User login
NIH to Begin Long COVID Trial Focused on Sleep, Exercise
The National institutes of Health will soon start a clinical trial in an attempt to find potential treatments for symptoms of long COVID, focusing on sleep disturbances, problems with exercise, and post-exertional malaise.
“When people can’t get reliable sleep, can’t exert themselves and feel sick following tasks that used to be simple, the physical and mental anguish can lead to feelings of utter helplessness,” Walter J. Koroshetz, MD, director of the NIH’s National Institute of Neurological Disorders and Stroke, said in a statement. “We urgently need to come up with answers to help those struggling with long COVID feel whole again.”
The new trials will be part of the NIH’s Researching COVID to Enhance Recovery initiative, known as RECOVER. Since beginning enrollment in July 2023 for four trials, RECOVER now features eight trials across the country looking at all parts of long COVID. RECOVER is part of a $1.15 billion nationwide program that Congress approved in 2020 for the NIH to research and test treatments for long COVID.
While focused on sleep disturbances, the trial will test two Food and Drug Administration–approved drugs currently used to treat people with hypersomnia. There will also be a trial to test if melatonin helps people with long COVID-related sleep problems. Light therapy will also be tested.
The trials that deal with problems people have had with exercise will focus on personalized cardiopulmonary rehabilitation, where patients experiment with exercise training, strength and flexibility training, education, and social support.
Another trial will look at structured pacing, which is designed to help people with exercise problems identify, control, and ease long COVID symptoms.
A version of this article appeared on WebMD.com.
The National institutes of Health will soon start a clinical trial in an attempt to find potential treatments for symptoms of long COVID, focusing on sleep disturbances, problems with exercise, and post-exertional malaise.
“When people can’t get reliable sleep, can’t exert themselves and feel sick following tasks that used to be simple, the physical and mental anguish can lead to feelings of utter helplessness,” Walter J. Koroshetz, MD, director of the NIH’s National Institute of Neurological Disorders and Stroke, said in a statement. “We urgently need to come up with answers to help those struggling with long COVID feel whole again.”
The new trials will be part of the NIH’s Researching COVID to Enhance Recovery initiative, known as RECOVER. Since beginning enrollment in July 2023 for four trials, RECOVER now features eight trials across the country looking at all parts of long COVID. RECOVER is part of a $1.15 billion nationwide program that Congress approved in 2020 for the NIH to research and test treatments for long COVID.
While focused on sleep disturbances, the trial will test two Food and Drug Administration–approved drugs currently used to treat people with hypersomnia. There will also be a trial to test if melatonin helps people with long COVID-related sleep problems. Light therapy will also be tested.
The trials that deal with problems people have had with exercise will focus on personalized cardiopulmonary rehabilitation, where patients experiment with exercise training, strength and flexibility training, education, and social support.
Another trial will look at structured pacing, which is designed to help people with exercise problems identify, control, and ease long COVID symptoms.
A version of this article appeared on WebMD.com.
The National institutes of Health will soon start a clinical trial in an attempt to find potential treatments for symptoms of long COVID, focusing on sleep disturbances, problems with exercise, and post-exertional malaise.
“When people can’t get reliable sleep, can’t exert themselves and feel sick following tasks that used to be simple, the physical and mental anguish can lead to feelings of utter helplessness,” Walter J. Koroshetz, MD, director of the NIH’s National Institute of Neurological Disorders and Stroke, said in a statement. “We urgently need to come up with answers to help those struggling with long COVID feel whole again.”
The new trials will be part of the NIH’s Researching COVID to Enhance Recovery initiative, known as RECOVER. Since beginning enrollment in July 2023 for four trials, RECOVER now features eight trials across the country looking at all parts of long COVID. RECOVER is part of a $1.15 billion nationwide program that Congress approved in 2020 for the NIH to research and test treatments for long COVID.
While focused on sleep disturbances, the trial will test two Food and Drug Administration–approved drugs currently used to treat people with hypersomnia. There will also be a trial to test if melatonin helps people with long COVID-related sleep problems. Light therapy will also be tested.
The trials that deal with problems people have had with exercise will focus on personalized cardiopulmonary rehabilitation, where patients experiment with exercise training, strength and flexibility training, education, and social support.
Another trial will look at structured pacing, which is designed to help people with exercise problems identify, control, and ease long COVID symptoms.
A version of this article appeared on WebMD.com.
Biden to end COVID emergencies in May
Doing so will have many effects, including the end of free vaccines and health services to fight the pandemic. The public health emergency has been renewed every 90 days since it was declared by the Trump administration in January 2020.
The declaration allowed major changes throughout the health care system to deal with the pandemic, including the free distribution of vaccines, testing, and treatments. In addition, telehealth services were expanded, and Medicaid and the Children’s Health Insurance Program were extended to millions more Americans.
Biden said the COVID-19 national emergency is set to expire March 1 while the declared public health emergency would currently expire on April 11. The president said both will be extended to end May 11.
There were nearly 300,000 newly reported COVID-19 cases in the United States for the week ending Jan. 25, according to CDC data, as well as more than 3,750 deaths.
A version of this article first appeared on WebMD.com.
Doing so will have many effects, including the end of free vaccines and health services to fight the pandemic. The public health emergency has been renewed every 90 days since it was declared by the Trump administration in January 2020.
The declaration allowed major changes throughout the health care system to deal with the pandemic, including the free distribution of vaccines, testing, and treatments. In addition, telehealth services were expanded, and Medicaid and the Children’s Health Insurance Program were extended to millions more Americans.
Biden said the COVID-19 national emergency is set to expire March 1 while the declared public health emergency would currently expire on April 11. The president said both will be extended to end May 11.
There were nearly 300,000 newly reported COVID-19 cases in the United States for the week ending Jan. 25, according to CDC data, as well as more than 3,750 deaths.
A version of this article first appeared on WebMD.com.
Doing so will have many effects, including the end of free vaccines and health services to fight the pandemic. The public health emergency has been renewed every 90 days since it was declared by the Trump administration in January 2020.
The declaration allowed major changes throughout the health care system to deal with the pandemic, including the free distribution of vaccines, testing, and treatments. In addition, telehealth services were expanded, and Medicaid and the Children’s Health Insurance Program were extended to millions more Americans.
Biden said the COVID-19 national emergency is set to expire March 1 while the declared public health emergency would currently expire on April 11. The president said both will be extended to end May 11.
There were nearly 300,000 newly reported COVID-19 cases in the United States for the week ending Jan. 25, according to CDC data, as well as more than 3,750 deaths.
A version of this article first appeared on WebMD.com.
FDA grants emergency authorization for Novavax COVID vaccine
on July 13.
The vaccine is authorized for adults only. Should the Centers for Disease Control and Prevention follow suit and approve its use, Novavax would join Moderna, Pfizer and Johnson & Johnson on the U.S. market. A CDC panel of advisors is expected to consider the new entry on July 19.
The Novavax vaccine is only for those who have not yet been vaccinated at all.
“Today’s authorization offers adults in the United States who have not yet received a COVID-19 vaccine another option that meets the FDA’s rigorous standards for safety, effectiveness and manufacturing quality needed to support emergency use authorization,” FDA Commissioner Robert Califf, MD, said in a statement. “COVID-19 vaccines remain the best preventive measure against severe disease caused by COVID-19 and I encourage anyone who is eligible for, but has not yet received a COVID-19 vaccine, to consider doing so.”
The Novavax vaccine is protein-based, making it different than mRNA vaccines from Pfizer and Moderna. It contains harmless elements of actual coronavirus spike protein and an ingredient known as a adjuvant that enhances the patient’s immune response.
Clinical trials found the vaccine to be 90.4% effective in preventing mild, moderate or severe COVID-19. Only 17 patients out of 17,200 developed COVID-19 after receiving both doses.
The FDA said, however, that Novavax’s vaccine did show evidence of increased risk of myocarditis – inflammation of the heart – and pericarditis, inflammation of tissue surrounding the heart. In most people both disorders began within 10 days.
A version of this article first appeared on WebMD.com.
on July 13.
The vaccine is authorized for adults only. Should the Centers for Disease Control and Prevention follow suit and approve its use, Novavax would join Moderna, Pfizer and Johnson & Johnson on the U.S. market. A CDC panel of advisors is expected to consider the new entry on July 19.
The Novavax vaccine is only for those who have not yet been vaccinated at all.
“Today’s authorization offers adults in the United States who have not yet received a COVID-19 vaccine another option that meets the FDA’s rigorous standards for safety, effectiveness and manufacturing quality needed to support emergency use authorization,” FDA Commissioner Robert Califf, MD, said in a statement. “COVID-19 vaccines remain the best preventive measure against severe disease caused by COVID-19 and I encourage anyone who is eligible for, but has not yet received a COVID-19 vaccine, to consider doing so.”
The Novavax vaccine is protein-based, making it different than mRNA vaccines from Pfizer and Moderna. It contains harmless elements of actual coronavirus spike protein and an ingredient known as a adjuvant that enhances the patient’s immune response.
Clinical trials found the vaccine to be 90.4% effective in preventing mild, moderate or severe COVID-19. Only 17 patients out of 17,200 developed COVID-19 after receiving both doses.
The FDA said, however, that Novavax’s vaccine did show evidence of increased risk of myocarditis – inflammation of the heart – and pericarditis, inflammation of tissue surrounding the heart. In most people both disorders began within 10 days.
A version of this article first appeared on WebMD.com.
on July 13.
The vaccine is authorized for adults only. Should the Centers for Disease Control and Prevention follow suit and approve its use, Novavax would join Moderna, Pfizer and Johnson & Johnson on the U.S. market. A CDC panel of advisors is expected to consider the new entry on July 19.
The Novavax vaccine is only for those who have not yet been vaccinated at all.
“Today’s authorization offers adults in the United States who have not yet received a COVID-19 vaccine another option that meets the FDA’s rigorous standards for safety, effectiveness and manufacturing quality needed to support emergency use authorization,” FDA Commissioner Robert Califf, MD, said in a statement. “COVID-19 vaccines remain the best preventive measure against severe disease caused by COVID-19 and I encourage anyone who is eligible for, but has not yet received a COVID-19 vaccine, to consider doing so.”
The Novavax vaccine is protein-based, making it different than mRNA vaccines from Pfizer and Moderna. It contains harmless elements of actual coronavirus spike protein and an ingredient known as a adjuvant that enhances the patient’s immune response.
Clinical trials found the vaccine to be 90.4% effective in preventing mild, moderate or severe COVID-19. Only 17 patients out of 17,200 developed COVID-19 after receiving both doses.
The FDA said, however, that Novavax’s vaccine did show evidence of increased risk of myocarditis – inflammation of the heart – and pericarditis, inflammation of tissue surrounding the heart. In most people both disorders began within 10 days.
A version of this article first appeared on WebMD.com.
Supreme Court appears ready to overturn Roe
to the news outlet Politico.
The draft opinion, written by Justice Samuel Alito, outlines ways a presumed majority of the nine justices believes the 1973 ruling in Roe v. Wade was incorrect. If signed by a majority of the court, the ruling would eliminate the protections for abortion rights that Roe provided and give the 50 states the power to legislate abortion.
“We hold that Roe and Casey must be overruled,” Justice Alito writes in the draft. “It is time to heed the Constitution and return the issue of abortion to the people’s elected representatives.”
While a final ruling was not expected from the court until June, the leaked draft – a nearly unprecedented breach of the court’s internal workings – gives a strong signal of the court’s five most conservative members’ decisions. During oral arguments in the case in December, conservative justices appeared prepared to undo at least part of the country’s abortion protections.
President Joe Biden said his administration was already preparing for a potential ruling that struck down federal abortion protections.
The White House, he said in a statement, is working on a “response to the continued attack on abortion and reproductive rights, under a variety of possible outcomes in the cases pending before the Supreme Court. We will be ready when any ruling is issued.”
But if the draft opinion becomes final, he said the fight will move to the states.
“It will fall on our nation’s elected officials at all levels of government to protect a woman’s right to choose,” he said. “And it will fall on voters to elect pro-choice officials this November.”
With more pro-abortion rights members of Congress, it would be possible to pass federal legislation protecting abortion rights, “which I will work to pass and sign into law.”
Should the Alito draft become law, its first impact would be to allow a Mississippi law that bans abortions after 15 weeks to take effect.
But quickly after that, abortions would become illegal in many states. Several conservative-leaning states, mostly in the South and Midwest, have already passed laws severely restricting abortions well beyond what Roe allowed. Should Roe be overturned then, those laws would take effect without the threat of lengthy lawsuits or rulings from lower-court judges who have blocked them.
Nearly half of the states, mostly in the Northeast and West, would likely allow abortion to continue in some way. In fact, several states, including Colorado and Vermont, have already passed laws granting the right to an abortion into state law.
The leaked draft, however, is still a draft, meaning it remains possible Roe survives. Anthony Kreis, PhD, a professor of law at Georgia State University, says that could have been the point of whoever leaked the draft.
“It suggests to me that whoever leaked it knew that public outrage was the last resort to stopping the court from overturning Roe v. Wade and letting states ban all abortions,” Dr. Kreis said. “The danger that abortions won’t be legal in most of the country is very real.”
A version of this article first appeared on WebMD.com.
This article was updated 5/3/22.
to the news outlet Politico.
The draft opinion, written by Justice Samuel Alito, outlines ways a presumed majority of the nine justices believes the 1973 ruling in Roe v. Wade was incorrect. If signed by a majority of the court, the ruling would eliminate the protections for abortion rights that Roe provided and give the 50 states the power to legislate abortion.
“We hold that Roe and Casey must be overruled,” Justice Alito writes in the draft. “It is time to heed the Constitution and return the issue of abortion to the people’s elected representatives.”
While a final ruling was not expected from the court until June, the leaked draft – a nearly unprecedented breach of the court’s internal workings – gives a strong signal of the court’s five most conservative members’ decisions. During oral arguments in the case in December, conservative justices appeared prepared to undo at least part of the country’s abortion protections.
President Joe Biden said his administration was already preparing for a potential ruling that struck down federal abortion protections.
The White House, he said in a statement, is working on a “response to the continued attack on abortion and reproductive rights, under a variety of possible outcomes in the cases pending before the Supreme Court. We will be ready when any ruling is issued.”
But if the draft opinion becomes final, he said the fight will move to the states.
“It will fall on our nation’s elected officials at all levels of government to protect a woman’s right to choose,” he said. “And it will fall on voters to elect pro-choice officials this November.”
With more pro-abortion rights members of Congress, it would be possible to pass federal legislation protecting abortion rights, “which I will work to pass and sign into law.”
Should the Alito draft become law, its first impact would be to allow a Mississippi law that bans abortions after 15 weeks to take effect.
But quickly after that, abortions would become illegal in many states. Several conservative-leaning states, mostly in the South and Midwest, have already passed laws severely restricting abortions well beyond what Roe allowed. Should Roe be overturned then, those laws would take effect without the threat of lengthy lawsuits or rulings from lower-court judges who have blocked them.
Nearly half of the states, mostly in the Northeast and West, would likely allow abortion to continue in some way. In fact, several states, including Colorado and Vermont, have already passed laws granting the right to an abortion into state law.
The leaked draft, however, is still a draft, meaning it remains possible Roe survives. Anthony Kreis, PhD, a professor of law at Georgia State University, says that could have been the point of whoever leaked the draft.
“It suggests to me that whoever leaked it knew that public outrage was the last resort to stopping the court from overturning Roe v. Wade and letting states ban all abortions,” Dr. Kreis said. “The danger that abortions won’t be legal in most of the country is very real.”
A version of this article first appeared on WebMD.com.
This article was updated 5/3/22.
to the news outlet Politico.
The draft opinion, written by Justice Samuel Alito, outlines ways a presumed majority of the nine justices believes the 1973 ruling in Roe v. Wade was incorrect. If signed by a majority of the court, the ruling would eliminate the protections for abortion rights that Roe provided and give the 50 states the power to legislate abortion.
“We hold that Roe and Casey must be overruled,” Justice Alito writes in the draft. “It is time to heed the Constitution and return the issue of abortion to the people’s elected representatives.”
While a final ruling was not expected from the court until June, the leaked draft – a nearly unprecedented breach of the court’s internal workings – gives a strong signal of the court’s five most conservative members’ decisions. During oral arguments in the case in December, conservative justices appeared prepared to undo at least part of the country’s abortion protections.
President Joe Biden said his administration was already preparing for a potential ruling that struck down federal abortion protections.
The White House, he said in a statement, is working on a “response to the continued attack on abortion and reproductive rights, under a variety of possible outcomes in the cases pending before the Supreme Court. We will be ready when any ruling is issued.”
But if the draft opinion becomes final, he said the fight will move to the states.
“It will fall on our nation’s elected officials at all levels of government to protect a woman’s right to choose,” he said. “And it will fall on voters to elect pro-choice officials this November.”
With more pro-abortion rights members of Congress, it would be possible to pass federal legislation protecting abortion rights, “which I will work to pass and sign into law.”
Should the Alito draft become law, its first impact would be to allow a Mississippi law that bans abortions after 15 weeks to take effect.
But quickly after that, abortions would become illegal in many states. Several conservative-leaning states, mostly in the South and Midwest, have already passed laws severely restricting abortions well beyond what Roe allowed. Should Roe be overturned then, those laws would take effect without the threat of lengthy lawsuits or rulings from lower-court judges who have blocked them.
Nearly half of the states, mostly in the Northeast and West, would likely allow abortion to continue in some way. In fact, several states, including Colorado and Vermont, have already passed laws granting the right to an abortion into state law.
The leaked draft, however, is still a draft, meaning it remains possible Roe survives. Anthony Kreis, PhD, a professor of law at Georgia State University, says that could have been the point of whoever leaked the draft.
“It suggests to me that whoever leaked it knew that public outrage was the last resort to stopping the court from overturning Roe v. Wade and letting states ban all abortions,” Dr. Kreis said. “The danger that abortions won’t be legal in most of the country is very real.”
A version of this article first appeared on WebMD.com.
This article was updated 5/3/22.
Judge strikes down Biden mask mandate for planes, transit
The mandate, enacted in February 2021, is unconstitutional because Congress never granted the Centers for Disease Control and Prevention the power to create such a requirement, U.S. District Judge Kathryn Kimball Mizelle said in her order issued April 18.
“Congress addressed whether the CDC may enact preventative measures that condition the interstate travel of an entire population to CDC dictates. It may not,” the order says.
While the government argued that the definition of “sanitation” in federal law allows it to create travel restrictions like the use of masks, Judge Mizelle disagreed.
“A power to improve ‘sanitation’ would easily extend to requiring vaccinations against COVID-19, the seasonal flu, or other diseases. Or to mandatory social distancing, coughing-into-elbows, and daily multivitamins,” she wrote.
The Biden administration has extended the mask mandate several times since it was first announced. Most recently, the mandate was extended last week and was set to end May 3.
The rule has been alternately praised and criticized by airlines, pilots, and flight attendants. Lawsuits have been filed over the mandate, but Judge Mizelle ruled in favor of two people and the Health Freedom Defense Fund, who filed suit in July 2021.
It is not yet clear if the Biden administration will appeal the decision.
A version of this article first appeared on WebMD.com.
The mandate, enacted in February 2021, is unconstitutional because Congress never granted the Centers for Disease Control and Prevention the power to create such a requirement, U.S. District Judge Kathryn Kimball Mizelle said in her order issued April 18.
“Congress addressed whether the CDC may enact preventative measures that condition the interstate travel of an entire population to CDC dictates. It may not,” the order says.
While the government argued that the definition of “sanitation” in federal law allows it to create travel restrictions like the use of masks, Judge Mizelle disagreed.
“A power to improve ‘sanitation’ would easily extend to requiring vaccinations against COVID-19, the seasonal flu, or other diseases. Or to mandatory social distancing, coughing-into-elbows, and daily multivitamins,” she wrote.
The Biden administration has extended the mask mandate several times since it was first announced. Most recently, the mandate was extended last week and was set to end May 3.
The rule has been alternately praised and criticized by airlines, pilots, and flight attendants. Lawsuits have been filed over the mandate, but Judge Mizelle ruled in favor of two people and the Health Freedom Defense Fund, who filed suit in July 2021.
It is not yet clear if the Biden administration will appeal the decision.
A version of this article first appeared on WebMD.com.
The mandate, enacted in February 2021, is unconstitutional because Congress never granted the Centers for Disease Control and Prevention the power to create such a requirement, U.S. District Judge Kathryn Kimball Mizelle said in her order issued April 18.
“Congress addressed whether the CDC may enact preventative measures that condition the interstate travel of an entire population to CDC dictates. It may not,” the order says.
While the government argued that the definition of “sanitation” in federal law allows it to create travel restrictions like the use of masks, Judge Mizelle disagreed.
“A power to improve ‘sanitation’ would easily extend to requiring vaccinations against COVID-19, the seasonal flu, or other diseases. Or to mandatory social distancing, coughing-into-elbows, and daily multivitamins,” she wrote.
The Biden administration has extended the mask mandate several times since it was first announced. Most recently, the mandate was extended last week and was set to end May 3.
The rule has been alternately praised and criticized by airlines, pilots, and flight attendants. Lawsuits have been filed over the mandate, but Judge Mizelle ruled in favor of two people and the Health Freedom Defense Fund, who filed suit in July 2021.
It is not yet clear if the Biden administration will appeal the decision.
A version of this article first appeared on WebMD.com.
FDA delays action on Pfizer vaccine for kids under 5
for younger children until data on the effects of three doses is available.
Peter Marks, MD, director of the FDA’s Center for Biologics Evaluation and Research, said the plan for a meeting the week of Feb. 14 of the FDA’s Vaccines and Related Biological Products Advisory Committee was to “understand if two doses would provide sufficient protection to move forward.”
Pfizer has asked the FDA to authorize the use of its mRNA vaccine for children under the age of 5. But, Dr. Marks said, “in looking through the data we realized now … that at this time it makes sense for us to wait until we have the data of the evaluation of a third dose before taking action.”
“If we feel something doesn’t meet (our) standard, we can’t go forward,” he said. “Rather than an issue of having anyone question the process, I hope this reassures people that the process has a standard.”
Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, predicted in January that the Pfizer vaccine for younger kids could be available this month. But, he also predicted three doses would be required.
Pfizer announced in mid-December that it planned to submit data to the FDA during the first half of 2022 if the three-dose study was successful. At that time, Pfizer said it didn’t identify any safety concerns with the 3-microgram dose for children ages 6 months to 4 years, which is much lower than the 30-microgram dose given to adults.
A version of this article first appeared on WebMD.com.
for younger children until data on the effects of three doses is available.
Peter Marks, MD, director of the FDA’s Center for Biologics Evaluation and Research, said the plan for a meeting the week of Feb. 14 of the FDA’s Vaccines and Related Biological Products Advisory Committee was to “understand if two doses would provide sufficient protection to move forward.”
Pfizer has asked the FDA to authorize the use of its mRNA vaccine for children under the age of 5. But, Dr. Marks said, “in looking through the data we realized now … that at this time it makes sense for us to wait until we have the data of the evaluation of a third dose before taking action.”
“If we feel something doesn’t meet (our) standard, we can’t go forward,” he said. “Rather than an issue of having anyone question the process, I hope this reassures people that the process has a standard.”
Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, predicted in January that the Pfizer vaccine for younger kids could be available this month. But, he also predicted three doses would be required.
Pfizer announced in mid-December that it planned to submit data to the FDA during the first half of 2022 if the three-dose study was successful. At that time, Pfizer said it didn’t identify any safety concerns with the 3-microgram dose for children ages 6 months to 4 years, which is much lower than the 30-microgram dose given to adults.
A version of this article first appeared on WebMD.com.
for younger children until data on the effects of three doses is available.
Peter Marks, MD, director of the FDA’s Center for Biologics Evaluation and Research, said the plan for a meeting the week of Feb. 14 of the FDA’s Vaccines and Related Biological Products Advisory Committee was to “understand if two doses would provide sufficient protection to move forward.”
Pfizer has asked the FDA to authorize the use of its mRNA vaccine for children under the age of 5. But, Dr. Marks said, “in looking through the data we realized now … that at this time it makes sense for us to wait until we have the data of the evaluation of a third dose before taking action.”
“If we feel something doesn’t meet (our) standard, we can’t go forward,” he said. “Rather than an issue of having anyone question the process, I hope this reassures people that the process has a standard.”
Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, predicted in January that the Pfizer vaccine for younger kids could be available this month. But, he also predicted three doses would be required.
Pfizer announced in mid-December that it planned to submit data to the FDA during the first half of 2022 if the three-dose study was successful. At that time, Pfizer said it didn’t identify any safety concerns with the 3-microgram dose for children ages 6 months to 4 years, which is much lower than the 30-microgram dose given to adults.
A version of this article first appeared on WebMD.com.
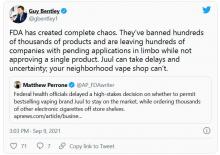
FDA moves to block some vape products, delays action on Juul
The agency had a court-ordered deadline of Sept. 9 to review more than 6.5 million applications for approval of what are considered new tobacco products – the vast majority of which are e-cigarettes and liquids, none of which have gone through FDA review before.
The FDA reviewed 93% of those applications in the past year, acting FDA Commissioner Janet Woodcock, MD, and Mitch Zeller, director of the FDA’s Center for Tobacco Products, said in a statement.
Of those reviewed, the agency rejected more than 946,000 flavored vape products, “because their applications lacked sufficient evidence that they have a benefit to adult smokers sufficient to overcome the public health threat posed by the well-documented, alarming levels of youth use of such products,” Dr. Woodcock and Mr. Zeller said.
The pair said more work is needed to finish the reviews to “ensure that we continue taking appropriate action to protect our nation’s youth from the dangers of all tobacco products, including e-cigarettes, which remain the most commonly used tobacco product by youth in the United States.”
No e-cigarette product has been given official FDA approval to be sold, meaning all e-cigarette products technically are on the market illegally, the agency said in 2020, but federal officials decided only to begin enforcing rules against flavored products, which surveys show are more often used by children. Tobacco-flavored and menthol e-cigarette products – which some adults use to quit smoking cigarettes – were exempted.
The American Cancer Society and other advocacy groups slammed the FDA’s decision to withhold action on major e-cigarette manufacturers, including Juul.
“The FDA’s failure today to act on applications by Juul, the manufacturer with the single biggest e-cigarette market share, is extremely disappointing and will allow the industry to further endanger public health and hook more kids on their highly addictive products,” Lisa Lacasse, president of ACS CAN, said in a statement, according to CNN.
“The FDA has had ample time to review the applications and allowing additional delays is unconscionable. There is overwhelming data to demonstrate the negative impact these kinds of flavored products have had on public health and their role in the youth e-cigarette epidemic. The time to act is now,” Ms. Lacasse added.
E-cigarette use among high school students rose from 11.7% in 2017 to 19.6% in 2020, the American Cancer Society said. Nearly 5% of middle schoolers reported using them in 2020.
A version of this article first appeared on WebMD.com.
The agency had a court-ordered deadline of Sept. 9 to review more than 6.5 million applications for approval of what are considered new tobacco products – the vast majority of which are e-cigarettes and liquids, none of which have gone through FDA review before.
The FDA reviewed 93% of those applications in the past year, acting FDA Commissioner Janet Woodcock, MD, and Mitch Zeller, director of the FDA’s Center for Tobacco Products, said in a statement.
Of those reviewed, the agency rejected more than 946,000 flavored vape products, “because their applications lacked sufficient evidence that they have a benefit to adult smokers sufficient to overcome the public health threat posed by the well-documented, alarming levels of youth use of such products,” Dr. Woodcock and Mr. Zeller said.
The pair said more work is needed to finish the reviews to “ensure that we continue taking appropriate action to protect our nation’s youth from the dangers of all tobacco products, including e-cigarettes, which remain the most commonly used tobacco product by youth in the United States.”
No e-cigarette product has been given official FDA approval to be sold, meaning all e-cigarette products technically are on the market illegally, the agency said in 2020, but federal officials decided only to begin enforcing rules against flavored products, which surveys show are more often used by children. Tobacco-flavored and menthol e-cigarette products – which some adults use to quit smoking cigarettes – were exempted.
The American Cancer Society and other advocacy groups slammed the FDA’s decision to withhold action on major e-cigarette manufacturers, including Juul.
“The FDA’s failure today to act on applications by Juul, the manufacturer with the single biggest e-cigarette market share, is extremely disappointing and will allow the industry to further endanger public health and hook more kids on their highly addictive products,” Lisa Lacasse, president of ACS CAN, said in a statement, according to CNN.
“The FDA has had ample time to review the applications and allowing additional delays is unconscionable. There is overwhelming data to demonstrate the negative impact these kinds of flavored products have had on public health and their role in the youth e-cigarette epidemic. The time to act is now,” Ms. Lacasse added.
E-cigarette use among high school students rose from 11.7% in 2017 to 19.6% in 2020, the American Cancer Society said. Nearly 5% of middle schoolers reported using them in 2020.
A version of this article first appeared on WebMD.com.
The agency had a court-ordered deadline of Sept. 9 to review more than 6.5 million applications for approval of what are considered new tobacco products – the vast majority of which are e-cigarettes and liquids, none of which have gone through FDA review before.
The FDA reviewed 93% of those applications in the past year, acting FDA Commissioner Janet Woodcock, MD, and Mitch Zeller, director of the FDA’s Center for Tobacco Products, said in a statement.
Of those reviewed, the agency rejected more than 946,000 flavored vape products, “because their applications lacked sufficient evidence that they have a benefit to adult smokers sufficient to overcome the public health threat posed by the well-documented, alarming levels of youth use of such products,” Dr. Woodcock and Mr. Zeller said.
The pair said more work is needed to finish the reviews to “ensure that we continue taking appropriate action to protect our nation’s youth from the dangers of all tobacco products, including e-cigarettes, which remain the most commonly used tobacco product by youth in the United States.”
No e-cigarette product has been given official FDA approval to be sold, meaning all e-cigarette products technically are on the market illegally, the agency said in 2020, but federal officials decided only to begin enforcing rules against flavored products, which surveys show are more often used by children. Tobacco-flavored and menthol e-cigarette products – which some adults use to quit smoking cigarettes – were exempted.
The American Cancer Society and other advocacy groups slammed the FDA’s decision to withhold action on major e-cigarette manufacturers, including Juul.
“The FDA’s failure today to act on applications by Juul, the manufacturer with the single biggest e-cigarette market share, is extremely disappointing and will allow the industry to further endanger public health and hook more kids on their highly addictive products,” Lisa Lacasse, president of ACS CAN, said in a statement, according to CNN.
“The FDA has had ample time to review the applications and allowing additional delays is unconscionable. There is overwhelming data to demonstrate the negative impact these kinds of flavored products have had on public health and their role in the youth e-cigarette epidemic. The time to act is now,” Ms. Lacasse added.
E-cigarette use among high school students rose from 11.7% in 2017 to 19.6% in 2020, the American Cancer Society said. Nearly 5% of middle schoolers reported using them in 2020.
A version of this article first appeared on WebMD.com.
CDC to show vaccinated people infected with Delta remain contagious
and infect others, the New York Times reported on July 29.
The revelation is one reason the agency reversed course this week and said fully vaccinated people should go back to wearing masks in many cases.
The new findings also are a reversal from what scientists had believed to be true about other variants of the virus, the New York Times said. The bottom line is that the CDC data shows people with so-called breakthrough cases of the Delta variant may be just as contagious as unvaccinated people, even if they do not show symptoms.
ABC News reported earlier on Jul 29 that the CDC’s updated mask guidance followed an outbreak on Cape Cod, where crowds gathered for the Fourth of July.
As of July 29, 882 people were tied to the outbreak centered in Provincetown, Mass. Of those who live in Massachusetts, 74% were unvaccinated. ABC said the majority were showing symptoms of COVID-19.
A version of this article first appeared on Medscape.com.
and infect others, the New York Times reported on July 29.
The revelation is one reason the agency reversed course this week and said fully vaccinated people should go back to wearing masks in many cases.
The new findings also are a reversal from what scientists had believed to be true about other variants of the virus, the New York Times said. The bottom line is that the CDC data shows people with so-called breakthrough cases of the Delta variant may be just as contagious as unvaccinated people, even if they do not show symptoms.
ABC News reported earlier on Jul 29 that the CDC’s updated mask guidance followed an outbreak on Cape Cod, where crowds gathered for the Fourth of July.
As of July 29, 882 people were tied to the outbreak centered in Provincetown, Mass. Of those who live in Massachusetts, 74% were unvaccinated. ABC said the majority were showing symptoms of COVID-19.
A version of this article first appeared on Medscape.com.
and infect others, the New York Times reported on July 29.
The revelation is one reason the agency reversed course this week and said fully vaccinated people should go back to wearing masks in many cases.
The new findings also are a reversal from what scientists had believed to be true about other variants of the virus, the New York Times said. The bottom line is that the CDC data shows people with so-called breakthrough cases of the Delta variant may be just as contagious as unvaccinated people, even if they do not show symptoms.
ABC News reported earlier on Jul 29 that the CDC’s updated mask guidance followed an outbreak on Cape Cod, where crowds gathered for the Fourth of July.
As of July 29, 882 people were tied to the outbreak centered in Provincetown, Mass. Of those who live in Massachusetts, 74% were unvaccinated. ABC said the majority were showing symptoms of COVID-19.
A version of this article first appeared on Medscape.com.
Record number of U.S. drug overdoses in 2020
More Americans died from drug overdoses in 2020 than in any other year, the CDC said July 14.
, according to the provisional data the National Center for Health Statistics reported.
The spikes are largely attributed to the rise in use of fentanyl and other synthetic opioids.
The Washington Post reported that more than 69,000 overdose deaths involved opioids, up from 50,963 in 2019.
Amid the crush of overdoses, the White House announced that President Joe Biden has nominated Rahul Gupta, MD, to lead the White House Office of National Drug Control Policy.
Dr. Gupta is a former health commissioner of West Virginia, and is chief medical and health officer for the March of Dimes.
“Dr. Gupta led efforts in West Virginia to address the opioid crisis, gaining national prominence as a leader in tackling this issue,” March of Dimes President and CEO Stacey Stewart said in a statement. “At March of Dimes, he has advocated for policies and programs to prevent and treat substance use, with a focus on the safety and care of pregnant women and infants.”
Healthday contributed to this report. A version of this article first appeared on WebMD.com.
More Americans died from drug overdoses in 2020 than in any other year, the CDC said July 14.
, according to the provisional data the National Center for Health Statistics reported.
The spikes are largely attributed to the rise in use of fentanyl and other synthetic opioids.
The Washington Post reported that more than 69,000 overdose deaths involved opioids, up from 50,963 in 2019.
Amid the crush of overdoses, the White House announced that President Joe Biden has nominated Rahul Gupta, MD, to lead the White House Office of National Drug Control Policy.
Dr. Gupta is a former health commissioner of West Virginia, and is chief medical and health officer for the March of Dimes.
“Dr. Gupta led efforts in West Virginia to address the opioid crisis, gaining national prominence as a leader in tackling this issue,” March of Dimes President and CEO Stacey Stewart said in a statement. “At March of Dimes, he has advocated for policies and programs to prevent and treat substance use, with a focus on the safety and care of pregnant women and infants.”
Healthday contributed to this report. A version of this article first appeared on WebMD.com.
More Americans died from drug overdoses in 2020 than in any other year, the CDC said July 14.
, according to the provisional data the National Center for Health Statistics reported.
The spikes are largely attributed to the rise in use of fentanyl and other synthetic opioids.
The Washington Post reported that more than 69,000 overdose deaths involved opioids, up from 50,963 in 2019.
Amid the crush of overdoses, the White House announced that President Joe Biden has nominated Rahul Gupta, MD, to lead the White House Office of National Drug Control Policy.
Dr. Gupta is a former health commissioner of West Virginia, and is chief medical and health officer for the March of Dimes.
“Dr. Gupta led efforts in West Virginia to address the opioid crisis, gaining national prominence as a leader in tackling this issue,” March of Dimes President and CEO Stacey Stewart said in a statement. “At March of Dimes, he has advocated for policies and programs to prevent and treat substance use, with a focus on the safety and care of pregnant women and infants.”
Healthday contributed to this report. A version of this article first appeared on WebMD.com.
Top JAMA editor on leave amid podcast investigation
The American Medical Association’s Joint Oversight Committee announced that Howard Bauchner, MD, is on leave beginning at the end of the day on March 25. Dr. Bauchner is the top editor at JAMA, the journal of the AMA.
“The decision to place the editor-in-chief on administrative leave neither implicates nor exonerates individuals and is standard operating procedure for such investigations,” the committee said in a statement.
More than 2,000 people signed a petition on Change.org calling for an investigation at JAMA over the February podcast episode, called “Structural Racism for Doctors: What Is It?”
Already, Edward H. Livingston, MD, the host of the podcast, has resigned as deputy editor of the journal.
During the podcast, Dr. Livingston, who is White, said, “Structural racism is an unfortunate term. Personally, I think taking racism out of the conversation will help. Many of us are offended by the concept that we are racist.”
The audio of the podcast has been deleted from JAMA’s website. In its place is audio of a statement from Dr. Bauchner. In his statement, which he released in the week prior to his being on leave, he said the comments in the podcast, which also featured Mitch Katz, MD, were “inaccurate, offensive, hurtful, and inconsistent with the standards of JAMA.”
Also deleted was a JAMA tweet promoting the podcast episode. The tweet said: “No physician is racist, so how can there be structural racism in health care? An explanation of the idea by doctors for doctors in this user-friendly podcast.”
This story will be updated.
A version of this article first appeared on WedMD.com.
The American Medical Association’s Joint Oversight Committee announced that Howard Bauchner, MD, is on leave beginning at the end of the day on March 25. Dr. Bauchner is the top editor at JAMA, the journal of the AMA.
“The decision to place the editor-in-chief on administrative leave neither implicates nor exonerates individuals and is standard operating procedure for such investigations,” the committee said in a statement.
More than 2,000 people signed a petition on Change.org calling for an investigation at JAMA over the February podcast episode, called “Structural Racism for Doctors: What Is It?”
Already, Edward H. Livingston, MD, the host of the podcast, has resigned as deputy editor of the journal.
During the podcast, Dr. Livingston, who is White, said, “Structural racism is an unfortunate term. Personally, I think taking racism out of the conversation will help. Many of us are offended by the concept that we are racist.”
The audio of the podcast has been deleted from JAMA’s website. In its place is audio of a statement from Dr. Bauchner. In his statement, which he released in the week prior to his being on leave, he said the comments in the podcast, which also featured Mitch Katz, MD, were “inaccurate, offensive, hurtful, and inconsistent with the standards of JAMA.”
Also deleted was a JAMA tweet promoting the podcast episode. The tweet said: “No physician is racist, so how can there be structural racism in health care? An explanation of the idea by doctors for doctors in this user-friendly podcast.”
This story will be updated.
A version of this article first appeared on WedMD.com.
The American Medical Association’s Joint Oversight Committee announced that Howard Bauchner, MD, is on leave beginning at the end of the day on March 25. Dr. Bauchner is the top editor at JAMA, the journal of the AMA.
“The decision to place the editor-in-chief on administrative leave neither implicates nor exonerates individuals and is standard operating procedure for such investigations,” the committee said in a statement.
More than 2,000 people signed a petition on Change.org calling for an investigation at JAMA over the February podcast episode, called “Structural Racism for Doctors: What Is It?”
Already, Edward H. Livingston, MD, the host of the podcast, has resigned as deputy editor of the journal.
During the podcast, Dr. Livingston, who is White, said, “Structural racism is an unfortunate term. Personally, I think taking racism out of the conversation will help. Many of us are offended by the concept that we are racist.”
The audio of the podcast has been deleted from JAMA’s website. In its place is audio of a statement from Dr. Bauchner. In his statement, which he released in the week prior to his being on leave, he said the comments in the podcast, which also featured Mitch Katz, MD, were “inaccurate, offensive, hurtful, and inconsistent with the standards of JAMA.”
Also deleted was a JAMA tweet promoting the podcast episode. The tweet said: “No physician is racist, so how can there be structural racism in health care? An explanation of the idea by doctors for doctors in this user-friendly podcast.”
This story will be updated.
A version of this article first appeared on WedMD.com.