User login
Erythema Nodosum Triggered by a Bite From a Copperhead Snake
The clinical manifestations of snakebites vary based on the species of snake, bite location, and amount and strength of the venom injected. Locally acting toxins in snake venom predominantly consist of enzymes, such as phospholipase A2, that cause local tissue destruction and can result in pain, swelling, blistering, ecchymosis, and tissue necrosis at the site of the bite within hours to days after the bite.1 Systemically acting toxins can target a wide variety of tissues and cause severe systemic complications including paralysis, rhabdomyolysis secondary to muscle damage, coagulopathy, sepsis, and cardiorespiratory failure.2
Although pain and swelling following snakebites typically resolve by 1 month after envenomation, copperhead snakes—a type of pit viper—may cause residual symptoms of pain and swelling lasting for a year or more.3 Additional cutaneous manifestations of copperhead snakebites include wound infections at the bite site, such as cellulitis and necrotizing fasciitis. More devastating complications that have been described following snake envenomation include tissue injury of an entire extremity and development of compartment syndrome, which requires urgent fasciotomy to prevent potential loss of the affected limb.4
Physicians should be aware of the potential complications of snakebites to properly manage and counsel their patients. We describe a 42-year-old woman with tender, erythematous, subcutaneous nodules persisting for 4 months following a copperhead snakebite. A biopsy confirmed the diagnosis of snakebite-associated erythema nodosum (EN).
Case Report
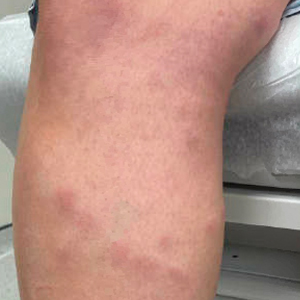
A 42-year-old woman presented to our clinic with progressive tender, pruritic, deep-seated, erythematous nodules in multiple locations on the legs after sustaining a bite by a copperhead snake on the left foot 4 months prior. The lesions tended to fluctuate in intensity. In the days following the bite, she initially developed painful red bumps on the left foot just proximal to the bite site with associated pain and swelling extending up to just below the left knee. She reported no other notable symptoms such as fever, arthralgia, fatigue, or gastrointestinal tract symptoms. Physical examination revealed bilateral pitting edema, which was worse in the left leg, along with multiple deep, palpable, tender subcutaneous nodules with erythematous surface change (Figure 1).
Workup performed by an outside provider over the previous month included 2 venous duplex ultrasounds of the left leg, which showed no signs of deep vein thrombosis. Additionally, the patient underwent lateral and anteroposterior radiographs of the left foot, tibia, and fibula, which showed no evidence of fracture.
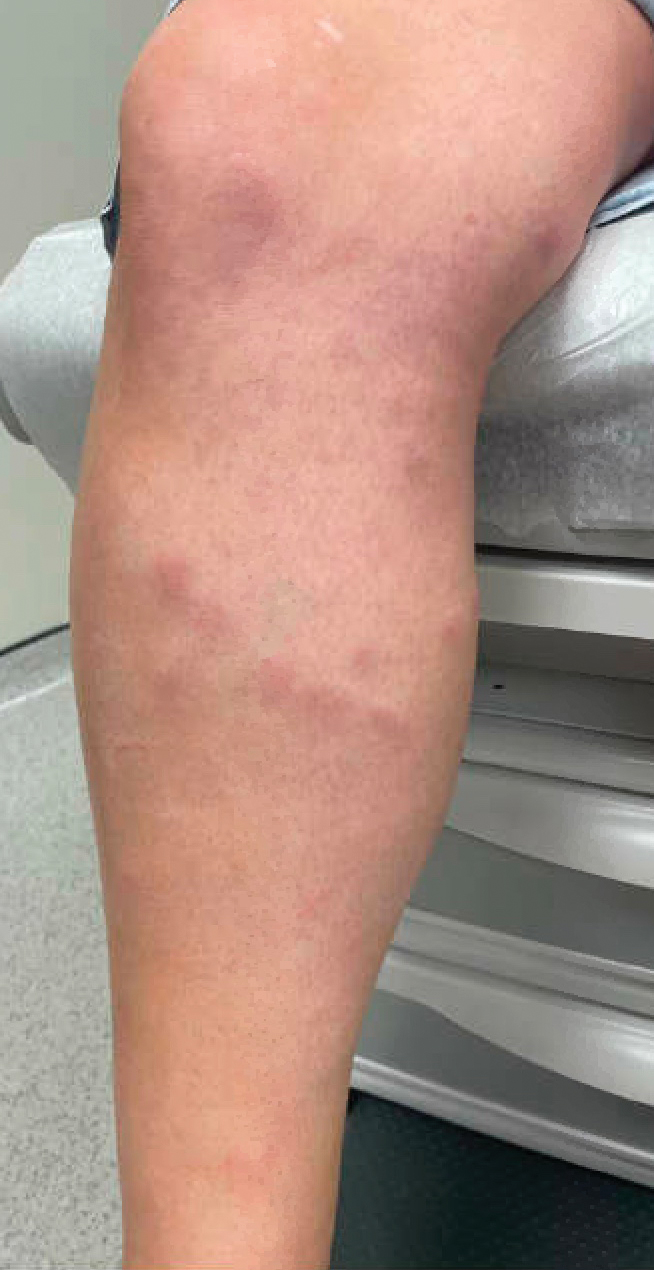
Given the morphology and distribution of the lesions (Figure 2), EN was strongly favored as the cause of the symptoms, and a biopsy confirmed the diagnosis. All immunohistochemical stains including auramine-rhodamine for acid-fast bacilli, Grocott-Gomori methenamine silver for fungal organisms, and Brown and Brenn were negative. Given the waxing and waning course of the lesions, which suggested an active neutrophilic rather than purely chronic granulomatous phase of EN, the patient was treated with colchicine 0.6 mg twice daily for 1 month.
Causes of EN and Clinical Manifestations
Erythema nodosum is a common form of septal panniculitis that can be precipitated by inflammatory conditions, infection, or medications (commonly oral contraceptive pills) but often is idiopathic.5 The acute phase is neutrophilic, with evolution over time to a granulomatous phase. Common etiologies include sarcoidosis; inflammatory bowel disease; and bacterial or fungal infections such as Streptococcus (especially common in children), histoplasmosis, and coccidioidomycosis. The patient was otherwise healthy and was not taking any medications that are known triggers of EN. A PubMed search of articles indexed for MEDLINE in the English-language literature using the terms copperhead snake bite, erythema nodosum snake, and copperhead snake erythema nodosum revealed no reports of EN following a bite from a copperhead snake; however, in one case, an adder bite led to erysipelas, likely due to disturbed blood and lymphatic flow, which then triggered EN.6 Additionally, EN has been reported as a delayed reaction to jellyfish stings.7
Clinical features of EN include the development of tender, erythematous, subcutaneous nodules and plaques most frequently over the pretibial region. Lesions typically evolve from raised, deep-seated nodules into flat indurated plaques over a span of weeks. Occasionally, there is a slight prodromal phase marked by nonspecific symptoms such as fever and arthralgia lasting for 3 to 6 days. Erythema nodosum typically results in spontaneous resolution after 4 to 8 weeks, and management involves treatment of any underlying condition with symptomatic care. Interestingly, our patient experienced persistent symptoms over the course of 4 months, with development of new nodular lesions throughout this time period. The most frequently used drugs for the management of symptomatic EN include nonsteroidal anti-inflammatory drugs, colchicine, and potassium iodide.8 A characteristic histologic finding of the granulomatous phase is the Miescher radial granuloma, which is a septal collection of histiocytes surrounding a cleft.9
Snakebite Reactions
Snakebites can result in a wide range of local and systemic manifestations, as snake venom may contain 20 or more toxins.10 Local complications of pit viper bites include pain, swelling, and fang marks; when examining fang marks, the presence of 2 distinct puncture wounds often indicates envenomation with a poisonous snake, whereas nonvenomous snakebites often result in smaller puncture wounds arranged in an arc. Following bites, pain can develop immediately and spread proximally up the affected limb, which occurred in our patient in the days following the bite. Intense local reactions can occur, as bites often result in intense edema of the affected limb spreading to the trunk in the days to weeks after the bite, occasionally accompanied by regional lymphadenopathy. Some bites can result in local necrosis and secondary bacterial infection caused by organisms in the oral cavity of the culprit snake.
Although they were not present in our patient, snakebites can result in a wide range of systemic toxicities ranging from clotting defects and hemolysis to neurotoxicity, myotoxicity, and nephrotoxicity.10 In severe cases, snake venom can result in disseminated intravascular coagulation, sepsis, and cardiorespiratory collapse.
The eastern copperhead (Agkistrodon contortrix) is a species of venomous snake that is endemic to eastern North America. Copperheads are members of the subfamily Crotalinae in the family Viperidae.11 Reported reactions to copperhead bites include cellulitis, necrotizing fasciitis, compartment syndrome, and tissue necrosis of an entire affected extremity.12,13 Our patient displayed no systemic symptoms to suggest envenomation.
Management of Snakebites
Treatment of snakebites varies based on the constellation and severity of symptoms as well as how recently the envenomation occurred. In urgent cases, antivenom may be administered to prevent further toxicity. In cases of progressive compartment syndrome, emergent surgical procedures such as fasciotomy or amputation are required to prevent further complications. When a superimposed bacterial infection is suspected, broad-spectrum antibiotics are required. Because our patient presented 4 months following the initial bite with isolated cutaneous manifestations, she was treated symptomatically with colchicine for EN.1,2
Final Thoughts
Our patient presented with EN following a bite from a copperhead snake. Physicians should be aware of possible etiologies of EN to evaluate patients who present with new-onset tender subcutaneous nodules. Additionally, physicians should be aware of venomous snakes endemic to their region and also understand the various complications that can result following a snakebite, with the potential for lingering cutaneous manifestations weeks to months following the initial bite.
- Warrell DA. Snake bite. Lancet. 2010;375:77-88. doi:10.1016/S0140-6736(09)61754-2
- White J. Overview of venomous snakes of the world. In: Dart RC, eds. Medical Toxicology. 3rd ed. Lippincott, Williams, & Wilkins; 2004:1543
- Spiller HA, Bosse GM. Prospective study of morbidity associated with snakebite envenomation. J Toxicol Clin Toxicol. 2003;41:125-130. doi:10.1081/clt-120019127
- Scharman EJ, Noffsinger VD. Copperhead snakebites: clinical severity of local effects. Ann Emerg Med. 2001;38:55-61. doi:10.1067/mem.2001.116148
- Hafsi W, Badri T. Erythema nodosum. In: StatPearls. StatPearls Publishing; November 28, 2022. Accessed July 22, 2024. https://www.ncbi.nlm.nih.gov/books/NBK470369/
- Nowowiejska J, Baran A, Flisiak I. Rare coexistence of unilateral erythema nodosum with erysipelas in the area of previous adder bite. Przegl Epidemiol. 2020;74:355-361. doi:10.32394/pe.74.28
- Auerbach PS, Hays JT. Erythema nodosum following a jellyfish sting. J Emerg Med. 1987;5:487-491. doi:10.1016/0736-4679(87)90211-3
- Gilchrist H, Patterson JW. Erythema nodosum and erythema induratum (nodular vasculitis): diagnosis and management. Dermatol Ther. 2010;23:320-327. doi:10.1111/j.1529-8019.2010.01332.x
- Sánchez Yus E, Sanz Vico MD, de Diego V. Miescher’s radial granuloma. a characteristic marker of erythema nodosum. Am J Dermatopathol. 1989;11:434-442. doi:10.1097/00000372-198910000-00005
- Mehta SR, Sashindran VK. Clinical features and management of snake bite. Med J Armed Forces India. 2002;58:247-249. doi:10.1016/S0377-1237(02)80140-X
- Brys AK, Gandolfi BM, Levinson H, et al. Copperhead envenomation resulting in a rare case of hand compartment syndrome and subsequent fasciotomy. Plast Reconstr Surg Glob Open. 2015;3:E396. doi:10.1097/GOX.0000000000000367
- Clark RF, Selden BS, Furbee B. The incidence of wound infection following crotalid envenomation. J Emerg Med. 1993;11:583-586. doi:10.1016/0736-4679(93)90313-v
- Buchanan JT, Thurman J. Crotalidae envenomation. In: StatPearls. StatPearls Publishing; October 3, 2022. Accessed July 22, 2024. https://www.ncbi.nlm.nih.gov/books/NBK551615/
The clinical manifestations of snakebites vary based on the species of snake, bite location, and amount and strength of the venom injected. Locally acting toxins in snake venom predominantly consist of enzymes, such as phospholipase A2, that cause local tissue destruction and can result in pain, swelling, blistering, ecchymosis, and tissue necrosis at the site of the bite within hours to days after the bite.1 Systemically acting toxins can target a wide variety of tissues and cause severe systemic complications including paralysis, rhabdomyolysis secondary to muscle damage, coagulopathy, sepsis, and cardiorespiratory failure.2
Although pain and swelling following snakebites typically resolve by 1 month after envenomation, copperhead snakes—a type of pit viper—may cause residual symptoms of pain and swelling lasting for a year or more.3 Additional cutaneous manifestations of copperhead snakebites include wound infections at the bite site, such as cellulitis and necrotizing fasciitis. More devastating complications that have been described following snake envenomation include tissue injury of an entire extremity and development of compartment syndrome, which requires urgent fasciotomy to prevent potential loss of the affected limb.4
Physicians should be aware of the potential complications of snakebites to properly manage and counsel their patients. We describe a 42-year-old woman with tender, erythematous, subcutaneous nodules persisting for 4 months following a copperhead snakebite. A biopsy confirmed the diagnosis of snakebite-associated erythema nodosum (EN).
Case Report
A 42-year-old woman presented to our clinic with progressive tender, pruritic, deep-seated, erythematous nodules in multiple locations on the legs after sustaining a bite by a copperhead snake on the left foot 4 months prior. The lesions tended to fluctuate in intensity. In the days following the bite, she initially developed painful red bumps on the left foot just proximal to the bite site with associated pain and swelling extending up to just below the left knee. She reported no other notable symptoms such as fever, arthralgia, fatigue, or gastrointestinal tract symptoms. Physical examination revealed bilateral pitting edema, which was worse in the left leg, along with multiple deep, palpable, tender subcutaneous nodules with erythematous surface change (Figure 1).

Workup performed by an outside provider over the previous month included 2 venous duplex ultrasounds of the left leg, which showed no signs of deep vein thrombosis. Additionally, the patient underwent lateral and anteroposterior radiographs of the left foot, tibia, and fibula, which showed no evidence of fracture.
Given the morphology and distribution of the lesions (Figure 2), EN was strongly favored as the cause of the symptoms, and a biopsy confirmed the diagnosis. All immunohistochemical stains including auramine-rhodamine for acid-fast bacilli, Grocott-Gomori methenamine silver for fungal organisms, and Brown and Brenn were negative. Given the waxing and waning course of the lesions, which suggested an active neutrophilic rather than purely chronic granulomatous phase of EN, the patient was treated with colchicine 0.6 mg twice daily for 1 month.

Causes of EN and Clinical Manifestations
Erythema nodosum is a common form of septal panniculitis that can be precipitated by inflammatory conditions, infection, or medications (commonly oral contraceptive pills) but often is idiopathic.5 The acute phase is neutrophilic, with evolution over time to a granulomatous phase. Common etiologies include sarcoidosis; inflammatory bowel disease; and bacterial or fungal infections such as Streptococcus (especially common in children), histoplasmosis, and coccidioidomycosis. The patient was otherwise healthy and was not taking any medications that are known triggers of EN. A PubMed search of articles indexed for MEDLINE in the English-language literature using the terms copperhead snake bite, erythema nodosum snake, and copperhead snake erythema nodosum revealed no reports of EN following a bite from a copperhead snake; however, in one case, an adder bite led to erysipelas, likely due to disturbed blood and lymphatic flow, which then triggered EN.6 Additionally, EN has been reported as a delayed reaction to jellyfish stings.7
Clinical features of EN include the development of tender, erythematous, subcutaneous nodules and plaques most frequently over the pretibial region. Lesions typically evolve from raised, deep-seated nodules into flat indurated plaques over a span of weeks. Occasionally, there is a slight prodromal phase marked by nonspecific symptoms such as fever and arthralgia lasting for 3 to 6 days. Erythema nodosum typically results in spontaneous resolution after 4 to 8 weeks, and management involves treatment of any underlying condition with symptomatic care. Interestingly, our patient experienced persistent symptoms over the course of 4 months, with development of new nodular lesions throughout this time period. The most frequently used drugs for the management of symptomatic EN include nonsteroidal anti-inflammatory drugs, colchicine, and potassium iodide.8 A characteristic histologic finding of the granulomatous phase is the Miescher radial granuloma, which is a septal collection of histiocytes surrounding a cleft.9
Snakebite Reactions
Snakebites can result in a wide range of local and systemic manifestations, as snake venom may contain 20 or more toxins.10 Local complications of pit viper bites include pain, swelling, and fang marks; when examining fang marks, the presence of 2 distinct puncture wounds often indicates envenomation with a poisonous snake, whereas nonvenomous snakebites often result in smaller puncture wounds arranged in an arc. Following bites, pain can develop immediately and spread proximally up the affected limb, which occurred in our patient in the days following the bite. Intense local reactions can occur, as bites often result in intense edema of the affected limb spreading to the trunk in the days to weeks after the bite, occasionally accompanied by regional lymphadenopathy. Some bites can result in local necrosis and secondary bacterial infection caused by organisms in the oral cavity of the culprit snake.
Although they were not present in our patient, snakebites can result in a wide range of systemic toxicities ranging from clotting defects and hemolysis to neurotoxicity, myotoxicity, and nephrotoxicity.10 In severe cases, snake venom can result in disseminated intravascular coagulation, sepsis, and cardiorespiratory collapse.
The eastern copperhead (Agkistrodon contortrix) is a species of venomous snake that is endemic to eastern North America. Copperheads are members of the subfamily Crotalinae in the family Viperidae.11 Reported reactions to copperhead bites include cellulitis, necrotizing fasciitis, compartment syndrome, and tissue necrosis of an entire affected extremity.12,13 Our patient displayed no systemic symptoms to suggest envenomation.
Management of Snakebites
Treatment of snakebites varies based on the constellation and severity of symptoms as well as how recently the envenomation occurred. In urgent cases, antivenom may be administered to prevent further toxicity. In cases of progressive compartment syndrome, emergent surgical procedures such as fasciotomy or amputation are required to prevent further complications. When a superimposed bacterial infection is suspected, broad-spectrum antibiotics are required. Because our patient presented 4 months following the initial bite with isolated cutaneous manifestations, she was treated symptomatically with colchicine for EN.1,2
Final Thoughts
Our patient presented with EN following a bite from a copperhead snake. Physicians should be aware of possible etiologies of EN to evaluate patients who present with new-onset tender subcutaneous nodules. Additionally, physicians should be aware of venomous snakes endemic to their region and also understand the various complications that can result following a snakebite, with the potential for lingering cutaneous manifestations weeks to months following the initial bite.
The clinical manifestations of snakebites vary based on the species of snake, bite location, and amount and strength of the venom injected. Locally acting toxins in snake venom predominantly consist of enzymes, such as phospholipase A2, that cause local tissue destruction and can result in pain, swelling, blistering, ecchymosis, and tissue necrosis at the site of the bite within hours to days after the bite.1 Systemically acting toxins can target a wide variety of tissues and cause severe systemic complications including paralysis, rhabdomyolysis secondary to muscle damage, coagulopathy, sepsis, and cardiorespiratory failure.2
Although pain and swelling following snakebites typically resolve by 1 month after envenomation, copperhead snakes—a type of pit viper—may cause residual symptoms of pain and swelling lasting for a year or more.3 Additional cutaneous manifestations of copperhead snakebites include wound infections at the bite site, such as cellulitis and necrotizing fasciitis. More devastating complications that have been described following snake envenomation include tissue injury of an entire extremity and development of compartment syndrome, which requires urgent fasciotomy to prevent potential loss of the affected limb.4
Physicians should be aware of the potential complications of snakebites to properly manage and counsel their patients. We describe a 42-year-old woman with tender, erythematous, subcutaneous nodules persisting for 4 months following a copperhead snakebite. A biopsy confirmed the diagnosis of snakebite-associated erythema nodosum (EN).
Case Report
A 42-year-old woman presented to our clinic with progressive tender, pruritic, deep-seated, erythematous nodules in multiple locations on the legs after sustaining a bite by a copperhead snake on the left foot 4 months prior. The lesions tended to fluctuate in intensity. In the days following the bite, she initially developed painful red bumps on the left foot just proximal to the bite site with associated pain and swelling extending up to just below the left knee. She reported no other notable symptoms such as fever, arthralgia, fatigue, or gastrointestinal tract symptoms. Physical examination revealed bilateral pitting edema, which was worse in the left leg, along with multiple deep, palpable, tender subcutaneous nodules with erythematous surface change (Figure 1).

Workup performed by an outside provider over the previous month included 2 venous duplex ultrasounds of the left leg, which showed no signs of deep vein thrombosis. Additionally, the patient underwent lateral and anteroposterior radiographs of the left foot, tibia, and fibula, which showed no evidence of fracture.
Given the morphology and distribution of the lesions (Figure 2), EN was strongly favored as the cause of the symptoms, and a biopsy confirmed the diagnosis. All immunohistochemical stains including auramine-rhodamine for acid-fast bacilli, Grocott-Gomori methenamine silver for fungal organisms, and Brown and Brenn were negative. Given the waxing and waning course of the lesions, which suggested an active neutrophilic rather than purely chronic granulomatous phase of EN, the patient was treated with colchicine 0.6 mg twice daily for 1 month.

Causes of EN and Clinical Manifestations
Erythema nodosum is a common form of septal panniculitis that can be precipitated by inflammatory conditions, infection, or medications (commonly oral contraceptive pills) but often is idiopathic.5 The acute phase is neutrophilic, with evolution over time to a granulomatous phase. Common etiologies include sarcoidosis; inflammatory bowel disease; and bacterial or fungal infections such as Streptococcus (especially common in children), histoplasmosis, and coccidioidomycosis. The patient was otherwise healthy and was not taking any medications that are known triggers of EN. A PubMed search of articles indexed for MEDLINE in the English-language literature using the terms copperhead snake bite, erythema nodosum snake, and copperhead snake erythema nodosum revealed no reports of EN following a bite from a copperhead snake; however, in one case, an adder bite led to erysipelas, likely due to disturbed blood and lymphatic flow, which then triggered EN.6 Additionally, EN has been reported as a delayed reaction to jellyfish stings.7
Clinical features of EN include the development of tender, erythematous, subcutaneous nodules and plaques most frequently over the pretibial region. Lesions typically evolve from raised, deep-seated nodules into flat indurated plaques over a span of weeks. Occasionally, there is a slight prodromal phase marked by nonspecific symptoms such as fever and arthralgia lasting for 3 to 6 days. Erythema nodosum typically results in spontaneous resolution after 4 to 8 weeks, and management involves treatment of any underlying condition with symptomatic care. Interestingly, our patient experienced persistent symptoms over the course of 4 months, with development of new nodular lesions throughout this time period. The most frequently used drugs for the management of symptomatic EN include nonsteroidal anti-inflammatory drugs, colchicine, and potassium iodide.8 A characteristic histologic finding of the granulomatous phase is the Miescher radial granuloma, which is a septal collection of histiocytes surrounding a cleft.9
Snakebite Reactions
Snakebites can result in a wide range of local and systemic manifestations, as snake venom may contain 20 or more toxins.10 Local complications of pit viper bites include pain, swelling, and fang marks; when examining fang marks, the presence of 2 distinct puncture wounds often indicates envenomation with a poisonous snake, whereas nonvenomous snakebites often result in smaller puncture wounds arranged in an arc. Following bites, pain can develop immediately and spread proximally up the affected limb, which occurred in our patient in the days following the bite. Intense local reactions can occur, as bites often result in intense edema of the affected limb spreading to the trunk in the days to weeks after the bite, occasionally accompanied by regional lymphadenopathy. Some bites can result in local necrosis and secondary bacterial infection caused by organisms in the oral cavity of the culprit snake.
Although they were not present in our patient, snakebites can result in a wide range of systemic toxicities ranging from clotting defects and hemolysis to neurotoxicity, myotoxicity, and nephrotoxicity.10 In severe cases, snake venom can result in disseminated intravascular coagulation, sepsis, and cardiorespiratory collapse.
The eastern copperhead (Agkistrodon contortrix) is a species of venomous snake that is endemic to eastern North America. Copperheads are members of the subfamily Crotalinae in the family Viperidae.11 Reported reactions to copperhead bites include cellulitis, necrotizing fasciitis, compartment syndrome, and tissue necrosis of an entire affected extremity.12,13 Our patient displayed no systemic symptoms to suggest envenomation.
Management of Snakebites
Treatment of snakebites varies based on the constellation and severity of symptoms as well as how recently the envenomation occurred. In urgent cases, antivenom may be administered to prevent further toxicity. In cases of progressive compartment syndrome, emergent surgical procedures such as fasciotomy or amputation are required to prevent further complications. When a superimposed bacterial infection is suspected, broad-spectrum antibiotics are required. Because our patient presented 4 months following the initial bite with isolated cutaneous manifestations, she was treated symptomatically with colchicine for EN.1,2
Final Thoughts
Our patient presented with EN following a bite from a copperhead snake. Physicians should be aware of possible etiologies of EN to evaluate patients who present with new-onset tender subcutaneous nodules. Additionally, physicians should be aware of venomous snakes endemic to their region and also understand the various complications that can result following a snakebite, with the potential for lingering cutaneous manifestations weeks to months following the initial bite.
- Warrell DA. Snake bite. Lancet. 2010;375:77-88. doi:10.1016/S0140-6736(09)61754-2
- White J. Overview of venomous snakes of the world. In: Dart RC, eds. Medical Toxicology. 3rd ed. Lippincott, Williams, & Wilkins; 2004:1543
- Spiller HA, Bosse GM. Prospective study of morbidity associated with snakebite envenomation. J Toxicol Clin Toxicol. 2003;41:125-130. doi:10.1081/clt-120019127
- Scharman EJ, Noffsinger VD. Copperhead snakebites: clinical severity of local effects. Ann Emerg Med. 2001;38:55-61. doi:10.1067/mem.2001.116148
- Hafsi W, Badri T. Erythema nodosum. In: StatPearls. StatPearls Publishing; November 28, 2022. Accessed July 22, 2024. https://www.ncbi.nlm.nih.gov/books/NBK470369/
- Nowowiejska J, Baran A, Flisiak I. Rare coexistence of unilateral erythema nodosum with erysipelas in the area of previous adder bite. Przegl Epidemiol. 2020;74:355-361. doi:10.32394/pe.74.28
- Auerbach PS, Hays JT. Erythema nodosum following a jellyfish sting. J Emerg Med. 1987;5:487-491. doi:10.1016/0736-4679(87)90211-3
- Gilchrist H, Patterson JW. Erythema nodosum and erythema induratum (nodular vasculitis): diagnosis and management. Dermatol Ther. 2010;23:320-327. doi:10.1111/j.1529-8019.2010.01332.x
- Sánchez Yus E, Sanz Vico MD, de Diego V. Miescher’s radial granuloma. a characteristic marker of erythema nodosum. Am J Dermatopathol. 1989;11:434-442. doi:10.1097/00000372-198910000-00005
- Mehta SR, Sashindran VK. Clinical features and management of snake bite. Med J Armed Forces India. 2002;58:247-249. doi:10.1016/S0377-1237(02)80140-X
- Brys AK, Gandolfi BM, Levinson H, et al. Copperhead envenomation resulting in a rare case of hand compartment syndrome and subsequent fasciotomy. Plast Reconstr Surg Glob Open. 2015;3:E396. doi:10.1097/GOX.0000000000000367
- Clark RF, Selden BS, Furbee B. The incidence of wound infection following crotalid envenomation. J Emerg Med. 1993;11:583-586. doi:10.1016/0736-4679(93)90313-v
- Buchanan JT, Thurman J. Crotalidae envenomation. In: StatPearls. StatPearls Publishing; October 3, 2022. Accessed July 22, 2024. https://www.ncbi.nlm.nih.gov/books/NBK551615/
- Warrell DA. Snake bite. Lancet. 2010;375:77-88. doi:10.1016/S0140-6736(09)61754-2
- White J. Overview of venomous snakes of the world. In: Dart RC, eds. Medical Toxicology. 3rd ed. Lippincott, Williams, & Wilkins; 2004:1543
- Spiller HA, Bosse GM. Prospective study of morbidity associated with snakebite envenomation. J Toxicol Clin Toxicol. 2003;41:125-130. doi:10.1081/clt-120019127
- Scharman EJ, Noffsinger VD. Copperhead snakebites: clinical severity of local effects. Ann Emerg Med. 2001;38:55-61. doi:10.1067/mem.2001.116148
- Hafsi W, Badri T. Erythema nodosum. In: StatPearls. StatPearls Publishing; November 28, 2022. Accessed July 22, 2024. https://www.ncbi.nlm.nih.gov/books/NBK470369/
- Nowowiejska J, Baran A, Flisiak I. Rare coexistence of unilateral erythema nodosum with erysipelas in the area of previous adder bite. Przegl Epidemiol. 2020;74:355-361. doi:10.32394/pe.74.28
- Auerbach PS, Hays JT. Erythema nodosum following a jellyfish sting. J Emerg Med. 1987;5:487-491. doi:10.1016/0736-4679(87)90211-3
- Gilchrist H, Patterson JW. Erythema nodosum and erythema induratum (nodular vasculitis): diagnosis and management. Dermatol Ther. 2010;23:320-327. doi:10.1111/j.1529-8019.2010.01332.x
- Sánchez Yus E, Sanz Vico MD, de Diego V. Miescher’s radial granuloma. a characteristic marker of erythema nodosum. Am J Dermatopathol. 1989;11:434-442. doi:10.1097/00000372-198910000-00005
- Mehta SR, Sashindran VK. Clinical features and management of snake bite. Med J Armed Forces India. 2002;58:247-249. doi:10.1016/S0377-1237(02)80140-X
- Brys AK, Gandolfi BM, Levinson H, et al. Copperhead envenomation resulting in a rare case of hand compartment syndrome and subsequent fasciotomy. Plast Reconstr Surg Glob Open. 2015;3:E396. doi:10.1097/GOX.0000000000000367
- Clark RF, Selden BS, Furbee B. The incidence of wound infection following crotalid envenomation. J Emerg Med. 1993;11:583-586. doi:10.1016/0736-4679(93)90313-v
- Buchanan JT, Thurman J. Crotalidae envenomation. In: StatPearls. StatPearls Publishing; October 3, 2022. Accessed July 22, 2024. https://www.ncbi.nlm.nih.gov/books/NBK551615/
Practice Points
- Erythema nodosum (EN) can occur following snakebites from pit vipers such as the eastern copperhead.
- The acute phase of EN is neutrophilic and responds to colchicine. The chronic phase of EN is granulomatous and responds best to rest and elevation as well as nonsteroidal anti-inflammatory drugs and iodides.
Brazilian Peppertree: Watch Out for This Lesser-Known Relative of Poison Ivy
Brazilian peppertree (Schinus terebinthifolia), a member of the Anacardiaceae family, is an internationally invasive plant that causes allergic contact dermatitis (ACD) in susceptible individuals. This noxious weed has settled into the landscape of the southern United States and continues to expand. Its key identifying features include its year-round white flowers as well as a peppery and turpentinelike aroma created by cracking its bright red berries. The ACD associated with contact—primarily with the plant’s sap—stems from known alkenyl phenols, cardol and cardanol. Treatment of Brazilian peppertree–associated ACD parallels that for poison ivy. As this pest increases its range, dermatologists living in endemic areas should familiarize themselves with Brazilian peppertree and its potential for harm.
Brazilian Peppertree Morphology and Geography
Plants in the Anacardiaceae family contribute to more ACD than any other family, and its 80 genera include most of the urushiol-containing plants, such as Toxicodendron (poison ivy, poison oak, poison sumac, Japanese lacquer tree), Anacardium (cashew tree), Mangifera (mango fruit), Semecarpus (India marking nut tree), and Schinus (Brazilian peppertree). Deciduous and evergreen tree members of the Anacardiaceae family grow primarily in tropical and subtropical locations and produce thick resins, 5-petalled flowers, and small fruit known as drupes. The genus name for Brazilian peppertree, Schinus, derives from Latin and Greek words meaning “mastic tree,” a relative of the pistachio tree that the Brazilian peppertree resembles.1 Brazilian peppertree leaves look and smell similar to Pistacia terebinthus (turpentine tree or terebinth), from which the species name terebinthifolia derives.2
Brazilian peppertree originated in South America, particularly Brazil, Paraguay, and Argentina.3 Since the 1840s,4 it has been an invasive weed in the United States, notably in Florida, California, Hawaii, Alabama, Georgia,5 Arizona,6 Nevada,3 and Texas.5,7 The plant also grows throughout the world, including parts of Africa, Asia, Central America, Europe,6 New Zealand,8 Australia, and various islands.9 The plant expertly outcompetes neighboring plants and has prompted control and eradication efforts in many locations.3
Identifying Features and Allergenic Plant Parts
Brazilian peppertree can be either a shrub or tree up to 30 feet tall.4 As an evergreen, it retains its leaves year-round. During fruiting seasons (primarily December through March7), bright red or pink (depending on the variety3) berries appear (Figure 1A) and contribute to its nickname “Florida holly.” Although generally considered an unwelcome guest in Florida, it does display white flowers (Figure 1B) year-round, especially from September to November.9 It characteristically exhibits 3 to 13 leaflets per leaf.10 The leaflets’ ovoid and ridged edges, netlike vasculature, shiny hue, and aroma can help identify the plant (Figure 2A). For decades, the sap of the Brazilian peppertree has been associated with skin irritation (Figure 2B).6 Although the sap of the plant serves as the main culprit of Brazilian peppertree–associated ACD, it appears that other parts of the plant, including the fruit, can cause irritating effects to skin on contact.11,12 The leaves, trunk, and fruit can be harmful to both humans and animals.6 Chemicals from flowers and crushed fruit also can lead to irritating effects in the respiratory tract if aspirated.13


Urushiol, an oily resin present in most plants of the Anacardiaceae family,14 contains many chemicals, including allergenic phenols, catechols, and resorcinols.15 Urushiol-allergic individuals develop dermatitis upon exposure to Brazilian peppertree sap.6 Alkenyl phenols found in Brazilian peppertree lead to the cutaneous manifestations in sensitized patients.11,12 In 1983, Stahl et al11 identified a phenol, cardanol (chemical name 3-pentadecylphenol16) C15:1, in Brazilian peppertree fruit. The group further tested this compound’s effect on skin via patch testing, which showed an allergic response.11 Cashew nut shells (Anacardium occidentale) contain cardanol, anacardic acid (a phenolic acid), and cardol (a phenol with the chemical name 5-pentadecylresorcinol),15,16 though Stahl et al11 were unable to extract these 2 substances (if present) from Brazilian peppertree fruit. When exposed to cardol and anacardic acid, those allergic to poison ivy often develop ACD,15 and these 2 substances are more irritating than cardanol.11 A later study did identify cardol in addition to cardanol in Brazilian peppertree.12
Cutaneous Manifestations
Brazilian peppertree–induced ACD appears similar to other plant-induced ACD with linear streaks of erythema, juicy papules, vesicles, coalescing erythematous plaques, and/or occasional edema and bullae accompanied by intense pruritus.
Treatment
Avoiding contact with Brazilian peppertree is the first line of defense, and treatment for a reaction associated with exposure is similar to that of poison ivy.17 Application of cool compresses, calamine lotion, and topical astringents offer symptom alleviation, and topical steroids (eg, clobetasol propionate 0.05% twice daily) can improve mild localized ACD when given prior to formation of blisters. For more severe and diffuse ACD, oral steroids (eg, prednisone 1 mg/kg/d tapered over 2–3 weeks) likely are necessary, though intramuscular options greatly alleviate discomfort in more severe cases (eg, intramuscular triamcinolone acetonide 1 mg/kg combined with betamethasone 0.1 mg/kg). Physicians should monitor sites for any signs of superimposed bacterial infection and initiate antibiotics as necessary.17
- Zona S. The correct gender of Schinus (Anacardiaceae). Phytotaxa. 2015;222:075-077.
- Terebinth. Encyclopedia.com website. Updated May 17, 2018. Accessed July 9, 2024. https://www.encyclopedia.com/plants-and-animals/plants/plants/terebinth
- Brazilian pepper tree. iNaturalist website. Accessed July 1, 2024. https://www.inaturalist.org/guide_taxa/841531#:~:text=Throughout% 20South%20and%20Central%20America,and%20as%20a%20topical%20antiseptic
- Center for Aquatic and Invasive Plants. Schinus terebinthifolia. Brazilian peppertree. Accessed July 1, 2024. https://plants.ifas.ufl.edu/plant-directory/schinus-terebinthifolia/#:~:text=Species%20Overview&text=People%20sensitive%20to%20poison%20ivy,associated%20with%20its%20bloom%20period
- Brazilian peppertree (Schinus terebinthifolia). Early Detection & Distribution Mapping System. Accessed July 4, 2024. https://www.eddmaps.org/distribution/usstate.cfm?sub=78819
- Morton F. Brazilian pepper: its impact on people, animals, and the environment. Econ Bot. 1978;32:353-359.
- Fire Effects Information System. Schinus terebinthifolius. US Department of Agriculture website. Accessed July 4, 2024. https://www.fs.usda.gov/database/feis/plants/shrub/schter/all.html
- New Zealand Plant Conservation Network. Schinus terebinthifolius. Accessed July 1, 2024. https://www.nzpcn.org.nz/flora/species/schinus-terebinthifolius
- Rojas-Sandoval J, Acevedo-Rodriguez P. Schinus terebinthifolius (Brazilian pepper tree). CABI Compendium. July 23, 2014. Accessed July 1, 2024. https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.49031
- Patocka J, Diz de Almeida J. Brazilian peppertree: review of pharmacology. Mil Med Sci Lett. 2017;86:32-41.
- Stahl E, Keller K, Blinn C. Cardanol, a skin irritant in pink pepper. Plant Medica. 1983;48:5-9.
- Skopp G, Opferkuch H-J, Schqenker G. n-Alkylphenols from Schinus terebinthifolius Raddi (Anacardiaceae). In German. Zeitschrift für Naturforschung C. 1987;42:1-16. https://doi.org/10.1515/znc-1987-1-203.
- Lloyd HA, Jaouni TM, Evans SL, et al. Terpenes of Schinus terebinthifolius. Phytochemistry. 1977;16:1301-1302.
- Goon ATJ, Goh CL. Plant dermatitis: Asian perspective. Indian J Dermatol. 2011;56:707-710.
- Rozas-Muñoz E, Lepoittevin JP, Pujol RM, et al. Allergic contact dermatitis to plants: understanding the chemistry will help our diagnostic approach. Actas Dermosifiliogr. 2012;103:456-477.
- Caillol S. Cardanol: a promising building block for biobased polymers and additives. Curr Opin Green Sustain Chem. 2018;14: 26-32.
- Prok L, McGovern T. Poison ivy (Toxicodendron) dermatitis. UpToDate. Updated June 21, 2024. Accessed July 7, 2024. https://www.uptodate.com/contents/poison-ivy-toxicodendron-dermatitis#
Brazilian peppertree (Schinus terebinthifolia), a member of the Anacardiaceae family, is an internationally invasive plant that causes allergic contact dermatitis (ACD) in susceptible individuals. This noxious weed has settled into the landscape of the southern United States and continues to expand. Its key identifying features include its year-round white flowers as well as a peppery and turpentinelike aroma created by cracking its bright red berries. The ACD associated with contact—primarily with the plant’s sap—stems from known alkenyl phenols, cardol and cardanol. Treatment of Brazilian peppertree–associated ACD parallels that for poison ivy. As this pest increases its range, dermatologists living in endemic areas should familiarize themselves with Brazilian peppertree and its potential for harm.
Brazilian Peppertree Morphology and Geography
Plants in the Anacardiaceae family contribute to more ACD than any other family, and its 80 genera include most of the urushiol-containing plants, such as Toxicodendron (poison ivy, poison oak, poison sumac, Japanese lacquer tree), Anacardium (cashew tree), Mangifera (mango fruit), Semecarpus (India marking nut tree), and Schinus (Brazilian peppertree). Deciduous and evergreen tree members of the Anacardiaceae family grow primarily in tropical and subtropical locations and produce thick resins, 5-petalled flowers, and small fruit known as drupes. The genus name for Brazilian peppertree, Schinus, derives from Latin and Greek words meaning “mastic tree,” a relative of the pistachio tree that the Brazilian peppertree resembles.1 Brazilian peppertree leaves look and smell similar to Pistacia terebinthus (turpentine tree or terebinth), from which the species name terebinthifolia derives.2
Brazilian peppertree originated in South America, particularly Brazil, Paraguay, and Argentina.3 Since the 1840s,4 it has been an invasive weed in the United States, notably in Florida, California, Hawaii, Alabama, Georgia,5 Arizona,6 Nevada,3 and Texas.5,7 The plant also grows throughout the world, including parts of Africa, Asia, Central America, Europe,6 New Zealand,8 Australia, and various islands.9 The plant expertly outcompetes neighboring plants and has prompted control and eradication efforts in many locations.3
Identifying Features and Allergenic Plant Parts
Brazilian peppertree can be either a shrub or tree up to 30 feet tall.4 As an evergreen, it retains its leaves year-round. During fruiting seasons (primarily December through March7), bright red or pink (depending on the variety3) berries appear (Figure 1A) and contribute to its nickname “Florida holly.” Although generally considered an unwelcome guest in Florida, it does display white flowers (Figure 1B) year-round, especially from September to November.9 It characteristically exhibits 3 to 13 leaflets per leaf.10 The leaflets’ ovoid and ridged edges, netlike vasculature, shiny hue, and aroma can help identify the plant (Figure 2A). For decades, the sap of the Brazilian peppertree has been associated with skin irritation (Figure 2B).6 Although the sap of the plant serves as the main culprit of Brazilian peppertree–associated ACD, it appears that other parts of the plant, including the fruit, can cause irritating effects to skin on contact.11,12 The leaves, trunk, and fruit can be harmful to both humans and animals.6 Chemicals from flowers and crushed fruit also can lead to irritating effects in the respiratory tract if aspirated.13


Urushiol, an oily resin present in most plants of the Anacardiaceae family,14 contains many chemicals, including allergenic phenols, catechols, and resorcinols.15 Urushiol-allergic individuals develop dermatitis upon exposure to Brazilian peppertree sap.6 Alkenyl phenols found in Brazilian peppertree lead to the cutaneous manifestations in sensitized patients.11,12 In 1983, Stahl et al11 identified a phenol, cardanol (chemical name 3-pentadecylphenol16) C15:1, in Brazilian peppertree fruit. The group further tested this compound’s effect on skin via patch testing, which showed an allergic response.11 Cashew nut shells (Anacardium occidentale) contain cardanol, anacardic acid (a phenolic acid), and cardol (a phenol with the chemical name 5-pentadecylresorcinol),15,16 though Stahl et al11 were unable to extract these 2 substances (if present) from Brazilian peppertree fruit. When exposed to cardol and anacardic acid, those allergic to poison ivy often develop ACD,15 and these 2 substances are more irritating than cardanol.11 A later study did identify cardol in addition to cardanol in Brazilian peppertree.12
Cutaneous Manifestations
Brazilian peppertree–induced ACD appears similar to other plant-induced ACD with linear streaks of erythema, juicy papules, vesicles, coalescing erythematous plaques, and/or occasional edema and bullae accompanied by intense pruritus.
Treatment
Avoiding contact with Brazilian peppertree is the first line of defense, and treatment for a reaction associated with exposure is similar to that of poison ivy.17 Application of cool compresses, calamine lotion, and topical astringents offer symptom alleviation, and topical steroids (eg, clobetasol propionate 0.05% twice daily) can improve mild localized ACD when given prior to formation of blisters. For more severe and diffuse ACD, oral steroids (eg, prednisone 1 mg/kg/d tapered over 2–3 weeks) likely are necessary, though intramuscular options greatly alleviate discomfort in more severe cases (eg, intramuscular triamcinolone acetonide 1 mg/kg combined with betamethasone 0.1 mg/kg). Physicians should monitor sites for any signs of superimposed bacterial infection and initiate antibiotics as necessary.17
Brazilian peppertree (Schinus terebinthifolia), a member of the Anacardiaceae family, is an internationally invasive plant that causes allergic contact dermatitis (ACD) in susceptible individuals. This noxious weed has settled into the landscape of the southern United States and continues to expand. Its key identifying features include its year-round white flowers as well as a peppery and turpentinelike aroma created by cracking its bright red berries. The ACD associated with contact—primarily with the plant’s sap—stems from known alkenyl phenols, cardol and cardanol. Treatment of Brazilian peppertree–associated ACD parallels that for poison ivy. As this pest increases its range, dermatologists living in endemic areas should familiarize themselves with Brazilian peppertree and its potential for harm.
Brazilian Peppertree Morphology and Geography
Plants in the Anacardiaceae family contribute to more ACD than any other family, and its 80 genera include most of the urushiol-containing plants, such as Toxicodendron (poison ivy, poison oak, poison sumac, Japanese lacquer tree), Anacardium (cashew tree), Mangifera (mango fruit), Semecarpus (India marking nut tree), and Schinus (Brazilian peppertree). Deciduous and evergreen tree members of the Anacardiaceae family grow primarily in tropical and subtropical locations and produce thick resins, 5-petalled flowers, and small fruit known as drupes. The genus name for Brazilian peppertree, Schinus, derives from Latin and Greek words meaning “mastic tree,” a relative of the pistachio tree that the Brazilian peppertree resembles.1 Brazilian peppertree leaves look and smell similar to Pistacia terebinthus (turpentine tree or terebinth), from which the species name terebinthifolia derives.2
Brazilian peppertree originated in South America, particularly Brazil, Paraguay, and Argentina.3 Since the 1840s,4 it has been an invasive weed in the United States, notably in Florida, California, Hawaii, Alabama, Georgia,5 Arizona,6 Nevada,3 and Texas.5,7 The plant also grows throughout the world, including parts of Africa, Asia, Central America, Europe,6 New Zealand,8 Australia, and various islands.9 The plant expertly outcompetes neighboring plants and has prompted control and eradication efforts in many locations.3
Identifying Features and Allergenic Plant Parts
Brazilian peppertree can be either a shrub or tree up to 30 feet tall.4 As an evergreen, it retains its leaves year-round. During fruiting seasons (primarily December through March7), bright red or pink (depending on the variety3) berries appear (Figure 1A) and contribute to its nickname “Florida holly.” Although generally considered an unwelcome guest in Florida, it does display white flowers (Figure 1B) year-round, especially from September to November.9 It characteristically exhibits 3 to 13 leaflets per leaf.10 The leaflets’ ovoid and ridged edges, netlike vasculature, shiny hue, and aroma can help identify the plant (Figure 2A). For decades, the sap of the Brazilian peppertree has been associated with skin irritation (Figure 2B).6 Although the sap of the plant serves as the main culprit of Brazilian peppertree–associated ACD, it appears that other parts of the plant, including the fruit, can cause irritating effects to skin on contact.11,12 The leaves, trunk, and fruit can be harmful to both humans and animals.6 Chemicals from flowers and crushed fruit also can lead to irritating effects in the respiratory tract if aspirated.13


Urushiol, an oily resin present in most plants of the Anacardiaceae family,14 contains many chemicals, including allergenic phenols, catechols, and resorcinols.15 Urushiol-allergic individuals develop dermatitis upon exposure to Brazilian peppertree sap.6 Alkenyl phenols found in Brazilian peppertree lead to the cutaneous manifestations in sensitized patients.11,12 In 1983, Stahl et al11 identified a phenol, cardanol (chemical name 3-pentadecylphenol16) C15:1, in Brazilian peppertree fruit. The group further tested this compound’s effect on skin via patch testing, which showed an allergic response.11 Cashew nut shells (Anacardium occidentale) contain cardanol, anacardic acid (a phenolic acid), and cardol (a phenol with the chemical name 5-pentadecylresorcinol),15,16 though Stahl et al11 were unable to extract these 2 substances (if present) from Brazilian peppertree fruit. When exposed to cardol and anacardic acid, those allergic to poison ivy often develop ACD,15 and these 2 substances are more irritating than cardanol.11 A later study did identify cardol in addition to cardanol in Brazilian peppertree.12
Cutaneous Manifestations
Brazilian peppertree–induced ACD appears similar to other plant-induced ACD with linear streaks of erythema, juicy papules, vesicles, coalescing erythematous plaques, and/or occasional edema and bullae accompanied by intense pruritus.
Treatment
Avoiding contact with Brazilian peppertree is the first line of defense, and treatment for a reaction associated with exposure is similar to that of poison ivy.17 Application of cool compresses, calamine lotion, and topical astringents offer symptom alleviation, and topical steroids (eg, clobetasol propionate 0.05% twice daily) can improve mild localized ACD when given prior to formation of blisters. For more severe and diffuse ACD, oral steroids (eg, prednisone 1 mg/kg/d tapered over 2–3 weeks) likely are necessary, though intramuscular options greatly alleviate discomfort in more severe cases (eg, intramuscular triamcinolone acetonide 1 mg/kg combined with betamethasone 0.1 mg/kg). Physicians should monitor sites for any signs of superimposed bacterial infection and initiate antibiotics as necessary.17
- Zona S. The correct gender of Schinus (Anacardiaceae). Phytotaxa. 2015;222:075-077.
- Terebinth. Encyclopedia.com website. Updated May 17, 2018. Accessed July 9, 2024. https://www.encyclopedia.com/plants-and-animals/plants/plants/terebinth
- Brazilian pepper tree. iNaturalist website. Accessed July 1, 2024. https://www.inaturalist.org/guide_taxa/841531#:~:text=Throughout% 20South%20and%20Central%20America,and%20as%20a%20topical%20antiseptic
- Center for Aquatic and Invasive Plants. Schinus terebinthifolia. Brazilian peppertree. Accessed July 1, 2024. https://plants.ifas.ufl.edu/plant-directory/schinus-terebinthifolia/#:~:text=Species%20Overview&text=People%20sensitive%20to%20poison%20ivy,associated%20with%20its%20bloom%20period
- Brazilian peppertree (Schinus terebinthifolia). Early Detection & Distribution Mapping System. Accessed July 4, 2024. https://www.eddmaps.org/distribution/usstate.cfm?sub=78819
- Morton F. Brazilian pepper: its impact on people, animals, and the environment. Econ Bot. 1978;32:353-359.
- Fire Effects Information System. Schinus terebinthifolius. US Department of Agriculture website. Accessed July 4, 2024. https://www.fs.usda.gov/database/feis/plants/shrub/schter/all.html
- New Zealand Plant Conservation Network. Schinus terebinthifolius. Accessed July 1, 2024. https://www.nzpcn.org.nz/flora/species/schinus-terebinthifolius
- Rojas-Sandoval J, Acevedo-Rodriguez P. Schinus terebinthifolius (Brazilian pepper tree). CABI Compendium. July 23, 2014. Accessed July 1, 2024. https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.49031
- Patocka J, Diz de Almeida J. Brazilian peppertree: review of pharmacology. Mil Med Sci Lett. 2017;86:32-41.
- Stahl E, Keller K, Blinn C. Cardanol, a skin irritant in pink pepper. Plant Medica. 1983;48:5-9.
- Skopp G, Opferkuch H-J, Schqenker G. n-Alkylphenols from Schinus terebinthifolius Raddi (Anacardiaceae). In German. Zeitschrift für Naturforschung C. 1987;42:1-16. https://doi.org/10.1515/znc-1987-1-203.
- Lloyd HA, Jaouni TM, Evans SL, et al. Terpenes of Schinus terebinthifolius. Phytochemistry. 1977;16:1301-1302.
- Goon ATJ, Goh CL. Plant dermatitis: Asian perspective. Indian J Dermatol. 2011;56:707-710.
- Rozas-Muñoz E, Lepoittevin JP, Pujol RM, et al. Allergic contact dermatitis to plants: understanding the chemistry will help our diagnostic approach. Actas Dermosifiliogr. 2012;103:456-477.
- Caillol S. Cardanol: a promising building block for biobased polymers and additives. Curr Opin Green Sustain Chem. 2018;14: 26-32.
- Prok L, McGovern T. Poison ivy (Toxicodendron) dermatitis. UpToDate. Updated June 21, 2024. Accessed July 7, 2024. https://www.uptodate.com/contents/poison-ivy-toxicodendron-dermatitis#
- Zona S. The correct gender of Schinus (Anacardiaceae). Phytotaxa. 2015;222:075-077.
- Terebinth. Encyclopedia.com website. Updated May 17, 2018. Accessed July 9, 2024. https://www.encyclopedia.com/plants-and-animals/plants/plants/terebinth
- Brazilian pepper tree. iNaturalist website. Accessed July 1, 2024. https://www.inaturalist.org/guide_taxa/841531#:~:text=Throughout% 20South%20and%20Central%20America,and%20as%20a%20topical%20antiseptic
- Center for Aquatic and Invasive Plants. Schinus terebinthifolia. Brazilian peppertree. Accessed July 1, 2024. https://plants.ifas.ufl.edu/plant-directory/schinus-terebinthifolia/#:~:text=Species%20Overview&text=People%20sensitive%20to%20poison%20ivy,associated%20with%20its%20bloom%20period
- Brazilian peppertree (Schinus terebinthifolia). Early Detection & Distribution Mapping System. Accessed July 4, 2024. https://www.eddmaps.org/distribution/usstate.cfm?sub=78819
- Morton F. Brazilian pepper: its impact on people, animals, and the environment. Econ Bot. 1978;32:353-359.
- Fire Effects Information System. Schinus terebinthifolius. US Department of Agriculture website. Accessed July 4, 2024. https://www.fs.usda.gov/database/feis/plants/shrub/schter/all.html
- New Zealand Plant Conservation Network. Schinus terebinthifolius. Accessed July 1, 2024. https://www.nzpcn.org.nz/flora/species/schinus-terebinthifolius
- Rojas-Sandoval J, Acevedo-Rodriguez P. Schinus terebinthifolius (Brazilian pepper tree). CABI Compendium. July 23, 2014. Accessed July 1, 2024. https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.49031
- Patocka J, Diz de Almeida J. Brazilian peppertree: review of pharmacology. Mil Med Sci Lett. 2017;86:32-41.
- Stahl E, Keller K, Blinn C. Cardanol, a skin irritant in pink pepper. Plant Medica. 1983;48:5-9.
- Skopp G, Opferkuch H-J, Schqenker G. n-Alkylphenols from Schinus terebinthifolius Raddi (Anacardiaceae). In German. Zeitschrift für Naturforschung C. 1987;42:1-16. https://doi.org/10.1515/znc-1987-1-203.
- Lloyd HA, Jaouni TM, Evans SL, et al. Terpenes of Schinus terebinthifolius. Phytochemistry. 1977;16:1301-1302.
- Goon ATJ, Goh CL. Plant dermatitis: Asian perspective. Indian J Dermatol. 2011;56:707-710.
- Rozas-Muñoz E, Lepoittevin JP, Pujol RM, et al. Allergic contact dermatitis to plants: understanding the chemistry will help our diagnostic approach. Actas Dermosifiliogr. 2012;103:456-477.
- Caillol S. Cardanol: a promising building block for biobased polymers and additives. Curr Opin Green Sustain Chem. 2018;14: 26-32.
- Prok L, McGovern T. Poison ivy (Toxicodendron) dermatitis. UpToDate. Updated June 21, 2024. Accessed July 7, 2024. https://www.uptodate.com/contents/poison-ivy-toxicodendron-dermatitis#
Practice Points
- The Anacardiaceae family contains several plants, including Brazilian peppertree and poison ivy, that have the potential to cause allergic contact dermatitis (ACD).
- Hot spots for Brazilian peppertree include Florida and California, though it also has been reported in Texas, Hawaii, Georgia, Alabama, Arkansas, Nevada, and Arizona.
- Alkenyl phenols (eg, cardol, cardanol) are the key sensitizers found in Brazilian peppertree.
- Treatment consists of supportive care and either topical, oral, or intramuscular steroids depending on the extent and severity of the ACD.
Aquatic Antagonists: Dermatologic Injuries From Sea Urchins (Echinoidea)
Sea urchins—members of the phylum Echinodermata and the class Echinoidea—are spiny marine invertebrates. Their consumption of fleshy algae makes them essential players in maintaining reef ecosystems.1,2 Echinoids, a class that includes heart urchins and sand dollars, are ubiquitous in benthic marine environments, both free floating and rock boring, and inhabit a wide range of latitudes spanning from polar oceans to warm seas.3 Despite their immobility and nonaggression, sea urchin puncture wounds are common among divers, snorkelers, swimmers, surfers, and fishers who accidentally come into contact with their sharp spines. Although the epidemiology of sea urchin exposure and injury is difficult to assess, the American Association of Poison Control Centers’ most recent annual report in 2022 documents approximately 1426 annual aquatic bites and/or envenomations.4
Sea Urchin Morphology and Toxicity
Echinoderms (a term of Greek origin meaning spiny skin) share a radially symmetric calcium carbonate skeleton (termed stereom) that is supported by collagenous ligaments.1 Sea urchins possess spines composed of calcite crystals, which radiate from their body and play a role in locomotion and defense against predators—namely sea otters, starfish/sea stars, wolf eels, and triggerfish, among others (Figure).5 These brittle spines can easily penetrate human skin and subsequently break off the sea urchin body. Most species of sea urchins possess solid spines, but a small percentage (80 of approximately 700 extant species) have hollow spines containing various toxic substances.6 Penetration and systemic absorption of the toxins within these spines can generate severe systemic responses.
The venomous flower urchin (Toxopneustes pileolus), found in the Indian and Pacific oceans, is one of the more common species known to produce a systemic reaction involving neuromuscular blockage.7-9 The most common species harvested off the Pacific coast of the United States—Strongylocentrotus purpuratus (purple sea urchin) and Strongylocentrotus franciscanus (red sea urchins)—are not inherently venomous.8

Both the sea urchin body and spines are covered in a unique epithelium thought to be responsible for the majority of their proinflammatory and pronociceptive properties. Epithelial compounds identified include serotonin, histamines, steroids, glycosides, hemolysins, proteases, and bradykininlike and cholinergic substances.5,7 Additionally, certain sea urchin species possess 3-pronged pincerlike organs at the base of spines called pedicellariae, which are used in feeding.10 Skin penetration by the pedicellariae is especially dangerous, as they tightly adhere to wounds and contain venom-producing organs that allow them to continue injecting toxins after their detachment from the sea urchin body.11
Presentation and Diagnosis of Sea Urchin Injuries
Sea urchin injuries have a wide range of manifestations depending on the number of spines involved, the presence of venom, the depth and location of spine penetration, the duration of spine retention in the skin, and the time before treatment initiation. The most common site of sea urchin injury unsurprisingly is the lower extremities and feet, often in the context of divers and swimmers walking across the sea floor. The hands are another frequently injured site, along with the legs, arms, back, scalp, and even oral mucosa.11
Although clinical history and presentation frequently reveal the mechanism of aquatic injury, patients often are unsure of the agent to which they were exposed and may be unaware of retained foreign bodies. Dermoscopy can distinguish the distinct lines radiating from the core of sea urchin spines from other foreign bodies lodged within the skin.6 It also can be used to locate spines for removal or for their analysis following punch biopsy.6,12 The radiopaque nature of sea urchin spines makes radiography and magnetic resonance imaging useful tools in assessment of periarticular soft-tissue damage and spine removal.8,11,13 Ultrasonography can reveal spines that no longer appear on radiography due to absorption by human tissue.14
Immediate Dermatologic Effects
Sea urchin injuries can be broadly categorized into immediate and delayed reactions. Immediate manifestations of contact with sea urchin spines include localized pain, bleeding, erythema, myalgia, and edema at the site of injury that can last from a few hours to 1 week without proper wound care and spine removal.5 Systemic symptoms ranging from dizziness, lightheadedness, paresthesia, aphonia, paralysis, coma, and death generally are only seen following injuries from venomous species, attachment of pedicellariae, injuries involving neurovascular structures, or penetration by more than 15 spines.7,11
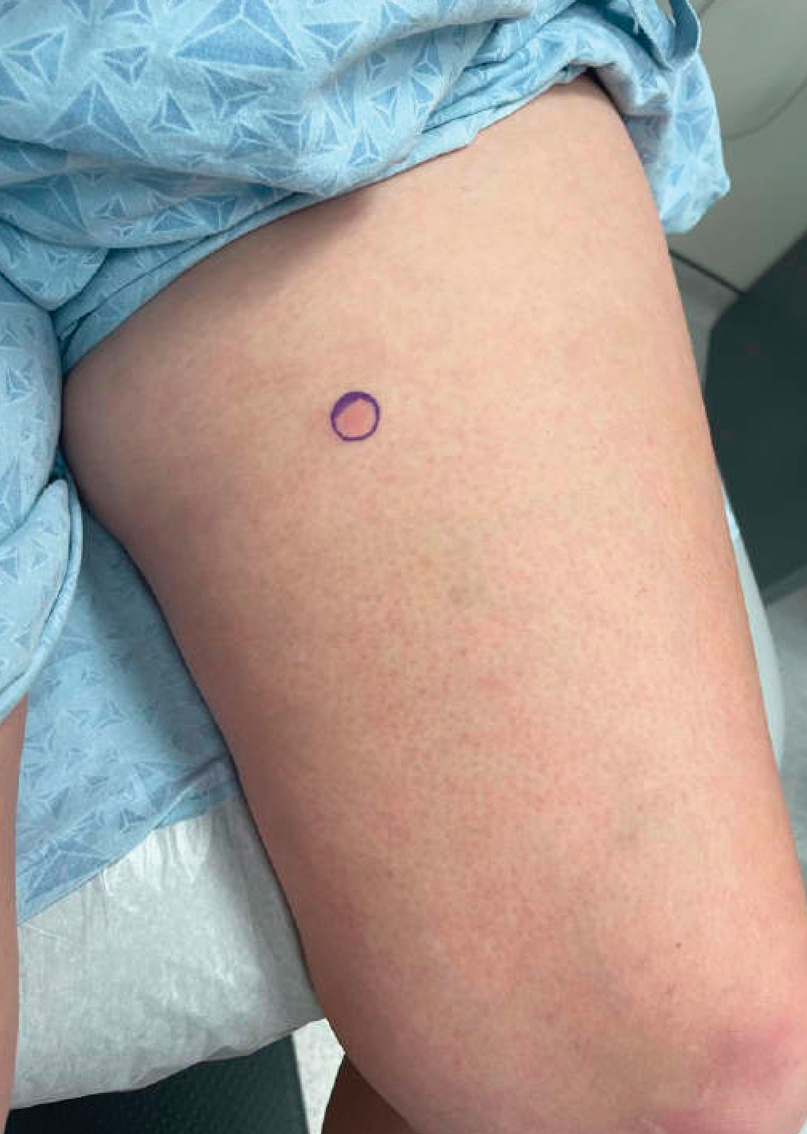
Initial treatment includes soaking the wound in hot water (113 °F [45 °C]) for 30 to 90 minutes and subsequently removing spines and pedicellariae to prevent development of delayed reactions.5,15,16 The compounds in the sea urchin epithelium are heat labile and will be inactivated upon soaking in hot water.16 Extraction of spines can be difficult, as they are brittle and easily break in the skin. Successful removal has been reported using forceps and a hypodermic needle as well as excision; both approaches may require local anesthesia.8,17 Another technique involves freezing the localized area with liquid nitrogen to allow easier removal upon skin blistering.18 Punch biopsy also has been utilized as an effective means of ensuring all spiny fragments are removed.9,19,20 These spines often cause black or purple tattoolike staining at the puncture site, which can persist for a few days after spine extraction.8 Ablation using the erbium-doped:YAG laser may be helpful for removal of associated pigment.21,22
Delayed Dermatologic Effects
Delayed reactions to sea urchin injuries often are attributable to prolonged retention of spines in the skin. Granulomatous reactions typically manifest 2 weeks after injury as firm nonsuppurative nodules with central umbilication and a hyperkeratotic surface.7 These nodules may or may not be painful. Histopathology most often reveals foreign body and sarcoidal-type granulomatous reactions. However, tuberculoid, necrobiotic, and suppurative granulomas also may develop.13 Other microscopic features include inflammatory reactions, suppurative dermatitis, focal necrosis, and microabscesses.23 Wounds with progression to granulomatous disease often require surgical debridement.
Other more serious sequalae can result from involvement of joint capsules, especially in the hands and feet. Sea urchin injury involving joint spaces should be treated aggressively, as progression to inflammatory or infectious synovitis and tenosynovitis can cause irreversible loss of joint function. Inflammatory synovitis occurs 1 to 2 months on average after injury following a period of minimal symptoms and begins as a gradual increase in joint swelling and decrease in range of motion.8 Infectious tenosynovitis manifests quite similarly. Although suppurative etiologies generally progress with a more acute onset, certain infectious organisms (eg, Mycobacterium) take on an indolent course and should not be overlooked as a cause of delayed symptoms.8 The Kavanel cardinal signs are a sensitive tool used in the diagnosis of infectious flexor sheath tenosynovitis.8,24 If suspicion for joint infection is high, emergency referral should be made for debridement and culture-guided antibiotic therapy. Left untreated, infectious tenosynovitis can result in tendon necrosis or rupture, digit necrosis, and systemic infection.24 Patients with joint involvement should be referred to specialty care (eg, hand surgeon), as they often require synovectomy and surgical removal of foreign material.8
From 1 month to 1 year after injury, prolonged granulomatous synovitis of the hand may eventually lead to joint destruction known as “sea urchin arthritis.” These patients present with decreased range of motion and numerous nodules on the hand with a hyperkeratotic surface. Radiography reveals joint space narrowing, osteolysis, subchondral sclerosis, and periosteal reaction. Synovectomy and debridement are necessary to prevent irreversible joint damage or the need for arthrodesis and bone grafting.24
Other Treatment Considerations
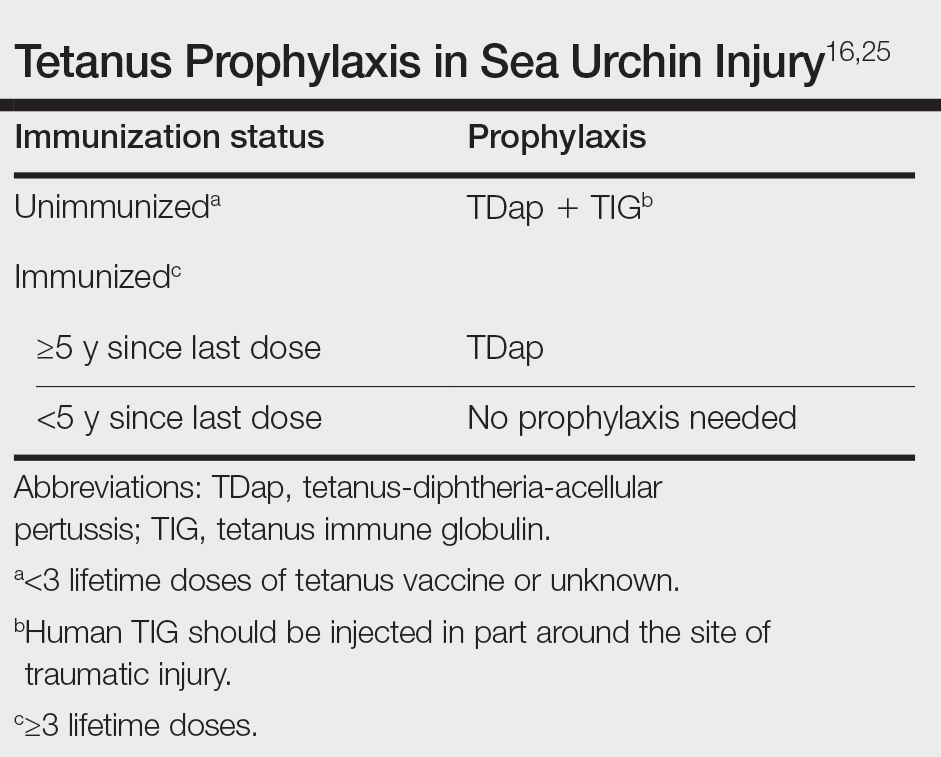
Other important considerations in the care of sea urchin spine injuries include assessment of tetanus immunization status and administration of necessary prophylaxis as soon as possible, even in delayed presentations (Table).16,25 Cultures should be taken only if infection is suspected. Prophylactic antibiotics are not recommended unless the patient is immunocompromised or otherwise has impaired wound healing. If a patient presents with systemic symptoms, they should be referred to an emergency care facility for further management.
Final Thoughts
Sea urchin injuries can lead to serious complications if not diagnosed quickly and treated properly. Retention of sea urchin spines in the deep tissues and joint spaces may lead to granulomas, inflammatory and infectious tenosynovitis (including mycobacterial infection), and sea urchin arthritis requiring surgical debridement and possible irreversible joint damage, up to a year after initial injury. Patients should be educated on the possibility of developing these delayed reactions and instructed to seek immediate care. Joint deformities, range-of-motion deficits, and involvement of neurovascular structures should be considered emergent and referred for proper management. Shoes and diving gear offer some protection but are easily penetrable by sharp sea urchin spines. Preventive focus should be aimed at educating patients and providers on the importance of prompt spine removal upon injury. Although dermatologic and systemic manifestations vary widely, a thorough history, physical examination, and appropriate use of imaging modalities can facilitate accurate diagnosis and guide treatment.
- Amemiya CT, Miyake T, Rast JP. Echinoderms. Curr Biol. 2005;15:R944-R946. doi:10.1016/j.cub.2005.11.026
- Koch NM, Coppard SE, Lessios HA, et al. A phylogenomic resolution of the sea urchin tree of life. BMC Evol Biol. 2018;18:189. doi:10.1186/s12862-018-1300-4
- Amir Y, Insler M, Giller A, et al. Senescence and longevity of sea urchins. Genes (Basel). 2020;11:573. doi:10.3390/genes11050573
- Gummin DD, Mowry JB, Beuhler MC, et al. 2022 Annual Report of the National Poison Data System® (NPDS) from America's Poison Centers®: 40th annual report. Clin Toxicol (Phila). 2023;61:717-939. doi:10.1080/15563650.2023.2268981
- Gelman Y, Kong EL, Murphy-Lavoie HM. Sea urchin toxicity. In: StatPearls [Internet]. StatPearls Publishing; 2021.
- Suarez-Conde MF, Vallone MG, González VM, et al. Sea urchin skin lesions: a case report. Dermatol Pract Concept. 2021;11:E2021009. doi:10.5826/dpc.1102a09
- Al-Kathiri L, Al-Najjar T, Sulaiman I. Sea urchin granuloma of the hands: a case report. Oman Med J. 2019;34:350-353. doi:10.5001/omj.2019.68
- Dahl WJ, Jebson P, Louis DS. Sea urchin injuries to the hand: a case report and review of the literature. Iowa Orthop J. 2010;30:153-156.
- Hatakeyama T, Ichise A, Unno H, et al. Carbohydrate recognition by the rhamnose-binding lectin SUL-I with a novel three-domain structure isolated from the venom of globiferous pedicellariae of the flower sea urchin Toxopneustes pileolus. Protein Sci. 2017;26:1574-1583. doi:10.1002/pro.3185
- Balhara KS, Stolbach A. Marine envenomations. Emerg Med Clin North Am. 2014;32:223-243. doi:10.1016/j.emc.2013.09.009
- Schwartz Z, Cohen M, Lipner SR. Sea urchin injuries: a review and clinical approach algorithm. J Dermatolog Treat. 2021;32:150-156. doi:10.1080/09546634.2019.1638884
- Park SJ, Park JW, Choi SY, et al. Use of dermoscopy after punch removal of a veiled sea urchin spine. Dermatol Ther. 2021;34:E14947. doi:10.1111/dth.14947
- Wada T, Soma T, Gaman K, et al. Sea urchin spine arthritis of the hand. J Hand Surg Am. 2008;33:398-401. doi:10.1016/j.jhsa.2007.11.016
- Groleau S, Chhem RK, Younge D, et al. Ultrasonography of foreign-body tenosynovitis. Can Assoc Radiol J. 1992;43:454-456.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337. doi:10.1016/j.emc.2016.12.004
- Noonburg GE. Management of extremity trauma and related infections occurring in the aquatic environment. J Am Acad Orthop Surg. 2005;13:243-253. doi:10.5435/00124635-200507000-00004
- Haddad Junior V. Observation of initial clinical manifestations and repercussions from the treatment of 314 human injuries caused by black sea urchins (Echinometra lucunter) on the southeastern Brazilian coast. Rev Soc Bras Med Trop. 2012;45:390-392. doi:10.1590/s0037-86822012000300021
- Gargus MD, Morohashi DK. A sea-urchin spine chilling remedy. N Engl J Med. 2012;367:1867-1868. doi:10.1056/NEJMc1209382
- Sjøberg T, de Weerd L. The usefulness of a skin biopsy punch to remove sea urchin spines. ANZ J Surg. 2010;80:383. doi:10.1111/j.1445-2197.2010.05296.x
- Cardenas-de la Garza JA, Cuellar-Barboza A, Ancer-Arellano J, et al. Classic dermatological tools: foreign body removal with punch biopsy.J Am Acad Dermatol. 2019;81:E93-E94. doi:10.1016/j.jaad.2018.10.038
- Gungor S, Tarikçi N, Gokdemir G. Removal of sea urchin spines using erbium-doped yttrium aluminum garnet ablation. Dermatol Surg. 2012;38:508-510. doi:10.1111/j.1524-4725.2011.02259.x
- Böer A, Ochsendorf FR, Beier C, et al. Effective removal of sea-urchin spines by erbium:YAG laser ablation. Br J Dermatol. 2001;145:169-170. doi:10.1046/j.1365-2133.2001.04306.x
- De La Torre C, Toribio J. Sea-urchin granuloma: histologic profile. a pathologic study of 50 biopsies. J Cutan Pathol. 2001;28:223-228. doi:10.1034/j.1600-0560.2001.028005223.x
- Yi A, Kennedy C, Chia B, et al. Radiographic soft tissue thickness differentiating pyogenic flexor tenosynovitis from other finger infections. J Hand Surg Am. 2019;44:394-399. doi:10.1016/j.jhsa.2019.01.013
- Callison C, Nguyen H. Tetanus prophylaxis. In: StatPearls [Internet]. StatPearls Publishing; 2022.
Sea urchins—members of the phylum Echinodermata and the class Echinoidea—are spiny marine invertebrates. Their consumption of fleshy algae makes them essential players in maintaining reef ecosystems.1,2 Echinoids, a class that includes heart urchins and sand dollars, are ubiquitous in benthic marine environments, both free floating and rock boring, and inhabit a wide range of latitudes spanning from polar oceans to warm seas.3 Despite their immobility and nonaggression, sea urchin puncture wounds are common among divers, snorkelers, swimmers, surfers, and fishers who accidentally come into contact with their sharp spines. Although the epidemiology of sea urchin exposure and injury is difficult to assess, the American Association of Poison Control Centers’ most recent annual report in 2022 documents approximately 1426 annual aquatic bites and/or envenomations.4
Sea Urchin Morphology and Toxicity
Echinoderms (a term of Greek origin meaning spiny skin) share a radially symmetric calcium carbonate skeleton (termed stereom) that is supported by collagenous ligaments.1 Sea urchins possess spines composed of calcite crystals, which radiate from their body and play a role in locomotion and defense against predators—namely sea otters, starfish/sea stars, wolf eels, and triggerfish, among others (Figure).5 These brittle spines can easily penetrate human skin and subsequently break off the sea urchin body. Most species of sea urchins possess solid spines, but a small percentage (80 of approximately 700 extant species) have hollow spines containing various toxic substances.6 Penetration and systemic absorption of the toxins within these spines can generate severe systemic responses.
The venomous flower urchin (Toxopneustes pileolus), found in the Indian and Pacific oceans, is one of the more common species known to produce a systemic reaction involving neuromuscular blockage.7-9 The most common species harvested off the Pacific coast of the United States—Strongylocentrotus purpuratus (purple sea urchin) and Strongylocentrotus franciscanus (red sea urchins)—are not inherently venomous.8

Both the sea urchin body and spines are covered in a unique epithelium thought to be responsible for the majority of their proinflammatory and pronociceptive properties. Epithelial compounds identified include serotonin, histamines, steroids, glycosides, hemolysins, proteases, and bradykininlike and cholinergic substances.5,7 Additionally, certain sea urchin species possess 3-pronged pincerlike organs at the base of spines called pedicellariae, which are used in feeding.10 Skin penetration by the pedicellariae is especially dangerous, as they tightly adhere to wounds and contain venom-producing organs that allow them to continue injecting toxins after their detachment from the sea urchin body.11
Presentation and Diagnosis of Sea Urchin Injuries
Sea urchin injuries have a wide range of manifestations depending on the number of spines involved, the presence of venom, the depth and location of spine penetration, the duration of spine retention in the skin, and the time before treatment initiation. The most common site of sea urchin injury unsurprisingly is the lower extremities and feet, often in the context of divers and swimmers walking across the sea floor. The hands are another frequently injured site, along with the legs, arms, back, scalp, and even oral mucosa.11
Although clinical history and presentation frequently reveal the mechanism of aquatic injury, patients often are unsure of the agent to which they were exposed and may be unaware of retained foreign bodies. Dermoscopy can distinguish the distinct lines radiating from the core of sea urchin spines from other foreign bodies lodged within the skin.6 It also can be used to locate spines for removal or for their analysis following punch biopsy.6,12 The radiopaque nature of sea urchin spines makes radiography and magnetic resonance imaging useful tools in assessment of periarticular soft-tissue damage and spine removal.8,11,13 Ultrasonography can reveal spines that no longer appear on radiography due to absorption by human tissue.14
Immediate Dermatologic Effects
Sea urchin injuries can be broadly categorized into immediate and delayed reactions. Immediate manifestations of contact with sea urchin spines include localized pain, bleeding, erythema, myalgia, and edema at the site of injury that can last from a few hours to 1 week without proper wound care and spine removal.5 Systemic symptoms ranging from dizziness, lightheadedness, paresthesia, aphonia, paralysis, coma, and death generally are only seen following injuries from venomous species, attachment of pedicellariae, injuries involving neurovascular structures, or penetration by more than 15 spines.7,11
Initial treatment includes soaking the wound in hot water (113 °F [45 °C]) for 30 to 90 minutes and subsequently removing spines and pedicellariae to prevent development of delayed reactions.5,15,16 The compounds in the sea urchin epithelium are heat labile and will be inactivated upon soaking in hot water.16 Extraction of spines can be difficult, as they are brittle and easily break in the skin. Successful removal has been reported using forceps and a hypodermic needle as well as excision; both approaches may require local anesthesia.8,17 Another technique involves freezing the localized area with liquid nitrogen to allow easier removal upon skin blistering.18 Punch biopsy also has been utilized as an effective means of ensuring all spiny fragments are removed.9,19,20 These spines often cause black or purple tattoolike staining at the puncture site, which can persist for a few days after spine extraction.8 Ablation using the erbium-doped:YAG laser may be helpful for removal of associated pigment.21,22
Delayed Dermatologic Effects
Delayed reactions to sea urchin injuries often are attributable to prolonged retention of spines in the skin. Granulomatous reactions typically manifest 2 weeks after injury as firm nonsuppurative nodules with central umbilication and a hyperkeratotic surface.7 These nodules may or may not be painful. Histopathology most often reveals foreign body and sarcoidal-type granulomatous reactions. However, tuberculoid, necrobiotic, and suppurative granulomas also may develop.13 Other microscopic features include inflammatory reactions, suppurative dermatitis, focal necrosis, and microabscesses.23 Wounds with progression to granulomatous disease often require surgical debridement.
Other more serious sequalae can result from involvement of joint capsules, especially in the hands and feet. Sea urchin injury involving joint spaces should be treated aggressively, as progression to inflammatory or infectious synovitis and tenosynovitis can cause irreversible loss of joint function. Inflammatory synovitis occurs 1 to 2 months on average after injury following a period of minimal symptoms and begins as a gradual increase in joint swelling and decrease in range of motion.8 Infectious tenosynovitis manifests quite similarly. Although suppurative etiologies generally progress with a more acute onset, certain infectious organisms (eg, Mycobacterium) take on an indolent course and should not be overlooked as a cause of delayed symptoms.8 The Kavanel cardinal signs are a sensitive tool used in the diagnosis of infectious flexor sheath tenosynovitis.8,24 If suspicion for joint infection is high, emergency referral should be made for debridement and culture-guided antibiotic therapy. Left untreated, infectious tenosynovitis can result in tendon necrosis or rupture, digit necrosis, and systemic infection.24 Patients with joint involvement should be referred to specialty care (eg, hand surgeon), as they often require synovectomy and surgical removal of foreign material.8
From 1 month to 1 year after injury, prolonged granulomatous synovitis of the hand may eventually lead to joint destruction known as “sea urchin arthritis.” These patients present with decreased range of motion and numerous nodules on the hand with a hyperkeratotic surface. Radiography reveals joint space narrowing, osteolysis, subchondral sclerosis, and periosteal reaction. Synovectomy and debridement are necessary to prevent irreversible joint damage or the need for arthrodesis and bone grafting.24
Other Treatment Considerations
Other important considerations in the care of sea urchin spine injuries include assessment of tetanus immunization status and administration of necessary prophylaxis as soon as possible, even in delayed presentations (Table).16,25 Cultures should be taken only if infection is suspected. Prophylactic antibiotics are not recommended unless the patient is immunocompromised or otherwise has impaired wound healing. If a patient presents with systemic symptoms, they should be referred to an emergency care facility for further management.
Final Thoughts
Sea urchin injuries can lead to serious complications if not diagnosed quickly and treated properly. Retention of sea urchin spines in the deep tissues and joint spaces may lead to granulomas, inflammatory and infectious tenosynovitis (including mycobacterial infection), and sea urchin arthritis requiring surgical debridement and possible irreversible joint damage, up to a year after initial injury. Patients should be educated on the possibility of developing these delayed reactions and instructed to seek immediate care. Joint deformities, range-of-motion deficits, and involvement of neurovascular structures should be considered emergent and referred for proper management. Shoes and diving gear offer some protection but are easily penetrable by sharp sea urchin spines. Preventive focus should be aimed at educating patients and providers on the importance of prompt spine removal upon injury. Although dermatologic and systemic manifestations vary widely, a thorough history, physical examination, and appropriate use of imaging modalities can facilitate accurate diagnosis and guide treatment.

Sea urchins—members of the phylum Echinodermata and the class Echinoidea—are spiny marine invertebrates. Their consumption of fleshy algae makes them essential players in maintaining reef ecosystems.1,2 Echinoids, a class that includes heart urchins and sand dollars, are ubiquitous in benthic marine environments, both free floating and rock boring, and inhabit a wide range of latitudes spanning from polar oceans to warm seas.3 Despite their immobility and nonaggression, sea urchin puncture wounds are common among divers, snorkelers, swimmers, surfers, and fishers who accidentally come into contact with their sharp spines. Although the epidemiology of sea urchin exposure and injury is difficult to assess, the American Association of Poison Control Centers’ most recent annual report in 2022 documents approximately 1426 annual aquatic bites and/or envenomations.4
Sea Urchin Morphology and Toxicity
Echinoderms (a term of Greek origin meaning spiny skin) share a radially symmetric calcium carbonate skeleton (termed stereom) that is supported by collagenous ligaments.1 Sea urchins possess spines composed of calcite crystals, which radiate from their body and play a role in locomotion and defense against predators—namely sea otters, starfish/sea stars, wolf eels, and triggerfish, among others (Figure).5 These brittle spines can easily penetrate human skin and subsequently break off the sea urchin body. Most species of sea urchins possess solid spines, but a small percentage (80 of approximately 700 extant species) have hollow spines containing various toxic substances.6 Penetration and systemic absorption of the toxins within these spines can generate severe systemic responses.
The venomous flower urchin (Toxopneustes pileolus), found in the Indian and Pacific oceans, is one of the more common species known to produce a systemic reaction involving neuromuscular blockage.7-9 The most common species harvested off the Pacific coast of the United States—Strongylocentrotus purpuratus (purple sea urchin) and Strongylocentrotus franciscanus (red sea urchins)—are not inherently venomous.8

Both the sea urchin body and spines are covered in a unique epithelium thought to be responsible for the majority of their proinflammatory and pronociceptive properties. Epithelial compounds identified include serotonin, histamines, steroids, glycosides, hemolysins, proteases, and bradykininlike and cholinergic substances.5,7 Additionally, certain sea urchin species possess 3-pronged pincerlike organs at the base of spines called pedicellariae, which are used in feeding.10 Skin penetration by the pedicellariae is especially dangerous, as they tightly adhere to wounds and contain venom-producing organs that allow them to continue injecting toxins after their detachment from the sea urchin body.11
Presentation and Diagnosis of Sea Urchin Injuries
Sea urchin injuries have a wide range of manifestations depending on the number of spines involved, the presence of venom, the depth and location of spine penetration, the duration of spine retention in the skin, and the time before treatment initiation. The most common site of sea urchin injury unsurprisingly is the lower extremities and feet, often in the context of divers and swimmers walking across the sea floor. The hands are another frequently injured site, along with the legs, arms, back, scalp, and even oral mucosa.11
Although clinical history and presentation frequently reveal the mechanism of aquatic injury, patients often are unsure of the agent to which they were exposed and may be unaware of retained foreign bodies. Dermoscopy can distinguish the distinct lines radiating from the core of sea urchin spines from other foreign bodies lodged within the skin.6 It also can be used to locate spines for removal or for their analysis following punch biopsy.6,12 The radiopaque nature of sea urchin spines makes radiography and magnetic resonance imaging useful tools in assessment of periarticular soft-tissue damage and spine removal.8,11,13 Ultrasonography can reveal spines that no longer appear on radiography due to absorption by human tissue.14
Immediate Dermatologic Effects
Sea urchin injuries can be broadly categorized into immediate and delayed reactions. Immediate manifestations of contact with sea urchin spines include localized pain, bleeding, erythema, myalgia, and edema at the site of injury that can last from a few hours to 1 week without proper wound care and spine removal.5 Systemic symptoms ranging from dizziness, lightheadedness, paresthesia, aphonia, paralysis, coma, and death generally are only seen following injuries from venomous species, attachment of pedicellariae, injuries involving neurovascular structures, or penetration by more than 15 spines.7,11
Initial treatment includes soaking the wound in hot water (113 °F [45 °C]) for 30 to 90 minutes and subsequently removing spines and pedicellariae to prevent development of delayed reactions.5,15,16 The compounds in the sea urchin epithelium are heat labile and will be inactivated upon soaking in hot water.16 Extraction of spines can be difficult, as they are brittle and easily break in the skin. Successful removal has been reported using forceps and a hypodermic needle as well as excision; both approaches may require local anesthesia.8,17 Another technique involves freezing the localized area with liquid nitrogen to allow easier removal upon skin blistering.18 Punch biopsy also has been utilized as an effective means of ensuring all spiny fragments are removed.9,19,20 These spines often cause black or purple tattoolike staining at the puncture site, which can persist for a few days after spine extraction.8 Ablation using the erbium-doped:YAG laser may be helpful for removal of associated pigment.21,22
Delayed Dermatologic Effects
Delayed reactions to sea urchin injuries often are attributable to prolonged retention of spines in the skin. Granulomatous reactions typically manifest 2 weeks after injury as firm nonsuppurative nodules with central umbilication and a hyperkeratotic surface.7 These nodules may or may not be painful. Histopathology most often reveals foreign body and sarcoidal-type granulomatous reactions. However, tuberculoid, necrobiotic, and suppurative granulomas also may develop.13 Other microscopic features include inflammatory reactions, suppurative dermatitis, focal necrosis, and microabscesses.23 Wounds with progression to granulomatous disease often require surgical debridement.
Other more serious sequalae can result from involvement of joint capsules, especially in the hands and feet. Sea urchin injury involving joint spaces should be treated aggressively, as progression to inflammatory or infectious synovitis and tenosynovitis can cause irreversible loss of joint function. Inflammatory synovitis occurs 1 to 2 months on average after injury following a period of minimal symptoms and begins as a gradual increase in joint swelling and decrease in range of motion.8 Infectious tenosynovitis manifests quite similarly. Although suppurative etiologies generally progress with a more acute onset, certain infectious organisms (eg, Mycobacterium) take on an indolent course and should not be overlooked as a cause of delayed symptoms.8 The Kavanel cardinal signs are a sensitive tool used in the diagnosis of infectious flexor sheath tenosynovitis.8,24 If suspicion for joint infection is high, emergency referral should be made for debridement and culture-guided antibiotic therapy. Left untreated, infectious tenosynovitis can result in tendon necrosis or rupture, digit necrosis, and systemic infection.24 Patients with joint involvement should be referred to specialty care (eg, hand surgeon), as they often require synovectomy and surgical removal of foreign material.8
From 1 month to 1 year after injury, prolonged granulomatous synovitis of the hand may eventually lead to joint destruction known as “sea urchin arthritis.” These patients present with decreased range of motion and numerous nodules on the hand with a hyperkeratotic surface. Radiography reveals joint space narrowing, osteolysis, subchondral sclerosis, and periosteal reaction. Synovectomy and debridement are necessary to prevent irreversible joint damage or the need for arthrodesis and bone grafting.24
Other Treatment Considerations
Other important considerations in the care of sea urchin spine injuries include assessment of tetanus immunization status and administration of necessary prophylaxis as soon as possible, even in delayed presentations (Table).16,25 Cultures should be taken only if infection is suspected. Prophylactic antibiotics are not recommended unless the patient is immunocompromised or otherwise has impaired wound healing. If a patient presents with systemic symptoms, they should be referred to an emergency care facility for further management.
Final Thoughts
Sea urchin injuries can lead to serious complications if not diagnosed quickly and treated properly. Retention of sea urchin spines in the deep tissues and joint spaces may lead to granulomas, inflammatory and infectious tenosynovitis (including mycobacterial infection), and sea urchin arthritis requiring surgical debridement and possible irreversible joint damage, up to a year after initial injury. Patients should be educated on the possibility of developing these delayed reactions and instructed to seek immediate care. Joint deformities, range-of-motion deficits, and involvement of neurovascular structures should be considered emergent and referred for proper management. Shoes and diving gear offer some protection but are easily penetrable by sharp sea urchin spines. Preventive focus should be aimed at educating patients and providers on the importance of prompt spine removal upon injury. Although dermatologic and systemic manifestations vary widely, a thorough history, physical examination, and appropriate use of imaging modalities can facilitate accurate diagnosis and guide treatment.

- Amemiya CT, Miyake T, Rast JP. Echinoderms. Curr Biol. 2005;15:R944-R946. doi:10.1016/j.cub.2005.11.026
- Koch NM, Coppard SE, Lessios HA, et al. A phylogenomic resolution of the sea urchin tree of life. BMC Evol Biol. 2018;18:189. doi:10.1186/s12862-018-1300-4
- Amir Y, Insler M, Giller A, et al. Senescence and longevity of sea urchins. Genes (Basel). 2020;11:573. doi:10.3390/genes11050573
- Gummin DD, Mowry JB, Beuhler MC, et al. 2022 Annual Report of the National Poison Data System® (NPDS) from America's Poison Centers®: 40th annual report. Clin Toxicol (Phila). 2023;61:717-939. doi:10.1080/15563650.2023.2268981
- Gelman Y, Kong EL, Murphy-Lavoie HM. Sea urchin toxicity. In: StatPearls [Internet]. StatPearls Publishing; 2021.
- Suarez-Conde MF, Vallone MG, González VM, et al. Sea urchin skin lesions: a case report. Dermatol Pract Concept. 2021;11:E2021009. doi:10.5826/dpc.1102a09
- Al-Kathiri L, Al-Najjar T, Sulaiman I. Sea urchin granuloma of the hands: a case report. Oman Med J. 2019;34:350-353. doi:10.5001/omj.2019.68
- Dahl WJ, Jebson P, Louis DS. Sea urchin injuries to the hand: a case report and review of the literature. Iowa Orthop J. 2010;30:153-156.
- Hatakeyama T, Ichise A, Unno H, et al. Carbohydrate recognition by the rhamnose-binding lectin SUL-I with a novel three-domain structure isolated from the venom of globiferous pedicellariae of the flower sea urchin Toxopneustes pileolus. Protein Sci. 2017;26:1574-1583. doi:10.1002/pro.3185
- Balhara KS, Stolbach A. Marine envenomations. Emerg Med Clin North Am. 2014;32:223-243. doi:10.1016/j.emc.2013.09.009
- Schwartz Z, Cohen M, Lipner SR. Sea urchin injuries: a review and clinical approach algorithm. J Dermatolog Treat. 2021;32:150-156. doi:10.1080/09546634.2019.1638884
- Park SJ, Park JW, Choi SY, et al. Use of dermoscopy after punch removal of a veiled sea urchin spine. Dermatol Ther. 2021;34:E14947. doi:10.1111/dth.14947
- Wada T, Soma T, Gaman K, et al. Sea urchin spine arthritis of the hand. J Hand Surg Am. 2008;33:398-401. doi:10.1016/j.jhsa.2007.11.016
- Groleau S, Chhem RK, Younge D, et al. Ultrasonography of foreign-body tenosynovitis. Can Assoc Radiol J. 1992;43:454-456.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337. doi:10.1016/j.emc.2016.12.004
- Noonburg GE. Management of extremity trauma and related infections occurring in the aquatic environment. J Am Acad Orthop Surg. 2005;13:243-253. doi:10.5435/00124635-200507000-00004
- Haddad Junior V. Observation of initial clinical manifestations and repercussions from the treatment of 314 human injuries caused by black sea urchins (Echinometra lucunter) on the southeastern Brazilian coast. Rev Soc Bras Med Trop. 2012;45:390-392. doi:10.1590/s0037-86822012000300021
- Gargus MD, Morohashi DK. A sea-urchin spine chilling remedy. N Engl J Med. 2012;367:1867-1868. doi:10.1056/NEJMc1209382
- Sjøberg T, de Weerd L. The usefulness of a skin biopsy punch to remove sea urchin spines. ANZ J Surg. 2010;80:383. doi:10.1111/j.1445-2197.2010.05296.x
- Cardenas-de la Garza JA, Cuellar-Barboza A, Ancer-Arellano J, et al. Classic dermatological tools: foreign body removal with punch biopsy.J Am Acad Dermatol. 2019;81:E93-E94. doi:10.1016/j.jaad.2018.10.038
- Gungor S, Tarikçi N, Gokdemir G. Removal of sea urchin spines using erbium-doped yttrium aluminum garnet ablation. Dermatol Surg. 2012;38:508-510. doi:10.1111/j.1524-4725.2011.02259.x
- Böer A, Ochsendorf FR, Beier C, et al. Effective removal of sea-urchin spines by erbium:YAG laser ablation. Br J Dermatol. 2001;145:169-170. doi:10.1046/j.1365-2133.2001.04306.x
- De La Torre C, Toribio J. Sea-urchin granuloma: histologic profile. a pathologic study of 50 biopsies. J Cutan Pathol. 2001;28:223-228. doi:10.1034/j.1600-0560.2001.028005223.x
- Yi A, Kennedy C, Chia B, et al. Radiographic soft tissue thickness differentiating pyogenic flexor tenosynovitis from other finger infections. J Hand Surg Am. 2019;44:394-399. doi:10.1016/j.jhsa.2019.01.013
- Callison C, Nguyen H. Tetanus prophylaxis. In: StatPearls [Internet]. StatPearls Publishing; 2022.
- Amemiya CT, Miyake T, Rast JP. Echinoderms. Curr Biol. 2005;15:R944-R946. doi:10.1016/j.cub.2005.11.026
- Koch NM, Coppard SE, Lessios HA, et al. A phylogenomic resolution of the sea urchin tree of life. BMC Evol Biol. 2018;18:189. doi:10.1186/s12862-018-1300-4
- Amir Y, Insler M, Giller A, et al. Senescence and longevity of sea urchins. Genes (Basel). 2020;11:573. doi:10.3390/genes11050573
- Gummin DD, Mowry JB, Beuhler MC, et al. 2022 Annual Report of the National Poison Data System® (NPDS) from America's Poison Centers®: 40th annual report. Clin Toxicol (Phila). 2023;61:717-939. doi:10.1080/15563650.2023.2268981
- Gelman Y, Kong EL, Murphy-Lavoie HM. Sea urchin toxicity. In: StatPearls [Internet]. StatPearls Publishing; 2021.
- Suarez-Conde MF, Vallone MG, González VM, et al. Sea urchin skin lesions: a case report. Dermatol Pract Concept. 2021;11:E2021009. doi:10.5826/dpc.1102a09
- Al-Kathiri L, Al-Najjar T, Sulaiman I. Sea urchin granuloma of the hands: a case report. Oman Med J. 2019;34:350-353. doi:10.5001/omj.2019.68
- Dahl WJ, Jebson P, Louis DS. Sea urchin injuries to the hand: a case report and review of the literature. Iowa Orthop J. 2010;30:153-156.
- Hatakeyama T, Ichise A, Unno H, et al. Carbohydrate recognition by the rhamnose-binding lectin SUL-I with a novel three-domain structure isolated from the venom of globiferous pedicellariae of the flower sea urchin Toxopneustes pileolus. Protein Sci. 2017;26:1574-1583. doi:10.1002/pro.3185
- Balhara KS, Stolbach A. Marine envenomations. Emerg Med Clin North Am. 2014;32:223-243. doi:10.1016/j.emc.2013.09.009
- Schwartz Z, Cohen M, Lipner SR. Sea urchin injuries: a review and clinical approach algorithm. J Dermatolog Treat. 2021;32:150-156. doi:10.1080/09546634.2019.1638884
- Park SJ, Park JW, Choi SY, et al. Use of dermoscopy after punch removal of a veiled sea urchin spine. Dermatol Ther. 2021;34:E14947. doi:10.1111/dth.14947
- Wada T, Soma T, Gaman K, et al. Sea urchin spine arthritis of the hand. J Hand Surg Am. 2008;33:398-401. doi:10.1016/j.jhsa.2007.11.016
- Groleau S, Chhem RK, Younge D, et al. Ultrasonography of foreign-body tenosynovitis. Can Assoc Radiol J. 1992;43:454-456.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337. doi:10.1016/j.emc.2016.12.004
- Noonburg GE. Management of extremity trauma and related infections occurring in the aquatic environment. J Am Acad Orthop Surg. 2005;13:243-253. doi:10.5435/00124635-200507000-00004
- Haddad Junior V. Observation of initial clinical manifestations and repercussions from the treatment of 314 human injuries caused by black sea urchins (Echinometra lucunter) on the southeastern Brazilian coast. Rev Soc Bras Med Trop. 2012;45:390-392. doi:10.1590/s0037-86822012000300021
- Gargus MD, Morohashi DK. A sea-urchin spine chilling remedy. N Engl J Med. 2012;367:1867-1868. doi:10.1056/NEJMc1209382
- Sjøberg T, de Weerd L. The usefulness of a skin biopsy punch to remove sea urchin spines. ANZ J Surg. 2010;80:383. doi:10.1111/j.1445-2197.2010.05296.x
- Cardenas-de la Garza JA, Cuellar-Barboza A, Ancer-Arellano J, et al. Classic dermatological tools: foreign body removal with punch biopsy.J Am Acad Dermatol. 2019;81:E93-E94. doi:10.1016/j.jaad.2018.10.038
- Gungor S, Tarikçi N, Gokdemir G. Removal of sea urchin spines using erbium-doped yttrium aluminum garnet ablation. Dermatol Surg. 2012;38:508-510. doi:10.1111/j.1524-4725.2011.02259.x
- Böer A, Ochsendorf FR, Beier C, et al. Effective removal of sea-urchin spines by erbium:YAG laser ablation. Br J Dermatol. 2001;145:169-170. doi:10.1046/j.1365-2133.2001.04306.x
- De La Torre C, Toribio J. Sea-urchin granuloma: histologic profile. a pathologic study of 50 biopsies. J Cutan Pathol. 2001;28:223-228. doi:10.1034/j.1600-0560.2001.028005223.x
- Yi A, Kennedy C, Chia B, et al. Radiographic soft tissue thickness differentiating pyogenic flexor tenosynovitis from other finger infections. J Hand Surg Am. 2019;44:394-399. doi:10.1016/j.jhsa.2019.01.013
- Callison C, Nguyen H. Tetanus prophylaxis. In: StatPearls [Internet]. StatPearls Publishing; 2022.
Practice Points
- Sea urchin spines easily become embedded in human skin upon contact and cause localized pain, edema, and black or purple pinpoint markings.
- Immediate treatment includes soaking in hot water (113 12°F [45 12°C]) for 30 to 90 minutes to inactivate proinflammatory compounds, followed by extraction of the spines.
- Successful methods of spine removal include the use of forceps and a hypodermic needle, as well as excision, liquid nitrogen, and punch biopsy.
- Prompt removal of the spines can reduce the incidence of delayed granulomatous reactions, synovitis, and sea urchin arthritis.
Aquatic Antagonists: Seaweed Dermatitis (Lyngbya majuscula)
Aquatic Antagonists: Seaweed Dermatitis (Lyngbya majuscula)
The filamentous cyanobacterium Lyngbya majuscula causes irritant contact dermatitis in beachgoers, fishers, and divers in tropical and subtropical marine environments worldwide.1 If fragments of L majuscula lodge in swimmers’ bathing suits, the toxins can become trapped against the skin and cause seaweed dermatitis.2 With climate change resulting in warmer oceans and more extreme storms, L majuscula blooms likely will become more frequent and widespread, thereby increasing the risk for human exposure.3,4 Herein, we describe the irritants that lead to dermatitis, clinical presentation, and prevention and management of seaweed dermatitis.
Identifying Features and Distribution of Plant
Lyngbya majuscula belongs to the family Oscillatoriaceae; these cyanobacteria grow as filaments and exhibit slow oscillating movements. Commonly referred to as blanketweed or mermaid’s hair due to its appearance, L majuscula grows fine hairlike clumps resembling a mass of olive-colored matted hair.1 Its thin filaments are 10- to 30-cm long and vary in color from red to white to brown.5 Microscopically, a rouleauxlike arrangement of discs provides the structure of each filament.6
First identified in Hawaii in 1912, L majuscula was not associated with seaweed dermatitis or dermatotoxicity by the medical community until the first outbreak occurred in Oahu in 1958, though fishermen and beachgoers previously had recognized a relationship between this particular seaweed and skin irritation.5,7 The first reporting included 125 confirmed cases, with many more mild unreported cases suspected.6 Now reported in about 100 locations worldwide, seaweed dermatitis outbreaks have occurred in Australia; Okinawa, Japan; Florida; and the Hawaiian and Marshall islands.1,2
Exposure to Seaweed
Lyngbya majuscula produces more than 70 biologically active compounds that irritate the skin, eyes, and respiratory system.2,8 It grows in marine and estuarine environments attached to seagrass, sand, and bedrock at depths of up to 30 m. Warm waters and maximal sunlight provide optimal growth conditions for L majuscula; therefore, the greatest risk for exposure occurs in the Northern and Southern hemispheres in the 1- to 2-month period following their summer solstices.5 Runoff during heavy rainfall, which is rich in soil extracts such as phosphorous, iron, and organic carbon, stimulates L majuscula growth and contributes to increased algal blooms.4
Dermatitis and Irritants
The dermatoxins Lyngbyatoxin A (LA) and debromoaplysiatoxin (DAT) cause the inflammatory and necrotic appearance of seaweed dermatitis.1,2,5,8 Lyngbyatoxin A is an indole alkaloid that is closely related to telocidin B, a poisonous compound associated with Streptomyces bacteria.9 Sampling of L majuscula and extraction of the dermatoxin, along with human and animal studies, confirmed DAT irritates the skin and induces dermatitis.5,6Stylocheilus longicauda (sea hare) feeds on L majuscula and contains isolates of DAT in its digestive tract.
Samples of L majuscula taken from several Hawaiian Islands where seaweed dermatitis outbreaks have occurred were examined for differences in toxicities via 6-hour patch tests on human skin.6 The samples obtained from the windward side of Oahu contained DAT and aplysiatoxin, while those obtained from the leeward side and Kahala Beach primarily contained LA. Although DAT and LA are vastly different in their molecular structures, testing elicited the same biologic response and induced the same level of skin irritation.6 Interestingly, not all strands of L majuscula produced LA and DAT and caused seaweed dermatitis; those that did lead to irritation were more red in color than nontoxic blooms.5,9
Cutaneous Manifestations
Seaweed dermatitis resembles chemical and thermal burns, ranging from a mild skin rash to severe contact dermatitis with itchy, swollen, ulcerated lesions.1,7 Patients typically develop a burning or itching sensation beneath their bathing suit or wetsuit that progresses to an erythematous papulovesicular eruption 2 to 24 hours after exposure.2,6 Within a week, vesicles and bullae desquamate, leaving behind tender erosions.1,2,6,8 Inframammary lesions are common in females and scrotal swelling in males.1,6 There is no known association between length of time spent in the water and severity of symptoms.5
Most reactions to L majuscula occur from exposure in the water; however, particles that become aerosolized during strong winds or storms can cause seaweed dermatitis on the face. Inhalation of L majuscula may lead to mucous membrane ulceration and pulmonary edema.1,5,6 Noncutaneous manifestations of seaweed dermatitis include headache, fatigue, and swelling of the eyes, nose, and throat (Figures 1 and 2).1,5
Prevention and Management
To prevent seaweed dermatitis, avoid swimming in ocean water during L majuscula blooms,10 which frequently occur following the summer solstices in the Northern and Southern hemispheres.5 The National Centers for Coastal Ocean Science Harmful Algae Bloom Monitoring System provides real-time access to algae bloom locations.11 Although this monitoring system is not specific to L majuscula, it may be helpful in determining where potential blooms are. Wearing protective clothing such as coveralls may benefit individuals who enter the water during blooms, but it does not guarantee protection.10
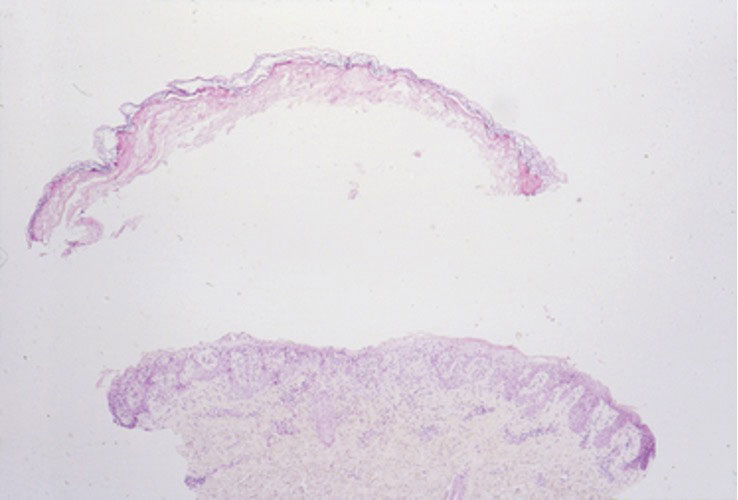
magnification ×40). Photograph courtesy of Scott Norton, MD, MPH, MSc (Washington, DC).
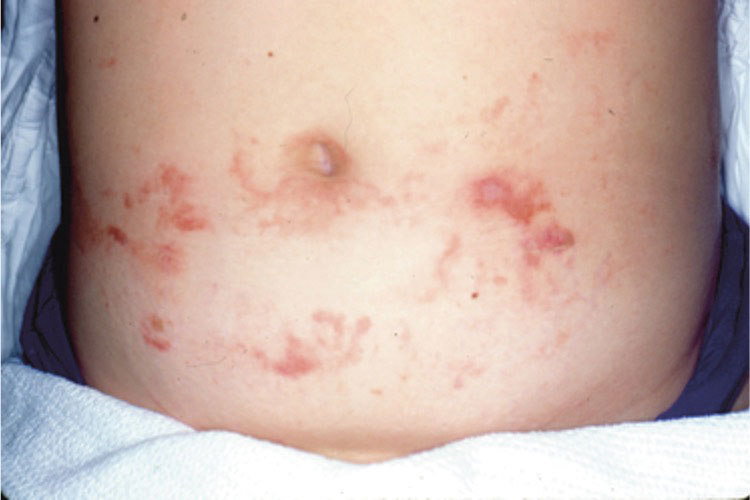
Currently, there is no treatment for seaweed dermatitis, but symptom management may reduce discomfort and pain. Washing affected skin with soap and water within an hour of exposure may help reduce the severity of seaweed dermatitis, though studies have shown mixed results.6,7 Application of cool compresses and soothing ointments (eg, calamine) provide symptomatic relief and promote healing.7 The dermatitis typically self-resolves within 1 week.
- Werner K, Marquart L, Norton S. Lyngbya dermatitis (toxic seaweed dermatitis). Int J Dermatol. 2011;51:59-62. doi:10.1111/j.1365-4632.2011.05042.x
- Osborne N, Shaw G. Dermatitis associated with exposure to a marine cyanobacterium during recreational water exposure. BMC Dermatol. 2008;8:5. doi:10.1186/1471-5945-8-5
- Hays G, Richardson A, Robinson C. Climate change and marine plankton. Trends Ecol Evol. 2005;20:337-344. doi:10.1016/j.tree.2005.03.004
- Albert S, O’Neil J, Udy J, et al. Blooms of the cyanobacterium Lyngbya majuscula in costal Queensland, Australia: disparate sites, common factors. Mar Pollut Bull. 2004;51:428-437. doi:10.1016/j.marpolbul.2004.10.016
- Osborne N, Webb P, Shaw G. The toxins of Lyngbya majuscula and their human and ecological health effects. Environ Int. 2001;27:381-392. doi:10.1016/s0160-4120(01)00098-8
- Izumi A, Moore R. Seaweed ( Lyngbya majuscula ) dermatitis . Clin Dermatol . 1987;5:92-100. doi:10.1016/s0738-081x(87)80014-7
- Grauer F, Arnold H. Seaweed dermatitis: first report of a dermatitis-producing marine alga. Arch Dermatol. 1961; 84:720-732. doi:10.1001/archderm.1961.01580170014003
- Taylor M, Stahl-Timmins W, Redshaw C, et al. Toxic alkaloids in Lyngbya majuscula and related tropical marine cyanobacteria. Harmful Algae . 2014;31:1-8. doi:10.1016/j.hal.2013.09.003
- Cardellina J, Marner F, Moore R. Seaweed dermatitis: structure of lyngbyatoxin A. Science. 1979;204:193-195. doi:10.1126/science.107586
- Osborne N. Occupational dermatitis caused by Lyngbya majuscule in Australia. Int J Dermatol . 2012;5:122-123. doi:10.1111/j.1365-4632.2009.04455.x
- Harmful Algal Bloom Monitoring System. National Centers for Coastal Ocean Science. Accessed May 23, 2024. https://coastalscience.noaa.gov/research/stressor-impacts-mitigation/hab-monitoring-system/
The filamentous cyanobacterium Lyngbya majuscula causes irritant contact dermatitis in beachgoers, fishers, and divers in tropical and subtropical marine environments worldwide.1 If fragments of L majuscula lodge in swimmers’ bathing suits, the toxins can become trapped against the skin and cause seaweed dermatitis.2 With climate change resulting in warmer oceans and more extreme storms, L majuscula blooms likely will become more frequent and widespread, thereby increasing the risk for human exposure.3,4 Herein, we describe the irritants that lead to dermatitis, clinical presentation, and prevention and management of seaweed dermatitis.
Identifying Features and Distribution of Plant
Lyngbya majuscula belongs to the family Oscillatoriaceae; these cyanobacteria grow as filaments and exhibit slow oscillating movements. Commonly referred to as blanketweed or mermaid’s hair due to its appearance, L majuscula grows fine hairlike clumps resembling a mass of olive-colored matted hair.1 Its thin filaments are 10- to 30-cm long and vary in color from red to white to brown.5 Microscopically, a rouleauxlike arrangement of discs provides the structure of each filament.6
First identified in Hawaii in 1912, L majuscula was not associated with seaweed dermatitis or dermatotoxicity by the medical community until the first outbreak occurred in Oahu in 1958, though fishermen and beachgoers previously had recognized a relationship between this particular seaweed and skin irritation.5,7 The first reporting included 125 confirmed cases, with many more mild unreported cases suspected.6 Now reported in about 100 locations worldwide, seaweed dermatitis outbreaks have occurred in Australia; Okinawa, Japan; Florida; and the Hawaiian and Marshall islands.1,2
Exposure to Seaweed
Lyngbya majuscula produces more than 70 biologically active compounds that irritate the skin, eyes, and respiratory system.2,8 It grows in marine and estuarine environments attached to seagrass, sand, and bedrock at depths of up to 30 m. Warm waters and maximal sunlight provide optimal growth conditions for L majuscula; therefore, the greatest risk for exposure occurs in the Northern and Southern hemispheres in the 1- to 2-month period following their summer solstices.5 Runoff during heavy rainfall, which is rich in soil extracts such as phosphorous, iron, and organic carbon, stimulates L majuscula growth and contributes to increased algal blooms.4
Dermatitis and Irritants
The dermatoxins Lyngbyatoxin A (LA) and debromoaplysiatoxin (DAT) cause the inflammatory and necrotic appearance of seaweed dermatitis.1,2,5,8 Lyngbyatoxin A is an indole alkaloid that is closely related to telocidin B, a poisonous compound associated with Streptomyces bacteria.9 Sampling of L majuscula and extraction of the dermatoxin, along with human and animal studies, confirmed DAT irritates the skin and induces dermatitis.5,6Stylocheilus longicauda (sea hare) feeds on L majuscula and contains isolates of DAT in its digestive tract.
Samples of L majuscula taken from several Hawaiian Islands where seaweed dermatitis outbreaks have occurred were examined for differences in toxicities via 6-hour patch tests on human skin.6 The samples obtained from the windward side of Oahu contained DAT and aplysiatoxin, while those obtained from the leeward side and Kahala Beach primarily contained LA. Although DAT and LA are vastly different in their molecular structures, testing elicited the same biologic response and induced the same level of skin irritation.6 Interestingly, not all strands of L majuscula produced LA and DAT and caused seaweed dermatitis; those that did lead to irritation were more red in color than nontoxic blooms.5,9
Cutaneous Manifestations
Seaweed dermatitis resembles chemical and thermal burns, ranging from a mild skin rash to severe contact dermatitis with itchy, swollen, ulcerated lesions.1,7 Patients typically develop a burning or itching sensation beneath their bathing suit or wetsuit that progresses to an erythematous papulovesicular eruption 2 to 24 hours after exposure.2,6 Within a week, vesicles and bullae desquamate, leaving behind tender erosions.1,2,6,8 Inframammary lesions are common in females and scrotal swelling in males.1,6 There is no known association between length of time spent in the water and severity of symptoms.5
Most reactions to L majuscula occur from exposure in the water; however, particles that become aerosolized during strong winds or storms can cause seaweed dermatitis on the face. Inhalation of L majuscula may lead to mucous membrane ulceration and pulmonary edema.1,5,6 Noncutaneous manifestations of seaweed dermatitis include headache, fatigue, and swelling of the eyes, nose, and throat (Figures 1 and 2).1,5
Prevention and Management
To prevent seaweed dermatitis, avoid swimming in ocean water during L majuscula blooms,10 which frequently occur following the summer solstices in the Northern and Southern hemispheres.5 The National Centers for Coastal Ocean Science Harmful Algae Bloom Monitoring System provides real-time access to algae bloom locations.11 Although this monitoring system is not specific to L majuscula, it may be helpful in determining where potential blooms are. Wearing protective clothing such as coveralls may benefit individuals who enter the water during blooms, but it does not guarantee protection.10

magnification ×40). Photograph courtesy of Scott Norton, MD, MPH, MSc (Washington, DC).

Currently, there is no treatment for seaweed dermatitis, but symptom management may reduce discomfort and pain. Washing affected skin with soap and water within an hour of exposure may help reduce the severity of seaweed dermatitis, though studies have shown mixed results.6,7 Application of cool compresses and soothing ointments (eg, calamine) provide symptomatic relief and promote healing.7 The dermatitis typically self-resolves within 1 week.
The filamentous cyanobacterium Lyngbya majuscula causes irritant contact dermatitis in beachgoers, fishers, and divers in tropical and subtropical marine environments worldwide.1 If fragments of L majuscula lodge in swimmers’ bathing suits, the toxins can become trapped against the skin and cause seaweed dermatitis.2 With climate change resulting in warmer oceans and more extreme storms, L majuscula blooms likely will become more frequent and widespread, thereby increasing the risk for human exposure.3,4 Herein, we describe the irritants that lead to dermatitis, clinical presentation, and prevention and management of seaweed dermatitis.
Identifying Features and Distribution of Plant
Lyngbya majuscula belongs to the family Oscillatoriaceae; these cyanobacteria grow as filaments and exhibit slow oscillating movements. Commonly referred to as blanketweed or mermaid’s hair due to its appearance, L majuscula grows fine hairlike clumps resembling a mass of olive-colored matted hair.1 Its thin filaments are 10- to 30-cm long and vary in color from red to white to brown.5 Microscopically, a rouleauxlike arrangement of discs provides the structure of each filament.6
First identified in Hawaii in 1912, L majuscula was not associated with seaweed dermatitis or dermatotoxicity by the medical community until the first outbreak occurred in Oahu in 1958, though fishermen and beachgoers previously had recognized a relationship between this particular seaweed and skin irritation.5,7 The first reporting included 125 confirmed cases, with many more mild unreported cases suspected.6 Now reported in about 100 locations worldwide, seaweed dermatitis outbreaks have occurred in Australia; Okinawa, Japan; Florida; and the Hawaiian and Marshall islands.1,2
Exposure to Seaweed
Lyngbya majuscula produces more than 70 biologically active compounds that irritate the skin, eyes, and respiratory system.2,8 It grows in marine and estuarine environments attached to seagrass, sand, and bedrock at depths of up to 30 m. Warm waters and maximal sunlight provide optimal growth conditions for L majuscula; therefore, the greatest risk for exposure occurs in the Northern and Southern hemispheres in the 1- to 2-month period following their summer solstices.5 Runoff during heavy rainfall, which is rich in soil extracts such as phosphorous, iron, and organic carbon, stimulates L majuscula growth and contributes to increased algal blooms.4
Dermatitis and Irritants
The dermatoxins Lyngbyatoxin A (LA) and debromoaplysiatoxin (DAT) cause the inflammatory and necrotic appearance of seaweed dermatitis.1,2,5,8 Lyngbyatoxin A is an indole alkaloid that is closely related to telocidin B, a poisonous compound associated with Streptomyces bacteria.9 Sampling of L majuscula and extraction of the dermatoxin, along with human and animal studies, confirmed DAT irritates the skin and induces dermatitis.5,6Stylocheilus longicauda (sea hare) feeds on L majuscula and contains isolates of DAT in its digestive tract.
Samples of L majuscula taken from several Hawaiian Islands where seaweed dermatitis outbreaks have occurred were examined for differences in toxicities via 6-hour patch tests on human skin.6 The samples obtained from the windward side of Oahu contained DAT and aplysiatoxin, while those obtained from the leeward side and Kahala Beach primarily contained LA. Although DAT and LA are vastly different in their molecular structures, testing elicited the same biologic response and induced the same level of skin irritation.6 Interestingly, not all strands of L majuscula produced LA and DAT and caused seaweed dermatitis; those that did lead to irritation were more red in color than nontoxic blooms.5,9
Cutaneous Manifestations
Seaweed dermatitis resembles chemical and thermal burns, ranging from a mild skin rash to severe contact dermatitis with itchy, swollen, ulcerated lesions.1,7 Patients typically develop a burning or itching sensation beneath their bathing suit or wetsuit that progresses to an erythematous papulovesicular eruption 2 to 24 hours after exposure.2,6 Within a week, vesicles and bullae desquamate, leaving behind tender erosions.1,2,6,8 Inframammary lesions are common in females and scrotal swelling in males.1,6 There is no known association between length of time spent in the water and severity of symptoms.5
Most reactions to L majuscula occur from exposure in the water; however, particles that become aerosolized during strong winds or storms can cause seaweed dermatitis on the face. Inhalation of L majuscula may lead to mucous membrane ulceration and pulmonary edema.1,5,6 Noncutaneous manifestations of seaweed dermatitis include headache, fatigue, and swelling of the eyes, nose, and throat (Figures 1 and 2).1,5
Prevention and Management
To prevent seaweed dermatitis, avoid swimming in ocean water during L majuscula blooms,10 which frequently occur following the summer solstices in the Northern and Southern hemispheres.5 The National Centers for Coastal Ocean Science Harmful Algae Bloom Monitoring System provides real-time access to algae bloom locations.11 Although this monitoring system is not specific to L majuscula, it may be helpful in determining where potential blooms are. Wearing protective clothing such as coveralls may benefit individuals who enter the water during blooms, but it does not guarantee protection.10

magnification ×40). Photograph courtesy of Scott Norton, MD, MPH, MSc (Washington, DC).

Currently, there is no treatment for seaweed dermatitis, but symptom management may reduce discomfort and pain. Washing affected skin with soap and water within an hour of exposure may help reduce the severity of seaweed dermatitis, though studies have shown mixed results.6,7 Application of cool compresses and soothing ointments (eg, calamine) provide symptomatic relief and promote healing.7 The dermatitis typically self-resolves within 1 week.
- Werner K, Marquart L, Norton S. Lyngbya dermatitis (toxic seaweed dermatitis). Int J Dermatol. 2011;51:59-62. doi:10.1111/j.1365-4632.2011.05042.x
- Osborne N, Shaw G. Dermatitis associated with exposure to a marine cyanobacterium during recreational water exposure. BMC Dermatol. 2008;8:5. doi:10.1186/1471-5945-8-5
- Hays G, Richardson A, Robinson C. Climate change and marine plankton. Trends Ecol Evol. 2005;20:337-344. doi:10.1016/j.tree.2005.03.004
- Albert S, O’Neil J, Udy J, et al. Blooms of the cyanobacterium Lyngbya majuscula in costal Queensland, Australia: disparate sites, common factors. Mar Pollut Bull. 2004;51:428-437. doi:10.1016/j.marpolbul.2004.10.016
- Osborne N, Webb P, Shaw G. The toxins of Lyngbya majuscula and their human and ecological health effects. Environ Int. 2001;27:381-392. doi:10.1016/s0160-4120(01)00098-8
- Izumi A, Moore R. Seaweed ( Lyngbya majuscula ) dermatitis . Clin Dermatol . 1987;5:92-100. doi:10.1016/s0738-081x(87)80014-7
- Grauer F, Arnold H. Seaweed dermatitis: first report of a dermatitis-producing marine alga. Arch Dermatol. 1961; 84:720-732. doi:10.1001/archderm.1961.01580170014003
- Taylor M, Stahl-Timmins W, Redshaw C, et al. Toxic alkaloids in Lyngbya majuscula and related tropical marine cyanobacteria. Harmful Algae . 2014;31:1-8. doi:10.1016/j.hal.2013.09.003
- Cardellina J, Marner F, Moore R. Seaweed dermatitis: structure of lyngbyatoxin A. Science. 1979;204:193-195. doi:10.1126/science.107586
- Osborne N. Occupational dermatitis caused by Lyngbya majuscule in Australia. Int J Dermatol . 2012;5:122-123. doi:10.1111/j.1365-4632.2009.04455.x
- Harmful Algal Bloom Monitoring System. National Centers for Coastal Ocean Science. Accessed May 23, 2024. https://coastalscience.noaa.gov/research/stressor-impacts-mitigation/hab-monitoring-system/
- Werner K, Marquart L, Norton S. Lyngbya dermatitis (toxic seaweed dermatitis). Int J Dermatol. 2011;51:59-62. doi:10.1111/j.1365-4632.2011.05042.x
- Osborne N, Shaw G. Dermatitis associated with exposure to a marine cyanobacterium during recreational water exposure. BMC Dermatol. 2008;8:5. doi:10.1186/1471-5945-8-5
- Hays G, Richardson A, Robinson C. Climate change and marine plankton. Trends Ecol Evol. 2005;20:337-344. doi:10.1016/j.tree.2005.03.004
- Albert S, O’Neil J, Udy J, et al. Blooms of the cyanobacterium Lyngbya majuscula in costal Queensland, Australia: disparate sites, common factors. Mar Pollut Bull. 2004;51:428-437. doi:10.1016/j.marpolbul.2004.10.016
- Osborne N, Webb P, Shaw G. The toxins of Lyngbya majuscula and their human and ecological health effects. Environ Int. 2001;27:381-392. doi:10.1016/s0160-4120(01)00098-8
- Izumi A, Moore R. Seaweed ( Lyngbya majuscula ) dermatitis . Clin Dermatol . 1987;5:92-100. doi:10.1016/s0738-081x(87)80014-7
- Grauer F, Arnold H. Seaweed dermatitis: first report of a dermatitis-producing marine alga. Arch Dermatol. 1961; 84:720-732. doi:10.1001/archderm.1961.01580170014003
- Taylor M, Stahl-Timmins W, Redshaw C, et al. Toxic alkaloids in Lyngbya majuscula and related tropical marine cyanobacteria. Harmful Algae . 2014;31:1-8. doi:10.1016/j.hal.2013.09.003
- Cardellina J, Marner F, Moore R. Seaweed dermatitis: structure of lyngbyatoxin A. Science. 1979;204:193-195. doi:10.1126/science.107586
- Osborne N. Occupational dermatitis caused by Lyngbya majuscule in Australia. Int J Dermatol . 2012;5:122-123. doi:10.1111/j.1365-4632.2009.04455.x
- Harmful Algal Bloom Monitoring System. National Centers for Coastal Ocean Science. Accessed May 23, 2024. https://coastalscience.noaa.gov/research/stressor-impacts-mitigation/hab-monitoring-system/
Aquatic Antagonists: Seaweed Dermatitis (Lyngbya majuscula)
Aquatic Antagonists: Seaweed Dermatitis (Lyngbya majuscula)
PRACTICE POINTS
- Lyngbya majuscula causes seaweed dermatitis in swimmers and can be prevented by avoiding rough turbid waters in areas known to have L majuscula blooms.
- Seaweed dermatitis should be included in the differential diagnosis for erythematous papulovesicular rashes manifesting in patients who recently have spent time in the ocean.
Botanical Briefs: Fig Phytophotodermatitis (Ficus carica)
Plant Parts and Nomenclature
Ficus carica (common fig) is a deciduous shrub or small tree with smooth gray bark that can grow up to 10 m in height (Figure 1). It is characterized by many spreading branches, but the trunk rarely grows beyond a diameter of 7 in. Its hairy leaves are coarse on the upper side and soft underneath with 3 to 7 deep lobes that can extend up to 25 cm in length or width; the leaves grow individually, alternating along the sides of the branches. Fig trees often can be seen adorning yards, gardens, and parks, especially in tropical and subtropical climates. Ficus carica should not be confused with Ficus benjamina (weeping fig), a common ornamental tree that also is used to provide shade in hot climates, though both can cause phototoxic skin eruptions.

The common fig tree originated in the Mediterranean and western Asia1 and has been cultivated by humans since the second and third millennia

Ficus carica is a member of the Moraceae family (derived from the Latin name for the mulberry tree), which includes 53 genera and approximately 1400 species, of which about 850 belong to the genus Ficus (the Latin name for a fig tree). The term carica likely comes from the Latin word carricare (to load) to describe a tree loaded with figs. Family members include trees, shrubs, lianas, and herbs that usually contain laticifers with a milky latex.
Traditional Uses
For centuries, components of the fig tree have been used in herbal teas and pastes to treat ailments ranging from sore throats to diarrhea, though there is no evidence to support their efficacy.4 Ancient Indians and Egyptians used plants such as the common fig tree containing furocoumarins to induce hyperpigmentation in vitiligo.5
Phototoxic Components
The leaves and sap of the common fig tree contain psoralens, which are members of the furocoumarin group of chemical compounds and are the source of its phototoxicity. The fruit does not contain psoralens.6-9 The tree also produces proteolytic enzymes such as protease, amylase, ficin, triterpenoids, and lipodiastase that enhance its phototoxic effects.8 Exposure to UV light between 320 and 400 nm following contact with these phototoxic components triggers a reaction in the skin over the course of 1 to 3 days.5 The psoralens bind in epidermal cells, cross-link the DNA, and cause cell-membrane destruction, leading to edema and necrosis.10 The delay in symptoms may be attributed to the time needed to synthesize acute-phase reaction proteins such as tumor necrosis factor α and IL-1.11 In spring and summer months, an increased concentration of psoralens in the leaves and sap contribute to an increased incidence of phytophotodermatitis.9 Humidity and sweat also increase the percutaneous absorption of psoralens.12,13
Allergens
Fig trees produce a latex protein that can cause cross-reactive hypersensitivity reactions in those allergic to F benjamina latex and rubber latex.6 The latex proteins in fig trees can act as airborne respiratory allergens. Ingestion of figs can produce anaphylactic reactions in those sensitized to rubber latex and F benjamina latex.7 Other plant families associated with phototoxic reactions include Rutaceae (lemon, lime, bitter orange), Apiaceae (formerly Umbelliferae)(carrot, parsnip, parsley, dill, celery, hogweed), and Fabaceae (prairie turnip).
Cutaneous Manifestations
Most cases of fig phytophotodermatitis begin with burning, pain, and/or itching within hours of sunlight exposure in areas of the skin that encountered components of the fig tree, often in a linear pattern. The affected areas become erythematous and edematous with formation of bullae and unilocular vesicles over the course of 1 to 3 days.12,14,15 Lesions may extend beyond the region of contact with the fig tree as they spread across the skin due to sweat or friction, and pain may linger even after the lesions resolve.12,13,16 Adults who handle fig trees (eg, pruning) are susceptible to phototoxic reactions, especially those using chain saws or other mechanisms that result in spray exposure, as the photosensitizing sap permeates the wood and bark of the entire tree.17 Similarly, children who handle fig leaves or sap during outdoor play can develop bullous eruptions. Severe cases have resulted in hospital admission after prolonged exposure.16 Additionally, irritant dermatitis may arise from contact with the trichomes or “hairs” on various parts of the plant.

Patients who use natural remedies containing components of the fig tree without the supervision of a medical provider put themselves at risk for unsafe or unwanted adverse effects, such as phytophotodermatitis.12,15,16,18 An entire family presented with burns after they applied fig leaf extract to the skin prior to tanning outside in the sun.19 A 42-year-old woman acquired a severe burn covering 81% of the body surface after topically applying fig leaf tea to the skin as a tanning agent.20 A subset of patients ingesting or applying fig tree components for conditions such as vitiligo, dermatitis, onychomycosis, and motor retardation developed similar cutaneous reactions.13,14,21,22 Lesions resembling finger marks can raise concerns for potential abuse or neglect in children.22
The differential diagnosis for fig phytophotodermatitis includes sunburn, chemical burns, drug-related photosensitivity, infectious lesions (eg, herpes simplex, bullous impetigo, Lyme disease, superficial lymphangitis), connective tissue disease (eg, systemic lupus erythematosus), contact dermatitis, and nonaccidental trauma.12,15,18 Compared to sunburn, phytophotodermatitis tends to increase in severity over days following exposure and heals with dramatic hyperpigmentation, which also prompts visits to dermatology.12
Treatment
Treatment of fig phytophotodermatitis chiefly is symptomatic, including analgesia, appropriate wound care, and infection prophylaxis. Topical and systemic corticosteroids may aid in the resolution of moderate to severe reactions.15,23,24 Even severe injuries over small areas or mild injuries to a high percentage of the total body surface area may require treatment in a burn unit. Patients should be encouraged to use mineral-based sunscreens on the affected areas to reduce the risk for hyperpigmentation. Individuals who regularly handle fig trees should use contact barriers including gloves and protective clothing (eg, long-sleeved shirts, long pants).
- Ikegami H, Nogata H, Hirashima K, et al. Analysis of genetic diversity among European and Asian fig varieties (Ficus carica L.) using ISSR, RAPD, and SSR markers. Genetic Resources and Crop Evolution. 2009;56:201-209.
- Zohary D, Spiegel-Roy P. Beginnings of fruit growing in the Old World. Science. 1975;187:319-327.
- Young R. Young’s Analytical Concordance. Thomas Nelson; 1982.
- Duke JA. Handbook of Medicinal Herbs. CRC Press; 2002.
- Pathak MA, Fitzpatrick TB. Bioassay of natural and synthetic furocoumarins (psoralens). J Invest Dermatol. 1959;32:509-518.
- Focke M, Hemmer W, Wöhrl S, et al. Cross-reactivity between Ficus benjamina latex and fig fruit in patients with clinical fig allergy. Clin Exp Allergy. 2003;33:971-977.
- Hemmer W, Focke M, Götz M, et al. Sensitization to Ficus benjamina: relationship to natural rubber latex allergy and identification of foods implicated in the Ficus-fruit syndrome. Clin Exp Allergy. 2004;34:1251-1258.
- Bonamonte D, Foti C, Lionetti N, et al. Photoallergic contact dermatitis to 8-methoxypsoralen in Ficus carica. Contact Dermatitis. 2010;62:343-348.
- Zaynoun ST, Aftimos BG, Abi Ali L, et al. Ficus carica; isolation and quantification of the photoactive components. Contact Dermatitis. 1984;11:21-25.
- Tessman JW, Isaacs ST, Hearst JE. Photochemistry of the furan-side 8-methoxypsoralen-thymidine monoadduct inside the DNA helix. conversion to diadduct and to pyrone-side monoadduct. Biochemistry. 1985;24:1669-1676.
- Geary P. Burns related to the use of psoralens as a tanning agent. Burns. 1996;22:636-637.
- Redgrave N, Solomon J. Severe phytophotodermatitis from fig sap: a little known phenomenon. BMJ Case Rep. 2021;14:E238745.
- Ozdamar E, Ozbek S, Akin S. An unusual cause of burn injury: fig leaf decoction used as a remedy for a dermatitis of unknown etiology. J Burn Care Rehabil. 2003;24:229-233; discussion 228.
- Berakha GJ, Lefkovits G. Psoralen phototherapy and phototoxicity. Ann Plast Surg. 1985;14:458-461.
- Papazoglou A, Mantadakis E. Fig tree leaves phytophotodermatitis. J Pediatr. 2021;239:244-245.
- Imen MS, Ahmadabadi A, Tavousi SH, et al. The curious cases of burn by fig tree leaves. Indian J Dermatol. 2019;64:71-73.
- Rouaiguia-Bouakkaz S, Amira-Guebailia H, Rivière C, et al. Identification and quantification of furanocoumarins in stem bark and wood of eight Algerian varieties of Ficus carica by RP-HPLC-DAD and RP-HPLC-DAD-MS. Nat Prod Commun. 2013;8:485-486.
- Oliveira AA, Morais J, Pires O, et al. Fig tree induced phytophotodermatitis. BMJ Case Rep. 2020;13:E233392.
- Bassioukas K, Stergiopoulou C, Hatzis J. Erythrodermic phytophotodermatitis after application of aqueous fig-leaf extract as an artificial suntan promoter and sunbathing. Contact Dermatitis. 2004;51:94-95.
- Sforza M, Andjelkov K, Zaccheddu R. Severe burn on 81% of body surface after sun tanning. Ulus Travma Acil Cerrahi Derg. 2013;19:383-384.
- Son JH, Jin H, You HS, et al. Five cases of phytophotodermatitis caused by fig leaves and relevant literature review. Ann Dermatol. 2017;29:86-90.
- Abali AE, Aka M, Aydogan C, et al. Burns or phytophotodermatitis, abuse or neglect: confusing aspects of skin lesions caused by the superstitious use of fig leaves. J Burn Care Res. 2012;33:E309-E312.
- Picard C, Morice C, Moreau A, et al. Phytophotodermatitis in children: a difficult diagnosis mimicking other dermatitis. 2017;5:1-3.
- Enjolras O, Soupre V, Picard A. Uncommon benign infantile vascular tumors. Adv Dermatol. 2008;24:105-124.
Plant Parts and Nomenclature
Ficus carica (common fig) is a deciduous shrub or small tree with smooth gray bark that can grow up to 10 m in height (Figure 1). It is characterized by many spreading branches, but the trunk rarely grows beyond a diameter of 7 in. Its hairy leaves are coarse on the upper side and soft underneath with 3 to 7 deep lobes that can extend up to 25 cm in length or width; the leaves grow individually, alternating along the sides of the branches. Fig trees often can be seen adorning yards, gardens, and parks, especially in tropical and subtropical climates. Ficus carica should not be confused with Ficus benjamina (weeping fig), a common ornamental tree that also is used to provide shade in hot climates, though both can cause phototoxic skin eruptions.

The common fig tree originated in the Mediterranean and western Asia1 and has been cultivated by humans since the second and third millennia

Ficus carica is a member of the Moraceae family (derived from the Latin name for the mulberry tree), which includes 53 genera and approximately 1400 species, of which about 850 belong to the genus Ficus (the Latin name for a fig tree). The term carica likely comes from the Latin word carricare (to load) to describe a tree loaded with figs. Family members include trees, shrubs, lianas, and herbs that usually contain laticifers with a milky latex.
Traditional Uses
For centuries, components of the fig tree have been used in herbal teas and pastes to treat ailments ranging from sore throats to diarrhea, though there is no evidence to support their efficacy.4 Ancient Indians and Egyptians used plants such as the common fig tree containing furocoumarins to induce hyperpigmentation in vitiligo.5
Phototoxic Components
The leaves and sap of the common fig tree contain psoralens, which are members of the furocoumarin group of chemical compounds and are the source of its phototoxicity. The fruit does not contain psoralens.6-9 The tree also produces proteolytic enzymes such as protease, amylase, ficin, triterpenoids, and lipodiastase that enhance its phototoxic effects.8 Exposure to UV light between 320 and 400 nm following contact with these phototoxic components triggers a reaction in the skin over the course of 1 to 3 days.5 The psoralens bind in epidermal cells, cross-link the DNA, and cause cell-membrane destruction, leading to edema and necrosis.10 The delay in symptoms may be attributed to the time needed to synthesize acute-phase reaction proteins such as tumor necrosis factor α and IL-1.11 In spring and summer months, an increased concentration of psoralens in the leaves and sap contribute to an increased incidence of phytophotodermatitis.9 Humidity and sweat also increase the percutaneous absorption of psoralens.12,13
Allergens
Fig trees produce a latex protein that can cause cross-reactive hypersensitivity reactions in those allergic to F benjamina latex and rubber latex.6 The latex proteins in fig trees can act as airborne respiratory allergens. Ingestion of figs can produce anaphylactic reactions in those sensitized to rubber latex and F benjamina latex.7 Other plant families associated with phototoxic reactions include Rutaceae (lemon, lime, bitter orange), Apiaceae (formerly Umbelliferae)(carrot, parsnip, parsley, dill, celery, hogweed), and Fabaceae (prairie turnip).
Cutaneous Manifestations
Most cases of fig phytophotodermatitis begin with burning, pain, and/or itching within hours of sunlight exposure in areas of the skin that encountered components of the fig tree, often in a linear pattern. The affected areas become erythematous and edematous with formation of bullae and unilocular vesicles over the course of 1 to 3 days.12,14,15 Lesions may extend beyond the region of contact with the fig tree as they spread across the skin due to sweat or friction, and pain may linger even after the lesions resolve.12,13,16 Adults who handle fig trees (eg, pruning) are susceptible to phototoxic reactions, especially those using chain saws or other mechanisms that result in spray exposure, as the photosensitizing sap permeates the wood and bark of the entire tree.17 Similarly, children who handle fig leaves or sap during outdoor play can develop bullous eruptions. Severe cases have resulted in hospital admission after prolonged exposure.16 Additionally, irritant dermatitis may arise from contact with the trichomes or “hairs” on various parts of the plant.

Patients who use natural remedies containing components of the fig tree without the supervision of a medical provider put themselves at risk for unsafe or unwanted adverse effects, such as phytophotodermatitis.12,15,16,18 An entire family presented with burns after they applied fig leaf extract to the skin prior to tanning outside in the sun.19 A 42-year-old woman acquired a severe burn covering 81% of the body surface after topically applying fig leaf tea to the skin as a tanning agent.20 A subset of patients ingesting or applying fig tree components for conditions such as vitiligo, dermatitis, onychomycosis, and motor retardation developed similar cutaneous reactions.13,14,21,22 Lesions resembling finger marks can raise concerns for potential abuse or neglect in children.22
The differential diagnosis for fig phytophotodermatitis includes sunburn, chemical burns, drug-related photosensitivity, infectious lesions (eg, herpes simplex, bullous impetigo, Lyme disease, superficial lymphangitis), connective tissue disease (eg, systemic lupus erythematosus), contact dermatitis, and nonaccidental trauma.12,15,18 Compared to sunburn, phytophotodermatitis tends to increase in severity over days following exposure and heals with dramatic hyperpigmentation, which also prompts visits to dermatology.12
Treatment
Treatment of fig phytophotodermatitis chiefly is symptomatic, including analgesia, appropriate wound care, and infection prophylaxis. Topical and systemic corticosteroids may aid in the resolution of moderate to severe reactions.15,23,24 Even severe injuries over small areas or mild injuries to a high percentage of the total body surface area may require treatment in a burn unit. Patients should be encouraged to use mineral-based sunscreens on the affected areas to reduce the risk for hyperpigmentation. Individuals who regularly handle fig trees should use contact barriers including gloves and protective clothing (eg, long-sleeved shirts, long pants).
Plant Parts and Nomenclature
Ficus carica (common fig) is a deciduous shrub or small tree with smooth gray bark that can grow up to 10 m in height (Figure 1). It is characterized by many spreading branches, but the trunk rarely grows beyond a diameter of 7 in. Its hairy leaves are coarse on the upper side and soft underneath with 3 to 7 deep lobes that can extend up to 25 cm in length or width; the leaves grow individually, alternating along the sides of the branches. Fig trees often can be seen adorning yards, gardens, and parks, especially in tropical and subtropical climates. Ficus carica should not be confused with Ficus benjamina (weeping fig), a common ornamental tree that also is used to provide shade in hot climates, though both can cause phototoxic skin eruptions.

The common fig tree originated in the Mediterranean and western Asia1 and has been cultivated by humans since the second and third millennia

Ficus carica is a member of the Moraceae family (derived from the Latin name for the mulberry tree), which includes 53 genera and approximately 1400 species, of which about 850 belong to the genus Ficus (the Latin name for a fig tree). The term carica likely comes from the Latin word carricare (to load) to describe a tree loaded with figs. Family members include trees, shrubs, lianas, and herbs that usually contain laticifers with a milky latex.
Traditional Uses
For centuries, components of the fig tree have been used in herbal teas and pastes to treat ailments ranging from sore throats to diarrhea, though there is no evidence to support their efficacy.4 Ancient Indians and Egyptians used plants such as the common fig tree containing furocoumarins to induce hyperpigmentation in vitiligo.5
Phototoxic Components
The leaves and sap of the common fig tree contain psoralens, which are members of the furocoumarin group of chemical compounds and are the source of its phototoxicity. The fruit does not contain psoralens.6-9 The tree also produces proteolytic enzymes such as protease, amylase, ficin, triterpenoids, and lipodiastase that enhance its phototoxic effects.8 Exposure to UV light between 320 and 400 nm following contact with these phototoxic components triggers a reaction in the skin over the course of 1 to 3 days.5 The psoralens bind in epidermal cells, cross-link the DNA, and cause cell-membrane destruction, leading to edema and necrosis.10 The delay in symptoms may be attributed to the time needed to synthesize acute-phase reaction proteins such as tumor necrosis factor α and IL-1.11 In spring and summer months, an increased concentration of psoralens in the leaves and sap contribute to an increased incidence of phytophotodermatitis.9 Humidity and sweat also increase the percutaneous absorption of psoralens.12,13
Allergens
Fig trees produce a latex protein that can cause cross-reactive hypersensitivity reactions in those allergic to F benjamina latex and rubber latex.6 The latex proteins in fig trees can act as airborne respiratory allergens. Ingestion of figs can produce anaphylactic reactions in those sensitized to rubber latex and F benjamina latex.7 Other plant families associated with phototoxic reactions include Rutaceae (lemon, lime, bitter orange), Apiaceae (formerly Umbelliferae)(carrot, parsnip, parsley, dill, celery, hogweed), and Fabaceae (prairie turnip).
Cutaneous Manifestations
Most cases of fig phytophotodermatitis begin with burning, pain, and/or itching within hours of sunlight exposure in areas of the skin that encountered components of the fig tree, often in a linear pattern. The affected areas become erythematous and edematous with formation of bullae and unilocular vesicles over the course of 1 to 3 days.12,14,15 Lesions may extend beyond the region of contact with the fig tree as they spread across the skin due to sweat or friction, and pain may linger even after the lesions resolve.12,13,16 Adults who handle fig trees (eg, pruning) are susceptible to phototoxic reactions, especially those using chain saws or other mechanisms that result in spray exposure, as the photosensitizing sap permeates the wood and bark of the entire tree.17 Similarly, children who handle fig leaves or sap during outdoor play can develop bullous eruptions. Severe cases have resulted in hospital admission after prolonged exposure.16 Additionally, irritant dermatitis may arise from contact with the trichomes or “hairs” on various parts of the plant.

Patients who use natural remedies containing components of the fig tree without the supervision of a medical provider put themselves at risk for unsafe or unwanted adverse effects, such as phytophotodermatitis.12,15,16,18 An entire family presented with burns after they applied fig leaf extract to the skin prior to tanning outside in the sun.19 A 42-year-old woman acquired a severe burn covering 81% of the body surface after topically applying fig leaf tea to the skin as a tanning agent.20 A subset of patients ingesting or applying fig tree components for conditions such as vitiligo, dermatitis, onychomycosis, and motor retardation developed similar cutaneous reactions.13,14,21,22 Lesions resembling finger marks can raise concerns for potential abuse or neglect in children.22
The differential diagnosis for fig phytophotodermatitis includes sunburn, chemical burns, drug-related photosensitivity, infectious lesions (eg, herpes simplex, bullous impetigo, Lyme disease, superficial lymphangitis), connective tissue disease (eg, systemic lupus erythematosus), contact dermatitis, and nonaccidental trauma.12,15,18 Compared to sunburn, phytophotodermatitis tends to increase in severity over days following exposure and heals with dramatic hyperpigmentation, which also prompts visits to dermatology.12
Treatment
Treatment of fig phytophotodermatitis chiefly is symptomatic, including analgesia, appropriate wound care, and infection prophylaxis. Topical and systemic corticosteroids may aid in the resolution of moderate to severe reactions.15,23,24 Even severe injuries over small areas or mild injuries to a high percentage of the total body surface area may require treatment in a burn unit. Patients should be encouraged to use mineral-based sunscreens on the affected areas to reduce the risk for hyperpigmentation. Individuals who regularly handle fig trees should use contact barriers including gloves and protective clothing (eg, long-sleeved shirts, long pants).
- Ikegami H, Nogata H, Hirashima K, et al. Analysis of genetic diversity among European and Asian fig varieties (Ficus carica L.) using ISSR, RAPD, and SSR markers. Genetic Resources and Crop Evolution. 2009;56:201-209.
- Zohary D, Spiegel-Roy P. Beginnings of fruit growing in the Old World. Science. 1975;187:319-327.
- Young R. Young’s Analytical Concordance. Thomas Nelson; 1982.
- Duke JA. Handbook of Medicinal Herbs. CRC Press; 2002.
- Pathak MA, Fitzpatrick TB. Bioassay of natural and synthetic furocoumarins (psoralens). J Invest Dermatol. 1959;32:509-518.
- Focke M, Hemmer W, Wöhrl S, et al. Cross-reactivity between Ficus benjamina latex and fig fruit in patients with clinical fig allergy. Clin Exp Allergy. 2003;33:971-977.
- Hemmer W, Focke M, Götz M, et al. Sensitization to Ficus benjamina: relationship to natural rubber latex allergy and identification of foods implicated in the Ficus-fruit syndrome. Clin Exp Allergy. 2004;34:1251-1258.
- Bonamonte D, Foti C, Lionetti N, et al. Photoallergic contact dermatitis to 8-methoxypsoralen in Ficus carica. Contact Dermatitis. 2010;62:343-348.
- Zaynoun ST, Aftimos BG, Abi Ali L, et al. Ficus carica; isolation and quantification of the photoactive components. Contact Dermatitis. 1984;11:21-25.
- Tessman JW, Isaacs ST, Hearst JE. Photochemistry of the furan-side 8-methoxypsoralen-thymidine monoadduct inside the DNA helix. conversion to diadduct and to pyrone-side monoadduct. Biochemistry. 1985;24:1669-1676.
- Geary P. Burns related to the use of psoralens as a tanning agent. Burns. 1996;22:636-637.
- Redgrave N, Solomon J. Severe phytophotodermatitis from fig sap: a little known phenomenon. BMJ Case Rep. 2021;14:E238745.
- Ozdamar E, Ozbek S, Akin S. An unusual cause of burn injury: fig leaf decoction used as a remedy for a dermatitis of unknown etiology. J Burn Care Rehabil. 2003;24:229-233; discussion 228.
- Berakha GJ, Lefkovits G. Psoralen phototherapy and phototoxicity. Ann Plast Surg. 1985;14:458-461.
- Papazoglou A, Mantadakis E. Fig tree leaves phytophotodermatitis. J Pediatr. 2021;239:244-245.
- Imen MS, Ahmadabadi A, Tavousi SH, et al. The curious cases of burn by fig tree leaves. Indian J Dermatol. 2019;64:71-73.
- Rouaiguia-Bouakkaz S, Amira-Guebailia H, Rivière C, et al. Identification and quantification of furanocoumarins in stem bark and wood of eight Algerian varieties of Ficus carica by RP-HPLC-DAD and RP-HPLC-DAD-MS. Nat Prod Commun. 2013;8:485-486.
- Oliveira AA, Morais J, Pires O, et al. Fig tree induced phytophotodermatitis. BMJ Case Rep. 2020;13:E233392.
- Bassioukas K, Stergiopoulou C, Hatzis J. Erythrodermic phytophotodermatitis after application of aqueous fig-leaf extract as an artificial suntan promoter and sunbathing. Contact Dermatitis. 2004;51:94-95.
- Sforza M, Andjelkov K, Zaccheddu R. Severe burn on 81% of body surface after sun tanning. Ulus Travma Acil Cerrahi Derg. 2013;19:383-384.
- Son JH, Jin H, You HS, et al. Five cases of phytophotodermatitis caused by fig leaves and relevant literature review. Ann Dermatol. 2017;29:86-90.
- Abali AE, Aka M, Aydogan C, et al. Burns or phytophotodermatitis, abuse or neglect: confusing aspects of skin lesions caused by the superstitious use of fig leaves. J Burn Care Res. 2012;33:E309-E312.
- Picard C, Morice C, Moreau A, et al. Phytophotodermatitis in children: a difficult diagnosis mimicking other dermatitis. 2017;5:1-3.
- Enjolras O, Soupre V, Picard A. Uncommon benign infantile vascular tumors. Adv Dermatol. 2008;24:105-124.
- Ikegami H, Nogata H, Hirashima K, et al. Analysis of genetic diversity among European and Asian fig varieties (Ficus carica L.) using ISSR, RAPD, and SSR markers. Genetic Resources and Crop Evolution. 2009;56:201-209.
- Zohary D, Spiegel-Roy P. Beginnings of fruit growing in the Old World. Science. 1975;187:319-327.
- Young R. Young’s Analytical Concordance. Thomas Nelson; 1982.
- Duke JA. Handbook of Medicinal Herbs. CRC Press; 2002.
- Pathak MA, Fitzpatrick TB. Bioassay of natural and synthetic furocoumarins (psoralens). J Invest Dermatol. 1959;32:509-518.
- Focke M, Hemmer W, Wöhrl S, et al. Cross-reactivity between Ficus benjamina latex and fig fruit in patients with clinical fig allergy. Clin Exp Allergy. 2003;33:971-977.
- Hemmer W, Focke M, Götz M, et al. Sensitization to Ficus benjamina: relationship to natural rubber latex allergy and identification of foods implicated in the Ficus-fruit syndrome. Clin Exp Allergy. 2004;34:1251-1258.
- Bonamonte D, Foti C, Lionetti N, et al. Photoallergic contact dermatitis to 8-methoxypsoralen in Ficus carica. Contact Dermatitis. 2010;62:343-348.
- Zaynoun ST, Aftimos BG, Abi Ali L, et al. Ficus carica; isolation and quantification of the photoactive components. Contact Dermatitis. 1984;11:21-25.
- Tessman JW, Isaacs ST, Hearst JE. Photochemistry of the furan-side 8-methoxypsoralen-thymidine monoadduct inside the DNA helix. conversion to diadduct and to pyrone-side monoadduct. Biochemistry. 1985;24:1669-1676.
- Geary P. Burns related to the use of psoralens as a tanning agent. Burns. 1996;22:636-637.
- Redgrave N, Solomon J. Severe phytophotodermatitis from fig sap: a little known phenomenon. BMJ Case Rep. 2021;14:E238745.
- Ozdamar E, Ozbek S, Akin S. An unusual cause of burn injury: fig leaf decoction used as a remedy for a dermatitis of unknown etiology. J Burn Care Rehabil. 2003;24:229-233; discussion 228.
- Berakha GJ, Lefkovits G. Psoralen phototherapy and phototoxicity. Ann Plast Surg. 1985;14:458-461.
- Papazoglou A, Mantadakis E. Fig tree leaves phytophotodermatitis. J Pediatr. 2021;239:244-245.
- Imen MS, Ahmadabadi A, Tavousi SH, et al. The curious cases of burn by fig tree leaves. Indian J Dermatol. 2019;64:71-73.
- Rouaiguia-Bouakkaz S, Amira-Guebailia H, Rivière C, et al. Identification and quantification of furanocoumarins in stem bark and wood of eight Algerian varieties of Ficus carica by RP-HPLC-DAD and RP-HPLC-DAD-MS. Nat Prod Commun. 2013;8:485-486.
- Oliveira AA, Morais J, Pires O, et al. Fig tree induced phytophotodermatitis. BMJ Case Rep. 2020;13:E233392.
- Bassioukas K, Stergiopoulou C, Hatzis J. Erythrodermic phytophotodermatitis after application of aqueous fig-leaf extract as an artificial suntan promoter and sunbathing. Contact Dermatitis. 2004;51:94-95.
- Sforza M, Andjelkov K, Zaccheddu R. Severe burn on 81% of body surface after sun tanning. Ulus Travma Acil Cerrahi Derg. 2013;19:383-384.
- Son JH, Jin H, You HS, et al. Five cases of phytophotodermatitis caused by fig leaves and relevant literature review. Ann Dermatol. 2017;29:86-90.
- Abali AE, Aka M, Aydogan C, et al. Burns or phytophotodermatitis, abuse or neglect: confusing aspects of skin lesions caused by the superstitious use of fig leaves. J Burn Care Res. 2012;33:E309-E312.
- Picard C, Morice C, Moreau A, et al. Phytophotodermatitis in children: a difficult diagnosis mimicking other dermatitis. 2017;5:1-3.
- Enjolras O, Soupre V, Picard A. Uncommon benign infantile vascular tumors. Adv Dermatol. 2008;24:105-124.
Practice Points
- Exposure to the components of the common fig tree (Ficus carica) can induce phytophotodermatitis.
- Notable postinflammatory hyperpigmentation typically occurs in the healing stage of fig phytophotodermatitis.
What’s Eating You? Carpet Beetles (Dermestidae)
Carpet beetle larvae of the family Dermestidae have been documented to cause both acute and delayed hypersensitivity reactions in susceptible individuals. These larvae have specialized horizontal rows of spear-shaped hairs called hastisetae, which detach easily into the surrounding environment and are small enough to travel by air. Exposure to hastisetae has been tied to adverse effects ranging from dermatitis to rhinoconjunctivitis and acute asthma, with treatment being mostly empiric and symptom based. Due to the pervasiveness of carpet beetles in homes, improved awareness of dermestid-induced manifestations is valuable for clinicians.
Beetles in the Dermestidae family do not bite humans but have been reported to cause skin reactions in addition to other symptoms typical of an allergic reaction. Skin contact with larval hairs (hastisetae) of these insects—known as carpet, larder, or hide beetles — may cause urticarial or edematous papules that are mistaken for papular urticaria or arthropod bites. 1 There are approximately 500 to 700 species of carpet beetles worldwide. Carpet beetles are a clinically underrecognized cause of allergic contact dermatitis given their frequent presence in homes across the world. 2 Carpet beetle larvae feed on shed skin, feathers, hair, wool, book bindings, felt, leather, wood, silk, and sometimes grains and thus can be found nearly anywhere. Most symptom-inducing exposures to Dermestidae beetles occur occupationally, such as in museum curators working hands-on with collection materials and workers handling infested materials such as wool. 3,4 In-home Dermestidae exposure may lead to symptoms, especially if regularly worn clothing and bedding materials are infested. The broad palate of dermestid members has resulted in substantial contamination of stored materials such as flour and fabric in addition to the destruction of museum collections. 5-7
The larvae of some dermestid species, most commonly of the genera Anthrenus and Dermestes, are 2 to 3 mm in length and have detachable hairlike hastisetae that shed into the surrounding environment throughout larval development (Figure 1).8 The hastisetae, located on the thoracic and abdominal segments (tergites), serve as a larval defense mechanism. When prodded, the round, hairy, wormlike larvae tense up and can raise their abdominal tergites while splaying the hastisetae out in a fanlike manner.9 Similar to porcupine quills, the hastisetae easily detach and can entrap the appendages of invertebrate predators. Hastisetae are not known to be sharp enough to puncture human skin, but friction and irritation from skin contact and superficial sticking of the hastisetae into mucous membranes and noncornified epithelium, such as in the bronchial airways, are thought to induce hypersensitivity reactions in susceptible individuals.

Additionally, hastisetae and the exoskeletons of both adult and larval dermestid beetles are composed mostly of chitin, which is highly allergenic. Chitin has been found to play a proinflammatory role in ocular inflammation, asthma, and bronchial reactivity via T helper cell (TH2)–mediated cellular interactions.10-12 Larvae shed their exoskeletons, including hastisetae, multiple times over the course of their development, which contributes to their potential allergen burden (Figure 2). Reports of positive prick and/or patch testing to larval components indicate some cases of both acute type 1 and delayed type 4 hypersensitivity reactions.4,8,13
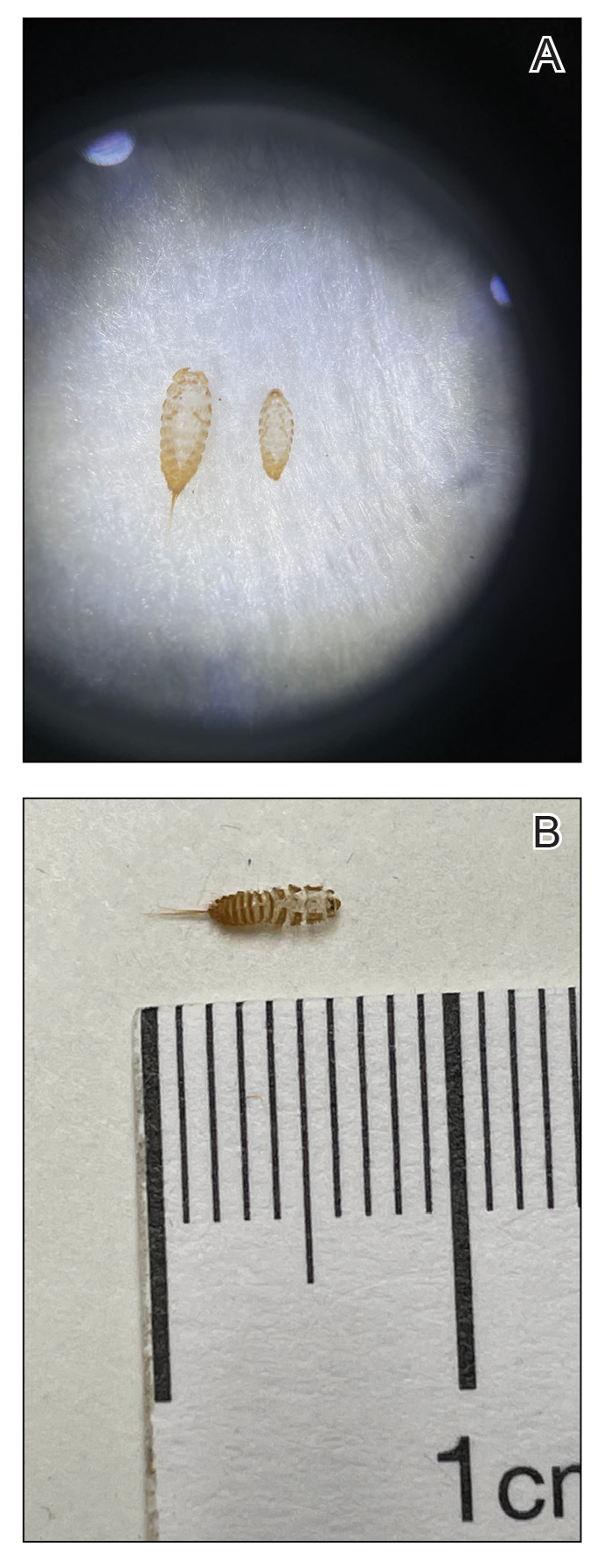
Clinical Presentation and Diagnosis
Multiple erythematous urticarial papules, papulopustules, and papulovesicles are the typical manifestations of dermestid dermatitis.3,4,13-16 Figure 3 demonstrates several characteristic edematous papules with background erythema. Unlike the clusters seen with flea and bed bug bites, dermestid-induced lesions typically are single and scattered, with a propensity for exposed limbs and the face. Exposure to hastisetae commonly results in classic allergic symptoms including rhinitis, conjunctivitis, coughing, wheezing, sneezing, and intranasal and periocular pruritus, even in those with no personal history of atopy.17-19 Lymphadenopathy, vasculitis, and allergic alveolitis also have been reported.20 A large infestation in which many individual beetles as well as larvae can be found in 1 or more areas of the inhabited structure has been reported to cause more severe symptoms, including acute eczema, otitis externa, lymphocytic vasculitis, and allergic alveolitis, all of which resolved within 3 months of thorough deinfestation cleaning.21

Skin-prick and/or patch testing is not necessary for this clinical diagnosis of dermestid-induced allergic contact dermatitis. This diagnosis is bolstered by (but does not require a history of) repeated symptom induction upon performing certain activities (eg, handling taxidermy specimens) and/or in certain environments (eg, only at home). Because of individual differences in hypersensitivity to dermestid parts, it is not typical for all members of a household to be affected.
When there are multiple potential suspected allergens or an unknown cause for symptoms despite a detailed history, allergy testing can be useful in confirming a diagnosis and directing management. Immediate-onset type 1 hypersensitivity reactions are evaluated using skin-prick testing or serum IgE levels, whereas delayed type 4 hypersensitivity reactions can be evaluated using patch testing. Type 1 reactions tend to present with classic allergy symptoms, especially where there are abundant mast cells to degranulate in the skin and mucosa of the gastrointestinal and respiratory tracts; these symptoms range from mild wheezing, urticaria, periorbital pruritus, and sneezing to outright asthma, diarrhea, rhinoconjunctivitis, and even anaphylaxis. With these reactions, initial exposure to an antigen such as chitin in the hastisetae leads to an asymptomatic sensitization against the antigen in which its introduction leads to a TH2-skewed cellular response, which promotes B-cell production of IgE antibodies. Upon subsequent exposure to this antigen, IgE antibodies bound to mast cells will lead them to degranulate with release of histamine and other proinflammatory molecules, resulting in clinical manifestations. The skin-prick test relies on introduction of potential antigens through the epidermis into the dermis with a sharp lancet to induce IgE antibody activation and then degranulation of the patient’s mast cells, resulting in a pruritic erythematous wheal. This IgE-mediated process has been shown to occur in response to dermestid larval parts among household dust, resulting in chronic coughing, sneezing, nasal pruritus, and asthma.15,17,22
Type 4 hypersensitivity reactions are T-cell mediated and also include a sensitization phase followed by symptom manifestation upon repeat exposure; however, these reactions usually are not immediate and can take up to 72 hours after exposure to manifest.23 This is because T cells specific to the antigen do not lead a process resulting in antibodies but instead recruit numerous other TH1-polarized mediators upon re-exposure to activate cytotoxic CD8+ T cells and macrophages to attempt to neutralize the antigen. Many type 4 reactions result in mostly cutaneous manifestations, such as contact dermatitis. Patch testing involves adhering potential allergens to the skin for a time with assessments at regular intervals to evaluate the level of reaction from weakly positive to severe. At minimum, most reports of dermestid-related manifestations include a rash such as erythematous papules, and several published cases involving patch testing have yielded positive results to various preparations of larval parts.3,14,21
Management and Treatment
Prevention of dermestid exposure is difficult given the myriad materials eaten by the larvae. An insect exterminator should verify and treat a carpet beetle infestation, while a dermatologist can treat symptomatic individuals. Treatment is driven by the severity of the patient’s discomfort and is aimed at both symptomatic relief and reducing dermestid exposure moving forward. Although in certain environments it will be nearly impossible to eradicate Dermestidae, cleaning thoroughly and regularly may go far to reduce exposure and associated symptoms.
Clothing and other materials such as bedding that will have direct skin contact should be washed to remove hastisetae and be stored in airtight containers in addition to items made with animal fibers, such as wool sweaters and down blankets. Mattresses, flooring, rugs, curtains, and other amenable areas should be vacuumed thoroughly, and the vacuum bag should be placed in the trash afterward. Protective pillow and mattress covers should be used. Stuffed animals in infested areas should be thrown away if not able to be completely washed and dried. Air conditioning systems may spread larval hairs away from the site of infestation and should be cleaned as much as possible. Surfaces where beetles and larvae also are commonly seen, such as windowsills, and hidden among closet and pantry items should also be wiped clean to remove both insects and potential substrate. In one case, scraping the wood flooring and applying a thick coat of varnish in addition to removing all stuffed animals from an affected individual’s home allowed for resolution of symptoms.17
Treatment for symptoms includes topical anti-inflammatory agents and/or oral antihistamines, with improvement in symptoms typically occurring within days and resolution dependent on level of exposure moving forward.
Final Thoughts
- Gumina ME, Yan AC. Carpet beetle dermatitis mimicking bullous impetigo. Pediatr Dermatol. 2021;38:329-331. doi:10.1111/pde.14453
- Bertone MA, Leong M, Bayless KM, et al. Arthropods of the great indoors: characterizing diversity inside urban and suburban homes. PeerJ. 2016;4:E1582. doi:10.7717/peerj.1582
- Siegel S, Lee N, Rohr A, et. al. Evaluation of dermestid sensitivity in museum personnel. J Allergy Clin Immunol. 1991;87:190. doi:10.1016/0091-6749(91)91488-F
- Brito FF, Mur P, Barber D, et al. Occupational rhinoconjunctivitis and asthma in a wool worker caused by Dermestidae spp. Allergy. 2002;57:1191-1194.
- Stengaard HL, Akerlund M, Grontoft T, et al. Future pest status of an insect pest in museums, Attagenus smirnovi: distribution and food consumption in relation to climate change. J Cult Herit. 2012;13:22l-227.
- Veer V, Negi BK, Rao KM. Dermestid beetles and some other insect pests associated with stored silkworm cocoons in India, including a world list of dermestid species found attacking this commodity. J Stored Products Research. 1996;32:69-89.
- Veer V, Prasad R, Rao KM. Taxonomic and biological notes on Attagenus and Anthrenus spp. (Coleoptera: Dermestidae) found damaging stored woolen fabrics in India. J Stored Products Research. 1991;27:189-198.
- Háva J. World Catalogue of Insects. Volume 13. Dermestidae (Coleoptera). Brill; 2015.
- Ruzzier E, Kadej M, Di Giulio A, et al. Entangling the enemy: ecological, systematic, and medical implications of dermestid beetle Hastisetae. Insects. 2021;12:436. doi:10.3390/insects12050436
- Arae K, Morita H, Unno H, et al. Chitin promotes antigen-specific Th2 cell-mediated murine asthma through induction of IL-33-mediated IL-1β production by DCs. Sci Rep. 2018;8:11721.
- Brinchmann BC, Bayat M, Brøgger T, et. al. A possible role of chitin in the pathogenesis of asthma and allergy. Ann Agric Environ Med. 2011;18:7-12.
- Bucolo C, Musumeci M, Musumeci S, et al. Acidic mammalian chitinase and the eye: implications for ocular inflammatory diseases. Front Pharmacol. 2011;2:1-4.
- Hoverson K, Wohltmann WE, Pollack RJ, et al. Dermestid dermatitis in a 2-year-old girl: case report and review of the literature. Pediatr Dermatol. 2015;32:E228-E233. doi:10.1111/pde.12641
- Simon L, Boukari F, Oumarou H, et al. Anthrenus sp. and an uncommon cluster of dermatitis. Emerg Infect Dis. 2021;27:1940-1943. doi:10.3201/eid2707.203245
- Ahmed R, Moy R, Barr R, et al. Carpet beetle dermatitis. J Am Acad Dermatol. 1981;5:428-432.
- MacArthur K, Richardson V, Novoa R, et al. Carpet beetle dermatitis: a possibly under-recognized entity. Int J Dermatol. 2016;55:577-579.
- Cuesta-Herranz J, de las Heras M, Sastre J, et al. Asthma caused by Dermestidae (black carpet beetle): a new allergen in house dust. J Allergy Clin Immunol. 1997;99(1 Pt 1):147-149.
- Bernstein J, Morgan M, Ghosh D, et al. Respiratory sensitization of a worker to the warehouse beetle Trogoderma variabile: an index case report. J Allergy Clin Immunol. 2009;123:1413-1416.
- Gorgojo IE, De Las Heras M, Pastor C, et al. Allergy to Dermestidae: a new indoor allergen? [abstract] J Allergy Clin Immunol. 2015;135:AB105.
- Ruzzier E, Kadej M, Battisti A. Occurrence, ecological function and medical importance of dermestid beetle hastisetae. PeerJ. 2020;8:E8340. doi:10.7717/peerj.8340
- Ramachandran J, Hern J, Almeyda J, et al. Contact dermatitis with cervical lymphadenopathy following exposure to the hide beetle, Dermestes peruvianus. Br J Dermatol. 1997;136:943-945.
- Horster S, Prinz J, Holm N, et al. Anthrenus-dermatitis. Hautarzt. 2002;53:328-331.
- Justiz Vaillant AA, Vashisht R, Zito PM. Immediate hypersensitivity reactions. In: StatPearls. StatPearls Publishing; 2023.
Carpet beetle larvae of the family Dermestidae have been documented to cause both acute and delayed hypersensitivity reactions in susceptible individuals. These larvae have specialized horizontal rows of spear-shaped hairs called hastisetae, which detach easily into the surrounding environment and are small enough to travel by air. Exposure to hastisetae has been tied to adverse effects ranging from dermatitis to rhinoconjunctivitis and acute asthma, with treatment being mostly empiric and symptom based. Due to the pervasiveness of carpet beetles in homes, improved awareness of dermestid-induced manifestations is valuable for clinicians.
Beetles in the Dermestidae family do not bite humans but have been reported to cause skin reactions in addition to other symptoms typical of an allergic reaction. Skin contact with larval hairs (hastisetae) of these insects—known as carpet, larder, or hide beetles — may cause urticarial or edematous papules that are mistaken for papular urticaria or arthropod bites. 1 There are approximately 500 to 700 species of carpet beetles worldwide. Carpet beetles are a clinically underrecognized cause of allergic contact dermatitis given their frequent presence in homes across the world. 2 Carpet beetle larvae feed on shed skin, feathers, hair, wool, book bindings, felt, leather, wood, silk, and sometimes grains and thus can be found nearly anywhere. Most symptom-inducing exposures to Dermestidae beetles occur occupationally, such as in museum curators working hands-on with collection materials and workers handling infested materials such as wool. 3,4 In-home Dermestidae exposure may lead to symptoms, especially if regularly worn clothing and bedding materials are infested. The broad palate of dermestid members has resulted in substantial contamination of stored materials such as flour and fabric in addition to the destruction of museum collections. 5-7
The larvae of some dermestid species, most commonly of the genera Anthrenus and Dermestes, are 2 to 3 mm in length and have detachable hairlike hastisetae that shed into the surrounding environment throughout larval development (Figure 1).8 The hastisetae, located on the thoracic and abdominal segments (tergites), serve as a larval defense mechanism. When prodded, the round, hairy, wormlike larvae tense up and can raise their abdominal tergites while splaying the hastisetae out in a fanlike manner.9 Similar to porcupine quills, the hastisetae easily detach and can entrap the appendages of invertebrate predators. Hastisetae are not known to be sharp enough to puncture human skin, but friction and irritation from skin contact and superficial sticking of the hastisetae into mucous membranes and noncornified epithelium, such as in the bronchial airways, are thought to induce hypersensitivity reactions in susceptible individuals.

Additionally, hastisetae and the exoskeletons of both adult and larval dermestid beetles are composed mostly of chitin, which is highly allergenic. Chitin has been found to play a proinflammatory role in ocular inflammation, asthma, and bronchial reactivity via T helper cell (TH2)–mediated cellular interactions.10-12 Larvae shed their exoskeletons, including hastisetae, multiple times over the course of their development, which contributes to their potential allergen burden (Figure 2). Reports of positive prick and/or patch testing to larval components indicate some cases of both acute type 1 and delayed type 4 hypersensitivity reactions.4,8,13

Clinical Presentation and Diagnosis
Multiple erythematous urticarial papules, papulopustules, and papulovesicles are the typical manifestations of dermestid dermatitis.3,4,13-16 Figure 3 demonstrates several characteristic edematous papules with background erythema. Unlike the clusters seen with flea and bed bug bites, dermestid-induced lesions typically are single and scattered, with a propensity for exposed limbs and the face. Exposure to hastisetae commonly results in classic allergic symptoms including rhinitis, conjunctivitis, coughing, wheezing, sneezing, and intranasal and periocular pruritus, even in those with no personal history of atopy.17-19 Lymphadenopathy, vasculitis, and allergic alveolitis also have been reported.20 A large infestation in which many individual beetles as well as larvae can be found in 1 or more areas of the inhabited structure has been reported to cause more severe symptoms, including acute eczema, otitis externa, lymphocytic vasculitis, and allergic alveolitis, all of which resolved within 3 months of thorough deinfestation cleaning.21

Skin-prick and/or patch testing is not necessary for this clinical diagnosis of dermestid-induced allergic contact dermatitis. This diagnosis is bolstered by (but does not require a history of) repeated symptom induction upon performing certain activities (eg, handling taxidermy specimens) and/or in certain environments (eg, only at home). Because of individual differences in hypersensitivity to dermestid parts, it is not typical for all members of a household to be affected.
When there are multiple potential suspected allergens or an unknown cause for symptoms despite a detailed history, allergy testing can be useful in confirming a diagnosis and directing management. Immediate-onset type 1 hypersensitivity reactions are evaluated using skin-prick testing or serum IgE levels, whereas delayed type 4 hypersensitivity reactions can be evaluated using patch testing. Type 1 reactions tend to present with classic allergy symptoms, especially where there are abundant mast cells to degranulate in the skin and mucosa of the gastrointestinal and respiratory tracts; these symptoms range from mild wheezing, urticaria, periorbital pruritus, and sneezing to outright asthma, diarrhea, rhinoconjunctivitis, and even anaphylaxis. With these reactions, initial exposure to an antigen such as chitin in the hastisetae leads to an asymptomatic sensitization against the antigen in which its introduction leads to a TH2-skewed cellular response, which promotes B-cell production of IgE antibodies. Upon subsequent exposure to this antigen, IgE antibodies bound to mast cells will lead them to degranulate with release of histamine and other proinflammatory molecules, resulting in clinical manifestations. The skin-prick test relies on introduction of potential antigens through the epidermis into the dermis with a sharp lancet to induce IgE antibody activation and then degranulation of the patient’s mast cells, resulting in a pruritic erythematous wheal. This IgE-mediated process has been shown to occur in response to dermestid larval parts among household dust, resulting in chronic coughing, sneezing, nasal pruritus, and asthma.15,17,22
Type 4 hypersensitivity reactions are T-cell mediated and also include a sensitization phase followed by symptom manifestation upon repeat exposure; however, these reactions usually are not immediate and can take up to 72 hours after exposure to manifest.23 This is because T cells specific to the antigen do not lead a process resulting in antibodies but instead recruit numerous other TH1-polarized mediators upon re-exposure to activate cytotoxic CD8+ T cells and macrophages to attempt to neutralize the antigen. Many type 4 reactions result in mostly cutaneous manifestations, such as contact dermatitis. Patch testing involves adhering potential allergens to the skin for a time with assessments at regular intervals to evaluate the level of reaction from weakly positive to severe. At minimum, most reports of dermestid-related manifestations include a rash such as erythematous papules, and several published cases involving patch testing have yielded positive results to various preparations of larval parts.3,14,21
Management and Treatment
Prevention of dermestid exposure is difficult given the myriad materials eaten by the larvae. An insect exterminator should verify and treat a carpet beetle infestation, while a dermatologist can treat symptomatic individuals. Treatment is driven by the severity of the patient’s discomfort and is aimed at both symptomatic relief and reducing dermestid exposure moving forward. Although in certain environments it will be nearly impossible to eradicate Dermestidae, cleaning thoroughly and regularly may go far to reduce exposure and associated symptoms.
Clothing and other materials such as bedding that will have direct skin contact should be washed to remove hastisetae and be stored in airtight containers in addition to items made with animal fibers, such as wool sweaters and down blankets. Mattresses, flooring, rugs, curtains, and other amenable areas should be vacuumed thoroughly, and the vacuum bag should be placed in the trash afterward. Protective pillow and mattress covers should be used. Stuffed animals in infested areas should be thrown away if not able to be completely washed and dried. Air conditioning systems may spread larval hairs away from the site of infestation and should be cleaned as much as possible. Surfaces where beetles and larvae also are commonly seen, such as windowsills, and hidden among closet and pantry items should also be wiped clean to remove both insects and potential substrate. In one case, scraping the wood flooring and applying a thick coat of varnish in addition to removing all stuffed animals from an affected individual’s home allowed for resolution of symptoms.17
Treatment for symptoms includes topical anti-inflammatory agents and/or oral antihistamines, with improvement in symptoms typically occurring within days and resolution dependent on level of exposure moving forward.
Final Thoughts
Carpet beetle larvae of the family Dermestidae have been documented to cause both acute and delayed hypersensitivity reactions in susceptible individuals. These larvae have specialized horizontal rows of spear-shaped hairs called hastisetae, which detach easily into the surrounding environment and are small enough to travel by air. Exposure to hastisetae has been tied to adverse effects ranging from dermatitis to rhinoconjunctivitis and acute asthma, with treatment being mostly empiric and symptom based. Due to the pervasiveness of carpet beetles in homes, improved awareness of dermestid-induced manifestations is valuable for clinicians.
Beetles in the Dermestidae family do not bite humans but have been reported to cause skin reactions in addition to other symptoms typical of an allergic reaction. Skin contact with larval hairs (hastisetae) of these insects—known as carpet, larder, or hide beetles — may cause urticarial or edematous papules that are mistaken for papular urticaria or arthropod bites. 1 There are approximately 500 to 700 species of carpet beetles worldwide. Carpet beetles are a clinically underrecognized cause of allergic contact dermatitis given their frequent presence in homes across the world. 2 Carpet beetle larvae feed on shed skin, feathers, hair, wool, book bindings, felt, leather, wood, silk, and sometimes grains and thus can be found nearly anywhere. Most symptom-inducing exposures to Dermestidae beetles occur occupationally, such as in museum curators working hands-on with collection materials and workers handling infested materials such as wool. 3,4 In-home Dermestidae exposure may lead to symptoms, especially if regularly worn clothing and bedding materials are infested. The broad palate of dermestid members has resulted in substantial contamination of stored materials such as flour and fabric in addition to the destruction of museum collections. 5-7
The larvae of some dermestid species, most commonly of the genera Anthrenus and Dermestes, are 2 to 3 mm in length and have detachable hairlike hastisetae that shed into the surrounding environment throughout larval development (Figure 1).8 The hastisetae, located on the thoracic and abdominal segments (tergites), serve as a larval defense mechanism. When prodded, the round, hairy, wormlike larvae tense up and can raise their abdominal tergites while splaying the hastisetae out in a fanlike manner.9 Similar to porcupine quills, the hastisetae easily detach and can entrap the appendages of invertebrate predators. Hastisetae are not known to be sharp enough to puncture human skin, but friction and irritation from skin contact and superficial sticking of the hastisetae into mucous membranes and noncornified epithelium, such as in the bronchial airways, are thought to induce hypersensitivity reactions in susceptible individuals.

Additionally, hastisetae and the exoskeletons of both adult and larval dermestid beetles are composed mostly of chitin, which is highly allergenic. Chitin has been found to play a proinflammatory role in ocular inflammation, asthma, and bronchial reactivity via T helper cell (TH2)–mediated cellular interactions.10-12 Larvae shed their exoskeletons, including hastisetae, multiple times over the course of their development, which contributes to their potential allergen burden (Figure 2). Reports of positive prick and/or patch testing to larval components indicate some cases of both acute type 1 and delayed type 4 hypersensitivity reactions.4,8,13

Clinical Presentation and Diagnosis
Multiple erythematous urticarial papules, papulopustules, and papulovesicles are the typical manifestations of dermestid dermatitis.3,4,13-16 Figure 3 demonstrates several characteristic edematous papules with background erythema. Unlike the clusters seen with flea and bed bug bites, dermestid-induced lesions typically are single and scattered, with a propensity for exposed limbs and the face. Exposure to hastisetae commonly results in classic allergic symptoms including rhinitis, conjunctivitis, coughing, wheezing, sneezing, and intranasal and periocular pruritus, even in those with no personal history of atopy.17-19 Lymphadenopathy, vasculitis, and allergic alveolitis also have been reported.20 A large infestation in which many individual beetles as well as larvae can be found in 1 or more areas of the inhabited structure has been reported to cause more severe symptoms, including acute eczema, otitis externa, lymphocytic vasculitis, and allergic alveolitis, all of which resolved within 3 months of thorough deinfestation cleaning.21

Skin-prick and/or patch testing is not necessary for this clinical diagnosis of dermestid-induced allergic contact dermatitis. This diagnosis is bolstered by (but does not require a history of) repeated symptom induction upon performing certain activities (eg, handling taxidermy specimens) and/or in certain environments (eg, only at home). Because of individual differences in hypersensitivity to dermestid parts, it is not typical for all members of a household to be affected.
When there are multiple potential suspected allergens or an unknown cause for symptoms despite a detailed history, allergy testing can be useful in confirming a diagnosis and directing management. Immediate-onset type 1 hypersensitivity reactions are evaluated using skin-prick testing or serum IgE levels, whereas delayed type 4 hypersensitivity reactions can be evaluated using patch testing. Type 1 reactions tend to present with classic allergy symptoms, especially where there are abundant mast cells to degranulate in the skin and mucosa of the gastrointestinal and respiratory tracts; these symptoms range from mild wheezing, urticaria, periorbital pruritus, and sneezing to outright asthma, diarrhea, rhinoconjunctivitis, and even anaphylaxis. With these reactions, initial exposure to an antigen such as chitin in the hastisetae leads to an asymptomatic sensitization against the antigen in which its introduction leads to a TH2-skewed cellular response, which promotes B-cell production of IgE antibodies. Upon subsequent exposure to this antigen, IgE antibodies bound to mast cells will lead them to degranulate with release of histamine and other proinflammatory molecules, resulting in clinical manifestations. The skin-prick test relies on introduction of potential antigens through the epidermis into the dermis with a sharp lancet to induce IgE antibody activation and then degranulation of the patient’s mast cells, resulting in a pruritic erythematous wheal. This IgE-mediated process has been shown to occur in response to dermestid larval parts among household dust, resulting in chronic coughing, sneezing, nasal pruritus, and asthma.15,17,22
Type 4 hypersensitivity reactions are T-cell mediated and also include a sensitization phase followed by symptom manifestation upon repeat exposure; however, these reactions usually are not immediate and can take up to 72 hours after exposure to manifest.23 This is because T cells specific to the antigen do not lead a process resulting in antibodies but instead recruit numerous other TH1-polarized mediators upon re-exposure to activate cytotoxic CD8+ T cells and macrophages to attempt to neutralize the antigen. Many type 4 reactions result in mostly cutaneous manifestations, such as contact dermatitis. Patch testing involves adhering potential allergens to the skin for a time with assessments at regular intervals to evaluate the level of reaction from weakly positive to severe. At minimum, most reports of dermestid-related manifestations include a rash such as erythematous papules, and several published cases involving patch testing have yielded positive results to various preparations of larval parts.3,14,21
Management and Treatment
Prevention of dermestid exposure is difficult given the myriad materials eaten by the larvae. An insect exterminator should verify and treat a carpet beetle infestation, while a dermatologist can treat symptomatic individuals. Treatment is driven by the severity of the patient’s discomfort and is aimed at both symptomatic relief and reducing dermestid exposure moving forward. Although in certain environments it will be nearly impossible to eradicate Dermestidae, cleaning thoroughly and regularly may go far to reduce exposure and associated symptoms.
Clothing and other materials such as bedding that will have direct skin contact should be washed to remove hastisetae and be stored in airtight containers in addition to items made with animal fibers, such as wool sweaters and down blankets. Mattresses, flooring, rugs, curtains, and other amenable areas should be vacuumed thoroughly, and the vacuum bag should be placed in the trash afterward. Protective pillow and mattress covers should be used. Stuffed animals in infested areas should be thrown away if not able to be completely washed and dried. Air conditioning systems may spread larval hairs away from the site of infestation and should be cleaned as much as possible. Surfaces where beetles and larvae also are commonly seen, such as windowsills, and hidden among closet and pantry items should also be wiped clean to remove both insects and potential substrate. In one case, scraping the wood flooring and applying a thick coat of varnish in addition to removing all stuffed animals from an affected individual’s home allowed for resolution of symptoms.17
Treatment for symptoms includes topical anti-inflammatory agents and/or oral antihistamines, with improvement in symptoms typically occurring within days and resolution dependent on level of exposure moving forward.
Final Thoughts
- Gumina ME, Yan AC. Carpet beetle dermatitis mimicking bullous impetigo. Pediatr Dermatol. 2021;38:329-331. doi:10.1111/pde.14453
- Bertone MA, Leong M, Bayless KM, et al. Arthropods of the great indoors: characterizing diversity inside urban and suburban homes. PeerJ. 2016;4:E1582. doi:10.7717/peerj.1582
- Siegel S, Lee N, Rohr A, et. al. Evaluation of dermestid sensitivity in museum personnel. J Allergy Clin Immunol. 1991;87:190. doi:10.1016/0091-6749(91)91488-F
- Brito FF, Mur P, Barber D, et al. Occupational rhinoconjunctivitis and asthma in a wool worker caused by Dermestidae spp. Allergy. 2002;57:1191-1194.
- Stengaard HL, Akerlund M, Grontoft T, et al. Future pest status of an insect pest in museums, Attagenus smirnovi: distribution and food consumption in relation to climate change. J Cult Herit. 2012;13:22l-227.
- Veer V, Negi BK, Rao KM. Dermestid beetles and some other insect pests associated with stored silkworm cocoons in India, including a world list of dermestid species found attacking this commodity. J Stored Products Research. 1996;32:69-89.
- Veer V, Prasad R, Rao KM. Taxonomic and biological notes on Attagenus and Anthrenus spp. (Coleoptera: Dermestidae) found damaging stored woolen fabrics in India. J Stored Products Research. 1991;27:189-198.
- Háva J. World Catalogue of Insects. Volume 13. Dermestidae (Coleoptera). Brill; 2015.
- Ruzzier E, Kadej M, Di Giulio A, et al. Entangling the enemy: ecological, systematic, and medical implications of dermestid beetle Hastisetae. Insects. 2021;12:436. doi:10.3390/insects12050436
- Arae K, Morita H, Unno H, et al. Chitin promotes antigen-specific Th2 cell-mediated murine asthma through induction of IL-33-mediated IL-1β production by DCs. Sci Rep. 2018;8:11721.
- Brinchmann BC, Bayat M, Brøgger T, et. al. A possible role of chitin in the pathogenesis of asthma and allergy. Ann Agric Environ Med. 2011;18:7-12.
- Bucolo C, Musumeci M, Musumeci S, et al. Acidic mammalian chitinase and the eye: implications for ocular inflammatory diseases. Front Pharmacol. 2011;2:1-4.
- Hoverson K, Wohltmann WE, Pollack RJ, et al. Dermestid dermatitis in a 2-year-old girl: case report and review of the literature. Pediatr Dermatol. 2015;32:E228-E233. doi:10.1111/pde.12641
- Simon L, Boukari F, Oumarou H, et al. Anthrenus sp. and an uncommon cluster of dermatitis. Emerg Infect Dis. 2021;27:1940-1943. doi:10.3201/eid2707.203245
- Ahmed R, Moy R, Barr R, et al. Carpet beetle dermatitis. J Am Acad Dermatol. 1981;5:428-432.
- MacArthur K, Richardson V, Novoa R, et al. Carpet beetle dermatitis: a possibly under-recognized entity. Int J Dermatol. 2016;55:577-579.
- Cuesta-Herranz J, de las Heras M, Sastre J, et al. Asthma caused by Dermestidae (black carpet beetle): a new allergen in house dust. J Allergy Clin Immunol. 1997;99(1 Pt 1):147-149.
- Bernstein J, Morgan M, Ghosh D, et al. Respiratory sensitization of a worker to the warehouse beetle Trogoderma variabile: an index case report. J Allergy Clin Immunol. 2009;123:1413-1416.
- Gorgojo IE, De Las Heras M, Pastor C, et al. Allergy to Dermestidae: a new indoor allergen? [abstract] J Allergy Clin Immunol. 2015;135:AB105.
- Ruzzier E, Kadej M, Battisti A. Occurrence, ecological function and medical importance of dermestid beetle hastisetae. PeerJ. 2020;8:E8340. doi:10.7717/peerj.8340
- Ramachandran J, Hern J, Almeyda J, et al. Contact dermatitis with cervical lymphadenopathy following exposure to the hide beetle, Dermestes peruvianus. Br J Dermatol. 1997;136:943-945.
- Horster S, Prinz J, Holm N, et al. Anthrenus-dermatitis. Hautarzt. 2002;53:328-331.
- Justiz Vaillant AA, Vashisht R, Zito PM. Immediate hypersensitivity reactions. In: StatPearls. StatPearls Publishing; 2023.
- Gumina ME, Yan AC. Carpet beetle dermatitis mimicking bullous impetigo. Pediatr Dermatol. 2021;38:329-331. doi:10.1111/pde.14453
- Bertone MA, Leong M, Bayless KM, et al. Arthropods of the great indoors: characterizing diversity inside urban and suburban homes. PeerJ. 2016;4:E1582. doi:10.7717/peerj.1582
- Siegel S, Lee N, Rohr A, et. al. Evaluation of dermestid sensitivity in museum personnel. J Allergy Clin Immunol. 1991;87:190. doi:10.1016/0091-6749(91)91488-F
- Brito FF, Mur P, Barber D, et al. Occupational rhinoconjunctivitis and asthma in a wool worker caused by Dermestidae spp. Allergy. 2002;57:1191-1194.
- Stengaard HL, Akerlund M, Grontoft T, et al. Future pest status of an insect pest in museums, Attagenus smirnovi: distribution and food consumption in relation to climate change. J Cult Herit. 2012;13:22l-227.
- Veer V, Negi BK, Rao KM. Dermestid beetles and some other insect pests associated with stored silkworm cocoons in India, including a world list of dermestid species found attacking this commodity. J Stored Products Research. 1996;32:69-89.
- Veer V, Prasad R, Rao KM. Taxonomic and biological notes on Attagenus and Anthrenus spp. (Coleoptera: Dermestidae) found damaging stored woolen fabrics in India. J Stored Products Research. 1991;27:189-198.
- Háva J. World Catalogue of Insects. Volume 13. Dermestidae (Coleoptera). Brill; 2015.
- Ruzzier E, Kadej M, Di Giulio A, et al. Entangling the enemy: ecological, systematic, and medical implications of dermestid beetle Hastisetae. Insects. 2021;12:436. doi:10.3390/insects12050436
- Arae K, Morita H, Unno H, et al. Chitin promotes antigen-specific Th2 cell-mediated murine asthma through induction of IL-33-mediated IL-1β production by DCs. Sci Rep. 2018;8:11721.
- Brinchmann BC, Bayat M, Brøgger T, et. al. A possible role of chitin in the pathogenesis of asthma and allergy. Ann Agric Environ Med. 2011;18:7-12.
- Bucolo C, Musumeci M, Musumeci S, et al. Acidic mammalian chitinase and the eye: implications for ocular inflammatory diseases. Front Pharmacol. 2011;2:1-4.
- Hoverson K, Wohltmann WE, Pollack RJ, et al. Dermestid dermatitis in a 2-year-old girl: case report and review of the literature. Pediatr Dermatol. 2015;32:E228-E233. doi:10.1111/pde.12641
- Simon L, Boukari F, Oumarou H, et al. Anthrenus sp. and an uncommon cluster of dermatitis. Emerg Infect Dis. 2021;27:1940-1943. doi:10.3201/eid2707.203245
- Ahmed R, Moy R, Barr R, et al. Carpet beetle dermatitis. J Am Acad Dermatol. 1981;5:428-432.
- MacArthur K, Richardson V, Novoa R, et al. Carpet beetle dermatitis: a possibly under-recognized entity. Int J Dermatol. 2016;55:577-579.
- Cuesta-Herranz J, de las Heras M, Sastre J, et al. Asthma caused by Dermestidae (black carpet beetle): a new allergen in house dust. J Allergy Clin Immunol. 1997;99(1 Pt 1):147-149.
- Bernstein J, Morgan M, Ghosh D, et al. Respiratory sensitization of a worker to the warehouse beetle Trogoderma variabile: an index case report. J Allergy Clin Immunol. 2009;123:1413-1416.
- Gorgojo IE, De Las Heras M, Pastor C, et al. Allergy to Dermestidae: a new indoor allergen? [abstract] J Allergy Clin Immunol. 2015;135:AB105.
- Ruzzier E, Kadej M, Battisti A. Occurrence, ecological function and medical importance of dermestid beetle hastisetae. PeerJ. 2020;8:E8340. doi:10.7717/peerj.8340
- Ramachandran J, Hern J, Almeyda J, et al. Contact dermatitis with cervical lymphadenopathy following exposure to the hide beetle, Dermestes peruvianus. Br J Dermatol. 1997;136:943-945.
- Horster S, Prinz J, Holm N, et al. Anthrenus-dermatitis. Hautarzt. 2002;53:328-331.
- Justiz Vaillant AA, Vashisht R, Zito PM. Immediate hypersensitivity reactions. In: StatPearls. StatPearls Publishing; 2023.
Practice Points
- Given their ubiquity, dermatologists should be aware of the potential for hypersensitivity reactions to carpet beetles (Dermestidae).
- Pruritic erythematous papules, pustules, and vesicles are the most common manifestations of exposure to larval hairs.
- Treatment is symptom based, and future exposure can be greatly diminished with thorough cleaning of the patient’s environment.
Aquatic Antagonists: Scorpionfish Envenomation
With the growing popularity of water sports and a proliferation of invasive species, human injuries from marine animal envenomation continue to rise.1-3 Members of the scorpionfish family Scorpaenidae are second only to stingrays as the leading cause of the 40,000 to 50,000 injuries annually from marine life worldwide.4 Because scorpionfish represent a growing threat and competition with native species, it has been suggested that they could replace endangered species on restaurant menus.5-8 Scorpionfish have been introduced by humans from tropical to temperate seas and are now common off the coast of California and the eastern coast from New York to Florida, as well as in the Caribbean, the Bahamas, and off the southern coast of Brazil. Victims of scorpionfish stings experience considerable pain and may require days to weeks to fully recover, highlighting the socioeconomic costs and burden of scorpionfish envenomation.9,10 Fishers, divers, swimmers, and aquarium owners are most often affected.
Family
The common term scorpionfish refers to both the family Scorpaenidae and the genus Scorpaena. Members of this family possess similar dorsal, anal, and pelvic fins, though they vary between genera in their size and the potency of the venom they insulate. Other familiar members include the genus Pterois (lionfish) and Synanceja (stonefish). Synanceja are the most venomous within the group, but scorpionfish stings more commonly arise from Pterois and Scorpaena.8 Because of the rare shapes and vibrant colors of scorpionfish, some traders and aquarium owners will seek and pay high prices for these fish, providing further opportunity for envenomation.11,12
Characteristics
Scorpionfish have with a high variation in color, ranging from lighter grays to intense reds depending on their geographic location and habitat. Synanceja are bland in coloration, blending in with rocks and gravel, but the more dramatic-appearing Scorpaena exhibit a large cranium and wide range of multicolored patterns (Figure 1).13Pterois serve as the most conspicuous member of the group with brightly colored red and white stripes (Figure 2). Scorpionfish commonly grow up to 19 inches long and boast 12 dorsal, 2 pelvic, and 3 anal spines housing 5 to 10 mg of venom.14 An integumentary sheath encapsulates each spine housing the glandular tissue that produces the potent venom.

Toxin Properties
Unlike Pterois and Synanceja, Scorpaena do not have venom ducts around their glands, complicating the work of marine biologists aiming to extract and study the venomous toxins. Several studies have managed to isolate scorpionfish venom and overcome its unstable heat-labile nature to investigate its biologic properties.15-20 Several high-molecular-weight proteins (50–800 kDa) comprise the venom, including hyaluronidase, integrin-inhibiting factors, capillary permeability factor, proteases, and some less-understood cytolytic toxins. These factors provoke the inflammatory, proteolytic, hemorrhagic, cardiovascular, and hemolytic biologic activities at both the local and systemic levels, directing damage to wounded tissues and inducing vascular and tissue permeability to reach cellular processes far and wide. Mediators of inflammation include tumor necrosis factor, IL-6, and monocyte chemoattractant protein 1, followed by neutrophils and other mononuclear cells, initiating the immune response at the wound site. Toxin potency remains for up to 2 days after fish death.1

Clinical Manifestation
Physicians may be guided by clinical symptoms in identifying scorpionfish stings, as the patient may not know the identity of their marine assailant. Initially, individuals punctured by scorpionfish spikes will experience an acute pain and burning sensation at the puncture site that may be accompanied by systemic symptoms such as nausea, vomiting, diarrhea, tachycardia, hypotension, loss of consciousness, difficulty breathing, and delirium.9,21-23 The pain will intensify and radiate distal to the site of envenomation, and the wound may exhibit vesiculation, erythema, bruising, pallor, and notable edema.4,24 Pain intensity peaks at 30 to 90 minutes after envenomation, and other systemic symptoms generally last for 24 to 48 hours.25 If patients do not seek prompt treatment, secondary infection may ensue, and the lingering venom in the blister may cause dermal necrosis, paresthesia, and anesthesia. Chronic sequelae may include joint contractures, compartment syndrome, necrotic ulcers, and chronic neuropathy.1
Management
Treatment of scorpionfish stings primarily is palliative and aimed at symptom reduction. Patients should immediately treat wounds with hot but not scalding water immersion.26,27 Given the thermolabile components of scorpionfish venom, the most effective treatment is to soak the affected limb in water 42 °C to 45 °C for 30 to 90 minutes. Any higher temperature may pose risk for scalding burns. Children should be monitored throughout treatment.28 If hot water immersion does not provide relief, oral analgesics may be considered. Stonefish antivenom is available and may be used for any scorpionfish sting given the shared biologic properties between genera. Providers evaluating stings could use sterile irrigation to clean wounds and search for foreign bodies including spine fragments; probing should be accomplished by instruments rather than a gloved finger. Providers should consider culturing wounds and prescribing antibiotics for suspected secondary infections. A tetanus toxoid history also should be elicited, and patients may have a booster administered, as indicated.29
- Rensch G, Murphy-Lavoie HM. Lionfish, scorpionfish, and stonefish toxicity. StatPearls. StatPearls Publishing; May 10, 2022.
- Cearnal L. Red lionfish and ciguatoxin: menace spreading through western hemisphere. Ann Emerg Med. 2012;60:21A-22A. doi:10.1016/j.annemergmed.2012.05.022
- Côté IM, Green SJ. Potential effects of climate change on a marine invasion: the importance of current context. Curr Zool. 2012;58:1-8. doi:10.1093/czoolo/58.1.1
- Venomology of scorpionfishes. In: Santhanam R. Biology and Ecology of Venomous Marine Scorpionfishes. Academic Press; 2019:263-278.
- Ferri J, Staglicˇic´ N, Matić-Skoko S. The black scorpionfish, Scorpaena porcus (Scorpaenidae): could it serve as reliable indicator of Mediterranean coastal communities’ health? Ecol Indicators. 2012;18:25-30. doi:10.1016/j.ecolind.2011.11.004
- Santhanam R. Biology and Ecology of Venomous Marine Scorpionfishes. Academic Press; 2019.
- Morris JA, Akins JL. Feeding ecology of invasive lionfish (Pterois volitans) in the Bahamian Archipelago. Environ Biol Fishes. 2009;86:389-398. doi:10.1007/s10641-009-9538-8
- Albins MA, Hixon MA. Worst case scenario: potential long-term effects of invasive predatory lionfish (Pterois volitans) on Atlantic and Caribbean coral-reef communities. Environ Biol Fishes. 2013;96:1151–1157. doi:10.1007/s10641-011-9795-1
- Haddad V Jr, Martins IA, Makyama HM. Injuries caused by scorpionfishes (Scorpaena plumieri Bloch, 1789 and Scorpaena brasiliensis Cuvier, 1829) in the Southwestern Atlantic Ocean (Brazilian coast): epidemiologic, clinic and therapeutic aspects of 23 stings in humans. Toxicon. 2003;42:79-83. doi:10.1016/s0041-0101(03)00103-x
- Campos FV, Menezes TN, Malacarne PF, et al. A review on the Scorpaena plumieri fish venom and its bioactive compounds. J Venom Anim Toxins Incl Trop Dis. 2016;22:35. doi:10.1186/s40409-016-0090-7
- Needleman RK, Neylan IP, Erickson TB. Environmental and ecological effects of climate change on venomous marine and amphibious species in the wilderness. Wilderness Environ Med. 2018;29:343-356. doi:10.1016/j.wem.2018.04.003
- Aldred B, Erickson T, Lipscomb J. Lionfish envenomations in an urban wilderness. Wilderness Environ Med. 1996;7:291-296. doi:10.1580/1080-6032(1996)007[0291:leiauw]2.3.co;2
- Stewart J, Hughes JM. Life-history traits of the southern hemisphere eastern red scorpionfish, Scorpaena cardinalis (Scorpaenidae: Scorpaeninae). Mar Freshw Res. 2010;61:1290-1297. doi:10.1071/MF10040
- Auerbach PS. Marine envenomations. N Engl J Med. 1991;325:486-493. doi:10.1056/NEJM199108153250707
- Andrich F, Carnielli JB, Cassoli JS, et al. A potent vasoactive cytolysin isolated from Scorpaena plumieri scorpionfish venom. Toxicon. 2010;56:487-496. doi:10.1016/j.toxicon.2010.05.003
- Gomes HL, Andrich F, Mauad H, et al. Cardiovascular effects of scorpionfish (Scorpaena plumieri) venom. Toxicon. 2010;55(2-3):580-589. doi:10.1016/j.toxicon.2009.10.012
- Menezes TN, Carnielli JB, Gomes HL, et al. Local inflammatory response induced by scorpionfish Scorpaena plumieri venom in mice. Toxicon. 2012;60:4-11. doi:10.1016/j.toxicon.2012.03.008
- Schaeffer RC Jr, Carlson RW, Russell FE. Some chemical properties of the venom of the scorpionfish Scorpaena guttata. Toxicon. 1971;9:69-78. doi:10.1016/0041-0101(71)90045-6
- Khalil AM, Wahsha MA, Abu Khadra KM, et al. Biochemical and histopathological effects of the stonefish (Synanceia verrucosa) venom in rats. Toxicon. 2018;142:45-51. doi:10.1016/j.toxicon.2017.12.052
- Mouchbahani-Constance S, Lesperance LS, Petitjean H, et al. Lionfish venom elicits pain predominantly through the activation of nonpeptidergic nociceptors. Pain. 2018;159:2255-2266. doi:10.1097/j.pain.0000000000001326
- Ottuso P. Aquatic dermatology: encounters with the denizens of the deep (and not so deep)—a review. part II: the vertebrates, single-celled organisms, and aquatic biotoxins. Int J Dermatol. 2013;52:268-278. doi:10.1111/j.1365-4632.2011.05426.x
- Bayley HH. Injuries caused by scorpion fish. Trans R Soc Trop Med Hyg. 1940;34:227-230. doi:10.1016/s0035-9203(40)90072-4
- González D. Epidemiological and clinical aspects of certain venomous animals of Spain. Toxicon. 1982;20:925-928. doi:10.1016/0041-0101(82)90080-0
- Halstead BW. Injurious effects from the sting of the scorpionfish, Scorpaena guttata. with report of a case. Calif Med. 1951;74:395-396.
- Vasievich MP, Villarreal JD, Tomecki KJ. Got the travel bug? a review of common infections, infestations, bites, and stings among returning travelers. Am J Clin Dermatol. 2016;17:451-462. doi:10.1007/s40257-016-0203-7
- Barnett S, Saggiomo S, Smout M, et al. Heat deactivation of the stonefish Synanceia horrida venom—implications for first-aid management. Diving Hyperb Med. 2017;47:155-158. doi:10.28920/dhm47.3.155-158
- Russell FE. Weever fish sting: the last word. Br Med J (Clin Res Ed). 1983;287:981-982. doi:10.1136/bmj.287.6397.981-c
- Tomlinson H, Elston DM. Aquatic antagonists: lionfish (Pterois volitans). Cutis. 2018;102:232-234.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337. doi:10.1016/j.emc.2016.12.004
With the growing popularity of water sports and a proliferation of invasive species, human injuries from marine animal envenomation continue to rise.1-3 Members of the scorpionfish family Scorpaenidae are second only to stingrays as the leading cause of the 40,000 to 50,000 injuries annually from marine life worldwide.4 Because scorpionfish represent a growing threat and competition with native species, it has been suggested that they could replace endangered species on restaurant menus.5-8 Scorpionfish have been introduced by humans from tropical to temperate seas and are now common off the coast of California and the eastern coast from New York to Florida, as well as in the Caribbean, the Bahamas, and off the southern coast of Brazil. Victims of scorpionfish stings experience considerable pain and may require days to weeks to fully recover, highlighting the socioeconomic costs and burden of scorpionfish envenomation.9,10 Fishers, divers, swimmers, and aquarium owners are most often affected.
Family
The common term scorpionfish refers to both the family Scorpaenidae and the genus Scorpaena. Members of this family possess similar dorsal, anal, and pelvic fins, though they vary between genera in their size and the potency of the venom they insulate. Other familiar members include the genus Pterois (lionfish) and Synanceja (stonefish). Synanceja are the most venomous within the group, but scorpionfish stings more commonly arise from Pterois and Scorpaena.8 Because of the rare shapes and vibrant colors of scorpionfish, some traders and aquarium owners will seek and pay high prices for these fish, providing further opportunity for envenomation.11,12
Characteristics
Scorpionfish have with a high variation in color, ranging from lighter grays to intense reds depending on their geographic location and habitat. Synanceja are bland in coloration, blending in with rocks and gravel, but the more dramatic-appearing Scorpaena exhibit a large cranium and wide range of multicolored patterns (Figure 1).13Pterois serve as the most conspicuous member of the group with brightly colored red and white stripes (Figure 2). Scorpionfish commonly grow up to 19 inches long and boast 12 dorsal, 2 pelvic, and 3 anal spines housing 5 to 10 mg of venom.14 An integumentary sheath encapsulates each spine housing the glandular tissue that produces the potent venom.

Toxin Properties
Unlike Pterois and Synanceja, Scorpaena do not have venom ducts around their glands, complicating the work of marine biologists aiming to extract and study the venomous toxins. Several studies have managed to isolate scorpionfish venom and overcome its unstable heat-labile nature to investigate its biologic properties.15-20 Several high-molecular-weight proteins (50–800 kDa) comprise the venom, including hyaluronidase, integrin-inhibiting factors, capillary permeability factor, proteases, and some less-understood cytolytic toxins. These factors provoke the inflammatory, proteolytic, hemorrhagic, cardiovascular, and hemolytic biologic activities at both the local and systemic levels, directing damage to wounded tissues and inducing vascular and tissue permeability to reach cellular processes far and wide. Mediators of inflammation include tumor necrosis factor, IL-6, and monocyte chemoattractant protein 1, followed by neutrophils and other mononuclear cells, initiating the immune response at the wound site. Toxin potency remains for up to 2 days after fish death.1

Clinical Manifestation
Physicians may be guided by clinical symptoms in identifying scorpionfish stings, as the patient may not know the identity of their marine assailant. Initially, individuals punctured by scorpionfish spikes will experience an acute pain and burning sensation at the puncture site that may be accompanied by systemic symptoms such as nausea, vomiting, diarrhea, tachycardia, hypotension, loss of consciousness, difficulty breathing, and delirium.9,21-23 The pain will intensify and radiate distal to the site of envenomation, and the wound may exhibit vesiculation, erythema, bruising, pallor, and notable edema.4,24 Pain intensity peaks at 30 to 90 minutes after envenomation, and other systemic symptoms generally last for 24 to 48 hours.25 If patients do not seek prompt treatment, secondary infection may ensue, and the lingering venom in the blister may cause dermal necrosis, paresthesia, and anesthesia. Chronic sequelae may include joint contractures, compartment syndrome, necrotic ulcers, and chronic neuropathy.1
Management
Treatment of scorpionfish stings primarily is palliative and aimed at symptom reduction. Patients should immediately treat wounds with hot but not scalding water immersion.26,27 Given the thermolabile components of scorpionfish venom, the most effective treatment is to soak the affected limb in water 42 °C to 45 °C for 30 to 90 minutes. Any higher temperature may pose risk for scalding burns. Children should be monitored throughout treatment.28 If hot water immersion does not provide relief, oral analgesics may be considered. Stonefish antivenom is available and may be used for any scorpionfish sting given the shared biologic properties between genera. Providers evaluating stings could use sterile irrigation to clean wounds and search for foreign bodies including spine fragments; probing should be accomplished by instruments rather than a gloved finger. Providers should consider culturing wounds and prescribing antibiotics for suspected secondary infections. A tetanus toxoid history also should be elicited, and patients may have a booster administered, as indicated.29
With the growing popularity of water sports and a proliferation of invasive species, human injuries from marine animal envenomation continue to rise.1-3 Members of the scorpionfish family Scorpaenidae are second only to stingrays as the leading cause of the 40,000 to 50,000 injuries annually from marine life worldwide.4 Because scorpionfish represent a growing threat and competition with native species, it has been suggested that they could replace endangered species on restaurant menus.5-8 Scorpionfish have been introduced by humans from tropical to temperate seas and are now common off the coast of California and the eastern coast from New York to Florida, as well as in the Caribbean, the Bahamas, and off the southern coast of Brazil. Victims of scorpionfish stings experience considerable pain and may require days to weeks to fully recover, highlighting the socioeconomic costs and burden of scorpionfish envenomation.9,10 Fishers, divers, swimmers, and aquarium owners are most often affected.
Family
The common term scorpionfish refers to both the family Scorpaenidae and the genus Scorpaena. Members of this family possess similar dorsal, anal, and pelvic fins, though they vary between genera in their size and the potency of the venom they insulate. Other familiar members include the genus Pterois (lionfish) and Synanceja (stonefish). Synanceja are the most venomous within the group, but scorpionfish stings more commonly arise from Pterois and Scorpaena.8 Because of the rare shapes and vibrant colors of scorpionfish, some traders and aquarium owners will seek and pay high prices for these fish, providing further opportunity for envenomation.11,12
Characteristics
Scorpionfish have with a high variation in color, ranging from lighter grays to intense reds depending on their geographic location and habitat. Synanceja are bland in coloration, blending in with rocks and gravel, but the more dramatic-appearing Scorpaena exhibit a large cranium and wide range of multicolored patterns (Figure 1).13Pterois serve as the most conspicuous member of the group with brightly colored red and white stripes (Figure 2). Scorpionfish commonly grow up to 19 inches long and boast 12 dorsal, 2 pelvic, and 3 anal spines housing 5 to 10 mg of venom.14 An integumentary sheath encapsulates each spine housing the glandular tissue that produces the potent venom.

Toxin Properties
Unlike Pterois and Synanceja, Scorpaena do not have venom ducts around their glands, complicating the work of marine biologists aiming to extract and study the venomous toxins. Several studies have managed to isolate scorpionfish venom and overcome its unstable heat-labile nature to investigate its biologic properties.15-20 Several high-molecular-weight proteins (50–800 kDa) comprise the venom, including hyaluronidase, integrin-inhibiting factors, capillary permeability factor, proteases, and some less-understood cytolytic toxins. These factors provoke the inflammatory, proteolytic, hemorrhagic, cardiovascular, and hemolytic biologic activities at both the local and systemic levels, directing damage to wounded tissues and inducing vascular and tissue permeability to reach cellular processes far and wide. Mediators of inflammation include tumor necrosis factor, IL-6, and monocyte chemoattractant protein 1, followed by neutrophils and other mononuclear cells, initiating the immune response at the wound site. Toxin potency remains for up to 2 days after fish death.1

Clinical Manifestation
Physicians may be guided by clinical symptoms in identifying scorpionfish stings, as the patient may not know the identity of their marine assailant. Initially, individuals punctured by scorpionfish spikes will experience an acute pain and burning sensation at the puncture site that may be accompanied by systemic symptoms such as nausea, vomiting, diarrhea, tachycardia, hypotension, loss of consciousness, difficulty breathing, and delirium.9,21-23 The pain will intensify and radiate distal to the site of envenomation, and the wound may exhibit vesiculation, erythema, bruising, pallor, and notable edema.4,24 Pain intensity peaks at 30 to 90 minutes after envenomation, and other systemic symptoms generally last for 24 to 48 hours.25 If patients do not seek prompt treatment, secondary infection may ensue, and the lingering venom in the blister may cause dermal necrosis, paresthesia, and anesthesia. Chronic sequelae may include joint contractures, compartment syndrome, necrotic ulcers, and chronic neuropathy.1
Management
Treatment of scorpionfish stings primarily is palliative and aimed at symptom reduction. Patients should immediately treat wounds with hot but not scalding water immersion.26,27 Given the thermolabile components of scorpionfish venom, the most effective treatment is to soak the affected limb in water 42 °C to 45 °C for 30 to 90 minutes. Any higher temperature may pose risk for scalding burns. Children should be monitored throughout treatment.28 If hot water immersion does not provide relief, oral analgesics may be considered. Stonefish antivenom is available and may be used for any scorpionfish sting given the shared biologic properties between genera. Providers evaluating stings could use sterile irrigation to clean wounds and search for foreign bodies including spine fragments; probing should be accomplished by instruments rather than a gloved finger. Providers should consider culturing wounds and prescribing antibiotics for suspected secondary infections. A tetanus toxoid history also should be elicited, and patients may have a booster administered, as indicated.29
- Rensch G, Murphy-Lavoie HM. Lionfish, scorpionfish, and stonefish toxicity. StatPearls. StatPearls Publishing; May 10, 2022.
- Cearnal L. Red lionfish and ciguatoxin: menace spreading through western hemisphere. Ann Emerg Med. 2012;60:21A-22A. doi:10.1016/j.annemergmed.2012.05.022
- Côté IM, Green SJ. Potential effects of climate change on a marine invasion: the importance of current context. Curr Zool. 2012;58:1-8. doi:10.1093/czoolo/58.1.1
- Venomology of scorpionfishes. In: Santhanam R. Biology and Ecology of Venomous Marine Scorpionfishes. Academic Press; 2019:263-278.
- Ferri J, Staglicˇic´ N, Matić-Skoko S. The black scorpionfish, Scorpaena porcus (Scorpaenidae): could it serve as reliable indicator of Mediterranean coastal communities’ health? Ecol Indicators. 2012;18:25-30. doi:10.1016/j.ecolind.2011.11.004
- Santhanam R. Biology and Ecology of Venomous Marine Scorpionfishes. Academic Press; 2019.
- Morris JA, Akins JL. Feeding ecology of invasive lionfish (Pterois volitans) in the Bahamian Archipelago. Environ Biol Fishes. 2009;86:389-398. doi:10.1007/s10641-009-9538-8
- Albins MA, Hixon MA. Worst case scenario: potential long-term effects of invasive predatory lionfish (Pterois volitans) on Atlantic and Caribbean coral-reef communities. Environ Biol Fishes. 2013;96:1151–1157. doi:10.1007/s10641-011-9795-1
- Haddad V Jr, Martins IA, Makyama HM. Injuries caused by scorpionfishes (Scorpaena plumieri Bloch, 1789 and Scorpaena brasiliensis Cuvier, 1829) in the Southwestern Atlantic Ocean (Brazilian coast): epidemiologic, clinic and therapeutic aspects of 23 stings in humans. Toxicon. 2003;42:79-83. doi:10.1016/s0041-0101(03)00103-x
- Campos FV, Menezes TN, Malacarne PF, et al. A review on the Scorpaena plumieri fish venom and its bioactive compounds. J Venom Anim Toxins Incl Trop Dis. 2016;22:35. doi:10.1186/s40409-016-0090-7
- Needleman RK, Neylan IP, Erickson TB. Environmental and ecological effects of climate change on venomous marine and amphibious species in the wilderness. Wilderness Environ Med. 2018;29:343-356. doi:10.1016/j.wem.2018.04.003
- Aldred B, Erickson T, Lipscomb J. Lionfish envenomations in an urban wilderness. Wilderness Environ Med. 1996;7:291-296. doi:10.1580/1080-6032(1996)007[0291:leiauw]2.3.co;2
- Stewart J, Hughes JM. Life-history traits of the southern hemisphere eastern red scorpionfish, Scorpaena cardinalis (Scorpaenidae: Scorpaeninae). Mar Freshw Res. 2010;61:1290-1297. doi:10.1071/MF10040
- Auerbach PS. Marine envenomations. N Engl J Med. 1991;325:486-493. doi:10.1056/NEJM199108153250707
- Andrich F, Carnielli JB, Cassoli JS, et al. A potent vasoactive cytolysin isolated from Scorpaena plumieri scorpionfish venom. Toxicon. 2010;56:487-496. doi:10.1016/j.toxicon.2010.05.003
- Gomes HL, Andrich F, Mauad H, et al. Cardiovascular effects of scorpionfish (Scorpaena plumieri) venom. Toxicon. 2010;55(2-3):580-589. doi:10.1016/j.toxicon.2009.10.012
- Menezes TN, Carnielli JB, Gomes HL, et al. Local inflammatory response induced by scorpionfish Scorpaena plumieri venom in mice. Toxicon. 2012;60:4-11. doi:10.1016/j.toxicon.2012.03.008
- Schaeffer RC Jr, Carlson RW, Russell FE. Some chemical properties of the venom of the scorpionfish Scorpaena guttata. Toxicon. 1971;9:69-78. doi:10.1016/0041-0101(71)90045-6
- Khalil AM, Wahsha MA, Abu Khadra KM, et al. Biochemical and histopathological effects of the stonefish (Synanceia verrucosa) venom in rats. Toxicon. 2018;142:45-51. doi:10.1016/j.toxicon.2017.12.052
- Mouchbahani-Constance S, Lesperance LS, Petitjean H, et al. Lionfish venom elicits pain predominantly through the activation of nonpeptidergic nociceptors. Pain. 2018;159:2255-2266. doi:10.1097/j.pain.0000000000001326
- Ottuso P. Aquatic dermatology: encounters with the denizens of the deep (and not so deep)—a review. part II: the vertebrates, single-celled organisms, and aquatic biotoxins. Int J Dermatol. 2013;52:268-278. doi:10.1111/j.1365-4632.2011.05426.x
- Bayley HH. Injuries caused by scorpion fish. Trans R Soc Trop Med Hyg. 1940;34:227-230. doi:10.1016/s0035-9203(40)90072-4
- González D. Epidemiological and clinical aspects of certain venomous animals of Spain. Toxicon. 1982;20:925-928. doi:10.1016/0041-0101(82)90080-0
- Halstead BW. Injurious effects from the sting of the scorpionfish, Scorpaena guttata. with report of a case. Calif Med. 1951;74:395-396.
- Vasievich MP, Villarreal JD, Tomecki KJ. Got the travel bug? a review of common infections, infestations, bites, and stings among returning travelers. Am J Clin Dermatol. 2016;17:451-462. doi:10.1007/s40257-016-0203-7
- Barnett S, Saggiomo S, Smout M, et al. Heat deactivation of the stonefish Synanceia horrida venom—implications for first-aid management. Diving Hyperb Med. 2017;47:155-158. doi:10.28920/dhm47.3.155-158
- Russell FE. Weever fish sting: the last word. Br Med J (Clin Res Ed). 1983;287:981-982. doi:10.1136/bmj.287.6397.981-c
- Tomlinson H, Elston DM. Aquatic antagonists: lionfish (Pterois volitans). Cutis. 2018;102:232-234.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337. doi:10.1016/j.emc.2016.12.004
- Rensch G, Murphy-Lavoie HM. Lionfish, scorpionfish, and stonefish toxicity. StatPearls. StatPearls Publishing; May 10, 2022.
- Cearnal L. Red lionfish and ciguatoxin: menace spreading through western hemisphere. Ann Emerg Med. 2012;60:21A-22A. doi:10.1016/j.annemergmed.2012.05.022
- Côté IM, Green SJ. Potential effects of climate change on a marine invasion: the importance of current context. Curr Zool. 2012;58:1-8. doi:10.1093/czoolo/58.1.1
- Venomology of scorpionfishes. In: Santhanam R. Biology and Ecology of Venomous Marine Scorpionfishes. Academic Press; 2019:263-278.
- Ferri J, Staglicˇic´ N, Matić-Skoko S. The black scorpionfish, Scorpaena porcus (Scorpaenidae): could it serve as reliable indicator of Mediterranean coastal communities’ health? Ecol Indicators. 2012;18:25-30. doi:10.1016/j.ecolind.2011.11.004
- Santhanam R. Biology and Ecology of Venomous Marine Scorpionfishes. Academic Press; 2019.
- Morris JA, Akins JL. Feeding ecology of invasive lionfish (Pterois volitans) in the Bahamian Archipelago. Environ Biol Fishes. 2009;86:389-398. doi:10.1007/s10641-009-9538-8
- Albins MA, Hixon MA. Worst case scenario: potential long-term effects of invasive predatory lionfish (Pterois volitans) on Atlantic and Caribbean coral-reef communities. Environ Biol Fishes. 2013;96:1151–1157. doi:10.1007/s10641-011-9795-1
- Haddad V Jr, Martins IA, Makyama HM. Injuries caused by scorpionfishes (Scorpaena plumieri Bloch, 1789 and Scorpaena brasiliensis Cuvier, 1829) in the Southwestern Atlantic Ocean (Brazilian coast): epidemiologic, clinic and therapeutic aspects of 23 stings in humans. Toxicon. 2003;42:79-83. doi:10.1016/s0041-0101(03)00103-x
- Campos FV, Menezes TN, Malacarne PF, et al. A review on the Scorpaena plumieri fish venom and its bioactive compounds. J Venom Anim Toxins Incl Trop Dis. 2016;22:35. doi:10.1186/s40409-016-0090-7
- Needleman RK, Neylan IP, Erickson TB. Environmental and ecological effects of climate change on venomous marine and amphibious species in the wilderness. Wilderness Environ Med. 2018;29:343-356. doi:10.1016/j.wem.2018.04.003
- Aldred B, Erickson T, Lipscomb J. Lionfish envenomations in an urban wilderness. Wilderness Environ Med. 1996;7:291-296. doi:10.1580/1080-6032(1996)007[0291:leiauw]2.3.co;2
- Stewart J, Hughes JM. Life-history traits of the southern hemisphere eastern red scorpionfish, Scorpaena cardinalis (Scorpaenidae: Scorpaeninae). Mar Freshw Res. 2010;61:1290-1297. doi:10.1071/MF10040
- Auerbach PS. Marine envenomations. N Engl J Med. 1991;325:486-493. doi:10.1056/NEJM199108153250707
- Andrich F, Carnielli JB, Cassoli JS, et al. A potent vasoactive cytolysin isolated from Scorpaena plumieri scorpionfish venom. Toxicon. 2010;56:487-496. doi:10.1016/j.toxicon.2010.05.003
- Gomes HL, Andrich F, Mauad H, et al. Cardiovascular effects of scorpionfish (Scorpaena plumieri) venom. Toxicon. 2010;55(2-3):580-589. doi:10.1016/j.toxicon.2009.10.012
- Menezes TN, Carnielli JB, Gomes HL, et al. Local inflammatory response induced by scorpionfish Scorpaena plumieri venom in mice. Toxicon. 2012;60:4-11. doi:10.1016/j.toxicon.2012.03.008
- Schaeffer RC Jr, Carlson RW, Russell FE. Some chemical properties of the venom of the scorpionfish Scorpaena guttata. Toxicon. 1971;9:69-78. doi:10.1016/0041-0101(71)90045-6
- Khalil AM, Wahsha MA, Abu Khadra KM, et al. Biochemical and histopathological effects of the stonefish (Synanceia verrucosa) venom in rats. Toxicon. 2018;142:45-51. doi:10.1016/j.toxicon.2017.12.052
- Mouchbahani-Constance S, Lesperance LS, Petitjean H, et al. Lionfish venom elicits pain predominantly through the activation of nonpeptidergic nociceptors. Pain. 2018;159:2255-2266. doi:10.1097/j.pain.0000000000001326
- Ottuso P. Aquatic dermatology: encounters with the denizens of the deep (and not so deep)—a review. part II: the vertebrates, single-celled organisms, and aquatic biotoxins. Int J Dermatol. 2013;52:268-278. doi:10.1111/j.1365-4632.2011.05426.x
- Bayley HH. Injuries caused by scorpion fish. Trans R Soc Trop Med Hyg. 1940;34:227-230. doi:10.1016/s0035-9203(40)90072-4
- González D. Epidemiological and clinical aspects of certain venomous animals of Spain. Toxicon. 1982;20:925-928. doi:10.1016/0041-0101(82)90080-0
- Halstead BW. Injurious effects from the sting of the scorpionfish, Scorpaena guttata. with report of a case. Calif Med. 1951;74:395-396.
- Vasievich MP, Villarreal JD, Tomecki KJ. Got the travel bug? a review of common infections, infestations, bites, and stings among returning travelers. Am J Clin Dermatol. 2016;17:451-462. doi:10.1007/s40257-016-0203-7
- Barnett S, Saggiomo S, Smout M, et al. Heat deactivation of the stonefish Synanceia horrida venom—implications for first-aid management. Diving Hyperb Med. 2017;47:155-158. doi:10.28920/dhm47.3.155-158
- Russell FE. Weever fish sting: the last word. Br Med J (Clin Res Ed). 1983;287:981-982. doi:10.1136/bmj.287.6397.981-c
- Tomlinson H, Elston DM. Aquatic antagonists: lionfish (Pterois volitans). Cutis. 2018;102:232-234.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337. doi:10.1016/j.emc.2016.12.004
Practice Points
- As some species of scorpionfish proliferate, providers may see an increase in envenomation cases.
- Physicians should suspect scorpionfish stings based on clinical symptoms and physical examination.
- Scorpionfish toxins are thermolabile, and patients can find symptom relief by immediately immersing the affected area in hot water (42 °C–45 °C) for 30 to 90 minutes.
What’s Eating You? Rhipicephalus Ticks Revisited
Characteristics
Rhipicephalus ticks belong to the Ixodidae family of hard-bodied ticks. They are large and teardrop shaped with an inornate scutum (hard dorsal plate) and relatively short mouthparts attached at a hexagonal basis capitulum (base of the head to which mouthparts are attached)(Figure).1 Widely spaced eyes and festoons also are present. The first pair of coxae—attachment base for the first pair of legs—are characteristically bifid; males have a pair of sclerotized adanal plates on the ventral surface adjacent to the anus as well as accessory adanal shields.2Rhipicephalus (formerly Boophilus) microplus (the so-called cattle tick) is a newly added species; it lacks posterior festoons, and the anal groove is absent.3

Almost all Rhipicephalus ticks, except for R microplus, are 3-host ticks in which a single blood meal is consumed from a vertebrate host at each active life stage—larva, nymph, and adult—to complete development.4,5 In contrast to most ixodid ticks, which are exophilic (living outside of human habitation), the Rhipicephalus sanguineus sensu lato species (the brown dog tick) is highly endophilic (adapted to indoor living) and often can be found hidden in cracks and crevices of walls in homes and peridomestic structures.6 It is predominately monotropic (all developmental stages feed on the same host species) and has a strong host preference for dogs, though it occasionally feeds on other hosts (eg, humans).7 Although most common in tropical and subtropical climates, they can be found anywhere there are dogs due to their ability to colonize indoor dwellings.8 In contrast, R microplus ticks have a predilection for cattle and livestock rather than humans, posing a notable concern to livestock worldwide. Infestation results in transmission of disease-causing pathogens, such as Babesia and Anaplasma species, which costs the cattle industry billions of dollars annually.9
Clinical Manifestations and Treatment
Tick bites usually manifest as intensely pruritic, erythematous papules at the site of tick attachment due to a local type IV hypersensitivity reaction to antigens in the tick’s saliva. This reaction can be long-lasting. In addition to pruritic papules following a bite, an attached tick can be mistaken for a skin neoplasm or nevus. Given that ticks are small, especially during the larval stage, dermoscopy may be helpful in making a diagnosis.10 Symptomatic relief usually can be achieved with topical antipruritics or oral antihistamines.
Of public health concern, brown dog ticks are important vectors of Rickettsia rickettsii (the causative organism of Rocky Mountain spotted fever [RMSF]) in the Western hemisphere, and Rickettsia conorii (the causative organism of Mediterranean spotted fever [MSF][also known as Boutonneuse fever]) in the Eastern hemisphere.11 Bites by ticks carrying rickettsial disease classically manifest with early symptoms of fever, headache, and myalgia, followed by a rash or by a localized eschar or tache noire (a black, necrotic, scabbed lesion) that represents direct endothelial invasion and vascular damage by Rickettsia.12 Rocky Mountain spotted fever and MSF are more prevalent during summer, likely due, in part, to the combination of increased outdoor activity and a higher rate of tick-questing (host-seeking) behavior in warmer climates.4,7
Rocky Mountain Spotted Fever—Dermacentor variabilis is the primary vector of RMSF in the southeastern United States; Dermacentor andersoni is the major vector of RMSF in Rocky Mountain states. Rhipicephalus sanguineus sensu lato is an important vector of RMSF in the southwestern United States, Mexico, and Central America.11,13
Early symptoms of RMSF are nonspecific and can include fever, headache, arthralgia, myalgia, and malaise. Gastrointestinal tract symptoms (eg, nausea, vomiting, anorexia) may occur; notable abdominal pain occurs in some patients, particularly children. A characteristic petechial rash occurs in as many as 90% of patients, typically at the third to fifth day of illness, and classically begins on the wrists and ankles, with progression to the palms and soles before spreading centripetally to the arms, legs, and trunk.14 An eschar at the inoculation site is uncommon in RMSF; when present, it is more suggestive of MSF.15
The classic triad of fever, headache, and rash is present in 3% of patients during the first 3 days after a tick bite and in 60% to 70% within 2 weeks.16 A rash often is absent when patients first seek medical attention and may not develop (absent in 9% to 12% of cases; so-called spotless RMSF). Therefore, absence of rash should not be a reason to withhold treatment.16 Empiric treatment with doxycycline should be started promptly for all suspected cases of RMSF because of the rapid progression of disease and an increased risk for morbidity and mortality with delayed diagnosis.
Patients do not become antibody positive until 7 to 10 days after symptoms begin; therefore, treatment should not be delayed while awaiting serologic test results. The case fatality rate in the United States is estimated to be 5% to 10% overall and as high as 40% to 50% among patients who are not treated until day 8 or 9 of illness.17
Cutaneous complications include skin necrosis and gangrene due to continuous tissue damage in severe cases.16 Severe infection also may manifest with signs of multiorgan system damage, including altered mental status, cerebral edema, meningismus, transient deafness, myocarditis, pulmonary hemorrhage and edema, conjunctivitis, retinal abnormalities, and acute renal failure.14,16 Risk factors for more severe illness include delayed treatment, age 40 years or older or younger than 10 years, and underlying medical conditions such as alcoholic liver disease and glucose-6-phosphate dehydrogenase deficiency. However, even some healthy young patients die of this disease.17
Mediterranean Spotted Fever—Rhipicephalus sanguineus sensu lato is the primary vector of MSF, which is prevalent in areas adjacent to the Mediterranean Sea, including southern Europe, Africa, and Central Asia; Sicily is the most highly affected region.18 Findings with MSF are nearly identical to those of RMSF, except that tache noire is more common, present in as many as 70% of cases at the site of the inoculating tick bite, and MSF typically follows a less severe clinical course.12 Similar to other rickettsial diseases, the pathogenesis of MSF involves direct injury to vascular endothelial cells, causing a vasculitis that is responsible for the clinical abnormalities observed.
Patients with severe MSF experience complications similar to severe RMSF, including neurologic manifestations and multiorgan damage.18 Risk factors include advanced age, immunocompromised state, cardiac disease, chronic alcoholism, diabetes mellitus, glucose-6-phosphate dehydrogenase deficiency, respiratory insufficiency, and delayed treatment.18
Treatment—For all spotted fever group rickettsial infections, doxycycline is the treatment of choice for all patients, including children and pregnant women. Treatment should be started without delay; recommended dosages are 100 mg twice daily for children weighing more than 45 kg and adults, and 2.2 mg/kg twice daily for children weighing 45 kg or less.12
Rhipicephalus tick bites rarely can result in paralysis; however, Dermacentor ticks are responsible for most cases of tick-related paralysis in North America. Other pathogens proven or reputed to be transmitted by Rhipicephalus sanguineus sensu lato with zoonotic potential include but are not limited to Rickettsia massiliae, Coxiella burnetti, Anaplasma platys, Leishmania infantum, and Crimean-Congo hemorrhagic fever virus (Nairovirus).19
Environmental Treatment and Prevention
The most effective way to prevent tick-borne illness is avoidance of tick bites. Primary prevention methods include vector control, use of repellents (eg, N,N-diethyl-meta-toluamide [DEET]), picaridin, permethrin), avoidance of areas with a high tick burden, use of protective clothing, and detection and removal of ticks as soon as possible.
Environmental and veterinary controls also are important methods of tick-bite prevention. A veterinarian can recommend a variety of agents for dogs and cats that prevent attachment of ticks. Environmental controls include synthetic or natural product-based chemical acaricides and nonchemical methods, such as landscape management (eg, sealing cracks and crevices in homes and controlling tall grasses, weeds, and leaf debris) to minimize potential tick habitat.20 Secondary prevention includes antibiotics for prophylaxis or for treatment of tick-borne disease, when indicated.
Numerous tick repellents are available commercially; others are being studied. DEET, the most widely used topical repellent, has a broad spectrum of activity against many tick species.21 In addition, DEET has a well-known safety and toxicity profile, with rare adverse effects, and is safe for use in pregnant women and children older than 2 years. Alternative repellents, such as those containing picaridin, ethyl butylacetylaminopropionate (IR3535 [Merck]), oil of lemon eucalyptus, and 2-undecanone can be effective; some show efficacy comparable to that of DEET.22 Permethrin, a synthetic pyrethroid, is a highly efficacious tick repellent and insecticide, especially when used in conjunction with a topical repellent such as DEET. Unlike topically applied repellents, permethrin spray is applied to fabric (eg, clothing, shoes, bed nets, camping gear), not to skin.
Indiscriminate use of acaricides worldwide has led to increasing selection of acaricide resistance in Rhipicephalus tick species, which is especially true with the use of acaricides in controlling R microplus livestock infestations; several tick populations now show resistance to all major classes of these compounds.23-25 For that reason, there has been an increasing effort to develop new chemical and nonchemical approaches to tick control that are more environmentally sustainable and strategies to minimize development and progression of resistance such as rotation of acaricides; reducing the frequency of their application; use of pesticide mixtures, synergists, or both; and increasing use of nonacaricidal methods of control.26
Prompt removal of ticks is important for preventing the transmission of tick-borne disease. Proper removal involves rubbing the tick in a circular motion with a moist gauze pad or using fine-tipped tweezers to grasp the tick as close to the skin surface as possible and pulling upward with a steady pressure.17,27 It is important not to jerk, twist, squeeze, smash, or burn the tick, as this can result in insufficient removal of mouthparts or spread contaminated tick fluids to mucous membranes, increasing the risk for infection. Application of petroleum jelly or nail polish to aid in tick removal have not been shown to be effective and are not recommended.16,28
- Dantas-Torres F. The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): from taxonomy to control. Vet Parasitol. 2008;152:173-185. doi:10.1016/j.vetpar.2007.12.030
- Madder M, Fourie JJ, Schetters TPM. Arachnida, Metastigmata, Ixodidae (except Ixodes holocyclus). In: Marchiondo AA, Cruthers LR, Fourie JJ, eds. Parasiticide Screening: In Vitro and In Vivo Tests With Relevant Parasite Rearing and Host Infection/Infestation Methods. Volume 1. Elsevier Academic Press; 2019:19-20.
- Burger TD, Shao R, Barker SC. Phylogenetic analysis of mitochondrial genome sequences indicates that the cattle tick, Rhipicephalus (Boophilus) microplus, contains a cryptic species. Mol Phylogenet Evol. 2014;76:241-253. doi:10.1016/j.ympev.2014.03.017
- Gray J, Dantas-Torres F, Estrada-Peña A, et al. Systematics and ecology of the brown dog tick, Rhipicephalus sanguineus. Ticks Tick Borne Dis. 2013;4:171-180. doi:10.1016/j.ttbdis.2012.12.003
- Tian Y, Lord CC, Kaufman PE. Brown dog tick, Rhipicephalus Sanguineus Latrielle (Arachnida: Acari: Ixodidae): EENY-221/IN378. EDIS. March 26, 2020. Accessed January 3, 2024. https://doi.org/10.32473/edis-in378-2020
- Saleh MN, Allen KE, Lineberry MW, et al. Ticks infesting dogs and cats in North America: biology, geographic distribution, and pathogen transmission. Vet Parasitol. 2021;294:109392. doi:10.1016/j.vetpar.2021.109392
- Dantas-Torres F. Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasit Vectors. 2010;3:26. doi:10.1186/1756-3305-3-26
- Dryden MW, Payne PA. Biology and control of ticks infesting dogs and cats in North America. Vet Ther. 2004;5:139-154.
- Nyangiwe N, Yawa M, Muchenje V. Driving forces for changes in geographic range of cattle ticks (Acari: Ixodidae) in Africa: a Review. S Afr J Anim Sci. 2018;48:829. doi:10.4314/sajas.v48i5.4
- Ramot Y, Zlotogorski A, Mumcuoglu KY. Brown dog tick (Rhipicephalus sanguineus) infestation of the penis detected by dermoscopy. Int J Dermatol. 2012;51:1402-1403. doi:10.1111/j.1365-4632.2010.04756.x
- Tucker NSG, Weeks ENI, Beati L, et al. Prevalence and distribution of pathogen infection and permethrin resistance in tropical and temperate populations of Rhipicephalus sanguineus s.l. collected worldwide. Med Vet Entomol. 2021;35:147-157. doi:10.1111/mve.12479
- McClain MT, Sexton DJ, Hall KK, eds. Other spotted fever group rickettsial infections. UpToDate. Updated October 10, 2022. Accessed January 3, 2024. https://www.uptodate.com/contents/other-spotted-fever-group-rickettsial-infections
- Ribeiro CM, Carvalho JLB, Bastos PAS, et al. Prevalence of Rickettsia rickettsii in ticks: systematic review and meta-analysis. Vector Borne Zoonotic Dis. 2021;21:557-565. doi:10.1089/vbz.2021.0004
- Pace EJ, O’Reilly M. Tickborne diseases: diagnosis and management. Am Fam Physician. 2020;101:530-540.
- Patterson JW. Weedon’s Skin Pathology. 5th ed. Elsevier; 2020.
- Dantas-Torres F. Rocky Mountain spotted fever. Lancet Infect Dis. 2007;7:724-732. doi:10.1016/S1473-3099(07)70261-X
- Biggs HM, Behravesh CB, Bradley KK, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis—United States. MMWR Recomm Rep. 2016;65:1-44. doi:10.15585/mmwr.rr6502a1
- Rossio R, Conalbi V, Castagna V, et al. Mediterranean spotted fever and hearing impairment: a rare complication. Int J Infect Dis. 2015;35:34-36. doi:10.1016/j.ijid.2015.04.005
- Dantas-Torres F, Otranto D. Further thoughts on the taxonomy and vector role of Rhipicephalus sanguineus group ticks. Vet Parasitol. 2015;208:9-13. doi:10.1016/j.vetpar.2014.12.014
- Eisen RJ, Kugeler KJ, Eisen L, et al. Tick-borne zoonoses in the United States: persistent and emerging threats to human health. ILAR J. 2017;58:319-335. doi:10.1093/ilar/ilx005
- Nguyen QD, Vu MN, Hebert AA. Insect repellents: an updated review for the clinician. J Am Acad Dermatol. 2018;88:123-130. doi:10.1016/j.jaad.2018.10.053
- Pages F, Dautel H, Duvallet G, et al. Tick repellents for human use: prevention of tick bites and tick-borne diseases. Vector Borne Zoonotic Dis. 2014;14:85-93. doi:10.1089/vbz.2013.1410
- Rodriguez-Vivas RI, Alonso-Díaz MA, et al. Prevalence and potential risk factors for organophosphate and pyrethroid resistance in Boophilus microplus ticks on cattle ranches from the State of Yucatan, Mexico. Vet Parasitol. 2006;136:335-342. doi:10.1016/j.vetpar.2005.05.069
- Rodríguez-Vivas RI, Rodríguez-Arevalo F, Alonso-Díaz MA, et al. Prevalence and potential risk factors for amitraz resistance in Boophilus microplus ticks in cattle farms in the State of Yucatan, Mexico. Prev Vet Med. 2006;75:280-286. doi:10.1016/j.prevetmed.2006.04.001
- Perez-Cogollo LC, Rodriguez-Vivas RI, Ramirez-Cruz GT, et al. First report of the cattle tick Rhipicephalus microplus resistant to ivermectin in Mexico. Vet Parasitol. 2010;168:165-169. doi:10.1016/j.vetpar.2009.10.021
- Rodriguez-Vivas RI, Jonsson NN, Bhushan C. Strategies for the control of Rhipicephalus microplus ticks in a world of conventional acaricide and macrocyclic lactone resistance. Parasitol Res.2018;117:3-29. doi:10.1007/s00436-017-5677-6
- Centers for Disease Control and Prevention. Tick removal. Updated May 13, 2022. Accessed January 3, 2024. https://www.cdc.gov/ticks/removing_a_tick.html
- Diaz JH. Chemical and plant-based insect repellents: efficacy, safety, and toxicity. Wilderness Environ Med. 2016;27:153-163. doi:10.1016/j.wem.2015.11.007
Characteristics
Rhipicephalus ticks belong to the Ixodidae family of hard-bodied ticks. They are large and teardrop shaped with an inornate scutum (hard dorsal plate) and relatively short mouthparts attached at a hexagonal basis capitulum (base of the head to which mouthparts are attached)(Figure).1 Widely spaced eyes and festoons also are present. The first pair of coxae—attachment base for the first pair of legs—are characteristically bifid; males have a pair of sclerotized adanal plates on the ventral surface adjacent to the anus as well as accessory adanal shields.2Rhipicephalus (formerly Boophilus) microplus (the so-called cattle tick) is a newly added species; it lacks posterior festoons, and the anal groove is absent.3

Almost all Rhipicephalus ticks, except for R microplus, are 3-host ticks in which a single blood meal is consumed from a vertebrate host at each active life stage—larva, nymph, and adult—to complete development.4,5 In contrast to most ixodid ticks, which are exophilic (living outside of human habitation), the Rhipicephalus sanguineus sensu lato species (the brown dog tick) is highly endophilic (adapted to indoor living) and often can be found hidden in cracks and crevices of walls in homes and peridomestic structures.6 It is predominately monotropic (all developmental stages feed on the same host species) and has a strong host preference for dogs, though it occasionally feeds on other hosts (eg, humans).7 Although most common in tropical and subtropical climates, they can be found anywhere there are dogs due to their ability to colonize indoor dwellings.8 In contrast, R microplus ticks have a predilection for cattle and livestock rather than humans, posing a notable concern to livestock worldwide. Infestation results in transmission of disease-causing pathogens, such as Babesia and Anaplasma species, which costs the cattle industry billions of dollars annually.9
Clinical Manifestations and Treatment
Tick bites usually manifest as intensely pruritic, erythematous papules at the site of tick attachment due to a local type IV hypersensitivity reaction to antigens in the tick’s saliva. This reaction can be long-lasting. In addition to pruritic papules following a bite, an attached tick can be mistaken for a skin neoplasm or nevus. Given that ticks are small, especially during the larval stage, dermoscopy may be helpful in making a diagnosis.10 Symptomatic relief usually can be achieved with topical antipruritics or oral antihistamines.
Of public health concern, brown dog ticks are important vectors of Rickettsia rickettsii (the causative organism of Rocky Mountain spotted fever [RMSF]) in the Western hemisphere, and Rickettsia conorii (the causative organism of Mediterranean spotted fever [MSF][also known as Boutonneuse fever]) in the Eastern hemisphere.11 Bites by ticks carrying rickettsial disease classically manifest with early symptoms of fever, headache, and myalgia, followed by a rash or by a localized eschar or tache noire (a black, necrotic, scabbed lesion) that represents direct endothelial invasion and vascular damage by Rickettsia.12 Rocky Mountain spotted fever and MSF are more prevalent during summer, likely due, in part, to the combination of increased outdoor activity and a higher rate of tick-questing (host-seeking) behavior in warmer climates.4,7
Rocky Mountain Spotted Fever—Dermacentor variabilis is the primary vector of RMSF in the southeastern United States; Dermacentor andersoni is the major vector of RMSF in Rocky Mountain states. Rhipicephalus sanguineus sensu lato is an important vector of RMSF in the southwestern United States, Mexico, and Central America.11,13
Early symptoms of RMSF are nonspecific and can include fever, headache, arthralgia, myalgia, and malaise. Gastrointestinal tract symptoms (eg, nausea, vomiting, anorexia) may occur; notable abdominal pain occurs in some patients, particularly children. A characteristic petechial rash occurs in as many as 90% of patients, typically at the third to fifth day of illness, and classically begins on the wrists and ankles, with progression to the palms and soles before spreading centripetally to the arms, legs, and trunk.14 An eschar at the inoculation site is uncommon in RMSF; when present, it is more suggestive of MSF.15
The classic triad of fever, headache, and rash is present in 3% of patients during the first 3 days after a tick bite and in 60% to 70% within 2 weeks.16 A rash often is absent when patients first seek medical attention and may not develop (absent in 9% to 12% of cases; so-called spotless RMSF). Therefore, absence of rash should not be a reason to withhold treatment.16 Empiric treatment with doxycycline should be started promptly for all suspected cases of RMSF because of the rapid progression of disease and an increased risk for morbidity and mortality with delayed diagnosis.
Patients do not become antibody positive until 7 to 10 days after symptoms begin; therefore, treatment should not be delayed while awaiting serologic test results. The case fatality rate in the United States is estimated to be 5% to 10% overall and as high as 40% to 50% among patients who are not treated until day 8 or 9 of illness.17
Cutaneous complications include skin necrosis and gangrene due to continuous tissue damage in severe cases.16 Severe infection also may manifest with signs of multiorgan system damage, including altered mental status, cerebral edema, meningismus, transient deafness, myocarditis, pulmonary hemorrhage and edema, conjunctivitis, retinal abnormalities, and acute renal failure.14,16 Risk factors for more severe illness include delayed treatment, age 40 years or older or younger than 10 years, and underlying medical conditions such as alcoholic liver disease and glucose-6-phosphate dehydrogenase deficiency. However, even some healthy young patients die of this disease.17
Mediterranean Spotted Fever—Rhipicephalus sanguineus sensu lato is the primary vector of MSF, which is prevalent in areas adjacent to the Mediterranean Sea, including southern Europe, Africa, and Central Asia; Sicily is the most highly affected region.18 Findings with MSF are nearly identical to those of RMSF, except that tache noire is more common, present in as many as 70% of cases at the site of the inoculating tick bite, and MSF typically follows a less severe clinical course.12 Similar to other rickettsial diseases, the pathogenesis of MSF involves direct injury to vascular endothelial cells, causing a vasculitis that is responsible for the clinical abnormalities observed.
Patients with severe MSF experience complications similar to severe RMSF, including neurologic manifestations and multiorgan damage.18 Risk factors include advanced age, immunocompromised state, cardiac disease, chronic alcoholism, diabetes mellitus, glucose-6-phosphate dehydrogenase deficiency, respiratory insufficiency, and delayed treatment.18
Treatment—For all spotted fever group rickettsial infections, doxycycline is the treatment of choice for all patients, including children and pregnant women. Treatment should be started without delay; recommended dosages are 100 mg twice daily for children weighing more than 45 kg and adults, and 2.2 mg/kg twice daily for children weighing 45 kg or less.12
Rhipicephalus tick bites rarely can result in paralysis; however, Dermacentor ticks are responsible for most cases of tick-related paralysis in North America. Other pathogens proven or reputed to be transmitted by Rhipicephalus sanguineus sensu lato with zoonotic potential include but are not limited to Rickettsia massiliae, Coxiella burnetti, Anaplasma platys, Leishmania infantum, and Crimean-Congo hemorrhagic fever virus (Nairovirus).19
Environmental Treatment and Prevention
The most effective way to prevent tick-borne illness is avoidance of tick bites. Primary prevention methods include vector control, use of repellents (eg, N,N-diethyl-meta-toluamide [DEET]), picaridin, permethrin), avoidance of areas with a high tick burden, use of protective clothing, and detection and removal of ticks as soon as possible.
Environmental and veterinary controls also are important methods of tick-bite prevention. A veterinarian can recommend a variety of agents for dogs and cats that prevent attachment of ticks. Environmental controls include synthetic or natural product-based chemical acaricides and nonchemical methods, such as landscape management (eg, sealing cracks and crevices in homes and controlling tall grasses, weeds, and leaf debris) to minimize potential tick habitat.20 Secondary prevention includes antibiotics for prophylaxis or for treatment of tick-borne disease, when indicated.
Numerous tick repellents are available commercially; others are being studied. DEET, the most widely used topical repellent, has a broad spectrum of activity against many tick species.21 In addition, DEET has a well-known safety and toxicity profile, with rare adverse effects, and is safe for use in pregnant women and children older than 2 years. Alternative repellents, such as those containing picaridin, ethyl butylacetylaminopropionate (IR3535 [Merck]), oil of lemon eucalyptus, and 2-undecanone can be effective; some show efficacy comparable to that of DEET.22 Permethrin, a synthetic pyrethroid, is a highly efficacious tick repellent and insecticide, especially when used in conjunction with a topical repellent such as DEET. Unlike topically applied repellents, permethrin spray is applied to fabric (eg, clothing, shoes, bed nets, camping gear), not to skin.
Indiscriminate use of acaricides worldwide has led to increasing selection of acaricide resistance in Rhipicephalus tick species, which is especially true with the use of acaricides in controlling R microplus livestock infestations; several tick populations now show resistance to all major classes of these compounds.23-25 For that reason, there has been an increasing effort to develop new chemical and nonchemical approaches to tick control that are more environmentally sustainable and strategies to minimize development and progression of resistance such as rotation of acaricides; reducing the frequency of their application; use of pesticide mixtures, synergists, or both; and increasing use of nonacaricidal methods of control.26
Prompt removal of ticks is important for preventing the transmission of tick-borne disease. Proper removal involves rubbing the tick in a circular motion with a moist gauze pad or using fine-tipped tweezers to grasp the tick as close to the skin surface as possible and pulling upward with a steady pressure.17,27 It is important not to jerk, twist, squeeze, smash, or burn the tick, as this can result in insufficient removal of mouthparts or spread contaminated tick fluids to mucous membranes, increasing the risk for infection. Application of petroleum jelly or nail polish to aid in tick removal have not been shown to be effective and are not recommended.16,28
Characteristics
Rhipicephalus ticks belong to the Ixodidae family of hard-bodied ticks. They are large and teardrop shaped with an inornate scutum (hard dorsal plate) and relatively short mouthparts attached at a hexagonal basis capitulum (base of the head to which mouthparts are attached)(Figure).1 Widely spaced eyes and festoons also are present. The first pair of coxae—attachment base for the first pair of legs—are characteristically bifid; males have a pair of sclerotized adanal plates on the ventral surface adjacent to the anus as well as accessory adanal shields.2Rhipicephalus (formerly Boophilus) microplus (the so-called cattle tick) is a newly added species; it lacks posterior festoons, and the anal groove is absent.3

Almost all Rhipicephalus ticks, except for R microplus, are 3-host ticks in which a single blood meal is consumed from a vertebrate host at each active life stage—larva, nymph, and adult—to complete development.4,5 In contrast to most ixodid ticks, which are exophilic (living outside of human habitation), the Rhipicephalus sanguineus sensu lato species (the brown dog tick) is highly endophilic (adapted to indoor living) and often can be found hidden in cracks and crevices of walls in homes and peridomestic structures.6 It is predominately monotropic (all developmental stages feed on the same host species) and has a strong host preference for dogs, though it occasionally feeds on other hosts (eg, humans).7 Although most common in tropical and subtropical climates, they can be found anywhere there are dogs due to their ability to colonize indoor dwellings.8 In contrast, R microplus ticks have a predilection for cattle and livestock rather than humans, posing a notable concern to livestock worldwide. Infestation results in transmission of disease-causing pathogens, such as Babesia and Anaplasma species, which costs the cattle industry billions of dollars annually.9
Clinical Manifestations and Treatment
Tick bites usually manifest as intensely pruritic, erythematous papules at the site of tick attachment due to a local type IV hypersensitivity reaction to antigens in the tick’s saliva. This reaction can be long-lasting. In addition to pruritic papules following a bite, an attached tick can be mistaken for a skin neoplasm or nevus. Given that ticks are small, especially during the larval stage, dermoscopy may be helpful in making a diagnosis.10 Symptomatic relief usually can be achieved with topical antipruritics or oral antihistamines.
Of public health concern, brown dog ticks are important vectors of Rickettsia rickettsii (the causative organism of Rocky Mountain spotted fever [RMSF]) in the Western hemisphere, and Rickettsia conorii (the causative organism of Mediterranean spotted fever [MSF][also known as Boutonneuse fever]) in the Eastern hemisphere.11 Bites by ticks carrying rickettsial disease classically manifest with early symptoms of fever, headache, and myalgia, followed by a rash or by a localized eschar or tache noire (a black, necrotic, scabbed lesion) that represents direct endothelial invasion and vascular damage by Rickettsia.12 Rocky Mountain spotted fever and MSF are more prevalent during summer, likely due, in part, to the combination of increased outdoor activity and a higher rate of tick-questing (host-seeking) behavior in warmer climates.4,7
Rocky Mountain Spotted Fever—Dermacentor variabilis is the primary vector of RMSF in the southeastern United States; Dermacentor andersoni is the major vector of RMSF in Rocky Mountain states. Rhipicephalus sanguineus sensu lato is an important vector of RMSF in the southwestern United States, Mexico, and Central America.11,13
Early symptoms of RMSF are nonspecific and can include fever, headache, arthralgia, myalgia, and malaise. Gastrointestinal tract symptoms (eg, nausea, vomiting, anorexia) may occur; notable abdominal pain occurs in some patients, particularly children. A characteristic petechial rash occurs in as many as 90% of patients, typically at the third to fifth day of illness, and classically begins on the wrists and ankles, with progression to the palms and soles before spreading centripetally to the arms, legs, and trunk.14 An eschar at the inoculation site is uncommon in RMSF; when present, it is more suggestive of MSF.15
The classic triad of fever, headache, and rash is present in 3% of patients during the first 3 days after a tick bite and in 60% to 70% within 2 weeks.16 A rash often is absent when patients first seek medical attention and may not develop (absent in 9% to 12% of cases; so-called spotless RMSF). Therefore, absence of rash should not be a reason to withhold treatment.16 Empiric treatment with doxycycline should be started promptly for all suspected cases of RMSF because of the rapid progression of disease and an increased risk for morbidity and mortality with delayed diagnosis.
Patients do not become antibody positive until 7 to 10 days after symptoms begin; therefore, treatment should not be delayed while awaiting serologic test results. The case fatality rate in the United States is estimated to be 5% to 10% overall and as high as 40% to 50% among patients who are not treated until day 8 or 9 of illness.17
Cutaneous complications include skin necrosis and gangrene due to continuous tissue damage in severe cases.16 Severe infection also may manifest with signs of multiorgan system damage, including altered mental status, cerebral edema, meningismus, transient deafness, myocarditis, pulmonary hemorrhage and edema, conjunctivitis, retinal abnormalities, and acute renal failure.14,16 Risk factors for more severe illness include delayed treatment, age 40 years or older or younger than 10 years, and underlying medical conditions such as alcoholic liver disease and glucose-6-phosphate dehydrogenase deficiency. However, even some healthy young patients die of this disease.17
Mediterranean Spotted Fever—Rhipicephalus sanguineus sensu lato is the primary vector of MSF, which is prevalent in areas adjacent to the Mediterranean Sea, including southern Europe, Africa, and Central Asia; Sicily is the most highly affected region.18 Findings with MSF are nearly identical to those of RMSF, except that tache noire is more common, present in as many as 70% of cases at the site of the inoculating tick bite, and MSF typically follows a less severe clinical course.12 Similar to other rickettsial diseases, the pathogenesis of MSF involves direct injury to vascular endothelial cells, causing a vasculitis that is responsible for the clinical abnormalities observed.
Patients with severe MSF experience complications similar to severe RMSF, including neurologic manifestations and multiorgan damage.18 Risk factors include advanced age, immunocompromised state, cardiac disease, chronic alcoholism, diabetes mellitus, glucose-6-phosphate dehydrogenase deficiency, respiratory insufficiency, and delayed treatment.18
Treatment—For all spotted fever group rickettsial infections, doxycycline is the treatment of choice for all patients, including children and pregnant women. Treatment should be started without delay; recommended dosages are 100 mg twice daily for children weighing more than 45 kg and adults, and 2.2 mg/kg twice daily for children weighing 45 kg or less.12
Rhipicephalus tick bites rarely can result in paralysis; however, Dermacentor ticks are responsible for most cases of tick-related paralysis in North America. Other pathogens proven or reputed to be transmitted by Rhipicephalus sanguineus sensu lato with zoonotic potential include but are not limited to Rickettsia massiliae, Coxiella burnetti, Anaplasma platys, Leishmania infantum, and Crimean-Congo hemorrhagic fever virus (Nairovirus).19
Environmental Treatment and Prevention
The most effective way to prevent tick-borne illness is avoidance of tick bites. Primary prevention methods include vector control, use of repellents (eg, N,N-diethyl-meta-toluamide [DEET]), picaridin, permethrin), avoidance of areas with a high tick burden, use of protective clothing, and detection and removal of ticks as soon as possible.
Environmental and veterinary controls also are important methods of tick-bite prevention. A veterinarian can recommend a variety of agents for dogs and cats that prevent attachment of ticks. Environmental controls include synthetic or natural product-based chemical acaricides and nonchemical methods, such as landscape management (eg, sealing cracks and crevices in homes and controlling tall grasses, weeds, and leaf debris) to minimize potential tick habitat.20 Secondary prevention includes antibiotics for prophylaxis or for treatment of tick-borne disease, when indicated.
Numerous tick repellents are available commercially; others are being studied. DEET, the most widely used topical repellent, has a broad spectrum of activity against many tick species.21 In addition, DEET has a well-known safety and toxicity profile, with rare adverse effects, and is safe for use in pregnant women and children older than 2 years. Alternative repellents, such as those containing picaridin, ethyl butylacetylaminopropionate (IR3535 [Merck]), oil of lemon eucalyptus, and 2-undecanone can be effective; some show efficacy comparable to that of DEET.22 Permethrin, a synthetic pyrethroid, is a highly efficacious tick repellent and insecticide, especially when used in conjunction with a topical repellent such as DEET. Unlike topically applied repellents, permethrin spray is applied to fabric (eg, clothing, shoes, bed nets, camping gear), not to skin.
Indiscriminate use of acaricides worldwide has led to increasing selection of acaricide resistance in Rhipicephalus tick species, which is especially true with the use of acaricides in controlling R microplus livestock infestations; several tick populations now show resistance to all major classes of these compounds.23-25 For that reason, there has been an increasing effort to develop new chemical and nonchemical approaches to tick control that are more environmentally sustainable and strategies to minimize development and progression of resistance such as rotation of acaricides; reducing the frequency of their application; use of pesticide mixtures, synergists, or both; and increasing use of nonacaricidal methods of control.26
Prompt removal of ticks is important for preventing the transmission of tick-borne disease. Proper removal involves rubbing the tick in a circular motion with a moist gauze pad or using fine-tipped tweezers to grasp the tick as close to the skin surface as possible and pulling upward with a steady pressure.17,27 It is important not to jerk, twist, squeeze, smash, or burn the tick, as this can result in insufficient removal of mouthparts or spread contaminated tick fluids to mucous membranes, increasing the risk for infection. Application of petroleum jelly or nail polish to aid in tick removal have not been shown to be effective and are not recommended.16,28
- Dantas-Torres F. The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): from taxonomy to control. Vet Parasitol. 2008;152:173-185. doi:10.1016/j.vetpar.2007.12.030
- Madder M, Fourie JJ, Schetters TPM. Arachnida, Metastigmata, Ixodidae (except Ixodes holocyclus). In: Marchiondo AA, Cruthers LR, Fourie JJ, eds. Parasiticide Screening: In Vitro and In Vivo Tests With Relevant Parasite Rearing and Host Infection/Infestation Methods. Volume 1. Elsevier Academic Press; 2019:19-20.
- Burger TD, Shao R, Barker SC. Phylogenetic analysis of mitochondrial genome sequences indicates that the cattle tick, Rhipicephalus (Boophilus) microplus, contains a cryptic species. Mol Phylogenet Evol. 2014;76:241-253. doi:10.1016/j.ympev.2014.03.017
- Gray J, Dantas-Torres F, Estrada-Peña A, et al. Systematics and ecology of the brown dog tick, Rhipicephalus sanguineus. Ticks Tick Borne Dis. 2013;4:171-180. doi:10.1016/j.ttbdis.2012.12.003
- Tian Y, Lord CC, Kaufman PE. Brown dog tick, Rhipicephalus Sanguineus Latrielle (Arachnida: Acari: Ixodidae): EENY-221/IN378. EDIS. March 26, 2020. Accessed January 3, 2024. https://doi.org/10.32473/edis-in378-2020
- Saleh MN, Allen KE, Lineberry MW, et al. Ticks infesting dogs and cats in North America: biology, geographic distribution, and pathogen transmission. Vet Parasitol. 2021;294:109392. doi:10.1016/j.vetpar.2021.109392
- Dantas-Torres F. Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasit Vectors. 2010;3:26. doi:10.1186/1756-3305-3-26
- Dryden MW, Payne PA. Biology and control of ticks infesting dogs and cats in North America. Vet Ther. 2004;5:139-154.
- Nyangiwe N, Yawa M, Muchenje V. Driving forces for changes in geographic range of cattle ticks (Acari: Ixodidae) in Africa: a Review. S Afr J Anim Sci. 2018;48:829. doi:10.4314/sajas.v48i5.4
- Ramot Y, Zlotogorski A, Mumcuoglu KY. Brown dog tick (Rhipicephalus sanguineus) infestation of the penis detected by dermoscopy. Int J Dermatol. 2012;51:1402-1403. doi:10.1111/j.1365-4632.2010.04756.x
- Tucker NSG, Weeks ENI, Beati L, et al. Prevalence and distribution of pathogen infection and permethrin resistance in tropical and temperate populations of Rhipicephalus sanguineus s.l. collected worldwide. Med Vet Entomol. 2021;35:147-157. doi:10.1111/mve.12479
- McClain MT, Sexton DJ, Hall KK, eds. Other spotted fever group rickettsial infections. UpToDate. Updated October 10, 2022. Accessed January 3, 2024. https://www.uptodate.com/contents/other-spotted-fever-group-rickettsial-infections
- Ribeiro CM, Carvalho JLB, Bastos PAS, et al. Prevalence of Rickettsia rickettsii in ticks: systematic review and meta-analysis. Vector Borne Zoonotic Dis. 2021;21:557-565. doi:10.1089/vbz.2021.0004
- Pace EJ, O’Reilly M. Tickborne diseases: diagnosis and management. Am Fam Physician. 2020;101:530-540.
- Patterson JW. Weedon’s Skin Pathology. 5th ed. Elsevier; 2020.
- Dantas-Torres F. Rocky Mountain spotted fever. Lancet Infect Dis. 2007;7:724-732. doi:10.1016/S1473-3099(07)70261-X
- Biggs HM, Behravesh CB, Bradley KK, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis—United States. MMWR Recomm Rep. 2016;65:1-44. doi:10.15585/mmwr.rr6502a1
- Rossio R, Conalbi V, Castagna V, et al. Mediterranean spotted fever and hearing impairment: a rare complication. Int J Infect Dis. 2015;35:34-36. doi:10.1016/j.ijid.2015.04.005
- Dantas-Torres F, Otranto D. Further thoughts on the taxonomy and vector role of Rhipicephalus sanguineus group ticks. Vet Parasitol. 2015;208:9-13. doi:10.1016/j.vetpar.2014.12.014
- Eisen RJ, Kugeler KJ, Eisen L, et al. Tick-borne zoonoses in the United States: persistent and emerging threats to human health. ILAR J. 2017;58:319-335. doi:10.1093/ilar/ilx005
- Nguyen QD, Vu MN, Hebert AA. Insect repellents: an updated review for the clinician. J Am Acad Dermatol. 2018;88:123-130. doi:10.1016/j.jaad.2018.10.053
- Pages F, Dautel H, Duvallet G, et al. Tick repellents for human use: prevention of tick bites and tick-borne diseases. Vector Borne Zoonotic Dis. 2014;14:85-93. doi:10.1089/vbz.2013.1410
- Rodriguez-Vivas RI, Alonso-Díaz MA, et al. Prevalence and potential risk factors for organophosphate and pyrethroid resistance in Boophilus microplus ticks on cattle ranches from the State of Yucatan, Mexico. Vet Parasitol. 2006;136:335-342. doi:10.1016/j.vetpar.2005.05.069
- Rodríguez-Vivas RI, Rodríguez-Arevalo F, Alonso-Díaz MA, et al. Prevalence and potential risk factors for amitraz resistance in Boophilus microplus ticks in cattle farms in the State of Yucatan, Mexico. Prev Vet Med. 2006;75:280-286. doi:10.1016/j.prevetmed.2006.04.001
- Perez-Cogollo LC, Rodriguez-Vivas RI, Ramirez-Cruz GT, et al. First report of the cattle tick Rhipicephalus microplus resistant to ivermectin in Mexico. Vet Parasitol. 2010;168:165-169. doi:10.1016/j.vetpar.2009.10.021
- Rodriguez-Vivas RI, Jonsson NN, Bhushan C. Strategies for the control of Rhipicephalus microplus ticks in a world of conventional acaricide and macrocyclic lactone resistance. Parasitol Res.2018;117:3-29. doi:10.1007/s00436-017-5677-6
- Centers for Disease Control and Prevention. Tick removal. Updated May 13, 2022. Accessed January 3, 2024. https://www.cdc.gov/ticks/removing_a_tick.html
- Diaz JH. Chemical and plant-based insect repellents: efficacy, safety, and toxicity. Wilderness Environ Med. 2016;27:153-163. doi:10.1016/j.wem.2015.11.007
- Dantas-Torres F. The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): from taxonomy to control. Vet Parasitol. 2008;152:173-185. doi:10.1016/j.vetpar.2007.12.030
- Madder M, Fourie JJ, Schetters TPM. Arachnida, Metastigmata, Ixodidae (except Ixodes holocyclus). In: Marchiondo AA, Cruthers LR, Fourie JJ, eds. Parasiticide Screening: In Vitro and In Vivo Tests With Relevant Parasite Rearing and Host Infection/Infestation Methods. Volume 1. Elsevier Academic Press; 2019:19-20.
- Burger TD, Shao R, Barker SC. Phylogenetic analysis of mitochondrial genome sequences indicates that the cattle tick, Rhipicephalus (Boophilus) microplus, contains a cryptic species. Mol Phylogenet Evol. 2014;76:241-253. doi:10.1016/j.ympev.2014.03.017
- Gray J, Dantas-Torres F, Estrada-Peña A, et al. Systematics and ecology of the brown dog tick, Rhipicephalus sanguineus. Ticks Tick Borne Dis. 2013;4:171-180. doi:10.1016/j.ttbdis.2012.12.003
- Tian Y, Lord CC, Kaufman PE. Brown dog tick, Rhipicephalus Sanguineus Latrielle (Arachnida: Acari: Ixodidae): EENY-221/IN378. EDIS. March 26, 2020. Accessed January 3, 2024. https://doi.org/10.32473/edis-in378-2020
- Saleh MN, Allen KE, Lineberry MW, et al. Ticks infesting dogs and cats in North America: biology, geographic distribution, and pathogen transmission. Vet Parasitol. 2021;294:109392. doi:10.1016/j.vetpar.2021.109392
- Dantas-Torres F. Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasit Vectors. 2010;3:26. doi:10.1186/1756-3305-3-26
- Dryden MW, Payne PA. Biology and control of ticks infesting dogs and cats in North America. Vet Ther. 2004;5:139-154.
- Nyangiwe N, Yawa M, Muchenje V. Driving forces for changes in geographic range of cattle ticks (Acari: Ixodidae) in Africa: a Review. S Afr J Anim Sci. 2018;48:829. doi:10.4314/sajas.v48i5.4
- Ramot Y, Zlotogorski A, Mumcuoglu KY. Brown dog tick (Rhipicephalus sanguineus) infestation of the penis detected by dermoscopy. Int J Dermatol. 2012;51:1402-1403. doi:10.1111/j.1365-4632.2010.04756.x
- Tucker NSG, Weeks ENI, Beati L, et al. Prevalence and distribution of pathogen infection and permethrin resistance in tropical and temperate populations of Rhipicephalus sanguineus s.l. collected worldwide. Med Vet Entomol. 2021;35:147-157. doi:10.1111/mve.12479
- McClain MT, Sexton DJ, Hall KK, eds. Other spotted fever group rickettsial infections. UpToDate. Updated October 10, 2022. Accessed January 3, 2024. https://www.uptodate.com/contents/other-spotted-fever-group-rickettsial-infections
- Ribeiro CM, Carvalho JLB, Bastos PAS, et al. Prevalence of Rickettsia rickettsii in ticks: systematic review and meta-analysis. Vector Borne Zoonotic Dis. 2021;21:557-565. doi:10.1089/vbz.2021.0004
- Pace EJ, O’Reilly M. Tickborne diseases: diagnosis and management. Am Fam Physician. 2020;101:530-540.
- Patterson JW. Weedon’s Skin Pathology. 5th ed. Elsevier; 2020.
- Dantas-Torres F. Rocky Mountain spotted fever. Lancet Infect Dis. 2007;7:724-732. doi:10.1016/S1473-3099(07)70261-X
- Biggs HM, Behravesh CB, Bradley KK, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis—United States. MMWR Recomm Rep. 2016;65:1-44. doi:10.15585/mmwr.rr6502a1
- Rossio R, Conalbi V, Castagna V, et al. Mediterranean spotted fever and hearing impairment: a rare complication. Int J Infect Dis. 2015;35:34-36. doi:10.1016/j.ijid.2015.04.005
- Dantas-Torres F, Otranto D. Further thoughts on the taxonomy and vector role of Rhipicephalus sanguineus group ticks. Vet Parasitol. 2015;208:9-13. doi:10.1016/j.vetpar.2014.12.014
- Eisen RJ, Kugeler KJ, Eisen L, et al. Tick-borne zoonoses in the United States: persistent and emerging threats to human health. ILAR J. 2017;58:319-335. doi:10.1093/ilar/ilx005
- Nguyen QD, Vu MN, Hebert AA. Insect repellents: an updated review for the clinician. J Am Acad Dermatol. 2018;88:123-130. doi:10.1016/j.jaad.2018.10.053
- Pages F, Dautel H, Duvallet G, et al. Tick repellents for human use: prevention of tick bites and tick-borne diseases. Vector Borne Zoonotic Dis. 2014;14:85-93. doi:10.1089/vbz.2013.1410
- Rodriguez-Vivas RI, Alonso-Díaz MA, et al. Prevalence and potential risk factors for organophosphate and pyrethroid resistance in Boophilus microplus ticks on cattle ranches from the State of Yucatan, Mexico. Vet Parasitol. 2006;136:335-342. doi:10.1016/j.vetpar.2005.05.069
- Rodríguez-Vivas RI, Rodríguez-Arevalo F, Alonso-Díaz MA, et al. Prevalence and potential risk factors for amitraz resistance in Boophilus microplus ticks in cattle farms in the State of Yucatan, Mexico. Prev Vet Med. 2006;75:280-286. doi:10.1016/j.prevetmed.2006.04.001
- Perez-Cogollo LC, Rodriguez-Vivas RI, Ramirez-Cruz GT, et al. First report of the cattle tick Rhipicephalus microplus resistant to ivermectin in Mexico. Vet Parasitol. 2010;168:165-169. doi:10.1016/j.vetpar.2009.10.021
- Rodriguez-Vivas RI, Jonsson NN, Bhushan C. Strategies for the control of Rhipicephalus microplus ticks in a world of conventional acaricide and macrocyclic lactone resistance. Parasitol Res.2018;117:3-29. doi:10.1007/s00436-017-5677-6
- Centers for Disease Control and Prevention. Tick removal. Updated May 13, 2022. Accessed January 3, 2024. https://www.cdc.gov/ticks/removing_a_tick.html
- Diaz JH. Chemical and plant-based insect repellents: efficacy, safety, and toxicity. Wilderness Environ Med. 2016;27:153-163. doi:10.1016/j.wem.2015.11.007
PRACTICE POINTS
- Rhipicephalus ticks are vectors of a variety of diseases, including the rickettsial diseases Rocky Mountain spotted fever and Mediterranean spotted fever.
- Presenting symptoms of a tick bite include intensely pruritic, erythematous papules and nodules at the site of tick attachment.
- If rickettsial disease is suspected, treatment with doxycycline should be initiated immediately; do not delay treatment to await results of confirmatory tests or because of the absence of a rash.
- Primary methods of prevention of tick-borne disease include repellents, protective clothing, vector control, and prompt removal of the tick.
Botanical Briefs: Neem Oil (Azadirachta indica)
Commonly known as neem or nimba, Azadirachta indica traditionally has been used as an oil or poultice to lighten skin pigment and reduce joint inflammation. Neem is a drought-resistant evergreen tree with thin serrated leaves, white fragrant flowers, and olivelike fruit (Figure 1). This plant is indigenous to India but also is readily found within tropical and semitropical environments throughout the Middle East, Southeast Asia, North Africa, and Australia.

Traditional Uses
For more than 4000 years, neem leaves, bark, fruit, and seeds have been used in food, insecticide, and herbal medicine cross-culturally in Indian Ayurvedic medicine and across Southeast Asia, particularly in Cambodia, Laos, Thailand, Myanmar, and Vietnam.1-3 Because of its many essential nutrients—oleic acid, palmitic acid, stearic acid, linoleic acid, behenic acid, arachidic acid, and palmitoleic acid—and readily available nature, some ethnic groups include neem in their diet.4 Neem commonly is used as a seasoning in soups and rice, eaten as a cooked vegetable, infused into teas and tonics, and pickled with other spices.5
All parts of the neem tree—both externally and internally—have been utilized in traditional medicine for the treatment of various diseases and ailments. The flowers have been used to treat eye diseases and dyspepsia, the fruit has been employed as an anthelmintic, the seeds and leaves have been used for malaria treatment and insecticide, the stem bark has been used for the treatment of diarrhea, and the root bark has been used for skin diseases and inflammation.6 Neem oil is a yellow-brown bitter substance that often is utilized to treat skin diseases such as psoriasis, eczema, fungal infections, and abscesses.
Case Report—A 77-year-old man presented with a diffuse rash across the lower back. He reported that he had been using topical neem oil to alleviate lower back pain and arthritis for the last 6 months with noted relief and improvement of back pain. After roughly 3 to 4 months of using neem oil, he noted a rash on the lower back, bilateral flanks, and buttocks (Figure 2). The rash was asymptomatic, and he denied any pruritus, scaling, pain, or burning. The patient was referred to dermatology and received a diagnosis of chemical leukoderma secondary to contact with A indica. The patient was advised to stop using the topical neem oil, and the rash was simply monitored, as it was asymptomatic.
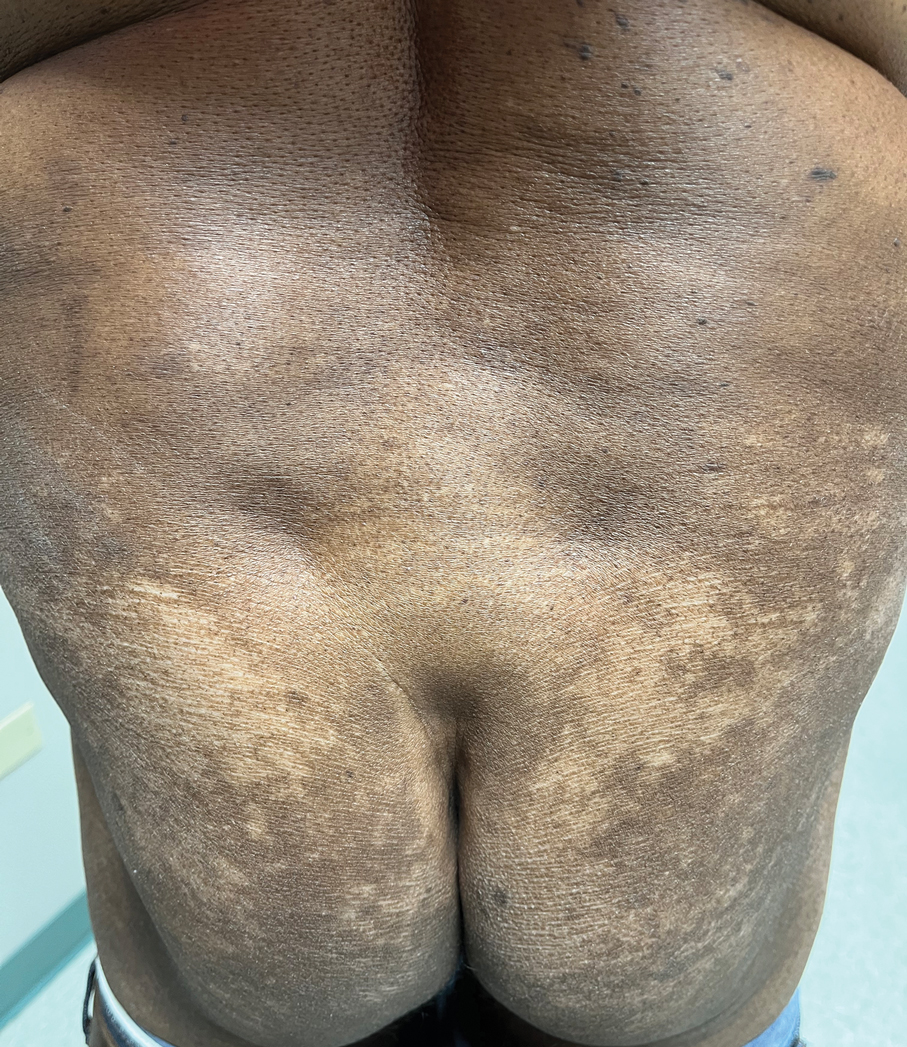
Bioactivity
Research has elucidated multiple bioactivity mechanisms of neem, including melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity.1,7-9 Literature on the diverse phytochemical components of A indica indicate high levels of limonoids, flavonoids, and triterpenoids that are responsible for much of its antioxidant, anti-inflammatory, and insecticide properties.1,10
Melanogenesis-Inhibitory Activity—To date, neem has been added to a number of cosmetic products used in Ayurvedic medicine. One study of isolated compounds in A indica showed superior inhibitory activities against melanogenesis with minimal toxicity to cells (86.5%–105.1% cell viability). Western blot analysis of samples extracted and isolated from neem root and bark showed melanogenesis-inhibitory activities in B16 melanoma cells through the inhibition of microphthalmia-associated transcription factor expression and decreased expression of tyrosinase, as well as tyrosinase-related proteins 1 and 2, which are largely responsible for melanin synthesis.11 In another study, A indica flowers and their extracted constituents—6-deacetylnimbin and kaempferide—suggest melanogenesis-inhibitory activities in B16 melanoma cells with little to no toxicity to the cells (81.0%–111.7% cell viability).1 In an evaluationof A indica seed extracts, some of the isolated limonoids and diterpenoids exhibited a marked melanogenesis-inhibitory effect (74%–91% reduction of melanin content) with no toxicity to the cell.5 All of these studies indicate that active compounds in neem root, bark, flowers, and seeds may be potential skin-lightening agents.
Toxicity Against Pests—Neem seeds have phytochemicals that convey some insecticidal properties. The seeds often are ground into a powder, combined with water, and sprayed onto crops to act as an insecticide. As a natural method of nonpesticidal management, A indica acts as an antifeedant, insect repellent, and egg-laying deterrent that protects crops from damage. Studies of A indica have noted effective nonpesticidal management against arthropod pests such as armyworm, termites, and the oriental fruit fly.7,12,13
Antimalarial Activity—One study indicated that nimbolide, a limonoid from the neem plant, demonstrated antimalarial activity against Plasmodium falciparum. In separate cultures of asexual parasites and mature gametocytes, parasite numbers were less than 50% of the number in control cultures (8.0% vs 8.5% parasitemia, respectively).14 Thus, the lower parasite numbers indicated by this study highlight the antimalarial utility of nimbolide and neem oil.
Antioxidant and Anti-inflammatory Activity—Neem bark has been reported to have considerable antioxidant activity due to its high phenolic content.1,15 One study showed that azadirachtin and nimbolide in neem exhibited concentration-dependent antiradical scavenging activity and antioxidant properties.16
The anti-inflammatory potential for neem may occur via the inhibition of the nuclear factor-κB signaling pathway, which is linked to cancer, inflammation, and apoptosis.17 It also has been observed that nimbidin within neem extracts—such as leaves, bark, and seed extract—suppresses the function of macrophages and neutrophils relevant to inflammation.16 Another study indicated neem’s anti-inflammatory activity due to the regulation of proinflammatory enzymes such as cyclooxygenase and lipoxygenase.18
Safety, Toxicity, and Risks
Ingestion—Although neem is safe to use in the general population, neem oil poisoning has been reported, particularly in young children. Ingesting large quantities of neem has resulted in vomiting, hepatic toxicity, metabolic acidosis, late neurologic sequelae, and encephalopathy in young children.19 The diagnosis of neem oil poisoning is based on patient history, clinical examination, and imaging findings. Poisoning can manifest as drowsiness, tachypnea, and generalized seizures.20
Topical Application—Topical use of neem appears to be safe if the substance is diluted with other ingredients. However, direct application to the skin is not advised, as it may cause leukoderma and could induce allergic contact dermatitis and other allergic reactions.4
Final Thoughts
The use of neem extract for disease prevention and treatment has been prevalent around the world since ancient times. Neem has been documented to possess melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity by means of tyrosinase inhibition, phytochemical production, limonoid expression, and nuclear factor-κB regulation, respectively. However, topical use of neem may trigger a cutaneous response, highlighting the importance of considering a diagnosis of neem oil–induced chemical leukoderma when patients present with a hypopigmented rash and relevant history.
- Kitdamrongtham W, Ishii K, Ebina K, et al. Limonoids and flavonoids from the flowers of Azadirachta indica var. siamensis, and their melanogenesis-inhibitory and cytotoxic activities. Chem Biodivers. 2014;11:73-84. doi:10.1002/cbdv.201300266
- Singh A, Srivastava PS, Lakshmikumaran M. Comparison of AFLP and SAMPL markers for assessment of intra-population genetic variation in Azadirachta indica A. Juss. Plant Sci. 2002;162:17-25. doi:10.1016/S0168-9452(01)00503-9
- Pandey G, Verma K, Singh M. Evaluation of phytochemical, antibacterial and free radical scavenging properties of Azadirachta Indica (neem) leaves. Int J Pharm Pharmaceut Sci. 2014;6:444-447.
- Romita P, Calogiuri G, Bellino M, et al. Allergic contact dermatitis caused by neem oil: an underrated allergen. Contact Dermatitis. 2019;81:133-134. doi:10.1111/cod. 13256
- Akihisa T, Noto T, Takahashi A, et al. Melanogenesis inhibitory, anti-inflammatory, and chemopreventive effects of limonoids from the seeds of Azadirachta indica A. Juss. (neem). J Oleo Sci. 2009;58:581-594.
- Subapriya R, Nagini S. Medicinal properties of neem leaves: a review. Curr Med Chem Anticancer Agents. 2005;5:149-156. doi:10.2174/1568011053174828
- Areekul S, Sinchaisri P, Tigvatananon S. Effect of Thai plant extracts on the Oriental fruit fly. I: toxicity test. Agriculture and Natural Resources. 1987;21:395-407.
- Rochanakij S, Thebtaranonth Y, Yenjai C, et al. Nimbolide, a constituent of Azadirachta indica, inhibits Plasmodium falciparum in culture. Southeast Asian J Trop Med Public Health. 1985;16:66-72.
- Sithisarn P, Supabphol R, Gritsanapan W. Antioxidant activity of Siamese neem tree (VP1209). J Ethnopharmacol. 2005;99:109-112. doi:10.1016/j.jep.2005.02.008
- Yin F, Lei XX, Cheng L, et al. Isolation and structure identification of the compounds from the seeds and leaves of Azadirachta indica A. Juss. J China Pharmaceut University. 2005;36:10-12.
- Su S, Cheng J, Zhang C, et al. Melanogenesis-inhibitory activities of limonoids and tricyclic diterpenoids from Azadirachta indica. Bioorganic Chemistry. 2020;100:103941. doi:j.bioorg.2020.103941
- Tulashie SK, Adjei F, Abraham J, et al. Potential of neem extracts as natural insecticide against fall armyworm (Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae). Case Stud Chem Environ Eng. 2021;4:100130. doi:10.1016/j.cscee.2021.100130
- Yashroy RC, Gupta PK. Neem-seed oil inhibits growth of termite surface-tunnels. Indian J Toxicol. 2000;7:49-50.
- Udeinya JI, Shu EN, Quakyi I, et al. An antimalarial neem leaf extract has both schizonticidal and gametocytocidal activities. Am J Therapeutics. 2008;15:108-110. doi:10.1097/MJT.0b013e31804c6d1d
- Bindurani R, Kumar K. Evaluation of antioxidant activity of hydro distilled extracts of leaf, heart wood and flower of Azadirachta indica. Int J Pharm Sci Rev Res. 2013;20:222.
- Alzohairy MA. Therapeutics role of Azadirachta indica (Neem) and their active constituents in diseases prevention and treatment [published online March 1, 2016]. Evid Based Complement Alternat Med. doi:10.1155/2016/7382506
- Schumacher M, Cerella C, Reuter S, et al. Anti-inflammatory, pro-apoptotic, and anti-proliferative effects of a methanolic neem (Azadirachta indica) leaf extract are mediated via modulation of the nuclear factor-κB pathway. Genes Nutr. 2011;6:149-160. doi:10.1007/s12263-010-0194-6
- Kaur G, Sarwar Alam M, Athar M. Nimbidin suppresses functions of macrophages and neutrophils: relevance to its anti-inflammatory mechanisms. Phytotherapy Res. 2004;18:419-424. doi:10.1002/ptr.1474
- Dhongade RK, Kavade SG, Damle RS. Neem oil poisoning. Indian Pediatr. 2008;45:56-57.
- Bhaskar MV, Pramod SJ, Jeevika MU, et al. MR imaging findings of neem oil poisoning. Am J Neuroradiol. 2010;31:E60-E61. doi:10.3174/ajnr.A2146
Commonly known as neem or nimba, Azadirachta indica traditionally has been used as an oil or poultice to lighten skin pigment and reduce joint inflammation. Neem is a drought-resistant evergreen tree with thin serrated leaves, white fragrant flowers, and olivelike fruit (Figure 1). This plant is indigenous to India but also is readily found within tropical and semitropical environments throughout the Middle East, Southeast Asia, North Africa, and Australia.

Traditional Uses
For more than 4000 years, neem leaves, bark, fruit, and seeds have been used in food, insecticide, and herbal medicine cross-culturally in Indian Ayurvedic medicine and across Southeast Asia, particularly in Cambodia, Laos, Thailand, Myanmar, and Vietnam.1-3 Because of its many essential nutrients—oleic acid, palmitic acid, stearic acid, linoleic acid, behenic acid, arachidic acid, and palmitoleic acid—and readily available nature, some ethnic groups include neem in their diet.4 Neem commonly is used as a seasoning in soups and rice, eaten as a cooked vegetable, infused into teas and tonics, and pickled with other spices.5
All parts of the neem tree—both externally and internally—have been utilized in traditional medicine for the treatment of various diseases and ailments. The flowers have been used to treat eye diseases and dyspepsia, the fruit has been employed as an anthelmintic, the seeds and leaves have been used for malaria treatment and insecticide, the stem bark has been used for the treatment of diarrhea, and the root bark has been used for skin diseases and inflammation.6 Neem oil is a yellow-brown bitter substance that often is utilized to treat skin diseases such as psoriasis, eczema, fungal infections, and abscesses.
Case Report—A 77-year-old man presented with a diffuse rash across the lower back. He reported that he had been using topical neem oil to alleviate lower back pain and arthritis for the last 6 months with noted relief and improvement of back pain. After roughly 3 to 4 months of using neem oil, he noted a rash on the lower back, bilateral flanks, and buttocks (Figure 2). The rash was asymptomatic, and he denied any pruritus, scaling, pain, or burning. The patient was referred to dermatology and received a diagnosis of chemical leukoderma secondary to contact with A indica. The patient was advised to stop using the topical neem oil, and the rash was simply monitored, as it was asymptomatic.

Bioactivity
Research has elucidated multiple bioactivity mechanisms of neem, including melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity.1,7-9 Literature on the diverse phytochemical components of A indica indicate high levels of limonoids, flavonoids, and triterpenoids that are responsible for much of its antioxidant, anti-inflammatory, and insecticide properties.1,10
Melanogenesis-Inhibitory Activity—To date, neem has been added to a number of cosmetic products used in Ayurvedic medicine. One study of isolated compounds in A indica showed superior inhibitory activities against melanogenesis with minimal toxicity to cells (86.5%–105.1% cell viability). Western blot analysis of samples extracted and isolated from neem root and bark showed melanogenesis-inhibitory activities in B16 melanoma cells through the inhibition of microphthalmia-associated transcription factor expression and decreased expression of tyrosinase, as well as tyrosinase-related proteins 1 and 2, which are largely responsible for melanin synthesis.11 In another study, A indica flowers and their extracted constituents—6-deacetylnimbin and kaempferide—suggest melanogenesis-inhibitory activities in B16 melanoma cells with little to no toxicity to the cells (81.0%–111.7% cell viability).1 In an evaluationof A indica seed extracts, some of the isolated limonoids and diterpenoids exhibited a marked melanogenesis-inhibitory effect (74%–91% reduction of melanin content) with no toxicity to the cell.5 All of these studies indicate that active compounds in neem root, bark, flowers, and seeds may be potential skin-lightening agents.
Toxicity Against Pests—Neem seeds have phytochemicals that convey some insecticidal properties. The seeds often are ground into a powder, combined with water, and sprayed onto crops to act as an insecticide. As a natural method of nonpesticidal management, A indica acts as an antifeedant, insect repellent, and egg-laying deterrent that protects crops from damage. Studies of A indica have noted effective nonpesticidal management against arthropod pests such as armyworm, termites, and the oriental fruit fly.7,12,13
Antimalarial Activity—One study indicated that nimbolide, a limonoid from the neem plant, demonstrated antimalarial activity against Plasmodium falciparum. In separate cultures of asexual parasites and mature gametocytes, parasite numbers were less than 50% of the number in control cultures (8.0% vs 8.5% parasitemia, respectively).14 Thus, the lower parasite numbers indicated by this study highlight the antimalarial utility of nimbolide and neem oil.
Antioxidant and Anti-inflammatory Activity—Neem bark has been reported to have considerable antioxidant activity due to its high phenolic content.1,15 One study showed that azadirachtin and nimbolide in neem exhibited concentration-dependent antiradical scavenging activity and antioxidant properties.16
The anti-inflammatory potential for neem may occur via the inhibition of the nuclear factor-κB signaling pathway, which is linked to cancer, inflammation, and apoptosis.17 It also has been observed that nimbidin within neem extracts—such as leaves, bark, and seed extract—suppresses the function of macrophages and neutrophils relevant to inflammation.16 Another study indicated neem’s anti-inflammatory activity due to the regulation of proinflammatory enzymes such as cyclooxygenase and lipoxygenase.18
Safety, Toxicity, and Risks
Ingestion—Although neem is safe to use in the general population, neem oil poisoning has been reported, particularly in young children. Ingesting large quantities of neem has resulted in vomiting, hepatic toxicity, metabolic acidosis, late neurologic sequelae, and encephalopathy in young children.19 The diagnosis of neem oil poisoning is based on patient history, clinical examination, and imaging findings. Poisoning can manifest as drowsiness, tachypnea, and generalized seizures.20
Topical Application—Topical use of neem appears to be safe if the substance is diluted with other ingredients. However, direct application to the skin is not advised, as it may cause leukoderma and could induce allergic contact dermatitis and other allergic reactions.4
Final Thoughts
The use of neem extract for disease prevention and treatment has been prevalent around the world since ancient times. Neem has been documented to possess melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity by means of tyrosinase inhibition, phytochemical production, limonoid expression, and nuclear factor-κB regulation, respectively. However, topical use of neem may trigger a cutaneous response, highlighting the importance of considering a diagnosis of neem oil–induced chemical leukoderma when patients present with a hypopigmented rash and relevant history.
Commonly known as neem or nimba, Azadirachta indica traditionally has been used as an oil or poultice to lighten skin pigment and reduce joint inflammation. Neem is a drought-resistant evergreen tree with thin serrated leaves, white fragrant flowers, and olivelike fruit (Figure 1). This plant is indigenous to India but also is readily found within tropical and semitropical environments throughout the Middle East, Southeast Asia, North Africa, and Australia.

Traditional Uses
For more than 4000 years, neem leaves, bark, fruit, and seeds have been used in food, insecticide, and herbal medicine cross-culturally in Indian Ayurvedic medicine and across Southeast Asia, particularly in Cambodia, Laos, Thailand, Myanmar, and Vietnam.1-3 Because of its many essential nutrients—oleic acid, palmitic acid, stearic acid, linoleic acid, behenic acid, arachidic acid, and palmitoleic acid—and readily available nature, some ethnic groups include neem in their diet.4 Neem commonly is used as a seasoning in soups and rice, eaten as a cooked vegetable, infused into teas and tonics, and pickled with other spices.5
All parts of the neem tree—both externally and internally—have been utilized in traditional medicine for the treatment of various diseases and ailments. The flowers have been used to treat eye diseases and dyspepsia, the fruit has been employed as an anthelmintic, the seeds and leaves have been used for malaria treatment and insecticide, the stem bark has been used for the treatment of diarrhea, and the root bark has been used for skin diseases and inflammation.6 Neem oil is a yellow-brown bitter substance that often is utilized to treat skin diseases such as psoriasis, eczema, fungal infections, and abscesses.
Case Report—A 77-year-old man presented with a diffuse rash across the lower back. He reported that he had been using topical neem oil to alleviate lower back pain and arthritis for the last 6 months with noted relief and improvement of back pain. After roughly 3 to 4 months of using neem oil, he noted a rash on the lower back, bilateral flanks, and buttocks (Figure 2). The rash was asymptomatic, and he denied any pruritus, scaling, pain, or burning. The patient was referred to dermatology and received a diagnosis of chemical leukoderma secondary to contact with A indica. The patient was advised to stop using the topical neem oil, and the rash was simply monitored, as it was asymptomatic.

Bioactivity
Research has elucidated multiple bioactivity mechanisms of neem, including melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity.1,7-9 Literature on the diverse phytochemical components of A indica indicate high levels of limonoids, flavonoids, and triterpenoids that are responsible for much of its antioxidant, anti-inflammatory, and insecticide properties.1,10
Melanogenesis-Inhibitory Activity—To date, neem has been added to a number of cosmetic products used in Ayurvedic medicine. One study of isolated compounds in A indica showed superior inhibitory activities against melanogenesis with minimal toxicity to cells (86.5%–105.1% cell viability). Western blot analysis of samples extracted and isolated from neem root and bark showed melanogenesis-inhibitory activities in B16 melanoma cells through the inhibition of microphthalmia-associated transcription factor expression and decreased expression of tyrosinase, as well as tyrosinase-related proteins 1 and 2, which are largely responsible for melanin synthesis.11 In another study, A indica flowers and their extracted constituents—6-deacetylnimbin and kaempferide—suggest melanogenesis-inhibitory activities in B16 melanoma cells with little to no toxicity to the cells (81.0%–111.7% cell viability).1 In an evaluationof A indica seed extracts, some of the isolated limonoids and diterpenoids exhibited a marked melanogenesis-inhibitory effect (74%–91% reduction of melanin content) with no toxicity to the cell.5 All of these studies indicate that active compounds in neem root, bark, flowers, and seeds may be potential skin-lightening agents.
Toxicity Against Pests—Neem seeds have phytochemicals that convey some insecticidal properties. The seeds often are ground into a powder, combined with water, and sprayed onto crops to act as an insecticide. As a natural method of nonpesticidal management, A indica acts as an antifeedant, insect repellent, and egg-laying deterrent that protects crops from damage. Studies of A indica have noted effective nonpesticidal management against arthropod pests such as armyworm, termites, and the oriental fruit fly.7,12,13
Antimalarial Activity—One study indicated that nimbolide, a limonoid from the neem plant, demonstrated antimalarial activity against Plasmodium falciparum. In separate cultures of asexual parasites and mature gametocytes, parasite numbers were less than 50% of the number in control cultures (8.0% vs 8.5% parasitemia, respectively).14 Thus, the lower parasite numbers indicated by this study highlight the antimalarial utility of nimbolide and neem oil.
Antioxidant and Anti-inflammatory Activity—Neem bark has been reported to have considerable antioxidant activity due to its high phenolic content.1,15 One study showed that azadirachtin and nimbolide in neem exhibited concentration-dependent antiradical scavenging activity and antioxidant properties.16
The anti-inflammatory potential for neem may occur via the inhibition of the nuclear factor-κB signaling pathway, which is linked to cancer, inflammation, and apoptosis.17 It also has been observed that nimbidin within neem extracts—such as leaves, bark, and seed extract—suppresses the function of macrophages and neutrophils relevant to inflammation.16 Another study indicated neem’s anti-inflammatory activity due to the regulation of proinflammatory enzymes such as cyclooxygenase and lipoxygenase.18
Safety, Toxicity, and Risks
Ingestion—Although neem is safe to use in the general population, neem oil poisoning has been reported, particularly in young children. Ingesting large quantities of neem has resulted in vomiting, hepatic toxicity, metabolic acidosis, late neurologic sequelae, and encephalopathy in young children.19 The diagnosis of neem oil poisoning is based on patient history, clinical examination, and imaging findings. Poisoning can manifest as drowsiness, tachypnea, and generalized seizures.20
Topical Application—Topical use of neem appears to be safe if the substance is diluted with other ingredients. However, direct application to the skin is not advised, as it may cause leukoderma and could induce allergic contact dermatitis and other allergic reactions.4
Final Thoughts
The use of neem extract for disease prevention and treatment has been prevalent around the world since ancient times. Neem has been documented to possess melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity by means of tyrosinase inhibition, phytochemical production, limonoid expression, and nuclear factor-κB regulation, respectively. However, topical use of neem may trigger a cutaneous response, highlighting the importance of considering a diagnosis of neem oil–induced chemical leukoderma when patients present with a hypopigmented rash and relevant history.
- Kitdamrongtham W, Ishii K, Ebina K, et al. Limonoids and flavonoids from the flowers of Azadirachta indica var. siamensis, and their melanogenesis-inhibitory and cytotoxic activities. Chem Biodivers. 2014;11:73-84. doi:10.1002/cbdv.201300266
- Singh A, Srivastava PS, Lakshmikumaran M. Comparison of AFLP and SAMPL markers for assessment of intra-population genetic variation in Azadirachta indica A. Juss. Plant Sci. 2002;162:17-25. doi:10.1016/S0168-9452(01)00503-9
- Pandey G, Verma K, Singh M. Evaluation of phytochemical, antibacterial and free radical scavenging properties of Azadirachta Indica (neem) leaves. Int J Pharm Pharmaceut Sci. 2014;6:444-447.
- Romita P, Calogiuri G, Bellino M, et al. Allergic contact dermatitis caused by neem oil: an underrated allergen. Contact Dermatitis. 2019;81:133-134. doi:10.1111/cod. 13256
- Akihisa T, Noto T, Takahashi A, et al. Melanogenesis inhibitory, anti-inflammatory, and chemopreventive effects of limonoids from the seeds of Azadirachta indica A. Juss. (neem). J Oleo Sci. 2009;58:581-594.
- Subapriya R, Nagini S. Medicinal properties of neem leaves: a review. Curr Med Chem Anticancer Agents. 2005;5:149-156. doi:10.2174/1568011053174828
- Areekul S, Sinchaisri P, Tigvatananon S. Effect of Thai plant extracts on the Oriental fruit fly. I: toxicity test. Agriculture and Natural Resources. 1987;21:395-407.
- Rochanakij S, Thebtaranonth Y, Yenjai C, et al. Nimbolide, a constituent of Azadirachta indica, inhibits Plasmodium falciparum in culture. Southeast Asian J Trop Med Public Health. 1985;16:66-72.
- Sithisarn P, Supabphol R, Gritsanapan W. Antioxidant activity of Siamese neem tree (VP1209). J Ethnopharmacol. 2005;99:109-112. doi:10.1016/j.jep.2005.02.008
- Yin F, Lei XX, Cheng L, et al. Isolation and structure identification of the compounds from the seeds and leaves of Azadirachta indica A. Juss. J China Pharmaceut University. 2005;36:10-12.
- Su S, Cheng J, Zhang C, et al. Melanogenesis-inhibitory activities of limonoids and tricyclic diterpenoids from Azadirachta indica. Bioorganic Chemistry. 2020;100:103941. doi:j.bioorg.2020.103941
- Tulashie SK, Adjei F, Abraham J, et al. Potential of neem extracts as natural insecticide against fall armyworm (Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae). Case Stud Chem Environ Eng. 2021;4:100130. doi:10.1016/j.cscee.2021.100130
- Yashroy RC, Gupta PK. Neem-seed oil inhibits growth of termite surface-tunnels. Indian J Toxicol. 2000;7:49-50.
- Udeinya JI, Shu EN, Quakyi I, et al. An antimalarial neem leaf extract has both schizonticidal and gametocytocidal activities. Am J Therapeutics. 2008;15:108-110. doi:10.1097/MJT.0b013e31804c6d1d
- Bindurani R, Kumar K. Evaluation of antioxidant activity of hydro distilled extracts of leaf, heart wood and flower of Azadirachta indica. Int J Pharm Sci Rev Res. 2013;20:222.
- Alzohairy MA. Therapeutics role of Azadirachta indica (Neem) and their active constituents in diseases prevention and treatment [published online March 1, 2016]. Evid Based Complement Alternat Med. doi:10.1155/2016/7382506
- Schumacher M, Cerella C, Reuter S, et al. Anti-inflammatory, pro-apoptotic, and anti-proliferative effects of a methanolic neem (Azadirachta indica) leaf extract are mediated via modulation of the nuclear factor-κB pathway. Genes Nutr. 2011;6:149-160. doi:10.1007/s12263-010-0194-6
- Kaur G, Sarwar Alam M, Athar M. Nimbidin suppresses functions of macrophages and neutrophils: relevance to its anti-inflammatory mechanisms. Phytotherapy Res. 2004;18:419-424. doi:10.1002/ptr.1474
- Dhongade RK, Kavade SG, Damle RS. Neem oil poisoning. Indian Pediatr. 2008;45:56-57.
- Bhaskar MV, Pramod SJ, Jeevika MU, et al. MR imaging findings of neem oil poisoning. Am J Neuroradiol. 2010;31:E60-E61. doi:10.3174/ajnr.A2146
- Kitdamrongtham W, Ishii K, Ebina K, et al. Limonoids and flavonoids from the flowers of Azadirachta indica var. siamensis, and their melanogenesis-inhibitory and cytotoxic activities. Chem Biodivers. 2014;11:73-84. doi:10.1002/cbdv.201300266
- Singh A, Srivastava PS, Lakshmikumaran M. Comparison of AFLP and SAMPL markers for assessment of intra-population genetic variation in Azadirachta indica A. Juss. Plant Sci. 2002;162:17-25. doi:10.1016/S0168-9452(01)00503-9
- Pandey G, Verma K, Singh M. Evaluation of phytochemical, antibacterial and free radical scavenging properties of Azadirachta Indica (neem) leaves. Int J Pharm Pharmaceut Sci. 2014;6:444-447.
- Romita P, Calogiuri G, Bellino M, et al. Allergic contact dermatitis caused by neem oil: an underrated allergen. Contact Dermatitis. 2019;81:133-134. doi:10.1111/cod. 13256
- Akihisa T, Noto T, Takahashi A, et al. Melanogenesis inhibitory, anti-inflammatory, and chemopreventive effects of limonoids from the seeds of Azadirachta indica A. Juss. (neem). J Oleo Sci. 2009;58:581-594.
- Subapriya R, Nagini S. Medicinal properties of neem leaves: a review. Curr Med Chem Anticancer Agents. 2005;5:149-156. doi:10.2174/1568011053174828
- Areekul S, Sinchaisri P, Tigvatananon S. Effect of Thai plant extracts on the Oriental fruit fly. I: toxicity test. Agriculture and Natural Resources. 1987;21:395-407.
- Rochanakij S, Thebtaranonth Y, Yenjai C, et al. Nimbolide, a constituent of Azadirachta indica, inhibits Plasmodium falciparum in culture. Southeast Asian J Trop Med Public Health. 1985;16:66-72.
- Sithisarn P, Supabphol R, Gritsanapan W. Antioxidant activity of Siamese neem tree (VP1209). J Ethnopharmacol. 2005;99:109-112. doi:10.1016/j.jep.2005.02.008
- Yin F, Lei XX, Cheng L, et al. Isolation and structure identification of the compounds from the seeds and leaves of Azadirachta indica A. Juss. J China Pharmaceut University. 2005;36:10-12.
- Su S, Cheng J, Zhang C, et al. Melanogenesis-inhibitory activities of limonoids and tricyclic diterpenoids from Azadirachta indica. Bioorganic Chemistry. 2020;100:103941. doi:j.bioorg.2020.103941
- Tulashie SK, Adjei F, Abraham J, et al. Potential of neem extracts as natural insecticide against fall armyworm (Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae). Case Stud Chem Environ Eng. 2021;4:100130. doi:10.1016/j.cscee.2021.100130
- Yashroy RC, Gupta PK. Neem-seed oil inhibits growth of termite surface-tunnels. Indian J Toxicol. 2000;7:49-50.
- Udeinya JI, Shu EN, Quakyi I, et al. An antimalarial neem leaf extract has both schizonticidal and gametocytocidal activities. Am J Therapeutics. 2008;15:108-110. doi:10.1097/MJT.0b013e31804c6d1d
- Bindurani R, Kumar K. Evaluation of antioxidant activity of hydro distilled extracts of leaf, heart wood and flower of Azadirachta indica. Int J Pharm Sci Rev Res. 2013;20:222.
- Alzohairy MA. Therapeutics role of Azadirachta indica (Neem) and their active constituents in diseases prevention and treatment [published online March 1, 2016]. Evid Based Complement Alternat Med. doi:10.1155/2016/7382506
- Schumacher M, Cerella C, Reuter S, et al. Anti-inflammatory, pro-apoptotic, and anti-proliferative effects of a methanolic neem (Azadirachta indica) leaf extract are mediated via modulation of the nuclear factor-κB pathway. Genes Nutr. 2011;6:149-160. doi:10.1007/s12263-010-0194-6
- Kaur G, Sarwar Alam M, Athar M. Nimbidin suppresses functions of macrophages and neutrophils: relevance to its anti-inflammatory mechanisms. Phytotherapy Res. 2004;18:419-424. doi:10.1002/ptr.1474
- Dhongade RK, Kavade SG, Damle RS. Neem oil poisoning. Indian Pediatr. 2008;45:56-57.
- Bhaskar MV, Pramod SJ, Jeevika MU, et al. MR imaging findings of neem oil poisoning. Am J Neuroradiol. 2010;31:E60-E61. doi:10.3174/ajnr.A2146
Practice Points
- Neem is a traditional herb with various bioactivities, such as melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity.
- Neem should be used with caution as a remedy because of its skin-lightening properties, which are attributed to melanogenesis-inhibitory activity via tyrosinase inhibition.
- Chemical leukoderma should be included in the differential diagnosis when a patient presents with a hypopigmented rash after topical use of neem products.
Botanical Briefs: Contact Dermatitis Induced by Western Poison Ivy (Toxicodendron rydbergii)
Clinical Importance
Western poison ivy (Toxicodendron rydbergii) is responsible for many of the cases of Toxicodendron contact dermatitis (TCD) reported in the western and northern United States. Toxicodendron plants cause more cases of allergic contact dermatitis (ACD) in North America than any other allergen1; 9 million Americans present to physician offices and 1.6 million present to emergency departments annually for ACD, emphasizing the notable medical burden of this condition.2,3 Exposure to urushiol, a plant resin containing potent allergens, precipitates this form of ACD.
An estimated 50% to 75% of adults in the United States demonstrate clinical sensitivity and exhibit ACD following contact with T rydbergii.4 Campers, hikers, firefighters, and forest workers often risk increased exposure through physical contact or aerosolized allergens in smoke. According to the Centers for Disease Control and Prevention, the incidence of visits to US emergency departments for TCD nearly doubled from 2002 to 2012,5 which may be explained by atmospheric CO2 levels that both promote increased growth of Toxicodendron species and augment their toxicity.6
Cutaneous Manifestations
The clinical presentation of T rydbergii contact dermatitis is similar to other allergenic members of the Toxicodendron genus. Patients sensitive to urushiol typically develop a pruritic erythematous rash within 1 to 2 days of exposure (range, 5 hours to 15 days).7 Erythematous and edematous streaks initially manifest on the extremities and often progress to bullae and oozing papulovesicles. In early disease, patients also may display black lesions on or near the rash8 (so-called black-dot dermatitis) caused by oxidized urushiol deposited on the skin—an uncommon yet classic presentation of TCD. Generally, symptoms resolve without complications and with few sequalae, though hyperpigmentation or a secondary infection can develop on or near affected areas.9,10
Taxonomy
The Toxicodendron genus belongs to the Anacardiaceae family, which includes pistachios, mangos, and cashews, and causes more cases of ACD than every other plant combined.4 (Shelled pistachios and cashews do not possess cross-reacting allergens and should not worry consumers; mango skin does contain urushiol.)
Toxicodendron (formerly part of the Rhus genus) includes several species of poison oak, poison ivy, and poison sumac and can be found in shrubs (T rydbergii and Toxicodendron diversilobum), vines (Toxicodendron radicans and Toxicodendron pubescens), and trees (Toxicodendron vernix). In addition, Toxicodendron taxa can hybridize with other taxa in close geographic proximity to form morphologic intermediates. Some individual plants have features of multiple species.11
Etymology
The common name of T rydbergii—western poison ivy—misleads the public; the plant contains no poison that can cause death and does not grow as ivy by wrapping around trees, as T radicans and English ivy (Hedera helix) do. Its formal genus, Toxicodendron, means “poison tree” in Greek and was given its generic name by the English botanist Phillip Miller in 1768,12 which caused the renaming of Rhus rydbergii as T rydbergii. The species name honors Per Axel Rydberg, a 19th and 20th century Swedish-American botanist.
Distribution
Toxicodendron rydbergii grows in California and other states in the western half of the United States as well as the states bordering Canada and Mexico. In Canada, it reigns as the most dominant form of poison ivy.13 Hikers and campers find T rydbergii in a variety of areas, including roadsides, river bottoms, sandy shores, talus slopes, precipices, and floodplains.11 This taxon grows under a variety of conditions and in distinct regions, and it thrives in both full sun or shade.
Identifying Features
Toxicodendron rydbergii turns red earlier than most plants; early red summer leaves should serve as a warning sign to hikers from a distance (Figure 1). It displays trifoliate ovate leaves (ie, each leaf contains 3 leaflets) on a dwarf nonclimbing shrub (Figure 2). Although the plant shares common features with its cousin T radicans (eastern poison ivy), T rydbergii is easily distinguished by its thicker stems, absence of aerial rootlets (abundant in T radicans), and short (approximately 1 meter) height.4

Curly hairs occupy the underside of T rydbergii leaflets and along the midrib; leaflet margins appear lobed or rounded. Lenticels appear as small holes in the bark that turn gray in the cold and become brighter come spring.13

The plant bears glabrous long petioles (leaf stems) and densely grouped clusters of yellow flowers. In autumn, the globose fruit—formed in clusters between each twig and leaf petiole (known as an axillary position)—change from yellow-green to tan (Figure 3). When urushiol exudes from damaged leaflets or other plant parts, it oxidizes on exposure to air and creates hardened black deposits on the plant. Even when grown in garden pots, T rydbergii maintains its distinguishing features.11

Dermatitis-Inducing Plant Parts
All parts of T rydbergii including leaves, stems, roots, and fruit contain the allergenic sap throughout the year.14 A person must damage or bruise the plant for urushiol to be released and produce its allergenic effects; softly brushing against undamaged plants typically does not induce dermatitis.4
Pathophysiology of Urushiol
Urushiol, a pale yellow, oily mixture of organic compounds conserved throughout all Toxicodendron species, contains highly allergenic alkyl catechols. These catechols possess hydroxyl groups at positions 1 and 2 on a benzene ring; the hydrocarbon side chain of poison ivies (typically 15–carbon atoms long) attaches at position 3.15 The catechols and the aliphatic side chain contribute to the plant’s antigenic and dermatitis-inducing properties.16
The high lipophilicity of urushiol allows for rapid and unforgiving absorption into the skin, notwithstanding attempts to wash it off. Upon direct contact, catechols of urushiol penetrate the epidermis and become oxidized to quinone intermediates that bind to antigen-presenting cells in the epidermis and dermis. Epidermal Langerhans cells and dermal macrophages internalize and present the antigen to CD4+ T cells in nearby lymph nodes. This sequence results in production of inflammatory mediators, clonal expansion of T-effector and T-memory cells specific to the allergenic catechols, and an ensuing cytotoxic response against epidermal cells and the dermal vasculature. Keratinocytes and monocytes mediate the inflammatory response by releasing other cytokines.4,17
Sensitization to urushiol generally occurs at 8 to 14 years of age; therefore, infants have lower susceptibility to dermatitis upon contact with T rydbergii.18 Most animals do not experience sensitization upon contact; in fact, birds and forest animals consume the urushiol-rich fruit of T rydbergii without harm.3
Prevention and Treatment
Toxicodendron dermatitis typically lasts 1 to 3 weeks but can remain for as long as 6 weeks without treatment.19 Recognition and physical avoidance of the plant provides the most promising preventive strategy. Immediate rinsing with soap and water can prevent TCD by breaking down urushiol and its allergenic components; however, this is an option for only a short time, as the skin absorbs 50% of urushiol within 10 minutes after contact.20 Nevertheless, patients must seize the earliest opportunity to wash off the affected area and remove any residual urushiol. Patients must be cautious when removing and washing clothing to prevent further contact.
Most health care providers treat TCD with a corticosteroid to reduce inflammation and intense pruritus. A high-potency topical corticosteroid (eg, clobetasol) may prove effective in providing early therapeutic relief in mild disease.21 A short course of a systemic steroid quickly and effectively quenches intense itching and should not be limited to what the clinician considers severe disease. Do not underestimate the patient’s symptoms with this eruption.
Prednisone dosing begins at 1 mg/kg daily and is then tapered slowly over 2 weeks (no shorter a time) for an optimal treatment course of 15 days.22 Prescribing an inadequate dosage and course of a corticosteroid leaves the patient susceptible to rebound dermatitis—and loss of trust in their provider.
Intramuscular injection of the long-acting corticosteroid triamcinolone acetonide with rapid-onset betamethasone provides rapid relief and fewer adverse effects than an oral corticosteroid.22 Despite the long-standing use of sedating oral antihistamines by clinicians, these drugs provide no benefit for pruritus or sleep because the histamine does not cause the itching of TCD, and antihistamines disrupt normal sleep architecture.23-25
Patients can consider several over-the-counter products that have varying degrees of efficacy.4,26 The few products for which prospective studies support their use include Tecnu (Tec Laboraties Inc), Zanfel (RhusTox), and the well-known soaps Dial (Henkel Corporation) and Goop (Critzas Industries, Inc).27,28
Aside from treating the direct effects of TCD, clinicians also must take note of any look for signs of secondary infection and occasionally should consider supplementing treatment with an antibiotic.
- Lofgran T, Mahabal GD. Toxicodendron toxicity. StatPearls [Internet]. Updated May 16, 2023. Accessed December 23, 2023. https://www.ncbi.nlm.nih.gov/books/NBK557866/
- The Lewin Group. The Burden of Skin Diseases 2005. Society for Investigative Dermatology and American Academy of Dermatology Association; 2005:37-40. Accessed December 26, 2023. https://www.lewin.com/content/dam/Lewin/Resources/Site_Sections/Publications/april2005skindisease.pdf
- Monroe J. Toxicodendron contact dermatitis: a case report and brief review. J Clin Aesthet Dermatol. 2020;13(9 Suppl 1):S29-S34.
- Gladman AC. Toxicodendron dermatitis: poison ivy, oak, and sumac. Wilderness Environ Med. 2006;17:120-128. doi:10.1580/pr31-05.1
- Fretwell S. Poison ivy cases on the rise. The State. Updated May 15,2017. Accessed December 26, 2023. https://www.thestate.com/news/local/article150403932.html
- Mohan JE, Ziska LH, Schlesinger WH, et al. Biomass and toxicity responses of poison ivy (Toxicodendron radicans) to elevated atmospheric CO2. Proc Natl Acad Sci U S A. 2006;103:9086-9089. doi:10.1073/pnas.0602392103
- Williams JV, Light J, Marks JG Jr. Individual variations in allergic contact dermatitis from urushiol. Arch Dermatol. 1999;135:1002-1003. doi:10.1001/archderm.135.8.1002
- Kurlan JG, Lucky AW. Black spot poison ivy: a report of 5 cases and a review of the literature. J Am Acad Dermatol. 2001;45:246-249. doi:10.1067/mjd.2001.114295
- Fisher AA. Poison ivy/oak/sumac. part II: specific features. Cutis. 1996;58:22-24.
- Brook I, Frazier EH, Yeager JK. Microbiology of infected poison ivy dermatitis. Br J Dermatol. 2000;142:943-946. doi:10.1046/j.1365-2133.2000.03475.x
- Gillis WT. The systematics and ecology of poison-ivy and the poison-oaks (Toxicodendron, Anacardiaceae). Rhodora. 1971;73:370-443.
- Reveal JL. Typification of six Philip Miller names of temperate North American Toxicodendron (Anacardiaceae) with proposals (999-1000) to reject T. crenatum and T. volubile. TAXON. 1991;40:333-335. doi:10.2307/1222994
- Guin JD, Gillis WT, Beaman JH. Recognizing the Toxicodendrons (poison ivy, poison oak, and poison sumac). J Am Acad Dermatol. 1981;4:99-114. doi:10.1016/s0190-9622(81)70014-8
- Lee NP, Arriola ER. Poison ivy, oak, and sumac dermatitis. West J Med. 1999;171:354-355.
- Marks JG Jr, Anderson BE, DeLeo VA, eds. Contact and Occupational Dermatology. Jaypee Brothers Medical Publishers Ltd; 2016.
- Dawson CR. The chemistry of poison ivy. Trans N Y Acad Sci. 1956;18:427-443. doi:10.1111/j.2164-0947.1956.tb00465.x
- Kalish RS. Recent developments in the pathogenesis of allergic contact dermatitis. Arch Dermatol. 1991;127:1558-1563.
- Fisher AA, Mitchell J. Toxicodendron plants and spices. In: Rietschel RL, Fowler JF Jr. Fisher’s Contact Dermatitis. 4th ed. Williams & Wilkins; 1995:461-523.
- Labib A, Yosipovitch G. Itchy Toxicodendron plant dermatitis. Allergies. 2022;2:16-22. doi:10.3390/allergies2010002
- Fisher AA. Poison ivy/oak dermatitis part I: prevention—soap and water, topical barriers, hyposensitization. Cutis. 1996;57:384-386.
- Kim Y, Flamm A, ElSohly MA, et al. Poison ivy, oak, and sumac dermatitis: what is known and what is new? 2019;30:183-190. doi:10.1097/DER.0000000000000472
- Prok L, McGovern T. Poison ivy (Toxicodendron) dermatitis. UpToDate. Updated October 16, 2023. Accessed December 26, 2023. https://www.uptodate.com/contents/poison-ivy-toxicodendron-dermatitis
- Klein PA, Clark RA. An evidence-based review of the efficacy of antihistamines in relieving pruritus in atopic dermatitis. Arch Dermatol. 1999;135:1522-1525. doi:10.1001/archderm.135.12.1522
- He A, Feldman SR, Fleischer AB Jr. An assessment of the use of antihistamines in the management of atopic dermatitis. J Am Acad Dermatol. 2018;79:92-96. doi:10.1016/j.jaad.2017.12.077
- van Zuuren EJ, Apfelbacher CJ, Fedorowicz Z, et al. No high level evidence to support the use of oral H1 antihistamines as monotherapy for eczema: a summary of a Cochrane systematic review. Syst Rev. 2014;3:25. doi:10.1186/2046-4053-3-25
- Neill BC, Neill JA, Brauker J, et al. Postexposure prevention of Toxicodendron dermatitis by early forceful unidirectional washing with liquid dishwashing soap. J Am Acad Dermatol. 2019;81:E25. doi:10.1016/j.jaad.2017.12.081
- Stibich AS, Yagan M, Sharma V, et al. Cost-effective post-exposure prevention of poison ivy dermatitis. Int J Dermatol. 2000;39:515-518. doi:10.1046/j.1365-4362.2000.00003.x
- Davila A, Laurora M, Fulton J, et al. A new topical agent, Zanfel, ameliorates urushiol-induced Toxicodendron allergic contact dermatitis [abstract]. Ann Emerg Med. 2003;42:S98.
Clinical Importance
Western poison ivy (Toxicodendron rydbergii) is responsible for many of the cases of Toxicodendron contact dermatitis (TCD) reported in the western and northern United States. Toxicodendron plants cause more cases of allergic contact dermatitis (ACD) in North America than any other allergen1; 9 million Americans present to physician offices and 1.6 million present to emergency departments annually for ACD, emphasizing the notable medical burden of this condition.2,3 Exposure to urushiol, a plant resin containing potent allergens, precipitates this form of ACD.
An estimated 50% to 75% of adults in the United States demonstrate clinical sensitivity and exhibit ACD following contact with T rydbergii.4 Campers, hikers, firefighters, and forest workers often risk increased exposure through physical contact or aerosolized allergens in smoke. According to the Centers for Disease Control and Prevention, the incidence of visits to US emergency departments for TCD nearly doubled from 2002 to 2012,5 which may be explained by atmospheric CO2 levels that both promote increased growth of Toxicodendron species and augment their toxicity.6
Cutaneous Manifestations
The clinical presentation of T rydbergii contact dermatitis is similar to other allergenic members of the Toxicodendron genus. Patients sensitive to urushiol typically develop a pruritic erythematous rash within 1 to 2 days of exposure (range, 5 hours to 15 days).7 Erythematous and edematous streaks initially manifest on the extremities and often progress to bullae and oozing papulovesicles. In early disease, patients also may display black lesions on or near the rash8 (so-called black-dot dermatitis) caused by oxidized urushiol deposited on the skin—an uncommon yet classic presentation of TCD. Generally, symptoms resolve without complications and with few sequalae, though hyperpigmentation or a secondary infection can develop on or near affected areas.9,10
Taxonomy
The Toxicodendron genus belongs to the Anacardiaceae family, which includes pistachios, mangos, and cashews, and causes more cases of ACD than every other plant combined.4 (Shelled pistachios and cashews do not possess cross-reacting allergens and should not worry consumers; mango skin does contain urushiol.)
Toxicodendron (formerly part of the Rhus genus) includes several species of poison oak, poison ivy, and poison sumac and can be found in shrubs (T rydbergii and Toxicodendron diversilobum), vines (Toxicodendron radicans and Toxicodendron pubescens), and trees (Toxicodendron vernix). In addition, Toxicodendron taxa can hybridize with other taxa in close geographic proximity to form morphologic intermediates. Some individual plants have features of multiple species.11
Etymology
The common name of T rydbergii—western poison ivy—misleads the public; the plant contains no poison that can cause death and does not grow as ivy by wrapping around trees, as T radicans and English ivy (Hedera helix) do. Its formal genus, Toxicodendron, means “poison tree” in Greek and was given its generic name by the English botanist Phillip Miller in 1768,12 which caused the renaming of Rhus rydbergii as T rydbergii. The species name honors Per Axel Rydberg, a 19th and 20th century Swedish-American botanist.
Distribution
Toxicodendron rydbergii grows in California and other states in the western half of the United States as well as the states bordering Canada and Mexico. In Canada, it reigns as the most dominant form of poison ivy.13 Hikers and campers find T rydbergii in a variety of areas, including roadsides, river bottoms, sandy shores, talus slopes, precipices, and floodplains.11 This taxon grows under a variety of conditions and in distinct regions, and it thrives in both full sun or shade.
Identifying Features
Toxicodendron rydbergii turns red earlier than most plants; early red summer leaves should serve as a warning sign to hikers from a distance (Figure 1). It displays trifoliate ovate leaves (ie, each leaf contains 3 leaflets) on a dwarf nonclimbing shrub (Figure 2). Although the plant shares common features with its cousin T radicans (eastern poison ivy), T rydbergii is easily distinguished by its thicker stems, absence of aerial rootlets (abundant in T radicans), and short (approximately 1 meter) height.4

Curly hairs occupy the underside of T rydbergii leaflets and along the midrib; leaflet margins appear lobed or rounded. Lenticels appear as small holes in the bark that turn gray in the cold and become brighter come spring.13

The plant bears glabrous long petioles (leaf stems) and densely grouped clusters of yellow flowers. In autumn, the globose fruit—formed in clusters between each twig and leaf petiole (known as an axillary position)—change from yellow-green to tan (Figure 3). When urushiol exudes from damaged leaflets or other plant parts, it oxidizes on exposure to air and creates hardened black deposits on the plant. Even when grown in garden pots, T rydbergii maintains its distinguishing features.11

Dermatitis-Inducing Plant Parts
All parts of T rydbergii including leaves, stems, roots, and fruit contain the allergenic sap throughout the year.14 A person must damage or bruise the plant for urushiol to be released and produce its allergenic effects; softly brushing against undamaged plants typically does not induce dermatitis.4
Pathophysiology of Urushiol
Urushiol, a pale yellow, oily mixture of organic compounds conserved throughout all Toxicodendron species, contains highly allergenic alkyl catechols. These catechols possess hydroxyl groups at positions 1 and 2 on a benzene ring; the hydrocarbon side chain of poison ivies (typically 15–carbon atoms long) attaches at position 3.15 The catechols and the aliphatic side chain contribute to the plant’s antigenic and dermatitis-inducing properties.16
The high lipophilicity of urushiol allows for rapid and unforgiving absorption into the skin, notwithstanding attempts to wash it off. Upon direct contact, catechols of urushiol penetrate the epidermis and become oxidized to quinone intermediates that bind to antigen-presenting cells in the epidermis and dermis. Epidermal Langerhans cells and dermal macrophages internalize and present the antigen to CD4+ T cells in nearby lymph nodes. This sequence results in production of inflammatory mediators, clonal expansion of T-effector and T-memory cells specific to the allergenic catechols, and an ensuing cytotoxic response against epidermal cells and the dermal vasculature. Keratinocytes and monocytes mediate the inflammatory response by releasing other cytokines.4,17
Sensitization to urushiol generally occurs at 8 to 14 years of age; therefore, infants have lower susceptibility to dermatitis upon contact with T rydbergii.18 Most animals do not experience sensitization upon contact; in fact, birds and forest animals consume the urushiol-rich fruit of T rydbergii without harm.3
Prevention and Treatment
Toxicodendron dermatitis typically lasts 1 to 3 weeks but can remain for as long as 6 weeks without treatment.19 Recognition and physical avoidance of the plant provides the most promising preventive strategy. Immediate rinsing with soap and water can prevent TCD by breaking down urushiol and its allergenic components; however, this is an option for only a short time, as the skin absorbs 50% of urushiol within 10 minutes after contact.20 Nevertheless, patients must seize the earliest opportunity to wash off the affected area and remove any residual urushiol. Patients must be cautious when removing and washing clothing to prevent further contact.
Most health care providers treat TCD with a corticosteroid to reduce inflammation and intense pruritus. A high-potency topical corticosteroid (eg, clobetasol) may prove effective in providing early therapeutic relief in mild disease.21 A short course of a systemic steroid quickly and effectively quenches intense itching and should not be limited to what the clinician considers severe disease. Do not underestimate the patient’s symptoms with this eruption.
Prednisone dosing begins at 1 mg/kg daily and is then tapered slowly over 2 weeks (no shorter a time) for an optimal treatment course of 15 days.22 Prescribing an inadequate dosage and course of a corticosteroid leaves the patient susceptible to rebound dermatitis—and loss of trust in their provider.
Intramuscular injection of the long-acting corticosteroid triamcinolone acetonide with rapid-onset betamethasone provides rapid relief and fewer adverse effects than an oral corticosteroid.22 Despite the long-standing use of sedating oral antihistamines by clinicians, these drugs provide no benefit for pruritus or sleep because the histamine does not cause the itching of TCD, and antihistamines disrupt normal sleep architecture.23-25
Patients can consider several over-the-counter products that have varying degrees of efficacy.4,26 The few products for which prospective studies support their use include Tecnu (Tec Laboraties Inc), Zanfel (RhusTox), and the well-known soaps Dial (Henkel Corporation) and Goop (Critzas Industries, Inc).27,28
Aside from treating the direct effects of TCD, clinicians also must take note of any look for signs of secondary infection and occasionally should consider supplementing treatment with an antibiotic.
Clinical Importance
Western poison ivy (Toxicodendron rydbergii) is responsible for many of the cases of Toxicodendron contact dermatitis (TCD) reported in the western and northern United States. Toxicodendron plants cause more cases of allergic contact dermatitis (ACD) in North America than any other allergen1; 9 million Americans present to physician offices and 1.6 million present to emergency departments annually for ACD, emphasizing the notable medical burden of this condition.2,3 Exposure to urushiol, a plant resin containing potent allergens, precipitates this form of ACD.
An estimated 50% to 75% of adults in the United States demonstrate clinical sensitivity and exhibit ACD following contact with T rydbergii.4 Campers, hikers, firefighters, and forest workers often risk increased exposure through physical contact or aerosolized allergens in smoke. According to the Centers for Disease Control and Prevention, the incidence of visits to US emergency departments for TCD nearly doubled from 2002 to 2012,5 which may be explained by atmospheric CO2 levels that both promote increased growth of Toxicodendron species and augment their toxicity.6
Cutaneous Manifestations
The clinical presentation of T rydbergii contact dermatitis is similar to other allergenic members of the Toxicodendron genus. Patients sensitive to urushiol typically develop a pruritic erythematous rash within 1 to 2 days of exposure (range, 5 hours to 15 days).7 Erythematous and edematous streaks initially manifest on the extremities and often progress to bullae and oozing papulovesicles. In early disease, patients also may display black lesions on or near the rash8 (so-called black-dot dermatitis) caused by oxidized urushiol deposited on the skin—an uncommon yet classic presentation of TCD. Generally, symptoms resolve without complications and with few sequalae, though hyperpigmentation or a secondary infection can develop on or near affected areas.9,10
Taxonomy
The Toxicodendron genus belongs to the Anacardiaceae family, which includes pistachios, mangos, and cashews, and causes more cases of ACD than every other plant combined.4 (Shelled pistachios and cashews do not possess cross-reacting allergens and should not worry consumers; mango skin does contain urushiol.)
Toxicodendron (formerly part of the Rhus genus) includes several species of poison oak, poison ivy, and poison sumac and can be found in shrubs (T rydbergii and Toxicodendron diversilobum), vines (Toxicodendron radicans and Toxicodendron pubescens), and trees (Toxicodendron vernix). In addition, Toxicodendron taxa can hybridize with other taxa in close geographic proximity to form morphologic intermediates. Some individual plants have features of multiple species.11
Etymology
The common name of T rydbergii—western poison ivy—misleads the public; the plant contains no poison that can cause death and does not grow as ivy by wrapping around trees, as T radicans and English ivy (Hedera helix) do. Its formal genus, Toxicodendron, means “poison tree” in Greek and was given its generic name by the English botanist Phillip Miller in 1768,12 which caused the renaming of Rhus rydbergii as T rydbergii. The species name honors Per Axel Rydberg, a 19th and 20th century Swedish-American botanist.
Distribution
Toxicodendron rydbergii grows in California and other states in the western half of the United States as well as the states bordering Canada and Mexico. In Canada, it reigns as the most dominant form of poison ivy.13 Hikers and campers find T rydbergii in a variety of areas, including roadsides, river bottoms, sandy shores, talus slopes, precipices, and floodplains.11 This taxon grows under a variety of conditions and in distinct regions, and it thrives in both full sun or shade.
Identifying Features
Toxicodendron rydbergii turns red earlier than most plants; early red summer leaves should serve as a warning sign to hikers from a distance (Figure 1). It displays trifoliate ovate leaves (ie, each leaf contains 3 leaflets) on a dwarf nonclimbing shrub (Figure 2). Although the plant shares common features with its cousin T radicans (eastern poison ivy), T rydbergii is easily distinguished by its thicker stems, absence of aerial rootlets (abundant in T radicans), and short (approximately 1 meter) height.4

Curly hairs occupy the underside of T rydbergii leaflets and along the midrib; leaflet margins appear lobed or rounded. Lenticels appear as small holes in the bark that turn gray in the cold and become brighter come spring.13

The plant bears glabrous long petioles (leaf stems) and densely grouped clusters of yellow flowers. In autumn, the globose fruit—formed in clusters between each twig and leaf petiole (known as an axillary position)—change from yellow-green to tan (Figure 3). When urushiol exudes from damaged leaflets or other plant parts, it oxidizes on exposure to air and creates hardened black deposits on the plant. Even when grown in garden pots, T rydbergii maintains its distinguishing features.11

Dermatitis-Inducing Plant Parts
All parts of T rydbergii including leaves, stems, roots, and fruit contain the allergenic sap throughout the year.14 A person must damage or bruise the plant for urushiol to be released and produce its allergenic effects; softly brushing against undamaged plants typically does not induce dermatitis.4
Pathophysiology of Urushiol
Urushiol, a pale yellow, oily mixture of organic compounds conserved throughout all Toxicodendron species, contains highly allergenic alkyl catechols. These catechols possess hydroxyl groups at positions 1 and 2 on a benzene ring; the hydrocarbon side chain of poison ivies (typically 15–carbon atoms long) attaches at position 3.15 The catechols and the aliphatic side chain contribute to the plant’s antigenic and dermatitis-inducing properties.16
The high lipophilicity of urushiol allows for rapid and unforgiving absorption into the skin, notwithstanding attempts to wash it off. Upon direct contact, catechols of urushiol penetrate the epidermis and become oxidized to quinone intermediates that bind to antigen-presenting cells in the epidermis and dermis. Epidermal Langerhans cells and dermal macrophages internalize and present the antigen to CD4+ T cells in nearby lymph nodes. This sequence results in production of inflammatory mediators, clonal expansion of T-effector and T-memory cells specific to the allergenic catechols, and an ensuing cytotoxic response against epidermal cells and the dermal vasculature. Keratinocytes and monocytes mediate the inflammatory response by releasing other cytokines.4,17
Sensitization to urushiol generally occurs at 8 to 14 years of age; therefore, infants have lower susceptibility to dermatitis upon contact with T rydbergii.18 Most animals do not experience sensitization upon contact; in fact, birds and forest animals consume the urushiol-rich fruit of T rydbergii without harm.3
Prevention and Treatment
Toxicodendron dermatitis typically lasts 1 to 3 weeks but can remain for as long as 6 weeks without treatment.19 Recognition and physical avoidance of the plant provides the most promising preventive strategy. Immediate rinsing with soap and water can prevent TCD by breaking down urushiol and its allergenic components; however, this is an option for only a short time, as the skin absorbs 50% of urushiol within 10 minutes after contact.20 Nevertheless, patients must seize the earliest opportunity to wash off the affected area and remove any residual urushiol. Patients must be cautious when removing and washing clothing to prevent further contact.
Most health care providers treat TCD with a corticosteroid to reduce inflammation and intense pruritus. A high-potency topical corticosteroid (eg, clobetasol) may prove effective in providing early therapeutic relief in mild disease.21 A short course of a systemic steroid quickly and effectively quenches intense itching and should not be limited to what the clinician considers severe disease. Do not underestimate the patient’s symptoms with this eruption.
Prednisone dosing begins at 1 mg/kg daily and is then tapered slowly over 2 weeks (no shorter a time) for an optimal treatment course of 15 days.22 Prescribing an inadequate dosage and course of a corticosteroid leaves the patient susceptible to rebound dermatitis—and loss of trust in their provider.
Intramuscular injection of the long-acting corticosteroid triamcinolone acetonide with rapid-onset betamethasone provides rapid relief and fewer adverse effects than an oral corticosteroid.22 Despite the long-standing use of sedating oral antihistamines by clinicians, these drugs provide no benefit for pruritus or sleep because the histamine does not cause the itching of TCD, and antihistamines disrupt normal sleep architecture.23-25
Patients can consider several over-the-counter products that have varying degrees of efficacy.4,26 The few products for which prospective studies support their use include Tecnu (Tec Laboraties Inc), Zanfel (RhusTox), and the well-known soaps Dial (Henkel Corporation) and Goop (Critzas Industries, Inc).27,28
Aside from treating the direct effects of TCD, clinicians also must take note of any look for signs of secondary infection and occasionally should consider supplementing treatment with an antibiotic.
- Lofgran T, Mahabal GD. Toxicodendron toxicity. StatPearls [Internet]. Updated May 16, 2023. Accessed December 23, 2023. https://www.ncbi.nlm.nih.gov/books/NBK557866/
- The Lewin Group. The Burden of Skin Diseases 2005. Society for Investigative Dermatology and American Academy of Dermatology Association; 2005:37-40. Accessed December 26, 2023. https://www.lewin.com/content/dam/Lewin/Resources/Site_Sections/Publications/april2005skindisease.pdf
- Monroe J. Toxicodendron contact dermatitis: a case report and brief review. J Clin Aesthet Dermatol. 2020;13(9 Suppl 1):S29-S34.
- Gladman AC. Toxicodendron dermatitis: poison ivy, oak, and sumac. Wilderness Environ Med. 2006;17:120-128. doi:10.1580/pr31-05.1
- Fretwell S. Poison ivy cases on the rise. The State. Updated May 15,2017. Accessed December 26, 2023. https://www.thestate.com/news/local/article150403932.html
- Mohan JE, Ziska LH, Schlesinger WH, et al. Biomass and toxicity responses of poison ivy (Toxicodendron radicans) to elevated atmospheric CO2. Proc Natl Acad Sci U S A. 2006;103:9086-9089. doi:10.1073/pnas.0602392103
- Williams JV, Light J, Marks JG Jr. Individual variations in allergic contact dermatitis from urushiol. Arch Dermatol. 1999;135:1002-1003. doi:10.1001/archderm.135.8.1002
- Kurlan JG, Lucky AW. Black spot poison ivy: a report of 5 cases and a review of the literature. J Am Acad Dermatol. 2001;45:246-249. doi:10.1067/mjd.2001.114295
- Fisher AA. Poison ivy/oak/sumac. part II: specific features. Cutis. 1996;58:22-24.
- Brook I, Frazier EH, Yeager JK. Microbiology of infected poison ivy dermatitis. Br J Dermatol. 2000;142:943-946. doi:10.1046/j.1365-2133.2000.03475.x
- Gillis WT. The systematics and ecology of poison-ivy and the poison-oaks (Toxicodendron, Anacardiaceae). Rhodora. 1971;73:370-443.
- Reveal JL. Typification of six Philip Miller names of temperate North American Toxicodendron (Anacardiaceae) with proposals (999-1000) to reject T. crenatum and T. volubile. TAXON. 1991;40:333-335. doi:10.2307/1222994
- Guin JD, Gillis WT, Beaman JH. Recognizing the Toxicodendrons (poison ivy, poison oak, and poison sumac). J Am Acad Dermatol. 1981;4:99-114. doi:10.1016/s0190-9622(81)70014-8
- Lee NP, Arriola ER. Poison ivy, oak, and sumac dermatitis. West J Med. 1999;171:354-355.
- Marks JG Jr, Anderson BE, DeLeo VA, eds. Contact and Occupational Dermatology. Jaypee Brothers Medical Publishers Ltd; 2016.
- Dawson CR. The chemistry of poison ivy. Trans N Y Acad Sci. 1956;18:427-443. doi:10.1111/j.2164-0947.1956.tb00465.x
- Kalish RS. Recent developments in the pathogenesis of allergic contact dermatitis. Arch Dermatol. 1991;127:1558-1563.
- Fisher AA, Mitchell J. Toxicodendron plants and spices. In: Rietschel RL, Fowler JF Jr. Fisher’s Contact Dermatitis. 4th ed. Williams & Wilkins; 1995:461-523.
- Labib A, Yosipovitch G. Itchy Toxicodendron plant dermatitis. Allergies. 2022;2:16-22. doi:10.3390/allergies2010002
- Fisher AA. Poison ivy/oak dermatitis part I: prevention—soap and water, topical barriers, hyposensitization. Cutis. 1996;57:384-386.
- Kim Y, Flamm A, ElSohly MA, et al. Poison ivy, oak, and sumac dermatitis: what is known and what is new? 2019;30:183-190. doi:10.1097/DER.0000000000000472
- Prok L, McGovern T. Poison ivy (Toxicodendron) dermatitis. UpToDate. Updated October 16, 2023. Accessed December 26, 2023. https://www.uptodate.com/contents/poison-ivy-toxicodendron-dermatitis
- Klein PA, Clark RA. An evidence-based review of the efficacy of antihistamines in relieving pruritus in atopic dermatitis. Arch Dermatol. 1999;135:1522-1525. doi:10.1001/archderm.135.12.1522
- He A, Feldman SR, Fleischer AB Jr. An assessment of the use of antihistamines in the management of atopic dermatitis. J Am Acad Dermatol. 2018;79:92-96. doi:10.1016/j.jaad.2017.12.077
- van Zuuren EJ, Apfelbacher CJ, Fedorowicz Z, et al. No high level evidence to support the use of oral H1 antihistamines as monotherapy for eczema: a summary of a Cochrane systematic review. Syst Rev. 2014;3:25. doi:10.1186/2046-4053-3-25
- Neill BC, Neill JA, Brauker J, et al. Postexposure prevention of Toxicodendron dermatitis by early forceful unidirectional washing with liquid dishwashing soap. J Am Acad Dermatol. 2019;81:E25. doi:10.1016/j.jaad.2017.12.081
- Stibich AS, Yagan M, Sharma V, et al. Cost-effective post-exposure prevention of poison ivy dermatitis. Int J Dermatol. 2000;39:515-518. doi:10.1046/j.1365-4362.2000.00003.x
- Davila A, Laurora M, Fulton J, et al. A new topical agent, Zanfel, ameliorates urushiol-induced Toxicodendron allergic contact dermatitis [abstract]. Ann Emerg Med. 2003;42:S98.
- Lofgran T, Mahabal GD. Toxicodendron toxicity. StatPearls [Internet]. Updated May 16, 2023. Accessed December 23, 2023. https://www.ncbi.nlm.nih.gov/books/NBK557866/
- The Lewin Group. The Burden of Skin Diseases 2005. Society for Investigative Dermatology and American Academy of Dermatology Association; 2005:37-40. Accessed December 26, 2023. https://www.lewin.com/content/dam/Lewin/Resources/Site_Sections/Publications/april2005skindisease.pdf
- Monroe J. Toxicodendron contact dermatitis: a case report and brief review. J Clin Aesthet Dermatol. 2020;13(9 Suppl 1):S29-S34.
- Gladman AC. Toxicodendron dermatitis: poison ivy, oak, and sumac. Wilderness Environ Med. 2006;17:120-128. doi:10.1580/pr31-05.1
- Fretwell S. Poison ivy cases on the rise. The State. Updated May 15,2017. Accessed December 26, 2023. https://www.thestate.com/news/local/article150403932.html
- Mohan JE, Ziska LH, Schlesinger WH, et al. Biomass and toxicity responses of poison ivy (Toxicodendron radicans) to elevated atmospheric CO2. Proc Natl Acad Sci U S A. 2006;103:9086-9089. doi:10.1073/pnas.0602392103
- Williams JV, Light J, Marks JG Jr. Individual variations in allergic contact dermatitis from urushiol. Arch Dermatol. 1999;135:1002-1003. doi:10.1001/archderm.135.8.1002
- Kurlan JG, Lucky AW. Black spot poison ivy: a report of 5 cases and a review of the literature. J Am Acad Dermatol. 2001;45:246-249. doi:10.1067/mjd.2001.114295
- Fisher AA. Poison ivy/oak/sumac. part II: specific features. Cutis. 1996;58:22-24.
- Brook I, Frazier EH, Yeager JK. Microbiology of infected poison ivy dermatitis. Br J Dermatol. 2000;142:943-946. doi:10.1046/j.1365-2133.2000.03475.x
- Gillis WT. The systematics and ecology of poison-ivy and the poison-oaks (Toxicodendron, Anacardiaceae). Rhodora. 1971;73:370-443.
- Reveal JL. Typification of six Philip Miller names of temperate North American Toxicodendron (Anacardiaceae) with proposals (999-1000) to reject T. crenatum and T. volubile. TAXON. 1991;40:333-335. doi:10.2307/1222994
- Guin JD, Gillis WT, Beaman JH. Recognizing the Toxicodendrons (poison ivy, poison oak, and poison sumac). J Am Acad Dermatol. 1981;4:99-114. doi:10.1016/s0190-9622(81)70014-8
- Lee NP, Arriola ER. Poison ivy, oak, and sumac dermatitis. West J Med. 1999;171:354-355.
- Marks JG Jr, Anderson BE, DeLeo VA, eds. Contact and Occupational Dermatology. Jaypee Brothers Medical Publishers Ltd; 2016.
- Dawson CR. The chemistry of poison ivy. Trans N Y Acad Sci. 1956;18:427-443. doi:10.1111/j.2164-0947.1956.tb00465.x
- Kalish RS. Recent developments in the pathogenesis of allergic contact dermatitis. Arch Dermatol. 1991;127:1558-1563.
- Fisher AA, Mitchell J. Toxicodendron plants and spices. In: Rietschel RL, Fowler JF Jr. Fisher’s Contact Dermatitis. 4th ed. Williams & Wilkins; 1995:461-523.
- Labib A, Yosipovitch G. Itchy Toxicodendron plant dermatitis. Allergies. 2022;2:16-22. doi:10.3390/allergies2010002
- Fisher AA. Poison ivy/oak dermatitis part I: prevention—soap and water, topical barriers, hyposensitization. Cutis. 1996;57:384-386.
- Kim Y, Flamm A, ElSohly MA, et al. Poison ivy, oak, and sumac dermatitis: what is known and what is new? 2019;30:183-190. doi:10.1097/DER.0000000000000472
- Prok L, McGovern T. Poison ivy (Toxicodendron) dermatitis. UpToDate. Updated October 16, 2023. Accessed December 26, 2023. https://www.uptodate.com/contents/poison-ivy-toxicodendron-dermatitis
- Klein PA, Clark RA. An evidence-based review of the efficacy of antihistamines in relieving pruritus in atopic dermatitis. Arch Dermatol. 1999;135:1522-1525. doi:10.1001/archderm.135.12.1522
- He A, Feldman SR, Fleischer AB Jr. An assessment of the use of antihistamines in the management of atopic dermatitis. J Am Acad Dermatol. 2018;79:92-96. doi:10.1016/j.jaad.2017.12.077
- van Zuuren EJ, Apfelbacher CJ, Fedorowicz Z, et al. No high level evidence to support the use of oral H1 antihistamines as monotherapy for eczema: a summary of a Cochrane systematic review. Syst Rev. 2014;3:25. doi:10.1186/2046-4053-3-25
- Neill BC, Neill JA, Brauker J, et al. Postexposure prevention of Toxicodendron dermatitis by early forceful unidirectional washing with liquid dishwashing soap. J Am Acad Dermatol. 2019;81:E25. doi:10.1016/j.jaad.2017.12.081
- Stibich AS, Yagan M, Sharma V, et al. Cost-effective post-exposure prevention of poison ivy dermatitis. Int J Dermatol. 2000;39:515-518. doi:10.1046/j.1365-4362.2000.00003.x
- Davila A, Laurora M, Fulton J, et al. A new topical agent, Zanfel, ameliorates urushiol-induced Toxicodendron allergic contact dermatitis [abstract]. Ann Emerg Med. 2003;42:S98.
PRACTICE POINTS
- Western poison ivy (Toxicodendron rydbergii) accounts for many of the cases of Toxicodendron contact dermatitis (TCD) in the western and northern United States. Individuals in these regions should be educated on how to identify T rydbergii to avoid TCD.
- Dermatologists should include TCD in the differential diagnosis when a patient presents with an erythematous pruritic rash in a linear pattern with sharp borders.
- Most patients who experience intense itching and pain from TCD benefit greatly from prompt treatment with an oral or intramuscular corticosteroid. Topical steroids rarely provide relief; oral antihistamines provide no benefit.