User login
Diabetic foot ulcers (DFUs) are devastating, common, and costly. This burden is borne disproportionately by veterans who have high prevalence of type 2 diabetes mellitus (T2DM) and other precipitating risk factors.1 The mortality of veterans following a DFU is sobering, and ulceration is recognized as a significant marker of disease severity.
A 2017 study by Brennan and colleagues reported a 19% mortality rate within 1 year, and only 29% survive past 5 years.2 DFUs are often complicated by peripheral arterial disease (PAD) and diabetic immune dysfunction, contributing to chronic wounds and infection.3,4 About 60% of all foot ulcers become infected, and > 20% of patients with a diabetic foot infection require amputation.5,6
A 2010 retrospective study reports that > 3,400 veterans have a diabetes-related lower extremity amputation annually, vastly surpassing the rate of amputation secondary to trauma in the Veterans Health Administration (VHA).7,8 The inpatient costs for each amputation exceeded $60,000 in fiscal year 2010, and these amputation-related costs represent only 1 component of the total expense to the VHA attributable to diabetic foot complications.7 A recent systematic review by Chan and colleagues estimated mean annual costs in the year following a foot ulcer to be $44,200 to the public payer.9 This implies that direct expenditures for treatment of DFUs within the VHA exceeds $3 billion annually.
Diabetic Foot Ulcer Prevention
Given the dramatic impact of diabetic foot complications to the veteran and the US health care system, the VHA has long recognized the importance of preventive care for those at risk. In 2017 US Department of Veterans Affairs (VA) and Department of Defense issued a clinical practice guideline for the management of T2DM that recommended prophylactic foot care for early identification of any deformity or skin breakdown.10 The guidelines note that a “person who has had a foot ulcer is at lifelong risk of further ulceration,” reflecting the high rate of recurrence among all patients, including veterans. Multiple studies suggest that as many as 40% of patients experience recidivism in the first year after healing from a wound.11-16
The VA is well equipped to deliver quality preventive care because of its innovative and long-standing PAVE (Prevention of Amputations for Veterans Everywhere) program.17 PAVE provides screening, education, appropriate footwear, and stratified care guidelines for veterans at risk for diabetes-related foot complications (Table 1). The practices encouraged by PAVE are evidence-based and synergistic with the objectives of the VA’s patient aligned care team (PACT) delivery approach.18 The granular data collected through PAVE are used to guide best practices and provide benchmarks for diabetic foot outcomes.
Unfortunately, despite PAVE guidelines requiring annual specialist foot care for at-risk veterans, a 2013 report by the VA Office of the Inspector General (OIG) found that one-third of all patients had no documentation of this minimal requirement of preventive foot care.19 Although the VA has worked to address this issue, the data hint at the missed opportunities for prevention of complications and the challenges of ensuring that a large at-risk veteran population has systematic and routine screening with access to specialist foot care.
Given the large proportion of veterans at high risk of chronic wound formation and the challenges of ensuring that this cohort receives good preventive foot care, expanding telemedicine has been suggested. Telemedicine solutions have the potential to reduce the impact of chronic wounds on overburdened clinic resources, schedules, and local and federal budgets.20 Interestingly, the only preventive practice for the diabetic foot that has been proven effective through multiple randomized controlled trials and national and international clinical guidance documents is once-daily foot temperature monitoring.21-26 Daily monitoring has the potential to reduce the burden of DFUs to veterans, improve veteran access to needed preventive care, and reduce costs incurred by the VHA treating diabetic foot complications. Yet despite a recent national guidance document detailing its appropriate use in PAVE 3 veterans, it remains underutilized.27
The purpose of this review is to: (1) discuss the evidence supporting once-daily remote temperature monitoring (RTM), a telemedicine approach critical to improving both veteran access to care and diabetic foot outcomes; (2) summarize a 2017 study that presented an advanced clinical understanding of RTM use among veterans; (3) provide previously unpublished data from this study comparing high-risk VA and non-VA cohorts, highlighting the opportunity for additional focus on foot ulcer prevention within the VA; and (4) report on recent VA utilization of a RTM technology based on this research, emphasizing lessons learned and best practices.
Remote Temperature Monitoring
The objective of daily foot temperature monitoring is to identify impending inflammatory foot conditions, such as DFUs, infection, and acute Charcot neuroarthropathy episodes. The patient and care team then act to resolve the cause of detected inflammation before clinical presentation (prevention) and begin treatment earlier than would otherwise be possible to avoid expensive complications, such as infection (early detection). Preventive therapies are low risk to the patient and inexpensive.
RTM is recommended by multiple clinical practice guidelines, including those of the International Working Group on the Diabetic Foot, the American College of Foot and Ankle Surgeons, and the Wound Healing Society.24-26 Its use is supported by evidence from 3 National Institutes of Health-funded and well-designed randomized controlled trials, 1 of which was additionally supported by a VA Health Services Research and Development Service Merit Award.21-23,28 Conducted between 2004 and 2007, these studies demonstrated the potential to reduce foot ulcer incidence by as much as 85% using a dermal thermometer to identify inflammation and prompt decreased ambulation. Investigators established a clinical monitoring protocol comparing the temperatures between 6 matched locations on the left and right feet. Persistent differences in contralateral temperatures exceeding 2.2°C (4.0°F) were used as a marker for elevated risk and to initiate preventive care. Based on the encouraging results from these studies, a 2017 effectiveness review prepared for the Agency for Healthcare Research and Quality concluded that “home monitoring of foot skin temperature is effective for reducing foot ulcer incidence and recurrence.”29
Accuracy of RTM
A 2017 longitudinal study (NCT02647346) has provided novel data to advance understanding of RTM for the prediction and prevention of DFUs.30 This study was the first to systematically analyze the accuracy of RTM over different monitoring thresholds. The results enable practitioners to deliver risk-stratified preventive care. Policy makers can use the data from this study to weigh the cost and benefits of RTM for population health.
The multicenter trials had 129 participants from 4 VA health care systems: VA Long Beach Healthcare System in California, Miami VA Healthcare System in Florida, Phoenix VA Healthcare System in Arizona, and VA West Los Angeles Healthcare System in California. Each participant was followed for 34 weeks under standard preventive foot care and was instructed to step on a telemedicine SmartMat (Podimetrics, Inc) RTM mat for 20 seconds daily. Participants and investigators were blinded to the temperature data so that the accuracy of temperature monitoring could be assessed. All participants had a history of T2DM and healed DFU. Principal exclusion criteria included unhealed plantar wound, history of proximal lower extremity amputation (ie, above ankle), active Charcot foot disease, and comorbidities that could potentially inhibit an inflammatory response, such as end-stage renal disease, active malignancy, and immunosuppressive diseases.
The investigators reported that RTM with the study mat detected 97% of nonacute plantar DFUs using the most commonly studied threshold (sustained 2.2°C temperature difference). The lead time averaged 37 days before clinical identification of the wound under standard care. Although the false-positive rate of 57% was high, corresponding to approximately 3.0 notifications per patient per year on average in the research setting, it is important to note that this study only considered the prediction of plantar DFUs. Thus, detection of foot inflammation secondary to other conditions, such as preulcerative lesion, dorsal wound, Charcot neuroarthropathy, or foot infection, were reported as a false positive per the study’s definitions. Further, Crisologo and Lavery noted in a translational medicine summary of this research, because the intervention is noninvasive and minimally impactful to the patient and the health care system, “the potential to arrest re-ulceration is worth the perceived inconvenience to the patient.”31
Secondary outcomes related to adherence and ease of use were encouraging. Eighty-eight percent of participants reported that the mat was “very easy to use,” the highest possible score, and 98% were able to set up the mat for home use without difficulty. At the end of the 34-week study, more than 74% of participants remained engaged in routine use of the mat under a per-protocol assessment of adherence. These results are especially impressive given the documented poor adherence of at-risk patients to routine use of therapeutic footwear, which has been reported to be as low as 15%.32
New Research
The data collected during this study has led to new research and advancements in RTM. A recent publication by Gordon and colleagues investigated whether RTM is less accurate in cohorts with perceived challenges.33 They include patients with recently healed wounds and those with a history of partial foot amputation. There was no difference in the accuracy or lead time for either cohort relative to the entire cohort, suggesting that RTM is appropriate for monitoring patients with recently healed DFUs or partial foot amputations.
In another recent study, the data were used to derive a novel approach to monitor a single at-risk foot.34 The practice of RTM has traditionally required comparing temperatures between contralaterally matched plantar locations on the feet, thus limiting its use in patients with a history of major lower extremity amputation and patients being treated for a wound, which may be bandaged or in an off-loading cast or boot. Because the risk factors for DFUs exist in both limbs, these patients are at high risk for developing complications to the contralateral foot and may benefit from preventive once-daily foot temperature monitoring. The investigators empirically derived a novel monitoring approach for patients without a contralateral control. This approach was found to predict 91% of impending plantar DFUs on average 41 days before clinical presentation with a false positive rate of 54%.
Additional Focus on Prevention
Table 2 shows previously unpublished data from a subgroup analysis between veteran and nonveteran participants in the study.25 These descriptive statistics reinforce some widely held assumptions regarding the high-risk veteran population and challenge others. For example, compared with the nonveteran participants, the veteran cohort unsurprisingly had a larger ratio of male participants (P < .01), had a higher rate of cigarette use (P < .01), and was more likely to live alone (although not at a statistically significant level). Veterans in the study had body mass index, rates of alcohol use, frequency of exercise, and glucose control comparable to that of nonveterans.
The potential impact of the PAVE program is clear in several of these comparisons. Although as few as 15% of patients use therapeutic shoes routinely, PAVE ensures that the majority of veterans receive them. Nearly 95% of veterans have therapeutic shoes compared with about 80% of nonveteran participants (P < .05). Veterans also had higher ankle-brachial index results (P < .05), although on average both cohorts were within normal clinical parameters. Veterans had a significantly longer duration since healing from the most recent wound, and fewer veteran participants had a wound that healed in the 3 months prior to the study. Despite this, during the study veterans had annualized DFU incidence equal to that of nonveterans. Furthermore, veterans also had significantly higher rates of amputation prior to participation. That these critical outcomes for veterans are no better than those observed in other care environments despite PAVE suggests that approaches recommended via PAVE alone are insufficient to significantly arrest DFU recurrence, and even more focus on prevention in the VA may be warranted.
From Research to Practice
Since the publication of the 2017 study, the VHA has been at the vanguard of translating the evidence and research underlying RTM into clinical practice. A clinical guidance document governing appropriate use of RTM with the study mat was recently published by the VA Prosthetic and Sensory Aids Service in collaboration with the National Podiatry Program office.27 This guidance document recommends once-daily RTM for at-risk veterans designated PAVE level 3. It defines roles and responsibilities required for the successful implementation of a RTM program with the study device. The document additionally presents various clinical monitoring protocols for veterans, although the protocol and thresholds used are at the discretion of the prescribing clinician and should reflect the risk profile of the veteran in question.
A staged response to inflammation has proven popular, whereby an initial high-sensitivity threshold is chosen for monitoring. The initial response is telephone outreach by a designee supplied by the clinic or device manufacturer, typically a trained registered nurse, to the veteran to collect subjective history and instruct off-loading and reduced ambulation, with a target of 50% baseline reduction in step count. Should the inflammation persist despite off-loading, an examination may be necessary to identify and resolve its cause. For recalcitrant inflammation, more targeted pressure off-loading of the affected area may be accomplished with custom orthotics, accommodative insoles, removable cast walkers, and total contact casting. After 2 to 4 weeks without signs of inflammation, the cause is deemed to have been resolved and lowered the acute risk for developing further diabetic foot complications.
More than 600 veterans have been monitored for > 1,000 patient-years—13 VA medical centers are practicing RTM with the study mat as of this writing. The monitoring program has been integrated into many veteran daily routines as evidenced by > 70% retaining full engagement after having been monitored for > 1 year. The total number of alerts/patient-years across these veterans has been 1.4, significantly lower than the 3.0 alerts/patient-year observed in the study. This is potentially due to successful interventions in response to detected inflammation, resolving inflammation, and avoiding unnecessary alerts occurring in the research setting, which did not employ interventions that resolved inflammation episodes. In the past 6 months, 68% of all inflammation detected resolved via off-loading alone without requiring further clinical intervention. In the cases that required an examination, 76% of patients reported clinically meaningful preventive care (eg, preulcerative callus was debrided, a subungual hemorrhage was treated, a foot ulcer was identified).
Organizational Best Practices
Several best practices have been cultivated related to initiating a RTM program at a new site, for promoting the success of a RTM program, and provisioning excellent preventive care to support the RTM program. Although we advise adhering to the recommendations in the VA guidance document,27 the authors have observed several additional organizational best practices that are not explicitly addressed.
Partnering with PACT. Collaboration between PAVE and PACT has the potential not only to improve outcomes for patients at risk for diabetic foot complications, but also can help identify appropriate high-risk veteran candidates for preventive care with RTM who may not be followed for routine care from a specialty provider, such as a podiatrist, as highlighted by the 2013 OIG report.
Prescreening eligible patients. Several programs have used PAVE data or appointment schedules to identify and target high-risk veterans proactively. This approach has several benefits. It simplifies clinical coordination and streamlines workflow for patient identification and onboarding. It also allows those veterans at highest risk to receive needed and recommended preventive care at their next scheduled appointment. Finally, if PAVE data are used to identify eligible patients, it has the added benefit of ensuring a baseline level of telemedicine preventive foot care for veterans who have become lost to follow-up and have not been seen recently for a routine foot examination.
Implementing foot monitoring during wound treatment. Recent research has expanded the reach of once-daily RTM with the mat to patients being treated for a wound to only 1 foot. This practice has 2 benefits: The patient is able to establish a preventive routine before healing, an important advantage because research strongly suggests that recurrence is most likely in the first months after healing. Second, 48% of patients with a history of DFUs will develop new wounds to the contralateral foot because risk factors, such as neuropathy and peripheral arterial disease, exist in both limbs.35 Furthermore, ongoing treatment for a wound to 1 foot may result in gait deviation and elevated pressure to the sound foot, additionally predisposing the veteran to complications, resulting in a high rate of wounds occurring to the unwounded foot during treatment (0.2 DFU/DFU-year).34 Thus, there is potential benefit in monitoring the sound foot while undergoing treatment for a wound; further, the patient will have immediate access to the device for prevention of recurrence once the wound has resolved.
Utilizing foot monitoring as an extension of telemedicine. Many VA facilities have large geographic catchment areas, making routine follow-up difficult for veterans living in rural areas. RTM serves as an extension of the patient’s daily self-examination and the clinician’s ability to monitor patients with objective information daily. The veterans using the system become more invested and feel as though they are taking an active role in their health care.
Investing in ongoing medical education. Multidisciplinary education sessions reviewing supporting clinical data and resultant clinical practice guidelines raise awareness for those providers and trainees unaware of preventive best practices for the diabetic foot, including those related to foot RTM. These sessions also are helpful for those familiar with foot temperature monitoring or who are responsible for administration of an ongoing program to remain current with contemporary best practices and to discuss improvements for patient care. Familiarity also can help address clinical inertia when benefits and evidence are clearly communicated with health care providers (HCPs).
Clinical Best Practices
Treating preulcerative lesions urgently and aggressively. Callus and other preulcerative lesions often cause progressive tissue damage and poor outcomes. When identified, these lesions should be promptly treated to ensure best outcomes.24
Recognizing the limits of patient self-examinations. Comorbidities such as visual impairment and reduced joint mobility often preclude patients from completing rigorous self-examinations of the foot, which is especially critical while collecting subjective history from the patient during triage of inflammation. A caregiver or spouse can help inspect the foot during outreach and provide additional context.36
Interpreting a benign foot on examination. Because RTM has been demonstrated to detect inflammation preceding a foot ulcer as many as 5 weeks before presentation to the clinic, some veterans may have few signs or symptoms of acute risk during examination. Often, the damage is to subcutaneous tissue resulting from repetitive microtrauma. Research suggests that clinical examination in these cases is often unreliable for identifying the earliest signs of risk, such as palpation to identify subtle temperature changes secondary to inflammation.37 If a patient has refractory inflammation requiring examination and presents with an otherwise unremarkable foot, it is an opportunity to evaluate whether the patient’s shoewear remains appropriate or has worn out, to communicate the veteran’s ongoing elevated risk, and to educate on the importance of diligence in daily foot self-examinations, daily use of the foot temperature monitoring, and continued off-loading until the inflammation resolves.
Communicating the distinction between healing and remission. Although healing is the goal of wound care, patients should be educated that the underlying disease remains after epithelialization. In some cases, tissue deep to the skin has not completed remodeling, and the patient is at acute risk of recurrence. Remission is a powerful metaphor that better describes the patient’s ongoing risk to encourage continued healthy routines and diligent self-care.38Considering the entirety of both feet for recurrence. Critical risk factors for diabetic foot complications, such as peripheral neuropathy and PAD, exist in both limbs, and patients with a history of wounds often develop new complications to different ipsilateral locations, or in as many as 48% of cases, to the contralateral foot.35 For best outcomes, detected inflammation should be treated aggressively independent of whether the location coincides with an area of previous concern.
Encouraging adherence, routine, and empowerment. Advanced diabetes mellitus and neuropathy may impact a patient’s executive function, and multiple studies have reported that patients at risk for inflammatory foot diseases exhibit fatalism toward their foot care and outcomes.39-41 Consistent education, encouragement, empowerment, and establishment of positive routines are needed to ensure high adherence with all preventive care regimens, including RTM.
Case Presentations
The following case series illustrates many of these clinical best practices and characterizes the potential benefits of RTM to veterans within the VA.
Case 1: Prevention After Healing
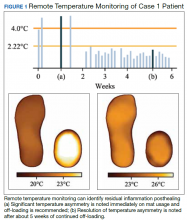
A veteran underwent a Chopart amputation and was recommended to use the mat after healing was perceived. Immediately on use of the study mat, the patient was found to have inflammation to the surgical incision (Figure 1). Clinical staff was alerted to the findings, and the patient was instructed to limit further walking and continue off-loading in his removable cast walker, per protocol. The inflammation of the operative foot quickly reduced, and the patient continued healing successfully, potentially avoiding incisional dehiscence and possible postoperative infection.
This case illustrates that patients’ wounds or surgical incisions may not be completely healed on epithelialization. In the immediate phase after closure, HCPs should consider additional protection to avoid complications. This case demonstrates that RTM can provide objective data to help guide care in that critical period.
Case 2: Identifying Preulcerative Lesions
An 88-year-old veteran had a chronic callus under the second metatarsal head. In addition to routine foot care and therapeutic shoes, he was followed with once-daily RTM. Inflammation was noted, and the veteran was seen in the podiatry clinic where debridement of the callus was performed. The difference in temperatures between feet detected by thermography prior to the clinic visits rapidly resolved after callus debridement, indicating that the underlying inflammation had subsided. RTM was used by the clinical staff to determine the appropriate time interval between clinic visits to avoid callus breakdown and subsequent ulceration.
Case 3: Extending the Clinic Into the Home
An 80-year-old veteran with T2DM and neuropathy was deemed a high-risk patient due to recurrent ulcerations to the left great toe. He was issued a RTM mat and was adherent with routine use. After nearly a year without hot-spot development, inflammation was noted (Figure 2).
Unfortunately, the patient had missed several routine foot care visits and likely that was the reason for the noted inflammation. The patient was called and became reengaged in regular visits for routine foot care. On debridement of his callus, a superficial, noninfected ulceration was discovered. Had remote monitoring not detected the inflammation and impending ulceration, the patient likely would not have been seen in the regular clinic and may have developed a wound infection, potentially resulting in a worse and more costly outcome.
Paradigm Shift to Prevention
Given the exceedingly high burden of diabetic foot complications in the VA, a paradigm shift is needed among HCPs from a culture of treatment to one of prevention. Bus and colleagues reported that in Europe, for every euro spent on ulcer prevention, 10 are spent on ulcer healing, and for every randomized clinical trial conducted on prevention, 10 are conducted on treatment.42-44 Hicks and colleagues showed that the cost of curative care for DFUs is 5 to 30 times greater than the cost of preventive care.45 For RTM in high-risk cohorts (ie, PAVE level 3), the number-needed-to-treat for DFU prevention may be as low as 6, assuming that a 70% reduction in incidence is possible, consistent with previous research. In the year following a DFU, costs exceed $44,000.9 Thus, it seems natural that future direction in diabetic foot care should emphasize prevention strategies.
Foot ulcers that become infected often lead to hospitalization and result in an increased burden to an already overburdened VA health care system. Research suggests that about two-thirds of all diabetic foot costs are attributable to inpatient management.46 The impact of diabetic foot complications on hospital resource utilization is staggering. A 2017 study by Skrepnik analyzed the risk of hospitalization for various diseases.47 The investigators found that the inpatient admission odds ratio (OR) for congestive heart failure was 2.6, surpassed only by DFUs (OR, 3.4) and diabetic foot infection (OR, 6.7). A 2019 point-prevalence study found that > 10% of hospital admissions have a foot-related condition as the primary or secondary reason, and the majority of these are due to foot diseases, such as ulcers, infections, and Charcot neuroarthropathy.48
It is therefore incumbent on VA HCPs to avert wound recurrence in the interest of avoiding veteran hospitalizations and for administrators to encourage and incentivize best practices for managing the diabetic foot, with an emphasis on prevention therapies. In evaluating the financial impact of prevention with foot RTM, administrators should consider that the cost benefit is likely to be realized across the medical center, with budgets related to inpatient management likely to receive the largest returns.
Prevention has the potential to rein in costs as well as reduce strain on the hospital and clinic by preventing outcomes that require frequent visits for treatment or hospitalization. Wound treatment is very burdensome to the clinic; patients require frequent (in many cases, weekly) examinations, and chronic wounds often require hospitalization, necessitating rounding and additional coordination in care. Thus, preventing wounds or reducing their severity at presentation substantially reduces burden on the clinic, even after accounting for the modest clinical resources needed to administer preventative care. For example, a brief examination may be necessary if the inflammation detected by the study mat is secondary to a callus that must be debrided. However, if the patient was not seen until the callus had progressed to a wound, weekly follow-up and substantial clinical and budgetary resources may be required to heal the wound. Preventive care allows for substantially better patient outcomes, and the minimal time invested prevents the clinical burden of extensive wound treatment.
The success of preventive efforts relies on multidisciplinary management of this high-risk patient cohort. Often, it is the responsibility of the primary care provider to follow diabetic foot clinical reminders and appropriately refer to specialty care. Successful, open communication between PACT, PAVE, and the Podiatry Service has been shown to reduce poor outcomes, including lower extremity amputations. Traditionally, the model of preventive care has included podiatrist-driven interventions, including integrated routine foot care and comprehensive diabetic foot education. Collaboration between routine evaluation and prompt referral of at-risk patients for specialist foot care, therapeutic footwear recommendations, daily self-foot examinations, and in-home temperature monitoring are critically effective when performed consistently.
When trying to translate research science to effective clinical practice for preventing lower extremity complication, there are several important concepts. First, given the frequency of examination for patients being treated for a wound, provision of good preventive care, such as RTM, can reduce overall burden to resource-constrained clinics and improve access for patients needing to be seen. Additionally, preventive efforts extend clinical practice into the home and may reduce the need for in-clinic examinations and routine follow-up visits. Finally, there may be a sense of trust established between the clinician and patient with a positive record of adherence with preventive practices. This may translate into more productive communication and less frequent routine visits to better accommodate urgent visits and ensure podiatric care is accessible to veterans.
Conclusions
There is a significant opportunity to shift diabetic foot care from treatment to prevention, improving veteran outcomes and reducing resource utilization. RTM is an evidence-based and recommended but underused telemedicine solution that can catalyze this needed paradigm shift. The VA has been at the forefront of preventive foot care through the PAVE program and more recently through research and clinical application of RTM for veterans. However, as the data presented suggest, more can be done to improve veteran outcomes. More widespread adoption of evidence-based preventive technologies for the diabetic foot, such as RTM, has the potential to dramatically improve the quality of and access to care and reduce costs and burden on resource-constrained clinics.
1. Liu Y, Sayam S, Shao X, et al. Prevalence of and trends in diabetes among veterans, United States, 2005-2014. Prev Chronic Dis. 2017;14:E135.
2. Brennan MB, Hess TM, Bartle B, et al. Diabetic foot ulcer severity predicts mortality among veterans with type 2 diabetes. J Diabetes Complications. 2017;31(3):556-561.
3. Prompers L, Schaper N, Apelqvist J, et al. Prediction of outcome in individuals with diabetic foot ulcers: focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE Study. Diabetologia. 2008;51(5):747-755.
4. Geerlings SE, Hoepelman AIM. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol Med Microbiol. 1999;26(3-4):259-265.
5. Prompers L, Huijberts M, Apelqvist J, et al. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia. 2007;50(1):18-25.
6. Glover JL, Weingarten MS, Buchbinder DS, Poucher RL, Deitrick GA 3rd, Fylling CP. A 4-year outcome-based retrospective study of wound healing and limb salvage in patients with chronic wounds. Adv Wound Care. 1997;10(1):33-38.
7. Franklin H, Rajan M, Tseng C-L, Pogach L, Sinha A. Cost of lower-limb amputation in U.S. veterans with diabetes using health services data in fiscal years 2004 and 2010. J Rehabil Res Dev. 2014;51(8):1325-1330.
8. Melcer T, Sechriest VF, Walker J, Galarneau M. A comparison of health outcomes for combat amputee and limb salvage patients injured in Iraq and Afghanistan wars. J Trauma Acute Care Surg. 2013;75(2)(suppl 2):S247-S254.
9. Chan B, Cadarette S, Wodchis W, Wong J, Mittmann N, Krahn M. Cost-of-illness studies in chronic ulcers: a systematic review. J Wound Care. 2017;26(suppl 4):S4-S14.
10. US Department of Veterans Affairs. VA/DoD clinical practice guideline for the management of type 2 diabetes mellitus in Primary Care. Version 5.0. https://www.healthquality.va.gov/guidelines/CD/diabetes/VADoDDMCPGFinal508.pdf. Published April 2017. Accessed January 31, 2020.
11. Morbach S, Furchert H, Gröblinghoff U, et al. Long-term prognosis of diabetic foot patients and their limbs: amputation and death over the course of a decade. Diabetes Care. 2012;35(10):2021-2027.
12. Apelqvist J, Larsson J, Agardh CD. Long-term prognosis for diabetic patients with foot ulcers. J Intern Med. 1993;233(6):485-491.
13. Pound N, Chipchase S, Treece K, Game F, Jeffcoate W. Ulcer-free survival following management of foot ulcers in diabetes. Diabet Med. 2005;22(10):1306-1309.
14. Dubský M, Jirkovská A, Bem R, et al. Risk factors for recurrence of diabetic foot ulcers: prospective follow-up analysis in the Eurodiale subgroup. Int Wound J. 2013;10(5):555-561.
15. Ulbrecht JS, Hurley T, Mauger DT, Cavanagh PR. Prevention of recurrent foot ulcers with plantar pressure-based in-shoe orthoses: the CareFUL prevention multicenter randomized controlled trial. Diabetes Care. 2014;37(7):1982-1989.
16. Waaijman R, de Haart M, Arts MLJ, et al. Risk factors for plantar foot ulcer recurrence in neuropathic diabetic patients. Diabetes Care. 2014;37(6):1697-1705.
17. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1410: Prevention of Amputations in Veterans Everywhere (PAVE) Program. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=5364. Published March 31, 2017. Accessed February 10, 2020.
18. Robbins JM, Wrobel JS, Kirsh S, Pogach L. Characteristics of high-functioning collaborations between primary care and podiatry in VHA patient aligned care teams. Fed Pract. 2016;33(8):32-36.
19. US Department of Veterans Affairs. Office of Inspector General. Healthcare inspection: foot care for patients with diabetes and additional risk factors for amputation. https://www.va.gov/oig/pubs/VAOIG-11-00711-74.pdf. Published January 17, 2013. Accessed February 3, 2020.
20. Kehle SM, Greer N, Rutks I, Wilt T. Interventions to improve veterans’ access to care: a systematic review of the literature. J Gen Intern Med. 2011;26(suppl 2):689-696.
21. Lavery LA, Higgins KR, Lanctot DR, et al. Home monitoring of foot skin temperatures to prevent ulceration. Diabetes Care. 2004;27(11):2642-2647.
22. Lavery LA, Higgins KR, Lanctot DR, et al. Preventing diabetic foot ulcer recurrence in high-risk patients: use of temperature monitoring as a self-assessment tool. Diabetes Care. 2007;30(1):14-20.
23. Armstrong DG, Holtz-Neiderer K, Wendel C, Mohler MJ, Kimbriel HR, Lavery LA. Skin temperature monitoring reduces the risk for diabetic foot ulceration in high-risk patients. Am J Med. 2007;120(12):1042-1046.
24. Bakker K, Apelqvist J, Lipsky BA, Van Netten JJ; International Working Group on the Diabetic Foot. The 2015 IWGDF guidance documents on prevention and management of foot problems in diabetes: development of an evidence-based global consensus. Diabetes Metab Res Rev. 2016;32 (suppl 1):2-6.
25. Frykberg RG, Zgonis T, Armstrong DG, et al; American College of Foot Ankle Surgeons. Diabetic foot disorders: a clinical practice guideline (2006 revision). J Foot Ankle Surg. 2006;45(suppl 5):S1-S66.
26. Lavery LA, Davis KE, Berriman SJ, et al. WHS guidelines update: diabetic foot ulcer treatment guidelines. Wound Repair Regen. 2016;24(1):112-126.
27. US Department of Veterans Affairs, VA National Prosthetics and Sensory Aids Service and National Podiatry Program Office. Podimetrics – TMD temperature monitoring devices. [Source not verified.]
28. Arad Y, Fonseca V, Peters A, Vinik A. Beyond the monofilament for the insensate diabetic foot: a systematic review of randomized trials to prevent the occurrence of plantar foot ulcers in patients with diabetes. Diabetes Care. 2011;34(4):1041-1046.
29. Dy SM, Bennett WL, Sharma R, et al. Preventing Complications and Treating Symptoms of Diabetic Peripheral Neuropathy. Rockville, MD: Agency for Healthcare Research and Quality US; 2017.
30. Frykberg RG, Gordon IL, Reyzelman AM, et al. Feasibility and efficacy of a SmartMat technology to predict development of diabetic plantar ulcers. Diabetes Care. 2017;40(7):973-980.
31. Crisologo PA, Lavery LA. Remote home monitoring to identify and prevent diabetic foot ulceration. Ann Transl Med. 2017;5(21):430.
32. Armstrong DG, Abu-Rumman PL, Nixon BP, Boulton AJ. Continuous activity monitoring in persons at high risk for diabetes-related lower-extremity amputation. J Am Podiatr Med Assoc. 2001;91(9):451-455.
33. Gordon IL, Rothenberg GM, Lepow BD, et al. Accuracy of a foot temperature monitoring mat for predicting diabetic foot ulcers in patients with recent wounds or partial foot amputation. Diabetes Res Clin Pract. 2020. [Online ahead of print.]
34. Lavery LA, Petersen BJ, Linders DR, Bloom JD, Rothenberg GM, Armstrong DG. Unilateral remote temperature monitoring to predict future ulceration for the diabetic foot in remission. BMJ Open Diabetes Res Care. 2019;7(1):e000696.
35. Petersen BJ, Rothenberg GM, Lakhani PJ, et al. Ulcer metastasis? Anatomical locations of recurrence for patients in diabetic foot remission. J Foot Ankle Res. 2020;13:1.
36. Killeen AL, Brock KM, Dancho JF, Walters JL. Remote temperature monitoring in patients with visual impairment due to diabetes mellitus, a proposed improvement to curren standard of care for prevention of diabetic foot ulcers. J Diabetes Sci Technol. 2020;14(1):37-45.
37. Murff RT, Armstrong DG, Lanctot D, Lavery LA, Athanasiou KA. How effective is manual palpation in detecting subtle temperature differences? Clin Podiatr Med Surg. 1998;15(1):151-154.
38. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376(24):2367-2375.
39. Natovich R, Kushnir T, Harman-Boehm I, et al. Cognitive dysfunction: part and parcel of the diabetic foot. Diabetes Care. 2016;39(7):1202-1207.
40. Zhong A, Li G, Wang D, Sun Y, Zou X, Li B. The risks and external effects of diabetic foot ulcer on diabetic patients: a hospital-based survey in Wuhan area, China. Wound Repair Regen. 2017;25(5):858-863.
41. Vileikyte L. Diabetic foot ulcers: a quality of life issue. Diabetes Metab Res Rev. 2001;17(4):246-249.
42. Van Acker K, Oleen-Burkey M, De Decker L, et al. Cost and resource utilization for prevention and treatment of foot lesions in a diabetic foot clinic in Belgium. Diabetes Res Clin Pract. 2000;50(2):87-95.
43. Kerr M, Rayman G, Jeffcoate WJ. Cost of diabetic foot disease to the National Health Service in England. Diabetes Med. 2014;31(12):1498-1504.
44. Bus SA, van Netten JJ. A shift in priority in diabetic foot care and research: 75% of foot ulcers are preventable. Diabetes Metab Res Rev. 2016;32(suppl 1):195-200.
45. Hicks CW, Selvarajah S, Mathioudakis N, et al. Burden of infected diabetic foot ulcers on hospital admissions and costs. Ann Vasc Surg. 2016;33:149-158.
46. Rice JB, Desai U, Cummings AKG, Birnbaum HG, Skornicki M, Parsons NB. Burden of diabetic foot ulcers for Medicare and private insurers. Diabetes Care. 2014;37(3):651-658.
47. Skrepnek GH, Mills JL Sr, Lavery LA, Armstrong DG. Health care service and outcomes among an estimated 6.7 million ambulatory care diabetic foot cases in the U.S. Diabetes Care. 2017;40(7):936-942.
48. Lazzarini PA, Hurn SE, Kuys SS, et al. Direct inpatient burden caused by foot-related conditions: a multisite point-prevalence study. BMJ Open. 2016;6(6):e010811.
Diabetic foot ulcers (DFUs) are devastating, common, and costly. This burden is borne disproportionately by veterans who have high prevalence of type 2 diabetes mellitus (T2DM) and other precipitating risk factors.1 The mortality of veterans following a DFU is sobering, and ulceration is recognized as a significant marker of disease severity.
A 2017 study by Brennan and colleagues reported a 19% mortality rate within 1 year, and only 29% survive past 5 years.2 DFUs are often complicated by peripheral arterial disease (PAD) and diabetic immune dysfunction, contributing to chronic wounds and infection.3,4 About 60% of all foot ulcers become infected, and > 20% of patients with a diabetic foot infection require amputation.5,6
A 2010 retrospective study reports that > 3,400 veterans have a diabetes-related lower extremity amputation annually, vastly surpassing the rate of amputation secondary to trauma in the Veterans Health Administration (VHA).7,8 The inpatient costs for each amputation exceeded $60,000 in fiscal year 2010, and these amputation-related costs represent only 1 component of the total expense to the VHA attributable to diabetic foot complications.7 A recent systematic review by Chan and colleagues estimated mean annual costs in the year following a foot ulcer to be $44,200 to the public payer.9 This implies that direct expenditures for treatment of DFUs within the VHA exceeds $3 billion annually.
Diabetic Foot Ulcer Prevention
Given the dramatic impact of diabetic foot complications to the veteran and the US health care system, the VHA has long recognized the importance of preventive care for those at risk. In 2017 US Department of Veterans Affairs (VA) and Department of Defense issued a clinical practice guideline for the management of T2DM that recommended prophylactic foot care for early identification of any deformity or skin breakdown.10 The guidelines note that a “person who has had a foot ulcer is at lifelong risk of further ulceration,” reflecting the high rate of recurrence among all patients, including veterans. Multiple studies suggest that as many as 40% of patients experience recidivism in the first year after healing from a wound.11-16
The VA is well equipped to deliver quality preventive care because of its innovative and long-standing PAVE (Prevention of Amputations for Veterans Everywhere) program.17 PAVE provides screening, education, appropriate footwear, and stratified care guidelines for veterans at risk for diabetes-related foot complications (Table 1). The practices encouraged by PAVE are evidence-based and synergistic with the objectives of the VA’s patient aligned care team (PACT) delivery approach.18 The granular data collected through PAVE are used to guide best practices and provide benchmarks for diabetic foot outcomes.
Unfortunately, despite PAVE guidelines requiring annual specialist foot care for at-risk veterans, a 2013 report by the VA Office of the Inspector General (OIG) found that one-third of all patients had no documentation of this minimal requirement of preventive foot care.19 Although the VA has worked to address this issue, the data hint at the missed opportunities for prevention of complications and the challenges of ensuring that a large at-risk veteran population has systematic and routine screening with access to specialist foot care.
Given the large proportion of veterans at high risk of chronic wound formation and the challenges of ensuring that this cohort receives good preventive foot care, expanding telemedicine has been suggested. Telemedicine solutions have the potential to reduce the impact of chronic wounds on overburdened clinic resources, schedules, and local and federal budgets.20 Interestingly, the only preventive practice for the diabetic foot that has been proven effective through multiple randomized controlled trials and national and international clinical guidance documents is once-daily foot temperature monitoring.21-26 Daily monitoring has the potential to reduce the burden of DFUs to veterans, improve veteran access to needed preventive care, and reduce costs incurred by the VHA treating diabetic foot complications. Yet despite a recent national guidance document detailing its appropriate use in PAVE 3 veterans, it remains underutilized.27
The purpose of this review is to: (1) discuss the evidence supporting once-daily remote temperature monitoring (RTM), a telemedicine approach critical to improving both veteran access to care and diabetic foot outcomes; (2) summarize a 2017 study that presented an advanced clinical understanding of RTM use among veterans; (3) provide previously unpublished data from this study comparing high-risk VA and non-VA cohorts, highlighting the opportunity for additional focus on foot ulcer prevention within the VA; and (4) report on recent VA utilization of a RTM technology based on this research, emphasizing lessons learned and best practices.
Remote Temperature Monitoring
The objective of daily foot temperature monitoring is to identify impending inflammatory foot conditions, such as DFUs, infection, and acute Charcot neuroarthropathy episodes. The patient and care team then act to resolve the cause of detected inflammation before clinical presentation (prevention) and begin treatment earlier than would otherwise be possible to avoid expensive complications, such as infection (early detection). Preventive therapies are low risk to the patient and inexpensive.
RTM is recommended by multiple clinical practice guidelines, including those of the International Working Group on the Diabetic Foot, the American College of Foot and Ankle Surgeons, and the Wound Healing Society.24-26 Its use is supported by evidence from 3 National Institutes of Health-funded and well-designed randomized controlled trials, 1 of which was additionally supported by a VA Health Services Research and Development Service Merit Award.21-23,28 Conducted between 2004 and 2007, these studies demonstrated the potential to reduce foot ulcer incidence by as much as 85% using a dermal thermometer to identify inflammation and prompt decreased ambulation. Investigators established a clinical monitoring protocol comparing the temperatures between 6 matched locations on the left and right feet. Persistent differences in contralateral temperatures exceeding 2.2°C (4.0°F) were used as a marker for elevated risk and to initiate preventive care. Based on the encouraging results from these studies, a 2017 effectiveness review prepared for the Agency for Healthcare Research and Quality concluded that “home monitoring of foot skin temperature is effective for reducing foot ulcer incidence and recurrence.”29
Accuracy of RTM
A 2017 longitudinal study (NCT02647346) has provided novel data to advance understanding of RTM for the prediction and prevention of DFUs.30 This study was the first to systematically analyze the accuracy of RTM over different monitoring thresholds. The results enable practitioners to deliver risk-stratified preventive care. Policy makers can use the data from this study to weigh the cost and benefits of RTM for population health.
The multicenter trials had 129 participants from 4 VA health care systems: VA Long Beach Healthcare System in California, Miami VA Healthcare System in Florida, Phoenix VA Healthcare System in Arizona, and VA West Los Angeles Healthcare System in California. Each participant was followed for 34 weeks under standard preventive foot care and was instructed to step on a telemedicine SmartMat (Podimetrics, Inc) RTM mat for 20 seconds daily. Participants and investigators were blinded to the temperature data so that the accuracy of temperature monitoring could be assessed. All participants had a history of T2DM and healed DFU. Principal exclusion criteria included unhealed plantar wound, history of proximal lower extremity amputation (ie, above ankle), active Charcot foot disease, and comorbidities that could potentially inhibit an inflammatory response, such as end-stage renal disease, active malignancy, and immunosuppressive diseases.
The investigators reported that RTM with the study mat detected 97% of nonacute plantar DFUs using the most commonly studied threshold (sustained 2.2°C temperature difference). The lead time averaged 37 days before clinical identification of the wound under standard care. Although the false-positive rate of 57% was high, corresponding to approximately 3.0 notifications per patient per year on average in the research setting, it is important to note that this study only considered the prediction of plantar DFUs. Thus, detection of foot inflammation secondary to other conditions, such as preulcerative lesion, dorsal wound, Charcot neuroarthropathy, or foot infection, were reported as a false positive per the study’s definitions. Further, Crisologo and Lavery noted in a translational medicine summary of this research, because the intervention is noninvasive and minimally impactful to the patient and the health care system, “the potential to arrest re-ulceration is worth the perceived inconvenience to the patient.”31
Secondary outcomes related to adherence and ease of use were encouraging. Eighty-eight percent of participants reported that the mat was “very easy to use,” the highest possible score, and 98% were able to set up the mat for home use without difficulty. At the end of the 34-week study, more than 74% of participants remained engaged in routine use of the mat under a per-protocol assessment of adherence. These results are especially impressive given the documented poor adherence of at-risk patients to routine use of therapeutic footwear, which has been reported to be as low as 15%.32
New Research
The data collected during this study has led to new research and advancements in RTM. A recent publication by Gordon and colleagues investigated whether RTM is less accurate in cohorts with perceived challenges.33 They include patients with recently healed wounds and those with a history of partial foot amputation. There was no difference in the accuracy or lead time for either cohort relative to the entire cohort, suggesting that RTM is appropriate for monitoring patients with recently healed DFUs or partial foot amputations.
In another recent study, the data were used to derive a novel approach to monitor a single at-risk foot.34 The practice of RTM has traditionally required comparing temperatures between contralaterally matched plantar locations on the feet, thus limiting its use in patients with a history of major lower extremity amputation and patients being treated for a wound, which may be bandaged or in an off-loading cast or boot. Because the risk factors for DFUs exist in both limbs, these patients are at high risk for developing complications to the contralateral foot and may benefit from preventive once-daily foot temperature monitoring. The investigators empirically derived a novel monitoring approach for patients without a contralateral control. This approach was found to predict 91% of impending plantar DFUs on average 41 days before clinical presentation with a false positive rate of 54%.
Additional Focus on Prevention
Table 2 shows previously unpublished data from a subgroup analysis between veteran and nonveteran participants in the study.25 These descriptive statistics reinforce some widely held assumptions regarding the high-risk veteran population and challenge others. For example, compared with the nonveteran participants, the veteran cohort unsurprisingly had a larger ratio of male participants (P < .01), had a higher rate of cigarette use (P < .01), and was more likely to live alone (although not at a statistically significant level). Veterans in the study had body mass index, rates of alcohol use, frequency of exercise, and glucose control comparable to that of nonveterans.
The potential impact of the PAVE program is clear in several of these comparisons. Although as few as 15% of patients use therapeutic shoes routinely, PAVE ensures that the majority of veterans receive them. Nearly 95% of veterans have therapeutic shoes compared with about 80% of nonveteran participants (P < .05). Veterans also had higher ankle-brachial index results (P < .05), although on average both cohorts were within normal clinical parameters. Veterans had a significantly longer duration since healing from the most recent wound, and fewer veteran participants had a wound that healed in the 3 months prior to the study. Despite this, during the study veterans had annualized DFU incidence equal to that of nonveterans. Furthermore, veterans also had significantly higher rates of amputation prior to participation. That these critical outcomes for veterans are no better than those observed in other care environments despite PAVE suggests that approaches recommended via PAVE alone are insufficient to significantly arrest DFU recurrence, and even more focus on prevention in the VA may be warranted.
From Research to Practice
Since the publication of the 2017 study, the VHA has been at the vanguard of translating the evidence and research underlying RTM into clinical practice. A clinical guidance document governing appropriate use of RTM with the study mat was recently published by the VA Prosthetic and Sensory Aids Service in collaboration with the National Podiatry Program office.27 This guidance document recommends once-daily RTM for at-risk veterans designated PAVE level 3. It defines roles and responsibilities required for the successful implementation of a RTM program with the study device. The document additionally presents various clinical monitoring protocols for veterans, although the protocol and thresholds used are at the discretion of the prescribing clinician and should reflect the risk profile of the veteran in question.
A staged response to inflammation has proven popular, whereby an initial high-sensitivity threshold is chosen for monitoring. The initial response is telephone outreach by a designee supplied by the clinic or device manufacturer, typically a trained registered nurse, to the veteran to collect subjective history and instruct off-loading and reduced ambulation, with a target of 50% baseline reduction in step count. Should the inflammation persist despite off-loading, an examination may be necessary to identify and resolve its cause. For recalcitrant inflammation, more targeted pressure off-loading of the affected area may be accomplished with custom orthotics, accommodative insoles, removable cast walkers, and total contact casting. After 2 to 4 weeks without signs of inflammation, the cause is deemed to have been resolved and lowered the acute risk for developing further diabetic foot complications.
More than 600 veterans have been monitored for > 1,000 patient-years—13 VA medical centers are practicing RTM with the study mat as of this writing. The monitoring program has been integrated into many veteran daily routines as evidenced by > 70% retaining full engagement after having been monitored for > 1 year. The total number of alerts/patient-years across these veterans has been 1.4, significantly lower than the 3.0 alerts/patient-year observed in the study. This is potentially due to successful interventions in response to detected inflammation, resolving inflammation, and avoiding unnecessary alerts occurring in the research setting, which did not employ interventions that resolved inflammation episodes. In the past 6 months, 68% of all inflammation detected resolved via off-loading alone without requiring further clinical intervention. In the cases that required an examination, 76% of patients reported clinically meaningful preventive care (eg, preulcerative callus was debrided, a subungual hemorrhage was treated, a foot ulcer was identified).
Organizational Best Practices
Several best practices have been cultivated related to initiating a RTM program at a new site, for promoting the success of a RTM program, and provisioning excellent preventive care to support the RTM program. Although we advise adhering to the recommendations in the VA guidance document,27 the authors have observed several additional organizational best practices that are not explicitly addressed.
Partnering with PACT. Collaboration between PAVE and PACT has the potential not only to improve outcomes for patients at risk for diabetic foot complications, but also can help identify appropriate high-risk veteran candidates for preventive care with RTM who may not be followed for routine care from a specialty provider, such as a podiatrist, as highlighted by the 2013 OIG report.
Prescreening eligible patients. Several programs have used PAVE data or appointment schedules to identify and target high-risk veterans proactively. This approach has several benefits. It simplifies clinical coordination and streamlines workflow for patient identification and onboarding. It also allows those veterans at highest risk to receive needed and recommended preventive care at their next scheduled appointment. Finally, if PAVE data are used to identify eligible patients, it has the added benefit of ensuring a baseline level of telemedicine preventive foot care for veterans who have become lost to follow-up and have not been seen recently for a routine foot examination.
Implementing foot monitoring during wound treatment. Recent research has expanded the reach of once-daily RTM with the mat to patients being treated for a wound to only 1 foot. This practice has 2 benefits: The patient is able to establish a preventive routine before healing, an important advantage because research strongly suggests that recurrence is most likely in the first months after healing. Second, 48% of patients with a history of DFUs will develop new wounds to the contralateral foot because risk factors, such as neuropathy and peripheral arterial disease, exist in both limbs.35 Furthermore, ongoing treatment for a wound to 1 foot may result in gait deviation and elevated pressure to the sound foot, additionally predisposing the veteran to complications, resulting in a high rate of wounds occurring to the unwounded foot during treatment (0.2 DFU/DFU-year).34 Thus, there is potential benefit in monitoring the sound foot while undergoing treatment for a wound; further, the patient will have immediate access to the device for prevention of recurrence once the wound has resolved.
Utilizing foot monitoring as an extension of telemedicine. Many VA facilities have large geographic catchment areas, making routine follow-up difficult for veterans living in rural areas. RTM serves as an extension of the patient’s daily self-examination and the clinician’s ability to monitor patients with objective information daily. The veterans using the system become more invested and feel as though they are taking an active role in their health care.
Investing in ongoing medical education. Multidisciplinary education sessions reviewing supporting clinical data and resultant clinical practice guidelines raise awareness for those providers and trainees unaware of preventive best practices for the diabetic foot, including those related to foot RTM. These sessions also are helpful for those familiar with foot temperature monitoring or who are responsible for administration of an ongoing program to remain current with contemporary best practices and to discuss improvements for patient care. Familiarity also can help address clinical inertia when benefits and evidence are clearly communicated with health care providers (HCPs).
Clinical Best Practices
Treating preulcerative lesions urgently and aggressively. Callus and other preulcerative lesions often cause progressive tissue damage and poor outcomes. When identified, these lesions should be promptly treated to ensure best outcomes.24
Recognizing the limits of patient self-examinations. Comorbidities such as visual impairment and reduced joint mobility often preclude patients from completing rigorous self-examinations of the foot, which is especially critical while collecting subjective history from the patient during triage of inflammation. A caregiver or spouse can help inspect the foot during outreach and provide additional context.36
Interpreting a benign foot on examination. Because RTM has been demonstrated to detect inflammation preceding a foot ulcer as many as 5 weeks before presentation to the clinic, some veterans may have few signs or symptoms of acute risk during examination. Often, the damage is to subcutaneous tissue resulting from repetitive microtrauma. Research suggests that clinical examination in these cases is often unreliable for identifying the earliest signs of risk, such as palpation to identify subtle temperature changes secondary to inflammation.37 If a patient has refractory inflammation requiring examination and presents with an otherwise unremarkable foot, it is an opportunity to evaluate whether the patient’s shoewear remains appropriate or has worn out, to communicate the veteran’s ongoing elevated risk, and to educate on the importance of diligence in daily foot self-examinations, daily use of the foot temperature monitoring, and continued off-loading until the inflammation resolves.
Communicating the distinction between healing and remission. Although healing is the goal of wound care, patients should be educated that the underlying disease remains after epithelialization. In some cases, tissue deep to the skin has not completed remodeling, and the patient is at acute risk of recurrence. Remission is a powerful metaphor that better describes the patient’s ongoing risk to encourage continued healthy routines and diligent self-care.38Considering the entirety of both feet for recurrence. Critical risk factors for diabetic foot complications, such as peripheral neuropathy and PAD, exist in both limbs, and patients with a history of wounds often develop new complications to different ipsilateral locations, or in as many as 48% of cases, to the contralateral foot.35 For best outcomes, detected inflammation should be treated aggressively independent of whether the location coincides with an area of previous concern.
Encouraging adherence, routine, and empowerment. Advanced diabetes mellitus and neuropathy may impact a patient’s executive function, and multiple studies have reported that patients at risk for inflammatory foot diseases exhibit fatalism toward their foot care and outcomes.39-41 Consistent education, encouragement, empowerment, and establishment of positive routines are needed to ensure high adherence with all preventive care regimens, including RTM.
Case Presentations
The following case series illustrates many of these clinical best practices and characterizes the potential benefits of RTM to veterans within the VA.
Case 1: Prevention After Healing
A veteran underwent a Chopart amputation and was recommended to use the mat after healing was perceived. Immediately on use of the study mat, the patient was found to have inflammation to the surgical incision (Figure 1). Clinical staff was alerted to the findings, and the patient was instructed to limit further walking and continue off-loading in his removable cast walker, per protocol. The inflammation of the operative foot quickly reduced, and the patient continued healing successfully, potentially avoiding incisional dehiscence and possible postoperative infection.
This case illustrates that patients’ wounds or surgical incisions may not be completely healed on epithelialization. In the immediate phase after closure, HCPs should consider additional protection to avoid complications. This case demonstrates that RTM can provide objective data to help guide care in that critical period.
Case 2: Identifying Preulcerative Lesions
An 88-year-old veteran had a chronic callus under the second metatarsal head. In addition to routine foot care and therapeutic shoes, he was followed with once-daily RTM. Inflammation was noted, and the veteran was seen in the podiatry clinic where debridement of the callus was performed. The difference in temperatures between feet detected by thermography prior to the clinic visits rapidly resolved after callus debridement, indicating that the underlying inflammation had subsided. RTM was used by the clinical staff to determine the appropriate time interval between clinic visits to avoid callus breakdown and subsequent ulceration.
Case 3: Extending the Clinic Into the Home
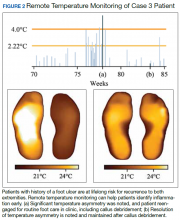
An 80-year-old veteran with T2DM and neuropathy was deemed a high-risk patient due to recurrent ulcerations to the left great toe. He was issued a RTM mat and was adherent with routine use. After nearly a year without hot-spot development, inflammation was noted (Figure 2).
Unfortunately, the patient had missed several routine foot care visits and likely that was the reason for the noted inflammation. The patient was called and became reengaged in regular visits for routine foot care. On debridement of his callus, a superficial, noninfected ulceration was discovered. Had remote monitoring not detected the inflammation and impending ulceration, the patient likely would not have been seen in the regular clinic and may have developed a wound infection, potentially resulting in a worse and more costly outcome.
Paradigm Shift to Prevention
Given the exceedingly high burden of diabetic foot complications in the VA, a paradigm shift is needed among HCPs from a culture of treatment to one of prevention. Bus and colleagues reported that in Europe, for every euro spent on ulcer prevention, 10 are spent on ulcer healing, and for every randomized clinical trial conducted on prevention, 10 are conducted on treatment.42-44 Hicks and colleagues showed that the cost of curative care for DFUs is 5 to 30 times greater than the cost of preventive care.45 For RTM in high-risk cohorts (ie, PAVE level 3), the number-needed-to-treat for DFU prevention may be as low as 6, assuming that a 70% reduction in incidence is possible, consistent with previous research. In the year following a DFU, costs exceed $44,000.9 Thus, it seems natural that future direction in diabetic foot care should emphasize prevention strategies.
Foot ulcers that become infected often lead to hospitalization and result in an increased burden to an already overburdened VA health care system. Research suggests that about two-thirds of all diabetic foot costs are attributable to inpatient management.46 The impact of diabetic foot complications on hospital resource utilization is staggering. A 2017 study by Skrepnik analyzed the risk of hospitalization for various diseases.47 The investigators found that the inpatient admission odds ratio (OR) for congestive heart failure was 2.6, surpassed only by DFUs (OR, 3.4) and diabetic foot infection (OR, 6.7). A 2019 point-prevalence study found that > 10% of hospital admissions have a foot-related condition as the primary or secondary reason, and the majority of these are due to foot diseases, such as ulcers, infections, and Charcot neuroarthropathy.48
It is therefore incumbent on VA HCPs to avert wound recurrence in the interest of avoiding veteran hospitalizations and for administrators to encourage and incentivize best practices for managing the diabetic foot, with an emphasis on prevention therapies. In evaluating the financial impact of prevention with foot RTM, administrators should consider that the cost benefit is likely to be realized across the medical center, with budgets related to inpatient management likely to receive the largest returns.
Prevention has the potential to rein in costs as well as reduce strain on the hospital and clinic by preventing outcomes that require frequent visits for treatment or hospitalization. Wound treatment is very burdensome to the clinic; patients require frequent (in many cases, weekly) examinations, and chronic wounds often require hospitalization, necessitating rounding and additional coordination in care. Thus, preventing wounds or reducing their severity at presentation substantially reduces burden on the clinic, even after accounting for the modest clinical resources needed to administer preventative care. For example, a brief examination may be necessary if the inflammation detected by the study mat is secondary to a callus that must be debrided. However, if the patient was not seen until the callus had progressed to a wound, weekly follow-up and substantial clinical and budgetary resources may be required to heal the wound. Preventive care allows for substantially better patient outcomes, and the minimal time invested prevents the clinical burden of extensive wound treatment.
The success of preventive efforts relies on multidisciplinary management of this high-risk patient cohort. Often, it is the responsibility of the primary care provider to follow diabetic foot clinical reminders and appropriately refer to specialty care. Successful, open communication between PACT, PAVE, and the Podiatry Service has been shown to reduce poor outcomes, including lower extremity amputations. Traditionally, the model of preventive care has included podiatrist-driven interventions, including integrated routine foot care and comprehensive diabetic foot education. Collaboration between routine evaluation and prompt referral of at-risk patients for specialist foot care, therapeutic footwear recommendations, daily self-foot examinations, and in-home temperature monitoring are critically effective when performed consistently.
When trying to translate research science to effective clinical practice for preventing lower extremity complication, there are several important concepts. First, given the frequency of examination for patients being treated for a wound, provision of good preventive care, such as RTM, can reduce overall burden to resource-constrained clinics and improve access for patients needing to be seen. Additionally, preventive efforts extend clinical practice into the home and may reduce the need for in-clinic examinations and routine follow-up visits. Finally, there may be a sense of trust established between the clinician and patient with a positive record of adherence with preventive practices. This may translate into more productive communication and less frequent routine visits to better accommodate urgent visits and ensure podiatric care is accessible to veterans.
Conclusions
There is a significant opportunity to shift diabetic foot care from treatment to prevention, improving veteran outcomes and reducing resource utilization. RTM is an evidence-based and recommended but underused telemedicine solution that can catalyze this needed paradigm shift. The VA has been at the forefront of preventive foot care through the PAVE program and more recently through research and clinical application of RTM for veterans. However, as the data presented suggest, more can be done to improve veteran outcomes. More widespread adoption of evidence-based preventive technologies for the diabetic foot, such as RTM, has the potential to dramatically improve the quality of and access to care and reduce costs and burden on resource-constrained clinics.
Diabetic foot ulcers (DFUs) are devastating, common, and costly. This burden is borne disproportionately by veterans who have high prevalence of type 2 diabetes mellitus (T2DM) and other precipitating risk factors.1 The mortality of veterans following a DFU is sobering, and ulceration is recognized as a significant marker of disease severity.
A 2017 study by Brennan and colleagues reported a 19% mortality rate within 1 year, and only 29% survive past 5 years.2 DFUs are often complicated by peripheral arterial disease (PAD) and diabetic immune dysfunction, contributing to chronic wounds and infection.3,4 About 60% of all foot ulcers become infected, and > 20% of patients with a diabetic foot infection require amputation.5,6
A 2010 retrospective study reports that > 3,400 veterans have a diabetes-related lower extremity amputation annually, vastly surpassing the rate of amputation secondary to trauma in the Veterans Health Administration (VHA).7,8 The inpatient costs for each amputation exceeded $60,000 in fiscal year 2010, and these amputation-related costs represent only 1 component of the total expense to the VHA attributable to diabetic foot complications.7 A recent systematic review by Chan and colleagues estimated mean annual costs in the year following a foot ulcer to be $44,200 to the public payer.9 This implies that direct expenditures for treatment of DFUs within the VHA exceeds $3 billion annually.
Diabetic Foot Ulcer Prevention
Given the dramatic impact of diabetic foot complications to the veteran and the US health care system, the VHA has long recognized the importance of preventive care for those at risk. In 2017 US Department of Veterans Affairs (VA) and Department of Defense issued a clinical practice guideline for the management of T2DM that recommended prophylactic foot care for early identification of any deformity or skin breakdown.10 The guidelines note that a “person who has had a foot ulcer is at lifelong risk of further ulceration,” reflecting the high rate of recurrence among all patients, including veterans. Multiple studies suggest that as many as 40% of patients experience recidivism in the first year after healing from a wound.11-16
The VA is well equipped to deliver quality preventive care because of its innovative and long-standing PAVE (Prevention of Amputations for Veterans Everywhere) program.17 PAVE provides screening, education, appropriate footwear, and stratified care guidelines for veterans at risk for diabetes-related foot complications (Table 1). The practices encouraged by PAVE are evidence-based and synergistic with the objectives of the VA’s patient aligned care team (PACT) delivery approach.18 The granular data collected through PAVE are used to guide best practices and provide benchmarks for diabetic foot outcomes.
Unfortunately, despite PAVE guidelines requiring annual specialist foot care for at-risk veterans, a 2013 report by the VA Office of the Inspector General (OIG) found that one-third of all patients had no documentation of this minimal requirement of preventive foot care.19 Although the VA has worked to address this issue, the data hint at the missed opportunities for prevention of complications and the challenges of ensuring that a large at-risk veteran population has systematic and routine screening with access to specialist foot care.
Given the large proportion of veterans at high risk of chronic wound formation and the challenges of ensuring that this cohort receives good preventive foot care, expanding telemedicine has been suggested. Telemedicine solutions have the potential to reduce the impact of chronic wounds on overburdened clinic resources, schedules, and local and federal budgets.20 Interestingly, the only preventive practice for the diabetic foot that has been proven effective through multiple randomized controlled trials and national and international clinical guidance documents is once-daily foot temperature monitoring.21-26 Daily monitoring has the potential to reduce the burden of DFUs to veterans, improve veteran access to needed preventive care, and reduce costs incurred by the VHA treating diabetic foot complications. Yet despite a recent national guidance document detailing its appropriate use in PAVE 3 veterans, it remains underutilized.27
The purpose of this review is to: (1) discuss the evidence supporting once-daily remote temperature monitoring (RTM), a telemedicine approach critical to improving both veteran access to care and diabetic foot outcomes; (2) summarize a 2017 study that presented an advanced clinical understanding of RTM use among veterans; (3) provide previously unpublished data from this study comparing high-risk VA and non-VA cohorts, highlighting the opportunity for additional focus on foot ulcer prevention within the VA; and (4) report on recent VA utilization of a RTM technology based on this research, emphasizing lessons learned and best practices.
Remote Temperature Monitoring
The objective of daily foot temperature monitoring is to identify impending inflammatory foot conditions, such as DFUs, infection, and acute Charcot neuroarthropathy episodes. The patient and care team then act to resolve the cause of detected inflammation before clinical presentation (prevention) and begin treatment earlier than would otherwise be possible to avoid expensive complications, such as infection (early detection). Preventive therapies are low risk to the patient and inexpensive.
RTM is recommended by multiple clinical practice guidelines, including those of the International Working Group on the Diabetic Foot, the American College of Foot and Ankle Surgeons, and the Wound Healing Society.24-26 Its use is supported by evidence from 3 National Institutes of Health-funded and well-designed randomized controlled trials, 1 of which was additionally supported by a VA Health Services Research and Development Service Merit Award.21-23,28 Conducted between 2004 and 2007, these studies demonstrated the potential to reduce foot ulcer incidence by as much as 85% using a dermal thermometer to identify inflammation and prompt decreased ambulation. Investigators established a clinical monitoring protocol comparing the temperatures between 6 matched locations on the left and right feet. Persistent differences in contralateral temperatures exceeding 2.2°C (4.0°F) were used as a marker for elevated risk and to initiate preventive care. Based on the encouraging results from these studies, a 2017 effectiveness review prepared for the Agency for Healthcare Research and Quality concluded that “home monitoring of foot skin temperature is effective for reducing foot ulcer incidence and recurrence.”29
Accuracy of RTM
A 2017 longitudinal study (NCT02647346) has provided novel data to advance understanding of RTM for the prediction and prevention of DFUs.30 This study was the first to systematically analyze the accuracy of RTM over different monitoring thresholds. The results enable practitioners to deliver risk-stratified preventive care. Policy makers can use the data from this study to weigh the cost and benefits of RTM for population health.
The multicenter trials had 129 participants from 4 VA health care systems: VA Long Beach Healthcare System in California, Miami VA Healthcare System in Florida, Phoenix VA Healthcare System in Arizona, and VA West Los Angeles Healthcare System in California. Each participant was followed for 34 weeks under standard preventive foot care and was instructed to step on a telemedicine SmartMat (Podimetrics, Inc) RTM mat for 20 seconds daily. Participants and investigators were blinded to the temperature data so that the accuracy of temperature monitoring could be assessed. All participants had a history of T2DM and healed DFU. Principal exclusion criteria included unhealed plantar wound, history of proximal lower extremity amputation (ie, above ankle), active Charcot foot disease, and comorbidities that could potentially inhibit an inflammatory response, such as end-stage renal disease, active malignancy, and immunosuppressive diseases.
The investigators reported that RTM with the study mat detected 97% of nonacute plantar DFUs using the most commonly studied threshold (sustained 2.2°C temperature difference). The lead time averaged 37 days before clinical identification of the wound under standard care. Although the false-positive rate of 57% was high, corresponding to approximately 3.0 notifications per patient per year on average in the research setting, it is important to note that this study only considered the prediction of plantar DFUs. Thus, detection of foot inflammation secondary to other conditions, such as preulcerative lesion, dorsal wound, Charcot neuroarthropathy, or foot infection, were reported as a false positive per the study’s definitions. Further, Crisologo and Lavery noted in a translational medicine summary of this research, because the intervention is noninvasive and minimally impactful to the patient and the health care system, “the potential to arrest re-ulceration is worth the perceived inconvenience to the patient.”31
Secondary outcomes related to adherence and ease of use were encouraging. Eighty-eight percent of participants reported that the mat was “very easy to use,” the highest possible score, and 98% were able to set up the mat for home use without difficulty. At the end of the 34-week study, more than 74% of participants remained engaged in routine use of the mat under a per-protocol assessment of adherence. These results are especially impressive given the documented poor adherence of at-risk patients to routine use of therapeutic footwear, which has been reported to be as low as 15%.32
New Research
The data collected during this study has led to new research and advancements in RTM. A recent publication by Gordon and colleagues investigated whether RTM is less accurate in cohorts with perceived challenges.33 They include patients with recently healed wounds and those with a history of partial foot amputation. There was no difference in the accuracy or lead time for either cohort relative to the entire cohort, suggesting that RTM is appropriate for monitoring patients with recently healed DFUs or partial foot amputations.
In another recent study, the data were used to derive a novel approach to monitor a single at-risk foot.34 The practice of RTM has traditionally required comparing temperatures between contralaterally matched plantar locations on the feet, thus limiting its use in patients with a history of major lower extremity amputation and patients being treated for a wound, which may be bandaged or in an off-loading cast or boot. Because the risk factors for DFUs exist in both limbs, these patients are at high risk for developing complications to the contralateral foot and may benefit from preventive once-daily foot temperature monitoring. The investigators empirically derived a novel monitoring approach for patients without a contralateral control. This approach was found to predict 91% of impending plantar DFUs on average 41 days before clinical presentation with a false positive rate of 54%.
Additional Focus on Prevention
Table 2 shows previously unpublished data from a subgroup analysis between veteran and nonveteran participants in the study.25 These descriptive statistics reinforce some widely held assumptions regarding the high-risk veteran population and challenge others. For example, compared with the nonveteran participants, the veteran cohort unsurprisingly had a larger ratio of male participants (P < .01), had a higher rate of cigarette use (P < .01), and was more likely to live alone (although not at a statistically significant level). Veterans in the study had body mass index, rates of alcohol use, frequency of exercise, and glucose control comparable to that of nonveterans.
The potential impact of the PAVE program is clear in several of these comparisons. Although as few as 15% of patients use therapeutic shoes routinely, PAVE ensures that the majority of veterans receive them. Nearly 95% of veterans have therapeutic shoes compared with about 80% of nonveteran participants (P < .05). Veterans also had higher ankle-brachial index results (P < .05), although on average both cohorts were within normal clinical parameters. Veterans had a significantly longer duration since healing from the most recent wound, and fewer veteran participants had a wound that healed in the 3 months prior to the study. Despite this, during the study veterans had annualized DFU incidence equal to that of nonveterans. Furthermore, veterans also had significantly higher rates of amputation prior to participation. That these critical outcomes for veterans are no better than those observed in other care environments despite PAVE suggests that approaches recommended via PAVE alone are insufficient to significantly arrest DFU recurrence, and even more focus on prevention in the VA may be warranted.
From Research to Practice
Since the publication of the 2017 study, the VHA has been at the vanguard of translating the evidence and research underlying RTM into clinical practice. A clinical guidance document governing appropriate use of RTM with the study mat was recently published by the VA Prosthetic and Sensory Aids Service in collaboration with the National Podiatry Program office.27 This guidance document recommends once-daily RTM for at-risk veterans designated PAVE level 3. It defines roles and responsibilities required for the successful implementation of a RTM program with the study device. The document additionally presents various clinical monitoring protocols for veterans, although the protocol and thresholds used are at the discretion of the prescribing clinician and should reflect the risk profile of the veteran in question.
A staged response to inflammation has proven popular, whereby an initial high-sensitivity threshold is chosen for monitoring. The initial response is telephone outreach by a designee supplied by the clinic or device manufacturer, typically a trained registered nurse, to the veteran to collect subjective history and instruct off-loading and reduced ambulation, with a target of 50% baseline reduction in step count. Should the inflammation persist despite off-loading, an examination may be necessary to identify and resolve its cause. For recalcitrant inflammation, more targeted pressure off-loading of the affected area may be accomplished with custom orthotics, accommodative insoles, removable cast walkers, and total contact casting. After 2 to 4 weeks without signs of inflammation, the cause is deemed to have been resolved and lowered the acute risk for developing further diabetic foot complications.
More than 600 veterans have been monitored for > 1,000 patient-years—13 VA medical centers are practicing RTM with the study mat as of this writing. The monitoring program has been integrated into many veteran daily routines as evidenced by > 70% retaining full engagement after having been monitored for > 1 year. The total number of alerts/patient-years across these veterans has been 1.4, significantly lower than the 3.0 alerts/patient-year observed in the study. This is potentially due to successful interventions in response to detected inflammation, resolving inflammation, and avoiding unnecessary alerts occurring in the research setting, which did not employ interventions that resolved inflammation episodes. In the past 6 months, 68% of all inflammation detected resolved via off-loading alone without requiring further clinical intervention. In the cases that required an examination, 76% of patients reported clinically meaningful preventive care (eg, preulcerative callus was debrided, a subungual hemorrhage was treated, a foot ulcer was identified).
Organizational Best Practices
Several best practices have been cultivated related to initiating a RTM program at a new site, for promoting the success of a RTM program, and provisioning excellent preventive care to support the RTM program. Although we advise adhering to the recommendations in the VA guidance document,27 the authors have observed several additional organizational best practices that are not explicitly addressed.
Partnering with PACT. Collaboration between PAVE and PACT has the potential not only to improve outcomes for patients at risk for diabetic foot complications, but also can help identify appropriate high-risk veteran candidates for preventive care with RTM who may not be followed for routine care from a specialty provider, such as a podiatrist, as highlighted by the 2013 OIG report.
Prescreening eligible patients. Several programs have used PAVE data or appointment schedules to identify and target high-risk veterans proactively. This approach has several benefits. It simplifies clinical coordination and streamlines workflow for patient identification and onboarding. It also allows those veterans at highest risk to receive needed and recommended preventive care at their next scheduled appointment. Finally, if PAVE data are used to identify eligible patients, it has the added benefit of ensuring a baseline level of telemedicine preventive foot care for veterans who have become lost to follow-up and have not been seen recently for a routine foot examination.
Implementing foot monitoring during wound treatment. Recent research has expanded the reach of once-daily RTM with the mat to patients being treated for a wound to only 1 foot. This practice has 2 benefits: The patient is able to establish a preventive routine before healing, an important advantage because research strongly suggests that recurrence is most likely in the first months after healing. Second, 48% of patients with a history of DFUs will develop new wounds to the contralateral foot because risk factors, such as neuropathy and peripheral arterial disease, exist in both limbs.35 Furthermore, ongoing treatment for a wound to 1 foot may result in gait deviation and elevated pressure to the sound foot, additionally predisposing the veteran to complications, resulting in a high rate of wounds occurring to the unwounded foot during treatment (0.2 DFU/DFU-year).34 Thus, there is potential benefit in monitoring the sound foot while undergoing treatment for a wound; further, the patient will have immediate access to the device for prevention of recurrence once the wound has resolved.
Utilizing foot monitoring as an extension of telemedicine. Many VA facilities have large geographic catchment areas, making routine follow-up difficult for veterans living in rural areas. RTM serves as an extension of the patient’s daily self-examination and the clinician’s ability to monitor patients with objective information daily. The veterans using the system become more invested and feel as though they are taking an active role in their health care.
Investing in ongoing medical education. Multidisciplinary education sessions reviewing supporting clinical data and resultant clinical practice guidelines raise awareness for those providers and trainees unaware of preventive best practices for the diabetic foot, including those related to foot RTM. These sessions also are helpful for those familiar with foot temperature monitoring or who are responsible for administration of an ongoing program to remain current with contemporary best practices and to discuss improvements for patient care. Familiarity also can help address clinical inertia when benefits and evidence are clearly communicated with health care providers (HCPs).
Clinical Best Practices
Treating preulcerative lesions urgently and aggressively. Callus and other preulcerative lesions often cause progressive tissue damage and poor outcomes. When identified, these lesions should be promptly treated to ensure best outcomes.24
Recognizing the limits of patient self-examinations. Comorbidities such as visual impairment and reduced joint mobility often preclude patients from completing rigorous self-examinations of the foot, which is especially critical while collecting subjective history from the patient during triage of inflammation. A caregiver or spouse can help inspect the foot during outreach and provide additional context.36
Interpreting a benign foot on examination. Because RTM has been demonstrated to detect inflammation preceding a foot ulcer as many as 5 weeks before presentation to the clinic, some veterans may have few signs or symptoms of acute risk during examination. Often, the damage is to subcutaneous tissue resulting from repetitive microtrauma. Research suggests that clinical examination in these cases is often unreliable for identifying the earliest signs of risk, such as palpation to identify subtle temperature changes secondary to inflammation.37 If a patient has refractory inflammation requiring examination and presents with an otherwise unremarkable foot, it is an opportunity to evaluate whether the patient’s shoewear remains appropriate or has worn out, to communicate the veteran’s ongoing elevated risk, and to educate on the importance of diligence in daily foot self-examinations, daily use of the foot temperature monitoring, and continued off-loading until the inflammation resolves.
Communicating the distinction between healing and remission. Although healing is the goal of wound care, patients should be educated that the underlying disease remains after epithelialization. In some cases, tissue deep to the skin has not completed remodeling, and the patient is at acute risk of recurrence. Remission is a powerful metaphor that better describes the patient’s ongoing risk to encourage continued healthy routines and diligent self-care.38Considering the entirety of both feet for recurrence. Critical risk factors for diabetic foot complications, such as peripheral neuropathy and PAD, exist in both limbs, and patients with a history of wounds often develop new complications to different ipsilateral locations, or in as many as 48% of cases, to the contralateral foot.35 For best outcomes, detected inflammation should be treated aggressively independent of whether the location coincides with an area of previous concern.
Encouraging adherence, routine, and empowerment. Advanced diabetes mellitus and neuropathy may impact a patient’s executive function, and multiple studies have reported that patients at risk for inflammatory foot diseases exhibit fatalism toward their foot care and outcomes.39-41 Consistent education, encouragement, empowerment, and establishment of positive routines are needed to ensure high adherence with all preventive care regimens, including RTM.
Case Presentations
The following case series illustrates many of these clinical best practices and characterizes the potential benefits of RTM to veterans within the VA.
Case 1: Prevention After Healing
A veteran underwent a Chopart amputation and was recommended to use the mat after healing was perceived. Immediately on use of the study mat, the patient was found to have inflammation to the surgical incision (Figure 1). Clinical staff was alerted to the findings, and the patient was instructed to limit further walking and continue off-loading in his removable cast walker, per protocol. The inflammation of the operative foot quickly reduced, and the patient continued healing successfully, potentially avoiding incisional dehiscence and possible postoperative infection.
This case illustrates that patients’ wounds or surgical incisions may not be completely healed on epithelialization. In the immediate phase after closure, HCPs should consider additional protection to avoid complications. This case demonstrates that RTM can provide objective data to help guide care in that critical period.
Case 2: Identifying Preulcerative Lesions
An 88-year-old veteran had a chronic callus under the second metatarsal head. In addition to routine foot care and therapeutic shoes, he was followed with once-daily RTM. Inflammation was noted, and the veteran was seen in the podiatry clinic where debridement of the callus was performed. The difference in temperatures between feet detected by thermography prior to the clinic visits rapidly resolved after callus debridement, indicating that the underlying inflammation had subsided. RTM was used by the clinical staff to determine the appropriate time interval between clinic visits to avoid callus breakdown and subsequent ulceration.
Case 3: Extending the Clinic Into the Home
An 80-year-old veteran with T2DM and neuropathy was deemed a high-risk patient due to recurrent ulcerations to the left great toe. He was issued a RTM mat and was adherent with routine use. After nearly a year without hot-spot development, inflammation was noted (Figure 2).
Unfortunately, the patient had missed several routine foot care visits and likely that was the reason for the noted inflammation. The patient was called and became reengaged in regular visits for routine foot care. On debridement of his callus, a superficial, noninfected ulceration was discovered. Had remote monitoring not detected the inflammation and impending ulceration, the patient likely would not have been seen in the regular clinic and may have developed a wound infection, potentially resulting in a worse and more costly outcome.
Paradigm Shift to Prevention
Given the exceedingly high burden of diabetic foot complications in the VA, a paradigm shift is needed among HCPs from a culture of treatment to one of prevention. Bus and colleagues reported that in Europe, for every euro spent on ulcer prevention, 10 are spent on ulcer healing, and for every randomized clinical trial conducted on prevention, 10 are conducted on treatment.42-44 Hicks and colleagues showed that the cost of curative care for DFUs is 5 to 30 times greater than the cost of preventive care.45 For RTM in high-risk cohorts (ie, PAVE level 3), the number-needed-to-treat for DFU prevention may be as low as 6, assuming that a 70% reduction in incidence is possible, consistent with previous research. In the year following a DFU, costs exceed $44,000.9 Thus, it seems natural that future direction in diabetic foot care should emphasize prevention strategies.
Foot ulcers that become infected often lead to hospitalization and result in an increased burden to an already overburdened VA health care system. Research suggests that about two-thirds of all diabetic foot costs are attributable to inpatient management.46 The impact of diabetic foot complications on hospital resource utilization is staggering. A 2017 study by Skrepnik analyzed the risk of hospitalization for various diseases.47 The investigators found that the inpatient admission odds ratio (OR) for congestive heart failure was 2.6, surpassed only by DFUs (OR, 3.4) and diabetic foot infection (OR, 6.7). A 2019 point-prevalence study found that > 10% of hospital admissions have a foot-related condition as the primary or secondary reason, and the majority of these are due to foot diseases, such as ulcers, infections, and Charcot neuroarthropathy.48
It is therefore incumbent on VA HCPs to avert wound recurrence in the interest of avoiding veteran hospitalizations and for administrators to encourage and incentivize best practices for managing the diabetic foot, with an emphasis on prevention therapies. In evaluating the financial impact of prevention with foot RTM, administrators should consider that the cost benefit is likely to be realized across the medical center, with budgets related to inpatient management likely to receive the largest returns.
Prevention has the potential to rein in costs as well as reduce strain on the hospital and clinic by preventing outcomes that require frequent visits for treatment or hospitalization. Wound treatment is very burdensome to the clinic; patients require frequent (in many cases, weekly) examinations, and chronic wounds often require hospitalization, necessitating rounding and additional coordination in care. Thus, preventing wounds or reducing their severity at presentation substantially reduces burden on the clinic, even after accounting for the modest clinical resources needed to administer preventative care. For example, a brief examination may be necessary if the inflammation detected by the study mat is secondary to a callus that must be debrided. However, if the patient was not seen until the callus had progressed to a wound, weekly follow-up and substantial clinical and budgetary resources may be required to heal the wound. Preventive care allows for substantially better patient outcomes, and the minimal time invested prevents the clinical burden of extensive wound treatment.
The success of preventive efforts relies on multidisciplinary management of this high-risk patient cohort. Often, it is the responsibility of the primary care provider to follow diabetic foot clinical reminders and appropriately refer to specialty care. Successful, open communication between PACT, PAVE, and the Podiatry Service has been shown to reduce poor outcomes, including lower extremity amputations. Traditionally, the model of preventive care has included podiatrist-driven interventions, including integrated routine foot care and comprehensive diabetic foot education. Collaboration between routine evaluation and prompt referral of at-risk patients for specialist foot care, therapeutic footwear recommendations, daily self-foot examinations, and in-home temperature monitoring are critically effective when performed consistently.
When trying to translate research science to effective clinical practice for preventing lower extremity complication, there are several important concepts. First, given the frequency of examination for patients being treated for a wound, provision of good preventive care, such as RTM, can reduce overall burden to resource-constrained clinics and improve access for patients needing to be seen. Additionally, preventive efforts extend clinical practice into the home and may reduce the need for in-clinic examinations and routine follow-up visits. Finally, there may be a sense of trust established between the clinician and patient with a positive record of adherence with preventive practices. This may translate into more productive communication and less frequent routine visits to better accommodate urgent visits and ensure podiatric care is accessible to veterans.
Conclusions
There is a significant opportunity to shift diabetic foot care from treatment to prevention, improving veteran outcomes and reducing resource utilization. RTM is an evidence-based and recommended but underused telemedicine solution that can catalyze this needed paradigm shift. The VA has been at the forefront of preventive foot care through the PAVE program and more recently through research and clinical application of RTM for veterans. However, as the data presented suggest, more can be done to improve veteran outcomes. More widespread adoption of evidence-based preventive technologies for the diabetic foot, such as RTM, has the potential to dramatically improve the quality of and access to care and reduce costs and burden on resource-constrained clinics.
1. Liu Y, Sayam S, Shao X, et al. Prevalence of and trends in diabetes among veterans, United States, 2005-2014. Prev Chronic Dis. 2017;14:E135.
2. Brennan MB, Hess TM, Bartle B, et al. Diabetic foot ulcer severity predicts mortality among veterans with type 2 diabetes. J Diabetes Complications. 2017;31(3):556-561.
3. Prompers L, Schaper N, Apelqvist J, et al. Prediction of outcome in individuals with diabetic foot ulcers: focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE Study. Diabetologia. 2008;51(5):747-755.
4. Geerlings SE, Hoepelman AIM. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol Med Microbiol. 1999;26(3-4):259-265.
5. Prompers L, Huijberts M, Apelqvist J, et al. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia. 2007;50(1):18-25.
6. Glover JL, Weingarten MS, Buchbinder DS, Poucher RL, Deitrick GA 3rd, Fylling CP. A 4-year outcome-based retrospective study of wound healing and limb salvage in patients with chronic wounds. Adv Wound Care. 1997;10(1):33-38.
7. Franklin H, Rajan M, Tseng C-L, Pogach L, Sinha A. Cost of lower-limb amputation in U.S. veterans with diabetes using health services data in fiscal years 2004 and 2010. J Rehabil Res Dev. 2014;51(8):1325-1330.
8. Melcer T, Sechriest VF, Walker J, Galarneau M. A comparison of health outcomes for combat amputee and limb salvage patients injured in Iraq and Afghanistan wars. J Trauma Acute Care Surg. 2013;75(2)(suppl 2):S247-S254.
9. Chan B, Cadarette S, Wodchis W, Wong J, Mittmann N, Krahn M. Cost-of-illness studies in chronic ulcers: a systematic review. J Wound Care. 2017;26(suppl 4):S4-S14.
10. US Department of Veterans Affairs. VA/DoD clinical practice guideline for the management of type 2 diabetes mellitus in Primary Care. Version 5.0. https://www.healthquality.va.gov/guidelines/CD/diabetes/VADoDDMCPGFinal508.pdf. Published April 2017. Accessed January 31, 2020.
11. Morbach S, Furchert H, Gröblinghoff U, et al. Long-term prognosis of diabetic foot patients and their limbs: amputation and death over the course of a decade. Diabetes Care. 2012;35(10):2021-2027.
12. Apelqvist J, Larsson J, Agardh CD. Long-term prognosis for diabetic patients with foot ulcers. J Intern Med. 1993;233(6):485-491.
13. Pound N, Chipchase S, Treece K, Game F, Jeffcoate W. Ulcer-free survival following management of foot ulcers in diabetes. Diabet Med. 2005;22(10):1306-1309.
14. Dubský M, Jirkovská A, Bem R, et al. Risk factors for recurrence of diabetic foot ulcers: prospective follow-up analysis in the Eurodiale subgroup. Int Wound J. 2013;10(5):555-561.
15. Ulbrecht JS, Hurley T, Mauger DT, Cavanagh PR. Prevention of recurrent foot ulcers with plantar pressure-based in-shoe orthoses: the CareFUL prevention multicenter randomized controlled trial. Diabetes Care. 2014;37(7):1982-1989.
16. Waaijman R, de Haart M, Arts MLJ, et al. Risk factors for plantar foot ulcer recurrence in neuropathic diabetic patients. Diabetes Care. 2014;37(6):1697-1705.
17. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1410: Prevention of Amputations in Veterans Everywhere (PAVE) Program. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=5364. Published March 31, 2017. Accessed February 10, 2020.
18. Robbins JM, Wrobel JS, Kirsh S, Pogach L. Characteristics of high-functioning collaborations between primary care and podiatry in VHA patient aligned care teams. Fed Pract. 2016;33(8):32-36.
19. US Department of Veterans Affairs. Office of Inspector General. Healthcare inspection: foot care for patients with diabetes and additional risk factors for amputation. https://www.va.gov/oig/pubs/VAOIG-11-00711-74.pdf. Published January 17, 2013. Accessed February 3, 2020.
20. Kehle SM, Greer N, Rutks I, Wilt T. Interventions to improve veterans’ access to care: a systematic review of the literature. J Gen Intern Med. 2011;26(suppl 2):689-696.
21. Lavery LA, Higgins KR, Lanctot DR, et al. Home monitoring of foot skin temperatures to prevent ulceration. Diabetes Care. 2004;27(11):2642-2647.
22. Lavery LA, Higgins KR, Lanctot DR, et al. Preventing diabetic foot ulcer recurrence in high-risk patients: use of temperature monitoring as a self-assessment tool. Diabetes Care. 2007;30(1):14-20.
23. Armstrong DG, Holtz-Neiderer K, Wendel C, Mohler MJ, Kimbriel HR, Lavery LA. Skin temperature monitoring reduces the risk for diabetic foot ulceration in high-risk patients. Am J Med. 2007;120(12):1042-1046.
24. Bakker K, Apelqvist J, Lipsky BA, Van Netten JJ; International Working Group on the Diabetic Foot. The 2015 IWGDF guidance documents on prevention and management of foot problems in diabetes: development of an evidence-based global consensus. Diabetes Metab Res Rev. 2016;32 (suppl 1):2-6.
25. Frykberg RG, Zgonis T, Armstrong DG, et al; American College of Foot Ankle Surgeons. Diabetic foot disorders: a clinical practice guideline (2006 revision). J Foot Ankle Surg. 2006;45(suppl 5):S1-S66.
26. Lavery LA, Davis KE, Berriman SJ, et al. WHS guidelines update: diabetic foot ulcer treatment guidelines. Wound Repair Regen. 2016;24(1):112-126.
27. US Department of Veterans Affairs, VA National Prosthetics and Sensory Aids Service and National Podiatry Program Office. Podimetrics – TMD temperature monitoring devices. [Source not verified.]
28. Arad Y, Fonseca V, Peters A, Vinik A. Beyond the monofilament for the insensate diabetic foot: a systematic review of randomized trials to prevent the occurrence of plantar foot ulcers in patients with diabetes. Diabetes Care. 2011;34(4):1041-1046.
29. Dy SM, Bennett WL, Sharma R, et al. Preventing Complications and Treating Symptoms of Diabetic Peripheral Neuropathy. Rockville, MD: Agency for Healthcare Research and Quality US; 2017.
30. Frykberg RG, Gordon IL, Reyzelman AM, et al. Feasibility and efficacy of a SmartMat technology to predict development of diabetic plantar ulcers. Diabetes Care. 2017;40(7):973-980.
31. Crisologo PA, Lavery LA. Remote home monitoring to identify and prevent diabetic foot ulceration. Ann Transl Med. 2017;5(21):430.
32. Armstrong DG, Abu-Rumman PL, Nixon BP, Boulton AJ. Continuous activity monitoring in persons at high risk for diabetes-related lower-extremity amputation. J Am Podiatr Med Assoc. 2001;91(9):451-455.
33. Gordon IL, Rothenberg GM, Lepow BD, et al. Accuracy of a foot temperature monitoring mat for predicting diabetic foot ulcers in patients with recent wounds or partial foot amputation. Diabetes Res Clin Pract. 2020. [Online ahead of print.]
34. Lavery LA, Petersen BJ, Linders DR, Bloom JD, Rothenberg GM, Armstrong DG. Unilateral remote temperature monitoring to predict future ulceration for the diabetic foot in remission. BMJ Open Diabetes Res Care. 2019;7(1):e000696.
35. Petersen BJ, Rothenberg GM, Lakhani PJ, et al. Ulcer metastasis? Anatomical locations of recurrence for patients in diabetic foot remission. J Foot Ankle Res. 2020;13:1.
36. Killeen AL, Brock KM, Dancho JF, Walters JL. Remote temperature monitoring in patients with visual impairment due to diabetes mellitus, a proposed improvement to curren standard of care for prevention of diabetic foot ulcers. J Diabetes Sci Technol. 2020;14(1):37-45.
37. Murff RT, Armstrong DG, Lanctot D, Lavery LA, Athanasiou KA. How effective is manual palpation in detecting subtle temperature differences? Clin Podiatr Med Surg. 1998;15(1):151-154.
38. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376(24):2367-2375.
39. Natovich R, Kushnir T, Harman-Boehm I, et al. Cognitive dysfunction: part and parcel of the diabetic foot. Diabetes Care. 2016;39(7):1202-1207.
40. Zhong A, Li G, Wang D, Sun Y, Zou X, Li B. The risks and external effects of diabetic foot ulcer on diabetic patients: a hospital-based survey in Wuhan area, China. Wound Repair Regen. 2017;25(5):858-863.
41. Vileikyte L. Diabetic foot ulcers: a quality of life issue. Diabetes Metab Res Rev. 2001;17(4):246-249.
42. Van Acker K, Oleen-Burkey M, De Decker L, et al. Cost and resource utilization for prevention and treatment of foot lesions in a diabetic foot clinic in Belgium. Diabetes Res Clin Pract. 2000;50(2):87-95.
43. Kerr M, Rayman G, Jeffcoate WJ. Cost of diabetic foot disease to the National Health Service in England. Diabetes Med. 2014;31(12):1498-1504.
44. Bus SA, van Netten JJ. A shift in priority in diabetic foot care and research: 75% of foot ulcers are preventable. Diabetes Metab Res Rev. 2016;32(suppl 1):195-200.
45. Hicks CW, Selvarajah S, Mathioudakis N, et al. Burden of infected diabetic foot ulcers on hospital admissions and costs. Ann Vasc Surg. 2016;33:149-158.
46. Rice JB, Desai U, Cummings AKG, Birnbaum HG, Skornicki M, Parsons NB. Burden of diabetic foot ulcers for Medicare and private insurers. Diabetes Care. 2014;37(3):651-658.
47. Skrepnek GH, Mills JL Sr, Lavery LA, Armstrong DG. Health care service and outcomes among an estimated 6.7 million ambulatory care diabetic foot cases in the U.S. Diabetes Care. 2017;40(7):936-942.
48. Lazzarini PA, Hurn SE, Kuys SS, et al. Direct inpatient burden caused by foot-related conditions: a multisite point-prevalence study. BMJ Open. 2016;6(6):e010811.
1. Liu Y, Sayam S, Shao X, et al. Prevalence of and trends in diabetes among veterans, United States, 2005-2014. Prev Chronic Dis. 2017;14:E135.
2. Brennan MB, Hess TM, Bartle B, et al. Diabetic foot ulcer severity predicts mortality among veterans with type 2 diabetes. J Diabetes Complications. 2017;31(3):556-561.
3. Prompers L, Schaper N, Apelqvist J, et al. Prediction of outcome in individuals with diabetic foot ulcers: focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE Study. Diabetologia. 2008;51(5):747-755.
4. Geerlings SE, Hoepelman AIM. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol Med Microbiol. 1999;26(3-4):259-265.
5. Prompers L, Huijberts M, Apelqvist J, et al. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia. 2007;50(1):18-25.
6. Glover JL, Weingarten MS, Buchbinder DS, Poucher RL, Deitrick GA 3rd, Fylling CP. A 4-year outcome-based retrospective study of wound healing and limb salvage in patients with chronic wounds. Adv Wound Care. 1997;10(1):33-38.
7. Franklin H, Rajan M, Tseng C-L, Pogach L, Sinha A. Cost of lower-limb amputation in U.S. veterans with diabetes using health services data in fiscal years 2004 and 2010. J Rehabil Res Dev. 2014;51(8):1325-1330.
8. Melcer T, Sechriest VF, Walker J, Galarneau M. A comparison of health outcomes for combat amputee and limb salvage patients injured in Iraq and Afghanistan wars. J Trauma Acute Care Surg. 2013;75(2)(suppl 2):S247-S254.
9. Chan B, Cadarette S, Wodchis W, Wong J, Mittmann N, Krahn M. Cost-of-illness studies in chronic ulcers: a systematic review. J Wound Care. 2017;26(suppl 4):S4-S14.
10. US Department of Veterans Affairs. VA/DoD clinical practice guideline for the management of type 2 diabetes mellitus in Primary Care. Version 5.0. https://www.healthquality.va.gov/guidelines/CD/diabetes/VADoDDMCPGFinal508.pdf. Published April 2017. Accessed January 31, 2020.
11. Morbach S, Furchert H, Gröblinghoff U, et al. Long-term prognosis of diabetic foot patients and their limbs: amputation and death over the course of a decade. Diabetes Care. 2012;35(10):2021-2027.
12. Apelqvist J, Larsson J, Agardh CD. Long-term prognosis for diabetic patients with foot ulcers. J Intern Med. 1993;233(6):485-491.
13. Pound N, Chipchase S, Treece K, Game F, Jeffcoate W. Ulcer-free survival following management of foot ulcers in diabetes. Diabet Med. 2005;22(10):1306-1309.
14. Dubský M, Jirkovská A, Bem R, et al. Risk factors for recurrence of diabetic foot ulcers: prospective follow-up analysis in the Eurodiale subgroup. Int Wound J. 2013;10(5):555-561.
15. Ulbrecht JS, Hurley T, Mauger DT, Cavanagh PR. Prevention of recurrent foot ulcers with plantar pressure-based in-shoe orthoses: the CareFUL prevention multicenter randomized controlled trial. Diabetes Care. 2014;37(7):1982-1989.
16. Waaijman R, de Haart M, Arts MLJ, et al. Risk factors for plantar foot ulcer recurrence in neuropathic diabetic patients. Diabetes Care. 2014;37(6):1697-1705.
17. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1410: Prevention of Amputations in Veterans Everywhere (PAVE) Program. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=5364. Published March 31, 2017. Accessed February 10, 2020.
18. Robbins JM, Wrobel JS, Kirsh S, Pogach L. Characteristics of high-functioning collaborations between primary care and podiatry in VHA patient aligned care teams. Fed Pract. 2016;33(8):32-36.
19. US Department of Veterans Affairs. Office of Inspector General. Healthcare inspection: foot care for patients with diabetes and additional risk factors for amputation. https://www.va.gov/oig/pubs/VAOIG-11-00711-74.pdf. Published January 17, 2013. Accessed February 3, 2020.
20. Kehle SM, Greer N, Rutks I, Wilt T. Interventions to improve veterans’ access to care: a systematic review of the literature. J Gen Intern Med. 2011;26(suppl 2):689-696.
21. Lavery LA, Higgins KR, Lanctot DR, et al. Home monitoring of foot skin temperatures to prevent ulceration. Diabetes Care. 2004;27(11):2642-2647.
22. Lavery LA, Higgins KR, Lanctot DR, et al. Preventing diabetic foot ulcer recurrence in high-risk patients: use of temperature monitoring as a self-assessment tool. Diabetes Care. 2007;30(1):14-20.
23. Armstrong DG, Holtz-Neiderer K, Wendel C, Mohler MJ, Kimbriel HR, Lavery LA. Skin temperature monitoring reduces the risk for diabetic foot ulceration in high-risk patients. Am J Med. 2007;120(12):1042-1046.
24. Bakker K, Apelqvist J, Lipsky BA, Van Netten JJ; International Working Group on the Diabetic Foot. The 2015 IWGDF guidance documents on prevention and management of foot problems in diabetes: development of an evidence-based global consensus. Diabetes Metab Res Rev. 2016;32 (suppl 1):2-6.
25. Frykberg RG, Zgonis T, Armstrong DG, et al; American College of Foot Ankle Surgeons. Diabetic foot disorders: a clinical practice guideline (2006 revision). J Foot Ankle Surg. 2006;45(suppl 5):S1-S66.
26. Lavery LA, Davis KE, Berriman SJ, et al. WHS guidelines update: diabetic foot ulcer treatment guidelines. Wound Repair Regen. 2016;24(1):112-126.
27. US Department of Veterans Affairs, VA National Prosthetics and Sensory Aids Service and National Podiatry Program Office. Podimetrics – TMD temperature monitoring devices. [Source not verified.]
28. Arad Y, Fonseca V, Peters A, Vinik A. Beyond the monofilament for the insensate diabetic foot: a systematic review of randomized trials to prevent the occurrence of plantar foot ulcers in patients with diabetes. Diabetes Care. 2011;34(4):1041-1046.
29. Dy SM, Bennett WL, Sharma R, et al. Preventing Complications and Treating Symptoms of Diabetic Peripheral Neuropathy. Rockville, MD: Agency for Healthcare Research and Quality US; 2017.
30. Frykberg RG, Gordon IL, Reyzelman AM, et al. Feasibility and efficacy of a SmartMat technology to predict development of diabetic plantar ulcers. Diabetes Care. 2017;40(7):973-980.
31. Crisologo PA, Lavery LA. Remote home monitoring to identify and prevent diabetic foot ulceration. Ann Transl Med. 2017;5(21):430.
32. Armstrong DG, Abu-Rumman PL, Nixon BP, Boulton AJ. Continuous activity monitoring in persons at high risk for diabetes-related lower-extremity amputation. J Am Podiatr Med Assoc. 2001;91(9):451-455.
33. Gordon IL, Rothenberg GM, Lepow BD, et al. Accuracy of a foot temperature monitoring mat for predicting diabetic foot ulcers in patients with recent wounds or partial foot amputation. Diabetes Res Clin Pract. 2020. [Online ahead of print.]
34. Lavery LA, Petersen BJ, Linders DR, Bloom JD, Rothenberg GM, Armstrong DG. Unilateral remote temperature monitoring to predict future ulceration for the diabetic foot in remission. BMJ Open Diabetes Res Care. 2019;7(1):e000696.
35. Petersen BJ, Rothenberg GM, Lakhani PJ, et al. Ulcer metastasis? Anatomical locations of recurrence for patients in diabetic foot remission. J Foot Ankle Res. 2020;13:1.
36. Killeen AL, Brock KM, Dancho JF, Walters JL. Remote temperature monitoring in patients with visual impairment due to diabetes mellitus, a proposed improvement to curren standard of care for prevention of diabetic foot ulcers. J Diabetes Sci Technol. 2020;14(1):37-45.
37. Murff RT, Armstrong DG, Lanctot D, Lavery LA, Athanasiou KA. How effective is manual palpation in detecting subtle temperature differences? Clin Podiatr Med Surg. 1998;15(1):151-154.
38. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376(24):2367-2375.
39. Natovich R, Kushnir T, Harman-Boehm I, et al. Cognitive dysfunction: part and parcel of the diabetic foot. Diabetes Care. 2016;39(7):1202-1207.
40. Zhong A, Li G, Wang D, Sun Y, Zou X, Li B. The risks and external effects of diabetic foot ulcer on diabetic patients: a hospital-based survey in Wuhan area, China. Wound Repair Regen. 2017;25(5):858-863.
41. Vileikyte L. Diabetic foot ulcers: a quality of life issue. Diabetes Metab Res Rev. 2001;17(4):246-249.
42. Van Acker K, Oleen-Burkey M, De Decker L, et al. Cost and resource utilization for prevention and treatment of foot lesions in a diabetic foot clinic in Belgium. Diabetes Res Clin Pract. 2000;50(2):87-95.
43. Kerr M, Rayman G, Jeffcoate WJ. Cost of diabetic foot disease to the National Health Service in England. Diabetes Med. 2014;31(12):1498-1504.
44. Bus SA, van Netten JJ. A shift in priority in diabetic foot care and research: 75% of foot ulcers are preventable. Diabetes Metab Res Rev. 2016;32(suppl 1):195-200.
45. Hicks CW, Selvarajah S, Mathioudakis N, et al. Burden of infected diabetic foot ulcers on hospital admissions and costs. Ann Vasc Surg. 2016;33:149-158.
46. Rice JB, Desai U, Cummings AKG, Birnbaum HG, Skornicki M, Parsons NB. Burden of diabetic foot ulcers for Medicare and private insurers. Diabetes Care. 2014;37(3):651-658.
47. Skrepnek GH, Mills JL Sr, Lavery LA, Armstrong DG. Health care service and outcomes among an estimated 6.7 million ambulatory care diabetic foot cases in the U.S. Diabetes Care. 2017;40(7):936-942.
48. Lazzarini PA, Hurn SE, Kuys SS, et al. Direct inpatient burden caused by foot-related conditions: a multisite point-prevalence study. BMJ Open. 2016;6(6):e010811.