highly visible clinical dermatology journal.
In thinking of new pathways and targets, Gupta et al published an article online in Archives of Dermatological Research on May 19 on the role of peroxisome proliferator-activated receptors (PPARs) and PPAR agonists in the treatment of multiple dermatologic diseases. For our purposes, I will highlight the section on acne and will start at the end: More research is needed. Peroxisome proliferator-activated receptors are nuclear hormone receptors that regulate gene expression, cell growth and differentiation, apoptosis, inflammatory responses, and tumorigenesis after binding with specific ligands. With respect to acne specifically, PPARs influence 2 of the pathophysiologic factors—sebum production and inflammation—due to their effect on lipid deposition in the sebocytes and inhibition of proinflammatory gene expression and downregulation of inflammatory cytokines. It appears that activation or inhibition of specific PPAR subtypes can either increase or decrease sebum production or be pro- or anti-inflammatory. The tough part is which receptors to activate and which to inhibit. This review related to an interesting clinical study that evaluated oral zileuton 600 mg administered 4 times daily for 3 months for acne. Zileuton inhibits leukotriene B4 production, which, as it turns out, is a natural ligand for PPARα. The idea here is that this blockade would be anti-inflammatory and indirectly inhibit the sebum production via PPARα suppression. The pilot study was reported as successful, with a decrease in the papulopustular acne severity index in a time-dependent manner in subjects evaluated.
What’s the issue?
So, what’s the point of this long-winded, double-paper review? We need to expand our acne horizons. We need new bench-to-bedside approaches. Which is your favorite target?
Acne at the AAD: Updates for 2015
At the recent 73rd Annual Meeting of the American Academy of Dermatology (AAD) in San Francisco, California, acne was once again a hot topic. Considerably more attention was paid to the role of androgens and, by default, antihormonal therapy as compared to prior years. Here are some of the highlights.
Spironolactone for Acne
Although not US Food and Drug Administration approved for the treatment of acne, spironolactone can be quite effective in the treatment of adult female acne due to its ability to block androgen receptors, decrease androgen production, inhibit 5α-reductase, and increase sexual hormone–binding protein. The question that often comes up is, how frequently do you need to check serum potassium levels, given that this drug is a potassium-sparing diuretic? According to both a poster at the AAD (P1296) and a study (the largest of its kind) published online on March 22 by Plovanich et al in JAMA Dermatology, the answer is not often or possibly not at all in young, otherwise-healthy women. Who should be checked then? Patients who have known cardiac and renal disease or impaired hepatic functioning as well as patients taking angiotensin-converting enzyme inhibitors, aldosterone blockers, angiotensin II antagonists, nonsteroidal anti-inflammatory drugs, or potassium supplementation. Be cautious with patients on digoxin and lithium, as spironolactone can increase their serum concentration and half-life.
Isotretinoin Risk
During a symposium (Acne Treatment Controversies), Dr. Diane M. Thiboutot discussed what’s new in the world of isotretinoin safety myths as well as useful tips to improve outcomes. The most recent data do not support a link between isotretinoin and inflammatory bowel disease. Prednisone and lower doses of isotretinoin can be used when initiating in a patient with severe inflammatory acne. Isotretinoin use also can be considered in patients with prior history of pseudotumor from tetracycline antibiotics with appropriate consultation. No significant issues regarding wound healing have been demonstrated or reported with isotretinoin, so the need to stop before cutaneous surgery is unlikely. Additionally, there are limited reports of complications with rhinoplasty, a possible slight increased risk for dry socket, and few cases of keloidal scarring with dermabrasion.
Updates in Pathophysiology
New immunologic targets for therapy and a better understanding of the role of the sebocyte in acne pathogenesis dominated the discussions at the AAD. In a workshop (Translating Evidence Into Practice: Acne Guidelines), Dr. Rachel V. Reynolds discussed the many hats worn by the sebocyte from an endocrine organ responsive to melanocortins, vitamin D, and corticotropin-releasing hormone to an immune cell responsible for secreting antimicrobial peptides and a broad array of inflammatory mediators implicated in the pathogenesis of acne. Fitting in with the recent excitement surrounding the identification of a key inflammasome (NLRP3 [NOD-like receptor family, pyrin domain containing 3]) in the IL-1β-induced subclinical inflammation in acne (Qin et al. J Invest Dermatol. 2014;134:381-388), it also was found that the sebocyte can participate in the same pathway. Propionibacterium acnes–induced NLRP3-derived IL-1β activation in sebocytes also has a role in acne pathogenesis (Li et al. J Invest Dermatol. 2014;134:2747-2756).
With respect to targeting elements of the inflammasome, during a forum (Treating Tumors and Inflammatory Skin Diseases With Immunomodulators and Biologics) I had the opportunity to discuss my collaboration with Dr. Jenny Kim of the University of California, Los Angeles, using nitric oxide–releasing nanoparticles (P1691). We found that not only do nitric oxide–releasing nanoparticles prevent the release of inflammatory cytokines from P acnes–stimulated human keratinocytes and monocytes, but even more importantly, this nanotechnology can inhibit multiple elements of the inflammasome cascade at the gene level. For example, caspase-1 and IL-1β suggests a mechanism(s) by which this technology could serve as not only a treatment of acne but of other inflammatory dermatoses.
That’s all for now! Stay tuned for more updates and hot topics in the world of acne.
At the recent 73rd Annual Meeting of the American Academy of Dermatology (AAD) in San Francisco, California, acne was once again a hot topic. Considerably more attention was paid to the role of androgens and, by default, antihormonal therapy as compared to prior years. Here are some of the highlights.
Spironolactone for Acne
Although not US Food and Drug Administration approved for the treatment of acne, spironolactone can be quite effective in the treatment of adult female acne due to its ability to block androgen receptors, decrease androgen production, inhibit 5α-reductase, and increase sexual hormone–binding protein. The question that often comes up is, how frequently do you need to check serum potassium levels, given that this drug is a potassium-sparing diuretic? According to both a poster at the AAD (P1296) and a study (the largest of its kind) published online on March 22 by Plovanich et al in JAMA Dermatology, the answer is not often or possibly not at all in young, otherwise-healthy women. Who should be checked then? Patients who have known cardiac and renal disease or impaired hepatic functioning as well as patients taking angiotensin-converting enzyme inhibitors, aldosterone blockers, angiotensin II antagonists, nonsteroidal anti-inflammatory drugs, or potassium supplementation. Be cautious with patients on digoxin and lithium, as spironolactone can increase their serum concentration and half-life.
Isotretinoin Risk
During a symposium (Acne Treatment Controversies), Dr. Diane M. Thiboutot discussed what’s new in the world of isotretinoin safety myths as well as useful tips to improve outcomes. The most recent data do not support a link between isotretinoin and inflammatory bowel disease. Prednisone and lower doses of isotretinoin can be used when initiating in a patient with severe inflammatory acne. Isotretinoin use also can be considered in patients with prior history of pseudotumor from tetracycline antibiotics with appropriate consultation. No significant issues regarding wound healing have been demonstrated or reported with isotretinoin, so the need to stop before cutaneous surgery is unlikely. Additionally, there are limited reports of complications with rhinoplasty, a possible slight increased risk for dry socket, and few cases of keloidal scarring with dermabrasion.
Updates in Pathophysiology
New immunologic targets for therapy and a better understanding of the role of the sebocyte in acne pathogenesis dominated the discussions at the AAD. In a workshop (Translating Evidence Into Practice: Acne Guidelines), Dr. Rachel V. Reynolds discussed the many hats worn by the sebocyte from an endocrine organ responsive to melanocortins, vitamin D, and corticotropin-releasing hormone to an immune cell responsible for secreting antimicrobial peptides and a broad array of inflammatory mediators implicated in the pathogenesis of acne. Fitting in with the recent excitement surrounding the identification of a key inflammasome (NLRP3 [NOD-like receptor family, pyrin domain containing 3]) in the IL-1β-induced subclinical inflammation in acne (Qin et al. J Invest Dermatol. 2014;134:381-388), it also was found that the sebocyte can participate in the same pathway. Propionibacterium acnes–induced NLRP3-derived IL-1β activation in sebocytes also has a role in acne pathogenesis (Li et al. J Invest Dermatol. 2014;134:2747-2756).
With respect to targeting elements of the inflammasome, during a forum (Treating Tumors and Inflammatory Skin Diseases With Immunomodulators and Biologics) I had the opportunity to discuss my collaboration with Dr. Jenny Kim of the University of California, Los Angeles, using nitric oxide–releasing nanoparticles (P1691). We found that not only do nitric oxide–releasing nanoparticles prevent the release of inflammatory cytokines from P acnes–stimulated human keratinocytes and monocytes, but even more importantly, this nanotechnology can inhibit multiple elements of the inflammasome cascade at the gene level. For example, caspase-1 and IL-1β suggests a mechanism(s) by which this technology could serve as not only a treatment of acne but of other inflammatory dermatoses.
That’s all for now! Stay tuned for more updates and hot topics in the world of acne.
At the recent 73rd Annual Meeting of the American Academy of Dermatology (AAD) in San Francisco, California, acne was once again a hot topic. Considerably more attention was paid to the role of androgens and, by default, antihormonal therapy as compared to prior years. Here are some of the highlights.
Spironolactone for Acne
Although not US Food and Drug Administration approved for the treatment of acne, spironolactone can be quite effective in the treatment of adult female acne due to its ability to block androgen receptors, decrease androgen production, inhibit 5α-reductase, and increase sexual hormone–binding protein. The question that often comes up is, how frequently do you need to check serum potassium levels, given that this drug is a potassium-sparing diuretic? According to both a poster at the AAD (P1296) and a study (the largest of its kind) published online on March 22 by Plovanich et al in JAMA Dermatology, the answer is not often or possibly not at all in young, otherwise-healthy women. Who should be checked then? Patients who have known cardiac and renal disease or impaired hepatic functioning as well as patients taking angiotensin-converting enzyme inhibitors, aldosterone blockers, angiotensin II antagonists, nonsteroidal anti-inflammatory drugs, or potassium supplementation. Be cautious with patients on digoxin and lithium, as spironolactone can increase their serum concentration and half-life.
Isotretinoin Risk
During a symposium (Acne Treatment Controversies), Dr. Diane M. Thiboutot discussed what’s new in the world of isotretinoin safety myths as well as useful tips to improve outcomes. The most recent data do not support a link between isotretinoin and inflammatory bowel disease. Prednisone and lower doses of isotretinoin can be used when initiating in a patient with severe inflammatory acne. Isotretinoin use also can be considered in patients with prior history of pseudotumor from tetracycline antibiotics with appropriate consultation. No significant issues regarding wound healing have been demonstrated or reported with isotretinoin, so the need to stop before cutaneous surgery is unlikely. Additionally, there are limited reports of complications with rhinoplasty, a possible slight increased risk for dry socket, and few cases of keloidal scarring with dermabrasion.
Updates in Pathophysiology
New immunologic targets for therapy and a better understanding of the role of the sebocyte in acne pathogenesis dominated the discussions at the AAD. In a workshop (Translating Evidence Into Practice: Acne Guidelines), Dr. Rachel V. Reynolds discussed the many hats worn by the sebocyte from an endocrine organ responsive to melanocortins, vitamin D, and corticotropin-releasing hormone to an immune cell responsible for secreting antimicrobial peptides and a broad array of inflammatory mediators implicated in the pathogenesis of acne. Fitting in with the recent excitement surrounding the identification of a key inflammasome (NLRP3 [NOD-like receptor family, pyrin domain containing 3]) in the IL-1β-induced subclinical inflammation in acne (Qin et al. J Invest Dermatol. 2014;134:381-388), it also was found that the sebocyte can participate in the same pathway. Propionibacterium acnes–induced NLRP3-derived IL-1β activation in sebocytes also has a role in acne pathogenesis (Li et al. J Invest Dermatol. 2014;134:2747-2756).
With respect to targeting elements of the inflammasome, during a forum (Treating Tumors and Inflammatory Skin Diseases With Immunomodulators and Biologics) I had the opportunity to discuss my collaboration with Dr. Jenny Kim of the University of California, Los Angeles, using nitric oxide–releasing nanoparticles (P1691). We found that not only do nitric oxide–releasing nanoparticles prevent the release of inflammatory cytokines from P acnes–stimulated human keratinocytes and monocytes, but even more importantly, this nanotechnology can inhibit multiple elements of the inflammasome cascade at the gene level. For example, caspase-1 and IL-1β suggests a mechanism(s) by which this technology could serve as not only a treatment of acne but of other inflammatory dermatoses.
That’s all for now! Stay tuned for more updates and hot topics in the world of acne.
The Role of Diet in Acne: We Get It, But What Should We Do About It?
The role of diet in acne, both as a causative agent and therapeutic intervention, has been the topic of discussion in both the dermatology community as well as the laypress for decades. There is ample evidence highlighting the association of acne and high glycemic loads, certain dairy products, and refined sugar product ingestion. In the most recent edition to the repository, Grossi et al (J Eur Acad Dermatol Venereol. doi:10.1111/jdv.12878) reanalyzed data from their case-control study among young patients (age range, 10–24 years; N=563) with a diagnosis of moderate to severe acne versus control (participants with no or mild acne) between March 2009 and February 2010 that was originally published in 2012 (J Am Acad Dermatol. 2012;67:1129-1135). The unique element was how they evaluated the data. The investigators utilized a semantic connectivity map approach derived from artificial neural network computational models, which allowed for a better understanding of the complex connections between all of the studied variables. (The assumption of a given relation between any variables would not influence the results.) The data were presented on an Auto Semantic Connectivity Map that resembled a 4-leaf clover, representing “explanatory” information pertaining to the cases and controls and “residual” information of less importance. It is worth seeing in the manuscript to better appreciate the data.
What did they find? There is a close association between moderate to severe acne and a high intake of milk, other dairy products, sweets, and chocolate. Obesity and the low consumption of fish were linked to the presence of moderate to severe acne, while high consumption of fish (1 d/wk or more), high intake of fruits and vegetables, and body mass index lower than 18.5 were all associated with limited or no acne.
What’s the issue?
By adopting a different analytic approach, it was shown once again that diet plays a substantial role in acne, indicating that some food items may stimulate selected acne-promoting pathways. But what now? Here is the evidence yet again, but where is the medicine of “evidence-based medicine”? It is time to recommend guidelines for screening and counseling. In a recent article, Bronsnick et al (J Am Acad Dermatol. 2014;71:1039.e1-1039.e12) found that the level of evidence supporting the benefit of a low-glycemic, low-carbohydrate diet was sufficient to recommend to acne patients. How many dermatologists feel comfortable providing dietary guidance to their acne patients? A consensus statement from relevant organizations such as the American Academy of Dermatology, the Society for Investigative Dermatology, and the American Acne & Rosacea Society that provides tangible and realistic screening tools to identify those who would benefit from dietary intervention and implementation and practice guidelines for Dr. Derm seeing 40 to 50 patients a day in Springfield, USA (homage to The Simpsons) based on the level of evidence available would be useful. What are your thoughts?
We want to know your views! Tell us what you think.
Suggested Readings
- Bowe WP, Joshi SS, Shalita AR. Diet and acne. J Am Acad Dermatol. 2010;63:124-141.
- Ferdowsian HR, Levin S. Does diet really affect acne? Skin Therapy Lett. 2010;15:1-2, 5.
- Melnik B. Dietary intervention in acne: attenuation of increased mTORC1 signaling promoted by Western diet. Dermatoendocrinol. 2012;4:20-32.
- Veith WB, Silverberg NB. The association of acne vulgaris with diet. Cutis. 2011;88:84-91.
The role of diet in acne, both as a causative agent and therapeutic intervention, has been the topic of discussion in both the dermatology community as well as the laypress for decades. There is ample evidence highlighting the association of acne and high glycemic loads, certain dairy products, and refined sugar product ingestion. In the most recent edition to the repository, Grossi et al (J Eur Acad Dermatol Venereol. doi:10.1111/jdv.12878) reanalyzed data from their case-control study among young patients (age range, 10–24 years; N=563) with a diagnosis of moderate to severe acne versus control (participants with no or mild acne) between March 2009 and February 2010 that was originally published in 2012 (J Am Acad Dermatol. 2012;67:1129-1135). The unique element was how they evaluated the data. The investigators utilized a semantic connectivity map approach derived from artificial neural network computational models, which allowed for a better understanding of the complex connections between all of the studied variables. (The assumption of a given relation between any variables would not influence the results.) The data were presented on an Auto Semantic Connectivity Map that resembled a 4-leaf clover, representing “explanatory” information pertaining to the cases and controls and “residual” information of less importance. It is worth seeing in the manuscript to better appreciate the data.
What did they find? There is a close association between moderate to severe acne and a high intake of milk, other dairy products, sweets, and chocolate. Obesity and the low consumption of fish were linked to the presence of moderate to severe acne, while high consumption of fish (1 d/wk or more), high intake of fruits and vegetables, and body mass index lower than 18.5 were all associated with limited or no acne.
What’s the issue?
By adopting a different analytic approach, it was shown once again that diet plays a substantial role in acne, indicating that some food items may stimulate selected acne-promoting pathways. But what now? Here is the evidence yet again, but where is the medicine of “evidence-based medicine”? It is time to recommend guidelines for screening and counseling. In a recent article, Bronsnick et al (J Am Acad Dermatol. 2014;71:1039.e1-1039.e12) found that the level of evidence supporting the benefit of a low-glycemic, low-carbohydrate diet was sufficient to recommend to acne patients. How many dermatologists feel comfortable providing dietary guidance to their acne patients? A consensus statement from relevant organizations such as the American Academy of Dermatology, the Society for Investigative Dermatology, and the American Acne & Rosacea Society that provides tangible and realistic screening tools to identify those who would benefit from dietary intervention and implementation and practice guidelines for Dr. Derm seeing 40 to 50 patients a day in Springfield, USA (homage to The Simpsons) based on the level of evidence available would be useful. What are your thoughts?
We want to know your views! Tell us what you think.
Suggested Readings
- Bowe WP, Joshi SS, Shalita AR. Diet and acne. J Am Acad Dermatol. 2010;63:124-141.
- Ferdowsian HR, Levin S. Does diet really affect acne? Skin Therapy Lett. 2010;15:1-2, 5.
- Melnik B. Dietary intervention in acne: attenuation of increased mTORC1 signaling promoted by Western diet. Dermatoendocrinol. 2012;4:20-32.
- Veith WB, Silverberg NB. The association of acne vulgaris with diet. Cutis. 2011;88:84-91.
The role of diet in acne, both as a causative agent and therapeutic intervention, has been the topic of discussion in both the dermatology community as well as the laypress for decades. There is ample evidence highlighting the association of acne and high glycemic loads, certain dairy products, and refined sugar product ingestion. In the most recent edition to the repository, Grossi et al (J Eur Acad Dermatol Venereol. doi:10.1111/jdv.12878) reanalyzed data from their case-control study among young patients (age range, 10–24 years; N=563) with a diagnosis of moderate to severe acne versus control (participants with no or mild acne) between March 2009 and February 2010 that was originally published in 2012 (J Am Acad Dermatol. 2012;67:1129-1135). The unique element was how they evaluated the data. The investigators utilized a semantic connectivity map approach derived from artificial neural network computational models, which allowed for a better understanding of the complex connections between all of the studied variables. (The assumption of a given relation between any variables would not influence the results.) The data were presented on an Auto Semantic Connectivity Map that resembled a 4-leaf clover, representing “explanatory” information pertaining to the cases and controls and “residual” information of less importance. It is worth seeing in the manuscript to better appreciate the data.
What did they find? There is a close association between moderate to severe acne and a high intake of milk, other dairy products, sweets, and chocolate. Obesity and the low consumption of fish were linked to the presence of moderate to severe acne, while high consumption of fish (1 d/wk or more), high intake of fruits and vegetables, and body mass index lower than 18.5 were all associated with limited or no acne.
What’s the issue?
By adopting a different analytic approach, it was shown once again that diet plays a substantial role in acne, indicating that some food items may stimulate selected acne-promoting pathways. But what now? Here is the evidence yet again, but where is the medicine of “evidence-based medicine”? It is time to recommend guidelines for screening and counseling. In a recent article, Bronsnick et al (J Am Acad Dermatol. 2014;71:1039.e1-1039.e12) found that the level of evidence supporting the benefit of a low-glycemic, low-carbohydrate diet was sufficient to recommend to acne patients. How many dermatologists feel comfortable providing dietary guidance to their acne patients? A consensus statement from relevant organizations such as the American Academy of Dermatology, the Society for Investigative Dermatology, and the American Acne & Rosacea Society that provides tangible and realistic screening tools to identify those who would benefit from dietary intervention and implementation and practice guidelines for Dr. Derm seeing 40 to 50 patients a day in Springfield, USA (homage to The Simpsons) based on the level of evidence available would be useful. What are your thoughts?
We want to know your views! Tell us what you think.
Suggested Readings
- Bowe WP, Joshi SS, Shalita AR. Diet and acne. J Am Acad Dermatol. 2010;63:124-141.
- Ferdowsian HR, Levin S. Does diet really affect acne? Skin Therapy Lett. 2010;15:1-2, 5.
- Melnik B. Dietary intervention in acne: attenuation of increased mTORC1 signaling promoted by Western diet. Dermatoendocrinol. 2012;4:20-32.
- Veith WB, Silverberg NB. The association of acne vulgaris with diet. Cutis. 2011;88:84-91.
Inflammatory Acne: New Developments in Pathogenesis and Treatment
Acne vulgaris is a chronic inflammatory disease that affects the majority of the population at some point in their lifetime. It is characterized by comedones, pustules, and papules. Acne pathogenesis is multifactorial with 4 primary factors that play a pivotal role in the formation of acne lesions: excess sebum production, abnormal keratinization, inflammation, and bacterial colonization of Propionibacterium acnes in the pilosebaceous unit.1 Although there is a general consensus on the pathogenic factors, the sequence of events in acne development is controversial. Traditionally it was believed that abnormal keratinization resulted in the creation of the microcomedone, the earliest subclinical acne lesion.2 Activation of sebaceous glands by androgens, excess sebum production, and keratin plug formation then were followed by P acnes colonization, with induction of the innate immune system culminating in inflammation.2 Androgen-induced sebum production and follicular hyperkeratinization and plugging have been cited as initial events that alter the pilosebaceous milieu, favoring the proliferation of P acnes1,3; however, evidence suggests inflammation as the inciting factor, with proof of significant inflammatory factors surrounding the pilosebaceous unit even in clinically uninvolved skin units in acne patients.4 Herein we will briefly review the most recent data and translational applications pertaining to the P acnes–triggered innate immune response via activation of toll-like receptor 2 (TLR2)5 and importantly the inflammasome.6,7
A new understanding of how P acnes induces the inflammatory cascade may represent a paradigm shift in the management of acne. Recognition of microbes, namely P acnes, by the innate immune system is the body’s first line of defense against pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs).6 Although these pathways combat infection and prevent foreign invasion, they also result in inflammation and tissue injury. The inflammatory response to PAMPs and DAMPs is mediated by the inflammasome, a caspase 1–activating cytoplasmic complex that induces the secretion of crucial proinflammatory cytokines.7 The exact mechanism by which P acnes exerts its proinflammatory activity has been somewhat unclear, though P acnes–induced inflammation has been shown to be mediated by proinflammatory cytokines tumor necrosis factor α, IL-1, IL-6, IL-8, and IL-12.5,8 However, remarkable evidence recently was presented regarding triggers of inflammation and the precise mechanism involved. Qin et al9 showed that P acnes is a potent trigger of IL-1β generation via activation of the NLRP3 (NOD-like receptor family, pyrin domain containing 3) inflammasome. Specifically, the study showed that human monocytes respond to P acnes by upregulating caspase 1, an inflammatory caspase required for proteolytic cleavage of IL-1β. The authors correlated their in vitro findings with clinical evidence of caspase 1 and NLRP3 expression in the dermis surrounding the pilosebaceous units of biopsied lesions.9 Kistowska et al6 confirmed and expanded on these data by showing the inability of NLRP3-deficient myeloid cells to secrete IL-1β and induce an inflammatory response in vivo. A recent investigation demonstrated that human sebocytes can function as constituents of the innate immune response, with P acnes triggering sebocyte NLRP3-inflammasome activation and subsequent IL-1β secretion. These observations were further confirmed in vivo with NLRP3-deficient mice displaying an impaired inflammatory response to P acnes.10
Our understanding of TLR2 signaling in the pathogenesis of acne also has expanded. It is well established that recognition of extracellular PAMPs and DAMPs is mediated by the expression of toll-like receptors on the surface of a variety of cells within the skin.11 Prior research demonstrated how P acnes increases TLR2 expression in keratinocytes, even in vivo.12 Stimulation by P acnes was shown to induce secretion of IL-8 (promoting a TH1 response) and IL-12 (promoting neutrophil chemotaxis) via TLR2 activation.5 Selway et al11 validated this finding by demonstrating that infundibular keratinocytes secrete IL-1α in response to the peptidoglycan cell wall of P acnes. Interestingly, Qin et al9 determined TLR2 inhibition resulted in partial suppression of IL-1β, possibly providing new evidence of TLR2-mediated activation of the NLRP3 inflammasome. Therefore, P acnes activates both extracellular and intracellular triggers of the innate immune response: TLR2 activation (requiring extracellular recognition of pathogens) and inflammasome-mediated activation (requiring internalization and access of the bacterium to the interior compartments of the cells).
Overall, these findings suggest that P acnes–induced inflammation can be selectively targeted by agents directed at inflammasome components, IL-1β, or toll-like receptors. A phase 2 double-blind, placebo-controlled trial assessing the efficacy of the anti–IL-1β monoclonal antibody gevokizumab found that 0.6 mg/kg administered subcutaneously resulted in a significant reduction in mean inflammatory lesion count compared to placebo (P=.077).13 The success of the IL-1 receptor antagonist anakinra against rare genetic autoinflammatory syndromes such as PAPA (pyogenic sterile arthritis, pyoderma gangrenosum, and acne) syndrome, an NLRP3 inflammasomopathy, sheds light onto new therapeutics that may be used to target acne vulgaris.14 Current topical therapies such as retinoids, which have already proven efficacious in the treatment of inflammatory acne, target these pathways. In vivo data revealed that treatment with isotretinoin significantly decreased TLR2 expression in monocytes (P<.001) and suppressed inflammatory cytokine responses to P acnes (P<.001).15 Adapalene, with or without benzoyl peroxide, also was shown to exert anti-inflammatory effects via TLR2 downregulation.16
These data and observations highlight a paradigm shift in our perception of acne. All acne is truly inflammatory, and by identifying aberrations in the immune response, we can develop targeted treatments for this chronic debilitating disease.
1. Kurokawa I, Danby FW, Ju Q, et al. New developments in our understanding of acne pathogenesis and treatment. Exp Dermatol. 2009;18:821-832.
2. Cunliffe WJ, Holland DB, Clark SM, et al. Comedogenesis: some aetiological, clinical and therapeutic strategies. Dermatology. 2003;206:11-16.
3. Bowe W, Kober M. Therapeutic update: acne. J Drugs Dermatol. 2014;13:235-238.
4. Jeremy AH, Holland DB, Roberts SG, et al. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003;121:20-27.
5. Kim J, Ochoa MT, Krutzik SR, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169:1535-1541.
6. Kistowska M, Gehrke S, Jankovic D, et al. IL-1beta drives inflammatory responses to Propionibacterium acnes in vitro and in vivo. J Invest Dermatol. 2014;134:677-685.
7. Contassot E, French LE. New insights into acne pathogenesis: Propionibacterium acnes activates the inflammasome. J Invest Dermatol. 2014;134:310-313.
8. Vowels BR, Yang S, Leyden JJ. Induction of proinflammatory cytokines by a soluble factor of Propionibacterium acnes: implications for chronic inflammatory acne. Infect Immun. 1995;63:3158-3165.
9. Qin M, Pirouz A, Kim MH, et al. Propionibacterium acnes induces IL-1beta secretion via the NLRP3 inflammasome in human monocytes. J Invest Dermatol. 2014;134:381-388.
10. Li ZJ, Choi DK, Sohn KC, et al. Propionibacterium acnes activates the NLRP3 inflammasome in human sebocytes. J Invest Dermatol. 2014;134:2747-2756.
11. Selway JL, Kurczab T, Kealey T, et al. Toll-like receptor 2 activation and comedogenesis: implications for the pathogenesis of acne. BMC Dermatol. 2013;13:10.
12. Jugeau S, Tenaud I, Knol AC, et al. Induction of toll-like receptors by Propionibacterium acnes. Br J Dermatol. 2005;153:1105-1113.
13. XOMA announces encouraging interim results from gevokizumab phase 2 study for moderate to severe acne vulgaris [press release]. Berkley, CA: XOMA Corporation; January 7, 2013. http://www.servier.com/content/xoma-announces-encouraging-interim-results-gevokizumab-phase-2-study-moderate-severe-acne. Accessed November 5, 2014.
14. Leemans JC, Cassel SL, Sutterwala FS. Sensing damage by the NLRP3 inflammasome. Immunol Rev. 2011;243:152-162.
15. Dispenza MC, Wolpert EB, Gilliland KL, et al. Systemic isotretinoin therapy normalizes exaggerated TLR-2-mediated innate immune responses in acne patients. J Invest Dermatol. 2012;132:2198-2205.
16. Zuliani T, Khammari A, Chaussy H, et al. Ex vivo demonstration of a synergistic effect of adapalene and benzoyl peroxide on inflammatory acne lesions. Exp Dermatol. 2011;20:850-853.
Acne vulgaris is a chronic inflammatory disease that affects the majority of the population at some point in their lifetime. It is characterized by comedones, pustules, and papules. Acne pathogenesis is multifactorial with 4 primary factors that play a pivotal role in the formation of acne lesions: excess sebum production, abnormal keratinization, inflammation, and bacterial colonization of Propionibacterium acnes in the pilosebaceous unit.1 Although there is a general consensus on the pathogenic factors, the sequence of events in acne development is controversial. Traditionally it was believed that abnormal keratinization resulted in the creation of the microcomedone, the earliest subclinical acne lesion.2 Activation of sebaceous glands by androgens, excess sebum production, and keratin plug formation then were followed by P acnes colonization, with induction of the innate immune system culminating in inflammation.2 Androgen-induced sebum production and follicular hyperkeratinization and plugging have been cited as initial events that alter the pilosebaceous milieu, favoring the proliferation of P acnes1,3; however, evidence suggests inflammation as the inciting factor, with proof of significant inflammatory factors surrounding the pilosebaceous unit even in clinically uninvolved skin units in acne patients.4 Herein we will briefly review the most recent data and translational applications pertaining to the P acnes–triggered innate immune response via activation of toll-like receptor 2 (TLR2)5 and importantly the inflammasome.6,7
A new understanding of how P acnes induces the inflammatory cascade may represent a paradigm shift in the management of acne. Recognition of microbes, namely P acnes, by the innate immune system is the body’s first line of defense against pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs).6 Although these pathways combat infection and prevent foreign invasion, they also result in inflammation and tissue injury. The inflammatory response to PAMPs and DAMPs is mediated by the inflammasome, a caspase 1–activating cytoplasmic complex that induces the secretion of crucial proinflammatory cytokines.7 The exact mechanism by which P acnes exerts its proinflammatory activity has been somewhat unclear, though P acnes–induced inflammation has been shown to be mediated by proinflammatory cytokines tumor necrosis factor α, IL-1, IL-6, IL-8, and IL-12.5,8 However, remarkable evidence recently was presented regarding triggers of inflammation and the precise mechanism involved. Qin et al9 showed that P acnes is a potent trigger of IL-1β generation via activation of the NLRP3 (NOD-like receptor family, pyrin domain containing 3) inflammasome. Specifically, the study showed that human monocytes respond to P acnes by upregulating caspase 1, an inflammatory caspase required for proteolytic cleavage of IL-1β. The authors correlated their in vitro findings with clinical evidence of caspase 1 and NLRP3 expression in the dermis surrounding the pilosebaceous units of biopsied lesions.9 Kistowska et al6 confirmed and expanded on these data by showing the inability of NLRP3-deficient myeloid cells to secrete IL-1β and induce an inflammatory response in vivo. A recent investigation demonstrated that human sebocytes can function as constituents of the innate immune response, with P acnes triggering sebocyte NLRP3-inflammasome activation and subsequent IL-1β secretion. These observations were further confirmed in vivo with NLRP3-deficient mice displaying an impaired inflammatory response to P acnes.10
Our understanding of TLR2 signaling in the pathogenesis of acne also has expanded. It is well established that recognition of extracellular PAMPs and DAMPs is mediated by the expression of toll-like receptors on the surface of a variety of cells within the skin.11 Prior research demonstrated how P acnes increases TLR2 expression in keratinocytes, even in vivo.12 Stimulation by P acnes was shown to induce secretion of IL-8 (promoting a TH1 response) and IL-12 (promoting neutrophil chemotaxis) via TLR2 activation.5 Selway et al11 validated this finding by demonstrating that infundibular keratinocytes secrete IL-1α in response to the peptidoglycan cell wall of P acnes. Interestingly, Qin et al9 determined TLR2 inhibition resulted in partial suppression of IL-1β, possibly providing new evidence of TLR2-mediated activation of the NLRP3 inflammasome. Therefore, P acnes activates both extracellular and intracellular triggers of the innate immune response: TLR2 activation (requiring extracellular recognition of pathogens) and inflammasome-mediated activation (requiring internalization and access of the bacterium to the interior compartments of the cells).
Overall, these findings suggest that P acnes–induced inflammation can be selectively targeted by agents directed at inflammasome components, IL-1β, or toll-like receptors. A phase 2 double-blind, placebo-controlled trial assessing the efficacy of the anti–IL-1β monoclonal antibody gevokizumab found that 0.6 mg/kg administered subcutaneously resulted in a significant reduction in mean inflammatory lesion count compared to placebo (P=.077).13 The success of the IL-1 receptor antagonist anakinra against rare genetic autoinflammatory syndromes such as PAPA (pyogenic sterile arthritis, pyoderma gangrenosum, and acne) syndrome, an NLRP3 inflammasomopathy, sheds light onto new therapeutics that may be used to target acne vulgaris.14 Current topical therapies such as retinoids, which have already proven efficacious in the treatment of inflammatory acne, target these pathways. In vivo data revealed that treatment with isotretinoin significantly decreased TLR2 expression in monocytes (P<.001) and suppressed inflammatory cytokine responses to P acnes (P<.001).15 Adapalene, with or without benzoyl peroxide, also was shown to exert anti-inflammatory effects via TLR2 downregulation.16
These data and observations highlight a paradigm shift in our perception of acne. All acne is truly inflammatory, and by identifying aberrations in the immune response, we can develop targeted treatments for this chronic debilitating disease.
Acne vulgaris is a chronic inflammatory disease that affects the majority of the population at some point in their lifetime. It is characterized by comedones, pustules, and papules. Acne pathogenesis is multifactorial with 4 primary factors that play a pivotal role in the formation of acne lesions: excess sebum production, abnormal keratinization, inflammation, and bacterial colonization of Propionibacterium acnes in the pilosebaceous unit.1 Although there is a general consensus on the pathogenic factors, the sequence of events in acne development is controversial. Traditionally it was believed that abnormal keratinization resulted in the creation of the microcomedone, the earliest subclinical acne lesion.2 Activation of sebaceous glands by androgens, excess sebum production, and keratin plug formation then were followed by P acnes colonization, with induction of the innate immune system culminating in inflammation.2 Androgen-induced sebum production and follicular hyperkeratinization and plugging have been cited as initial events that alter the pilosebaceous milieu, favoring the proliferation of P acnes1,3; however, evidence suggests inflammation as the inciting factor, with proof of significant inflammatory factors surrounding the pilosebaceous unit even in clinically uninvolved skin units in acne patients.4 Herein we will briefly review the most recent data and translational applications pertaining to the P acnes–triggered innate immune response via activation of toll-like receptor 2 (TLR2)5 and importantly the inflammasome.6,7
A new understanding of how P acnes induces the inflammatory cascade may represent a paradigm shift in the management of acne. Recognition of microbes, namely P acnes, by the innate immune system is the body’s first line of defense against pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs).6 Although these pathways combat infection and prevent foreign invasion, they also result in inflammation and tissue injury. The inflammatory response to PAMPs and DAMPs is mediated by the inflammasome, a caspase 1–activating cytoplasmic complex that induces the secretion of crucial proinflammatory cytokines.7 The exact mechanism by which P acnes exerts its proinflammatory activity has been somewhat unclear, though P acnes–induced inflammation has been shown to be mediated by proinflammatory cytokines tumor necrosis factor α, IL-1, IL-6, IL-8, and IL-12.5,8 However, remarkable evidence recently was presented regarding triggers of inflammation and the precise mechanism involved. Qin et al9 showed that P acnes is a potent trigger of IL-1β generation via activation of the NLRP3 (NOD-like receptor family, pyrin domain containing 3) inflammasome. Specifically, the study showed that human monocytes respond to P acnes by upregulating caspase 1, an inflammatory caspase required for proteolytic cleavage of IL-1β. The authors correlated their in vitro findings with clinical evidence of caspase 1 and NLRP3 expression in the dermis surrounding the pilosebaceous units of biopsied lesions.9 Kistowska et al6 confirmed and expanded on these data by showing the inability of NLRP3-deficient myeloid cells to secrete IL-1β and induce an inflammatory response in vivo. A recent investigation demonstrated that human sebocytes can function as constituents of the innate immune response, with P acnes triggering sebocyte NLRP3-inflammasome activation and subsequent IL-1β secretion. These observations were further confirmed in vivo with NLRP3-deficient mice displaying an impaired inflammatory response to P acnes.10
Our understanding of TLR2 signaling in the pathogenesis of acne also has expanded. It is well established that recognition of extracellular PAMPs and DAMPs is mediated by the expression of toll-like receptors on the surface of a variety of cells within the skin.11 Prior research demonstrated how P acnes increases TLR2 expression in keratinocytes, even in vivo.12 Stimulation by P acnes was shown to induce secretion of IL-8 (promoting a TH1 response) and IL-12 (promoting neutrophil chemotaxis) via TLR2 activation.5 Selway et al11 validated this finding by demonstrating that infundibular keratinocytes secrete IL-1α in response to the peptidoglycan cell wall of P acnes. Interestingly, Qin et al9 determined TLR2 inhibition resulted in partial suppression of IL-1β, possibly providing new evidence of TLR2-mediated activation of the NLRP3 inflammasome. Therefore, P acnes activates both extracellular and intracellular triggers of the innate immune response: TLR2 activation (requiring extracellular recognition of pathogens) and inflammasome-mediated activation (requiring internalization and access of the bacterium to the interior compartments of the cells).
Overall, these findings suggest that P acnes–induced inflammation can be selectively targeted by agents directed at inflammasome components, IL-1β, or toll-like receptors. A phase 2 double-blind, placebo-controlled trial assessing the efficacy of the anti–IL-1β monoclonal antibody gevokizumab found that 0.6 mg/kg administered subcutaneously resulted in a significant reduction in mean inflammatory lesion count compared to placebo (P=.077).13 The success of the IL-1 receptor antagonist anakinra against rare genetic autoinflammatory syndromes such as PAPA (pyogenic sterile arthritis, pyoderma gangrenosum, and acne) syndrome, an NLRP3 inflammasomopathy, sheds light onto new therapeutics that may be used to target acne vulgaris.14 Current topical therapies such as retinoids, which have already proven efficacious in the treatment of inflammatory acne, target these pathways. In vivo data revealed that treatment with isotretinoin significantly decreased TLR2 expression in monocytes (P<.001) and suppressed inflammatory cytokine responses to P acnes (P<.001).15 Adapalene, with or without benzoyl peroxide, also was shown to exert anti-inflammatory effects via TLR2 downregulation.16
These data and observations highlight a paradigm shift in our perception of acne. All acne is truly inflammatory, and by identifying aberrations in the immune response, we can develop targeted treatments for this chronic debilitating disease.
1. Kurokawa I, Danby FW, Ju Q, et al. New developments in our understanding of acne pathogenesis and treatment. Exp Dermatol. 2009;18:821-832.
2. Cunliffe WJ, Holland DB, Clark SM, et al. Comedogenesis: some aetiological, clinical and therapeutic strategies. Dermatology. 2003;206:11-16.
3. Bowe W, Kober M. Therapeutic update: acne. J Drugs Dermatol. 2014;13:235-238.
4. Jeremy AH, Holland DB, Roberts SG, et al. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003;121:20-27.
5. Kim J, Ochoa MT, Krutzik SR, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169:1535-1541.
6. Kistowska M, Gehrke S, Jankovic D, et al. IL-1beta drives inflammatory responses to Propionibacterium acnes in vitro and in vivo. J Invest Dermatol. 2014;134:677-685.
7. Contassot E, French LE. New insights into acne pathogenesis: Propionibacterium acnes activates the inflammasome. J Invest Dermatol. 2014;134:310-313.
8. Vowels BR, Yang S, Leyden JJ. Induction of proinflammatory cytokines by a soluble factor of Propionibacterium acnes: implications for chronic inflammatory acne. Infect Immun. 1995;63:3158-3165.
9. Qin M, Pirouz A, Kim MH, et al. Propionibacterium acnes induces IL-1beta secretion via the NLRP3 inflammasome in human monocytes. J Invest Dermatol. 2014;134:381-388.
10. Li ZJ, Choi DK, Sohn KC, et al. Propionibacterium acnes activates the NLRP3 inflammasome in human sebocytes. J Invest Dermatol. 2014;134:2747-2756.
11. Selway JL, Kurczab T, Kealey T, et al. Toll-like receptor 2 activation and comedogenesis: implications for the pathogenesis of acne. BMC Dermatol. 2013;13:10.
12. Jugeau S, Tenaud I, Knol AC, et al. Induction of toll-like receptors by Propionibacterium acnes. Br J Dermatol. 2005;153:1105-1113.
13. XOMA announces encouraging interim results from gevokizumab phase 2 study for moderate to severe acne vulgaris [press release]. Berkley, CA: XOMA Corporation; January 7, 2013. http://www.servier.com/content/xoma-announces-encouraging-interim-results-gevokizumab-phase-2-study-moderate-severe-acne. Accessed November 5, 2014.
14. Leemans JC, Cassel SL, Sutterwala FS. Sensing damage by the NLRP3 inflammasome. Immunol Rev. 2011;243:152-162.
15. Dispenza MC, Wolpert EB, Gilliland KL, et al. Systemic isotretinoin therapy normalizes exaggerated TLR-2-mediated innate immune responses in acne patients. J Invest Dermatol. 2012;132:2198-2205.
16. Zuliani T, Khammari A, Chaussy H, et al. Ex vivo demonstration of a synergistic effect of adapalene and benzoyl peroxide on inflammatory acne lesions. Exp Dermatol. 2011;20:850-853.
1. Kurokawa I, Danby FW, Ju Q, et al. New developments in our understanding of acne pathogenesis and treatment. Exp Dermatol. 2009;18:821-832.
2. Cunliffe WJ, Holland DB, Clark SM, et al. Comedogenesis: some aetiological, clinical and therapeutic strategies. Dermatology. 2003;206:11-16.
3. Bowe W, Kober M. Therapeutic update: acne. J Drugs Dermatol. 2014;13:235-238.
4. Jeremy AH, Holland DB, Roberts SG, et al. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003;121:20-27.
5. Kim J, Ochoa MT, Krutzik SR, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169:1535-1541.
6. Kistowska M, Gehrke S, Jankovic D, et al. IL-1beta drives inflammatory responses to Propionibacterium acnes in vitro and in vivo. J Invest Dermatol. 2014;134:677-685.
7. Contassot E, French LE. New insights into acne pathogenesis: Propionibacterium acnes activates the inflammasome. J Invest Dermatol. 2014;134:310-313.
8. Vowels BR, Yang S, Leyden JJ. Induction of proinflammatory cytokines by a soluble factor of Propionibacterium acnes: implications for chronic inflammatory acne. Infect Immun. 1995;63:3158-3165.
9. Qin M, Pirouz A, Kim MH, et al. Propionibacterium acnes induces IL-1beta secretion via the NLRP3 inflammasome in human monocytes. J Invest Dermatol. 2014;134:381-388.
10. Li ZJ, Choi DK, Sohn KC, et al. Propionibacterium acnes activates the NLRP3 inflammasome in human sebocytes. J Invest Dermatol. 2014;134:2747-2756.
11. Selway JL, Kurczab T, Kealey T, et al. Toll-like receptor 2 activation and comedogenesis: implications for the pathogenesis of acne. BMC Dermatol. 2013;13:10.
12. Jugeau S, Tenaud I, Knol AC, et al. Induction of toll-like receptors by Propionibacterium acnes. Br J Dermatol. 2005;153:1105-1113.
13. XOMA announces encouraging interim results from gevokizumab phase 2 study for moderate to severe acne vulgaris [press release]. Berkley, CA: XOMA Corporation; January 7, 2013. http://www.servier.com/content/xoma-announces-encouraging-interim-results-gevokizumab-phase-2-study-moderate-severe-acne. Accessed November 5, 2014.
14. Leemans JC, Cassel SL, Sutterwala FS. Sensing damage by the NLRP3 inflammasome. Immunol Rev. 2011;243:152-162.
15. Dispenza MC, Wolpert EB, Gilliland KL, et al. Systemic isotretinoin therapy normalizes exaggerated TLR-2-mediated innate immune responses in acne patients. J Invest Dermatol. 2012;132:2198-2205.
16. Zuliani T, Khammari A, Chaussy H, et al. Ex vivo demonstration of a synergistic effect of adapalene and benzoyl peroxide on inflammatory acne lesions. Exp Dermatol. 2011;20:850-853.
The Cutting Edge: New Research and Developments From the AAD Annual Meeting
As one of the most common skin diseases, acne research, clinical guidelines, and therapeutic innovation are always a hot topic at the Annual Meeting of the American Academy of Dermatology (March 21-25, 2014). A new dimension in the chicken and egg, or rather microcomedone and inflammation, story of acne pathogenesis emerged in a recent Journal of Investigative Dermatology (2014;134:381-388) article, which demonstrated that Propionibacterium acnes triggers a key inflammatory mediator, IL-1β, via the inflammasome (a compilation of inflammatory proteins such as caspases and NOD-like receptors) activation, suggesting a role for inflammasome-mediated inflammation in acne pathogenesis in addition to and independent of toll-like receptor activation. A potential therapeutic target, perhaps?
Drs. Ted Rosen and Joshua Zeichner in 2 independent sessions (Treating Tumors and Inflammatory Skin Diseases With Immunomodulators and Biologics [Rosen] and Acne Treatment Controversies [Zeichner]) discussed the importance of purposeful utilization of oral antibiotics—less is more—to prevent the continued emergence of antimicrobial resistance. Dr. Rosen commented that doxycycline at doses ≥50 mg daily provides serum levels that have an impact on commensal or colonized organisms, while lower doses provide only the anti-inflammatory effects without any bacteriostatic impact. This finding highlights the importance of low-dose controlled-release formulations. Dr. Zeichner also stressed the importance of knowing when to quit; if a patient does not improve in 6 to 8 weeks of therapy, move on. He also commented on the importance of multimechanistic therapy (solo is a no-go), utilizing benzoyl peroxide–containing products and most importantly retinoids from day 1. Dr. Zeichner also stressed the importance of recognizing acne mimics, such as gram-negative folliculitis, and made it clear that hormonally driven acne must not be missed, especially in the adult female population.
Lastly, new directions in acne are emerging, utilizing the science of nanotechnology (nano is equivalent to 1 billionth of a part). Drug delivery with nanomaterials is being fervently pursued across the globe in the field of acne. Nanoparticles can allow for sustained and controlled release of established products, increasing efficacy and stability, compliance due to decreased dosing, and safety by limiting associated irritation and dryness. In a session on nanotechnology, Dr. Rox Anderson presented his work utilizing gold nanoparticles to selectively destroy sebaceous glands via selective photothermolysis. He commented, “Do we really need our sebaceous glands,” citing that babies and infants do just fine without their activity. This work is currently in clinical trials in Europe. Nanotechnology also can be used to deliver previously undeliverable actives, such as the gaseous molecule nitric oxide. It was shown that a nitric oxide–releasing nanoparticle technology effectively penetrated the pilosebaceous unit, killed P acnes in culture, and inhibited inflammatory cytokine production by keratinocytes exposed to P acnes.
Stay tuned for more innovation coming soon!
As one of the most common skin diseases, acne research, clinical guidelines, and therapeutic innovation are always a hot topic at the Annual Meeting of the American Academy of Dermatology (March 21-25, 2014). A new dimension in the chicken and egg, or rather microcomedone and inflammation, story of acne pathogenesis emerged in a recent Journal of Investigative Dermatology (2014;134:381-388) article, which demonstrated that Propionibacterium acnes triggers a key inflammatory mediator, IL-1β, via the inflammasome (a compilation of inflammatory proteins such as caspases and NOD-like receptors) activation, suggesting a role for inflammasome-mediated inflammation in acne pathogenesis in addition to and independent of toll-like receptor activation. A potential therapeutic target, perhaps?
Drs. Ted Rosen and Joshua Zeichner in 2 independent sessions (Treating Tumors and Inflammatory Skin Diseases With Immunomodulators and Biologics [Rosen] and Acne Treatment Controversies [Zeichner]) discussed the importance of purposeful utilization of oral antibiotics—less is more—to prevent the continued emergence of antimicrobial resistance. Dr. Rosen commented that doxycycline at doses ≥50 mg daily provides serum levels that have an impact on commensal or colonized organisms, while lower doses provide only the anti-inflammatory effects without any bacteriostatic impact. This finding highlights the importance of low-dose controlled-release formulations. Dr. Zeichner also stressed the importance of knowing when to quit; if a patient does not improve in 6 to 8 weeks of therapy, move on. He also commented on the importance of multimechanistic therapy (solo is a no-go), utilizing benzoyl peroxide–containing products and most importantly retinoids from day 1. Dr. Zeichner also stressed the importance of recognizing acne mimics, such as gram-negative folliculitis, and made it clear that hormonally driven acne must not be missed, especially in the adult female population.
Lastly, new directions in acne are emerging, utilizing the science of nanotechnology (nano is equivalent to 1 billionth of a part). Drug delivery with nanomaterials is being fervently pursued across the globe in the field of acne. Nanoparticles can allow for sustained and controlled release of established products, increasing efficacy and stability, compliance due to decreased dosing, and safety by limiting associated irritation and dryness. In a session on nanotechnology, Dr. Rox Anderson presented his work utilizing gold nanoparticles to selectively destroy sebaceous glands via selective photothermolysis. He commented, “Do we really need our sebaceous glands,” citing that babies and infants do just fine without their activity. This work is currently in clinical trials in Europe. Nanotechnology also can be used to deliver previously undeliverable actives, such as the gaseous molecule nitric oxide. It was shown that a nitric oxide–releasing nanoparticle technology effectively penetrated the pilosebaceous unit, killed P acnes in culture, and inhibited inflammatory cytokine production by keratinocytes exposed to P acnes.
Stay tuned for more innovation coming soon!
As one of the most common skin diseases, acne research, clinical guidelines, and therapeutic innovation are always a hot topic at the Annual Meeting of the American Academy of Dermatology (March 21-25, 2014). A new dimension in the chicken and egg, or rather microcomedone and inflammation, story of acne pathogenesis emerged in a recent Journal of Investigative Dermatology (2014;134:381-388) article, which demonstrated that Propionibacterium acnes triggers a key inflammatory mediator, IL-1β, via the inflammasome (a compilation of inflammatory proteins such as caspases and NOD-like receptors) activation, suggesting a role for inflammasome-mediated inflammation in acne pathogenesis in addition to and independent of toll-like receptor activation. A potential therapeutic target, perhaps?
Drs. Ted Rosen and Joshua Zeichner in 2 independent sessions (Treating Tumors and Inflammatory Skin Diseases With Immunomodulators and Biologics [Rosen] and Acne Treatment Controversies [Zeichner]) discussed the importance of purposeful utilization of oral antibiotics—less is more—to prevent the continued emergence of antimicrobial resistance. Dr. Rosen commented that doxycycline at doses ≥50 mg daily provides serum levels that have an impact on commensal or colonized organisms, while lower doses provide only the anti-inflammatory effects without any bacteriostatic impact. This finding highlights the importance of low-dose controlled-release formulations. Dr. Zeichner also stressed the importance of knowing when to quit; if a patient does not improve in 6 to 8 weeks of therapy, move on. He also commented on the importance of multimechanistic therapy (solo is a no-go), utilizing benzoyl peroxide–containing products and most importantly retinoids from day 1. Dr. Zeichner also stressed the importance of recognizing acne mimics, such as gram-negative folliculitis, and made it clear that hormonally driven acne must not be missed, especially in the adult female population.
Lastly, new directions in acne are emerging, utilizing the science of nanotechnology (nano is equivalent to 1 billionth of a part). Drug delivery with nanomaterials is being fervently pursued across the globe in the field of acne. Nanoparticles can allow for sustained and controlled release of established products, increasing efficacy and stability, compliance due to decreased dosing, and safety by limiting associated irritation and dryness. In a session on nanotechnology, Dr. Rox Anderson presented his work utilizing gold nanoparticles to selectively destroy sebaceous glands via selective photothermolysis. He commented, “Do we really need our sebaceous glands,” citing that babies and infants do just fine without their activity. This work is currently in clinical trials in Europe. Nanotechnology also can be used to deliver previously undeliverable actives, such as the gaseous molecule nitric oxide. It was shown that a nitric oxide–releasing nanoparticle technology effectively penetrated the pilosebaceous unit, killed P acnes in culture, and inhibited inflammatory cytokine production by keratinocytes exposed to P acnes.
Stay tuned for more innovation coming soon!