User login
Building Tailored Resource Guides to Address Social Risks and Advance Health Equity in the Veterans Health Administration
Social risk factors and social needs have significant, often cumulative, impacts on health outcomes and are closely tied to health inequities. Defined as the individual-level adverse social conditions associated with poor health, social risk factors broadly include experiences such as food insecurity and housing instability; whereas the term social needs incorporates a person’s perceptions of and priorities related to their health-related needs.1 One recent study examining data from the Veterans Health Administration (VHA) found a 27% higher odds of mortality with each additional identified social risk, underscoring the critical link between social risks and veteran health outcomes.2
Assessing Circumstances and Offering Resources for Needs (ACORN), a collaborative quality improvement initiative conducted in partnership with the VHA Office of Health Equity and VHA National Social Work Program, Care Management and Social Work Services, is a social risk screening and referral program that aims to systematically identify and address unmet social needs among veterans to improve health and advance health equity.3,4 ACORN consists of 2 components: (1) a veteran-tailored screener to identify social risks within 9 domains; and (2) provision of relevant VA and community resources and referrals to address identified needs.3,5 Veterans who screen positive for ≥ 1 need receive referrals to a social worker or other relevant services, such as nutrition and food services or mental health, support navigating resources, and/or geographically tailored resource guides. This article describes the development and use of resource guides as a cross-cutting intervention component to address unmet social needs in diverse clinical settings and shares lessons learned from implementation in VHA outpatient clinics.
BACKGROUND
Unequal distribution of resources combined with historical discriminatory policies and practices, often linked to institutionalized racism, create inequities that lead to health disparities and hinder advancements in population health.6,7 Although health care systems alone cannot eliminate all health inequities, they can implement programs to identify social risks and address individual-level needs as 1 component of the multilevel approach needed to achieve health equity.8
As a national health care system serving > 9 million veterans, the VHA is well positioned to address social needs as an essential part of health. The VHA routinely screens for certain social risks, including housing instability, food insecurity, and intimate partner violence, and has a robust system of supports to address these and other needs among veterans, such as supportive housing services, vocational rehabilitation, assistance for justice-involved veterans, technology access support, and peer-support services.9-11 However, the VHA lacks a systematic approach to broader screening for social risks.
To address this gap, ACORN was developed in 2018 by an advisory board of subject matter experts, including clinical leaders, clinical psychologists, social workers, and health services researchers with content expertise in social risks and social needs.3 This interprofessional team sought to develop a veteran-tailored screener and resource referral initiative that could be scaled efficiently across VHA clinical settings.
Although health care organizations are increasingly implementing screening and interventions for social risks within clinical care, best practices and evidence-based tools to support clinical staff in these efforts are limited.12 Resource guides—curated lists of supportive services and organizations—may serve as a scalable “low-touch” intervention to help clinical staff address needs either alone or with more intensive interventions, such as social worker case management or patient navigation services.13
RESOURCE GUIDES—A Cross-Cutting Tool
The VHA has a uniquely robust network of nearly 18,000 social workers with clinical expertise in identifying, comprehensively assessing, and addressing social risks and needs among veterans. Interprofessional patient aligned care teams (PACTs)—a patient-centered medical home initiative that includes embedding social workers into primary care teams—facilitate the VHA’s capacity to address both medical and social needs.14 Social workers in PACTs and other care settings provide in-depth assessment and case management services to veterans who have a range of complex social needs. However, despite these comprehensive social services, in the setting of universal screening with a tool such as ACORN, it may not be feasible or practical to refer all patients who screen positive to a social worker for immediate follow-up, particularly in settings with capacity or resource limitations. For example, rates of screening positive on ACORN for ≥ 1 social risk have ranged from 48% of veterans in primary care sites and 80% in social work sites to nearly 100% in a PACT clinic for veterans experiencing homelessness.15
Additionally, a key challenge in the design of social needs interventions is determining how to optimize intervention intensity based on individual patient needs, acuity, and preferences. A substantial proportion of individuals who screen positive for social risks decline offered assistance, such as referrals.16 Resource guides are a cross-cutting tool that can be offered to veterans across a variety of settings, including primary care, specialty clinics, or emergency departments, as either a standalone intervention or one provided in combination with other resources or services. For patients who may not be interested in or feel comfortable accepting assistance at the time of screening or for those who prefer to research and navigate resources on their own, tailored resource guides can serve as a lower touch intervention to ensure interprofessional clinical staff across a range of settings and specialties have accessible, reliable, and up-to-date information to give to patients at the point of clinical care.17
Resource guides also can be used with higher touch clinical social work interventions, such as crisis management, supportive counseling, and case management. For example, social workers can use resource guides to provide education on VHA or community resources during clinical encounters with veterans and/or provide the guides to veterans to reference for future needs. Resource guides can further be used as a tool to support community resource navigation provided by nonclinical staff, such as peer specialists or community health workers.
How to BUILD RESOURCE GUIDES
Our team created resource guides (Figure) to provide veterans with concise, geographically tailored lists of VHA and other federal, state, and community services for the social risk domains included on the ACORN screener. To inform and develop a framework for building and maintaining ACORN guides, we first reviewed existing models that use this approach, including Boston Medical Center's WE CARE (Well Child Care, Evaluation, Community Resources, Advocacy, Referral, Education) and Thrive programs. We provide an overview of our process, which can be applied to clinical settings both within and outside the VHA.18,19
Partnerships
Active collaboration with frontline clinical social workers and local social work leadership is a critical part of identifying and prioritizing quality resources. Equipped with the knowledge of the local resource landscape, social workers can provide recommendations pertaining to national or federal, state, and local programs that have a history of being responsive to patients’ expressed needs.20 VHA social workers have robust knowledge of the veteran-specific resources available at VHA medical centers and nationally, and their clinical training equips them with the expertise to provide guidance about which resources to prioritize for inclusion in the guides.20
After receiving initial guidance from clinical social workers, our team began outreach to compile detailed program information, gauge program serviceability, and build relationships with both VHA and community-based services. Aligning with programs that share a similar mission in addressing social needs has proved crucial when developing the resource guides. Beyond ensuring the accuracy of program information, regular contact provides an opportunity to address capacity and workflow concerns that may arise from increased referrals. Additionally, open lines of communication with various supportive services facilitate connections with additional organizations and resources within the area.
The value of these relationships was evident at the onset of the COVID-19 pandemic, when the ACORN resource guides in use by our clinical site partners required frequent modifications to reflect rapid changes in services (eg, closures, transition to fully virtual programs, social distancing and masking requirements). Having established connections with community organizations was essential to navigating the evolving landscape of available programs and supports.
Curating Quality Resources
ACORN currently screens for social risks in 9 domains: food, housing, utilities, transportation, education, employment, legal, social isolation/loneliness, and digital needs. Each resource guide pertains to a specific social risk domain and associated question(s) in the screener, allowing staff to quickly identify which guides a veteran may benefit from based on their screening responses. The guides are meant to be short and comprehensive but not exhaustive lists of programs and services. We limit the length of the guides to one single-sided page to provide high-yield, geographically tailored resources in an easy-to-use format. The guides should reflect the geographic area served by the VHA medical center or the community-based outpatient clinic (CBOC) where a veteran receives clinical care, but they also may include national- and state-level resources that provide services and programs to veterans.
Although there is local variation in the availability and accessibility of services across social risk domains, some domains have an abundance of resources and organizations at federal or national, state, and/or community levels. To narrow the list of resources to the highest yield programs, we developed a series of questions that serve as selection criteria to inform resource inclusion (Table).
Because the resource guides are intended to be broadly applicable to a large number of veterans, we prioritize generalizable resources over those with narrow eligibility criteria and/or services. When more intensive support is needed, social workers and other VHA clinical and nonclinical staff can supplement the resources on the guides with additional, more tailored resources that are based on individual factors, such as physical residence, income, transportation access, or household composition (eg, veteran families with children or older adults).
Formatting Resource Guides
Along with relevant information, such as the program name, location, and a specific point of contact, brief program descriptions provide information about services offered, eligibility criteria, application requirements, alternate contacts and locations, and website links. At the bottom of each guide, a section is included with the name and direct contact information for a social worker (often the individual assigned to the clinic where the veteran completed the ACORN screener) or another VHA staff member who can be reached for further assistance. These staff members are familiar with the content included on the guides and provide veterans with additional information or higher touch support as necessary. This contact information is useful for veterans who initially decline assistance or referrals but later want to follow up with staff for support or questions.
Visual consistency is a key feature of the ACORN resource guides, and layout and design elements are used to maximize space and enhance usability. Corresponding font colors for all program titles, contact information, and website links assist in visually separating and drawing attention to pertinent contact information. QR codes linked to program websites also are incorporated for veterans to easily access resource information from their smartphone or other electronic device.
Maintaining Resource Guides
To ensure continued accuracy, resource guides are updated about every 6 months to reflect changes in closures, transitions to virtual/in-person services, changes in location, new points of contact, and modifications to services or eligibility requirements. Notations also are made if any changes to services or eligibility are temporary or permanent. Recording these temporary adjustments was critical early in the COVID-19 pandemic as service offerings, eligibility requirements, and application processes changed often.
Updating the guides also facilitates continuous relationship building and connections with VHA and community-based services. Resource guides are living documents: they maintain lines of communication with designated contacts, allow for opportunities to improve the presentation of evolving program information in the guides, and offer the chance to learn about additional programs in the area that may meet veterans' needs.
Creating a Manual for ACORN Resource Guide Development
To facilitate dissemination of ACORN across VHA clinical settings and locations, our team developed a Resource Guide Manual to aid ACORN clinical sites in developing resource guides.21 The manual provides step-by-step guidance from recommendations for identifying resources to formatting and layout considerations. Supplemental materials include a checklist to ensure each program description includes the necessary information for veterans to successfully access the resource, as well as page templates and style suggestions to maximize usability. These templates standardize formatting across the social risk domain guides and include options for electronic and paper distribution.
RESOURCE GUIDE LIMITATIONS
The labor involved in building and maintaining multiple guides is considerable and requires a time investment both upfront and long term, which may not be feasible for clinical sites with limited staff. However, many VHA social workers maintain lists of resource and referral services for veterans as part of their routine clinical case management. These lists can serve as a valuable and timesaving starting point in curating high-yield resources for formal resource guides. To further reduce the time needed to develop guides, sites can use ACORN resource guide templates rather than designing and formatting guides from scratch. In addition to informing veterans of relevant services and programs, resource guides also can be provided to new staff, such as social workers or peer specialists, during onboarding to help familiarize them with available services to address veterans’ unmet needs.
Resources included on the guides also are geographically tailored, based on the physical location of the VHA medical center or CBOC. Some community-based services listed may not be as relevant, accessible, available, or convenient to veterans who live far from the clinic, which is relevant for nearly 25% of veterans who live in rural communities.22 This is a circumstance in which the expertise of VHA social workers should be used to recommend more appropriately tailored resources to a veteran. Use of free, publicly available electronic resource databases (eg, 211 Helpline Center) also can provide social workers and patients with an overview of all available resources within their community. There are paid referral platform services that health systems can contract with as well.23 However, the potential drawbacks to these alternative platforms include high startup costs or costly user-license fees for medical centers or clinics, inconsistent updates to resource information, and lack of compatibility with some electronic health record systems.23
Resource guides are not intended to take the place of a clinical social worker or other health professional but rather to serve in a supplemental capacity to clinical services. Certain circumstances necessitate a more comprehensive clinical assessment and/or a warm handoff to a social worker, including assistance with urgent food or housing needs, and ACORN workflows are created with urgent needs pathways in mind. Determining how to optimize intervention intensity based on individual patients’ expressed needs, preferences, and acuity remains a challenge for health care organizations conducting social risk screening.12 While distribution of geographically tailored resource guides can be a useful low touch intervention for some veterans, others will require more intensive case management to address or meet their needs. Some veterans also may fall in the middle of this spectrum, where a resource guide is not enough but intensive case management services facilitated by social workers are not needed or wanted by the patient. Integration of peer specialists, patient navigators, or community health workers who can work with veterans to support them in identifying, connecting with, and receiving support from relevant programs may help fill this gap. Given their knowledge and lived experience, these professionals also can promote patient-centered care as part of the health care team.
CONCLUSIONS
Whether used as a low-touch, standalone intervention or in combination with higher touch services (eg, case management or resource navigation), resource guides are a valuable tool for health care organizations working to address social needs as a component of efforts to advance health equity, reduce health disparities, and promote population health. We provide a pragmatic framework for developing and maintaining resource guides used in the ACORN initiative. However, additional work is needed to optimize the design, content, and format of resource guides for both usability and effectiveness as a social risk intervention across health care settings.
Acknowledgments
We express our gratitude for the Veterans Health Administration (VHA) Office of Health Equity and the VHA National Social Work Program, Care Management and Social Work Services for their support of the Assessing Circumstances and Offering Resources for Needs (ACORN) initiative. We also express our appreciation for those who supported the initial screener development as part of the ACORN Advisory Board, including Stacey Curran, BA; Charles Drebing, PhD; J. Stewart Evans, MD, MSc; Edward Federman, PhD; Maneesha Gulati, LICSW, ACSW; Nancy Kressin, PhD; Kenneth Link, LICSW; Monica Sharma, MD; and Jacqueline Spencer, MD, MPH. We also express our appreciation for those who supported the initial ACORN resource guide development, including Chuck Drebing, PhD, Ed Federman, PhD, and Ken Link, LICSW, and for the clinical care team members, especially the social workers and nurses, at our ACORN partner sites as well as the community-based partners who have helped us develop comprehensive resource guides for veterans. This work was supported by funding from the VHA Office of Health Equity and by resources and use of facilities at the VA Bedford Healthcare System, VA New England Healthcare System, and VA Providence Healthcare System. Alicia J. Cohen was additionally supported by a VA HSR&D Career Development Award (CDA 20-037).
1. Alderwick H, Gottlieb LM. Meanings and misunderstandings: a social determinants of health lexicon for health care systems. Milbank Q. 2019;97(2):407-419. doi:10.1111/1468-0009.12390
2. Blosnich JM, Montgomery AE, Taylor LD, Dichter ME. Adverse social factors and all-cause mortality among male and female patients receiving care in the Veterans Health Administration. Prev Med. 2020;141:106272. doi:10.1016/j.ypmed.2020.106272
3. Russell LE, Cohen AJ, Chrzas S, et al. Implementing a social needs screening and referral program among veterans: Assessing Circumstances & Offering Resources for Needs (ACORN). J Gen Intern Med. 2023;38(13):2906-2913. doi:10.1007/s11606-023-08181-9
4. Cohen AJ, Russell LE, Elwy AR, et al. Adaptation of a social risk screening and referral initiative across clinical populations, settings, and contexts in the Department of Veterans Affairs Health System. Front Health Serv. 2023;2. doi:10.3389/frhs.2022.958969
5. Cohen AJ, Kennedy MA, Mitchell KM, Russell LE. The Assessing Circumstances & Offering Resources for Needs (ACORN) initiative. Updated September 2022. Accessed December 4, 2023. https://www.va.gov/HEALTHEQUITY/docs/ACORN_Screening_Tool.pdf
6. Jones CP. Levels of racism: a theoretic framework and a gardener’s tale. Am J Public Health. 2000;90(8):1212-1215. doi:10.2105/ajph.90.8.1212
7. American Public Health Association. Creating the healthiest nation: advancing health equity. Accessed November 28, 2023. https://www.apha.org/-/media/files/pdf/factsheets/advancing_health_equity.ashx?la=en&hash=9144021FDA33B4E7E02447CB28CA3F9D4BE5EF18
8. Castrucci B, Auerbach J. Meeting individual social needs falls short of addressing social determinants of health. Health Aff. Published January 16, 2019. doi:10.1377/hblog20190115.234942
9. Montgomery AE, Fargo JD, Byrne TH, Kane VR, Culhane DP. Universal screening for homelessness and risk for homelessness in the Veterans Health Administration. Am J Public Health. 2013;103(suppl 2):S210-211. doi:10.2105/AJPH.2013.301398
10. Cohen AJ, Rudolph JL, Thomas KS, et al. Food insecurity among veterans: resources to screen and intervene. Fed Pract. 2020;37(1):16-23.
11. Iverson KM, Adjognon O, Grillo AR, et al. Intimate partner violence screening programs in the Veterans Health Administration: informing scale-up of successful practices. J Gen Intern Med. 2019;34(11):2435-2442. doi:10.1007/s11606-019-05240-y
12. National Academies of Sciences, Engineering, and Medicine. Integrating Social Care into the Delivery of Health Care: Moving Upstream to Improve the Nation’s Health. The National Academies Press; 2019. Accessed November 28, 2023. https://nap.nationalacademies.org/catalog/25467/integrating-social-care-into-the-delivery-of-health-care-moving
13. Gottlieb LM, Adler NE, Wing H, et al. Effects of in-person assistance vs personalized written resources about social services on household social risks and child and caregiver health: a randomized clinical trial. JAMA Netw Open. 2020;3(3):e200701. doi:10.1001/jamanetworkopen.2020.0701
14. Cornell PY, Halladay CW, Ader J, et al. Embedding social workers in Veterans Health Administration primary care teams reduces emergency department visits. Health Aff (Millwood). 2020;39(4):603-612. doi:10.1377/hlthaff.2019.01589
15. Cohen AJ, Bruton M, Hooshyar D. US Department of Veterans Affairs, Office of Health Services Research and Development. The WHO’s greatest ICD-10 hits for fiscal year 2022: social determinants of health. Published March 9, 2022. Updated November 6, 2023. Accessed December 4, 2023. https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/video_archive.cfm?SessionID=4125

16. De Marchis EH, Alderwick H, Gottlieb LM. Do patients want help addressing social risks? J Am Board Fam Med. 2020;33(2):170-175. doi:10.3122/jabfm.2020.02.190309
17. Cohen AJ, Isaacson N, Torby M, Smith A, Zhang G, Patel MR. Motivators, barriers, and preferences to engagement with offered social care assistance among people with diabetes: a mixed methods study. Am J Prev Med. 2022;63(3, suppl 2):S152-S163. doi:10.1016/j.amepre.2022.02.022
18. Buitron de la Vega P, Losi S, Sprague Martinez L, et al. Implementing an EHR-based screening and referral system to address social determinants of health in primary care. Med Care. 2019;57(suppl 6, suppl 2):S133-S139. doi:10.1097/MLR.0000000000001029
19. Boston Medical Center. The WE CARE Model. Accessed November 28, 2023. https://www.bmc.org/pediatrics-primary-care/we-care/we-care-model
20. US Department of Veterans Affairs, Office of Rural Health. VA social work. Updated July 11, 2023. Accessed December 4, 2023. https://www.socialwork.va.gov
21. Mitchell KM, Russell LE, Cohen AJ, Kennedy MA. Building ACORN resource guides for veterans. Accessed November 28, 2023. https://www.va.gov/HEALTHEQUITY/docs/ACORN_Resource_Guide_Manual.pdf
22. US Department of Veterans Affairs, Veterans Health Administration, Office of Rural Health. Rural Veterans. Accessed November 28, 2023. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp
23. Cartier Y, Fichtenberg C, Gottlieb L. Community resource referral platforms: a guide for health care organizations. Published 2019. Accessed December 4, 2023. https://sirenetwork.ucsf.edu/tools-resources/resources/community-resource-referral-platforms-guide-health-care-organizations
Social risk factors and social needs have significant, often cumulative, impacts on health outcomes and are closely tied to health inequities. Defined as the individual-level adverse social conditions associated with poor health, social risk factors broadly include experiences such as food insecurity and housing instability; whereas the term social needs incorporates a person’s perceptions of and priorities related to their health-related needs.1 One recent study examining data from the Veterans Health Administration (VHA) found a 27% higher odds of mortality with each additional identified social risk, underscoring the critical link between social risks and veteran health outcomes.2
Assessing Circumstances and Offering Resources for Needs (ACORN), a collaborative quality improvement initiative conducted in partnership with the VHA Office of Health Equity and VHA National Social Work Program, Care Management and Social Work Services, is a social risk screening and referral program that aims to systematically identify and address unmet social needs among veterans to improve health and advance health equity.3,4 ACORN consists of 2 components: (1) a veteran-tailored screener to identify social risks within 9 domains; and (2) provision of relevant VA and community resources and referrals to address identified needs.3,5 Veterans who screen positive for ≥ 1 need receive referrals to a social worker or other relevant services, such as nutrition and food services or mental health, support navigating resources, and/or geographically tailored resource guides. This article describes the development and use of resource guides as a cross-cutting intervention component to address unmet social needs in diverse clinical settings and shares lessons learned from implementation in VHA outpatient clinics.
BACKGROUND
Unequal distribution of resources combined with historical discriminatory policies and practices, often linked to institutionalized racism, create inequities that lead to health disparities and hinder advancements in population health.6,7 Although health care systems alone cannot eliminate all health inequities, they can implement programs to identify social risks and address individual-level needs as 1 component of the multilevel approach needed to achieve health equity.8
As a national health care system serving > 9 million veterans, the VHA is well positioned to address social needs as an essential part of health. The VHA routinely screens for certain social risks, including housing instability, food insecurity, and intimate partner violence, and has a robust system of supports to address these and other needs among veterans, such as supportive housing services, vocational rehabilitation, assistance for justice-involved veterans, technology access support, and peer-support services.9-11 However, the VHA lacks a systematic approach to broader screening for social risks.
To address this gap, ACORN was developed in 2018 by an advisory board of subject matter experts, including clinical leaders, clinical psychologists, social workers, and health services researchers with content expertise in social risks and social needs.3 This interprofessional team sought to develop a veteran-tailored screener and resource referral initiative that could be scaled efficiently across VHA clinical settings.
Although health care organizations are increasingly implementing screening and interventions for social risks within clinical care, best practices and evidence-based tools to support clinical staff in these efforts are limited.12 Resource guides—curated lists of supportive services and organizations—may serve as a scalable “low-touch” intervention to help clinical staff address needs either alone or with more intensive interventions, such as social worker case management or patient navigation services.13
RESOURCE GUIDES—A Cross-Cutting Tool
The VHA has a uniquely robust network of nearly 18,000 social workers with clinical expertise in identifying, comprehensively assessing, and addressing social risks and needs among veterans. Interprofessional patient aligned care teams (PACTs)—a patient-centered medical home initiative that includes embedding social workers into primary care teams—facilitate the VHA’s capacity to address both medical and social needs.14 Social workers in PACTs and other care settings provide in-depth assessment and case management services to veterans who have a range of complex social needs. However, despite these comprehensive social services, in the setting of universal screening with a tool such as ACORN, it may not be feasible or practical to refer all patients who screen positive to a social worker for immediate follow-up, particularly in settings with capacity or resource limitations. For example, rates of screening positive on ACORN for ≥ 1 social risk have ranged from 48% of veterans in primary care sites and 80% in social work sites to nearly 100% in a PACT clinic for veterans experiencing homelessness.15
Additionally, a key challenge in the design of social needs interventions is determining how to optimize intervention intensity based on individual patient needs, acuity, and preferences. A substantial proportion of individuals who screen positive for social risks decline offered assistance, such as referrals.16 Resource guides are a cross-cutting tool that can be offered to veterans across a variety of settings, including primary care, specialty clinics, or emergency departments, as either a standalone intervention or one provided in combination with other resources or services. For patients who may not be interested in or feel comfortable accepting assistance at the time of screening or for those who prefer to research and navigate resources on their own, tailored resource guides can serve as a lower touch intervention to ensure interprofessional clinical staff across a range of settings and specialties have accessible, reliable, and up-to-date information to give to patients at the point of clinical care.17
Resource guides also can be used with higher touch clinical social work interventions, such as crisis management, supportive counseling, and case management. For example, social workers can use resource guides to provide education on VHA or community resources during clinical encounters with veterans and/or provide the guides to veterans to reference for future needs. Resource guides can further be used as a tool to support community resource navigation provided by nonclinical staff, such as peer specialists or community health workers.
How to BUILD RESOURCE GUIDES
Our team created resource guides (Figure) to provide veterans with concise, geographically tailored lists of VHA and other federal, state, and community services for the social risk domains included on the ACORN screener. To inform and develop a framework for building and maintaining ACORN guides, we first reviewed existing models that use this approach, including Boston Medical Center's WE CARE (Well Child Care, Evaluation, Community Resources, Advocacy, Referral, Education) and Thrive programs. We provide an overview of our process, which can be applied to clinical settings both within and outside the VHA.18,19
Partnerships
Active collaboration with frontline clinical social workers and local social work leadership is a critical part of identifying and prioritizing quality resources. Equipped with the knowledge of the local resource landscape, social workers can provide recommendations pertaining to national or federal, state, and local programs that have a history of being responsive to patients’ expressed needs.20 VHA social workers have robust knowledge of the veteran-specific resources available at VHA medical centers and nationally, and their clinical training equips them with the expertise to provide guidance about which resources to prioritize for inclusion in the guides.20
After receiving initial guidance from clinical social workers, our team began outreach to compile detailed program information, gauge program serviceability, and build relationships with both VHA and community-based services. Aligning with programs that share a similar mission in addressing social needs has proved crucial when developing the resource guides. Beyond ensuring the accuracy of program information, regular contact provides an opportunity to address capacity and workflow concerns that may arise from increased referrals. Additionally, open lines of communication with various supportive services facilitate connections with additional organizations and resources within the area.
The value of these relationships was evident at the onset of the COVID-19 pandemic, when the ACORN resource guides in use by our clinical site partners required frequent modifications to reflect rapid changes in services (eg, closures, transition to fully virtual programs, social distancing and masking requirements). Having established connections with community organizations was essential to navigating the evolving landscape of available programs and supports.
Curating Quality Resources
ACORN currently screens for social risks in 9 domains: food, housing, utilities, transportation, education, employment, legal, social isolation/loneliness, and digital needs. Each resource guide pertains to a specific social risk domain and associated question(s) in the screener, allowing staff to quickly identify which guides a veteran may benefit from based on their screening responses. The guides are meant to be short and comprehensive but not exhaustive lists of programs and services. We limit the length of the guides to one single-sided page to provide high-yield, geographically tailored resources in an easy-to-use format. The guides should reflect the geographic area served by the VHA medical center or the community-based outpatient clinic (CBOC) where a veteran receives clinical care, but they also may include national- and state-level resources that provide services and programs to veterans.
Although there is local variation in the availability and accessibility of services across social risk domains, some domains have an abundance of resources and organizations at federal or national, state, and/or community levels. To narrow the list of resources to the highest yield programs, we developed a series of questions that serve as selection criteria to inform resource inclusion (Table).
Because the resource guides are intended to be broadly applicable to a large number of veterans, we prioritize generalizable resources over those with narrow eligibility criteria and/or services. When more intensive support is needed, social workers and other VHA clinical and nonclinical staff can supplement the resources on the guides with additional, more tailored resources that are based on individual factors, such as physical residence, income, transportation access, or household composition (eg, veteran families with children or older adults).
Formatting Resource Guides
Along with relevant information, such as the program name, location, and a specific point of contact, brief program descriptions provide information about services offered, eligibility criteria, application requirements, alternate contacts and locations, and website links. At the bottom of each guide, a section is included with the name and direct contact information for a social worker (often the individual assigned to the clinic where the veteran completed the ACORN screener) or another VHA staff member who can be reached for further assistance. These staff members are familiar with the content included on the guides and provide veterans with additional information or higher touch support as necessary. This contact information is useful for veterans who initially decline assistance or referrals but later want to follow up with staff for support or questions.
Visual consistency is a key feature of the ACORN resource guides, and layout and design elements are used to maximize space and enhance usability. Corresponding font colors for all program titles, contact information, and website links assist in visually separating and drawing attention to pertinent contact information. QR codes linked to program websites also are incorporated for veterans to easily access resource information from their smartphone or other electronic device.
Maintaining Resource Guides
To ensure continued accuracy, resource guides are updated about every 6 months to reflect changes in closures, transitions to virtual/in-person services, changes in location, new points of contact, and modifications to services or eligibility requirements. Notations also are made if any changes to services or eligibility are temporary or permanent. Recording these temporary adjustments was critical early in the COVID-19 pandemic as service offerings, eligibility requirements, and application processes changed often.
Updating the guides also facilitates continuous relationship building and connections with VHA and community-based services. Resource guides are living documents: they maintain lines of communication with designated contacts, allow for opportunities to improve the presentation of evolving program information in the guides, and offer the chance to learn about additional programs in the area that may meet veterans' needs.
Creating a Manual for ACORN Resource Guide Development
To facilitate dissemination of ACORN across VHA clinical settings and locations, our team developed a Resource Guide Manual to aid ACORN clinical sites in developing resource guides.21 The manual provides step-by-step guidance from recommendations for identifying resources to formatting and layout considerations. Supplemental materials include a checklist to ensure each program description includes the necessary information for veterans to successfully access the resource, as well as page templates and style suggestions to maximize usability. These templates standardize formatting across the social risk domain guides and include options for electronic and paper distribution.
RESOURCE GUIDE LIMITATIONS
The labor involved in building and maintaining multiple guides is considerable and requires a time investment both upfront and long term, which may not be feasible for clinical sites with limited staff. However, many VHA social workers maintain lists of resource and referral services for veterans as part of their routine clinical case management. These lists can serve as a valuable and timesaving starting point in curating high-yield resources for formal resource guides. To further reduce the time needed to develop guides, sites can use ACORN resource guide templates rather than designing and formatting guides from scratch. In addition to informing veterans of relevant services and programs, resource guides also can be provided to new staff, such as social workers or peer specialists, during onboarding to help familiarize them with available services to address veterans’ unmet needs.
Resources included on the guides also are geographically tailored, based on the physical location of the VHA medical center or CBOC. Some community-based services listed may not be as relevant, accessible, available, or convenient to veterans who live far from the clinic, which is relevant for nearly 25% of veterans who live in rural communities.22 This is a circumstance in which the expertise of VHA social workers should be used to recommend more appropriately tailored resources to a veteran. Use of free, publicly available electronic resource databases (eg, 211 Helpline Center) also can provide social workers and patients with an overview of all available resources within their community. There are paid referral platform services that health systems can contract with as well.23 However, the potential drawbacks to these alternative platforms include high startup costs or costly user-license fees for medical centers or clinics, inconsistent updates to resource information, and lack of compatibility with some electronic health record systems.23
Resource guides are not intended to take the place of a clinical social worker or other health professional but rather to serve in a supplemental capacity to clinical services. Certain circumstances necessitate a more comprehensive clinical assessment and/or a warm handoff to a social worker, including assistance with urgent food or housing needs, and ACORN workflows are created with urgent needs pathways in mind. Determining how to optimize intervention intensity based on individual patients’ expressed needs, preferences, and acuity remains a challenge for health care organizations conducting social risk screening.12 While distribution of geographically tailored resource guides can be a useful low touch intervention for some veterans, others will require more intensive case management to address or meet their needs. Some veterans also may fall in the middle of this spectrum, where a resource guide is not enough but intensive case management services facilitated by social workers are not needed or wanted by the patient. Integration of peer specialists, patient navigators, or community health workers who can work with veterans to support them in identifying, connecting with, and receiving support from relevant programs may help fill this gap. Given their knowledge and lived experience, these professionals also can promote patient-centered care as part of the health care team.
CONCLUSIONS
Whether used as a low-touch, standalone intervention or in combination with higher touch services (eg, case management or resource navigation), resource guides are a valuable tool for health care organizations working to address social needs as a component of efforts to advance health equity, reduce health disparities, and promote population health. We provide a pragmatic framework for developing and maintaining resource guides used in the ACORN initiative. However, additional work is needed to optimize the design, content, and format of resource guides for both usability and effectiveness as a social risk intervention across health care settings.
Acknowledgments
We express our gratitude for the Veterans Health Administration (VHA) Office of Health Equity and the VHA National Social Work Program, Care Management and Social Work Services for their support of the Assessing Circumstances and Offering Resources for Needs (ACORN) initiative. We also express our appreciation for those who supported the initial screener development as part of the ACORN Advisory Board, including Stacey Curran, BA; Charles Drebing, PhD; J. Stewart Evans, MD, MSc; Edward Federman, PhD; Maneesha Gulati, LICSW, ACSW; Nancy Kressin, PhD; Kenneth Link, LICSW; Monica Sharma, MD; and Jacqueline Spencer, MD, MPH. We also express our appreciation for those who supported the initial ACORN resource guide development, including Chuck Drebing, PhD, Ed Federman, PhD, and Ken Link, LICSW, and for the clinical care team members, especially the social workers and nurses, at our ACORN partner sites as well as the community-based partners who have helped us develop comprehensive resource guides for veterans. This work was supported by funding from the VHA Office of Health Equity and by resources and use of facilities at the VA Bedford Healthcare System, VA New England Healthcare System, and VA Providence Healthcare System. Alicia J. Cohen was additionally supported by a VA HSR&D Career Development Award (CDA 20-037).
Social risk factors and social needs have significant, often cumulative, impacts on health outcomes and are closely tied to health inequities. Defined as the individual-level adverse social conditions associated with poor health, social risk factors broadly include experiences such as food insecurity and housing instability; whereas the term social needs incorporates a person’s perceptions of and priorities related to their health-related needs.1 One recent study examining data from the Veterans Health Administration (VHA) found a 27% higher odds of mortality with each additional identified social risk, underscoring the critical link between social risks and veteran health outcomes.2
Assessing Circumstances and Offering Resources for Needs (ACORN), a collaborative quality improvement initiative conducted in partnership with the VHA Office of Health Equity and VHA National Social Work Program, Care Management and Social Work Services, is a social risk screening and referral program that aims to systematically identify and address unmet social needs among veterans to improve health and advance health equity.3,4 ACORN consists of 2 components: (1) a veteran-tailored screener to identify social risks within 9 domains; and (2) provision of relevant VA and community resources and referrals to address identified needs.3,5 Veterans who screen positive for ≥ 1 need receive referrals to a social worker or other relevant services, such as nutrition and food services or mental health, support navigating resources, and/or geographically tailored resource guides. This article describes the development and use of resource guides as a cross-cutting intervention component to address unmet social needs in diverse clinical settings and shares lessons learned from implementation in VHA outpatient clinics.
BACKGROUND
Unequal distribution of resources combined with historical discriminatory policies and practices, often linked to institutionalized racism, create inequities that lead to health disparities and hinder advancements in population health.6,7 Although health care systems alone cannot eliminate all health inequities, they can implement programs to identify social risks and address individual-level needs as 1 component of the multilevel approach needed to achieve health equity.8
As a national health care system serving > 9 million veterans, the VHA is well positioned to address social needs as an essential part of health. The VHA routinely screens for certain social risks, including housing instability, food insecurity, and intimate partner violence, and has a robust system of supports to address these and other needs among veterans, such as supportive housing services, vocational rehabilitation, assistance for justice-involved veterans, technology access support, and peer-support services.9-11 However, the VHA lacks a systematic approach to broader screening for social risks.
To address this gap, ACORN was developed in 2018 by an advisory board of subject matter experts, including clinical leaders, clinical psychologists, social workers, and health services researchers with content expertise in social risks and social needs.3 This interprofessional team sought to develop a veteran-tailored screener and resource referral initiative that could be scaled efficiently across VHA clinical settings.
Although health care organizations are increasingly implementing screening and interventions for social risks within clinical care, best practices and evidence-based tools to support clinical staff in these efforts are limited.12 Resource guides—curated lists of supportive services and organizations—may serve as a scalable “low-touch” intervention to help clinical staff address needs either alone or with more intensive interventions, such as social worker case management or patient navigation services.13
RESOURCE GUIDES—A Cross-Cutting Tool
The VHA has a uniquely robust network of nearly 18,000 social workers with clinical expertise in identifying, comprehensively assessing, and addressing social risks and needs among veterans. Interprofessional patient aligned care teams (PACTs)—a patient-centered medical home initiative that includes embedding social workers into primary care teams—facilitate the VHA’s capacity to address both medical and social needs.14 Social workers in PACTs and other care settings provide in-depth assessment and case management services to veterans who have a range of complex social needs. However, despite these comprehensive social services, in the setting of universal screening with a tool such as ACORN, it may not be feasible or practical to refer all patients who screen positive to a social worker for immediate follow-up, particularly in settings with capacity or resource limitations. For example, rates of screening positive on ACORN for ≥ 1 social risk have ranged from 48% of veterans in primary care sites and 80% in social work sites to nearly 100% in a PACT clinic for veterans experiencing homelessness.15
Additionally, a key challenge in the design of social needs interventions is determining how to optimize intervention intensity based on individual patient needs, acuity, and preferences. A substantial proportion of individuals who screen positive for social risks decline offered assistance, such as referrals.16 Resource guides are a cross-cutting tool that can be offered to veterans across a variety of settings, including primary care, specialty clinics, or emergency departments, as either a standalone intervention or one provided in combination with other resources or services. For patients who may not be interested in or feel comfortable accepting assistance at the time of screening or for those who prefer to research and navigate resources on their own, tailored resource guides can serve as a lower touch intervention to ensure interprofessional clinical staff across a range of settings and specialties have accessible, reliable, and up-to-date information to give to patients at the point of clinical care.17
Resource guides also can be used with higher touch clinical social work interventions, such as crisis management, supportive counseling, and case management. For example, social workers can use resource guides to provide education on VHA or community resources during clinical encounters with veterans and/or provide the guides to veterans to reference for future needs. Resource guides can further be used as a tool to support community resource navigation provided by nonclinical staff, such as peer specialists or community health workers.
How to BUILD RESOURCE GUIDES
Our team created resource guides (Figure) to provide veterans with concise, geographically tailored lists of VHA and other federal, state, and community services for the social risk domains included on the ACORN screener. To inform and develop a framework for building and maintaining ACORN guides, we first reviewed existing models that use this approach, including Boston Medical Center's WE CARE (Well Child Care, Evaluation, Community Resources, Advocacy, Referral, Education) and Thrive programs. We provide an overview of our process, which can be applied to clinical settings both within and outside the VHA.18,19
Partnerships
Active collaboration with frontline clinical social workers and local social work leadership is a critical part of identifying and prioritizing quality resources. Equipped with the knowledge of the local resource landscape, social workers can provide recommendations pertaining to national or federal, state, and local programs that have a history of being responsive to patients’ expressed needs.20 VHA social workers have robust knowledge of the veteran-specific resources available at VHA medical centers and nationally, and their clinical training equips them with the expertise to provide guidance about which resources to prioritize for inclusion in the guides.20
After receiving initial guidance from clinical social workers, our team began outreach to compile detailed program information, gauge program serviceability, and build relationships with both VHA and community-based services. Aligning with programs that share a similar mission in addressing social needs has proved crucial when developing the resource guides. Beyond ensuring the accuracy of program information, regular contact provides an opportunity to address capacity and workflow concerns that may arise from increased referrals. Additionally, open lines of communication with various supportive services facilitate connections with additional organizations and resources within the area.
The value of these relationships was evident at the onset of the COVID-19 pandemic, when the ACORN resource guides in use by our clinical site partners required frequent modifications to reflect rapid changes in services (eg, closures, transition to fully virtual programs, social distancing and masking requirements). Having established connections with community organizations was essential to navigating the evolving landscape of available programs and supports.
Curating Quality Resources
ACORN currently screens for social risks in 9 domains: food, housing, utilities, transportation, education, employment, legal, social isolation/loneliness, and digital needs. Each resource guide pertains to a specific social risk domain and associated question(s) in the screener, allowing staff to quickly identify which guides a veteran may benefit from based on their screening responses. The guides are meant to be short and comprehensive but not exhaustive lists of programs and services. We limit the length of the guides to one single-sided page to provide high-yield, geographically tailored resources in an easy-to-use format. The guides should reflect the geographic area served by the VHA medical center or the community-based outpatient clinic (CBOC) where a veteran receives clinical care, but they also may include national- and state-level resources that provide services and programs to veterans.
Although there is local variation in the availability and accessibility of services across social risk domains, some domains have an abundance of resources and organizations at federal or national, state, and/or community levels. To narrow the list of resources to the highest yield programs, we developed a series of questions that serve as selection criteria to inform resource inclusion (Table).
Because the resource guides are intended to be broadly applicable to a large number of veterans, we prioritize generalizable resources over those with narrow eligibility criteria and/or services. When more intensive support is needed, social workers and other VHA clinical and nonclinical staff can supplement the resources on the guides with additional, more tailored resources that are based on individual factors, such as physical residence, income, transportation access, or household composition (eg, veteran families with children or older adults).
Formatting Resource Guides
Along with relevant information, such as the program name, location, and a specific point of contact, brief program descriptions provide information about services offered, eligibility criteria, application requirements, alternate contacts and locations, and website links. At the bottom of each guide, a section is included with the name and direct contact information for a social worker (often the individual assigned to the clinic where the veteran completed the ACORN screener) or another VHA staff member who can be reached for further assistance. These staff members are familiar with the content included on the guides and provide veterans with additional information or higher touch support as necessary. This contact information is useful for veterans who initially decline assistance or referrals but later want to follow up with staff for support or questions.
Visual consistency is a key feature of the ACORN resource guides, and layout and design elements are used to maximize space and enhance usability. Corresponding font colors for all program titles, contact information, and website links assist in visually separating and drawing attention to pertinent contact information. QR codes linked to program websites also are incorporated for veterans to easily access resource information from their smartphone or other electronic device.
Maintaining Resource Guides
To ensure continued accuracy, resource guides are updated about every 6 months to reflect changes in closures, transitions to virtual/in-person services, changes in location, new points of contact, and modifications to services or eligibility requirements. Notations also are made if any changes to services or eligibility are temporary or permanent. Recording these temporary adjustments was critical early in the COVID-19 pandemic as service offerings, eligibility requirements, and application processes changed often.
Updating the guides also facilitates continuous relationship building and connections with VHA and community-based services. Resource guides are living documents: they maintain lines of communication with designated contacts, allow for opportunities to improve the presentation of evolving program information in the guides, and offer the chance to learn about additional programs in the area that may meet veterans' needs.
Creating a Manual for ACORN Resource Guide Development
To facilitate dissemination of ACORN across VHA clinical settings and locations, our team developed a Resource Guide Manual to aid ACORN clinical sites in developing resource guides.21 The manual provides step-by-step guidance from recommendations for identifying resources to formatting and layout considerations. Supplemental materials include a checklist to ensure each program description includes the necessary information for veterans to successfully access the resource, as well as page templates and style suggestions to maximize usability. These templates standardize formatting across the social risk domain guides and include options for electronic and paper distribution.
RESOURCE GUIDE LIMITATIONS
The labor involved in building and maintaining multiple guides is considerable and requires a time investment both upfront and long term, which may not be feasible for clinical sites with limited staff. However, many VHA social workers maintain lists of resource and referral services for veterans as part of their routine clinical case management. These lists can serve as a valuable and timesaving starting point in curating high-yield resources for formal resource guides. To further reduce the time needed to develop guides, sites can use ACORN resource guide templates rather than designing and formatting guides from scratch. In addition to informing veterans of relevant services and programs, resource guides also can be provided to new staff, such as social workers or peer specialists, during onboarding to help familiarize them with available services to address veterans’ unmet needs.
Resources included on the guides also are geographically tailored, based on the physical location of the VHA medical center or CBOC. Some community-based services listed may not be as relevant, accessible, available, or convenient to veterans who live far from the clinic, which is relevant for nearly 25% of veterans who live in rural communities.22 This is a circumstance in which the expertise of VHA social workers should be used to recommend more appropriately tailored resources to a veteran. Use of free, publicly available electronic resource databases (eg, 211 Helpline Center) also can provide social workers and patients with an overview of all available resources within their community. There are paid referral platform services that health systems can contract with as well.23 However, the potential drawbacks to these alternative platforms include high startup costs or costly user-license fees for medical centers or clinics, inconsistent updates to resource information, and lack of compatibility with some electronic health record systems.23
Resource guides are not intended to take the place of a clinical social worker or other health professional but rather to serve in a supplemental capacity to clinical services. Certain circumstances necessitate a more comprehensive clinical assessment and/or a warm handoff to a social worker, including assistance with urgent food or housing needs, and ACORN workflows are created with urgent needs pathways in mind. Determining how to optimize intervention intensity based on individual patients’ expressed needs, preferences, and acuity remains a challenge for health care organizations conducting social risk screening.12 While distribution of geographically tailored resource guides can be a useful low touch intervention for some veterans, others will require more intensive case management to address or meet their needs. Some veterans also may fall in the middle of this spectrum, where a resource guide is not enough but intensive case management services facilitated by social workers are not needed or wanted by the patient. Integration of peer specialists, patient navigators, or community health workers who can work with veterans to support them in identifying, connecting with, and receiving support from relevant programs may help fill this gap. Given their knowledge and lived experience, these professionals also can promote patient-centered care as part of the health care team.
CONCLUSIONS
Whether used as a low-touch, standalone intervention or in combination with higher touch services (eg, case management or resource navigation), resource guides are a valuable tool for health care organizations working to address social needs as a component of efforts to advance health equity, reduce health disparities, and promote population health. We provide a pragmatic framework for developing and maintaining resource guides used in the ACORN initiative. However, additional work is needed to optimize the design, content, and format of resource guides for both usability and effectiveness as a social risk intervention across health care settings.
Acknowledgments
We express our gratitude for the Veterans Health Administration (VHA) Office of Health Equity and the VHA National Social Work Program, Care Management and Social Work Services for their support of the Assessing Circumstances and Offering Resources for Needs (ACORN) initiative. We also express our appreciation for those who supported the initial screener development as part of the ACORN Advisory Board, including Stacey Curran, BA; Charles Drebing, PhD; J. Stewart Evans, MD, MSc; Edward Federman, PhD; Maneesha Gulati, LICSW, ACSW; Nancy Kressin, PhD; Kenneth Link, LICSW; Monica Sharma, MD; and Jacqueline Spencer, MD, MPH. We also express our appreciation for those who supported the initial ACORN resource guide development, including Chuck Drebing, PhD, Ed Federman, PhD, and Ken Link, LICSW, and for the clinical care team members, especially the social workers and nurses, at our ACORN partner sites as well as the community-based partners who have helped us develop comprehensive resource guides for veterans. This work was supported by funding from the VHA Office of Health Equity and by resources and use of facilities at the VA Bedford Healthcare System, VA New England Healthcare System, and VA Providence Healthcare System. Alicia J. Cohen was additionally supported by a VA HSR&D Career Development Award (CDA 20-037).
1. Alderwick H, Gottlieb LM. Meanings and misunderstandings: a social determinants of health lexicon for health care systems. Milbank Q. 2019;97(2):407-419. doi:10.1111/1468-0009.12390
2. Blosnich JM, Montgomery AE, Taylor LD, Dichter ME. Adverse social factors and all-cause mortality among male and female patients receiving care in the Veterans Health Administration. Prev Med. 2020;141:106272. doi:10.1016/j.ypmed.2020.106272
3. Russell LE, Cohen AJ, Chrzas S, et al. Implementing a social needs screening and referral program among veterans: Assessing Circumstances & Offering Resources for Needs (ACORN). J Gen Intern Med. 2023;38(13):2906-2913. doi:10.1007/s11606-023-08181-9
4. Cohen AJ, Russell LE, Elwy AR, et al. Adaptation of a social risk screening and referral initiative across clinical populations, settings, and contexts in the Department of Veterans Affairs Health System. Front Health Serv. 2023;2. doi:10.3389/frhs.2022.958969
5. Cohen AJ, Kennedy MA, Mitchell KM, Russell LE. The Assessing Circumstances & Offering Resources for Needs (ACORN) initiative. Updated September 2022. Accessed December 4, 2023. https://www.va.gov/HEALTHEQUITY/docs/ACORN_Screening_Tool.pdf
6. Jones CP. Levels of racism: a theoretic framework and a gardener’s tale. Am J Public Health. 2000;90(8):1212-1215. doi:10.2105/ajph.90.8.1212
7. American Public Health Association. Creating the healthiest nation: advancing health equity. Accessed November 28, 2023. https://www.apha.org/-/media/files/pdf/factsheets/advancing_health_equity.ashx?la=en&hash=9144021FDA33B4E7E02447CB28CA3F9D4BE5EF18
8. Castrucci B, Auerbach J. Meeting individual social needs falls short of addressing social determinants of health. Health Aff. Published January 16, 2019. doi:10.1377/hblog20190115.234942
9. Montgomery AE, Fargo JD, Byrne TH, Kane VR, Culhane DP. Universal screening for homelessness and risk for homelessness in the Veterans Health Administration. Am J Public Health. 2013;103(suppl 2):S210-211. doi:10.2105/AJPH.2013.301398
10. Cohen AJ, Rudolph JL, Thomas KS, et al. Food insecurity among veterans: resources to screen and intervene. Fed Pract. 2020;37(1):16-23.
11. Iverson KM, Adjognon O, Grillo AR, et al. Intimate partner violence screening programs in the Veterans Health Administration: informing scale-up of successful practices. J Gen Intern Med. 2019;34(11):2435-2442. doi:10.1007/s11606-019-05240-y
12. National Academies of Sciences, Engineering, and Medicine. Integrating Social Care into the Delivery of Health Care: Moving Upstream to Improve the Nation’s Health. The National Academies Press; 2019. Accessed November 28, 2023. https://nap.nationalacademies.org/catalog/25467/integrating-social-care-into-the-delivery-of-health-care-moving
13. Gottlieb LM, Adler NE, Wing H, et al. Effects of in-person assistance vs personalized written resources about social services on household social risks and child and caregiver health: a randomized clinical trial. JAMA Netw Open. 2020;3(3):e200701. doi:10.1001/jamanetworkopen.2020.0701
14. Cornell PY, Halladay CW, Ader J, et al. Embedding social workers in Veterans Health Administration primary care teams reduces emergency department visits. Health Aff (Millwood). 2020;39(4):603-612. doi:10.1377/hlthaff.2019.01589
15. Cohen AJ, Bruton M, Hooshyar D. US Department of Veterans Affairs, Office of Health Services Research and Development. The WHO’s greatest ICD-10 hits for fiscal year 2022: social determinants of health. Published March 9, 2022. Updated November 6, 2023. Accessed December 4, 2023. https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/video_archive.cfm?SessionID=4125

16. De Marchis EH, Alderwick H, Gottlieb LM. Do patients want help addressing social risks? J Am Board Fam Med. 2020;33(2):170-175. doi:10.3122/jabfm.2020.02.190309
17. Cohen AJ, Isaacson N, Torby M, Smith A, Zhang G, Patel MR. Motivators, barriers, and preferences to engagement with offered social care assistance among people with diabetes: a mixed methods study. Am J Prev Med. 2022;63(3, suppl 2):S152-S163. doi:10.1016/j.amepre.2022.02.022
18. Buitron de la Vega P, Losi S, Sprague Martinez L, et al. Implementing an EHR-based screening and referral system to address social determinants of health in primary care. Med Care. 2019;57(suppl 6, suppl 2):S133-S139. doi:10.1097/MLR.0000000000001029
19. Boston Medical Center. The WE CARE Model. Accessed November 28, 2023. https://www.bmc.org/pediatrics-primary-care/we-care/we-care-model
20. US Department of Veterans Affairs, Office of Rural Health. VA social work. Updated July 11, 2023. Accessed December 4, 2023. https://www.socialwork.va.gov
21. Mitchell KM, Russell LE, Cohen AJ, Kennedy MA. Building ACORN resource guides for veterans. Accessed November 28, 2023. https://www.va.gov/HEALTHEQUITY/docs/ACORN_Resource_Guide_Manual.pdf
22. US Department of Veterans Affairs, Veterans Health Administration, Office of Rural Health. Rural Veterans. Accessed November 28, 2023. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp
23. Cartier Y, Fichtenberg C, Gottlieb L. Community resource referral platforms: a guide for health care organizations. Published 2019. Accessed December 4, 2023. https://sirenetwork.ucsf.edu/tools-resources/resources/community-resource-referral-platforms-guide-health-care-organizations
1. Alderwick H, Gottlieb LM. Meanings and misunderstandings: a social determinants of health lexicon for health care systems. Milbank Q. 2019;97(2):407-419. doi:10.1111/1468-0009.12390
2. Blosnich JM, Montgomery AE, Taylor LD, Dichter ME. Adverse social factors and all-cause mortality among male and female patients receiving care in the Veterans Health Administration. Prev Med. 2020;141:106272. doi:10.1016/j.ypmed.2020.106272
3. Russell LE, Cohen AJ, Chrzas S, et al. Implementing a social needs screening and referral program among veterans: Assessing Circumstances & Offering Resources for Needs (ACORN). J Gen Intern Med. 2023;38(13):2906-2913. doi:10.1007/s11606-023-08181-9
4. Cohen AJ, Russell LE, Elwy AR, et al. Adaptation of a social risk screening and referral initiative across clinical populations, settings, and contexts in the Department of Veterans Affairs Health System. Front Health Serv. 2023;2. doi:10.3389/frhs.2022.958969
5. Cohen AJ, Kennedy MA, Mitchell KM, Russell LE. The Assessing Circumstances & Offering Resources for Needs (ACORN) initiative. Updated September 2022. Accessed December 4, 2023. https://www.va.gov/HEALTHEQUITY/docs/ACORN_Screening_Tool.pdf
6. Jones CP. Levels of racism: a theoretic framework and a gardener’s tale. Am J Public Health. 2000;90(8):1212-1215. doi:10.2105/ajph.90.8.1212
7. American Public Health Association. Creating the healthiest nation: advancing health equity. Accessed November 28, 2023. https://www.apha.org/-/media/files/pdf/factsheets/advancing_health_equity.ashx?la=en&hash=9144021FDA33B4E7E02447CB28CA3F9D4BE5EF18
8. Castrucci B, Auerbach J. Meeting individual social needs falls short of addressing social determinants of health. Health Aff. Published January 16, 2019. doi:10.1377/hblog20190115.234942
9. Montgomery AE, Fargo JD, Byrne TH, Kane VR, Culhane DP. Universal screening for homelessness and risk for homelessness in the Veterans Health Administration. Am J Public Health. 2013;103(suppl 2):S210-211. doi:10.2105/AJPH.2013.301398
10. Cohen AJ, Rudolph JL, Thomas KS, et al. Food insecurity among veterans: resources to screen and intervene. Fed Pract. 2020;37(1):16-23.
11. Iverson KM, Adjognon O, Grillo AR, et al. Intimate partner violence screening programs in the Veterans Health Administration: informing scale-up of successful practices. J Gen Intern Med. 2019;34(11):2435-2442. doi:10.1007/s11606-019-05240-y
12. National Academies of Sciences, Engineering, and Medicine. Integrating Social Care into the Delivery of Health Care: Moving Upstream to Improve the Nation’s Health. The National Academies Press; 2019. Accessed November 28, 2023. https://nap.nationalacademies.org/catalog/25467/integrating-social-care-into-the-delivery-of-health-care-moving
13. Gottlieb LM, Adler NE, Wing H, et al. Effects of in-person assistance vs personalized written resources about social services on household social risks and child and caregiver health: a randomized clinical trial. JAMA Netw Open. 2020;3(3):e200701. doi:10.1001/jamanetworkopen.2020.0701
14. Cornell PY, Halladay CW, Ader J, et al. Embedding social workers in Veterans Health Administration primary care teams reduces emergency department visits. Health Aff (Millwood). 2020;39(4):603-612. doi:10.1377/hlthaff.2019.01589
15. Cohen AJ, Bruton M, Hooshyar D. US Department of Veterans Affairs, Office of Health Services Research and Development. The WHO’s greatest ICD-10 hits for fiscal year 2022: social determinants of health. Published March 9, 2022. Updated November 6, 2023. Accessed December 4, 2023. https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/video_archive.cfm?SessionID=4125

16. De Marchis EH, Alderwick H, Gottlieb LM. Do patients want help addressing social risks? J Am Board Fam Med. 2020;33(2):170-175. doi:10.3122/jabfm.2020.02.190309
17. Cohen AJ, Isaacson N, Torby M, Smith A, Zhang G, Patel MR. Motivators, barriers, and preferences to engagement with offered social care assistance among people with diabetes: a mixed methods study. Am J Prev Med. 2022;63(3, suppl 2):S152-S163. doi:10.1016/j.amepre.2022.02.022
18. Buitron de la Vega P, Losi S, Sprague Martinez L, et al. Implementing an EHR-based screening and referral system to address social determinants of health in primary care. Med Care. 2019;57(suppl 6, suppl 2):S133-S139. doi:10.1097/MLR.0000000000001029
19. Boston Medical Center. The WE CARE Model. Accessed November 28, 2023. https://www.bmc.org/pediatrics-primary-care/we-care/we-care-model
20. US Department of Veterans Affairs, Office of Rural Health. VA social work. Updated July 11, 2023. Accessed December 4, 2023. https://www.socialwork.va.gov
21. Mitchell KM, Russell LE, Cohen AJ, Kennedy MA. Building ACORN resource guides for veterans. Accessed November 28, 2023. https://www.va.gov/HEALTHEQUITY/docs/ACORN_Resource_Guide_Manual.pdf
22. US Department of Veterans Affairs, Veterans Health Administration, Office of Rural Health. Rural Veterans. Accessed November 28, 2023. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp
23. Cartier Y, Fichtenberg C, Gottlieb L. Community resource referral platforms: a guide for health care organizations. Published 2019. Accessed December 4, 2023. https://sirenetwork.ucsf.edu/tools-resources/resources/community-resource-referral-platforms-guide-health-care-organizations
Enhancing Diabetes Self-Management Education and Psychological Services for Veterans With Comorbid Chronic Health and Mental Health Conditions
Veterans have a higher prevalence of type 2 diabetes mellitus (T2DM) when compared with their civilian counterparts with an overall prevalence rate of 25%.1 This higher prevalence is similar to other major chronic health conditions, including heart disease and arthritis, with additional costs for disease self-management.2 Psychological and behavioral change strategies are a principal means of limiting the severity and even restoring function once T2DM is diagnosed.3 More broadly, there is mounting evidence that addressing distress and behavior change are important across many conditions, particularly T2DM.4 Therefore, the US Department of Veterans Affairs (VA) has established patient education and multidisciplinary interventions to optimize engagement in T2DM self-management and health behavior change.5
Traditional T2DM education programs aim to meet the American Diabetes Association (ADA) standards of medical care and include a T2DM educator and other allied health professionals. ADA Standard 1.2 emphasizes “productive interactions between a prepared, proactive care team and an informed, activated patient.”6 Thus, to attain ADA accreditation, educational programs require instructors to teach about T2DM while engaging patients to help them set and achieve recommended changes. The requirements emphasize setting specific goals, (ie, eating wisely, being physically active, monitoring blood sugars or taking medications). The care team also helps to identify barriers, and at a required follow-up class, patients evaluate how well they met goals and make modifications if needed. The impact of traditional patient education programs to improve glycemic levels is well established.7 Importantly, veterans with comorbid mental health conditions may not experience the same beneficial outcomes if or when they participate in traditional diabetes or self-management programs.8,9 Veterans with T2DM may be particularly vulnerable to chronic stress and effects of comorbid mental health diagnoses.10 Furthermore, when individuals experience T2DM-related distress, associations with poor health outcomes, including elevated hemoglobin A1c (HbA1c), are observed independent of depression.11
Health psychology services integrate into medical settings and strive to reach veterans who may not engage in traditional mental health clinical offerings.12 These collaborative interventions focus less on diagnostic or screening procedures and more on a patient’s understanding of illness and ability and willingness to carry out treatment regimens. Given the significant roles of distress and co-occurring conditions, health psychology services further aim to provide psychoeducation about stress management in order to explore and enhance motivation for making a wide range of health behavior changes.
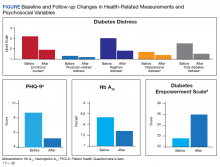
The purpose of this study was to evaluate baseline and follow-up HbA1c, weight, and psychosocial measures, namely, health-related self-efficacy and T2DM-related distress among a small sample that engaged in integrated health psychology services. The focus of this evidence-based psychotherapy service was to improve T2DM self-care and physical health. The participants were offered cognitive and behavioral strategies for setting and meeting personalized T2DM self-management goals. Importantly, motivational interviewing was used throughout to adapt to the participants’ preferences and needs as well as to maintain engagement.
Methods
Primary care providers referred veterans with T2DM to the Health Psychology service at VA Ann Arbor Healthcare System (VAAAHS). A T2DM diagnosis was verified through electronic health record review. Most common referrals included addressing coping with chronic illness and improving glycemic levels. Veterans were invited to participate in a program evaluation project to monitor health-related changes. All participants provided written informed consent and did not receive incentive or payment for participating. The VAAAHS Institutional Review Board reviewed and approved this study.
Intervention
Veterans met individually with a health psychologist or health psychology trainee to create personalized health and behavioral goals for improving T2DM self-management, overall health, and psychological well-being. This intervention included motivational interviewing, SMART (specific, measurable, action-oriented, realistic, timely) goal setting, behavioral activation, acceptance of T2DM-related physical changes, problem-solving therapy, challenging maladaptive disease-related cognitions, and incorporating values to help find motivation for change. Int
Data Collection
Participants completed study measures at the beginning and end of the T2DM-focused intervention sessions. Demographic variables collected included age, sex, race/ethnicity, highest educational attainment, and whether a veteran was prescribed insulin, service connected for T2DM, concurrent enrollment in other educational programs, and time since T2DM diagnosis. Measures were selected based on their relevance to T2DM psychosocial care and diabetes health outcomes.13
Body mass index, low-density lipoprotein cholesterol, blood pressure (BP), HbA1c within 3 months of the pre- and postmeasures were collected by reviewing medical records. T2DM complications were collected by self-report, and comorbid physical and mental health conditions were collected by review of the most recent primary care note. The Diabetes Empowerment Scale-Short Form (DES-SF) is a well-validated measure that was used to measure T2DM-related psychosocial self-efficacy.14 Scores ranged from 8 to 40 with higher scores indicating higher diabetes T2DM empowerment. The Patient Health Questionnaire 9-item (PHQ-9) was used to assess the frequency of somatic (fatigue, appetite, psychomotor) and cognitive symptoms (anhedonia, low mood) of depression over the past 2 weeks.15 The Generalized Anxiety Disorder 7-item (GAD-7) was used to assess the frequency of common anxiety symptoms, including feelings of worry, difficulty controlling worry, and trouble relaxing.16 Veterans were also asked to rate their general health on a 5-point Likert scale. Self-rated health is a well-established indicator of disability and risk of future T2DM complications in older adults.17,18 The Diabetes Distress Scale (DDS) was used to measure emotional burden, physician-related distress, regimen-related distress, and T2DM-related interpersonal distress.19 Scores > 2.0 suggest clinical significant diabetes distress.20 Medication questionnaires were adapted from Wilson and colleagues, 2013.21
Statistical Analyses
Descriptive statistics, including mean and standard deviation (SD) or frequency distributions, as appropriate, were used to characterize the sample. For pre- and postintervention within-group comparisons, a paired samples Student t test analysis was used to evaluate baseline and follow-up measures for statistically significant differences between continuous variables; scores also were evaluated for clinically meaningful change.
Results
This sample (N = 13) of older adults was predominately male, white, with HbA1c > 7.0, and prescribed insulin (Table). On average, participants were at higher risk for future complications due to high BP, hyperlipidemia, and BMI > 30.0. Regarding participation, veterans were seen for an average of 7.8 sessions (range, 4-13) with 46% service connected for T2DM. Of note, 4 veterans received other T2DM-specific self-management support within the same year of their participation with health psychology, such as attending a T2DM education class or T2DM shared medical appointment.22 Reliability in the current sample for the DES-SF was high (Cronbach α = 0.90), PHQ-9 was good (Cronbach α = 0.81), and GAD-7 was very good (Cronbach α = 0.86).
Among the 13 older adults, the most common T2DM-related complications included peripheral neuropathy (n = 7), heart pain or heart attack (n = 5), and retinopathy (n = 4). Recent primary care notes showed a mean (SD) 7 (2.2) comorbid chronic medical conditions with a high prevalence of cardiometabolic illnesses including hypertension, hyperlipidemia, obstructive sleep apnea, and a diagnosis of chronic pain. Eleven veterans were diagnosed with a mental health condition, including bipolar disorder, depression, anxiety, trauma-related disorder, and sleep disorders. Veterans reported high T2DM emotional distress (mean [SD] 3.1 [1.2]), moderate regimen-related distress (mean [SD] 2.9 [1.1]), and moderate total T2DM distress (mean [SD] 2.4 [0.7]). Physician distress (mean [SD] 1.3 [0.55]) and interpersonal T2DM distress (mean [SD] 1.6 [0.9]) subscales indicated little to no distress. The sample reported mild symptoms of depression (PHQ-9 mean [SD] 8.8 [4.6]); mild symptoms of anxiety (GAD-7 mean, 7.1; SD, 4.4), and Diabetes Empowerment (mean, 31.2; SD, 6.0). Participants described missing an average of 2.4 days within the past 30 days of their T2DM oral medications.
Twelve veterans (92.7%) completed the Follow-up questionnaires. The Figure illustrates statistically significant changes in patient-reported outcomes between baseline and follow-up. Clinically meaningful reductions were shown in total T2DM distress (t11 = 5.03, P < .01), T2DM emotional burden (t11 = 4.83, P = .01), and T2DM regimen-related distress (t11 = 5.14, P < .01). There was a significant increase in T2DM self-efficacy (t11 = 0.32, P = .008) as well. A statistically significant reduction was seen in depressive symptoms (t11 = 2.22, P = .048). While HbA1c fell by .56 percentage points (standard error of the mean [SEM], 31; P = .10), this change was not statistically significant. Follow-up analyses also showed a clinically, though not statistically, significant reduction in weight loss by 6.9 lb. (SEM, 3.8; P = .20), and reductions of generalized anxiety by 1.2 points (SEM, 1.4; P = .42). Pre- and postanalyses did not show differences among self-rated health, physician-related burden, interpersonal-related burden, and indicators of medication taking behavior.
Discussion
This observational study evaluated change among patient-reported T2DM-specific and general distress measures and health outcomes among a small sample of veterans at VAAAHS medical center that engaged in an episode of individual care with health psychology. Statistically significant decreases were observed in T2DM-related distress. Noteworthy, these decreases were observed for the emotional burden and regimen subscales, and each of these was clinically meaningful, falling below a score of 2.0 on the T2DM-specific scale. This is important given that T2DM distress may interfere with the ability to understand and find motivation for engaging in health behavior change. Incorporating stress management interventions into interdisciplinary health programs has been demonstrated to improve not only levels of distress, but also other health outcomes, such as health related quality of life and cardiac events in heart disease.23 Thus, behavioral health interventions that incorporate cognitive-behavioral strategies to enhance distress-specific coping may prove important to include among individuals with T2DM.
Reductions in T2DM-related distress also converged with increases observed in the T2DM empowerment scale. These significant improvements in perceived ability suggest increased self-efficacy and willingness to follow a daily T2DM regimen. This finding aligns with the social support literature that demonstrates how instrumental and other aspects of autonomous social support mediate improvements in health-related outcomes and reduced T2DM distress.24,25 Health psychology interventions strive to both provide social support as well as enhance participants’ perceptions and use of existing support as a cognitive-behavioral strategy. Adding in assessments of social support could shed light on such mediating factors.
The ADA standards of care encourage heath care providers to engage patients in conversations in order to better understand the barriers of T2DM self-care.13 How to best support patients within a primary care multidisciplinary team remains unclear.26 T2DM distress and negative reactions to T2DM, including symptoms of anxiety and depression, are common and may require specific referral to a mental health provider if repeated attempts at T2DM education do not improve self-management and illness biomarkers.27 Thus, integrating these providers and services within the medical setting aims to reach more veterans and potentially meet these standards of care. With our health psychology integrated services, clinically significant decreases in anxiety and statistically significant decreases in depressive symptoms were observed that approached “mild to no” symptoms. Although this was not measured formally, the veterans were not engaging in mental health specialty care historically or during the year of the health psychology intervention. This suggests that health psychology services helped bridge the gap and address these psychosocial needs within the small sample.
For clinical measures, modest decreases were observed for HbA1c and weight. The authors recognize that these changes may not be optimal in terms of health status. A review of the specific patient-centered goals may illuminate this finding. For example, 1 participant had a goal to consume fewer sugary beverages and achieved this behavior change. Yet this change alone may not equate to actual weight loss or a lower HbA1c. Furthermore, in the context of T2DM-related distress, maintaining current weight and/or blood sugar levels may be a more realistic goal. An evaluation of the specific patient-oriented action goals and observed progress may be important outcomes to include in larger studies. Moreover, while not significant, the average HbA1c decrease of about 1% is comparable with traditional T2DM education and should be considered in light of the sample’s significant mental health comorbidities. While landmark intensive glucose control trials illustrate significant benefits in reductions of hyperglycemia and nonfatal cardiovascular disease, these reductions are associated with an approximate 2-fold risk of hypoglycemia.28-30 Thus, the focus on improved glycemic control has been criticized as lacking meaning to patients in contrast to preventing T2DM complications and persevering quality of life.31
Limitations and Future Directions
Noted limitations include small sample size, the range of time, and a broad number of sessions given that the intervention was tailored to each veteran. Conclusions drawn from a small sample may be influenced by individual outliers. Given co-occurring conditions and moderate levels of distress, all participants may benefit from additional support resources.
In addition to these considerations, having a comparison group could further strengthen the study as part of an observational database. A between-group comparison could help clinicians better understand what the interventions offer as well as some individual factors that relate to participation and success with behavior change. In the future, studies with a priori hypotheses could also consider the trajectories of weight and blood sugar levels for extended periods; for example, 6 months before the intervention and 6 months following.32 Given the complexity of comorbid mental health and chronic medical conditions in this sample, it also may be important to measure the relationships between chronic physical symptoms as an additional barrier for veterans to make health behavior changes.
Conclusions
The authors believe that the health psychology interventions offered important support and motivation for engagement in health behavior change that led to reduced distress in this patient group. It remains a challenge to engage veterans with psychiatric conditions in mental health care, and simultaneously for health care systems that strive to reduce costs and complications associated with chronic illness management.33 Aligned with these broader health care goals, the ADA aims to reduce complications and cost and improve outcomes for T2DM with guidelines requiring mental and behavioral health interventions. The authors believe that health psychology interventions are a personalized and feasible bridge to address engagement, illness-related distress while improving patient-satisfaction and T2DM self-management.
Acknowledgments
The authors thank the veterans who participated in the observational study. We thank the VA Ann Arbor Healthcare System Institutional Review Board. For instrumental support for health psychology integrated services, we acknowledge Adam Tremblay, MD, Primary Care Chief, and R.J. Schildhouse, MD, Acting Associate Chief of Staff, Ambulatory Care. The work was supported by the Ambulatory Care Service at the VA Ann Arbor Healthcare System and the VA Office of Academic Affiliations.
1. Liu Y, Sayam S, Shao X, et al. Prevalence of and trends in diabetes among veterans, United States, 2005-2014. Prev Chronic Dis. 2017;14(12):E135, 1-5. doi:10.5888/pcd14.170230
2. Yu W, Ravelo A, Wagner TH, et al. Prevalence and costs of chronic conditions in the VA health care system. Med Care Res Rev. 2003;60(3)(suppl):146S-167S. doi:10.1177/1077558703257000
3. American Psychological Association. Psychology and Health in Action. Updated 2016. Accessed February 10, 2021. https://www.apa.org/health/fall-2016-updates.pdf
4. The US Burden of Disease Collaborators. The state of US health, 1990-2016. JAMA. 2018;319(14):1444-1472. doi:10.1001/jama.2018.0158
5. Piette JD, Kerr E, Richardson C, Heisler M. Veterans Affairs research on health information technologies for diabetes self-management support. J Diabetes Sci Technol. 2008;2(1):15-23. doi:10.1177/193229680800200104
6. American Diabetes Association. 1. Improving care and promoting health in populations: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S7-S12. doi:10.2337/dc19-S001
7. Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self-management education for adults with type 2 diabetes. A meta-analysis of the effect on glycemic control. Diabetes Care. 2002;25(7):1159-1171. doi:10.2337/diacare.25.7.1159
8. Janney CA, Owen R, Bowersox NW, Ratz D, Kilbourne EA. Bipolar disorder influences weight loss in the nationally implemented MOVE! program for veterans. Bipolar Disord. 2015;17:87.
9. Piette JD, Kerr EA. The impact of comorbid chronic conditions on diabetes care. Diabetes Care. 2006;29(3):725-731. doi:10.2337/diacare.29.03.06.dc05-2078
10. Trief PM, Ouimette P, Wade M, Shanahan P, Weinstock RS. Post-traumatic stress disorder and diabetes: Co-morbidity and outcomes in a male veterans sample. J Behav Med. 2006;29(5):411-418. doi:10.1007/s10865-006-9067-2
11. Fisher L, Mullan JT, Arean P, Glasgow RE, Hessler D, Masharani U. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care. 2010;33(1):23-28. doi:10.2337/dc09-1238
12. Bohnert KM, Pfeiffer PN, Szymanski BR, McCarthy JF. Continuation of care following an initial primary care visit with a mental health diagnosis: differences by receipt of VHA Primary Care-Mental Health Integration services. Gen Hosp Psychiatry. 2013;35(1):66-70. doi:10.1016/j.genhosppsych.2012.09.002
13. Young-Hyman D, De Groot M, Hill-Briggs F, Gonzalez JS, Hood K, Peyrot M. Psychosocial care for people with diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39(12):2126-2140. doi:10.2337/dc16-2053
14. Anderson R, Fitzgerald J, Gruppen L, Funnell M, Oh M. The diabetes empowerment scale-short form (DES-SF). Diabetes Care. 2003;26(5):1641-1642. doi:10.2337/diacare.26.5.1641-a
15. Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.doi:10.1046/j.1525-1497.2001.016009606.x
16. Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097. doi:10.1001/archinte.166.10.1092
17. Pinquart M. Correlates of subjective health in older adults: a meta-analysis. Psychol Aging. 2001;16(3):414. doi:10.1037/0882-7974.16.3.414
18. Hayes AJ, Clarke PM, Glasziou PG, Simes RJ, Drury PL, Keech AC. Can self-rated health scores be used for risk prediction in patients with type 2 diabetes? Diabetes Care. 2008;31(4):795-797. doi:10.2337/dc07-1391
19. Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28(3):626-631. doi:10.2337/diacare.28.3.626
20. Fisher L, Hessler DDM, Polonsky WH, Mullan J. When is diabetes distress meaningful?: Establishing cut points for the Diabetes Distress Scale. Diabetes Care. 2012;35(2):259-264. doi:10.2337/dc11-1572
21. Wilson IB, Fowler FJ Jr, Cosenza CA, et al. Cognitive and field testing of a new set of medication adherence self-report items for HIV care. AIDS Behav. 2013;18(12):2349-2358. doi:10.1007/s10461-013-0610-1
22. Heisler M, Burgess J, Cass J, et al. The Shared Health Appointments and Reciprocal Enhanced Support (SHARES) study: study protocol for a randomized trial. Trials. 2017;18(1):239. doi:10.1186/s13063-017-1959-7
23. Blumenthal JA, Babyak MA, Carney RM, et al. Exercise, depression, and mortality after myocardial infarction in the ENRICHD Trial. Med Sci Sports Exerc. 2004;36(5):746-755. doi:10.1249/01.MSS.0000125997.63493.13
24. Lee AA, Piette JD, Heisler M, Rosland AM. Diabetes distress and glycemic control: the buffering effect of autonomy support from important family members and friends. Diabetes Care. 2018;41(6):1157-1163. doi:10.2337/dc17-2396
25. Baek RN, Tanenbaum ML, Gonzalez JS. Diabetes burden and diabetes distress: the buffering effect of social support. Ann Behav Med. 2014;48(2):1-11.doi:10.1007/s12160-013-9585-4
26. Jortberg BT, Miller BF, Gabbay RA, Sparling K, Dickinson WP. Patient-centered medical home: how it affects psychosocial outcomes for diabetes. Curr Diab Rep. 2012;12(6):721-728. doi:10.1007/s11892-012-0316-1
27. American Diabetes Association. Lifestyle management: standards of medical care in diabetes-2019. Diabetes Care. 2019;41(suppl 1):S38-S50. doi:10.2337/dc19-S005
28. UK Prospective Diabetes Study Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes. Lancet. 1998;352(9131):854-865.
29. The Diabetes Control and Complications Trial Research Group, Control TD, Trial C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329(14):977-986. doi:10.1056/NEJM199309303291401
30. Kelly TN, Bazzano LA, Fonseca VA, Thethi TK, Reynolds K, He J. Systematic review: glucose control and cardiovascular disease in type 2 diabetes. Ann Intern Med. 2009;151(6):394-403. doi:10.1037/1072-5245.13.1.64
31. Yudkin JS, Lipska KJ, Montori VM. The idolatry of the surrogate. BMJ. 2012;344(7839):8-10. doi:10.1136/bmj.d7995
32. Lutes LD, Damschroder LJ, Masheb R, et al. Behavioral treatment for veterans with obesity: 24-month weight outcomes from the ASPIRE-VA Small Changes Randomized Trial. J Gen Intern Med. 2017;32(1):40-47. doi:10.1007/s11606-017-3987-0
33. Krejci LP, Carter K, Gaudet T. The vision and implementation of personalized, proactive, patient-driven health care for veterans. Med Care. 2014;52(12)(suppl 5):S5-S8. doi:10.1097/MLR.0000000000000226
Veterans have a higher prevalence of type 2 diabetes mellitus (T2DM) when compared with their civilian counterparts with an overall prevalence rate of 25%.1 This higher prevalence is similar to other major chronic health conditions, including heart disease and arthritis, with additional costs for disease self-management.2 Psychological and behavioral change strategies are a principal means of limiting the severity and even restoring function once T2DM is diagnosed.3 More broadly, there is mounting evidence that addressing distress and behavior change are important across many conditions, particularly T2DM.4 Therefore, the US Department of Veterans Affairs (VA) has established patient education and multidisciplinary interventions to optimize engagement in T2DM self-management and health behavior change.5
Traditional T2DM education programs aim to meet the American Diabetes Association (ADA) standards of medical care and include a T2DM educator and other allied health professionals. ADA Standard 1.2 emphasizes “productive interactions between a prepared, proactive care team and an informed, activated patient.”6 Thus, to attain ADA accreditation, educational programs require instructors to teach about T2DM while engaging patients to help them set and achieve recommended changes. The requirements emphasize setting specific goals, (ie, eating wisely, being physically active, monitoring blood sugars or taking medications). The care team also helps to identify barriers, and at a required follow-up class, patients evaluate how well they met goals and make modifications if needed. The impact of traditional patient education programs to improve glycemic levels is well established.7 Importantly, veterans with comorbid mental health conditions may not experience the same beneficial outcomes if or when they participate in traditional diabetes or self-management programs.8,9 Veterans with T2DM may be particularly vulnerable to chronic stress and effects of comorbid mental health diagnoses.10 Furthermore, when individuals experience T2DM-related distress, associations with poor health outcomes, including elevated hemoglobin A1c (HbA1c), are observed independent of depression.11
Health psychology services integrate into medical settings and strive to reach veterans who may not engage in traditional mental health clinical offerings.12 These collaborative interventions focus less on diagnostic or screening procedures and more on a patient’s understanding of illness and ability and willingness to carry out treatment regimens. Given the significant roles of distress and co-occurring conditions, health psychology services further aim to provide psychoeducation about stress management in order to explore and enhance motivation for making a wide range of health behavior changes.
The purpose of this study was to evaluate baseline and follow-up HbA1c, weight, and psychosocial measures, namely, health-related self-efficacy and T2DM-related distress among a small sample that engaged in integrated health psychology services. The focus of this evidence-based psychotherapy service was to improve T2DM self-care and physical health. The participants were offered cognitive and behavioral strategies for setting and meeting personalized T2DM self-management goals. Importantly, motivational interviewing was used throughout to adapt to the participants’ preferences and needs as well as to maintain engagement.
Methods
Primary care providers referred veterans with T2DM to the Health Psychology service at VA Ann Arbor Healthcare System (VAAAHS). A T2DM diagnosis was verified through electronic health record review. Most common referrals included addressing coping with chronic illness and improving glycemic levels. Veterans were invited to participate in a program evaluation project to monitor health-related changes. All participants provided written informed consent and did not receive incentive or payment for participating. The VAAAHS Institutional Review Board reviewed and approved this study.
Intervention
Veterans met individually with a health psychologist or health psychology trainee to create personalized health and behavioral goals for improving T2DM self-management, overall health, and psychological well-being. This intervention included motivational interviewing, SMART (specific, measurable, action-oriented, realistic, timely) goal setting, behavioral activation, acceptance of T2DM-related physical changes, problem-solving therapy, challenging maladaptive disease-related cognitions, and incorporating values to help find motivation for change. Int
Data Collection
Participants completed study measures at the beginning and end of the T2DM-focused intervention sessions. Demographic variables collected included age, sex, race/ethnicity, highest educational attainment, and whether a veteran was prescribed insulin, service connected for T2DM, concurrent enrollment in other educational programs, and time since T2DM diagnosis. Measures were selected based on their relevance to T2DM psychosocial care and diabetes health outcomes.13
Body mass index, low-density lipoprotein cholesterol, blood pressure (BP), HbA1c within 3 months of the pre- and postmeasures were collected by reviewing medical records. T2DM complications were collected by self-report, and comorbid physical and mental health conditions were collected by review of the most recent primary care note. The Diabetes Empowerment Scale-Short Form (DES-SF) is a well-validated measure that was used to measure T2DM-related psychosocial self-efficacy.14 Scores ranged from 8 to 40 with higher scores indicating higher diabetes T2DM empowerment. The Patient Health Questionnaire 9-item (PHQ-9) was used to assess the frequency of somatic (fatigue, appetite, psychomotor) and cognitive symptoms (anhedonia, low mood) of depression over the past 2 weeks.15 The Generalized Anxiety Disorder 7-item (GAD-7) was used to assess the frequency of common anxiety symptoms, including feelings of worry, difficulty controlling worry, and trouble relaxing.16 Veterans were also asked to rate their general health on a 5-point Likert scale. Self-rated health is a well-established indicator of disability and risk of future T2DM complications in older adults.17,18 The Diabetes Distress Scale (DDS) was used to measure emotional burden, physician-related distress, regimen-related distress, and T2DM-related interpersonal distress.19 Scores > 2.0 suggest clinical significant diabetes distress.20 Medication questionnaires were adapted from Wilson and colleagues, 2013.21
Statistical Analyses
Descriptive statistics, including mean and standard deviation (SD) or frequency distributions, as appropriate, were used to characterize the sample. For pre- and postintervention within-group comparisons, a paired samples Student t test analysis was used to evaluate baseline and follow-up measures for statistically significant differences between continuous variables; scores also were evaluated for clinically meaningful change.
Results
This sample (N = 13) of older adults was predominately male, white, with HbA1c > 7.0, and prescribed insulin (Table). On average, participants were at higher risk for future complications due to high BP, hyperlipidemia, and BMI > 30.0. Regarding participation, veterans were seen for an average of 7.8 sessions (range, 4-13) with 46% service connected for T2DM. Of note, 4 veterans received other T2DM-specific self-management support within the same year of their participation with health psychology, such as attending a T2DM education class or T2DM shared medical appointment.22 Reliability in the current sample for the DES-SF was high (Cronbach α = 0.90), PHQ-9 was good (Cronbach α = 0.81), and GAD-7 was very good (Cronbach α = 0.86).
Among the 13 older adults, the most common T2DM-related complications included peripheral neuropathy (n = 7), heart pain or heart attack (n = 5), and retinopathy (n = 4). Recent primary care notes showed a mean (SD) 7 (2.2) comorbid chronic medical conditions with a high prevalence of cardiometabolic illnesses including hypertension, hyperlipidemia, obstructive sleep apnea, and a diagnosis of chronic pain. Eleven veterans were diagnosed with a mental health condition, including bipolar disorder, depression, anxiety, trauma-related disorder, and sleep disorders. Veterans reported high T2DM emotional distress (mean [SD] 3.1 [1.2]), moderate regimen-related distress (mean [SD] 2.9 [1.1]), and moderate total T2DM distress (mean [SD] 2.4 [0.7]). Physician distress (mean [SD] 1.3 [0.55]) and interpersonal T2DM distress (mean [SD] 1.6 [0.9]) subscales indicated little to no distress. The sample reported mild symptoms of depression (PHQ-9 mean [SD] 8.8 [4.6]); mild symptoms of anxiety (GAD-7 mean, 7.1; SD, 4.4), and Diabetes Empowerment (mean, 31.2; SD, 6.0). Participants described missing an average of 2.4 days within the past 30 days of their T2DM oral medications.
Twelve veterans (92.7%) completed the Follow-up questionnaires. The Figure illustrates statistically significant changes in patient-reported outcomes between baseline and follow-up. Clinically meaningful reductions were shown in total T2DM distress (t11 = 5.03, P < .01), T2DM emotional burden (t11 = 4.83, P = .01), and T2DM regimen-related distress (t11 = 5.14, P < .01). There was a significant increase in T2DM self-efficacy (t11 = 0.32, P = .008) as well. A statistically significant reduction was seen in depressive symptoms (t11 = 2.22, P = .048). While HbA1c fell by .56 percentage points (standard error of the mean [SEM], 31; P = .10), this change was not statistically significant. Follow-up analyses also showed a clinically, though not statistically, significant reduction in weight loss by 6.9 lb. (SEM, 3.8; P = .20), and reductions of generalized anxiety by 1.2 points (SEM, 1.4; P = .42). Pre- and postanalyses did not show differences among self-rated health, physician-related burden, interpersonal-related burden, and indicators of medication taking behavior.
Discussion
This observational study evaluated change among patient-reported T2DM-specific and general distress measures and health outcomes among a small sample of veterans at VAAAHS medical center that engaged in an episode of individual care with health psychology. Statistically significant decreases were observed in T2DM-related distress. Noteworthy, these decreases were observed for the emotional burden and regimen subscales, and each of these was clinically meaningful, falling below a score of 2.0 on the T2DM-specific scale. This is important given that T2DM distress may interfere with the ability to understand and find motivation for engaging in health behavior change. Incorporating stress management interventions into interdisciplinary health programs has been demonstrated to improve not only levels of distress, but also other health outcomes, such as health related quality of life and cardiac events in heart disease.23 Thus, behavioral health interventions that incorporate cognitive-behavioral strategies to enhance distress-specific coping may prove important to include among individuals with T2DM.
Reductions in T2DM-related distress also converged with increases observed in the T2DM empowerment scale. These significant improvements in perceived ability suggest increased self-efficacy and willingness to follow a daily T2DM regimen. This finding aligns with the social support literature that demonstrates how instrumental and other aspects of autonomous social support mediate improvements in health-related outcomes and reduced T2DM distress.24,25 Health psychology interventions strive to both provide social support as well as enhance participants’ perceptions and use of existing support as a cognitive-behavioral strategy. Adding in assessments of social support could shed light on such mediating factors.
The ADA standards of care encourage heath care providers to engage patients in conversations in order to better understand the barriers of T2DM self-care.13 How to best support patients within a primary care multidisciplinary team remains unclear.26 T2DM distress and negative reactions to T2DM, including symptoms of anxiety and depression, are common and may require specific referral to a mental health provider if repeated attempts at T2DM education do not improve self-management and illness biomarkers.27 Thus, integrating these providers and services within the medical setting aims to reach more veterans and potentially meet these standards of care. With our health psychology integrated services, clinically significant decreases in anxiety and statistically significant decreases in depressive symptoms were observed that approached “mild to no” symptoms. Although this was not measured formally, the veterans were not engaging in mental health specialty care historically or during the year of the health psychology intervention. This suggests that health psychology services helped bridge the gap and address these psychosocial needs within the small sample.
For clinical measures, modest decreases were observed for HbA1c and weight. The authors recognize that these changes may not be optimal in terms of health status. A review of the specific patient-centered goals may illuminate this finding. For example, 1 participant had a goal to consume fewer sugary beverages and achieved this behavior change. Yet this change alone may not equate to actual weight loss or a lower HbA1c. Furthermore, in the context of T2DM-related distress, maintaining current weight and/or blood sugar levels may be a more realistic goal. An evaluation of the specific patient-oriented action goals and observed progress may be important outcomes to include in larger studies. Moreover, while not significant, the average HbA1c decrease of about 1% is comparable with traditional T2DM education and should be considered in light of the sample’s significant mental health comorbidities. While landmark intensive glucose control trials illustrate significant benefits in reductions of hyperglycemia and nonfatal cardiovascular disease, these reductions are associated with an approximate 2-fold risk of hypoglycemia.28-30 Thus, the focus on improved glycemic control has been criticized as lacking meaning to patients in contrast to preventing T2DM complications and persevering quality of life.31
Limitations and Future Directions
Noted limitations include small sample size, the range of time, and a broad number of sessions given that the intervention was tailored to each veteran. Conclusions drawn from a small sample may be influenced by individual outliers. Given co-occurring conditions and moderate levels of distress, all participants may benefit from additional support resources.
In addition to these considerations, having a comparison group could further strengthen the study as part of an observational database. A between-group comparison could help clinicians better understand what the interventions offer as well as some individual factors that relate to participation and success with behavior change. In the future, studies with a priori hypotheses could also consider the trajectories of weight and blood sugar levels for extended periods; for example, 6 months before the intervention and 6 months following.32 Given the complexity of comorbid mental health and chronic medical conditions in this sample, it also may be important to measure the relationships between chronic physical symptoms as an additional barrier for veterans to make health behavior changes.
Conclusions
The authors believe that the health psychology interventions offered important support and motivation for engagement in health behavior change that led to reduced distress in this patient group. It remains a challenge to engage veterans with psychiatric conditions in mental health care, and simultaneously for health care systems that strive to reduce costs and complications associated with chronic illness management.33 Aligned with these broader health care goals, the ADA aims to reduce complications and cost and improve outcomes for T2DM with guidelines requiring mental and behavioral health interventions. The authors believe that health psychology interventions are a personalized and feasible bridge to address engagement, illness-related distress while improving patient-satisfaction and T2DM self-management.
Acknowledgments
The authors thank the veterans who participated in the observational study. We thank the VA Ann Arbor Healthcare System Institutional Review Board. For instrumental support for health psychology integrated services, we acknowledge Adam Tremblay, MD, Primary Care Chief, and R.J. Schildhouse, MD, Acting Associate Chief of Staff, Ambulatory Care. The work was supported by the Ambulatory Care Service at the VA Ann Arbor Healthcare System and the VA Office of Academic Affiliations.
Veterans have a higher prevalence of type 2 diabetes mellitus (T2DM) when compared with their civilian counterparts with an overall prevalence rate of 25%.1 This higher prevalence is similar to other major chronic health conditions, including heart disease and arthritis, with additional costs for disease self-management.2 Psychological and behavioral change strategies are a principal means of limiting the severity and even restoring function once T2DM is diagnosed.3 More broadly, there is mounting evidence that addressing distress and behavior change are important across many conditions, particularly T2DM.4 Therefore, the US Department of Veterans Affairs (VA) has established patient education and multidisciplinary interventions to optimize engagement in T2DM self-management and health behavior change.5
Traditional T2DM education programs aim to meet the American Diabetes Association (ADA) standards of medical care and include a T2DM educator and other allied health professionals. ADA Standard 1.2 emphasizes “productive interactions between a prepared, proactive care team and an informed, activated patient.”6 Thus, to attain ADA accreditation, educational programs require instructors to teach about T2DM while engaging patients to help them set and achieve recommended changes. The requirements emphasize setting specific goals, (ie, eating wisely, being physically active, monitoring blood sugars or taking medications). The care team also helps to identify barriers, and at a required follow-up class, patients evaluate how well they met goals and make modifications if needed. The impact of traditional patient education programs to improve glycemic levels is well established.7 Importantly, veterans with comorbid mental health conditions may not experience the same beneficial outcomes if or when they participate in traditional diabetes or self-management programs.8,9 Veterans with T2DM may be particularly vulnerable to chronic stress and effects of comorbid mental health diagnoses.10 Furthermore, when individuals experience T2DM-related distress, associations with poor health outcomes, including elevated hemoglobin A1c (HbA1c), are observed independent of depression.11
Health psychology services integrate into medical settings and strive to reach veterans who may not engage in traditional mental health clinical offerings.12 These collaborative interventions focus less on diagnostic or screening procedures and more on a patient’s understanding of illness and ability and willingness to carry out treatment regimens. Given the significant roles of distress and co-occurring conditions, health psychology services further aim to provide psychoeducation about stress management in order to explore and enhance motivation for making a wide range of health behavior changes.
The purpose of this study was to evaluate baseline and follow-up HbA1c, weight, and psychosocial measures, namely, health-related self-efficacy and T2DM-related distress among a small sample that engaged in integrated health psychology services. The focus of this evidence-based psychotherapy service was to improve T2DM self-care and physical health. The participants were offered cognitive and behavioral strategies for setting and meeting personalized T2DM self-management goals. Importantly, motivational interviewing was used throughout to adapt to the participants’ preferences and needs as well as to maintain engagement.
Methods
Primary care providers referred veterans with T2DM to the Health Psychology service at VA Ann Arbor Healthcare System (VAAAHS). A T2DM diagnosis was verified through electronic health record review. Most common referrals included addressing coping with chronic illness and improving glycemic levels. Veterans were invited to participate in a program evaluation project to monitor health-related changes. All participants provided written informed consent and did not receive incentive or payment for participating. The VAAAHS Institutional Review Board reviewed and approved this study.
Intervention
Veterans met individually with a health psychologist or health psychology trainee to create personalized health and behavioral goals for improving T2DM self-management, overall health, and psychological well-being. This intervention included motivational interviewing, SMART (specific, measurable, action-oriented, realistic, timely) goal setting, behavioral activation, acceptance of T2DM-related physical changes, problem-solving therapy, challenging maladaptive disease-related cognitions, and incorporating values to help find motivation for change. Int
Data Collection
Participants completed study measures at the beginning and end of the T2DM-focused intervention sessions. Demographic variables collected included age, sex, race/ethnicity, highest educational attainment, and whether a veteran was prescribed insulin, service connected for T2DM, concurrent enrollment in other educational programs, and time since T2DM diagnosis. Measures were selected based on their relevance to T2DM psychosocial care and diabetes health outcomes.13
Body mass index, low-density lipoprotein cholesterol, blood pressure (BP), HbA1c within 3 months of the pre- and postmeasures were collected by reviewing medical records. T2DM complications were collected by self-report, and comorbid physical and mental health conditions were collected by review of the most recent primary care note. The Diabetes Empowerment Scale-Short Form (DES-SF) is a well-validated measure that was used to measure T2DM-related psychosocial self-efficacy.14 Scores ranged from 8 to 40 with higher scores indicating higher diabetes T2DM empowerment. The Patient Health Questionnaire 9-item (PHQ-9) was used to assess the frequency of somatic (fatigue, appetite, psychomotor) and cognitive symptoms (anhedonia, low mood) of depression over the past 2 weeks.15 The Generalized Anxiety Disorder 7-item (GAD-7) was used to assess the frequency of common anxiety symptoms, including feelings of worry, difficulty controlling worry, and trouble relaxing.16 Veterans were also asked to rate their general health on a 5-point Likert scale. Self-rated health is a well-established indicator of disability and risk of future T2DM complications in older adults.17,18 The Diabetes Distress Scale (DDS) was used to measure emotional burden, physician-related distress, regimen-related distress, and T2DM-related interpersonal distress.19 Scores > 2.0 suggest clinical significant diabetes distress.20 Medication questionnaires were adapted from Wilson and colleagues, 2013.21
Statistical Analyses
Descriptive statistics, including mean and standard deviation (SD) or frequency distributions, as appropriate, were used to characterize the sample. For pre- and postintervention within-group comparisons, a paired samples Student t test analysis was used to evaluate baseline and follow-up measures for statistically significant differences between continuous variables; scores also were evaluated for clinically meaningful change.
Results
This sample (N = 13) of older adults was predominately male, white, with HbA1c > 7.0, and prescribed insulin (Table). On average, participants were at higher risk for future complications due to high BP, hyperlipidemia, and BMI > 30.0. Regarding participation, veterans were seen for an average of 7.8 sessions (range, 4-13) with 46% service connected for T2DM. Of note, 4 veterans received other T2DM-specific self-management support within the same year of their participation with health psychology, such as attending a T2DM education class or T2DM shared medical appointment.22 Reliability in the current sample for the DES-SF was high (Cronbach α = 0.90), PHQ-9 was good (Cronbach α = 0.81), and GAD-7 was very good (Cronbach α = 0.86).
Among the 13 older adults, the most common T2DM-related complications included peripheral neuropathy (n = 7), heart pain or heart attack (n = 5), and retinopathy (n = 4). Recent primary care notes showed a mean (SD) 7 (2.2) comorbid chronic medical conditions with a high prevalence of cardiometabolic illnesses including hypertension, hyperlipidemia, obstructive sleep apnea, and a diagnosis of chronic pain. Eleven veterans were diagnosed with a mental health condition, including bipolar disorder, depression, anxiety, trauma-related disorder, and sleep disorders. Veterans reported high T2DM emotional distress (mean [SD] 3.1 [1.2]), moderate regimen-related distress (mean [SD] 2.9 [1.1]), and moderate total T2DM distress (mean [SD] 2.4 [0.7]). Physician distress (mean [SD] 1.3 [0.55]) and interpersonal T2DM distress (mean [SD] 1.6 [0.9]) subscales indicated little to no distress. The sample reported mild symptoms of depression (PHQ-9 mean [SD] 8.8 [4.6]); mild symptoms of anxiety (GAD-7 mean, 7.1; SD, 4.4), and Diabetes Empowerment (mean, 31.2; SD, 6.0). Participants described missing an average of 2.4 days within the past 30 days of their T2DM oral medications.
Twelve veterans (92.7%) completed the Follow-up questionnaires. The Figure illustrates statistically significant changes in patient-reported outcomes between baseline and follow-up. Clinically meaningful reductions were shown in total T2DM distress (t11 = 5.03, P < .01), T2DM emotional burden (t11 = 4.83, P = .01), and T2DM regimen-related distress (t11 = 5.14, P < .01). There was a significant increase in T2DM self-efficacy (t11 = 0.32, P = .008) as well. A statistically significant reduction was seen in depressive symptoms (t11 = 2.22, P = .048). While HbA1c fell by .56 percentage points (standard error of the mean [SEM], 31; P = .10), this change was not statistically significant. Follow-up analyses also showed a clinically, though not statistically, significant reduction in weight loss by 6.9 lb. (SEM, 3.8; P = .20), and reductions of generalized anxiety by 1.2 points (SEM, 1.4; P = .42). Pre- and postanalyses did not show differences among self-rated health, physician-related burden, interpersonal-related burden, and indicators of medication taking behavior.
Discussion
This observational study evaluated change among patient-reported T2DM-specific and general distress measures and health outcomes among a small sample of veterans at VAAAHS medical center that engaged in an episode of individual care with health psychology. Statistically significant decreases were observed in T2DM-related distress. Noteworthy, these decreases were observed for the emotional burden and regimen subscales, and each of these was clinically meaningful, falling below a score of 2.0 on the T2DM-specific scale. This is important given that T2DM distress may interfere with the ability to understand and find motivation for engaging in health behavior change. Incorporating stress management interventions into interdisciplinary health programs has been demonstrated to improve not only levels of distress, but also other health outcomes, such as health related quality of life and cardiac events in heart disease.23 Thus, behavioral health interventions that incorporate cognitive-behavioral strategies to enhance distress-specific coping may prove important to include among individuals with T2DM.
Reductions in T2DM-related distress also converged with increases observed in the T2DM empowerment scale. These significant improvements in perceived ability suggest increased self-efficacy and willingness to follow a daily T2DM regimen. This finding aligns with the social support literature that demonstrates how instrumental and other aspects of autonomous social support mediate improvements in health-related outcomes and reduced T2DM distress.24,25 Health psychology interventions strive to both provide social support as well as enhance participants’ perceptions and use of existing support as a cognitive-behavioral strategy. Adding in assessments of social support could shed light on such mediating factors.
The ADA standards of care encourage heath care providers to engage patients in conversations in order to better understand the barriers of T2DM self-care.13 How to best support patients within a primary care multidisciplinary team remains unclear.26 T2DM distress and negative reactions to T2DM, including symptoms of anxiety and depression, are common and may require specific referral to a mental health provider if repeated attempts at T2DM education do not improve self-management and illness biomarkers.27 Thus, integrating these providers and services within the medical setting aims to reach more veterans and potentially meet these standards of care. With our health psychology integrated services, clinically significant decreases in anxiety and statistically significant decreases in depressive symptoms were observed that approached “mild to no” symptoms. Although this was not measured formally, the veterans were not engaging in mental health specialty care historically or during the year of the health psychology intervention. This suggests that health psychology services helped bridge the gap and address these psychosocial needs within the small sample.
For clinical measures, modest decreases were observed for HbA1c and weight. The authors recognize that these changes may not be optimal in terms of health status. A review of the specific patient-centered goals may illuminate this finding. For example, 1 participant had a goal to consume fewer sugary beverages and achieved this behavior change. Yet this change alone may not equate to actual weight loss or a lower HbA1c. Furthermore, in the context of T2DM-related distress, maintaining current weight and/or blood sugar levels may be a more realistic goal. An evaluation of the specific patient-oriented action goals and observed progress may be important outcomes to include in larger studies. Moreover, while not significant, the average HbA1c decrease of about 1% is comparable with traditional T2DM education and should be considered in light of the sample’s significant mental health comorbidities. While landmark intensive glucose control trials illustrate significant benefits in reductions of hyperglycemia and nonfatal cardiovascular disease, these reductions are associated with an approximate 2-fold risk of hypoglycemia.28-30 Thus, the focus on improved glycemic control has been criticized as lacking meaning to patients in contrast to preventing T2DM complications and persevering quality of life.31
Limitations and Future Directions
Noted limitations include small sample size, the range of time, and a broad number of sessions given that the intervention was tailored to each veteran. Conclusions drawn from a small sample may be influenced by individual outliers. Given co-occurring conditions and moderate levels of distress, all participants may benefit from additional support resources.
In addition to these considerations, having a comparison group could further strengthen the study as part of an observational database. A between-group comparison could help clinicians better understand what the interventions offer as well as some individual factors that relate to participation and success with behavior change. In the future, studies with a priori hypotheses could also consider the trajectories of weight and blood sugar levels for extended periods; for example, 6 months before the intervention and 6 months following.32 Given the complexity of comorbid mental health and chronic medical conditions in this sample, it also may be important to measure the relationships between chronic physical symptoms as an additional barrier for veterans to make health behavior changes.
Conclusions
The authors believe that the health psychology interventions offered important support and motivation for engagement in health behavior change that led to reduced distress in this patient group. It remains a challenge to engage veterans with psychiatric conditions in mental health care, and simultaneously for health care systems that strive to reduce costs and complications associated with chronic illness management.33 Aligned with these broader health care goals, the ADA aims to reduce complications and cost and improve outcomes for T2DM with guidelines requiring mental and behavioral health interventions. The authors believe that health psychology interventions are a personalized and feasible bridge to address engagement, illness-related distress while improving patient-satisfaction and T2DM self-management.
Acknowledgments
The authors thank the veterans who participated in the observational study. We thank the VA Ann Arbor Healthcare System Institutional Review Board. For instrumental support for health psychology integrated services, we acknowledge Adam Tremblay, MD, Primary Care Chief, and R.J. Schildhouse, MD, Acting Associate Chief of Staff, Ambulatory Care. The work was supported by the Ambulatory Care Service at the VA Ann Arbor Healthcare System and the VA Office of Academic Affiliations.
1. Liu Y, Sayam S, Shao X, et al. Prevalence of and trends in diabetes among veterans, United States, 2005-2014. Prev Chronic Dis. 2017;14(12):E135, 1-5. doi:10.5888/pcd14.170230
2. Yu W, Ravelo A, Wagner TH, et al. Prevalence and costs of chronic conditions in the VA health care system. Med Care Res Rev. 2003;60(3)(suppl):146S-167S. doi:10.1177/1077558703257000
3. American Psychological Association. Psychology and Health in Action. Updated 2016. Accessed February 10, 2021. https://www.apa.org/health/fall-2016-updates.pdf
4. The US Burden of Disease Collaborators. The state of US health, 1990-2016. JAMA. 2018;319(14):1444-1472. doi:10.1001/jama.2018.0158
5. Piette JD, Kerr E, Richardson C, Heisler M. Veterans Affairs research on health information technologies for diabetes self-management support. J Diabetes Sci Technol. 2008;2(1):15-23. doi:10.1177/193229680800200104
6. American Diabetes Association. 1. Improving care and promoting health in populations: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S7-S12. doi:10.2337/dc19-S001
7. Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self-management education for adults with type 2 diabetes. A meta-analysis of the effect on glycemic control. Diabetes Care. 2002;25(7):1159-1171. doi:10.2337/diacare.25.7.1159
8. Janney CA, Owen R, Bowersox NW, Ratz D, Kilbourne EA. Bipolar disorder influences weight loss in the nationally implemented MOVE! program for veterans. Bipolar Disord. 2015;17:87.
9. Piette JD, Kerr EA. The impact of comorbid chronic conditions on diabetes care. Diabetes Care. 2006;29(3):725-731. doi:10.2337/diacare.29.03.06.dc05-2078
10. Trief PM, Ouimette P, Wade M, Shanahan P, Weinstock RS. Post-traumatic stress disorder and diabetes: Co-morbidity and outcomes in a male veterans sample. J Behav Med. 2006;29(5):411-418. doi:10.1007/s10865-006-9067-2
11. Fisher L, Mullan JT, Arean P, Glasgow RE, Hessler D, Masharani U. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care. 2010;33(1):23-28. doi:10.2337/dc09-1238
12. Bohnert KM, Pfeiffer PN, Szymanski BR, McCarthy JF. Continuation of care following an initial primary care visit with a mental health diagnosis: differences by receipt of VHA Primary Care-Mental Health Integration services. Gen Hosp Psychiatry. 2013;35(1):66-70. doi:10.1016/j.genhosppsych.2012.09.002
13. Young-Hyman D, De Groot M, Hill-Briggs F, Gonzalez JS, Hood K, Peyrot M. Psychosocial care for people with diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39(12):2126-2140. doi:10.2337/dc16-2053
14. Anderson R, Fitzgerald J, Gruppen L, Funnell M, Oh M. The diabetes empowerment scale-short form (DES-SF). Diabetes Care. 2003;26(5):1641-1642. doi:10.2337/diacare.26.5.1641-a
15. Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.doi:10.1046/j.1525-1497.2001.016009606.x
16. Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097. doi:10.1001/archinte.166.10.1092
17. Pinquart M. Correlates of subjective health in older adults: a meta-analysis. Psychol Aging. 2001;16(3):414. doi:10.1037/0882-7974.16.3.414
18. Hayes AJ, Clarke PM, Glasziou PG, Simes RJ, Drury PL, Keech AC. Can self-rated health scores be used for risk prediction in patients with type 2 diabetes? Diabetes Care. 2008;31(4):795-797. doi:10.2337/dc07-1391
19. Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28(3):626-631. doi:10.2337/diacare.28.3.626
20. Fisher L, Hessler DDM, Polonsky WH, Mullan J. When is diabetes distress meaningful?: Establishing cut points for the Diabetes Distress Scale. Diabetes Care. 2012;35(2):259-264. doi:10.2337/dc11-1572
21. Wilson IB, Fowler FJ Jr, Cosenza CA, et al. Cognitive and field testing of a new set of medication adherence self-report items for HIV care. AIDS Behav. 2013;18(12):2349-2358. doi:10.1007/s10461-013-0610-1
22. Heisler M, Burgess J, Cass J, et al. The Shared Health Appointments and Reciprocal Enhanced Support (SHARES) study: study protocol for a randomized trial. Trials. 2017;18(1):239. doi:10.1186/s13063-017-1959-7
23. Blumenthal JA, Babyak MA, Carney RM, et al. Exercise, depression, and mortality after myocardial infarction in the ENRICHD Trial. Med Sci Sports Exerc. 2004;36(5):746-755. doi:10.1249/01.MSS.0000125997.63493.13
24. Lee AA, Piette JD, Heisler M, Rosland AM. Diabetes distress and glycemic control: the buffering effect of autonomy support from important family members and friends. Diabetes Care. 2018;41(6):1157-1163. doi:10.2337/dc17-2396
25. Baek RN, Tanenbaum ML, Gonzalez JS. Diabetes burden and diabetes distress: the buffering effect of social support. Ann Behav Med. 2014;48(2):1-11.doi:10.1007/s12160-013-9585-4
26. Jortberg BT, Miller BF, Gabbay RA, Sparling K, Dickinson WP. Patient-centered medical home: how it affects psychosocial outcomes for diabetes. Curr Diab Rep. 2012;12(6):721-728. doi:10.1007/s11892-012-0316-1
27. American Diabetes Association. Lifestyle management: standards of medical care in diabetes-2019. Diabetes Care. 2019;41(suppl 1):S38-S50. doi:10.2337/dc19-S005
28. UK Prospective Diabetes Study Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes. Lancet. 1998;352(9131):854-865.
29. The Diabetes Control and Complications Trial Research Group, Control TD, Trial C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329(14):977-986. doi:10.1056/NEJM199309303291401
30. Kelly TN, Bazzano LA, Fonseca VA, Thethi TK, Reynolds K, He J. Systematic review: glucose control and cardiovascular disease in type 2 diabetes. Ann Intern Med. 2009;151(6):394-403. doi:10.1037/1072-5245.13.1.64
31. Yudkin JS, Lipska KJ, Montori VM. The idolatry of the surrogate. BMJ. 2012;344(7839):8-10. doi:10.1136/bmj.d7995
32. Lutes LD, Damschroder LJ, Masheb R, et al. Behavioral treatment for veterans with obesity: 24-month weight outcomes from the ASPIRE-VA Small Changes Randomized Trial. J Gen Intern Med. 2017;32(1):40-47. doi:10.1007/s11606-017-3987-0
33. Krejci LP, Carter K, Gaudet T. The vision and implementation of personalized, proactive, patient-driven health care for veterans. Med Care. 2014;52(12)(suppl 5):S5-S8. doi:10.1097/MLR.0000000000000226
1. Liu Y, Sayam S, Shao X, et al. Prevalence of and trends in diabetes among veterans, United States, 2005-2014. Prev Chronic Dis. 2017;14(12):E135, 1-5. doi:10.5888/pcd14.170230
2. Yu W, Ravelo A, Wagner TH, et al. Prevalence and costs of chronic conditions in the VA health care system. Med Care Res Rev. 2003;60(3)(suppl):146S-167S. doi:10.1177/1077558703257000
3. American Psychological Association. Psychology and Health in Action. Updated 2016. Accessed February 10, 2021. https://www.apa.org/health/fall-2016-updates.pdf
4. The US Burden of Disease Collaborators. The state of US health, 1990-2016. JAMA. 2018;319(14):1444-1472. doi:10.1001/jama.2018.0158
5. Piette JD, Kerr E, Richardson C, Heisler M. Veterans Affairs research on health information technologies for diabetes self-management support. J Diabetes Sci Technol. 2008;2(1):15-23. doi:10.1177/193229680800200104
6. American Diabetes Association. 1. Improving care and promoting health in populations: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S7-S12. doi:10.2337/dc19-S001
7. Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self-management education for adults with type 2 diabetes. A meta-analysis of the effect on glycemic control. Diabetes Care. 2002;25(7):1159-1171. doi:10.2337/diacare.25.7.1159
8. Janney CA, Owen R, Bowersox NW, Ratz D, Kilbourne EA. Bipolar disorder influences weight loss in the nationally implemented MOVE! program for veterans. Bipolar Disord. 2015;17:87.
9. Piette JD, Kerr EA. The impact of comorbid chronic conditions on diabetes care. Diabetes Care. 2006;29(3):725-731. doi:10.2337/diacare.29.03.06.dc05-2078
10. Trief PM, Ouimette P, Wade M, Shanahan P, Weinstock RS. Post-traumatic stress disorder and diabetes: Co-morbidity and outcomes in a male veterans sample. J Behav Med. 2006;29(5):411-418. doi:10.1007/s10865-006-9067-2
11. Fisher L, Mullan JT, Arean P, Glasgow RE, Hessler D, Masharani U. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care. 2010;33(1):23-28. doi:10.2337/dc09-1238
12. Bohnert KM, Pfeiffer PN, Szymanski BR, McCarthy JF. Continuation of care following an initial primary care visit with a mental health diagnosis: differences by receipt of VHA Primary Care-Mental Health Integration services. Gen Hosp Psychiatry. 2013;35(1):66-70. doi:10.1016/j.genhosppsych.2012.09.002
13. Young-Hyman D, De Groot M, Hill-Briggs F, Gonzalez JS, Hood K, Peyrot M. Psychosocial care for people with diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39(12):2126-2140. doi:10.2337/dc16-2053
14. Anderson R, Fitzgerald J, Gruppen L, Funnell M, Oh M. The diabetes empowerment scale-short form (DES-SF). Diabetes Care. 2003;26(5):1641-1642. doi:10.2337/diacare.26.5.1641-a
15. Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.doi:10.1046/j.1525-1497.2001.016009606.x
16. Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097. doi:10.1001/archinte.166.10.1092
17. Pinquart M. Correlates of subjective health in older adults: a meta-analysis. Psychol Aging. 2001;16(3):414. doi:10.1037/0882-7974.16.3.414
18. Hayes AJ, Clarke PM, Glasziou PG, Simes RJ, Drury PL, Keech AC. Can self-rated health scores be used for risk prediction in patients with type 2 diabetes? Diabetes Care. 2008;31(4):795-797. doi:10.2337/dc07-1391
19. Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28(3):626-631. doi:10.2337/diacare.28.3.626
20. Fisher L, Hessler DDM, Polonsky WH, Mullan J. When is diabetes distress meaningful?: Establishing cut points for the Diabetes Distress Scale. Diabetes Care. 2012;35(2):259-264. doi:10.2337/dc11-1572
21. Wilson IB, Fowler FJ Jr, Cosenza CA, et al. Cognitive and field testing of a new set of medication adherence self-report items for HIV care. AIDS Behav. 2013;18(12):2349-2358. doi:10.1007/s10461-013-0610-1
22. Heisler M, Burgess J, Cass J, et al. The Shared Health Appointments and Reciprocal Enhanced Support (SHARES) study: study protocol for a randomized trial. Trials. 2017;18(1):239. doi:10.1186/s13063-017-1959-7
23. Blumenthal JA, Babyak MA, Carney RM, et al. Exercise, depression, and mortality after myocardial infarction in the ENRICHD Trial. Med Sci Sports Exerc. 2004;36(5):746-755. doi:10.1249/01.MSS.0000125997.63493.13
24. Lee AA, Piette JD, Heisler M, Rosland AM. Diabetes distress and glycemic control: the buffering effect of autonomy support from important family members and friends. Diabetes Care. 2018;41(6):1157-1163. doi:10.2337/dc17-2396
25. Baek RN, Tanenbaum ML, Gonzalez JS. Diabetes burden and diabetes distress: the buffering effect of social support. Ann Behav Med. 2014;48(2):1-11.doi:10.1007/s12160-013-9585-4
26. Jortberg BT, Miller BF, Gabbay RA, Sparling K, Dickinson WP. Patient-centered medical home: how it affects psychosocial outcomes for diabetes. Curr Diab Rep. 2012;12(6):721-728. doi:10.1007/s11892-012-0316-1
27. American Diabetes Association. Lifestyle management: standards of medical care in diabetes-2019. Diabetes Care. 2019;41(suppl 1):S38-S50. doi:10.2337/dc19-S005
28. UK Prospective Diabetes Study Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes. Lancet. 1998;352(9131):854-865.
29. The Diabetes Control and Complications Trial Research Group, Control TD, Trial C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329(14):977-986. doi:10.1056/NEJM199309303291401
30. Kelly TN, Bazzano LA, Fonseca VA, Thethi TK, Reynolds K, He J. Systematic review: glucose control and cardiovascular disease in type 2 diabetes. Ann Intern Med. 2009;151(6):394-403. doi:10.1037/1072-5245.13.1.64
31. Yudkin JS, Lipska KJ, Montori VM. The idolatry of the surrogate. BMJ. 2012;344(7839):8-10. doi:10.1136/bmj.d7995
32. Lutes LD, Damschroder LJ, Masheb R, et al. Behavioral treatment for veterans with obesity: 24-month weight outcomes from the ASPIRE-VA Small Changes Randomized Trial. J Gen Intern Med. 2017;32(1):40-47. doi:10.1007/s11606-017-3987-0
33. Krejci LP, Carter K, Gaudet T. The vision and implementation of personalized, proactive, patient-driven health care for veterans. Med Care. 2014;52(12)(suppl 5):S5-S8. doi:10.1097/MLR.0000000000000226
Behavioral Interventions in Multiple Sclerosis
Multiple Sclerosis (MS) is a chronic demyelinating disease of the central nervous system that affects nearly 1 million people in the US.1 In addition to the accumulation of functional limitations, patients with MS commonly experience mental health and physical symptoms such as depression, anxiety, stress, fatigue, and pain. Day-to-day life with MS requires adaptation to challenges and active maintenance of health and well-being over time. Behavioral intervention and treatment, whether in the form of psychotherapy, health behavior coaching, or the promotion of active self-management, is an integral component of interprofessional care and key aspect of living well with MS.
Behavioral Comorbidities
Depression
Depression is a common concern among individuals with MS. Population-based studies suggest that individuals with MS have a roughly 1 in 4 chance of developing major depressive disorder over their lifetime.2 However, at any given time, between 40% and 60% of individuals with MS report clinically meaningful levels of depressive symptoms.3 Although the relationship between MS disease characteristics and depression is unclear, some evidence suggests that depressive symptoms are more common at certain points in illness, such as early in the disease process as individuals grapple with the onset of new symptoms, late in the disease process as they accumulate greater disability, and during active clinical relapses.3-5
Depression often is comorbid with, and adds to the symptom burden of, other common conditions in MS such as fatigue and cognitive dysfunction.6-8 Thus, it is not surprising that it associated with poorer overall quality of life (QOL).9 Depression also is a risk factor for suicidal ideation and suicide for patients with MS.10,11
Fortunately, several behavioral interventions show promise in treating depression in patients with MS. Both individual and group formats of cognitive behavioral therapy (CBT), a treatment focused on challenging maladaptive patterns of thought and behavior, have been shown to improve depressive symptoms for people with MS.12,13 Several brief and efficient group-based programs grounded in CBT and focused on the development of specific skills, including problem solving, goal setting, relationship management, and managing emotions, have been shown to reduce depressive symptoms.13,14 CBT for depression in MS has been shown to be effective when delivered via telephone.15,16
Anxiety
Anxiety is common among individuals with MS. Existing data suggest more than one-third of individuals with MS will qualify for a diagnosis of anxiety disorder during their lifetime.17 The characteristics of anxiety disorders are broad and heterogenous, including generalized anxiety disorder, panic disorder, obsessive compulsive disorders, and health-specific phobias such as needle/injection anxiety. Some estimates suggest a point prevalence of 34% for the presence of clinically meaningful symptoms.18 Similar to depression, anxiety symptoms can be more common during periods of stress, threat, and transition including early in the disease course while adapting to new diagnosis, late in the disease course with increasing disability, and during clinical relapses.19-21
The efficacy of behavioral interventions for anxiety in MS is less well established than it is for depression, but some preliminary evidence suggests that individual CBT may be effective for reducing general symptoms of anxiety as well as health-related anxiety.22,23 Brief, targeted CBT also has been shown to improve injection anxiety, removing a barrier to self-care including the administration of MS disease modifying therapies (DMTs).24
Stress
Stress is commonly conceptualized as a person’s perception that efforts to manage internal and external demands exceed available coping resources.25 Such demands involve both psychological and physiological processes and come in many forms for people with MS and can include daily hassles, major life events, traumatic stress, and perceptions of global nonspecific stress. The relationship between stress and MS remains complex and poorly understood. Nonetheless, individuals with MS frequently report that stress exacerbates their symptoms.26
Some evidence also suggests stress may exacerbate the MS disease process, resulting in more frequent relapses and increased lesion activity visible on MRI.27,28 In addition to mindfulness (described below), stress inoculation training (CBT and relaxation training), and stress-focused group-based self-management have been shown to be beneficial.29,30 In an intriguing and rigorous trial, a 24-week stress management therapy based on CBT was associated with the development of fewer new MS lesions visible on MRI.31
Adaptation to Illness
MS presents challenges that vary between patients and over time. Individuals may confront new physical and cognitive limitations that inhibit the completion of daily tasks, reduce independence, and limit participation in valued and meaningful activities. In addition, the unpredictability of the disease contributes to perceptions of uncertainty and uncontrollability, which in turn result in higher illness impact and poorer psychological outcomes.32 Building cognitive and behavioral skills to address these challenges can promote adaptation to illness and reduce overall distress associated with chronic illness.33 Psychosocial intervention also can address the uncertainty commonly experienced by individuals with MS.34
Self-Management
As with any chronic illness, living well with MS requires ongoing commitment and active engagement with health and personal care over time. The process of building knowledge and skills to manage the day-to-day physical, emotional, and social aspects of living with illness often is referred to as self-management.35 For individuals with MS, this may take the form of participation in programs that address adaptation and psychological distress like those described above, but it also may include improving health behavior (eg, physical activity, DMT adherence, modification of maladaptive habits like smoking or hazardous alcohol use) and symptom management (eg, fatigue, pain). Self-management programs typically include education, the practice of identifying, problem solving, and following through with specific and realistic health and wellness goals, as well as the bolstering of self-efficacy.
Physical Activity
Once discouraged for patients with MS, physical activity is now considered a cornerstone of health and wellness. Physical activity and interventions that target various forms of exercise have been shown to improve strength and endurance, reduce functional decline, enhance QOL, and likely reduce mortality.35-39 A variety of brief behavioral interventions have been shown to improve physical activity in MS. Structured group-based exercise classes focusing on various activities such as aerobic training (eg, cycling) or resistance training (eg, lower extremity strengthening) have demonstrated improvements in various measures of fitness and mood states such as depression and QOL. Brief home-based telephone counseling interventions based in social cognitive theory (eg, goal setting, navigating obstacles) and motivational interviewing strategies (eg, open-ended questions, affirmation, reflective listening, summarizing) also have been shown to be effective not only at increasing physical activity and improving depression and fatigue.40,41
Adherence to Treatment
One primary focus of adherence to treatment is medication management. For individuals with MS, DMTs represent a primary means of reducing disease burden and delaying functional decline. Many DMTs require consistent self-administration over time. Some evidence suggests that poorer adherence is associated with a greater risk of relapse and more rapid disease progression.42,43 Brief telephone counseling, again based on social cognitive theory, and principles of motivational interviewing combined with home telehealth monitoring by a care coordinator has been shown to improve adherence to DMTs.44
Mindfulness
In recent years, mindfulness training has emerged as a popular and common behavioral intervention among individuals with MS. Programs like Mindfulness-Based Stress Reduction (MBSR) provide training in meditation techniques designed to promote mindfulness, which is defined as paying attention to present moment experience, including sensations, thoughts, and emotions, without judgment or attachment.45 Cultivating mindfulness helps people with MS cope with and adapt to symptoms and stressors.46 Mindfulness interventions typically are delivered in a group format. For example, MBSR consists of 8 in-person group sessions with daily meditation practice homework. Mindfulness interventions also have been delivered effectively with smartphone apps.47 Mindfulness programs have been shown to improve depression, anxiety, fatigue, stress, and QOL for patients with MS.48-50
Fatigue
More than 90% of individuals with MS report fatigue, and many identify it as their most disabling symptom.51 Often defined as “a subjective lack of physical and/or mental energy that is perceived by the individual or caregiver to interfere with usual and desired activities,” fatigue has been shown to be associated with longer disease duration, greater physical disability, progressive subtype, and depressive symptoms, although the relative and possibly overlapping impact of these issues is only partially understood.52,53 Fatigue is associated with poorer overall mental health and negatively impacts work and social roles.54
Several behavioral interventions have been developed to address fatigue in MS. Using both individual and group based formats and across several modalities (eg, in-person, telephone, online modules, or a combination), behavioral fatigue interventions most commonly combine traditional general CBT skills (eg, addressing maladaptive thoughts and behaviors) with a variety of fatigue-specific skill building exercises that may include fatigue education, energy conservation strategies, improving sleep, enlisting social support, and self-management goal setting strategies.35,55-57
Pain
Chronic pain is common and disabling in people with MS.58,59 Nearly 50% report experiencing moderate to severe chronic pain.59,60 Individuals with MS reporting pain often are older, more disabled (higher Expanded Disability Status Scale score), and have longer disease duration that those who are not experiencing chronic pain.61 Patients report various types of pain in the following order of frequency: dysesthetic pain (18.1%), back pain (16.4%), painful tonic spasms (11.0%), Lhermitte sign (9.0%), visceral pain (2.9%), and trigeminal neuralgia (2.0%).61 Chronic pain has a negative impact on QOL in the areas of sleep, work, maintaining relationships, recreational activities, and overall life enjoyment.59 Additionally, research has shown that greater pain intensity and pain-related interference with activities of daily living are both associated with greater depression severity.62,63
The literature supports the use of behavioral interventions for pain in people with MS.61 Behavioral interventions include in-person exercise interventions (eg, water aerobics, cycling, rowing ergometer, treadmill walking, and resistance training), self-hypnosis, and telephone-based self-management programs based on CBT.35,64,65 As described above, CBT-based self-management programs combine learning CBT skills (eg, modifying maladaptive thoughts) with pain-specific skill building such as pain education, pacing activities, and improving sleep. Of note, MS education including, but not limited to, pain was as effective as a CBT-based self-management program in reducing pain intensity and interference.35 In addition, there is evidence to support acceptance- and mindfulness-based interventions for chronic pain, and online mindfulness-based cognitive therapy for MS related pain is currently being tested in a randomized controlled trial.35,66
Conclusion
People with MS face significant challenges in coping with and adapting to a chronic and unpredictable disease. However, there is considerable evidence that behavioral interventions can improve many of the most common and disabling symptoms in MS including depression, anxiety, stress, fatigue, and pain as well as health behavior and self-care. Research also suggests that improvements in one of these problems (eg, physical inactivity) can influence improvement in other symptoms (eg, depression and fatigue). Unlike other treatment options, behavioral interventions can be delivered in various formats (eg, in-person and electronic health), are time-limited, and cause few (if any) undesirable systemic adverse effects. Behavioral interventions are therefore, an essential part of interprofessional care and rehabilitation for patients with MS.
1. Wallin MT, Culpepper WJ, Campbell JD, et al; US Multiple Sclerosis Workgroup. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology. 2019;92(10):e1029-e1040.
2. Marrie RA, Reingold S, Cohen J, et al. The incidence and prevalence of psychiatric disorders in multiple sclerosis: a systematic review. Mult Scler. 2015;21(3):305-317.
3. Chwastiak L, Ehde DM, Gibbons LE, Sullivan M, Bowen JD, Kraft GH. Depressive symptoms and severity of illness in multiple sclerosis: epidemiologic study of a large community sample. Am J Psychiatry. 2002;159(11):1862-1868.
4. Williams RM, Turner AP, Hatzakis M Jr, Bowen JD, Rodriquez AA, Haselkorn JK. Prevalence and correlates of depression among veterans with multiple sclerosis. Neurology. 2005;64(1):75-80.
5. Moore P, Hirst C, Harding KE, Clarkson H, Pickersgill TP, Robertson NP. Multiple sclerosis relapses and depression. J Psychosom Res. 2012;73(4):272-276.
6. Wood B, van der Mei IA, Ponsonby AL, et al. Prevalence and concurrence of anxiety, depression and fatigue over time in multiple sclerosis. Mult Scler. 2013;19(2):217-224.
7. Arnett PA, Higginson CI, Voss WD, et al. Depressed mood in multiple sclerosis: relationship to capacity-demanding memory and attentional functioning. Neuropsychology. 1999;13(3):434-446.
8. Diamond BJ, Johnson SK, Kaufman M, Graves L. Relationships between information processing, depression, fatigue and cognition in multiple sclerosis. Arch Clin Neuropsychol. 2008;23(2):189-199.
9. Benedict RH, Wahlig E, Bakshi R, et al. Predicting quality of life in multiple sclerosis: accounting for physical disability, fatigue, cognition, mood disorder, personality, and behavior change. J Neurol Sci. 2005;231(1-2):29-34.
10. Turner AP, Williams RM, Bowen JD, Kivlahan DR, Haselkorn JK. Suicidal ideation in multiple sclerosis. Arch Phys Med Rehabil. 2006;87(8):1073-1078.
11. Stenager EN, Koch-Henriksen N, Stenager E. Risk factors for suicide in multiple sclerosis. Psychother Psychosom. 1996;65(2):86-90.
12. Mohr DC, Boudewyn AC, Goodkin DE, Bostrom A, Epstein L. Comparative outcomes for individual cognitive-behavior therapy, supportive-expressive group psychotherapy, and sertraline for the treatment of depression in multiple sclerosis. J Consult Clin Psychol. 2001;69(6):942-949.
13. Larcombe NA, Wilson PH. An evaluation of cognitive-behaviour therapy for depression in patients with multiple sclerosis. Br J Psychiatry. 1984;145:366-371.
14. Lincoln NB, Yuill F, Holmes J, et al. Evaluation of an adjustment group for people with multiple sclerosis and low mood: a randomized controlled trial. Mult Scler. 2011;17(10):1250-1257.
15. Mohr DC, Likosky W, Bertagnolli A, et al. Telephone-administered cognitive-behavioral therapy for the treatment of depressive symptoms in multiple sclerosis. J Consult Clin Psychol. 2000;68(2):356-361.
16. Mohr DC, Hart SL, Julian L, et al. Telephone-administered psychotherapy for depression. Arch Gen Psychiatry. 2005;62(9):1007-1014.
17. Korostil M, Feinstein A. Anxiety disorders and their clinical correlates in multiple sclerosis patients. Mult Scler. 2007;13(1):67-72.
18. Boeschoten RE, Braamse AMJ, Beekman ATF, et al. Prevalence of depression and anxiety in multiple sclerosis: a systematic review and meta-analysis. J Neurol Sci. 2017;372:331-341.
19. Dahl OP, Stordal E, Lydersen S, Midgard R. Anxiety and depression in multiple sclerosis. A comparative population-based study in Nord-Trøndelag County, Norway. Mult Scler. 2009;15(12):1495-1501.
20. Burns MN, Nawacki E, Siddique J, Pelletier D, Mohr DC. Prospective examination of anxiety and depression before and during confirmed and pseudoexacerbations in patients with multiple sclerosis. Psychosom Med. 2013;75(1):76-82.
21. Uguz F, Akpinar Z, Ozkan I, Tokgoz S. Mood and anxiety disorders in patients with multiple sclerosis. Int J Psychiatry Clin Pract. 2008;12(1):19-24.
22. Askey-Jones S, David AS, Silber E, Shaw P, Chalder T. Cognitive behaviour therapy for common mental disorders in people with multiple sclerosis: a bench marking study. Behav Res Ther. 2013;51(10):648-655.
23. Carrigan N, Dysch L, Salkovskis PM. The impact of health anxiety in multiple sclerosis: a replication and treatment case series. Behav Cogn Psychother. 2018;46(2):148-167.
24. Mohr DC, Cox D, Merluzzi N. Self-injection anxiety training: a treatment for patients unable to self-inject injectable medications. Mult Scler. 2005;11(2):182-185.
25. Lazarus RS, Folkman S. Stress, Appraisal, and Coping. New York, NY: Springer; 1984.
26. Ackerman KD, Heyman R, Rabin BS, et al. Stressful life events precede exacerbations of multiple sclerosis. Psychosom Med. 2002;64(6):916-920.
27. Mohr DC, Hart SL, Julian L, Cox D, Pelletier D. Association between stressful life events and exacerbation in multiple sclerosis: a meta-analysis. BMJ. 2004;328(7442):731.
28. Mohr DC, Goodkin DE, Bacchetti P, et al. Psychological stress and the subsequent appearance of new brain MRI lesions in MS. Neurology. 2000;55(1):55-61.
29. Foley FW, Bedell JR, LaRocca NG, Scheinberg LC, Reznikoff M. Efficacy of stress-inoculation training in coping with multiple sclerosis. J Consult Clin Psychol. 1987;55(6):919-922.
30. Hughes RB, Robinson-Whelen S, Taylor HB, Hall JW. Stress self-management: an intervention for women with physical disabilities. Womens Health Issues. 2006;16(6):389-399.
31. Mohr DC, Lovera J, Brown T, et al. A randomized trial of stress management for the prevention of new brain lesions in MS. Neurology. 2012;79(5):412-419.
32. Dennison L, Moss-Morris R, Chalder T. A review of psychological correlates of adjustment in patients with multiple sclerosis. Clin Psychol Rev. 2009;29(2):141-153.
33. Moss-Morris R, Dennison L, Landau S, Yardley L, Silber E, Chalder T. A randomized controlled trial of cognitive behavioral therapy (CBT) for adjusting to multiple sclerosis (the saMS trial): does CBT work and for whom does it work? J Consult Clin Psychol. 2013;81(2):251-262.
34. Molton IR, Koelmel E, Curran M, von Geldern G, Ordway A, Alschuler KN. Pilot intervention to promote tolerance for uncertainty in early multiple sclerosis. Rehabil Psychol. 2019;64(3):339-350.
35. Ehde DM, Elzea JL, Verrall AM, Gibbons LE, Smith AE, Amtmann D. Efficacy of a telephone-delivered self-management intervention for persons with multiple sclerosis: a randomized controlled trial with a one-year follow-up. Arch Phys Med Rehabil. 2015;96(11):1945-1958.e2.
36. DeBolt LS, McCubbin JA. The effects of home-based resistance exercise on balance, power, and mobility in adults with multiple sclerosis. Arch Phys Med Rehabil. 2004;85(2):290-297.
37. Stuifbergen AK, Blozis SA, Harrison TC, Becker HA. Exercise, functional limitations, and quality of life: a longitudinal study of persons with multiple sclerosis. Arch Phys Med Rehabil. 2006;87(7):935-943.
38. Turner AP, Hartoonian N, Maynard C, Leipertz SL, Haselkorn JK. Smoking and physical activity: examining health behaviors and 15-year mortality among individuals with multiple sclerosis. Arch Phys Med Rehabil. 2015;96(3):402-409.
39. Turner AP, Kivlahan DR, Haselkorn JK. Exercise and quality of life among people with multiple sclerosis: looking beyond physical functioning to mental health and participation in life. Arch Phys Med Rehabil. 2009;90(3):420-428.
40. Turner AP, Hartoonian N, Sloan AP, et al. Improving fatigue and depression in individuals with multiple sclerosis using telephone-administered physical activity counseling. J Consult Clin Psychol. 2016;84(4):297-309.
41. Bombardier CH, Ehde DM, Gibbons LE, et al. Telephone-based physical activity counseling for major depression in people with multiple sclerosis. J Consult Clin Psychol. 2013;81(1):89-99.
42. Burks J, Marshall TS, Ye X. Adherence to disease-modifying therapies and its impact on relapse, health resource utilization, and costs among patients with multiple sclerosis. Clinicoecon Outcomes Res. 2017;9:251-260.
43. Freedman MS. Disease-modifying drugs for multiple sclerosis: current and future aspects. Expert Opin Pharmacother. 2006;7 Suppl 1:S1-S9.
44. Turner AP, Sloan AP, Kivlahan DR, Haselkorn JK. Telephone counseling and home telehealth monitoring to improve medication adherence: results of a pilot trial among individuals with multiple sclerosis. Rehabil Psychol. 2014;59(2):136-146.
45. Kabat-Zinn J. Full Catastrophe Living. London, UK: Piatkus; 2013.
46. Bishop SR. What do we really know about mindfulness-based stress reduction? [published correction appears in Psychosom Med. 2002;64(3):449]. Psychosom Med. 2002;64(1):71-83.
47. Lindsay EK, Young S, Smyth JM, Brown KW, Creswell JD. Acceptance lowers stress reactivity: dismantling mindfulness training in a randomized controlled trial. Psychoneuroendocrinology. 2018;87:63-73.
48. Simpson R, Mair FS, Mercer SW. Mindfulness-based stress reduction for people with multiple sclerosis - a feasibility randomised controlled trial. BMC Neurol. 2017;17(1):94.
49. Cavalera C, Rovaris M, Mendozzi L, et al. Online meditation training for people with multiple sclerosis: a randomized controlled trial. Mult Scler. 2019;25(4):610-617.
50. Grossman P, Kappos L, Gensicke H, et al. MS quality of life, depression, and fatigue improve after mindfulness training: a randomized trial. Neurology. 2010;75(13):1141-1149.
51. Shah A. Fatigue in multiple sclerosis. Phys Med Rehabil Clin N Am. 2009;20(2):363-372.
52. Guidelines MSCfCP. Fatigue and Multiple Sclerosis: Evidence-based Management Strategies for Fatigue in Multiple Sclerosis. Washington, DC: Paralyzed Veterans of America; 1998.
53. Krupp LB. Fatigue in multiple sclerosis: definition, pathophysiology and treatment. CNS Drugs. 2003;17(4):225-234.
54. Schwartz CE, Coulthard-Morris L, Zeng Q. Psychosocial correlates of fatigue in multiple sclerosis. Arch Phys Med Rehabil. 1996;77(2):165-170.
55. Moss-Morris R, McCrone P, Yardley L, van Kessel K, Wills G, Dennison L. A pilot randomised controlled trial of an Internet-based cognitive behavioural therapy self-management programme (MS Invigor8) for multiple sclerosis fatigue. Behav Res Ther. 2012;50(6):415-421.
56. Thomas PW, Thomas S, Kersten P, et al. Multi-centre parallel arm randomised controlled trial to assess the effectiveness and cost-effectiveness of a group-based cognitive behavioural approach to managing fatigue in people with multiple sclerosis. BMC Neurol. 2010;10:43.
57. van Kessel K, Moss-Morris R, Willoughby E, Chalder T, Johnson MH, Robinson E. A randomized controlled trial of cognitive behavior therapy for multiple sclerosis fatigue. Psychosom Med. 2008;70(2):205-213.
58. Foley PL, Vesterinen HM, Laird BJ, et al. Prevalence and natural history of pain in adults with multiple sclerosis: systematic review and meta-analysis. Pain. 2013;154(5):632-642.
59. O’Connor AB, Schwid SR, Herrmann DN, Markman JD, Dworkin RH. Pain associated with multiple sclerosis: systematic review and proposed classification. Pain. 2008;137(1):96-111.
60. Ehde DM, Osborne TL, Hanley MA, Jensen MP, Kraft GH. The scope and nature of pain in persons with multiple sclerosis. Mult Scler. 2006;12(5):629-638.
61. Aboud T, Schuster NM. Pain management in multiple sclerosis: a review of available treatment options. Curr Treat Options Neurol. 2019;21(12):62.
62. Amtmann D, Askew RL, Kim J, et al. Pain affects depression through anxiety, fatigue, and sleep in multiple sclerosis. Rehabil Psychol. 2015;60(1):81-90.
63. Arewasikporn A, Turner AP, Alschuler KN, Hughes AJ, Ehde DM. Cognitive and affective mechanisms of pain and fatigue in multiple sclerosis. Health Psychol. 2018;37(6):544-552.
64. Demaneuf T, Aitken Z, Karahalios A, et al. Effectiveness of exercise interventions for pain reduction in people with multiple sclerosis: a systematic review and meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. 2019;100(1):128-139.
65. Jensen MP, Barber J, Romano JM, et al. A comparison of self-hypnosis versus progressive muscle relaxation in patients with multiple sclerosis and chronic pain. Int J Clin Exp Hypn. 2009;57(2):198-221.
66. Veehof MM, Oskam MJ, Schreurs KM, Bohlmeijer ET. Acceptance-based interventions for the treatment of chronic pain: a systematic review and meta-analysis. Pain. 2011;152(3):533-542.
Multiple Sclerosis (MS) is a chronic demyelinating disease of the central nervous system that affects nearly 1 million people in the US.1 In addition to the accumulation of functional limitations, patients with MS commonly experience mental health and physical symptoms such as depression, anxiety, stress, fatigue, and pain. Day-to-day life with MS requires adaptation to challenges and active maintenance of health and well-being over time. Behavioral intervention and treatment, whether in the form of psychotherapy, health behavior coaching, or the promotion of active self-management, is an integral component of interprofessional care and key aspect of living well with MS.
Behavioral Comorbidities
Depression
Depression is a common concern among individuals with MS. Population-based studies suggest that individuals with MS have a roughly 1 in 4 chance of developing major depressive disorder over their lifetime.2 However, at any given time, between 40% and 60% of individuals with MS report clinically meaningful levels of depressive symptoms.3 Although the relationship between MS disease characteristics and depression is unclear, some evidence suggests that depressive symptoms are more common at certain points in illness, such as early in the disease process as individuals grapple with the onset of new symptoms, late in the disease process as they accumulate greater disability, and during active clinical relapses.3-5
Depression often is comorbid with, and adds to the symptom burden of, other common conditions in MS such as fatigue and cognitive dysfunction.6-8 Thus, it is not surprising that it associated with poorer overall quality of life (QOL).9 Depression also is a risk factor for suicidal ideation and suicide for patients with MS.10,11
Fortunately, several behavioral interventions show promise in treating depression in patients with MS. Both individual and group formats of cognitive behavioral therapy (CBT), a treatment focused on challenging maladaptive patterns of thought and behavior, have been shown to improve depressive symptoms for people with MS.12,13 Several brief and efficient group-based programs grounded in CBT and focused on the development of specific skills, including problem solving, goal setting, relationship management, and managing emotions, have been shown to reduce depressive symptoms.13,14 CBT for depression in MS has been shown to be effective when delivered via telephone.15,16
Anxiety
Anxiety is common among individuals with MS. Existing data suggest more than one-third of individuals with MS will qualify for a diagnosis of anxiety disorder during their lifetime.17 The characteristics of anxiety disorders are broad and heterogenous, including generalized anxiety disorder, panic disorder, obsessive compulsive disorders, and health-specific phobias such as needle/injection anxiety. Some estimates suggest a point prevalence of 34% for the presence of clinically meaningful symptoms.18 Similar to depression, anxiety symptoms can be more common during periods of stress, threat, and transition including early in the disease course while adapting to new diagnosis, late in the disease course with increasing disability, and during clinical relapses.19-21
The efficacy of behavioral interventions for anxiety in MS is less well established than it is for depression, but some preliminary evidence suggests that individual CBT may be effective for reducing general symptoms of anxiety as well as health-related anxiety.22,23 Brief, targeted CBT also has been shown to improve injection anxiety, removing a barrier to self-care including the administration of MS disease modifying therapies (DMTs).24
Stress
Stress is commonly conceptualized as a person’s perception that efforts to manage internal and external demands exceed available coping resources.25 Such demands involve both psychological and physiological processes and come in many forms for people with MS and can include daily hassles, major life events, traumatic stress, and perceptions of global nonspecific stress. The relationship between stress and MS remains complex and poorly understood. Nonetheless, individuals with MS frequently report that stress exacerbates their symptoms.26
Some evidence also suggests stress may exacerbate the MS disease process, resulting in more frequent relapses and increased lesion activity visible on MRI.27,28 In addition to mindfulness (described below), stress inoculation training (CBT and relaxation training), and stress-focused group-based self-management have been shown to be beneficial.29,30 In an intriguing and rigorous trial, a 24-week stress management therapy based on CBT was associated with the development of fewer new MS lesions visible on MRI.31
Adaptation to Illness
MS presents challenges that vary between patients and over time. Individuals may confront new physical and cognitive limitations that inhibit the completion of daily tasks, reduce independence, and limit participation in valued and meaningful activities. In addition, the unpredictability of the disease contributes to perceptions of uncertainty and uncontrollability, which in turn result in higher illness impact and poorer psychological outcomes.32 Building cognitive and behavioral skills to address these challenges can promote adaptation to illness and reduce overall distress associated with chronic illness.33 Psychosocial intervention also can address the uncertainty commonly experienced by individuals with MS.34
Self-Management
As with any chronic illness, living well with MS requires ongoing commitment and active engagement with health and personal care over time. The process of building knowledge and skills to manage the day-to-day physical, emotional, and social aspects of living with illness often is referred to as self-management.35 For individuals with MS, this may take the form of participation in programs that address adaptation and psychological distress like those described above, but it also may include improving health behavior (eg, physical activity, DMT adherence, modification of maladaptive habits like smoking or hazardous alcohol use) and symptom management (eg, fatigue, pain). Self-management programs typically include education, the practice of identifying, problem solving, and following through with specific and realistic health and wellness goals, as well as the bolstering of self-efficacy.
Physical Activity
Once discouraged for patients with MS, physical activity is now considered a cornerstone of health and wellness. Physical activity and interventions that target various forms of exercise have been shown to improve strength and endurance, reduce functional decline, enhance QOL, and likely reduce mortality.35-39 A variety of brief behavioral interventions have been shown to improve physical activity in MS. Structured group-based exercise classes focusing on various activities such as aerobic training (eg, cycling) or resistance training (eg, lower extremity strengthening) have demonstrated improvements in various measures of fitness and mood states such as depression and QOL. Brief home-based telephone counseling interventions based in social cognitive theory (eg, goal setting, navigating obstacles) and motivational interviewing strategies (eg, open-ended questions, affirmation, reflective listening, summarizing) also have been shown to be effective not only at increasing physical activity and improving depression and fatigue.40,41
Adherence to Treatment
One primary focus of adherence to treatment is medication management. For individuals with MS, DMTs represent a primary means of reducing disease burden and delaying functional decline. Many DMTs require consistent self-administration over time. Some evidence suggests that poorer adherence is associated with a greater risk of relapse and more rapid disease progression.42,43 Brief telephone counseling, again based on social cognitive theory, and principles of motivational interviewing combined with home telehealth monitoring by a care coordinator has been shown to improve adherence to DMTs.44
Mindfulness
In recent years, mindfulness training has emerged as a popular and common behavioral intervention among individuals with MS. Programs like Mindfulness-Based Stress Reduction (MBSR) provide training in meditation techniques designed to promote mindfulness, which is defined as paying attention to present moment experience, including sensations, thoughts, and emotions, without judgment or attachment.45 Cultivating mindfulness helps people with MS cope with and adapt to symptoms and stressors.46 Mindfulness interventions typically are delivered in a group format. For example, MBSR consists of 8 in-person group sessions with daily meditation practice homework. Mindfulness interventions also have been delivered effectively with smartphone apps.47 Mindfulness programs have been shown to improve depression, anxiety, fatigue, stress, and QOL for patients with MS.48-50
Fatigue
More than 90% of individuals with MS report fatigue, and many identify it as their most disabling symptom.51 Often defined as “a subjective lack of physical and/or mental energy that is perceived by the individual or caregiver to interfere with usual and desired activities,” fatigue has been shown to be associated with longer disease duration, greater physical disability, progressive subtype, and depressive symptoms, although the relative and possibly overlapping impact of these issues is only partially understood.52,53 Fatigue is associated with poorer overall mental health and negatively impacts work and social roles.54
Several behavioral interventions have been developed to address fatigue in MS. Using both individual and group based formats and across several modalities (eg, in-person, telephone, online modules, or a combination), behavioral fatigue interventions most commonly combine traditional general CBT skills (eg, addressing maladaptive thoughts and behaviors) with a variety of fatigue-specific skill building exercises that may include fatigue education, energy conservation strategies, improving sleep, enlisting social support, and self-management goal setting strategies.35,55-57
Pain
Chronic pain is common and disabling in people with MS.58,59 Nearly 50% report experiencing moderate to severe chronic pain.59,60 Individuals with MS reporting pain often are older, more disabled (higher Expanded Disability Status Scale score), and have longer disease duration that those who are not experiencing chronic pain.61 Patients report various types of pain in the following order of frequency: dysesthetic pain (18.1%), back pain (16.4%), painful tonic spasms (11.0%), Lhermitte sign (9.0%), visceral pain (2.9%), and trigeminal neuralgia (2.0%).61 Chronic pain has a negative impact on QOL in the areas of sleep, work, maintaining relationships, recreational activities, and overall life enjoyment.59 Additionally, research has shown that greater pain intensity and pain-related interference with activities of daily living are both associated with greater depression severity.62,63
The literature supports the use of behavioral interventions for pain in people with MS.61 Behavioral interventions include in-person exercise interventions (eg, water aerobics, cycling, rowing ergometer, treadmill walking, and resistance training), self-hypnosis, and telephone-based self-management programs based on CBT.35,64,65 As described above, CBT-based self-management programs combine learning CBT skills (eg, modifying maladaptive thoughts) with pain-specific skill building such as pain education, pacing activities, and improving sleep. Of note, MS education including, but not limited to, pain was as effective as a CBT-based self-management program in reducing pain intensity and interference.35 In addition, there is evidence to support acceptance- and mindfulness-based interventions for chronic pain, and online mindfulness-based cognitive therapy for MS related pain is currently being tested in a randomized controlled trial.35,66
Conclusion
People with MS face significant challenges in coping with and adapting to a chronic and unpredictable disease. However, there is considerable evidence that behavioral interventions can improve many of the most common and disabling symptoms in MS including depression, anxiety, stress, fatigue, and pain as well as health behavior and self-care. Research also suggests that improvements in one of these problems (eg, physical inactivity) can influence improvement in other symptoms (eg, depression and fatigue). Unlike other treatment options, behavioral interventions can be delivered in various formats (eg, in-person and electronic health), are time-limited, and cause few (if any) undesirable systemic adverse effects. Behavioral interventions are therefore, an essential part of interprofessional care and rehabilitation for patients with MS.
Multiple Sclerosis (MS) is a chronic demyelinating disease of the central nervous system that affects nearly 1 million people in the US.1 In addition to the accumulation of functional limitations, patients with MS commonly experience mental health and physical symptoms such as depression, anxiety, stress, fatigue, and pain. Day-to-day life with MS requires adaptation to challenges and active maintenance of health and well-being over time. Behavioral intervention and treatment, whether in the form of psychotherapy, health behavior coaching, or the promotion of active self-management, is an integral component of interprofessional care and key aspect of living well with MS.
Behavioral Comorbidities
Depression
Depression is a common concern among individuals with MS. Population-based studies suggest that individuals with MS have a roughly 1 in 4 chance of developing major depressive disorder over their lifetime.2 However, at any given time, between 40% and 60% of individuals with MS report clinically meaningful levels of depressive symptoms.3 Although the relationship between MS disease characteristics and depression is unclear, some evidence suggests that depressive symptoms are more common at certain points in illness, such as early in the disease process as individuals grapple with the onset of new symptoms, late in the disease process as they accumulate greater disability, and during active clinical relapses.3-5
Depression often is comorbid with, and adds to the symptom burden of, other common conditions in MS such as fatigue and cognitive dysfunction.6-8 Thus, it is not surprising that it associated with poorer overall quality of life (QOL).9 Depression also is a risk factor for suicidal ideation and suicide for patients with MS.10,11
Fortunately, several behavioral interventions show promise in treating depression in patients with MS. Both individual and group formats of cognitive behavioral therapy (CBT), a treatment focused on challenging maladaptive patterns of thought and behavior, have been shown to improve depressive symptoms for people with MS.12,13 Several brief and efficient group-based programs grounded in CBT and focused on the development of specific skills, including problem solving, goal setting, relationship management, and managing emotions, have been shown to reduce depressive symptoms.13,14 CBT for depression in MS has been shown to be effective when delivered via telephone.15,16
Anxiety
Anxiety is common among individuals with MS. Existing data suggest more than one-third of individuals with MS will qualify for a diagnosis of anxiety disorder during their lifetime.17 The characteristics of anxiety disorders are broad and heterogenous, including generalized anxiety disorder, panic disorder, obsessive compulsive disorders, and health-specific phobias such as needle/injection anxiety. Some estimates suggest a point prevalence of 34% for the presence of clinically meaningful symptoms.18 Similar to depression, anxiety symptoms can be more common during periods of stress, threat, and transition including early in the disease course while adapting to new diagnosis, late in the disease course with increasing disability, and during clinical relapses.19-21
The efficacy of behavioral interventions for anxiety in MS is less well established than it is for depression, but some preliminary evidence suggests that individual CBT may be effective for reducing general symptoms of anxiety as well as health-related anxiety.22,23 Brief, targeted CBT also has been shown to improve injection anxiety, removing a barrier to self-care including the administration of MS disease modifying therapies (DMTs).24
Stress
Stress is commonly conceptualized as a person’s perception that efforts to manage internal and external demands exceed available coping resources.25 Such demands involve both psychological and physiological processes and come in many forms for people with MS and can include daily hassles, major life events, traumatic stress, and perceptions of global nonspecific stress. The relationship between stress and MS remains complex and poorly understood. Nonetheless, individuals with MS frequently report that stress exacerbates their symptoms.26
Some evidence also suggests stress may exacerbate the MS disease process, resulting in more frequent relapses and increased lesion activity visible on MRI.27,28 In addition to mindfulness (described below), stress inoculation training (CBT and relaxation training), and stress-focused group-based self-management have been shown to be beneficial.29,30 In an intriguing and rigorous trial, a 24-week stress management therapy based on CBT was associated with the development of fewer new MS lesions visible on MRI.31
Adaptation to Illness
MS presents challenges that vary between patients and over time. Individuals may confront new physical and cognitive limitations that inhibit the completion of daily tasks, reduce independence, and limit participation in valued and meaningful activities. In addition, the unpredictability of the disease contributes to perceptions of uncertainty and uncontrollability, which in turn result in higher illness impact and poorer psychological outcomes.32 Building cognitive and behavioral skills to address these challenges can promote adaptation to illness and reduce overall distress associated with chronic illness.33 Psychosocial intervention also can address the uncertainty commonly experienced by individuals with MS.34
Self-Management
As with any chronic illness, living well with MS requires ongoing commitment and active engagement with health and personal care over time. The process of building knowledge and skills to manage the day-to-day physical, emotional, and social aspects of living with illness often is referred to as self-management.35 For individuals with MS, this may take the form of participation in programs that address adaptation and psychological distress like those described above, but it also may include improving health behavior (eg, physical activity, DMT adherence, modification of maladaptive habits like smoking or hazardous alcohol use) and symptom management (eg, fatigue, pain). Self-management programs typically include education, the practice of identifying, problem solving, and following through with specific and realistic health and wellness goals, as well as the bolstering of self-efficacy.
Physical Activity
Once discouraged for patients with MS, physical activity is now considered a cornerstone of health and wellness. Physical activity and interventions that target various forms of exercise have been shown to improve strength and endurance, reduce functional decline, enhance QOL, and likely reduce mortality.35-39 A variety of brief behavioral interventions have been shown to improve physical activity in MS. Structured group-based exercise classes focusing on various activities such as aerobic training (eg, cycling) or resistance training (eg, lower extremity strengthening) have demonstrated improvements in various measures of fitness and mood states such as depression and QOL. Brief home-based telephone counseling interventions based in social cognitive theory (eg, goal setting, navigating obstacles) and motivational interviewing strategies (eg, open-ended questions, affirmation, reflective listening, summarizing) also have been shown to be effective not only at increasing physical activity and improving depression and fatigue.40,41
Adherence to Treatment
One primary focus of adherence to treatment is medication management. For individuals with MS, DMTs represent a primary means of reducing disease burden and delaying functional decline. Many DMTs require consistent self-administration over time. Some evidence suggests that poorer adherence is associated with a greater risk of relapse and more rapid disease progression.42,43 Brief telephone counseling, again based on social cognitive theory, and principles of motivational interviewing combined with home telehealth monitoring by a care coordinator has been shown to improve adherence to DMTs.44
Mindfulness
In recent years, mindfulness training has emerged as a popular and common behavioral intervention among individuals with MS. Programs like Mindfulness-Based Stress Reduction (MBSR) provide training in meditation techniques designed to promote mindfulness, which is defined as paying attention to present moment experience, including sensations, thoughts, and emotions, without judgment or attachment.45 Cultivating mindfulness helps people with MS cope with and adapt to symptoms and stressors.46 Mindfulness interventions typically are delivered in a group format. For example, MBSR consists of 8 in-person group sessions with daily meditation practice homework. Mindfulness interventions also have been delivered effectively with smartphone apps.47 Mindfulness programs have been shown to improve depression, anxiety, fatigue, stress, and QOL for patients with MS.48-50
Fatigue
More than 90% of individuals with MS report fatigue, and many identify it as their most disabling symptom.51 Often defined as “a subjective lack of physical and/or mental energy that is perceived by the individual or caregiver to interfere with usual and desired activities,” fatigue has been shown to be associated with longer disease duration, greater physical disability, progressive subtype, and depressive symptoms, although the relative and possibly overlapping impact of these issues is only partially understood.52,53 Fatigue is associated with poorer overall mental health and negatively impacts work and social roles.54
Several behavioral interventions have been developed to address fatigue in MS. Using both individual and group based formats and across several modalities (eg, in-person, telephone, online modules, or a combination), behavioral fatigue interventions most commonly combine traditional general CBT skills (eg, addressing maladaptive thoughts and behaviors) with a variety of fatigue-specific skill building exercises that may include fatigue education, energy conservation strategies, improving sleep, enlisting social support, and self-management goal setting strategies.35,55-57
Pain
Chronic pain is common and disabling in people with MS.58,59 Nearly 50% report experiencing moderate to severe chronic pain.59,60 Individuals with MS reporting pain often are older, more disabled (higher Expanded Disability Status Scale score), and have longer disease duration that those who are not experiencing chronic pain.61 Patients report various types of pain in the following order of frequency: dysesthetic pain (18.1%), back pain (16.4%), painful tonic spasms (11.0%), Lhermitte sign (9.0%), visceral pain (2.9%), and trigeminal neuralgia (2.0%).61 Chronic pain has a negative impact on QOL in the areas of sleep, work, maintaining relationships, recreational activities, and overall life enjoyment.59 Additionally, research has shown that greater pain intensity and pain-related interference with activities of daily living are both associated with greater depression severity.62,63
The literature supports the use of behavioral interventions for pain in people with MS.61 Behavioral interventions include in-person exercise interventions (eg, water aerobics, cycling, rowing ergometer, treadmill walking, and resistance training), self-hypnosis, and telephone-based self-management programs based on CBT.35,64,65 As described above, CBT-based self-management programs combine learning CBT skills (eg, modifying maladaptive thoughts) with pain-specific skill building such as pain education, pacing activities, and improving sleep. Of note, MS education including, but not limited to, pain was as effective as a CBT-based self-management program in reducing pain intensity and interference.35 In addition, there is evidence to support acceptance- and mindfulness-based interventions for chronic pain, and online mindfulness-based cognitive therapy for MS related pain is currently being tested in a randomized controlled trial.35,66
Conclusion
People with MS face significant challenges in coping with and adapting to a chronic and unpredictable disease. However, there is considerable evidence that behavioral interventions can improve many of the most common and disabling symptoms in MS including depression, anxiety, stress, fatigue, and pain as well as health behavior and self-care. Research also suggests that improvements in one of these problems (eg, physical inactivity) can influence improvement in other symptoms (eg, depression and fatigue). Unlike other treatment options, behavioral interventions can be delivered in various formats (eg, in-person and electronic health), are time-limited, and cause few (if any) undesirable systemic adverse effects. Behavioral interventions are therefore, an essential part of interprofessional care and rehabilitation for patients with MS.
1. Wallin MT, Culpepper WJ, Campbell JD, et al; US Multiple Sclerosis Workgroup. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology. 2019;92(10):e1029-e1040.
2. Marrie RA, Reingold S, Cohen J, et al. The incidence and prevalence of psychiatric disorders in multiple sclerosis: a systematic review. Mult Scler. 2015;21(3):305-317.
3. Chwastiak L, Ehde DM, Gibbons LE, Sullivan M, Bowen JD, Kraft GH. Depressive symptoms and severity of illness in multiple sclerosis: epidemiologic study of a large community sample. Am J Psychiatry. 2002;159(11):1862-1868.
4. Williams RM, Turner AP, Hatzakis M Jr, Bowen JD, Rodriquez AA, Haselkorn JK. Prevalence and correlates of depression among veterans with multiple sclerosis. Neurology. 2005;64(1):75-80.
5. Moore P, Hirst C, Harding KE, Clarkson H, Pickersgill TP, Robertson NP. Multiple sclerosis relapses and depression. J Psychosom Res. 2012;73(4):272-276.
6. Wood B, van der Mei IA, Ponsonby AL, et al. Prevalence and concurrence of anxiety, depression and fatigue over time in multiple sclerosis. Mult Scler. 2013;19(2):217-224.
7. Arnett PA, Higginson CI, Voss WD, et al. Depressed mood in multiple sclerosis: relationship to capacity-demanding memory and attentional functioning. Neuropsychology. 1999;13(3):434-446.
8. Diamond BJ, Johnson SK, Kaufman M, Graves L. Relationships between information processing, depression, fatigue and cognition in multiple sclerosis. Arch Clin Neuropsychol. 2008;23(2):189-199.
9. Benedict RH, Wahlig E, Bakshi R, et al. Predicting quality of life in multiple sclerosis: accounting for physical disability, fatigue, cognition, mood disorder, personality, and behavior change. J Neurol Sci. 2005;231(1-2):29-34.
10. Turner AP, Williams RM, Bowen JD, Kivlahan DR, Haselkorn JK. Suicidal ideation in multiple sclerosis. Arch Phys Med Rehabil. 2006;87(8):1073-1078.
11. Stenager EN, Koch-Henriksen N, Stenager E. Risk factors for suicide in multiple sclerosis. Psychother Psychosom. 1996;65(2):86-90.
12. Mohr DC, Boudewyn AC, Goodkin DE, Bostrom A, Epstein L. Comparative outcomes for individual cognitive-behavior therapy, supportive-expressive group psychotherapy, and sertraline for the treatment of depression in multiple sclerosis. J Consult Clin Psychol. 2001;69(6):942-949.
13. Larcombe NA, Wilson PH. An evaluation of cognitive-behaviour therapy for depression in patients with multiple sclerosis. Br J Psychiatry. 1984;145:366-371.
14. Lincoln NB, Yuill F, Holmes J, et al. Evaluation of an adjustment group for people with multiple sclerosis and low mood: a randomized controlled trial. Mult Scler. 2011;17(10):1250-1257.
15. Mohr DC, Likosky W, Bertagnolli A, et al. Telephone-administered cognitive-behavioral therapy for the treatment of depressive symptoms in multiple sclerosis. J Consult Clin Psychol. 2000;68(2):356-361.
16. Mohr DC, Hart SL, Julian L, et al. Telephone-administered psychotherapy for depression. Arch Gen Psychiatry. 2005;62(9):1007-1014.
17. Korostil M, Feinstein A. Anxiety disorders and their clinical correlates in multiple sclerosis patients. Mult Scler. 2007;13(1):67-72.
18. Boeschoten RE, Braamse AMJ, Beekman ATF, et al. Prevalence of depression and anxiety in multiple sclerosis: a systematic review and meta-analysis. J Neurol Sci. 2017;372:331-341.
19. Dahl OP, Stordal E, Lydersen S, Midgard R. Anxiety and depression in multiple sclerosis. A comparative population-based study in Nord-Trøndelag County, Norway. Mult Scler. 2009;15(12):1495-1501.
20. Burns MN, Nawacki E, Siddique J, Pelletier D, Mohr DC. Prospective examination of anxiety and depression before and during confirmed and pseudoexacerbations in patients with multiple sclerosis. Psychosom Med. 2013;75(1):76-82.
21. Uguz F, Akpinar Z, Ozkan I, Tokgoz S. Mood and anxiety disorders in patients with multiple sclerosis. Int J Psychiatry Clin Pract. 2008;12(1):19-24.
22. Askey-Jones S, David AS, Silber E, Shaw P, Chalder T. Cognitive behaviour therapy for common mental disorders in people with multiple sclerosis: a bench marking study. Behav Res Ther. 2013;51(10):648-655.
23. Carrigan N, Dysch L, Salkovskis PM. The impact of health anxiety in multiple sclerosis: a replication and treatment case series. Behav Cogn Psychother. 2018;46(2):148-167.
24. Mohr DC, Cox D, Merluzzi N. Self-injection anxiety training: a treatment for patients unable to self-inject injectable medications. Mult Scler. 2005;11(2):182-185.
25. Lazarus RS, Folkman S. Stress, Appraisal, and Coping. New York, NY: Springer; 1984.
26. Ackerman KD, Heyman R, Rabin BS, et al. Stressful life events precede exacerbations of multiple sclerosis. Psychosom Med. 2002;64(6):916-920.
27. Mohr DC, Hart SL, Julian L, Cox D, Pelletier D. Association between stressful life events and exacerbation in multiple sclerosis: a meta-analysis. BMJ. 2004;328(7442):731.
28. Mohr DC, Goodkin DE, Bacchetti P, et al. Psychological stress and the subsequent appearance of new brain MRI lesions in MS. Neurology. 2000;55(1):55-61.
29. Foley FW, Bedell JR, LaRocca NG, Scheinberg LC, Reznikoff M. Efficacy of stress-inoculation training in coping with multiple sclerosis. J Consult Clin Psychol. 1987;55(6):919-922.
30. Hughes RB, Robinson-Whelen S, Taylor HB, Hall JW. Stress self-management: an intervention for women with physical disabilities. Womens Health Issues. 2006;16(6):389-399.
31. Mohr DC, Lovera J, Brown T, et al. A randomized trial of stress management for the prevention of new brain lesions in MS. Neurology. 2012;79(5):412-419.
32. Dennison L, Moss-Morris R, Chalder T. A review of psychological correlates of adjustment in patients with multiple sclerosis. Clin Psychol Rev. 2009;29(2):141-153.
33. Moss-Morris R, Dennison L, Landau S, Yardley L, Silber E, Chalder T. A randomized controlled trial of cognitive behavioral therapy (CBT) for adjusting to multiple sclerosis (the saMS trial): does CBT work and for whom does it work? J Consult Clin Psychol. 2013;81(2):251-262.
34. Molton IR, Koelmel E, Curran M, von Geldern G, Ordway A, Alschuler KN. Pilot intervention to promote tolerance for uncertainty in early multiple sclerosis. Rehabil Psychol. 2019;64(3):339-350.
35. Ehde DM, Elzea JL, Verrall AM, Gibbons LE, Smith AE, Amtmann D. Efficacy of a telephone-delivered self-management intervention for persons with multiple sclerosis: a randomized controlled trial with a one-year follow-up. Arch Phys Med Rehabil. 2015;96(11):1945-1958.e2.
36. DeBolt LS, McCubbin JA. The effects of home-based resistance exercise on balance, power, and mobility in adults with multiple sclerosis. Arch Phys Med Rehabil. 2004;85(2):290-297.
37. Stuifbergen AK, Blozis SA, Harrison TC, Becker HA. Exercise, functional limitations, and quality of life: a longitudinal study of persons with multiple sclerosis. Arch Phys Med Rehabil. 2006;87(7):935-943.
38. Turner AP, Hartoonian N, Maynard C, Leipertz SL, Haselkorn JK. Smoking and physical activity: examining health behaviors and 15-year mortality among individuals with multiple sclerosis. Arch Phys Med Rehabil. 2015;96(3):402-409.
39. Turner AP, Kivlahan DR, Haselkorn JK. Exercise and quality of life among people with multiple sclerosis: looking beyond physical functioning to mental health and participation in life. Arch Phys Med Rehabil. 2009;90(3):420-428.
40. Turner AP, Hartoonian N, Sloan AP, et al. Improving fatigue and depression in individuals with multiple sclerosis using telephone-administered physical activity counseling. J Consult Clin Psychol. 2016;84(4):297-309.
41. Bombardier CH, Ehde DM, Gibbons LE, et al. Telephone-based physical activity counseling for major depression in people with multiple sclerosis. J Consult Clin Psychol. 2013;81(1):89-99.
42. Burks J, Marshall TS, Ye X. Adherence to disease-modifying therapies and its impact on relapse, health resource utilization, and costs among patients with multiple sclerosis. Clinicoecon Outcomes Res. 2017;9:251-260.
43. Freedman MS. Disease-modifying drugs for multiple sclerosis: current and future aspects. Expert Opin Pharmacother. 2006;7 Suppl 1:S1-S9.
44. Turner AP, Sloan AP, Kivlahan DR, Haselkorn JK. Telephone counseling and home telehealth monitoring to improve medication adherence: results of a pilot trial among individuals with multiple sclerosis. Rehabil Psychol. 2014;59(2):136-146.
45. Kabat-Zinn J. Full Catastrophe Living. London, UK: Piatkus; 2013.
46. Bishop SR. What do we really know about mindfulness-based stress reduction? [published correction appears in Psychosom Med. 2002;64(3):449]. Psychosom Med. 2002;64(1):71-83.
47. Lindsay EK, Young S, Smyth JM, Brown KW, Creswell JD. Acceptance lowers stress reactivity: dismantling mindfulness training in a randomized controlled trial. Psychoneuroendocrinology. 2018;87:63-73.
48. Simpson R, Mair FS, Mercer SW. Mindfulness-based stress reduction for people with multiple sclerosis - a feasibility randomised controlled trial. BMC Neurol. 2017;17(1):94.
49. Cavalera C, Rovaris M, Mendozzi L, et al. Online meditation training for people with multiple sclerosis: a randomized controlled trial. Mult Scler. 2019;25(4):610-617.
50. Grossman P, Kappos L, Gensicke H, et al. MS quality of life, depression, and fatigue improve after mindfulness training: a randomized trial. Neurology. 2010;75(13):1141-1149.
51. Shah A. Fatigue in multiple sclerosis. Phys Med Rehabil Clin N Am. 2009;20(2):363-372.
52. Guidelines MSCfCP. Fatigue and Multiple Sclerosis: Evidence-based Management Strategies for Fatigue in Multiple Sclerosis. Washington, DC: Paralyzed Veterans of America; 1998.
53. Krupp LB. Fatigue in multiple sclerosis: definition, pathophysiology and treatment. CNS Drugs. 2003;17(4):225-234.
54. Schwartz CE, Coulthard-Morris L, Zeng Q. Psychosocial correlates of fatigue in multiple sclerosis. Arch Phys Med Rehabil. 1996;77(2):165-170.
55. Moss-Morris R, McCrone P, Yardley L, van Kessel K, Wills G, Dennison L. A pilot randomised controlled trial of an Internet-based cognitive behavioural therapy self-management programme (MS Invigor8) for multiple sclerosis fatigue. Behav Res Ther. 2012;50(6):415-421.
56. Thomas PW, Thomas S, Kersten P, et al. Multi-centre parallel arm randomised controlled trial to assess the effectiveness and cost-effectiveness of a group-based cognitive behavioural approach to managing fatigue in people with multiple sclerosis. BMC Neurol. 2010;10:43.
57. van Kessel K, Moss-Morris R, Willoughby E, Chalder T, Johnson MH, Robinson E. A randomized controlled trial of cognitive behavior therapy for multiple sclerosis fatigue. Psychosom Med. 2008;70(2):205-213.
58. Foley PL, Vesterinen HM, Laird BJ, et al. Prevalence and natural history of pain in adults with multiple sclerosis: systematic review and meta-analysis. Pain. 2013;154(5):632-642.
59. O’Connor AB, Schwid SR, Herrmann DN, Markman JD, Dworkin RH. Pain associated with multiple sclerosis: systematic review and proposed classification. Pain. 2008;137(1):96-111.
60. Ehde DM, Osborne TL, Hanley MA, Jensen MP, Kraft GH. The scope and nature of pain in persons with multiple sclerosis. Mult Scler. 2006;12(5):629-638.
61. Aboud T, Schuster NM. Pain management in multiple sclerosis: a review of available treatment options. Curr Treat Options Neurol. 2019;21(12):62.
62. Amtmann D, Askew RL, Kim J, et al. Pain affects depression through anxiety, fatigue, and sleep in multiple sclerosis. Rehabil Psychol. 2015;60(1):81-90.
63. Arewasikporn A, Turner AP, Alschuler KN, Hughes AJ, Ehde DM. Cognitive and affective mechanisms of pain and fatigue in multiple sclerosis. Health Psychol. 2018;37(6):544-552.
64. Demaneuf T, Aitken Z, Karahalios A, et al. Effectiveness of exercise interventions for pain reduction in people with multiple sclerosis: a systematic review and meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. 2019;100(1):128-139.
65. Jensen MP, Barber J, Romano JM, et al. A comparison of self-hypnosis versus progressive muscle relaxation in patients with multiple sclerosis and chronic pain. Int J Clin Exp Hypn. 2009;57(2):198-221.
66. Veehof MM, Oskam MJ, Schreurs KM, Bohlmeijer ET. Acceptance-based interventions for the treatment of chronic pain: a systematic review and meta-analysis. Pain. 2011;152(3):533-542.
1. Wallin MT, Culpepper WJ, Campbell JD, et al; US Multiple Sclerosis Workgroup. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology. 2019;92(10):e1029-e1040.
2. Marrie RA, Reingold S, Cohen J, et al. The incidence and prevalence of psychiatric disorders in multiple sclerosis: a systematic review. Mult Scler. 2015;21(3):305-317.
3. Chwastiak L, Ehde DM, Gibbons LE, Sullivan M, Bowen JD, Kraft GH. Depressive symptoms and severity of illness in multiple sclerosis: epidemiologic study of a large community sample. Am J Psychiatry. 2002;159(11):1862-1868.
4. Williams RM, Turner AP, Hatzakis M Jr, Bowen JD, Rodriquez AA, Haselkorn JK. Prevalence and correlates of depression among veterans with multiple sclerosis. Neurology. 2005;64(1):75-80.
5. Moore P, Hirst C, Harding KE, Clarkson H, Pickersgill TP, Robertson NP. Multiple sclerosis relapses and depression. J Psychosom Res. 2012;73(4):272-276.
6. Wood B, van der Mei IA, Ponsonby AL, et al. Prevalence and concurrence of anxiety, depression and fatigue over time in multiple sclerosis. Mult Scler. 2013;19(2):217-224.
7. Arnett PA, Higginson CI, Voss WD, et al. Depressed mood in multiple sclerosis: relationship to capacity-demanding memory and attentional functioning. Neuropsychology. 1999;13(3):434-446.
8. Diamond BJ, Johnson SK, Kaufman M, Graves L. Relationships between information processing, depression, fatigue and cognition in multiple sclerosis. Arch Clin Neuropsychol. 2008;23(2):189-199.
9. Benedict RH, Wahlig E, Bakshi R, et al. Predicting quality of life in multiple sclerosis: accounting for physical disability, fatigue, cognition, mood disorder, personality, and behavior change. J Neurol Sci. 2005;231(1-2):29-34.
10. Turner AP, Williams RM, Bowen JD, Kivlahan DR, Haselkorn JK. Suicidal ideation in multiple sclerosis. Arch Phys Med Rehabil. 2006;87(8):1073-1078.
11. Stenager EN, Koch-Henriksen N, Stenager E. Risk factors for suicide in multiple sclerosis. Psychother Psychosom. 1996;65(2):86-90.
12. Mohr DC, Boudewyn AC, Goodkin DE, Bostrom A, Epstein L. Comparative outcomes for individual cognitive-behavior therapy, supportive-expressive group psychotherapy, and sertraline for the treatment of depression in multiple sclerosis. J Consult Clin Psychol. 2001;69(6):942-949.
13. Larcombe NA, Wilson PH. An evaluation of cognitive-behaviour therapy for depression in patients with multiple sclerosis. Br J Psychiatry. 1984;145:366-371.
14. Lincoln NB, Yuill F, Holmes J, et al. Evaluation of an adjustment group for people with multiple sclerosis and low mood: a randomized controlled trial. Mult Scler. 2011;17(10):1250-1257.
15. Mohr DC, Likosky W, Bertagnolli A, et al. Telephone-administered cognitive-behavioral therapy for the treatment of depressive symptoms in multiple sclerosis. J Consult Clin Psychol. 2000;68(2):356-361.
16. Mohr DC, Hart SL, Julian L, et al. Telephone-administered psychotherapy for depression. Arch Gen Psychiatry. 2005;62(9):1007-1014.
17. Korostil M, Feinstein A. Anxiety disorders and their clinical correlates in multiple sclerosis patients. Mult Scler. 2007;13(1):67-72.
18. Boeschoten RE, Braamse AMJ, Beekman ATF, et al. Prevalence of depression and anxiety in multiple sclerosis: a systematic review and meta-analysis. J Neurol Sci. 2017;372:331-341.
19. Dahl OP, Stordal E, Lydersen S, Midgard R. Anxiety and depression in multiple sclerosis. A comparative population-based study in Nord-Trøndelag County, Norway. Mult Scler. 2009;15(12):1495-1501.
20. Burns MN, Nawacki E, Siddique J, Pelletier D, Mohr DC. Prospective examination of anxiety and depression before and during confirmed and pseudoexacerbations in patients with multiple sclerosis. Psychosom Med. 2013;75(1):76-82.
21. Uguz F, Akpinar Z, Ozkan I, Tokgoz S. Mood and anxiety disorders in patients with multiple sclerosis. Int J Psychiatry Clin Pract. 2008;12(1):19-24.
22. Askey-Jones S, David AS, Silber E, Shaw P, Chalder T. Cognitive behaviour therapy for common mental disorders in people with multiple sclerosis: a bench marking study. Behav Res Ther. 2013;51(10):648-655.
23. Carrigan N, Dysch L, Salkovskis PM. The impact of health anxiety in multiple sclerosis: a replication and treatment case series. Behav Cogn Psychother. 2018;46(2):148-167.
24. Mohr DC, Cox D, Merluzzi N. Self-injection anxiety training: a treatment for patients unable to self-inject injectable medications. Mult Scler. 2005;11(2):182-185.
25. Lazarus RS, Folkman S. Stress, Appraisal, and Coping. New York, NY: Springer; 1984.
26. Ackerman KD, Heyman R, Rabin BS, et al. Stressful life events precede exacerbations of multiple sclerosis. Psychosom Med. 2002;64(6):916-920.
27. Mohr DC, Hart SL, Julian L, Cox D, Pelletier D. Association between stressful life events and exacerbation in multiple sclerosis: a meta-analysis. BMJ. 2004;328(7442):731.
28. Mohr DC, Goodkin DE, Bacchetti P, et al. Psychological stress and the subsequent appearance of new brain MRI lesions in MS. Neurology. 2000;55(1):55-61.
29. Foley FW, Bedell JR, LaRocca NG, Scheinberg LC, Reznikoff M. Efficacy of stress-inoculation training in coping with multiple sclerosis. J Consult Clin Psychol. 1987;55(6):919-922.
30. Hughes RB, Robinson-Whelen S, Taylor HB, Hall JW. Stress self-management: an intervention for women with physical disabilities. Womens Health Issues. 2006;16(6):389-399.
31. Mohr DC, Lovera J, Brown T, et al. A randomized trial of stress management for the prevention of new brain lesions in MS. Neurology. 2012;79(5):412-419.
32. Dennison L, Moss-Morris R, Chalder T. A review of psychological correlates of adjustment in patients with multiple sclerosis. Clin Psychol Rev. 2009;29(2):141-153.
33. Moss-Morris R, Dennison L, Landau S, Yardley L, Silber E, Chalder T. A randomized controlled trial of cognitive behavioral therapy (CBT) for adjusting to multiple sclerosis (the saMS trial): does CBT work and for whom does it work? J Consult Clin Psychol. 2013;81(2):251-262.
34. Molton IR, Koelmel E, Curran M, von Geldern G, Ordway A, Alschuler KN. Pilot intervention to promote tolerance for uncertainty in early multiple sclerosis. Rehabil Psychol. 2019;64(3):339-350.
35. Ehde DM, Elzea JL, Verrall AM, Gibbons LE, Smith AE, Amtmann D. Efficacy of a telephone-delivered self-management intervention for persons with multiple sclerosis: a randomized controlled trial with a one-year follow-up. Arch Phys Med Rehabil. 2015;96(11):1945-1958.e2.
36. DeBolt LS, McCubbin JA. The effects of home-based resistance exercise on balance, power, and mobility in adults with multiple sclerosis. Arch Phys Med Rehabil. 2004;85(2):290-297.
37. Stuifbergen AK, Blozis SA, Harrison TC, Becker HA. Exercise, functional limitations, and quality of life: a longitudinal study of persons with multiple sclerosis. Arch Phys Med Rehabil. 2006;87(7):935-943.
38. Turner AP, Hartoonian N, Maynard C, Leipertz SL, Haselkorn JK. Smoking and physical activity: examining health behaviors and 15-year mortality among individuals with multiple sclerosis. Arch Phys Med Rehabil. 2015;96(3):402-409.
39. Turner AP, Kivlahan DR, Haselkorn JK. Exercise and quality of life among people with multiple sclerosis: looking beyond physical functioning to mental health and participation in life. Arch Phys Med Rehabil. 2009;90(3):420-428.
40. Turner AP, Hartoonian N, Sloan AP, et al. Improving fatigue and depression in individuals with multiple sclerosis using telephone-administered physical activity counseling. J Consult Clin Psychol. 2016;84(4):297-309.
41. Bombardier CH, Ehde DM, Gibbons LE, et al. Telephone-based physical activity counseling for major depression in people with multiple sclerosis. J Consult Clin Psychol. 2013;81(1):89-99.
42. Burks J, Marshall TS, Ye X. Adherence to disease-modifying therapies and its impact on relapse, health resource utilization, and costs among patients with multiple sclerosis. Clinicoecon Outcomes Res. 2017;9:251-260.
43. Freedman MS. Disease-modifying drugs for multiple sclerosis: current and future aspects. Expert Opin Pharmacother. 2006;7 Suppl 1:S1-S9.
44. Turner AP, Sloan AP, Kivlahan DR, Haselkorn JK. Telephone counseling and home telehealth monitoring to improve medication adherence: results of a pilot trial among individuals with multiple sclerosis. Rehabil Psychol. 2014;59(2):136-146.
45. Kabat-Zinn J. Full Catastrophe Living. London, UK: Piatkus; 2013.
46. Bishop SR. What do we really know about mindfulness-based stress reduction? [published correction appears in Psychosom Med. 2002;64(3):449]. Psychosom Med. 2002;64(1):71-83.
47. Lindsay EK, Young S, Smyth JM, Brown KW, Creswell JD. Acceptance lowers stress reactivity: dismantling mindfulness training in a randomized controlled trial. Psychoneuroendocrinology. 2018;87:63-73.
48. Simpson R, Mair FS, Mercer SW. Mindfulness-based stress reduction for people with multiple sclerosis - a feasibility randomised controlled trial. BMC Neurol. 2017;17(1):94.
49. Cavalera C, Rovaris M, Mendozzi L, et al. Online meditation training for people with multiple sclerosis: a randomized controlled trial. Mult Scler. 2019;25(4):610-617.
50. Grossman P, Kappos L, Gensicke H, et al. MS quality of life, depression, and fatigue improve after mindfulness training: a randomized trial. Neurology. 2010;75(13):1141-1149.
51. Shah A. Fatigue in multiple sclerosis. Phys Med Rehabil Clin N Am. 2009;20(2):363-372.
52. Guidelines MSCfCP. Fatigue and Multiple Sclerosis: Evidence-based Management Strategies for Fatigue in Multiple Sclerosis. Washington, DC: Paralyzed Veterans of America; 1998.
53. Krupp LB. Fatigue in multiple sclerosis: definition, pathophysiology and treatment. CNS Drugs. 2003;17(4):225-234.
54. Schwartz CE, Coulthard-Morris L, Zeng Q. Psychosocial correlates of fatigue in multiple sclerosis. Arch Phys Med Rehabil. 1996;77(2):165-170.
55. Moss-Morris R, McCrone P, Yardley L, van Kessel K, Wills G, Dennison L. A pilot randomised controlled trial of an Internet-based cognitive behavioural therapy self-management programme (MS Invigor8) for multiple sclerosis fatigue. Behav Res Ther. 2012;50(6):415-421.
56. Thomas PW, Thomas S, Kersten P, et al. Multi-centre parallel arm randomised controlled trial to assess the effectiveness and cost-effectiveness of a group-based cognitive behavioural approach to managing fatigue in people with multiple sclerosis. BMC Neurol. 2010;10:43.
57. van Kessel K, Moss-Morris R, Willoughby E, Chalder T, Johnson MH, Robinson E. A randomized controlled trial of cognitive behavior therapy for multiple sclerosis fatigue. Psychosom Med. 2008;70(2):205-213.
58. Foley PL, Vesterinen HM, Laird BJ, et al. Prevalence and natural history of pain in adults with multiple sclerosis: systematic review and meta-analysis. Pain. 2013;154(5):632-642.
59. O’Connor AB, Schwid SR, Herrmann DN, Markman JD, Dworkin RH. Pain associated with multiple sclerosis: systematic review and proposed classification. Pain. 2008;137(1):96-111.
60. Ehde DM, Osborne TL, Hanley MA, Jensen MP, Kraft GH. The scope and nature of pain in persons with multiple sclerosis. Mult Scler. 2006;12(5):629-638.
61. Aboud T, Schuster NM. Pain management in multiple sclerosis: a review of available treatment options. Curr Treat Options Neurol. 2019;21(12):62.
62. Amtmann D, Askew RL, Kim J, et al. Pain affects depression through anxiety, fatigue, and sleep in multiple sclerosis. Rehabil Psychol. 2015;60(1):81-90.
63. Arewasikporn A, Turner AP, Alschuler KN, Hughes AJ, Ehde DM. Cognitive and affective mechanisms of pain and fatigue in multiple sclerosis. Health Psychol. 2018;37(6):544-552.
64. Demaneuf T, Aitken Z, Karahalios A, et al. Effectiveness of exercise interventions for pain reduction in people with multiple sclerosis: a systematic review and meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. 2019;100(1):128-139.
65. Jensen MP, Barber J, Romano JM, et al. A comparison of self-hypnosis versus progressive muscle relaxation in patients with multiple sclerosis and chronic pain. Int J Clin Exp Hypn. 2009;57(2):198-221.
66. Veehof MM, Oskam MJ, Schreurs KM, Bohlmeijer ET. Acceptance-based interventions for the treatment of chronic pain: a systematic review and meta-analysis. Pain. 2011;152(3):533-542.
Telehealth Pulmonary Rehabilitation for Patients With Severe Chronic Obstructive Pulmonary Disease
According to World Health Organization estimates, 65 million people have moderate-to-severe chronic obstructive pulmonary disease (COPD) globally, and > 20 million patients with COPD are living in the US.1 COPD is a progressive respiratory disease with a poor prognosis and a significant cause of morbidity and mortality in the US, especially within the Veterans Health Administration (VHA).2 The prevalence of COPD is higher in veterans than it is in the general population. COPD prevalence in the adult US population has been estimated to be between 5% and 15%, whereas in veterans, prevalence estimates have ranged from about 5% to 43%.3-5
COPD is associated with disabling dyspnea, muscle weakness, exercise intolerance, morbidity, and mortality. These symptoms and complications gradually and progressively compromise mobility, ability to perform daily functions, and decrease quality of life (QOL). Dyspnea, fatigue, and discomfort are the principal symptoms that negatively impact exercise tolerance.6,7 Therefore, patients often intentionally limit their activities to avoid these uncomfortable feelings and adopt a more sedentary behavior. As the disease progresses, individuals with COPD will gradually need assistance in performing activities of daily living, which eventually leads to functional dependence.
Pulmonary rehabilitation (PR) is an essential component of the management of symptomatic patients with COPD. PR is an evidence-based, multidisciplinary, comprehensive intervention that includes exercise and education for patients with chronic respiratory disease.8 The key benefits of PR are clinical improvements in dyspnea, physical capacity, QOL, and reduced disability in patients with COPD and other respiratory diseases.9-11 PR was found to improve respiratory health in veterans with COPD and decrease respiratory-related health care utilization.12
Despite the known benefits of PR, many patients with chronic respiratory diseases are not referred or do not have access to rehabilitation. Also, uptake of PR is low due to patient frailty, transportation issues, and other health care access problems.13-15 Unfortunately, in the US health care system, access to PR and other nonpharmacologic treatments can be challenging due to a shortage of available PR programs, limited physician referral to existing programs, and lack of family and social support.16
There are only a few accredited PR programs in VHA facilities, and they tend to be located in urban areas.12,17 Many patients have limited access to the PR programs due to geographic distance to the programs and transportation challenges (eg, limited ability to drive, cost of transportation). Moreover, veterans with COPD are likely to have limited mobility or are homebound due to experiencing shortness of breath with minimal exertion. Given the clear benefits of PR and the increasing impact of COPD on morbidity and mortality of the patients with COPD, strategies to improve the access and capacity of PR are needed. VA telehealth services allow for distribution of health care services in different geographic locations by providing access for the veterans who live in rural and highly rural areas. The most recent implementation of VA Video Connect (VVC) by the VHA provides a new avenue for clinicians to deliver much needed medical care into the veterans’ home.
COPD Telehealth Program
In this article, we describe the processes for developing and delivering an in-home, interactive, supervised PR program for veterans with severe COPD through VA telehealth service. The program consists of 18 sessions delivered over 6 weeks by a licensed physical therapist (PT) and a respiratory therapist (RT). The aims of the telehealth PR are to improve exercise tolerance, reduce dyspnea and fatigue, improve QOL, improve accessibility, and decrease costs and transportation burdens for patients with COPD. The program was developed, implemented and delivered by an interdisciplinary team, including a pulmonologist, PT, RT, physiatrist, and nonclinical supporting staff.
Patient Assessment
To be eligible to participate in the program the patient must: (1) have a forced expiratory volume (FEV1) < 60%; ( 2) be medically stable and be receiving optimal medical management; (3) have no severe cognitive impairments; (4) be able to use a computer and e-mail; (5) be able to ambulate with or without a walking device; (6) be willing to enroll in a smoking cessation program or to stop smoking; (7) be willing to participate without prolonged interruption; and (8) have all visual and auditory impairments corrected with medical devices.
After referral and enrollment, patients receive medical and physical examinations by the PR team, including a pulmonologist, a PT, and a RT, to ensure that the patients are medically stable to undergo rehabilitation and to develop a tailored exercise program while being mindful of the comorbidities, limitations, and precautions, (eg, loss of balance, risk of fall, limited range of motion). The preprogram assessment includes a pulmonary function test, arterial blood gas test, Montreal Cognitive Assessment, Modified Medical Research Council Scale, St. George Respiratory Questionnaire, the COPD Assessment Test, Patient Health Questionnaire-9,Generalized Anxiety Disorder Assessment-7, Epworth Sleepiness Scale, Katz Index of Independence of Activities of Daily Living, medications and inhaler use, oxygen use, breathing pattern, coughing, 6-minute walk test, Modified Borg Dyspnea Scale, grip strength, 5 Times Sit to Stand Test, manual muscle test, gait measure, Timed Up & Go test, clinical balance tests, range of motion, flexibility, sensation, pain, and fall history.18-32 Educational needs (eg, respiratory hygiene, nutrition, infection control, sleep, disease/symptom management) also are evaluated.
This thorough assessment is performed in a face-to-face outpatient visit. During the program participation, a physiatrist may be consulted for additional needs (eg, wheelchair assessment, home safety evaluation/ modifications, and mobility/disability issues). After completing the 6-week program, patients are scheduled for the postprogram evaluation in a face-to-face outpatient visit with the clinicians.
Equipment
Both clinician and the patient are equipped with a computer with Wi-Fi connectivity, a webcam, and a microphone. Patients are provided an exercise pictorial booklet, an exercise compact disk (audio and video), small exercise apparatuses (eg, assorted colors of resistance bands, hand grip exerciser, hand putty, ergometer, harmonica, and pedometer), incentive spirometer, pulse oximeter, cough assistive device (as needed), blood pressure monitor, COPD information booklets, and a diary to use at home during the program.33
Technology Preparation
Prior to starting the telehealth program, the patient is contacted 1 or 2 days before the first session for technical preparation and familiarization of the VA telehealth connection process. Either the PT or RT provides step-by-step instructions for the patient to practice connecting through VVC during this preparatory phone call. The patient also practices using the computer webcam, speaker, and microphone; checks the telehealth scheduling e-mail; and learns how to solve possible common technical issues (eg, adjusting volume and position of webcam). The patient is asked to set up a table close to the computer and to place all exercise apparatuses and respiratory devices on the table surface.
Program Delivery
A secure online VVC is used for connection during the telehealth session. The patient received an e-mail from the telehealth scheduling system with a link for VVC before each session. During the 6-week program, each telehealth session is conducted by a PT and a RT concurrently for 120 minutes, 3 days per week. The PT provides exercises for the patient to attempt, and the RT provides breathing training and monitoring during the session. After a successful connection to VVC, the therapist verifies the patient’s identity and confirms patient consent for the telehealth session.
After this check-in process, the patient performs a self-measure of resting blood pressure (BP), heart rate, respiratory rate, and blood oxygen saturation and reports to the therapists. During the exercise session, fatigue/exertion, dyspnea (Modified Borg Dyspnea Scale; Borg CR10 Scale), BP, heart rate, oxygen saturation, and other clinical symptoms and responses to exercise are monitored by the therapists, using both patient-reported measures and clinical observation by the therapists.34,35 Any medical emergency during the session is reported immediately to the pulmonologist for further management.
Structure
Prior to each exercise session, exercise precautions, fall prevention, good posture, pursed-lip breathing, pacing, and coordinated breathing are discussed with the patient. The PT demonstrates stretching and warm-up exercises in front of the webcam for the patient to follow. Then the patient performs all exercises in view of the webcam during the session (Figure 1). A RT monitors breathing patterns and corrects with verbal instructions if not properly performed.
Loss of skeletal muscle mass and cachexia are highly prevalent comorbidities of COPD and have been associated with breathlessness, functional limitation, and poor prognosis.36 To address these comorbidities, our program consists of progressive strengthening, aerobic, balance, and flexibility exercises. Resistance bands and tubes are used for strengthening exercises. Callisthenic exercises (eg, chair squat, chair stand, knee marching, bridging, single limb stances, and lunge) are used for progressive strengthening and balance exercises. Progression of strengthening and balance exercises are done through increasing the volume of exercise (ie, numbers of sets and repetitions) and increased load and level of difficulty based on the patient’s progress and comorbidity. The exercise program focuses on strengthening muscles, especially large muscle groups, to improve overall muscle strength and performance of functional activities.37
Arm/pedal ergometer and daily walking are used for daily aerobic exercise. In a study of patients with COPD by the PAC-COPD Study Group, step counter use was found to increase physical activity and improve exercise capacity, which supports its use in COPD management.38 During program participation, the patient is asked to wear a pedometer to monitor the number of steps taken per day and to report step data to the therapists during the telehealth session. The pedometer stores the previous 41 calendar days of data and displays the most recent 7 calendar days of data.
The patient is encouraged to set a realistic daily step goal. The general program goal is to increase at least 1000 steps per day. However, this goal can be adjusted depending on the patient’s health status and comorbid conditions. The PAC-COPD Study Group found that for every additional 1000 daily steps at low intensity, COPD hospitalization risk decreased by 20%.39 A magnitude of 2000 steps or about 1 mile of walking per day was found to be associated with increased physical activity and health benefits in the general population.40
Respiratory muscle training and breathing exercise are provided by the RT, using breathing and incentive spirometer techniques (Figure 2). Huff coughing, diaphragmatic deep breathing, and pursed-lip breathing are instructed by the RT during the session. Effective coughing technique with a cough assistive device is also provided during breathing training if needed.
Patient Education
In patients with COPD, there are numerous positive health benefits associated with education, including assisting the patients to become active participants in the PR program leading to satisfying outcomes; assisting the patients to better understand the lung health, disease processes, physical and psychological changes that occur with COPD; assisting the patients to explore coping strategies for those changes; building lifelong behavioral changes; and developing the self-management skills for sustainability. Through the educational process, patients with COPD can become more skilled at collaborative self-management and improve adherence to their treatment plan, which in turn can result in a reduction in hospital admissions and reduced health care costs.8,41
Education is provided with every session after the patient completes the exercise. Patients are required to record their COPD symptoms, daily activity, home exercise program, sleep, food intake, and additional physical or social activity in their COPD diary and to report during the session (Figure 3). A COPD diary assists patients in self-monitoring their COPD symptoms and provides the therapists with information about clinical changes, behavioral changes, and/or specific unmet needs for education. Several topics related to COPD are included in the education session: lung or respiratory disease/condition and self-management; smoking cessation; physical activity; energy-conserving techniques; breathing and coughing techniques; smoking cessation; nutrition/healthy eating and weight counseling; sex and intimacy; psychological counseling and/or group support; emergency planning (eg, medical, travel, and inclement weather); correct use of inhaler and medications; home oxygen; sleep and sleep hygiene; palliative care and advanced directive; infection control; and sputum clearance.42,43
Program Maintenance
After successfully completing the 6-week program, patients are referred to the VA TeleMOVE! Program or MOVE! Weight Management Program for continuous, long-term management of weight, nutrition, physical activity/exercise, and social activity needs or goals. The patients are scheduled for monthly follow-up phone visits for 6 months with the telerehabilitation team for enforcing sustainability. The phone call visit consists of reviewing breathing techniques, exercise program, physical activity, education, encouragement, and addressing any issues that arise during the self-maintained period.
Limitations
There are several issues of concern and precautions when delivering PR through telehealth into the home. First, the patient performs exercises independently without being manually guarded by the therapists. Risk of falls are a major concern due to impaired balance, poor vision, and other possible unusual physiologic responses to exercise (eg, drop in BP, dizziness, loss of balance). The area in front of the computer needs to be cleared of fall hazards (ie, area rug, wires, objects on the floor). The patient also needs to be educated on self-measurements of BP and oxygen saturation and reports to the therapists. The therapists provide detailed instructions on how to obtain these measures correctly; otherwise, the values may not be valid for a clinical judgment during the exercise session or for other clinical management. In a home environment, there is a limited use of exercise apparatuses. For this program, we only used resistance bands/tubes, small arm/leg ergometer, hand grip, and hand putty for the exercise program. We feel that dumbbell and weight plates are not suitable due to a possible risk of injury if the patient accidently drops them.
Advanced balance training is not suitable due to an increased risk for falls. Without the presence of the PT, level of challenge/difficulty is somewhat limited for this telehealth supervision exercise program. In addition, visual and audio quality are necessary for the session. The patient and the therapists need to see each other clearly to ensure correct methods and forms of each exercise. Furthermore, rehearsal of technical skills with the therapists is very important because this population is older and often has limited computer skills. Any technical difficulty or failure can lead to undesirable situations (eg, anxiety episodes, worries, shortness of breath, upset), which compromise exercise performance during the session. Finally, a phone is needed as an alternative in case of a poor VVC connection.
Conclusion
COPD symptoms and complications greatly affect patients’ ability to perform daily activities, decrease QOL and functional ability, and result in extensive use of health services. Many patients have limited access to a PR program at hospitals or rehabilitation centers due to health conditions, lack of transportation, and/or family support. This home-based, interactive telehealth PR program can break down the geographic barriers, solve poor program accessibility, potentially increase the utilization of PR, and reduce the cost and travel required by the patients.
Acknowledgments
The Telehealth Pulmonary Rehabilitation Program was originally funded by the Veterans Health Administration VA ACCESS Program (AS, CL, HKH). We thank all the veterans for their time and effort in participating in this newly developed rehabilitation program.
1. World Health Organization. Chronic obstructive pulmonary disease (COPD). http://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd). Published December 1, 2017. Accessed August 7, 2019.
2. Yu W, Ravelo A, Wagner TH, et al. Prevalence and costs of chronic conditions in the VA health care system. Med Care Res Rev. 2003;60(suppl 3):146S-167S.
3. Doney B, Hnizdo E, Dillon CF, et al. Prevalence of airflow obstruction in U.S. adults aged 40-79 years: NHANES data 1988-1994 and 2007-2010. COPD. 2015;12(4):355-365.
4. Murphy DE, Chaudhry Z, Almoosa KF, Panos RJ. High prevalence of chronic obstructive pulmonary disease among veterans in the urban midwest. Mil Med. 2011;176(5):552-560.
5. Cypel YS, Hines SE, Davey VJ, Eber SM, Schneiderman AI. Self-reported physician-diagnosed chronic obstructive pulmonary disease and spirometry patterns in Vietnam Era US Army Chemical Corps veterans: a retrospective cohort study. Am J Ind Med. 2018;61(10):802-814.
6. Rochester CL. Exercise training in chronic obstructive pulmonary disease. J Rehabil Res Dev. 2003;40(5)(suppl 2):59-80.
7. Cortopassi F, Gurung P, Pinto-Plata V. Chronic obstructive pulmonary disease in elderly patients. Clin Geriatr Med. 2017;33(4):539-552.
8. Spruit MA, Singh SJ, Garvey C, et al; ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13-e64.
9. Robinson H, Williams V, Curtis F, Bridle C, Jones AW. Facilitators and barriers to physical activity following pulmonary rehabilitation in COPD: a systematic review of qualitative studies. NPJ Prim Care Respir Med. 2018;28(1):19.
10. McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;(2):CD003793.
11. Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary rehabilitation: joint AACP/AACVPR evidence-based clinical practice guidelines. Chest. 2007;131(suppl 5):4S-42S.
12. Major S, Moreno M, Shelton J, Panos RJ. Veterans with chronic obstructive pulmonary disease achieve clinically relevant improvements in respiratory health after pulmonary rehabilitation. J Cardiopulm Rehabil Prev. 2014;34(6):420-429.
13. Liu Y, Dickerson T, Early F, Fuld J, Clarkson PJ. Understanding influences on the uptake of pulmonary rehabilitation in the East of England: an inclusive design/mixed methods study protocol. BMJ Open. 2018;8(4):e020750.
14. Harris D, Hayter M, Allender S. Factors affecting the offer of pulmonary rehabilitation to patients with chronic obstructive pulmonary disease by primary care professionals: a qualitative study. Prim Health Care Res Dev. 2008;9(4):280-290.
15. Mathar H, Fastholm P, Hansen IR, Larsen NS. Why do patients with COPD decline rehabilitation. Scand J Caring Sci. 2016;30(3):432-441.
16. Han MK, Martinez CH, Au DH, et al. Meeting the challenge of COPD care delivery in the USA: a multiprovider perspective. Lancet Respir Med. 2016;4(6):473-526.
17. American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR). Online searchable program directory. https://www.aacvpr.org/Resources/Program-Directory Accessed July 19, 2018.
18. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699.
19. Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J. 1959;2(5147):257-266.
20. O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2007 update. Can Respir J. 2007;14(suppl B):5B-32B.
21. Jones PW, Quirk FH, Baveystock CM. The St George’s Respiratory Questionnaire. Respir Med. 1991;85(suppl B):25-31.
22. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648-654.
23. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
24. Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097.
25. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540-545.
26. Katz S. Assessing self-maintenance: activities of daily living, mobility and instrumental activities of daily living. J Am Geriatr Soc. 1983;31(12):721-727.
27. Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428-1446.
28. Mahler DA, Horowitz MB. Perception of breathlessness during exercise in patients with respiratory disease. Med Sci Sports Exerc. 1994;26(9):1078-1081.
29. Liao WC, Wang CH, Yu SY, Chen LY, Wang CY. Grip strength measurement in older adults in Taiwan: a comparison of three testing positions. Australas J Ageing. 2014;33(4):278-282.
30. Buatois S, Miljkovic D, Manckoundia P, et al. Five times sit to stand test is a predictor of recurrent falls in healthy community-living subjects aged 65 and older. J Am Geriatr Soc. 2008;56(8):1575-1577.
31. Bryant MS, Workman CD, Jackson GR. Multidirectional walk test in persons with Parkinson’s disease: a validity study. Int J Rehabil Res. 2015;38(1):88-91.
32. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142-148.
33. University of Nebraska Medical Center. Timed Up and Go (TUG) Test. https://www.unmc.edu/media/intmed/geriatrics/nebgec/pdf/frailelderlyjuly09/toolkits/timedupandgo_w_norms.pdf. Accessed August 13, 2019.
34. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377-381.
35. Mahler DA, Horowitz MB. Clinical evaluation of exertional dyspnea. Clin Chest Med. 1994;15(2):259-269.
36. Dudgeon D, Baracos VE. Physiological and functional failure in chronic obstructive pulmonary disease, congestive heart failure and cancer: a debilitating intersection of sarcopenia, cachexia and breathlessness. Curr Opin Support Palliat Care. 2016;10(3):236-241.
37. Lee AL, Holland AE. Time to adapt exercise training regimens in pulmonary rehabilitation—a review of the literature. Int J Chron Obstruct Pulmon Dis. 2014;9:1275-1288.
38. Qiu S, Cai X, Wang X, et al. Using step counters to promote physical activity and exercise capacity in patients with chronic obstructive pulmonary disease: a meta-analysis. Ther Adv Respir Dis. 2018;12:1753466618787386.
39. Donaire-Gonzalez D, Gimeno-Santos E, Balcells E, et al; PAC-COPD Study Group. Benefits of physical activity on COPD hospitalization depend on intensity. Eur Respir J. 2015;46(5):1281-1289.
40. Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298(19):2296-2304.
41. Zwerink M, Brusse-Keizer M, van der Valk PD, et al. Self-management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;19(3):CD002990.
42. Wilson JS, O’Neill B, Reilly J, MacMahon J, Bradley JM. Education in pulmonary rehabilitation: the patient’s perspective. Arch Phys Med Rehabil. 2007;88(12):1704-1709.
43. Bourbeau J, Nault D, Dang-Tan T. Self-management and behaviour modification in COPD. Patient Educ Couns. 2004;52(3):271-277.
According to World Health Organization estimates, 65 million people have moderate-to-severe chronic obstructive pulmonary disease (COPD) globally, and > 20 million patients with COPD are living in the US.1 COPD is a progressive respiratory disease with a poor prognosis and a significant cause of morbidity and mortality in the US, especially within the Veterans Health Administration (VHA).2 The prevalence of COPD is higher in veterans than it is in the general population. COPD prevalence in the adult US population has been estimated to be between 5% and 15%, whereas in veterans, prevalence estimates have ranged from about 5% to 43%.3-5
COPD is associated with disabling dyspnea, muscle weakness, exercise intolerance, morbidity, and mortality. These symptoms and complications gradually and progressively compromise mobility, ability to perform daily functions, and decrease quality of life (QOL). Dyspnea, fatigue, and discomfort are the principal symptoms that negatively impact exercise tolerance.6,7 Therefore, patients often intentionally limit their activities to avoid these uncomfortable feelings and adopt a more sedentary behavior. As the disease progresses, individuals with COPD will gradually need assistance in performing activities of daily living, which eventually leads to functional dependence.
Pulmonary rehabilitation (PR) is an essential component of the management of symptomatic patients with COPD. PR is an evidence-based, multidisciplinary, comprehensive intervention that includes exercise and education for patients with chronic respiratory disease.8 The key benefits of PR are clinical improvements in dyspnea, physical capacity, QOL, and reduced disability in patients with COPD and other respiratory diseases.9-11 PR was found to improve respiratory health in veterans with COPD and decrease respiratory-related health care utilization.12
Despite the known benefits of PR, many patients with chronic respiratory diseases are not referred or do not have access to rehabilitation. Also, uptake of PR is low due to patient frailty, transportation issues, and other health care access problems.13-15 Unfortunately, in the US health care system, access to PR and other nonpharmacologic treatments can be challenging due to a shortage of available PR programs, limited physician referral to existing programs, and lack of family and social support.16
There are only a few accredited PR programs in VHA facilities, and they tend to be located in urban areas.12,17 Many patients have limited access to the PR programs due to geographic distance to the programs and transportation challenges (eg, limited ability to drive, cost of transportation). Moreover, veterans with COPD are likely to have limited mobility or are homebound due to experiencing shortness of breath with minimal exertion. Given the clear benefits of PR and the increasing impact of COPD on morbidity and mortality of the patients with COPD, strategies to improve the access and capacity of PR are needed. VA telehealth services allow for distribution of health care services in different geographic locations by providing access for the veterans who live in rural and highly rural areas. The most recent implementation of VA Video Connect (VVC) by the VHA provides a new avenue for clinicians to deliver much needed medical care into the veterans’ home.
COPD Telehealth Program
In this article, we describe the processes for developing and delivering an in-home, interactive, supervised PR program for veterans with severe COPD through VA telehealth service. The program consists of 18 sessions delivered over 6 weeks by a licensed physical therapist (PT) and a respiratory therapist (RT). The aims of the telehealth PR are to improve exercise tolerance, reduce dyspnea and fatigue, improve QOL, improve accessibility, and decrease costs and transportation burdens for patients with COPD. The program was developed, implemented and delivered by an interdisciplinary team, including a pulmonologist, PT, RT, physiatrist, and nonclinical supporting staff.
Patient Assessment
To be eligible to participate in the program the patient must: (1) have a forced expiratory volume (FEV1) < 60%; ( 2) be medically stable and be receiving optimal medical management; (3) have no severe cognitive impairments; (4) be able to use a computer and e-mail; (5) be able to ambulate with or without a walking device; (6) be willing to enroll in a smoking cessation program or to stop smoking; (7) be willing to participate without prolonged interruption; and (8) have all visual and auditory impairments corrected with medical devices.
After referral and enrollment, patients receive medical and physical examinations by the PR team, including a pulmonologist, a PT, and a RT, to ensure that the patients are medically stable to undergo rehabilitation and to develop a tailored exercise program while being mindful of the comorbidities, limitations, and precautions, (eg, loss of balance, risk of fall, limited range of motion). The preprogram assessment includes a pulmonary function test, arterial blood gas test, Montreal Cognitive Assessment, Modified Medical Research Council Scale, St. George Respiratory Questionnaire, the COPD Assessment Test, Patient Health Questionnaire-9,Generalized Anxiety Disorder Assessment-7, Epworth Sleepiness Scale, Katz Index of Independence of Activities of Daily Living, medications and inhaler use, oxygen use, breathing pattern, coughing, 6-minute walk test, Modified Borg Dyspnea Scale, grip strength, 5 Times Sit to Stand Test, manual muscle test, gait measure, Timed Up & Go test, clinical balance tests, range of motion, flexibility, sensation, pain, and fall history.18-32 Educational needs (eg, respiratory hygiene, nutrition, infection control, sleep, disease/symptom management) also are evaluated.
This thorough assessment is performed in a face-to-face outpatient visit. During the program participation, a physiatrist may be consulted for additional needs (eg, wheelchair assessment, home safety evaluation/ modifications, and mobility/disability issues). After completing the 6-week program, patients are scheduled for the postprogram evaluation in a face-to-face outpatient visit with the clinicians.
Equipment
Both clinician and the patient are equipped with a computer with Wi-Fi connectivity, a webcam, and a microphone. Patients are provided an exercise pictorial booklet, an exercise compact disk (audio and video), small exercise apparatuses (eg, assorted colors of resistance bands, hand grip exerciser, hand putty, ergometer, harmonica, and pedometer), incentive spirometer, pulse oximeter, cough assistive device (as needed), blood pressure monitor, COPD information booklets, and a diary to use at home during the program.33
Technology Preparation
Prior to starting the telehealth program, the patient is contacted 1 or 2 days before the first session for technical preparation and familiarization of the VA telehealth connection process. Either the PT or RT provides step-by-step instructions for the patient to practice connecting through VVC during this preparatory phone call. The patient also practices using the computer webcam, speaker, and microphone; checks the telehealth scheduling e-mail; and learns how to solve possible common technical issues (eg, adjusting volume and position of webcam). The patient is asked to set up a table close to the computer and to place all exercise apparatuses and respiratory devices on the table surface.
Program Delivery
A secure online VVC is used for connection during the telehealth session. The patient received an e-mail from the telehealth scheduling system with a link for VVC before each session. During the 6-week program, each telehealth session is conducted by a PT and a RT concurrently for 120 minutes, 3 days per week. The PT provides exercises for the patient to attempt, and the RT provides breathing training and monitoring during the session. After a successful connection to VVC, the therapist verifies the patient’s identity and confirms patient consent for the telehealth session.
After this check-in process, the patient performs a self-measure of resting blood pressure (BP), heart rate, respiratory rate, and blood oxygen saturation and reports to the therapists. During the exercise session, fatigue/exertion, dyspnea (Modified Borg Dyspnea Scale; Borg CR10 Scale), BP, heart rate, oxygen saturation, and other clinical symptoms and responses to exercise are monitored by the therapists, using both patient-reported measures and clinical observation by the therapists.34,35 Any medical emergency during the session is reported immediately to the pulmonologist for further management.
Structure
Prior to each exercise session, exercise precautions, fall prevention, good posture, pursed-lip breathing, pacing, and coordinated breathing are discussed with the patient. The PT demonstrates stretching and warm-up exercises in front of the webcam for the patient to follow. Then the patient performs all exercises in view of the webcam during the session (Figure 1). A RT monitors breathing patterns and corrects with verbal instructions if not properly performed.
Loss of skeletal muscle mass and cachexia are highly prevalent comorbidities of COPD and have been associated with breathlessness, functional limitation, and poor prognosis.36 To address these comorbidities, our program consists of progressive strengthening, aerobic, balance, and flexibility exercises. Resistance bands and tubes are used for strengthening exercises. Callisthenic exercises (eg, chair squat, chair stand, knee marching, bridging, single limb stances, and lunge) are used for progressive strengthening and balance exercises. Progression of strengthening and balance exercises are done through increasing the volume of exercise (ie, numbers of sets and repetitions) and increased load and level of difficulty based on the patient’s progress and comorbidity. The exercise program focuses on strengthening muscles, especially large muscle groups, to improve overall muscle strength and performance of functional activities.37
Arm/pedal ergometer and daily walking are used for daily aerobic exercise. In a study of patients with COPD by the PAC-COPD Study Group, step counter use was found to increase physical activity and improve exercise capacity, which supports its use in COPD management.38 During program participation, the patient is asked to wear a pedometer to monitor the number of steps taken per day and to report step data to the therapists during the telehealth session. The pedometer stores the previous 41 calendar days of data and displays the most recent 7 calendar days of data.
The patient is encouraged to set a realistic daily step goal. The general program goal is to increase at least 1000 steps per day. However, this goal can be adjusted depending on the patient’s health status and comorbid conditions. The PAC-COPD Study Group found that for every additional 1000 daily steps at low intensity, COPD hospitalization risk decreased by 20%.39 A magnitude of 2000 steps or about 1 mile of walking per day was found to be associated with increased physical activity and health benefits in the general population.40
Respiratory muscle training and breathing exercise are provided by the RT, using breathing and incentive spirometer techniques (Figure 2). Huff coughing, diaphragmatic deep breathing, and pursed-lip breathing are instructed by the RT during the session. Effective coughing technique with a cough assistive device is also provided during breathing training if needed.
Patient Education
In patients with COPD, there are numerous positive health benefits associated with education, including assisting the patients to become active participants in the PR program leading to satisfying outcomes; assisting the patients to better understand the lung health, disease processes, physical and psychological changes that occur with COPD; assisting the patients to explore coping strategies for those changes; building lifelong behavioral changes; and developing the self-management skills for sustainability. Through the educational process, patients with COPD can become more skilled at collaborative self-management and improve adherence to their treatment plan, which in turn can result in a reduction in hospital admissions and reduced health care costs.8,41
Education is provided with every session after the patient completes the exercise. Patients are required to record their COPD symptoms, daily activity, home exercise program, sleep, food intake, and additional physical or social activity in their COPD diary and to report during the session (Figure 3). A COPD diary assists patients in self-monitoring their COPD symptoms and provides the therapists with information about clinical changes, behavioral changes, and/or specific unmet needs for education. Several topics related to COPD are included in the education session: lung or respiratory disease/condition and self-management; smoking cessation; physical activity; energy-conserving techniques; breathing and coughing techniques; smoking cessation; nutrition/healthy eating and weight counseling; sex and intimacy; psychological counseling and/or group support; emergency planning (eg, medical, travel, and inclement weather); correct use of inhaler and medications; home oxygen; sleep and sleep hygiene; palliative care and advanced directive; infection control; and sputum clearance.42,43
Program Maintenance
After successfully completing the 6-week program, patients are referred to the VA TeleMOVE! Program or MOVE! Weight Management Program for continuous, long-term management of weight, nutrition, physical activity/exercise, and social activity needs or goals. The patients are scheduled for monthly follow-up phone visits for 6 months with the telerehabilitation team for enforcing sustainability. The phone call visit consists of reviewing breathing techniques, exercise program, physical activity, education, encouragement, and addressing any issues that arise during the self-maintained period.
Limitations
There are several issues of concern and precautions when delivering PR through telehealth into the home. First, the patient performs exercises independently without being manually guarded by the therapists. Risk of falls are a major concern due to impaired balance, poor vision, and other possible unusual physiologic responses to exercise (eg, drop in BP, dizziness, loss of balance). The area in front of the computer needs to be cleared of fall hazards (ie, area rug, wires, objects on the floor). The patient also needs to be educated on self-measurements of BP and oxygen saturation and reports to the therapists. The therapists provide detailed instructions on how to obtain these measures correctly; otherwise, the values may not be valid for a clinical judgment during the exercise session or for other clinical management. In a home environment, there is a limited use of exercise apparatuses. For this program, we only used resistance bands/tubes, small arm/leg ergometer, hand grip, and hand putty for the exercise program. We feel that dumbbell and weight plates are not suitable due to a possible risk of injury if the patient accidently drops them.
Advanced balance training is not suitable due to an increased risk for falls. Without the presence of the PT, level of challenge/difficulty is somewhat limited for this telehealth supervision exercise program. In addition, visual and audio quality are necessary for the session. The patient and the therapists need to see each other clearly to ensure correct methods and forms of each exercise. Furthermore, rehearsal of technical skills with the therapists is very important because this population is older and often has limited computer skills. Any technical difficulty or failure can lead to undesirable situations (eg, anxiety episodes, worries, shortness of breath, upset), which compromise exercise performance during the session. Finally, a phone is needed as an alternative in case of a poor VVC connection.
Conclusion
COPD symptoms and complications greatly affect patients’ ability to perform daily activities, decrease QOL and functional ability, and result in extensive use of health services. Many patients have limited access to a PR program at hospitals or rehabilitation centers due to health conditions, lack of transportation, and/or family support. This home-based, interactive telehealth PR program can break down the geographic barriers, solve poor program accessibility, potentially increase the utilization of PR, and reduce the cost and travel required by the patients.
Acknowledgments
The Telehealth Pulmonary Rehabilitation Program was originally funded by the Veterans Health Administration VA ACCESS Program (AS, CL, HKH). We thank all the veterans for their time and effort in participating in this newly developed rehabilitation program.
According to World Health Organization estimates, 65 million people have moderate-to-severe chronic obstructive pulmonary disease (COPD) globally, and > 20 million patients with COPD are living in the US.1 COPD is a progressive respiratory disease with a poor prognosis and a significant cause of morbidity and mortality in the US, especially within the Veterans Health Administration (VHA).2 The prevalence of COPD is higher in veterans than it is in the general population. COPD prevalence in the adult US population has been estimated to be between 5% and 15%, whereas in veterans, prevalence estimates have ranged from about 5% to 43%.3-5
COPD is associated with disabling dyspnea, muscle weakness, exercise intolerance, morbidity, and mortality. These symptoms and complications gradually and progressively compromise mobility, ability to perform daily functions, and decrease quality of life (QOL). Dyspnea, fatigue, and discomfort are the principal symptoms that negatively impact exercise tolerance.6,7 Therefore, patients often intentionally limit their activities to avoid these uncomfortable feelings and adopt a more sedentary behavior. As the disease progresses, individuals with COPD will gradually need assistance in performing activities of daily living, which eventually leads to functional dependence.
Pulmonary rehabilitation (PR) is an essential component of the management of symptomatic patients with COPD. PR is an evidence-based, multidisciplinary, comprehensive intervention that includes exercise and education for patients with chronic respiratory disease.8 The key benefits of PR are clinical improvements in dyspnea, physical capacity, QOL, and reduced disability in patients with COPD and other respiratory diseases.9-11 PR was found to improve respiratory health in veterans with COPD and decrease respiratory-related health care utilization.12
Despite the known benefits of PR, many patients with chronic respiratory diseases are not referred or do not have access to rehabilitation. Also, uptake of PR is low due to patient frailty, transportation issues, and other health care access problems.13-15 Unfortunately, in the US health care system, access to PR and other nonpharmacologic treatments can be challenging due to a shortage of available PR programs, limited physician referral to existing programs, and lack of family and social support.16
There are only a few accredited PR programs in VHA facilities, and they tend to be located in urban areas.12,17 Many patients have limited access to the PR programs due to geographic distance to the programs and transportation challenges (eg, limited ability to drive, cost of transportation). Moreover, veterans with COPD are likely to have limited mobility or are homebound due to experiencing shortness of breath with minimal exertion. Given the clear benefits of PR and the increasing impact of COPD on morbidity and mortality of the patients with COPD, strategies to improve the access and capacity of PR are needed. VA telehealth services allow for distribution of health care services in different geographic locations by providing access for the veterans who live in rural and highly rural areas. The most recent implementation of VA Video Connect (VVC) by the VHA provides a new avenue for clinicians to deliver much needed medical care into the veterans’ home.
COPD Telehealth Program
In this article, we describe the processes for developing and delivering an in-home, interactive, supervised PR program for veterans with severe COPD through VA telehealth service. The program consists of 18 sessions delivered over 6 weeks by a licensed physical therapist (PT) and a respiratory therapist (RT). The aims of the telehealth PR are to improve exercise tolerance, reduce dyspnea and fatigue, improve QOL, improve accessibility, and decrease costs and transportation burdens for patients with COPD. The program was developed, implemented and delivered by an interdisciplinary team, including a pulmonologist, PT, RT, physiatrist, and nonclinical supporting staff.
Patient Assessment
To be eligible to participate in the program the patient must: (1) have a forced expiratory volume (FEV1) < 60%; ( 2) be medically stable and be receiving optimal medical management; (3) have no severe cognitive impairments; (4) be able to use a computer and e-mail; (5) be able to ambulate with or without a walking device; (6) be willing to enroll in a smoking cessation program or to stop smoking; (7) be willing to participate without prolonged interruption; and (8) have all visual and auditory impairments corrected with medical devices.
After referral and enrollment, patients receive medical and physical examinations by the PR team, including a pulmonologist, a PT, and a RT, to ensure that the patients are medically stable to undergo rehabilitation and to develop a tailored exercise program while being mindful of the comorbidities, limitations, and precautions, (eg, loss of balance, risk of fall, limited range of motion). The preprogram assessment includes a pulmonary function test, arterial blood gas test, Montreal Cognitive Assessment, Modified Medical Research Council Scale, St. George Respiratory Questionnaire, the COPD Assessment Test, Patient Health Questionnaire-9,Generalized Anxiety Disorder Assessment-7, Epworth Sleepiness Scale, Katz Index of Independence of Activities of Daily Living, medications and inhaler use, oxygen use, breathing pattern, coughing, 6-minute walk test, Modified Borg Dyspnea Scale, grip strength, 5 Times Sit to Stand Test, manual muscle test, gait measure, Timed Up & Go test, clinical balance tests, range of motion, flexibility, sensation, pain, and fall history.18-32 Educational needs (eg, respiratory hygiene, nutrition, infection control, sleep, disease/symptom management) also are evaluated.
This thorough assessment is performed in a face-to-face outpatient visit. During the program participation, a physiatrist may be consulted for additional needs (eg, wheelchair assessment, home safety evaluation/ modifications, and mobility/disability issues). After completing the 6-week program, patients are scheduled for the postprogram evaluation in a face-to-face outpatient visit with the clinicians.
Equipment
Both clinician and the patient are equipped with a computer with Wi-Fi connectivity, a webcam, and a microphone. Patients are provided an exercise pictorial booklet, an exercise compact disk (audio and video), small exercise apparatuses (eg, assorted colors of resistance bands, hand grip exerciser, hand putty, ergometer, harmonica, and pedometer), incentive spirometer, pulse oximeter, cough assistive device (as needed), blood pressure monitor, COPD information booklets, and a diary to use at home during the program.33
Technology Preparation
Prior to starting the telehealth program, the patient is contacted 1 or 2 days before the first session for technical preparation and familiarization of the VA telehealth connection process. Either the PT or RT provides step-by-step instructions for the patient to practice connecting through VVC during this preparatory phone call. The patient also practices using the computer webcam, speaker, and microphone; checks the telehealth scheduling e-mail; and learns how to solve possible common technical issues (eg, adjusting volume and position of webcam). The patient is asked to set up a table close to the computer and to place all exercise apparatuses and respiratory devices on the table surface.
Program Delivery
A secure online VVC is used for connection during the telehealth session. The patient received an e-mail from the telehealth scheduling system with a link for VVC before each session. During the 6-week program, each telehealth session is conducted by a PT and a RT concurrently for 120 minutes, 3 days per week. The PT provides exercises for the patient to attempt, and the RT provides breathing training and monitoring during the session. After a successful connection to VVC, the therapist verifies the patient’s identity and confirms patient consent for the telehealth session.
After this check-in process, the patient performs a self-measure of resting blood pressure (BP), heart rate, respiratory rate, and blood oxygen saturation and reports to the therapists. During the exercise session, fatigue/exertion, dyspnea (Modified Borg Dyspnea Scale; Borg CR10 Scale), BP, heart rate, oxygen saturation, and other clinical symptoms and responses to exercise are monitored by the therapists, using both patient-reported measures and clinical observation by the therapists.34,35 Any medical emergency during the session is reported immediately to the pulmonologist for further management.
Structure
Prior to each exercise session, exercise precautions, fall prevention, good posture, pursed-lip breathing, pacing, and coordinated breathing are discussed with the patient. The PT demonstrates stretching and warm-up exercises in front of the webcam for the patient to follow. Then the patient performs all exercises in view of the webcam during the session (Figure 1). A RT monitors breathing patterns and corrects with verbal instructions if not properly performed.
Loss of skeletal muscle mass and cachexia are highly prevalent comorbidities of COPD and have been associated with breathlessness, functional limitation, and poor prognosis.36 To address these comorbidities, our program consists of progressive strengthening, aerobic, balance, and flexibility exercises. Resistance bands and tubes are used for strengthening exercises. Callisthenic exercises (eg, chair squat, chair stand, knee marching, bridging, single limb stances, and lunge) are used for progressive strengthening and balance exercises. Progression of strengthening and balance exercises are done through increasing the volume of exercise (ie, numbers of sets and repetitions) and increased load and level of difficulty based on the patient’s progress and comorbidity. The exercise program focuses on strengthening muscles, especially large muscle groups, to improve overall muscle strength and performance of functional activities.37
Arm/pedal ergometer and daily walking are used for daily aerobic exercise. In a study of patients with COPD by the PAC-COPD Study Group, step counter use was found to increase physical activity and improve exercise capacity, which supports its use in COPD management.38 During program participation, the patient is asked to wear a pedometer to monitor the number of steps taken per day and to report step data to the therapists during the telehealth session. The pedometer stores the previous 41 calendar days of data and displays the most recent 7 calendar days of data.
The patient is encouraged to set a realistic daily step goal. The general program goal is to increase at least 1000 steps per day. However, this goal can be adjusted depending on the patient’s health status and comorbid conditions. The PAC-COPD Study Group found that for every additional 1000 daily steps at low intensity, COPD hospitalization risk decreased by 20%.39 A magnitude of 2000 steps or about 1 mile of walking per day was found to be associated with increased physical activity and health benefits in the general population.40
Respiratory muscle training and breathing exercise are provided by the RT, using breathing and incentive spirometer techniques (Figure 2). Huff coughing, diaphragmatic deep breathing, and pursed-lip breathing are instructed by the RT during the session. Effective coughing technique with a cough assistive device is also provided during breathing training if needed.
Patient Education
In patients with COPD, there are numerous positive health benefits associated with education, including assisting the patients to become active participants in the PR program leading to satisfying outcomes; assisting the patients to better understand the lung health, disease processes, physical and psychological changes that occur with COPD; assisting the patients to explore coping strategies for those changes; building lifelong behavioral changes; and developing the self-management skills for sustainability. Through the educational process, patients with COPD can become more skilled at collaborative self-management and improve adherence to their treatment plan, which in turn can result in a reduction in hospital admissions and reduced health care costs.8,41
Education is provided with every session after the patient completes the exercise. Patients are required to record their COPD symptoms, daily activity, home exercise program, sleep, food intake, and additional physical or social activity in their COPD diary and to report during the session (Figure 3). A COPD diary assists patients in self-monitoring their COPD symptoms and provides the therapists with information about clinical changes, behavioral changes, and/or specific unmet needs for education. Several topics related to COPD are included in the education session: lung or respiratory disease/condition and self-management; smoking cessation; physical activity; energy-conserving techniques; breathing and coughing techniques; smoking cessation; nutrition/healthy eating and weight counseling; sex and intimacy; psychological counseling and/or group support; emergency planning (eg, medical, travel, and inclement weather); correct use of inhaler and medications; home oxygen; sleep and sleep hygiene; palliative care and advanced directive; infection control; and sputum clearance.42,43
Program Maintenance
After successfully completing the 6-week program, patients are referred to the VA TeleMOVE! Program or MOVE! Weight Management Program for continuous, long-term management of weight, nutrition, physical activity/exercise, and social activity needs or goals. The patients are scheduled for monthly follow-up phone visits for 6 months with the telerehabilitation team for enforcing sustainability. The phone call visit consists of reviewing breathing techniques, exercise program, physical activity, education, encouragement, and addressing any issues that arise during the self-maintained period.
Limitations
There are several issues of concern and precautions when delivering PR through telehealth into the home. First, the patient performs exercises independently without being manually guarded by the therapists. Risk of falls are a major concern due to impaired balance, poor vision, and other possible unusual physiologic responses to exercise (eg, drop in BP, dizziness, loss of balance). The area in front of the computer needs to be cleared of fall hazards (ie, area rug, wires, objects on the floor). The patient also needs to be educated on self-measurements of BP and oxygen saturation and reports to the therapists. The therapists provide detailed instructions on how to obtain these measures correctly; otherwise, the values may not be valid for a clinical judgment during the exercise session or for other clinical management. In a home environment, there is a limited use of exercise apparatuses. For this program, we only used resistance bands/tubes, small arm/leg ergometer, hand grip, and hand putty for the exercise program. We feel that dumbbell and weight plates are not suitable due to a possible risk of injury if the patient accidently drops them.
Advanced balance training is not suitable due to an increased risk for falls. Without the presence of the PT, level of challenge/difficulty is somewhat limited for this telehealth supervision exercise program. In addition, visual and audio quality are necessary for the session. The patient and the therapists need to see each other clearly to ensure correct methods and forms of each exercise. Furthermore, rehearsal of technical skills with the therapists is very important because this population is older and often has limited computer skills. Any technical difficulty or failure can lead to undesirable situations (eg, anxiety episodes, worries, shortness of breath, upset), which compromise exercise performance during the session. Finally, a phone is needed as an alternative in case of a poor VVC connection.
Conclusion
COPD symptoms and complications greatly affect patients’ ability to perform daily activities, decrease QOL and functional ability, and result in extensive use of health services. Many patients have limited access to a PR program at hospitals or rehabilitation centers due to health conditions, lack of transportation, and/or family support. This home-based, interactive telehealth PR program can break down the geographic barriers, solve poor program accessibility, potentially increase the utilization of PR, and reduce the cost and travel required by the patients.
Acknowledgments
The Telehealth Pulmonary Rehabilitation Program was originally funded by the Veterans Health Administration VA ACCESS Program (AS, CL, HKH). We thank all the veterans for their time and effort in participating in this newly developed rehabilitation program.
1. World Health Organization. Chronic obstructive pulmonary disease (COPD). http://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd). Published December 1, 2017. Accessed August 7, 2019.
2. Yu W, Ravelo A, Wagner TH, et al. Prevalence and costs of chronic conditions in the VA health care system. Med Care Res Rev. 2003;60(suppl 3):146S-167S.
3. Doney B, Hnizdo E, Dillon CF, et al. Prevalence of airflow obstruction in U.S. adults aged 40-79 years: NHANES data 1988-1994 and 2007-2010. COPD. 2015;12(4):355-365.
4. Murphy DE, Chaudhry Z, Almoosa KF, Panos RJ. High prevalence of chronic obstructive pulmonary disease among veterans in the urban midwest. Mil Med. 2011;176(5):552-560.
5. Cypel YS, Hines SE, Davey VJ, Eber SM, Schneiderman AI. Self-reported physician-diagnosed chronic obstructive pulmonary disease and spirometry patterns in Vietnam Era US Army Chemical Corps veterans: a retrospective cohort study. Am J Ind Med. 2018;61(10):802-814.
6. Rochester CL. Exercise training in chronic obstructive pulmonary disease. J Rehabil Res Dev. 2003;40(5)(suppl 2):59-80.
7. Cortopassi F, Gurung P, Pinto-Plata V. Chronic obstructive pulmonary disease in elderly patients. Clin Geriatr Med. 2017;33(4):539-552.
8. Spruit MA, Singh SJ, Garvey C, et al; ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13-e64.
9. Robinson H, Williams V, Curtis F, Bridle C, Jones AW. Facilitators and barriers to physical activity following pulmonary rehabilitation in COPD: a systematic review of qualitative studies. NPJ Prim Care Respir Med. 2018;28(1):19.
10. McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;(2):CD003793.
11. Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary rehabilitation: joint AACP/AACVPR evidence-based clinical practice guidelines. Chest. 2007;131(suppl 5):4S-42S.
12. Major S, Moreno M, Shelton J, Panos RJ. Veterans with chronic obstructive pulmonary disease achieve clinically relevant improvements in respiratory health after pulmonary rehabilitation. J Cardiopulm Rehabil Prev. 2014;34(6):420-429.
13. Liu Y, Dickerson T, Early F, Fuld J, Clarkson PJ. Understanding influences on the uptake of pulmonary rehabilitation in the East of England: an inclusive design/mixed methods study protocol. BMJ Open. 2018;8(4):e020750.
14. Harris D, Hayter M, Allender S. Factors affecting the offer of pulmonary rehabilitation to patients with chronic obstructive pulmonary disease by primary care professionals: a qualitative study. Prim Health Care Res Dev. 2008;9(4):280-290.
15. Mathar H, Fastholm P, Hansen IR, Larsen NS. Why do patients with COPD decline rehabilitation. Scand J Caring Sci. 2016;30(3):432-441.
16. Han MK, Martinez CH, Au DH, et al. Meeting the challenge of COPD care delivery in the USA: a multiprovider perspective. Lancet Respir Med. 2016;4(6):473-526.
17. American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR). Online searchable program directory. https://www.aacvpr.org/Resources/Program-Directory Accessed July 19, 2018.
18. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699.
19. Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J. 1959;2(5147):257-266.
20. O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2007 update. Can Respir J. 2007;14(suppl B):5B-32B.
21. Jones PW, Quirk FH, Baveystock CM. The St George’s Respiratory Questionnaire. Respir Med. 1991;85(suppl B):25-31.
22. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648-654.
23. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
24. Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097.
25. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540-545.
26. Katz S. Assessing self-maintenance: activities of daily living, mobility and instrumental activities of daily living. J Am Geriatr Soc. 1983;31(12):721-727.
27. Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428-1446.
28. Mahler DA, Horowitz MB. Perception of breathlessness during exercise in patients with respiratory disease. Med Sci Sports Exerc. 1994;26(9):1078-1081.
29. Liao WC, Wang CH, Yu SY, Chen LY, Wang CY. Grip strength measurement in older adults in Taiwan: a comparison of three testing positions. Australas J Ageing. 2014;33(4):278-282.
30. Buatois S, Miljkovic D, Manckoundia P, et al. Five times sit to stand test is a predictor of recurrent falls in healthy community-living subjects aged 65 and older. J Am Geriatr Soc. 2008;56(8):1575-1577.
31. Bryant MS, Workman CD, Jackson GR. Multidirectional walk test in persons with Parkinson’s disease: a validity study. Int J Rehabil Res. 2015;38(1):88-91.
32. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142-148.
33. University of Nebraska Medical Center. Timed Up and Go (TUG) Test. https://www.unmc.edu/media/intmed/geriatrics/nebgec/pdf/frailelderlyjuly09/toolkits/timedupandgo_w_norms.pdf. Accessed August 13, 2019.
34. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377-381.
35. Mahler DA, Horowitz MB. Clinical evaluation of exertional dyspnea. Clin Chest Med. 1994;15(2):259-269.
36. Dudgeon D, Baracos VE. Physiological and functional failure in chronic obstructive pulmonary disease, congestive heart failure and cancer: a debilitating intersection of sarcopenia, cachexia and breathlessness. Curr Opin Support Palliat Care. 2016;10(3):236-241.
37. Lee AL, Holland AE. Time to adapt exercise training regimens in pulmonary rehabilitation—a review of the literature. Int J Chron Obstruct Pulmon Dis. 2014;9:1275-1288.
38. Qiu S, Cai X, Wang X, et al. Using step counters to promote physical activity and exercise capacity in patients with chronic obstructive pulmonary disease: a meta-analysis. Ther Adv Respir Dis. 2018;12:1753466618787386.
39. Donaire-Gonzalez D, Gimeno-Santos E, Balcells E, et al; PAC-COPD Study Group. Benefits of physical activity on COPD hospitalization depend on intensity. Eur Respir J. 2015;46(5):1281-1289.
40. Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298(19):2296-2304.
41. Zwerink M, Brusse-Keizer M, van der Valk PD, et al. Self-management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;19(3):CD002990.
42. Wilson JS, O’Neill B, Reilly J, MacMahon J, Bradley JM. Education in pulmonary rehabilitation: the patient’s perspective. Arch Phys Med Rehabil. 2007;88(12):1704-1709.
43. Bourbeau J, Nault D, Dang-Tan T. Self-management and behaviour modification in COPD. Patient Educ Couns. 2004;52(3):271-277.
1. World Health Organization. Chronic obstructive pulmonary disease (COPD). http://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd). Published December 1, 2017. Accessed August 7, 2019.
2. Yu W, Ravelo A, Wagner TH, et al. Prevalence and costs of chronic conditions in the VA health care system. Med Care Res Rev. 2003;60(suppl 3):146S-167S.
3. Doney B, Hnizdo E, Dillon CF, et al. Prevalence of airflow obstruction in U.S. adults aged 40-79 years: NHANES data 1988-1994 and 2007-2010. COPD. 2015;12(4):355-365.
4. Murphy DE, Chaudhry Z, Almoosa KF, Panos RJ. High prevalence of chronic obstructive pulmonary disease among veterans in the urban midwest. Mil Med. 2011;176(5):552-560.
5. Cypel YS, Hines SE, Davey VJ, Eber SM, Schneiderman AI. Self-reported physician-diagnosed chronic obstructive pulmonary disease and spirometry patterns in Vietnam Era US Army Chemical Corps veterans: a retrospective cohort study. Am J Ind Med. 2018;61(10):802-814.
6. Rochester CL. Exercise training in chronic obstructive pulmonary disease. J Rehabil Res Dev. 2003;40(5)(suppl 2):59-80.
7. Cortopassi F, Gurung P, Pinto-Plata V. Chronic obstructive pulmonary disease in elderly patients. Clin Geriatr Med. 2017;33(4):539-552.
8. Spruit MA, Singh SJ, Garvey C, et al; ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13-e64.
9. Robinson H, Williams V, Curtis F, Bridle C, Jones AW. Facilitators and barriers to physical activity following pulmonary rehabilitation in COPD: a systematic review of qualitative studies. NPJ Prim Care Respir Med. 2018;28(1):19.
10. McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;(2):CD003793.
11. Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary rehabilitation: joint AACP/AACVPR evidence-based clinical practice guidelines. Chest. 2007;131(suppl 5):4S-42S.
12. Major S, Moreno M, Shelton J, Panos RJ. Veterans with chronic obstructive pulmonary disease achieve clinically relevant improvements in respiratory health after pulmonary rehabilitation. J Cardiopulm Rehabil Prev. 2014;34(6):420-429.
13. Liu Y, Dickerson T, Early F, Fuld J, Clarkson PJ. Understanding influences on the uptake of pulmonary rehabilitation in the East of England: an inclusive design/mixed methods study protocol. BMJ Open. 2018;8(4):e020750.
14. Harris D, Hayter M, Allender S. Factors affecting the offer of pulmonary rehabilitation to patients with chronic obstructive pulmonary disease by primary care professionals: a qualitative study. Prim Health Care Res Dev. 2008;9(4):280-290.
15. Mathar H, Fastholm P, Hansen IR, Larsen NS. Why do patients with COPD decline rehabilitation. Scand J Caring Sci. 2016;30(3):432-441.
16. Han MK, Martinez CH, Au DH, et al. Meeting the challenge of COPD care delivery in the USA: a multiprovider perspective. Lancet Respir Med. 2016;4(6):473-526.
17. American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR). Online searchable program directory. https://www.aacvpr.org/Resources/Program-Directory Accessed July 19, 2018.
18. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699.
19. Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J. 1959;2(5147):257-266.
20. O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2007 update. Can Respir J. 2007;14(suppl B):5B-32B.
21. Jones PW, Quirk FH, Baveystock CM. The St George’s Respiratory Questionnaire. Respir Med. 1991;85(suppl B):25-31.
22. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648-654.
23. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
24. Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097.
25. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540-545.
26. Katz S. Assessing self-maintenance: activities of daily living, mobility and instrumental activities of daily living. J Am Geriatr Soc. 1983;31(12):721-727.
27. Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428-1446.
28. Mahler DA, Horowitz MB. Perception of breathlessness during exercise in patients with respiratory disease. Med Sci Sports Exerc. 1994;26(9):1078-1081.
29. Liao WC, Wang CH, Yu SY, Chen LY, Wang CY. Grip strength measurement in older adults in Taiwan: a comparison of three testing positions. Australas J Ageing. 2014;33(4):278-282.
30. Buatois S, Miljkovic D, Manckoundia P, et al. Five times sit to stand test is a predictor of recurrent falls in healthy community-living subjects aged 65 and older. J Am Geriatr Soc. 2008;56(8):1575-1577.
31. Bryant MS, Workman CD, Jackson GR. Multidirectional walk test in persons with Parkinson’s disease: a validity study. Int J Rehabil Res. 2015;38(1):88-91.
32. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142-148.
33. University of Nebraska Medical Center. Timed Up and Go (TUG) Test. https://www.unmc.edu/media/intmed/geriatrics/nebgec/pdf/frailelderlyjuly09/toolkits/timedupandgo_w_norms.pdf. Accessed August 13, 2019.
34. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377-381.
35. Mahler DA, Horowitz MB. Clinical evaluation of exertional dyspnea. Clin Chest Med. 1994;15(2):259-269.
36. Dudgeon D, Baracos VE. Physiological and functional failure in chronic obstructive pulmonary disease, congestive heart failure and cancer: a debilitating intersection of sarcopenia, cachexia and breathlessness. Curr Opin Support Palliat Care. 2016;10(3):236-241.
37. Lee AL, Holland AE. Time to adapt exercise training regimens in pulmonary rehabilitation—a review of the literature. Int J Chron Obstruct Pulmon Dis. 2014;9:1275-1288.
38. Qiu S, Cai X, Wang X, et al. Using step counters to promote physical activity and exercise capacity in patients with chronic obstructive pulmonary disease: a meta-analysis. Ther Adv Respir Dis. 2018;12:1753466618787386.
39. Donaire-Gonzalez D, Gimeno-Santos E, Balcells E, et al; PAC-COPD Study Group. Benefits of physical activity on COPD hospitalization depend on intensity. Eur Respir J. 2015;46(5):1281-1289.
40. Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298(19):2296-2304.
41. Zwerink M, Brusse-Keizer M, van der Valk PD, et al. Self-management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;19(3):CD002990.
42. Wilson JS, O’Neill B, Reilly J, MacMahon J, Bradley JM. Education in pulmonary rehabilitation: the patient’s perspective. Arch Phys Med Rehabil. 2007;88(12):1704-1709.
43. Bourbeau J, Nault D, Dang-Tan T. Self-management and behaviour modification in COPD. Patient Educ Couns. 2004;52(3):271-277.
Patient Knowledge of and Barriers to Breast, Colon, and Cervical Cancer Screenings: A Cross-Sectional Survey of TRICARE Beneficiaries (FULL)
The National Defense Appropriations Act for fiscal year 2009, Subtitle B, waived copayments for preventive cancer screening services for all TRICARE beneficiaries, excluding Medicare-eligible beneficiaries.1 These preventive services include screening for colorectal cancer (CRC), breast cancer, and cervical cancer based on current guidelines (eAppendix1).
Despite having unrestricted access to these cancer screenings, TRICARE Prime beneficiaries report overall screening completion rates that are below the national commercial benchmarks established by the Healthcare Effectiveness Data and Information Set (HEDIS) for all 3 cancer types.2 Specifically, among TRICARE Prime beneficiaries enrolled in the western region of the U.S. in October 2013, the reported breast cancer screening rate was 61.6% (43,138/69,976) for women aged 42 to 69 years, which is well below the HEDIS 75th percentile of 76%. Similarly, the reported rate of cervical cancer screening among women aged 24 to 64 years was 68.3% (63,523/92,946), well below the HEDIS 75th percentile of 79%. Last, the reported rate of CRC screening among male and female TRICARE Prime members aged 51 to 75 years was 61.6% (52,860/85,827), also below the 2013 HEDIS 75th percentile of 63% based on internal review of TRICARE data used for HEDIS reporting.
Given the reported low screening rates, the Defense Health Agency (DHA) performed a cross-sectional survey to assess TRICARE Prime West region beneficiaries’ knowledge and understanding of preventive health screening, specifically for breast cancer, cervical cancer, and CRC, and to identify any potential barriers to access for these screenings.
Methods
A mostly closed-ended, 42-item telephone survey was designed and conducted (eAppendix2)
All women participating in the survey, regardless of age, were asked questions regarding cervical cancer screening. Women aged ≥ 42 years additionally were asked a second set of survey questions specific to breast cancer screening, and women aged between 51 and 64 years were asked a third set of questions related to CRC screening. The ages selected were 1 to 2 years after the recommended age for the respective screening to ensure adequate follow-up time for the member to obtain the screening. Men included in the survey were asked questions related only to CRC screening.
The target survey sample was 3,500 beneficiaries, separated into the following 4 strata: women aged 21 to 64 years of age enrolled in the direct care system (n = 1,250); women aged 21 to 64 years enrolled in the purchased (commercial) care network (n = 1,250); men aged 51 to 64 years enrolled in the direct care system (n = 500); and men aged 51 to 64 years enrolled in the purchased care network (n = 500). The random sample was drawn from an overall population of about 35,000 members. Sampling was performed without replacement until the target number of surveys was achieved. Survey completion was defined as the respondent having reached the end of the survey questionnaire but not necessarily having answered every question.
Data Elements
The preventive health survey collected information on beneficiaries’ knowledge of and satisfaction with their PCM, the primary location where they sought health care in the previous 12 months, preference for scheduling cancer screening tests, and general knowledge about the frequency and type of screening for breast, cervical, and colorectal cancers. Responses were scored based on guidelines effective as of 2009. In addition, the survey collected information on the beneficiary’s overall health status, current age, highest level of education achieved, current employment status, place of residence (on or off a military installation), race, and whether the beneficiary carried other health insurance aside from TRICARE.
Survey Mode and Fielding
A sampling population of eligible beneficiaries was created from a database of all TRICARE Prime beneficiaries. An automated system was used to randomly draw potential participants from the sample. Survey interviewers were given the beneficiary’s name and telephone number but no other identifiable information. Phone numbers from the sample were dialed up to 6 times before the number was classified as a “no answer.” Interviewers read to each beneficiary a statement describing the survey and participation risk and benefits and explained that participation was voluntary and the participant could end the survey at any time without penalty or prejudice. The survey commenced only after verbal consent was obtained.
Sample Weighting and Statistical Analysis
Each survey record was weighted to control for potential bias associated with unequal rates of noncoverage and nonresponse in the sampled population. A design weight was calculated as the ratio of the frame size and the sample size in each stratum. For each stratum, an adjusted response rate (RR) was calculated as the number of completed surveys divided by the number of eligible respondents. Since all respondents were eligible, the RR was not adjusted. The ratio of the design weight to the adjusted RR was calculated and assigned to each survey.
Frequency distributions and descriptive statistics were calculated for all close-ended survey items. Open-ended survey items were summarized and assessed qualitatively. When appropriate, open-ended responses were categorized and included in descriptive analyses. No formal statistical testing was performed.
Results
A total of 6,563 beneficiaries were contacted, and 3,688 agreed to participate (56%), resulting in 3,500 TRICARE beneficiaries completing the survey (95% completion rate), of whom 71% (2,500) were female. The overall cooperation rates were similar across the 4 strata. Interviews ceased once 3,500 surveys were completed. The largest distribution of respondents was aged between 55 and 64 years (37%) (Table 1). Respondents aged 21 to 24 years comprised the smallest percentage of the sample (7%). Nearly a third of respondents were dependents of ADSMs (30%), another 30% were retirees, and most respondents self-identified as white (Table 1).
Barriers to Screening
A series of survey questions was asked about specific barriers to cancer screening, including the convenience of appointment times for the respondent’s last cancer screening. The majority (69%, 2,415 of 3,500) responded that the appointment times were convenient. Among those who stated that times were not convenient and those who had not scheduled an examination, 66% responded that they did not know or were not sure how to schedule a cancer screening test.
Screening Preferences
Less than half of survey respondents (48%) reported that they received screening guideline information from their physician or provider; 24% reported that they performed their own research. Only 9% reported that they learned about the guidelines through TRICARE materials, and 7% of respondents indicated that media, family, or friends were their source of screening information.
The survey respondents who indicated that they had not scheduled a screening examination were asked when (time of day) they preferred to have a screening. Less than half (47%) reported that varying available appointment times would not affect their ability to obtain screening. One-quarter preferred times for screening during working hours, 20% preferred times after working hours, 6% preferred times before working hours, and 2% responded that they were unsure or did not know. The majority (89%) reported that they would prefer to receive all available screenings on the same day if possible.
Breast Cancer Screening
Nearly all (98%) of the 1,100 women aged between 42 and 64 years reported having received a mammogram. These women were asked a specific subset of questions related to breast cancer screening. Respondents were asked to state the recommended age at which women should begin receiving mammogram screenings. More than half (55%) provided the correct response (40 years old, per the U.S. Preventive Services Task Force guidelines).3,4 About three-quarters of respondents (789) correctly responded annually to the question regarding how often women should receive mammograms.
The survey also sought to identify barriers that prevented women from obtaining necessary breast cancer screening. However, the majority surveyed (85%) noted that the question was not applicable because they typically scheduled screening appointments. Only a few (3%) reported factors such as either themselves or someone they know having had a negative experience, discomfort, pain, or concerns of a falsepositive result as reasons for not obtaining breast cancer screening. Of the 112 respondents to the open-ended question, 25% reported that their schedules prevented them from scheduling a mammogram in the past; 12% reported that an inconvenient clinic location, appointment time, or process prevented them from receiving a screening; and 13% reported forgetting to schedule the screening (Table 2).
Cervical Cancer Screening
Female respondents aged between 21 and 64 years (n = 2,432) were asked about the recommended age at which women should begin receiving cervical cancer screening. Only 1% of respondents provided the correct response (that screening begins at 21 years of age per the U.S. Preventive Services Task Force Report guidelines), while 88% provided an incorrect response, and 11% were unsure or did not provide any response.5 Among all respondents, 98% reported having had a cervical cancer screening.
Respondents were asked how frequently women should have a Papanicolaou (Pap) test. Responses such as “2 to 3 years,” “2 years,” or “every other year” were labeled as correct, whereas responses such as “every 6 months” or “greater than 3 years” were labeled as incorrect. Just 12% of respondents provided a correct response, whereas 86% answered incorrectly, and 2% did not answer or did not know. Of those who answered incorrectly, the most common response was “annually” or “every year,” with no notable differences according to race, age, or beneficiary category.
To better understand barriers to screening, respondents were asked to identify reasons they might not have sought cervical cancer screening. The majority (84%) reported that they typically scheduled appointments and that the question was not applicable. However, among 228 respondents who provided an open-ended response and who had not previously undergone a hysterectomy, 8% stated that they had received no reminder or that they lacked sufficient information to schedule the appointment, 21% forgot to schedule, 18% reported a scheduling conflict or difficulty in receiving care, and 13% noted that they did not believe in annual screening (Table 2).
Colorectal Cancer Screening
Eighty-seven percent of eligible respondents (n = 1,734) reported having ever had a sigmoidoscopy and/or colonoscopy. Respondents were asked for their understanding of the recommended age for men and women to begin CRC screening.6 Nearly three-quarters of respondents provided a correct response (n = 1,225), compared with 23% of respondents (n = 407) who answered incorrectly and 6% (n = 102) who did not provide a response or stated they did not know. Correct responses were numerically higher among white respondents (73%) compared with black (62%) and other (62%) respondents as well as among persons aged < 60 years (73%) vs those aged > 60 years (67%).
Respondents aged between 51 and 64 years were asked how often the average person should receive colon cancer screenings. The most common response was that screening should occur every 5 years (33%) followed by every 10 years (26%). This aligns with the U.S. Preventive Services Task Force’s recommendations for flexible sigmoidoscopy every 5 years or colonoscopy every 10 years.
Eligible respondents were asked to identify reasons they did not seek CRC screening. Eighty-six percent of respondents indicated that they typically scheduled CRC screening and that the question was not applicable. Among respondents who provided an open-ended response, 26% cited feeling uncomfortable with the procedure, 15% cited forgetting to schedule a screening, 15% noted a lack of information on screening, and 11% reported no need for screening (Table 2). Among the 1,734 respondents, 80% reported that they would prefer a fecal occult blood test (FOBT) over either a colonoscopy or a sigmoidoscopy. Only 51% reported that their PCM had previously discussed the different types of CRC screenings at some point.
Discussion
The purpose of this large, representative survey was to obtain information on beneficiaries’ knowledge, perceived barriers, and beliefs regarding breast, cervical, and colorectal cancer screenings to identify factors contributing to low completion rates. As far as is known, this is the first study to address these questions in a TRICARE population. Overall, the findings suggest that beneficiaries consider cancer screening important, largely relying on their PCM or their research to better understand how and when to obtain such screenings. The majority received 1 or more screenings prior to the survey, but there were some common knowledge gaps about how to schedule screening appointments, relevant TRICARE medical benefits, and the current recommendations regarding screening timing and frequency. A commonly reported issue across all surveyed groups was inconvenient screening times.
More than half (55%) of respondents correctly noted that breast cancer screening begins at age 40 years (based on recommendations at the time the survey was conducted), and 72% understood when screening should occur. Despite access to care, inconvenient schedules and testing locations were considered the biggest barriers to regularly obtaining a mammogram. There are few studies on knowledge of breast cancer screening in an insured population available for comparison.7-10 One study of medically insured black and non-Hispanic women aged 43 to 49 years showed that lack of reminders or knowledge about the need for mammograms, cost, being too busy, and forgetting to schedule appointments were all factors associated with nonadherence to repeat mammography examinations.8 In an integrative review published in 2000, authors cited that among 8 of 13 relevant studies, the major barrier to receiving a recommended mammogram was lack of physician recommendation.7
For cervical cancer screening, few respondents (1%) correctly identified the age for initiation of screening, and just 12% correctly identified the frequency of screening. These findings are consistent with those of other studies, suggesting a general misunderstanding
about Pap tests in the U.S. and among low-income women.11,12 Reported barriers to screening were uncommon but included scheduling conflicts and lack of reminders or information and were consistent with barriers cited in prior studies.13,14 A few respondents (13%) noted that they did not believe in annual screening, which is similar to the findings of Decker and colleagues who cited lack of knowledge about the test and belief that screening is of no benefit as reasons for failure to get a recommended Pap test.13 These findings suggest a need to improve patientprovider communication and to provide more patient educational materials about the importance of cervical cancer screening.
A large proportion (71%) gave the correct response regarding the appropriate age to initiate CRC screening. Discomfort with the procedure, belief that the screening is unnecessary, or lack of physician’s recommendation were noted barriers to CRC screening. These findings are similar to those reported elsewhere in non-TRICARE populations.15-20 Two focus groups included participants with little knowledge about CRC screening, such as risk factors and symptoms, and expressed fear and embarrassment about CRC and screening. Few of the focus group participants were aware of the available options for screening, and some were confused about the purpose and benefits of the various screening modalities.16
A Health Information National Trends survey reported that 24% participants had not received a colonoscopy or a sigmoidoscopy because their PCM did not order it or say that it was necessary.15 The reported perceived barriers included fear of an adverse finding, injury to the colon from screening, and embarrassment. A study performed in 1,901 Medicare-insured individuals with no history of CRC cited lack of knowledge/awareness and no physician order as the most common reasons for not undergoing CRC screening.18
Strengths and Limitations
A major strength of the current survey is the 56% completion rate, which far exceeds other survey participation rates that were as low as 9%.21 A second strength is the scope of the survey to capture information on not 1 but 3 different cancer screening practices in a unique population who receive preventive screenings at low to no cost.
There are a few study limitations. The majority of respondents identified as white (80%), which does not fully align with the racial distribution of the TRICARE Prime population in the West Region, which is about 68% white. This higher proportion of white respondents may affect the ability to generalize findings to other populations. However, given the open access to care, race should not be a major factor contributing to screening decisions. Another potential limitation to the generalizability of the study is that the age of the respondents was capped at 64 years. Considering that some of the reported barriers to screening were “too busy” or “scheduling conflict,” a study population that included respondents aged ≥ 65 years (who might be more likely to be retired) might report lower rates of these schedule-related barriers.
A third limitation is that most questions about prior screenings pertained to any time in the past, and, therefore, limited the ability to identify current factors leading to lower screening rates. Last, the survey was developed prior to the 2012 changes in cervical and breast cancer screening recommendations and was therefore scored based on prior recommendations. Given that the goal was to assess knowledge and barriers, results are not expected to differ greatly if they are scored using the newer guidelines.
Conclusion
Findings from this cross-sectional survey indicate high levels of knowledge among TRICARE West Region beneficiaries regarding when and how often screening for breast cancer, cervical cancer, and CRC should occur. To encourage TRICARE beneficiaries to seek and obtain recommended and covered cancer screenings, further efforts are needed, including more education about the importance of screening and how to obtain screening. The survey results suggest that TRICARE Prime beneficiaries view cancer screening as important for overall health but they require (and also may desire) more frequent scheduling reminders, education, and more options for scheduling. Newer modalities for communicating with beneficiaries, such as automated telephone appointment reminders, reminder texts, online appointment scheduling, educational blogs, podcasts on cancer screening, extended appointment hours, or unconventional strategies to bundle screening services, are tools that could be used by providers to achieve greater compliance with cancer screening recommendations.
Author Disclosure
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Click here to read the digital edition.
1. TRICARE. TRICARE policy manual 6010.57-M. http://manuals.tricare.osd.mil/pages/DisplayManualaspx?SeriesId=POLICY. Published February 1, 2008. Accessed March 9, 2017.
2. National Committee for Quality Assurance. 2013 accreditation benchmarks and thresholds—mid-year update. http://www.ncqa.org/Portals/0/PolicyUpdates/Trending %20and%20Benchmarks/archives/2013_BENCHMARKS ANDTHRESHOLDS_for%20MidYear%20Update_Final.pdf. Published July 24, 2013. Accessed March 9, 2017.
3. U.S. Preventative Services Task Force. Archived final recommendation statement: breast cancer: screening, 2002. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/breast-cancer-screening-2002. Published December 30, 2013. Accessed March 9, 2017.
4. Smith RA, Saslow D, Sawyer KA, et al; American Cancer Society High-Risk Work Group; American Cancer Society Screening Older Women Work Group; American Cancer Society Mammography Work Group; American Cancer Society Physical Examination Work Group; American Cancer Society New Technologies Work Group; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast cancer screening: update 2003. CA Cancer J Clin. 2003;53(3):141-169.
5. Moyer VA; U.S. Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156(12):880-891, W312.
6. U.S. Preventive Services Task Force. Archived: colorectal cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/colorectal-cancer-screening. Published October 2008. Accessed March 9, 2017.
7. George SA. Barriers to breast cancer screening: an integrative review. Health Care Women Int. 2000;21(1):53-65.
8. Gierisch JM, O’Neill SC, Rimer BK, DeFrank JT, Bowling JM, Skinner CS. Factors associated with annual-interval mammography for women in their 40s. Cancer Epidemiol. 2009;33(1):72-78.
9. Peppercorn J, Houck K, Beri N, et al. Breast cancer screening utilization and understanding of current guidelines among rural U.S. women with private insurance. Breast Cancer Res Treat. 2015;153(3):659-667.
10. Sarma EA. Barriers to screening mammography. Health Psychol Rev. 2015;9(1):42-62.
11. Hawkins NA, Benard VB, Greek A, Roland KB, Manninen D, Saraiya M. Patient knowledge and beliefs as barriers to extending cervical cancer screening intervals in federally qualified health centers. Prev Med. 2013;57(5):641-645.
12. Hawkins NA, Cooper CP, Saraiya M, Gelb CA, Polonec L. Why the Pap test? Awareness and use of the Pap test among women in the United States. J Womens Health (Larchmt). 2011;20(4):511-515.
13. Decker KM, Turner D, Demers AA, Martens PJ, Lambert P, Chateau D. Evaluating the effectiveness of cervical cancer screening invitation letters. J Womens Health (Larchmt). 2013;22(8):687-693.
14. Yao X, Dembe AE, Wickizer T, Lu B. Does time pressure create barriers for people to receive preventive health services? Prev Med. 2015;74:55-58.
15. Geiger TM, Miedema BW, Geana MV, Thaler K, Rangnekar NJ, Cameron GT. Improving rates for screening colonoscopy: analysis of the Health Information National Trends Survey (HINTS I) data. Surgical Endoscopy. 2008;22(2):527-533.
16. Greisinger A, Hawley ST, Bettencourt JL, Perz CA, Vernon SW. Primary care patients’ understanding of colorectal cancer screening. Cancer Detect Prev. 2006;30(1):67-74.
17. Janz NK, Wren PA, Schottenfeld D, Guire KE. Colorectal cancer screening attitudes and behavior: a populationbased study. Prev Med. 2003;37(6, pt 1):627-634.
18. Klabunde CN, Schenck AP, Davis WW. Barriers to colorectal cancer screening among Medicare consumers. Am J Prev Med. 2006;30(4):313-319.
19. Klabunde CN, Vernon SW, Nadel MR, Breen N, Seeff LC, Brown ML. Barriers to colorectal cancer screening: a comparison of reports from primary care physicians and average-risk adults. Med Care. 2005;43(9):939-944.
20. Berkowitz Z, Hawkins NA, Peipins LA, White MC, Nadel MR. Beliefs, risk perceptions, and gaps in knowledge as barriers to colorectal cancer screening in older adults. J Am Geriatr Soc. 2008;56(2):307-314.
21. Pew Research Center. Assessing the representativeness of public opinion surveys. http://www.people-press.org/2012/05/15/assessing-the-representativeness-of-public-opinion-surveys/. Published May 15, 2012. Accessed March 9, 2017.
The National Defense Appropriations Act for fiscal year 2009, Subtitle B, waived copayments for preventive cancer screening services for all TRICARE beneficiaries, excluding Medicare-eligible beneficiaries.1 These preventive services include screening for colorectal cancer (CRC), breast cancer, and cervical cancer based on current guidelines (eAppendix1).
Despite having unrestricted access to these cancer screenings, TRICARE Prime beneficiaries report overall screening completion rates that are below the national commercial benchmarks established by the Healthcare Effectiveness Data and Information Set (HEDIS) for all 3 cancer types.2 Specifically, among TRICARE Prime beneficiaries enrolled in the western region of the U.S. in October 2013, the reported breast cancer screening rate was 61.6% (43,138/69,976) for women aged 42 to 69 years, which is well below the HEDIS 75th percentile of 76%. Similarly, the reported rate of cervical cancer screening among women aged 24 to 64 years was 68.3% (63,523/92,946), well below the HEDIS 75th percentile of 79%. Last, the reported rate of CRC screening among male and female TRICARE Prime members aged 51 to 75 years was 61.6% (52,860/85,827), also below the 2013 HEDIS 75th percentile of 63% based on internal review of TRICARE data used for HEDIS reporting.
Given the reported low screening rates, the Defense Health Agency (DHA) performed a cross-sectional survey to assess TRICARE Prime West region beneficiaries’ knowledge and understanding of preventive health screening, specifically for breast cancer, cervical cancer, and CRC, and to identify any potential barriers to access for these screenings.
Methods
A mostly closed-ended, 42-item telephone survey was designed and conducted (eAppendix2)
All women participating in the survey, regardless of age, were asked questions regarding cervical cancer screening. Women aged ≥ 42 years additionally were asked a second set of survey questions specific to breast cancer screening, and women aged between 51 and 64 years were asked a third set of questions related to CRC screening. The ages selected were 1 to 2 years after the recommended age for the respective screening to ensure adequate follow-up time for the member to obtain the screening. Men included in the survey were asked questions related only to CRC screening.
The target survey sample was 3,500 beneficiaries, separated into the following 4 strata: women aged 21 to 64 years of age enrolled in the direct care system (n = 1,250); women aged 21 to 64 years enrolled in the purchased (commercial) care network (n = 1,250); men aged 51 to 64 years enrolled in the direct care system (n = 500); and men aged 51 to 64 years enrolled in the purchased care network (n = 500). The random sample was drawn from an overall population of about 35,000 members. Sampling was performed without replacement until the target number of surveys was achieved. Survey completion was defined as the respondent having reached the end of the survey questionnaire but not necessarily having answered every question.
Data Elements
The preventive health survey collected information on beneficiaries’ knowledge of and satisfaction with their PCM, the primary location where they sought health care in the previous 12 months, preference for scheduling cancer screening tests, and general knowledge about the frequency and type of screening for breast, cervical, and colorectal cancers. Responses were scored based on guidelines effective as of 2009. In addition, the survey collected information on the beneficiary’s overall health status, current age, highest level of education achieved, current employment status, place of residence (on or off a military installation), race, and whether the beneficiary carried other health insurance aside from TRICARE.
Survey Mode and Fielding
A sampling population of eligible beneficiaries was created from a database of all TRICARE Prime beneficiaries. An automated system was used to randomly draw potential participants from the sample. Survey interviewers were given the beneficiary’s name and telephone number but no other identifiable information. Phone numbers from the sample were dialed up to 6 times before the number was classified as a “no answer.” Interviewers read to each beneficiary a statement describing the survey and participation risk and benefits and explained that participation was voluntary and the participant could end the survey at any time without penalty or prejudice. The survey commenced only after verbal consent was obtained.
Sample Weighting and Statistical Analysis
Each survey record was weighted to control for potential bias associated with unequal rates of noncoverage and nonresponse in the sampled population. A design weight was calculated as the ratio of the frame size and the sample size in each stratum. For each stratum, an adjusted response rate (RR) was calculated as the number of completed surveys divided by the number of eligible respondents. Since all respondents were eligible, the RR was not adjusted. The ratio of the design weight to the adjusted RR was calculated and assigned to each survey.
Frequency distributions and descriptive statistics were calculated for all close-ended survey items. Open-ended survey items were summarized and assessed qualitatively. When appropriate, open-ended responses were categorized and included in descriptive analyses. No formal statistical testing was performed.
Results
A total of 6,563 beneficiaries were contacted, and 3,688 agreed to participate (56%), resulting in 3,500 TRICARE beneficiaries completing the survey (95% completion rate), of whom 71% (2,500) were female. The overall cooperation rates were similar across the 4 strata. Interviews ceased once 3,500 surveys were completed. The largest distribution of respondents was aged between 55 and 64 years (37%) (Table 1). Respondents aged 21 to 24 years comprised the smallest percentage of the sample (7%). Nearly a third of respondents were dependents of ADSMs (30%), another 30% were retirees, and most respondents self-identified as white (Table 1).
Barriers to Screening
A series of survey questions was asked about specific barriers to cancer screening, including the convenience of appointment times for the respondent’s last cancer screening. The majority (69%, 2,415 of 3,500) responded that the appointment times were convenient. Among those who stated that times were not convenient and those who had not scheduled an examination, 66% responded that they did not know or were not sure how to schedule a cancer screening test.
Screening Preferences
Less than half of survey respondents (48%) reported that they received screening guideline information from their physician or provider; 24% reported that they performed their own research. Only 9% reported that they learned about the guidelines through TRICARE materials, and 7% of respondents indicated that media, family, or friends were their source of screening information.
The survey respondents who indicated that they had not scheduled a screening examination were asked when (time of day) they preferred to have a screening. Less than half (47%) reported that varying available appointment times would not affect their ability to obtain screening. One-quarter preferred times for screening during working hours, 20% preferred times after working hours, 6% preferred times before working hours, and 2% responded that they were unsure or did not know. The majority (89%) reported that they would prefer to receive all available screenings on the same day if possible.
Breast Cancer Screening
Nearly all (98%) of the 1,100 women aged between 42 and 64 years reported having received a mammogram. These women were asked a specific subset of questions related to breast cancer screening. Respondents were asked to state the recommended age at which women should begin receiving mammogram screenings. More than half (55%) provided the correct response (40 years old, per the U.S. Preventive Services Task Force guidelines).3,4 About three-quarters of respondents (789) correctly responded annually to the question regarding how often women should receive mammograms.
The survey also sought to identify barriers that prevented women from obtaining necessary breast cancer screening. However, the majority surveyed (85%) noted that the question was not applicable because they typically scheduled screening appointments. Only a few (3%) reported factors such as either themselves or someone they know having had a negative experience, discomfort, pain, or concerns of a falsepositive result as reasons for not obtaining breast cancer screening. Of the 112 respondents to the open-ended question, 25% reported that their schedules prevented them from scheduling a mammogram in the past; 12% reported that an inconvenient clinic location, appointment time, or process prevented them from receiving a screening; and 13% reported forgetting to schedule the screening (Table 2).
Cervical Cancer Screening
Female respondents aged between 21 and 64 years (n = 2,432) were asked about the recommended age at which women should begin receiving cervical cancer screening. Only 1% of respondents provided the correct response (that screening begins at 21 years of age per the U.S. Preventive Services Task Force Report guidelines), while 88% provided an incorrect response, and 11% were unsure or did not provide any response.5 Among all respondents, 98% reported having had a cervical cancer screening.
Respondents were asked how frequently women should have a Papanicolaou (Pap) test. Responses such as “2 to 3 years,” “2 years,” or “every other year” were labeled as correct, whereas responses such as “every 6 months” or “greater than 3 years” were labeled as incorrect. Just 12% of respondents provided a correct response, whereas 86% answered incorrectly, and 2% did not answer or did not know. Of those who answered incorrectly, the most common response was “annually” or “every year,” with no notable differences according to race, age, or beneficiary category.
To better understand barriers to screening, respondents were asked to identify reasons they might not have sought cervical cancer screening. The majority (84%) reported that they typically scheduled appointments and that the question was not applicable. However, among 228 respondents who provided an open-ended response and who had not previously undergone a hysterectomy, 8% stated that they had received no reminder or that they lacked sufficient information to schedule the appointment, 21% forgot to schedule, 18% reported a scheduling conflict or difficulty in receiving care, and 13% noted that they did not believe in annual screening (Table 2).
Colorectal Cancer Screening
Eighty-seven percent of eligible respondents (n = 1,734) reported having ever had a sigmoidoscopy and/or colonoscopy. Respondents were asked for their understanding of the recommended age for men and women to begin CRC screening.6 Nearly three-quarters of respondents provided a correct response (n = 1,225), compared with 23% of respondents (n = 407) who answered incorrectly and 6% (n = 102) who did not provide a response or stated they did not know. Correct responses were numerically higher among white respondents (73%) compared with black (62%) and other (62%) respondents as well as among persons aged < 60 years (73%) vs those aged > 60 years (67%).
Respondents aged between 51 and 64 years were asked how often the average person should receive colon cancer screenings. The most common response was that screening should occur every 5 years (33%) followed by every 10 years (26%). This aligns with the U.S. Preventive Services Task Force’s recommendations for flexible sigmoidoscopy every 5 years or colonoscopy every 10 years.
Eligible respondents were asked to identify reasons they did not seek CRC screening. Eighty-six percent of respondents indicated that they typically scheduled CRC screening and that the question was not applicable. Among respondents who provided an open-ended response, 26% cited feeling uncomfortable with the procedure, 15% cited forgetting to schedule a screening, 15% noted a lack of information on screening, and 11% reported no need for screening (Table 2). Among the 1,734 respondents, 80% reported that they would prefer a fecal occult blood test (FOBT) over either a colonoscopy or a sigmoidoscopy. Only 51% reported that their PCM had previously discussed the different types of CRC screenings at some point.
Discussion
The purpose of this large, representative survey was to obtain information on beneficiaries’ knowledge, perceived barriers, and beliefs regarding breast, cervical, and colorectal cancer screenings to identify factors contributing to low completion rates. As far as is known, this is the first study to address these questions in a TRICARE population. Overall, the findings suggest that beneficiaries consider cancer screening important, largely relying on their PCM or their research to better understand how and when to obtain such screenings. The majority received 1 or more screenings prior to the survey, but there were some common knowledge gaps about how to schedule screening appointments, relevant TRICARE medical benefits, and the current recommendations regarding screening timing and frequency. A commonly reported issue across all surveyed groups was inconvenient screening times.
More than half (55%) of respondents correctly noted that breast cancer screening begins at age 40 years (based on recommendations at the time the survey was conducted), and 72% understood when screening should occur. Despite access to care, inconvenient schedules and testing locations were considered the biggest barriers to regularly obtaining a mammogram. There are few studies on knowledge of breast cancer screening in an insured population available for comparison.7-10 One study of medically insured black and non-Hispanic women aged 43 to 49 years showed that lack of reminders or knowledge about the need for mammograms, cost, being too busy, and forgetting to schedule appointments were all factors associated with nonadherence to repeat mammography examinations.8 In an integrative review published in 2000, authors cited that among 8 of 13 relevant studies, the major barrier to receiving a recommended mammogram was lack of physician recommendation.7
For cervical cancer screening, few respondents (1%) correctly identified the age for initiation of screening, and just 12% correctly identified the frequency of screening. These findings are consistent with those of other studies, suggesting a general misunderstanding
about Pap tests in the U.S. and among low-income women.11,12 Reported barriers to screening were uncommon but included scheduling conflicts and lack of reminders or information and were consistent with barriers cited in prior studies.13,14 A few respondents (13%) noted that they did not believe in annual screening, which is similar to the findings of Decker and colleagues who cited lack of knowledge about the test and belief that screening is of no benefit as reasons for failure to get a recommended Pap test.13 These findings suggest a need to improve patientprovider communication and to provide more patient educational materials about the importance of cervical cancer screening.
A large proportion (71%) gave the correct response regarding the appropriate age to initiate CRC screening. Discomfort with the procedure, belief that the screening is unnecessary, or lack of physician’s recommendation were noted barriers to CRC screening. These findings are similar to those reported elsewhere in non-TRICARE populations.15-20 Two focus groups included participants with little knowledge about CRC screening, such as risk factors and symptoms, and expressed fear and embarrassment about CRC and screening. Few of the focus group participants were aware of the available options for screening, and some were confused about the purpose and benefits of the various screening modalities.16
A Health Information National Trends survey reported that 24% participants had not received a colonoscopy or a sigmoidoscopy because their PCM did not order it or say that it was necessary.15 The reported perceived barriers included fear of an adverse finding, injury to the colon from screening, and embarrassment. A study performed in 1,901 Medicare-insured individuals with no history of CRC cited lack of knowledge/awareness and no physician order as the most common reasons for not undergoing CRC screening.18
Strengths and Limitations
A major strength of the current survey is the 56% completion rate, which far exceeds other survey participation rates that were as low as 9%.21 A second strength is the scope of the survey to capture information on not 1 but 3 different cancer screening practices in a unique population who receive preventive screenings at low to no cost.
There are a few study limitations. The majority of respondents identified as white (80%), which does not fully align with the racial distribution of the TRICARE Prime population in the West Region, which is about 68% white. This higher proportion of white respondents may affect the ability to generalize findings to other populations. However, given the open access to care, race should not be a major factor contributing to screening decisions. Another potential limitation to the generalizability of the study is that the age of the respondents was capped at 64 years. Considering that some of the reported barriers to screening were “too busy” or “scheduling conflict,” a study population that included respondents aged ≥ 65 years (who might be more likely to be retired) might report lower rates of these schedule-related barriers.
A third limitation is that most questions about prior screenings pertained to any time in the past, and, therefore, limited the ability to identify current factors leading to lower screening rates. Last, the survey was developed prior to the 2012 changes in cervical and breast cancer screening recommendations and was therefore scored based on prior recommendations. Given that the goal was to assess knowledge and barriers, results are not expected to differ greatly if they are scored using the newer guidelines.
Conclusion
Findings from this cross-sectional survey indicate high levels of knowledge among TRICARE West Region beneficiaries regarding when and how often screening for breast cancer, cervical cancer, and CRC should occur. To encourage TRICARE beneficiaries to seek and obtain recommended and covered cancer screenings, further efforts are needed, including more education about the importance of screening and how to obtain screening. The survey results suggest that TRICARE Prime beneficiaries view cancer screening as important for overall health but they require (and also may desire) more frequent scheduling reminders, education, and more options for scheduling. Newer modalities for communicating with beneficiaries, such as automated telephone appointment reminders, reminder texts, online appointment scheduling, educational blogs, podcasts on cancer screening, extended appointment hours, or unconventional strategies to bundle screening services, are tools that could be used by providers to achieve greater compliance with cancer screening recommendations.
Author Disclosure
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Click here to read the digital edition.
The National Defense Appropriations Act for fiscal year 2009, Subtitle B, waived copayments for preventive cancer screening services for all TRICARE beneficiaries, excluding Medicare-eligible beneficiaries.1 These preventive services include screening for colorectal cancer (CRC), breast cancer, and cervical cancer based on current guidelines (eAppendix1).
Despite having unrestricted access to these cancer screenings, TRICARE Prime beneficiaries report overall screening completion rates that are below the national commercial benchmarks established by the Healthcare Effectiveness Data and Information Set (HEDIS) for all 3 cancer types.2 Specifically, among TRICARE Prime beneficiaries enrolled in the western region of the U.S. in October 2013, the reported breast cancer screening rate was 61.6% (43,138/69,976) for women aged 42 to 69 years, which is well below the HEDIS 75th percentile of 76%. Similarly, the reported rate of cervical cancer screening among women aged 24 to 64 years was 68.3% (63,523/92,946), well below the HEDIS 75th percentile of 79%. Last, the reported rate of CRC screening among male and female TRICARE Prime members aged 51 to 75 years was 61.6% (52,860/85,827), also below the 2013 HEDIS 75th percentile of 63% based on internal review of TRICARE data used for HEDIS reporting.
Given the reported low screening rates, the Defense Health Agency (DHA) performed a cross-sectional survey to assess TRICARE Prime West region beneficiaries’ knowledge and understanding of preventive health screening, specifically for breast cancer, cervical cancer, and CRC, and to identify any potential barriers to access for these screenings.
Methods
A mostly closed-ended, 42-item telephone survey was designed and conducted (eAppendix2)
All women participating in the survey, regardless of age, were asked questions regarding cervical cancer screening. Women aged ≥ 42 years additionally were asked a second set of survey questions specific to breast cancer screening, and women aged between 51 and 64 years were asked a third set of questions related to CRC screening. The ages selected were 1 to 2 years after the recommended age for the respective screening to ensure adequate follow-up time for the member to obtain the screening. Men included in the survey were asked questions related only to CRC screening.
The target survey sample was 3,500 beneficiaries, separated into the following 4 strata: women aged 21 to 64 years of age enrolled in the direct care system (n = 1,250); women aged 21 to 64 years enrolled in the purchased (commercial) care network (n = 1,250); men aged 51 to 64 years enrolled in the direct care system (n = 500); and men aged 51 to 64 years enrolled in the purchased care network (n = 500). The random sample was drawn from an overall population of about 35,000 members. Sampling was performed without replacement until the target number of surveys was achieved. Survey completion was defined as the respondent having reached the end of the survey questionnaire but not necessarily having answered every question.
Data Elements
The preventive health survey collected information on beneficiaries’ knowledge of and satisfaction with their PCM, the primary location where they sought health care in the previous 12 months, preference for scheduling cancer screening tests, and general knowledge about the frequency and type of screening for breast, cervical, and colorectal cancers. Responses were scored based on guidelines effective as of 2009. In addition, the survey collected information on the beneficiary’s overall health status, current age, highest level of education achieved, current employment status, place of residence (on or off a military installation), race, and whether the beneficiary carried other health insurance aside from TRICARE.
Survey Mode and Fielding
A sampling population of eligible beneficiaries was created from a database of all TRICARE Prime beneficiaries. An automated system was used to randomly draw potential participants from the sample. Survey interviewers were given the beneficiary’s name and telephone number but no other identifiable information. Phone numbers from the sample were dialed up to 6 times before the number was classified as a “no answer.” Interviewers read to each beneficiary a statement describing the survey and participation risk and benefits and explained that participation was voluntary and the participant could end the survey at any time without penalty or prejudice. The survey commenced only after verbal consent was obtained.
Sample Weighting and Statistical Analysis
Each survey record was weighted to control for potential bias associated with unequal rates of noncoverage and nonresponse in the sampled population. A design weight was calculated as the ratio of the frame size and the sample size in each stratum. For each stratum, an adjusted response rate (RR) was calculated as the number of completed surveys divided by the number of eligible respondents. Since all respondents were eligible, the RR was not adjusted. The ratio of the design weight to the adjusted RR was calculated and assigned to each survey.
Frequency distributions and descriptive statistics were calculated for all close-ended survey items. Open-ended survey items were summarized and assessed qualitatively. When appropriate, open-ended responses were categorized and included in descriptive analyses. No formal statistical testing was performed.
Results
A total of 6,563 beneficiaries were contacted, and 3,688 agreed to participate (56%), resulting in 3,500 TRICARE beneficiaries completing the survey (95% completion rate), of whom 71% (2,500) were female. The overall cooperation rates were similar across the 4 strata. Interviews ceased once 3,500 surveys were completed. The largest distribution of respondents was aged between 55 and 64 years (37%) (Table 1). Respondents aged 21 to 24 years comprised the smallest percentage of the sample (7%). Nearly a third of respondents were dependents of ADSMs (30%), another 30% were retirees, and most respondents self-identified as white (Table 1).
Barriers to Screening
A series of survey questions was asked about specific barriers to cancer screening, including the convenience of appointment times for the respondent’s last cancer screening. The majority (69%, 2,415 of 3,500) responded that the appointment times were convenient. Among those who stated that times were not convenient and those who had not scheduled an examination, 66% responded that they did not know or were not sure how to schedule a cancer screening test.
Screening Preferences
Less than half of survey respondents (48%) reported that they received screening guideline information from their physician or provider; 24% reported that they performed their own research. Only 9% reported that they learned about the guidelines through TRICARE materials, and 7% of respondents indicated that media, family, or friends were their source of screening information.
The survey respondents who indicated that they had not scheduled a screening examination were asked when (time of day) they preferred to have a screening. Less than half (47%) reported that varying available appointment times would not affect their ability to obtain screening. One-quarter preferred times for screening during working hours, 20% preferred times after working hours, 6% preferred times before working hours, and 2% responded that they were unsure or did not know. The majority (89%) reported that they would prefer to receive all available screenings on the same day if possible.
Breast Cancer Screening
Nearly all (98%) of the 1,100 women aged between 42 and 64 years reported having received a mammogram. These women were asked a specific subset of questions related to breast cancer screening. Respondents were asked to state the recommended age at which women should begin receiving mammogram screenings. More than half (55%) provided the correct response (40 years old, per the U.S. Preventive Services Task Force guidelines).3,4 About three-quarters of respondents (789) correctly responded annually to the question regarding how often women should receive mammograms.
The survey also sought to identify barriers that prevented women from obtaining necessary breast cancer screening. However, the majority surveyed (85%) noted that the question was not applicable because they typically scheduled screening appointments. Only a few (3%) reported factors such as either themselves or someone they know having had a negative experience, discomfort, pain, or concerns of a falsepositive result as reasons for not obtaining breast cancer screening. Of the 112 respondents to the open-ended question, 25% reported that their schedules prevented them from scheduling a mammogram in the past; 12% reported that an inconvenient clinic location, appointment time, or process prevented them from receiving a screening; and 13% reported forgetting to schedule the screening (Table 2).
Cervical Cancer Screening
Female respondents aged between 21 and 64 years (n = 2,432) were asked about the recommended age at which women should begin receiving cervical cancer screening. Only 1% of respondents provided the correct response (that screening begins at 21 years of age per the U.S. Preventive Services Task Force Report guidelines), while 88% provided an incorrect response, and 11% were unsure or did not provide any response.5 Among all respondents, 98% reported having had a cervical cancer screening.
Respondents were asked how frequently women should have a Papanicolaou (Pap) test. Responses such as “2 to 3 years,” “2 years,” or “every other year” were labeled as correct, whereas responses such as “every 6 months” or “greater than 3 years” were labeled as incorrect. Just 12% of respondents provided a correct response, whereas 86% answered incorrectly, and 2% did not answer or did not know. Of those who answered incorrectly, the most common response was “annually” or “every year,” with no notable differences according to race, age, or beneficiary category.
To better understand barriers to screening, respondents were asked to identify reasons they might not have sought cervical cancer screening. The majority (84%) reported that they typically scheduled appointments and that the question was not applicable. However, among 228 respondents who provided an open-ended response and who had not previously undergone a hysterectomy, 8% stated that they had received no reminder or that they lacked sufficient information to schedule the appointment, 21% forgot to schedule, 18% reported a scheduling conflict or difficulty in receiving care, and 13% noted that they did not believe in annual screening (Table 2).
Colorectal Cancer Screening
Eighty-seven percent of eligible respondents (n = 1,734) reported having ever had a sigmoidoscopy and/or colonoscopy. Respondents were asked for their understanding of the recommended age for men and women to begin CRC screening.6 Nearly three-quarters of respondents provided a correct response (n = 1,225), compared with 23% of respondents (n = 407) who answered incorrectly and 6% (n = 102) who did not provide a response or stated they did not know. Correct responses were numerically higher among white respondents (73%) compared with black (62%) and other (62%) respondents as well as among persons aged < 60 years (73%) vs those aged > 60 years (67%).
Respondents aged between 51 and 64 years were asked how often the average person should receive colon cancer screenings. The most common response was that screening should occur every 5 years (33%) followed by every 10 years (26%). This aligns with the U.S. Preventive Services Task Force’s recommendations for flexible sigmoidoscopy every 5 years or colonoscopy every 10 years.
Eligible respondents were asked to identify reasons they did not seek CRC screening. Eighty-six percent of respondents indicated that they typically scheduled CRC screening and that the question was not applicable. Among respondents who provided an open-ended response, 26% cited feeling uncomfortable with the procedure, 15% cited forgetting to schedule a screening, 15% noted a lack of information on screening, and 11% reported no need for screening (Table 2). Among the 1,734 respondents, 80% reported that they would prefer a fecal occult blood test (FOBT) over either a colonoscopy or a sigmoidoscopy. Only 51% reported that their PCM had previously discussed the different types of CRC screenings at some point.
Discussion
The purpose of this large, representative survey was to obtain information on beneficiaries’ knowledge, perceived barriers, and beliefs regarding breast, cervical, and colorectal cancer screenings to identify factors contributing to low completion rates. As far as is known, this is the first study to address these questions in a TRICARE population. Overall, the findings suggest that beneficiaries consider cancer screening important, largely relying on their PCM or their research to better understand how and when to obtain such screenings. The majority received 1 or more screenings prior to the survey, but there were some common knowledge gaps about how to schedule screening appointments, relevant TRICARE medical benefits, and the current recommendations regarding screening timing and frequency. A commonly reported issue across all surveyed groups was inconvenient screening times.
More than half (55%) of respondents correctly noted that breast cancer screening begins at age 40 years (based on recommendations at the time the survey was conducted), and 72% understood when screening should occur. Despite access to care, inconvenient schedules and testing locations were considered the biggest barriers to regularly obtaining a mammogram. There are few studies on knowledge of breast cancer screening in an insured population available for comparison.7-10 One study of medically insured black and non-Hispanic women aged 43 to 49 years showed that lack of reminders or knowledge about the need for mammograms, cost, being too busy, and forgetting to schedule appointments were all factors associated with nonadherence to repeat mammography examinations.8 In an integrative review published in 2000, authors cited that among 8 of 13 relevant studies, the major barrier to receiving a recommended mammogram was lack of physician recommendation.7
For cervical cancer screening, few respondents (1%) correctly identified the age for initiation of screening, and just 12% correctly identified the frequency of screening. These findings are consistent with those of other studies, suggesting a general misunderstanding
about Pap tests in the U.S. and among low-income women.11,12 Reported barriers to screening were uncommon but included scheduling conflicts and lack of reminders or information and were consistent with barriers cited in prior studies.13,14 A few respondents (13%) noted that they did not believe in annual screening, which is similar to the findings of Decker and colleagues who cited lack of knowledge about the test and belief that screening is of no benefit as reasons for failure to get a recommended Pap test.13 These findings suggest a need to improve patientprovider communication and to provide more patient educational materials about the importance of cervical cancer screening.
A large proportion (71%) gave the correct response regarding the appropriate age to initiate CRC screening. Discomfort with the procedure, belief that the screening is unnecessary, or lack of physician’s recommendation were noted barriers to CRC screening. These findings are similar to those reported elsewhere in non-TRICARE populations.15-20 Two focus groups included participants with little knowledge about CRC screening, such as risk factors and symptoms, and expressed fear and embarrassment about CRC and screening. Few of the focus group participants were aware of the available options for screening, and some were confused about the purpose and benefits of the various screening modalities.16
A Health Information National Trends survey reported that 24% participants had not received a colonoscopy or a sigmoidoscopy because their PCM did not order it or say that it was necessary.15 The reported perceived barriers included fear of an adverse finding, injury to the colon from screening, and embarrassment. A study performed in 1,901 Medicare-insured individuals with no history of CRC cited lack of knowledge/awareness and no physician order as the most common reasons for not undergoing CRC screening.18
Strengths and Limitations
A major strength of the current survey is the 56% completion rate, which far exceeds other survey participation rates that were as low as 9%.21 A second strength is the scope of the survey to capture information on not 1 but 3 different cancer screening practices in a unique population who receive preventive screenings at low to no cost.
There are a few study limitations. The majority of respondents identified as white (80%), which does not fully align with the racial distribution of the TRICARE Prime population in the West Region, which is about 68% white. This higher proportion of white respondents may affect the ability to generalize findings to other populations. However, given the open access to care, race should not be a major factor contributing to screening decisions. Another potential limitation to the generalizability of the study is that the age of the respondents was capped at 64 years. Considering that some of the reported barriers to screening were “too busy” or “scheduling conflict,” a study population that included respondents aged ≥ 65 years (who might be more likely to be retired) might report lower rates of these schedule-related barriers.
A third limitation is that most questions about prior screenings pertained to any time in the past, and, therefore, limited the ability to identify current factors leading to lower screening rates. Last, the survey was developed prior to the 2012 changes in cervical and breast cancer screening recommendations and was therefore scored based on prior recommendations. Given that the goal was to assess knowledge and barriers, results are not expected to differ greatly if they are scored using the newer guidelines.
Conclusion
Findings from this cross-sectional survey indicate high levels of knowledge among TRICARE West Region beneficiaries regarding when and how often screening for breast cancer, cervical cancer, and CRC should occur. To encourage TRICARE beneficiaries to seek and obtain recommended and covered cancer screenings, further efforts are needed, including more education about the importance of screening and how to obtain screening. The survey results suggest that TRICARE Prime beneficiaries view cancer screening as important for overall health but they require (and also may desire) more frequent scheduling reminders, education, and more options for scheduling. Newer modalities for communicating with beneficiaries, such as automated telephone appointment reminders, reminder texts, online appointment scheduling, educational blogs, podcasts on cancer screening, extended appointment hours, or unconventional strategies to bundle screening services, are tools that could be used by providers to achieve greater compliance with cancer screening recommendations.
Author Disclosure
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Click here to read the digital edition.
1. TRICARE. TRICARE policy manual 6010.57-M. http://manuals.tricare.osd.mil/pages/DisplayManualaspx?SeriesId=POLICY. Published February 1, 2008. Accessed March 9, 2017.
2. National Committee for Quality Assurance. 2013 accreditation benchmarks and thresholds—mid-year update. http://www.ncqa.org/Portals/0/PolicyUpdates/Trending %20and%20Benchmarks/archives/2013_BENCHMARKS ANDTHRESHOLDS_for%20MidYear%20Update_Final.pdf. Published July 24, 2013. Accessed March 9, 2017.
3. U.S. Preventative Services Task Force. Archived final recommendation statement: breast cancer: screening, 2002. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/breast-cancer-screening-2002. Published December 30, 2013. Accessed March 9, 2017.
4. Smith RA, Saslow D, Sawyer KA, et al; American Cancer Society High-Risk Work Group; American Cancer Society Screening Older Women Work Group; American Cancer Society Mammography Work Group; American Cancer Society Physical Examination Work Group; American Cancer Society New Technologies Work Group; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast cancer screening: update 2003. CA Cancer J Clin. 2003;53(3):141-169.
5. Moyer VA; U.S. Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156(12):880-891, W312.
6. U.S. Preventive Services Task Force. Archived: colorectal cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/colorectal-cancer-screening. Published October 2008. Accessed March 9, 2017.
7. George SA. Barriers to breast cancer screening: an integrative review. Health Care Women Int. 2000;21(1):53-65.
8. Gierisch JM, O’Neill SC, Rimer BK, DeFrank JT, Bowling JM, Skinner CS. Factors associated with annual-interval mammography for women in their 40s. Cancer Epidemiol. 2009;33(1):72-78.
9. Peppercorn J, Houck K, Beri N, et al. Breast cancer screening utilization and understanding of current guidelines among rural U.S. women with private insurance. Breast Cancer Res Treat. 2015;153(3):659-667.
10. Sarma EA. Barriers to screening mammography. Health Psychol Rev. 2015;9(1):42-62.
11. Hawkins NA, Benard VB, Greek A, Roland KB, Manninen D, Saraiya M. Patient knowledge and beliefs as barriers to extending cervical cancer screening intervals in federally qualified health centers. Prev Med. 2013;57(5):641-645.
12. Hawkins NA, Cooper CP, Saraiya M, Gelb CA, Polonec L. Why the Pap test? Awareness and use of the Pap test among women in the United States. J Womens Health (Larchmt). 2011;20(4):511-515.
13. Decker KM, Turner D, Demers AA, Martens PJ, Lambert P, Chateau D. Evaluating the effectiveness of cervical cancer screening invitation letters. J Womens Health (Larchmt). 2013;22(8):687-693.
14. Yao X, Dembe AE, Wickizer T, Lu B. Does time pressure create barriers for people to receive preventive health services? Prev Med. 2015;74:55-58.
15. Geiger TM, Miedema BW, Geana MV, Thaler K, Rangnekar NJ, Cameron GT. Improving rates for screening colonoscopy: analysis of the Health Information National Trends Survey (HINTS I) data. Surgical Endoscopy. 2008;22(2):527-533.
16. Greisinger A, Hawley ST, Bettencourt JL, Perz CA, Vernon SW. Primary care patients’ understanding of colorectal cancer screening. Cancer Detect Prev. 2006;30(1):67-74.
17. Janz NK, Wren PA, Schottenfeld D, Guire KE. Colorectal cancer screening attitudes and behavior: a populationbased study. Prev Med. 2003;37(6, pt 1):627-634.
18. Klabunde CN, Schenck AP, Davis WW. Barriers to colorectal cancer screening among Medicare consumers. Am J Prev Med. 2006;30(4):313-319.
19. Klabunde CN, Vernon SW, Nadel MR, Breen N, Seeff LC, Brown ML. Barriers to colorectal cancer screening: a comparison of reports from primary care physicians and average-risk adults. Med Care. 2005;43(9):939-944.
20. Berkowitz Z, Hawkins NA, Peipins LA, White MC, Nadel MR. Beliefs, risk perceptions, and gaps in knowledge as barriers to colorectal cancer screening in older adults. J Am Geriatr Soc. 2008;56(2):307-314.
21. Pew Research Center. Assessing the representativeness of public opinion surveys. http://www.people-press.org/2012/05/15/assessing-the-representativeness-of-public-opinion-surveys/. Published May 15, 2012. Accessed March 9, 2017.
1. TRICARE. TRICARE policy manual 6010.57-M. http://manuals.tricare.osd.mil/pages/DisplayManualaspx?SeriesId=POLICY. Published February 1, 2008. Accessed March 9, 2017.
2. National Committee for Quality Assurance. 2013 accreditation benchmarks and thresholds—mid-year update. http://www.ncqa.org/Portals/0/PolicyUpdates/Trending %20and%20Benchmarks/archives/2013_BENCHMARKS ANDTHRESHOLDS_for%20MidYear%20Update_Final.pdf. Published July 24, 2013. Accessed March 9, 2017.
3. U.S. Preventative Services Task Force. Archived final recommendation statement: breast cancer: screening, 2002. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/breast-cancer-screening-2002. Published December 30, 2013. Accessed March 9, 2017.
4. Smith RA, Saslow D, Sawyer KA, et al; American Cancer Society High-Risk Work Group; American Cancer Society Screening Older Women Work Group; American Cancer Society Mammography Work Group; American Cancer Society Physical Examination Work Group; American Cancer Society New Technologies Work Group; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast cancer screening: update 2003. CA Cancer J Clin. 2003;53(3):141-169.
5. Moyer VA; U.S. Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156(12):880-891, W312.
6. U.S. Preventive Services Task Force. Archived: colorectal cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/colorectal-cancer-screening. Published October 2008. Accessed March 9, 2017.
7. George SA. Barriers to breast cancer screening: an integrative review. Health Care Women Int. 2000;21(1):53-65.
8. Gierisch JM, O’Neill SC, Rimer BK, DeFrank JT, Bowling JM, Skinner CS. Factors associated with annual-interval mammography for women in their 40s. Cancer Epidemiol. 2009;33(1):72-78.
9. Peppercorn J, Houck K, Beri N, et al. Breast cancer screening utilization and understanding of current guidelines among rural U.S. women with private insurance. Breast Cancer Res Treat. 2015;153(3):659-667.
10. Sarma EA. Barriers to screening mammography. Health Psychol Rev. 2015;9(1):42-62.
11. Hawkins NA, Benard VB, Greek A, Roland KB, Manninen D, Saraiya M. Patient knowledge and beliefs as barriers to extending cervical cancer screening intervals in federally qualified health centers. Prev Med. 2013;57(5):641-645.
12. Hawkins NA, Cooper CP, Saraiya M, Gelb CA, Polonec L. Why the Pap test? Awareness and use of the Pap test among women in the United States. J Womens Health (Larchmt). 2011;20(4):511-515.
13. Decker KM, Turner D, Demers AA, Martens PJ, Lambert P, Chateau D. Evaluating the effectiveness of cervical cancer screening invitation letters. J Womens Health (Larchmt). 2013;22(8):687-693.
14. Yao X, Dembe AE, Wickizer T, Lu B. Does time pressure create barriers for people to receive preventive health services? Prev Med. 2015;74:55-58.
15. Geiger TM, Miedema BW, Geana MV, Thaler K, Rangnekar NJ, Cameron GT. Improving rates for screening colonoscopy: analysis of the Health Information National Trends Survey (HINTS I) data. Surgical Endoscopy. 2008;22(2):527-533.
16. Greisinger A, Hawley ST, Bettencourt JL, Perz CA, Vernon SW. Primary care patients’ understanding of colorectal cancer screening. Cancer Detect Prev. 2006;30(1):67-74.
17. Janz NK, Wren PA, Schottenfeld D, Guire KE. Colorectal cancer screening attitudes and behavior: a populationbased study. Prev Med. 2003;37(6, pt 1):627-634.
18. Klabunde CN, Schenck AP, Davis WW. Barriers to colorectal cancer screening among Medicare consumers. Am J Prev Med. 2006;30(4):313-319.
19. Klabunde CN, Vernon SW, Nadel MR, Breen N, Seeff LC, Brown ML. Barriers to colorectal cancer screening: a comparison of reports from primary care physicians and average-risk adults. Med Care. 2005;43(9):939-944.
20. Berkowitz Z, Hawkins NA, Peipins LA, White MC, Nadel MR. Beliefs, risk perceptions, and gaps in knowledge as barriers to colorectal cancer screening in older adults. J Am Geriatr Soc. 2008;56(2):307-314.
21. Pew Research Center. Assessing the representativeness of public opinion surveys. http://www.people-press.org/2012/05/15/assessing-the-representativeness-of-public-opinion-surveys/. Published May 15, 2012. Accessed March 9, 2017.
Tips for Living With Tourette Syndrome
Click here to download the PDF.
Click here to download the PDF.
Click here to download the PDF.
Tips for Preventing Encephalitis
Click here to download the PDF.
Click here to download the PDF.
Click here to download the PDF.
Tips for Living With Ataxia
Click here to download the PDF.
Click here to download the PDF.
Click here to download the PDF.
Tips for Living With Bipolar Disorder
Click here to download the PDF.
Click here to download the PDF.
Click here to download the PDF.
Tips for Living With Narcolepsy
Click here to download the PDF.
Click here to download the PDF.
Click here to download the PDF.