User login
Quality of Life of Children with NI after Fundoplication for GERD
Aspiration pneumonia is the most common cause of death in children with severe neurological impairment (NI).13 For several reasons (eg, improved survival of extremely low‐birth‐weight infants, technological advances, and changing societal attitudes), the number of children with severe NI is increasing. Many children with severe NI have dysfunctional swallowing and gastroesophageal reflux disease (GERD).46 This combination places them at high risk for recurrent aspiration that, in turn, leads to aspiration pneumonia, repeated hospitalization, respiratory failure, compromised quality of life, and death.7, 8
The most common treatment approach for the combination of dysfunctional swallowing and GERD is surgical fundoplication with a gastrostomy feeding tube. Fundoplication is the third most common procedure performed in children by pediatric surgeons in the United States.9 Fifty percent of the children who receive a fundoplication have neurological impairment.10, 11 The goals of the surgery to treat GERD unresponsive to medical management are to reduce the risk of aspiration pneumonia, improve nutritional status, and improve the quality of life of the children and their families. However, few prospective longitudinal studies have determined whether the quality of life of the children or their caregivers actually improves over time.
The importance of caregiver and child quality of life is increasingly recognized as a critical outcome of any intervention in this population.12, 13 Previous studies of fundoplication in children with NI, GERD, and dysfunctional swallowing reported surgical mortality rates between 1% and 3% and other complications between 4% and 39%, reflecting the medical fragility of these children.5, 1418 Some of these studies had longitudinal follow‐up and reported long‐term data. Recurrence of symptoms was reported in up to 56% of patients, recurrence of AP in up to 39%, further surgical procedures in up to 19%, and mortality in up to 17%.14, 1921 Few case series of children with neurological impairment who have had a fundoplication have addressed child and caregiver quality of life following either a fundoplication or placement of a feeding tube.2224 In their study of 16 patients who had a fundoplication and gastrostomy tube placed, Tawfik et al. found improvements in children's happiness, ease of giving medicines, and time to devote to other children. Sullivan et al. found improvement in caregiver quality of life following placement of a gastrostomy tube in a child; however, they did not specifically identify those children who had been treated with a fundoplication. In their retrospective study, O'Neill et al. found improved child and caregiver quality of life following a fundoplication. Collectively, these studies have found that parents report improvement of both their own and their child's quality of life after either intervention. However, not having baseline measurements, not controlling for degree of functional impairment of the children, small sample sizes, and large loss to follow‐up limit the utility of these studies. In this ongoing, long‐term prospective longitudinal study, we report the initial impact of a fundoplication on the quality of life of both children and their caregivers.
The primary objective of this study was to determine change over time in the quality of life of children with neurological impairment who received surgical treatment of their GERD and of the caregivers of these children, controlling for the degree of functional impairment of the children. We hypothesized that child and caregiver quality of life would both improve after primary fundoplication and gastrostomy tube placement. Secondary objectives included describing rates of complications in this population.
METHODS
Setting and Study Population
We enrolled patients from newborn to 18 years of age who had a diagnosis compatible with neurological impairment and who received their first fundoplication for GERD between January 2005 and July 2006 at Primary Children's Medical Center (PCMC), in Salt Lake City, Utah. PCMC is a 232‐bed children's hospital in the Intermountain West owned and operated by Intermountain Healthcare, Inc., a large vertically integrated health care delivery system that serves as both the primary hospital for Salt Lake County and as the tertiary‐care hospital for 4 additional states (Wyoming, Nevada, Idaho, and Montana).25 Patients who had a previous gastrojejunal feeding tube were excluded as were patients who had a previous fundoplication, as these procedures may have biased their reported quality of life, our main outcome measure.
Patients were included in the study if they had GERD (based on clinical history or testing) that had been refractory to medical management (defined as continued gastroesophageal reflux symptoms despite antireflux medications). GERD was defined using the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) criteria.26 These include: the presence of clinical symptoms and at least 1 abnormal result from an upper gastrointestinal x‐ray series (recognizing that this test is neither sensitive nor specific for reflux), pH probe, upper gastrointestinal endoscopy with biopsy, nuclear medicine scan, or a modified barium swallow. As this was a prospective observational study, physicians were allowed to order testing as their practice dictated. Patients were excluded if they had neurological impairment but lacked objective documentation of GERD using the NASPGHAN recommendations, unless there were obvious clinical indications such as witnessed vomiting and aspiration (N = 3). No patient received a prophylactic fundoplication (fundoplication without documented GERD).
Study Design
This is an ongoing prospective longitudinal study. Patients who had a first fundoplication at PCMC were identified by the surgical service, with weekly lists shared with the research team. Patients were approached by a research assistant during that initial hospitalization to see if they met inclusion criteria for the study using data from the medical records and surgical team when necessary.
Data Variables and Sources
Indications for the fundoplication, performance and results of diagnostic testing for GERD, complications of the fundoplication, and reasons for the neurological impairment were obtained through review of the electronic and paper medical records. Mortality data, subsequent emergency department visits, and admissions to the hospital were obtained using Intermountain Healthcare's electronic data warehouse, which merges clinical, financial, and administrative data including the Utah Vital Statistics database.
Neurological impairment was defined from 2 sources: (1) clinical diagnoses as identified by providers and (2) International Classification of Diseases Codes Modified, version 9 (ICD‐9 CM) identified a priori as indicating neurological impairment.
Instruments and Study Outcomes
Functional status was measured using the WeeFIM. This instrument has been tested and shown to be valid and reliable for children more than 6 months old with neurodevelopmental disabilities including spina bifida and Down syndrome.2732 WeeFIM is a self‐administered parent instrument composed of 18 items and 6 domains (self‐care, sphincter control, transfers, locomotion, communication, and social cognition).33 The WeeFIM allows patients to be stratified into areas of function from severely impaired to normal.
The primary outcome was child quality of life as measured by the Child Health Questionnaire Parental Form 50 (CHQ‐PF50). Caregiver quality of life was measured using the Short‐Form Health Status Survey (SF‐36) and Parenting Stress Index/Short Form (PSI/SF). The CHQ‐PF50 is a self‐administered parent questionnaire of 50 questions that measures 6 domains, including physical function and abilities, pain and discomfort, general health perception, behavior, temperament and moods, and satisfaction with growth and development.34 This instrument has been tested for validity and reliability in children with cerebral palsy.35 The SF‐36 is a widely accepted measure of health status that measures 8 domains of health: physical functioning, role limitations due to physical problems, bodily pain, general health, vitality, social functioning, role limitations due to emotional problems, and mental health. The SF‐36 has been well studied and has been used to measure the effect on a caregiver's quality of life associated with caring for a chronically ill child with significant medical problems.36, 37 Higher scores in each domain of both the CHQPF50 and the SF‐36 reflect better quality of life. Caregiver stress was measured using the PSI/SF (Psychological Assessment Resources Inc, Odessa, FL).38 In the PSI/SF a parent rates the parentchild dyad on 36 items that are summarized in 3 subscales: parental distress, parentchild dysfunctional interaction, and difficult child. A parent scores each item as strongly agree, agree, not sure, disagree, or strongly disagree. The sum of the 3 subscale scores is the total stress score. Higher scores denote a greater degree of stress. The PSI/SF has been validated in several studies for caregivers of children with chronic diseases.3943
The CHQ‐PF50, SF‐36, and PSI/SF questionnaires and the WeeFIM functional status measure were administered to each study patient and caregiver in person at enrollment (baseline) and by mail 1 month after fundoplication. A follow‐up postcard reminder was mailed 1 week after the initial mailing. Second and final mailings were sent to nonresponders 3 and 5 weeks, respectively, after the initial mailing.
Secondary outcomes included rates of complications including failure of the fundoplication. Complications were defined as a subsequent emergency department visit, hospitalization, or death related to the surgery, gastrostomy tube, or aspiration pneumonia. Failures were defined as a second fundoplication or the insertion of a gastrojejunal feeding tube as nonsurgical management of recurrent GERD and/or paraesophageal hernia. Secondary outcomes were followed from the time of the first fundoplication until 1 month after surgery.
Analyses
The differential effect of the fundoplication on the quality of life measures was assessed and quantified through statistical analysis. Because the primary interest was to measure change in baseline characteristics over time, repeated‐measures models were used to compare the group relative to changes in functional status. In particular, changes from baseline values were modeled 1 month after the procedure. The Kenward‐Roger approximation of degrees of freedom was used to compute P values from the overall tests.44 Repeated‐measures models were fit to the data using restricted maximum likelihood estimation. An autoregressive covariance matrix was assumed for the multiple measurements of each individual, thus limiting the number of restrictions forced by the model on the data. The repeated‐measures models used all the available data on participants, including those who dropped out of the study. To obtain the most accurate comparison of the study group, the covariate of functional status at baseline was taken into account in the fitted models. Statistical analyses were performed with SAS statistical software (version 9.13; SAS Institute, Cary NC). Student t tests were performed for comparison of means of the quality‐of‐life domains for the study cohort compared to either the general population or specific groups of patients for comparative purposes.
The study was approved by the institutional review boards of the University of Utah Health Sciences Center and Primary Children's Medical Center.
RESULTS
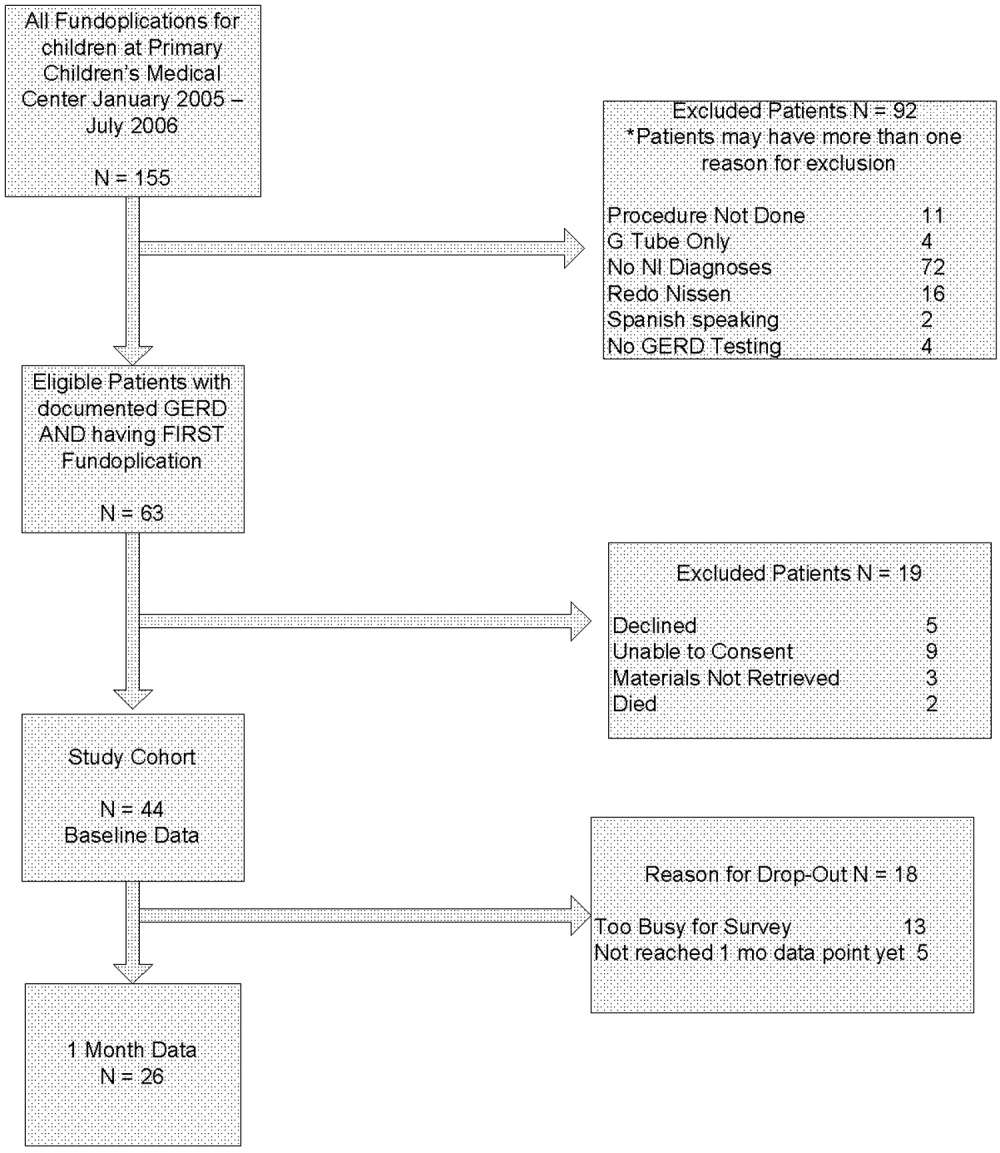
Sixty‐three children met eligibility criteria. Forty‐four families (70%) initially agreed to participate in the study and completed the baseline questionnaires (see Fig. 1). The mean age of the children was 2.2 years. Twenty‐six parents of children completed the 1‐month postfundoplication quality‐of‐life questionnaires. Thirteen patients were lost to follow‐up, 5 of whom had not reached the 1‐month postfundoplication time point. The median WeeFIM (functional status) score of the whole group was 31.2 (95% confidence interval [CI] 11‐71) compared with a childhood matched‐age norm of 83 (95% CI 60‐110), P = .001. WeeFIM scores did not change significantly from baseline to 1 month, P = .98 (Kruskall‐Wallis test).
Data for the 13 parents and children (30%) who gave baseline data but were subsequently lost to follow‐up are shown in Table 1. Reasons for loss to follow‐up were caregiver reporting being too busy to fill out the questionnaires (n = 8) and no reason stated (n = 5).
In addition to the diagnosis of GERD, clinical indications for fundoplication were vomiting (55%), feeding‐related issues (47%), and failure to thrive (39%). Diagnosis of GERD was confirmed for 41 of 44 patients77% had an abnormal upper GI, 26% an abnormal pH probe, 14% an abnormal endoscopy, and 24% an abnormal swallow study. The remaining 3 had obvious clinical symptoms for GERD and did not require further testing according to their attending surgeon (2 with witnessed vomiting leading to aspiration and 1 who was exclusively gastrostomy‐fed and was witnessed having feeds coming from the tracheostomy). Various medications had been tried and were considered to have failed in these patients: 39% had been treated with acid‐suppressive agents; 80% with acid blocking agents; and 61% with prokinetic agents. Fourteen patients (32%) had cerebral palsy, and 14 (32%) had a brain or spinal cord abnormality (see Table 2).
| Diagnostic category | ICD‐9 codes used |
|---|---|
| |
| Brain or spinal cord anomaly | 335.22, 742.0, 742.1, 742.2, 742.4, 742.53 |
| Cerebral palsy | 343.0, 343.1, 343.2, 343.8, 343.9, 344.00 |
| Hydrocephalus | 331.3, 331.4, 742.3 |
| Down syndrome | 758.0 |
| Seizures | 345.10, 345.11, 345.3, 345.41, 345.50, 345.81, 345.90, 345.91 |
| Muscular dystrophy or myopathy | 359.0, 359.1, 359.2, 359.9 |
| Nervous system anomaly | 742.8, 742.9 |
| Cerebral degeneration | 330.8, 331.9 |
| Chromosomal anomaly | 758.2, 758.3, 758.5, 758.89 |
| Infantile spasms | 345.60, 345.61 |
| Menial retardation | 317.0, 318.1, 318.2 |
| Spinal muscle atrophy | 335.0, 335.10 |
Thirty‐four children underwent a laparoscopic Nissen fundoplication, and 10 had an open Nissen fundoplication. All had gastrostomy tubes placed or replaced at the time of surgery.
Analysis of the mean bodily pain scores from the CHQ‐PF50 revealed that bodily pain of patients in the study cohort had improved from baseline after 1 month of follow‐up (mean score at baseline, 32.8; after 1 month of follow‐up, 47.5; P = .01), after adjusting for functional status. However, these mean bodily pain scores were significantly lower than those of children with cerebral palsy (mean score, 73.9; P < .001).34, 35 After adjusting for functional status, scores were improved for role/social‐physical limitations (mean baseline score, 30.6; 1‐month follow‐up score, 56.6; P = .01), mental health (mean baseline score, 62.7; 1‐month follow‐up score, 70.6; P = .01), family limitation of activities (mean baseline score, 43.3; 1‐month follow‐up score, 55.1; P = .03), and parental time (mean baseline score, 43.0; 1‐month follow‐up score, 55.3; P = .03). Scores were unchanged for physical function, global health, general health perception, physical summary, role/social‐emotional, mental health, self‐esteem, and psychological summary (see Table 3).
| Domain of Quality of Life | Baseline (Mean and SD) | 1‐Month Follow‐Up (Mean and SD) | P Value |
|---|---|---|---|
| Physical functioning | 19.3 (34.1) | 16.7 (30.8) | 0.77 |
| Role physical* | 30.6 (44.4) | 56.6 (40.5) | 0.01 |
| Bodily pain* | 32.8 (24.4) | 47.5 (25.7) | 0.01 |
| Global health | 42.0 (23.7) | 44.1 (22.6) | 0.19 |
| General behavior | 72.1 (29.3) | 78.7 (14.5) | 0.21 |
| Self‐esteem | 39.9 (21.1) | 32.8 (19.4) | 0.36 |
| Mental health | 62.7 (15.9) | 70.6 (16.6) | 0.01 |
| Family limitation of activity* | 43.3 (23.7) | 55.1 (21.3) | 0.03 |
| Parental time* | 43.0 (35.5) | 55.3 (32.5) | 0.03 |
| Physical summary | 23.1 (21.2) | 17.8 (13.9) | 0.17 |
| Psychological summary | 39.0 (11.8) | 39.6 (10.8) | 0.76 |
Analysis of the SF‐36 of the parents of these children revealed mean scores significantly lower than those in general U.S. population for all quality‐of‐life domains except physical function (see Table 4). Many baseline domain scores were similar to those of adults with clinical depression. The only domain that showed improvement in quality of life of the caregivers over the 1‐month follow‐up period was vitality (mean baseline score, 41.3; 1‐month follow‐up score, 48.2; P = .001).
| Quality‐of‐life domain | Study group mean (SD) | U.S. population norm mean (SD) | P value |
|---|---|---|---|
| |||
| Physical functioning | 89.35 (14.60) | 84.15 (23.26) | 0.10 |
| Role physical | 71.02 (39.96) | 80.96 (34.00) | 0.05 |
| Bodily pain | 82.50 (24.00) | 75.15 (23.69) | 0.04 |
| General Health* | 59.07 (18.75) | 71.95 (20.34) | 0.001 |
| Vitality* | 41.33 (19.49) | 60.86 (33.04) | 0.001 |
| Social functioning* | 63.33 (34.48) | 83.28 (22.69) | 0.001 |
| Role emotional | 60.60 (40.20) | 81.26 (33.04) | 0.001 |
| Mental health | 67.00 (19.61) | 74.74 (18.05) | 0.004 |
Total stress as measured by the PSI/SF mean was 79.1 at baseline and 77.6 1 month after fundoplication (P = .54). This was significantly higher stress than the parental norm of 71.0 (P = .01). One in 4 parents expressed clinically significant levels of stress (scores > 90, 90th percentile).
Patients suffered the following complications in the month after fundoplication. Eight children had at least 1 subsequent emergency department visit related to a complication of the gastrostomy tube (8 visits), to respiratory distress (1 visit), or tovomiting (1 visit). Seven children had a subsequent admission to the hospital related to a complication of the gastrostomy tube (4 admissions), complication of surgery (2 admissions), or aspiration pneumonia (1 admission). None of the children had a repeat fundoplication or subsequently underwent placement of a gastrojejunal feeding tube. One patient died. She was 10 months old when she died, which was 3 weeks after she had received a fundoplication. She had obstructive hydrocephalus, cortical blindness, and developmental delay, and respiratory arrest and subsequent tonsillar herniation led to her death.
DISCUSSION
Parents of children with neurological impairment and GERD who underwent their first fundoplication reported improved quality of life of their children in the domains of bodily pain, role/social‐physical limitations, mental health, family limitation of activities, and parental time over the first month after surgery, when controlling for the children's degree of functional impairment. The only significant similar improvement in the parent self‐reported quality of life was in the domain of vitality.
This study had several limitations. Loss to follow‐up may have led to a bias reflecting the phenomenon that patients who have poorer quality of life are less likely to report this, or even to be able to participate in the follow‐up component of a study like this. In survival analyses, this incomplete follow‐up of patients is called informative dropout and may be minimized by applying a statistical technique that accounts for this, using the Q‐TWiST.45 However, our current study design and analysis plan precluded using this methodology. As shown in Table 1, we did not find any differences between those patients who stayed in the study and those who dropped out. Also, we were able to contact most parents who reported being too busy to fill out the surveys. Patient heterogeneity is also a concern: Table 2 shows the wide array of diagnoses responsible for the children's neurological impairment. However, we used a standardized functional status measure to ensure we were analyzing similarly disabled patients. Also, the standard deviation of the mean WeeFIM score was small, implying little variability in the study cohort. Our study analyzed data from a single center, which reflects care in the western United States. However, our hospital is similar to other medium and large children's hospitals and our patient population similar to others that perform fundoplication for children with neurological impairment.46 We believe our findings are generalizable to other surgical centers that perform a similar volume of fundoplications in such children with NI.
Our study findings are similar to those reported by O'Neill et al., whose study found that parents reported improved quality of life of their children in ease of feedings, physical comfort during feeding, and ability of the child to enjoy life.23 The CHQPF50 does not specifically ask about feeding, but we did find similar improvement in the domain of role/social‐physical limitations. O'Neill et al. also found that after the children in their study received a fundoplication, caregivers reported their own quality of life improved in the areas of being able to spend more time caring for their child's needs, which is similar to our findings of fewer family limitations of activities and more parental time. Our findings were somewhat dissimilar to the O'Neill et al. study, as parents in their study found several additional areas of improvement in caregiver own quality of life. One explanation for the differing results may be differences in the populations studied. Parents in our study had SF‐36 scores for general health, vitality, and social functioning that were similar to those of adults with depression,47 whereas parents in the O'Neill study did not. Although the O'Neill et al. study was the first to examine these critical quality‐of‐life outcomes for children with NI who have received fundoplication, it had several methodological limitations. We have had the opportunity to build on the work of O'Neill et al. and in a prospective study to capture standardized baseline data (therefore not subject to recall bias, as was likely in the O'Neill et al. study) and collect long‐term data on this population. We also controlled for functional status, which did not improve over the 1 month and by itself could be responsible for the already poor caregiver quality of life. Some aspects of the children's care did improve, but perhaps not enough to overcome the severe disabilities the children and their caregivers live with on a daily basis. We found some evidence to support that the parents' PSI/SF scores were similar to those of parents of children with heart disease, other enterally fed children, and children with traumatic brain injury (who make up between 1 in 3 and 1 in 5 parents with severe stress).39, 41, 43 Future interventions should address the stress and quality of life of these caregivers, especially if surgery does not improve caregiver quality of life or decrease stress.
Contrary to an emerging body of literature in pediatrics that describes a positive correlation between the health of children with chronic illnesses and their caregivers' quality of life,12, 42 we did not find large immediate improvements in caregiver quality of life and decrease in stress as their children's quality of life improved. This may be related to the number of parents in our sample being too small to detect such changes or that changes in longer‐term (greater than 6 months or 1 year) quality of life not being reflected by short‐term assessment. Caregiver and child quality of life following fundoplication needs to be studied over the long term (eg, over many years). We are continuing to follow these patients and their families and will repeat the quality‐of‐life measures 6 and 12 months after fundoplication and report these findings.
Additional studies of treatments for neurologically impaired children with GERD are needed. Randomized trials of alternatives to fundoplication such as gastrojejunal feeding tubes have been proposed, with which we strongly agree.46, 48 We believe that any randomized, controlled trial of children with neurological impairment and GERD must measure child and caregiver quality of life and functional status outcomes. 0
| Variables | Study patients at baseline (N = 44) | Study patients at 1‐month follow‐up (N = 26) | P value |
|---|---|---|---|
| Functional Status Measure | |||
| WeeFIM Score | 24 | 36 | NS |
| Child CHQ‐PF50 Quality‐of‐Life Scores | |||
| Role physical | 30.6 | 56.6 | 0.01 |
| Bodily pain | 32.8 | 47.5 | 0.01 |
| Mental health | 62.7 | 70.6 | 0.01 |
| Family limitation of activity | 43.3 | 55.1 | 0.03 |
| Parental time | 43.0 | 55.3 | 0.03 |
| Global health | 42.0 | 44.1 | NS |
| Physical functioning | 19.3 | 16.7 | NS |
| General behavior | 72.1 | 78.7 | NS |
| Self‐esteem | 39.9 | 32.8 | NS |
| Role emotional | 27.1 | 37.1 | NS |
| Physical summary | 23.1 | 17.8 | NS |
| Psychological summary | 39.0 | 39.6 | NS |
| Caregiver SF‐36 Quality‐of‐Life Scores | |||
| Vitality | 41.3 | 46.9 | 0.001 |
| Role physical | 89.9 | 92.5 | NS |
| Bodily pain | 71.0 | 78.7 | NS |
| General health | 82.5 | 81.1 | NS |
| Social functioning | 59.1 | 59.5 | NS |
| Role emotional | 60.6 | 65.6 | NS |
| Mental health | 67.0 | 73.5 | NS |
| Parenting stress index | 79.1 | 77.7 | NS |
Acknowledgements
The authors thank Tanner Coleman and Matthew Swenson for their invaluable help in recruiting patients. Dr. Srivastava was supported in part by the Children's Health Research Center, University of Utah and Primary Children's Medical Center Foundation.
- ,,.Comparison of respiratory mortality in the profoundly mentally retarded and in the less retarded.J Ment Defic Res.1979;23(1):1–7.
- ,,.Cause of death in cerebral palsy: a descriptive study.Arch Dis Child.1999;81:390–394.
- .Survival rates of children with severe neurologic disabilities: a review.Semin Pediatr Neurol.2003;10(2):120–129.
- ,.Gastroesophageal reflux among severely retarded children.J Pediatr.1979;94:710–714.
- ,,,,,.Operation for gastro‐oesophageal reflux associated with severe mental retardation.Arch Dis Child.1993;68:347–351.
- ,,,,,.Prevalence and severity of feeding and nutritional problems in children with neurological impairment: Oxford Feeding Study.Dev Med Child Neurol.2000;42:674–680.
- ,,, et al.Aspiration pneumonia in pediatric age group: etiology, predisposing factors and clinical outcome.J Pak Med Assoc.1999;49(4):105–108.
- ,.Respiratory problems in children with neurological impairment.Arch Dis Child.2003;88(1):75–78.
- ,.Gastroesophageal reflux in childhood.Curr Probl Surg.1996;33(1):1–70.
- ,.Minimally invasive surgical techniques in reoperative surgery for gastroesophageal reflux disease in infants and children.Am Surg.2002;68:989–992.
- .Laparoscopic Nissen procedure in children.Semin Laparosc Surg.2002;9(3):146–152.
- ,,, et al.Caregiving process and caregiver burden: conceptual models to guide research and practice.BMC Pediatr.2004;4(1):1.
- ,.Theoretical and psychometric analysis of caregiver strain.Res Nurs Health.1996;19:499–510.
- ,,,,,.Complications and reoperation after Nissen fundoplication in childhood.Am J Surg.1987;153(2):177–183.
- ,,, et al.Surgical treatment of gastroesophageal reflux in children: a combined hospital study of 7467 patients.Pediatrics.1998;101:419–422.
- ,,,,,.Outcomes of surgical fundoplication in children.Clin Gastroenterol Hepatol.2004;2:978–984.
- ,,, et al.The respiratory advantage of laparoscopic Nissen fundoplication.J Pediatr Surg.2003;38:886–891.
- ,,,.Laparoscopic Nissen fundoplication in children: 2‐5‐year follow‐up.Pediatr Surg Int.2003;19:537–539.
- ,,.Recognition of recurrent gastroesophageal reflux following antireflux surgery in the neurologically disabled child: high index of suspicion and definitive evaluation.J Pediatr Surg.1992;27:983–988; discussion988–990.
- ,,.Sequelae of antireflux surgery in profoundly disabled children.J Pediatr Surg.1992;27(2):267–271; discussion271–263.
- ,,.Efficacy of the Nissen fundoplication in the management of gastroesophageal reflux following esophageal atresia repair.J Pediatr Surg.1993;28(1):53–55.
- ,,,.Caregivers' perceptions following gastrostomy in severely disabled children with feeding problems.Dev Med Child Neurol.1997;39:746–751.
- ,,,,.Care‐giver evaluation of anti‐gastroesophageal reflux procedures in neurologically impaired children: what is the real‐life outcome?J Pediatr Surg.1996;31:375–380.
- ,,, et al.Impact of gastrostomy tube feeding on the quality of life of carers of children with cerebral palsy.Dev Med Child Neurol.2004;46:796–800.
- ,.Organizational responses to managed care: issues for academic health centers and implications for pediatric programs.Pediatrics.1998;101:805–811; discussion811–802.
- Children's Digestive Health and Nutrition Foundation Website. Gastroesophageal Reflux Disease in the Neurologically Impaired Child. Available at: http://www.cdhnf.org/PractitionerSeries.asp. Accessed August 30,2006.
- ,,,,.Functional status of school‐aged children with Down syndrome.J Paediatr Child Health.2002;38(2):160–165.
- ,,,,,.Concurrent validity of the Functional Independence Measure for Children (WeeFIM) and the Pediatric Evaluation of Disabilities Inventory in children with developmental disabilities and acquired brain injuries.Phys Occup Ther Pediatr.2001;21(2–3):91–101.
- ,,, et al.The WeeFIM instrument: its utility in detecting change in children with developmental disabilities.Arch Phys Med Rehabil.2000;81:1317–1326.
- ,,, et al.Functional assessment and care of children with neurodevelopmental disabilities.Am J Phys Med Rehabil.2000;79(2):114–123.
- ,,,,,.Predictors of mortality, morbidity, and disability in a cohort of infants < or = 28 weeks' gestation.Clin Pediatr (Phila).1993;32:521–527.
- ,.Content validity of a pediatric functional independence measure.Appl Nurs Res.1990;3(3):120–122.
- ,,, et al.The Functional Independence Measure for Children (WeeFIM). Conceptual basis and pilot use in children with developmental disabilities.Clin Pediatr (Phila). Jul1994;33:421–430.
- ,,.The CHQ User's Manual.1st ed.Boston, MA:The Health Institute, New England Medical Center,1996.
- ,,,,,.Comparing reliability and validity of pediatric instruments for measuring health and well‐being of children with spastic cerebral palsy.Dev Med Child Neurol.2002;44:468–476.
- ,,,,.Needs of carers of severely disabled people: are they identified and met adequately?Health Soc Care Community.2001;9(4):235–243.
- ,,.The realities of postoperative disability and the carer's burden.Ann R Coll Surg Engl.2001;83(3):215–218.
- Abdidin.Parenting Stress Index.3rd ed.Lutz, FL:Psychological Assessment Resources, Inc.;1995.
- ,,,.Parental stress and burden following traumatic brain injury amongst children and adolescents.Brain Inj. Jan2003;17(1):1–23.
- ,,.Comparing stress levels of parents of children with cancer and parents of children with physical disabilities.Psychooncology. Dec2004;13(12):898–903.
- ,,.Stress levels experienced by the parents of enterally fed children.Child Care Health Dev.2004;30:507–513.
- ,,, et al.The health and well‐being of caregivers of children with cerebral palsy.Pediatrics.2005;115:e626–e636.
- ,.Parenting stress and children with heart disease.J Pediatr Health Care.2003;17(4):163–168.
- ,.Small sample inference for fixed effects from restricted maximum likelihood.Biometrics.1997;53:983–997.
- ,,.Methods for the analysis of quality‐of‐life and survival data in health technology assessment.Health Technol Assess.1999;3(10):1–152.
- ,,, et al.Fundoplication and gastrostomy versus image‐guided gastrojejunal tube for enteral feeding in neurologically impaired children with gastroesophageal reflux.J Pediatr Surg.2002;37:407–412.
- SF‐36 Health Survey: Manual and Interpretation Guide.Lincoln, RI:QualityMetric Inc.;1993,year="2000"2000.
- ,,.The role of protective antireflux procedures in neurologically impaired children: a decision analysis.J Pediatr Surg. Mar2002;37:500–506.
Aspiration pneumonia is the most common cause of death in children with severe neurological impairment (NI).13 For several reasons (eg, improved survival of extremely low‐birth‐weight infants, technological advances, and changing societal attitudes), the number of children with severe NI is increasing. Many children with severe NI have dysfunctional swallowing and gastroesophageal reflux disease (GERD).46 This combination places them at high risk for recurrent aspiration that, in turn, leads to aspiration pneumonia, repeated hospitalization, respiratory failure, compromised quality of life, and death.7, 8
The most common treatment approach for the combination of dysfunctional swallowing and GERD is surgical fundoplication with a gastrostomy feeding tube. Fundoplication is the third most common procedure performed in children by pediatric surgeons in the United States.9 Fifty percent of the children who receive a fundoplication have neurological impairment.10, 11 The goals of the surgery to treat GERD unresponsive to medical management are to reduce the risk of aspiration pneumonia, improve nutritional status, and improve the quality of life of the children and their families. However, few prospective longitudinal studies have determined whether the quality of life of the children or their caregivers actually improves over time.
The importance of caregiver and child quality of life is increasingly recognized as a critical outcome of any intervention in this population.12, 13 Previous studies of fundoplication in children with NI, GERD, and dysfunctional swallowing reported surgical mortality rates between 1% and 3% and other complications between 4% and 39%, reflecting the medical fragility of these children.5, 1418 Some of these studies had longitudinal follow‐up and reported long‐term data. Recurrence of symptoms was reported in up to 56% of patients, recurrence of AP in up to 39%, further surgical procedures in up to 19%, and mortality in up to 17%.14, 1921 Few case series of children with neurological impairment who have had a fundoplication have addressed child and caregiver quality of life following either a fundoplication or placement of a feeding tube.2224 In their study of 16 patients who had a fundoplication and gastrostomy tube placed, Tawfik et al. found improvements in children's happiness, ease of giving medicines, and time to devote to other children. Sullivan et al. found improvement in caregiver quality of life following placement of a gastrostomy tube in a child; however, they did not specifically identify those children who had been treated with a fundoplication. In their retrospective study, O'Neill et al. found improved child and caregiver quality of life following a fundoplication. Collectively, these studies have found that parents report improvement of both their own and their child's quality of life after either intervention. However, not having baseline measurements, not controlling for degree of functional impairment of the children, small sample sizes, and large loss to follow‐up limit the utility of these studies. In this ongoing, long‐term prospective longitudinal study, we report the initial impact of a fundoplication on the quality of life of both children and their caregivers.
The primary objective of this study was to determine change over time in the quality of life of children with neurological impairment who received surgical treatment of their GERD and of the caregivers of these children, controlling for the degree of functional impairment of the children. We hypothesized that child and caregiver quality of life would both improve after primary fundoplication and gastrostomy tube placement. Secondary objectives included describing rates of complications in this population.
METHODS
Setting and Study Population
We enrolled patients from newborn to 18 years of age who had a diagnosis compatible with neurological impairment and who received their first fundoplication for GERD between January 2005 and July 2006 at Primary Children's Medical Center (PCMC), in Salt Lake City, Utah. PCMC is a 232‐bed children's hospital in the Intermountain West owned and operated by Intermountain Healthcare, Inc., a large vertically integrated health care delivery system that serves as both the primary hospital for Salt Lake County and as the tertiary‐care hospital for 4 additional states (Wyoming, Nevada, Idaho, and Montana).25 Patients who had a previous gastrojejunal feeding tube were excluded as were patients who had a previous fundoplication, as these procedures may have biased their reported quality of life, our main outcome measure.
Patients were included in the study if they had GERD (based on clinical history or testing) that had been refractory to medical management (defined as continued gastroesophageal reflux symptoms despite antireflux medications). GERD was defined using the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) criteria.26 These include: the presence of clinical symptoms and at least 1 abnormal result from an upper gastrointestinal x‐ray series (recognizing that this test is neither sensitive nor specific for reflux), pH probe, upper gastrointestinal endoscopy with biopsy, nuclear medicine scan, or a modified barium swallow. As this was a prospective observational study, physicians were allowed to order testing as their practice dictated. Patients were excluded if they had neurological impairment but lacked objective documentation of GERD using the NASPGHAN recommendations, unless there were obvious clinical indications such as witnessed vomiting and aspiration (N = 3). No patient received a prophylactic fundoplication (fundoplication without documented GERD).
Study Design
This is an ongoing prospective longitudinal study. Patients who had a first fundoplication at PCMC were identified by the surgical service, with weekly lists shared with the research team. Patients were approached by a research assistant during that initial hospitalization to see if they met inclusion criteria for the study using data from the medical records and surgical team when necessary.
Data Variables and Sources
Indications for the fundoplication, performance and results of diagnostic testing for GERD, complications of the fundoplication, and reasons for the neurological impairment were obtained through review of the electronic and paper medical records. Mortality data, subsequent emergency department visits, and admissions to the hospital were obtained using Intermountain Healthcare's electronic data warehouse, which merges clinical, financial, and administrative data including the Utah Vital Statistics database.
Neurological impairment was defined from 2 sources: (1) clinical diagnoses as identified by providers and (2) International Classification of Diseases Codes Modified, version 9 (ICD‐9 CM) identified a priori as indicating neurological impairment.
Instruments and Study Outcomes
Functional status was measured using the WeeFIM. This instrument has been tested and shown to be valid and reliable for children more than 6 months old with neurodevelopmental disabilities including spina bifida and Down syndrome.2732 WeeFIM is a self‐administered parent instrument composed of 18 items and 6 domains (self‐care, sphincter control, transfers, locomotion, communication, and social cognition).33 The WeeFIM allows patients to be stratified into areas of function from severely impaired to normal.
The primary outcome was child quality of life as measured by the Child Health Questionnaire Parental Form 50 (CHQ‐PF50). Caregiver quality of life was measured using the Short‐Form Health Status Survey (SF‐36) and Parenting Stress Index/Short Form (PSI/SF). The CHQ‐PF50 is a self‐administered parent questionnaire of 50 questions that measures 6 domains, including physical function and abilities, pain and discomfort, general health perception, behavior, temperament and moods, and satisfaction with growth and development.34 This instrument has been tested for validity and reliability in children with cerebral palsy.35 The SF‐36 is a widely accepted measure of health status that measures 8 domains of health: physical functioning, role limitations due to physical problems, bodily pain, general health, vitality, social functioning, role limitations due to emotional problems, and mental health. The SF‐36 has been well studied and has been used to measure the effect on a caregiver's quality of life associated with caring for a chronically ill child with significant medical problems.36, 37 Higher scores in each domain of both the CHQPF50 and the SF‐36 reflect better quality of life. Caregiver stress was measured using the PSI/SF (Psychological Assessment Resources Inc, Odessa, FL).38 In the PSI/SF a parent rates the parentchild dyad on 36 items that are summarized in 3 subscales: parental distress, parentchild dysfunctional interaction, and difficult child. A parent scores each item as strongly agree, agree, not sure, disagree, or strongly disagree. The sum of the 3 subscale scores is the total stress score. Higher scores denote a greater degree of stress. The PSI/SF has been validated in several studies for caregivers of children with chronic diseases.3943
The CHQ‐PF50, SF‐36, and PSI/SF questionnaires and the WeeFIM functional status measure were administered to each study patient and caregiver in person at enrollment (baseline) and by mail 1 month after fundoplication. A follow‐up postcard reminder was mailed 1 week after the initial mailing. Second and final mailings were sent to nonresponders 3 and 5 weeks, respectively, after the initial mailing.
Secondary outcomes included rates of complications including failure of the fundoplication. Complications were defined as a subsequent emergency department visit, hospitalization, or death related to the surgery, gastrostomy tube, or aspiration pneumonia. Failures were defined as a second fundoplication or the insertion of a gastrojejunal feeding tube as nonsurgical management of recurrent GERD and/or paraesophageal hernia. Secondary outcomes were followed from the time of the first fundoplication until 1 month after surgery.
Analyses
The differential effect of the fundoplication on the quality of life measures was assessed and quantified through statistical analysis. Because the primary interest was to measure change in baseline characteristics over time, repeated‐measures models were used to compare the group relative to changes in functional status. In particular, changes from baseline values were modeled 1 month after the procedure. The Kenward‐Roger approximation of degrees of freedom was used to compute P values from the overall tests.44 Repeated‐measures models were fit to the data using restricted maximum likelihood estimation. An autoregressive covariance matrix was assumed for the multiple measurements of each individual, thus limiting the number of restrictions forced by the model on the data. The repeated‐measures models used all the available data on participants, including those who dropped out of the study. To obtain the most accurate comparison of the study group, the covariate of functional status at baseline was taken into account in the fitted models. Statistical analyses were performed with SAS statistical software (version 9.13; SAS Institute, Cary NC). Student t tests were performed for comparison of means of the quality‐of‐life domains for the study cohort compared to either the general population or specific groups of patients for comparative purposes.
The study was approved by the institutional review boards of the University of Utah Health Sciences Center and Primary Children's Medical Center.
RESULTS
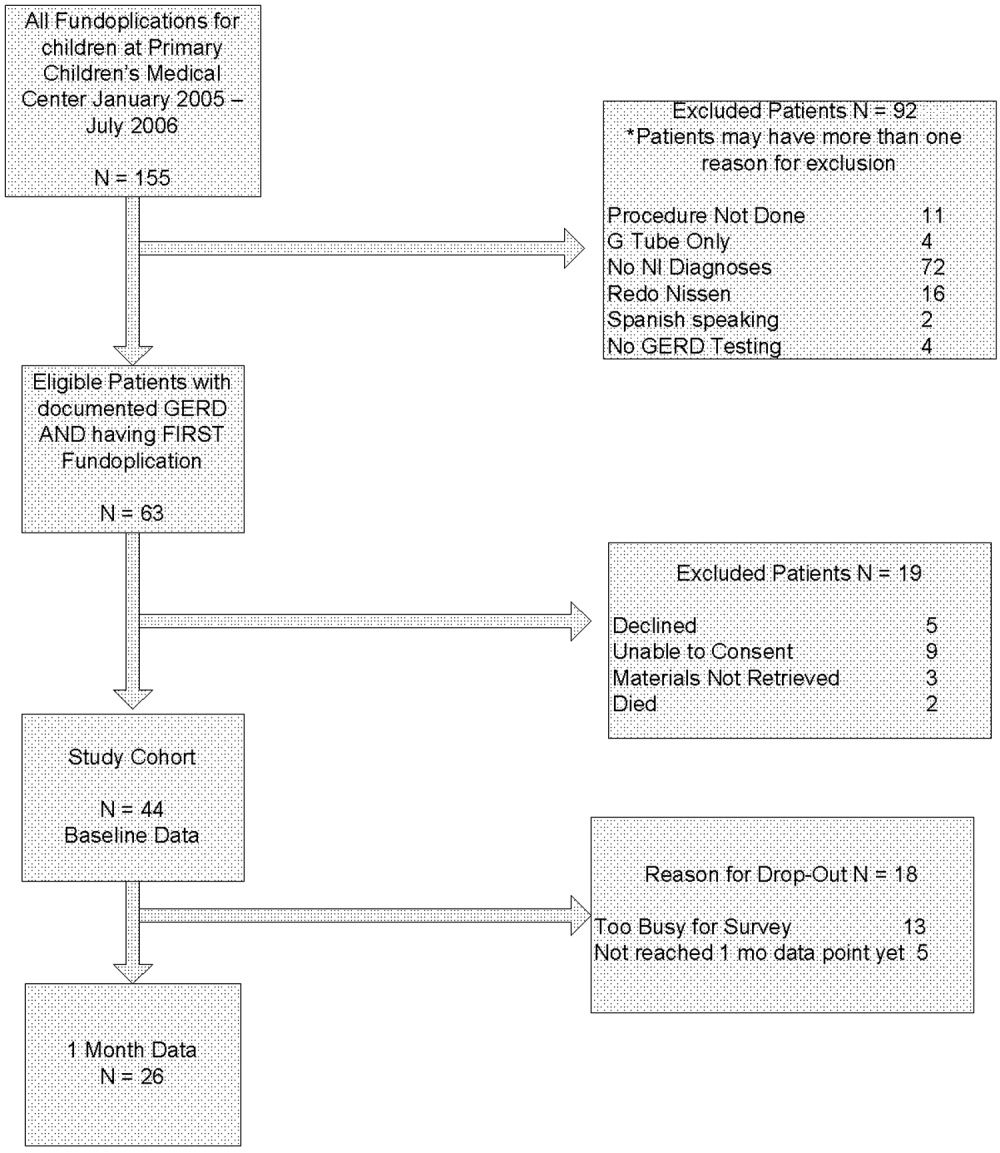
Sixty‐three children met eligibility criteria. Forty‐four families (70%) initially agreed to participate in the study and completed the baseline questionnaires (see Fig. 1). The mean age of the children was 2.2 years. Twenty‐six parents of children completed the 1‐month postfundoplication quality‐of‐life questionnaires. Thirteen patients were lost to follow‐up, 5 of whom had not reached the 1‐month postfundoplication time point. The median WeeFIM (functional status) score of the whole group was 31.2 (95% confidence interval [CI] 11‐71) compared with a childhood matched‐age norm of 83 (95% CI 60‐110), P = .001. WeeFIM scores did not change significantly from baseline to 1 month, P = .98 (Kruskall‐Wallis test).

Data for the 13 parents and children (30%) who gave baseline data but were subsequently lost to follow‐up are shown in Table 1. Reasons for loss to follow‐up were caregiver reporting being too busy to fill out the questionnaires (n = 8) and no reason stated (n = 5).
In addition to the diagnosis of GERD, clinical indications for fundoplication were vomiting (55%), feeding‐related issues (47%), and failure to thrive (39%). Diagnosis of GERD was confirmed for 41 of 44 patients77% had an abnormal upper GI, 26% an abnormal pH probe, 14% an abnormal endoscopy, and 24% an abnormal swallow study. The remaining 3 had obvious clinical symptoms for GERD and did not require further testing according to their attending surgeon (2 with witnessed vomiting leading to aspiration and 1 who was exclusively gastrostomy‐fed and was witnessed having feeds coming from the tracheostomy). Various medications had been tried and were considered to have failed in these patients: 39% had been treated with acid‐suppressive agents; 80% with acid blocking agents; and 61% with prokinetic agents. Fourteen patients (32%) had cerebral palsy, and 14 (32%) had a brain or spinal cord abnormality (see Table 2).
| Diagnostic category | ICD‐9 codes used |
|---|---|
| |
| Brain or spinal cord anomaly | 335.22, 742.0, 742.1, 742.2, 742.4, 742.53 |
| Cerebral palsy | 343.0, 343.1, 343.2, 343.8, 343.9, 344.00 |
| Hydrocephalus | 331.3, 331.4, 742.3 |
| Down syndrome | 758.0 |
| Seizures | 345.10, 345.11, 345.3, 345.41, 345.50, 345.81, 345.90, 345.91 |
| Muscular dystrophy or myopathy | 359.0, 359.1, 359.2, 359.9 |
| Nervous system anomaly | 742.8, 742.9 |
| Cerebral degeneration | 330.8, 331.9 |
| Chromosomal anomaly | 758.2, 758.3, 758.5, 758.89 |
| Infantile spasms | 345.60, 345.61 |
| Menial retardation | 317.0, 318.1, 318.2 |
| Spinal muscle atrophy | 335.0, 335.10 |
Thirty‐four children underwent a laparoscopic Nissen fundoplication, and 10 had an open Nissen fundoplication. All had gastrostomy tubes placed or replaced at the time of surgery.
Analysis of the mean bodily pain scores from the CHQ‐PF50 revealed that bodily pain of patients in the study cohort had improved from baseline after 1 month of follow‐up (mean score at baseline, 32.8; after 1 month of follow‐up, 47.5; P = .01), after adjusting for functional status. However, these mean bodily pain scores were significantly lower than those of children with cerebral palsy (mean score, 73.9; P < .001).34, 35 After adjusting for functional status, scores were improved for role/social‐physical limitations (mean baseline score, 30.6; 1‐month follow‐up score, 56.6; P = .01), mental health (mean baseline score, 62.7; 1‐month follow‐up score, 70.6; P = .01), family limitation of activities (mean baseline score, 43.3; 1‐month follow‐up score, 55.1; P = .03), and parental time (mean baseline score, 43.0; 1‐month follow‐up score, 55.3; P = .03). Scores were unchanged for physical function, global health, general health perception, physical summary, role/social‐emotional, mental health, self‐esteem, and psychological summary (see Table 3).
| Domain of Quality of Life | Baseline (Mean and SD) | 1‐Month Follow‐Up (Mean and SD) | P Value |
|---|---|---|---|
| Physical functioning | 19.3 (34.1) | 16.7 (30.8) | 0.77 |
| Role physical* | 30.6 (44.4) | 56.6 (40.5) | 0.01 |
| Bodily pain* | 32.8 (24.4) | 47.5 (25.7) | 0.01 |
| Global health | 42.0 (23.7) | 44.1 (22.6) | 0.19 |
| General behavior | 72.1 (29.3) | 78.7 (14.5) | 0.21 |
| Self‐esteem | 39.9 (21.1) | 32.8 (19.4) | 0.36 |
| Mental health | 62.7 (15.9) | 70.6 (16.6) | 0.01 |
| Family limitation of activity* | 43.3 (23.7) | 55.1 (21.3) | 0.03 |
| Parental time* | 43.0 (35.5) | 55.3 (32.5) | 0.03 |
| Physical summary | 23.1 (21.2) | 17.8 (13.9) | 0.17 |
| Psychological summary | 39.0 (11.8) | 39.6 (10.8) | 0.76 |
Analysis of the SF‐36 of the parents of these children revealed mean scores significantly lower than those in general U.S. population for all quality‐of‐life domains except physical function (see Table 4). Many baseline domain scores were similar to those of adults with clinical depression. The only domain that showed improvement in quality of life of the caregivers over the 1‐month follow‐up period was vitality (mean baseline score, 41.3; 1‐month follow‐up score, 48.2; P = .001).
| Quality‐of‐life domain | Study group mean (SD) | U.S. population norm mean (SD) | P value |
|---|---|---|---|
| |||
| Physical functioning | 89.35 (14.60) | 84.15 (23.26) | 0.10 |
| Role physical | 71.02 (39.96) | 80.96 (34.00) | 0.05 |
| Bodily pain | 82.50 (24.00) | 75.15 (23.69) | 0.04 |
| General Health* | 59.07 (18.75) | 71.95 (20.34) | 0.001 |
| Vitality* | 41.33 (19.49) | 60.86 (33.04) | 0.001 |
| Social functioning* | 63.33 (34.48) | 83.28 (22.69) | 0.001 |
| Role emotional | 60.60 (40.20) | 81.26 (33.04) | 0.001 |
| Mental health | 67.00 (19.61) | 74.74 (18.05) | 0.004 |
Total stress as measured by the PSI/SF mean was 79.1 at baseline and 77.6 1 month after fundoplication (P = .54). This was significantly higher stress than the parental norm of 71.0 (P = .01). One in 4 parents expressed clinically significant levels of stress (scores > 90, 90th percentile).
Patients suffered the following complications in the month after fundoplication. Eight children had at least 1 subsequent emergency department visit related to a complication of the gastrostomy tube (8 visits), to respiratory distress (1 visit), or tovomiting (1 visit). Seven children had a subsequent admission to the hospital related to a complication of the gastrostomy tube (4 admissions), complication of surgery (2 admissions), or aspiration pneumonia (1 admission). None of the children had a repeat fundoplication or subsequently underwent placement of a gastrojejunal feeding tube. One patient died. She was 10 months old when she died, which was 3 weeks after she had received a fundoplication. She had obstructive hydrocephalus, cortical blindness, and developmental delay, and respiratory arrest and subsequent tonsillar herniation led to her death.
DISCUSSION
Parents of children with neurological impairment and GERD who underwent their first fundoplication reported improved quality of life of their children in the domains of bodily pain, role/social‐physical limitations, mental health, family limitation of activities, and parental time over the first month after surgery, when controlling for the children's degree of functional impairment. The only significant similar improvement in the parent self‐reported quality of life was in the domain of vitality.
This study had several limitations. Loss to follow‐up may have led to a bias reflecting the phenomenon that patients who have poorer quality of life are less likely to report this, or even to be able to participate in the follow‐up component of a study like this. In survival analyses, this incomplete follow‐up of patients is called informative dropout and may be minimized by applying a statistical technique that accounts for this, using the Q‐TWiST.45 However, our current study design and analysis plan precluded using this methodology. As shown in Table 1, we did not find any differences between those patients who stayed in the study and those who dropped out. Also, we were able to contact most parents who reported being too busy to fill out the surveys. Patient heterogeneity is also a concern: Table 2 shows the wide array of diagnoses responsible for the children's neurological impairment. However, we used a standardized functional status measure to ensure we were analyzing similarly disabled patients. Also, the standard deviation of the mean WeeFIM score was small, implying little variability in the study cohort. Our study analyzed data from a single center, which reflects care in the western United States. However, our hospital is similar to other medium and large children's hospitals and our patient population similar to others that perform fundoplication for children with neurological impairment.46 We believe our findings are generalizable to other surgical centers that perform a similar volume of fundoplications in such children with NI.
Our study findings are similar to those reported by O'Neill et al., whose study found that parents reported improved quality of life of their children in ease of feedings, physical comfort during feeding, and ability of the child to enjoy life.23 The CHQPF50 does not specifically ask about feeding, but we did find similar improvement in the domain of role/social‐physical limitations. O'Neill et al. also found that after the children in their study received a fundoplication, caregivers reported their own quality of life improved in the areas of being able to spend more time caring for their child's needs, which is similar to our findings of fewer family limitations of activities and more parental time. Our findings were somewhat dissimilar to the O'Neill et al. study, as parents in their study found several additional areas of improvement in caregiver own quality of life. One explanation for the differing results may be differences in the populations studied. Parents in our study had SF‐36 scores for general health, vitality, and social functioning that were similar to those of adults with depression,47 whereas parents in the O'Neill study did not. Although the O'Neill et al. study was the first to examine these critical quality‐of‐life outcomes for children with NI who have received fundoplication, it had several methodological limitations. We have had the opportunity to build on the work of O'Neill et al. and in a prospective study to capture standardized baseline data (therefore not subject to recall bias, as was likely in the O'Neill et al. study) and collect long‐term data on this population. We also controlled for functional status, which did not improve over the 1 month and by itself could be responsible for the already poor caregiver quality of life. Some aspects of the children's care did improve, but perhaps not enough to overcome the severe disabilities the children and their caregivers live with on a daily basis. We found some evidence to support that the parents' PSI/SF scores were similar to those of parents of children with heart disease, other enterally fed children, and children with traumatic brain injury (who make up between 1 in 3 and 1 in 5 parents with severe stress).39, 41, 43 Future interventions should address the stress and quality of life of these caregivers, especially if surgery does not improve caregiver quality of life or decrease stress.
Contrary to an emerging body of literature in pediatrics that describes a positive correlation between the health of children with chronic illnesses and their caregivers' quality of life,12, 42 we did not find large immediate improvements in caregiver quality of life and decrease in stress as their children's quality of life improved. This may be related to the number of parents in our sample being too small to detect such changes or that changes in longer‐term (greater than 6 months or 1 year) quality of life not being reflected by short‐term assessment. Caregiver and child quality of life following fundoplication needs to be studied over the long term (eg, over many years). We are continuing to follow these patients and their families and will repeat the quality‐of‐life measures 6 and 12 months after fundoplication and report these findings.
Additional studies of treatments for neurologically impaired children with GERD are needed. Randomized trials of alternatives to fundoplication such as gastrojejunal feeding tubes have been proposed, with which we strongly agree.46, 48 We believe that any randomized, controlled trial of children with neurological impairment and GERD must measure child and caregiver quality of life and functional status outcomes. 0
| Variables | Study patients at baseline (N = 44) | Study patients at 1‐month follow‐up (N = 26) | P value |
|---|---|---|---|
| Functional Status Measure | |||
| WeeFIM Score | 24 | 36 | NS |
| Child CHQ‐PF50 Quality‐of‐Life Scores | |||
| Role physical | 30.6 | 56.6 | 0.01 |
| Bodily pain | 32.8 | 47.5 | 0.01 |
| Mental health | 62.7 | 70.6 | 0.01 |
| Family limitation of activity | 43.3 | 55.1 | 0.03 |
| Parental time | 43.0 | 55.3 | 0.03 |
| Global health | 42.0 | 44.1 | NS |
| Physical functioning | 19.3 | 16.7 | NS |
| General behavior | 72.1 | 78.7 | NS |
| Self‐esteem | 39.9 | 32.8 | NS |
| Role emotional | 27.1 | 37.1 | NS |
| Physical summary | 23.1 | 17.8 | NS |
| Psychological summary | 39.0 | 39.6 | NS |
| Caregiver SF‐36 Quality‐of‐Life Scores | |||
| Vitality | 41.3 | 46.9 | 0.001 |
| Role physical | 89.9 | 92.5 | NS |
| Bodily pain | 71.0 | 78.7 | NS |
| General health | 82.5 | 81.1 | NS |
| Social functioning | 59.1 | 59.5 | NS |
| Role emotional | 60.6 | 65.6 | NS |
| Mental health | 67.0 | 73.5 | NS |
| Parenting stress index | 79.1 | 77.7 | NS |
Acknowledgements
The authors thank Tanner Coleman and Matthew Swenson for their invaluable help in recruiting patients. Dr. Srivastava was supported in part by the Children's Health Research Center, University of Utah and Primary Children's Medical Center Foundation.
Aspiration pneumonia is the most common cause of death in children with severe neurological impairment (NI).13 For several reasons (eg, improved survival of extremely low‐birth‐weight infants, technological advances, and changing societal attitudes), the number of children with severe NI is increasing. Many children with severe NI have dysfunctional swallowing and gastroesophageal reflux disease (GERD).46 This combination places them at high risk for recurrent aspiration that, in turn, leads to aspiration pneumonia, repeated hospitalization, respiratory failure, compromised quality of life, and death.7, 8
The most common treatment approach for the combination of dysfunctional swallowing and GERD is surgical fundoplication with a gastrostomy feeding tube. Fundoplication is the third most common procedure performed in children by pediatric surgeons in the United States.9 Fifty percent of the children who receive a fundoplication have neurological impairment.10, 11 The goals of the surgery to treat GERD unresponsive to medical management are to reduce the risk of aspiration pneumonia, improve nutritional status, and improve the quality of life of the children and their families. However, few prospective longitudinal studies have determined whether the quality of life of the children or their caregivers actually improves over time.
The importance of caregiver and child quality of life is increasingly recognized as a critical outcome of any intervention in this population.12, 13 Previous studies of fundoplication in children with NI, GERD, and dysfunctional swallowing reported surgical mortality rates between 1% and 3% and other complications between 4% and 39%, reflecting the medical fragility of these children.5, 1418 Some of these studies had longitudinal follow‐up and reported long‐term data. Recurrence of symptoms was reported in up to 56% of patients, recurrence of AP in up to 39%, further surgical procedures in up to 19%, and mortality in up to 17%.14, 1921 Few case series of children with neurological impairment who have had a fundoplication have addressed child and caregiver quality of life following either a fundoplication or placement of a feeding tube.2224 In their study of 16 patients who had a fundoplication and gastrostomy tube placed, Tawfik et al. found improvements in children's happiness, ease of giving medicines, and time to devote to other children. Sullivan et al. found improvement in caregiver quality of life following placement of a gastrostomy tube in a child; however, they did not specifically identify those children who had been treated with a fundoplication. In their retrospective study, O'Neill et al. found improved child and caregiver quality of life following a fundoplication. Collectively, these studies have found that parents report improvement of both their own and their child's quality of life after either intervention. However, not having baseline measurements, not controlling for degree of functional impairment of the children, small sample sizes, and large loss to follow‐up limit the utility of these studies. In this ongoing, long‐term prospective longitudinal study, we report the initial impact of a fundoplication on the quality of life of both children and their caregivers.
The primary objective of this study was to determine change over time in the quality of life of children with neurological impairment who received surgical treatment of their GERD and of the caregivers of these children, controlling for the degree of functional impairment of the children. We hypothesized that child and caregiver quality of life would both improve after primary fundoplication and gastrostomy tube placement. Secondary objectives included describing rates of complications in this population.
METHODS
Setting and Study Population
We enrolled patients from newborn to 18 years of age who had a diagnosis compatible with neurological impairment and who received their first fundoplication for GERD between January 2005 and July 2006 at Primary Children's Medical Center (PCMC), in Salt Lake City, Utah. PCMC is a 232‐bed children's hospital in the Intermountain West owned and operated by Intermountain Healthcare, Inc., a large vertically integrated health care delivery system that serves as both the primary hospital for Salt Lake County and as the tertiary‐care hospital for 4 additional states (Wyoming, Nevada, Idaho, and Montana).25 Patients who had a previous gastrojejunal feeding tube were excluded as were patients who had a previous fundoplication, as these procedures may have biased their reported quality of life, our main outcome measure.
Patients were included in the study if they had GERD (based on clinical history or testing) that had been refractory to medical management (defined as continued gastroesophageal reflux symptoms despite antireflux medications). GERD was defined using the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) criteria.26 These include: the presence of clinical symptoms and at least 1 abnormal result from an upper gastrointestinal x‐ray series (recognizing that this test is neither sensitive nor specific for reflux), pH probe, upper gastrointestinal endoscopy with biopsy, nuclear medicine scan, or a modified barium swallow. As this was a prospective observational study, physicians were allowed to order testing as their practice dictated. Patients were excluded if they had neurological impairment but lacked objective documentation of GERD using the NASPGHAN recommendations, unless there were obvious clinical indications such as witnessed vomiting and aspiration (N = 3). No patient received a prophylactic fundoplication (fundoplication without documented GERD).
Study Design
This is an ongoing prospective longitudinal study. Patients who had a first fundoplication at PCMC were identified by the surgical service, with weekly lists shared with the research team. Patients were approached by a research assistant during that initial hospitalization to see if they met inclusion criteria for the study using data from the medical records and surgical team when necessary.
Data Variables and Sources
Indications for the fundoplication, performance and results of diagnostic testing for GERD, complications of the fundoplication, and reasons for the neurological impairment were obtained through review of the electronic and paper medical records. Mortality data, subsequent emergency department visits, and admissions to the hospital were obtained using Intermountain Healthcare's electronic data warehouse, which merges clinical, financial, and administrative data including the Utah Vital Statistics database.
Neurological impairment was defined from 2 sources: (1) clinical diagnoses as identified by providers and (2) International Classification of Diseases Codes Modified, version 9 (ICD‐9 CM) identified a priori as indicating neurological impairment.
Instruments and Study Outcomes
Functional status was measured using the WeeFIM. This instrument has been tested and shown to be valid and reliable for children more than 6 months old with neurodevelopmental disabilities including spina bifida and Down syndrome.2732 WeeFIM is a self‐administered parent instrument composed of 18 items and 6 domains (self‐care, sphincter control, transfers, locomotion, communication, and social cognition).33 The WeeFIM allows patients to be stratified into areas of function from severely impaired to normal.
The primary outcome was child quality of life as measured by the Child Health Questionnaire Parental Form 50 (CHQ‐PF50). Caregiver quality of life was measured using the Short‐Form Health Status Survey (SF‐36) and Parenting Stress Index/Short Form (PSI/SF). The CHQ‐PF50 is a self‐administered parent questionnaire of 50 questions that measures 6 domains, including physical function and abilities, pain and discomfort, general health perception, behavior, temperament and moods, and satisfaction with growth and development.34 This instrument has been tested for validity and reliability in children with cerebral palsy.35 The SF‐36 is a widely accepted measure of health status that measures 8 domains of health: physical functioning, role limitations due to physical problems, bodily pain, general health, vitality, social functioning, role limitations due to emotional problems, and mental health. The SF‐36 has been well studied and has been used to measure the effect on a caregiver's quality of life associated with caring for a chronically ill child with significant medical problems.36, 37 Higher scores in each domain of both the CHQPF50 and the SF‐36 reflect better quality of life. Caregiver stress was measured using the PSI/SF (Psychological Assessment Resources Inc, Odessa, FL).38 In the PSI/SF a parent rates the parentchild dyad on 36 items that are summarized in 3 subscales: parental distress, parentchild dysfunctional interaction, and difficult child. A parent scores each item as strongly agree, agree, not sure, disagree, or strongly disagree. The sum of the 3 subscale scores is the total stress score. Higher scores denote a greater degree of stress. The PSI/SF has been validated in several studies for caregivers of children with chronic diseases.3943
The CHQ‐PF50, SF‐36, and PSI/SF questionnaires and the WeeFIM functional status measure were administered to each study patient and caregiver in person at enrollment (baseline) and by mail 1 month after fundoplication. A follow‐up postcard reminder was mailed 1 week after the initial mailing. Second and final mailings were sent to nonresponders 3 and 5 weeks, respectively, after the initial mailing.
Secondary outcomes included rates of complications including failure of the fundoplication. Complications were defined as a subsequent emergency department visit, hospitalization, or death related to the surgery, gastrostomy tube, or aspiration pneumonia. Failures were defined as a second fundoplication or the insertion of a gastrojejunal feeding tube as nonsurgical management of recurrent GERD and/or paraesophageal hernia. Secondary outcomes were followed from the time of the first fundoplication until 1 month after surgery.
Analyses
The differential effect of the fundoplication on the quality of life measures was assessed and quantified through statistical analysis. Because the primary interest was to measure change in baseline characteristics over time, repeated‐measures models were used to compare the group relative to changes in functional status. In particular, changes from baseline values were modeled 1 month after the procedure. The Kenward‐Roger approximation of degrees of freedom was used to compute P values from the overall tests.44 Repeated‐measures models were fit to the data using restricted maximum likelihood estimation. An autoregressive covariance matrix was assumed for the multiple measurements of each individual, thus limiting the number of restrictions forced by the model on the data. The repeated‐measures models used all the available data on participants, including those who dropped out of the study. To obtain the most accurate comparison of the study group, the covariate of functional status at baseline was taken into account in the fitted models. Statistical analyses were performed with SAS statistical software (version 9.13; SAS Institute, Cary NC). Student t tests were performed for comparison of means of the quality‐of‐life domains for the study cohort compared to either the general population or specific groups of patients for comparative purposes.
The study was approved by the institutional review boards of the University of Utah Health Sciences Center and Primary Children's Medical Center.
RESULTS
Sixty‐three children met eligibility criteria. Forty‐four families (70%) initially agreed to participate in the study and completed the baseline questionnaires (see Fig. 1). The mean age of the children was 2.2 years. Twenty‐six parents of children completed the 1‐month postfundoplication quality‐of‐life questionnaires. Thirteen patients were lost to follow‐up, 5 of whom had not reached the 1‐month postfundoplication time point. The median WeeFIM (functional status) score of the whole group was 31.2 (95% confidence interval [CI] 11‐71) compared with a childhood matched‐age norm of 83 (95% CI 60‐110), P = .001. WeeFIM scores did not change significantly from baseline to 1 month, P = .98 (Kruskall‐Wallis test).

Data for the 13 parents and children (30%) who gave baseline data but were subsequently lost to follow‐up are shown in Table 1. Reasons for loss to follow‐up were caregiver reporting being too busy to fill out the questionnaires (n = 8) and no reason stated (n = 5).
In addition to the diagnosis of GERD, clinical indications for fundoplication were vomiting (55%), feeding‐related issues (47%), and failure to thrive (39%). Diagnosis of GERD was confirmed for 41 of 44 patients77% had an abnormal upper GI, 26% an abnormal pH probe, 14% an abnormal endoscopy, and 24% an abnormal swallow study. The remaining 3 had obvious clinical symptoms for GERD and did not require further testing according to their attending surgeon (2 with witnessed vomiting leading to aspiration and 1 who was exclusively gastrostomy‐fed and was witnessed having feeds coming from the tracheostomy). Various medications had been tried and were considered to have failed in these patients: 39% had been treated with acid‐suppressive agents; 80% with acid blocking agents; and 61% with prokinetic agents. Fourteen patients (32%) had cerebral palsy, and 14 (32%) had a brain or spinal cord abnormality (see Table 2).
| Diagnostic category | ICD‐9 codes used |
|---|---|
| |
| Brain or spinal cord anomaly | 335.22, 742.0, 742.1, 742.2, 742.4, 742.53 |
| Cerebral palsy | 343.0, 343.1, 343.2, 343.8, 343.9, 344.00 |
| Hydrocephalus | 331.3, 331.4, 742.3 |
| Down syndrome | 758.0 |
| Seizures | 345.10, 345.11, 345.3, 345.41, 345.50, 345.81, 345.90, 345.91 |
| Muscular dystrophy or myopathy | 359.0, 359.1, 359.2, 359.9 |
| Nervous system anomaly | 742.8, 742.9 |
| Cerebral degeneration | 330.8, 331.9 |
| Chromosomal anomaly | 758.2, 758.3, 758.5, 758.89 |
| Infantile spasms | 345.60, 345.61 |
| Menial retardation | 317.0, 318.1, 318.2 |
| Spinal muscle atrophy | 335.0, 335.10 |
Thirty‐four children underwent a laparoscopic Nissen fundoplication, and 10 had an open Nissen fundoplication. All had gastrostomy tubes placed or replaced at the time of surgery.
Analysis of the mean bodily pain scores from the CHQ‐PF50 revealed that bodily pain of patients in the study cohort had improved from baseline after 1 month of follow‐up (mean score at baseline, 32.8; after 1 month of follow‐up, 47.5; P = .01), after adjusting for functional status. However, these mean bodily pain scores were significantly lower than those of children with cerebral palsy (mean score, 73.9; P < .001).34, 35 After adjusting for functional status, scores were improved for role/social‐physical limitations (mean baseline score, 30.6; 1‐month follow‐up score, 56.6; P = .01), mental health (mean baseline score, 62.7; 1‐month follow‐up score, 70.6; P = .01), family limitation of activities (mean baseline score, 43.3; 1‐month follow‐up score, 55.1; P = .03), and parental time (mean baseline score, 43.0; 1‐month follow‐up score, 55.3; P = .03). Scores were unchanged for physical function, global health, general health perception, physical summary, role/social‐emotional, mental health, self‐esteem, and psychological summary (see Table 3).
| Domain of Quality of Life | Baseline (Mean and SD) | 1‐Month Follow‐Up (Mean and SD) | P Value |
|---|---|---|---|
| Physical functioning | 19.3 (34.1) | 16.7 (30.8) | 0.77 |
| Role physical* | 30.6 (44.4) | 56.6 (40.5) | 0.01 |
| Bodily pain* | 32.8 (24.4) | 47.5 (25.7) | 0.01 |
| Global health | 42.0 (23.7) | 44.1 (22.6) | 0.19 |
| General behavior | 72.1 (29.3) | 78.7 (14.5) | 0.21 |
| Self‐esteem | 39.9 (21.1) | 32.8 (19.4) | 0.36 |
| Mental health | 62.7 (15.9) | 70.6 (16.6) | 0.01 |
| Family limitation of activity* | 43.3 (23.7) | 55.1 (21.3) | 0.03 |
| Parental time* | 43.0 (35.5) | 55.3 (32.5) | 0.03 |
| Physical summary | 23.1 (21.2) | 17.8 (13.9) | 0.17 |
| Psychological summary | 39.0 (11.8) | 39.6 (10.8) | 0.76 |
Analysis of the SF‐36 of the parents of these children revealed mean scores significantly lower than those in general U.S. population for all quality‐of‐life domains except physical function (see Table 4). Many baseline domain scores were similar to those of adults with clinical depression. The only domain that showed improvement in quality of life of the caregivers over the 1‐month follow‐up period was vitality (mean baseline score, 41.3; 1‐month follow‐up score, 48.2; P = .001).
| Quality‐of‐life domain | Study group mean (SD) | U.S. population norm mean (SD) | P value |
|---|---|---|---|
| |||
| Physical functioning | 89.35 (14.60) | 84.15 (23.26) | 0.10 |
| Role physical | 71.02 (39.96) | 80.96 (34.00) | 0.05 |
| Bodily pain | 82.50 (24.00) | 75.15 (23.69) | 0.04 |
| General Health* | 59.07 (18.75) | 71.95 (20.34) | 0.001 |
| Vitality* | 41.33 (19.49) | 60.86 (33.04) | 0.001 |
| Social functioning* | 63.33 (34.48) | 83.28 (22.69) | 0.001 |
| Role emotional | 60.60 (40.20) | 81.26 (33.04) | 0.001 |
| Mental health | 67.00 (19.61) | 74.74 (18.05) | 0.004 |
Total stress as measured by the PSI/SF mean was 79.1 at baseline and 77.6 1 month after fundoplication (P = .54). This was significantly higher stress than the parental norm of 71.0 (P = .01). One in 4 parents expressed clinically significant levels of stress (scores > 90, 90th percentile).
Patients suffered the following complications in the month after fundoplication. Eight children had at least 1 subsequent emergency department visit related to a complication of the gastrostomy tube (8 visits), to respiratory distress (1 visit), or tovomiting (1 visit). Seven children had a subsequent admission to the hospital related to a complication of the gastrostomy tube (4 admissions), complication of surgery (2 admissions), or aspiration pneumonia (1 admission). None of the children had a repeat fundoplication or subsequently underwent placement of a gastrojejunal feeding tube. One patient died. She was 10 months old when she died, which was 3 weeks after she had received a fundoplication. She had obstructive hydrocephalus, cortical blindness, and developmental delay, and respiratory arrest and subsequent tonsillar herniation led to her death.
DISCUSSION
Parents of children with neurological impairment and GERD who underwent their first fundoplication reported improved quality of life of their children in the domains of bodily pain, role/social‐physical limitations, mental health, family limitation of activities, and parental time over the first month after surgery, when controlling for the children's degree of functional impairment. The only significant similar improvement in the parent self‐reported quality of life was in the domain of vitality.
This study had several limitations. Loss to follow‐up may have led to a bias reflecting the phenomenon that patients who have poorer quality of life are less likely to report this, or even to be able to participate in the follow‐up component of a study like this. In survival analyses, this incomplete follow‐up of patients is called informative dropout and may be minimized by applying a statistical technique that accounts for this, using the Q‐TWiST.45 However, our current study design and analysis plan precluded using this methodology. As shown in Table 1, we did not find any differences between those patients who stayed in the study and those who dropped out. Also, we were able to contact most parents who reported being too busy to fill out the surveys. Patient heterogeneity is also a concern: Table 2 shows the wide array of diagnoses responsible for the children's neurological impairment. However, we used a standardized functional status measure to ensure we were analyzing similarly disabled patients. Also, the standard deviation of the mean WeeFIM score was small, implying little variability in the study cohort. Our study analyzed data from a single center, which reflects care in the western United States. However, our hospital is similar to other medium and large children's hospitals and our patient population similar to others that perform fundoplication for children with neurological impairment.46 We believe our findings are generalizable to other surgical centers that perform a similar volume of fundoplications in such children with NI.
Our study findings are similar to those reported by O'Neill et al., whose study found that parents reported improved quality of life of their children in ease of feedings, physical comfort during feeding, and ability of the child to enjoy life.23 The CHQPF50 does not specifically ask about feeding, but we did find similar improvement in the domain of role/social‐physical limitations. O'Neill et al. also found that after the children in their study received a fundoplication, caregivers reported their own quality of life improved in the areas of being able to spend more time caring for their child's needs, which is similar to our findings of fewer family limitations of activities and more parental time. Our findings were somewhat dissimilar to the O'Neill et al. study, as parents in their study found several additional areas of improvement in caregiver own quality of life. One explanation for the differing results may be differences in the populations studied. Parents in our study had SF‐36 scores for general health, vitality, and social functioning that were similar to those of adults with depression,47 whereas parents in the O'Neill study did not. Although the O'Neill et al. study was the first to examine these critical quality‐of‐life outcomes for children with NI who have received fundoplication, it had several methodological limitations. We have had the opportunity to build on the work of O'Neill et al. and in a prospective study to capture standardized baseline data (therefore not subject to recall bias, as was likely in the O'Neill et al. study) and collect long‐term data on this population. We also controlled for functional status, which did not improve over the 1 month and by itself could be responsible for the already poor caregiver quality of life. Some aspects of the children's care did improve, but perhaps not enough to overcome the severe disabilities the children and their caregivers live with on a daily basis. We found some evidence to support that the parents' PSI/SF scores were similar to those of parents of children with heart disease, other enterally fed children, and children with traumatic brain injury (who make up between 1 in 3 and 1 in 5 parents with severe stress).39, 41, 43 Future interventions should address the stress and quality of life of these caregivers, especially if surgery does not improve caregiver quality of life or decrease stress.
Contrary to an emerging body of literature in pediatrics that describes a positive correlation between the health of children with chronic illnesses and their caregivers' quality of life,12, 42 we did not find large immediate improvements in caregiver quality of life and decrease in stress as their children's quality of life improved. This may be related to the number of parents in our sample being too small to detect such changes or that changes in longer‐term (greater than 6 months or 1 year) quality of life not being reflected by short‐term assessment. Caregiver and child quality of life following fundoplication needs to be studied over the long term (eg, over many years). We are continuing to follow these patients and their families and will repeat the quality‐of‐life measures 6 and 12 months after fundoplication and report these findings.
Additional studies of treatments for neurologically impaired children with GERD are needed. Randomized trials of alternatives to fundoplication such as gastrojejunal feeding tubes have been proposed, with which we strongly agree.46, 48 We believe that any randomized, controlled trial of children with neurological impairment and GERD must measure child and caregiver quality of life and functional status outcomes. 0
| Variables | Study patients at baseline (N = 44) | Study patients at 1‐month follow‐up (N = 26) | P value |
|---|---|---|---|
| Functional Status Measure | |||
| WeeFIM Score | 24 | 36 | NS |
| Child CHQ‐PF50 Quality‐of‐Life Scores | |||
| Role physical | 30.6 | 56.6 | 0.01 |
| Bodily pain | 32.8 | 47.5 | 0.01 |
| Mental health | 62.7 | 70.6 | 0.01 |
| Family limitation of activity | 43.3 | 55.1 | 0.03 |
| Parental time | 43.0 | 55.3 | 0.03 |
| Global health | 42.0 | 44.1 | NS |
| Physical functioning | 19.3 | 16.7 | NS |
| General behavior | 72.1 | 78.7 | NS |
| Self‐esteem | 39.9 | 32.8 | NS |
| Role emotional | 27.1 | 37.1 | NS |
| Physical summary | 23.1 | 17.8 | NS |
| Psychological summary | 39.0 | 39.6 | NS |
| Caregiver SF‐36 Quality‐of‐Life Scores | |||
| Vitality | 41.3 | 46.9 | 0.001 |
| Role physical | 89.9 | 92.5 | NS |
| Bodily pain | 71.0 | 78.7 | NS |
| General health | 82.5 | 81.1 | NS |
| Social functioning | 59.1 | 59.5 | NS |
| Role emotional | 60.6 | 65.6 | NS |
| Mental health | 67.0 | 73.5 | NS |
| Parenting stress index | 79.1 | 77.7 | NS |
Acknowledgements
The authors thank Tanner Coleman and Matthew Swenson for their invaluable help in recruiting patients. Dr. Srivastava was supported in part by the Children's Health Research Center, University of Utah and Primary Children's Medical Center Foundation.
- ,,.Comparison of respiratory mortality in the profoundly mentally retarded and in the less retarded.J Ment Defic Res.1979;23(1):1–7.
- ,,.Cause of death in cerebral palsy: a descriptive study.Arch Dis Child.1999;81:390–394.
- .Survival rates of children with severe neurologic disabilities: a review.Semin Pediatr Neurol.2003;10(2):120–129.
- ,.Gastroesophageal reflux among severely retarded children.J Pediatr.1979;94:710–714.
- ,,,,,.Operation for gastro‐oesophageal reflux associated with severe mental retardation.Arch Dis Child.1993;68:347–351.
- ,,,,,.Prevalence and severity of feeding and nutritional problems in children with neurological impairment: Oxford Feeding Study.Dev Med Child Neurol.2000;42:674–680.
- ,,, et al.Aspiration pneumonia in pediatric age group: etiology, predisposing factors and clinical outcome.J Pak Med Assoc.1999;49(4):105–108.
- ,.Respiratory problems in children with neurological impairment.Arch Dis Child.2003;88(1):75–78.
- ,.Gastroesophageal reflux in childhood.Curr Probl Surg.1996;33(1):1–70.
- ,.Minimally invasive surgical techniques in reoperative surgery for gastroesophageal reflux disease in infants and children.Am Surg.2002;68:989–992.
- .Laparoscopic Nissen procedure in children.Semin Laparosc Surg.2002;9(3):146–152.
- ,,, et al.Caregiving process and caregiver burden: conceptual models to guide research and practice.BMC Pediatr.2004;4(1):1.
- ,.Theoretical and psychometric analysis of caregiver strain.Res Nurs Health.1996;19:499–510.
- ,,,,,.Complications and reoperation after Nissen fundoplication in childhood.Am J Surg.1987;153(2):177–183.
- ,,, et al.Surgical treatment of gastroesophageal reflux in children: a combined hospital study of 7467 patients.Pediatrics.1998;101:419–422.
- ,,,,,.Outcomes of surgical fundoplication in children.Clin Gastroenterol Hepatol.2004;2:978–984.
- ,,, et al.The respiratory advantage of laparoscopic Nissen fundoplication.J Pediatr Surg.2003;38:886–891.
- ,,,.Laparoscopic Nissen fundoplication in children: 2‐5‐year follow‐up.Pediatr Surg Int.2003;19:537–539.
- ,,.Recognition of recurrent gastroesophageal reflux following antireflux surgery in the neurologically disabled child: high index of suspicion and definitive evaluation.J Pediatr Surg.1992;27:983–988; discussion988–990.
- ,,.Sequelae of antireflux surgery in profoundly disabled children.J Pediatr Surg.1992;27(2):267–271; discussion271–263.
- ,,.Efficacy of the Nissen fundoplication in the management of gastroesophageal reflux following esophageal atresia repair.J Pediatr Surg.1993;28(1):53–55.
- ,,,.Caregivers' perceptions following gastrostomy in severely disabled children with feeding problems.Dev Med Child Neurol.1997;39:746–751.
- ,,,,.Care‐giver evaluation of anti‐gastroesophageal reflux procedures in neurologically impaired children: what is the real‐life outcome?J Pediatr Surg.1996;31:375–380.
- ,,, et al.Impact of gastrostomy tube feeding on the quality of life of carers of children with cerebral palsy.Dev Med Child Neurol.2004;46:796–800.
- ,.Organizational responses to managed care: issues for academic health centers and implications for pediatric programs.Pediatrics.1998;101:805–811; discussion811–802.
- Children's Digestive Health and Nutrition Foundation Website. Gastroesophageal Reflux Disease in the Neurologically Impaired Child. Available at: http://www.cdhnf.org/PractitionerSeries.asp. Accessed August 30,2006.
- ,,,,.Functional status of school‐aged children with Down syndrome.J Paediatr Child Health.2002;38(2):160–165.
- ,,,,,.Concurrent validity of the Functional Independence Measure for Children (WeeFIM) and the Pediatric Evaluation of Disabilities Inventory in children with developmental disabilities and acquired brain injuries.Phys Occup Ther Pediatr.2001;21(2–3):91–101.
- ,,, et al.The WeeFIM instrument: its utility in detecting change in children with developmental disabilities.Arch Phys Med Rehabil.2000;81:1317–1326.
- ,,, et al.Functional assessment and care of children with neurodevelopmental disabilities.Am J Phys Med Rehabil.2000;79(2):114–123.
- ,,,,,.Predictors of mortality, morbidity, and disability in a cohort of infants < or = 28 weeks' gestation.Clin Pediatr (Phila).1993;32:521–527.
- ,.Content validity of a pediatric functional independence measure.Appl Nurs Res.1990;3(3):120–122.
- ,,, et al.The Functional Independence Measure for Children (WeeFIM). Conceptual basis and pilot use in children with developmental disabilities.Clin Pediatr (Phila). Jul1994;33:421–430.
- ,,.The CHQ User's Manual.1st ed.Boston, MA:The Health Institute, New England Medical Center,1996.
- ,,,,,.Comparing reliability and validity of pediatric instruments for measuring health and well‐being of children with spastic cerebral palsy.Dev Med Child Neurol.2002;44:468–476.
- ,,,,.Needs of carers of severely disabled people: are they identified and met adequately?Health Soc Care Community.2001;9(4):235–243.
- ,,.The realities of postoperative disability and the carer's burden.Ann R Coll Surg Engl.2001;83(3):215–218.
- Abdidin.Parenting Stress Index.3rd ed.Lutz, FL:Psychological Assessment Resources, Inc.;1995.
- ,,,.Parental stress and burden following traumatic brain injury amongst children and adolescents.Brain Inj. Jan2003;17(1):1–23.
- ,,.Comparing stress levels of parents of children with cancer and parents of children with physical disabilities.Psychooncology. Dec2004;13(12):898–903.
- ,,.Stress levels experienced by the parents of enterally fed children.Child Care Health Dev.2004;30:507–513.
- ,,, et al.The health and well‐being of caregivers of children with cerebral palsy.Pediatrics.2005;115:e626–e636.
- ,.Parenting stress and children with heart disease.J Pediatr Health Care.2003;17(4):163–168.
- ,.Small sample inference for fixed effects from restricted maximum likelihood.Biometrics.1997;53:983–997.
- ,,.Methods for the analysis of quality‐of‐life and survival data in health technology assessment.Health Technol Assess.1999;3(10):1–152.
- ,,, et al.Fundoplication and gastrostomy versus image‐guided gastrojejunal tube for enteral feeding in neurologically impaired children with gastroesophageal reflux.J Pediatr Surg.2002;37:407–412.
- SF‐36 Health Survey: Manual and Interpretation Guide.Lincoln, RI:QualityMetric Inc.;1993,year="2000"2000.
- ,,.The role of protective antireflux procedures in neurologically impaired children: a decision analysis.J Pediatr Surg. Mar2002;37:500–506.
- ,,.Comparison of respiratory mortality in the profoundly mentally retarded and in the less retarded.J Ment Defic Res.1979;23(1):1–7.
- ,,.Cause of death in cerebral palsy: a descriptive study.Arch Dis Child.1999;81:390–394.
- .Survival rates of children with severe neurologic disabilities: a review.Semin Pediatr Neurol.2003;10(2):120–129.
- ,.Gastroesophageal reflux among severely retarded children.J Pediatr.1979;94:710–714.
- ,,,,,.Operation for gastro‐oesophageal reflux associated with severe mental retardation.Arch Dis Child.1993;68:347–351.
- ,,,,,.Prevalence and severity of feeding and nutritional problems in children with neurological impairment: Oxford Feeding Study.Dev Med Child Neurol.2000;42:674–680.
- ,,, et al.Aspiration pneumonia in pediatric age group: etiology, predisposing factors and clinical outcome.J Pak Med Assoc.1999;49(4):105–108.
- ,.Respiratory problems in children with neurological impairment.Arch Dis Child.2003;88(1):75–78.
- ,.Gastroesophageal reflux in childhood.Curr Probl Surg.1996;33(1):1–70.
- ,.Minimally invasive surgical techniques in reoperative surgery for gastroesophageal reflux disease in infants and children.Am Surg.2002;68:989–992.
- .Laparoscopic Nissen procedure in children.Semin Laparosc Surg.2002;9(3):146–152.
- ,,, et al.Caregiving process and caregiver burden: conceptual models to guide research and practice.BMC Pediatr.2004;4(1):1.
- ,.Theoretical and psychometric analysis of caregiver strain.Res Nurs Health.1996;19:499–510.
- ,,,,,.Complications and reoperation after Nissen fundoplication in childhood.Am J Surg.1987;153(2):177–183.
- ,,, et al.Surgical treatment of gastroesophageal reflux in children: a combined hospital study of 7467 patients.Pediatrics.1998;101:419–422.
- ,,,,,.Outcomes of surgical fundoplication in children.Clin Gastroenterol Hepatol.2004;2:978–984.
- ,,, et al.The respiratory advantage of laparoscopic Nissen fundoplication.J Pediatr Surg.2003;38:886–891.
- ,,,.Laparoscopic Nissen fundoplication in children: 2‐5‐year follow‐up.Pediatr Surg Int.2003;19:537–539.
- ,,.Recognition of recurrent gastroesophageal reflux following antireflux surgery in the neurologically disabled child: high index of suspicion and definitive evaluation.J Pediatr Surg.1992;27:983–988; discussion988–990.
- ,,.Sequelae of antireflux surgery in profoundly disabled children.J Pediatr Surg.1992;27(2):267–271; discussion271–263.
- ,,.Efficacy of the Nissen fundoplication in the management of gastroesophageal reflux following esophageal atresia repair.J Pediatr Surg.1993;28(1):53–55.
- ,,,.Caregivers' perceptions following gastrostomy in severely disabled children with feeding problems.Dev Med Child Neurol.1997;39:746–751.
- ,,,,.Care‐giver evaluation of anti‐gastroesophageal reflux procedures in neurologically impaired children: what is the real‐life outcome?J Pediatr Surg.1996;31:375–380.
- ,,, et al.Impact of gastrostomy tube feeding on the quality of life of carers of children with cerebral palsy.Dev Med Child Neurol.2004;46:796–800.
- ,.Organizational responses to managed care: issues for academic health centers and implications for pediatric programs.Pediatrics.1998;101:805–811; discussion811–802.
- Children's Digestive Health and Nutrition Foundation Website. Gastroesophageal Reflux Disease in the Neurologically Impaired Child. Available at: http://www.cdhnf.org/PractitionerSeries.asp. Accessed August 30,2006.
- ,,,,.Functional status of school‐aged children with Down syndrome.J Paediatr Child Health.2002;38(2):160–165.
- ,,,,,.Concurrent validity of the Functional Independence Measure for Children (WeeFIM) and the Pediatric Evaluation of Disabilities Inventory in children with developmental disabilities and acquired brain injuries.Phys Occup Ther Pediatr.2001;21(2–3):91–101.
- ,,, et al.The WeeFIM instrument: its utility in detecting change in children with developmental disabilities.Arch Phys Med Rehabil.2000;81:1317–1326.
- ,,, et al.Functional assessment and care of children with neurodevelopmental disabilities.Am J Phys Med Rehabil.2000;79(2):114–123.
- ,,,,,.Predictors of mortality, morbidity, and disability in a cohort of infants < or = 28 weeks' gestation.Clin Pediatr (Phila).1993;32:521–527.
- ,.Content validity of a pediatric functional independence measure.Appl Nurs Res.1990;3(3):120–122.
- ,,, et al.The Functional Independence Measure for Children (WeeFIM). Conceptual basis and pilot use in children with developmental disabilities.Clin Pediatr (Phila). Jul1994;33:421–430.
- ,,.The CHQ User's Manual.1st ed.Boston, MA:The Health Institute, New England Medical Center,1996.
- ,,,,,.Comparing reliability and validity of pediatric instruments for measuring health and well‐being of children with spastic cerebral palsy.Dev Med Child Neurol.2002;44:468–476.
- ,,,,.Needs of carers of severely disabled people: are they identified and met adequately?Health Soc Care Community.2001;9(4):235–243.
- ,,.The realities of postoperative disability and the carer's burden.Ann R Coll Surg Engl.2001;83(3):215–218.
- Abdidin.Parenting Stress Index.3rd ed.Lutz, FL:Psychological Assessment Resources, Inc.;1995.
- ,,,.Parental stress and burden following traumatic brain injury amongst children and adolescents.Brain Inj. Jan2003;17(1):1–23.
- ,,.Comparing stress levels of parents of children with cancer and parents of children with physical disabilities.Psychooncology. Dec2004;13(12):898–903.
- ,,.Stress levels experienced by the parents of enterally fed children.Child Care Health Dev.2004;30:507–513.
- ,,, et al.The health and well‐being of caregivers of children with cerebral palsy.Pediatrics.2005;115:e626–e636.
- ,.Parenting stress and children with heart disease.J Pediatr Health Care.2003;17(4):163–168.
- ,.Small sample inference for fixed effects from restricted maximum likelihood.Biometrics.1997;53:983–997.
- ,,.Methods for the analysis of quality‐of‐life and survival data in health technology assessment.Health Technol Assess.1999;3(10):1–152.
- ,,, et al.Fundoplication and gastrostomy versus image‐guided gastrojejunal tube for enteral feeding in neurologically impaired children with gastroesophageal reflux.J Pediatr Surg.2002;37:407–412.
- SF‐36 Health Survey: Manual and Interpretation Guide.Lincoln, RI:QualityMetric Inc.;1993,year="2000"2000.
- ,,.The role of protective antireflux procedures in neurologically impaired children: a decision analysis.J Pediatr Surg. Mar2002;37:500–506.