User login
Psoriasis-Associated Fatigue: Pathogenesis, Metrics, and Treatment
Fatigue is defined as “an overwhelming, sustained sense of exhaustion and decreased capacity for physical and mental work,”1 and it is experienced by most patients with chronic disease. There are 2 types of fatigue: acute and chronic.2 Acute fatigue typically is caused by an identified insult (ie, injury), is self-limited, and is relieved by rest. Chronic fatigue, which may have multiple unknown causes, may accompany chronic illness and lasts longer than 6 months.2 In chronic disease, fatigue can originate peripherally (neu romuscular dysfunction outside of the central nervous system) or centrally (neurotransmitter activity within the central nervous system). Generally, central fatigue is more relevant in patients with chronic disease; however, both central and peripheral fatigue frequently coexist.
Fatigue, even with its accepted definition, is a nonspecific symptom, making it difficult to measure. Because of its subjective nature and the lack of effective therapies, clinicians often ignore fatigue. Still, patients with chronic disease continue to cite fatigue as one of the most challenging aspects of their disease that frequently decreases their quality of life (QOL).2
Fatigue has been well recognized in a number of chronic inflammatory diseases such as rheumatoid arthritis,3,4 systemic lupus erythematosus,5 fibromyalgia,6 and primary Sjögren syndrome.7 Similarly, fatigue is a frequent concern among patients with psoriasis and psoriatic arthritis.8 Given the prevalence and significance of psoriasis-associated fatigue,9 new efforts are needed to understand its pathophysiology, to develop new metrics for its evaluation, and to investigate therapeutic strategies to target it clinically. The following discussion provides an overview of the association between fatigue and psoriatic disease as well as the commonly used metrics for evaluating fatigue. Possible therapeutic agents with which to manage fatigue in this patient population also are provided.
Pathogenesis of Psoriasis-Associated Fatigue
Immunologic/Molecular Basis for Psoriasis-Associated Fatigue
Several theories aim to explain the pathophysiology of fatigue in patients with psoriatic disease. Psoriasis is a chronic inflammatory disease characterized by sharply demarcated erythematous plaques with adherent scale (Figure 1). Many in vitro studies have demonstrated the complex cytokine network that underlies the histopathologic alterations we observe in psoriatic lesions.10,11 Until recently, psoriasis was considered a type I autoimmune disease with strong TH1 signaling, influenced by IFN-γ, IL-2, and IL-12.12 TH1-producing proinflammatory cytokines, tumor necrosis factor α (TNF-α), and IFN-γ are elevated in psoriatic lesions.13 Studies on the efficacy of ustekinumab, a monoclonal antibody targeting IL-12 and IL-23, demonstrate the integral role of the immune system in psoriasis pathogenesis as the production of IL-12 polarizes T cells into TH1 cells.14,15 However, in recent years, TH17 cells have been linked to autoimmune inflammation16 and have been localized to the dermis in psoriatic lesions.17


Among a milieu of inflammatory cytokines, IL-1 is crucial for the early differentiation of TH17 cells.18 The IL-1 family of cytokines serve as primary mediators of inflammation with members including the IL-1 agonists (IL-1α, IL-1β),19 IL-1 receptor antagonist (IL-1RA),20 and IL-1 receptor type II (IL-1RII).20 The latter two inhibit IL-1 agonists from binding to their receptor (IL-1RI).19,20 A study by Yoshinaga et al21 investigated the level of inflammatory cytokines within lesional and nonlesional psoriatic skin, finding elevated levels of IL-1β in lesional skin. Another study found that IL-1β expression was increased 357% within biopsied psoriasiform lesions from flaky-skin mice, a useful model to examine the hyperproliferative alterations in the skin. This same study revealed that in vivo IL-1β neutralization alleviated the psoriasiform features in these same mice, suggesting IL-1β is integral to psoriasis pathogenesis.22
Evidence indicates that the aforementioned inflammatory mediators may contribute to psoriasis-associated fatigue. When the peripheral immune system is continuously activated, such as in psoriasis, the peripherally produced proinflammatory cytokines and subsequent immune signaling are monitored by the brain via afferent nerves, cytokine transporters at the blood-brain barrier, and IL-1 receptors on macrophages and endothelial cells of brain venules.23 For example, subseptic doses of lipopolysaccharide injected into rats induced messenger RNA expression of IL-1β in the choroid plexus, circumventricular organs, and the meninges,24 sites where cytokines can enter the blood-brain barrier via diffusion or cytokine transporters.23 These results may suggest a pathway that relays the peripheral immune signals that underlie psoriatic disease to the brain, resulting in activation of brain circuitry that mediates various negative behavioral responses, including fatigue.23 Following a central IL-1β infusion in mice, investigators found a significant decrease in the running performance (P<.01)25; the same infusion increased lethargy, malaise, and fatigue in rats.26 Interestingly, administration of IL-1RA significantly increased run time to fatigue (P<.05), supporting the hypothesis that IL-1β plays an important role in fatigue.25 Other investigators found that administration of many cytokines (IL-1β, IL-6, TNF-α) into rats induced depressivelike behaviors27 and suppressed locomotor activity.28 Lastly, another investigation found that IL-1RI knockout mice were resistant to symptoms of sickness, such as social exploration, anorexia, immobility, and weight loss, following IL-1β injections.29 Although the translatability of these studies to humans is not entirely clear, one study found that the proinflammatory cytokines IL-1 and TNF-α were elevated in patients with chronic fatigue syndrome.30 Furthermore, a 2013 systematic review found that several serum inflammatory markers including IL-6 and TNF-α were elevated in patients with severe plaque psoriasis compared to healthy controls.31 Therefore, these shared inflammatory cytokines may contribute to and explain the pathogenesis of both fatigue and psoriasis.
Confounding Factors
Although fatigue may be partially explained by the joint effect of inflammatory mediators on both the skin and the brain, there is evidence to suggest that other confounding factors may modify this association and affect its clinical presentation. The pathophysiology of fatigue in psoriasis may not be strictly immunologic; the environmental, psychological, and physical effects of psoriasis may all contribute to and perpetuate fatigue.9,32,33 Interestingly, the pathophysiology of psoriasis involves many cytokines also known to contribute to features of the metabolic syndrome.34 For example, elevated levels of free fatty acids, TNF-α, and IL-6 act in concert to promote inflammation, alter glucose metabolism, and dysregulate endothelial cell function, contributing to dyslipidemia, insulin resistance, and cardiovascular disease.35 A systematic review found a high prevalence of metabolic syndrome in patients with psoriasis and have found that those with more severe disease have an even greater risk for developing metabolic syndrome.34
Numerous studies have documented that upward of 80% of patients consider psoriasis to have a major impact on their QOL.36-38 The National Psoriasis Foundation assessed patients’ perspectives on the social, physical, and psychological aspects of their disease, finding that health-related QOL is impaired in patients with psoriatic disease.36,39 Patients reported their disease interfered with overall emotional well-being and life enjoyment and cited feelings of anger, frustration, helplessness, embarrassment, and self-consciousness, all of which can influence fatigue.36,39 Pain and pruritus (Figure 2) can interrupt sleep and thus may also contribute to symptoms of fatigue.40 Patients with psoriatic disease have a higher incidence of both depression and anxiety compared with the general population. Another study found that patient-reported factors of disability, pain, and fatigue were associated with clinical depression and anxiety; however, these factors are commonly observed in this cohort of patients and thus it is unclear whether they are predictors of or the result of depression.38
Furthermore, psoriatic disease leads to considerable economic burdens; one study (N=5604) found that among respondents who were not employed, 92% reported they were unemployed solely due to their psoriatic disease.36 One study explored the relationship between fatigue, work disability, and psoriatic arthritis, finding that the association between fatigue and work productivity loss persisted after controlling for cutaneous/musculoskeletal activity.41 However, another investigation revealed contradicting results, finding that improvements in fatigue correlated with improvements in joint and skin pain.9
Therefore, we can conclude that the pathogenesis of psoriasis-associated fatigue is the result of a multifactorial immunologic, psychologic, and physiologic pathway that triggers symptoms of exhaustion and lethargy. Fatigue is a complex multidimensional symptom activated by psoriatic disease, directly by shared inflammatory cytokines and indirectly by factors of disease activity and psychiatric distress that inherently influence somatic manifestations of fatigue. Regardless of its pathogenesis, these data and observations highlight the importance of fatigue symptoms and the need for new therapeutics to target this debilitating disease.
Measurement of Fatigue in Psoriasis
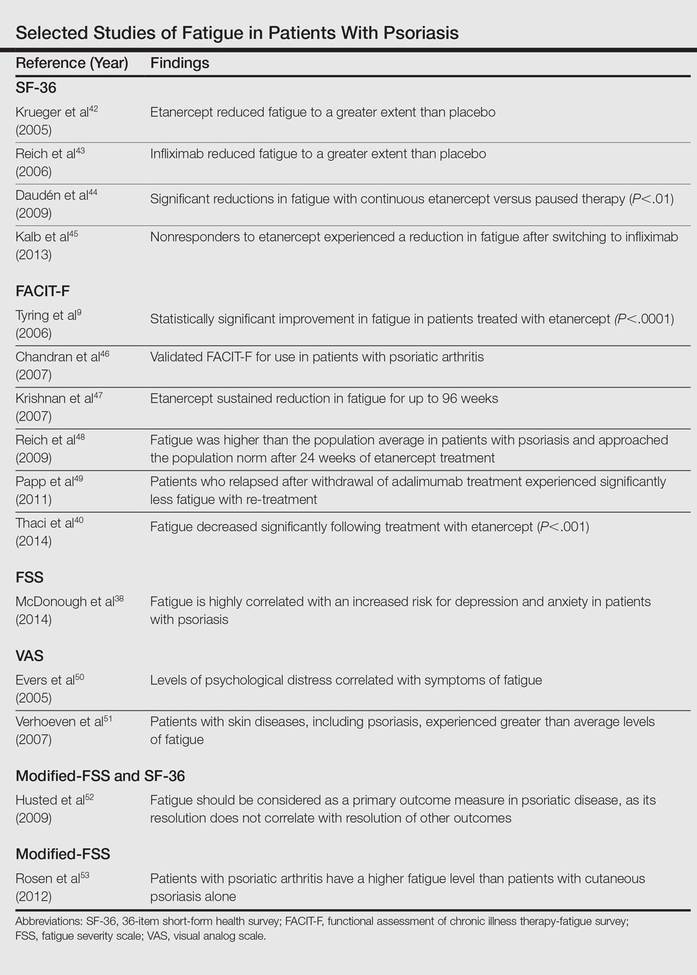
A patient’s level of fatigue is not objectively quantifiable. For this reason, clinicians and investigators have relied on self-report instruments to gauge fatigue (Table).9,38,40,42-53 These survey instruments each have distinct advantages and disadvantages, though all are subject to common difficulties. Many rely on the literacy of patients and their interpretation of each item, which can make completing the survey difficult and yield variability between subjects. Patients are inaccurate in self-reporting even measurable characteristics such as height and weight,54 which introduces an element of uncertainty in the reporting of subjective symptoms (ie, fatigue). Lastly, there are several biases implicit in self-reporting including recall bias, selective recall, and digit preference.55
When analyzing fatigue due to a chronic disease, several symptoms may be misconstrued or interfere with the interpretation of fatigue. For instance, patients with multiple sclerosis may confuse neuropathy-associated muscle weakness with fatigue. These interactions can be controlled for in self-report instruments and validated through careful study of many patients. Disease-specific questionnaires have been validated for use in several diseases,56-58 though none have been validated for cutaneous psoriasis in the absence of psoriatic arthritis. The need for validated instruments in psoriasis is great, as symptoms such as sleep disturbance and arthralgia may confound metrics of fatigue.
Thus far, 4 self-report instruments have been used to study fatigue in psoriasis: the medical outcomes 36-item short-form health survey (SF-36), the functional assessment of chronic illness therapy-fatigue, the fatigue severity scale (FSS), and the visual analog scale (VAS) for fatigue.
The SF-36 is a 36-item survey designed to measure 8 dimensions of health status in patients with chronic disease.59 Items are answered using a 3- to 6-point Likert scale, or in a yes/no format. Although the SF-36 is typically administered by a trained interviewer, it relies on a patient’s interpretation of language that must be used to describe their level of fatigue, which may not capture the full range of symptoms. Also, the length of the survey makes it impractical for use in clinical practice.
The functional assessment of chronic illness therapy-fatigue survey is validated for use in psoriatic arthritis. It is similar to the SF-36 in its use of a 5-point Likert scale to answer each of 13 items. It improves on the SF-36 model by including questions about associated symptoms (ie, pain, medication side effects) that may interfere with the measurement of fatigue. It also investigates the impact of fatigue on several areas of functioning. However, it is subject to the same pitfalls of interpretation and a rigid scale with which to answer questions.
The FSS is another Likert scale–based instrument that gauges both level of fatigue and its impact using 9 items and a 7-point scale. Investigators used the FSS to uncover an association between increasing fatigue scores and depression in patients with psoriatic disease.38
The VAS overcomes many of the language and interpretation issues inherent in Likert scale–based instruments. Patients are presented with a single item in which they are asked to plot their level of fatigue on a continuous line, with one end representing no fatigue and the other end the worst possible fatigue. Although VAS adds simplicity of response and removes some ambiguity from surveying, it provides no information about the functional impact of fatigue on patients. It also does not provide a method to control for other symptoms.
Treatment of Psoriasis-Associated Fatigue
Much of our understanding of psoriasis-associated fatigue arises from therapeutic clinical trials. Because increased concentrations of proinflammatory cytokines are associated with fatigue, it has been suggested that blocking these cytokines with biologic agents may relieve fatigue symptoms. For example, investigators found that patients treated with etanercept, a soluble TNF-α receptor fusion protein, had clinically meaningful improvement in fatigue compared to those receiving placebo, with sustained improvements at 96 weeks.9,47 We must note, however, that the decrease in fatigue correlated with improvements in cutaneous/arthritic pain. Nevertheless, another study found that treatment with the same drug decreased fatigue in patients with psoriasis, even after controlling for improvements in the psoriasis area severity index score.40 Adalimumab is another monoclonal antibody for TNF-α that has caused a notable decline in fatigue symptoms.49
These data suggest that biologic agents are useful in the treatment of fatigue. Biologic agents are frequently administered to patients with moderate to severe psoriasis in whom more conservative treatments previously failed. However, cutaneous/arthritic disease severity is not always correlated with fatigue, so these data may urge clinicians to lower their threshold for treatment with biologics in patients with substantial fatigue symptoms. Although further investigations are necessary, we may even consider using a biologic therapy for severe fatigue in those without severe psoriatic disease.
Conclusion
Fatigue is a multidimensional symptom, impacted both directly and indirectly by psoriasis pathophysiology. The prevalence of fatigue within this patient population suggests that clinicians need to recognize the symptom as a core domain in psoriasis evaluation. Although a host of metrics have been used to quantify/qualify fatigue, there remains a need for a validated instrument for assessing fatigue in patients with psoriatic disease.
Biologic agents have proven useful in the treatment of psoriasis-associated fatigue. The central role of proinflammatory cytokines to both fatigue and psoriasis pathogenesis provide insight into potential treatment targets. Understanding the overlapping pathophysiology of psoriasis and fatigue provides an avenue for developing innovative strategies to target molecules implicated in the activation of the immune system. In the future, it may be possible to predict the severity of fatigue by measuring the levels of serum inflammatory cytokines; in fact, a new study aims to identify a panel of soluble biomarkers that can predict joint damage in psoriatic arthritis.60 Taken together, the findings described suggest that further study is needed to characterize, measure, and treat psoriasis-associated fatigue.
- NANDA Nursing Diagnoses: Definitions and Classification, 1999-2000. Philadelphia, PA: NANDA International; 1999.
- Swain MG. Fatigue in chronic disease. Clin Sci (Lond). 2000;99:1-8.
- Wolfe F, Hawley DJ, Wilson K. The prevalence and meaning of fatigue in rheumatic disease. J Rheumatol. 1996;23:1407-1417.
- van Hoogmoed D, Fransen J, Bleijenberg G, et al. Physical and psychosocial correlates of severe fatigue in rheumatoid arthritis. Rheumatology (Oxford). 2010;49:1294-1302.
- Cleanthous S, Tyagi M, Isenberg DA, et al. What do we know about self-reported fatigue in systemic lupus erythematosus? Lupus. 2012;21:465-476.
- Ulus Y, Akyol Y, Tander B, et al. Sleep quality in fibromyalgia and rheumatoid arthritis: associations with pain, fatigue, depression, and disease activity. Clin Exp Rheumatol. 2011;29(6, suppl 69):S92-S96.
- Segal B, Thomas W, Rogers T, et al. Prevalence, severity, and predictors of fatigue in subjects with primary Sjögren’s syndrome. Arthritis Rheum. 2008;59:1780-1787.
- Gladman DD, Mease PJ, Strand V, et al. Consensus on a core set of domains for psoriatic arthritis. J Rheumatol. 2007;34:1167-1170.
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
- De Rosa G, Mignogna C. The histopathology of psoriasis. Reumatismo. 2007;59(suppl 1):46-48.
- Nickoloff BJ, Xin H, Nestle FO, et al. The cytokine and chemokine network in psoriasis. Clin Dermatol. 2007;25:568-573.
- Zaba LC, Fuentes-Duculan J, Eungdamrong NJ, et al. Psoriasis is characterized by accumulation of immunostimulatory and Th1/Th17 cell-polarizing myeloid dendritic cells. J Invest Dermatol. 2009;129:79-88.
- Austin LM, Ozawa M, Kikuchi T, et al. The majority of epidermal T cells in psoriasis vulgaris lesions can produce type 1 cytokines, interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J Invest Dermatol. 1999;113:752-759.
- Lebwohl M, Papp K, Han C, et al. Ustekinumab improves health-related quality of life in patients with moderate-to-severe psoriasis: results from the PHOENIX 1 trial. Br J Dermatol. 2010;162:137-146.
- Sabat R, Wolk K. Pathogenesis of psoriasis. In: Sterry W, Sabat R, Philipp S, eds. Psoriasis: Diagnosis and Management. Chichester, UK: John Wiley & Sons, Ltd; 2014:28-48.
- Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345-350.
- Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207-1211.
- Chung Y, Chang SH, Martinez GJ, et al. Critical regulation of early Th17 cell differentiation by IL-1 signaling. Immunity. 2009;30:576-587.
- Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720-3732.
- Jensen LE. Targeting the IL-1 family members in skin inflammation. Curr Opin Investig Drugs. 2010;11:1211-1220.
- Yoshinaga Y, Higaki M, Terajima S, et al. Detection of inflammatory cytokines in psoriatic skin. Arch Dermatol Res. 1995;287:158-164.
- Schon M, Behmenburg C, Denzer D, et al. Pathogenic function of IL-1 beta in psoriasiform skin lesions of flaky skin (fsn/fsn) mice. Clin Exp Immunol. 2001;123:505-510.
- Dantzer R, O’Connor JC, Freund GG, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46-56.
- Quan N, Stern EL, Whiteside MB, et al. Induction of pro-inflammatory cytokine mRNAs in the brain after peripheral injection of subseptic doses of lipopolysaccharide in the rat. J Neuroimmunol. 1999;93:72-80.
- Carmichael MD, Davis JM, Murphy EA, et al. Role of brain IL-1beta on fatigue after exercise-induced muscle damage. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1344-R1348.
- Swain MG, Beck P, Rioux K, et al. Augmented interleukin-1beta-induced depression of locomotor activity in cholestatic rats. Hepatology. 1998;28:1561-1565.
- Kent S, Bluthé RM, Kelley KW, et al. Sickness behavior as a new target for drug development. Trends Pharmacol Sci. 1992;13:24-28.
- Lacosta S, Merali Z, Anisman H. Influence of interleukin-1beta on exploratory behaviors, plasma ACTH, corticosterone, and central biogenic amines in mice. Psychopharmacology. 1998;137:351-361.
- Bluthé RM, Laye S, Michaud B, et al. Role of interleukin-1beta and tumour necrosis factor-alpha in lipopolysaccharide-induced sickness behaviour: a study with interleukin-1 type I receptor-deficient mice. Eur J Neurosci. 2000;12:4447-4456.
- Maes M, Twisk FN, Ringel K. Inflammatory and cell-mediated immune biomarkers in myalgic encephalomyelitis/chronic fatigue syndrome and depression: inflammatory markers are higher in myalgic encephalomyelitis/chronic fatigue syndrome than in depression. Psychother Psychosom. 2012;81:286-295.
- Dowlatshahi EA, van der Voort EAM, Arends LR, et al. Markers of systemic inflammation in psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2013;169:266-282.
- Jankovic S, Raznatovic M, Marinkovic J, et al. Health-related quality of life in patients with psoriasis.J Cutan Med Surg. 2011;15:29-36.
- Carneiro C, Chaves M, Verardino G, et al. Fatigue in psoriasis with arthritis. Skinmed. 2011;9:34-37.
- Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and metabolic syndrome: a systematic review and meta-analysis of observational studies. J Am Acad Dermatol. 2013;68:654-662.
- Sterry W, Strober BE, Menter A. Obesity in psoriasis: the metabolic, clinical and therapeutic implications. report of an interdisciplinary conference and review. Br J Dermatol. 2007;157:649-655.
- Armstrong AW, Schupp C, Wu J, et al. Quality of life and work productivity impairment among psoriasis patients: findings from the National Psoriasis Foundation survey data 2003-2011. PloS One. 2012;7:e52935.
- de Korte J, Sprangers MA, Mombers FM, et al. Quality of life in patients with psoriasis: a systematic literature review. J Invest Dermatol. 2004;9:140-147.
- McDonough E, Ayearst R, Eder L, et al. Depression and anxiety in psoriatic disease: prevalence and associated factors. J Rheumatol. 2014;41:887-896.
- Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280-284.
- Thaci D, Galimberti R, Amaya-Guerra M, et al. Improvement in aspects of sleep with etanercept and optional adjunctive topical therapy in patients with moderate-to-severe psoriasis: results from the PRISTINE trial. J Eur Acad Dermatol Venereol. 2014;28:900-906.
- Walsh JA, McFadden ML, Morgan MD, et al. Work productivity loss and fatigue in psoriatic arthritis. J Rheumatol. 2014;41:1670-1674.
- Krueger GG, Langley RG, Finlay AY, et al. Patient-reported outcomes of psoriasis improvement with etanercept therapy: results of a randomized phase III trial. Br J Dermatol. 2005;153:1192-1199.
- Reich K, Nestle FO, Papp K, et al. Improvement in quality of life with infliximab induction and maintenance therapy in patients with moderate-to-severe psoriasis: a randomized controlled trial. Br J Dermatol. 2006;154:1161-1168.
- Daudén E, Griffiths CE, Ortonne JP, et al. Improvements in patient-reported outcomes in moderate-to-severe psoriasis patients receiving continuous or paused etanercept treatment over 54 weeks: the CRYSTEL study. J Eur Acad Dermatol Venereol. 2009;23:1374-1382.
- Kalb RE, Blauvelt A, Sofen HL, et al. Effect of infliximab on health-related quality of life and disease activity by body region in patients with moderate-to-severe psoriasis and inadequate response to etanercept: results from the PSUNRISE trial. J Drugs Dermatol. 2013;12:874-880.
- Chandran V, Bhella S, Schentag C, et al. Functional assessment of chronic illness therapy-fatigue scale is valid in patients with psoriatic arthritis. Ann Rheumatic Dis. 2007;66:936-939.
- Krishnan R, Cella D, Leonardi C, et al. Effects of etanercept therapy on fatigue and symptoms of depression in subjects treated for moderate to severe plaque psoriasis for up to 96 weeks. Br J Dermatol. 2007;157:1275-1277.
- Reich K, Segaert S, Van de Kerkhof P, et al. Once-weekly administration of etanercept 50 mgimproves patient-reported outcomes in patients with moderate-to-severe plaque psoriasis. Dermatology. 2009;219:239-249.
- Papp K, Crowley J, Ortonne JP, et al. Adalimumab for moderate to severe chronic plaque psoriasis: efficacy and safety of retreatment and disease recurrence following withdrawal from therapy. Br J Dermatol. 2011;164:434-441.
- Evers AW, Lu Y, Duller P, et al. Common burden of chronic skin diseases? contributors to psychological distress in adults with psoriasis and atopic dermatitis. Br J Dermatol. 2005;152:1275-1281.
- Verhoeven EW, Kraaimaat FW, van de Kerkhof PC, et al. Prevalence of physical symptoms of itch, pain and fatigue in patients with skin diseases in general practice. Br J Dermatol. 2007;156:1346-1349.
- Husted JA, Tom BD, Schentag CT, et al. Occurrence and correlates of fatigue in psoriatic arthritis. Ann Rheum Dis. 2009;68:1553-1558.
- Rosen CF, Mussani F, Chandran V, et al. Patients with psoriatic arthritis have worse quality of life than those with psoriasis alone. Rheumatology. 2012;51:571-576.
- Gorber SC, Tremblay M, Moher D, et al. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: a systematic review. Obes Rev. 2007;8:307-326.
- Fadnes LT, Taube A, Tylleskär T. How to identify information bias due to self-reporting in epidemiological research. Int J Epidemiol. 2009;7:3.
- Brown RG, Dittner A, Findley L, et al. The Parkinson fatigue scale. Parkinsonism Relat Disord. 2005;11:49-55.
- Fisk JD, Ritvo PG, Ross L, et al. Measuring the functional impact of fatigue: initial validation of the fatigue impact scale. Clin Infect Dis. 1994;18(suppl 1):S79-S83.
- Bowman SJ, Booth DA, Platts RG. Measurement of fatigue and discomfort in primary Sjögren’s syndrome using a new questionnaire tool. Rheumatology. 2004;43:758-764.
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. conceptual framework and item selection. Med Care. 1992;30:473-483.
- FitzGerald O, Mease PJ. Biomarkers: project update from the GRAPPA 2012 annual meeting. J Rheumatol. 2013;40:1453-1454.
Fatigue is defined as “an overwhelming, sustained sense of exhaustion and decreased capacity for physical and mental work,”1 and it is experienced by most patients with chronic disease. There are 2 types of fatigue: acute and chronic.2 Acute fatigue typically is caused by an identified insult (ie, injury), is self-limited, and is relieved by rest. Chronic fatigue, which may have multiple unknown causes, may accompany chronic illness and lasts longer than 6 months.2 In chronic disease, fatigue can originate peripherally (neu romuscular dysfunction outside of the central nervous system) or centrally (neurotransmitter activity within the central nervous system). Generally, central fatigue is more relevant in patients with chronic disease; however, both central and peripheral fatigue frequently coexist.
Fatigue, even with its accepted definition, is a nonspecific symptom, making it difficult to measure. Because of its subjective nature and the lack of effective therapies, clinicians often ignore fatigue. Still, patients with chronic disease continue to cite fatigue as one of the most challenging aspects of their disease that frequently decreases their quality of life (QOL).2
Fatigue has been well recognized in a number of chronic inflammatory diseases such as rheumatoid arthritis,3,4 systemic lupus erythematosus,5 fibromyalgia,6 and primary Sjögren syndrome.7 Similarly, fatigue is a frequent concern among patients with psoriasis and psoriatic arthritis.8 Given the prevalence and significance of psoriasis-associated fatigue,9 new efforts are needed to understand its pathophysiology, to develop new metrics for its evaluation, and to investigate therapeutic strategies to target it clinically. The following discussion provides an overview of the association between fatigue and psoriatic disease as well as the commonly used metrics for evaluating fatigue. Possible therapeutic agents with which to manage fatigue in this patient population also are provided.
Pathogenesis of Psoriasis-Associated Fatigue
Immunologic/Molecular Basis for Psoriasis-Associated Fatigue
Several theories aim to explain the pathophysiology of fatigue in patients with psoriatic disease. Psoriasis is a chronic inflammatory disease characterized by sharply demarcated erythematous plaques with adherent scale (Figure 1). Many in vitro studies have demonstrated the complex cytokine network that underlies the histopathologic alterations we observe in psoriatic lesions.10,11 Until recently, psoriasis was considered a type I autoimmune disease with strong TH1 signaling, influenced by IFN-γ, IL-2, and IL-12.12 TH1-producing proinflammatory cytokines, tumor necrosis factor α (TNF-α), and IFN-γ are elevated in psoriatic lesions.13 Studies on the efficacy of ustekinumab, a monoclonal antibody targeting IL-12 and IL-23, demonstrate the integral role of the immune system in psoriasis pathogenesis as the production of IL-12 polarizes T cells into TH1 cells.14,15 However, in recent years, TH17 cells have been linked to autoimmune inflammation16 and have been localized to the dermis in psoriatic lesions.17


Among a milieu of inflammatory cytokines, IL-1 is crucial for the early differentiation of TH17 cells.18 The IL-1 family of cytokines serve as primary mediators of inflammation with members including the IL-1 agonists (IL-1α, IL-1β),19 IL-1 receptor antagonist (IL-1RA),20 and IL-1 receptor type II (IL-1RII).20 The latter two inhibit IL-1 agonists from binding to their receptor (IL-1RI).19,20 A study by Yoshinaga et al21 investigated the level of inflammatory cytokines within lesional and nonlesional psoriatic skin, finding elevated levels of IL-1β in lesional skin. Another study found that IL-1β expression was increased 357% within biopsied psoriasiform lesions from flaky-skin mice, a useful model to examine the hyperproliferative alterations in the skin. This same study revealed that in vivo IL-1β neutralization alleviated the psoriasiform features in these same mice, suggesting IL-1β is integral to psoriasis pathogenesis.22
Evidence indicates that the aforementioned inflammatory mediators may contribute to psoriasis-associated fatigue. When the peripheral immune system is continuously activated, such as in psoriasis, the peripherally produced proinflammatory cytokines and subsequent immune signaling are monitored by the brain via afferent nerves, cytokine transporters at the blood-brain barrier, and IL-1 receptors on macrophages and endothelial cells of brain venules.23 For example, subseptic doses of lipopolysaccharide injected into rats induced messenger RNA expression of IL-1β in the choroid plexus, circumventricular organs, and the meninges,24 sites where cytokines can enter the blood-brain barrier via diffusion or cytokine transporters.23 These results may suggest a pathway that relays the peripheral immune signals that underlie psoriatic disease to the brain, resulting in activation of brain circuitry that mediates various negative behavioral responses, including fatigue.23 Following a central IL-1β infusion in mice, investigators found a significant decrease in the running performance (P<.01)25; the same infusion increased lethargy, malaise, and fatigue in rats.26 Interestingly, administration of IL-1RA significantly increased run time to fatigue (P<.05), supporting the hypothesis that IL-1β plays an important role in fatigue.25 Other investigators found that administration of many cytokines (IL-1β, IL-6, TNF-α) into rats induced depressivelike behaviors27 and suppressed locomotor activity.28 Lastly, another investigation found that IL-1RI knockout mice were resistant to symptoms of sickness, such as social exploration, anorexia, immobility, and weight loss, following IL-1β injections.29 Although the translatability of these studies to humans is not entirely clear, one study found that the proinflammatory cytokines IL-1 and TNF-α were elevated in patients with chronic fatigue syndrome.30 Furthermore, a 2013 systematic review found that several serum inflammatory markers including IL-6 and TNF-α were elevated in patients with severe plaque psoriasis compared to healthy controls.31 Therefore, these shared inflammatory cytokines may contribute to and explain the pathogenesis of both fatigue and psoriasis.
Confounding Factors
Although fatigue may be partially explained by the joint effect of inflammatory mediators on both the skin and the brain, there is evidence to suggest that other confounding factors may modify this association and affect its clinical presentation. The pathophysiology of fatigue in psoriasis may not be strictly immunologic; the environmental, psychological, and physical effects of psoriasis may all contribute to and perpetuate fatigue.9,32,33 Interestingly, the pathophysiology of psoriasis involves many cytokines also known to contribute to features of the metabolic syndrome.34 For example, elevated levels of free fatty acids, TNF-α, and IL-6 act in concert to promote inflammation, alter glucose metabolism, and dysregulate endothelial cell function, contributing to dyslipidemia, insulin resistance, and cardiovascular disease.35 A systematic review found a high prevalence of metabolic syndrome in patients with psoriasis and have found that those with more severe disease have an even greater risk for developing metabolic syndrome.34
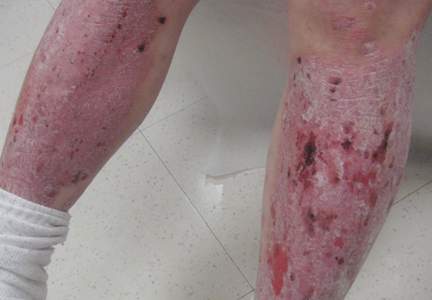
Numerous studies have documented that upward of 80% of patients consider psoriasis to have a major impact on their QOL.36-38 The National Psoriasis Foundation assessed patients’ perspectives on the social, physical, and psychological aspects of their disease, finding that health-related QOL is impaired in patients with psoriatic disease.36,39 Patients reported their disease interfered with overall emotional well-being and life enjoyment and cited feelings of anger, frustration, helplessness, embarrassment, and self-consciousness, all of which can influence fatigue.36,39 Pain and pruritus (Figure 2) can interrupt sleep and thus may also contribute to symptoms of fatigue.40 Patients with psoriatic disease have a higher incidence of both depression and anxiety compared with the general population. Another study found that patient-reported factors of disability, pain, and fatigue were associated with clinical depression and anxiety; however, these factors are commonly observed in this cohort of patients and thus it is unclear whether they are predictors of or the result of depression.38
Furthermore, psoriatic disease leads to considerable economic burdens; one study (N=5604) found that among respondents who were not employed, 92% reported they were unemployed solely due to their psoriatic disease.36 One study explored the relationship between fatigue, work disability, and psoriatic arthritis, finding that the association between fatigue and work productivity loss persisted after controlling for cutaneous/musculoskeletal activity.41 However, another investigation revealed contradicting results, finding that improvements in fatigue correlated with improvements in joint and skin pain.9
Therefore, we can conclude that the pathogenesis of psoriasis-associated fatigue is the result of a multifactorial immunologic, psychologic, and physiologic pathway that triggers symptoms of exhaustion and lethargy. Fatigue is a complex multidimensional symptom activated by psoriatic disease, directly by shared inflammatory cytokines and indirectly by factors of disease activity and psychiatric distress that inherently influence somatic manifestations of fatigue. Regardless of its pathogenesis, these data and observations highlight the importance of fatigue symptoms and the need for new therapeutics to target this debilitating disease.
Measurement of Fatigue in Psoriasis
A patient’s level of fatigue is not objectively quantifiable. For this reason, clinicians and investigators have relied on self-report instruments to gauge fatigue (Table).9,38,40,42-53 These survey instruments each have distinct advantages and disadvantages, though all are subject to common difficulties. Many rely on the literacy of patients and their interpretation of each item, which can make completing the survey difficult and yield variability between subjects. Patients are inaccurate in self-reporting even measurable characteristics such as height and weight,54 which introduces an element of uncertainty in the reporting of subjective symptoms (ie, fatigue). Lastly, there are several biases implicit in self-reporting including recall bias, selective recall, and digit preference.55
When analyzing fatigue due to a chronic disease, several symptoms may be misconstrued or interfere with the interpretation of fatigue. For instance, patients with multiple sclerosis may confuse neuropathy-associated muscle weakness with fatigue. These interactions can be controlled for in self-report instruments and validated through careful study of many patients. Disease-specific questionnaires have been validated for use in several diseases,56-58 though none have been validated for cutaneous psoriasis in the absence of psoriatic arthritis. The need for validated instruments in psoriasis is great, as symptoms such as sleep disturbance and arthralgia may confound metrics of fatigue.
Thus far, 4 self-report instruments have been used to study fatigue in psoriasis: the medical outcomes 36-item short-form health survey (SF-36), the functional assessment of chronic illness therapy-fatigue, the fatigue severity scale (FSS), and the visual analog scale (VAS) for fatigue.
The SF-36 is a 36-item survey designed to measure 8 dimensions of health status in patients with chronic disease.59 Items are answered using a 3- to 6-point Likert scale, or in a yes/no format. Although the SF-36 is typically administered by a trained interviewer, it relies on a patient’s interpretation of language that must be used to describe their level of fatigue, which may not capture the full range of symptoms. Also, the length of the survey makes it impractical for use in clinical practice.
The functional assessment of chronic illness therapy-fatigue survey is validated for use in psoriatic arthritis. It is similar to the SF-36 in its use of a 5-point Likert scale to answer each of 13 items. It improves on the SF-36 model by including questions about associated symptoms (ie, pain, medication side effects) that may interfere with the measurement of fatigue. It also investigates the impact of fatigue on several areas of functioning. However, it is subject to the same pitfalls of interpretation and a rigid scale with which to answer questions.
The FSS is another Likert scale–based instrument that gauges both level of fatigue and its impact using 9 items and a 7-point scale. Investigators used the FSS to uncover an association between increasing fatigue scores and depression in patients with psoriatic disease.38
The VAS overcomes many of the language and interpretation issues inherent in Likert scale–based instruments. Patients are presented with a single item in which they are asked to plot their level of fatigue on a continuous line, with one end representing no fatigue and the other end the worst possible fatigue. Although VAS adds simplicity of response and removes some ambiguity from surveying, it provides no information about the functional impact of fatigue on patients. It also does not provide a method to control for other symptoms.
Treatment of Psoriasis-Associated Fatigue
Much of our understanding of psoriasis-associated fatigue arises from therapeutic clinical trials. Because increased concentrations of proinflammatory cytokines are associated with fatigue, it has been suggested that blocking these cytokines with biologic agents may relieve fatigue symptoms. For example, investigators found that patients treated with etanercept, a soluble TNF-α receptor fusion protein, had clinically meaningful improvement in fatigue compared to those receiving placebo, with sustained improvements at 96 weeks.9,47 We must note, however, that the decrease in fatigue correlated with improvements in cutaneous/arthritic pain. Nevertheless, another study found that treatment with the same drug decreased fatigue in patients with psoriasis, even after controlling for improvements in the psoriasis area severity index score.40 Adalimumab is another monoclonal antibody for TNF-α that has caused a notable decline in fatigue symptoms.49
These data suggest that biologic agents are useful in the treatment of fatigue. Biologic agents are frequently administered to patients with moderate to severe psoriasis in whom more conservative treatments previously failed. However, cutaneous/arthritic disease severity is not always correlated with fatigue, so these data may urge clinicians to lower their threshold for treatment with biologics in patients with substantial fatigue symptoms. Although further investigations are necessary, we may even consider using a biologic therapy for severe fatigue in those without severe psoriatic disease.
Conclusion
Fatigue is a multidimensional symptom, impacted both directly and indirectly by psoriasis pathophysiology. The prevalence of fatigue within this patient population suggests that clinicians need to recognize the symptom as a core domain in psoriasis evaluation. Although a host of metrics have been used to quantify/qualify fatigue, there remains a need for a validated instrument for assessing fatigue in patients with psoriatic disease.
Biologic agents have proven useful in the treatment of psoriasis-associated fatigue. The central role of proinflammatory cytokines to both fatigue and psoriasis pathogenesis provide insight into potential treatment targets. Understanding the overlapping pathophysiology of psoriasis and fatigue provides an avenue for developing innovative strategies to target molecules implicated in the activation of the immune system. In the future, it may be possible to predict the severity of fatigue by measuring the levels of serum inflammatory cytokines; in fact, a new study aims to identify a panel of soluble biomarkers that can predict joint damage in psoriatic arthritis.60 Taken together, the findings described suggest that further study is needed to characterize, measure, and treat psoriasis-associated fatigue.
Fatigue is defined as “an overwhelming, sustained sense of exhaustion and decreased capacity for physical and mental work,”1 and it is experienced by most patients with chronic disease. There are 2 types of fatigue: acute and chronic.2 Acute fatigue typically is caused by an identified insult (ie, injury), is self-limited, and is relieved by rest. Chronic fatigue, which may have multiple unknown causes, may accompany chronic illness and lasts longer than 6 months.2 In chronic disease, fatigue can originate peripherally (neu romuscular dysfunction outside of the central nervous system) or centrally (neurotransmitter activity within the central nervous system). Generally, central fatigue is more relevant in patients with chronic disease; however, both central and peripheral fatigue frequently coexist.
Fatigue, even with its accepted definition, is a nonspecific symptom, making it difficult to measure. Because of its subjective nature and the lack of effective therapies, clinicians often ignore fatigue. Still, patients with chronic disease continue to cite fatigue as one of the most challenging aspects of their disease that frequently decreases their quality of life (QOL).2
Fatigue has been well recognized in a number of chronic inflammatory diseases such as rheumatoid arthritis,3,4 systemic lupus erythematosus,5 fibromyalgia,6 and primary Sjögren syndrome.7 Similarly, fatigue is a frequent concern among patients with psoriasis and psoriatic arthritis.8 Given the prevalence and significance of psoriasis-associated fatigue,9 new efforts are needed to understand its pathophysiology, to develop new metrics for its evaluation, and to investigate therapeutic strategies to target it clinically. The following discussion provides an overview of the association between fatigue and psoriatic disease as well as the commonly used metrics for evaluating fatigue. Possible therapeutic agents with which to manage fatigue in this patient population also are provided.
Pathogenesis of Psoriasis-Associated Fatigue
Immunologic/Molecular Basis for Psoriasis-Associated Fatigue
Several theories aim to explain the pathophysiology of fatigue in patients with psoriatic disease. Psoriasis is a chronic inflammatory disease characterized by sharply demarcated erythematous plaques with adherent scale (Figure 1). Many in vitro studies have demonstrated the complex cytokine network that underlies the histopathologic alterations we observe in psoriatic lesions.10,11 Until recently, psoriasis was considered a type I autoimmune disease with strong TH1 signaling, influenced by IFN-γ, IL-2, and IL-12.12 TH1-producing proinflammatory cytokines, tumor necrosis factor α (TNF-α), and IFN-γ are elevated in psoriatic lesions.13 Studies on the efficacy of ustekinumab, a monoclonal antibody targeting IL-12 and IL-23, demonstrate the integral role of the immune system in psoriasis pathogenesis as the production of IL-12 polarizes T cells into TH1 cells.14,15 However, in recent years, TH17 cells have been linked to autoimmune inflammation16 and have been localized to the dermis in psoriatic lesions.17


Among a milieu of inflammatory cytokines, IL-1 is crucial for the early differentiation of TH17 cells.18 The IL-1 family of cytokines serve as primary mediators of inflammation with members including the IL-1 agonists (IL-1α, IL-1β),19 IL-1 receptor antagonist (IL-1RA),20 and IL-1 receptor type II (IL-1RII).20 The latter two inhibit IL-1 agonists from binding to their receptor (IL-1RI).19,20 A study by Yoshinaga et al21 investigated the level of inflammatory cytokines within lesional and nonlesional psoriatic skin, finding elevated levels of IL-1β in lesional skin. Another study found that IL-1β expression was increased 357% within biopsied psoriasiform lesions from flaky-skin mice, a useful model to examine the hyperproliferative alterations in the skin. This same study revealed that in vivo IL-1β neutralization alleviated the psoriasiform features in these same mice, suggesting IL-1β is integral to psoriasis pathogenesis.22
Evidence indicates that the aforementioned inflammatory mediators may contribute to psoriasis-associated fatigue. When the peripheral immune system is continuously activated, such as in psoriasis, the peripherally produced proinflammatory cytokines and subsequent immune signaling are monitored by the brain via afferent nerves, cytokine transporters at the blood-brain barrier, and IL-1 receptors on macrophages and endothelial cells of brain venules.23 For example, subseptic doses of lipopolysaccharide injected into rats induced messenger RNA expression of IL-1β in the choroid plexus, circumventricular organs, and the meninges,24 sites where cytokines can enter the blood-brain barrier via diffusion or cytokine transporters.23 These results may suggest a pathway that relays the peripheral immune signals that underlie psoriatic disease to the brain, resulting in activation of brain circuitry that mediates various negative behavioral responses, including fatigue.23 Following a central IL-1β infusion in mice, investigators found a significant decrease in the running performance (P<.01)25; the same infusion increased lethargy, malaise, and fatigue in rats.26 Interestingly, administration of IL-1RA significantly increased run time to fatigue (P<.05), supporting the hypothesis that IL-1β plays an important role in fatigue.25 Other investigators found that administration of many cytokines (IL-1β, IL-6, TNF-α) into rats induced depressivelike behaviors27 and suppressed locomotor activity.28 Lastly, another investigation found that IL-1RI knockout mice were resistant to symptoms of sickness, such as social exploration, anorexia, immobility, and weight loss, following IL-1β injections.29 Although the translatability of these studies to humans is not entirely clear, one study found that the proinflammatory cytokines IL-1 and TNF-α were elevated in patients with chronic fatigue syndrome.30 Furthermore, a 2013 systematic review found that several serum inflammatory markers including IL-6 and TNF-α were elevated in patients with severe plaque psoriasis compared to healthy controls.31 Therefore, these shared inflammatory cytokines may contribute to and explain the pathogenesis of both fatigue and psoriasis.
Confounding Factors
Although fatigue may be partially explained by the joint effect of inflammatory mediators on both the skin and the brain, there is evidence to suggest that other confounding factors may modify this association and affect its clinical presentation. The pathophysiology of fatigue in psoriasis may not be strictly immunologic; the environmental, psychological, and physical effects of psoriasis may all contribute to and perpetuate fatigue.9,32,33 Interestingly, the pathophysiology of psoriasis involves many cytokines also known to contribute to features of the metabolic syndrome.34 For example, elevated levels of free fatty acids, TNF-α, and IL-6 act in concert to promote inflammation, alter glucose metabolism, and dysregulate endothelial cell function, contributing to dyslipidemia, insulin resistance, and cardiovascular disease.35 A systematic review found a high prevalence of metabolic syndrome in patients with psoriasis and have found that those with more severe disease have an even greater risk for developing metabolic syndrome.34
Numerous studies have documented that upward of 80% of patients consider psoriasis to have a major impact on their QOL.36-38 The National Psoriasis Foundation assessed patients’ perspectives on the social, physical, and psychological aspects of their disease, finding that health-related QOL is impaired in patients with psoriatic disease.36,39 Patients reported their disease interfered with overall emotional well-being and life enjoyment and cited feelings of anger, frustration, helplessness, embarrassment, and self-consciousness, all of which can influence fatigue.36,39 Pain and pruritus (Figure 2) can interrupt sleep and thus may also contribute to symptoms of fatigue.40 Patients with psoriatic disease have a higher incidence of both depression and anxiety compared with the general population. Another study found that patient-reported factors of disability, pain, and fatigue were associated with clinical depression and anxiety; however, these factors are commonly observed in this cohort of patients and thus it is unclear whether they are predictors of or the result of depression.38
Furthermore, psoriatic disease leads to considerable economic burdens; one study (N=5604) found that among respondents who were not employed, 92% reported they were unemployed solely due to their psoriatic disease.36 One study explored the relationship between fatigue, work disability, and psoriatic arthritis, finding that the association between fatigue and work productivity loss persisted after controlling for cutaneous/musculoskeletal activity.41 However, another investigation revealed contradicting results, finding that improvements in fatigue correlated with improvements in joint and skin pain.9
Therefore, we can conclude that the pathogenesis of psoriasis-associated fatigue is the result of a multifactorial immunologic, psychologic, and physiologic pathway that triggers symptoms of exhaustion and lethargy. Fatigue is a complex multidimensional symptom activated by psoriatic disease, directly by shared inflammatory cytokines and indirectly by factors of disease activity and psychiatric distress that inherently influence somatic manifestations of fatigue. Regardless of its pathogenesis, these data and observations highlight the importance of fatigue symptoms and the need for new therapeutics to target this debilitating disease.
Measurement of Fatigue in Psoriasis
A patient’s level of fatigue is not objectively quantifiable. For this reason, clinicians and investigators have relied on self-report instruments to gauge fatigue (Table).9,38,40,42-53 These survey instruments each have distinct advantages and disadvantages, though all are subject to common difficulties. Many rely on the literacy of patients and their interpretation of each item, which can make completing the survey difficult and yield variability between subjects. Patients are inaccurate in self-reporting even measurable characteristics such as height and weight,54 which introduces an element of uncertainty in the reporting of subjective symptoms (ie, fatigue). Lastly, there are several biases implicit in self-reporting including recall bias, selective recall, and digit preference.55
When analyzing fatigue due to a chronic disease, several symptoms may be misconstrued or interfere with the interpretation of fatigue. For instance, patients with multiple sclerosis may confuse neuropathy-associated muscle weakness with fatigue. These interactions can be controlled for in self-report instruments and validated through careful study of many patients. Disease-specific questionnaires have been validated for use in several diseases,56-58 though none have been validated for cutaneous psoriasis in the absence of psoriatic arthritis. The need for validated instruments in psoriasis is great, as symptoms such as sleep disturbance and arthralgia may confound metrics of fatigue.
Thus far, 4 self-report instruments have been used to study fatigue in psoriasis: the medical outcomes 36-item short-form health survey (SF-36), the functional assessment of chronic illness therapy-fatigue, the fatigue severity scale (FSS), and the visual analog scale (VAS) for fatigue.
The SF-36 is a 36-item survey designed to measure 8 dimensions of health status in patients with chronic disease.59 Items are answered using a 3- to 6-point Likert scale, or in a yes/no format. Although the SF-36 is typically administered by a trained interviewer, it relies on a patient’s interpretation of language that must be used to describe their level of fatigue, which may not capture the full range of symptoms. Also, the length of the survey makes it impractical for use in clinical practice.
The functional assessment of chronic illness therapy-fatigue survey is validated for use in psoriatic arthritis. It is similar to the SF-36 in its use of a 5-point Likert scale to answer each of 13 items. It improves on the SF-36 model by including questions about associated symptoms (ie, pain, medication side effects) that may interfere with the measurement of fatigue. It also investigates the impact of fatigue on several areas of functioning. However, it is subject to the same pitfalls of interpretation and a rigid scale with which to answer questions.
The FSS is another Likert scale–based instrument that gauges both level of fatigue and its impact using 9 items and a 7-point scale. Investigators used the FSS to uncover an association between increasing fatigue scores and depression in patients with psoriatic disease.38
The VAS overcomes many of the language and interpretation issues inherent in Likert scale–based instruments. Patients are presented with a single item in which they are asked to plot their level of fatigue on a continuous line, with one end representing no fatigue and the other end the worst possible fatigue. Although VAS adds simplicity of response and removes some ambiguity from surveying, it provides no information about the functional impact of fatigue on patients. It also does not provide a method to control for other symptoms.
Treatment of Psoriasis-Associated Fatigue
Much of our understanding of psoriasis-associated fatigue arises from therapeutic clinical trials. Because increased concentrations of proinflammatory cytokines are associated with fatigue, it has been suggested that blocking these cytokines with biologic agents may relieve fatigue symptoms. For example, investigators found that patients treated with etanercept, a soluble TNF-α receptor fusion protein, had clinically meaningful improvement in fatigue compared to those receiving placebo, with sustained improvements at 96 weeks.9,47 We must note, however, that the decrease in fatigue correlated with improvements in cutaneous/arthritic pain. Nevertheless, another study found that treatment with the same drug decreased fatigue in patients with psoriasis, even after controlling for improvements in the psoriasis area severity index score.40 Adalimumab is another monoclonal antibody for TNF-α that has caused a notable decline in fatigue symptoms.49
These data suggest that biologic agents are useful in the treatment of fatigue. Biologic agents are frequently administered to patients with moderate to severe psoriasis in whom more conservative treatments previously failed. However, cutaneous/arthritic disease severity is not always correlated with fatigue, so these data may urge clinicians to lower their threshold for treatment with biologics in patients with substantial fatigue symptoms. Although further investigations are necessary, we may even consider using a biologic therapy for severe fatigue in those without severe psoriatic disease.
Conclusion
Fatigue is a multidimensional symptom, impacted both directly and indirectly by psoriasis pathophysiology. The prevalence of fatigue within this patient population suggests that clinicians need to recognize the symptom as a core domain in psoriasis evaluation. Although a host of metrics have been used to quantify/qualify fatigue, there remains a need for a validated instrument for assessing fatigue in patients with psoriatic disease.
Biologic agents have proven useful in the treatment of psoriasis-associated fatigue. The central role of proinflammatory cytokines to both fatigue and psoriasis pathogenesis provide insight into potential treatment targets. Understanding the overlapping pathophysiology of psoriasis and fatigue provides an avenue for developing innovative strategies to target molecules implicated in the activation of the immune system. In the future, it may be possible to predict the severity of fatigue by measuring the levels of serum inflammatory cytokines; in fact, a new study aims to identify a panel of soluble biomarkers that can predict joint damage in psoriatic arthritis.60 Taken together, the findings described suggest that further study is needed to characterize, measure, and treat psoriasis-associated fatigue.
- NANDA Nursing Diagnoses: Definitions and Classification, 1999-2000. Philadelphia, PA: NANDA International; 1999.
- Swain MG. Fatigue in chronic disease. Clin Sci (Lond). 2000;99:1-8.
- Wolfe F, Hawley DJ, Wilson K. The prevalence and meaning of fatigue in rheumatic disease. J Rheumatol. 1996;23:1407-1417.
- van Hoogmoed D, Fransen J, Bleijenberg G, et al. Physical and psychosocial correlates of severe fatigue in rheumatoid arthritis. Rheumatology (Oxford). 2010;49:1294-1302.
- Cleanthous S, Tyagi M, Isenberg DA, et al. What do we know about self-reported fatigue in systemic lupus erythematosus? Lupus. 2012;21:465-476.
- Ulus Y, Akyol Y, Tander B, et al. Sleep quality in fibromyalgia and rheumatoid arthritis: associations with pain, fatigue, depression, and disease activity. Clin Exp Rheumatol. 2011;29(6, suppl 69):S92-S96.
- Segal B, Thomas W, Rogers T, et al. Prevalence, severity, and predictors of fatigue in subjects with primary Sjögren’s syndrome. Arthritis Rheum. 2008;59:1780-1787.
- Gladman DD, Mease PJ, Strand V, et al. Consensus on a core set of domains for psoriatic arthritis. J Rheumatol. 2007;34:1167-1170.
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
- De Rosa G, Mignogna C. The histopathology of psoriasis. Reumatismo. 2007;59(suppl 1):46-48.
- Nickoloff BJ, Xin H, Nestle FO, et al. The cytokine and chemokine network in psoriasis. Clin Dermatol. 2007;25:568-573.
- Zaba LC, Fuentes-Duculan J, Eungdamrong NJ, et al. Psoriasis is characterized by accumulation of immunostimulatory and Th1/Th17 cell-polarizing myeloid dendritic cells. J Invest Dermatol. 2009;129:79-88.
- Austin LM, Ozawa M, Kikuchi T, et al. The majority of epidermal T cells in psoriasis vulgaris lesions can produce type 1 cytokines, interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J Invest Dermatol. 1999;113:752-759.
- Lebwohl M, Papp K, Han C, et al. Ustekinumab improves health-related quality of life in patients with moderate-to-severe psoriasis: results from the PHOENIX 1 trial. Br J Dermatol. 2010;162:137-146.
- Sabat R, Wolk K. Pathogenesis of psoriasis. In: Sterry W, Sabat R, Philipp S, eds. Psoriasis: Diagnosis and Management. Chichester, UK: John Wiley & Sons, Ltd; 2014:28-48.
- Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345-350.
- Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207-1211.
- Chung Y, Chang SH, Martinez GJ, et al. Critical regulation of early Th17 cell differentiation by IL-1 signaling. Immunity. 2009;30:576-587.
- Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720-3732.
- Jensen LE. Targeting the IL-1 family members in skin inflammation. Curr Opin Investig Drugs. 2010;11:1211-1220.
- Yoshinaga Y, Higaki M, Terajima S, et al. Detection of inflammatory cytokines in psoriatic skin. Arch Dermatol Res. 1995;287:158-164.
- Schon M, Behmenburg C, Denzer D, et al. Pathogenic function of IL-1 beta in psoriasiform skin lesions of flaky skin (fsn/fsn) mice. Clin Exp Immunol. 2001;123:505-510.
- Dantzer R, O’Connor JC, Freund GG, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46-56.
- Quan N, Stern EL, Whiteside MB, et al. Induction of pro-inflammatory cytokine mRNAs in the brain after peripheral injection of subseptic doses of lipopolysaccharide in the rat. J Neuroimmunol. 1999;93:72-80.
- Carmichael MD, Davis JM, Murphy EA, et al. Role of brain IL-1beta on fatigue after exercise-induced muscle damage. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1344-R1348.
- Swain MG, Beck P, Rioux K, et al. Augmented interleukin-1beta-induced depression of locomotor activity in cholestatic rats. Hepatology. 1998;28:1561-1565.
- Kent S, Bluthé RM, Kelley KW, et al. Sickness behavior as a new target for drug development. Trends Pharmacol Sci. 1992;13:24-28.
- Lacosta S, Merali Z, Anisman H. Influence of interleukin-1beta on exploratory behaviors, plasma ACTH, corticosterone, and central biogenic amines in mice. Psychopharmacology. 1998;137:351-361.
- Bluthé RM, Laye S, Michaud B, et al. Role of interleukin-1beta and tumour necrosis factor-alpha in lipopolysaccharide-induced sickness behaviour: a study with interleukin-1 type I receptor-deficient mice. Eur J Neurosci. 2000;12:4447-4456.
- Maes M, Twisk FN, Ringel K. Inflammatory and cell-mediated immune biomarkers in myalgic encephalomyelitis/chronic fatigue syndrome and depression: inflammatory markers are higher in myalgic encephalomyelitis/chronic fatigue syndrome than in depression. Psychother Psychosom. 2012;81:286-295.
- Dowlatshahi EA, van der Voort EAM, Arends LR, et al. Markers of systemic inflammation in psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2013;169:266-282.
- Jankovic S, Raznatovic M, Marinkovic J, et al. Health-related quality of life in patients with psoriasis.J Cutan Med Surg. 2011;15:29-36.
- Carneiro C, Chaves M, Verardino G, et al. Fatigue in psoriasis with arthritis. Skinmed. 2011;9:34-37.
- Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and metabolic syndrome: a systematic review and meta-analysis of observational studies. J Am Acad Dermatol. 2013;68:654-662.
- Sterry W, Strober BE, Menter A. Obesity in psoriasis: the metabolic, clinical and therapeutic implications. report of an interdisciplinary conference and review. Br J Dermatol. 2007;157:649-655.
- Armstrong AW, Schupp C, Wu J, et al. Quality of life and work productivity impairment among psoriasis patients: findings from the National Psoriasis Foundation survey data 2003-2011. PloS One. 2012;7:e52935.
- de Korte J, Sprangers MA, Mombers FM, et al. Quality of life in patients with psoriasis: a systematic literature review. J Invest Dermatol. 2004;9:140-147.
- McDonough E, Ayearst R, Eder L, et al. Depression and anxiety in psoriatic disease: prevalence and associated factors. J Rheumatol. 2014;41:887-896.
- Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280-284.
- Thaci D, Galimberti R, Amaya-Guerra M, et al. Improvement in aspects of sleep with etanercept and optional adjunctive topical therapy in patients with moderate-to-severe psoriasis: results from the PRISTINE trial. J Eur Acad Dermatol Venereol. 2014;28:900-906.
- Walsh JA, McFadden ML, Morgan MD, et al. Work productivity loss and fatigue in psoriatic arthritis. J Rheumatol. 2014;41:1670-1674.
- Krueger GG, Langley RG, Finlay AY, et al. Patient-reported outcomes of psoriasis improvement with etanercept therapy: results of a randomized phase III trial. Br J Dermatol. 2005;153:1192-1199.
- Reich K, Nestle FO, Papp K, et al. Improvement in quality of life with infliximab induction and maintenance therapy in patients with moderate-to-severe psoriasis: a randomized controlled trial. Br J Dermatol. 2006;154:1161-1168.
- Daudén E, Griffiths CE, Ortonne JP, et al. Improvements in patient-reported outcomes in moderate-to-severe psoriasis patients receiving continuous or paused etanercept treatment over 54 weeks: the CRYSTEL study. J Eur Acad Dermatol Venereol. 2009;23:1374-1382.
- Kalb RE, Blauvelt A, Sofen HL, et al. Effect of infliximab on health-related quality of life and disease activity by body region in patients with moderate-to-severe psoriasis and inadequate response to etanercept: results from the PSUNRISE trial. J Drugs Dermatol. 2013;12:874-880.
- Chandran V, Bhella S, Schentag C, et al. Functional assessment of chronic illness therapy-fatigue scale is valid in patients with psoriatic arthritis. Ann Rheumatic Dis. 2007;66:936-939.
- Krishnan R, Cella D, Leonardi C, et al. Effects of etanercept therapy on fatigue and symptoms of depression in subjects treated for moderate to severe plaque psoriasis for up to 96 weeks. Br J Dermatol. 2007;157:1275-1277.
- Reich K, Segaert S, Van de Kerkhof P, et al. Once-weekly administration of etanercept 50 mgimproves patient-reported outcomes in patients with moderate-to-severe plaque psoriasis. Dermatology. 2009;219:239-249.
- Papp K, Crowley J, Ortonne JP, et al. Adalimumab for moderate to severe chronic plaque psoriasis: efficacy and safety of retreatment and disease recurrence following withdrawal from therapy. Br J Dermatol. 2011;164:434-441.
- Evers AW, Lu Y, Duller P, et al. Common burden of chronic skin diseases? contributors to psychological distress in adults with psoriasis and atopic dermatitis. Br J Dermatol. 2005;152:1275-1281.
- Verhoeven EW, Kraaimaat FW, van de Kerkhof PC, et al. Prevalence of physical symptoms of itch, pain and fatigue in patients with skin diseases in general practice. Br J Dermatol. 2007;156:1346-1349.
- Husted JA, Tom BD, Schentag CT, et al. Occurrence and correlates of fatigue in psoriatic arthritis. Ann Rheum Dis. 2009;68:1553-1558.
- Rosen CF, Mussani F, Chandran V, et al. Patients with psoriatic arthritis have worse quality of life than those with psoriasis alone. Rheumatology. 2012;51:571-576.
- Gorber SC, Tremblay M, Moher D, et al. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: a systematic review. Obes Rev. 2007;8:307-326.
- Fadnes LT, Taube A, Tylleskär T. How to identify information bias due to self-reporting in epidemiological research. Int J Epidemiol. 2009;7:3.
- Brown RG, Dittner A, Findley L, et al. The Parkinson fatigue scale. Parkinsonism Relat Disord. 2005;11:49-55.
- Fisk JD, Ritvo PG, Ross L, et al. Measuring the functional impact of fatigue: initial validation of the fatigue impact scale. Clin Infect Dis. 1994;18(suppl 1):S79-S83.
- Bowman SJ, Booth DA, Platts RG. Measurement of fatigue and discomfort in primary Sjögren’s syndrome using a new questionnaire tool. Rheumatology. 2004;43:758-764.
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. conceptual framework and item selection. Med Care. 1992;30:473-483.
- FitzGerald O, Mease PJ. Biomarkers: project update from the GRAPPA 2012 annual meeting. J Rheumatol. 2013;40:1453-1454.
- NANDA Nursing Diagnoses: Definitions and Classification, 1999-2000. Philadelphia, PA: NANDA International; 1999.
- Swain MG. Fatigue in chronic disease. Clin Sci (Lond). 2000;99:1-8.
- Wolfe F, Hawley DJ, Wilson K. The prevalence and meaning of fatigue in rheumatic disease. J Rheumatol. 1996;23:1407-1417.
- van Hoogmoed D, Fransen J, Bleijenberg G, et al. Physical and psychosocial correlates of severe fatigue in rheumatoid arthritis. Rheumatology (Oxford). 2010;49:1294-1302.
- Cleanthous S, Tyagi M, Isenberg DA, et al. What do we know about self-reported fatigue in systemic lupus erythematosus? Lupus. 2012;21:465-476.
- Ulus Y, Akyol Y, Tander B, et al. Sleep quality in fibromyalgia and rheumatoid arthritis: associations with pain, fatigue, depression, and disease activity. Clin Exp Rheumatol. 2011;29(6, suppl 69):S92-S96.
- Segal B, Thomas W, Rogers T, et al. Prevalence, severity, and predictors of fatigue in subjects with primary Sjögren’s syndrome. Arthritis Rheum. 2008;59:1780-1787.
- Gladman DD, Mease PJ, Strand V, et al. Consensus on a core set of domains for psoriatic arthritis. J Rheumatol. 2007;34:1167-1170.
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
- De Rosa G, Mignogna C. The histopathology of psoriasis. Reumatismo. 2007;59(suppl 1):46-48.
- Nickoloff BJ, Xin H, Nestle FO, et al. The cytokine and chemokine network in psoriasis. Clin Dermatol. 2007;25:568-573.
- Zaba LC, Fuentes-Duculan J, Eungdamrong NJ, et al. Psoriasis is characterized by accumulation of immunostimulatory and Th1/Th17 cell-polarizing myeloid dendritic cells. J Invest Dermatol. 2009;129:79-88.
- Austin LM, Ozawa M, Kikuchi T, et al. The majority of epidermal T cells in psoriasis vulgaris lesions can produce type 1 cytokines, interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J Invest Dermatol. 1999;113:752-759.
- Lebwohl M, Papp K, Han C, et al. Ustekinumab improves health-related quality of life in patients with moderate-to-severe psoriasis: results from the PHOENIX 1 trial. Br J Dermatol. 2010;162:137-146.
- Sabat R, Wolk K. Pathogenesis of psoriasis. In: Sterry W, Sabat R, Philipp S, eds. Psoriasis: Diagnosis and Management. Chichester, UK: John Wiley & Sons, Ltd; 2014:28-48.
- Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345-350.
- Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207-1211.
- Chung Y, Chang SH, Martinez GJ, et al. Critical regulation of early Th17 cell differentiation by IL-1 signaling. Immunity. 2009;30:576-587.
- Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720-3732.
- Jensen LE. Targeting the IL-1 family members in skin inflammation. Curr Opin Investig Drugs. 2010;11:1211-1220.
- Yoshinaga Y, Higaki M, Terajima S, et al. Detection of inflammatory cytokines in psoriatic skin. Arch Dermatol Res. 1995;287:158-164.
- Schon M, Behmenburg C, Denzer D, et al. Pathogenic function of IL-1 beta in psoriasiform skin lesions of flaky skin (fsn/fsn) mice. Clin Exp Immunol. 2001;123:505-510.
- Dantzer R, O’Connor JC, Freund GG, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46-56.
- Quan N, Stern EL, Whiteside MB, et al. Induction of pro-inflammatory cytokine mRNAs in the brain after peripheral injection of subseptic doses of lipopolysaccharide in the rat. J Neuroimmunol. 1999;93:72-80.
- Carmichael MD, Davis JM, Murphy EA, et al. Role of brain IL-1beta on fatigue after exercise-induced muscle damage. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1344-R1348.
- Swain MG, Beck P, Rioux K, et al. Augmented interleukin-1beta-induced depression of locomotor activity in cholestatic rats. Hepatology. 1998;28:1561-1565.
- Kent S, Bluthé RM, Kelley KW, et al. Sickness behavior as a new target for drug development. Trends Pharmacol Sci. 1992;13:24-28.
- Lacosta S, Merali Z, Anisman H. Influence of interleukin-1beta on exploratory behaviors, plasma ACTH, corticosterone, and central biogenic amines in mice. Psychopharmacology. 1998;137:351-361.
- Bluthé RM, Laye S, Michaud B, et al. Role of interleukin-1beta and tumour necrosis factor-alpha in lipopolysaccharide-induced sickness behaviour: a study with interleukin-1 type I receptor-deficient mice. Eur J Neurosci. 2000;12:4447-4456.
- Maes M, Twisk FN, Ringel K. Inflammatory and cell-mediated immune biomarkers in myalgic encephalomyelitis/chronic fatigue syndrome and depression: inflammatory markers are higher in myalgic encephalomyelitis/chronic fatigue syndrome than in depression. Psychother Psychosom. 2012;81:286-295.
- Dowlatshahi EA, van der Voort EAM, Arends LR, et al. Markers of systemic inflammation in psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2013;169:266-282.
- Jankovic S, Raznatovic M, Marinkovic J, et al. Health-related quality of life in patients with psoriasis.J Cutan Med Surg. 2011;15:29-36.
- Carneiro C, Chaves M, Verardino G, et al. Fatigue in psoriasis with arthritis. Skinmed. 2011;9:34-37.
- Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and metabolic syndrome: a systematic review and meta-analysis of observational studies. J Am Acad Dermatol. 2013;68:654-662.
- Sterry W, Strober BE, Menter A. Obesity in psoriasis: the metabolic, clinical and therapeutic implications. report of an interdisciplinary conference and review. Br J Dermatol. 2007;157:649-655.
- Armstrong AW, Schupp C, Wu J, et al. Quality of life and work productivity impairment among psoriasis patients: findings from the National Psoriasis Foundation survey data 2003-2011. PloS One. 2012;7:e52935.
- de Korte J, Sprangers MA, Mombers FM, et al. Quality of life in patients with psoriasis: a systematic literature review. J Invest Dermatol. 2004;9:140-147.
- McDonough E, Ayearst R, Eder L, et al. Depression and anxiety in psoriatic disease: prevalence and associated factors. J Rheumatol. 2014;41:887-896.
- Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137:280-284.
- Thaci D, Galimberti R, Amaya-Guerra M, et al. Improvement in aspects of sleep with etanercept and optional adjunctive topical therapy in patients with moderate-to-severe psoriasis: results from the PRISTINE trial. J Eur Acad Dermatol Venereol. 2014;28:900-906.
- Walsh JA, McFadden ML, Morgan MD, et al. Work productivity loss and fatigue in psoriatic arthritis. J Rheumatol. 2014;41:1670-1674.
- Krueger GG, Langley RG, Finlay AY, et al. Patient-reported outcomes of psoriasis improvement with etanercept therapy: results of a randomized phase III trial. Br J Dermatol. 2005;153:1192-1199.
- Reich K, Nestle FO, Papp K, et al. Improvement in quality of life with infliximab induction and maintenance therapy in patients with moderate-to-severe psoriasis: a randomized controlled trial. Br J Dermatol. 2006;154:1161-1168.
- Daudén E, Griffiths CE, Ortonne JP, et al. Improvements in patient-reported outcomes in moderate-to-severe psoriasis patients receiving continuous or paused etanercept treatment over 54 weeks: the CRYSTEL study. J Eur Acad Dermatol Venereol. 2009;23:1374-1382.
- Kalb RE, Blauvelt A, Sofen HL, et al. Effect of infliximab on health-related quality of life and disease activity by body region in patients with moderate-to-severe psoriasis and inadequate response to etanercept: results from the PSUNRISE trial. J Drugs Dermatol. 2013;12:874-880.
- Chandran V, Bhella S, Schentag C, et al. Functional assessment of chronic illness therapy-fatigue scale is valid in patients with psoriatic arthritis. Ann Rheumatic Dis. 2007;66:936-939.
- Krishnan R, Cella D, Leonardi C, et al. Effects of etanercept therapy on fatigue and symptoms of depression in subjects treated for moderate to severe plaque psoriasis for up to 96 weeks. Br J Dermatol. 2007;157:1275-1277.
- Reich K, Segaert S, Van de Kerkhof P, et al. Once-weekly administration of etanercept 50 mgimproves patient-reported outcomes in patients with moderate-to-severe plaque psoriasis. Dermatology. 2009;219:239-249.
- Papp K, Crowley J, Ortonne JP, et al. Adalimumab for moderate to severe chronic plaque psoriasis: efficacy and safety of retreatment and disease recurrence following withdrawal from therapy. Br J Dermatol. 2011;164:434-441.
- Evers AW, Lu Y, Duller P, et al. Common burden of chronic skin diseases? contributors to psychological distress in adults with psoriasis and atopic dermatitis. Br J Dermatol. 2005;152:1275-1281.
- Verhoeven EW, Kraaimaat FW, van de Kerkhof PC, et al. Prevalence of physical symptoms of itch, pain and fatigue in patients with skin diseases in general practice. Br J Dermatol. 2007;156:1346-1349.
- Husted JA, Tom BD, Schentag CT, et al. Occurrence and correlates of fatigue in psoriatic arthritis. Ann Rheum Dis. 2009;68:1553-1558.
- Rosen CF, Mussani F, Chandran V, et al. Patients with psoriatic arthritis have worse quality of life than those with psoriasis alone. Rheumatology. 2012;51:571-576.
- Gorber SC, Tremblay M, Moher D, et al. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: a systematic review. Obes Rev. 2007;8:307-326.
- Fadnes LT, Taube A, Tylleskär T. How to identify information bias due to self-reporting in epidemiological research. Int J Epidemiol. 2009;7:3.
- Brown RG, Dittner A, Findley L, et al. The Parkinson fatigue scale. Parkinsonism Relat Disord. 2005;11:49-55.
- Fisk JD, Ritvo PG, Ross L, et al. Measuring the functional impact of fatigue: initial validation of the fatigue impact scale. Clin Infect Dis. 1994;18(suppl 1):S79-S83.
- Bowman SJ, Booth DA, Platts RG. Measurement of fatigue and discomfort in primary Sjögren’s syndrome using a new questionnaire tool. Rheumatology. 2004;43:758-764.
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. conceptual framework and item selection. Med Care. 1992;30:473-483.
- FitzGerald O, Mease PJ. Biomarkers: project update from the GRAPPA 2012 annual meeting. J Rheumatol. 2013;40:1453-1454.
Practice Points
- Psoriasis-associated fatigue results from the impact of the inflammatory cascade on the central nervous system and from the negative influences of disease on patients.
- Although psoriasis-associated fatigue is common, there is a lack of validated systems to quantify its severity and guide therapy.
- Given the overlapping pathophysiology of psoriasis and fatigue, biologic agents may be beneficial for treating psoriasis-associated fatigue.
Inflammatory Acne: New Developments in Pathogenesis and Treatment
Acne vulgaris is a chronic inflammatory disease that affects the majority of the population at some point in their lifetime. It is characterized by comedones, pustules, and papules. Acne pathogenesis is multifactorial with 4 primary factors that play a pivotal role in the formation of acne lesions: excess sebum production, abnormal keratinization, inflammation, and bacterial colonization of Propionibacterium acnes in the pilosebaceous unit.1 Although there is a general consensus on the pathogenic factors, the sequence of events in acne development is controversial. Traditionally it was believed that abnormal keratinization resulted in the creation of the microcomedone, the earliest subclinical acne lesion.2 Activation of sebaceous glands by androgens, excess sebum production, and keratin plug formation then were followed by P acnes colonization, with induction of the innate immune system culminating in inflammation.2 Androgen-induced sebum production and follicular hyperkeratinization and plugging have been cited as initial events that alter the pilosebaceous milieu, favoring the proliferation of P acnes1,3; however, evidence suggests inflammation as the inciting factor, with proof of significant inflammatory factors surrounding the pilosebaceous unit even in clinically uninvolved skin units in acne patients.4 Herein we will briefly review the most recent data and translational applications pertaining to the P acnes–triggered innate immune response via activation of toll-like receptor 2 (TLR2)5 and importantly the inflammasome.6,7
A new understanding of how P acnes induces the inflammatory cascade may represent a paradigm shift in the management of acne. Recognition of microbes, namely P acnes, by the innate immune system is the body’s first line of defense against pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs).6 Although these pathways combat infection and prevent foreign invasion, they also result in inflammation and tissue injury. The inflammatory response to PAMPs and DAMPs is mediated by the inflammasome, a caspase 1–activating cytoplasmic complex that induces the secretion of crucial proinflammatory cytokines.7 The exact mechanism by which P acnes exerts its proinflammatory activity has been somewhat unclear, though P acnes–induced inflammation has been shown to be mediated by proinflammatory cytokines tumor necrosis factor α, IL-1, IL-6, IL-8, and IL-12.5,8 However, remarkable evidence recently was presented regarding triggers of inflammation and the precise mechanism involved. Qin et al9 showed that P acnes is a potent trigger of IL-1β generation via activation of the NLRP3 (NOD-like receptor family, pyrin domain containing 3) inflammasome. Specifically, the study showed that human monocytes respond to P acnes by upregulating caspase 1, an inflammatory caspase required for proteolytic cleavage of IL-1β. The authors correlated their in vitro findings with clinical evidence of caspase 1 and NLRP3 expression in the dermis surrounding the pilosebaceous units of biopsied lesions.9 Kistowska et al6 confirmed and expanded on these data by showing the inability of NLRP3-deficient myeloid cells to secrete IL-1β and induce an inflammatory response in vivo. A recent investigation demonstrated that human sebocytes can function as constituents of the innate immune response, with P acnes triggering sebocyte NLRP3-inflammasome activation and subsequent IL-1β secretion. These observations were further confirmed in vivo with NLRP3-deficient mice displaying an impaired inflammatory response to P acnes.10
Our understanding of TLR2 signaling in the pathogenesis of acne also has expanded. It is well established that recognition of extracellular PAMPs and DAMPs is mediated by the expression of toll-like receptors on the surface of a variety of cells within the skin.11 Prior research demonstrated how P acnes increases TLR2 expression in keratinocytes, even in vivo.12 Stimulation by P acnes was shown to induce secretion of IL-8 (promoting a TH1 response) and IL-12 (promoting neutrophil chemotaxis) via TLR2 activation.5 Selway et al11 validated this finding by demonstrating that infundibular keratinocytes secrete IL-1α in response to the peptidoglycan cell wall of P acnes. Interestingly, Qin et al9 determined TLR2 inhibition resulted in partial suppression of IL-1β, possibly providing new evidence of TLR2-mediated activation of the NLRP3 inflammasome. Therefore, P acnes activates both extracellular and intracellular triggers of the innate immune response: TLR2 activation (requiring extracellular recognition of pathogens) and inflammasome-mediated activation (requiring internalization and access of the bacterium to the interior compartments of the cells).
Overall, these findings suggest that P acnes–induced inflammation can be selectively targeted by agents directed at inflammasome components, IL-1β, or toll-like receptors. A phase 2 double-blind, placebo-controlled trial assessing the efficacy of the anti–IL-1β monoclonal antibody gevokizumab found that 0.6 mg/kg administered subcutaneously resulted in a significant reduction in mean inflammatory lesion count compared to placebo (P=.077).13 The success of the IL-1 receptor antagonist anakinra against rare genetic autoinflammatory syndromes such as PAPA (pyogenic sterile arthritis, pyoderma gangrenosum, and acne) syndrome, an NLRP3 inflammasomopathy, sheds light onto new therapeutics that may be used to target acne vulgaris.14 Current topical therapies such as retinoids, which have already proven efficacious in the treatment of inflammatory acne, target these pathways. In vivo data revealed that treatment with isotretinoin significantly decreased TLR2 expression in monocytes (P<.001) and suppressed inflammatory cytokine responses to P acnes (P<.001).15 Adapalene, with or without benzoyl peroxide, also was shown to exert anti-inflammatory effects via TLR2 downregulation.16
These data and observations highlight a paradigm shift in our perception of acne. All acne is truly inflammatory, and by identifying aberrations in the immune response, we can develop targeted treatments for this chronic debilitating disease.
1. Kurokawa I, Danby FW, Ju Q, et al. New developments in our understanding of acne pathogenesis and treatment. Exp Dermatol. 2009;18:821-832.
2. Cunliffe WJ, Holland DB, Clark SM, et al. Comedogenesis: some aetiological, clinical and therapeutic strategies. Dermatology. 2003;206:11-16.
3. Bowe W, Kober M. Therapeutic update: acne. J Drugs Dermatol. 2014;13:235-238.
4. Jeremy AH, Holland DB, Roberts SG, et al. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003;121:20-27.
5. Kim J, Ochoa MT, Krutzik SR, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169:1535-1541.
6. Kistowska M, Gehrke S, Jankovic D, et al. IL-1beta drives inflammatory responses to Propionibacterium acnes in vitro and in vivo. J Invest Dermatol. 2014;134:677-685.
7. Contassot E, French LE. New insights into acne pathogenesis: Propionibacterium acnes activates the inflammasome. J Invest Dermatol. 2014;134:310-313.
8. Vowels BR, Yang S, Leyden JJ. Induction of proinflammatory cytokines by a soluble factor of Propionibacterium acnes: implications for chronic inflammatory acne. Infect Immun. 1995;63:3158-3165.
9. Qin M, Pirouz A, Kim MH, et al. Propionibacterium acnes induces IL-1beta secretion via the NLRP3 inflammasome in human monocytes. J Invest Dermatol. 2014;134:381-388.
10. Li ZJ, Choi DK, Sohn KC, et al. Propionibacterium acnes activates the NLRP3 inflammasome in human sebocytes. J Invest Dermatol. 2014;134:2747-2756.
11. Selway JL, Kurczab T, Kealey T, et al. Toll-like receptor 2 activation and comedogenesis: implications for the pathogenesis of acne. BMC Dermatol. 2013;13:10.
12. Jugeau S, Tenaud I, Knol AC, et al. Induction of toll-like receptors by Propionibacterium acnes. Br J Dermatol. 2005;153:1105-1113.
13. XOMA announces encouraging interim results from gevokizumab phase 2 study for moderate to severe acne vulgaris [press release]. Berkley, CA: XOMA Corporation; January 7, 2013. http://www.servier.com/content/xoma-announces-encouraging-interim-results-gevokizumab-phase-2-study-moderate-severe-acne. Accessed November 5, 2014.
14. Leemans JC, Cassel SL, Sutterwala FS. Sensing damage by the NLRP3 inflammasome. Immunol Rev. 2011;243:152-162.
15. Dispenza MC, Wolpert EB, Gilliland KL, et al. Systemic isotretinoin therapy normalizes exaggerated TLR-2-mediated innate immune responses in acne patients. J Invest Dermatol. 2012;132:2198-2205.
16. Zuliani T, Khammari A, Chaussy H, et al. Ex vivo demonstration of a synergistic effect of adapalene and benzoyl peroxide on inflammatory acne lesions. Exp Dermatol. 2011;20:850-853.
Acne vulgaris is a chronic inflammatory disease that affects the majority of the population at some point in their lifetime. It is characterized by comedones, pustules, and papules. Acne pathogenesis is multifactorial with 4 primary factors that play a pivotal role in the formation of acne lesions: excess sebum production, abnormal keratinization, inflammation, and bacterial colonization of Propionibacterium acnes in the pilosebaceous unit.1 Although there is a general consensus on the pathogenic factors, the sequence of events in acne development is controversial. Traditionally it was believed that abnormal keratinization resulted in the creation of the microcomedone, the earliest subclinical acne lesion.2 Activation of sebaceous glands by androgens, excess sebum production, and keratin plug formation then were followed by P acnes colonization, with induction of the innate immune system culminating in inflammation.2 Androgen-induced sebum production and follicular hyperkeratinization and plugging have been cited as initial events that alter the pilosebaceous milieu, favoring the proliferation of P acnes1,3; however, evidence suggests inflammation as the inciting factor, with proof of significant inflammatory factors surrounding the pilosebaceous unit even in clinically uninvolved skin units in acne patients.4 Herein we will briefly review the most recent data and translational applications pertaining to the P acnes–triggered innate immune response via activation of toll-like receptor 2 (TLR2)5 and importantly the inflammasome.6,7
A new understanding of how P acnes induces the inflammatory cascade may represent a paradigm shift in the management of acne. Recognition of microbes, namely P acnes, by the innate immune system is the body’s first line of defense against pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs).6 Although these pathways combat infection and prevent foreign invasion, they also result in inflammation and tissue injury. The inflammatory response to PAMPs and DAMPs is mediated by the inflammasome, a caspase 1–activating cytoplasmic complex that induces the secretion of crucial proinflammatory cytokines.7 The exact mechanism by which P acnes exerts its proinflammatory activity has been somewhat unclear, though P acnes–induced inflammation has been shown to be mediated by proinflammatory cytokines tumor necrosis factor α, IL-1, IL-6, IL-8, and IL-12.5,8 However, remarkable evidence recently was presented regarding triggers of inflammation and the precise mechanism involved. Qin et al9 showed that P acnes is a potent trigger of IL-1β generation via activation of the NLRP3 (NOD-like receptor family, pyrin domain containing 3) inflammasome. Specifically, the study showed that human monocytes respond to P acnes by upregulating caspase 1, an inflammatory caspase required for proteolytic cleavage of IL-1β. The authors correlated their in vitro findings with clinical evidence of caspase 1 and NLRP3 expression in the dermis surrounding the pilosebaceous units of biopsied lesions.9 Kistowska et al6 confirmed and expanded on these data by showing the inability of NLRP3-deficient myeloid cells to secrete IL-1β and induce an inflammatory response in vivo. A recent investigation demonstrated that human sebocytes can function as constituents of the innate immune response, with P acnes triggering sebocyte NLRP3-inflammasome activation and subsequent IL-1β secretion. These observations were further confirmed in vivo with NLRP3-deficient mice displaying an impaired inflammatory response to P acnes.10
Our understanding of TLR2 signaling in the pathogenesis of acne also has expanded. It is well established that recognition of extracellular PAMPs and DAMPs is mediated by the expression of toll-like receptors on the surface of a variety of cells within the skin.11 Prior research demonstrated how P acnes increases TLR2 expression in keratinocytes, even in vivo.12 Stimulation by P acnes was shown to induce secretion of IL-8 (promoting a TH1 response) and IL-12 (promoting neutrophil chemotaxis) via TLR2 activation.5 Selway et al11 validated this finding by demonstrating that infundibular keratinocytes secrete IL-1α in response to the peptidoglycan cell wall of P acnes. Interestingly, Qin et al9 determined TLR2 inhibition resulted in partial suppression of IL-1β, possibly providing new evidence of TLR2-mediated activation of the NLRP3 inflammasome. Therefore, P acnes activates both extracellular and intracellular triggers of the innate immune response: TLR2 activation (requiring extracellular recognition of pathogens) and inflammasome-mediated activation (requiring internalization and access of the bacterium to the interior compartments of the cells).
Overall, these findings suggest that P acnes–induced inflammation can be selectively targeted by agents directed at inflammasome components, IL-1β, or toll-like receptors. A phase 2 double-blind, placebo-controlled trial assessing the efficacy of the anti–IL-1β monoclonal antibody gevokizumab found that 0.6 mg/kg administered subcutaneously resulted in a significant reduction in mean inflammatory lesion count compared to placebo (P=.077).13 The success of the IL-1 receptor antagonist anakinra against rare genetic autoinflammatory syndromes such as PAPA (pyogenic sterile arthritis, pyoderma gangrenosum, and acne) syndrome, an NLRP3 inflammasomopathy, sheds light onto new therapeutics that may be used to target acne vulgaris.14 Current topical therapies such as retinoids, which have already proven efficacious in the treatment of inflammatory acne, target these pathways. In vivo data revealed that treatment with isotretinoin significantly decreased TLR2 expression in monocytes (P<.001) and suppressed inflammatory cytokine responses to P acnes (P<.001).15 Adapalene, with or without benzoyl peroxide, also was shown to exert anti-inflammatory effects via TLR2 downregulation.16
These data and observations highlight a paradigm shift in our perception of acne. All acne is truly inflammatory, and by identifying aberrations in the immune response, we can develop targeted treatments for this chronic debilitating disease.
Acne vulgaris is a chronic inflammatory disease that affects the majority of the population at some point in their lifetime. It is characterized by comedones, pustules, and papules. Acne pathogenesis is multifactorial with 4 primary factors that play a pivotal role in the formation of acne lesions: excess sebum production, abnormal keratinization, inflammation, and bacterial colonization of Propionibacterium acnes in the pilosebaceous unit.1 Although there is a general consensus on the pathogenic factors, the sequence of events in acne development is controversial. Traditionally it was believed that abnormal keratinization resulted in the creation of the microcomedone, the earliest subclinical acne lesion.2 Activation of sebaceous glands by androgens, excess sebum production, and keratin plug formation then were followed by P acnes colonization, with induction of the innate immune system culminating in inflammation.2 Androgen-induced sebum production and follicular hyperkeratinization and plugging have been cited as initial events that alter the pilosebaceous milieu, favoring the proliferation of P acnes1,3; however, evidence suggests inflammation as the inciting factor, with proof of significant inflammatory factors surrounding the pilosebaceous unit even in clinically uninvolved skin units in acne patients.4 Herein we will briefly review the most recent data and translational applications pertaining to the P acnes–triggered innate immune response via activation of toll-like receptor 2 (TLR2)5 and importantly the inflammasome.6,7
A new understanding of how P acnes induces the inflammatory cascade may represent a paradigm shift in the management of acne. Recognition of microbes, namely P acnes, by the innate immune system is the body’s first line of defense against pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs).6 Although these pathways combat infection and prevent foreign invasion, they also result in inflammation and tissue injury. The inflammatory response to PAMPs and DAMPs is mediated by the inflammasome, a caspase 1–activating cytoplasmic complex that induces the secretion of crucial proinflammatory cytokines.7 The exact mechanism by which P acnes exerts its proinflammatory activity has been somewhat unclear, though P acnes–induced inflammation has been shown to be mediated by proinflammatory cytokines tumor necrosis factor α, IL-1, IL-6, IL-8, and IL-12.5,8 However, remarkable evidence recently was presented regarding triggers of inflammation and the precise mechanism involved. Qin et al9 showed that P acnes is a potent trigger of IL-1β generation via activation of the NLRP3 (NOD-like receptor family, pyrin domain containing 3) inflammasome. Specifically, the study showed that human monocytes respond to P acnes by upregulating caspase 1, an inflammatory caspase required for proteolytic cleavage of IL-1β. The authors correlated their in vitro findings with clinical evidence of caspase 1 and NLRP3 expression in the dermis surrounding the pilosebaceous units of biopsied lesions.9 Kistowska et al6 confirmed and expanded on these data by showing the inability of NLRP3-deficient myeloid cells to secrete IL-1β and induce an inflammatory response in vivo. A recent investigation demonstrated that human sebocytes can function as constituents of the innate immune response, with P acnes triggering sebocyte NLRP3-inflammasome activation and subsequent IL-1β secretion. These observations were further confirmed in vivo with NLRP3-deficient mice displaying an impaired inflammatory response to P acnes.10
Our understanding of TLR2 signaling in the pathogenesis of acne also has expanded. It is well established that recognition of extracellular PAMPs and DAMPs is mediated by the expression of toll-like receptors on the surface of a variety of cells within the skin.11 Prior research demonstrated how P acnes increases TLR2 expression in keratinocytes, even in vivo.12 Stimulation by P acnes was shown to induce secretion of IL-8 (promoting a TH1 response) and IL-12 (promoting neutrophil chemotaxis) via TLR2 activation.5 Selway et al11 validated this finding by demonstrating that infundibular keratinocytes secrete IL-1α in response to the peptidoglycan cell wall of P acnes. Interestingly, Qin et al9 determined TLR2 inhibition resulted in partial suppression of IL-1β, possibly providing new evidence of TLR2-mediated activation of the NLRP3 inflammasome. Therefore, P acnes activates both extracellular and intracellular triggers of the innate immune response: TLR2 activation (requiring extracellular recognition of pathogens) and inflammasome-mediated activation (requiring internalization and access of the bacterium to the interior compartments of the cells).
Overall, these findings suggest that P acnes–induced inflammation can be selectively targeted by agents directed at inflammasome components, IL-1β, or toll-like receptors. A phase 2 double-blind, placebo-controlled trial assessing the efficacy of the anti–IL-1β monoclonal antibody gevokizumab found that 0.6 mg/kg administered subcutaneously resulted in a significant reduction in mean inflammatory lesion count compared to placebo (P=.077).13 The success of the IL-1 receptor antagonist anakinra against rare genetic autoinflammatory syndromes such as PAPA (pyogenic sterile arthritis, pyoderma gangrenosum, and acne) syndrome, an NLRP3 inflammasomopathy, sheds light onto new therapeutics that may be used to target acne vulgaris.14 Current topical therapies such as retinoids, which have already proven efficacious in the treatment of inflammatory acne, target these pathways. In vivo data revealed that treatment with isotretinoin significantly decreased TLR2 expression in monocytes (P<.001) and suppressed inflammatory cytokine responses to P acnes (P<.001).15 Adapalene, with or without benzoyl peroxide, also was shown to exert anti-inflammatory effects via TLR2 downregulation.16
These data and observations highlight a paradigm shift in our perception of acne. All acne is truly inflammatory, and by identifying aberrations in the immune response, we can develop targeted treatments for this chronic debilitating disease.
1. Kurokawa I, Danby FW, Ju Q, et al. New developments in our understanding of acne pathogenesis and treatment. Exp Dermatol. 2009;18:821-832.
2. Cunliffe WJ, Holland DB, Clark SM, et al. Comedogenesis: some aetiological, clinical and therapeutic strategies. Dermatology. 2003;206:11-16.
3. Bowe W, Kober M. Therapeutic update: acne. J Drugs Dermatol. 2014;13:235-238.
4. Jeremy AH, Holland DB, Roberts SG, et al. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003;121:20-27.
5. Kim J, Ochoa MT, Krutzik SR, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169:1535-1541.
6. Kistowska M, Gehrke S, Jankovic D, et al. IL-1beta drives inflammatory responses to Propionibacterium acnes in vitro and in vivo. J Invest Dermatol. 2014;134:677-685.
7. Contassot E, French LE. New insights into acne pathogenesis: Propionibacterium acnes activates the inflammasome. J Invest Dermatol. 2014;134:310-313.
8. Vowels BR, Yang S, Leyden JJ. Induction of proinflammatory cytokines by a soluble factor of Propionibacterium acnes: implications for chronic inflammatory acne. Infect Immun. 1995;63:3158-3165.
9. Qin M, Pirouz A, Kim MH, et al. Propionibacterium acnes induces IL-1beta secretion via the NLRP3 inflammasome in human monocytes. J Invest Dermatol. 2014;134:381-388.
10. Li ZJ, Choi DK, Sohn KC, et al. Propionibacterium acnes activates the NLRP3 inflammasome in human sebocytes. J Invest Dermatol. 2014;134:2747-2756.
11. Selway JL, Kurczab T, Kealey T, et al. Toll-like receptor 2 activation and comedogenesis: implications for the pathogenesis of acne. BMC Dermatol. 2013;13:10.
12. Jugeau S, Tenaud I, Knol AC, et al. Induction of toll-like receptors by Propionibacterium acnes. Br J Dermatol. 2005;153:1105-1113.
13. XOMA announces encouraging interim results from gevokizumab phase 2 study for moderate to severe acne vulgaris [press release]. Berkley, CA: XOMA Corporation; January 7, 2013. http://www.servier.com/content/xoma-announces-encouraging-interim-results-gevokizumab-phase-2-study-moderate-severe-acne. Accessed November 5, 2014.
14. Leemans JC, Cassel SL, Sutterwala FS. Sensing damage by the NLRP3 inflammasome. Immunol Rev. 2011;243:152-162.
15. Dispenza MC, Wolpert EB, Gilliland KL, et al. Systemic isotretinoin therapy normalizes exaggerated TLR-2-mediated innate immune responses in acne patients. J Invest Dermatol. 2012;132:2198-2205.
16. Zuliani T, Khammari A, Chaussy H, et al. Ex vivo demonstration of a synergistic effect of adapalene and benzoyl peroxide on inflammatory acne lesions. Exp Dermatol. 2011;20:850-853.
1. Kurokawa I, Danby FW, Ju Q, et al. New developments in our understanding of acne pathogenesis and treatment. Exp Dermatol. 2009;18:821-832.
2. Cunliffe WJ, Holland DB, Clark SM, et al. Comedogenesis: some aetiological, clinical and therapeutic strategies. Dermatology. 2003;206:11-16.
3. Bowe W, Kober M. Therapeutic update: acne. J Drugs Dermatol. 2014;13:235-238.
4. Jeremy AH, Holland DB, Roberts SG, et al. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003;121:20-27.
5. Kim J, Ochoa MT, Krutzik SR, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169:1535-1541.
6. Kistowska M, Gehrke S, Jankovic D, et al. IL-1beta drives inflammatory responses to Propionibacterium acnes in vitro and in vivo. J Invest Dermatol. 2014;134:677-685.
7. Contassot E, French LE. New insights into acne pathogenesis: Propionibacterium acnes activates the inflammasome. J Invest Dermatol. 2014;134:310-313.
8. Vowels BR, Yang S, Leyden JJ. Induction of proinflammatory cytokines by a soluble factor of Propionibacterium acnes: implications for chronic inflammatory acne. Infect Immun. 1995;63:3158-3165.
9. Qin M, Pirouz A, Kim MH, et al. Propionibacterium acnes induces IL-1beta secretion via the NLRP3 inflammasome in human monocytes. J Invest Dermatol. 2014;134:381-388.
10. Li ZJ, Choi DK, Sohn KC, et al. Propionibacterium acnes activates the NLRP3 inflammasome in human sebocytes. J Invest Dermatol. 2014;134:2747-2756.
11. Selway JL, Kurczab T, Kealey T, et al. Toll-like receptor 2 activation and comedogenesis: implications for the pathogenesis of acne. BMC Dermatol. 2013;13:10.
12. Jugeau S, Tenaud I, Knol AC, et al. Induction of toll-like receptors by Propionibacterium acnes. Br J Dermatol. 2005;153:1105-1113.
13. XOMA announces encouraging interim results from gevokizumab phase 2 study for moderate to severe acne vulgaris [press release]. Berkley, CA: XOMA Corporation; January 7, 2013. http://www.servier.com/content/xoma-announces-encouraging-interim-results-gevokizumab-phase-2-study-moderate-severe-acne. Accessed November 5, 2014.
14. Leemans JC, Cassel SL, Sutterwala FS. Sensing damage by the NLRP3 inflammasome. Immunol Rev. 2011;243:152-162.
15. Dispenza MC, Wolpert EB, Gilliland KL, et al. Systemic isotretinoin therapy normalizes exaggerated TLR-2-mediated innate immune responses in acne patients. J Invest Dermatol. 2012;132:2198-2205.
16. Zuliani T, Khammari A, Chaussy H, et al. Ex vivo demonstration of a synergistic effect of adapalene and benzoyl peroxide on inflammatory acne lesions. Exp Dermatol. 2011;20:850-853.