User login
Paradoxical Reaction to TNF-α Inhibitor Therapy in a Patient With Hidradenitis Suppurativa
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the pilosebaceous unit that occurs in concert with elevations of various cytokines, including tumor necrosis factor α (TNF-α), IL-1β, IL-10, and IL-17.1,2 Adalimumab is a TNF-α inhibitor approved by the US Food and Drug Administration for the treatment of HS. Although TNF-α inhibitors are effective for many immune-mediated inflammatory disorders, paradoxical drug reactions have been reported following treatment with these agents.3-6 True paradoxical drug reactions likely are immune mediated and directly lead to new onset of a pathologic condition that would otherwise respond to that drug. For example, there are reports of rheumatoid arthritis patients who were treated with a TNF-α inhibitor and developed psoriatic skin lesions.3,6 Paradoxical drug reactions also have been reported with acute-onset inflammatory bowel disease and HS or less commonly pyoderma gangrenosum (PG), uveitis, granulomatous reactions, and vasculitis.4,5 We present the case of a patient with HS who was treated with a TNF-α inhibitor and developed 2 distinct paradoxical drug reactions. We also provide an overview of paradoxical drug reactions associated with TNF-α inhibitors.
A 38-year-old woman developed a painful “boil” on the right leg that was previously treated in the emergency department with incision and drainage as well as oral clindamycin for 7 days, but the lesion spread and continued to worsen. She had a history of HS in the axillae and groin region that had been present since 12 years of age. The condition was poorly controlled despite multiple courses of oral antibiotics and surgical resections. An oral contraceptive also was attempted, but the patient discontinued treatment when liver enzyme levels became elevated. The patient had no other notable medical history, including skin disease. There was a family history of HS in her father and a sibling. Seeking more effective treatment, the patient was offered adalimumab approximately 4 months prior to clinical presentation and agreed to start a course of the drug. She received a loading dose of 160 mg on day 1 and 80 mg on day 15 followed by a maintenance dosage of 40 mg weekly. She experienced improvement in HS symptoms after 3 months on adalimumab; however, she developed scaly pruritic patches on the scalp, arms, and legs that were consistent with psoriasis. Because of the absence of a personal or family history of psoriasis, the patient was informed of the probability of paradoxical psoriasis resulting from adalimumab. She elected to continue adalimumab because of the improvement in HS symptoms, and the psoriatic lesions were mild and adequately controlled with a topical steroid.
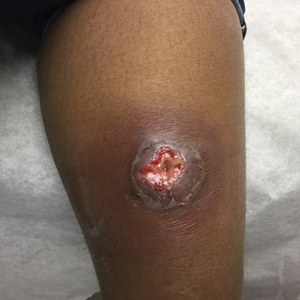
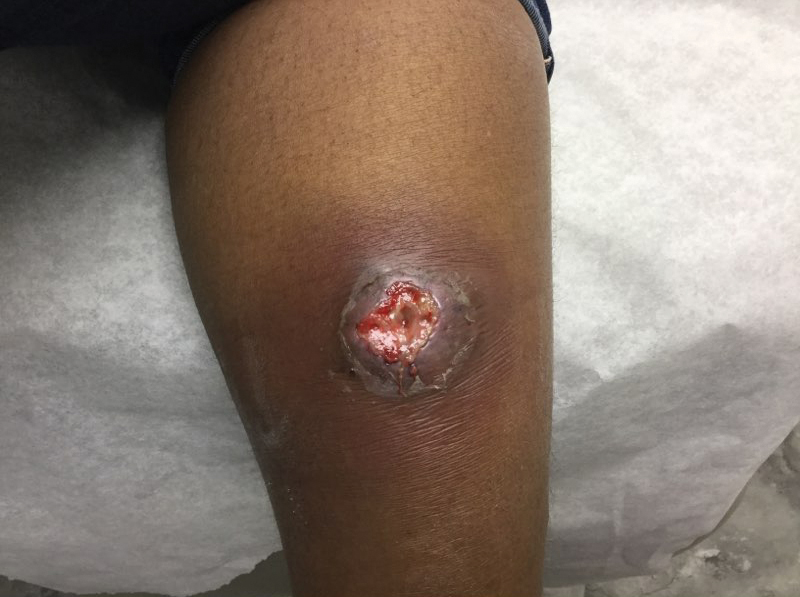
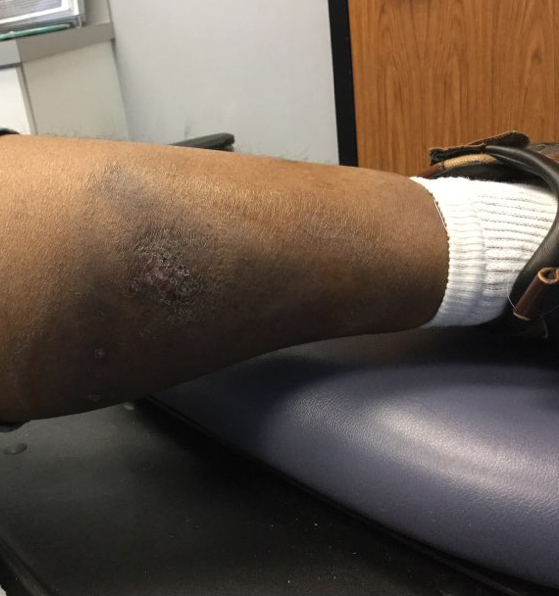
At the current presentation 1 month later, physical examination revealed a large indurated and ulcerated area with jagged edges at the incision and drainage site (Figure 1). Pyoderma gangrenosum was clinically suspected; a biopsy was performed, and the patient was started on oral prednisone. At 2-week follow-up, the ulcer was found to be rapidly resolving with prednisone and healing with cribriform scarring (Figure 2). Histopathology revealed an undermining neutrophilic inflammatory process that was consistent with PG. A diagnosis of PG was made based on previously published criteria7 and the following major/minor criteria in the patient: pathology; absence of infection on histologic analysis; history of pathergy related to worsening ulceration at the site of incision and drainage of the initial boil; clinical findings of an ulcer with peripheral violaceous erythema; undermined borders and tenderness at the site; and rapid resolution of the ulcer with prednisone.
Cessation of adalimumab gradually led to clearance of both psoriasiform lesions and PG; however, HS lesions persisted.
Although the precise pathogenesis of HS is unclear, both genetic abnormalities of the pilosebaceous unit and a dysregulated immune reaction appear to lead to the clinical characteristics of chronic inflammation and scarring seen in HS. A key effector appears to be helper T-cell (TH17) lymphocyte activation, with increased secretion of TNF-α, IL-1β, and IL-17.1,2 In turn, IL-17 induces higher expression of TNF-α, leading to a persistent cycle of inflammation. Peripheral recruitment of IL-17–producing neutrophils also may contribute to chronic inflammation.8
Adalimumab is the only US Food and Drug Administration–approved biologic indicated for the treatment of HS. Our patient initially responded to adalimumab with improvement of HS; however, treatment had to be discontinued because of the unusual occurrence of 2 distinct paradoxical reactions in a short span of time. Psoriasis and PG are both considered true paradoxical reactions because primary occurrences of both diseases usually are responsive to treatment with adalimumab.
Tumor necrosis factor α inhibitor–induced psoriasis arises de novo and is estimated to occur in approximately 5% of patients with rheumatoid arthritis.3,6 Palmoplantar pustular psoriasiform reactions are the most common form of paradoxical psoriasis. Topical medications can be used to treat skin lesions, but systemic treatment is required in many cases. Switching to an alternate class of a biologic, such as an IL-17, IL-12/23, or IL-23 inhibitor, can improve the skin reaction; however, such treatment is inconsistently successful, and paradoxical drug reactions also have been seen with these other classes of biologics.4,9
Recent studies support distinct immune causes for classical and paradoxical psoriasis. In classical psoriasis, plasmacytoid dendritic cells (pDCs) produce IFN-α, which stimulates conventional dendritic cells to produce TNF-α. However, TNF-α matures both pDCs and conventional dendritic cells; upon maturation, both types of dendritic cells lose the ability to produce IFN-α, thus allowing TNF-α to become dominant.10 The blockade of TNF-α prevents pDC maturation, leading to uninhibited IFN-α, which appears to drive inflammation in paradoxical psoriasis. In classical psoriasis, oligoclonal dermal CD4+ T cells and epidermal CD8+ T cells remain, even in resolved skin lesions, and can cause disease recurrence through reactivation of skin-resident memory T cells.11 No relapse of paradoxical psoriasis occurs with discontinuation of anti-TNF-α therapy, which supports the notion of an absence of memory T cells.
The incidence of paradoxical psoriasis in patients receiving a TNF-α inhibitor for HS is unclear.12 There are case series in which patients who had concurrent psoriasis and HS were successfully treated with a TNF-α inhibitor.13 A recently recognized condition—PASH syndrome—encompasses the clinical triad of PG, acne, and HS.10
Our patient had no history of acne or PG, only a long-standing history of HS. New-onset PG occurred only after a TNF-α inhibitor was initiated. Notably, PASH syndrome has been successfully treated with TNF-α inhibitors, highlighting the shared inflammatory etiology of HS and PG.14 In patients with concurrent PG and HS, TNF-α inhibitors were more effective for treating PG than for HS.
Pyoderma gangrenosum is an inflammatory disorder that often occurs concomitantly with other conditions, such as inflammatory bowel disease. The exact underlying cause of PG is unclear, but there appears to be both neutrophil and T-cell dysfunction in PG, with excess inflammatory cytokine production (eg, IL-1β, TNF-α, IL-17).15
The mainstay of treatment of PG is systemic corticosteroids and immunosuppressives, such as cyclosporine. Tumor necrosis factor α inhibitors as well as other interleukin inhibitors are increasingly utilized as potential therapeutic alternatives for PG.16,17
Unlike paradoxical psoriasis, the underlying cause of paradoxical PG is unclear.18,19 A similar mechanism may be postulated whereby inhibition of TNF-α leads to excessive activation of alternative inflammatory pathways that result in paradoxical PG. In one study, the prevalence of PG among 68,232 patients with HS was 0.18% compared with 0.01% among those without HS; therefore, patients with HS appear to be more predisposed to PG.20
This case illustrates the complex, often conflicting effects of cytokine inhibition in the paradoxical elicitation of alternative inflammatory disorders as an unintended consequence of the initial cytokine blockade. It is likely that genetic predisposition allows for paradoxical reactions in some patients when there is predominant inhibition of one cytokine in the inflammatory pathway. In rare cases, multiple paradoxical reactions are possible.
1. Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2018;9:2965. doi:10.3389/fimmu.2018.02965
2. Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation and pathogenesis. J Am Acad Dermatol. 2020; 82:1045-1058. doi:10.1016/j.jaad.2019.08.090
3. Brown G, Wang E, Leon A, et al. Tumor necrosis factor-α inhibitor-induced psoriasis: systematic review of clinical features, histopathological findings, and management experience. J Am Acad Dermatol. 2017;76:334-341. doi:10.1016/j.jaad.2016.08.012
4. Puig L. Paradoxical reactions: anti-tumor necrosis factor alpha agents, ustekinumab, secukinumab, ixekizumab and others. Curr Prob Dermatol. 2018;53:49-63. doi:10.1159/000479475
5. Faivre C, Villani AP, Aubin F, et al; . Hidradenitis suppurativa (HS): an unrecognized paradoxical effect of biologic agents (BA) used in chronic inflammatory diseases. J Am Acad Dermatol. 2016;74:1153-1159. doi:10.1016/j.jaad.2016.01.018
6. Ko JM, Gottlieb AB, Kerbleski JF. Induction and exacerbation of psoriasis with TNF-blockade therapy: a review and analysis of 127 cases. J Dermatolog Treat. 2009;20:100-108. doi:10.1080/09546630802441234
7. Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a delphi consensus of international experts. JAMA Dermatol. 2018;154:461-466. doi:10.1001/jamadermatol.2017.5980
8. Lima AL, Karl I, Giner T, et al. Keratinocytes and neutrophils are important sources of proinflammatory molecules in hidradenitis suppurativa. Br J Dermatol. 2016;174:514-521. doi:10.1111/bjd.14214
9. Li SJ, Perez-Chada LM, Merola JF. TNF inhibitor-induced psoriasis: proposed algorithm for treatment and management. J Psoriasis Psoriatic Arthritis. 2019;4:70-80. doi:10.1177/2475530318810851
10. Conrad C, Di Domizio J, Mylonas A, et al. TNF blockade induces a dysregulated type I interferon response without autoimmunity in paradoxical psoriasis. Nat Commun. 2018;9:25. doi:10.1038/s41467-017-02466-4
11. Matos TR, O’Malley JT, Lowry EL, et al. Clinically resolved psoriatic lesions contain psoriasis-specific IL-17-producing αβ T cell clones. J Clin Invest. 2017;127:4031-4041. doi:10.1172/JCI93396
12. Faivre C, Villani AP, Aubin F, et al. Hidradenitis suppurativa (HS): an unrecognized paradoxical effect of biologic agents (BA) used in chronic inflammatory diseases. J Am Acad Dermatol. 2016;74:1153-1159. doi:10.1016/j.jaad.2016.01.018
13. Marzano AV, Damiani G, Ceccherini I, et al. Autoinflammation in pyoderma gangrenosum and its syndromic form (pyoderma gangrenosum, acne and suppurative hidradenitis). Br J Dermatol. 2017;176:1588-1598. doi:10.1111/bjd.15226
14. Cugno M, Borghi A, Marzano AV. PAPA, PASH, PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562. doi:10.1007/s40257-017-0265-1
15. Wang EA, Steel A, Luxardi G, et al. Classic ulcerative pyoderma gangrenosum is a T cell-mediated disease targeting follicular adnexal structures: a hypothesis based on molecular and clinicopathologic studies. Front Immunol. 2018;8:1980. doi:10.3389/fimmu.2017.01980
16. Patel F, Fitzmaurice S, Duong C, et al. Effective strategies for the management of pyoderma gangrenosum: a comprehensive review. Acta Derm Venereol. 2015;95:525-531. doi:10.2340/00015555-2008
17. Partridge ACR, Bai JW, Rosen CF, et al. Effectiveness of systemic treatments for pyoderma gangrenosum: a systematic review of observational studies and clinical trials. Br J Dermatol. 2018;179:290-295. doi:10.1111/bjd.16485
18. Benzaquen M, Monnier J, Beaussault Y, et al. Pyoderma gangrenosum arising during treatment of psoriasis with adalimumab: effectiveness of ustekinumab. Australas J Dermatol. 2017;58:e270-e271. doi:10.1111/ajd.12545
19. Fujimoto N, Yamasaki Y, Watanabe RJ. Paradoxical uveitis and pyoderma gangrenosum in a patient with psoriatic arthritis under infliximab treatment. J Dtsch Dermatol Ges. 2018;16:1139-1140. doi:10.1111/ddg.13632
20. Tannenbaum R, Strunk A, Garg A. Overall and subgroup prevalence of pyoderma gangrenosum among patients with hidradenitis suppurativa: a population-based analysis in the United States. J Am Acad Dermatol. 2019;80:1533-1537. doi:10.1016/j.jaad.2019.02.004
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the pilosebaceous unit that occurs in concert with elevations of various cytokines, including tumor necrosis factor α (TNF-α), IL-1β, IL-10, and IL-17.1,2 Adalimumab is a TNF-α inhibitor approved by the US Food and Drug Administration for the treatment of HS. Although TNF-α inhibitors are effective for many immune-mediated inflammatory disorders, paradoxical drug reactions have been reported following treatment with these agents.3-6 True paradoxical drug reactions likely are immune mediated and directly lead to new onset of a pathologic condition that would otherwise respond to that drug. For example, there are reports of rheumatoid arthritis patients who were treated with a TNF-α inhibitor and developed psoriatic skin lesions.3,6 Paradoxical drug reactions also have been reported with acute-onset inflammatory bowel disease and HS or less commonly pyoderma gangrenosum (PG), uveitis, granulomatous reactions, and vasculitis.4,5 We present the case of a patient with HS who was treated with a TNF-α inhibitor and developed 2 distinct paradoxical drug reactions. We also provide an overview of paradoxical drug reactions associated with TNF-α inhibitors.
A 38-year-old woman developed a painful “boil” on the right leg that was previously treated in the emergency department with incision and drainage as well as oral clindamycin for 7 days, but the lesion spread and continued to worsen. She had a history of HS in the axillae and groin region that had been present since 12 years of age. The condition was poorly controlled despite multiple courses of oral antibiotics and surgical resections. An oral contraceptive also was attempted, but the patient discontinued treatment when liver enzyme levels became elevated. The patient had no other notable medical history, including skin disease. There was a family history of HS in her father and a sibling. Seeking more effective treatment, the patient was offered adalimumab approximately 4 months prior to clinical presentation and agreed to start a course of the drug. She received a loading dose of 160 mg on day 1 and 80 mg on day 15 followed by a maintenance dosage of 40 mg weekly. She experienced improvement in HS symptoms after 3 months on adalimumab; however, she developed scaly pruritic patches on the scalp, arms, and legs that were consistent with psoriasis. Because of the absence of a personal or family history of psoriasis, the patient was informed of the probability of paradoxical psoriasis resulting from adalimumab. She elected to continue adalimumab because of the improvement in HS symptoms, and the psoriatic lesions were mild and adequately controlled with a topical steroid.
At the current presentation 1 month later, physical examination revealed a large indurated and ulcerated area with jagged edges at the incision and drainage site (Figure 1). Pyoderma gangrenosum was clinically suspected; a biopsy was performed, and the patient was started on oral prednisone. At 2-week follow-up, the ulcer was found to be rapidly resolving with prednisone and healing with cribriform scarring (Figure 2). Histopathology revealed an undermining neutrophilic inflammatory process that was consistent with PG. A diagnosis of PG was made based on previously published criteria7 and the following major/minor criteria in the patient: pathology; absence of infection on histologic analysis; history of pathergy related to worsening ulceration at the site of incision and drainage of the initial boil; clinical findings of an ulcer with peripheral violaceous erythema; undermined borders and tenderness at the site; and rapid resolution of the ulcer with prednisone.

Cessation of adalimumab gradually led to clearance of both psoriasiform lesions and PG; however, HS lesions persisted.

Although the precise pathogenesis of HS is unclear, both genetic abnormalities of the pilosebaceous unit and a dysregulated immune reaction appear to lead to the clinical characteristics of chronic inflammation and scarring seen in HS. A key effector appears to be helper T-cell (TH17) lymphocyte activation, with increased secretion of TNF-α, IL-1β, and IL-17.1,2 In turn, IL-17 induces higher expression of TNF-α, leading to a persistent cycle of inflammation. Peripheral recruitment of IL-17–producing neutrophils also may contribute to chronic inflammation.8
Adalimumab is the only US Food and Drug Administration–approved biologic indicated for the treatment of HS. Our patient initially responded to adalimumab with improvement of HS; however, treatment had to be discontinued because of the unusual occurrence of 2 distinct paradoxical reactions in a short span of time. Psoriasis and PG are both considered true paradoxical reactions because primary occurrences of both diseases usually are responsive to treatment with adalimumab.
Tumor necrosis factor α inhibitor–induced psoriasis arises de novo and is estimated to occur in approximately 5% of patients with rheumatoid arthritis.3,6 Palmoplantar pustular psoriasiform reactions are the most common form of paradoxical psoriasis. Topical medications can be used to treat skin lesions, but systemic treatment is required in many cases. Switching to an alternate class of a biologic, such as an IL-17, IL-12/23, or IL-23 inhibitor, can improve the skin reaction; however, such treatment is inconsistently successful, and paradoxical drug reactions also have been seen with these other classes of biologics.4,9
Recent studies support distinct immune causes for classical and paradoxical psoriasis. In classical psoriasis, plasmacytoid dendritic cells (pDCs) produce IFN-α, which stimulates conventional dendritic cells to produce TNF-α. However, TNF-α matures both pDCs and conventional dendritic cells; upon maturation, both types of dendritic cells lose the ability to produce IFN-α, thus allowing TNF-α to become dominant.10 The blockade of TNF-α prevents pDC maturation, leading to uninhibited IFN-α, which appears to drive inflammation in paradoxical psoriasis. In classical psoriasis, oligoclonal dermal CD4+ T cells and epidermal CD8+ T cells remain, even in resolved skin lesions, and can cause disease recurrence through reactivation of skin-resident memory T cells.11 No relapse of paradoxical psoriasis occurs with discontinuation of anti-TNF-α therapy, which supports the notion of an absence of memory T cells.
The incidence of paradoxical psoriasis in patients receiving a TNF-α inhibitor for HS is unclear.12 There are case series in which patients who had concurrent psoriasis and HS were successfully treated with a TNF-α inhibitor.13 A recently recognized condition—PASH syndrome—encompasses the clinical triad of PG, acne, and HS.10
Our patient had no history of acne or PG, only a long-standing history of HS. New-onset PG occurred only after a TNF-α inhibitor was initiated. Notably, PASH syndrome has been successfully treated with TNF-α inhibitors, highlighting the shared inflammatory etiology of HS and PG.14 In patients with concurrent PG and HS, TNF-α inhibitors were more effective for treating PG than for HS.
Pyoderma gangrenosum is an inflammatory disorder that often occurs concomitantly with other conditions, such as inflammatory bowel disease. The exact underlying cause of PG is unclear, but there appears to be both neutrophil and T-cell dysfunction in PG, with excess inflammatory cytokine production (eg, IL-1β, TNF-α, IL-17).15
The mainstay of treatment of PG is systemic corticosteroids and immunosuppressives, such as cyclosporine. Tumor necrosis factor α inhibitors as well as other interleukin inhibitors are increasingly utilized as potential therapeutic alternatives for PG.16,17
Unlike paradoxical psoriasis, the underlying cause of paradoxical PG is unclear.18,19 A similar mechanism may be postulated whereby inhibition of TNF-α leads to excessive activation of alternative inflammatory pathways that result in paradoxical PG. In one study, the prevalence of PG among 68,232 patients with HS was 0.18% compared with 0.01% among those without HS; therefore, patients with HS appear to be more predisposed to PG.20
This case illustrates the complex, often conflicting effects of cytokine inhibition in the paradoxical elicitation of alternative inflammatory disorders as an unintended consequence of the initial cytokine blockade. It is likely that genetic predisposition allows for paradoxical reactions in some patients when there is predominant inhibition of one cytokine in the inflammatory pathway. In rare cases, multiple paradoxical reactions are possible.
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the pilosebaceous unit that occurs in concert with elevations of various cytokines, including tumor necrosis factor α (TNF-α), IL-1β, IL-10, and IL-17.1,2 Adalimumab is a TNF-α inhibitor approved by the US Food and Drug Administration for the treatment of HS. Although TNF-α inhibitors are effective for many immune-mediated inflammatory disorders, paradoxical drug reactions have been reported following treatment with these agents.3-6 True paradoxical drug reactions likely are immune mediated and directly lead to new onset of a pathologic condition that would otherwise respond to that drug. For example, there are reports of rheumatoid arthritis patients who were treated with a TNF-α inhibitor and developed psoriatic skin lesions.3,6 Paradoxical drug reactions also have been reported with acute-onset inflammatory bowel disease and HS or less commonly pyoderma gangrenosum (PG), uveitis, granulomatous reactions, and vasculitis.4,5 We present the case of a patient with HS who was treated with a TNF-α inhibitor and developed 2 distinct paradoxical drug reactions. We also provide an overview of paradoxical drug reactions associated with TNF-α inhibitors.
A 38-year-old woman developed a painful “boil” on the right leg that was previously treated in the emergency department with incision and drainage as well as oral clindamycin for 7 days, but the lesion spread and continued to worsen. She had a history of HS in the axillae and groin region that had been present since 12 years of age. The condition was poorly controlled despite multiple courses of oral antibiotics and surgical resections. An oral contraceptive also was attempted, but the patient discontinued treatment when liver enzyme levels became elevated. The patient had no other notable medical history, including skin disease. There was a family history of HS in her father and a sibling. Seeking more effective treatment, the patient was offered adalimumab approximately 4 months prior to clinical presentation and agreed to start a course of the drug. She received a loading dose of 160 mg on day 1 and 80 mg on day 15 followed by a maintenance dosage of 40 mg weekly. She experienced improvement in HS symptoms after 3 months on adalimumab; however, she developed scaly pruritic patches on the scalp, arms, and legs that were consistent with psoriasis. Because of the absence of a personal or family history of psoriasis, the patient was informed of the probability of paradoxical psoriasis resulting from adalimumab. She elected to continue adalimumab because of the improvement in HS symptoms, and the psoriatic lesions were mild and adequately controlled with a topical steroid.
At the current presentation 1 month later, physical examination revealed a large indurated and ulcerated area with jagged edges at the incision and drainage site (Figure 1). Pyoderma gangrenosum was clinically suspected; a biopsy was performed, and the patient was started on oral prednisone. At 2-week follow-up, the ulcer was found to be rapidly resolving with prednisone and healing with cribriform scarring (Figure 2). Histopathology revealed an undermining neutrophilic inflammatory process that was consistent with PG. A diagnosis of PG was made based on previously published criteria7 and the following major/minor criteria in the patient: pathology; absence of infection on histologic analysis; history of pathergy related to worsening ulceration at the site of incision and drainage of the initial boil; clinical findings of an ulcer with peripheral violaceous erythema; undermined borders and tenderness at the site; and rapid resolution of the ulcer with prednisone.

Cessation of adalimumab gradually led to clearance of both psoriasiform lesions and PG; however, HS lesions persisted.

Although the precise pathogenesis of HS is unclear, both genetic abnormalities of the pilosebaceous unit and a dysregulated immune reaction appear to lead to the clinical characteristics of chronic inflammation and scarring seen in HS. A key effector appears to be helper T-cell (TH17) lymphocyte activation, with increased secretion of TNF-α, IL-1β, and IL-17.1,2 In turn, IL-17 induces higher expression of TNF-α, leading to a persistent cycle of inflammation. Peripheral recruitment of IL-17–producing neutrophils also may contribute to chronic inflammation.8
Adalimumab is the only US Food and Drug Administration–approved biologic indicated for the treatment of HS. Our patient initially responded to adalimumab with improvement of HS; however, treatment had to be discontinued because of the unusual occurrence of 2 distinct paradoxical reactions in a short span of time. Psoriasis and PG are both considered true paradoxical reactions because primary occurrences of both diseases usually are responsive to treatment with adalimumab.
Tumor necrosis factor α inhibitor–induced psoriasis arises de novo and is estimated to occur in approximately 5% of patients with rheumatoid arthritis.3,6 Palmoplantar pustular psoriasiform reactions are the most common form of paradoxical psoriasis. Topical medications can be used to treat skin lesions, but systemic treatment is required in many cases. Switching to an alternate class of a biologic, such as an IL-17, IL-12/23, or IL-23 inhibitor, can improve the skin reaction; however, such treatment is inconsistently successful, and paradoxical drug reactions also have been seen with these other classes of biologics.4,9
Recent studies support distinct immune causes for classical and paradoxical psoriasis. In classical psoriasis, plasmacytoid dendritic cells (pDCs) produce IFN-α, which stimulates conventional dendritic cells to produce TNF-α. However, TNF-α matures both pDCs and conventional dendritic cells; upon maturation, both types of dendritic cells lose the ability to produce IFN-α, thus allowing TNF-α to become dominant.10 The blockade of TNF-α prevents pDC maturation, leading to uninhibited IFN-α, which appears to drive inflammation in paradoxical psoriasis. In classical psoriasis, oligoclonal dermal CD4+ T cells and epidermal CD8+ T cells remain, even in resolved skin lesions, and can cause disease recurrence through reactivation of skin-resident memory T cells.11 No relapse of paradoxical psoriasis occurs with discontinuation of anti-TNF-α therapy, which supports the notion of an absence of memory T cells.
The incidence of paradoxical psoriasis in patients receiving a TNF-α inhibitor for HS is unclear.12 There are case series in which patients who had concurrent psoriasis and HS were successfully treated with a TNF-α inhibitor.13 A recently recognized condition—PASH syndrome—encompasses the clinical triad of PG, acne, and HS.10
Our patient had no history of acne or PG, only a long-standing history of HS. New-onset PG occurred only after a TNF-α inhibitor was initiated. Notably, PASH syndrome has been successfully treated with TNF-α inhibitors, highlighting the shared inflammatory etiology of HS and PG.14 In patients with concurrent PG and HS, TNF-α inhibitors were more effective for treating PG than for HS.
Pyoderma gangrenosum is an inflammatory disorder that often occurs concomitantly with other conditions, such as inflammatory bowel disease. The exact underlying cause of PG is unclear, but there appears to be both neutrophil and T-cell dysfunction in PG, with excess inflammatory cytokine production (eg, IL-1β, TNF-α, IL-17).15
The mainstay of treatment of PG is systemic corticosteroids and immunosuppressives, such as cyclosporine. Tumor necrosis factor α inhibitors as well as other interleukin inhibitors are increasingly utilized as potential therapeutic alternatives for PG.16,17
Unlike paradoxical psoriasis, the underlying cause of paradoxical PG is unclear.18,19 A similar mechanism may be postulated whereby inhibition of TNF-α leads to excessive activation of alternative inflammatory pathways that result in paradoxical PG. In one study, the prevalence of PG among 68,232 patients with HS was 0.18% compared with 0.01% among those without HS; therefore, patients with HS appear to be more predisposed to PG.20
This case illustrates the complex, often conflicting effects of cytokine inhibition in the paradoxical elicitation of alternative inflammatory disorders as an unintended consequence of the initial cytokine blockade. It is likely that genetic predisposition allows for paradoxical reactions in some patients when there is predominant inhibition of one cytokine in the inflammatory pathway. In rare cases, multiple paradoxical reactions are possible.
1. Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2018;9:2965. doi:10.3389/fimmu.2018.02965
2. Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation and pathogenesis. J Am Acad Dermatol. 2020; 82:1045-1058. doi:10.1016/j.jaad.2019.08.090
3. Brown G, Wang E, Leon A, et al. Tumor necrosis factor-α inhibitor-induced psoriasis: systematic review of clinical features, histopathological findings, and management experience. J Am Acad Dermatol. 2017;76:334-341. doi:10.1016/j.jaad.2016.08.012
4. Puig L. Paradoxical reactions: anti-tumor necrosis factor alpha agents, ustekinumab, secukinumab, ixekizumab and others. Curr Prob Dermatol. 2018;53:49-63. doi:10.1159/000479475
5. Faivre C, Villani AP, Aubin F, et al; . Hidradenitis suppurativa (HS): an unrecognized paradoxical effect of biologic agents (BA) used in chronic inflammatory diseases. J Am Acad Dermatol. 2016;74:1153-1159. doi:10.1016/j.jaad.2016.01.018
6. Ko JM, Gottlieb AB, Kerbleski JF. Induction and exacerbation of psoriasis with TNF-blockade therapy: a review and analysis of 127 cases. J Dermatolog Treat. 2009;20:100-108. doi:10.1080/09546630802441234
7. Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a delphi consensus of international experts. JAMA Dermatol. 2018;154:461-466. doi:10.1001/jamadermatol.2017.5980
8. Lima AL, Karl I, Giner T, et al. Keratinocytes and neutrophils are important sources of proinflammatory molecules in hidradenitis suppurativa. Br J Dermatol. 2016;174:514-521. doi:10.1111/bjd.14214
9. Li SJ, Perez-Chada LM, Merola JF. TNF inhibitor-induced psoriasis: proposed algorithm for treatment and management. J Psoriasis Psoriatic Arthritis. 2019;4:70-80. doi:10.1177/2475530318810851
10. Conrad C, Di Domizio J, Mylonas A, et al. TNF blockade induces a dysregulated type I interferon response without autoimmunity in paradoxical psoriasis. Nat Commun. 2018;9:25. doi:10.1038/s41467-017-02466-4
11. Matos TR, O’Malley JT, Lowry EL, et al. Clinically resolved psoriatic lesions contain psoriasis-specific IL-17-producing αβ T cell clones. J Clin Invest. 2017;127:4031-4041. doi:10.1172/JCI93396
12. Faivre C, Villani AP, Aubin F, et al. Hidradenitis suppurativa (HS): an unrecognized paradoxical effect of biologic agents (BA) used in chronic inflammatory diseases. J Am Acad Dermatol. 2016;74:1153-1159. doi:10.1016/j.jaad.2016.01.018
13. Marzano AV, Damiani G, Ceccherini I, et al. Autoinflammation in pyoderma gangrenosum and its syndromic form (pyoderma gangrenosum, acne and suppurative hidradenitis). Br J Dermatol. 2017;176:1588-1598. doi:10.1111/bjd.15226
14. Cugno M, Borghi A, Marzano AV. PAPA, PASH, PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562. doi:10.1007/s40257-017-0265-1
15. Wang EA, Steel A, Luxardi G, et al. Classic ulcerative pyoderma gangrenosum is a T cell-mediated disease targeting follicular adnexal structures: a hypothesis based on molecular and clinicopathologic studies. Front Immunol. 2018;8:1980. doi:10.3389/fimmu.2017.01980
16. Patel F, Fitzmaurice S, Duong C, et al. Effective strategies for the management of pyoderma gangrenosum: a comprehensive review. Acta Derm Venereol. 2015;95:525-531. doi:10.2340/00015555-2008
17. Partridge ACR, Bai JW, Rosen CF, et al. Effectiveness of systemic treatments for pyoderma gangrenosum: a systematic review of observational studies and clinical trials. Br J Dermatol. 2018;179:290-295. doi:10.1111/bjd.16485
18. Benzaquen M, Monnier J, Beaussault Y, et al. Pyoderma gangrenosum arising during treatment of psoriasis with adalimumab: effectiveness of ustekinumab. Australas J Dermatol. 2017;58:e270-e271. doi:10.1111/ajd.12545
19. Fujimoto N, Yamasaki Y, Watanabe RJ. Paradoxical uveitis and pyoderma gangrenosum in a patient with psoriatic arthritis under infliximab treatment. J Dtsch Dermatol Ges. 2018;16:1139-1140. doi:10.1111/ddg.13632
20. Tannenbaum R, Strunk A, Garg A. Overall and subgroup prevalence of pyoderma gangrenosum among patients with hidradenitis suppurativa: a population-based analysis in the United States. J Am Acad Dermatol. 2019;80:1533-1537. doi:10.1016/j.jaad.2019.02.004
1. Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2018;9:2965. doi:10.3389/fimmu.2018.02965
2. Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation and pathogenesis. J Am Acad Dermatol. 2020; 82:1045-1058. doi:10.1016/j.jaad.2019.08.090
3. Brown G, Wang E, Leon A, et al. Tumor necrosis factor-α inhibitor-induced psoriasis: systematic review of clinical features, histopathological findings, and management experience. J Am Acad Dermatol. 2017;76:334-341. doi:10.1016/j.jaad.2016.08.012
4. Puig L. Paradoxical reactions: anti-tumor necrosis factor alpha agents, ustekinumab, secukinumab, ixekizumab and others. Curr Prob Dermatol. 2018;53:49-63. doi:10.1159/000479475
5. Faivre C, Villani AP, Aubin F, et al; . Hidradenitis suppurativa (HS): an unrecognized paradoxical effect of biologic agents (BA) used in chronic inflammatory diseases. J Am Acad Dermatol. 2016;74:1153-1159. doi:10.1016/j.jaad.2016.01.018
6. Ko JM, Gottlieb AB, Kerbleski JF. Induction and exacerbation of psoriasis with TNF-blockade therapy: a review and analysis of 127 cases. J Dermatolog Treat. 2009;20:100-108. doi:10.1080/09546630802441234
7. Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a delphi consensus of international experts. JAMA Dermatol. 2018;154:461-466. doi:10.1001/jamadermatol.2017.5980
8. Lima AL, Karl I, Giner T, et al. Keratinocytes and neutrophils are important sources of proinflammatory molecules in hidradenitis suppurativa. Br J Dermatol. 2016;174:514-521. doi:10.1111/bjd.14214
9. Li SJ, Perez-Chada LM, Merola JF. TNF inhibitor-induced psoriasis: proposed algorithm for treatment and management. J Psoriasis Psoriatic Arthritis. 2019;4:70-80. doi:10.1177/2475530318810851
10. Conrad C, Di Domizio J, Mylonas A, et al. TNF blockade induces a dysregulated type I interferon response without autoimmunity in paradoxical psoriasis. Nat Commun. 2018;9:25. doi:10.1038/s41467-017-02466-4
11. Matos TR, O’Malley JT, Lowry EL, et al. Clinically resolved psoriatic lesions contain psoriasis-specific IL-17-producing αβ T cell clones. J Clin Invest. 2017;127:4031-4041. doi:10.1172/JCI93396
12. Faivre C, Villani AP, Aubin F, et al. Hidradenitis suppurativa (HS): an unrecognized paradoxical effect of biologic agents (BA) used in chronic inflammatory diseases. J Am Acad Dermatol. 2016;74:1153-1159. doi:10.1016/j.jaad.2016.01.018
13. Marzano AV, Damiani G, Ceccherini I, et al. Autoinflammation in pyoderma gangrenosum and its syndromic form (pyoderma gangrenosum, acne and suppurative hidradenitis). Br J Dermatol. 2017;176:1588-1598. doi:10.1111/bjd.15226
14. Cugno M, Borghi A, Marzano AV. PAPA, PASH, PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562. doi:10.1007/s40257-017-0265-1
15. Wang EA, Steel A, Luxardi G, et al. Classic ulcerative pyoderma gangrenosum is a T cell-mediated disease targeting follicular adnexal structures: a hypothesis based on molecular and clinicopathologic studies. Front Immunol. 2018;8:1980. doi:10.3389/fimmu.2017.01980
16. Patel F, Fitzmaurice S, Duong C, et al. Effective strategies for the management of pyoderma gangrenosum: a comprehensive review. Acta Derm Venereol. 2015;95:525-531. doi:10.2340/00015555-2008
17. Partridge ACR, Bai JW, Rosen CF, et al. Effectiveness of systemic treatments for pyoderma gangrenosum: a systematic review of observational studies and clinical trials. Br J Dermatol. 2018;179:290-295. doi:10.1111/bjd.16485
18. Benzaquen M, Monnier J, Beaussault Y, et al. Pyoderma gangrenosum arising during treatment of psoriasis with adalimumab: effectiveness of ustekinumab. Australas J Dermatol. 2017;58:e270-e271. doi:10.1111/ajd.12545
19. Fujimoto N, Yamasaki Y, Watanabe RJ. Paradoxical uveitis and pyoderma gangrenosum in a patient with psoriatic arthritis under infliximab treatment. J Dtsch Dermatol Ges. 2018;16:1139-1140. doi:10.1111/ddg.13632
20. Tannenbaum R, Strunk A, Garg A. Overall and subgroup prevalence of pyoderma gangrenosum among patients with hidradenitis suppurativa: a population-based analysis in the United States. J Am Acad Dermatol. 2019;80:1533-1537. doi:10.1016/j.jaad.2019.02.004
Practice Points
- Clinicians need to be aware of the potential risk for a paradoxical reaction in patients receiving a tumor necrosis factor α (TNF-α) inhibitor for hidradenitis suppurativa.
- Although uncommon, developing more than 1 type of paradoxical skin reaction is possible with a TNF-α inhibitor.
- Early recognition and appropriate management of these paradoxical reactions are critical.
TNF blockers not associated with poorer pregnancy outcomes
SAN DIEGO – Continuing a tumor necrosis factor inhibitor (TNFi) during pregnancy does not increase risk of worse fetal or obstetric outcomes, according to new research presented at the annual meeting of the American College of Rheumatology.
Patients who continued a TNFi also had fewer severe infections requiring hospitalization, compared with those who stopped taking the medication during their pregnancy.
“The main message is that patients continuing were not doing worse than the patients stopping. It’s an important clinical message for rheumatologists who are not really confident in dealing with these drugs during pregnancy,” said Anna Moltó, MD, PhD, a rheumatologist at Cochin Hospital, Paris, who led the research. “It adds to the data that it seems to be safe,” she added in an interview.
Previous research, largely from pregnant patients with inflammatory bowel disease, suggests that taking a TNFi during pregnancy is safe, and 2020 ACR guidelines conditionally recommend continuing therapy prior to and during pregnancy; however, many people still stop taking the drugs during pregnancy for fear of potentially harming the fetus.
To better understand how TNFi use affected pregnancy outcomes, Dr. Moltó and colleagues analyzed data from a French nationwide health insurance database to identify adult women with chronic rheumatic inflammatory disease. All women included in the cohort had a singleton pregnancy between 2008 and 2017 and were taking a TNFi upon pregnancy diagnosis.
Patients who restarted TNFi after initially pausing because of pregnancy were included in the continuation group.
Researchers identified more than 2,000 pregnancies, including 1,503 in individuals with spondyloarthritis and 579 individuals with rheumatoid arthritis. Patients were, on average, 31 years old and were diagnosed with a rheumatic disease 4 years prior to their pregnancy.
About 72% (n = 1,497) discontinued TNFi after learning they were pregnant, and 584 individuals continued treatment. Dr. Moltó noted that data from more recent years might have captured lower discontinuation rates among pregnant individuals, but those data were not available for the study.
There was no difference in unfavorable obstetrical or infant outcomes, including spontaneous abortion, preeclampsia, gestational diabetes, major congenital malformation, and severe infection of the infant requiring hospitalization. Somewhat surprisingly, the data showed that women who discontinued a TNFi were more likely to be hospitalized for infection either during their pregnancy or up to 6 weeks after delivery, compared with those who continued therapy (1.3% vs. 0.2%, respectively).
Dr. Moltó is currently looking into what could be behind this counterintuitive result, but she hypothesizes that patients who had stopped TNFi may have been taking more glucocorticoids.
“At our institution, there is generally a comfort level with continuing TNF inhibitors during pregnancy, at least until about 36 weeks,” said Sara K. Tedeschi, MD, MPH, a rheumatologist at Brigham and Women’s Hospital and assistant professor of medicine at Harvard Medical School, both in Boston. Sometimes, there is concern for risk of infection to the infant, depending on the type of TNFi being used, she added during a press conference.
“I think that these are really informative and supportive data to let women know that they probably have a really good chance of doing very well during the pregnancy if they continue” their TNFi, said Dr. Tedeschi, who was not involved with the study.
TNF discontinuation on the decline
In a related study, researchers at McGill University, Montreal, found that TNFi discontinuation prior to pregnancy had decreased over time in individuals with chronic inflammatory diseases.
Using a database of U.S. insurance claims, they identified 3,372 women with RA, ankylosing spondylitis (AS), psoriasis/psoriatic arthritis (PsA), and/or inflammatory bowel disease (IBD) who previously used a TNFi and gave birth between 2011 and 2019. A patient was considered to have used a TNFi if she had filled a prescription or had an infusion procedure insurance claim within 12 weeks before the gestational period or anytime during pregnancy. Researchers did not have time-specific data to account for women who stopped treatment at pregnancy diagnosis.
Nearly half (47%) of all identified pregnancies were in individuals with IBD, and the rest included patients with RA (24%), psoriasis or PsA (16%), AS (3%), or more than one diagnosis (10%).
In total, 14% of women discontinued TNFi use in the 12 weeks before becoming pregnant and did not restart. From 2011 to 2013, 19% of patients stopped their TNFi, but this proportion decreased overtime, with 10% of patients stopping therapy from 2017 to 2019 (P < .0001).
This decline “possibly reflects the increase in real-world evidence about the safety of TNFi in pregnancy. That research, in turn, led to new guidelines recommending the continuation of TNFi during pregnancy,” first author Leah Flatman, a PhD candidate in epidemiology at McGill, said in an interview. “I think we can see this potentially as good news.”
More patients with RA, psoriasis/PsA, and AS discontinued TNFi therapy prior to conception (23%-25%), compared with those with IBD (5%).
Ms. Flatman noted that her study and Moltó’s study complement each other by providing data on individuals stopping TNFi prior to conception versus those stopping treatment after pregnancy diagnosis.
“These findings demonstrate that continuing TNFi during pregnancy appears not to be associated with an increase in adverse obstetrical or infant outcomes,” Ms. Flatman said of Dr. Moltó’s study. “As guidelines currently recommend continuing TNFi, studies like this help demonstrate that the guideline changes do not appear to be associated with an increase in adverse events.”
Dr. Moltó and Ms. Flatman disclosed no relevant financial relationships. Dr. Tedeschi has worked as a consultant for Novartis.
A version of this article appeared on Medscape.com.
SAN DIEGO – Continuing a tumor necrosis factor inhibitor (TNFi) during pregnancy does not increase risk of worse fetal or obstetric outcomes, according to new research presented at the annual meeting of the American College of Rheumatology.
Patients who continued a TNFi also had fewer severe infections requiring hospitalization, compared with those who stopped taking the medication during their pregnancy.
“The main message is that patients continuing were not doing worse than the patients stopping. It’s an important clinical message for rheumatologists who are not really confident in dealing with these drugs during pregnancy,” said Anna Moltó, MD, PhD, a rheumatologist at Cochin Hospital, Paris, who led the research. “It adds to the data that it seems to be safe,” she added in an interview.
Previous research, largely from pregnant patients with inflammatory bowel disease, suggests that taking a TNFi during pregnancy is safe, and 2020 ACR guidelines conditionally recommend continuing therapy prior to and during pregnancy; however, many people still stop taking the drugs during pregnancy for fear of potentially harming the fetus.
To better understand how TNFi use affected pregnancy outcomes, Dr. Moltó and colleagues analyzed data from a French nationwide health insurance database to identify adult women with chronic rheumatic inflammatory disease. All women included in the cohort had a singleton pregnancy between 2008 and 2017 and were taking a TNFi upon pregnancy diagnosis.
Patients who restarted TNFi after initially pausing because of pregnancy were included in the continuation group.
Researchers identified more than 2,000 pregnancies, including 1,503 in individuals with spondyloarthritis and 579 individuals with rheumatoid arthritis. Patients were, on average, 31 years old and were diagnosed with a rheumatic disease 4 years prior to their pregnancy.
About 72% (n = 1,497) discontinued TNFi after learning they were pregnant, and 584 individuals continued treatment. Dr. Moltó noted that data from more recent years might have captured lower discontinuation rates among pregnant individuals, but those data were not available for the study.
There was no difference in unfavorable obstetrical or infant outcomes, including spontaneous abortion, preeclampsia, gestational diabetes, major congenital malformation, and severe infection of the infant requiring hospitalization. Somewhat surprisingly, the data showed that women who discontinued a TNFi were more likely to be hospitalized for infection either during their pregnancy or up to 6 weeks after delivery, compared with those who continued therapy (1.3% vs. 0.2%, respectively).
Dr. Moltó is currently looking into what could be behind this counterintuitive result, but she hypothesizes that patients who had stopped TNFi may have been taking more glucocorticoids.
“At our institution, there is generally a comfort level with continuing TNF inhibitors during pregnancy, at least until about 36 weeks,” said Sara K. Tedeschi, MD, MPH, a rheumatologist at Brigham and Women’s Hospital and assistant professor of medicine at Harvard Medical School, both in Boston. Sometimes, there is concern for risk of infection to the infant, depending on the type of TNFi being used, she added during a press conference.
“I think that these are really informative and supportive data to let women know that they probably have a really good chance of doing very well during the pregnancy if they continue” their TNFi, said Dr. Tedeschi, who was not involved with the study.
TNF discontinuation on the decline
In a related study, researchers at McGill University, Montreal, found that TNFi discontinuation prior to pregnancy had decreased over time in individuals with chronic inflammatory diseases.
Using a database of U.S. insurance claims, they identified 3,372 women with RA, ankylosing spondylitis (AS), psoriasis/psoriatic arthritis (PsA), and/or inflammatory bowel disease (IBD) who previously used a TNFi and gave birth between 2011 and 2019. A patient was considered to have used a TNFi if she had filled a prescription or had an infusion procedure insurance claim within 12 weeks before the gestational period or anytime during pregnancy. Researchers did not have time-specific data to account for women who stopped treatment at pregnancy diagnosis.
Nearly half (47%) of all identified pregnancies were in individuals with IBD, and the rest included patients with RA (24%), psoriasis or PsA (16%), AS (3%), or more than one diagnosis (10%).
In total, 14% of women discontinued TNFi use in the 12 weeks before becoming pregnant and did not restart. From 2011 to 2013, 19% of patients stopped their TNFi, but this proportion decreased overtime, with 10% of patients stopping therapy from 2017 to 2019 (P < .0001).
This decline “possibly reflects the increase in real-world evidence about the safety of TNFi in pregnancy. That research, in turn, led to new guidelines recommending the continuation of TNFi during pregnancy,” first author Leah Flatman, a PhD candidate in epidemiology at McGill, said in an interview. “I think we can see this potentially as good news.”
More patients with RA, psoriasis/PsA, and AS discontinued TNFi therapy prior to conception (23%-25%), compared with those with IBD (5%).
Ms. Flatman noted that her study and Moltó’s study complement each other by providing data on individuals stopping TNFi prior to conception versus those stopping treatment after pregnancy diagnosis.
“These findings demonstrate that continuing TNFi during pregnancy appears not to be associated with an increase in adverse obstetrical or infant outcomes,” Ms. Flatman said of Dr. Moltó’s study. “As guidelines currently recommend continuing TNFi, studies like this help demonstrate that the guideline changes do not appear to be associated with an increase in adverse events.”
Dr. Moltó and Ms. Flatman disclosed no relevant financial relationships. Dr. Tedeschi has worked as a consultant for Novartis.
A version of this article appeared on Medscape.com.
SAN DIEGO – Continuing a tumor necrosis factor inhibitor (TNFi) during pregnancy does not increase risk of worse fetal or obstetric outcomes, according to new research presented at the annual meeting of the American College of Rheumatology.
Patients who continued a TNFi also had fewer severe infections requiring hospitalization, compared with those who stopped taking the medication during their pregnancy.
“The main message is that patients continuing were not doing worse than the patients stopping. It’s an important clinical message for rheumatologists who are not really confident in dealing with these drugs during pregnancy,” said Anna Moltó, MD, PhD, a rheumatologist at Cochin Hospital, Paris, who led the research. “It adds to the data that it seems to be safe,” she added in an interview.
Previous research, largely from pregnant patients with inflammatory bowel disease, suggests that taking a TNFi during pregnancy is safe, and 2020 ACR guidelines conditionally recommend continuing therapy prior to and during pregnancy; however, many people still stop taking the drugs during pregnancy for fear of potentially harming the fetus.
To better understand how TNFi use affected pregnancy outcomes, Dr. Moltó and colleagues analyzed data from a French nationwide health insurance database to identify adult women with chronic rheumatic inflammatory disease. All women included in the cohort had a singleton pregnancy between 2008 and 2017 and were taking a TNFi upon pregnancy diagnosis.
Patients who restarted TNFi after initially pausing because of pregnancy were included in the continuation group.
Researchers identified more than 2,000 pregnancies, including 1,503 in individuals with spondyloarthritis and 579 individuals with rheumatoid arthritis. Patients were, on average, 31 years old and were diagnosed with a rheumatic disease 4 years prior to their pregnancy.
About 72% (n = 1,497) discontinued TNFi after learning they were pregnant, and 584 individuals continued treatment. Dr. Moltó noted that data from more recent years might have captured lower discontinuation rates among pregnant individuals, but those data were not available for the study.
There was no difference in unfavorable obstetrical or infant outcomes, including spontaneous abortion, preeclampsia, gestational diabetes, major congenital malformation, and severe infection of the infant requiring hospitalization. Somewhat surprisingly, the data showed that women who discontinued a TNFi were more likely to be hospitalized for infection either during their pregnancy or up to 6 weeks after delivery, compared with those who continued therapy (1.3% vs. 0.2%, respectively).
Dr. Moltó is currently looking into what could be behind this counterintuitive result, but she hypothesizes that patients who had stopped TNFi may have been taking more glucocorticoids.
“At our institution, there is generally a comfort level with continuing TNF inhibitors during pregnancy, at least until about 36 weeks,” said Sara K. Tedeschi, MD, MPH, a rheumatologist at Brigham and Women’s Hospital and assistant professor of medicine at Harvard Medical School, both in Boston. Sometimes, there is concern for risk of infection to the infant, depending on the type of TNFi being used, she added during a press conference.
“I think that these are really informative and supportive data to let women know that they probably have a really good chance of doing very well during the pregnancy if they continue” their TNFi, said Dr. Tedeschi, who was not involved with the study.
TNF discontinuation on the decline
In a related study, researchers at McGill University, Montreal, found that TNFi discontinuation prior to pregnancy had decreased over time in individuals with chronic inflammatory diseases.
Using a database of U.S. insurance claims, they identified 3,372 women with RA, ankylosing spondylitis (AS), psoriasis/psoriatic arthritis (PsA), and/or inflammatory bowel disease (IBD) who previously used a TNFi and gave birth between 2011 and 2019. A patient was considered to have used a TNFi if she had filled a prescription or had an infusion procedure insurance claim within 12 weeks before the gestational period or anytime during pregnancy. Researchers did not have time-specific data to account for women who stopped treatment at pregnancy diagnosis.
Nearly half (47%) of all identified pregnancies were in individuals with IBD, and the rest included patients with RA (24%), psoriasis or PsA (16%), AS (3%), or more than one diagnosis (10%).
In total, 14% of women discontinued TNFi use in the 12 weeks before becoming pregnant and did not restart. From 2011 to 2013, 19% of patients stopped their TNFi, but this proportion decreased overtime, with 10% of patients stopping therapy from 2017 to 2019 (P < .0001).
This decline “possibly reflects the increase in real-world evidence about the safety of TNFi in pregnancy. That research, in turn, led to new guidelines recommending the continuation of TNFi during pregnancy,” first author Leah Flatman, a PhD candidate in epidemiology at McGill, said in an interview. “I think we can see this potentially as good news.”
More patients with RA, psoriasis/PsA, and AS discontinued TNFi therapy prior to conception (23%-25%), compared with those with IBD (5%).
Ms. Flatman noted that her study and Moltó’s study complement each other by providing data on individuals stopping TNFi prior to conception versus those stopping treatment after pregnancy diagnosis.
“These findings demonstrate that continuing TNFi during pregnancy appears not to be associated with an increase in adverse obstetrical or infant outcomes,” Ms. Flatman said of Dr. Moltó’s study. “As guidelines currently recommend continuing TNFi, studies like this help demonstrate that the guideline changes do not appear to be associated with an increase in adverse events.”
Dr. Moltó and Ms. Flatman disclosed no relevant financial relationships. Dr. Tedeschi has worked as a consultant for Novartis.
A version of this article appeared on Medscape.com.
AT ACR 2023
The challenges of palmoplantar pustulosis and other acral psoriatic disease
WASHINGTON – The approval last year of the interleukin (IL)-36 receptor antagonist spesolimab for treating generalized pustular psoriasis flares brightened the treatment landscape for this rare condition, and a recently published phase 2 study suggests a potential role of spesolimab for flare prevention. , according to speakers at the annual research symposium of the National Psoriasis Foundation.
“The IL-36 receptor antagonists don’t seem to be quite the answer for [palmoplantar pustulosis] that they are for generalized pustular psoriasis [GPP],” Megan H. Noe, MD, MPH, assistant professor of dermatology at Harvard Medical School and a dermatologist at Brigham and Women’s Hospital, Boston, said at the meeting.
Psoriasis affecting the hands and feet – both pustular and nonpustular – has a higher impact on quality of life and higher functional disability than does non-acral psoriasis, is less responsive to treatment, and has a “very confusing nomenclature” that complicates research and thus management, said Jason Ezra Hawkes, MD, a dermatologist in Rocklin, Calif., and former faculty member of several departments of dermatology. Both he and Dr. Noe spoke during a tough-to-treat session at the NPF meeting.
IL-17 and IL-23 blockade, as well as tumor necrosis factor (TNF) inhibition, are effective overall for palmoplantar psoriasis (nonpustular), but in general, responses are lower than for plaque psoriasis. Apremilast (Otezla), a phosphodiesterase-4 inhibitor, has some efficacy for pustular variants, but for hyperkeratotic variants it “does not perform as well as more selective inhibition of IL-17 and IL-23 blockade,” he said.
In general, ”what’s happening in the acral sites is different from an immune perspective than what’s happening in the non-acral sites,” and more research utilizing a clearer, descriptive nomenclature is needed to tease out differing immunophenotypes, explained Dr. Hawkes, who has led multiple clinical trials of treatments for psoriasis and other inflammatory skin conditions.
Palmoplantar pustulosis, and a word on generalized disease
Dermatologists are using a variety of treatments for palmoplantar pustulosis, with no clear first-line choices, Dr. Noe said. In a case series of almost 200 patients with palmoplantar pustulosis across 20 dermatology practices, published in JAMA Dermatology, 35% of patients received a systemic therapy prescription at their initial encounter – most commonly acitretin, followed by methotrexate and phototherapy. “Biologics were used, but use was varied and not as often as with oral agents,” said Dr. Noe, a coauthor of the study.
TNF blockers led to improvements ranging from 57% to 84%, depending on the agent, in a 2020 retrospective study of patients with palmoplantar pustulosis or acrodermatitis continua of Hallopeau, Dr. Noe noted. However, rates of complete clearance were only 20%-29%.
Apremilast showed modest efficacy after 5 months of treatment, with 62% of patients achieving at least a 50% improvement in the Palmoplantar Pustulosis Psoriasis Area and Severity Index (PPPASI) in a 2021 open-label, phase 2 study involving 21 patients. “This may represent a potential treatment option,” Dr. Noe said. “It’s something, but not what we’re used to seeing in our plaque psoriasis patients.”
A 2021 phase 2a, double-blind, randomized, placebo-controlled study of spesolimab in patients with palmoplantar pustulosis, meanwhile, failed to meet its primary endpoint, with only 32% of patients achieving a 50% improvement at 16 weeks, compared with 24% of patients in the placebo arm. And a recently published network meta-analysis found that none of the five drugs studied in seven randomized controlled trials – biologic or oral – was more effective than placebo for clearance or improvement of palmoplantar pustulosis.
The spesolimab (Spevigo) results have been disappointing considering the biologic’s newfound efficacy and role as the first Food and Drug Administration–approved therapy for generalized pustular disease, according to Dr. Noe. The ability of a single 900-mg intravenous dose of the IL-36 receptor antagonist to completely clear pustules at 1 week in 54% of patients with generalized disease, compared with 6% of the placebo group, was “groundbreaking,” she said, referring to results of the pivotal trial published in the New England Journal of Medicine.
And given that “preventing GPP flares is ultimately what we want,” she said, more good news was reported this year in The Lancet: The finding from an international, randomized, placebo-controlled study that high-dose subcutaneous spesolimab significantly reduced the risk of a flare over 48 weeks. “There are lots of ongoing studies right now to understand the best way to dose spesolimab,” she said.
Moreover, another IL-36 receptor antagonist, imsidolimab, is being investigated in a phase 3 trial for generalized pustular disease, she noted. A phase 2, open-label study of patients with GPP found that “more than half of patients were very much improved at 4 weeks, and some patients started showing improvement at day 3,” Dr. Noe said.
An area of research she is interested in is the potential for Janus kinase (JAK) inhibitors as a treatment for palmoplantar pustulosis. For pustulosis on the hands and feet, recent case reports describing the efficacy of JAK inhibitors have caught her eye. “Right now, all we have is this case report data, mostly with tofacitinib, but I think it’s exciting,” she said, noting a recently published report in the British Journal of Dermatology.
Palmoplantar psoriasis
Pustular psoriatic disease can be localized to the hand and/or feet only, or can co-occur with generalized pustular disease, just as palmoplantar psoriasis can be localized to the hands and/or feet or, more commonly, can co-occur with widespread plaque psoriasis. Research has shown, Dr. Hawkes said, that with both types of acral disease, many patients have or have had plaque psoriasis outside of acral sites.
The nomenclature and acronyms for palmoplantar psoriatic disease have complicated patient education, communication, and research, Dr. Hawkes said. Does PPP refer to palmoplantar psoriasis, or palmoplantar pustulosis, for instance? What is the difference between palmoplantar pustulosis (coined PPP) and palmoplantar pustular psoriasis (referred to as PPPP)?
What if disease is only on the hands, only on the feet, or only on the backs of the hands? And at what point is disease not classified as palmoplantar psoriasis, but plaque psoriasis with involvement of the hands and feet? Inconsistencies and lack of clarification lead to “confusing” literature, he said.
Heterogeneity in populations across trials resulting from “inconsistent categorization and phenotype inclusion” may partly account for the recalcitrance to treatment reported in the literature, he said. Misdiagnosis as psoriasis in cases of localized disease (confusion with eczema, for instance), and the fact that hands and feet are subject to increased trauma and injury, compared with non-acral sites, are also at play.
Trials may also allow insufficient time for improvement, compared with non-acral sites. “What we’ve learned about the hands and feet is that it takes a much longer time for disease to improve,” Dr. Hawkes said, so primary endpoints must take this into account.
There is unique immunologic signaling in palmoplantar disease that differs from the predominant signaling in traditional plaque psoriasis, he emphasized, and “mixed immunophenotypes” that need to be unraveled.
Dr. Hawkes disclosed ties with AbbVie, Arcutis, Bristol-Myers Squibb, Boehringer Ingelheim, Janssen, LEO, Lilly, Novartis, Pfizer, Regeneron, Sanofi, Sun Pharma, and UCB. Dr. Noe disclosed ties to Bristol-Myers Squibb and Boehringer Ingelheim.
WASHINGTON – The approval last year of the interleukin (IL)-36 receptor antagonist spesolimab for treating generalized pustular psoriasis flares brightened the treatment landscape for this rare condition, and a recently published phase 2 study suggests a potential role of spesolimab for flare prevention. , according to speakers at the annual research symposium of the National Psoriasis Foundation.
“The IL-36 receptor antagonists don’t seem to be quite the answer for [palmoplantar pustulosis] that they are for generalized pustular psoriasis [GPP],” Megan H. Noe, MD, MPH, assistant professor of dermatology at Harvard Medical School and a dermatologist at Brigham and Women’s Hospital, Boston, said at the meeting.
Psoriasis affecting the hands and feet – both pustular and nonpustular – has a higher impact on quality of life and higher functional disability than does non-acral psoriasis, is less responsive to treatment, and has a “very confusing nomenclature” that complicates research and thus management, said Jason Ezra Hawkes, MD, a dermatologist in Rocklin, Calif., and former faculty member of several departments of dermatology. Both he and Dr. Noe spoke during a tough-to-treat session at the NPF meeting.
IL-17 and IL-23 blockade, as well as tumor necrosis factor (TNF) inhibition, are effective overall for palmoplantar psoriasis (nonpustular), but in general, responses are lower than for plaque psoriasis. Apremilast (Otezla), a phosphodiesterase-4 inhibitor, has some efficacy for pustular variants, but for hyperkeratotic variants it “does not perform as well as more selective inhibition of IL-17 and IL-23 blockade,” he said.
In general, ”what’s happening in the acral sites is different from an immune perspective than what’s happening in the non-acral sites,” and more research utilizing a clearer, descriptive nomenclature is needed to tease out differing immunophenotypes, explained Dr. Hawkes, who has led multiple clinical trials of treatments for psoriasis and other inflammatory skin conditions.
Palmoplantar pustulosis, and a word on generalized disease
Dermatologists are using a variety of treatments for palmoplantar pustulosis, with no clear first-line choices, Dr. Noe said. In a case series of almost 200 patients with palmoplantar pustulosis across 20 dermatology practices, published in JAMA Dermatology, 35% of patients received a systemic therapy prescription at their initial encounter – most commonly acitretin, followed by methotrexate and phototherapy. “Biologics were used, but use was varied and not as often as with oral agents,” said Dr. Noe, a coauthor of the study.
TNF blockers led to improvements ranging from 57% to 84%, depending on the agent, in a 2020 retrospective study of patients with palmoplantar pustulosis or acrodermatitis continua of Hallopeau, Dr. Noe noted. However, rates of complete clearance were only 20%-29%.
Apremilast showed modest efficacy after 5 months of treatment, with 62% of patients achieving at least a 50% improvement in the Palmoplantar Pustulosis Psoriasis Area and Severity Index (PPPASI) in a 2021 open-label, phase 2 study involving 21 patients. “This may represent a potential treatment option,” Dr. Noe said. “It’s something, but not what we’re used to seeing in our plaque psoriasis patients.”
A 2021 phase 2a, double-blind, randomized, placebo-controlled study of spesolimab in patients with palmoplantar pustulosis, meanwhile, failed to meet its primary endpoint, with only 32% of patients achieving a 50% improvement at 16 weeks, compared with 24% of patients in the placebo arm. And a recently published network meta-analysis found that none of the five drugs studied in seven randomized controlled trials – biologic or oral – was more effective than placebo for clearance or improvement of palmoplantar pustulosis.
The spesolimab (Spevigo) results have been disappointing considering the biologic’s newfound efficacy and role as the first Food and Drug Administration–approved therapy for generalized pustular disease, according to Dr. Noe. The ability of a single 900-mg intravenous dose of the IL-36 receptor antagonist to completely clear pustules at 1 week in 54% of patients with generalized disease, compared with 6% of the placebo group, was “groundbreaking,” she said, referring to results of the pivotal trial published in the New England Journal of Medicine.
And given that “preventing GPP flares is ultimately what we want,” she said, more good news was reported this year in The Lancet: The finding from an international, randomized, placebo-controlled study that high-dose subcutaneous spesolimab significantly reduced the risk of a flare over 48 weeks. “There are lots of ongoing studies right now to understand the best way to dose spesolimab,” she said.
Moreover, another IL-36 receptor antagonist, imsidolimab, is being investigated in a phase 3 trial for generalized pustular disease, she noted. A phase 2, open-label study of patients with GPP found that “more than half of patients were very much improved at 4 weeks, and some patients started showing improvement at day 3,” Dr. Noe said.
An area of research she is interested in is the potential for Janus kinase (JAK) inhibitors as a treatment for palmoplantar pustulosis. For pustulosis on the hands and feet, recent case reports describing the efficacy of JAK inhibitors have caught her eye. “Right now, all we have is this case report data, mostly with tofacitinib, but I think it’s exciting,” she said, noting a recently published report in the British Journal of Dermatology.
Palmoplantar psoriasis
Pustular psoriatic disease can be localized to the hand and/or feet only, or can co-occur with generalized pustular disease, just as palmoplantar psoriasis can be localized to the hands and/or feet or, more commonly, can co-occur with widespread plaque psoriasis. Research has shown, Dr. Hawkes said, that with both types of acral disease, many patients have or have had plaque psoriasis outside of acral sites.
The nomenclature and acronyms for palmoplantar psoriatic disease have complicated patient education, communication, and research, Dr. Hawkes said. Does PPP refer to palmoplantar psoriasis, or palmoplantar pustulosis, for instance? What is the difference between palmoplantar pustulosis (coined PPP) and palmoplantar pustular psoriasis (referred to as PPPP)?
What if disease is only on the hands, only on the feet, or only on the backs of the hands? And at what point is disease not classified as palmoplantar psoriasis, but plaque psoriasis with involvement of the hands and feet? Inconsistencies and lack of clarification lead to “confusing” literature, he said.
Heterogeneity in populations across trials resulting from “inconsistent categorization and phenotype inclusion” may partly account for the recalcitrance to treatment reported in the literature, he said. Misdiagnosis as psoriasis in cases of localized disease (confusion with eczema, for instance), and the fact that hands and feet are subject to increased trauma and injury, compared with non-acral sites, are also at play.
Trials may also allow insufficient time for improvement, compared with non-acral sites. “What we’ve learned about the hands and feet is that it takes a much longer time for disease to improve,” Dr. Hawkes said, so primary endpoints must take this into account.
There is unique immunologic signaling in palmoplantar disease that differs from the predominant signaling in traditional plaque psoriasis, he emphasized, and “mixed immunophenotypes” that need to be unraveled.
Dr. Hawkes disclosed ties with AbbVie, Arcutis, Bristol-Myers Squibb, Boehringer Ingelheim, Janssen, LEO, Lilly, Novartis, Pfizer, Regeneron, Sanofi, Sun Pharma, and UCB. Dr. Noe disclosed ties to Bristol-Myers Squibb and Boehringer Ingelheim.
WASHINGTON – The approval last year of the interleukin (IL)-36 receptor antagonist spesolimab for treating generalized pustular psoriasis flares brightened the treatment landscape for this rare condition, and a recently published phase 2 study suggests a potential role of spesolimab for flare prevention. , according to speakers at the annual research symposium of the National Psoriasis Foundation.
“The IL-36 receptor antagonists don’t seem to be quite the answer for [palmoplantar pustulosis] that they are for generalized pustular psoriasis [GPP],” Megan H. Noe, MD, MPH, assistant professor of dermatology at Harvard Medical School and a dermatologist at Brigham and Women’s Hospital, Boston, said at the meeting.
Psoriasis affecting the hands and feet – both pustular and nonpustular – has a higher impact on quality of life and higher functional disability than does non-acral psoriasis, is less responsive to treatment, and has a “very confusing nomenclature” that complicates research and thus management, said Jason Ezra Hawkes, MD, a dermatologist in Rocklin, Calif., and former faculty member of several departments of dermatology. Both he and Dr. Noe spoke during a tough-to-treat session at the NPF meeting.
IL-17 and IL-23 blockade, as well as tumor necrosis factor (TNF) inhibition, are effective overall for palmoplantar psoriasis (nonpustular), but in general, responses are lower than for plaque psoriasis. Apremilast (Otezla), a phosphodiesterase-4 inhibitor, has some efficacy for pustular variants, but for hyperkeratotic variants it “does not perform as well as more selective inhibition of IL-17 and IL-23 blockade,” he said.
In general, ”what’s happening in the acral sites is different from an immune perspective than what’s happening in the non-acral sites,” and more research utilizing a clearer, descriptive nomenclature is needed to tease out differing immunophenotypes, explained Dr. Hawkes, who has led multiple clinical trials of treatments for psoriasis and other inflammatory skin conditions.
Palmoplantar pustulosis, and a word on generalized disease
Dermatologists are using a variety of treatments for palmoplantar pustulosis, with no clear first-line choices, Dr. Noe said. In a case series of almost 200 patients with palmoplantar pustulosis across 20 dermatology practices, published in JAMA Dermatology, 35% of patients received a systemic therapy prescription at their initial encounter – most commonly acitretin, followed by methotrexate and phototherapy. “Biologics were used, but use was varied and not as often as with oral agents,” said Dr. Noe, a coauthor of the study.
TNF blockers led to improvements ranging from 57% to 84%, depending on the agent, in a 2020 retrospective study of patients with palmoplantar pustulosis or acrodermatitis continua of Hallopeau, Dr. Noe noted. However, rates of complete clearance were only 20%-29%.
Apremilast showed modest efficacy after 5 months of treatment, with 62% of patients achieving at least a 50% improvement in the Palmoplantar Pustulosis Psoriasis Area and Severity Index (PPPASI) in a 2021 open-label, phase 2 study involving 21 patients. “This may represent a potential treatment option,” Dr. Noe said. “It’s something, but not what we’re used to seeing in our plaque psoriasis patients.”
A 2021 phase 2a, double-blind, randomized, placebo-controlled study of spesolimab in patients with palmoplantar pustulosis, meanwhile, failed to meet its primary endpoint, with only 32% of patients achieving a 50% improvement at 16 weeks, compared with 24% of patients in the placebo arm. And a recently published network meta-analysis found that none of the five drugs studied in seven randomized controlled trials – biologic or oral – was more effective than placebo for clearance or improvement of palmoplantar pustulosis.
The spesolimab (Spevigo) results have been disappointing considering the biologic’s newfound efficacy and role as the first Food and Drug Administration–approved therapy for generalized pustular disease, according to Dr. Noe. The ability of a single 900-mg intravenous dose of the IL-36 receptor antagonist to completely clear pustules at 1 week in 54% of patients with generalized disease, compared with 6% of the placebo group, was “groundbreaking,” she said, referring to results of the pivotal trial published in the New England Journal of Medicine.
And given that “preventing GPP flares is ultimately what we want,” she said, more good news was reported this year in The Lancet: The finding from an international, randomized, placebo-controlled study that high-dose subcutaneous spesolimab significantly reduced the risk of a flare over 48 weeks. “There are lots of ongoing studies right now to understand the best way to dose spesolimab,” she said.
Moreover, another IL-36 receptor antagonist, imsidolimab, is being investigated in a phase 3 trial for generalized pustular disease, she noted. A phase 2, open-label study of patients with GPP found that “more than half of patients were very much improved at 4 weeks, and some patients started showing improvement at day 3,” Dr. Noe said.
An area of research she is interested in is the potential for Janus kinase (JAK) inhibitors as a treatment for palmoplantar pustulosis. For pustulosis on the hands and feet, recent case reports describing the efficacy of JAK inhibitors have caught her eye. “Right now, all we have is this case report data, mostly with tofacitinib, but I think it’s exciting,” she said, noting a recently published report in the British Journal of Dermatology.
Palmoplantar psoriasis
Pustular psoriatic disease can be localized to the hand and/or feet only, or can co-occur with generalized pustular disease, just as palmoplantar psoriasis can be localized to the hands and/or feet or, more commonly, can co-occur with widespread plaque psoriasis. Research has shown, Dr. Hawkes said, that with both types of acral disease, many patients have or have had plaque psoriasis outside of acral sites.
The nomenclature and acronyms for palmoplantar psoriatic disease have complicated patient education, communication, and research, Dr. Hawkes said. Does PPP refer to palmoplantar psoriasis, or palmoplantar pustulosis, for instance? What is the difference between palmoplantar pustulosis (coined PPP) and palmoplantar pustular psoriasis (referred to as PPPP)?
What if disease is only on the hands, only on the feet, or only on the backs of the hands? And at what point is disease not classified as palmoplantar psoriasis, but plaque psoriasis with involvement of the hands and feet? Inconsistencies and lack of clarification lead to “confusing” literature, he said.
Heterogeneity in populations across trials resulting from “inconsistent categorization and phenotype inclusion” may partly account for the recalcitrance to treatment reported in the literature, he said. Misdiagnosis as psoriasis in cases of localized disease (confusion with eczema, for instance), and the fact that hands and feet are subject to increased trauma and injury, compared with non-acral sites, are also at play.
Trials may also allow insufficient time for improvement, compared with non-acral sites. “What we’ve learned about the hands and feet is that it takes a much longer time for disease to improve,” Dr. Hawkes said, so primary endpoints must take this into account.
There is unique immunologic signaling in palmoplantar disease that differs from the predominant signaling in traditional plaque psoriasis, he emphasized, and “mixed immunophenotypes” that need to be unraveled.
Dr. Hawkes disclosed ties with AbbVie, Arcutis, Bristol-Myers Squibb, Boehringer Ingelheim, Janssen, LEO, Lilly, Novartis, Pfizer, Regeneron, Sanofi, Sun Pharma, and UCB. Dr. Noe disclosed ties to Bristol-Myers Squibb and Boehringer Ingelheim.
AT THE NPF RESEARCH SYMPOSIUM 2023
Review estimates acne risk with JAK inhibitor therapy
TOPLINE:
, according to an analysis of 25 JAK inhibitor studies.
METHODOLOGY:
- Acne has been reported to be an adverse effect of JAK inhibitors, but not much is known about how common acne is overall and how incidence differs between different JAK inhibitors and the disease being treated.
- For the systematic review and meta-analysis, researchers identified 25 phase 2 or 3 randomized, controlled trials that reported acne as an adverse event associated with the use of JAK inhibitors.
- The study population included 10,839 participants (54% male, 46% female).
- The primary outcome was the incidence of acne following a period of JAK inhibitor use.
TAKEAWAY:
- Overall, the risk of acne was significantly higher among those treated with JAK inhibitors in comparison with patients given placebo in a pooled analysis (odds ratio [OR], 3.83).
- The risk of acne was highest with abrocitinib (OR, 13.47), followed by baricitinib (OR, 4.96), upadacitinib (OR, 4.79), deuruxolitinib (OR, 3.30), and deucravacitinib (OR, 2.64). By JAK inhibitor class, results were as follows: JAK1-specific inhibitors (OR, 4.69), combined JAK1 and JAK2 inhibitors (OR, 3.43), and tyrosine kinase 2 inhibitors (OR, 2.64).
- In a subgroup analysis, risk of acne was higher among patients using JAK inhibitors for dermatologic conditions in comparison with those using JAK inhibitors for nondermatologic conditions (OR, 4.67 vs 1.18).
- Age and gender had no apparent impact on the effect of JAK inhibitor use on acne risk.
IN PRACTICE:
“The occurrence of acne following treatment with certain classes of JAK inhibitors is of potential concern, as this adverse effect may jeopardize treatment adherence among some patients,” the researchers wrote. More studies are needed “to characterize the underlying mechanism of acne with JAK inhibitor use and to identify best practices for treatment,” they added.
SOURCE:
The lead author was Jeremy Martinez, MPH, of Harvard Medical School, Boston. The study was published online in JAMA Dermatology.
LIMITATIONS:
The review was limited by the variable classification and reporting of acne across studies, the potential exclusion of relevant studies, and the small number of studies for certain drugs.
DISCLOSURES:
The studies were mainly funded by the pharmaceutical industry. Mr. Martinez disclosed no relevant financial relationships. Several coauthors have ties with Dexcel Pharma Technologies, AbbVie, Concert, Pfizer, 3Derm Systems, Incyte, Aclaris, Eli Lilly, Concert, Equillium, ASLAN, ACOM, and Boehringer Ingelheim.
A version of this article appeared on Medscape.com.
TOPLINE:
, according to an analysis of 25 JAK inhibitor studies.
METHODOLOGY:
- Acne has been reported to be an adverse effect of JAK inhibitors, but not much is known about how common acne is overall and how incidence differs between different JAK inhibitors and the disease being treated.
- For the systematic review and meta-analysis, researchers identified 25 phase 2 or 3 randomized, controlled trials that reported acne as an adverse event associated with the use of JAK inhibitors.
- The study population included 10,839 participants (54% male, 46% female).
- The primary outcome was the incidence of acne following a period of JAK inhibitor use.
TAKEAWAY:
- Overall, the risk of acne was significantly higher among those treated with JAK inhibitors in comparison with patients given placebo in a pooled analysis (odds ratio [OR], 3.83).
- The risk of acne was highest with abrocitinib (OR, 13.47), followed by baricitinib (OR, 4.96), upadacitinib (OR, 4.79), deuruxolitinib (OR, 3.30), and deucravacitinib (OR, 2.64). By JAK inhibitor class, results were as follows: JAK1-specific inhibitors (OR, 4.69), combined JAK1 and JAK2 inhibitors (OR, 3.43), and tyrosine kinase 2 inhibitors (OR, 2.64).
- In a subgroup analysis, risk of acne was higher among patients using JAK inhibitors for dermatologic conditions in comparison with those using JAK inhibitors for nondermatologic conditions (OR, 4.67 vs 1.18).
- Age and gender had no apparent impact on the effect of JAK inhibitor use on acne risk.
IN PRACTICE:
“The occurrence of acne following treatment with certain classes of JAK inhibitors is of potential concern, as this adverse effect may jeopardize treatment adherence among some patients,” the researchers wrote. More studies are needed “to characterize the underlying mechanism of acne with JAK inhibitor use and to identify best practices for treatment,” they added.
SOURCE:
The lead author was Jeremy Martinez, MPH, of Harvard Medical School, Boston. The study was published online in JAMA Dermatology.
LIMITATIONS:
The review was limited by the variable classification and reporting of acne across studies, the potential exclusion of relevant studies, and the small number of studies for certain drugs.
DISCLOSURES:
The studies were mainly funded by the pharmaceutical industry. Mr. Martinez disclosed no relevant financial relationships. Several coauthors have ties with Dexcel Pharma Technologies, AbbVie, Concert, Pfizer, 3Derm Systems, Incyte, Aclaris, Eli Lilly, Concert, Equillium, ASLAN, ACOM, and Boehringer Ingelheim.
A version of this article appeared on Medscape.com.
TOPLINE:
, according to an analysis of 25 JAK inhibitor studies.
METHODOLOGY:
- Acne has been reported to be an adverse effect of JAK inhibitors, but not much is known about how common acne is overall and how incidence differs between different JAK inhibitors and the disease being treated.
- For the systematic review and meta-analysis, researchers identified 25 phase 2 or 3 randomized, controlled trials that reported acne as an adverse event associated with the use of JAK inhibitors.
- The study population included 10,839 participants (54% male, 46% female).
- The primary outcome was the incidence of acne following a period of JAK inhibitor use.
TAKEAWAY:
- Overall, the risk of acne was significantly higher among those treated with JAK inhibitors in comparison with patients given placebo in a pooled analysis (odds ratio [OR], 3.83).
- The risk of acne was highest with abrocitinib (OR, 13.47), followed by baricitinib (OR, 4.96), upadacitinib (OR, 4.79), deuruxolitinib (OR, 3.30), and deucravacitinib (OR, 2.64). By JAK inhibitor class, results were as follows: JAK1-specific inhibitors (OR, 4.69), combined JAK1 and JAK2 inhibitors (OR, 3.43), and tyrosine kinase 2 inhibitors (OR, 2.64).
- In a subgroup analysis, risk of acne was higher among patients using JAK inhibitors for dermatologic conditions in comparison with those using JAK inhibitors for nondermatologic conditions (OR, 4.67 vs 1.18).
- Age and gender had no apparent impact on the effect of JAK inhibitor use on acne risk.
IN PRACTICE:
“The occurrence of acne following treatment with certain classes of JAK inhibitors is of potential concern, as this adverse effect may jeopardize treatment adherence among some patients,” the researchers wrote. More studies are needed “to characterize the underlying mechanism of acne with JAK inhibitor use and to identify best practices for treatment,” they added.
SOURCE:
The lead author was Jeremy Martinez, MPH, of Harvard Medical School, Boston. The study was published online in JAMA Dermatology.
LIMITATIONS:
The review was limited by the variable classification and reporting of acne across studies, the potential exclusion of relevant studies, and the small number of studies for certain drugs.
DISCLOSURES:
The studies were mainly funded by the pharmaceutical industry. Mr. Martinez disclosed no relevant financial relationships. Several coauthors have ties with Dexcel Pharma Technologies, AbbVie, Concert, Pfizer, 3Derm Systems, Incyte, Aclaris, Eli Lilly, Concert, Equillium, ASLAN, ACOM, and Boehringer Ingelheim.
A version of this article appeared on Medscape.com.
Researchers tease apart multiple biologic failure in psoriasis, PsA
WASHINGTON – Multiple biologic failure in a minority of patients with psoriasis may have several causes, from genetic endotypes and immunologic factors to lower serum drug levels, the presence of anti-drug antibody levels, female sex, and certain comorbidities, Wilson Liao, MD, said at the annual research symposium of the National Psoriasis Foundation.
“Tough-to-treat psoriasis remains a challenge despite newer therapies ... Why do we still have this sub-population of patients who seem to be refractory?” said Dr. Liao, professor and associate vice chair of research in the department of dermatology at the University of California, San Francisco, who coauthored a 2015-2022 prospective cohort analysis that documented about 6% of patients failing two or more biologic agents of different mechanistic classes.
“These patients are really suffering,” he said. “We need to have better guidelines and treatment algorithms for these patients.”
A significant number of patients with psoriatic arthritis (PsA), meanwhile, are inadequate responders to tumor necrosis factor (TNF) inhibition, Christopher T. Ritchlin, MD, PhD, professor of medicine in the division of allergy/immunology and rheumatology and the Center of Musculoskeletal Research at the University of Rochester (N.Y.), said during another session at the meeting.
The long-term “persistence,” or usage, of first-line biologics in patients with PsA – and of second-line biologics in patients who failed one TNF-inhibitor – is low, but the literature offers little information on the reasons for TNF-inhibitor discontinuation, said Dr. Ritchlin, who coauthored a perspective piece in Arthritis & Rheumatology on managing the patient with PsA who fails one TNF inhibitor.
Dr. Ritchlin and his coauthors were asked to provide evidence-informed advice and algorithms, but the task was difficult. “It’s hard to know what to recommend for the next step if we don’t know why patients failed the first,” he said. “The point is, we need more data. [Clinical trials] are not recording the kind of information we need.”
Anti-drug antibodies, genetics, other factors in psoriasis
Research shows that in large cohorts, “all the biologics do seem to lose efficacy over time,” said Dr. Liao, who directs the UCSF Psoriasis and Skin Treatment Center. “Some are better than others, but we do see a loss of effectiveness over time.”
A cohort study published in 2022 in JAMA Dermatology, for instance, documented declining “drug survival” associated with ineffectiveness during 2 years of treatment for each of five biologics studied (adalimumab [Humira], ustekinumab [Stelara], secukinumab [Cosentyx], guselkumab [Tremfya], and ixekizumab [Taltz]).
“There have been a number of theories put forward” as to why that’s the case, including lower serum drug levels, “which of course can be related to anti-drug antibody production,” he said.
He pointed to two studies of ustekinumab: One prospective observational cohort study that reported an association of lower early drug levels of the IL-12/23 receptor antagonist with lower Psoriasis Area and Severity Index (PASI) scores, and another observational study that documented an association between anti-drug antibody positivity with lower ustekinumab levels and impaired clinical response.
“We also now know ... that there are genetic endotypes in psoriasis, and that patients who are [HLA-C*06:02]-positive tend to respond a little better to drugs like ustekinumab, and those who are [HLA-C*06:02]-negative tend to do a little better with the TNF inhibitors,” Dr. Liao said. The human leukocyte antigen (HLA) allele HLA-C*06:02 is associated with susceptibility to psoriasis.
In a study using a national psoriasis registry, HLA-C*06:02-negative patients were 3 times more likely to achieve PASI90 status in response to adalimumab, a TNF-alpha inhibitor, than with ustekinumab treatment. And in a meta-analysis covering eight studies with more than 1,000 patients with psoriasis, the median PASI75 response rate after 6 months of ustekinumab therapy was 92% in the HLA-C*06:02-positive group and 67% in HLA-C*06:02-negative patients.
The recently published cohort study showing a 6% rate of multiple biologic failure evaluated patients in the multicenter CorEvitas Psoriasis Registry who initiated their first biologic between 2015 and 2020 and were followed for 2 or more years. Investigators looked for sociodemographic and clinical differences between the patients who continued use of their first biologic for at least 2 years (“good response”), and those who discontinued two or more biologics of different classes, each used for at least 90 days, because of inadequate efficacy.
Of 1,039 evaluated patients, 490 (47.2%) had good clinical response to their first biologic and 65 (6.3%) had multiple biologic failure. All biologic classes were represented among those who failed multiple biologics. The first and second biologic classes used were attempted for a mean duration of 10 months – “an adequate trial” of each, Dr. Liao said.
In multivariable regression analysis, six variables were significantly associated with multiple biologic failure: female sex at birth, shorter disease duration, earlier year of biologic initiation, prior nonbiologic systemic therapy, having Medicaid insurance, and a history of hyperlipidemia. The latter is “interesting because other studies have shown that metabolic syndrome, of which hyperlipidemia is a component, can also relate to poor response to biologics,” Dr. Liao said.
The most common sequences of first-to-second biologics among those with multiple biologic failure were TNF inhibitor to IL-17 inhibitor (30.8%); IL-12/23 inhibitor to IL-17 inhibitor (21.5%); TNF inhibitor to IL-12/23 inhibitor (12.3%); and IL-17 inhibitor to IL-23 inhibitor (10.8%).
The vast majority of patients failed more than two biologics, however, and “more than 20% had five or more biologics tried over a relatively short period,” Dr. Liao said.
Comorbidities and biologic failure in psoriasis, PsA
In practice, it was said during a discussion period, biologic failures in psoriasis can be of two types: a primary inadequate response or initial failure, or a secondary failure with initial improvement followed by declining or no response. “I agree 100% that these probably represent two different endotypes,” Dr. Liao said. “There’s research emerging that psoriasis isn’t necessarily a clean phenotype.”
The option of focusing on comorbidities in the face of biologic failure was another point of discussion. “Maybe the next biologic is not the answer,” a meeting participant said. “Maybe we should focus on metabolic syndrome.”
“I agree,” Dr. Liao said. “In clinic, there are people who may not respond to therapies but have other comorbidities and factors that make it difficult to manage [their psoriasis] ... that may be causative for psoriasis. Maybe if we treat the comorbidities, it will make it easier to treat the psoriasis.”
Addressing comorbidities and “extra-articular traits” such as poorly controlled diabetes, centralized pain, anxiety and depression, and obesity is something Dr. Ritchlin advocates for PsA. “Centralized pain, I believe, is a major driver of nonresponse,” he said at the meeting. “We have to be careful about blaming nonresponse and lack of efficacy of biologics when it could be a wholly different mechanism the biologic won’t treat ... for example, centralized pain.”
As with psoriasis, the emergence of antidrug antibodies may be one reason for the secondary failure of biologic agents for PsA, Dr. Ritchlin and his coauthors wrote in their paper on management of PsA after failure of one TNF inhibitor. Other areas to consider in evaluating failure, they wrote, are compliance and time of dosing, and financial barriers.
Low long-term persistence of second-line biologics for patients with PsA was demonstrated in a national cohort study utilizing the French health insurance database, Dr. Ritchlin noted at the research meeting.
The French study covered almost 3,000 patients who started a second biologic after discontinuing a TNF inhibitor during 2015-2020. Overall, 1-year and 3-year persistence rates were 42% and 17%, respectively.
Dr. Liao disclosed research grant funding from AbbVie, Amgen, Janssen, Leo, Novartis, Pfizer, Regeneron, and Trex Bio. Dr. Ritchlin reported no disclosures.
WASHINGTON – Multiple biologic failure in a minority of patients with psoriasis may have several causes, from genetic endotypes and immunologic factors to lower serum drug levels, the presence of anti-drug antibody levels, female sex, and certain comorbidities, Wilson Liao, MD, said at the annual research symposium of the National Psoriasis Foundation.
“Tough-to-treat psoriasis remains a challenge despite newer therapies ... Why do we still have this sub-population of patients who seem to be refractory?” said Dr. Liao, professor and associate vice chair of research in the department of dermatology at the University of California, San Francisco, who coauthored a 2015-2022 prospective cohort analysis that documented about 6% of patients failing two or more biologic agents of different mechanistic classes.
“These patients are really suffering,” he said. “We need to have better guidelines and treatment algorithms for these patients.”
A significant number of patients with psoriatic arthritis (PsA), meanwhile, are inadequate responders to tumor necrosis factor (TNF) inhibition, Christopher T. Ritchlin, MD, PhD, professor of medicine in the division of allergy/immunology and rheumatology and the Center of Musculoskeletal Research at the University of Rochester (N.Y.), said during another session at the meeting.
The long-term “persistence,” or usage, of first-line biologics in patients with PsA – and of second-line biologics in patients who failed one TNF-inhibitor – is low, but the literature offers little information on the reasons for TNF-inhibitor discontinuation, said Dr. Ritchlin, who coauthored a perspective piece in Arthritis & Rheumatology on managing the patient with PsA who fails one TNF inhibitor.
Dr. Ritchlin and his coauthors were asked to provide evidence-informed advice and algorithms, but the task was difficult. “It’s hard to know what to recommend for the next step if we don’t know why patients failed the first,” he said. “The point is, we need more data. [Clinical trials] are not recording the kind of information we need.”
Anti-drug antibodies, genetics, other factors in psoriasis
Research shows that in large cohorts, “all the biologics do seem to lose efficacy over time,” said Dr. Liao, who directs the UCSF Psoriasis and Skin Treatment Center. “Some are better than others, but we do see a loss of effectiveness over time.”
A cohort study published in 2022 in JAMA Dermatology, for instance, documented declining “drug survival” associated with ineffectiveness during 2 years of treatment for each of five biologics studied (adalimumab [Humira], ustekinumab [Stelara], secukinumab [Cosentyx], guselkumab [Tremfya], and ixekizumab [Taltz]).
“There have been a number of theories put forward” as to why that’s the case, including lower serum drug levels, “which of course can be related to anti-drug antibody production,” he said.
He pointed to two studies of ustekinumab: One prospective observational cohort study that reported an association of lower early drug levels of the IL-12/23 receptor antagonist with lower Psoriasis Area and Severity Index (PASI) scores, and another observational study that documented an association between anti-drug antibody positivity with lower ustekinumab levels and impaired clinical response.
“We also now know ... that there are genetic endotypes in psoriasis, and that patients who are [HLA-C*06:02]-positive tend to respond a little better to drugs like ustekinumab, and those who are [HLA-C*06:02]-negative tend to do a little better with the TNF inhibitors,” Dr. Liao said. The human leukocyte antigen (HLA) allele HLA-C*06:02 is associated with susceptibility to psoriasis.
In a study using a national psoriasis registry, HLA-C*06:02-negative patients were 3 times more likely to achieve PASI90 status in response to adalimumab, a TNF-alpha inhibitor, than with ustekinumab treatment. And in a meta-analysis covering eight studies with more than 1,000 patients with psoriasis, the median PASI75 response rate after 6 months of ustekinumab therapy was 92% in the HLA-C*06:02-positive group and 67% in HLA-C*06:02-negative patients.
The recently published cohort study showing a 6% rate of multiple biologic failure evaluated patients in the multicenter CorEvitas Psoriasis Registry who initiated their first biologic between 2015 and 2020 and were followed for 2 or more years. Investigators looked for sociodemographic and clinical differences between the patients who continued use of their first biologic for at least 2 years (“good response”), and those who discontinued two or more biologics of different classes, each used for at least 90 days, because of inadequate efficacy.
Of 1,039 evaluated patients, 490 (47.2%) had good clinical response to their first biologic and 65 (6.3%) had multiple biologic failure. All biologic classes were represented among those who failed multiple biologics. The first and second biologic classes used were attempted for a mean duration of 10 months – “an adequate trial” of each, Dr. Liao said.
In multivariable regression analysis, six variables were significantly associated with multiple biologic failure: female sex at birth, shorter disease duration, earlier year of biologic initiation, prior nonbiologic systemic therapy, having Medicaid insurance, and a history of hyperlipidemia. The latter is “interesting because other studies have shown that metabolic syndrome, of which hyperlipidemia is a component, can also relate to poor response to biologics,” Dr. Liao said.
The most common sequences of first-to-second biologics among those with multiple biologic failure were TNF inhibitor to IL-17 inhibitor (30.8%); IL-12/23 inhibitor to IL-17 inhibitor (21.5%); TNF inhibitor to IL-12/23 inhibitor (12.3%); and IL-17 inhibitor to IL-23 inhibitor (10.8%).
The vast majority of patients failed more than two biologics, however, and “more than 20% had five or more biologics tried over a relatively short period,” Dr. Liao said.
Comorbidities and biologic failure in psoriasis, PsA
In practice, it was said during a discussion period, biologic failures in psoriasis can be of two types: a primary inadequate response or initial failure, or a secondary failure with initial improvement followed by declining or no response. “I agree 100% that these probably represent two different endotypes,” Dr. Liao said. “There’s research emerging that psoriasis isn’t necessarily a clean phenotype.”
The option of focusing on comorbidities in the face of biologic failure was another point of discussion. “Maybe the next biologic is not the answer,” a meeting participant said. “Maybe we should focus on metabolic syndrome.”
“I agree,” Dr. Liao said. “In clinic, there are people who may not respond to therapies but have other comorbidities and factors that make it difficult to manage [their psoriasis] ... that may be causative for psoriasis. Maybe if we treat the comorbidities, it will make it easier to treat the psoriasis.”
Addressing comorbidities and “extra-articular traits” such as poorly controlled diabetes, centralized pain, anxiety and depression, and obesity is something Dr. Ritchlin advocates for PsA. “Centralized pain, I believe, is a major driver of nonresponse,” he said at the meeting. “We have to be careful about blaming nonresponse and lack of efficacy of biologics when it could be a wholly different mechanism the biologic won’t treat ... for example, centralized pain.”
As with psoriasis, the emergence of antidrug antibodies may be one reason for the secondary failure of biologic agents for PsA, Dr. Ritchlin and his coauthors wrote in their paper on management of PsA after failure of one TNF inhibitor. Other areas to consider in evaluating failure, they wrote, are compliance and time of dosing, and financial barriers.
Low long-term persistence of second-line biologics for patients with PsA was demonstrated in a national cohort study utilizing the French health insurance database, Dr. Ritchlin noted at the research meeting.
The French study covered almost 3,000 patients who started a second biologic after discontinuing a TNF inhibitor during 2015-2020. Overall, 1-year and 3-year persistence rates were 42% and 17%, respectively.
Dr. Liao disclosed research grant funding from AbbVie, Amgen, Janssen, Leo, Novartis, Pfizer, Regeneron, and Trex Bio. Dr. Ritchlin reported no disclosures.
WASHINGTON – Multiple biologic failure in a minority of patients with psoriasis may have several causes, from genetic endotypes and immunologic factors to lower serum drug levels, the presence of anti-drug antibody levels, female sex, and certain comorbidities, Wilson Liao, MD, said at the annual research symposium of the National Psoriasis Foundation.
“Tough-to-treat psoriasis remains a challenge despite newer therapies ... Why do we still have this sub-population of patients who seem to be refractory?” said Dr. Liao, professor and associate vice chair of research in the department of dermatology at the University of California, San Francisco, who coauthored a 2015-2022 prospective cohort analysis that documented about 6% of patients failing two or more biologic agents of different mechanistic classes.
“These patients are really suffering,” he said. “We need to have better guidelines and treatment algorithms for these patients.”
A significant number of patients with psoriatic arthritis (PsA), meanwhile, are inadequate responders to tumor necrosis factor (TNF) inhibition, Christopher T. Ritchlin, MD, PhD, professor of medicine in the division of allergy/immunology and rheumatology and the Center of Musculoskeletal Research at the University of Rochester (N.Y.), said during another session at the meeting.
The long-term “persistence,” or usage, of first-line biologics in patients with PsA – and of second-line biologics in patients who failed one TNF-inhibitor – is low, but the literature offers little information on the reasons for TNF-inhibitor discontinuation, said Dr. Ritchlin, who coauthored a perspective piece in Arthritis & Rheumatology on managing the patient with PsA who fails one TNF inhibitor.
Dr. Ritchlin and his coauthors were asked to provide evidence-informed advice and algorithms, but the task was difficult. “It’s hard to know what to recommend for the next step if we don’t know why patients failed the first,” he said. “The point is, we need more data. [Clinical trials] are not recording the kind of information we need.”
Anti-drug antibodies, genetics, other factors in psoriasis
Research shows that in large cohorts, “all the biologics do seem to lose efficacy over time,” said Dr. Liao, who directs the UCSF Psoriasis and Skin Treatment Center. “Some are better than others, but we do see a loss of effectiveness over time.”
A cohort study published in 2022 in JAMA Dermatology, for instance, documented declining “drug survival” associated with ineffectiveness during 2 years of treatment for each of five biologics studied (adalimumab [Humira], ustekinumab [Stelara], secukinumab [Cosentyx], guselkumab [Tremfya], and ixekizumab [Taltz]).
“There have been a number of theories put forward” as to why that’s the case, including lower serum drug levels, “which of course can be related to anti-drug antibody production,” he said.
He pointed to two studies of ustekinumab: One prospective observational cohort study that reported an association of lower early drug levels of the IL-12/23 receptor antagonist with lower Psoriasis Area and Severity Index (PASI) scores, and another observational study that documented an association between anti-drug antibody positivity with lower ustekinumab levels and impaired clinical response.
“We also now know ... that there are genetic endotypes in psoriasis, and that patients who are [HLA-C*06:02]-positive tend to respond a little better to drugs like ustekinumab, and those who are [HLA-C*06:02]-negative tend to do a little better with the TNF inhibitors,” Dr. Liao said. The human leukocyte antigen (HLA) allele HLA-C*06:02 is associated with susceptibility to psoriasis.
In a study using a national psoriasis registry, HLA-C*06:02-negative patients were 3 times more likely to achieve PASI90 status in response to adalimumab, a TNF-alpha inhibitor, than with ustekinumab treatment. And in a meta-analysis covering eight studies with more than 1,000 patients with psoriasis, the median PASI75 response rate after 6 months of ustekinumab therapy was 92% in the HLA-C*06:02-positive group and 67% in HLA-C*06:02-negative patients.
The recently published cohort study showing a 6% rate of multiple biologic failure evaluated patients in the multicenter CorEvitas Psoriasis Registry who initiated their first biologic between 2015 and 2020 and were followed for 2 or more years. Investigators looked for sociodemographic and clinical differences between the patients who continued use of their first biologic for at least 2 years (“good response”), and those who discontinued two or more biologics of different classes, each used for at least 90 days, because of inadequate efficacy.
Of 1,039 evaluated patients, 490 (47.2%) had good clinical response to their first biologic and 65 (6.3%) had multiple biologic failure. All biologic classes were represented among those who failed multiple biologics. The first and second biologic classes used were attempted for a mean duration of 10 months – “an adequate trial” of each, Dr. Liao said.
In multivariable regression analysis, six variables were significantly associated with multiple biologic failure: female sex at birth, shorter disease duration, earlier year of biologic initiation, prior nonbiologic systemic therapy, having Medicaid insurance, and a history of hyperlipidemia. The latter is “interesting because other studies have shown that metabolic syndrome, of which hyperlipidemia is a component, can also relate to poor response to biologics,” Dr. Liao said.
The most common sequences of first-to-second biologics among those with multiple biologic failure were TNF inhibitor to IL-17 inhibitor (30.8%); IL-12/23 inhibitor to IL-17 inhibitor (21.5%); TNF inhibitor to IL-12/23 inhibitor (12.3%); and IL-17 inhibitor to IL-23 inhibitor (10.8%).
The vast majority of patients failed more than two biologics, however, and “more than 20% had five or more biologics tried over a relatively short period,” Dr. Liao said.
Comorbidities and biologic failure in psoriasis, PsA
In practice, it was said during a discussion period, biologic failures in psoriasis can be of two types: a primary inadequate response or initial failure, or a secondary failure with initial improvement followed by declining or no response. “I agree 100% that these probably represent two different endotypes,” Dr. Liao said. “There’s research emerging that psoriasis isn’t necessarily a clean phenotype.”
The option of focusing on comorbidities in the face of biologic failure was another point of discussion. “Maybe the next biologic is not the answer,” a meeting participant said. “Maybe we should focus on metabolic syndrome.”
“I agree,” Dr. Liao said. “In clinic, there are people who may not respond to therapies but have other comorbidities and factors that make it difficult to manage [their psoriasis] ... that may be causative for psoriasis. Maybe if we treat the comorbidities, it will make it easier to treat the psoriasis.”
Addressing comorbidities and “extra-articular traits” such as poorly controlled diabetes, centralized pain, anxiety and depression, and obesity is something Dr. Ritchlin advocates for PsA. “Centralized pain, I believe, is a major driver of nonresponse,” he said at the meeting. “We have to be careful about blaming nonresponse and lack of efficacy of biologics when it could be a wholly different mechanism the biologic won’t treat ... for example, centralized pain.”
As with psoriasis, the emergence of antidrug antibodies may be one reason for the secondary failure of biologic agents for PsA, Dr. Ritchlin and his coauthors wrote in their paper on management of PsA after failure of one TNF inhibitor. Other areas to consider in evaluating failure, they wrote, are compliance and time of dosing, and financial barriers.
Low long-term persistence of second-line biologics for patients with PsA was demonstrated in a national cohort study utilizing the French health insurance database, Dr. Ritchlin noted at the research meeting.
The French study covered almost 3,000 patients who started a second biologic after discontinuing a TNF inhibitor during 2015-2020. Overall, 1-year and 3-year persistence rates were 42% and 17%, respectively.
Dr. Liao disclosed research grant funding from AbbVie, Amgen, Janssen, Leo, Novartis, Pfizer, Regeneron, and Trex Bio. Dr. Ritchlin reported no disclosures.
AT THE NPF RESEARCH SYMPOSIUM 2023
Most patients with psoriasis not engaged in highly shared decision-making
TOPLINE:
METHODOLOGY:
- Researchers drew from the 2014-2017 and 2019 Medical Expenditure Panel Survey (MEPS) to identify 3,715,027 patients with psoriasis, to evaluate the association between SDM (a patient-centered approach to selecting treatment on the basis of a discussion between the clinician and patient) and satisfaction with care.
- SDM was determined by patient responses on a 4-point Likert scale to seven MEPS variables, including the question, “How often did doctors or other health providers listen carefully to you?”
- Patient satisfaction with care was measured with a MEPS variable that asked respondents to rate their health care providers on a scale of 1-10.
- Researchers used multiple logistic regression to assess the association between SDM and demographic and clinical characteristics in patients with psoriasis, and multiple linear regression analysis to assess the association between SDM and patient satisfaction with care.
TAKEAWAY:
- The average SDM score was 3.6 out of 4, and the average satisfaction with care score was 8.6 out of 10.
- However, only about 42% of the cohort reported a high SDM, defined as a score of 3.9 or greater.
- After adjusting for covariates, the researchers found that patients who had high SDM had, on average, 85% higher satisfaction with care (P < .001).
- Compared with men, women had about 27% higher satisfaction with care (P = .023), whereas non-Hispanic patients had lower satisfaction with care compared with Hispanic patients (P = .037).
IN PRACTICE:
“It is important to construct a framework for carrying out SDM with patients with psoriasis to enhance clinician-patient communication and improve patient outcomes,” the authors concluded.
SOURCE:
April W. Armstrong, MD, MPH, chief of dermatology at the University of California, Los Angeles, led the research. The study was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The ability to measure SDM in patients with psoriasis was limited by the seven items from MEPS. The diagnosis of psoriasis was based on self-report.
DISCLOSURES:
The study was funded by the National Psoriasis Foundation. Dr. Armstrong disclosed that she has served as a research investigator and/or scientific adviser to AbbVie, Almirall, Arcutis, ASLAN, Beiersdorf, Boehringer Ingelheim, Bristol Myers Squibb, EPI, Incyte, Leo, UCB, Janssen, Lilly, Nimbus, Novartis, Ortho Dermatologics, Sun, Dermavant, Dermira, Sanofi, Regeneron, Pfizer, and Modmed.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers drew from the 2014-2017 and 2019 Medical Expenditure Panel Survey (MEPS) to identify 3,715,027 patients with psoriasis, to evaluate the association between SDM (a patient-centered approach to selecting treatment on the basis of a discussion between the clinician and patient) and satisfaction with care.
- SDM was determined by patient responses on a 4-point Likert scale to seven MEPS variables, including the question, “How often did doctors or other health providers listen carefully to you?”
- Patient satisfaction with care was measured with a MEPS variable that asked respondents to rate their health care providers on a scale of 1-10.
- Researchers used multiple logistic regression to assess the association between SDM and demographic and clinical characteristics in patients with psoriasis, and multiple linear regression analysis to assess the association between SDM and patient satisfaction with care.
TAKEAWAY:
- The average SDM score was 3.6 out of 4, and the average satisfaction with care score was 8.6 out of 10.
- However, only about 42% of the cohort reported a high SDM, defined as a score of 3.9 or greater.
- After adjusting for covariates, the researchers found that patients who had high SDM had, on average, 85% higher satisfaction with care (P < .001).
- Compared with men, women had about 27% higher satisfaction with care (P = .023), whereas non-Hispanic patients had lower satisfaction with care compared with Hispanic patients (P = .037).
IN PRACTICE:
“It is important to construct a framework for carrying out SDM with patients with psoriasis to enhance clinician-patient communication and improve patient outcomes,” the authors concluded.
SOURCE:
April W. Armstrong, MD, MPH, chief of dermatology at the University of California, Los Angeles, led the research. The study was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The ability to measure SDM in patients with psoriasis was limited by the seven items from MEPS. The diagnosis of psoriasis was based on self-report.
DISCLOSURES:
The study was funded by the National Psoriasis Foundation. Dr. Armstrong disclosed that she has served as a research investigator and/or scientific adviser to AbbVie, Almirall, Arcutis, ASLAN, Beiersdorf, Boehringer Ingelheim, Bristol Myers Squibb, EPI, Incyte, Leo, UCB, Janssen, Lilly, Nimbus, Novartis, Ortho Dermatologics, Sun, Dermavant, Dermira, Sanofi, Regeneron, Pfizer, and Modmed.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers drew from the 2014-2017 and 2019 Medical Expenditure Panel Survey (MEPS) to identify 3,715,027 patients with psoriasis, to evaluate the association between SDM (a patient-centered approach to selecting treatment on the basis of a discussion between the clinician and patient) and satisfaction with care.
- SDM was determined by patient responses on a 4-point Likert scale to seven MEPS variables, including the question, “How often did doctors or other health providers listen carefully to you?”
- Patient satisfaction with care was measured with a MEPS variable that asked respondents to rate their health care providers on a scale of 1-10.
- Researchers used multiple logistic regression to assess the association between SDM and demographic and clinical characteristics in patients with psoriasis, and multiple linear regression analysis to assess the association between SDM and patient satisfaction with care.
TAKEAWAY:
- The average SDM score was 3.6 out of 4, and the average satisfaction with care score was 8.6 out of 10.
- However, only about 42% of the cohort reported a high SDM, defined as a score of 3.9 or greater.
- After adjusting for covariates, the researchers found that patients who had high SDM had, on average, 85% higher satisfaction with care (P < .001).
- Compared with men, women had about 27% higher satisfaction with care (P = .023), whereas non-Hispanic patients had lower satisfaction with care compared with Hispanic patients (P = .037).
IN PRACTICE:
“It is important to construct a framework for carrying out SDM with patients with psoriasis to enhance clinician-patient communication and improve patient outcomes,” the authors concluded.
SOURCE:
April W. Armstrong, MD, MPH, chief of dermatology at the University of California, Los Angeles, led the research. The study was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The ability to measure SDM in patients with psoriasis was limited by the seven items from MEPS. The diagnosis of psoriasis was based on self-report.
DISCLOSURES:
The study was funded by the National Psoriasis Foundation. Dr. Armstrong disclosed that she has served as a research investigator and/or scientific adviser to AbbVie, Almirall, Arcutis, ASLAN, Beiersdorf, Boehringer Ingelheim, Bristol Myers Squibb, EPI, Incyte, Leo, UCB, Janssen, Lilly, Nimbus, Novartis, Ortho Dermatologics, Sun, Dermavant, Dermira, Sanofi, Regeneron, Pfizer, and Modmed.
A version of this article first appeared on Medscape.com.
FDA OKs first ustekinumab biosimilar
The U.S. Food and Drug Administration has approved ustekinumab-auub (Wezlana) as a biosimilar to ustekinumab (Stelara) for the treatment of multiple inflammatory conditions. This is the first approval for a ustekinumab biosimilar in the United States.
Ustekinumab-auub was also granted an interchangeability designation, meaning that, depending on state law, a pharmacist may substitute the biosimilar for the reference product without consulting the prescribing provider.
“Today’s approval exemplifies the FDA’s longstanding commitment to support a competitive marketplace for biological products,” Sarah Yim, MD, director of the Office of Therapeutic Biologics and Biosimilars in the FDA’s Center for Drug Evaluation and Research, said in a statement. “This approval can empower patients by helping to increase access to safe, effective, and high-quality medications at potentially lower cost.”
Ustekinumab, manufactured by Johnson & Johnson, targets interleukin-12 and IL-23 and was first approved in 2009. Ustekinumab-auub was developed by Amgen.
Ustekinumab-auub is approved for the treatment of adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy, active psoriatic arthritis, moderate to severely active Crohn’s disease, and moderate to severely active ulcerative colitis. It is also approved for pediatric patients aged 6 years and older with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy and active psoriatic arthritis.
The approval was based on “comprehensive review of scientific evidence,” including “comparisons of the products on an analytical level using an extensive battery of chemical and biological tests and biological assays that confirmed similarity in the structural and functional features of Wezlana and Stelara (including those known to impact safety and efficacy), and comparative human pharmacokinetic data, clinical immunogenicity data, and other clinical safety and effectiveness data,” the FDA said.
Some common side effects of ustekinumab-auub include nasopharyngitis, upper respiratory tract infection, headache, fatigue, and nausea. The most severe side effect of the biosimilar, as with the reference drug ustekinumab, is infection.
The product launch of ustekinumab-auub will be delayed as a part of a settlement of Johnson & Johnson’s lawsuit against Amgen, according to Reuters. The details of the settlement are confidential, but it was stated that the biosimilar would be available by Jan. 1, 2025.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved ustekinumab-auub (Wezlana) as a biosimilar to ustekinumab (Stelara) for the treatment of multiple inflammatory conditions. This is the first approval for a ustekinumab biosimilar in the United States.
Ustekinumab-auub was also granted an interchangeability designation, meaning that, depending on state law, a pharmacist may substitute the biosimilar for the reference product without consulting the prescribing provider.
“Today’s approval exemplifies the FDA’s longstanding commitment to support a competitive marketplace for biological products,” Sarah Yim, MD, director of the Office of Therapeutic Biologics and Biosimilars in the FDA’s Center for Drug Evaluation and Research, said in a statement. “This approval can empower patients by helping to increase access to safe, effective, and high-quality medications at potentially lower cost.”
Ustekinumab, manufactured by Johnson & Johnson, targets interleukin-12 and IL-23 and was first approved in 2009. Ustekinumab-auub was developed by Amgen.
Ustekinumab-auub is approved for the treatment of adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy, active psoriatic arthritis, moderate to severely active Crohn’s disease, and moderate to severely active ulcerative colitis. It is also approved for pediatric patients aged 6 years and older with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy and active psoriatic arthritis.
The approval was based on “comprehensive review of scientific evidence,” including “comparisons of the products on an analytical level using an extensive battery of chemical and biological tests and biological assays that confirmed similarity in the structural and functional features of Wezlana and Stelara (including those known to impact safety and efficacy), and comparative human pharmacokinetic data, clinical immunogenicity data, and other clinical safety and effectiveness data,” the FDA said.
Some common side effects of ustekinumab-auub include nasopharyngitis, upper respiratory tract infection, headache, fatigue, and nausea. The most severe side effect of the biosimilar, as with the reference drug ustekinumab, is infection.
The product launch of ustekinumab-auub will be delayed as a part of a settlement of Johnson & Johnson’s lawsuit against Amgen, according to Reuters. The details of the settlement are confidential, but it was stated that the biosimilar would be available by Jan. 1, 2025.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved ustekinumab-auub (Wezlana) as a biosimilar to ustekinumab (Stelara) for the treatment of multiple inflammatory conditions. This is the first approval for a ustekinumab biosimilar in the United States.
Ustekinumab-auub was also granted an interchangeability designation, meaning that, depending on state law, a pharmacist may substitute the biosimilar for the reference product without consulting the prescribing provider.
“Today’s approval exemplifies the FDA’s longstanding commitment to support a competitive marketplace for biological products,” Sarah Yim, MD, director of the Office of Therapeutic Biologics and Biosimilars in the FDA’s Center for Drug Evaluation and Research, said in a statement. “This approval can empower patients by helping to increase access to safe, effective, and high-quality medications at potentially lower cost.”
Ustekinumab, manufactured by Johnson & Johnson, targets interleukin-12 and IL-23 and was first approved in 2009. Ustekinumab-auub was developed by Amgen.
Ustekinumab-auub is approved for the treatment of adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy, active psoriatic arthritis, moderate to severely active Crohn’s disease, and moderate to severely active ulcerative colitis. It is also approved for pediatric patients aged 6 years and older with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy and active psoriatic arthritis.
The approval was based on “comprehensive review of scientific evidence,” including “comparisons of the products on an analytical level using an extensive battery of chemical and biological tests and biological assays that confirmed similarity in the structural and functional features of Wezlana and Stelara (including those known to impact safety and efficacy), and comparative human pharmacokinetic data, clinical immunogenicity data, and other clinical safety and effectiveness data,” the FDA said.
Some common side effects of ustekinumab-auub include nasopharyngitis, upper respiratory tract infection, headache, fatigue, and nausea. The most severe side effect of the biosimilar, as with the reference drug ustekinumab, is infection.
The product launch of ustekinumab-auub will be delayed as a part of a settlement of Johnson & Johnson’s lawsuit against Amgen, according to Reuters. The details of the settlement are confidential, but it was stated that the biosimilar would be available by Jan. 1, 2025.
A version of this article first appeared on Medscape.com.
Review finds no CV or VTE risk signal with use of JAK inhibitors for skin indications
, results from a systematic literature review, and meta-analysis showed.
“There remains a knowledge gap regarding the risk of JAK inhibitor use and VTE and/or MACE in the dermatologic population,” researchers led by Michael S. Garshick, MD, a cardiologist at New York University Langone Health, wrote in their study, which was published online in JAMA Dermatology . “Pooled safety studies suggest that the risk of MACE and VTE may be lower in patients treated with JAK inhibitors for a dermatologic indication than the risk observed in the ORAL Surveillance study, which may be related to the younger age and better health status of those enrolled in trials for dermatologic indications.” The results of that study, which included patients with rheumatoid arthritis only, resulted in the addition of a boxed warning in the labels for topical and oral JAK inhibitors regarding the increased risk of MACE, VTE, serious infections, malignancies, and death .
For the review – thought to be the first to specifically evaluate these risks for dermatologic indications – the researchers searched PubMed and ClinicalTrials.gov from inception through April 1, 2023, for phase 3 dermatology randomized clinical trials (RCTs) to evaluate the risk of MACE, VTE, and all-cause mortality with JAK inhibitors, compared with placebo or an active comparator in the treatment of immune-mediated inflammatory skin diseases. They followed Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines and used a random-effects model and the DerSimonian-Laird method to calculate adverse events with odds ratios.
The database search yielded 35 RCTs with a total of 20,651 patients. Their mean age was 38.5 years, 54% were male, and the mean follow-up time was 4.9 months. Of the 35 trials, most (21) involved patients with atopic dermatitis, followed by psoriasis/psoriatic arthritis (9 trials), alopecia areata (3 trials) and vitiligo (2 trials).
The researchers found no significant difference between JAK inhibitors and placebo/active comparator in composite MACE and all-cause mortality (odds ratio, 0.83; 95% confidence interval, 0.44-1.57) or in VTE (OR, 0.52; 95% CI, 0.26-1.04).
In a secondary analysis, which included additional psoriatic arthritis RCTs, no significant differences between the treatment and placebo/active comparator groups were observed. Similarly, subgroup analyses of oral versus topical JAK inhibitors and a sensitivity analysis that excluded pediatric trials showed no significant differences between patients exposed to JAK inhibitors and those not exposed.
The researchers acknowledged certain limitations of the review, including the lack of access to patient-level data, the fact that most trials only included short-term follow-up, and that the findings have limited generalizability to an older patient population. “It remains unclear if the cardiovascular risks of JAK inhibitors are primarily due to patient level cardiovascular risk factors or are drug mediated,” they concluded. “Dermatologists should carefully select patients and assess baseline cardiovascular risk factors when considering JAK therapy. Cardiovascular risk assessment should continue for the duration of treatment.”
Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the center for eczema and itch at Northwestern University, Chicago, who was asked to comment on the study results, characterized the findings as reassuring to dermatologists who may be reluctant to initiate therapy with JAK inhibitors based on concerns about safety signals for MACE, VTE, and all-cause mortality.
“These data systematically show that across medications and across conditions, there doesn’t appear to be an increased signal for these events during the short-term, placebo-controlled period which generally spans a few months in most studies,” he told this news organization. The findings, he added, “align well with our clinical experience to date for JAK inhibitor use in inflammatory skin disease. Short-term safety, particularly in relation to boxed warning events such MACE, VTE, and all-cause mortality, have generally been favorable with real-world use. It’s good to have a rigorous statistical analysis to refer to when setting patient expectations.”
However, he noted that these data only examined short-term safety during the placebo or active comparator-controlled periods. “Considering that events like MACE or VTE may take many months or years to manifest, continued long-term data generation is needed to fully answer the question of risk,” he said.
Dr. Garshick disclosed that he received grants from Pfizer and personal fees from Bristol Myers Squibb during the conduct of the study and personal fees from Kiniksa Pharmaceuticals outside the submitted work. Several other coauthors reported having advisory board roles and/or having received funding or support from several pharmaceutical companies. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, investigator, and/or a member of the advisory board for several pharmaceutical companies, including those that develop JAK inhibitors.
, results from a systematic literature review, and meta-analysis showed.
“There remains a knowledge gap regarding the risk of JAK inhibitor use and VTE and/or MACE in the dermatologic population,” researchers led by Michael S. Garshick, MD, a cardiologist at New York University Langone Health, wrote in their study, which was published online in JAMA Dermatology . “Pooled safety studies suggest that the risk of MACE and VTE may be lower in patients treated with JAK inhibitors for a dermatologic indication than the risk observed in the ORAL Surveillance study, which may be related to the younger age and better health status of those enrolled in trials for dermatologic indications.” The results of that study, which included patients with rheumatoid arthritis only, resulted in the addition of a boxed warning in the labels for topical and oral JAK inhibitors regarding the increased risk of MACE, VTE, serious infections, malignancies, and death .
For the review – thought to be the first to specifically evaluate these risks for dermatologic indications – the researchers searched PubMed and ClinicalTrials.gov from inception through April 1, 2023, for phase 3 dermatology randomized clinical trials (RCTs) to evaluate the risk of MACE, VTE, and all-cause mortality with JAK inhibitors, compared with placebo or an active comparator in the treatment of immune-mediated inflammatory skin diseases. They followed Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines and used a random-effects model and the DerSimonian-Laird method to calculate adverse events with odds ratios.
The database search yielded 35 RCTs with a total of 20,651 patients. Their mean age was 38.5 years, 54% were male, and the mean follow-up time was 4.9 months. Of the 35 trials, most (21) involved patients with atopic dermatitis, followed by psoriasis/psoriatic arthritis (9 trials), alopecia areata (3 trials) and vitiligo (2 trials).
The researchers found no significant difference between JAK inhibitors and placebo/active comparator in composite MACE and all-cause mortality (odds ratio, 0.83; 95% confidence interval, 0.44-1.57) or in VTE (OR, 0.52; 95% CI, 0.26-1.04).
In a secondary analysis, which included additional psoriatic arthritis RCTs, no significant differences between the treatment and placebo/active comparator groups were observed. Similarly, subgroup analyses of oral versus topical JAK inhibitors and a sensitivity analysis that excluded pediatric trials showed no significant differences between patients exposed to JAK inhibitors and those not exposed.
The researchers acknowledged certain limitations of the review, including the lack of access to patient-level data, the fact that most trials only included short-term follow-up, and that the findings have limited generalizability to an older patient population. “It remains unclear if the cardiovascular risks of JAK inhibitors are primarily due to patient level cardiovascular risk factors or are drug mediated,” they concluded. “Dermatologists should carefully select patients and assess baseline cardiovascular risk factors when considering JAK therapy. Cardiovascular risk assessment should continue for the duration of treatment.”
Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the center for eczema and itch at Northwestern University, Chicago, who was asked to comment on the study results, characterized the findings as reassuring to dermatologists who may be reluctant to initiate therapy with JAK inhibitors based on concerns about safety signals for MACE, VTE, and all-cause mortality.
“These data systematically show that across medications and across conditions, there doesn’t appear to be an increased signal for these events during the short-term, placebo-controlled period which generally spans a few months in most studies,” he told this news organization. The findings, he added, “align well with our clinical experience to date for JAK inhibitor use in inflammatory skin disease. Short-term safety, particularly in relation to boxed warning events such MACE, VTE, and all-cause mortality, have generally been favorable with real-world use. It’s good to have a rigorous statistical analysis to refer to when setting patient expectations.”
However, he noted that these data only examined short-term safety during the placebo or active comparator-controlled periods. “Considering that events like MACE or VTE may take many months or years to manifest, continued long-term data generation is needed to fully answer the question of risk,” he said.
Dr. Garshick disclosed that he received grants from Pfizer and personal fees from Bristol Myers Squibb during the conduct of the study and personal fees from Kiniksa Pharmaceuticals outside the submitted work. Several other coauthors reported having advisory board roles and/or having received funding or support from several pharmaceutical companies. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, investigator, and/or a member of the advisory board for several pharmaceutical companies, including those that develop JAK inhibitors.
, results from a systematic literature review, and meta-analysis showed.
“There remains a knowledge gap regarding the risk of JAK inhibitor use and VTE and/or MACE in the dermatologic population,” researchers led by Michael S. Garshick, MD, a cardiologist at New York University Langone Health, wrote in their study, which was published online in JAMA Dermatology . “Pooled safety studies suggest that the risk of MACE and VTE may be lower in patients treated with JAK inhibitors for a dermatologic indication than the risk observed in the ORAL Surveillance study, which may be related to the younger age and better health status of those enrolled in trials for dermatologic indications.” The results of that study, which included patients with rheumatoid arthritis only, resulted in the addition of a boxed warning in the labels for topical and oral JAK inhibitors regarding the increased risk of MACE, VTE, serious infections, malignancies, and death .
For the review – thought to be the first to specifically evaluate these risks for dermatologic indications – the researchers searched PubMed and ClinicalTrials.gov from inception through April 1, 2023, for phase 3 dermatology randomized clinical trials (RCTs) to evaluate the risk of MACE, VTE, and all-cause mortality with JAK inhibitors, compared with placebo or an active comparator in the treatment of immune-mediated inflammatory skin diseases. They followed Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines and used a random-effects model and the DerSimonian-Laird method to calculate adverse events with odds ratios.
The database search yielded 35 RCTs with a total of 20,651 patients. Their mean age was 38.5 years, 54% were male, and the mean follow-up time was 4.9 months. Of the 35 trials, most (21) involved patients with atopic dermatitis, followed by psoriasis/psoriatic arthritis (9 trials), alopecia areata (3 trials) and vitiligo (2 trials).
The researchers found no significant difference between JAK inhibitors and placebo/active comparator in composite MACE and all-cause mortality (odds ratio, 0.83; 95% confidence interval, 0.44-1.57) or in VTE (OR, 0.52; 95% CI, 0.26-1.04).
In a secondary analysis, which included additional psoriatic arthritis RCTs, no significant differences between the treatment and placebo/active comparator groups were observed. Similarly, subgroup analyses of oral versus topical JAK inhibitors and a sensitivity analysis that excluded pediatric trials showed no significant differences between patients exposed to JAK inhibitors and those not exposed.
The researchers acknowledged certain limitations of the review, including the lack of access to patient-level data, the fact that most trials only included short-term follow-up, and that the findings have limited generalizability to an older patient population. “It remains unclear if the cardiovascular risks of JAK inhibitors are primarily due to patient level cardiovascular risk factors or are drug mediated,” they concluded. “Dermatologists should carefully select patients and assess baseline cardiovascular risk factors when considering JAK therapy. Cardiovascular risk assessment should continue for the duration of treatment.”
Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the center for eczema and itch at Northwestern University, Chicago, who was asked to comment on the study results, characterized the findings as reassuring to dermatologists who may be reluctant to initiate therapy with JAK inhibitors based on concerns about safety signals for MACE, VTE, and all-cause mortality.
“These data systematically show that across medications and across conditions, there doesn’t appear to be an increased signal for these events during the short-term, placebo-controlled period which generally spans a few months in most studies,” he told this news organization. The findings, he added, “align well with our clinical experience to date for JAK inhibitor use in inflammatory skin disease. Short-term safety, particularly in relation to boxed warning events such MACE, VTE, and all-cause mortality, have generally been favorable with real-world use. It’s good to have a rigorous statistical analysis to refer to when setting patient expectations.”
However, he noted that these data only examined short-term safety during the placebo or active comparator-controlled periods. “Considering that events like MACE or VTE may take many months or years to manifest, continued long-term data generation is needed to fully answer the question of risk,” he said.
Dr. Garshick disclosed that he received grants from Pfizer and personal fees from Bristol Myers Squibb during the conduct of the study and personal fees from Kiniksa Pharmaceuticals outside the submitted work. Several other coauthors reported having advisory board roles and/or having received funding or support from several pharmaceutical companies. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, investigator, and/or a member of the advisory board for several pharmaceutical companies, including those that develop JAK inhibitors.
FROM JAMA DERMATOLOGY
Hospital Dermatology: Review of Research in 2022-2023
Dermatologists improve the diagnostic accuracy and quality of care of patients in the hospital setting. They help shorten the length of stay, improve outpatient follow-up, and reduce the rate of hospital readmission.1 Medicare beneficiaries hospitalized with skin conditions at institutions with a dermatology hospitalist—a provider with a specialty interest in inpatient dermatology—have 24% lower odds of risk-adjusted 30-day mortality and 12% lower odds of risk-adjusted 30-day readmissions.2
In the last year, research among the dermatology hospitalist community has actively contributed to our understanding of challenging inpatient skin diseases and has identified new ways in which dermatologists can contribute to the care of hospitalized patients. In this review, we highlight 4 areas of focus from the published literature in 2022-2023—severe cutaneous adverse reactions, supportive oncodermatology, cost of inpatient services, and teledermatology.
Severe Cutaneous Adverse Reactions: Old and New
Severe cutaneous adverse reactions to medications frequently are encountered in the inpatient setting. Dermatology hospitalists are well positioned to phenotype these reactions, drawing insights that aid in identifying, characterizing, risk stratifying, and managing these conditions, which have considerable morbidity and mortality.
A recent 20-year retrospective review of cases of acute generalized exanthematous pustulosis (N=340) across 10 academic systems—the largest to date—improves our understanding of the features of this rare entity.3 The authors found that acute generalized exanthematous pustulosis most often is triggered by β-lactam and other antibiotics (75.5%) and is accompanied by fever (49.7%), neutrophilia (85.1%), and eosinophilia (52.1%). Kidney and liver involvement occur in less than 10% of cases, and mortality rates are low but not zero, with an all-cause 30-day mortality rate of 3.5%.3
In a multi-institutional retrospective study of 68 patients diagnosed with DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome, Sharma et al4 developed a scoring system to identify those at greatest risk for DRESS recurrence. Variables associated with recurrence including younger age, female sex, and features considered atypical for DRESS syndrome—nonmorbilliform rash; absence of facial edema; antinuclear antibody positivity; medication class other than antibiotic, antigout, or antiseizure—were used to develop a “ReDRESS” score. This predictive model had a sensitivity of 73% and specificity of 83% for predicting DRESS recurrence.4
Another case series characterized SCoRCH (sudden conjunctivitis, lymphopenia, sunburnlike rash, and hemodynamic changes), a newly described hypersensitivity reaction to trimethoprim-sulfamethoxazole.5 The onset of this reaction typically occurs 4 to 11 days after initiation of trimethoprim-sulfamethoxazole but can occur as quickly as 1 day following re-exposure. Patients are systemically ill with fever, hypotension, tachycardia, acute renal insufficiency, and transaminitis, and they have a diffuse sunburnlike erythema without scale, facial edema, and conjunctivitis. It is thought this distinct hypersensitivity reaction may be mediated by IL-6, which has a role in triggering a sepsislike physiology, with vasodilation, hypotension, and edema.5
A systematic review and meta-analysis found that sulfonamides remain the most prominent cause of Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN).6 A case-control study described SJS/TEN presentations triggered by Mycoplasma, advocating for routine Mycoplasma screening, especially in patients without a clear medication culprit. Mycoplasma-induced cases carried statistically lower rates of mortality (0%) compared with medication-induced cases (22.5%).7 Another prospective open-label study evaluated SJS/TEN management by randomizing 25 patients to receive either combination therapy with methylprednisolone plus a tumor necrosis factor α inhibitor or methylprednisolone alone.8 Anti–tumor necrosis factor therapy was associated with a shorter length of initial steroid treatment and duration of the acute stage, hospitalization, and time to re-epithelialization8; however, as in a prior randomized unblinded trial,9 there was no difference in mortality between the 2 groups.
There is limited high-quality evidence to support the use of any systemic immunomodulator to decrease SJS/TEN–related mortality.10 A Cochrane systematic review highlighted the many limitations of the available data due to variations in presentation, assessment, and management.11 Because SJS/TEN is rare, powering studies based on mortality is infeasible; the authors calculated that 2872 participants were needed to detect a 50% mortality reduction among those with SCORTEN (severity-of-illness score for TEN) scores of 0 to 1.11 Therefore, collaborative efforts using appropriate outcomes measures (eg, time to re-epithelialization, length of hospital stay), standardized terminology and dosing regimens, and adaptive trial designs are needed. Consensus-derived assessment and treatment protocols could help account for variation, ensure consistency in treatment, and enable head-to-head comparisons. Members of the Society of Dermatology Hospitalists are working on efforts to standardize terminology and validate outcomes measures needed for future studies.12
Supportive Oncodermatology: A New Frontier
With the advent of immune checkpoint inhibitors (ICIs) for a growing number of cancers, dermatologists have become critical to identifying and managing cutaneous immune-related adverse events (cirAEs). Recent findings have demonstrated that dermatology input improves patient outcomes, not only regarding the treatment of dermatoses but also by augmenting cancer-related survival. One group found that patients with cirAEs who were evaluated by a dermatologist had improved progression-free (hazard ratio, 0.69; 95% CI, 0.54-0.87; P=.002) and overall survival rates (hazard ratio, 0.62; 95% CI, 0.45-0.84; P=.002), controlling for cirAE severity, age, sex, cancer type, and ICI subtype. Patients who were under the care of a dermatologist also were more likely to resume ICI therapy following an interruption (odds ratio, 10.52; 95% CI, 5.15-21.48; P<.001).13 Dermatologists help to optimize skin-directed and targeted therapies, such as dupilumab, minimizing exposure to systemic immunosuppression in these complex patients.14
Supportive oncodermatologists also have made important observations on how cirAEs relate to other adverse events and prognosis. A review of 628 patients found that almost half of those with cirAEs had co-occurring noncutaneous immune-related adverse events, most commonly pulmonary. Psoriasiform eruptions were most frequently associated with noncutaneous immune-related adverse events, and cutaneous reactions frequently preceded the development of systemic manifestations, serving as a clinical biomarker to provide prognostic information.15 A review of 95 patients found that spongiotic and lichenoid interface reactions were associated with decreased mortality rates, whereas vacuolar interface and perivascular dermatitis were associated with increased mortality.16
As with severe cutaneous adverse events, dermatology input has been critical for accurately phenotyping and risk stratifying these novel reactions. The dermatologist’s skill set is necessary for optimizing skin-directed and targeted therapies while minimizing systemic immunosuppression, thereby improving patient outcomes with respect to rash, cancer response, and survival.
The Cost of Inpatient Skin Disease
Hospitalizations account for approximately half of all health care expenditures, and hospital readmission, seen as a measure of the quality of health care delivery, can double this cost.17 Identifying and developing protocols for addressing patients with complex chronic inflammatory disorders is one strategy for improving outcomes and reducing financial burden. Inpatient dermatologists have identified hidradenitis suppurativa as one disease that can benefit from early intervention by dermatologists in the hospital, with its 30-day (17.8%) and 180-day (48.6%) readmission rates being comparable to those of heart failure.18
Following an index emergency department (ED) visit, 17.2% (3484/20,269) of patients with HS have at least 1 return ED visit within 30 days, while only 2.4% (483/20,269) have a dermatology visit within the same time frame.19 Understanding the risk factors for hospital readmission and ED utilization, including severity of illness, the presence of medical comorbidities, health coverage under Medicaid, and receipt of opioids, can allow dermatologists to anticipate those at greatest risk.19 Opportunities exist for cross-specialty interventions to anticipate and address modifiable risk factors. Shorter time to dermatology outpatient follow-up leads to improved clinic attendance and may help reduce ED utilization and hospital readmission.20
Teledermatology: Leveraging Inpatient Expertise
Although the benefit of inpatient dermatologic care is substantial, access to that care is finite. Following the COVID-19 pandemic, there is an increased acceptance of telemedicine and the long-term role it can play in leveraging dermatologic expertise, including meeting the increasing demand for inpatient dermatology care in rural and resource-poor communities.21
Recent studies conducted by dermatology hospitalists have illustrated the value of asynchronous store-and-forward technology in settings lacking access to consultative dermatology.22,23 Stephens et al22 found that expanding provider-to-provider electronic consultation (e-consultation) capacity to an inpatient rehabilitation facility resulted in completed consultations within 1.5 days compared with a 7- to 14-day wait time for patients attending an in-person urgent access dermatology clinic. In another study, the implementation of asynchronous dermatology e-consultations for immunobullous diseases, vasculitis, and herpes zoster resulted in a change in diagnosis 86% of the time, accompanied by at least 1 new systemic or topical therapy recommendation.23
Researchers also identified ways in which teledermatology can be inelegant and proposed specific supplemental data to aid in diagnosis. A review of 126 inpatient e-consultations demonstrated limitations related to the diagnosis of skin and soft-tissue infections. In two-thirds to three-quarters of cases, potentially useful descriptive information was missing, and in 70% (88/126), images were not appropriately focused. The authors developed a detailed checklist to help primary medical teams focus their differential diagnoses.24 A recent pilot study found that supplementation of clinical information with a standardized questionnaire and thermal images improved the accuracy of cellulitis diagnosis. Using this method, there was no difference in accuracy between dermatology hospitalists and other board-certified dermatologists, supporting the notion that any dermatologist can fulfill this need successfully, even without specific inpatient experience.25 Due to the high incidence and cost of cellulitis and related hospital admissions,26 such an intervention could have a considerable financial and patient safety impact.
Final Thoughts
This last year brought many changes to the health care landscape, the recession of a global pandemic, and an increasingly complex health care delivery system. Inpatient dermatologists met these challenges by providing high-quality dermatologic care and practice-modifying research in the areas of severe cutaneous adverse reactions, supportive oncodermatology, hospital readmission, telemedicine, and more, demonstrating the value of dermatologic expertise in the hospital setting.
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528.
- Puri P, Pollock BD, Yousif M, et al. Association of Society of Dermatology hospitalist institutions with improved outcomes in Medicare beneficiaries hospitalized for skin disease. J Am Acad Dermatol. 2023;88:1372-1375.
- Creadore A, Desai S, Alloo A, et al. Clinical characteristics, disease course, and outcomes of patients with acute generalized exanthematous pustulosis in the US. JAMA Dermatol. 2022;158:176-183.
- Sharma AN, Murphy K, Shwe S, et al. Predicting DRESS syndrome recurrence—the ReDRESS score. JAMA Dermatol. 2022;158:1445-1447.
- Brian M, Rose EK, Mauskar MM, et al. Sudden conjunctivitis, lymphopenia, and rash combined with hemodynamic changes (SCoRCH) after trimethoprim-sulfamethoxazole use: a case series study of a hypersensitivity reaction. JAMA Dermatol. 2023;159:73-78.
- Lee EY, Knox C, Phillips EJ. Worldwide prevalence of antibiotic-associated Stevens-Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. JAMA Dermatol. 2023;159:384-392.
- Liew YCC, Choo KJL, Oh CC, et al. Mycoplasma-induced Stevens-Johnson syndrome/toxic epidermal necrolysis: case-control analysis of a cohort managed in a specialized center. J Am Acad Dermatol. 2022;86:811-817.
- Ao S, Gao X, Zhan J, et al. Inhibition of tumor necrosis factor improves conventional steroid therapy for Stevens-Johnson syndrome/toxic epidermal necrolysis in a cohort of patients. J Am Acad Dermatol. 2022;86:1236-1245.
- Wang CW, Yang LY, Chen CB, et al; the Taiwan Severe Cutaneous Adverse Reaction (TSCAR) Consortium. Randomized, controlled trial of TNF-α antagonist in CTL-mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128:985-996.
- Han JJ, Creadore A, Seminario-Vidal L, et al. Medical management of Stevens-Johnson syndrome/toxic epidermal necrolysis among North American dermatologists. J Am Acad Dermatol. 2022;87:429-431.
- Noe MH, Micheletti RG. Systemic interventions for treatment of Stevens-Johnson syndrome/toxic epidermal necrolysis: summary of a Cochrane review. JAMA Dermatol. 2022;158:1436-1437.
- Waters M, Dobry A, Le ST, et al. Development of a skin-directed scoring system for Stevens-Johnson syndrome and epidermal necrolysis: a Delphi consensus exercise. JAMA Dermatol. 2023;159:772-777.
- Jacoby TV, Shah N, Asdourian MS, et al. Dermatology evaluation for cutaneous immune-related adverse events is associated with improved survival in cancer patients treated with checkpoint inhibition. J Am Acad Dermatol. 2023;88:711-714.
- Said JT, Elman SA, Perez-Chada LM, et al. Treatment of immune checkpoint inhibitor-mediated psoriasis: a systematic review. J Am Acad Dermatol. 2022;87:399-400.
- Asdourian MS, Shah N, Jacoby TV, et al. Evaluating patterns of co-occurrence between cutaneous and noncutaneous immune-related adverse events after immune checkpoint inhibitor therapy. J Am Acad Dermatol. 2023;88:246-249.
- Hirotsu KE, Scott MKD, Marquez C, et al. Histologic subtype of cutaneous immune-related adverse events predicts overall survival in patients receiving immune checkpoint inhibitors. J Am Acad Dermatol. 2022;87:651-653.
- Benbassat J, Taragin M. Hospital readmissions as a measure of quality of health care: advantages and limitations. Arch Intern Med. 2000;160:1074-1081.
- Edigin E, Kaul S, Eseaton PO, et al. At 180 days hidradenitis suppurativa readmission rate is comparable to heart failure: analysis of the nationwide readmissions database. J Am Acad Dermatol. 2022;87:188-192.
- Wang CX, Buss JL, Keller M, et al. Factors associated with dermatologic follow-up vs emergency department return in patients with hidradenitis suppurativa after an initial emergency department visit. JAMA Dermatol. 2022;158:1378-1386.
- Zakaria A, Chang AY, Kim-Lim P, et al. Predictors of postdischarge follow-up attendance among hospitalized dermatology patients: disparities and potential interventions. J Am Acad Dermatol. 2022;87:186-188.
- Arnold JD, Yoon S, Kirkorian AY. The national burden of inpatient dermatology in adults. J Am Acad Dermatol. 2019;80:425-432. doi:10.1016/j.jaad.2018.06.070
- Stephens MR, Das S, Smith GP. Utilization and outcomes of an asynchronous teledermatology pilot for an inpatient rehabilitation hospital. J Am Acad Dermatol. 2022;87:421-423.
- Ortiz C, Khosravi H, Kettering C, et al. Concordance data for inpatient asynchronous eDermatology consultation for immunobullous disease, zoster, and vasculitis. J Am Acad Dermatol. 2022;86:918-920.
- Salle R, Hua C, Mongereau M, et al. Challenges and limitations of teledermatology for skin and soft-tissue infections: a real-world study of an expert center. J Am Acad Dermatol. 2023;88:457-459.
- Creadore A, Manjaly P, Tkachenko E, et al. The utility of augmented teledermatology to improve dermatologist diagnosis of cellulitis: a cross-sectional study. Arch Dermatol Res. 2023;315:1347-1353.
- Weng QY, Raff AB, Cohen JM, et al. Costs and consequences associated with misdiagnosed lower extremity cellulitis. JAMA Dermatol. 2017;153:141-146.
Dermatologists improve the diagnostic accuracy and quality of care of patients in the hospital setting. They help shorten the length of stay, improve outpatient follow-up, and reduce the rate of hospital readmission.1 Medicare beneficiaries hospitalized with skin conditions at institutions with a dermatology hospitalist—a provider with a specialty interest in inpatient dermatology—have 24% lower odds of risk-adjusted 30-day mortality and 12% lower odds of risk-adjusted 30-day readmissions.2
In the last year, research among the dermatology hospitalist community has actively contributed to our understanding of challenging inpatient skin diseases and has identified new ways in which dermatologists can contribute to the care of hospitalized patients. In this review, we highlight 4 areas of focus from the published literature in 2022-2023—severe cutaneous adverse reactions, supportive oncodermatology, cost of inpatient services, and teledermatology.
Severe Cutaneous Adverse Reactions: Old and New
Severe cutaneous adverse reactions to medications frequently are encountered in the inpatient setting. Dermatology hospitalists are well positioned to phenotype these reactions, drawing insights that aid in identifying, characterizing, risk stratifying, and managing these conditions, which have considerable morbidity and mortality.
A recent 20-year retrospective review of cases of acute generalized exanthematous pustulosis (N=340) across 10 academic systems—the largest to date—improves our understanding of the features of this rare entity.3 The authors found that acute generalized exanthematous pustulosis most often is triggered by β-lactam and other antibiotics (75.5%) and is accompanied by fever (49.7%), neutrophilia (85.1%), and eosinophilia (52.1%). Kidney and liver involvement occur in less than 10% of cases, and mortality rates are low but not zero, with an all-cause 30-day mortality rate of 3.5%.3
In a multi-institutional retrospective study of 68 patients diagnosed with DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome, Sharma et al4 developed a scoring system to identify those at greatest risk for DRESS recurrence. Variables associated with recurrence including younger age, female sex, and features considered atypical for DRESS syndrome—nonmorbilliform rash; absence of facial edema; antinuclear antibody positivity; medication class other than antibiotic, antigout, or antiseizure—were used to develop a “ReDRESS” score. This predictive model had a sensitivity of 73% and specificity of 83% for predicting DRESS recurrence.4
Another case series characterized SCoRCH (sudden conjunctivitis, lymphopenia, sunburnlike rash, and hemodynamic changes), a newly described hypersensitivity reaction to trimethoprim-sulfamethoxazole.5 The onset of this reaction typically occurs 4 to 11 days after initiation of trimethoprim-sulfamethoxazole but can occur as quickly as 1 day following re-exposure. Patients are systemically ill with fever, hypotension, tachycardia, acute renal insufficiency, and transaminitis, and they have a diffuse sunburnlike erythema without scale, facial edema, and conjunctivitis. It is thought this distinct hypersensitivity reaction may be mediated by IL-6, which has a role in triggering a sepsislike physiology, with vasodilation, hypotension, and edema.5
A systematic review and meta-analysis found that sulfonamides remain the most prominent cause of Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN).6 A case-control study described SJS/TEN presentations triggered by Mycoplasma, advocating for routine Mycoplasma screening, especially in patients without a clear medication culprit. Mycoplasma-induced cases carried statistically lower rates of mortality (0%) compared with medication-induced cases (22.5%).7 Another prospective open-label study evaluated SJS/TEN management by randomizing 25 patients to receive either combination therapy with methylprednisolone plus a tumor necrosis factor α inhibitor or methylprednisolone alone.8 Anti–tumor necrosis factor therapy was associated with a shorter length of initial steroid treatment and duration of the acute stage, hospitalization, and time to re-epithelialization8; however, as in a prior randomized unblinded trial,9 there was no difference in mortality between the 2 groups.
There is limited high-quality evidence to support the use of any systemic immunomodulator to decrease SJS/TEN–related mortality.10 A Cochrane systematic review highlighted the many limitations of the available data due to variations in presentation, assessment, and management.11 Because SJS/TEN is rare, powering studies based on mortality is infeasible; the authors calculated that 2872 participants were needed to detect a 50% mortality reduction among those with SCORTEN (severity-of-illness score for TEN) scores of 0 to 1.11 Therefore, collaborative efforts using appropriate outcomes measures (eg, time to re-epithelialization, length of hospital stay), standardized terminology and dosing regimens, and adaptive trial designs are needed. Consensus-derived assessment and treatment protocols could help account for variation, ensure consistency in treatment, and enable head-to-head comparisons. Members of the Society of Dermatology Hospitalists are working on efforts to standardize terminology and validate outcomes measures needed for future studies.12
Supportive Oncodermatology: A New Frontier
With the advent of immune checkpoint inhibitors (ICIs) for a growing number of cancers, dermatologists have become critical to identifying and managing cutaneous immune-related adverse events (cirAEs). Recent findings have demonstrated that dermatology input improves patient outcomes, not only regarding the treatment of dermatoses but also by augmenting cancer-related survival. One group found that patients with cirAEs who were evaluated by a dermatologist had improved progression-free (hazard ratio, 0.69; 95% CI, 0.54-0.87; P=.002) and overall survival rates (hazard ratio, 0.62; 95% CI, 0.45-0.84; P=.002), controlling for cirAE severity, age, sex, cancer type, and ICI subtype. Patients who were under the care of a dermatologist also were more likely to resume ICI therapy following an interruption (odds ratio, 10.52; 95% CI, 5.15-21.48; P<.001).13 Dermatologists help to optimize skin-directed and targeted therapies, such as dupilumab, minimizing exposure to systemic immunosuppression in these complex patients.14
Supportive oncodermatologists also have made important observations on how cirAEs relate to other adverse events and prognosis. A review of 628 patients found that almost half of those with cirAEs had co-occurring noncutaneous immune-related adverse events, most commonly pulmonary. Psoriasiform eruptions were most frequently associated with noncutaneous immune-related adverse events, and cutaneous reactions frequently preceded the development of systemic manifestations, serving as a clinical biomarker to provide prognostic information.15 A review of 95 patients found that spongiotic and lichenoid interface reactions were associated with decreased mortality rates, whereas vacuolar interface and perivascular dermatitis were associated with increased mortality.16
As with severe cutaneous adverse events, dermatology input has been critical for accurately phenotyping and risk stratifying these novel reactions. The dermatologist’s skill set is necessary for optimizing skin-directed and targeted therapies while minimizing systemic immunosuppression, thereby improving patient outcomes with respect to rash, cancer response, and survival.
The Cost of Inpatient Skin Disease
Hospitalizations account for approximately half of all health care expenditures, and hospital readmission, seen as a measure of the quality of health care delivery, can double this cost.17 Identifying and developing protocols for addressing patients with complex chronic inflammatory disorders is one strategy for improving outcomes and reducing financial burden. Inpatient dermatologists have identified hidradenitis suppurativa as one disease that can benefit from early intervention by dermatologists in the hospital, with its 30-day (17.8%) and 180-day (48.6%) readmission rates being comparable to those of heart failure.18
Following an index emergency department (ED) visit, 17.2% (3484/20,269) of patients with HS have at least 1 return ED visit within 30 days, while only 2.4% (483/20,269) have a dermatology visit within the same time frame.19 Understanding the risk factors for hospital readmission and ED utilization, including severity of illness, the presence of medical comorbidities, health coverage under Medicaid, and receipt of opioids, can allow dermatologists to anticipate those at greatest risk.19 Opportunities exist for cross-specialty interventions to anticipate and address modifiable risk factors. Shorter time to dermatology outpatient follow-up leads to improved clinic attendance and may help reduce ED utilization and hospital readmission.20
Teledermatology: Leveraging Inpatient Expertise
Although the benefit of inpatient dermatologic care is substantial, access to that care is finite. Following the COVID-19 pandemic, there is an increased acceptance of telemedicine and the long-term role it can play in leveraging dermatologic expertise, including meeting the increasing demand for inpatient dermatology care in rural and resource-poor communities.21
Recent studies conducted by dermatology hospitalists have illustrated the value of asynchronous store-and-forward technology in settings lacking access to consultative dermatology.22,23 Stephens et al22 found that expanding provider-to-provider electronic consultation (e-consultation) capacity to an inpatient rehabilitation facility resulted in completed consultations within 1.5 days compared with a 7- to 14-day wait time for patients attending an in-person urgent access dermatology clinic. In another study, the implementation of asynchronous dermatology e-consultations for immunobullous diseases, vasculitis, and herpes zoster resulted in a change in diagnosis 86% of the time, accompanied by at least 1 new systemic or topical therapy recommendation.23
Researchers also identified ways in which teledermatology can be inelegant and proposed specific supplemental data to aid in diagnosis. A review of 126 inpatient e-consultations demonstrated limitations related to the diagnosis of skin and soft-tissue infections. In two-thirds to three-quarters of cases, potentially useful descriptive information was missing, and in 70% (88/126), images were not appropriately focused. The authors developed a detailed checklist to help primary medical teams focus their differential diagnoses.24 A recent pilot study found that supplementation of clinical information with a standardized questionnaire and thermal images improved the accuracy of cellulitis diagnosis. Using this method, there was no difference in accuracy between dermatology hospitalists and other board-certified dermatologists, supporting the notion that any dermatologist can fulfill this need successfully, even without specific inpatient experience.25 Due to the high incidence and cost of cellulitis and related hospital admissions,26 such an intervention could have a considerable financial and patient safety impact.
Final Thoughts
This last year brought many changes to the health care landscape, the recession of a global pandemic, and an increasingly complex health care delivery system. Inpatient dermatologists met these challenges by providing high-quality dermatologic care and practice-modifying research in the areas of severe cutaneous adverse reactions, supportive oncodermatology, hospital readmission, telemedicine, and more, demonstrating the value of dermatologic expertise in the hospital setting.
Dermatologists improve the diagnostic accuracy and quality of care of patients in the hospital setting. They help shorten the length of stay, improve outpatient follow-up, and reduce the rate of hospital readmission.1 Medicare beneficiaries hospitalized with skin conditions at institutions with a dermatology hospitalist—a provider with a specialty interest in inpatient dermatology—have 24% lower odds of risk-adjusted 30-day mortality and 12% lower odds of risk-adjusted 30-day readmissions.2
In the last year, research among the dermatology hospitalist community has actively contributed to our understanding of challenging inpatient skin diseases and has identified new ways in which dermatologists can contribute to the care of hospitalized patients. In this review, we highlight 4 areas of focus from the published literature in 2022-2023—severe cutaneous adverse reactions, supportive oncodermatology, cost of inpatient services, and teledermatology.
Severe Cutaneous Adverse Reactions: Old and New
Severe cutaneous adverse reactions to medications frequently are encountered in the inpatient setting. Dermatology hospitalists are well positioned to phenotype these reactions, drawing insights that aid in identifying, characterizing, risk stratifying, and managing these conditions, which have considerable morbidity and mortality.
A recent 20-year retrospective review of cases of acute generalized exanthematous pustulosis (N=340) across 10 academic systems—the largest to date—improves our understanding of the features of this rare entity.3 The authors found that acute generalized exanthematous pustulosis most often is triggered by β-lactam and other antibiotics (75.5%) and is accompanied by fever (49.7%), neutrophilia (85.1%), and eosinophilia (52.1%). Kidney and liver involvement occur in less than 10% of cases, and mortality rates are low but not zero, with an all-cause 30-day mortality rate of 3.5%.3
In a multi-institutional retrospective study of 68 patients diagnosed with DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome, Sharma et al4 developed a scoring system to identify those at greatest risk for DRESS recurrence. Variables associated with recurrence including younger age, female sex, and features considered atypical for DRESS syndrome—nonmorbilliform rash; absence of facial edema; antinuclear antibody positivity; medication class other than antibiotic, antigout, or antiseizure—were used to develop a “ReDRESS” score. This predictive model had a sensitivity of 73% and specificity of 83% for predicting DRESS recurrence.4
Another case series characterized SCoRCH (sudden conjunctivitis, lymphopenia, sunburnlike rash, and hemodynamic changes), a newly described hypersensitivity reaction to trimethoprim-sulfamethoxazole.5 The onset of this reaction typically occurs 4 to 11 days after initiation of trimethoprim-sulfamethoxazole but can occur as quickly as 1 day following re-exposure. Patients are systemically ill with fever, hypotension, tachycardia, acute renal insufficiency, and transaminitis, and they have a diffuse sunburnlike erythema without scale, facial edema, and conjunctivitis. It is thought this distinct hypersensitivity reaction may be mediated by IL-6, which has a role in triggering a sepsislike physiology, with vasodilation, hypotension, and edema.5
A systematic review and meta-analysis found that sulfonamides remain the most prominent cause of Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN).6 A case-control study described SJS/TEN presentations triggered by Mycoplasma, advocating for routine Mycoplasma screening, especially in patients without a clear medication culprit. Mycoplasma-induced cases carried statistically lower rates of mortality (0%) compared with medication-induced cases (22.5%).7 Another prospective open-label study evaluated SJS/TEN management by randomizing 25 patients to receive either combination therapy with methylprednisolone plus a tumor necrosis factor α inhibitor or methylprednisolone alone.8 Anti–tumor necrosis factor therapy was associated with a shorter length of initial steroid treatment and duration of the acute stage, hospitalization, and time to re-epithelialization8; however, as in a prior randomized unblinded trial,9 there was no difference in mortality between the 2 groups.
There is limited high-quality evidence to support the use of any systemic immunomodulator to decrease SJS/TEN–related mortality.10 A Cochrane systematic review highlighted the many limitations of the available data due to variations in presentation, assessment, and management.11 Because SJS/TEN is rare, powering studies based on mortality is infeasible; the authors calculated that 2872 participants were needed to detect a 50% mortality reduction among those with SCORTEN (severity-of-illness score for TEN) scores of 0 to 1.11 Therefore, collaborative efforts using appropriate outcomes measures (eg, time to re-epithelialization, length of hospital stay), standardized terminology and dosing regimens, and adaptive trial designs are needed. Consensus-derived assessment and treatment protocols could help account for variation, ensure consistency in treatment, and enable head-to-head comparisons. Members of the Society of Dermatology Hospitalists are working on efforts to standardize terminology and validate outcomes measures needed for future studies.12
Supportive Oncodermatology: A New Frontier
With the advent of immune checkpoint inhibitors (ICIs) for a growing number of cancers, dermatologists have become critical to identifying and managing cutaneous immune-related adverse events (cirAEs). Recent findings have demonstrated that dermatology input improves patient outcomes, not only regarding the treatment of dermatoses but also by augmenting cancer-related survival. One group found that patients with cirAEs who were evaluated by a dermatologist had improved progression-free (hazard ratio, 0.69; 95% CI, 0.54-0.87; P=.002) and overall survival rates (hazard ratio, 0.62; 95% CI, 0.45-0.84; P=.002), controlling for cirAE severity, age, sex, cancer type, and ICI subtype. Patients who were under the care of a dermatologist also were more likely to resume ICI therapy following an interruption (odds ratio, 10.52; 95% CI, 5.15-21.48; P<.001).13 Dermatologists help to optimize skin-directed and targeted therapies, such as dupilumab, minimizing exposure to systemic immunosuppression in these complex patients.14
Supportive oncodermatologists also have made important observations on how cirAEs relate to other adverse events and prognosis. A review of 628 patients found that almost half of those with cirAEs had co-occurring noncutaneous immune-related adverse events, most commonly pulmonary. Psoriasiform eruptions were most frequently associated with noncutaneous immune-related adverse events, and cutaneous reactions frequently preceded the development of systemic manifestations, serving as a clinical biomarker to provide prognostic information.15 A review of 95 patients found that spongiotic and lichenoid interface reactions were associated with decreased mortality rates, whereas vacuolar interface and perivascular dermatitis were associated with increased mortality.16
As with severe cutaneous adverse events, dermatology input has been critical for accurately phenotyping and risk stratifying these novel reactions. The dermatologist’s skill set is necessary for optimizing skin-directed and targeted therapies while minimizing systemic immunosuppression, thereby improving patient outcomes with respect to rash, cancer response, and survival.
The Cost of Inpatient Skin Disease
Hospitalizations account for approximately half of all health care expenditures, and hospital readmission, seen as a measure of the quality of health care delivery, can double this cost.17 Identifying and developing protocols for addressing patients with complex chronic inflammatory disorders is one strategy for improving outcomes and reducing financial burden. Inpatient dermatologists have identified hidradenitis suppurativa as one disease that can benefit from early intervention by dermatologists in the hospital, with its 30-day (17.8%) and 180-day (48.6%) readmission rates being comparable to those of heart failure.18
Following an index emergency department (ED) visit, 17.2% (3484/20,269) of patients with HS have at least 1 return ED visit within 30 days, while only 2.4% (483/20,269) have a dermatology visit within the same time frame.19 Understanding the risk factors for hospital readmission and ED utilization, including severity of illness, the presence of medical comorbidities, health coverage under Medicaid, and receipt of opioids, can allow dermatologists to anticipate those at greatest risk.19 Opportunities exist for cross-specialty interventions to anticipate and address modifiable risk factors. Shorter time to dermatology outpatient follow-up leads to improved clinic attendance and may help reduce ED utilization and hospital readmission.20
Teledermatology: Leveraging Inpatient Expertise
Although the benefit of inpatient dermatologic care is substantial, access to that care is finite. Following the COVID-19 pandemic, there is an increased acceptance of telemedicine and the long-term role it can play in leveraging dermatologic expertise, including meeting the increasing demand for inpatient dermatology care in rural and resource-poor communities.21
Recent studies conducted by dermatology hospitalists have illustrated the value of asynchronous store-and-forward technology in settings lacking access to consultative dermatology.22,23 Stephens et al22 found that expanding provider-to-provider electronic consultation (e-consultation) capacity to an inpatient rehabilitation facility resulted in completed consultations within 1.5 days compared with a 7- to 14-day wait time for patients attending an in-person urgent access dermatology clinic. In another study, the implementation of asynchronous dermatology e-consultations for immunobullous diseases, vasculitis, and herpes zoster resulted in a change in diagnosis 86% of the time, accompanied by at least 1 new systemic or topical therapy recommendation.23
Researchers also identified ways in which teledermatology can be inelegant and proposed specific supplemental data to aid in diagnosis. A review of 126 inpatient e-consultations demonstrated limitations related to the diagnosis of skin and soft-tissue infections. In two-thirds to three-quarters of cases, potentially useful descriptive information was missing, and in 70% (88/126), images were not appropriately focused. The authors developed a detailed checklist to help primary medical teams focus their differential diagnoses.24 A recent pilot study found that supplementation of clinical information with a standardized questionnaire and thermal images improved the accuracy of cellulitis diagnosis. Using this method, there was no difference in accuracy between dermatology hospitalists and other board-certified dermatologists, supporting the notion that any dermatologist can fulfill this need successfully, even without specific inpatient experience.25 Due to the high incidence and cost of cellulitis and related hospital admissions,26 such an intervention could have a considerable financial and patient safety impact.
Final Thoughts
This last year brought many changes to the health care landscape, the recession of a global pandemic, and an increasingly complex health care delivery system. Inpatient dermatologists met these challenges by providing high-quality dermatologic care and practice-modifying research in the areas of severe cutaneous adverse reactions, supportive oncodermatology, hospital readmission, telemedicine, and more, demonstrating the value of dermatologic expertise in the hospital setting.
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528.
- Puri P, Pollock BD, Yousif M, et al. Association of Society of Dermatology hospitalist institutions with improved outcomes in Medicare beneficiaries hospitalized for skin disease. J Am Acad Dermatol. 2023;88:1372-1375.
- Creadore A, Desai S, Alloo A, et al. Clinical characteristics, disease course, and outcomes of patients with acute generalized exanthematous pustulosis in the US. JAMA Dermatol. 2022;158:176-183.
- Sharma AN, Murphy K, Shwe S, et al. Predicting DRESS syndrome recurrence—the ReDRESS score. JAMA Dermatol. 2022;158:1445-1447.
- Brian M, Rose EK, Mauskar MM, et al. Sudden conjunctivitis, lymphopenia, and rash combined with hemodynamic changes (SCoRCH) after trimethoprim-sulfamethoxazole use: a case series study of a hypersensitivity reaction. JAMA Dermatol. 2023;159:73-78.
- Lee EY, Knox C, Phillips EJ. Worldwide prevalence of antibiotic-associated Stevens-Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. JAMA Dermatol. 2023;159:384-392.
- Liew YCC, Choo KJL, Oh CC, et al. Mycoplasma-induced Stevens-Johnson syndrome/toxic epidermal necrolysis: case-control analysis of a cohort managed in a specialized center. J Am Acad Dermatol. 2022;86:811-817.
- Ao S, Gao X, Zhan J, et al. Inhibition of tumor necrosis factor improves conventional steroid therapy for Stevens-Johnson syndrome/toxic epidermal necrolysis in a cohort of patients. J Am Acad Dermatol. 2022;86:1236-1245.
- Wang CW, Yang LY, Chen CB, et al; the Taiwan Severe Cutaneous Adverse Reaction (TSCAR) Consortium. Randomized, controlled trial of TNF-α antagonist in CTL-mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128:985-996.
- Han JJ, Creadore A, Seminario-Vidal L, et al. Medical management of Stevens-Johnson syndrome/toxic epidermal necrolysis among North American dermatologists. J Am Acad Dermatol. 2022;87:429-431.
- Noe MH, Micheletti RG. Systemic interventions for treatment of Stevens-Johnson syndrome/toxic epidermal necrolysis: summary of a Cochrane review. JAMA Dermatol. 2022;158:1436-1437.
- Waters M, Dobry A, Le ST, et al. Development of a skin-directed scoring system for Stevens-Johnson syndrome and epidermal necrolysis: a Delphi consensus exercise. JAMA Dermatol. 2023;159:772-777.
- Jacoby TV, Shah N, Asdourian MS, et al. Dermatology evaluation for cutaneous immune-related adverse events is associated with improved survival in cancer patients treated with checkpoint inhibition. J Am Acad Dermatol. 2023;88:711-714.
- Said JT, Elman SA, Perez-Chada LM, et al. Treatment of immune checkpoint inhibitor-mediated psoriasis: a systematic review. J Am Acad Dermatol. 2022;87:399-400.
- Asdourian MS, Shah N, Jacoby TV, et al. Evaluating patterns of co-occurrence between cutaneous and noncutaneous immune-related adverse events after immune checkpoint inhibitor therapy. J Am Acad Dermatol. 2023;88:246-249.
- Hirotsu KE, Scott MKD, Marquez C, et al. Histologic subtype of cutaneous immune-related adverse events predicts overall survival in patients receiving immune checkpoint inhibitors. J Am Acad Dermatol. 2022;87:651-653.
- Benbassat J, Taragin M. Hospital readmissions as a measure of quality of health care: advantages and limitations. Arch Intern Med. 2000;160:1074-1081.
- Edigin E, Kaul S, Eseaton PO, et al. At 180 days hidradenitis suppurativa readmission rate is comparable to heart failure: analysis of the nationwide readmissions database. J Am Acad Dermatol. 2022;87:188-192.
- Wang CX, Buss JL, Keller M, et al. Factors associated with dermatologic follow-up vs emergency department return in patients with hidradenitis suppurativa after an initial emergency department visit. JAMA Dermatol. 2022;158:1378-1386.
- Zakaria A, Chang AY, Kim-Lim P, et al. Predictors of postdischarge follow-up attendance among hospitalized dermatology patients: disparities and potential interventions. J Am Acad Dermatol. 2022;87:186-188.
- Arnold JD, Yoon S, Kirkorian AY. The national burden of inpatient dermatology in adults. J Am Acad Dermatol. 2019;80:425-432. doi:10.1016/j.jaad.2018.06.070
- Stephens MR, Das S, Smith GP. Utilization and outcomes of an asynchronous teledermatology pilot for an inpatient rehabilitation hospital. J Am Acad Dermatol. 2022;87:421-423.
- Ortiz C, Khosravi H, Kettering C, et al. Concordance data for inpatient asynchronous eDermatology consultation for immunobullous disease, zoster, and vasculitis. J Am Acad Dermatol. 2022;86:918-920.
- Salle R, Hua C, Mongereau M, et al. Challenges and limitations of teledermatology for skin and soft-tissue infections: a real-world study of an expert center. J Am Acad Dermatol. 2023;88:457-459.
- Creadore A, Manjaly P, Tkachenko E, et al. The utility of augmented teledermatology to improve dermatologist diagnosis of cellulitis: a cross-sectional study. Arch Dermatol Res. 2023;315:1347-1353.
- Weng QY, Raff AB, Cohen JM, et al. Costs and consequences associated with misdiagnosed lower extremity cellulitis. JAMA Dermatol. 2017;153:141-146.
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528.
- Puri P, Pollock BD, Yousif M, et al. Association of Society of Dermatology hospitalist institutions with improved outcomes in Medicare beneficiaries hospitalized for skin disease. J Am Acad Dermatol. 2023;88:1372-1375.
- Creadore A, Desai S, Alloo A, et al. Clinical characteristics, disease course, and outcomes of patients with acute generalized exanthematous pustulosis in the US. JAMA Dermatol. 2022;158:176-183.
- Sharma AN, Murphy K, Shwe S, et al. Predicting DRESS syndrome recurrence—the ReDRESS score. JAMA Dermatol. 2022;158:1445-1447.
- Brian M, Rose EK, Mauskar MM, et al. Sudden conjunctivitis, lymphopenia, and rash combined with hemodynamic changes (SCoRCH) after trimethoprim-sulfamethoxazole use: a case series study of a hypersensitivity reaction. JAMA Dermatol. 2023;159:73-78.
- Lee EY, Knox C, Phillips EJ. Worldwide prevalence of antibiotic-associated Stevens-Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. JAMA Dermatol. 2023;159:384-392.
- Liew YCC, Choo KJL, Oh CC, et al. Mycoplasma-induced Stevens-Johnson syndrome/toxic epidermal necrolysis: case-control analysis of a cohort managed in a specialized center. J Am Acad Dermatol. 2022;86:811-817.
- Ao S, Gao X, Zhan J, et al. Inhibition of tumor necrosis factor improves conventional steroid therapy for Stevens-Johnson syndrome/toxic epidermal necrolysis in a cohort of patients. J Am Acad Dermatol. 2022;86:1236-1245.
- Wang CW, Yang LY, Chen CB, et al; the Taiwan Severe Cutaneous Adverse Reaction (TSCAR) Consortium. Randomized, controlled trial of TNF-α antagonist in CTL-mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128:985-996.
- Han JJ, Creadore A, Seminario-Vidal L, et al. Medical management of Stevens-Johnson syndrome/toxic epidermal necrolysis among North American dermatologists. J Am Acad Dermatol. 2022;87:429-431.
- Noe MH, Micheletti RG. Systemic interventions for treatment of Stevens-Johnson syndrome/toxic epidermal necrolysis: summary of a Cochrane review. JAMA Dermatol. 2022;158:1436-1437.
- Waters M, Dobry A, Le ST, et al. Development of a skin-directed scoring system for Stevens-Johnson syndrome and epidermal necrolysis: a Delphi consensus exercise. JAMA Dermatol. 2023;159:772-777.
- Jacoby TV, Shah N, Asdourian MS, et al. Dermatology evaluation for cutaneous immune-related adverse events is associated with improved survival in cancer patients treated with checkpoint inhibition. J Am Acad Dermatol. 2023;88:711-714.
- Said JT, Elman SA, Perez-Chada LM, et al. Treatment of immune checkpoint inhibitor-mediated psoriasis: a systematic review. J Am Acad Dermatol. 2022;87:399-400.
- Asdourian MS, Shah N, Jacoby TV, et al. Evaluating patterns of co-occurrence between cutaneous and noncutaneous immune-related adverse events after immune checkpoint inhibitor therapy. J Am Acad Dermatol. 2023;88:246-249.
- Hirotsu KE, Scott MKD, Marquez C, et al. Histologic subtype of cutaneous immune-related adverse events predicts overall survival in patients receiving immune checkpoint inhibitors. J Am Acad Dermatol. 2022;87:651-653.
- Benbassat J, Taragin M. Hospital readmissions as a measure of quality of health care: advantages and limitations. Arch Intern Med. 2000;160:1074-1081.
- Edigin E, Kaul S, Eseaton PO, et al. At 180 days hidradenitis suppurativa readmission rate is comparable to heart failure: analysis of the nationwide readmissions database. J Am Acad Dermatol. 2022;87:188-192.
- Wang CX, Buss JL, Keller M, et al. Factors associated with dermatologic follow-up vs emergency department return in patients with hidradenitis suppurativa after an initial emergency department visit. JAMA Dermatol. 2022;158:1378-1386.
- Zakaria A, Chang AY, Kim-Lim P, et al. Predictors of postdischarge follow-up attendance among hospitalized dermatology patients: disparities and potential interventions. J Am Acad Dermatol. 2022;87:186-188.
- Arnold JD, Yoon S, Kirkorian AY. The national burden of inpatient dermatology in adults. J Am Acad Dermatol. 2019;80:425-432. doi:10.1016/j.jaad.2018.06.070
- Stephens MR, Das S, Smith GP. Utilization and outcomes of an asynchronous teledermatology pilot for an inpatient rehabilitation hospital. J Am Acad Dermatol. 2022;87:421-423.
- Ortiz C, Khosravi H, Kettering C, et al. Concordance data for inpatient asynchronous eDermatology consultation for immunobullous disease, zoster, and vasculitis. J Am Acad Dermatol. 2022;86:918-920.
- Salle R, Hua C, Mongereau M, et al. Challenges and limitations of teledermatology for skin and soft-tissue infections: a real-world study of an expert center. J Am Acad Dermatol. 2023;88:457-459.
- Creadore A, Manjaly P, Tkachenko E, et al. The utility of augmented teledermatology to improve dermatologist diagnosis of cellulitis: a cross-sectional study. Arch Dermatol Res. 2023;315:1347-1353.
- Weng QY, Raff AB, Cohen JM, et al. Costs and consequences associated with misdiagnosed lower extremity cellulitis. JAMA Dermatol. 2017;153:141-146.
Practice Points
- A severe hypersensitivity reaction to trimethoprim-sulfamethoxazole—sudden conjunctivitis, lymphopenia, sunburnlike rash, and hemodynamic changes (SCoRCH)—has been described.
- Patients experiencing cutaneous reactions to immune checkpoint inhibitors have improved progression-free and overall survival rates if evaluated by a dermatologist who can optimize skin-directed and targeted therapies.
- Interventions, including shorter time to dermatology outpatient follow-up, are needed to reduce emergency department utilization by patients with hidradenitis suppurativa.
- Asynchronous store-and-forward dermatology e-consultation is effective for immunobullous diseases, vasculitis, herpes zoster, and cellulitis, demonstrating the utility of teledermatology in the inpatient setting, particularly when standardized data capture tools are used.
Potential Uses of Nonthermal Atmospheric Pressure Technology for Dermatologic Conditions in Children
Nonthermal atmospheric plasma (NTAP)(or cold atmospheric plasma [CAP]) is a rapidly developing treatment modality for a wide range of dermatologic conditions. Plasma (or ionized gas) refers to a state of matter composed of electrons, protons, and neutral atoms that generate reactive oxygen and nitrogen species.1 Plasma previously was created using thermal energy, but recent advances have allowed the creation of plasma using atmospheric pressure and room temperature; thus, NTAP can be used without causing damage to living tissue through heat.1 Plasma technology varies greatly, but it generally can be classified as either direct or indirect therapy; direct therapy uses the human body as an electrode, whereas indirect therapy creates plasma through the interaction between 2 electrode devices.1,2 When used on the skin, important dose-dependent relationships have been observed, with CAP application longer than 2 minutes being associated with increased keratinocyte and fibroblast apoptosis.2 Thus, CAP can cause diverse changes to the skin depending on application time and methodology. At adequate yet low concentrations, plasma can promote fibroblast proliferation and upregulate genes involved in collagen and transforming growth factor synthesis.1 Additionally, the reactive oxygen and nitrogen species created by NTAP have been shown to inactivate microorganisms through the destruction of biofilms, lead to diminished immune cell infiltration and cytokine release in autoimmune dermatologic conditions, and exert antitumor properties through cellular DNA damage.1-3 In dermatology, these properties can be harvested to promote wound healing at low doses and the treatment of proliferative skin conditions at high doses.1
Because of its novelty, the safety profile of NTAP is still under investigation, but preliminary studies are promising and show no damage to the skin barrier when excessive plasma exposure is avoided.4 However, dose- and time-dependent damage to cells has been shown. As a result, the exact dose of plasma considered safe is highly variable depending on the vessel, technique, and user, and future clinical research is needed to guide this methodology.4 Additionally, CAP has been shown to cause little pain at the skin surface and may lead to decreased levels of pain in healing wound sites.5 Given this promising safety profile and minimal discomfort to patients, NTAP technology remains promising for use in pediatric dermatology, but there are limited data to characterize its potential use in this population. In this systematic review, we aimed to elucidate reported applications of NTAP for skin conditions in children and discuss the trajectory of this technology in the future of pediatric dermatology.
Methodology
A comprehensive literature review was conducted to identify studies evaluating NTAP technology in pediatric populations using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines. A search of PubMed, Embase, and Web of Science articles was conducted in April 2023 using the terms nonthermal atmospheric plasma or cold atmospheric plasma. All English-language articles that described the use of NTAP as a treatment in pediatric populations or articles that described NTAP use in the treatment of common conditions in this patient group were included based on a review of the article titles and abstracts by 2 independent reviewers, followed by full-text review of relevant articles (M.G., C.L.). Any discrepancies in eligible articles were settled by a third independent researcher (M.V.). One hundred twenty studies were identified, and 95 were screened for inclusion; 9 studies met inclusion criteria and were summarized in this review.
Results
A total of 9 studies were included in this review: 3 describing the success of NTAP in pediatric populations6-8 and 6 describing the potential success of NTAP for dermatologic conditions commonly seen in children (Table).9-14
Studies Describing Success of NTAP—Three clinical reports described the efficacy of NTAP in pediatric dermatology. A case series from 2020 showed full clearance of warts in 100% of patients (n=5) with a 0% recurrence rate when NTAP treatment was applied for 2 minutes to each lesion during each treatment session with the electrode held 1 mm from the lesional surface.6 Each patient was followed up at 3 to 4 weeks, and treatment was repeated if lesions persisted. Patients reported no pain during the procedure, and no adverse effects were noted over the course of treatment.6 Second, a case report described full clearance of diaper dermatitis with no recurrence after 6 months following 6 treatments with NTAP in a 14-month-old girl.7 After treatment with econazole nitrate cream, oral antibiotics, and prednisone failed, CAP treatment was initiated. Each treatment lasted 15 minutes with 3-day time intervals between each of the 6 treatments. There were no adverse events or recurrence of rash at 6-month follow-up.7 A final case report described full clearance of molluscum contagiosum (MC), with no recurrence after 2 months following 4 treatments with NTAP in a 12-year-old boy.8 The patient had untreated MC on the face, neck, shoulder, and thighs. Lesions of the face were treated with CAP, while the other sites were treated with cantharidin using a 0.7% collodion-based solution. Four CAP treatments were performed at 1-month intervals, with CAP applied 1 mm from the lesional surfaces in a circular pattern for 2 minutes. At follow-up 2 months after the final treatment, the patient had no adverse effects and showed no pigmentary changes or scarring.8
Studies Describing the Potential Success of NTAP—Beyond these studies, limited research has been done on NTAP in pediatric populations. The Table summarizes 6 additional studies completed with promising treatment results for dermatologic conditions commonly seen in children: striae distensae, keloids, atopic dermatitis, psoriasis, inverse psoriasis, and acne vulgaris. Across all reports and studies, patients showed significant improvement in their dermatologic conditions following the use of NTAP technology with limited adverse effects reported (P<.05). Suwanchinda and Nararatwanchai9 studied the use of CAP for the treatment of striae distensae. They recruited 23 patients and treated half the body with CAP biweekly for 5 sessions; the other half was left untreated. At follow-up 30 days after the final treatment, striae distensae had improved for both patient and observer assessment scores.9 Another study performed by Suwanchinda and Nararatwanchai10 looked at the efficacy of CAP in treating keloids. They recruited 18 patients, and keloid scars were treated in halves—one half treated with CAP biweekly for 5 sessions and the other left untreated. At follow-up 30 days after the final treatment, keloids significantly improved in color, melanin, texture, and hemoglobin based on assessment by the Antera 3D imaging system (Miravex Limited)(P<.05).10
Kim et al11 studied the efficacy of CAP for the treatment of atopic dermatitis in 22 patients. Each patient had mild to moderate atopic dermatitis that had not been treated with topical agents or antibiotics for at least 2 weeks prior to beginning the study. Additionally, only patients with symmetric lesions—meaning only patients with lesions on both sides of the anatomical extremities—were included. Each patient then received CAP on 1 symmetric lesion and placebo on the other. Cold atmospheric plasma treatment was done 5 mm away from the lesion, and each treatment lasted for 5 minutes. Treatments were done at weeks 0, 1, and 2, with follow-up 4 weeks after the final treatment. The clinical severity of disease was assessed at weeks 0, 1, 2, and 4. Results showed that at week 4, the mean (SD) modified Atopic Dermatitis Antecubital Severity score decreased from 33.73 (21.21) at week 0 to 13.12 (15.92). Additionally, the pruritic visual analog scale showed significant improvement with treatment vs baseline (P≤.0001).11
Two studies examined how NTAP can be used in the treatment of psoriasis. First, Gareri et al12 used CAP to treat a psoriatic plaque in a 20-year-old woman. These plaques on the left hand previously had been unresponsive to topical psoriasis treatments. The patient received 2 treatments with CAP on days 0 and 3; at 14 days, the plaque completely resolved with an itch score of 0.12 Next, Zheng et al13 treated 2 patients with NTAP for inverse psoriasis. The first patient was a 26-year-old woman with plaques in the axilla and buttocks as well as inframammary lesions that failed to respond to treatment with topicals and vitamin D analogues. She received CAP treatments 2 to 3 times weekly for 5 total treatments with application to each region occurring 1 mm from the skin surface. The lesions completely resolved with no recurrence at 6 weeks. The second patient was a 38-year-old woman with inverse psoriasis in the axilla and groin; she received treatment every 3 days for 8 total treatments, which led to complete remission, with no recurrence noted at 1 month.13
Arisi et al14 used NTAP to treat acne vulgaris in 2 patients. The first patient was a 24-year-old man with moderate acne on the face that did not improve with topicals or oral antibiotics. The patient received 5 CAP treatments with no adverse events noted. The patient discontinued treatment on his own, but the number of lesions decreased after the fifth treatment. The second patient was a 21-year-old woman with moderate facial acne that failed to respond to treatment with topicals and oral tetracycline. The patient received 8 CAP treatments and experienced a reduction in the number of lesions during treatment. There were no adverse events, and improvement was maintained at 3-month follow-up.14
Comment
Although the use of NTAP in pediatric dermatology is scarcely described in the literature, the technology will certainly have applications in the future treatment of a wide variety of pediatric disorders. In addition to the clinical success shown in several studies,6-14 this technology has been shown to cause minimal damage to skin when application time is minimized. One study conducted on ex vivo skin showed that NTAP technology can safely be used for up to 2 minutes without major DNA damage.15 Through its diverse mechanisms of action, NTAP can induce modification of proteins and cell membranes in a noninvasive manner.2 In conditions with impaired barrier function, such as atopic and diaper dermatitis, studies in mouse models have shown improvement in lesions via upregulation of mesencephalic astrocyte-derived neurotrophic factor that contributes to decreased inflammation and cell apoptosis.16 Additionally, the generation of reactive oxygen and nitrogen species has been shown to decrease Staphylococcus aureus colonization to improve atopic dermatitis lesions in patients.11
Many other proposed benefits of NTAP in dermatologic disease also have been proposed. Nonthermal atmospheric plasma has been shown to increase messenger RNA expression of proinflammatory cytokines (IL-1, IL-6) and upregulate type III collagen production in early stages of wound healing.17 Furthermore, NTAP has been shown to stimulate nuclear factor erythroid 2–related pathways involved in antioxidant production in keratinocytes, further promoting wound healing.18 Additionally, CAP has been shown to increase expression of caspases and induce mitochondrial dysfunction that promotes cell death in different cancer cell lines.19 It is clear that the exact breadth of NTAP’s biochemical effects are unknown, but the current literature shows promise for its use in cutaneous healing and cancer treatment.
Beyond its diverse applications, treatment with NTAP yields a unique advantage to pharmacologic therapies in that there is no risk for medication interactions or risk for pharmacologic adverse effects. Cantharidin is not approved by the US Food and Drug Administration but commonly is used to treat MC. It is a blister beetle extract that causes a blister to form when applied to the skin. When orally ingested, the drug is toxic to the gastrointestinal tract and kidneys because of its phosphodiesterase inhibition, a feared complication in pediatric patients who may inadvertently ingest it during treatment.20 This utility extends beyond MC, such as the beneficial outcomes described by Suwanchinda and Nararatwanchai10 in using NTAP for keloid scars. Treatment with NTAP may replace triamcinolone injections, which are commonly associated with skin atrophy and ulceration. In addition, NTAP application to the skin has been reported to be relatively painless.5 Thus, NTAP maintains a distinct advantage over other commonly used nonpharmacologic treatment options, including curettage and cryosurgery. Curettage has widely been noted to be traumatic for the patient, may be more likely to leave a mark, and is prone to user error.20 Cryosurgery is a common form of treatment for MC because it is cost-effective and has good cosmetic results; however, it is more painful than cantharidin or anesthetized curettage.21 Treatment with NTAP is an emerging therapeutic tool with an expanding role in the treatment of dermatologic patients because it provides advantages over many standard therapies due to its minimal side-effect profile involving pain and nonpharmacologic nature.
Limitations of this report include exclusion of non–English-language articles and lack of control or comparison groups to standard therapies across studies. Additionally, reports of NTAP success occurred in many conditions that are self-limited and may have resolved on their own. Regardless, we aimed to summarize how NTAP currently is being used in pediatric populations and highlight its potential uses moving forward. Given its promising safety profile and painless nature, future clinical trials should prioritize the investigation of NTAP use in common pediatric dermatologic conditions to determine if they are equal or superior to current standards of care.
- Gan L, Zhang S, Poorun D, et al. Medical applications of nonthermal atmospheric pressure plasma in dermatology. J Dtsch Dermatol Ges. 2018;16:7-13. doi:https://doi.org/10.1111/ddg.13373
- Gay-Mimbrera J, García MC, Isla-Tejera B, et al. Clinical and biological principles of cold atmospheric plasma application in skin cancer. Adv Ther. 2016;33:894-909. doi:10.1007/s12325-016-0338-1. Published correction appears in Adv Ther. 2017;34:280. doi:10.1007/s12325-016-0437-z
- Zhai SY, Kong MG, Xia YM. Cold atmospheric plasma ameliorates skin diseases involving reactive oxygen/nitrogen species-mediated functions. Front Immunol. 2022;13:868386. doi:10.3389/fimmu.2022.868386
- Tan F, Wang Y, Zhang S, et al. Plasma dermatology: skin therapy using cold atmospheric plasma. Front Oncol. 2022;12:918484. doi:10.3389/fonc.2022.918484
- van Welzen A, Hoch M, Wahl P, et al. The response and tolerability of a novel cold atmospheric plasma wound dressing for the healing of split skin graft donor sites: a controlled pilot study. Skin Pharmacol Physiol. 2021;34:328-336. doi:10.1159/000517524
- Friedman PC, Fridman G, Fridman A. Using cold plasma to treat warts in children: a case series. Pediatr Dermatol. 2020;37:706-709. doi:10.1111/pde.14180
- Zhang C, Zhao J, Gao Y, et al. Cold atmospheric plasma treatment for diaper dermatitis: a case report [published online January 27, 2021]. Dermatol Ther. 2021;34:E14739. doi:10.1111/dth.14739
- Friedman PC, Fridman G, Fridman A. Cold atmospheric pressure plasma clears molluscum contagiosum. Exp Dermatol. 2023;32:562-563. doi:10.1111/exd.14695
- Suwanchinda A, Nararatwanchai T. The efficacy and safety of the innovative cold atmospheric-pressure plasma technology in the treatment of striae distensae: a randomized controlled trial. J Cosmet Dermatol. 2022;21:6805-6814. doi:10.1111/jocd.15458
- Suwanchinda A, Nararatwanchai T. Efficacy and safety of the innovative cold atmospheric-pressure plasma technology in the treatment of keloid: a randomized controlled trial. J Cosmet Dermatol. 2022;21:6788-6797. doi:10.1111/jocd.15397
- Kim YJ, Lim DJ, Lee MY, et al. Prospective, comparative clinical pilot study of cold atmospheric plasma device in the treatment of atopic dermatitis. Sci Rep. 2021;11:14461. doi:10.1038/s41598-021-93941-y
- Gareri C, Bennardo L, De Masi G. Use of a new cold plasma tool for psoriasis treatment: a case report. SAGE Open Med Case Rep. 2020;8:2050313X20922709. doi:10.1177/2050313X20922709
- Zheng L, Gao J, Cao Y, et al. Two case reports of inverse psoriasis treated with cold atmospheric plasma. Dermatol Ther. 2020;33:E14257. doi:10.1111/dth.14257
- Arisi M, Venturuzzo A, Gelmetti A, et al. Cold atmospheric plasma (CAP) as a promising therapeutic option for mild to moderate acne vulgaris: clinical and non-invasive evaluation of two cases. Clin Plasma Med. 2020;19-20:100110.
- Isbary G, Köritzer J, Mitra A, et al. Ex vivo human skin experiments for the evaluation of safety of new cold atmospheric plasma devices. Clin Plasma Med. 2013;1:36-44.
- Sun T, Zhang X, Hou C, et al. Cold plasma irradiation attenuates atopic dermatitis via enhancing HIF-1α-induced MANF transcription expression. Front Immunol. 2022;13:941219. doi:10.3389/fimmu.2022.941219
- Eggers B, Marciniak J, Memmert S, et al. The beneficial effect of cold atmospheric plasma on parameters of molecules and cell function involved in wound healing in human osteoblast-like cells in vitro. Odontology. 2020;108:607-616. doi:10.1007/s10266-020-00487-y
- Conway GE, He Z, Hutanu AL, et al. Cold atmospheric plasma induces accumulation of lysosomes and caspase-independent cell death in U373MG glioblastoma multiforme cells. Sci Rep. 2019;9:12891. doi:10.1038/s41598-019-49013-3
- Schmidt A, Dietrich S, Steuer A, et al. Non-thermal plasma activates human keratinocytes by stimulation of antioxidant and phase II pathways. J Biol Chem. 2015;290:6731-6750. doi:10.1074/jbc.M114.603555
- Silverberg NB. Pediatric molluscum contagiosum. Pediatr Drugs. 2003;5:505-511. doi:10.2165/00148581-200305080-00001
- Cotton DW, Cooper C, Barrett DF, et al. Severe atypical molluscum contagiosum infection in an immunocompromised host. Br J Dermatol. 1987;116:871-876. doi:10.1111/j.1365-2133.1987.tb04908.x
Nonthermal atmospheric plasma (NTAP)(or cold atmospheric plasma [CAP]) is a rapidly developing treatment modality for a wide range of dermatologic conditions. Plasma (or ionized gas) refers to a state of matter composed of electrons, protons, and neutral atoms that generate reactive oxygen and nitrogen species.1 Plasma previously was created using thermal energy, but recent advances have allowed the creation of plasma using atmospheric pressure and room temperature; thus, NTAP can be used without causing damage to living tissue through heat.1 Plasma technology varies greatly, but it generally can be classified as either direct or indirect therapy; direct therapy uses the human body as an electrode, whereas indirect therapy creates plasma through the interaction between 2 electrode devices.1,2 When used on the skin, important dose-dependent relationships have been observed, with CAP application longer than 2 minutes being associated with increased keratinocyte and fibroblast apoptosis.2 Thus, CAP can cause diverse changes to the skin depending on application time and methodology. At adequate yet low concentrations, plasma can promote fibroblast proliferation and upregulate genes involved in collagen and transforming growth factor synthesis.1 Additionally, the reactive oxygen and nitrogen species created by NTAP have been shown to inactivate microorganisms through the destruction of biofilms, lead to diminished immune cell infiltration and cytokine release in autoimmune dermatologic conditions, and exert antitumor properties through cellular DNA damage.1-3 In dermatology, these properties can be harvested to promote wound healing at low doses and the treatment of proliferative skin conditions at high doses.1
Because of its novelty, the safety profile of NTAP is still under investigation, but preliminary studies are promising and show no damage to the skin barrier when excessive plasma exposure is avoided.4 However, dose- and time-dependent damage to cells has been shown. As a result, the exact dose of plasma considered safe is highly variable depending on the vessel, technique, and user, and future clinical research is needed to guide this methodology.4 Additionally, CAP has been shown to cause little pain at the skin surface and may lead to decreased levels of pain in healing wound sites.5 Given this promising safety profile and minimal discomfort to patients, NTAP technology remains promising for use in pediatric dermatology, but there are limited data to characterize its potential use in this population. In this systematic review, we aimed to elucidate reported applications of NTAP for skin conditions in children and discuss the trajectory of this technology in the future of pediatric dermatology.
Methodology
A comprehensive literature review was conducted to identify studies evaluating NTAP technology in pediatric populations using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines. A search of PubMed, Embase, and Web of Science articles was conducted in April 2023 using the terms nonthermal atmospheric plasma or cold atmospheric plasma. All English-language articles that described the use of NTAP as a treatment in pediatric populations or articles that described NTAP use in the treatment of common conditions in this patient group were included based on a review of the article titles and abstracts by 2 independent reviewers, followed by full-text review of relevant articles (M.G., C.L.). Any discrepancies in eligible articles were settled by a third independent researcher (M.V.). One hundred twenty studies were identified, and 95 were screened for inclusion; 9 studies met inclusion criteria and were summarized in this review.
Results
A total of 9 studies were included in this review: 3 describing the success of NTAP in pediatric populations6-8 and 6 describing the potential success of NTAP for dermatologic conditions commonly seen in children (Table).9-14

Studies Describing Success of NTAP—Three clinical reports described the efficacy of NTAP in pediatric dermatology. A case series from 2020 showed full clearance of warts in 100% of patients (n=5) with a 0% recurrence rate when NTAP treatment was applied for 2 minutes to each lesion during each treatment session with the electrode held 1 mm from the lesional surface.6 Each patient was followed up at 3 to 4 weeks, and treatment was repeated if lesions persisted. Patients reported no pain during the procedure, and no adverse effects were noted over the course of treatment.6 Second, a case report described full clearance of diaper dermatitis with no recurrence after 6 months following 6 treatments with NTAP in a 14-month-old girl.7 After treatment with econazole nitrate cream, oral antibiotics, and prednisone failed, CAP treatment was initiated. Each treatment lasted 15 minutes with 3-day time intervals between each of the 6 treatments. There were no adverse events or recurrence of rash at 6-month follow-up.7 A final case report described full clearance of molluscum contagiosum (MC), with no recurrence after 2 months following 4 treatments with NTAP in a 12-year-old boy.8 The patient had untreated MC on the face, neck, shoulder, and thighs. Lesions of the face were treated with CAP, while the other sites were treated with cantharidin using a 0.7% collodion-based solution. Four CAP treatments were performed at 1-month intervals, with CAP applied 1 mm from the lesional surfaces in a circular pattern for 2 minutes. At follow-up 2 months after the final treatment, the patient had no adverse effects and showed no pigmentary changes or scarring.8
Studies Describing the Potential Success of NTAP—Beyond these studies, limited research has been done on NTAP in pediatric populations. The Table summarizes 6 additional studies completed with promising treatment results for dermatologic conditions commonly seen in children: striae distensae, keloids, atopic dermatitis, psoriasis, inverse psoriasis, and acne vulgaris. Across all reports and studies, patients showed significant improvement in their dermatologic conditions following the use of NTAP technology with limited adverse effects reported (P<.05). Suwanchinda and Nararatwanchai9 studied the use of CAP for the treatment of striae distensae. They recruited 23 patients and treated half the body with CAP biweekly for 5 sessions; the other half was left untreated. At follow-up 30 days after the final treatment, striae distensae had improved for both patient and observer assessment scores.9 Another study performed by Suwanchinda and Nararatwanchai10 looked at the efficacy of CAP in treating keloids. They recruited 18 patients, and keloid scars were treated in halves—one half treated with CAP biweekly for 5 sessions and the other left untreated. At follow-up 30 days after the final treatment, keloids significantly improved in color, melanin, texture, and hemoglobin based on assessment by the Antera 3D imaging system (Miravex Limited)(P<.05).10
Kim et al11 studied the efficacy of CAP for the treatment of atopic dermatitis in 22 patients. Each patient had mild to moderate atopic dermatitis that had not been treated with topical agents or antibiotics for at least 2 weeks prior to beginning the study. Additionally, only patients with symmetric lesions—meaning only patients with lesions on both sides of the anatomical extremities—were included. Each patient then received CAP on 1 symmetric lesion and placebo on the other. Cold atmospheric plasma treatment was done 5 mm away from the lesion, and each treatment lasted for 5 minutes. Treatments were done at weeks 0, 1, and 2, with follow-up 4 weeks after the final treatment. The clinical severity of disease was assessed at weeks 0, 1, 2, and 4. Results showed that at week 4, the mean (SD) modified Atopic Dermatitis Antecubital Severity score decreased from 33.73 (21.21) at week 0 to 13.12 (15.92). Additionally, the pruritic visual analog scale showed significant improvement with treatment vs baseline (P≤.0001).11
Two studies examined how NTAP can be used in the treatment of psoriasis. First, Gareri et al12 used CAP to treat a psoriatic plaque in a 20-year-old woman. These plaques on the left hand previously had been unresponsive to topical psoriasis treatments. The patient received 2 treatments with CAP on days 0 and 3; at 14 days, the plaque completely resolved with an itch score of 0.12 Next, Zheng et al13 treated 2 patients with NTAP for inverse psoriasis. The first patient was a 26-year-old woman with plaques in the axilla and buttocks as well as inframammary lesions that failed to respond to treatment with topicals and vitamin D analogues. She received CAP treatments 2 to 3 times weekly for 5 total treatments with application to each region occurring 1 mm from the skin surface. The lesions completely resolved with no recurrence at 6 weeks. The second patient was a 38-year-old woman with inverse psoriasis in the axilla and groin; she received treatment every 3 days for 8 total treatments, which led to complete remission, with no recurrence noted at 1 month.13
Arisi et al14 used NTAP to treat acne vulgaris in 2 patients. The first patient was a 24-year-old man with moderate acne on the face that did not improve with topicals or oral antibiotics. The patient received 5 CAP treatments with no adverse events noted. The patient discontinued treatment on his own, but the number of lesions decreased after the fifth treatment. The second patient was a 21-year-old woman with moderate facial acne that failed to respond to treatment with topicals and oral tetracycline. The patient received 8 CAP treatments and experienced a reduction in the number of lesions during treatment. There were no adverse events, and improvement was maintained at 3-month follow-up.14
Comment
Although the use of NTAP in pediatric dermatology is scarcely described in the literature, the technology will certainly have applications in the future treatment of a wide variety of pediatric disorders. In addition to the clinical success shown in several studies,6-14 this technology has been shown to cause minimal damage to skin when application time is minimized. One study conducted on ex vivo skin showed that NTAP technology can safely be used for up to 2 minutes without major DNA damage.15 Through its diverse mechanisms of action, NTAP can induce modification of proteins and cell membranes in a noninvasive manner.2 In conditions with impaired barrier function, such as atopic and diaper dermatitis, studies in mouse models have shown improvement in lesions via upregulation of mesencephalic astrocyte-derived neurotrophic factor that contributes to decreased inflammation and cell apoptosis.16 Additionally, the generation of reactive oxygen and nitrogen species has been shown to decrease Staphylococcus aureus colonization to improve atopic dermatitis lesions in patients.11
Many other proposed benefits of NTAP in dermatologic disease also have been proposed. Nonthermal atmospheric plasma has been shown to increase messenger RNA expression of proinflammatory cytokines (IL-1, IL-6) and upregulate type III collagen production in early stages of wound healing.17 Furthermore, NTAP has been shown to stimulate nuclear factor erythroid 2–related pathways involved in antioxidant production in keratinocytes, further promoting wound healing.18 Additionally, CAP has been shown to increase expression of caspases and induce mitochondrial dysfunction that promotes cell death in different cancer cell lines.19 It is clear that the exact breadth of NTAP’s biochemical effects are unknown, but the current literature shows promise for its use in cutaneous healing and cancer treatment.
Beyond its diverse applications, treatment with NTAP yields a unique advantage to pharmacologic therapies in that there is no risk for medication interactions or risk for pharmacologic adverse effects. Cantharidin is not approved by the US Food and Drug Administration but commonly is used to treat MC. It is a blister beetle extract that causes a blister to form when applied to the skin. When orally ingested, the drug is toxic to the gastrointestinal tract and kidneys because of its phosphodiesterase inhibition, a feared complication in pediatric patients who may inadvertently ingest it during treatment.20 This utility extends beyond MC, such as the beneficial outcomes described by Suwanchinda and Nararatwanchai10 in using NTAP for keloid scars. Treatment with NTAP may replace triamcinolone injections, which are commonly associated with skin atrophy and ulceration. In addition, NTAP application to the skin has been reported to be relatively painless.5 Thus, NTAP maintains a distinct advantage over other commonly used nonpharmacologic treatment options, including curettage and cryosurgery. Curettage has widely been noted to be traumatic for the patient, may be more likely to leave a mark, and is prone to user error.20 Cryosurgery is a common form of treatment for MC because it is cost-effective and has good cosmetic results; however, it is more painful than cantharidin or anesthetized curettage.21 Treatment with NTAP is an emerging therapeutic tool with an expanding role in the treatment of dermatologic patients because it provides advantages over many standard therapies due to its minimal side-effect profile involving pain and nonpharmacologic nature.
Limitations of this report include exclusion of non–English-language articles and lack of control or comparison groups to standard therapies across studies. Additionally, reports of NTAP success occurred in many conditions that are self-limited and may have resolved on their own. Regardless, we aimed to summarize how NTAP currently is being used in pediatric populations and highlight its potential uses moving forward. Given its promising safety profile and painless nature, future clinical trials should prioritize the investigation of NTAP use in common pediatric dermatologic conditions to determine if they are equal or superior to current standards of care.
Nonthermal atmospheric plasma (NTAP)(or cold atmospheric plasma [CAP]) is a rapidly developing treatment modality for a wide range of dermatologic conditions. Plasma (or ionized gas) refers to a state of matter composed of electrons, protons, and neutral atoms that generate reactive oxygen and nitrogen species.1 Plasma previously was created using thermal energy, but recent advances have allowed the creation of plasma using atmospheric pressure and room temperature; thus, NTAP can be used without causing damage to living tissue through heat.1 Plasma technology varies greatly, but it generally can be classified as either direct or indirect therapy; direct therapy uses the human body as an electrode, whereas indirect therapy creates plasma through the interaction between 2 electrode devices.1,2 When used on the skin, important dose-dependent relationships have been observed, with CAP application longer than 2 minutes being associated with increased keratinocyte and fibroblast apoptosis.2 Thus, CAP can cause diverse changes to the skin depending on application time and methodology. At adequate yet low concentrations, plasma can promote fibroblast proliferation and upregulate genes involved in collagen and transforming growth factor synthesis.1 Additionally, the reactive oxygen and nitrogen species created by NTAP have been shown to inactivate microorganisms through the destruction of biofilms, lead to diminished immune cell infiltration and cytokine release in autoimmune dermatologic conditions, and exert antitumor properties through cellular DNA damage.1-3 In dermatology, these properties can be harvested to promote wound healing at low doses and the treatment of proliferative skin conditions at high doses.1
Because of its novelty, the safety profile of NTAP is still under investigation, but preliminary studies are promising and show no damage to the skin barrier when excessive plasma exposure is avoided.4 However, dose- and time-dependent damage to cells has been shown. As a result, the exact dose of plasma considered safe is highly variable depending on the vessel, technique, and user, and future clinical research is needed to guide this methodology.4 Additionally, CAP has been shown to cause little pain at the skin surface and may lead to decreased levels of pain in healing wound sites.5 Given this promising safety profile and minimal discomfort to patients, NTAP technology remains promising for use in pediatric dermatology, but there are limited data to characterize its potential use in this population. In this systematic review, we aimed to elucidate reported applications of NTAP for skin conditions in children and discuss the trajectory of this technology in the future of pediatric dermatology.
Methodology
A comprehensive literature review was conducted to identify studies evaluating NTAP technology in pediatric populations using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines. A search of PubMed, Embase, and Web of Science articles was conducted in April 2023 using the terms nonthermal atmospheric plasma or cold atmospheric plasma. All English-language articles that described the use of NTAP as a treatment in pediatric populations or articles that described NTAP use in the treatment of common conditions in this patient group were included based on a review of the article titles and abstracts by 2 independent reviewers, followed by full-text review of relevant articles (M.G., C.L.). Any discrepancies in eligible articles were settled by a third independent researcher (M.V.). One hundred twenty studies were identified, and 95 were screened for inclusion; 9 studies met inclusion criteria and were summarized in this review.
Results
A total of 9 studies were included in this review: 3 describing the success of NTAP in pediatric populations6-8 and 6 describing the potential success of NTAP for dermatologic conditions commonly seen in children (Table).9-14

Studies Describing Success of NTAP—Three clinical reports described the efficacy of NTAP in pediatric dermatology. A case series from 2020 showed full clearance of warts in 100% of patients (n=5) with a 0% recurrence rate when NTAP treatment was applied for 2 minutes to each lesion during each treatment session with the electrode held 1 mm from the lesional surface.6 Each patient was followed up at 3 to 4 weeks, and treatment was repeated if lesions persisted. Patients reported no pain during the procedure, and no adverse effects were noted over the course of treatment.6 Second, a case report described full clearance of diaper dermatitis with no recurrence after 6 months following 6 treatments with NTAP in a 14-month-old girl.7 After treatment with econazole nitrate cream, oral antibiotics, and prednisone failed, CAP treatment was initiated. Each treatment lasted 15 minutes with 3-day time intervals between each of the 6 treatments. There were no adverse events or recurrence of rash at 6-month follow-up.7 A final case report described full clearance of molluscum contagiosum (MC), with no recurrence after 2 months following 4 treatments with NTAP in a 12-year-old boy.8 The patient had untreated MC on the face, neck, shoulder, and thighs. Lesions of the face were treated with CAP, while the other sites were treated with cantharidin using a 0.7% collodion-based solution. Four CAP treatments were performed at 1-month intervals, with CAP applied 1 mm from the lesional surfaces in a circular pattern for 2 minutes. At follow-up 2 months after the final treatment, the patient had no adverse effects and showed no pigmentary changes or scarring.8
Studies Describing the Potential Success of NTAP—Beyond these studies, limited research has been done on NTAP in pediatric populations. The Table summarizes 6 additional studies completed with promising treatment results for dermatologic conditions commonly seen in children: striae distensae, keloids, atopic dermatitis, psoriasis, inverse psoriasis, and acne vulgaris. Across all reports and studies, patients showed significant improvement in their dermatologic conditions following the use of NTAP technology with limited adverse effects reported (P<.05). Suwanchinda and Nararatwanchai9 studied the use of CAP for the treatment of striae distensae. They recruited 23 patients and treated half the body with CAP biweekly for 5 sessions; the other half was left untreated. At follow-up 30 days after the final treatment, striae distensae had improved for both patient and observer assessment scores.9 Another study performed by Suwanchinda and Nararatwanchai10 looked at the efficacy of CAP in treating keloids. They recruited 18 patients, and keloid scars were treated in halves—one half treated with CAP biweekly for 5 sessions and the other left untreated. At follow-up 30 days after the final treatment, keloids significantly improved in color, melanin, texture, and hemoglobin based on assessment by the Antera 3D imaging system (Miravex Limited)(P<.05).10
Kim et al11 studied the efficacy of CAP for the treatment of atopic dermatitis in 22 patients. Each patient had mild to moderate atopic dermatitis that had not been treated with topical agents or antibiotics for at least 2 weeks prior to beginning the study. Additionally, only patients with symmetric lesions—meaning only patients with lesions on both sides of the anatomical extremities—were included. Each patient then received CAP on 1 symmetric lesion and placebo on the other. Cold atmospheric plasma treatment was done 5 mm away from the lesion, and each treatment lasted for 5 minutes. Treatments were done at weeks 0, 1, and 2, with follow-up 4 weeks after the final treatment. The clinical severity of disease was assessed at weeks 0, 1, 2, and 4. Results showed that at week 4, the mean (SD) modified Atopic Dermatitis Antecubital Severity score decreased from 33.73 (21.21) at week 0 to 13.12 (15.92). Additionally, the pruritic visual analog scale showed significant improvement with treatment vs baseline (P≤.0001).11
Two studies examined how NTAP can be used in the treatment of psoriasis. First, Gareri et al12 used CAP to treat a psoriatic plaque in a 20-year-old woman. These plaques on the left hand previously had been unresponsive to topical psoriasis treatments. The patient received 2 treatments with CAP on days 0 and 3; at 14 days, the plaque completely resolved with an itch score of 0.12 Next, Zheng et al13 treated 2 patients with NTAP for inverse psoriasis. The first patient was a 26-year-old woman with plaques in the axilla and buttocks as well as inframammary lesions that failed to respond to treatment with topicals and vitamin D analogues. She received CAP treatments 2 to 3 times weekly for 5 total treatments with application to each region occurring 1 mm from the skin surface. The lesions completely resolved with no recurrence at 6 weeks. The second patient was a 38-year-old woman with inverse psoriasis in the axilla and groin; she received treatment every 3 days for 8 total treatments, which led to complete remission, with no recurrence noted at 1 month.13
Arisi et al14 used NTAP to treat acne vulgaris in 2 patients. The first patient was a 24-year-old man with moderate acne on the face that did not improve with topicals or oral antibiotics. The patient received 5 CAP treatments with no adverse events noted. The patient discontinued treatment on his own, but the number of lesions decreased after the fifth treatment. The second patient was a 21-year-old woman with moderate facial acne that failed to respond to treatment with topicals and oral tetracycline. The patient received 8 CAP treatments and experienced a reduction in the number of lesions during treatment. There were no adverse events, and improvement was maintained at 3-month follow-up.14
Comment
Although the use of NTAP in pediatric dermatology is scarcely described in the literature, the technology will certainly have applications in the future treatment of a wide variety of pediatric disorders. In addition to the clinical success shown in several studies,6-14 this technology has been shown to cause minimal damage to skin when application time is minimized. One study conducted on ex vivo skin showed that NTAP technology can safely be used for up to 2 minutes without major DNA damage.15 Through its diverse mechanisms of action, NTAP can induce modification of proteins and cell membranes in a noninvasive manner.2 In conditions with impaired barrier function, such as atopic and diaper dermatitis, studies in mouse models have shown improvement in lesions via upregulation of mesencephalic astrocyte-derived neurotrophic factor that contributes to decreased inflammation and cell apoptosis.16 Additionally, the generation of reactive oxygen and nitrogen species has been shown to decrease Staphylococcus aureus colonization to improve atopic dermatitis lesions in patients.11
Many other proposed benefits of NTAP in dermatologic disease also have been proposed. Nonthermal atmospheric plasma has been shown to increase messenger RNA expression of proinflammatory cytokines (IL-1, IL-6) and upregulate type III collagen production in early stages of wound healing.17 Furthermore, NTAP has been shown to stimulate nuclear factor erythroid 2–related pathways involved in antioxidant production in keratinocytes, further promoting wound healing.18 Additionally, CAP has been shown to increase expression of caspases and induce mitochondrial dysfunction that promotes cell death in different cancer cell lines.19 It is clear that the exact breadth of NTAP’s biochemical effects are unknown, but the current literature shows promise for its use in cutaneous healing and cancer treatment.
Beyond its diverse applications, treatment with NTAP yields a unique advantage to pharmacologic therapies in that there is no risk for medication interactions or risk for pharmacologic adverse effects. Cantharidin is not approved by the US Food and Drug Administration but commonly is used to treat MC. It is a blister beetle extract that causes a blister to form when applied to the skin. When orally ingested, the drug is toxic to the gastrointestinal tract and kidneys because of its phosphodiesterase inhibition, a feared complication in pediatric patients who may inadvertently ingest it during treatment.20 This utility extends beyond MC, such as the beneficial outcomes described by Suwanchinda and Nararatwanchai10 in using NTAP for keloid scars. Treatment with NTAP may replace triamcinolone injections, which are commonly associated with skin atrophy and ulceration. In addition, NTAP application to the skin has been reported to be relatively painless.5 Thus, NTAP maintains a distinct advantage over other commonly used nonpharmacologic treatment options, including curettage and cryosurgery. Curettage has widely been noted to be traumatic for the patient, may be more likely to leave a mark, and is prone to user error.20 Cryosurgery is a common form of treatment for MC because it is cost-effective and has good cosmetic results; however, it is more painful than cantharidin or anesthetized curettage.21 Treatment with NTAP is an emerging therapeutic tool with an expanding role in the treatment of dermatologic patients because it provides advantages over many standard therapies due to its minimal side-effect profile involving pain and nonpharmacologic nature.
Limitations of this report include exclusion of non–English-language articles and lack of control or comparison groups to standard therapies across studies. Additionally, reports of NTAP success occurred in many conditions that are self-limited and may have resolved on their own. Regardless, we aimed to summarize how NTAP currently is being used in pediatric populations and highlight its potential uses moving forward. Given its promising safety profile and painless nature, future clinical trials should prioritize the investigation of NTAP use in common pediatric dermatologic conditions to determine if they are equal or superior to current standards of care.
- Gan L, Zhang S, Poorun D, et al. Medical applications of nonthermal atmospheric pressure plasma in dermatology. J Dtsch Dermatol Ges. 2018;16:7-13. doi:https://doi.org/10.1111/ddg.13373
- Gay-Mimbrera J, García MC, Isla-Tejera B, et al. Clinical and biological principles of cold atmospheric plasma application in skin cancer. Adv Ther. 2016;33:894-909. doi:10.1007/s12325-016-0338-1. Published correction appears in Adv Ther. 2017;34:280. doi:10.1007/s12325-016-0437-z
- Zhai SY, Kong MG, Xia YM. Cold atmospheric plasma ameliorates skin diseases involving reactive oxygen/nitrogen species-mediated functions. Front Immunol. 2022;13:868386. doi:10.3389/fimmu.2022.868386
- Tan F, Wang Y, Zhang S, et al. Plasma dermatology: skin therapy using cold atmospheric plasma. Front Oncol. 2022;12:918484. doi:10.3389/fonc.2022.918484
- van Welzen A, Hoch M, Wahl P, et al. The response and tolerability of a novel cold atmospheric plasma wound dressing for the healing of split skin graft donor sites: a controlled pilot study. Skin Pharmacol Physiol. 2021;34:328-336. doi:10.1159/000517524
- Friedman PC, Fridman G, Fridman A. Using cold plasma to treat warts in children: a case series. Pediatr Dermatol. 2020;37:706-709. doi:10.1111/pde.14180
- Zhang C, Zhao J, Gao Y, et al. Cold atmospheric plasma treatment for diaper dermatitis: a case report [published online January 27, 2021]. Dermatol Ther. 2021;34:E14739. doi:10.1111/dth.14739
- Friedman PC, Fridman G, Fridman A. Cold atmospheric pressure plasma clears molluscum contagiosum. Exp Dermatol. 2023;32:562-563. doi:10.1111/exd.14695
- Suwanchinda A, Nararatwanchai T. The efficacy and safety of the innovative cold atmospheric-pressure plasma technology in the treatment of striae distensae: a randomized controlled trial. J Cosmet Dermatol. 2022;21:6805-6814. doi:10.1111/jocd.15458
- Suwanchinda A, Nararatwanchai T. Efficacy and safety of the innovative cold atmospheric-pressure plasma technology in the treatment of keloid: a randomized controlled trial. J Cosmet Dermatol. 2022;21:6788-6797. doi:10.1111/jocd.15397
- Kim YJ, Lim DJ, Lee MY, et al. Prospective, comparative clinical pilot study of cold atmospheric plasma device in the treatment of atopic dermatitis. Sci Rep. 2021;11:14461. doi:10.1038/s41598-021-93941-y
- Gareri C, Bennardo L, De Masi G. Use of a new cold plasma tool for psoriasis treatment: a case report. SAGE Open Med Case Rep. 2020;8:2050313X20922709. doi:10.1177/2050313X20922709
- Zheng L, Gao J, Cao Y, et al. Two case reports of inverse psoriasis treated with cold atmospheric plasma. Dermatol Ther. 2020;33:E14257. doi:10.1111/dth.14257
- Arisi M, Venturuzzo A, Gelmetti A, et al. Cold atmospheric plasma (CAP) as a promising therapeutic option for mild to moderate acne vulgaris: clinical and non-invasive evaluation of two cases. Clin Plasma Med. 2020;19-20:100110.
- Isbary G, Köritzer J, Mitra A, et al. Ex vivo human skin experiments for the evaluation of safety of new cold atmospheric plasma devices. Clin Plasma Med. 2013;1:36-44.
- Sun T, Zhang X, Hou C, et al. Cold plasma irradiation attenuates atopic dermatitis via enhancing HIF-1α-induced MANF transcription expression. Front Immunol. 2022;13:941219. doi:10.3389/fimmu.2022.941219
- Eggers B, Marciniak J, Memmert S, et al. The beneficial effect of cold atmospheric plasma on parameters of molecules and cell function involved in wound healing in human osteoblast-like cells in vitro. Odontology. 2020;108:607-616. doi:10.1007/s10266-020-00487-y
- Conway GE, He Z, Hutanu AL, et al. Cold atmospheric plasma induces accumulation of lysosomes and caspase-independent cell death in U373MG glioblastoma multiforme cells. Sci Rep. 2019;9:12891. doi:10.1038/s41598-019-49013-3
- Schmidt A, Dietrich S, Steuer A, et al. Non-thermal plasma activates human keratinocytes by stimulation of antioxidant and phase II pathways. J Biol Chem. 2015;290:6731-6750. doi:10.1074/jbc.M114.603555
- Silverberg NB. Pediatric molluscum contagiosum. Pediatr Drugs. 2003;5:505-511. doi:10.2165/00148581-200305080-00001
- Cotton DW, Cooper C, Barrett DF, et al. Severe atypical molluscum contagiosum infection in an immunocompromised host. Br J Dermatol. 1987;116:871-876. doi:10.1111/j.1365-2133.1987.tb04908.x
- Gan L, Zhang S, Poorun D, et al. Medical applications of nonthermal atmospheric pressure plasma in dermatology. J Dtsch Dermatol Ges. 2018;16:7-13. doi:https://doi.org/10.1111/ddg.13373
- Gay-Mimbrera J, García MC, Isla-Tejera B, et al. Clinical and biological principles of cold atmospheric plasma application in skin cancer. Adv Ther. 2016;33:894-909. doi:10.1007/s12325-016-0338-1. Published correction appears in Adv Ther. 2017;34:280. doi:10.1007/s12325-016-0437-z
- Zhai SY, Kong MG, Xia YM. Cold atmospheric plasma ameliorates skin diseases involving reactive oxygen/nitrogen species-mediated functions. Front Immunol. 2022;13:868386. doi:10.3389/fimmu.2022.868386
- Tan F, Wang Y, Zhang S, et al. Plasma dermatology: skin therapy using cold atmospheric plasma. Front Oncol. 2022;12:918484. doi:10.3389/fonc.2022.918484
- van Welzen A, Hoch M, Wahl P, et al. The response and tolerability of a novel cold atmospheric plasma wound dressing for the healing of split skin graft donor sites: a controlled pilot study. Skin Pharmacol Physiol. 2021;34:328-336. doi:10.1159/000517524
- Friedman PC, Fridman G, Fridman A. Using cold plasma to treat warts in children: a case series. Pediatr Dermatol. 2020;37:706-709. doi:10.1111/pde.14180
- Zhang C, Zhao J, Gao Y, et al. Cold atmospheric plasma treatment for diaper dermatitis: a case report [published online January 27, 2021]. Dermatol Ther. 2021;34:E14739. doi:10.1111/dth.14739
- Friedman PC, Fridman G, Fridman A. Cold atmospheric pressure plasma clears molluscum contagiosum. Exp Dermatol. 2023;32:562-563. doi:10.1111/exd.14695
- Suwanchinda A, Nararatwanchai T. The efficacy and safety of the innovative cold atmospheric-pressure plasma technology in the treatment of striae distensae: a randomized controlled trial. J Cosmet Dermatol. 2022;21:6805-6814. doi:10.1111/jocd.15458
- Suwanchinda A, Nararatwanchai T. Efficacy and safety of the innovative cold atmospheric-pressure plasma technology in the treatment of keloid: a randomized controlled trial. J Cosmet Dermatol. 2022;21:6788-6797. doi:10.1111/jocd.15397
- Kim YJ, Lim DJ, Lee MY, et al. Prospective, comparative clinical pilot study of cold atmospheric plasma device in the treatment of atopic dermatitis. Sci Rep. 2021;11:14461. doi:10.1038/s41598-021-93941-y
- Gareri C, Bennardo L, De Masi G. Use of a new cold plasma tool for psoriasis treatment: a case report. SAGE Open Med Case Rep. 2020;8:2050313X20922709. doi:10.1177/2050313X20922709
- Zheng L, Gao J, Cao Y, et al. Two case reports of inverse psoriasis treated with cold atmospheric plasma. Dermatol Ther. 2020;33:E14257. doi:10.1111/dth.14257
- Arisi M, Venturuzzo A, Gelmetti A, et al. Cold atmospheric plasma (CAP) as a promising therapeutic option for mild to moderate acne vulgaris: clinical and non-invasive evaluation of two cases. Clin Plasma Med. 2020;19-20:100110.
- Isbary G, Köritzer J, Mitra A, et al. Ex vivo human skin experiments for the evaluation of safety of new cold atmospheric plasma devices. Clin Plasma Med. 2013;1:36-44.
- Sun T, Zhang X, Hou C, et al. Cold plasma irradiation attenuates atopic dermatitis via enhancing HIF-1α-induced MANF transcription expression. Front Immunol. 2022;13:941219. doi:10.3389/fimmu.2022.941219
- Eggers B, Marciniak J, Memmert S, et al. The beneficial effect of cold atmospheric plasma on parameters of molecules and cell function involved in wound healing in human osteoblast-like cells in vitro. Odontology. 2020;108:607-616. doi:10.1007/s10266-020-00487-y
- Conway GE, He Z, Hutanu AL, et al. Cold atmospheric plasma induces accumulation of lysosomes and caspase-independent cell death in U373MG glioblastoma multiforme cells. Sci Rep. 2019;9:12891. doi:10.1038/s41598-019-49013-3
- Schmidt A, Dietrich S, Steuer A, et al. Non-thermal plasma activates human keratinocytes by stimulation of antioxidant and phase II pathways. J Biol Chem. 2015;290:6731-6750. doi:10.1074/jbc.M114.603555
- Silverberg NB. Pediatric molluscum contagiosum. Pediatr Drugs. 2003;5:505-511. doi:10.2165/00148581-200305080-00001
- Cotton DW, Cooper C, Barrett DF, et al. Severe atypical molluscum contagiosum infection in an immunocompromised host. Br J Dermatol. 1987;116:871-876. doi:10.1111/j.1365-2133.1987.tb04908.x
Practice Points
- Nonthermal atmospheric plasma (NTAP)(also known as cold atmospheric plasma) has been shown to cause minimal damage to skin when application time is minimized.
- Beyond its diverse applications, treatment with NTAP yields a unique advantage to pharmacologic therapies in that there is no risk for medication interactions or pharmacologic adverse effects.
- Although the use of NTAP in pediatric dermatology is scarcely described in the literature, the technology will certainly have applications in the future treatment of a wide variety of pediatric disorders.