User login
Adverse GERD Outcomes Rare After ALTE
Apparent life‐threatening events (ALTEs) are frightening for the parent/guardian and represent a challenge for the healthcare provider. ALTEs are defined as worrisome episodes of any combination of apnea, color change, change in muscle tone, choking or gagging.1 ALTEs account for 0.6% to 0.8% of emergency department (ED) visits for children <12 months old,2, 3 have an average length of stay (LOS) of 4.4 days and an average cost of $15,000 per hospitalization.4
Gastroesophageal reflux disease (GERD) is common in infancy11 and also is the most commonly (in 31%55% of ALTE cases) attributed cause of ALTE.2, 4, 5 It has been speculated that chemosensitivity to gastric acid results in laryngospasm, bronchospasm, and apnea. However, several small studies have failed to prove a causal link between reflux episodes and apnea.69 Furthermore, although consensus guidelines for GERD have been developed,14 the clinical use of testing for GERD remains highly variable. A study of infants discharged with an ALTE (n = 12,067) from 36 children's hospitals in the United States revealed extensive variability in the use of pH probes and upper gastrointestinal x‐ray series to diagnose GERD.4
The incidence of adverse outcomes associated with GERD after an ALTE remains unknown. It is also unknown whether an association exists between long‐term gastrointestinal (GI) outcomes and testing demonstrative of GERD or a diagnosis of GERD during hospitalization for ALTE. The primary objective of our study was to determine, in patients with an ALTE, the adverse outcomes associated with GERD (failure‐to‐thrive, aspiration pneumonia, and/or anti‐reflux surgery), the incidence of readmission for second ALTE, and death. Our secondary objective was to determine risk factors for adverse outcomes associated with GERD following an ALTE.
METHODS
Design
This was a retrospective cohort study. We reviewed electronic and paper medical charts of all infants <12 months of age admitted for ALTE between January 1, 1999 and December 31, 2003 to Primary Children's Medical Center in Salt Lake City, UT, which serves as the tertiary pediatric center for >1 million children and the primary facility for >270,000 children in Salt Lake County, UT.10 Primary Children's Medical Center is operated by a vertically integrated not‐for‐profit healthcare system (Intermountain Healthcare), which has 20 affiliated hospitals and EDs. The study was approved by the institutional review boards of the University of Utah and Intermountain Healthcare, and the privacy board of Intermountain Healthcare.
Participants
Patients were included if a computer search of ED chief complaint or hospital discharge diagnoses found one or more of the following keywords (or corresponding International Classification of Diseases, Ninth Revision [ICD‐9] codes if applicable): ALTE, altered mental status, apnea, breath‐holding spell, choking, GERD, hypotonia, lethargy, other convulsions, other neurologic diagnosis, other respiratory diagnosis, pallor, seizures, sleep apnea, stiff, syncope, and unresponsiveness. These diagnoses were chosen as potential proxy diagnoses or codes for possible ALTE, as ALTE did not have a corresponding ICD‐9 code at the time of this study.
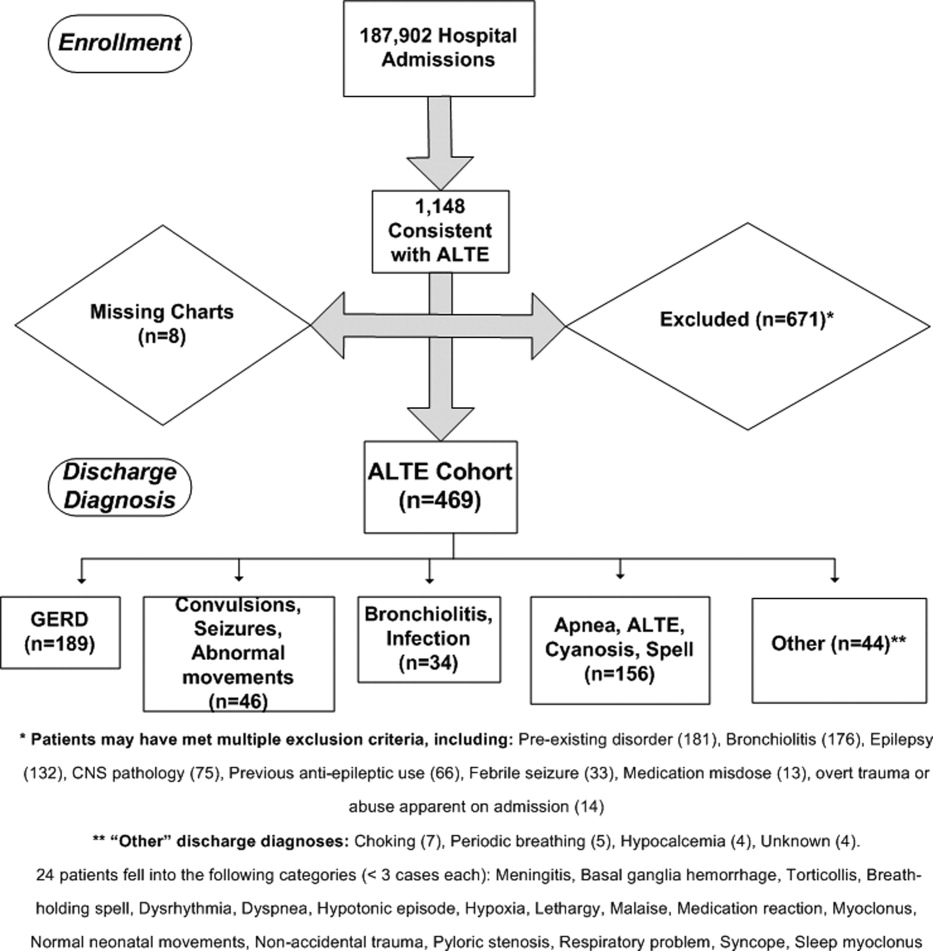
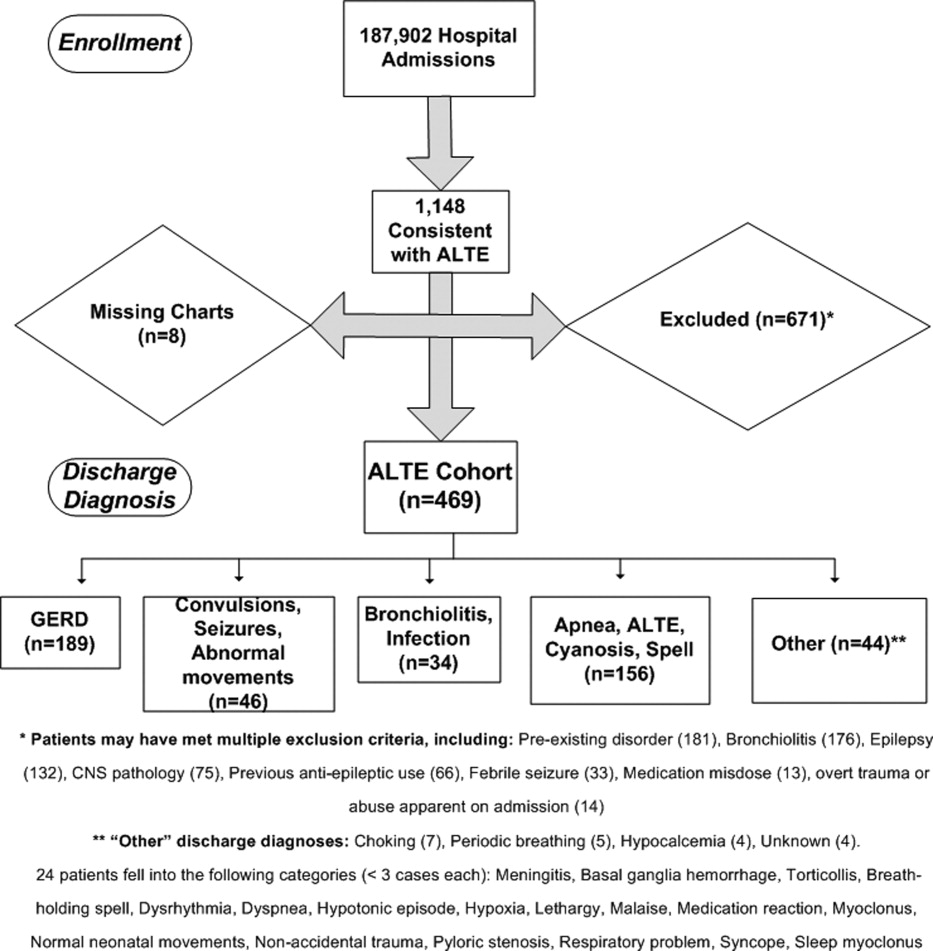
Detailed review of the medical record included infants who were <12 months old at admission with a history consistent with ALTE, defined as an episode frightening to the observer with any combination of apnea, color change, change in muscle tone, choking, or gagging. Infants were excluded from the study if they had a previously documented underlying medical condition to explain the ALTE (such as a known seizure disorder) or had a clearly apparent diagnosis upon initial history and physical examination (such as bronchiolitis diagnosed in the emergency department) that would explain the event. Patients with unstable vital signs (eg, hypotension), trauma clearly apparent on admission, documented medication dosing error, or febrile seizure were also excluded. A complete list of exclusion criteria is found in Figure 1.
All hospital admissions, ED visits, and Pediatric GI clinic notes were reviewed for adverse outcomes associated with GERD, second ALTE admission, and death. The follow‐up time period included the original enrollment period (January 1, 1999 through December 31, 2003) through August 31, 2009.
Outcomes
Adverse outcomes associated with GERD were defined as aspiration pneumonia, failure‐to‐thrive (FTT; either admission or discharge diagnosis of FTT at another hospitalization, or follow‐up to gastroenterology clinic for FTT) and/or anti‐reflux surgery (Nissen fundoplication or gastrojejunal tube placement) as these are potential clinical consequences of having severe and uncontrolled GERD. We further collected readmission data for a second ALTE. Deaths and the attributed reasons were also collected.
Risk Factors
Potential risk factors for adverse outcomes associated with GERD (all during index hospitalization) included: age; prematurity; gender; previous event (as described by the parent, without previous ALTE hospitalization); primary discharge diagnosis of GERD; testing positive for reflux on index ALTE admission (upper GI x‐ray series, esophageal pH probe, swallow study, endoscopy, and/or consultation of pediatric gastroenterologist with results or assessments indicating gastroesophageal reflux); any anti‐reflux medication prescribed upon discharge; and LOS. We also considered diagnosis of neurologic impairment during follow‐up, which was defined as seizures or diagnosis of developmental delay from any etiology not recognized on index ALTE admission. We examined these risk factors as we postulated they might indicate higher risk for both ALTE and adverse outcomes associated with GERD, or might indicate a higher severity of initial event.
Analyses
Summary statistics were performed for adverse outcomes associated with GERD, readmission, and death. Univariate analyses were performed for risk factors using chi‐square tests for dichotomous predictors and Wilcoxon rank sum tests for nonparametric continuous predictors for any of the 3 adverse outcomes (FTT, aspiration pneumonia [AP], and/or anti‐reflux surgery) associated with GERD. All analyses were performed using SAS 9.13 (Carey, NC).
RESULTS
Eleven hundred forty‐eight infants with ALTE met inclusion criteria, from 187,903 patients meeting initial search criteria. Six hundred seventy‐one patients were excluded and 8 patients had missing charts. The study population of the 469‐patient cohort is shown in Figure 1.
Demographics are displayed in Table 1. The mean age was 65 days. One hundred three (22%) were premature. One hundred eighty‐nine patients (40%) had a primary discharge diagnosis of GERD; details of the diagnoses for the remaining patients are in Figure 1. Median length of follow‐up for the cohort was 7.8 years. The entire study period was 10.7 years.
| ALTE Cohort | |
|---|---|
| N = 469 | |
| |
| Female | 233 (49.7%) |
| Race | |
| Caucasian | 371 (79.1%) |
| Hispanic | 64 (13.6%) |
| Pacific Islander | 6 (1.3%) |
| Black | 4 (0.8%) |
| Other/unknown | 24 (5.1%) |
| Mean age in days (SD) | 65.2 (69.5) |
| Prematurity | 103 (22%) |
| Underwent testing for gastroesophageal reflux | 214 (45.6%) |
| Discharged on anti‐reflux medication | 238 (50.7%) |
| Previous event | 127 (27.1%) |
| Mean length of stay in days (SD) | 2.4 (2.4) |
| Later neurologic impairment (seizures or developmental delay) | 23 (4.9%) |
| Primary discharge diagnosis of GERD | 189 (40%) |
Eighteen patients (3.8%) had an adverse outcome associated with GERD. Four (0.9%) had aspiration pneumonia, 9 (1.9%) had failure‐to‐thrive, and 7 (1.5%) had a Nissen fundoplication (no patients had a gastrojejunal tube placed). Five patients had a gastrostomy tube placed at the time of fundoplication. Two patients had more than 1 adverse GI outcome; 1 patient had aspiration pneumonia and another had failure‐to‐thrive prior to their Nissen fundoplications.
Fifty‐six patients (11.9%) were readmitted for a second ALTE. Median time from index ALTE to second ALTE admission was 16.5 days (interquartile range: Q1, 8Q3, 32). Two (0.4%) patients died. Both (occurring at 18 months and 5.5 years after the initial ALTE hospitalization) were related to the children first developing seizure disorders and severe developmental delay. Neither of the patients who died had an index discharge diagnoses of GERD.
There was no significance of the following variables in predicting adverse outcomes associated with GERD: age, prematurity, gender, previous event, testing positive for reflux, primary discharge diagnosis of GERD, or discharge on anti‐reflux medications (see Table 2). Patients with adverse outcomes associated with GERD had longer mean LOS on the index ALTE hospitalization (4.3 days vs 2.4 days; P = 0.03) and a higher rate of neurologic impairment diagnosed in follow‐up (16.7% vs 4.4%; P = 0.02) than patients without long‐term adverse GI outcomes. Patients with neurological impairment diagnosed in follow‐up were more likely to eventually develop an adverse outcome associated with GERD, compared to patients without neurological impairment (odds ratio 8.4; 95% confidence interval 1.1516.1).
| AP, FTT, or Surgery | No Long‐Term Adverse GI Outcome | P Value | |
|---|---|---|---|
| N = 18 | N = 451 | ||
| |||
| Mean age in days (SD) | 51.9 (76.4) | 65.8 (69.3) | 0.27 |
| Prematurity | 1 (5.6%) | 102 (22.6%) | 0.08 |
| Male gender | 12 (66.7%) | 220 (48.8%) | 0.14 |
| Previous ALTE‐like event (no hospitalization) | 8 (44.4%) | 119 (26.4%) | 0.09 |
| Testing positive for reflux | 9 (50%) | 177 (39.3%) | 0.36 |
| Discharge diagnosis GERD | 9 (50%) | 180 (39.9%) | 0.39 |
| Discharged on anti‐reflux medication | 9 (50%) | 229 (50.7%) | 0.95 |
| Mean length of stay in days (SD) | 4.3 (4.7) | 2.4 (2.3) | 0.03 |
| Neurologic impairment diagnosed in follow‐up | 3 (16.7%) | 20 (4.4%) | 0.02 |
DISCUSSION
Our study had 2 main findings. First, infants admitted for an ALTE had a low percentage (3.8%) of adverse outcomes associated with GERD. Review of the literature provides little context to interpret this percentage. One study reports 9 per 100,000 of the general population <18 years of age having anti‐reflux surgery.24 The percentage of adverse outcomes associated with GERD converted to a rate in our study would likely reflect the bias of our center serving as a referral population for Utah and 5 surrounding states. Furthermore, there may be an additional bias of ALTE being a potential indication for anti‐reflux surgery for some clinicians, as well as confounding additional diagnoses (such as later neurologic impairment which might independently increase risk of study outcomes).
The second main finding of our study is that the development of neurologic impairment was predictive of developing adverse GI outcomes. As previous studies have shown that neurological impairment cannot be predicted during the initial ALTE hospitalization,14 adverse outcomes associated with GERD are similarly not predictable with current clinical approaches. Furthermore, the exact nature of the relationship between neurologic impairment and ALTEs remains unclear. While previous studies have described the increased prevalence of adverse neurological outcomes (such as seizures, developmental delay) in children who have had an ALTE, it is unclear what the precipitating reason for ALTE is in these infants (seizure, central apnea, GERD, etc).14
There is ongoing debate in the literature surrounding the optimal diagnosis of GERD in infants with ALTE. Recent guidelines state that investigations aimed to prove GERD causing an ALTE should include pH probe or impedance monitor testing, in combination with a sleep study, and discourages a GERD diagnosis based on upper GI‐series alone.14 Given the low use of pH probe and impedance monitoring at our institution during the study period (86% of the patients who had GI‐related testing had an upper GI‐series), we did not attempt to find the sensitivity or specificity of the different GI testing modalities for GERD in the setting of ALTE. The high use of upper‐GI series is not unique to our institutionone large study examining practice variation from 12,067 ALTE admissions in 36 children's hospitals, with 36.9% of infants (n = 4453) having a primary discharge diagnosis of gastroesophageal reflux, revealed that only 8.9% (SD 28%) received an esophageal pH probe, while 25.6% (SD 43.6%) had an upper GI‐series or swallow study.4
Given the difficulties in assigning a GERD diagnosis for ALTE infants, we focused on the long‐term adverse outcomes associated with GERD for the entire ALTE infant cohort. The 3 adverse outcomes we chose deserve some mention. Aspiration pneumonia is generally due to either primary aspiration (from dysfunctional swallowing) or secondary aspiration (from GERD). Failure‐to‐thrive can be due to ongoing GERD. Anti‐reflux surgery is often performed for severe GERD. While we believe that a prospective study with better diagnostic evaluations for GERD and apnea (such as pH probe or impedance monitor in combination with a sleep study) might help elucidate the unclear relationship between reflux episodes and ALTE, the low percentage of adverse outcomes associated with GERD after ALTE may suggest that such a study would be difficult both in terms of sample size and an unnecessary use of resources.
We also found that a high percentage (11.9%) of all patients had readmission for a second ALTE. This was substantially higher than the 2.5% readmission rate for second ALTE reported in a previous study of short‐term follow‐up (30 days).4 The number of readmissions in our study might be higher due to our comprehensive follow‐up, both in length of time and number of additional EDs and hospitals captured. Unfortunately, the retrospective nature of our study makes it difficult to determine if any interventions (prescription of anti‐reflux medication, education on reflux precautions) impacted the rate of readmission, as compliance was not measurable. Further studies should address why patients return with recurrent ALTE.
Interestingly, several potential risk factors did not predict long‐term adverse GI outcomes. For example, prematurity, a discharge diagnosis of GERD, or prescription of an anti‐reflux medication, were not associated with adverse GI outcomes. These findings support the concept that a diagnosis of GERD, at least as is commonly applied, is not meaningful in the setting of an ALTE. We did find associations with longer length of stay (LOS), and with eventual development of neurological impairment. Longer LOS might be a proxy for other subtle predictors that could influence adverse outcomes, such as requiring additional diagnostic tests prolonging hospitalization, or continued ALTEs while inpatient. The neurologic outcomes of patients with ALTE have been previously published, and the strong correlation between neurologic impairment and GERD has been well described.14, 25
There are several strengths of this study. This is the first study, to our knowledge, to look at adverse outcomes associated with GERD following ALTE, despite GERD being the most commonly attributed cause. The use of Intermountain Healthcare's electronic medical record system allowed for comprehensive tracking, over an extensive follow‐up period (median of 7.8 years), across 20 hospitals and EDs which care for the vast majority of pediatric patients in Utah. Finally, this large cohort of ALTE patients used clinical data from medical records and not only administrative data.
There are limitations of this study. This is a retrospective cohort study. Some of our study outcomes may be a result of pathophysiology other than GERD and, conversely, GERD may be a result of other issues (neurologic impairment). The small sample size and low percentage of the study outcomes make it possible that we did not detect true risk factors. Patients were lost to follow‐up if they moved or presented to a hospital not within the Intermountain Healthcare system. This study has slightly different patient numbers from 3 previously published studies for different outcomes on this cohort, as exclusion criteria for the different cohorts were different.14, 26, 27 Six patients had only their electronic medical record reviewed because the paper chart was missing.
IMPLICATIONS
The results of this study extend previous work of various outcomes regarding well‐appearing infants following an ALTE.14, 26, 27 In these studies, 3.9% and 3% were ultimately diagnosed with epilepsy and developmental delay, respectively; 1.4% were diagnosed with abusive head trauma; and 0.6% required otolaryngologic surgical intervention. In these previous studies, there were few predictors of these outcomes, with testing demonstrating largely normal results during the index ALTE admission.
Our study helps clinicians place the outcomes of aspiration pneumonia, failure‐to‐thrive, and anti‐reflux surgery into the context of these other studies when discharging infants from the hospital after an ALTE. Collectively, these studies provide clinicians with the information that, in the setting of a well‐appearing infant, few diagnostic tests in their ALTE patients will yield a definitive diagnosis. Ultimately, close follow‐up with further investigations if symptoms recur will be an important part of diagnosing the etiology of the ALTE in these infants.
We found that well‐appearing infants with ALTE, regardless of attributed cause, are at low risk for adverse outcomes associated with GERD. Only the eventual development of neurologic impairment or an increased length of stay during index ALTE hospitalization was found to be predictive of these outcomes.
Acknowledgements
The following individuals have made substantive intellectual contributions to this study: conception and design (G.Z., J.L.B., W.D.J., C.G.M., R.S.), acquisition of data (G.Z., J.L.B.), analysis (G.Z., RS) and interpretation of data (G.Z., J.L.B., W.D.J., C.G.M., R.S.). In addition, all listed authors have contributed to either drafting the article or revising it critically for important intellectual content. Finally, all listed authors have given final approval of this version submitted for publication. The authors also acknowledge Chelsea Welch for her assistance in data collection.
Disclosures: This study was presented in part at the national Pediatric Academic Societies meetings in Vancouver, Canada, May 2010 and in Denver, CO, May 2011. This study was supported by a National Institutes of Child Health and Human Development (NICHD) grant for Dr Srivastava (K23 HD052553), and a National Institute on Drug Abuse (NIDA) grant for Dr Bonkowsky (K08 DA24753). This research was supported in part by the Children's Health Research Center, University of Utah. There are no conflicts of interest.
- Infantile apnea and home monitoring.NIH Consensus Statement 1986 Sep 29‐Oct 1.Pediatrics.1987;79(2):292–299.
- ,.Causes of apparent life threatening events in infants: a systematic review.Arch Dis Child.2004;89:1043–1048.
- ,.Parental reported apnea, admissions to hospital and sudden infant death syndrome.Acta Paediatr.2001;90(4):417–422.
- ,,,.Variation in inpatient resource utilization and management of apparent life‐threatening events.J Pediatr.2008;152(5):629–635.
- ,,,,,.Discharge diagnoses in infants with apparent life‐threatening event.Pediatr Int.2003;45:560–563.
- ,,,,,.Lack of temporal relation between acid reflux in the proximal oesophagus and cardiorespiratory events in sleeping infants.Eur J Pediatr.1992;151(3):208–212.
- ,,, et al.Patterns of gastroesophageal reflux (GER) in patients with apparent life‐threatening events.J Pediatr Gastroenterol Nutr.1989;8(2):157–160.
- ,,, et al.Characteristics of continuous esophageal pH‐metering in infants with gastroesophageal reflux and apparent life‐threatening events.Eur J Pediatr Surg.1995;5(3);136–138.
- ,,,,.Apnea is not prolonged by acid gastroesophageal reflux in preterm infants.Pediatrics.2005;116:1059–1063.
- ,.Organizational responses to managed care: issues for academic health centers and implications for pediatric programs.Pediatrics.1998;101(4):805–811.
- ,,, et al.Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN).J Pediatr Gastroenterol Nutr.2009;49:498–547.
- ,,, et al.Prevalence and natural history of gastroesophageal reflux: pediatric prospective study.Pediatrics.2009;123(3):779–783.
- ,.Systematic review: the estra‐oesophageal symptoms of gastro‐oesophageal reflux disease in children.Aliment Pharmacol Ther.2009;29:258–272.
- ,,,.Death, child abuse, and adverse neurological outcomes of infants after an apparent life‐threatening event.Pediatrics.2008;122:125–131.
- ,,, et al.Abusive head injury as a cause of apparent life‐threatening events in infancy.Arch Pediatr Adolesc Med.2003;157:1011–1015.
- ,,.Accidental and nonaccidental poisonings as a cause of apparent life‐threatening events in infants.Pediatrics.2008;122:e359–e362.
- ,,,.Yield of diagnostic testing in infants who have had an apparent life‐threatening event.Pediatrics.2005;115:885–893.
- ,,, et al.Guidelines for evaluation and treatment of gastroesophageal reflux in infants and children: recommendations of the North American Society for Pediatric Gastroenterology and Nutrition.J Pediatr Gastroenterol Nutr.2001;32(suppl 2):S1–S31.
- ,,,.Comparison of barium radiology with esophageal pH monitoring in the diagnosis of gastroesophageal reflux disease.Am J Gastroenterol.1996;91(6):1181–1195.
- ,,, et al.Gastroesophageal reflux disease: correlation of esophageal pH testing and radiographic finding.Radiology.1992;185:483–486.
- ,,, et al.Gastro‐esophageal reflux demonstrated by radiography in infants less than 1 year of age. Comparison with pH monitoring.Acta Radiol.2003;44:136–138.
- ,,, et al.Double‐blind placebo controlled trial of omeprazole in irritable infants with gastroesophageal reflux.J Pediatr.2003;143:219–223.
- ,,, et al.Clinical predictors of pathological gastro‐oesophageal reflux in infants with persistant distress.J Paediatr Child Health.2006;42:134–139.
- ,,.National trends in the use of anti‐reflux procedures for children.Pediatrics.2006;118:1828–1835.
- ,,, et al.Prevalence and severity of feeding and nutritional problems in children with neurological impairment: Oxford feeding study.Dev Med Child Neurol.2000;42:674–680.
- ,,.Abusive head trauma in children presenting with an apparent life‐threatening event.J Pediatr.2010;157(5):821–825.
- ,,.Usefulness of airway evaluation in children initially seen with apparent life‐threatening event.Arch Otolaryngol Head Neck Surg.2011;137(4):359–362.
Apparent life‐threatening events (ALTEs) are frightening for the parent/guardian and represent a challenge for the healthcare provider. ALTEs are defined as worrisome episodes of any combination of apnea, color change, change in muscle tone, choking or gagging.1 ALTEs account for 0.6% to 0.8% of emergency department (ED) visits for children <12 months old,2, 3 have an average length of stay (LOS) of 4.4 days and an average cost of $15,000 per hospitalization.4
Gastroesophageal reflux disease (GERD) is common in infancy11 and also is the most commonly (in 31%55% of ALTE cases) attributed cause of ALTE.2, 4, 5 It has been speculated that chemosensitivity to gastric acid results in laryngospasm, bronchospasm, and apnea. However, several small studies have failed to prove a causal link between reflux episodes and apnea.69 Furthermore, although consensus guidelines for GERD have been developed,14 the clinical use of testing for GERD remains highly variable. A study of infants discharged with an ALTE (n = 12,067) from 36 children's hospitals in the United States revealed extensive variability in the use of pH probes and upper gastrointestinal x‐ray series to diagnose GERD.4
The incidence of adverse outcomes associated with GERD after an ALTE remains unknown. It is also unknown whether an association exists between long‐term gastrointestinal (GI) outcomes and testing demonstrative of GERD or a diagnosis of GERD during hospitalization for ALTE. The primary objective of our study was to determine, in patients with an ALTE, the adverse outcomes associated with GERD (failure‐to‐thrive, aspiration pneumonia, and/or anti‐reflux surgery), the incidence of readmission for second ALTE, and death. Our secondary objective was to determine risk factors for adverse outcomes associated with GERD following an ALTE.
METHODS
Design
This was a retrospective cohort study. We reviewed electronic and paper medical charts of all infants <12 months of age admitted for ALTE between January 1, 1999 and December 31, 2003 to Primary Children's Medical Center in Salt Lake City, UT, which serves as the tertiary pediatric center for >1 million children and the primary facility for >270,000 children in Salt Lake County, UT.10 Primary Children's Medical Center is operated by a vertically integrated not‐for‐profit healthcare system (Intermountain Healthcare), which has 20 affiliated hospitals and EDs. The study was approved by the institutional review boards of the University of Utah and Intermountain Healthcare, and the privacy board of Intermountain Healthcare.
Participants
Patients were included if a computer search of ED chief complaint or hospital discharge diagnoses found one or more of the following keywords (or corresponding International Classification of Diseases, Ninth Revision [ICD‐9] codes if applicable): ALTE, altered mental status, apnea, breath‐holding spell, choking, GERD, hypotonia, lethargy, other convulsions, other neurologic diagnosis, other respiratory diagnosis, pallor, seizures, sleep apnea, stiff, syncope, and unresponsiveness. These diagnoses were chosen as potential proxy diagnoses or codes for possible ALTE, as ALTE did not have a corresponding ICD‐9 code at the time of this study.
Detailed review of the medical record included infants who were <12 months old at admission with a history consistent with ALTE, defined as an episode frightening to the observer with any combination of apnea, color change, change in muscle tone, choking, or gagging. Infants were excluded from the study if they had a previously documented underlying medical condition to explain the ALTE (such as a known seizure disorder) or had a clearly apparent diagnosis upon initial history and physical examination (such as bronchiolitis diagnosed in the emergency department) that would explain the event. Patients with unstable vital signs (eg, hypotension), trauma clearly apparent on admission, documented medication dosing error, or febrile seizure were also excluded. A complete list of exclusion criteria is found in Figure 1.

All hospital admissions, ED visits, and Pediatric GI clinic notes were reviewed for adverse outcomes associated with GERD, second ALTE admission, and death. The follow‐up time period included the original enrollment period (January 1, 1999 through December 31, 2003) through August 31, 2009.
Outcomes
Adverse outcomes associated with GERD were defined as aspiration pneumonia, failure‐to‐thrive (FTT; either admission or discharge diagnosis of FTT at another hospitalization, or follow‐up to gastroenterology clinic for FTT) and/or anti‐reflux surgery (Nissen fundoplication or gastrojejunal tube placement) as these are potential clinical consequences of having severe and uncontrolled GERD. We further collected readmission data for a second ALTE. Deaths and the attributed reasons were also collected.
Risk Factors
Potential risk factors for adverse outcomes associated with GERD (all during index hospitalization) included: age; prematurity; gender; previous event (as described by the parent, without previous ALTE hospitalization); primary discharge diagnosis of GERD; testing positive for reflux on index ALTE admission (upper GI x‐ray series, esophageal pH probe, swallow study, endoscopy, and/or consultation of pediatric gastroenterologist with results or assessments indicating gastroesophageal reflux); any anti‐reflux medication prescribed upon discharge; and LOS. We also considered diagnosis of neurologic impairment during follow‐up, which was defined as seizures or diagnosis of developmental delay from any etiology not recognized on index ALTE admission. We examined these risk factors as we postulated they might indicate higher risk for both ALTE and adverse outcomes associated with GERD, or might indicate a higher severity of initial event.
Analyses
Summary statistics were performed for adverse outcomes associated with GERD, readmission, and death. Univariate analyses were performed for risk factors using chi‐square tests for dichotomous predictors and Wilcoxon rank sum tests for nonparametric continuous predictors for any of the 3 adverse outcomes (FTT, aspiration pneumonia [AP], and/or anti‐reflux surgery) associated with GERD. All analyses were performed using SAS 9.13 (Carey, NC).
RESULTS
Eleven hundred forty‐eight infants with ALTE met inclusion criteria, from 187,903 patients meeting initial search criteria. Six hundred seventy‐one patients were excluded and 8 patients had missing charts. The study population of the 469‐patient cohort is shown in Figure 1.
Demographics are displayed in Table 1. The mean age was 65 days. One hundred three (22%) were premature. One hundred eighty‐nine patients (40%) had a primary discharge diagnosis of GERD; details of the diagnoses for the remaining patients are in Figure 1. Median length of follow‐up for the cohort was 7.8 years. The entire study period was 10.7 years.
| ALTE Cohort | |
|---|---|
| N = 469 | |
| |
| Female | 233 (49.7%) |
| Race | |
| Caucasian | 371 (79.1%) |
| Hispanic | 64 (13.6%) |
| Pacific Islander | 6 (1.3%) |
| Black | 4 (0.8%) |
| Other/unknown | 24 (5.1%) |
| Mean age in days (SD) | 65.2 (69.5) |
| Prematurity | 103 (22%) |
| Underwent testing for gastroesophageal reflux | 214 (45.6%) |
| Discharged on anti‐reflux medication | 238 (50.7%) |
| Previous event | 127 (27.1%) |
| Mean length of stay in days (SD) | 2.4 (2.4) |
| Later neurologic impairment (seizures or developmental delay) | 23 (4.9%) |
| Primary discharge diagnosis of GERD | 189 (40%) |
Eighteen patients (3.8%) had an adverse outcome associated with GERD. Four (0.9%) had aspiration pneumonia, 9 (1.9%) had failure‐to‐thrive, and 7 (1.5%) had a Nissen fundoplication (no patients had a gastrojejunal tube placed). Five patients had a gastrostomy tube placed at the time of fundoplication. Two patients had more than 1 adverse GI outcome; 1 patient had aspiration pneumonia and another had failure‐to‐thrive prior to their Nissen fundoplications.
Fifty‐six patients (11.9%) were readmitted for a second ALTE. Median time from index ALTE to second ALTE admission was 16.5 days (interquartile range: Q1, 8Q3, 32). Two (0.4%) patients died. Both (occurring at 18 months and 5.5 years after the initial ALTE hospitalization) were related to the children first developing seizure disorders and severe developmental delay. Neither of the patients who died had an index discharge diagnoses of GERD.
There was no significance of the following variables in predicting adverse outcomes associated with GERD: age, prematurity, gender, previous event, testing positive for reflux, primary discharge diagnosis of GERD, or discharge on anti‐reflux medications (see Table 2). Patients with adverse outcomes associated with GERD had longer mean LOS on the index ALTE hospitalization (4.3 days vs 2.4 days; P = 0.03) and a higher rate of neurologic impairment diagnosed in follow‐up (16.7% vs 4.4%; P = 0.02) than patients without long‐term adverse GI outcomes. Patients with neurological impairment diagnosed in follow‐up were more likely to eventually develop an adverse outcome associated with GERD, compared to patients without neurological impairment (odds ratio 8.4; 95% confidence interval 1.1516.1).
| AP, FTT, or Surgery | No Long‐Term Adverse GI Outcome | P Value | |
|---|---|---|---|
| N = 18 | N = 451 | ||
| |||
| Mean age in days (SD) | 51.9 (76.4) | 65.8 (69.3) | 0.27 |
| Prematurity | 1 (5.6%) | 102 (22.6%) | 0.08 |
| Male gender | 12 (66.7%) | 220 (48.8%) | 0.14 |
| Previous ALTE‐like event (no hospitalization) | 8 (44.4%) | 119 (26.4%) | 0.09 |
| Testing positive for reflux | 9 (50%) | 177 (39.3%) | 0.36 |
| Discharge diagnosis GERD | 9 (50%) | 180 (39.9%) | 0.39 |
| Discharged on anti‐reflux medication | 9 (50%) | 229 (50.7%) | 0.95 |
| Mean length of stay in days (SD) | 4.3 (4.7) | 2.4 (2.3) | 0.03 |
| Neurologic impairment diagnosed in follow‐up | 3 (16.7%) | 20 (4.4%) | 0.02 |
DISCUSSION
Our study had 2 main findings. First, infants admitted for an ALTE had a low percentage (3.8%) of adverse outcomes associated with GERD. Review of the literature provides little context to interpret this percentage. One study reports 9 per 100,000 of the general population <18 years of age having anti‐reflux surgery.24 The percentage of adverse outcomes associated with GERD converted to a rate in our study would likely reflect the bias of our center serving as a referral population for Utah and 5 surrounding states. Furthermore, there may be an additional bias of ALTE being a potential indication for anti‐reflux surgery for some clinicians, as well as confounding additional diagnoses (such as later neurologic impairment which might independently increase risk of study outcomes).
The second main finding of our study is that the development of neurologic impairment was predictive of developing adverse GI outcomes. As previous studies have shown that neurological impairment cannot be predicted during the initial ALTE hospitalization,14 adverse outcomes associated with GERD are similarly not predictable with current clinical approaches. Furthermore, the exact nature of the relationship between neurologic impairment and ALTEs remains unclear. While previous studies have described the increased prevalence of adverse neurological outcomes (such as seizures, developmental delay) in children who have had an ALTE, it is unclear what the precipitating reason for ALTE is in these infants (seizure, central apnea, GERD, etc).14
There is ongoing debate in the literature surrounding the optimal diagnosis of GERD in infants with ALTE. Recent guidelines state that investigations aimed to prove GERD causing an ALTE should include pH probe or impedance monitor testing, in combination with a sleep study, and discourages a GERD diagnosis based on upper GI‐series alone.14 Given the low use of pH probe and impedance monitoring at our institution during the study period (86% of the patients who had GI‐related testing had an upper GI‐series), we did not attempt to find the sensitivity or specificity of the different GI testing modalities for GERD in the setting of ALTE. The high use of upper‐GI series is not unique to our institutionone large study examining practice variation from 12,067 ALTE admissions in 36 children's hospitals, with 36.9% of infants (n = 4453) having a primary discharge diagnosis of gastroesophageal reflux, revealed that only 8.9% (SD 28%) received an esophageal pH probe, while 25.6% (SD 43.6%) had an upper GI‐series or swallow study.4
Given the difficulties in assigning a GERD diagnosis for ALTE infants, we focused on the long‐term adverse outcomes associated with GERD for the entire ALTE infant cohort. The 3 adverse outcomes we chose deserve some mention. Aspiration pneumonia is generally due to either primary aspiration (from dysfunctional swallowing) or secondary aspiration (from GERD). Failure‐to‐thrive can be due to ongoing GERD. Anti‐reflux surgery is often performed for severe GERD. While we believe that a prospective study with better diagnostic evaluations for GERD and apnea (such as pH probe or impedance monitor in combination with a sleep study) might help elucidate the unclear relationship between reflux episodes and ALTE, the low percentage of adverse outcomes associated with GERD after ALTE may suggest that such a study would be difficult both in terms of sample size and an unnecessary use of resources.
We also found that a high percentage (11.9%) of all patients had readmission for a second ALTE. This was substantially higher than the 2.5% readmission rate for second ALTE reported in a previous study of short‐term follow‐up (30 days).4 The number of readmissions in our study might be higher due to our comprehensive follow‐up, both in length of time and number of additional EDs and hospitals captured. Unfortunately, the retrospective nature of our study makes it difficult to determine if any interventions (prescription of anti‐reflux medication, education on reflux precautions) impacted the rate of readmission, as compliance was not measurable. Further studies should address why patients return with recurrent ALTE.
Interestingly, several potential risk factors did not predict long‐term adverse GI outcomes. For example, prematurity, a discharge diagnosis of GERD, or prescription of an anti‐reflux medication, were not associated with adverse GI outcomes. These findings support the concept that a diagnosis of GERD, at least as is commonly applied, is not meaningful in the setting of an ALTE. We did find associations with longer length of stay (LOS), and with eventual development of neurological impairment. Longer LOS might be a proxy for other subtle predictors that could influence adverse outcomes, such as requiring additional diagnostic tests prolonging hospitalization, or continued ALTEs while inpatient. The neurologic outcomes of patients with ALTE have been previously published, and the strong correlation between neurologic impairment and GERD has been well described.14, 25
There are several strengths of this study. This is the first study, to our knowledge, to look at adverse outcomes associated with GERD following ALTE, despite GERD being the most commonly attributed cause. The use of Intermountain Healthcare's electronic medical record system allowed for comprehensive tracking, over an extensive follow‐up period (median of 7.8 years), across 20 hospitals and EDs which care for the vast majority of pediatric patients in Utah. Finally, this large cohort of ALTE patients used clinical data from medical records and not only administrative data.
There are limitations of this study. This is a retrospective cohort study. Some of our study outcomes may be a result of pathophysiology other than GERD and, conversely, GERD may be a result of other issues (neurologic impairment). The small sample size and low percentage of the study outcomes make it possible that we did not detect true risk factors. Patients were lost to follow‐up if they moved or presented to a hospital not within the Intermountain Healthcare system. This study has slightly different patient numbers from 3 previously published studies for different outcomes on this cohort, as exclusion criteria for the different cohorts were different.14, 26, 27 Six patients had only their electronic medical record reviewed because the paper chart was missing.
IMPLICATIONS
The results of this study extend previous work of various outcomes regarding well‐appearing infants following an ALTE.14, 26, 27 In these studies, 3.9% and 3% were ultimately diagnosed with epilepsy and developmental delay, respectively; 1.4% were diagnosed with abusive head trauma; and 0.6% required otolaryngologic surgical intervention. In these previous studies, there were few predictors of these outcomes, with testing demonstrating largely normal results during the index ALTE admission.
Our study helps clinicians place the outcomes of aspiration pneumonia, failure‐to‐thrive, and anti‐reflux surgery into the context of these other studies when discharging infants from the hospital after an ALTE. Collectively, these studies provide clinicians with the information that, in the setting of a well‐appearing infant, few diagnostic tests in their ALTE patients will yield a definitive diagnosis. Ultimately, close follow‐up with further investigations if symptoms recur will be an important part of diagnosing the etiology of the ALTE in these infants.
We found that well‐appearing infants with ALTE, regardless of attributed cause, are at low risk for adverse outcomes associated with GERD. Only the eventual development of neurologic impairment or an increased length of stay during index ALTE hospitalization was found to be predictive of these outcomes.
Acknowledgements
The following individuals have made substantive intellectual contributions to this study: conception and design (G.Z., J.L.B., W.D.J., C.G.M., R.S.), acquisition of data (G.Z., J.L.B.), analysis (G.Z., RS) and interpretation of data (G.Z., J.L.B., W.D.J., C.G.M., R.S.). In addition, all listed authors have contributed to either drafting the article or revising it critically for important intellectual content. Finally, all listed authors have given final approval of this version submitted for publication. The authors also acknowledge Chelsea Welch for her assistance in data collection.
Disclosures: This study was presented in part at the national Pediatric Academic Societies meetings in Vancouver, Canada, May 2010 and in Denver, CO, May 2011. This study was supported by a National Institutes of Child Health and Human Development (NICHD) grant for Dr Srivastava (K23 HD052553), and a National Institute on Drug Abuse (NIDA) grant for Dr Bonkowsky (K08 DA24753). This research was supported in part by the Children's Health Research Center, University of Utah. There are no conflicts of interest.
Apparent life‐threatening events (ALTEs) are frightening for the parent/guardian and represent a challenge for the healthcare provider. ALTEs are defined as worrisome episodes of any combination of apnea, color change, change in muscle tone, choking or gagging.1 ALTEs account for 0.6% to 0.8% of emergency department (ED) visits for children <12 months old,2, 3 have an average length of stay (LOS) of 4.4 days and an average cost of $15,000 per hospitalization.4
Gastroesophageal reflux disease (GERD) is common in infancy11 and also is the most commonly (in 31%55% of ALTE cases) attributed cause of ALTE.2, 4, 5 It has been speculated that chemosensitivity to gastric acid results in laryngospasm, bronchospasm, and apnea. However, several small studies have failed to prove a causal link between reflux episodes and apnea.69 Furthermore, although consensus guidelines for GERD have been developed,14 the clinical use of testing for GERD remains highly variable. A study of infants discharged with an ALTE (n = 12,067) from 36 children's hospitals in the United States revealed extensive variability in the use of pH probes and upper gastrointestinal x‐ray series to diagnose GERD.4
The incidence of adverse outcomes associated with GERD after an ALTE remains unknown. It is also unknown whether an association exists between long‐term gastrointestinal (GI) outcomes and testing demonstrative of GERD or a diagnosis of GERD during hospitalization for ALTE. The primary objective of our study was to determine, in patients with an ALTE, the adverse outcomes associated with GERD (failure‐to‐thrive, aspiration pneumonia, and/or anti‐reflux surgery), the incidence of readmission for second ALTE, and death. Our secondary objective was to determine risk factors for adverse outcomes associated with GERD following an ALTE.
METHODS
Design
This was a retrospective cohort study. We reviewed electronic and paper medical charts of all infants <12 months of age admitted for ALTE between January 1, 1999 and December 31, 2003 to Primary Children's Medical Center in Salt Lake City, UT, which serves as the tertiary pediatric center for >1 million children and the primary facility for >270,000 children in Salt Lake County, UT.10 Primary Children's Medical Center is operated by a vertically integrated not‐for‐profit healthcare system (Intermountain Healthcare), which has 20 affiliated hospitals and EDs. The study was approved by the institutional review boards of the University of Utah and Intermountain Healthcare, and the privacy board of Intermountain Healthcare.
Participants
Patients were included if a computer search of ED chief complaint or hospital discharge diagnoses found one or more of the following keywords (or corresponding International Classification of Diseases, Ninth Revision [ICD‐9] codes if applicable): ALTE, altered mental status, apnea, breath‐holding spell, choking, GERD, hypotonia, lethargy, other convulsions, other neurologic diagnosis, other respiratory diagnosis, pallor, seizures, sleep apnea, stiff, syncope, and unresponsiveness. These diagnoses were chosen as potential proxy diagnoses or codes for possible ALTE, as ALTE did not have a corresponding ICD‐9 code at the time of this study.
Detailed review of the medical record included infants who were <12 months old at admission with a history consistent with ALTE, defined as an episode frightening to the observer with any combination of apnea, color change, change in muscle tone, choking, or gagging. Infants were excluded from the study if they had a previously documented underlying medical condition to explain the ALTE (such as a known seizure disorder) or had a clearly apparent diagnosis upon initial history and physical examination (such as bronchiolitis diagnosed in the emergency department) that would explain the event. Patients with unstable vital signs (eg, hypotension), trauma clearly apparent on admission, documented medication dosing error, or febrile seizure were also excluded. A complete list of exclusion criteria is found in Figure 1.

All hospital admissions, ED visits, and Pediatric GI clinic notes were reviewed for adverse outcomes associated with GERD, second ALTE admission, and death. The follow‐up time period included the original enrollment period (January 1, 1999 through December 31, 2003) through August 31, 2009.
Outcomes
Adverse outcomes associated with GERD were defined as aspiration pneumonia, failure‐to‐thrive (FTT; either admission or discharge diagnosis of FTT at another hospitalization, or follow‐up to gastroenterology clinic for FTT) and/or anti‐reflux surgery (Nissen fundoplication or gastrojejunal tube placement) as these are potential clinical consequences of having severe and uncontrolled GERD. We further collected readmission data for a second ALTE. Deaths and the attributed reasons were also collected.
Risk Factors
Potential risk factors for adverse outcomes associated with GERD (all during index hospitalization) included: age; prematurity; gender; previous event (as described by the parent, without previous ALTE hospitalization); primary discharge diagnosis of GERD; testing positive for reflux on index ALTE admission (upper GI x‐ray series, esophageal pH probe, swallow study, endoscopy, and/or consultation of pediatric gastroenterologist with results or assessments indicating gastroesophageal reflux); any anti‐reflux medication prescribed upon discharge; and LOS. We also considered diagnosis of neurologic impairment during follow‐up, which was defined as seizures or diagnosis of developmental delay from any etiology not recognized on index ALTE admission. We examined these risk factors as we postulated they might indicate higher risk for both ALTE and adverse outcomes associated with GERD, or might indicate a higher severity of initial event.
Analyses
Summary statistics were performed for adverse outcomes associated with GERD, readmission, and death. Univariate analyses were performed for risk factors using chi‐square tests for dichotomous predictors and Wilcoxon rank sum tests for nonparametric continuous predictors for any of the 3 adverse outcomes (FTT, aspiration pneumonia [AP], and/or anti‐reflux surgery) associated with GERD. All analyses were performed using SAS 9.13 (Carey, NC).
RESULTS
Eleven hundred forty‐eight infants with ALTE met inclusion criteria, from 187,903 patients meeting initial search criteria. Six hundred seventy‐one patients were excluded and 8 patients had missing charts. The study population of the 469‐patient cohort is shown in Figure 1.
Demographics are displayed in Table 1. The mean age was 65 days. One hundred three (22%) were premature. One hundred eighty‐nine patients (40%) had a primary discharge diagnosis of GERD; details of the diagnoses for the remaining patients are in Figure 1. Median length of follow‐up for the cohort was 7.8 years. The entire study period was 10.7 years.
| ALTE Cohort | |
|---|---|
| N = 469 | |
| |
| Female | 233 (49.7%) |
| Race | |
| Caucasian | 371 (79.1%) |
| Hispanic | 64 (13.6%) |
| Pacific Islander | 6 (1.3%) |
| Black | 4 (0.8%) |
| Other/unknown | 24 (5.1%) |
| Mean age in days (SD) | 65.2 (69.5) |
| Prematurity | 103 (22%) |
| Underwent testing for gastroesophageal reflux | 214 (45.6%) |
| Discharged on anti‐reflux medication | 238 (50.7%) |
| Previous event | 127 (27.1%) |
| Mean length of stay in days (SD) | 2.4 (2.4) |
| Later neurologic impairment (seizures or developmental delay) | 23 (4.9%) |
| Primary discharge diagnosis of GERD | 189 (40%) |
Eighteen patients (3.8%) had an adverse outcome associated with GERD. Four (0.9%) had aspiration pneumonia, 9 (1.9%) had failure‐to‐thrive, and 7 (1.5%) had a Nissen fundoplication (no patients had a gastrojejunal tube placed). Five patients had a gastrostomy tube placed at the time of fundoplication. Two patients had more than 1 adverse GI outcome; 1 patient had aspiration pneumonia and another had failure‐to‐thrive prior to their Nissen fundoplications.
Fifty‐six patients (11.9%) were readmitted for a second ALTE. Median time from index ALTE to second ALTE admission was 16.5 days (interquartile range: Q1, 8Q3, 32). Two (0.4%) patients died. Both (occurring at 18 months and 5.5 years after the initial ALTE hospitalization) were related to the children first developing seizure disorders and severe developmental delay. Neither of the patients who died had an index discharge diagnoses of GERD.
There was no significance of the following variables in predicting adverse outcomes associated with GERD: age, prematurity, gender, previous event, testing positive for reflux, primary discharge diagnosis of GERD, or discharge on anti‐reflux medications (see Table 2). Patients with adverse outcomes associated with GERD had longer mean LOS on the index ALTE hospitalization (4.3 days vs 2.4 days; P = 0.03) and a higher rate of neurologic impairment diagnosed in follow‐up (16.7% vs 4.4%; P = 0.02) than patients without long‐term adverse GI outcomes. Patients with neurological impairment diagnosed in follow‐up were more likely to eventually develop an adverse outcome associated with GERD, compared to patients without neurological impairment (odds ratio 8.4; 95% confidence interval 1.1516.1).
| AP, FTT, or Surgery | No Long‐Term Adverse GI Outcome | P Value | |
|---|---|---|---|
| N = 18 | N = 451 | ||
| |||
| Mean age in days (SD) | 51.9 (76.4) | 65.8 (69.3) | 0.27 |
| Prematurity | 1 (5.6%) | 102 (22.6%) | 0.08 |
| Male gender | 12 (66.7%) | 220 (48.8%) | 0.14 |
| Previous ALTE‐like event (no hospitalization) | 8 (44.4%) | 119 (26.4%) | 0.09 |
| Testing positive for reflux | 9 (50%) | 177 (39.3%) | 0.36 |
| Discharge diagnosis GERD | 9 (50%) | 180 (39.9%) | 0.39 |
| Discharged on anti‐reflux medication | 9 (50%) | 229 (50.7%) | 0.95 |
| Mean length of stay in days (SD) | 4.3 (4.7) | 2.4 (2.3) | 0.03 |
| Neurologic impairment diagnosed in follow‐up | 3 (16.7%) | 20 (4.4%) | 0.02 |
DISCUSSION
Our study had 2 main findings. First, infants admitted for an ALTE had a low percentage (3.8%) of adverse outcomes associated with GERD. Review of the literature provides little context to interpret this percentage. One study reports 9 per 100,000 of the general population <18 years of age having anti‐reflux surgery.24 The percentage of adverse outcomes associated with GERD converted to a rate in our study would likely reflect the bias of our center serving as a referral population for Utah and 5 surrounding states. Furthermore, there may be an additional bias of ALTE being a potential indication for anti‐reflux surgery for some clinicians, as well as confounding additional diagnoses (such as later neurologic impairment which might independently increase risk of study outcomes).
The second main finding of our study is that the development of neurologic impairment was predictive of developing adverse GI outcomes. As previous studies have shown that neurological impairment cannot be predicted during the initial ALTE hospitalization,14 adverse outcomes associated with GERD are similarly not predictable with current clinical approaches. Furthermore, the exact nature of the relationship between neurologic impairment and ALTEs remains unclear. While previous studies have described the increased prevalence of adverse neurological outcomes (such as seizures, developmental delay) in children who have had an ALTE, it is unclear what the precipitating reason for ALTE is in these infants (seizure, central apnea, GERD, etc).14
There is ongoing debate in the literature surrounding the optimal diagnosis of GERD in infants with ALTE. Recent guidelines state that investigations aimed to prove GERD causing an ALTE should include pH probe or impedance monitor testing, in combination with a sleep study, and discourages a GERD diagnosis based on upper GI‐series alone.14 Given the low use of pH probe and impedance monitoring at our institution during the study period (86% of the patients who had GI‐related testing had an upper GI‐series), we did not attempt to find the sensitivity or specificity of the different GI testing modalities for GERD in the setting of ALTE. The high use of upper‐GI series is not unique to our institutionone large study examining practice variation from 12,067 ALTE admissions in 36 children's hospitals, with 36.9% of infants (n = 4453) having a primary discharge diagnosis of gastroesophageal reflux, revealed that only 8.9% (SD 28%) received an esophageal pH probe, while 25.6% (SD 43.6%) had an upper GI‐series or swallow study.4
Given the difficulties in assigning a GERD diagnosis for ALTE infants, we focused on the long‐term adverse outcomes associated with GERD for the entire ALTE infant cohort. The 3 adverse outcomes we chose deserve some mention. Aspiration pneumonia is generally due to either primary aspiration (from dysfunctional swallowing) or secondary aspiration (from GERD). Failure‐to‐thrive can be due to ongoing GERD. Anti‐reflux surgery is often performed for severe GERD. While we believe that a prospective study with better diagnostic evaluations for GERD and apnea (such as pH probe or impedance monitor in combination with a sleep study) might help elucidate the unclear relationship between reflux episodes and ALTE, the low percentage of adverse outcomes associated with GERD after ALTE may suggest that such a study would be difficult both in terms of sample size and an unnecessary use of resources.
We also found that a high percentage (11.9%) of all patients had readmission for a second ALTE. This was substantially higher than the 2.5% readmission rate for second ALTE reported in a previous study of short‐term follow‐up (30 days).4 The number of readmissions in our study might be higher due to our comprehensive follow‐up, both in length of time and number of additional EDs and hospitals captured. Unfortunately, the retrospective nature of our study makes it difficult to determine if any interventions (prescription of anti‐reflux medication, education on reflux precautions) impacted the rate of readmission, as compliance was not measurable. Further studies should address why patients return with recurrent ALTE.
Interestingly, several potential risk factors did not predict long‐term adverse GI outcomes. For example, prematurity, a discharge diagnosis of GERD, or prescription of an anti‐reflux medication, were not associated with adverse GI outcomes. These findings support the concept that a diagnosis of GERD, at least as is commonly applied, is not meaningful in the setting of an ALTE. We did find associations with longer length of stay (LOS), and with eventual development of neurological impairment. Longer LOS might be a proxy for other subtle predictors that could influence adverse outcomes, such as requiring additional diagnostic tests prolonging hospitalization, or continued ALTEs while inpatient. The neurologic outcomes of patients with ALTE have been previously published, and the strong correlation between neurologic impairment and GERD has been well described.14, 25
There are several strengths of this study. This is the first study, to our knowledge, to look at adverse outcomes associated with GERD following ALTE, despite GERD being the most commonly attributed cause. The use of Intermountain Healthcare's electronic medical record system allowed for comprehensive tracking, over an extensive follow‐up period (median of 7.8 years), across 20 hospitals and EDs which care for the vast majority of pediatric patients in Utah. Finally, this large cohort of ALTE patients used clinical data from medical records and not only administrative data.
There are limitations of this study. This is a retrospective cohort study. Some of our study outcomes may be a result of pathophysiology other than GERD and, conversely, GERD may be a result of other issues (neurologic impairment). The small sample size and low percentage of the study outcomes make it possible that we did not detect true risk factors. Patients were lost to follow‐up if they moved or presented to a hospital not within the Intermountain Healthcare system. This study has slightly different patient numbers from 3 previously published studies for different outcomes on this cohort, as exclusion criteria for the different cohorts were different.14, 26, 27 Six patients had only their electronic medical record reviewed because the paper chart was missing.
IMPLICATIONS
The results of this study extend previous work of various outcomes regarding well‐appearing infants following an ALTE.14, 26, 27 In these studies, 3.9% and 3% were ultimately diagnosed with epilepsy and developmental delay, respectively; 1.4% were diagnosed with abusive head trauma; and 0.6% required otolaryngologic surgical intervention. In these previous studies, there were few predictors of these outcomes, with testing demonstrating largely normal results during the index ALTE admission.
Our study helps clinicians place the outcomes of aspiration pneumonia, failure‐to‐thrive, and anti‐reflux surgery into the context of these other studies when discharging infants from the hospital after an ALTE. Collectively, these studies provide clinicians with the information that, in the setting of a well‐appearing infant, few diagnostic tests in their ALTE patients will yield a definitive diagnosis. Ultimately, close follow‐up with further investigations if symptoms recur will be an important part of diagnosing the etiology of the ALTE in these infants.
We found that well‐appearing infants with ALTE, regardless of attributed cause, are at low risk for adverse outcomes associated with GERD. Only the eventual development of neurologic impairment or an increased length of stay during index ALTE hospitalization was found to be predictive of these outcomes.
Acknowledgements
The following individuals have made substantive intellectual contributions to this study: conception and design (G.Z., J.L.B., W.D.J., C.G.M., R.S.), acquisition of data (G.Z., J.L.B.), analysis (G.Z., RS) and interpretation of data (G.Z., J.L.B., W.D.J., C.G.M., R.S.). In addition, all listed authors have contributed to either drafting the article or revising it critically for important intellectual content. Finally, all listed authors have given final approval of this version submitted for publication. The authors also acknowledge Chelsea Welch for her assistance in data collection.
Disclosures: This study was presented in part at the national Pediatric Academic Societies meetings in Vancouver, Canada, May 2010 and in Denver, CO, May 2011. This study was supported by a National Institutes of Child Health and Human Development (NICHD) grant for Dr Srivastava (K23 HD052553), and a National Institute on Drug Abuse (NIDA) grant for Dr Bonkowsky (K08 DA24753). This research was supported in part by the Children's Health Research Center, University of Utah. There are no conflicts of interest.
- Infantile apnea and home monitoring.NIH Consensus Statement 1986 Sep 29‐Oct 1.Pediatrics.1987;79(2):292–299.
- ,.Causes of apparent life threatening events in infants: a systematic review.Arch Dis Child.2004;89:1043–1048.
- ,.Parental reported apnea, admissions to hospital and sudden infant death syndrome.Acta Paediatr.2001;90(4):417–422.
- ,,,.Variation in inpatient resource utilization and management of apparent life‐threatening events.J Pediatr.2008;152(5):629–635.
- ,,,,,.Discharge diagnoses in infants with apparent life‐threatening event.Pediatr Int.2003;45:560–563.
- ,,,,,.Lack of temporal relation between acid reflux in the proximal oesophagus and cardiorespiratory events in sleeping infants.Eur J Pediatr.1992;151(3):208–212.
- ,,, et al.Patterns of gastroesophageal reflux (GER) in patients with apparent life‐threatening events.J Pediatr Gastroenterol Nutr.1989;8(2):157–160.
- ,,, et al.Characteristics of continuous esophageal pH‐metering in infants with gastroesophageal reflux and apparent life‐threatening events.Eur J Pediatr Surg.1995;5(3);136–138.
- ,,,,.Apnea is not prolonged by acid gastroesophageal reflux in preterm infants.Pediatrics.2005;116:1059–1063.
- ,.Organizational responses to managed care: issues for academic health centers and implications for pediatric programs.Pediatrics.1998;101(4):805–811.
- ,,, et al.Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN).J Pediatr Gastroenterol Nutr.2009;49:498–547.
- ,,, et al.Prevalence and natural history of gastroesophageal reflux: pediatric prospective study.Pediatrics.2009;123(3):779–783.
- ,.Systematic review: the estra‐oesophageal symptoms of gastro‐oesophageal reflux disease in children.Aliment Pharmacol Ther.2009;29:258–272.
- ,,,.Death, child abuse, and adverse neurological outcomes of infants after an apparent life‐threatening event.Pediatrics.2008;122:125–131.
- ,,, et al.Abusive head injury as a cause of apparent life‐threatening events in infancy.Arch Pediatr Adolesc Med.2003;157:1011–1015.
- ,,.Accidental and nonaccidental poisonings as a cause of apparent life‐threatening events in infants.Pediatrics.2008;122:e359–e362.
- ,,,.Yield of diagnostic testing in infants who have had an apparent life‐threatening event.Pediatrics.2005;115:885–893.
- ,,, et al.Guidelines for evaluation and treatment of gastroesophageal reflux in infants and children: recommendations of the North American Society for Pediatric Gastroenterology and Nutrition.J Pediatr Gastroenterol Nutr.2001;32(suppl 2):S1–S31.
- ,,,.Comparison of barium radiology with esophageal pH monitoring in the diagnosis of gastroesophageal reflux disease.Am J Gastroenterol.1996;91(6):1181–1195.
- ,,, et al.Gastroesophageal reflux disease: correlation of esophageal pH testing and radiographic finding.Radiology.1992;185:483–486.
- ,,, et al.Gastro‐esophageal reflux demonstrated by radiography in infants less than 1 year of age. Comparison with pH monitoring.Acta Radiol.2003;44:136–138.
- ,,, et al.Double‐blind placebo controlled trial of omeprazole in irritable infants with gastroesophageal reflux.J Pediatr.2003;143:219–223.
- ,,, et al.Clinical predictors of pathological gastro‐oesophageal reflux in infants with persistant distress.J Paediatr Child Health.2006;42:134–139.
- ,,.National trends in the use of anti‐reflux procedures for children.Pediatrics.2006;118:1828–1835.
- ,,, et al.Prevalence and severity of feeding and nutritional problems in children with neurological impairment: Oxford feeding study.Dev Med Child Neurol.2000;42:674–680.
- ,,.Abusive head trauma in children presenting with an apparent life‐threatening event.J Pediatr.2010;157(5):821–825.
- ,,.Usefulness of airway evaluation in children initially seen with apparent life‐threatening event.Arch Otolaryngol Head Neck Surg.2011;137(4):359–362.
- Infantile apnea and home monitoring.NIH Consensus Statement 1986 Sep 29‐Oct 1.Pediatrics.1987;79(2):292–299.
- ,.Causes of apparent life threatening events in infants: a systematic review.Arch Dis Child.2004;89:1043–1048.
- ,.Parental reported apnea, admissions to hospital and sudden infant death syndrome.Acta Paediatr.2001;90(4):417–422.
- ,,,.Variation in inpatient resource utilization and management of apparent life‐threatening events.J Pediatr.2008;152(5):629–635.
- ,,,,,.Discharge diagnoses in infants with apparent life‐threatening event.Pediatr Int.2003;45:560–563.
- ,,,,,.Lack of temporal relation between acid reflux in the proximal oesophagus and cardiorespiratory events in sleeping infants.Eur J Pediatr.1992;151(3):208–212.
- ,,, et al.Patterns of gastroesophageal reflux (GER) in patients with apparent life‐threatening events.J Pediatr Gastroenterol Nutr.1989;8(2):157–160.
- ,,, et al.Characteristics of continuous esophageal pH‐metering in infants with gastroesophageal reflux and apparent life‐threatening events.Eur J Pediatr Surg.1995;5(3);136–138.
- ,,,,.Apnea is not prolonged by acid gastroesophageal reflux in preterm infants.Pediatrics.2005;116:1059–1063.
- ,.Organizational responses to managed care: issues for academic health centers and implications for pediatric programs.Pediatrics.1998;101(4):805–811.
- ,,, et al.Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN).J Pediatr Gastroenterol Nutr.2009;49:498–547.
- ,,, et al.Prevalence and natural history of gastroesophageal reflux: pediatric prospective study.Pediatrics.2009;123(3):779–783.
- ,.Systematic review: the estra‐oesophageal symptoms of gastro‐oesophageal reflux disease in children.Aliment Pharmacol Ther.2009;29:258–272.
- ,,,.Death, child abuse, and adverse neurological outcomes of infants after an apparent life‐threatening event.Pediatrics.2008;122:125–131.
- ,,, et al.Abusive head injury as a cause of apparent life‐threatening events in infancy.Arch Pediatr Adolesc Med.2003;157:1011–1015.
- ,,.Accidental and nonaccidental poisonings as a cause of apparent life‐threatening events in infants.Pediatrics.2008;122:e359–e362.
- ,,,.Yield of diagnostic testing in infants who have had an apparent life‐threatening event.Pediatrics.2005;115:885–893.
- ,,, et al.Guidelines for evaluation and treatment of gastroesophageal reflux in infants and children: recommendations of the North American Society for Pediatric Gastroenterology and Nutrition.J Pediatr Gastroenterol Nutr.2001;32(suppl 2):S1–S31.
- ,,,.Comparison of barium radiology with esophageal pH monitoring in the diagnosis of gastroesophageal reflux disease.Am J Gastroenterol.1996;91(6):1181–1195.
- ,,, et al.Gastroesophageal reflux disease: correlation of esophageal pH testing and radiographic finding.Radiology.1992;185:483–486.
- ,,, et al.Gastro‐esophageal reflux demonstrated by radiography in infants less than 1 year of age. Comparison with pH monitoring.Acta Radiol.2003;44:136–138.
- ,,, et al.Double‐blind placebo controlled trial of omeprazole in irritable infants with gastroesophageal reflux.J Pediatr.2003;143:219–223.
- ,,, et al.Clinical predictors of pathological gastro‐oesophageal reflux in infants with persistant distress.J Paediatr Child Health.2006;42:134–139.
- ,,.National trends in the use of anti‐reflux procedures for children.Pediatrics.2006;118:1828–1835.
- ,,, et al.Prevalence and severity of feeding and nutritional problems in children with neurological impairment: Oxford feeding study.Dev Med Child Neurol.2000;42:674–680.
- ,,.Abusive head trauma in children presenting with an apparent life‐threatening event.J Pediatr.2010;157(5):821–825.
- ,,.Usefulness of airway evaluation in children initially seen with apparent life‐threatening event.Arch Otolaryngol Head Neck Surg.2011;137(4):359–362.
Copyright © 2012 Society of Hospital Medicine