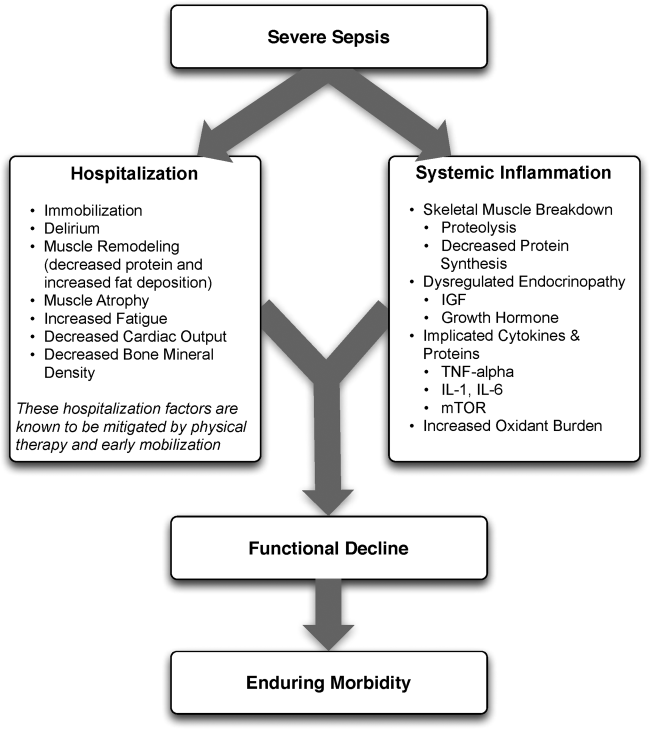
User login
Patterns and Predictors of Short-Term Peripherally Inserted Central Catheter Use: A Multicenter Prospective Cohort Study
Peripherally inserted central catheters (PICCs) are integral to the care of hospitalized patients in the United States.1 Consequently, utilization of these devices in acutely ill patients has steadily increased in the past decade.2 Although originally designed to support the delivery of total parenteral nutrition, PICCs have found broader applications in the hospital setting given the ease and safety of placement, the advances in technology that facilitate insertion, and the growing availability of specially trained vascular nurses that place these devices at the bedside.3 Furthermore, because they are placed in deeper veins of the arm, PICCs are more durable than peripheral catheters and can support venous access for extended durations.4-6
However, the growing use of PICCs has led to the realization that these devices are not without attendant risks. For example, PICCs are associated with venous thromboembolism (VTE) and central-line associated blood stream infection (CLABSI).7,8 Additionally, complications such as catheter occlusion and tip migration commonly occur and may interrupt care or necessitate device removal.9-11 Hence, thoughtful weighing of the risks against the benefits of PICC use prior to placement is necessary. To facilitate such decision-making, we developed the Michigan Appropriateness Guide for Intravenous (IV) Catheters (MAGIC) criteria,12 which is an evidence-based tool that defines when the use of a PICC is appropriate in hospitalized adults.
The use of PICCs for infusion of peripherally compatible therapies for 5 or fewer days is rated as inappropriate by MAGIC.12 This strategy is also endorsed by the Centers for Disease Control and Prevention’s (CDC) guidelines for the prevention of catheter-related infections.13 Despite these recommendations, short-term PICC use remains common. For example, a study conducted at a tertiary pediatric care center reported a trend toward shorter PICC dwell times and increasing rates of early removal.2 However, factors that prompt such short-term PICC use are poorly understood. Without understanding drivers and outcomes of short-term PICC use, interventions to prevent such practice are unlikely to succeed.
Therefore, by using data from a multicenter cohort study, we examined patterns of short-term PICC use and sought to identify which patient, provider, and device factors were associated with such use. We hypothesized that short-term placement would be associated with difficult venous access and would also be associated with the risk of major and minor complications.
METHODS
Study Setting and Design
We used data from the Michigan Hospital Medicine Safety (HMS) Consortium to examine patterns and predictors of short-term PICC use.14 As a multi-institutional clinical quality initiative sponsored by Blue Cross Blue Shield of Michigan and Blue Care Network, HMS aims to improve the quality of care by preventing adverse events in hospitalized medical patients.4,15-17 In January of 2014, dedicated, trained abstractors started collecting data on PICC placements at participating HMS hospitals by using a standard protocol and template for data collection. Patients who received PICCs while admitted to either a general medicine unit or an intensive care unit (ICU) during clinical care were eligible for inclusion. Patients were excluded if they were (a) under the age of 18 years, (b) pregnant, (c) admitted to a nonmedical service (eg, surgery), or (d) admitted under observation status.
Every 14 days, each hospital collected data on the first 17 eligible patients that received a PICC, with at least 7 of these placements occurring in an ICU setting. All patients were prospectively followed until the PICC was removed, death, or until 70 days after insertion, whichever occurred first. For patients who had their PICC removed prior to hospital discharge, follow-up occurred via a review of medical records. For those discharged with a PICC in place, both medical record review and telephone follow-up were performed. To ensure data quality, annual random audits at each participating hospital were performed by the coordinating center at the University of Michigan.
For this analysis, we included all available data as of June 30, 2016. However, HMS hospitals continue to collect data on PICC use and outcomes as part of an ongoing clinical quality initiative to reduce the incidence of PICC-related complications.
Patient, Provider, and Device Data
Patient characteristics, including demographics, detailed medical history, comorbidities, physical findings, laboratory results, and medications were abstracted directly from medical records. To estimate the comorbidity burden, the Charlson-Deyo comorbidity score was calculated for each patient by using data available in the medical record at the time of PICC placement.18 Data, such as the documented indication for PICC insertion and the reason for removal, were obtained directly from medical records. Provider characteristics, including the specialty of the attending physician at the time of insertion and the type of operator who inserted the PICC, were also collected. Institutional characteristics, such as total number of beds, teaching versus nonteaching, and urban versus rural, were obtained from hospital publicly reported data and semiannual surveys of HMS sites.19,20 Data on device characteristics, such as catheter gauge, coating, insertion attempts, tip location, and number of lumens, were abstracted from PICC insertion notes.
Outcomes of Interest
The outcome of interest was short-term PICC use, defined as PICCs removed within 5 days of insertion. Patients who expired with a PICC in situ were excluded. Secondary outcomes of interest included PICC-related complications, categorized as major (eg, symptomatic VTE and CLABSI) or minor (eg, catheter occlusion, superficial thrombosis, mechanical complications [kinking, coiling], exit site infection, and tip migration). Symptomatic VTE was defined as clinically diagnosed deep venous thrombosis (DVT) and/or pulmonary embolism (PE) not present at the time of PICC placement and confirmed via imaging (ultrasound or venogram for DVT; computed tomography scan, ventilation perfusion scan, or pulmonary angiogram for PE). CLABSI was defined in accordance with the CDC’s National Healthcare Safety Network criteria or according to Infectious Diseases Society of America recommendations.21,22 All minor PICC complications were defined in accordance with prior published definitions.4
Statistical Analysis
Cases of short-term PICC use were identified and compared with patients with a PICC dwell time of 6 or more days by patient, provider, and device characteristics. The initial analyses for the associations of putative factors with short-term PICC use were performed using χ2 or Wilcoxon tests for categorical and continuous variables, respectively. Univariable mixed effect logistic regression models (with a random hospital-specific intercept) were then used to control for hospital-level clustering. Next, a mixed effects multivariable logistic regression model was used to identify factors associated with short-term PICC use. Variables with P ≤ .25 were considered as candidate predictors for the final multivariable model, which was chosen through a stepwise variable selection algorithm performed on 1000 bootstrapped data sets.23 Variables in the final model were retained based on their frequency of selection in the bootstrapped samples, significance level, and contribution to the overall model likelihood. Results were expressed as odds ratios (OR) with corresponding 95% confidence intervals (CI). SAS for Windows (version 9.3, SAS Institute Inc., Cary, NC) was used for analyses.
Ethical and Regulatory Oversight
The study was classified as “not regulated” by the Institutional Review Board at the University of Michigan (HUM00078730).
RESULTS
Overall Characteristics of the Study Cohort
Characteristics of Short-Term Peripherally Inserted Central Catheter Use
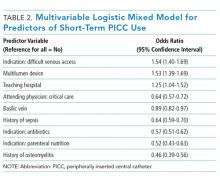
Of the 15,397 PICCs included, we identified 3902 PICCs (25.3%) with a dwell time of ≤5 days (median = 3 days; IQR, 2-4 days). When compared to PICCs that were in place for longer durations, no significant differences in age or comorbidity scores were observed. Importantly, despite recommendations to avoid PICCs in patients with moderate to severe chronic kidney disease (glomerular filtration rate [GFR] ≤ 59 ml/min), 1292 (33.1%) short-term PICCs occurred in patients that met such criteria.
Among short-term PICCs, 3618 (92.7%) were power-capable devices, 2785 (71.4%) were 5-French, and 2813 (72.1%) were multilumen. Indications for the use of short-term PICCs differed from longer term devices in important ways (P < .001). For example, the most common documented indication for short-term PICC use was difficult venous access (28.2%), while for long-term PICCs, it was antibiotic administration (39.8%). General internists and hospitalists were the most common attending physicians for patients with short-term and long-term PICCs (65.1% and 65.5%, respectively [P = .73]). Also, the proportion of critical care physicians responsible for patients with short versus long-term PICC use was similar (14.0% vs 15.0%, respectively [P = .123]). Of the short-term PICCs, 2583 (66.2%) were inserted by vascular access nurses, 795 (20.4%) by interventional radiologists, and 439 (11.3%) by advance practice professionals. Almost all of the PICCs placed ≤5 days (95.5%) were removed during hospitalization.
Complications Associated with Short-Term Peripherally Inserted Central Catheter Use
DISCUSSION
This large, multisite prospective cohort study is the first to examine patterns and predictors of short-term PICC use in hospitalized adults. By examining clinically granular data derived from the medical records of patients across 52 hospitals, we found that short-term use was common, representing 25% of all PICCs placed. Almost all such PICCs were removed prior to discharge, suggesting that they were placed primarily to meet acute needs during hospitalization. Multivariable models indicated that patients with difficult venous access, multilumen devices, and teaching hospital settings were associated with short-term use. Given that (a) short term PICC use is not recommended by published evidence-based guidelines,12,13 (b) both major and minor complications were not uncommon despite brief exposure, and (c) specific factors might be targeted to avoid such use, strategies to improve PICC decision-making in the hospital appear increasingly necessary.
In our study, difficult venous access was the most common documented indication for short-term PICC placement. For patients in whom an anticipated catheter dwell time of 5 days or less is expected, MAGIC recommends the consideration of midline or peripheral IV catheters placed under ultrasound guidance.12 A midline is a type of peripheral IV catheter that is about 7.5 cm to 25 cm in length and is typically inserted in the larger diameter veins of the upper extremity, such as the cephalic or basilic veins, with the tip terminating distal to the subclavian vein.7,12 While there is a paucity of information that directly compares PICCs to midlines, some data suggest a lower risk of bloodstream infection and thrombosis associated with the latter.24-26 For example, at one quaternary teaching hospital, house staff who are trained to insert midline catheters under ultrasound guidance in critically ill patients with difficult venous access reported no CLABSI and DVT events.26
Interestingly, multilumen catheters were used twice as often as single lumen catheters in patients with short-term PICCs. In these instances, the use of additional lumens is questionable, as infusion of multiple incompatible fluids was not commonly listed as an indication prompting PICC use. Because multilumen PICCs are associated with higher risks of both VTE and CLABSI compared to single lumen devices, such use represents an important safety concern.27-29 Institutional efforts that not only limit the use of multilumen PICCs but also fundamentally define when use of a PICC is appropriate may substantially improve outcomes related to vascular access.28,30,31We observed that short-term PICCs were more common in teaching compared to nonteaching hospitals. While the design of the present study precludes understanding the reasons for such a difference, some plausible theories include the presence of physician trainees who may not appreciate the risks of PICC use, diminishing peripheral IV access securement skills, and the lack of alternatives to PICC use. Educating trainees who most often order PICCs in teaching settings as to when they should or should not consider this device may represent an important quality improvement opportunity.32 Similarly, auditing and assessing the clinical skills of those entrusted to place peripheral IVs might prove helpful.33,34 Finally, the introduction of a midline program, or similar programs that expand the scope of vascular access teams to place alternative devices, should be explored as a means to improve PICC use and patient safety.
Our study also found that a third of patients who received PICCs for 5 or fewer days had moderate to severe chronic kidney disease. In these patients who may require renal replacement therapy, prior PICC placement is among the strongest predictors of arteriovenous fistula failure.35,36 Therefore, even though national guidelines discourage the use of PICCs in these patients and recommend alternative routes of venous access,12,37,38 such practice is clearly not happening. System-based interventions that begin by identifying patients who require vein preservation (eg, those with a GFR < 45 ml/min) and are therefore not appropriate for a PICC would be a welcomed first step in improving care for such patients.37,38Our study has limitations. First, the observational nature of the study limits the ability to assess for causality or to account for the effects of unmeasured confounders. Second, while the use of medical records to collect granular data is valuable, differences in documentation patterns within and across hospitals, including patterns of missing data, may produce a misclassification of covariates or outcomes. Third, while we found that higher rates of short-term PICC use were associated with teaching hospitals and patients with difficult venous access, we were unable to determine the precise reasons for this practice trend. Qualitative or mixed-methods approaches to understand provider decision-making in these settings would be welcomed.
Our study also has several strengths. First, to our knowledge, this is the first study to systematically describe and evaluate patterns and predictors of short-term PICC use. The finding that PICCs placed for difficult venous access is a dominant category of short-term placement confirms clinical suspicions regarding inappropriate use and strengthens the need for pathways or protocols to manage such patients. Second, the inclusion of medical patients in diverse institutions offers not only real-world insights related to PICC use, but also offers findings that should be generalizable to other hospitals and health systems. Third, the use of a robust data collection strategy that emphasized standardized data collection, dedicated trained abstractors, and random audits to ensure data quality strengthen the findings of this work. Finally, our findings highlight an urgent need to develop policies related to PICC use, including limiting the use of multiple lumens and avoidance in patients with moderate to severe kidney disease.
In conclusion, short-term use of PICCs is prevalent and associated with key patient, provider, and device factors. Such use is also associated with complications, such as catheter occlusion, tip migration, VTE, and CLABSI. Limiting the use of multiple-lumen PICCs, enhancing education for when a PICC should be used, and defining strategies for patients with difficult access may help reduce inappropriate PICC use and improve patient safety. Future studies to examine implementation of such interventions would be welcomed.
Disclosure: Drs. Paje, Conlon, Swaminathan, and Boldenow disclose no conflicts of interest. Dr. Chopra has received honoraria for talks at hospitals as a visiting professor. Dr. Flanders discloses consultancies for the Institute for Healthcare Improvement and the Society of Hospital Medicine, royalties from Wiley Publishing, honoraria for various talks at hospitals as a visiting professor, grants from the CDC Foundation, Agency for Healthcare Research and Quality, Blue Cross Blue Shield of Michigan (BCBSM), and Michigan Hospital Association, and expert witness testimony. Dr. Bernstein discloses consultancies for Blue Care Network and grants from BCBSM, Department of Veterans Affairs, and National Institutes of Health. Dr. Kaatz discloses no relevant conflicts of interest. BCBSM and Blue Care Network provided support for the Michigan HMS Consortium as part of the BCBSM Value Partnerships program. Although BCBSM and HMS work collaboratively, the opinions, beliefs, and viewpoints expressed by the author do not necessarily reflect the opinions, beliefs, and viewpoints of BCBSM or any of its employees. Dr. Chopra is supported by a career development award from the Agency for Healthcare Research and Quality (1-K08-HS022835-01). BCBSM and Blue Care Network supported data collection at each participating site and funded the data coordinating center but had no role in study concept, interpretation of findings, or in the preparation, final approval, or decision to submit the manuscript.
1. Al Raiy B, Fakih MG, Bryan-Nomides N, et al. Peripherally inserted central venous catheters in the acute care setting: A safe alternative to high-risk short-term central venous catheters. Am J Infect Control. 2010;38(2):149-153. PubMed
2. Gibson C, Connolly BL, Moineddin R, Mahant S, Filipescu D, Amaral JG. Peripherally inserted central catheters: use at a tertiary care pediatric center. J Vasc Interv Radiol. 2013;24(9):1323-1331. PubMed
3. Chopra V, Flanders SA, Saint S. The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527-1528. PubMed
4. Chopra V, Smith S, Swaminathan L, et al. Variations in Peripherally Inserted Central Catheter Use and Outcomes in Michigan Hospitals. JAMA Intern Med. 2016;176(4):548-551. PubMed
5. Cowl CT, Weinstock JV, Al-Jurf A, Ephgrave K, Murray JA, Dillon K. Complications and cost associated with parenteral nutrition delivered to hospitalized patients through either subclavian or peripherally-inserted central catheters. Clin Nutr. 2000;19(4):237-243. PubMed
6. MacDonald AS, Master SK, Moffitt EA. A comparative study of peripherally inserted silicone catheters for parenteral nutrition. Can J Anaesth. 1977;24(2):263-269. PubMed
7. Chopra V, O’Horo JC, Rogers MA, Maki DG, Safdar N. The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2013;34(9):908-918. PubMed
8. Chopra V, Anand S, Hickner A, et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet. 2013;382(9889):311-325. PubMed
9. Beccaria P, Silvetti S, Mucci M, Battini I, Brambilla P, Zangrillo A. Contributing factors for a late spontaneous peripherally inserted central catheter migration: a case report and review of literature. J Vasc Access. 2015;16(3):178-182. PubMed
10. Turcotte S, Dube S, Beauchamp G. Peripherally inserted central venous catheters are not superior to central venous catheters in the acute care of surgical patients on the ward. World J Surg. 2006;30(8):1605-1619. PubMed
11. Pikwer A, Akeson J, Lindgren S. Complications associated with peripheral or central routes for central venous cannulation. Anaesthesia. 2012;67(1):65-71. PubMed
12. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): Results From a Multispecialty Panel Using the RAND/UCLA Appropriateness Method. Ann Intern Med. 15 2015;163(6 Suppl):S1-S40. PubMed
13. O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2011;39(4 Suppl 1):S1-S34. PubMed
14. Michigan Hospital Medicine Safety Consortium. 2016; http://mi-hms.org/. Accessed November 11, 2016.
15. Greene MT, Spyropoulos AC, Chopra V, et al. Validation of Risk Assessment Models of Venous Thromboembolism in Hospitalized Medical Patients. Am J Med. 2016;129(9):1001.e1009-1001.e1018. PubMed
16. Greene MT, Flanders SA, Woller SC, Bernstein SJ, Chopra V. The Association Between PICC Use and Venous Thromboembolism in Upper and Lower Extremities. Am J Med. 2015;128(9):986-993. PubMed
17. Flanders SA, Greene MT, Grant P, et al. Hospital performance for pharmacologic venous thromboembolism prophylaxis and rate of venous thromboembolism : a cohort study. JAMA Intern Med. 2014;174(10):1577-1584. PubMed
18. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619. PubMed
19. Hospital Bed Inventory. 2016; http://www.michigan.gov/documents/mdhhs/HOSPBEDINV_October_3__2016_536834_7.pdf. Accessed November 22, 2016.
20. Compare Hospitals. 2016; http://www.leapfroggroup.org/compare-hospitals. Accessed November 22, 2016.
21. NHSN Patient Safety Component Manual. 2016; http://www.cdc.gov/nhsn/pdfs/pscmanual/pcsmanual_current.pdf. Accessed November 22, 2016.
22. Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1-45. PubMed
23. Austin PC, Tu JV. Bootstrap Methods for Developing Predictive Models. Am Stat. 2004;58(2):131-137.
24. Pathak R, Patel A, Enuh H, Adekunle O, Shrisgantharajah V, Diaz K. The Incidence of Central Line-Associated Bacteremia After the Introduction of Midline Catheters in a Ventilator Unit Population. Infect Dis Clin Pract. 2015;23(3):131-134. PubMed
25. Adams DZ, Little A, Vinsant C, Khandelwal S. The Midline Catheter: A Clinical Review. J Emerg Med. 2016;51(3):252-258. PubMed
26. Deutsch GB, Sathyanarayana SA, Singh N, Nicastro J. Ultrasound-guided placement of midline catheters in the surgical intensive care unit: a cost-effective proposal for timely central line removal. J Surg Res. 2014;191(1):1-5. PubMed
27. Chopra V, Anand S, Krein SL, Chenoweth C, Saint S. Bloodstream Infection, Venous Thrombosis, and Peripherally Inserted Central Catheters: Reappraising the Evidence. Am J Med. 2012;125(8):733-741. PubMed
28. Ratz D, Hofer T, Flanders SA, Saint S, Chopra V. Limiting the Number of Lumens in Peripherally Inserted Central Catheters to Improve Outcomes and Reduce Cost: A Simulation Study. Infect Control Hosp Epidemiol. 2016;37(7):811-817. PubMed
29. Pongruangporn M, Ajenjo MC, Russo AJ, et al. Patient- and device-specific risk factors for peripherally inserted central venous catheter-related bloodstream infections. Infect Control Hosp Epidemiol. 2013;34(2):184-189. PubMed
30. Shannon RP, Patel B, Cummins D, Shannon AH, Ganguli G, Lu Y. Economics of central line--associated bloodstream infections. Am J Med Qual. 2006;21(6 Suppl):7S-16S. PubMed
31. O’Brien J, Paquet F, Lindsay R, Valenti D. Insertion of PICCs with minimum number of lumens reduces complications and costs. J AmColl Radiol. 2013;10(11):864-868. PubMed
32. Wong BM, Etchells EE, Kuper A, Levinson W, Shojania KG. Teaching quality improvement and patient safety to trainees: a systematic review. Acad Med. 2010;85(9):1425-1439. PubMed
33. Conlon T, Himebauch A, Marie Cahill A, et al. 1246: Bedside Picc Placement by Pediatric Icu Providers Is Feasible and Safe. Crit Care Med. 2016;44(12 Suppl 1):387.
34. Moran J, Colbert CY, Song J, et al. Screening for novel risk factors related to peripherally inserted central catheter-associated complications. J Hosp Med. 2014;9(8):481-489. PubMed
35. Gonsalves CF, Eschelman DJ, Sullivan KL, DuBois N, Bonn J. Incidence of central vein stenosis and occlusion following upper extremity PICC and port placement. Cardiovasc Intervent Radiol. 2003;26(2):123-127. PubMed
36. El Ters M, Schears GJ, Taler SJ, et al. Association between prior peripherally inserted central catheters and lack of functioning arteriovenous fistulas: a case-control study in hemodialysis patients. Am J Kidney Dis. 2012;60(4):601-608. PubMed
37. Vascular Access 2006 Work Group. Clinical practice guidelines for vascular access. Am J Kidney Dis. 2006;48 Suppl 1:S248-S273. PubMed
38. Hoggard J, Saad T, Schon D, et al. Guidelines for venous access in patients with chronic kidney disease. A Position Statement from the American Society of Diagnostic and Interventional Nephrology, Clinical Practice Committee and the Association for Vascular Access. Semin Dial. 2008;21(2):186-191. PubMed
Peripherally inserted central catheters (PICCs) are integral to the care of hospitalized patients in the United States.1 Consequently, utilization of these devices in acutely ill patients has steadily increased in the past decade.2 Although originally designed to support the delivery of total parenteral nutrition, PICCs have found broader applications in the hospital setting given the ease and safety of placement, the advances in technology that facilitate insertion, and the growing availability of specially trained vascular nurses that place these devices at the bedside.3 Furthermore, because they are placed in deeper veins of the arm, PICCs are more durable than peripheral catheters and can support venous access for extended durations.4-6
However, the growing use of PICCs has led to the realization that these devices are not without attendant risks. For example, PICCs are associated with venous thromboembolism (VTE) and central-line associated blood stream infection (CLABSI).7,8 Additionally, complications such as catheter occlusion and tip migration commonly occur and may interrupt care or necessitate device removal.9-11 Hence, thoughtful weighing of the risks against the benefits of PICC use prior to placement is necessary. To facilitate such decision-making, we developed the Michigan Appropriateness Guide for Intravenous (IV) Catheters (MAGIC) criteria,12 which is an evidence-based tool that defines when the use of a PICC is appropriate in hospitalized adults.
The use of PICCs for infusion of peripherally compatible therapies for 5 or fewer days is rated as inappropriate by MAGIC.12 This strategy is also endorsed by the Centers for Disease Control and Prevention’s (CDC) guidelines for the prevention of catheter-related infections.13 Despite these recommendations, short-term PICC use remains common. For example, a study conducted at a tertiary pediatric care center reported a trend toward shorter PICC dwell times and increasing rates of early removal.2 However, factors that prompt such short-term PICC use are poorly understood. Without understanding drivers and outcomes of short-term PICC use, interventions to prevent such practice are unlikely to succeed.
Therefore, by using data from a multicenter cohort study, we examined patterns of short-term PICC use and sought to identify which patient, provider, and device factors were associated with such use. We hypothesized that short-term placement would be associated with difficult venous access and would also be associated with the risk of major and minor complications.
METHODS
Study Setting and Design
We used data from the Michigan Hospital Medicine Safety (HMS) Consortium to examine patterns and predictors of short-term PICC use.14 As a multi-institutional clinical quality initiative sponsored by Blue Cross Blue Shield of Michigan and Blue Care Network, HMS aims to improve the quality of care by preventing adverse events in hospitalized medical patients.4,15-17 In January of 2014, dedicated, trained abstractors started collecting data on PICC placements at participating HMS hospitals by using a standard protocol and template for data collection. Patients who received PICCs while admitted to either a general medicine unit or an intensive care unit (ICU) during clinical care were eligible for inclusion. Patients were excluded if they were (a) under the age of 18 years, (b) pregnant, (c) admitted to a nonmedical service (eg, surgery), or (d) admitted under observation status.
Every 14 days, each hospital collected data on the first 17 eligible patients that received a PICC, with at least 7 of these placements occurring in an ICU setting. All patients were prospectively followed until the PICC was removed, death, or until 70 days after insertion, whichever occurred first. For patients who had their PICC removed prior to hospital discharge, follow-up occurred via a review of medical records. For those discharged with a PICC in place, both medical record review and telephone follow-up were performed. To ensure data quality, annual random audits at each participating hospital were performed by the coordinating center at the University of Michigan.
For this analysis, we included all available data as of June 30, 2016. However, HMS hospitals continue to collect data on PICC use and outcomes as part of an ongoing clinical quality initiative to reduce the incidence of PICC-related complications.
Patient, Provider, and Device Data
Patient characteristics, including demographics, detailed medical history, comorbidities, physical findings, laboratory results, and medications were abstracted directly from medical records. To estimate the comorbidity burden, the Charlson-Deyo comorbidity score was calculated for each patient by using data available in the medical record at the time of PICC placement.18 Data, such as the documented indication for PICC insertion and the reason for removal, were obtained directly from medical records. Provider characteristics, including the specialty of the attending physician at the time of insertion and the type of operator who inserted the PICC, were also collected. Institutional characteristics, such as total number of beds, teaching versus nonteaching, and urban versus rural, were obtained from hospital publicly reported data and semiannual surveys of HMS sites.19,20 Data on device characteristics, such as catheter gauge, coating, insertion attempts, tip location, and number of lumens, were abstracted from PICC insertion notes.
Outcomes of Interest
The outcome of interest was short-term PICC use, defined as PICCs removed within 5 days of insertion. Patients who expired with a PICC in situ were excluded. Secondary outcomes of interest included PICC-related complications, categorized as major (eg, symptomatic VTE and CLABSI) or minor (eg, catheter occlusion, superficial thrombosis, mechanical complications [kinking, coiling], exit site infection, and tip migration). Symptomatic VTE was defined as clinically diagnosed deep venous thrombosis (DVT) and/or pulmonary embolism (PE) not present at the time of PICC placement and confirmed via imaging (ultrasound or venogram for DVT; computed tomography scan, ventilation perfusion scan, or pulmonary angiogram for PE). CLABSI was defined in accordance with the CDC’s National Healthcare Safety Network criteria or according to Infectious Diseases Society of America recommendations.21,22 All minor PICC complications were defined in accordance with prior published definitions.4
Statistical Analysis
Cases of short-term PICC use were identified and compared with patients with a PICC dwell time of 6 or more days by patient, provider, and device characteristics. The initial analyses for the associations of putative factors with short-term PICC use were performed using χ2 or Wilcoxon tests for categorical and continuous variables, respectively. Univariable mixed effect logistic regression models (with a random hospital-specific intercept) were then used to control for hospital-level clustering. Next, a mixed effects multivariable logistic regression model was used to identify factors associated with short-term PICC use. Variables with P ≤ .25 were considered as candidate predictors for the final multivariable model, which was chosen through a stepwise variable selection algorithm performed on 1000 bootstrapped data sets.23 Variables in the final model were retained based on their frequency of selection in the bootstrapped samples, significance level, and contribution to the overall model likelihood. Results were expressed as odds ratios (OR) with corresponding 95% confidence intervals (CI). SAS for Windows (version 9.3, SAS Institute Inc., Cary, NC) was used for analyses.
Ethical and Regulatory Oversight
The study was classified as “not regulated” by the Institutional Review Board at the University of Michigan (HUM00078730).
RESULTS
Overall Characteristics of the Study Cohort
Characteristics of Short-Term Peripherally Inserted Central Catheter Use
Of the 15,397 PICCs included, we identified 3902 PICCs (25.3%) with a dwell time of ≤5 days (median = 3 days; IQR, 2-4 days). When compared to PICCs that were in place for longer durations, no significant differences in age or comorbidity scores were observed. Importantly, despite recommendations to avoid PICCs in patients with moderate to severe chronic kidney disease (glomerular filtration rate [GFR] ≤ 59 ml/min), 1292 (33.1%) short-term PICCs occurred in patients that met such criteria.
Among short-term PICCs, 3618 (92.7%) were power-capable devices, 2785 (71.4%) were 5-French, and 2813 (72.1%) were multilumen. Indications for the use of short-term PICCs differed from longer term devices in important ways (P < .001). For example, the most common documented indication for short-term PICC use was difficult venous access (28.2%), while for long-term PICCs, it was antibiotic administration (39.8%). General internists and hospitalists were the most common attending physicians for patients with short-term and long-term PICCs (65.1% and 65.5%, respectively [P = .73]). Also, the proportion of critical care physicians responsible for patients with short versus long-term PICC use was similar (14.0% vs 15.0%, respectively [P = .123]). Of the short-term PICCs, 2583 (66.2%) were inserted by vascular access nurses, 795 (20.4%) by interventional radiologists, and 439 (11.3%) by advance practice professionals. Almost all of the PICCs placed ≤5 days (95.5%) were removed during hospitalization.
Complications Associated with Short-Term Peripherally Inserted Central Catheter Use
DISCUSSION
This large, multisite prospective cohort study is the first to examine patterns and predictors of short-term PICC use in hospitalized adults. By examining clinically granular data derived from the medical records of patients across 52 hospitals, we found that short-term use was common, representing 25% of all PICCs placed. Almost all such PICCs were removed prior to discharge, suggesting that they were placed primarily to meet acute needs during hospitalization. Multivariable models indicated that patients with difficult venous access, multilumen devices, and teaching hospital settings were associated with short-term use. Given that (a) short term PICC use is not recommended by published evidence-based guidelines,12,13 (b) both major and minor complications were not uncommon despite brief exposure, and (c) specific factors might be targeted to avoid such use, strategies to improve PICC decision-making in the hospital appear increasingly necessary.
In our study, difficult venous access was the most common documented indication for short-term PICC placement. For patients in whom an anticipated catheter dwell time of 5 days or less is expected, MAGIC recommends the consideration of midline or peripheral IV catheters placed under ultrasound guidance.12 A midline is a type of peripheral IV catheter that is about 7.5 cm to 25 cm in length and is typically inserted in the larger diameter veins of the upper extremity, such as the cephalic or basilic veins, with the tip terminating distal to the subclavian vein.7,12 While there is a paucity of information that directly compares PICCs to midlines, some data suggest a lower risk of bloodstream infection and thrombosis associated with the latter.24-26 For example, at one quaternary teaching hospital, house staff who are trained to insert midline catheters under ultrasound guidance in critically ill patients with difficult venous access reported no CLABSI and DVT events.26
Interestingly, multilumen catheters were used twice as often as single lumen catheters in patients with short-term PICCs. In these instances, the use of additional lumens is questionable, as infusion of multiple incompatible fluids was not commonly listed as an indication prompting PICC use. Because multilumen PICCs are associated with higher risks of both VTE and CLABSI compared to single lumen devices, such use represents an important safety concern.27-29 Institutional efforts that not only limit the use of multilumen PICCs but also fundamentally define when use of a PICC is appropriate may substantially improve outcomes related to vascular access.28,30,31We observed that short-term PICCs were more common in teaching compared to nonteaching hospitals. While the design of the present study precludes understanding the reasons for such a difference, some plausible theories include the presence of physician trainees who may not appreciate the risks of PICC use, diminishing peripheral IV access securement skills, and the lack of alternatives to PICC use. Educating trainees who most often order PICCs in teaching settings as to when they should or should not consider this device may represent an important quality improvement opportunity.32 Similarly, auditing and assessing the clinical skills of those entrusted to place peripheral IVs might prove helpful.33,34 Finally, the introduction of a midline program, or similar programs that expand the scope of vascular access teams to place alternative devices, should be explored as a means to improve PICC use and patient safety.
Our study also found that a third of patients who received PICCs for 5 or fewer days had moderate to severe chronic kidney disease. In these patients who may require renal replacement therapy, prior PICC placement is among the strongest predictors of arteriovenous fistula failure.35,36 Therefore, even though national guidelines discourage the use of PICCs in these patients and recommend alternative routes of venous access,12,37,38 such practice is clearly not happening. System-based interventions that begin by identifying patients who require vein preservation (eg, those with a GFR < 45 ml/min) and are therefore not appropriate for a PICC would be a welcomed first step in improving care for such patients.37,38Our study has limitations. First, the observational nature of the study limits the ability to assess for causality or to account for the effects of unmeasured confounders. Second, while the use of medical records to collect granular data is valuable, differences in documentation patterns within and across hospitals, including patterns of missing data, may produce a misclassification of covariates or outcomes. Third, while we found that higher rates of short-term PICC use were associated with teaching hospitals and patients with difficult venous access, we were unable to determine the precise reasons for this practice trend. Qualitative or mixed-methods approaches to understand provider decision-making in these settings would be welcomed.
Our study also has several strengths. First, to our knowledge, this is the first study to systematically describe and evaluate patterns and predictors of short-term PICC use. The finding that PICCs placed for difficult venous access is a dominant category of short-term placement confirms clinical suspicions regarding inappropriate use and strengthens the need for pathways or protocols to manage such patients. Second, the inclusion of medical patients in diverse institutions offers not only real-world insights related to PICC use, but also offers findings that should be generalizable to other hospitals and health systems. Third, the use of a robust data collection strategy that emphasized standardized data collection, dedicated trained abstractors, and random audits to ensure data quality strengthen the findings of this work. Finally, our findings highlight an urgent need to develop policies related to PICC use, including limiting the use of multiple lumens and avoidance in patients with moderate to severe kidney disease.
In conclusion, short-term use of PICCs is prevalent and associated with key patient, provider, and device factors. Such use is also associated with complications, such as catheter occlusion, tip migration, VTE, and CLABSI. Limiting the use of multiple-lumen PICCs, enhancing education for when a PICC should be used, and defining strategies for patients with difficult access may help reduce inappropriate PICC use and improve patient safety. Future studies to examine implementation of such interventions would be welcomed.
Disclosure: Drs. Paje, Conlon, Swaminathan, and Boldenow disclose no conflicts of interest. Dr. Chopra has received honoraria for talks at hospitals as a visiting professor. Dr. Flanders discloses consultancies for the Institute for Healthcare Improvement and the Society of Hospital Medicine, royalties from Wiley Publishing, honoraria for various talks at hospitals as a visiting professor, grants from the CDC Foundation, Agency for Healthcare Research and Quality, Blue Cross Blue Shield of Michigan (BCBSM), and Michigan Hospital Association, and expert witness testimony. Dr. Bernstein discloses consultancies for Blue Care Network and grants from BCBSM, Department of Veterans Affairs, and National Institutes of Health. Dr. Kaatz discloses no relevant conflicts of interest. BCBSM and Blue Care Network provided support for the Michigan HMS Consortium as part of the BCBSM Value Partnerships program. Although BCBSM and HMS work collaboratively, the opinions, beliefs, and viewpoints expressed by the author do not necessarily reflect the opinions, beliefs, and viewpoints of BCBSM or any of its employees. Dr. Chopra is supported by a career development award from the Agency for Healthcare Research and Quality (1-K08-HS022835-01). BCBSM and Blue Care Network supported data collection at each participating site and funded the data coordinating center but had no role in study concept, interpretation of findings, or in the preparation, final approval, or decision to submit the manuscript.
Peripherally inserted central catheters (PICCs) are integral to the care of hospitalized patients in the United States.1 Consequently, utilization of these devices in acutely ill patients has steadily increased in the past decade.2 Although originally designed to support the delivery of total parenteral nutrition, PICCs have found broader applications in the hospital setting given the ease and safety of placement, the advances in technology that facilitate insertion, and the growing availability of specially trained vascular nurses that place these devices at the bedside.3 Furthermore, because they are placed in deeper veins of the arm, PICCs are more durable than peripheral catheters and can support venous access for extended durations.4-6
However, the growing use of PICCs has led to the realization that these devices are not without attendant risks. For example, PICCs are associated with venous thromboembolism (VTE) and central-line associated blood stream infection (CLABSI).7,8 Additionally, complications such as catheter occlusion and tip migration commonly occur and may interrupt care or necessitate device removal.9-11 Hence, thoughtful weighing of the risks against the benefits of PICC use prior to placement is necessary. To facilitate such decision-making, we developed the Michigan Appropriateness Guide for Intravenous (IV) Catheters (MAGIC) criteria,12 which is an evidence-based tool that defines when the use of a PICC is appropriate in hospitalized adults.
The use of PICCs for infusion of peripherally compatible therapies for 5 or fewer days is rated as inappropriate by MAGIC.12 This strategy is also endorsed by the Centers for Disease Control and Prevention’s (CDC) guidelines for the prevention of catheter-related infections.13 Despite these recommendations, short-term PICC use remains common. For example, a study conducted at a tertiary pediatric care center reported a trend toward shorter PICC dwell times and increasing rates of early removal.2 However, factors that prompt such short-term PICC use are poorly understood. Without understanding drivers and outcomes of short-term PICC use, interventions to prevent such practice are unlikely to succeed.
Therefore, by using data from a multicenter cohort study, we examined patterns of short-term PICC use and sought to identify which patient, provider, and device factors were associated with such use. We hypothesized that short-term placement would be associated with difficult venous access and would also be associated with the risk of major and minor complications.
METHODS
Study Setting and Design
We used data from the Michigan Hospital Medicine Safety (HMS) Consortium to examine patterns and predictors of short-term PICC use.14 As a multi-institutional clinical quality initiative sponsored by Blue Cross Blue Shield of Michigan and Blue Care Network, HMS aims to improve the quality of care by preventing adverse events in hospitalized medical patients.4,15-17 In January of 2014, dedicated, trained abstractors started collecting data on PICC placements at participating HMS hospitals by using a standard protocol and template for data collection. Patients who received PICCs while admitted to either a general medicine unit or an intensive care unit (ICU) during clinical care were eligible for inclusion. Patients were excluded if they were (a) under the age of 18 years, (b) pregnant, (c) admitted to a nonmedical service (eg, surgery), or (d) admitted under observation status.
Every 14 days, each hospital collected data on the first 17 eligible patients that received a PICC, with at least 7 of these placements occurring in an ICU setting. All patients were prospectively followed until the PICC was removed, death, or until 70 days after insertion, whichever occurred first. For patients who had their PICC removed prior to hospital discharge, follow-up occurred via a review of medical records. For those discharged with a PICC in place, both medical record review and telephone follow-up were performed. To ensure data quality, annual random audits at each participating hospital were performed by the coordinating center at the University of Michigan.
For this analysis, we included all available data as of June 30, 2016. However, HMS hospitals continue to collect data on PICC use and outcomes as part of an ongoing clinical quality initiative to reduce the incidence of PICC-related complications.
Patient, Provider, and Device Data
Patient characteristics, including demographics, detailed medical history, comorbidities, physical findings, laboratory results, and medications were abstracted directly from medical records. To estimate the comorbidity burden, the Charlson-Deyo comorbidity score was calculated for each patient by using data available in the medical record at the time of PICC placement.18 Data, such as the documented indication for PICC insertion and the reason for removal, were obtained directly from medical records. Provider characteristics, including the specialty of the attending physician at the time of insertion and the type of operator who inserted the PICC, were also collected. Institutional characteristics, such as total number of beds, teaching versus nonteaching, and urban versus rural, were obtained from hospital publicly reported data and semiannual surveys of HMS sites.19,20 Data on device characteristics, such as catheter gauge, coating, insertion attempts, tip location, and number of lumens, were abstracted from PICC insertion notes.
Outcomes of Interest
The outcome of interest was short-term PICC use, defined as PICCs removed within 5 days of insertion. Patients who expired with a PICC in situ were excluded. Secondary outcomes of interest included PICC-related complications, categorized as major (eg, symptomatic VTE and CLABSI) or minor (eg, catheter occlusion, superficial thrombosis, mechanical complications [kinking, coiling], exit site infection, and tip migration). Symptomatic VTE was defined as clinically diagnosed deep venous thrombosis (DVT) and/or pulmonary embolism (PE) not present at the time of PICC placement and confirmed via imaging (ultrasound or venogram for DVT; computed tomography scan, ventilation perfusion scan, or pulmonary angiogram for PE). CLABSI was defined in accordance with the CDC’s National Healthcare Safety Network criteria or according to Infectious Diseases Society of America recommendations.21,22 All minor PICC complications were defined in accordance with prior published definitions.4
Statistical Analysis
Cases of short-term PICC use were identified and compared with patients with a PICC dwell time of 6 or more days by patient, provider, and device characteristics. The initial analyses for the associations of putative factors with short-term PICC use were performed using χ2 or Wilcoxon tests for categorical and continuous variables, respectively. Univariable mixed effect logistic regression models (with a random hospital-specific intercept) were then used to control for hospital-level clustering. Next, a mixed effects multivariable logistic regression model was used to identify factors associated with short-term PICC use. Variables with P ≤ .25 were considered as candidate predictors for the final multivariable model, which was chosen through a stepwise variable selection algorithm performed on 1000 bootstrapped data sets.23 Variables in the final model were retained based on their frequency of selection in the bootstrapped samples, significance level, and contribution to the overall model likelihood. Results were expressed as odds ratios (OR) with corresponding 95% confidence intervals (CI). SAS for Windows (version 9.3, SAS Institute Inc., Cary, NC) was used for analyses.
Ethical and Regulatory Oversight
The study was classified as “not regulated” by the Institutional Review Board at the University of Michigan (HUM00078730).
RESULTS
Overall Characteristics of the Study Cohort
Characteristics of Short-Term Peripherally Inserted Central Catheter Use
Of the 15,397 PICCs included, we identified 3902 PICCs (25.3%) with a dwell time of ≤5 days (median = 3 days; IQR, 2-4 days). When compared to PICCs that were in place for longer durations, no significant differences in age or comorbidity scores were observed. Importantly, despite recommendations to avoid PICCs in patients with moderate to severe chronic kidney disease (glomerular filtration rate [GFR] ≤ 59 ml/min), 1292 (33.1%) short-term PICCs occurred in patients that met such criteria.
Among short-term PICCs, 3618 (92.7%) were power-capable devices, 2785 (71.4%) were 5-French, and 2813 (72.1%) were multilumen. Indications for the use of short-term PICCs differed from longer term devices in important ways (P < .001). For example, the most common documented indication for short-term PICC use was difficult venous access (28.2%), while for long-term PICCs, it was antibiotic administration (39.8%). General internists and hospitalists were the most common attending physicians for patients with short-term and long-term PICCs (65.1% and 65.5%, respectively [P = .73]). Also, the proportion of critical care physicians responsible for patients with short versus long-term PICC use was similar (14.0% vs 15.0%, respectively [P = .123]). Of the short-term PICCs, 2583 (66.2%) were inserted by vascular access nurses, 795 (20.4%) by interventional radiologists, and 439 (11.3%) by advance practice professionals. Almost all of the PICCs placed ≤5 days (95.5%) were removed during hospitalization.
Complications Associated with Short-Term Peripherally Inserted Central Catheter Use
DISCUSSION
This large, multisite prospective cohort study is the first to examine patterns and predictors of short-term PICC use in hospitalized adults. By examining clinically granular data derived from the medical records of patients across 52 hospitals, we found that short-term use was common, representing 25% of all PICCs placed. Almost all such PICCs were removed prior to discharge, suggesting that they were placed primarily to meet acute needs during hospitalization. Multivariable models indicated that patients with difficult venous access, multilumen devices, and teaching hospital settings were associated with short-term use. Given that (a) short term PICC use is not recommended by published evidence-based guidelines,12,13 (b) both major and minor complications were not uncommon despite brief exposure, and (c) specific factors might be targeted to avoid such use, strategies to improve PICC decision-making in the hospital appear increasingly necessary.
In our study, difficult venous access was the most common documented indication for short-term PICC placement. For patients in whom an anticipated catheter dwell time of 5 days or less is expected, MAGIC recommends the consideration of midline or peripheral IV catheters placed under ultrasound guidance.12 A midline is a type of peripheral IV catheter that is about 7.5 cm to 25 cm in length and is typically inserted in the larger diameter veins of the upper extremity, such as the cephalic or basilic veins, with the tip terminating distal to the subclavian vein.7,12 While there is a paucity of information that directly compares PICCs to midlines, some data suggest a lower risk of bloodstream infection and thrombosis associated with the latter.24-26 For example, at one quaternary teaching hospital, house staff who are trained to insert midline catheters under ultrasound guidance in critically ill patients with difficult venous access reported no CLABSI and DVT events.26
Interestingly, multilumen catheters were used twice as often as single lumen catheters in patients with short-term PICCs. In these instances, the use of additional lumens is questionable, as infusion of multiple incompatible fluids was not commonly listed as an indication prompting PICC use. Because multilumen PICCs are associated with higher risks of both VTE and CLABSI compared to single lumen devices, such use represents an important safety concern.27-29 Institutional efforts that not only limit the use of multilumen PICCs but also fundamentally define when use of a PICC is appropriate may substantially improve outcomes related to vascular access.28,30,31We observed that short-term PICCs were more common in teaching compared to nonteaching hospitals. While the design of the present study precludes understanding the reasons for such a difference, some plausible theories include the presence of physician trainees who may not appreciate the risks of PICC use, diminishing peripheral IV access securement skills, and the lack of alternatives to PICC use. Educating trainees who most often order PICCs in teaching settings as to when they should or should not consider this device may represent an important quality improvement opportunity.32 Similarly, auditing and assessing the clinical skills of those entrusted to place peripheral IVs might prove helpful.33,34 Finally, the introduction of a midline program, or similar programs that expand the scope of vascular access teams to place alternative devices, should be explored as a means to improve PICC use and patient safety.
Our study also found that a third of patients who received PICCs for 5 or fewer days had moderate to severe chronic kidney disease. In these patients who may require renal replacement therapy, prior PICC placement is among the strongest predictors of arteriovenous fistula failure.35,36 Therefore, even though national guidelines discourage the use of PICCs in these patients and recommend alternative routes of venous access,12,37,38 such practice is clearly not happening. System-based interventions that begin by identifying patients who require vein preservation (eg, those with a GFR < 45 ml/min) and are therefore not appropriate for a PICC would be a welcomed first step in improving care for such patients.37,38Our study has limitations. First, the observational nature of the study limits the ability to assess for causality or to account for the effects of unmeasured confounders. Second, while the use of medical records to collect granular data is valuable, differences in documentation patterns within and across hospitals, including patterns of missing data, may produce a misclassification of covariates or outcomes. Third, while we found that higher rates of short-term PICC use were associated with teaching hospitals and patients with difficult venous access, we were unable to determine the precise reasons for this practice trend. Qualitative or mixed-methods approaches to understand provider decision-making in these settings would be welcomed.
Our study also has several strengths. First, to our knowledge, this is the first study to systematically describe and evaluate patterns and predictors of short-term PICC use. The finding that PICCs placed for difficult venous access is a dominant category of short-term placement confirms clinical suspicions regarding inappropriate use and strengthens the need for pathways or protocols to manage such patients. Second, the inclusion of medical patients in diverse institutions offers not only real-world insights related to PICC use, but also offers findings that should be generalizable to other hospitals and health systems. Third, the use of a robust data collection strategy that emphasized standardized data collection, dedicated trained abstractors, and random audits to ensure data quality strengthen the findings of this work. Finally, our findings highlight an urgent need to develop policies related to PICC use, including limiting the use of multiple lumens and avoidance in patients with moderate to severe kidney disease.
In conclusion, short-term use of PICCs is prevalent and associated with key patient, provider, and device factors. Such use is also associated with complications, such as catheter occlusion, tip migration, VTE, and CLABSI. Limiting the use of multiple-lumen PICCs, enhancing education for when a PICC should be used, and defining strategies for patients with difficult access may help reduce inappropriate PICC use and improve patient safety. Future studies to examine implementation of such interventions would be welcomed.
Disclosure: Drs. Paje, Conlon, Swaminathan, and Boldenow disclose no conflicts of interest. Dr. Chopra has received honoraria for talks at hospitals as a visiting professor. Dr. Flanders discloses consultancies for the Institute for Healthcare Improvement and the Society of Hospital Medicine, royalties from Wiley Publishing, honoraria for various talks at hospitals as a visiting professor, grants from the CDC Foundation, Agency for Healthcare Research and Quality, Blue Cross Blue Shield of Michigan (BCBSM), and Michigan Hospital Association, and expert witness testimony. Dr. Bernstein discloses consultancies for Blue Care Network and grants from BCBSM, Department of Veterans Affairs, and National Institutes of Health. Dr. Kaatz discloses no relevant conflicts of interest. BCBSM and Blue Care Network provided support for the Michigan HMS Consortium as part of the BCBSM Value Partnerships program. Although BCBSM and HMS work collaboratively, the opinions, beliefs, and viewpoints expressed by the author do not necessarily reflect the opinions, beliefs, and viewpoints of BCBSM or any of its employees. Dr. Chopra is supported by a career development award from the Agency for Healthcare Research and Quality (1-K08-HS022835-01). BCBSM and Blue Care Network supported data collection at each participating site and funded the data coordinating center but had no role in study concept, interpretation of findings, or in the preparation, final approval, or decision to submit the manuscript.
1. Al Raiy B, Fakih MG, Bryan-Nomides N, et al. Peripherally inserted central venous catheters in the acute care setting: A safe alternative to high-risk short-term central venous catheters. Am J Infect Control. 2010;38(2):149-153. PubMed
2. Gibson C, Connolly BL, Moineddin R, Mahant S, Filipescu D, Amaral JG. Peripherally inserted central catheters: use at a tertiary care pediatric center. J Vasc Interv Radiol. 2013;24(9):1323-1331. PubMed
3. Chopra V, Flanders SA, Saint S. The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527-1528. PubMed
4. Chopra V, Smith S, Swaminathan L, et al. Variations in Peripherally Inserted Central Catheter Use and Outcomes in Michigan Hospitals. JAMA Intern Med. 2016;176(4):548-551. PubMed
5. Cowl CT, Weinstock JV, Al-Jurf A, Ephgrave K, Murray JA, Dillon K. Complications and cost associated with parenteral nutrition delivered to hospitalized patients through either subclavian or peripherally-inserted central catheters. Clin Nutr. 2000;19(4):237-243. PubMed
6. MacDonald AS, Master SK, Moffitt EA. A comparative study of peripherally inserted silicone catheters for parenteral nutrition. Can J Anaesth. 1977;24(2):263-269. PubMed
7. Chopra V, O’Horo JC, Rogers MA, Maki DG, Safdar N. The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2013;34(9):908-918. PubMed
8. Chopra V, Anand S, Hickner A, et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet. 2013;382(9889):311-325. PubMed
9. Beccaria P, Silvetti S, Mucci M, Battini I, Brambilla P, Zangrillo A. Contributing factors for a late spontaneous peripherally inserted central catheter migration: a case report and review of literature. J Vasc Access. 2015;16(3):178-182. PubMed
10. Turcotte S, Dube S, Beauchamp G. Peripherally inserted central venous catheters are not superior to central venous catheters in the acute care of surgical patients on the ward. World J Surg. 2006;30(8):1605-1619. PubMed
11. Pikwer A, Akeson J, Lindgren S. Complications associated with peripheral or central routes for central venous cannulation. Anaesthesia. 2012;67(1):65-71. PubMed
12. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): Results From a Multispecialty Panel Using the RAND/UCLA Appropriateness Method. Ann Intern Med. 15 2015;163(6 Suppl):S1-S40. PubMed
13. O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2011;39(4 Suppl 1):S1-S34. PubMed
14. Michigan Hospital Medicine Safety Consortium. 2016; http://mi-hms.org/. Accessed November 11, 2016.
15. Greene MT, Spyropoulos AC, Chopra V, et al. Validation of Risk Assessment Models of Venous Thromboembolism in Hospitalized Medical Patients. Am J Med. 2016;129(9):1001.e1009-1001.e1018. PubMed
16. Greene MT, Flanders SA, Woller SC, Bernstein SJ, Chopra V. The Association Between PICC Use and Venous Thromboembolism in Upper and Lower Extremities. Am J Med. 2015;128(9):986-993. PubMed
17. Flanders SA, Greene MT, Grant P, et al. Hospital performance for pharmacologic venous thromboembolism prophylaxis and rate of venous thromboembolism : a cohort study. JAMA Intern Med. 2014;174(10):1577-1584. PubMed
18. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619. PubMed
19. Hospital Bed Inventory. 2016; http://www.michigan.gov/documents/mdhhs/HOSPBEDINV_October_3__2016_536834_7.pdf. Accessed November 22, 2016.
20. Compare Hospitals. 2016; http://www.leapfroggroup.org/compare-hospitals. Accessed November 22, 2016.
21. NHSN Patient Safety Component Manual. 2016; http://www.cdc.gov/nhsn/pdfs/pscmanual/pcsmanual_current.pdf. Accessed November 22, 2016.
22. Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1-45. PubMed
23. Austin PC, Tu JV. Bootstrap Methods for Developing Predictive Models. Am Stat. 2004;58(2):131-137.
24. Pathak R, Patel A, Enuh H, Adekunle O, Shrisgantharajah V, Diaz K. The Incidence of Central Line-Associated Bacteremia After the Introduction of Midline Catheters in a Ventilator Unit Population. Infect Dis Clin Pract. 2015;23(3):131-134. PubMed
25. Adams DZ, Little A, Vinsant C, Khandelwal S. The Midline Catheter: A Clinical Review. J Emerg Med. 2016;51(3):252-258. PubMed
26. Deutsch GB, Sathyanarayana SA, Singh N, Nicastro J. Ultrasound-guided placement of midline catheters in the surgical intensive care unit: a cost-effective proposal for timely central line removal. J Surg Res. 2014;191(1):1-5. PubMed
27. Chopra V, Anand S, Krein SL, Chenoweth C, Saint S. Bloodstream Infection, Venous Thrombosis, and Peripherally Inserted Central Catheters: Reappraising the Evidence. Am J Med. 2012;125(8):733-741. PubMed
28. Ratz D, Hofer T, Flanders SA, Saint S, Chopra V. Limiting the Number of Lumens in Peripherally Inserted Central Catheters to Improve Outcomes and Reduce Cost: A Simulation Study. Infect Control Hosp Epidemiol. 2016;37(7):811-817. PubMed
29. Pongruangporn M, Ajenjo MC, Russo AJ, et al. Patient- and device-specific risk factors for peripherally inserted central venous catheter-related bloodstream infections. Infect Control Hosp Epidemiol. 2013;34(2):184-189. PubMed
30. Shannon RP, Patel B, Cummins D, Shannon AH, Ganguli G, Lu Y. Economics of central line--associated bloodstream infections. Am J Med Qual. 2006;21(6 Suppl):7S-16S. PubMed
31. O’Brien J, Paquet F, Lindsay R, Valenti D. Insertion of PICCs with minimum number of lumens reduces complications and costs. J AmColl Radiol. 2013;10(11):864-868. PubMed
32. Wong BM, Etchells EE, Kuper A, Levinson W, Shojania KG. Teaching quality improvement and patient safety to trainees: a systematic review. Acad Med. 2010;85(9):1425-1439. PubMed
33. Conlon T, Himebauch A, Marie Cahill A, et al. 1246: Bedside Picc Placement by Pediatric Icu Providers Is Feasible and Safe. Crit Care Med. 2016;44(12 Suppl 1):387.
34. Moran J, Colbert CY, Song J, et al. Screening for novel risk factors related to peripherally inserted central catheter-associated complications. J Hosp Med. 2014;9(8):481-489. PubMed
35. Gonsalves CF, Eschelman DJ, Sullivan KL, DuBois N, Bonn J. Incidence of central vein stenosis and occlusion following upper extremity PICC and port placement. Cardiovasc Intervent Radiol. 2003;26(2):123-127. PubMed
36. El Ters M, Schears GJ, Taler SJ, et al. Association between prior peripherally inserted central catheters and lack of functioning arteriovenous fistulas: a case-control study in hemodialysis patients. Am J Kidney Dis. 2012;60(4):601-608. PubMed
37. Vascular Access 2006 Work Group. Clinical practice guidelines for vascular access. Am J Kidney Dis. 2006;48 Suppl 1:S248-S273. PubMed
38. Hoggard J, Saad T, Schon D, et al. Guidelines for venous access in patients with chronic kidney disease. A Position Statement from the American Society of Diagnostic and Interventional Nephrology, Clinical Practice Committee and the Association for Vascular Access. Semin Dial. 2008;21(2):186-191. PubMed
1. Al Raiy B, Fakih MG, Bryan-Nomides N, et al. Peripherally inserted central venous catheters in the acute care setting: A safe alternative to high-risk short-term central venous catheters. Am J Infect Control. 2010;38(2):149-153. PubMed
2. Gibson C, Connolly BL, Moineddin R, Mahant S, Filipescu D, Amaral JG. Peripherally inserted central catheters: use at a tertiary care pediatric center. J Vasc Interv Radiol. 2013;24(9):1323-1331. PubMed
3. Chopra V, Flanders SA, Saint S. The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527-1528. PubMed
4. Chopra V, Smith S, Swaminathan L, et al. Variations in Peripherally Inserted Central Catheter Use and Outcomes in Michigan Hospitals. JAMA Intern Med. 2016;176(4):548-551. PubMed
5. Cowl CT, Weinstock JV, Al-Jurf A, Ephgrave K, Murray JA, Dillon K. Complications and cost associated with parenteral nutrition delivered to hospitalized patients through either subclavian or peripherally-inserted central catheters. Clin Nutr. 2000;19(4):237-243. PubMed
6. MacDonald AS, Master SK, Moffitt EA. A comparative study of peripherally inserted silicone catheters for parenteral nutrition. Can J Anaesth. 1977;24(2):263-269. PubMed
7. Chopra V, O’Horo JC, Rogers MA, Maki DG, Safdar N. The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2013;34(9):908-918. PubMed
8. Chopra V, Anand S, Hickner A, et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet. 2013;382(9889):311-325. PubMed
9. Beccaria P, Silvetti S, Mucci M, Battini I, Brambilla P, Zangrillo A. Contributing factors for a late spontaneous peripherally inserted central catheter migration: a case report and review of literature. J Vasc Access. 2015;16(3):178-182. PubMed
10. Turcotte S, Dube S, Beauchamp G. Peripherally inserted central venous catheters are not superior to central venous catheters in the acute care of surgical patients on the ward. World J Surg. 2006;30(8):1605-1619. PubMed
11. Pikwer A, Akeson J, Lindgren S. Complications associated with peripheral or central routes for central venous cannulation. Anaesthesia. 2012;67(1):65-71. PubMed
12. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): Results From a Multispecialty Panel Using the RAND/UCLA Appropriateness Method. Ann Intern Med. 15 2015;163(6 Suppl):S1-S40. PubMed
13. O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2011;39(4 Suppl 1):S1-S34. PubMed
14. Michigan Hospital Medicine Safety Consortium. 2016; http://mi-hms.org/. Accessed November 11, 2016.
15. Greene MT, Spyropoulos AC, Chopra V, et al. Validation of Risk Assessment Models of Venous Thromboembolism in Hospitalized Medical Patients. Am J Med. 2016;129(9):1001.e1009-1001.e1018. PubMed
16. Greene MT, Flanders SA, Woller SC, Bernstein SJ, Chopra V. The Association Between PICC Use and Venous Thromboembolism in Upper and Lower Extremities. Am J Med. 2015;128(9):986-993. PubMed
17. Flanders SA, Greene MT, Grant P, et al. Hospital performance for pharmacologic venous thromboembolism prophylaxis and rate of venous thromboembolism : a cohort study. JAMA Intern Med. 2014;174(10):1577-1584. PubMed
18. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619. PubMed
19. Hospital Bed Inventory. 2016; http://www.michigan.gov/documents/mdhhs/HOSPBEDINV_October_3__2016_536834_7.pdf. Accessed November 22, 2016.
20. Compare Hospitals. 2016; http://www.leapfroggroup.org/compare-hospitals. Accessed November 22, 2016.
21. NHSN Patient Safety Component Manual. 2016; http://www.cdc.gov/nhsn/pdfs/pscmanual/pcsmanual_current.pdf. Accessed November 22, 2016.
22. Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1-45. PubMed
23. Austin PC, Tu JV. Bootstrap Methods for Developing Predictive Models. Am Stat. 2004;58(2):131-137.
24. Pathak R, Patel A, Enuh H, Adekunle O, Shrisgantharajah V, Diaz K. The Incidence of Central Line-Associated Bacteremia After the Introduction of Midline Catheters in a Ventilator Unit Population. Infect Dis Clin Pract. 2015;23(3):131-134. PubMed
25. Adams DZ, Little A, Vinsant C, Khandelwal S. The Midline Catheter: A Clinical Review. J Emerg Med. 2016;51(3):252-258. PubMed
26. Deutsch GB, Sathyanarayana SA, Singh N, Nicastro J. Ultrasound-guided placement of midline catheters in the surgical intensive care unit: a cost-effective proposal for timely central line removal. J Surg Res. 2014;191(1):1-5. PubMed
27. Chopra V, Anand S, Krein SL, Chenoweth C, Saint S. Bloodstream Infection, Venous Thrombosis, and Peripherally Inserted Central Catheters: Reappraising the Evidence. Am J Med. 2012;125(8):733-741. PubMed
28. Ratz D, Hofer T, Flanders SA, Saint S, Chopra V. Limiting the Number of Lumens in Peripherally Inserted Central Catheters to Improve Outcomes and Reduce Cost: A Simulation Study. Infect Control Hosp Epidemiol. 2016;37(7):811-817. PubMed
29. Pongruangporn M, Ajenjo MC, Russo AJ, et al. Patient- and device-specific risk factors for peripherally inserted central venous catheter-related bloodstream infections. Infect Control Hosp Epidemiol. 2013;34(2):184-189. PubMed
30. Shannon RP, Patel B, Cummins D, Shannon AH, Ganguli G, Lu Y. Economics of central line--associated bloodstream infections. Am J Med Qual. 2006;21(6 Suppl):7S-16S. PubMed
31. O’Brien J, Paquet F, Lindsay R, Valenti D. Insertion of PICCs with minimum number of lumens reduces complications and costs. J AmColl Radiol. 2013;10(11):864-868. PubMed
32. Wong BM, Etchells EE, Kuper A, Levinson W, Shojania KG. Teaching quality improvement and patient safety to trainees: a systematic review. Acad Med. 2010;85(9):1425-1439. PubMed
33. Conlon T, Himebauch A, Marie Cahill A, et al. 1246: Bedside Picc Placement by Pediatric Icu Providers Is Feasible and Safe. Crit Care Med. 2016;44(12 Suppl 1):387.
34. Moran J, Colbert CY, Song J, et al. Screening for novel risk factors related to peripherally inserted central catheter-associated complications. J Hosp Med. 2014;9(8):481-489. PubMed
35. Gonsalves CF, Eschelman DJ, Sullivan KL, DuBois N, Bonn J. Incidence of central vein stenosis and occlusion following upper extremity PICC and port placement. Cardiovasc Intervent Radiol. 2003;26(2):123-127. PubMed
36. El Ters M, Schears GJ, Taler SJ, et al. Association between prior peripherally inserted central catheters and lack of functioning arteriovenous fistulas: a case-control study in hemodialysis patients. Am J Kidney Dis. 2012;60(4):601-608. PubMed
37. Vascular Access 2006 Work Group. Clinical practice guidelines for vascular access. Am J Kidney Dis. 2006;48 Suppl 1:S248-S273. PubMed
38. Hoggard J, Saad T, Schon D, et al. Guidelines for venous access in patients with chronic kidney disease. A Position Statement from the American Society of Diagnostic and Interventional Nephrology, Clinical Practice Committee and the Association for Vascular Access. Semin Dial. 2008;21(2):186-191. PubMed
© 2018 Society of Hospital Medicine
Hospitalists in the ICU: Necessary But Not Sufficient
In the United States, up to 6 million patients are admitted to intensive care units (ICUs) annually at a cost estimated to exceed $80 billion or about 13% of total hospital costs.1,2 It also appears that as our population ages and illness severity increases, demand for ICU care is increasing.3 Given its importance, the organization and delivery of critical care has been extensively studied. High-intensity physician staffing by an intensivist (all patients managed or comanaged by an intensivist), while inconsistently shown to be associated with improved outcomes, has been endorsed as a high-quality care model by professional societies and the Leapfrog group. Despite its adoption by many hospitals, widespread implementation has been hampered by a national shortage of intensivists that continues to worsen over time. Hospitals, by necessity, look to alternative models to care for critically ill patients, and one such model is the use of hospitalists.
The Society of Hospital Medicine estimates that there are nearly 50,000 hospitalists practicing in the United States, and several studies show they routinely provide care in the nation’s ICUs.4 While in some ICUs hospitalists work alongside intensivists, in many, they work without intensivist support, and regardless of the model, they often serve as the primary attending physician. There is good reason to think this model of care would be effective. Most hospitalists are internists, graduating from training programs that tend to emphasize care of acutely ill hospitalized patients. Hospitalists are often present in the hospital 24/7, are comfortable working in multidisciplinary teams, and routinely engage in quality improvement, which are all characteristics common in highly functioning ICUs. Yet, a study in this issue of the Journal of Hospital Medicine raises some concern.
Sweigart and colleagues5 surveyed 425 hospitalists to understand the structure and perception of their ICU practices. Consistent with prior studies, 77% provided ICU care with 66% serving as the primary attending. A novel finding is the high level of angst and lack of support hospitalists perceived in caring for these critically ill patients. Among rural hospitalists, 43% reported they were expected to practice beyond their perceived scope of practice, and almost a third reported they never had sufficient intensivist support. Even more concerning is that among hospitalists serving as the primary attending, over two-thirds reported difficulty transferring patients to a higher level of care (Sweigart et al.5). While we have concerns over how representative this sample is of hospitalist practice (the survey response rate was only about 10%), it does appear that many hospitalists feel very uncomfortable with the ICU care they are providing and perceive barriers to moving their patients to a potentially safer care setting.
While one might argue more intensivists would solve this problem, calls for more intensivists are shortsighted, as there are compelling reasons to believe that such efforts will do little to address the mismatch between patient need and provider supply. Graduate medical education slots for intensivists cannot be easily and affordably increased, and even if more intensivists could be trained, there are few incentives to encourage them to work where they are needed most. Prioritization of intensivist training also diverts resources from training demands in equally important undersupplied specialties such as primary care.6 Finally, simply increasing intensivist supply fails to attend to important issues surrounding the multidisciplinary nature of care in an ICU, which relies heavily on multiple providers communicating and collaborating to provide optimal care. As noted in the study by Sweigart and colleagues,5 even in settings where intensivists were available 24 hours per day or made all major decisions, nearly one-third of hospitalists felt they practiced beyond their scope of expertise, suggesting that more intensivists may do little to improve hospitalists’ comfort in caring for patients in the ICU.
In lieu of increasing intensivist numbers, policymakers should consider several strategies that have the potential to improve the quality of care delivered to patients in the ICU without increasing intensivists. Recent data suggest that some ICU patients can be safely managed by physician assistants and nurse practitioners.7,8 Care models involving such providers may free up overworked intensivists and hospitalists, allowing them to focus their efforts on the sickest patients. ICU telemedicine has also emerged as a promising tool that can bring the expertise of intensivists to hospitals where they are needed. Beyond the additional oversight of routine care practices it provides, telemedicine could allow rapid and real time consultation with intensivists for clinicians at the bedside facing difficult management decisions, potentially saving lives.9 The rapid growth of clinically integrated networks, which often include large well-staffed medical centers surrounded by many smaller regional hospitals, might facilitate faster implementation of innovative telemedicine models. Regionalization of care is a third strategy that may improve the quality of care for the critically ill without increasing intensivist supply. Regionalization seeks to selectively transfer the most ill patients to high-volume centers with the greatest expertise in critical care, a practice associated with reduced mortality.10 Of course, for regionalization to be successful, front-line providers like hospitalists need to be able to orchestrate the transfer to the referral center, a process that, as noted by Sweigart and others, is neither easy nor universally successful.11
A final strategy would be to reduce the demand for intensivists through limiting the number of individuals in an ICU. While policies that explicitly ration ICU beds for individuals who have the greatest ability to benefit are ethically problematic, reductions in ICU beds would force providers to implicitly allocate beds more efficiently. There are a multitude of studies showing that our nation’s ICUs are often filled with patients who derive little benefit from intensive care.12,13 Further research on ethically sound strategies to avoid ICU admission for patients unlikely to benefit is desperately needed. With fewer patients in an ICU, the busy intensivist could focus on the sickest patients and spend more time communicating with hospitalists about patients they are managing together.
Regardless of the care models that develop, hospitalists will increasingly be called upon to staff ICUs. Hospitalists are necessary, but as the study by Sweigart et al.5 suggests, just throwing them into our current ICU models with little support from their critical care colleagues is not sufficient. In the absence of a major influx of new intensivists, hospital medicine and critical care professional societies need to actively collaborate to develop creative training and educational models that provide hospitalists with the necessary skills to care for the critically ill and to lead the multidisciplinary care teams they will work within. More importantly, these professional societies must advocate together for more substantial reform to our current ICU care models. Novel solutions that prioritize the efficient use of existing ICU beds for those individuals with the greatest ability to benefit, but also capitalize on emerging technologies and regional centers of excellence, have great potential to address the mismatch between supply and demand. Given the increasing demand and substantial cost for ICU care, we can’t afford to continue with business as usual.
Disclosure
The authors declared no conflicts of interest.
1. Pastores SM, Dakwar J, Halpern NA. Costs of critical care medicine. Crit Care Clin. 2012;28(1):1-10, v. PubMed
2. Nguyen YL, Kahn JM, Angus DC. Reorganizing adult critical care delivery: the role of regionalization, telemedicine, and community outreach. Am J Respir Crit Care Med. 2010;181(11):1164-1169. PubMed
3. Halpern NA, Goldman DA, Tan KS, Pastores SM. Trends in Critical Care Beds and Use Among Population Groups and Medicare and Medicaid Beneficiaries in the United States: 2000-2010. Crit Care Med. 2016;44(8):1490-1499. PubMed
4. Hyzy RC, Flanders SA, Pronovost PJ, et al. Characteristics of intensive care units in Michigan: Not an open and closed case. J Hosp Med. 2010;5(1):4-9. PubMed
5. Sweigart JR, Aymond D, Burger A, et al. Characterizing Hospitalist Practice and Perceptions of Critical Care Delivery. J Hosp Med. In press. PubMed
6. Kahn JM, Rubenfeld GD. The myth of the workforce crisis. Why the United States does not need more intensivist physicians. Am J Respir Crit Care Med. 2015;191(2):128-134. PubMed
7. Gershengorn HB, Johnson MP, Factor P. The use of nonphysician providers in adult intensive care units. Am J Respir Crit Care Med. 2012;185(6):600-605. PubMed
8. Gershengorn HB, Wunsch H, Wahab R, et al. Impact of nonphysician staffing on outcomes in a medical ICU. Chest. 2011;139(6):1347-1353. PubMed
9. Kahn JM, Le TQ, Barnato AE, et al. ICU Telemedicine and Critical Care Mortality: A National Effectiveness Study. Med Care. 2016;54(3):319-325. PubMed
10. Kahn JM, Linde-Zwirble WT, Wunsch H, et al. Potential value of regionalized intensive care for mechanically ventilated medical patients. Am J Respir Crit Care Med. 2008;177(3):285-291. PubMed
11. Bosk EA, Veinot T, Iwashyna TJ. Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49(6):592-598. PubMed
12. Admon AJ, Wunsch H, Iwashyna TJ, Cooke CR. Hospital Contributions to Variability in the Use of ICUs Among Elderly Medicare Recipients. Crit Care Med. 2017;45(1):75-84. PubMed
13. Seymour CW, Iwashyna TJ, Ehlenbach WJ, Wunsch H, Cooke CR. Hospital-level variation in the use of intensive care. Health Serv Res. 2012;47(5):2060-2080. PubMed
In the United States, up to 6 million patients are admitted to intensive care units (ICUs) annually at a cost estimated to exceed $80 billion or about 13% of total hospital costs.1,2 It also appears that as our population ages and illness severity increases, demand for ICU care is increasing.3 Given its importance, the organization and delivery of critical care has been extensively studied. High-intensity physician staffing by an intensivist (all patients managed or comanaged by an intensivist), while inconsistently shown to be associated with improved outcomes, has been endorsed as a high-quality care model by professional societies and the Leapfrog group. Despite its adoption by many hospitals, widespread implementation has been hampered by a national shortage of intensivists that continues to worsen over time. Hospitals, by necessity, look to alternative models to care for critically ill patients, and one such model is the use of hospitalists.
The Society of Hospital Medicine estimates that there are nearly 50,000 hospitalists practicing in the United States, and several studies show they routinely provide care in the nation’s ICUs.4 While in some ICUs hospitalists work alongside intensivists, in many, they work without intensivist support, and regardless of the model, they often serve as the primary attending physician. There is good reason to think this model of care would be effective. Most hospitalists are internists, graduating from training programs that tend to emphasize care of acutely ill hospitalized patients. Hospitalists are often present in the hospital 24/7, are comfortable working in multidisciplinary teams, and routinely engage in quality improvement, which are all characteristics common in highly functioning ICUs. Yet, a study in this issue of the Journal of Hospital Medicine raises some concern.
Sweigart and colleagues5 surveyed 425 hospitalists to understand the structure and perception of their ICU practices. Consistent with prior studies, 77% provided ICU care with 66% serving as the primary attending. A novel finding is the high level of angst and lack of support hospitalists perceived in caring for these critically ill patients. Among rural hospitalists, 43% reported they were expected to practice beyond their perceived scope of practice, and almost a third reported they never had sufficient intensivist support. Even more concerning is that among hospitalists serving as the primary attending, over two-thirds reported difficulty transferring patients to a higher level of care (Sweigart et al.5). While we have concerns over how representative this sample is of hospitalist practice (the survey response rate was only about 10%), it does appear that many hospitalists feel very uncomfortable with the ICU care they are providing and perceive barriers to moving their patients to a potentially safer care setting.
While one might argue more intensivists would solve this problem, calls for more intensivists are shortsighted, as there are compelling reasons to believe that such efforts will do little to address the mismatch between patient need and provider supply. Graduate medical education slots for intensivists cannot be easily and affordably increased, and even if more intensivists could be trained, there are few incentives to encourage them to work where they are needed most. Prioritization of intensivist training also diverts resources from training demands in equally important undersupplied specialties such as primary care.6 Finally, simply increasing intensivist supply fails to attend to important issues surrounding the multidisciplinary nature of care in an ICU, which relies heavily on multiple providers communicating and collaborating to provide optimal care. As noted in the study by Sweigart and colleagues,5 even in settings where intensivists were available 24 hours per day or made all major decisions, nearly one-third of hospitalists felt they practiced beyond their scope of expertise, suggesting that more intensivists may do little to improve hospitalists’ comfort in caring for patients in the ICU.
In lieu of increasing intensivist numbers, policymakers should consider several strategies that have the potential to improve the quality of care delivered to patients in the ICU without increasing intensivists. Recent data suggest that some ICU patients can be safely managed by physician assistants and nurse practitioners.7,8 Care models involving such providers may free up overworked intensivists and hospitalists, allowing them to focus their efforts on the sickest patients. ICU telemedicine has also emerged as a promising tool that can bring the expertise of intensivists to hospitals where they are needed. Beyond the additional oversight of routine care practices it provides, telemedicine could allow rapid and real time consultation with intensivists for clinicians at the bedside facing difficult management decisions, potentially saving lives.9 The rapid growth of clinically integrated networks, which often include large well-staffed medical centers surrounded by many smaller regional hospitals, might facilitate faster implementation of innovative telemedicine models. Regionalization of care is a third strategy that may improve the quality of care for the critically ill without increasing intensivist supply. Regionalization seeks to selectively transfer the most ill patients to high-volume centers with the greatest expertise in critical care, a practice associated with reduced mortality.10 Of course, for regionalization to be successful, front-line providers like hospitalists need to be able to orchestrate the transfer to the referral center, a process that, as noted by Sweigart and others, is neither easy nor universally successful.11
A final strategy would be to reduce the demand for intensivists through limiting the number of individuals in an ICU. While policies that explicitly ration ICU beds for individuals who have the greatest ability to benefit are ethically problematic, reductions in ICU beds would force providers to implicitly allocate beds more efficiently. There are a multitude of studies showing that our nation’s ICUs are often filled with patients who derive little benefit from intensive care.12,13 Further research on ethically sound strategies to avoid ICU admission for patients unlikely to benefit is desperately needed. With fewer patients in an ICU, the busy intensivist could focus on the sickest patients and spend more time communicating with hospitalists about patients they are managing together.
Regardless of the care models that develop, hospitalists will increasingly be called upon to staff ICUs. Hospitalists are necessary, but as the study by Sweigart et al.5 suggests, just throwing them into our current ICU models with little support from their critical care colleagues is not sufficient. In the absence of a major influx of new intensivists, hospital medicine and critical care professional societies need to actively collaborate to develop creative training and educational models that provide hospitalists with the necessary skills to care for the critically ill and to lead the multidisciplinary care teams they will work within. More importantly, these professional societies must advocate together for more substantial reform to our current ICU care models. Novel solutions that prioritize the efficient use of existing ICU beds for those individuals with the greatest ability to benefit, but also capitalize on emerging technologies and regional centers of excellence, have great potential to address the mismatch between supply and demand. Given the increasing demand and substantial cost for ICU care, we can’t afford to continue with business as usual.
Disclosure
The authors declared no conflicts of interest.
In the United States, up to 6 million patients are admitted to intensive care units (ICUs) annually at a cost estimated to exceed $80 billion or about 13% of total hospital costs.1,2 It also appears that as our population ages and illness severity increases, demand for ICU care is increasing.3 Given its importance, the organization and delivery of critical care has been extensively studied. High-intensity physician staffing by an intensivist (all patients managed or comanaged by an intensivist), while inconsistently shown to be associated with improved outcomes, has been endorsed as a high-quality care model by professional societies and the Leapfrog group. Despite its adoption by many hospitals, widespread implementation has been hampered by a national shortage of intensivists that continues to worsen over time. Hospitals, by necessity, look to alternative models to care for critically ill patients, and one such model is the use of hospitalists.
The Society of Hospital Medicine estimates that there are nearly 50,000 hospitalists practicing in the United States, and several studies show they routinely provide care in the nation’s ICUs.4 While in some ICUs hospitalists work alongside intensivists, in many, they work without intensivist support, and regardless of the model, they often serve as the primary attending physician. There is good reason to think this model of care would be effective. Most hospitalists are internists, graduating from training programs that tend to emphasize care of acutely ill hospitalized patients. Hospitalists are often present in the hospital 24/7, are comfortable working in multidisciplinary teams, and routinely engage in quality improvement, which are all characteristics common in highly functioning ICUs. Yet, a study in this issue of the Journal of Hospital Medicine raises some concern.
Sweigart and colleagues5 surveyed 425 hospitalists to understand the structure and perception of their ICU practices. Consistent with prior studies, 77% provided ICU care with 66% serving as the primary attending. A novel finding is the high level of angst and lack of support hospitalists perceived in caring for these critically ill patients. Among rural hospitalists, 43% reported they were expected to practice beyond their perceived scope of practice, and almost a third reported they never had sufficient intensivist support. Even more concerning is that among hospitalists serving as the primary attending, over two-thirds reported difficulty transferring patients to a higher level of care (Sweigart et al.5). While we have concerns over how representative this sample is of hospitalist practice (the survey response rate was only about 10%), it does appear that many hospitalists feel very uncomfortable with the ICU care they are providing and perceive barriers to moving their patients to a potentially safer care setting.
While one might argue more intensivists would solve this problem, calls for more intensivists are shortsighted, as there are compelling reasons to believe that such efforts will do little to address the mismatch between patient need and provider supply. Graduate medical education slots for intensivists cannot be easily and affordably increased, and even if more intensivists could be trained, there are few incentives to encourage them to work where they are needed most. Prioritization of intensivist training also diverts resources from training demands in equally important undersupplied specialties such as primary care.6 Finally, simply increasing intensivist supply fails to attend to important issues surrounding the multidisciplinary nature of care in an ICU, which relies heavily on multiple providers communicating and collaborating to provide optimal care. As noted in the study by Sweigart and colleagues,5 even in settings where intensivists were available 24 hours per day or made all major decisions, nearly one-third of hospitalists felt they practiced beyond their scope of expertise, suggesting that more intensivists may do little to improve hospitalists’ comfort in caring for patients in the ICU.
In lieu of increasing intensivist numbers, policymakers should consider several strategies that have the potential to improve the quality of care delivered to patients in the ICU without increasing intensivists. Recent data suggest that some ICU patients can be safely managed by physician assistants and nurse practitioners.7,8 Care models involving such providers may free up overworked intensivists and hospitalists, allowing them to focus their efforts on the sickest patients. ICU telemedicine has also emerged as a promising tool that can bring the expertise of intensivists to hospitals where they are needed. Beyond the additional oversight of routine care practices it provides, telemedicine could allow rapid and real time consultation with intensivists for clinicians at the bedside facing difficult management decisions, potentially saving lives.9 The rapid growth of clinically integrated networks, which often include large well-staffed medical centers surrounded by many smaller regional hospitals, might facilitate faster implementation of innovative telemedicine models. Regionalization of care is a third strategy that may improve the quality of care for the critically ill without increasing intensivist supply. Regionalization seeks to selectively transfer the most ill patients to high-volume centers with the greatest expertise in critical care, a practice associated with reduced mortality.10 Of course, for regionalization to be successful, front-line providers like hospitalists need to be able to orchestrate the transfer to the referral center, a process that, as noted by Sweigart and others, is neither easy nor universally successful.11
A final strategy would be to reduce the demand for intensivists through limiting the number of individuals in an ICU. While policies that explicitly ration ICU beds for individuals who have the greatest ability to benefit are ethically problematic, reductions in ICU beds would force providers to implicitly allocate beds more efficiently. There are a multitude of studies showing that our nation’s ICUs are often filled with patients who derive little benefit from intensive care.12,13 Further research on ethically sound strategies to avoid ICU admission for patients unlikely to benefit is desperately needed. With fewer patients in an ICU, the busy intensivist could focus on the sickest patients and spend more time communicating with hospitalists about patients they are managing together.
Regardless of the care models that develop, hospitalists will increasingly be called upon to staff ICUs. Hospitalists are necessary, but as the study by Sweigart et al.5 suggests, just throwing them into our current ICU models with little support from their critical care colleagues is not sufficient. In the absence of a major influx of new intensivists, hospital medicine and critical care professional societies need to actively collaborate to develop creative training and educational models that provide hospitalists with the necessary skills to care for the critically ill and to lead the multidisciplinary care teams they will work within. More importantly, these professional societies must advocate together for more substantial reform to our current ICU care models. Novel solutions that prioritize the efficient use of existing ICU beds for those individuals with the greatest ability to benefit, but also capitalize on emerging technologies and regional centers of excellence, have great potential to address the mismatch between supply and demand. Given the increasing demand and substantial cost for ICU care, we can’t afford to continue with business as usual.
Disclosure
The authors declared no conflicts of interest.
1. Pastores SM, Dakwar J, Halpern NA. Costs of critical care medicine. Crit Care Clin. 2012;28(1):1-10, v. PubMed
2. Nguyen YL, Kahn JM, Angus DC. Reorganizing adult critical care delivery: the role of regionalization, telemedicine, and community outreach. Am J Respir Crit Care Med. 2010;181(11):1164-1169. PubMed
3. Halpern NA, Goldman DA, Tan KS, Pastores SM. Trends in Critical Care Beds and Use Among Population Groups and Medicare and Medicaid Beneficiaries in the United States: 2000-2010. Crit Care Med. 2016;44(8):1490-1499. PubMed
4. Hyzy RC, Flanders SA, Pronovost PJ, et al. Characteristics of intensive care units in Michigan: Not an open and closed case. J Hosp Med. 2010;5(1):4-9. PubMed
5. Sweigart JR, Aymond D, Burger A, et al. Characterizing Hospitalist Practice and Perceptions of Critical Care Delivery. J Hosp Med. In press. PubMed
6. Kahn JM, Rubenfeld GD. The myth of the workforce crisis. Why the United States does not need more intensivist physicians. Am J Respir Crit Care Med. 2015;191(2):128-134. PubMed
7. Gershengorn HB, Johnson MP, Factor P. The use of nonphysician providers in adult intensive care units. Am J Respir Crit Care Med. 2012;185(6):600-605. PubMed
8. Gershengorn HB, Wunsch H, Wahab R, et al. Impact of nonphysician staffing on outcomes in a medical ICU. Chest. 2011;139(6):1347-1353. PubMed
9. Kahn JM, Le TQ, Barnato AE, et al. ICU Telemedicine and Critical Care Mortality: A National Effectiveness Study. Med Care. 2016;54(3):319-325. PubMed
10. Kahn JM, Linde-Zwirble WT, Wunsch H, et al. Potential value of regionalized intensive care for mechanically ventilated medical patients. Am J Respir Crit Care Med. 2008;177(3):285-291. PubMed
11. Bosk EA, Veinot T, Iwashyna TJ. Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49(6):592-598. PubMed
12. Admon AJ, Wunsch H, Iwashyna TJ, Cooke CR. Hospital Contributions to Variability in the Use of ICUs Among Elderly Medicare Recipients. Crit Care Med. 2017;45(1):75-84. PubMed
13. Seymour CW, Iwashyna TJ, Ehlenbach WJ, Wunsch H, Cooke CR. Hospital-level variation in the use of intensive care. Health Serv Res. 2012;47(5):2060-2080. PubMed
1. Pastores SM, Dakwar J, Halpern NA. Costs of critical care medicine. Crit Care Clin. 2012;28(1):1-10, v. PubMed
2. Nguyen YL, Kahn JM, Angus DC. Reorganizing adult critical care delivery: the role of regionalization, telemedicine, and community outreach. Am J Respir Crit Care Med. 2010;181(11):1164-1169. PubMed
3. Halpern NA, Goldman DA, Tan KS, Pastores SM. Trends in Critical Care Beds and Use Among Population Groups and Medicare and Medicaid Beneficiaries in the United States: 2000-2010. Crit Care Med. 2016;44(8):1490-1499. PubMed
4. Hyzy RC, Flanders SA, Pronovost PJ, et al. Characteristics of intensive care units in Michigan: Not an open and closed case. J Hosp Med. 2010;5(1):4-9. PubMed
5. Sweigart JR, Aymond D, Burger A, et al. Characterizing Hospitalist Practice and Perceptions of Critical Care Delivery. J Hosp Med. In press. PubMed
6. Kahn JM, Rubenfeld GD. The myth of the workforce crisis. Why the United States does not need more intensivist physicians. Am J Respir Crit Care Med. 2015;191(2):128-134. PubMed
7. Gershengorn HB, Johnson MP, Factor P. The use of nonphysician providers in adult intensive care units. Am J Respir Crit Care Med. 2012;185(6):600-605. PubMed
8. Gershengorn HB, Wunsch H, Wahab R, et al. Impact of nonphysician staffing on outcomes in a medical ICU. Chest. 2011;139(6):1347-1353. PubMed
9. Kahn JM, Le TQ, Barnato AE, et al. ICU Telemedicine and Critical Care Mortality: A National Effectiveness Study. Med Care. 2016;54(3):319-325. PubMed
10. Kahn JM, Linde-Zwirble WT, Wunsch H, et al. Potential value of regionalized intensive care for mechanically ventilated medical patients. Am J Respir Crit Care Med. 2008;177(3):285-291. PubMed
11. Bosk EA, Veinot T, Iwashyna TJ. Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49(6):592-598. PubMed
12. Admon AJ, Wunsch H, Iwashyna TJ, Cooke CR. Hospital Contributions to Variability in the Use of ICUs Among Elderly Medicare Recipients. Crit Care Med. 2017;45(1):75-84. PubMed
13. Seymour CW, Iwashyna TJ, Ehlenbach WJ, Wunsch H, Cooke CR. Hospital-level variation in the use of intensive care. Health Serv Res. 2012;47(5):2060-2080. PubMed
© 2018 Society of Hospital Medicine
Do Bedside Visual Tools Improve Patient and Caregiver Satisfaction? A Systematic Review of the Literature
Patient satisfaction with medical care during hospitalization is a common quality metric.1,2 Studies showing higher patient satisfaction have reported lower 30-day hospital readmissions3 and improved overall health.4,5 Conversely, communication failures are associated with dissatisfaction among hospitalized patients and adverse outcomes.6,7 A lack of familiarity with hospital providers weakens collaborative decision making and prevents high-quality patient care.8,9
Bedside visual tools, such as whiteboards and pictures of medical staff, have been widely used to enhance communication between patients, families, and providers.10,11 Results of studies evaluating these tools are varied. For example, 1 study found that 98% of patients were better able to identify physicians when their names were written on whiteboards.12 Yet in another, only 21.1% of patients were more likely to correctly identify ≥1 physicians using pictures.13 Thus, despite widespread use,11 whether visual tools improve patient satisfaction and patient care more broadly remains unclear.14,15
We performed a systematic review to answer the following 3 questions: first, what is the effect of visual tools on outcomes (ie, provider identification, understanding of providers’ roles, patient–provider communication, and satisfaction); second, does impact vary by type of visual tool (eg, whiteboards vs pictures of providers); and third, what factors (eg, study design, patient population) are associated with provider identification, communication, and patient satisfaction?
METHODS
Search Strategy
We used the Preferred Reporting Items for Systematic Reviews and Meta-Analysis when performing this review.16 A research librarian (WT) conducted serial searches for studies reporting the use of bedside visual tools for hospitalized patients in Medline (via OVID), Embase, SCOPUS, Web of Science, CINAHL, and Cochrane DSR and CENTRAL. Controlled vocabularies (ie, Medical Subject Headings terms) were used to identify synonyms for visual tools of interest. Additional studies were identified manually through bibliographies and meeting abstracts. No study design, publication date, or language restrictions were placed on the search, which was conducted between April 2016 and February 2017 (see supplementary Appendix A).
Study Selection
Two reviewers (AG and KT) independently assessed study eligibility; discrepancies were resolved by a third reviewer (VC). We included all adult or pediatric English language studies in which the effect of visual tool(s) on patient outcomes was reported. Visual tools were defined as the bedside display of information or an instrument given to patients to convey information regarding providers or medical care. Patient-reported outcomes included the following: (a) physician identification, (b) understanding of provider roles, (c) patient–provider communication, and (d) patient satisfaction with care. Providers were defined as physicians, residents, interns, medical students, nurse practitioners, or nurses. We excluded studies that were not original research (eg, conference abstracts, not peer reviewed), reported qualitative data without quantitative outcomes, or did not include a bedside visual tool. Given our interest in hospitalized general medicine patients, studies conducted in emergency departments, surgical units, obstetrics and gynecology wards, and intensive care units were excluded.
Data Extraction and Analysis
Data were extracted independently and in duplicate from all studies by using a template adapted from the Cochrane Collaboration.17 For all studies, we abstracted study design, type of visual tool (eg, whiteboards), unit setting (eg, medical), population studied (eg, adult vs pediatric), and outcomes reported (ie, physician identification, understanding of provider roles, communication, and satisfaction with care). Reviewers independently assessed and categorized the impact of tools on reported outcomes.
To standardize and compare outcomes across studies, the following were used to denote a positive association between visual tools and relevant outcomes: a greater number of physicians correctly identified by name/picture or title/role; the use of terms such as “high,” “agreed,” or “significant” on surveys; or ≥4 Likert scores for domains of identification, understanding of roles, communication, and satisfaction with care. Conversely, the inability to identify providers compared to the control/baseline; poor recall of titles/roles; lower Likert-scale scores (ie, ≤2); or survey terms such as “poor,” “disagreed,” or “insignificant” were considered to connote negative impact. Studies in which Likert scores were rated neither high nor low (ie, 3), or in which patients neither agreed nor disagreed on value were considered neutral.
Owing to clinical heterogeneity within studies, meta-analyses were not performed. Descriptive statistics were used to describe study outcomes. A priori18 studies were evaluated according to the following categories: design (eg, randomized vs observational), outcomes (eg, patient satisfaction), intervention (type of visual tool), and patient population (adult or pediatric). Because pediatric patients have underdeveloped communication skills and include parents and/or guardians, data from pediatric studies were tabulated and reported separately to those from adult studies.
Quality Assessment
As recommended by the Cochrane Collaboration, 2 reviewers (AG, KT) assessed the risk of study bias by using the Downs and Black Scale.17,19 Discrepancies in assessment were resolved by a third reviewer (VC). This instrument uses a point-based system to estimate the quality of a study by rating domains such as internal and external validity, bias, and confounding. In keeping with prior systematic reviews,18,20,21 studies with a score of ≥18 were considered high quality. Interrater agreement for the adjudication of study quality was calculated using the Cohen κ statistic.
RESULTS
STUDIES OF ADULT HOSPITALIZED PATIENTS
Eleven studies were conducted on adult hospitalized patients 12-14,22-24,26,27,29,30,33 and included 3 randomized controlled studies.14,27,33
Results by Outcomes Provider Identification Nine studies measured patients’ ability to identify providers with the use of visual aids, and all 9 reported improvements in this outcome. Visual tools used to measure provider identification included pictures (n = 5),13,14,23,27,33 whiteboards (n = 3),12,22,30 and patient portals (n = 1).26 Within studies that used pictures, individual pictures (n = 2)13,23 and handouts with pictures of multiple providers (n = 3) were used.14,27,33 In 2 studies, care team members such as a dietitian, physiotherapist or pharmacist, were included when measuring identification.14,33
Understanding Providers’ RolesSix studies assessed the effect of visual tools on patients’ understanding of provider roles.13,14,22,26,27,33 Four studies reported a positive effect with the use of pictures,27,33 whiteboards,22 and patient portals.26 However, 2 studies reported either no difference or negative impressions. Appel et al.14 reported no difference in the understanding of physician roles using a handout of providers’ pictures and titles. Arora et al.13 used individual pictures of physicians with descriptions of roles and found a negative association, as demonstrated by fewer patients rating their understanding of physicians’ roles as excellent or very good in the intervention period (45.6%) compared with the baseline (55.3%).
Patient–Provider Communication
Three studies evaluated the influence of visual tools on communication.14,24,29 Using pictures, Appel et al.14 found no difference in the perceived quality of communication. Singh et al.29 used whiteboards and reported improved communication scores for physicians and nurses. With notepads, patients surveyed by Farberg et al.24 stated that the tool improved provider communication.
Patient Satisfaction
Five studies assessed patient satisfaction related to the use of visual tools. 22,23,27,30,33 One study reported satisfaction as positive with the use of individual pictures.23 Two studies that used handouts with pictures of all team members reported either a positive33 or neutral27 impact on satisfaction. Studies that used whiteboards reported a positive association with satisfaction22,30 despite differences in content, such as the inclusion of prewritten prompts for writing goals of care and scheduled tests30 versus the name of the nurse and their education level.22
Results by Type of Visual Tool Pictures
Five studies that used pictures reported a positive effect on provider identification.13,14,23,27,33 Two27,33 of 4 studies13,14,27,33 that assessed patients’ understanding of team member roles reported a positive influence, while 1 reported no difference.14 A fourth study demonstrated a negative association, perhaps due to differences in the description of providers’ roles listed on the tool.13 Only 1 study examined the influence of pictures on patient–provider communication, and this study found no difference.14 Satisfaction with care via the use of pictures varied between positive (2 studies)23,33 and neutral (1 study).27
Whiteboards
Four studies tested the use of whiteboards; of these, 3 reported a positive influence on provider identification.12,22,30 One study reported a positive impact on patient–provider communication.29 Two studies noted a positive effect on patient satisfaction.22,30 Notably, the responsibility for updating whiteboards differed between the studies (ie, nurses only22 vs residents, medical students, and nurses).30
Patient Portal
In 1 study, an electronic portal that included names with pictures of providers, descriptions of their roles, lists of medications, and scheduled tests and/or procedures was used as a visual tool. The portal improved patients’ identification of physicians and patients’ understanding of roles. However, improvements in the knowledge of medication changes and planned tests and/or procedures during hospitalization were not observed.26 This finding would suggest limitations in the hospitalized patient’s knowledge of the plan of care, which could potentially weaken patient–provider communication.
Notepads
Only 1 study assessed the use of formatted notepads on patient–provider communication and noted a positive association. Notepads used prompts for different categories (eg, diagnosis/treatment, medications, etc) to encourage patient questions for providers.24
STUDIES OF PEDIATRIC HOSPITALIZED PATIENTS
Five studies were conducted on hospitalized pediatric units.15,25,28,31,32 All studies surveyed the parents, guardians, or caregivers of pediatric patients. One study excluded patients ≥12 years of age because of legal differences in access to adolescent health information,32 while another interviewed parents and/or guardians of teenagers.15
Results by Outcomes Provider Identification and Understanding of Physicians’ Roles
Four studies that assessed the influence of visual tools on provider identification and understanding of roles reported a positive association.15,25,28,31 Visual tools varied between pictures (n = 2),15,31 patient portal (n = 1),28 and whiteboards and pictures combined (n = 1).25 The measurement of outcomes varied between surveys with free text responses,28 multiple choice questions,25 and 1-5 Likert scales.15,31
Patient–Provider Communication
Two studies assessed the impact of patient portal use on communication and reported a positive association.28,32 The 2 portals autopopulated names, pictures, and roles of providers from electronic medical records. Singh et al.28 used a portal that was also available in Spanish and accommodated for non-English speakers. Kelly et al.32 reported that 90% of parents perceived that portal use was associated with reduced errors in care, with 8% finding errors in their child’s medication list.
Patient Satisfaction
Three studies assessed patient satisfaction via the use of visual tools.15,28,31 Singh et al.28 noted a positive influence on satisfaction via a patient portal. Dudas et al.15 used a single-page handout with names and pictures of each provider, along with information regarding the training and roles of each provider. Distribution of these handouts to patients by investigators led to a positive influence on satisfaction. While Unaka et al.31 used a similar handout, they asked residents to distribute them and found no significant difference in satisfaction scores between the intervention (66%) and control group (62%).
Results by Type of Visual Tool Pictures
Two studies reported a positive impact on provider identification and understanding of roles with the use of pictures.15,31 Dudas et al.15 demonstrated a 4.8-fold increase in the odds of parents identifying a medical student, as compared with the control. Similarly, after adjusting for length of stay and prior hospitalization, Unaka et al.31 reported that a higher percentage of patients correctly identified providers using this approach.
Whiteboard and Picture
One study evaluated the simultaneous use of whiteboards and pictures to improve the identification of providers. The study noted improved identification of supervising doctors and increased recognition of roles for supervising doctors, residents, and medical students.25
Patient Portal
Two studies used patient portals as visual tools. Singh et al.28 assessed the use of a patient portal with names, roles, and pictures of treatment team members. Use of this tool was positively associated with provider identification, understanding of roles, communication, and satisfaction. Kelly et al.32 noted that 60% of parents felt that portal use improved healthcare team communication.
RISK OF STUDY BIAS
The risk of bias was assessed for both adult and pediatric studies in aggregate. The average risk of bias using the Downs and Black Scale was 17.81 (range 14-22, standard deviation [SD] 2.20). Of the 16 included studies, 9 were rated at a low risk of bias (score
- >
18).13-15,26-31 Risk of bias was greatest for measures of external validity (mean 2.88, range 2-3, SD 0.34), internal validity (mean 4.06, range 3-6, SD 1.00), and confounding (mean 2.69, range 1-6, SD 1.35). Two of 3 randomized controlled trials had a low risk of bias.14,27 Interrater reliability for study quality adjudication was 0.90, suggesting excellent agreement (see supplementary Appendix B).
DISCUSSION
In this systematic review, the effects of visual tools on outcomes, such as provider identification, understanding of roles, patient–provider communication, and satisfaction with care, were variable. The majority of included studies were conducted on adult patients (n = 11).12-14,22-24,26,27,29,30,33 Pictures were the most frequently used tool (n = 7)13-15,23,27,31,33 and consequently had the greatest sample size across the review (n = 1297). While pictures had a positive influence on provider identification in all studies, comprehension of provider roles and satisfaction were variable. Although the content of whiteboards varied between studies, they showed favorable effects on provider identification (3 of 4 studies)12,22,30 and satisfaction (2 of 2 studies).22,30 While electronic medical record-based tools had a positive influence on outcomes,26,28 only 1 accounted for language preferences.28 Formatted notepads positively influenced patient–provider communication, but their use was limited by literacy.24 Collectively, these data suggest that visual tools have varying effects on patient-reported outcomes, likely owing to differences in study design, interventions, and evaluation methods.
Theoretically, visual tools should facilitate easier identification of providers and engender collaborative relationships. However, such tools do not replace face-to-face patient–provider and family discussions. Rather, these enhancements best serve as a medium to asynchronously display information to patients and family members. Indeed, within the included studies, we found that the use of visual tools was effective in improving satisfaction (6/8 studies), identification (13/13 studies), and understanding of provider roles (8/10 studies). Thus, it is reasonable to say that, in conjunction with excellent clinical care, these tools have an important role in improving care delivery in the hospital.
Despite this promise, we noted that the effectiveness of individual tools varied, a fact that may relate to differences across studies. First, inconsistencies in the format and/or content of the tools were noted. For example, within studies using pictures, tools varied from individual photographs of each team member13,23 to 1-page handouts with pictures of all team members.14,15,31 Such differences in presentation could affect spatial recognition in identifying providers, as single photos are known to be easier to process than multiple images at the same time.34 Second, no study evaluated patient preference of a visual tool. Thus, personal preferences for pictures versus whiteboards versus electronic modalities or a combination of tools might affect outcomes. Additionally, the utility of visual tools in visually impaired, confused, or non-English-speaking patients may limit effectiveness. Future studies that address these aspects and account for patient preferences may better elucidate the role of visual tools in hospitals.
Our results should be considered in the context of several limitations. First, only 3 studies used randomized trial designs; thus, confounding from unmeasured variables inherent to observational designs is possible. Second, none of the interventions tested were blinded to providers, raising the possibility of a Hawthorne effect (ie, alteration of provider behavior in response to awareness of being observed).35 Third, all studies were conducted at single centers, and only 9 of 16 studies were rated at a low risk of bias; thus, caution in broad extrapolations of this literature is necessary.
However, our study has several strengths, including a thorough search of heterogeneous literature, inclusion of both adult and pediatric populations, and a focus on myriad patient-reported outcomes. Second, by contrasting outcomes and measurement strategies across studies, our review helps explicate differences in results related to variation in outcome measurement or presentation of visual data. Third, because we frame results by outcome and type of visual tool used, we are able to identify strengths and weaknesses of individual tools in novel ways. Finally, our data suggest that the use of picture-based techniques and whiteboards are among the most promising visual interventions. Future studies that pair graphic designers with patients to improve the layout of these tools might prove valuable. Additionally, because the measurement of outcomes is confounded by aspects such as lack of controls, severity of illness, and language barriers, a randomized design would help provide greater clarity regarding effectiveness.
In conclusion, we found that visual tools appear to foster recognition of providers and understanding of their roles. However, variability of format, content, and measurement of outcomes hinders the identification of a single optimal approach. Future work using randomized controlled trial designs and standardized tools and measurements would be welcomed.
Acknowledgments
The authors thank Laura Appel, Kevin O’Leary, and Siddharth Singh for providing unpublished data and clarifications to help these analyses.
Disclosure
Anupama Goyal is the guarantor. Anupama Goyal and Komalpreet Tur performed primary data abstraction and analysis. Anupama Goyal, Scott Flanders, Jason Mann, and Vineet Chopra drafted the manuscript. All authors contributed to the development of the selection criteria, the risk of bias assessment strategy, and the data extraction criteria. Anupama Goyal, Jason Mann, Whitney Townsend, and Vineet Chopra developed the search strategy. Vineet Chopra provided systematic review expertise. All authors read, provided feedback, and approved the final manuscript. The authors declare that they have no conflicts of interest.
1. Berwick DM. A user’s manual for the IOM’s ‘Quality Chasm’ report. Health Aff (Millwood). 2002;21(3):80-90. PubMed
2. Jha AK, Orav EJ, Zheng J, Epstein AM. Patients’ perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921-1931. PubMed
3. Boulding W, Glickman SW, Manary MP, Schulman KA, Staelin R. Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17(1):41-48. PubMed
4. Little P, Everitt H, Williamson I, et al. Observational study of effect of patient centredness and positive approach on outcomes of general practice consultations. BMJ. 2001;323(7318):908-911. PubMed
5. Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152(9):1422-1433. PubMed
6. Arora V, Johnson J, Lovinger D, Humphrey HJ, Meltzer DO. Communication failures in patient sign-out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care. 2005;14(6):401-407. PubMed
7. Leonard M, Graham S, Bonacum D. The human factor: the critical importance of effective teamwork and communication in providing safe care. Qual Saf Health Care. 2004;13 Suppl 1:i85-i90. PubMed
8. Alam M, Lee A, Ibrahimi OA, et al. A multistep approach to improving biopsy site identification in dermatology: physician, staff, and patient roles based on a Delphi consensus. JAMA Dermatol. 2014;150(5):550-558. PubMed
9. Arora V, Gangireddy S, Mehrotra A, Ginde R, Tormey M, Meltzer D. Ability of hospitalized patients to identify their in-hospital physicians. Arch Intern Med. 2009;169(2):199-201. PubMed
10. Makaryus AN, Friedman EA. Does your patient know your name? An approach to enhancing patients’ awareness of their caretaker’s name. J Healthc Qual. 2005;27(4):53-56. PubMed
11. Sehgal NL, Green A, Vidyarthi AR, Blegen MA, Wachter RM. Patient whiteboards as a communication tool in the hospital setting: a survey of practices and recommendations. J Hosp Med. 2010;5(4):234-239. PubMed
12. Maniaci MJ, Heckman MG, Dawson NL. Increasing a patient’s ability to identify his or her attending physician using a patient room display. Arch Intern Med. 2010;170:1084-1085. PubMed
13. Arora VM, Schaninger C, D’Arcy M, et al. Improving inpatients’ identification of their doctors: Use of FACE™ cards. Jt Comm J Qual Patient Saf. 2009;35(12):613-619. PubMed
14. Appel L, Abrams H, Morra D, Wu RC. Put a face to a name: a randomized controlled trial evaluating the impact of providing clinician photographs on inpatients’ recall. Am J Med. 2015;128(1):82-89. PubMed
15. Dudas RA, Lemerman H, Barone M, Serwint JR. PHACES (Photographs of Academic Clinicians and Their Educational Status): a tool to improve delivery of family-centered care. Acad Pediatr. 2010;10(2):138-145. PubMed
16. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-269. PubMed
17. Higgins JP, Green S, editors. Cochrane handbook for systematic reviews of interventions. West Sussex, UK: The Cochrane Collaboration and Wiley Online Library; 2008.
18. Petrilli CM, Mack M, Petrilli JJ, Hickner A, Saint S, Chopra V. Understanding the role of physician attire on patient perceptions: a systematic review of the literature—targeting attire to improve likelihood of rapport (TAILOR) investigators. BMJ Open. 2015;5(1):e006578. PubMed
19. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377-384. PubMed
20. Seyffert M, Lagisetty P, Landgraf J, et al. Internet-delivered cognitive behavioral therapy to treat insomnia: a systematic review and meta-analysis. PLoS One. 2016;11(2):e0149139. PubMed
21. Patel R, Chang T, Greysen SR, Chopra V. Social media use in chronic disease: a systematic review and novel taxonomy. Am J Med. 2015;128(12):1335-1350. PubMed
22. Carlin BJ. Using whiteboards: fixed identities. Am J Nurs. 2008;108(11):72A-72B, 72D-72E. PubMed
23. Francis JJ, Pankratz VS, Huddleston JM. Patient satisfaction associated with correct identification of physician’s photographs. Mayo Clin Proc. 2001;76(6):604-608. PubMed
24. Farberg AS, Lin AM, Kuhn L, Flanders SA, Kim CS. Dear Doctor: a tool to facilitate patient-centered communication. J Hosp Med. 2013;8(10):553-558. PubMed
25. Hayes RM, Wickline A, Hensley C, et al. A quality improvement project to improve family recognition of medical team member roles. Hosp Pediatr. 2015;5(9):480-486. PubMed
26. O’Leary KJ, Lohman ME, Culver E, Killarney A, Randy Smith G Jr, Liebovitz DM. The effect of tablet computers with a mobile patient portal application on hospitalized patients’ knowledge and activation. J Am Med Inform Assoc. 2016;23(1):159-165. PubMed
27. Simons Y, Caprio T, Furiasse N, Kriss M, Williams MV, O’Leary KJ. The impact of facecards on patients’ knowledge, satisfaction, trust, and agreement with hospital physicians: a pilot study. J Hosp Med. 2014;9(3):137-141. PubMed
28. Singh A, Rhee KE, Brennan JJ, Kuelbs C, El-Kareh R, Fisher ES. Who’s my doctor? Using an electronic tool to improve team member identification on an inpatient pediatrics team. Hosp Pediatr. 2016;6(3):157-165. PubMed
29. Singh S, Fletcher KE, Pandl GJ, et al. It’s the writing on the wall: whiteboards improve inpatient satisfaction with provider communication. Am J Med Qual. 2011;26(2):127-131. PubMed
30. Tan M, Hooper Evans K, Braddock CH 3rd, Shieh L. Patient whiteboards to improve patient-centred care in the hospital. Postgrad Med J. 2013;89(1056):604-609. PubMed
31. Unaka NI, White CM, Sucharew HJ, Yau C, Clark SL, Brady PW. Effect of a face sheet tool on medical team provider identification and family satisfaction. J Hosp Med. 2014;9(3):186-188. PubMed
32. Kelly MM, Hoonakker PL, Dean SM. Using an inpatient portal to engage families in pediatric hospital care. J Am Med Inform Assoc. 2017;24(1):153-161. PubMed
33. Brener MI, Epstein JA, Cho J, Yeh HC, Dudas RA, Feldman L. Faces of all clinically engaged staff: a quality improvement project that enhances the hospitalised patient experience. Int J Clin Pract. 2016;70(11):923-929. PubMed
34. De Valois RL, De Valois KK. Spatial vision. Annu Rev Psychol. 1980;31:309-341. PubMed
Patient satisfaction with medical care during hospitalization is a common quality metric.1,2 Studies showing higher patient satisfaction have reported lower 30-day hospital readmissions3 and improved overall health.4,5 Conversely, communication failures are associated with dissatisfaction among hospitalized patients and adverse outcomes.6,7 A lack of familiarity with hospital providers weakens collaborative decision making and prevents high-quality patient care.8,9
Bedside visual tools, such as whiteboards and pictures of medical staff, have been widely used to enhance communication between patients, families, and providers.10,11 Results of studies evaluating these tools are varied. For example, 1 study found that 98% of patients were better able to identify physicians when their names were written on whiteboards.12 Yet in another, only 21.1% of patients were more likely to correctly identify ≥1 physicians using pictures.13 Thus, despite widespread use,11 whether visual tools improve patient satisfaction and patient care more broadly remains unclear.14,15
We performed a systematic review to answer the following 3 questions: first, what is the effect of visual tools on outcomes (ie, provider identification, understanding of providers’ roles, patient–provider communication, and satisfaction); second, does impact vary by type of visual tool (eg, whiteboards vs pictures of providers); and third, what factors (eg, study design, patient population) are associated with provider identification, communication, and patient satisfaction?
METHODS
Search Strategy
We used the Preferred Reporting Items for Systematic Reviews and Meta-Analysis when performing this review.16 A research librarian (WT) conducted serial searches for studies reporting the use of bedside visual tools for hospitalized patients in Medline (via OVID), Embase, SCOPUS, Web of Science, CINAHL, and Cochrane DSR and CENTRAL. Controlled vocabularies (ie, Medical Subject Headings terms) were used to identify synonyms for visual tools of interest. Additional studies were identified manually through bibliographies and meeting abstracts. No study design, publication date, or language restrictions were placed on the search, which was conducted between April 2016 and February 2017 (see supplementary Appendix A).
Study Selection
Two reviewers (AG and KT) independently assessed study eligibility; discrepancies were resolved by a third reviewer (VC). We included all adult or pediatric English language studies in which the effect of visual tool(s) on patient outcomes was reported. Visual tools were defined as the bedside display of information or an instrument given to patients to convey information regarding providers or medical care. Patient-reported outcomes included the following: (a) physician identification, (b) understanding of provider roles, (c) patient–provider communication, and (d) patient satisfaction with care. Providers were defined as physicians, residents, interns, medical students, nurse practitioners, or nurses. We excluded studies that were not original research (eg, conference abstracts, not peer reviewed), reported qualitative data without quantitative outcomes, or did not include a bedside visual tool. Given our interest in hospitalized general medicine patients, studies conducted in emergency departments, surgical units, obstetrics and gynecology wards, and intensive care units were excluded.
Data Extraction and Analysis
Data were extracted independently and in duplicate from all studies by using a template adapted from the Cochrane Collaboration.17 For all studies, we abstracted study design, type of visual tool (eg, whiteboards), unit setting (eg, medical), population studied (eg, adult vs pediatric), and outcomes reported (ie, physician identification, understanding of provider roles, communication, and satisfaction with care). Reviewers independently assessed and categorized the impact of tools on reported outcomes.
To standardize and compare outcomes across studies, the following were used to denote a positive association between visual tools and relevant outcomes: a greater number of physicians correctly identified by name/picture or title/role; the use of terms such as “high,” “agreed,” or “significant” on surveys; or ≥4 Likert scores for domains of identification, understanding of roles, communication, and satisfaction with care. Conversely, the inability to identify providers compared to the control/baseline; poor recall of titles/roles; lower Likert-scale scores (ie, ≤2); or survey terms such as “poor,” “disagreed,” or “insignificant” were considered to connote negative impact. Studies in which Likert scores were rated neither high nor low (ie, 3), or in which patients neither agreed nor disagreed on value were considered neutral.
Owing to clinical heterogeneity within studies, meta-analyses were not performed. Descriptive statistics were used to describe study outcomes. A priori18 studies were evaluated according to the following categories: design (eg, randomized vs observational), outcomes (eg, patient satisfaction), intervention (type of visual tool), and patient population (adult or pediatric). Because pediatric patients have underdeveloped communication skills and include parents and/or guardians, data from pediatric studies were tabulated and reported separately to those from adult studies.
Quality Assessment
As recommended by the Cochrane Collaboration, 2 reviewers (AG, KT) assessed the risk of study bias by using the Downs and Black Scale.17,19 Discrepancies in assessment were resolved by a third reviewer (VC). This instrument uses a point-based system to estimate the quality of a study by rating domains such as internal and external validity, bias, and confounding. In keeping with prior systematic reviews,18,20,21 studies with a score of ≥18 were considered high quality. Interrater agreement for the adjudication of study quality was calculated using the Cohen κ statistic.
RESULTS
STUDIES OF ADULT HOSPITALIZED PATIENTS
Eleven studies were conducted on adult hospitalized patients 12-14,22-24,26,27,29,30,33 and included 3 randomized controlled studies.14,27,33
Results by Outcomes Provider Identification Nine studies measured patients’ ability to identify providers with the use of visual aids, and all 9 reported improvements in this outcome. Visual tools used to measure provider identification included pictures (n = 5),13,14,23,27,33 whiteboards (n = 3),12,22,30 and patient portals (n = 1).26 Within studies that used pictures, individual pictures (n = 2)13,23 and handouts with pictures of multiple providers (n = 3) were used.14,27,33 In 2 studies, care team members such as a dietitian, physiotherapist or pharmacist, were included when measuring identification.14,33
Understanding Providers’ RolesSix studies assessed the effect of visual tools on patients’ understanding of provider roles.13,14,22,26,27,33 Four studies reported a positive effect with the use of pictures,27,33 whiteboards,22 and patient portals.26 However, 2 studies reported either no difference or negative impressions. Appel et al.14 reported no difference in the understanding of physician roles using a handout of providers’ pictures and titles. Arora et al.13 used individual pictures of physicians with descriptions of roles and found a negative association, as demonstrated by fewer patients rating their understanding of physicians’ roles as excellent or very good in the intervention period (45.6%) compared with the baseline (55.3%).
Patient–Provider Communication
Three studies evaluated the influence of visual tools on communication.14,24,29 Using pictures, Appel et al.14 found no difference in the perceived quality of communication. Singh et al.29 used whiteboards and reported improved communication scores for physicians and nurses. With notepads, patients surveyed by Farberg et al.24 stated that the tool improved provider communication.
Patient Satisfaction
Five studies assessed patient satisfaction related to the use of visual tools. 22,23,27,30,33 One study reported satisfaction as positive with the use of individual pictures.23 Two studies that used handouts with pictures of all team members reported either a positive33 or neutral27 impact on satisfaction. Studies that used whiteboards reported a positive association with satisfaction22,30 despite differences in content, such as the inclusion of prewritten prompts for writing goals of care and scheduled tests30 versus the name of the nurse and their education level.22
Results by Type of Visual Tool Pictures
Five studies that used pictures reported a positive effect on provider identification.13,14,23,27,33 Two27,33 of 4 studies13,14,27,33 that assessed patients’ understanding of team member roles reported a positive influence, while 1 reported no difference.14 A fourth study demonstrated a negative association, perhaps due to differences in the description of providers’ roles listed on the tool.13 Only 1 study examined the influence of pictures on patient–provider communication, and this study found no difference.14 Satisfaction with care via the use of pictures varied between positive (2 studies)23,33 and neutral (1 study).27
Whiteboards
Four studies tested the use of whiteboards; of these, 3 reported a positive influence on provider identification.12,22,30 One study reported a positive impact on patient–provider communication.29 Two studies noted a positive effect on patient satisfaction.22,30 Notably, the responsibility for updating whiteboards differed between the studies (ie, nurses only22 vs residents, medical students, and nurses).30
Patient Portal
In 1 study, an electronic portal that included names with pictures of providers, descriptions of their roles, lists of medications, and scheduled tests and/or procedures was used as a visual tool. The portal improved patients’ identification of physicians and patients’ understanding of roles. However, improvements in the knowledge of medication changes and planned tests and/or procedures during hospitalization were not observed.26 This finding would suggest limitations in the hospitalized patient’s knowledge of the plan of care, which could potentially weaken patient–provider communication.
Notepads
Only 1 study assessed the use of formatted notepads on patient–provider communication and noted a positive association. Notepads used prompts for different categories (eg, diagnosis/treatment, medications, etc) to encourage patient questions for providers.24
STUDIES OF PEDIATRIC HOSPITALIZED PATIENTS
Five studies were conducted on hospitalized pediatric units.15,25,28,31,32 All studies surveyed the parents, guardians, or caregivers of pediatric patients. One study excluded patients ≥12 years of age because of legal differences in access to adolescent health information,32 while another interviewed parents and/or guardians of teenagers.15
Results by Outcomes Provider Identification and Understanding of Physicians’ Roles
Four studies that assessed the influence of visual tools on provider identification and understanding of roles reported a positive association.15,25,28,31 Visual tools varied between pictures (n = 2),15,31 patient portal (n = 1),28 and whiteboards and pictures combined (n = 1).25 The measurement of outcomes varied between surveys with free text responses,28 multiple choice questions,25 and 1-5 Likert scales.15,31
Patient–Provider Communication
Two studies assessed the impact of patient portal use on communication and reported a positive association.28,32 The 2 portals autopopulated names, pictures, and roles of providers from electronic medical records. Singh et al.28 used a portal that was also available in Spanish and accommodated for non-English speakers. Kelly et al.32 reported that 90% of parents perceived that portal use was associated with reduced errors in care, with 8% finding errors in their child’s medication list.
Patient Satisfaction
Three studies assessed patient satisfaction via the use of visual tools.15,28,31 Singh et al.28 noted a positive influence on satisfaction via a patient portal. Dudas et al.15 used a single-page handout with names and pictures of each provider, along with information regarding the training and roles of each provider. Distribution of these handouts to patients by investigators led to a positive influence on satisfaction. While Unaka et al.31 used a similar handout, they asked residents to distribute them and found no significant difference in satisfaction scores between the intervention (66%) and control group (62%).
Results by Type of Visual Tool Pictures
Two studies reported a positive impact on provider identification and understanding of roles with the use of pictures.15,31 Dudas et al.15 demonstrated a 4.8-fold increase in the odds of parents identifying a medical student, as compared with the control. Similarly, after adjusting for length of stay and prior hospitalization, Unaka et al.31 reported that a higher percentage of patients correctly identified providers using this approach.
Whiteboard and Picture
One study evaluated the simultaneous use of whiteboards and pictures to improve the identification of providers. The study noted improved identification of supervising doctors and increased recognition of roles for supervising doctors, residents, and medical students.25
Patient Portal
Two studies used patient portals as visual tools. Singh et al.28 assessed the use of a patient portal with names, roles, and pictures of treatment team members. Use of this tool was positively associated with provider identification, understanding of roles, communication, and satisfaction. Kelly et al.32 noted that 60% of parents felt that portal use improved healthcare team communication.
RISK OF STUDY BIAS
The risk of bias was assessed for both adult and pediatric studies in aggregate. The average risk of bias using the Downs and Black Scale was 17.81 (range 14-22, standard deviation [SD] 2.20). Of the 16 included studies, 9 were rated at a low risk of bias (score
- >
18).13-15,26-31 Risk of bias was greatest for measures of external validity (mean 2.88, range 2-3, SD 0.34), internal validity (mean 4.06, range 3-6, SD 1.00), and confounding (mean 2.69, range 1-6, SD 1.35). Two of 3 randomized controlled trials had a low risk of bias.14,27 Interrater reliability for study quality adjudication was 0.90, suggesting excellent agreement (see supplementary Appendix B).
DISCUSSION
In this systematic review, the effects of visual tools on outcomes, such as provider identification, understanding of roles, patient–provider communication, and satisfaction with care, were variable. The majority of included studies were conducted on adult patients (n = 11).12-14,22-24,26,27,29,30,33 Pictures were the most frequently used tool (n = 7)13-15,23,27,31,33 and consequently had the greatest sample size across the review (n = 1297). While pictures had a positive influence on provider identification in all studies, comprehension of provider roles and satisfaction were variable. Although the content of whiteboards varied between studies, they showed favorable effects on provider identification (3 of 4 studies)12,22,30 and satisfaction (2 of 2 studies).22,30 While electronic medical record-based tools had a positive influence on outcomes,26,28 only 1 accounted for language preferences.28 Formatted notepads positively influenced patient–provider communication, but their use was limited by literacy.24 Collectively, these data suggest that visual tools have varying effects on patient-reported outcomes, likely owing to differences in study design, interventions, and evaluation methods.
Theoretically, visual tools should facilitate easier identification of providers and engender collaborative relationships. However, such tools do not replace face-to-face patient–provider and family discussions. Rather, these enhancements best serve as a medium to asynchronously display information to patients and family members. Indeed, within the included studies, we found that the use of visual tools was effective in improving satisfaction (6/8 studies), identification (13/13 studies), and understanding of provider roles (8/10 studies). Thus, it is reasonable to say that, in conjunction with excellent clinical care, these tools have an important role in improving care delivery in the hospital.
Despite this promise, we noted that the effectiveness of individual tools varied, a fact that may relate to differences across studies. First, inconsistencies in the format and/or content of the tools were noted. For example, within studies using pictures, tools varied from individual photographs of each team member13,23 to 1-page handouts with pictures of all team members.14,15,31 Such differences in presentation could affect spatial recognition in identifying providers, as single photos are known to be easier to process than multiple images at the same time.34 Second, no study evaluated patient preference of a visual tool. Thus, personal preferences for pictures versus whiteboards versus electronic modalities or a combination of tools might affect outcomes. Additionally, the utility of visual tools in visually impaired, confused, or non-English-speaking patients may limit effectiveness. Future studies that address these aspects and account for patient preferences may better elucidate the role of visual tools in hospitals.
Our results should be considered in the context of several limitations. First, only 3 studies used randomized trial designs; thus, confounding from unmeasured variables inherent to observational designs is possible. Second, none of the interventions tested were blinded to providers, raising the possibility of a Hawthorne effect (ie, alteration of provider behavior in response to awareness of being observed).35 Third, all studies were conducted at single centers, and only 9 of 16 studies were rated at a low risk of bias; thus, caution in broad extrapolations of this literature is necessary.
However, our study has several strengths, including a thorough search of heterogeneous literature, inclusion of both adult and pediatric populations, and a focus on myriad patient-reported outcomes. Second, by contrasting outcomes and measurement strategies across studies, our review helps explicate differences in results related to variation in outcome measurement or presentation of visual data. Third, because we frame results by outcome and type of visual tool used, we are able to identify strengths and weaknesses of individual tools in novel ways. Finally, our data suggest that the use of picture-based techniques and whiteboards are among the most promising visual interventions. Future studies that pair graphic designers with patients to improve the layout of these tools might prove valuable. Additionally, because the measurement of outcomes is confounded by aspects such as lack of controls, severity of illness, and language barriers, a randomized design would help provide greater clarity regarding effectiveness.
In conclusion, we found that visual tools appear to foster recognition of providers and understanding of their roles. However, variability of format, content, and measurement of outcomes hinders the identification of a single optimal approach. Future work using randomized controlled trial designs and standardized tools and measurements would be welcomed.
Acknowledgments
The authors thank Laura Appel, Kevin O’Leary, and Siddharth Singh for providing unpublished data and clarifications to help these analyses.
Disclosure
Anupama Goyal is the guarantor. Anupama Goyal and Komalpreet Tur performed primary data abstraction and analysis. Anupama Goyal, Scott Flanders, Jason Mann, and Vineet Chopra drafted the manuscript. All authors contributed to the development of the selection criteria, the risk of bias assessment strategy, and the data extraction criteria. Anupama Goyal, Jason Mann, Whitney Townsend, and Vineet Chopra developed the search strategy. Vineet Chopra provided systematic review expertise. All authors read, provided feedback, and approved the final manuscript. The authors declare that they have no conflicts of interest.
Patient satisfaction with medical care during hospitalization is a common quality metric.1,2 Studies showing higher patient satisfaction have reported lower 30-day hospital readmissions3 and improved overall health.4,5 Conversely, communication failures are associated with dissatisfaction among hospitalized patients and adverse outcomes.6,7 A lack of familiarity with hospital providers weakens collaborative decision making and prevents high-quality patient care.8,9
Bedside visual tools, such as whiteboards and pictures of medical staff, have been widely used to enhance communication between patients, families, and providers.10,11 Results of studies evaluating these tools are varied. For example, 1 study found that 98% of patients were better able to identify physicians when their names were written on whiteboards.12 Yet in another, only 21.1% of patients were more likely to correctly identify ≥1 physicians using pictures.13 Thus, despite widespread use,11 whether visual tools improve patient satisfaction and patient care more broadly remains unclear.14,15
We performed a systematic review to answer the following 3 questions: first, what is the effect of visual tools on outcomes (ie, provider identification, understanding of providers’ roles, patient–provider communication, and satisfaction); second, does impact vary by type of visual tool (eg, whiteboards vs pictures of providers); and third, what factors (eg, study design, patient population) are associated with provider identification, communication, and patient satisfaction?
METHODS
Search Strategy
We used the Preferred Reporting Items for Systematic Reviews and Meta-Analysis when performing this review.16 A research librarian (WT) conducted serial searches for studies reporting the use of bedside visual tools for hospitalized patients in Medline (via OVID), Embase, SCOPUS, Web of Science, CINAHL, and Cochrane DSR and CENTRAL. Controlled vocabularies (ie, Medical Subject Headings terms) were used to identify synonyms for visual tools of interest. Additional studies were identified manually through bibliographies and meeting abstracts. No study design, publication date, or language restrictions were placed on the search, which was conducted between April 2016 and February 2017 (see supplementary Appendix A).
Study Selection
Two reviewers (AG and KT) independently assessed study eligibility; discrepancies were resolved by a third reviewer (VC). We included all adult or pediatric English language studies in which the effect of visual tool(s) on patient outcomes was reported. Visual tools were defined as the bedside display of information or an instrument given to patients to convey information regarding providers or medical care. Patient-reported outcomes included the following: (a) physician identification, (b) understanding of provider roles, (c) patient–provider communication, and (d) patient satisfaction with care. Providers were defined as physicians, residents, interns, medical students, nurse practitioners, or nurses. We excluded studies that were not original research (eg, conference abstracts, not peer reviewed), reported qualitative data without quantitative outcomes, or did not include a bedside visual tool. Given our interest in hospitalized general medicine patients, studies conducted in emergency departments, surgical units, obstetrics and gynecology wards, and intensive care units were excluded.
Data Extraction and Analysis
Data were extracted independently and in duplicate from all studies by using a template adapted from the Cochrane Collaboration.17 For all studies, we abstracted study design, type of visual tool (eg, whiteboards), unit setting (eg, medical), population studied (eg, adult vs pediatric), and outcomes reported (ie, physician identification, understanding of provider roles, communication, and satisfaction with care). Reviewers independently assessed and categorized the impact of tools on reported outcomes.
To standardize and compare outcomes across studies, the following were used to denote a positive association between visual tools and relevant outcomes: a greater number of physicians correctly identified by name/picture or title/role; the use of terms such as “high,” “agreed,” or “significant” on surveys; or ≥4 Likert scores for domains of identification, understanding of roles, communication, and satisfaction with care. Conversely, the inability to identify providers compared to the control/baseline; poor recall of titles/roles; lower Likert-scale scores (ie, ≤2); or survey terms such as “poor,” “disagreed,” or “insignificant” were considered to connote negative impact. Studies in which Likert scores were rated neither high nor low (ie, 3), or in which patients neither agreed nor disagreed on value were considered neutral.
Owing to clinical heterogeneity within studies, meta-analyses were not performed. Descriptive statistics were used to describe study outcomes. A priori18 studies were evaluated according to the following categories: design (eg, randomized vs observational), outcomes (eg, patient satisfaction), intervention (type of visual tool), and patient population (adult or pediatric). Because pediatric patients have underdeveloped communication skills and include parents and/or guardians, data from pediatric studies were tabulated and reported separately to those from adult studies.
Quality Assessment
As recommended by the Cochrane Collaboration, 2 reviewers (AG, KT) assessed the risk of study bias by using the Downs and Black Scale.17,19 Discrepancies in assessment were resolved by a third reviewer (VC). This instrument uses a point-based system to estimate the quality of a study by rating domains such as internal and external validity, bias, and confounding. In keeping with prior systematic reviews,18,20,21 studies with a score of ≥18 were considered high quality. Interrater agreement for the adjudication of study quality was calculated using the Cohen κ statistic.
RESULTS
STUDIES OF ADULT HOSPITALIZED PATIENTS
Eleven studies were conducted on adult hospitalized patients 12-14,22-24,26,27,29,30,33 and included 3 randomized controlled studies.14,27,33
Results by Outcomes Provider Identification Nine studies measured patients’ ability to identify providers with the use of visual aids, and all 9 reported improvements in this outcome. Visual tools used to measure provider identification included pictures (n = 5),13,14,23,27,33 whiteboards (n = 3),12,22,30 and patient portals (n = 1).26 Within studies that used pictures, individual pictures (n = 2)13,23 and handouts with pictures of multiple providers (n = 3) were used.14,27,33 In 2 studies, care team members such as a dietitian, physiotherapist or pharmacist, were included when measuring identification.14,33
Understanding Providers’ RolesSix studies assessed the effect of visual tools on patients’ understanding of provider roles.13,14,22,26,27,33 Four studies reported a positive effect with the use of pictures,27,33 whiteboards,22 and patient portals.26 However, 2 studies reported either no difference or negative impressions. Appel et al.14 reported no difference in the understanding of physician roles using a handout of providers’ pictures and titles. Arora et al.13 used individual pictures of physicians with descriptions of roles and found a negative association, as demonstrated by fewer patients rating their understanding of physicians’ roles as excellent or very good in the intervention period (45.6%) compared with the baseline (55.3%).
Patient–Provider Communication
Three studies evaluated the influence of visual tools on communication.14,24,29 Using pictures, Appel et al.14 found no difference in the perceived quality of communication. Singh et al.29 used whiteboards and reported improved communication scores for physicians and nurses. With notepads, patients surveyed by Farberg et al.24 stated that the tool improved provider communication.
Patient Satisfaction
Five studies assessed patient satisfaction related to the use of visual tools. 22,23,27,30,33 One study reported satisfaction as positive with the use of individual pictures.23 Two studies that used handouts with pictures of all team members reported either a positive33 or neutral27 impact on satisfaction. Studies that used whiteboards reported a positive association with satisfaction22,30 despite differences in content, such as the inclusion of prewritten prompts for writing goals of care and scheduled tests30 versus the name of the nurse and their education level.22
Results by Type of Visual Tool Pictures
Five studies that used pictures reported a positive effect on provider identification.13,14,23,27,33 Two27,33 of 4 studies13,14,27,33 that assessed patients’ understanding of team member roles reported a positive influence, while 1 reported no difference.14 A fourth study demonstrated a negative association, perhaps due to differences in the description of providers’ roles listed on the tool.13 Only 1 study examined the influence of pictures on patient–provider communication, and this study found no difference.14 Satisfaction with care via the use of pictures varied between positive (2 studies)23,33 and neutral (1 study).27
Whiteboards
Four studies tested the use of whiteboards; of these, 3 reported a positive influence on provider identification.12,22,30 One study reported a positive impact on patient–provider communication.29 Two studies noted a positive effect on patient satisfaction.22,30 Notably, the responsibility for updating whiteboards differed between the studies (ie, nurses only22 vs residents, medical students, and nurses).30
Patient Portal
In 1 study, an electronic portal that included names with pictures of providers, descriptions of their roles, lists of medications, and scheduled tests and/or procedures was used as a visual tool. The portal improved patients’ identification of physicians and patients’ understanding of roles. However, improvements in the knowledge of medication changes and planned tests and/or procedures during hospitalization were not observed.26 This finding would suggest limitations in the hospitalized patient’s knowledge of the plan of care, which could potentially weaken patient–provider communication.
Notepads
Only 1 study assessed the use of formatted notepads on patient–provider communication and noted a positive association. Notepads used prompts for different categories (eg, diagnosis/treatment, medications, etc) to encourage patient questions for providers.24
STUDIES OF PEDIATRIC HOSPITALIZED PATIENTS
Five studies were conducted on hospitalized pediatric units.15,25,28,31,32 All studies surveyed the parents, guardians, or caregivers of pediatric patients. One study excluded patients ≥12 years of age because of legal differences in access to adolescent health information,32 while another interviewed parents and/or guardians of teenagers.15
Results by Outcomes Provider Identification and Understanding of Physicians’ Roles
Four studies that assessed the influence of visual tools on provider identification and understanding of roles reported a positive association.15,25,28,31 Visual tools varied between pictures (n = 2),15,31 patient portal (n = 1),28 and whiteboards and pictures combined (n = 1).25 The measurement of outcomes varied between surveys with free text responses,28 multiple choice questions,25 and 1-5 Likert scales.15,31
Patient–Provider Communication
Two studies assessed the impact of patient portal use on communication and reported a positive association.28,32 The 2 portals autopopulated names, pictures, and roles of providers from electronic medical records. Singh et al.28 used a portal that was also available in Spanish and accommodated for non-English speakers. Kelly et al.32 reported that 90% of parents perceived that portal use was associated with reduced errors in care, with 8% finding errors in their child’s medication list.
Patient Satisfaction
Three studies assessed patient satisfaction via the use of visual tools.15,28,31 Singh et al.28 noted a positive influence on satisfaction via a patient portal. Dudas et al.15 used a single-page handout with names and pictures of each provider, along with information regarding the training and roles of each provider. Distribution of these handouts to patients by investigators led to a positive influence on satisfaction. While Unaka et al.31 used a similar handout, they asked residents to distribute them and found no significant difference in satisfaction scores between the intervention (66%) and control group (62%).
Results by Type of Visual Tool Pictures
Two studies reported a positive impact on provider identification and understanding of roles with the use of pictures.15,31 Dudas et al.15 demonstrated a 4.8-fold increase in the odds of parents identifying a medical student, as compared with the control. Similarly, after adjusting for length of stay and prior hospitalization, Unaka et al.31 reported that a higher percentage of patients correctly identified providers using this approach.
Whiteboard and Picture
One study evaluated the simultaneous use of whiteboards and pictures to improve the identification of providers. The study noted improved identification of supervising doctors and increased recognition of roles for supervising doctors, residents, and medical students.25
Patient Portal
Two studies used patient portals as visual tools. Singh et al.28 assessed the use of a patient portal with names, roles, and pictures of treatment team members. Use of this tool was positively associated with provider identification, understanding of roles, communication, and satisfaction. Kelly et al.32 noted that 60% of parents felt that portal use improved healthcare team communication.
RISK OF STUDY BIAS
The risk of bias was assessed for both adult and pediatric studies in aggregate. The average risk of bias using the Downs and Black Scale was 17.81 (range 14-22, standard deviation [SD] 2.20). Of the 16 included studies, 9 were rated at a low risk of bias (score
- >
18).13-15,26-31 Risk of bias was greatest for measures of external validity (mean 2.88, range 2-3, SD 0.34), internal validity (mean 4.06, range 3-6, SD 1.00), and confounding (mean 2.69, range 1-6, SD 1.35). Two of 3 randomized controlled trials had a low risk of bias.14,27 Interrater reliability for study quality adjudication was 0.90, suggesting excellent agreement (see supplementary Appendix B).
DISCUSSION
In this systematic review, the effects of visual tools on outcomes, such as provider identification, understanding of roles, patient–provider communication, and satisfaction with care, were variable. The majority of included studies were conducted on adult patients (n = 11).12-14,22-24,26,27,29,30,33 Pictures were the most frequently used tool (n = 7)13-15,23,27,31,33 and consequently had the greatest sample size across the review (n = 1297). While pictures had a positive influence on provider identification in all studies, comprehension of provider roles and satisfaction were variable. Although the content of whiteboards varied between studies, they showed favorable effects on provider identification (3 of 4 studies)12,22,30 and satisfaction (2 of 2 studies).22,30 While electronic medical record-based tools had a positive influence on outcomes,26,28 only 1 accounted for language preferences.28 Formatted notepads positively influenced patient–provider communication, but their use was limited by literacy.24 Collectively, these data suggest that visual tools have varying effects on patient-reported outcomes, likely owing to differences in study design, interventions, and evaluation methods.
Theoretically, visual tools should facilitate easier identification of providers and engender collaborative relationships. However, such tools do not replace face-to-face patient–provider and family discussions. Rather, these enhancements best serve as a medium to asynchronously display information to patients and family members. Indeed, within the included studies, we found that the use of visual tools was effective in improving satisfaction (6/8 studies), identification (13/13 studies), and understanding of provider roles (8/10 studies). Thus, it is reasonable to say that, in conjunction with excellent clinical care, these tools have an important role in improving care delivery in the hospital.
Despite this promise, we noted that the effectiveness of individual tools varied, a fact that may relate to differences across studies. First, inconsistencies in the format and/or content of the tools were noted. For example, within studies using pictures, tools varied from individual photographs of each team member13,23 to 1-page handouts with pictures of all team members.14,15,31 Such differences in presentation could affect spatial recognition in identifying providers, as single photos are known to be easier to process than multiple images at the same time.34 Second, no study evaluated patient preference of a visual tool. Thus, personal preferences for pictures versus whiteboards versus electronic modalities or a combination of tools might affect outcomes. Additionally, the utility of visual tools in visually impaired, confused, or non-English-speaking patients may limit effectiveness. Future studies that address these aspects and account for patient preferences may better elucidate the role of visual tools in hospitals.
Our results should be considered in the context of several limitations. First, only 3 studies used randomized trial designs; thus, confounding from unmeasured variables inherent to observational designs is possible. Second, none of the interventions tested were blinded to providers, raising the possibility of a Hawthorne effect (ie, alteration of provider behavior in response to awareness of being observed).35 Third, all studies were conducted at single centers, and only 9 of 16 studies were rated at a low risk of bias; thus, caution in broad extrapolations of this literature is necessary.
However, our study has several strengths, including a thorough search of heterogeneous literature, inclusion of both adult and pediatric populations, and a focus on myriad patient-reported outcomes. Second, by contrasting outcomes and measurement strategies across studies, our review helps explicate differences in results related to variation in outcome measurement or presentation of visual data. Third, because we frame results by outcome and type of visual tool used, we are able to identify strengths and weaknesses of individual tools in novel ways. Finally, our data suggest that the use of picture-based techniques and whiteboards are among the most promising visual interventions. Future studies that pair graphic designers with patients to improve the layout of these tools might prove valuable. Additionally, because the measurement of outcomes is confounded by aspects such as lack of controls, severity of illness, and language barriers, a randomized design would help provide greater clarity regarding effectiveness.
In conclusion, we found that visual tools appear to foster recognition of providers and understanding of their roles. However, variability of format, content, and measurement of outcomes hinders the identification of a single optimal approach. Future work using randomized controlled trial designs and standardized tools and measurements would be welcomed.
Acknowledgments
The authors thank Laura Appel, Kevin O’Leary, and Siddharth Singh for providing unpublished data and clarifications to help these analyses.
Disclosure
Anupama Goyal is the guarantor. Anupama Goyal and Komalpreet Tur performed primary data abstraction and analysis. Anupama Goyal, Scott Flanders, Jason Mann, and Vineet Chopra drafted the manuscript. All authors contributed to the development of the selection criteria, the risk of bias assessment strategy, and the data extraction criteria. Anupama Goyal, Jason Mann, Whitney Townsend, and Vineet Chopra developed the search strategy. Vineet Chopra provided systematic review expertise. All authors read, provided feedback, and approved the final manuscript. The authors declare that they have no conflicts of interest.
1. Berwick DM. A user’s manual for the IOM’s ‘Quality Chasm’ report. Health Aff (Millwood). 2002;21(3):80-90. PubMed
2. Jha AK, Orav EJ, Zheng J, Epstein AM. Patients’ perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921-1931. PubMed
3. Boulding W, Glickman SW, Manary MP, Schulman KA, Staelin R. Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17(1):41-48. PubMed
4. Little P, Everitt H, Williamson I, et al. Observational study of effect of patient centredness and positive approach on outcomes of general practice consultations. BMJ. 2001;323(7318):908-911. PubMed
5. Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152(9):1422-1433. PubMed
6. Arora V, Johnson J, Lovinger D, Humphrey HJ, Meltzer DO. Communication failures in patient sign-out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care. 2005;14(6):401-407. PubMed
7. Leonard M, Graham S, Bonacum D. The human factor: the critical importance of effective teamwork and communication in providing safe care. Qual Saf Health Care. 2004;13 Suppl 1:i85-i90. PubMed
8. Alam M, Lee A, Ibrahimi OA, et al. A multistep approach to improving biopsy site identification in dermatology: physician, staff, and patient roles based on a Delphi consensus. JAMA Dermatol. 2014;150(5):550-558. PubMed
9. Arora V, Gangireddy S, Mehrotra A, Ginde R, Tormey M, Meltzer D. Ability of hospitalized patients to identify their in-hospital physicians. Arch Intern Med. 2009;169(2):199-201. PubMed
10. Makaryus AN, Friedman EA. Does your patient know your name? An approach to enhancing patients’ awareness of their caretaker’s name. J Healthc Qual. 2005;27(4):53-56. PubMed
11. Sehgal NL, Green A, Vidyarthi AR, Blegen MA, Wachter RM. Patient whiteboards as a communication tool in the hospital setting: a survey of practices and recommendations. J Hosp Med. 2010;5(4):234-239. PubMed
12. Maniaci MJ, Heckman MG, Dawson NL. Increasing a patient’s ability to identify his or her attending physician using a patient room display. Arch Intern Med. 2010;170:1084-1085. PubMed
13. Arora VM, Schaninger C, D’Arcy M, et al. Improving inpatients’ identification of their doctors: Use of FACE™ cards. Jt Comm J Qual Patient Saf. 2009;35(12):613-619. PubMed
14. Appel L, Abrams H, Morra D, Wu RC. Put a face to a name: a randomized controlled trial evaluating the impact of providing clinician photographs on inpatients’ recall. Am J Med. 2015;128(1):82-89. PubMed
15. Dudas RA, Lemerman H, Barone M, Serwint JR. PHACES (Photographs of Academic Clinicians and Their Educational Status): a tool to improve delivery of family-centered care. Acad Pediatr. 2010;10(2):138-145. PubMed
16. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-269. PubMed
17. Higgins JP, Green S, editors. Cochrane handbook for systematic reviews of interventions. West Sussex, UK: The Cochrane Collaboration and Wiley Online Library; 2008.
18. Petrilli CM, Mack M, Petrilli JJ, Hickner A, Saint S, Chopra V. Understanding the role of physician attire on patient perceptions: a systematic review of the literature—targeting attire to improve likelihood of rapport (TAILOR) investigators. BMJ Open. 2015;5(1):e006578. PubMed
19. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377-384. PubMed
20. Seyffert M, Lagisetty P, Landgraf J, et al. Internet-delivered cognitive behavioral therapy to treat insomnia: a systematic review and meta-analysis. PLoS One. 2016;11(2):e0149139. PubMed
21. Patel R, Chang T, Greysen SR, Chopra V. Social media use in chronic disease: a systematic review and novel taxonomy. Am J Med. 2015;128(12):1335-1350. PubMed
22. Carlin BJ. Using whiteboards: fixed identities. Am J Nurs. 2008;108(11):72A-72B, 72D-72E. PubMed
23. Francis JJ, Pankratz VS, Huddleston JM. Patient satisfaction associated with correct identification of physician’s photographs. Mayo Clin Proc. 2001;76(6):604-608. PubMed
24. Farberg AS, Lin AM, Kuhn L, Flanders SA, Kim CS. Dear Doctor: a tool to facilitate patient-centered communication. J Hosp Med. 2013;8(10):553-558. PubMed
25. Hayes RM, Wickline A, Hensley C, et al. A quality improvement project to improve family recognition of medical team member roles. Hosp Pediatr. 2015;5(9):480-486. PubMed
26. O’Leary KJ, Lohman ME, Culver E, Killarney A, Randy Smith G Jr, Liebovitz DM. The effect of tablet computers with a mobile patient portal application on hospitalized patients’ knowledge and activation. J Am Med Inform Assoc. 2016;23(1):159-165. PubMed
27. Simons Y, Caprio T, Furiasse N, Kriss M, Williams MV, O’Leary KJ. The impact of facecards on patients’ knowledge, satisfaction, trust, and agreement with hospital physicians: a pilot study. J Hosp Med. 2014;9(3):137-141. PubMed
28. Singh A, Rhee KE, Brennan JJ, Kuelbs C, El-Kareh R, Fisher ES. Who’s my doctor? Using an electronic tool to improve team member identification on an inpatient pediatrics team. Hosp Pediatr. 2016;6(3):157-165. PubMed
29. Singh S, Fletcher KE, Pandl GJ, et al. It’s the writing on the wall: whiteboards improve inpatient satisfaction with provider communication. Am J Med Qual. 2011;26(2):127-131. PubMed
30. Tan M, Hooper Evans K, Braddock CH 3rd, Shieh L. Patient whiteboards to improve patient-centred care in the hospital. Postgrad Med J. 2013;89(1056):604-609. PubMed
31. Unaka NI, White CM, Sucharew HJ, Yau C, Clark SL, Brady PW. Effect of a face sheet tool on medical team provider identification and family satisfaction. J Hosp Med. 2014;9(3):186-188. PubMed
32. Kelly MM, Hoonakker PL, Dean SM. Using an inpatient portal to engage families in pediatric hospital care. J Am Med Inform Assoc. 2017;24(1):153-161. PubMed
33. Brener MI, Epstein JA, Cho J, Yeh HC, Dudas RA, Feldman L. Faces of all clinically engaged staff: a quality improvement project that enhances the hospitalised patient experience. Int J Clin Pract. 2016;70(11):923-929. PubMed
34. De Valois RL, De Valois KK. Spatial vision. Annu Rev Psychol. 1980;31:309-341. PubMed
1. Berwick DM. A user’s manual for the IOM’s ‘Quality Chasm’ report. Health Aff (Millwood). 2002;21(3):80-90. PubMed
2. Jha AK, Orav EJ, Zheng J, Epstein AM. Patients’ perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921-1931. PubMed
3. Boulding W, Glickman SW, Manary MP, Schulman KA, Staelin R. Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care. 2011;17(1):41-48. PubMed
4. Little P, Everitt H, Williamson I, et al. Observational study of effect of patient centredness and positive approach on outcomes of general practice consultations. BMJ. 2001;323(7318):908-911. PubMed
5. Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152(9):1422-1433. PubMed
6. Arora V, Johnson J, Lovinger D, Humphrey HJ, Meltzer DO. Communication failures in patient sign-out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care. 2005;14(6):401-407. PubMed
7. Leonard M, Graham S, Bonacum D. The human factor: the critical importance of effective teamwork and communication in providing safe care. Qual Saf Health Care. 2004;13 Suppl 1:i85-i90. PubMed
8. Alam M, Lee A, Ibrahimi OA, et al. A multistep approach to improving biopsy site identification in dermatology: physician, staff, and patient roles based on a Delphi consensus. JAMA Dermatol. 2014;150(5):550-558. PubMed
9. Arora V, Gangireddy S, Mehrotra A, Ginde R, Tormey M, Meltzer D. Ability of hospitalized patients to identify their in-hospital physicians. Arch Intern Med. 2009;169(2):199-201. PubMed
10. Makaryus AN, Friedman EA. Does your patient know your name? An approach to enhancing patients’ awareness of their caretaker’s name. J Healthc Qual. 2005;27(4):53-56. PubMed
11. Sehgal NL, Green A, Vidyarthi AR, Blegen MA, Wachter RM. Patient whiteboards as a communication tool in the hospital setting: a survey of practices and recommendations. J Hosp Med. 2010;5(4):234-239. PubMed
12. Maniaci MJ, Heckman MG, Dawson NL. Increasing a patient’s ability to identify his or her attending physician using a patient room display. Arch Intern Med. 2010;170:1084-1085. PubMed
13. Arora VM, Schaninger C, D’Arcy M, et al. Improving inpatients’ identification of their doctors: Use of FACE™ cards. Jt Comm J Qual Patient Saf. 2009;35(12):613-619. PubMed
14. Appel L, Abrams H, Morra D, Wu RC. Put a face to a name: a randomized controlled trial evaluating the impact of providing clinician photographs on inpatients’ recall. Am J Med. 2015;128(1):82-89. PubMed
15. Dudas RA, Lemerman H, Barone M, Serwint JR. PHACES (Photographs of Academic Clinicians and Their Educational Status): a tool to improve delivery of family-centered care. Acad Pediatr. 2010;10(2):138-145. PubMed
16. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-269. PubMed
17. Higgins JP, Green S, editors. Cochrane handbook for systematic reviews of interventions. West Sussex, UK: The Cochrane Collaboration and Wiley Online Library; 2008.
18. Petrilli CM, Mack M, Petrilli JJ, Hickner A, Saint S, Chopra V. Understanding the role of physician attire on patient perceptions: a systematic review of the literature—targeting attire to improve likelihood of rapport (TAILOR) investigators. BMJ Open. 2015;5(1):e006578. PubMed
19. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377-384. PubMed
20. Seyffert M, Lagisetty P, Landgraf J, et al. Internet-delivered cognitive behavioral therapy to treat insomnia: a systematic review and meta-analysis. PLoS One. 2016;11(2):e0149139. PubMed
21. Patel R, Chang T, Greysen SR, Chopra V. Social media use in chronic disease: a systematic review and novel taxonomy. Am J Med. 2015;128(12):1335-1350. PubMed
22. Carlin BJ. Using whiteboards: fixed identities. Am J Nurs. 2008;108(11):72A-72B, 72D-72E. PubMed
23. Francis JJ, Pankratz VS, Huddleston JM. Patient satisfaction associated with correct identification of physician’s photographs. Mayo Clin Proc. 2001;76(6):604-608. PubMed
24. Farberg AS, Lin AM, Kuhn L, Flanders SA, Kim CS. Dear Doctor: a tool to facilitate patient-centered communication. J Hosp Med. 2013;8(10):553-558. PubMed
25. Hayes RM, Wickline A, Hensley C, et al. A quality improvement project to improve family recognition of medical team member roles. Hosp Pediatr. 2015;5(9):480-486. PubMed
26. O’Leary KJ, Lohman ME, Culver E, Killarney A, Randy Smith G Jr, Liebovitz DM. The effect of tablet computers with a mobile patient portal application on hospitalized patients’ knowledge and activation. J Am Med Inform Assoc. 2016;23(1):159-165. PubMed
27. Simons Y, Caprio T, Furiasse N, Kriss M, Williams MV, O’Leary KJ. The impact of facecards on patients’ knowledge, satisfaction, trust, and agreement with hospital physicians: a pilot study. J Hosp Med. 2014;9(3):137-141. PubMed
28. Singh A, Rhee KE, Brennan JJ, Kuelbs C, El-Kareh R, Fisher ES. Who’s my doctor? Using an electronic tool to improve team member identification on an inpatient pediatrics team. Hosp Pediatr. 2016;6(3):157-165. PubMed
29. Singh S, Fletcher KE, Pandl GJ, et al. It’s the writing on the wall: whiteboards improve inpatient satisfaction with provider communication. Am J Med Qual. 2011;26(2):127-131. PubMed
30. Tan M, Hooper Evans K, Braddock CH 3rd, Shieh L. Patient whiteboards to improve patient-centred care in the hospital. Postgrad Med J. 2013;89(1056):604-609. PubMed
31. Unaka NI, White CM, Sucharew HJ, Yau C, Clark SL, Brady PW. Effect of a face sheet tool on medical team provider identification and family satisfaction. J Hosp Med. 2014;9(3):186-188. PubMed
32. Kelly MM, Hoonakker PL, Dean SM. Using an inpatient portal to engage families in pediatric hospital care. J Am Med Inform Assoc. 2017;24(1):153-161. PubMed
33. Brener MI, Epstein JA, Cho J, Yeh HC, Dudas RA, Feldman L. Faces of all clinically engaged staff: a quality improvement project that enhances the hospitalised patient experience. Int J Clin Pract. 2016;70(11):923-929. PubMed
34. De Valois RL, De Valois KK. Spatial vision. Annu Rev Psychol. 1980;31:309-341. PubMed
© 2017 Society of Hospital Medicine
PICC Use in Adults With Pneumonia
Pneumonia is the most common cause of unplanned hospitalization in the United States.[1] Despite its clinical toll, the management of this disease has evolved markedly. Expanding vaccination programs, efforts to improve timeliness of antibiotic therapy, and improved processes of care are but a few developments that have improved outcomes for patients afflicted with this illness.[2, 3]
Use of peripherally inserted central catheters (PICCs) is an example of a modern development in the management of patients with pneumonia.[4, 5, 6, 7] PICCs provide many of the benefits associated with central venous catheters (CVCs) including reliable venous access for delivery of antibiotics, phlebotomy, and invasive hemodynamic monitoring. However, as they are placed in veins of the upper extremity, PICCs bypass insertion risks (eg, injury to the carotid vessels or pneumothorax) associated with placement of traditional CVCs.[8] Because they offer durable venous access, PICCs also facilitate care transitions while continuing intravenous antimicrobial therapy in patients with pneumonia.
However, accumulating evidence also suggests that PICCs are associated with important complications, including central lineassociated bloodstream infectionand venous thromboembolism.[9, 10] Furthermore, knowledge gaps in clinicians regarding indications for appropriate use and management of complications associated with PICCs have been recognized.[10, 11] These elements are problematic because reports of unjustified and inappropriate PICC use are growing in the literature.[12, 13] Such concerns have prompted a number of policy calls to improve PICC use, including Choosing Wisely recommendations by various professional societies.[14, 15]
As little is known about the prevalence or patterns of PICC use in adults hospitalized with pneumonia, we conducted a retrospective cohort study using data from a large network of US hospitals.
METHODS
Setting and Participants
We included patients from hospitals that participated in Premier's inpatient dataset, a large, fee‐supported, multipayer administrative database that has been used extensively in health services research to measure quality of care and comparative effectiveness of interventions.[16] Participating hospitals represent all regions of the United States and include teaching and nonteaching facilities in rural and urban locations. In addition to variables found in the uniform billing form, the Premier inpatient database also includes a date‐stamped list of charges for procedures conducted during hospitalization such as PICC placement. As PICC‐specific data are not available in most nationally representative datasets, Premier offers unique insights into utilization, timing, and factors associated with use of PICCs in hospitalized settings.
We included adult patients aged 18 years who were (1) admitted with a principal diagnosis of pneumonia present on admission, or secondary diagnosis of pneumonia if paired with a principal diagnosis of sepsis, respiratory failure, or influenza; (2) received at least 1 day of antibiotics between July 1, 2007 and November 30, 2011, and (3) underwent chest x‐ray or computed tomography (CT) at the time of admission. International Classification of Disease, 9th Revision, Clinical Modification (ICD‐9‐CM) codes were used for patient selection. Patients who were not admitted (eg, observation cases), had cystic fibrosis, or marked as pneumonia not present on admission were excluded. For patients who had more than 1 hospitalization during the study period, a single admission was randomly selected for inclusion.
Patient, Physician, and Hospital Data
For all patients, age, gender, marital status, insurance, race, and ethnicity were captured. Using software provided by the Healthcare Costs and Utilization Project, we categorized information on 29 comorbid conditions and computed a combined comorbidity score as described by Gagne et al.[17] Patients were considered to have healthcare‐associated pneumonia (HCAP) if they were: (1) admitted from a skilled nursing or a long‐term care facility, (2) hospitalized in the previous 90 days, (3) on dialysis, or (4) receiving immunosuppressing medications (eg, chemotherapy or steroids equivalent to at least 20 mg of prednisone per day) at the time of admission. Information on specialty of the admitting physician and hospital characteristics (eg, size, location, teaching status) were sourced through Premier data.
Receipt of PICCs and Related Therapies
Among eligible adult patients hospitalized with pneumonia, we identified patients who received a PICC at any time during hospitalization via PICC‐specific billing codes. Non‐PICC devices (eg, midlines, Hickman catheters) were not included. For all insertions, we assessed day of PICC placement relative to admission date. Data on type of PICC (eg, power‐injection capable, antibiotic coating) or PICC characteristics (size, number of lumens) were not available. We used billing codes to assess use of invasive or noninvasive ventilation, vasopressors, and administration of pneumonia‐specific antibiotics (eg, ‐lactams, macrolides, fluoroquinolones). Early exposure was defined when a billing code appeared within 2 days of hospital admission.
Outcomes of Interest
The primary outcome of interest was receipt of a PICC. Additionally, we assessed factors associated with PICC placement and variation in risk‐standardized rates of PICC use between hospitals.
Statistical Analyses
Patient and hospital characteristics were summarized using frequencies for categorical variables and medians with interquartile ranges for continuous variables. We examined association of individual patient and hospital characteristics with use of PICCs using generalized estimating equations models with a logit link for categorical variables and identity link for continuous variables, accounting for patient clustering within hospitals.
| Characteristic | Total, No. (%) | No PICC, No. (%) | PICC, No. (%) | P Value* |
|---|---|---|---|---|
| ||||
| 545,250 (100) | 503,401 (92.3) | 41,849 (7.7) | ||
| Demographics | ||||
| Age, median (Q1Q3), y | 71 (5782) | 72 (5782) | 69 (5780) | <0.001 |
| Gender | <0.001 | |||
| Male | 256,448 (47.0) | 237,232 (47.1) | 19,216 (45.9) | |
| Female | 288,802 (53.0) | 266,169 (52.9) | 22,633 (54.1) | |
| Race/ethnicity | <0.001 | |||
| White | 377,255 (69.2) | 346,689 (68.9) | 30,566 (73.0) | |
| Black | 63,345 (11.6) | 58,407 (11.6) | 4,938 (11.8) | |
| Hispanic | 22,855 (4.2) | 21,716 (4.3) | 1,139 (2.7) | |
| Other | 81,795 (15.0) | 76,589 (15.2) | 5,206 (12.4) | |
| Admitting specialty | <0.001 | |||
| Internal medicine | 236,859 (43.4) | 218,689 (43.4) | 18,170 (43.4) | |
| Hospital medicine | 116,499 (21.4) | 107,671 (21.4) | 8,828 (21.1) | |
| Family practice | 80,388 (14.7) | 75,482 (15.0) | 4,906 (11.7) | |
| Critical care and pulmonary | 35,670 (6.5) | 30,529 (6.1) | 41,849 (12.3) | |
| Geriatrics | 4,812 (0.9) | 4,098 (0.8) | 714 (1.7) | |
| Other | 71,022 (13.0) | 66,932 (13.3) | 4,090 (9.8) | |
| Insurance | <0.001 | |||
| Medicare | 370,303 (67.9) | 341,379 (67.8) | 28,924 (69.1) | |
| Medicaid | 45,505 (8.3) | 41,100 (8.2) | 4,405 (10.5) | |
| Managed care | 69,984 (12.8) | 65,280 (13.0) | 4,704 (11.2) | |
| Commercialindemnity | 20,672 (3.8) | 19,251 (3.8) | 1,421 (3.4) | |
| Other | 38,786 (7.1) | 36,391 (7.2) | 2,395 (5.7) | |
| Comorbidities | ||||
| Gagne combined comorbidity score, median (Q1Q3) | 2 (15) | 2 (14) | 4 (26) | <0.001 |
| Hypertension | 332,347 (60.9) | 306,964 (61.0) | 25,383 (60.7) | 0.13 |
| Chronic pulmonary disease | 255,403 (46.8) | 234,619 (46.6) | 20,784 (49.7) | <0.001 |
| Diabetes | 171,247 (31.4) | 155,540 (30.9) | 15,707 (37.5) | <0.001 |
| Congestive heart failure | 146,492 (26.9) | 131,041 (26.0) | 15,451 (36.9) | <0.001 |
| Atrial fibrillation | 108,405 (19.9) | 97,124 (19.3) | 11,281 (27.0) | <0.001 |
| Renal failure | 104,404 (19.1) | 94,277 (18.7) | 10,127 (24.2) | <0.001 |
| Nicotine replacement therapy/tobacco use | 89,938 (16.5) | 83,247 (16.5) | 6,691 (16.0) | <0.001 |
| Obesity | 60,242 (11.0) | 53,268 (10.6) | 6,974 (16.7) | <0.001 |
| Coagulopathy | 41,717 (7.6) | 35,371 (7.0) | 6,346 (15.2) | <0.001 |
| Prior stroke (1 year) | 26,787 (4.9) | 24,046 (4.78) | 2,741 (6.55) | <0.001 |
| Metastatic cancer | 21,868 (4.0) | 20,244 (4.0) | 1,624 (3.9) | 0.16 |
| Solid tumor w/out metastasis | 21,083 (3.9) | 19,380 (3.8) | 1,703 (4.1) | 0.12 |
| Prior VTE (1 year) | 19,090 (3.5) | 16,906 (3.4) | 2,184 (5.2) | <0.001 |
| Chronic liver disease | 16,273 (3.0) | 14,207 (2.8) | 2,066 (4.9) | <0.001 |
| Prior bacteremia (1 year) | 4,106 (0.7) | 3,584 (0.7) | 522 (1.2) | <0.001 |
| Nephrotic syndrome | 671 (0.1) | 607 (0.1) | 64 (0.2) | 0.03 |
| Morbidity markers | ||||
| Type of pneumonia | <0.001 | |||
| CAP | 376,370 (69.1) | 352,900 (70.1) | 23,830 (56.9) | |
| HCAP | 168,520 (30.9) | 150,501 (29.9) | 18,019 (43.1) | |
| Sepsis present on admission | 114,578 (21.0) | 96,467 (19.2) | 18,111 (43.3) | <0.001 |
| Non‐invasive ventilation | 47,913(8.8) | 40,599 (8.1) | 7,314 (17.5) | <0.001 |
| Invasive mechanical ventilation | 56,179 (10.3) | 44,228 (8.8) | 11,951 (28.6) | <0.001 |
| ICU status | 97,703 (17.9) | 80,380 (16.0) | 17,323 (41.4) | <0.001 |
| Vasopressor use | 48,353 (8.9) | 38,030 (7.6) | 10,323 (24.7) | <0.001 |
| Antibiotic/medication use | ||||
| Anti‐MRSA agent (vancomycin) | 146,068 (26.8) | 123,327 (24.5) | 22,741 (54.3) | <0.001 |
| Third‐generation cephalosporin | 250,782 (46.0) | 235,556 (46.8) | 15,226 (36.4) | <0.001 |
| Anti‐Pseudomonal cephalosporin | 41,798 (7.7) | 36,982 (7.3) | 4,816 (11.5) | <0.001 |
| Anti‐Pseudomonal ‐lactam | 122,215 (22.4) | 105,741 (21.0) | 16,474 (39.4) | <0.001 |
| Fluroquinolone | 288,051 (52.8) | 267,131 (53.1) | 20,920 (50.0) | <0.001 |
| Macrolide | 223,737 (41.0) | 210,954 (41.9) | 12,783 (30.5) | <0.001 |
| Aminoglycoside | 15,415 (2.8) | 12,661 (2.5) | 2,754 (6.6) | <0.001 |
| Oral steroids | 44,486 (8.2) | 41,586 (8.3) | 2,900 (6.9) | <0.001 |
| Intravenous steroids | 146,308 (26.8) | 133,920 (26.6) | 12,388 (29.6) | <0.001 |
| VTE prophylaxis with LMWH | 190,735 (35.0) | 174,612 (34.7) | 16,123 (38.5) | 0.01 |
| Discharge disposition | ||||
| Home | 282,146 (51.7) | 272,604(54.1) | 9,542 (22.8) | <0.001 |
| Home with home health | 71,977 (13.2) | 65,289 (13.0) | 6,688 (16.0) | <0.001 |
| Skilled nursing facility | 111,541 (20.5) | 97,113 (19.3) | 14,428 (34.5) | <0.001 |
| Hospice | 20,428 (3.7) | 17,902 (3.6) | 2,526 (6.0) | <0.001 |
| Expired | 47,733 (8.7) | 40,768 (8.1) | 6,965 (16.6) | <0.001 |
| Other | 11,425 (2.1) | 9,725 (1.9) | 1,700 (4.1) | <0.001 |
We then developed a multivariable hierarchical generalized linear model (HGLM) for PICC placement with a random effect for hospital. In this model, we included patient demographics, comorbidities, sepsis on admission, type of pneumonia (eg, HCAP vs community‐associated pneumonia [CAP]), admitting physician specialty, and indicators for early receipt of specific treatments such as guideline‐recommended antibiotics, vasopressors, ventilation (invasive or noninvasive), and pneumatic compression devices for prophylaxis of deep vein thrombosis.
To understand and estimate between‐hospital variation in PICC use, we calculated risk‐standardized rates of PICC use (RSPICC) across hospitals using HGLM methods. These methods are also employed by the Centers for Medicare and Medicaid Services to calculate risk‐standardized measures for public reporting.[18] Because hospital rates of PICC use were highly skewed (21.2% [n = 105] of hospitals had no patients with PICCs), we restricted this model to the 343 hospitals that had at least 5 patients with a PICC to obtain stable estimates. For each hospital, we estimated a predicted rate of PICC use (pPICC) as the sum of predicted probabilities of PICC receipt from patient factors and the random intercept for hospital in which they were admitted. We then calculated an expected rate of PICC use (ePICC) per hospital as the sum of expected probabilities of PICC receipt from patient factors only. RSPICC for each hospital was then computed as the product of the overall unadjusted mean PICC rate (PICC) from all patients and the ratio of the predicted to expected PICC rate (uPICC*[pPICC/ePICC]).[19] Kruskal‐Wallis tests were used to evaluate the association between hospital characteristics with RSPICC rates. To evaluate the impact of the hospital in variation in PICC use, we assessed the change in likelihood ratio of a hierarchical model with hospital random effects compared to a logistic regression model with patient factors only. In addition, we estimated the intraclass correlation (ICC) to assess the proportion of variation in PICC use associated with the hospital, and the median odds ratio (MOR) from the hierarchical model. The MOR is the median of a set of odds ratios comparing 2 patients with the same set of characteristics treated at 2 randomly selected hospitals.[20, 21, 22] All analyses were performed using the Statistical Analysis System version 9.3 (SAS Institute, Inc., Cary, NC) and Stata 13 (StataCorp Inc., College Station, TX).
Ethical and Regulatory Oversight
Permission to conduct this study was obtained from the institutional review board at Baystate Medical Center, Springfield, Massachusetts. The study did not qualify as human subjects research and made use of fully deidentified data.
RESULTS
Between July 2007 and November 2011, 634,285 admissions representing 545,250 unique patients from 495 hospitals met eligibility criteria and were included in the study (Figure 1). Included patients had a median age of 71 years (interquartile range [IQR]: 5782), and 53.0% were female. Most patients were Caucasian (69.2%), unmarried (51.6%), and insured by Medicare (67.9%). Patients were admitted to the hospital by internal medicine providers (43.4%), hospitalists (21.4%), and family practice providers (14.7%); notably, critical care and pulmonary medicine providers admitted 6.5% of patients. The median Gagne comorbidity score was 2 (IQR: 15). Hypertension, chronic obstructive pulmonary disease, diabetes, and congestive heart failure were among the most common comorbidities observed (Table 1).
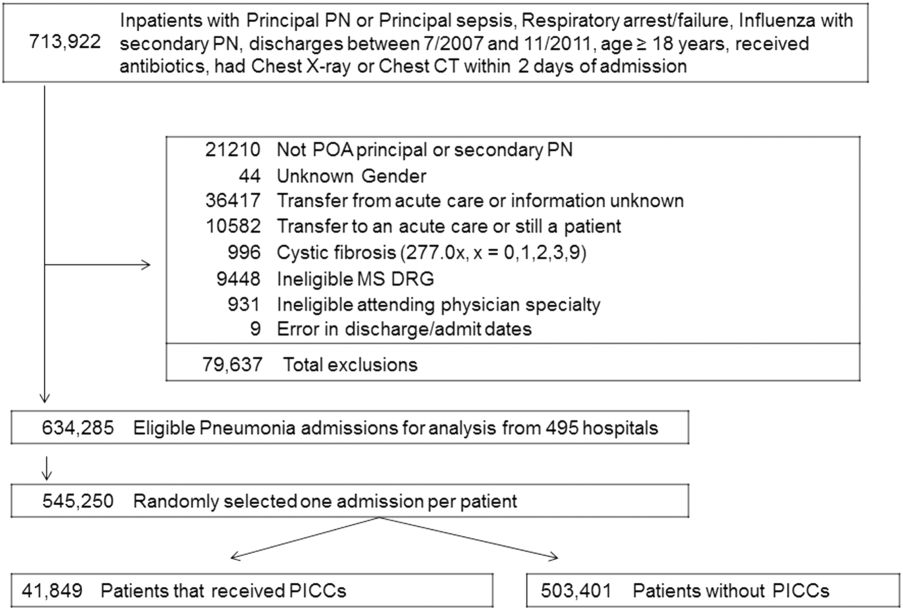
Among eligible patients, 41,849 (7.7%) received a PICC during hospitalization. Approximately a quarter of all patients who received PICCs did so by hospital day 2; 90% underwent insertion by hospital day 11 (mean = 5.4 days, median = 4 days). Patients who received PICCs were younger (median IQR: 69 years, 5780 years) but otherwise demographically similar to those that did not receive PICCs (median IQR: 72 years, 5782 years). Compared to other specialties, patients admitted by critical care/pulmonary providers were twice as likely to receive PICCs (12.3% vs 6.1%, P < .001). Patients who received PICCs had higher comorbidity scores than those who did not (median Gagne comorbidity score 4 vs 2, P < 0.001) and were more likely to be diagnosed with HCAP (43.1% vs 29.9%, P < 0.001) than CAP (56.9% vs 70.1%, P < 0.001).
PICC recipients were also more likely to receive intensive care unit (ICU) level of care (41.4% vs 16%, P < 0.001) and both noninvasive (17.5% vs 8.1%, P < 0.001) and invasive ventilation (28.6% vs 8.8%, P < 0.001) upon admission. Vasopressor use was also significantly more frequent in patients who received PICCs (24.7% vs 7.6%, P < 0.001) compared to those who did not receive these devices. Patients with PICCs were more often discharged to skilled nursing facilities (34.5% vs 19.3%) than those without PICCs.
Characteristics Associated With PICC Use Following Multivariable Modeling
Using HGLM with a random hospital effect, multiple patient characteristics were associated with PICC use (Table 2). Patients 65 years of age were less likely to receive a PICC compared to younger patients (odds ratio [OR]: 0.81, 95% confidence interval [CI]: 0.79‐0.84). Weight loss (OR: 2.03, 95% CI: 1.97‐2.10), sepsis on admission (OR: 1.80, 95% CI: 1.75‐1.85), and ICU status on hospital day 1 or 2 (OR: 1.70, 95% CI: 1.64‐1.75) represented 3 factors most strongly associated with PICC use.
| Patient Characteristic | Odds Ratio | 95% Confidence Intervals |
|---|---|---|
| ||
| Age group (>66 vs 65 years) | 0.82 | 0.790.84 |
| Race/ethnicity | ||
| Other | 1.02 | 0.971.06 |
| Black | 0.99 | 0.951.03 |
| Hispanic | 0.82 | 0.760.88 |
| White | Referent | |
| Marital status | ||
| Other/missing | 1.07 | 1.011.14 |
| Single | 1.02 | 1.001.05 |
| Married | Referent | |
| Insurance payor | ||
| Other | 0.85 | 0.800.89 |
| Medicaid | 1.13 | 1.081.18 |
| Managed care | 0.95 | 0.910.99 |
| Commercialindemnity | 0.93 | 0.871.00 |
| Medicare | Referent | |
| Admitting physician specialty | ||
| Pulmonary/critical care medicine | 1.18 | 1.131.24 |
| Family practice | 1.01 | 0.971.05 |
| Geriatric medicine (FP and IM) | 1.85 | 1.662.05 |
| Hospitalist | 0.94 | 0.910.98 |
| Other specialties | 1.02 | 0.971.06 |
| Internal medicine | Referent | |
| Comorbidities | ||
| Congestive heart failure | 1.27 | 1.241.31 |
| Valvular disease | 1.11 | 1.071.15 |
| Pulmonary circulation disorders | 1.37 | 1.321.42 |
| Peripheral vascular disease | 1.09 | 1.051.13 |
| Hypertension | 0.94 | 0.920.97 |
| Paralysis | 1.59 | 1.511.67 |
| Other neurological disorders | 1.20 | 1.161.23 |
| Chronic lung disease | 1.10 | 1.071.12 |
| Diabetes | 1.13 | 1.101.16 |
| Hypothyroidism | 1.03 | 1.001.06 |
| Liver disease | 1.16 | 1.101.23 |
| Ulcer | 1.86 | 1.153.02 |
| Lymphoma | 0.88 | 0.810.96 |
| Metastatic cancer | 0.75 | 0.710.80 |
| Solid tumor without metastasis | 0.93 | 0.880.98 |
| Arthritis | 1.22 | 1.161.28 |
| Obesity | 1.47 | 1.421.52 |
| Weight loss | 2.03 | 1.972.10 |
| Blood loss | 1.69 | 1.551.85 |
| Deficiency anemias | 1.40 | 1.371.44 |
| Alcohol abuse | 1.19 | 1.131.26 |
| Drug abuse | 1.31 | 1.231.39 |
| Psychoses | 1.16 | 1.111.21 |
| Depression | 1.10 | 1.061.13 |
| Renal failure | 0.96 | 0.930.98 |
| Type of pneumonia | ||
| HCAP | 1.03 | 1.011.06 |
| CAP | Referent | |
| Sepsis (POA) | 1.80 | 1.751.85 |
| Antibiotic exposure | ||
| Anti‐MRSA agent | 1.72 | 1.671.76 |
| Anti‐Pseudomonal carbapenem | 1.37 | 1.311.44 |
| Non‐Pseudomonal carbapenem | 1.48 | 1.331.66 |
| Third‐generation cephalosporin | 1.04 | 1.011.07 |
| Anti‐Pseudomonal cephalosporin | 1.25 | 1.201.30 |
| Anti‐Pseudomonal ‐lactam | 1.27 | 1.231.31 |
| Aztreonam | 1.31 | 1.231.40 |
| Non‐Pseudomonal ‐lactam | 1.36 | 1.231.50 |
| ‐lactam | 1.55 | 1.261.90 |
| Respiratory quinolone | 0.90 | 0.870.92 |
| Macrolide | 0.85 | 0.820.88 |
| Doxycycline | 0.94 | 0.871.01 |
| Aminoglycoside | 1.21 | 1.141.27 |
| Vasopressors | 1.06 | 1.031.10 |
| Noninvasive ventilation | 1.29 | 1.251.34 |
| Invasive ventilation | 1.66 | 1.611.72 |
| Intensive care unit on admission | 1.70 | 1.641.75 |
| Atrial fibrillation | 1.26 | 1.221.29 |
| Upper extremity chronic DVT | 1.61 | 1.132.28 |
| Nicotine replacement therapy/tobacco abuse | 0.91 | 0.880.94 |
| Aspirin | 0.94 | 0.920.97 |
| Warfarin | 0.90 | 0.860.94 |
| LMWH, prophylactic dose | 1.10 | 1.081.13 |
| LMWH, treatment dose | 1.22 | 1.161.29 |
| Intravenous steroids | 1.05 | 1.021.08 |
| Bacteremia (prior year) | 1.14 | 1.021.27 |
| VTE (prior year) | 1.11 | 1.061.18 |
| Pneumatic compression device | 1.25 | 1.081.45 |
| Invasive ventilation (prior year) | 1.17 | 1.111.24 |
| Irritable bowel disease | 1.19 | 1.051.36 |
Therapy with potent parenteral antimicrobials including antimethicillin‐resistant Staphylococcus aureus agents (OR: 1.72, 95% CI: 1.67‐1.76), antipseudomonal ‐lactamases (OR: 1.27, 95% CI: 1.23‐1.31), and carbapenems (OR: 1.37, 95% CI: 1.31‐1.44) were significantly associated with PICC use. Conversely, use of macrolides (OR: 0.85, 95% CI: 0.82‐0.88) or respiratory fluoroquinolones (OR: 0.90, 95% CI: 0.87‐0.92) were associated with lower likelihood of PICC use. After adjusting for antimicrobial therapy, HCAP was only slightly more likely to result in PICC use than CAP (OR: 1.03, 95% CI: 1.01‐1.06). Compared to internal medicine providers, admission by geriatricians and critical care/pulmonary specialists was associated with greater likelihood of PICC use (OR: 1.85, 95% CI: 1.66‐2.05 and OR: 1.18, 95% CI: =1.13‐1.24, respectively). Admission by hospitalists was associated with a modestly lower likelihood of PICC placement (OR: 0.94, 95% CI: 0.91‐0.98).
Hospital Level Variation in PICC Use
To ensure stable estimates of hospital PICC use, we excluded 152 facilities (31%): 10% had no patients with PICCs and 21% had <5 patients who received a PICC. Therefore, RSPICC was estimated for 343 of 495 facilities (69%) (Figure 2). In these facilities, RSPICC varied from 0.3% to 41.7%. Hospital RSPICC was significantly associated with hospital location (median 11.9% vs 7.8% for urban vs rural hospitals respectively, P = 0.05). RSPICCs were also greater among hospitals in Southern (11.3%), Western (12.7%), and Midwest (12.0%) regions of the nation compared to those in the Northeast (8.4%) (P = 0.02) (Table 3).
| Hospital Characteristic (No.) | Median (IQR), % | P Value |
|---|---|---|
| ||
| Bed size | 0.12 | |
| 200 beds (106) | 9.1 (4.816.3) | |
| 201 beds (237) | 11.6 (5.817.6) | |
| Rural/urban | 0.05 | |
| Urban (275) | 11.9 (5.517.4) | |
| Rural (68) | 7.8 (5.014.0) | |
| Region | 0.02 | |
| Northeast (50) | 8.4 (3.913.0) | |
| Midwest (69) | 12.0 (5.817.4) | |
| West (57) | 12.7 (7.617.0) | |
| South (167) | 11.3 (4.817.8) | |
| Teaching status | 0.77 | |
| Nonteaching (246) | 10.9 (5.017.4) | |
| Teaching (97) | 12.0 (5.816.9) | |
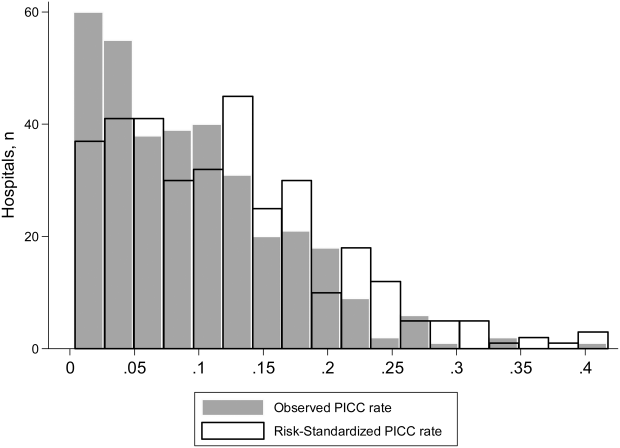
A likelihood ratio test comparing the hierarchical model to a logistic model with patient factors only was highly significant (P < 0.001), indicating that the hospital where the patient was treated had a major impact on receipt of PICC after accounting for patient factors. The MOR was 2.71, which is a larger effect than we found for any of the individual patient characteristics. The proportion of variance explained by hospitals was 25% (95% CI: 22%‐28%), as measured by the ICC.
DISCUSSION
In this study of 545,250 adults hospitalized with pneumonia, we found that approximately 8% of patients received a PICC. Patients who received PICCs had more comorbidities, were more frequently diagnosed with HCAP, and were more often admitted to the ICU, where they experienced greater rates of mechanical ventilation, noninvasive ventilation, and vasopressor use compared to those who did not receive a PICC. Additionally, risk‐adjusted rates of PICC use varied as much as 10‐fold across institutions. In fact, almost 70% of the total variation in rates of PICC use remained unexplained by hospital or patient characteristics. Although use of PICCs is often clinically nuanced in ways that are difficult to capture in large datasets (eg, difficult venous access or inability to tolerate oral medications), the substantial variation of PICC use observed suggests that physician and institutional practice styles are the major determinants of PICC placement during a hospitalization for pneumonia. Because PICCs are associated with serious complications, and evidence regarding discretionary use is accumulating, a research agenda examining reasons for such use and related outcomes appears necessary.
The placement of PICCs has grown substantially in hospitalized patients all over the world.[23, 24] Although originally developed for total parenteral nutrition in surgical patients,[25] contemporary reports of PICC use in critical illness,[26] diseases such as cystic fibrosis,[27] and even pregnancy[28] are now common. Although PICCs are clinically invaluable in many of these conditions, growing use of these devices has led to the realization that benefits may be offset by complications.[9, 10, 29, 30] Additionally, recent data suggest that not all PICCs may be used for appropriate reasons. For instance, in a decade‐long study at a tertiary care center, changes in patterns of PICC use including shortened dwell times, multiple insertions in a single patient, and unclear indications for use were reported.[11] In another study at an academic medical center, a substantial proportion of PICCs were found to be idle or unjustified.[12] It comes as little surprise, then, that a recent multicenter study found that 1 out of every 5 clinicians did not even know that their patient had a PICC.[29] Although calls to improve PICC use in the hospital setting have emerged, strategies to do so are limited by data that emanate from single‐center reports or retrospective designs. No other studies reporting use of PICCs across US hospitals for any clinical condition currently exist.[31]
We found that patients with weight loss, those with greater combined comorbidity scores, and those who were critically ill or diagnosed with sepsis were more likely to receive PICCs than others. These observations suggest that PICC use may reflect underlying severity of illness, as advanced care such as ventilator support was often associated with PICC use. Additionally, discharge to a skilled nursing facility was frequently associated with PICC placement, a finding consistent with a recent study evaluating the use of PICCs in these settings.[32] However, a substantial proportion of PICC use remained unexplained by available patient or hospital factors. Although our study was not specifically designed to examine this question, a possible reason may relate to unmeasured institutional factors that influence the propensity to use a PICC, recently termed as PICC culture.[33] For example, it is plausible that hospitals with nursing‐led PICC teams or interventional radiology (such as teaching hospitals) are more likely to use PICCs than those without such operators. This hypothesis may explain why urban, larger, and teaching hospitals exhibited higher rates of PICC use. Conversely, providers may have an affinity toward PICC use that is predicated not just by operator availability, but also local hospital norms. Understanding why some facilities use PICCs at higher rates than others and implications of such variation with respect to patient safety, cost, and outcomes is important. Study designs that use mixed‐methods approaches or seek to qualitatively understand reasons behind PICC use are likely to be valuable in this enquiry.
Our study has limitations. First, we used an administrative dataset and ICD‐9‐CM codes rather than clinical data from medical records to identify cases of pneumonia or comorbidities. Our estimates of PICC use across hospitals thus may not fully account for differences in severity of illness, and it is possible that patients needed a PICC for reasons that we could not observe. However, the substantial variation observed in rates of PICC use across hospitals is unlikely to be explained by differences in patient severity of illness, documentation, or coding practices. Second, as PICC removal codes were not available, we are unable to comment on how often hospitalized pneumonia patients were discharged with PICCs or received antimicrobial therapy beyond their inpatient stay. Third, although we observed that a number of patient and hospital factors were associated with PICC receipt, our study was not designed to determine the reasons underlying these patterns.
These limitations aside, our study has important strengths. To our knowledge, this is the first study to report utilization and outcomes associated with PICC use among those hospitalized with pneumonia across the United States. The inclusion of a large number of patients receiving care in diverse facilities lends a high degree of external validity to our findings. Second, we used advanced modeling to identify factors associated with PICC use in hospitalized patients with pneumonia, producing innovative and novel findings. Third, our study is the first to show the existence of substantial variation in rates of PICC use across US hospitals within the single disease state of pneumonia. Understanding the drivers of this variability is important as it may inform future studies, policies, and practices to improve PICC use in hospitalized patients.
In conclusion, we found that PICC use in patients hospitalized with pneumonia is common and highly variable. Future studies examining the contextual factors behind PICC use and their association with outcomes are needed to facilitate efforts to standardize PICC use across hospitals.
Disclosures
Dr. Chopra is supported by a career development award (1‐K08‐HS022835‐01) from the Agency of Healthcare Research and Quality. The authors report no conflicts of interest.
- , . Reasons for being admitted to the hospital through the emergency department, 2003. Healthcare Cost and Utilization Project Statistical Brief 2. Rockville, MD: Agency for Healthcare Research and Quality. Available at: www.hcup‐us.ahrq.gov/reports/statbriefs/sb2.pdf. Published February 2006. Accessed June 27, 2014.
- , , , et al. National patterns of risk‐standardized mortality and readmission after hospitalization for acute myocardial infarction, heart failure, and pneumonia: update on publicly reported outcomes measures based on the 2013 release. J Gen Intern Med. 2014;29(10):1333–1340.
- , , , et al. Quality of care for elderly patients hospitalized for pneumonia in the United States, 2006 to 2010. JAMA Intern Med. 2014;174(11):1806–1814.
- , . PICC lines: the latest home care challenge. RN. 1990;53(1):44–51.
- , , , . Peripherally inserted central catheters in an acute‐care hospital. Arch Intern Med. 1994;154(16):1833–1837.
- , . The peripherally inserted central catheter: a retrospective look at three years of insertions. J Intraven Nurs. 1993;16(2):92–103.
- , , , . Peripherally inserted central catheters in general medicine. Mayo Clin Proc. 1997;72(3):225–233.
- , , . Two‐year trends of peripherally inserted central catheter‐line complications at a tertiary‐care hospital: role of nursing expertise. Infect Control Hosp Epidemiol. 2001;22(6):377–379.
- , , , , , . PICC‐associated bloodstream infections: prevalence, patterns, and predictors. Am J Med. 2014;127(4):319–328.
- , , , , . The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta‐analysis. Infect Control Hosp Epidemiol. 2013;34(9):908–918.
- , , , , , . Peripherally inserted central catheters: use at a tertiary care pediatric center. J Vasc Interv Radiol. 2013;24(9):1323–1331.
- , , , et al. Temporary central venous catheter utilization patterns in a large tertiary care center: tracking the “idle central venous catheter”. Infect Control Hosp Epidemiol. 2012;33(1):50–57.
- , , , , . Inappropriate intravascular device use: a prospective study. Journal Hosp Infect. 2011;78(2):128–132.
- , , , et al. Enhancing patient‐centered care: SGIM and choosing wisely. J Gen Intern Med. 2014;29(3):432–433.
- , , , et al. Critical and honest conversations: the evidence behind the “Choosing Wisely” campaign recommendations by the American Society of Nephrology. Clin J Am Soc Nephrol. 2012;7(10):1664–1672.
- , , , et al. Using highly detailed administrative data to predict pneumonia mortality. PLoS One. 2014;9(1):e87382.
- , , , , . A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749–759.
- , , , , . Hospitals with the highest intensive care utilization provide lower quality pneumonia care to the elderly. Crit Care Med. 2015;43(6):1178–1186.
- , . Statistical and clinical aspects of hospital outcomes profiling. Stat Sci. 2007;22(2):206–226.
- , . Appropriate assessment of neighborhood effects on individual health: integrating random and fixed effects in multilevel logistic regression. Am J Epidemiol. 2005;161(1):81–88.
- , , , . Interpreting parameters in the logistic regression model with random effects. Biometrics. 2000;56(3):909–914.
- , , , . Hospital‐level associations with 30‐day patient mortality after cardiac surgery: a tutorial on the application and interpretation of marginal and multilevel logistic regression. BMC Med Res Methodol. 2012;12:28.
- , , , . Experiences of the first PICC team in the Czech Republic. Br J Nurs. 2015;24(suppl 2):S4–S10.
- , , , et al. Greece reports prototype intervention with first peripherally inserted central catheter: case report and literature review. J Vasc Nurs. 2012;30(3):88–93.
- Total intravenous nutrition with peripherally inserted silicone elastomer central venous catheters. Arch Surg. 1975;110(5):644–646.
- , . Focus on peripherally inserted central catheters in critically ill patients. World J Crit Care Med. 2014;3(4):80–94.
- , , , et al. Quality improvement initiative to reduce deep vein thrombosis associated with peripherally inserted central catheters in adults with cystic fibrosis. Ann Am Thorac Soc. 2014;11(9):1404–1410.
- , , , . Peripherally Inserted central catheter (PICC) complications during pregnancy. JPEN J Parenter Enteral Nutr. 2013;38(5):595–601.
- , , , et al. Do clinicians know which of their patients have central venous catheters?: a multicenter observational study. Ann Intern Med. 2014;161(8):562–567.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013;382(9889):311–325.
- , , . The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527–1528.
- , , , et al. Peripherally inserted central catheter use in skilled nursing facilities: a pilot study. J Am Geriatr Soc. 2015;63(9):1894–1899.
- , , , , . Inpatient venous access practices: PICC culture and the kidney patient. J Vasc Access. 2015;16(3):206–210.
Pneumonia is the most common cause of unplanned hospitalization in the United States.[1] Despite its clinical toll, the management of this disease has evolved markedly. Expanding vaccination programs, efforts to improve timeliness of antibiotic therapy, and improved processes of care are but a few developments that have improved outcomes for patients afflicted with this illness.[2, 3]
Use of peripherally inserted central catheters (PICCs) is an example of a modern development in the management of patients with pneumonia.[4, 5, 6, 7] PICCs provide many of the benefits associated with central venous catheters (CVCs) including reliable venous access for delivery of antibiotics, phlebotomy, and invasive hemodynamic monitoring. However, as they are placed in veins of the upper extremity, PICCs bypass insertion risks (eg, injury to the carotid vessels or pneumothorax) associated with placement of traditional CVCs.[8] Because they offer durable venous access, PICCs also facilitate care transitions while continuing intravenous antimicrobial therapy in patients with pneumonia.
However, accumulating evidence also suggests that PICCs are associated with important complications, including central lineassociated bloodstream infectionand venous thromboembolism.[9, 10] Furthermore, knowledge gaps in clinicians regarding indications for appropriate use and management of complications associated with PICCs have been recognized.[10, 11] These elements are problematic because reports of unjustified and inappropriate PICC use are growing in the literature.[12, 13] Such concerns have prompted a number of policy calls to improve PICC use, including Choosing Wisely recommendations by various professional societies.[14, 15]
As little is known about the prevalence or patterns of PICC use in adults hospitalized with pneumonia, we conducted a retrospective cohort study using data from a large network of US hospitals.
METHODS
Setting and Participants
We included patients from hospitals that participated in Premier's inpatient dataset, a large, fee‐supported, multipayer administrative database that has been used extensively in health services research to measure quality of care and comparative effectiveness of interventions.[16] Participating hospitals represent all regions of the United States and include teaching and nonteaching facilities in rural and urban locations. In addition to variables found in the uniform billing form, the Premier inpatient database also includes a date‐stamped list of charges for procedures conducted during hospitalization such as PICC placement. As PICC‐specific data are not available in most nationally representative datasets, Premier offers unique insights into utilization, timing, and factors associated with use of PICCs in hospitalized settings.
We included adult patients aged 18 years who were (1) admitted with a principal diagnosis of pneumonia present on admission, or secondary diagnosis of pneumonia if paired with a principal diagnosis of sepsis, respiratory failure, or influenza; (2) received at least 1 day of antibiotics between July 1, 2007 and November 30, 2011, and (3) underwent chest x‐ray or computed tomography (CT) at the time of admission. International Classification of Disease, 9th Revision, Clinical Modification (ICD‐9‐CM) codes were used for patient selection. Patients who were not admitted (eg, observation cases), had cystic fibrosis, or marked as pneumonia not present on admission were excluded. For patients who had more than 1 hospitalization during the study period, a single admission was randomly selected for inclusion.
Patient, Physician, and Hospital Data
For all patients, age, gender, marital status, insurance, race, and ethnicity were captured. Using software provided by the Healthcare Costs and Utilization Project, we categorized information on 29 comorbid conditions and computed a combined comorbidity score as described by Gagne et al.[17] Patients were considered to have healthcare‐associated pneumonia (HCAP) if they were: (1) admitted from a skilled nursing or a long‐term care facility, (2) hospitalized in the previous 90 days, (3) on dialysis, or (4) receiving immunosuppressing medications (eg, chemotherapy or steroids equivalent to at least 20 mg of prednisone per day) at the time of admission. Information on specialty of the admitting physician and hospital characteristics (eg, size, location, teaching status) were sourced through Premier data.
Receipt of PICCs and Related Therapies
Among eligible adult patients hospitalized with pneumonia, we identified patients who received a PICC at any time during hospitalization via PICC‐specific billing codes. Non‐PICC devices (eg, midlines, Hickman catheters) were not included. For all insertions, we assessed day of PICC placement relative to admission date. Data on type of PICC (eg, power‐injection capable, antibiotic coating) or PICC characteristics (size, number of lumens) were not available. We used billing codes to assess use of invasive or noninvasive ventilation, vasopressors, and administration of pneumonia‐specific antibiotics (eg, ‐lactams, macrolides, fluoroquinolones). Early exposure was defined when a billing code appeared within 2 days of hospital admission.
Outcomes of Interest
The primary outcome of interest was receipt of a PICC. Additionally, we assessed factors associated with PICC placement and variation in risk‐standardized rates of PICC use between hospitals.
Statistical Analyses
Patient and hospital characteristics were summarized using frequencies for categorical variables and medians with interquartile ranges for continuous variables. We examined association of individual patient and hospital characteristics with use of PICCs using generalized estimating equations models with a logit link for categorical variables and identity link for continuous variables, accounting for patient clustering within hospitals.
| Characteristic | Total, No. (%) | No PICC, No. (%) | PICC, No. (%) | P Value* |
|---|---|---|---|---|
| ||||
| 545,250 (100) | 503,401 (92.3) | 41,849 (7.7) | ||
| Demographics | ||||
| Age, median (Q1Q3), y | 71 (5782) | 72 (5782) | 69 (5780) | <0.001 |
| Gender | <0.001 | |||
| Male | 256,448 (47.0) | 237,232 (47.1) | 19,216 (45.9) | |
| Female | 288,802 (53.0) | 266,169 (52.9) | 22,633 (54.1) | |
| Race/ethnicity | <0.001 | |||
| White | 377,255 (69.2) | 346,689 (68.9) | 30,566 (73.0) | |
| Black | 63,345 (11.6) | 58,407 (11.6) | 4,938 (11.8) | |
| Hispanic | 22,855 (4.2) | 21,716 (4.3) | 1,139 (2.7) | |
| Other | 81,795 (15.0) | 76,589 (15.2) | 5,206 (12.4) | |
| Admitting specialty | <0.001 | |||
| Internal medicine | 236,859 (43.4) | 218,689 (43.4) | 18,170 (43.4) | |
| Hospital medicine | 116,499 (21.4) | 107,671 (21.4) | 8,828 (21.1) | |
| Family practice | 80,388 (14.7) | 75,482 (15.0) | 4,906 (11.7) | |
| Critical care and pulmonary | 35,670 (6.5) | 30,529 (6.1) | 41,849 (12.3) | |
| Geriatrics | 4,812 (0.9) | 4,098 (0.8) | 714 (1.7) | |
| Other | 71,022 (13.0) | 66,932 (13.3) | 4,090 (9.8) | |
| Insurance | <0.001 | |||
| Medicare | 370,303 (67.9) | 341,379 (67.8) | 28,924 (69.1) | |
| Medicaid | 45,505 (8.3) | 41,100 (8.2) | 4,405 (10.5) | |
| Managed care | 69,984 (12.8) | 65,280 (13.0) | 4,704 (11.2) | |
| Commercialindemnity | 20,672 (3.8) | 19,251 (3.8) | 1,421 (3.4) | |
| Other | 38,786 (7.1) | 36,391 (7.2) | 2,395 (5.7) | |
| Comorbidities | ||||
| Gagne combined comorbidity score, median (Q1Q3) | 2 (15) | 2 (14) | 4 (26) | <0.001 |
| Hypertension | 332,347 (60.9) | 306,964 (61.0) | 25,383 (60.7) | 0.13 |
| Chronic pulmonary disease | 255,403 (46.8) | 234,619 (46.6) | 20,784 (49.7) | <0.001 |
| Diabetes | 171,247 (31.4) | 155,540 (30.9) | 15,707 (37.5) | <0.001 |
| Congestive heart failure | 146,492 (26.9) | 131,041 (26.0) | 15,451 (36.9) | <0.001 |
| Atrial fibrillation | 108,405 (19.9) | 97,124 (19.3) | 11,281 (27.0) | <0.001 |
| Renal failure | 104,404 (19.1) | 94,277 (18.7) | 10,127 (24.2) | <0.001 |
| Nicotine replacement therapy/tobacco use | 89,938 (16.5) | 83,247 (16.5) | 6,691 (16.0) | <0.001 |
| Obesity | 60,242 (11.0) | 53,268 (10.6) | 6,974 (16.7) | <0.001 |
| Coagulopathy | 41,717 (7.6) | 35,371 (7.0) | 6,346 (15.2) | <0.001 |
| Prior stroke (1 year) | 26,787 (4.9) | 24,046 (4.78) | 2,741 (6.55) | <0.001 |
| Metastatic cancer | 21,868 (4.0) | 20,244 (4.0) | 1,624 (3.9) | 0.16 |
| Solid tumor w/out metastasis | 21,083 (3.9) | 19,380 (3.8) | 1,703 (4.1) | 0.12 |
| Prior VTE (1 year) | 19,090 (3.5) | 16,906 (3.4) | 2,184 (5.2) | <0.001 |
| Chronic liver disease | 16,273 (3.0) | 14,207 (2.8) | 2,066 (4.9) | <0.001 |
| Prior bacteremia (1 year) | 4,106 (0.7) | 3,584 (0.7) | 522 (1.2) | <0.001 |
| Nephrotic syndrome | 671 (0.1) | 607 (0.1) | 64 (0.2) | 0.03 |
| Morbidity markers | ||||
| Type of pneumonia | <0.001 | |||
| CAP | 376,370 (69.1) | 352,900 (70.1) | 23,830 (56.9) | |
| HCAP | 168,520 (30.9) | 150,501 (29.9) | 18,019 (43.1) | |
| Sepsis present on admission | 114,578 (21.0) | 96,467 (19.2) | 18,111 (43.3) | <0.001 |
| Non‐invasive ventilation | 47,913(8.8) | 40,599 (8.1) | 7,314 (17.5) | <0.001 |
| Invasive mechanical ventilation | 56,179 (10.3) | 44,228 (8.8) | 11,951 (28.6) | <0.001 |
| ICU status | 97,703 (17.9) | 80,380 (16.0) | 17,323 (41.4) | <0.001 |
| Vasopressor use | 48,353 (8.9) | 38,030 (7.6) | 10,323 (24.7) | <0.001 |
| Antibiotic/medication use | ||||
| Anti‐MRSA agent (vancomycin) | 146,068 (26.8) | 123,327 (24.5) | 22,741 (54.3) | <0.001 |
| Third‐generation cephalosporin | 250,782 (46.0) | 235,556 (46.8) | 15,226 (36.4) | <0.001 |
| Anti‐Pseudomonal cephalosporin | 41,798 (7.7) | 36,982 (7.3) | 4,816 (11.5) | <0.001 |
| Anti‐Pseudomonal ‐lactam | 122,215 (22.4) | 105,741 (21.0) | 16,474 (39.4) | <0.001 |
| Fluroquinolone | 288,051 (52.8) | 267,131 (53.1) | 20,920 (50.0) | <0.001 |
| Macrolide | 223,737 (41.0) | 210,954 (41.9) | 12,783 (30.5) | <0.001 |
| Aminoglycoside | 15,415 (2.8) | 12,661 (2.5) | 2,754 (6.6) | <0.001 |
| Oral steroids | 44,486 (8.2) | 41,586 (8.3) | 2,900 (6.9) | <0.001 |
| Intravenous steroids | 146,308 (26.8) | 133,920 (26.6) | 12,388 (29.6) | <0.001 |
| VTE prophylaxis with LMWH | 190,735 (35.0) | 174,612 (34.7) | 16,123 (38.5) | 0.01 |
| Discharge disposition | ||||
| Home | 282,146 (51.7) | 272,604(54.1) | 9,542 (22.8) | <0.001 |
| Home with home health | 71,977 (13.2) | 65,289 (13.0) | 6,688 (16.0) | <0.001 |
| Skilled nursing facility | 111,541 (20.5) | 97,113 (19.3) | 14,428 (34.5) | <0.001 |
| Hospice | 20,428 (3.7) | 17,902 (3.6) | 2,526 (6.0) | <0.001 |
| Expired | 47,733 (8.7) | 40,768 (8.1) | 6,965 (16.6) | <0.001 |
| Other | 11,425 (2.1) | 9,725 (1.9) | 1,700 (4.1) | <0.001 |
We then developed a multivariable hierarchical generalized linear model (HGLM) for PICC placement with a random effect for hospital. In this model, we included patient demographics, comorbidities, sepsis on admission, type of pneumonia (eg, HCAP vs community‐associated pneumonia [CAP]), admitting physician specialty, and indicators for early receipt of specific treatments such as guideline‐recommended antibiotics, vasopressors, ventilation (invasive or noninvasive), and pneumatic compression devices for prophylaxis of deep vein thrombosis.
To understand and estimate between‐hospital variation in PICC use, we calculated risk‐standardized rates of PICC use (RSPICC) across hospitals using HGLM methods. These methods are also employed by the Centers for Medicare and Medicaid Services to calculate risk‐standardized measures for public reporting.[18] Because hospital rates of PICC use were highly skewed (21.2% [n = 105] of hospitals had no patients with PICCs), we restricted this model to the 343 hospitals that had at least 5 patients with a PICC to obtain stable estimates. For each hospital, we estimated a predicted rate of PICC use (pPICC) as the sum of predicted probabilities of PICC receipt from patient factors and the random intercept for hospital in which they were admitted. We then calculated an expected rate of PICC use (ePICC) per hospital as the sum of expected probabilities of PICC receipt from patient factors only. RSPICC for each hospital was then computed as the product of the overall unadjusted mean PICC rate (PICC) from all patients and the ratio of the predicted to expected PICC rate (uPICC*[pPICC/ePICC]).[19] Kruskal‐Wallis tests were used to evaluate the association between hospital characteristics with RSPICC rates. To evaluate the impact of the hospital in variation in PICC use, we assessed the change in likelihood ratio of a hierarchical model with hospital random effects compared to a logistic regression model with patient factors only. In addition, we estimated the intraclass correlation (ICC) to assess the proportion of variation in PICC use associated with the hospital, and the median odds ratio (MOR) from the hierarchical model. The MOR is the median of a set of odds ratios comparing 2 patients with the same set of characteristics treated at 2 randomly selected hospitals.[20, 21, 22] All analyses were performed using the Statistical Analysis System version 9.3 (SAS Institute, Inc., Cary, NC) and Stata 13 (StataCorp Inc., College Station, TX).
Ethical and Regulatory Oversight
Permission to conduct this study was obtained from the institutional review board at Baystate Medical Center, Springfield, Massachusetts. The study did not qualify as human subjects research and made use of fully deidentified data.
RESULTS
Between July 2007 and November 2011, 634,285 admissions representing 545,250 unique patients from 495 hospitals met eligibility criteria and were included in the study (Figure 1). Included patients had a median age of 71 years (interquartile range [IQR]: 5782), and 53.0% were female. Most patients were Caucasian (69.2%), unmarried (51.6%), and insured by Medicare (67.9%). Patients were admitted to the hospital by internal medicine providers (43.4%), hospitalists (21.4%), and family practice providers (14.7%); notably, critical care and pulmonary medicine providers admitted 6.5% of patients. The median Gagne comorbidity score was 2 (IQR: 15). Hypertension, chronic obstructive pulmonary disease, diabetes, and congestive heart failure were among the most common comorbidities observed (Table 1).

Among eligible patients, 41,849 (7.7%) received a PICC during hospitalization. Approximately a quarter of all patients who received PICCs did so by hospital day 2; 90% underwent insertion by hospital day 11 (mean = 5.4 days, median = 4 days). Patients who received PICCs were younger (median IQR: 69 years, 5780 years) but otherwise demographically similar to those that did not receive PICCs (median IQR: 72 years, 5782 years). Compared to other specialties, patients admitted by critical care/pulmonary providers were twice as likely to receive PICCs (12.3% vs 6.1%, P < .001). Patients who received PICCs had higher comorbidity scores than those who did not (median Gagne comorbidity score 4 vs 2, P < 0.001) and were more likely to be diagnosed with HCAP (43.1% vs 29.9%, P < 0.001) than CAP (56.9% vs 70.1%, P < 0.001).
PICC recipients were also more likely to receive intensive care unit (ICU) level of care (41.4% vs 16%, P < 0.001) and both noninvasive (17.5% vs 8.1%, P < 0.001) and invasive ventilation (28.6% vs 8.8%, P < 0.001) upon admission. Vasopressor use was also significantly more frequent in patients who received PICCs (24.7% vs 7.6%, P < 0.001) compared to those who did not receive these devices. Patients with PICCs were more often discharged to skilled nursing facilities (34.5% vs 19.3%) than those without PICCs.
Characteristics Associated With PICC Use Following Multivariable Modeling
Using HGLM with a random hospital effect, multiple patient characteristics were associated with PICC use (Table 2). Patients 65 years of age were less likely to receive a PICC compared to younger patients (odds ratio [OR]: 0.81, 95% confidence interval [CI]: 0.79‐0.84). Weight loss (OR: 2.03, 95% CI: 1.97‐2.10), sepsis on admission (OR: 1.80, 95% CI: 1.75‐1.85), and ICU status on hospital day 1 or 2 (OR: 1.70, 95% CI: 1.64‐1.75) represented 3 factors most strongly associated with PICC use.
| Patient Characteristic | Odds Ratio | 95% Confidence Intervals |
|---|---|---|
| ||
| Age group (>66 vs 65 years) | 0.82 | 0.790.84 |
| Race/ethnicity | ||
| Other | 1.02 | 0.971.06 |
| Black | 0.99 | 0.951.03 |
| Hispanic | 0.82 | 0.760.88 |
| White | Referent | |
| Marital status | ||
| Other/missing | 1.07 | 1.011.14 |
| Single | 1.02 | 1.001.05 |
| Married | Referent | |
| Insurance payor | ||
| Other | 0.85 | 0.800.89 |
| Medicaid | 1.13 | 1.081.18 |
| Managed care | 0.95 | 0.910.99 |
| Commercialindemnity | 0.93 | 0.871.00 |
| Medicare | Referent | |
| Admitting physician specialty | ||
| Pulmonary/critical care medicine | 1.18 | 1.131.24 |
| Family practice | 1.01 | 0.971.05 |
| Geriatric medicine (FP and IM) | 1.85 | 1.662.05 |
| Hospitalist | 0.94 | 0.910.98 |
| Other specialties | 1.02 | 0.971.06 |
| Internal medicine | Referent | |
| Comorbidities | ||
| Congestive heart failure | 1.27 | 1.241.31 |
| Valvular disease | 1.11 | 1.071.15 |
| Pulmonary circulation disorders | 1.37 | 1.321.42 |
| Peripheral vascular disease | 1.09 | 1.051.13 |
| Hypertension | 0.94 | 0.920.97 |
| Paralysis | 1.59 | 1.511.67 |
| Other neurological disorders | 1.20 | 1.161.23 |
| Chronic lung disease | 1.10 | 1.071.12 |
| Diabetes | 1.13 | 1.101.16 |
| Hypothyroidism | 1.03 | 1.001.06 |
| Liver disease | 1.16 | 1.101.23 |
| Ulcer | 1.86 | 1.153.02 |
| Lymphoma | 0.88 | 0.810.96 |
| Metastatic cancer | 0.75 | 0.710.80 |
| Solid tumor without metastasis | 0.93 | 0.880.98 |
| Arthritis | 1.22 | 1.161.28 |
| Obesity | 1.47 | 1.421.52 |
| Weight loss | 2.03 | 1.972.10 |
| Blood loss | 1.69 | 1.551.85 |
| Deficiency anemias | 1.40 | 1.371.44 |
| Alcohol abuse | 1.19 | 1.131.26 |
| Drug abuse | 1.31 | 1.231.39 |
| Psychoses | 1.16 | 1.111.21 |
| Depression | 1.10 | 1.061.13 |
| Renal failure | 0.96 | 0.930.98 |
| Type of pneumonia | ||
| HCAP | 1.03 | 1.011.06 |
| CAP | Referent | |
| Sepsis (POA) | 1.80 | 1.751.85 |
| Antibiotic exposure | ||
| Anti‐MRSA agent | 1.72 | 1.671.76 |
| Anti‐Pseudomonal carbapenem | 1.37 | 1.311.44 |
| Non‐Pseudomonal carbapenem | 1.48 | 1.331.66 |
| Third‐generation cephalosporin | 1.04 | 1.011.07 |
| Anti‐Pseudomonal cephalosporin | 1.25 | 1.201.30 |
| Anti‐Pseudomonal ‐lactam | 1.27 | 1.231.31 |
| Aztreonam | 1.31 | 1.231.40 |
| Non‐Pseudomonal ‐lactam | 1.36 | 1.231.50 |
| ‐lactam | 1.55 | 1.261.90 |
| Respiratory quinolone | 0.90 | 0.870.92 |
| Macrolide | 0.85 | 0.820.88 |
| Doxycycline | 0.94 | 0.871.01 |
| Aminoglycoside | 1.21 | 1.141.27 |
| Vasopressors | 1.06 | 1.031.10 |
| Noninvasive ventilation | 1.29 | 1.251.34 |
| Invasive ventilation | 1.66 | 1.611.72 |
| Intensive care unit on admission | 1.70 | 1.641.75 |
| Atrial fibrillation | 1.26 | 1.221.29 |
| Upper extremity chronic DVT | 1.61 | 1.132.28 |
| Nicotine replacement therapy/tobacco abuse | 0.91 | 0.880.94 |
| Aspirin | 0.94 | 0.920.97 |
| Warfarin | 0.90 | 0.860.94 |
| LMWH, prophylactic dose | 1.10 | 1.081.13 |
| LMWH, treatment dose | 1.22 | 1.161.29 |
| Intravenous steroids | 1.05 | 1.021.08 |
| Bacteremia (prior year) | 1.14 | 1.021.27 |
| VTE (prior year) | 1.11 | 1.061.18 |
| Pneumatic compression device | 1.25 | 1.081.45 |
| Invasive ventilation (prior year) | 1.17 | 1.111.24 |
| Irritable bowel disease | 1.19 | 1.051.36 |
Therapy with potent parenteral antimicrobials including antimethicillin‐resistant Staphylococcus aureus agents (OR: 1.72, 95% CI: 1.67‐1.76), antipseudomonal ‐lactamases (OR: 1.27, 95% CI: 1.23‐1.31), and carbapenems (OR: 1.37, 95% CI: 1.31‐1.44) were significantly associated with PICC use. Conversely, use of macrolides (OR: 0.85, 95% CI: 0.82‐0.88) or respiratory fluoroquinolones (OR: 0.90, 95% CI: 0.87‐0.92) were associated with lower likelihood of PICC use. After adjusting for antimicrobial therapy, HCAP was only slightly more likely to result in PICC use than CAP (OR: 1.03, 95% CI: 1.01‐1.06). Compared to internal medicine providers, admission by geriatricians and critical care/pulmonary specialists was associated with greater likelihood of PICC use (OR: 1.85, 95% CI: 1.66‐2.05 and OR: 1.18, 95% CI: =1.13‐1.24, respectively). Admission by hospitalists was associated with a modestly lower likelihood of PICC placement (OR: 0.94, 95% CI: 0.91‐0.98).
Hospital Level Variation in PICC Use
To ensure stable estimates of hospital PICC use, we excluded 152 facilities (31%): 10% had no patients with PICCs and 21% had <5 patients who received a PICC. Therefore, RSPICC was estimated for 343 of 495 facilities (69%) (Figure 2). In these facilities, RSPICC varied from 0.3% to 41.7%. Hospital RSPICC was significantly associated with hospital location (median 11.9% vs 7.8% for urban vs rural hospitals respectively, P = 0.05). RSPICCs were also greater among hospitals in Southern (11.3%), Western (12.7%), and Midwest (12.0%) regions of the nation compared to those in the Northeast (8.4%) (P = 0.02) (Table 3).
| Hospital Characteristic (No.) | Median (IQR), % | P Value |
|---|---|---|
| ||
| Bed size | 0.12 | |
| 200 beds (106) | 9.1 (4.816.3) | |
| 201 beds (237) | 11.6 (5.817.6) | |
| Rural/urban | 0.05 | |
| Urban (275) | 11.9 (5.517.4) | |
| Rural (68) | 7.8 (5.014.0) | |
| Region | 0.02 | |
| Northeast (50) | 8.4 (3.913.0) | |
| Midwest (69) | 12.0 (5.817.4) | |
| West (57) | 12.7 (7.617.0) | |
| South (167) | 11.3 (4.817.8) | |
| Teaching status | 0.77 | |
| Nonteaching (246) | 10.9 (5.017.4) | |
| Teaching (97) | 12.0 (5.816.9) | |

A likelihood ratio test comparing the hierarchical model to a logistic model with patient factors only was highly significant (P < 0.001), indicating that the hospital where the patient was treated had a major impact on receipt of PICC after accounting for patient factors. The MOR was 2.71, which is a larger effect than we found for any of the individual patient characteristics. The proportion of variance explained by hospitals was 25% (95% CI: 22%‐28%), as measured by the ICC.
DISCUSSION
In this study of 545,250 adults hospitalized with pneumonia, we found that approximately 8% of patients received a PICC. Patients who received PICCs had more comorbidities, were more frequently diagnosed with HCAP, and were more often admitted to the ICU, where they experienced greater rates of mechanical ventilation, noninvasive ventilation, and vasopressor use compared to those who did not receive a PICC. Additionally, risk‐adjusted rates of PICC use varied as much as 10‐fold across institutions. In fact, almost 70% of the total variation in rates of PICC use remained unexplained by hospital or patient characteristics. Although use of PICCs is often clinically nuanced in ways that are difficult to capture in large datasets (eg, difficult venous access or inability to tolerate oral medications), the substantial variation of PICC use observed suggests that physician and institutional practice styles are the major determinants of PICC placement during a hospitalization for pneumonia. Because PICCs are associated with serious complications, and evidence regarding discretionary use is accumulating, a research agenda examining reasons for such use and related outcomes appears necessary.
The placement of PICCs has grown substantially in hospitalized patients all over the world.[23, 24] Although originally developed for total parenteral nutrition in surgical patients,[25] contemporary reports of PICC use in critical illness,[26] diseases such as cystic fibrosis,[27] and even pregnancy[28] are now common. Although PICCs are clinically invaluable in many of these conditions, growing use of these devices has led to the realization that benefits may be offset by complications.[9, 10, 29, 30] Additionally, recent data suggest that not all PICCs may be used for appropriate reasons. For instance, in a decade‐long study at a tertiary care center, changes in patterns of PICC use including shortened dwell times, multiple insertions in a single patient, and unclear indications for use were reported.[11] In another study at an academic medical center, a substantial proportion of PICCs were found to be idle or unjustified.[12] It comes as little surprise, then, that a recent multicenter study found that 1 out of every 5 clinicians did not even know that their patient had a PICC.[29] Although calls to improve PICC use in the hospital setting have emerged, strategies to do so are limited by data that emanate from single‐center reports or retrospective designs. No other studies reporting use of PICCs across US hospitals for any clinical condition currently exist.[31]
We found that patients with weight loss, those with greater combined comorbidity scores, and those who were critically ill or diagnosed with sepsis were more likely to receive PICCs than others. These observations suggest that PICC use may reflect underlying severity of illness, as advanced care such as ventilator support was often associated with PICC use. Additionally, discharge to a skilled nursing facility was frequently associated with PICC placement, a finding consistent with a recent study evaluating the use of PICCs in these settings.[32] However, a substantial proportion of PICC use remained unexplained by available patient or hospital factors. Although our study was not specifically designed to examine this question, a possible reason may relate to unmeasured institutional factors that influence the propensity to use a PICC, recently termed as PICC culture.[33] For example, it is plausible that hospitals with nursing‐led PICC teams or interventional radiology (such as teaching hospitals) are more likely to use PICCs than those without such operators. This hypothesis may explain why urban, larger, and teaching hospitals exhibited higher rates of PICC use. Conversely, providers may have an affinity toward PICC use that is predicated not just by operator availability, but also local hospital norms. Understanding why some facilities use PICCs at higher rates than others and implications of such variation with respect to patient safety, cost, and outcomes is important. Study designs that use mixed‐methods approaches or seek to qualitatively understand reasons behind PICC use are likely to be valuable in this enquiry.
Our study has limitations. First, we used an administrative dataset and ICD‐9‐CM codes rather than clinical data from medical records to identify cases of pneumonia or comorbidities. Our estimates of PICC use across hospitals thus may not fully account for differences in severity of illness, and it is possible that patients needed a PICC for reasons that we could not observe. However, the substantial variation observed in rates of PICC use across hospitals is unlikely to be explained by differences in patient severity of illness, documentation, or coding practices. Second, as PICC removal codes were not available, we are unable to comment on how often hospitalized pneumonia patients were discharged with PICCs or received antimicrobial therapy beyond their inpatient stay. Third, although we observed that a number of patient and hospital factors were associated with PICC receipt, our study was not designed to determine the reasons underlying these patterns.
These limitations aside, our study has important strengths. To our knowledge, this is the first study to report utilization and outcomes associated with PICC use among those hospitalized with pneumonia across the United States. The inclusion of a large number of patients receiving care in diverse facilities lends a high degree of external validity to our findings. Second, we used advanced modeling to identify factors associated with PICC use in hospitalized patients with pneumonia, producing innovative and novel findings. Third, our study is the first to show the existence of substantial variation in rates of PICC use across US hospitals within the single disease state of pneumonia. Understanding the drivers of this variability is important as it may inform future studies, policies, and practices to improve PICC use in hospitalized patients.
In conclusion, we found that PICC use in patients hospitalized with pneumonia is common and highly variable. Future studies examining the contextual factors behind PICC use and their association with outcomes are needed to facilitate efforts to standardize PICC use across hospitals.
Disclosures
Dr. Chopra is supported by a career development award (1‐K08‐HS022835‐01) from the Agency of Healthcare Research and Quality. The authors report no conflicts of interest.
Pneumonia is the most common cause of unplanned hospitalization in the United States.[1] Despite its clinical toll, the management of this disease has evolved markedly. Expanding vaccination programs, efforts to improve timeliness of antibiotic therapy, and improved processes of care are but a few developments that have improved outcomes for patients afflicted with this illness.[2, 3]
Use of peripherally inserted central catheters (PICCs) is an example of a modern development in the management of patients with pneumonia.[4, 5, 6, 7] PICCs provide many of the benefits associated with central venous catheters (CVCs) including reliable venous access for delivery of antibiotics, phlebotomy, and invasive hemodynamic monitoring. However, as they are placed in veins of the upper extremity, PICCs bypass insertion risks (eg, injury to the carotid vessels or pneumothorax) associated with placement of traditional CVCs.[8] Because they offer durable venous access, PICCs also facilitate care transitions while continuing intravenous antimicrobial therapy in patients with pneumonia.
However, accumulating evidence also suggests that PICCs are associated with important complications, including central lineassociated bloodstream infectionand venous thromboembolism.[9, 10] Furthermore, knowledge gaps in clinicians regarding indications for appropriate use and management of complications associated with PICCs have been recognized.[10, 11] These elements are problematic because reports of unjustified and inappropriate PICC use are growing in the literature.[12, 13] Such concerns have prompted a number of policy calls to improve PICC use, including Choosing Wisely recommendations by various professional societies.[14, 15]
As little is known about the prevalence or patterns of PICC use in adults hospitalized with pneumonia, we conducted a retrospective cohort study using data from a large network of US hospitals.
METHODS
Setting and Participants
We included patients from hospitals that participated in Premier's inpatient dataset, a large, fee‐supported, multipayer administrative database that has been used extensively in health services research to measure quality of care and comparative effectiveness of interventions.[16] Participating hospitals represent all regions of the United States and include teaching and nonteaching facilities in rural and urban locations. In addition to variables found in the uniform billing form, the Premier inpatient database also includes a date‐stamped list of charges for procedures conducted during hospitalization such as PICC placement. As PICC‐specific data are not available in most nationally representative datasets, Premier offers unique insights into utilization, timing, and factors associated with use of PICCs in hospitalized settings.
We included adult patients aged 18 years who were (1) admitted with a principal diagnosis of pneumonia present on admission, or secondary diagnosis of pneumonia if paired with a principal diagnosis of sepsis, respiratory failure, or influenza; (2) received at least 1 day of antibiotics between July 1, 2007 and November 30, 2011, and (3) underwent chest x‐ray or computed tomography (CT) at the time of admission. International Classification of Disease, 9th Revision, Clinical Modification (ICD‐9‐CM) codes were used for patient selection. Patients who were not admitted (eg, observation cases), had cystic fibrosis, or marked as pneumonia not present on admission were excluded. For patients who had more than 1 hospitalization during the study period, a single admission was randomly selected for inclusion.
Patient, Physician, and Hospital Data
For all patients, age, gender, marital status, insurance, race, and ethnicity were captured. Using software provided by the Healthcare Costs and Utilization Project, we categorized information on 29 comorbid conditions and computed a combined comorbidity score as described by Gagne et al.[17] Patients were considered to have healthcare‐associated pneumonia (HCAP) if they were: (1) admitted from a skilled nursing or a long‐term care facility, (2) hospitalized in the previous 90 days, (3) on dialysis, or (4) receiving immunosuppressing medications (eg, chemotherapy or steroids equivalent to at least 20 mg of prednisone per day) at the time of admission. Information on specialty of the admitting physician and hospital characteristics (eg, size, location, teaching status) were sourced through Premier data.
Receipt of PICCs and Related Therapies
Among eligible adult patients hospitalized with pneumonia, we identified patients who received a PICC at any time during hospitalization via PICC‐specific billing codes. Non‐PICC devices (eg, midlines, Hickman catheters) were not included. For all insertions, we assessed day of PICC placement relative to admission date. Data on type of PICC (eg, power‐injection capable, antibiotic coating) or PICC characteristics (size, number of lumens) were not available. We used billing codes to assess use of invasive or noninvasive ventilation, vasopressors, and administration of pneumonia‐specific antibiotics (eg, ‐lactams, macrolides, fluoroquinolones). Early exposure was defined when a billing code appeared within 2 days of hospital admission.
Outcomes of Interest
The primary outcome of interest was receipt of a PICC. Additionally, we assessed factors associated with PICC placement and variation in risk‐standardized rates of PICC use between hospitals.
Statistical Analyses
Patient and hospital characteristics were summarized using frequencies for categorical variables and medians with interquartile ranges for continuous variables. We examined association of individual patient and hospital characteristics with use of PICCs using generalized estimating equations models with a logit link for categorical variables and identity link for continuous variables, accounting for patient clustering within hospitals.
| Characteristic | Total, No. (%) | No PICC, No. (%) | PICC, No. (%) | P Value* |
|---|---|---|---|---|
| ||||
| 545,250 (100) | 503,401 (92.3) | 41,849 (7.7) | ||
| Demographics | ||||
| Age, median (Q1Q3), y | 71 (5782) | 72 (5782) | 69 (5780) | <0.001 |
| Gender | <0.001 | |||
| Male | 256,448 (47.0) | 237,232 (47.1) | 19,216 (45.9) | |
| Female | 288,802 (53.0) | 266,169 (52.9) | 22,633 (54.1) | |
| Race/ethnicity | <0.001 | |||
| White | 377,255 (69.2) | 346,689 (68.9) | 30,566 (73.0) | |
| Black | 63,345 (11.6) | 58,407 (11.6) | 4,938 (11.8) | |
| Hispanic | 22,855 (4.2) | 21,716 (4.3) | 1,139 (2.7) | |
| Other | 81,795 (15.0) | 76,589 (15.2) | 5,206 (12.4) | |
| Admitting specialty | <0.001 | |||
| Internal medicine | 236,859 (43.4) | 218,689 (43.4) | 18,170 (43.4) | |
| Hospital medicine | 116,499 (21.4) | 107,671 (21.4) | 8,828 (21.1) | |
| Family practice | 80,388 (14.7) | 75,482 (15.0) | 4,906 (11.7) | |
| Critical care and pulmonary | 35,670 (6.5) | 30,529 (6.1) | 41,849 (12.3) | |
| Geriatrics | 4,812 (0.9) | 4,098 (0.8) | 714 (1.7) | |
| Other | 71,022 (13.0) | 66,932 (13.3) | 4,090 (9.8) | |
| Insurance | <0.001 | |||
| Medicare | 370,303 (67.9) | 341,379 (67.8) | 28,924 (69.1) | |
| Medicaid | 45,505 (8.3) | 41,100 (8.2) | 4,405 (10.5) | |
| Managed care | 69,984 (12.8) | 65,280 (13.0) | 4,704 (11.2) | |
| Commercialindemnity | 20,672 (3.8) | 19,251 (3.8) | 1,421 (3.4) | |
| Other | 38,786 (7.1) | 36,391 (7.2) | 2,395 (5.7) | |
| Comorbidities | ||||
| Gagne combined comorbidity score, median (Q1Q3) | 2 (15) | 2 (14) | 4 (26) | <0.001 |
| Hypertension | 332,347 (60.9) | 306,964 (61.0) | 25,383 (60.7) | 0.13 |
| Chronic pulmonary disease | 255,403 (46.8) | 234,619 (46.6) | 20,784 (49.7) | <0.001 |
| Diabetes | 171,247 (31.4) | 155,540 (30.9) | 15,707 (37.5) | <0.001 |
| Congestive heart failure | 146,492 (26.9) | 131,041 (26.0) | 15,451 (36.9) | <0.001 |
| Atrial fibrillation | 108,405 (19.9) | 97,124 (19.3) | 11,281 (27.0) | <0.001 |
| Renal failure | 104,404 (19.1) | 94,277 (18.7) | 10,127 (24.2) | <0.001 |
| Nicotine replacement therapy/tobacco use | 89,938 (16.5) | 83,247 (16.5) | 6,691 (16.0) | <0.001 |
| Obesity | 60,242 (11.0) | 53,268 (10.6) | 6,974 (16.7) | <0.001 |
| Coagulopathy | 41,717 (7.6) | 35,371 (7.0) | 6,346 (15.2) | <0.001 |
| Prior stroke (1 year) | 26,787 (4.9) | 24,046 (4.78) | 2,741 (6.55) | <0.001 |
| Metastatic cancer | 21,868 (4.0) | 20,244 (4.0) | 1,624 (3.9) | 0.16 |
| Solid tumor w/out metastasis | 21,083 (3.9) | 19,380 (3.8) | 1,703 (4.1) | 0.12 |
| Prior VTE (1 year) | 19,090 (3.5) | 16,906 (3.4) | 2,184 (5.2) | <0.001 |
| Chronic liver disease | 16,273 (3.0) | 14,207 (2.8) | 2,066 (4.9) | <0.001 |
| Prior bacteremia (1 year) | 4,106 (0.7) | 3,584 (0.7) | 522 (1.2) | <0.001 |
| Nephrotic syndrome | 671 (0.1) | 607 (0.1) | 64 (0.2) | 0.03 |
| Morbidity markers | ||||
| Type of pneumonia | <0.001 | |||
| CAP | 376,370 (69.1) | 352,900 (70.1) | 23,830 (56.9) | |
| HCAP | 168,520 (30.9) | 150,501 (29.9) | 18,019 (43.1) | |
| Sepsis present on admission | 114,578 (21.0) | 96,467 (19.2) | 18,111 (43.3) | <0.001 |
| Non‐invasive ventilation | 47,913(8.8) | 40,599 (8.1) | 7,314 (17.5) | <0.001 |
| Invasive mechanical ventilation | 56,179 (10.3) | 44,228 (8.8) | 11,951 (28.6) | <0.001 |
| ICU status | 97,703 (17.9) | 80,380 (16.0) | 17,323 (41.4) | <0.001 |
| Vasopressor use | 48,353 (8.9) | 38,030 (7.6) | 10,323 (24.7) | <0.001 |
| Antibiotic/medication use | ||||
| Anti‐MRSA agent (vancomycin) | 146,068 (26.8) | 123,327 (24.5) | 22,741 (54.3) | <0.001 |
| Third‐generation cephalosporin | 250,782 (46.0) | 235,556 (46.8) | 15,226 (36.4) | <0.001 |
| Anti‐Pseudomonal cephalosporin | 41,798 (7.7) | 36,982 (7.3) | 4,816 (11.5) | <0.001 |
| Anti‐Pseudomonal ‐lactam | 122,215 (22.4) | 105,741 (21.0) | 16,474 (39.4) | <0.001 |
| Fluroquinolone | 288,051 (52.8) | 267,131 (53.1) | 20,920 (50.0) | <0.001 |
| Macrolide | 223,737 (41.0) | 210,954 (41.9) | 12,783 (30.5) | <0.001 |
| Aminoglycoside | 15,415 (2.8) | 12,661 (2.5) | 2,754 (6.6) | <0.001 |
| Oral steroids | 44,486 (8.2) | 41,586 (8.3) | 2,900 (6.9) | <0.001 |
| Intravenous steroids | 146,308 (26.8) | 133,920 (26.6) | 12,388 (29.6) | <0.001 |
| VTE prophylaxis with LMWH | 190,735 (35.0) | 174,612 (34.7) | 16,123 (38.5) | 0.01 |
| Discharge disposition | ||||
| Home | 282,146 (51.7) | 272,604(54.1) | 9,542 (22.8) | <0.001 |
| Home with home health | 71,977 (13.2) | 65,289 (13.0) | 6,688 (16.0) | <0.001 |
| Skilled nursing facility | 111,541 (20.5) | 97,113 (19.3) | 14,428 (34.5) | <0.001 |
| Hospice | 20,428 (3.7) | 17,902 (3.6) | 2,526 (6.0) | <0.001 |
| Expired | 47,733 (8.7) | 40,768 (8.1) | 6,965 (16.6) | <0.001 |
| Other | 11,425 (2.1) | 9,725 (1.9) | 1,700 (4.1) | <0.001 |
We then developed a multivariable hierarchical generalized linear model (HGLM) for PICC placement with a random effect for hospital. In this model, we included patient demographics, comorbidities, sepsis on admission, type of pneumonia (eg, HCAP vs community‐associated pneumonia [CAP]), admitting physician specialty, and indicators for early receipt of specific treatments such as guideline‐recommended antibiotics, vasopressors, ventilation (invasive or noninvasive), and pneumatic compression devices for prophylaxis of deep vein thrombosis.
To understand and estimate between‐hospital variation in PICC use, we calculated risk‐standardized rates of PICC use (RSPICC) across hospitals using HGLM methods. These methods are also employed by the Centers for Medicare and Medicaid Services to calculate risk‐standardized measures for public reporting.[18] Because hospital rates of PICC use were highly skewed (21.2% [n = 105] of hospitals had no patients with PICCs), we restricted this model to the 343 hospitals that had at least 5 patients with a PICC to obtain stable estimates. For each hospital, we estimated a predicted rate of PICC use (pPICC) as the sum of predicted probabilities of PICC receipt from patient factors and the random intercept for hospital in which they were admitted. We then calculated an expected rate of PICC use (ePICC) per hospital as the sum of expected probabilities of PICC receipt from patient factors only. RSPICC for each hospital was then computed as the product of the overall unadjusted mean PICC rate (PICC) from all patients and the ratio of the predicted to expected PICC rate (uPICC*[pPICC/ePICC]).[19] Kruskal‐Wallis tests were used to evaluate the association between hospital characteristics with RSPICC rates. To evaluate the impact of the hospital in variation in PICC use, we assessed the change in likelihood ratio of a hierarchical model with hospital random effects compared to a logistic regression model with patient factors only. In addition, we estimated the intraclass correlation (ICC) to assess the proportion of variation in PICC use associated with the hospital, and the median odds ratio (MOR) from the hierarchical model. The MOR is the median of a set of odds ratios comparing 2 patients with the same set of characteristics treated at 2 randomly selected hospitals.[20, 21, 22] All analyses were performed using the Statistical Analysis System version 9.3 (SAS Institute, Inc., Cary, NC) and Stata 13 (StataCorp Inc., College Station, TX).
Ethical and Regulatory Oversight
Permission to conduct this study was obtained from the institutional review board at Baystate Medical Center, Springfield, Massachusetts. The study did not qualify as human subjects research and made use of fully deidentified data.
RESULTS
Between July 2007 and November 2011, 634,285 admissions representing 545,250 unique patients from 495 hospitals met eligibility criteria and were included in the study (Figure 1). Included patients had a median age of 71 years (interquartile range [IQR]: 5782), and 53.0% were female. Most patients were Caucasian (69.2%), unmarried (51.6%), and insured by Medicare (67.9%). Patients were admitted to the hospital by internal medicine providers (43.4%), hospitalists (21.4%), and family practice providers (14.7%); notably, critical care and pulmonary medicine providers admitted 6.5% of patients. The median Gagne comorbidity score was 2 (IQR: 15). Hypertension, chronic obstructive pulmonary disease, diabetes, and congestive heart failure were among the most common comorbidities observed (Table 1).

Among eligible patients, 41,849 (7.7%) received a PICC during hospitalization. Approximately a quarter of all patients who received PICCs did so by hospital day 2; 90% underwent insertion by hospital day 11 (mean = 5.4 days, median = 4 days). Patients who received PICCs were younger (median IQR: 69 years, 5780 years) but otherwise demographically similar to those that did not receive PICCs (median IQR: 72 years, 5782 years). Compared to other specialties, patients admitted by critical care/pulmonary providers were twice as likely to receive PICCs (12.3% vs 6.1%, P < .001). Patients who received PICCs had higher comorbidity scores than those who did not (median Gagne comorbidity score 4 vs 2, P < 0.001) and were more likely to be diagnosed with HCAP (43.1% vs 29.9%, P < 0.001) than CAP (56.9% vs 70.1%, P < 0.001).
PICC recipients were also more likely to receive intensive care unit (ICU) level of care (41.4% vs 16%, P < 0.001) and both noninvasive (17.5% vs 8.1%, P < 0.001) and invasive ventilation (28.6% vs 8.8%, P < 0.001) upon admission. Vasopressor use was also significantly more frequent in patients who received PICCs (24.7% vs 7.6%, P < 0.001) compared to those who did not receive these devices. Patients with PICCs were more often discharged to skilled nursing facilities (34.5% vs 19.3%) than those without PICCs.
Characteristics Associated With PICC Use Following Multivariable Modeling
Using HGLM with a random hospital effect, multiple patient characteristics were associated with PICC use (Table 2). Patients 65 years of age were less likely to receive a PICC compared to younger patients (odds ratio [OR]: 0.81, 95% confidence interval [CI]: 0.79‐0.84). Weight loss (OR: 2.03, 95% CI: 1.97‐2.10), sepsis on admission (OR: 1.80, 95% CI: 1.75‐1.85), and ICU status on hospital day 1 or 2 (OR: 1.70, 95% CI: 1.64‐1.75) represented 3 factors most strongly associated with PICC use.
| Patient Characteristic | Odds Ratio | 95% Confidence Intervals |
|---|---|---|
| ||
| Age group (>66 vs 65 years) | 0.82 | 0.790.84 |
| Race/ethnicity | ||
| Other | 1.02 | 0.971.06 |
| Black | 0.99 | 0.951.03 |
| Hispanic | 0.82 | 0.760.88 |
| White | Referent | |
| Marital status | ||
| Other/missing | 1.07 | 1.011.14 |
| Single | 1.02 | 1.001.05 |
| Married | Referent | |
| Insurance payor | ||
| Other | 0.85 | 0.800.89 |
| Medicaid | 1.13 | 1.081.18 |
| Managed care | 0.95 | 0.910.99 |
| Commercialindemnity | 0.93 | 0.871.00 |
| Medicare | Referent | |
| Admitting physician specialty | ||
| Pulmonary/critical care medicine | 1.18 | 1.131.24 |
| Family practice | 1.01 | 0.971.05 |
| Geriatric medicine (FP and IM) | 1.85 | 1.662.05 |
| Hospitalist | 0.94 | 0.910.98 |
| Other specialties | 1.02 | 0.971.06 |
| Internal medicine | Referent | |
| Comorbidities | ||
| Congestive heart failure | 1.27 | 1.241.31 |
| Valvular disease | 1.11 | 1.071.15 |
| Pulmonary circulation disorders | 1.37 | 1.321.42 |
| Peripheral vascular disease | 1.09 | 1.051.13 |
| Hypertension | 0.94 | 0.920.97 |
| Paralysis | 1.59 | 1.511.67 |
| Other neurological disorders | 1.20 | 1.161.23 |
| Chronic lung disease | 1.10 | 1.071.12 |
| Diabetes | 1.13 | 1.101.16 |
| Hypothyroidism | 1.03 | 1.001.06 |
| Liver disease | 1.16 | 1.101.23 |
| Ulcer | 1.86 | 1.153.02 |
| Lymphoma | 0.88 | 0.810.96 |
| Metastatic cancer | 0.75 | 0.710.80 |
| Solid tumor without metastasis | 0.93 | 0.880.98 |
| Arthritis | 1.22 | 1.161.28 |
| Obesity | 1.47 | 1.421.52 |
| Weight loss | 2.03 | 1.972.10 |
| Blood loss | 1.69 | 1.551.85 |
| Deficiency anemias | 1.40 | 1.371.44 |
| Alcohol abuse | 1.19 | 1.131.26 |
| Drug abuse | 1.31 | 1.231.39 |
| Psychoses | 1.16 | 1.111.21 |
| Depression | 1.10 | 1.061.13 |
| Renal failure | 0.96 | 0.930.98 |
| Type of pneumonia | ||
| HCAP | 1.03 | 1.011.06 |
| CAP | Referent | |
| Sepsis (POA) | 1.80 | 1.751.85 |
| Antibiotic exposure | ||
| Anti‐MRSA agent | 1.72 | 1.671.76 |
| Anti‐Pseudomonal carbapenem | 1.37 | 1.311.44 |
| Non‐Pseudomonal carbapenem | 1.48 | 1.331.66 |
| Third‐generation cephalosporin | 1.04 | 1.011.07 |
| Anti‐Pseudomonal cephalosporin | 1.25 | 1.201.30 |
| Anti‐Pseudomonal ‐lactam | 1.27 | 1.231.31 |
| Aztreonam | 1.31 | 1.231.40 |
| Non‐Pseudomonal ‐lactam | 1.36 | 1.231.50 |
| ‐lactam | 1.55 | 1.261.90 |
| Respiratory quinolone | 0.90 | 0.870.92 |
| Macrolide | 0.85 | 0.820.88 |
| Doxycycline | 0.94 | 0.871.01 |
| Aminoglycoside | 1.21 | 1.141.27 |
| Vasopressors | 1.06 | 1.031.10 |
| Noninvasive ventilation | 1.29 | 1.251.34 |
| Invasive ventilation | 1.66 | 1.611.72 |
| Intensive care unit on admission | 1.70 | 1.641.75 |
| Atrial fibrillation | 1.26 | 1.221.29 |
| Upper extremity chronic DVT | 1.61 | 1.132.28 |
| Nicotine replacement therapy/tobacco abuse | 0.91 | 0.880.94 |
| Aspirin | 0.94 | 0.920.97 |
| Warfarin | 0.90 | 0.860.94 |
| LMWH, prophylactic dose | 1.10 | 1.081.13 |
| LMWH, treatment dose | 1.22 | 1.161.29 |
| Intravenous steroids | 1.05 | 1.021.08 |
| Bacteremia (prior year) | 1.14 | 1.021.27 |
| VTE (prior year) | 1.11 | 1.061.18 |
| Pneumatic compression device | 1.25 | 1.081.45 |
| Invasive ventilation (prior year) | 1.17 | 1.111.24 |
| Irritable bowel disease | 1.19 | 1.051.36 |
Therapy with potent parenteral antimicrobials including antimethicillin‐resistant Staphylococcus aureus agents (OR: 1.72, 95% CI: 1.67‐1.76), antipseudomonal ‐lactamases (OR: 1.27, 95% CI: 1.23‐1.31), and carbapenems (OR: 1.37, 95% CI: 1.31‐1.44) were significantly associated with PICC use. Conversely, use of macrolides (OR: 0.85, 95% CI: 0.82‐0.88) or respiratory fluoroquinolones (OR: 0.90, 95% CI: 0.87‐0.92) were associated with lower likelihood of PICC use. After adjusting for antimicrobial therapy, HCAP was only slightly more likely to result in PICC use than CAP (OR: 1.03, 95% CI: 1.01‐1.06). Compared to internal medicine providers, admission by geriatricians and critical care/pulmonary specialists was associated with greater likelihood of PICC use (OR: 1.85, 95% CI: 1.66‐2.05 and OR: 1.18, 95% CI: =1.13‐1.24, respectively). Admission by hospitalists was associated with a modestly lower likelihood of PICC placement (OR: 0.94, 95% CI: 0.91‐0.98).
Hospital Level Variation in PICC Use
To ensure stable estimates of hospital PICC use, we excluded 152 facilities (31%): 10% had no patients with PICCs and 21% had <5 patients who received a PICC. Therefore, RSPICC was estimated for 343 of 495 facilities (69%) (Figure 2). In these facilities, RSPICC varied from 0.3% to 41.7%. Hospital RSPICC was significantly associated with hospital location (median 11.9% vs 7.8% for urban vs rural hospitals respectively, P = 0.05). RSPICCs were also greater among hospitals in Southern (11.3%), Western (12.7%), and Midwest (12.0%) regions of the nation compared to those in the Northeast (8.4%) (P = 0.02) (Table 3).
| Hospital Characteristic (No.) | Median (IQR), % | P Value |
|---|---|---|
| ||
| Bed size | 0.12 | |
| 200 beds (106) | 9.1 (4.816.3) | |
| 201 beds (237) | 11.6 (5.817.6) | |
| Rural/urban | 0.05 | |
| Urban (275) | 11.9 (5.517.4) | |
| Rural (68) | 7.8 (5.014.0) | |
| Region | 0.02 | |
| Northeast (50) | 8.4 (3.913.0) | |
| Midwest (69) | 12.0 (5.817.4) | |
| West (57) | 12.7 (7.617.0) | |
| South (167) | 11.3 (4.817.8) | |
| Teaching status | 0.77 | |
| Nonteaching (246) | 10.9 (5.017.4) | |
| Teaching (97) | 12.0 (5.816.9) | |

A likelihood ratio test comparing the hierarchical model to a logistic model with patient factors only was highly significant (P < 0.001), indicating that the hospital where the patient was treated had a major impact on receipt of PICC after accounting for patient factors. The MOR was 2.71, which is a larger effect than we found for any of the individual patient characteristics. The proportion of variance explained by hospitals was 25% (95% CI: 22%‐28%), as measured by the ICC.
DISCUSSION
In this study of 545,250 adults hospitalized with pneumonia, we found that approximately 8% of patients received a PICC. Patients who received PICCs had more comorbidities, were more frequently diagnosed with HCAP, and were more often admitted to the ICU, where they experienced greater rates of mechanical ventilation, noninvasive ventilation, and vasopressor use compared to those who did not receive a PICC. Additionally, risk‐adjusted rates of PICC use varied as much as 10‐fold across institutions. In fact, almost 70% of the total variation in rates of PICC use remained unexplained by hospital or patient characteristics. Although use of PICCs is often clinically nuanced in ways that are difficult to capture in large datasets (eg, difficult venous access or inability to tolerate oral medications), the substantial variation of PICC use observed suggests that physician and institutional practice styles are the major determinants of PICC placement during a hospitalization for pneumonia. Because PICCs are associated with serious complications, and evidence regarding discretionary use is accumulating, a research agenda examining reasons for such use and related outcomes appears necessary.
The placement of PICCs has grown substantially in hospitalized patients all over the world.[23, 24] Although originally developed for total parenteral nutrition in surgical patients,[25] contemporary reports of PICC use in critical illness,[26] diseases such as cystic fibrosis,[27] and even pregnancy[28] are now common. Although PICCs are clinically invaluable in many of these conditions, growing use of these devices has led to the realization that benefits may be offset by complications.[9, 10, 29, 30] Additionally, recent data suggest that not all PICCs may be used for appropriate reasons. For instance, in a decade‐long study at a tertiary care center, changes in patterns of PICC use including shortened dwell times, multiple insertions in a single patient, and unclear indications for use were reported.[11] In another study at an academic medical center, a substantial proportion of PICCs were found to be idle or unjustified.[12] It comes as little surprise, then, that a recent multicenter study found that 1 out of every 5 clinicians did not even know that their patient had a PICC.[29] Although calls to improve PICC use in the hospital setting have emerged, strategies to do so are limited by data that emanate from single‐center reports or retrospective designs. No other studies reporting use of PICCs across US hospitals for any clinical condition currently exist.[31]
We found that patients with weight loss, those with greater combined comorbidity scores, and those who were critically ill or diagnosed with sepsis were more likely to receive PICCs than others. These observations suggest that PICC use may reflect underlying severity of illness, as advanced care such as ventilator support was often associated with PICC use. Additionally, discharge to a skilled nursing facility was frequently associated with PICC placement, a finding consistent with a recent study evaluating the use of PICCs in these settings.[32] However, a substantial proportion of PICC use remained unexplained by available patient or hospital factors. Although our study was not specifically designed to examine this question, a possible reason may relate to unmeasured institutional factors that influence the propensity to use a PICC, recently termed as PICC culture.[33] For example, it is plausible that hospitals with nursing‐led PICC teams or interventional radiology (such as teaching hospitals) are more likely to use PICCs than those without such operators. This hypothesis may explain why urban, larger, and teaching hospitals exhibited higher rates of PICC use. Conversely, providers may have an affinity toward PICC use that is predicated not just by operator availability, but also local hospital norms. Understanding why some facilities use PICCs at higher rates than others and implications of such variation with respect to patient safety, cost, and outcomes is important. Study designs that use mixed‐methods approaches or seek to qualitatively understand reasons behind PICC use are likely to be valuable in this enquiry.
Our study has limitations. First, we used an administrative dataset and ICD‐9‐CM codes rather than clinical data from medical records to identify cases of pneumonia or comorbidities. Our estimates of PICC use across hospitals thus may not fully account for differences in severity of illness, and it is possible that patients needed a PICC for reasons that we could not observe. However, the substantial variation observed in rates of PICC use across hospitals is unlikely to be explained by differences in patient severity of illness, documentation, or coding practices. Second, as PICC removal codes were not available, we are unable to comment on how often hospitalized pneumonia patients were discharged with PICCs or received antimicrobial therapy beyond their inpatient stay. Third, although we observed that a number of patient and hospital factors were associated with PICC receipt, our study was not designed to determine the reasons underlying these patterns.
These limitations aside, our study has important strengths. To our knowledge, this is the first study to report utilization and outcomes associated with PICC use among those hospitalized with pneumonia across the United States. The inclusion of a large number of patients receiving care in diverse facilities lends a high degree of external validity to our findings. Second, we used advanced modeling to identify factors associated with PICC use in hospitalized patients with pneumonia, producing innovative and novel findings. Third, our study is the first to show the existence of substantial variation in rates of PICC use across US hospitals within the single disease state of pneumonia. Understanding the drivers of this variability is important as it may inform future studies, policies, and practices to improve PICC use in hospitalized patients.
In conclusion, we found that PICC use in patients hospitalized with pneumonia is common and highly variable. Future studies examining the contextual factors behind PICC use and their association with outcomes are needed to facilitate efforts to standardize PICC use across hospitals.
Disclosures
Dr. Chopra is supported by a career development award (1‐K08‐HS022835‐01) from the Agency of Healthcare Research and Quality. The authors report no conflicts of interest.
- , . Reasons for being admitted to the hospital through the emergency department, 2003. Healthcare Cost and Utilization Project Statistical Brief 2. Rockville, MD: Agency for Healthcare Research and Quality. Available at: www.hcup‐us.ahrq.gov/reports/statbriefs/sb2.pdf. Published February 2006. Accessed June 27, 2014.
- , , , et al. National patterns of risk‐standardized mortality and readmission after hospitalization for acute myocardial infarction, heart failure, and pneumonia: update on publicly reported outcomes measures based on the 2013 release. J Gen Intern Med. 2014;29(10):1333–1340.
- , , , et al. Quality of care for elderly patients hospitalized for pneumonia in the United States, 2006 to 2010. JAMA Intern Med. 2014;174(11):1806–1814.
- , . PICC lines: the latest home care challenge. RN. 1990;53(1):44–51.
- , , , . Peripherally inserted central catheters in an acute‐care hospital. Arch Intern Med. 1994;154(16):1833–1837.
- , . The peripherally inserted central catheter: a retrospective look at three years of insertions. J Intraven Nurs. 1993;16(2):92–103.
- , , , . Peripherally inserted central catheters in general medicine. Mayo Clin Proc. 1997;72(3):225–233.
- , , . Two‐year trends of peripherally inserted central catheter‐line complications at a tertiary‐care hospital: role of nursing expertise. Infect Control Hosp Epidemiol. 2001;22(6):377–379.
- , , , , , . PICC‐associated bloodstream infections: prevalence, patterns, and predictors. Am J Med. 2014;127(4):319–328.
- , , , , . The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta‐analysis. Infect Control Hosp Epidemiol. 2013;34(9):908–918.
- , , , , , . Peripherally inserted central catheters: use at a tertiary care pediatric center. J Vasc Interv Radiol. 2013;24(9):1323–1331.
- , , , et al. Temporary central venous catheter utilization patterns in a large tertiary care center: tracking the “idle central venous catheter”. Infect Control Hosp Epidemiol. 2012;33(1):50–57.
- , , , , . Inappropriate intravascular device use: a prospective study. Journal Hosp Infect. 2011;78(2):128–132.
- , , , et al. Enhancing patient‐centered care: SGIM and choosing wisely. J Gen Intern Med. 2014;29(3):432–433.
- , , , et al. Critical and honest conversations: the evidence behind the “Choosing Wisely” campaign recommendations by the American Society of Nephrology. Clin J Am Soc Nephrol. 2012;7(10):1664–1672.
- , , , et al. Using highly detailed administrative data to predict pneumonia mortality. PLoS One. 2014;9(1):e87382.
- , , , , . A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749–759.
- , , , , . Hospitals with the highest intensive care utilization provide lower quality pneumonia care to the elderly. Crit Care Med. 2015;43(6):1178–1186.
- , . Statistical and clinical aspects of hospital outcomes profiling. Stat Sci. 2007;22(2):206–226.
- , . Appropriate assessment of neighborhood effects on individual health: integrating random and fixed effects in multilevel logistic regression. Am J Epidemiol. 2005;161(1):81–88.
- , , , . Interpreting parameters in the logistic regression model with random effects. Biometrics. 2000;56(3):909–914.
- , , , . Hospital‐level associations with 30‐day patient mortality after cardiac surgery: a tutorial on the application and interpretation of marginal and multilevel logistic regression. BMC Med Res Methodol. 2012;12:28.
- , , , . Experiences of the first PICC team in the Czech Republic. Br J Nurs. 2015;24(suppl 2):S4–S10.
- , , , et al. Greece reports prototype intervention with first peripherally inserted central catheter: case report and literature review. J Vasc Nurs. 2012;30(3):88–93.
- Total intravenous nutrition with peripherally inserted silicone elastomer central venous catheters. Arch Surg. 1975;110(5):644–646.
- , . Focus on peripherally inserted central catheters in critically ill patients. World J Crit Care Med. 2014;3(4):80–94.
- , , , et al. Quality improvement initiative to reduce deep vein thrombosis associated with peripherally inserted central catheters in adults with cystic fibrosis. Ann Am Thorac Soc. 2014;11(9):1404–1410.
- , , , . Peripherally Inserted central catheter (PICC) complications during pregnancy. JPEN J Parenter Enteral Nutr. 2013;38(5):595–601.
- , , , et al. Do clinicians know which of their patients have central venous catheters?: a multicenter observational study. Ann Intern Med. 2014;161(8):562–567.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013;382(9889):311–325.
- , , . The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527–1528.
- , , , et al. Peripherally inserted central catheter use in skilled nursing facilities: a pilot study. J Am Geriatr Soc. 2015;63(9):1894–1899.
- , , , , . Inpatient venous access practices: PICC culture and the kidney patient. J Vasc Access. 2015;16(3):206–210.
- , . Reasons for being admitted to the hospital through the emergency department, 2003. Healthcare Cost and Utilization Project Statistical Brief 2. Rockville, MD: Agency for Healthcare Research and Quality. Available at: www.hcup‐us.ahrq.gov/reports/statbriefs/sb2.pdf. Published February 2006. Accessed June 27, 2014.
- , , , et al. National patterns of risk‐standardized mortality and readmission after hospitalization for acute myocardial infarction, heart failure, and pneumonia: update on publicly reported outcomes measures based on the 2013 release. J Gen Intern Med. 2014;29(10):1333–1340.
- , , , et al. Quality of care for elderly patients hospitalized for pneumonia in the United States, 2006 to 2010. JAMA Intern Med. 2014;174(11):1806–1814.
- , . PICC lines: the latest home care challenge. RN. 1990;53(1):44–51.
- , , , . Peripherally inserted central catheters in an acute‐care hospital. Arch Intern Med. 1994;154(16):1833–1837.
- , . The peripherally inserted central catheter: a retrospective look at three years of insertions. J Intraven Nurs. 1993;16(2):92–103.
- , , , . Peripherally inserted central catheters in general medicine. Mayo Clin Proc. 1997;72(3):225–233.
- , , . Two‐year trends of peripherally inserted central catheter‐line complications at a tertiary‐care hospital: role of nursing expertise. Infect Control Hosp Epidemiol. 2001;22(6):377–379.
- , , , , , . PICC‐associated bloodstream infections: prevalence, patterns, and predictors. Am J Med. 2014;127(4):319–328.
- , , , , . The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta‐analysis. Infect Control Hosp Epidemiol. 2013;34(9):908–918.
- , , , , , . Peripherally inserted central catheters: use at a tertiary care pediatric center. J Vasc Interv Radiol. 2013;24(9):1323–1331.
- , , , et al. Temporary central venous catheter utilization patterns in a large tertiary care center: tracking the “idle central venous catheter”. Infect Control Hosp Epidemiol. 2012;33(1):50–57.
- , , , , . Inappropriate intravascular device use: a prospective study. Journal Hosp Infect. 2011;78(2):128–132.
- , , , et al. Enhancing patient‐centered care: SGIM and choosing wisely. J Gen Intern Med. 2014;29(3):432–433.
- , , , et al. Critical and honest conversations: the evidence behind the “Choosing Wisely” campaign recommendations by the American Society of Nephrology. Clin J Am Soc Nephrol. 2012;7(10):1664–1672.
- , , , et al. Using highly detailed administrative data to predict pneumonia mortality. PLoS One. 2014;9(1):e87382.
- , , , , . A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749–759.
- , , , , . Hospitals with the highest intensive care utilization provide lower quality pneumonia care to the elderly. Crit Care Med. 2015;43(6):1178–1186.
- , . Statistical and clinical aspects of hospital outcomes profiling. Stat Sci. 2007;22(2):206–226.
- , . Appropriate assessment of neighborhood effects on individual health: integrating random and fixed effects in multilevel logistic regression. Am J Epidemiol. 2005;161(1):81–88.
- , , , . Interpreting parameters in the logistic regression model with random effects. Biometrics. 2000;56(3):909–914.
- , , , . Hospital‐level associations with 30‐day patient mortality after cardiac surgery: a tutorial on the application and interpretation of marginal and multilevel logistic regression. BMC Med Res Methodol. 2012;12:28.
- , , , . Experiences of the first PICC team in the Czech Republic. Br J Nurs. 2015;24(suppl 2):S4–S10.
- , , , et al. Greece reports prototype intervention with first peripherally inserted central catheter: case report and literature review. J Vasc Nurs. 2012;30(3):88–93.
- Total intravenous nutrition with peripherally inserted silicone elastomer central venous catheters. Arch Surg. 1975;110(5):644–646.
- , . Focus on peripherally inserted central catheters in critically ill patients. World J Crit Care Med. 2014;3(4):80–94.
- , , , et al. Quality improvement initiative to reduce deep vein thrombosis associated with peripherally inserted central catheters in adults with cystic fibrosis. Ann Am Thorac Soc. 2014;11(9):1404–1410.
- , , , . Peripherally Inserted central catheter (PICC) complications during pregnancy. JPEN J Parenter Enteral Nutr. 2013;38(5):595–601.
- , , , et al. Do clinicians know which of their patients have central venous catheters?: a multicenter observational study. Ann Intern Med. 2014;161(8):562–567.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013;382(9889):311–325.
- , , . The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527–1528.
- , , , et al. Peripherally inserted central catheter use in skilled nursing facilities: a pilot study. J Am Geriatr Soc. 2015;63(9):1894–1899.
- , , , , . Inpatient venous access practices: PICC culture and the kidney patient. J Vasc Access. 2015;16(3):206–210.
Assessing Vascular Nursing Experience
Peripherally inserted central catheters (PICCs) are among the most prevalent of venous access devices in hospitalized patients.[1, 2] Although growing use of these devices reflects clinical advantages, such as a reduced risk of complications during insertion and durable venous access, use of PICCs is also likely related to the growth of vascular access nursing.[3, 4] A relatively new specialty, vascular access nurses obtain, maintain, and manage venous access in hospitalized patients.[4, 5] Depending on their scope of practice, these professionals are responsible not only for insertion of devices, such as peripheral intravenous catheters and PICCs, but also nontunneled central venous catheters and arterial catheters in some settings.[6]
Although a growing number of US hospitals have introduced vascular nursing teams,[7] little is known about the experience, practice, knowledge, and beliefs of vascular access nurses. This knowledge gap is relevant for hospitalists and hospital medicine as (1) vascular access nurses increasingly represent a key partner in the care of hospitalized patients; (2) the knowledge and practice of these individuals directly affects patient safety and clinical outcomes; and (3) understanding experience, practice, and beliefs of these specialists can help inform decision making and quality‐improvement efforts related to PICCs. As hospitalists increasingly order the placement of and care for patients with PICCs, they are also well suited to improve PICC practice.
Therefore, we conducted a survey of vascular access nurses employed by hospitals that participate in the Michigan Hospital Medicine Safety (HMS) Consortium, a Blue Cross Blue Shield of Michiganfunded collaborative quality initiative.[6] We aimed to understand experience, practice, knowledge, and beliefs related to PICC care and use.
METHODS
Study Setting and Participants
To quantify vascular nursing experience, practice, knowledge, and beliefs, we conducted a Web‐based survey of vascular nurses across 47 Michigan hospitals that participate in HMS. A statewide quality‐improvement initiative, HMS aims to prevent adverse events in hospitalized medical patients through the creation of a data registry and sharing of best practices. The setting and design of this multicenter initiative have been previously described.[8, 9] Although participation is voluntary, each hospital receives payment for participating in the consortium and for data collection. Because HMS has an ongoing initiative aimed at identifying and preventing PICC‐related complications, this study was particularly relevant for participating hospitals and nurses.
Each HMS site has a designated quality‐improvement lead, physician champion, and data abstractor. To coordinate distribution and dissemination of the survey, we contacted the quality‐improvement leads at each site and enquired whether their hospital employed vascular access nurses who placed PICCs. Because we were only interested in responses from vascular access nurses, HMS hospitals that did not have these providers or stated PICCs were placed by other specialists (eg, interventional radiology) were excluded. At eligible sites, we obtained the total number of vascular nurses employed so as to determine the number of eligible respondents. In this manner, a purposeful sample of vascular nurses at participating HMS hospitals was constituted.
Participation in the survey was solicited through hospital quality leads that either distributed an electronic survey link to vascular nurses at their facilities or sent us individual email addresses to contact them directly. A cover letter explaining the rationale and the purpose of the survey along with the survey link was then sent to respondents through either of these routes. The survey was administered at all HMS sites contemporaneously and kept open for a period of 5 weeks. During the 5‐week period, 2 e‐mail reminders were sent to encourage participation. As a token of appreciation, a $10 Amazon gift card was offered to those who took the survey.
Development and Validation of the Survey
We developed the survey instrument (which we call PICC1 as we hope to administer longitudinally to track changes over time) by first conducting a literature search to identify relevant evidence‐based guidelines and studies regarding vascular access nursing practices and experiences.[10, 11, 12, 13] In addition, we consulted and involved national and international leaders in vascular access nursing to ensure validity and representativeness of the questions posed. We were specifically interested in nursing background, hospital practices, types of PICCs used, use of various technologies, relationships with healthcare providers, and management of complications. To understand participant characteristics and quantify potential variation in responses, we collected basic participant data including demographics, years in practice, number of PICCs placed, leadership roles, and vascular access certification status. Based on clinical reasoning and existing studies,[14, 15] we hypothesized that responses regarding certain practices (ultrasound use, electrocardiography [ECG] guidance system use), management of complications, or perceptions regarding leadership might vary based on years of experience, number of PICCs placed, or certification status. We therefore examined these associations as prespecified subgroup analyses.
The initial survey instrument was pilot tested with vascular nurses outside of the sampling frame. Based on feedback from the pilot testers, the instrument was refined and edited to improve clarity of the questions. In addition, specific skip patterns and logic were programmed into the final survey to reduce respondent burden and allow participants to seamlessly bypass questions that were contingent on a prior response (eg, use of ECG to place PICCs would lead to a series of questions about ECG‐assisted placement only for those respondents who used the technology). This final version of the survey was tested by members of the study team (V.C., L.K., S.L.K.) and then posted to SurveyMonkey for dissemination.
Statistical Analysis
Descriptive statistics (percentage, n/N) were used to tabulate results. In accordance with our a priori hypothesis that variation to responses might be associated with respondent characteristics, responses to questions regarding insertion practice (eg, use of ultrasound, measurement of catheter:vein ratio, trimming of catheters) and approach to complications (eg, catheter occlusion, deep vein thrombosis [DVT] notification, and PICC removal in the setting of fever) were compared by respondent years in practice (dichotomized to <5 vs >5 years), volume of PICCs placed (<999 vs 1000), and certification status (yes/no). Bivariate comparisons were made using 2 or Fisher exact tests based on the number of responses in a cell as appropriate; 2‐sided with a P value <0.05 was considered statistically significant. All statistical analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC).
Ethical and Regulatory Oversight
Because our study sought to describe existing practice without collecting any individual or facility level identifiable information, the project received a Not Regulated status by the University of Michigan Medical School Institutional Review Board (HUM00088351).
RESULTS
Of 172 vascular nurses who received invitations, 140 completed the survey for a response rate of 81%. Respondents reported working in not‐for‐profit hospitals (36%), academic medical centers (29%), and for‐profit hospitals (21%). Although multiple providers (eg, interventional radiology staff and providers, physicians) placed PICCs, 95% of those surveyed reported that they placed the majority of the PICCs at their institutions. Although most respondents placed PICCs in adult patients (86%), a few also placed PICCs in pediatric populations (17%). Vascular nursing programs were largely housed in their own department, but some reported to general nursing or subspecialties such as interventional radiology, cardiology, and critical care. Most respondents indicated their facilities had written policies regarding standard insertion and care practices (87% and 95%, respectively), but only 30% had policies regarding the necessity or appropriateness of PICCs.
Experience among respondents was variable: approximately a third had placed PICCs for <5 years (28.6%), whereas 58% reported placing PICCs for 5 years Correspondingly, 26% reported having placed 100 to 500 PICCs, whereas 34% had placed 1000 or more PICCs. Only 23% of those surveyed held a dedicated vascular access certification, such as board certified in vascular access or certified registered nurse infusion, whereas 16% indicated that they served as the vascular access lead nurse for their facility. Following placement, 94% of respondents reported that their facilities tracked the number of PICCs inserted, but only 40% indicated that dwell times of devices were also recorded. Only 30% of nurses reported that their hospitals had a written policy to evaluate PICC necessity or appropriateness following placement (Table 1).
| No.* | % | |
|---|---|---|
| ||
| Participant characteristics | ||
| For how many years have you been inserting PICCs? | ||
| <5 years | 40 | 28.6% |
| 5 years | 81 | 57.9% |
| Missing | ||
| In which of the following populations do you insert PICCs? | ||
| Adult patients | 121 | 86.4% |
| Pediatric patients | 24 | 17.1% |
| Neonatal patients | 1 | 0.7% |
| In which of the following locations do you place PICCs? (Select all that apply.) | ||
| Adult medical ward | 115 | 82.1% |
| General adult surgical ward | 110 | 78.6% |
| General pediatric medical ward | 34 | 24.3% |
| General pediatric surgical ward | 24 | 17.1% |
| Adult intensive care unit | 114 | 81.4% |
| Pediatric intensive care unit | 19 | 13.6% |
| Neonatal intensive care unit | 3 | 2.1% |
| Other intensive care unit | 59 | 42.1% |
| Outpatient clinic or emergency department | 17 | 12.1% |
| Other | 10 | 7.1% |
| Approximately how many PICCs may you have placed in your career? | ||
| 099 | 15 | 10.7% |
| 100499 | 36 | 25.7% |
| 500999 | 23 | 16.4% |
| 1,000 | 47 | 33.6% |
| Are you the vascular access lead nurse for your facility or organization? | ||
| Yes | 22 | 15.7% |
| No | 98 | 70.0% |
| Do you currently hold a dedicated vascular access certification (BC‐VA, CRNI, etc.)? | ||
| Yes | 32 | 22.9% |
| No | 89 | 63.6% |
| Facility characteristics | ||
| Which of the following best describes your primary work location? | ||
| Academic medical center | 41 | 29.3% |
| For‐profit community‐based hospital or medical center | 30 | 21.4% |
| Not‐for‐profit community‐based hospital or medical center | 50 | 35.7% |
| Who inserts the most PICCs in your facility? | ||
| Vascular access nurses | 133 | 95.0% |
| Interventional radiology or other providers | 7 | 5.0% |
| In which department is vascular access nursing located? | ||
| Vascular nursing | 76 | 54.3% |
| General nursing | 38 | 27.1% |
| Interventional radiology | 15 | 10.7% |
| Other | 11 | 7.9% |
| Using your best guess, how many PICCs do you think your facility inserts each month? | ||
| <25 | 5 | 3.6% |
| 2549 | 13 | 9.3% |
| 50100 | 39 | 27.9% |
| >100 | 78 | 55.7% |
| Unknown | 2 | 1.4% |
| How many vascular access nurses are employed by your facility? | ||
| <4 | 14 | 10.0% |
| 46 | 33 | 23.6% |
| 79 | 15 | 10.7% |
| 1015 | 25 | 17.9% |
| >15 | 53 | 37.9% |
| Does your facility track the number of PICCs placed? | ||
| Yes | 132 | 94.3% |
| No | 5 | 3.6% |
| Unknown | 3 | 2.1% |
| Does your facility track the duration or dwell time of PICCs? | ||
| Yes | 56 | 40.0% |
| No | 60 | 42.9% |
| Unknown | 24 | 17.1% |
| Does your facility have a written policy regarding standard PICC insertion practices? | ||
| Yes | 122 | 87.1% |
| No | 8 | 5.7% |
| Unknown | 7 | 5.0% |
| Does your facility have a written policy regarding standard PICC care and maintenance? | ||
| Yes | 133 | 95.0% |
| No | 3 | 2.1% |
| Unknown | 1 | 0.7% |
| Does your facility have a written process to review the necessity or appropriateness of a PICC? | ||
| Yes | 42 | 30.0% |
| No | 63 | 45.0% |
| Unknown | 20 | 14.3% |
The most commonly reported indications for PICC placement included intravenous antibiotics at discharge, difficult venous access, and placement for chemotherapy in patients with cancer. Forty‐six percent of nurses indicated they had placed a PICC in a patient receiving some form of dialysis in the past several months; however, 91% of these respondents reported receiving approval from nephrology prior to placement in these patients. Although almost all nurses (91%) used ultrasound to find a suitable vein for PICC placement, a smaller percentage used ultrasound to estimate the catheter‐to‐vein ratio to prevent thrombosis (79%), and only a few (14%) documented this figure in the medical record. Three‐quarters of those surveyed (76%) indicated they used ECG‐based systems to position PICC tips at the cavoatrial junction to prevent thrombosis. Of those who used this technology, 36% still obtained chest x‐rays to verify the position of the PICC tip. According to 84% of respondents, flushing of PICCs was performed mainly by bedside nurses, whereas scheduled weekly dressing changes were most often performed by vascular access nurses (Table 2).
| Question | No. | % |
|---|---|---|
| ||
| Do you use ultrasound to find a suitable vein prior to PICC insertion? | ||
| Yes | 128 | 91.4% |
| No | 0 | 0.0% |
| Do you use ultrasound to estimate the catheter‐to‐vein ratio prior to PICC insertion? | ||
| Yes | 110 | 78.6% |
| No | 18 | 12.9% |
| When using ultrasound, do you document the catheter‐to‐vein ratio in the PICC insertion note? | ||
| Yes | 20 | 14.3% |
| No | 89 | 63.6% |
| Do you use ECG guidance‐assisted systems to place PICCs? | ||
| Yes | 106 | 75.7% |
| No | 21 | 15.0% |
| If using ECG guidance, do you still routinely obtain a chest x‐ray to verify PICC tip position after placing the PICC using ECG guidance? | ||
| Yes | 38 | 27.1% |
| No | 68 | 48.6% |
| Who is primarily responsible for administering and adhering to a flushing protocol after PICC insertion at your facility? | ||
| Bedside nurses | 118 | 83.6% |
| Patients | 1 | 0.7% |
| Vascular access nurses | 8 | 5.7% |
| Which of the following agents are most often used to flush PICCs? | ||
| Both heparin and normal saline flushes | 61 | 43.6% |
| Normal saline only | 63 | 45.0% |
| Heparin only | 3 | 2.1% |
| Who is responsible for scheduled weekly dressing changes for PICCs? | ||
| Vascular access nurses | 110 | 78.6% |
| Bedside nurses | 14 | 10.0% |
| Other (eg, IR staff, ICU staff) | 3 | 2.1% |
| In the past few months, have you placed a PICC in a patient who was receiving a form of dialysis (eg, peritoneal or hemodialysis)? | ||
| Yes | 65 | 46.4% |
| No | 64 | 45.7% |
| If you have placed PICCs in patients on dialysis, do you discuss PICC placement or receive approval from nephrology prior to inserting the PICC? | ||
| Yes | 59 | 90.8% |
| No | 6 | 9.2% |
With respect to complications, catheter occlusion, migration, and DVT were reported as the 3 most prevalent adverse events. Interestingly, respondents did not report central lineassociated bloodstream infection (CLABSI) as a common complication. Additionally, 51% of those surveyed indicated that physicians unnecessarily removed PICCs when CLABSI was suspected but not confirmed. When managing catheter occlusion, 50% of respondents began with normal saline flushes but used tissue‐plasminogen activator if saline failed to resolve occlusion. Management of catheter migration varied based on degree of device movement: when the PICC had migrated <5 cm, most respondents (77%) indicated they would first obtain a chest x‐ray to determine the position of the PICC tip, with few (4%) performing catheter exchange. However, if the PICC had migrated more than 5 cm, a significantly greater proportion of respondents (21%) indicated they would perform a catheter exchange. With regard to managing DVT, most vascular nurses reported they notified nurses and physicians to continue using the PICC but recommended tests to confirm the diagnosis.
To better understand the experiences of vascular nurses, we asked for their perceptions regarding appropriateness of PICC use and relationships with bedside nurses, physicians, and leadership. Over a third of respondents (36%) felt that <5% of all PICCs may be inappropriate in their facility, whereas 1 in 5 indicated that 10% to 24% of PICCs placed in their facilities may be inappropriate or could have been avoided. Almost all (98%) of the nurses stated they were not empowered to remove idle or clinically unnecessary PICCs without physician authorization. Although 51% of nurses described the support received from hospital leadership as excellent, very good, or good, 43% described leadership support as either fair or poor. Conversely, relationships with bedside nurses and physicians were rated as being very good or good by nearly two‐thirds of those surveyed (64% and 65%, respectively) (Table 3).
| Question | No. | % |
|---|---|---|
| ||
| Which of the following PICC‐related complications have you most frequently encountered in your practice? | ||
| Catheter occlusion | 81 | 57.9% |
| Catheter migration | 27 | 19.3% |
| PICC‐associated DVT | 6 | 4.3% |
| Catheter fracture or embolization | 3 | 2.1% |
| Exit site infection | 3 | 2.1% |
| Coiling or kinking after insertion | 2 | 1.4% |
| If you suspect a patient has catheter occlusion, which of the following best describes your approach to resolving this problem? | ||
| Begin with normal saline but use a tPA product if this fails to restore patency | 70 | 50.0% |
| Use a tPA product (eg, Cathflo, Activase, or Retavase) to restore patency | 44 | 31.4% |
| Begin with heparin‐based flushes but use a tPA product if this fails to restore | 7 | 5.0% |
| Use only normal saline flushes to restore patency | 3 | 2.1% |
| If you find a PICC that has migrated out or has been accidentally dislodged <5 cm in a patient without symptoms, and the device is still clinically needed, which of the following best describes your practice? | ||
| Obtain a chest x‐ray to verify tip position | 108 | 77.1% |
| Perform a complete catheter exchange over a guidewire if possible | 5 | 3.6% |
| Notify/discuss next steps with physician | 5 | 3.6% |
| Other | 6 | 4.3% |
| If you find a PICC that has migrated out or has been accidentally dislodged >5 cm in a patient without symptoms, and the device is still clinically needed, which of the following best describes your practice? | ||
| Obtain a chest x‐ray to verify tip position | 72 | 51.4% |
| Perform a catheter exchange over a guidewire if possible | 30 | 21.4% |
| Notify/discuss next steps with physician | 10 | 7.1% |
| Other | 12 | 8.6% |
| Which of the following best describes your first approach when you suspect a patient has PICC‐associated phlebitis? | ||
| Discuss best course of action with physician or nurse | 79 | 56.4% |
| Supportive measures (eg, warm compresses, analgesics, monitoring) | 25 | 17.9% |
| Remove the PICC | 15 | 10.7% |
| Other | 5 | 3.6% |
| Which of the following best describes your first approach when you suspect a patient has a PICC‐related DVT? | ||
| Notify caregivers to continue using PICC and consider tests such as ultrasound | 82 | 58.6% |
| Notify bedside nurse and physician not to continue use of the PICC and consider tests such as ultrasound | 42 | 30.0% |
| PICCs are often removed when physicians suspect, but have not yet confirmed, CLABSI. Considering your experiences, what percentage of PICCs may have been removed in this manner at your facility? | ||
| <5% | 11 | 7.9% |
| 59% | 16 | 11.4% |
| 1024% | 24 | 17.1% |
| 25% | 71 | 50.7% |
| Based on your experience, what percentage of PICCs do you think are inappropriate or could have been avoided at your facility? | ||
| <5% | 51 | 36.4% |
| 59% | 25 | 17.9% |
| 1024% | 28 | 20.0% |
| 2550% | 13 | 9.3% |
| >50% | 5 | 3.6% |
| Are vascular access nurses empowered to remove PICCs that are idle or clinically unnecessary without physician authorization? | ||
| Yes | 3 | 2.1% |
| No | 122 | 87.1% |
| How would you rank the overall support your vascular access service receives from hospital leadership? | ||
| Excellent | 5 | 3.6% |
| Very good | 32 | 22.9% |
| Good | 40 | 28.6% |
| Fair | 35 | 25.0% |
| Poor | 25 | 17.9% |
| How would you describe your relationship with physicians at your facility when it comes to communicating recommendations or management of PICCs? | ||
| Very good | 28 | 20.0% |
| Good | 63 | 45.0% |
| Fair | 35 | 25.0% |
| Poor | 7 | 5.0% |
| Very poor | 4 | 2.9% |
| How would you describe your relationship with bedside nurses at your facility when it comes to communicating recommendations or management of PICCs? | ||
| Very good | 32 | 22.9% |
| Good | 58 | 41.4% |
| Fair | 38 | 27.1% |
| Poor | 7 | 5.0% |
| Very poor | 2 | 1.4% |
Variation in Responses Based on Years in Practice or Certification
We initially hypothesized that responses regarding practice (ultrasound use, ECG guidance system use), management of complications, or perceptions regarding leadership might vary based on years of experience, number of PICCs placed, or certification status. However, no statistically significant associations with these factors and individual responses were identified.
DISCUSSION
In this survey of 140 vascular access nurses in hospitals across Michigan, new insights regarding the experience, practice, knowledge, and beliefs of this group of providers were obtained. We found that vascular access nurses varied with respect to years in practice, volume of PICCs placed, and certification status, reflecting heterogeneity in this provider group. Variation in insertion techniques, such as use of ultrasound to examine catheter‐to‐vein ratio (a key way to prevent thrombosis) or newer ECG technology to position the PICC, was also noted. Although indications for PICC insertion appeared consistent with published literature, the frequency with which these devices were placed in patients receiving dialysis (reportedly with nephrology approval) was surprising given national calls to avoid such use.[16] Opportunities to improve hospital practices, such as tracking PICC dwell times and PICC necessity, as well as the potential need to better educate physicians on when to remove PICCs for suspected CLABSI, were also identified. Collectively, these data are highly relevant to hospitalists and health systems as they help to identify areas for quality improvement and inform clinical practice regarding the use of PICCs in hospitalized patients. As hospitalists increasingly order PICCs and manage complications associated with these devices, they are well suited to use these data so as to improve patient safety and clinical outcomes.
Venous access is the most common medical procedure performed in hospitalized medical patients. Although a number of devices including peripheral intravenous catheters, central venous catheters, and PICCs are used for this purpose, the growing use of PICCs to secure venous access has been documented in several studies.[17] Such growth, in part, undoubtedly reflects increasing availability of vascular access nurses. Traditionally placed by interventional radiologists, the creation of dedicated vascular nursing teams has resulted in these subspecialists now serving in more of a backup or trouble‐shooting role rather than that of primary operator.[4, 14] This paradigm shift is well illustrated in a recent survey of infection preventionists, where over 60% of respondents reported that they had a vascular nursing team in their facility.[7] The growth of these nursing‐led vascular access teams has produced not only high rates of insertion success and low rates of complications, but also greater cost‐effectiveness when compared to interventional radiologybased insertion.[18]
Nonetheless, our survey also identified a number of important concerns regarding PICC practices and vascular nursing providers. First, we found variation in areas such as insertion practices and management of complications. Such variability highlights the importance of both growing and disseminating the evidence base for consistent practice in vascular nursing. Through their close clinical affiliation with vascular nurses and shared interests in obtaining safe and appropriate venous access for patients, hospitalists are ideally poised to lead this effort. Second, similarities between vascular nurse opinions regarding appropriateness of PICCs and those of hospitalists from a prior survey were noted.[19] Namely, a substantial proportion of both vascular nurses and hospitalists felt that some PICCs were inappropriate and could be avoided. Third, although relationships between vascular access nurses and leadership were reported as being variable, the survey responses suggested relatively good interprovider relationships with bedside nurses and physicians. Such relationships likely reflect the close clinical ties that emerge from being in the trenches of patient care and suggest that interventions to improve care in partnership with these providers are highly viable.
Our study has some limitations. First, despite a high response rate, our study used a survey design and reports findings from a convenience sample of vascular access nurses in a single state. Thus, nonrespondent and selection biases remain threats to our conclusions. Additionally, some respondents did not complete all responses, perhaps due to nonapplicability to practice or other unknown reasons. The pattern of missingness observed, however, suggested that such responses were missing at random. Second, we surveyed vascular nurses in hospitals that are actively engaged in improving PICC practices; our findings may therefore not be representative of vascular nursing professionals as a whole and may instead reflect those of a highly motivated group of individuals. Relatedly, the underlying reasons for adoption of specific practices or techniques cannot be discerned from our study. Third, although we did not find differences based on years in practice or certification status, our sample size was relatively small and likely underpowered for these comparisons. Finally, our study sample consists of vascular nurses who are clustered within hospitals in which they are employed. Therefore, overlap between reported practices and those required by the facility are possible.
Despite these limitations, our study has important strengths. First, this is among the most comprehensive of surveys examining vascular nursing experience, practice, knowledge, and beliefs. The growing presence of these providers across US hospitals, coupled with limited insight regarding their clinical practices, highlight the importance and utility of these data. Second, we noted important differences in experience, practices, and interprovider relationships between vascular providers in this field. Although we are unable to ascertain the drivers or significance of such variation, hospitals and health systems focused on improving patient safety should consider quantifying and exploring these factors. Third, findings from our survey within Michigan suggest the need for similar, larger studies across the country. Partnerships with nursing organizations or larger professional groups that represent vascular nursing specialists may be helpful in this regard.
In conclusion, we found important similarities and differences in vascular nursing experience, practice, knowledge, and beliefs in Michigan. These data are useful as they help provide context regarding the constitution of these teams, current practices, and opportunities for improving care. Hospitalists seeking to improve patient safety may use these data to better inform vascular access practice in hospitalized patients.
Acknowledgements
The authors thank Claire Rickard, PhD, RN, Britt Meyer, RN, Peter Carr, PhD, and David Dempsey, RN for their assistance in developing the survey instrument used in this study.
Disclosures: This project was funded through an Investigator Initiated Research Grant from the Blue Cross Blue Shield of Michigan (BCBSM) Foundation (grant number 2140.II). The funding source played no role in study design, data acquisition, analysis, or reporting of the data. Support for the Hospital Medicine Safety (HMS) Consortium is provided by BCBSM and the Blue Care Network as part of the BCBSM Value Partnerships program. Although BCBSM and HMS work collaboratively, the opinions, beliefs, and viewpoints expressed by the authors do not necessarily reflect the opinions, beliefs, and viewpoints of BCBSM or any of its employees. This work was also supported with resources from the Veterans Affairs Ann Arbor Healthcare System. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
- , , , et al. Peripherally inserted central venous catheters in the acute care setting: a safe alternative to high‐risk short‐term central venous catheters. Am J Infect Control. 2010;38(2):149–153.
- , , , , . Risk of venous thromboembolism in hospitalized patients with peripherally inserted central catheters. J Hosp Med. 2009;4(7):417–422.
- , , , , , . Central venous catheter placement by advanced practice nurses demonstrates low procedural complication and infection rates‐‐a report from 13 years of service. Crit Care Med. 2014;42(3):536–543.
- . Developing an alternative workflow model for peripherally inserted central catheter placement. J Infus Nurs. 2012;34(1):34–42.
- , . Facility wide benefits of radiology vascular access teams. Radiol Manage. 2010;32(1):28–32; quiz 33–34.
- , . Moving the needle forward: the imperative for collaboration in vascular access. J Infus Nurs. 2015;38(2):100–102.
- , , , . Use of designated PICC teams by U.S. hospitals: a survey‐based study [published online November 10, 2015]. J Patient Saf. doi: 10.1097/PTS.0000000000000246
- , , , , . The association between PICC use and venous thromboembolism in upper and lower extremities. American J Med. 2015;128(9):986–993.e1.
- , , , et al. Hospital performance for pharmacologic venous thromboembolism prophylaxis and rate of venous thromboembolism: a cohort study. JAMA Intern Med. 2014;174(10):1577–1584.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013;382(9889):311–325.
- Infusion Nurses Society. Infusion nursing standards of practice. J Infus Nurs. 2006;29(1 suppl):S1–S92.
- , , , et al. Guidelines for the prevention of intravascular catheter‐related infections. Am J Infect Control. 2011;39(4 suppl 1):S1–S34.
- , , , et al. International evidence‐based recommendations on ultrasound‐guided vascular access. Intensive Care Med. 2012;38(7):1105–1117.
- , , . A single institution experience of seven hundred consecutively placed peripherally inserted central venous catheters. J Vasc Access. 2014;15(6):498–502.
- , , . Central venous access devices site care practices: an international survey of 34 countries [published online September 3, 2015]. J Vasc Access. doi: 10.5301/jva.5000450
- American Society of Nephrology. World's Leading Kidney Society Joins Effort to Reduce Unnecessary Medical Tests and Procedures. Available at: https://www.asn‐online.org/policy/choosingwisely/PressReleaseChoosingWisely.pdf. Accessed September 4, 2015.
- , , , . A survey of the current use of peripherally inserted central venous catheter (PICC) in Swedish oncology departments. Acta Oncol. 2013;52(6):1241–1242.
- , . Nurse‐led PICC insertion: is it cost effective? Br J Nurs. 2013;22(19):S9–S15.
- , , , et al. Hospitalist experiences, practice, opinions, and knowledge regarding peripherally inserted central catheters: a Michigan survey. J Hosp Med. 2013;8(6):309–314.
Peripherally inserted central catheters (PICCs) are among the most prevalent of venous access devices in hospitalized patients.[1, 2] Although growing use of these devices reflects clinical advantages, such as a reduced risk of complications during insertion and durable venous access, use of PICCs is also likely related to the growth of vascular access nursing.[3, 4] A relatively new specialty, vascular access nurses obtain, maintain, and manage venous access in hospitalized patients.[4, 5] Depending on their scope of practice, these professionals are responsible not only for insertion of devices, such as peripheral intravenous catheters and PICCs, but also nontunneled central venous catheters and arterial catheters in some settings.[6]
Although a growing number of US hospitals have introduced vascular nursing teams,[7] little is known about the experience, practice, knowledge, and beliefs of vascular access nurses. This knowledge gap is relevant for hospitalists and hospital medicine as (1) vascular access nurses increasingly represent a key partner in the care of hospitalized patients; (2) the knowledge and practice of these individuals directly affects patient safety and clinical outcomes; and (3) understanding experience, practice, and beliefs of these specialists can help inform decision making and quality‐improvement efforts related to PICCs. As hospitalists increasingly order the placement of and care for patients with PICCs, they are also well suited to improve PICC practice.
Therefore, we conducted a survey of vascular access nurses employed by hospitals that participate in the Michigan Hospital Medicine Safety (HMS) Consortium, a Blue Cross Blue Shield of Michiganfunded collaborative quality initiative.[6] We aimed to understand experience, practice, knowledge, and beliefs related to PICC care and use.
METHODS
Study Setting and Participants
To quantify vascular nursing experience, practice, knowledge, and beliefs, we conducted a Web‐based survey of vascular nurses across 47 Michigan hospitals that participate in HMS. A statewide quality‐improvement initiative, HMS aims to prevent adverse events in hospitalized medical patients through the creation of a data registry and sharing of best practices. The setting and design of this multicenter initiative have been previously described.[8, 9] Although participation is voluntary, each hospital receives payment for participating in the consortium and for data collection. Because HMS has an ongoing initiative aimed at identifying and preventing PICC‐related complications, this study was particularly relevant for participating hospitals and nurses.
Each HMS site has a designated quality‐improvement lead, physician champion, and data abstractor. To coordinate distribution and dissemination of the survey, we contacted the quality‐improvement leads at each site and enquired whether their hospital employed vascular access nurses who placed PICCs. Because we were only interested in responses from vascular access nurses, HMS hospitals that did not have these providers or stated PICCs were placed by other specialists (eg, interventional radiology) were excluded. At eligible sites, we obtained the total number of vascular nurses employed so as to determine the number of eligible respondents. In this manner, a purposeful sample of vascular nurses at participating HMS hospitals was constituted.
Participation in the survey was solicited through hospital quality leads that either distributed an electronic survey link to vascular nurses at their facilities or sent us individual email addresses to contact them directly. A cover letter explaining the rationale and the purpose of the survey along with the survey link was then sent to respondents through either of these routes. The survey was administered at all HMS sites contemporaneously and kept open for a period of 5 weeks. During the 5‐week period, 2 e‐mail reminders were sent to encourage participation. As a token of appreciation, a $10 Amazon gift card was offered to those who took the survey.
Development and Validation of the Survey
We developed the survey instrument (which we call PICC1 as we hope to administer longitudinally to track changes over time) by first conducting a literature search to identify relevant evidence‐based guidelines and studies regarding vascular access nursing practices and experiences.[10, 11, 12, 13] In addition, we consulted and involved national and international leaders in vascular access nursing to ensure validity and representativeness of the questions posed. We were specifically interested in nursing background, hospital practices, types of PICCs used, use of various technologies, relationships with healthcare providers, and management of complications. To understand participant characteristics and quantify potential variation in responses, we collected basic participant data including demographics, years in practice, number of PICCs placed, leadership roles, and vascular access certification status. Based on clinical reasoning and existing studies,[14, 15] we hypothesized that responses regarding certain practices (ultrasound use, electrocardiography [ECG] guidance system use), management of complications, or perceptions regarding leadership might vary based on years of experience, number of PICCs placed, or certification status. We therefore examined these associations as prespecified subgroup analyses.
The initial survey instrument was pilot tested with vascular nurses outside of the sampling frame. Based on feedback from the pilot testers, the instrument was refined and edited to improve clarity of the questions. In addition, specific skip patterns and logic were programmed into the final survey to reduce respondent burden and allow participants to seamlessly bypass questions that were contingent on a prior response (eg, use of ECG to place PICCs would lead to a series of questions about ECG‐assisted placement only for those respondents who used the technology). This final version of the survey was tested by members of the study team (V.C., L.K., S.L.K.) and then posted to SurveyMonkey for dissemination.
Statistical Analysis
Descriptive statistics (percentage, n/N) were used to tabulate results. In accordance with our a priori hypothesis that variation to responses might be associated with respondent characteristics, responses to questions regarding insertion practice (eg, use of ultrasound, measurement of catheter:vein ratio, trimming of catheters) and approach to complications (eg, catheter occlusion, deep vein thrombosis [DVT] notification, and PICC removal in the setting of fever) were compared by respondent years in practice (dichotomized to <5 vs >5 years), volume of PICCs placed (<999 vs 1000), and certification status (yes/no). Bivariate comparisons were made using 2 or Fisher exact tests based on the number of responses in a cell as appropriate; 2‐sided with a P value <0.05 was considered statistically significant. All statistical analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC).
Ethical and Regulatory Oversight
Because our study sought to describe existing practice without collecting any individual or facility level identifiable information, the project received a Not Regulated status by the University of Michigan Medical School Institutional Review Board (HUM00088351).
RESULTS
Of 172 vascular nurses who received invitations, 140 completed the survey for a response rate of 81%. Respondents reported working in not‐for‐profit hospitals (36%), academic medical centers (29%), and for‐profit hospitals (21%). Although multiple providers (eg, interventional radiology staff and providers, physicians) placed PICCs, 95% of those surveyed reported that they placed the majority of the PICCs at their institutions. Although most respondents placed PICCs in adult patients (86%), a few also placed PICCs in pediatric populations (17%). Vascular nursing programs were largely housed in their own department, but some reported to general nursing or subspecialties such as interventional radiology, cardiology, and critical care. Most respondents indicated their facilities had written policies regarding standard insertion and care practices (87% and 95%, respectively), but only 30% had policies regarding the necessity or appropriateness of PICCs.
Experience among respondents was variable: approximately a third had placed PICCs for <5 years (28.6%), whereas 58% reported placing PICCs for 5 years Correspondingly, 26% reported having placed 100 to 500 PICCs, whereas 34% had placed 1000 or more PICCs. Only 23% of those surveyed held a dedicated vascular access certification, such as board certified in vascular access or certified registered nurse infusion, whereas 16% indicated that they served as the vascular access lead nurse for their facility. Following placement, 94% of respondents reported that their facilities tracked the number of PICCs inserted, but only 40% indicated that dwell times of devices were also recorded. Only 30% of nurses reported that their hospitals had a written policy to evaluate PICC necessity or appropriateness following placement (Table 1).
| No.* | % | |
|---|---|---|
| ||
| Participant characteristics | ||
| For how many years have you been inserting PICCs? | ||
| <5 years | 40 | 28.6% |
| 5 years | 81 | 57.9% |
| Missing | ||
| In which of the following populations do you insert PICCs? | ||
| Adult patients | 121 | 86.4% |
| Pediatric patients | 24 | 17.1% |
| Neonatal patients | 1 | 0.7% |
| In which of the following locations do you place PICCs? (Select all that apply.) | ||
| Adult medical ward | 115 | 82.1% |
| General adult surgical ward | 110 | 78.6% |
| General pediatric medical ward | 34 | 24.3% |
| General pediatric surgical ward | 24 | 17.1% |
| Adult intensive care unit | 114 | 81.4% |
| Pediatric intensive care unit | 19 | 13.6% |
| Neonatal intensive care unit | 3 | 2.1% |
| Other intensive care unit | 59 | 42.1% |
| Outpatient clinic or emergency department | 17 | 12.1% |
| Other | 10 | 7.1% |
| Approximately how many PICCs may you have placed in your career? | ||
| 099 | 15 | 10.7% |
| 100499 | 36 | 25.7% |
| 500999 | 23 | 16.4% |
| 1,000 | 47 | 33.6% |
| Are you the vascular access lead nurse for your facility or organization? | ||
| Yes | 22 | 15.7% |
| No | 98 | 70.0% |
| Do you currently hold a dedicated vascular access certification (BC‐VA, CRNI, etc.)? | ||
| Yes | 32 | 22.9% |
| No | 89 | 63.6% |
| Facility characteristics | ||
| Which of the following best describes your primary work location? | ||
| Academic medical center | 41 | 29.3% |
| For‐profit community‐based hospital or medical center | 30 | 21.4% |
| Not‐for‐profit community‐based hospital or medical center | 50 | 35.7% |
| Who inserts the most PICCs in your facility? | ||
| Vascular access nurses | 133 | 95.0% |
| Interventional radiology or other providers | 7 | 5.0% |
| In which department is vascular access nursing located? | ||
| Vascular nursing | 76 | 54.3% |
| General nursing | 38 | 27.1% |
| Interventional radiology | 15 | 10.7% |
| Other | 11 | 7.9% |
| Using your best guess, how many PICCs do you think your facility inserts each month? | ||
| <25 | 5 | 3.6% |
| 2549 | 13 | 9.3% |
| 50100 | 39 | 27.9% |
| >100 | 78 | 55.7% |
| Unknown | 2 | 1.4% |
| How many vascular access nurses are employed by your facility? | ||
| <4 | 14 | 10.0% |
| 46 | 33 | 23.6% |
| 79 | 15 | 10.7% |
| 1015 | 25 | 17.9% |
| >15 | 53 | 37.9% |
| Does your facility track the number of PICCs placed? | ||
| Yes | 132 | 94.3% |
| No | 5 | 3.6% |
| Unknown | 3 | 2.1% |
| Does your facility track the duration or dwell time of PICCs? | ||
| Yes | 56 | 40.0% |
| No | 60 | 42.9% |
| Unknown | 24 | 17.1% |
| Does your facility have a written policy regarding standard PICC insertion practices? | ||
| Yes | 122 | 87.1% |
| No | 8 | 5.7% |
| Unknown | 7 | 5.0% |
| Does your facility have a written policy regarding standard PICC care and maintenance? | ||
| Yes | 133 | 95.0% |
| No | 3 | 2.1% |
| Unknown | 1 | 0.7% |
| Does your facility have a written process to review the necessity or appropriateness of a PICC? | ||
| Yes | 42 | 30.0% |
| No | 63 | 45.0% |
| Unknown | 20 | 14.3% |
The most commonly reported indications for PICC placement included intravenous antibiotics at discharge, difficult venous access, and placement for chemotherapy in patients with cancer. Forty‐six percent of nurses indicated they had placed a PICC in a patient receiving some form of dialysis in the past several months; however, 91% of these respondents reported receiving approval from nephrology prior to placement in these patients. Although almost all nurses (91%) used ultrasound to find a suitable vein for PICC placement, a smaller percentage used ultrasound to estimate the catheter‐to‐vein ratio to prevent thrombosis (79%), and only a few (14%) documented this figure in the medical record. Three‐quarters of those surveyed (76%) indicated they used ECG‐based systems to position PICC tips at the cavoatrial junction to prevent thrombosis. Of those who used this technology, 36% still obtained chest x‐rays to verify the position of the PICC tip. According to 84% of respondents, flushing of PICCs was performed mainly by bedside nurses, whereas scheduled weekly dressing changes were most often performed by vascular access nurses (Table 2).
| Question | No. | % |
|---|---|---|
| ||
| Do you use ultrasound to find a suitable vein prior to PICC insertion? | ||
| Yes | 128 | 91.4% |
| No | 0 | 0.0% |
| Do you use ultrasound to estimate the catheter‐to‐vein ratio prior to PICC insertion? | ||
| Yes | 110 | 78.6% |
| No | 18 | 12.9% |
| When using ultrasound, do you document the catheter‐to‐vein ratio in the PICC insertion note? | ||
| Yes | 20 | 14.3% |
| No | 89 | 63.6% |
| Do you use ECG guidance‐assisted systems to place PICCs? | ||
| Yes | 106 | 75.7% |
| No | 21 | 15.0% |
| If using ECG guidance, do you still routinely obtain a chest x‐ray to verify PICC tip position after placing the PICC using ECG guidance? | ||
| Yes | 38 | 27.1% |
| No | 68 | 48.6% |
| Who is primarily responsible for administering and adhering to a flushing protocol after PICC insertion at your facility? | ||
| Bedside nurses | 118 | 83.6% |
| Patients | 1 | 0.7% |
| Vascular access nurses | 8 | 5.7% |
| Which of the following agents are most often used to flush PICCs? | ||
| Both heparin and normal saline flushes | 61 | 43.6% |
| Normal saline only | 63 | 45.0% |
| Heparin only | 3 | 2.1% |
| Who is responsible for scheduled weekly dressing changes for PICCs? | ||
| Vascular access nurses | 110 | 78.6% |
| Bedside nurses | 14 | 10.0% |
| Other (eg, IR staff, ICU staff) | 3 | 2.1% |
| In the past few months, have you placed a PICC in a patient who was receiving a form of dialysis (eg, peritoneal or hemodialysis)? | ||
| Yes | 65 | 46.4% |
| No | 64 | 45.7% |
| If you have placed PICCs in patients on dialysis, do you discuss PICC placement or receive approval from nephrology prior to inserting the PICC? | ||
| Yes | 59 | 90.8% |
| No | 6 | 9.2% |
With respect to complications, catheter occlusion, migration, and DVT were reported as the 3 most prevalent adverse events. Interestingly, respondents did not report central lineassociated bloodstream infection (CLABSI) as a common complication. Additionally, 51% of those surveyed indicated that physicians unnecessarily removed PICCs when CLABSI was suspected but not confirmed. When managing catheter occlusion, 50% of respondents began with normal saline flushes but used tissue‐plasminogen activator if saline failed to resolve occlusion. Management of catheter migration varied based on degree of device movement: when the PICC had migrated <5 cm, most respondents (77%) indicated they would first obtain a chest x‐ray to determine the position of the PICC tip, with few (4%) performing catheter exchange. However, if the PICC had migrated more than 5 cm, a significantly greater proportion of respondents (21%) indicated they would perform a catheter exchange. With regard to managing DVT, most vascular nurses reported they notified nurses and physicians to continue using the PICC but recommended tests to confirm the diagnosis.
To better understand the experiences of vascular nurses, we asked for their perceptions regarding appropriateness of PICC use and relationships with bedside nurses, physicians, and leadership. Over a third of respondents (36%) felt that <5% of all PICCs may be inappropriate in their facility, whereas 1 in 5 indicated that 10% to 24% of PICCs placed in their facilities may be inappropriate or could have been avoided. Almost all (98%) of the nurses stated they were not empowered to remove idle or clinically unnecessary PICCs without physician authorization. Although 51% of nurses described the support received from hospital leadership as excellent, very good, or good, 43% described leadership support as either fair or poor. Conversely, relationships with bedside nurses and physicians were rated as being very good or good by nearly two‐thirds of those surveyed (64% and 65%, respectively) (Table 3).
| Question | No. | % |
|---|---|---|
| ||
| Which of the following PICC‐related complications have you most frequently encountered in your practice? | ||
| Catheter occlusion | 81 | 57.9% |
| Catheter migration | 27 | 19.3% |
| PICC‐associated DVT | 6 | 4.3% |
| Catheter fracture or embolization | 3 | 2.1% |
| Exit site infection | 3 | 2.1% |
| Coiling or kinking after insertion | 2 | 1.4% |
| If you suspect a patient has catheter occlusion, which of the following best describes your approach to resolving this problem? | ||
| Begin with normal saline but use a tPA product if this fails to restore patency | 70 | 50.0% |
| Use a tPA product (eg, Cathflo, Activase, or Retavase) to restore patency | 44 | 31.4% |
| Begin with heparin‐based flushes but use a tPA product if this fails to restore | 7 | 5.0% |
| Use only normal saline flushes to restore patency | 3 | 2.1% |
| If you find a PICC that has migrated out or has been accidentally dislodged <5 cm in a patient without symptoms, and the device is still clinically needed, which of the following best describes your practice? | ||
| Obtain a chest x‐ray to verify tip position | 108 | 77.1% |
| Perform a complete catheter exchange over a guidewire if possible | 5 | 3.6% |
| Notify/discuss next steps with physician | 5 | 3.6% |
| Other | 6 | 4.3% |
| If you find a PICC that has migrated out or has been accidentally dislodged >5 cm in a patient without symptoms, and the device is still clinically needed, which of the following best describes your practice? | ||
| Obtain a chest x‐ray to verify tip position | 72 | 51.4% |
| Perform a catheter exchange over a guidewire if possible | 30 | 21.4% |
| Notify/discuss next steps with physician | 10 | 7.1% |
| Other | 12 | 8.6% |
| Which of the following best describes your first approach when you suspect a patient has PICC‐associated phlebitis? | ||
| Discuss best course of action with physician or nurse | 79 | 56.4% |
| Supportive measures (eg, warm compresses, analgesics, monitoring) | 25 | 17.9% |
| Remove the PICC | 15 | 10.7% |
| Other | 5 | 3.6% |
| Which of the following best describes your first approach when you suspect a patient has a PICC‐related DVT? | ||
| Notify caregivers to continue using PICC and consider tests such as ultrasound | 82 | 58.6% |
| Notify bedside nurse and physician not to continue use of the PICC and consider tests such as ultrasound | 42 | 30.0% |
| PICCs are often removed when physicians suspect, but have not yet confirmed, CLABSI. Considering your experiences, what percentage of PICCs may have been removed in this manner at your facility? | ||
| <5% | 11 | 7.9% |
| 59% | 16 | 11.4% |
| 1024% | 24 | 17.1% |
| 25% | 71 | 50.7% |
| Based on your experience, what percentage of PICCs do you think are inappropriate or could have been avoided at your facility? | ||
| <5% | 51 | 36.4% |
| 59% | 25 | 17.9% |
| 1024% | 28 | 20.0% |
| 2550% | 13 | 9.3% |
| >50% | 5 | 3.6% |
| Are vascular access nurses empowered to remove PICCs that are idle or clinically unnecessary without physician authorization? | ||
| Yes | 3 | 2.1% |
| No | 122 | 87.1% |
| How would you rank the overall support your vascular access service receives from hospital leadership? | ||
| Excellent | 5 | 3.6% |
| Very good | 32 | 22.9% |
| Good | 40 | 28.6% |
| Fair | 35 | 25.0% |
| Poor | 25 | 17.9% |
| How would you describe your relationship with physicians at your facility when it comes to communicating recommendations or management of PICCs? | ||
| Very good | 28 | 20.0% |
| Good | 63 | 45.0% |
| Fair | 35 | 25.0% |
| Poor | 7 | 5.0% |
| Very poor | 4 | 2.9% |
| How would you describe your relationship with bedside nurses at your facility when it comes to communicating recommendations or management of PICCs? | ||
| Very good | 32 | 22.9% |
| Good | 58 | 41.4% |
| Fair | 38 | 27.1% |
| Poor | 7 | 5.0% |
| Very poor | 2 | 1.4% |
Variation in Responses Based on Years in Practice or Certification
We initially hypothesized that responses regarding practice (ultrasound use, ECG guidance system use), management of complications, or perceptions regarding leadership might vary based on years of experience, number of PICCs placed, or certification status. However, no statistically significant associations with these factors and individual responses were identified.
DISCUSSION
In this survey of 140 vascular access nurses in hospitals across Michigan, new insights regarding the experience, practice, knowledge, and beliefs of this group of providers were obtained. We found that vascular access nurses varied with respect to years in practice, volume of PICCs placed, and certification status, reflecting heterogeneity in this provider group. Variation in insertion techniques, such as use of ultrasound to examine catheter‐to‐vein ratio (a key way to prevent thrombosis) or newer ECG technology to position the PICC, was also noted. Although indications for PICC insertion appeared consistent with published literature, the frequency with which these devices were placed in patients receiving dialysis (reportedly with nephrology approval) was surprising given national calls to avoid such use.[16] Opportunities to improve hospital practices, such as tracking PICC dwell times and PICC necessity, as well as the potential need to better educate physicians on when to remove PICCs for suspected CLABSI, were also identified. Collectively, these data are highly relevant to hospitalists and health systems as they help to identify areas for quality improvement and inform clinical practice regarding the use of PICCs in hospitalized patients. As hospitalists increasingly order PICCs and manage complications associated with these devices, they are well suited to use these data so as to improve patient safety and clinical outcomes.
Venous access is the most common medical procedure performed in hospitalized medical patients. Although a number of devices including peripheral intravenous catheters, central venous catheters, and PICCs are used for this purpose, the growing use of PICCs to secure venous access has been documented in several studies.[17] Such growth, in part, undoubtedly reflects increasing availability of vascular access nurses. Traditionally placed by interventional radiologists, the creation of dedicated vascular nursing teams has resulted in these subspecialists now serving in more of a backup or trouble‐shooting role rather than that of primary operator.[4, 14] This paradigm shift is well illustrated in a recent survey of infection preventionists, where over 60% of respondents reported that they had a vascular nursing team in their facility.[7] The growth of these nursing‐led vascular access teams has produced not only high rates of insertion success and low rates of complications, but also greater cost‐effectiveness when compared to interventional radiologybased insertion.[18]
Nonetheless, our survey also identified a number of important concerns regarding PICC practices and vascular nursing providers. First, we found variation in areas such as insertion practices and management of complications. Such variability highlights the importance of both growing and disseminating the evidence base for consistent practice in vascular nursing. Through their close clinical affiliation with vascular nurses and shared interests in obtaining safe and appropriate venous access for patients, hospitalists are ideally poised to lead this effort. Second, similarities between vascular nurse opinions regarding appropriateness of PICCs and those of hospitalists from a prior survey were noted.[19] Namely, a substantial proportion of both vascular nurses and hospitalists felt that some PICCs were inappropriate and could be avoided. Third, although relationships between vascular access nurses and leadership were reported as being variable, the survey responses suggested relatively good interprovider relationships with bedside nurses and physicians. Such relationships likely reflect the close clinical ties that emerge from being in the trenches of patient care and suggest that interventions to improve care in partnership with these providers are highly viable.
Our study has some limitations. First, despite a high response rate, our study used a survey design and reports findings from a convenience sample of vascular access nurses in a single state. Thus, nonrespondent and selection biases remain threats to our conclusions. Additionally, some respondents did not complete all responses, perhaps due to nonapplicability to practice or other unknown reasons. The pattern of missingness observed, however, suggested that such responses were missing at random. Second, we surveyed vascular nurses in hospitals that are actively engaged in improving PICC practices; our findings may therefore not be representative of vascular nursing professionals as a whole and may instead reflect those of a highly motivated group of individuals. Relatedly, the underlying reasons for adoption of specific practices or techniques cannot be discerned from our study. Third, although we did not find differences based on years in practice or certification status, our sample size was relatively small and likely underpowered for these comparisons. Finally, our study sample consists of vascular nurses who are clustered within hospitals in which they are employed. Therefore, overlap between reported practices and those required by the facility are possible.
Despite these limitations, our study has important strengths. First, this is among the most comprehensive of surveys examining vascular nursing experience, practice, knowledge, and beliefs. The growing presence of these providers across US hospitals, coupled with limited insight regarding their clinical practices, highlight the importance and utility of these data. Second, we noted important differences in experience, practices, and interprovider relationships between vascular providers in this field. Although we are unable to ascertain the drivers or significance of such variation, hospitals and health systems focused on improving patient safety should consider quantifying and exploring these factors. Third, findings from our survey within Michigan suggest the need for similar, larger studies across the country. Partnerships with nursing organizations or larger professional groups that represent vascular nursing specialists may be helpful in this regard.
In conclusion, we found important similarities and differences in vascular nursing experience, practice, knowledge, and beliefs in Michigan. These data are useful as they help provide context regarding the constitution of these teams, current practices, and opportunities for improving care. Hospitalists seeking to improve patient safety may use these data to better inform vascular access practice in hospitalized patients.
Acknowledgements
The authors thank Claire Rickard, PhD, RN, Britt Meyer, RN, Peter Carr, PhD, and David Dempsey, RN for their assistance in developing the survey instrument used in this study.
Disclosures: This project was funded through an Investigator Initiated Research Grant from the Blue Cross Blue Shield of Michigan (BCBSM) Foundation (grant number 2140.II). The funding source played no role in study design, data acquisition, analysis, or reporting of the data. Support for the Hospital Medicine Safety (HMS) Consortium is provided by BCBSM and the Blue Care Network as part of the BCBSM Value Partnerships program. Although BCBSM and HMS work collaboratively, the opinions, beliefs, and viewpoints expressed by the authors do not necessarily reflect the opinions, beliefs, and viewpoints of BCBSM or any of its employees. This work was also supported with resources from the Veterans Affairs Ann Arbor Healthcare System. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Peripherally inserted central catheters (PICCs) are among the most prevalent of venous access devices in hospitalized patients.[1, 2] Although growing use of these devices reflects clinical advantages, such as a reduced risk of complications during insertion and durable venous access, use of PICCs is also likely related to the growth of vascular access nursing.[3, 4] A relatively new specialty, vascular access nurses obtain, maintain, and manage venous access in hospitalized patients.[4, 5] Depending on their scope of practice, these professionals are responsible not only for insertion of devices, such as peripheral intravenous catheters and PICCs, but also nontunneled central venous catheters and arterial catheters in some settings.[6]
Although a growing number of US hospitals have introduced vascular nursing teams,[7] little is known about the experience, practice, knowledge, and beliefs of vascular access nurses. This knowledge gap is relevant for hospitalists and hospital medicine as (1) vascular access nurses increasingly represent a key partner in the care of hospitalized patients; (2) the knowledge and practice of these individuals directly affects patient safety and clinical outcomes; and (3) understanding experience, practice, and beliefs of these specialists can help inform decision making and quality‐improvement efforts related to PICCs. As hospitalists increasingly order the placement of and care for patients with PICCs, they are also well suited to improve PICC practice.
Therefore, we conducted a survey of vascular access nurses employed by hospitals that participate in the Michigan Hospital Medicine Safety (HMS) Consortium, a Blue Cross Blue Shield of Michiganfunded collaborative quality initiative.[6] We aimed to understand experience, practice, knowledge, and beliefs related to PICC care and use.
METHODS
Study Setting and Participants
To quantify vascular nursing experience, practice, knowledge, and beliefs, we conducted a Web‐based survey of vascular nurses across 47 Michigan hospitals that participate in HMS. A statewide quality‐improvement initiative, HMS aims to prevent adverse events in hospitalized medical patients through the creation of a data registry and sharing of best practices. The setting and design of this multicenter initiative have been previously described.[8, 9] Although participation is voluntary, each hospital receives payment for participating in the consortium and for data collection. Because HMS has an ongoing initiative aimed at identifying and preventing PICC‐related complications, this study was particularly relevant for participating hospitals and nurses.
Each HMS site has a designated quality‐improvement lead, physician champion, and data abstractor. To coordinate distribution and dissemination of the survey, we contacted the quality‐improvement leads at each site and enquired whether their hospital employed vascular access nurses who placed PICCs. Because we were only interested in responses from vascular access nurses, HMS hospitals that did not have these providers or stated PICCs were placed by other specialists (eg, interventional radiology) were excluded. At eligible sites, we obtained the total number of vascular nurses employed so as to determine the number of eligible respondents. In this manner, a purposeful sample of vascular nurses at participating HMS hospitals was constituted.
Participation in the survey was solicited through hospital quality leads that either distributed an electronic survey link to vascular nurses at their facilities or sent us individual email addresses to contact them directly. A cover letter explaining the rationale and the purpose of the survey along with the survey link was then sent to respondents through either of these routes. The survey was administered at all HMS sites contemporaneously and kept open for a period of 5 weeks. During the 5‐week period, 2 e‐mail reminders were sent to encourage participation. As a token of appreciation, a $10 Amazon gift card was offered to those who took the survey.
Development and Validation of the Survey
We developed the survey instrument (which we call PICC1 as we hope to administer longitudinally to track changes over time) by first conducting a literature search to identify relevant evidence‐based guidelines and studies regarding vascular access nursing practices and experiences.[10, 11, 12, 13] In addition, we consulted and involved national and international leaders in vascular access nursing to ensure validity and representativeness of the questions posed. We were specifically interested in nursing background, hospital practices, types of PICCs used, use of various technologies, relationships with healthcare providers, and management of complications. To understand participant characteristics and quantify potential variation in responses, we collected basic participant data including demographics, years in practice, number of PICCs placed, leadership roles, and vascular access certification status. Based on clinical reasoning and existing studies,[14, 15] we hypothesized that responses regarding certain practices (ultrasound use, electrocardiography [ECG] guidance system use), management of complications, or perceptions regarding leadership might vary based on years of experience, number of PICCs placed, or certification status. We therefore examined these associations as prespecified subgroup analyses.
The initial survey instrument was pilot tested with vascular nurses outside of the sampling frame. Based on feedback from the pilot testers, the instrument was refined and edited to improve clarity of the questions. In addition, specific skip patterns and logic were programmed into the final survey to reduce respondent burden and allow participants to seamlessly bypass questions that were contingent on a prior response (eg, use of ECG to place PICCs would lead to a series of questions about ECG‐assisted placement only for those respondents who used the technology). This final version of the survey was tested by members of the study team (V.C., L.K., S.L.K.) and then posted to SurveyMonkey for dissemination.
Statistical Analysis
Descriptive statistics (percentage, n/N) were used to tabulate results. In accordance with our a priori hypothesis that variation to responses might be associated with respondent characteristics, responses to questions regarding insertion practice (eg, use of ultrasound, measurement of catheter:vein ratio, trimming of catheters) and approach to complications (eg, catheter occlusion, deep vein thrombosis [DVT] notification, and PICC removal in the setting of fever) were compared by respondent years in practice (dichotomized to <5 vs >5 years), volume of PICCs placed (<999 vs 1000), and certification status (yes/no). Bivariate comparisons were made using 2 or Fisher exact tests based on the number of responses in a cell as appropriate; 2‐sided with a P value <0.05 was considered statistically significant. All statistical analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC).
Ethical and Regulatory Oversight
Because our study sought to describe existing practice without collecting any individual or facility level identifiable information, the project received a Not Regulated status by the University of Michigan Medical School Institutional Review Board (HUM00088351).
RESULTS
Of 172 vascular nurses who received invitations, 140 completed the survey for a response rate of 81%. Respondents reported working in not‐for‐profit hospitals (36%), academic medical centers (29%), and for‐profit hospitals (21%). Although multiple providers (eg, interventional radiology staff and providers, physicians) placed PICCs, 95% of those surveyed reported that they placed the majority of the PICCs at their institutions. Although most respondents placed PICCs in adult patients (86%), a few also placed PICCs in pediatric populations (17%). Vascular nursing programs were largely housed in their own department, but some reported to general nursing or subspecialties such as interventional radiology, cardiology, and critical care. Most respondents indicated their facilities had written policies regarding standard insertion and care practices (87% and 95%, respectively), but only 30% had policies regarding the necessity or appropriateness of PICCs.
Experience among respondents was variable: approximately a third had placed PICCs for <5 years (28.6%), whereas 58% reported placing PICCs for 5 years Correspondingly, 26% reported having placed 100 to 500 PICCs, whereas 34% had placed 1000 or more PICCs. Only 23% of those surveyed held a dedicated vascular access certification, such as board certified in vascular access or certified registered nurse infusion, whereas 16% indicated that they served as the vascular access lead nurse for their facility. Following placement, 94% of respondents reported that their facilities tracked the number of PICCs inserted, but only 40% indicated that dwell times of devices were also recorded. Only 30% of nurses reported that their hospitals had a written policy to evaluate PICC necessity or appropriateness following placement (Table 1).
| No.* | % | |
|---|---|---|
| ||
| Participant characteristics | ||
| For how many years have you been inserting PICCs? | ||
| <5 years | 40 | 28.6% |
| 5 years | 81 | 57.9% |
| Missing | ||
| In which of the following populations do you insert PICCs? | ||
| Adult patients | 121 | 86.4% |
| Pediatric patients | 24 | 17.1% |
| Neonatal patients | 1 | 0.7% |
| In which of the following locations do you place PICCs? (Select all that apply.) | ||
| Adult medical ward | 115 | 82.1% |
| General adult surgical ward | 110 | 78.6% |
| General pediatric medical ward | 34 | 24.3% |
| General pediatric surgical ward | 24 | 17.1% |
| Adult intensive care unit | 114 | 81.4% |
| Pediatric intensive care unit | 19 | 13.6% |
| Neonatal intensive care unit | 3 | 2.1% |
| Other intensive care unit | 59 | 42.1% |
| Outpatient clinic or emergency department | 17 | 12.1% |
| Other | 10 | 7.1% |
| Approximately how many PICCs may you have placed in your career? | ||
| 099 | 15 | 10.7% |
| 100499 | 36 | 25.7% |
| 500999 | 23 | 16.4% |
| 1,000 | 47 | 33.6% |
| Are you the vascular access lead nurse for your facility or organization? | ||
| Yes | 22 | 15.7% |
| No | 98 | 70.0% |
| Do you currently hold a dedicated vascular access certification (BC‐VA, CRNI, etc.)? | ||
| Yes | 32 | 22.9% |
| No | 89 | 63.6% |
| Facility characteristics | ||
| Which of the following best describes your primary work location? | ||
| Academic medical center | 41 | 29.3% |
| For‐profit community‐based hospital or medical center | 30 | 21.4% |
| Not‐for‐profit community‐based hospital or medical center | 50 | 35.7% |
| Who inserts the most PICCs in your facility? | ||
| Vascular access nurses | 133 | 95.0% |
| Interventional radiology or other providers | 7 | 5.0% |
| In which department is vascular access nursing located? | ||
| Vascular nursing | 76 | 54.3% |
| General nursing | 38 | 27.1% |
| Interventional radiology | 15 | 10.7% |
| Other | 11 | 7.9% |
| Using your best guess, how many PICCs do you think your facility inserts each month? | ||
| <25 | 5 | 3.6% |
| 2549 | 13 | 9.3% |
| 50100 | 39 | 27.9% |
| >100 | 78 | 55.7% |
| Unknown | 2 | 1.4% |
| How many vascular access nurses are employed by your facility? | ||
| <4 | 14 | 10.0% |
| 46 | 33 | 23.6% |
| 79 | 15 | 10.7% |
| 1015 | 25 | 17.9% |
| >15 | 53 | 37.9% |
| Does your facility track the number of PICCs placed? | ||
| Yes | 132 | 94.3% |
| No | 5 | 3.6% |
| Unknown | 3 | 2.1% |
| Does your facility track the duration or dwell time of PICCs? | ||
| Yes | 56 | 40.0% |
| No | 60 | 42.9% |
| Unknown | 24 | 17.1% |
| Does your facility have a written policy regarding standard PICC insertion practices? | ||
| Yes | 122 | 87.1% |
| No | 8 | 5.7% |
| Unknown | 7 | 5.0% |
| Does your facility have a written policy regarding standard PICC care and maintenance? | ||
| Yes | 133 | 95.0% |
| No | 3 | 2.1% |
| Unknown | 1 | 0.7% |
| Does your facility have a written process to review the necessity or appropriateness of a PICC? | ||
| Yes | 42 | 30.0% |
| No | 63 | 45.0% |
| Unknown | 20 | 14.3% |
The most commonly reported indications for PICC placement included intravenous antibiotics at discharge, difficult venous access, and placement for chemotherapy in patients with cancer. Forty‐six percent of nurses indicated they had placed a PICC in a patient receiving some form of dialysis in the past several months; however, 91% of these respondents reported receiving approval from nephrology prior to placement in these patients. Although almost all nurses (91%) used ultrasound to find a suitable vein for PICC placement, a smaller percentage used ultrasound to estimate the catheter‐to‐vein ratio to prevent thrombosis (79%), and only a few (14%) documented this figure in the medical record. Three‐quarters of those surveyed (76%) indicated they used ECG‐based systems to position PICC tips at the cavoatrial junction to prevent thrombosis. Of those who used this technology, 36% still obtained chest x‐rays to verify the position of the PICC tip. According to 84% of respondents, flushing of PICCs was performed mainly by bedside nurses, whereas scheduled weekly dressing changes were most often performed by vascular access nurses (Table 2).
| Question | No. | % |
|---|---|---|
| ||
| Do you use ultrasound to find a suitable vein prior to PICC insertion? | ||
| Yes | 128 | 91.4% |
| No | 0 | 0.0% |
| Do you use ultrasound to estimate the catheter‐to‐vein ratio prior to PICC insertion? | ||
| Yes | 110 | 78.6% |
| No | 18 | 12.9% |
| When using ultrasound, do you document the catheter‐to‐vein ratio in the PICC insertion note? | ||
| Yes | 20 | 14.3% |
| No | 89 | 63.6% |
| Do you use ECG guidance‐assisted systems to place PICCs? | ||
| Yes | 106 | 75.7% |
| No | 21 | 15.0% |
| If using ECG guidance, do you still routinely obtain a chest x‐ray to verify PICC tip position after placing the PICC using ECG guidance? | ||
| Yes | 38 | 27.1% |
| No | 68 | 48.6% |
| Who is primarily responsible for administering and adhering to a flushing protocol after PICC insertion at your facility? | ||
| Bedside nurses | 118 | 83.6% |
| Patients | 1 | 0.7% |
| Vascular access nurses | 8 | 5.7% |
| Which of the following agents are most often used to flush PICCs? | ||
| Both heparin and normal saline flushes | 61 | 43.6% |
| Normal saline only | 63 | 45.0% |
| Heparin only | 3 | 2.1% |
| Who is responsible for scheduled weekly dressing changes for PICCs? | ||
| Vascular access nurses | 110 | 78.6% |
| Bedside nurses | 14 | 10.0% |
| Other (eg, IR staff, ICU staff) | 3 | 2.1% |
| In the past few months, have you placed a PICC in a patient who was receiving a form of dialysis (eg, peritoneal or hemodialysis)? | ||
| Yes | 65 | 46.4% |
| No | 64 | 45.7% |
| If you have placed PICCs in patients on dialysis, do you discuss PICC placement or receive approval from nephrology prior to inserting the PICC? | ||
| Yes | 59 | 90.8% |
| No | 6 | 9.2% |
With respect to complications, catheter occlusion, migration, and DVT were reported as the 3 most prevalent adverse events. Interestingly, respondents did not report central lineassociated bloodstream infection (CLABSI) as a common complication. Additionally, 51% of those surveyed indicated that physicians unnecessarily removed PICCs when CLABSI was suspected but not confirmed. When managing catheter occlusion, 50% of respondents began with normal saline flushes but used tissue‐plasminogen activator if saline failed to resolve occlusion. Management of catheter migration varied based on degree of device movement: when the PICC had migrated <5 cm, most respondents (77%) indicated they would first obtain a chest x‐ray to determine the position of the PICC tip, with few (4%) performing catheter exchange. However, if the PICC had migrated more than 5 cm, a significantly greater proportion of respondents (21%) indicated they would perform a catheter exchange. With regard to managing DVT, most vascular nurses reported they notified nurses and physicians to continue using the PICC but recommended tests to confirm the diagnosis.
To better understand the experiences of vascular nurses, we asked for their perceptions regarding appropriateness of PICC use and relationships with bedside nurses, physicians, and leadership. Over a third of respondents (36%) felt that <5% of all PICCs may be inappropriate in their facility, whereas 1 in 5 indicated that 10% to 24% of PICCs placed in their facilities may be inappropriate or could have been avoided. Almost all (98%) of the nurses stated they were not empowered to remove idle or clinically unnecessary PICCs without physician authorization. Although 51% of nurses described the support received from hospital leadership as excellent, very good, or good, 43% described leadership support as either fair or poor. Conversely, relationships with bedside nurses and physicians were rated as being very good or good by nearly two‐thirds of those surveyed (64% and 65%, respectively) (Table 3).
| Question | No. | % |
|---|---|---|
| ||
| Which of the following PICC‐related complications have you most frequently encountered in your practice? | ||
| Catheter occlusion | 81 | 57.9% |
| Catheter migration | 27 | 19.3% |
| PICC‐associated DVT | 6 | 4.3% |
| Catheter fracture or embolization | 3 | 2.1% |
| Exit site infection | 3 | 2.1% |
| Coiling or kinking after insertion | 2 | 1.4% |
| If you suspect a patient has catheter occlusion, which of the following best describes your approach to resolving this problem? | ||
| Begin with normal saline but use a tPA product if this fails to restore patency | 70 | 50.0% |
| Use a tPA product (eg, Cathflo, Activase, or Retavase) to restore patency | 44 | 31.4% |
| Begin with heparin‐based flushes but use a tPA product if this fails to restore | 7 | 5.0% |
| Use only normal saline flushes to restore patency | 3 | 2.1% |
| If you find a PICC that has migrated out or has been accidentally dislodged <5 cm in a patient without symptoms, and the device is still clinically needed, which of the following best describes your practice? | ||
| Obtain a chest x‐ray to verify tip position | 108 | 77.1% |
| Perform a complete catheter exchange over a guidewire if possible | 5 | 3.6% |
| Notify/discuss next steps with physician | 5 | 3.6% |
| Other | 6 | 4.3% |
| If you find a PICC that has migrated out or has been accidentally dislodged >5 cm in a patient without symptoms, and the device is still clinically needed, which of the following best describes your practice? | ||
| Obtain a chest x‐ray to verify tip position | 72 | 51.4% |
| Perform a catheter exchange over a guidewire if possible | 30 | 21.4% |
| Notify/discuss next steps with physician | 10 | 7.1% |
| Other | 12 | 8.6% |
| Which of the following best describes your first approach when you suspect a patient has PICC‐associated phlebitis? | ||
| Discuss best course of action with physician or nurse | 79 | 56.4% |
| Supportive measures (eg, warm compresses, analgesics, monitoring) | 25 | 17.9% |
| Remove the PICC | 15 | 10.7% |
| Other | 5 | 3.6% |
| Which of the following best describes your first approach when you suspect a patient has a PICC‐related DVT? | ||
| Notify caregivers to continue using PICC and consider tests such as ultrasound | 82 | 58.6% |
| Notify bedside nurse and physician not to continue use of the PICC and consider tests such as ultrasound | 42 | 30.0% |
| PICCs are often removed when physicians suspect, but have not yet confirmed, CLABSI. Considering your experiences, what percentage of PICCs may have been removed in this manner at your facility? | ||
| <5% | 11 | 7.9% |
| 59% | 16 | 11.4% |
| 1024% | 24 | 17.1% |
| 25% | 71 | 50.7% |
| Based on your experience, what percentage of PICCs do you think are inappropriate or could have been avoided at your facility? | ||
| <5% | 51 | 36.4% |
| 59% | 25 | 17.9% |
| 1024% | 28 | 20.0% |
| 2550% | 13 | 9.3% |
| >50% | 5 | 3.6% |
| Are vascular access nurses empowered to remove PICCs that are idle or clinically unnecessary without physician authorization? | ||
| Yes | 3 | 2.1% |
| No | 122 | 87.1% |
| How would you rank the overall support your vascular access service receives from hospital leadership? | ||
| Excellent | 5 | 3.6% |
| Very good | 32 | 22.9% |
| Good | 40 | 28.6% |
| Fair | 35 | 25.0% |
| Poor | 25 | 17.9% |
| How would you describe your relationship with physicians at your facility when it comes to communicating recommendations or management of PICCs? | ||
| Very good | 28 | 20.0% |
| Good | 63 | 45.0% |
| Fair | 35 | 25.0% |
| Poor | 7 | 5.0% |
| Very poor | 4 | 2.9% |
| How would you describe your relationship with bedside nurses at your facility when it comes to communicating recommendations or management of PICCs? | ||
| Very good | 32 | 22.9% |
| Good | 58 | 41.4% |
| Fair | 38 | 27.1% |
| Poor | 7 | 5.0% |
| Very poor | 2 | 1.4% |
Variation in Responses Based on Years in Practice or Certification
We initially hypothesized that responses regarding practice (ultrasound use, ECG guidance system use), management of complications, or perceptions regarding leadership might vary based on years of experience, number of PICCs placed, or certification status. However, no statistically significant associations with these factors and individual responses were identified.
DISCUSSION
In this survey of 140 vascular access nurses in hospitals across Michigan, new insights regarding the experience, practice, knowledge, and beliefs of this group of providers were obtained. We found that vascular access nurses varied with respect to years in practice, volume of PICCs placed, and certification status, reflecting heterogeneity in this provider group. Variation in insertion techniques, such as use of ultrasound to examine catheter‐to‐vein ratio (a key way to prevent thrombosis) or newer ECG technology to position the PICC, was also noted. Although indications for PICC insertion appeared consistent with published literature, the frequency with which these devices were placed in patients receiving dialysis (reportedly with nephrology approval) was surprising given national calls to avoid such use.[16] Opportunities to improve hospital practices, such as tracking PICC dwell times and PICC necessity, as well as the potential need to better educate physicians on when to remove PICCs for suspected CLABSI, were also identified. Collectively, these data are highly relevant to hospitalists and health systems as they help to identify areas for quality improvement and inform clinical practice regarding the use of PICCs in hospitalized patients. As hospitalists increasingly order PICCs and manage complications associated with these devices, they are well suited to use these data so as to improve patient safety and clinical outcomes.
Venous access is the most common medical procedure performed in hospitalized medical patients. Although a number of devices including peripheral intravenous catheters, central venous catheters, and PICCs are used for this purpose, the growing use of PICCs to secure venous access has been documented in several studies.[17] Such growth, in part, undoubtedly reflects increasing availability of vascular access nurses. Traditionally placed by interventional radiologists, the creation of dedicated vascular nursing teams has resulted in these subspecialists now serving in more of a backup or trouble‐shooting role rather than that of primary operator.[4, 14] This paradigm shift is well illustrated in a recent survey of infection preventionists, where over 60% of respondents reported that they had a vascular nursing team in their facility.[7] The growth of these nursing‐led vascular access teams has produced not only high rates of insertion success and low rates of complications, but also greater cost‐effectiveness when compared to interventional radiologybased insertion.[18]
Nonetheless, our survey also identified a number of important concerns regarding PICC practices and vascular nursing providers. First, we found variation in areas such as insertion practices and management of complications. Such variability highlights the importance of both growing and disseminating the evidence base for consistent practice in vascular nursing. Through their close clinical affiliation with vascular nurses and shared interests in obtaining safe and appropriate venous access for patients, hospitalists are ideally poised to lead this effort. Second, similarities between vascular nurse opinions regarding appropriateness of PICCs and those of hospitalists from a prior survey were noted.[19] Namely, a substantial proportion of both vascular nurses and hospitalists felt that some PICCs were inappropriate and could be avoided. Third, although relationships between vascular access nurses and leadership were reported as being variable, the survey responses suggested relatively good interprovider relationships with bedside nurses and physicians. Such relationships likely reflect the close clinical ties that emerge from being in the trenches of patient care and suggest that interventions to improve care in partnership with these providers are highly viable.
Our study has some limitations. First, despite a high response rate, our study used a survey design and reports findings from a convenience sample of vascular access nurses in a single state. Thus, nonrespondent and selection biases remain threats to our conclusions. Additionally, some respondents did not complete all responses, perhaps due to nonapplicability to practice or other unknown reasons. The pattern of missingness observed, however, suggested that such responses were missing at random. Second, we surveyed vascular nurses in hospitals that are actively engaged in improving PICC practices; our findings may therefore not be representative of vascular nursing professionals as a whole and may instead reflect those of a highly motivated group of individuals. Relatedly, the underlying reasons for adoption of specific practices or techniques cannot be discerned from our study. Third, although we did not find differences based on years in practice or certification status, our sample size was relatively small and likely underpowered for these comparisons. Finally, our study sample consists of vascular nurses who are clustered within hospitals in which they are employed. Therefore, overlap between reported practices and those required by the facility are possible.
Despite these limitations, our study has important strengths. First, this is among the most comprehensive of surveys examining vascular nursing experience, practice, knowledge, and beliefs. The growing presence of these providers across US hospitals, coupled with limited insight regarding their clinical practices, highlight the importance and utility of these data. Second, we noted important differences in experience, practices, and interprovider relationships between vascular providers in this field. Although we are unable to ascertain the drivers or significance of such variation, hospitals and health systems focused on improving patient safety should consider quantifying and exploring these factors. Third, findings from our survey within Michigan suggest the need for similar, larger studies across the country. Partnerships with nursing organizations or larger professional groups that represent vascular nursing specialists may be helpful in this regard.
In conclusion, we found important similarities and differences in vascular nursing experience, practice, knowledge, and beliefs in Michigan. These data are useful as they help provide context regarding the constitution of these teams, current practices, and opportunities for improving care. Hospitalists seeking to improve patient safety may use these data to better inform vascular access practice in hospitalized patients.
Acknowledgements
The authors thank Claire Rickard, PhD, RN, Britt Meyer, RN, Peter Carr, PhD, and David Dempsey, RN for their assistance in developing the survey instrument used in this study.
Disclosures: This project was funded through an Investigator Initiated Research Grant from the Blue Cross Blue Shield of Michigan (BCBSM) Foundation (grant number 2140.II). The funding source played no role in study design, data acquisition, analysis, or reporting of the data. Support for the Hospital Medicine Safety (HMS) Consortium is provided by BCBSM and the Blue Care Network as part of the BCBSM Value Partnerships program. Although BCBSM and HMS work collaboratively, the opinions, beliefs, and viewpoints expressed by the authors do not necessarily reflect the opinions, beliefs, and viewpoints of BCBSM or any of its employees. This work was also supported with resources from the Veterans Affairs Ann Arbor Healthcare System. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
- , , , et al. Peripherally inserted central venous catheters in the acute care setting: a safe alternative to high‐risk short‐term central venous catheters. Am J Infect Control. 2010;38(2):149–153.
- , , , , . Risk of venous thromboembolism in hospitalized patients with peripherally inserted central catheters. J Hosp Med. 2009;4(7):417–422.
- , , , , , . Central venous catheter placement by advanced practice nurses demonstrates low procedural complication and infection rates‐‐a report from 13 years of service. Crit Care Med. 2014;42(3):536–543.
- . Developing an alternative workflow model for peripherally inserted central catheter placement. J Infus Nurs. 2012;34(1):34–42.
- , . Facility wide benefits of radiology vascular access teams. Radiol Manage. 2010;32(1):28–32; quiz 33–34.
- , . Moving the needle forward: the imperative for collaboration in vascular access. J Infus Nurs. 2015;38(2):100–102.
- , , , . Use of designated PICC teams by U.S. hospitals: a survey‐based study [published online November 10, 2015]. J Patient Saf. doi: 10.1097/PTS.0000000000000246
- , , , , . The association between PICC use and venous thromboembolism in upper and lower extremities. American J Med. 2015;128(9):986–993.e1.
- , , , et al. Hospital performance for pharmacologic venous thromboembolism prophylaxis and rate of venous thromboembolism: a cohort study. JAMA Intern Med. 2014;174(10):1577–1584.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013;382(9889):311–325.
- Infusion Nurses Society. Infusion nursing standards of practice. J Infus Nurs. 2006;29(1 suppl):S1–S92.
- , , , et al. Guidelines for the prevention of intravascular catheter‐related infections. Am J Infect Control. 2011;39(4 suppl 1):S1–S34.
- , , , et al. International evidence‐based recommendations on ultrasound‐guided vascular access. Intensive Care Med. 2012;38(7):1105–1117.
- , , . A single institution experience of seven hundred consecutively placed peripherally inserted central venous catheters. J Vasc Access. 2014;15(6):498–502.
- , , . Central venous access devices site care practices: an international survey of 34 countries [published online September 3, 2015]. J Vasc Access. doi: 10.5301/jva.5000450
- American Society of Nephrology. World's Leading Kidney Society Joins Effort to Reduce Unnecessary Medical Tests and Procedures. Available at: https://www.asn‐online.org/policy/choosingwisely/PressReleaseChoosingWisely.pdf. Accessed September 4, 2015.
- , , , . A survey of the current use of peripherally inserted central venous catheter (PICC) in Swedish oncology departments. Acta Oncol. 2013;52(6):1241–1242.
- , . Nurse‐led PICC insertion: is it cost effective? Br J Nurs. 2013;22(19):S9–S15.
- , , , et al. Hospitalist experiences, practice, opinions, and knowledge regarding peripherally inserted central catheters: a Michigan survey. J Hosp Med. 2013;8(6):309–314.
- , , , et al. Peripherally inserted central venous catheters in the acute care setting: a safe alternative to high‐risk short‐term central venous catheters. Am J Infect Control. 2010;38(2):149–153.
- , , , , . Risk of venous thromboembolism in hospitalized patients with peripherally inserted central catheters. J Hosp Med. 2009;4(7):417–422.
- , , , , , . Central venous catheter placement by advanced practice nurses demonstrates low procedural complication and infection rates‐‐a report from 13 years of service. Crit Care Med. 2014;42(3):536–543.
- . Developing an alternative workflow model for peripherally inserted central catheter placement. J Infus Nurs. 2012;34(1):34–42.
- , . Facility wide benefits of radiology vascular access teams. Radiol Manage. 2010;32(1):28–32; quiz 33–34.
- , . Moving the needle forward: the imperative for collaboration in vascular access. J Infus Nurs. 2015;38(2):100–102.
- , , , . Use of designated PICC teams by U.S. hospitals: a survey‐based study [published online November 10, 2015]. J Patient Saf. doi: 10.1097/PTS.0000000000000246
- , , , , . The association between PICC use and venous thromboembolism in upper and lower extremities. American J Med. 2015;128(9):986–993.e1.
- , , , et al. Hospital performance for pharmacologic venous thromboembolism prophylaxis and rate of venous thromboembolism: a cohort study. JAMA Intern Med. 2014;174(10):1577–1584.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013;382(9889):311–325.
- Infusion Nurses Society. Infusion nursing standards of practice. J Infus Nurs. 2006;29(1 suppl):S1–S92.
- , , , et al. Guidelines for the prevention of intravascular catheter‐related infections. Am J Infect Control. 2011;39(4 suppl 1):S1–S34.
- , , , et al. International evidence‐based recommendations on ultrasound‐guided vascular access. Intensive Care Med. 2012;38(7):1105–1117.
- , , . A single institution experience of seven hundred consecutively placed peripherally inserted central venous catheters. J Vasc Access. 2014;15(6):498–502.
- , , . Central venous access devices site care practices: an international survey of 34 countries [published online September 3, 2015]. J Vasc Access. doi: 10.5301/jva.5000450
- American Society of Nephrology. World's Leading Kidney Society Joins Effort to Reduce Unnecessary Medical Tests and Procedures. Available at: https://www.asn‐online.org/policy/choosingwisely/PressReleaseChoosingWisely.pdf. Accessed September 4, 2015.
- , , , . A survey of the current use of peripherally inserted central venous catheter (PICC) in Swedish oncology departments. Acta Oncol. 2013;52(6):1241–1242.
- , . Nurse‐led PICC insertion: is it cost effective? Br J Nurs. 2013;22(19):S9–S15.
- , , , et al. Hospitalist experiences, practice, opinions, and knowledge regarding peripherally inserted central catheters: a Michigan survey. J Hosp Med. 2013;8(6):309–314.
© 2015 Society of Hospital Medicine
Mobilization in Severe Sepsis
Severe sepsis, defined as an infection leading to systemic inflammatory response and acute organ dysfunction, is a significant cause of morbidity and mortality.[1, 2, 3] Although it has been a condition classically attributed to patients in the intensive care unit (ICU), accumulating data suggest that a substantial proportion of patients with severe sepsis are managed by hospitalists and floor teams in non‐ICU, general ward settings.[1, 4, 5] Although the incidence of severe sepsis continues to rise both in the United States and other developed nations,[2, 6, 7] advances in early recognition, management, and care of this condition have resulted in improved rates of survival.[8] The resultant increase in a severe sepsis survivor population[6] make the long‐term sequelae of this condition an important public health problem.[9]
In both the ICU and on general wards, severe sepsis survivors suffer from decreased functional status, worsened quality of life, increased cognitive dysfunction, and sarcopenia.[4, 6, 10, 11, 12, 13, 14] Not surprisingly, many such patients are discharged to long‐term care facilities for physical rehabilitation,[15] with escalating utilization of resources[16] and cost.[17, 18] Inexpensive interventions that improve outcomes following sepsis would thus be welcomed.
It is well known that physical therapy (PT) and early mobilization are beneficial in mitigating functional decline in a number of conditions.[19, 20, 21, 22] PT can improve outcomes in several ways: prevention of bed rest deconditioning, mitigation of mechanisms that lead to sarcopenia, increased pulmonary and tissue aerobic capacity, and improved sense of well‐being. Indeed, among the population cared for in ICU settings, early mobility and PT lead to more ventilator‐free days, better functional status at discharge, shorter duration of delirium, and even a potentially reduced risk of central line‐associated bloodstream infection (CLABSI).[23, 24] However, whether initiating early PT can improve outcomes in patients with severe sepsis treated by either intensivists or hospitalists/floor teams outside the ICU is unknown.
Therefore, to better understand this phenomenon, we systematically reviewed and integrated the literature regarding early mobilization and PT for severe sepsis outside the ICU. To be more inclusive, a secondary review including populations with any infectious etiology and severe sepsis treated within the ICU was also conducted. Our review begins by providing an overview of the pathophysiology behind functional decline in severe sepsis, along with existing evidence on early mobilization efficacy in other patient populations. We then proceed with a review of the extant literature on the aforementioned topic. We conclude with an evaluation of the current evidence on the subject, along with assertions regarding future research in the area.
PATHOPHYSIOLOGY OF DISABILITY FOLLOWING HOSPITALIZATION FOR SEVERE SEPSIS
The pathophysiology behind functional decline in patients hospitalized with severe sepsis is multifactorial (Figure 1). During hospitalization, it is well known that patients suffer from restricted mobility,[25] and that this impediment is linked to poor functional outcomes.[26] Described as far back as Hippocrates,[27] more recent studies have elucidated how prolonged bed rest leads to a multitude of physiological changes that promote deconditioning.[28] Specifically, skeletal muscle atrophy and decreased protein synthesis, independent of ongoing disease processes and acute illness, have been demonstrated in both animal and human models of prolonged inactivity.[29, 30] Additionally, bed rest leading to insensible fluid losses, a decline in stroke volume and effective cardiac output, bone loss, and decreased insulin sensitivity has been reported.[28, 31] There is little doubt that the aforementioned issues pertain to severe sepsis patients outside the ICU. In fact, nearly all of the acute mechanisms driving Creditor's hazards of hospitalization are noted among patients with severe sepsis.[32]
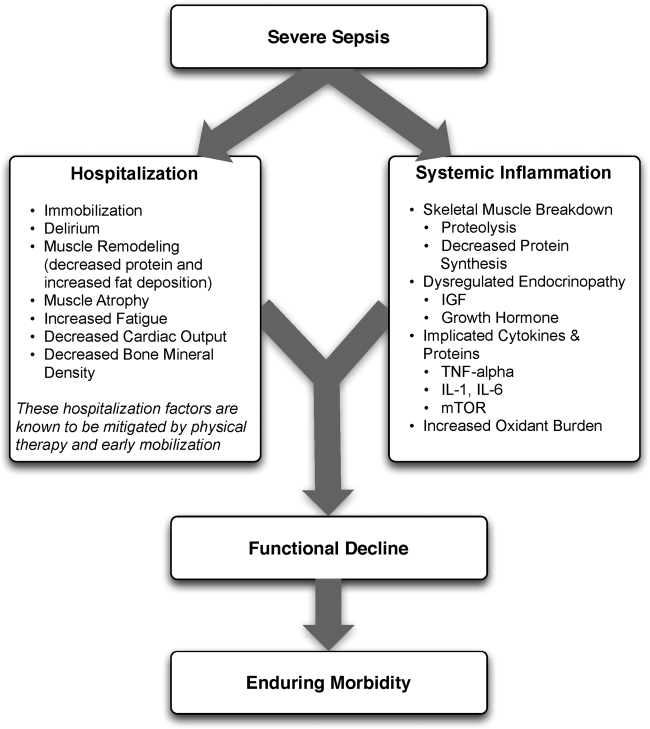
Furthermore, several factors preceding hospitalization may increase risk of disability. For example, Covinsky et al. described a number of risk factors, such as comorbid conditions, cognitive impairment, and various psychosocial aspects such as depression and limited social support, as being associated with increased risk of functional decline.[33] Thus, both in‐hospital and prehospital factors likely combine within an individual patient's context to determine risk of physical decline.
On this backdrop and the inherent immobilization associated with hospitalization, sepsis and inflammation catalyze physiologic changes that further propagate deconditioning.[7] Implicated pathways and proteins for this process include the mammalian target of rapamycin, human growth hormone, insulin‐like growth factors, interleukin‐1, and tumor necrosis factor‐. Through several metabolic alterations, sepsis independently promotes skeletal muscle breakdown and impairs skeletal muscle synthesis.[34, 35, 36] Inflammation associated with sepsis also increases oxidant burden, further leading to muscle dysfunction and dysregulation.[7, 31, 37, 38]
EFFECTS OF PHYSICAL THERAPY AND MOBILIZATION ON CLINICAL OUTCOMES
In patients with nonsepsis conditions who are at risk for functional decline, the effectiveness of physical therapy has been studied in multiple settings with positive outcomes. For example, in hospitalized elderly patients with general deconditioning, PT‐based interventions have demonstrated reductions in length of hospital stay.[39] Additionally, exercise in healthy subjects who have been subjected to bed rest has been shown to attenuate physiological changes, and maintain plasma and red cell volume and work capacity.[40] Adequate safety and improved outcomes have also been demonstrated in the general population of critically ill patients who receive early PT and mobilization. Improved functional capacity at discharge, decreases in duration of delirium, increased ventilator‐free days, decreased risk for CLABSI, and a better general sense of well‐being following these interventions have been widely reported in the literature.[14, 19, 23, 24, 41, 42, 43, 44, 45] Interestingly, critically ill patients may have a dose‐ and time‐dependent response to PT; that is, high intensity and early onset mobility‐based interventions are often associated with more ventilator‐free time and improved functional outcomes, resulting in shorter ICU and hospital length of stay.[42, 46, 47, 48]
Moderate intensity exercise has also been shown to improve 6‐minute walking distance in patients convalescing from coronary artery bypass grafting surgery.[49] Furthermore, in the postoperative setting, patients suffering traumatic hip fractures are known to benefit from physical and occupational therapies with shorter time to ambulation and improved locomotion in the recovery period.[21, 50, 51] Among patients with stroke, PT and gait training has led to improvements in speed, gait, independence during walking, activities of daily living, and extended activities of daily living.[52, 53, 54] A recent meta‐analysis also suggested that extra PT compared to regular treatment in patients with acute and subacute conditions such as stroke and postoperative states improved mobility and quality of life, while reducing length of hospital stay.[22]
Although this evidence suggests potential benefits for PT and mobilization, it is important to note that the effect of these treatments in dissimilar populations is unknown and may not necessarily be positive. For example, a recent study examining PT and its impact on patients with hip osteoarthritis showed no clinical benefit.[55] Mobilizing patients in severe illness may be associated with important risks, including falls, worsening of their clinical status, or moral discouragement in the setting of limited capacity. Therefore, understanding which elements of mobilization efforts create the greatest impact in the context of delivery of the intervention is critical to assessing the risk, benefit, and efficacy of PT‐based interventions.
EARLY PHYSICAL THERAPY FOR SEVERE SEPSIS OUTSIDE THE ICU: LITERATURE REVIEW
Given the functional decline associated with severe sepsis and the evidence of PT efficacy in other populations, we reviewed the current literature for studies evaluating physical therapy in severe sepsis patients outside the ICU. With the assistance of medical reference librarians, we searched MEDLINE via PubMed (1950present), EMBASE (1946present), Cochrane CENTRAL Register of Controlled Trials, and the Cochrane Database of Reviews of Effectiveness (1960present via Ovid). The search was last updated in June 2014.
We searched for studies that (1) involved human patients 18 years of age, (2) included patients with a primary diagnosis of sepsis or severe sepsis being treated outside the ICU, (3) featured a primary intervention that included PT or an early mobilization‐based initiative, and (4) reported a primary clinical or functional outcome of interest. Early was defined based on the included studies' definition. To be fully inclusive, we also conducted a secondary review with inclusion criteria expanded to studies of either any infectious pathology or severe sepsis patient in the ICU that employed PT interventions.
Our electronic search retrieved 815 records (Figure 2). Despite this approach, no publications met our primary inclusion criteria as we found no study that implemented a mobility intervention directed toward patients with sepsis treated outside the ICU. Our expanded secondary review included patients with any infectious pathology or those with severe sepsis in the ICU treated with PT; in this review, 2 studies met eligibility criteria.[56] In a 2003 cluster‐randomized trial, Mundy and colleagues randomized patients admitted with pneumonia to receive early PT or usual care. The outcomes of interest were hospital length of stay, mortality, number of chest radiographs, emergency department visits, and readmissions at 30 and 90 days after hospital admission. Although the study has important limitations (including patient‐level difference between trial arms, subjective definition of early mobilization), the authors found a significant decrease in length of stay among patients with pneumonia who received early PT compared to controls (5.8 vs 6.9 days, absolute difference 1.1 days, 95% confidence interval: 02.2 days). The study also reported a substantial decrease in adjusted mean hospital charges for the early mobilization group versus the usual care group ($10,159 per patient vs. $12,868 per patient, P=0.05). In the second study, Sossdorf et al. retrospectively evaluated a cohort of 999 patients with severe sepsis and septic shock and assessed whether onset and frequency of PT‐based interventions was associated with clinical benefit. After multivariate analysis, the authors reported a small mortality benefit associated with the relative number of PT interventions (hazard ratio: 0.982, P<0.001).[45]
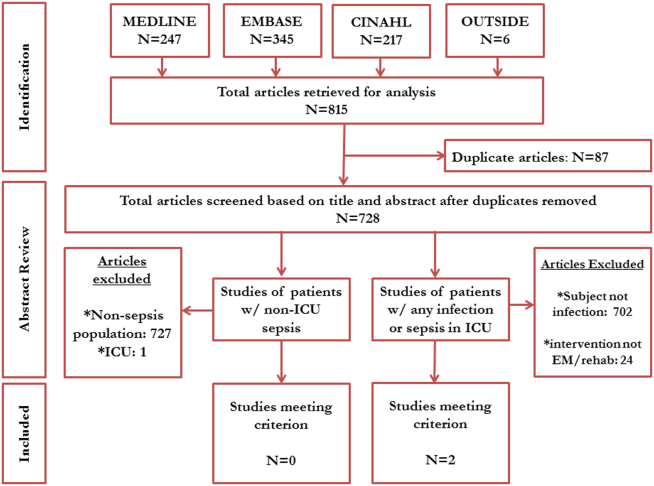
EXPLAINING THE VOID
Our integrative review of the current literature reveals a gap in our understanding of the role of early mobilization in severe sepsis both within and beyond the ICU. Given the promise of PT‐based interventions and the toll of severe sepsis, one must ask: why may this be so?
First, the understanding that severe sepsis leads to significant, long‐term consequences for survivors has only been identified recently. Thus, it is possible that the burden and consequences related to this condition have not been fully recognized in clinical settings, leading to a paucity of research and interventions. Although the association between sepsis and mortality has been known since the 1990s,[57] long‐term complications and enduring morbidity of this disease continue to be realized. Indeed, many studies delineating the longer‐term effects of sepsis have been only recently published.[6, 10, 11, 12, 13]
Second, it is likely that many clinicians ascribe to the viewpoint that severe sepsis is an ICU‐only condition, a myth that has been discounted by multiple studies.[1, 4, 5] Although our study shows a paucity of evidence in both ICU and nonICU‐based severe sepsis, almost half of severe sepsis occurs outside the ICU, carrying with it many of the same clinical implications. Additionally, increased morbidity, mortality, and resource utilization are known to be true in all patients with severe sepsis, irrespective of where they receive treatment in the hospital.[4, 5, 6] Recent evidence has also shown that severe sepsis treated on the floor may be clinically, epidemiologically, and even prognostically unique from its ICU counterpart.[5, 58, 59] Therefore, it appears that research domains with tailored interventions to both ICU and non‐ICU severe sepsis patients are important areas of inquiry for clinicians. Such research may serve the purpose of assessing impact of early mobilization and unmasking any treatment heterogeneity that may exist when dealing with severe sepsis. Though trials of PT in ICU‐based severe sepsis are underway,[60] it is prudent that these also extend beyond the ICU‐setting.
Third, variability in early mobility practices and billing documentation for severe sepsis patients may exist, adding barriers to performing high‐quality research on this topic. In fact, administrative billing records for PT may offer insufficient granularity about services provided or therapies administered, particularly in the ICU where variability in early mobilization practices have been shown despite common employment of physiotherapists.[61]
Finally, many hospitalists may believe that patients with severe sepsis are simply too sick for early mobilization or PT, possibly limiting their participation in clinical or research‐based interventions. This perception has been well described in ICU populations, where it has been well studied and shown to be false.[41, 42, 43] Nevertheless, if severe sepsis patients are viewed as relatively sick hospitalized patients, it is plausible that resistance against early mobilization interventions may exist.[62] Understanding these biases and being mindful of such barriers when conducting studies in this area would be important.
CONCLUSION AND FUTURE DIRECTIONS
The cost burdens of severe sepsis are substantial. Elixhauser et al. suggest that it is currently the single most expensive cause of acute hospitalization in the United States.[63] Importantly, a large proportion of patients with severe sepsis receive care from hospitalists and/or floor teams on the general wards. Our integrative review has demonstrated a knowledge gap when it comes to rigorous assessments of PT and mobilization treatments in patients with severe sepsis within and beyond the ICU. Existing evidence provides a strong rationale for why functional decline occurs in patients with severe sepsis. A reasonable argument for PT‐based interventions to mitigate functional decline in this subset exists, but rigorous evaluation of such interventions is necessary. Physical and mobilization‐based treatments are routinely available and efficacious in several other settings and populations. It could be rapidly deployed and potentially improve outcomes in those with severe sepsis. Research would be welcomed to establish optimal dosing, efficacy, and cost effectiveness of PT and early mobilization for severe sepsis, particularly in patients treated on the general wards by hospitalists and floor teams.
How may such a research agenda be launched? A balanced multipronged approach is necessary. First, large‐scale epidemiological data to understand variation in practice are needed. Focused studies carried out by community and academic hospitalists on septic patients treated outside the ICU are the call of the hour. These data, in turn, can help create registries that assess for risk factors, quality of treatment, and long‐term outcomes among survivors of this condition. Second, evaluation and improvement of the coding and precision of physical and occupational therapy billing records is necessary so that their added value can be assessed and tracked using administrative data. Third, targeted prospective studies and clinical trials to directly evaluate the effect of PT in well‐defined patient populations with sepsis outside the ICU are needed. In this arena, hospitalist expertise and trained physical therapists will be crucial. The focus of this work should be directed toward both short‐term and long‐term functional outcomes, as well as mortality and morbidity assessments. Fourth, these patient‐centered efforts should loop back and inform the foundational biology of severe sepsis, thus illuminating patient‐centered end points, from biomarker analysis to physiometric measurements in basic and translational research.
In conclusion, this review sheds light on the fact that interventions that may mitigate the functional and cognitive decline in survivors of severe sepsis appear underdeveloped. Although the precise benefit of such interventions remains unclear, the low‐cost, widespread availability and generalizability of PT‐based interventions make it a worthy candidate for future research. As the numbers of survivors of sepsis expand, an unmet public health need for interventions to improve the long‐term outcomes of this population exists. Hospitalists and intensivists caring for severe sepsis patients must rise to meet this need. Together, we can help improve the lives of patients afflicted with severe sepsis, wherever they may receive care in the hospital.
Acknowledgements
The authors acknowledge the efforts of medical research librarians Andy Hickner, MSI, and Marissa Conte, MSI, on this project.
Disclosures
This work was supported by the National Institutes of HealthK08, HL091249 (T.J.I.) and VA HSR&D IIR‐11109 (T.J.I.). The views expressed here are the authors' own and do not necessarily represent the views of the US government or the Department of Veterans' Affairs. The authors report no conflicts of interest.
- , . Epidemiology of sepsis: an update. Crit Care Med. 2001;29:S109–S116.
- , , , et al. Nationwide trends of severe sepsis in the 21st century (2000–2007). Chest. 2011;140:1223–1231.
- , , , et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554.
- , , , et al. Functional outcomes of general medical patients with severe sepsis. BMC Infect Dis. 2013;13:588.
- , , , et al. The epidemiology of acute organ system dysfunction from severe sepsis outside of the intensive care unit. J Hosp Med. 2013;8:243–247.
- , , , et al. Population burden of long‐term survivorship after severe sepsis in older Americans. J Am Geriatr Soc. 2012;60:1070–1077.
- , , , et al. Systemic inflammatory response syndrome increases immobility‐induced neuromuscular weakness. Crit Care Med. 2008;36:910–916.
- , , , et al. Early goal‐directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377.
- . The lingering consequences of sepsis: a hidden public health disaster? JAMA. 2010;304:1833–1834.
- , , , et al. Long‐term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794.
- , , , et al. Spurious inferences about long‐term outcomes: the case of severe sepsis and geriatric conditions. Am J Respir Crit Care Med. 2012;185:835–841.
- , , , et al. Long‐term outcome and quality‐adjusted life years after severe sepsis. Crit Care Med. 2009;37:1268–1274.
- , , , et al. Long‐term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38:1276–1283.
- , , , et al. Improving post‐intensive care unit neuropsychiatric outcomes: understanding cognitive effects of physical activity. Am J Respir Crit Care Med. 2012;186:1220–1228.
- , , , et al. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2012;40:754–761.
- , , , et al. Long‐term acute care hospital utilization after critical illness. JAMA. 2010;303:2253–2259.
- , , , et al. Long‐term survival and healthcare utilization outcomes attributable to sepsis and pneumonia. BMC Health Serv Res. 2012;12:432.
- , , , et al. Long‐term mortality and medical care charges in patients with severe sepsis. Crit Care Med. 2003;31:2316–2323.
- , , , et al. Early exercise in critically ill patients enhances short‐term functional recovery. Crit Care Med. 2009;37:2499–2505.
- , , , et al. Exercise‐based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2011;(7):CD001800.
- , , , et al. What is the role of timing in the surgical and rehabilitative care of community‐dwelling older persons with acute hip fracture? Arch Intern Med. 1997;157:513–520.
- , , . Extra physical therapy reduces patient length of stay and improves functional outcomes and quality of life in people with acute or subacute conditions: a systematic review. Arch Phys Med Rehabilil. 2011;92:1490–1500.
- , , . Reduction of intensive care unit length of stay: the case of early mobilization. Health Care Manag (Frederick). 2014;33:128–135.
- , , , et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–1882.
- , , , et al. Hospitalization, restricted activity, and the development of disability among older persons. JAMA. 2004;292:2115–2124.
- , , , et al. Low mobility during hospitalization and functional decline in older adults. J Am Geriatr Soc. 2011;59:266–273.
- , . The Medical Works of Hippocrates. Oxford, United Kingdom: Blackwell; 1950.
- , , . An overview of the issues: physiological effects of bed rest and restricted physical activity. Med Sci Sports Exerc. 1997;29:187–190.
- , , , et al. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol. 1996;270:E627–E633.
- , . Metabolic consequences of muscle disuse atrophy. J Nutr. 2005;135:1824S–1828S.
- . Inactivity and inflammation in the critically ill patient. Crit Care Clin. 2007;23:21–34.
- . Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118:219–223.
- , , . Hospitalization‐associated disability: “She was probably able to ambulate, but I'm not sure”. JAMA. 2011;306:1782–1793.
- , , , et al. A sustained rat model for studying the long‐lasting catabolic state of sepsis. Infect Immun. 1999;67:1079–1085.
- . Regulation of skeletal muscle protein turnover during sepsis. Curr Opin Clin Nutr. Metab Care. 1998;1:217–224.
- , , . Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab. 2007;293:E453–E459.
- , . From muscle disuse to myopathy in COPD: potential contribution of oxidative stress. Eur Respir J. 2005;26:703–719.
- , , . Oxidative stress and gene expression in sepsis. Br J Anaesth. 2003;90:221–232.
- , , , et al. Early ambulation and length of stay in older adults hospitalized for acute illness. Arch Intern Med. 2010;170:1942–1943.
- . Intensive exercise training during bed rest attenuates deconditioning. Med Sci Sports Exerc. 1997;29:207–215.
- , , , et al. Early activity is feasible and safe in respiratory failure patients. Crit Care Med. 2007;35:139–145.
- , , , et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36:2238–2243.
- . Clinical trials of early mobilization of critically ill patients. Crit Care Med. 2009;37:S442–S447.
- . Mobilizing patients in the intensive care unit: improving neuromuscular weakness and physical function. JAMA. 2008;300:1685–1690.
- , , , et al. Potential effect of physiotherapeutic treatment on mortality rate in patients with severe sepsis and septic shock: a retrospective cohort analysis. J Crit Care. 2013;28:954–958.
- , , , et al. Effects of physical training on functional status in patients with prolonged mechanical ventilation. Phys Ther. 2006;86:1271–1281.
- , , , et al. Impact of whole‐body rehabilitation in patients receiving chronic mechanical ventilation. Crit Care Med. 2005;33:2259–2265.
- . Rehabilitation of patients admitted to a respiratory intensive care unit. Arch Phys Med Rehabil. 1998;79:849–854.
- , , , et al. Supervised moderate intensity exercise improves distance walked at hospital discharge following coronary artery bypass graft surgery—a randomised controlled trial. Heart Lung Circ. 2008;17:129–138.
- , , , et al. Systematic review of hip fracture rehabilitation practices in the elderly. Arch Phys Med Rehabil. 2009;90:246–262.
- , , , et al. Physical therapy and mobility 2 and 6 months after hip fracture. J Am Geriatr Soc. 2004;52:1114–1120.
- , , , et al. Physical fitness training for stroke patients. Cochrane Database Syst Rev. 2011;(11):CD003316.
- , , , et al. Effects of augmented exercise therapy on outcome of gait and gait‐related activities in the first 6 months after stroke: a meta‐analysis. Stroke. 2011;42:3311–3315.
- , , , et al. Effects of augmented exercise therapy time after stroke: a meta‐analysis. Stroke. 2004;35:2529–2539.
- , , , et al. Effect of physical therapy on pain and function in patients with hip osteoarthritis: a randomized clinical trial. JAMA. 2014;311:1987–1997.
- , , , et al. Early mobilization of patients hospitalized with community‐acquired pneumonia. Chest. 2003;124:883–889.
- , , , et al. Magnitude and duration of the effect of sepsis on survival. Department of Veterans Affairs Systemic Sepsis Cooperative Studies Group. JAMA. 1997;277:1058–1063.
- , , , et al. Epidemiology of sepsis in Victoria, Australia. Crit Care Med. 2005;33:71–80.
- , , , et al. Sepsis incidence and outcome: contrasting the intensive care unit with the hospital ward. Crit Care Med. 2007;35:1284–1289.
- , , . Early rehabilitation in sepsis: a prospective randomised controlled trial investigating functional and physiological outcomes The i‐PERFORM Trial (Protocol Article). BMC Anesthesiol. 2011;11:21.
- , , , et al. TEAM: a prospective multi‐centre cohort study of early activity and mobilisation in ICU. In: American Thoracic Society 2013 International Conference; May 17–22, 2013; Philadelphia, PA. Am J Respir Crit Care Med. 2013;187:A3625.
- , , , et al. Improving long‐term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Crit Care Med. 2012;40:502–509.
- , , . Septicemia in U.S. hospitals, 2009: statistical brief #122. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD; 2006.
Severe sepsis, defined as an infection leading to systemic inflammatory response and acute organ dysfunction, is a significant cause of morbidity and mortality.[1, 2, 3] Although it has been a condition classically attributed to patients in the intensive care unit (ICU), accumulating data suggest that a substantial proportion of patients with severe sepsis are managed by hospitalists and floor teams in non‐ICU, general ward settings.[1, 4, 5] Although the incidence of severe sepsis continues to rise both in the United States and other developed nations,[2, 6, 7] advances in early recognition, management, and care of this condition have resulted in improved rates of survival.[8] The resultant increase in a severe sepsis survivor population[6] make the long‐term sequelae of this condition an important public health problem.[9]
In both the ICU and on general wards, severe sepsis survivors suffer from decreased functional status, worsened quality of life, increased cognitive dysfunction, and sarcopenia.[4, 6, 10, 11, 12, 13, 14] Not surprisingly, many such patients are discharged to long‐term care facilities for physical rehabilitation,[15] with escalating utilization of resources[16] and cost.[17, 18] Inexpensive interventions that improve outcomes following sepsis would thus be welcomed.
It is well known that physical therapy (PT) and early mobilization are beneficial in mitigating functional decline in a number of conditions.[19, 20, 21, 22] PT can improve outcomes in several ways: prevention of bed rest deconditioning, mitigation of mechanisms that lead to sarcopenia, increased pulmonary and tissue aerobic capacity, and improved sense of well‐being. Indeed, among the population cared for in ICU settings, early mobility and PT lead to more ventilator‐free days, better functional status at discharge, shorter duration of delirium, and even a potentially reduced risk of central line‐associated bloodstream infection (CLABSI).[23, 24] However, whether initiating early PT can improve outcomes in patients with severe sepsis treated by either intensivists or hospitalists/floor teams outside the ICU is unknown.
Therefore, to better understand this phenomenon, we systematically reviewed and integrated the literature regarding early mobilization and PT for severe sepsis outside the ICU. To be more inclusive, a secondary review including populations with any infectious etiology and severe sepsis treated within the ICU was also conducted. Our review begins by providing an overview of the pathophysiology behind functional decline in severe sepsis, along with existing evidence on early mobilization efficacy in other patient populations. We then proceed with a review of the extant literature on the aforementioned topic. We conclude with an evaluation of the current evidence on the subject, along with assertions regarding future research in the area.
PATHOPHYSIOLOGY OF DISABILITY FOLLOWING HOSPITALIZATION FOR SEVERE SEPSIS
The pathophysiology behind functional decline in patients hospitalized with severe sepsis is multifactorial (Figure 1). During hospitalization, it is well known that patients suffer from restricted mobility,[25] and that this impediment is linked to poor functional outcomes.[26] Described as far back as Hippocrates,[27] more recent studies have elucidated how prolonged bed rest leads to a multitude of physiological changes that promote deconditioning.[28] Specifically, skeletal muscle atrophy and decreased protein synthesis, independent of ongoing disease processes and acute illness, have been demonstrated in both animal and human models of prolonged inactivity.[29, 30] Additionally, bed rest leading to insensible fluid losses, a decline in stroke volume and effective cardiac output, bone loss, and decreased insulin sensitivity has been reported.[28, 31] There is little doubt that the aforementioned issues pertain to severe sepsis patients outside the ICU. In fact, nearly all of the acute mechanisms driving Creditor's hazards of hospitalization are noted among patients with severe sepsis.[32]

Furthermore, several factors preceding hospitalization may increase risk of disability. For example, Covinsky et al. described a number of risk factors, such as comorbid conditions, cognitive impairment, and various psychosocial aspects such as depression and limited social support, as being associated with increased risk of functional decline.[33] Thus, both in‐hospital and prehospital factors likely combine within an individual patient's context to determine risk of physical decline.
On this backdrop and the inherent immobilization associated with hospitalization, sepsis and inflammation catalyze physiologic changes that further propagate deconditioning.[7] Implicated pathways and proteins for this process include the mammalian target of rapamycin, human growth hormone, insulin‐like growth factors, interleukin‐1, and tumor necrosis factor‐. Through several metabolic alterations, sepsis independently promotes skeletal muscle breakdown and impairs skeletal muscle synthesis.[34, 35, 36] Inflammation associated with sepsis also increases oxidant burden, further leading to muscle dysfunction and dysregulation.[7, 31, 37, 38]
EFFECTS OF PHYSICAL THERAPY AND MOBILIZATION ON CLINICAL OUTCOMES
In patients with nonsepsis conditions who are at risk for functional decline, the effectiveness of physical therapy has been studied in multiple settings with positive outcomes. For example, in hospitalized elderly patients with general deconditioning, PT‐based interventions have demonstrated reductions in length of hospital stay.[39] Additionally, exercise in healthy subjects who have been subjected to bed rest has been shown to attenuate physiological changes, and maintain plasma and red cell volume and work capacity.[40] Adequate safety and improved outcomes have also been demonstrated in the general population of critically ill patients who receive early PT and mobilization. Improved functional capacity at discharge, decreases in duration of delirium, increased ventilator‐free days, decreased risk for CLABSI, and a better general sense of well‐being following these interventions have been widely reported in the literature.[14, 19, 23, 24, 41, 42, 43, 44, 45] Interestingly, critically ill patients may have a dose‐ and time‐dependent response to PT; that is, high intensity and early onset mobility‐based interventions are often associated with more ventilator‐free time and improved functional outcomes, resulting in shorter ICU and hospital length of stay.[42, 46, 47, 48]
Moderate intensity exercise has also been shown to improve 6‐minute walking distance in patients convalescing from coronary artery bypass grafting surgery.[49] Furthermore, in the postoperative setting, patients suffering traumatic hip fractures are known to benefit from physical and occupational therapies with shorter time to ambulation and improved locomotion in the recovery period.[21, 50, 51] Among patients with stroke, PT and gait training has led to improvements in speed, gait, independence during walking, activities of daily living, and extended activities of daily living.[52, 53, 54] A recent meta‐analysis also suggested that extra PT compared to regular treatment in patients with acute and subacute conditions such as stroke and postoperative states improved mobility and quality of life, while reducing length of hospital stay.[22]
Although this evidence suggests potential benefits for PT and mobilization, it is important to note that the effect of these treatments in dissimilar populations is unknown and may not necessarily be positive. For example, a recent study examining PT and its impact on patients with hip osteoarthritis showed no clinical benefit.[55] Mobilizing patients in severe illness may be associated with important risks, including falls, worsening of their clinical status, or moral discouragement in the setting of limited capacity. Therefore, understanding which elements of mobilization efforts create the greatest impact in the context of delivery of the intervention is critical to assessing the risk, benefit, and efficacy of PT‐based interventions.
EARLY PHYSICAL THERAPY FOR SEVERE SEPSIS OUTSIDE THE ICU: LITERATURE REVIEW
Given the functional decline associated with severe sepsis and the evidence of PT efficacy in other populations, we reviewed the current literature for studies evaluating physical therapy in severe sepsis patients outside the ICU. With the assistance of medical reference librarians, we searched MEDLINE via PubMed (1950present), EMBASE (1946present), Cochrane CENTRAL Register of Controlled Trials, and the Cochrane Database of Reviews of Effectiveness (1960present via Ovid). The search was last updated in June 2014.
We searched for studies that (1) involved human patients 18 years of age, (2) included patients with a primary diagnosis of sepsis or severe sepsis being treated outside the ICU, (3) featured a primary intervention that included PT or an early mobilization‐based initiative, and (4) reported a primary clinical or functional outcome of interest. Early was defined based on the included studies' definition. To be fully inclusive, we also conducted a secondary review with inclusion criteria expanded to studies of either any infectious pathology or severe sepsis patient in the ICU that employed PT interventions.
Our electronic search retrieved 815 records (Figure 2). Despite this approach, no publications met our primary inclusion criteria as we found no study that implemented a mobility intervention directed toward patients with sepsis treated outside the ICU. Our expanded secondary review included patients with any infectious pathology or those with severe sepsis in the ICU treated with PT; in this review, 2 studies met eligibility criteria.[56] In a 2003 cluster‐randomized trial, Mundy and colleagues randomized patients admitted with pneumonia to receive early PT or usual care. The outcomes of interest were hospital length of stay, mortality, number of chest radiographs, emergency department visits, and readmissions at 30 and 90 days after hospital admission. Although the study has important limitations (including patient‐level difference between trial arms, subjective definition of early mobilization), the authors found a significant decrease in length of stay among patients with pneumonia who received early PT compared to controls (5.8 vs 6.9 days, absolute difference 1.1 days, 95% confidence interval: 02.2 days). The study also reported a substantial decrease in adjusted mean hospital charges for the early mobilization group versus the usual care group ($10,159 per patient vs. $12,868 per patient, P=0.05). In the second study, Sossdorf et al. retrospectively evaluated a cohort of 999 patients with severe sepsis and septic shock and assessed whether onset and frequency of PT‐based interventions was associated with clinical benefit. After multivariate analysis, the authors reported a small mortality benefit associated with the relative number of PT interventions (hazard ratio: 0.982, P<0.001).[45]

EXPLAINING THE VOID
Our integrative review of the current literature reveals a gap in our understanding of the role of early mobilization in severe sepsis both within and beyond the ICU. Given the promise of PT‐based interventions and the toll of severe sepsis, one must ask: why may this be so?
First, the understanding that severe sepsis leads to significant, long‐term consequences for survivors has only been identified recently. Thus, it is possible that the burden and consequences related to this condition have not been fully recognized in clinical settings, leading to a paucity of research and interventions. Although the association between sepsis and mortality has been known since the 1990s,[57] long‐term complications and enduring morbidity of this disease continue to be realized. Indeed, many studies delineating the longer‐term effects of sepsis have been only recently published.[6, 10, 11, 12, 13]
Second, it is likely that many clinicians ascribe to the viewpoint that severe sepsis is an ICU‐only condition, a myth that has been discounted by multiple studies.[1, 4, 5] Although our study shows a paucity of evidence in both ICU and nonICU‐based severe sepsis, almost half of severe sepsis occurs outside the ICU, carrying with it many of the same clinical implications. Additionally, increased morbidity, mortality, and resource utilization are known to be true in all patients with severe sepsis, irrespective of where they receive treatment in the hospital.[4, 5, 6] Recent evidence has also shown that severe sepsis treated on the floor may be clinically, epidemiologically, and even prognostically unique from its ICU counterpart.[5, 58, 59] Therefore, it appears that research domains with tailored interventions to both ICU and non‐ICU severe sepsis patients are important areas of inquiry for clinicians. Such research may serve the purpose of assessing impact of early mobilization and unmasking any treatment heterogeneity that may exist when dealing with severe sepsis. Though trials of PT in ICU‐based severe sepsis are underway,[60] it is prudent that these also extend beyond the ICU‐setting.
Third, variability in early mobility practices and billing documentation for severe sepsis patients may exist, adding barriers to performing high‐quality research on this topic. In fact, administrative billing records for PT may offer insufficient granularity about services provided or therapies administered, particularly in the ICU where variability in early mobilization practices have been shown despite common employment of physiotherapists.[61]
Finally, many hospitalists may believe that patients with severe sepsis are simply too sick for early mobilization or PT, possibly limiting their participation in clinical or research‐based interventions. This perception has been well described in ICU populations, where it has been well studied and shown to be false.[41, 42, 43] Nevertheless, if severe sepsis patients are viewed as relatively sick hospitalized patients, it is plausible that resistance against early mobilization interventions may exist.[62] Understanding these biases and being mindful of such barriers when conducting studies in this area would be important.
CONCLUSION AND FUTURE DIRECTIONS
The cost burdens of severe sepsis are substantial. Elixhauser et al. suggest that it is currently the single most expensive cause of acute hospitalization in the United States.[63] Importantly, a large proportion of patients with severe sepsis receive care from hospitalists and/or floor teams on the general wards. Our integrative review has demonstrated a knowledge gap when it comes to rigorous assessments of PT and mobilization treatments in patients with severe sepsis within and beyond the ICU. Existing evidence provides a strong rationale for why functional decline occurs in patients with severe sepsis. A reasonable argument for PT‐based interventions to mitigate functional decline in this subset exists, but rigorous evaluation of such interventions is necessary. Physical and mobilization‐based treatments are routinely available and efficacious in several other settings and populations. It could be rapidly deployed and potentially improve outcomes in those with severe sepsis. Research would be welcomed to establish optimal dosing, efficacy, and cost effectiveness of PT and early mobilization for severe sepsis, particularly in patients treated on the general wards by hospitalists and floor teams.
How may such a research agenda be launched? A balanced multipronged approach is necessary. First, large‐scale epidemiological data to understand variation in practice are needed. Focused studies carried out by community and academic hospitalists on septic patients treated outside the ICU are the call of the hour. These data, in turn, can help create registries that assess for risk factors, quality of treatment, and long‐term outcomes among survivors of this condition. Second, evaluation and improvement of the coding and precision of physical and occupational therapy billing records is necessary so that their added value can be assessed and tracked using administrative data. Third, targeted prospective studies and clinical trials to directly evaluate the effect of PT in well‐defined patient populations with sepsis outside the ICU are needed. In this arena, hospitalist expertise and trained physical therapists will be crucial. The focus of this work should be directed toward both short‐term and long‐term functional outcomes, as well as mortality and morbidity assessments. Fourth, these patient‐centered efforts should loop back and inform the foundational biology of severe sepsis, thus illuminating patient‐centered end points, from biomarker analysis to physiometric measurements in basic and translational research.
In conclusion, this review sheds light on the fact that interventions that may mitigate the functional and cognitive decline in survivors of severe sepsis appear underdeveloped. Although the precise benefit of such interventions remains unclear, the low‐cost, widespread availability and generalizability of PT‐based interventions make it a worthy candidate for future research. As the numbers of survivors of sepsis expand, an unmet public health need for interventions to improve the long‐term outcomes of this population exists. Hospitalists and intensivists caring for severe sepsis patients must rise to meet this need. Together, we can help improve the lives of patients afflicted with severe sepsis, wherever they may receive care in the hospital.
Acknowledgements
The authors acknowledge the efforts of medical research librarians Andy Hickner, MSI, and Marissa Conte, MSI, on this project.
Disclosures
This work was supported by the National Institutes of HealthK08, HL091249 (T.J.I.) and VA HSR&D IIR‐11109 (T.J.I.). The views expressed here are the authors' own and do not necessarily represent the views of the US government or the Department of Veterans' Affairs. The authors report no conflicts of interest.
Severe sepsis, defined as an infection leading to systemic inflammatory response and acute organ dysfunction, is a significant cause of morbidity and mortality.[1, 2, 3] Although it has been a condition classically attributed to patients in the intensive care unit (ICU), accumulating data suggest that a substantial proportion of patients with severe sepsis are managed by hospitalists and floor teams in non‐ICU, general ward settings.[1, 4, 5] Although the incidence of severe sepsis continues to rise both in the United States and other developed nations,[2, 6, 7] advances in early recognition, management, and care of this condition have resulted in improved rates of survival.[8] The resultant increase in a severe sepsis survivor population[6] make the long‐term sequelae of this condition an important public health problem.[9]
In both the ICU and on general wards, severe sepsis survivors suffer from decreased functional status, worsened quality of life, increased cognitive dysfunction, and sarcopenia.[4, 6, 10, 11, 12, 13, 14] Not surprisingly, many such patients are discharged to long‐term care facilities for physical rehabilitation,[15] with escalating utilization of resources[16] and cost.[17, 18] Inexpensive interventions that improve outcomes following sepsis would thus be welcomed.
It is well known that physical therapy (PT) and early mobilization are beneficial in mitigating functional decline in a number of conditions.[19, 20, 21, 22] PT can improve outcomes in several ways: prevention of bed rest deconditioning, mitigation of mechanisms that lead to sarcopenia, increased pulmonary and tissue aerobic capacity, and improved sense of well‐being. Indeed, among the population cared for in ICU settings, early mobility and PT lead to more ventilator‐free days, better functional status at discharge, shorter duration of delirium, and even a potentially reduced risk of central line‐associated bloodstream infection (CLABSI).[23, 24] However, whether initiating early PT can improve outcomes in patients with severe sepsis treated by either intensivists or hospitalists/floor teams outside the ICU is unknown.
Therefore, to better understand this phenomenon, we systematically reviewed and integrated the literature regarding early mobilization and PT for severe sepsis outside the ICU. To be more inclusive, a secondary review including populations with any infectious etiology and severe sepsis treated within the ICU was also conducted. Our review begins by providing an overview of the pathophysiology behind functional decline in severe sepsis, along with existing evidence on early mobilization efficacy in other patient populations. We then proceed with a review of the extant literature on the aforementioned topic. We conclude with an evaluation of the current evidence on the subject, along with assertions regarding future research in the area.
PATHOPHYSIOLOGY OF DISABILITY FOLLOWING HOSPITALIZATION FOR SEVERE SEPSIS
The pathophysiology behind functional decline in patients hospitalized with severe sepsis is multifactorial (Figure 1). During hospitalization, it is well known that patients suffer from restricted mobility,[25] and that this impediment is linked to poor functional outcomes.[26] Described as far back as Hippocrates,[27] more recent studies have elucidated how prolonged bed rest leads to a multitude of physiological changes that promote deconditioning.[28] Specifically, skeletal muscle atrophy and decreased protein synthesis, independent of ongoing disease processes and acute illness, have been demonstrated in both animal and human models of prolonged inactivity.[29, 30] Additionally, bed rest leading to insensible fluid losses, a decline in stroke volume and effective cardiac output, bone loss, and decreased insulin sensitivity has been reported.[28, 31] There is little doubt that the aforementioned issues pertain to severe sepsis patients outside the ICU. In fact, nearly all of the acute mechanisms driving Creditor's hazards of hospitalization are noted among patients with severe sepsis.[32]

Furthermore, several factors preceding hospitalization may increase risk of disability. For example, Covinsky et al. described a number of risk factors, such as comorbid conditions, cognitive impairment, and various psychosocial aspects such as depression and limited social support, as being associated with increased risk of functional decline.[33] Thus, both in‐hospital and prehospital factors likely combine within an individual patient's context to determine risk of physical decline.
On this backdrop and the inherent immobilization associated with hospitalization, sepsis and inflammation catalyze physiologic changes that further propagate deconditioning.[7] Implicated pathways and proteins for this process include the mammalian target of rapamycin, human growth hormone, insulin‐like growth factors, interleukin‐1, and tumor necrosis factor‐. Through several metabolic alterations, sepsis independently promotes skeletal muscle breakdown and impairs skeletal muscle synthesis.[34, 35, 36] Inflammation associated with sepsis also increases oxidant burden, further leading to muscle dysfunction and dysregulation.[7, 31, 37, 38]
EFFECTS OF PHYSICAL THERAPY AND MOBILIZATION ON CLINICAL OUTCOMES
In patients with nonsepsis conditions who are at risk for functional decline, the effectiveness of physical therapy has been studied in multiple settings with positive outcomes. For example, in hospitalized elderly patients with general deconditioning, PT‐based interventions have demonstrated reductions in length of hospital stay.[39] Additionally, exercise in healthy subjects who have been subjected to bed rest has been shown to attenuate physiological changes, and maintain plasma and red cell volume and work capacity.[40] Adequate safety and improved outcomes have also been demonstrated in the general population of critically ill patients who receive early PT and mobilization. Improved functional capacity at discharge, decreases in duration of delirium, increased ventilator‐free days, decreased risk for CLABSI, and a better general sense of well‐being following these interventions have been widely reported in the literature.[14, 19, 23, 24, 41, 42, 43, 44, 45] Interestingly, critically ill patients may have a dose‐ and time‐dependent response to PT; that is, high intensity and early onset mobility‐based interventions are often associated with more ventilator‐free time and improved functional outcomes, resulting in shorter ICU and hospital length of stay.[42, 46, 47, 48]
Moderate intensity exercise has also been shown to improve 6‐minute walking distance in patients convalescing from coronary artery bypass grafting surgery.[49] Furthermore, in the postoperative setting, patients suffering traumatic hip fractures are known to benefit from physical and occupational therapies with shorter time to ambulation and improved locomotion in the recovery period.[21, 50, 51] Among patients with stroke, PT and gait training has led to improvements in speed, gait, independence during walking, activities of daily living, and extended activities of daily living.[52, 53, 54] A recent meta‐analysis also suggested that extra PT compared to regular treatment in patients with acute and subacute conditions such as stroke and postoperative states improved mobility and quality of life, while reducing length of hospital stay.[22]
Although this evidence suggests potential benefits for PT and mobilization, it is important to note that the effect of these treatments in dissimilar populations is unknown and may not necessarily be positive. For example, a recent study examining PT and its impact on patients with hip osteoarthritis showed no clinical benefit.[55] Mobilizing patients in severe illness may be associated with important risks, including falls, worsening of their clinical status, or moral discouragement in the setting of limited capacity. Therefore, understanding which elements of mobilization efforts create the greatest impact in the context of delivery of the intervention is critical to assessing the risk, benefit, and efficacy of PT‐based interventions.
EARLY PHYSICAL THERAPY FOR SEVERE SEPSIS OUTSIDE THE ICU: LITERATURE REVIEW
Given the functional decline associated with severe sepsis and the evidence of PT efficacy in other populations, we reviewed the current literature for studies evaluating physical therapy in severe sepsis patients outside the ICU. With the assistance of medical reference librarians, we searched MEDLINE via PubMed (1950present), EMBASE (1946present), Cochrane CENTRAL Register of Controlled Trials, and the Cochrane Database of Reviews of Effectiveness (1960present via Ovid). The search was last updated in June 2014.
We searched for studies that (1) involved human patients 18 years of age, (2) included patients with a primary diagnosis of sepsis or severe sepsis being treated outside the ICU, (3) featured a primary intervention that included PT or an early mobilization‐based initiative, and (4) reported a primary clinical or functional outcome of interest. Early was defined based on the included studies' definition. To be fully inclusive, we also conducted a secondary review with inclusion criteria expanded to studies of either any infectious pathology or severe sepsis patient in the ICU that employed PT interventions.
Our electronic search retrieved 815 records (Figure 2). Despite this approach, no publications met our primary inclusion criteria as we found no study that implemented a mobility intervention directed toward patients with sepsis treated outside the ICU. Our expanded secondary review included patients with any infectious pathology or those with severe sepsis in the ICU treated with PT; in this review, 2 studies met eligibility criteria.[56] In a 2003 cluster‐randomized trial, Mundy and colleagues randomized patients admitted with pneumonia to receive early PT or usual care. The outcomes of interest were hospital length of stay, mortality, number of chest radiographs, emergency department visits, and readmissions at 30 and 90 days after hospital admission. Although the study has important limitations (including patient‐level difference between trial arms, subjective definition of early mobilization), the authors found a significant decrease in length of stay among patients with pneumonia who received early PT compared to controls (5.8 vs 6.9 days, absolute difference 1.1 days, 95% confidence interval: 02.2 days). The study also reported a substantial decrease in adjusted mean hospital charges for the early mobilization group versus the usual care group ($10,159 per patient vs. $12,868 per patient, P=0.05). In the second study, Sossdorf et al. retrospectively evaluated a cohort of 999 patients with severe sepsis and septic shock and assessed whether onset and frequency of PT‐based interventions was associated with clinical benefit. After multivariate analysis, the authors reported a small mortality benefit associated with the relative number of PT interventions (hazard ratio: 0.982, P<0.001).[45]

EXPLAINING THE VOID
Our integrative review of the current literature reveals a gap in our understanding of the role of early mobilization in severe sepsis both within and beyond the ICU. Given the promise of PT‐based interventions and the toll of severe sepsis, one must ask: why may this be so?
First, the understanding that severe sepsis leads to significant, long‐term consequences for survivors has only been identified recently. Thus, it is possible that the burden and consequences related to this condition have not been fully recognized in clinical settings, leading to a paucity of research and interventions. Although the association between sepsis and mortality has been known since the 1990s,[57] long‐term complications and enduring morbidity of this disease continue to be realized. Indeed, many studies delineating the longer‐term effects of sepsis have been only recently published.[6, 10, 11, 12, 13]
Second, it is likely that many clinicians ascribe to the viewpoint that severe sepsis is an ICU‐only condition, a myth that has been discounted by multiple studies.[1, 4, 5] Although our study shows a paucity of evidence in both ICU and nonICU‐based severe sepsis, almost half of severe sepsis occurs outside the ICU, carrying with it many of the same clinical implications. Additionally, increased morbidity, mortality, and resource utilization are known to be true in all patients with severe sepsis, irrespective of where they receive treatment in the hospital.[4, 5, 6] Recent evidence has also shown that severe sepsis treated on the floor may be clinically, epidemiologically, and even prognostically unique from its ICU counterpart.[5, 58, 59] Therefore, it appears that research domains with tailored interventions to both ICU and non‐ICU severe sepsis patients are important areas of inquiry for clinicians. Such research may serve the purpose of assessing impact of early mobilization and unmasking any treatment heterogeneity that may exist when dealing with severe sepsis. Though trials of PT in ICU‐based severe sepsis are underway,[60] it is prudent that these also extend beyond the ICU‐setting.
Third, variability in early mobility practices and billing documentation for severe sepsis patients may exist, adding barriers to performing high‐quality research on this topic. In fact, administrative billing records for PT may offer insufficient granularity about services provided or therapies administered, particularly in the ICU where variability in early mobilization practices have been shown despite common employment of physiotherapists.[61]
Finally, many hospitalists may believe that patients with severe sepsis are simply too sick for early mobilization or PT, possibly limiting their participation in clinical or research‐based interventions. This perception has been well described in ICU populations, where it has been well studied and shown to be false.[41, 42, 43] Nevertheless, if severe sepsis patients are viewed as relatively sick hospitalized patients, it is plausible that resistance against early mobilization interventions may exist.[62] Understanding these biases and being mindful of such barriers when conducting studies in this area would be important.
CONCLUSION AND FUTURE DIRECTIONS
The cost burdens of severe sepsis are substantial. Elixhauser et al. suggest that it is currently the single most expensive cause of acute hospitalization in the United States.[63] Importantly, a large proportion of patients with severe sepsis receive care from hospitalists and/or floor teams on the general wards. Our integrative review has demonstrated a knowledge gap when it comes to rigorous assessments of PT and mobilization treatments in patients with severe sepsis within and beyond the ICU. Existing evidence provides a strong rationale for why functional decline occurs in patients with severe sepsis. A reasonable argument for PT‐based interventions to mitigate functional decline in this subset exists, but rigorous evaluation of such interventions is necessary. Physical and mobilization‐based treatments are routinely available and efficacious in several other settings and populations. It could be rapidly deployed and potentially improve outcomes in those with severe sepsis. Research would be welcomed to establish optimal dosing, efficacy, and cost effectiveness of PT and early mobilization for severe sepsis, particularly in patients treated on the general wards by hospitalists and floor teams.
How may such a research agenda be launched? A balanced multipronged approach is necessary. First, large‐scale epidemiological data to understand variation in practice are needed. Focused studies carried out by community and academic hospitalists on septic patients treated outside the ICU are the call of the hour. These data, in turn, can help create registries that assess for risk factors, quality of treatment, and long‐term outcomes among survivors of this condition. Second, evaluation and improvement of the coding and precision of physical and occupational therapy billing records is necessary so that their added value can be assessed and tracked using administrative data. Third, targeted prospective studies and clinical trials to directly evaluate the effect of PT in well‐defined patient populations with sepsis outside the ICU are needed. In this arena, hospitalist expertise and trained physical therapists will be crucial. The focus of this work should be directed toward both short‐term and long‐term functional outcomes, as well as mortality and morbidity assessments. Fourth, these patient‐centered efforts should loop back and inform the foundational biology of severe sepsis, thus illuminating patient‐centered end points, from biomarker analysis to physiometric measurements in basic and translational research.
In conclusion, this review sheds light on the fact that interventions that may mitigate the functional and cognitive decline in survivors of severe sepsis appear underdeveloped. Although the precise benefit of such interventions remains unclear, the low‐cost, widespread availability and generalizability of PT‐based interventions make it a worthy candidate for future research. As the numbers of survivors of sepsis expand, an unmet public health need for interventions to improve the long‐term outcomes of this population exists. Hospitalists and intensivists caring for severe sepsis patients must rise to meet this need. Together, we can help improve the lives of patients afflicted with severe sepsis, wherever they may receive care in the hospital.
Acknowledgements
The authors acknowledge the efforts of medical research librarians Andy Hickner, MSI, and Marissa Conte, MSI, on this project.
Disclosures
This work was supported by the National Institutes of HealthK08, HL091249 (T.J.I.) and VA HSR&D IIR‐11109 (T.J.I.). The views expressed here are the authors' own and do not necessarily represent the views of the US government or the Department of Veterans' Affairs. The authors report no conflicts of interest.
- , . Epidemiology of sepsis: an update. Crit Care Med. 2001;29:S109–S116.
- , , , et al. Nationwide trends of severe sepsis in the 21st century (2000–2007). Chest. 2011;140:1223–1231.
- , , , et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554.
- , , , et al. Functional outcomes of general medical patients with severe sepsis. BMC Infect Dis. 2013;13:588.
- , , , et al. The epidemiology of acute organ system dysfunction from severe sepsis outside of the intensive care unit. J Hosp Med. 2013;8:243–247.
- , , , et al. Population burden of long‐term survivorship after severe sepsis in older Americans. J Am Geriatr Soc. 2012;60:1070–1077.
- , , , et al. Systemic inflammatory response syndrome increases immobility‐induced neuromuscular weakness. Crit Care Med. 2008;36:910–916.
- , , , et al. Early goal‐directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377.
- . The lingering consequences of sepsis: a hidden public health disaster? JAMA. 2010;304:1833–1834.
- , , , et al. Long‐term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794.
- , , , et al. Spurious inferences about long‐term outcomes: the case of severe sepsis and geriatric conditions. Am J Respir Crit Care Med. 2012;185:835–841.
- , , , et al. Long‐term outcome and quality‐adjusted life years after severe sepsis. Crit Care Med. 2009;37:1268–1274.
- , , , et al. Long‐term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38:1276–1283.
- , , , et al. Improving post‐intensive care unit neuropsychiatric outcomes: understanding cognitive effects of physical activity. Am J Respir Crit Care Med. 2012;186:1220–1228.
- , , , et al. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2012;40:754–761.
- , , , et al. Long‐term acute care hospital utilization after critical illness. JAMA. 2010;303:2253–2259.
- , , , et al. Long‐term survival and healthcare utilization outcomes attributable to sepsis and pneumonia. BMC Health Serv Res. 2012;12:432.
- , , , et al. Long‐term mortality and medical care charges in patients with severe sepsis. Crit Care Med. 2003;31:2316–2323.
- , , , et al. Early exercise in critically ill patients enhances short‐term functional recovery. Crit Care Med. 2009;37:2499–2505.
- , , , et al. Exercise‐based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2011;(7):CD001800.
- , , , et al. What is the role of timing in the surgical and rehabilitative care of community‐dwelling older persons with acute hip fracture? Arch Intern Med. 1997;157:513–520.
- , , . Extra physical therapy reduces patient length of stay and improves functional outcomes and quality of life in people with acute or subacute conditions: a systematic review. Arch Phys Med Rehabilil. 2011;92:1490–1500.
- , , . Reduction of intensive care unit length of stay: the case of early mobilization. Health Care Manag (Frederick). 2014;33:128–135.
- , , , et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–1882.
- , , , et al. Hospitalization, restricted activity, and the development of disability among older persons. JAMA. 2004;292:2115–2124.
- , , , et al. Low mobility during hospitalization and functional decline in older adults. J Am Geriatr Soc. 2011;59:266–273.
- , . The Medical Works of Hippocrates. Oxford, United Kingdom: Blackwell; 1950.
- , , . An overview of the issues: physiological effects of bed rest and restricted physical activity. Med Sci Sports Exerc. 1997;29:187–190.
- , , , et al. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol. 1996;270:E627–E633.
- , . Metabolic consequences of muscle disuse atrophy. J Nutr. 2005;135:1824S–1828S.
- . Inactivity and inflammation in the critically ill patient. Crit Care Clin. 2007;23:21–34.
- . Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118:219–223.
- , , . Hospitalization‐associated disability: “She was probably able to ambulate, but I'm not sure”. JAMA. 2011;306:1782–1793.
- , , , et al. A sustained rat model for studying the long‐lasting catabolic state of sepsis. Infect Immun. 1999;67:1079–1085.
- . Regulation of skeletal muscle protein turnover during sepsis. Curr Opin Clin Nutr. Metab Care. 1998;1:217–224.
- , , . Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab. 2007;293:E453–E459.
- , . From muscle disuse to myopathy in COPD: potential contribution of oxidative stress. Eur Respir J. 2005;26:703–719.
- , , . Oxidative stress and gene expression in sepsis. Br J Anaesth. 2003;90:221–232.
- , , , et al. Early ambulation and length of stay in older adults hospitalized for acute illness. Arch Intern Med. 2010;170:1942–1943.
- . Intensive exercise training during bed rest attenuates deconditioning. Med Sci Sports Exerc. 1997;29:207–215.
- , , , et al. Early activity is feasible and safe in respiratory failure patients. Crit Care Med. 2007;35:139–145.
- , , , et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36:2238–2243.
- . Clinical trials of early mobilization of critically ill patients. Crit Care Med. 2009;37:S442–S447.
- . Mobilizing patients in the intensive care unit: improving neuromuscular weakness and physical function. JAMA. 2008;300:1685–1690.
- , , , et al. Potential effect of physiotherapeutic treatment on mortality rate in patients with severe sepsis and septic shock: a retrospective cohort analysis. J Crit Care. 2013;28:954–958.
- , , , et al. Effects of physical training on functional status in patients with prolonged mechanical ventilation. Phys Ther. 2006;86:1271–1281.
- , , , et al. Impact of whole‐body rehabilitation in patients receiving chronic mechanical ventilation. Crit Care Med. 2005;33:2259–2265.
- . Rehabilitation of patients admitted to a respiratory intensive care unit. Arch Phys Med Rehabil. 1998;79:849–854.
- , , , et al. Supervised moderate intensity exercise improves distance walked at hospital discharge following coronary artery bypass graft surgery—a randomised controlled trial. Heart Lung Circ. 2008;17:129–138.
- , , , et al. Systematic review of hip fracture rehabilitation practices in the elderly. Arch Phys Med Rehabil. 2009;90:246–262.
- , , , et al. Physical therapy and mobility 2 and 6 months after hip fracture. J Am Geriatr Soc. 2004;52:1114–1120.
- , , , et al. Physical fitness training for stroke patients. Cochrane Database Syst Rev. 2011;(11):CD003316.
- , , , et al. Effects of augmented exercise therapy on outcome of gait and gait‐related activities in the first 6 months after stroke: a meta‐analysis. Stroke. 2011;42:3311–3315.
- , , , et al. Effects of augmented exercise therapy time after stroke: a meta‐analysis. Stroke. 2004;35:2529–2539.
- , , , et al. Effect of physical therapy on pain and function in patients with hip osteoarthritis: a randomized clinical trial. JAMA. 2014;311:1987–1997.
- , , , et al. Early mobilization of patients hospitalized with community‐acquired pneumonia. Chest. 2003;124:883–889.
- , , , et al. Magnitude and duration of the effect of sepsis on survival. Department of Veterans Affairs Systemic Sepsis Cooperative Studies Group. JAMA. 1997;277:1058–1063.
- , , , et al. Epidemiology of sepsis in Victoria, Australia. Crit Care Med. 2005;33:71–80.
- , , , et al. Sepsis incidence and outcome: contrasting the intensive care unit with the hospital ward. Crit Care Med. 2007;35:1284–1289.
- , , . Early rehabilitation in sepsis: a prospective randomised controlled trial investigating functional and physiological outcomes The i‐PERFORM Trial (Protocol Article). BMC Anesthesiol. 2011;11:21.
- , , , et al. TEAM: a prospective multi‐centre cohort study of early activity and mobilisation in ICU. In: American Thoracic Society 2013 International Conference; May 17–22, 2013; Philadelphia, PA. Am J Respir Crit Care Med. 2013;187:A3625.
- , , , et al. Improving long‐term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Crit Care Med. 2012;40:502–509.
- , , . Septicemia in U.S. hospitals, 2009: statistical brief #122. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD; 2006.
- , . Epidemiology of sepsis: an update. Crit Care Med. 2001;29:S109–S116.
- , , , et al. Nationwide trends of severe sepsis in the 21st century (2000–2007). Chest. 2011;140:1223–1231.
- , , , et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554.
- , , , et al. Functional outcomes of general medical patients with severe sepsis. BMC Infect Dis. 2013;13:588.
- , , , et al. The epidemiology of acute organ system dysfunction from severe sepsis outside of the intensive care unit. J Hosp Med. 2013;8:243–247.
- , , , et al. Population burden of long‐term survivorship after severe sepsis in older Americans. J Am Geriatr Soc. 2012;60:1070–1077.
- , , , et al. Systemic inflammatory response syndrome increases immobility‐induced neuromuscular weakness. Crit Care Med. 2008;36:910–916.
- , , , et al. Early goal‐directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377.
- . The lingering consequences of sepsis: a hidden public health disaster? JAMA. 2010;304:1833–1834.
- , , , et al. Long‐term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794.
- , , , et al. Spurious inferences about long‐term outcomes: the case of severe sepsis and geriatric conditions. Am J Respir Crit Care Med. 2012;185:835–841.
- , , , et al. Long‐term outcome and quality‐adjusted life years after severe sepsis. Crit Care Med. 2009;37:1268–1274.
- , , , et al. Long‐term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38:1276–1283.
- , , , et al. Improving post‐intensive care unit neuropsychiatric outcomes: understanding cognitive effects of physical activity. Am J Respir Crit Care Med. 2012;186:1220–1228.
- , , , et al. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2012;40:754–761.
- , , , et al. Long‐term acute care hospital utilization after critical illness. JAMA. 2010;303:2253–2259.
- , , , et al. Long‐term survival and healthcare utilization outcomes attributable to sepsis and pneumonia. BMC Health Serv Res. 2012;12:432.
- , , , et al. Long‐term mortality and medical care charges in patients with severe sepsis. Crit Care Med. 2003;31:2316–2323.
- , , , et al. Early exercise in critically ill patients enhances short‐term functional recovery. Crit Care Med. 2009;37:2499–2505.
- , , , et al. Exercise‐based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2011;(7):CD001800.
- , , , et al. What is the role of timing in the surgical and rehabilitative care of community‐dwelling older persons with acute hip fracture? Arch Intern Med. 1997;157:513–520.
- , , . Extra physical therapy reduces patient length of stay and improves functional outcomes and quality of life in people with acute or subacute conditions: a systematic review. Arch Phys Med Rehabilil. 2011;92:1490–1500.
- , , . Reduction of intensive care unit length of stay: the case of early mobilization. Health Care Manag (Frederick). 2014;33:128–135.
- , , , et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–1882.
- , , , et al. Hospitalization, restricted activity, and the development of disability among older persons. JAMA. 2004;292:2115–2124.
- , , , et al. Low mobility during hospitalization and functional decline in older adults. J Am Geriatr Soc. 2011;59:266–273.
- , . The Medical Works of Hippocrates. Oxford, United Kingdom: Blackwell; 1950.
- , , . An overview of the issues: physiological effects of bed rest and restricted physical activity. Med Sci Sports Exerc. 1997;29:187–190.
- , , , et al. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol. 1996;270:E627–E633.
- , . Metabolic consequences of muscle disuse atrophy. J Nutr. 2005;135:1824S–1828S.
- . Inactivity and inflammation in the critically ill patient. Crit Care Clin. 2007;23:21–34.
- . Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118:219–223.
- , , . Hospitalization‐associated disability: “She was probably able to ambulate, but I'm not sure”. JAMA. 2011;306:1782–1793.
- , , , et al. A sustained rat model for studying the long‐lasting catabolic state of sepsis. Infect Immun. 1999;67:1079–1085.
- . Regulation of skeletal muscle protein turnover during sepsis. Curr Opin Clin Nutr. Metab Care. 1998;1:217–224.
- , , . Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab. 2007;293:E453–E459.
- , . From muscle disuse to myopathy in COPD: potential contribution of oxidative stress. Eur Respir J. 2005;26:703–719.
- , , . Oxidative stress and gene expression in sepsis. Br J Anaesth. 2003;90:221–232.
- , , , et al. Early ambulation and length of stay in older adults hospitalized for acute illness. Arch Intern Med. 2010;170:1942–1943.
- . Intensive exercise training during bed rest attenuates deconditioning. Med Sci Sports Exerc. 1997;29:207–215.
- , , , et al. Early activity is feasible and safe in respiratory failure patients. Crit Care Med. 2007;35:139–145.
- , , , et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36:2238–2243.
- . Clinical trials of early mobilization of critically ill patients. Crit Care Med. 2009;37:S442–S447.
- . Mobilizing patients in the intensive care unit: improving neuromuscular weakness and physical function. JAMA. 2008;300:1685–1690.
- , , , et al. Potential effect of physiotherapeutic treatment on mortality rate in patients with severe sepsis and septic shock: a retrospective cohort analysis. J Crit Care. 2013;28:954–958.
- , , , et al. Effects of physical training on functional status in patients with prolonged mechanical ventilation. Phys Ther. 2006;86:1271–1281.
- , , , et al. Impact of whole‐body rehabilitation in patients receiving chronic mechanical ventilation. Crit Care Med. 2005;33:2259–2265.
- . Rehabilitation of patients admitted to a respiratory intensive care unit. Arch Phys Med Rehabil. 1998;79:849–854.
- , , , et al. Supervised moderate intensity exercise improves distance walked at hospital discharge following coronary artery bypass graft surgery—a randomised controlled trial. Heart Lung Circ. 2008;17:129–138.
- , , , et al. Systematic review of hip fracture rehabilitation practices in the elderly. Arch Phys Med Rehabil. 2009;90:246–262.
- , , , et al. Physical therapy and mobility 2 and 6 months after hip fracture. J Am Geriatr Soc. 2004;52:1114–1120.
- , , , et al. Physical fitness training for stroke patients. Cochrane Database Syst Rev. 2011;(11):CD003316.
- , , , et al. Effects of augmented exercise therapy on outcome of gait and gait‐related activities in the first 6 months after stroke: a meta‐analysis. Stroke. 2011;42:3311–3315.
- , , , et al. Effects of augmented exercise therapy time after stroke: a meta‐analysis. Stroke. 2004;35:2529–2539.
- , , , et al. Effect of physical therapy on pain and function in patients with hip osteoarthritis: a randomized clinical trial. JAMA. 2014;311:1987–1997.
- , , , et al. Early mobilization of patients hospitalized with community‐acquired pneumonia. Chest. 2003;124:883–889.
- , , , et al. Magnitude and duration of the effect of sepsis on survival. Department of Veterans Affairs Systemic Sepsis Cooperative Studies Group. JAMA. 1997;277:1058–1063.
- , , , et al. Epidemiology of sepsis in Victoria, Australia. Crit Care Med. 2005;33:71–80.
- , , , et al. Sepsis incidence and outcome: contrasting the intensive care unit with the hospital ward. Crit Care Med. 2007;35:1284–1289.
- , , . Early rehabilitation in sepsis: a prospective randomised controlled trial investigating functional and physiological outcomes The i‐PERFORM Trial (Protocol Article). BMC Anesthesiol. 2011;11:21.
- , , , et al. TEAM: a prospective multi‐centre cohort study of early activity and mobilisation in ICU. In: American Thoracic Society 2013 International Conference; May 17–22, 2013; Philadelphia, PA. Am J Respir Crit Care Med. 2013;187:A3625.
- , , , et al. Improving long‐term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Crit Care Med. 2012;40:502–509.
- , , . Septicemia in U.S. hospitals, 2009: statistical brief #122. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD; 2006.
Evaluating an Academic Hospitalist Service
Improving quality while reducing costs remains important for hospitals across the United States, including the approximately 150 hospitals that are part of the Veterans Affairs (VA) healthcare system.[1, 2] The field of hospital medicine has grown rapidly, leading to predictions that the majority of inpatient care in the United States eventually will be delivered by hospitalists.[3, 4] In 2010, 57% of US hospitals had hospitalists on staff, including 87% of hospitals with 200 beds,[5] and nearly 80% of VA hospitals.[6]
The demand for hospitalists within teaching hospitals has grown in part as a response to the mandate to reduce residency work hours.[7] Furthermore, previous research has found that hospitalist care is associated with modest reductions in length of stay (LOS) and weak but inconsistent differences in quality.[8] The educational effect of hospitalists has been far less examined. The limited number of studies published to date suggests that hospitalists may improve resident learning and house‐officer satisfaction in academic medical centers and community teaching hospitals[9, 10, 11] and provide positive experiences for medical students12,13; however, Wachter et al reported no significant changes in clinical outcomes or patient, faculty, and house‐staff satisfaction in a newly designed hospital medicine service in San Francisco.[14] Additionally, whether using hospitalists influences nurse‐physician communication[15] is unknown.
Recognizing the limited and sometimes conflicting evidence about the hospitalist model, we report the results of a 3‐year quasi‐experimental evaluation of the experience at our medical center with academic hospitalists. As part of a VA Systems Redesign Improvement Capability Grantknown as the Hospital Outcomes Program of Excellence (HOPE) Initiativewe created a hospitalist‐based medicine team focused on quality improvement, medical education, and patient outcomes.
METHODS
Setting and Design
The main hospital of the VA Ann Arbor Healthcare System, located in Ann Arbor, Michigan, operates 105 acute‐care beds and 40 extended‐care beds. At the time of this evaluation, the medicine service consisted of 4 internal medicine teamsGold, Silver, Burgundy, and Yelloweach of which was responsible for admitting patients on a rotating basis every fourth day, with limited numbers of admissions occurring between each team's primary admitting day. Each team is led by an attending physician, a board‐certified (or board‐eligible) general internist or subspecialist who is also a faculty member at the University of Michigan Medical School. Each team has a senior medical resident, 2 to 3 interns, and 3 to 5 medical students (mostly third‐year students). In total, there are approximately 50 senior medical residents, 60 interns, and 170 medical students who rotate through the medicine service each year. Traditional rounding involves the medical students and interns receiving sign‐out from the overnight team in the morning, then pre‐rounding on each patient by obtaining an interval history, performing an exam, and checking any test results. A tentative plan of care is formed with the senior medical resident, usually by discussing each patient very quickly in the team room. Attending rounds are then conducted, with the physician team visiting each patient one by one to review and plan all aspects of care in detail. When time allows, small segments of teaching may occur during these attending work rounds. This system had been in place for >20 years.
Resulting in part from a grant received from the VA Systems Redesign Central Office (ie, the HOPE Initiative), the Gold team was modified in July 2009 and an academic hospitalist (S.S.) was assigned to head this team. Specific hospitalists were selected by the Associate Chief of Medicine (S.S.) and the Chief of Medicine (R.H.M.) to serve as Gold team attendings on a regular basis. The other teams continued to be overseen by the Chief of Medicine, and the Gold team remained within the medicine service. Characteristics of the Gold and nonGold team attendings can be found in Table 1. The 3 other teams initially were noninterventional concurrent control groups. However, during the second year of the evaluation, the Silver team adopted some of the initiatives as a result of the preliminary findings observed on Gold. Specifically, in the second year of the evaluation, approximately 42% of attendings on the Silver team were from the Gold team. This increased in the third year to 67% of coverage by Gold team attendings on the Silver team. The evaluation of the Gold team ended in June 2012.
| Characteristic | Gold Team | Non‐Gold Teams |
|---|---|---|
| Total number of attendings | 14 | 57 |
| Sex, % | ||
| Male | 79 | 58 |
| Female | 21 | 42 |
| Median years postresidency (range) | 10 (130) | 7 (141) |
| Subspecialists, % | 14 | 40 |
| Median days on service per year (range) | 53 (574) | 30 (592) |
The clinical interventions implemented on the Gold team were quality‐improvement work and were therefore exempt from institutional review board review. Human subjects' approval was, however, received to conduct interviews as part of a qualitative assessment.
Clinical Interventions
Several interventions involving the clinical care delivered were introduced on the Gold team, with a focus on improving communication among healthcare workers (Table 2).
| Clinical Interventions | Educational Interventions |
|---|---|
| Modified structure of attending rounds | Modified structure of attending rounds |
| Circle of Concern rounds | Attending reading list |
| Clinical Care Coordinator | Nifty Fifty reading list for learners |
| Regular attending team meetings | Website to provide expectations to learners |
| Two‐month per year commitment by attendings |
Structure of Attending Rounds
The structure of morning rounds was modified on the Gold team. Similar to the traditional structure, medical students and interns on the Gold team receive sign‐out from the overnight team in the morning. However, interns and students may or may not conduct pre‐rounds on each patient. The majority of time between sign‐out and the arrival of the attending physician is spent on work rounds. The senior resident leads rounds with the interns and students, discussing each patient while focusing on overnight events and current symptoms, new physical‐examination findings, and laboratory and test data. The plan of care to be presented to the attending is then formulated with the senior resident. The attending physician then leads Circle of Concern rounds with an expanded team, including a charge nurse, a clinical pharmacist, and a nurse Clinical Care Coordinator. Attending rounds tend to use an E‐AP format: significant Events overnight are discussed, followed by an Assessment & Plan by problem for the top active problems. Using this model, the attendings are able to focus more on teaching and discussing the patient plan than in the traditional model (in which the learner presents the details of the subjective, objective, laboratory, and radiographic data, with limited time left for the assessment and plan for each problem).
Circle of Concern Rounds
Suzanne Gordon described the Circle of Concern in her book Nursing Against the Odds.[16] From her observations, she noted that physicians typically form a circle to discuss patient care during rounds. The circle expands when another physician joins the group; however, the circle does not similarly expand to include nurses when they approach the group. Instead, nurses typically remain on the periphery, listening silently or trying to communicate to physicians' backs.[16] Thus, to promote nurse‐physician communication, Circle of Concern rounds were formally introduced on the Gold team. Each morning, the charge nurse rounds with the team and is encouraged to bring up nursing concerns. The inpatient clinical pharmacist is also included 2 to 3 times per week to help provide education to residents and students and perform medication reconciliation.
Clinical Care Coordinator
The role of the nurse Clinical Care Coordinatoralso introduced on the Gold teamis to provide continuity of patient care, facilitate interdisciplinary communication, facilitate patient discharge, ensure appropriate appointments are scheduled, communicate with the ambulatory care service to ensure proper transition between inpatient and outpatient care, and help educate residents and students on VA procedures and resources.
Regular Gold Team Meetings
All Gold team attendings are expected to dedicate 2 months per year to inpatient service (divided into half‐month blocks), instead of the average 1 month per year for attendings on the other teams. The Gold team attendings, unlike the other teams, also attend bimonthly meetings to discuss strategies for running the team.
Educational Interventions
Given the high number of learners on the medicine service, we wanted to enhance the educational experience for our learners. We thus implemented various interventions, in addition to the change in the structure of rounds, as described below.
Reading List for Learners: The Nifty Fifty
Because reading about clinical medicine is an integral part of medical education, we make explicit our expectation that residents and students read something clinically relevant every day. To promote this, we have provided a Nifty Fifty reading list of key articles. The PDF of each article is provided, along with a brief summary highlighting key points.
Reading List for Gold Attendings and Support Staff
To promote a common understanding of leadership techniques, management books are provided to Gold attending physicians and other members of the team (eg, Care Coordinator, nurse researcher, systems redesign engineer). One book is discussed at each Gold team meeting (Table 3), with participants taking turns leading the discussion.
| Book Title | Author(s) |
|---|---|
| The One Minute Manager | Ken Blanchard and Spencer Johnson |
| Good to Great | Jim Collins |
| Good to Great and the Social Sectors | Jim Collins |
| The Checklist Manifesto: How to Get Things Right | Atul Gawande |
| The Five Dysfunctions of a Team: A Leadership Fable | Patrick Lencioni |
| Getting to Yes: Negotiating Agreement Without Giving In | Roger Fisher, William Ury, and Bruce Patton |
| The Effective Executive: The Definitive Guide to Getting the Right Things Done | Peter Drucker |
| A Sense of Urgency | John Kotter |
| The Power of Positive Deviance: How Unlikely Innovators Solve the World's Toughest Problems | Richard Pascale, Jerry Sternin, and Monique Sternin |
| On the Mend: Revolutionizing Healthcare to Save Lives and Transform the Industry | John Toussaint and Roger Gerard |
| Outliers: The Story of Success | Malcolm Gladwell |
| Nursing Against the Odds: How Health Care Cost Cutting, Media Stereotypes, and Medical Hubris Undermine Nurses and Patient Care | Suzanne Gordon |
| How the Mighty Fall and Why Some Companies Never Give In | Jim Collins |
| What the Best College Teachers Do | Ken Bain |
| The Creative Destruction of Medicine | Eric Topol |
| What Got You Here Won't Get You There: How Successful People Become Even More Successful! | Marshall Goldsmith |
Website
A HOPE Initiative website was created (
Qualitative Assessment
To evaluate our efforts, we conducted a thorough qualitative assessment during the third year of the program. A total of 35 semistructured qualitative interviews were conducted with patients and staff from all levels of the organization, including senior leadership. The qualitative assessment was led by research staff from the Center for Clinical Management Research, who were minimally involved in the redesign effort and could provide an unbiased view of the initiative. Field notes from the semistructured interviews were analyzed, with themes developed using a descriptive approach and through discussion by a multidisciplinary team, which included building team consensus on findings that were supported by clear evidence in the data.[17]
Quantitative Outcome Measures
Clinical Outcomes
To determine if our communication and educational interventions had an impact on patient care, we used hospital administrative data to evaluate admission rates, LOS, and readmission rates for all 4 of the medicine teams. Additional clinical measures were assessed as needed. For example, we monitored the impact of the clinical pharmacist during a 4‐week pilot study by asking the Clinical Care Coordinator to track the proportion of patient encounters (n=170) in which the clinical pharmacist changed management or provided education to team members. Additionally, 2 staff surveys were conducted. The first survey focused on healthcare‐worker communication and was given to inpatient nurses and physicians (including attendings, residents, and medical students) who were recently on an inpatient medical service rotation. The survey included questions from previously validated communication measures,[18, 19, 20] as well as study‐specific questions. The second survey evaluated the new role of the Clinical Care Coordinator (Appendix). Both physicians and nurses who interacted with the Gold team's Clinical Care Coordinator were asked to complete this survey.
Educational Outcomes
To assess the educational interventions, we used learner evaluations of attendings, by both residents and medical students, and standardized internal medicine National Board of Medical Examiners Subject Examination (or shelf) scores for third‐year medical students. A separate evaluation of medical student perceptions of the rounding structure introduced on the Gold team using survey design has already been published.[21]
Statistical Analyses
Data from all sources were analyzed using SAS 9.3 (SAS Institute, Inc., Cary, NC). Outliers for the LOS variable were removed from the analysis. Means and frequency distributions were examined for all variables. Student t tests and [2] tests of independence were used to compare data between groups. Multivariable linear regression models controlling for time (preintervention vs postintervention) were used to assess the effect of the HOPE Initiative on patient LOS and readmission rates. In all cases, 2‐tailed P values of 0.05 or less were considered statistically significant.
Role of the Funding Source
The VA Office of Systems Redesign provided funding but was not involved in the design or conduct of the study, data analysis, or preparation of the manuscript.
RESULTS
Clinical Outcomes
Patient Outcomes
Our multivariable linear regression analysis, controlling for time, showed a significant reduction in LOS of approximately 0.3 days on all teams after the HOPE Initiative began (P=0.004). There were no significant differences between the Gold and non‐Gold teams in the multivariate models when controlling for time for any of the patient‐outcome measures. The number of admissions increased for all 4 medical teams (Figure 1), but, as shown in Figures 2 and 3, the readmission rates for all teams remained relatively stable over this same period of time.

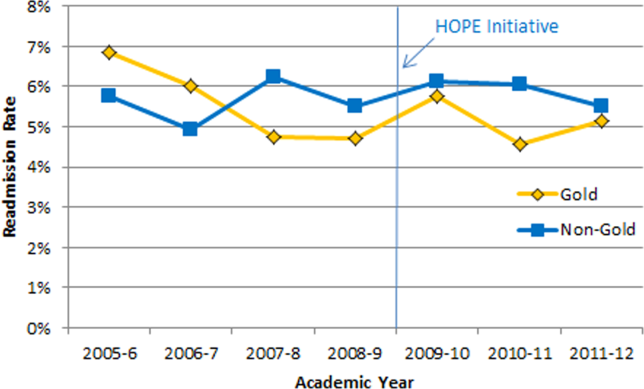

Clinical Pharmacist on Gold Team Rounds
The inpatient clinical pharmacist changed the management plan for 22% of the patients seen on rounds. Contributions from the clinical pharmacist included adjusting the dosing of ordered medication and correcting medication reconciliation. Education and pharmaceutical information was provided to the team in another 6% of the 170 consecutive patient encounters evaluated.
Perception of Circle of Concern Rounds
Circle of Concern rounds were generally well‐received by both nurses and physicians. In a healthcare‐worker communication survey, completed by 38 physicians (62% response rate) and 48 nurses (54% response rate), the majority of both physicians (83%) and nurses (68%) felt Circle of Concern rounds improved communication.
Nurse Perception of Communication
The healthcare‐worker communication survey asked inpatient nurses to rate communication between nurses and physicians on each of the 4 medicine teams. Significantly more nurses were satisfied with communication with the Gold team (71%) compared with the other 3 medicine teams (53%; P=0.02) (Figure 4).
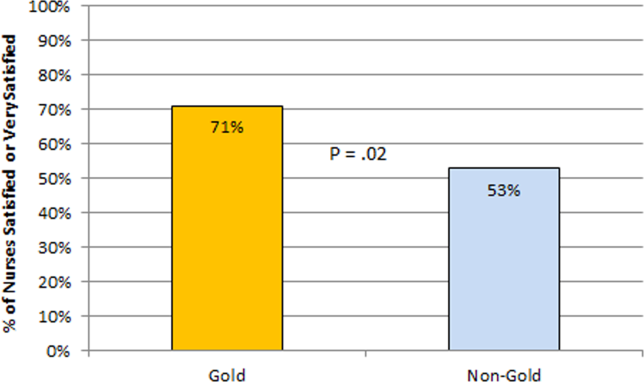
Perception of the Clinical Care Coordinator
In total, 20 physicians (87% response rate) and 10 nurses (56% response rate) completed the Clinical Care Coordinator survey. The physician results were overwhelmingly positive: 100% were satisfied or very satisfied with the role; 100% felt each team should have a Clinical Care Coordinator; and 100% agreed or strongly agreed that the Clinical Care Coordinator ensures that appropriate follow‐up is arranged, provides continuity of care, assists with interdisciplinary communication, and helps facilitate discharge. The majority of nurses was also satisfied or very satisfied with the Clinical Care Coordinator role and felt each team should have one.
Educational Outcomes
House Officer Evaluation of Attendings
Monthly evaluations of attending physicians by house officers (Figure 5) revealed that prior to the HOPE Initiative, little differences were observed between teams, as would be expected because attending assignment was largely random. After the intervention date of July 2009, however, significant differences were noted, with Gold team attendings receiving significantly higher teaching evaluations immediately after the introduction of the HOPE Initiative. Although ratings for Gold attendings remained more favorable, the difference was no longer statistically significant in the second and third year of the initiative, likely due to Gold attendings serving on other medicine teams, which contributed to an improvement in ratings of all attendings.
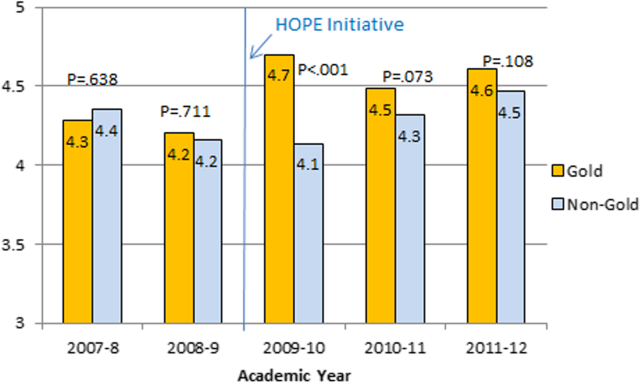
Medical Student Evaluation of Attendings
Monthly evaluations of attending physicians by third‐year medical students (Figure 6) revealed differences between the Gold attendings and all others, with the attendings that joined the Gold team in 2009 receiving higher teaching evaluations even before the HOPE Initiative started. However, this difference remained statistically significant in years 2 and 3 postinitiative, despite the addition of 4 new junior attendings.
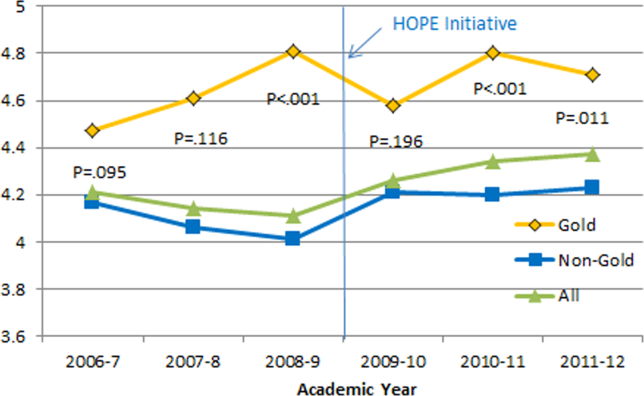
Medical Student Medicine Shelf Scores
The national average on the shelf exam, which reflects learning after the internal medicine third‐year clerkship, has ranged from 75 to 78 for the past several years, with University of Michigan students averaging significantly higher scores prior to and after the HOPE Initiative. However, following the HOPE Initiative, third‐year medical students on the Gold team scored significantly higher on the shelf exam compared with their colleagues on the non‐Gold teams (84 vs 82; P=0.006). This difference in the shelf exam scores, although small, is statistically significant. It represents a measurable improvement in shelf scores in our system and demonstrates the potential educational benefit for the students. Over this same time period, scores on the United States Medical Licensing Exam, given to medical students at the beginning of their third year, remained stable (233 preHOPE Initiative; 234 postHOPE Initiative).
Qualitative Assessment
Qualitative data collected as part of our evaluation of the HOPE Initiative also suggested that nurse‐physician communication had improved since the start of the project. In particular, they reported positively on the Gold team in general, the Circle of Concern rounds, and the Clinical Care Coordinator (Table 4).
| Staff Type | Statement1 |
|---|---|
| |
| Nurse | [Gold is] above and beyond other [teams]. Other teams don't run as smoothly. |
| Nurse | There has been a difference in communication [on Gold]. You can tell the difference in how they communicate with staff. We know the Clinical Care Coordinator or charge nurse is rounding with that team, so there is more communication. |
| Nurse | The most important thing that has improved communication is the Circle of Concern rounds. |
| Physician | [The Gold Clinical Care Coordinator] expedites care, not only what to do but who to call. She can convey the urgency. On rounds she is able to break off, put in an order, place a call, talk to a patient. Things that we would do at 11 AM she gets to at 9 AM. A couple of hours may not seem like much, but sometimes it can make the difference between things happening that day instead of the next. |
| Physician | The Clinical Care Coordinator is completely indispensable. Major benefit to providing care to Veterans. |
| Physician | I like to think Gold has lifted all of the teams to a higher level. |
| Medical student | It may be due to personalities vs the Gold [team] itself, but there is more emphasis on best practices. Are we following guidelines even if it is not related to the primary reason for admission? |
| Medical student | Gold is very collegial and nurses/physicians know one another by name. Physicians request rather than order; this sets a good example to me on how to approach the nurses. |
| Chief resident | [Gold attendings] encourage senior residents to take charge and run the team, although the attending is there for back‐up and support. This provides great learning for the residents. Interns and medical students also are affected because they have to step up their game as well. |
DISCUSSION
Within academic medical centers, hospitalists are expected to care for patients, teach, and help improve the quality and efficiency of hospital‐based care.[7] The Department of Veterans Affairs runs the largest integrated healthcare system in the United States, with approximately 80% of VA hospitals having hospital medicine programs. Overall, one‐third of US residents perform part of their residency training at a VA hospital.[22, 23] Thus, the effects of a system‐wide change at a VA hospital may have implications throughout the country. We studied one such intervention. Our primary findings are that we were able to improve communication and learner education with minimal effects on patient outcomes. While overall LOS decreased slightly postintervention, after taking into account secular trends, readmission rates did not.
We are not the first to evaluate a hospital medicine team using a quasi‐experimental design. For example, Meltzer and colleagues evaluated a hospitalist program at the University of Chicago Medical Center and found that, by the second year of operation, hospitalist care was associated with significantly shorter LOS (0.49 days), reduced costs, and decreased mortality.[24] Auerbach also evaluated a newly created hospital medicine service, finding decreased LOS (0.61 days), lower costs, and lower risk of mortality by the second year of the program.[25]
Improving nurse‐physician communication is considered important for avoiding medical error,[26] yet there has been limited empirical study of methods to improve communication within the medical profession.[27] Based both on our surveys and qualitative interviews, healthcare‐worker communication appeared to improve on the Gold team during the study. A key component of this improvement is likely related to instituting Circle of Concern rounds, in which nurses joined the medical team during attending rounds. Such an intervention likely helped to address organizational silence[28] and enhance the psychological safety of the nursing staff, because the attending physician was proactive about soliciting the input of nurses during rounds.[29] Such leader inclusivenesswords and deeds exhibited by leaders that invite and appreciate others' contributionscan aid interdisciplinary teams in overcoming the negative effects of status differences, thereby promoting collaboration.[29] The inclusion of nurses on rounds is also relationship‐building, which Gotlib Conn and colleagues found was important to improved interprofessional communication and collaboration.[30] In the future, using a tool such as the Teamwork Effectiveness Assessment Module (TEAM) developed by the American Board of Internal Medicine[31] could provide further evaluation of the impact on interprofessional teamwork and communication.
The focus on learner education, though evaluated in prior studies, is also novel. One previous survey of medical students showed that engaging students in substantive discussions is associated with greater student satisfaction.[32] Another survey of medical students found that attendings who were enthusiastic about teaching, inspired confidence in knowledge and skills, provided useful feedback, and encouraged increased student responsibility were viewed as more effective teachers.[33] No previous study that we are aware of, however, has looked at actual educational outcomes, such as shelf scores. The National Board of Medical Examiners reports that the Medicine subject exam is scaled to have a mean of 70 and a standard deviation of 8.[34] Thus, a mean increase in score of 2 points is small, but not trivial. This shows improvement in a hard educational outcome. Additionally, 2 points, although small in the context of total score and standard deviation, may make a substantial difference to an individual student in terms of overall grade, and, thus, residency applications. Our finding that third‐year medical students on the Gold team performed significantly better than University of Michigan third‐year medical students on other teams is an intriguing finding that warrants confirmation. On the other hand, this finding is consistent with a previous report evaluating learner satisfaction in which Bodnar et al found improved ratings of quantity and quality of teaching on teams with a nontraditional structure (Gold team).[21] Moreover, despite relatively few studies, the reason underlying the educational benefit of hospitalists should surprise few. The hospitalist model ensures that learners are supervised by physicians who are experts in the care of hospitalized patients.[35] Hospitalists hired at teaching hospitals to work on services with learners are generally chosen because they possess superior educational skills.[7]
Our findings should be interpreted in the context of the following limitations. First, our study focused on a single academically affiliated VA hospital. As other VA hospitals are pursuing a similar approach (eg, the Houston and Detroit VA medical centers), replicating our results will be important. Second, the VA system, although the largest integrated healthcare system in the United States, has unique characteristicssuch as an integrated electronic health record and predominantly male patient populationthat may make generalizations to the larger US healthcare system challenging. Third, there was a slightly lower response rate among nurses on a few of the surveys to evaluate our efforts; however, this rate of response is standard at our facility. Finally, our evaluation lacks an empirical measure of healthcare‐worker communication, such as incident reports.
Despite these limitations, our results have important implications. Using both quantitative and qualitative assessment, we found that academic hospitalists have the ability to improve healthcare‐worker communication and enhance learner education without increasing LOS. These findings are directly applicable to VA medical centers and potentially applicable to other academic medical centers.
Acknowledgments
The authors thank Milisa Manojlovich, PhD, RN, Edward Kennedy, MS, and Andrew Hickner, MSI, for help with preparation of this manuscript.
Disclosures: This work was funded by a US Department of Veterans Affairs, Office of Systems Redesign Improvement Capability grant. The findings and conclusions in this report are those of the authors and do not necessarily represent the position or policy of the Department of Veterans Affairs. Dr. Saint reports receiving travel reimbursement for giving invited talks at the Society of Hospital Medicine's National Meeting, as well as serving on the advisory boards of Doximity and Jvion.
APPENDIX
Survey to Evaluate the Care Coordinator Position
| Yes | No | Not Sure | |
| Q1. Are you familiar with the role of the Care Coordinator on the Gold Service (Susan Lee)? | 1 | 2 | 3 |
Please indicate how much you agree or disagree with the statements below.
| Strongly Agree | Agree | Neutral | Disagree | Strongly Disagree | Don't Know | |
| Q2. The Care Coordinator ensures that appropriate primary care follow‐up and any other appropriate services are arranged. | 1 | 2 | 3 | 4 | 5 | 9 |
| Q3. The Care Coordinator provides continuity of patient care on the Gold Service. | 1 | 2 | 3 | 4 | 5 | 9 |
| Q4. The Care Coordinator helps educate House Officers and Medical Students on VA processes (e.g., CPRS). | 1 | 2 | 3 | 4 | 5 | 9 |
| Q5. The Care Coordinator assists with interdisciplinary communication between the medical team and other services (e.g., nursing, ambulatory care, pharmacy, social work) | 1 | 2 | 3 | 4 | 5 | 9 |
| Q6. The Care Coordinator helps facilitate patient discharge. | 1 | 2 | 3 | 4 | 5 | 9 |
| Q7. The Care Coordinator initiates communication with the ambulatory care teams to coordinate care. | 1 | 2 | 3 | 4 | 5 | 9 |
| Yes | No | |
| Q8. Are you a physician (attending or resident), or medical student who has been on more than one medical team at the VA (Gold, Silver, Burgundy, or Yellow)? | 1 | 2 |
If no, please skip to Q13
If yes, comparing your experience on the Gold Service (with the Care Coordinator) to your experience on any of the other services (Silver, Burgundy, or Yellow):
| Not at All | Very Little | Somewhat | To a Great Extent | |
| Q9. To what extent does the presence of a Care Coordinator affect patient care? | 1 | 2 | 3 | 4 |
| Q10. To what extent does the presence of a Care Coordinator improve patient flow? | 1 | 2 | 3 | 4 |
| Q11. To what extent does the presence of a Care Coordinator assist with education? | 1 | 2 | 3 | 4 |
| Q12. To what extent does the presence of a Care Coordinator contribute to attending rounds? | 1 | 2 | 3 | 4 |
| Yes | No | |
| Q13. Do you work [as a nurse] in ambulatory care? | 1 | 2 |
If no, please skip to Q17.
If yes, comparing your experience with the Gold Service (with the Care Coordinator) to the other services (Silver, Burgundy, or Yellow):
| Not at All | Very Little | Somewhat | To a Great Extent | |
| Q14. To what extent does the presence of a Care Coordinator improve coordination of care between inpatient and outpatient services? | 1 | 2 | 3 | 4 |
| Q15. To what extent does the presence of a Care Coordinator help identify high risk patients who require follow‐up? | 1 | 2 | 3 | 4 |
| Q16. To what extent does the presence of a Care Coordinator ensure follow‐up appointments are scheduled? | 1 | 2 | 3 | 4 |
| Yes | No | Not Sure | |
| Q17. Do you think each medical team should have a Care Coordinator? | 1 | 2 | 3 |
| Q18. Are there any additional tasks or duties you think would improve the effectiveness of the Care Coordinator? |
| Very Satisfied | Satisfied | Neutral | Dissatisfied | Very Dissatisfied | |
| Q19. Overall how satisfied are you with the role of the Care Coordinator on the Gold Service? | 1 | 2 | 3 | 4 | 5 |
| Q20. Do you have any other comments about the role of the Care Coordinator? |
| Q21. What is your position? |
| 1. Physician (attending or resident) or medical student |
| 2. Nurse (inpatient or ambulatory care) |
- Kohn LT, Corrigan JM, Donaldson MS, eds. To Err Is Human: Building a Safer Health System. Washington, D.C.: National Academies Press; 2000.
- Institute of Medicine of the National Academies. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, D.C.: National Academies Press; 2001.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- . Growth in care provided by hospitalists. N Engl J Med. 2009;360(26):2789–2791.
- American Hospital Association. AHA Annual Survey of Hospitals, 2010. Chicago, IL: Health Forum, LLC; 2010.
- , , , . Preventing hospital‐acquired infections: a national survey of practices reported by U.S. hospitals in 2005 and 2009. J Gen Intern Med. 2012;27(7):773–779.
- , . Hospitalists in teaching hospitals: opportunities but not without danger. J Gen Intern Med. 2004;19(4):392–393.
- , . Do hospitalist physicians improve the quality of inpatient care delivery? A systematic review of process, efficiency and outcome measures. BMC Med. 2011;9:58.
- , , , . Effect of hospitalist attending physicians on trainee educational experiences: a systematic review. J Hosp Med. 2009;4(8):490–498.
- , , , , , . Resident satisfaction on an academic hospitalist service: time to teach. Am J Med. 2002;112(7):597–601.
- , , , et al. The positive impact of initiation of hospitalist clinician educators. J Gen Intern Med. 2004;19(4):293–301.
- , . Third‐year medical students' evaluation of hospitalist and nonhospitalist faculty during the inpatient portion of their pediatrics clerkships. J Hosp Med. 2007;2(1):17–22.
- , , , . Medical student evaluation of the quality of hospitalist and nonhospitalist teaching faculty on inpatient medicine rotations. Acad Med. 2004;79(1):78–82.
- , , , , . Reorganizing an academic medical service: impact on cost, quality, patient satisfaction, and education. JAMA. 1998;279(19):1560–1565.
- . Nurse/physician communication through a sensemaking lens: shifting the paradigm to improve patient safety. Med Care. 2010;48(11):941–946.
- . Nursing Against the Odds: How Health Care Cost Cutting, Media Stereotypes, and Medical Hubris Undermine Nurses and Patient Care. Ithaca, NY: Cornell University Press; 2005.
- . Focus on research methods: whatever happened to qualitative description? Res Nurs Health. 2000;23:334–340.
- , , , , . Organizational assessment in intensive care units (ICUs): construct development, reliability, and validity of the ICU nurse‐physician questionnaire. Med Care. 1991;29(8):709–726.
- . Development of an instrument to measure collaboration and satisfaction about care decisions. J Adv Nurs. 1994;20(1):176–182.
- . Development of the practice environment scale of the Nursing Work Index. Res Nurs Health. 2002;25(3):176–188.
- , , . Does the structure of inpatient rounds affect medical student education? Int J Med Educ. 2013;4:96–100.
- U.S. Department of Veterans Affairs, Office of Academic Affiliations. Medical and Dental Education Program. Available at: http://www.va. gov/oaa/GME_default.asp. Published 2012. Accessed May 08, 2013.
- , . Graduate medical education, 2011–2012. JAMA. 2012;308(21):2264–2279.
- , , , et al. Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists. Ann Intern Med. 2002;137(11):866–874.
- , , , , , . Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes. Ann Intern Med. 2002;137(11):859–865.
- , , . Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79(2):186–194.
- , , . ‘It depends': medical residents' perspectives on working with nurses. Am J Nurs. 2009;109(7):34–44.
- , . Organizational silence: a barrier to change and development in a pluralistic world. Acad Manage Rev. 2000;25(4):706–725.
- , . Making it safe: the effects of leader inclusiveness and professional status on psychological safety and improvement efforts in health care teams. J Organiz Behav. 2006;27:941–966.
- , , , , . Interprofessional communication with hospitalist and consultant physicians in general internal medicine: a qualitative study. BMC Health Serv Res. 2012;12:437.
- , , , , , . A new tool to give hospitalists feedback to improve interprofessional teamwork and advance patient care. Health Aff (Millwood). 2012;31(11):2485–2492.
- , , , , , . Impact of instructional practices on student satisfaction with attendings' teaching in the inpatient component of internal medicine clerkships. J Gen Intern Med. 2006;21(1):7–12.
- , . Medical students' perceptions of the elements of effective inpatient teaching by attending physicians and housestaff. J Gen Intern Med. 2005;20(7):635–639.
- National Board of Medical Examiners Subject Examination Program. Internal Medicine Advanced Clinical Examination, score interpretation guide. Available at: http://www.nbme.org/PDF/SampleScoreReports/Internal_Medicine_ACE_Score_Report.pdf. Published 2011. Accessed September 13, 2013.
- . The impact of hospitalists on medical education and the academic health system. Ann Intern Med. 1999;130(4 part 2):364–367.
Improving quality while reducing costs remains important for hospitals across the United States, including the approximately 150 hospitals that are part of the Veterans Affairs (VA) healthcare system.[1, 2] The field of hospital medicine has grown rapidly, leading to predictions that the majority of inpatient care in the United States eventually will be delivered by hospitalists.[3, 4] In 2010, 57% of US hospitals had hospitalists on staff, including 87% of hospitals with 200 beds,[5] and nearly 80% of VA hospitals.[6]
The demand for hospitalists within teaching hospitals has grown in part as a response to the mandate to reduce residency work hours.[7] Furthermore, previous research has found that hospitalist care is associated with modest reductions in length of stay (LOS) and weak but inconsistent differences in quality.[8] The educational effect of hospitalists has been far less examined. The limited number of studies published to date suggests that hospitalists may improve resident learning and house‐officer satisfaction in academic medical centers and community teaching hospitals[9, 10, 11] and provide positive experiences for medical students12,13; however, Wachter et al reported no significant changes in clinical outcomes or patient, faculty, and house‐staff satisfaction in a newly designed hospital medicine service in San Francisco.[14] Additionally, whether using hospitalists influences nurse‐physician communication[15] is unknown.
Recognizing the limited and sometimes conflicting evidence about the hospitalist model, we report the results of a 3‐year quasi‐experimental evaluation of the experience at our medical center with academic hospitalists. As part of a VA Systems Redesign Improvement Capability Grantknown as the Hospital Outcomes Program of Excellence (HOPE) Initiativewe created a hospitalist‐based medicine team focused on quality improvement, medical education, and patient outcomes.
METHODS
Setting and Design
The main hospital of the VA Ann Arbor Healthcare System, located in Ann Arbor, Michigan, operates 105 acute‐care beds and 40 extended‐care beds. At the time of this evaluation, the medicine service consisted of 4 internal medicine teamsGold, Silver, Burgundy, and Yelloweach of which was responsible for admitting patients on a rotating basis every fourth day, with limited numbers of admissions occurring between each team's primary admitting day. Each team is led by an attending physician, a board‐certified (or board‐eligible) general internist or subspecialist who is also a faculty member at the University of Michigan Medical School. Each team has a senior medical resident, 2 to 3 interns, and 3 to 5 medical students (mostly third‐year students). In total, there are approximately 50 senior medical residents, 60 interns, and 170 medical students who rotate through the medicine service each year. Traditional rounding involves the medical students and interns receiving sign‐out from the overnight team in the morning, then pre‐rounding on each patient by obtaining an interval history, performing an exam, and checking any test results. A tentative plan of care is formed with the senior medical resident, usually by discussing each patient very quickly in the team room. Attending rounds are then conducted, with the physician team visiting each patient one by one to review and plan all aspects of care in detail. When time allows, small segments of teaching may occur during these attending work rounds. This system had been in place for >20 years.
Resulting in part from a grant received from the VA Systems Redesign Central Office (ie, the HOPE Initiative), the Gold team was modified in July 2009 and an academic hospitalist (S.S.) was assigned to head this team. Specific hospitalists were selected by the Associate Chief of Medicine (S.S.) and the Chief of Medicine (R.H.M.) to serve as Gold team attendings on a regular basis. The other teams continued to be overseen by the Chief of Medicine, and the Gold team remained within the medicine service. Characteristics of the Gold and nonGold team attendings can be found in Table 1. The 3 other teams initially were noninterventional concurrent control groups. However, during the second year of the evaluation, the Silver team adopted some of the initiatives as a result of the preliminary findings observed on Gold. Specifically, in the second year of the evaluation, approximately 42% of attendings on the Silver team were from the Gold team. This increased in the third year to 67% of coverage by Gold team attendings on the Silver team. The evaluation of the Gold team ended in June 2012.
| Characteristic | Gold Team | Non‐Gold Teams |
|---|---|---|
| Total number of attendings | 14 | 57 |
| Sex, % | ||
| Male | 79 | 58 |
| Female | 21 | 42 |
| Median years postresidency (range) | 10 (130) | 7 (141) |
| Subspecialists, % | 14 | 40 |
| Median days on service per year (range) | 53 (574) | 30 (592) |
The clinical interventions implemented on the Gold team were quality‐improvement work and were therefore exempt from institutional review board review. Human subjects' approval was, however, received to conduct interviews as part of a qualitative assessment.
Clinical Interventions
Several interventions involving the clinical care delivered were introduced on the Gold team, with a focus on improving communication among healthcare workers (Table 2).
| Clinical Interventions | Educational Interventions |
|---|---|
| Modified structure of attending rounds | Modified structure of attending rounds |
| Circle of Concern rounds | Attending reading list |
| Clinical Care Coordinator | Nifty Fifty reading list for learners |
| Regular attending team meetings | Website to provide expectations to learners |
| Two‐month per year commitment by attendings |
Structure of Attending Rounds
The structure of morning rounds was modified on the Gold team. Similar to the traditional structure, medical students and interns on the Gold team receive sign‐out from the overnight team in the morning. However, interns and students may or may not conduct pre‐rounds on each patient. The majority of time between sign‐out and the arrival of the attending physician is spent on work rounds. The senior resident leads rounds with the interns and students, discussing each patient while focusing on overnight events and current symptoms, new physical‐examination findings, and laboratory and test data. The plan of care to be presented to the attending is then formulated with the senior resident. The attending physician then leads Circle of Concern rounds with an expanded team, including a charge nurse, a clinical pharmacist, and a nurse Clinical Care Coordinator. Attending rounds tend to use an E‐AP format: significant Events overnight are discussed, followed by an Assessment & Plan by problem for the top active problems. Using this model, the attendings are able to focus more on teaching and discussing the patient plan than in the traditional model (in which the learner presents the details of the subjective, objective, laboratory, and radiographic data, with limited time left for the assessment and plan for each problem).
Circle of Concern Rounds
Suzanne Gordon described the Circle of Concern in her book Nursing Against the Odds.[16] From her observations, she noted that physicians typically form a circle to discuss patient care during rounds. The circle expands when another physician joins the group; however, the circle does not similarly expand to include nurses when they approach the group. Instead, nurses typically remain on the periphery, listening silently or trying to communicate to physicians' backs.[16] Thus, to promote nurse‐physician communication, Circle of Concern rounds were formally introduced on the Gold team. Each morning, the charge nurse rounds with the team and is encouraged to bring up nursing concerns. The inpatient clinical pharmacist is also included 2 to 3 times per week to help provide education to residents and students and perform medication reconciliation.
Clinical Care Coordinator
The role of the nurse Clinical Care Coordinatoralso introduced on the Gold teamis to provide continuity of patient care, facilitate interdisciplinary communication, facilitate patient discharge, ensure appropriate appointments are scheduled, communicate with the ambulatory care service to ensure proper transition between inpatient and outpatient care, and help educate residents and students on VA procedures and resources.
Regular Gold Team Meetings
All Gold team attendings are expected to dedicate 2 months per year to inpatient service (divided into half‐month blocks), instead of the average 1 month per year for attendings on the other teams. The Gold team attendings, unlike the other teams, also attend bimonthly meetings to discuss strategies for running the team.
Educational Interventions
Given the high number of learners on the medicine service, we wanted to enhance the educational experience for our learners. We thus implemented various interventions, in addition to the change in the structure of rounds, as described below.
Reading List for Learners: The Nifty Fifty
Because reading about clinical medicine is an integral part of medical education, we make explicit our expectation that residents and students read something clinically relevant every day. To promote this, we have provided a Nifty Fifty reading list of key articles. The PDF of each article is provided, along with a brief summary highlighting key points.
Reading List for Gold Attendings and Support Staff
To promote a common understanding of leadership techniques, management books are provided to Gold attending physicians and other members of the team (eg, Care Coordinator, nurse researcher, systems redesign engineer). One book is discussed at each Gold team meeting (Table 3), with participants taking turns leading the discussion.
| Book Title | Author(s) |
|---|---|
| The One Minute Manager | Ken Blanchard and Spencer Johnson |
| Good to Great | Jim Collins |
| Good to Great and the Social Sectors | Jim Collins |
| The Checklist Manifesto: How to Get Things Right | Atul Gawande |
| The Five Dysfunctions of a Team: A Leadership Fable | Patrick Lencioni |
| Getting to Yes: Negotiating Agreement Without Giving In | Roger Fisher, William Ury, and Bruce Patton |
| The Effective Executive: The Definitive Guide to Getting the Right Things Done | Peter Drucker |
| A Sense of Urgency | John Kotter |
| The Power of Positive Deviance: How Unlikely Innovators Solve the World's Toughest Problems | Richard Pascale, Jerry Sternin, and Monique Sternin |
| On the Mend: Revolutionizing Healthcare to Save Lives and Transform the Industry | John Toussaint and Roger Gerard |
| Outliers: The Story of Success | Malcolm Gladwell |
| Nursing Against the Odds: How Health Care Cost Cutting, Media Stereotypes, and Medical Hubris Undermine Nurses and Patient Care | Suzanne Gordon |
| How the Mighty Fall and Why Some Companies Never Give In | Jim Collins |
| What the Best College Teachers Do | Ken Bain |
| The Creative Destruction of Medicine | Eric Topol |
| What Got You Here Won't Get You There: How Successful People Become Even More Successful! | Marshall Goldsmith |
Website
A HOPE Initiative website was created (
Qualitative Assessment
To evaluate our efforts, we conducted a thorough qualitative assessment during the third year of the program. A total of 35 semistructured qualitative interviews were conducted with patients and staff from all levels of the organization, including senior leadership. The qualitative assessment was led by research staff from the Center for Clinical Management Research, who were minimally involved in the redesign effort and could provide an unbiased view of the initiative. Field notes from the semistructured interviews were analyzed, with themes developed using a descriptive approach and through discussion by a multidisciplinary team, which included building team consensus on findings that were supported by clear evidence in the data.[17]
Quantitative Outcome Measures
Clinical Outcomes
To determine if our communication and educational interventions had an impact on patient care, we used hospital administrative data to evaluate admission rates, LOS, and readmission rates for all 4 of the medicine teams. Additional clinical measures were assessed as needed. For example, we monitored the impact of the clinical pharmacist during a 4‐week pilot study by asking the Clinical Care Coordinator to track the proportion of patient encounters (n=170) in which the clinical pharmacist changed management or provided education to team members. Additionally, 2 staff surveys were conducted. The first survey focused on healthcare‐worker communication and was given to inpatient nurses and physicians (including attendings, residents, and medical students) who were recently on an inpatient medical service rotation. The survey included questions from previously validated communication measures,[18, 19, 20] as well as study‐specific questions. The second survey evaluated the new role of the Clinical Care Coordinator (Appendix). Both physicians and nurses who interacted with the Gold team's Clinical Care Coordinator were asked to complete this survey.
Educational Outcomes
To assess the educational interventions, we used learner evaluations of attendings, by both residents and medical students, and standardized internal medicine National Board of Medical Examiners Subject Examination (or shelf) scores for third‐year medical students. A separate evaluation of medical student perceptions of the rounding structure introduced on the Gold team using survey design has already been published.[21]
Statistical Analyses
Data from all sources were analyzed using SAS 9.3 (SAS Institute, Inc., Cary, NC). Outliers for the LOS variable were removed from the analysis. Means and frequency distributions were examined for all variables. Student t tests and [2] tests of independence were used to compare data between groups. Multivariable linear regression models controlling for time (preintervention vs postintervention) were used to assess the effect of the HOPE Initiative on patient LOS and readmission rates. In all cases, 2‐tailed P values of 0.05 or less were considered statistically significant.
Role of the Funding Source
The VA Office of Systems Redesign provided funding but was not involved in the design or conduct of the study, data analysis, or preparation of the manuscript.
RESULTS
Clinical Outcomes
Patient Outcomes
Our multivariable linear regression analysis, controlling for time, showed a significant reduction in LOS of approximately 0.3 days on all teams after the HOPE Initiative began (P=0.004). There were no significant differences between the Gold and non‐Gold teams in the multivariate models when controlling for time for any of the patient‐outcome measures. The number of admissions increased for all 4 medical teams (Figure 1), but, as shown in Figures 2 and 3, the readmission rates for all teams remained relatively stable over this same period of time.



Clinical Pharmacist on Gold Team Rounds
The inpatient clinical pharmacist changed the management plan for 22% of the patients seen on rounds. Contributions from the clinical pharmacist included adjusting the dosing of ordered medication and correcting medication reconciliation. Education and pharmaceutical information was provided to the team in another 6% of the 170 consecutive patient encounters evaluated.
Perception of Circle of Concern Rounds
Circle of Concern rounds were generally well‐received by both nurses and physicians. In a healthcare‐worker communication survey, completed by 38 physicians (62% response rate) and 48 nurses (54% response rate), the majority of both physicians (83%) and nurses (68%) felt Circle of Concern rounds improved communication.
Nurse Perception of Communication
The healthcare‐worker communication survey asked inpatient nurses to rate communication between nurses and physicians on each of the 4 medicine teams. Significantly more nurses were satisfied with communication with the Gold team (71%) compared with the other 3 medicine teams (53%; P=0.02) (Figure 4).

Perception of the Clinical Care Coordinator
In total, 20 physicians (87% response rate) and 10 nurses (56% response rate) completed the Clinical Care Coordinator survey. The physician results were overwhelmingly positive: 100% were satisfied or very satisfied with the role; 100% felt each team should have a Clinical Care Coordinator; and 100% agreed or strongly agreed that the Clinical Care Coordinator ensures that appropriate follow‐up is arranged, provides continuity of care, assists with interdisciplinary communication, and helps facilitate discharge. The majority of nurses was also satisfied or very satisfied with the Clinical Care Coordinator role and felt each team should have one.
Educational Outcomes
House Officer Evaluation of Attendings
Monthly evaluations of attending physicians by house officers (Figure 5) revealed that prior to the HOPE Initiative, little differences were observed between teams, as would be expected because attending assignment was largely random. After the intervention date of July 2009, however, significant differences were noted, with Gold team attendings receiving significantly higher teaching evaluations immediately after the introduction of the HOPE Initiative. Although ratings for Gold attendings remained more favorable, the difference was no longer statistically significant in the second and third year of the initiative, likely due to Gold attendings serving on other medicine teams, which contributed to an improvement in ratings of all attendings.

Medical Student Evaluation of Attendings
Monthly evaluations of attending physicians by third‐year medical students (Figure 6) revealed differences between the Gold attendings and all others, with the attendings that joined the Gold team in 2009 receiving higher teaching evaluations even before the HOPE Initiative started. However, this difference remained statistically significant in years 2 and 3 postinitiative, despite the addition of 4 new junior attendings.

Medical Student Medicine Shelf Scores
The national average on the shelf exam, which reflects learning after the internal medicine third‐year clerkship, has ranged from 75 to 78 for the past several years, with University of Michigan students averaging significantly higher scores prior to and after the HOPE Initiative. However, following the HOPE Initiative, third‐year medical students on the Gold team scored significantly higher on the shelf exam compared with their colleagues on the non‐Gold teams (84 vs 82; P=0.006). This difference in the shelf exam scores, although small, is statistically significant. It represents a measurable improvement in shelf scores in our system and demonstrates the potential educational benefit for the students. Over this same time period, scores on the United States Medical Licensing Exam, given to medical students at the beginning of their third year, remained stable (233 preHOPE Initiative; 234 postHOPE Initiative).
Qualitative Assessment
Qualitative data collected as part of our evaluation of the HOPE Initiative also suggested that nurse‐physician communication had improved since the start of the project. In particular, they reported positively on the Gold team in general, the Circle of Concern rounds, and the Clinical Care Coordinator (Table 4).
| Staff Type | Statement1 |
|---|---|
| |
| Nurse | [Gold is] above and beyond other [teams]. Other teams don't run as smoothly. |
| Nurse | There has been a difference in communication [on Gold]. You can tell the difference in how they communicate with staff. We know the Clinical Care Coordinator or charge nurse is rounding with that team, so there is more communication. |
| Nurse | The most important thing that has improved communication is the Circle of Concern rounds. |
| Physician | [The Gold Clinical Care Coordinator] expedites care, not only what to do but who to call. She can convey the urgency. On rounds she is able to break off, put in an order, place a call, talk to a patient. Things that we would do at 11 AM she gets to at 9 AM. A couple of hours may not seem like much, but sometimes it can make the difference between things happening that day instead of the next. |
| Physician | The Clinical Care Coordinator is completely indispensable. Major benefit to providing care to Veterans. |
| Physician | I like to think Gold has lifted all of the teams to a higher level. |
| Medical student | It may be due to personalities vs the Gold [team] itself, but there is more emphasis on best practices. Are we following guidelines even if it is not related to the primary reason for admission? |
| Medical student | Gold is very collegial and nurses/physicians know one another by name. Physicians request rather than order; this sets a good example to me on how to approach the nurses. |
| Chief resident | [Gold attendings] encourage senior residents to take charge and run the team, although the attending is there for back‐up and support. This provides great learning for the residents. Interns and medical students also are affected because they have to step up their game as well. |
DISCUSSION
Within academic medical centers, hospitalists are expected to care for patients, teach, and help improve the quality and efficiency of hospital‐based care.[7] The Department of Veterans Affairs runs the largest integrated healthcare system in the United States, with approximately 80% of VA hospitals having hospital medicine programs. Overall, one‐third of US residents perform part of their residency training at a VA hospital.[22, 23] Thus, the effects of a system‐wide change at a VA hospital may have implications throughout the country. We studied one such intervention. Our primary findings are that we were able to improve communication and learner education with minimal effects on patient outcomes. While overall LOS decreased slightly postintervention, after taking into account secular trends, readmission rates did not.
We are not the first to evaluate a hospital medicine team using a quasi‐experimental design. For example, Meltzer and colleagues evaluated a hospitalist program at the University of Chicago Medical Center and found that, by the second year of operation, hospitalist care was associated with significantly shorter LOS (0.49 days), reduced costs, and decreased mortality.[24] Auerbach also evaluated a newly created hospital medicine service, finding decreased LOS (0.61 days), lower costs, and lower risk of mortality by the second year of the program.[25]
Improving nurse‐physician communication is considered important for avoiding medical error,[26] yet there has been limited empirical study of methods to improve communication within the medical profession.[27] Based both on our surveys and qualitative interviews, healthcare‐worker communication appeared to improve on the Gold team during the study. A key component of this improvement is likely related to instituting Circle of Concern rounds, in which nurses joined the medical team during attending rounds. Such an intervention likely helped to address organizational silence[28] and enhance the psychological safety of the nursing staff, because the attending physician was proactive about soliciting the input of nurses during rounds.[29] Such leader inclusivenesswords and deeds exhibited by leaders that invite and appreciate others' contributionscan aid interdisciplinary teams in overcoming the negative effects of status differences, thereby promoting collaboration.[29] The inclusion of nurses on rounds is also relationship‐building, which Gotlib Conn and colleagues found was important to improved interprofessional communication and collaboration.[30] In the future, using a tool such as the Teamwork Effectiveness Assessment Module (TEAM) developed by the American Board of Internal Medicine[31] could provide further evaluation of the impact on interprofessional teamwork and communication.
The focus on learner education, though evaluated in prior studies, is also novel. One previous survey of medical students showed that engaging students in substantive discussions is associated with greater student satisfaction.[32] Another survey of medical students found that attendings who were enthusiastic about teaching, inspired confidence in knowledge and skills, provided useful feedback, and encouraged increased student responsibility were viewed as more effective teachers.[33] No previous study that we are aware of, however, has looked at actual educational outcomes, such as shelf scores. The National Board of Medical Examiners reports that the Medicine subject exam is scaled to have a mean of 70 and a standard deviation of 8.[34] Thus, a mean increase in score of 2 points is small, but not trivial. This shows improvement in a hard educational outcome. Additionally, 2 points, although small in the context of total score and standard deviation, may make a substantial difference to an individual student in terms of overall grade, and, thus, residency applications. Our finding that third‐year medical students on the Gold team performed significantly better than University of Michigan third‐year medical students on other teams is an intriguing finding that warrants confirmation. On the other hand, this finding is consistent with a previous report evaluating learner satisfaction in which Bodnar et al found improved ratings of quantity and quality of teaching on teams with a nontraditional structure (Gold team).[21] Moreover, despite relatively few studies, the reason underlying the educational benefit of hospitalists should surprise few. The hospitalist model ensures that learners are supervised by physicians who are experts in the care of hospitalized patients.[35] Hospitalists hired at teaching hospitals to work on services with learners are generally chosen because they possess superior educational skills.[7]
Our findings should be interpreted in the context of the following limitations. First, our study focused on a single academically affiliated VA hospital. As other VA hospitals are pursuing a similar approach (eg, the Houston and Detroit VA medical centers), replicating our results will be important. Second, the VA system, although the largest integrated healthcare system in the United States, has unique characteristicssuch as an integrated electronic health record and predominantly male patient populationthat may make generalizations to the larger US healthcare system challenging. Third, there was a slightly lower response rate among nurses on a few of the surveys to evaluate our efforts; however, this rate of response is standard at our facility. Finally, our evaluation lacks an empirical measure of healthcare‐worker communication, such as incident reports.
Despite these limitations, our results have important implications. Using both quantitative and qualitative assessment, we found that academic hospitalists have the ability to improve healthcare‐worker communication and enhance learner education without increasing LOS. These findings are directly applicable to VA medical centers and potentially applicable to other academic medical centers.
Acknowledgments
The authors thank Milisa Manojlovich, PhD, RN, Edward Kennedy, MS, and Andrew Hickner, MSI, for help with preparation of this manuscript.
Disclosures: This work was funded by a US Department of Veterans Affairs, Office of Systems Redesign Improvement Capability grant. The findings and conclusions in this report are those of the authors and do not necessarily represent the position or policy of the Department of Veterans Affairs. Dr. Saint reports receiving travel reimbursement for giving invited talks at the Society of Hospital Medicine's National Meeting, as well as serving on the advisory boards of Doximity and Jvion.
APPENDIX
Survey to Evaluate the Care Coordinator Position
| Yes | No | Not Sure | |
| Q1. Are you familiar with the role of the Care Coordinator on the Gold Service (Susan Lee)? | 1 | 2 | 3 |
Please indicate how much you agree or disagree with the statements below.
| Strongly Agree | Agree | Neutral | Disagree | Strongly Disagree | Don't Know | |
| Q2. The Care Coordinator ensures that appropriate primary care follow‐up and any other appropriate services are arranged. | 1 | 2 | 3 | 4 | 5 | 9 |
| Q3. The Care Coordinator provides continuity of patient care on the Gold Service. | 1 | 2 | 3 | 4 | 5 | 9 |
| Q4. The Care Coordinator helps educate House Officers and Medical Students on VA processes (e.g., CPRS). | 1 | 2 | 3 | 4 | 5 | 9 |
| Q5. The Care Coordinator assists with interdisciplinary communication between the medical team and other services (e.g., nursing, ambulatory care, pharmacy, social work) | 1 | 2 | 3 | 4 | 5 | 9 |
| Q6. The Care Coordinator helps facilitate patient discharge. | 1 | 2 | 3 | 4 | 5 | 9 |
| Q7. The Care Coordinator initiates communication with the ambulatory care teams to coordinate care. | 1 | 2 | 3 | 4 | 5 | 9 |
| Yes | No | |
| Q8. Are you a physician (attending or resident), or medical student who has been on more than one medical team at the VA (Gold, Silver, Burgundy, or Yellow)? | 1 | 2 |
If no, please skip to Q13
If yes, comparing your experience on the Gold Service (with the Care Coordinator) to your experience on any of the other services (Silver, Burgundy, or Yellow):
| Not at All | Very Little | Somewhat | To a Great Extent | |
| Q9. To what extent does the presence of a Care Coordinator affect patient care? | 1 | 2 | 3 | 4 |
| Q10. To what extent does the presence of a Care Coordinator improve patient flow? | 1 | 2 | 3 | 4 |
| Q11. To what extent does the presence of a Care Coordinator assist with education? | 1 | 2 | 3 | 4 |
| Q12. To what extent does the presence of a Care Coordinator contribute to attending rounds? | 1 | 2 | 3 | 4 |
| Yes | No | |
| Q13. Do you work [as a nurse] in ambulatory care? | 1 | 2 |
If no, please skip to Q17.
If yes, comparing your experience with the Gold Service (with the Care Coordinator) to the other services (Silver, Burgundy, or Yellow):
| Not at All | Very Little | Somewhat | To a Great Extent | |
| Q14. To what extent does the presence of a Care Coordinator improve coordination of care between inpatient and outpatient services? | 1 | 2 | 3 | 4 |
| Q15. To what extent does the presence of a Care Coordinator help identify high risk patients who require follow‐up? | 1 | 2 | 3 | 4 |
| Q16. To what extent does the presence of a Care Coordinator ensure follow‐up appointments are scheduled? | 1 | 2 | 3 | 4 |
| Yes | No | Not Sure | |
| Q17. Do you think each medical team should have a Care Coordinator? | 1 | 2 | 3 |
| Q18. Are there any additional tasks or duties you think would improve the effectiveness of the Care Coordinator? |
| Very Satisfied | Satisfied | Neutral | Dissatisfied | Very Dissatisfied | |
| Q19. Overall how satisfied are you with the role of the Care Coordinator on the Gold Service? | 1 | 2 | 3 | 4 | 5 |
| Q20. Do you have any other comments about the role of the Care Coordinator? |
| Q21. What is your position? |
| 1. Physician (attending or resident) or medical student |
| 2. Nurse (inpatient or ambulatory care) |
Improving quality while reducing costs remains important for hospitals across the United States, including the approximately 150 hospitals that are part of the Veterans Affairs (VA) healthcare system.[1, 2] The field of hospital medicine has grown rapidly, leading to predictions that the majority of inpatient care in the United States eventually will be delivered by hospitalists.[3, 4] In 2010, 57% of US hospitals had hospitalists on staff, including 87% of hospitals with 200 beds,[5] and nearly 80% of VA hospitals.[6]
The demand for hospitalists within teaching hospitals has grown in part as a response to the mandate to reduce residency work hours.[7] Furthermore, previous research has found that hospitalist care is associated with modest reductions in length of stay (LOS) and weak but inconsistent differences in quality.[8] The educational effect of hospitalists has been far less examined. The limited number of studies published to date suggests that hospitalists may improve resident learning and house‐officer satisfaction in academic medical centers and community teaching hospitals[9, 10, 11] and provide positive experiences for medical students12,13; however, Wachter et al reported no significant changes in clinical outcomes or patient, faculty, and house‐staff satisfaction in a newly designed hospital medicine service in San Francisco.[14] Additionally, whether using hospitalists influences nurse‐physician communication[15] is unknown.
Recognizing the limited and sometimes conflicting evidence about the hospitalist model, we report the results of a 3‐year quasi‐experimental evaluation of the experience at our medical center with academic hospitalists. As part of a VA Systems Redesign Improvement Capability Grantknown as the Hospital Outcomes Program of Excellence (HOPE) Initiativewe created a hospitalist‐based medicine team focused on quality improvement, medical education, and patient outcomes.
METHODS
Setting and Design
The main hospital of the VA Ann Arbor Healthcare System, located in Ann Arbor, Michigan, operates 105 acute‐care beds and 40 extended‐care beds. At the time of this evaluation, the medicine service consisted of 4 internal medicine teamsGold, Silver, Burgundy, and Yelloweach of which was responsible for admitting patients on a rotating basis every fourth day, with limited numbers of admissions occurring between each team's primary admitting day. Each team is led by an attending physician, a board‐certified (or board‐eligible) general internist or subspecialist who is also a faculty member at the University of Michigan Medical School. Each team has a senior medical resident, 2 to 3 interns, and 3 to 5 medical students (mostly third‐year students). In total, there are approximately 50 senior medical residents, 60 interns, and 170 medical students who rotate through the medicine service each year. Traditional rounding involves the medical students and interns receiving sign‐out from the overnight team in the morning, then pre‐rounding on each patient by obtaining an interval history, performing an exam, and checking any test results. A tentative plan of care is formed with the senior medical resident, usually by discussing each patient very quickly in the team room. Attending rounds are then conducted, with the physician team visiting each patient one by one to review and plan all aspects of care in detail. When time allows, small segments of teaching may occur during these attending work rounds. This system had been in place for >20 years.
Resulting in part from a grant received from the VA Systems Redesign Central Office (ie, the HOPE Initiative), the Gold team was modified in July 2009 and an academic hospitalist (S.S.) was assigned to head this team. Specific hospitalists were selected by the Associate Chief of Medicine (S.S.) and the Chief of Medicine (R.H.M.) to serve as Gold team attendings on a regular basis. The other teams continued to be overseen by the Chief of Medicine, and the Gold team remained within the medicine service. Characteristics of the Gold and nonGold team attendings can be found in Table 1. The 3 other teams initially were noninterventional concurrent control groups. However, during the second year of the evaluation, the Silver team adopted some of the initiatives as a result of the preliminary findings observed on Gold. Specifically, in the second year of the evaluation, approximately 42% of attendings on the Silver team were from the Gold team. This increased in the third year to 67% of coverage by Gold team attendings on the Silver team. The evaluation of the Gold team ended in June 2012.
| Characteristic | Gold Team | Non‐Gold Teams |
|---|---|---|
| Total number of attendings | 14 | 57 |
| Sex, % | ||
| Male | 79 | 58 |
| Female | 21 | 42 |
| Median years postresidency (range) | 10 (130) | 7 (141) |
| Subspecialists, % | 14 | 40 |
| Median days on service per year (range) | 53 (574) | 30 (592) |
The clinical interventions implemented on the Gold team were quality‐improvement work and were therefore exempt from institutional review board review. Human subjects' approval was, however, received to conduct interviews as part of a qualitative assessment.
Clinical Interventions
Several interventions involving the clinical care delivered were introduced on the Gold team, with a focus on improving communication among healthcare workers (Table 2).
| Clinical Interventions | Educational Interventions |
|---|---|
| Modified structure of attending rounds | Modified structure of attending rounds |
| Circle of Concern rounds | Attending reading list |
| Clinical Care Coordinator | Nifty Fifty reading list for learners |
| Regular attending team meetings | Website to provide expectations to learners |
| Two‐month per year commitment by attendings |
Structure of Attending Rounds
The structure of morning rounds was modified on the Gold team. Similar to the traditional structure, medical students and interns on the Gold team receive sign‐out from the overnight team in the morning. However, interns and students may or may not conduct pre‐rounds on each patient. The majority of time between sign‐out and the arrival of the attending physician is spent on work rounds. The senior resident leads rounds with the interns and students, discussing each patient while focusing on overnight events and current symptoms, new physical‐examination findings, and laboratory and test data. The plan of care to be presented to the attending is then formulated with the senior resident. The attending physician then leads Circle of Concern rounds with an expanded team, including a charge nurse, a clinical pharmacist, and a nurse Clinical Care Coordinator. Attending rounds tend to use an E‐AP format: significant Events overnight are discussed, followed by an Assessment & Plan by problem for the top active problems. Using this model, the attendings are able to focus more on teaching and discussing the patient plan than in the traditional model (in which the learner presents the details of the subjective, objective, laboratory, and radiographic data, with limited time left for the assessment and plan for each problem).
Circle of Concern Rounds
Suzanne Gordon described the Circle of Concern in her book Nursing Against the Odds.[16] From her observations, she noted that physicians typically form a circle to discuss patient care during rounds. The circle expands when another physician joins the group; however, the circle does not similarly expand to include nurses when they approach the group. Instead, nurses typically remain on the periphery, listening silently or trying to communicate to physicians' backs.[16] Thus, to promote nurse‐physician communication, Circle of Concern rounds were formally introduced on the Gold team. Each morning, the charge nurse rounds with the team and is encouraged to bring up nursing concerns. The inpatient clinical pharmacist is also included 2 to 3 times per week to help provide education to residents and students and perform medication reconciliation.
Clinical Care Coordinator
The role of the nurse Clinical Care Coordinatoralso introduced on the Gold teamis to provide continuity of patient care, facilitate interdisciplinary communication, facilitate patient discharge, ensure appropriate appointments are scheduled, communicate with the ambulatory care service to ensure proper transition between inpatient and outpatient care, and help educate residents and students on VA procedures and resources.
Regular Gold Team Meetings
All Gold team attendings are expected to dedicate 2 months per year to inpatient service (divided into half‐month blocks), instead of the average 1 month per year for attendings on the other teams. The Gold team attendings, unlike the other teams, also attend bimonthly meetings to discuss strategies for running the team.
Educational Interventions
Given the high number of learners on the medicine service, we wanted to enhance the educational experience for our learners. We thus implemented various interventions, in addition to the change in the structure of rounds, as described below.
Reading List for Learners: The Nifty Fifty
Because reading about clinical medicine is an integral part of medical education, we make explicit our expectation that residents and students read something clinically relevant every day. To promote this, we have provided a Nifty Fifty reading list of key articles. The PDF of each article is provided, along with a brief summary highlighting key points.
Reading List for Gold Attendings and Support Staff
To promote a common understanding of leadership techniques, management books are provided to Gold attending physicians and other members of the team (eg, Care Coordinator, nurse researcher, systems redesign engineer). One book is discussed at each Gold team meeting (Table 3), with participants taking turns leading the discussion.
| Book Title | Author(s) |
|---|---|
| The One Minute Manager | Ken Blanchard and Spencer Johnson |
| Good to Great | Jim Collins |
| Good to Great and the Social Sectors | Jim Collins |
| The Checklist Manifesto: How to Get Things Right | Atul Gawande |
| The Five Dysfunctions of a Team: A Leadership Fable | Patrick Lencioni |
| Getting to Yes: Negotiating Agreement Without Giving In | Roger Fisher, William Ury, and Bruce Patton |
| The Effective Executive: The Definitive Guide to Getting the Right Things Done | Peter Drucker |
| A Sense of Urgency | John Kotter |
| The Power of Positive Deviance: How Unlikely Innovators Solve the World's Toughest Problems | Richard Pascale, Jerry Sternin, and Monique Sternin |
| On the Mend: Revolutionizing Healthcare to Save Lives and Transform the Industry | John Toussaint and Roger Gerard |
| Outliers: The Story of Success | Malcolm Gladwell |
| Nursing Against the Odds: How Health Care Cost Cutting, Media Stereotypes, and Medical Hubris Undermine Nurses and Patient Care | Suzanne Gordon |
| How the Mighty Fall and Why Some Companies Never Give In | Jim Collins |
| What the Best College Teachers Do | Ken Bain |
| The Creative Destruction of Medicine | Eric Topol |
| What Got You Here Won't Get You There: How Successful People Become Even More Successful! | Marshall Goldsmith |
Website
A HOPE Initiative website was created (
Qualitative Assessment
To evaluate our efforts, we conducted a thorough qualitative assessment during the third year of the program. A total of 35 semistructured qualitative interviews were conducted with patients and staff from all levels of the organization, including senior leadership. The qualitative assessment was led by research staff from the Center for Clinical Management Research, who were minimally involved in the redesign effort and could provide an unbiased view of the initiative. Field notes from the semistructured interviews were analyzed, with themes developed using a descriptive approach and through discussion by a multidisciplinary team, which included building team consensus on findings that were supported by clear evidence in the data.[17]
Quantitative Outcome Measures
Clinical Outcomes
To determine if our communication and educational interventions had an impact on patient care, we used hospital administrative data to evaluate admission rates, LOS, and readmission rates for all 4 of the medicine teams. Additional clinical measures were assessed as needed. For example, we monitored the impact of the clinical pharmacist during a 4‐week pilot study by asking the Clinical Care Coordinator to track the proportion of patient encounters (n=170) in which the clinical pharmacist changed management or provided education to team members. Additionally, 2 staff surveys were conducted. The first survey focused on healthcare‐worker communication and was given to inpatient nurses and physicians (including attendings, residents, and medical students) who were recently on an inpatient medical service rotation. The survey included questions from previously validated communication measures,[18, 19, 20] as well as study‐specific questions. The second survey evaluated the new role of the Clinical Care Coordinator (Appendix). Both physicians and nurses who interacted with the Gold team's Clinical Care Coordinator were asked to complete this survey.
Educational Outcomes
To assess the educational interventions, we used learner evaluations of attendings, by both residents and medical students, and standardized internal medicine National Board of Medical Examiners Subject Examination (or shelf) scores for third‐year medical students. A separate evaluation of medical student perceptions of the rounding structure introduced on the Gold team using survey design has already been published.[21]
Statistical Analyses
Data from all sources were analyzed using SAS 9.3 (SAS Institute, Inc., Cary, NC). Outliers for the LOS variable were removed from the analysis. Means and frequency distributions were examined for all variables. Student t tests and [2] tests of independence were used to compare data between groups. Multivariable linear regression models controlling for time (preintervention vs postintervention) were used to assess the effect of the HOPE Initiative on patient LOS and readmission rates. In all cases, 2‐tailed P values of 0.05 or less were considered statistically significant.
Role of the Funding Source
The VA Office of Systems Redesign provided funding but was not involved in the design or conduct of the study, data analysis, or preparation of the manuscript.
RESULTS
Clinical Outcomes
Patient Outcomes
Our multivariable linear regression analysis, controlling for time, showed a significant reduction in LOS of approximately 0.3 days on all teams after the HOPE Initiative began (P=0.004). There were no significant differences between the Gold and non‐Gold teams in the multivariate models when controlling for time for any of the patient‐outcome measures. The number of admissions increased for all 4 medical teams (Figure 1), but, as shown in Figures 2 and 3, the readmission rates for all teams remained relatively stable over this same period of time.



Clinical Pharmacist on Gold Team Rounds
The inpatient clinical pharmacist changed the management plan for 22% of the patients seen on rounds. Contributions from the clinical pharmacist included adjusting the dosing of ordered medication and correcting medication reconciliation. Education and pharmaceutical information was provided to the team in another 6% of the 170 consecutive patient encounters evaluated.
Perception of Circle of Concern Rounds
Circle of Concern rounds were generally well‐received by both nurses and physicians. In a healthcare‐worker communication survey, completed by 38 physicians (62% response rate) and 48 nurses (54% response rate), the majority of both physicians (83%) and nurses (68%) felt Circle of Concern rounds improved communication.
Nurse Perception of Communication
The healthcare‐worker communication survey asked inpatient nurses to rate communication between nurses and physicians on each of the 4 medicine teams. Significantly more nurses were satisfied with communication with the Gold team (71%) compared with the other 3 medicine teams (53%; P=0.02) (Figure 4).

Perception of the Clinical Care Coordinator
In total, 20 physicians (87% response rate) and 10 nurses (56% response rate) completed the Clinical Care Coordinator survey. The physician results were overwhelmingly positive: 100% were satisfied or very satisfied with the role; 100% felt each team should have a Clinical Care Coordinator; and 100% agreed or strongly agreed that the Clinical Care Coordinator ensures that appropriate follow‐up is arranged, provides continuity of care, assists with interdisciplinary communication, and helps facilitate discharge. The majority of nurses was also satisfied or very satisfied with the Clinical Care Coordinator role and felt each team should have one.
Educational Outcomes
House Officer Evaluation of Attendings
Monthly evaluations of attending physicians by house officers (Figure 5) revealed that prior to the HOPE Initiative, little differences were observed between teams, as would be expected because attending assignment was largely random. After the intervention date of July 2009, however, significant differences were noted, with Gold team attendings receiving significantly higher teaching evaluations immediately after the introduction of the HOPE Initiative. Although ratings for Gold attendings remained more favorable, the difference was no longer statistically significant in the second and third year of the initiative, likely due to Gold attendings serving on other medicine teams, which contributed to an improvement in ratings of all attendings.

Medical Student Evaluation of Attendings
Monthly evaluations of attending physicians by third‐year medical students (Figure 6) revealed differences between the Gold attendings and all others, with the attendings that joined the Gold team in 2009 receiving higher teaching evaluations even before the HOPE Initiative started. However, this difference remained statistically significant in years 2 and 3 postinitiative, despite the addition of 4 new junior attendings.

Medical Student Medicine Shelf Scores
The national average on the shelf exam, which reflects learning after the internal medicine third‐year clerkship, has ranged from 75 to 78 for the past several years, with University of Michigan students averaging significantly higher scores prior to and after the HOPE Initiative. However, following the HOPE Initiative, third‐year medical students on the Gold team scored significantly higher on the shelf exam compared with their colleagues on the non‐Gold teams (84 vs 82; P=0.006). This difference in the shelf exam scores, although small, is statistically significant. It represents a measurable improvement in shelf scores in our system and demonstrates the potential educational benefit for the students. Over this same time period, scores on the United States Medical Licensing Exam, given to medical students at the beginning of their third year, remained stable (233 preHOPE Initiative; 234 postHOPE Initiative).
Qualitative Assessment
Qualitative data collected as part of our evaluation of the HOPE Initiative also suggested that nurse‐physician communication had improved since the start of the project. In particular, they reported positively on the Gold team in general, the Circle of Concern rounds, and the Clinical Care Coordinator (Table 4).
| Staff Type | Statement1 |
|---|---|
| |
| Nurse | [Gold is] above and beyond other [teams]. Other teams don't run as smoothly. |
| Nurse | There has been a difference in communication [on Gold]. You can tell the difference in how they communicate with staff. We know the Clinical Care Coordinator or charge nurse is rounding with that team, so there is more communication. |
| Nurse | The most important thing that has improved communication is the Circle of Concern rounds. |
| Physician | [The Gold Clinical Care Coordinator] expedites care, not only what to do but who to call. She can convey the urgency. On rounds she is able to break off, put in an order, place a call, talk to a patient. Things that we would do at 11 AM she gets to at 9 AM. A couple of hours may not seem like much, but sometimes it can make the difference between things happening that day instead of the next. |
| Physician | The Clinical Care Coordinator is completely indispensable. Major benefit to providing care to Veterans. |
| Physician | I like to think Gold has lifted all of the teams to a higher level. |
| Medical student | It may be due to personalities vs the Gold [team] itself, but there is more emphasis on best practices. Are we following guidelines even if it is not related to the primary reason for admission? |
| Medical student | Gold is very collegial and nurses/physicians know one another by name. Physicians request rather than order; this sets a good example to me on how to approach the nurses. |
| Chief resident | [Gold attendings] encourage senior residents to take charge and run the team, although the attending is there for back‐up and support. This provides great learning for the residents. Interns and medical students also are affected because they have to step up their game as well. |
DISCUSSION
Within academic medical centers, hospitalists are expected to care for patients, teach, and help improve the quality and efficiency of hospital‐based care.[7] The Department of Veterans Affairs runs the largest integrated healthcare system in the United States, with approximately 80% of VA hospitals having hospital medicine programs. Overall, one‐third of US residents perform part of their residency training at a VA hospital.[22, 23] Thus, the effects of a system‐wide change at a VA hospital may have implications throughout the country. We studied one such intervention. Our primary findings are that we were able to improve communication and learner education with minimal effects on patient outcomes. While overall LOS decreased slightly postintervention, after taking into account secular trends, readmission rates did not.
We are not the first to evaluate a hospital medicine team using a quasi‐experimental design. For example, Meltzer and colleagues evaluated a hospitalist program at the University of Chicago Medical Center and found that, by the second year of operation, hospitalist care was associated with significantly shorter LOS (0.49 days), reduced costs, and decreased mortality.[24] Auerbach also evaluated a newly created hospital medicine service, finding decreased LOS (0.61 days), lower costs, and lower risk of mortality by the second year of the program.[25]
Improving nurse‐physician communication is considered important for avoiding medical error,[26] yet there has been limited empirical study of methods to improve communication within the medical profession.[27] Based both on our surveys and qualitative interviews, healthcare‐worker communication appeared to improve on the Gold team during the study. A key component of this improvement is likely related to instituting Circle of Concern rounds, in which nurses joined the medical team during attending rounds. Such an intervention likely helped to address organizational silence[28] and enhance the psychological safety of the nursing staff, because the attending physician was proactive about soliciting the input of nurses during rounds.[29] Such leader inclusivenesswords and deeds exhibited by leaders that invite and appreciate others' contributionscan aid interdisciplinary teams in overcoming the negative effects of status differences, thereby promoting collaboration.[29] The inclusion of nurses on rounds is also relationship‐building, which Gotlib Conn and colleagues found was important to improved interprofessional communication and collaboration.[30] In the future, using a tool such as the Teamwork Effectiveness Assessment Module (TEAM) developed by the American Board of Internal Medicine[31] could provide further evaluation of the impact on interprofessional teamwork and communication.
The focus on learner education, though evaluated in prior studies, is also novel. One previous survey of medical students showed that engaging students in substantive discussions is associated with greater student satisfaction.[32] Another survey of medical students found that attendings who were enthusiastic about teaching, inspired confidence in knowledge and skills, provided useful feedback, and encouraged increased student responsibility were viewed as more effective teachers.[33] No previous study that we are aware of, however, has looked at actual educational outcomes, such as shelf scores. The National Board of Medical Examiners reports that the Medicine subject exam is scaled to have a mean of 70 and a standard deviation of 8.[34] Thus, a mean increase in score of 2 points is small, but not trivial. This shows improvement in a hard educational outcome. Additionally, 2 points, although small in the context of total score and standard deviation, may make a substantial difference to an individual student in terms of overall grade, and, thus, residency applications. Our finding that third‐year medical students on the Gold team performed significantly better than University of Michigan third‐year medical students on other teams is an intriguing finding that warrants confirmation. On the other hand, this finding is consistent with a previous report evaluating learner satisfaction in which Bodnar et al found improved ratings of quantity and quality of teaching on teams with a nontraditional structure (Gold team).[21] Moreover, despite relatively few studies, the reason underlying the educational benefit of hospitalists should surprise few. The hospitalist model ensures that learners are supervised by physicians who are experts in the care of hospitalized patients.[35] Hospitalists hired at teaching hospitals to work on services with learners are generally chosen because they possess superior educational skills.[7]
Our findings should be interpreted in the context of the following limitations. First, our study focused on a single academically affiliated VA hospital. As other VA hospitals are pursuing a similar approach (eg, the Houston and Detroit VA medical centers), replicating our results will be important. Second, the VA system, although the largest integrated healthcare system in the United States, has unique characteristicssuch as an integrated electronic health record and predominantly male patient populationthat may make generalizations to the larger US healthcare system challenging. Third, there was a slightly lower response rate among nurses on a few of the surveys to evaluate our efforts; however, this rate of response is standard at our facility. Finally, our evaluation lacks an empirical measure of healthcare‐worker communication, such as incident reports.
Despite these limitations, our results have important implications. Using both quantitative and qualitative assessment, we found that academic hospitalists have the ability to improve healthcare‐worker communication and enhance learner education without increasing LOS. These findings are directly applicable to VA medical centers and potentially applicable to other academic medical centers.
Acknowledgments
The authors thank Milisa Manojlovich, PhD, RN, Edward Kennedy, MS, and Andrew Hickner, MSI, for help with preparation of this manuscript.
Disclosures: This work was funded by a US Department of Veterans Affairs, Office of Systems Redesign Improvement Capability grant. The findings and conclusions in this report are those of the authors and do not necessarily represent the position or policy of the Department of Veterans Affairs. Dr. Saint reports receiving travel reimbursement for giving invited talks at the Society of Hospital Medicine's National Meeting, as well as serving on the advisory boards of Doximity and Jvion.
APPENDIX
Survey to Evaluate the Care Coordinator Position
| Yes | No | Not Sure | |
| Q1. Are you familiar with the role of the Care Coordinator on the Gold Service (Susan Lee)? | 1 | 2 | 3 |
Please indicate how much you agree or disagree with the statements below.
| Strongly Agree | Agree | Neutral | Disagree | Strongly Disagree | Don't Know | |
| Q2. The Care Coordinator ensures that appropriate primary care follow‐up and any other appropriate services are arranged. | 1 | 2 | 3 | 4 | 5 | 9 |
| Q3. The Care Coordinator provides continuity of patient care on the Gold Service. | 1 | 2 | 3 | 4 | 5 | 9 |
| Q4. The Care Coordinator helps educate House Officers and Medical Students on VA processes (e.g., CPRS). | 1 | 2 | 3 | 4 | 5 | 9 |
| Q5. The Care Coordinator assists with interdisciplinary communication between the medical team and other services (e.g., nursing, ambulatory care, pharmacy, social work) | 1 | 2 | 3 | 4 | 5 | 9 |
| Q6. The Care Coordinator helps facilitate patient discharge. | 1 | 2 | 3 | 4 | 5 | 9 |
| Q7. The Care Coordinator initiates communication with the ambulatory care teams to coordinate care. | 1 | 2 | 3 | 4 | 5 | 9 |
| Yes | No | |
| Q8. Are you a physician (attending or resident), or medical student who has been on more than one medical team at the VA (Gold, Silver, Burgundy, or Yellow)? | 1 | 2 |
If no, please skip to Q13
If yes, comparing your experience on the Gold Service (with the Care Coordinator) to your experience on any of the other services (Silver, Burgundy, or Yellow):
| Not at All | Very Little | Somewhat | To a Great Extent | |
| Q9. To what extent does the presence of a Care Coordinator affect patient care? | 1 | 2 | 3 | 4 |
| Q10. To what extent does the presence of a Care Coordinator improve patient flow? | 1 | 2 | 3 | 4 |
| Q11. To what extent does the presence of a Care Coordinator assist with education? | 1 | 2 | 3 | 4 |
| Q12. To what extent does the presence of a Care Coordinator contribute to attending rounds? | 1 | 2 | 3 | 4 |
| Yes | No | |
| Q13. Do you work [as a nurse] in ambulatory care? | 1 | 2 |
If no, please skip to Q17.
If yes, comparing your experience with the Gold Service (with the Care Coordinator) to the other services (Silver, Burgundy, or Yellow):
| Not at All | Very Little | Somewhat | To a Great Extent | |
| Q14. To what extent does the presence of a Care Coordinator improve coordination of care between inpatient and outpatient services? | 1 | 2 | 3 | 4 |
| Q15. To what extent does the presence of a Care Coordinator help identify high risk patients who require follow‐up? | 1 | 2 | 3 | 4 |
| Q16. To what extent does the presence of a Care Coordinator ensure follow‐up appointments are scheduled? | 1 | 2 | 3 | 4 |
| Yes | No | Not Sure | |
| Q17. Do you think each medical team should have a Care Coordinator? | 1 | 2 | 3 |
| Q18. Are there any additional tasks or duties you think would improve the effectiveness of the Care Coordinator? |
| Very Satisfied | Satisfied | Neutral | Dissatisfied | Very Dissatisfied | |
| Q19. Overall how satisfied are you with the role of the Care Coordinator on the Gold Service? | 1 | 2 | 3 | 4 | 5 |
| Q20. Do you have any other comments about the role of the Care Coordinator? |
| Q21. What is your position? |
| 1. Physician (attending or resident) or medical student |
| 2. Nurse (inpatient or ambulatory care) |
- Kohn LT, Corrigan JM, Donaldson MS, eds. To Err Is Human: Building a Safer Health System. Washington, D.C.: National Academies Press; 2000.
- Institute of Medicine of the National Academies. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, D.C.: National Academies Press; 2001.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- . Growth in care provided by hospitalists. N Engl J Med. 2009;360(26):2789–2791.
- American Hospital Association. AHA Annual Survey of Hospitals, 2010. Chicago, IL: Health Forum, LLC; 2010.
- , , , . Preventing hospital‐acquired infections: a national survey of practices reported by U.S. hospitals in 2005 and 2009. J Gen Intern Med. 2012;27(7):773–779.
- , . Hospitalists in teaching hospitals: opportunities but not without danger. J Gen Intern Med. 2004;19(4):392–393.
- , . Do hospitalist physicians improve the quality of inpatient care delivery? A systematic review of process, efficiency and outcome measures. BMC Med. 2011;9:58.
- , , , . Effect of hospitalist attending physicians on trainee educational experiences: a systematic review. J Hosp Med. 2009;4(8):490–498.
- , , , , , . Resident satisfaction on an academic hospitalist service: time to teach. Am J Med. 2002;112(7):597–601.
- , , , et al. The positive impact of initiation of hospitalist clinician educators. J Gen Intern Med. 2004;19(4):293–301.
- , . Third‐year medical students' evaluation of hospitalist and nonhospitalist faculty during the inpatient portion of their pediatrics clerkships. J Hosp Med. 2007;2(1):17–22.
- , , , . Medical student evaluation of the quality of hospitalist and nonhospitalist teaching faculty on inpatient medicine rotations. Acad Med. 2004;79(1):78–82.
- , , , , . Reorganizing an academic medical service: impact on cost, quality, patient satisfaction, and education. JAMA. 1998;279(19):1560–1565.
- . Nurse/physician communication through a sensemaking lens: shifting the paradigm to improve patient safety. Med Care. 2010;48(11):941–946.
- . Nursing Against the Odds: How Health Care Cost Cutting, Media Stereotypes, and Medical Hubris Undermine Nurses and Patient Care. Ithaca, NY: Cornell University Press; 2005.
- . Focus on research methods: whatever happened to qualitative description? Res Nurs Health. 2000;23:334–340.
- , , , , . Organizational assessment in intensive care units (ICUs): construct development, reliability, and validity of the ICU nurse‐physician questionnaire. Med Care. 1991;29(8):709–726.
- . Development of an instrument to measure collaboration and satisfaction about care decisions. J Adv Nurs. 1994;20(1):176–182.
- . Development of the practice environment scale of the Nursing Work Index. Res Nurs Health. 2002;25(3):176–188.
- , , . Does the structure of inpatient rounds affect medical student education? Int J Med Educ. 2013;4:96–100.
- U.S. Department of Veterans Affairs, Office of Academic Affiliations. Medical and Dental Education Program. Available at: http://www.va. gov/oaa/GME_default.asp. Published 2012. Accessed May 08, 2013.
- , . Graduate medical education, 2011–2012. JAMA. 2012;308(21):2264–2279.
- , , , et al. Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists. Ann Intern Med. 2002;137(11):866–874.
- , , , , , . Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes. Ann Intern Med. 2002;137(11):859–865.
- , , . Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79(2):186–194.
- , , . ‘It depends': medical residents' perspectives on working with nurses. Am J Nurs. 2009;109(7):34–44.
- , . Organizational silence: a barrier to change and development in a pluralistic world. Acad Manage Rev. 2000;25(4):706–725.
- , . Making it safe: the effects of leader inclusiveness and professional status on psychological safety and improvement efforts in health care teams. J Organiz Behav. 2006;27:941–966.
- , , , , . Interprofessional communication with hospitalist and consultant physicians in general internal medicine: a qualitative study. BMC Health Serv Res. 2012;12:437.
- , , , , , . A new tool to give hospitalists feedback to improve interprofessional teamwork and advance patient care. Health Aff (Millwood). 2012;31(11):2485–2492.
- , , , , , . Impact of instructional practices on student satisfaction with attendings' teaching in the inpatient component of internal medicine clerkships. J Gen Intern Med. 2006;21(1):7–12.
- , . Medical students' perceptions of the elements of effective inpatient teaching by attending physicians and housestaff. J Gen Intern Med. 2005;20(7):635–639.
- National Board of Medical Examiners Subject Examination Program. Internal Medicine Advanced Clinical Examination, score interpretation guide. Available at: http://www.nbme.org/PDF/SampleScoreReports/Internal_Medicine_ACE_Score_Report.pdf. Published 2011. Accessed September 13, 2013.
- . The impact of hospitalists on medical education and the academic health system. Ann Intern Med. 1999;130(4 part 2):364–367.
- Kohn LT, Corrigan JM, Donaldson MS, eds. To Err Is Human: Building a Safer Health System. Washington, D.C.: National Academies Press; 2000.
- Institute of Medicine of the National Academies. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, D.C.: National Academies Press; 2001.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- . Growth in care provided by hospitalists. N Engl J Med. 2009;360(26):2789–2791.
- American Hospital Association. AHA Annual Survey of Hospitals, 2010. Chicago, IL: Health Forum, LLC; 2010.
- , , , . Preventing hospital‐acquired infections: a national survey of practices reported by U.S. hospitals in 2005 and 2009. J Gen Intern Med. 2012;27(7):773–779.
- , . Hospitalists in teaching hospitals: opportunities but not without danger. J Gen Intern Med. 2004;19(4):392–393.
- , . Do hospitalist physicians improve the quality of inpatient care delivery? A systematic review of process, efficiency and outcome measures. BMC Med. 2011;9:58.
- , , , . Effect of hospitalist attending physicians on trainee educational experiences: a systematic review. J Hosp Med. 2009;4(8):490–498.
- , , , , , . Resident satisfaction on an academic hospitalist service: time to teach. Am J Med. 2002;112(7):597–601.
- , , , et al. The positive impact of initiation of hospitalist clinician educators. J Gen Intern Med. 2004;19(4):293–301.
- , . Third‐year medical students' evaluation of hospitalist and nonhospitalist faculty during the inpatient portion of their pediatrics clerkships. J Hosp Med. 2007;2(1):17–22.
- , , , . Medical student evaluation of the quality of hospitalist and nonhospitalist teaching faculty on inpatient medicine rotations. Acad Med. 2004;79(1):78–82.
- , , , , . Reorganizing an academic medical service: impact on cost, quality, patient satisfaction, and education. JAMA. 1998;279(19):1560–1565.
- . Nurse/physician communication through a sensemaking lens: shifting the paradigm to improve patient safety. Med Care. 2010;48(11):941–946.
- . Nursing Against the Odds: How Health Care Cost Cutting, Media Stereotypes, and Medical Hubris Undermine Nurses and Patient Care. Ithaca, NY: Cornell University Press; 2005.
- . Focus on research methods: whatever happened to qualitative description? Res Nurs Health. 2000;23:334–340.
- , , , , . Organizational assessment in intensive care units (ICUs): construct development, reliability, and validity of the ICU nurse‐physician questionnaire. Med Care. 1991;29(8):709–726.
- . Development of an instrument to measure collaboration and satisfaction about care decisions. J Adv Nurs. 1994;20(1):176–182.
- . Development of the practice environment scale of the Nursing Work Index. Res Nurs Health. 2002;25(3):176–188.
- , , . Does the structure of inpatient rounds affect medical student education? Int J Med Educ. 2013;4:96–100.
- U.S. Department of Veterans Affairs, Office of Academic Affiliations. Medical and Dental Education Program. Available at: http://www.va. gov/oaa/GME_default.asp. Published 2012. Accessed May 08, 2013.
- , . Graduate medical education, 2011–2012. JAMA. 2012;308(21):2264–2279.
- , , , et al. Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists. Ann Intern Med. 2002;137(11):866–874.
- , , , , , . Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes. Ann Intern Med. 2002;137(11):859–865.
- , , . Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79(2):186–194.
- , , . ‘It depends': medical residents' perspectives on working with nurses. Am J Nurs. 2009;109(7):34–44.
- , . Organizational silence: a barrier to change and development in a pluralistic world. Acad Manage Rev. 2000;25(4):706–725.
- , . Making it safe: the effects of leader inclusiveness and professional status on psychological safety and improvement efforts in health care teams. J Organiz Behav. 2006;27:941–966.
- , , , , . Interprofessional communication with hospitalist and consultant physicians in general internal medicine: a qualitative study. BMC Health Serv Res. 2012;12:437.
- , , , , , . A new tool to give hospitalists feedback to improve interprofessional teamwork and advance patient care. Health Aff (Millwood). 2012;31(11):2485–2492.
- , , , , , . Impact of instructional practices on student satisfaction with attendings' teaching in the inpatient component of internal medicine clerkships. J Gen Intern Med. 2006;21(1):7–12.
- , . Medical students' perceptions of the elements of effective inpatient teaching by attending physicians and housestaff. J Gen Intern Med. 2005;20(7):635–639.
- National Board of Medical Examiners Subject Examination Program. Internal Medicine Advanced Clinical Examination, score interpretation guide. Available at: http://www.nbme.org/PDF/SampleScoreReports/Internal_Medicine_ACE_Score_Report.pdf. Published 2011. Accessed September 13, 2013.
- . The impact of hospitalists on medical education and the academic health system. Ann Intern Med. 1999;130(4 part 2):364–367.
© 2013 Society of The Authors. Journal of Hospital Medicine published by Wiley Periodicals, Inc. on behalf of Society of Hospital Medicine.
Hospitalist Experiences With PICCs
Peripherally inserted central catheters (PICCs) are central venous catheters that are inserted through peripheral veins of the upper extremities in adults. Because they are safer to insert than central venous catheters (CVCs) and have become increasingly available at the bedside through the advent of specially trained vascular access nurses,[1] the use of PICCs in hospitalized patients has risen across the United States.[2] As the largest group of inpatient providers, hospitalists play a key role in the decision to insert and subsequently manage PICCs in hospitalized patients. Unfortunately, little is known about national hospitalist experiences, practice patterns, or knowledge when it comes to these commonly used devices. Therefore, we designed a 10‐question survey to investigate PICC‐related practices and knowledge among adult hospitalists practicing throughout the United States.
PATIENTS AND METHODS
Questions for this survey were derived from a previously published study conducted across 10 hospitals in the state of Michigan.[3] To assess external validity and test specific hypotheses formulated from the Michigan study, those questions with the greatest variation in response or those most amenable to interventions were chosen for inclusion in this survey.
To reach a national audience of practicing adult hospitalists, we submitted a survey proposal to the Society of Hospital Medicine's (SHM) Research Committee. The SHM Research Committee reviews such proposals using a peer‐review process to ensure both scientific integrity and validity of the survey instrument. Because the survey was already distributed to many hospitalists in Michigan, we requested that only hospitalists outside of Michigan be invited to participate in the national survey. All responses were collected anonymously, and no identifiable data were collected from respondents. Between February 1, 2013 and March 15, 2013, data were collected via an e‐mail sent directly from the SHM to members that contained a link to the study survey administered using SurveyMonkey. To augment data collection, nonresponders to the original e‐mail invitation were sent a second reminder e‐mail midway through the study. Descriptive statistics (percentages) were used to tabulate responses. The institutional review board at the University of Michigan Health System provided ethical and regulatory approval for this study.
RESULTS
A total of 2112 electronic survey invitations were sent to non‐Michigan adult hospitalists, with 381 completing the online survey (response rate 18%). Among respondents to the national survey, 86% reported having placed a PICC solely to obtain venous access in a hospitalized patient (rather than for specific indications such as long‐term intravenous antibiotics, chemotherapy, or parenteral nutrition), whereas 82% reported having cared for a patient who specifically requested a PICC (Table 1). PICC‐related deep vein thrombosis (DVT) and bloodstream infections were reported as being the most frequent PICC complications encountered by hospitalists, followed by superficial thrombophlebitis and mechanical complications such as coiling, kinking, and migration of the PICC tip.
| Total (N=381) | |
|---|---|
| |
| Hospitalist experiences related to PICCs | |
| Among hospitalized patients you have cared for, have any of your patients ever had a PICC placed solely to obtain venous access (eg, not for an indication such as long‐term IV antibiotics, chemotherapy, or TPN)? | |
| Yes | 328 (86.1%) |
| No | 53 (13.9%) |
| Have you ever cared for a patient who specifically requested a PICC because of prior experience with this device? | |
| Yes | 311 (81.6%) |
| No | 70 (18.4%) |
| Most frequently encountered PICC complications | |
| Upper‐extremity DVT or PE | 48 (12.6%) |
| Bloodstream infection | 41 (10.8%) |
| Superficial thrombophlebitis | 34 (8.9%) |
| Cellulitis/exit site erythema | 26 (6.8%) |
| Coiling, kinking of the PICC | 14 (3.7%) |
| Migration of the PICC tip | 9 (2.4%) |
| Breakage of PICC (anywhere) | 6 (1.6%) |
| Hospitalist practice related to PICCs | |
| During patient rounds, do you routinely examine PICCs for external problems (eg, cracks, breaks, leaks, or redness at the insertion site)? | |
| Yes, daily | 97 (25.5%) |
| Yes, but only if the nurse or patient alerts me to a problem with the PICC | 190 (49.9%) |
| No, I don't routinely examine the PICC for external problems | 94 (24.7%) |
| Have you ever forgotten or been unaware of the presence of a PICC? | |
| Yes | 216 (56.7%) |
| No | 165 (43.3%) |
| Assuming no contraindications exist, do you anticoagulate patients who develop a PICC‐associated DVT? | |
| Yes, for at least 1 month | 41(10.8%) |
| Yes, for at least 3 months* | 198 (52.0%) |
| Yes, for at least 6 months | 11 (2.9%) |
| Yes, I anticoagulate for as long as the line remains in place. Once the line is removed, I stop anticoagulation | 30 (7.9%) |
| Yes, I anticoagulate for as long as the line remains in place followed by another 4 weeks of therapy | 72 (18.9%) |
| I don't usually anticoagulate patients who develop a PICC‐related DVT | 29 (7.6%) |
| When a hospitalized patient develops a PICC‐related DVT, do you routinely remove the PICC? | |
| Yes | 271 (71.1%) |
| No | 110 (28.9%) |
| Hospitalist opinions related to PICCs | |
| Thinking about your hospital and your experiences, what percentage of PICC insertions may represent inappropriate use (eg, PICC placed for short‐term venous access for a presumed infection that could be treated with oral antibiotic or PICCs that were promptly removed as the patient no longer needed it for clinical management)? | |
| <10% | 192 (50.4%) |
| 10%25% | 160 (42.0%) |
| 26%50% | 22 (5.8%) |
| >50% | 7 (1.8%) |
| Do you think hospitalists should be trained to insert PICCs? | |
| Yes | 162 (42.5%) |
| No | 219 (57.5%) |
| Hospitalist knowledge related to PICCs | |
| Why is the position of the PICC‐tip checked following bedside PICC insertion? | |
| To decrease the risk of arrhythmia from tip placement in the right atrial | 267 (70.1%) |
| To ensure it is not accidentally placed into an artery | 44 (11.5%) |
| To minimize the risk of venous thrombosis* | 33 (8.7%) |
| For documentation purposes (to reduce the risk of lawsuits related tocomplications) | 16 (4.2%) |
| I don't know | 21 (5.5%) |
Several potentially important safety concerns regarding hospitalist PICC practices were observed in this survey. For instance, only 25% of hospitalists reported examining PICCs on daily rounds for external problems. When alerted by nurses or patients about problems with the device, this number doubled to 50%. In addition, 57% of respondents admitted to having at least once forgotten about the presence of a PICC in their hospitalized patient.
Participants also reported significant variation in duration of anticoagulation therapy for PICC‐related DVT, with only half of all respondents selecting the guideline‐recommended 3 months of anticoagulation.[4, 5] With respect to knowledge regarding PICCs, only 9% of respondents recognized that tip verification performed after PICC insertion was conducted to lower risk of venous thromboembolism, not that of arrhythmia.[6] Hospitalists were ambivalent about being trained on how to place PICCs, with only 43% indicating this skill was necessary. Finally, as many as 10% to 25% of PICCs inserted in their hospitals were felt to be inappropriately placed and/or avoidable by 42% of those surveyed.
DISCUSSION
As the use of PICCs rises in hospitalized patients, variability in practices associated with the use of these indwelling vascular catheters is being increasingly recognized. For instance, Tejedor and colleagues reported that PICCs placed in hospitalized patients at their academic medical center were often idle or inserted in patients who simultaneously have peripheral intravenous catheters.[7] Recent data from a tertiary care pediatric center found significantly greater PICC utilization rates over the past decade in association with shorter dwell times, suggesting important and dynamic changes in patterns of use of these devices.[2] Our prior survey of hospitalists in 10 Michigan hospitals also found variations in reported hospitalist practices, knowledge, and experiences related to PICCs.[3] However, the extent to which the Michigan experience portrayed a national trend remained unclear and was the impetus behind this survey. Results from this study appear to support findings from Michigan and highlight several potential opportunities to improve hospitalist PICC practices on a national scale.
In particular, 57% of respondents in this study (compared to 51% of Michigan hospitalists) stated they had at least once forgotten that their patient had a PICC. As early removal of PICCs that are clinically no longer necessary is a cornerstone to preventing thrombosis and infection,[4, 5, 6, 8] the potential impact of such forgetfulness on clinical outcomes and patient safety is of concern. Notably, PICC‐related DVT and bloodstream infection remained the 2 most commonly encountered complications in this survey, just as in the Michigan study.
Reported variations in treatment duration for PICC‐related DVT were also common in this study, with only half of all respondents in both surveys selecting the guideline‐recommended minimum of 3 months of anticoagulation. Finally, a substantial proportion (42%) of participants felt that 10% to 25% of PICCs placed in their hospitals might be inappropriately placed and avoidable, again echoing the sentiments of 51% of the participants in the Michigan survey. These findings strengthen the call to develop a research agenda focused on PICC use in hospitalized patients across the United States.
Why may hospitalists across the country demonstrate such variability when it comes to these indwelling vascular devices? PICCs have historically been viewed as safer with respect to complications such as infection and thrombosis than other central venous catheters, a viewpoint that has likely promulgated their use in the inpatient setting. However, as we and others have shown,[8, 9, 10, 11, 12] this notion is rapidly vanishing and being replaced by the recognition that severity of illness and patient comorbidities are more important determinants of complications than the device itself. Additionally, important knowledge gaps exist when it comes to the safe use of PICCs in hospitalized patients, contributing to variation in indications for insertion, removal, and treatment of complications related to these devices.
Our study is notably limited by a low response rate. Because the survey was administered directly by SHM without collection of respondent data (eg, practice location, years in practice), we are unable to adjust or weight these data to represent a national cohort of adult hospitalists. However, as responses to questions are consistent with our findings from Michigan, and the response rates of this survey are comparable to observed response rates from prior SHM‐administered nationwide surveys (10%40%),[13, 14, 15] we do not believe our findings necessarily represent systematic deviations from the truth and assumed that these responses were missing at random. In addition, owing to use of a survey‐based design, our study is inherently limited by a number of biases, including the use of a convenience sample of SHM members, nonresponse bias, and recall bias. Given these limitations, the association between the available responses and real‐world clinical practice is unclear and deserving of further investigation.
These limitations notwithstanding, our study has several strengths. We found important national variations in reported practices and knowledge related to PICCs, affirming the need to develop a research agenda to improve practice. Further, because a significant proportion of hospitalists may forget their patients have PICCs, our study supports the role of technologies such as catheter reminder systems, computerized decision aids, and automatic stop orders to improve PICC use. These technologies, if utilized in a workflow‐sensitive fashion, could improve PICC safety in hospitalized settings and merit exploration. In addition, our study highlights the growing need for criteria to guide the use of PICCs in hospital settings. Although the Infusion Nursing Society of America has published indications and guidelines for use of vascular devices,[6] these do not always incorporate clinical nuances such as necessity of intravenous therapy or duration of treatment in decision making. The development of evidence‐based appropriateness criteria to guide clinical decision making is thus critical to improving use of PICCs in inpatient settings.[16]
With growing recognition of PICC‐related complications in hospitalized patients, an urgent need to improve practice related to these devices exists. This study begins to define the scope of such work across the United States. Until more rigorous evidence becomes available to guide clinical practice, hospitals and hospitalists should begin to carefully monitor PICC use to safeguard and improve patient safety.
Disclosures
The Blue Cross/Blue Shield of Michigan Foundation funded this study through an investigator‐initiated research proposal (1931‐PIRAP to Dr. Chopra). The funding source played no role in study design, acquisition of data, data analysis, or reporting of these results. The authors report no conflicts of interest.
- , . Peripherally inserted central catheter: compliance with evidence‐based indications for insertion in an inpatient setting. J Infus Nurs. 2013;36(4):291–296.
- , , , , , . Peripherally inserted central catheters: use at a tertiary care pediatric center. J Vasc Interv Radiol. 2013;24(9):1323–1331.
- , , , et al. Hospitalist experiences, practice, opinions, and knowledge regarding peripherally inserted central catheters: a Michigan survey. J Hosp Med. 2013;8(6):309–314.
- , , , et al. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 suppl):7S–47S.
- , , , et al. Quality improvement guidelines for central venous access. J Vasc Interv Radiol. 2010;21(7):976–981.
- , , , et al. Infusion nursing standards of practice. J Infus Nurs. 2011;34(1S):1–115.
- , , , et al. Temporary central venous catheter utilization patterns in a large tertiary care center: tracking the “idle central venous catheter”. Infect Control Hosp Epidemiol. 2012;33(1):50–57.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013;382(9889):311–325.
- , , , , . Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429–435.
- , , , et al. Patient‐ and device‐specific risk factors for peripherally inserted central venous catheter‐related bloodstream infections. Infect Control Hosp Epidemiol. 2013;34(2):184–189.
- , , , , . The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta‐analysis. Infect Control Hosp Epidemiol. 2013;34(9):908–918.
- , . Risk of catheter‐related bloodstream infection with peripherally inserted central venous catheters used in hospitalized patients. Chest. 2005;128(2):489–495.
- , , , , ; Society of Hospital Medicine Career Satisfaction Task Force. Job characteristics, satisfaction, and burnout across hospitalist practice models. J Hosp Med. 2012;7(5):402–410.
- , . Clinical hospital medicine fellowships: perspectives of employers, hospitalists, and medicine residents. J Hosp Med. 2008;3(1):28–34.
- , , , . Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5–9.
- , , . The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527–1528.
Peripherally inserted central catheters (PICCs) are central venous catheters that are inserted through peripheral veins of the upper extremities in adults. Because they are safer to insert than central venous catheters (CVCs) and have become increasingly available at the bedside through the advent of specially trained vascular access nurses,[1] the use of PICCs in hospitalized patients has risen across the United States.[2] As the largest group of inpatient providers, hospitalists play a key role in the decision to insert and subsequently manage PICCs in hospitalized patients. Unfortunately, little is known about national hospitalist experiences, practice patterns, or knowledge when it comes to these commonly used devices. Therefore, we designed a 10‐question survey to investigate PICC‐related practices and knowledge among adult hospitalists practicing throughout the United States.
PATIENTS AND METHODS
Questions for this survey were derived from a previously published study conducted across 10 hospitals in the state of Michigan.[3] To assess external validity and test specific hypotheses formulated from the Michigan study, those questions with the greatest variation in response or those most amenable to interventions were chosen for inclusion in this survey.
To reach a national audience of practicing adult hospitalists, we submitted a survey proposal to the Society of Hospital Medicine's (SHM) Research Committee. The SHM Research Committee reviews such proposals using a peer‐review process to ensure both scientific integrity and validity of the survey instrument. Because the survey was already distributed to many hospitalists in Michigan, we requested that only hospitalists outside of Michigan be invited to participate in the national survey. All responses were collected anonymously, and no identifiable data were collected from respondents. Between February 1, 2013 and March 15, 2013, data were collected via an e‐mail sent directly from the SHM to members that contained a link to the study survey administered using SurveyMonkey. To augment data collection, nonresponders to the original e‐mail invitation were sent a second reminder e‐mail midway through the study. Descriptive statistics (percentages) were used to tabulate responses. The institutional review board at the University of Michigan Health System provided ethical and regulatory approval for this study.
RESULTS
A total of 2112 electronic survey invitations were sent to non‐Michigan adult hospitalists, with 381 completing the online survey (response rate 18%). Among respondents to the national survey, 86% reported having placed a PICC solely to obtain venous access in a hospitalized patient (rather than for specific indications such as long‐term intravenous antibiotics, chemotherapy, or parenteral nutrition), whereas 82% reported having cared for a patient who specifically requested a PICC (Table 1). PICC‐related deep vein thrombosis (DVT) and bloodstream infections were reported as being the most frequent PICC complications encountered by hospitalists, followed by superficial thrombophlebitis and mechanical complications such as coiling, kinking, and migration of the PICC tip.
| Total (N=381) | |
|---|---|
| |
| Hospitalist experiences related to PICCs | |
| Among hospitalized patients you have cared for, have any of your patients ever had a PICC placed solely to obtain venous access (eg, not for an indication such as long‐term IV antibiotics, chemotherapy, or TPN)? | |
| Yes | 328 (86.1%) |
| No | 53 (13.9%) |
| Have you ever cared for a patient who specifically requested a PICC because of prior experience with this device? | |
| Yes | 311 (81.6%) |
| No | 70 (18.4%) |
| Most frequently encountered PICC complications | |
| Upper‐extremity DVT or PE | 48 (12.6%) |
| Bloodstream infection | 41 (10.8%) |
| Superficial thrombophlebitis | 34 (8.9%) |
| Cellulitis/exit site erythema | 26 (6.8%) |
| Coiling, kinking of the PICC | 14 (3.7%) |
| Migration of the PICC tip | 9 (2.4%) |
| Breakage of PICC (anywhere) | 6 (1.6%) |
| Hospitalist practice related to PICCs | |
| During patient rounds, do you routinely examine PICCs for external problems (eg, cracks, breaks, leaks, or redness at the insertion site)? | |
| Yes, daily | 97 (25.5%) |
| Yes, but only if the nurse or patient alerts me to a problem with the PICC | 190 (49.9%) |
| No, I don't routinely examine the PICC for external problems | 94 (24.7%) |
| Have you ever forgotten or been unaware of the presence of a PICC? | |
| Yes | 216 (56.7%) |
| No | 165 (43.3%) |
| Assuming no contraindications exist, do you anticoagulate patients who develop a PICC‐associated DVT? | |
| Yes, for at least 1 month | 41(10.8%) |
| Yes, for at least 3 months* | 198 (52.0%) |
| Yes, for at least 6 months | 11 (2.9%) |
| Yes, I anticoagulate for as long as the line remains in place. Once the line is removed, I stop anticoagulation | 30 (7.9%) |
| Yes, I anticoagulate for as long as the line remains in place followed by another 4 weeks of therapy | 72 (18.9%) |
| I don't usually anticoagulate patients who develop a PICC‐related DVT | 29 (7.6%) |
| When a hospitalized patient develops a PICC‐related DVT, do you routinely remove the PICC? | |
| Yes | 271 (71.1%) |
| No | 110 (28.9%) |
| Hospitalist opinions related to PICCs | |
| Thinking about your hospital and your experiences, what percentage of PICC insertions may represent inappropriate use (eg, PICC placed for short‐term venous access for a presumed infection that could be treated with oral antibiotic or PICCs that were promptly removed as the patient no longer needed it for clinical management)? | |
| <10% | 192 (50.4%) |
| 10%25% | 160 (42.0%) |
| 26%50% | 22 (5.8%) |
| >50% | 7 (1.8%) |
| Do you think hospitalists should be trained to insert PICCs? | |
| Yes | 162 (42.5%) |
| No | 219 (57.5%) |
| Hospitalist knowledge related to PICCs | |
| Why is the position of the PICC‐tip checked following bedside PICC insertion? | |
| To decrease the risk of arrhythmia from tip placement in the right atrial | 267 (70.1%) |
| To ensure it is not accidentally placed into an artery | 44 (11.5%) |
| To minimize the risk of venous thrombosis* | 33 (8.7%) |
| For documentation purposes (to reduce the risk of lawsuits related tocomplications) | 16 (4.2%) |
| I don't know | 21 (5.5%) |
Several potentially important safety concerns regarding hospitalist PICC practices were observed in this survey. For instance, only 25% of hospitalists reported examining PICCs on daily rounds for external problems. When alerted by nurses or patients about problems with the device, this number doubled to 50%. In addition, 57% of respondents admitted to having at least once forgotten about the presence of a PICC in their hospitalized patient.
Participants also reported significant variation in duration of anticoagulation therapy for PICC‐related DVT, with only half of all respondents selecting the guideline‐recommended 3 months of anticoagulation.[4, 5] With respect to knowledge regarding PICCs, only 9% of respondents recognized that tip verification performed after PICC insertion was conducted to lower risk of venous thromboembolism, not that of arrhythmia.[6] Hospitalists were ambivalent about being trained on how to place PICCs, with only 43% indicating this skill was necessary. Finally, as many as 10% to 25% of PICCs inserted in their hospitals were felt to be inappropriately placed and/or avoidable by 42% of those surveyed.
DISCUSSION
As the use of PICCs rises in hospitalized patients, variability in practices associated with the use of these indwelling vascular catheters is being increasingly recognized. For instance, Tejedor and colleagues reported that PICCs placed in hospitalized patients at their academic medical center were often idle or inserted in patients who simultaneously have peripheral intravenous catheters.[7] Recent data from a tertiary care pediatric center found significantly greater PICC utilization rates over the past decade in association with shorter dwell times, suggesting important and dynamic changes in patterns of use of these devices.[2] Our prior survey of hospitalists in 10 Michigan hospitals also found variations in reported hospitalist practices, knowledge, and experiences related to PICCs.[3] However, the extent to which the Michigan experience portrayed a national trend remained unclear and was the impetus behind this survey. Results from this study appear to support findings from Michigan and highlight several potential opportunities to improve hospitalist PICC practices on a national scale.
In particular, 57% of respondents in this study (compared to 51% of Michigan hospitalists) stated they had at least once forgotten that their patient had a PICC. As early removal of PICCs that are clinically no longer necessary is a cornerstone to preventing thrombosis and infection,[4, 5, 6, 8] the potential impact of such forgetfulness on clinical outcomes and patient safety is of concern. Notably, PICC‐related DVT and bloodstream infection remained the 2 most commonly encountered complications in this survey, just as in the Michigan study.
Reported variations in treatment duration for PICC‐related DVT were also common in this study, with only half of all respondents in both surveys selecting the guideline‐recommended minimum of 3 months of anticoagulation. Finally, a substantial proportion (42%) of participants felt that 10% to 25% of PICCs placed in their hospitals might be inappropriately placed and avoidable, again echoing the sentiments of 51% of the participants in the Michigan survey. These findings strengthen the call to develop a research agenda focused on PICC use in hospitalized patients across the United States.
Why may hospitalists across the country demonstrate such variability when it comes to these indwelling vascular devices? PICCs have historically been viewed as safer with respect to complications such as infection and thrombosis than other central venous catheters, a viewpoint that has likely promulgated their use in the inpatient setting. However, as we and others have shown,[8, 9, 10, 11, 12] this notion is rapidly vanishing and being replaced by the recognition that severity of illness and patient comorbidities are more important determinants of complications than the device itself. Additionally, important knowledge gaps exist when it comes to the safe use of PICCs in hospitalized patients, contributing to variation in indications for insertion, removal, and treatment of complications related to these devices.
Our study is notably limited by a low response rate. Because the survey was administered directly by SHM without collection of respondent data (eg, practice location, years in practice), we are unable to adjust or weight these data to represent a national cohort of adult hospitalists. However, as responses to questions are consistent with our findings from Michigan, and the response rates of this survey are comparable to observed response rates from prior SHM‐administered nationwide surveys (10%40%),[13, 14, 15] we do not believe our findings necessarily represent systematic deviations from the truth and assumed that these responses were missing at random. In addition, owing to use of a survey‐based design, our study is inherently limited by a number of biases, including the use of a convenience sample of SHM members, nonresponse bias, and recall bias. Given these limitations, the association between the available responses and real‐world clinical practice is unclear and deserving of further investigation.
These limitations notwithstanding, our study has several strengths. We found important national variations in reported practices and knowledge related to PICCs, affirming the need to develop a research agenda to improve practice. Further, because a significant proportion of hospitalists may forget their patients have PICCs, our study supports the role of technologies such as catheter reminder systems, computerized decision aids, and automatic stop orders to improve PICC use. These technologies, if utilized in a workflow‐sensitive fashion, could improve PICC safety in hospitalized settings and merit exploration. In addition, our study highlights the growing need for criteria to guide the use of PICCs in hospital settings. Although the Infusion Nursing Society of America has published indications and guidelines for use of vascular devices,[6] these do not always incorporate clinical nuances such as necessity of intravenous therapy or duration of treatment in decision making. The development of evidence‐based appropriateness criteria to guide clinical decision making is thus critical to improving use of PICCs in inpatient settings.[16]
With growing recognition of PICC‐related complications in hospitalized patients, an urgent need to improve practice related to these devices exists. This study begins to define the scope of such work across the United States. Until more rigorous evidence becomes available to guide clinical practice, hospitals and hospitalists should begin to carefully monitor PICC use to safeguard and improve patient safety.
Disclosures
The Blue Cross/Blue Shield of Michigan Foundation funded this study through an investigator‐initiated research proposal (1931‐PIRAP to Dr. Chopra). The funding source played no role in study design, acquisition of data, data analysis, or reporting of these results. The authors report no conflicts of interest.
Peripherally inserted central catheters (PICCs) are central venous catheters that are inserted through peripheral veins of the upper extremities in adults. Because they are safer to insert than central venous catheters (CVCs) and have become increasingly available at the bedside through the advent of specially trained vascular access nurses,[1] the use of PICCs in hospitalized patients has risen across the United States.[2] As the largest group of inpatient providers, hospitalists play a key role in the decision to insert and subsequently manage PICCs in hospitalized patients. Unfortunately, little is known about national hospitalist experiences, practice patterns, or knowledge when it comes to these commonly used devices. Therefore, we designed a 10‐question survey to investigate PICC‐related practices and knowledge among adult hospitalists practicing throughout the United States.
PATIENTS AND METHODS
Questions for this survey were derived from a previously published study conducted across 10 hospitals in the state of Michigan.[3] To assess external validity and test specific hypotheses formulated from the Michigan study, those questions with the greatest variation in response or those most amenable to interventions were chosen for inclusion in this survey.
To reach a national audience of practicing adult hospitalists, we submitted a survey proposal to the Society of Hospital Medicine's (SHM) Research Committee. The SHM Research Committee reviews such proposals using a peer‐review process to ensure both scientific integrity and validity of the survey instrument. Because the survey was already distributed to many hospitalists in Michigan, we requested that only hospitalists outside of Michigan be invited to participate in the national survey. All responses were collected anonymously, and no identifiable data were collected from respondents. Between February 1, 2013 and March 15, 2013, data were collected via an e‐mail sent directly from the SHM to members that contained a link to the study survey administered using SurveyMonkey. To augment data collection, nonresponders to the original e‐mail invitation were sent a second reminder e‐mail midway through the study. Descriptive statistics (percentages) were used to tabulate responses. The institutional review board at the University of Michigan Health System provided ethical and regulatory approval for this study.
RESULTS
A total of 2112 electronic survey invitations were sent to non‐Michigan adult hospitalists, with 381 completing the online survey (response rate 18%). Among respondents to the national survey, 86% reported having placed a PICC solely to obtain venous access in a hospitalized patient (rather than for specific indications such as long‐term intravenous antibiotics, chemotherapy, or parenteral nutrition), whereas 82% reported having cared for a patient who specifically requested a PICC (Table 1). PICC‐related deep vein thrombosis (DVT) and bloodstream infections were reported as being the most frequent PICC complications encountered by hospitalists, followed by superficial thrombophlebitis and mechanical complications such as coiling, kinking, and migration of the PICC tip.
| Total (N=381) | |
|---|---|
| |
| Hospitalist experiences related to PICCs | |
| Among hospitalized patients you have cared for, have any of your patients ever had a PICC placed solely to obtain venous access (eg, not for an indication such as long‐term IV antibiotics, chemotherapy, or TPN)? | |
| Yes | 328 (86.1%) |
| No | 53 (13.9%) |
| Have you ever cared for a patient who specifically requested a PICC because of prior experience with this device? | |
| Yes | 311 (81.6%) |
| No | 70 (18.4%) |
| Most frequently encountered PICC complications | |
| Upper‐extremity DVT or PE | 48 (12.6%) |
| Bloodstream infection | 41 (10.8%) |
| Superficial thrombophlebitis | 34 (8.9%) |
| Cellulitis/exit site erythema | 26 (6.8%) |
| Coiling, kinking of the PICC | 14 (3.7%) |
| Migration of the PICC tip | 9 (2.4%) |
| Breakage of PICC (anywhere) | 6 (1.6%) |
| Hospitalist practice related to PICCs | |
| During patient rounds, do you routinely examine PICCs for external problems (eg, cracks, breaks, leaks, or redness at the insertion site)? | |
| Yes, daily | 97 (25.5%) |
| Yes, but only if the nurse or patient alerts me to a problem with the PICC | 190 (49.9%) |
| No, I don't routinely examine the PICC for external problems | 94 (24.7%) |
| Have you ever forgotten or been unaware of the presence of a PICC? | |
| Yes | 216 (56.7%) |
| No | 165 (43.3%) |
| Assuming no contraindications exist, do you anticoagulate patients who develop a PICC‐associated DVT? | |
| Yes, for at least 1 month | 41(10.8%) |
| Yes, for at least 3 months* | 198 (52.0%) |
| Yes, for at least 6 months | 11 (2.9%) |
| Yes, I anticoagulate for as long as the line remains in place. Once the line is removed, I stop anticoagulation | 30 (7.9%) |
| Yes, I anticoagulate for as long as the line remains in place followed by another 4 weeks of therapy | 72 (18.9%) |
| I don't usually anticoagulate patients who develop a PICC‐related DVT | 29 (7.6%) |
| When a hospitalized patient develops a PICC‐related DVT, do you routinely remove the PICC? | |
| Yes | 271 (71.1%) |
| No | 110 (28.9%) |
| Hospitalist opinions related to PICCs | |
| Thinking about your hospital and your experiences, what percentage of PICC insertions may represent inappropriate use (eg, PICC placed for short‐term venous access for a presumed infection that could be treated with oral antibiotic or PICCs that were promptly removed as the patient no longer needed it for clinical management)? | |
| <10% | 192 (50.4%) |
| 10%25% | 160 (42.0%) |
| 26%50% | 22 (5.8%) |
| >50% | 7 (1.8%) |
| Do you think hospitalists should be trained to insert PICCs? | |
| Yes | 162 (42.5%) |
| No | 219 (57.5%) |
| Hospitalist knowledge related to PICCs | |
| Why is the position of the PICC‐tip checked following bedside PICC insertion? | |
| To decrease the risk of arrhythmia from tip placement in the right atrial | 267 (70.1%) |
| To ensure it is not accidentally placed into an artery | 44 (11.5%) |
| To minimize the risk of venous thrombosis* | 33 (8.7%) |
| For documentation purposes (to reduce the risk of lawsuits related tocomplications) | 16 (4.2%) |
| I don't know | 21 (5.5%) |
Several potentially important safety concerns regarding hospitalist PICC practices were observed in this survey. For instance, only 25% of hospitalists reported examining PICCs on daily rounds for external problems. When alerted by nurses or patients about problems with the device, this number doubled to 50%. In addition, 57% of respondents admitted to having at least once forgotten about the presence of a PICC in their hospitalized patient.
Participants also reported significant variation in duration of anticoagulation therapy for PICC‐related DVT, with only half of all respondents selecting the guideline‐recommended 3 months of anticoagulation.[4, 5] With respect to knowledge regarding PICCs, only 9% of respondents recognized that tip verification performed after PICC insertion was conducted to lower risk of venous thromboembolism, not that of arrhythmia.[6] Hospitalists were ambivalent about being trained on how to place PICCs, with only 43% indicating this skill was necessary. Finally, as many as 10% to 25% of PICCs inserted in their hospitals were felt to be inappropriately placed and/or avoidable by 42% of those surveyed.
DISCUSSION
As the use of PICCs rises in hospitalized patients, variability in practices associated with the use of these indwelling vascular catheters is being increasingly recognized. For instance, Tejedor and colleagues reported that PICCs placed in hospitalized patients at their academic medical center were often idle or inserted in patients who simultaneously have peripheral intravenous catheters.[7] Recent data from a tertiary care pediatric center found significantly greater PICC utilization rates over the past decade in association with shorter dwell times, suggesting important and dynamic changes in patterns of use of these devices.[2] Our prior survey of hospitalists in 10 Michigan hospitals also found variations in reported hospitalist practices, knowledge, and experiences related to PICCs.[3] However, the extent to which the Michigan experience portrayed a national trend remained unclear and was the impetus behind this survey. Results from this study appear to support findings from Michigan and highlight several potential opportunities to improve hospitalist PICC practices on a national scale.
In particular, 57% of respondents in this study (compared to 51% of Michigan hospitalists) stated they had at least once forgotten that their patient had a PICC. As early removal of PICCs that are clinically no longer necessary is a cornerstone to preventing thrombosis and infection,[4, 5, 6, 8] the potential impact of such forgetfulness on clinical outcomes and patient safety is of concern. Notably, PICC‐related DVT and bloodstream infection remained the 2 most commonly encountered complications in this survey, just as in the Michigan study.
Reported variations in treatment duration for PICC‐related DVT were also common in this study, with only half of all respondents in both surveys selecting the guideline‐recommended minimum of 3 months of anticoagulation. Finally, a substantial proportion (42%) of participants felt that 10% to 25% of PICCs placed in their hospitals might be inappropriately placed and avoidable, again echoing the sentiments of 51% of the participants in the Michigan survey. These findings strengthen the call to develop a research agenda focused on PICC use in hospitalized patients across the United States.
Why may hospitalists across the country demonstrate such variability when it comes to these indwelling vascular devices? PICCs have historically been viewed as safer with respect to complications such as infection and thrombosis than other central venous catheters, a viewpoint that has likely promulgated their use in the inpatient setting. However, as we and others have shown,[8, 9, 10, 11, 12] this notion is rapidly vanishing and being replaced by the recognition that severity of illness and patient comorbidities are more important determinants of complications than the device itself. Additionally, important knowledge gaps exist when it comes to the safe use of PICCs in hospitalized patients, contributing to variation in indications for insertion, removal, and treatment of complications related to these devices.
Our study is notably limited by a low response rate. Because the survey was administered directly by SHM without collection of respondent data (eg, practice location, years in practice), we are unable to adjust or weight these data to represent a national cohort of adult hospitalists. However, as responses to questions are consistent with our findings from Michigan, and the response rates of this survey are comparable to observed response rates from prior SHM‐administered nationwide surveys (10%40%),[13, 14, 15] we do not believe our findings necessarily represent systematic deviations from the truth and assumed that these responses were missing at random. In addition, owing to use of a survey‐based design, our study is inherently limited by a number of biases, including the use of a convenience sample of SHM members, nonresponse bias, and recall bias. Given these limitations, the association between the available responses and real‐world clinical practice is unclear and deserving of further investigation.
These limitations notwithstanding, our study has several strengths. We found important national variations in reported practices and knowledge related to PICCs, affirming the need to develop a research agenda to improve practice. Further, because a significant proportion of hospitalists may forget their patients have PICCs, our study supports the role of technologies such as catheter reminder systems, computerized decision aids, and automatic stop orders to improve PICC use. These technologies, if utilized in a workflow‐sensitive fashion, could improve PICC safety in hospitalized settings and merit exploration. In addition, our study highlights the growing need for criteria to guide the use of PICCs in hospital settings. Although the Infusion Nursing Society of America has published indications and guidelines for use of vascular devices,[6] these do not always incorporate clinical nuances such as necessity of intravenous therapy or duration of treatment in decision making. The development of evidence‐based appropriateness criteria to guide clinical decision making is thus critical to improving use of PICCs in inpatient settings.[16]
With growing recognition of PICC‐related complications in hospitalized patients, an urgent need to improve practice related to these devices exists. This study begins to define the scope of such work across the United States. Until more rigorous evidence becomes available to guide clinical practice, hospitals and hospitalists should begin to carefully monitor PICC use to safeguard and improve patient safety.
Disclosures
The Blue Cross/Blue Shield of Michigan Foundation funded this study through an investigator‐initiated research proposal (1931‐PIRAP to Dr. Chopra). The funding source played no role in study design, acquisition of data, data analysis, or reporting of these results. The authors report no conflicts of interest.
- , . Peripherally inserted central catheter: compliance with evidence‐based indications for insertion in an inpatient setting. J Infus Nurs. 2013;36(4):291–296.
- , , , , , . Peripherally inserted central catheters: use at a tertiary care pediatric center. J Vasc Interv Radiol. 2013;24(9):1323–1331.
- , , , et al. Hospitalist experiences, practice, opinions, and knowledge regarding peripherally inserted central catheters: a Michigan survey. J Hosp Med. 2013;8(6):309–314.
- , , , et al. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 suppl):7S–47S.
- , , , et al. Quality improvement guidelines for central venous access. J Vasc Interv Radiol. 2010;21(7):976–981.
- , , , et al. Infusion nursing standards of practice. J Infus Nurs. 2011;34(1S):1–115.
- , , , et al. Temporary central venous catheter utilization patterns in a large tertiary care center: tracking the “idle central venous catheter”. Infect Control Hosp Epidemiol. 2012;33(1):50–57.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013;382(9889):311–325.
- , , , , . Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429–435.
- , , , et al. Patient‐ and device‐specific risk factors for peripherally inserted central venous catheter‐related bloodstream infections. Infect Control Hosp Epidemiol. 2013;34(2):184–189.
- , , , , . The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta‐analysis. Infect Control Hosp Epidemiol. 2013;34(9):908–918.
- , . Risk of catheter‐related bloodstream infection with peripherally inserted central venous catheters used in hospitalized patients. Chest. 2005;128(2):489–495.
- , , , , ; Society of Hospital Medicine Career Satisfaction Task Force. Job characteristics, satisfaction, and burnout across hospitalist practice models. J Hosp Med. 2012;7(5):402–410.
- , . Clinical hospital medicine fellowships: perspectives of employers, hospitalists, and medicine residents. J Hosp Med. 2008;3(1):28–34.
- , , , . Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5–9.
- , , . The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527–1528.
- , . Peripherally inserted central catheter: compliance with evidence‐based indications for insertion in an inpatient setting. J Infus Nurs. 2013;36(4):291–296.
- , , , , , . Peripherally inserted central catheters: use at a tertiary care pediatric center. J Vasc Interv Radiol. 2013;24(9):1323–1331.
- , , , et al. Hospitalist experiences, practice, opinions, and knowledge regarding peripherally inserted central catheters: a Michigan survey. J Hosp Med. 2013;8(6):309–314.
- , , , et al. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 suppl):7S–47S.
- , , , et al. Quality improvement guidelines for central venous access. J Vasc Interv Radiol. 2010;21(7):976–981.
- , , , et al. Infusion nursing standards of practice. J Infus Nurs. 2011;34(1S):1–115.
- , , , et al. Temporary central venous catheter utilization patterns in a large tertiary care center: tracking the “idle central venous catheter”. Infect Control Hosp Epidemiol. 2012;33(1):50–57.
- , , , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta‐analysis. Lancet. 2013;382(9889):311–325.
- , , , , . Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429–435.
- , , , et al. Patient‐ and device‐specific risk factors for peripherally inserted central venous catheter‐related bloodstream infections. Infect Control Hosp Epidemiol. 2013;34(2):184–189.
- , , , , . The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta‐analysis. Infect Control Hosp Epidemiol. 2013;34(9):908–918.
- , . Risk of catheter‐related bloodstream infection with peripherally inserted central venous catheters used in hospitalized patients. Chest. 2005;128(2):489–495.
- , , , , ; Society of Hospital Medicine Career Satisfaction Task Force. Job characteristics, satisfaction, and burnout across hospitalist practice models. J Hosp Med. 2012;7(5):402–410.
- , . Clinical hospital medicine fellowships: perspectives of employers, hospitalists, and medicine residents. J Hosp Med. 2008;3(1):28–34.
- , , , . Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5–9.
- , , . The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527–1528.
Dear Doctor: A Patient‐Centered Tool
In their seminal report Crossing the Quality Chasm, the Institute of Medicine outlined patient‐centered care as 1 of its 6 aims to improve the healthcare delivery system.[1] Patients who are more involved in their diagnosis and treatment plan are more likely to feel respected, be satisfied with their healthcare experience, and ultimately have better outcomes.[2, 3] In a study of hospitalized patients, only 42% were able to state their diagnosis at the time of discharge, suggesting that hospital providers could communicate better with patients about their hospital care.[4] Additionally, only 28% of hospitalized patients were able to list their medications, and only 37% were able to state the purpose of their medications. Although hospitals have taken great strides to improve the quality of patient care, publicly reported patient care surveys, such as the Hospital Consumer Assessment of Hospitals and Health Systems (HCAHPS), suggest that physician communication with patients could be further improved.[1, 5] Furthermore, a recent report by the Institute of Medicine stresses the need to get patients and families involved in their care.[6] Thus, hospital‐based providers should seek to enhance the quality of their communication with patients.
With greater emphasis placed on patient‐ and family‐centered care at many health systems, simple and easy‐to‐implement strategies to improve communication with patients need to be developed and tested.[7, 8] Patients who actively participate in their healthcare by asking questions of their doctor are able to control the focus of their interaction and adjust the amount of information provided.[9] Simply asking questions can have a critical impact, as 1 study found that the frequency with which patients asked questions was significantly related to the amount of information received about general and specific medical matters.[10] The notepad is a common tool for reminders and personal interactions that is used in everyday life, but has not been formalized in the hospital. We introduced Dear Doctor (DD) notes, a bedside notepad designed to prompt patient questions, with the goal of facilitating patient communication with their hospitalist physicians (Figure 1). As hospitalists provide direct and indirect care to a growing number of hospitalized patients, they are likely to be asked questions and opinions about the patients' diagnoses and plans. Furthermore, hospitalists are poised to lead institutional quality, safety, efficiency, and service improvement efforts in the inpatient setting. Becoming familiar with communication‐enhancing tools, such as the DD notes, may help hospitalists in their improvement team roles.

METHODS
Setting
We conducted a study between July 2009 and September 2009 on inpatient medical wards at a large academic medical center with 610 beds and over 44,000 annual discharges.[11] The internal medicine services served by attending physicians and residents comprise a large proportion of hospitalized patients, accounting for over 17,000 discharges per year. Each medical unit includes 32 beds.
Population
Patients over the age of 18 years admitted to a general medicine or cardiology unit and who were able to verbally communicate in English were eligible to be surveyed in the study. Patients with a length of stay <24 hours were excluded. A total of 664 patients were surveyed for inclusion in the study, 440 patients in the intervention group and 224 patients in the control group.
Intervention
The DD notepad included sample questions and informational prompts derived with input from a community focus group. The community focus group consisted of current and formerly hospitalized patients and family members who were asked by members of the study group what they thought would be important to include on a notepad provided to patients. From their answers the study team developed the DD notepad prototype. The DD notepad included 3 general categories of questions: (1) diagnosis and treatment, (2) tests and procedures, and (3) medications. To address other miscellaneous topics such as discharge and posthospital care needs, a section was designated for the patient to check off as I have a few more questions (Use the back of the sheet).
All patients admitted to the study units were intended to receive the DD notepad and pen, which were placed on the bedside table during the room change by our custodial staff. Patients who did not receive DD notepads in the intervention group during their first hospitalization day were provided with 1 by the clinical assistants working with the hospitalists. These patients did not initially receive the notepad due to logistical reasons from temporary rotating staff who were not instructed to provide the notepads. Patients were not formally prompted to use the notepad. Hospitalists, residents, and nurses on the study units were informed about the distribution of DD notes to patients on these units; however, they were not provided with any specific instructions on how they should incorporate the DD notes into their interactions with their patients. The use of the DD notepad was left to each healthcare professional's own discretion.
Members of the study team surveyed patients who had been in the hospital for a minimum of 24 hours in the intervention and control groups twice weekly. All responses were deidentified of any personal or health information. Patients were asked to rate on a scale from 1 to 5 their use of the DD notepads, their perceived value, the circumstances in which the notepads were used, and their level of satisfaction with how their physicians communicated and answered their questions (1 = no improvement, 5 = significant improvement). For control patients, questions pertaining to DD notepads were not applicable and were therefore excluded.
Statistical Analysis
The data were analyzed in an intention‐to‐treat analysis of all 440 patients in the intervention group. Intervention and control groups were compared using 2, rank sum, and Fisher exact statistical tests, with significance assigned as P < 0.05, using SPSS software version 17.0 (SPSS, Inc., Chicago, IL). Our project was approved by the University of Michigan's institutional review board.
RESULTS
Of the 440 patients surveyed in the intervention group (1 general medicine and 1 cardiology unit), 343 (78%) received the notepads in their rooms and 207 (47%) used them (Figure 2). Not every patient in the intervention group received DD notepads due to inconsistent placement of DD notepads upon every room turnover. Of the patients admitted to the control group (1 general medicine and 1 cardiology unit), 224 were surveyed. Fifty‐four percent of the 440 patients in the intervention group reported that they took notes related to their hospital care, compared to only 22% of the 224 patients in the control group (P < 0.001). Of the patients who took notes within the intervention group (n = 207), 91% of them utilized the DD notepads.
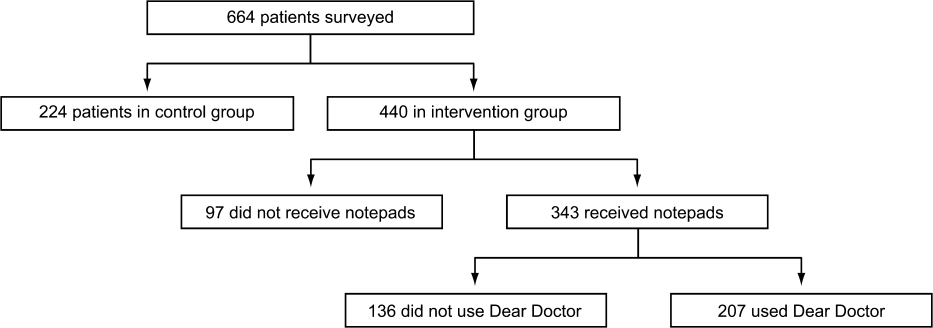
Patients in the intervention group who received and used the DD notes (n = 207) compared to patients in the control group (n = 224) were more likely to report that their questions were answered by their physicians (4.63 vs 4.45, P < 0.001). In an intention‐to‐treat analysis of all 440 patients in the intervention group, the overall satisfaction with physician communication was not significantly different between the intervention and control groups as measured on a 5‐point Likert scale (4.55 vs 4.55, P = 0.89). However, 89% of the patients in the intervention group who used the notepads felt that DD notepads either moderately or significantly improved their communication with their providers (Figure 3).

When the 207 patients who received DD notepads were asked how they used this tool, 99% of these patients used DD to write down questions, 82% to keep track of tests and procedures, and 54% indicated that their family and friends also used the notepads during the hospital stay (Table 1). Among these patients who utilized the DD notepads, 93% reported that they would use them again in the future.
| Wrote Notes? (P < 0.001) (%) | Used DD? (%) | Use in Future? (%) | Frequency of Questions Answered (P < 0.001) | DD Improved Communication | Satisfied With Communication? (P = 0.89) | |
|---|---|---|---|---|---|---|
| ||||||
| Intervention (n = 440) | 54 | 91 | 93.2 | 4.63 | 3.76 | 4.55 |
| Control (n = 224) | 22 | 4.45 | 4.55 | |||
Of the 97 patients in the intervention group who did not receive a DD notepad, we asked if they would use the DD notepad if they were made aware of such a tool. Of these patients, 77% agreed that they would use DD notes if they were made available in the future, 100% of them said that they would use DD to write down questions, 97% indicated that they would write notes about tests and procedures, and 88% of them believed that their families and friends would use DD notes.
DISCUSSION
As hospitals place greater emphasis and value on patient‐centered care as part of their clinical mission, it is important to develop tools to help facilitate the doctor‐patient relationship. We found that patients who were provided the Dear Doctor notepad were more satisfied that their doctors answered their questions and felt this tool enhanced their ability to communicate with their physicians. Employing the use of a familiar tool such as the notepad to remind patients about specific issues in their interactions with their providers can be a powerful intervention. Our study demonstrated that the DD notepad was widely accepted by patients, and that almost all of them would use this tool if it were made available to them in the future.
Other tools and methods to enhance the quality of communication between patients and their healthcare providers have included using whiteboards in the patients' rooms to relay the care plan to the patients, implementing bedside rounds by the healthcare team, and multidisciplinary huddling to coordinate information to the patient.[12, 13] Studies of these communication tools have shown potential to improve teamwork, interaction, and patient care. All of these have their own merit and value, and our DD notepad should be considered an adjunct to existing methods to enhance the patient care experience. A bedside tool that is familiar in form to most patients also needs to have the feature of easy access and use. Once this barrier has been removed for the patient and their family members, tools such as the DD notepad can impact the patient‐centeredness by fostering increased and better quality dialogue between the patients, their family members, and healthcare providers.
The DD notepad represents a means of communication that may have the potential to empower patients. It is possible that through question prompts, the DD notepad stimulates the patient to be an active partner with his or her healthcare team. This may enable patients to have some sense of control and accountability of their care in a setting where they would otherwise feel overwhelmed or powerless. The 3 general categories to help patients write down their questions included diagnosis, treatment plan, and medications. In the inpatient setting, where patient‐care activities can be fast paced, and patients are unable to recall some details when speaking to the healthcare team, these notes may remind the patient to write their thoughts down so that they may be remembered for a future time. In situations where patients may not know which questions to ask, the question prompts may be particularly helpful. We did not assess whether our particular question prompts were the key elements that resulted in their perceived value, or whether simply placing a blank notepad at the bedside would also have been successful. However, the specific questions were suggested by the focus groups. Enhanced communication, focusing on the patients' understanding of their condition, and the need to pursue certain diagnostic or therapeutic interventions, may help patients to be better prepared for the next course of plan. These topics of reasons for hospitalization, treatment plan, and medication changes are also important for patients to be active participants in their care, in particular as they transition from 1 site of care to the next, and their healthcare will be delivered by different providers.
There are several limitations to our study. First, this intervention was performed at a single hospital site with only 2 clinical services (general medicine, cardiology) represented in the study groups. Although we do not have any causal reasons to believe this tool would be looked upon differently by patients on other clinical services, it is possible that patients on a different clinical unit or service may view this tool as less or more useful. Second, as the patients were not randomized to intervention, but rather based on the units to which they were admitted, it is possible that other variables, such as the experience of the unit staff, the patient's condition, and housestaff‐based service versus hospitalist‐based service may have played a role in how the patients perceived the use of DD notes. Third, patients were only surveyed if they were able to verbally communicate in English. These notes may not be as useful in hospital settings to populations with language or literacy barriers. Fourth, the logistical implementation of DD notes limited our ability to deliver the DD notepads in every patient's rooms, where only 78% of the intervention group received the DD notepads. This may be the reason that we did not find that overall satisfaction with physician communication differed between intervention and control groups. Nonetheless, we performed an intention‐to‐treat analysis to minimize any biases in our analysis. Last, although our survey of patients asked about their satisfaction in using the DD note pads, we did not compare these results with those of Press‐Ganey or HCAHPS scores of patients on the intervention group versus the control group. Additionally, lack of data about type, quality, and quantity of questions asked by a control group to see if the notepads actually improved quality of questions asked is a limitation; however, we believe our outcomes of interest were most specifically evaluated through our survey questions.
DD notes show that the majority of patients who use this tool feel a modest to significant improvement in communication with their providers. Although the quality of medical care is undoubtedly the first priority, the patients' view of their care, which includes communication, is arguably just as important. An often‐forgotten goal of hospitals and clinics is to provide service excellence along with high‐quality care. Thus, it is imperative for hospitals and their care providers to not only focus on the quality and safety of the clinical care, but also be mindful of the patient's entire experience throughout their hospital stay. Many of the categories of questions asked in the HCAHPS address the patient's experience and perspectives of hospital care. Furthermore, the role of the HCAHPS survey in the Value‐Based Purchasing rules may enhance the importance of these notepads. As the results of HCAHPS are becoming more transparent and available to the public, the impact of such results will have a greater significance to the future of the hospital's clinical mission.
CONCLUSION
DD notepads are a simple, low‐cost, patient‐centered tool that can be an effective reminder for patients to ask their healthcare providers questions related to their hospital care. Utilizing a common tool such as the notepad, redesigned for the healthcare setting, can serve to help healthcare providers interact with their patients. Patient satisfaction may be higher in patients who use the DD notepad.
Disclosures: Aaron S. Farberg, MD, and Andrew M. Lin, MD, contributed equally in every way and should be considered co‐first authors. This work was supported by a University of Michigan Fostering Innovations Grant. The authors have no conflicting financial interests.
- Committee on Quality of Health Care in America, Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
- , , , . An evidence base for patient‐centered cancer care: a meta‐analysis of studies of observed communication between cancer specialists and their patients. Patient Educ Couns. 2009;77(3):379–383.
- . Effective physician‐patient communication and health outcomes: a review. Can Med Assoc J. 1995;152:1423–1433.
- , . Patients' understanding of their treatment plans and diagnosis at discharge. Mayo Clin Proc. 2005;80(8):991–994.
- , , , . Patients' perception of hospital care in the United States. N Engl J Med. 2008;359:1921–1931.
- Institute of Medicine of the National Academies.Best care at lower cost: the path to continuously learning health care in America. Available at: http://www.iom.edu/Reports/2012/Best‐Care‐at‐Lower‐Cost‐The‐Path‐to‐Continuously‐Learning‐Health‐Care‐in‐America.aspx. Accessed October 5, 2012.
- , . An introduction to technology for patient‐centered collaborative care. J Ambul Care Manage. 2006;29:195–198.
- , , , , , . Effect on health‐related outcome of interventions to alter the interaction between patients and practitioners: a systematic review of trials. Ann Fam Med. 2004;2(6):595–608
- , , , , . Characteristics of physicians with participatory decision‐making styles. Ann Intern Med. 1996;124(5):497–504.
- . Information‐giving consultations: the influence of patients' communicative styles and personal characteristics. Soc Sci Med. 1991:32(5):541–548.
- University of Michigan Health System.Patient care and University of Michigan Health System. Available at: http://www.uofmhealth.org/about%2Bumhs/about‐clinical‐care. Accessed August 31, 2012.
- , , , et al. It's the writing on the wall: whiteboards improve inpatient satisfaction with provider communication. Am J Med Qual. 2011;26(2):127–131
- , , , , . Patient whiteboards as a communication tool in the hospital setting: a survey of practices and recommendations. J Hosp Med. 2010;5(4):234–239.
In their seminal report Crossing the Quality Chasm, the Institute of Medicine outlined patient‐centered care as 1 of its 6 aims to improve the healthcare delivery system.[1] Patients who are more involved in their diagnosis and treatment plan are more likely to feel respected, be satisfied with their healthcare experience, and ultimately have better outcomes.[2, 3] In a study of hospitalized patients, only 42% were able to state their diagnosis at the time of discharge, suggesting that hospital providers could communicate better with patients about their hospital care.[4] Additionally, only 28% of hospitalized patients were able to list their medications, and only 37% were able to state the purpose of their medications. Although hospitals have taken great strides to improve the quality of patient care, publicly reported patient care surveys, such as the Hospital Consumer Assessment of Hospitals and Health Systems (HCAHPS), suggest that physician communication with patients could be further improved.[1, 5] Furthermore, a recent report by the Institute of Medicine stresses the need to get patients and families involved in their care.[6] Thus, hospital‐based providers should seek to enhance the quality of their communication with patients.
With greater emphasis placed on patient‐ and family‐centered care at many health systems, simple and easy‐to‐implement strategies to improve communication with patients need to be developed and tested.[7, 8] Patients who actively participate in their healthcare by asking questions of their doctor are able to control the focus of their interaction and adjust the amount of information provided.[9] Simply asking questions can have a critical impact, as 1 study found that the frequency with which patients asked questions was significantly related to the amount of information received about general and specific medical matters.[10] The notepad is a common tool for reminders and personal interactions that is used in everyday life, but has not been formalized in the hospital. We introduced Dear Doctor (DD) notes, a bedside notepad designed to prompt patient questions, with the goal of facilitating patient communication with their hospitalist physicians (Figure 1). As hospitalists provide direct and indirect care to a growing number of hospitalized patients, they are likely to be asked questions and opinions about the patients' diagnoses and plans. Furthermore, hospitalists are poised to lead institutional quality, safety, efficiency, and service improvement efforts in the inpatient setting. Becoming familiar with communication‐enhancing tools, such as the DD notes, may help hospitalists in their improvement team roles.

METHODS
Setting
We conducted a study between July 2009 and September 2009 on inpatient medical wards at a large academic medical center with 610 beds and over 44,000 annual discharges.[11] The internal medicine services served by attending physicians and residents comprise a large proportion of hospitalized patients, accounting for over 17,000 discharges per year. Each medical unit includes 32 beds.
Population
Patients over the age of 18 years admitted to a general medicine or cardiology unit and who were able to verbally communicate in English were eligible to be surveyed in the study. Patients with a length of stay <24 hours were excluded. A total of 664 patients were surveyed for inclusion in the study, 440 patients in the intervention group and 224 patients in the control group.
Intervention
The DD notepad included sample questions and informational prompts derived with input from a community focus group. The community focus group consisted of current and formerly hospitalized patients and family members who were asked by members of the study group what they thought would be important to include on a notepad provided to patients. From their answers the study team developed the DD notepad prototype. The DD notepad included 3 general categories of questions: (1) diagnosis and treatment, (2) tests and procedures, and (3) medications. To address other miscellaneous topics such as discharge and posthospital care needs, a section was designated for the patient to check off as I have a few more questions (Use the back of the sheet).
All patients admitted to the study units were intended to receive the DD notepad and pen, which were placed on the bedside table during the room change by our custodial staff. Patients who did not receive DD notepads in the intervention group during their first hospitalization day were provided with 1 by the clinical assistants working with the hospitalists. These patients did not initially receive the notepad due to logistical reasons from temporary rotating staff who were not instructed to provide the notepads. Patients were not formally prompted to use the notepad. Hospitalists, residents, and nurses on the study units were informed about the distribution of DD notes to patients on these units; however, they were not provided with any specific instructions on how they should incorporate the DD notes into their interactions with their patients. The use of the DD notepad was left to each healthcare professional's own discretion.
Members of the study team surveyed patients who had been in the hospital for a minimum of 24 hours in the intervention and control groups twice weekly. All responses were deidentified of any personal or health information. Patients were asked to rate on a scale from 1 to 5 their use of the DD notepads, their perceived value, the circumstances in which the notepads were used, and their level of satisfaction with how their physicians communicated and answered their questions (1 = no improvement, 5 = significant improvement). For control patients, questions pertaining to DD notepads were not applicable and were therefore excluded.
Statistical Analysis
The data were analyzed in an intention‐to‐treat analysis of all 440 patients in the intervention group. Intervention and control groups were compared using 2, rank sum, and Fisher exact statistical tests, with significance assigned as P < 0.05, using SPSS software version 17.0 (SPSS, Inc., Chicago, IL). Our project was approved by the University of Michigan's institutional review board.
RESULTS
Of the 440 patients surveyed in the intervention group (1 general medicine and 1 cardiology unit), 343 (78%) received the notepads in their rooms and 207 (47%) used them (Figure 2). Not every patient in the intervention group received DD notepads due to inconsistent placement of DD notepads upon every room turnover. Of the patients admitted to the control group (1 general medicine and 1 cardiology unit), 224 were surveyed. Fifty‐four percent of the 440 patients in the intervention group reported that they took notes related to their hospital care, compared to only 22% of the 224 patients in the control group (P < 0.001). Of the patients who took notes within the intervention group (n = 207), 91% of them utilized the DD notepads.

Patients in the intervention group who received and used the DD notes (n = 207) compared to patients in the control group (n = 224) were more likely to report that their questions were answered by their physicians (4.63 vs 4.45, P < 0.001). In an intention‐to‐treat analysis of all 440 patients in the intervention group, the overall satisfaction with physician communication was not significantly different between the intervention and control groups as measured on a 5‐point Likert scale (4.55 vs 4.55, P = 0.89). However, 89% of the patients in the intervention group who used the notepads felt that DD notepads either moderately or significantly improved their communication with their providers (Figure 3).

When the 207 patients who received DD notepads were asked how they used this tool, 99% of these patients used DD to write down questions, 82% to keep track of tests and procedures, and 54% indicated that their family and friends also used the notepads during the hospital stay (Table 1). Among these patients who utilized the DD notepads, 93% reported that they would use them again in the future.
| Wrote Notes? (P < 0.001) (%) | Used DD? (%) | Use in Future? (%) | Frequency of Questions Answered (P < 0.001) | DD Improved Communication | Satisfied With Communication? (P = 0.89) | |
|---|---|---|---|---|---|---|
| ||||||
| Intervention (n = 440) | 54 | 91 | 93.2 | 4.63 | 3.76 | 4.55 |
| Control (n = 224) | 22 | 4.45 | 4.55 | |||
Of the 97 patients in the intervention group who did not receive a DD notepad, we asked if they would use the DD notepad if they were made aware of such a tool. Of these patients, 77% agreed that they would use DD notes if they were made available in the future, 100% of them said that they would use DD to write down questions, 97% indicated that they would write notes about tests and procedures, and 88% of them believed that their families and friends would use DD notes.
DISCUSSION
As hospitals place greater emphasis and value on patient‐centered care as part of their clinical mission, it is important to develop tools to help facilitate the doctor‐patient relationship. We found that patients who were provided the Dear Doctor notepad were more satisfied that their doctors answered their questions and felt this tool enhanced their ability to communicate with their physicians. Employing the use of a familiar tool such as the notepad to remind patients about specific issues in their interactions with their providers can be a powerful intervention. Our study demonstrated that the DD notepad was widely accepted by patients, and that almost all of them would use this tool if it were made available to them in the future.
Other tools and methods to enhance the quality of communication between patients and their healthcare providers have included using whiteboards in the patients' rooms to relay the care plan to the patients, implementing bedside rounds by the healthcare team, and multidisciplinary huddling to coordinate information to the patient.[12, 13] Studies of these communication tools have shown potential to improve teamwork, interaction, and patient care. All of these have their own merit and value, and our DD notepad should be considered an adjunct to existing methods to enhance the patient care experience. A bedside tool that is familiar in form to most patients also needs to have the feature of easy access and use. Once this barrier has been removed for the patient and their family members, tools such as the DD notepad can impact the patient‐centeredness by fostering increased and better quality dialogue between the patients, their family members, and healthcare providers.
The DD notepad represents a means of communication that may have the potential to empower patients. It is possible that through question prompts, the DD notepad stimulates the patient to be an active partner with his or her healthcare team. This may enable patients to have some sense of control and accountability of their care in a setting where they would otherwise feel overwhelmed or powerless. The 3 general categories to help patients write down their questions included diagnosis, treatment plan, and medications. In the inpatient setting, where patient‐care activities can be fast paced, and patients are unable to recall some details when speaking to the healthcare team, these notes may remind the patient to write their thoughts down so that they may be remembered for a future time. In situations where patients may not know which questions to ask, the question prompts may be particularly helpful. We did not assess whether our particular question prompts were the key elements that resulted in their perceived value, or whether simply placing a blank notepad at the bedside would also have been successful. However, the specific questions were suggested by the focus groups. Enhanced communication, focusing on the patients' understanding of their condition, and the need to pursue certain diagnostic or therapeutic interventions, may help patients to be better prepared for the next course of plan. These topics of reasons for hospitalization, treatment plan, and medication changes are also important for patients to be active participants in their care, in particular as they transition from 1 site of care to the next, and their healthcare will be delivered by different providers.
There are several limitations to our study. First, this intervention was performed at a single hospital site with only 2 clinical services (general medicine, cardiology) represented in the study groups. Although we do not have any causal reasons to believe this tool would be looked upon differently by patients on other clinical services, it is possible that patients on a different clinical unit or service may view this tool as less or more useful. Second, as the patients were not randomized to intervention, but rather based on the units to which they were admitted, it is possible that other variables, such as the experience of the unit staff, the patient's condition, and housestaff‐based service versus hospitalist‐based service may have played a role in how the patients perceived the use of DD notes. Third, patients were only surveyed if they were able to verbally communicate in English. These notes may not be as useful in hospital settings to populations with language or literacy barriers. Fourth, the logistical implementation of DD notes limited our ability to deliver the DD notepads in every patient's rooms, where only 78% of the intervention group received the DD notepads. This may be the reason that we did not find that overall satisfaction with physician communication differed between intervention and control groups. Nonetheless, we performed an intention‐to‐treat analysis to minimize any biases in our analysis. Last, although our survey of patients asked about their satisfaction in using the DD note pads, we did not compare these results with those of Press‐Ganey or HCAHPS scores of patients on the intervention group versus the control group. Additionally, lack of data about type, quality, and quantity of questions asked by a control group to see if the notepads actually improved quality of questions asked is a limitation; however, we believe our outcomes of interest were most specifically evaluated through our survey questions.
DD notes show that the majority of patients who use this tool feel a modest to significant improvement in communication with their providers. Although the quality of medical care is undoubtedly the first priority, the patients' view of their care, which includes communication, is arguably just as important. An often‐forgotten goal of hospitals and clinics is to provide service excellence along with high‐quality care. Thus, it is imperative for hospitals and their care providers to not only focus on the quality and safety of the clinical care, but also be mindful of the patient's entire experience throughout their hospital stay. Many of the categories of questions asked in the HCAHPS address the patient's experience and perspectives of hospital care. Furthermore, the role of the HCAHPS survey in the Value‐Based Purchasing rules may enhance the importance of these notepads. As the results of HCAHPS are becoming more transparent and available to the public, the impact of such results will have a greater significance to the future of the hospital's clinical mission.
CONCLUSION
DD notepads are a simple, low‐cost, patient‐centered tool that can be an effective reminder for patients to ask their healthcare providers questions related to their hospital care. Utilizing a common tool such as the notepad, redesigned for the healthcare setting, can serve to help healthcare providers interact with their patients. Patient satisfaction may be higher in patients who use the DD notepad.
Disclosures: Aaron S. Farberg, MD, and Andrew M. Lin, MD, contributed equally in every way and should be considered co‐first authors. This work was supported by a University of Michigan Fostering Innovations Grant. The authors have no conflicting financial interests.
In their seminal report Crossing the Quality Chasm, the Institute of Medicine outlined patient‐centered care as 1 of its 6 aims to improve the healthcare delivery system.[1] Patients who are more involved in their diagnosis and treatment plan are more likely to feel respected, be satisfied with their healthcare experience, and ultimately have better outcomes.[2, 3] In a study of hospitalized patients, only 42% were able to state their diagnosis at the time of discharge, suggesting that hospital providers could communicate better with patients about their hospital care.[4] Additionally, only 28% of hospitalized patients were able to list their medications, and only 37% were able to state the purpose of their medications. Although hospitals have taken great strides to improve the quality of patient care, publicly reported patient care surveys, such as the Hospital Consumer Assessment of Hospitals and Health Systems (HCAHPS), suggest that physician communication with patients could be further improved.[1, 5] Furthermore, a recent report by the Institute of Medicine stresses the need to get patients and families involved in their care.[6] Thus, hospital‐based providers should seek to enhance the quality of their communication with patients.
With greater emphasis placed on patient‐ and family‐centered care at many health systems, simple and easy‐to‐implement strategies to improve communication with patients need to be developed and tested.[7, 8] Patients who actively participate in their healthcare by asking questions of their doctor are able to control the focus of their interaction and adjust the amount of information provided.[9] Simply asking questions can have a critical impact, as 1 study found that the frequency with which patients asked questions was significantly related to the amount of information received about general and specific medical matters.[10] The notepad is a common tool for reminders and personal interactions that is used in everyday life, but has not been formalized in the hospital. We introduced Dear Doctor (DD) notes, a bedside notepad designed to prompt patient questions, with the goal of facilitating patient communication with their hospitalist physicians (Figure 1). As hospitalists provide direct and indirect care to a growing number of hospitalized patients, they are likely to be asked questions and opinions about the patients' diagnoses and plans. Furthermore, hospitalists are poised to lead institutional quality, safety, efficiency, and service improvement efforts in the inpatient setting. Becoming familiar with communication‐enhancing tools, such as the DD notes, may help hospitalists in their improvement team roles.

METHODS
Setting
We conducted a study between July 2009 and September 2009 on inpatient medical wards at a large academic medical center with 610 beds and over 44,000 annual discharges.[11] The internal medicine services served by attending physicians and residents comprise a large proportion of hospitalized patients, accounting for over 17,000 discharges per year. Each medical unit includes 32 beds.
Population
Patients over the age of 18 years admitted to a general medicine or cardiology unit and who were able to verbally communicate in English were eligible to be surveyed in the study. Patients with a length of stay <24 hours were excluded. A total of 664 patients were surveyed for inclusion in the study, 440 patients in the intervention group and 224 patients in the control group.
Intervention
The DD notepad included sample questions and informational prompts derived with input from a community focus group. The community focus group consisted of current and formerly hospitalized patients and family members who were asked by members of the study group what they thought would be important to include on a notepad provided to patients. From their answers the study team developed the DD notepad prototype. The DD notepad included 3 general categories of questions: (1) diagnosis and treatment, (2) tests and procedures, and (3) medications. To address other miscellaneous topics such as discharge and posthospital care needs, a section was designated for the patient to check off as I have a few more questions (Use the back of the sheet).
All patients admitted to the study units were intended to receive the DD notepad and pen, which were placed on the bedside table during the room change by our custodial staff. Patients who did not receive DD notepads in the intervention group during their first hospitalization day were provided with 1 by the clinical assistants working with the hospitalists. These patients did not initially receive the notepad due to logistical reasons from temporary rotating staff who were not instructed to provide the notepads. Patients were not formally prompted to use the notepad. Hospitalists, residents, and nurses on the study units were informed about the distribution of DD notes to patients on these units; however, they were not provided with any specific instructions on how they should incorporate the DD notes into their interactions with their patients. The use of the DD notepad was left to each healthcare professional's own discretion.
Members of the study team surveyed patients who had been in the hospital for a minimum of 24 hours in the intervention and control groups twice weekly. All responses were deidentified of any personal or health information. Patients were asked to rate on a scale from 1 to 5 their use of the DD notepads, their perceived value, the circumstances in which the notepads were used, and their level of satisfaction with how their physicians communicated and answered their questions (1 = no improvement, 5 = significant improvement). For control patients, questions pertaining to DD notepads were not applicable and were therefore excluded.
Statistical Analysis
The data were analyzed in an intention‐to‐treat analysis of all 440 patients in the intervention group. Intervention and control groups were compared using 2, rank sum, and Fisher exact statistical tests, with significance assigned as P < 0.05, using SPSS software version 17.0 (SPSS, Inc., Chicago, IL). Our project was approved by the University of Michigan's institutional review board.
RESULTS
Of the 440 patients surveyed in the intervention group (1 general medicine and 1 cardiology unit), 343 (78%) received the notepads in their rooms and 207 (47%) used them (Figure 2). Not every patient in the intervention group received DD notepads due to inconsistent placement of DD notepads upon every room turnover. Of the patients admitted to the control group (1 general medicine and 1 cardiology unit), 224 were surveyed. Fifty‐four percent of the 440 patients in the intervention group reported that they took notes related to their hospital care, compared to only 22% of the 224 patients in the control group (P < 0.001). Of the patients who took notes within the intervention group (n = 207), 91% of them utilized the DD notepads.

Patients in the intervention group who received and used the DD notes (n = 207) compared to patients in the control group (n = 224) were more likely to report that their questions were answered by their physicians (4.63 vs 4.45, P < 0.001). In an intention‐to‐treat analysis of all 440 patients in the intervention group, the overall satisfaction with physician communication was not significantly different between the intervention and control groups as measured on a 5‐point Likert scale (4.55 vs 4.55, P = 0.89). However, 89% of the patients in the intervention group who used the notepads felt that DD notepads either moderately or significantly improved their communication with their providers (Figure 3).

When the 207 patients who received DD notepads were asked how they used this tool, 99% of these patients used DD to write down questions, 82% to keep track of tests and procedures, and 54% indicated that their family and friends also used the notepads during the hospital stay (Table 1). Among these patients who utilized the DD notepads, 93% reported that they would use them again in the future.
| Wrote Notes? (P < 0.001) (%) | Used DD? (%) | Use in Future? (%) | Frequency of Questions Answered (P < 0.001) | DD Improved Communication | Satisfied With Communication? (P = 0.89) | |
|---|---|---|---|---|---|---|
| ||||||
| Intervention (n = 440) | 54 | 91 | 93.2 | 4.63 | 3.76 | 4.55 |
| Control (n = 224) | 22 | 4.45 | 4.55 | |||
Of the 97 patients in the intervention group who did not receive a DD notepad, we asked if they would use the DD notepad if they were made aware of such a tool. Of these patients, 77% agreed that they would use DD notes if they were made available in the future, 100% of them said that they would use DD to write down questions, 97% indicated that they would write notes about tests and procedures, and 88% of them believed that their families and friends would use DD notes.
DISCUSSION
As hospitals place greater emphasis and value on patient‐centered care as part of their clinical mission, it is important to develop tools to help facilitate the doctor‐patient relationship. We found that patients who were provided the Dear Doctor notepad were more satisfied that their doctors answered their questions and felt this tool enhanced their ability to communicate with their physicians. Employing the use of a familiar tool such as the notepad to remind patients about specific issues in their interactions with their providers can be a powerful intervention. Our study demonstrated that the DD notepad was widely accepted by patients, and that almost all of them would use this tool if it were made available to them in the future.
Other tools and methods to enhance the quality of communication between patients and their healthcare providers have included using whiteboards in the patients' rooms to relay the care plan to the patients, implementing bedside rounds by the healthcare team, and multidisciplinary huddling to coordinate information to the patient.[12, 13] Studies of these communication tools have shown potential to improve teamwork, interaction, and patient care. All of these have their own merit and value, and our DD notepad should be considered an adjunct to existing methods to enhance the patient care experience. A bedside tool that is familiar in form to most patients also needs to have the feature of easy access and use. Once this barrier has been removed for the patient and their family members, tools such as the DD notepad can impact the patient‐centeredness by fostering increased and better quality dialogue between the patients, their family members, and healthcare providers.
The DD notepad represents a means of communication that may have the potential to empower patients. It is possible that through question prompts, the DD notepad stimulates the patient to be an active partner with his or her healthcare team. This may enable patients to have some sense of control and accountability of their care in a setting where they would otherwise feel overwhelmed or powerless. The 3 general categories to help patients write down their questions included diagnosis, treatment plan, and medications. In the inpatient setting, where patient‐care activities can be fast paced, and patients are unable to recall some details when speaking to the healthcare team, these notes may remind the patient to write their thoughts down so that they may be remembered for a future time. In situations where patients may not know which questions to ask, the question prompts may be particularly helpful. We did not assess whether our particular question prompts were the key elements that resulted in their perceived value, or whether simply placing a blank notepad at the bedside would also have been successful. However, the specific questions were suggested by the focus groups. Enhanced communication, focusing on the patients' understanding of their condition, and the need to pursue certain diagnostic or therapeutic interventions, may help patients to be better prepared for the next course of plan. These topics of reasons for hospitalization, treatment plan, and medication changes are also important for patients to be active participants in their care, in particular as they transition from 1 site of care to the next, and their healthcare will be delivered by different providers.
There are several limitations to our study. First, this intervention was performed at a single hospital site with only 2 clinical services (general medicine, cardiology) represented in the study groups. Although we do not have any causal reasons to believe this tool would be looked upon differently by patients on other clinical services, it is possible that patients on a different clinical unit or service may view this tool as less or more useful. Second, as the patients were not randomized to intervention, but rather based on the units to which they were admitted, it is possible that other variables, such as the experience of the unit staff, the patient's condition, and housestaff‐based service versus hospitalist‐based service may have played a role in how the patients perceived the use of DD notes. Third, patients were only surveyed if they were able to verbally communicate in English. These notes may not be as useful in hospital settings to populations with language or literacy barriers. Fourth, the logistical implementation of DD notes limited our ability to deliver the DD notepads in every patient's rooms, where only 78% of the intervention group received the DD notepads. This may be the reason that we did not find that overall satisfaction with physician communication differed between intervention and control groups. Nonetheless, we performed an intention‐to‐treat analysis to minimize any biases in our analysis. Last, although our survey of patients asked about their satisfaction in using the DD note pads, we did not compare these results with those of Press‐Ganey or HCAHPS scores of patients on the intervention group versus the control group. Additionally, lack of data about type, quality, and quantity of questions asked by a control group to see if the notepads actually improved quality of questions asked is a limitation; however, we believe our outcomes of interest were most specifically evaluated through our survey questions.
DD notes show that the majority of patients who use this tool feel a modest to significant improvement in communication with their providers. Although the quality of medical care is undoubtedly the first priority, the patients' view of their care, which includes communication, is arguably just as important. An often‐forgotten goal of hospitals and clinics is to provide service excellence along with high‐quality care. Thus, it is imperative for hospitals and their care providers to not only focus on the quality and safety of the clinical care, but also be mindful of the patient's entire experience throughout their hospital stay. Many of the categories of questions asked in the HCAHPS address the patient's experience and perspectives of hospital care. Furthermore, the role of the HCAHPS survey in the Value‐Based Purchasing rules may enhance the importance of these notepads. As the results of HCAHPS are becoming more transparent and available to the public, the impact of such results will have a greater significance to the future of the hospital's clinical mission.
CONCLUSION
DD notepads are a simple, low‐cost, patient‐centered tool that can be an effective reminder for patients to ask their healthcare providers questions related to their hospital care. Utilizing a common tool such as the notepad, redesigned for the healthcare setting, can serve to help healthcare providers interact with their patients. Patient satisfaction may be higher in patients who use the DD notepad.
Disclosures: Aaron S. Farberg, MD, and Andrew M. Lin, MD, contributed equally in every way and should be considered co‐first authors. This work was supported by a University of Michigan Fostering Innovations Grant. The authors have no conflicting financial interests.
- Committee on Quality of Health Care in America, Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
- , , , . An evidence base for patient‐centered cancer care: a meta‐analysis of studies of observed communication between cancer specialists and their patients. Patient Educ Couns. 2009;77(3):379–383.
- . Effective physician‐patient communication and health outcomes: a review. Can Med Assoc J. 1995;152:1423–1433.
- , . Patients' understanding of their treatment plans and diagnosis at discharge. Mayo Clin Proc. 2005;80(8):991–994.
- , , , . Patients' perception of hospital care in the United States. N Engl J Med. 2008;359:1921–1931.
- Institute of Medicine of the National Academies.Best care at lower cost: the path to continuously learning health care in America. Available at: http://www.iom.edu/Reports/2012/Best‐Care‐at‐Lower‐Cost‐The‐Path‐to‐Continuously‐Learning‐Health‐Care‐in‐America.aspx. Accessed October 5, 2012.
- , . An introduction to technology for patient‐centered collaborative care. J Ambul Care Manage. 2006;29:195–198.
- , , , , , . Effect on health‐related outcome of interventions to alter the interaction between patients and practitioners: a systematic review of trials. Ann Fam Med. 2004;2(6):595–608
- , , , , . Characteristics of physicians with participatory decision‐making styles. Ann Intern Med. 1996;124(5):497–504.
- . Information‐giving consultations: the influence of patients' communicative styles and personal characteristics. Soc Sci Med. 1991:32(5):541–548.
- University of Michigan Health System.Patient care and University of Michigan Health System. Available at: http://www.uofmhealth.org/about%2Bumhs/about‐clinical‐care. Accessed August 31, 2012.
- , , , et al. It's the writing on the wall: whiteboards improve inpatient satisfaction with provider communication. Am J Med Qual. 2011;26(2):127–131
- , , , , . Patient whiteboards as a communication tool in the hospital setting: a survey of practices and recommendations. J Hosp Med. 2010;5(4):234–239.
- Committee on Quality of Health Care in America, Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
- , , , . An evidence base for patient‐centered cancer care: a meta‐analysis of studies of observed communication between cancer specialists and their patients. Patient Educ Couns. 2009;77(3):379–383.
- . Effective physician‐patient communication and health outcomes: a review. Can Med Assoc J. 1995;152:1423–1433.
- , . Patients' understanding of their treatment plans and diagnosis at discharge. Mayo Clin Proc. 2005;80(8):991–994.
- , , , . Patients' perception of hospital care in the United States. N Engl J Med. 2008;359:1921–1931.
- Institute of Medicine of the National Academies.Best care at lower cost: the path to continuously learning health care in America. Available at: http://www.iom.edu/Reports/2012/Best‐Care‐at‐Lower‐Cost‐The‐Path‐to‐Continuously‐Learning‐Health‐Care‐in‐America.aspx. Accessed October 5, 2012.
- , . An introduction to technology for patient‐centered collaborative care. J Ambul Care Manage. 2006;29:195–198.
- , , , , , . Effect on health‐related outcome of interventions to alter the interaction between patients and practitioners: a systematic review of trials. Ann Fam Med. 2004;2(6):595–608
- , , , , . Characteristics of physicians with participatory decision‐making styles. Ann Intern Med. 1996;124(5):497–504.
- . Information‐giving consultations: the influence of patients' communicative styles and personal characteristics. Soc Sci Med. 1991:32(5):541–548.
- University of Michigan Health System.Patient care and University of Michigan Health System. Available at: http://www.uofmhealth.org/about%2Bumhs/about‐clinical‐care. Accessed August 31, 2012.
- , , , et al. It's the writing on the wall: whiteboards improve inpatient satisfaction with provider communication. Am J Med Qual. 2011;26(2):127–131
- , , , , . Patient whiteboards as a communication tool in the hospital setting: a survey of practices and recommendations. J Hosp Med. 2010;5(4):234–239.
© 2013 Society of Hospital Medicine
Hospitalist Experiences Regarding PICCs
Peripherally inserted central catheters (PICCs) have become among the most common central venous catheters (CVCs) used in contemporary medical practice.[1] Although they were originally developed for delivery of parenteral nutrition, the use of PICCs has expanded to include chemotherapy administration, long‐term intravenous (IV) antibiotic treatment, and venous access when obtaining peripheral veins is difficult (eg, occluded peripheral veins, unusual venous anatomies).[2] Despite these roles, little is known about PICC use in hospitalized patients. This knowledge gap is important, as PICCs are placed in inpatient settings for a variety of reasons. Some of these reasons may not be appropriate, and inappropriate PICC use may worsen outcomes and increase healthcare costs.[3] In addition, PICCs are not innocuous and are frequently associated with important complications including thrombophlebitis, central‐lineassociated bloodstream infection and venous thromboembolism.[4, 5, 6] Therefore, understanding patterns and knowledge associated with PICC use is also an important patient safety concern.
As the main providers of inpatient care, hospitalists frequently order the insertion of PICCs and treat PICC‐related complications. Unfortunately, to date, no study has surveyed hospitalists regarding management or use of PICCs. Understanding hospitalist experiences, practice, opinions, and knowledge related to PICCs is therefore of significant interest when examining present‐day PICC use. To bridge this important knowledge gap and better understand these practices, we conducted a Web‐based survey of hospitalists in 5 healthcare systems in the state of Michigan.
METHODS
A convenience sample of hospitalists (N=227) was assembled from 5 large healthcare systems (representing 10 hospitals) that participate in the Hospital Medicine Safety (HMS) Consortium, a Blue Cross/Blue Shield of Michiganfunded statewide collaborative quality initiative. Individuals engaged in research, quality improvement, or leadership at HMS sites were invited to serve as site principal investigators (site PIs). Site PIs were responsible for obtaining regulatory approval at their parent facilities and disseminating the survey to providers in their group. Participation in the survey was solicited via e‐mail invitations from site PIs to hospitalists within their provider group. To encourage participation, a $10 electronic gift card was offered to respondents who successfully completed the survey. Reminder e‐mails were also sent each week by site PIs to augment participation. To enhance study recruitment, all responses were collected anonymously. The survey was administered between August 2012 and September 2012; data collection occurred for 5 weeks during this interval.
Survey questions were derived from our published, evidence‐based conceptual framework of PICC‐related complications. Briefly, this model identifies complications related to PICCs as arising from domains related to patient‐, provider‐, and device‐related characteristics based on existing evidence.[2] For our survey, questions were sourced from each of these domains so as to improve understanding of hospitalist experience, practice, opinions, and knowledge regarding PICC use. To ensure clarity of the survey questions, all questions were first pilot‐tested with a group of randomly selected hospitalist respondents at the University of Michigan Health System. Direct feedback obtained from these respondents was then used to iteratively improve each question. In order to generate holistic responses, questions were designed to generate a response reflective of the participants typical PICC use/subenario. We used SurveyMonkey to collect and manage survey data.
Statistical Analyses
Variation in hospitalist experience, reported practice, opinions, and knowledge regarding PICCs was assessed by hospitalist type (full time vs part time), years of practice (<1, 15, >5), and care‐delivery model (direct care vs learner‐based care). Bivariate comparisons were made using the 2 or Fisher exact tests as appropriate; 2‐sided with a P value <0.05 was considered statistically significant. All analyses were conducted using Stata version 11 (StataCorp, College Station, TX). Local institutional review board approval was obtained at each site participating in the survey.
RESULTS
A total of 227 surveys were administered and 144 responses collected, for a survey response rate of 63%. Each participating site had unique characteristics including size, number of hospitalists, and modality of PICC insertion (Table 1). Of the hospitalists who completed the survey, 81% held full‐time clinical positions and had been in practice an average of 5.6 years. Surveyed hospitalists reported caring for an average of 40.6 patients per week and ordering a mean of 2.9 (range, 015) PICCs per week of clinical service. Among survey respondents, 36% provided direct patient care, 34% provided care either directly or through mid‐level providers and housestaff, and 9% delivered care exclusively through mid‐level providers or housestaff (Table 2). As our survey was conducted anonymously, potential identifying information such as age, race, and sex of those responding was not collected.
| Survey Site | No. of Hospitals | No. of Inpatient Beds | No. of Annual Inpatient Encounters | No. of Hospitalists | Full‐Time Hospitalists, % | Avg. No. Weeks/Year on Service | Avg. Years of Experience | No. PICCs/Week, 2012 | Modality of PICC Insertion Available |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| University of Michigan Health System | 1 | 900+ | 5,775 | 46 | 100 | 25 | 6 | 42 | Vascular access nurse |
| Ann Arbor VA Medical Center | 1 | 135 | 825 | 16 | 50 | 17.6 | 5.1 | 12 | Vascular access nurse |
| Spectrum Health System | 2 | 800 | 14,000 | 47 | 80 | 34 | 3.75 | 56 | Interventional radiology |
| Trinity Health System | 3 | 634 | 2,300 | 67 | 80 | 24 | 4 | 31 | Interventional radiology and hospitalists |
| Henry Ford Health System | 3 | 1,150 | 1,450 | 51 | 100 | 20.4 | 5.6 | 15 | Vascular access nurse |
| Characteristic | Total (N=144) |
|---|---|
| |
| Hospitalist type, n (%) | |
| Full time | 117 (81) |
| Part time | 19 (13) |
| Unknown | 8 (6) |
| Weeks/year on a clinical service, n (%) | |
| <20 | 24 (17) |
| 20 | 107 (74) |
| Unknown | 13 (9) |
| Mean (SD) | 25.5 (10.7) |
| Median | 26 |
| Type of patients treated, n (%) | |
| Adults only | 129 (90) |
| Adults and children | 7 (5) |
| Unknown | 8 (6) |
| Years in practice as a hospitalist, n (%) | |
| 5 | 81 (56) |
| >5 | 54 (38) |
| Unknown | 9 (6) |
| Model of care delivery, n (%) | |
| Direct | 52 (36) |
| Some midlevel or housestaff providers (<50% of all encounters) | 49 (34) |
| Mostly midlevel or housestaff providers (>50% of all encounters) | 22 (15) |
| Only midlevel or housestaff providers | 13 (9) |
| Unknown | 8 (6) |
| Location of practice | |
| Trinity Health System | 39 (27) |
| University of Michigan Health System | 37 (26) |
| Henry Ford Health System | 28 (19) |
| Spectrum Health System | 21 (15) |
| Ann Arbor VA Medical Center | 11 (8) |
| Unknown | 8 (6) |
Hospitalist Experiences and Practice Related to Peripherally Inserted Central Catheters
According to responding hospitalists, the most common indications for PICC placement were long‐term IV antibiotic treatment (64%), followed by inability to obtain peripheral venous access (24%). Hospitalists reported an average duration of PICC placement of 17 days (range, 342 days). A significant percentage of hospitalists (93%) stated that they had cared for patients where a PICC was placed only for use during hospitalization, with the most common reason for such insertion being difficulty in otherwise securing venous access (67%). Respondents also reported caring for patients who had both PICCs and peripheral IV catheters in place at the same time; 49% stated that they had experienced this <5 times, whereas 33% stated they had experienced this 510 times. Furthermore, 87% of respondents indicated having admitted a patient who specifically requested a PICC due to prior difficulties with venous access. More than half of surveyed hospitalists (63%) admitted to having been contacted by a PICC nurse enquiring as to whether their patient might benefit from PICC insertion.
The majority of hospitalists (66%) reported that they specified the number of lumens when ordering PICCs. Thirty‐eight percent indicated that this decision was based on type of medication, whereas 35% selected the lowest number of lumens possible. A power PICC (specialized PICCs that are designed to withstand high‐pressure contrast injections), was specifically requested for radiographic studies (56%), infusion of large volume of fluids (10%), or was the default PICC type at their facility (34%).
A majority (74%) of survey respondents also reported that once inserted, PICCs were always used to obtain blood for routine laboratory testing. Moreover, 41% indicated that PICCs were also always used to obtain blood for microbiological cultures. The 3 most frequently encountered PICC‐related complications reported by hospitalists in our survey were blockage of a PICC lumen, bloodstream infection, and venous thromboembolism (VTE; Table 3).
| Hospitalist Experiences With PICCs | Total (N=144) |
|---|---|
| |
| Primary indication for PICC placement* | |
| Long‐term IV antibiotics | 64 |
| Venous access in a patient with poor peripheral veins | 24 |
| Parenteral nutrition | 5 |
| Chemotherapy | 4 |
| Patient specifically requested a PICC | 1 |
| Unknown/other | 2 |
| PICC placed only for venous access, n (%) | |
| Yes | 135 (94) |
| No | 9 (6) |
| PICC placed only during hospitalization, n (%) | |
| Yes | 134 (93) |
| No | 10 (7) |
| Notified by a PICC nurse (or other provider) that patient may need or benefit from a PICC, n (%) | |
| Yes | 91 (63) |
| No | 53 (37) |
| How frequently PICCs are used to obtain blood for routine laboratory testing, n (%) | |
| Always | 106 (74) |
| Unknown/other | 38 (26) |
| How frequently PICCs are used to obtain blood for blood cultures, n (%) | |
| Always | 59 (41) |
| Unknown/other | 85 (59) |
| Hospitalist Opinions on PICCs | Total (N=144) |
| In your opinion, is it appropriate to place a vascular in a hospitalized patient if other forms of peripheral access cannot be obtained? n (%) | |
| Yes | 121 (84) |
| No | 21 (15) |
| Unknown | 2 (1) |
| In your opinion, should hospitalists be trained to insert PICCs? n (%) | |
| No | 57 (40) |
| Yes, this is an important skill set for hospitalists | 46 (32) |
| Unsure | 39 (27) |
| Unknown/other | 2 (1) |
| Do you think the increasing number of vascular nurses and PICC nursing teams has influenced the use of PICCs in hospitalized patients? n (%) | |
| Yes | 112 (78) |
| No | 30 (21) |
| Unknown | 2 (1) |
| What % of PICC insertions do you think may represent inappropriate use in your hospital? n (%) | |
| <10 | 53 (37) |
| 1025 | 68 (47) |
| 2550 | 18 (13) |
| >50 | 3 (2) |
| Unknown/other | 2 (1) |
Hospitalist Opinions Regarding Peripherally Inserted Central Catheters
Compared with CVCs, 69% of hospitalists felt that PICCs were safer and more efficient because they could stay in place longer and were less likely to cause infection. Most (65%) also agreed that PICCs were more convenient than CVCs because they were inserted by PICC teams. Additionally, 74% of hospitalists felt that their patients preferred PICCs because they minimize pain from routine peripheral IV changes and phlebotomy. A majority of respondents (84%) indicated that it was appropriate to place a PICC if other forms of peripheral venous access could not be obtained. However, when specifically questioned, 47% of hospitalists indicated that at least 10%25% of PICCs placed in their hospitals might represent inappropriate use. A majority (78%) agreed with the statement that the increase in numbers of vascular nurses had influenced use of PICCs in hospitalized patients, but most (45%) were neutral when asked if PICCs were more cost‐effective than traditional CVCs.
Hospitalist Knowledge Regarding Risk of Peripherally Inserted Central CatheterRelated Venous Thromboembolism and Bloodstream Infection
Although 65% of responding hospitalists disagreed with the statement that PICCs were less likely to lead to VTE, important knowledge gaps regarding PICCs and VTE were identified (Table 4). For instance, only 4% of hospitalists were correctly aware that the PICC‐tip position is checked to reduce risk of PICC‐related VTE, and only 12% knew that the site of PICC insertion has also been associated with VTE risk. Although 85% of respondents stated they would prescribe a therapeutic dose of an anticoagulant in the case of PICC‐associated VTE, deviations from the guideline‐recommended 3‐month treatment period were noted. For example, 6% of hospitalists reported treating with anticoagulation for 6 months, and 19% stated they would treat as long as the PICC remained in place, plus an additional period of time (eg, 24 weeks) after removal. With respect to bloodstream infection, 92% of responding hospitalists correctly identified PICC duration and prompt removal as factors promoting PICC‐related bloodstream infection and 78% accurately identified components of the catheter‐associated bloodstream infection bundle. When specifically asked about factors associated with risk of PICC‐related bloodstream infection, only half of respondents recognized the number of PICC lumens as being associated with this outcome.
| Total (N=144) | |
|---|---|
| |
| Why is the position of the PICC tip checked after bedside PICC insertion? n (%) | |
| To decrease the risk of arrhythmia related to right‐atrial positioning | 108 (75) |
| To minimize the risk of VTEa | 6 (4) |
| To ensure it is not accidentally placed into an artery | 16 (11) |
| For documentation purposes (to reduce the risk of lawsuits related to line‐insertion complications) | 6 (4) |
| Unsure/Unknown | 8 (6) |
| According to the 2012 ACCP Guidelines on VTE prevention, is pharmacologic prophylaxis for DVT recommended in patients who receive long‐term PICCs? n (%) | |
| No; no anticoagulant prophylaxis is recommended for patients who receive long‐term PICCsa | 107 (74) |
| Yes, but the choice and duration of anticoagulant is at the discretion of the provider | 23 (16) |
| Yes; aspirin is recommended for 3 months | 4 (3) |
| Yes; anticoagulation with warfarin or enoxaparin is recommended for 3 months | 3 (2) |
| Yes; anticoagulation with warfarin or enoxaparin is recommended for 6 months | 2 (1) |
| Unknown | 5 (4) |
| Assuming no contraindications exist, do you anticoagulate patients who develop a PICC‐associated DVT (with any therapeutic anticoagulant)? n (%) | |
| Yesa | 122 (85) |
| No | 16 (11) |
| Unknown | 6 (4) |
| How long do you usually prescribe anticoagulation for patients who develop PICC‐associated DVT? n (%) | |
| I don't prescribe anticoagulation | 12 (8) |
| 1 month | 4 (3) |
| 3 monthsa | 84 (58) |
| 6 months | 8 (6) |
| As long as the line remains in place; I stop anticoagulation once the PICC comes out | 3 (2) |
| As long as the line remains in place and for an additional specified period of time after line removal, such as 2 or 4 weeks | 27 (19) |
| Unknown | 6 (4) |
| As part of the treatment of PICC‐related DVT, do you routinely remove the PICC?b n (%) | |
| Yes | 102 (71) |
| No | 36 (25) |
| Unknown | 6 (4) |
Variation in Hospitalist Knowledge, Experience, or Opinions
We assessed whether any of our findings varied according to hospitalist type (full time versus part time), years of practice (<1, 15, >5), and model of care delivery (direct care vs learner‐based care). Our analyses suggested that part‐time hospitalists were more likely to select rarely when it came to finding patients with a PICC and a working peripheral IV at the same time (74% vs 45%, P=0.02). Interestingly, a higher percentage of those in practice <5 years indicated that 10%25% of PICCs represented inappropriate placement (58% vs 33%, P<0.01) and that vascular nurses had influenced the use of PICCs in hospitalized patients (88% vs 69%, P=0.01). Lastly, a higher percentage of hospitalists who provided direct patient care reported that PICCs were always used to obtain blood for microbiological culture (54% vs 37%, P=0.05).
DISCUSSION
In this survey of hospitalists practicing at 5 large healthcare systems in Michigan, we observed significant variation in experience, reported practice, opinions, and knowledge related to PICCs. Our findings highlight important concerns related to inpatient PICC use and suggest a need for greater scrutiny related to these devices in these settings.
The use of PICCs in hospitalized patients has risen dramatically over the past decade. Though such growth is multifactorial and relates in part to increasing inpatient volume and complexity, hospitalists have increasingly turned to PICCs as a convenient and reliable tool to obtain venous access.[7] Indeed, in our survey, PICCs that were only used during hospitalization were most likely to be placed for this very reason. Because PICCs are safer to insert than CVCs and the original evidence regarding PICC‐related VTE or bloodstream infection suggested low rates of these events,[8, 9, 10, 11, 12, 13, 14] many hospitalists may not perceive these devices as being associated with significant risks. In fact, some have suggested that hospitalists be specifically trained to insert these devices, given their safety compared with traditional CVCs.[7]
However, accumulating evidence suggests that PICCs are associated with important complications.[5, 15, 16] In studies examining risk of bloodstream infection, PICCs were associated with significant risk of this outcome.[6, 17, 18] Recently, the presence of a PICC was identified as an independent predictor of VTE in hospitalized patients.[19] Several studies and systematic reviews have repeatedly demonstrated these findings.[19, 20, 21, 22] A recent systematic review examining nonpharmacologic methods to prevent catheter‐related thrombosis specifically called for avoidance of PICC insertion to prevent thrombosis in hospitalized patients.[23] Despite this growing evidence base, the use of PICCs in the inpatient setting is likely to rise, and our survey highlights several practices that may contribute to adverse outcomes. For instance, hospitalists in our survey were unlikely to remove a PICC until a patient was discharged, irrespective of the need for this device. As each day with a PICC increases the risk of complications, such practice poses potential patient safety concerns. Similarly, many hospitalists believe that PICCs are safer than CVCs, a viewpoint that does not stand up to increasing scrutiny and highlights important knowledge gaps. The risk of PICC‐related complications appears not to be a stationary target, but rather a dynamic balance that is influenced by patient‐, provider‐, and device‐specific characteristics.[2] Increasing discretionary use (especially for patients with poor peripheral venous access), forgetting at times that a patient has a PICC, and the finding that up to 25% of PICCs placed in their hospitals may be unnecessary underscore concerns regarding the safety of current practice trends. Interestingly, the viewpoints of hospitalists in practice <5 years and those providing direct patient care were more likely to reflect concerns regarding inappropriate placement, influence of vascular nurses, and use of PICCs for blood culture. This finding may reflect that these nuances are more recent phenomena or perhaps most apparent when care is delivered directly.
Our study must be interpreted in the context of several limitations. First, as this was a survey‐based study of a small, convenience sample of hospitalists in a single state, recall, respondent, and systematic biases remain threats to our findings. However, all site PIs encouraged survey participation and (through local dialogue) none were aware of material differences between those who did or did not participate in the study. Similarly, Michigan is a diverse and relatively large state, and our results should be generalizable to other settings; however, national studies are necessary to confirm our findings. Second, our response rate may be perceived as low; however, our rates are in accordance with, and, in fact, superior to those of many existing physician surveys.[24] Finally, only 1 federal facility was included in this study; thus, this care‐delivery model is underrepresented, limiting generalization of findings to other such sites.
However, our study also has important strengths. First, this is the only survey that specifically examines hospitalist viewpoints when it comes to PICCs. As hospitalists frequently order and/or insert these devices, their perspectives are highly pertinent to discussions regarding current PICC use. Second, our survey highlights several instances that may be associated with preventable patient harm and identifies areas where interventions may be valuable. For example, forgetting the presence of a device, keeping PICCs in place throughout hospitalization, and rendering treatment for PICC‐related VTE not in accordance with accepted guidelines are remediable practices that may lead to poor outcomes. Interventions such as device‐reminder alerts, provider education regarding complications from PICCs, and systematic efforts to identify and remove unnecessary PICCs may mitigate these problems. Finally, our findings highlight the need for data repositories that track PICC use and hospitalist practice on a national scale. Given the risk and significance of the complications associated with these devices, understanding the epidemiology, use, and potential misuse of PICCs are important areas for hospitalist research.
In conclusion, our study of hospitalist experience, practice, opinions, and knowledge related to PICCs suggests important gaps between available evidence and current practice. There is growing need for the development of appropriateness criteria to guide vascular access in inpatient settings.[25, 26] Such criteria should consider not only type of venous access device, but granular details including rationale for venous access, nature of the infusate, optimal number of lumens, and safest gauge when recommending devices. Until such criteria and comparative studies become available, hospitals should consider instituting policies to monitor PICC use with specific attention to indication for insertion, duration of placement, and complications. These interventions represent a first and necessary step in improving patient safety when it comes to preventing PICC‐related complications.
Disclosures
The Blue Cross/Blue Shield of Michigan Foundation in Detroit funded this study through an investigator‐initiated research proposal (1931‐PIRAP). The funding source, however, played no role in study design, acquisition of data, data analysis, or reporting of these results. The authors report no conflicts of interest.
- , , , et al. Hospital‐wide survey of the use of central venous catheters. J Hosp Infect. 2011;77(4):304–308.
- , , , , . Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med. 2012;125(8):733–741.
- , , . The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527–1528.
- , , , et al. Reduction of peripherally inserted central catheter associated deep venous thrombosis [published online ahead of print August 1, 2012]. Chest. doi: 10.1378/chest.12–0923.
- , , . Complications associated with peripheral or central routes for central venous cannulation. Anaesthesia. 2012;67(1):65–71.
- , , , et al. Patient‐ and device‐specific risk factors for peripherally inserted central venous catheter‐related bloodstream infections. Infect Control Hosp Epidemiol. 2013;34(2):184–189.
- , . Peripherally inserted central catheter use in the hospitalized patient: is there a role for the hospitalist? J Hosp Med. 2009;4(6):E1–E4.
- , , , , , . Peripherally inserted central catheter (PICC)‐associated upper‐extremity deep venous thrombosis (UEDVT) in critical‐care setting. Chest. 2005;128(4 suppl S):193S–194S.
- , , , , , . Complications and cost associated with parenteral nutrition delivered to hospitalized patients through either subclavian or peripherally inserted central catheters. Clin Nutr. 2000;19(4):237–243.
- , . Risk of catheter‐related bloodstream infection with peripherally inserted central venous catheters used in hospitalized patients. Chest. 2005;128(2):489–495.
- , , , . Long‐term intravenous therapy with peripherally inserted silicone elastomer central venous catheters in patients with malignant diseases. Cancer. 1979;43(5):1937–1943.
- , , , , . Central vs peripheral venous catheters in critically ill patients. Chest. 1986;90(6):806–809.
- , , , , . Infectious complications among patients receiving home intravenous therapy with peripheral, central, or peripherally placed central venous catheters. Am J Med. 1991;91(3B):95S–100S.
- , , , , . Upper‐extremity deep venous thrombosis and pulmonary embolism: a prospective study. Chest. 1991;99(2):280–283.
- , , , et al. Risk factors for catheter‐related thrombosis (CRT) in cancer patients: a patient‐level data (IPD) meta‐analysis of clinical trials and prospective studies. J Thromb Haemost. 2011;9(2):312–319.
- , , , et al. Venous thrombosis associated with peripherally inserted central catheters: a retrospective analysis of the Cleveland Clinic experience. Clin Infect Dis. 2002;34(9):1179–1183.
- , , , et al. Peripherally inserted central venous catheter–associated bloodstream infections in hospitalized adult patients. Infect Control Hosp Epidemiol. 2011;32(2):125–130.
- , , . Peripherally inserted central catheter bloodstream infection surveillance rates in an acute care setting in Saudi Arabia. Ann Saudi Med. 2012;32(2):169–173.
- , , , et al. Derivation and validation of a simple model to identify venous thromboembolism risk in medical patients. Am J Med. 2011;124(10):947.e942–954.e942.
- , , , et al. Risk of symptomatic DVT associated with peripherally inserted central catheters. Chest. 2010;138(4):803–810.
- , , . The clinical significance of peripherally inserted central venous catheter‐related deep vein thrombosis. Neurocrit Care. 2011;15(3):454–460.
- , , , et al. Catheter‐associated bloodstream infection incidence and risk factors in adults with cancer: a prospective cohort study. J Hosp Infect. 2011;78(1):26–30.
- , , , . Nonpharmacologic interventions for prevention of catheter‐related thrombosis: a systematic review [published online ahead of print September 13, 2012]. J Crit Care. doi: 10.1016/j.jcrc.2012.07.007.
- , , . Why are response rates in clinician surveys declining? Can Fam Physician. 2012;58(4):e225–e228.
- , , , , , . Sensitivity and specificity of the RAND/UCLA Appropriateness Method to identify the overuse and underuse of coronary revascularization and hysterectomy. J Clin Epidemiol. 2001;54(10):1004–1010.
- , , , et al. Variations by specialty in physician ratings of the appropriateness and necessity of indications for procedures. Med Care. 1996;34(6):512–523.
Peripherally inserted central catheters (PICCs) have become among the most common central venous catheters (CVCs) used in contemporary medical practice.[1] Although they were originally developed for delivery of parenteral nutrition, the use of PICCs has expanded to include chemotherapy administration, long‐term intravenous (IV) antibiotic treatment, and venous access when obtaining peripheral veins is difficult (eg, occluded peripheral veins, unusual venous anatomies).[2] Despite these roles, little is known about PICC use in hospitalized patients. This knowledge gap is important, as PICCs are placed in inpatient settings for a variety of reasons. Some of these reasons may not be appropriate, and inappropriate PICC use may worsen outcomes and increase healthcare costs.[3] In addition, PICCs are not innocuous and are frequently associated with important complications including thrombophlebitis, central‐lineassociated bloodstream infection and venous thromboembolism.[4, 5, 6] Therefore, understanding patterns and knowledge associated with PICC use is also an important patient safety concern.
As the main providers of inpatient care, hospitalists frequently order the insertion of PICCs and treat PICC‐related complications. Unfortunately, to date, no study has surveyed hospitalists regarding management or use of PICCs. Understanding hospitalist experiences, practice, opinions, and knowledge related to PICCs is therefore of significant interest when examining present‐day PICC use. To bridge this important knowledge gap and better understand these practices, we conducted a Web‐based survey of hospitalists in 5 healthcare systems in the state of Michigan.
METHODS
A convenience sample of hospitalists (N=227) was assembled from 5 large healthcare systems (representing 10 hospitals) that participate in the Hospital Medicine Safety (HMS) Consortium, a Blue Cross/Blue Shield of Michiganfunded statewide collaborative quality initiative. Individuals engaged in research, quality improvement, or leadership at HMS sites were invited to serve as site principal investigators (site PIs). Site PIs were responsible for obtaining regulatory approval at their parent facilities and disseminating the survey to providers in their group. Participation in the survey was solicited via e‐mail invitations from site PIs to hospitalists within their provider group. To encourage participation, a $10 electronic gift card was offered to respondents who successfully completed the survey. Reminder e‐mails were also sent each week by site PIs to augment participation. To enhance study recruitment, all responses were collected anonymously. The survey was administered between August 2012 and September 2012; data collection occurred for 5 weeks during this interval.
Survey questions were derived from our published, evidence‐based conceptual framework of PICC‐related complications. Briefly, this model identifies complications related to PICCs as arising from domains related to patient‐, provider‐, and device‐related characteristics based on existing evidence.[2] For our survey, questions were sourced from each of these domains so as to improve understanding of hospitalist experience, practice, opinions, and knowledge regarding PICC use. To ensure clarity of the survey questions, all questions were first pilot‐tested with a group of randomly selected hospitalist respondents at the University of Michigan Health System. Direct feedback obtained from these respondents was then used to iteratively improve each question. In order to generate holistic responses, questions were designed to generate a response reflective of the participants typical PICC use/subenario. We used SurveyMonkey to collect and manage survey data.
Statistical Analyses
Variation in hospitalist experience, reported practice, opinions, and knowledge regarding PICCs was assessed by hospitalist type (full time vs part time), years of practice (<1, 15, >5), and care‐delivery model (direct care vs learner‐based care). Bivariate comparisons were made using the 2 or Fisher exact tests as appropriate; 2‐sided with a P value <0.05 was considered statistically significant. All analyses were conducted using Stata version 11 (StataCorp, College Station, TX). Local institutional review board approval was obtained at each site participating in the survey.
RESULTS
A total of 227 surveys were administered and 144 responses collected, for a survey response rate of 63%. Each participating site had unique characteristics including size, number of hospitalists, and modality of PICC insertion (Table 1). Of the hospitalists who completed the survey, 81% held full‐time clinical positions and had been in practice an average of 5.6 years. Surveyed hospitalists reported caring for an average of 40.6 patients per week and ordering a mean of 2.9 (range, 015) PICCs per week of clinical service. Among survey respondents, 36% provided direct patient care, 34% provided care either directly or through mid‐level providers and housestaff, and 9% delivered care exclusively through mid‐level providers or housestaff (Table 2). As our survey was conducted anonymously, potential identifying information such as age, race, and sex of those responding was not collected.
| Survey Site | No. of Hospitals | No. of Inpatient Beds | No. of Annual Inpatient Encounters | No. of Hospitalists | Full‐Time Hospitalists, % | Avg. No. Weeks/Year on Service | Avg. Years of Experience | No. PICCs/Week, 2012 | Modality of PICC Insertion Available |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| University of Michigan Health System | 1 | 900+ | 5,775 | 46 | 100 | 25 | 6 | 42 | Vascular access nurse |
| Ann Arbor VA Medical Center | 1 | 135 | 825 | 16 | 50 | 17.6 | 5.1 | 12 | Vascular access nurse |
| Spectrum Health System | 2 | 800 | 14,000 | 47 | 80 | 34 | 3.75 | 56 | Interventional radiology |
| Trinity Health System | 3 | 634 | 2,300 | 67 | 80 | 24 | 4 | 31 | Interventional radiology and hospitalists |
| Henry Ford Health System | 3 | 1,150 | 1,450 | 51 | 100 | 20.4 | 5.6 | 15 | Vascular access nurse |
| Characteristic | Total (N=144) |
|---|---|
| |
| Hospitalist type, n (%) | |
| Full time | 117 (81) |
| Part time | 19 (13) |
| Unknown | 8 (6) |
| Weeks/year on a clinical service, n (%) | |
| <20 | 24 (17) |
| 20 | 107 (74) |
| Unknown | 13 (9) |
| Mean (SD) | 25.5 (10.7) |
| Median | 26 |
| Type of patients treated, n (%) | |
| Adults only | 129 (90) |
| Adults and children | 7 (5) |
| Unknown | 8 (6) |
| Years in practice as a hospitalist, n (%) | |
| 5 | 81 (56) |
| >5 | 54 (38) |
| Unknown | 9 (6) |
| Model of care delivery, n (%) | |
| Direct | 52 (36) |
| Some midlevel or housestaff providers (<50% of all encounters) | 49 (34) |
| Mostly midlevel or housestaff providers (>50% of all encounters) | 22 (15) |
| Only midlevel or housestaff providers | 13 (9) |
| Unknown | 8 (6) |
| Location of practice | |
| Trinity Health System | 39 (27) |
| University of Michigan Health System | 37 (26) |
| Henry Ford Health System | 28 (19) |
| Spectrum Health System | 21 (15) |
| Ann Arbor VA Medical Center | 11 (8) |
| Unknown | 8 (6) |
Hospitalist Experiences and Practice Related to Peripherally Inserted Central Catheters
According to responding hospitalists, the most common indications for PICC placement were long‐term IV antibiotic treatment (64%), followed by inability to obtain peripheral venous access (24%). Hospitalists reported an average duration of PICC placement of 17 days (range, 342 days). A significant percentage of hospitalists (93%) stated that they had cared for patients where a PICC was placed only for use during hospitalization, with the most common reason for such insertion being difficulty in otherwise securing venous access (67%). Respondents also reported caring for patients who had both PICCs and peripheral IV catheters in place at the same time; 49% stated that they had experienced this <5 times, whereas 33% stated they had experienced this 510 times. Furthermore, 87% of respondents indicated having admitted a patient who specifically requested a PICC due to prior difficulties with venous access. More than half of surveyed hospitalists (63%) admitted to having been contacted by a PICC nurse enquiring as to whether their patient might benefit from PICC insertion.
The majority of hospitalists (66%) reported that they specified the number of lumens when ordering PICCs. Thirty‐eight percent indicated that this decision was based on type of medication, whereas 35% selected the lowest number of lumens possible. A power PICC (specialized PICCs that are designed to withstand high‐pressure contrast injections), was specifically requested for radiographic studies (56%), infusion of large volume of fluids (10%), or was the default PICC type at their facility (34%).
A majority (74%) of survey respondents also reported that once inserted, PICCs were always used to obtain blood for routine laboratory testing. Moreover, 41% indicated that PICCs were also always used to obtain blood for microbiological cultures. The 3 most frequently encountered PICC‐related complications reported by hospitalists in our survey were blockage of a PICC lumen, bloodstream infection, and venous thromboembolism (VTE; Table 3).
| Hospitalist Experiences With PICCs | Total (N=144) |
|---|---|
| |
| Primary indication for PICC placement* | |
| Long‐term IV antibiotics | 64 |
| Venous access in a patient with poor peripheral veins | 24 |
| Parenteral nutrition | 5 |
| Chemotherapy | 4 |
| Patient specifically requested a PICC | 1 |
| Unknown/other | 2 |
| PICC placed only for venous access, n (%) | |
| Yes | 135 (94) |
| No | 9 (6) |
| PICC placed only during hospitalization, n (%) | |
| Yes | 134 (93) |
| No | 10 (7) |
| Notified by a PICC nurse (or other provider) that patient may need or benefit from a PICC, n (%) | |
| Yes | 91 (63) |
| No | 53 (37) |
| How frequently PICCs are used to obtain blood for routine laboratory testing, n (%) | |
| Always | 106 (74) |
| Unknown/other | 38 (26) |
| How frequently PICCs are used to obtain blood for blood cultures, n (%) | |
| Always | 59 (41) |
| Unknown/other | 85 (59) |
| Hospitalist Opinions on PICCs | Total (N=144) |
| In your opinion, is it appropriate to place a vascular in a hospitalized patient if other forms of peripheral access cannot be obtained? n (%) | |
| Yes | 121 (84) |
| No | 21 (15) |
| Unknown | 2 (1) |
| In your opinion, should hospitalists be trained to insert PICCs? n (%) | |
| No | 57 (40) |
| Yes, this is an important skill set for hospitalists | 46 (32) |
| Unsure | 39 (27) |
| Unknown/other | 2 (1) |
| Do you think the increasing number of vascular nurses and PICC nursing teams has influenced the use of PICCs in hospitalized patients? n (%) | |
| Yes | 112 (78) |
| No | 30 (21) |
| Unknown | 2 (1) |
| What % of PICC insertions do you think may represent inappropriate use in your hospital? n (%) | |
| <10 | 53 (37) |
| 1025 | 68 (47) |
| 2550 | 18 (13) |
| >50 | 3 (2) |
| Unknown/other | 2 (1) |
Hospitalist Opinions Regarding Peripherally Inserted Central Catheters
Compared with CVCs, 69% of hospitalists felt that PICCs were safer and more efficient because they could stay in place longer and were less likely to cause infection. Most (65%) also agreed that PICCs were more convenient than CVCs because they were inserted by PICC teams. Additionally, 74% of hospitalists felt that their patients preferred PICCs because they minimize pain from routine peripheral IV changes and phlebotomy. A majority of respondents (84%) indicated that it was appropriate to place a PICC if other forms of peripheral venous access could not be obtained. However, when specifically questioned, 47% of hospitalists indicated that at least 10%25% of PICCs placed in their hospitals might represent inappropriate use. A majority (78%) agreed with the statement that the increase in numbers of vascular nurses had influenced use of PICCs in hospitalized patients, but most (45%) were neutral when asked if PICCs were more cost‐effective than traditional CVCs.
Hospitalist Knowledge Regarding Risk of Peripherally Inserted Central CatheterRelated Venous Thromboembolism and Bloodstream Infection
Although 65% of responding hospitalists disagreed with the statement that PICCs were less likely to lead to VTE, important knowledge gaps regarding PICCs and VTE were identified (Table 4). For instance, only 4% of hospitalists were correctly aware that the PICC‐tip position is checked to reduce risk of PICC‐related VTE, and only 12% knew that the site of PICC insertion has also been associated with VTE risk. Although 85% of respondents stated they would prescribe a therapeutic dose of an anticoagulant in the case of PICC‐associated VTE, deviations from the guideline‐recommended 3‐month treatment period were noted. For example, 6% of hospitalists reported treating with anticoagulation for 6 months, and 19% stated they would treat as long as the PICC remained in place, plus an additional period of time (eg, 24 weeks) after removal. With respect to bloodstream infection, 92% of responding hospitalists correctly identified PICC duration and prompt removal as factors promoting PICC‐related bloodstream infection and 78% accurately identified components of the catheter‐associated bloodstream infection bundle. When specifically asked about factors associated with risk of PICC‐related bloodstream infection, only half of respondents recognized the number of PICC lumens as being associated with this outcome.
| Total (N=144) | |
|---|---|
| |
| Why is the position of the PICC tip checked after bedside PICC insertion? n (%) | |
| To decrease the risk of arrhythmia related to right‐atrial positioning | 108 (75) |
| To minimize the risk of VTEa | 6 (4) |
| To ensure it is not accidentally placed into an artery | 16 (11) |
| For documentation purposes (to reduce the risk of lawsuits related to line‐insertion complications) | 6 (4) |
| Unsure/Unknown | 8 (6) |
| According to the 2012 ACCP Guidelines on VTE prevention, is pharmacologic prophylaxis for DVT recommended in patients who receive long‐term PICCs? n (%) | |
| No; no anticoagulant prophylaxis is recommended for patients who receive long‐term PICCsa | 107 (74) |
| Yes, but the choice and duration of anticoagulant is at the discretion of the provider | 23 (16) |
| Yes; aspirin is recommended for 3 months | 4 (3) |
| Yes; anticoagulation with warfarin or enoxaparin is recommended for 3 months | 3 (2) |
| Yes; anticoagulation with warfarin or enoxaparin is recommended for 6 months | 2 (1) |
| Unknown | 5 (4) |
| Assuming no contraindications exist, do you anticoagulate patients who develop a PICC‐associated DVT (with any therapeutic anticoagulant)? n (%) | |
| Yesa | 122 (85) |
| No | 16 (11) |
| Unknown | 6 (4) |
| How long do you usually prescribe anticoagulation for patients who develop PICC‐associated DVT? n (%) | |
| I don't prescribe anticoagulation | 12 (8) |
| 1 month | 4 (3) |
| 3 monthsa | 84 (58) |
| 6 months | 8 (6) |
| As long as the line remains in place; I stop anticoagulation once the PICC comes out | 3 (2) |
| As long as the line remains in place and for an additional specified period of time after line removal, such as 2 or 4 weeks | 27 (19) |
| Unknown | 6 (4) |
| As part of the treatment of PICC‐related DVT, do you routinely remove the PICC?b n (%) | |
| Yes | 102 (71) |
| No | 36 (25) |
| Unknown | 6 (4) |
Variation in Hospitalist Knowledge, Experience, or Opinions
We assessed whether any of our findings varied according to hospitalist type (full time versus part time), years of practice (<1, 15, >5), and model of care delivery (direct care vs learner‐based care). Our analyses suggested that part‐time hospitalists were more likely to select rarely when it came to finding patients with a PICC and a working peripheral IV at the same time (74% vs 45%, P=0.02). Interestingly, a higher percentage of those in practice <5 years indicated that 10%25% of PICCs represented inappropriate placement (58% vs 33%, P<0.01) and that vascular nurses had influenced the use of PICCs in hospitalized patients (88% vs 69%, P=0.01). Lastly, a higher percentage of hospitalists who provided direct patient care reported that PICCs were always used to obtain blood for microbiological culture (54% vs 37%, P=0.05).
DISCUSSION
In this survey of hospitalists practicing at 5 large healthcare systems in Michigan, we observed significant variation in experience, reported practice, opinions, and knowledge related to PICCs. Our findings highlight important concerns related to inpatient PICC use and suggest a need for greater scrutiny related to these devices in these settings.
The use of PICCs in hospitalized patients has risen dramatically over the past decade. Though such growth is multifactorial and relates in part to increasing inpatient volume and complexity, hospitalists have increasingly turned to PICCs as a convenient and reliable tool to obtain venous access.[7] Indeed, in our survey, PICCs that were only used during hospitalization were most likely to be placed for this very reason. Because PICCs are safer to insert than CVCs and the original evidence regarding PICC‐related VTE or bloodstream infection suggested low rates of these events,[8, 9, 10, 11, 12, 13, 14] many hospitalists may not perceive these devices as being associated with significant risks. In fact, some have suggested that hospitalists be specifically trained to insert these devices, given their safety compared with traditional CVCs.[7]
However, accumulating evidence suggests that PICCs are associated with important complications.[5, 15, 16] In studies examining risk of bloodstream infection, PICCs were associated with significant risk of this outcome.[6, 17, 18] Recently, the presence of a PICC was identified as an independent predictor of VTE in hospitalized patients.[19] Several studies and systematic reviews have repeatedly demonstrated these findings.[19, 20, 21, 22] A recent systematic review examining nonpharmacologic methods to prevent catheter‐related thrombosis specifically called for avoidance of PICC insertion to prevent thrombosis in hospitalized patients.[23] Despite this growing evidence base, the use of PICCs in the inpatient setting is likely to rise, and our survey highlights several practices that may contribute to adverse outcomes. For instance, hospitalists in our survey were unlikely to remove a PICC until a patient was discharged, irrespective of the need for this device. As each day with a PICC increases the risk of complications, such practice poses potential patient safety concerns. Similarly, many hospitalists believe that PICCs are safer than CVCs, a viewpoint that does not stand up to increasing scrutiny and highlights important knowledge gaps. The risk of PICC‐related complications appears not to be a stationary target, but rather a dynamic balance that is influenced by patient‐, provider‐, and device‐specific characteristics.[2] Increasing discretionary use (especially for patients with poor peripheral venous access), forgetting at times that a patient has a PICC, and the finding that up to 25% of PICCs placed in their hospitals may be unnecessary underscore concerns regarding the safety of current practice trends. Interestingly, the viewpoints of hospitalists in practice <5 years and those providing direct patient care were more likely to reflect concerns regarding inappropriate placement, influence of vascular nurses, and use of PICCs for blood culture. This finding may reflect that these nuances are more recent phenomena or perhaps most apparent when care is delivered directly.
Our study must be interpreted in the context of several limitations. First, as this was a survey‐based study of a small, convenience sample of hospitalists in a single state, recall, respondent, and systematic biases remain threats to our findings. However, all site PIs encouraged survey participation and (through local dialogue) none were aware of material differences between those who did or did not participate in the study. Similarly, Michigan is a diverse and relatively large state, and our results should be generalizable to other settings; however, national studies are necessary to confirm our findings. Second, our response rate may be perceived as low; however, our rates are in accordance with, and, in fact, superior to those of many existing physician surveys.[24] Finally, only 1 federal facility was included in this study; thus, this care‐delivery model is underrepresented, limiting generalization of findings to other such sites.
However, our study also has important strengths. First, this is the only survey that specifically examines hospitalist viewpoints when it comes to PICCs. As hospitalists frequently order and/or insert these devices, their perspectives are highly pertinent to discussions regarding current PICC use. Second, our survey highlights several instances that may be associated with preventable patient harm and identifies areas where interventions may be valuable. For example, forgetting the presence of a device, keeping PICCs in place throughout hospitalization, and rendering treatment for PICC‐related VTE not in accordance with accepted guidelines are remediable practices that may lead to poor outcomes. Interventions such as device‐reminder alerts, provider education regarding complications from PICCs, and systematic efforts to identify and remove unnecessary PICCs may mitigate these problems. Finally, our findings highlight the need for data repositories that track PICC use and hospitalist practice on a national scale. Given the risk and significance of the complications associated with these devices, understanding the epidemiology, use, and potential misuse of PICCs are important areas for hospitalist research.
In conclusion, our study of hospitalist experience, practice, opinions, and knowledge related to PICCs suggests important gaps between available evidence and current practice. There is growing need for the development of appropriateness criteria to guide vascular access in inpatient settings.[25, 26] Such criteria should consider not only type of venous access device, but granular details including rationale for venous access, nature of the infusate, optimal number of lumens, and safest gauge when recommending devices. Until such criteria and comparative studies become available, hospitals should consider instituting policies to monitor PICC use with specific attention to indication for insertion, duration of placement, and complications. These interventions represent a first and necessary step in improving patient safety when it comes to preventing PICC‐related complications.
Disclosures
The Blue Cross/Blue Shield of Michigan Foundation in Detroit funded this study through an investigator‐initiated research proposal (1931‐PIRAP). The funding source, however, played no role in study design, acquisition of data, data analysis, or reporting of these results. The authors report no conflicts of interest.
Peripherally inserted central catheters (PICCs) have become among the most common central venous catheters (CVCs) used in contemporary medical practice.[1] Although they were originally developed for delivery of parenteral nutrition, the use of PICCs has expanded to include chemotherapy administration, long‐term intravenous (IV) antibiotic treatment, and venous access when obtaining peripheral veins is difficult (eg, occluded peripheral veins, unusual venous anatomies).[2] Despite these roles, little is known about PICC use in hospitalized patients. This knowledge gap is important, as PICCs are placed in inpatient settings for a variety of reasons. Some of these reasons may not be appropriate, and inappropriate PICC use may worsen outcomes and increase healthcare costs.[3] In addition, PICCs are not innocuous and are frequently associated with important complications including thrombophlebitis, central‐lineassociated bloodstream infection and venous thromboembolism.[4, 5, 6] Therefore, understanding patterns and knowledge associated with PICC use is also an important patient safety concern.
As the main providers of inpatient care, hospitalists frequently order the insertion of PICCs and treat PICC‐related complications. Unfortunately, to date, no study has surveyed hospitalists regarding management or use of PICCs. Understanding hospitalist experiences, practice, opinions, and knowledge related to PICCs is therefore of significant interest when examining present‐day PICC use. To bridge this important knowledge gap and better understand these practices, we conducted a Web‐based survey of hospitalists in 5 healthcare systems in the state of Michigan.
METHODS
A convenience sample of hospitalists (N=227) was assembled from 5 large healthcare systems (representing 10 hospitals) that participate in the Hospital Medicine Safety (HMS) Consortium, a Blue Cross/Blue Shield of Michiganfunded statewide collaborative quality initiative. Individuals engaged in research, quality improvement, or leadership at HMS sites were invited to serve as site principal investigators (site PIs). Site PIs were responsible for obtaining regulatory approval at their parent facilities and disseminating the survey to providers in their group. Participation in the survey was solicited via e‐mail invitations from site PIs to hospitalists within their provider group. To encourage participation, a $10 electronic gift card was offered to respondents who successfully completed the survey. Reminder e‐mails were also sent each week by site PIs to augment participation. To enhance study recruitment, all responses were collected anonymously. The survey was administered between August 2012 and September 2012; data collection occurred for 5 weeks during this interval.
Survey questions were derived from our published, evidence‐based conceptual framework of PICC‐related complications. Briefly, this model identifies complications related to PICCs as arising from domains related to patient‐, provider‐, and device‐related characteristics based on existing evidence.[2] For our survey, questions were sourced from each of these domains so as to improve understanding of hospitalist experience, practice, opinions, and knowledge regarding PICC use. To ensure clarity of the survey questions, all questions were first pilot‐tested with a group of randomly selected hospitalist respondents at the University of Michigan Health System. Direct feedback obtained from these respondents was then used to iteratively improve each question. In order to generate holistic responses, questions were designed to generate a response reflective of the participants typical PICC use/subenario. We used SurveyMonkey to collect and manage survey data.
Statistical Analyses
Variation in hospitalist experience, reported practice, opinions, and knowledge regarding PICCs was assessed by hospitalist type (full time vs part time), years of practice (<1, 15, >5), and care‐delivery model (direct care vs learner‐based care). Bivariate comparisons were made using the 2 or Fisher exact tests as appropriate; 2‐sided with a P value <0.05 was considered statistically significant. All analyses were conducted using Stata version 11 (StataCorp, College Station, TX). Local institutional review board approval was obtained at each site participating in the survey.
RESULTS
A total of 227 surveys were administered and 144 responses collected, for a survey response rate of 63%. Each participating site had unique characteristics including size, number of hospitalists, and modality of PICC insertion (Table 1). Of the hospitalists who completed the survey, 81% held full‐time clinical positions and had been in practice an average of 5.6 years. Surveyed hospitalists reported caring for an average of 40.6 patients per week and ordering a mean of 2.9 (range, 015) PICCs per week of clinical service. Among survey respondents, 36% provided direct patient care, 34% provided care either directly or through mid‐level providers and housestaff, and 9% delivered care exclusively through mid‐level providers or housestaff (Table 2). As our survey was conducted anonymously, potential identifying information such as age, race, and sex of those responding was not collected.
| Survey Site | No. of Hospitals | No. of Inpatient Beds | No. of Annual Inpatient Encounters | No. of Hospitalists | Full‐Time Hospitalists, % | Avg. No. Weeks/Year on Service | Avg. Years of Experience | No. PICCs/Week, 2012 | Modality of PICC Insertion Available |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| University of Michigan Health System | 1 | 900+ | 5,775 | 46 | 100 | 25 | 6 | 42 | Vascular access nurse |
| Ann Arbor VA Medical Center | 1 | 135 | 825 | 16 | 50 | 17.6 | 5.1 | 12 | Vascular access nurse |
| Spectrum Health System | 2 | 800 | 14,000 | 47 | 80 | 34 | 3.75 | 56 | Interventional radiology |
| Trinity Health System | 3 | 634 | 2,300 | 67 | 80 | 24 | 4 | 31 | Interventional radiology and hospitalists |
| Henry Ford Health System | 3 | 1,150 | 1,450 | 51 | 100 | 20.4 | 5.6 | 15 | Vascular access nurse |
| Characteristic | Total (N=144) |
|---|---|
| |
| Hospitalist type, n (%) | |
| Full time | 117 (81) |
| Part time | 19 (13) |
| Unknown | 8 (6) |
| Weeks/year on a clinical service, n (%) | |
| <20 | 24 (17) |
| 20 | 107 (74) |
| Unknown | 13 (9) |
| Mean (SD) | 25.5 (10.7) |
| Median | 26 |
| Type of patients treated, n (%) | |
| Adults only | 129 (90) |
| Adults and children | 7 (5) |
| Unknown | 8 (6) |
| Years in practice as a hospitalist, n (%) | |
| 5 | 81 (56) |
| >5 | 54 (38) |
| Unknown | 9 (6) |
| Model of care delivery, n (%) | |
| Direct | 52 (36) |
| Some midlevel or housestaff providers (<50% of all encounters) | 49 (34) |
| Mostly midlevel or housestaff providers (>50% of all encounters) | 22 (15) |
| Only midlevel or housestaff providers | 13 (9) |
| Unknown | 8 (6) |
| Location of practice | |
| Trinity Health System | 39 (27) |
| University of Michigan Health System | 37 (26) |
| Henry Ford Health System | 28 (19) |
| Spectrum Health System | 21 (15) |
| Ann Arbor VA Medical Center | 11 (8) |
| Unknown | 8 (6) |
Hospitalist Experiences and Practice Related to Peripherally Inserted Central Catheters
According to responding hospitalists, the most common indications for PICC placement were long‐term IV antibiotic treatment (64%), followed by inability to obtain peripheral venous access (24%). Hospitalists reported an average duration of PICC placement of 17 days (range, 342 days). A significant percentage of hospitalists (93%) stated that they had cared for patients where a PICC was placed only for use during hospitalization, with the most common reason for such insertion being difficulty in otherwise securing venous access (67%). Respondents also reported caring for patients who had both PICCs and peripheral IV catheters in place at the same time; 49% stated that they had experienced this <5 times, whereas 33% stated they had experienced this 510 times. Furthermore, 87% of respondents indicated having admitted a patient who specifically requested a PICC due to prior difficulties with venous access. More than half of surveyed hospitalists (63%) admitted to having been contacted by a PICC nurse enquiring as to whether their patient might benefit from PICC insertion.
The majority of hospitalists (66%) reported that they specified the number of lumens when ordering PICCs. Thirty‐eight percent indicated that this decision was based on type of medication, whereas 35% selected the lowest number of lumens possible. A power PICC (specialized PICCs that are designed to withstand high‐pressure contrast injections), was specifically requested for radiographic studies (56%), infusion of large volume of fluids (10%), or was the default PICC type at their facility (34%).
A majority (74%) of survey respondents also reported that once inserted, PICCs were always used to obtain blood for routine laboratory testing. Moreover, 41% indicated that PICCs were also always used to obtain blood for microbiological cultures. The 3 most frequently encountered PICC‐related complications reported by hospitalists in our survey were blockage of a PICC lumen, bloodstream infection, and venous thromboembolism (VTE; Table 3).
| Hospitalist Experiences With PICCs | Total (N=144) |
|---|---|
| |
| Primary indication for PICC placement* | |
| Long‐term IV antibiotics | 64 |
| Venous access in a patient with poor peripheral veins | 24 |
| Parenteral nutrition | 5 |
| Chemotherapy | 4 |
| Patient specifically requested a PICC | 1 |
| Unknown/other | 2 |
| PICC placed only for venous access, n (%) | |
| Yes | 135 (94) |
| No | 9 (6) |
| PICC placed only during hospitalization, n (%) | |
| Yes | 134 (93) |
| No | 10 (7) |
| Notified by a PICC nurse (or other provider) that patient may need or benefit from a PICC, n (%) | |
| Yes | 91 (63) |
| No | 53 (37) |
| How frequently PICCs are used to obtain blood for routine laboratory testing, n (%) | |
| Always | 106 (74) |
| Unknown/other | 38 (26) |
| How frequently PICCs are used to obtain blood for blood cultures, n (%) | |
| Always | 59 (41) |
| Unknown/other | 85 (59) |
| Hospitalist Opinions on PICCs | Total (N=144) |
| In your opinion, is it appropriate to place a vascular in a hospitalized patient if other forms of peripheral access cannot be obtained? n (%) | |
| Yes | 121 (84) |
| No | 21 (15) |
| Unknown | 2 (1) |
| In your opinion, should hospitalists be trained to insert PICCs? n (%) | |
| No | 57 (40) |
| Yes, this is an important skill set for hospitalists | 46 (32) |
| Unsure | 39 (27) |
| Unknown/other | 2 (1) |
| Do you think the increasing number of vascular nurses and PICC nursing teams has influenced the use of PICCs in hospitalized patients? n (%) | |
| Yes | 112 (78) |
| No | 30 (21) |
| Unknown | 2 (1) |
| What % of PICC insertions do you think may represent inappropriate use in your hospital? n (%) | |
| <10 | 53 (37) |
| 1025 | 68 (47) |
| 2550 | 18 (13) |
| >50 | 3 (2) |
| Unknown/other | 2 (1) |
Hospitalist Opinions Regarding Peripherally Inserted Central Catheters
Compared with CVCs, 69% of hospitalists felt that PICCs were safer and more efficient because they could stay in place longer and were less likely to cause infection. Most (65%) also agreed that PICCs were more convenient than CVCs because they were inserted by PICC teams. Additionally, 74% of hospitalists felt that their patients preferred PICCs because they minimize pain from routine peripheral IV changes and phlebotomy. A majority of respondents (84%) indicated that it was appropriate to place a PICC if other forms of peripheral venous access could not be obtained. However, when specifically questioned, 47% of hospitalists indicated that at least 10%25% of PICCs placed in their hospitals might represent inappropriate use. A majority (78%) agreed with the statement that the increase in numbers of vascular nurses had influenced use of PICCs in hospitalized patients, but most (45%) were neutral when asked if PICCs were more cost‐effective than traditional CVCs.
Hospitalist Knowledge Regarding Risk of Peripherally Inserted Central CatheterRelated Venous Thromboembolism and Bloodstream Infection
Although 65% of responding hospitalists disagreed with the statement that PICCs were less likely to lead to VTE, important knowledge gaps regarding PICCs and VTE were identified (Table 4). For instance, only 4% of hospitalists were correctly aware that the PICC‐tip position is checked to reduce risk of PICC‐related VTE, and only 12% knew that the site of PICC insertion has also been associated with VTE risk. Although 85% of respondents stated they would prescribe a therapeutic dose of an anticoagulant in the case of PICC‐associated VTE, deviations from the guideline‐recommended 3‐month treatment period were noted. For example, 6% of hospitalists reported treating with anticoagulation for 6 months, and 19% stated they would treat as long as the PICC remained in place, plus an additional period of time (eg, 24 weeks) after removal. With respect to bloodstream infection, 92% of responding hospitalists correctly identified PICC duration and prompt removal as factors promoting PICC‐related bloodstream infection and 78% accurately identified components of the catheter‐associated bloodstream infection bundle. When specifically asked about factors associated with risk of PICC‐related bloodstream infection, only half of respondents recognized the number of PICC lumens as being associated with this outcome.
| Total (N=144) | |
|---|---|
| |
| Why is the position of the PICC tip checked after bedside PICC insertion? n (%) | |
| To decrease the risk of arrhythmia related to right‐atrial positioning | 108 (75) |
| To minimize the risk of VTEa | 6 (4) |
| To ensure it is not accidentally placed into an artery | 16 (11) |
| For documentation purposes (to reduce the risk of lawsuits related to line‐insertion complications) | 6 (4) |
| Unsure/Unknown | 8 (6) |
| According to the 2012 ACCP Guidelines on VTE prevention, is pharmacologic prophylaxis for DVT recommended in patients who receive long‐term PICCs? n (%) | |
| No; no anticoagulant prophylaxis is recommended for patients who receive long‐term PICCsa | 107 (74) |
| Yes, but the choice and duration of anticoagulant is at the discretion of the provider | 23 (16) |
| Yes; aspirin is recommended for 3 months | 4 (3) |
| Yes; anticoagulation with warfarin or enoxaparin is recommended for 3 months | 3 (2) |
| Yes; anticoagulation with warfarin or enoxaparin is recommended for 6 months | 2 (1) |
| Unknown | 5 (4) |
| Assuming no contraindications exist, do you anticoagulate patients who develop a PICC‐associated DVT (with any therapeutic anticoagulant)? n (%) | |
| Yesa | 122 (85) |
| No | 16 (11) |
| Unknown | 6 (4) |
| How long do you usually prescribe anticoagulation for patients who develop PICC‐associated DVT? n (%) | |
| I don't prescribe anticoagulation | 12 (8) |
| 1 month | 4 (3) |
| 3 monthsa | 84 (58) |
| 6 months | 8 (6) |
| As long as the line remains in place; I stop anticoagulation once the PICC comes out | 3 (2) |
| As long as the line remains in place and for an additional specified period of time after line removal, such as 2 or 4 weeks | 27 (19) |
| Unknown | 6 (4) |
| As part of the treatment of PICC‐related DVT, do you routinely remove the PICC?b n (%) | |
| Yes | 102 (71) |
| No | 36 (25) |
| Unknown | 6 (4) |
Variation in Hospitalist Knowledge, Experience, or Opinions
We assessed whether any of our findings varied according to hospitalist type (full time versus part time), years of practice (<1, 15, >5), and model of care delivery (direct care vs learner‐based care). Our analyses suggested that part‐time hospitalists were more likely to select rarely when it came to finding patients with a PICC and a working peripheral IV at the same time (74% vs 45%, P=0.02). Interestingly, a higher percentage of those in practice <5 years indicated that 10%25% of PICCs represented inappropriate placement (58% vs 33%, P<0.01) and that vascular nurses had influenced the use of PICCs in hospitalized patients (88% vs 69%, P=0.01). Lastly, a higher percentage of hospitalists who provided direct patient care reported that PICCs were always used to obtain blood for microbiological culture (54% vs 37%, P=0.05).
DISCUSSION
In this survey of hospitalists practicing at 5 large healthcare systems in Michigan, we observed significant variation in experience, reported practice, opinions, and knowledge related to PICCs. Our findings highlight important concerns related to inpatient PICC use and suggest a need for greater scrutiny related to these devices in these settings.
The use of PICCs in hospitalized patients has risen dramatically over the past decade. Though such growth is multifactorial and relates in part to increasing inpatient volume and complexity, hospitalists have increasingly turned to PICCs as a convenient and reliable tool to obtain venous access.[7] Indeed, in our survey, PICCs that were only used during hospitalization were most likely to be placed for this very reason. Because PICCs are safer to insert than CVCs and the original evidence regarding PICC‐related VTE or bloodstream infection suggested low rates of these events,[8, 9, 10, 11, 12, 13, 14] many hospitalists may not perceive these devices as being associated with significant risks. In fact, some have suggested that hospitalists be specifically trained to insert these devices, given their safety compared with traditional CVCs.[7]
However, accumulating evidence suggests that PICCs are associated with important complications.[5, 15, 16] In studies examining risk of bloodstream infection, PICCs were associated with significant risk of this outcome.[6, 17, 18] Recently, the presence of a PICC was identified as an independent predictor of VTE in hospitalized patients.[19] Several studies and systematic reviews have repeatedly demonstrated these findings.[19, 20, 21, 22] A recent systematic review examining nonpharmacologic methods to prevent catheter‐related thrombosis specifically called for avoidance of PICC insertion to prevent thrombosis in hospitalized patients.[23] Despite this growing evidence base, the use of PICCs in the inpatient setting is likely to rise, and our survey highlights several practices that may contribute to adverse outcomes. For instance, hospitalists in our survey were unlikely to remove a PICC until a patient was discharged, irrespective of the need for this device. As each day with a PICC increases the risk of complications, such practice poses potential patient safety concerns. Similarly, many hospitalists believe that PICCs are safer than CVCs, a viewpoint that does not stand up to increasing scrutiny and highlights important knowledge gaps. The risk of PICC‐related complications appears not to be a stationary target, but rather a dynamic balance that is influenced by patient‐, provider‐, and device‐specific characteristics.[2] Increasing discretionary use (especially for patients with poor peripheral venous access), forgetting at times that a patient has a PICC, and the finding that up to 25% of PICCs placed in their hospitals may be unnecessary underscore concerns regarding the safety of current practice trends. Interestingly, the viewpoints of hospitalists in practice <5 years and those providing direct patient care were more likely to reflect concerns regarding inappropriate placement, influence of vascular nurses, and use of PICCs for blood culture. This finding may reflect that these nuances are more recent phenomena or perhaps most apparent when care is delivered directly.
Our study must be interpreted in the context of several limitations. First, as this was a survey‐based study of a small, convenience sample of hospitalists in a single state, recall, respondent, and systematic biases remain threats to our findings. However, all site PIs encouraged survey participation and (through local dialogue) none were aware of material differences between those who did or did not participate in the study. Similarly, Michigan is a diverse and relatively large state, and our results should be generalizable to other settings; however, national studies are necessary to confirm our findings. Second, our response rate may be perceived as low; however, our rates are in accordance with, and, in fact, superior to those of many existing physician surveys.[24] Finally, only 1 federal facility was included in this study; thus, this care‐delivery model is underrepresented, limiting generalization of findings to other such sites.
However, our study also has important strengths. First, this is the only survey that specifically examines hospitalist viewpoints when it comes to PICCs. As hospitalists frequently order and/or insert these devices, their perspectives are highly pertinent to discussions regarding current PICC use. Second, our survey highlights several instances that may be associated with preventable patient harm and identifies areas where interventions may be valuable. For example, forgetting the presence of a device, keeping PICCs in place throughout hospitalization, and rendering treatment for PICC‐related VTE not in accordance with accepted guidelines are remediable practices that may lead to poor outcomes. Interventions such as device‐reminder alerts, provider education regarding complications from PICCs, and systematic efforts to identify and remove unnecessary PICCs may mitigate these problems. Finally, our findings highlight the need for data repositories that track PICC use and hospitalist practice on a national scale. Given the risk and significance of the complications associated with these devices, understanding the epidemiology, use, and potential misuse of PICCs are important areas for hospitalist research.
In conclusion, our study of hospitalist experience, practice, opinions, and knowledge related to PICCs suggests important gaps between available evidence and current practice. There is growing need for the development of appropriateness criteria to guide vascular access in inpatient settings.[25, 26] Such criteria should consider not only type of venous access device, but granular details including rationale for venous access, nature of the infusate, optimal number of lumens, and safest gauge when recommending devices. Until such criteria and comparative studies become available, hospitals should consider instituting policies to monitor PICC use with specific attention to indication for insertion, duration of placement, and complications. These interventions represent a first and necessary step in improving patient safety when it comes to preventing PICC‐related complications.
Disclosures
The Blue Cross/Blue Shield of Michigan Foundation in Detroit funded this study through an investigator‐initiated research proposal (1931‐PIRAP). The funding source, however, played no role in study design, acquisition of data, data analysis, or reporting of these results. The authors report no conflicts of interest.
- , , , et al. Hospital‐wide survey of the use of central venous catheters. J Hosp Infect. 2011;77(4):304–308.
- , , , , . Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med. 2012;125(8):733–741.
- , , . The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527–1528.
- , , , et al. Reduction of peripherally inserted central catheter associated deep venous thrombosis [published online ahead of print August 1, 2012]. Chest. doi: 10.1378/chest.12–0923.
- , , . Complications associated with peripheral or central routes for central venous cannulation. Anaesthesia. 2012;67(1):65–71.
- , , , et al. Patient‐ and device‐specific risk factors for peripherally inserted central venous catheter‐related bloodstream infections. Infect Control Hosp Epidemiol. 2013;34(2):184–189.
- , . Peripherally inserted central catheter use in the hospitalized patient: is there a role for the hospitalist? J Hosp Med. 2009;4(6):E1–E4.
- , , , , , . Peripherally inserted central catheter (PICC)‐associated upper‐extremity deep venous thrombosis (UEDVT) in critical‐care setting. Chest. 2005;128(4 suppl S):193S–194S.
- , , , , , . Complications and cost associated with parenteral nutrition delivered to hospitalized patients through either subclavian or peripherally inserted central catheters. Clin Nutr. 2000;19(4):237–243.
- , . Risk of catheter‐related bloodstream infection with peripherally inserted central venous catheters used in hospitalized patients. Chest. 2005;128(2):489–495.
- , , , . Long‐term intravenous therapy with peripherally inserted silicone elastomer central venous catheters in patients with malignant diseases. Cancer. 1979;43(5):1937–1943.
- , , , , . Central vs peripheral venous catheters in critically ill patients. Chest. 1986;90(6):806–809.
- , , , , . Infectious complications among patients receiving home intravenous therapy with peripheral, central, or peripherally placed central venous catheters. Am J Med. 1991;91(3B):95S–100S.
- , , , , . Upper‐extremity deep venous thrombosis and pulmonary embolism: a prospective study. Chest. 1991;99(2):280–283.
- , , , et al. Risk factors for catheter‐related thrombosis (CRT) in cancer patients: a patient‐level data (IPD) meta‐analysis of clinical trials and prospective studies. J Thromb Haemost. 2011;9(2):312–319.
- , , , et al. Venous thrombosis associated with peripherally inserted central catheters: a retrospective analysis of the Cleveland Clinic experience. Clin Infect Dis. 2002;34(9):1179–1183.
- , , , et al. Peripherally inserted central venous catheter–associated bloodstream infections in hospitalized adult patients. Infect Control Hosp Epidemiol. 2011;32(2):125–130.
- , , . Peripherally inserted central catheter bloodstream infection surveillance rates in an acute care setting in Saudi Arabia. Ann Saudi Med. 2012;32(2):169–173.
- , , , et al. Derivation and validation of a simple model to identify venous thromboembolism risk in medical patients. Am J Med. 2011;124(10):947.e942–954.e942.
- , , , et al. Risk of symptomatic DVT associated with peripherally inserted central catheters. Chest. 2010;138(4):803–810.
- , , . The clinical significance of peripherally inserted central venous catheter‐related deep vein thrombosis. Neurocrit Care. 2011;15(3):454–460.
- , , , et al. Catheter‐associated bloodstream infection incidence and risk factors in adults with cancer: a prospective cohort study. J Hosp Infect. 2011;78(1):26–30.
- , , , . Nonpharmacologic interventions for prevention of catheter‐related thrombosis: a systematic review [published online ahead of print September 13, 2012]. J Crit Care. doi: 10.1016/j.jcrc.2012.07.007.
- , , . Why are response rates in clinician surveys declining? Can Fam Physician. 2012;58(4):e225–e228.
- , , , , , . Sensitivity and specificity of the RAND/UCLA Appropriateness Method to identify the overuse and underuse of coronary revascularization and hysterectomy. J Clin Epidemiol. 2001;54(10):1004–1010.
- , , , et al. Variations by specialty in physician ratings of the appropriateness and necessity of indications for procedures. Med Care. 1996;34(6):512–523.
- , , , et al. Hospital‐wide survey of the use of central venous catheters. J Hosp Infect. 2011;77(4):304–308.
- , , , , . Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med. 2012;125(8):733–741.
- , , . The problem with peripherally inserted central catheters. JAMA. 2012;308(15):1527–1528.
- , , , et al. Reduction of peripherally inserted central catheter associated deep venous thrombosis [published online ahead of print August 1, 2012]. Chest. doi: 10.1378/chest.12–0923.
- , , . Complications associated with peripheral or central routes for central venous cannulation. Anaesthesia. 2012;67(1):65–71.
- , , , et al. Patient‐ and device‐specific risk factors for peripherally inserted central venous catheter‐related bloodstream infections. Infect Control Hosp Epidemiol. 2013;34(2):184–189.
- , . Peripherally inserted central catheter use in the hospitalized patient: is there a role for the hospitalist? J Hosp Med. 2009;4(6):E1–E4.
- , , , , , . Peripherally inserted central catheter (PICC)‐associated upper‐extremity deep venous thrombosis (UEDVT) in critical‐care setting. Chest. 2005;128(4 suppl S):193S–194S.
- , , , , , . Complications and cost associated with parenteral nutrition delivered to hospitalized patients through either subclavian or peripherally inserted central catheters. Clin Nutr. 2000;19(4):237–243.
- , . Risk of catheter‐related bloodstream infection with peripherally inserted central venous catheters used in hospitalized patients. Chest. 2005;128(2):489–495.
- , , , . Long‐term intravenous therapy with peripherally inserted silicone elastomer central venous catheters in patients with malignant diseases. Cancer. 1979;43(5):1937–1943.
- , , , , . Central vs peripheral venous catheters in critically ill patients. Chest. 1986;90(6):806–809.
- , , , , . Infectious complications among patients receiving home intravenous therapy with peripheral, central, or peripherally placed central venous catheters. Am J Med. 1991;91(3B):95S–100S.
- , , , , . Upper‐extremity deep venous thrombosis and pulmonary embolism: a prospective study. Chest. 1991;99(2):280–283.
- , , , et al. Risk factors for catheter‐related thrombosis (CRT) in cancer patients: a patient‐level data (IPD) meta‐analysis of clinical trials and prospective studies. J Thromb Haemost. 2011;9(2):312–319.
- , , , et al. Venous thrombosis associated with peripherally inserted central catheters: a retrospective analysis of the Cleveland Clinic experience. Clin Infect Dis. 2002;34(9):1179–1183.
- , , , et al. Peripherally inserted central venous catheter–associated bloodstream infections in hospitalized adult patients. Infect Control Hosp Epidemiol. 2011;32(2):125–130.
- , , . Peripherally inserted central catheter bloodstream infection surveillance rates in an acute care setting in Saudi Arabia. Ann Saudi Med. 2012;32(2):169–173.
- , , , et al. Derivation and validation of a simple model to identify venous thromboembolism risk in medical patients. Am J Med. 2011;124(10):947.e942–954.e942.
- , , , et al. Risk of symptomatic DVT associated with peripherally inserted central catheters. Chest. 2010;138(4):803–810.
- , , . The clinical significance of peripherally inserted central venous catheter‐related deep vein thrombosis. Neurocrit Care. 2011;15(3):454–460.
- , , , et al. Catheter‐associated bloodstream infection incidence and risk factors in adults with cancer: a prospective cohort study. J Hosp Infect. 2011;78(1):26–30.
- , , , . Nonpharmacologic interventions for prevention of catheter‐related thrombosis: a systematic review [published online ahead of print September 13, 2012]. J Crit Care. doi: 10.1016/j.jcrc.2012.07.007.
- , , . Why are response rates in clinician surveys declining? Can Fam Physician. 2012;58(4):e225–e228.
- , , , , , . Sensitivity and specificity of the RAND/UCLA Appropriateness Method to identify the overuse and underuse of coronary revascularization and hysterectomy. J Clin Epidemiol. 2001;54(10):1004–1010.
- , , , et al. Variations by specialty in physician ratings of the appropriateness and necessity of indications for procedures. Med Care. 1996;34(6):512–523.
Copyright © 2013 Society of Hospital Medicine