User login
Endoscopy-related infections found higher than expected, prophylaxis overused
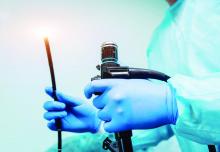
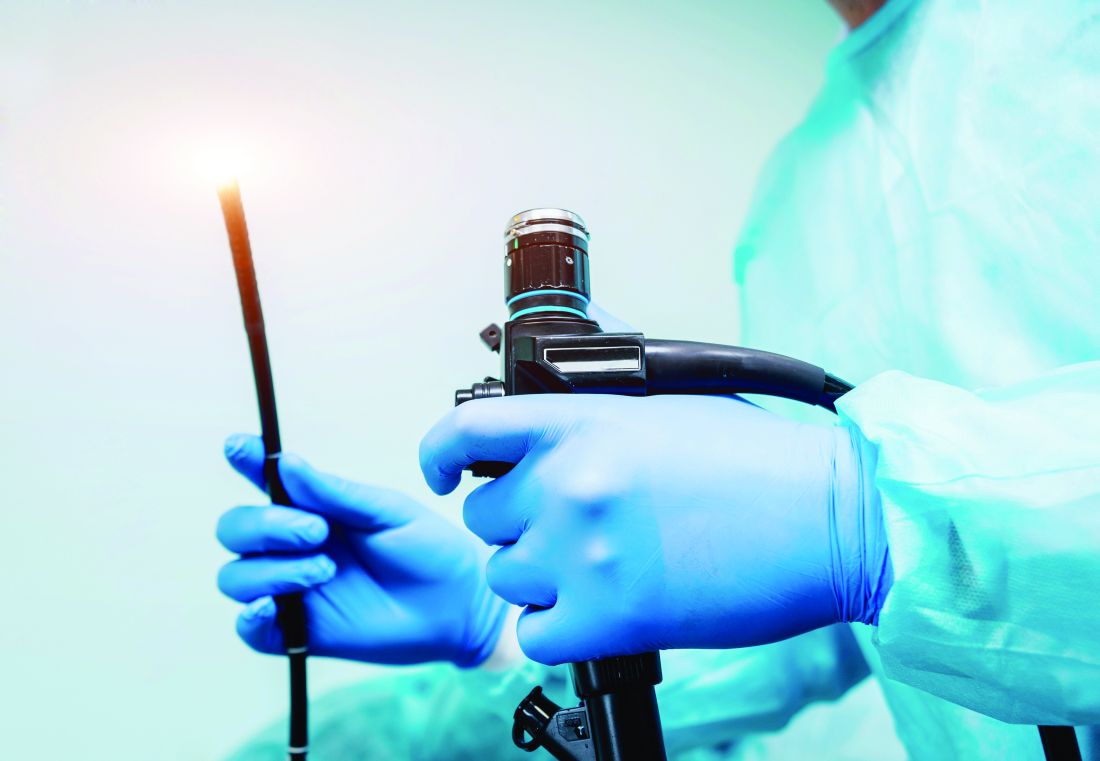
ATLANTA – The risk of infection from flexible endoscopes is far greater than generally believed, despite the excessive use of prophylactic antimicrobials in patients undergoing endoscopy, recent studies show.
Many gastroenterologists and guidelines from professional organizations use a reference point of “less than one per million” regarding the risk of infection from scopes, but a Johns Hopkins University study of more than 2.3 million patients in 6 states showed that the infection risk with colonoscopy is about 1 per 1,000, the risk for upper gastrointestinal endoscopy is about 3 per 1,000, and the risk with cystoscopy is about 4 per 1,000, Cori Ofstead said at the International Conference on Emerging Infectious Diseases.
“For bronchoscopy [the infection risk] was 15.6 in 1,000, which is 1.6% – not anywhere in the 1 in a million range,” said Ms. Ofstead, president and chief executive officer of Ofstead & Associates, a St. Paul, Minn. health care research firm.
It also turns out that prophylactic antibiotics are frequently given to patients undergoing routine endoscopy procedures, she said, noting that four major associations – two gastroenterology associations and two urology associations in the United States and Europe – recommend that prophylactic antimicrobials be given with routine endoscopies for certain patients undergoing certain types of procedures.
One U.S. organization is recommending prophylactic antimicrobials for every patient undergoing ureteroscopy, she added.
A Cleveland Clinic study looking at the impact of those American Urological Association guidelines for prophylactic antimicrobials showed that in a subset of patients with negative urine cultures before ureteroscopy, 100% received the prophylaxis, and 68% were also given other antimicrobials to take home.
“So the question, of course, is how well does this work...,” Ms. Ofstead said. “They found 3%-4% infection, with the rates exactly the same – no statistically significant differences – between patients who got prophylaxis just in the hospital or who went home with prophylactic meds, and they concluded that there was no benefit to the extra take-home antimicrobials.”
Others studies in multiple countries show either no impact or only minor impact of this prophylaxis on infection rates, and yet all show infection rates after endoscopy that are not one in a million, but in “the percentage point range,” she said.
“As we move toward more of these minimally invasive procedures, we need to be aware that we’re using extremely complex instruments that are very difficult to clean and disinfect or sterilize,” she said, adding that “in the field we’re seeing that improper reprocessing is actually business as usual.”
Infections have been seen with all kinds of scopes, Ms. Ofstead noted.
“The potential for this becoming a bit of a monster is enhanced by the widespread use of prophylactic antimicrobials during endoscopy, and I’m also troubled by the quick reaction of giving people antimicrobials when they have a positive culture from a scope rather than making sure the scope is clean,” she said, explaining that while most scopes have microbes and patients could be getting infections, they also may be reacting to soil and endotoxins in the scope rather than microbes.
“In any case, to reduce risks there are a number of things people can do,” she said. When using reusable scopes, proper cleaning is essential. “I think we should be moving toward scopes that can be disassembled so we can see inside and get those channels clean,” adding that efforts should also be made to move toward single-use scopes.
“Particularly in these outbreak situations where we’re using bronchoscopy on multiple patients, there’s just no excuse for reusing bronchoscopes and not sterilizing them between uses and making darn sure that they’re not full of whatever our outbreak pathogen is,” Ms. Ofstead said. “And lastly, I’m hoping that some folks here can talk some sense into people at the professional associations who are recommending prophylactic antimicrobial use, because if we don’t get some stewardship going, we’re going to be in big trouble.”
The guidelines create a conundrum for doctors who are torn between that stewardship and a failure to follow the recommendations.
“Their professional organization is telling them to give prophylactic antimicrobials. If they don’t do it and a patients gets an infection, that’s a malpractice issue. So we’ve got to go through those associations and get them to stop recommending prophylactic antimicrobials when there is no evidence of their effectiveness,” she said.
Ms. Ofstead has been a consultant for 3M Company, Ambu, Auris, Boston Scientific, Cogentix, Convergascent, Healthmark, Invendo Medical, Nanosonics, and Advanced Sterilization Products, and has received grant/research support from 3M Company, Advanced Sterilization Products, Ambu, Boston Scientific, Cogentix, Healthmark, Invendo Medical, Medivators, and Steris.
sworcester@frontlinemedcom.com
SOURCE: Ofstead C., ICEID 2018 Presentation.
ATLANTA – The risk of infection from flexible endoscopes is far greater than generally believed, despite the excessive use of prophylactic antimicrobials in patients undergoing endoscopy, recent studies show.
Many gastroenterologists and guidelines from professional organizations use a reference point of “less than one per million” regarding the risk of infection from scopes, but a Johns Hopkins University study of more than 2.3 million patients in 6 states showed that the infection risk with colonoscopy is about 1 per 1,000, the risk for upper gastrointestinal endoscopy is about 3 per 1,000, and the risk with cystoscopy is about 4 per 1,000, Cori Ofstead said at the International Conference on Emerging Infectious Diseases.
“For bronchoscopy [the infection risk] was 15.6 in 1,000, which is 1.6% – not anywhere in the 1 in a million range,” said Ms. Ofstead, president and chief executive officer of Ofstead & Associates, a St. Paul, Minn. health care research firm.
It also turns out that prophylactic antibiotics are frequently given to patients undergoing routine endoscopy procedures, she said, noting that four major associations – two gastroenterology associations and two urology associations in the United States and Europe – recommend that prophylactic antimicrobials be given with routine endoscopies for certain patients undergoing certain types of procedures.
One U.S. organization is recommending prophylactic antimicrobials for every patient undergoing ureteroscopy, she added.
A Cleveland Clinic study looking at the impact of those American Urological Association guidelines for prophylactic antimicrobials showed that in a subset of patients with negative urine cultures before ureteroscopy, 100% received the prophylaxis, and 68% were also given other antimicrobials to take home.
“So the question, of course, is how well does this work...,” Ms. Ofstead said. “They found 3%-4% infection, with the rates exactly the same – no statistically significant differences – between patients who got prophylaxis just in the hospital or who went home with prophylactic meds, and they concluded that there was no benefit to the extra take-home antimicrobials.”
Others studies in multiple countries show either no impact or only minor impact of this prophylaxis on infection rates, and yet all show infection rates after endoscopy that are not one in a million, but in “the percentage point range,” she said.
“As we move toward more of these minimally invasive procedures, we need to be aware that we’re using extremely complex instruments that are very difficult to clean and disinfect or sterilize,” she said, adding that “in the field we’re seeing that improper reprocessing is actually business as usual.”
Infections have been seen with all kinds of scopes, Ms. Ofstead noted.
“The potential for this becoming a bit of a monster is enhanced by the widespread use of prophylactic antimicrobials during endoscopy, and I’m also troubled by the quick reaction of giving people antimicrobials when they have a positive culture from a scope rather than making sure the scope is clean,” she said, explaining that while most scopes have microbes and patients could be getting infections, they also may be reacting to soil and endotoxins in the scope rather than microbes.
“In any case, to reduce risks there are a number of things people can do,” she said. When using reusable scopes, proper cleaning is essential. “I think we should be moving toward scopes that can be disassembled so we can see inside and get those channels clean,” adding that efforts should also be made to move toward single-use scopes.
“Particularly in these outbreak situations where we’re using bronchoscopy on multiple patients, there’s just no excuse for reusing bronchoscopes and not sterilizing them between uses and making darn sure that they’re not full of whatever our outbreak pathogen is,” Ms. Ofstead said. “And lastly, I’m hoping that some folks here can talk some sense into people at the professional associations who are recommending prophylactic antimicrobial use, because if we don’t get some stewardship going, we’re going to be in big trouble.”
The guidelines create a conundrum for doctors who are torn between that stewardship and a failure to follow the recommendations.
“Their professional organization is telling them to give prophylactic antimicrobials. If they don’t do it and a patients gets an infection, that’s a malpractice issue. So we’ve got to go through those associations and get them to stop recommending prophylactic antimicrobials when there is no evidence of their effectiveness,” she said.
Ms. Ofstead has been a consultant for 3M Company, Ambu, Auris, Boston Scientific, Cogentix, Convergascent, Healthmark, Invendo Medical, Nanosonics, and Advanced Sterilization Products, and has received grant/research support from 3M Company, Advanced Sterilization Products, Ambu, Boston Scientific, Cogentix, Healthmark, Invendo Medical, Medivators, and Steris.
sworcester@frontlinemedcom.com
SOURCE: Ofstead C., ICEID 2018 Presentation.
ATLANTA – The risk of infection from flexible endoscopes is far greater than generally believed, despite the excessive use of prophylactic antimicrobials in patients undergoing endoscopy, recent studies show.
Many gastroenterologists and guidelines from professional organizations use a reference point of “less than one per million” regarding the risk of infection from scopes, but a Johns Hopkins University study of more than 2.3 million patients in 6 states showed that the infection risk with colonoscopy is about 1 per 1,000, the risk for upper gastrointestinal endoscopy is about 3 per 1,000, and the risk with cystoscopy is about 4 per 1,000, Cori Ofstead said at the International Conference on Emerging Infectious Diseases.
“For bronchoscopy [the infection risk] was 15.6 in 1,000, which is 1.6% – not anywhere in the 1 in a million range,” said Ms. Ofstead, president and chief executive officer of Ofstead & Associates, a St. Paul, Minn. health care research firm.
It also turns out that prophylactic antibiotics are frequently given to patients undergoing routine endoscopy procedures, she said, noting that four major associations – two gastroenterology associations and two urology associations in the United States and Europe – recommend that prophylactic antimicrobials be given with routine endoscopies for certain patients undergoing certain types of procedures.
One U.S. organization is recommending prophylactic antimicrobials for every patient undergoing ureteroscopy, she added.
A Cleveland Clinic study looking at the impact of those American Urological Association guidelines for prophylactic antimicrobials showed that in a subset of patients with negative urine cultures before ureteroscopy, 100% received the prophylaxis, and 68% were also given other antimicrobials to take home.
“So the question, of course, is how well does this work...,” Ms. Ofstead said. “They found 3%-4% infection, with the rates exactly the same – no statistically significant differences – between patients who got prophylaxis just in the hospital or who went home with prophylactic meds, and they concluded that there was no benefit to the extra take-home antimicrobials.”
Others studies in multiple countries show either no impact or only minor impact of this prophylaxis on infection rates, and yet all show infection rates after endoscopy that are not one in a million, but in “the percentage point range,” she said.
“As we move toward more of these minimally invasive procedures, we need to be aware that we’re using extremely complex instruments that are very difficult to clean and disinfect or sterilize,” she said, adding that “in the field we’re seeing that improper reprocessing is actually business as usual.”
Infections have been seen with all kinds of scopes, Ms. Ofstead noted.
“The potential for this becoming a bit of a monster is enhanced by the widespread use of prophylactic antimicrobials during endoscopy, and I’m also troubled by the quick reaction of giving people antimicrobials when they have a positive culture from a scope rather than making sure the scope is clean,” she said, explaining that while most scopes have microbes and patients could be getting infections, they also may be reacting to soil and endotoxins in the scope rather than microbes.
“In any case, to reduce risks there are a number of things people can do,” she said. When using reusable scopes, proper cleaning is essential. “I think we should be moving toward scopes that can be disassembled so we can see inside and get those channels clean,” adding that efforts should also be made to move toward single-use scopes.
“Particularly in these outbreak situations where we’re using bronchoscopy on multiple patients, there’s just no excuse for reusing bronchoscopes and not sterilizing them between uses and making darn sure that they’re not full of whatever our outbreak pathogen is,” Ms. Ofstead said. “And lastly, I’m hoping that some folks here can talk some sense into people at the professional associations who are recommending prophylactic antimicrobial use, because if we don’t get some stewardship going, we’re going to be in big trouble.”
The guidelines create a conundrum for doctors who are torn between that stewardship and a failure to follow the recommendations.
“Their professional organization is telling them to give prophylactic antimicrobials. If they don’t do it and a patients gets an infection, that’s a malpractice issue. So we’ve got to go through those associations and get them to stop recommending prophylactic antimicrobials when there is no evidence of their effectiveness,” she said.
Ms. Ofstead has been a consultant for 3M Company, Ambu, Auris, Boston Scientific, Cogentix, Convergascent, Healthmark, Invendo Medical, Nanosonics, and Advanced Sterilization Products, and has received grant/research support from 3M Company, Advanced Sterilization Products, Ambu, Boston Scientific, Cogentix, Healthmark, Invendo Medical, Medivators, and Steris.
sworcester@frontlinemedcom.com
SOURCE: Ofstead C., ICEID 2018 Presentation.
REPORTING FROM ICEID 2018
Key clinical point:
Major finding: Infection risk is about 1 per 1,000 with colonoscopy; 3 per 1,000 with upper gastrointestinal endoscopy; and 4 per 1,000 with cystoscopy.
Study details: Endoscopic procedures performed at ASCs in 2014 all-payer claims data from 6 U.S. states.
Disclosures: Ms. Ofstead has been a consultant for 3M Company, Ambu, Auris, Boston Scientific, Cogentix, Convergascent, Healthmark, Invendo Medical, Nanosonics, and Advanced Sterilization Products, and has received grant/research support from 3M Company, Advanced Sterilization Products, Ambu, Boston Scientific, Cogentix, Healthmark, Invendo Medical, Medivators, and Steris.
Source: Ofstead C et al. ICEID 2018 Presentation.
CDC: Trivalent adjuvanted influenza vaccine aIIV3 safe in elderly adults
ATLANTA – according to an analysis of reports to the Vaccine Adverse Event Reporting System (VAERS) during July 2016 through March 2018.
VAERS received 630 reports related to the vaccine (aIIV3; FLUAD) during the study period, of which 521 involved adults aged 65 years and older.
“Eighteen (3%) were serious reports, including two death reports (0.4%), all in adults aged [at least] 65 years,” Penina Haber and her colleagues at the Immunization Safety Office at the Centers for Disease Control and Prevention reported in a poster at the International Conference on Emerging Infectious Diseases.
The deaths included a 75-year-old man who died from Sjögren’s syndrome and a 65-year-old man who died from a myocardial infarction. The other serious events included five neurologic disorders (two cases of Guillain-Barré syndrome and one each of Bell’s palsy, Bickerstaff encephalitis, and lower-extremity weakness), five musculoskeletal and connective tissue disorders (three with shoulder pain and two with arm pain), three general disorders and administration site conditions (two cases of fever/chills and one case of cellulitis/bursitis), and one case each of a gastrointestinal disorder (acute diarrhea/gastroenteritis), an injury (a fall), and a skin/subcutaneous tissue disorder (keratosis pilaris rubra), according to the investigators.
There were no reports of anaphylaxis.
For the sake of comparison, the investigators also looked at reports associated with IIV3-HD and IIV3/IIV4 vaccines in adults aged 65 years and older during the same time period; they found that patient characteristics and reported events were similar for all the vaccines. For example, the percentages of reports involving patients aged 65 years and older were 65% or 66% for each, and those involving patients aged 75-84 years were 27%-29%. Further, 0.2%-0.6% of reports for each vaccine involved death.
The most frequently reported events for aIIV3, IIV3-HD, and IIV3/IIV4, respectively, were extremity pain (21%, 17%, and 15%, respectively), injection site erythema (18%, 19%, and 15%), and injection site pain (15%, 16%, and 16%), they said.
The aIIV3 vaccine – the first seasonal inactivated trivalent influenza vaccine produced from three influenza virus strains (two subtype A strains and one type B strain) – was approved by the Food and Drug Administration in 2015 for adults aged 65 years and older. It was the first influenza vaccine containing the adjuvant MF59 – a purified oil-in-water emulsion of squalene oil added to boost immune response in that population. Its safety was assessed in 15 randomized, controlled clinical studies, and several trials in older adults supported its efficacy and safety over nonadjuvanted influenza vaccines, the investigators reported. They noted that the Advisory Committee on Immunization Practices (ACIP) recommended the vaccine as an option for routine use in adults aged 65 years and older during the 2016-2017 flu seasons.
For the 2018-2019 flu season, ACIP determined that “For persons aged ≥65 years, any age-appropriate IIV formulation (standard-dose or high-dose, trivalent or quadrivalent, unadjuvanted or adjuvanted) or RIV4 are acceptable options.”
The findings of the analysis of the 2017-2018 flu season data are consistent with prelicensure studies, Ms. Haber and her colleagues concluded, noting that data mining did not detect disproportional reporting of any unexpected adverse event.
“[There were] no safety concerns following aIIV3 when compared to the nonadjuvanted influenza vaccines (IIV3-HD or IIV3/IIV4),” they wrote, adding that the “CDC and FDA will continue to monitor and ensure the safety of aIIV3.”
Ms. Haber reported having no disclosures
sworcester@frontlinemedcom.com
SOURCE: Haber P et al. ICEID 2018, Board 320.
ATLANTA – according to an analysis of reports to the Vaccine Adverse Event Reporting System (VAERS) during July 2016 through March 2018.
VAERS received 630 reports related to the vaccine (aIIV3; FLUAD) during the study period, of which 521 involved adults aged 65 years and older.
“Eighteen (3%) were serious reports, including two death reports (0.4%), all in adults aged [at least] 65 years,” Penina Haber and her colleagues at the Immunization Safety Office at the Centers for Disease Control and Prevention reported in a poster at the International Conference on Emerging Infectious Diseases.
The deaths included a 75-year-old man who died from Sjögren’s syndrome and a 65-year-old man who died from a myocardial infarction. The other serious events included five neurologic disorders (two cases of Guillain-Barré syndrome and one each of Bell’s palsy, Bickerstaff encephalitis, and lower-extremity weakness), five musculoskeletal and connective tissue disorders (three with shoulder pain and two with arm pain), three general disorders and administration site conditions (two cases of fever/chills and one case of cellulitis/bursitis), and one case each of a gastrointestinal disorder (acute diarrhea/gastroenteritis), an injury (a fall), and a skin/subcutaneous tissue disorder (keratosis pilaris rubra), according to the investigators.
There were no reports of anaphylaxis.
For the sake of comparison, the investigators also looked at reports associated with IIV3-HD and IIV3/IIV4 vaccines in adults aged 65 years and older during the same time period; they found that patient characteristics and reported events were similar for all the vaccines. For example, the percentages of reports involving patients aged 65 years and older were 65% or 66% for each, and those involving patients aged 75-84 years were 27%-29%. Further, 0.2%-0.6% of reports for each vaccine involved death.
The most frequently reported events for aIIV3, IIV3-HD, and IIV3/IIV4, respectively, were extremity pain (21%, 17%, and 15%, respectively), injection site erythema (18%, 19%, and 15%), and injection site pain (15%, 16%, and 16%), they said.
The aIIV3 vaccine – the first seasonal inactivated trivalent influenza vaccine produced from three influenza virus strains (two subtype A strains and one type B strain) – was approved by the Food and Drug Administration in 2015 for adults aged 65 years and older. It was the first influenza vaccine containing the adjuvant MF59 – a purified oil-in-water emulsion of squalene oil added to boost immune response in that population. Its safety was assessed in 15 randomized, controlled clinical studies, and several trials in older adults supported its efficacy and safety over nonadjuvanted influenza vaccines, the investigators reported. They noted that the Advisory Committee on Immunization Practices (ACIP) recommended the vaccine as an option for routine use in adults aged 65 years and older during the 2016-2017 flu seasons.
For the 2018-2019 flu season, ACIP determined that “For persons aged ≥65 years, any age-appropriate IIV formulation (standard-dose or high-dose, trivalent or quadrivalent, unadjuvanted or adjuvanted) or RIV4 are acceptable options.”
The findings of the analysis of the 2017-2018 flu season data are consistent with prelicensure studies, Ms. Haber and her colleagues concluded, noting that data mining did not detect disproportional reporting of any unexpected adverse event.
“[There were] no safety concerns following aIIV3 when compared to the nonadjuvanted influenza vaccines (IIV3-HD or IIV3/IIV4),” they wrote, adding that the “CDC and FDA will continue to monitor and ensure the safety of aIIV3.”
Ms. Haber reported having no disclosures
sworcester@frontlinemedcom.com
SOURCE: Haber P et al. ICEID 2018, Board 320.
ATLANTA – according to an analysis of reports to the Vaccine Adverse Event Reporting System (VAERS) during July 2016 through March 2018.
VAERS received 630 reports related to the vaccine (aIIV3; FLUAD) during the study period, of which 521 involved adults aged 65 years and older.
“Eighteen (3%) were serious reports, including two death reports (0.4%), all in adults aged [at least] 65 years,” Penina Haber and her colleagues at the Immunization Safety Office at the Centers for Disease Control and Prevention reported in a poster at the International Conference on Emerging Infectious Diseases.
The deaths included a 75-year-old man who died from Sjögren’s syndrome and a 65-year-old man who died from a myocardial infarction. The other serious events included five neurologic disorders (two cases of Guillain-Barré syndrome and one each of Bell’s palsy, Bickerstaff encephalitis, and lower-extremity weakness), five musculoskeletal and connective tissue disorders (three with shoulder pain and two with arm pain), three general disorders and administration site conditions (two cases of fever/chills and one case of cellulitis/bursitis), and one case each of a gastrointestinal disorder (acute diarrhea/gastroenteritis), an injury (a fall), and a skin/subcutaneous tissue disorder (keratosis pilaris rubra), according to the investigators.
There were no reports of anaphylaxis.
For the sake of comparison, the investigators also looked at reports associated with IIV3-HD and IIV3/IIV4 vaccines in adults aged 65 years and older during the same time period; they found that patient characteristics and reported events were similar for all the vaccines. For example, the percentages of reports involving patients aged 65 years and older were 65% or 66% for each, and those involving patients aged 75-84 years were 27%-29%. Further, 0.2%-0.6% of reports for each vaccine involved death.
The most frequently reported events for aIIV3, IIV3-HD, and IIV3/IIV4, respectively, were extremity pain (21%, 17%, and 15%, respectively), injection site erythema (18%, 19%, and 15%), and injection site pain (15%, 16%, and 16%), they said.
The aIIV3 vaccine – the first seasonal inactivated trivalent influenza vaccine produced from three influenza virus strains (two subtype A strains and one type B strain) – was approved by the Food and Drug Administration in 2015 for adults aged 65 years and older. It was the first influenza vaccine containing the adjuvant MF59 – a purified oil-in-water emulsion of squalene oil added to boost immune response in that population. Its safety was assessed in 15 randomized, controlled clinical studies, and several trials in older adults supported its efficacy and safety over nonadjuvanted influenza vaccines, the investigators reported. They noted that the Advisory Committee on Immunization Practices (ACIP) recommended the vaccine as an option for routine use in adults aged 65 years and older during the 2016-2017 flu seasons.
For the 2018-2019 flu season, ACIP determined that “For persons aged ≥65 years, any age-appropriate IIV formulation (standard-dose or high-dose, trivalent or quadrivalent, unadjuvanted or adjuvanted) or RIV4 are acceptable options.”
The findings of the analysis of the 2017-2018 flu season data are consistent with prelicensure studies, Ms. Haber and her colleagues concluded, noting that data mining did not detect disproportional reporting of any unexpected adverse event.
“[There were] no safety concerns following aIIV3 when compared to the nonadjuvanted influenza vaccines (IIV3-HD or IIV3/IIV4),” they wrote, adding that the “CDC and FDA will continue to monitor and ensure the safety of aIIV3.”
Ms. Haber reported having no disclosures
sworcester@frontlinemedcom.com
SOURCE: Haber P et al. ICEID 2018, Board 320.
REPORTING FROM ICEID 2018
Key clinical point: No new or unexpected adverse events were reported among the 630 reports related to the vaccine during the study period, of which 521 involved adults aged 65 years and older.
Major finding: Of 521 reports, 18 were serious, and there were two deaths.
Study details: A review of 521 reports to the Vaccine Adverse Event Reporting System in 2017-2018.
Disclosures: Ms. Haber reported having no disclosures.
Source: Haber P et al. ICEID 2018, Board 320.
PCV13 moderately effective in older adults
ATLANTA – (IPD) caused by PCV13 vaccine serotypes in adults aged 65 years and older, according to a case-control study involving Medicare beneficiaries.
Conversely, the 23-valent pneumococcal polysaccharide vaccine (PPSV23) showed limited effectiveness against serotypes unique to that vaccine in the study, which included 699 cases and more than 10,000 controls, Olivia Almendares, an epidemiologist at the Centers for Disease Control and Prevention, Atlanta, and her colleagues reported in a poster at the International Conference on Emerging Infectious Diseases.
“Vaccine efficacy against PCV13 [plus 6C type, which has cross-reactivity with serotype 6A] was 47% in those who received PCV13 vaccine only,” Ms. Almendares said in an interview, noting that efficacy was 26% against serotype 3 and 67% against other PCV13 serotypes (plus 6C). “Vaccine efficacy against PPSV23-unique types was 36% for those who received only PPSV23.”
Neither vaccine showed effectiveness against serotypes not included in the respective vaccines, she said.
The findings are timely given that the Advisory Committee on Immunization Practices (ACIP) is reevaluating its PCV13 recommendation for adults aged 65 years and older, she added.
“Specifically, ACIP is addressing whether PCV13 should be recommended routinely for all immunocompetent adults aged 65 and older given sustained indirect effects,” she said, explaining that, in 2014 when ACIP recommended routine use of the vaccine in series with PPSV23 for adults aged 65 years and older, the committee recognized that herd immunity effects from PCV13 use in children might eventually limit the utility of this recommendation, and therefore it proposed reevaluation and revision as needed after 4 years.
For the current study, she and her colleagues linked IPD cases in persons aged 65 years and older, which were identified through Active Bacterial Core surveillance during 2015-2016, to records for Centers for Medicare & Medicaid Services (CMS) beneficiaries. Vaccination and medical histories were obtained through medical records, and vaccine effectiveness was estimated as one minus the odds ratio for vaccination with PCV13 only or PPSV23 only versus neither vaccine using conditional logistic regression, with adjustment for sex and underlying medical conditions.
Of 2,246 IPD cases, 1,017 (45%) were matched to Medicare beneficiaries, and 699 were included in the analysis after those with noncontinuous enrollment in Medicare, long-term care residence, and missing census tract data were excluded. The cases were matched based on age, census tract of residence, and length of Medicare enrollment to 10,152 matched controls identified through CMS.
IPD associated with PCV13 (plus type 6C) accounted for 164 (23% of cases), of which 88 (12% of cases) involved serotype 3, and invasive pneumococcal disease associated with PPSV23 accounted for 350 cases (50%), she said.
PCV13 vaccine was given alone in 14% and 18% of cases and controls, respectively; PPSV23 alone was given in 22% and 21% of case patients and controls, respectively; and both vaccines were given in 8% of cases and controls.
Compared with controls, case patients were more likely to be of nonwhite race (16% vs. 11%), to have more than one chronic medical condition (88% vs. 58%), and to have one or more immunocompromising conditions (54% vs. 32%), she and her colleagues reported.
“PCV13 showed moderate overall effectiveness in preventing IPD caused by PCV13 (including 6C), but effectiveness may be lower for serotype 3 than for other PCV13 types,” she said.
“These results are in agreement with those from CAPiTA – a large clinical trial conducted in the Netherlands, which showed PCV13 to be effective against IPD caused by vaccine serotypes among community-dwelling adults aged 65 and older,” she noted. “Additionally, data from CDC surveillance suggest that PCV13-serotype [invasive pneumococcal disease] among children and adults aged 65 and older has declined dramatically following PCV13 introduction for children in 2010, as predicted.”
In fact, among adults aged 65 years and older, PCV13-serotype invasive pneumococcal disease declined by 40% after the vaccine was introduced in children. This corresponds to a change in the annual PCV13-serotype incidence from 14 cases per 100,000 population in 2010 to five cases per 100,000 population in 2014, she said; she added that IPD incidence plateaued in 2014-2016 with vaccine serotypes contributing to a small proportion of overall IPD burden among adults aged 65 years and older.
ACIP’s reevaluation of the PCV13 recommendation is ongoing and will be addressed at upcoming meetings.
“As part of the review process, we look at changes in disease incidence focusing primarily on invasive pneumococcal disease and noninvasive pneumonia, vaccine efficacy and effectiveness, and vaccine safety,” she said. She noted that ACIP currently has no plans to consider revising PCV13 recommendations for adults who have immunocompromising conditions, for whom PCV13 has been recommended since 2012.
Ms. Almendares reported having no disclosures.
SOURCE: Almendares O et al. ICEID 2018, Board 376.
ATLANTA – (IPD) caused by PCV13 vaccine serotypes in adults aged 65 years and older, according to a case-control study involving Medicare beneficiaries.
Conversely, the 23-valent pneumococcal polysaccharide vaccine (PPSV23) showed limited effectiveness against serotypes unique to that vaccine in the study, which included 699 cases and more than 10,000 controls, Olivia Almendares, an epidemiologist at the Centers for Disease Control and Prevention, Atlanta, and her colleagues reported in a poster at the International Conference on Emerging Infectious Diseases.
“Vaccine efficacy against PCV13 [plus 6C type, which has cross-reactivity with serotype 6A] was 47% in those who received PCV13 vaccine only,” Ms. Almendares said in an interview, noting that efficacy was 26% against serotype 3 and 67% against other PCV13 serotypes (plus 6C). “Vaccine efficacy against PPSV23-unique types was 36% for those who received only PPSV23.”
Neither vaccine showed effectiveness against serotypes not included in the respective vaccines, she said.
The findings are timely given that the Advisory Committee on Immunization Practices (ACIP) is reevaluating its PCV13 recommendation for adults aged 65 years and older, she added.
“Specifically, ACIP is addressing whether PCV13 should be recommended routinely for all immunocompetent adults aged 65 and older given sustained indirect effects,” she said, explaining that, in 2014 when ACIP recommended routine use of the vaccine in series with PPSV23 for adults aged 65 years and older, the committee recognized that herd immunity effects from PCV13 use in children might eventually limit the utility of this recommendation, and therefore it proposed reevaluation and revision as needed after 4 years.
For the current study, she and her colleagues linked IPD cases in persons aged 65 years and older, which were identified through Active Bacterial Core surveillance during 2015-2016, to records for Centers for Medicare & Medicaid Services (CMS) beneficiaries. Vaccination and medical histories were obtained through medical records, and vaccine effectiveness was estimated as one minus the odds ratio for vaccination with PCV13 only or PPSV23 only versus neither vaccine using conditional logistic regression, with adjustment for sex and underlying medical conditions.
Of 2,246 IPD cases, 1,017 (45%) were matched to Medicare beneficiaries, and 699 were included in the analysis after those with noncontinuous enrollment in Medicare, long-term care residence, and missing census tract data were excluded. The cases were matched based on age, census tract of residence, and length of Medicare enrollment to 10,152 matched controls identified through CMS.
IPD associated with PCV13 (plus type 6C) accounted for 164 (23% of cases), of which 88 (12% of cases) involved serotype 3, and invasive pneumococcal disease associated with PPSV23 accounted for 350 cases (50%), she said.
PCV13 vaccine was given alone in 14% and 18% of cases and controls, respectively; PPSV23 alone was given in 22% and 21% of case patients and controls, respectively; and both vaccines were given in 8% of cases and controls.
Compared with controls, case patients were more likely to be of nonwhite race (16% vs. 11%), to have more than one chronic medical condition (88% vs. 58%), and to have one or more immunocompromising conditions (54% vs. 32%), she and her colleagues reported.
“PCV13 showed moderate overall effectiveness in preventing IPD caused by PCV13 (including 6C), but effectiveness may be lower for serotype 3 than for other PCV13 types,” she said.
“These results are in agreement with those from CAPiTA – a large clinical trial conducted in the Netherlands, which showed PCV13 to be effective against IPD caused by vaccine serotypes among community-dwelling adults aged 65 and older,” she noted. “Additionally, data from CDC surveillance suggest that PCV13-serotype [invasive pneumococcal disease] among children and adults aged 65 and older has declined dramatically following PCV13 introduction for children in 2010, as predicted.”
In fact, among adults aged 65 years and older, PCV13-serotype invasive pneumococcal disease declined by 40% after the vaccine was introduced in children. This corresponds to a change in the annual PCV13-serotype incidence from 14 cases per 100,000 population in 2010 to five cases per 100,000 population in 2014, she said; she added that IPD incidence plateaued in 2014-2016 with vaccine serotypes contributing to a small proportion of overall IPD burden among adults aged 65 years and older.
ACIP’s reevaluation of the PCV13 recommendation is ongoing and will be addressed at upcoming meetings.
“As part of the review process, we look at changes in disease incidence focusing primarily on invasive pneumococcal disease and noninvasive pneumonia, vaccine efficacy and effectiveness, and vaccine safety,” she said. She noted that ACIP currently has no plans to consider revising PCV13 recommendations for adults who have immunocompromising conditions, for whom PCV13 has been recommended since 2012.
Ms. Almendares reported having no disclosures.
SOURCE: Almendares O et al. ICEID 2018, Board 376.
ATLANTA – (IPD) caused by PCV13 vaccine serotypes in adults aged 65 years and older, according to a case-control study involving Medicare beneficiaries.
Conversely, the 23-valent pneumococcal polysaccharide vaccine (PPSV23) showed limited effectiveness against serotypes unique to that vaccine in the study, which included 699 cases and more than 10,000 controls, Olivia Almendares, an epidemiologist at the Centers for Disease Control and Prevention, Atlanta, and her colleagues reported in a poster at the International Conference on Emerging Infectious Diseases.
“Vaccine efficacy against PCV13 [plus 6C type, which has cross-reactivity with serotype 6A] was 47% in those who received PCV13 vaccine only,” Ms. Almendares said in an interview, noting that efficacy was 26% against serotype 3 and 67% against other PCV13 serotypes (plus 6C). “Vaccine efficacy against PPSV23-unique types was 36% for those who received only PPSV23.”
Neither vaccine showed effectiveness against serotypes not included in the respective vaccines, she said.
The findings are timely given that the Advisory Committee on Immunization Practices (ACIP) is reevaluating its PCV13 recommendation for adults aged 65 years and older, she added.
“Specifically, ACIP is addressing whether PCV13 should be recommended routinely for all immunocompetent adults aged 65 and older given sustained indirect effects,” she said, explaining that, in 2014 when ACIP recommended routine use of the vaccine in series with PPSV23 for adults aged 65 years and older, the committee recognized that herd immunity effects from PCV13 use in children might eventually limit the utility of this recommendation, and therefore it proposed reevaluation and revision as needed after 4 years.
For the current study, she and her colleagues linked IPD cases in persons aged 65 years and older, which were identified through Active Bacterial Core surveillance during 2015-2016, to records for Centers for Medicare & Medicaid Services (CMS) beneficiaries. Vaccination and medical histories were obtained through medical records, and vaccine effectiveness was estimated as one minus the odds ratio for vaccination with PCV13 only or PPSV23 only versus neither vaccine using conditional logistic regression, with adjustment for sex and underlying medical conditions.
Of 2,246 IPD cases, 1,017 (45%) were matched to Medicare beneficiaries, and 699 were included in the analysis after those with noncontinuous enrollment in Medicare, long-term care residence, and missing census tract data were excluded. The cases were matched based on age, census tract of residence, and length of Medicare enrollment to 10,152 matched controls identified through CMS.
IPD associated with PCV13 (plus type 6C) accounted for 164 (23% of cases), of which 88 (12% of cases) involved serotype 3, and invasive pneumococcal disease associated with PPSV23 accounted for 350 cases (50%), she said.
PCV13 vaccine was given alone in 14% and 18% of cases and controls, respectively; PPSV23 alone was given in 22% and 21% of case patients and controls, respectively; and both vaccines were given in 8% of cases and controls.
Compared with controls, case patients were more likely to be of nonwhite race (16% vs. 11%), to have more than one chronic medical condition (88% vs. 58%), and to have one or more immunocompromising conditions (54% vs. 32%), she and her colleagues reported.
“PCV13 showed moderate overall effectiveness in preventing IPD caused by PCV13 (including 6C), but effectiveness may be lower for serotype 3 than for other PCV13 types,” she said.
“These results are in agreement with those from CAPiTA – a large clinical trial conducted in the Netherlands, which showed PCV13 to be effective against IPD caused by vaccine serotypes among community-dwelling adults aged 65 and older,” she noted. “Additionally, data from CDC surveillance suggest that PCV13-serotype [invasive pneumococcal disease] among children and adults aged 65 and older has declined dramatically following PCV13 introduction for children in 2010, as predicted.”
In fact, among adults aged 65 years and older, PCV13-serotype invasive pneumococcal disease declined by 40% after the vaccine was introduced in children. This corresponds to a change in the annual PCV13-serotype incidence from 14 cases per 100,000 population in 2010 to five cases per 100,000 population in 2014, she said; she added that IPD incidence plateaued in 2014-2016 with vaccine serotypes contributing to a small proportion of overall IPD burden among adults aged 65 years and older.
ACIP’s reevaluation of the PCV13 recommendation is ongoing and will be addressed at upcoming meetings.
“As part of the review process, we look at changes in disease incidence focusing primarily on invasive pneumococcal disease and noninvasive pneumonia, vaccine efficacy and effectiveness, and vaccine safety,” she said. She noted that ACIP currently has no plans to consider revising PCV13 recommendations for adults who have immunocompromising conditions, for whom PCV13 has been recommended since 2012.
Ms. Almendares reported having no disclosures.
SOURCE: Almendares O et al. ICEID 2018, Board 376.
REPORTING FROM ICEID 2018
Natural history is comparable for astrovirus, sapovirus, norovirus
ATLANTA – Astrovirus and sapovirus in children visiting the ED with acute gastroenteritis have a similar natural history, which is also similar to that of norovirus, according to findings from a prospective cohort study.
Thanks to the increased use of molecular diagnostic testing, astrovirus and sapovirus (AsV and SaV) recently gained new appreciation as a cause of gastroenteritis, but little is known about their natural history.
The current findings from the Alberta Provincial Pediatric Enteric Infection Team (APPETITE) study provide some longitudinal insight regarding that history, Gillian A. M. Tarr, PhD, of the University of Calgary (Alta.), and her colleagues noted in a poster presentation at the International Conference on Emerging Infectious Diseases.
The investigators tested rectal swabs and stool samples from 2,590 children under age 18 years who had experienced at least three episodes of diarrhea and/or vomiting in 24 hours. AsV was detected in 84 children (3.2%), SaV was detected in 229 (8.8%), and norovirus (NoV) was detected in 655 (25%). After excluding those with coinfection, a total of 54, 144, and 478 children with AsV, SaV, and NoV, respectively, were included.
Vomiting occurred in 83%, 92%, and 98%; diarrhea occurred in 94%, 79% and 75%; and blood was present in the stool in 4.0%, 7.0%, and 3.5%, of those with AsV, SaV, and NoV, respectively, they reported.
The median maximal vomiting episodes per day was slightly lower for AsV versus SaV and NoV (about 3 vs. 5 and 7, respectively); the median maximal diarrhea episodes was slightly higher for AsV (about 5 vs. 4 for both SaV and NoV).
Vomiting duration was similar for AsV and SaV (about 3 days) and slightly longer than for NoV (about 2 days); median diarrhea duration was about 5 days for all three viruses.
“Most AsV cases present with both vomiting and diarrhea; most SaV cases present with vomiting and only approximately half with diarrhea,” the investigators wrote.
APPETITE study participants were enrolled from the EDs of two large children’s hospitals from December 2014 to May 2018. All were tested for 18 enteric pathogens and information on patient symptoms, medications, and potential exposures at enrollment and at 14 days was provided by patients and parents/guardians.
“We compared the natural histories of AsV and SaV to that of NoV, and found that all are comparable. These data provide a clearer picture than previously available of the duration and intensity of cardinal gastroenteritis symptoms associated with AsV and SaV infections,” they concluded.
Dr. Tarr reported having no financial disclosures.
ATLANTA – Astrovirus and sapovirus in children visiting the ED with acute gastroenteritis have a similar natural history, which is also similar to that of norovirus, according to findings from a prospective cohort study.
Thanks to the increased use of molecular diagnostic testing, astrovirus and sapovirus (AsV and SaV) recently gained new appreciation as a cause of gastroenteritis, but little is known about their natural history.
The current findings from the Alberta Provincial Pediatric Enteric Infection Team (APPETITE) study provide some longitudinal insight regarding that history, Gillian A. M. Tarr, PhD, of the University of Calgary (Alta.), and her colleagues noted in a poster presentation at the International Conference on Emerging Infectious Diseases.
The investigators tested rectal swabs and stool samples from 2,590 children under age 18 years who had experienced at least three episodes of diarrhea and/or vomiting in 24 hours. AsV was detected in 84 children (3.2%), SaV was detected in 229 (8.8%), and norovirus (NoV) was detected in 655 (25%). After excluding those with coinfection, a total of 54, 144, and 478 children with AsV, SaV, and NoV, respectively, were included.
Vomiting occurred in 83%, 92%, and 98%; diarrhea occurred in 94%, 79% and 75%; and blood was present in the stool in 4.0%, 7.0%, and 3.5%, of those with AsV, SaV, and NoV, respectively, they reported.
The median maximal vomiting episodes per day was slightly lower for AsV versus SaV and NoV (about 3 vs. 5 and 7, respectively); the median maximal diarrhea episodes was slightly higher for AsV (about 5 vs. 4 for both SaV and NoV).
Vomiting duration was similar for AsV and SaV (about 3 days) and slightly longer than for NoV (about 2 days); median diarrhea duration was about 5 days for all three viruses.
“Most AsV cases present with both vomiting and diarrhea; most SaV cases present with vomiting and only approximately half with diarrhea,” the investigators wrote.
APPETITE study participants were enrolled from the EDs of two large children’s hospitals from December 2014 to May 2018. All were tested for 18 enteric pathogens and information on patient symptoms, medications, and potential exposures at enrollment and at 14 days was provided by patients and parents/guardians.
“We compared the natural histories of AsV and SaV to that of NoV, and found that all are comparable. These data provide a clearer picture than previously available of the duration and intensity of cardinal gastroenteritis symptoms associated with AsV and SaV infections,” they concluded.
Dr. Tarr reported having no financial disclosures.
ATLANTA – Astrovirus and sapovirus in children visiting the ED with acute gastroenteritis have a similar natural history, which is also similar to that of norovirus, according to findings from a prospective cohort study.
Thanks to the increased use of molecular diagnostic testing, astrovirus and sapovirus (AsV and SaV) recently gained new appreciation as a cause of gastroenteritis, but little is known about their natural history.
The current findings from the Alberta Provincial Pediatric Enteric Infection Team (APPETITE) study provide some longitudinal insight regarding that history, Gillian A. M. Tarr, PhD, of the University of Calgary (Alta.), and her colleagues noted in a poster presentation at the International Conference on Emerging Infectious Diseases.
The investigators tested rectal swabs and stool samples from 2,590 children under age 18 years who had experienced at least three episodes of diarrhea and/or vomiting in 24 hours. AsV was detected in 84 children (3.2%), SaV was detected in 229 (8.8%), and norovirus (NoV) was detected in 655 (25%). After excluding those with coinfection, a total of 54, 144, and 478 children with AsV, SaV, and NoV, respectively, were included.
Vomiting occurred in 83%, 92%, and 98%; diarrhea occurred in 94%, 79% and 75%; and blood was present in the stool in 4.0%, 7.0%, and 3.5%, of those with AsV, SaV, and NoV, respectively, they reported.
The median maximal vomiting episodes per day was slightly lower for AsV versus SaV and NoV (about 3 vs. 5 and 7, respectively); the median maximal diarrhea episodes was slightly higher for AsV (about 5 vs. 4 for both SaV and NoV).
Vomiting duration was similar for AsV and SaV (about 3 days) and slightly longer than for NoV (about 2 days); median diarrhea duration was about 5 days for all three viruses.
“Most AsV cases present with both vomiting and diarrhea; most SaV cases present with vomiting and only approximately half with diarrhea,” the investigators wrote.
APPETITE study participants were enrolled from the EDs of two large children’s hospitals from December 2014 to May 2018. All were tested for 18 enteric pathogens and information on patient symptoms, medications, and potential exposures at enrollment and at 14 days was provided by patients and parents/guardians.
“We compared the natural histories of AsV and SaV to that of NoV, and found that all are comparable. These data provide a clearer picture than previously available of the duration and intensity of cardinal gastroenteritis symptoms associated with AsV and SaV infections,” they concluded.
Dr. Tarr reported having no financial disclosures.
REPORTING FROM ICEID 2018
Key clinical point:
Major finding: Vomiting lasted about 3 days for astrovirus and sapovirus and 2 days for norovirus; diarrhea lasted about 5 days for all three viruses.
Study details: A prospective cohort study of 2,590 children.
Disclosures: Dr. Tarr reported having no financial disclosures.
Zika virus infection: Novel assay extends diagnostic window
ATLANTA – A novel pyrosequencing (PSQ)–based reverse-transcription polymerase chain reaction (RT-PCR) assay improves and expands diagnostic capabilities for Zika virus infection, according to findings in 60 patients diagnosed with the virus in 2016 and 2017.
The PSQ assay provides rapid, specific, and cost-effective detection of the virus in tissues of congenital and pregnancy-associated infections, and, compared with serum-based assays, extends the time frame for Zika virus detection, Julu Bhatnagar, PhD, reported in a presentation at the International Conference on Emerging Infectious Diseases.
Dr. Bhatnagar and her colleagues from the Centers for Disease Control and Prevention in Atlanta developed the assay and evaluated it using RNA extracted from formalin-fixed, paraffin-embedded placental/fetal tissues from 53 women with varying pregnancy outcomes, and brain tissues from seven infants with microcephaly who died. In all of the tissue samples, which were received between January 2016 and August 2017, Zika virus was previously identified by conventional RT-PCR and Sanger sequencing.
The PSQ assay detected and sequence confirmed Zika virus in tissues from all 60 patients, whereas 40 negative control samples, including tissues from dengue- and chikungunya virus–confirmed cases, all tested negative.
In addition, the PSQ assay detected Zika virus in placental tissues from three other cases that were previously negative by the conventional tissue-based RT-PCR, thereby demonstrating better sensitivity of the PSQ assay in comparison to conventional tissue RT-PCR, said Dr. Bhatnagar, who is molecular pathology team leader in the Infectious Diseases Pathology Branch, Division of High-Consequence Pathogens and Pathology at the CDC’s National Center for Emerging and Zoonotic Infectious Diseases.
“Importantly, PSQ results can be obtained in 1 day and at half the cost of Sanger sequencing,” she said.
The findings are important because Zika virus infection during pregnancy can cause microcephaly and is associated with pregnancy loss. Laboratory diagnosis of the virus is challenging for pregnancy-associated infections because of the short duration of viremia, she explained.
However, prolonged detection of Zika virus RNA in placental, fetal, and neonatal brain tissue has been reported.
Dr. Bhatnagar was the first author on a 2016 study published in Emerging Infectious Diseases that provided confirmation of the linkage of Zika virus with microcephaly and that suggested its association with adverse pregnancy outcomes and provided evidence of Zika virus replication and persistence in fetal brain and placenta.
“This article highlights the value of tissue analysis to expand opportunities to diagnose Zika virus congenital and pregnancy-associated infections and to enhance the understanding of mechanism of Zika virus intrauterine transmission and pathogenesis,” she and her colleagues wrote in that article. “In addition, the tissue-based RT-PCRs extend the time frame for Zika virus detection and particularly help to establish a diagnosis retrospectively, enabling pregnant women and their health care providers to identify the cause of severe microcephaly or fetal loss.”
Those findings led to the hypothesis that the PSQ assay evaluated in the current study would provide better opportunities for detection, particularly in cases where serum RT-PCR or serologic testing is negative because of testing performed outside the optimal testing window, she said.
Indeed, the novel assay not only allows for an extended time frame for Zika virus detection, it also provides insights into viral tissue tropism and persistence, she noted.
According to the CDC, no local mosquito-borne Zika virus transmissions have been reported in the continental United States in 2018, but transmission is still a threat internationally, and those traveling outside of the continental United States should find out if they are traveling to an area with risk of Zika.
Dr. Bhatnagar reported having no disclosures.
SOURCE: Bhatnagar J et al. ICEID 2018, Abstract O1.
ATLANTA – A novel pyrosequencing (PSQ)–based reverse-transcription polymerase chain reaction (RT-PCR) assay improves and expands diagnostic capabilities for Zika virus infection, according to findings in 60 patients diagnosed with the virus in 2016 and 2017.
The PSQ assay provides rapid, specific, and cost-effective detection of the virus in tissues of congenital and pregnancy-associated infections, and, compared with serum-based assays, extends the time frame for Zika virus detection, Julu Bhatnagar, PhD, reported in a presentation at the International Conference on Emerging Infectious Diseases.
Dr. Bhatnagar and her colleagues from the Centers for Disease Control and Prevention in Atlanta developed the assay and evaluated it using RNA extracted from formalin-fixed, paraffin-embedded placental/fetal tissues from 53 women with varying pregnancy outcomes, and brain tissues from seven infants with microcephaly who died. In all of the tissue samples, which were received between January 2016 and August 2017, Zika virus was previously identified by conventional RT-PCR and Sanger sequencing.
The PSQ assay detected and sequence confirmed Zika virus in tissues from all 60 patients, whereas 40 negative control samples, including tissues from dengue- and chikungunya virus–confirmed cases, all tested negative.
In addition, the PSQ assay detected Zika virus in placental tissues from three other cases that were previously negative by the conventional tissue-based RT-PCR, thereby demonstrating better sensitivity of the PSQ assay in comparison to conventional tissue RT-PCR, said Dr. Bhatnagar, who is molecular pathology team leader in the Infectious Diseases Pathology Branch, Division of High-Consequence Pathogens and Pathology at the CDC’s National Center for Emerging and Zoonotic Infectious Diseases.
“Importantly, PSQ results can be obtained in 1 day and at half the cost of Sanger sequencing,” she said.
The findings are important because Zika virus infection during pregnancy can cause microcephaly and is associated with pregnancy loss. Laboratory diagnosis of the virus is challenging for pregnancy-associated infections because of the short duration of viremia, she explained.
However, prolonged detection of Zika virus RNA in placental, fetal, and neonatal brain tissue has been reported.
Dr. Bhatnagar was the first author on a 2016 study published in Emerging Infectious Diseases that provided confirmation of the linkage of Zika virus with microcephaly and that suggested its association with adverse pregnancy outcomes and provided evidence of Zika virus replication and persistence in fetal brain and placenta.
“This article highlights the value of tissue analysis to expand opportunities to diagnose Zika virus congenital and pregnancy-associated infections and to enhance the understanding of mechanism of Zika virus intrauterine transmission and pathogenesis,” she and her colleagues wrote in that article. “In addition, the tissue-based RT-PCRs extend the time frame for Zika virus detection and particularly help to establish a diagnosis retrospectively, enabling pregnant women and their health care providers to identify the cause of severe microcephaly or fetal loss.”
Those findings led to the hypothesis that the PSQ assay evaluated in the current study would provide better opportunities for detection, particularly in cases where serum RT-PCR or serologic testing is negative because of testing performed outside the optimal testing window, she said.
Indeed, the novel assay not only allows for an extended time frame for Zika virus detection, it also provides insights into viral tissue tropism and persistence, she noted.
According to the CDC, no local mosquito-borne Zika virus transmissions have been reported in the continental United States in 2018, but transmission is still a threat internationally, and those traveling outside of the continental United States should find out if they are traveling to an area with risk of Zika.
Dr. Bhatnagar reported having no disclosures.
SOURCE: Bhatnagar J et al. ICEID 2018, Abstract O1.
ATLANTA – A novel pyrosequencing (PSQ)–based reverse-transcription polymerase chain reaction (RT-PCR) assay improves and expands diagnostic capabilities for Zika virus infection, according to findings in 60 patients diagnosed with the virus in 2016 and 2017.
The PSQ assay provides rapid, specific, and cost-effective detection of the virus in tissues of congenital and pregnancy-associated infections, and, compared with serum-based assays, extends the time frame for Zika virus detection, Julu Bhatnagar, PhD, reported in a presentation at the International Conference on Emerging Infectious Diseases.
Dr. Bhatnagar and her colleagues from the Centers for Disease Control and Prevention in Atlanta developed the assay and evaluated it using RNA extracted from formalin-fixed, paraffin-embedded placental/fetal tissues from 53 women with varying pregnancy outcomes, and brain tissues from seven infants with microcephaly who died. In all of the tissue samples, which were received between January 2016 and August 2017, Zika virus was previously identified by conventional RT-PCR and Sanger sequencing.
The PSQ assay detected and sequence confirmed Zika virus in tissues from all 60 patients, whereas 40 negative control samples, including tissues from dengue- and chikungunya virus–confirmed cases, all tested negative.
In addition, the PSQ assay detected Zika virus in placental tissues from three other cases that were previously negative by the conventional tissue-based RT-PCR, thereby demonstrating better sensitivity of the PSQ assay in comparison to conventional tissue RT-PCR, said Dr. Bhatnagar, who is molecular pathology team leader in the Infectious Diseases Pathology Branch, Division of High-Consequence Pathogens and Pathology at the CDC’s National Center for Emerging and Zoonotic Infectious Diseases.
“Importantly, PSQ results can be obtained in 1 day and at half the cost of Sanger sequencing,” she said.
The findings are important because Zika virus infection during pregnancy can cause microcephaly and is associated with pregnancy loss. Laboratory diagnosis of the virus is challenging for pregnancy-associated infections because of the short duration of viremia, she explained.
However, prolonged detection of Zika virus RNA in placental, fetal, and neonatal brain tissue has been reported.
Dr. Bhatnagar was the first author on a 2016 study published in Emerging Infectious Diseases that provided confirmation of the linkage of Zika virus with microcephaly and that suggested its association with adverse pregnancy outcomes and provided evidence of Zika virus replication and persistence in fetal brain and placenta.
“This article highlights the value of tissue analysis to expand opportunities to diagnose Zika virus congenital and pregnancy-associated infections and to enhance the understanding of mechanism of Zika virus intrauterine transmission and pathogenesis,” she and her colleagues wrote in that article. “In addition, the tissue-based RT-PCRs extend the time frame for Zika virus detection and particularly help to establish a diagnosis retrospectively, enabling pregnant women and their health care providers to identify the cause of severe microcephaly or fetal loss.”
Those findings led to the hypothesis that the PSQ assay evaluated in the current study would provide better opportunities for detection, particularly in cases where serum RT-PCR or serologic testing is negative because of testing performed outside the optimal testing window, she said.
Indeed, the novel assay not only allows for an extended time frame for Zika virus detection, it also provides insights into viral tissue tropism and persistence, she noted.
According to the CDC, no local mosquito-borne Zika virus transmissions have been reported in the continental United States in 2018, but transmission is still a threat internationally, and those traveling outside of the continental United States should find out if they are traveling to an area with risk of Zika.
Dr. Bhatnagar reported having no disclosures.
SOURCE: Bhatnagar J et al. ICEID 2018, Abstract O1.
REPORTING FROM ICEID 2018
Key clinical point: A novel assay extends the time frame for diagnosing Zika virus infection.
Major finding: The PSQ assay detected and sequence confirmed Zika virus in all 60 samples; 40 negative control samples all tested negative.
Study details: An analysis of 60 tissue samples using a novel assay.
Disclosures: Dr. Bhatnagar reported having no disclosures.
Source: Bhatnagar J et al. ICEID 2018, Abstract O1.
Review protocols, follow reprocessing guidelines to cut device-related HAIs
ATLANTA – Ongoing vigilance regarding the role of and transmission of antimicrobial-resistant pathogen is needed, according to Isaac Benowitz, MD, of the Centers for Disease Control and Prevention’s Division of Healthcare Quality Promotion (DHQP).
A review of records from the DHQP, which investigates and responds to infections and related adverse events in health care settings upon invitation, showed that in 2017 environmental pathogens were most often the triggers for these investigations, said Dr. Benowitz, a medical epidemiologist.
He reviewed internal records for consultations with state and local health departments involving medical devices and collected data on health care setting, pathogen, investigation findings including possible exposure or transmission, and public health actions.
Of 285 consultations, 48 involved a specific medical device or general medical device reprocessing, he said, noting that most of those 48 were in an acute care hospital (63%) or clinic (19%).
“The most frequent pathogens noted in these consultations were nontuberculous mycobacteria at 21%, Candida species ... at 10%, and Burkholderia species ... at 8%,” he said, noting that a wide variety of devices were implicated.
In the inpatient setting these devices included ventilators, dialysis machines, breast pumps, central lines, and respiratory therapy equipment. In the outpatient setting they included glucometers and opthalmic equipment.
“In many settings we saw issues with endoscopes, including duodenoscopes, but also bronchoscopes,” he added.
Actions taken as part of the investigations included medical device recalls, improved infection control and reprocessing procedures, and patient notification, education, guidance, testing, and treatment.
In some cases there was disciplinary action or oversight for health care professionals, he added.
Investigations identified medical devices contaminated in manufacturing, incorrect reprocessing of endoscopes or ventilators, and inappropriate medical device use or reuse, he said.
A number of lessons can be learned from these and other investigations, he added.
“First, devices can be reservoirs and transmission vectors for health care–associated infections. Second, health care facilities, health care facility staff, and public health partners should take opportunities to review protocols and the practices within those protocol,” he said. “These are opportunities to strengthen infection control practices even in the absence of documented transmission.”
In fact, in most of the investigations he discussed, transmission was rarely confirmed to be associated with a medical device. This was largely because of a lack of “epidemiological rigor,” but associations between health care–associated infections and medical devices “are still quite meaningful and often actionable,” he said.
Dr. Benowitz stressed the importance of engaging public health partners to discuss findings and actions, explaining that “what may look like a single-facility issue may have a very different perspective when you realize that there’s a similar issue at another facility elsewhere.”
“For all devices, it’s important to ensure adherence to the device reprocessing guidelines, “ he added, noting that these include a combination of facility protocols, manufacturer instructions for use, and guidance from organizations like the Food and Drug Administration and the CDC.
Dr. Benowitz reported having no disclosures.
sworcester@mdedge.com
SOURCE: Benowitz I et al. ICEID 2018, Oral Abstract Presentation E2.
ATLANTA – Ongoing vigilance regarding the role of and transmission of antimicrobial-resistant pathogen is needed, according to Isaac Benowitz, MD, of the Centers for Disease Control and Prevention’s Division of Healthcare Quality Promotion (DHQP).
A review of records from the DHQP, which investigates and responds to infections and related adverse events in health care settings upon invitation, showed that in 2017 environmental pathogens were most often the triggers for these investigations, said Dr. Benowitz, a medical epidemiologist.
He reviewed internal records for consultations with state and local health departments involving medical devices and collected data on health care setting, pathogen, investigation findings including possible exposure or transmission, and public health actions.
Of 285 consultations, 48 involved a specific medical device or general medical device reprocessing, he said, noting that most of those 48 were in an acute care hospital (63%) or clinic (19%).
“The most frequent pathogens noted in these consultations were nontuberculous mycobacteria at 21%, Candida species ... at 10%, and Burkholderia species ... at 8%,” he said, noting that a wide variety of devices were implicated.
In the inpatient setting these devices included ventilators, dialysis machines, breast pumps, central lines, and respiratory therapy equipment. In the outpatient setting they included glucometers and opthalmic equipment.
“In many settings we saw issues with endoscopes, including duodenoscopes, but also bronchoscopes,” he added.
Actions taken as part of the investigations included medical device recalls, improved infection control and reprocessing procedures, and patient notification, education, guidance, testing, and treatment.
In some cases there was disciplinary action or oversight for health care professionals, he added.
Investigations identified medical devices contaminated in manufacturing, incorrect reprocessing of endoscopes or ventilators, and inappropriate medical device use or reuse, he said.
A number of lessons can be learned from these and other investigations, he added.
“First, devices can be reservoirs and transmission vectors for health care–associated infections. Second, health care facilities, health care facility staff, and public health partners should take opportunities to review protocols and the practices within those protocol,” he said. “These are opportunities to strengthen infection control practices even in the absence of documented transmission.”
In fact, in most of the investigations he discussed, transmission was rarely confirmed to be associated with a medical device. This was largely because of a lack of “epidemiological rigor,” but associations between health care–associated infections and medical devices “are still quite meaningful and often actionable,” he said.
Dr. Benowitz stressed the importance of engaging public health partners to discuss findings and actions, explaining that “what may look like a single-facility issue may have a very different perspective when you realize that there’s a similar issue at another facility elsewhere.”
“For all devices, it’s important to ensure adherence to the device reprocessing guidelines, “ he added, noting that these include a combination of facility protocols, manufacturer instructions for use, and guidance from organizations like the Food and Drug Administration and the CDC.
Dr. Benowitz reported having no disclosures.
sworcester@mdedge.com
SOURCE: Benowitz I et al. ICEID 2018, Oral Abstract Presentation E2.
ATLANTA – Ongoing vigilance regarding the role of and transmission of antimicrobial-resistant pathogen is needed, according to Isaac Benowitz, MD, of the Centers for Disease Control and Prevention’s Division of Healthcare Quality Promotion (DHQP).
A review of records from the DHQP, which investigates and responds to infections and related adverse events in health care settings upon invitation, showed that in 2017 environmental pathogens were most often the triggers for these investigations, said Dr. Benowitz, a medical epidemiologist.
He reviewed internal records for consultations with state and local health departments involving medical devices and collected data on health care setting, pathogen, investigation findings including possible exposure or transmission, and public health actions.
Of 285 consultations, 48 involved a specific medical device or general medical device reprocessing, he said, noting that most of those 48 were in an acute care hospital (63%) or clinic (19%).
“The most frequent pathogens noted in these consultations were nontuberculous mycobacteria at 21%, Candida species ... at 10%, and Burkholderia species ... at 8%,” he said, noting that a wide variety of devices were implicated.
In the inpatient setting these devices included ventilators, dialysis machines, breast pumps, central lines, and respiratory therapy equipment. In the outpatient setting they included glucometers and opthalmic equipment.
“In many settings we saw issues with endoscopes, including duodenoscopes, but also bronchoscopes,” he added.
Actions taken as part of the investigations included medical device recalls, improved infection control and reprocessing procedures, and patient notification, education, guidance, testing, and treatment.
In some cases there was disciplinary action or oversight for health care professionals, he added.
Investigations identified medical devices contaminated in manufacturing, incorrect reprocessing of endoscopes or ventilators, and inappropriate medical device use or reuse, he said.
A number of lessons can be learned from these and other investigations, he added.
“First, devices can be reservoirs and transmission vectors for health care–associated infections. Second, health care facilities, health care facility staff, and public health partners should take opportunities to review protocols and the practices within those protocol,” he said. “These are opportunities to strengthen infection control practices even in the absence of documented transmission.”
In fact, in most of the investigations he discussed, transmission was rarely confirmed to be associated with a medical device. This was largely because of a lack of “epidemiological rigor,” but associations between health care–associated infections and medical devices “are still quite meaningful and often actionable,” he said.
Dr. Benowitz stressed the importance of engaging public health partners to discuss findings and actions, explaining that “what may look like a single-facility issue may have a very different perspective when you realize that there’s a similar issue at another facility elsewhere.”
“For all devices, it’s important to ensure adherence to the device reprocessing guidelines, “ he added, noting that these include a combination of facility protocols, manufacturer instructions for use, and guidance from organizations like the Food and Drug Administration and the CDC.
Dr. Benowitz reported having no disclosures.
sworcester@mdedge.com
SOURCE: Benowitz I et al. ICEID 2018, Oral Abstract Presentation E2.
REPORTING FROM ICEID 2018
Key clinical point: Medical devices can be reservoirs and transmission vectors for health care–associated infections.
Major finding: Of 285 consultations, 48 involved medical devices or device reprocessing.
Study details: A review of records from 285 consultations
Disclosures: Dr. Benowitz reported having no disclosures
Source: Benowitz I et al. ICEID 2018, Oral Abstract Presentation E2.
Cutaneous lesions? Consider C. diphtheriae in those with foreign travel
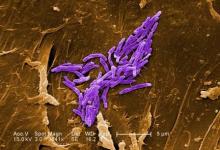
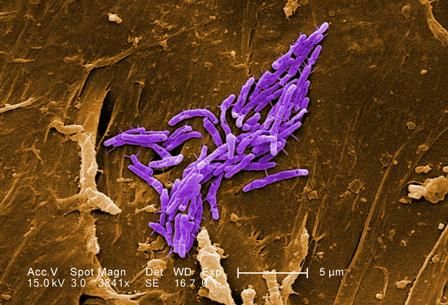
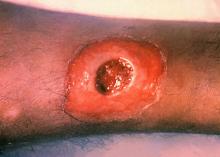
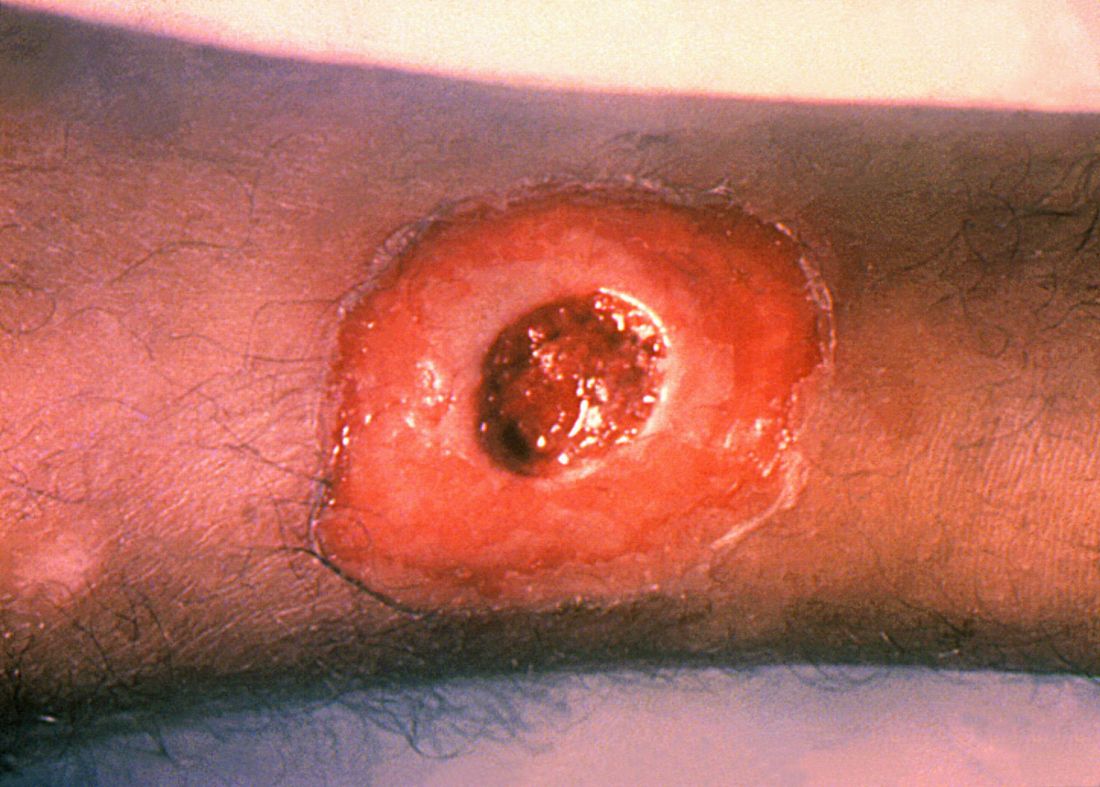
ATLANTA – Seven cases of imported Corynebacterium diphtheriae in Minnesota highlight the importance of maintaining suspicion that cutaneous lesions in individuals with recent travel to endemic countries might be associated with C. diphtheriae infection.
The cases also underscore the importance of referring C. diphtheriae isolates to state health departments for confirmatory testing, Jayne Griffith, of the Minnesota Department of Health, and her colleagues reported in a poster at the International Conference on Emerging Infectious Diseases.
“C. diphtheriae infections was not clinically suspected in any of these case-patients. All infections were initially identified solely by [matrix-assisted laser desorption/ionization time-of-flight spectrometry] testing performed at the clinical institutions,” the investigators wrote. “Confirmation and further toxigenicity testing allowed for prompt case investigation and public health response, preventing disease spread.”
Infections caused by toxigenic C. diphtheriae are rare in the United States because of widespread vaccination, but remain endemic in countries with suboptimal vaccine coverage. For this reason, infection is a concern for unvaccinated individuals traveling to diphtheria-endemic countries as well as for those who have contact with people from these areas. The investigators noted that “infections are primarily respiratory or cutaneous; respiratory infections can be life-threatening and cutaneous wounds may serve as a reservoir from which bacteria can be transmitted to susceptible contacts.”
The Minnesota cases involved patients who presented with cutaneous ulcers between 2014 and 2017. The Minnesota Department of Health confirmed C. diphtheriae status by culture after the initial identification at private institutions or providers using matrix-assisted laser desorption/ionization time-of-flight spectrometry. Isolates were sent to the Centers for Disease Control and Prevention Pertussis and Diphtheria Laboratory for biotyping and confirmation of toxigenicity.
The CDC confirmed that isolates from two patients were toxigenic C. diphtheriae biotype mitis. The remaining cases were nontoxigenic diphtheria, including C. diphtheriae mitis (three case-patients, including one who also had Staphylococcus aureus, and another who also had methicillin-resistant S. aureus) and two case-patients with C. diphtheriae belfanti.
The patients with toxigenic infection included a 35-year-old woman who developed an abdominal boil after spending months in Somalia, and a 48-year-old man who cut his leg while in Ethiopia.
The patients with nontoxigenic infection included four foreign-born individuals ranging in age from 7 to 66 years and one 24-year-old man from the United States. One of the foreign-born individuals had immigrated from a diphtheria-endemic country 3 months prior to his diagnosis, but none of the remaining four had traveled outside the United States in the 6 months prior to infection onset. One, however, lived in a home with family members who traveled frequently to eastern Africa. The vaccination status of these patients was unknown.
Contact tracing was conducted and prophylactic antibiotics were recommended as appropriate. Vaccination was recommended when a case-patient and/or contact was inadequately immunized.
Both patients with toxigenic infection experienced wound healing after appropriate antibiotic therapy.
Ms. Griffith reported having no disclosures.
ATLANTA – Seven cases of imported Corynebacterium diphtheriae in Minnesota highlight the importance of maintaining suspicion that cutaneous lesions in individuals with recent travel to endemic countries might be associated with C. diphtheriae infection.
The cases also underscore the importance of referring C. diphtheriae isolates to state health departments for confirmatory testing, Jayne Griffith, of the Minnesota Department of Health, and her colleagues reported in a poster at the International Conference on Emerging Infectious Diseases.
“C. diphtheriae infections was not clinically suspected in any of these case-patients. All infections were initially identified solely by [matrix-assisted laser desorption/ionization time-of-flight spectrometry] testing performed at the clinical institutions,” the investigators wrote. “Confirmation and further toxigenicity testing allowed for prompt case investigation and public health response, preventing disease spread.”
Infections caused by toxigenic C. diphtheriae are rare in the United States because of widespread vaccination, but remain endemic in countries with suboptimal vaccine coverage. For this reason, infection is a concern for unvaccinated individuals traveling to diphtheria-endemic countries as well as for those who have contact with people from these areas. The investigators noted that “infections are primarily respiratory or cutaneous; respiratory infections can be life-threatening and cutaneous wounds may serve as a reservoir from which bacteria can be transmitted to susceptible contacts.”
The Minnesota cases involved patients who presented with cutaneous ulcers between 2014 and 2017. The Minnesota Department of Health confirmed C. diphtheriae status by culture after the initial identification at private institutions or providers using matrix-assisted laser desorption/ionization time-of-flight spectrometry. Isolates were sent to the Centers for Disease Control and Prevention Pertussis and Diphtheria Laboratory for biotyping and confirmation of toxigenicity.
The CDC confirmed that isolates from two patients were toxigenic C. diphtheriae biotype mitis. The remaining cases were nontoxigenic diphtheria, including C. diphtheriae mitis (three case-patients, including one who also had Staphylococcus aureus, and another who also had methicillin-resistant S. aureus) and two case-patients with C. diphtheriae belfanti.
The patients with toxigenic infection included a 35-year-old woman who developed an abdominal boil after spending months in Somalia, and a 48-year-old man who cut his leg while in Ethiopia.
The patients with nontoxigenic infection included four foreign-born individuals ranging in age from 7 to 66 years and one 24-year-old man from the United States. One of the foreign-born individuals had immigrated from a diphtheria-endemic country 3 months prior to his diagnosis, but none of the remaining four had traveled outside the United States in the 6 months prior to infection onset. One, however, lived in a home with family members who traveled frequently to eastern Africa. The vaccination status of these patients was unknown.
Contact tracing was conducted and prophylactic antibiotics were recommended as appropriate. Vaccination was recommended when a case-patient and/or contact was inadequately immunized.
Both patients with toxigenic infection experienced wound healing after appropriate antibiotic therapy.
Ms. Griffith reported having no disclosures.
ATLANTA – Seven cases of imported Corynebacterium diphtheriae in Minnesota highlight the importance of maintaining suspicion that cutaneous lesions in individuals with recent travel to endemic countries might be associated with C. diphtheriae infection.
The cases also underscore the importance of referring C. diphtheriae isolates to state health departments for confirmatory testing, Jayne Griffith, of the Minnesota Department of Health, and her colleagues reported in a poster at the International Conference on Emerging Infectious Diseases.
“C. diphtheriae infections was not clinically suspected in any of these case-patients. All infections were initially identified solely by [matrix-assisted laser desorption/ionization time-of-flight spectrometry] testing performed at the clinical institutions,” the investigators wrote. “Confirmation and further toxigenicity testing allowed for prompt case investigation and public health response, preventing disease spread.”
Infections caused by toxigenic C. diphtheriae are rare in the United States because of widespread vaccination, but remain endemic in countries with suboptimal vaccine coverage. For this reason, infection is a concern for unvaccinated individuals traveling to diphtheria-endemic countries as well as for those who have contact with people from these areas. The investigators noted that “infections are primarily respiratory or cutaneous; respiratory infections can be life-threatening and cutaneous wounds may serve as a reservoir from which bacteria can be transmitted to susceptible contacts.”
The Minnesota cases involved patients who presented with cutaneous ulcers between 2014 and 2017. The Minnesota Department of Health confirmed C. diphtheriae status by culture after the initial identification at private institutions or providers using matrix-assisted laser desorption/ionization time-of-flight spectrometry. Isolates were sent to the Centers for Disease Control and Prevention Pertussis and Diphtheria Laboratory for biotyping and confirmation of toxigenicity.
The CDC confirmed that isolates from two patients were toxigenic C. diphtheriae biotype mitis. The remaining cases were nontoxigenic diphtheria, including C. diphtheriae mitis (three case-patients, including one who also had Staphylococcus aureus, and another who also had methicillin-resistant S. aureus) and two case-patients with C. diphtheriae belfanti.
The patients with toxigenic infection included a 35-year-old woman who developed an abdominal boil after spending months in Somalia, and a 48-year-old man who cut his leg while in Ethiopia.
The patients with nontoxigenic infection included four foreign-born individuals ranging in age from 7 to 66 years and one 24-year-old man from the United States. One of the foreign-born individuals had immigrated from a diphtheria-endemic country 3 months prior to his diagnosis, but none of the remaining four had traveled outside the United States in the 6 months prior to infection onset. One, however, lived in a home with family members who traveled frequently to eastern Africa. The vaccination status of these patients was unknown.
Contact tracing was conducted and prophylactic antibiotics were recommended as appropriate. Vaccination was recommended when a case-patient and/or contact was inadequately immunized.
Both patients with toxigenic infection experienced wound healing after appropriate antibiotic therapy.
Ms. Griffith reported having no disclosures.
REPORTING FROM ICEID 2018
Key clinical point: Corynebacterium diphtheriae should be considered in individuals who present with cutaneous lesions after traveling to diphtheria-endemic countries.
Major finding: Refer suspect C. diphtheriae isolates to state health departments.
Study details: A review of seven C. diphtheriae cases.
Disclosures: Ms. Griffith reported having no disclosures.
United Kingdom experience provides important lessons for controlling C. auris outbreaks
ATLANTA – The persistence and transmission of Candida auris in health care settings appears to be dependent on environmental survival, underscoring the need for careful investigation of the environment – and, in particular, multiuse patient equipment.
That’s the key lesson from one of the largest outbreaks of the emerging, multidrug-resistant pathogen to date, David Eyre, DPhil, said at the International Conference on Emerging Infectious Diseases.
“Our experience at Oxford began with a Public Health England alert, which closely followed a similar alert from the [Centers for Disease Control and Prevention] in the summer of 2016,” Dr. Eyre of the University of Oxford (England) said during an update on the epidemiology of the outbreak and the successful, multipronged effort to control it.
The outbreak, which occurred in the neurosciences intensive care unit of Oxford University Hospitals beginning in early 2015, was detected in 2016 when a cluster of C. auris infections was identified and traced to the unit. An intensive patient and environmental screening program was established, isolation protocols were used for patients who tested positive, enhanced cleaning processes were initiated, and equipment was removed and replaced with single-use equipment when possible.
“We also worked quite closely with our staff to raise awareness,” he said, adding that colonized patients who were undergoing a surgical procedure received single-dose antifungal prophylaxis prior to the procedure.
A case-control study was conducted, and after the researchers used multivariate logistic regression to control for length of stay, patient physiology, and biomarkers, exposure to multiuse skin surface axillary temperature monitoring was shown to be one of the strongest independent predictors of C. auris colonization and infection (odds ratio 6.80), he said, adding that antifungal exposure was also a significant risk factor, but only 5% of patients had received antifungals.
The axillary probes were then removed from the environment. As of April 2017 (when the probes were removed), 66 patients had been colonized or infected, and an additional 10 cases occurred after the probes were removed, with the last case occurring in November 2017.
Seven of the 76 cases involved invasive infection, and 1 patient died several months after hospital discharge, Dr. Eyre said.
The patient screening processes allowed for estimation of colonization time (approximately 2 months), and also allowed for whole-genome sequencing of 79 samples from 43 patients, 6 environmental isolates, and 2 isolates from regional surveillance, Dr. Eyre said.
All outbreak sequences formed a single genetic cluster within the C. auris South African clade, and were found to have been introduced to Oxford around 2012 or 2013, with about six mutations per year, or “roughly 12 million base pairs in total,” he said, adding that both patients and temperature probes were colonized with multiple strains, and there was “close mixing” between the two.
This pattern changed following removal of the temperature probes, but it took some time.
“However, from November [2017] onward – so that’s now 291 days ... we’ve not had another new patient isolate, and that’s not only no invasive infection, but also no colonization despite continuing the screening program,” he said.
According to the CDC, C. auris is “an emerging fungus that presents a serious global health threat” because of its often multidrug-resistant nature, difficulty identifying the pathogen using standard laboratory methods, and the risk for misidentification in labs without specific technology, which could lead to inappropriate management.
“It has caused outbreaks in health care settings. For this reason, it is important to quickly identify C. auris in a hospitalized patient so that health care facilities can take special precautions to stop its spread,” a CDC page on C. auris states. “CDC encourages all U.S. laboratory staff who identify C. auris to notify their state or local public health authorities and CDC at candidaauris@cdc.gov.”
Dr. Eyre reported having no disclosures.
SOURCE: Eyre D et al. ICEID 2018 Oral Abstract Presentation.
ATLANTA – The persistence and transmission of Candida auris in health care settings appears to be dependent on environmental survival, underscoring the need for careful investigation of the environment – and, in particular, multiuse patient equipment.
That’s the key lesson from one of the largest outbreaks of the emerging, multidrug-resistant pathogen to date, David Eyre, DPhil, said at the International Conference on Emerging Infectious Diseases.
“Our experience at Oxford began with a Public Health England alert, which closely followed a similar alert from the [Centers for Disease Control and Prevention] in the summer of 2016,” Dr. Eyre of the University of Oxford (England) said during an update on the epidemiology of the outbreak and the successful, multipronged effort to control it.
The outbreak, which occurred in the neurosciences intensive care unit of Oxford University Hospitals beginning in early 2015, was detected in 2016 when a cluster of C. auris infections was identified and traced to the unit. An intensive patient and environmental screening program was established, isolation protocols were used for patients who tested positive, enhanced cleaning processes were initiated, and equipment was removed and replaced with single-use equipment when possible.
“We also worked quite closely with our staff to raise awareness,” he said, adding that colonized patients who were undergoing a surgical procedure received single-dose antifungal prophylaxis prior to the procedure.
A case-control study was conducted, and after the researchers used multivariate logistic regression to control for length of stay, patient physiology, and biomarkers, exposure to multiuse skin surface axillary temperature monitoring was shown to be one of the strongest independent predictors of C. auris colonization and infection (odds ratio 6.80), he said, adding that antifungal exposure was also a significant risk factor, but only 5% of patients had received antifungals.
The axillary probes were then removed from the environment. As of April 2017 (when the probes were removed), 66 patients had been colonized or infected, and an additional 10 cases occurred after the probes were removed, with the last case occurring in November 2017.
Seven of the 76 cases involved invasive infection, and 1 patient died several months after hospital discharge, Dr. Eyre said.
The patient screening processes allowed for estimation of colonization time (approximately 2 months), and also allowed for whole-genome sequencing of 79 samples from 43 patients, 6 environmental isolates, and 2 isolates from regional surveillance, Dr. Eyre said.
All outbreak sequences formed a single genetic cluster within the C. auris South African clade, and were found to have been introduced to Oxford around 2012 or 2013, with about six mutations per year, or “roughly 12 million base pairs in total,” he said, adding that both patients and temperature probes were colonized with multiple strains, and there was “close mixing” between the two.
This pattern changed following removal of the temperature probes, but it took some time.
“However, from November [2017] onward – so that’s now 291 days ... we’ve not had another new patient isolate, and that’s not only no invasive infection, but also no colonization despite continuing the screening program,” he said.
According to the CDC, C. auris is “an emerging fungus that presents a serious global health threat” because of its often multidrug-resistant nature, difficulty identifying the pathogen using standard laboratory methods, and the risk for misidentification in labs without specific technology, which could lead to inappropriate management.
“It has caused outbreaks in health care settings. For this reason, it is important to quickly identify C. auris in a hospitalized patient so that health care facilities can take special precautions to stop its spread,” a CDC page on C. auris states. “CDC encourages all U.S. laboratory staff who identify C. auris to notify their state or local public health authorities and CDC at candidaauris@cdc.gov.”
Dr. Eyre reported having no disclosures.
SOURCE: Eyre D et al. ICEID 2018 Oral Abstract Presentation.
ATLANTA – The persistence and transmission of Candida auris in health care settings appears to be dependent on environmental survival, underscoring the need for careful investigation of the environment – and, in particular, multiuse patient equipment.
That’s the key lesson from one of the largest outbreaks of the emerging, multidrug-resistant pathogen to date, David Eyre, DPhil, said at the International Conference on Emerging Infectious Diseases.
“Our experience at Oxford began with a Public Health England alert, which closely followed a similar alert from the [Centers for Disease Control and Prevention] in the summer of 2016,” Dr. Eyre of the University of Oxford (England) said during an update on the epidemiology of the outbreak and the successful, multipronged effort to control it.
The outbreak, which occurred in the neurosciences intensive care unit of Oxford University Hospitals beginning in early 2015, was detected in 2016 when a cluster of C. auris infections was identified and traced to the unit. An intensive patient and environmental screening program was established, isolation protocols were used for patients who tested positive, enhanced cleaning processes were initiated, and equipment was removed and replaced with single-use equipment when possible.
“We also worked quite closely with our staff to raise awareness,” he said, adding that colonized patients who were undergoing a surgical procedure received single-dose antifungal prophylaxis prior to the procedure.
A case-control study was conducted, and after the researchers used multivariate logistic regression to control for length of stay, patient physiology, and biomarkers, exposure to multiuse skin surface axillary temperature monitoring was shown to be one of the strongest independent predictors of C. auris colonization and infection (odds ratio 6.80), he said, adding that antifungal exposure was also a significant risk factor, but only 5% of patients had received antifungals.
The axillary probes were then removed from the environment. As of April 2017 (when the probes were removed), 66 patients had been colonized or infected, and an additional 10 cases occurred after the probes were removed, with the last case occurring in November 2017.
Seven of the 76 cases involved invasive infection, and 1 patient died several months after hospital discharge, Dr. Eyre said.
The patient screening processes allowed for estimation of colonization time (approximately 2 months), and also allowed for whole-genome sequencing of 79 samples from 43 patients, 6 environmental isolates, and 2 isolates from regional surveillance, Dr. Eyre said.
All outbreak sequences formed a single genetic cluster within the C. auris South African clade, and were found to have been introduced to Oxford around 2012 or 2013, with about six mutations per year, or “roughly 12 million base pairs in total,” he said, adding that both patients and temperature probes were colonized with multiple strains, and there was “close mixing” between the two.
This pattern changed following removal of the temperature probes, but it took some time.
“However, from November [2017] onward – so that’s now 291 days ... we’ve not had another new patient isolate, and that’s not only no invasive infection, but also no colonization despite continuing the screening program,” he said.
According to the CDC, C. auris is “an emerging fungus that presents a serious global health threat” because of its often multidrug-resistant nature, difficulty identifying the pathogen using standard laboratory methods, and the risk for misidentification in labs without specific technology, which could lead to inappropriate management.
“It has caused outbreaks in health care settings. For this reason, it is important to quickly identify C. auris in a hospitalized patient so that health care facilities can take special precautions to stop its spread,” a CDC page on C. auris states. “CDC encourages all U.S. laboratory staff who identify C. auris to notify their state or local public health authorities and CDC at candidaauris@cdc.gov.”
Dr. Eyre reported having no disclosures.
SOURCE: Eyre D et al. ICEID 2018 Oral Abstract Presentation.
REPORTING FROM ICEID 2018
Key clinical point:
Major finding: Ten additional cases occurred in the 7 months after the axillary probes were removed from the environment.
Study details: A review of the epidemiology and control of a C. auris outbreak affecting 76 patients.
Disclosures: Dr. Eyre reported having no disclosures.
Source: Eyre D et al. ICEID 2018 Oral Abstract Presentation.
Patient transfers between hospitals contribute substantially to CDI burden
ATLANTA – Patient sharing among hospital facilities contributed substantially to the overall Clostridium difficile infection rate, an analysis of interhospital contamination effects showed.
In fact, 7.6% of all Clostridium difficile infection (CDI) cases at the nearly 400 California hospitals included in the study were directly attributable to the patient-sharing network, Daniel Sewell, PhD, reported at the International Conference on Emerging Infectious Diseases.
“The methods that we employed allowed us to estimate the expected increase in CDI cases due to transfers as a function of the CDI rate at the hospital from which those patients were brought. These transfer patients were responsible for about 3.06 times the number of CDI cases as a normal patient,” said Dr. Sewell, a biostatistician at the University of Iowa, Iowa City.
The findings, which underscored the importance of regional (rather than local) efforts to minimize the spread of health care–associated infections, are based on an analysis of 27,200,873 hospital admissions and 532,320 same-day patient transfers identified from the Healthcare Cost and Utilization Project California State Inpatient Database for 2005-2011.
Transfer networks based on the monthly average number of patients discharged from one hospital and admitted to another on the same day were constructed, and the monthly average number of CDI cases per hospital were considered, along with hospital-level characteristics such as patient length of stay, age, and number of diagnoses. Network autocorrelation models that help eliminate bias were then used to assess the contamination effects between hospitals, he explained.
This led to development of an equation that can be used to determine the expected number of CDI cases in a hospital as a function of the number of transfers coming in and the contamination level of the source hospitals. The ability to calculate the expected number of CDI cases in this fashion is an important factor for the success of regional versus local intervention efforts, which are increasingly thought to be important for reducing health care–associated infections.
“If we want to design a coordinated or regional approach, we’ve got to have a much better understanding of the role that patient transfers have in these diseases,” Dr. Sewell said.
As most hospitals included in the study had a low CDI rate and a low transfer rate, the CDIs attributable to transfers represent a minority of cases, but they are a substantial minority, he said, noting that the main concern is with the “perfect storm” of high CDI rate plus high transfer rate.
The methodological approach used in this study to estimate CDI rates can be used for any health care–associated infection of interest, he added.
Dr. Sewell reported that he had no disclosures.
The AGA Fecal Microbiota Transplantation (FMT) National Registry will assess short- and long-term patient outcomes associated with FMT. Learn more at http://ow.ly/WdQE30lBuSu
ATLANTA – Patient sharing among hospital facilities contributed substantially to the overall Clostridium difficile infection rate, an analysis of interhospital contamination effects showed.
In fact, 7.6% of all Clostridium difficile infection (CDI) cases at the nearly 400 California hospitals included in the study were directly attributable to the patient-sharing network, Daniel Sewell, PhD, reported at the International Conference on Emerging Infectious Diseases.
“The methods that we employed allowed us to estimate the expected increase in CDI cases due to transfers as a function of the CDI rate at the hospital from which those patients were brought. These transfer patients were responsible for about 3.06 times the number of CDI cases as a normal patient,” said Dr. Sewell, a biostatistician at the University of Iowa, Iowa City.
The findings, which underscored the importance of regional (rather than local) efforts to minimize the spread of health care–associated infections, are based on an analysis of 27,200,873 hospital admissions and 532,320 same-day patient transfers identified from the Healthcare Cost and Utilization Project California State Inpatient Database for 2005-2011.
Transfer networks based on the monthly average number of patients discharged from one hospital and admitted to another on the same day were constructed, and the monthly average number of CDI cases per hospital were considered, along with hospital-level characteristics such as patient length of stay, age, and number of diagnoses. Network autocorrelation models that help eliminate bias were then used to assess the contamination effects between hospitals, he explained.
This led to development of an equation that can be used to determine the expected number of CDI cases in a hospital as a function of the number of transfers coming in and the contamination level of the source hospitals. The ability to calculate the expected number of CDI cases in this fashion is an important factor for the success of regional versus local intervention efforts, which are increasingly thought to be important for reducing health care–associated infections.
“If we want to design a coordinated or regional approach, we’ve got to have a much better understanding of the role that patient transfers have in these diseases,” Dr. Sewell said.
As most hospitals included in the study had a low CDI rate and a low transfer rate, the CDIs attributable to transfers represent a minority of cases, but they are a substantial minority, he said, noting that the main concern is with the “perfect storm” of high CDI rate plus high transfer rate.
The methodological approach used in this study to estimate CDI rates can be used for any health care–associated infection of interest, he added.
Dr. Sewell reported that he had no disclosures.
The AGA Fecal Microbiota Transplantation (FMT) National Registry will assess short- and long-term patient outcomes associated with FMT. Learn more at http://ow.ly/WdQE30lBuSu
ATLANTA – Patient sharing among hospital facilities contributed substantially to the overall Clostridium difficile infection rate, an analysis of interhospital contamination effects showed.
In fact, 7.6% of all Clostridium difficile infection (CDI) cases at the nearly 400 California hospitals included in the study were directly attributable to the patient-sharing network, Daniel Sewell, PhD, reported at the International Conference on Emerging Infectious Diseases.
“The methods that we employed allowed us to estimate the expected increase in CDI cases due to transfers as a function of the CDI rate at the hospital from which those patients were brought. These transfer patients were responsible for about 3.06 times the number of CDI cases as a normal patient,” said Dr. Sewell, a biostatistician at the University of Iowa, Iowa City.
The findings, which underscored the importance of regional (rather than local) efforts to minimize the spread of health care–associated infections, are based on an analysis of 27,200,873 hospital admissions and 532,320 same-day patient transfers identified from the Healthcare Cost and Utilization Project California State Inpatient Database for 2005-2011.
Transfer networks based on the monthly average number of patients discharged from one hospital and admitted to another on the same day were constructed, and the monthly average number of CDI cases per hospital were considered, along with hospital-level characteristics such as patient length of stay, age, and number of diagnoses. Network autocorrelation models that help eliminate bias were then used to assess the contamination effects between hospitals, he explained.
This led to development of an equation that can be used to determine the expected number of CDI cases in a hospital as a function of the number of transfers coming in and the contamination level of the source hospitals. The ability to calculate the expected number of CDI cases in this fashion is an important factor for the success of regional versus local intervention efforts, which are increasingly thought to be important for reducing health care–associated infections.
“If we want to design a coordinated or regional approach, we’ve got to have a much better understanding of the role that patient transfers have in these diseases,” Dr. Sewell said.
As most hospitals included in the study had a low CDI rate and a low transfer rate, the CDIs attributable to transfers represent a minority of cases, but they are a substantial minority, he said, noting that the main concern is with the “perfect storm” of high CDI rate plus high transfer rate.
The methodological approach used in this study to estimate CDI rates can be used for any health care–associated infection of interest, he added.
Dr. Sewell reported that he had no disclosures.
The AGA Fecal Microbiota Transplantation (FMT) National Registry will assess short- and long-term patient outcomes associated with FMT. Learn more at http://ow.ly/WdQE30lBuSu
REPORTING FROM ICEID 2018
Key clinical point: Patient sharing among hospitals contributes substantially to Clostridium difficile infection (CDI) rates.
Major finding: Patient transfers account for 7.6% of the overall CDI burden.
Study details: A statistical analysis to estimate interhospital CDI transmissions.
Disclosures: Dr. Sewell reported that he had no disclosures.
Patient transfers between hospitals contribute substantially to CDI burden
ATLANTA – Patient sharing among hospital facilities contributed substantially to the overall Clostridium difficile infection rate, an analysis of interhospital contamination effects showed.
In fact, 7.6% of all Clostridium difficile infection (CDI) cases at the nearly 400 California hospitals included in the study were directly attributable to the patient-sharing network, Daniel Sewell, PhD, reported at the International Conference on Emerging Infectious Diseases.
“The methods that we employed allowed us to estimate the expected increase in CDI cases due to transfers as a function of the CDI rate at the hospital from which those patients were brought. These transfer patients were responsible for about 3.06 times the number of CDI cases as a normal patient,” said Dr. Sewell, a biostatistician at the University of Iowa, Iowa City.
The findings, which underscored the importance of regional (rather than local) efforts to minimize the spread of health care–associated infections, are based on an analysis of 27,200,873 hospital admissions and 532,320 same-day patient transfers identified from the Healthcare Cost and Utilization Project California State Inpatient Database for 2005-2011.
Transfer networks based on the monthly average number of patients discharged from one hospital and admitted to another on the same day were constructed, and the monthly average number of CDI cases per hospital were considered, along with hospital-level characteristics such as patient length of stay, age, and number of diagnoses. Network autocorrelation models that help eliminate bias were then used to assess the contamination effects between hospitals, he explained.
This led to development of an equation that can be used to determine the expected number of CDI cases in a hospital as a function of the number of transfers coming in and the contamination level of the source hospitals. The ability to calculate the expected number of CDI cases in this fashion is an important factor for the success of regional versus local intervention efforts, which are increasingly thought to be important for reducing health care–associated infections.
“If we want to design a coordinated or regional approach, we’ve got to have a much better understanding of the role that patient transfers have in these diseases,” Dr. Sewell said.
As most hospitals included in the study had a low CDI rate and a low transfer rate, the CDIs attributable to transfers represent a minority of cases, but they are a substantial minority, he said, noting that the main concern is with the “perfect storm” of high CDI rate plus high transfer rate.
The methodological approach used in this study to estimate CDI rates can be used for any health care–associated infection of interest, he added.
Dr. Sewell reported that he had no disclosures.
ATLANTA – Patient sharing among hospital facilities contributed substantially to the overall Clostridium difficile infection rate, an analysis of interhospital contamination effects showed.
In fact, 7.6% of all Clostridium difficile infection (CDI) cases at the nearly 400 California hospitals included in the study were directly attributable to the patient-sharing network, Daniel Sewell, PhD, reported at the International Conference on Emerging Infectious Diseases.
“The methods that we employed allowed us to estimate the expected increase in CDI cases due to transfers as a function of the CDI rate at the hospital from which those patients were brought. These transfer patients were responsible for about 3.06 times the number of CDI cases as a normal patient,” said Dr. Sewell, a biostatistician at the University of Iowa, Iowa City.
The findings, which underscored the importance of regional (rather than local) efforts to minimize the spread of health care–associated infections, are based on an analysis of 27,200,873 hospital admissions and 532,320 same-day patient transfers identified from the Healthcare Cost and Utilization Project California State Inpatient Database for 2005-2011.
Transfer networks based on the monthly average number of patients discharged from one hospital and admitted to another on the same day were constructed, and the monthly average number of CDI cases per hospital were considered, along with hospital-level characteristics such as patient length of stay, age, and number of diagnoses. Network autocorrelation models that help eliminate bias were then used to assess the contamination effects between hospitals, he explained.
This led to development of an equation that can be used to determine the expected number of CDI cases in a hospital as a function of the number of transfers coming in and the contamination level of the source hospitals. The ability to calculate the expected number of CDI cases in this fashion is an important factor for the success of regional versus local intervention efforts, which are increasingly thought to be important for reducing health care–associated infections.
“If we want to design a coordinated or regional approach, we’ve got to have a much better understanding of the role that patient transfers have in these diseases,” Dr. Sewell said.
As most hospitals included in the study had a low CDI rate and a low transfer rate, the CDIs attributable to transfers represent a minority of cases, but they are a substantial minority, he said, noting that the main concern is with the “perfect storm” of high CDI rate plus high transfer rate.
The methodological approach used in this study to estimate CDI rates can be used for any health care–associated infection of interest, he added.
Dr. Sewell reported that he had no disclosures.
ATLANTA – Patient sharing among hospital facilities contributed substantially to the overall Clostridium difficile infection rate, an analysis of interhospital contamination effects showed.
In fact, 7.6% of all Clostridium difficile infection (CDI) cases at the nearly 400 California hospitals included in the study were directly attributable to the patient-sharing network, Daniel Sewell, PhD, reported at the International Conference on Emerging Infectious Diseases.
“The methods that we employed allowed us to estimate the expected increase in CDI cases due to transfers as a function of the CDI rate at the hospital from which those patients were brought. These transfer patients were responsible for about 3.06 times the number of CDI cases as a normal patient,” said Dr. Sewell, a biostatistician at the University of Iowa, Iowa City.
The findings, which underscored the importance of regional (rather than local) efforts to minimize the spread of health care–associated infections, are based on an analysis of 27,200,873 hospital admissions and 532,320 same-day patient transfers identified from the Healthcare Cost and Utilization Project California State Inpatient Database for 2005-2011.
Transfer networks based on the monthly average number of patients discharged from one hospital and admitted to another on the same day were constructed, and the monthly average number of CDI cases per hospital were considered, along with hospital-level characteristics such as patient length of stay, age, and number of diagnoses. Network autocorrelation models that help eliminate bias were then used to assess the contamination effects between hospitals, he explained.
This led to development of an equation that can be used to determine the expected number of CDI cases in a hospital as a function of the number of transfers coming in and the contamination level of the source hospitals. The ability to calculate the expected number of CDI cases in this fashion is an important factor for the success of regional versus local intervention efforts, which are increasingly thought to be important for reducing health care–associated infections.
“If we want to design a coordinated or regional approach, we’ve got to have a much better understanding of the role that patient transfers have in these diseases,” Dr. Sewell said.
As most hospitals included in the study had a low CDI rate and a low transfer rate, the CDIs attributable to transfers represent a minority of cases, but they are a substantial minority, he said, noting that the main concern is with the “perfect storm” of high CDI rate plus high transfer rate.
The methodological approach used in this study to estimate CDI rates can be used for any health care–associated infection of interest, he added.
Dr. Sewell reported that he had no disclosures.
REPORTING FROM ICEID 2018
Key clinical point: Patient sharing among hospitals contributes substantially to Clostridium difficile infection (CDI) rates.
Major finding: Patient transfers account for 7.6% of the overall CDI burden.
Study details: A statistical analysis to estimate interhospital CDI transmissions.
Disclosures: Dr. Sewell reported that he had no disclosures.