User login
Implementation of a Virtual Huddle to Support Patient Care During the COVID-19 Pandemic
The COVID-19 pandemic challenged hospital medicine teams to care for patients with complex respiratory needs, comply with evolving protocols, and remain abreast of new therapies.1,2 Pulmonary and critical care medicine (PCCM) faculty grappled with similar issues, acknowledging that their critical care expertise could be beneficial outside of the intensive care unit (ICU). Clinical pharmacists managed the procurement, allocation, and monitoring of complex (and sometimes limited) pharmacologic therapies. Although strategies used by health care systems to prepare and restructure for COVID-19 are reported, processes to enhance multidisciplinary care are limited.3,4 Therefore, we developed the COVID-19 Tele-Huddle Program using video conference to support hospital medicine teams caring for patients with COVID-19 and high disease severity.
Program Description
The Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, is a 349-bed, level 1A federal health care facility serving more than 113,000 veterans in southeast Texas.5 The COVID-19 Tele-Huddle Program took place over a 4-week period from July 6 to August 2, 2020. By the end of the 4-week period, there was a decline in the number of COVID patient admissions and thus the need for the huddle. Participation in the huddle also declined, likely reflecting the end of the surge and an increase in knowledge about COVID management acquired by the teams. Each COVID-19 Tele-Huddle Program consultation session consisted of at least 1 member from each hospital medicine team, 1 to 2 PCCM faculty members, and 1 to 2 clinical pharmacy specialists (Figure). The consultation team members included 4 PCCM faculty members and 2 clinical pharmacy specialists. The internal medicine (IM) participants included 10 ward teams with a total of 20 interns (PGY1), 12 upper-level residents (PGY2 and PGY 3), and 10 attending physicians.
The COVID-19 Tele-Huddle Program was a daily (including weekends) video conference. The hospital medicine team members joined the huddle from team workrooms, using webcams supplied by the MEDVAMC information technology department. The COVID-19 Tele-Huddle Program consultation team members joined remotely. Each hospital medicine team joined the huddle at a pre-assigned 15- to 30-minute time allotment, which varied based on patient volume. Participation in the huddle was mandatory for the first week and became optional thereafter. This was in recognition of the steep learning curve and provided the teams both basic knowledge of COVID management and a shared understanding of when a multidisciplinary consultation would be critical. Mandatory daily participation was challenging due to the pressures of patient volume during the surge.
COVID-19 patients with high disease severity were discussed during huddles based on specific criteria: all newly admitted COVID-19 patients, patients requiring step-down level of care, those with increasing oxygen requirements, and/or patients requiring authorization of remdesivir therapy, which required clinical pharmacy authorization at MEDVAMC. The hospital medicine teams reported the patients’ oxygen requirements, comorbid medical conditions, current and prior therapies, fluid status, and relevant laboratory values. A dashboard using the Premier Inc. TheraDoc clinical decision support system was developed to display patient vital signs, laboratory values, and medications. The PCCM faculty and clinical pharmacists listened to inpatient medicine teams presentations and used the dashboard and radiographic images to formulate clinical decisions. Discussion of a patient at the huddle did not preclude in-person consultation at any time.
Tele-Huddles were not recorded, and all protected health information discussed was accessed through the electronic health record using a secure network. Data on length of the meeting, number of patients discussed, and management decisions were recorded daily in a spreadsheet. At the end of the 4-week surge, participants in the program completed a survey, which assessed participant demographics, prior experience with COVID-19, and satisfaction with the program based on a series of agree/disagree questions.
Program Metrics
During the COVID-19 Tele-Huddle Program 4-week evaluation period, 323 encounters were discussed with 117 unique patients with COVID-19. A median (IQR) of 5 (4-8) hospital medicine teams discussed 15 (9-18) patients. The COVID-19 Tele-Huddle Program lasted a median (IQR) 74 (53-94) minutes. A mean (SD) 27% (13) of patients with COVID-19 admitted to the acute care services were discussed.
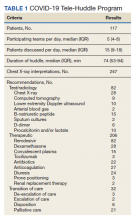
The multidisciplinary team provided 247 chest X-ray interpretations, 82 diagnostic recommendations, 206 therapeutic recommendations, and 32 transition of care recommendations (Table 1). A total of 55 (47%) patients were given remdesivir with first dose authorized by clinical pharmacy and given within a median (IQR) 6 (3-10) hours after the order was placed. Oxygen therapy, including titration and de-escalation of high-flow nasal cannula and noninvasive positive pressure ventilation (NIPPV), was used for 26 (22.2%) patients. Additional interventions included the review of imaging, the assessment of volume status to guide diuretic recommendations, and the discussion of goals of care.
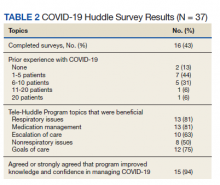
Of the participating IM trainees and attendings, 16 of 37 (43%) completed the user survey (Table 2). Prior experience with COVID-19 patients varied, with 7 of 16 respondents indicating experience with ≥ 5 patients with COVID-19 prior to the intervention period. Respondents believed that the huddle was helpful in management of respiratory issues (13 of 16), management of medications (13 of 16), escalation of care to ICU (10 of 16), and management of nonrespiratory issues (8 of 16) and goals of care (12 of 16). Fifteen of 16 participants strongly agreed or agreed that the COVID-19 Tele-Huddle Program improved their knowledge and confidence in managing patients. One participant commented, “Getting interdisciplinary help on COVID patients has really helped our team feel confident in our decisions about some of these very complex patients.” Another respondent commented, “Reliability was very helpful for planning how to discuss updates with our patients rather than the formal consultative process.”
Discussion
During the unprecedented COVID-19 pandemic, health care systems have been challenged to manage a large volume of patients, often with high disease severity, in non-ICU settings. This surge in cases has placed strain on hospital medicine teams. There is a subset of patients with COVID-19 with high disease severity that may be managed safely by hospital medicine teams, provided the accessibility and support of consultants, such as PCCM faculty and clinical pharmacists.
Huddles are defined as functional groups of people focused on enhancing communication and care coordination to benefit patient safety. While often brief in nature, huddles can encompass a variety of structures, agendas, and outcome measures.6,7 We implemented a modified huddle using video conferencing to provide important aspects of critical care for patients with COVID-19. Face-to-face evaluation of about 15 patients each day would have strained an already burdened PCCM faculty who were providing additional critical care services as part of the surge response. Conversion of in-person consultations to the COVID-19 Tele-Huddle Program allowed for mitigation of COVID-19 transmission risk for additional clinicians, conservation of personal protective equipment, and more effective communication between acute inpatient practitioners and clinical services. The huddle model expedited the authorization and delivery of therapeutics, including remdesivir, which was prescribed for many patients discussed. Clinical pharmacists provided a review of all medications with input on escalation, de-escalation, dosing, drug-drug interactions, and emergency use authorization therapies.
Our experience resonates with previously described advantages of a huddle model, including the reliability of the consultation, empowerment for all members with a de-emphasis on hierarchy and accountability expected by all.8 The huddle provided situational awareness about patients that may require escalation of care to the ICU and/or further goals of care conversations. Assistance with these transitions of care was highly appreciated by the hospital medicine teams who voiced that these decisions were quite challenging. COVID-19 patients at risk for decompensation were referred for in-person consultation and follow-up if required.
addition, the COVID-19 Tele-Huddle Program allowed for a safe and dependable venue for IM trainees and attending physicians to voice questions and concerns about their patients. We observed the development of a shared mental model among all huddle participants, in the face of a steep learning curve on the management of patients with complex respiratory needs. This was reflected in the survey: Most respondents reported improved knowledge and confidence in managing these patients. Situational awareness that arose from the huddle provided the PCCM faculty the opportunity to guide the inpatient ward teams on next steps whether it be escalation to the ICU and/or further goals of care conversations. Facilitation of transitions of care were voiced as challenging decisions faced by the inpatient ward teams, and there was appreciation for additional support from the PCCM faculty in making these difficult decisions.
Challenges and Opportunities
This was a single-center experience caring for veterans. Challenges with having virtual huddles during the COVID-19 surge involved both time for the health care practitioners and technology. This was recognized early by the educational leaders at our facility, and headsets and cameras were purchased for the team rooms and made available as quickly as possible. Another limitation was the unpredictability and variability of patient volume for specific teams that sometimes would affect the efficiency of the huddle. The number of teams who attended the COVID-19 huddle was highest for the first 2 weeks (maximum of 9 teams) but declined to a nadir of 3 at the end of the month. This reflected the increase in knowledge about COVID-19 and respiratory disease that the teams acquired initially as well as a decline in COVID-19 patient admissions over those weeks.
The COVID-19 Tele-Huddle Program model also can be expanded to include other frontline clinicians, including nurses and respiratory therapists. For example, case management huddles were performed in a similar way during the COVID-19 surge to allow for efficient and effective multidisciplinary conversations about patients
Conclusions
Given the rise of telemedicine and availability of video conferencing services, virtual huddles can be implemented in institutions with appropriate staff and remote access to health records. Multidisciplinary consultation services using video conferencing can serve as an adjunct to the traditional, in-person consultation service model for patients with complex needs.
Acknowledgments
The authors acknowledge all of the Baylor Internal Medicine house staff and internal medicine attendings who participated in our huddle and more importantly, cared for our veterans during this COVID-19 surge.
1. Heymann DL, Shindo N; WHO Scientific and Technical Advisory Group for Infectious Hazards. COVID-19: what is next for public health?. Lancet. 2020;395(10224):542-545. doi:10.1016/S0140-6736(20)30374-3
2. Dichter JR, Kanter RK, Dries D, et al; Task Force for Mass Critical Care. System-level planning, coordination, and communication: care of the critically ill and injured during pandemics and disasters: CHEST consensus statement. Chest. 2014;146(suppl 4):e87S-e102S. doi:10.1378/chest.14-0738
3. Chowdhury JM, Patel M, Zheng M, Abramian O, Criner GJ. Mobilization and preparation of a large urban academic center during the COVID-19 pandemic. Ann Am Thorac Soc. 2020;17(8):922-925. doi:10.1513/AnnalsATS.202003-259PS
4. Uppal A, Silvestri DM, Siegler M, et al. Critical care and emergency department response at the epicenter of the COVID-19 pandemic. Health Aff (Millwood). 2020;39(8):1443-1449. doi:10.1377/hlthaff.2020.00901
5. US Department of Veterans Affairs. Michael E. DeBakey VA Medical Center- Houston, Texas. Accessed December 10, 2020. https://www.houston.va.gov/about
6. Provost SM, Lanham HJ, Leykum LK, McDaniel RR Jr, Pugh J. Health care huddles: managing complexity to achieve high reliability. Health Care Manage Rev. 2015;40(1):2-12. doi:10.1097/HMR.0000000000000009
7. Franklin BJ, Gandhi TK, Bates DW, et al. Impact of multidisciplinary team huddles on patient safety: a systematic review and proposed taxonomy. BMJ Qual Saf. 2020;29(10):1-2. doi:10.1136/bmjqs-2019-009911
8. Goldenhar LM, Brady PW, Sutcliffe KM, Muething SE. Huddling for high reliability and situation awareness. BMJ Qual Saf. 2013;22(11):899-906. doi:10.1136/bmjqs-2012-001467
The COVID-19 pandemic challenged hospital medicine teams to care for patients with complex respiratory needs, comply with evolving protocols, and remain abreast of new therapies.1,2 Pulmonary and critical care medicine (PCCM) faculty grappled with similar issues, acknowledging that their critical care expertise could be beneficial outside of the intensive care unit (ICU). Clinical pharmacists managed the procurement, allocation, and monitoring of complex (and sometimes limited) pharmacologic therapies. Although strategies used by health care systems to prepare and restructure for COVID-19 are reported, processes to enhance multidisciplinary care are limited.3,4 Therefore, we developed the COVID-19 Tele-Huddle Program using video conference to support hospital medicine teams caring for patients with COVID-19 and high disease severity.
Program Description
The Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, is a 349-bed, level 1A federal health care facility serving more than 113,000 veterans in southeast Texas.5 The COVID-19 Tele-Huddle Program took place over a 4-week period from July 6 to August 2, 2020. By the end of the 4-week period, there was a decline in the number of COVID patient admissions and thus the need for the huddle. Participation in the huddle also declined, likely reflecting the end of the surge and an increase in knowledge about COVID management acquired by the teams. Each COVID-19 Tele-Huddle Program consultation session consisted of at least 1 member from each hospital medicine team, 1 to 2 PCCM faculty members, and 1 to 2 clinical pharmacy specialists (Figure). The consultation team members included 4 PCCM faculty members and 2 clinical pharmacy specialists. The internal medicine (IM) participants included 10 ward teams with a total of 20 interns (PGY1), 12 upper-level residents (PGY2 and PGY 3), and 10 attending physicians.
The COVID-19 Tele-Huddle Program was a daily (including weekends) video conference. The hospital medicine team members joined the huddle from team workrooms, using webcams supplied by the MEDVAMC information technology department. The COVID-19 Tele-Huddle Program consultation team members joined remotely. Each hospital medicine team joined the huddle at a pre-assigned 15- to 30-minute time allotment, which varied based on patient volume. Participation in the huddle was mandatory for the first week and became optional thereafter. This was in recognition of the steep learning curve and provided the teams both basic knowledge of COVID management and a shared understanding of when a multidisciplinary consultation would be critical. Mandatory daily participation was challenging due to the pressures of patient volume during the surge.
COVID-19 patients with high disease severity were discussed during huddles based on specific criteria: all newly admitted COVID-19 patients, patients requiring step-down level of care, those with increasing oxygen requirements, and/or patients requiring authorization of remdesivir therapy, which required clinical pharmacy authorization at MEDVAMC. The hospital medicine teams reported the patients’ oxygen requirements, comorbid medical conditions, current and prior therapies, fluid status, and relevant laboratory values. A dashboard using the Premier Inc. TheraDoc clinical decision support system was developed to display patient vital signs, laboratory values, and medications. The PCCM faculty and clinical pharmacists listened to inpatient medicine teams presentations and used the dashboard and radiographic images to formulate clinical decisions. Discussion of a patient at the huddle did not preclude in-person consultation at any time.
Tele-Huddles were not recorded, and all protected health information discussed was accessed through the electronic health record using a secure network. Data on length of the meeting, number of patients discussed, and management decisions were recorded daily in a spreadsheet. At the end of the 4-week surge, participants in the program completed a survey, which assessed participant demographics, prior experience with COVID-19, and satisfaction with the program based on a series of agree/disagree questions.
Program Metrics
During the COVID-19 Tele-Huddle Program 4-week evaluation period, 323 encounters were discussed with 117 unique patients with COVID-19. A median (IQR) of 5 (4-8) hospital medicine teams discussed 15 (9-18) patients. The COVID-19 Tele-Huddle Program lasted a median (IQR) 74 (53-94) minutes. A mean (SD) 27% (13) of patients with COVID-19 admitted to the acute care services were discussed.
The multidisciplinary team provided 247 chest X-ray interpretations, 82 diagnostic recommendations, 206 therapeutic recommendations, and 32 transition of care recommendations (Table 1). A total of 55 (47%) patients were given remdesivir with first dose authorized by clinical pharmacy and given within a median (IQR) 6 (3-10) hours after the order was placed. Oxygen therapy, including titration and de-escalation of high-flow nasal cannula and noninvasive positive pressure ventilation (NIPPV), was used for 26 (22.2%) patients. Additional interventions included the review of imaging, the assessment of volume status to guide diuretic recommendations, and the discussion of goals of care.
Of the participating IM trainees and attendings, 16 of 37 (43%) completed the user survey (Table 2). Prior experience with COVID-19 patients varied, with 7 of 16 respondents indicating experience with ≥ 5 patients with COVID-19 prior to the intervention period. Respondents believed that the huddle was helpful in management of respiratory issues (13 of 16), management of medications (13 of 16), escalation of care to ICU (10 of 16), and management of nonrespiratory issues (8 of 16) and goals of care (12 of 16). Fifteen of 16 participants strongly agreed or agreed that the COVID-19 Tele-Huddle Program improved their knowledge and confidence in managing patients. One participant commented, “Getting interdisciplinary help on COVID patients has really helped our team feel confident in our decisions about some of these very complex patients.” Another respondent commented, “Reliability was very helpful for planning how to discuss updates with our patients rather than the formal consultative process.”
Discussion
During the unprecedented COVID-19 pandemic, health care systems have been challenged to manage a large volume of patients, often with high disease severity, in non-ICU settings. This surge in cases has placed strain on hospital medicine teams. There is a subset of patients with COVID-19 with high disease severity that may be managed safely by hospital medicine teams, provided the accessibility and support of consultants, such as PCCM faculty and clinical pharmacists.
Huddles are defined as functional groups of people focused on enhancing communication and care coordination to benefit patient safety. While often brief in nature, huddles can encompass a variety of structures, agendas, and outcome measures.6,7 We implemented a modified huddle using video conferencing to provide important aspects of critical care for patients with COVID-19. Face-to-face evaluation of about 15 patients each day would have strained an already burdened PCCM faculty who were providing additional critical care services as part of the surge response. Conversion of in-person consultations to the COVID-19 Tele-Huddle Program allowed for mitigation of COVID-19 transmission risk for additional clinicians, conservation of personal protective equipment, and more effective communication between acute inpatient practitioners and clinical services. The huddle model expedited the authorization and delivery of therapeutics, including remdesivir, which was prescribed for many patients discussed. Clinical pharmacists provided a review of all medications with input on escalation, de-escalation, dosing, drug-drug interactions, and emergency use authorization therapies.
Our experience resonates with previously described advantages of a huddle model, including the reliability of the consultation, empowerment for all members with a de-emphasis on hierarchy and accountability expected by all.8 The huddle provided situational awareness about patients that may require escalation of care to the ICU and/or further goals of care conversations. Assistance with these transitions of care was highly appreciated by the hospital medicine teams who voiced that these decisions were quite challenging. COVID-19 patients at risk for decompensation were referred for in-person consultation and follow-up if required.
addition, the COVID-19 Tele-Huddle Program allowed for a safe and dependable venue for IM trainees and attending physicians to voice questions and concerns about their patients. We observed the development of a shared mental model among all huddle participants, in the face of a steep learning curve on the management of patients with complex respiratory needs. This was reflected in the survey: Most respondents reported improved knowledge and confidence in managing these patients. Situational awareness that arose from the huddle provided the PCCM faculty the opportunity to guide the inpatient ward teams on next steps whether it be escalation to the ICU and/or further goals of care conversations. Facilitation of transitions of care were voiced as challenging decisions faced by the inpatient ward teams, and there was appreciation for additional support from the PCCM faculty in making these difficult decisions.
Challenges and Opportunities
This was a single-center experience caring for veterans. Challenges with having virtual huddles during the COVID-19 surge involved both time for the health care practitioners and technology. This was recognized early by the educational leaders at our facility, and headsets and cameras were purchased for the team rooms and made available as quickly as possible. Another limitation was the unpredictability and variability of patient volume for specific teams that sometimes would affect the efficiency of the huddle. The number of teams who attended the COVID-19 huddle was highest for the first 2 weeks (maximum of 9 teams) but declined to a nadir of 3 at the end of the month. This reflected the increase in knowledge about COVID-19 and respiratory disease that the teams acquired initially as well as a decline in COVID-19 patient admissions over those weeks.
The COVID-19 Tele-Huddle Program model also can be expanded to include other frontline clinicians, including nurses and respiratory therapists. For example, case management huddles were performed in a similar way during the COVID-19 surge to allow for efficient and effective multidisciplinary conversations about patients
Conclusions
Given the rise of telemedicine and availability of video conferencing services, virtual huddles can be implemented in institutions with appropriate staff and remote access to health records. Multidisciplinary consultation services using video conferencing can serve as an adjunct to the traditional, in-person consultation service model for patients with complex needs.
Acknowledgments
The authors acknowledge all of the Baylor Internal Medicine house staff and internal medicine attendings who participated in our huddle and more importantly, cared for our veterans during this COVID-19 surge.
The COVID-19 pandemic challenged hospital medicine teams to care for patients with complex respiratory needs, comply with evolving protocols, and remain abreast of new therapies.1,2 Pulmonary and critical care medicine (PCCM) faculty grappled with similar issues, acknowledging that their critical care expertise could be beneficial outside of the intensive care unit (ICU). Clinical pharmacists managed the procurement, allocation, and monitoring of complex (and sometimes limited) pharmacologic therapies. Although strategies used by health care systems to prepare and restructure for COVID-19 are reported, processes to enhance multidisciplinary care are limited.3,4 Therefore, we developed the COVID-19 Tele-Huddle Program using video conference to support hospital medicine teams caring for patients with COVID-19 and high disease severity.
Program Description
The Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, is a 349-bed, level 1A federal health care facility serving more than 113,000 veterans in southeast Texas.5 The COVID-19 Tele-Huddle Program took place over a 4-week period from July 6 to August 2, 2020. By the end of the 4-week period, there was a decline in the number of COVID patient admissions and thus the need for the huddle. Participation in the huddle also declined, likely reflecting the end of the surge and an increase in knowledge about COVID management acquired by the teams. Each COVID-19 Tele-Huddle Program consultation session consisted of at least 1 member from each hospital medicine team, 1 to 2 PCCM faculty members, and 1 to 2 clinical pharmacy specialists (Figure). The consultation team members included 4 PCCM faculty members and 2 clinical pharmacy specialists. The internal medicine (IM) participants included 10 ward teams with a total of 20 interns (PGY1), 12 upper-level residents (PGY2 and PGY 3), and 10 attending physicians.
The COVID-19 Tele-Huddle Program was a daily (including weekends) video conference. The hospital medicine team members joined the huddle from team workrooms, using webcams supplied by the MEDVAMC information technology department. The COVID-19 Tele-Huddle Program consultation team members joined remotely. Each hospital medicine team joined the huddle at a pre-assigned 15- to 30-minute time allotment, which varied based on patient volume. Participation in the huddle was mandatory for the first week and became optional thereafter. This was in recognition of the steep learning curve and provided the teams both basic knowledge of COVID management and a shared understanding of when a multidisciplinary consultation would be critical. Mandatory daily participation was challenging due to the pressures of patient volume during the surge.
COVID-19 patients with high disease severity were discussed during huddles based on specific criteria: all newly admitted COVID-19 patients, patients requiring step-down level of care, those with increasing oxygen requirements, and/or patients requiring authorization of remdesivir therapy, which required clinical pharmacy authorization at MEDVAMC. The hospital medicine teams reported the patients’ oxygen requirements, comorbid medical conditions, current and prior therapies, fluid status, and relevant laboratory values. A dashboard using the Premier Inc. TheraDoc clinical decision support system was developed to display patient vital signs, laboratory values, and medications. The PCCM faculty and clinical pharmacists listened to inpatient medicine teams presentations and used the dashboard and radiographic images to formulate clinical decisions. Discussion of a patient at the huddle did not preclude in-person consultation at any time.
Tele-Huddles were not recorded, and all protected health information discussed was accessed through the electronic health record using a secure network. Data on length of the meeting, number of patients discussed, and management decisions were recorded daily in a spreadsheet. At the end of the 4-week surge, participants in the program completed a survey, which assessed participant demographics, prior experience with COVID-19, and satisfaction with the program based on a series of agree/disagree questions.
Program Metrics
During the COVID-19 Tele-Huddle Program 4-week evaluation period, 323 encounters were discussed with 117 unique patients with COVID-19. A median (IQR) of 5 (4-8) hospital medicine teams discussed 15 (9-18) patients. The COVID-19 Tele-Huddle Program lasted a median (IQR) 74 (53-94) minutes. A mean (SD) 27% (13) of patients with COVID-19 admitted to the acute care services were discussed.
The multidisciplinary team provided 247 chest X-ray interpretations, 82 diagnostic recommendations, 206 therapeutic recommendations, and 32 transition of care recommendations (Table 1). A total of 55 (47%) patients were given remdesivir with first dose authorized by clinical pharmacy and given within a median (IQR) 6 (3-10) hours after the order was placed. Oxygen therapy, including titration and de-escalation of high-flow nasal cannula and noninvasive positive pressure ventilation (NIPPV), was used for 26 (22.2%) patients. Additional interventions included the review of imaging, the assessment of volume status to guide diuretic recommendations, and the discussion of goals of care.
Of the participating IM trainees and attendings, 16 of 37 (43%) completed the user survey (Table 2). Prior experience with COVID-19 patients varied, with 7 of 16 respondents indicating experience with ≥ 5 patients with COVID-19 prior to the intervention period. Respondents believed that the huddle was helpful in management of respiratory issues (13 of 16), management of medications (13 of 16), escalation of care to ICU (10 of 16), and management of nonrespiratory issues (8 of 16) and goals of care (12 of 16). Fifteen of 16 participants strongly agreed or agreed that the COVID-19 Tele-Huddle Program improved their knowledge and confidence in managing patients. One participant commented, “Getting interdisciplinary help on COVID patients has really helped our team feel confident in our decisions about some of these very complex patients.” Another respondent commented, “Reliability was very helpful for planning how to discuss updates with our patients rather than the formal consultative process.”
Discussion
During the unprecedented COVID-19 pandemic, health care systems have been challenged to manage a large volume of patients, often with high disease severity, in non-ICU settings. This surge in cases has placed strain on hospital medicine teams. There is a subset of patients with COVID-19 with high disease severity that may be managed safely by hospital medicine teams, provided the accessibility and support of consultants, such as PCCM faculty and clinical pharmacists.
Huddles are defined as functional groups of people focused on enhancing communication and care coordination to benefit patient safety. While often brief in nature, huddles can encompass a variety of structures, agendas, and outcome measures.6,7 We implemented a modified huddle using video conferencing to provide important aspects of critical care for patients with COVID-19. Face-to-face evaluation of about 15 patients each day would have strained an already burdened PCCM faculty who were providing additional critical care services as part of the surge response. Conversion of in-person consultations to the COVID-19 Tele-Huddle Program allowed for mitigation of COVID-19 transmission risk for additional clinicians, conservation of personal protective equipment, and more effective communication between acute inpatient practitioners and clinical services. The huddle model expedited the authorization and delivery of therapeutics, including remdesivir, which was prescribed for many patients discussed. Clinical pharmacists provided a review of all medications with input on escalation, de-escalation, dosing, drug-drug interactions, and emergency use authorization therapies.
Our experience resonates with previously described advantages of a huddle model, including the reliability of the consultation, empowerment for all members with a de-emphasis on hierarchy and accountability expected by all.8 The huddle provided situational awareness about patients that may require escalation of care to the ICU and/or further goals of care conversations. Assistance with these transitions of care was highly appreciated by the hospital medicine teams who voiced that these decisions were quite challenging. COVID-19 patients at risk for decompensation were referred for in-person consultation and follow-up if required.
addition, the COVID-19 Tele-Huddle Program allowed for a safe and dependable venue for IM trainees and attending physicians to voice questions and concerns about their patients. We observed the development of a shared mental model among all huddle participants, in the face of a steep learning curve on the management of patients with complex respiratory needs. This was reflected in the survey: Most respondents reported improved knowledge and confidence in managing these patients. Situational awareness that arose from the huddle provided the PCCM faculty the opportunity to guide the inpatient ward teams on next steps whether it be escalation to the ICU and/or further goals of care conversations. Facilitation of transitions of care were voiced as challenging decisions faced by the inpatient ward teams, and there was appreciation for additional support from the PCCM faculty in making these difficult decisions.
Challenges and Opportunities
This was a single-center experience caring for veterans. Challenges with having virtual huddles during the COVID-19 surge involved both time for the health care practitioners and technology. This was recognized early by the educational leaders at our facility, and headsets and cameras were purchased for the team rooms and made available as quickly as possible. Another limitation was the unpredictability and variability of patient volume for specific teams that sometimes would affect the efficiency of the huddle. The number of teams who attended the COVID-19 huddle was highest for the first 2 weeks (maximum of 9 teams) but declined to a nadir of 3 at the end of the month. This reflected the increase in knowledge about COVID-19 and respiratory disease that the teams acquired initially as well as a decline in COVID-19 patient admissions over those weeks.
The COVID-19 Tele-Huddle Program model also can be expanded to include other frontline clinicians, including nurses and respiratory therapists. For example, case management huddles were performed in a similar way during the COVID-19 surge to allow for efficient and effective multidisciplinary conversations about patients
Conclusions
Given the rise of telemedicine and availability of video conferencing services, virtual huddles can be implemented in institutions with appropriate staff and remote access to health records. Multidisciplinary consultation services using video conferencing can serve as an adjunct to the traditional, in-person consultation service model for patients with complex needs.
Acknowledgments
The authors acknowledge all of the Baylor Internal Medicine house staff and internal medicine attendings who participated in our huddle and more importantly, cared for our veterans during this COVID-19 surge.
1. Heymann DL, Shindo N; WHO Scientific and Technical Advisory Group for Infectious Hazards. COVID-19: what is next for public health?. Lancet. 2020;395(10224):542-545. doi:10.1016/S0140-6736(20)30374-3
2. Dichter JR, Kanter RK, Dries D, et al; Task Force for Mass Critical Care. System-level planning, coordination, and communication: care of the critically ill and injured during pandemics and disasters: CHEST consensus statement. Chest. 2014;146(suppl 4):e87S-e102S. doi:10.1378/chest.14-0738
3. Chowdhury JM, Patel M, Zheng M, Abramian O, Criner GJ. Mobilization and preparation of a large urban academic center during the COVID-19 pandemic. Ann Am Thorac Soc. 2020;17(8):922-925. doi:10.1513/AnnalsATS.202003-259PS
4. Uppal A, Silvestri DM, Siegler M, et al. Critical care and emergency department response at the epicenter of the COVID-19 pandemic. Health Aff (Millwood). 2020;39(8):1443-1449. doi:10.1377/hlthaff.2020.00901
5. US Department of Veterans Affairs. Michael E. DeBakey VA Medical Center- Houston, Texas. Accessed December 10, 2020. https://www.houston.va.gov/about
6. Provost SM, Lanham HJ, Leykum LK, McDaniel RR Jr, Pugh J. Health care huddles: managing complexity to achieve high reliability. Health Care Manage Rev. 2015;40(1):2-12. doi:10.1097/HMR.0000000000000009
7. Franklin BJ, Gandhi TK, Bates DW, et al. Impact of multidisciplinary team huddles on patient safety: a systematic review and proposed taxonomy. BMJ Qual Saf. 2020;29(10):1-2. doi:10.1136/bmjqs-2019-009911
8. Goldenhar LM, Brady PW, Sutcliffe KM, Muething SE. Huddling for high reliability and situation awareness. BMJ Qual Saf. 2013;22(11):899-906. doi:10.1136/bmjqs-2012-001467
1. Heymann DL, Shindo N; WHO Scientific and Technical Advisory Group for Infectious Hazards. COVID-19: what is next for public health?. Lancet. 2020;395(10224):542-545. doi:10.1016/S0140-6736(20)30374-3
2. Dichter JR, Kanter RK, Dries D, et al; Task Force for Mass Critical Care. System-level planning, coordination, and communication: care of the critically ill and injured during pandemics and disasters: CHEST consensus statement. Chest. 2014;146(suppl 4):e87S-e102S. doi:10.1378/chest.14-0738
3. Chowdhury JM, Patel M, Zheng M, Abramian O, Criner GJ. Mobilization and preparation of a large urban academic center during the COVID-19 pandemic. Ann Am Thorac Soc. 2020;17(8):922-925. doi:10.1513/AnnalsATS.202003-259PS
4. Uppal A, Silvestri DM, Siegler M, et al. Critical care and emergency department response at the epicenter of the COVID-19 pandemic. Health Aff (Millwood). 2020;39(8):1443-1449. doi:10.1377/hlthaff.2020.00901
5. US Department of Veterans Affairs. Michael E. DeBakey VA Medical Center- Houston, Texas. Accessed December 10, 2020. https://www.houston.va.gov/about
6. Provost SM, Lanham HJ, Leykum LK, McDaniel RR Jr, Pugh J. Health care huddles: managing complexity to achieve high reliability. Health Care Manage Rev. 2015;40(1):2-12. doi:10.1097/HMR.0000000000000009
7. Franklin BJ, Gandhi TK, Bates DW, et al. Impact of multidisciplinary team huddles on patient safety: a systematic review and proposed taxonomy. BMJ Qual Saf. 2020;29(10):1-2. doi:10.1136/bmjqs-2019-009911
8. Goldenhar LM, Brady PW, Sutcliffe KM, Muething SE. Huddling for high reliability and situation awareness. BMJ Qual Saf. 2013;22(11):899-906. doi:10.1136/bmjqs-2012-001467
Telehealth Pulmonary Rehabilitation for Patients With Severe Chronic Obstructive Pulmonary Disease
According to World Health Organization estimates, 65 million people have moderate-to-severe chronic obstructive pulmonary disease (COPD) globally, and > 20 million patients with COPD are living in the US.1 COPD is a progressive respiratory disease with a poor prognosis and a significant cause of morbidity and mortality in the US, especially within the Veterans Health Administration (VHA).2 The prevalence of COPD is higher in veterans than it is in the general population. COPD prevalence in the adult US population has been estimated to be between 5% and 15%, whereas in veterans, prevalence estimates have ranged from about 5% to 43%.3-5
COPD is associated with disabling dyspnea, muscle weakness, exercise intolerance, morbidity, and mortality. These symptoms and complications gradually and progressively compromise mobility, ability to perform daily functions, and decrease quality of life (QOL). Dyspnea, fatigue, and discomfort are the principal symptoms that negatively impact exercise tolerance.6,7 Therefore, patients often intentionally limit their activities to avoid these uncomfortable feelings and adopt a more sedentary behavior. As the disease progresses, individuals with COPD will gradually need assistance in performing activities of daily living, which eventually leads to functional dependence.
Pulmonary rehabilitation (PR) is an essential component of the management of symptomatic patients with COPD. PR is an evidence-based, multidisciplinary, comprehensive intervention that includes exercise and education for patients with chronic respiratory disease.8 The key benefits of PR are clinical improvements in dyspnea, physical capacity, QOL, and reduced disability in patients with COPD and other respiratory diseases.9-11 PR was found to improve respiratory health in veterans with COPD and decrease respiratory-related health care utilization.12
Despite the known benefits of PR, many patients with chronic respiratory diseases are not referred or do not have access to rehabilitation. Also, uptake of PR is low due to patient frailty, transportation issues, and other health care access problems.13-15 Unfortunately, in the US health care system, access to PR and other nonpharmacologic treatments can be challenging due to a shortage of available PR programs, limited physician referral to existing programs, and lack of family and social support.16
There are only a few accredited PR programs in VHA facilities, and they tend to be located in urban areas.12,17 Many patients have limited access to the PR programs due to geographic distance to the programs and transportation challenges (eg, limited ability to drive, cost of transportation). Moreover, veterans with COPD are likely to have limited mobility or are homebound due to experiencing shortness of breath with minimal exertion. Given the clear benefits of PR and the increasing impact of COPD on morbidity and mortality of the patients with COPD, strategies to improve the access and capacity of PR are needed. VA telehealth services allow for distribution of health care services in different geographic locations by providing access for the veterans who live in rural and highly rural areas. The most recent implementation of VA Video Connect (VVC) by the VHA provides a new avenue for clinicians to deliver much needed medical care into the veterans’ home.
COPD Telehealth Program
In this article, we describe the processes for developing and delivering an in-home, interactive, supervised PR program for veterans with severe COPD through VA telehealth service. The program consists of 18 sessions delivered over 6 weeks by a licensed physical therapist (PT) and a respiratory therapist (RT). The aims of the telehealth PR are to improve exercise tolerance, reduce dyspnea and fatigue, improve QOL, improve accessibility, and decrease costs and transportation burdens for patients with COPD. The program was developed, implemented and delivered by an interdisciplinary team, including a pulmonologist, PT, RT, physiatrist, and nonclinical supporting staff.
Patient Assessment
To be eligible to participate in the program the patient must: (1) have a forced expiratory volume (FEV1) < 60%; ( 2) be medically stable and be receiving optimal medical management; (3) have no severe cognitive impairments; (4) be able to use a computer and e-mail; (5) be able to ambulate with or without a walking device; (6) be willing to enroll in a smoking cessation program or to stop smoking; (7) be willing to participate without prolonged interruption; and (8) have all visual and auditory impairments corrected with medical devices.
After referral and enrollment, patients receive medical and physical examinations by the PR team, including a pulmonologist, a PT, and a RT, to ensure that the patients are medically stable to undergo rehabilitation and to develop a tailored exercise program while being mindful of the comorbidities, limitations, and precautions, (eg, loss of balance, risk of fall, limited range of motion). The preprogram assessment includes a pulmonary function test, arterial blood gas test, Montreal Cognitive Assessment, Modified Medical Research Council Scale, St. George Respiratory Questionnaire, the COPD Assessment Test, Patient Health Questionnaire-9,Generalized Anxiety Disorder Assessment-7, Epworth Sleepiness Scale, Katz Index of Independence of Activities of Daily Living, medications and inhaler use, oxygen use, breathing pattern, coughing, 6-minute walk test, Modified Borg Dyspnea Scale, grip strength, 5 Times Sit to Stand Test, manual muscle test, gait measure, Timed Up & Go test, clinical balance tests, range of motion, flexibility, sensation, pain, and fall history.18-32 Educational needs (eg, respiratory hygiene, nutrition, infection control, sleep, disease/symptom management) also are evaluated.
This thorough assessment is performed in a face-to-face outpatient visit. During the program participation, a physiatrist may be consulted for additional needs (eg, wheelchair assessment, home safety evaluation/ modifications, and mobility/disability issues). After completing the 6-week program, patients are scheduled for the postprogram evaluation in a face-to-face outpatient visit with the clinicians.
Equipment
Both clinician and the patient are equipped with a computer with Wi-Fi connectivity, a webcam, and a microphone. Patients are provided an exercise pictorial booklet, an exercise compact disk (audio and video), small exercise apparatuses (eg, assorted colors of resistance bands, hand grip exerciser, hand putty, ergometer, harmonica, and pedometer), incentive spirometer, pulse oximeter, cough assistive device (as needed), blood pressure monitor, COPD information booklets, and a diary to use at home during the program.33
Technology Preparation
Prior to starting the telehealth program, the patient is contacted 1 or 2 days before the first session for technical preparation and familiarization of the VA telehealth connection process. Either the PT or RT provides step-by-step instructions for the patient to practice connecting through VVC during this preparatory phone call. The patient also practices using the computer webcam, speaker, and microphone; checks the telehealth scheduling e-mail; and learns how to solve possible common technical issues (eg, adjusting volume and position of webcam). The patient is asked to set up a table close to the computer and to place all exercise apparatuses and respiratory devices on the table surface.
Program Delivery
A secure online VVC is used for connection during the telehealth session. The patient received an e-mail from the telehealth scheduling system with a link for VVC before each session. During the 6-week program, each telehealth session is conducted by a PT and a RT concurrently for 120 minutes, 3 days per week. The PT provides exercises for the patient to attempt, and the RT provides breathing training and monitoring during the session. After a successful connection to VVC, the therapist verifies the patient’s identity and confirms patient consent for the telehealth session.
After this check-in process, the patient performs a self-measure of resting blood pressure (BP), heart rate, respiratory rate, and blood oxygen saturation and reports to the therapists. During the exercise session, fatigue/exertion, dyspnea (Modified Borg Dyspnea Scale; Borg CR10 Scale), BP, heart rate, oxygen saturation, and other clinical symptoms and responses to exercise are monitored by the therapists, using both patient-reported measures and clinical observation by the therapists.34,35 Any medical emergency during the session is reported immediately to the pulmonologist for further management.
Structure
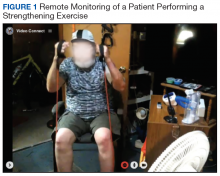
Prior to each exercise session, exercise precautions, fall prevention, good posture, pursed-lip breathing, pacing, and coordinated breathing are discussed with the patient. The PT demonstrates stretching and warm-up exercises in front of the webcam for the patient to follow. Then the patient performs all exercises in view of the webcam during the session (Figure 1). A RT monitors breathing patterns and corrects with verbal instructions if not properly performed.
Loss of skeletal muscle mass and cachexia are highly prevalent comorbidities of COPD and have been associated with breathlessness, functional limitation, and poor prognosis.36 To address these comorbidities, our program consists of progressive strengthening, aerobic, balance, and flexibility exercises. Resistance bands and tubes are used for strengthening exercises. Callisthenic exercises (eg, chair squat, chair stand, knee marching, bridging, single limb stances, and lunge) are used for progressive strengthening and balance exercises. Progression of strengthening and balance exercises are done through increasing the volume of exercise (ie, numbers of sets and repetitions) and increased load and level of difficulty based on the patient’s progress and comorbidity. The exercise program focuses on strengthening muscles, especially large muscle groups, to improve overall muscle strength and performance of functional activities.37
Arm/pedal ergometer and daily walking are used for daily aerobic exercise. In a study of patients with COPD by the PAC-COPD Study Group, step counter use was found to increase physical activity and improve exercise capacity, which supports its use in COPD management.38 During program participation, the patient is asked to wear a pedometer to monitor the number of steps taken per day and to report step data to the therapists during the telehealth session. The pedometer stores the previous 41 calendar days of data and displays the most recent 7 calendar days of data.
The patient is encouraged to set a realistic daily step goal. The general program goal is to increase at least 1000 steps per day. However, this goal can be adjusted depending on the patient’s health status and comorbid conditions. The PAC-COPD Study Group found that for every additional 1000 daily steps at low intensity, COPD hospitalization risk decreased by 20%.39 A magnitude of 2000 steps or about 1 mile of walking per day was found to be associated with increased physical activity and health benefits in the general population.40
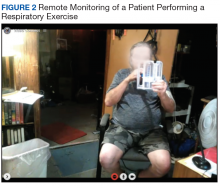
Respiratory muscle training and breathing exercise are provided by the RT, using breathing and incentive spirometer techniques (Figure 2). Huff coughing, diaphragmatic deep breathing, and pursed-lip breathing are instructed by the RT during the session. Effective coughing technique with a cough assistive device is also provided during breathing training if needed.
Patient Education
In patients with COPD, there are numerous positive health benefits associated with education, including assisting the patients to become active participants in the PR program leading to satisfying outcomes; assisting the patients to better understand the lung health, disease processes, physical and psychological changes that occur with COPD; assisting the patients to explore coping strategies for those changes; building lifelong behavioral changes; and developing the self-management skills for sustainability. Through the educational process, patients with COPD can become more skilled at collaborative self-management and improve adherence to their treatment plan, which in turn can result in a reduction in hospital admissions and reduced health care costs.8,41
Education is provided with every session after the patient completes the exercise. Patients are required to record their COPD symptoms, daily activity, home exercise program, sleep, food intake, and additional physical or social activity in their COPD diary and to report during the session (Figure 3). A COPD diary assists patients in self-monitoring their COPD symptoms and provides the therapists with information about clinical changes, behavioral changes, and/or specific unmet needs for education. Several topics related to COPD are included in the education session: lung or respiratory disease/condition and self-management; smoking cessation; physical activity; energy-conserving techniques; breathing and coughing techniques; smoking cessation; nutrition/healthy eating and weight counseling; sex and intimacy; psychological counseling and/or group support; emergency planning (eg, medical, travel, and inclement weather); correct use of inhaler and medications; home oxygen; sleep and sleep hygiene; palliative care and advanced directive; infection control; and sputum clearance.42,43
Program Maintenance
After successfully completing the 6-week program, patients are referred to the VA TeleMOVE! Program or MOVE! Weight Management Program for continuous, long-term management of weight, nutrition, physical activity/exercise, and social activity needs or goals. The patients are scheduled for monthly follow-up phone visits for 6 months with the telerehabilitation team for enforcing sustainability. The phone call visit consists of reviewing breathing techniques, exercise program, physical activity, education, encouragement, and addressing any issues that arise during the self-maintained period.
Limitations
There are several issues of concern and precautions when delivering PR through telehealth into the home. First, the patient performs exercises independently without being manually guarded by the therapists. Risk of falls are a major concern due to impaired balance, poor vision, and other possible unusual physiologic responses to exercise (eg, drop in BP, dizziness, loss of balance). The area in front of the computer needs to be cleared of fall hazards (ie, area rug, wires, objects on the floor). The patient also needs to be educated on self-measurements of BP and oxygen saturation and reports to the therapists. The therapists provide detailed instructions on how to obtain these measures correctly; otherwise, the values may not be valid for a clinical judgment during the exercise session or for other clinical management. In a home environment, there is a limited use of exercise apparatuses. For this program, we only used resistance bands/tubes, small arm/leg ergometer, hand grip, and hand putty for the exercise program. We feel that dumbbell and weight plates are not suitable due to a possible risk of injury if the patient accidently drops them.
Advanced balance training is not suitable due to an increased risk for falls. Without the presence of the PT, level of challenge/difficulty is somewhat limited for this telehealth supervision exercise program. In addition, visual and audio quality are necessary for the session. The patient and the therapists need to see each other clearly to ensure correct methods and forms of each exercise. Furthermore, rehearsal of technical skills with the therapists is very important because this population is older and often has limited computer skills. Any technical difficulty or failure can lead to undesirable situations (eg, anxiety episodes, worries, shortness of breath, upset), which compromise exercise performance during the session. Finally, a phone is needed as an alternative in case of a poor VVC connection.
Conclusion
COPD symptoms and complications greatly affect patients’ ability to perform daily activities, decrease QOL and functional ability, and result in extensive use of health services. Many patients have limited access to a PR program at hospitals or rehabilitation centers due to health conditions, lack of transportation, and/or family support. This home-based, interactive telehealth PR program can break down the geographic barriers, solve poor program accessibility, potentially increase the utilization of PR, and reduce the cost and travel required by the patients.
Acknowledgments
The Telehealth Pulmonary Rehabilitation Program was originally funded by the Veterans Health Administration VA ACCESS Program (AS, CL, HKH). We thank all the veterans for their time and effort in participating in this newly developed rehabilitation program.
1. World Health Organization. Chronic obstructive pulmonary disease (COPD). http://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd). Published December 1, 2017. Accessed August 7, 2019.
2. Yu W, Ravelo A, Wagner TH, et al. Prevalence and costs of chronic conditions in the VA health care system. Med Care Res Rev. 2003;60(suppl 3):146S-167S.
3. Doney B, Hnizdo E, Dillon CF, et al. Prevalence of airflow obstruction in U.S. adults aged 40-79 years: NHANES data 1988-1994 and 2007-2010. COPD. 2015;12(4):355-365.
4. Murphy DE, Chaudhry Z, Almoosa KF, Panos RJ. High prevalence of chronic obstructive pulmonary disease among veterans in the urban midwest. Mil Med. 2011;176(5):552-560.
5. Cypel YS, Hines SE, Davey VJ, Eber SM, Schneiderman AI. Self-reported physician-diagnosed chronic obstructive pulmonary disease and spirometry patterns in Vietnam Era US Army Chemical Corps veterans: a retrospective cohort study. Am J Ind Med. 2018;61(10):802-814.
6. Rochester CL. Exercise training in chronic obstructive pulmonary disease. J Rehabil Res Dev. 2003;40(5)(suppl 2):59-80.
7. Cortopassi F, Gurung P, Pinto-Plata V. Chronic obstructive pulmonary disease in elderly patients. Clin Geriatr Med. 2017;33(4):539-552.
8. Spruit MA, Singh SJ, Garvey C, et al; ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13-e64.
9. Robinson H, Williams V, Curtis F, Bridle C, Jones AW. Facilitators and barriers to physical activity following pulmonary rehabilitation in COPD: a systematic review of qualitative studies. NPJ Prim Care Respir Med. 2018;28(1):19.
10. McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;(2):CD003793.
11. Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary rehabilitation: joint AACP/AACVPR evidence-based clinical practice guidelines. Chest. 2007;131(suppl 5):4S-42S.
12. Major S, Moreno M, Shelton J, Panos RJ. Veterans with chronic obstructive pulmonary disease achieve clinically relevant improvements in respiratory health after pulmonary rehabilitation. J Cardiopulm Rehabil Prev. 2014;34(6):420-429.
13. Liu Y, Dickerson T, Early F, Fuld J, Clarkson PJ. Understanding influences on the uptake of pulmonary rehabilitation in the East of England: an inclusive design/mixed methods study protocol. BMJ Open. 2018;8(4):e020750.
14. Harris D, Hayter M, Allender S. Factors affecting the offer of pulmonary rehabilitation to patients with chronic obstructive pulmonary disease by primary care professionals: a qualitative study. Prim Health Care Res Dev. 2008;9(4):280-290.
15. Mathar H, Fastholm P, Hansen IR, Larsen NS. Why do patients with COPD decline rehabilitation. Scand J Caring Sci. 2016;30(3):432-441.
16. Han MK, Martinez CH, Au DH, et al. Meeting the challenge of COPD care delivery in the USA: a multiprovider perspective. Lancet Respir Med. 2016;4(6):473-526.
17. American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR). Online searchable program directory. https://www.aacvpr.org/Resources/Program-Directory Accessed July 19, 2018.
18. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699.
19. Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J. 1959;2(5147):257-266.
20. O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2007 update. Can Respir J. 2007;14(suppl B):5B-32B.
21. Jones PW, Quirk FH, Baveystock CM. The St George’s Respiratory Questionnaire. Respir Med. 1991;85(suppl B):25-31.
22. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648-654.
23. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
24. Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097.
25. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540-545.
26. Katz S. Assessing self-maintenance: activities of daily living, mobility and instrumental activities of daily living. J Am Geriatr Soc. 1983;31(12):721-727.
27. Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428-1446.
28. Mahler DA, Horowitz MB. Perception of breathlessness during exercise in patients with respiratory disease. Med Sci Sports Exerc. 1994;26(9):1078-1081.
29. Liao WC, Wang CH, Yu SY, Chen LY, Wang CY. Grip strength measurement in older adults in Taiwan: a comparison of three testing positions. Australas J Ageing. 2014;33(4):278-282.
30. Buatois S, Miljkovic D, Manckoundia P, et al. Five times sit to stand test is a predictor of recurrent falls in healthy community-living subjects aged 65 and older. J Am Geriatr Soc. 2008;56(8):1575-1577.
31. Bryant MS, Workman CD, Jackson GR. Multidirectional walk test in persons with Parkinson’s disease: a validity study. Int J Rehabil Res. 2015;38(1):88-91.
32. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142-148.
33. University of Nebraska Medical Center. Timed Up and Go (TUG) Test. https://www.unmc.edu/media/intmed/geriatrics/nebgec/pdf/frailelderlyjuly09/toolkits/timedupandgo_w_norms.pdf. Accessed August 13, 2019.
34. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377-381.
35. Mahler DA, Horowitz MB. Clinical evaluation of exertional dyspnea. Clin Chest Med. 1994;15(2):259-269.
36. Dudgeon D, Baracos VE. Physiological and functional failure in chronic obstructive pulmonary disease, congestive heart failure and cancer: a debilitating intersection of sarcopenia, cachexia and breathlessness. Curr Opin Support Palliat Care. 2016;10(3):236-241.
37. Lee AL, Holland AE. Time to adapt exercise training regimens in pulmonary rehabilitation—a review of the literature. Int J Chron Obstruct Pulmon Dis. 2014;9:1275-1288.
38. Qiu S, Cai X, Wang X, et al. Using step counters to promote physical activity and exercise capacity in patients with chronic obstructive pulmonary disease: a meta-analysis. Ther Adv Respir Dis. 2018;12:1753466618787386.
39. Donaire-Gonzalez D, Gimeno-Santos E, Balcells E, et al; PAC-COPD Study Group. Benefits of physical activity on COPD hospitalization depend on intensity. Eur Respir J. 2015;46(5):1281-1289.
40. Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298(19):2296-2304.
41. Zwerink M, Brusse-Keizer M, van der Valk PD, et al. Self-management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;19(3):CD002990.
42. Wilson JS, O’Neill B, Reilly J, MacMahon J, Bradley JM. Education in pulmonary rehabilitation: the patient’s perspective. Arch Phys Med Rehabil. 2007;88(12):1704-1709.
43. Bourbeau J, Nault D, Dang-Tan T. Self-management and behaviour modification in COPD. Patient Educ Couns. 2004;52(3):271-277.
According to World Health Organization estimates, 65 million people have moderate-to-severe chronic obstructive pulmonary disease (COPD) globally, and > 20 million patients with COPD are living in the US.1 COPD is a progressive respiratory disease with a poor prognosis and a significant cause of morbidity and mortality in the US, especially within the Veterans Health Administration (VHA).2 The prevalence of COPD is higher in veterans than it is in the general population. COPD prevalence in the adult US population has been estimated to be between 5% and 15%, whereas in veterans, prevalence estimates have ranged from about 5% to 43%.3-5
COPD is associated with disabling dyspnea, muscle weakness, exercise intolerance, morbidity, and mortality. These symptoms and complications gradually and progressively compromise mobility, ability to perform daily functions, and decrease quality of life (QOL). Dyspnea, fatigue, and discomfort are the principal symptoms that negatively impact exercise tolerance.6,7 Therefore, patients often intentionally limit their activities to avoid these uncomfortable feelings and adopt a more sedentary behavior. As the disease progresses, individuals with COPD will gradually need assistance in performing activities of daily living, which eventually leads to functional dependence.
Pulmonary rehabilitation (PR) is an essential component of the management of symptomatic patients with COPD. PR is an evidence-based, multidisciplinary, comprehensive intervention that includes exercise and education for patients with chronic respiratory disease.8 The key benefits of PR are clinical improvements in dyspnea, physical capacity, QOL, and reduced disability in patients with COPD and other respiratory diseases.9-11 PR was found to improve respiratory health in veterans with COPD and decrease respiratory-related health care utilization.12
Despite the known benefits of PR, many patients with chronic respiratory diseases are not referred or do not have access to rehabilitation. Also, uptake of PR is low due to patient frailty, transportation issues, and other health care access problems.13-15 Unfortunately, in the US health care system, access to PR and other nonpharmacologic treatments can be challenging due to a shortage of available PR programs, limited physician referral to existing programs, and lack of family and social support.16
There are only a few accredited PR programs in VHA facilities, and they tend to be located in urban areas.12,17 Many patients have limited access to the PR programs due to geographic distance to the programs and transportation challenges (eg, limited ability to drive, cost of transportation). Moreover, veterans with COPD are likely to have limited mobility or are homebound due to experiencing shortness of breath with minimal exertion. Given the clear benefits of PR and the increasing impact of COPD on morbidity and mortality of the patients with COPD, strategies to improve the access and capacity of PR are needed. VA telehealth services allow for distribution of health care services in different geographic locations by providing access for the veterans who live in rural and highly rural areas. The most recent implementation of VA Video Connect (VVC) by the VHA provides a new avenue for clinicians to deliver much needed medical care into the veterans’ home.
COPD Telehealth Program
In this article, we describe the processes for developing and delivering an in-home, interactive, supervised PR program for veterans with severe COPD through VA telehealth service. The program consists of 18 sessions delivered over 6 weeks by a licensed physical therapist (PT) and a respiratory therapist (RT). The aims of the telehealth PR are to improve exercise tolerance, reduce dyspnea and fatigue, improve QOL, improve accessibility, and decrease costs and transportation burdens for patients with COPD. The program was developed, implemented and delivered by an interdisciplinary team, including a pulmonologist, PT, RT, physiatrist, and nonclinical supporting staff.
Patient Assessment
To be eligible to participate in the program the patient must: (1) have a forced expiratory volume (FEV1) < 60%; ( 2) be medically stable and be receiving optimal medical management; (3) have no severe cognitive impairments; (4) be able to use a computer and e-mail; (5) be able to ambulate with or without a walking device; (6) be willing to enroll in a smoking cessation program or to stop smoking; (7) be willing to participate without prolonged interruption; and (8) have all visual and auditory impairments corrected with medical devices.
After referral and enrollment, patients receive medical and physical examinations by the PR team, including a pulmonologist, a PT, and a RT, to ensure that the patients are medically stable to undergo rehabilitation and to develop a tailored exercise program while being mindful of the comorbidities, limitations, and precautions, (eg, loss of balance, risk of fall, limited range of motion). The preprogram assessment includes a pulmonary function test, arterial blood gas test, Montreal Cognitive Assessment, Modified Medical Research Council Scale, St. George Respiratory Questionnaire, the COPD Assessment Test, Patient Health Questionnaire-9,Generalized Anxiety Disorder Assessment-7, Epworth Sleepiness Scale, Katz Index of Independence of Activities of Daily Living, medications and inhaler use, oxygen use, breathing pattern, coughing, 6-minute walk test, Modified Borg Dyspnea Scale, grip strength, 5 Times Sit to Stand Test, manual muscle test, gait measure, Timed Up & Go test, clinical balance tests, range of motion, flexibility, sensation, pain, and fall history.18-32 Educational needs (eg, respiratory hygiene, nutrition, infection control, sleep, disease/symptom management) also are evaluated.
This thorough assessment is performed in a face-to-face outpatient visit. During the program participation, a physiatrist may be consulted for additional needs (eg, wheelchair assessment, home safety evaluation/ modifications, and mobility/disability issues). After completing the 6-week program, patients are scheduled for the postprogram evaluation in a face-to-face outpatient visit with the clinicians.
Equipment
Both clinician and the patient are equipped with a computer with Wi-Fi connectivity, a webcam, and a microphone. Patients are provided an exercise pictorial booklet, an exercise compact disk (audio and video), small exercise apparatuses (eg, assorted colors of resistance bands, hand grip exerciser, hand putty, ergometer, harmonica, and pedometer), incentive spirometer, pulse oximeter, cough assistive device (as needed), blood pressure monitor, COPD information booklets, and a diary to use at home during the program.33
Technology Preparation
Prior to starting the telehealth program, the patient is contacted 1 or 2 days before the first session for technical preparation and familiarization of the VA telehealth connection process. Either the PT or RT provides step-by-step instructions for the patient to practice connecting through VVC during this preparatory phone call. The patient also practices using the computer webcam, speaker, and microphone; checks the telehealth scheduling e-mail; and learns how to solve possible common technical issues (eg, adjusting volume and position of webcam). The patient is asked to set up a table close to the computer and to place all exercise apparatuses and respiratory devices on the table surface.
Program Delivery
A secure online VVC is used for connection during the telehealth session. The patient received an e-mail from the telehealth scheduling system with a link for VVC before each session. During the 6-week program, each telehealth session is conducted by a PT and a RT concurrently for 120 minutes, 3 days per week. The PT provides exercises for the patient to attempt, and the RT provides breathing training and monitoring during the session. After a successful connection to VVC, the therapist verifies the patient’s identity and confirms patient consent for the telehealth session.
After this check-in process, the patient performs a self-measure of resting blood pressure (BP), heart rate, respiratory rate, and blood oxygen saturation and reports to the therapists. During the exercise session, fatigue/exertion, dyspnea (Modified Borg Dyspnea Scale; Borg CR10 Scale), BP, heart rate, oxygen saturation, and other clinical symptoms and responses to exercise are monitored by the therapists, using both patient-reported measures and clinical observation by the therapists.34,35 Any medical emergency during the session is reported immediately to the pulmonologist for further management.
Structure
Prior to each exercise session, exercise precautions, fall prevention, good posture, pursed-lip breathing, pacing, and coordinated breathing are discussed with the patient. The PT demonstrates stretching and warm-up exercises in front of the webcam for the patient to follow. Then the patient performs all exercises in view of the webcam during the session (Figure 1). A RT monitors breathing patterns and corrects with verbal instructions if not properly performed.
Loss of skeletal muscle mass and cachexia are highly prevalent comorbidities of COPD and have been associated with breathlessness, functional limitation, and poor prognosis.36 To address these comorbidities, our program consists of progressive strengthening, aerobic, balance, and flexibility exercises. Resistance bands and tubes are used for strengthening exercises. Callisthenic exercises (eg, chair squat, chair stand, knee marching, bridging, single limb stances, and lunge) are used for progressive strengthening and balance exercises. Progression of strengthening and balance exercises are done through increasing the volume of exercise (ie, numbers of sets and repetitions) and increased load and level of difficulty based on the patient’s progress and comorbidity. The exercise program focuses on strengthening muscles, especially large muscle groups, to improve overall muscle strength and performance of functional activities.37
Arm/pedal ergometer and daily walking are used for daily aerobic exercise. In a study of patients with COPD by the PAC-COPD Study Group, step counter use was found to increase physical activity and improve exercise capacity, which supports its use in COPD management.38 During program participation, the patient is asked to wear a pedometer to monitor the number of steps taken per day and to report step data to the therapists during the telehealth session. The pedometer stores the previous 41 calendar days of data and displays the most recent 7 calendar days of data.
The patient is encouraged to set a realistic daily step goal. The general program goal is to increase at least 1000 steps per day. However, this goal can be adjusted depending on the patient’s health status and comorbid conditions. The PAC-COPD Study Group found that for every additional 1000 daily steps at low intensity, COPD hospitalization risk decreased by 20%.39 A magnitude of 2000 steps or about 1 mile of walking per day was found to be associated with increased physical activity and health benefits in the general population.40
Respiratory muscle training and breathing exercise are provided by the RT, using breathing and incentive spirometer techniques (Figure 2). Huff coughing, diaphragmatic deep breathing, and pursed-lip breathing are instructed by the RT during the session. Effective coughing technique with a cough assistive device is also provided during breathing training if needed.
Patient Education
In patients with COPD, there are numerous positive health benefits associated with education, including assisting the patients to become active participants in the PR program leading to satisfying outcomes; assisting the patients to better understand the lung health, disease processes, physical and psychological changes that occur with COPD; assisting the patients to explore coping strategies for those changes; building lifelong behavioral changes; and developing the self-management skills for sustainability. Through the educational process, patients with COPD can become more skilled at collaborative self-management and improve adherence to their treatment plan, which in turn can result in a reduction in hospital admissions and reduced health care costs.8,41
Education is provided with every session after the patient completes the exercise. Patients are required to record their COPD symptoms, daily activity, home exercise program, sleep, food intake, and additional physical or social activity in their COPD diary and to report during the session (Figure 3). A COPD diary assists patients in self-monitoring their COPD symptoms and provides the therapists with information about clinical changes, behavioral changes, and/or specific unmet needs for education. Several topics related to COPD are included in the education session: lung or respiratory disease/condition and self-management; smoking cessation; physical activity; energy-conserving techniques; breathing and coughing techniques; smoking cessation; nutrition/healthy eating and weight counseling; sex and intimacy; psychological counseling and/or group support; emergency planning (eg, medical, travel, and inclement weather); correct use of inhaler and medications; home oxygen; sleep and sleep hygiene; palliative care and advanced directive; infection control; and sputum clearance.42,43
Program Maintenance
After successfully completing the 6-week program, patients are referred to the VA TeleMOVE! Program or MOVE! Weight Management Program for continuous, long-term management of weight, nutrition, physical activity/exercise, and social activity needs or goals. The patients are scheduled for monthly follow-up phone visits for 6 months with the telerehabilitation team for enforcing sustainability. The phone call visit consists of reviewing breathing techniques, exercise program, physical activity, education, encouragement, and addressing any issues that arise during the self-maintained period.
Limitations
There are several issues of concern and precautions when delivering PR through telehealth into the home. First, the patient performs exercises independently without being manually guarded by the therapists. Risk of falls are a major concern due to impaired balance, poor vision, and other possible unusual physiologic responses to exercise (eg, drop in BP, dizziness, loss of balance). The area in front of the computer needs to be cleared of fall hazards (ie, area rug, wires, objects on the floor). The patient also needs to be educated on self-measurements of BP and oxygen saturation and reports to the therapists. The therapists provide detailed instructions on how to obtain these measures correctly; otherwise, the values may not be valid for a clinical judgment during the exercise session or for other clinical management. In a home environment, there is a limited use of exercise apparatuses. For this program, we only used resistance bands/tubes, small arm/leg ergometer, hand grip, and hand putty for the exercise program. We feel that dumbbell and weight plates are not suitable due to a possible risk of injury if the patient accidently drops them.
Advanced balance training is not suitable due to an increased risk for falls. Without the presence of the PT, level of challenge/difficulty is somewhat limited for this telehealth supervision exercise program. In addition, visual and audio quality are necessary for the session. The patient and the therapists need to see each other clearly to ensure correct methods and forms of each exercise. Furthermore, rehearsal of technical skills with the therapists is very important because this population is older and often has limited computer skills. Any technical difficulty or failure can lead to undesirable situations (eg, anxiety episodes, worries, shortness of breath, upset), which compromise exercise performance during the session. Finally, a phone is needed as an alternative in case of a poor VVC connection.
Conclusion
COPD symptoms and complications greatly affect patients’ ability to perform daily activities, decrease QOL and functional ability, and result in extensive use of health services. Many patients have limited access to a PR program at hospitals or rehabilitation centers due to health conditions, lack of transportation, and/or family support. This home-based, interactive telehealth PR program can break down the geographic barriers, solve poor program accessibility, potentially increase the utilization of PR, and reduce the cost and travel required by the patients.
Acknowledgments
The Telehealth Pulmonary Rehabilitation Program was originally funded by the Veterans Health Administration VA ACCESS Program (AS, CL, HKH). We thank all the veterans for their time and effort in participating in this newly developed rehabilitation program.
According to World Health Organization estimates, 65 million people have moderate-to-severe chronic obstructive pulmonary disease (COPD) globally, and > 20 million patients with COPD are living in the US.1 COPD is a progressive respiratory disease with a poor prognosis and a significant cause of morbidity and mortality in the US, especially within the Veterans Health Administration (VHA).2 The prevalence of COPD is higher in veterans than it is in the general population. COPD prevalence in the adult US population has been estimated to be between 5% and 15%, whereas in veterans, prevalence estimates have ranged from about 5% to 43%.3-5
COPD is associated with disabling dyspnea, muscle weakness, exercise intolerance, morbidity, and mortality. These symptoms and complications gradually and progressively compromise mobility, ability to perform daily functions, and decrease quality of life (QOL). Dyspnea, fatigue, and discomfort are the principal symptoms that negatively impact exercise tolerance.6,7 Therefore, patients often intentionally limit their activities to avoid these uncomfortable feelings and adopt a more sedentary behavior. As the disease progresses, individuals with COPD will gradually need assistance in performing activities of daily living, which eventually leads to functional dependence.
Pulmonary rehabilitation (PR) is an essential component of the management of symptomatic patients with COPD. PR is an evidence-based, multidisciplinary, comprehensive intervention that includes exercise and education for patients with chronic respiratory disease.8 The key benefits of PR are clinical improvements in dyspnea, physical capacity, QOL, and reduced disability in patients with COPD and other respiratory diseases.9-11 PR was found to improve respiratory health in veterans with COPD and decrease respiratory-related health care utilization.12
Despite the known benefits of PR, many patients with chronic respiratory diseases are not referred or do not have access to rehabilitation. Also, uptake of PR is low due to patient frailty, transportation issues, and other health care access problems.13-15 Unfortunately, in the US health care system, access to PR and other nonpharmacologic treatments can be challenging due to a shortage of available PR programs, limited physician referral to existing programs, and lack of family and social support.16
There are only a few accredited PR programs in VHA facilities, and they tend to be located in urban areas.12,17 Many patients have limited access to the PR programs due to geographic distance to the programs and transportation challenges (eg, limited ability to drive, cost of transportation). Moreover, veterans with COPD are likely to have limited mobility or are homebound due to experiencing shortness of breath with minimal exertion. Given the clear benefits of PR and the increasing impact of COPD on morbidity and mortality of the patients with COPD, strategies to improve the access and capacity of PR are needed. VA telehealth services allow for distribution of health care services in different geographic locations by providing access for the veterans who live in rural and highly rural areas. The most recent implementation of VA Video Connect (VVC) by the VHA provides a new avenue for clinicians to deliver much needed medical care into the veterans’ home.
COPD Telehealth Program
In this article, we describe the processes for developing and delivering an in-home, interactive, supervised PR program for veterans with severe COPD through VA telehealth service. The program consists of 18 sessions delivered over 6 weeks by a licensed physical therapist (PT) and a respiratory therapist (RT). The aims of the telehealth PR are to improve exercise tolerance, reduce dyspnea and fatigue, improve QOL, improve accessibility, and decrease costs and transportation burdens for patients with COPD. The program was developed, implemented and delivered by an interdisciplinary team, including a pulmonologist, PT, RT, physiatrist, and nonclinical supporting staff.
Patient Assessment
To be eligible to participate in the program the patient must: (1) have a forced expiratory volume (FEV1) < 60%; ( 2) be medically stable and be receiving optimal medical management; (3) have no severe cognitive impairments; (4) be able to use a computer and e-mail; (5) be able to ambulate with or without a walking device; (6) be willing to enroll in a smoking cessation program or to stop smoking; (7) be willing to participate without prolonged interruption; and (8) have all visual and auditory impairments corrected with medical devices.
After referral and enrollment, patients receive medical and physical examinations by the PR team, including a pulmonologist, a PT, and a RT, to ensure that the patients are medically stable to undergo rehabilitation and to develop a tailored exercise program while being mindful of the comorbidities, limitations, and precautions, (eg, loss of balance, risk of fall, limited range of motion). The preprogram assessment includes a pulmonary function test, arterial blood gas test, Montreal Cognitive Assessment, Modified Medical Research Council Scale, St. George Respiratory Questionnaire, the COPD Assessment Test, Patient Health Questionnaire-9,Generalized Anxiety Disorder Assessment-7, Epworth Sleepiness Scale, Katz Index of Independence of Activities of Daily Living, medications and inhaler use, oxygen use, breathing pattern, coughing, 6-minute walk test, Modified Borg Dyspnea Scale, grip strength, 5 Times Sit to Stand Test, manual muscle test, gait measure, Timed Up & Go test, clinical balance tests, range of motion, flexibility, sensation, pain, and fall history.18-32 Educational needs (eg, respiratory hygiene, nutrition, infection control, sleep, disease/symptom management) also are evaluated.
This thorough assessment is performed in a face-to-face outpatient visit. During the program participation, a physiatrist may be consulted for additional needs (eg, wheelchair assessment, home safety evaluation/ modifications, and mobility/disability issues). After completing the 6-week program, patients are scheduled for the postprogram evaluation in a face-to-face outpatient visit with the clinicians.
Equipment
Both clinician and the patient are equipped with a computer with Wi-Fi connectivity, a webcam, and a microphone. Patients are provided an exercise pictorial booklet, an exercise compact disk (audio and video), small exercise apparatuses (eg, assorted colors of resistance bands, hand grip exerciser, hand putty, ergometer, harmonica, and pedometer), incentive spirometer, pulse oximeter, cough assistive device (as needed), blood pressure monitor, COPD information booklets, and a diary to use at home during the program.33
Technology Preparation
Prior to starting the telehealth program, the patient is contacted 1 or 2 days before the first session for technical preparation and familiarization of the VA telehealth connection process. Either the PT or RT provides step-by-step instructions for the patient to practice connecting through VVC during this preparatory phone call. The patient also practices using the computer webcam, speaker, and microphone; checks the telehealth scheduling e-mail; and learns how to solve possible common technical issues (eg, adjusting volume and position of webcam). The patient is asked to set up a table close to the computer and to place all exercise apparatuses and respiratory devices on the table surface.
Program Delivery
A secure online VVC is used for connection during the telehealth session. The patient received an e-mail from the telehealth scheduling system with a link for VVC before each session. During the 6-week program, each telehealth session is conducted by a PT and a RT concurrently for 120 minutes, 3 days per week. The PT provides exercises for the patient to attempt, and the RT provides breathing training and monitoring during the session. After a successful connection to VVC, the therapist verifies the patient’s identity and confirms patient consent for the telehealth session.
After this check-in process, the patient performs a self-measure of resting blood pressure (BP), heart rate, respiratory rate, and blood oxygen saturation and reports to the therapists. During the exercise session, fatigue/exertion, dyspnea (Modified Borg Dyspnea Scale; Borg CR10 Scale), BP, heart rate, oxygen saturation, and other clinical symptoms and responses to exercise are monitored by the therapists, using both patient-reported measures and clinical observation by the therapists.34,35 Any medical emergency during the session is reported immediately to the pulmonologist for further management.
Structure
Prior to each exercise session, exercise precautions, fall prevention, good posture, pursed-lip breathing, pacing, and coordinated breathing are discussed with the patient. The PT demonstrates stretching and warm-up exercises in front of the webcam for the patient to follow. Then the patient performs all exercises in view of the webcam during the session (Figure 1). A RT monitors breathing patterns and corrects with verbal instructions if not properly performed.
Loss of skeletal muscle mass and cachexia are highly prevalent comorbidities of COPD and have been associated with breathlessness, functional limitation, and poor prognosis.36 To address these comorbidities, our program consists of progressive strengthening, aerobic, balance, and flexibility exercises. Resistance bands and tubes are used for strengthening exercises. Callisthenic exercises (eg, chair squat, chair stand, knee marching, bridging, single limb stances, and lunge) are used for progressive strengthening and balance exercises. Progression of strengthening and balance exercises are done through increasing the volume of exercise (ie, numbers of sets and repetitions) and increased load and level of difficulty based on the patient’s progress and comorbidity. The exercise program focuses on strengthening muscles, especially large muscle groups, to improve overall muscle strength and performance of functional activities.37
Arm/pedal ergometer and daily walking are used for daily aerobic exercise. In a study of patients with COPD by the PAC-COPD Study Group, step counter use was found to increase physical activity and improve exercise capacity, which supports its use in COPD management.38 During program participation, the patient is asked to wear a pedometer to monitor the number of steps taken per day and to report step data to the therapists during the telehealth session. The pedometer stores the previous 41 calendar days of data and displays the most recent 7 calendar days of data.
The patient is encouraged to set a realistic daily step goal. The general program goal is to increase at least 1000 steps per day. However, this goal can be adjusted depending on the patient’s health status and comorbid conditions. The PAC-COPD Study Group found that for every additional 1000 daily steps at low intensity, COPD hospitalization risk decreased by 20%.39 A magnitude of 2000 steps or about 1 mile of walking per day was found to be associated with increased physical activity and health benefits in the general population.40
Respiratory muscle training and breathing exercise are provided by the RT, using breathing and incentive spirometer techniques (Figure 2). Huff coughing, diaphragmatic deep breathing, and pursed-lip breathing are instructed by the RT during the session. Effective coughing technique with a cough assistive device is also provided during breathing training if needed.
Patient Education
In patients with COPD, there are numerous positive health benefits associated with education, including assisting the patients to become active participants in the PR program leading to satisfying outcomes; assisting the patients to better understand the lung health, disease processes, physical and psychological changes that occur with COPD; assisting the patients to explore coping strategies for those changes; building lifelong behavioral changes; and developing the self-management skills for sustainability. Through the educational process, patients with COPD can become more skilled at collaborative self-management and improve adherence to their treatment plan, which in turn can result in a reduction in hospital admissions and reduced health care costs.8,41
Education is provided with every session after the patient completes the exercise. Patients are required to record their COPD symptoms, daily activity, home exercise program, sleep, food intake, and additional physical or social activity in their COPD diary and to report during the session (Figure 3). A COPD diary assists patients in self-monitoring their COPD symptoms and provides the therapists with information about clinical changes, behavioral changes, and/or specific unmet needs for education. Several topics related to COPD are included in the education session: lung or respiratory disease/condition and self-management; smoking cessation; physical activity; energy-conserving techniques; breathing and coughing techniques; smoking cessation; nutrition/healthy eating and weight counseling; sex and intimacy; psychological counseling and/or group support; emergency planning (eg, medical, travel, and inclement weather); correct use of inhaler and medications; home oxygen; sleep and sleep hygiene; palliative care and advanced directive; infection control; and sputum clearance.42,43
Program Maintenance
After successfully completing the 6-week program, patients are referred to the VA TeleMOVE! Program or MOVE! Weight Management Program for continuous, long-term management of weight, nutrition, physical activity/exercise, and social activity needs or goals. The patients are scheduled for monthly follow-up phone visits for 6 months with the telerehabilitation team for enforcing sustainability. The phone call visit consists of reviewing breathing techniques, exercise program, physical activity, education, encouragement, and addressing any issues that arise during the self-maintained period.
Limitations
There are several issues of concern and precautions when delivering PR through telehealth into the home. First, the patient performs exercises independently without being manually guarded by the therapists. Risk of falls are a major concern due to impaired balance, poor vision, and other possible unusual physiologic responses to exercise (eg, drop in BP, dizziness, loss of balance). The area in front of the computer needs to be cleared of fall hazards (ie, area rug, wires, objects on the floor). The patient also needs to be educated on self-measurements of BP and oxygen saturation and reports to the therapists. The therapists provide detailed instructions on how to obtain these measures correctly; otherwise, the values may not be valid for a clinical judgment during the exercise session or for other clinical management. In a home environment, there is a limited use of exercise apparatuses. For this program, we only used resistance bands/tubes, small arm/leg ergometer, hand grip, and hand putty for the exercise program. We feel that dumbbell and weight plates are not suitable due to a possible risk of injury if the patient accidently drops them.
Advanced balance training is not suitable due to an increased risk for falls. Without the presence of the PT, level of challenge/difficulty is somewhat limited for this telehealth supervision exercise program. In addition, visual and audio quality are necessary for the session. The patient and the therapists need to see each other clearly to ensure correct methods and forms of each exercise. Furthermore, rehearsal of technical skills with the therapists is very important because this population is older and often has limited computer skills. Any technical difficulty or failure can lead to undesirable situations (eg, anxiety episodes, worries, shortness of breath, upset), which compromise exercise performance during the session. Finally, a phone is needed as an alternative in case of a poor VVC connection.
Conclusion
COPD symptoms and complications greatly affect patients’ ability to perform daily activities, decrease QOL and functional ability, and result in extensive use of health services. Many patients have limited access to a PR program at hospitals or rehabilitation centers due to health conditions, lack of transportation, and/or family support. This home-based, interactive telehealth PR program can break down the geographic barriers, solve poor program accessibility, potentially increase the utilization of PR, and reduce the cost and travel required by the patients.
Acknowledgments
The Telehealth Pulmonary Rehabilitation Program was originally funded by the Veterans Health Administration VA ACCESS Program (AS, CL, HKH). We thank all the veterans for their time and effort in participating in this newly developed rehabilitation program.
1. World Health Organization. Chronic obstructive pulmonary disease (COPD). http://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd). Published December 1, 2017. Accessed August 7, 2019.
2. Yu W, Ravelo A, Wagner TH, et al. Prevalence and costs of chronic conditions in the VA health care system. Med Care Res Rev. 2003;60(suppl 3):146S-167S.
3. Doney B, Hnizdo E, Dillon CF, et al. Prevalence of airflow obstruction in U.S. adults aged 40-79 years: NHANES data 1988-1994 and 2007-2010. COPD. 2015;12(4):355-365.
4. Murphy DE, Chaudhry Z, Almoosa KF, Panos RJ. High prevalence of chronic obstructive pulmonary disease among veterans in the urban midwest. Mil Med. 2011;176(5):552-560.
5. Cypel YS, Hines SE, Davey VJ, Eber SM, Schneiderman AI. Self-reported physician-diagnosed chronic obstructive pulmonary disease and spirometry patterns in Vietnam Era US Army Chemical Corps veterans: a retrospective cohort study. Am J Ind Med. 2018;61(10):802-814.
6. Rochester CL. Exercise training in chronic obstructive pulmonary disease. J Rehabil Res Dev. 2003;40(5)(suppl 2):59-80.
7. Cortopassi F, Gurung P, Pinto-Plata V. Chronic obstructive pulmonary disease in elderly patients. Clin Geriatr Med. 2017;33(4):539-552.
8. Spruit MA, Singh SJ, Garvey C, et al; ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13-e64.
9. Robinson H, Williams V, Curtis F, Bridle C, Jones AW. Facilitators and barriers to physical activity following pulmonary rehabilitation in COPD: a systematic review of qualitative studies. NPJ Prim Care Respir Med. 2018;28(1):19.
10. McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;(2):CD003793.
11. Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary rehabilitation: joint AACP/AACVPR evidence-based clinical practice guidelines. Chest. 2007;131(suppl 5):4S-42S.
12. Major S, Moreno M, Shelton J, Panos RJ. Veterans with chronic obstructive pulmonary disease achieve clinically relevant improvements in respiratory health after pulmonary rehabilitation. J Cardiopulm Rehabil Prev. 2014;34(6):420-429.
13. Liu Y, Dickerson T, Early F, Fuld J, Clarkson PJ. Understanding influences on the uptake of pulmonary rehabilitation in the East of England: an inclusive design/mixed methods study protocol. BMJ Open. 2018;8(4):e020750.
14. Harris D, Hayter M, Allender S. Factors affecting the offer of pulmonary rehabilitation to patients with chronic obstructive pulmonary disease by primary care professionals: a qualitative study. Prim Health Care Res Dev. 2008;9(4):280-290.
15. Mathar H, Fastholm P, Hansen IR, Larsen NS. Why do patients with COPD decline rehabilitation. Scand J Caring Sci. 2016;30(3):432-441.
16. Han MK, Martinez CH, Au DH, et al. Meeting the challenge of COPD care delivery in the USA: a multiprovider perspective. Lancet Respir Med. 2016;4(6):473-526.
17. American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR). Online searchable program directory. https://www.aacvpr.org/Resources/Program-Directory Accessed July 19, 2018.
18. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699.
19. Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J. 1959;2(5147):257-266.
20. O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2007 update. Can Respir J. 2007;14(suppl B):5B-32B.
21. Jones PW, Quirk FH, Baveystock CM. The St George’s Respiratory Questionnaire. Respir Med. 1991;85(suppl B):25-31.
22. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648-654.
23. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
24. Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097.
25. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540-545.
26. Katz S. Assessing self-maintenance: activities of daily living, mobility and instrumental activities of daily living. J Am Geriatr Soc. 1983;31(12):721-727.
27. Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428-1446.
28. Mahler DA, Horowitz MB. Perception of breathlessness during exercise in patients with respiratory disease. Med Sci Sports Exerc. 1994;26(9):1078-1081.
29. Liao WC, Wang CH, Yu SY, Chen LY, Wang CY. Grip strength measurement in older adults in Taiwan: a comparison of three testing positions. Australas J Ageing. 2014;33(4):278-282.
30. Buatois S, Miljkovic D, Manckoundia P, et al. Five times sit to stand test is a predictor of recurrent falls in healthy community-living subjects aged 65 and older. J Am Geriatr Soc. 2008;56(8):1575-1577.
31. Bryant MS, Workman CD, Jackson GR. Multidirectional walk test in persons with Parkinson’s disease: a validity study. Int J Rehabil Res. 2015;38(1):88-91.
32. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142-148.
33. University of Nebraska Medical Center. Timed Up and Go (TUG) Test. https://www.unmc.edu/media/intmed/geriatrics/nebgec/pdf/frailelderlyjuly09/toolkits/timedupandgo_w_norms.pdf. Accessed August 13, 2019.
34. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377-381.
35. Mahler DA, Horowitz MB. Clinical evaluation of exertional dyspnea. Clin Chest Med. 1994;15(2):259-269.
36. Dudgeon D, Baracos VE. Physiological and functional failure in chronic obstructive pulmonary disease, congestive heart failure and cancer: a debilitating intersection of sarcopenia, cachexia and breathlessness. Curr Opin Support Palliat Care. 2016;10(3):236-241.
37. Lee AL, Holland AE. Time to adapt exercise training regimens in pulmonary rehabilitation—a review of the literature. Int J Chron Obstruct Pulmon Dis. 2014;9:1275-1288.
38. Qiu S, Cai X, Wang X, et al. Using step counters to promote physical activity and exercise capacity in patients with chronic obstructive pulmonary disease: a meta-analysis. Ther Adv Respir Dis. 2018;12:1753466618787386.
39. Donaire-Gonzalez D, Gimeno-Santos E, Balcells E, et al; PAC-COPD Study Group. Benefits of physical activity on COPD hospitalization depend on intensity. Eur Respir J. 2015;46(5):1281-1289.
40. Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298(19):2296-2304.
41. Zwerink M, Brusse-Keizer M, van der Valk PD, et al. Self-management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;19(3):CD002990.
42. Wilson JS, O’Neill B, Reilly J, MacMahon J, Bradley JM. Education in pulmonary rehabilitation: the patient’s perspective. Arch Phys Med Rehabil. 2007;88(12):1704-1709.
43. Bourbeau J, Nault D, Dang-Tan T. Self-management and behaviour modification in COPD. Patient Educ Couns. 2004;52(3):271-277.
1. World Health Organization. Chronic obstructive pulmonary disease (COPD). http://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd). Published December 1, 2017. Accessed August 7, 2019.
2. Yu W, Ravelo A, Wagner TH, et al. Prevalence and costs of chronic conditions in the VA health care system. Med Care Res Rev. 2003;60(suppl 3):146S-167S.
3. Doney B, Hnizdo E, Dillon CF, et al. Prevalence of airflow obstruction in U.S. adults aged 40-79 years: NHANES data 1988-1994 and 2007-2010. COPD. 2015;12(4):355-365.
4. Murphy DE, Chaudhry Z, Almoosa KF, Panos RJ. High prevalence of chronic obstructive pulmonary disease among veterans in the urban midwest. Mil Med. 2011;176(5):552-560.
5. Cypel YS, Hines SE, Davey VJ, Eber SM, Schneiderman AI. Self-reported physician-diagnosed chronic obstructive pulmonary disease and spirometry patterns in Vietnam Era US Army Chemical Corps veterans: a retrospective cohort study. Am J Ind Med. 2018;61(10):802-814.
6. Rochester CL. Exercise training in chronic obstructive pulmonary disease. J Rehabil Res Dev. 2003;40(5)(suppl 2):59-80.
7. Cortopassi F, Gurung P, Pinto-Plata V. Chronic obstructive pulmonary disease in elderly patients. Clin Geriatr Med. 2017;33(4):539-552.
8. Spruit MA, Singh SJ, Garvey C, et al; ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13-e64.
9. Robinson H, Williams V, Curtis F, Bridle C, Jones AW. Facilitators and barriers to physical activity following pulmonary rehabilitation in COPD: a systematic review of qualitative studies. NPJ Prim Care Respir Med. 2018;28(1):19.
10. McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;(2):CD003793.
11. Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary rehabilitation: joint AACP/AACVPR evidence-based clinical practice guidelines. Chest. 2007;131(suppl 5):4S-42S.
12. Major S, Moreno M, Shelton J, Panos RJ. Veterans with chronic obstructive pulmonary disease achieve clinically relevant improvements in respiratory health after pulmonary rehabilitation. J Cardiopulm Rehabil Prev. 2014;34(6):420-429.
13. Liu Y, Dickerson T, Early F, Fuld J, Clarkson PJ. Understanding influences on the uptake of pulmonary rehabilitation in the East of England: an inclusive design/mixed methods study protocol. BMJ Open. 2018;8(4):e020750.
14. Harris D, Hayter M, Allender S. Factors affecting the offer of pulmonary rehabilitation to patients with chronic obstructive pulmonary disease by primary care professionals: a qualitative study. Prim Health Care Res Dev. 2008;9(4):280-290.
15. Mathar H, Fastholm P, Hansen IR, Larsen NS. Why do patients with COPD decline rehabilitation. Scand J Caring Sci. 2016;30(3):432-441.
16. Han MK, Martinez CH, Au DH, et al. Meeting the challenge of COPD care delivery in the USA: a multiprovider perspective. Lancet Respir Med. 2016;4(6):473-526.
17. American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR). Online searchable program directory. https://www.aacvpr.org/Resources/Program-Directory Accessed July 19, 2018.
18. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699.
19. Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J. 1959;2(5147):257-266.
20. O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2007 update. Can Respir J. 2007;14(suppl B):5B-32B.
21. Jones PW, Quirk FH, Baveystock CM. The St George’s Respiratory Questionnaire. Respir Med. 1991;85(suppl B):25-31.
22. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648-654.
23. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
24. Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097.
25. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540-545.
26. Katz S. Assessing self-maintenance: activities of daily living, mobility and instrumental activities of daily living. J Am Geriatr Soc. 1983;31(12):721-727.
27. Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428-1446.
28. Mahler DA, Horowitz MB. Perception of breathlessness during exercise in patients with respiratory disease. Med Sci Sports Exerc. 1994;26(9):1078-1081.
29. Liao WC, Wang CH, Yu SY, Chen LY, Wang CY. Grip strength measurement in older adults in Taiwan: a comparison of three testing positions. Australas J Ageing. 2014;33(4):278-282.
30. Buatois S, Miljkovic D, Manckoundia P, et al. Five times sit to stand test is a predictor of recurrent falls in healthy community-living subjects aged 65 and older. J Am Geriatr Soc. 2008;56(8):1575-1577.
31. Bryant MS, Workman CD, Jackson GR. Multidirectional walk test in persons with Parkinson’s disease: a validity study. Int J Rehabil Res. 2015;38(1):88-91.
32. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142-148.
33. University of Nebraska Medical Center. Timed Up and Go (TUG) Test. https://www.unmc.edu/media/intmed/geriatrics/nebgec/pdf/frailelderlyjuly09/toolkits/timedupandgo_w_norms.pdf. Accessed August 13, 2019.
34. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377-381.
35. Mahler DA, Horowitz MB. Clinical evaluation of exertional dyspnea. Clin Chest Med. 1994;15(2):259-269.
36. Dudgeon D, Baracos VE. Physiological and functional failure in chronic obstructive pulmonary disease, congestive heart failure and cancer: a debilitating intersection of sarcopenia, cachexia and breathlessness. Curr Opin Support Palliat Care. 2016;10(3):236-241.
37. Lee AL, Holland AE. Time to adapt exercise training regimens in pulmonary rehabilitation—a review of the literature. Int J Chron Obstruct Pulmon Dis. 2014;9:1275-1288.
38. Qiu S, Cai X, Wang X, et al. Using step counters to promote physical activity and exercise capacity in patients with chronic obstructive pulmonary disease: a meta-analysis. Ther Adv Respir Dis. 2018;12:1753466618787386.
39. Donaire-Gonzalez D, Gimeno-Santos E, Balcells E, et al; PAC-COPD Study Group. Benefits of physical activity on COPD hospitalization depend on intensity. Eur Respir J. 2015;46(5):1281-1289.
40. Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298(19):2296-2304.
41. Zwerink M, Brusse-Keizer M, van der Valk PD, et al. Self-management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;19(3):CD002990.
42. Wilson JS, O’Neill B, Reilly J, MacMahon J, Bradley JM. Education in pulmonary rehabilitation: the patient’s perspective. Arch Phys Med Rehabil. 2007;88(12):1704-1709.
43. Bourbeau J, Nault D, Dang-Tan T. Self-management and behaviour modification in COPD. Patient Educ Couns. 2004;52(3):271-277.