User login
test publish
test for unpublish
test for unpublish
test for unpublish
headline
new article test
new article test
Association Between Pruritus and Fibromyalgia: Results of a Population-Based, Cross-Sectional Study
Pruritus, which is defined as an itching sensation that elicits a desire to scratch, is the most common cutaneous condition. Pruritus is considered chronic when it lasts for more than 6 weeks.1 Etiologies implicated in chronic pruritus include but are not limited to primary skin diseases such as atopic dermatitis, systemic causes, neuropathic disorders, and psychogenic reasons.2 In approximately 8% to 35% of patients, the cause of pruritus remains elusive despite intensive investigation.3 The mechanisms of itch are multifaceted and include complex neural pathways.4 Although itch and pain share many similarities, they have distinct pathways based on their spinal connections.5 Nevertheless, both conditions show a wide overlap of receptors on peripheral nerve endings and activated brain parts.6,7 Fibromyalgia, the third most common musculoskeletal condition, affects 2% to 3% of the population worldwide and is at least 7 times more common in females.8,9 Its pathogenesis is not entirely clear but is thought to involve neurogenic inflammation, aberrations in peripheral nerves, and central pain mechanisms. Fibromyalgia is characterized by a plethora of symptoms including chronic widespread pain, autonomic disturbances, persistent fatigue and sleep disturbances, and hyperalgesia, as well as somatic and psychiatric symptoms.10
Fibromyalgia is accompanied by altered skin features including increased counts of mast cells and excessive degranulation,11 neurogenic inflammation with elevated cytokine expression,12 disrupted collagen metabolism,13 and microcirculation abnormalities.14 There has been limited research exploring the dermatologic manifestations of fibromyalgia. One retrospective study that included 845 patients with fibromyalgia reported increased occurrence of “neurodermatoses,” including pruritus, neurotic excoriations, prurigo nodules, and lichen simplex chronicus (LSC), among other cutaneous comorbidities.15 Another small study demonstrated an increased incidence of xerosis and neurotic excoriations in females with fibromyalgia.16 A paucity of large epidemiologic studies demonstrating the fibromyalgia-pruritus connection may lead to misdiagnosis, misinterpretation, and undertreatment of these patients.
Up to 49% of fibromyalgia patients experience small-fiber neuropathy.17 Electrophysiologic measurements, quantitative sensory testing, pain-related evoked potentials, and skin biopsies showed that patients with fibromyalgia have compromised small-fiber function, impaired pathways carrying fiber pain signals, and reduced skin innervation and regenerating fibers.18,19 Accordingly, pruritus that has been reported in fibromyalgia is believed to be of neuropathic origin.15 Overall, it is suspected that the same mechanism that causes hypersensitivity and pain in fibromyalgia patients also predisposes them to pruritus. Similar systemic treatments (eg, analgesics, antidepressants, anticonvulsants) prescribed for both conditions support this theory.20-25
Our large cross-sectional study sought to establish the association between fibromyalgia and pruritus as well as related pruritic conditions.
Methods
Study Design and Setting—We conducted a cross-sectional retrospective study using data-mining techniques to access information from the Clalit Health Services (CHS) database. Clalit Health Services is the largest health maintenance organization in Israel. It encompasses an extensive database with continuous real-time input from medical, administrative, and pharmaceutical computerized operating systems, which helps facilitate data collection for epidemiologic studies. A chronic disease register is gathered from these data sources and continuously updated and validated through logistic checks. The current study was approved by the institutional review board of the CHS (approval #0212-17-com2). Informed consent was not required because the data were de-identified and this was a noninterventional observational study.
Study Population and Covariates—Medical records of CHS enrollees were screened for the diagnosis of fibromyalgia, and data on prevalent cases of fibromyalgia were retrieved. The diagnosis of fibromyalgia was based on the documentation of a fibromyalgia-specific diagnostic code registered by a board-certified rheumatologist. A control group of individuals without fibromyalgia was selected through 1:2 matching based on age, sex, and primary care clinic. The control group was randomly selected from the list of CHS members frequency-matched to cases, excluding case patients with fibromyalgia. Age matching was grounded on the exact year of birth (1-year strata).
Other covariates in the analysis included pruritus-related skin disorders, including prurigo nodularis, neurotic excoriations, and LSC. There were 3 socioeconomic status categories according to patients' poverty index: low, intermediate, and high.26
Statistical Analysis—The distribution of sociodemographic and clinical features was compared between patients with fibromyalgia and controls using the χ2 test for sex and socioeconomic status and the t test for age. Conditional logistic regression then was used to calculate adjusted odds ratio (OR) and 95% CI to compare patients with fibromyalgia and controls with respect to the presence of pruritic comorbidities. All statistical analyses were performed using SPSS software (version 26). P<.05 was considered statistically significant in all tests.
Results
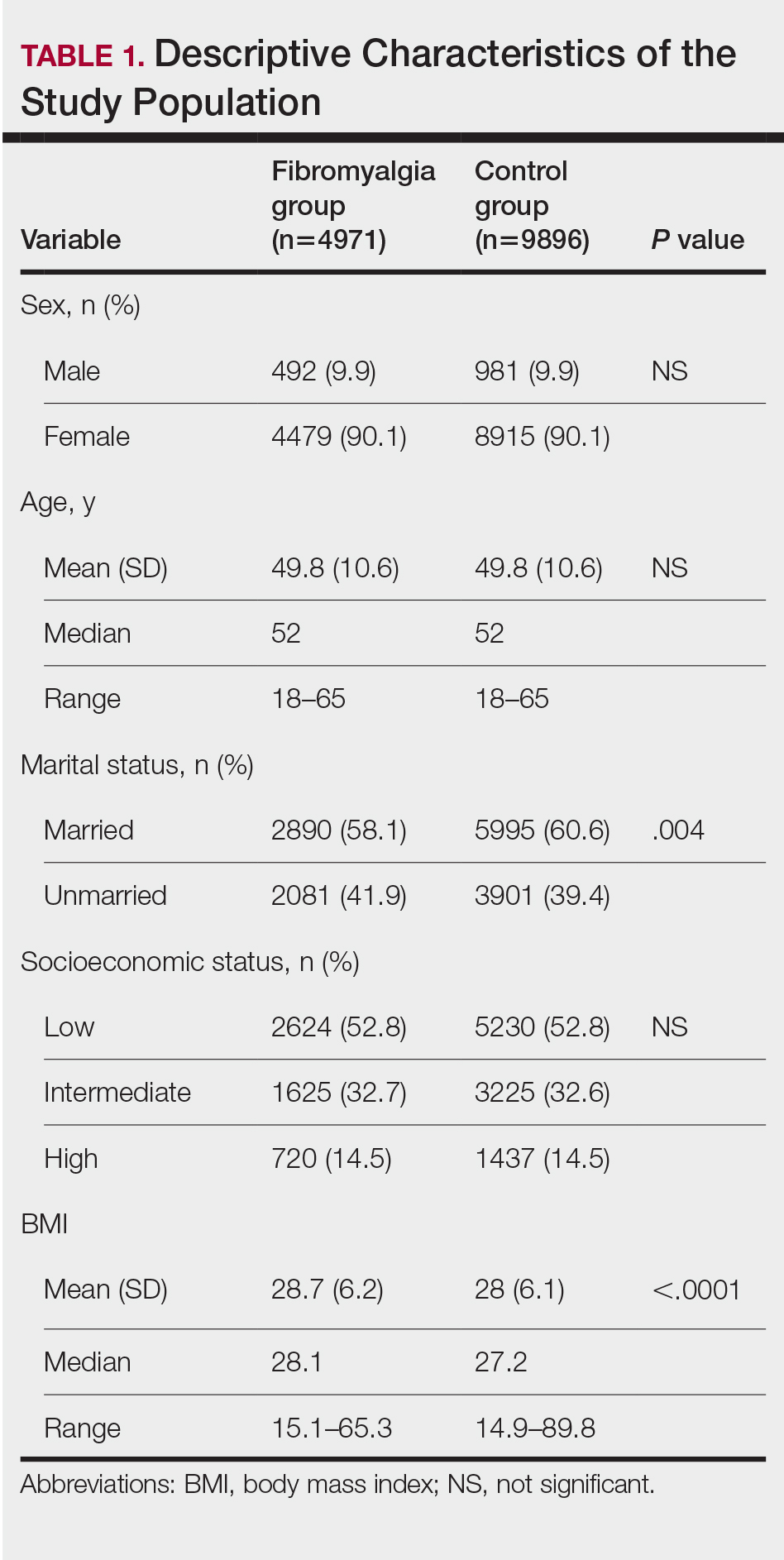
Our study population comprised 4971 patients with fibromyalgia and 9896 age- and sex-matched controls. Proportional to the reported female predominance among patients with fibromyalgia,27 4479 (90.1%) patients with fibromyalgia were females and a similar proportion was documented among controls (P=.99). There was a slightly higher proportion of unmarried patients among those with fibromyalgia compared with controls (41.9% vs 39.4%; P=.004). Socioeconomic status was matched between patients and controls (P=.99). Descriptive characteristics of the study population are presented in Table 1.
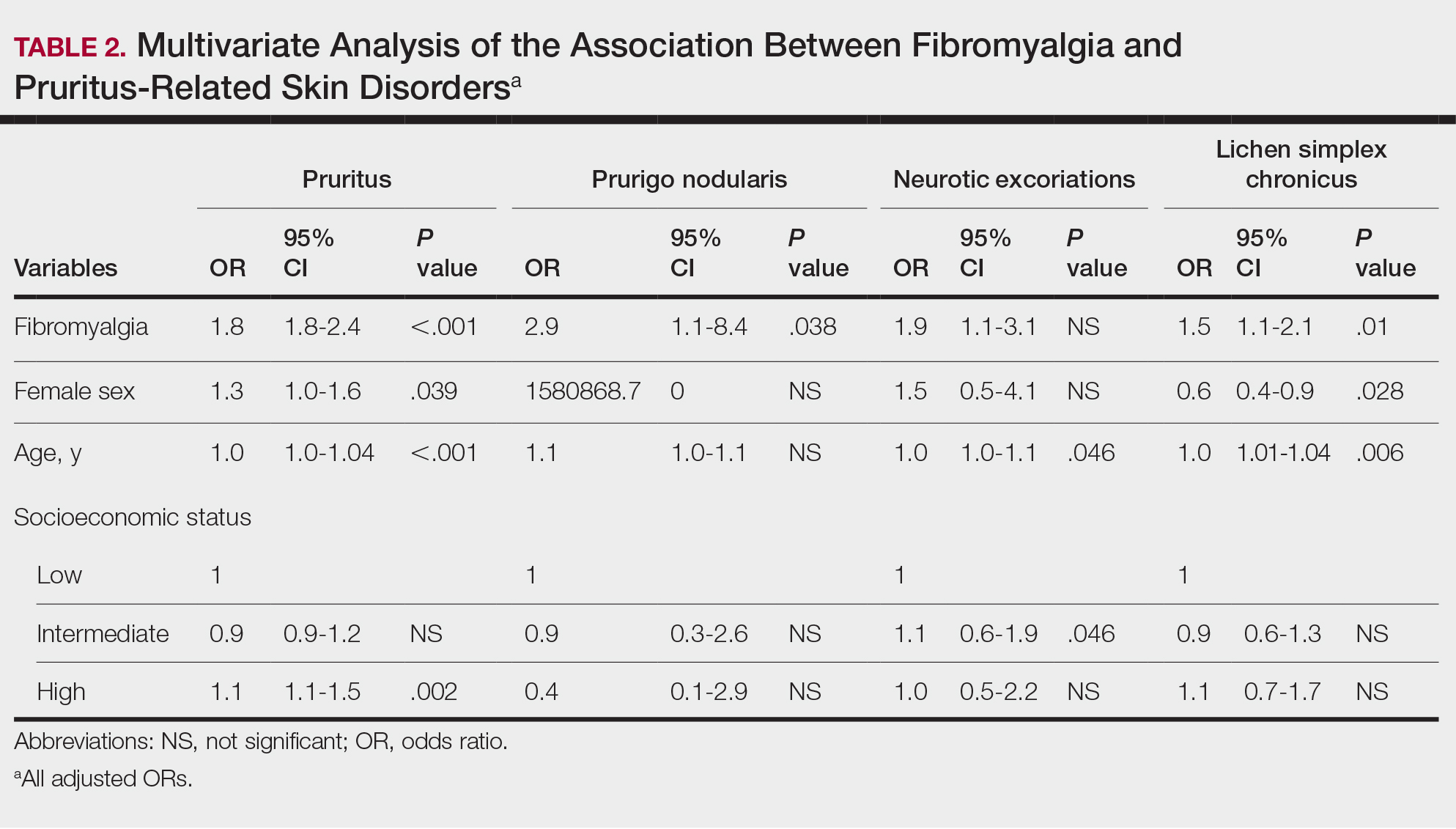
We assessed the presence of pruritus as well as 3 other pruritus-related skin disorders—prurigo nodularis, neurotic excoriations, and LSC—among patients with fibromyalgia and controls. Logistic regression was used to evaluate the independent association between fibromyalgia and pruritus. Table 2 presents the results of multivariate logistic regression models and summarizes the adjusted ORs for pruritic conditions in patients with fibromyalgia and different demographic features across the entire study sample. Fibromyalgia demonstrated strong independent associations with pruritus (OR, 1.8; 95% CI, 1.8-2.4; P<.001), prurigo nodularis (OR, 2.9; 95% CI, 1.1-8.4; P=.038), and LSC (OR, 1.5; 95% CI, 1.1-2.1; P=.01); the association with neurotic excoriations was not significant. Female sex significantly increased the risk for pruritus (OR 1.3; 95% CI, 1.0-1.6; P=.039), while age slightly increased the odds for pruritus (OR, 1.0; 95% CI, 1.0-1.04; P<.001), neurotic excoriations (OR, 1.0; 95% CI, 1.0-1.1; P=.046), and LSC (OR, 1.0; 95% CI, 1.01-1.04; P=.006). Finally, socioeconomic status was inversely correlated with pruritus (OR, 1.1; 95% CI, 1.1-1.5; P=.002).
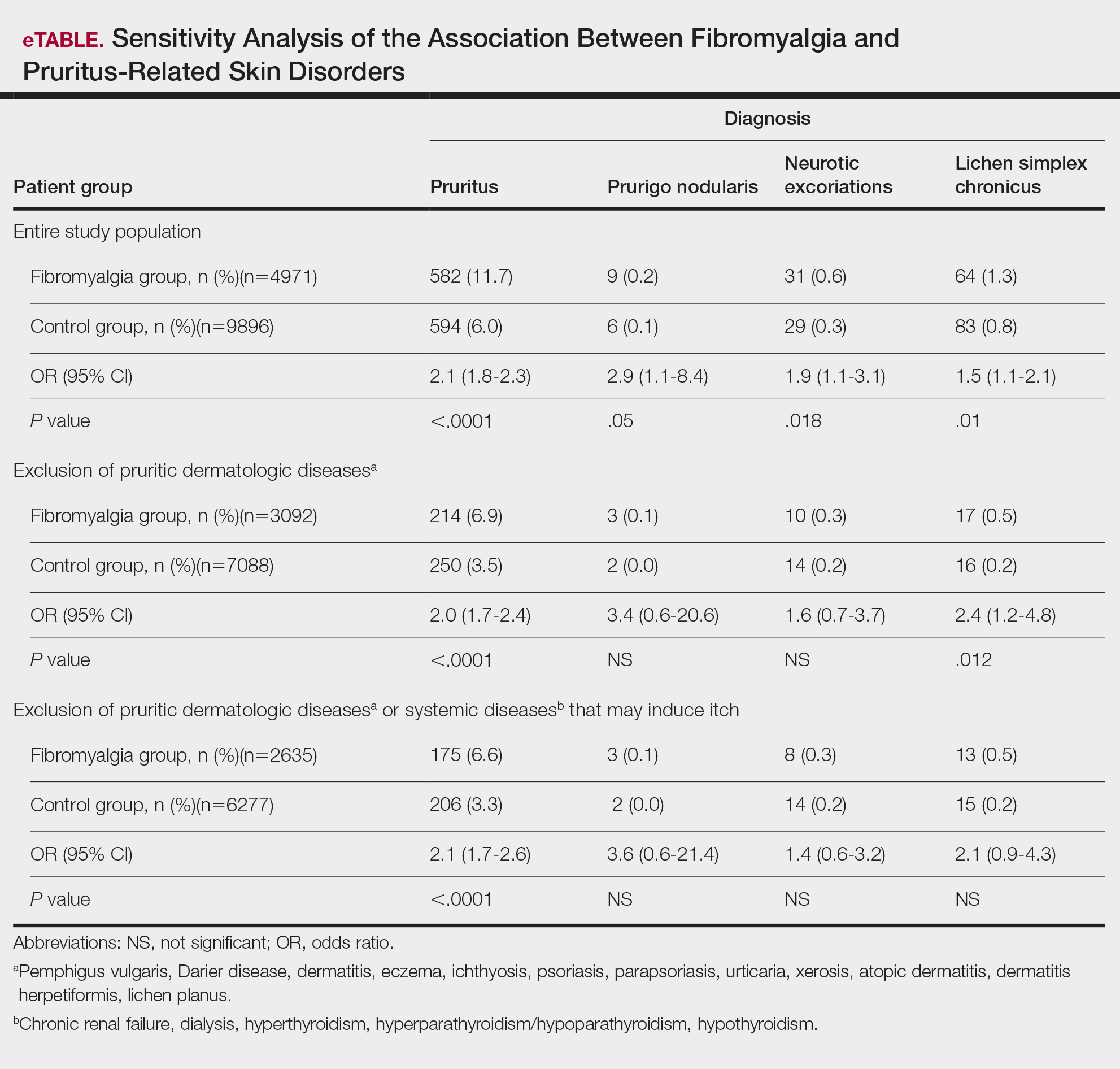
Frequencies and ORs for the association between fibromyalgia and pruritus with associated pruritic disorders stratified by exclusion of pruritic dermatologic and/or systemic diseases that may induce itch are presented in the eTable. Analyzing the entire study cohort, significant increases were observed in the odds of all 4 pruritic disorders analyzed. The frequency of pruritus was almost double in patients with fibromyalgia compared with controls (11.7% vs 6.0%; OR, 2.1; 95% CI, 1.8-2.3; P<.0001). Prurigo nodularis (0.2% vs 0.1%; OR, 2.9; 95% CI, 1.1-8.4; P=.05), neurotic excoriations (0.6% vs 0.3%; OR, 1.9; 95% CI, 1.1-3.1; P=.018), and LSC (1.3% vs 0.8%; OR, 1.5; 95% CI, 1.1-2.1; P=.01) frequencies were all higher in patients with fibromyalgia than controls. When primary skin disorders that may cause itch (eg, pemphigus vulgaris, Darier disease, dermatitis, eczema, ichthyosis, psoriasis, parapsoriasis, urticaria, xerosis, atopic dermatitis, dermatitis herpetiformis, lichen planus) were excluded, the prevalence of pruritus in patients with fibromyalgia was still 1.97 times greater than in the controls (6.9% vs. 3.5%; OR, 2.0; 95% CI, 1.7-2.4; P<.0001). These results remained unchanged even when excluding pruritic dermatologic disorders as well as systemic diseases associated with pruritus (eg, chronic renal failure, dialysis, hyperthyroidism, hyperparathyroidism/hypoparathyroidism, hypothyroidism). Patients with fibromyalgia still displayed a significantly higher prevalence of pruritus compared with the control group (6.6% vs 3.3%; OR, 2.1; 95% CI, 1.7-2.6; P<.0001).
Comment
A wide range of skin manifestations have been associated with fibromyalgia, but the exact mechanisms remain unclear. Nevertheless, it is conceivable that autonomic nervous system dysfunction,28-31 amplified cutaneous opioid receptor activity,32 and an elevated presence of cutaneous mast cells with excessive degranulation may partially explain the frequent occurrence of pruritus and related skin disorders such as neurotic excoriations, prurigo nodularis, and LSC in individuals with fibromyalgia.15,16 In line with these findings, our study—which was based on data from the largest health maintenance organization in Israel—demonstrated an increased prevalence of pruritus and related pruritic disorders among individuals diagnosed with fibromyalgia.
This cross-sectional study links pruritus with fibromyalgia. Few preliminary epidemiologic studies have shown an increased occurrence of cutaneous manifestations in patients with fibromyalgia. One chart review that looked at skin findings in patients with fibromyalgia revealed 32 distinct cutaneous manifestations, and pruritus was the major concern in 3.3% of 845 patients.15
A focused cross-sectional study involving only women (66 with fibromyalgia and 79 healthy controls) discovered 14 skin conditions that were more common in those with fibromyalgia. Notably, xerosis and neurotic excoriations were more prevalent compared to the control group.16
The brain and the skin—both derivatives of the embryonic ectoderm33,34—are linked by pruritus. Although itch has its dedicated neurons, there is a wide-ranging overlap of brain-activated areas between pain and itch,6 and the neural anatomy of pain and itch are closely related in both the peripheral and central nervous systems35-37; for example, diseases of the central nervous system are accompanied by pruritus in as many as 15% of cases, while postherpetic neuralgia can result in chronic pain, itching, or a combination of both.38,39 Other instances include notalgia paresthetica and brachioradial pruritus.38 Additionally, there is a noteworthy psychologic impact associated with both itch and pain,40,41 with both psychosomatic and psychologic factors implicated in chronic pruritus and in fibromyalgia.42 Lastly, the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system are altered in both fibromyalgia and pruritus.43-45
Tey et al45 characterized the itch experienced in fibromyalgia as functional, which is described as pruritus associated with a somatoform disorder. In our study, we found a higher prevalence of pruritus among patients with fibromyalgia, and this association remained significant (P<.05) even when excluding other pruritic skin conditions and systemic diseases that can trigger itching. In addition, our logistic regression analyses revealed independent associations between fibromyalgia and pruritus, prurigo nodularis, and LSC.
According to Twycross et al,46 there are 4 clinical categories of itch, which may coexist7: pruritoceptive (originating in the skin), neuropathic (originating in pathology located along the afferent pathway), neurogenic (central origin but lacks a neural pathology), and psychogenic.47 Skin biopsy findings in patients with fibromyalgia include increased mast cell counts11 and degranulation,48 increased expression of δ and κ opioid receptors,32 vasoconstriction within tender points,49 and elevated IL-1β, IL-6, or tumor necrosis factor α by reverse transcriptase-polymerase chain reaction.12 A case recently was presented by Görg et al50 involving a female patient with fibromyalgia who had been experiencing chronic pruritus, which the authors attributed to small-fiber neuropathy based on evidence from a skin biopsy indicating a reduced number of intraepidermal nerves and the fact that the itching originated around tender points. Altogether, the observed alterations may work together to make patients with fibromyalgia more susceptible to various skin-related comorbidities in general, especially those related to pruritus. Eventually, it might be the case that several itch categories and related pathomechanisms are involved in the pruritus phenotype of patients with fibromyalgia.
Age-related alterations in nerve fibers, lower immune function, xerosis, polypharmacy, and increased frequency of systemic diseases with age are just a few of the factors that may predispose older individuals to pruritus.51,52 Indeed, our logistic regression model showed that age was significantly and independently associated with pruritus (P<.001), neurotic excoriations (P=.046), and LSC (P=.006). Female sex also was significantly linked with pruritus (P=.039). Intriguingly, high socioeconomic status was significantly associated with the diagnosis of pruritus (P=.002), possibly due to easier access to medical care.
There is a considerable overlap between the therapeutic approaches used in pruritus, pruritus-related skin disorders, and fibromyalgia. Antidepressants, anxiolytics, analgesics, and antiepileptics have been used to address both conditions.45 The association between these conditions advocates for a multidisciplinary approach in patients with fibromyalgia and potentially supports the rationale for unified therapeutics for both conditions.
Conclusion
Our findings indicate an association between fibromyalgia and pruritus as well as associated pruritic skin disorders. Given the convoluted and largely undiscovered mechanisms underlying fibromyalgia, managing patients with this condition may present substantial challenges.53 The data presented here support the implementation of a multidisciplinary treatment approach for patients with fibromyalgia. This approach should focus on managing fibromyalgia pain as well as addressing its concurrent skin-related conditions. It is advisable to consider treatments such as antiepileptics (eg, pregabalin, gabapentin) that specifically target neuropathic disorders in affected patients. These treatments may hold promise for alleviating fibromyalgia-related pain54 and mitigating its related cutaneous comorbidities, especially pruritus.
- Stander S, Weisshaar E, Mettang T, et al. Clinical classification of itch: a position paper of the International Forum for the Study of Itch. Acta Derm Venereol. 2007; 87:291-294.
- Yosipovitch G, Bernhard JD. Clinical practice. chronic pruritus. N Engl J Med. 2013;368:1625-1634.
- Song J, Xian D, Yang L, et al. Pruritus: progress toward pathogenesis and treatment. Biomed Res Int. 2018;2018:9625936.
- Potenzieri C, Undem BJ. Basic mechanisms of itch. Clin Exp Allergy. 2012;42:8-19.
- McMahon SB, Koltzenburg M. Itching for an explanation. Trends Neurosci. 1992;15:497-501.
- Drzezga A, Darsow U, Treede RD, et al. Central activation by histamine-induced itch: analogies to pain processing: a correlational analysis of O-15 H2O positron emission tomography studies. Pain. 2001; 92:295-305.
- Yosipovitch G, Greaves MW, Schmelz M. Itch. Lancet. 2003;361:690-694.
- Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. part I. Arthritis Rheum. 2008; 58:15-25.
- Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. part II. Arthritis Rheum. 2008; 58:26-35.
- Sarzi-Puttini P, Giorgi V, Marotto D, et al. Fibromyalgia: an update on clinical characteristics, aetiopathogenesis and treatment. Nat Rev Rheumatol. 2020;16:645-660.
- Blanco I, Beritze N, Arguelles M, et al. Abnormal overexpression of mastocytes in skin biopsies of fibromyalgia patients. Clin Rheumatol. 2010;29:1403-1412.
- Salemi S, Rethage J, Wollina U, et al. Detection of interleukin 1beta (IL-1beta), IL-6, and tumor necrosis factor-alpha in skin of patients with fibromyalgia. J Rheumatol. 2003;30:146-150.
- Sprott H, Muller A, Heine H. Collagen cross-links in fibromyalgia syndrome. Z Rheumatol. 1998;57(suppl 2):52-55.
- Morf S, Amann-Vesti B, Forster A, et al. Microcirculation abnormalities in patients with fibromyalgia—measured by capillary microscopy and laser fluxmetry. Arthritis Res Ther. 2005;7:R209-R216.
- Laniosz V, Wetter DA, Godar DA. Dermatologic manifestations of fibromyalgia. Clin Rheumatol. 2014;33:1009-1013.
- Dogramaci AC, Yalcinkaya EY. Skin problems in fibromyalgia. Nobel Med. 2009;5:50-52.
- Grayston R, Czanner G, Elhadd K, et al. A systematic review and meta-analysis of the prevalence of small fiber pathology in fibromyalgia: implications for a new paradigm in fibromyalgia etiopathogenesis. Semin Arthritis Rheum. 2019;48:933-940.
- Uceyler N, Zeller D, Kahn AK, et al. Small fibre pathology in patients with fibromyalgia syndrome. Brain. 2013;136:1857-1867.
- Devigili G, Tugnoli V, Penza P, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain. 2008; 131:1912- 1925.
- Reed C, Birnbaum HG, Ivanova JI, et al. Real-world role of tricyclic antidepressants in the treatment of fibromyalgia. Pain Pract. 2012; 12:533-540.
- Moret C, Briley M. Antidepressants in the treatment of fibromyalgia. Neuropsychiatr Dis Treat. 2006;2:537-548.
- Arnold LM, Keck PE Jr, Welge JA. Antidepressant treatment of fibromyalgia. a meta-analysis and review. Psychosomatics. 2000;41:104-113.
- Moore A, Wiffen P, Kalso E. Antiepileptic drugs for neuropathic pain and fibromyalgia. JAMA. 2014;312:182-183.
- Shevchenko A, Valdes-Rodriguez R, Yosipovitch G. Causes, pathophysiology, and treatment of pruritus in the mature patient. Clin Dermatol. 2018;36:140-151.
- Scheinfeld N. The role of gabapentin in treating diseases with cutaneous manifestations and pain. Int J Dermatol. 2003;42:491-495.
- Points Location Intelligence. Accessed July 30, 2024. https://points.co.il/en/points-location-intelligence/
- Yunus MB. The role of gender in fibromyalgia syndrome. Curr Rheumatol Rep. 2001;3:128-134.
- Cakir T, Evcik D, Dundar U, et al. Evaluation of sympathetic skin response and f wave in fibromyalgia syndrome patients. Turk J Rheumatol. 2011;26:38-43.
- Ozkan O, Yildiz M, Koklukaya E. The correlation of laboratory tests and sympathetic skin response parameters by using artificial neural networks in fibromyalgia patients. J Med Syst. 2012;36:1841-1848.
- Ozkan O, Yildiz M, Arslan E, et al. A study on the effects of sympathetic skin response parameters in diagnosis of fibromyalgia using artificial neural networks. J Med Syst. 2016;40:54.
- Ulas UH, Unlu E, Hamamcioglu K, et al. Dysautonomia in fibromyalgia syndrome: sympathetic skin responses and RR interval analysis. Rheumatol Int. 2006;26:383-387.
- Salemi S, Aeschlimann A, Wollina U, et al. Up-regulation of delta-opioid receptors and kappa-opioid receptors in the skin of fibromyalgia patients. Arthritis Rheum. 2007;56:2464-2466.
- Elshazzly M, Lopez MJ, Reddy V, et al. Central nervous system. StatPearls. StatPearls Publishing; 2022.
- Hu MS, Borrelli MR, Hong WX, et al. Embryonic skin development and repair. Organogenesis. 2018;14:46-63.
- Davidson S, Zhang X, Yoon CH, et al. The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci. 2007;27:10007-10014.
- Sikand P, Shimada SG, Green BG, et al. Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain. 2009;144:66-75.
- Davidson S, Giesler GJ. The multiple pathways for itch and their interactions with pain. Trends Neurosci. 2010;33:550-558.
- Dhand A, Aminoff MJ. The neurology of itch. Brain. 2014;137:313-322.
- Binder A, Koroschetz J, Baron R. Disease mechanisms in neuropathic itch. Nat Clin Pract Neurol. 2008;4:329-337.
- Fjellner B, Arnetz BB. Psychological predictors of pruritus during mental stress. Acta Derm Venereol. 1985;65:504-508.
- Papoiu AD, Wang H, Coghill RC, et al. Contagious itch in humans: a study of visual ‘transmission’ of itch in atopic dermatitis and healthy subjects. Br J Dermatol. 2011;164:1299-1303.
- Stumpf A, Schneider G, Stander S. Psychosomatic and psychiatric disorders and psychologic factors in pruritus. Clin Dermatol. 2018;36:704-708.
- Herman JP, McKlveen JM, Ghosal S, et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. 2016;6:603-621.
- Brown ED, Micozzi MS, Craft NE, et al. Plasma carotenoids in normal men after a single ingestion of vegetables or purified beta-carotene. Am J Clin Nutr. 1989;49:1258-1265.
- Tey HL, Wallengren J, Yosipovitch G. Psychosomatic factors in pruritus. Clin Dermatol. 2013;31:31-40.
- Twycross R, Greaves MW, Handwerker H, et al. Itch: scratching more than the surface. QJM. 2003;96:7-26.
- Bernhard JD. Itch and pruritus: what are they, and how should itches be classified? Dermatol Ther. 2005;18:288-291.
- Enestrom S, Bengtsson A, Frodin T. Dermal IgG deposits and increase of mast cells in patients with fibromyalgia—relevant findings or epiphenomena? Scand J Rheumatol. 1997;26:308-313.
- Jeschonneck M, Grohmann G, Hein G, et al. Abnormal microcirculation and temperature in skin above tender points in patients with fibromyalgia. Rheumatology (Oxford). 2000;39:917-921.
- Görg M, Zeidler C, Pereira MP, et al. Generalized chronic pruritus with fibromyalgia. J Dtsch Dermatol Ges. 2021;19:909-911.
- Garibyan L, Chiou AS, Elmariah SB. Advanced aging skin and itch: addressing an unmet need. Dermatol Ther. 2013;26:92-103.
- Cohen KR, Frank J, Salbu RL, et al. Pruritus in the elderly: clinical approaches to the improvement of quality of life. P T. 2012;37:227-239.
- Tzadok R, Ablin JN. Current and emerging pharmacotherapy for fibromyalgia. Pain Res Manag. 2020; 2020:6541798.
- Wiffen PJ, Derry S, Moore RA, et al. Antiepileptic drugs for neuropathic pain and fibromyalgia—an overview of Cochrane reviews. Cochrane Database Syst Rev. 2013:CD010567.
Pruritus, which is defined as an itching sensation that elicits a desire to scratch, is the most common cutaneous condition. Pruritus is considered chronic when it lasts for more than 6 weeks.1 Etiologies implicated in chronic pruritus include but are not limited to primary skin diseases such as atopic dermatitis, systemic causes, neuropathic disorders, and psychogenic reasons.2 In approximately 8% to 35% of patients, the cause of pruritus remains elusive despite intensive investigation.3 The mechanisms of itch are multifaceted and include complex neural pathways.4 Although itch and pain share many similarities, they have distinct pathways based on their spinal connections.5 Nevertheless, both conditions show a wide overlap of receptors on peripheral nerve endings and activated brain parts.6,7 Fibromyalgia, the third most common musculoskeletal condition, affects 2% to 3% of the population worldwide and is at least 7 times more common in females.8,9 Its pathogenesis is not entirely clear but is thought to involve neurogenic inflammation, aberrations in peripheral nerves, and central pain mechanisms. Fibromyalgia is characterized by a plethora of symptoms including chronic widespread pain, autonomic disturbances, persistent fatigue and sleep disturbances, and hyperalgesia, as well as somatic and psychiatric symptoms.10
Fibromyalgia is accompanied by altered skin features including increased counts of mast cells and excessive degranulation,11 neurogenic inflammation with elevated cytokine expression,12 disrupted collagen metabolism,13 and microcirculation abnormalities.14 There has been limited research exploring the dermatologic manifestations of fibromyalgia. One retrospective study that included 845 patients with fibromyalgia reported increased occurrence of “neurodermatoses,” including pruritus, neurotic excoriations, prurigo nodules, and lichen simplex chronicus (LSC), among other cutaneous comorbidities.15 Another small study demonstrated an increased incidence of xerosis and neurotic excoriations in females with fibromyalgia.16 A paucity of large epidemiologic studies demonstrating the fibromyalgia-pruritus connection may lead to misdiagnosis, misinterpretation, and undertreatment of these patients.
Up to 49% of fibromyalgia patients experience small-fiber neuropathy.17 Electrophysiologic measurements, quantitative sensory testing, pain-related evoked potentials, and skin biopsies showed that patients with fibromyalgia have compromised small-fiber function, impaired pathways carrying fiber pain signals, and reduced skin innervation and regenerating fibers.18,19 Accordingly, pruritus that has been reported in fibromyalgia is believed to be of neuropathic origin.15 Overall, it is suspected that the same mechanism that causes hypersensitivity and pain in fibromyalgia patients also predisposes them to pruritus. Similar systemic treatments (eg, analgesics, antidepressants, anticonvulsants) prescribed for both conditions support this theory.20-25
Our large cross-sectional study sought to establish the association between fibromyalgia and pruritus as well as related pruritic conditions.
Methods
Study Design and Setting—We conducted a cross-sectional retrospective study using data-mining techniques to access information from the Clalit Health Services (CHS) database. Clalit Health Services is the largest health maintenance organization in Israel. It encompasses an extensive database with continuous real-time input from medical, administrative, and pharmaceutical computerized operating systems, which helps facilitate data collection for epidemiologic studies. A chronic disease register is gathered from these data sources and continuously updated and validated through logistic checks. The current study was approved by the institutional review board of the CHS (approval #0212-17-com2). Informed consent was not required because the data were de-identified and this was a noninterventional observational study.
Study Population and Covariates—Medical records of CHS enrollees were screened for the diagnosis of fibromyalgia, and data on prevalent cases of fibromyalgia were retrieved. The diagnosis of fibromyalgia was based on the documentation of a fibromyalgia-specific diagnostic code registered by a board-certified rheumatologist. A control group of individuals without fibromyalgia was selected through 1:2 matching based on age, sex, and primary care clinic. The control group was randomly selected from the list of CHS members frequency-matched to cases, excluding case patients with fibromyalgia. Age matching was grounded on the exact year of birth (1-year strata).
Other covariates in the analysis included pruritus-related skin disorders, including prurigo nodularis, neurotic excoriations, and LSC. There were 3 socioeconomic status categories according to patients' poverty index: low, intermediate, and high.26
Statistical Analysis—The distribution of sociodemographic and clinical features was compared between patients with fibromyalgia and controls using the χ2 test for sex and socioeconomic status and the t test for age. Conditional logistic regression then was used to calculate adjusted odds ratio (OR) and 95% CI to compare patients with fibromyalgia and controls with respect to the presence of pruritic comorbidities. All statistical analyses were performed using SPSS software (version 26). P<.05 was considered statistically significant in all tests.
Results
Our study population comprised 4971 patients with fibromyalgia and 9896 age- and sex-matched controls. Proportional to the reported female predominance among patients with fibromyalgia,27 4479 (90.1%) patients with fibromyalgia were females and a similar proportion was documented among controls (P=.99). There was a slightly higher proportion of unmarried patients among those with fibromyalgia compared with controls (41.9% vs 39.4%; P=.004). Socioeconomic status was matched between patients and controls (P=.99). Descriptive characteristics of the study population are presented in Table 1.

We assessed the presence of pruritus as well as 3 other pruritus-related skin disorders—prurigo nodularis, neurotic excoriations, and LSC—among patients with fibromyalgia and controls. Logistic regression was used to evaluate the independent association between fibromyalgia and pruritus. Table 2 presents the results of multivariate logistic regression models and summarizes the adjusted ORs for pruritic conditions in patients with fibromyalgia and different demographic features across the entire study sample. Fibromyalgia demonstrated strong independent associations with pruritus (OR, 1.8; 95% CI, 1.8-2.4; P<.001), prurigo nodularis (OR, 2.9; 95% CI, 1.1-8.4; P=.038), and LSC (OR, 1.5; 95% CI, 1.1-2.1; P=.01); the association with neurotic excoriations was not significant. Female sex significantly increased the risk for pruritus (OR 1.3; 95% CI, 1.0-1.6; P=.039), while age slightly increased the odds for pruritus (OR, 1.0; 95% CI, 1.0-1.04; P<.001), neurotic excoriations (OR, 1.0; 95% CI, 1.0-1.1; P=.046), and LSC (OR, 1.0; 95% CI, 1.01-1.04; P=.006). Finally, socioeconomic status was inversely correlated with pruritus (OR, 1.1; 95% CI, 1.1-1.5; P=.002).

Frequencies and ORs for the association between fibromyalgia and pruritus with associated pruritic disorders stratified by exclusion of pruritic dermatologic and/or systemic diseases that may induce itch are presented in the eTable. Analyzing the entire study cohort, significant increases were observed in the odds of all 4 pruritic disorders analyzed. The frequency of pruritus was almost double in patients with fibromyalgia compared with controls (11.7% vs 6.0%; OR, 2.1; 95% CI, 1.8-2.3; P<.0001). Prurigo nodularis (0.2% vs 0.1%; OR, 2.9; 95% CI, 1.1-8.4; P=.05), neurotic excoriations (0.6% vs 0.3%; OR, 1.9; 95% CI, 1.1-3.1; P=.018), and LSC (1.3% vs 0.8%; OR, 1.5; 95% CI, 1.1-2.1; P=.01) frequencies were all higher in patients with fibromyalgia than controls. When primary skin disorders that may cause itch (eg, pemphigus vulgaris, Darier disease, dermatitis, eczema, ichthyosis, psoriasis, parapsoriasis, urticaria, xerosis, atopic dermatitis, dermatitis herpetiformis, lichen planus) were excluded, the prevalence of pruritus in patients with fibromyalgia was still 1.97 times greater than in the controls (6.9% vs. 3.5%; OR, 2.0; 95% CI, 1.7-2.4; P<.0001). These results remained unchanged even when excluding pruritic dermatologic disorders as well as systemic diseases associated with pruritus (eg, chronic renal failure, dialysis, hyperthyroidism, hyperparathyroidism/hypoparathyroidism, hypothyroidism). Patients with fibromyalgia still displayed a significantly higher prevalence of pruritus compared with the control group (6.6% vs 3.3%; OR, 2.1; 95% CI, 1.7-2.6; P<.0001).

Comment
A wide range of skin manifestations have been associated with fibromyalgia, but the exact mechanisms remain unclear. Nevertheless, it is conceivable that autonomic nervous system dysfunction,28-31 amplified cutaneous opioid receptor activity,32 and an elevated presence of cutaneous mast cells with excessive degranulation may partially explain the frequent occurrence of pruritus and related skin disorders such as neurotic excoriations, prurigo nodularis, and LSC in individuals with fibromyalgia.15,16 In line with these findings, our study—which was based on data from the largest health maintenance organization in Israel—demonstrated an increased prevalence of pruritus and related pruritic disorders among individuals diagnosed with fibromyalgia.
This cross-sectional study links pruritus with fibromyalgia. Few preliminary epidemiologic studies have shown an increased occurrence of cutaneous manifestations in patients with fibromyalgia. One chart review that looked at skin findings in patients with fibromyalgia revealed 32 distinct cutaneous manifestations, and pruritus was the major concern in 3.3% of 845 patients.15
A focused cross-sectional study involving only women (66 with fibromyalgia and 79 healthy controls) discovered 14 skin conditions that were more common in those with fibromyalgia. Notably, xerosis and neurotic excoriations were more prevalent compared to the control group.16
The brain and the skin—both derivatives of the embryonic ectoderm33,34—are linked by pruritus. Although itch has its dedicated neurons, there is a wide-ranging overlap of brain-activated areas between pain and itch,6 and the neural anatomy of pain and itch are closely related in both the peripheral and central nervous systems35-37; for example, diseases of the central nervous system are accompanied by pruritus in as many as 15% of cases, while postherpetic neuralgia can result in chronic pain, itching, or a combination of both.38,39 Other instances include notalgia paresthetica and brachioradial pruritus.38 Additionally, there is a noteworthy psychologic impact associated with both itch and pain,40,41 with both psychosomatic and psychologic factors implicated in chronic pruritus and in fibromyalgia.42 Lastly, the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system are altered in both fibromyalgia and pruritus.43-45
Tey et al45 characterized the itch experienced in fibromyalgia as functional, which is described as pruritus associated with a somatoform disorder. In our study, we found a higher prevalence of pruritus among patients with fibromyalgia, and this association remained significant (P<.05) even when excluding other pruritic skin conditions and systemic diseases that can trigger itching. In addition, our logistic regression analyses revealed independent associations between fibromyalgia and pruritus, prurigo nodularis, and LSC.
According to Twycross et al,46 there are 4 clinical categories of itch, which may coexist7: pruritoceptive (originating in the skin), neuropathic (originating in pathology located along the afferent pathway), neurogenic (central origin but lacks a neural pathology), and psychogenic.47 Skin biopsy findings in patients with fibromyalgia include increased mast cell counts11 and degranulation,48 increased expression of δ and κ opioid receptors,32 vasoconstriction within tender points,49 and elevated IL-1β, IL-6, or tumor necrosis factor α by reverse transcriptase-polymerase chain reaction.12 A case recently was presented by Görg et al50 involving a female patient with fibromyalgia who had been experiencing chronic pruritus, which the authors attributed to small-fiber neuropathy based on evidence from a skin biopsy indicating a reduced number of intraepidermal nerves and the fact that the itching originated around tender points. Altogether, the observed alterations may work together to make patients with fibromyalgia more susceptible to various skin-related comorbidities in general, especially those related to pruritus. Eventually, it might be the case that several itch categories and related pathomechanisms are involved in the pruritus phenotype of patients with fibromyalgia.
Age-related alterations in nerve fibers, lower immune function, xerosis, polypharmacy, and increased frequency of systemic diseases with age are just a few of the factors that may predispose older individuals to pruritus.51,52 Indeed, our logistic regression model showed that age was significantly and independently associated with pruritus (P<.001), neurotic excoriations (P=.046), and LSC (P=.006). Female sex also was significantly linked with pruritus (P=.039). Intriguingly, high socioeconomic status was significantly associated with the diagnosis of pruritus (P=.002), possibly due to easier access to medical care.
There is a considerable overlap between the therapeutic approaches used in pruritus, pruritus-related skin disorders, and fibromyalgia. Antidepressants, anxiolytics, analgesics, and antiepileptics have been used to address both conditions.45 The association between these conditions advocates for a multidisciplinary approach in patients with fibromyalgia and potentially supports the rationale for unified therapeutics for both conditions.
Conclusion
Our findings indicate an association between fibromyalgia and pruritus as well as associated pruritic skin disorders. Given the convoluted and largely undiscovered mechanisms underlying fibromyalgia, managing patients with this condition may present substantial challenges.53 The data presented here support the implementation of a multidisciplinary treatment approach for patients with fibromyalgia. This approach should focus on managing fibromyalgia pain as well as addressing its concurrent skin-related conditions. It is advisable to consider treatments such as antiepileptics (eg, pregabalin, gabapentin) that specifically target neuropathic disorders in affected patients. These treatments may hold promise for alleviating fibromyalgia-related pain54 and mitigating its related cutaneous comorbidities, especially pruritus.
Pruritus, which is defined as an itching sensation that elicits a desire to scratch, is the most common cutaneous condition. Pruritus is considered chronic when it lasts for more than 6 weeks.1 Etiologies implicated in chronic pruritus include but are not limited to primary skin diseases such as atopic dermatitis, systemic causes, neuropathic disorders, and psychogenic reasons.2 In approximately 8% to 35% of patients, the cause of pruritus remains elusive despite intensive investigation.3 The mechanisms of itch are multifaceted and include complex neural pathways.4 Although itch and pain share many similarities, they have distinct pathways based on their spinal connections.5 Nevertheless, both conditions show a wide overlap of receptors on peripheral nerve endings and activated brain parts.6,7 Fibromyalgia, the third most common musculoskeletal condition, affects 2% to 3% of the population worldwide and is at least 7 times more common in females.8,9 Its pathogenesis is not entirely clear but is thought to involve neurogenic inflammation, aberrations in peripheral nerves, and central pain mechanisms. Fibromyalgia is characterized by a plethora of symptoms including chronic widespread pain, autonomic disturbances, persistent fatigue and sleep disturbances, and hyperalgesia, as well as somatic and psychiatric symptoms.10
Fibromyalgia is accompanied by altered skin features including increased counts of mast cells and excessive degranulation,11 neurogenic inflammation with elevated cytokine expression,12 disrupted collagen metabolism,13 and microcirculation abnormalities.14 There has been limited research exploring the dermatologic manifestations of fibromyalgia. One retrospective study that included 845 patients with fibromyalgia reported increased occurrence of “neurodermatoses,” including pruritus, neurotic excoriations, prurigo nodules, and lichen simplex chronicus (LSC), among other cutaneous comorbidities.15 Another small study demonstrated an increased incidence of xerosis and neurotic excoriations in females with fibromyalgia.16 A paucity of large epidemiologic studies demonstrating the fibromyalgia-pruritus connection may lead to misdiagnosis, misinterpretation, and undertreatment of these patients.
Up to 49% of fibromyalgia patients experience small-fiber neuropathy.17 Electrophysiologic measurements, quantitative sensory testing, pain-related evoked potentials, and skin biopsies showed that patients with fibromyalgia have compromised small-fiber function, impaired pathways carrying fiber pain signals, and reduced skin innervation and regenerating fibers.18,19 Accordingly, pruritus that has been reported in fibromyalgia is believed to be of neuropathic origin.15 Overall, it is suspected that the same mechanism that causes hypersensitivity and pain in fibromyalgia patients also predisposes them to pruritus. Similar systemic treatments (eg, analgesics, antidepressants, anticonvulsants) prescribed for both conditions support this theory.20-25
Our large cross-sectional study sought to establish the association between fibromyalgia and pruritus as well as related pruritic conditions.
Methods
Study Design and Setting—We conducted a cross-sectional retrospective study using data-mining techniques to access information from the Clalit Health Services (CHS) database. Clalit Health Services is the largest health maintenance organization in Israel. It encompasses an extensive database with continuous real-time input from medical, administrative, and pharmaceutical computerized operating systems, which helps facilitate data collection for epidemiologic studies. A chronic disease register is gathered from these data sources and continuously updated and validated through logistic checks. The current study was approved by the institutional review board of the CHS (approval #0212-17-com2). Informed consent was not required because the data were de-identified and this was a noninterventional observational study.
Study Population and Covariates—Medical records of CHS enrollees were screened for the diagnosis of fibromyalgia, and data on prevalent cases of fibromyalgia were retrieved. The diagnosis of fibromyalgia was based on the documentation of a fibromyalgia-specific diagnostic code registered by a board-certified rheumatologist. A control group of individuals without fibromyalgia was selected through 1:2 matching based on age, sex, and primary care clinic. The control group was randomly selected from the list of CHS members frequency-matched to cases, excluding case patients with fibromyalgia. Age matching was grounded on the exact year of birth (1-year strata).
Other covariates in the analysis included pruritus-related skin disorders, including prurigo nodularis, neurotic excoriations, and LSC. There were 3 socioeconomic status categories according to patients' poverty index: low, intermediate, and high.26
Statistical Analysis—The distribution of sociodemographic and clinical features was compared between patients with fibromyalgia and controls using the χ2 test for sex and socioeconomic status and the t test for age. Conditional logistic regression then was used to calculate adjusted odds ratio (OR) and 95% CI to compare patients with fibromyalgia and controls with respect to the presence of pruritic comorbidities. All statistical analyses were performed using SPSS software (version 26). P<.05 was considered statistically significant in all tests.
Results
Our study population comprised 4971 patients with fibromyalgia and 9896 age- and sex-matched controls. Proportional to the reported female predominance among patients with fibromyalgia,27 4479 (90.1%) patients with fibromyalgia were females and a similar proportion was documented among controls (P=.99). There was a slightly higher proportion of unmarried patients among those with fibromyalgia compared with controls (41.9% vs 39.4%; P=.004). Socioeconomic status was matched between patients and controls (P=.99). Descriptive characteristics of the study population are presented in Table 1.

We assessed the presence of pruritus as well as 3 other pruritus-related skin disorders—prurigo nodularis, neurotic excoriations, and LSC—among patients with fibromyalgia and controls. Logistic regression was used to evaluate the independent association between fibromyalgia and pruritus. Table 2 presents the results of multivariate logistic regression models and summarizes the adjusted ORs for pruritic conditions in patients with fibromyalgia and different demographic features across the entire study sample. Fibromyalgia demonstrated strong independent associations with pruritus (OR, 1.8; 95% CI, 1.8-2.4; P<.001), prurigo nodularis (OR, 2.9; 95% CI, 1.1-8.4; P=.038), and LSC (OR, 1.5; 95% CI, 1.1-2.1; P=.01); the association with neurotic excoriations was not significant. Female sex significantly increased the risk for pruritus (OR 1.3; 95% CI, 1.0-1.6; P=.039), while age slightly increased the odds for pruritus (OR, 1.0; 95% CI, 1.0-1.04; P<.001), neurotic excoriations (OR, 1.0; 95% CI, 1.0-1.1; P=.046), and LSC (OR, 1.0; 95% CI, 1.01-1.04; P=.006). Finally, socioeconomic status was inversely correlated with pruritus (OR, 1.1; 95% CI, 1.1-1.5; P=.002).

Frequencies and ORs for the association between fibromyalgia and pruritus with associated pruritic disorders stratified by exclusion of pruritic dermatologic and/or systemic diseases that may induce itch are presented in the eTable. Analyzing the entire study cohort, significant increases were observed in the odds of all 4 pruritic disorders analyzed. The frequency of pruritus was almost double in patients with fibromyalgia compared with controls (11.7% vs 6.0%; OR, 2.1; 95% CI, 1.8-2.3; P<.0001). Prurigo nodularis (0.2% vs 0.1%; OR, 2.9; 95% CI, 1.1-8.4; P=.05), neurotic excoriations (0.6% vs 0.3%; OR, 1.9; 95% CI, 1.1-3.1; P=.018), and LSC (1.3% vs 0.8%; OR, 1.5; 95% CI, 1.1-2.1; P=.01) frequencies were all higher in patients with fibromyalgia than controls. When primary skin disorders that may cause itch (eg, pemphigus vulgaris, Darier disease, dermatitis, eczema, ichthyosis, psoriasis, parapsoriasis, urticaria, xerosis, atopic dermatitis, dermatitis herpetiformis, lichen planus) were excluded, the prevalence of pruritus in patients with fibromyalgia was still 1.97 times greater than in the controls (6.9% vs. 3.5%; OR, 2.0; 95% CI, 1.7-2.4; P<.0001). These results remained unchanged even when excluding pruritic dermatologic disorders as well as systemic diseases associated with pruritus (eg, chronic renal failure, dialysis, hyperthyroidism, hyperparathyroidism/hypoparathyroidism, hypothyroidism). Patients with fibromyalgia still displayed a significantly higher prevalence of pruritus compared with the control group (6.6% vs 3.3%; OR, 2.1; 95% CI, 1.7-2.6; P<.0001).

Comment
A wide range of skin manifestations have been associated with fibromyalgia, but the exact mechanisms remain unclear. Nevertheless, it is conceivable that autonomic nervous system dysfunction,28-31 amplified cutaneous opioid receptor activity,32 and an elevated presence of cutaneous mast cells with excessive degranulation may partially explain the frequent occurrence of pruritus and related skin disorders such as neurotic excoriations, prurigo nodularis, and LSC in individuals with fibromyalgia.15,16 In line with these findings, our study—which was based on data from the largest health maintenance organization in Israel—demonstrated an increased prevalence of pruritus and related pruritic disorders among individuals diagnosed with fibromyalgia.
This cross-sectional study links pruritus with fibromyalgia. Few preliminary epidemiologic studies have shown an increased occurrence of cutaneous manifestations in patients with fibromyalgia. One chart review that looked at skin findings in patients with fibromyalgia revealed 32 distinct cutaneous manifestations, and pruritus was the major concern in 3.3% of 845 patients.15
A focused cross-sectional study involving only women (66 with fibromyalgia and 79 healthy controls) discovered 14 skin conditions that were more common in those with fibromyalgia. Notably, xerosis and neurotic excoriations were more prevalent compared to the control group.16
The brain and the skin—both derivatives of the embryonic ectoderm33,34—are linked by pruritus. Although itch has its dedicated neurons, there is a wide-ranging overlap of brain-activated areas between pain and itch,6 and the neural anatomy of pain and itch are closely related in both the peripheral and central nervous systems35-37; for example, diseases of the central nervous system are accompanied by pruritus in as many as 15% of cases, while postherpetic neuralgia can result in chronic pain, itching, or a combination of both.38,39 Other instances include notalgia paresthetica and brachioradial pruritus.38 Additionally, there is a noteworthy psychologic impact associated with both itch and pain,40,41 with both psychosomatic and psychologic factors implicated in chronic pruritus and in fibromyalgia.42 Lastly, the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system are altered in both fibromyalgia and pruritus.43-45
Tey et al45 characterized the itch experienced in fibromyalgia as functional, which is described as pruritus associated with a somatoform disorder. In our study, we found a higher prevalence of pruritus among patients with fibromyalgia, and this association remained significant (P<.05) even when excluding other pruritic skin conditions and systemic diseases that can trigger itching. In addition, our logistic regression analyses revealed independent associations between fibromyalgia and pruritus, prurigo nodularis, and LSC.
According to Twycross et al,46 there are 4 clinical categories of itch, which may coexist7: pruritoceptive (originating in the skin), neuropathic (originating in pathology located along the afferent pathway), neurogenic (central origin but lacks a neural pathology), and psychogenic.47 Skin biopsy findings in patients with fibromyalgia include increased mast cell counts11 and degranulation,48 increased expression of δ and κ opioid receptors,32 vasoconstriction within tender points,49 and elevated IL-1β, IL-6, or tumor necrosis factor α by reverse transcriptase-polymerase chain reaction.12 A case recently was presented by Görg et al50 involving a female patient with fibromyalgia who had been experiencing chronic pruritus, which the authors attributed to small-fiber neuropathy based on evidence from a skin biopsy indicating a reduced number of intraepidermal nerves and the fact that the itching originated around tender points. Altogether, the observed alterations may work together to make patients with fibromyalgia more susceptible to various skin-related comorbidities in general, especially those related to pruritus. Eventually, it might be the case that several itch categories and related pathomechanisms are involved in the pruritus phenotype of patients with fibromyalgia.
Age-related alterations in nerve fibers, lower immune function, xerosis, polypharmacy, and increased frequency of systemic diseases with age are just a few of the factors that may predispose older individuals to pruritus.51,52 Indeed, our logistic regression model showed that age was significantly and independently associated with pruritus (P<.001), neurotic excoriations (P=.046), and LSC (P=.006). Female sex also was significantly linked with pruritus (P=.039). Intriguingly, high socioeconomic status was significantly associated with the diagnosis of pruritus (P=.002), possibly due to easier access to medical care.
There is a considerable overlap between the therapeutic approaches used in pruritus, pruritus-related skin disorders, and fibromyalgia. Antidepressants, anxiolytics, analgesics, and antiepileptics have been used to address both conditions.45 The association between these conditions advocates for a multidisciplinary approach in patients with fibromyalgia and potentially supports the rationale for unified therapeutics for both conditions.
Conclusion
Our findings indicate an association between fibromyalgia and pruritus as well as associated pruritic skin disorders. Given the convoluted and largely undiscovered mechanisms underlying fibromyalgia, managing patients with this condition may present substantial challenges.53 The data presented here support the implementation of a multidisciplinary treatment approach for patients with fibromyalgia. This approach should focus on managing fibromyalgia pain as well as addressing its concurrent skin-related conditions. It is advisable to consider treatments such as antiepileptics (eg, pregabalin, gabapentin) that specifically target neuropathic disorders in affected patients. These treatments may hold promise for alleviating fibromyalgia-related pain54 and mitigating its related cutaneous comorbidities, especially pruritus.
- Stander S, Weisshaar E, Mettang T, et al. Clinical classification of itch: a position paper of the International Forum for the Study of Itch. Acta Derm Venereol. 2007; 87:291-294.
- Yosipovitch G, Bernhard JD. Clinical practice. chronic pruritus. N Engl J Med. 2013;368:1625-1634.
- Song J, Xian D, Yang L, et al. Pruritus: progress toward pathogenesis and treatment. Biomed Res Int. 2018;2018:9625936.
- Potenzieri C, Undem BJ. Basic mechanisms of itch. Clin Exp Allergy. 2012;42:8-19.
- McMahon SB, Koltzenburg M. Itching for an explanation. Trends Neurosci. 1992;15:497-501.
- Drzezga A, Darsow U, Treede RD, et al. Central activation by histamine-induced itch: analogies to pain processing: a correlational analysis of O-15 H2O positron emission tomography studies. Pain. 2001; 92:295-305.
- Yosipovitch G, Greaves MW, Schmelz M. Itch. Lancet. 2003;361:690-694.
- Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. part I. Arthritis Rheum. 2008; 58:15-25.
- Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. part II. Arthritis Rheum. 2008; 58:26-35.
- Sarzi-Puttini P, Giorgi V, Marotto D, et al. Fibromyalgia: an update on clinical characteristics, aetiopathogenesis and treatment. Nat Rev Rheumatol. 2020;16:645-660.
- Blanco I, Beritze N, Arguelles M, et al. Abnormal overexpression of mastocytes in skin biopsies of fibromyalgia patients. Clin Rheumatol. 2010;29:1403-1412.
- Salemi S, Rethage J, Wollina U, et al. Detection of interleukin 1beta (IL-1beta), IL-6, and tumor necrosis factor-alpha in skin of patients with fibromyalgia. J Rheumatol. 2003;30:146-150.
- Sprott H, Muller A, Heine H. Collagen cross-links in fibromyalgia syndrome. Z Rheumatol. 1998;57(suppl 2):52-55.
- Morf S, Amann-Vesti B, Forster A, et al. Microcirculation abnormalities in patients with fibromyalgia—measured by capillary microscopy and laser fluxmetry. Arthritis Res Ther. 2005;7:R209-R216.
- Laniosz V, Wetter DA, Godar DA. Dermatologic manifestations of fibromyalgia. Clin Rheumatol. 2014;33:1009-1013.
- Dogramaci AC, Yalcinkaya EY. Skin problems in fibromyalgia. Nobel Med. 2009;5:50-52.
- Grayston R, Czanner G, Elhadd K, et al. A systematic review and meta-analysis of the prevalence of small fiber pathology in fibromyalgia: implications for a new paradigm in fibromyalgia etiopathogenesis. Semin Arthritis Rheum. 2019;48:933-940.
- Uceyler N, Zeller D, Kahn AK, et al. Small fibre pathology in patients with fibromyalgia syndrome. Brain. 2013;136:1857-1867.
- Devigili G, Tugnoli V, Penza P, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain. 2008; 131:1912- 1925.
- Reed C, Birnbaum HG, Ivanova JI, et al. Real-world role of tricyclic antidepressants in the treatment of fibromyalgia. Pain Pract. 2012; 12:533-540.
- Moret C, Briley M. Antidepressants in the treatment of fibromyalgia. Neuropsychiatr Dis Treat. 2006;2:537-548.
- Arnold LM, Keck PE Jr, Welge JA. Antidepressant treatment of fibromyalgia. a meta-analysis and review. Psychosomatics. 2000;41:104-113.
- Moore A, Wiffen P, Kalso E. Antiepileptic drugs for neuropathic pain and fibromyalgia. JAMA. 2014;312:182-183.
- Shevchenko A, Valdes-Rodriguez R, Yosipovitch G. Causes, pathophysiology, and treatment of pruritus in the mature patient. Clin Dermatol. 2018;36:140-151.
- Scheinfeld N. The role of gabapentin in treating diseases with cutaneous manifestations and pain. Int J Dermatol. 2003;42:491-495.
- Points Location Intelligence. Accessed July 30, 2024. https://points.co.il/en/points-location-intelligence/
- Yunus MB. The role of gender in fibromyalgia syndrome. Curr Rheumatol Rep. 2001;3:128-134.
- Cakir T, Evcik D, Dundar U, et al. Evaluation of sympathetic skin response and f wave in fibromyalgia syndrome patients. Turk J Rheumatol. 2011;26:38-43.
- Ozkan O, Yildiz M, Koklukaya E. The correlation of laboratory tests and sympathetic skin response parameters by using artificial neural networks in fibromyalgia patients. J Med Syst. 2012;36:1841-1848.
- Ozkan O, Yildiz M, Arslan E, et al. A study on the effects of sympathetic skin response parameters in diagnosis of fibromyalgia using artificial neural networks. J Med Syst. 2016;40:54.
- Ulas UH, Unlu E, Hamamcioglu K, et al. Dysautonomia in fibromyalgia syndrome: sympathetic skin responses and RR interval analysis. Rheumatol Int. 2006;26:383-387.
- Salemi S, Aeschlimann A, Wollina U, et al. Up-regulation of delta-opioid receptors and kappa-opioid receptors in the skin of fibromyalgia patients. Arthritis Rheum. 2007;56:2464-2466.
- Elshazzly M, Lopez MJ, Reddy V, et al. Central nervous system. StatPearls. StatPearls Publishing; 2022.
- Hu MS, Borrelli MR, Hong WX, et al. Embryonic skin development and repair. Organogenesis. 2018;14:46-63.
- Davidson S, Zhang X, Yoon CH, et al. The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci. 2007;27:10007-10014.
- Sikand P, Shimada SG, Green BG, et al. Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain. 2009;144:66-75.
- Davidson S, Giesler GJ. The multiple pathways for itch and their interactions with pain. Trends Neurosci. 2010;33:550-558.
- Dhand A, Aminoff MJ. The neurology of itch. Brain. 2014;137:313-322.
- Binder A, Koroschetz J, Baron R. Disease mechanisms in neuropathic itch. Nat Clin Pract Neurol. 2008;4:329-337.
- Fjellner B, Arnetz BB. Psychological predictors of pruritus during mental stress. Acta Derm Venereol. 1985;65:504-508.
- Papoiu AD, Wang H, Coghill RC, et al. Contagious itch in humans: a study of visual ‘transmission’ of itch in atopic dermatitis and healthy subjects. Br J Dermatol. 2011;164:1299-1303.
- Stumpf A, Schneider G, Stander S. Psychosomatic and psychiatric disorders and psychologic factors in pruritus. Clin Dermatol. 2018;36:704-708.
- Herman JP, McKlveen JM, Ghosal S, et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. 2016;6:603-621.
- Brown ED, Micozzi MS, Craft NE, et al. Plasma carotenoids in normal men after a single ingestion of vegetables or purified beta-carotene. Am J Clin Nutr. 1989;49:1258-1265.
- Tey HL, Wallengren J, Yosipovitch G. Psychosomatic factors in pruritus. Clin Dermatol. 2013;31:31-40.
- Twycross R, Greaves MW, Handwerker H, et al. Itch: scratching more than the surface. QJM. 2003;96:7-26.
- Bernhard JD. Itch and pruritus: what are they, and how should itches be classified? Dermatol Ther. 2005;18:288-291.
- Enestrom S, Bengtsson A, Frodin T. Dermal IgG deposits and increase of mast cells in patients with fibromyalgia—relevant findings or epiphenomena? Scand J Rheumatol. 1997;26:308-313.
- Jeschonneck M, Grohmann G, Hein G, et al. Abnormal microcirculation and temperature in skin above tender points in patients with fibromyalgia. Rheumatology (Oxford). 2000;39:917-921.
- Görg M, Zeidler C, Pereira MP, et al. Generalized chronic pruritus with fibromyalgia. J Dtsch Dermatol Ges. 2021;19:909-911.
- Garibyan L, Chiou AS, Elmariah SB. Advanced aging skin and itch: addressing an unmet need. Dermatol Ther. 2013;26:92-103.
- Cohen KR, Frank J, Salbu RL, et al. Pruritus in the elderly: clinical approaches to the improvement of quality of life. P T. 2012;37:227-239.
- Tzadok R, Ablin JN. Current and emerging pharmacotherapy for fibromyalgia. Pain Res Manag. 2020; 2020:6541798.
- Wiffen PJ, Derry S, Moore RA, et al. Antiepileptic drugs for neuropathic pain and fibromyalgia—an overview of Cochrane reviews. Cochrane Database Syst Rev. 2013:CD010567.
- Stander S, Weisshaar E, Mettang T, et al. Clinical classification of itch: a position paper of the International Forum for the Study of Itch. Acta Derm Venereol. 2007; 87:291-294.
- Yosipovitch G, Bernhard JD. Clinical practice. chronic pruritus. N Engl J Med. 2013;368:1625-1634.
- Song J, Xian D, Yang L, et al. Pruritus: progress toward pathogenesis and treatment. Biomed Res Int. 2018;2018:9625936.
- Potenzieri C, Undem BJ. Basic mechanisms of itch. Clin Exp Allergy. 2012;42:8-19.
- McMahon SB, Koltzenburg M. Itching for an explanation. Trends Neurosci. 1992;15:497-501.
- Drzezga A, Darsow U, Treede RD, et al. Central activation by histamine-induced itch: analogies to pain processing: a correlational analysis of O-15 H2O positron emission tomography studies. Pain. 2001; 92:295-305.
- Yosipovitch G, Greaves MW, Schmelz M. Itch. Lancet. 2003;361:690-694.
- Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. part I. Arthritis Rheum. 2008; 58:15-25.
- Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. part II. Arthritis Rheum. 2008; 58:26-35.
- Sarzi-Puttini P, Giorgi V, Marotto D, et al. Fibromyalgia: an update on clinical characteristics, aetiopathogenesis and treatment. Nat Rev Rheumatol. 2020;16:645-660.
- Blanco I, Beritze N, Arguelles M, et al. Abnormal overexpression of mastocytes in skin biopsies of fibromyalgia patients. Clin Rheumatol. 2010;29:1403-1412.
- Salemi S, Rethage J, Wollina U, et al. Detection of interleukin 1beta (IL-1beta), IL-6, and tumor necrosis factor-alpha in skin of patients with fibromyalgia. J Rheumatol. 2003;30:146-150.
- Sprott H, Muller A, Heine H. Collagen cross-links in fibromyalgia syndrome. Z Rheumatol. 1998;57(suppl 2):52-55.
- Morf S, Amann-Vesti B, Forster A, et al. Microcirculation abnormalities in patients with fibromyalgia—measured by capillary microscopy and laser fluxmetry. Arthritis Res Ther. 2005;7:R209-R216.
- Laniosz V, Wetter DA, Godar DA. Dermatologic manifestations of fibromyalgia. Clin Rheumatol. 2014;33:1009-1013.
- Dogramaci AC, Yalcinkaya EY. Skin problems in fibromyalgia. Nobel Med. 2009;5:50-52.
- Grayston R, Czanner G, Elhadd K, et al. A systematic review and meta-analysis of the prevalence of small fiber pathology in fibromyalgia: implications for a new paradigm in fibromyalgia etiopathogenesis. Semin Arthritis Rheum. 2019;48:933-940.
- Uceyler N, Zeller D, Kahn AK, et al. Small fibre pathology in patients with fibromyalgia syndrome. Brain. 2013;136:1857-1867.
- Devigili G, Tugnoli V, Penza P, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain. 2008; 131:1912- 1925.
- Reed C, Birnbaum HG, Ivanova JI, et al. Real-world role of tricyclic antidepressants in the treatment of fibromyalgia. Pain Pract. 2012; 12:533-540.
- Moret C, Briley M. Antidepressants in the treatment of fibromyalgia. Neuropsychiatr Dis Treat. 2006;2:537-548.
- Arnold LM, Keck PE Jr, Welge JA. Antidepressant treatment of fibromyalgia. a meta-analysis and review. Psychosomatics. 2000;41:104-113.
- Moore A, Wiffen P, Kalso E. Antiepileptic drugs for neuropathic pain and fibromyalgia. JAMA. 2014;312:182-183.
- Shevchenko A, Valdes-Rodriguez R, Yosipovitch G. Causes, pathophysiology, and treatment of pruritus in the mature patient. Clin Dermatol. 2018;36:140-151.
- Scheinfeld N. The role of gabapentin in treating diseases with cutaneous manifestations and pain. Int J Dermatol. 2003;42:491-495.
- Points Location Intelligence. Accessed July 30, 2024. https://points.co.il/en/points-location-intelligence/
- Yunus MB. The role of gender in fibromyalgia syndrome. Curr Rheumatol Rep. 2001;3:128-134.
- Cakir T, Evcik D, Dundar U, et al. Evaluation of sympathetic skin response and f wave in fibromyalgia syndrome patients. Turk J Rheumatol. 2011;26:38-43.
- Ozkan O, Yildiz M, Koklukaya E. The correlation of laboratory tests and sympathetic skin response parameters by using artificial neural networks in fibromyalgia patients. J Med Syst. 2012;36:1841-1848.
- Ozkan O, Yildiz M, Arslan E, et al. A study on the effects of sympathetic skin response parameters in diagnosis of fibromyalgia using artificial neural networks. J Med Syst. 2016;40:54.
- Ulas UH, Unlu E, Hamamcioglu K, et al. Dysautonomia in fibromyalgia syndrome: sympathetic skin responses and RR interval analysis. Rheumatol Int. 2006;26:383-387.
- Salemi S, Aeschlimann A, Wollina U, et al. Up-regulation of delta-opioid receptors and kappa-opioid receptors in the skin of fibromyalgia patients. Arthritis Rheum. 2007;56:2464-2466.
- Elshazzly M, Lopez MJ, Reddy V, et al. Central nervous system. StatPearls. StatPearls Publishing; 2022.
- Hu MS, Borrelli MR, Hong WX, et al. Embryonic skin development and repair. Organogenesis. 2018;14:46-63.
- Davidson S, Zhang X, Yoon CH, et al. The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci. 2007;27:10007-10014.
- Sikand P, Shimada SG, Green BG, et al. Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain. 2009;144:66-75.
- Davidson S, Giesler GJ. The multiple pathways for itch and their interactions with pain. Trends Neurosci. 2010;33:550-558.
- Dhand A, Aminoff MJ. The neurology of itch. Brain. 2014;137:313-322.
- Binder A, Koroschetz J, Baron R. Disease mechanisms in neuropathic itch. Nat Clin Pract Neurol. 2008;4:329-337.
- Fjellner B, Arnetz BB. Psychological predictors of pruritus during mental stress. Acta Derm Venereol. 1985;65:504-508.
- Papoiu AD, Wang H, Coghill RC, et al. Contagious itch in humans: a study of visual ‘transmission’ of itch in atopic dermatitis and healthy subjects. Br J Dermatol. 2011;164:1299-1303.
- Stumpf A, Schneider G, Stander S. Psychosomatic and psychiatric disorders and psychologic factors in pruritus. Clin Dermatol. 2018;36:704-708.
- Herman JP, McKlveen JM, Ghosal S, et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. 2016;6:603-621.
- Brown ED, Micozzi MS, Craft NE, et al. Plasma carotenoids in normal men after a single ingestion of vegetables or purified beta-carotene. Am J Clin Nutr. 1989;49:1258-1265.
- Tey HL, Wallengren J, Yosipovitch G. Psychosomatic factors in pruritus. Clin Dermatol. 2013;31:31-40.
- Twycross R, Greaves MW, Handwerker H, et al. Itch: scratching more than the surface. QJM. 2003;96:7-26.
- Bernhard JD. Itch and pruritus: what are they, and how should itches be classified? Dermatol Ther. 2005;18:288-291.
- Enestrom S, Bengtsson A, Frodin T. Dermal IgG deposits and increase of mast cells in patients with fibromyalgia—relevant findings or epiphenomena? Scand J Rheumatol. 1997;26:308-313.
- Jeschonneck M, Grohmann G, Hein G, et al. Abnormal microcirculation and temperature in skin above tender points in patients with fibromyalgia. Rheumatology (Oxford). 2000;39:917-921.
- Görg M, Zeidler C, Pereira MP, et al. Generalized chronic pruritus with fibromyalgia. J Dtsch Dermatol Ges. 2021;19:909-911.
- Garibyan L, Chiou AS, Elmariah SB. Advanced aging skin and itch: addressing an unmet need. Dermatol Ther. 2013;26:92-103.
- Cohen KR, Frank J, Salbu RL, et al. Pruritus in the elderly: clinical approaches to the improvement of quality of life. P T. 2012;37:227-239.
- Tzadok R, Ablin JN. Current and emerging pharmacotherapy for fibromyalgia. Pain Res Manag. 2020; 2020:6541798.
- Wiffen PJ, Derry S, Moore RA, et al. Antiepileptic drugs for neuropathic pain and fibromyalgia—an overview of Cochrane reviews. Cochrane Database Syst Rev. 2013:CD010567.
Practice Points
- Dermatologists should be aware of the connection between fibromyalgia, pruritus, and related conditions to improve patient care.
- The association between fibromyalgia and pruritus underscores the importance of employing multidisciplinary treatment strategies for managing these conditions.
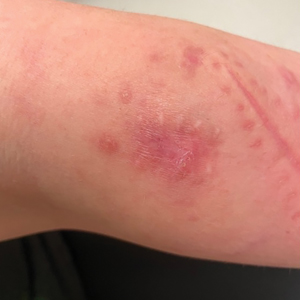
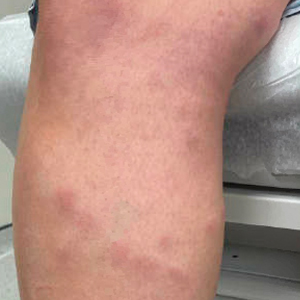
Painful Plaque on the Forearm
The Diagnosis: Mycobacterium marinum Infection
A repeat excisional biopsy showed suppurative granulomatous dermatitis with negative stains for infectious organisms; however, tissue culture grew Mycobacterium marinum. The patient had a history of exposure to fish tanks, which are a potential habitat for nontuberculous mycobacteria. These bacteria can enter the body through a minor laceration or cut in the skin, which was likely due to her occupation and pet care activities.1 Her fish tank exposure combined with the cutaneous findings of a long-standing indurated plaque with proximal nodular lymphangitis made M marinum infection the most likely diagnosis.2
Due to the limited specificity and sensitivity of patient symptoms, histologic staining, and direct microscopy, the gold standard for diagnosing acid-fast bacilli is tissue culture. 3 Tissue polymerase chain reaction testing is most useful in identifying the species of mycobacteria when histologic stains identify acid-fast bacilli but repeated tissue cultures are negative.4 With M marinum, a high clinical suspicion is needed to acquire a positive tissue culture because it needs to be grown for several weeks and at a temperature of 30 °C.5 Therefore, the physician should inform the laboratory if there is any suspicion for M marinum to increase the likelihood of obtaining a positive culture.
The differential diagnosis for M marinum infection includes other skin diseases that can cause nodular lymphangitis (also known as sporotrichoid spread) such as sporotrichosis, leishmaniasis, and certain bacterial and fungal infections. Although cat scratch disease, which is caused by Bartonella henselae, can appear similar to M marinum on histopathology, it clinically manifests with a single papulovesicular lesion at the site of inoculation that then forms a central eschar and resolves within a few weeks. Cat scratch disease typically causes painful lymphadenopathy, but it does not cause nodular lymphangitis or sporotrichoid spread.6 Sporotrichosis can have a similar clinical and histologic manifestation to M marinum infection, but the patient history typically includes exposure to Sporothrix schenckii through gardening or other contact with thorns, plants, or soil.2 Cutaneous sarcoidosis can have a similar clinical appearance to M marinum infection, but nodular lymphangitis does not occur and histopathology would demonstrate noncaseating epithelioid cell granulomas.7 Lastly, although vegetative pyogenic granuloma can have some of the same histologic findings as M marinum, it typically also demonstrates sinus tract formation, which was not present in our case. Additionally, vegetative pyogenic granuloma manifests with a verrucous and pustular plaque that would not have lymphocutaneous spread.8
Treatment of cutaneous M marinum infection is guided by antibiotic susceptibility testing. One regimen is clarithromycin (500 mg twice daily9) plus ethambutol. 10 Treatment often entails a multidrug combination due to the high rates of antibiotic resistance. Other antibiotics that potentially can be used include rifampin, trimethoprim-sulfamethoxazole, minocycline, and quinolones. The treatment duration typically is more than 3 months, and therapy is continued for 4 to 6 weeks after the skin lesions resolve.11 Excision of the lesion is reserved for patients with M marinum infection that fails to respond to antibiotic therapy.5
- Wayne LG, Sramek HA. Agents of newly recognized or infrequently encountered mycobacterial diseases. Clin Microbiol Rev. 1992;5:1-25. doi:10.1128/CMR.5.1.1
- Tobin EH, Jih WW. Sporotrichoid lymphocutaneous infections: etiology, diagnosis and therapy. Am Fam Physician. 2001;63:326-332.
- van Ingen J. Diagnosis of nontuberculous mycobacterial infections. Semin Respir Crit Care Med. 2013;34:103-109. doi:10.1055/s-0033-1333569
- Williamson H, Phillips R, Sarfo S, et al. Genetic diversity of PCR-positive, culture-negative and culture-positive Mycobacterium ulcerans isolated from Buruli ulcer patients in Ghana. PLoS One. 2014;9:E88007. doi:10.1371/journal.pone.0088007
- Aubry A, Mougari F, Reibel F, et al. Mycobacterium marinum. Microbiol Spectr. 2017;5. doi:10.1128/microbiolspec.TNMI7-0038-2016
- Baranowski K, Huang B. Cat scratch disease. StatPearls [Internet]. Updated June 12, 2023. Accessed July 15, 2024. https://www.ncbi.nlm .nih.gov/books/NBK482139/
- Sanchez M, Haimovic A, Prystowsky S. Sarcoidosis. Dermatol Clin. 2015;33:389-416. doi:10.1016/j.det.2015.03.006
- Borg Grech S, Vella Baldacchino A, Corso R, et al. Superficial granulomatous pyoderma successfully treated with intravenous immunoglobulin. Eur J Case Rep Intern Med. 2021;8:002656. doi:10.12890/2021_002656
- Krooks J, Weatherall A, Markowitz S. Complete resolution of Mycobacterium marinum infection with clarithromycin and ethambutol: a case report and a review of the literature. J Clin Aesthet Dermatol. 2018;11:48-51.
- Medel-Plaza M., Esteban J. Current treatment options for Mycobacterium marinum cutaneous infections. Expert Opin Pharmacother. 2023;24:1113-1123. doi:10.1080/14656566.2023.2211258
- Tirado-Sánchez A, Bonifaz A. Nodular lymphangitis (sporotrichoid lymphocutaneous infections): clues to differential diagnosis. J Fungi (Basel). 2018;4:56. doi:10.3390/jof4020056
The Diagnosis: Mycobacterium marinum Infection
A repeat excisional biopsy showed suppurative granulomatous dermatitis with negative stains for infectious organisms; however, tissue culture grew Mycobacterium marinum. The patient had a history of exposure to fish tanks, which are a potential habitat for nontuberculous mycobacteria. These bacteria can enter the body through a minor laceration or cut in the skin, which was likely due to her occupation and pet care activities.1 Her fish tank exposure combined with the cutaneous findings of a long-standing indurated plaque with proximal nodular lymphangitis made M marinum infection the most likely diagnosis.2
Due to the limited specificity and sensitivity of patient symptoms, histologic staining, and direct microscopy, the gold standard for diagnosing acid-fast bacilli is tissue culture. 3 Tissue polymerase chain reaction testing is most useful in identifying the species of mycobacteria when histologic stains identify acid-fast bacilli but repeated tissue cultures are negative.4 With M marinum, a high clinical suspicion is needed to acquire a positive tissue culture because it needs to be grown for several weeks and at a temperature of 30 °C.5 Therefore, the physician should inform the laboratory if there is any suspicion for M marinum to increase the likelihood of obtaining a positive culture.
The differential diagnosis for M marinum infection includes other skin diseases that can cause nodular lymphangitis (also known as sporotrichoid spread) such as sporotrichosis, leishmaniasis, and certain bacterial and fungal infections. Although cat scratch disease, which is caused by Bartonella henselae, can appear similar to M marinum on histopathology, it clinically manifests with a single papulovesicular lesion at the site of inoculation that then forms a central eschar and resolves within a few weeks. Cat scratch disease typically causes painful lymphadenopathy, but it does not cause nodular lymphangitis or sporotrichoid spread.6 Sporotrichosis can have a similar clinical and histologic manifestation to M marinum infection, but the patient history typically includes exposure to Sporothrix schenckii through gardening or other contact with thorns, plants, or soil.2 Cutaneous sarcoidosis can have a similar clinical appearance to M marinum infection, but nodular lymphangitis does not occur and histopathology would demonstrate noncaseating epithelioid cell granulomas.7 Lastly, although vegetative pyogenic granuloma can have some of the same histologic findings as M marinum, it typically also demonstrates sinus tract formation, which was not present in our case. Additionally, vegetative pyogenic granuloma manifests with a verrucous and pustular plaque that would not have lymphocutaneous spread.8
Treatment of cutaneous M marinum infection is guided by antibiotic susceptibility testing. One regimen is clarithromycin (500 mg twice daily9) plus ethambutol. 10 Treatment often entails a multidrug combination due to the high rates of antibiotic resistance. Other antibiotics that potentially can be used include rifampin, trimethoprim-sulfamethoxazole, minocycline, and quinolones. The treatment duration typically is more than 3 months, and therapy is continued for 4 to 6 weeks after the skin lesions resolve.11 Excision of the lesion is reserved for patients with M marinum infection that fails to respond to antibiotic therapy.5
The Diagnosis: Mycobacterium marinum Infection
A repeat excisional biopsy showed suppurative granulomatous dermatitis with negative stains for infectious organisms; however, tissue culture grew Mycobacterium marinum. The patient had a history of exposure to fish tanks, which are a potential habitat for nontuberculous mycobacteria. These bacteria can enter the body through a minor laceration or cut in the skin, which was likely due to her occupation and pet care activities.1 Her fish tank exposure combined with the cutaneous findings of a long-standing indurated plaque with proximal nodular lymphangitis made M marinum infection the most likely diagnosis.2
Due to the limited specificity and sensitivity of patient symptoms, histologic staining, and direct microscopy, the gold standard for diagnosing acid-fast bacilli is tissue culture. 3 Tissue polymerase chain reaction testing is most useful in identifying the species of mycobacteria when histologic stains identify acid-fast bacilli but repeated tissue cultures are negative.4 With M marinum, a high clinical suspicion is needed to acquire a positive tissue culture because it needs to be grown for several weeks and at a temperature of 30 °C.5 Therefore, the physician should inform the laboratory if there is any suspicion for M marinum to increase the likelihood of obtaining a positive culture.
The differential diagnosis for M marinum infection includes other skin diseases that can cause nodular lymphangitis (also known as sporotrichoid spread) such as sporotrichosis, leishmaniasis, and certain bacterial and fungal infections. Although cat scratch disease, which is caused by Bartonella henselae, can appear similar to M marinum on histopathology, it clinically manifests with a single papulovesicular lesion at the site of inoculation that then forms a central eschar and resolves within a few weeks. Cat scratch disease typically causes painful lymphadenopathy, but it does not cause nodular lymphangitis or sporotrichoid spread.6 Sporotrichosis can have a similar clinical and histologic manifestation to M marinum infection, but the patient history typically includes exposure to Sporothrix schenckii through gardening or other contact with thorns, plants, or soil.2 Cutaneous sarcoidosis can have a similar clinical appearance to M marinum infection, but nodular lymphangitis does not occur and histopathology would demonstrate noncaseating epithelioid cell granulomas.7 Lastly, although vegetative pyogenic granuloma can have some of the same histologic findings as M marinum, it typically also demonstrates sinus tract formation, which was not present in our case. Additionally, vegetative pyogenic granuloma manifests with a verrucous and pustular plaque that would not have lymphocutaneous spread.8
Treatment of cutaneous M marinum infection is guided by antibiotic susceptibility testing. One regimen is clarithromycin (500 mg twice daily9) plus ethambutol. 10 Treatment often entails a multidrug combination due to the high rates of antibiotic resistance. Other antibiotics that potentially can be used include rifampin, trimethoprim-sulfamethoxazole, minocycline, and quinolones. The treatment duration typically is more than 3 months, and therapy is continued for 4 to 6 weeks after the skin lesions resolve.11 Excision of the lesion is reserved for patients with M marinum infection that fails to respond to antibiotic therapy.5
- Wayne LG, Sramek HA. Agents of newly recognized or infrequently encountered mycobacterial diseases. Clin Microbiol Rev. 1992;5:1-25. doi:10.1128/CMR.5.1.1
- Tobin EH, Jih WW. Sporotrichoid lymphocutaneous infections: etiology, diagnosis and therapy. Am Fam Physician. 2001;63:326-332.
- van Ingen J. Diagnosis of nontuberculous mycobacterial infections. Semin Respir Crit Care Med. 2013;34:103-109. doi:10.1055/s-0033-1333569
- Williamson H, Phillips R, Sarfo S, et al. Genetic diversity of PCR-positive, culture-negative and culture-positive Mycobacterium ulcerans isolated from Buruli ulcer patients in Ghana. PLoS One. 2014;9:E88007. doi:10.1371/journal.pone.0088007
- Aubry A, Mougari F, Reibel F, et al. Mycobacterium marinum. Microbiol Spectr. 2017;5. doi:10.1128/microbiolspec.TNMI7-0038-2016
- Baranowski K, Huang B. Cat scratch disease. StatPearls [Internet]. Updated June 12, 2023. Accessed July 15, 2024. https://www.ncbi.nlm .nih.gov/books/NBK482139/
- Sanchez M, Haimovic A, Prystowsky S. Sarcoidosis. Dermatol Clin. 2015;33:389-416. doi:10.1016/j.det.2015.03.006
- Borg Grech S, Vella Baldacchino A, Corso R, et al. Superficial granulomatous pyoderma successfully treated with intravenous immunoglobulin. Eur J Case Rep Intern Med. 2021;8:002656. doi:10.12890/2021_002656
- Krooks J, Weatherall A, Markowitz S. Complete resolution of Mycobacterium marinum infection with clarithromycin and ethambutol: a case report and a review of the literature. J Clin Aesthet Dermatol. 2018;11:48-51.
- Medel-Plaza M., Esteban J. Current treatment options for Mycobacterium marinum cutaneous infections. Expert Opin Pharmacother. 2023;24:1113-1123. doi:10.1080/14656566.2023.2211258
- Tirado-Sánchez A, Bonifaz A. Nodular lymphangitis (sporotrichoid lymphocutaneous infections): clues to differential diagnosis. J Fungi (Basel). 2018;4:56. doi:10.3390/jof4020056
- Wayne LG, Sramek HA. Agents of newly recognized or infrequently encountered mycobacterial diseases. Clin Microbiol Rev. 1992;5:1-25. doi:10.1128/CMR.5.1.1
- Tobin EH, Jih WW. Sporotrichoid lymphocutaneous infections: etiology, diagnosis and therapy. Am Fam Physician. 2001;63:326-332.
- van Ingen J. Diagnosis of nontuberculous mycobacterial infections. Semin Respir Crit Care Med. 2013;34:103-109. doi:10.1055/s-0033-1333569
- Williamson H, Phillips R, Sarfo S, et al. Genetic diversity of PCR-positive, culture-negative and culture-positive Mycobacterium ulcerans isolated from Buruli ulcer patients in Ghana. PLoS One. 2014;9:E88007. doi:10.1371/journal.pone.0088007
- Aubry A, Mougari F, Reibel F, et al. Mycobacterium marinum. Microbiol Spectr. 2017;5. doi:10.1128/microbiolspec.TNMI7-0038-2016
- Baranowski K, Huang B. Cat scratch disease. StatPearls [Internet]. Updated June 12, 2023. Accessed July 15, 2024. https://www.ncbi.nlm .nih.gov/books/NBK482139/
- Sanchez M, Haimovic A, Prystowsky S. Sarcoidosis. Dermatol Clin. 2015;33:389-416. doi:10.1016/j.det.2015.03.006
- Borg Grech S, Vella Baldacchino A, Corso R, et al. Superficial granulomatous pyoderma successfully treated with intravenous immunoglobulin. Eur J Case Rep Intern Med. 2021;8:002656. doi:10.12890/2021_002656
- Krooks J, Weatherall A, Markowitz S. Complete resolution of Mycobacterium marinum infection with clarithromycin and ethambutol: a case report and a review of the literature. J Clin Aesthet Dermatol. 2018;11:48-51.
- Medel-Plaza M., Esteban J. Current treatment options for Mycobacterium marinum cutaneous infections. Expert Opin Pharmacother. 2023;24:1113-1123. doi:10.1080/14656566.2023.2211258
- Tirado-Sánchez A, Bonifaz A. Nodular lymphangitis (sporotrichoid lymphocutaneous infections): clues to differential diagnosis. J Fungi (Basel). 2018;4:56. doi:10.3390/jof4020056
A 30-year-old woman presented to the dermatology clinic with lesions on the right forearm of 2 years’ duration. Her medical history was unremarkable. She reported working as a chef and caring for multiple pets in her home, including 3 cats, 6 fish tanks, 3 dogs, and 3 lizards. Physical examination revealed a painful, indurated, red-violaceous plaque on the right forearm with satellite pink nodules that had been slowly migrating proximally up the forearm. An outside excisional biopsy performed 1 year prior had shown suppurative granulomatous dermatitis with negative stains for infectious organisms and negative tissue cultures. At that time, the patient was diagnosed with ruptured folliculitis; however, a subsequent lack of clinical improvement prompted her to seek a second opinion at our clinic.
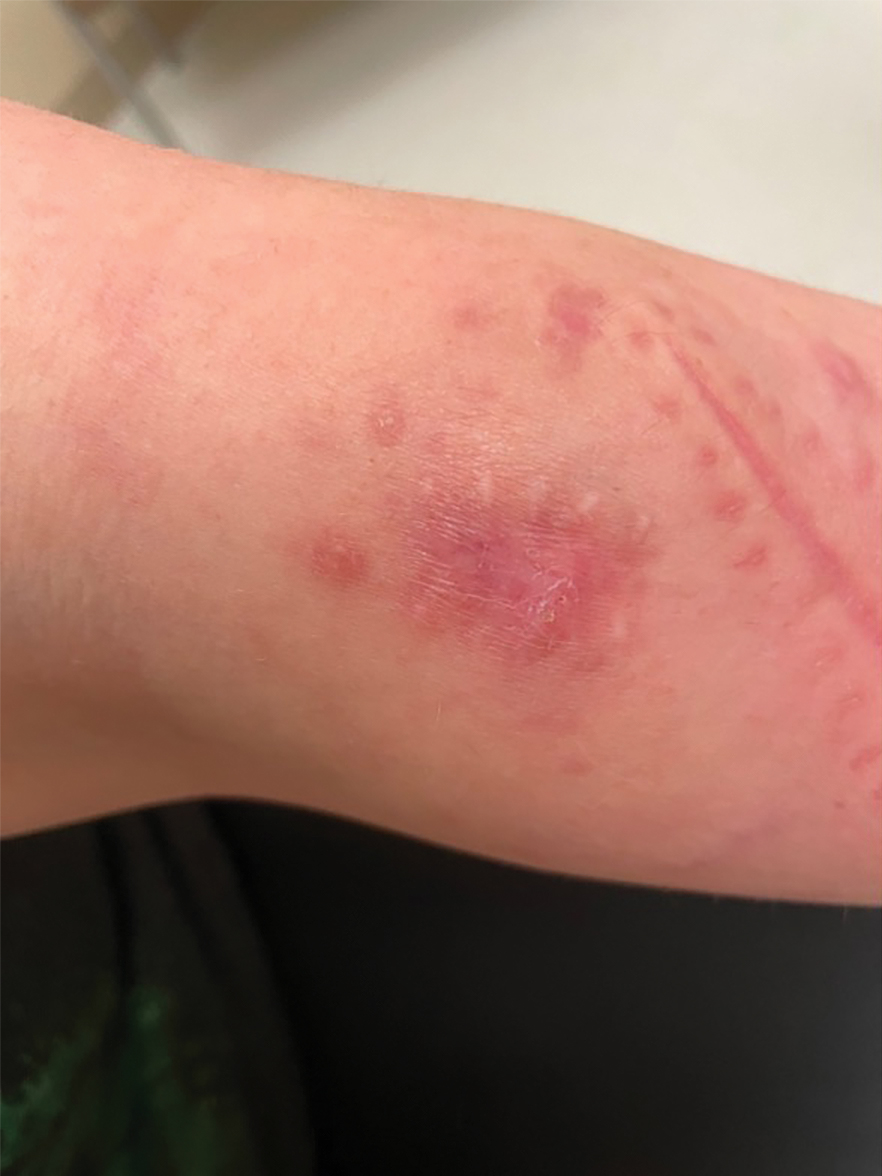
Erythema Nodosum Triggered by a Bite From a Copperhead Snake
The clinical manifestations of snakebites vary based on the species of snake, bite location, and amount and strength of the venom injected. Locally acting toxins in snake venom predominantly consist of enzymes, such as phospholipase A2, that cause local tissue destruction and can result in pain, swelling, blistering, ecchymosis, and tissue necrosis at the site of the bite within hours to days after the bite.1 Systemically acting toxins can target a wide variety of tissues and cause severe systemic complications including paralysis, rhabdomyolysis secondary to muscle damage, coagulopathy, sepsis, and cardiorespiratory failure.2
Although pain and swelling following snakebites typically resolve by 1 month after envenomation, copperhead snakes—a type of pit viper—may cause residual symptoms of pain and swelling lasting for a year or more.3 Additional cutaneous manifestations of copperhead snakebites include wound infections at the bite site, such as cellulitis and necrotizing fasciitis. More devastating complications that have been described following snake envenomation include tissue injury of an entire extremity and development of compartment syndrome, which requires urgent fasciotomy to prevent potential loss of the affected limb.4
Physicians should be aware of the potential complications of snakebites to properly manage and counsel their patients. We describe a 42-year-old woman with tender, erythematous, subcutaneous nodules persisting for 4 months following a copperhead snakebite. A biopsy confirmed the diagnosis of snakebite-associated erythema nodosum (EN).
Case Report
A 42-year-old woman presented to our clinic with progressive tender, pruritic, deep-seated, erythematous nodules in multiple locations on the legs after sustaining a bite by a copperhead snake on the left foot 4 months prior. The lesions tended to fluctuate in intensity. In the days following the bite, she initially developed painful red bumps on the left foot just proximal to the bite site with associated pain and swelling extending up to just below the left knee. She reported no other notable symptoms such as fever, arthralgia, fatigue, or gastrointestinal tract symptoms. Physical examination revealed bilateral pitting edema, which was worse in the left leg, along with multiple deep, palpable, tender subcutaneous nodules with erythematous surface change (Figure 1).
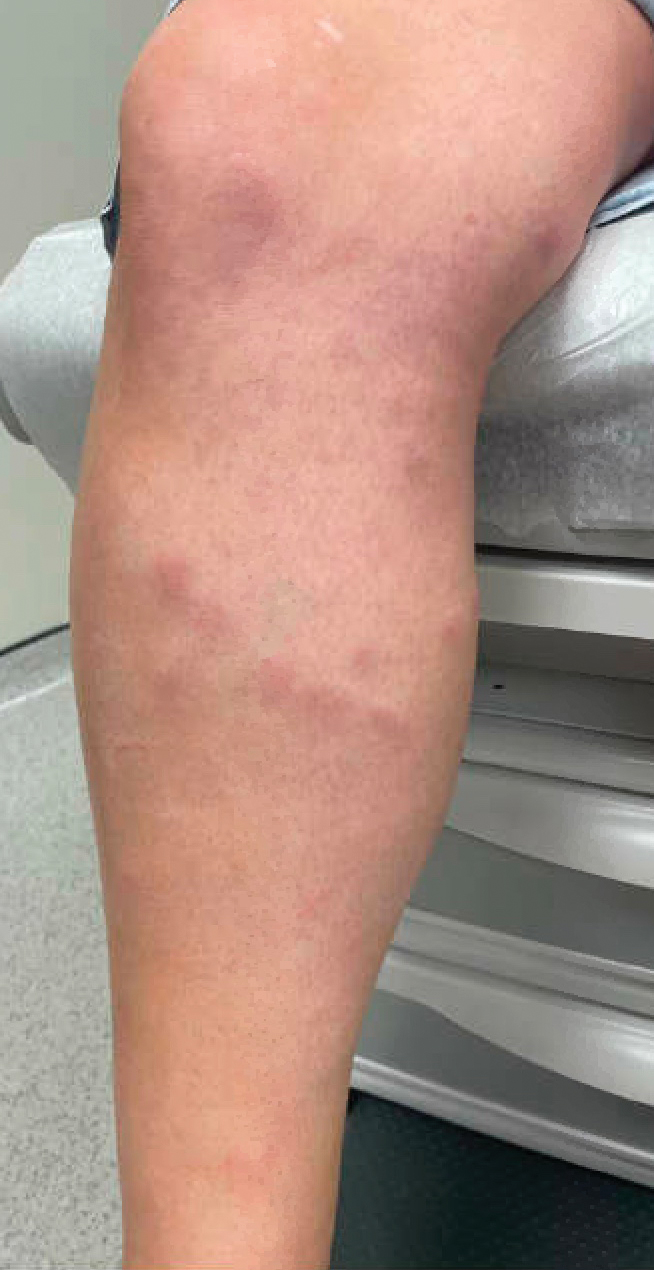
Workup performed by an outside provider over the previous month included 2 venous duplex ultrasounds of the left leg, which showed no signs of deep vein thrombosis. Additionally, the patient underwent lateral and anteroposterior radiographs of the left foot, tibia, and fibula, which showed no evidence of fracture.
Given the morphology and distribution of the lesions (Figure 2), EN was strongly favored as the cause of the symptoms, and a biopsy confirmed the diagnosis. All immunohistochemical stains including auramine-rhodamine for acid-fast bacilli, Grocott-Gomori methenamine silver for fungal organisms, and Brown and Brenn were negative. Given the waxing and waning course of the lesions, which suggested an active neutrophilic rather than purely chronic granulomatous phase of EN, the patient was treated with colchicine 0.6 mg twice daily for 1 month.
Causes of EN and Clinical Manifestations
Erythema nodosum is a common form of septal panniculitis that can be precipitated by inflammatory conditions, infection, or medications (commonly oral contraceptive pills) but often is idiopathic.5 The acute phase is neutrophilic, with evolution over time to a granulomatous phase. Common etiologies include sarcoidosis; inflammatory bowel disease; and bacterial or fungal infections such as Streptococcus (especially common in children), histoplasmosis, and coccidioidomycosis. The patient was otherwise healthy and was not taking any medications that are known triggers of EN. A PubMed search of articles indexed for MEDLINE in the English-language literature using the terms copperhead snake bite, erythema nodosum snake, and copperhead snake erythema nodosum revealed no reports of EN following a bite from a copperhead snake; however, in one case, an adder bite led to erysipelas, likely due to disturbed blood and lymphatic flow, which then triggered EN.6 Additionally, EN has been reported as a delayed reaction to jellyfish stings.7
Clinical features of EN include the development of tender, erythematous, subcutaneous nodules and plaques most frequently over the pretibial region. Lesions typically evolve from raised, deep-seated nodules into flat indurated plaques over a span of weeks. Occasionally, there is a slight prodromal phase marked by nonspecific symptoms such as fever and arthralgia lasting for 3 to 6 days. Erythema nodosum typically results in spontaneous resolution after 4 to 8 weeks, and management involves treatment of any underlying condition with symptomatic care. Interestingly, our patient experienced persistent symptoms over the course of 4 months, with development of new nodular lesions throughout this time period. The most frequently used drugs for the management of symptomatic EN include nonsteroidal anti-inflammatory drugs, colchicine, and potassium iodide.8 A characteristic histologic finding of the granulomatous phase is the Miescher radial granuloma, which is a septal collection of histiocytes surrounding a cleft.9
Snakebite Reactions
Snakebites can result in a wide range of local and systemic manifestations, as snake venom may contain 20 or more toxins.10 Local complications of pit viper bites include pain, swelling, and fang marks; when examining fang marks, the presence of 2 distinct puncture wounds often indicates envenomation with a poisonous snake, whereas nonvenomous snakebites often result in smaller puncture wounds arranged in an arc. Following bites, pain can develop immediately and spread proximally up the affected limb, which occurred in our patient in the days following the bite. Intense local reactions can occur, as bites often result in intense edema of the affected limb spreading to the trunk in the days to weeks after the bite, occasionally accompanied by regional lymphadenopathy. Some bites can result in local necrosis and secondary bacterial infection caused by organisms in the oral cavity of the culprit snake.
Although they were not present in our patient, snakebites can result in a wide range of systemic toxicities ranging from clotting defects and hemolysis to neurotoxicity, myotoxicity, and nephrotoxicity.10 In severe cases, snake venom can result in disseminated intravascular coagulation, sepsis, and cardiorespiratory collapse.
The eastern copperhead (Agkistrodon contortrix) is a species of venomous snake that is endemic to eastern North America. Copperheads are members of the subfamily Crotalinae in the family Viperidae.11 Reported reactions to copperhead bites include cellulitis, necrotizing fasciitis, compartment syndrome, and tissue necrosis of an entire affected extremity.12,13 Our patient displayed no systemic symptoms to suggest envenomation.
Management of Snakebites
Treatment of snakebites varies based on the constellation and severity of symptoms as well as how recently the envenomation occurred. In urgent cases, antivenom may be administered to prevent further toxicity. In cases of progressive compartment syndrome, emergent surgical procedures such as fasciotomy or amputation are required to prevent further complications. When a superimposed bacterial infection is suspected, broad-spectrum antibiotics are required. Because our patient presented 4 months following the initial bite with isolated cutaneous manifestations, she was treated symptomatically with colchicine for EN.1,2
Final Thoughts
Our patient presented with EN following a bite from a copperhead snake. Physicians should be aware of possible etiologies of EN to evaluate patients who present with new-onset tender subcutaneous nodules. Additionally, physicians should be aware of venomous snakes endemic to their region and also understand the various complications that can result following a snakebite, with the potential for lingering cutaneous manifestations weeks to months following the initial bite.
- Warrell DA. Snake bite. Lancet. 2010;375:77-88. doi:10.1016/S0140-6736(09)61754-2
- White J. Overview of venomous snakes of the world. In: Dart RC, eds. Medical Toxicology. 3rd ed. Lippincott, Williams, & Wilkins; 2004:1543
- Spiller HA, Bosse GM. Prospective study of morbidity associated with snakebite envenomation. J Toxicol Clin Toxicol. 2003;41:125-130. doi:10.1081/clt-120019127
- Scharman EJ, Noffsinger VD. Copperhead snakebites: clinical severity of local effects. Ann Emerg Med. 2001;38:55-61. doi:10.1067/mem.2001.116148
- Hafsi W, Badri T. Erythema nodosum. In: StatPearls. StatPearls Publishing; November 28, 2022. Accessed July 22, 2024. https://www.ncbi.nlm.nih.gov/books/NBK470369/
- Nowowiejska J, Baran A, Flisiak I. Rare coexistence of unilateral erythema nodosum with erysipelas in the area of previous adder bite. Przegl Epidemiol. 2020;74:355-361. doi:10.32394/pe.74.28
- Auerbach PS, Hays JT. Erythema nodosum following a jellyfish sting. J Emerg Med. 1987;5:487-491. doi:10.1016/0736-4679(87)90211-3
- Gilchrist H, Patterson JW. Erythema nodosum and erythema induratum (nodular vasculitis): diagnosis and management. Dermatol Ther. 2010;23:320-327. doi:10.1111/j.1529-8019.2010.01332.x
- Sánchez Yus E, Sanz Vico MD, de Diego V. Miescher’s radial granuloma. a characteristic marker of erythema nodosum. Am J Dermatopathol. 1989;11:434-442. doi:10.1097/00000372-198910000-00005
- Mehta SR, Sashindran VK. Clinical features and management of snake bite. Med J Armed Forces India. 2002;58:247-249. doi:10.1016/S0377-1237(02)80140-X
- Brys AK, Gandolfi BM, Levinson H, et al. Copperhead envenomation resulting in a rare case of hand compartment syndrome and subsequent fasciotomy. Plast Reconstr Surg Glob Open. 2015;3:E396. doi:10.1097/GOX.0000000000000367
- Clark RF, Selden BS, Furbee B. The incidence of wound infection following crotalid envenomation. J Emerg Med. 1993;11:583-586. doi:10.1016/0736-4679(93)90313-v
- Buchanan JT, Thurman J. Crotalidae envenomation. In: StatPearls. StatPearls Publishing; October 3, 2022. Accessed July 22, 2024. https://www.ncbi.nlm.nih.gov/books/NBK551615/
The clinical manifestations of snakebites vary based on the species of snake, bite location, and amount and strength of the venom injected. Locally acting toxins in snake venom predominantly consist of enzymes, such as phospholipase A2, that cause local tissue destruction and can result in pain, swelling, blistering, ecchymosis, and tissue necrosis at the site of the bite within hours to days after the bite.1 Systemically acting toxins can target a wide variety of tissues and cause severe systemic complications including paralysis, rhabdomyolysis secondary to muscle damage, coagulopathy, sepsis, and cardiorespiratory failure.2
Although pain and swelling following snakebites typically resolve by 1 month after envenomation, copperhead snakes—a type of pit viper—may cause residual symptoms of pain and swelling lasting for a year or more.3 Additional cutaneous manifestations of copperhead snakebites include wound infections at the bite site, such as cellulitis and necrotizing fasciitis. More devastating complications that have been described following snake envenomation include tissue injury of an entire extremity and development of compartment syndrome, which requires urgent fasciotomy to prevent potential loss of the affected limb.4
Physicians should be aware of the potential complications of snakebites to properly manage and counsel their patients. We describe a 42-year-old woman with tender, erythematous, subcutaneous nodules persisting for 4 months following a copperhead snakebite. A biopsy confirmed the diagnosis of snakebite-associated erythema nodosum (EN).
Case Report
A 42-year-old woman presented to our clinic with progressive tender, pruritic, deep-seated, erythematous nodules in multiple locations on the legs after sustaining a bite by a copperhead snake on the left foot 4 months prior. The lesions tended to fluctuate in intensity. In the days following the bite, she initially developed painful red bumps on the left foot just proximal to the bite site with associated pain and swelling extending up to just below the left knee. She reported no other notable symptoms such as fever, arthralgia, fatigue, or gastrointestinal tract symptoms. Physical examination revealed bilateral pitting edema, which was worse in the left leg, along with multiple deep, palpable, tender subcutaneous nodules with erythematous surface change (Figure 1).

Workup performed by an outside provider over the previous month included 2 venous duplex ultrasounds of the left leg, which showed no signs of deep vein thrombosis. Additionally, the patient underwent lateral and anteroposterior radiographs of the left foot, tibia, and fibula, which showed no evidence of fracture.
Given the morphology and distribution of the lesions (Figure 2), EN was strongly favored as the cause of the symptoms, and a biopsy confirmed the diagnosis. All immunohistochemical stains including auramine-rhodamine for acid-fast bacilli, Grocott-Gomori methenamine silver for fungal organisms, and Brown and Brenn were negative. Given the waxing and waning course of the lesions, which suggested an active neutrophilic rather than purely chronic granulomatous phase of EN, the patient was treated with colchicine 0.6 mg twice daily for 1 month.

Causes of EN and Clinical Manifestations
Erythema nodosum is a common form of septal panniculitis that can be precipitated by inflammatory conditions, infection, or medications (commonly oral contraceptive pills) but often is idiopathic.5 The acute phase is neutrophilic, with evolution over time to a granulomatous phase. Common etiologies include sarcoidosis; inflammatory bowel disease; and bacterial or fungal infections such as Streptococcus (especially common in children), histoplasmosis, and coccidioidomycosis. The patient was otherwise healthy and was not taking any medications that are known triggers of EN. A PubMed search of articles indexed for MEDLINE in the English-language literature using the terms copperhead snake bite, erythema nodosum snake, and copperhead snake erythema nodosum revealed no reports of EN following a bite from a copperhead snake; however, in one case, an adder bite led to erysipelas, likely due to disturbed blood and lymphatic flow, which then triggered EN.6 Additionally, EN has been reported as a delayed reaction to jellyfish stings.7
Clinical features of EN include the development of tender, erythematous, subcutaneous nodules and plaques most frequently over the pretibial region. Lesions typically evolve from raised, deep-seated nodules into flat indurated plaques over a span of weeks. Occasionally, there is a slight prodromal phase marked by nonspecific symptoms such as fever and arthralgia lasting for 3 to 6 days. Erythema nodosum typically results in spontaneous resolution after 4 to 8 weeks, and management involves treatment of any underlying condition with symptomatic care. Interestingly, our patient experienced persistent symptoms over the course of 4 months, with development of new nodular lesions throughout this time period. The most frequently used drugs for the management of symptomatic EN include nonsteroidal anti-inflammatory drugs, colchicine, and potassium iodide.8 A characteristic histologic finding of the granulomatous phase is the Miescher radial granuloma, which is a septal collection of histiocytes surrounding a cleft.9
Snakebite Reactions
Snakebites can result in a wide range of local and systemic manifestations, as snake venom may contain 20 or more toxins.10 Local complications of pit viper bites include pain, swelling, and fang marks; when examining fang marks, the presence of 2 distinct puncture wounds often indicates envenomation with a poisonous snake, whereas nonvenomous snakebites often result in smaller puncture wounds arranged in an arc. Following bites, pain can develop immediately and spread proximally up the affected limb, which occurred in our patient in the days following the bite. Intense local reactions can occur, as bites often result in intense edema of the affected limb spreading to the trunk in the days to weeks after the bite, occasionally accompanied by regional lymphadenopathy. Some bites can result in local necrosis and secondary bacterial infection caused by organisms in the oral cavity of the culprit snake.
Although they were not present in our patient, snakebites can result in a wide range of systemic toxicities ranging from clotting defects and hemolysis to neurotoxicity, myotoxicity, and nephrotoxicity.10 In severe cases, snake venom can result in disseminated intravascular coagulation, sepsis, and cardiorespiratory collapse.
The eastern copperhead (Agkistrodon contortrix) is a species of venomous snake that is endemic to eastern North America. Copperheads are members of the subfamily Crotalinae in the family Viperidae.11 Reported reactions to copperhead bites include cellulitis, necrotizing fasciitis, compartment syndrome, and tissue necrosis of an entire affected extremity.12,13 Our patient displayed no systemic symptoms to suggest envenomation.
Management of Snakebites
Treatment of snakebites varies based on the constellation and severity of symptoms as well as how recently the envenomation occurred. In urgent cases, antivenom may be administered to prevent further toxicity. In cases of progressive compartment syndrome, emergent surgical procedures such as fasciotomy or amputation are required to prevent further complications. When a superimposed bacterial infection is suspected, broad-spectrum antibiotics are required. Because our patient presented 4 months following the initial bite with isolated cutaneous manifestations, she was treated symptomatically with colchicine for EN.1,2
Final Thoughts
Our patient presented with EN following a bite from a copperhead snake. Physicians should be aware of possible etiologies of EN to evaluate patients who present with new-onset tender subcutaneous nodules. Additionally, physicians should be aware of venomous snakes endemic to their region and also understand the various complications that can result following a snakebite, with the potential for lingering cutaneous manifestations weeks to months following the initial bite.
The clinical manifestations of snakebites vary based on the species of snake, bite location, and amount and strength of the venom injected. Locally acting toxins in snake venom predominantly consist of enzymes, such as phospholipase A2, that cause local tissue destruction and can result in pain, swelling, blistering, ecchymosis, and tissue necrosis at the site of the bite within hours to days after the bite.1 Systemically acting toxins can target a wide variety of tissues and cause severe systemic complications including paralysis, rhabdomyolysis secondary to muscle damage, coagulopathy, sepsis, and cardiorespiratory failure.2
Although pain and swelling following snakebites typically resolve by 1 month after envenomation, copperhead snakes—a type of pit viper—may cause residual symptoms of pain and swelling lasting for a year or more.3 Additional cutaneous manifestations of copperhead snakebites include wound infections at the bite site, such as cellulitis and necrotizing fasciitis. More devastating complications that have been described following snake envenomation include tissue injury of an entire extremity and development of compartment syndrome, which requires urgent fasciotomy to prevent potential loss of the affected limb.4
Physicians should be aware of the potential complications of snakebites to properly manage and counsel their patients. We describe a 42-year-old woman with tender, erythematous, subcutaneous nodules persisting for 4 months following a copperhead snakebite. A biopsy confirmed the diagnosis of snakebite-associated erythema nodosum (EN).
Case Report
A 42-year-old woman presented to our clinic with progressive tender, pruritic, deep-seated, erythematous nodules in multiple locations on the legs after sustaining a bite by a copperhead snake on the left foot 4 months prior. The lesions tended to fluctuate in intensity. In the days following the bite, she initially developed painful red bumps on the left foot just proximal to the bite site with associated pain and swelling extending up to just below the left knee. She reported no other notable symptoms such as fever, arthralgia, fatigue, or gastrointestinal tract symptoms. Physical examination revealed bilateral pitting edema, which was worse in the left leg, along with multiple deep, palpable, tender subcutaneous nodules with erythematous surface change (Figure 1).

Workup performed by an outside provider over the previous month included 2 venous duplex ultrasounds of the left leg, which showed no signs of deep vein thrombosis. Additionally, the patient underwent lateral and anteroposterior radiographs of the left foot, tibia, and fibula, which showed no evidence of fracture.
Given the morphology and distribution of the lesions (Figure 2), EN was strongly favored as the cause of the symptoms, and a biopsy confirmed the diagnosis. All immunohistochemical stains including auramine-rhodamine for acid-fast bacilli, Grocott-Gomori methenamine silver for fungal organisms, and Brown and Brenn were negative. Given the waxing and waning course of the lesions, which suggested an active neutrophilic rather than purely chronic granulomatous phase of EN, the patient was treated with colchicine 0.6 mg twice daily for 1 month.

Causes of EN and Clinical Manifestations
Erythema nodosum is a common form of septal panniculitis that can be precipitated by inflammatory conditions, infection, or medications (commonly oral contraceptive pills) but often is idiopathic.5 The acute phase is neutrophilic, with evolution over time to a granulomatous phase. Common etiologies include sarcoidosis; inflammatory bowel disease; and bacterial or fungal infections such as Streptococcus (especially common in children), histoplasmosis, and coccidioidomycosis. The patient was otherwise healthy and was not taking any medications that are known triggers of EN. A PubMed search of articles indexed for MEDLINE in the English-language literature using the terms copperhead snake bite, erythema nodosum snake, and copperhead snake erythema nodosum revealed no reports of EN following a bite from a copperhead snake; however, in one case, an adder bite led to erysipelas, likely due to disturbed blood and lymphatic flow, which then triggered EN.6 Additionally, EN has been reported as a delayed reaction to jellyfish stings.7
Clinical features of EN include the development of tender, erythematous, subcutaneous nodules and plaques most frequently over the pretibial region. Lesions typically evolve from raised, deep-seated nodules into flat indurated plaques over a span of weeks. Occasionally, there is a slight prodromal phase marked by nonspecific symptoms such as fever and arthralgia lasting for 3 to 6 days. Erythema nodosum typically results in spontaneous resolution after 4 to 8 weeks, and management involves treatment of any underlying condition with symptomatic care. Interestingly, our patient experienced persistent symptoms over the course of 4 months, with development of new nodular lesions throughout this time period. The most frequently used drugs for the management of symptomatic EN include nonsteroidal anti-inflammatory drugs, colchicine, and potassium iodide.8 A characteristic histologic finding of the granulomatous phase is the Miescher radial granuloma, which is a septal collection of histiocytes surrounding a cleft.9
Snakebite Reactions
Snakebites can result in a wide range of local and systemic manifestations, as snake venom may contain 20 or more toxins.10 Local complications of pit viper bites include pain, swelling, and fang marks; when examining fang marks, the presence of 2 distinct puncture wounds often indicates envenomation with a poisonous snake, whereas nonvenomous snakebites often result in smaller puncture wounds arranged in an arc. Following bites, pain can develop immediately and spread proximally up the affected limb, which occurred in our patient in the days following the bite. Intense local reactions can occur, as bites often result in intense edema of the affected limb spreading to the trunk in the days to weeks after the bite, occasionally accompanied by regional lymphadenopathy. Some bites can result in local necrosis and secondary bacterial infection caused by organisms in the oral cavity of the culprit snake.
Although they were not present in our patient, snakebites can result in a wide range of systemic toxicities ranging from clotting defects and hemolysis to neurotoxicity, myotoxicity, and nephrotoxicity.10 In severe cases, snake venom can result in disseminated intravascular coagulation, sepsis, and cardiorespiratory collapse.
The eastern copperhead (Agkistrodon contortrix) is a species of venomous snake that is endemic to eastern North America. Copperheads are members of the subfamily Crotalinae in the family Viperidae.11 Reported reactions to copperhead bites include cellulitis, necrotizing fasciitis, compartment syndrome, and tissue necrosis of an entire affected extremity.12,13 Our patient displayed no systemic symptoms to suggest envenomation.
Management of Snakebites
Treatment of snakebites varies based on the constellation and severity of symptoms as well as how recently the envenomation occurred. In urgent cases, antivenom may be administered to prevent further toxicity. In cases of progressive compartment syndrome, emergent surgical procedures such as fasciotomy or amputation are required to prevent further complications. When a superimposed bacterial infection is suspected, broad-spectrum antibiotics are required. Because our patient presented 4 months following the initial bite with isolated cutaneous manifestations, she was treated symptomatically with colchicine for EN.1,2
Final Thoughts
Our patient presented with EN following a bite from a copperhead snake. Physicians should be aware of possible etiologies of EN to evaluate patients who present with new-onset tender subcutaneous nodules. Additionally, physicians should be aware of venomous snakes endemic to their region and also understand the various complications that can result following a snakebite, with the potential for lingering cutaneous manifestations weeks to months following the initial bite.
- Warrell DA. Snake bite. Lancet. 2010;375:77-88. doi:10.1016/S0140-6736(09)61754-2
- White J. Overview of venomous snakes of the world. In: Dart RC, eds. Medical Toxicology. 3rd ed. Lippincott, Williams, & Wilkins; 2004:1543
- Spiller HA, Bosse GM. Prospective study of morbidity associated with snakebite envenomation. J Toxicol Clin Toxicol. 2003;41:125-130. doi:10.1081/clt-120019127
- Scharman EJ, Noffsinger VD. Copperhead snakebites: clinical severity of local effects. Ann Emerg Med. 2001;38:55-61. doi:10.1067/mem.2001.116148
- Hafsi W, Badri T. Erythema nodosum. In: StatPearls. StatPearls Publishing; November 28, 2022. Accessed July 22, 2024. https://www.ncbi.nlm.nih.gov/books/NBK470369/
- Nowowiejska J, Baran A, Flisiak I. Rare coexistence of unilateral erythema nodosum with erysipelas in the area of previous adder bite. Przegl Epidemiol. 2020;74:355-361. doi:10.32394/pe.74.28
- Auerbach PS, Hays JT. Erythema nodosum following a jellyfish sting. J Emerg Med. 1987;5:487-491. doi:10.1016/0736-4679(87)90211-3
- Gilchrist H, Patterson JW. Erythema nodosum and erythema induratum (nodular vasculitis): diagnosis and management. Dermatol Ther. 2010;23:320-327. doi:10.1111/j.1529-8019.2010.01332.x
- Sánchez Yus E, Sanz Vico MD, de Diego V. Miescher’s radial granuloma. a characteristic marker of erythema nodosum. Am J Dermatopathol. 1989;11:434-442. doi:10.1097/00000372-198910000-00005
- Mehta SR, Sashindran VK. Clinical features and management of snake bite. Med J Armed Forces India. 2002;58:247-249. doi:10.1016/S0377-1237(02)80140-X
- Brys AK, Gandolfi BM, Levinson H, et al. Copperhead envenomation resulting in a rare case of hand compartment syndrome and subsequent fasciotomy. Plast Reconstr Surg Glob Open. 2015;3:E396. doi:10.1097/GOX.0000000000000367
- Clark RF, Selden BS, Furbee B. The incidence of wound infection following crotalid envenomation. J Emerg Med. 1993;11:583-586. doi:10.1016/0736-4679(93)90313-v
- Buchanan JT, Thurman J. Crotalidae envenomation. In: StatPearls. StatPearls Publishing; October 3, 2022. Accessed July 22, 2024. https://www.ncbi.nlm.nih.gov/books/NBK551615/
- Warrell DA. Snake bite. Lancet. 2010;375:77-88. doi:10.1016/S0140-6736(09)61754-2
- White J. Overview of venomous snakes of the world. In: Dart RC, eds. Medical Toxicology. 3rd ed. Lippincott, Williams, & Wilkins; 2004:1543
- Spiller HA, Bosse GM. Prospective study of morbidity associated with snakebite envenomation. J Toxicol Clin Toxicol. 2003;41:125-130. doi:10.1081/clt-120019127
- Scharman EJ, Noffsinger VD. Copperhead snakebites: clinical severity of local effects. Ann Emerg Med. 2001;38:55-61. doi:10.1067/mem.2001.116148
- Hafsi W, Badri T. Erythema nodosum. In: StatPearls. StatPearls Publishing; November 28, 2022. Accessed July 22, 2024. https://www.ncbi.nlm.nih.gov/books/NBK470369/
- Nowowiejska J, Baran A, Flisiak I. Rare coexistence of unilateral erythema nodosum with erysipelas in the area of previous adder bite. Przegl Epidemiol. 2020;74:355-361. doi:10.32394/pe.74.28
- Auerbach PS, Hays JT. Erythema nodosum following a jellyfish sting. J Emerg Med. 1987;5:487-491. doi:10.1016/0736-4679(87)90211-3
- Gilchrist H, Patterson JW. Erythema nodosum and erythema induratum (nodular vasculitis): diagnosis and management. Dermatol Ther. 2010;23:320-327. doi:10.1111/j.1529-8019.2010.01332.x
- Sánchez Yus E, Sanz Vico MD, de Diego V. Miescher’s radial granuloma. a characteristic marker of erythema nodosum. Am J Dermatopathol. 1989;11:434-442. doi:10.1097/00000372-198910000-00005
- Mehta SR, Sashindran VK. Clinical features and management of snake bite. Med J Armed Forces India. 2002;58:247-249. doi:10.1016/S0377-1237(02)80140-X
- Brys AK, Gandolfi BM, Levinson H, et al. Copperhead envenomation resulting in a rare case of hand compartment syndrome and subsequent fasciotomy. Plast Reconstr Surg Glob Open. 2015;3:E396. doi:10.1097/GOX.0000000000000367
- Clark RF, Selden BS, Furbee B. The incidence of wound infection following crotalid envenomation. J Emerg Med. 1993;11:583-586. doi:10.1016/0736-4679(93)90313-v
- Buchanan JT, Thurman J. Crotalidae envenomation. In: StatPearls. StatPearls Publishing; October 3, 2022. Accessed July 22, 2024. https://www.ncbi.nlm.nih.gov/books/NBK551615/
Practice Points
- Erythema nodosum (EN) can occur following snakebites from pit vipers such as the eastern copperhead.
- The acute phase of EN is neutrophilic and responds to colchicine. The chronic phase of EN is granulomatous and responds best to rest and elevation as well as nonsteroidal anti-inflammatory drugs and iodides.
Distinguishing Generalized Bullous Fixed Drug Eruption From SJS/TEN: A Retrospective Study on Clinical and Demographic Features
To the Editor:
Generalized bullous fixed drug eruption (GBFDE) is a rare subtype of fixed drug eruption (FDE) that manifests as widespread blisters and erosions following exposure to a causative drug.1 Diagnostic criteria include involvement of at least 3 to 6 anatomic sites—head and neck, anterior trunk, posterior trunk, upper extremities, lower extremities, or genitalia—and more than 10% of the body surface area. It can be challenging to differentiate GBFDE from severe drug rashes such as Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) due to extensive body surface area involvement of blisters and erosions. Specific features distinguishing GBFDE from SJS/TEN include primary lesions consisting of larger erythematous to dusky, circular plaques that progress to bullae and coalesce into widespread erosions; history of FDE; lack of severe mucosal involvement; and better overall prognosis.2 Treatment typically involves discontinuation of the culprit medication and supportive care; evidence for systemic therapies is not well established.
Our study aimed to characterize the clinical and demographic features of GBFDE in our institution to highlight potential key differences between this diagnosis and SJS/TEN. An electronic medical record search was performed to identify patients who were clinically diagnosed with GBFDE at New York-Presbyterian/Weill Cornell Medical Center (New York, New York) in both outpatient and inpatient settings from January 2015 to December 2022. This retrospective study was approved by the Weill Cornell Medicine institutional review board (#22-05024777).
Ten patients were identified and included in the analysis (eTable). The mean age of the patients was 56 years (range, 39–76 years). Seven (70%) patients had skin of color (non-White) and 6 (60%) were female. The mean body mass index was 35 (range, 20–57), and 7 (70%) patients were clinically obese (body mass index >30). Only 2 (20%) patients had a history of a documented drug eruption (hives and erythema multiforme), and no patients had a history of FDE. Erythematous dusky patches followed by rapid development of blisters were noted within 3 days of drug initiation in 40% (4/10) and within 5 days in 80% (8/10) of patients. Antibiotics were identified as likely inciting agents in 8 (80%) patients. Biopsies were obtained in 3 (30%) patients and all 3 demonstrated cytotoxic CD8+ interface dermatitis with marked epithelial necrosis, neutrophilia, eosinophilia, and melanophage accumulation. Fever was present at initial presentation in only 4 (40%) patients, and only 1 (10%) patient had oral mucosal involvement. All 10 patients had intertriginous involvement (axillae, 90% [9/10]; gluteal cleft, 80% [8/10]; groin, 80% [8/10]; inframammary folds, 20% [2/10]), and there was considerable flank involvement in 9 (90%) patients. All 10 patients had initial erythematous to dusky, circular patches on the trunk and proximal extremities that then denuded most dramatically in the intertriginous areas (Figure). Six (60%) patients received systemic therapy, including 5 patients treated with a single dose of etanercept 50 mg. In patients with continued progression, 1 or 2 additional doses of etanercept 50 mg were administered at 48- to 72-hour intervals until blistering halted. Treatment with etanercept resulted in clinical improvement in all 5 patients, and there were no identifiable adverse events. The mean hospital stay was 19.7 days (range, 1–63 days).
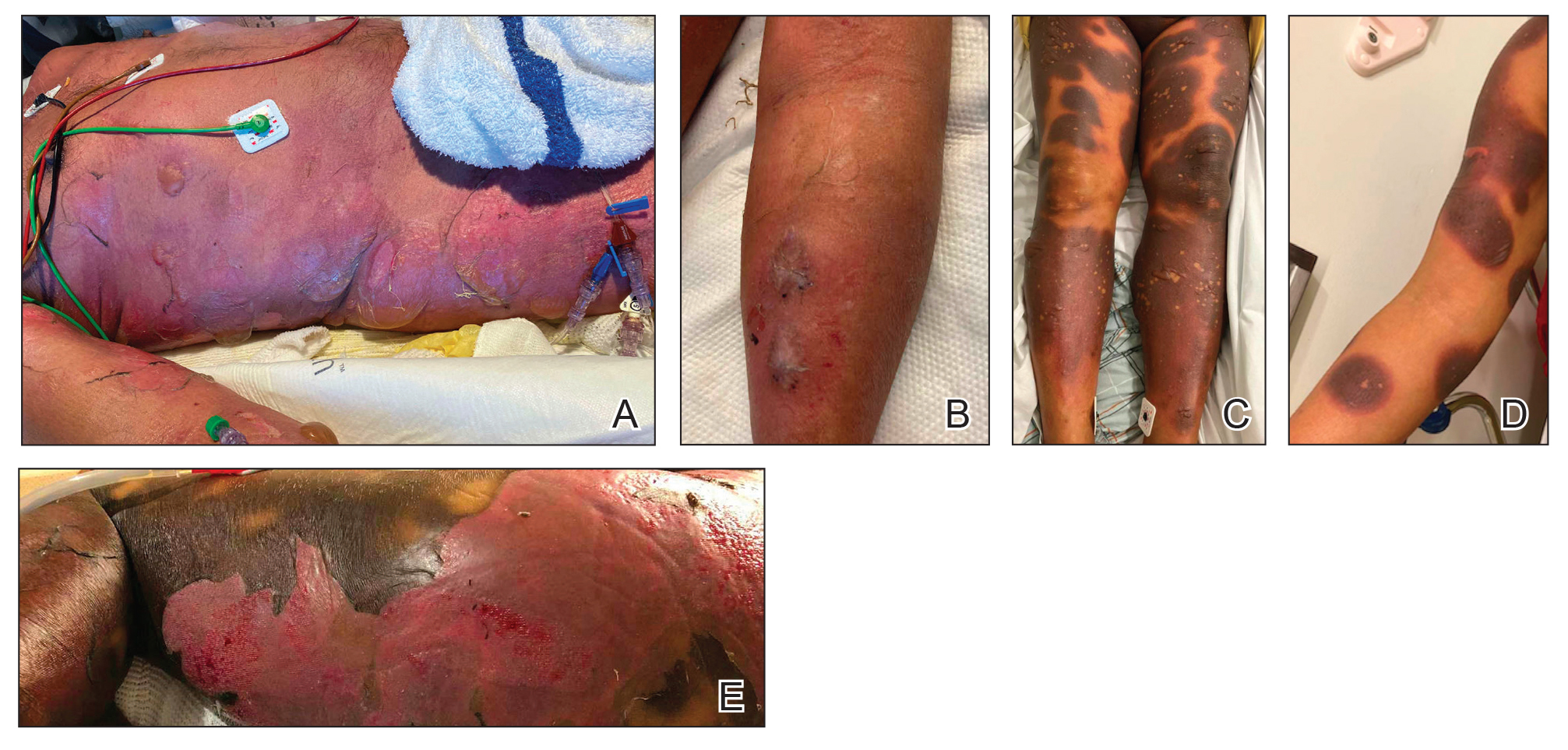
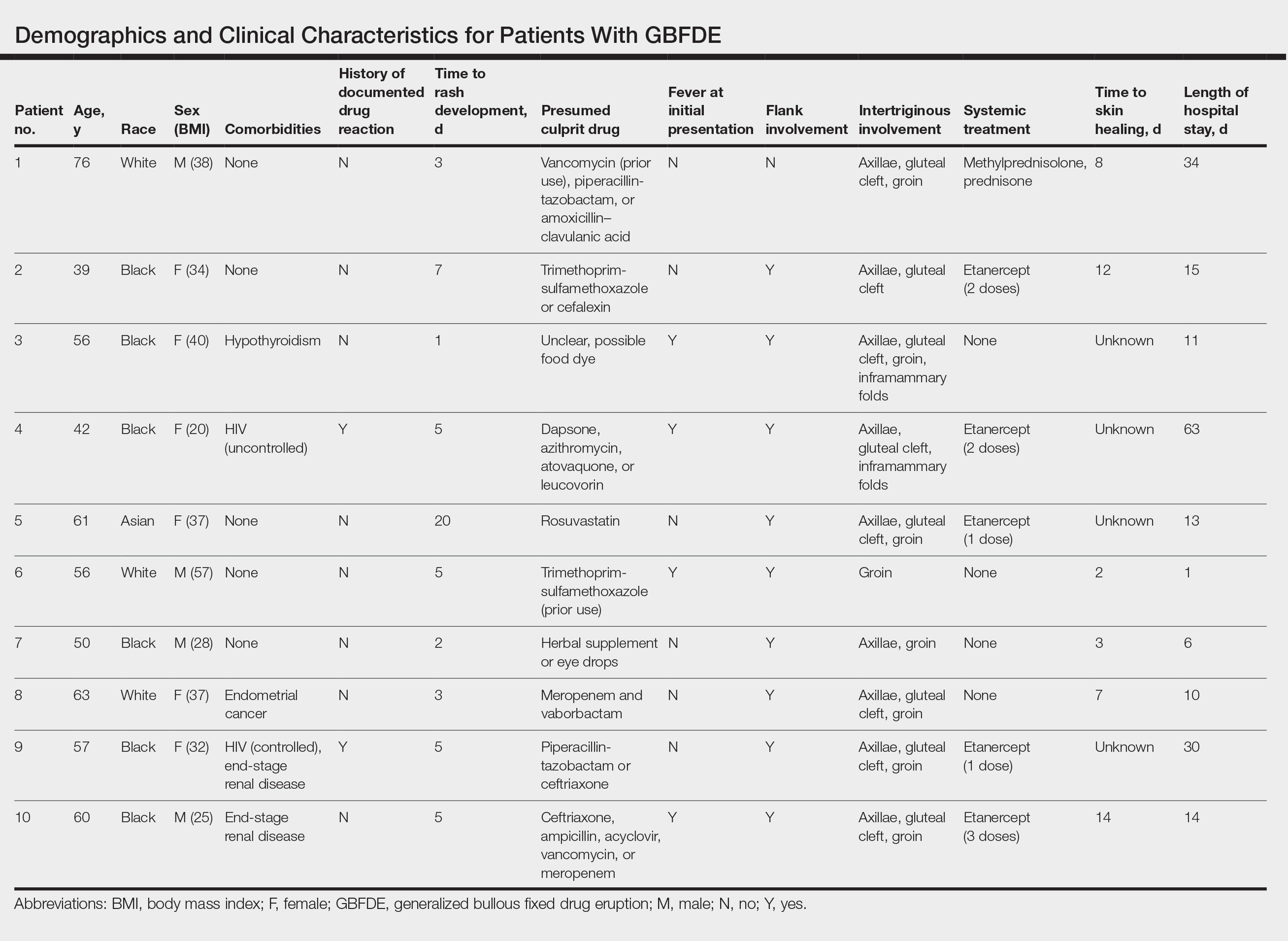
This study highlights notable demographic and clinical features of GBFDE that have not been widely described in the literature. Large erythematous and dusky patches with broad zones of blistering with particular localization to the neck, intertriginous areas, and flanks typically are not described in SJS/TEN and may be helpful in distinguishing these conditions from GBFDE. Mild or complete lack of mucosal and facial involvement as well as more rapid time from drug initiation to rash (as rapid as 1 day) were key factors that aided in distinguishing GBFDE from SJS/TEN in our patients. Although a history of FDE is considered a key characteristic in the diagnosis of GBFDE, none of our patients had a known history of FDE, suggesting GBFDE may be the initial manifestation of FDE in some patients. Histopathology showed similar findings consistent with FDE in the 3 patients in whom a biopsy was performed. The remaining patients were diagnosed clinically based on the presence of distinctive, perfectly circular, dusky plaques present at the periphery of larger denuded areas, which are characteristic of GBFDE. Lower levels of serum granulysin3 have been shown to help distinguish GBFDE from SJS/TEN, but this test is not readily available with time-sensitive results at most institutions, and exact diagnostic ranges for GBFDE vs SJS/TEN are not yet known.
Our study was limited by a small number of patients at a single institution. Another limitation was the retrospective design.
Interestingly, a high proportion of our patients were non-White and clinically obese, which are factors that should be considered for future research. Sixty percent (6/10) of the patients in our study were Black, which is a notable difference from our hospital’s general admission demographics with Black individuals constituting 12% of patients.4 Our study also highlighted the utility of etanercept, which has reported mortality benefits and decreased time to re-epithelialization in other severe blistering cutaneous drug reactions including SJS/TEN,5 as a potential therapeutic option in GBFDE.
It is imperative that clinicians recognize the differences between GBFDE and SJS/TEN, as correct diagnosis is crucial for identifying the most likely causative drug as well as providing accurate prognostic information and may have future therapeutic implications as we further understand the immunologic profiles of these severe blistering drug reactions.
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399. doi:10.1007/s40257-020-00505-3
- Anderson HJ, Lee JB. A review of fixed drug eruption with a special focus on generalized bullous fixed drug eruption. Medicina (Kaunas). 2021;57:925. doi:10.3390/medicina57090925
- Cho YT, Lin JW, Chen YC, et al. Generalized bullous fixed drug eruption is distinct from Stevens-Johnson syndrome/toxic epidermal necrolysis by immunohistopathological features. J Am Acad Dermatol. 2014;70:539-548. doi:10.1016/j.jaad.2013.11.015
- Tran T, Shapiro A. New York-Presbyterian 2022 Health Equity Report. New York-Presbyterian; 2023. Accessed July 22, 2024. https://nyp.widen.net/s/jqfbrvrf9p/dalio-center-2022-health-equity-report
- Dreyer SD, Torres J, Stoddard M, et al. Efficacy of etanercept in the treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis. Cutis. 2021;107:E22-E28. doi:10.12788/cutis.0288
To the Editor:
Generalized bullous fixed drug eruption (GBFDE) is a rare subtype of fixed drug eruption (FDE) that manifests as widespread blisters and erosions following exposure to a causative drug.1 Diagnostic criteria include involvement of at least 3 to 6 anatomic sites—head and neck, anterior trunk, posterior trunk, upper extremities, lower extremities, or genitalia—and more than 10% of the body surface area. It can be challenging to differentiate GBFDE from severe drug rashes such as Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) due to extensive body surface area involvement of blisters and erosions. Specific features distinguishing GBFDE from SJS/TEN include primary lesions consisting of larger erythematous to dusky, circular plaques that progress to bullae and coalesce into widespread erosions; history of FDE; lack of severe mucosal involvement; and better overall prognosis.2 Treatment typically involves discontinuation of the culprit medication and supportive care; evidence for systemic therapies is not well established.
Our study aimed to characterize the clinical and demographic features of GBFDE in our institution to highlight potential key differences between this diagnosis and SJS/TEN. An electronic medical record search was performed to identify patients who were clinically diagnosed with GBFDE at New York-Presbyterian/Weill Cornell Medical Center (New York, New York) in both outpatient and inpatient settings from January 2015 to December 2022. This retrospective study was approved by the Weill Cornell Medicine institutional review board (#22-05024777).
Ten patients were identified and included in the analysis (eTable). The mean age of the patients was 56 years (range, 39–76 years). Seven (70%) patients had skin of color (non-White) and 6 (60%) were female. The mean body mass index was 35 (range, 20–57), and 7 (70%) patients were clinically obese (body mass index >30). Only 2 (20%) patients had a history of a documented drug eruption (hives and erythema multiforme), and no patients had a history of FDE. Erythematous dusky patches followed by rapid development of blisters were noted within 3 days of drug initiation in 40% (4/10) and within 5 days in 80% (8/10) of patients. Antibiotics were identified as likely inciting agents in 8 (80%) patients. Biopsies were obtained in 3 (30%) patients and all 3 demonstrated cytotoxic CD8+ interface dermatitis with marked epithelial necrosis, neutrophilia, eosinophilia, and melanophage accumulation. Fever was present at initial presentation in only 4 (40%) patients, and only 1 (10%) patient had oral mucosal involvement. All 10 patients had intertriginous involvement (axillae, 90% [9/10]; gluteal cleft, 80% [8/10]; groin, 80% [8/10]; inframammary folds, 20% [2/10]), and there was considerable flank involvement in 9 (90%) patients. All 10 patients had initial erythematous to dusky, circular patches on the trunk and proximal extremities that then denuded most dramatically in the intertriginous areas (Figure). Six (60%) patients received systemic therapy, including 5 patients treated with a single dose of etanercept 50 mg. In patients with continued progression, 1 or 2 additional doses of etanercept 50 mg were administered at 48- to 72-hour intervals until blistering halted. Treatment with etanercept resulted in clinical improvement in all 5 patients, and there were no identifiable adverse events. The mean hospital stay was 19.7 days (range, 1–63 days).


This study highlights notable demographic and clinical features of GBFDE that have not been widely described in the literature. Large erythematous and dusky patches with broad zones of blistering with particular localization to the neck, intertriginous areas, and flanks typically are not described in SJS/TEN and may be helpful in distinguishing these conditions from GBFDE. Mild or complete lack of mucosal and facial involvement as well as more rapid time from drug initiation to rash (as rapid as 1 day) were key factors that aided in distinguishing GBFDE from SJS/TEN in our patients. Although a history of FDE is considered a key characteristic in the diagnosis of GBFDE, none of our patients had a known history of FDE, suggesting GBFDE may be the initial manifestation of FDE in some patients. Histopathology showed similar findings consistent with FDE in the 3 patients in whom a biopsy was performed. The remaining patients were diagnosed clinically based on the presence of distinctive, perfectly circular, dusky plaques present at the periphery of larger denuded areas, which are characteristic of GBFDE. Lower levels of serum granulysin3 have been shown to help distinguish GBFDE from SJS/TEN, but this test is not readily available with time-sensitive results at most institutions, and exact diagnostic ranges for GBFDE vs SJS/TEN are not yet known.
Our study was limited by a small number of patients at a single institution. Another limitation was the retrospective design.
Interestingly, a high proportion of our patients were non-White and clinically obese, which are factors that should be considered for future research. Sixty percent (6/10) of the patients in our study were Black, which is a notable difference from our hospital’s general admission demographics with Black individuals constituting 12% of patients.4 Our study also highlighted the utility of etanercept, which has reported mortality benefits and decreased time to re-epithelialization in other severe blistering cutaneous drug reactions including SJS/TEN,5 as a potential therapeutic option in GBFDE.
It is imperative that clinicians recognize the differences between GBFDE and SJS/TEN, as correct diagnosis is crucial for identifying the most likely causative drug as well as providing accurate prognostic information and may have future therapeutic implications as we further understand the immunologic profiles of these severe blistering drug reactions.
To the Editor:
Generalized bullous fixed drug eruption (GBFDE) is a rare subtype of fixed drug eruption (FDE) that manifests as widespread blisters and erosions following exposure to a causative drug.1 Diagnostic criteria include involvement of at least 3 to 6 anatomic sites—head and neck, anterior trunk, posterior trunk, upper extremities, lower extremities, or genitalia—and more than 10% of the body surface area. It can be challenging to differentiate GBFDE from severe drug rashes such as Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) due to extensive body surface area involvement of blisters and erosions. Specific features distinguishing GBFDE from SJS/TEN include primary lesions consisting of larger erythematous to dusky, circular plaques that progress to bullae and coalesce into widespread erosions; history of FDE; lack of severe mucosal involvement; and better overall prognosis.2 Treatment typically involves discontinuation of the culprit medication and supportive care; evidence for systemic therapies is not well established.
Our study aimed to characterize the clinical and demographic features of GBFDE in our institution to highlight potential key differences between this diagnosis and SJS/TEN. An electronic medical record search was performed to identify patients who were clinically diagnosed with GBFDE at New York-Presbyterian/Weill Cornell Medical Center (New York, New York) in both outpatient and inpatient settings from January 2015 to December 2022. This retrospective study was approved by the Weill Cornell Medicine institutional review board (#22-05024777).
Ten patients were identified and included in the analysis (eTable). The mean age of the patients was 56 years (range, 39–76 years). Seven (70%) patients had skin of color (non-White) and 6 (60%) were female. The mean body mass index was 35 (range, 20–57), and 7 (70%) patients were clinically obese (body mass index >30). Only 2 (20%) patients had a history of a documented drug eruption (hives and erythema multiforme), and no patients had a history of FDE. Erythematous dusky patches followed by rapid development of blisters were noted within 3 days of drug initiation in 40% (4/10) and within 5 days in 80% (8/10) of patients. Antibiotics were identified as likely inciting agents in 8 (80%) patients. Biopsies were obtained in 3 (30%) patients and all 3 demonstrated cytotoxic CD8+ interface dermatitis with marked epithelial necrosis, neutrophilia, eosinophilia, and melanophage accumulation. Fever was present at initial presentation in only 4 (40%) patients, and only 1 (10%) patient had oral mucosal involvement. All 10 patients had intertriginous involvement (axillae, 90% [9/10]; gluteal cleft, 80% [8/10]; groin, 80% [8/10]; inframammary folds, 20% [2/10]), and there was considerable flank involvement in 9 (90%) patients. All 10 patients had initial erythematous to dusky, circular patches on the trunk and proximal extremities that then denuded most dramatically in the intertriginous areas (Figure). Six (60%) patients received systemic therapy, including 5 patients treated with a single dose of etanercept 50 mg. In patients with continued progression, 1 or 2 additional doses of etanercept 50 mg were administered at 48- to 72-hour intervals until blistering halted. Treatment with etanercept resulted in clinical improvement in all 5 patients, and there were no identifiable adverse events. The mean hospital stay was 19.7 days (range, 1–63 days).


This study highlights notable demographic and clinical features of GBFDE that have not been widely described in the literature. Large erythematous and dusky patches with broad zones of blistering with particular localization to the neck, intertriginous areas, and flanks typically are not described in SJS/TEN and may be helpful in distinguishing these conditions from GBFDE. Mild or complete lack of mucosal and facial involvement as well as more rapid time from drug initiation to rash (as rapid as 1 day) were key factors that aided in distinguishing GBFDE from SJS/TEN in our patients. Although a history of FDE is considered a key characteristic in the diagnosis of GBFDE, none of our patients had a known history of FDE, suggesting GBFDE may be the initial manifestation of FDE in some patients. Histopathology showed similar findings consistent with FDE in the 3 patients in whom a biopsy was performed. The remaining patients were diagnosed clinically based on the presence of distinctive, perfectly circular, dusky plaques present at the periphery of larger denuded areas, which are characteristic of GBFDE. Lower levels of serum granulysin3 have been shown to help distinguish GBFDE from SJS/TEN, but this test is not readily available with time-sensitive results at most institutions, and exact diagnostic ranges for GBFDE vs SJS/TEN are not yet known.
Our study was limited by a small number of patients at a single institution. Another limitation was the retrospective design.
Interestingly, a high proportion of our patients were non-White and clinically obese, which are factors that should be considered for future research. Sixty percent (6/10) of the patients in our study were Black, which is a notable difference from our hospital’s general admission demographics with Black individuals constituting 12% of patients.4 Our study also highlighted the utility of etanercept, which has reported mortality benefits and decreased time to re-epithelialization in other severe blistering cutaneous drug reactions including SJS/TEN,5 as a potential therapeutic option in GBFDE.
It is imperative that clinicians recognize the differences between GBFDE and SJS/TEN, as correct diagnosis is crucial for identifying the most likely causative drug as well as providing accurate prognostic information and may have future therapeutic implications as we further understand the immunologic profiles of these severe blistering drug reactions.
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399. doi:10.1007/s40257-020-00505-3
- Anderson HJ, Lee JB. A review of fixed drug eruption with a special focus on generalized bullous fixed drug eruption. Medicina (Kaunas). 2021;57:925. doi:10.3390/medicina57090925
- Cho YT, Lin JW, Chen YC, et al. Generalized bullous fixed drug eruption is distinct from Stevens-Johnson syndrome/toxic epidermal necrolysis by immunohistopathological features. J Am Acad Dermatol. 2014;70:539-548. doi:10.1016/j.jaad.2013.11.015
- Tran T, Shapiro A. New York-Presbyterian 2022 Health Equity Report. New York-Presbyterian; 2023. Accessed July 22, 2024. https://nyp.widen.net/s/jqfbrvrf9p/dalio-center-2022-health-equity-report
- Dreyer SD, Torres J, Stoddard M, et al. Efficacy of etanercept in the treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis. Cutis. 2021;107:E22-E28. doi:10.12788/cutis.0288
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399. doi:10.1007/s40257-020-00505-3
- Anderson HJ, Lee JB. A review of fixed drug eruption with a special focus on generalized bullous fixed drug eruption. Medicina (Kaunas). 2021;57:925. doi:10.3390/medicina57090925
- Cho YT, Lin JW, Chen YC, et al. Generalized bullous fixed drug eruption is distinct from Stevens-Johnson syndrome/toxic epidermal necrolysis by immunohistopathological features. J Am Acad Dermatol. 2014;70:539-548. doi:10.1016/j.jaad.2013.11.015
- Tran T, Shapiro A. New York-Presbyterian 2022 Health Equity Report. New York-Presbyterian; 2023. Accessed July 22, 2024. https://nyp.widen.net/s/jqfbrvrf9p/dalio-center-2022-health-equity-report
- Dreyer SD, Torres J, Stoddard M, et al. Efficacy of etanercept in the treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis. Cutis. 2021;107:E22-E28. doi:10.12788/cutis.0288
PRACTICE POINTS
- Distinguishing features of generalized bullous fixed
drug eruption (GBFDE) may include truncal and proximal predilection with early intertriginous blistering. - Etanercept is a viable treatment option for GBFDE.
Comment on “Erythrodermic Pityriasis Rubra Pilaris Following COVID-19 Vaccination”
To the Editor:
We read with interest the case report from Abdelkader et al1 (Cutis. 2024;113:E22-E24) of a 32-year-old man who received the Sinopharm BBIBP COVID-19 vaccine (BBIBP-CorV) and experienced acute-onset erythroderma and severe itching. The patient did not disclose any recent medication intake and had no noteworthy medical history. Physical examination revealed palmoplantar keratoderma, keratotic follicular papules on the legs and feet, and typical orange-red erythroderma. The laboratory workup was normal, including a negative test result for HIV infection.
The absence of details regarding the patient’s history of allergic reactions or sensitivities is one possible shortcoming in this case report and may have given important information about the possible reason for the erythroderma that occurred following vaccination. Furthermore, more research into the precise Sinopharm BBIBP vaccine ingredients that may have caused the skin reaction would have been helpful in deciphering the underlying mechanisms.
Larger-scale studies examining the frequency of cutaneous reactions following COVID-19 vaccination with various vaccine formulations may be the focus of future research efforts and could assist in determining the risk factors for experiencing such reactions, which would enable health care providers to offer advice on vaccination alternatives or preventative measures for those who are more vulnerable. Furthermore, collaboration among dermatologists and allergists could improve patient outcomes and improve management.
By highlighting an uncommon but noteworthy dermatologic manifestation following COVID-19 immunization, this case report emphasizes how crucial it is to keep an eye out for and report any possible side effects linked to vaccinations to protect patient safety. Subsequent investigations should concentrate on enhancing comprehension of the pathophysiology of cutaneous reactions following immunization and devising tactics to alleviate these hazards. Working together, researchers and health care professionals can effectively tackle the issues raised by these newly discovered vaccine-related skin responses.
1. Abdelkader HA, Khedr H, El-Komy MH. Erythrodermic pityriasis rubra pilaris following COVID-19 vaccination. Cutis. 2024;113:E22-E24. doi:10.12788/cutis.1010
To the Editor:
We read with interest the case report from Abdelkader et al1 (Cutis. 2024;113:E22-E24) of a 32-year-old man who received the Sinopharm BBIBP COVID-19 vaccine (BBIBP-CorV) and experienced acute-onset erythroderma and severe itching. The patient did not disclose any recent medication intake and had no noteworthy medical history. Physical examination revealed palmoplantar keratoderma, keratotic follicular papules on the legs and feet, and typical orange-red erythroderma. The laboratory workup was normal, including a negative test result for HIV infection.
The absence of details regarding the patient’s history of allergic reactions or sensitivities is one possible shortcoming in this case report and may have given important information about the possible reason for the erythroderma that occurred following vaccination. Furthermore, more research into the precise Sinopharm BBIBP vaccine ingredients that may have caused the skin reaction would have been helpful in deciphering the underlying mechanisms.
Larger-scale studies examining the frequency of cutaneous reactions following COVID-19 vaccination with various vaccine formulations may be the focus of future research efforts and could assist in determining the risk factors for experiencing such reactions, which would enable health care providers to offer advice on vaccination alternatives or preventative measures for those who are more vulnerable. Furthermore, collaboration among dermatologists and allergists could improve patient outcomes and improve management.
By highlighting an uncommon but noteworthy dermatologic manifestation following COVID-19 immunization, this case report emphasizes how crucial it is to keep an eye out for and report any possible side effects linked to vaccinations to protect patient safety. Subsequent investigations should concentrate on enhancing comprehension of the pathophysiology of cutaneous reactions following immunization and devising tactics to alleviate these hazards. Working together, researchers and health care professionals can effectively tackle the issues raised by these newly discovered vaccine-related skin responses.
To the Editor:
We read with interest the case report from Abdelkader et al1 (Cutis. 2024;113:E22-E24) of a 32-year-old man who received the Sinopharm BBIBP COVID-19 vaccine (BBIBP-CorV) and experienced acute-onset erythroderma and severe itching. The patient did not disclose any recent medication intake and had no noteworthy medical history. Physical examination revealed palmoplantar keratoderma, keratotic follicular papules on the legs and feet, and typical orange-red erythroderma. The laboratory workup was normal, including a negative test result for HIV infection.
The absence of details regarding the patient’s history of allergic reactions or sensitivities is one possible shortcoming in this case report and may have given important information about the possible reason for the erythroderma that occurred following vaccination. Furthermore, more research into the precise Sinopharm BBIBP vaccine ingredients that may have caused the skin reaction would have been helpful in deciphering the underlying mechanisms.
Larger-scale studies examining the frequency of cutaneous reactions following COVID-19 vaccination with various vaccine formulations may be the focus of future research efforts and could assist in determining the risk factors for experiencing such reactions, which would enable health care providers to offer advice on vaccination alternatives or preventative measures for those who are more vulnerable. Furthermore, collaboration among dermatologists and allergists could improve patient outcomes and improve management.
By highlighting an uncommon but noteworthy dermatologic manifestation following COVID-19 immunization, this case report emphasizes how crucial it is to keep an eye out for and report any possible side effects linked to vaccinations to protect patient safety. Subsequent investigations should concentrate on enhancing comprehension of the pathophysiology of cutaneous reactions following immunization and devising tactics to alleviate these hazards. Working together, researchers and health care professionals can effectively tackle the issues raised by these newly discovered vaccine-related skin responses.
1. Abdelkader HA, Khedr H, El-Komy MH. Erythrodermic pityriasis rubra pilaris following COVID-19 vaccination. Cutis. 2024;113:E22-E24. doi:10.12788/cutis.1010
1. Abdelkader HA, Khedr H, El-Komy MH. Erythrodermic pityriasis rubra pilaris following COVID-19 vaccination. Cutis. 2024;113:E22-E24. doi:10.12788/cutis.1010
A Whiff of Trouble: Navigating Allergic Contact Dermatitis to Fragrance
Fragrances are complex organic compounds that are sufficiently volatile to produce an odor—most often a pleasant one—or at times intended to neutralize unpleasant odors. They can be further divided into natural fragrances (eg, essential oils) and synthetic ones. Fragrances are found in abundance in our daily lives: in perfumes; colognes; lotions; shampoos; and an array of other personal, household, and even industrial products (Table). These exposures include products directly applied to the skin, rinsed off, or aerosolized. A single product often contains a multitude of different fragrances to create the scents we know and love. To many, fragrances can be an important part of everyday life or even a part of one’s identity. But that once-intoxicating aroma can transform into an itchy skin nightmare; fragrances are among the most common contact allergens.

Given the widespread prevalence of fragrances in so many products, understanding fragrance allergy and skillful avoidance is imperative. In this review, we explore important aspects of fragrance allergic contact dermatitis (ACD), including chemistry, epidemiology, patch test considerations, and management strategies for patients, with the goal of providing valuable clinical insights for treating physicians on how patients can embrace a fragrance-free lifestyle.
How Fragrances Act as Allergens
A plethora of chemicals emit odors, of which more than 2000 are used to create the fragranced products we see on our shelves today.1 For many of these fragrances, contact allergy develops because the fragrance acts as a hapten (ie, a small molecule that combines with a carrier protein to elicit an immune response).2 Some fragrance molecules require “activation” to be able to bind to proteins; these are known as prehaptens.3 For example, the natural fragrance linalool is generally considered nonallergenic in its initial form. However, once it is exposed to air, it may undergo oxidation to become linalool hydroperoxides, a well-established contact allergen. Some fragrances can become allergenic in the skin itself, often secondary to enzymatic reactions—these are known as prohaptens.3 However, most fragrances are directly reactive to skin proteins on the basis of chemical reactions such as Michael addition and Schiff base formation.4 In either case, the end result is that fragrance allergens, including essential oils, may cause skin sensitization and subsequent ACD.5,6
Epidemiology
Contact allergy to fragrances is not uncommon; in a multicenter cross-sectional study conducted in 5 European countries, the prevalence in the general population was estimated to be as high as 2.6% and 1.9% among 3119 patients patch tested to fragrance mix I (FMI) and fragrance mix II (FMII), respectively.7 Studies in patients referred for patch testing have shown a higher 5% to 25% prevalence of fragrance allergy, largely depending on what population was evaluated.1 Factors such as sociocultural differences in frequency and types of fragrances used could contribute to this variation.
During patch testing, the primary fragrance screening allergens are FMI, FMII, and balsam of Peru (BOP)(Myroxylon pereirae resin).7 In recent years, hydroperoxides of linalool and limonene also have emerged as potentially important fragrance allergens.8 The frequencies of patch-test positivity of these allergens can be quite high in referral-based populations. In a study performed by the North American Contact Dermatitis Group (NACDG) from 2019 to 2020, frequencies of fragrance allergen positivity were 12.8% for FMI, 5.2% for FMII, 7.4% for BOP, 11.1% for hydroperoxides of linalool, and 3.5% for hydroperoxides of limonene.8 Additionally, it was noted that FMI and hydroperoxides of linalool were among the top 10 most frequently positive allergens.9 It should be kept in mind that NACDG studies are drawn from a referral population and not representative of the general population.
Allergic contact dermatitis to fragrances can manifest anywhere on the body, but certain patterns are characteristic. A study by the NACDG analyzed fragrance and botanical patch test results in 24,246 patients and found that fragrance/botanical-sensitive patients more commonly had dermatitis involving the face (odds ratio [OR], 1.12; 95% CI, 1.03-1.21), legs (OR, 1.22; 95% CI, 1.06-1.41), and anal/genital areas (OR, 1.26; 95% CI, 1.04-1.52) and were less likely to have hand dermatitis (OR, 0.88; 95% CI, 0.82-0.95) compared with non–fragrance/botanical-sensitive patients.10 However, other studies have found that hand dermatitis is common among fragrance-allergic individuals.11-13
Fragrance allergy tends to be more common in women than men, which likely is attributable to differences in product use and exposure.10 The prevalence of fragrance allergy increases with age in both men and women, peaking at approximately 50 years of age, likely due to repeat exposure or age-related changes to the skin barrier or immune system.14
Occupational fragrance exposures are important to consider, and fragrance ACD is associated with hairdressers, beauticians, office workers exposed to aromatherapy diffusers, and food handlers.15 Less-obvious professions that involve exposure to fragrances used to cover up unwanted odors—such as working with industrial and cleaning chemicals or even metalworking—also have been reported to be associated with ACD.16
Patch Test Considerations
Patch testing is essential to confirm fragrance allergy and guide treatment, but because there are so many potential fragrance allergens, there is no perfect patch test strategy. In a standard patch test series, the most important screening allergens are considered to be FMI, FMII, and BOP; tested together, they are thought to detect a large proportion of cases of fragrance allergy. Strikingly, in a large European study (N=1951), patch testing with the fragrance markers in the baseline panel failed to detect more than 40% of cases of allergy compared to testing with 26 individual fragrance allergens.17 Other studies have reported that a smaller proportion of fragrance allergies are missed by using baseline screening allergens alone.18,19 Limonene and linalool hydroperoxides also are potentially important fragrance allergens to consider adding to the patch test panel, as unoxidized limonene and linalool commonly are used in many products and could theoretically undergo auto-oxidation under use conditions.8 However, because of the high number of irritant, questionable, and potentially false-positive reactions, the Information Network of Departments of Dermatology has recommended against adding these hydroperoxides to a standard screening tray for patch testing.20 It must be remembered that a positive patch test to a fragrance does not necessarily represent ACD unless the patient has a clinically relevant exposure to the allergen.21
In patients who test negative to the baseline fragrance-screening allergens and in whom a high degree of suspicion remains, further testing with supplemental fragrance allergens (commercially available from patch test suppliers) is warranted.17 The thin-layer rapid use epicutaneous (T.R.U.E.) test (SmartPractice) includes FMI and BOP but not FMII or linalool or limonene hydroperoxides. More comprehensive patch test panels are available that include additional fragrances, such as the North American 80 Comprehensive Series and the American Contact Dermatitis Society Core Allergen Series.22-24 It is important to remain vigilant and consider expanded patch testing if patients initially test negative but suspicion remains.
Furthermore, patch testing with the patient’s own products is an important consideration. Uter et al25 evaluated patch testing using patients’ perfumes, deodorants, and shaving lotions, and approximately 41% (53/129) of patients who tested positive to their own product tested negative for fragrance-screening allergens. Although it can be difficult to ascertain which exact component of a commercial product is the culprit, a positive patch test may still provide clinically relevant information for patients and treating physicians. In cases of questionable or weak-positive results, repeat testing or repeated open application tests can help re-evaluate suspected products.
Cross-reactivity should be considered when patch testing for fragrances. Atwater et al10 found that cross-reactivity between FMI, FMII, and BOP was common; for instance, approximately 40% of patients testing positive to FMII or BOP also had positive reactions to FMI (522/1182 and 768/1942, respectively). Understanding this concept is important because in some cases (as detailed below) patients will need to avoid all fragrances, not just the ones to which they have previously been exposed, given the limitations on fragrance labeling in the United States. However, this may change with the Modernization of Cosmetic Regulation Act of 2022.26
Avoiding Fragrances: Improving Patient Education and Outcomes
Once a relevant contact allergy to fragrance is established after patch testing, successful avoidance is critical but challenging, as there are numerous potential pitfalls. Missing just 1 hidden source of fragrance exposure will often be the difference between success or failure. Dermatologists play a crucial role in guiding patients through the intricate process of identifying and avoiding potential allergens.
Optimal Safety: Embracing a Fragrance-Free Lifestyle
For fragrance-allergic patients, it generally is safest to completely avoid fragrance.
First, if a patient only shows positive patch-test reactions to fragrance screening mixes (and not to the particular fragrances in these mixes), there is no way to be certain which fragrances the patient needs to avoid.
Second, even if specific fragrance allergens are identified, numerous chemically related fragrances to which the patient may be allergic are not commercially available for patch testing. One review provided evidence of 162 fragrance allergens that have been documented to cause contact allergy.1 Dermatologists generally patch test to screening mixtures and/or the 26 fragrance chemicals required on labels in European products (European Directive fragrance).27 Therefore, there are more than 100 known fragrance allergens that are not routinely tested to which patients could be allergic.
Third, certain fragrances, such as limonene and linalool, are found in many products with fragrance, and it is difficult to find products without these substances. Limonene and linalool themselves are not potent allergens; however, upon air exposure, they may auto-oxidize to hydroperoxides of limonene and linalool, which are increasingly common positive patch tests.19
Additionally, patients should be advised that many products labeled “fragrance free,” “unscented,” or “free and clear” are not truly fragrance free, and patients should not choose products based on these claims. There are no legal definitions for these claims in the United States, and industries are allowed to choose the definition they prefer. Numerous products labeled “unscented” use this term to indicate that the product had an odor, the company used a masking fragrance to hide the odor, and then the product can be considered unscented. In many holistic stores, most products labeled “fragrance free” are only free of artificial fragrances but contain essential oils. Of the 162 documented fragrance allergens, 80 are essential oils.6 Essential oils are perceived to be safe by the vast majority of the population because they are viewed as “natural” and “unprocessed” sources of fragrance.28 However, numerous allergenic terpenes have been discovered in essential oils, including functionalized variations of alcohols (eg, geraniol, bisabolol) and aldehydes (eg, citronellal).6 Essential oils also consist of nonterpenic compounds produced through the phenylpropanoids pathway, including eugenol and cinnamaldehyde. One review showed that most essential oils contain one or more European Directive fragrance.29 Therefore, many products labeled “unscented,” “fragrance free,” or “natural” are not free of fragrance and may be unsafe for fragrance-allergic patients.
Although not required, manufacturers sometimes voluntarily list one or more of the 162 currently identified fragrance allergens on product labels. Also, there are more than 50 potentially allergenic essential oils that can be listed on labels by their common names or by genus or species. In addition, there are synonyms for fragrance, such as aroma, parfum, perfume, and scent. Therefore, there are several hundred different ingredient names on labels that indicate the presence of fragrance, and patients are very unlikely to successfully identify fragrance-free products by trying to read product labels on their own.
Lastly, in the United States product labels only require products to state that they contain “fragrance” and do not mandate the listing of specific fragrances. If a patient is allergic to a specific fragrance, there is no way to determine if that fragrance is present in these products. This will change with the enactment of Modernization of Cosmetics Regulation Act of 2022, which empowers the US Food and Drug Administration to require manufacturers to disclose many, but not all, fragrance allergens on the labels of cosmetic and topical products.26
For all these reasons, patients should be advised to use a medical database to choose safe alternative products instead of trying to read labels themselves to avoid fragrance. The American Contact Dermatitis Society’s Contact Allergen Management Program (CAMP) database (https://www.contactderm.org/resources/acds-camp) is designed to identify safe alternative products for patients with contact allergies. When CAMP is programmed to avoid “fragrance,” it will list only “safe” products free of all fragrances found in a comprehensive fragrance cross-reactor group.30 This customizable database is available as an application that can be downloaded onto a patient’s mobile device. Fragrance-allergic patients should be encouraged to use the CAMP application or other similar applications (eg, SkinSAFE)(https://www.skinsafeproducts.com/) to find all the products they use.
Potential Pitfalls in Fragrance Avoidance
Most physicians, even dermatologists, will not know which products on the market are fragrance free from a contact allergy standpoint. Patients should instruct their physicians to use the allergen-avoidance application of choice whenever recommending new topical products, whether prescription or nonprescription. In 2009, Nardelli and colleagues31 found that 10% of topical pharmaceutical products contained a total of 66 different fragrance substances.
Individuals who are allergic to fragrance also can react to fragrances used by close contacts (ie, consort dermatitis).32 Therefore, fragrance-allergic individuals who do not improve after changing their personal products should consider urging their spouses or significant others to choose their personal care products using an allergen-avoidance application. Also, physical contact with pets can cause reactions, and the use of a fragrance-free pet shampoo is recommended. Additionally, allergic individuals who are providing care for small children should select fragrance-free products for them.
Some of the most heavily fragranced products on the market are found at hair salons. One exposure to an allergen often can keep patients broken out for up to 4 weeks and occasionally longer, a typical frequency for salon visits—even if the individual is taking great care to avoid fragrance at home. Patients should be instructed to bring their own shampoo, conditioner, and styling products to the salon. These patients also should bring safe moisturizer and nail polish remover for manicures. Additionally, aromatherapy used in most massages can cause flare-ups, and it is recommended that allergic patients purchase fragrance-free massage oil to bring to their sessions.
Fragranced soaps and cleansers can leave a residue on the palmar surface of the hands and fingers. This residue may not meet the threshold for causing a reaction on the thick skin of these surfaces, but it is sufficient to passively transfer fragrance to other more sensitive areas, such as the eyelids. Passive transfer of fragrance can be a major source of allergen exposure and should not be overlooked. Allergic patients should be instructed to bring safe hand cleansers to friends’ houses, restaurants, or work.
Airborne fragrances in a patient’s environment can reach sufficient concentration to cause airborne contact dermatitis. In one case report, an Uber driver developed facial airborne ACD from a fragrance diffuser in his vehicle and his condition improved upon removing the diffuser.33 Therefore, patients should be instructed to avoid fragranced diffusers, scented candles, room deodorizers, incense, and wax melts.
Fragrance in household products also can be an issue. Fragrance-allergic patients should be instructed to choose fragrance-free cleaning products and to avoid fragranced wipes on surfaces that may be touched. In addition, they should be instructed to use fragrance-free laundry products. It is not required for household products in the United States to list their ingredients, and the majority do not have complete ingredient lists. Therefore, it is imperative that the patient use an allergen-avoidance application that identifies products that have full ingredient disclosure and are free of fragrance.
For individuals who enjoy perfume and/or cologne, it may be possible for them to resume use of these products in some cases after their condition has fully cleared with complete fragrance avoidance. They should avoid spraying products into the air or applying them directly onto the skin and should instead dip a cotton swab into the perfume/cologne and dab a small amount onto their clothing. This technique can sometimes satisfy the patient and improve compliance.
If a patient who is allergic to fragrance does not clear after 6 weeks of complete fragrance avoidance, it is worth considering systemic contact dermatitis due to ingestion of fragrance-related substances in foods.34 A large number of fragrance materials also are food flavorings. For patients allergic to a specific fragrance(s), systemic avoidance needs to be specific to the allergen, and the Flavor and Extract Manufacturers Association’s flavor ingredient library is most helpful (https://www.femaflavor.org/flavor-library). If the patient is allergic to the complex mixture BOP, a balsam-free diet can be attempted.35,36
Final Thoughts
Dermatologists must equip themselves with the knowledge to educate fragrance-allergic patients on proper avoidance. The multifaceted nature of fragrance avoidance requires a personalized approach, combining label scrutiny, utilization of a safe-product application, and tailored recommendations for specific situations. By guiding patients through these complexities, dermatologists can empower patients to manage their fragrance allergy and enhance their quality of life.
- de Groot AC. Fragrances: contact allergy and other adverse effects. Dermatitis. 2020;31:13-35.
- Uter W. Contact allergy to fragrances: current clinical and regulatory trends. Allergol Select. 2017;1:190-199.
- Karlberg AT, Börje A, Duus Johansen J, et al. Activation of non-sensitizing or low-sensitizing fragrance substances into potent sensitizers - prehaptens and prohaptens. Contact Dermatitis. 2013;69:323-334.
- Patlewicz GY, Wright ZM, Basketter DA, et al. Structure-activity relationships for selected fragrance allergens. Contact Dermatitis. 2002;47:219-226. doi:10.1034/j.1600-0536.2002.470406
- Ward JM, Reeder M, Atwater AR. Essential oils debunked: separating fact from myth. Cutis. 2020;105:174-176.
- de Groot AC, Schmidt E. Essential oils, part IV: contact allergy. Dermatitis. 2016;27:170-175.
- Diepgen TL, Ofenloch R, Bruze M, et al. Prevalence of fragrance contact allergy in the general population of five European countries: a cross-sectional study. Br J Dermatol. 2015;173:1411-1419
- Ogueta IA, Brared Christensson J, Giménez-Arnau E, et al. Limonene and linalool hydroperoxides review: pros and cons for routine patch testing. Contact Dermatitis. 2022;87:1-12.
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group Patch Test Results: 2019-2020. Dermatitis. 2023;34:90-104.
- Atwater AR, Ward JM, Liu B, et al. Fragrance- and botanical-related allergy and associated concomitant reactions: a retrospective analysis of the North American Contact Dermatitis Group Data 2007-2016. Dermatitis. 2021;32:42-52.
- Tai V, Sharifah Rosniza SNC, Tang MM. Contact sensitization to fragrance allergen: a 5-year review in the Department of Dermatology, Hospital Kuala Lumpur. Med J Malaysia. 2023;78:583-588.
- Periyasamy MK, Sekar SC, Rai R. Analysis of hypersensitivity in fragrance series by patch testing. Indian Dermatol Online J. 2019;10:657-662.
- Heydorn S, Menné T, Johansen JD. Fragrance allergy and hand eczema - a review. Contact Dermatitis. 2003;48:59-66.
- Buckley DA, Rycroft RJG, White IR, et al. The frequency of fragrance allergy in patch-tested patients increases with their age. Br J Dermatol. 2003;149:986-989.
- Montgomery RL, Agius R, Wilkinson SM, et al. UK trends of allergic occupational skin disease attributed to fragrances 1996-2015. Contact Dermatitis. 2018;78:33-40.
- Reeder MJ. Allergic contact dermatitis to fragrances. Dermatol Clin. 2020;38:371-377.
- Mann J, McFadden JP, White JML, et al. Baseline series fragrance markers fail to predict contact allergy. Contact Dermatitis. 2014;70:276-281.
- Vejanurug P, Tresukosol P, Sajjachareonpong P, et al. Fragrance allergy could be missed without patch testing with 26 individual fragrance allergens. Contact Dermatitis. 2016;74:230-235.
- Sukakul T, Bruze M, Mowitz M, et al. Simultaneous patch testing with fragrance markers in the baseline series and the ingredients of fragrance mixes: an update from southern Sweden. Contact Dermatitis. 2022;86:514-523.
- Schubert S, Geier J, Brans R, et al; IVDK. Patch testing hydroperoxides of limonene and linalool in consecutive patients-results of the IVDK 2018-2020. Contact Dermatitis. 2023;89:85-94. doi:10.1111/cod.14332
- Storrs FJ. Fragrance. Dermatitis. 2007;18:3-7.
- T.R.U.E. test. SmartPractice website. Accessed July 24, 2024. https://www.smartpractice.com/shop/category?id=581719&m=SPA ACDS
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2020 update. Dermatitis. 2020;31:279-282. https://pubmed.ncbi.nlm.nih.gov/32947457/
- North American 80 Comprehensive Series NAC-80. Chemotechnique MB Diagnostics AB website. Accessed July 24, 2024. https://www.chemotechnique.se/products/national-series/north-american-80-comprehensive-series/
- Uter W, Geier J, Schnuch A, et al. Patch test results with patients’ own perfumes, deodorants and shaving lotions: results of the IVDK 1998-2002. J Eur Acad Dermatol Venereol. 2007;21:374-379.
- Filley AR, Woodruff CM. The Modernization of Cosmetics Regulation Act of 2022: what dermatologists need to know. J Am Acad Dermatol. 2023;89:629-631.
- European Parliament and the Council of the European Union. Directive 2003/15/EC of the European Parliament and of the Council of 27 February 2003 amending Council Directive 76/768/EEC on the approximation of the laws of the Member States relating to cosmetic products (text with EEA relevance). November 3, 2003. Accessed June 7, 2024. https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2003:066:0026:0035:en:PDF
- Sharmeen JB, Mahomoodally FM, Zengin G, et al. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules. 2021;26:666.
- Scheman A, Scheman N, Rakowski EM. European Directive fragrances in natural products. Dermatitis. 2014;25:51-55.
- Scheman A, Hipolito R, Severson D, et al. Contact allergy cross-reactions: retrospective clinical data and review of the literature. Dermatitis. 2017;28:128-140.
- Nardelli A, D’Hooghe E, Drieghe J, et al. Allergic contact dermatitis from fragrance components in specific topical pharmaceutical products in Belgium. Contact Dermatitis. 2009;60:303-313.
- Lee J, Guo S, Dinalo J, et al. Consort allergic contact dermatitis: a systematic review. Dermatitis. 2022;33:181-186.
- Perper M, Cervantes J, Eber AE, et al. Airborne contact dermatitis caused by fragrance diffusers in Uber cars. Contact Dermatitis. 2017;77:116-117.
- Nijhawan RI, Molenda M, Zirwas MJ, et al. Systemic contact dermatitis. Dermatol Clin. 2009;27:355-364.
- Salam TN, Fowler JF. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
- Scheman A, Rakowski EM, Chou V, et al. Balsam of Peru: past and future. Dermatitis. 2013;24:153-160.
Fragrances are complex organic compounds that are sufficiently volatile to produce an odor—most often a pleasant one—or at times intended to neutralize unpleasant odors. They can be further divided into natural fragrances (eg, essential oils) and synthetic ones. Fragrances are found in abundance in our daily lives: in perfumes; colognes; lotions; shampoos; and an array of other personal, household, and even industrial products (Table). These exposures include products directly applied to the skin, rinsed off, or aerosolized. A single product often contains a multitude of different fragrances to create the scents we know and love. To many, fragrances can be an important part of everyday life or even a part of one’s identity. But that once-intoxicating aroma can transform into an itchy skin nightmare; fragrances are among the most common contact allergens.

Given the widespread prevalence of fragrances in so many products, understanding fragrance allergy and skillful avoidance is imperative. In this review, we explore important aspects of fragrance allergic contact dermatitis (ACD), including chemistry, epidemiology, patch test considerations, and management strategies for patients, with the goal of providing valuable clinical insights for treating physicians on how patients can embrace a fragrance-free lifestyle.
How Fragrances Act as Allergens
A plethora of chemicals emit odors, of which more than 2000 are used to create the fragranced products we see on our shelves today.1 For many of these fragrances, contact allergy develops because the fragrance acts as a hapten (ie, a small molecule that combines with a carrier protein to elicit an immune response).2 Some fragrance molecules require “activation” to be able to bind to proteins; these are known as prehaptens.3 For example, the natural fragrance linalool is generally considered nonallergenic in its initial form. However, once it is exposed to air, it may undergo oxidation to become linalool hydroperoxides, a well-established contact allergen. Some fragrances can become allergenic in the skin itself, often secondary to enzymatic reactions—these are known as prohaptens.3 However, most fragrances are directly reactive to skin proteins on the basis of chemical reactions such as Michael addition and Schiff base formation.4 In either case, the end result is that fragrance allergens, including essential oils, may cause skin sensitization and subsequent ACD.5,6
Epidemiology
Contact allergy to fragrances is not uncommon; in a multicenter cross-sectional study conducted in 5 European countries, the prevalence in the general population was estimated to be as high as 2.6% and 1.9% among 3119 patients patch tested to fragrance mix I (FMI) and fragrance mix II (FMII), respectively.7 Studies in patients referred for patch testing have shown a higher 5% to 25% prevalence of fragrance allergy, largely depending on what population was evaluated.1 Factors such as sociocultural differences in frequency and types of fragrances used could contribute to this variation.
During patch testing, the primary fragrance screening allergens are FMI, FMII, and balsam of Peru (BOP)(Myroxylon pereirae resin).7 In recent years, hydroperoxides of linalool and limonene also have emerged as potentially important fragrance allergens.8 The frequencies of patch-test positivity of these allergens can be quite high in referral-based populations. In a study performed by the North American Contact Dermatitis Group (NACDG) from 2019 to 2020, frequencies of fragrance allergen positivity were 12.8% for FMI, 5.2% for FMII, 7.4% for BOP, 11.1% for hydroperoxides of linalool, and 3.5% for hydroperoxides of limonene.8 Additionally, it was noted that FMI and hydroperoxides of linalool were among the top 10 most frequently positive allergens.9 It should be kept in mind that NACDG studies are drawn from a referral population and not representative of the general population.
Allergic contact dermatitis to fragrances can manifest anywhere on the body, but certain patterns are characteristic. A study by the NACDG analyzed fragrance and botanical patch test results in 24,246 patients and found that fragrance/botanical-sensitive patients more commonly had dermatitis involving the face (odds ratio [OR], 1.12; 95% CI, 1.03-1.21), legs (OR, 1.22; 95% CI, 1.06-1.41), and anal/genital areas (OR, 1.26; 95% CI, 1.04-1.52) and were less likely to have hand dermatitis (OR, 0.88; 95% CI, 0.82-0.95) compared with non–fragrance/botanical-sensitive patients.10 However, other studies have found that hand dermatitis is common among fragrance-allergic individuals.11-13
Fragrance allergy tends to be more common in women than men, which likely is attributable to differences in product use and exposure.10 The prevalence of fragrance allergy increases with age in both men and women, peaking at approximately 50 years of age, likely due to repeat exposure or age-related changes to the skin barrier or immune system.14
Occupational fragrance exposures are important to consider, and fragrance ACD is associated with hairdressers, beauticians, office workers exposed to aromatherapy diffusers, and food handlers.15 Less-obvious professions that involve exposure to fragrances used to cover up unwanted odors—such as working with industrial and cleaning chemicals or even metalworking—also have been reported to be associated with ACD.16
Patch Test Considerations
Patch testing is essential to confirm fragrance allergy and guide treatment, but because there are so many potential fragrance allergens, there is no perfect patch test strategy. In a standard patch test series, the most important screening allergens are considered to be FMI, FMII, and BOP; tested together, they are thought to detect a large proportion of cases of fragrance allergy. Strikingly, in a large European study (N=1951), patch testing with the fragrance markers in the baseline panel failed to detect more than 40% of cases of allergy compared to testing with 26 individual fragrance allergens.17 Other studies have reported that a smaller proportion of fragrance allergies are missed by using baseline screening allergens alone.18,19 Limonene and linalool hydroperoxides also are potentially important fragrance allergens to consider adding to the patch test panel, as unoxidized limonene and linalool commonly are used in many products and could theoretically undergo auto-oxidation under use conditions.8 However, because of the high number of irritant, questionable, and potentially false-positive reactions, the Information Network of Departments of Dermatology has recommended against adding these hydroperoxides to a standard screening tray for patch testing.20 It must be remembered that a positive patch test to a fragrance does not necessarily represent ACD unless the patient has a clinically relevant exposure to the allergen.21
In patients who test negative to the baseline fragrance-screening allergens and in whom a high degree of suspicion remains, further testing with supplemental fragrance allergens (commercially available from patch test suppliers) is warranted.17 The thin-layer rapid use epicutaneous (T.R.U.E.) test (SmartPractice) includes FMI and BOP but not FMII or linalool or limonene hydroperoxides. More comprehensive patch test panels are available that include additional fragrances, such as the North American 80 Comprehensive Series and the American Contact Dermatitis Society Core Allergen Series.22-24 It is important to remain vigilant and consider expanded patch testing if patients initially test negative but suspicion remains.
Furthermore, patch testing with the patient’s own products is an important consideration. Uter et al25 evaluated patch testing using patients’ perfumes, deodorants, and shaving lotions, and approximately 41% (53/129) of patients who tested positive to their own product tested negative for fragrance-screening allergens. Although it can be difficult to ascertain which exact component of a commercial product is the culprit, a positive patch test may still provide clinically relevant information for patients and treating physicians. In cases of questionable or weak-positive results, repeat testing or repeated open application tests can help re-evaluate suspected products.
Cross-reactivity should be considered when patch testing for fragrances. Atwater et al10 found that cross-reactivity between FMI, FMII, and BOP was common; for instance, approximately 40% of patients testing positive to FMII or BOP also had positive reactions to FMI (522/1182 and 768/1942, respectively). Understanding this concept is important because in some cases (as detailed below) patients will need to avoid all fragrances, not just the ones to which they have previously been exposed, given the limitations on fragrance labeling in the United States. However, this may change with the Modernization of Cosmetic Regulation Act of 2022.26
Avoiding Fragrances: Improving Patient Education and Outcomes
Once a relevant contact allergy to fragrance is established after patch testing, successful avoidance is critical but challenging, as there are numerous potential pitfalls. Missing just 1 hidden source of fragrance exposure will often be the difference between success or failure. Dermatologists play a crucial role in guiding patients through the intricate process of identifying and avoiding potential allergens.
Optimal Safety: Embracing a Fragrance-Free Lifestyle
For fragrance-allergic patients, it generally is safest to completely avoid fragrance.
First, if a patient only shows positive patch-test reactions to fragrance screening mixes (and not to the particular fragrances in these mixes), there is no way to be certain which fragrances the patient needs to avoid.
Second, even if specific fragrance allergens are identified, numerous chemically related fragrances to which the patient may be allergic are not commercially available for patch testing. One review provided evidence of 162 fragrance allergens that have been documented to cause contact allergy.1 Dermatologists generally patch test to screening mixtures and/or the 26 fragrance chemicals required on labels in European products (European Directive fragrance).27 Therefore, there are more than 100 known fragrance allergens that are not routinely tested to which patients could be allergic.
Third, certain fragrances, such as limonene and linalool, are found in many products with fragrance, and it is difficult to find products without these substances. Limonene and linalool themselves are not potent allergens; however, upon air exposure, they may auto-oxidize to hydroperoxides of limonene and linalool, which are increasingly common positive patch tests.19
Additionally, patients should be advised that many products labeled “fragrance free,” “unscented,” or “free and clear” are not truly fragrance free, and patients should not choose products based on these claims. There are no legal definitions for these claims in the United States, and industries are allowed to choose the definition they prefer. Numerous products labeled “unscented” use this term to indicate that the product had an odor, the company used a masking fragrance to hide the odor, and then the product can be considered unscented. In many holistic stores, most products labeled “fragrance free” are only free of artificial fragrances but contain essential oils. Of the 162 documented fragrance allergens, 80 are essential oils.6 Essential oils are perceived to be safe by the vast majority of the population because they are viewed as “natural” and “unprocessed” sources of fragrance.28 However, numerous allergenic terpenes have been discovered in essential oils, including functionalized variations of alcohols (eg, geraniol, bisabolol) and aldehydes (eg, citronellal).6 Essential oils also consist of nonterpenic compounds produced through the phenylpropanoids pathway, including eugenol and cinnamaldehyde. One review showed that most essential oils contain one or more European Directive fragrance.29 Therefore, many products labeled “unscented,” “fragrance free,” or “natural” are not free of fragrance and may be unsafe for fragrance-allergic patients.
Although not required, manufacturers sometimes voluntarily list one or more of the 162 currently identified fragrance allergens on product labels. Also, there are more than 50 potentially allergenic essential oils that can be listed on labels by their common names or by genus or species. In addition, there are synonyms for fragrance, such as aroma, parfum, perfume, and scent. Therefore, there are several hundred different ingredient names on labels that indicate the presence of fragrance, and patients are very unlikely to successfully identify fragrance-free products by trying to read product labels on their own.
Lastly, in the United States product labels only require products to state that they contain “fragrance” and do not mandate the listing of specific fragrances. If a patient is allergic to a specific fragrance, there is no way to determine if that fragrance is present in these products. This will change with the enactment of Modernization of Cosmetics Regulation Act of 2022, which empowers the US Food and Drug Administration to require manufacturers to disclose many, but not all, fragrance allergens on the labels of cosmetic and topical products.26
For all these reasons, patients should be advised to use a medical database to choose safe alternative products instead of trying to read labels themselves to avoid fragrance. The American Contact Dermatitis Society’s Contact Allergen Management Program (CAMP) database (https://www.contactderm.org/resources/acds-camp) is designed to identify safe alternative products for patients with contact allergies. When CAMP is programmed to avoid “fragrance,” it will list only “safe” products free of all fragrances found in a comprehensive fragrance cross-reactor group.30 This customizable database is available as an application that can be downloaded onto a patient’s mobile device. Fragrance-allergic patients should be encouraged to use the CAMP application or other similar applications (eg, SkinSAFE)(https://www.skinsafeproducts.com/) to find all the products they use.
Potential Pitfalls in Fragrance Avoidance
Most physicians, even dermatologists, will not know which products on the market are fragrance free from a contact allergy standpoint. Patients should instruct their physicians to use the allergen-avoidance application of choice whenever recommending new topical products, whether prescription or nonprescription. In 2009, Nardelli and colleagues31 found that 10% of topical pharmaceutical products contained a total of 66 different fragrance substances.
Individuals who are allergic to fragrance also can react to fragrances used by close contacts (ie, consort dermatitis).32 Therefore, fragrance-allergic individuals who do not improve after changing their personal products should consider urging their spouses or significant others to choose their personal care products using an allergen-avoidance application. Also, physical contact with pets can cause reactions, and the use of a fragrance-free pet shampoo is recommended. Additionally, allergic individuals who are providing care for small children should select fragrance-free products for them.
Some of the most heavily fragranced products on the market are found at hair salons. One exposure to an allergen often can keep patients broken out for up to 4 weeks and occasionally longer, a typical frequency for salon visits—even if the individual is taking great care to avoid fragrance at home. Patients should be instructed to bring their own shampoo, conditioner, and styling products to the salon. These patients also should bring safe moisturizer and nail polish remover for manicures. Additionally, aromatherapy used in most massages can cause flare-ups, and it is recommended that allergic patients purchase fragrance-free massage oil to bring to their sessions.
Fragranced soaps and cleansers can leave a residue on the palmar surface of the hands and fingers. This residue may not meet the threshold for causing a reaction on the thick skin of these surfaces, but it is sufficient to passively transfer fragrance to other more sensitive areas, such as the eyelids. Passive transfer of fragrance can be a major source of allergen exposure and should not be overlooked. Allergic patients should be instructed to bring safe hand cleansers to friends’ houses, restaurants, or work.
Airborne fragrances in a patient’s environment can reach sufficient concentration to cause airborne contact dermatitis. In one case report, an Uber driver developed facial airborne ACD from a fragrance diffuser in his vehicle and his condition improved upon removing the diffuser.33 Therefore, patients should be instructed to avoid fragranced diffusers, scented candles, room deodorizers, incense, and wax melts.
Fragrance in household products also can be an issue. Fragrance-allergic patients should be instructed to choose fragrance-free cleaning products and to avoid fragranced wipes on surfaces that may be touched. In addition, they should be instructed to use fragrance-free laundry products. It is not required for household products in the United States to list their ingredients, and the majority do not have complete ingredient lists. Therefore, it is imperative that the patient use an allergen-avoidance application that identifies products that have full ingredient disclosure and are free of fragrance.
For individuals who enjoy perfume and/or cologne, it may be possible for them to resume use of these products in some cases after their condition has fully cleared with complete fragrance avoidance. They should avoid spraying products into the air or applying them directly onto the skin and should instead dip a cotton swab into the perfume/cologne and dab a small amount onto their clothing. This technique can sometimes satisfy the patient and improve compliance.
If a patient who is allergic to fragrance does not clear after 6 weeks of complete fragrance avoidance, it is worth considering systemic contact dermatitis due to ingestion of fragrance-related substances in foods.34 A large number of fragrance materials also are food flavorings. For patients allergic to a specific fragrance(s), systemic avoidance needs to be specific to the allergen, and the Flavor and Extract Manufacturers Association’s flavor ingredient library is most helpful (https://www.femaflavor.org/flavor-library). If the patient is allergic to the complex mixture BOP, a balsam-free diet can be attempted.35,36
Final Thoughts
Dermatologists must equip themselves with the knowledge to educate fragrance-allergic patients on proper avoidance. The multifaceted nature of fragrance avoidance requires a personalized approach, combining label scrutiny, utilization of a safe-product application, and tailored recommendations for specific situations. By guiding patients through these complexities, dermatologists can empower patients to manage their fragrance allergy and enhance their quality of life.
Fragrances are complex organic compounds that are sufficiently volatile to produce an odor—most often a pleasant one—or at times intended to neutralize unpleasant odors. They can be further divided into natural fragrances (eg, essential oils) and synthetic ones. Fragrances are found in abundance in our daily lives: in perfumes; colognes; lotions; shampoos; and an array of other personal, household, and even industrial products (Table). These exposures include products directly applied to the skin, rinsed off, or aerosolized. A single product often contains a multitude of different fragrances to create the scents we know and love. To many, fragrances can be an important part of everyday life or even a part of one’s identity. But that once-intoxicating aroma can transform into an itchy skin nightmare; fragrances are among the most common contact allergens.

Given the widespread prevalence of fragrances in so many products, understanding fragrance allergy and skillful avoidance is imperative. In this review, we explore important aspects of fragrance allergic contact dermatitis (ACD), including chemistry, epidemiology, patch test considerations, and management strategies for patients, with the goal of providing valuable clinical insights for treating physicians on how patients can embrace a fragrance-free lifestyle.
How Fragrances Act as Allergens
A plethora of chemicals emit odors, of which more than 2000 are used to create the fragranced products we see on our shelves today.1 For many of these fragrances, contact allergy develops because the fragrance acts as a hapten (ie, a small molecule that combines with a carrier protein to elicit an immune response).2 Some fragrance molecules require “activation” to be able to bind to proteins; these are known as prehaptens.3 For example, the natural fragrance linalool is generally considered nonallergenic in its initial form. However, once it is exposed to air, it may undergo oxidation to become linalool hydroperoxides, a well-established contact allergen. Some fragrances can become allergenic in the skin itself, often secondary to enzymatic reactions—these are known as prohaptens.3 However, most fragrances are directly reactive to skin proteins on the basis of chemical reactions such as Michael addition and Schiff base formation.4 In either case, the end result is that fragrance allergens, including essential oils, may cause skin sensitization and subsequent ACD.5,6
Epidemiology
Contact allergy to fragrances is not uncommon; in a multicenter cross-sectional study conducted in 5 European countries, the prevalence in the general population was estimated to be as high as 2.6% and 1.9% among 3119 patients patch tested to fragrance mix I (FMI) and fragrance mix II (FMII), respectively.7 Studies in patients referred for patch testing have shown a higher 5% to 25% prevalence of fragrance allergy, largely depending on what population was evaluated.1 Factors such as sociocultural differences in frequency and types of fragrances used could contribute to this variation.
During patch testing, the primary fragrance screening allergens are FMI, FMII, and balsam of Peru (BOP)(Myroxylon pereirae resin).7 In recent years, hydroperoxides of linalool and limonene also have emerged as potentially important fragrance allergens.8 The frequencies of patch-test positivity of these allergens can be quite high in referral-based populations. In a study performed by the North American Contact Dermatitis Group (NACDG) from 2019 to 2020, frequencies of fragrance allergen positivity were 12.8% for FMI, 5.2% for FMII, 7.4% for BOP, 11.1% for hydroperoxides of linalool, and 3.5% for hydroperoxides of limonene.8 Additionally, it was noted that FMI and hydroperoxides of linalool were among the top 10 most frequently positive allergens.9 It should be kept in mind that NACDG studies are drawn from a referral population and not representative of the general population.
Allergic contact dermatitis to fragrances can manifest anywhere on the body, but certain patterns are characteristic. A study by the NACDG analyzed fragrance and botanical patch test results in 24,246 patients and found that fragrance/botanical-sensitive patients more commonly had dermatitis involving the face (odds ratio [OR], 1.12; 95% CI, 1.03-1.21), legs (OR, 1.22; 95% CI, 1.06-1.41), and anal/genital areas (OR, 1.26; 95% CI, 1.04-1.52) and were less likely to have hand dermatitis (OR, 0.88; 95% CI, 0.82-0.95) compared with non–fragrance/botanical-sensitive patients.10 However, other studies have found that hand dermatitis is common among fragrance-allergic individuals.11-13
Fragrance allergy tends to be more common in women than men, which likely is attributable to differences in product use and exposure.10 The prevalence of fragrance allergy increases with age in both men and women, peaking at approximately 50 years of age, likely due to repeat exposure or age-related changes to the skin barrier or immune system.14
Occupational fragrance exposures are important to consider, and fragrance ACD is associated with hairdressers, beauticians, office workers exposed to aromatherapy diffusers, and food handlers.15 Less-obvious professions that involve exposure to fragrances used to cover up unwanted odors—such as working with industrial and cleaning chemicals or even metalworking—also have been reported to be associated with ACD.16
Patch Test Considerations
Patch testing is essential to confirm fragrance allergy and guide treatment, but because there are so many potential fragrance allergens, there is no perfect patch test strategy. In a standard patch test series, the most important screening allergens are considered to be FMI, FMII, and BOP; tested together, they are thought to detect a large proportion of cases of fragrance allergy. Strikingly, in a large European study (N=1951), patch testing with the fragrance markers in the baseline panel failed to detect more than 40% of cases of allergy compared to testing with 26 individual fragrance allergens.17 Other studies have reported that a smaller proportion of fragrance allergies are missed by using baseline screening allergens alone.18,19 Limonene and linalool hydroperoxides also are potentially important fragrance allergens to consider adding to the patch test panel, as unoxidized limonene and linalool commonly are used in many products and could theoretically undergo auto-oxidation under use conditions.8 However, because of the high number of irritant, questionable, and potentially false-positive reactions, the Information Network of Departments of Dermatology has recommended against adding these hydroperoxides to a standard screening tray for patch testing.20 It must be remembered that a positive patch test to a fragrance does not necessarily represent ACD unless the patient has a clinically relevant exposure to the allergen.21
In patients who test negative to the baseline fragrance-screening allergens and in whom a high degree of suspicion remains, further testing with supplemental fragrance allergens (commercially available from patch test suppliers) is warranted.17 The thin-layer rapid use epicutaneous (T.R.U.E.) test (SmartPractice) includes FMI and BOP but not FMII or linalool or limonene hydroperoxides. More comprehensive patch test panels are available that include additional fragrances, such as the North American 80 Comprehensive Series and the American Contact Dermatitis Society Core Allergen Series.22-24 It is important to remain vigilant and consider expanded patch testing if patients initially test negative but suspicion remains.
Furthermore, patch testing with the patient’s own products is an important consideration. Uter et al25 evaluated patch testing using patients’ perfumes, deodorants, and shaving lotions, and approximately 41% (53/129) of patients who tested positive to their own product tested negative for fragrance-screening allergens. Although it can be difficult to ascertain which exact component of a commercial product is the culprit, a positive patch test may still provide clinically relevant information for patients and treating physicians. In cases of questionable or weak-positive results, repeat testing or repeated open application tests can help re-evaluate suspected products.
Cross-reactivity should be considered when patch testing for fragrances. Atwater et al10 found that cross-reactivity between FMI, FMII, and BOP was common; for instance, approximately 40% of patients testing positive to FMII or BOP also had positive reactions to FMI (522/1182 and 768/1942, respectively). Understanding this concept is important because in some cases (as detailed below) patients will need to avoid all fragrances, not just the ones to which they have previously been exposed, given the limitations on fragrance labeling in the United States. However, this may change with the Modernization of Cosmetic Regulation Act of 2022.26
Avoiding Fragrances: Improving Patient Education and Outcomes
Once a relevant contact allergy to fragrance is established after patch testing, successful avoidance is critical but challenging, as there are numerous potential pitfalls. Missing just 1 hidden source of fragrance exposure will often be the difference between success or failure. Dermatologists play a crucial role in guiding patients through the intricate process of identifying and avoiding potential allergens.
Optimal Safety: Embracing a Fragrance-Free Lifestyle
For fragrance-allergic patients, it generally is safest to completely avoid fragrance.
First, if a patient only shows positive patch-test reactions to fragrance screening mixes (and not to the particular fragrances in these mixes), there is no way to be certain which fragrances the patient needs to avoid.
Second, even if specific fragrance allergens are identified, numerous chemically related fragrances to which the patient may be allergic are not commercially available for patch testing. One review provided evidence of 162 fragrance allergens that have been documented to cause contact allergy.1 Dermatologists generally patch test to screening mixtures and/or the 26 fragrance chemicals required on labels in European products (European Directive fragrance).27 Therefore, there are more than 100 known fragrance allergens that are not routinely tested to which patients could be allergic.
Third, certain fragrances, such as limonene and linalool, are found in many products with fragrance, and it is difficult to find products without these substances. Limonene and linalool themselves are not potent allergens; however, upon air exposure, they may auto-oxidize to hydroperoxides of limonene and linalool, which are increasingly common positive patch tests.19
Additionally, patients should be advised that many products labeled “fragrance free,” “unscented,” or “free and clear” are not truly fragrance free, and patients should not choose products based on these claims. There are no legal definitions for these claims in the United States, and industries are allowed to choose the definition they prefer. Numerous products labeled “unscented” use this term to indicate that the product had an odor, the company used a masking fragrance to hide the odor, and then the product can be considered unscented. In many holistic stores, most products labeled “fragrance free” are only free of artificial fragrances but contain essential oils. Of the 162 documented fragrance allergens, 80 are essential oils.6 Essential oils are perceived to be safe by the vast majority of the population because they are viewed as “natural” and “unprocessed” sources of fragrance.28 However, numerous allergenic terpenes have been discovered in essential oils, including functionalized variations of alcohols (eg, geraniol, bisabolol) and aldehydes (eg, citronellal).6 Essential oils also consist of nonterpenic compounds produced through the phenylpropanoids pathway, including eugenol and cinnamaldehyde. One review showed that most essential oils contain one or more European Directive fragrance.29 Therefore, many products labeled “unscented,” “fragrance free,” or “natural” are not free of fragrance and may be unsafe for fragrance-allergic patients.
Although not required, manufacturers sometimes voluntarily list one or more of the 162 currently identified fragrance allergens on product labels. Also, there are more than 50 potentially allergenic essential oils that can be listed on labels by their common names or by genus or species. In addition, there are synonyms for fragrance, such as aroma, parfum, perfume, and scent. Therefore, there are several hundred different ingredient names on labels that indicate the presence of fragrance, and patients are very unlikely to successfully identify fragrance-free products by trying to read product labels on their own.
Lastly, in the United States product labels only require products to state that they contain “fragrance” and do not mandate the listing of specific fragrances. If a patient is allergic to a specific fragrance, there is no way to determine if that fragrance is present in these products. This will change with the enactment of Modernization of Cosmetics Regulation Act of 2022, which empowers the US Food and Drug Administration to require manufacturers to disclose many, but not all, fragrance allergens on the labels of cosmetic and topical products.26
For all these reasons, patients should be advised to use a medical database to choose safe alternative products instead of trying to read labels themselves to avoid fragrance. The American Contact Dermatitis Society’s Contact Allergen Management Program (CAMP) database (https://www.contactderm.org/resources/acds-camp) is designed to identify safe alternative products for patients with contact allergies. When CAMP is programmed to avoid “fragrance,” it will list only “safe” products free of all fragrances found in a comprehensive fragrance cross-reactor group.30 This customizable database is available as an application that can be downloaded onto a patient’s mobile device. Fragrance-allergic patients should be encouraged to use the CAMP application or other similar applications (eg, SkinSAFE)(https://www.skinsafeproducts.com/) to find all the products they use.
Potential Pitfalls in Fragrance Avoidance
Most physicians, even dermatologists, will not know which products on the market are fragrance free from a contact allergy standpoint. Patients should instruct their physicians to use the allergen-avoidance application of choice whenever recommending new topical products, whether prescription or nonprescription. In 2009, Nardelli and colleagues31 found that 10% of topical pharmaceutical products contained a total of 66 different fragrance substances.
Individuals who are allergic to fragrance also can react to fragrances used by close contacts (ie, consort dermatitis).32 Therefore, fragrance-allergic individuals who do not improve after changing their personal products should consider urging their spouses or significant others to choose their personal care products using an allergen-avoidance application. Also, physical contact with pets can cause reactions, and the use of a fragrance-free pet shampoo is recommended. Additionally, allergic individuals who are providing care for small children should select fragrance-free products for them.
Some of the most heavily fragranced products on the market are found at hair salons. One exposure to an allergen often can keep patients broken out for up to 4 weeks and occasionally longer, a typical frequency for salon visits—even if the individual is taking great care to avoid fragrance at home. Patients should be instructed to bring their own shampoo, conditioner, and styling products to the salon. These patients also should bring safe moisturizer and nail polish remover for manicures. Additionally, aromatherapy used in most massages can cause flare-ups, and it is recommended that allergic patients purchase fragrance-free massage oil to bring to their sessions.
Fragranced soaps and cleansers can leave a residue on the palmar surface of the hands and fingers. This residue may not meet the threshold for causing a reaction on the thick skin of these surfaces, but it is sufficient to passively transfer fragrance to other more sensitive areas, such as the eyelids. Passive transfer of fragrance can be a major source of allergen exposure and should not be overlooked. Allergic patients should be instructed to bring safe hand cleansers to friends’ houses, restaurants, or work.
Airborne fragrances in a patient’s environment can reach sufficient concentration to cause airborne contact dermatitis. In one case report, an Uber driver developed facial airborne ACD from a fragrance diffuser in his vehicle and his condition improved upon removing the diffuser.33 Therefore, patients should be instructed to avoid fragranced diffusers, scented candles, room deodorizers, incense, and wax melts.
Fragrance in household products also can be an issue. Fragrance-allergic patients should be instructed to choose fragrance-free cleaning products and to avoid fragranced wipes on surfaces that may be touched. In addition, they should be instructed to use fragrance-free laundry products. It is not required for household products in the United States to list their ingredients, and the majority do not have complete ingredient lists. Therefore, it is imperative that the patient use an allergen-avoidance application that identifies products that have full ingredient disclosure and are free of fragrance.
For individuals who enjoy perfume and/or cologne, it may be possible for them to resume use of these products in some cases after their condition has fully cleared with complete fragrance avoidance. They should avoid spraying products into the air or applying them directly onto the skin and should instead dip a cotton swab into the perfume/cologne and dab a small amount onto their clothing. This technique can sometimes satisfy the patient and improve compliance.
If a patient who is allergic to fragrance does not clear after 6 weeks of complete fragrance avoidance, it is worth considering systemic contact dermatitis due to ingestion of fragrance-related substances in foods.34 A large number of fragrance materials also are food flavorings. For patients allergic to a specific fragrance(s), systemic avoidance needs to be specific to the allergen, and the Flavor and Extract Manufacturers Association’s flavor ingredient library is most helpful (https://www.femaflavor.org/flavor-library). If the patient is allergic to the complex mixture BOP, a balsam-free diet can be attempted.35,36
Final Thoughts
Dermatologists must equip themselves with the knowledge to educate fragrance-allergic patients on proper avoidance. The multifaceted nature of fragrance avoidance requires a personalized approach, combining label scrutiny, utilization of a safe-product application, and tailored recommendations for specific situations. By guiding patients through these complexities, dermatologists can empower patients to manage their fragrance allergy and enhance their quality of life.
- de Groot AC. Fragrances: contact allergy and other adverse effects. Dermatitis. 2020;31:13-35.
- Uter W. Contact allergy to fragrances: current clinical and regulatory trends. Allergol Select. 2017;1:190-199.
- Karlberg AT, Börje A, Duus Johansen J, et al. Activation of non-sensitizing or low-sensitizing fragrance substances into potent sensitizers - prehaptens and prohaptens. Contact Dermatitis. 2013;69:323-334.
- Patlewicz GY, Wright ZM, Basketter DA, et al. Structure-activity relationships for selected fragrance allergens. Contact Dermatitis. 2002;47:219-226. doi:10.1034/j.1600-0536.2002.470406
- Ward JM, Reeder M, Atwater AR. Essential oils debunked: separating fact from myth. Cutis. 2020;105:174-176.
- de Groot AC, Schmidt E. Essential oils, part IV: contact allergy. Dermatitis. 2016;27:170-175.
- Diepgen TL, Ofenloch R, Bruze M, et al. Prevalence of fragrance contact allergy in the general population of five European countries: a cross-sectional study. Br J Dermatol. 2015;173:1411-1419
- Ogueta IA, Brared Christensson J, Giménez-Arnau E, et al. Limonene and linalool hydroperoxides review: pros and cons for routine patch testing. Contact Dermatitis. 2022;87:1-12.
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group Patch Test Results: 2019-2020. Dermatitis. 2023;34:90-104.
- Atwater AR, Ward JM, Liu B, et al. Fragrance- and botanical-related allergy and associated concomitant reactions: a retrospective analysis of the North American Contact Dermatitis Group Data 2007-2016. Dermatitis. 2021;32:42-52.
- Tai V, Sharifah Rosniza SNC, Tang MM. Contact sensitization to fragrance allergen: a 5-year review in the Department of Dermatology, Hospital Kuala Lumpur. Med J Malaysia. 2023;78:583-588.
- Periyasamy MK, Sekar SC, Rai R. Analysis of hypersensitivity in fragrance series by patch testing. Indian Dermatol Online J. 2019;10:657-662.
- Heydorn S, Menné T, Johansen JD. Fragrance allergy and hand eczema - a review. Contact Dermatitis. 2003;48:59-66.
- Buckley DA, Rycroft RJG, White IR, et al. The frequency of fragrance allergy in patch-tested patients increases with their age. Br J Dermatol. 2003;149:986-989.
- Montgomery RL, Agius R, Wilkinson SM, et al. UK trends of allergic occupational skin disease attributed to fragrances 1996-2015. Contact Dermatitis. 2018;78:33-40.
- Reeder MJ. Allergic contact dermatitis to fragrances. Dermatol Clin. 2020;38:371-377.
- Mann J, McFadden JP, White JML, et al. Baseline series fragrance markers fail to predict contact allergy. Contact Dermatitis. 2014;70:276-281.
- Vejanurug P, Tresukosol P, Sajjachareonpong P, et al. Fragrance allergy could be missed without patch testing with 26 individual fragrance allergens. Contact Dermatitis. 2016;74:230-235.
- Sukakul T, Bruze M, Mowitz M, et al. Simultaneous patch testing with fragrance markers in the baseline series and the ingredients of fragrance mixes: an update from southern Sweden. Contact Dermatitis. 2022;86:514-523.
- Schubert S, Geier J, Brans R, et al; IVDK. Patch testing hydroperoxides of limonene and linalool in consecutive patients-results of the IVDK 2018-2020. Contact Dermatitis. 2023;89:85-94. doi:10.1111/cod.14332
- Storrs FJ. Fragrance. Dermatitis. 2007;18:3-7.
- T.R.U.E. test. SmartPractice website. Accessed July 24, 2024. https://www.smartpractice.com/shop/category?id=581719&m=SPA ACDS
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2020 update. Dermatitis. 2020;31:279-282. https://pubmed.ncbi.nlm.nih.gov/32947457/
- North American 80 Comprehensive Series NAC-80. Chemotechnique MB Diagnostics AB website. Accessed July 24, 2024. https://www.chemotechnique.se/products/national-series/north-american-80-comprehensive-series/
- Uter W, Geier J, Schnuch A, et al. Patch test results with patients’ own perfumes, deodorants and shaving lotions: results of the IVDK 1998-2002. J Eur Acad Dermatol Venereol. 2007;21:374-379.
- Filley AR, Woodruff CM. The Modernization of Cosmetics Regulation Act of 2022: what dermatologists need to know. J Am Acad Dermatol. 2023;89:629-631.
- European Parliament and the Council of the European Union. Directive 2003/15/EC of the European Parliament and of the Council of 27 February 2003 amending Council Directive 76/768/EEC on the approximation of the laws of the Member States relating to cosmetic products (text with EEA relevance). November 3, 2003. Accessed June 7, 2024. https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2003:066:0026:0035:en:PDF
- Sharmeen JB, Mahomoodally FM, Zengin G, et al. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules. 2021;26:666.
- Scheman A, Scheman N, Rakowski EM. European Directive fragrances in natural products. Dermatitis. 2014;25:51-55.
- Scheman A, Hipolito R, Severson D, et al. Contact allergy cross-reactions: retrospective clinical data and review of the literature. Dermatitis. 2017;28:128-140.
- Nardelli A, D’Hooghe E, Drieghe J, et al. Allergic contact dermatitis from fragrance components in specific topical pharmaceutical products in Belgium. Contact Dermatitis. 2009;60:303-313.
- Lee J, Guo S, Dinalo J, et al. Consort allergic contact dermatitis: a systematic review. Dermatitis. 2022;33:181-186.
- Perper M, Cervantes J, Eber AE, et al. Airborne contact dermatitis caused by fragrance diffusers in Uber cars. Contact Dermatitis. 2017;77:116-117.
- Nijhawan RI, Molenda M, Zirwas MJ, et al. Systemic contact dermatitis. Dermatol Clin. 2009;27:355-364.
- Salam TN, Fowler JF. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
- Scheman A, Rakowski EM, Chou V, et al. Balsam of Peru: past and future. Dermatitis. 2013;24:153-160.
- de Groot AC. Fragrances: contact allergy and other adverse effects. Dermatitis. 2020;31:13-35.
- Uter W. Contact allergy to fragrances: current clinical and regulatory trends. Allergol Select. 2017;1:190-199.
- Karlberg AT, Börje A, Duus Johansen J, et al. Activation of non-sensitizing or low-sensitizing fragrance substances into potent sensitizers - prehaptens and prohaptens. Contact Dermatitis. 2013;69:323-334.
- Patlewicz GY, Wright ZM, Basketter DA, et al. Structure-activity relationships for selected fragrance allergens. Contact Dermatitis. 2002;47:219-226. doi:10.1034/j.1600-0536.2002.470406
- Ward JM, Reeder M, Atwater AR. Essential oils debunked: separating fact from myth. Cutis. 2020;105:174-176.
- de Groot AC, Schmidt E. Essential oils, part IV: contact allergy. Dermatitis. 2016;27:170-175.
- Diepgen TL, Ofenloch R, Bruze M, et al. Prevalence of fragrance contact allergy in the general population of five European countries: a cross-sectional study. Br J Dermatol. 2015;173:1411-1419
- Ogueta IA, Brared Christensson J, Giménez-Arnau E, et al. Limonene and linalool hydroperoxides review: pros and cons for routine patch testing. Contact Dermatitis. 2022;87:1-12.
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group Patch Test Results: 2019-2020. Dermatitis. 2023;34:90-104.
- Atwater AR, Ward JM, Liu B, et al. Fragrance- and botanical-related allergy and associated concomitant reactions: a retrospective analysis of the North American Contact Dermatitis Group Data 2007-2016. Dermatitis. 2021;32:42-52.
- Tai V, Sharifah Rosniza SNC, Tang MM. Contact sensitization to fragrance allergen: a 5-year review in the Department of Dermatology, Hospital Kuala Lumpur. Med J Malaysia. 2023;78:583-588.
- Periyasamy MK, Sekar SC, Rai R. Analysis of hypersensitivity in fragrance series by patch testing. Indian Dermatol Online J. 2019;10:657-662.
- Heydorn S, Menné T, Johansen JD. Fragrance allergy and hand eczema - a review. Contact Dermatitis. 2003;48:59-66.
- Buckley DA, Rycroft RJG, White IR, et al. The frequency of fragrance allergy in patch-tested patients increases with their age. Br J Dermatol. 2003;149:986-989.
- Montgomery RL, Agius R, Wilkinson SM, et al. UK trends of allergic occupational skin disease attributed to fragrances 1996-2015. Contact Dermatitis. 2018;78:33-40.
- Reeder MJ. Allergic contact dermatitis to fragrances. Dermatol Clin. 2020;38:371-377.
- Mann J, McFadden JP, White JML, et al. Baseline series fragrance markers fail to predict contact allergy. Contact Dermatitis. 2014;70:276-281.
- Vejanurug P, Tresukosol P, Sajjachareonpong P, et al. Fragrance allergy could be missed without patch testing with 26 individual fragrance allergens. Contact Dermatitis. 2016;74:230-235.
- Sukakul T, Bruze M, Mowitz M, et al. Simultaneous patch testing with fragrance markers in the baseline series and the ingredients of fragrance mixes: an update from southern Sweden. Contact Dermatitis. 2022;86:514-523.
- Schubert S, Geier J, Brans R, et al; IVDK. Patch testing hydroperoxides of limonene and linalool in consecutive patients-results of the IVDK 2018-2020. Contact Dermatitis. 2023;89:85-94. doi:10.1111/cod.14332
- Storrs FJ. Fragrance. Dermatitis. 2007;18:3-7.
- T.R.U.E. test. SmartPractice website. Accessed July 24, 2024. https://www.smartpractice.com/shop/category?id=581719&m=SPA ACDS
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2020 update. Dermatitis. 2020;31:279-282. https://pubmed.ncbi.nlm.nih.gov/32947457/
- North American 80 Comprehensive Series NAC-80. Chemotechnique MB Diagnostics AB website. Accessed July 24, 2024. https://www.chemotechnique.se/products/national-series/north-american-80-comprehensive-series/
- Uter W, Geier J, Schnuch A, et al. Patch test results with patients’ own perfumes, deodorants and shaving lotions: results of the IVDK 1998-2002. J Eur Acad Dermatol Venereol. 2007;21:374-379.
- Filley AR, Woodruff CM. The Modernization of Cosmetics Regulation Act of 2022: what dermatologists need to know. J Am Acad Dermatol. 2023;89:629-631.
- European Parliament and the Council of the European Union. Directive 2003/15/EC of the European Parliament and of the Council of 27 February 2003 amending Council Directive 76/768/EEC on the approximation of the laws of the Member States relating to cosmetic products (text with EEA relevance). November 3, 2003. Accessed June 7, 2024. https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2003:066:0026:0035:en:PDF
- Sharmeen JB, Mahomoodally FM, Zengin G, et al. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules. 2021;26:666.
- Scheman A, Scheman N, Rakowski EM. European Directive fragrances in natural products. Dermatitis. 2014;25:51-55.
- Scheman A, Hipolito R, Severson D, et al. Contact allergy cross-reactions: retrospective clinical data and review of the literature. Dermatitis. 2017;28:128-140.
- Nardelli A, D’Hooghe E, Drieghe J, et al. Allergic contact dermatitis from fragrance components in specific topical pharmaceutical products in Belgium. Contact Dermatitis. 2009;60:303-313.
- Lee J, Guo S, Dinalo J, et al. Consort allergic contact dermatitis: a systematic review. Dermatitis. 2022;33:181-186.
- Perper M, Cervantes J, Eber AE, et al. Airborne contact dermatitis caused by fragrance diffusers in Uber cars. Contact Dermatitis. 2017;77:116-117.
- Nijhawan RI, Molenda M, Zirwas MJ, et al. Systemic contact dermatitis. Dermatol Clin. 2009;27:355-364.
- Salam TN, Fowler JF. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
- Scheman A, Rakowski EM, Chou V, et al. Balsam of Peru: past and future. Dermatitis. 2013;24:153-160.
Practice Points
- Fragrance allergy is common due to daily exposure from many sources, ranging from personal care products and cosmetics to cleaning products, foods/spices, and workplace materials.
- More than 100 different fragrances can cause contact allergy, but patch testing in routine practice usually is limited to a few key screening allergens with important limitations.
- Fragrance avoidance is challenging, and comprehensive patient education is critical, including the provision of a list of safe products that are truly fragrance free.