User login
4.16 Healthcare Systems: Research
Introduction
Research is a rapidly growing aspect of inpatient medicine. The practice of evidence-based medicine and the acute need for more evidence on inpatient conditions require that pediatric hospitalists understand and participate in research related activities. Pediatric hospitalists’ role in research will vary depending on their setting and job description. This role may include many facets, from reviewing relevant patient-based articles, to participating in multi-institutional studies requiring enrollment of patients, to leading local or national studies. Pediatric hospitalists should have a basic understanding of research methods and processes in order to participate in and benefit from research. Pediatric hospitalists are well positioned to promote research to patients, the family/caregivers, colleagues, and other healthcare providers and through this, to contribute to the effective care of hospitalized patients.
Knowledge
Pediatric hospitalists should be able to:
- Compare and contrast the advantages and disadvantages of experimental (such as randomized control trials) and observational (such as descriptive, cohort, or case control) study designs, including meta-analyses and systematic reviews.
- Define common sources of bias, including information bias, selection bias, and uncontrolled confounding, and describe how each may impact a study.
- Define basic statistical terms such as sample, discrete and continuous data variables, measures of central tendency (mean, median, and mode), and variability (variance, standard deviation, range).
- List resources available to access current or proposed studies including The Pediatric Health Information System (PHIS), the Healthcare Cost and Utilization Project (HCUP), the Kids’ Inpatient Database (KID), clinicaltrials.gov, and others.
- Name potential research funding sources, such as the Agency for Healthcare Research and Quality (AHRQ), the National Institutes of Health (NIH), the Patient-Centered Outcomes Research Institute (PCORI), the Robert Wood Johnson Foundation, local and state funding sources, and others.
- Summarize the goals of pediatric hospital medicine-specific research networks, including the Pediatric Research in the Inpatient Setting (PRIS) network and the Value in Pediatrics (VIP) network.
- Discuss the basic resources commonly required to support research components, including data collection, data analysis, abstract and manuscript preparation, grant funding, and others.
- Review the aspects of the research process that relate to protection of participants, including informed consent and/or assent, the institutional review boards (IRB) review, and HIPAA (Health Insurance Portability and Accountability Act) forms.
- Discuss special protections needed when conducting research with vulnerable populations.
- Define “minimal risk” for a healthy child and for a child with an illness.
- Discuss why common training that addresses ethics, vulnerable populations, consenting, data safety, and other items is required prior to participating as a research team member for a research study.
- Compare and contrast the goals, intent, study focus, and IRB requirements for quality improvement studies from those of traditional clinical research.
- Cite the steps needed to obtain approval for a QI study within the local context.
- Compare and contrast the goals, intent, study focus, and IRB requirements for education studies to those of traditional clinical research.
- Cite the steps needed to obtain approval for a study focused on educational outcomes.
- List common barriers to implementation of clinical studies and describe the pediatric hospitalist’s role in overcoming these barriers.
Skills
Pediatric hospitalists should be able to:
- Utilize a format such as PICO (Population, Intervention, Comparison, Outcome) to generate an answerable patient-centered clinical question that is relevant to improving patient care.
- Demonstrate proficiency in systematic searching of the primary medical literature using online search engines.
- Perform critical appraisal of the literature, including identifying threats to study validity, determining if study subjects were similar to local patients, and determining if all clinically important outcomes were considered.
- Apply and integrate the results of studies to clinical practice.
- Determine if the likely benefits noted in a treatment study are worth the potential harm and cost.
- Determine whether a test noted in a diagnostic study is available, affordable, accurate, and precise in the present clinical setting and determine whether the results of the test will change the management of patients being treated.
- Determine if the magnitude of risk warrants an attempt to stop the exposure for a given study on harm.
- Identify if the results of a given study on disease prognosis will lead directly to selecting therapy and/or are useful for counseling patients.
- Participate in educating learners and junior faculty about research and research methodologies, within the local context.
- Determine the relevance of potential research studies with regards to impact on patient care.
- Perform effective informed consent or assent for patients participating in research studies, as appropriate.
- Identify and resolve conflict of interest or potential conflict of interest when participating in research studies.
- Demonstrate basic skills in acquiring, managing, and sharing data collected for research purposes in a responsible and professional manner.
- Adhere to standards for protecting confidentiality, avoiding unjustified exclusions, sharing data, and adhering to copyright law.
- Perform peer-review of a manuscript, abstract, or other research-based work, in collaboration with colleagues as appropriate.
- Demonstrate basic skills in communicating about research opportunities with patients and the family/caregivers within the local context.
Attitudes
Pediatric hospitalists should be able to:
- Recognize the value of seeking the research that supports clinical care decisions and how research fills knowledge gaps and challenges the field to advance.
- Realize the importance of informed consent for patient participation in clinical research.
- Reflect on the importance of patient assent, even in the presence of legal guardian informed consent, when involving children in clinical research.
- Exemplify highly ethical behaviors when promoting or participating in research studies.
- Realize the value of and exemplify a willingness to perform journal-requested peer review of manuscripts, conference abstracts, or other research-based work.
- Reflect on and provide support and education for patients and the family/caregivers on the benefits of research for hospitalized children.
Systems Organization and Improvement
In order to improve efficiency and quality within their organizations, pediatric hospitalists should:
- Lead, coordinate, or participate in interdisciplinary initiatives to develop and sustain participation of interdisciplinary teams in performance of research.
- Collaborate with colleagues, hospital administration, and community leaders for thoughtful application of research findings to improve systems of healthcare delivery.
- Lead, coordinate, or participate in national multi-center research efforts that improve the evidence base in inpatient pediatrics, within local context.
- Collaborate with leaders in the university department of pediatrics and school of medicine, hospital administration, and medical staff to encourage local hospital participation in national multi-center research efforts.
- Collaborate with research team members to educate colleagues, hospital staff, and others on the importance of research in improving child health outcomes.
1. Hulley SB, Cummins SR, Browner WS, Grady DG, Newman TB. Designing Clinical Research, 4th ed. Philadelphia, PA: Wolters Kluwer; 2013.
Introduction
Research is a rapidly growing aspect of inpatient medicine. The practice of evidence-based medicine and the acute need for more evidence on inpatient conditions require that pediatric hospitalists understand and participate in research related activities. Pediatric hospitalists’ role in research will vary depending on their setting and job description. This role may include many facets, from reviewing relevant patient-based articles, to participating in multi-institutional studies requiring enrollment of patients, to leading local or national studies. Pediatric hospitalists should have a basic understanding of research methods and processes in order to participate in and benefit from research. Pediatric hospitalists are well positioned to promote research to patients, the family/caregivers, colleagues, and other healthcare providers and through this, to contribute to the effective care of hospitalized patients.
Knowledge
Pediatric hospitalists should be able to:
- Compare and contrast the advantages and disadvantages of experimental (such as randomized control trials) and observational (such as descriptive, cohort, or case control) study designs, including meta-analyses and systematic reviews.
- Define common sources of bias, including information bias, selection bias, and uncontrolled confounding, and describe how each may impact a study.
- Define basic statistical terms such as sample, discrete and continuous data variables, measures of central tendency (mean, median, and mode), and variability (variance, standard deviation, range).
- List resources available to access current or proposed studies including The Pediatric Health Information System (PHIS), the Healthcare Cost and Utilization Project (HCUP), the Kids’ Inpatient Database (KID), clinicaltrials.gov, and others.
- Name potential research funding sources, such as the Agency for Healthcare Research and Quality (AHRQ), the National Institutes of Health (NIH), the Patient-Centered Outcomes Research Institute (PCORI), the Robert Wood Johnson Foundation, local and state funding sources, and others.
- Summarize the goals of pediatric hospital medicine-specific research networks, including the Pediatric Research in the Inpatient Setting (PRIS) network and the Value in Pediatrics (VIP) network.
- Discuss the basic resources commonly required to support research components, including data collection, data analysis, abstract and manuscript preparation, grant funding, and others.
- Review the aspects of the research process that relate to protection of participants, including informed consent and/or assent, the institutional review boards (IRB) review, and HIPAA (Health Insurance Portability and Accountability Act) forms.
- Discuss special protections needed when conducting research with vulnerable populations.
- Define “minimal risk” for a healthy child and for a child with an illness.
- Discuss why common training that addresses ethics, vulnerable populations, consenting, data safety, and other items is required prior to participating as a research team member for a research study.
- Compare and contrast the goals, intent, study focus, and IRB requirements for quality improvement studies from those of traditional clinical research.
- Cite the steps needed to obtain approval for a QI study within the local context.
- Compare and contrast the goals, intent, study focus, and IRB requirements for education studies to those of traditional clinical research.
- Cite the steps needed to obtain approval for a study focused on educational outcomes.
- List common barriers to implementation of clinical studies and describe the pediatric hospitalist’s role in overcoming these barriers.
Skills
Pediatric hospitalists should be able to:
- Utilize a format such as PICO (Population, Intervention, Comparison, Outcome) to generate an answerable patient-centered clinical question that is relevant to improving patient care.
- Demonstrate proficiency in systematic searching of the primary medical literature using online search engines.
- Perform critical appraisal of the literature, including identifying threats to study validity, determining if study subjects were similar to local patients, and determining if all clinically important outcomes were considered.
- Apply and integrate the results of studies to clinical practice.
- Determine if the likely benefits noted in a treatment study are worth the potential harm and cost.
- Determine whether a test noted in a diagnostic study is available, affordable, accurate, and precise in the present clinical setting and determine whether the results of the test will change the management of patients being treated.
- Determine if the magnitude of risk warrants an attempt to stop the exposure for a given study on harm.
- Identify if the results of a given study on disease prognosis will lead directly to selecting therapy and/or are useful for counseling patients.
- Participate in educating learners and junior faculty about research and research methodologies, within the local context.
- Determine the relevance of potential research studies with regards to impact on patient care.
- Perform effective informed consent or assent for patients participating in research studies, as appropriate.
- Identify and resolve conflict of interest or potential conflict of interest when participating in research studies.
- Demonstrate basic skills in acquiring, managing, and sharing data collected for research purposes in a responsible and professional manner.
- Adhere to standards for protecting confidentiality, avoiding unjustified exclusions, sharing data, and adhering to copyright law.
- Perform peer-review of a manuscript, abstract, or other research-based work, in collaboration with colleagues as appropriate.
- Demonstrate basic skills in communicating about research opportunities with patients and the family/caregivers within the local context.
Attitudes
Pediatric hospitalists should be able to:
- Recognize the value of seeking the research that supports clinical care decisions and how research fills knowledge gaps and challenges the field to advance.
- Realize the importance of informed consent for patient participation in clinical research.
- Reflect on the importance of patient assent, even in the presence of legal guardian informed consent, when involving children in clinical research.
- Exemplify highly ethical behaviors when promoting or participating in research studies.
- Realize the value of and exemplify a willingness to perform journal-requested peer review of manuscripts, conference abstracts, or other research-based work.
- Reflect on and provide support and education for patients and the family/caregivers on the benefits of research for hospitalized children.
Systems Organization and Improvement
In order to improve efficiency and quality within their organizations, pediatric hospitalists should:
- Lead, coordinate, or participate in interdisciplinary initiatives to develop and sustain participation of interdisciplinary teams in performance of research.
- Collaborate with colleagues, hospital administration, and community leaders for thoughtful application of research findings to improve systems of healthcare delivery.
- Lead, coordinate, or participate in national multi-center research efforts that improve the evidence base in inpatient pediatrics, within local context.
- Collaborate with leaders in the university department of pediatrics and school of medicine, hospital administration, and medical staff to encourage local hospital participation in national multi-center research efforts.
- Collaborate with research team members to educate colleagues, hospital staff, and others on the importance of research in improving child health outcomes.
Introduction
Research is a rapidly growing aspect of inpatient medicine. The practice of evidence-based medicine and the acute need for more evidence on inpatient conditions require that pediatric hospitalists understand and participate in research related activities. Pediatric hospitalists’ role in research will vary depending on their setting and job description. This role may include many facets, from reviewing relevant patient-based articles, to participating in multi-institutional studies requiring enrollment of patients, to leading local or national studies. Pediatric hospitalists should have a basic understanding of research methods and processes in order to participate in and benefit from research. Pediatric hospitalists are well positioned to promote research to patients, the family/caregivers, colleagues, and other healthcare providers and through this, to contribute to the effective care of hospitalized patients.
Knowledge
Pediatric hospitalists should be able to:
- Compare and contrast the advantages and disadvantages of experimental (such as randomized control trials) and observational (such as descriptive, cohort, or case control) study designs, including meta-analyses and systematic reviews.
- Define common sources of bias, including information bias, selection bias, and uncontrolled confounding, and describe how each may impact a study.
- Define basic statistical terms such as sample, discrete and continuous data variables, measures of central tendency (mean, median, and mode), and variability (variance, standard deviation, range).
- List resources available to access current or proposed studies including The Pediatric Health Information System (PHIS), the Healthcare Cost and Utilization Project (HCUP), the Kids’ Inpatient Database (KID), clinicaltrials.gov, and others.
- Name potential research funding sources, such as the Agency for Healthcare Research and Quality (AHRQ), the National Institutes of Health (NIH), the Patient-Centered Outcomes Research Institute (PCORI), the Robert Wood Johnson Foundation, local and state funding sources, and others.
- Summarize the goals of pediatric hospital medicine-specific research networks, including the Pediatric Research in the Inpatient Setting (PRIS) network and the Value in Pediatrics (VIP) network.
- Discuss the basic resources commonly required to support research components, including data collection, data analysis, abstract and manuscript preparation, grant funding, and others.
- Review the aspects of the research process that relate to protection of participants, including informed consent and/or assent, the institutional review boards (IRB) review, and HIPAA (Health Insurance Portability and Accountability Act) forms.
- Discuss special protections needed when conducting research with vulnerable populations.
- Define “minimal risk” for a healthy child and for a child with an illness.
- Discuss why common training that addresses ethics, vulnerable populations, consenting, data safety, and other items is required prior to participating as a research team member for a research study.
- Compare and contrast the goals, intent, study focus, and IRB requirements for quality improvement studies from those of traditional clinical research.
- Cite the steps needed to obtain approval for a QI study within the local context.
- Compare and contrast the goals, intent, study focus, and IRB requirements for education studies to those of traditional clinical research.
- Cite the steps needed to obtain approval for a study focused on educational outcomes.
- List common barriers to implementation of clinical studies and describe the pediatric hospitalist’s role in overcoming these barriers.
Skills
Pediatric hospitalists should be able to:
- Utilize a format such as PICO (Population, Intervention, Comparison, Outcome) to generate an answerable patient-centered clinical question that is relevant to improving patient care.
- Demonstrate proficiency in systematic searching of the primary medical literature using online search engines.
- Perform critical appraisal of the literature, including identifying threats to study validity, determining if study subjects were similar to local patients, and determining if all clinically important outcomes were considered.
- Apply and integrate the results of studies to clinical practice.
- Determine if the likely benefits noted in a treatment study are worth the potential harm and cost.
- Determine whether a test noted in a diagnostic study is available, affordable, accurate, and precise in the present clinical setting and determine whether the results of the test will change the management of patients being treated.
- Determine if the magnitude of risk warrants an attempt to stop the exposure for a given study on harm.
- Identify if the results of a given study on disease prognosis will lead directly to selecting therapy and/or are useful for counseling patients.
- Participate in educating learners and junior faculty about research and research methodologies, within the local context.
- Determine the relevance of potential research studies with regards to impact on patient care.
- Perform effective informed consent or assent for patients participating in research studies, as appropriate.
- Identify and resolve conflict of interest or potential conflict of interest when participating in research studies.
- Demonstrate basic skills in acquiring, managing, and sharing data collected for research purposes in a responsible and professional manner.
- Adhere to standards for protecting confidentiality, avoiding unjustified exclusions, sharing data, and adhering to copyright law.
- Perform peer-review of a manuscript, abstract, or other research-based work, in collaboration with colleagues as appropriate.
- Demonstrate basic skills in communicating about research opportunities with patients and the family/caregivers within the local context.
Attitudes
Pediatric hospitalists should be able to:
- Recognize the value of seeking the research that supports clinical care decisions and how research fills knowledge gaps and challenges the field to advance.
- Realize the importance of informed consent for patient participation in clinical research.
- Reflect on the importance of patient assent, even in the presence of legal guardian informed consent, when involving children in clinical research.
- Exemplify highly ethical behaviors when promoting or participating in research studies.
- Realize the value of and exemplify a willingness to perform journal-requested peer review of manuscripts, conference abstracts, or other research-based work.
- Reflect on and provide support and education for patients and the family/caregivers on the benefits of research for hospitalized children.
Systems Organization and Improvement
In order to improve efficiency and quality within their organizations, pediatric hospitalists should:
- Lead, coordinate, or participate in interdisciplinary initiatives to develop and sustain participation of interdisciplinary teams in performance of research.
- Collaborate with colleagues, hospital administration, and community leaders for thoughtful application of research findings to improve systems of healthcare delivery.
- Lead, coordinate, or participate in national multi-center research efforts that improve the evidence base in inpatient pediatrics, within local context.
- Collaborate with leaders in the university department of pediatrics and school of medicine, hospital administration, and medical staff to encourage local hospital participation in national multi-center research efforts.
- Collaborate with research team members to educate colleagues, hospital staff, and others on the importance of research in improving child health outcomes.
1. Hulley SB, Cummins SR, Browner WS, Grady DG, Newman TB. Designing Clinical Research, 4th ed. Philadelphia, PA: Wolters Kluwer; 2013.
1. Hulley SB, Cummins SR, Browner WS, Grady DG, Newman TB. Designing Clinical Research, 4th ed. Philadelphia, PA: Wolters Kluwer; 2013.
Development of the PRIS Network
Since the term hospitalist was coined in 1996,1 the field of hospital medicine has grown exponentially. Hospitalists are caring for increasing numbers of adultsincluding Medicare beneficiaries in hospitals across the United States.2 Pediatric hospital medicine has grown in parallel. By 1998, 50% of pediatric department chairs across the US and Canada had implemented hospitalist programs, with another 27% reporting they were soon to do so.3 A bit more than a decade later, pediatric hospitalists can be found in nearly every major academic medical center, and in a large proportion of community hospitals throughout the US and Canada.
In the past several years, major advances have begun to occur in the manner in which hospital medicine research is conducted. In this article, we will describe the manner in which pediatric hospital medicine research has advanced over the past several years, culminating in the conduct of several large multicenter research projects through the Pediatric Research in Inpatient Settings (PRIS) Network. We believe that lessons learned in the development of PRIS could help foster the growth of other current and future networks of hospitalist researchers, and lay the groundwork for national improvement efforts.
HOSPITAL MEDICINE RESEARCH: GROWTH AND DEVELOPMENT
In 2001, a small group of thought leaders in pediatric hospital medicine (see Acknowledgements) conceived the notion of starting a hospitalist research network, which they named the Pediatric Research in Inpatient Settings (PRIS) Network.4 PRIS was modeled in part after a successful pediatric primary care network.5 Since hospitalists in institutions across the country were being tasked to improve the care of hospitalized patients, and to lead diverse quality and safety initiatives, why not create a network to facilitate identification of high priority problems and evidence‐based approaches to them, and coordinate improvement efforts? The ambitious goal of the fledgling network was to conduct transformative research into inpatient healthcare delivery and discover both condition‐dependent and condition‐independent processes of care that were linked to patient outcomes.
PRIS began as (and remains) an open research networkfrom the outset, any hospitalist could join. The notion of this network, even in its earliest stages, was sufficiently appealing to professional societies that the Society of Hospital Medicine (SHM), the Academic Pediatric Association (APA), and the American Academy of Pediatrics (AAP) agreed to cosponsor the network, fostering its early growth. The community of pediatric hospitalists was tremendously supportive as well; over 300 hospitalists initially signed up to participate. Initial studies were generated through surveys of members, through which variability in systemic organization and variation in the management of clinical conditions and systems‐based issues across inpatient settings was identified and quantified.68
In the 2000s, as PRIS grew as a network, the research capacity of individuals within the field also grew. An increasing number of hospitalists began dedicating their academic careers to pursuing rigorous methodological training and conducting pediatric hospital medicine research. A series of studies began to emerge analyzing data from large administrative datasets that described the variation in hospital care (but lack clinical results and clinical outcomes outside of the hospital setting), such as the Pediatric Health Information Systems (PHIS) database operated by the Children's Hospital Association (formerly known as the Child Health Corporation of America).913 Pediatric hospital medicine fellowships began to appear,14 and over time, a cohort of hospitalist investigators with sufficient independence to mentor others arose.
THE REDESIGN OF PRIS
In 2009, a Pediatric Hospital Medicine Roundtable of 22 international leaders was convened under the guidance of SHM, APA, and AAP.15 This initiative, roughly a decade after the inception of the field, was critical to bringing pediatric hospitalist research and PRIS to the next level. It was recognized in that meeting that while PRIS had made a good start, it would not be possible to grow the network to the point of conducting top quality multicenter studies without the active involvement of a larger number of rigorously trained hospitalist researchers. To stimulate the network's growth, the existing PRIS Steering Committeea diverse group of clinical, educational, administrative, and research leaders in the fieldfacilitated the transfer of leadership to a new Executive Council led entirely by trained researchers (see Table 1), with the support of the APA. The Executive Council subsequently developed a series of standard operating procedures (see Table 2) that have created a transparent process to deal with important, but often difficult, academic issues that networks face.
|
| Published papers, total number of papers: 150 |
| Grants awarded, funding $3.7 million |
| Grants pending, funding $3.3 million |
| Research positions included director of research center, NIH study sections, national research committees, journal editorial experience |
| Mentors to junior faculty, fellows, and housestaff |
| However, no division chief or professor rank at the time of the executive council creation (this has since changed) |
|
| Mission |
| Vision |
| Values |
| Objectives (first 5 years) |
| Organizational structure (executive council, ex officio members, advisory group, staff and participant organizations/member hospitalist groups) |
| Authorship and publication |
| Institutional review board approval |
| Protocol selection and review |
| Network funding |
| Ancillary studies |
| Adverse event reporting |
| Site monitoring |
DEVELOPMENT OF MULTICENTER RESEARCH PROJECTS
The redesign of PRIS did not alter its objective: to build the evidence base regarding the optimal inpatient management of children. Evidence on how best to care for many pediatric conditions remains lacking, largely due to the facts that: a) death, the most definitive and readily measured of outcomes, is rare in pediatric hospitals; b) many pediatric conditions are relatively uncommon in any single hospital; and c) few validated, well‐developed metrics of inpatient pediatric quality exist.
As PRIS sought to launch multicenter studies of inpatient care quality, it continued to receive strong support from the APA, SHM, and AAP, and gained the support of a new partner, the Children's Hospital Association, which is comprised of a large group of children's hospitals across Canada and the US. The membership of PRIS grew to involve over 600 pediatric hospitalists from more than 75 hospitals.4 With a core group of funded hospitalist investigators, and strong support from partner organizations, the network sought and received funding for 3 major studies that are currently underway. Release of the federal government's Affordable Care Act and Comparative Effectiveness Research portfolio stimulated much of this work, stimulating the network to reach out to existing and new stakeholders and successfully compete for several multicenter studies.
Prioritization Project
Through its Prioritization Project ($1.6 million over 3 years, Children's Hospital Association), PRIS is using data on over 3.5 million hospitalizations in the PHIS database to identify conditions that are prevalent and costly, and whose management varies highly across institutions.16 After identifying the top ranked medical and surgical conditions for further study, the project is conducting drill downs in which the reasons for variation are being sought. By partnering with hospital and clinical leadership at these hospitals, and producing a data‐driven approach to prioritization, PRIS aims to conduct collaborative research and improvement work across hospitals that aim to understand and reduce the unwarranted variation in resource utilization for several of these conditions, and measure the impact of such efforts on patient and cost outcomes.
PHIS+
PHIS+ ($9 million over 3 years, Agency for Healthcare Research and Quality) is a project that is taking electronically stored laboratory, microbiology, and radiology data from 6 children's hospitals, with diverse electronic health record systems, to build a robust new database.17 The project also funds several comparative effectiveness projects (several of which are either high prevalence, high cost, or exhibit high variation in resource utilization, as demonstrated in the Prioritization Project) that are being carried out using this new database. This PHIS+ database will serve as an ongoing resource for hospitalist and subspecialist investigators interested in evaluating and improving the care of hospitalized children across multiple medical centers at once.
I‐PASS
Innovation in Pediatric Education (IIPE)‐PRIS Accelerating Safe Sign‐outs (I‐PASS) ($3 million over 3 years, Department of Health and Human Services) is a research and improvement project that is evaluating the effects on patient safety, resident experience, and diverse care processes of implementing a bundle of interventions designed to improve handoffs at change of shift.18, 19 It is one of the first multicenter educational improvement projects of its kind. Given the commonalities between change‐of‐shift handoffs in pediatrics and other fields, and the commonalities between different types of handoffs in the inpatient and outpatient setting, I‐PASS may yield communication and improvement lessons that extend beyond the confines of the study population itself.
The strategic focus of these 3 grants was to develop studies that are relevant for both the membership of practicing hospitalists and appealing to the stakeholders of the network. PRIS intends that these 3 projects will be but the first few in a long series of studies led by investigators nationwide who are interested in better understanding, and advancing the care of hospitalized children.
RELEVANCE TO OTHER NETWORKS
We believe that the story of PRIS' development, current studies, and future plans has relevance to other adult, as well as pediatric, hospital medicine networks (see Table 3). As in pediatrics, a growing group of midcareer adult hospital medicine investigators has emerged, with proven track records in attracting federal funding and conducting research germane to our field. Some have previously worked together on large‐scale multisite studies.2023 A core group have come together to form the HOspital MEdicine Reengineering Network (HOMERUN).24 HOMERUN has recently secured funding from the Association of American Medical Colleges (AAMC) for a project that is linking clinical data from several hospitals to a centralized database, a project analogous to PHIS+, and will allow for Comparative Effectiveness Research studies that have more accurate case ascertainment (by using clinical data to build cohorts) and ensuring additional power by securing a larger number of cases. Defining which clinical questions to address first will help establish this new entity as a leader in hospital medicine research. Attracting stakeholder involvement will help make these endeavors successful. In recent months, PRIS and HOMERUN jointly collaborated on the submission of a large Centers for Medicare and Medicaid Innovation (CMMI) proposal to extend the work of I‐PASS to include several internal medicine and additional pediatric resident and hospitalist care settings. Future collaborations between networks may help foster more rapid advances in care.
| Governance involves hospitalist investigators |
| In‐person governance meetings to ensure/gauge buy‐in |
| Stable infrastructure critical for success |
| Mentoring important for succession |
| Grants to fund large‐scale projects demonstrate track record for network |
| MembershipWhat do members want/need? |
Another pediatric hospitalist network has also emerged in the past few years, with a focus on quality improvement across inpatient pediatric settings, the Value in Pediatrics (VIP) Network.25 Although still early in its development, VIP has already successfully engaged in national quality improvement work regarding benchmarking care provided for children with bronchiolitis, reducing patient identification (ID) band errors, and improving discharge communications. VIP recently became part of the AAP's Quality Improvement Innovation Network (QuINN) group through which it is receiving infrastructure support.
As they develop, hospital medicine research and improvement networks will seek to systematically design and rigorously execute multicenter projects that provide answers to those clinical questions which practicing hospitalists face on a daily basis. As they do so, mentoring of both junior investigators and novice investigators will be necessary for the longevity of networks. To foster junior investigators, PRIS has undertaken a series of workshops presented at various national conferences, in addition to working with junior investigators directly on its currently funded studies.
CONCLUSION
Hospitalists' engagement in research and quality improvement networks builds upon their already successful engagement in clinical care, education, and quality improvement at a local level. A research and improvement mission that is tightly coupled with the day‐to‐day needs of these other important hospitalist activities creates a synergy with the potential to lead to transformative advances in patient care. If hospitalists can discover how best to deliver care, train the next generation of providers, and work to implement needed improvements at a local and national level, they will have an unprecedented opportunity to improve the care and health of children and adults.
Acknowledgements
The authors acknowledge the PRIS Network. They offer profound thanks to the members of the PRIS Steering Committee who founded the network and served throughout its initial 8 years (20012009), without whom the network would never have been launched: Mary Ottolini, Jack Percelay, Dan Rauch, Erin Stucky, and David Zipes (in addition to C.P.L.); and the current PRIS Executive Council who are leading the network: Patrick Conway, Ron Keren, Sanjay Mahant, Samir Shah, Tamara Simon, Joel Tieder, and Karen Wilson (in addition to C.P.L. and R.S.).
Note Added in Proof
Disclosures: I‐PASS is funded by grant 1R18AE00002901, from the Department of Health and Human Resources (DHHR). PHIS+ is funded by grant 1R01HSO986201, from the Agency for Healthcare Research and Quality (AHRQ). The Prioritization Project is funded by a grant from the Children's Hospital Association (CHA). The PRIS Network has received support from CHA, APA, AAP, and SHM. C.P.L. and R.S. are both Executive Council members of the PRIS Network and receive support from CHA.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335(7):514–517.
- ,,,.Growth in the care of older patients by hospitalists in the United States.N Engl J Med.2009;360(11):1102–1112.
- ,,,,,.Pediatric hospitalists in Canada and the United States: a survey of pediatric academic department chairs.Ambul Pediatr.2001;1(6):338–339.
- Pediatric Research in Inpatient Settings. Available at: http://www.prisnetwork.org. Accessed June 21, 2012.
- ,,, et al.Pediatric research in office settings (PROS): a national practice‐based research network to improve children's health care.Pediatrics.1998;102(6):1350–1357.
- ,,,,.Variation in pediatric hospitalists' use of proven and unproven therapies: a study from the Pediatric Research in Inpatient Settings (PRIS) network.J Hosp Med.2008;3(4):292–298.
- ,,,,,.Variations in management of common inpatient pediatric illnesses: hospitalists and community pediatricians.Pediatrics.2006;118(2):441–447.
- ,,, et al.Family‐centered rounds on pediatric wards: a PRIS network survey of US and Canadian hospitalists.Pediatrics.2010;126(1):37–43.
- ,,,,.Primary early thoracoscopy and reduction in length of hospital stay and additional procedures among children with complicated pneumonia: results of a multicenter retrospective cohort study.Arch Pediatr Adolesc Med.2008;162(7):675–681.
- ,,, et al.Infection rates following initial cerebrospinal fluid shunt placement across pediatric hospitals in the United States. Clinical article.J Neurosurg Pediatr.2009;4(2):156–165.
- ,,, et al.Reflux related hospital admissions after fundoplication in children with neurological impairment: retrospective cohort study.BMJ.2009;339:b4411.
- ,,.Pediatric hospital adherence to the standard of care for acute gastroenteritis.Pediatrics.2009;124(6):e1081–1087.
- ,,,,,.Prolonged intravenous therapy versus early transition to oral antimicrobial therapy for acute osteomyelitis in children.Pediatrics.2009;123(2):636–642.
- ,.Characteristics of pediatric hospital medicine fellowships and training programs.J Hosp Med.2009;4(3):157–163.
- ,,, et al.Pediatric hospital medicine: a strategic planning roundtable to chart the future.J Hosp Med.2012;7(4):329–334.
- ,,, et al.A novel method for prioritizating comparative effectiveness research topics.Arch Pediatr Adolesc Med. In press.
- ,,, et al.Federating clinical data from six pediatric hospitals: process and initial results from the PHIS+ Consortium. In:Improving Health: Informatics and IT Changing the World. Proceedings of the AMIA 2011 Annual Symposium,Washington, DC, October 22–26,2011:994–1003. Epub 2011 October 22.
- ,,,.Establishing a multisite education and research project requires leadership, expertise, collaboration, and an important aim.Pediatrics.2010;126(4):619–622.
- ,,,,,.I‐PASS, a mnemonic to standardize verbal handoffs.Pediatrics.2012;129(2):201–204.
- ,,, et al.Factors associated with discussion of care plans and code status at the time of hospital admission: results from the Multicenter Hospitalist Study.J Hosp Med.2008;3(6):437–445.
- ,,, et al.Do hospitalists affect clinical outcomes and efficiency for patients with acute upper gastrointestinal hemorrhage (UGIH)?J Hosp Med.2010;5(3):133–139.
- ,,, et al.Hospital readmission in general medicine patients: a prediction model.J Gen Intern Med.2010;25(3):211–219.
- ,,, et al.Code status discussions at hospital admission are not associated with patient and surrogate satisfaction with hospital care: results from the Multicenter Hospitalist Study.Am J Hosp Palliat Care.2011;28(2):102–108.
- HOMERUN. i2b2 Wiki, HOMERUN page. Available at: https://community.i2b2.org/wiki/display/HOMERUN/HOMERUN+Home. Accessed March 9, 2011.
- Value in Pediatrics Network Homepage. Available at: http://www.phm‐vipnetwork.com. Accessed June 21, 2012.
Since the term hospitalist was coined in 1996,1 the field of hospital medicine has grown exponentially. Hospitalists are caring for increasing numbers of adultsincluding Medicare beneficiaries in hospitals across the United States.2 Pediatric hospital medicine has grown in parallel. By 1998, 50% of pediatric department chairs across the US and Canada had implemented hospitalist programs, with another 27% reporting they were soon to do so.3 A bit more than a decade later, pediatric hospitalists can be found in nearly every major academic medical center, and in a large proportion of community hospitals throughout the US and Canada.
In the past several years, major advances have begun to occur in the manner in which hospital medicine research is conducted. In this article, we will describe the manner in which pediatric hospital medicine research has advanced over the past several years, culminating in the conduct of several large multicenter research projects through the Pediatric Research in Inpatient Settings (PRIS) Network. We believe that lessons learned in the development of PRIS could help foster the growth of other current and future networks of hospitalist researchers, and lay the groundwork for national improvement efforts.
HOSPITAL MEDICINE RESEARCH: GROWTH AND DEVELOPMENT
In 2001, a small group of thought leaders in pediatric hospital medicine (see Acknowledgements) conceived the notion of starting a hospitalist research network, which they named the Pediatric Research in Inpatient Settings (PRIS) Network.4 PRIS was modeled in part after a successful pediatric primary care network.5 Since hospitalists in institutions across the country were being tasked to improve the care of hospitalized patients, and to lead diverse quality and safety initiatives, why not create a network to facilitate identification of high priority problems and evidence‐based approaches to them, and coordinate improvement efforts? The ambitious goal of the fledgling network was to conduct transformative research into inpatient healthcare delivery and discover both condition‐dependent and condition‐independent processes of care that were linked to patient outcomes.
PRIS began as (and remains) an open research networkfrom the outset, any hospitalist could join. The notion of this network, even in its earliest stages, was sufficiently appealing to professional societies that the Society of Hospital Medicine (SHM), the Academic Pediatric Association (APA), and the American Academy of Pediatrics (AAP) agreed to cosponsor the network, fostering its early growth. The community of pediatric hospitalists was tremendously supportive as well; over 300 hospitalists initially signed up to participate. Initial studies were generated through surveys of members, through which variability in systemic organization and variation in the management of clinical conditions and systems‐based issues across inpatient settings was identified and quantified.68
In the 2000s, as PRIS grew as a network, the research capacity of individuals within the field also grew. An increasing number of hospitalists began dedicating their academic careers to pursuing rigorous methodological training and conducting pediatric hospital medicine research. A series of studies began to emerge analyzing data from large administrative datasets that described the variation in hospital care (but lack clinical results and clinical outcomes outside of the hospital setting), such as the Pediatric Health Information Systems (PHIS) database operated by the Children's Hospital Association (formerly known as the Child Health Corporation of America).913 Pediatric hospital medicine fellowships began to appear,14 and over time, a cohort of hospitalist investigators with sufficient independence to mentor others arose.
THE REDESIGN OF PRIS
In 2009, a Pediatric Hospital Medicine Roundtable of 22 international leaders was convened under the guidance of SHM, APA, and AAP.15 This initiative, roughly a decade after the inception of the field, was critical to bringing pediatric hospitalist research and PRIS to the next level. It was recognized in that meeting that while PRIS had made a good start, it would not be possible to grow the network to the point of conducting top quality multicenter studies without the active involvement of a larger number of rigorously trained hospitalist researchers. To stimulate the network's growth, the existing PRIS Steering Committeea diverse group of clinical, educational, administrative, and research leaders in the fieldfacilitated the transfer of leadership to a new Executive Council led entirely by trained researchers (see Table 1), with the support of the APA. The Executive Council subsequently developed a series of standard operating procedures (see Table 2) that have created a transparent process to deal with important, but often difficult, academic issues that networks face.
|
| Published papers, total number of papers: 150 |
| Grants awarded, funding $3.7 million |
| Grants pending, funding $3.3 million |
| Research positions included director of research center, NIH study sections, national research committees, journal editorial experience |
| Mentors to junior faculty, fellows, and housestaff |
| However, no division chief or professor rank at the time of the executive council creation (this has since changed) |
|
| Mission |
| Vision |
| Values |
| Objectives (first 5 years) |
| Organizational structure (executive council, ex officio members, advisory group, staff and participant organizations/member hospitalist groups) |
| Authorship and publication |
| Institutional review board approval |
| Protocol selection and review |
| Network funding |
| Ancillary studies |
| Adverse event reporting |
| Site monitoring |
DEVELOPMENT OF MULTICENTER RESEARCH PROJECTS
The redesign of PRIS did not alter its objective: to build the evidence base regarding the optimal inpatient management of children. Evidence on how best to care for many pediatric conditions remains lacking, largely due to the facts that: a) death, the most definitive and readily measured of outcomes, is rare in pediatric hospitals; b) many pediatric conditions are relatively uncommon in any single hospital; and c) few validated, well‐developed metrics of inpatient pediatric quality exist.
As PRIS sought to launch multicenter studies of inpatient care quality, it continued to receive strong support from the APA, SHM, and AAP, and gained the support of a new partner, the Children's Hospital Association, which is comprised of a large group of children's hospitals across Canada and the US. The membership of PRIS grew to involve over 600 pediatric hospitalists from more than 75 hospitals.4 With a core group of funded hospitalist investigators, and strong support from partner organizations, the network sought and received funding for 3 major studies that are currently underway. Release of the federal government's Affordable Care Act and Comparative Effectiveness Research portfolio stimulated much of this work, stimulating the network to reach out to existing and new stakeholders and successfully compete for several multicenter studies.
Prioritization Project
Through its Prioritization Project ($1.6 million over 3 years, Children's Hospital Association), PRIS is using data on over 3.5 million hospitalizations in the PHIS database to identify conditions that are prevalent and costly, and whose management varies highly across institutions.16 After identifying the top ranked medical and surgical conditions for further study, the project is conducting drill downs in which the reasons for variation are being sought. By partnering with hospital and clinical leadership at these hospitals, and producing a data‐driven approach to prioritization, PRIS aims to conduct collaborative research and improvement work across hospitals that aim to understand and reduce the unwarranted variation in resource utilization for several of these conditions, and measure the impact of such efforts on patient and cost outcomes.
PHIS+
PHIS+ ($9 million over 3 years, Agency for Healthcare Research and Quality) is a project that is taking electronically stored laboratory, microbiology, and radiology data from 6 children's hospitals, with diverse electronic health record systems, to build a robust new database.17 The project also funds several comparative effectiveness projects (several of which are either high prevalence, high cost, or exhibit high variation in resource utilization, as demonstrated in the Prioritization Project) that are being carried out using this new database. This PHIS+ database will serve as an ongoing resource for hospitalist and subspecialist investigators interested in evaluating and improving the care of hospitalized children across multiple medical centers at once.
I‐PASS
Innovation in Pediatric Education (IIPE)‐PRIS Accelerating Safe Sign‐outs (I‐PASS) ($3 million over 3 years, Department of Health and Human Services) is a research and improvement project that is evaluating the effects on patient safety, resident experience, and diverse care processes of implementing a bundle of interventions designed to improve handoffs at change of shift.18, 19 It is one of the first multicenter educational improvement projects of its kind. Given the commonalities between change‐of‐shift handoffs in pediatrics and other fields, and the commonalities between different types of handoffs in the inpatient and outpatient setting, I‐PASS may yield communication and improvement lessons that extend beyond the confines of the study population itself.
The strategic focus of these 3 grants was to develop studies that are relevant for both the membership of practicing hospitalists and appealing to the stakeholders of the network. PRIS intends that these 3 projects will be but the first few in a long series of studies led by investigators nationwide who are interested in better understanding, and advancing the care of hospitalized children.
RELEVANCE TO OTHER NETWORKS
We believe that the story of PRIS' development, current studies, and future plans has relevance to other adult, as well as pediatric, hospital medicine networks (see Table 3). As in pediatrics, a growing group of midcareer adult hospital medicine investigators has emerged, with proven track records in attracting federal funding and conducting research germane to our field. Some have previously worked together on large‐scale multisite studies.2023 A core group have come together to form the HOspital MEdicine Reengineering Network (HOMERUN).24 HOMERUN has recently secured funding from the Association of American Medical Colleges (AAMC) for a project that is linking clinical data from several hospitals to a centralized database, a project analogous to PHIS+, and will allow for Comparative Effectiveness Research studies that have more accurate case ascertainment (by using clinical data to build cohorts) and ensuring additional power by securing a larger number of cases. Defining which clinical questions to address first will help establish this new entity as a leader in hospital medicine research. Attracting stakeholder involvement will help make these endeavors successful. In recent months, PRIS and HOMERUN jointly collaborated on the submission of a large Centers for Medicare and Medicaid Innovation (CMMI) proposal to extend the work of I‐PASS to include several internal medicine and additional pediatric resident and hospitalist care settings. Future collaborations between networks may help foster more rapid advances in care.
| Governance involves hospitalist investigators |
| In‐person governance meetings to ensure/gauge buy‐in |
| Stable infrastructure critical for success |
| Mentoring important for succession |
| Grants to fund large‐scale projects demonstrate track record for network |
| MembershipWhat do members want/need? |
Another pediatric hospitalist network has also emerged in the past few years, with a focus on quality improvement across inpatient pediatric settings, the Value in Pediatrics (VIP) Network.25 Although still early in its development, VIP has already successfully engaged in national quality improvement work regarding benchmarking care provided for children with bronchiolitis, reducing patient identification (ID) band errors, and improving discharge communications. VIP recently became part of the AAP's Quality Improvement Innovation Network (QuINN) group through which it is receiving infrastructure support.
As they develop, hospital medicine research and improvement networks will seek to systematically design and rigorously execute multicenter projects that provide answers to those clinical questions which practicing hospitalists face on a daily basis. As they do so, mentoring of both junior investigators and novice investigators will be necessary for the longevity of networks. To foster junior investigators, PRIS has undertaken a series of workshops presented at various national conferences, in addition to working with junior investigators directly on its currently funded studies.
CONCLUSION
Hospitalists' engagement in research and quality improvement networks builds upon their already successful engagement in clinical care, education, and quality improvement at a local level. A research and improvement mission that is tightly coupled with the day‐to‐day needs of these other important hospitalist activities creates a synergy with the potential to lead to transformative advances in patient care. If hospitalists can discover how best to deliver care, train the next generation of providers, and work to implement needed improvements at a local and national level, they will have an unprecedented opportunity to improve the care and health of children and adults.
Acknowledgements
The authors acknowledge the PRIS Network. They offer profound thanks to the members of the PRIS Steering Committee who founded the network and served throughout its initial 8 years (20012009), without whom the network would never have been launched: Mary Ottolini, Jack Percelay, Dan Rauch, Erin Stucky, and David Zipes (in addition to C.P.L.); and the current PRIS Executive Council who are leading the network: Patrick Conway, Ron Keren, Sanjay Mahant, Samir Shah, Tamara Simon, Joel Tieder, and Karen Wilson (in addition to C.P.L. and R.S.).
Note Added in Proof
Disclosures: I‐PASS is funded by grant 1R18AE00002901, from the Department of Health and Human Resources (DHHR). PHIS+ is funded by grant 1R01HSO986201, from the Agency for Healthcare Research and Quality (AHRQ). The Prioritization Project is funded by a grant from the Children's Hospital Association (CHA). The PRIS Network has received support from CHA, APA, AAP, and SHM. C.P.L. and R.S. are both Executive Council members of the PRIS Network and receive support from CHA.
Since the term hospitalist was coined in 1996,1 the field of hospital medicine has grown exponentially. Hospitalists are caring for increasing numbers of adultsincluding Medicare beneficiaries in hospitals across the United States.2 Pediatric hospital medicine has grown in parallel. By 1998, 50% of pediatric department chairs across the US and Canada had implemented hospitalist programs, with another 27% reporting they were soon to do so.3 A bit more than a decade later, pediatric hospitalists can be found in nearly every major academic medical center, and in a large proportion of community hospitals throughout the US and Canada.
In the past several years, major advances have begun to occur in the manner in which hospital medicine research is conducted. In this article, we will describe the manner in which pediatric hospital medicine research has advanced over the past several years, culminating in the conduct of several large multicenter research projects through the Pediatric Research in Inpatient Settings (PRIS) Network. We believe that lessons learned in the development of PRIS could help foster the growth of other current and future networks of hospitalist researchers, and lay the groundwork for national improvement efforts.
HOSPITAL MEDICINE RESEARCH: GROWTH AND DEVELOPMENT
In 2001, a small group of thought leaders in pediatric hospital medicine (see Acknowledgements) conceived the notion of starting a hospitalist research network, which they named the Pediatric Research in Inpatient Settings (PRIS) Network.4 PRIS was modeled in part after a successful pediatric primary care network.5 Since hospitalists in institutions across the country were being tasked to improve the care of hospitalized patients, and to lead diverse quality and safety initiatives, why not create a network to facilitate identification of high priority problems and evidence‐based approaches to them, and coordinate improvement efforts? The ambitious goal of the fledgling network was to conduct transformative research into inpatient healthcare delivery and discover both condition‐dependent and condition‐independent processes of care that were linked to patient outcomes.
PRIS began as (and remains) an open research networkfrom the outset, any hospitalist could join. The notion of this network, even in its earliest stages, was sufficiently appealing to professional societies that the Society of Hospital Medicine (SHM), the Academic Pediatric Association (APA), and the American Academy of Pediatrics (AAP) agreed to cosponsor the network, fostering its early growth. The community of pediatric hospitalists was tremendously supportive as well; over 300 hospitalists initially signed up to participate. Initial studies were generated through surveys of members, through which variability in systemic organization and variation in the management of clinical conditions and systems‐based issues across inpatient settings was identified and quantified.68
In the 2000s, as PRIS grew as a network, the research capacity of individuals within the field also grew. An increasing number of hospitalists began dedicating their academic careers to pursuing rigorous methodological training and conducting pediatric hospital medicine research. A series of studies began to emerge analyzing data from large administrative datasets that described the variation in hospital care (but lack clinical results and clinical outcomes outside of the hospital setting), such as the Pediatric Health Information Systems (PHIS) database operated by the Children's Hospital Association (formerly known as the Child Health Corporation of America).913 Pediatric hospital medicine fellowships began to appear,14 and over time, a cohort of hospitalist investigators with sufficient independence to mentor others arose.
THE REDESIGN OF PRIS
In 2009, a Pediatric Hospital Medicine Roundtable of 22 international leaders was convened under the guidance of SHM, APA, and AAP.15 This initiative, roughly a decade after the inception of the field, was critical to bringing pediatric hospitalist research and PRIS to the next level. It was recognized in that meeting that while PRIS had made a good start, it would not be possible to grow the network to the point of conducting top quality multicenter studies without the active involvement of a larger number of rigorously trained hospitalist researchers. To stimulate the network's growth, the existing PRIS Steering Committeea diverse group of clinical, educational, administrative, and research leaders in the fieldfacilitated the transfer of leadership to a new Executive Council led entirely by trained researchers (see Table 1), with the support of the APA. The Executive Council subsequently developed a series of standard operating procedures (see Table 2) that have created a transparent process to deal with important, but often difficult, academic issues that networks face.
|
| Published papers, total number of papers: 150 |
| Grants awarded, funding $3.7 million |
| Grants pending, funding $3.3 million |
| Research positions included director of research center, NIH study sections, national research committees, journal editorial experience |
| Mentors to junior faculty, fellows, and housestaff |
| However, no division chief or professor rank at the time of the executive council creation (this has since changed) |
|
| Mission |
| Vision |
| Values |
| Objectives (first 5 years) |
| Organizational structure (executive council, ex officio members, advisory group, staff and participant organizations/member hospitalist groups) |
| Authorship and publication |
| Institutional review board approval |
| Protocol selection and review |
| Network funding |
| Ancillary studies |
| Adverse event reporting |
| Site monitoring |
DEVELOPMENT OF MULTICENTER RESEARCH PROJECTS
The redesign of PRIS did not alter its objective: to build the evidence base regarding the optimal inpatient management of children. Evidence on how best to care for many pediatric conditions remains lacking, largely due to the facts that: a) death, the most definitive and readily measured of outcomes, is rare in pediatric hospitals; b) many pediatric conditions are relatively uncommon in any single hospital; and c) few validated, well‐developed metrics of inpatient pediatric quality exist.
As PRIS sought to launch multicenter studies of inpatient care quality, it continued to receive strong support from the APA, SHM, and AAP, and gained the support of a new partner, the Children's Hospital Association, which is comprised of a large group of children's hospitals across Canada and the US. The membership of PRIS grew to involve over 600 pediatric hospitalists from more than 75 hospitals.4 With a core group of funded hospitalist investigators, and strong support from partner organizations, the network sought and received funding for 3 major studies that are currently underway. Release of the federal government's Affordable Care Act and Comparative Effectiveness Research portfolio stimulated much of this work, stimulating the network to reach out to existing and new stakeholders and successfully compete for several multicenter studies.
Prioritization Project
Through its Prioritization Project ($1.6 million over 3 years, Children's Hospital Association), PRIS is using data on over 3.5 million hospitalizations in the PHIS database to identify conditions that are prevalent and costly, and whose management varies highly across institutions.16 After identifying the top ranked medical and surgical conditions for further study, the project is conducting drill downs in which the reasons for variation are being sought. By partnering with hospital and clinical leadership at these hospitals, and producing a data‐driven approach to prioritization, PRIS aims to conduct collaborative research and improvement work across hospitals that aim to understand and reduce the unwarranted variation in resource utilization for several of these conditions, and measure the impact of such efforts on patient and cost outcomes.
PHIS+
PHIS+ ($9 million over 3 years, Agency for Healthcare Research and Quality) is a project that is taking electronically stored laboratory, microbiology, and radiology data from 6 children's hospitals, with diverse electronic health record systems, to build a robust new database.17 The project also funds several comparative effectiveness projects (several of which are either high prevalence, high cost, or exhibit high variation in resource utilization, as demonstrated in the Prioritization Project) that are being carried out using this new database. This PHIS+ database will serve as an ongoing resource for hospitalist and subspecialist investigators interested in evaluating and improving the care of hospitalized children across multiple medical centers at once.
I‐PASS
Innovation in Pediatric Education (IIPE)‐PRIS Accelerating Safe Sign‐outs (I‐PASS) ($3 million over 3 years, Department of Health and Human Services) is a research and improvement project that is evaluating the effects on patient safety, resident experience, and diverse care processes of implementing a bundle of interventions designed to improve handoffs at change of shift.18, 19 It is one of the first multicenter educational improvement projects of its kind. Given the commonalities between change‐of‐shift handoffs in pediatrics and other fields, and the commonalities between different types of handoffs in the inpatient and outpatient setting, I‐PASS may yield communication and improvement lessons that extend beyond the confines of the study population itself.
The strategic focus of these 3 grants was to develop studies that are relevant for both the membership of practicing hospitalists and appealing to the stakeholders of the network. PRIS intends that these 3 projects will be but the first few in a long series of studies led by investigators nationwide who are interested in better understanding, and advancing the care of hospitalized children.
RELEVANCE TO OTHER NETWORKS
We believe that the story of PRIS' development, current studies, and future plans has relevance to other adult, as well as pediatric, hospital medicine networks (see Table 3). As in pediatrics, a growing group of midcareer adult hospital medicine investigators has emerged, with proven track records in attracting federal funding and conducting research germane to our field. Some have previously worked together on large‐scale multisite studies.2023 A core group have come together to form the HOspital MEdicine Reengineering Network (HOMERUN).24 HOMERUN has recently secured funding from the Association of American Medical Colleges (AAMC) for a project that is linking clinical data from several hospitals to a centralized database, a project analogous to PHIS+, and will allow for Comparative Effectiveness Research studies that have more accurate case ascertainment (by using clinical data to build cohorts) and ensuring additional power by securing a larger number of cases. Defining which clinical questions to address first will help establish this new entity as a leader in hospital medicine research. Attracting stakeholder involvement will help make these endeavors successful. In recent months, PRIS and HOMERUN jointly collaborated on the submission of a large Centers for Medicare and Medicaid Innovation (CMMI) proposal to extend the work of I‐PASS to include several internal medicine and additional pediatric resident and hospitalist care settings. Future collaborations between networks may help foster more rapid advances in care.
| Governance involves hospitalist investigators |
| In‐person governance meetings to ensure/gauge buy‐in |
| Stable infrastructure critical for success |
| Mentoring important for succession |
| Grants to fund large‐scale projects demonstrate track record for network |
| MembershipWhat do members want/need? |
Another pediatric hospitalist network has also emerged in the past few years, with a focus on quality improvement across inpatient pediatric settings, the Value in Pediatrics (VIP) Network.25 Although still early in its development, VIP has already successfully engaged in national quality improvement work regarding benchmarking care provided for children with bronchiolitis, reducing patient identification (ID) band errors, and improving discharge communications. VIP recently became part of the AAP's Quality Improvement Innovation Network (QuINN) group through which it is receiving infrastructure support.
As they develop, hospital medicine research and improvement networks will seek to systematically design and rigorously execute multicenter projects that provide answers to those clinical questions which practicing hospitalists face on a daily basis. As they do so, mentoring of both junior investigators and novice investigators will be necessary for the longevity of networks. To foster junior investigators, PRIS has undertaken a series of workshops presented at various national conferences, in addition to working with junior investigators directly on its currently funded studies.
CONCLUSION
Hospitalists' engagement in research and quality improvement networks builds upon their already successful engagement in clinical care, education, and quality improvement at a local level. A research and improvement mission that is tightly coupled with the day‐to‐day needs of these other important hospitalist activities creates a synergy with the potential to lead to transformative advances in patient care. If hospitalists can discover how best to deliver care, train the next generation of providers, and work to implement needed improvements at a local and national level, they will have an unprecedented opportunity to improve the care and health of children and adults.
Acknowledgements
The authors acknowledge the PRIS Network. They offer profound thanks to the members of the PRIS Steering Committee who founded the network and served throughout its initial 8 years (20012009), without whom the network would never have been launched: Mary Ottolini, Jack Percelay, Dan Rauch, Erin Stucky, and David Zipes (in addition to C.P.L.); and the current PRIS Executive Council who are leading the network: Patrick Conway, Ron Keren, Sanjay Mahant, Samir Shah, Tamara Simon, Joel Tieder, and Karen Wilson (in addition to C.P.L. and R.S.).
Note Added in Proof
Disclosures: I‐PASS is funded by grant 1R18AE00002901, from the Department of Health and Human Resources (DHHR). PHIS+ is funded by grant 1R01HSO986201, from the Agency for Healthcare Research and Quality (AHRQ). The Prioritization Project is funded by a grant from the Children's Hospital Association (CHA). The PRIS Network has received support from CHA, APA, AAP, and SHM. C.P.L. and R.S. are both Executive Council members of the PRIS Network and receive support from CHA.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335(7):514–517.
- ,,,.Growth in the care of older patients by hospitalists in the United States.N Engl J Med.2009;360(11):1102–1112.
- ,,,,,.Pediatric hospitalists in Canada and the United States: a survey of pediatric academic department chairs.Ambul Pediatr.2001;1(6):338–339.
- Pediatric Research in Inpatient Settings. Available at: http://www.prisnetwork.org. Accessed June 21, 2012.
- ,,, et al.Pediatric research in office settings (PROS): a national practice‐based research network to improve children's health care.Pediatrics.1998;102(6):1350–1357.
- ,,,,.Variation in pediatric hospitalists' use of proven and unproven therapies: a study from the Pediatric Research in Inpatient Settings (PRIS) network.J Hosp Med.2008;3(4):292–298.
- ,,,,,.Variations in management of common inpatient pediatric illnesses: hospitalists and community pediatricians.Pediatrics.2006;118(2):441–447.
- ,,, et al.Family‐centered rounds on pediatric wards: a PRIS network survey of US and Canadian hospitalists.Pediatrics.2010;126(1):37–43.
- ,,,,.Primary early thoracoscopy and reduction in length of hospital stay and additional procedures among children with complicated pneumonia: results of a multicenter retrospective cohort study.Arch Pediatr Adolesc Med.2008;162(7):675–681.
- ,,, et al.Infection rates following initial cerebrospinal fluid shunt placement across pediatric hospitals in the United States. Clinical article.J Neurosurg Pediatr.2009;4(2):156–165.
- ,,, et al.Reflux related hospital admissions after fundoplication in children with neurological impairment: retrospective cohort study.BMJ.2009;339:b4411.
- ,,.Pediatric hospital adherence to the standard of care for acute gastroenteritis.Pediatrics.2009;124(6):e1081–1087.
- ,,,,,.Prolonged intravenous therapy versus early transition to oral antimicrobial therapy for acute osteomyelitis in children.Pediatrics.2009;123(2):636–642.
- ,.Characteristics of pediatric hospital medicine fellowships and training programs.J Hosp Med.2009;4(3):157–163.
- ,,, et al.Pediatric hospital medicine: a strategic planning roundtable to chart the future.J Hosp Med.2012;7(4):329–334.
- ,,, et al.A novel method for prioritizating comparative effectiveness research topics.Arch Pediatr Adolesc Med. In press.
- ,,, et al.Federating clinical data from six pediatric hospitals: process and initial results from the PHIS+ Consortium. In:Improving Health: Informatics and IT Changing the World. Proceedings of the AMIA 2011 Annual Symposium,Washington, DC, October 22–26,2011:994–1003. Epub 2011 October 22.
- ,,,.Establishing a multisite education and research project requires leadership, expertise, collaboration, and an important aim.Pediatrics.2010;126(4):619–622.
- ,,,,,.I‐PASS, a mnemonic to standardize verbal handoffs.Pediatrics.2012;129(2):201–204.
- ,,, et al.Factors associated with discussion of care plans and code status at the time of hospital admission: results from the Multicenter Hospitalist Study.J Hosp Med.2008;3(6):437–445.
- ,,, et al.Do hospitalists affect clinical outcomes and efficiency for patients with acute upper gastrointestinal hemorrhage (UGIH)?J Hosp Med.2010;5(3):133–139.
- ,,, et al.Hospital readmission in general medicine patients: a prediction model.J Gen Intern Med.2010;25(3):211–219.
- ,,, et al.Code status discussions at hospital admission are not associated with patient and surrogate satisfaction with hospital care: results from the Multicenter Hospitalist Study.Am J Hosp Palliat Care.2011;28(2):102–108.
- HOMERUN. i2b2 Wiki, HOMERUN page. Available at: https://community.i2b2.org/wiki/display/HOMERUN/HOMERUN+Home. Accessed March 9, 2011.
- Value in Pediatrics Network Homepage. Available at: http://www.phm‐vipnetwork.com. Accessed June 21, 2012.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335(7):514–517.
- ,,,.Growth in the care of older patients by hospitalists in the United States.N Engl J Med.2009;360(11):1102–1112.
- ,,,,,.Pediatric hospitalists in Canada and the United States: a survey of pediatric academic department chairs.Ambul Pediatr.2001;1(6):338–339.
- Pediatric Research in Inpatient Settings. Available at: http://www.prisnetwork.org. Accessed June 21, 2012.
- ,,, et al.Pediatric research in office settings (PROS): a national practice‐based research network to improve children's health care.Pediatrics.1998;102(6):1350–1357.
- ,,,,.Variation in pediatric hospitalists' use of proven and unproven therapies: a study from the Pediatric Research in Inpatient Settings (PRIS) network.J Hosp Med.2008;3(4):292–298.
- ,,,,,.Variations in management of common inpatient pediatric illnesses: hospitalists and community pediatricians.Pediatrics.2006;118(2):441–447.
- ,,, et al.Family‐centered rounds on pediatric wards: a PRIS network survey of US and Canadian hospitalists.Pediatrics.2010;126(1):37–43.
- ,,,,.Primary early thoracoscopy and reduction in length of hospital stay and additional procedures among children with complicated pneumonia: results of a multicenter retrospective cohort study.Arch Pediatr Adolesc Med.2008;162(7):675–681.
- ,,, et al.Infection rates following initial cerebrospinal fluid shunt placement across pediatric hospitals in the United States. Clinical article.J Neurosurg Pediatr.2009;4(2):156–165.
- ,,, et al.Reflux related hospital admissions after fundoplication in children with neurological impairment: retrospective cohort study.BMJ.2009;339:b4411.
- ,,.Pediatric hospital adherence to the standard of care for acute gastroenteritis.Pediatrics.2009;124(6):e1081–1087.
- ,,,,,.Prolonged intravenous therapy versus early transition to oral antimicrobial therapy for acute osteomyelitis in children.Pediatrics.2009;123(2):636–642.
- ,.Characteristics of pediatric hospital medicine fellowships and training programs.J Hosp Med.2009;4(3):157–163.
- ,,, et al.Pediatric hospital medicine: a strategic planning roundtable to chart the future.J Hosp Med.2012;7(4):329–334.
- ,,, et al.A novel method for prioritizating comparative effectiveness research topics.Arch Pediatr Adolesc Med. In press.
- ,,, et al.Federating clinical data from six pediatric hospitals: process and initial results from the PHIS+ Consortium. In:Improving Health: Informatics and IT Changing the World. Proceedings of the AMIA 2011 Annual Symposium,Washington, DC, October 22–26,2011:994–1003. Epub 2011 October 22.
- ,,,.Establishing a multisite education and research project requires leadership, expertise, collaboration, and an important aim.Pediatrics.2010;126(4):619–622.
- ,,,,,.I‐PASS, a mnemonic to standardize verbal handoffs.Pediatrics.2012;129(2):201–204.
- ,,, et al.Factors associated with discussion of care plans and code status at the time of hospital admission: results from the Multicenter Hospitalist Study.J Hosp Med.2008;3(6):437–445.
- ,,, et al.Do hospitalists affect clinical outcomes and efficiency for patients with acute upper gastrointestinal hemorrhage (UGIH)?J Hosp Med.2010;5(3):133–139.
- ,,, et al.Hospital readmission in general medicine patients: a prediction model.J Gen Intern Med.2010;25(3):211–219.
- ,,, et al.Code status discussions at hospital admission are not associated with patient and surrogate satisfaction with hospital care: results from the Multicenter Hospitalist Study.Am J Hosp Palliat Care.2011;28(2):102–108.
- HOMERUN. i2b2 Wiki, HOMERUN page. Available at: https://community.i2b2.org/wiki/display/HOMERUN/HOMERUN+Home. Accessed March 9, 2011.
- Value in Pediatrics Network Homepage. Available at: http://www.phm‐vipnetwork.com. Accessed June 21, 2012.
Adverse GERD Outcomes Rare After ALTE
Apparent life‐threatening events (ALTEs) are frightening for the parent/guardian and represent a challenge for the healthcare provider. ALTEs are defined as worrisome episodes of any combination of apnea, color change, change in muscle tone, choking or gagging.1 ALTEs account for 0.6% to 0.8% of emergency department (ED) visits for children <12 months old,2, 3 have an average length of stay (LOS) of 4.4 days and an average cost of $15,000 per hospitalization.4
Gastroesophageal reflux disease (GERD) is common in infancy11 and also is the most commonly (in 31%55% of ALTE cases) attributed cause of ALTE.2, 4, 5 It has been speculated that chemosensitivity to gastric acid results in laryngospasm, bronchospasm, and apnea. However, several small studies have failed to prove a causal link between reflux episodes and apnea.69 Furthermore, although consensus guidelines for GERD have been developed,14 the clinical use of testing for GERD remains highly variable. A study of infants discharged with an ALTE (n = 12,067) from 36 children's hospitals in the United States revealed extensive variability in the use of pH probes and upper gastrointestinal x‐ray series to diagnose GERD.4
The incidence of adverse outcomes associated with GERD after an ALTE remains unknown. It is also unknown whether an association exists between long‐term gastrointestinal (GI) outcomes and testing demonstrative of GERD or a diagnosis of GERD during hospitalization for ALTE. The primary objective of our study was to determine, in patients with an ALTE, the adverse outcomes associated with GERD (failure‐to‐thrive, aspiration pneumonia, and/or anti‐reflux surgery), the incidence of readmission for second ALTE, and death. Our secondary objective was to determine risk factors for adverse outcomes associated with GERD following an ALTE.
METHODS
Design
This was a retrospective cohort study. We reviewed electronic and paper medical charts of all infants <12 months of age admitted for ALTE between January 1, 1999 and December 31, 2003 to Primary Children's Medical Center in Salt Lake City, UT, which serves as the tertiary pediatric center for >1 million children and the primary facility for >270,000 children in Salt Lake County, UT.10 Primary Children's Medical Center is operated by a vertically integrated not‐for‐profit healthcare system (Intermountain Healthcare), which has 20 affiliated hospitals and EDs. The study was approved by the institutional review boards of the University of Utah and Intermountain Healthcare, and the privacy board of Intermountain Healthcare.
Participants
Patients were included if a computer search of ED chief complaint or hospital discharge diagnoses found one or more of the following keywords (or corresponding International Classification of Diseases, Ninth Revision [ICD‐9] codes if applicable): ALTE, altered mental status, apnea, breath‐holding spell, choking, GERD, hypotonia, lethargy, other convulsions, other neurologic diagnosis, other respiratory diagnosis, pallor, seizures, sleep apnea, stiff, syncope, and unresponsiveness. These diagnoses were chosen as potential proxy diagnoses or codes for possible ALTE, as ALTE did not have a corresponding ICD‐9 code at the time of this study.
Detailed review of the medical record included infants who were <12 months old at admission with a history consistent with ALTE, defined as an episode frightening to the observer with any combination of apnea, color change, change in muscle tone, choking, or gagging. Infants were excluded from the study if they had a previously documented underlying medical condition to explain the ALTE (such as a known seizure disorder) or had a clearly apparent diagnosis upon initial history and physical examination (such as bronchiolitis diagnosed in the emergency department) that would explain the event. Patients with unstable vital signs (eg, hypotension), trauma clearly apparent on admission, documented medication dosing error, or febrile seizure were also excluded. A complete list of exclusion criteria is found in Figure 1.
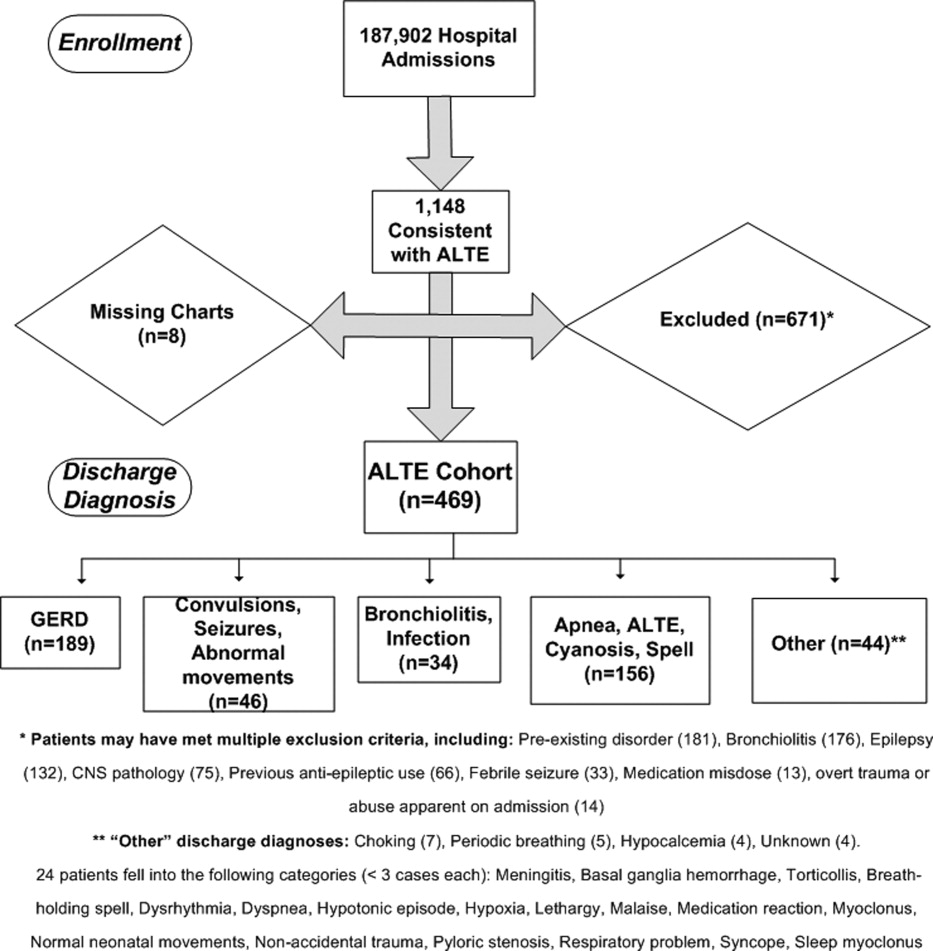
All hospital admissions, ED visits, and Pediatric GI clinic notes were reviewed for adverse outcomes associated with GERD, second ALTE admission, and death. The follow‐up time period included the original enrollment period (January 1, 1999 through December 31, 2003) through August 31, 2009.
Outcomes
Adverse outcomes associated with GERD were defined as aspiration pneumonia, failure‐to‐thrive (FTT; either admission or discharge diagnosis of FTT at another hospitalization, or follow‐up to gastroenterology clinic for FTT) and/or anti‐reflux surgery (Nissen fundoplication or gastrojejunal tube placement) as these are potential clinical consequences of having severe and uncontrolled GERD. We further collected readmission data for a second ALTE. Deaths and the attributed reasons were also collected.
Risk Factors
Potential risk factors for adverse outcomes associated with GERD (all during index hospitalization) included: age; prematurity; gender; previous event (as described by the parent, without previous ALTE hospitalization); primary discharge diagnosis of GERD; testing positive for reflux on index ALTE admission (upper GI x‐ray series, esophageal pH probe, swallow study, endoscopy, and/or consultation of pediatric gastroenterologist with results or assessments indicating gastroesophageal reflux); any anti‐reflux medication prescribed upon discharge; and LOS. We also considered diagnosis of neurologic impairment during follow‐up, which was defined as seizures or diagnosis of developmental delay from any etiology not recognized on index ALTE admission. We examined these risk factors as we postulated they might indicate higher risk for both ALTE and adverse outcomes associated with GERD, or might indicate a higher severity of initial event.
Analyses
Summary statistics were performed for adverse outcomes associated with GERD, readmission, and death. Univariate analyses were performed for risk factors using chi‐square tests for dichotomous predictors and Wilcoxon rank sum tests for nonparametric continuous predictors for any of the 3 adverse outcomes (FTT, aspiration pneumonia [AP], and/or anti‐reflux surgery) associated with GERD. All analyses were performed using SAS 9.13 (Carey, NC).
RESULTS
Eleven hundred forty‐eight infants with ALTE met inclusion criteria, from 187,903 patients meeting initial search criteria. Six hundred seventy‐one patients were excluded and 8 patients had missing charts. The study population of the 469‐patient cohort is shown in Figure 1.
Demographics are displayed in Table 1. The mean age was 65 days. One hundred three (22%) were premature. One hundred eighty‐nine patients (40%) had a primary discharge diagnosis of GERD; details of the diagnoses for the remaining patients are in Figure 1. Median length of follow‐up for the cohort was 7.8 years. The entire study period was 10.7 years.
| ALTE Cohort | |
|---|---|
| N = 469 | |
| |
| Female | 233 (49.7%) |
| Race | |
| Caucasian | 371 (79.1%) |
| Hispanic | 64 (13.6%) |
| Pacific Islander | 6 (1.3%) |
| Black | 4 (0.8%) |
| Other/unknown | 24 (5.1%) |
| Mean age in days (SD) | 65.2 (69.5) |
| Prematurity | 103 (22%) |
| Underwent testing for gastroesophageal reflux | 214 (45.6%) |
| Discharged on anti‐reflux medication | 238 (50.7%) |
| Previous event | 127 (27.1%) |
| Mean length of stay in days (SD) | 2.4 (2.4) |
| Later neurologic impairment (seizures or developmental delay) | 23 (4.9%) |
| Primary discharge diagnosis of GERD | 189 (40%) |
Eighteen patients (3.8%) had an adverse outcome associated with GERD. Four (0.9%) had aspiration pneumonia, 9 (1.9%) had failure‐to‐thrive, and 7 (1.5%) had a Nissen fundoplication (no patients had a gastrojejunal tube placed). Five patients had a gastrostomy tube placed at the time of fundoplication. Two patients had more than 1 adverse GI outcome; 1 patient had aspiration pneumonia and another had failure‐to‐thrive prior to their Nissen fundoplications.
Fifty‐six patients (11.9%) were readmitted for a second ALTE. Median time from index ALTE to second ALTE admission was 16.5 days (interquartile range: Q1, 8Q3, 32). Two (0.4%) patients died. Both (occurring at 18 months and 5.5 years after the initial ALTE hospitalization) were related to the children first developing seizure disorders and severe developmental delay. Neither of the patients who died had an index discharge diagnoses of GERD.
There was no significance of the following variables in predicting adverse outcomes associated with GERD: age, prematurity, gender, previous event, testing positive for reflux, primary discharge diagnosis of GERD, or discharge on anti‐reflux medications (see Table 2). Patients with adverse outcomes associated with GERD had longer mean LOS on the index ALTE hospitalization (4.3 days vs 2.4 days; P = 0.03) and a higher rate of neurologic impairment diagnosed in follow‐up (16.7% vs 4.4%; P = 0.02) than patients without long‐term adverse GI outcomes. Patients with neurological impairment diagnosed in follow‐up were more likely to eventually develop an adverse outcome associated with GERD, compared to patients without neurological impairment (odds ratio 8.4; 95% confidence interval 1.1516.1).
| AP, FTT, or Surgery | No Long‐Term Adverse GI Outcome | P Value | |
|---|---|---|---|
| N = 18 | N = 451 | ||
| |||
| Mean age in days (SD) | 51.9 (76.4) | 65.8 (69.3) | 0.27 |
| Prematurity | 1 (5.6%) | 102 (22.6%) | 0.08 |
| Male gender | 12 (66.7%) | 220 (48.8%) | 0.14 |
| Previous ALTE‐like event (no hospitalization) | 8 (44.4%) | 119 (26.4%) | 0.09 |
| Testing positive for reflux | 9 (50%) | 177 (39.3%) | 0.36 |
| Discharge diagnosis GERD | 9 (50%) | 180 (39.9%) | 0.39 |
| Discharged on anti‐reflux medication | 9 (50%) | 229 (50.7%) | 0.95 |
| Mean length of stay in days (SD) | 4.3 (4.7) | 2.4 (2.3) | 0.03 |
| Neurologic impairment diagnosed in follow‐up | 3 (16.7%) | 20 (4.4%) | 0.02 |
DISCUSSION
Our study had 2 main findings. First, infants admitted for an ALTE had a low percentage (3.8%) of adverse outcomes associated with GERD. Review of the literature provides little context to interpret this percentage. One study reports 9 per 100,000 of the general population <18 years of age having anti‐reflux surgery.24 The percentage of adverse outcomes associated with GERD converted to a rate in our study would likely reflect the bias of our center serving as a referral population for Utah and 5 surrounding states. Furthermore, there may be an additional bias of ALTE being a potential indication for anti‐reflux surgery for some clinicians, as well as confounding additional diagnoses (such as later neurologic impairment which might independently increase risk of study outcomes).
The second main finding of our study is that the development of neurologic impairment was predictive of developing adverse GI outcomes. As previous studies have shown that neurological impairment cannot be predicted during the initial ALTE hospitalization,14 adverse outcomes associated with GERD are similarly not predictable with current clinical approaches. Furthermore, the exact nature of the relationship between neurologic impairment and ALTEs remains unclear. While previous studies have described the increased prevalence of adverse neurological outcomes (such as seizures, developmental delay) in children who have had an ALTE, it is unclear what the precipitating reason for ALTE is in these infants (seizure, central apnea, GERD, etc).14
There is ongoing debate in the literature surrounding the optimal diagnosis of GERD in infants with ALTE. Recent guidelines state that investigations aimed to prove GERD causing an ALTE should include pH probe or impedance monitor testing, in combination with a sleep study, and discourages a GERD diagnosis based on upper GI‐series alone.14 Given the low use of pH probe and impedance monitoring at our institution during the study period (86% of the patients who had GI‐related testing had an upper GI‐series), we did not attempt to find the sensitivity or specificity of the different GI testing modalities for GERD in the setting of ALTE. The high use of upper‐GI series is not unique to our institutionone large study examining practice variation from 12,067 ALTE admissions in 36 children's hospitals, with 36.9% of infants (n = 4453) having a primary discharge diagnosis of gastroesophageal reflux, revealed that only 8.9% (SD 28%) received an esophageal pH probe, while 25.6% (SD 43.6%) had an upper GI‐series or swallow study.4
Given the difficulties in assigning a GERD diagnosis for ALTE infants, we focused on the long‐term adverse outcomes associated with GERD for the entire ALTE infant cohort. The 3 adverse outcomes we chose deserve some mention. Aspiration pneumonia is generally due to either primary aspiration (from dysfunctional swallowing) or secondary aspiration (from GERD). Failure‐to‐thrive can be due to ongoing GERD. Anti‐reflux surgery is often performed for severe GERD. While we believe that a prospective study with better diagnostic evaluations for GERD and apnea (such as pH probe or impedance monitor in combination with a sleep study) might help elucidate the unclear relationship between reflux episodes and ALTE, the low percentage of adverse outcomes associated with GERD after ALTE may suggest that such a study would be difficult both in terms of sample size and an unnecessary use of resources.
We also found that a high percentage (11.9%) of all patients had readmission for a second ALTE. This was substantially higher than the 2.5% readmission rate for second ALTE reported in a previous study of short‐term follow‐up (30 days).4 The number of readmissions in our study might be higher due to our comprehensive follow‐up, both in length of time and number of additional EDs and hospitals captured. Unfortunately, the retrospective nature of our study makes it difficult to determine if any interventions (prescription of anti‐reflux medication, education on reflux precautions) impacted the rate of readmission, as compliance was not measurable. Further studies should address why patients return with recurrent ALTE.
Interestingly, several potential risk factors did not predict long‐term adverse GI outcomes. For example, prematurity, a discharge diagnosis of GERD, or prescription of an anti‐reflux medication, were not associated with adverse GI outcomes. These findings support the concept that a diagnosis of GERD, at least as is commonly applied, is not meaningful in the setting of an ALTE. We did find associations with longer length of stay (LOS), and with eventual development of neurological impairment. Longer LOS might be a proxy for other subtle predictors that could influence adverse outcomes, such as requiring additional diagnostic tests prolonging hospitalization, or continued ALTEs while inpatient. The neurologic outcomes of patients with ALTE have been previously published, and the strong correlation between neurologic impairment and GERD has been well described.14, 25
There are several strengths of this study. This is the first study, to our knowledge, to look at adverse outcomes associated with GERD following ALTE, despite GERD being the most commonly attributed cause. The use of Intermountain Healthcare's electronic medical record system allowed for comprehensive tracking, over an extensive follow‐up period (median of 7.8 years), across 20 hospitals and EDs which care for the vast majority of pediatric patients in Utah. Finally, this large cohort of ALTE patients used clinical data from medical records and not only administrative data.
There are limitations of this study. This is a retrospective cohort study. Some of our study outcomes may be a result of pathophysiology other than GERD and, conversely, GERD may be a result of other issues (neurologic impairment). The small sample size and low percentage of the study outcomes make it possible that we did not detect true risk factors. Patients were lost to follow‐up if they moved or presented to a hospital not within the Intermountain Healthcare system. This study has slightly different patient numbers from 3 previously published studies for different outcomes on this cohort, as exclusion criteria for the different cohorts were different.14, 26, 27 Six patients had only their electronic medical record reviewed because the paper chart was missing.
IMPLICATIONS
The results of this study extend previous work of various outcomes regarding well‐appearing infants following an ALTE.14, 26, 27 In these studies, 3.9% and 3% were ultimately diagnosed with epilepsy and developmental delay, respectively; 1.4% were diagnosed with abusive head trauma; and 0.6% required otolaryngologic surgical intervention. In these previous studies, there were few predictors of these outcomes, with testing demonstrating largely normal results during the index ALTE admission.
Our study helps clinicians place the outcomes of aspiration pneumonia, failure‐to‐thrive, and anti‐reflux surgery into the context of these other studies when discharging infants from the hospital after an ALTE. Collectively, these studies provide clinicians with the information that, in the setting of a well‐appearing infant, few diagnostic tests in their ALTE patients will yield a definitive diagnosis. Ultimately, close follow‐up with further investigations if symptoms recur will be an important part of diagnosing the etiology of the ALTE in these infants.
We found that well‐appearing infants with ALTE, regardless of attributed cause, are at low risk for adverse outcomes associated with GERD. Only the eventual development of neurologic impairment or an increased length of stay during index ALTE hospitalization was found to be predictive of these outcomes.
Acknowledgements
The following individuals have made substantive intellectual contributions to this study: conception and design (G.Z., J.L.B., W.D.J., C.G.M., R.S.), acquisition of data (G.Z., J.L.B.), analysis (G.Z., RS) and interpretation of data (G.Z., J.L.B., W.D.J., C.G.M., R.S.). In addition, all listed authors have contributed to either drafting the article or revising it critically for important intellectual content. Finally, all listed authors have given final approval of this version submitted for publication. The authors also acknowledge Chelsea Welch for her assistance in data collection.
Disclosures: This study was presented in part at the national Pediatric Academic Societies meetings in Vancouver, Canada, May 2010 and in Denver, CO, May 2011. This study was supported by a National Institutes of Child Health and Human Development (NICHD) grant for Dr Srivastava (K23 HD052553), and a National Institute on Drug Abuse (NIDA) grant for Dr Bonkowsky (K08 DA24753). This research was supported in part by the Children's Health Research Center, University of Utah. There are no conflicts of interest.
- Infantile apnea and home monitoring.NIH Consensus Statement 1986 Sep 29‐Oct 1.Pediatrics.1987;79(2):292–299.
- ,.Causes of apparent life threatening events in infants: a systematic review.Arch Dis Child.2004;89:1043–1048.
- ,.Parental reported apnea, admissions to hospital and sudden infant death syndrome.Acta Paediatr.2001;90(4):417–422.
- ,,,.Variation in inpatient resource utilization and management of apparent life‐threatening events.J Pediatr.2008;152(5):629–635.
- ,,,,,.Discharge diagnoses in infants with apparent life‐threatening event.Pediatr Int.2003;45:560–563.
- ,,,,,.Lack of temporal relation between acid reflux in the proximal oesophagus and cardiorespiratory events in sleeping infants.Eur J Pediatr.1992;151(3):208–212.
- ,,, et al.Patterns of gastroesophageal reflux (GER) in patients with apparent life‐threatening events.J Pediatr Gastroenterol Nutr.1989;8(2):157–160.
- ,,, et al.Characteristics of continuous esophageal pH‐metering in infants with gastroesophageal reflux and apparent life‐threatening events.Eur J Pediatr Surg.1995;5(3);136–138.
- ,,,,.Apnea is not prolonged by acid gastroesophageal reflux in preterm infants.Pediatrics.2005;116:1059–1063.
- ,.Organizational responses to managed care: issues for academic health centers and implications for pediatric programs.Pediatrics.1998;101(4):805–811.
- ,,, et al.Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN).J Pediatr Gastroenterol Nutr.2009;49:498–547.
- ,,, et al.Prevalence and natural history of gastroesophageal reflux: pediatric prospective study.Pediatrics.2009;123(3):779–783.
- ,.Systematic review: the estra‐oesophageal symptoms of gastro‐oesophageal reflux disease in children.Aliment Pharmacol Ther.2009;29:258–272.
- ,,,.Death, child abuse, and adverse neurological outcomes of infants after an apparent life‐threatening event.Pediatrics.2008;122:125–131.
- ,,, et al.Abusive head injury as a cause of apparent life‐threatening events in infancy.Arch Pediatr Adolesc Med.2003;157:1011–1015.
- ,,.Accidental and nonaccidental poisonings as a cause of apparent life‐threatening events in infants.Pediatrics.2008;122:e359–e362.
- ,,,.Yield of diagnostic testing in infants who have had an apparent life‐threatening event.Pediatrics.2005;115:885–893.
- ,,, et al.Guidelines for evaluation and treatment of gastroesophageal reflux in infants and children: recommendations of the North American Society for Pediatric Gastroenterology and Nutrition.J Pediatr Gastroenterol Nutr.2001;32(suppl 2):S1–S31.
- ,,,.Comparison of barium radiology with esophageal pH monitoring in the diagnosis of gastroesophageal reflux disease.Am J Gastroenterol.1996;91(6):1181–1195.
- ,,, et al.Gastroesophageal reflux disease: correlation of esophageal pH testing and radiographic finding.Radiology.1992;185:483–486.
- ,,, et al.Gastro‐esophageal reflux demonstrated by radiography in infants less than 1 year of age. Comparison with pH monitoring.Acta Radiol.2003;44:136–138.
- ,,, et al.Double‐blind placebo controlled trial of omeprazole in irritable infants with gastroesophageal reflux.J Pediatr.2003;143:219–223.
- ,,, et al.Clinical predictors of pathological gastro‐oesophageal reflux in infants with persistant distress.J Paediatr Child Health.2006;42:134–139.
- ,,.National trends in the use of anti‐reflux procedures for children.Pediatrics.2006;118:1828–1835.
- ,,, et al.Prevalence and severity of feeding and nutritional problems in children with neurological impairment: Oxford feeding study.Dev Med Child Neurol.2000;42:674–680.
- ,,.Abusive head trauma in children presenting with an apparent life‐threatening event.J Pediatr.2010;157(5):821–825.
- ,,.Usefulness of airway evaluation in children initially seen with apparent life‐threatening event.Arch Otolaryngol Head Neck Surg.2011;137(4):359–362.
Apparent life‐threatening events (ALTEs) are frightening for the parent/guardian and represent a challenge for the healthcare provider. ALTEs are defined as worrisome episodes of any combination of apnea, color change, change in muscle tone, choking or gagging.1 ALTEs account for 0.6% to 0.8% of emergency department (ED) visits for children <12 months old,2, 3 have an average length of stay (LOS) of 4.4 days and an average cost of $15,000 per hospitalization.4
Gastroesophageal reflux disease (GERD) is common in infancy11 and also is the most commonly (in 31%55% of ALTE cases) attributed cause of ALTE.2, 4, 5 It has been speculated that chemosensitivity to gastric acid results in laryngospasm, bronchospasm, and apnea. However, several small studies have failed to prove a causal link between reflux episodes and apnea.69 Furthermore, although consensus guidelines for GERD have been developed,14 the clinical use of testing for GERD remains highly variable. A study of infants discharged with an ALTE (n = 12,067) from 36 children's hospitals in the United States revealed extensive variability in the use of pH probes and upper gastrointestinal x‐ray series to diagnose GERD.4
The incidence of adverse outcomes associated with GERD after an ALTE remains unknown. It is also unknown whether an association exists between long‐term gastrointestinal (GI) outcomes and testing demonstrative of GERD or a diagnosis of GERD during hospitalization for ALTE. The primary objective of our study was to determine, in patients with an ALTE, the adverse outcomes associated with GERD (failure‐to‐thrive, aspiration pneumonia, and/or anti‐reflux surgery), the incidence of readmission for second ALTE, and death. Our secondary objective was to determine risk factors for adverse outcomes associated with GERD following an ALTE.
METHODS
Design
This was a retrospective cohort study. We reviewed electronic and paper medical charts of all infants <12 months of age admitted for ALTE between January 1, 1999 and December 31, 2003 to Primary Children's Medical Center in Salt Lake City, UT, which serves as the tertiary pediatric center for >1 million children and the primary facility for >270,000 children in Salt Lake County, UT.10 Primary Children's Medical Center is operated by a vertically integrated not‐for‐profit healthcare system (Intermountain Healthcare), which has 20 affiliated hospitals and EDs. The study was approved by the institutional review boards of the University of Utah and Intermountain Healthcare, and the privacy board of Intermountain Healthcare.
Participants
Patients were included if a computer search of ED chief complaint or hospital discharge diagnoses found one or more of the following keywords (or corresponding International Classification of Diseases, Ninth Revision [ICD‐9] codes if applicable): ALTE, altered mental status, apnea, breath‐holding spell, choking, GERD, hypotonia, lethargy, other convulsions, other neurologic diagnosis, other respiratory diagnosis, pallor, seizures, sleep apnea, stiff, syncope, and unresponsiveness. These diagnoses were chosen as potential proxy diagnoses or codes for possible ALTE, as ALTE did not have a corresponding ICD‐9 code at the time of this study.
Detailed review of the medical record included infants who were <12 months old at admission with a history consistent with ALTE, defined as an episode frightening to the observer with any combination of apnea, color change, change in muscle tone, choking, or gagging. Infants were excluded from the study if they had a previously documented underlying medical condition to explain the ALTE (such as a known seizure disorder) or had a clearly apparent diagnosis upon initial history and physical examination (such as bronchiolitis diagnosed in the emergency department) that would explain the event. Patients with unstable vital signs (eg, hypotension), trauma clearly apparent on admission, documented medication dosing error, or febrile seizure were also excluded. A complete list of exclusion criteria is found in Figure 1.

All hospital admissions, ED visits, and Pediatric GI clinic notes were reviewed for adverse outcomes associated with GERD, second ALTE admission, and death. The follow‐up time period included the original enrollment period (January 1, 1999 through December 31, 2003) through August 31, 2009.
Outcomes
Adverse outcomes associated with GERD were defined as aspiration pneumonia, failure‐to‐thrive (FTT; either admission or discharge diagnosis of FTT at another hospitalization, or follow‐up to gastroenterology clinic for FTT) and/or anti‐reflux surgery (Nissen fundoplication or gastrojejunal tube placement) as these are potential clinical consequences of having severe and uncontrolled GERD. We further collected readmission data for a second ALTE. Deaths and the attributed reasons were also collected.
Risk Factors
Potential risk factors for adverse outcomes associated with GERD (all during index hospitalization) included: age; prematurity; gender; previous event (as described by the parent, without previous ALTE hospitalization); primary discharge diagnosis of GERD; testing positive for reflux on index ALTE admission (upper GI x‐ray series, esophageal pH probe, swallow study, endoscopy, and/or consultation of pediatric gastroenterologist with results or assessments indicating gastroesophageal reflux); any anti‐reflux medication prescribed upon discharge; and LOS. We also considered diagnosis of neurologic impairment during follow‐up, which was defined as seizures or diagnosis of developmental delay from any etiology not recognized on index ALTE admission. We examined these risk factors as we postulated they might indicate higher risk for both ALTE and adverse outcomes associated with GERD, or might indicate a higher severity of initial event.
Analyses
Summary statistics were performed for adverse outcomes associated with GERD, readmission, and death. Univariate analyses were performed for risk factors using chi‐square tests for dichotomous predictors and Wilcoxon rank sum tests for nonparametric continuous predictors for any of the 3 adverse outcomes (FTT, aspiration pneumonia [AP], and/or anti‐reflux surgery) associated with GERD. All analyses were performed using SAS 9.13 (Carey, NC).
RESULTS
Eleven hundred forty‐eight infants with ALTE met inclusion criteria, from 187,903 patients meeting initial search criteria. Six hundred seventy‐one patients were excluded and 8 patients had missing charts. The study population of the 469‐patient cohort is shown in Figure 1.
Demographics are displayed in Table 1. The mean age was 65 days. One hundred three (22%) were premature. One hundred eighty‐nine patients (40%) had a primary discharge diagnosis of GERD; details of the diagnoses for the remaining patients are in Figure 1. Median length of follow‐up for the cohort was 7.8 years. The entire study period was 10.7 years.
| ALTE Cohort | |
|---|---|
| N = 469 | |
| |
| Female | 233 (49.7%) |
| Race | |
| Caucasian | 371 (79.1%) |
| Hispanic | 64 (13.6%) |
| Pacific Islander | 6 (1.3%) |
| Black | 4 (0.8%) |
| Other/unknown | 24 (5.1%) |
| Mean age in days (SD) | 65.2 (69.5) |
| Prematurity | 103 (22%) |
| Underwent testing for gastroesophageal reflux | 214 (45.6%) |
| Discharged on anti‐reflux medication | 238 (50.7%) |
| Previous event | 127 (27.1%) |
| Mean length of stay in days (SD) | 2.4 (2.4) |
| Later neurologic impairment (seizures or developmental delay) | 23 (4.9%) |
| Primary discharge diagnosis of GERD | 189 (40%) |
Eighteen patients (3.8%) had an adverse outcome associated with GERD. Four (0.9%) had aspiration pneumonia, 9 (1.9%) had failure‐to‐thrive, and 7 (1.5%) had a Nissen fundoplication (no patients had a gastrojejunal tube placed). Five patients had a gastrostomy tube placed at the time of fundoplication. Two patients had more than 1 adverse GI outcome; 1 patient had aspiration pneumonia and another had failure‐to‐thrive prior to their Nissen fundoplications.
Fifty‐six patients (11.9%) were readmitted for a second ALTE. Median time from index ALTE to second ALTE admission was 16.5 days (interquartile range: Q1, 8Q3, 32). Two (0.4%) patients died. Both (occurring at 18 months and 5.5 years after the initial ALTE hospitalization) were related to the children first developing seizure disorders and severe developmental delay. Neither of the patients who died had an index discharge diagnoses of GERD.
There was no significance of the following variables in predicting adverse outcomes associated with GERD: age, prematurity, gender, previous event, testing positive for reflux, primary discharge diagnosis of GERD, or discharge on anti‐reflux medications (see Table 2). Patients with adverse outcomes associated with GERD had longer mean LOS on the index ALTE hospitalization (4.3 days vs 2.4 days; P = 0.03) and a higher rate of neurologic impairment diagnosed in follow‐up (16.7% vs 4.4%; P = 0.02) than patients without long‐term adverse GI outcomes. Patients with neurological impairment diagnosed in follow‐up were more likely to eventually develop an adverse outcome associated with GERD, compared to patients without neurological impairment (odds ratio 8.4; 95% confidence interval 1.1516.1).
| AP, FTT, or Surgery | No Long‐Term Adverse GI Outcome | P Value | |
|---|---|---|---|
| N = 18 | N = 451 | ||
| |||
| Mean age in days (SD) | 51.9 (76.4) | 65.8 (69.3) | 0.27 |
| Prematurity | 1 (5.6%) | 102 (22.6%) | 0.08 |
| Male gender | 12 (66.7%) | 220 (48.8%) | 0.14 |
| Previous ALTE‐like event (no hospitalization) | 8 (44.4%) | 119 (26.4%) | 0.09 |
| Testing positive for reflux | 9 (50%) | 177 (39.3%) | 0.36 |
| Discharge diagnosis GERD | 9 (50%) | 180 (39.9%) | 0.39 |
| Discharged on anti‐reflux medication | 9 (50%) | 229 (50.7%) | 0.95 |
| Mean length of stay in days (SD) | 4.3 (4.7) | 2.4 (2.3) | 0.03 |
| Neurologic impairment diagnosed in follow‐up | 3 (16.7%) | 20 (4.4%) | 0.02 |
DISCUSSION
Our study had 2 main findings. First, infants admitted for an ALTE had a low percentage (3.8%) of adverse outcomes associated with GERD. Review of the literature provides little context to interpret this percentage. One study reports 9 per 100,000 of the general population <18 years of age having anti‐reflux surgery.24 The percentage of adverse outcomes associated with GERD converted to a rate in our study would likely reflect the bias of our center serving as a referral population for Utah and 5 surrounding states. Furthermore, there may be an additional bias of ALTE being a potential indication for anti‐reflux surgery for some clinicians, as well as confounding additional diagnoses (such as later neurologic impairment which might independently increase risk of study outcomes).
The second main finding of our study is that the development of neurologic impairment was predictive of developing adverse GI outcomes. As previous studies have shown that neurological impairment cannot be predicted during the initial ALTE hospitalization,14 adverse outcomes associated with GERD are similarly not predictable with current clinical approaches. Furthermore, the exact nature of the relationship between neurologic impairment and ALTEs remains unclear. While previous studies have described the increased prevalence of adverse neurological outcomes (such as seizures, developmental delay) in children who have had an ALTE, it is unclear what the precipitating reason for ALTE is in these infants (seizure, central apnea, GERD, etc).14
There is ongoing debate in the literature surrounding the optimal diagnosis of GERD in infants with ALTE. Recent guidelines state that investigations aimed to prove GERD causing an ALTE should include pH probe or impedance monitor testing, in combination with a sleep study, and discourages a GERD diagnosis based on upper GI‐series alone.14 Given the low use of pH probe and impedance monitoring at our institution during the study period (86% of the patients who had GI‐related testing had an upper GI‐series), we did not attempt to find the sensitivity or specificity of the different GI testing modalities for GERD in the setting of ALTE. The high use of upper‐GI series is not unique to our institutionone large study examining practice variation from 12,067 ALTE admissions in 36 children's hospitals, with 36.9% of infants (n = 4453) having a primary discharge diagnosis of gastroesophageal reflux, revealed that only 8.9% (SD 28%) received an esophageal pH probe, while 25.6% (SD 43.6%) had an upper GI‐series or swallow study.4
Given the difficulties in assigning a GERD diagnosis for ALTE infants, we focused on the long‐term adverse outcomes associated with GERD for the entire ALTE infant cohort. The 3 adverse outcomes we chose deserve some mention. Aspiration pneumonia is generally due to either primary aspiration (from dysfunctional swallowing) or secondary aspiration (from GERD). Failure‐to‐thrive can be due to ongoing GERD. Anti‐reflux surgery is often performed for severe GERD. While we believe that a prospective study with better diagnostic evaluations for GERD and apnea (such as pH probe or impedance monitor in combination with a sleep study) might help elucidate the unclear relationship between reflux episodes and ALTE, the low percentage of adverse outcomes associated with GERD after ALTE may suggest that such a study would be difficult both in terms of sample size and an unnecessary use of resources.
We also found that a high percentage (11.9%) of all patients had readmission for a second ALTE. This was substantially higher than the 2.5% readmission rate for second ALTE reported in a previous study of short‐term follow‐up (30 days).4 The number of readmissions in our study might be higher due to our comprehensive follow‐up, both in length of time and number of additional EDs and hospitals captured. Unfortunately, the retrospective nature of our study makes it difficult to determine if any interventions (prescription of anti‐reflux medication, education on reflux precautions) impacted the rate of readmission, as compliance was not measurable. Further studies should address why patients return with recurrent ALTE.
Interestingly, several potential risk factors did not predict long‐term adverse GI outcomes. For example, prematurity, a discharge diagnosis of GERD, or prescription of an anti‐reflux medication, were not associated with adverse GI outcomes. These findings support the concept that a diagnosis of GERD, at least as is commonly applied, is not meaningful in the setting of an ALTE. We did find associations with longer length of stay (LOS), and with eventual development of neurological impairment. Longer LOS might be a proxy for other subtle predictors that could influence adverse outcomes, such as requiring additional diagnostic tests prolonging hospitalization, or continued ALTEs while inpatient. The neurologic outcomes of patients with ALTE have been previously published, and the strong correlation between neurologic impairment and GERD has been well described.14, 25
There are several strengths of this study. This is the first study, to our knowledge, to look at adverse outcomes associated with GERD following ALTE, despite GERD being the most commonly attributed cause. The use of Intermountain Healthcare's electronic medical record system allowed for comprehensive tracking, over an extensive follow‐up period (median of 7.8 years), across 20 hospitals and EDs which care for the vast majority of pediatric patients in Utah. Finally, this large cohort of ALTE patients used clinical data from medical records and not only administrative data.
There are limitations of this study. This is a retrospective cohort study. Some of our study outcomes may be a result of pathophysiology other than GERD and, conversely, GERD may be a result of other issues (neurologic impairment). The small sample size and low percentage of the study outcomes make it possible that we did not detect true risk factors. Patients were lost to follow‐up if they moved or presented to a hospital not within the Intermountain Healthcare system. This study has slightly different patient numbers from 3 previously published studies for different outcomes on this cohort, as exclusion criteria for the different cohorts were different.14, 26, 27 Six patients had only their electronic medical record reviewed because the paper chart was missing.
IMPLICATIONS
The results of this study extend previous work of various outcomes regarding well‐appearing infants following an ALTE.14, 26, 27 In these studies, 3.9% and 3% were ultimately diagnosed with epilepsy and developmental delay, respectively; 1.4% were diagnosed with abusive head trauma; and 0.6% required otolaryngologic surgical intervention. In these previous studies, there were few predictors of these outcomes, with testing demonstrating largely normal results during the index ALTE admission.
Our study helps clinicians place the outcomes of aspiration pneumonia, failure‐to‐thrive, and anti‐reflux surgery into the context of these other studies when discharging infants from the hospital after an ALTE. Collectively, these studies provide clinicians with the information that, in the setting of a well‐appearing infant, few diagnostic tests in their ALTE patients will yield a definitive diagnosis. Ultimately, close follow‐up with further investigations if symptoms recur will be an important part of diagnosing the etiology of the ALTE in these infants.
We found that well‐appearing infants with ALTE, regardless of attributed cause, are at low risk for adverse outcomes associated with GERD. Only the eventual development of neurologic impairment or an increased length of stay during index ALTE hospitalization was found to be predictive of these outcomes.
Acknowledgements
The following individuals have made substantive intellectual contributions to this study: conception and design (G.Z., J.L.B., W.D.J., C.G.M., R.S.), acquisition of data (G.Z., J.L.B.), analysis (G.Z., RS) and interpretation of data (G.Z., J.L.B., W.D.J., C.G.M., R.S.). In addition, all listed authors have contributed to either drafting the article or revising it critically for important intellectual content. Finally, all listed authors have given final approval of this version submitted for publication. The authors also acknowledge Chelsea Welch for her assistance in data collection.
Disclosures: This study was presented in part at the national Pediatric Academic Societies meetings in Vancouver, Canada, May 2010 and in Denver, CO, May 2011. This study was supported by a National Institutes of Child Health and Human Development (NICHD) grant for Dr Srivastava (K23 HD052553), and a National Institute on Drug Abuse (NIDA) grant for Dr Bonkowsky (K08 DA24753). This research was supported in part by the Children's Health Research Center, University of Utah. There are no conflicts of interest.
Apparent life‐threatening events (ALTEs) are frightening for the parent/guardian and represent a challenge for the healthcare provider. ALTEs are defined as worrisome episodes of any combination of apnea, color change, change in muscle tone, choking or gagging.1 ALTEs account for 0.6% to 0.8% of emergency department (ED) visits for children <12 months old,2, 3 have an average length of stay (LOS) of 4.4 days and an average cost of $15,000 per hospitalization.4
Gastroesophageal reflux disease (GERD) is common in infancy11 and also is the most commonly (in 31%55% of ALTE cases) attributed cause of ALTE.2, 4, 5 It has been speculated that chemosensitivity to gastric acid results in laryngospasm, bronchospasm, and apnea. However, several small studies have failed to prove a causal link between reflux episodes and apnea.69 Furthermore, although consensus guidelines for GERD have been developed,14 the clinical use of testing for GERD remains highly variable. A study of infants discharged with an ALTE (n = 12,067) from 36 children's hospitals in the United States revealed extensive variability in the use of pH probes and upper gastrointestinal x‐ray series to diagnose GERD.4
The incidence of adverse outcomes associated with GERD after an ALTE remains unknown. It is also unknown whether an association exists between long‐term gastrointestinal (GI) outcomes and testing demonstrative of GERD or a diagnosis of GERD during hospitalization for ALTE. The primary objective of our study was to determine, in patients with an ALTE, the adverse outcomes associated with GERD (failure‐to‐thrive, aspiration pneumonia, and/or anti‐reflux surgery), the incidence of readmission for second ALTE, and death. Our secondary objective was to determine risk factors for adverse outcomes associated with GERD following an ALTE.
METHODS
Design
This was a retrospective cohort study. We reviewed electronic and paper medical charts of all infants <12 months of age admitted for ALTE between January 1, 1999 and December 31, 2003 to Primary Children's Medical Center in Salt Lake City, UT, which serves as the tertiary pediatric center for >1 million children and the primary facility for >270,000 children in Salt Lake County, UT.10 Primary Children's Medical Center is operated by a vertically integrated not‐for‐profit healthcare system (Intermountain Healthcare), which has 20 affiliated hospitals and EDs. The study was approved by the institutional review boards of the University of Utah and Intermountain Healthcare, and the privacy board of Intermountain Healthcare.
Participants
Patients were included if a computer search of ED chief complaint or hospital discharge diagnoses found one or more of the following keywords (or corresponding International Classification of Diseases, Ninth Revision [ICD‐9] codes if applicable): ALTE, altered mental status, apnea, breath‐holding spell, choking, GERD, hypotonia, lethargy, other convulsions, other neurologic diagnosis, other respiratory diagnosis, pallor, seizures, sleep apnea, stiff, syncope, and unresponsiveness. These diagnoses were chosen as potential proxy diagnoses or codes for possible ALTE, as ALTE did not have a corresponding ICD‐9 code at the time of this study.
Detailed review of the medical record included infants who were <12 months old at admission with a history consistent with ALTE, defined as an episode frightening to the observer with any combination of apnea, color change, change in muscle tone, choking, or gagging. Infants were excluded from the study if they had a previously documented underlying medical condition to explain the ALTE (such as a known seizure disorder) or had a clearly apparent diagnosis upon initial history and physical examination (such as bronchiolitis diagnosed in the emergency department) that would explain the event. Patients with unstable vital signs (eg, hypotension), trauma clearly apparent on admission, documented medication dosing error, or febrile seizure were also excluded. A complete list of exclusion criteria is found in Figure 1.

All hospital admissions, ED visits, and Pediatric GI clinic notes were reviewed for adverse outcomes associated with GERD, second ALTE admission, and death. The follow‐up time period included the original enrollment period (January 1, 1999 through December 31, 2003) through August 31, 2009.
Outcomes
Adverse outcomes associated with GERD were defined as aspiration pneumonia, failure‐to‐thrive (FTT; either admission or discharge diagnosis of FTT at another hospitalization, or follow‐up to gastroenterology clinic for FTT) and/or anti‐reflux surgery (Nissen fundoplication or gastrojejunal tube placement) as these are potential clinical consequences of having severe and uncontrolled GERD. We further collected readmission data for a second ALTE. Deaths and the attributed reasons were also collected.
Risk Factors
Potential risk factors for adverse outcomes associated with GERD (all during index hospitalization) included: age; prematurity; gender; previous event (as described by the parent, without previous ALTE hospitalization); primary discharge diagnosis of GERD; testing positive for reflux on index ALTE admission (upper GI x‐ray series, esophageal pH probe, swallow study, endoscopy, and/or consultation of pediatric gastroenterologist with results or assessments indicating gastroesophageal reflux); any anti‐reflux medication prescribed upon discharge; and LOS. We also considered diagnosis of neurologic impairment during follow‐up, which was defined as seizures or diagnosis of developmental delay from any etiology not recognized on index ALTE admission. We examined these risk factors as we postulated they might indicate higher risk for both ALTE and adverse outcomes associated with GERD, or might indicate a higher severity of initial event.
Analyses
Summary statistics were performed for adverse outcomes associated with GERD, readmission, and death. Univariate analyses were performed for risk factors using chi‐square tests for dichotomous predictors and Wilcoxon rank sum tests for nonparametric continuous predictors for any of the 3 adverse outcomes (FTT, aspiration pneumonia [AP], and/or anti‐reflux surgery) associated with GERD. All analyses were performed using SAS 9.13 (Carey, NC).
RESULTS
Eleven hundred forty‐eight infants with ALTE met inclusion criteria, from 187,903 patients meeting initial search criteria. Six hundred seventy‐one patients were excluded and 8 patients had missing charts. The study population of the 469‐patient cohort is shown in Figure 1.
Demographics are displayed in Table 1. The mean age was 65 days. One hundred three (22%) were premature. One hundred eighty‐nine patients (40%) had a primary discharge diagnosis of GERD; details of the diagnoses for the remaining patients are in Figure 1. Median length of follow‐up for the cohort was 7.8 years. The entire study period was 10.7 years.
| ALTE Cohort | |
|---|---|
| N = 469 | |
| |
| Female | 233 (49.7%) |
| Race | |
| Caucasian | 371 (79.1%) |
| Hispanic | 64 (13.6%) |
| Pacific Islander | 6 (1.3%) |
| Black | 4 (0.8%) |
| Other/unknown | 24 (5.1%) |
| Mean age in days (SD) | 65.2 (69.5) |
| Prematurity | 103 (22%) |
| Underwent testing for gastroesophageal reflux | 214 (45.6%) |
| Discharged on anti‐reflux medication | 238 (50.7%) |
| Previous event | 127 (27.1%) |
| Mean length of stay in days (SD) | 2.4 (2.4) |
| Later neurologic impairment (seizures or developmental delay) | 23 (4.9%) |
| Primary discharge diagnosis of GERD | 189 (40%) |
Eighteen patients (3.8%) had an adverse outcome associated with GERD. Four (0.9%) had aspiration pneumonia, 9 (1.9%) had failure‐to‐thrive, and 7 (1.5%) had a Nissen fundoplication (no patients had a gastrojejunal tube placed). Five patients had a gastrostomy tube placed at the time of fundoplication. Two patients had more than 1 adverse GI outcome; 1 patient had aspiration pneumonia and another had failure‐to‐thrive prior to their Nissen fundoplications.
Fifty‐six patients (11.9%) were readmitted for a second ALTE. Median time from index ALTE to second ALTE admission was 16.5 days (interquartile range: Q1, 8Q3, 32). Two (0.4%) patients died. Both (occurring at 18 months and 5.5 years after the initial ALTE hospitalization) were related to the children first developing seizure disorders and severe developmental delay. Neither of the patients who died had an index discharge diagnoses of GERD.
There was no significance of the following variables in predicting adverse outcomes associated with GERD: age, prematurity, gender, previous event, testing positive for reflux, primary discharge diagnosis of GERD, or discharge on anti‐reflux medications (see Table 2). Patients with adverse outcomes associated with GERD had longer mean LOS on the index ALTE hospitalization (4.3 days vs 2.4 days; P = 0.03) and a higher rate of neurologic impairment diagnosed in follow‐up (16.7% vs 4.4%; P = 0.02) than patients without long‐term adverse GI outcomes. Patients with neurological impairment diagnosed in follow‐up were more likely to eventually develop an adverse outcome associated with GERD, compared to patients without neurological impairment (odds ratio 8.4; 95% confidence interval 1.1516.1).
| AP, FTT, or Surgery | No Long‐Term Adverse GI Outcome | P Value | |
|---|---|---|---|
| N = 18 | N = 451 | ||
| |||
| Mean age in days (SD) | 51.9 (76.4) | 65.8 (69.3) | 0.27 |
| Prematurity | 1 (5.6%) | 102 (22.6%) | 0.08 |
| Male gender | 12 (66.7%) | 220 (48.8%) | 0.14 |
| Previous ALTE‐like event (no hospitalization) | 8 (44.4%) | 119 (26.4%) | 0.09 |
| Testing positive for reflux | 9 (50%) | 177 (39.3%) | 0.36 |
| Discharge diagnosis GERD | 9 (50%) | 180 (39.9%) | 0.39 |
| Discharged on anti‐reflux medication | 9 (50%) | 229 (50.7%) | 0.95 |
| Mean length of stay in days (SD) | 4.3 (4.7) | 2.4 (2.3) | 0.03 |
| Neurologic impairment diagnosed in follow‐up | 3 (16.7%) | 20 (4.4%) | 0.02 |
DISCUSSION
Our study had 2 main findings. First, infants admitted for an ALTE had a low percentage (3.8%) of adverse outcomes associated with GERD. Review of the literature provides little context to interpret this percentage. One study reports 9 per 100,000 of the general population <18 years of age having anti‐reflux surgery.24 The percentage of adverse outcomes associated with GERD converted to a rate in our study would likely reflect the bias of our center serving as a referral population for Utah and 5 surrounding states. Furthermore, there may be an additional bias of ALTE being a potential indication for anti‐reflux surgery for some clinicians, as well as confounding additional diagnoses (such as later neurologic impairment which might independently increase risk of study outcomes).
The second main finding of our study is that the development of neurologic impairment was predictive of developing adverse GI outcomes. As previous studies have shown that neurological impairment cannot be predicted during the initial ALTE hospitalization,14 adverse outcomes associated with GERD are similarly not predictable with current clinical approaches. Furthermore, the exact nature of the relationship between neurologic impairment and ALTEs remains unclear. While previous studies have described the increased prevalence of adverse neurological outcomes (such as seizures, developmental delay) in children who have had an ALTE, it is unclear what the precipitating reason for ALTE is in these infants (seizure, central apnea, GERD, etc).14
There is ongoing debate in the literature surrounding the optimal diagnosis of GERD in infants with ALTE. Recent guidelines state that investigations aimed to prove GERD causing an ALTE should include pH probe or impedance monitor testing, in combination with a sleep study, and discourages a GERD diagnosis based on upper GI‐series alone.14 Given the low use of pH probe and impedance monitoring at our institution during the study period (86% of the patients who had GI‐related testing had an upper GI‐series), we did not attempt to find the sensitivity or specificity of the different GI testing modalities for GERD in the setting of ALTE. The high use of upper‐GI series is not unique to our institutionone large study examining practice variation from 12,067 ALTE admissions in 36 children's hospitals, with 36.9% of infants (n = 4453) having a primary discharge diagnosis of gastroesophageal reflux, revealed that only 8.9% (SD 28%) received an esophageal pH probe, while 25.6% (SD 43.6%) had an upper GI‐series or swallow study.4
Given the difficulties in assigning a GERD diagnosis for ALTE infants, we focused on the long‐term adverse outcomes associated with GERD for the entire ALTE infant cohort. The 3 adverse outcomes we chose deserve some mention. Aspiration pneumonia is generally due to either primary aspiration (from dysfunctional swallowing) or secondary aspiration (from GERD). Failure‐to‐thrive can be due to ongoing GERD. Anti‐reflux surgery is often performed for severe GERD. While we believe that a prospective study with better diagnostic evaluations for GERD and apnea (such as pH probe or impedance monitor in combination with a sleep study) might help elucidate the unclear relationship between reflux episodes and ALTE, the low percentage of adverse outcomes associated with GERD after ALTE may suggest that such a study would be difficult both in terms of sample size and an unnecessary use of resources.
We also found that a high percentage (11.9%) of all patients had readmission for a second ALTE. This was substantially higher than the 2.5% readmission rate for second ALTE reported in a previous study of short‐term follow‐up (30 days).4 The number of readmissions in our study might be higher due to our comprehensive follow‐up, both in length of time and number of additional EDs and hospitals captured. Unfortunately, the retrospective nature of our study makes it difficult to determine if any interventions (prescription of anti‐reflux medication, education on reflux precautions) impacted the rate of readmission, as compliance was not measurable. Further studies should address why patients return with recurrent ALTE.
Interestingly, several potential risk factors did not predict long‐term adverse GI outcomes. For example, prematurity, a discharge diagnosis of GERD, or prescription of an anti‐reflux medication, were not associated with adverse GI outcomes. These findings support the concept that a diagnosis of GERD, at least as is commonly applied, is not meaningful in the setting of an ALTE. We did find associations with longer length of stay (LOS), and with eventual development of neurological impairment. Longer LOS might be a proxy for other subtle predictors that could influence adverse outcomes, such as requiring additional diagnostic tests prolonging hospitalization, or continued ALTEs while inpatient. The neurologic outcomes of patients with ALTE have been previously published, and the strong correlation between neurologic impairment and GERD has been well described.14, 25
There are several strengths of this study. This is the first study, to our knowledge, to look at adverse outcomes associated with GERD following ALTE, despite GERD being the most commonly attributed cause. The use of Intermountain Healthcare's electronic medical record system allowed for comprehensive tracking, over an extensive follow‐up period (median of 7.8 years), across 20 hospitals and EDs which care for the vast majority of pediatric patients in Utah. Finally, this large cohort of ALTE patients used clinical data from medical records and not only administrative data.
There are limitations of this study. This is a retrospective cohort study. Some of our study outcomes may be a result of pathophysiology other than GERD and, conversely, GERD may be a result of other issues (neurologic impairment). The small sample size and low percentage of the study outcomes make it possible that we did not detect true risk factors. Patients were lost to follow‐up if they moved or presented to a hospital not within the Intermountain Healthcare system. This study has slightly different patient numbers from 3 previously published studies for different outcomes on this cohort, as exclusion criteria for the different cohorts were different.14, 26, 27 Six patients had only their electronic medical record reviewed because the paper chart was missing.
IMPLICATIONS
The results of this study extend previous work of various outcomes regarding well‐appearing infants following an ALTE.14, 26, 27 In these studies, 3.9% and 3% were ultimately diagnosed with epilepsy and developmental delay, respectively; 1.4% were diagnosed with abusive head trauma; and 0.6% required otolaryngologic surgical intervention. In these previous studies, there were few predictors of these outcomes, with testing demonstrating largely normal results during the index ALTE admission.
Our study helps clinicians place the outcomes of aspiration pneumonia, failure‐to‐thrive, and anti‐reflux surgery into the context of these other studies when discharging infants from the hospital after an ALTE. Collectively, these studies provide clinicians with the information that, in the setting of a well‐appearing infant, few diagnostic tests in their ALTE patients will yield a definitive diagnosis. Ultimately, close follow‐up with further investigations if symptoms recur will be an important part of diagnosing the etiology of the ALTE in these infants.
We found that well‐appearing infants with ALTE, regardless of attributed cause, are at low risk for adverse outcomes associated with GERD. Only the eventual development of neurologic impairment or an increased length of stay during index ALTE hospitalization was found to be predictive of these outcomes.
Acknowledgements
The following individuals have made substantive intellectual contributions to this study: conception and design (G.Z., J.L.B., W.D.J., C.G.M., R.S.), acquisition of data (G.Z., J.L.B.), analysis (G.Z., RS) and interpretation of data (G.Z., J.L.B., W.D.J., C.G.M., R.S.). In addition, all listed authors have contributed to either drafting the article or revising it critically for important intellectual content. Finally, all listed authors have given final approval of this version submitted for publication. The authors also acknowledge Chelsea Welch for her assistance in data collection.
Disclosures: This study was presented in part at the national Pediatric Academic Societies meetings in Vancouver, Canada, May 2010 and in Denver, CO, May 2011. This study was supported by a National Institutes of Child Health and Human Development (NICHD) grant for Dr Srivastava (K23 HD052553), and a National Institute on Drug Abuse (NIDA) grant for Dr Bonkowsky (K08 DA24753). This research was supported in part by the Children's Health Research Center, University of Utah. There are no conflicts of interest.
- Infantile apnea and home monitoring.NIH Consensus Statement 1986 Sep 29‐Oct 1.Pediatrics.1987;79(2):292–299.
- ,.Causes of apparent life threatening events in infants: a systematic review.Arch Dis Child.2004;89:1043–1048.
- ,.Parental reported apnea, admissions to hospital and sudden infant death syndrome.Acta Paediatr.2001;90(4):417–422.
- ,,,.Variation in inpatient resource utilization and management of apparent life‐threatening events.J Pediatr.2008;152(5):629–635.
- ,,,,,.Discharge diagnoses in infants with apparent life‐threatening event.Pediatr Int.2003;45:560–563.
- ,,,,,.Lack of temporal relation between acid reflux in the proximal oesophagus and cardiorespiratory events in sleeping infants.Eur J Pediatr.1992;151(3):208–212.
- ,,, et al.Patterns of gastroesophageal reflux (GER) in patients with apparent life‐threatening events.J Pediatr Gastroenterol Nutr.1989;8(2):157–160.
- ,,, et al.Characteristics of continuous esophageal pH‐metering in infants with gastroesophageal reflux and apparent life‐threatening events.Eur J Pediatr Surg.1995;5(3);136–138.
- ,,,,.Apnea is not prolonged by acid gastroesophageal reflux in preterm infants.Pediatrics.2005;116:1059–1063.
- ,.Organizational responses to managed care: issues for academic health centers and implications for pediatric programs.Pediatrics.1998;101(4):805–811.
- ,,, et al.Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN).J Pediatr Gastroenterol Nutr.2009;49:498–547.
- ,,, et al.Prevalence and natural history of gastroesophageal reflux: pediatric prospective study.Pediatrics.2009;123(3):779–783.
- ,.Systematic review: the estra‐oesophageal symptoms of gastro‐oesophageal reflux disease in children.Aliment Pharmacol Ther.2009;29:258–272.
- ,,,.Death, child abuse, and adverse neurological outcomes of infants after an apparent life‐threatening event.Pediatrics.2008;122:125–131.
- ,,, et al.Abusive head injury as a cause of apparent life‐threatening events in infancy.Arch Pediatr Adolesc Med.2003;157:1011–1015.
- ,,.Accidental and nonaccidental poisonings as a cause of apparent life‐threatening events in infants.Pediatrics.2008;122:e359–e362.
- ,,,.Yield of diagnostic testing in infants who have had an apparent life‐threatening event.Pediatrics.2005;115:885–893.
- ,,, et al.Guidelines for evaluation and treatment of gastroesophageal reflux in infants and children: recommendations of the North American Society for Pediatric Gastroenterology and Nutrition.J Pediatr Gastroenterol Nutr.2001;32(suppl 2):S1–S31.
- ,,,.Comparison of barium radiology with esophageal pH monitoring in the diagnosis of gastroesophageal reflux disease.Am J Gastroenterol.1996;91(6):1181–1195.
- ,,, et al.Gastroesophageal reflux disease: correlation of esophageal pH testing and radiographic finding.Radiology.1992;185:483–486.
- ,,, et al.Gastro‐esophageal reflux demonstrated by radiography in infants less than 1 year of age. Comparison with pH monitoring.Acta Radiol.2003;44:136–138.
- ,,, et al.Double‐blind placebo controlled trial of omeprazole in irritable infants with gastroesophageal reflux.J Pediatr.2003;143:219–223.
- ,,, et al.Clinical predictors of pathological gastro‐oesophageal reflux in infants with persistant distress.J Paediatr Child Health.2006;42:134–139.
- ,,.National trends in the use of anti‐reflux procedures for children.Pediatrics.2006;118:1828–1835.
- ,,, et al.Prevalence and severity of feeding and nutritional problems in children with neurological impairment: Oxford feeding study.Dev Med Child Neurol.2000;42:674–680.
- ,,.Abusive head trauma in children presenting with an apparent life‐threatening event.J Pediatr.2010;157(5):821–825.
- ,,.Usefulness of airway evaluation in children initially seen with apparent life‐threatening event.Arch Otolaryngol Head Neck Surg.2011;137(4):359–362.
- Infantile apnea and home monitoring.NIH Consensus Statement 1986 Sep 29‐Oct 1.Pediatrics.1987;79(2):292–299.
- ,.Causes of apparent life threatening events in infants: a systematic review.Arch Dis Child.2004;89:1043–1048.
- ,.Parental reported apnea, admissions to hospital and sudden infant death syndrome.Acta Paediatr.2001;90(4):417–422.
- ,,,.Variation in inpatient resource utilization and management of apparent life‐threatening events.J Pediatr.2008;152(5):629–635.
- ,,,,,.Discharge diagnoses in infants with apparent life‐threatening event.Pediatr Int.2003;45:560–563.
- ,,,,,.Lack of temporal relation between acid reflux in the proximal oesophagus and cardiorespiratory events in sleeping infants.Eur J Pediatr.1992;151(3):208–212.
- ,,, et al.Patterns of gastroesophageal reflux (GER) in patients with apparent life‐threatening events.J Pediatr Gastroenterol Nutr.1989;8(2):157–160.
- ,,, et al.Characteristics of continuous esophageal pH‐metering in infants with gastroesophageal reflux and apparent life‐threatening events.Eur J Pediatr Surg.1995;5(3);136–138.
- ,,,,.Apnea is not prolonged by acid gastroesophageal reflux in preterm infants.Pediatrics.2005;116:1059–1063.
- ,.Organizational responses to managed care: issues for academic health centers and implications for pediatric programs.Pediatrics.1998;101(4):805–811.
- ,,, et al.Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN).J Pediatr Gastroenterol Nutr.2009;49:498–547.
- ,,, et al.Prevalence and natural history of gastroesophageal reflux: pediatric prospective study.Pediatrics.2009;123(3):779–783.
- ,.Systematic review: the estra‐oesophageal symptoms of gastro‐oesophageal reflux disease in children.Aliment Pharmacol Ther.2009;29:258–272.
- ,,,.Death, child abuse, and adverse neurological outcomes of infants after an apparent life‐threatening event.Pediatrics.2008;122:125–131.
- ,,, et al.Abusive head injury as a cause of apparent life‐threatening events in infancy.Arch Pediatr Adolesc Med.2003;157:1011–1015.
- ,,.Accidental and nonaccidental poisonings as a cause of apparent life‐threatening events in infants.Pediatrics.2008;122:e359–e362.
- ,,,.Yield of diagnostic testing in infants who have had an apparent life‐threatening event.Pediatrics.2005;115:885–893.
- ,,, et al.Guidelines for evaluation and treatment of gastroesophageal reflux in infants and children: recommendations of the North American Society for Pediatric Gastroenterology and Nutrition.J Pediatr Gastroenterol Nutr.2001;32(suppl 2):S1–S31.
- ,,,.Comparison of barium radiology with esophageal pH monitoring in the diagnosis of gastroesophageal reflux disease.Am J Gastroenterol.1996;91(6):1181–1195.
- ,,, et al.Gastroesophageal reflux disease: correlation of esophageal pH testing and radiographic finding.Radiology.1992;185:483–486.
- ,,, et al.Gastro‐esophageal reflux demonstrated by radiography in infants less than 1 year of age. Comparison with pH monitoring.Acta Radiol.2003;44:136–138.
- ,,, et al.Double‐blind placebo controlled trial of omeprazole in irritable infants with gastroesophageal reflux.J Pediatr.2003;143:219–223.
- ,,, et al.Clinical predictors of pathological gastro‐oesophageal reflux in infants with persistant distress.J Paediatr Child Health.2006;42:134–139.
- ,,.National trends in the use of anti‐reflux procedures for children.Pediatrics.2006;118:1828–1835.
- ,,, et al.Prevalence and severity of feeding and nutritional problems in children with neurological impairment: Oxford feeding study.Dev Med Child Neurol.2000;42:674–680.
- ,,.Abusive head trauma in children presenting with an apparent life‐threatening event.J Pediatr.2010;157(5):821–825.
- ,,.Usefulness of airway evaluation in children initially seen with apparent life‐threatening event.Arch Otolaryngol Head Neck Surg.2011;137(4):359–362.
Copyright © 2012 Society of Hospital Medicine
PHM Strategic Planning Roundtable
Hospitalists are the fastest growing segment of physicians in the United States.1 Given the growing field of Pediatric Hospital Medicine (PHM) and the need to define strategic direction, the Society of Hospital Medicine (SHM), the American Academy of Pediatrics (AAP), and the Academic Pediatric Association (APA) sponsored a strategic planning meeting in February 2009 that brought together 22 PHM leaders to discuss the future of the field.
PHM is at a critical juncture in terms of clinical practice, research, workforce issues, and quality improvement. The field has developed sufficiently to produce leaders capable of setting an agenda and moving forward. A discussion with the American Board of Pediatrics (ABP) by PHM leaders from the AAP, APA, and SHM at the Pediatric Hospital Medicine 2007 Conference regarding subspecialty designation stimulated convening the PHM Strategic Planning Roundtable to address the task of coordinating further development of PHM (Table 1).
|
| Develop a strategic vision for the role of PHM in the future of children's health care |
| Describe the current gaps between the vision and today's reality |
| Develop a common understanding regarding current initiatives in PHM domains of clinical practice, quality, research, and workforce |
| Determine the method(s) by which participants can be organized to accomplish additional initiatives to implement the vision |
| Identify and prioritize key strategic initiatives |
| Assign accountability and determine next steps and timeline to implement the selected initiatives |
The objective of this article is to describe: (1) the Strategic Planning Roundtable's vision for the field of pediatric hospital medicine; (2) the generation and progress on specific initiatives in clinical practice, quality, research, and workforce identified by the Strategic Planning Roundtable; and (3) issues in the designation of PHM as a subspecialty.
METHODS
The PHM Strategic Planning Roundtable was conducted by a facilitator (S.M.) during a 2‐day retreat using established healthcare strategic planning methods.2
Participants were the existing PHM leaders from the AAP, APA, and SHM, as well as other national leaders in clinical practice, quality, research, and workforce. Development of the vision statement was a key step in which the participants developed a consensus‐based aspirational view of the future. The draft version of the vision statement was initially developed after extensive interviews with key stakeholders and experts in PHM, and was revised by the participants in the course of a facilitated group discussion during the retreat. Following creation of the vision statement, the group then defined the elements of transformation pertaining to PHM and detailed the components of the vision.
Analysis of internal and external environmental factors was critical in the strategic planning process. This type of analysis, detailing the current state of PHM practice, permitted the strategic planners to understand the gaps that existed between the aspirational vision statement and today's reality, and set the stage to identify and implement initiatives to achieve the vision. Several months before the meeting, 4 expert panels comprised of PHM specialists representing a variety of academic and clinical practice settings were brought together via e‐mail and conference calls to focus on 4 domains of PHM: clinical practice, quality of care, research, and workforce. These groups were asked to describe the current status, challenges, and opportunities in these areas. Combining literature review and key stakeholder interviews, their findings and recommendations were distilled into brief summaries that were presented at the Roundtable meeting. Following the presentations, the participants, working in small groups representing all areas of focus,provided additional feedback.
Following the creation of a consensus vision statement and review of internal and external factors, the participants worked to identify specific initiatives in the 4 domains that would advance the field towards the goals contained in the vision statement. These initiatives were grouped into categories. Initiatives by category were scored and prioritized according to predetermined criteria including potential impact, cost, operational complexity, and achievability.
For each initiative selected, the group developed targets and metrics that would be used to track progress. Assigning leadership, accountability, and a timeline to each of the selected projects completed the implementation plan. In addition, the group developed an organizational structure to provide oversight for the overall process, and designated individuals representing the sponsoring organizations into those roles. In conclusion, the group discussed potential structures to guide the future of PHM.
CLINICAL PRACTICE
The Roundtable defined clinical practice for PHM as the general medical care of the hospitalized child, including direct patient care and leadership of the inpatient service. Clinical practice is affected by a number of current national trends including: fewer primary care providers interested in, or with the time to provide, inpatient care; resident work hour restrictions; increasing complexity of clinical issues; and increasing availability of pediatric hospitalists. At the hospital level, clinical practice is affected by increasing need for quality and safety measures, electronic health records and computerized physician order entry, and mounting financial pressures on the hospital system. Hospitalists are assuming more roles in leading quality and safety initiatives, creating computerized systems that address children's needs, and creating financially viable systems of quality pediatric care.3 Hospitalists' clinical care and leadership roles are emerging, and therefore the field faces training and mentorship issues.
Progress to date in this area includes 2 textbooks that define a scope of knowledge and practice, and a newly developed journal in PHM. Core competencies in PHM have been published and provide further refinement of scope and a template for future training.4
Multiple opportunities exist for hospitalists to establish themselves as clinical leaders. Hospitalists can become the preferred providers for hospitalized chronically ill children, with specific initiatives to improve care coordination and multidisciplinary communication. In addition to care coordination and decreasing length of stay, hospitalists, with their intimate knowledge of hospital operations, can be leaders in hospital capacity management and patient flow to increase operational efficiency. Hospitalists can expand evidence‐based guidelines for, and data about, inpatient conditions, and explore the effect of workload and hours on patient care. In addition, there is an expanding role into administrative areas, as well as alternate care arenas, such as: intensive care support (pediatric and neonatal), transport, sedation, palliative care, and pain management. Activities in administrative and alternate care areas have profound direct affects on patient care, as well as providing value added services and additional revenue streams which can further support clinical needs. Finally, achieving quality targets will likely be increasingly linked to payment, so hospitalists may play a key role in the incentives paid to their hospitals. Meeting these challenges will further solidify the standing of hospitalists in the clinical realm.
QUALITY
National and governmental agencies have influenced quality and performance improvement measurements in adult healthcare, resulting in improvements in adult healthcare quality measurement.5 There is limited similar influence or measure development in pediatric medicine, so the quality chasm between adult and child healthcare has widened. Few resources are invested in improving quality and safety of pediatric inpatient care. Of the 18 private health insurance plans' quality and pay for performance programs identified by Leapfrog, only 17% developed pediatric‐specific inpatient measures.6 Only 5 of 40 controlled trials of quality improvement efforts for children published between 1980 and 1998 addressed inpatient problems.7
There have been recent efforts at the national level addressing these issues, highlighted by the introduction of The Children's Health Care Quality Act, in 2007. Early studies in PHM systems focused on overall operational efficiency, documenting 9% to 16% decreases in length of stay and cost compared to traditional models of care.8 Conway et al. identified higher reported adherence to evidence‐based care for hospitalists compared to community pediatricians.9 However, Landrigan et al. demonstrated that there is still large variation in care that exists in the management of common inpatient diagnoses, lacking strong evidence‐based guidelines even among pediatric hospitalists.10 Moreover, there have been no significant studies reviewing the impact of pediatric hospitalists on safety of inpatient care. Magnifying these challenges is the reality that our healthcare system is fragmented with various entities scrambling to define, measure, and compare the effectiveness and safety of pediatric healthcare.
These challenges create an opportunity for PHM to develop a model of how to deliver the highest quality and safest care to our patients. The solution is complex and will take cooperation at many levels of our healthcare system. Improving the safety and quality of care for children in all settings of inpatient care in the United States may best be accomplished via an effective collaborative. This collaborative should be comprehensive and inclusive, and focused on demonstrating and disseminating how standardized, evidence‐based care in both clinical and safety domains can lead to high‐value and high‐quality outcomes. The success of PHM will be measured by its ability to deliver a clear value proposition to all consumers and payers of healthcare. The creation of a robust national collaborative network is a first step towards meeting this goal and will take an extraordinary effort. A PHM Quality Improvement (QI) Collaborative workgroup was created in August 2009. Three collaboratives have been commissioned: (1) Reduction of patient identification errors; (2) Improving discharge communication to referring primary care providers for pediatric hospitalist programs, and (3) Reducing the misuse and overuse of bronchodilators for bronchiolitis. All the collaborative groups have effectively engaged key groups of stakeholders and utilized standard QI tools, demonstrating improvement by the fall of 2010 (unpublished data, S.N.).
RESEARCH
Despite being a relatively young field, there is a critical mass of pediatric hospitalist‐investigators who are establishing research career paths for themselves by securing external grant funding for their work, publishing, and receiving mentorship from largely non‐hospitalist mentors. Some hospitalists are now in a position to mentor junior investigators. These hospitalist‐investigators identified a collective goal of working together across multiple sites in a clinical research network. The goal is to conduct high‐quality studies and provide the necessary clinical information to allow practicing hospitalists to make better decisions regarding patient care. This new inpatient evidence‐base will have the added advantage of helping further define the field of PHM.
The Pediatric Research in Inpatient Settings Network (PRIS) was identified as the vehicle to accomplish these goals. A series of objectives were identified to redesign PRIS in order to accommodate and organize this new influx of hospitalist‐investigators. These objectives included having hospitalist‐investigators commit their time to the prioritization, design, and execution of multicenter studies, drafting new governance documents for PRIS, securing external funding, redefining the relationships of the 3 existing organizations that formed PRIS (AAP, APA, SHM), defining how new clinical sites could be added to PRIS, creating a pipeline for junior hospitalist‐investigators to transition to leadership roles, securing a data coordinating center with established expertise in conducting multicenter studies, and establishing an external research advisory committee of leaders in pediatric clinical research and QI.
Several critical issues were identified, but funding remained a priority for the sustainability of PRIS. Comparative effectiveness (CE) was recognized as a potential important source of future funding. Pediatric studies on CE (eg, surgery vs medical management) conducted by PRIS would provide important new data to allow hospitalists to practice evidence‐based medicine and to improve quality.
A Research Leadership Task Force was created with 4 members of the PHM Strategic Planning Roundtable to work on the identified issues. The APA leadership worked with PRIS to establish a new Executive Council (comprised of additional qualified hospitalist‐investigators). The Executive Council was charged with accomplishing the tasks outlined from the Strategic Planning Roundtable. They have created the governance documents and standard operating procedures necessary for PRIS to conduct multicenter studies, defined a strategic framework for PRIS including the mission, vision and values, and funding strategy. In February 2010, PRIS received a 3‐year award for over $1 million from the Child Health Corporation of America to both fund the infrastructure of PRIS and to conduct a Prioritization Project. The Prioritization Project seeks to identify the conditions that are costly, prevalent, and demonstrate high inter‐hospital variation in resource utilization, which signals either lack of high‐quality data upon which to base medical decisions, and/or an opportunity to standardize care across hospitals. Some of these conditions will warrant further investigation to define the evidence base, whereas other conditions may require implementation studies to reliably introduce evidence into practice. Members of the Executive Council received additional funding to investigate community settings, as most children are hospitalized outside of large children's hospitals. PRIS also reengaged all 3 societies (APA, AAP, and SHM) for support for the first face‐to‐face meeting of the Executive Council. PRIS applied for 2 Recovery Act stimulus grants, and received funding for both of approximately $12 million. The processes used to design, provide feedback, and shepherd these initial studies formed the basis for the standard operating procedures for the Network. PRIS is now reengaging its membership to establish how sites may be able to conduct research, and receive new ideas to be considered for study in PRIS.
Although much work remains to be done, the Executive Council is continuing the charge with quarterly face‐to‐face meetings, hiring of a full‐time PRIS Coordinator, and carrying out these initial projects, while maintaining the goal of meeting the needs of the membership and PHM. If PRIS is to accomplish its mission of improving the health of, and healthcare delivery to, hospitalized children and their families, then the types of studies undertaken will include not only original research questions, but also comparative implementation methods to better understand how hospitalists in a variety of settings can best translate research findings into clinical practice and ultimately improve patient outcomes.
WORKFORCE
The current number of pediatric hospitalists is difficult to gauge11; estimates range from 1500 to 3000 physicians. There are groups of pediatric hospitalists within several national organizations including the AAP, APA, and SHM, in addition to a very active listserve community. It is likely that only a portion of pediatric hospitalists are represented by membership in these organizations.
Most physicians entering the field of PHM come directly out of residency. A recent survey by Freed et al.12 reported that 3% of current pediatric residents are interested in PHM as a career. In another survey by Freed et al., about 6% of recent pediatric residency graduates reported currently practicing as pediatric hospitalists.13 This difference may indicate a number of pediatricians practicing transiently as pediatric hospitalists.
There are numerous issues that will affect the growth and sustainability of PHM. A large number of pediatric residents entering the field will be needed to maintain current numbers. With 45% of hospitalists in practice less than 3 years,11 the growth of PHM in both numbers and influence will require an increasing number of hospitalists with sustained careers in the field. Recognition as experts in inpatient care, as well as expansion of the role of hospitalists beyond the clinical realm to education, research, and hospital leadership, will foster long‐term career satisfaction. The increasingly common stature of hospital medicine as an independent division, equivalent to general pediatrics and subspecialty divisions within a department, may further bolster the perception of hospital medicine as a career.
The majority of pediatric hospitalists believe that current pediatric residency training does not provide all of the skills necessary to practice as a pediatric hospitalist,14 though there is disagreement regarding how additional training in pediatric hospital medicine should be achieved: a dedicated fellowship versus continuing medical education (CME). There are several initiatives with the potential to transform the way pediatric hospitalists are trained and certified. The Residency Review and Redesign Project indicates that pediatric residency is likely to be reformed to better meet the training demands of the individual resident's chosen career path. Changing residency to better prepare pediatric residents to take positions in pediatric hospital medicine will certainly affect the workforce emerging from residency programs and their subsequent training needs.15 The American Board of Internal Medicine and the American Board of Family Medicine have approved a Recognition of Focused Practice in Hospital Medicine. This recognition is gained through the Maintenance of Certification (MOC) Program of the respective boards after a minimum of 3 years of practice. SHM is offering fellow recognition in tiered designations of Fellow of Hospital Medicine (FHM), Senior Fellow of Hospital Medicine, and Master of Hospital Medicine. Five hundred hospitalists, including many pediatric hospitalists, received the inaugural FHM designation in 2009. Organizational recognition is a common process in many other medical fields, although previously limited in pediatrics to Fellow of the AAP. FHM is an important step, but cannot substitute for specific training and certification.
Academic fellowships in PHM will aid in the training of hospitalists with scholarly skills and will help produce more pediatric hospitalists with clinical, quality, administrative, and leadership skills. A model of subspecialty fellowship training and certification of all PHM physicians would require a several‐fold increase in available fellowships, currently approximately 15.
Ongoing CME offerings are also critical to sustaining and developing the workforce. The annual national meetings of the APA, AAP, and SHM all offer PHM‐dedicated content, and there is an annual PHM conference sponsored by these 3 organizations. There are now multiple additional national and regional meetings focused on PHM, reflecting the growing audience for PHM CME content. The AAP offers a PHM study guide and an Education in quality improvement for pediatric practice (eQIPP) module on inpatient asthma, specifically designed to facilitate the MOC process for pediatric hospitalists.
Some form of ABP recognition may be necessary to provide the status for PHM to be widely recognized as a viable academic career in the larger pediatric community. This would entail standardized fellowships that will ensure graduates have demonstrated proficiency in the core competencies. PHM leaders have engaged the ABP to better understand the subspecialty approval process and thoughtfully examine the ramifications of subspecialty status, specifically what subspecialty certification would mean for PHM providers and hospitals. Achieving ABP certification may create a new standard of care meaning that noncertified PHM providers will be at a disadvantage. It is unknown what the impact on pediatric inpatient care would be if a PHM standard was set without the supply of practitioners to provide that care.
STRUCTURE
The efforts of the Roundtable demonstrate the potential effectiveness of the current structure that guides the field: that of the cooperative interchange between the PHM leaders within the APA, AAP, and SHM. It may be that, similar to Pediatric Emergency Medicine (PEM), no formal, unifying structure is necessary. Alternatively, both Adolescent Medicine and Behavioral and Developmental Pediatrics (BDP) have their own organizations that guide their respective fields. A hybrid model is that of Pediatric Cardiology which has the Joint Council on Congenital Heart Disease. This structure assures that the leaders of the various organizations concerned with congenital heart disease meet at least annually to report on their activities and coordinate future efforts. Its makeup is similar to how the planning committee of the annual national PHM conference is constructed. Although PHM has largely succeeded with the current organizational structure, it is possible that a more formal structure is needed to continue forward.
CONCLUSION
The Roundtable members developed the following vision for PHM: Pediatric hospitalists will transform the delivery of hospital care for children. This will be done by achieving 7 goals (Table 2).
|
| We will ensure that care for hospitalized children is fully integrated and includes the medical home |
| We will design and support systems for children that eliminate harm associated with hospital care |
| We will develop a skilled and stable workforce that is the preferred provider of care for most hospitalized children |
| We will use collaborative research models to answer questions of clinical efficacy, comparative effectiveness, and quality improvement, and we will deliver care based on that knowledge |
| We will provide the expertise that supports continuing education in the care of the hospitalized child for pediatric hospitalists, trainees, midlevel providers, and hospital staff |
| We will create value for our patients and organizations in which we work based on our unique expertise in PHM clinical care, research, and education |
| We will be leaders and influential agents in national health care policies that impact hospital care |
Attaining this vision will take tremendous dedication, effort, and collaboration. As a starting point, the following initiatives were proposed and implemented as noted:
Clinical
-
Develop an educational plan supporting the PHM Core Competencies, addressing both hospitalist training needs and the role as formal educators.
-
Create a clinical practice monitoring dashboard template for use at PHM hospitals and practices (implemented July 2010).
Quality
-
Undertake environmental assessment of PHM participation on key quality and safety committees, societies, and agencies to ensure appropriate PHM representation in liaison and/or leadership positions.
-
Create a plan for a QI collaborative by assessing the needs and resources available; draft plans for 2 projects (1 safety and 1 quality) which will improve care for children hospitalized with common conditions (started July 2009).
Research
-
Create a collaborative research entity by restructuring the existing research network and formalizing relationships with affiliated networks.
-
Create a pipeline/mentorship system to increase the number of PHM researchers.
Workforce
-
Develop a descriptive statement that can be used by any PHM physician that defines the field of PHM and answers the question who are we?
-
Develop a communications tool describing value added of PHM.
-
Develop a tool to assess career satisfaction among PHM physicians, with links to current SHM work in this area.
Structure
-
Formalize an organizational infrastructure for oversight and guidance of PHM Strategic Planning Roundtable efforts, with clear delineation of the relationships with the AAP, APA, and SHM.
This review demonstrates the work that needs to be done to close the gaps between the current state of affairs and the full vision of the potential impact of PHM. Harm is still common in hospitalized children, and, as a group of physicians, we do not consistently provide evidence‐based care. Quality and safety activities are currently dispersed throughout multiple national entities often working in silos. Much of our PHM research is fragmented, with a lack of effective research networks and collaborative efforts. We also found that while our workforce has many strengths, it is not yet stable.
We believe the Roundtable was successful in describing the current state of PHM and laying a course for the future. We developed a series of deliverable products that have already seen success on many fronts, and that will serve as the foundation for further maturation of the field. We hope to engage the pediatric community, within and without PHM, to comment, advise, and foster PHM so that these efforts are not static but ongoing and evolving. Already, new challenges have arisen not addressed at the Roundtable, such as further resident work restrictions, and healthcare reform with its potential effects on hospital finances. This is truly an exciting and dynamic time, and we know that this is just the beginning.
Acknowledgements
The authors acknowledge the contribution of all members of the roundtable: Douglas Carlson, Vincent Chiang, Patrick Conway, Jennifer Daru, Matthew Garber, Christopher Landrigan, Patricia Lye, Sanjay Mahant, Jennifer Maniscalco, Sanford Melzer, Stephen Muething, Steve Narang, Mary Ottolini, Jack Percelay, Daniel Rauch, Mario Reyes, Beth Robbins, Jeff Sperring, Rajendu Srivastava, Erin Stucky, Lisa Zaoutis, and David Zipes. The authors thank David Zipes for his help in reviewing the manuscript.
- ,.The hospitalist movement 5 years later.JAMA.2002;287(4):487–494.
- ,,.The Physician Strategist: Setting Strategic Direction for Your Practice; Chicago, Irwin Professional Pub,1996.
- ,.Pediatric hospitalists: training, current practice, and career goals.J Hosp Med.2009;4(3):179–186.
- The Pediatric Core Competencies Supplement.J Hosp Med.2010;5(suppl 2):1–114.
- ,,,.Reauthorizing SCHIP: Opportunities for Promoting Effective Health Coverage and High Quality Care for Children and Adolescents. Publication 1051.New York, NY:The Commonwealth Fund; August2007:4.
- ,.National Association of Children's Hospitals. Quality Performance Measurement in Medicaid and SCHIP: Result of a 2006 National Survey of State Officials.Lansing, MI:Health Management Associates; August2006.
- ,,,.A report card on quality improvement for children's health care.Pediatrics.2001;107:143–155.
- ,,, et al.Impact of a hospitalist system on length of stay and cost for children with common conditions.Pediatrics.2007;120(2):267–274.
- ,,,,,.Variations in management of common inpatient pediatric illnesses: hospitalists and community pediatricians.Pediatrics.2006;118:441–447.
- ,,,,.Variation in pediatric hospitalists' use of unproven therapies: a study from the Pediatric Research in Inpatient Settings (PRIS) network.J Hosp Med.2008;3(4):292–298.
- ,,,.Characteristics of the pediatric hospitalist workforce: its roles and work environment.Pediatrics.2007;120(1):33–39.
- ,,,,.General pediatrics resident perspectives on training decisions and career choice.Pediatrics.2009;123(suppl 1):S26–S30.
- ,,,,.Recently trained general pediatricians: perspectives on residency training and scope of practice.Pediatrics.2009;123(suppl 1):S38–S43.
- ,,,.PRIS survey: pediatric hospitalist roles and training needs [abstract].Pediatr Res.2004(55):1.
- ,,.The Residency Review and Redesign in Pediatrics (R3P) Project: roots and branches.Pediatrics.2009;123(suppl 1):S8–S11.
Hospitalists are the fastest growing segment of physicians in the United States.1 Given the growing field of Pediatric Hospital Medicine (PHM) and the need to define strategic direction, the Society of Hospital Medicine (SHM), the American Academy of Pediatrics (AAP), and the Academic Pediatric Association (APA) sponsored a strategic planning meeting in February 2009 that brought together 22 PHM leaders to discuss the future of the field.
PHM is at a critical juncture in terms of clinical practice, research, workforce issues, and quality improvement. The field has developed sufficiently to produce leaders capable of setting an agenda and moving forward. A discussion with the American Board of Pediatrics (ABP) by PHM leaders from the AAP, APA, and SHM at the Pediatric Hospital Medicine 2007 Conference regarding subspecialty designation stimulated convening the PHM Strategic Planning Roundtable to address the task of coordinating further development of PHM (Table 1).
|
| Develop a strategic vision for the role of PHM in the future of children's health care |
| Describe the current gaps between the vision and today's reality |
| Develop a common understanding regarding current initiatives in PHM domains of clinical practice, quality, research, and workforce |
| Determine the method(s) by which participants can be organized to accomplish additional initiatives to implement the vision |
| Identify and prioritize key strategic initiatives |
| Assign accountability and determine next steps and timeline to implement the selected initiatives |
The objective of this article is to describe: (1) the Strategic Planning Roundtable's vision for the field of pediatric hospital medicine; (2) the generation and progress on specific initiatives in clinical practice, quality, research, and workforce identified by the Strategic Planning Roundtable; and (3) issues in the designation of PHM as a subspecialty.
METHODS
The PHM Strategic Planning Roundtable was conducted by a facilitator (S.M.) during a 2‐day retreat using established healthcare strategic planning methods.2
Participants were the existing PHM leaders from the AAP, APA, and SHM, as well as other national leaders in clinical practice, quality, research, and workforce. Development of the vision statement was a key step in which the participants developed a consensus‐based aspirational view of the future. The draft version of the vision statement was initially developed after extensive interviews with key stakeholders and experts in PHM, and was revised by the participants in the course of a facilitated group discussion during the retreat. Following creation of the vision statement, the group then defined the elements of transformation pertaining to PHM and detailed the components of the vision.
Analysis of internal and external environmental factors was critical in the strategic planning process. This type of analysis, detailing the current state of PHM practice, permitted the strategic planners to understand the gaps that existed between the aspirational vision statement and today's reality, and set the stage to identify and implement initiatives to achieve the vision. Several months before the meeting, 4 expert panels comprised of PHM specialists representing a variety of academic and clinical practice settings were brought together via e‐mail and conference calls to focus on 4 domains of PHM: clinical practice, quality of care, research, and workforce. These groups were asked to describe the current status, challenges, and opportunities in these areas. Combining literature review and key stakeholder interviews, their findings and recommendations were distilled into brief summaries that were presented at the Roundtable meeting. Following the presentations, the participants, working in small groups representing all areas of focus,provided additional feedback.
Following the creation of a consensus vision statement and review of internal and external factors, the participants worked to identify specific initiatives in the 4 domains that would advance the field towards the goals contained in the vision statement. These initiatives were grouped into categories. Initiatives by category were scored and prioritized according to predetermined criteria including potential impact, cost, operational complexity, and achievability.
For each initiative selected, the group developed targets and metrics that would be used to track progress. Assigning leadership, accountability, and a timeline to each of the selected projects completed the implementation plan. In addition, the group developed an organizational structure to provide oversight for the overall process, and designated individuals representing the sponsoring organizations into those roles. In conclusion, the group discussed potential structures to guide the future of PHM.
CLINICAL PRACTICE
The Roundtable defined clinical practice for PHM as the general medical care of the hospitalized child, including direct patient care and leadership of the inpatient service. Clinical practice is affected by a number of current national trends including: fewer primary care providers interested in, or with the time to provide, inpatient care; resident work hour restrictions; increasing complexity of clinical issues; and increasing availability of pediatric hospitalists. At the hospital level, clinical practice is affected by increasing need for quality and safety measures, electronic health records and computerized physician order entry, and mounting financial pressures on the hospital system. Hospitalists are assuming more roles in leading quality and safety initiatives, creating computerized systems that address children's needs, and creating financially viable systems of quality pediatric care.3 Hospitalists' clinical care and leadership roles are emerging, and therefore the field faces training and mentorship issues.
Progress to date in this area includes 2 textbooks that define a scope of knowledge and practice, and a newly developed journal in PHM. Core competencies in PHM have been published and provide further refinement of scope and a template for future training.4
Multiple opportunities exist for hospitalists to establish themselves as clinical leaders. Hospitalists can become the preferred providers for hospitalized chronically ill children, with specific initiatives to improve care coordination and multidisciplinary communication. In addition to care coordination and decreasing length of stay, hospitalists, with their intimate knowledge of hospital operations, can be leaders in hospital capacity management and patient flow to increase operational efficiency. Hospitalists can expand evidence‐based guidelines for, and data about, inpatient conditions, and explore the effect of workload and hours on patient care. In addition, there is an expanding role into administrative areas, as well as alternate care arenas, such as: intensive care support (pediatric and neonatal), transport, sedation, palliative care, and pain management. Activities in administrative and alternate care areas have profound direct affects on patient care, as well as providing value added services and additional revenue streams which can further support clinical needs. Finally, achieving quality targets will likely be increasingly linked to payment, so hospitalists may play a key role in the incentives paid to their hospitals. Meeting these challenges will further solidify the standing of hospitalists in the clinical realm.
QUALITY
National and governmental agencies have influenced quality and performance improvement measurements in adult healthcare, resulting in improvements in adult healthcare quality measurement.5 There is limited similar influence or measure development in pediatric medicine, so the quality chasm between adult and child healthcare has widened. Few resources are invested in improving quality and safety of pediatric inpatient care. Of the 18 private health insurance plans' quality and pay for performance programs identified by Leapfrog, only 17% developed pediatric‐specific inpatient measures.6 Only 5 of 40 controlled trials of quality improvement efforts for children published between 1980 and 1998 addressed inpatient problems.7
There have been recent efforts at the national level addressing these issues, highlighted by the introduction of The Children's Health Care Quality Act, in 2007. Early studies in PHM systems focused on overall operational efficiency, documenting 9% to 16% decreases in length of stay and cost compared to traditional models of care.8 Conway et al. identified higher reported adherence to evidence‐based care for hospitalists compared to community pediatricians.9 However, Landrigan et al. demonstrated that there is still large variation in care that exists in the management of common inpatient diagnoses, lacking strong evidence‐based guidelines even among pediatric hospitalists.10 Moreover, there have been no significant studies reviewing the impact of pediatric hospitalists on safety of inpatient care. Magnifying these challenges is the reality that our healthcare system is fragmented with various entities scrambling to define, measure, and compare the effectiveness and safety of pediatric healthcare.
These challenges create an opportunity for PHM to develop a model of how to deliver the highest quality and safest care to our patients. The solution is complex and will take cooperation at many levels of our healthcare system. Improving the safety and quality of care for children in all settings of inpatient care in the United States may best be accomplished via an effective collaborative. This collaborative should be comprehensive and inclusive, and focused on demonstrating and disseminating how standardized, evidence‐based care in both clinical and safety domains can lead to high‐value and high‐quality outcomes. The success of PHM will be measured by its ability to deliver a clear value proposition to all consumers and payers of healthcare. The creation of a robust national collaborative network is a first step towards meeting this goal and will take an extraordinary effort. A PHM Quality Improvement (QI) Collaborative workgroup was created in August 2009. Three collaboratives have been commissioned: (1) Reduction of patient identification errors; (2) Improving discharge communication to referring primary care providers for pediatric hospitalist programs, and (3) Reducing the misuse and overuse of bronchodilators for bronchiolitis. All the collaborative groups have effectively engaged key groups of stakeholders and utilized standard QI tools, demonstrating improvement by the fall of 2010 (unpublished data, S.N.).
RESEARCH
Despite being a relatively young field, there is a critical mass of pediatric hospitalist‐investigators who are establishing research career paths for themselves by securing external grant funding for their work, publishing, and receiving mentorship from largely non‐hospitalist mentors. Some hospitalists are now in a position to mentor junior investigators. These hospitalist‐investigators identified a collective goal of working together across multiple sites in a clinical research network. The goal is to conduct high‐quality studies and provide the necessary clinical information to allow practicing hospitalists to make better decisions regarding patient care. This new inpatient evidence‐base will have the added advantage of helping further define the field of PHM.
The Pediatric Research in Inpatient Settings Network (PRIS) was identified as the vehicle to accomplish these goals. A series of objectives were identified to redesign PRIS in order to accommodate and organize this new influx of hospitalist‐investigators. These objectives included having hospitalist‐investigators commit their time to the prioritization, design, and execution of multicenter studies, drafting new governance documents for PRIS, securing external funding, redefining the relationships of the 3 existing organizations that formed PRIS (AAP, APA, SHM), defining how new clinical sites could be added to PRIS, creating a pipeline for junior hospitalist‐investigators to transition to leadership roles, securing a data coordinating center with established expertise in conducting multicenter studies, and establishing an external research advisory committee of leaders in pediatric clinical research and QI.
Several critical issues were identified, but funding remained a priority for the sustainability of PRIS. Comparative effectiveness (CE) was recognized as a potential important source of future funding. Pediatric studies on CE (eg, surgery vs medical management) conducted by PRIS would provide important new data to allow hospitalists to practice evidence‐based medicine and to improve quality.
A Research Leadership Task Force was created with 4 members of the PHM Strategic Planning Roundtable to work on the identified issues. The APA leadership worked with PRIS to establish a new Executive Council (comprised of additional qualified hospitalist‐investigators). The Executive Council was charged with accomplishing the tasks outlined from the Strategic Planning Roundtable. They have created the governance documents and standard operating procedures necessary for PRIS to conduct multicenter studies, defined a strategic framework for PRIS including the mission, vision and values, and funding strategy. In February 2010, PRIS received a 3‐year award for over $1 million from the Child Health Corporation of America to both fund the infrastructure of PRIS and to conduct a Prioritization Project. The Prioritization Project seeks to identify the conditions that are costly, prevalent, and demonstrate high inter‐hospital variation in resource utilization, which signals either lack of high‐quality data upon which to base medical decisions, and/or an opportunity to standardize care across hospitals. Some of these conditions will warrant further investigation to define the evidence base, whereas other conditions may require implementation studies to reliably introduce evidence into practice. Members of the Executive Council received additional funding to investigate community settings, as most children are hospitalized outside of large children's hospitals. PRIS also reengaged all 3 societies (APA, AAP, and SHM) for support for the first face‐to‐face meeting of the Executive Council. PRIS applied for 2 Recovery Act stimulus grants, and received funding for both of approximately $12 million. The processes used to design, provide feedback, and shepherd these initial studies formed the basis for the standard operating procedures for the Network. PRIS is now reengaging its membership to establish how sites may be able to conduct research, and receive new ideas to be considered for study in PRIS.
Although much work remains to be done, the Executive Council is continuing the charge with quarterly face‐to‐face meetings, hiring of a full‐time PRIS Coordinator, and carrying out these initial projects, while maintaining the goal of meeting the needs of the membership and PHM. If PRIS is to accomplish its mission of improving the health of, and healthcare delivery to, hospitalized children and their families, then the types of studies undertaken will include not only original research questions, but also comparative implementation methods to better understand how hospitalists in a variety of settings can best translate research findings into clinical practice and ultimately improve patient outcomes.
WORKFORCE
The current number of pediatric hospitalists is difficult to gauge11; estimates range from 1500 to 3000 physicians. There are groups of pediatric hospitalists within several national organizations including the AAP, APA, and SHM, in addition to a very active listserve community. It is likely that only a portion of pediatric hospitalists are represented by membership in these organizations.
Most physicians entering the field of PHM come directly out of residency. A recent survey by Freed et al.12 reported that 3% of current pediatric residents are interested in PHM as a career. In another survey by Freed et al., about 6% of recent pediatric residency graduates reported currently practicing as pediatric hospitalists.13 This difference may indicate a number of pediatricians practicing transiently as pediatric hospitalists.
There are numerous issues that will affect the growth and sustainability of PHM. A large number of pediatric residents entering the field will be needed to maintain current numbers. With 45% of hospitalists in practice less than 3 years,11 the growth of PHM in both numbers and influence will require an increasing number of hospitalists with sustained careers in the field. Recognition as experts in inpatient care, as well as expansion of the role of hospitalists beyond the clinical realm to education, research, and hospital leadership, will foster long‐term career satisfaction. The increasingly common stature of hospital medicine as an independent division, equivalent to general pediatrics and subspecialty divisions within a department, may further bolster the perception of hospital medicine as a career.
The majority of pediatric hospitalists believe that current pediatric residency training does not provide all of the skills necessary to practice as a pediatric hospitalist,14 though there is disagreement regarding how additional training in pediatric hospital medicine should be achieved: a dedicated fellowship versus continuing medical education (CME). There are several initiatives with the potential to transform the way pediatric hospitalists are trained and certified. The Residency Review and Redesign Project indicates that pediatric residency is likely to be reformed to better meet the training demands of the individual resident's chosen career path. Changing residency to better prepare pediatric residents to take positions in pediatric hospital medicine will certainly affect the workforce emerging from residency programs and their subsequent training needs.15 The American Board of Internal Medicine and the American Board of Family Medicine have approved a Recognition of Focused Practice in Hospital Medicine. This recognition is gained through the Maintenance of Certification (MOC) Program of the respective boards after a minimum of 3 years of practice. SHM is offering fellow recognition in tiered designations of Fellow of Hospital Medicine (FHM), Senior Fellow of Hospital Medicine, and Master of Hospital Medicine. Five hundred hospitalists, including many pediatric hospitalists, received the inaugural FHM designation in 2009. Organizational recognition is a common process in many other medical fields, although previously limited in pediatrics to Fellow of the AAP. FHM is an important step, but cannot substitute for specific training and certification.
Academic fellowships in PHM will aid in the training of hospitalists with scholarly skills and will help produce more pediatric hospitalists with clinical, quality, administrative, and leadership skills. A model of subspecialty fellowship training and certification of all PHM physicians would require a several‐fold increase in available fellowships, currently approximately 15.
Ongoing CME offerings are also critical to sustaining and developing the workforce. The annual national meetings of the APA, AAP, and SHM all offer PHM‐dedicated content, and there is an annual PHM conference sponsored by these 3 organizations. There are now multiple additional national and regional meetings focused on PHM, reflecting the growing audience for PHM CME content. The AAP offers a PHM study guide and an Education in quality improvement for pediatric practice (eQIPP) module on inpatient asthma, specifically designed to facilitate the MOC process for pediatric hospitalists.
Some form of ABP recognition may be necessary to provide the status for PHM to be widely recognized as a viable academic career in the larger pediatric community. This would entail standardized fellowships that will ensure graduates have demonstrated proficiency in the core competencies. PHM leaders have engaged the ABP to better understand the subspecialty approval process and thoughtfully examine the ramifications of subspecialty status, specifically what subspecialty certification would mean for PHM providers and hospitals. Achieving ABP certification may create a new standard of care meaning that noncertified PHM providers will be at a disadvantage. It is unknown what the impact on pediatric inpatient care would be if a PHM standard was set without the supply of practitioners to provide that care.
STRUCTURE
The efforts of the Roundtable demonstrate the potential effectiveness of the current structure that guides the field: that of the cooperative interchange between the PHM leaders within the APA, AAP, and SHM. It may be that, similar to Pediatric Emergency Medicine (PEM), no formal, unifying structure is necessary. Alternatively, both Adolescent Medicine and Behavioral and Developmental Pediatrics (BDP) have their own organizations that guide their respective fields. A hybrid model is that of Pediatric Cardiology which has the Joint Council on Congenital Heart Disease. This structure assures that the leaders of the various organizations concerned with congenital heart disease meet at least annually to report on their activities and coordinate future efforts. Its makeup is similar to how the planning committee of the annual national PHM conference is constructed. Although PHM has largely succeeded with the current organizational structure, it is possible that a more formal structure is needed to continue forward.
CONCLUSION
The Roundtable members developed the following vision for PHM: Pediatric hospitalists will transform the delivery of hospital care for children. This will be done by achieving 7 goals (Table 2).
|
| We will ensure that care for hospitalized children is fully integrated and includes the medical home |
| We will design and support systems for children that eliminate harm associated with hospital care |
| We will develop a skilled and stable workforce that is the preferred provider of care for most hospitalized children |
| We will use collaborative research models to answer questions of clinical efficacy, comparative effectiveness, and quality improvement, and we will deliver care based on that knowledge |
| We will provide the expertise that supports continuing education in the care of the hospitalized child for pediatric hospitalists, trainees, midlevel providers, and hospital staff |
| We will create value for our patients and organizations in which we work based on our unique expertise in PHM clinical care, research, and education |
| We will be leaders and influential agents in national health care policies that impact hospital care |
Attaining this vision will take tremendous dedication, effort, and collaboration. As a starting point, the following initiatives were proposed and implemented as noted:
Clinical
-
Develop an educational plan supporting the PHM Core Competencies, addressing both hospitalist training needs and the role as formal educators.
-
Create a clinical practice monitoring dashboard template for use at PHM hospitals and practices (implemented July 2010).
Quality
-
Undertake environmental assessment of PHM participation on key quality and safety committees, societies, and agencies to ensure appropriate PHM representation in liaison and/or leadership positions.
-
Create a plan for a QI collaborative by assessing the needs and resources available; draft plans for 2 projects (1 safety and 1 quality) which will improve care for children hospitalized with common conditions (started July 2009).
Research
-
Create a collaborative research entity by restructuring the existing research network and formalizing relationships with affiliated networks.
-
Create a pipeline/mentorship system to increase the number of PHM researchers.
Workforce
-
Develop a descriptive statement that can be used by any PHM physician that defines the field of PHM and answers the question who are we?
-
Develop a communications tool describing value added of PHM.
-
Develop a tool to assess career satisfaction among PHM physicians, with links to current SHM work in this area.
Structure
-
Formalize an organizational infrastructure for oversight and guidance of PHM Strategic Planning Roundtable efforts, with clear delineation of the relationships with the AAP, APA, and SHM.
This review demonstrates the work that needs to be done to close the gaps between the current state of affairs and the full vision of the potential impact of PHM. Harm is still common in hospitalized children, and, as a group of physicians, we do not consistently provide evidence‐based care. Quality and safety activities are currently dispersed throughout multiple national entities often working in silos. Much of our PHM research is fragmented, with a lack of effective research networks and collaborative efforts. We also found that while our workforce has many strengths, it is not yet stable.
We believe the Roundtable was successful in describing the current state of PHM and laying a course for the future. We developed a series of deliverable products that have already seen success on many fronts, and that will serve as the foundation for further maturation of the field. We hope to engage the pediatric community, within and without PHM, to comment, advise, and foster PHM so that these efforts are not static but ongoing and evolving. Already, new challenges have arisen not addressed at the Roundtable, such as further resident work restrictions, and healthcare reform with its potential effects on hospital finances. This is truly an exciting and dynamic time, and we know that this is just the beginning.
Acknowledgements
The authors acknowledge the contribution of all members of the roundtable: Douglas Carlson, Vincent Chiang, Patrick Conway, Jennifer Daru, Matthew Garber, Christopher Landrigan, Patricia Lye, Sanjay Mahant, Jennifer Maniscalco, Sanford Melzer, Stephen Muething, Steve Narang, Mary Ottolini, Jack Percelay, Daniel Rauch, Mario Reyes, Beth Robbins, Jeff Sperring, Rajendu Srivastava, Erin Stucky, Lisa Zaoutis, and David Zipes. The authors thank David Zipes for his help in reviewing the manuscript.
Hospitalists are the fastest growing segment of physicians in the United States.1 Given the growing field of Pediatric Hospital Medicine (PHM) and the need to define strategic direction, the Society of Hospital Medicine (SHM), the American Academy of Pediatrics (AAP), and the Academic Pediatric Association (APA) sponsored a strategic planning meeting in February 2009 that brought together 22 PHM leaders to discuss the future of the field.
PHM is at a critical juncture in terms of clinical practice, research, workforce issues, and quality improvement. The field has developed sufficiently to produce leaders capable of setting an agenda and moving forward. A discussion with the American Board of Pediatrics (ABP) by PHM leaders from the AAP, APA, and SHM at the Pediatric Hospital Medicine 2007 Conference regarding subspecialty designation stimulated convening the PHM Strategic Planning Roundtable to address the task of coordinating further development of PHM (Table 1).
|
| Develop a strategic vision for the role of PHM in the future of children's health care |
| Describe the current gaps between the vision and today's reality |
| Develop a common understanding regarding current initiatives in PHM domains of clinical practice, quality, research, and workforce |
| Determine the method(s) by which participants can be organized to accomplish additional initiatives to implement the vision |
| Identify and prioritize key strategic initiatives |
| Assign accountability and determine next steps and timeline to implement the selected initiatives |
The objective of this article is to describe: (1) the Strategic Planning Roundtable's vision for the field of pediatric hospital medicine; (2) the generation and progress on specific initiatives in clinical practice, quality, research, and workforce identified by the Strategic Planning Roundtable; and (3) issues in the designation of PHM as a subspecialty.
METHODS
The PHM Strategic Planning Roundtable was conducted by a facilitator (S.M.) during a 2‐day retreat using established healthcare strategic planning methods.2
Participants were the existing PHM leaders from the AAP, APA, and SHM, as well as other national leaders in clinical practice, quality, research, and workforce. Development of the vision statement was a key step in which the participants developed a consensus‐based aspirational view of the future. The draft version of the vision statement was initially developed after extensive interviews with key stakeholders and experts in PHM, and was revised by the participants in the course of a facilitated group discussion during the retreat. Following creation of the vision statement, the group then defined the elements of transformation pertaining to PHM and detailed the components of the vision.
Analysis of internal and external environmental factors was critical in the strategic planning process. This type of analysis, detailing the current state of PHM practice, permitted the strategic planners to understand the gaps that existed between the aspirational vision statement and today's reality, and set the stage to identify and implement initiatives to achieve the vision. Several months before the meeting, 4 expert panels comprised of PHM specialists representing a variety of academic and clinical practice settings were brought together via e‐mail and conference calls to focus on 4 domains of PHM: clinical practice, quality of care, research, and workforce. These groups were asked to describe the current status, challenges, and opportunities in these areas. Combining literature review and key stakeholder interviews, their findings and recommendations were distilled into brief summaries that were presented at the Roundtable meeting. Following the presentations, the participants, working in small groups representing all areas of focus,provided additional feedback.
Following the creation of a consensus vision statement and review of internal and external factors, the participants worked to identify specific initiatives in the 4 domains that would advance the field towards the goals contained in the vision statement. These initiatives were grouped into categories. Initiatives by category were scored and prioritized according to predetermined criteria including potential impact, cost, operational complexity, and achievability.
For each initiative selected, the group developed targets and metrics that would be used to track progress. Assigning leadership, accountability, and a timeline to each of the selected projects completed the implementation plan. In addition, the group developed an organizational structure to provide oversight for the overall process, and designated individuals representing the sponsoring organizations into those roles. In conclusion, the group discussed potential structures to guide the future of PHM.
CLINICAL PRACTICE
The Roundtable defined clinical practice for PHM as the general medical care of the hospitalized child, including direct patient care and leadership of the inpatient service. Clinical practice is affected by a number of current national trends including: fewer primary care providers interested in, or with the time to provide, inpatient care; resident work hour restrictions; increasing complexity of clinical issues; and increasing availability of pediatric hospitalists. At the hospital level, clinical practice is affected by increasing need for quality and safety measures, electronic health records and computerized physician order entry, and mounting financial pressures on the hospital system. Hospitalists are assuming more roles in leading quality and safety initiatives, creating computerized systems that address children's needs, and creating financially viable systems of quality pediatric care.3 Hospitalists' clinical care and leadership roles are emerging, and therefore the field faces training and mentorship issues.
Progress to date in this area includes 2 textbooks that define a scope of knowledge and practice, and a newly developed journal in PHM. Core competencies in PHM have been published and provide further refinement of scope and a template for future training.4
Multiple opportunities exist for hospitalists to establish themselves as clinical leaders. Hospitalists can become the preferred providers for hospitalized chronically ill children, with specific initiatives to improve care coordination and multidisciplinary communication. In addition to care coordination and decreasing length of stay, hospitalists, with their intimate knowledge of hospital operations, can be leaders in hospital capacity management and patient flow to increase operational efficiency. Hospitalists can expand evidence‐based guidelines for, and data about, inpatient conditions, and explore the effect of workload and hours on patient care. In addition, there is an expanding role into administrative areas, as well as alternate care arenas, such as: intensive care support (pediatric and neonatal), transport, sedation, palliative care, and pain management. Activities in administrative and alternate care areas have profound direct affects on patient care, as well as providing value added services and additional revenue streams which can further support clinical needs. Finally, achieving quality targets will likely be increasingly linked to payment, so hospitalists may play a key role in the incentives paid to their hospitals. Meeting these challenges will further solidify the standing of hospitalists in the clinical realm.
QUALITY
National and governmental agencies have influenced quality and performance improvement measurements in adult healthcare, resulting in improvements in adult healthcare quality measurement.5 There is limited similar influence or measure development in pediatric medicine, so the quality chasm between adult and child healthcare has widened. Few resources are invested in improving quality and safety of pediatric inpatient care. Of the 18 private health insurance plans' quality and pay for performance programs identified by Leapfrog, only 17% developed pediatric‐specific inpatient measures.6 Only 5 of 40 controlled trials of quality improvement efforts for children published between 1980 and 1998 addressed inpatient problems.7
There have been recent efforts at the national level addressing these issues, highlighted by the introduction of The Children's Health Care Quality Act, in 2007. Early studies in PHM systems focused on overall operational efficiency, documenting 9% to 16% decreases in length of stay and cost compared to traditional models of care.8 Conway et al. identified higher reported adherence to evidence‐based care for hospitalists compared to community pediatricians.9 However, Landrigan et al. demonstrated that there is still large variation in care that exists in the management of common inpatient diagnoses, lacking strong evidence‐based guidelines even among pediatric hospitalists.10 Moreover, there have been no significant studies reviewing the impact of pediatric hospitalists on safety of inpatient care. Magnifying these challenges is the reality that our healthcare system is fragmented with various entities scrambling to define, measure, and compare the effectiveness and safety of pediatric healthcare.
These challenges create an opportunity for PHM to develop a model of how to deliver the highest quality and safest care to our patients. The solution is complex and will take cooperation at many levels of our healthcare system. Improving the safety and quality of care for children in all settings of inpatient care in the United States may best be accomplished via an effective collaborative. This collaborative should be comprehensive and inclusive, and focused on demonstrating and disseminating how standardized, evidence‐based care in both clinical and safety domains can lead to high‐value and high‐quality outcomes. The success of PHM will be measured by its ability to deliver a clear value proposition to all consumers and payers of healthcare. The creation of a robust national collaborative network is a first step towards meeting this goal and will take an extraordinary effort. A PHM Quality Improvement (QI) Collaborative workgroup was created in August 2009. Three collaboratives have been commissioned: (1) Reduction of patient identification errors; (2) Improving discharge communication to referring primary care providers for pediatric hospitalist programs, and (3) Reducing the misuse and overuse of bronchodilators for bronchiolitis. All the collaborative groups have effectively engaged key groups of stakeholders and utilized standard QI tools, demonstrating improvement by the fall of 2010 (unpublished data, S.N.).
RESEARCH
Despite being a relatively young field, there is a critical mass of pediatric hospitalist‐investigators who are establishing research career paths for themselves by securing external grant funding for their work, publishing, and receiving mentorship from largely non‐hospitalist mentors. Some hospitalists are now in a position to mentor junior investigators. These hospitalist‐investigators identified a collective goal of working together across multiple sites in a clinical research network. The goal is to conduct high‐quality studies and provide the necessary clinical information to allow practicing hospitalists to make better decisions regarding patient care. This new inpatient evidence‐base will have the added advantage of helping further define the field of PHM.
The Pediatric Research in Inpatient Settings Network (PRIS) was identified as the vehicle to accomplish these goals. A series of objectives were identified to redesign PRIS in order to accommodate and organize this new influx of hospitalist‐investigators. These objectives included having hospitalist‐investigators commit their time to the prioritization, design, and execution of multicenter studies, drafting new governance documents for PRIS, securing external funding, redefining the relationships of the 3 existing organizations that formed PRIS (AAP, APA, SHM), defining how new clinical sites could be added to PRIS, creating a pipeline for junior hospitalist‐investigators to transition to leadership roles, securing a data coordinating center with established expertise in conducting multicenter studies, and establishing an external research advisory committee of leaders in pediatric clinical research and QI.
Several critical issues were identified, but funding remained a priority for the sustainability of PRIS. Comparative effectiveness (CE) was recognized as a potential important source of future funding. Pediatric studies on CE (eg, surgery vs medical management) conducted by PRIS would provide important new data to allow hospitalists to practice evidence‐based medicine and to improve quality.
A Research Leadership Task Force was created with 4 members of the PHM Strategic Planning Roundtable to work on the identified issues. The APA leadership worked with PRIS to establish a new Executive Council (comprised of additional qualified hospitalist‐investigators). The Executive Council was charged with accomplishing the tasks outlined from the Strategic Planning Roundtable. They have created the governance documents and standard operating procedures necessary for PRIS to conduct multicenter studies, defined a strategic framework for PRIS including the mission, vision and values, and funding strategy. In February 2010, PRIS received a 3‐year award for over $1 million from the Child Health Corporation of America to both fund the infrastructure of PRIS and to conduct a Prioritization Project. The Prioritization Project seeks to identify the conditions that are costly, prevalent, and demonstrate high inter‐hospital variation in resource utilization, which signals either lack of high‐quality data upon which to base medical decisions, and/or an opportunity to standardize care across hospitals. Some of these conditions will warrant further investigation to define the evidence base, whereas other conditions may require implementation studies to reliably introduce evidence into practice. Members of the Executive Council received additional funding to investigate community settings, as most children are hospitalized outside of large children's hospitals. PRIS also reengaged all 3 societies (APA, AAP, and SHM) for support for the first face‐to‐face meeting of the Executive Council. PRIS applied for 2 Recovery Act stimulus grants, and received funding for both of approximately $12 million. The processes used to design, provide feedback, and shepherd these initial studies formed the basis for the standard operating procedures for the Network. PRIS is now reengaging its membership to establish how sites may be able to conduct research, and receive new ideas to be considered for study in PRIS.
Although much work remains to be done, the Executive Council is continuing the charge with quarterly face‐to‐face meetings, hiring of a full‐time PRIS Coordinator, and carrying out these initial projects, while maintaining the goal of meeting the needs of the membership and PHM. If PRIS is to accomplish its mission of improving the health of, and healthcare delivery to, hospitalized children and their families, then the types of studies undertaken will include not only original research questions, but also comparative implementation methods to better understand how hospitalists in a variety of settings can best translate research findings into clinical practice and ultimately improve patient outcomes.
WORKFORCE
The current number of pediatric hospitalists is difficult to gauge11; estimates range from 1500 to 3000 physicians. There are groups of pediatric hospitalists within several national organizations including the AAP, APA, and SHM, in addition to a very active listserve community. It is likely that only a portion of pediatric hospitalists are represented by membership in these organizations.
Most physicians entering the field of PHM come directly out of residency. A recent survey by Freed et al.12 reported that 3% of current pediatric residents are interested in PHM as a career. In another survey by Freed et al., about 6% of recent pediatric residency graduates reported currently practicing as pediatric hospitalists.13 This difference may indicate a number of pediatricians practicing transiently as pediatric hospitalists.
There are numerous issues that will affect the growth and sustainability of PHM. A large number of pediatric residents entering the field will be needed to maintain current numbers. With 45% of hospitalists in practice less than 3 years,11 the growth of PHM in both numbers and influence will require an increasing number of hospitalists with sustained careers in the field. Recognition as experts in inpatient care, as well as expansion of the role of hospitalists beyond the clinical realm to education, research, and hospital leadership, will foster long‐term career satisfaction. The increasingly common stature of hospital medicine as an independent division, equivalent to general pediatrics and subspecialty divisions within a department, may further bolster the perception of hospital medicine as a career.
The majority of pediatric hospitalists believe that current pediatric residency training does not provide all of the skills necessary to practice as a pediatric hospitalist,14 though there is disagreement regarding how additional training in pediatric hospital medicine should be achieved: a dedicated fellowship versus continuing medical education (CME). There are several initiatives with the potential to transform the way pediatric hospitalists are trained and certified. The Residency Review and Redesign Project indicates that pediatric residency is likely to be reformed to better meet the training demands of the individual resident's chosen career path. Changing residency to better prepare pediatric residents to take positions in pediatric hospital medicine will certainly affect the workforce emerging from residency programs and their subsequent training needs.15 The American Board of Internal Medicine and the American Board of Family Medicine have approved a Recognition of Focused Practice in Hospital Medicine. This recognition is gained through the Maintenance of Certification (MOC) Program of the respective boards after a minimum of 3 years of practice. SHM is offering fellow recognition in tiered designations of Fellow of Hospital Medicine (FHM), Senior Fellow of Hospital Medicine, and Master of Hospital Medicine. Five hundred hospitalists, including many pediatric hospitalists, received the inaugural FHM designation in 2009. Organizational recognition is a common process in many other medical fields, although previously limited in pediatrics to Fellow of the AAP. FHM is an important step, but cannot substitute for specific training and certification.
Academic fellowships in PHM will aid in the training of hospitalists with scholarly skills and will help produce more pediatric hospitalists with clinical, quality, administrative, and leadership skills. A model of subspecialty fellowship training and certification of all PHM physicians would require a several‐fold increase in available fellowships, currently approximately 15.
Ongoing CME offerings are also critical to sustaining and developing the workforce. The annual national meetings of the APA, AAP, and SHM all offer PHM‐dedicated content, and there is an annual PHM conference sponsored by these 3 organizations. There are now multiple additional national and regional meetings focused on PHM, reflecting the growing audience for PHM CME content. The AAP offers a PHM study guide and an Education in quality improvement for pediatric practice (eQIPP) module on inpatient asthma, specifically designed to facilitate the MOC process for pediatric hospitalists.
Some form of ABP recognition may be necessary to provide the status for PHM to be widely recognized as a viable academic career in the larger pediatric community. This would entail standardized fellowships that will ensure graduates have demonstrated proficiency in the core competencies. PHM leaders have engaged the ABP to better understand the subspecialty approval process and thoughtfully examine the ramifications of subspecialty status, specifically what subspecialty certification would mean for PHM providers and hospitals. Achieving ABP certification may create a new standard of care meaning that noncertified PHM providers will be at a disadvantage. It is unknown what the impact on pediatric inpatient care would be if a PHM standard was set without the supply of practitioners to provide that care.
STRUCTURE
The efforts of the Roundtable demonstrate the potential effectiveness of the current structure that guides the field: that of the cooperative interchange between the PHM leaders within the APA, AAP, and SHM. It may be that, similar to Pediatric Emergency Medicine (PEM), no formal, unifying structure is necessary. Alternatively, both Adolescent Medicine and Behavioral and Developmental Pediatrics (BDP) have their own organizations that guide their respective fields. A hybrid model is that of Pediatric Cardiology which has the Joint Council on Congenital Heart Disease. This structure assures that the leaders of the various organizations concerned with congenital heart disease meet at least annually to report on their activities and coordinate future efforts. Its makeup is similar to how the planning committee of the annual national PHM conference is constructed. Although PHM has largely succeeded with the current organizational structure, it is possible that a more formal structure is needed to continue forward.
CONCLUSION
The Roundtable members developed the following vision for PHM: Pediatric hospitalists will transform the delivery of hospital care for children. This will be done by achieving 7 goals (Table 2).
|
| We will ensure that care for hospitalized children is fully integrated and includes the medical home |
| We will design and support systems for children that eliminate harm associated with hospital care |
| We will develop a skilled and stable workforce that is the preferred provider of care for most hospitalized children |
| We will use collaborative research models to answer questions of clinical efficacy, comparative effectiveness, and quality improvement, and we will deliver care based on that knowledge |
| We will provide the expertise that supports continuing education in the care of the hospitalized child for pediatric hospitalists, trainees, midlevel providers, and hospital staff |
| We will create value for our patients and organizations in which we work based on our unique expertise in PHM clinical care, research, and education |
| We will be leaders and influential agents in national health care policies that impact hospital care |
Attaining this vision will take tremendous dedication, effort, and collaboration. As a starting point, the following initiatives were proposed and implemented as noted:
Clinical
-
Develop an educational plan supporting the PHM Core Competencies, addressing both hospitalist training needs and the role as formal educators.
-
Create a clinical practice monitoring dashboard template for use at PHM hospitals and practices (implemented July 2010).
Quality
-
Undertake environmental assessment of PHM participation on key quality and safety committees, societies, and agencies to ensure appropriate PHM representation in liaison and/or leadership positions.
-
Create a plan for a QI collaborative by assessing the needs and resources available; draft plans for 2 projects (1 safety and 1 quality) which will improve care for children hospitalized with common conditions (started July 2009).
Research
-
Create a collaborative research entity by restructuring the existing research network and formalizing relationships with affiliated networks.
-
Create a pipeline/mentorship system to increase the number of PHM researchers.
Workforce
-
Develop a descriptive statement that can be used by any PHM physician that defines the field of PHM and answers the question who are we?
-
Develop a communications tool describing value added of PHM.
-
Develop a tool to assess career satisfaction among PHM physicians, with links to current SHM work in this area.
Structure
-
Formalize an organizational infrastructure for oversight and guidance of PHM Strategic Planning Roundtable efforts, with clear delineation of the relationships with the AAP, APA, and SHM.
This review demonstrates the work that needs to be done to close the gaps between the current state of affairs and the full vision of the potential impact of PHM. Harm is still common in hospitalized children, and, as a group of physicians, we do not consistently provide evidence‐based care. Quality and safety activities are currently dispersed throughout multiple national entities often working in silos. Much of our PHM research is fragmented, with a lack of effective research networks and collaborative efforts. We also found that while our workforce has many strengths, it is not yet stable.
We believe the Roundtable was successful in describing the current state of PHM and laying a course for the future. We developed a series of deliverable products that have already seen success on many fronts, and that will serve as the foundation for further maturation of the field. We hope to engage the pediatric community, within and without PHM, to comment, advise, and foster PHM so that these efforts are not static but ongoing and evolving. Already, new challenges have arisen not addressed at the Roundtable, such as further resident work restrictions, and healthcare reform with its potential effects on hospital finances. This is truly an exciting and dynamic time, and we know that this is just the beginning.
Acknowledgements
The authors acknowledge the contribution of all members of the roundtable: Douglas Carlson, Vincent Chiang, Patrick Conway, Jennifer Daru, Matthew Garber, Christopher Landrigan, Patricia Lye, Sanjay Mahant, Jennifer Maniscalco, Sanford Melzer, Stephen Muething, Steve Narang, Mary Ottolini, Jack Percelay, Daniel Rauch, Mario Reyes, Beth Robbins, Jeff Sperring, Rajendu Srivastava, Erin Stucky, Lisa Zaoutis, and David Zipes. The authors thank David Zipes for his help in reviewing the manuscript.
- ,.The hospitalist movement 5 years later.JAMA.2002;287(4):487–494.
- ,,.The Physician Strategist: Setting Strategic Direction for Your Practice; Chicago, Irwin Professional Pub,1996.
- ,.Pediatric hospitalists: training, current practice, and career goals.J Hosp Med.2009;4(3):179–186.
- The Pediatric Core Competencies Supplement.J Hosp Med.2010;5(suppl 2):1–114.
- ,,,.Reauthorizing SCHIP: Opportunities for Promoting Effective Health Coverage and High Quality Care for Children and Adolescents. Publication 1051.New York, NY:The Commonwealth Fund; August2007:4.
- ,.National Association of Children's Hospitals. Quality Performance Measurement in Medicaid and SCHIP: Result of a 2006 National Survey of State Officials.Lansing, MI:Health Management Associates; August2006.
- ,,,.A report card on quality improvement for children's health care.Pediatrics.2001;107:143–155.
- ,,, et al.Impact of a hospitalist system on length of stay and cost for children with common conditions.Pediatrics.2007;120(2):267–274.
- ,,,,,.Variations in management of common inpatient pediatric illnesses: hospitalists and community pediatricians.Pediatrics.2006;118:441–447.
- ,,,,.Variation in pediatric hospitalists' use of unproven therapies: a study from the Pediatric Research in Inpatient Settings (PRIS) network.J Hosp Med.2008;3(4):292–298.
- ,,,.Characteristics of the pediatric hospitalist workforce: its roles and work environment.Pediatrics.2007;120(1):33–39.
- ,,,,.General pediatrics resident perspectives on training decisions and career choice.Pediatrics.2009;123(suppl 1):S26–S30.
- ,,,,.Recently trained general pediatricians: perspectives on residency training and scope of practice.Pediatrics.2009;123(suppl 1):S38–S43.
- ,,,.PRIS survey: pediatric hospitalist roles and training needs [abstract].Pediatr Res.2004(55):1.
- ,,.The Residency Review and Redesign in Pediatrics (R3P) Project: roots and branches.Pediatrics.2009;123(suppl 1):S8–S11.
- ,.The hospitalist movement 5 years later.JAMA.2002;287(4):487–494.
- ,,.The Physician Strategist: Setting Strategic Direction for Your Practice; Chicago, Irwin Professional Pub,1996.
- ,.Pediatric hospitalists: training, current practice, and career goals.J Hosp Med.2009;4(3):179–186.
- The Pediatric Core Competencies Supplement.J Hosp Med.2010;5(suppl 2):1–114.
- ,,,.Reauthorizing SCHIP: Opportunities for Promoting Effective Health Coverage and High Quality Care for Children and Adolescents. Publication 1051.New York, NY:The Commonwealth Fund; August2007:4.
- ,.National Association of Children's Hospitals. Quality Performance Measurement in Medicaid and SCHIP: Result of a 2006 National Survey of State Officials.Lansing, MI:Health Management Associates; August2006.
- ,,,.A report card on quality improvement for children's health care.Pediatrics.2001;107:143–155.
- ,,, et al.Impact of a hospitalist system on length of stay and cost for children with common conditions.Pediatrics.2007;120(2):267–274.
- ,,,,,.Variations in management of common inpatient pediatric illnesses: hospitalists and community pediatricians.Pediatrics.2006;118:441–447.
- ,,,,.Variation in pediatric hospitalists' use of unproven therapies: a study from the Pediatric Research in Inpatient Settings (PRIS) network.J Hosp Med.2008;3(4):292–298.
- ,,,.Characteristics of the pediatric hospitalist workforce: its roles and work environment.Pediatrics.2007;120(1):33–39.
- ,,,,.General pediatrics resident perspectives on training decisions and career choice.Pediatrics.2009;123(suppl 1):S26–S30.
- ,,,,.Recently trained general pediatricians: perspectives on residency training and scope of practice.Pediatrics.2009;123(suppl 1):S38–S43.
- ,,,.PRIS survey: pediatric hospitalist roles and training needs [abstract].Pediatr Res.2004(55):1.
- ,,.The Residency Review and Redesign in Pediatrics (R3P) Project: roots and branches.Pediatrics.2009;123(suppl 1):S8–S11.
Delays in Pediatric Discharge
Inpatient pediatrics is undergoing a paradigm shift in at least 3 ways. First, more children with chronic disease are being cared for in the hospital over time.1 Second, previous inpatient conditions are treated at home with advancing technology such as peripherally‐inserted catheters.2 Third, there are new areas of growing specialization, such as hospital medicine, in which the practitioners deliver more efficient care.3, 4
Nationwide, there is increasing pressure to improve inpatient quality of care. The Institute of Medicine defines 6 aims for improvement, including timeliness (reducing waits and sometimes harmful delays for both those who receive and those who give care) and efficiency of care (avoiding waste, including waste of equipment, supplies, ideas, and energy).5 Reducing unnecessary stays in the hospital is a potential quality measure that hospitals may use to address the timeliness and efficiency of care delivered to hospitalized children.
Delays in discharge have been used as markers of unnecessary stays in the hospital for inpatient adult and pediatric care,6, 7 but these are limited to inpatient systems from almost 20 years ago. Current reasons why patients are delayed from discharge, if at all, are not well described. We undertook this study to describe delays in hospital discharges at a tertiary‐care children's hospital in terms of number of patients, length of days of delay, and type of delay. In addition, we sought to characterize the impact of discharge delays on overall length of stay (LOS) and costs.
Methods
Patient Population/Study Design
All children cared for on 2 pediatric medical teams at Primary Children's Medical Center during the month of August 2004 were eligible for the study. Two research assistants independently attended team rounds and collected data relating to: the reasons for ongoing hospitalization, pending items (eg, consultations, tests), and the plan of care for that day. The research assistants each attended daily team rounds for the entire month of August (1 for each team, switching to the opposite team after 2 weeks). This was combined with information available in the Patient Tracker, a software tool developed to improve communication between caregivers and improve discharge efficiency.8 This software tool details diagnoses, daily medical care plans, discharge criteria, and ongoing medical interventions while tracking daily changes in interventions and the medical care plan for each patient cared for on a pediatric medical team.
The research assistants subsequently presented their observations along with information from Patient Tracker to 2 experienced physicians (R.S. and B.S.) who independently determined if a delay occurred, the number of delay days extending discharge, and the cause of the delay, if present, categorized according to the taxonomy of the Delay Tool.6, 7 If there was not enough information for either of the physicians to identify and classify a delay, the electronic medical record of the patient was also reviewed. Discrepancies between physicians assigning delays were discussed until consensus was reached.
The study was approved by the Institutional Review Board of the University of Utah Health Sciences Center and Primary Children's Medical Center (PCMC).
Setting
PCMC is a 233‐bed tertiary‐care children's hospital, owned and operated by Intermountain Healthcare (a not‐for‐profit vertically integrated managed care organization) in the Intermountain West, which serves as both the primary hospital for Salt Lake County and as a tertiary‐care children's hospital for 5 states (UT, MT, WY, ID, and NV).9
Study Definitions
Delay and Length of Delay
Delays in discharge were measured using a validated and reliable instrument, the Delay Tool.6, 7 A discharge was classified as delayed if there was no medical reason for the patient to be in the hospital on a given day, identical to the definition used in the original studies to validate the tool. Delays were recorded as whole days, not fractions of days or hours, as described in the original validation of the tool. For example, if the medical team requested a consultation, and the consultant's opinion was rendered late, but the patient would have remained in the hospital anyway, then this period of time would not count as a delay. However, if the medical team did not receive a consultant's opinion within the standard time (24 hours as defined for this study and in validating studies for the Delay Tool), and the patient's sole reason for being in the hospital during that day was waiting for that opinion, then that period of time would count toward a delay due to a late consultative opinion. Delays of less than 1 day, due to the mechanics of discharging a patient from the hospital (providing prescriptions, follow up, communication, arranging home health, and transportation) were not measured in this study, to match the original methodology of the Delay Tool.
Type of Delay
Primary reason for delay was assigned according to the taxonomy of the Delay Tool.6, 7 Delays were categorized to 1 of the following: (1) test scheduling; (2) obtaining test results; (3) surgery; (4) consultation; (5) patient (eg, family unavailable for decision‐making); (6) physician responsibility; (7) education, training. or research; (8) discharge planning or scheduling; and (9) availability of outside care and resources. There are 166 subcategories that clarify why a delay occurred. For example, within the main category of obtaining test results (2), there are 3 subcategories of delays related to problem in executing the test (2.1), return of results is delayed (2.2), and test results not reviewed within standard time of return (2.3). Subcategories are further divided to provide detail on the cause of delay. For example, a delay categorized as a 2.1:1 [(2) obtaining test results; (2.1) problem in executing the test; and 2.1:1 test to be done by MD is delayed beyond day desired], or 2.3:1 [(2) obtaining test results; (2.3) test results not reviewed within standard time of return; and 2.3:1 delay because physician did not review results]) both relate to physician causes of delays within the general category of obtaining test results. Some delays had more than 1 cause. A secondary cause of delay was assigned if applicable; however, the number of days delayed was attributed to the primary cause for analysis purposes.
Exemptions to Delay and Special Populations
Certain subpopulations of patients presented unique issues that led to them being unlikely to be classified as having a delay. For example, patients with a diagnosis of new onset of type 1 diabetes are historically admitted for 3 days at our hospital, which includes a specific education program; delays were not considered until this minimum period had passed. Children with medically complex care (eg, multisystem disease, multiple specialists involved, multiple medications) were included in this study.10 However, these children with frequent hospital admission were often fragile at discharge, and could meet criteria for readmission even on the date of their discharge, hence assigning a delay day was usually not indicated because of easily justified ongoing medical need for hospitalization.
Study Variables
The LOS, total costs, and routine demographic and administrative data for each study patient were extracted from Intermountain Healthcare's Enterprise Data Warehouse (EDW). The EDW contains detailed data about the cost of providing health care. Costs were derived from the hospital's cost accounting program, the Standard Cost Master, which is a transaction‐based microcosting accounting system.1113
For patients whose LOS extended before August 1 or after August 31, total hospital costs were averaged per day, and only days falling inside the month of August were counted in calculating the impact the delays in discharge had on the total costs of hospitalization. Hospital days that extended outside of August were not counted in either the numerator for potential days of delay or in the denominator for total days in the hospital.
Analyses
Descriptive statistics were calculated for the number, length of days of delay, and type of delay. Interrater reliability to assign a delay was ascertained for the 2 physicians. Mean LOS, mean total costs, and standard deviations (SDs) were calculated. All analyses were performed using Statistical Analytical Software version 9.13 (SAS Institute, Cary, NC).
Results
During the 31 days of the study, 171 patients occupied hospital beds an average of 7.3 days on the 2 inpatient medical teams, for a total of 911 inpatient days. Seven patients were admitted prior to August 1; 6 of these were discharged during the month of August and 1 stayed through the entire month and was discharged in September. Three additional patients were admitted in August and discharged in September. There were 6 readmissions during the month of August, and 1 patient was excluded from the study because of lack of sufficient information. All patients with delays were able to be classified according to the Delay Tool taxonomy. Interrater reliability for the 2 study physicians was 98%.
The characteristics between the patients who did and did not experience a delay in discharge are shown in Table 1. Thirty‐nine of 171 patients (22.8%), experienced at least 1 delay day. Eighteen of 39 patients had only 1 delay day (46.2%) and 11 patients experienced 2 delayed days (28.2%) (Figure 1). The average length of delay was 2.1 days.
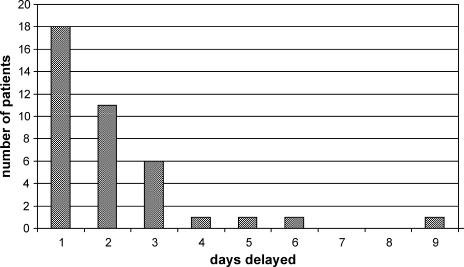
| Nondelayed Patients (N = 132) | Delayed Patients (N = 39) | P Value | |
|---|---|---|---|
| |||
| Age (months), mean (SD)* | 22.6 (14.4) | 15.0 (14.6) | 0.009 |
| LOS during August (days), mean (SD)* | 4.64 (6.1) | 7.64 (7.15) | <0.001 |
| Total costs during August ($), mean (SD)* | 10,451 (19,254) | 14,341 (16,241) | 0.002 |
| Number of ICD‐9 CM diagnoses codes, mean (SD)* | 7.1 (7.4) | 8.5 (7.3) | 0.056 |
| Number of ICD‐9 CM procedure codes, mean (SD)* | 1.7 (3.8) | 1.6 (2.6) | 0.068 |
| Number of Patients with APR‐DRG SOI 3 (%) | 59 (44.7%) | 19 (48.7%) | 0.65 |
Delays attributed to physician responsibility accounted for 42.3% (16.5/39) of patient delays (conservative management or clinical decision‐making), with discharge planning delays accounting for 21.8% (family‐related, patient‐related, and hospital‐related problems), consultation for 14.1% (delay in obtaining or lack of follow‐up), test scheduling for 12.8%, and obtaining test results for 5.1% (ordering and weekend scheduling). There were no primary delays due to surgery, education and research, or unavailability of outside resources such as a skilled nursing bed. Four patients had a single additional secondary cause of delay assigned to them, related to physician responsibility, consultation, surgery and test scheduling; these were split, attributing 0.5 patients to each delay type (thus, the 17/39 patients delayed for physician responsibility was analyzed as 16.5/39) (Table 2).
| Delay Category | Number of Patients Experiencing Delays* | Percentage of All Patients Experiencing Delays (%) | Percentage of Study Patients Observed (%) | Total Delay Days | Average Length of Delays (days) | Percentage of Hospital Days That Were Delay Days (%) |
|---|---|---|---|---|---|---|
| ||||||
| 1. Scheduling | 5 | 12.8 | 2.92 | 16 | 3.20 | 1.76 |
| 2. Obtaining results | 2 | 5.1 | 1.17 | 3 | 1.50 | 0.33 |
| 3. Surgery | 0.5 | 1.3 | 0.29 | 1.5 | 3.00 | 0.16 |
| 4. Consultation | 5.5 | 14.1 | 3.22 | 10.5 | 1.91 | 1.15 |
| 5. Patient | 1 | 2.6 | 0.58 | 2 | 2.00 | 0.22 |
| 6. Physician | 16.5 | 42.3 | 9.65 | 33.5 | 2.03 | 3.68 |
| 7. Education | 0 | 0 | 0.00 | 0 | 0 | 0.00 |
| 8. Discharge | 8.5 | 21.8 | 4.97 | 15.5 | 1.82 | 1.70 |
| 9. Outside | 0 | 0 | 0.00 | 0 | 0 | 0.00 |
| Total | 39 | 100 | 22.81 | 82 | 2.10 | 9.00 |
There were 82 delay‐related hospital days of 911 total inpatient days on the 2 medical teams for August 2004 (9%). More than $170,000 in excess costs was incurred due to delay days from a total of approximately 1.9 million dollars in patient costs for the month (8.9%).
Discussion
This study finds that discharge delays in a tertiary care children's hospital are common; almost 1 in 4 patients experienced a medically unnecessary excess hospital stay of at least 1 day. The average length of a delay was 2.1 days, and overall, delays consumed 9% of pediatric hospital days and 8.9% of total costs. The most common reason for a delay was related to physician clinical care, including excessively conservative management and variability in clinical decision‐making.
Our study results are similar to the other 2 published studies that use the Delay Tool. In the adult and pediatric studies, between 10% and 30% of patients experienced a delay in discharge, with the average length of delay between 2.9 and 3 days.6, 7 Although both studies were conducted at teaching hospitals, what is particularly interesting is that they were conducted almost 20 years ago. During this period of time, there has been a shift in the inpatient pediatric patient population. In recent years, children who are cared for in the hospital have more chronic illnesses.1 In addition, there has been a shift in the types of conditions that may be cared for at home and those that now require inpatient stay.2 Despite this, delays continue at a similar proportion, but the cause of delays have shifted from scheduling and consultation to physician responsibility.
There is another tool in the literature which is more widely used, the Pediatric Appropriateness Evaluation Protocol (PAEP), which is based on the Appropriateness Evaluation Protocol for adults.1417 This tool is used to determine the appropriateness of ongoing hospitalization, not the cause of delay if ongoing hospitalization is inappropriate. The 3 areas that are evaluated (medical services, nursing and ancillary services, and patient's condition) have objective criteria that dictate if the hospitalization is appropriate or not (eg, parenteral (intravenous) therapy for at least 8 hours on that day, under nursing and ancillary services). The PAEP may be less sensitive given today's healthcare resource utilization climate. Many clinicians and families would agree that insertion of a peripheral central catheter is an acceptable form of outpatient treatment for many pediatric conditions. In conjunction with the Delay Tool, the PAEP could be used to determine if a delay occurred, then the Delay Tool used to categorize the cause of the delay. We choose to use expert clinician judgment to determine if a delay had occurred. We were more interested in why patients who are admitted (appropriately or inappropriately) cannot be discharged sooner, thus allowing for future intervention studies targeted to impact delays in discharge, as elucidated in this study. The Delay Tool specifically allowed us to categorize the reasons for delays. Given that the average LOS for patients in the nondelayed group was over 4 days, despite not using a tool such as the PAEP, we believe that these were likely to be appropriate admissions.
A recent study reported the first use of the Medical Care Appropriateness Protocol (MCAP) in a tertiary‐care children's hospital. The authors used the MCAP to determine the impact of an intervention on reducing inappropriate hospitals days for children. This tool is similarly labor‐intensive to the Delay Tool. Interestingly, this Canadian study found a high rate of inappropriate hospital days (47%), which may be in part attributable to a different outcome measurement tool and/or a different health care system.18
There are several limitations to our study that deserve mention. The Delay Tool requires clinician judgment regarding whether or not there was a delay in discharge for that day. We may have introduced some bias in our study, as hospitalist investigators assigned the delay and blinding to the attending physician specialty of record was not feasible. However, our results are similar to the other 2 published studies that have used this tool, and we specifically chose not to analyze or report results in terms of hospitalist and nonhospitalist attending physicians. The Delay Tool is not designed to differentiate shorter delays in terms of hours instead of days (eg, due to the inability for the patient to get a ride home). Shorter delays may be of particular importance depending on the occupancy rate of the hospital, the demand for beds, and other patient and hospital factors. We could not capture these shorter delays (although they did occur frequently) due to the original description of the Delay Tool. In addition, we would not have been able to report data on the impact on LOS and costs, as these are attributed to whole days in the hospital. However, if we had been able to differentiate shorter delays, this would bias our results to show a greater percentage of delays over smaller increments of time. Generalizability is an issue, given that this was a single‐center study. This study sample included over 80 different attending physicians participating in community pediatrician, subspecialty, and hospitalist practice groups. However, the patient population at PCMC is similar to other medium and large children's hospitals in the United States. The month observed may not reflect the entire year of hospitalizationsthere may be seasonal variations with delays depending on the volume and type of illness seen. The study was conducted in August, when there are newer house staff present. However, physician responsibility, which was the largest source of delays in our study, had little attribution to house staff. Most of the decisions were those of attending physicians, which would largely be unaffected by the time of year of the study. Finally, we were unable to assess the safety of the potential earlier discharge, as this was an observational study. However, in any future intervention studies examining processes to discharge patients sooner, measures of safety to the patient are a necessity. Finally, given the potential of ongoing admission, even on the date of discharge of our most fragile patients, this approach to discovering causes of delay may not apply to this important group, which is responsible for significant and growing resource utilization.
Despite these limitations, our findings demonstrate that in an era of children staying in the hospital less, and more medically‐complex children being admitted,10 a substantial number of children who are hospitalized at a children's hospital may have been discharged sooner. The majority of these decisions were directly related to physician responsibility. As consumers, providers, and hospitals work together to develop quality measures that are reflective of inpatient pediatric care, the Delay Tool may be able to highlight 2 aims of quality (ie, timeliness and efficiency of care) that could be used to assess the impact of interventions designed to safely discharge patients sooner. Interventions such as audit‐feedback,18 clinical guideline deployment,19 and hospitalist systems of care4 continue to hold the promise of earlier discharge; however, tools designed to measure inappropriate use of hospital days should be employed to demonstrate their effectiveness. Our study demonstrates ongoing waste in children's hospitals.
Conclusions
Almost 1 out of 4 patients in this 1‐month period could have been discharged sooner than they were. The impact of delays on costs and LOS are substantial and should provide strong incentives to develop effective interventions. Such interventions will need to address variations in physician criteria for discharge, more efficient discharge planning, and timely scheduling of consultation and diagnostic testing.
Acknowledgements
The authors thank Joni R. Beshanky and Harry Selker for their help and training in the use of the Delay Tool.
- .The transformation of child health in the United States: social disparities in child health persistent despite dramatic improvement in child health overall.Health Aff (Millwood).2004;23(5):9–25.
- , , , .Hospitalist care of medically complex children.Pediatr Res.2004;55(4):314A–315A.
- , , , .Pediatric hospitalists: a systematic review of the literature.Pediatrics.2006;117(5):1736–1744.
- , , , et al.Pediatric hospitalists: report of a leadership conference.Pediatrics.2006;117(4):1122–1130.
- Institute of Medicine.Crossing the Quality Chasm: A New Health System for the Twenty‐first Century.Washington, DC:National Academy Press;2001.
- , , .Using the Delay Tool to attribute causes for unnecessary pediatric hospital days.Med Care.1990;28(10):982–989.
- , , , .The epidemiology of delays in a teaching hospital. The development and use of a tool that detects unnecessary hospital days.Med Care.1989;27(2):112–129.
- , , , , .A tool for improving patient discharge process and hospital communication practices: the Patient Tracker.AMIA Annu Symp Proc.2007;11:493–497.
- , .Organizational responses to managed care: issues for academic health centers and implications for pediatric programs.Pediatrics.1998;101(4 Pt 2):805–811; discussion 811–802.
- , , .Hospitalist care of the medically complex child.Pediatr Clin North Am.2005;52(4):1165–1187.
- , , , et al.Epidemiology, complications, and cost of hospitalization in children with laboratory‐confirmed influenza infection.Pediatrics.2006;118(6):2409–2417.
- , , , , , .Clinical and economic outcomes of conventional amphotericin B‐associated nephrotoxicity.Clin Infect Dis.2002;35(12):e120–e127.
- , , , et al.Using a hospital information system to assess the effects of adverse drug events.Proc Annu Symp Comput Appl Med Care.1993;1993:161–165.
- , , .Appropriateness of hospitalization in a Canadian pediatric hospital.Pediatrics.1993;91(1):70–74.
- , , , .The appropriateness evaluation protocol: application in an Australian children's hospital.Aust Clin Rev.1991;11(4):123–131.
- , .Assessing the need to hospitalize children: pediatric appropriateness evaluation protocol.Pediatrics.1989;84(2):242–247.
- .Medically inappropriate hospital use in a pediatric population.N Engl J Med.1988;318(16):1033–1037.
- , , , , .Reducing inappropriate hospital use on a general pediatric inpatient unit.Pediatrics.2008;121(5):e1068–e1073.
- , .Reliable implementation of clinical pathways: what will it take—that is the question.J Pediatr.2008;152(3):303–304.
Inpatient pediatrics is undergoing a paradigm shift in at least 3 ways. First, more children with chronic disease are being cared for in the hospital over time.1 Second, previous inpatient conditions are treated at home with advancing technology such as peripherally‐inserted catheters.2 Third, there are new areas of growing specialization, such as hospital medicine, in which the practitioners deliver more efficient care.3, 4
Nationwide, there is increasing pressure to improve inpatient quality of care. The Institute of Medicine defines 6 aims for improvement, including timeliness (reducing waits and sometimes harmful delays for both those who receive and those who give care) and efficiency of care (avoiding waste, including waste of equipment, supplies, ideas, and energy).5 Reducing unnecessary stays in the hospital is a potential quality measure that hospitals may use to address the timeliness and efficiency of care delivered to hospitalized children.
Delays in discharge have been used as markers of unnecessary stays in the hospital for inpatient adult and pediatric care,6, 7 but these are limited to inpatient systems from almost 20 years ago. Current reasons why patients are delayed from discharge, if at all, are not well described. We undertook this study to describe delays in hospital discharges at a tertiary‐care children's hospital in terms of number of patients, length of days of delay, and type of delay. In addition, we sought to characterize the impact of discharge delays on overall length of stay (LOS) and costs.
Methods
Patient Population/Study Design
All children cared for on 2 pediatric medical teams at Primary Children's Medical Center during the month of August 2004 were eligible for the study. Two research assistants independently attended team rounds and collected data relating to: the reasons for ongoing hospitalization, pending items (eg, consultations, tests), and the plan of care for that day. The research assistants each attended daily team rounds for the entire month of August (1 for each team, switching to the opposite team after 2 weeks). This was combined with information available in the Patient Tracker, a software tool developed to improve communication between caregivers and improve discharge efficiency.8 This software tool details diagnoses, daily medical care plans, discharge criteria, and ongoing medical interventions while tracking daily changes in interventions and the medical care plan for each patient cared for on a pediatric medical team.
The research assistants subsequently presented their observations along with information from Patient Tracker to 2 experienced physicians (R.S. and B.S.) who independently determined if a delay occurred, the number of delay days extending discharge, and the cause of the delay, if present, categorized according to the taxonomy of the Delay Tool.6, 7 If there was not enough information for either of the physicians to identify and classify a delay, the electronic medical record of the patient was also reviewed. Discrepancies between physicians assigning delays were discussed until consensus was reached.
The study was approved by the Institutional Review Board of the University of Utah Health Sciences Center and Primary Children's Medical Center (PCMC).
Setting
PCMC is a 233‐bed tertiary‐care children's hospital, owned and operated by Intermountain Healthcare (a not‐for‐profit vertically integrated managed care organization) in the Intermountain West, which serves as both the primary hospital for Salt Lake County and as a tertiary‐care children's hospital for 5 states (UT, MT, WY, ID, and NV).9
Study Definitions
Delay and Length of Delay
Delays in discharge were measured using a validated and reliable instrument, the Delay Tool.6, 7 A discharge was classified as delayed if there was no medical reason for the patient to be in the hospital on a given day, identical to the definition used in the original studies to validate the tool. Delays were recorded as whole days, not fractions of days or hours, as described in the original validation of the tool. For example, if the medical team requested a consultation, and the consultant's opinion was rendered late, but the patient would have remained in the hospital anyway, then this period of time would not count as a delay. However, if the medical team did not receive a consultant's opinion within the standard time (24 hours as defined for this study and in validating studies for the Delay Tool), and the patient's sole reason for being in the hospital during that day was waiting for that opinion, then that period of time would count toward a delay due to a late consultative opinion. Delays of less than 1 day, due to the mechanics of discharging a patient from the hospital (providing prescriptions, follow up, communication, arranging home health, and transportation) were not measured in this study, to match the original methodology of the Delay Tool.
Type of Delay
Primary reason for delay was assigned according to the taxonomy of the Delay Tool.6, 7 Delays were categorized to 1 of the following: (1) test scheduling; (2) obtaining test results; (3) surgery; (4) consultation; (5) patient (eg, family unavailable for decision‐making); (6) physician responsibility; (7) education, training. or research; (8) discharge planning or scheduling; and (9) availability of outside care and resources. There are 166 subcategories that clarify why a delay occurred. For example, within the main category of obtaining test results (2), there are 3 subcategories of delays related to problem in executing the test (2.1), return of results is delayed (2.2), and test results not reviewed within standard time of return (2.3). Subcategories are further divided to provide detail on the cause of delay. For example, a delay categorized as a 2.1:1 [(2) obtaining test results; (2.1) problem in executing the test; and 2.1:1 test to be done by MD is delayed beyond day desired], or 2.3:1 [(2) obtaining test results; (2.3) test results not reviewed within standard time of return; and 2.3:1 delay because physician did not review results]) both relate to physician causes of delays within the general category of obtaining test results. Some delays had more than 1 cause. A secondary cause of delay was assigned if applicable; however, the number of days delayed was attributed to the primary cause for analysis purposes.
Exemptions to Delay and Special Populations
Certain subpopulations of patients presented unique issues that led to them being unlikely to be classified as having a delay. For example, patients with a diagnosis of new onset of type 1 diabetes are historically admitted for 3 days at our hospital, which includes a specific education program; delays were not considered until this minimum period had passed. Children with medically complex care (eg, multisystem disease, multiple specialists involved, multiple medications) were included in this study.10 However, these children with frequent hospital admission were often fragile at discharge, and could meet criteria for readmission even on the date of their discharge, hence assigning a delay day was usually not indicated because of easily justified ongoing medical need for hospitalization.
Study Variables
The LOS, total costs, and routine demographic and administrative data for each study patient were extracted from Intermountain Healthcare's Enterprise Data Warehouse (EDW). The EDW contains detailed data about the cost of providing health care. Costs were derived from the hospital's cost accounting program, the Standard Cost Master, which is a transaction‐based microcosting accounting system.1113
For patients whose LOS extended before August 1 or after August 31, total hospital costs were averaged per day, and only days falling inside the month of August were counted in calculating the impact the delays in discharge had on the total costs of hospitalization. Hospital days that extended outside of August were not counted in either the numerator for potential days of delay or in the denominator for total days in the hospital.
Analyses
Descriptive statistics were calculated for the number, length of days of delay, and type of delay. Interrater reliability to assign a delay was ascertained for the 2 physicians. Mean LOS, mean total costs, and standard deviations (SDs) were calculated. All analyses were performed using Statistical Analytical Software version 9.13 (SAS Institute, Cary, NC).
Results
During the 31 days of the study, 171 patients occupied hospital beds an average of 7.3 days on the 2 inpatient medical teams, for a total of 911 inpatient days. Seven patients were admitted prior to August 1; 6 of these were discharged during the month of August and 1 stayed through the entire month and was discharged in September. Three additional patients were admitted in August and discharged in September. There were 6 readmissions during the month of August, and 1 patient was excluded from the study because of lack of sufficient information. All patients with delays were able to be classified according to the Delay Tool taxonomy. Interrater reliability for the 2 study physicians was 98%.
The characteristics between the patients who did and did not experience a delay in discharge are shown in Table 1. Thirty‐nine of 171 patients (22.8%), experienced at least 1 delay day. Eighteen of 39 patients had only 1 delay day (46.2%) and 11 patients experienced 2 delayed days (28.2%) (Figure 1). The average length of delay was 2.1 days.

| Nondelayed Patients (N = 132) | Delayed Patients (N = 39) | P Value | |
|---|---|---|---|
| |||
| Age (months), mean (SD)* | 22.6 (14.4) | 15.0 (14.6) | 0.009 |
| LOS during August (days), mean (SD)* | 4.64 (6.1) | 7.64 (7.15) | <0.001 |
| Total costs during August ($), mean (SD)* | 10,451 (19,254) | 14,341 (16,241) | 0.002 |
| Number of ICD‐9 CM diagnoses codes, mean (SD)* | 7.1 (7.4) | 8.5 (7.3) | 0.056 |
| Number of ICD‐9 CM procedure codes, mean (SD)* | 1.7 (3.8) | 1.6 (2.6) | 0.068 |
| Number of Patients with APR‐DRG SOI 3 (%) | 59 (44.7%) | 19 (48.7%) | 0.65 |
Delays attributed to physician responsibility accounted for 42.3% (16.5/39) of patient delays (conservative management or clinical decision‐making), with discharge planning delays accounting for 21.8% (family‐related, patient‐related, and hospital‐related problems), consultation for 14.1% (delay in obtaining or lack of follow‐up), test scheduling for 12.8%, and obtaining test results for 5.1% (ordering and weekend scheduling). There were no primary delays due to surgery, education and research, or unavailability of outside resources such as a skilled nursing bed. Four patients had a single additional secondary cause of delay assigned to them, related to physician responsibility, consultation, surgery and test scheduling; these were split, attributing 0.5 patients to each delay type (thus, the 17/39 patients delayed for physician responsibility was analyzed as 16.5/39) (Table 2).
| Delay Category | Number of Patients Experiencing Delays* | Percentage of All Patients Experiencing Delays (%) | Percentage of Study Patients Observed (%) | Total Delay Days | Average Length of Delays (days) | Percentage of Hospital Days That Were Delay Days (%) |
|---|---|---|---|---|---|---|
| ||||||
| 1. Scheduling | 5 | 12.8 | 2.92 | 16 | 3.20 | 1.76 |
| 2. Obtaining results | 2 | 5.1 | 1.17 | 3 | 1.50 | 0.33 |
| 3. Surgery | 0.5 | 1.3 | 0.29 | 1.5 | 3.00 | 0.16 |
| 4. Consultation | 5.5 | 14.1 | 3.22 | 10.5 | 1.91 | 1.15 |
| 5. Patient | 1 | 2.6 | 0.58 | 2 | 2.00 | 0.22 |
| 6. Physician | 16.5 | 42.3 | 9.65 | 33.5 | 2.03 | 3.68 |
| 7. Education | 0 | 0 | 0.00 | 0 | 0 | 0.00 |
| 8. Discharge | 8.5 | 21.8 | 4.97 | 15.5 | 1.82 | 1.70 |
| 9. Outside | 0 | 0 | 0.00 | 0 | 0 | 0.00 |
| Total | 39 | 100 | 22.81 | 82 | 2.10 | 9.00 |
There were 82 delay‐related hospital days of 911 total inpatient days on the 2 medical teams for August 2004 (9%). More than $170,000 in excess costs was incurred due to delay days from a total of approximately 1.9 million dollars in patient costs for the month (8.9%).
Discussion
This study finds that discharge delays in a tertiary care children's hospital are common; almost 1 in 4 patients experienced a medically unnecessary excess hospital stay of at least 1 day. The average length of a delay was 2.1 days, and overall, delays consumed 9% of pediatric hospital days and 8.9% of total costs. The most common reason for a delay was related to physician clinical care, including excessively conservative management and variability in clinical decision‐making.
Our study results are similar to the other 2 published studies that use the Delay Tool. In the adult and pediatric studies, between 10% and 30% of patients experienced a delay in discharge, with the average length of delay between 2.9 and 3 days.6, 7 Although both studies were conducted at teaching hospitals, what is particularly interesting is that they were conducted almost 20 years ago. During this period of time, there has been a shift in the inpatient pediatric patient population. In recent years, children who are cared for in the hospital have more chronic illnesses.1 In addition, there has been a shift in the types of conditions that may be cared for at home and those that now require inpatient stay.2 Despite this, delays continue at a similar proportion, but the cause of delays have shifted from scheduling and consultation to physician responsibility.
There is another tool in the literature which is more widely used, the Pediatric Appropriateness Evaluation Protocol (PAEP), which is based on the Appropriateness Evaluation Protocol for adults.1417 This tool is used to determine the appropriateness of ongoing hospitalization, not the cause of delay if ongoing hospitalization is inappropriate. The 3 areas that are evaluated (medical services, nursing and ancillary services, and patient's condition) have objective criteria that dictate if the hospitalization is appropriate or not (eg, parenteral (intravenous) therapy for at least 8 hours on that day, under nursing and ancillary services). The PAEP may be less sensitive given today's healthcare resource utilization climate. Many clinicians and families would agree that insertion of a peripheral central catheter is an acceptable form of outpatient treatment for many pediatric conditions. In conjunction with the Delay Tool, the PAEP could be used to determine if a delay occurred, then the Delay Tool used to categorize the cause of the delay. We choose to use expert clinician judgment to determine if a delay had occurred. We were more interested in why patients who are admitted (appropriately or inappropriately) cannot be discharged sooner, thus allowing for future intervention studies targeted to impact delays in discharge, as elucidated in this study. The Delay Tool specifically allowed us to categorize the reasons for delays. Given that the average LOS for patients in the nondelayed group was over 4 days, despite not using a tool such as the PAEP, we believe that these were likely to be appropriate admissions.
A recent study reported the first use of the Medical Care Appropriateness Protocol (MCAP) in a tertiary‐care children's hospital. The authors used the MCAP to determine the impact of an intervention on reducing inappropriate hospitals days for children. This tool is similarly labor‐intensive to the Delay Tool. Interestingly, this Canadian study found a high rate of inappropriate hospital days (47%), which may be in part attributable to a different outcome measurement tool and/or a different health care system.18
There are several limitations to our study that deserve mention. The Delay Tool requires clinician judgment regarding whether or not there was a delay in discharge for that day. We may have introduced some bias in our study, as hospitalist investigators assigned the delay and blinding to the attending physician specialty of record was not feasible. However, our results are similar to the other 2 published studies that have used this tool, and we specifically chose not to analyze or report results in terms of hospitalist and nonhospitalist attending physicians. The Delay Tool is not designed to differentiate shorter delays in terms of hours instead of days (eg, due to the inability for the patient to get a ride home). Shorter delays may be of particular importance depending on the occupancy rate of the hospital, the demand for beds, and other patient and hospital factors. We could not capture these shorter delays (although they did occur frequently) due to the original description of the Delay Tool. In addition, we would not have been able to report data on the impact on LOS and costs, as these are attributed to whole days in the hospital. However, if we had been able to differentiate shorter delays, this would bias our results to show a greater percentage of delays over smaller increments of time. Generalizability is an issue, given that this was a single‐center study. This study sample included over 80 different attending physicians participating in community pediatrician, subspecialty, and hospitalist practice groups. However, the patient population at PCMC is similar to other medium and large children's hospitals in the United States. The month observed may not reflect the entire year of hospitalizationsthere may be seasonal variations with delays depending on the volume and type of illness seen. The study was conducted in August, when there are newer house staff present. However, physician responsibility, which was the largest source of delays in our study, had little attribution to house staff. Most of the decisions were those of attending physicians, which would largely be unaffected by the time of year of the study. Finally, we were unable to assess the safety of the potential earlier discharge, as this was an observational study. However, in any future intervention studies examining processes to discharge patients sooner, measures of safety to the patient are a necessity. Finally, given the potential of ongoing admission, even on the date of discharge of our most fragile patients, this approach to discovering causes of delay may not apply to this important group, which is responsible for significant and growing resource utilization.
Despite these limitations, our findings demonstrate that in an era of children staying in the hospital less, and more medically‐complex children being admitted,10 a substantial number of children who are hospitalized at a children's hospital may have been discharged sooner. The majority of these decisions were directly related to physician responsibility. As consumers, providers, and hospitals work together to develop quality measures that are reflective of inpatient pediatric care, the Delay Tool may be able to highlight 2 aims of quality (ie, timeliness and efficiency of care) that could be used to assess the impact of interventions designed to safely discharge patients sooner. Interventions such as audit‐feedback,18 clinical guideline deployment,19 and hospitalist systems of care4 continue to hold the promise of earlier discharge; however, tools designed to measure inappropriate use of hospital days should be employed to demonstrate their effectiveness. Our study demonstrates ongoing waste in children's hospitals.
Conclusions
Almost 1 out of 4 patients in this 1‐month period could have been discharged sooner than they were. The impact of delays on costs and LOS are substantial and should provide strong incentives to develop effective interventions. Such interventions will need to address variations in physician criteria for discharge, more efficient discharge planning, and timely scheduling of consultation and diagnostic testing.
Acknowledgements
The authors thank Joni R. Beshanky and Harry Selker for their help and training in the use of the Delay Tool.
Inpatient pediatrics is undergoing a paradigm shift in at least 3 ways. First, more children with chronic disease are being cared for in the hospital over time.1 Second, previous inpatient conditions are treated at home with advancing technology such as peripherally‐inserted catheters.2 Third, there are new areas of growing specialization, such as hospital medicine, in which the practitioners deliver more efficient care.3, 4
Nationwide, there is increasing pressure to improve inpatient quality of care. The Institute of Medicine defines 6 aims for improvement, including timeliness (reducing waits and sometimes harmful delays for both those who receive and those who give care) and efficiency of care (avoiding waste, including waste of equipment, supplies, ideas, and energy).5 Reducing unnecessary stays in the hospital is a potential quality measure that hospitals may use to address the timeliness and efficiency of care delivered to hospitalized children.
Delays in discharge have been used as markers of unnecessary stays in the hospital for inpatient adult and pediatric care,6, 7 but these are limited to inpatient systems from almost 20 years ago. Current reasons why patients are delayed from discharge, if at all, are not well described. We undertook this study to describe delays in hospital discharges at a tertiary‐care children's hospital in terms of number of patients, length of days of delay, and type of delay. In addition, we sought to characterize the impact of discharge delays on overall length of stay (LOS) and costs.
Methods
Patient Population/Study Design
All children cared for on 2 pediatric medical teams at Primary Children's Medical Center during the month of August 2004 were eligible for the study. Two research assistants independently attended team rounds and collected data relating to: the reasons for ongoing hospitalization, pending items (eg, consultations, tests), and the plan of care for that day. The research assistants each attended daily team rounds for the entire month of August (1 for each team, switching to the opposite team after 2 weeks). This was combined with information available in the Patient Tracker, a software tool developed to improve communication between caregivers and improve discharge efficiency.8 This software tool details diagnoses, daily medical care plans, discharge criteria, and ongoing medical interventions while tracking daily changes in interventions and the medical care plan for each patient cared for on a pediatric medical team.
The research assistants subsequently presented their observations along with information from Patient Tracker to 2 experienced physicians (R.S. and B.S.) who independently determined if a delay occurred, the number of delay days extending discharge, and the cause of the delay, if present, categorized according to the taxonomy of the Delay Tool.6, 7 If there was not enough information for either of the physicians to identify and classify a delay, the electronic medical record of the patient was also reviewed. Discrepancies between physicians assigning delays were discussed until consensus was reached.
The study was approved by the Institutional Review Board of the University of Utah Health Sciences Center and Primary Children's Medical Center (PCMC).
Setting
PCMC is a 233‐bed tertiary‐care children's hospital, owned and operated by Intermountain Healthcare (a not‐for‐profit vertically integrated managed care organization) in the Intermountain West, which serves as both the primary hospital for Salt Lake County and as a tertiary‐care children's hospital for 5 states (UT, MT, WY, ID, and NV).9
Study Definitions
Delay and Length of Delay
Delays in discharge were measured using a validated and reliable instrument, the Delay Tool.6, 7 A discharge was classified as delayed if there was no medical reason for the patient to be in the hospital on a given day, identical to the definition used in the original studies to validate the tool. Delays were recorded as whole days, not fractions of days or hours, as described in the original validation of the tool. For example, if the medical team requested a consultation, and the consultant's opinion was rendered late, but the patient would have remained in the hospital anyway, then this period of time would not count as a delay. However, if the medical team did not receive a consultant's opinion within the standard time (24 hours as defined for this study and in validating studies for the Delay Tool), and the patient's sole reason for being in the hospital during that day was waiting for that opinion, then that period of time would count toward a delay due to a late consultative opinion. Delays of less than 1 day, due to the mechanics of discharging a patient from the hospital (providing prescriptions, follow up, communication, arranging home health, and transportation) were not measured in this study, to match the original methodology of the Delay Tool.
Type of Delay
Primary reason for delay was assigned according to the taxonomy of the Delay Tool.6, 7 Delays were categorized to 1 of the following: (1) test scheduling; (2) obtaining test results; (3) surgery; (4) consultation; (5) patient (eg, family unavailable for decision‐making); (6) physician responsibility; (7) education, training. or research; (8) discharge planning or scheduling; and (9) availability of outside care and resources. There are 166 subcategories that clarify why a delay occurred. For example, within the main category of obtaining test results (2), there are 3 subcategories of delays related to problem in executing the test (2.1), return of results is delayed (2.2), and test results not reviewed within standard time of return (2.3). Subcategories are further divided to provide detail on the cause of delay. For example, a delay categorized as a 2.1:1 [(2) obtaining test results; (2.1) problem in executing the test; and 2.1:1 test to be done by MD is delayed beyond day desired], or 2.3:1 [(2) obtaining test results; (2.3) test results not reviewed within standard time of return; and 2.3:1 delay because physician did not review results]) both relate to physician causes of delays within the general category of obtaining test results. Some delays had more than 1 cause. A secondary cause of delay was assigned if applicable; however, the number of days delayed was attributed to the primary cause for analysis purposes.
Exemptions to Delay and Special Populations
Certain subpopulations of patients presented unique issues that led to them being unlikely to be classified as having a delay. For example, patients with a diagnosis of new onset of type 1 diabetes are historically admitted for 3 days at our hospital, which includes a specific education program; delays were not considered until this minimum period had passed. Children with medically complex care (eg, multisystem disease, multiple specialists involved, multiple medications) were included in this study.10 However, these children with frequent hospital admission were often fragile at discharge, and could meet criteria for readmission even on the date of their discharge, hence assigning a delay day was usually not indicated because of easily justified ongoing medical need for hospitalization.
Study Variables
The LOS, total costs, and routine demographic and administrative data for each study patient were extracted from Intermountain Healthcare's Enterprise Data Warehouse (EDW). The EDW contains detailed data about the cost of providing health care. Costs were derived from the hospital's cost accounting program, the Standard Cost Master, which is a transaction‐based microcosting accounting system.1113
For patients whose LOS extended before August 1 or after August 31, total hospital costs were averaged per day, and only days falling inside the month of August were counted in calculating the impact the delays in discharge had on the total costs of hospitalization. Hospital days that extended outside of August were not counted in either the numerator for potential days of delay or in the denominator for total days in the hospital.
Analyses
Descriptive statistics were calculated for the number, length of days of delay, and type of delay. Interrater reliability to assign a delay was ascertained for the 2 physicians. Mean LOS, mean total costs, and standard deviations (SDs) were calculated. All analyses were performed using Statistical Analytical Software version 9.13 (SAS Institute, Cary, NC).
Results
During the 31 days of the study, 171 patients occupied hospital beds an average of 7.3 days on the 2 inpatient medical teams, for a total of 911 inpatient days. Seven patients were admitted prior to August 1; 6 of these were discharged during the month of August and 1 stayed through the entire month and was discharged in September. Three additional patients were admitted in August and discharged in September. There were 6 readmissions during the month of August, and 1 patient was excluded from the study because of lack of sufficient information. All patients with delays were able to be classified according to the Delay Tool taxonomy. Interrater reliability for the 2 study physicians was 98%.
The characteristics between the patients who did and did not experience a delay in discharge are shown in Table 1. Thirty‐nine of 171 patients (22.8%), experienced at least 1 delay day. Eighteen of 39 patients had only 1 delay day (46.2%) and 11 patients experienced 2 delayed days (28.2%) (Figure 1). The average length of delay was 2.1 days.

| Nondelayed Patients (N = 132) | Delayed Patients (N = 39) | P Value | |
|---|---|---|---|
| |||
| Age (months), mean (SD)* | 22.6 (14.4) | 15.0 (14.6) | 0.009 |
| LOS during August (days), mean (SD)* | 4.64 (6.1) | 7.64 (7.15) | <0.001 |
| Total costs during August ($), mean (SD)* | 10,451 (19,254) | 14,341 (16,241) | 0.002 |
| Number of ICD‐9 CM diagnoses codes, mean (SD)* | 7.1 (7.4) | 8.5 (7.3) | 0.056 |
| Number of ICD‐9 CM procedure codes, mean (SD)* | 1.7 (3.8) | 1.6 (2.6) | 0.068 |
| Number of Patients with APR‐DRG SOI 3 (%) | 59 (44.7%) | 19 (48.7%) | 0.65 |
Delays attributed to physician responsibility accounted for 42.3% (16.5/39) of patient delays (conservative management or clinical decision‐making), with discharge planning delays accounting for 21.8% (family‐related, patient‐related, and hospital‐related problems), consultation for 14.1% (delay in obtaining or lack of follow‐up), test scheduling for 12.8%, and obtaining test results for 5.1% (ordering and weekend scheduling). There were no primary delays due to surgery, education and research, or unavailability of outside resources such as a skilled nursing bed. Four patients had a single additional secondary cause of delay assigned to them, related to physician responsibility, consultation, surgery and test scheduling; these were split, attributing 0.5 patients to each delay type (thus, the 17/39 patients delayed for physician responsibility was analyzed as 16.5/39) (Table 2).
| Delay Category | Number of Patients Experiencing Delays* | Percentage of All Patients Experiencing Delays (%) | Percentage of Study Patients Observed (%) | Total Delay Days | Average Length of Delays (days) | Percentage of Hospital Days That Were Delay Days (%) |
|---|---|---|---|---|---|---|
| ||||||
| 1. Scheduling | 5 | 12.8 | 2.92 | 16 | 3.20 | 1.76 |
| 2. Obtaining results | 2 | 5.1 | 1.17 | 3 | 1.50 | 0.33 |
| 3. Surgery | 0.5 | 1.3 | 0.29 | 1.5 | 3.00 | 0.16 |
| 4. Consultation | 5.5 | 14.1 | 3.22 | 10.5 | 1.91 | 1.15 |
| 5. Patient | 1 | 2.6 | 0.58 | 2 | 2.00 | 0.22 |
| 6. Physician | 16.5 | 42.3 | 9.65 | 33.5 | 2.03 | 3.68 |
| 7. Education | 0 | 0 | 0.00 | 0 | 0 | 0.00 |
| 8. Discharge | 8.5 | 21.8 | 4.97 | 15.5 | 1.82 | 1.70 |
| 9. Outside | 0 | 0 | 0.00 | 0 | 0 | 0.00 |
| Total | 39 | 100 | 22.81 | 82 | 2.10 | 9.00 |
There were 82 delay‐related hospital days of 911 total inpatient days on the 2 medical teams for August 2004 (9%). More than $170,000 in excess costs was incurred due to delay days from a total of approximately 1.9 million dollars in patient costs for the month (8.9%).
Discussion
This study finds that discharge delays in a tertiary care children's hospital are common; almost 1 in 4 patients experienced a medically unnecessary excess hospital stay of at least 1 day. The average length of a delay was 2.1 days, and overall, delays consumed 9% of pediatric hospital days and 8.9% of total costs. The most common reason for a delay was related to physician clinical care, including excessively conservative management and variability in clinical decision‐making.
Our study results are similar to the other 2 published studies that use the Delay Tool. In the adult and pediatric studies, between 10% and 30% of patients experienced a delay in discharge, with the average length of delay between 2.9 and 3 days.6, 7 Although both studies were conducted at teaching hospitals, what is particularly interesting is that they were conducted almost 20 years ago. During this period of time, there has been a shift in the inpatient pediatric patient population. In recent years, children who are cared for in the hospital have more chronic illnesses.1 In addition, there has been a shift in the types of conditions that may be cared for at home and those that now require inpatient stay.2 Despite this, delays continue at a similar proportion, but the cause of delays have shifted from scheduling and consultation to physician responsibility.
There is another tool in the literature which is more widely used, the Pediatric Appropriateness Evaluation Protocol (PAEP), which is based on the Appropriateness Evaluation Protocol for adults.1417 This tool is used to determine the appropriateness of ongoing hospitalization, not the cause of delay if ongoing hospitalization is inappropriate. The 3 areas that are evaluated (medical services, nursing and ancillary services, and patient's condition) have objective criteria that dictate if the hospitalization is appropriate or not (eg, parenteral (intravenous) therapy for at least 8 hours on that day, under nursing and ancillary services). The PAEP may be less sensitive given today's healthcare resource utilization climate. Many clinicians and families would agree that insertion of a peripheral central catheter is an acceptable form of outpatient treatment for many pediatric conditions. In conjunction with the Delay Tool, the PAEP could be used to determine if a delay occurred, then the Delay Tool used to categorize the cause of the delay. We choose to use expert clinician judgment to determine if a delay had occurred. We were more interested in why patients who are admitted (appropriately or inappropriately) cannot be discharged sooner, thus allowing for future intervention studies targeted to impact delays in discharge, as elucidated in this study. The Delay Tool specifically allowed us to categorize the reasons for delays. Given that the average LOS for patients in the nondelayed group was over 4 days, despite not using a tool such as the PAEP, we believe that these were likely to be appropriate admissions.
A recent study reported the first use of the Medical Care Appropriateness Protocol (MCAP) in a tertiary‐care children's hospital. The authors used the MCAP to determine the impact of an intervention on reducing inappropriate hospitals days for children. This tool is similarly labor‐intensive to the Delay Tool. Interestingly, this Canadian study found a high rate of inappropriate hospital days (47%), which may be in part attributable to a different outcome measurement tool and/or a different health care system.18
There are several limitations to our study that deserve mention. The Delay Tool requires clinician judgment regarding whether or not there was a delay in discharge for that day. We may have introduced some bias in our study, as hospitalist investigators assigned the delay and blinding to the attending physician specialty of record was not feasible. However, our results are similar to the other 2 published studies that have used this tool, and we specifically chose not to analyze or report results in terms of hospitalist and nonhospitalist attending physicians. The Delay Tool is not designed to differentiate shorter delays in terms of hours instead of days (eg, due to the inability for the patient to get a ride home). Shorter delays may be of particular importance depending on the occupancy rate of the hospital, the demand for beds, and other patient and hospital factors. We could not capture these shorter delays (although they did occur frequently) due to the original description of the Delay Tool. In addition, we would not have been able to report data on the impact on LOS and costs, as these are attributed to whole days in the hospital. However, if we had been able to differentiate shorter delays, this would bias our results to show a greater percentage of delays over smaller increments of time. Generalizability is an issue, given that this was a single‐center study. This study sample included over 80 different attending physicians participating in community pediatrician, subspecialty, and hospitalist practice groups. However, the patient population at PCMC is similar to other medium and large children's hospitals in the United States. The month observed may not reflect the entire year of hospitalizationsthere may be seasonal variations with delays depending on the volume and type of illness seen. The study was conducted in August, when there are newer house staff present. However, physician responsibility, which was the largest source of delays in our study, had little attribution to house staff. Most of the decisions were those of attending physicians, which would largely be unaffected by the time of year of the study. Finally, we were unable to assess the safety of the potential earlier discharge, as this was an observational study. However, in any future intervention studies examining processes to discharge patients sooner, measures of safety to the patient are a necessity. Finally, given the potential of ongoing admission, even on the date of discharge of our most fragile patients, this approach to discovering causes of delay may not apply to this important group, which is responsible for significant and growing resource utilization.
Despite these limitations, our findings demonstrate that in an era of children staying in the hospital less, and more medically‐complex children being admitted,10 a substantial number of children who are hospitalized at a children's hospital may have been discharged sooner. The majority of these decisions were directly related to physician responsibility. As consumers, providers, and hospitals work together to develop quality measures that are reflective of inpatient pediatric care, the Delay Tool may be able to highlight 2 aims of quality (ie, timeliness and efficiency of care) that could be used to assess the impact of interventions designed to safely discharge patients sooner. Interventions such as audit‐feedback,18 clinical guideline deployment,19 and hospitalist systems of care4 continue to hold the promise of earlier discharge; however, tools designed to measure inappropriate use of hospital days should be employed to demonstrate their effectiveness. Our study demonstrates ongoing waste in children's hospitals.
Conclusions
Almost 1 out of 4 patients in this 1‐month period could have been discharged sooner than they were. The impact of delays on costs and LOS are substantial and should provide strong incentives to develop effective interventions. Such interventions will need to address variations in physician criteria for discharge, more efficient discharge planning, and timely scheduling of consultation and diagnostic testing.
Acknowledgements
The authors thank Joni R. Beshanky and Harry Selker for their help and training in the use of the Delay Tool.
- .The transformation of child health in the United States: social disparities in child health persistent despite dramatic improvement in child health overall.Health Aff (Millwood).2004;23(5):9–25.
- , , , .Hospitalist care of medically complex children.Pediatr Res.2004;55(4):314A–315A.
- , , , .Pediatric hospitalists: a systematic review of the literature.Pediatrics.2006;117(5):1736–1744.
- , , , et al.Pediatric hospitalists: report of a leadership conference.Pediatrics.2006;117(4):1122–1130.
- Institute of Medicine.Crossing the Quality Chasm: A New Health System for the Twenty‐first Century.Washington, DC:National Academy Press;2001.
- , , .Using the Delay Tool to attribute causes for unnecessary pediatric hospital days.Med Care.1990;28(10):982–989.
- , , , .The epidemiology of delays in a teaching hospital. The development and use of a tool that detects unnecessary hospital days.Med Care.1989;27(2):112–129.
- , , , , .A tool for improving patient discharge process and hospital communication practices: the Patient Tracker.AMIA Annu Symp Proc.2007;11:493–497.
- , .Organizational responses to managed care: issues for academic health centers and implications for pediatric programs.Pediatrics.1998;101(4 Pt 2):805–811; discussion 811–802.
- , , .Hospitalist care of the medically complex child.Pediatr Clin North Am.2005;52(4):1165–1187.
- , , , et al.Epidemiology, complications, and cost of hospitalization in children with laboratory‐confirmed influenza infection.Pediatrics.2006;118(6):2409–2417.
- , , , , , .Clinical and economic outcomes of conventional amphotericin B‐associated nephrotoxicity.Clin Infect Dis.2002;35(12):e120–e127.
- , , , et al.Using a hospital information system to assess the effects of adverse drug events.Proc Annu Symp Comput Appl Med Care.1993;1993:161–165.
- , , .Appropriateness of hospitalization in a Canadian pediatric hospital.Pediatrics.1993;91(1):70–74.
- , , , .The appropriateness evaluation protocol: application in an Australian children's hospital.Aust Clin Rev.1991;11(4):123–131.
- , .Assessing the need to hospitalize children: pediatric appropriateness evaluation protocol.Pediatrics.1989;84(2):242–247.
- .Medically inappropriate hospital use in a pediatric population.N Engl J Med.1988;318(16):1033–1037.
- , , , , .Reducing inappropriate hospital use on a general pediatric inpatient unit.Pediatrics.2008;121(5):e1068–e1073.
- , .Reliable implementation of clinical pathways: what will it take—that is the question.J Pediatr.2008;152(3):303–304.
- .The transformation of child health in the United States: social disparities in child health persistent despite dramatic improvement in child health overall.Health Aff (Millwood).2004;23(5):9–25.
- , , , .Hospitalist care of medically complex children.Pediatr Res.2004;55(4):314A–315A.
- , , , .Pediatric hospitalists: a systematic review of the literature.Pediatrics.2006;117(5):1736–1744.
- , , , et al.Pediatric hospitalists: report of a leadership conference.Pediatrics.2006;117(4):1122–1130.
- Institute of Medicine.Crossing the Quality Chasm: A New Health System for the Twenty‐first Century.Washington, DC:National Academy Press;2001.
- , , .Using the Delay Tool to attribute causes for unnecessary pediatric hospital days.Med Care.1990;28(10):982–989.
- , , , .The epidemiology of delays in a teaching hospital. The development and use of a tool that detects unnecessary hospital days.Med Care.1989;27(2):112–129.
- , , , , .A tool for improving patient discharge process and hospital communication practices: the Patient Tracker.AMIA Annu Symp Proc.2007;11:493–497.
- , .Organizational responses to managed care: issues for academic health centers and implications for pediatric programs.Pediatrics.1998;101(4 Pt 2):805–811; discussion 811–802.
- , , .Hospitalist care of the medically complex child.Pediatr Clin North Am.2005;52(4):1165–1187.
- , , , et al.Epidemiology, complications, and cost of hospitalization in children with laboratory‐confirmed influenza infection.Pediatrics.2006;118(6):2409–2417.
- , , , , , .Clinical and economic outcomes of conventional amphotericin B‐associated nephrotoxicity.Clin Infect Dis.2002;35(12):e120–e127.
- , , , et al.Using a hospital information system to assess the effects of adverse drug events.Proc Annu Symp Comput Appl Med Care.1993;1993:161–165.
- , , .Appropriateness of hospitalization in a Canadian pediatric hospital.Pediatrics.1993;91(1):70–74.
- , , , .The appropriateness evaluation protocol: application in an Australian children's hospital.Aust Clin Rev.1991;11(4):123–131.
- , .Assessing the need to hospitalize children: pediatric appropriateness evaluation protocol.Pediatrics.1989;84(2):242–247.
- .Medically inappropriate hospital use in a pediatric population.N Engl J Med.1988;318(16):1033–1037.
- , , , , .Reducing inappropriate hospital use on a general pediatric inpatient unit.Pediatrics.2008;121(5):e1068–e1073.
- , .Reliable implementation of clinical pathways: what will it take—that is the question.J Pediatr.2008;152(3):303–304.
Copyright © 2009 Society of Hospital Medicine
Hospitalist‐PCP Communication Needs
Hospitalist systems focus on providing acute treatments to patients and expediting hospital discharge, sometimes without regard for the need to work in concert with community providers, leading to fragmentation of care.1 This fragmentation, particularly at the transitions of care, such as when patients move from the outpatient setting to a hospitalist system and then back to their primary care providers (PCPs), can lead to communication breakdowns and delays in care, and may compromise patient outcomes.2 Suboptimal treatments, such as medication errors and the ordering of redundant tests can occur in either setting if prior treatment information is not relayed in a timely and accurate fashion. Landrigan et al.3 described a conceptual model in 2001 that recognized the complexity of the hospitalist‐PCP communication system. Specifically, optimal care of hospitalized children includes PCPs, family members, hospitalists, and support staff while meeting the communication needs of families and PCPs. Additionally, mediating a smooth transition into and out of the hospital needs to be measured carefully.3
Previous adult studies have reported that hospitalist systems sometimes create discontinuity of patient care, which can have a negative impact on the quality of care provided to patients if there is poor communication between hospitalists and PCPs.2, 4, 5 Existing research on hospitalist‐PCP communication focuses mainly on adult hospitalist models with little known about the quality of current pediatric hospitalist‐PCP communication.
The objective of this study was to qualitatively explore issues around communication between pediatric hospitalists and PCPs. Specifically, we sought to explore the quality of communication practices and barriers to optimal communication within the hospitalist‐PCP model at a tertiary care children's hospital. The results are serving as a needs assessment to guide the design of a quality improvement project with the aim of improving pediatric hospitalist‐PCP communication.
METHODS
Study Design
Phone interviews of pediatric hospitalists and PCPs were conducted. The study was approved by the University of Utah and Primary Children's Medical Center (PCMC) Institutional Review Boards.
Setting
PCMC is a 232‐bed, tertiary‐care referral center and community hospital in Salt Lake City, UT, which serves a catchment area of approximately 1,000,000 children in 5 Intermountain West states (Utah, Idaho, Nevada, Montana, and Wyoming). In 2005, there were more than 40,000 emergency department visits and more than 11,000 hospital admissions. At the time of this study, the Division of Pediatric Inpatient Medicine (hospitalist division) included 11 full‐time equivalents. All hospitalists play a teaching role and are on faculty at the University of Utah School of Medicine. In 2005, approximately 45% of medical inpatients at PCMC were cared for by the hospitalist division, with approximately 95% cared for by resident teams.
Participants
Ten University of Utah pediatric hospitalists and 12 PCPs within our catchment area completed interviews. Verbal consent was given before study participants began the phone interview. All hospitalists from the hospitalist division, excluding the first author, completed an interview. PCPs who had referred patients to the hospitalist division in the year preceding this study (2004) were identified through a referring database kept by the hospitalist division. An attempt was made to interview physicians in multiple practice settings and geographic locations.
Inclusion criteria for PCPs included their willingness to complete the interview as well as having had patients cared for by the hospitalist division in the preceding year (2004). There was no preference given to any physicians, including physicians well‐known by the research team or more frequent users of the hospitalist division.
Instrument
To develop our questionnaire, we conducted a detailed literature search to identify issues surrounding hospitalist‐PCP communication in the adult and pediatric hospitalist literature. Search terms included: hospitalists, interprofessional relations, patient discharge, communication, follow‐up care, transitions, and primary care provider using the PubMed database, limited to English language articles from 1990 to 2005. The 6 issues for the final questionnaire were identified from published hospitalist survey questions (in both adult and pediatric literature) and published articles addressing themes regarding hospitalist and PCP attitudes (specifically in regard to the communication process).1, 2, 4, 6 These 6 issues (quality of communication, barriers to communication, methods of information sharing, key data element requirements, critical timing, and perceived benefits) were incorporated into the open‐ended and closed‐ended questionnaire (Table 1). The original draft of the questionnaire was pretested on 2 hospitalists and 3 PCPs by L.H., who has graduate level formal training and experience in the design, refinement, implementation, and evaluation of questionnaires.
| Questions |
|---|
| 1. Do you use the hospitalist system at PCMC? yes/no |
| 1a. If yes: For what % of your patients that are hospitalized do you use the hospitalist system? |
| 2. How would you rate the quality of communications between hospitalists and Primary Care Providers? |
| a: excellent; b: very good; c: good; d: fair; e: poor. |
| 2a. Why did you give it that rating? |
| 3. What barriers, if any, have you experienced in communicating with hospitalists/Primary Care Providers? |
| 4. What communication methods have been effective in the past? What suggestions do you have for improving communication methods? |
| 5. What information would you like to receive from hospitalists/Primary Care Providers regarding your patients' hospital care? |
| 6. At what points in the care process would you like to receive communications from hospitalists/Primary Care Providers? |
| 7. What suggestions do you have for improving overall communications between hospitalists and Primary Care Providers? |
| 8. Do you have access to e‐mail and use it regularly in your practice? |
| 8a. Do you have access to a fax machine and use it regularly in your practice? |
| 8b. Do you have access to a telephone and use it regularly in your practice? |
| 8c. Considering e‐mail, fax, and telephone, which of these methods do you think would be the most effective for communicating with hospitalists/Primary Care Providers? |
| 9. Do you believe that improving communications between hospitalists and Primary Care Providers would improve the quality of patient care? |
| 9a. If yes: How? |
| 9b. If no: Why not? |
| 10. Any other comments/feedback? |
Data Collection/Analysis
After consent, participants were administered the phone questionnaire by L.H. during April, May, and June 2005. Interviews were transcribed verbatim into a Microsoft Word document by a trained transcriptionist. Responses were openly coded and then grouped into the respective main topics of interest. No further interviews were conducted when theoretical saturation was obtained (ie, respondents did not identify any new themes). Themes were compared using qualitative methods.7, 8
RESULTS
Only 1 physician per practice was interviewed. No PCP who was able to be contacted declined an interview, although some did require multiple phone attempts to schedule the interview. PCPs were located in Salt Lake County (n = 6), in other Utah counties (n = 3), and in surrounding Intermountain West states (n = 3). From January 1, 2004 to December 31, 2004, we estimate that the hospitalist division cared for patients from approximately 35 practices (50% in Salt Lake County, 30% in other Utah counties, and 20% in surrounding Intermountain West states).
Hospitalists and PCPs agreed that overall quality of communication ranged from poor to very good (Table 2). Both parties acknowledged that significant barriers to optimal communication exist, yet the barriers differed for each group. Hospitalists and PCPs also agreed that optimal communication could improve many aspects of patient care and should take place upon discharge and admission of patients and with major clinical changes. Both hospitalists and PCPs also wanted accurate and timely information. One priority that the participants emphasized is the timely transfer of admission notification and the receipt of accurate and timely discharge summaries by PCPs.
| Hospitalists | Primary Care Providers | |
|---|---|---|
| Quality of communication | ||
| Poor | 0% | 33% |
| Fair | 50% | 17% |
| Good | 40% | 8% |
| Very good | 10% | 42% |
| Excellent | 0% | 0% |
| Barriers to communication | Lack of PCP directory; | Not knowing name and contact information of hospitalist taking care of their patient; |
| Lack of access to patients' medication or problem list; | Teaching hospital with numerous residents and students | |
| Lack of standardized system | ||
| Methods of information sharing | Electronic medical record ideal for sharing information | Electronic medical record ideal for sharing information; |
| Phone calls and faxes effective, especially if pager numbers were included | ||
| Key data elements | Diagnoses; | Diagnoses; |
| Medications; | Medications; | |
| Follow‐up plans | Follow‐up plans | |
| Critical timing | At discharge; | At discharge; |
| After admission; | After admission; | |
| Major clinical changes | Major clinical changes | |
| Perceived benefits | Improved patient satisfaction; | Improved patient satisfaction; |
| Improved follow‐up; | Improved follow‐up; | |
| Decreased medication errors; | Decreased medication errors; | |
| Increased efficiency | Increased efficiency |
Quality of Communication
Overall, both groups rated communication quality from poor to very good (Table 2). Notably, no hospitalists or PCPs rated overall quality as excellent, but 33% of PCPs rated it as poor compared to 0% of the hospitalists. Fifty‐eight percent (7/12) of PCPs used the hospitalists for 80% of their admissions to the hospital.
For hospitalists, lack of communication stemmed from busy schedules, not knowing who the PCP was, or not having the PCP contact information. Similarly, PCPs commented that they often found out their patient was admitted to the hospital only when the patient showed up in their office for a follow‐up visit. Both hospitalists and PCPs felt it was the hospitalist's job to inform and update PCPs on their patient's status while hospitalized. However, if the patient was admitted via the emergency department (ED), hospitalists felt that it was the ED's responsibility to inform the PCPs of their patients' admission.
Barriers to Communication
PCPs and hospitalists noted different barriers to optimal communication. Hospitalists identified the lack of a PCP directory, the lack of access to patients' medication and problem lists, and the lack of a standardized system to communicate with PCPs as major barriers. The delayed receipt of the discharge summary by PCPs was also viewed as a barrier by hospitalists. Pediatric hospitalists found the large variation in PCP availability as well as the variation in PCP preferred methods of communication (phone call, fax, or e‐mail) to be additional barriers. PCPs, on the other hand, struggled with the complexity of the hospital system. The fact that PCMC is a teaching hospital with numerous residents and students assisting in their patients' care, as well as not knowing the names and contact information of the hospitalists taking care of their patients, served as barriers to optimal communication. Additionally, PCPs noted the delay in receiving discharge summaries as a barrier and a source of frustration.
Methods of Information Sharing
All PCPs and hospitalists had access to telephones and faxes and used them regularly in their practices (100% for both groups). A majority of PCPs believed phone calls and faxes were effective means of information sharing, especially if pager numbers of the hospitalists were included. Some PCPs and a larger number of hospitalists thought an electronic medical record was an ideal tool for sharing information. However, PCPs appeared to have a lower rate of e‐mail access and usage compared with hospitalists.
Key Data Elements
There was agreement among PCPs and hospitalists regarding which data elements were important to be relayed among providers. PCPs and hospitalists were most interested in the following data elements upon patient discharge: diagnoses from the hospitalization, medications the patient was to take, and follow‐up plans for the patient. Hospitalists also thought PCPs could help by providing a list of current medications and a detailed past medical and social history upon admission. This information could be easily provided to the accepting hospitalist attending by phone or fax from the PCP.
Critical Timing and Perceived Benefits
Hospitalists and PCPs agreed that the most critical times for optimal hospitalist‐PCP communication were primarily at time of discharge from the hospital, after admission to the hospital, and when major clinical changes occurred. The majority of hospitalists and PCPs thought that improved communication would improve the quality of patient care through: (1) improved patient satisfaction; (2) improved quality and quantity of follow‐up; (3) decreased medication errors; and (4) increased efficiency for the PCPs and hospitalists.
DISCUSSION
Both pediatric hospitalists and PCPs agree on what information is important to transmit (diagnoses, medications, follow‐up needs, and pending laboratory test results) and critical times for communication during the hospitalization (at discharge, admission, and during major clinical changes). However, there was discrepancy in the barriers to optimal communication for each group. Identifying and addressing these barriers can help both hospitalists and PCPs implement targeted interventions aimed at improving communication. As the number of pediatric hospitalist programs increases, the risk for hospitalist‐PCP communication breakdowns, which can have a negative impact on patient care, also increases.
Previous adult studies describe the scope of the problem around poor communication between hospitalists and PCPs.1, 912 Kripalani et al.10 reported recently that delays and omissions in communication are common at hospital discharge among adult hospitalists and that computer‐generated summaries, educational interventions, and standardized formats may facilitate more timely transfer of pertinent information. However, there is limited data on pediatric hospitalist‐PCP communication. Srivastava et al.5 found that 60% of community physicians thought hospitalist systems may impair communication with PCPs when evaluating community and hospital‐based physicians' attitudes regarding pediatric hospitalist systems.
PCPs can feel left out when their patients are cared for by hospitalists.13 One PCP in our study commented: Include the referral doc as part of the team. We're the ones who will take care of them after discharge. It seems like an autonomous thing down there and we're excluded from the patient care team. Additionally, patients want their PCPs to remain involved in their care as they transition into and out of the hospital setting.1, 13
The continuity visit model has been proposed by Wachter and Pantilat14 to describe a clinical encounter between the primary physician and hospitalized patient, when the patient has a different physician of record. In this model, the PCP can endorse the hospitalist model and the individual hospitalist, notice subtle findings that differ from the patient's baseline, and help clarify patient preferences regarding difficult situations by drawing on their previous relationship with the patient. This visit may also benefit the PCP by providing insights into the patient's illness, personality, or social support that he or she was unaware of previously. However, in order for the continuity visit to exist, the PCP has to be informed of their patient's admission in the first place. Ethical dilemmas also have been raised regarding who bears primary responsibility for maintaining open lines of communication when patients are hospitalized.15 Lo15 advocates that PCPs can and should be involved in meaningful ways in the inpatient care of their patients even when they are not acting as the treating physicians. Specifically, he suggests that PCPs personally visit particularly ill patients or those with difficult diagnoses and use frequent phone calls to all admitted patients.
Beyond telephone calls and continuity visits, hospitalists and PCPs rely on discharge summaries as a key part of the information transfer about a patient's hospitalization.1, 16, 17 These documents are rendered useless if they are inaccurate, illegible, or not delivered in a timely manner.18 In a study of California family physicians, discharge summaries were thought to be too detailed by 84% of PCPs, and reportedly arrived before the patient's first follow‐up appointment only 33% of the time.1 O'Leary et al.19 found that 41% of the Department of Medicine physicians surveyed believed that at least 1 of their patients hospitalized in the previous 6 months had experienced a preventable adverse event related to poor transfer of information at discharge. In our study, PCPs noted that discharge summaries often arrived in their offices well after the patient had been seen for their follow‐up appointment.
Both hospitalists and PCPs agree that a concise and precise discharge summary should include an overview of the hospitalization with important details highlighted. Similar to the findings of Pantilat et al.,1 in our study PCPs specifically want detailed information with regard to diagnoses, discharge medications, and what to expect when they see the patient in their clinic. Follow‐up phone calls to PCPs to see that they received written information and if they require further details is 1 solution to ensuring good follow‐up, yet this adds to the burden of communication and could be an additional barrier.
The teaching institutions in which physicians train also pose unique obstacles to optimal communication. In academic medical centers, medical students and residents perform a majority of the discharge duties (eg, writing prescriptions, dictating discharge summaries, making follow‐up appointments, and calling PCPs), and teaching these trainees the importance of timely and accurate communication becomes an added challenge. Educators have to find novel ways of providing incentives to residents and medical students to get them to effectively participate in this process. Plauth et al.20 reports that hospitalists feel they needed better training in residency around communicating, noting a meaningful underemphasis during residency training in regard to communication with referring physicians. These skills should be taught in medical school and supported by both hospitalists and PCPs throughout residency training.
Both hospitalists and PCPs also want easy and reliable ways to access their colleagues, which ideally would be automatic. One PCP commented: a weekly or semiweekly phone call would be nice. Another suggested to: fax a short note. One hospitalist acknowledged: a systematic approach would be betterwhether a fax or telephone call and make sure there is a way of checking to make sure the communication has happened. Another hospitalist simply remarked: it needs to be done on every patient.
Thus, it seems an improved communication system should be flexible enough to accommodate unique provider preferences, such as communication via phone, fax, or e‐mail. This is demonstrated by 1 PCP who preferred the phone, but most convenient is the periodic fax updates. I don't have to be taken away from seeing patients.
Lo15 calls for a standard to be established for delivering care within the patient‐PCP‐hospitalist triad. Phone calls and faxes are 2 readily available methods of communication. However, the frequent back‐and‐forth of missed calls, unreturned calls, and days‐off is certainly a factor in determining efficiency and effectiveness of phone calls.
E‐mail, if it is widely used by all participants, may be an effective option for delivery that could provide confirmation of receipt. However, the lack of universal e‐mail usage by all providers remains a barrier. Questions as to which method is more time consuming and for whom, need to be studied further. Patient confidentiality also requires that this protected health information arrive in the proper hands. Personal relationships can also contribute to successful communication. One provider may be more likely to contact another if they know each other through some personal connection, such as medical school, residency, or a social group.
Our study has several limitations. The sample size was small. We obtained responses from a sample of key stakeholders in the hospitalist‐PCP communication process. We were limited by the number of hospitalists at our institution as well as the interest and availability of PCPs to respond. We are unable to determine the total number of patients by respondent PCP practice cared for by the hospitalist division. This could influence the results depending on whether the respondent PCP was a frequent or infrequent utilizer of the hospitalist system. However, we feel reassured that we are not missing important information, because in our methods, a priori, we had intended to stop interviewing PCPs once theoretical saturation had been reached (ie, respondents did not identify any new information). In our study, that occurred with 12 PCPs.
We attempted to interview a single physician in a number of different practice settings in order to gain insight into the perceptions of that individual as well as those of their partners. The views expressed by these individuals may not represent the views of hospitalists and PCPs outside of our practice area. Furthermore, PCMC serves as both a community pediatric hospital and a tertiary‐referral center for a large area, yet the current experience of 1 hospitalist division and 1 cohort of referring PCPs may contain regional variation that contributed bias to the responses.
Selection bias may have been introduced in our study by the inherent nature of phone interviews. We interviewed only providers with previous communication experience with our hospitalist division. These providers may have had a vested interest in the communication process. We did not interview those PCPs who did not have any communication with our hospitalist division or those who may have used the hospitalist division previously and decided to no longer use the division. Interviewing these groups may have provided additional insight into the communication issues mentioned here. Additionally, useful information could have been gleaned from trying to find out more from the 33% of PCPs who felt communication was poor. We anticipate further studies exploring this issue in more depth.
Future Directions
As a result of this study, we have implemented several interventions to improve information sharing between hospitalists and PCPs, including: 1) we updated current contact information (including names of physicians, office addresses, phone numbers, fax numbers, and e‐mail addresses) for all PCPs in our catchment area along with their preferred methods of communication; 2) we worked with the transcription services to automatically add PCP addresses, phone numbers, and fax numbers to dictated notes, eliminating time wasted searching for contact information; and 3) we standardized key data elements in admission history and physicals and discharge notes to increase the efficiency of the communication process.
Furthermore, we have implemented a standardized system to facilitate communication with PCPs. This system includes an automated process to notify PCPs of their patient's hospital admission, including the admission date, preliminary diagnoses, and responsible physician's contact information. We are currently undertaking a quality improvement project aimed at achieving timely transfer of discharge information to PCPs, including medications, follow‐up appointments, and a succinct hospital summary. Finally, establishing an evaluation process to monitor both successes and failures will be paramount to any interventions.
CONCLUSIONS
Hospitalists and PCPs agree that overall quality of communication ranges from poor to very good. Both PCPs and pediatric hospitalists acknowledge that significant barriers to optimal communication exist, yet the barriers differ for each group. They also agree that optimal communication would improve many aspects of patient care and should take place upon discharge and admission of patients and with major clinical changes.
Pediatric hospitalists and PCPs identified issues around optimal communication similar to those noted in the adult hospital medicine literature. Interventions to improve pediatric hospitalist‐PCP communication should at least address these 6 issues: (1) quality of communication; (2) barriers to communication; (3) methods of information sharing; (4) key data element requirements; (5) critical timing; and (6) perceived benefits. Such interventions will likely improve hospitalist‐PCP communication and potentially improve the quality of patient care. However, future studies will need to demonstrate the link between improved hospitalist‐PCP communication and improved patient care and outcomes.
Acknowledgements
The authors are indebted to Flory Nkoy, MD, MPH, MS, for his help in manuscript preparation and critical review.
- ,,,.Primary care physician attitudes regarding communication with hospitalists.Am J Med.2001;111(9B):15S–20S.
- ,,,,,.Physician attitudes toward and prevalence of the hospitalist model of care: results of a national survey.Am J Med.2000;109(8):648–653.
- ,,,,,.Pediatric hospitalists: what do we know, and where do we go from here?Ambul Pediatr.2001;1(6):340–345.
- ,,.Physician views on caring for hospitalized patients and the hospitalist model of inpatient care.J Gen Intern Med.2001;16(2):116–119.
- ,,,,,.Community and hospital‐based physicians' attitudes regarding pediatric hospitalist systems.Pediatrics.2005;115(1):34–38.
- ,.The patient provider relationship and the hospitalist movement. Introduction.Dis Mon.2002;48(4):189–190.
- ,.Rigour and qualitative research.BMJ.1995;311(6997):109–112.
- ,.Needs assessment in post graduate medical education: a review.Med Educ Online.2001;7:1–8.
- ,,,.How physicians perceive hospitalist services after implementation: anticipation vs reality.Arch Intern Med.2003;163(19):2330–2336.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297(8):831–841.
- ,,.Generalist‐subspecialist communication for children with chronic conditions: a regional physician survey.Pediatrics.2003;112(6 Pt 1):1314–1320.
- ,,,,,.Communication breakdown in the outpatient referral process.J Gen Intern Med.2000;15(9):626–631.
- ,,.How do patients view the role of the primary care physician in inpatient care?Dis Mon.2002;48(4):230–238.
- ,.The “continuity visit” and the hospitalist model of care.Dis Mon.2002;48(4):267–272.
- .Ethical and policy implications of hospitalist systems.Dis Mon.2002;48(4):281–290.
- ,,, et al.From a paper‐based transmission of discharge summaries to electronic communication in health care regions.Int J Med Inform.2006;75(3‐4):209–215.
- ,,.Tying up loose ends: discharging patients with unresolved medical issues.Arch Intern Med.2007;167(12):1305–1311.
- ,.Improving communication between hospital and community physicians. Feasibility study of a handwritten, faxed hospital discharge summary. Discharge Summary Study Group.Can Fam Physician.1999;45:2893–2899.
- ,,,,.Outpatient physicians' satisfaction with discharge summaries and perceived need for an electronic discharge summary.J Hosp Med.2006;1(5):317–320.
- ,,,.Hospitalists' perceptions of their residency training needs: results of a national survey.Am J Med.2001;111(3):247–254.
Hospitalist systems focus on providing acute treatments to patients and expediting hospital discharge, sometimes without regard for the need to work in concert with community providers, leading to fragmentation of care.1 This fragmentation, particularly at the transitions of care, such as when patients move from the outpatient setting to a hospitalist system and then back to their primary care providers (PCPs), can lead to communication breakdowns and delays in care, and may compromise patient outcomes.2 Suboptimal treatments, such as medication errors and the ordering of redundant tests can occur in either setting if prior treatment information is not relayed in a timely and accurate fashion. Landrigan et al.3 described a conceptual model in 2001 that recognized the complexity of the hospitalist‐PCP communication system. Specifically, optimal care of hospitalized children includes PCPs, family members, hospitalists, and support staff while meeting the communication needs of families and PCPs. Additionally, mediating a smooth transition into and out of the hospital needs to be measured carefully.3
Previous adult studies have reported that hospitalist systems sometimes create discontinuity of patient care, which can have a negative impact on the quality of care provided to patients if there is poor communication between hospitalists and PCPs.2, 4, 5 Existing research on hospitalist‐PCP communication focuses mainly on adult hospitalist models with little known about the quality of current pediatric hospitalist‐PCP communication.
The objective of this study was to qualitatively explore issues around communication between pediatric hospitalists and PCPs. Specifically, we sought to explore the quality of communication practices and barriers to optimal communication within the hospitalist‐PCP model at a tertiary care children's hospital. The results are serving as a needs assessment to guide the design of a quality improvement project with the aim of improving pediatric hospitalist‐PCP communication.
METHODS
Study Design
Phone interviews of pediatric hospitalists and PCPs were conducted. The study was approved by the University of Utah and Primary Children's Medical Center (PCMC) Institutional Review Boards.
Setting
PCMC is a 232‐bed, tertiary‐care referral center and community hospital in Salt Lake City, UT, which serves a catchment area of approximately 1,000,000 children in 5 Intermountain West states (Utah, Idaho, Nevada, Montana, and Wyoming). In 2005, there were more than 40,000 emergency department visits and more than 11,000 hospital admissions. At the time of this study, the Division of Pediatric Inpatient Medicine (hospitalist division) included 11 full‐time equivalents. All hospitalists play a teaching role and are on faculty at the University of Utah School of Medicine. In 2005, approximately 45% of medical inpatients at PCMC were cared for by the hospitalist division, with approximately 95% cared for by resident teams.
Participants
Ten University of Utah pediatric hospitalists and 12 PCPs within our catchment area completed interviews. Verbal consent was given before study participants began the phone interview. All hospitalists from the hospitalist division, excluding the first author, completed an interview. PCPs who had referred patients to the hospitalist division in the year preceding this study (2004) were identified through a referring database kept by the hospitalist division. An attempt was made to interview physicians in multiple practice settings and geographic locations.
Inclusion criteria for PCPs included their willingness to complete the interview as well as having had patients cared for by the hospitalist division in the preceding year (2004). There was no preference given to any physicians, including physicians well‐known by the research team or more frequent users of the hospitalist division.
Instrument
To develop our questionnaire, we conducted a detailed literature search to identify issues surrounding hospitalist‐PCP communication in the adult and pediatric hospitalist literature. Search terms included: hospitalists, interprofessional relations, patient discharge, communication, follow‐up care, transitions, and primary care provider using the PubMed database, limited to English language articles from 1990 to 2005. The 6 issues for the final questionnaire were identified from published hospitalist survey questions (in both adult and pediatric literature) and published articles addressing themes regarding hospitalist and PCP attitudes (specifically in regard to the communication process).1, 2, 4, 6 These 6 issues (quality of communication, barriers to communication, methods of information sharing, key data element requirements, critical timing, and perceived benefits) were incorporated into the open‐ended and closed‐ended questionnaire (Table 1). The original draft of the questionnaire was pretested on 2 hospitalists and 3 PCPs by L.H., who has graduate level formal training and experience in the design, refinement, implementation, and evaluation of questionnaires.
| Questions |
|---|
| 1. Do you use the hospitalist system at PCMC? yes/no |
| 1a. If yes: For what % of your patients that are hospitalized do you use the hospitalist system? |
| 2. How would you rate the quality of communications between hospitalists and Primary Care Providers? |
| a: excellent; b: very good; c: good; d: fair; e: poor. |
| 2a. Why did you give it that rating? |
| 3. What barriers, if any, have you experienced in communicating with hospitalists/Primary Care Providers? |
| 4. What communication methods have been effective in the past? What suggestions do you have for improving communication methods? |
| 5. What information would you like to receive from hospitalists/Primary Care Providers regarding your patients' hospital care? |
| 6. At what points in the care process would you like to receive communications from hospitalists/Primary Care Providers? |
| 7. What suggestions do you have for improving overall communications between hospitalists and Primary Care Providers? |
| 8. Do you have access to e‐mail and use it regularly in your practice? |
| 8a. Do you have access to a fax machine and use it regularly in your practice? |
| 8b. Do you have access to a telephone and use it regularly in your practice? |
| 8c. Considering e‐mail, fax, and telephone, which of these methods do you think would be the most effective for communicating with hospitalists/Primary Care Providers? |
| 9. Do you believe that improving communications between hospitalists and Primary Care Providers would improve the quality of patient care? |
| 9a. If yes: How? |
| 9b. If no: Why not? |
| 10. Any other comments/feedback? |
Data Collection/Analysis
After consent, participants were administered the phone questionnaire by L.H. during April, May, and June 2005. Interviews were transcribed verbatim into a Microsoft Word document by a trained transcriptionist. Responses were openly coded and then grouped into the respective main topics of interest. No further interviews were conducted when theoretical saturation was obtained (ie, respondents did not identify any new themes). Themes were compared using qualitative methods.7, 8
RESULTS
Only 1 physician per practice was interviewed. No PCP who was able to be contacted declined an interview, although some did require multiple phone attempts to schedule the interview. PCPs were located in Salt Lake County (n = 6), in other Utah counties (n = 3), and in surrounding Intermountain West states (n = 3). From January 1, 2004 to December 31, 2004, we estimate that the hospitalist division cared for patients from approximately 35 practices (50% in Salt Lake County, 30% in other Utah counties, and 20% in surrounding Intermountain West states).
Hospitalists and PCPs agreed that overall quality of communication ranged from poor to very good (Table 2). Both parties acknowledged that significant barriers to optimal communication exist, yet the barriers differed for each group. Hospitalists and PCPs also agreed that optimal communication could improve many aspects of patient care and should take place upon discharge and admission of patients and with major clinical changes. Both hospitalists and PCPs also wanted accurate and timely information. One priority that the participants emphasized is the timely transfer of admission notification and the receipt of accurate and timely discharge summaries by PCPs.
| Hospitalists | Primary Care Providers | |
|---|---|---|
| Quality of communication | ||
| Poor | 0% | 33% |
| Fair | 50% | 17% |
| Good | 40% | 8% |
| Very good | 10% | 42% |
| Excellent | 0% | 0% |
| Barriers to communication | Lack of PCP directory; | Not knowing name and contact information of hospitalist taking care of their patient; |
| Lack of access to patients' medication or problem list; | Teaching hospital with numerous residents and students | |
| Lack of standardized system | ||
| Methods of information sharing | Electronic medical record ideal for sharing information | Electronic medical record ideal for sharing information; |
| Phone calls and faxes effective, especially if pager numbers were included | ||
| Key data elements | Diagnoses; | Diagnoses; |
| Medications; | Medications; | |
| Follow‐up plans | Follow‐up plans | |
| Critical timing | At discharge; | At discharge; |
| After admission; | After admission; | |
| Major clinical changes | Major clinical changes | |
| Perceived benefits | Improved patient satisfaction; | Improved patient satisfaction; |
| Improved follow‐up; | Improved follow‐up; | |
| Decreased medication errors; | Decreased medication errors; | |
| Increased efficiency | Increased efficiency |
Quality of Communication
Overall, both groups rated communication quality from poor to very good (Table 2). Notably, no hospitalists or PCPs rated overall quality as excellent, but 33% of PCPs rated it as poor compared to 0% of the hospitalists. Fifty‐eight percent (7/12) of PCPs used the hospitalists for 80% of their admissions to the hospital.
For hospitalists, lack of communication stemmed from busy schedules, not knowing who the PCP was, or not having the PCP contact information. Similarly, PCPs commented that they often found out their patient was admitted to the hospital only when the patient showed up in their office for a follow‐up visit. Both hospitalists and PCPs felt it was the hospitalist's job to inform and update PCPs on their patient's status while hospitalized. However, if the patient was admitted via the emergency department (ED), hospitalists felt that it was the ED's responsibility to inform the PCPs of their patients' admission.
Barriers to Communication
PCPs and hospitalists noted different barriers to optimal communication. Hospitalists identified the lack of a PCP directory, the lack of access to patients' medication and problem lists, and the lack of a standardized system to communicate with PCPs as major barriers. The delayed receipt of the discharge summary by PCPs was also viewed as a barrier by hospitalists. Pediatric hospitalists found the large variation in PCP availability as well as the variation in PCP preferred methods of communication (phone call, fax, or e‐mail) to be additional barriers. PCPs, on the other hand, struggled with the complexity of the hospital system. The fact that PCMC is a teaching hospital with numerous residents and students assisting in their patients' care, as well as not knowing the names and contact information of the hospitalists taking care of their patients, served as barriers to optimal communication. Additionally, PCPs noted the delay in receiving discharge summaries as a barrier and a source of frustration.
Methods of Information Sharing
All PCPs and hospitalists had access to telephones and faxes and used them regularly in their practices (100% for both groups). A majority of PCPs believed phone calls and faxes were effective means of information sharing, especially if pager numbers of the hospitalists were included. Some PCPs and a larger number of hospitalists thought an electronic medical record was an ideal tool for sharing information. However, PCPs appeared to have a lower rate of e‐mail access and usage compared with hospitalists.
Key Data Elements
There was agreement among PCPs and hospitalists regarding which data elements were important to be relayed among providers. PCPs and hospitalists were most interested in the following data elements upon patient discharge: diagnoses from the hospitalization, medications the patient was to take, and follow‐up plans for the patient. Hospitalists also thought PCPs could help by providing a list of current medications and a detailed past medical and social history upon admission. This information could be easily provided to the accepting hospitalist attending by phone or fax from the PCP.
Critical Timing and Perceived Benefits
Hospitalists and PCPs agreed that the most critical times for optimal hospitalist‐PCP communication were primarily at time of discharge from the hospital, after admission to the hospital, and when major clinical changes occurred. The majority of hospitalists and PCPs thought that improved communication would improve the quality of patient care through: (1) improved patient satisfaction; (2) improved quality and quantity of follow‐up; (3) decreased medication errors; and (4) increased efficiency for the PCPs and hospitalists.
DISCUSSION
Both pediatric hospitalists and PCPs agree on what information is important to transmit (diagnoses, medications, follow‐up needs, and pending laboratory test results) and critical times for communication during the hospitalization (at discharge, admission, and during major clinical changes). However, there was discrepancy in the barriers to optimal communication for each group. Identifying and addressing these barriers can help both hospitalists and PCPs implement targeted interventions aimed at improving communication. As the number of pediatric hospitalist programs increases, the risk for hospitalist‐PCP communication breakdowns, which can have a negative impact on patient care, also increases.
Previous adult studies describe the scope of the problem around poor communication between hospitalists and PCPs.1, 912 Kripalani et al.10 reported recently that delays and omissions in communication are common at hospital discharge among adult hospitalists and that computer‐generated summaries, educational interventions, and standardized formats may facilitate more timely transfer of pertinent information. However, there is limited data on pediatric hospitalist‐PCP communication. Srivastava et al.5 found that 60% of community physicians thought hospitalist systems may impair communication with PCPs when evaluating community and hospital‐based physicians' attitudes regarding pediatric hospitalist systems.
PCPs can feel left out when their patients are cared for by hospitalists.13 One PCP in our study commented: Include the referral doc as part of the team. We're the ones who will take care of them after discharge. It seems like an autonomous thing down there and we're excluded from the patient care team. Additionally, patients want their PCPs to remain involved in their care as they transition into and out of the hospital setting.1, 13
The continuity visit model has been proposed by Wachter and Pantilat14 to describe a clinical encounter between the primary physician and hospitalized patient, when the patient has a different physician of record. In this model, the PCP can endorse the hospitalist model and the individual hospitalist, notice subtle findings that differ from the patient's baseline, and help clarify patient preferences regarding difficult situations by drawing on their previous relationship with the patient. This visit may also benefit the PCP by providing insights into the patient's illness, personality, or social support that he or she was unaware of previously. However, in order for the continuity visit to exist, the PCP has to be informed of their patient's admission in the first place. Ethical dilemmas also have been raised regarding who bears primary responsibility for maintaining open lines of communication when patients are hospitalized.15 Lo15 advocates that PCPs can and should be involved in meaningful ways in the inpatient care of their patients even when they are not acting as the treating physicians. Specifically, he suggests that PCPs personally visit particularly ill patients or those with difficult diagnoses and use frequent phone calls to all admitted patients.
Beyond telephone calls and continuity visits, hospitalists and PCPs rely on discharge summaries as a key part of the information transfer about a patient's hospitalization.1, 16, 17 These documents are rendered useless if they are inaccurate, illegible, or not delivered in a timely manner.18 In a study of California family physicians, discharge summaries were thought to be too detailed by 84% of PCPs, and reportedly arrived before the patient's first follow‐up appointment only 33% of the time.1 O'Leary et al.19 found that 41% of the Department of Medicine physicians surveyed believed that at least 1 of their patients hospitalized in the previous 6 months had experienced a preventable adverse event related to poor transfer of information at discharge. In our study, PCPs noted that discharge summaries often arrived in their offices well after the patient had been seen for their follow‐up appointment.
Both hospitalists and PCPs agree that a concise and precise discharge summary should include an overview of the hospitalization with important details highlighted. Similar to the findings of Pantilat et al.,1 in our study PCPs specifically want detailed information with regard to diagnoses, discharge medications, and what to expect when they see the patient in their clinic. Follow‐up phone calls to PCPs to see that they received written information and if they require further details is 1 solution to ensuring good follow‐up, yet this adds to the burden of communication and could be an additional barrier.
The teaching institutions in which physicians train also pose unique obstacles to optimal communication. In academic medical centers, medical students and residents perform a majority of the discharge duties (eg, writing prescriptions, dictating discharge summaries, making follow‐up appointments, and calling PCPs), and teaching these trainees the importance of timely and accurate communication becomes an added challenge. Educators have to find novel ways of providing incentives to residents and medical students to get them to effectively participate in this process. Plauth et al.20 reports that hospitalists feel they needed better training in residency around communicating, noting a meaningful underemphasis during residency training in regard to communication with referring physicians. These skills should be taught in medical school and supported by both hospitalists and PCPs throughout residency training.
Both hospitalists and PCPs also want easy and reliable ways to access their colleagues, which ideally would be automatic. One PCP commented: a weekly or semiweekly phone call would be nice. Another suggested to: fax a short note. One hospitalist acknowledged: a systematic approach would be betterwhether a fax or telephone call and make sure there is a way of checking to make sure the communication has happened. Another hospitalist simply remarked: it needs to be done on every patient.
Thus, it seems an improved communication system should be flexible enough to accommodate unique provider preferences, such as communication via phone, fax, or e‐mail. This is demonstrated by 1 PCP who preferred the phone, but most convenient is the periodic fax updates. I don't have to be taken away from seeing patients.
Lo15 calls for a standard to be established for delivering care within the patient‐PCP‐hospitalist triad. Phone calls and faxes are 2 readily available methods of communication. However, the frequent back‐and‐forth of missed calls, unreturned calls, and days‐off is certainly a factor in determining efficiency and effectiveness of phone calls.
E‐mail, if it is widely used by all participants, may be an effective option for delivery that could provide confirmation of receipt. However, the lack of universal e‐mail usage by all providers remains a barrier. Questions as to which method is more time consuming and for whom, need to be studied further. Patient confidentiality also requires that this protected health information arrive in the proper hands. Personal relationships can also contribute to successful communication. One provider may be more likely to contact another if they know each other through some personal connection, such as medical school, residency, or a social group.
Our study has several limitations. The sample size was small. We obtained responses from a sample of key stakeholders in the hospitalist‐PCP communication process. We were limited by the number of hospitalists at our institution as well as the interest and availability of PCPs to respond. We are unable to determine the total number of patients by respondent PCP practice cared for by the hospitalist division. This could influence the results depending on whether the respondent PCP was a frequent or infrequent utilizer of the hospitalist system. However, we feel reassured that we are not missing important information, because in our methods, a priori, we had intended to stop interviewing PCPs once theoretical saturation had been reached (ie, respondents did not identify any new information). In our study, that occurred with 12 PCPs.
We attempted to interview a single physician in a number of different practice settings in order to gain insight into the perceptions of that individual as well as those of their partners. The views expressed by these individuals may not represent the views of hospitalists and PCPs outside of our practice area. Furthermore, PCMC serves as both a community pediatric hospital and a tertiary‐referral center for a large area, yet the current experience of 1 hospitalist division and 1 cohort of referring PCPs may contain regional variation that contributed bias to the responses.
Selection bias may have been introduced in our study by the inherent nature of phone interviews. We interviewed only providers with previous communication experience with our hospitalist division. These providers may have had a vested interest in the communication process. We did not interview those PCPs who did not have any communication with our hospitalist division or those who may have used the hospitalist division previously and decided to no longer use the division. Interviewing these groups may have provided additional insight into the communication issues mentioned here. Additionally, useful information could have been gleaned from trying to find out more from the 33% of PCPs who felt communication was poor. We anticipate further studies exploring this issue in more depth.
Future Directions
As a result of this study, we have implemented several interventions to improve information sharing between hospitalists and PCPs, including: 1) we updated current contact information (including names of physicians, office addresses, phone numbers, fax numbers, and e‐mail addresses) for all PCPs in our catchment area along with their preferred methods of communication; 2) we worked with the transcription services to automatically add PCP addresses, phone numbers, and fax numbers to dictated notes, eliminating time wasted searching for contact information; and 3) we standardized key data elements in admission history and physicals and discharge notes to increase the efficiency of the communication process.
Furthermore, we have implemented a standardized system to facilitate communication with PCPs. This system includes an automated process to notify PCPs of their patient's hospital admission, including the admission date, preliminary diagnoses, and responsible physician's contact information. We are currently undertaking a quality improvement project aimed at achieving timely transfer of discharge information to PCPs, including medications, follow‐up appointments, and a succinct hospital summary. Finally, establishing an evaluation process to monitor both successes and failures will be paramount to any interventions.
CONCLUSIONS
Hospitalists and PCPs agree that overall quality of communication ranges from poor to very good. Both PCPs and pediatric hospitalists acknowledge that significant barriers to optimal communication exist, yet the barriers differ for each group. They also agree that optimal communication would improve many aspects of patient care and should take place upon discharge and admission of patients and with major clinical changes.
Pediatric hospitalists and PCPs identified issues around optimal communication similar to those noted in the adult hospital medicine literature. Interventions to improve pediatric hospitalist‐PCP communication should at least address these 6 issues: (1) quality of communication; (2) barriers to communication; (3) methods of information sharing; (4) key data element requirements; (5) critical timing; and (6) perceived benefits. Such interventions will likely improve hospitalist‐PCP communication and potentially improve the quality of patient care. However, future studies will need to demonstrate the link between improved hospitalist‐PCP communication and improved patient care and outcomes.
Acknowledgements
The authors are indebted to Flory Nkoy, MD, MPH, MS, for his help in manuscript preparation and critical review.
Hospitalist systems focus on providing acute treatments to patients and expediting hospital discharge, sometimes without regard for the need to work in concert with community providers, leading to fragmentation of care.1 This fragmentation, particularly at the transitions of care, such as when patients move from the outpatient setting to a hospitalist system and then back to their primary care providers (PCPs), can lead to communication breakdowns and delays in care, and may compromise patient outcomes.2 Suboptimal treatments, such as medication errors and the ordering of redundant tests can occur in either setting if prior treatment information is not relayed in a timely and accurate fashion. Landrigan et al.3 described a conceptual model in 2001 that recognized the complexity of the hospitalist‐PCP communication system. Specifically, optimal care of hospitalized children includes PCPs, family members, hospitalists, and support staff while meeting the communication needs of families and PCPs. Additionally, mediating a smooth transition into and out of the hospital needs to be measured carefully.3
Previous adult studies have reported that hospitalist systems sometimes create discontinuity of patient care, which can have a negative impact on the quality of care provided to patients if there is poor communication between hospitalists and PCPs.2, 4, 5 Existing research on hospitalist‐PCP communication focuses mainly on adult hospitalist models with little known about the quality of current pediatric hospitalist‐PCP communication.
The objective of this study was to qualitatively explore issues around communication between pediatric hospitalists and PCPs. Specifically, we sought to explore the quality of communication practices and barriers to optimal communication within the hospitalist‐PCP model at a tertiary care children's hospital. The results are serving as a needs assessment to guide the design of a quality improvement project with the aim of improving pediatric hospitalist‐PCP communication.
METHODS
Study Design
Phone interviews of pediatric hospitalists and PCPs were conducted. The study was approved by the University of Utah and Primary Children's Medical Center (PCMC) Institutional Review Boards.
Setting
PCMC is a 232‐bed, tertiary‐care referral center and community hospital in Salt Lake City, UT, which serves a catchment area of approximately 1,000,000 children in 5 Intermountain West states (Utah, Idaho, Nevada, Montana, and Wyoming). In 2005, there were more than 40,000 emergency department visits and more than 11,000 hospital admissions. At the time of this study, the Division of Pediatric Inpatient Medicine (hospitalist division) included 11 full‐time equivalents. All hospitalists play a teaching role and are on faculty at the University of Utah School of Medicine. In 2005, approximately 45% of medical inpatients at PCMC were cared for by the hospitalist division, with approximately 95% cared for by resident teams.
Participants
Ten University of Utah pediatric hospitalists and 12 PCPs within our catchment area completed interviews. Verbal consent was given before study participants began the phone interview. All hospitalists from the hospitalist division, excluding the first author, completed an interview. PCPs who had referred patients to the hospitalist division in the year preceding this study (2004) were identified through a referring database kept by the hospitalist division. An attempt was made to interview physicians in multiple practice settings and geographic locations.
Inclusion criteria for PCPs included their willingness to complete the interview as well as having had patients cared for by the hospitalist division in the preceding year (2004). There was no preference given to any physicians, including physicians well‐known by the research team or more frequent users of the hospitalist division.
Instrument
To develop our questionnaire, we conducted a detailed literature search to identify issues surrounding hospitalist‐PCP communication in the adult and pediatric hospitalist literature. Search terms included: hospitalists, interprofessional relations, patient discharge, communication, follow‐up care, transitions, and primary care provider using the PubMed database, limited to English language articles from 1990 to 2005. The 6 issues for the final questionnaire were identified from published hospitalist survey questions (in both adult and pediatric literature) and published articles addressing themes regarding hospitalist and PCP attitudes (specifically in regard to the communication process).1, 2, 4, 6 These 6 issues (quality of communication, barriers to communication, methods of information sharing, key data element requirements, critical timing, and perceived benefits) were incorporated into the open‐ended and closed‐ended questionnaire (Table 1). The original draft of the questionnaire was pretested on 2 hospitalists and 3 PCPs by L.H., who has graduate level formal training and experience in the design, refinement, implementation, and evaluation of questionnaires.
| Questions |
|---|
| 1. Do you use the hospitalist system at PCMC? yes/no |
| 1a. If yes: For what % of your patients that are hospitalized do you use the hospitalist system? |
| 2. How would you rate the quality of communications between hospitalists and Primary Care Providers? |
| a: excellent; b: very good; c: good; d: fair; e: poor. |
| 2a. Why did you give it that rating? |
| 3. What barriers, if any, have you experienced in communicating with hospitalists/Primary Care Providers? |
| 4. What communication methods have been effective in the past? What suggestions do you have for improving communication methods? |
| 5. What information would you like to receive from hospitalists/Primary Care Providers regarding your patients' hospital care? |
| 6. At what points in the care process would you like to receive communications from hospitalists/Primary Care Providers? |
| 7. What suggestions do you have for improving overall communications between hospitalists and Primary Care Providers? |
| 8. Do you have access to e‐mail and use it regularly in your practice? |
| 8a. Do you have access to a fax machine and use it regularly in your practice? |
| 8b. Do you have access to a telephone and use it regularly in your practice? |
| 8c. Considering e‐mail, fax, and telephone, which of these methods do you think would be the most effective for communicating with hospitalists/Primary Care Providers? |
| 9. Do you believe that improving communications between hospitalists and Primary Care Providers would improve the quality of patient care? |
| 9a. If yes: How? |
| 9b. If no: Why not? |
| 10. Any other comments/feedback? |
Data Collection/Analysis
After consent, participants were administered the phone questionnaire by L.H. during April, May, and June 2005. Interviews were transcribed verbatim into a Microsoft Word document by a trained transcriptionist. Responses were openly coded and then grouped into the respective main topics of interest. No further interviews were conducted when theoretical saturation was obtained (ie, respondents did not identify any new themes). Themes were compared using qualitative methods.7, 8
RESULTS
Only 1 physician per practice was interviewed. No PCP who was able to be contacted declined an interview, although some did require multiple phone attempts to schedule the interview. PCPs were located in Salt Lake County (n = 6), in other Utah counties (n = 3), and in surrounding Intermountain West states (n = 3). From January 1, 2004 to December 31, 2004, we estimate that the hospitalist division cared for patients from approximately 35 practices (50% in Salt Lake County, 30% in other Utah counties, and 20% in surrounding Intermountain West states).
Hospitalists and PCPs agreed that overall quality of communication ranged from poor to very good (Table 2). Both parties acknowledged that significant barriers to optimal communication exist, yet the barriers differed for each group. Hospitalists and PCPs also agreed that optimal communication could improve many aspects of patient care and should take place upon discharge and admission of patients and with major clinical changes. Both hospitalists and PCPs also wanted accurate and timely information. One priority that the participants emphasized is the timely transfer of admission notification and the receipt of accurate and timely discharge summaries by PCPs.
| Hospitalists | Primary Care Providers | |
|---|---|---|
| Quality of communication | ||
| Poor | 0% | 33% |
| Fair | 50% | 17% |
| Good | 40% | 8% |
| Very good | 10% | 42% |
| Excellent | 0% | 0% |
| Barriers to communication | Lack of PCP directory; | Not knowing name and contact information of hospitalist taking care of their patient; |
| Lack of access to patients' medication or problem list; | Teaching hospital with numerous residents and students | |
| Lack of standardized system | ||
| Methods of information sharing | Electronic medical record ideal for sharing information | Electronic medical record ideal for sharing information; |
| Phone calls and faxes effective, especially if pager numbers were included | ||
| Key data elements | Diagnoses; | Diagnoses; |
| Medications; | Medications; | |
| Follow‐up plans | Follow‐up plans | |
| Critical timing | At discharge; | At discharge; |
| After admission; | After admission; | |
| Major clinical changes | Major clinical changes | |
| Perceived benefits | Improved patient satisfaction; | Improved patient satisfaction; |
| Improved follow‐up; | Improved follow‐up; | |
| Decreased medication errors; | Decreased medication errors; | |
| Increased efficiency | Increased efficiency |
Quality of Communication
Overall, both groups rated communication quality from poor to very good (Table 2). Notably, no hospitalists or PCPs rated overall quality as excellent, but 33% of PCPs rated it as poor compared to 0% of the hospitalists. Fifty‐eight percent (7/12) of PCPs used the hospitalists for 80% of their admissions to the hospital.
For hospitalists, lack of communication stemmed from busy schedules, not knowing who the PCP was, or not having the PCP contact information. Similarly, PCPs commented that they often found out their patient was admitted to the hospital only when the patient showed up in their office for a follow‐up visit. Both hospitalists and PCPs felt it was the hospitalist's job to inform and update PCPs on their patient's status while hospitalized. However, if the patient was admitted via the emergency department (ED), hospitalists felt that it was the ED's responsibility to inform the PCPs of their patients' admission.
Barriers to Communication
PCPs and hospitalists noted different barriers to optimal communication. Hospitalists identified the lack of a PCP directory, the lack of access to patients' medication and problem lists, and the lack of a standardized system to communicate with PCPs as major barriers. The delayed receipt of the discharge summary by PCPs was also viewed as a barrier by hospitalists. Pediatric hospitalists found the large variation in PCP availability as well as the variation in PCP preferred methods of communication (phone call, fax, or e‐mail) to be additional barriers. PCPs, on the other hand, struggled with the complexity of the hospital system. The fact that PCMC is a teaching hospital with numerous residents and students assisting in their patients' care, as well as not knowing the names and contact information of the hospitalists taking care of their patients, served as barriers to optimal communication. Additionally, PCPs noted the delay in receiving discharge summaries as a barrier and a source of frustration.
Methods of Information Sharing
All PCPs and hospitalists had access to telephones and faxes and used them regularly in their practices (100% for both groups). A majority of PCPs believed phone calls and faxes were effective means of information sharing, especially if pager numbers of the hospitalists were included. Some PCPs and a larger number of hospitalists thought an electronic medical record was an ideal tool for sharing information. However, PCPs appeared to have a lower rate of e‐mail access and usage compared with hospitalists.
Key Data Elements
There was agreement among PCPs and hospitalists regarding which data elements were important to be relayed among providers. PCPs and hospitalists were most interested in the following data elements upon patient discharge: diagnoses from the hospitalization, medications the patient was to take, and follow‐up plans for the patient. Hospitalists also thought PCPs could help by providing a list of current medications and a detailed past medical and social history upon admission. This information could be easily provided to the accepting hospitalist attending by phone or fax from the PCP.
Critical Timing and Perceived Benefits
Hospitalists and PCPs agreed that the most critical times for optimal hospitalist‐PCP communication were primarily at time of discharge from the hospital, after admission to the hospital, and when major clinical changes occurred. The majority of hospitalists and PCPs thought that improved communication would improve the quality of patient care through: (1) improved patient satisfaction; (2) improved quality and quantity of follow‐up; (3) decreased medication errors; and (4) increased efficiency for the PCPs and hospitalists.
DISCUSSION
Both pediatric hospitalists and PCPs agree on what information is important to transmit (diagnoses, medications, follow‐up needs, and pending laboratory test results) and critical times for communication during the hospitalization (at discharge, admission, and during major clinical changes). However, there was discrepancy in the barriers to optimal communication for each group. Identifying and addressing these barriers can help both hospitalists and PCPs implement targeted interventions aimed at improving communication. As the number of pediatric hospitalist programs increases, the risk for hospitalist‐PCP communication breakdowns, which can have a negative impact on patient care, also increases.
Previous adult studies describe the scope of the problem around poor communication between hospitalists and PCPs.1, 912 Kripalani et al.10 reported recently that delays and omissions in communication are common at hospital discharge among adult hospitalists and that computer‐generated summaries, educational interventions, and standardized formats may facilitate more timely transfer of pertinent information. However, there is limited data on pediatric hospitalist‐PCP communication. Srivastava et al.5 found that 60% of community physicians thought hospitalist systems may impair communication with PCPs when evaluating community and hospital‐based physicians' attitudes regarding pediatric hospitalist systems.
PCPs can feel left out when their patients are cared for by hospitalists.13 One PCP in our study commented: Include the referral doc as part of the team. We're the ones who will take care of them after discharge. It seems like an autonomous thing down there and we're excluded from the patient care team. Additionally, patients want their PCPs to remain involved in their care as they transition into and out of the hospital setting.1, 13
The continuity visit model has been proposed by Wachter and Pantilat14 to describe a clinical encounter between the primary physician and hospitalized patient, when the patient has a different physician of record. In this model, the PCP can endorse the hospitalist model and the individual hospitalist, notice subtle findings that differ from the patient's baseline, and help clarify patient preferences regarding difficult situations by drawing on their previous relationship with the patient. This visit may also benefit the PCP by providing insights into the patient's illness, personality, or social support that he or she was unaware of previously. However, in order for the continuity visit to exist, the PCP has to be informed of their patient's admission in the first place. Ethical dilemmas also have been raised regarding who bears primary responsibility for maintaining open lines of communication when patients are hospitalized.15 Lo15 advocates that PCPs can and should be involved in meaningful ways in the inpatient care of their patients even when they are not acting as the treating physicians. Specifically, he suggests that PCPs personally visit particularly ill patients or those with difficult diagnoses and use frequent phone calls to all admitted patients.
Beyond telephone calls and continuity visits, hospitalists and PCPs rely on discharge summaries as a key part of the information transfer about a patient's hospitalization.1, 16, 17 These documents are rendered useless if they are inaccurate, illegible, or not delivered in a timely manner.18 In a study of California family physicians, discharge summaries were thought to be too detailed by 84% of PCPs, and reportedly arrived before the patient's first follow‐up appointment only 33% of the time.1 O'Leary et al.19 found that 41% of the Department of Medicine physicians surveyed believed that at least 1 of their patients hospitalized in the previous 6 months had experienced a preventable adverse event related to poor transfer of information at discharge. In our study, PCPs noted that discharge summaries often arrived in their offices well after the patient had been seen for their follow‐up appointment.
Both hospitalists and PCPs agree that a concise and precise discharge summary should include an overview of the hospitalization with important details highlighted. Similar to the findings of Pantilat et al.,1 in our study PCPs specifically want detailed information with regard to diagnoses, discharge medications, and what to expect when they see the patient in their clinic. Follow‐up phone calls to PCPs to see that they received written information and if they require further details is 1 solution to ensuring good follow‐up, yet this adds to the burden of communication and could be an additional barrier.
The teaching institutions in which physicians train also pose unique obstacles to optimal communication. In academic medical centers, medical students and residents perform a majority of the discharge duties (eg, writing prescriptions, dictating discharge summaries, making follow‐up appointments, and calling PCPs), and teaching these trainees the importance of timely and accurate communication becomes an added challenge. Educators have to find novel ways of providing incentives to residents and medical students to get them to effectively participate in this process. Plauth et al.20 reports that hospitalists feel they needed better training in residency around communicating, noting a meaningful underemphasis during residency training in regard to communication with referring physicians. These skills should be taught in medical school and supported by both hospitalists and PCPs throughout residency training.
Both hospitalists and PCPs also want easy and reliable ways to access their colleagues, which ideally would be automatic. One PCP commented: a weekly or semiweekly phone call would be nice. Another suggested to: fax a short note. One hospitalist acknowledged: a systematic approach would be betterwhether a fax or telephone call and make sure there is a way of checking to make sure the communication has happened. Another hospitalist simply remarked: it needs to be done on every patient.
Thus, it seems an improved communication system should be flexible enough to accommodate unique provider preferences, such as communication via phone, fax, or e‐mail. This is demonstrated by 1 PCP who preferred the phone, but most convenient is the periodic fax updates. I don't have to be taken away from seeing patients.
Lo15 calls for a standard to be established for delivering care within the patient‐PCP‐hospitalist triad. Phone calls and faxes are 2 readily available methods of communication. However, the frequent back‐and‐forth of missed calls, unreturned calls, and days‐off is certainly a factor in determining efficiency and effectiveness of phone calls.
E‐mail, if it is widely used by all participants, may be an effective option for delivery that could provide confirmation of receipt. However, the lack of universal e‐mail usage by all providers remains a barrier. Questions as to which method is more time consuming and for whom, need to be studied further. Patient confidentiality also requires that this protected health information arrive in the proper hands. Personal relationships can also contribute to successful communication. One provider may be more likely to contact another if they know each other through some personal connection, such as medical school, residency, or a social group.
Our study has several limitations. The sample size was small. We obtained responses from a sample of key stakeholders in the hospitalist‐PCP communication process. We were limited by the number of hospitalists at our institution as well as the interest and availability of PCPs to respond. We are unable to determine the total number of patients by respondent PCP practice cared for by the hospitalist division. This could influence the results depending on whether the respondent PCP was a frequent or infrequent utilizer of the hospitalist system. However, we feel reassured that we are not missing important information, because in our methods, a priori, we had intended to stop interviewing PCPs once theoretical saturation had been reached (ie, respondents did not identify any new information). In our study, that occurred with 12 PCPs.
We attempted to interview a single physician in a number of different practice settings in order to gain insight into the perceptions of that individual as well as those of their partners. The views expressed by these individuals may not represent the views of hospitalists and PCPs outside of our practice area. Furthermore, PCMC serves as both a community pediatric hospital and a tertiary‐referral center for a large area, yet the current experience of 1 hospitalist division and 1 cohort of referring PCPs may contain regional variation that contributed bias to the responses.
Selection bias may have been introduced in our study by the inherent nature of phone interviews. We interviewed only providers with previous communication experience with our hospitalist division. These providers may have had a vested interest in the communication process. We did not interview those PCPs who did not have any communication with our hospitalist division or those who may have used the hospitalist division previously and decided to no longer use the division. Interviewing these groups may have provided additional insight into the communication issues mentioned here. Additionally, useful information could have been gleaned from trying to find out more from the 33% of PCPs who felt communication was poor. We anticipate further studies exploring this issue in more depth.
Future Directions
As a result of this study, we have implemented several interventions to improve information sharing between hospitalists and PCPs, including: 1) we updated current contact information (including names of physicians, office addresses, phone numbers, fax numbers, and e‐mail addresses) for all PCPs in our catchment area along with their preferred methods of communication; 2) we worked with the transcription services to automatically add PCP addresses, phone numbers, and fax numbers to dictated notes, eliminating time wasted searching for contact information; and 3) we standardized key data elements in admission history and physicals and discharge notes to increase the efficiency of the communication process.
Furthermore, we have implemented a standardized system to facilitate communication with PCPs. This system includes an automated process to notify PCPs of their patient's hospital admission, including the admission date, preliminary diagnoses, and responsible physician's contact information. We are currently undertaking a quality improvement project aimed at achieving timely transfer of discharge information to PCPs, including medications, follow‐up appointments, and a succinct hospital summary. Finally, establishing an evaluation process to monitor both successes and failures will be paramount to any interventions.
CONCLUSIONS
Hospitalists and PCPs agree that overall quality of communication ranges from poor to very good. Both PCPs and pediatric hospitalists acknowledge that significant barriers to optimal communication exist, yet the barriers differ for each group. They also agree that optimal communication would improve many aspects of patient care and should take place upon discharge and admission of patients and with major clinical changes.
Pediatric hospitalists and PCPs identified issues around optimal communication similar to those noted in the adult hospital medicine literature. Interventions to improve pediatric hospitalist‐PCP communication should at least address these 6 issues: (1) quality of communication; (2) barriers to communication; (3) methods of information sharing; (4) key data element requirements; (5) critical timing; and (6) perceived benefits. Such interventions will likely improve hospitalist‐PCP communication and potentially improve the quality of patient care. However, future studies will need to demonstrate the link between improved hospitalist‐PCP communication and improved patient care and outcomes.
Acknowledgements
The authors are indebted to Flory Nkoy, MD, MPH, MS, for his help in manuscript preparation and critical review.
- ,,,.Primary care physician attitudes regarding communication with hospitalists.Am J Med.2001;111(9B):15S–20S.
- ,,,,,.Physician attitudes toward and prevalence of the hospitalist model of care: results of a national survey.Am J Med.2000;109(8):648–653.
- ,,,,,.Pediatric hospitalists: what do we know, and where do we go from here?Ambul Pediatr.2001;1(6):340–345.
- ,,.Physician views on caring for hospitalized patients and the hospitalist model of inpatient care.J Gen Intern Med.2001;16(2):116–119.
- ,,,,,.Community and hospital‐based physicians' attitudes regarding pediatric hospitalist systems.Pediatrics.2005;115(1):34–38.
- ,.The patient provider relationship and the hospitalist movement. Introduction.Dis Mon.2002;48(4):189–190.
- ,.Rigour and qualitative research.BMJ.1995;311(6997):109–112.
- ,.Needs assessment in post graduate medical education: a review.Med Educ Online.2001;7:1–8.
- ,,,.How physicians perceive hospitalist services after implementation: anticipation vs reality.Arch Intern Med.2003;163(19):2330–2336.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297(8):831–841.
- ,,.Generalist‐subspecialist communication for children with chronic conditions: a regional physician survey.Pediatrics.2003;112(6 Pt 1):1314–1320.
- ,,,,,.Communication breakdown in the outpatient referral process.J Gen Intern Med.2000;15(9):626–631.
- ,,.How do patients view the role of the primary care physician in inpatient care?Dis Mon.2002;48(4):230–238.
- ,.The “continuity visit” and the hospitalist model of care.Dis Mon.2002;48(4):267–272.
- .Ethical and policy implications of hospitalist systems.Dis Mon.2002;48(4):281–290.
- ,,, et al.From a paper‐based transmission of discharge summaries to electronic communication in health care regions.Int J Med Inform.2006;75(3‐4):209–215.
- ,,.Tying up loose ends: discharging patients with unresolved medical issues.Arch Intern Med.2007;167(12):1305–1311.
- ,.Improving communication between hospital and community physicians. Feasibility study of a handwritten, faxed hospital discharge summary. Discharge Summary Study Group.Can Fam Physician.1999;45:2893–2899.
- ,,,,.Outpatient physicians' satisfaction with discharge summaries and perceived need for an electronic discharge summary.J Hosp Med.2006;1(5):317–320.
- ,,,.Hospitalists' perceptions of their residency training needs: results of a national survey.Am J Med.2001;111(3):247–254.
- ,,,.Primary care physician attitudes regarding communication with hospitalists.Am J Med.2001;111(9B):15S–20S.
- ,,,,,.Physician attitudes toward and prevalence of the hospitalist model of care: results of a national survey.Am J Med.2000;109(8):648–653.
- ,,,,,.Pediatric hospitalists: what do we know, and where do we go from here?Ambul Pediatr.2001;1(6):340–345.
- ,,.Physician views on caring for hospitalized patients and the hospitalist model of inpatient care.J Gen Intern Med.2001;16(2):116–119.
- ,,,,,.Community and hospital‐based physicians' attitudes regarding pediatric hospitalist systems.Pediatrics.2005;115(1):34–38.
- ,.The patient provider relationship and the hospitalist movement. Introduction.Dis Mon.2002;48(4):189–190.
- ,.Rigour and qualitative research.BMJ.1995;311(6997):109–112.
- ,.Needs assessment in post graduate medical education: a review.Med Educ Online.2001;7:1–8.
- ,,,.How physicians perceive hospitalist services after implementation: anticipation vs reality.Arch Intern Med.2003;163(19):2330–2336.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297(8):831–841.
- ,,.Generalist‐subspecialist communication for children with chronic conditions: a regional physician survey.Pediatrics.2003;112(6 Pt 1):1314–1320.
- ,,,,,.Communication breakdown in the outpatient referral process.J Gen Intern Med.2000;15(9):626–631.
- ,,.How do patients view the role of the primary care physician in inpatient care?Dis Mon.2002;48(4):230–238.
- ,.The “continuity visit” and the hospitalist model of care.Dis Mon.2002;48(4):267–272.
- .Ethical and policy implications of hospitalist systems.Dis Mon.2002;48(4):281–290.
- ,,, et al.From a paper‐based transmission of discharge summaries to electronic communication in health care regions.Int J Med Inform.2006;75(3‐4):209–215.
- ,,.Tying up loose ends: discharging patients with unresolved medical issues.Arch Intern Med.2007;167(12):1305–1311.
- ,.Improving communication between hospital and community physicians. Feasibility study of a handwritten, faxed hospital discharge summary. Discharge Summary Study Group.Can Fam Physician.1999;45:2893–2899.
- ,,,,.Outpatient physicians' satisfaction with discharge summaries and perceived need for an electronic discharge summary.J Hosp Med.2006;1(5):317–320.
- ,,,.Hospitalists' perceptions of their residency training needs: results of a national survey.Am J Med.2001;111(3):247–254.
Copyright © 2009 Society of Hospital Medicine
Quality of Life of Children with NI after Fundoplication for GERD
Aspiration pneumonia is the most common cause of death in children with severe neurological impairment (NI).13 For several reasons (eg, improved survival of extremely low‐birth‐weight infants, technological advances, and changing societal attitudes), the number of children with severe NI is increasing. Many children with severe NI have dysfunctional swallowing and gastroesophageal reflux disease (GERD).46 This combination places them at high risk for recurrent aspiration that, in turn, leads to aspiration pneumonia, repeated hospitalization, respiratory failure, compromised quality of life, and death.7, 8
The most common treatment approach for the combination of dysfunctional swallowing and GERD is surgical fundoplication with a gastrostomy feeding tube. Fundoplication is the third most common procedure performed in children by pediatric surgeons in the United States.9 Fifty percent of the children who receive a fundoplication have neurological impairment.10, 11 The goals of the surgery to treat GERD unresponsive to medical management are to reduce the risk of aspiration pneumonia, improve nutritional status, and improve the quality of life of the children and their families. However, few prospective longitudinal studies have determined whether the quality of life of the children or their caregivers actually improves over time.
The importance of caregiver and child quality of life is increasingly recognized as a critical outcome of any intervention in this population.12, 13 Previous studies of fundoplication in children with NI, GERD, and dysfunctional swallowing reported surgical mortality rates between 1% and 3% and other complications between 4% and 39%, reflecting the medical fragility of these children.5, 1418 Some of these studies had longitudinal follow‐up and reported long‐term data. Recurrence of symptoms was reported in up to 56% of patients, recurrence of AP in up to 39%, further surgical procedures in up to 19%, and mortality in up to 17%.14, 1921 Few case series of children with neurological impairment who have had a fundoplication have addressed child and caregiver quality of life following either a fundoplication or placement of a feeding tube.2224 In their study of 16 patients who had a fundoplication and gastrostomy tube placed, Tawfik et al. found improvements in children's happiness, ease of giving medicines, and time to devote to other children. Sullivan et al. found improvement in caregiver quality of life following placement of a gastrostomy tube in a child; however, they did not specifically identify those children who had been treated with a fundoplication. In their retrospective study, O'Neill et al. found improved child and caregiver quality of life following a fundoplication. Collectively, these studies have found that parents report improvement of both their own and their child's quality of life after either intervention. However, not having baseline measurements, not controlling for degree of functional impairment of the children, small sample sizes, and large loss to follow‐up limit the utility of these studies. In this ongoing, long‐term prospective longitudinal study, we report the initial impact of a fundoplication on the quality of life of both children and their caregivers.
The primary objective of this study was to determine change over time in the quality of life of children with neurological impairment who received surgical treatment of their GERD and of the caregivers of these children, controlling for the degree of functional impairment of the children. We hypothesized that child and caregiver quality of life would both improve after primary fundoplication and gastrostomy tube placement. Secondary objectives included describing rates of complications in this population.
METHODS
Setting and Study Population
We enrolled patients from newborn to 18 years of age who had a diagnosis compatible with neurological impairment and who received their first fundoplication for GERD between January 2005 and July 2006 at Primary Children's Medical Center (PCMC), in Salt Lake City, Utah. PCMC is a 232‐bed children's hospital in the Intermountain West owned and operated by Intermountain Healthcare, Inc., a large vertically integrated health care delivery system that serves as both the primary hospital for Salt Lake County and as the tertiary‐care hospital for 4 additional states (Wyoming, Nevada, Idaho, and Montana).25 Patients who had a previous gastrojejunal feeding tube were excluded as were patients who had a previous fundoplication, as these procedures may have biased their reported quality of life, our main outcome measure.
Patients were included in the study if they had GERD (based on clinical history or testing) that had been refractory to medical management (defined as continued gastroesophageal reflux symptoms despite antireflux medications). GERD was defined using the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) criteria.26 These include: the presence of clinical symptoms and at least 1 abnormal result from an upper gastrointestinal x‐ray series (recognizing that this test is neither sensitive nor specific for reflux), pH probe, upper gastrointestinal endoscopy with biopsy, nuclear medicine scan, or a modified barium swallow. As this was a prospective observational study, physicians were allowed to order testing as their practice dictated. Patients were excluded if they had neurological impairment but lacked objective documentation of GERD using the NASPGHAN recommendations, unless there were obvious clinical indications such as witnessed vomiting and aspiration (N = 3). No patient received a prophylactic fundoplication (fundoplication without documented GERD).
Study Design
This is an ongoing prospective longitudinal study. Patients who had a first fundoplication at PCMC were identified by the surgical service, with weekly lists shared with the research team. Patients were approached by a research assistant during that initial hospitalization to see if they met inclusion criteria for the study using data from the medical records and surgical team when necessary.
Data Variables and Sources
Indications for the fundoplication, performance and results of diagnostic testing for GERD, complications of the fundoplication, and reasons for the neurological impairment were obtained through review of the electronic and paper medical records. Mortality data, subsequent emergency department visits, and admissions to the hospital were obtained using Intermountain Healthcare's electronic data warehouse, which merges clinical, financial, and administrative data including the Utah Vital Statistics database.
Neurological impairment was defined from 2 sources: (1) clinical diagnoses as identified by providers and (2) International Classification of Diseases Codes Modified, version 9 (ICD‐9 CM) identified a priori as indicating neurological impairment.
Instruments and Study Outcomes
Functional status was measured using the WeeFIM. This instrument has been tested and shown to be valid and reliable for children more than 6 months old with neurodevelopmental disabilities including spina bifida and Down syndrome.2732 WeeFIM is a self‐administered parent instrument composed of 18 items and 6 domains (self‐care, sphincter control, transfers, locomotion, communication, and social cognition).33 The WeeFIM allows patients to be stratified into areas of function from severely impaired to normal.
The primary outcome was child quality of life as measured by the Child Health Questionnaire Parental Form 50 (CHQ‐PF50). Caregiver quality of life was measured using the Short‐Form Health Status Survey (SF‐36) and Parenting Stress Index/Short Form (PSI/SF). The CHQ‐PF50 is a self‐administered parent questionnaire of 50 questions that measures 6 domains, including physical function and abilities, pain and discomfort, general health perception, behavior, temperament and moods, and satisfaction with growth and development.34 This instrument has been tested for validity and reliability in children with cerebral palsy.35 The SF‐36 is a widely accepted measure of health status that measures 8 domains of health: physical functioning, role limitations due to physical problems, bodily pain, general health, vitality, social functioning, role limitations due to emotional problems, and mental health. The SF‐36 has been well studied and has been used to measure the effect on a caregiver's quality of life associated with caring for a chronically ill child with significant medical problems.36, 37 Higher scores in each domain of both the CHQPF50 and the SF‐36 reflect better quality of life. Caregiver stress was measured using the PSI/SF (Psychological Assessment Resources Inc, Odessa, FL).38 In the PSI/SF a parent rates the parentchild dyad on 36 items that are summarized in 3 subscales: parental distress, parentchild dysfunctional interaction, and difficult child. A parent scores each item as strongly agree, agree, not sure, disagree, or strongly disagree. The sum of the 3 subscale scores is the total stress score. Higher scores denote a greater degree of stress. The PSI/SF has been validated in several studies for caregivers of children with chronic diseases.3943
The CHQ‐PF50, SF‐36, and PSI/SF questionnaires and the WeeFIM functional status measure were administered to each study patient and caregiver in person at enrollment (baseline) and by mail 1 month after fundoplication. A follow‐up postcard reminder was mailed 1 week after the initial mailing. Second and final mailings were sent to nonresponders 3 and 5 weeks, respectively, after the initial mailing.
Secondary outcomes included rates of complications including failure of the fundoplication. Complications were defined as a subsequent emergency department visit, hospitalization, or death related to the surgery, gastrostomy tube, or aspiration pneumonia. Failures were defined as a second fundoplication or the insertion of a gastrojejunal feeding tube as nonsurgical management of recurrent GERD and/or paraesophageal hernia. Secondary outcomes were followed from the time of the first fundoplication until 1 month after surgery.
Analyses
The differential effect of the fundoplication on the quality of life measures was assessed and quantified through statistical analysis. Because the primary interest was to measure change in baseline characteristics over time, repeated‐measures models were used to compare the group relative to changes in functional status. In particular, changes from baseline values were modeled 1 month after the procedure. The Kenward‐Roger approximation of degrees of freedom was used to compute P values from the overall tests.44 Repeated‐measures models were fit to the data using restricted maximum likelihood estimation. An autoregressive covariance matrix was assumed for the multiple measurements of each individual, thus limiting the number of restrictions forced by the model on the data. The repeated‐measures models used all the available data on participants, including those who dropped out of the study. To obtain the most accurate comparison of the study group, the covariate of functional status at baseline was taken into account in the fitted models. Statistical analyses were performed with SAS statistical software (version 9.13; SAS Institute, Cary NC). Student t tests were performed for comparison of means of the quality‐of‐life domains for the study cohort compared to either the general population or specific groups of patients for comparative purposes.
The study was approved by the institutional review boards of the University of Utah Health Sciences Center and Primary Children's Medical Center.
RESULTS
Sixty‐three children met eligibility criteria. Forty‐four families (70%) initially agreed to participate in the study and completed the baseline questionnaires (see Fig. 1). The mean age of the children was 2.2 years. Twenty‐six parents of children completed the 1‐month postfundoplication quality‐of‐life questionnaires. Thirteen patients were lost to follow‐up, 5 of whom had not reached the 1‐month postfundoplication time point. The median WeeFIM (functional status) score of the whole group was 31.2 (95% confidence interval [CI] 11‐71) compared with a childhood matched‐age norm of 83 (95% CI 60‐110), P = .001. WeeFIM scores did not change significantly from baseline to 1 month, P = .98 (Kruskall‐Wallis test).
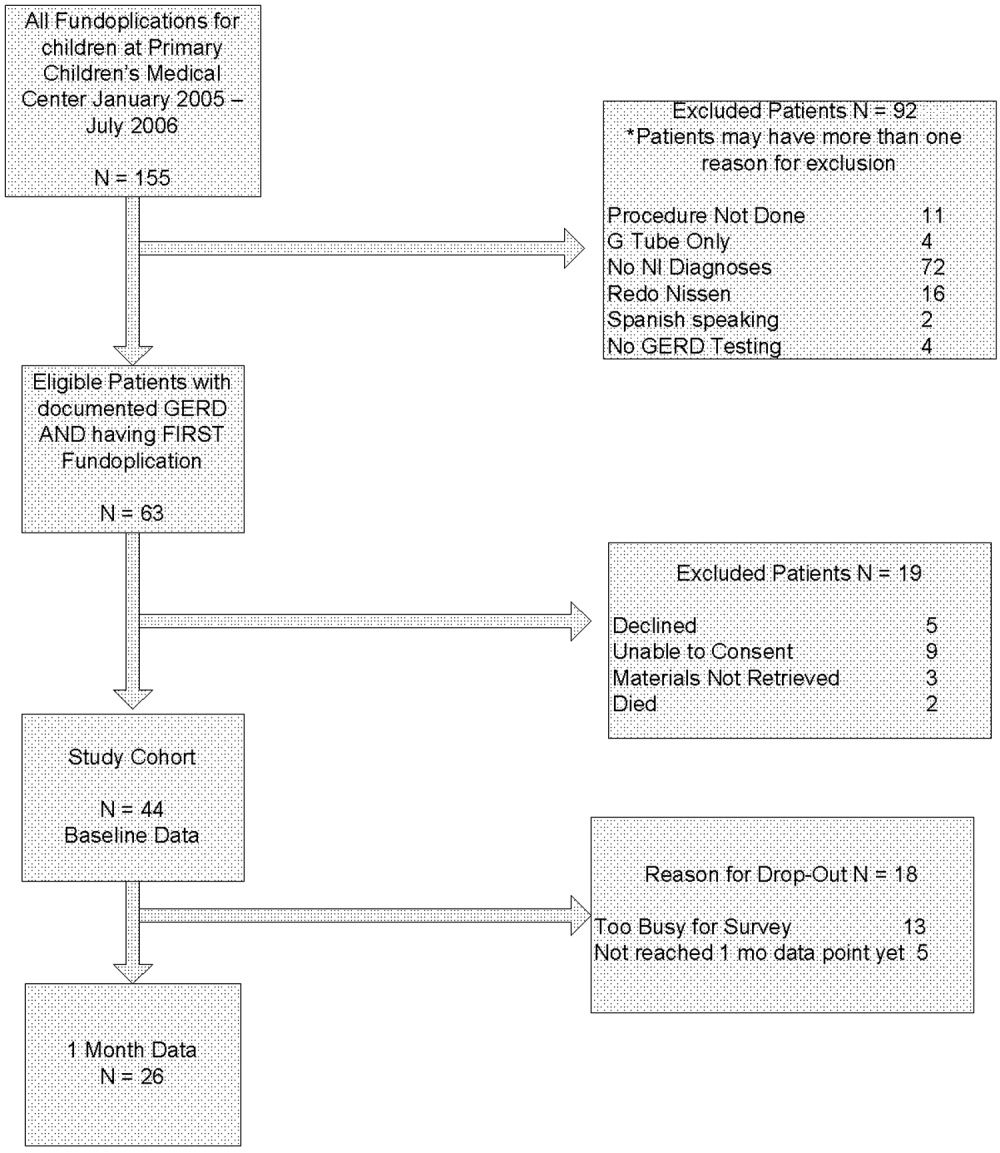
Data for the 13 parents and children (30%) who gave baseline data but were subsequently lost to follow‐up are shown in Table 1. Reasons for loss to follow‐up were caregiver reporting being too busy to fill out the questionnaires (n = 8) and no reason stated (n = 5).
In addition to the diagnosis of GERD, clinical indications for fundoplication were vomiting (55%), feeding‐related issues (47%), and failure to thrive (39%). Diagnosis of GERD was confirmed for 41 of 44 patients77% had an abnormal upper GI, 26% an abnormal pH probe, 14% an abnormal endoscopy, and 24% an abnormal swallow study. The remaining 3 had obvious clinical symptoms for GERD and did not require further testing according to their attending surgeon (2 with witnessed vomiting leading to aspiration and 1 who was exclusively gastrostomy‐fed and was witnessed having feeds coming from the tracheostomy). Various medications had been tried and were considered to have failed in these patients: 39% had been treated with acid‐suppressive agents; 80% with acid blocking agents; and 61% with prokinetic agents. Fourteen patients (32%) had cerebral palsy, and 14 (32%) had a brain or spinal cord abnormality (see Table 2).
| Diagnostic category | ICD‐9 codes used |
|---|---|
| |
| Brain or spinal cord anomaly | 335.22, 742.0, 742.1, 742.2, 742.4, 742.53 |
| Cerebral palsy | 343.0, 343.1, 343.2, 343.8, 343.9, 344.00 |
| Hydrocephalus | 331.3, 331.4, 742.3 |
| Down syndrome | 758.0 |
| Seizures | 345.10, 345.11, 345.3, 345.41, 345.50, 345.81, 345.90, 345.91 |
| Muscular dystrophy or myopathy | 359.0, 359.1, 359.2, 359.9 |
| Nervous system anomaly | 742.8, 742.9 |
| Cerebral degeneration | 330.8, 331.9 |
| Chromosomal anomaly | 758.2, 758.3, 758.5, 758.89 |
| Infantile spasms | 345.60, 345.61 |
| Menial retardation | 317.0, 318.1, 318.2 |
| Spinal muscle atrophy | 335.0, 335.10 |
Thirty‐four children underwent a laparoscopic Nissen fundoplication, and 10 had an open Nissen fundoplication. All had gastrostomy tubes placed or replaced at the time of surgery.
Analysis of the mean bodily pain scores from the CHQ‐PF50 revealed that bodily pain of patients in the study cohort had improved from baseline after 1 month of follow‐up (mean score at baseline, 32.8; after 1 month of follow‐up, 47.5; P = .01), after adjusting for functional status. However, these mean bodily pain scores were significantly lower than those of children with cerebral palsy (mean score, 73.9; P < .001).34, 35 After adjusting for functional status, scores were improved for role/social‐physical limitations (mean baseline score, 30.6; 1‐month follow‐up score, 56.6; P = .01), mental health (mean baseline score, 62.7; 1‐month follow‐up score, 70.6; P = .01), family limitation of activities (mean baseline score, 43.3; 1‐month follow‐up score, 55.1; P = .03), and parental time (mean baseline score, 43.0; 1‐month follow‐up score, 55.3; P = .03). Scores were unchanged for physical function, global health, general health perception, physical summary, role/social‐emotional, mental health, self‐esteem, and psychological summary (see Table 3).
| Domain of Quality of Life | Baseline (Mean and SD) | 1‐Month Follow‐Up (Mean and SD) | P Value |
|---|---|---|---|
| Physical functioning | 19.3 (34.1) | 16.7 (30.8) | 0.77 |
| Role physical* | 30.6 (44.4) | 56.6 (40.5) | 0.01 |
| Bodily pain* | 32.8 (24.4) | 47.5 (25.7) | 0.01 |
| Global health | 42.0 (23.7) | 44.1 (22.6) | 0.19 |
| General behavior | 72.1 (29.3) | 78.7 (14.5) | 0.21 |
| Self‐esteem | 39.9 (21.1) | 32.8 (19.4) | 0.36 |
| Mental health | 62.7 (15.9) | 70.6 (16.6) | 0.01 |
| Family limitation of activity* | 43.3 (23.7) | 55.1 (21.3) | 0.03 |
| Parental time* | 43.0 (35.5) | 55.3 (32.5) | 0.03 |
| Physical summary | 23.1 (21.2) | 17.8 (13.9) | 0.17 |
| Psychological summary | 39.0 (11.8) | 39.6 (10.8) | 0.76 |
Analysis of the SF‐36 of the parents of these children revealed mean scores significantly lower than those in general U.S. population for all quality‐of‐life domains except physical function (see Table 4). Many baseline domain scores were similar to those of adults with clinical depression. The only domain that showed improvement in quality of life of the caregivers over the 1‐month follow‐up period was vitality (mean baseline score, 41.3; 1‐month follow‐up score, 48.2; P = .001).
| Quality‐of‐life domain | Study group mean (SD) | U.S. population norm mean (SD) | P value |
|---|---|---|---|
| |||
| Physical functioning | 89.35 (14.60) | 84.15 (23.26) | 0.10 |
| Role physical | 71.02 (39.96) | 80.96 (34.00) | 0.05 |
| Bodily pain | 82.50 (24.00) | 75.15 (23.69) | 0.04 |
| General Health* | 59.07 (18.75) | 71.95 (20.34) | 0.001 |
| Vitality* | 41.33 (19.49) | 60.86 (33.04) | 0.001 |
| Social functioning* | 63.33 (34.48) | 83.28 (22.69) | 0.001 |
| Role emotional | 60.60 (40.20) | 81.26 (33.04) | 0.001 |
| Mental health | 67.00 (19.61) | 74.74 (18.05) | 0.004 |
Total stress as measured by the PSI/SF mean was 79.1 at baseline and 77.6 1 month after fundoplication (P = .54). This was significantly higher stress than the parental norm of 71.0 (P = .01). One in 4 parents expressed clinically significant levels of stress (scores > 90, 90th percentile).
Patients suffered the following complications in the month after fundoplication. Eight children had at least 1 subsequent emergency department visit related to a complication of the gastrostomy tube (8 visits), to respiratory distress (1 visit), or tovomiting (1 visit). Seven children had a subsequent admission to the hospital related to a complication of the gastrostomy tube (4 admissions), complication of surgery (2 admissions), or aspiration pneumonia (1 admission). None of the children had a repeat fundoplication or subsequently underwent placement of a gastrojejunal feeding tube. One patient died. She was 10 months old when she died, which was 3 weeks after she had received a fundoplication. She had obstructive hydrocephalus, cortical blindness, and developmental delay, and respiratory arrest and subsequent tonsillar herniation led to her death.
DISCUSSION
Parents of children with neurological impairment and GERD who underwent their first fundoplication reported improved quality of life of their children in the domains of bodily pain, role/social‐physical limitations, mental health, family limitation of activities, and parental time over the first month after surgery, when controlling for the children's degree of functional impairment. The only significant similar improvement in the parent self‐reported quality of life was in the domain of vitality.
This study had several limitations. Loss to follow‐up may have led to a bias reflecting the phenomenon that patients who have poorer quality of life are less likely to report this, or even to be able to participate in the follow‐up component of a study like this. In survival analyses, this incomplete follow‐up of patients is called informative dropout and may be minimized by applying a statistical technique that accounts for this, using the Q‐TWiST.45 However, our current study design and analysis plan precluded using this methodology. As shown in Table 1, we did not find any differences between those patients who stayed in the study and those who dropped out. Also, we were able to contact most parents who reported being too busy to fill out the surveys. Patient heterogeneity is also a concern: Table 2 shows the wide array of diagnoses responsible for the children's neurological impairment. However, we used a standardized functional status measure to ensure we were analyzing similarly disabled patients. Also, the standard deviation of the mean WeeFIM score was small, implying little variability in the study cohort. Our study analyzed data from a single center, which reflects care in the western United States. However, our hospital is similar to other medium and large children's hospitals and our patient population similar to others that perform fundoplication for children with neurological impairment.46 We believe our findings are generalizable to other surgical centers that perform a similar volume of fundoplications in such children with NI.
Our study findings are similar to those reported by O'Neill et al., whose study found that parents reported improved quality of life of their children in ease of feedings, physical comfort during feeding, and ability of the child to enjoy life.23 The CHQPF50 does not specifically ask about feeding, but we did find similar improvement in the domain of role/social‐physical limitations. O'Neill et al. also found that after the children in their study received a fundoplication, caregivers reported their own quality of life improved in the areas of being able to spend more time caring for their child's needs, which is similar to our findings of fewer family limitations of activities and more parental time. Our findings were somewhat dissimilar to the O'Neill et al. study, as parents in their study found several additional areas of improvement in caregiver own quality of life. One explanation for the differing results may be differences in the populations studied. Parents in our study had SF‐36 scores for general health, vitality, and social functioning that were similar to those of adults with depression,47 whereas parents in the O'Neill study did not. Although the O'Neill et al. study was the first to examine these critical quality‐of‐life outcomes for children with NI who have received fundoplication, it had several methodological limitations. We have had the opportunity to build on the work of O'Neill et al. and in a prospective study to capture standardized baseline data (therefore not subject to recall bias, as was likely in the O'Neill et al. study) and collect long‐term data on this population. We also controlled for functional status, which did not improve over the 1 month and by itself could be responsible for the already poor caregiver quality of life. Some aspects of the children's care did improve, but perhaps not enough to overcome the severe disabilities the children and their caregivers live with on a daily basis. We found some evidence to support that the parents' PSI/SF scores were similar to those of parents of children with heart disease, other enterally fed children, and children with traumatic brain injury (who make up between 1 in 3 and 1 in 5 parents with severe stress).39, 41, 43 Future interventions should address the stress and quality of life of these caregivers, especially if surgery does not improve caregiver quality of life or decrease stress.
Contrary to an emerging body of literature in pediatrics that describes a positive correlation between the health of children with chronic illnesses and their caregivers' quality of life,12, 42 we did not find large immediate improvements in caregiver quality of life and decrease in stress as their children's quality of life improved. This may be related to the number of parents in our sample being too small to detect such changes or that changes in longer‐term (greater than 6 months or 1 year) quality of life not being reflected by short‐term assessment. Caregiver and child quality of life following fundoplication needs to be studied over the long term (eg, over many years). We are continuing to follow these patients and their families and will repeat the quality‐of‐life measures 6 and 12 months after fundoplication and report these findings.
Additional studies of treatments for neurologically impaired children with GERD are needed. Randomized trials of alternatives to fundoplication such as gastrojejunal feeding tubes have been proposed, with which we strongly agree.46, 48 We believe that any randomized, controlled trial of children with neurological impairment and GERD must measure child and caregiver quality of life and functional status outcomes. 0
| Variables | Study patients at baseline (N = 44) | Study patients at 1‐month follow‐up (N = 26) | P value |
|---|---|---|---|
| Functional Status Measure | |||
| WeeFIM Score | 24 | 36 | NS |
| Child CHQ‐PF50 Quality‐of‐Life Scores | |||
| Role physical | 30.6 | 56.6 | 0.01 |
| Bodily pain | 32.8 | 47.5 | 0.01 |
| Mental health | 62.7 | 70.6 | 0.01 |
| Family limitation of activity | 43.3 | 55.1 | 0.03 |
| Parental time | 43.0 | 55.3 | 0.03 |
| Global health | 42.0 | 44.1 | NS |
| Physical functioning | 19.3 | 16.7 | NS |
| General behavior | 72.1 | 78.7 | NS |
| Self‐esteem | 39.9 | 32.8 | NS |
| Role emotional | 27.1 | 37.1 | NS |
| Physical summary | 23.1 | 17.8 | NS |
| Psychological summary | 39.0 | 39.6 | NS |
| Caregiver SF‐36 Quality‐of‐Life Scores | |||
| Vitality | 41.3 | 46.9 | 0.001 |
| Role physical | 89.9 | 92.5 | NS |
| Bodily pain | 71.0 | 78.7 | NS |
| General health | 82.5 | 81.1 | NS |
| Social functioning | 59.1 | 59.5 | NS |
| Role emotional | 60.6 | 65.6 | NS |
| Mental health | 67.0 | 73.5 | NS |
| Parenting stress index | 79.1 | 77.7 | NS |
Acknowledgements
The authors thank Tanner Coleman and Matthew Swenson for their invaluable help in recruiting patients. Dr. Srivastava was supported in part by the Children's Health Research Center, University of Utah and Primary Children's Medical Center Foundation.
- ,,.Comparison of respiratory mortality in the profoundly mentally retarded and in the less retarded.J Ment Defic Res.1979;23(1):1–7.
- ,,.Cause of death in cerebral palsy: a descriptive study.Arch Dis Child.1999;81:390–394.
- .Survival rates of children with severe neurologic disabilities: a review.Semin Pediatr Neurol.2003;10(2):120–129.
- ,.Gastroesophageal reflux among severely retarded children.J Pediatr.1979;94:710–714.
- ,,,,,.Operation for gastro‐oesophageal reflux associated with severe mental retardation.Arch Dis Child.1993;68:347–351.
- ,,,,,.Prevalence and severity of feeding and nutritional problems in children with neurological impairment: Oxford Feeding Study.Dev Med Child Neurol.2000;42:674–680.
- ,,, et al.Aspiration pneumonia in pediatric age group: etiology, predisposing factors and clinical outcome.J Pak Med Assoc.1999;49(4):105–108.
- ,.Respiratory problems in children with neurological impairment.Arch Dis Child.2003;88(1):75–78.
- ,.Gastroesophageal reflux in childhood.Curr Probl Surg.1996;33(1):1–70.
- ,.Minimally invasive surgical techniques in reoperative surgery for gastroesophageal reflux disease in infants and children.Am Surg.2002;68:989–992.
- .Laparoscopic Nissen procedure in children.Semin Laparosc Surg.2002;9(3):146–152.
- ,,, et al.Caregiving process and caregiver burden: conceptual models to guide research and practice.BMC Pediatr.2004;4(1):1.
- ,.Theoretical and psychometric analysis of caregiver strain.Res Nurs Health.1996;19:499–510.
- ,,,,,.Complications and reoperation after Nissen fundoplication in childhood.Am J Surg.1987;153(2):177–183.
- ,,, et al.Surgical treatment of gastroesophageal reflux in children: a combined hospital study of 7467 patients.Pediatrics.1998;101:419–422.
- ,,,,,.Outcomes of surgical fundoplication in children.Clin Gastroenterol Hepatol.2004;2:978–984.
- ,,, et al.The respiratory advantage of laparoscopic Nissen fundoplication.J Pediatr Surg.2003;38:886–891.
- ,,,.Laparoscopic Nissen fundoplication in children: 2‐5‐year follow‐up.Pediatr Surg Int.2003;19:537–539.
- ,,.Recognition of recurrent gastroesophageal reflux following antireflux surgery in the neurologically disabled child: high index of suspicion and definitive evaluation.J Pediatr Surg.1992;27:983–988; discussion988–990.
- ,,.Sequelae of antireflux surgery in profoundly disabled children.J Pediatr Surg.1992;27(2):267–271; discussion271–263.
- ,,.Efficacy of the Nissen fundoplication in the management of gastroesophageal reflux following esophageal atresia repair.J Pediatr Surg.1993;28(1):53–55.
- ,,,.Caregivers' perceptions following gastrostomy in severely disabled children with feeding problems.Dev Med Child Neurol.1997;39:746–751.
- ,,,,.Care‐giver evaluation of anti‐gastroesophageal reflux procedures in neurologically impaired children: what is the real‐life outcome?J Pediatr Surg.1996;31:375–380.
- ,,, et al.Impact of gastrostomy tube feeding on the quality of life of carers of children with cerebral palsy.Dev Med Child Neurol.2004;46:796–800.
- ,.Organizational responses to managed care: issues for academic health centers and implications for pediatric programs.Pediatrics.1998;101:805–811; discussion811–802.
- Children's Digestive Health and Nutrition Foundation Website. Gastroesophageal Reflux Disease in the Neurologically Impaired Child. Available at: http://www.cdhnf.org/PractitionerSeries.asp. Accessed August 30,2006.
- ,,,,.Functional status of school‐aged children with Down syndrome.J Paediatr Child Health.2002;38(2):160–165.
- ,,,,,.Concurrent validity of the Functional Independence Measure for Children (WeeFIM) and the Pediatric Evaluation of Disabilities Inventory in children with developmental disabilities and acquired brain injuries.Phys Occup Ther Pediatr.2001;21(2–3):91–101.
- ,,, et al.The WeeFIM instrument: its utility in detecting change in children with developmental disabilities.Arch Phys Med Rehabil.2000;81:1317–1326.
- ,,, et al.Functional assessment and care of children with neurodevelopmental disabilities.Am J Phys Med Rehabil.2000;79(2):114–123.
- ,,,,,.Predictors of mortality, morbidity, and disability in a cohort of infants < or = 28 weeks' gestation.Clin Pediatr (Phila).1993;32:521–527.
- ,.Content validity of a pediatric functional independence measure.Appl Nurs Res.1990;3(3):120–122.
- ,,, et al.The Functional Independence Measure for Children (WeeFIM). Conceptual basis and pilot use in children with developmental disabilities.Clin Pediatr (Phila). Jul1994;33:421–430.
- ,,.The CHQ User's Manual.1st ed.Boston, MA:The Health Institute, New England Medical Center,1996.
- ,,,,,.Comparing reliability and validity of pediatric instruments for measuring health and well‐being of children with spastic cerebral palsy.Dev Med Child Neurol.2002;44:468–476.
- ,,,,.Needs of carers of severely disabled people: are they identified and met adequately?Health Soc Care Community.2001;9(4):235–243.
- ,,.The realities of postoperative disability and the carer's burden.Ann R Coll Surg Engl.2001;83(3):215–218.
- Abdidin.Parenting Stress Index.3rd ed.Lutz, FL:Psychological Assessment Resources, Inc.;1995.
- ,,,.Parental stress and burden following traumatic brain injury amongst children and adolescents.Brain Inj. Jan2003;17(1):1–23.
- ,,.Comparing stress levels of parents of children with cancer and parents of children with physical disabilities.Psychooncology. Dec2004;13(12):898–903.
- ,,.Stress levels experienced by the parents of enterally fed children.Child Care Health Dev.2004;30:507–513.
- ,,, et al.The health and well‐being of caregivers of children with cerebral palsy.Pediatrics.2005;115:e626–e636.
- ,.Parenting stress and children with heart disease.J Pediatr Health Care.2003;17(4):163–168.
- ,.Small sample inference for fixed effects from restricted maximum likelihood.Biometrics.1997;53:983–997.
- ,,.Methods for the analysis of quality‐of‐life and survival data in health technology assessment.Health Technol Assess.1999;3(10):1–152.
- ,,, et al.Fundoplication and gastrostomy versus image‐guided gastrojejunal tube for enteral feeding in neurologically impaired children with gastroesophageal reflux.J Pediatr Surg.2002;37:407–412.
- SF‐36 Health Survey: Manual and Interpretation Guide.Lincoln, RI:QualityMetric Inc.;1993,year="2000"2000.
- ,,.The role of protective antireflux procedures in neurologically impaired children: a decision analysis.J Pediatr Surg. Mar2002;37:500–506.
Aspiration pneumonia is the most common cause of death in children with severe neurological impairment (NI).13 For several reasons (eg, improved survival of extremely low‐birth‐weight infants, technological advances, and changing societal attitudes), the number of children with severe NI is increasing. Many children with severe NI have dysfunctional swallowing and gastroesophageal reflux disease (GERD).46 This combination places them at high risk for recurrent aspiration that, in turn, leads to aspiration pneumonia, repeated hospitalization, respiratory failure, compromised quality of life, and death.7, 8
The most common treatment approach for the combination of dysfunctional swallowing and GERD is surgical fundoplication with a gastrostomy feeding tube. Fundoplication is the third most common procedure performed in children by pediatric surgeons in the United States.9 Fifty percent of the children who receive a fundoplication have neurological impairment.10, 11 The goals of the surgery to treat GERD unresponsive to medical management are to reduce the risk of aspiration pneumonia, improve nutritional status, and improve the quality of life of the children and their families. However, few prospective longitudinal studies have determined whether the quality of life of the children or their caregivers actually improves over time.
The importance of caregiver and child quality of life is increasingly recognized as a critical outcome of any intervention in this population.12, 13 Previous studies of fundoplication in children with NI, GERD, and dysfunctional swallowing reported surgical mortality rates between 1% and 3% and other complications between 4% and 39%, reflecting the medical fragility of these children.5, 1418 Some of these studies had longitudinal follow‐up and reported long‐term data. Recurrence of symptoms was reported in up to 56% of patients, recurrence of AP in up to 39%, further surgical procedures in up to 19%, and mortality in up to 17%.14, 1921 Few case series of children with neurological impairment who have had a fundoplication have addressed child and caregiver quality of life following either a fundoplication or placement of a feeding tube.2224 In their study of 16 patients who had a fundoplication and gastrostomy tube placed, Tawfik et al. found improvements in children's happiness, ease of giving medicines, and time to devote to other children. Sullivan et al. found improvement in caregiver quality of life following placement of a gastrostomy tube in a child; however, they did not specifically identify those children who had been treated with a fundoplication. In their retrospective study, O'Neill et al. found improved child and caregiver quality of life following a fundoplication. Collectively, these studies have found that parents report improvement of both their own and their child's quality of life after either intervention. However, not having baseline measurements, not controlling for degree of functional impairment of the children, small sample sizes, and large loss to follow‐up limit the utility of these studies. In this ongoing, long‐term prospective longitudinal study, we report the initial impact of a fundoplication on the quality of life of both children and their caregivers.
The primary objective of this study was to determine change over time in the quality of life of children with neurological impairment who received surgical treatment of their GERD and of the caregivers of these children, controlling for the degree of functional impairment of the children. We hypothesized that child and caregiver quality of life would both improve after primary fundoplication and gastrostomy tube placement. Secondary objectives included describing rates of complications in this population.
METHODS
Setting and Study Population
We enrolled patients from newborn to 18 years of age who had a diagnosis compatible with neurological impairment and who received their first fundoplication for GERD between January 2005 and July 2006 at Primary Children's Medical Center (PCMC), in Salt Lake City, Utah. PCMC is a 232‐bed children's hospital in the Intermountain West owned and operated by Intermountain Healthcare, Inc., a large vertically integrated health care delivery system that serves as both the primary hospital for Salt Lake County and as the tertiary‐care hospital for 4 additional states (Wyoming, Nevada, Idaho, and Montana).25 Patients who had a previous gastrojejunal feeding tube were excluded as were patients who had a previous fundoplication, as these procedures may have biased their reported quality of life, our main outcome measure.
Patients were included in the study if they had GERD (based on clinical history or testing) that had been refractory to medical management (defined as continued gastroesophageal reflux symptoms despite antireflux medications). GERD was defined using the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) criteria.26 These include: the presence of clinical symptoms and at least 1 abnormal result from an upper gastrointestinal x‐ray series (recognizing that this test is neither sensitive nor specific for reflux), pH probe, upper gastrointestinal endoscopy with biopsy, nuclear medicine scan, or a modified barium swallow. As this was a prospective observational study, physicians were allowed to order testing as their practice dictated. Patients were excluded if they had neurological impairment but lacked objective documentation of GERD using the NASPGHAN recommendations, unless there were obvious clinical indications such as witnessed vomiting and aspiration (N = 3). No patient received a prophylactic fundoplication (fundoplication without documented GERD).
Study Design
This is an ongoing prospective longitudinal study. Patients who had a first fundoplication at PCMC were identified by the surgical service, with weekly lists shared with the research team. Patients were approached by a research assistant during that initial hospitalization to see if they met inclusion criteria for the study using data from the medical records and surgical team when necessary.
Data Variables and Sources
Indications for the fundoplication, performance and results of diagnostic testing for GERD, complications of the fundoplication, and reasons for the neurological impairment were obtained through review of the electronic and paper medical records. Mortality data, subsequent emergency department visits, and admissions to the hospital were obtained using Intermountain Healthcare's electronic data warehouse, which merges clinical, financial, and administrative data including the Utah Vital Statistics database.
Neurological impairment was defined from 2 sources: (1) clinical diagnoses as identified by providers and (2) International Classification of Diseases Codes Modified, version 9 (ICD‐9 CM) identified a priori as indicating neurological impairment.
Instruments and Study Outcomes
Functional status was measured using the WeeFIM. This instrument has been tested and shown to be valid and reliable for children more than 6 months old with neurodevelopmental disabilities including spina bifida and Down syndrome.2732 WeeFIM is a self‐administered parent instrument composed of 18 items and 6 domains (self‐care, sphincter control, transfers, locomotion, communication, and social cognition).33 The WeeFIM allows patients to be stratified into areas of function from severely impaired to normal.
The primary outcome was child quality of life as measured by the Child Health Questionnaire Parental Form 50 (CHQ‐PF50). Caregiver quality of life was measured using the Short‐Form Health Status Survey (SF‐36) and Parenting Stress Index/Short Form (PSI/SF). The CHQ‐PF50 is a self‐administered parent questionnaire of 50 questions that measures 6 domains, including physical function and abilities, pain and discomfort, general health perception, behavior, temperament and moods, and satisfaction with growth and development.34 This instrument has been tested for validity and reliability in children with cerebral palsy.35 The SF‐36 is a widely accepted measure of health status that measures 8 domains of health: physical functioning, role limitations due to physical problems, bodily pain, general health, vitality, social functioning, role limitations due to emotional problems, and mental health. The SF‐36 has been well studied and has been used to measure the effect on a caregiver's quality of life associated with caring for a chronically ill child with significant medical problems.36, 37 Higher scores in each domain of both the CHQPF50 and the SF‐36 reflect better quality of life. Caregiver stress was measured using the PSI/SF (Psychological Assessment Resources Inc, Odessa, FL).38 In the PSI/SF a parent rates the parentchild dyad on 36 items that are summarized in 3 subscales: parental distress, parentchild dysfunctional interaction, and difficult child. A parent scores each item as strongly agree, agree, not sure, disagree, or strongly disagree. The sum of the 3 subscale scores is the total stress score. Higher scores denote a greater degree of stress. The PSI/SF has been validated in several studies for caregivers of children with chronic diseases.3943
The CHQ‐PF50, SF‐36, and PSI/SF questionnaires and the WeeFIM functional status measure were administered to each study patient and caregiver in person at enrollment (baseline) and by mail 1 month after fundoplication. A follow‐up postcard reminder was mailed 1 week after the initial mailing. Second and final mailings were sent to nonresponders 3 and 5 weeks, respectively, after the initial mailing.
Secondary outcomes included rates of complications including failure of the fundoplication. Complications were defined as a subsequent emergency department visit, hospitalization, or death related to the surgery, gastrostomy tube, or aspiration pneumonia. Failures were defined as a second fundoplication or the insertion of a gastrojejunal feeding tube as nonsurgical management of recurrent GERD and/or paraesophageal hernia. Secondary outcomes were followed from the time of the first fundoplication until 1 month after surgery.
Analyses
The differential effect of the fundoplication on the quality of life measures was assessed and quantified through statistical analysis. Because the primary interest was to measure change in baseline characteristics over time, repeated‐measures models were used to compare the group relative to changes in functional status. In particular, changes from baseline values were modeled 1 month after the procedure. The Kenward‐Roger approximation of degrees of freedom was used to compute P values from the overall tests.44 Repeated‐measures models were fit to the data using restricted maximum likelihood estimation. An autoregressive covariance matrix was assumed for the multiple measurements of each individual, thus limiting the number of restrictions forced by the model on the data. The repeated‐measures models used all the available data on participants, including those who dropped out of the study. To obtain the most accurate comparison of the study group, the covariate of functional status at baseline was taken into account in the fitted models. Statistical analyses were performed with SAS statistical software (version 9.13; SAS Institute, Cary NC). Student t tests were performed for comparison of means of the quality‐of‐life domains for the study cohort compared to either the general population or specific groups of patients for comparative purposes.
The study was approved by the institutional review boards of the University of Utah Health Sciences Center and Primary Children's Medical Center.
RESULTS
Sixty‐three children met eligibility criteria. Forty‐four families (70%) initially agreed to participate in the study and completed the baseline questionnaires (see Fig. 1). The mean age of the children was 2.2 years. Twenty‐six parents of children completed the 1‐month postfundoplication quality‐of‐life questionnaires. Thirteen patients were lost to follow‐up, 5 of whom had not reached the 1‐month postfundoplication time point. The median WeeFIM (functional status) score of the whole group was 31.2 (95% confidence interval [CI] 11‐71) compared with a childhood matched‐age norm of 83 (95% CI 60‐110), P = .001. WeeFIM scores did not change significantly from baseline to 1 month, P = .98 (Kruskall‐Wallis test).

Data for the 13 parents and children (30%) who gave baseline data but were subsequently lost to follow‐up are shown in Table 1. Reasons for loss to follow‐up were caregiver reporting being too busy to fill out the questionnaires (n = 8) and no reason stated (n = 5).
In addition to the diagnosis of GERD, clinical indications for fundoplication were vomiting (55%), feeding‐related issues (47%), and failure to thrive (39%). Diagnosis of GERD was confirmed for 41 of 44 patients77% had an abnormal upper GI, 26% an abnormal pH probe, 14% an abnormal endoscopy, and 24% an abnormal swallow study. The remaining 3 had obvious clinical symptoms for GERD and did not require further testing according to their attending surgeon (2 with witnessed vomiting leading to aspiration and 1 who was exclusively gastrostomy‐fed and was witnessed having feeds coming from the tracheostomy). Various medications had been tried and were considered to have failed in these patients: 39% had been treated with acid‐suppressive agents; 80% with acid blocking agents; and 61% with prokinetic agents. Fourteen patients (32%) had cerebral palsy, and 14 (32%) had a brain or spinal cord abnormality (see Table 2).
| Diagnostic category | ICD‐9 codes used |
|---|---|
| |
| Brain or spinal cord anomaly | 335.22, 742.0, 742.1, 742.2, 742.4, 742.53 |
| Cerebral palsy | 343.0, 343.1, 343.2, 343.8, 343.9, 344.00 |
| Hydrocephalus | 331.3, 331.4, 742.3 |
| Down syndrome | 758.0 |
| Seizures | 345.10, 345.11, 345.3, 345.41, 345.50, 345.81, 345.90, 345.91 |
| Muscular dystrophy or myopathy | 359.0, 359.1, 359.2, 359.9 |
| Nervous system anomaly | 742.8, 742.9 |
| Cerebral degeneration | 330.8, 331.9 |
| Chromosomal anomaly | 758.2, 758.3, 758.5, 758.89 |
| Infantile spasms | 345.60, 345.61 |
| Menial retardation | 317.0, 318.1, 318.2 |
| Spinal muscle atrophy | 335.0, 335.10 |
Thirty‐four children underwent a laparoscopic Nissen fundoplication, and 10 had an open Nissen fundoplication. All had gastrostomy tubes placed or replaced at the time of surgery.
Analysis of the mean bodily pain scores from the CHQ‐PF50 revealed that bodily pain of patients in the study cohort had improved from baseline after 1 month of follow‐up (mean score at baseline, 32.8; after 1 month of follow‐up, 47.5; P = .01), after adjusting for functional status. However, these mean bodily pain scores were significantly lower than those of children with cerebral palsy (mean score, 73.9; P < .001).34, 35 After adjusting for functional status, scores were improved for role/social‐physical limitations (mean baseline score, 30.6; 1‐month follow‐up score, 56.6; P = .01), mental health (mean baseline score, 62.7; 1‐month follow‐up score, 70.6; P = .01), family limitation of activities (mean baseline score, 43.3; 1‐month follow‐up score, 55.1; P = .03), and parental time (mean baseline score, 43.0; 1‐month follow‐up score, 55.3; P = .03). Scores were unchanged for physical function, global health, general health perception, physical summary, role/social‐emotional, mental health, self‐esteem, and psychological summary (see Table 3).
| Domain of Quality of Life | Baseline (Mean and SD) | 1‐Month Follow‐Up (Mean and SD) | P Value |
|---|---|---|---|
| Physical functioning | 19.3 (34.1) | 16.7 (30.8) | 0.77 |
| Role physical* | 30.6 (44.4) | 56.6 (40.5) | 0.01 |
| Bodily pain* | 32.8 (24.4) | 47.5 (25.7) | 0.01 |
| Global health | 42.0 (23.7) | 44.1 (22.6) | 0.19 |
| General behavior | 72.1 (29.3) | 78.7 (14.5) | 0.21 |
| Self‐esteem | 39.9 (21.1) | 32.8 (19.4) | 0.36 |
| Mental health | 62.7 (15.9) | 70.6 (16.6) | 0.01 |
| Family limitation of activity* | 43.3 (23.7) | 55.1 (21.3) | 0.03 |
| Parental time* | 43.0 (35.5) | 55.3 (32.5) | 0.03 |
| Physical summary | 23.1 (21.2) | 17.8 (13.9) | 0.17 |
| Psychological summary | 39.0 (11.8) | 39.6 (10.8) | 0.76 |
Analysis of the SF‐36 of the parents of these children revealed mean scores significantly lower than those in general U.S. population for all quality‐of‐life domains except physical function (see Table 4). Many baseline domain scores were similar to those of adults with clinical depression. The only domain that showed improvement in quality of life of the caregivers over the 1‐month follow‐up period was vitality (mean baseline score, 41.3; 1‐month follow‐up score, 48.2; P = .001).
| Quality‐of‐life domain | Study group mean (SD) | U.S. population norm mean (SD) | P value |
|---|---|---|---|
| |||
| Physical functioning | 89.35 (14.60) | 84.15 (23.26) | 0.10 |
| Role physical | 71.02 (39.96) | 80.96 (34.00) | 0.05 |
| Bodily pain | 82.50 (24.00) | 75.15 (23.69) | 0.04 |
| General Health* | 59.07 (18.75) | 71.95 (20.34) | 0.001 |
| Vitality* | 41.33 (19.49) | 60.86 (33.04) | 0.001 |
| Social functioning* | 63.33 (34.48) | 83.28 (22.69) | 0.001 |
| Role emotional | 60.60 (40.20) | 81.26 (33.04) | 0.001 |
| Mental health | 67.00 (19.61) | 74.74 (18.05) | 0.004 |
Total stress as measured by the PSI/SF mean was 79.1 at baseline and 77.6 1 month after fundoplication (P = .54). This was significantly higher stress than the parental norm of 71.0 (P = .01). One in 4 parents expressed clinically significant levels of stress (scores > 90, 90th percentile).
Patients suffered the following complications in the month after fundoplication. Eight children had at least 1 subsequent emergency department visit related to a complication of the gastrostomy tube (8 visits), to respiratory distress (1 visit), or tovomiting (1 visit). Seven children had a subsequent admission to the hospital related to a complication of the gastrostomy tube (4 admissions), complication of surgery (2 admissions), or aspiration pneumonia (1 admission). None of the children had a repeat fundoplication or subsequently underwent placement of a gastrojejunal feeding tube. One patient died. She was 10 months old when she died, which was 3 weeks after she had received a fundoplication. She had obstructive hydrocephalus, cortical blindness, and developmental delay, and respiratory arrest and subsequent tonsillar herniation led to her death.
DISCUSSION
Parents of children with neurological impairment and GERD who underwent their first fundoplication reported improved quality of life of their children in the domains of bodily pain, role/social‐physical limitations, mental health, family limitation of activities, and parental time over the first month after surgery, when controlling for the children's degree of functional impairment. The only significant similar improvement in the parent self‐reported quality of life was in the domain of vitality.
This study had several limitations. Loss to follow‐up may have led to a bias reflecting the phenomenon that patients who have poorer quality of life are less likely to report this, or even to be able to participate in the follow‐up component of a study like this. In survival analyses, this incomplete follow‐up of patients is called informative dropout and may be minimized by applying a statistical technique that accounts for this, using the Q‐TWiST.45 However, our current study design and analysis plan precluded using this methodology. As shown in Table 1, we did not find any differences between those patients who stayed in the study and those who dropped out. Also, we were able to contact most parents who reported being too busy to fill out the surveys. Patient heterogeneity is also a concern: Table 2 shows the wide array of diagnoses responsible for the children's neurological impairment. However, we used a standardized functional status measure to ensure we were analyzing similarly disabled patients. Also, the standard deviation of the mean WeeFIM score was small, implying little variability in the study cohort. Our study analyzed data from a single center, which reflects care in the western United States. However, our hospital is similar to other medium and large children's hospitals and our patient population similar to others that perform fundoplication for children with neurological impairment.46 We believe our findings are generalizable to other surgical centers that perform a similar volume of fundoplications in such children with NI.
Our study findings are similar to those reported by O'Neill et al., whose study found that parents reported improved quality of life of their children in ease of feedings, physical comfort during feeding, and ability of the child to enjoy life.23 The CHQPF50 does not specifically ask about feeding, but we did find similar improvement in the domain of role/social‐physical limitations. O'Neill et al. also found that after the children in their study received a fundoplication, caregivers reported their own quality of life improved in the areas of being able to spend more time caring for their child's needs, which is similar to our findings of fewer family limitations of activities and more parental time. Our findings were somewhat dissimilar to the O'Neill et al. study, as parents in their study found several additional areas of improvement in caregiver own quality of life. One explanation for the differing results may be differences in the populations studied. Parents in our study had SF‐36 scores for general health, vitality, and social functioning that were similar to those of adults with depression,47 whereas parents in the O'Neill study did not. Although the O'Neill et al. study was the first to examine these critical quality‐of‐life outcomes for children with NI who have received fundoplication, it had several methodological limitations. We have had the opportunity to build on the work of O'Neill et al. and in a prospective study to capture standardized baseline data (therefore not subject to recall bias, as was likely in the O'Neill et al. study) and collect long‐term data on this population. We also controlled for functional status, which did not improve over the 1 month and by itself could be responsible for the already poor caregiver quality of life. Some aspects of the children's care did improve, but perhaps not enough to overcome the severe disabilities the children and their caregivers live with on a daily basis. We found some evidence to support that the parents' PSI/SF scores were similar to those of parents of children with heart disease, other enterally fed children, and children with traumatic brain injury (who make up between 1 in 3 and 1 in 5 parents with severe stress).39, 41, 43 Future interventions should address the stress and quality of life of these caregivers, especially if surgery does not improve caregiver quality of life or decrease stress.
Contrary to an emerging body of literature in pediatrics that describes a positive correlation between the health of children with chronic illnesses and their caregivers' quality of life,12, 42 we did not find large immediate improvements in caregiver quality of life and decrease in stress as their children's quality of life improved. This may be related to the number of parents in our sample being too small to detect such changes or that changes in longer‐term (greater than 6 months or 1 year) quality of life not being reflected by short‐term assessment. Caregiver and child quality of life following fundoplication needs to be studied over the long term (eg, over many years). We are continuing to follow these patients and their families and will repeat the quality‐of‐life measures 6 and 12 months after fundoplication and report these findings.
Additional studies of treatments for neurologically impaired children with GERD are needed. Randomized trials of alternatives to fundoplication such as gastrojejunal feeding tubes have been proposed, with which we strongly agree.46, 48 We believe that any randomized, controlled trial of children with neurological impairment and GERD must measure child and caregiver quality of life and functional status outcomes. 0
| Variables | Study patients at baseline (N = 44) | Study patients at 1‐month follow‐up (N = 26) | P value |
|---|---|---|---|
| Functional Status Measure | |||
| WeeFIM Score | 24 | 36 | NS |
| Child CHQ‐PF50 Quality‐of‐Life Scores | |||
| Role physical | 30.6 | 56.6 | 0.01 |
| Bodily pain | 32.8 | 47.5 | 0.01 |
| Mental health | 62.7 | 70.6 | 0.01 |
| Family limitation of activity | 43.3 | 55.1 | 0.03 |
| Parental time | 43.0 | 55.3 | 0.03 |
| Global health | 42.0 | 44.1 | NS |
| Physical functioning | 19.3 | 16.7 | NS |
| General behavior | 72.1 | 78.7 | NS |
| Self‐esteem | 39.9 | 32.8 | NS |
| Role emotional | 27.1 | 37.1 | NS |
| Physical summary | 23.1 | 17.8 | NS |
| Psychological summary | 39.0 | 39.6 | NS |
| Caregiver SF‐36 Quality‐of‐Life Scores | |||
| Vitality | 41.3 | 46.9 | 0.001 |
| Role physical | 89.9 | 92.5 | NS |
| Bodily pain | 71.0 | 78.7 | NS |
| General health | 82.5 | 81.1 | NS |
| Social functioning | 59.1 | 59.5 | NS |
| Role emotional | 60.6 | 65.6 | NS |
| Mental health | 67.0 | 73.5 | NS |
| Parenting stress index | 79.1 | 77.7 | NS |
Acknowledgements
The authors thank Tanner Coleman and Matthew Swenson for their invaluable help in recruiting patients. Dr. Srivastava was supported in part by the Children's Health Research Center, University of Utah and Primary Children's Medical Center Foundation.
Aspiration pneumonia is the most common cause of death in children with severe neurological impairment (NI).13 For several reasons (eg, improved survival of extremely low‐birth‐weight infants, technological advances, and changing societal attitudes), the number of children with severe NI is increasing. Many children with severe NI have dysfunctional swallowing and gastroesophageal reflux disease (GERD).46 This combination places them at high risk for recurrent aspiration that, in turn, leads to aspiration pneumonia, repeated hospitalization, respiratory failure, compromised quality of life, and death.7, 8
The most common treatment approach for the combination of dysfunctional swallowing and GERD is surgical fundoplication with a gastrostomy feeding tube. Fundoplication is the third most common procedure performed in children by pediatric surgeons in the United States.9 Fifty percent of the children who receive a fundoplication have neurological impairment.10, 11 The goals of the surgery to treat GERD unresponsive to medical management are to reduce the risk of aspiration pneumonia, improve nutritional status, and improve the quality of life of the children and their families. However, few prospective longitudinal studies have determined whether the quality of life of the children or their caregivers actually improves over time.
The importance of caregiver and child quality of life is increasingly recognized as a critical outcome of any intervention in this population.12, 13 Previous studies of fundoplication in children with NI, GERD, and dysfunctional swallowing reported surgical mortality rates between 1% and 3% and other complications between 4% and 39%, reflecting the medical fragility of these children.5, 1418 Some of these studies had longitudinal follow‐up and reported long‐term data. Recurrence of symptoms was reported in up to 56% of patients, recurrence of AP in up to 39%, further surgical procedures in up to 19%, and mortality in up to 17%.14, 1921 Few case series of children with neurological impairment who have had a fundoplication have addressed child and caregiver quality of life following either a fundoplication or placement of a feeding tube.2224 In their study of 16 patients who had a fundoplication and gastrostomy tube placed, Tawfik et al. found improvements in children's happiness, ease of giving medicines, and time to devote to other children. Sullivan et al. found improvement in caregiver quality of life following placement of a gastrostomy tube in a child; however, they did not specifically identify those children who had been treated with a fundoplication. In their retrospective study, O'Neill et al. found improved child and caregiver quality of life following a fundoplication. Collectively, these studies have found that parents report improvement of both their own and their child's quality of life after either intervention. However, not having baseline measurements, not controlling for degree of functional impairment of the children, small sample sizes, and large loss to follow‐up limit the utility of these studies. In this ongoing, long‐term prospective longitudinal study, we report the initial impact of a fundoplication on the quality of life of both children and their caregivers.
The primary objective of this study was to determine change over time in the quality of life of children with neurological impairment who received surgical treatment of their GERD and of the caregivers of these children, controlling for the degree of functional impairment of the children. We hypothesized that child and caregiver quality of life would both improve after primary fundoplication and gastrostomy tube placement. Secondary objectives included describing rates of complications in this population.
METHODS
Setting and Study Population
We enrolled patients from newborn to 18 years of age who had a diagnosis compatible with neurological impairment and who received their first fundoplication for GERD between January 2005 and July 2006 at Primary Children's Medical Center (PCMC), in Salt Lake City, Utah. PCMC is a 232‐bed children's hospital in the Intermountain West owned and operated by Intermountain Healthcare, Inc., a large vertically integrated health care delivery system that serves as both the primary hospital for Salt Lake County and as the tertiary‐care hospital for 4 additional states (Wyoming, Nevada, Idaho, and Montana).25 Patients who had a previous gastrojejunal feeding tube were excluded as were patients who had a previous fundoplication, as these procedures may have biased their reported quality of life, our main outcome measure.
Patients were included in the study if they had GERD (based on clinical history or testing) that had been refractory to medical management (defined as continued gastroesophageal reflux symptoms despite antireflux medications). GERD was defined using the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) criteria.26 These include: the presence of clinical symptoms and at least 1 abnormal result from an upper gastrointestinal x‐ray series (recognizing that this test is neither sensitive nor specific for reflux), pH probe, upper gastrointestinal endoscopy with biopsy, nuclear medicine scan, or a modified barium swallow. As this was a prospective observational study, physicians were allowed to order testing as their practice dictated. Patients were excluded if they had neurological impairment but lacked objective documentation of GERD using the NASPGHAN recommendations, unless there were obvious clinical indications such as witnessed vomiting and aspiration (N = 3). No patient received a prophylactic fundoplication (fundoplication without documented GERD).
Study Design
This is an ongoing prospective longitudinal study. Patients who had a first fundoplication at PCMC were identified by the surgical service, with weekly lists shared with the research team. Patients were approached by a research assistant during that initial hospitalization to see if they met inclusion criteria for the study using data from the medical records and surgical team when necessary.
Data Variables and Sources
Indications for the fundoplication, performance and results of diagnostic testing for GERD, complications of the fundoplication, and reasons for the neurological impairment were obtained through review of the electronic and paper medical records. Mortality data, subsequent emergency department visits, and admissions to the hospital were obtained using Intermountain Healthcare's electronic data warehouse, which merges clinical, financial, and administrative data including the Utah Vital Statistics database.
Neurological impairment was defined from 2 sources: (1) clinical diagnoses as identified by providers and (2) International Classification of Diseases Codes Modified, version 9 (ICD‐9 CM) identified a priori as indicating neurological impairment.
Instruments and Study Outcomes
Functional status was measured using the WeeFIM. This instrument has been tested and shown to be valid and reliable for children more than 6 months old with neurodevelopmental disabilities including spina bifida and Down syndrome.2732 WeeFIM is a self‐administered parent instrument composed of 18 items and 6 domains (self‐care, sphincter control, transfers, locomotion, communication, and social cognition).33 The WeeFIM allows patients to be stratified into areas of function from severely impaired to normal.
The primary outcome was child quality of life as measured by the Child Health Questionnaire Parental Form 50 (CHQ‐PF50). Caregiver quality of life was measured using the Short‐Form Health Status Survey (SF‐36) and Parenting Stress Index/Short Form (PSI/SF). The CHQ‐PF50 is a self‐administered parent questionnaire of 50 questions that measures 6 domains, including physical function and abilities, pain and discomfort, general health perception, behavior, temperament and moods, and satisfaction with growth and development.34 This instrument has been tested for validity and reliability in children with cerebral palsy.35 The SF‐36 is a widely accepted measure of health status that measures 8 domains of health: physical functioning, role limitations due to physical problems, bodily pain, general health, vitality, social functioning, role limitations due to emotional problems, and mental health. The SF‐36 has been well studied and has been used to measure the effect on a caregiver's quality of life associated with caring for a chronically ill child with significant medical problems.36, 37 Higher scores in each domain of both the CHQPF50 and the SF‐36 reflect better quality of life. Caregiver stress was measured using the PSI/SF (Psychological Assessment Resources Inc, Odessa, FL).38 In the PSI/SF a parent rates the parentchild dyad on 36 items that are summarized in 3 subscales: parental distress, parentchild dysfunctional interaction, and difficult child. A parent scores each item as strongly agree, agree, not sure, disagree, or strongly disagree. The sum of the 3 subscale scores is the total stress score. Higher scores denote a greater degree of stress. The PSI/SF has been validated in several studies for caregivers of children with chronic diseases.3943
The CHQ‐PF50, SF‐36, and PSI/SF questionnaires and the WeeFIM functional status measure were administered to each study patient and caregiver in person at enrollment (baseline) and by mail 1 month after fundoplication. A follow‐up postcard reminder was mailed 1 week after the initial mailing. Second and final mailings were sent to nonresponders 3 and 5 weeks, respectively, after the initial mailing.
Secondary outcomes included rates of complications including failure of the fundoplication. Complications were defined as a subsequent emergency department visit, hospitalization, or death related to the surgery, gastrostomy tube, or aspiration pneumonia. Failures were defined as a second fundoplication or the insertion of a gastrojejunal feeding tube as nonsurgical management of recurrent GERD and/or paraesophageal hernia. Secondary outcomes were followed from the time of the first fundoplication until 1 month after surgery.
Analyses
The differential effect of the fundoplication on the quality of life measures was assessed and quantified through statistical analysis. Because the primary interest was to measure change in baseline characteristics over time, repeated‐measures models were used to compare the group relative to changes in functional status. In particular, changes from baseline values were modeled 1 month after the procedure. The Kenward‐Roger approximation of degrees of freedom was used to compute P values from the overall tests.44 Repeated‐measures models were fit to the data using restricted maximum likelihood estimation. An autoregressive covariance matrix was assumed for the multiple measurements of each individual, thus limiting the number of restrictions forced by the model on the data. The repeated‐measures models used all the available data on participants, including those who dropped out of the study. To obtain the most accurate comparison of the study group, the covariate of functional status at baseline was taken into account in the fitted models. Statistical analyses were performed with SAS statistical software (version 9.13; SAS Institute, Cary NC). Student t tests were performed for comparison of means of the quality‐of‐life domains for the study cohort compared to either the general population or specific groups of patients for comparative purposes.
The study was approved by the institutional review boards of the University of Utah Health Sciences Center and Primary Children's Medical Center.
RESULTS
Sixty‐three children met eligibility criteria. Forty‐four families (70%) initially agreed to participate in the study and completed the baseline questionnaires (see Fig. 1). The mean age of the children was 2.2 years. Twenty‐six parents of children completed the 1‐month postfundoplication quality‐of‐life questionnaires. Thirteen patients were lost to follow‐up, 5 of whom had not reached the 1‐month postfundoplication time point. The median WeeFIM (functional status) score of the whole group was 31.2 (95% confidence interval [CI] 11‐71) compared with a childhood matched‐age norm of 83 (95% CI 60‐110), P = .001. WeeFIM scores did not change significantly from baseline to 1 month, P = .98 (Kruskall‐Wallis test).

Data for the 13 parents and children (30%) who gave baseline data but were subsequently lost to follow‐up are shown in Table 1. Reasons for loss to follow‐up were caregiver reporting being too busy to fill out the questionnaires (n = 8) and no reason stated (n = 5).
In addition to the diagnosis of GERD, clinical indications for fundoplication were vomiting (55%), feeding‐related issues (47%), and failure to thrive (39%). Diagnosis of GERD was confirmed for 41 of 44 patients77% had an abnormal upper GI, 26% an abnormal pH probe, 14% an abnormal endoscopy, and 24% an abnormal swallow study. The remaining 3 had obvious clinical symptoms for GERD and did not require further testing according to their attending surgeon (2 with witnessed vomiting leading to aspiration and 1 who was exclusively gastrostomy‐fed and was witnessed having feeds coming from the tracheostomy). Various medications had been tried and were considered to have failed in these patients: 39% had been treated with acid‐suppressive agents; 80% with acid blocking agents; and 61% with prokinetic agents. Fourteen patients (32%) had cerebral palsy, and 14 (32%) had a brain or spinal cord abnormality (see Table 2).
| Diagnostic category | ICD‐9 codes used |
|---|---|
| |
| Brain or spinal cord anomaly | 335.22, 742.0, 742.1, 742.2, 742.4, 742.53 |
| Cerebral palsy | 343.0, 343.1, 343.2, 343.8, 343.9, 344.00 |
| Hydrocephalus | 331.3, 331.4, 742.3 |
| Down syndrome | 758.0 |
| Seizures | 345.10, 345.11, 345.3, 345.41, 345.50, 345.81, 345.90, 345.91 |
| Muscular dystrophy or myopathy | 359.0, 359.1, 359.2, 359.9 |
| Nervous system anomaly | 742.8, 742.9 |
| Cerebral degeneration | 330.8, 331.9 |
| Chromosomal anomaly | 758.2, 758.3, 758.5, 758.89 |
| Infantile spasms | 345.60, 345.61 |
| Menial retardation | 317.0, 318.1, 318.2 |
| Spinal muscle atrophy | 335.0, 335.10 |
Thirty‐four children underwent a laparoscopic Nissen fundoplication, and 10 had an open Nissen fundoplication. All had gastrostomy tubes placed or replaced at the time of surgery.
Analysis of the mean bodily pain scores from the CHQ‐PF50 revealed that bodily pain of patients in the study cohort had improved from baseline after 1 month of follow‐up (mean score at baseline, 32.8; after 1 month of follow‐up, 47.5; P = .01), after adjusting for functional status. However, these mean bodily pain scores were significantly lower than those of children with cerebral palsy (mean score, 73.9; P < .001).34, 35 After adjusting for functional status, scores were improved for role/social‐physical limitations (mean baseline score, 30.6; 1‐month follow‐up score, 56.6; P = .01), mental health (mean baseline score, 62.7; 1‐month follow‐up score, 70.6; P = .01), family limitation of activities (mean baseline score, 43.3; 1‐month follow‐up score, 55.1; P = .03), and parental time (mean baseline score, 43.0; 1‐month follow‐up score, 55.3; P = .03). Scores were unchanged for physical function, global health, general health perception, physical summary, role/social‐emotional, mental health, self‐esteem, and psychological summary (see Table 3).
| Domain of Quality of Life | Baseline (Mean and SD) | 1‐Month Follow‐Up (Mean and SD) | P Value |
|---|---|---|---|
| Physical functioning | 19.3 (34.1) | 16.7 (30.8) | 0.77 |
| Role physical* | 30.6 (44.4) | 56.6 (40.5) | 0.01 |
| Bodily pain* | 32.8 (24.4) | 47.5 (25.7) | 0.01 |
| Global health | 42.0 (23.7) | 44.1 (22.6) | 0.19 |
| General behavior | 72.1 (29.3) | 78.7 (14.5) | 0.21 |
| Self‐esteem | 39.9 (21.1) | 32.8 (19.4) | 0.36 |
| Mental health | 62.7 (15.9) | 70.6 (16.6) | 0.01 |
| Family limitation of activity* | 43.3 (23.7) | 55.1 (21.3) | 0.03 |
| Parental time* | 43.0 (35.5) | 55.3 (32.5) | 0.03 |
| Physical summary | 23.1 (21.2) | 17.8 (13.9) | 0.17 |
| Psychological summary | 39.0 (11.8) | 39.6 (10.8) | 0.76 |
Analysis of the SF‐36 of the parents of these children revealed mean scores significantly lower than those in general U.S. population for all quality‐of‐life domains except physical function (see Table 4). Many baseline domain scores were similar to those of adults with clinical depression. The only domain that showed improvement in quality of life of the caregivers over the 1‐month follow‐up period was vitality (mean baseline score, 41.3; 1‐month follow‐up score, 48.2; P = .001).
| Quality‐of‐life domain | Study group mean (SD) | U.S. population norm mean (SD) | P value |
|---|---|---|---|
| |||
| Physical functioning | 89.35 (14.60) | 84.15 (23.26) | 0.10 |
| Role physical | 71.02 (39.96) | 80.96 (34.00) | 0.05 |
| Bodily pain | 82.50 (24.00) | 75.15 (23.69) | 0.04 |
| General Health* | 59.07 (18.75) | 71.95 (20.34) | 0.001 |
| Vitality* | 41.33 (19.49) | 60.86 (33.04) | 0.001 |
| Social functioning* | 63.33 (34.48) | 83.28 (22.69) | 0.001 |
| Role emotional | 60.60 (40.20) | 81.26 (33.04) | 0.001 |
| Mental health | 67.00 (19.61) | 74.74 (18.05) | 0.004 |
Total stress as measured by the PSI/SF mean was 79.1 at baseline and 77.6 1 month after fundoplication (P = .54). This was significantly higher stress than the parental norm of 71.0 (P = .01). One in 4 parents expressed clinically significant levels of stress (scores > 90, 90th percentile).
Patients suffered the following complications in the month after fundoplication. Eight children had at least 1 subsequent emergency department visit related to a complication of the gastrostomy tube (8 visits), to respiratory distress (1 visit), or tovomiting (1 visit). Seven children had a subsequent admission to the hospital related to a complication of the gastrostomy tube (4 admissions), complication of surgery (2 admissions), or aspiration pneumonia (1 admission). None of the children had a repeat fundoplication or subsequently underwent placement of a gastrojejunal feeding tube. One patient died. She was 10 months old when she died, which was 3 weeks after she had received a fundoplication. She had obstructive hydrocephalus, cortical blindness, and developmental delay, and respiratory arrest and subsequent tonsillar herniation led to her death.
DISCUSSION
Parents of children with neurological impairment and GERD who underwent their first fundoplication reported improved quality of life of their children in the domains of bodily pain, role/social‐physical limitations, mental health, family limitation of activities, and parental time over the first month after surgery, when controlling for the children's degree of functional impairment. The only significant similar improvement in the parent self‐reported quality of life was in the domain of vitality.
This study had several limitations. Loss to follow‐up may have led to a bias reflecting the phenomenon that patients who have poorer quality of life are less likely to report this, or even to be able to participate in the follow‐up component of a study like this. In survival analyses, this incomplete follow‐up of patients is called informative dropout and may be minimized by applying a statistical technique that accounts for this, using the Q‐TWiST.45 However, our current study design and analysis plan precluded using this methodology. As shown in Table 1, we did not find any differences between those patients who stayed in the study and those who dropped out. Also, we were able to contact most parents who reported being too busy to fill out the surveys. Patient heterogeneity is also a concern: Table 2 shows the wide array of diagnoses responsible for the children's neurological impairment. However, we used a standardized functional status measure to ensure we were analyzing similarly disabled patients. Also, the standard deviation of the mean WeeFIM score was small, implying little variability in the study cohort. Our study analyzed data from a single center, which reflects care in the western United States. However, our hospital is similar to other medium and large children's hospitals and our patient population similar to others that perform fundoplication for children with neurological impairment.46 We believe our findings are generalizable to other surgical centers that perform a similar volume of fundoplications in such children with NI.
Our study findings are similar to those reported by O'Neill et al., whose study found that parents reported improved quality of life of their children in ease of feedings, physical comfort during feeding, and ability of the child to enjoy life.23 The CHQPF50 does not specifically ask about feeding, but we did find similar improvement in the domain of role/social‐physical limitations. O'Neill et al. also found that after the children in their study received a fundoplication, caregivers reported their own quality of life improved in the areas of being able to spend more time caring for their child's needs, which is similar to our findings of fewer family limitations of activities and more parental time. Our findings were somewhat dissimilar to the O'Neill et al. study, as parents in their study found several additional areas of improvement in caregiver own quality of life. One explanation for the differing results may be differences in the populations studied. Parents in our study had SF‐36 scores for general health, vitality, and social functioning that were similar to those of adults with depression,47 whereas parents in the O'Neill study did not. Although the O'Neill et al. study was the first to examine these critical quality‐of‐life outcomes for children with NI who have received fundoplication, it had several methodological limitations. We have had the opportunity to build on the work of O'Neill et al. and in a prospective study to capture standardized baseline data (therefore not subject to recall bias, as was likely in the O'Neill et al. study) and collect long‐term data on this population. We also controlled for functional status, which did not improve over the 1 month and by itself could be responsible for the already poor caregiver quality of life. Some aspects of the children's care did improve, but perhaps not enough to overcome the severe disabilities the children and their caregivers live with on a daily basis. We found some evidence to support that the parents' PSI/SF scores were similar to those of parents of children with heart disease, other enterally fed children, and children with traumatic brain injury (who make up between 1 in 3 and 1 in 5 parents with severe stress).39, 41, 43 Future interventions should address the stress and quality of life of these caregivers, especially if surgery does not improve caregiver quality of life or decrease stress.
Contrary to an emerging body of literature in pediatrics that describes a positive correlation between the health of children with chronic illnesses and their caregivers' quality of life,12, 42 we did not find large immediate improvements in caregiver quality of life and decrease in stress as their children's quality of life improved. This may be related to the number of parents in our sample being too small to detect such changes or that changes in longer‐term (greater than 6 months or 1 year) quality of life not being reflected by short‐term assessment. Caregiver and child quality of life following fundoplication needs to be studied over the long term (eg, over many years). We are continuing to follow these patients and their families and will repeat the quality‐of‐life measures 6 and 12 months after fundoplication and report these findings.
Additional studies of treatments for neurologically impaired children with GERD are needed. Randomized trials of alternatives to fundoplication such as gastrojejunal feeding tubes have been proposed, with which we strongly agree.46, 48 We believe that any randomized, controlled trial of children with neurological impairment and GERD must measure child and caregiver quality of life and functional status outcomes. 0
| Variables | Study patients at baseline (N = 44) | Study patients at 1‐month follow‐up (N = 26) | P value |
|---|---|---|---|
| Functional Status Measure | |||
| WeeFIM Score | 24 | 36 | NS |
| Child CHQ‐PF50 Quality‐of‐Life Scores | |||
| Role physical | 30.6 | 56.6 | 0.01 |
| Bodily pain | 32.8 | 47.5 | 0.01 |
| Mental health | 62.7 | 70.6 | 0.01 |
| Family limitation of activity | 43.3 | 55.1 | 0.03 |
| Parental time | 43.0 | 55.3 | 0.03 |
| Global health | 42.0 | 44.1 | NS |
| Physical functioning | 19.3 | 16.7 | NS |
| General behavior | 72.1 | 78.7 | NS |
| Self‐esteem | 39.9 | 32.8 | NS |
| Role emotional | 27.1 | 37.1 | NS |
| Physical summary | 23.1 | 17.8 | NS |
| Psychological summary | 39.0 | 39.6 | NS |
| Caregiver SF‐36 Quality‐of‐Life Scores | |||
| Vitality | 41.3 | 46.9 | 0.001 |
| Role physical | 89.9 | 92.5 | NS |
| Bodily pain | 71.0 | 78.7 | NS |
| General health | 82.5 | 81.1 | NS |
| Social functioning | 59.1 | 59.5 | NS |
| Role emotional | 60.6 | 65.6 | NS |
| Mental health | 67.0 | 73.5 | NS |
| Parenting stress index | 79.1 | 77.7 | NS |
Acknowledgements
The authors thank Tanner Coleman and Matthew Swenson for their invaluable help in recruiting patients. Dr. Srivastava was supported in part by the Children's Health Research Center, University of Utah and Primary Children's Medical Center Foundation.
- ,,.Comparison of respiratory mortality in the profoundly mentally retarded and in the less retarded.J Ment Defic Res.1979;23(1):1–7.
- ,,.Cause of death in cerebral palsy: a descriptive study.Arch Dis Child.1999;81:390–394.
- .Survival rates of children with severe neurologic disabilities: a review.Semin Pediatr Neurol.2003;10(2):120–129.
- ,.Gastroesophageal reflux among severely retarded children.J Pediatr.1979;94:710–714.
- ,,,,,.Operation for gastro‐oesophageal reflux associated with severe mental retardation.Arch Dis Child.1993;68:347–351.
- ,,,,,.Prevalence and severity of feeding and nutritional problems in children with neurological impairment: Oxford Feeding Study.Dev Med Child Neurol.2000;42:674–680.
- ,,, et al.Aspiration pneumonia in pediatric age group: etiology, predisposing factors and clinical outcome.J Pak Med Assoc.1999;49(4):105–108.
- ,.Respiratory problems in children with neurological impairment.Arch Dis Child.2003;88(1):75–78.
- ,.Gastroesophageal reflux in childhood.Curr Probl Surg.1996;33(1):1–70.
- ,.Minimally invasive surgical techniques in reoperative surgery for gastroesophageal reflux disease in infants and children.Am Surg.2002;68:989–992.
- .Laparoscopic Nissen procedure in children.Semin Laparosc Surg.2002;9(3):146–152.
- ,,, et al.Caregiving process and caregiver burden: conceptual models to guide research and practice.BMC Pediatr.2004;4(1):1.
- ,.Theoretical and psychometric analysis of caregiver strain.Res Nurs Health.1996;19:499–510.
- ,,,,,.Complications and reoperation after Nissen fundoplication in childhood.Am J Surg.1987;153(2):177–183.
- ,,, et al.Surgical treatment of gastroesophageal reflux in children: a combined hospital study of 7467 patients.Pediatrics.1998;101:419–422.
- ,,,,,.Outcomes of surgical fundoplication in children.Clin Gastroenterol Hepatol.2004;2:978–984.
- ,,, et al.The respiratory advantage of laparoscopic Nissen fundoplication.J Pediatr Surg.2003;38:886–891.
- ,,,.Laparoscopic Nissen fundoplication in children: 2‐5‐year follow‐up.Pediatr Surg Int.2003;19:537–539.
- ,,.Recognition of recurrent gastroesophageal reflux following antireflux surgery in the neurologically disabled child: high index of suspicion and definitive evaluation.J Pediatr Surg.1992;27:983–988; discussion988–990.
- ,,.Sequelae of antireflux surgery in profoundly disabled children.J Pediatr Surg.1992;27(2):267–271; discussion271–263.
- ,,.Efficacy of the Nissen fundoplication in the management of gastroesophageal reflux following esophageal atresia repair.J Pediatr Surg.1993;28(1):53–55.
- ,,,.Caregivers' perceptions following gastrostomy in severely disabled children with feeding problems.Dev Med Child Neurol.1997;39:746–751.
- ,,,,.Care‐giver evaluation of anti‐gastroesophageal reflux procedures in neurologically impaired children: what is the real‐life outcome?J Pediatr Surg.1996;31:375–380.
- ,,, et al.Impact of gastrostomy tube feeding on the quality of life of carers of children with cerebral palsy.Dev Med Child Neurol.2004;46:796–800.
- ,.Organizational responses to managed care: issues for academic health centers and implications for pediatric programs.Pediatrics.1998;101:805–811; discussion811–802.
- Children's Digestive Health and Nutrition Foundation Website. Gastroesophageal Reflux Disease in the Neurologically Impaired Child. Available at: http://www.cdhnf.org/PractitionerSeries.asp. Accessed August 30,2006.
- ,,,,.Functional status of school‐aged children with Down syndrome.J Paediatr Child Health.2002;38(2):160–165.
- ,,,,,.Concurrent validity of the Functional Independence Measure for Children (WeeFIM) and the Pediatric Evaluation of Disabilities Inventory in children with developmental disabilities and acquired brain injuries.Phys Occup Ther Pediatr.2001;21(2–3):91–101.
- ,,, et al.The WeeFIM instrument: its utility in detecting change in children with developmental disabilities.Arch Phys Med Rehabil.2000;81:1317–1326.
- ,,, et al.Functional assessment and care of children with neurodevelopmental disabilities.Am J Phys Med Rehabil.2000;79(2):114–123.
- ,,,,,.Predictors of mortality, morbidity, and disability in a cohort of infants < or = 28 weeks' gestation.Clin Pediatr (Phila).1993;32:521–527.
- ,.Content validity of a pediatric functional independence measure.Appl Nurs Res.1990;3(3):120–122.
- ,,, et al.The Functional Independence Measure for Children (WeeFIM). Conceptual basis and pilot use in children with developmental disabilities.Clin Pediatr (Phila). Jul1994;33:421–430.
- ,,.The CHQ User's Manual.1st ed.Boston, MA:The Health Institute, New England Medical Center,1996.
- ,,,,,.Comparing reliability and validity of pediatric instruments for measuring health and well‐being of children with spastic cerebral palsy.Dev Med Child Neurol.2002;44:468–476.
- ,,,,.Needs of carers of severely disabled people: are they identified and met adequately?Health Soc Care Community.2001;9(4):235–243.
- ,,.The realities of postoperative disability and the carer's burden.Ann R Coll Surg Engl.2001;83(3):215–218.
- Abdidin.Parenting Stress Index.3rd ed.Lutz, FL:Psychological Assessment Resources, Inc.;1995.
- ,,,.Parental stress and burden following traumatic brain injury amongst children and adolescents.Brain Inj. Jan2003;17(1):1–23.
- ,,.Comparing stress levels of parents of children with cancer and parents of children with physical disabilities.Psychooncology. Dec2004;13(12):898–903.
- ,,.Stress levels experienced by the parents of enterally fed children.Child Care Health Dev.2004;30:507–513.
- ,,, et al.The health and well‐being of caregivers of children with cerebral palsy.Pediatrics.2005;115:e626–e636.
- ,.Parenting stress and children with heart disease.J Pediatr Health Care.2003;17(4):163–168.
- ,.Small sample inference for fixed effects from restricted maximum likelihood.Biometrics.1997;53:983–997.
- ,,.Methods for the analysis of quality‐of‐life and survival data in health technology assessment.Health Technol Assess.1999;3(10):1–152.
- ,,, et al.Fundoplication and gastrostomy versus image‐guided gastrojejunal tube for enteral feeding in neurologically impaired children with gastroesophageal reflux.J Pediatr Surg.2002;37:407–412.
- SF‐36 Health Survey: Manual and Interpretation Guide.Lincoln, RI:QualityMetric Inc.;1993,year="2000"2000.
- ,,.The role of protective antireflux procedures in neurologically impaired children: a decision analysis.J Pediatr Surg. Mar2002;37:500–506.
- ,,.Comparison of respiratory mortality in the profoundly mentally retarded and in the less retarded.J Ment Defic Res.1979;23(1):1–7.
- ,,.Cause of death in cerebral palsy: a descriptive study.Arch Dis Child.1999;81:390–394.
- .Survival rates of children with severe neurologic disabilities: a review.Semin Pediatr Neurol.2003;10(2):120–129.
- ,.Gastroesophageal reflux among severely retarded children.J Pediatr.1979;94:710–714.
- ,,,,,.Operation for gastro‐oesophageal reflux associated with severe mental retardation.Arch Dis Child.1993;68:347–351.
- ,,,,,.Prevalence and severity of feeding and nutritional problems in children with neurological impairment: Oxford Feeding Study.Dev Med Child Neurol.2000;42:674–680.
- ,,, et al.Aspiration pneumonia in pediatric age group: etiology, predisposing factors and clinical outcome.J Pak Med Assoc.1999;49(4):105–108.
- ,.Respiratory problems in children with neurological impairment.Arch Dis Child.2003;88(1):75–78.
- ,.Gastroesophageal reflux in childhood.Curr Probl Surg.1996;33(1):1–70.
- ,.Minimally invasive surgical techniques in reoperative surgery for gastroesophageal reflux disease in infants and children.Am Surg.2002;68:989–992.
- .Laparoscopic Nissen procedure in children.Semin Laparosc Surg.2002;9(3):146–152.
- ,,, et al.Caregiving process and caregiver burden: conceptual models to guide research and practice.BMC Pediatr.2004;4(1):1.
- ,.Theoretical and psychometric analysis of caregiver strain.Res Nurs Health.1996;19:499–510.
- ,,,,,.Complications and reoperation after Nissen fundoplication in childhood.Am J Surg.1987;153(2):177–183.
- ,,, et al.Surgical treatment of gastroesophageal reflux in children: a combined hospital study of 7467 patients.Pediatrics.1998;101:419–422.
- ,,,,,.Outcomes of surgical fundoplication in children.Clin Gastroenterol Hepatol.2004;2:978–984.
- ,,, et al.The respiratory advantage of laparoscopic Nissen fundoplication.J Pediatr Surg.2003;38:886–891.
- ,,,.Laparoscopic Nissen fundoplication in children: 2‐5‐year follow‐up.Pediatr Surg Int.2003;19:537–539.
- ,,.Recognition of recurrent gastroesophageal reflux following antireflux surgery in the neurologically disabled child: high index of suspicion and definitive evaluation.J Pediatr Surg.1992;27:983–988; discussion988–990.
- ,,.Sequelae of antireflux surgery in profoundly disabled children.J Pediatr Surg.1992;27(2):267–271; discussion271–263.
- ,,.Efficacy of the Nissen fundoplication in the management of gastroesophageal reflux following esophageal atresia repair.J Pediatr Surg.1993;28(1):53–55.
- ,,,.Caregivers' perceptions following gastrostomy in severely disabled children with feeding problems.Dev Med Child Neurol.1997;39:746–751.
- ,,,,.Care‐giver evaluation of anti‐gastroesophageal reflux procedures in neurologically impaired children: what is the real‐life outcome?J Pediatr Surg.1996;31:375–380.
- ,,, et al.Impact of gastrostomy tube feeding on the quality of life of carers of children with cerebral palsy.Dev Med Child Neurol.2004;46:796–800.
- ,.Organizational responses to managed care: issues for academic health centers and implications for pediatric programs.Pediatrics.1998;101:805–811; discussion811–802.
- Children's Digestive Health and Nutrition Foundation Website. Gastroesophageal Reflux Disease in the Neurologically Impaired Child. Available at: http://www.cdhnf.org/PractitionerSeries.asp. Accessed August 30,2006.
- ,,,,.Functional status of school‐aged children with Down syndrome.J Paediatr Child Health.2002;38(2):160–165.
- ,,,,,.Concurrent validity of the Functional Independence Measure for Children (WeeFIM) and the Pediatric Evaluation of Disabilities Inventory in children with developmental disabilities and acquired brain injuries.Phys Occup Ther Pediatr.2001;21(2–3):91–101.
- ,,, et al.The WeeFIM instrument: its utility in detecting change in children with developmental disabilities.Arch Phys Med Rehabil.2000;81:1317–1326.
- ,,, et al.Functional assessment and care of children with neurodevelopmental disabilities.Am J Phys Med Rehabil.2000;79(2):114–123.
- ,,,,,.Predictors of mortality, morbidity, and disability in a cohort of infants < or = 28 weeks' gestation.Clin Pediatr (Phila).1993;32:521–527.
- ,.Content validity of a pediatric functional independence measure.Appl Nurs Res.1990;3(3):120–122.
- ,,, et al.The Functional Independence Measure for Children (WeeFIM). Conceptual basis and pilot use in children with developmental disabilities.Clin Pediatr (Phila). Jul1994;33:421–430.
- ,,.The CHQ User's Manual.1st ed.Boston, MA:The Health Institute, New England Medical Center,1996.
- ,,,,,.Comparing reliability and validity of pediatric instruments for measuring health and well‐being of children with spastic cerebral palsy.Dev Med Child Neurol.2002;44:468–476.
- ,,,,.Needs of carers of severely disabled people: are they identified and met adequately?Health Soc Care Community.2001;9(4):235–243.
- ,,.The realities of postoperative disability and the carer's burden.Ann R Coll Surg Engl.2001;83(3):215–218.
- Abdidin.Parenting Stress Index.3rd ed.Lutz, FL:Psychological Assessment Resources, Inc.;1995.
- ,,,.Parental stress and burden following traumatic brain injury amongst children and adolescents.Brain Inj. Jan2003;17(1):1–23.
- ,,.Comparing stress levels of parents of children with cancer and parents of children with physical disabilities.Psychooncology. Dec2004;13(12):898–903.
- ,,.Stress levels experienced by the parents of enterally fed children.Child Care Health Dev.2004;30:507–513.
- ,,, et al.The health and well‐being of caregivers of children with cerebral palsy.Pediatrics.2005;115:e626–e636.
- ,.Parenting stress and children with heart disease.J Pediatr Health Care.2003;17(4):163–168.
- ,.Small sample inference for fixed effects from restricted maximum likelihood.Biometrics.1997;53:983–997.
- ,,.Methods for the analysis of quality‐of‐life and survival data in health technology assessment.Health Technol Assess.1999;3(10):1–152.
- ,,, et al.Fundoplication and gastrostomy versus image‐guided gastrojejunal tube for enteral feeding in neurologically impaired children with gastroesophageal reflux.J Pediatr Surg.2002;37:407–412.
- SF‐36 Health Survey: Manual and Interpretation Guide.Lincoln, RI:QualityMetric Inc.;1993,year="2000"2000.
- ,,.The role of protective antireflux procedures in neurologically impaired children: a decision analysis.J Pediatr Surg. Mar2002;37:500–506.