User login
Potential Uses of Nonthermal Atmospheric Pressure Technology for Dermatologic Conditions in Children
Nonthermal atmospheric plasma (NTAP)(or cold atmospheric plasma [CAP]) is a rapidly developing treatment modality for a wide range of dermatologic conditions. Plasma (or ionized gas) refers to a state of matter composed of electrons, protons, and neutral atoms that generate reactive oxygen and nitrogen species.1 Plasma previously was created using thermal energy, but recent advances have allowed the creation of plasma using atmospheric pressure and room temperature; thus, NTAP can be used without causing damage to living tissue through heat.1 Plasma technology varies greatly, but it generally can be classified as either direct or indirect therapy; direct therapy uses the human body as an electrode, whereas indirect therapy creates plasma through the interaction between 2 electrode devices.1,2 When used on the skin, important dose-dependent relationships have been observed, with CAP application longer than 2 minutes being associated with increased keratinocyte and fibroblast apoptosis.2 Thus, CAP can cause diverse changes to the skin depending on application time and methodology. At adequate yet low concentrations, plasma can promote fibroblast proliferation and upregulate genes involved in collagen and transforming growth factor synthesis.1 Additionally, the reactive oxygen and nitrogen species created by NTAP have been shown to inactivate microorganisms through the destruction of biofilms, lead to diminished immune cell infiltration and cytokine release in autoimmune dermatologic conditions, and exert antitumor properties through cellular DNA damage.1-3 In dermatology, these properties can be harvested to promote wound healing at low doses and the treatment of proliferative skin conditions at high doses.1
Because of its novelty, the safety profile of NTAP is still under investigation, but preliminary studies are promising and show no damage to the skin barrier when excessive plasma exposure is avoided.4 However, dose- and time-dependent damage to cells has been shown. As a result, the exact dose of plasma considered safe is highly variable depending on the vessel, technique, and user, and future clinical research is needed to guide this methodology.4 Additionally, CAP has been shown to cause little pain at the skin surface and may lead to decreased levels of pain in healing wound sites.5 Given this promising safety profile and minimal discomfort to patients, NTAP technology remains promising for use in pediatric dermatology, but there are limited data to characterize its potential use in this population. In this systematic review, we aimed to elucidate reported applications of NTAP for skin conditions in children and discuss the trajectory of this technology in the future of pediatric dermatology.
Methodology
A comprehensive literature review was conducted to identify studies evaluating NTAP technology in pediatric populations using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines. A search of PubMed, Embase, and Web of Science articles was conducted in April 2023 using the terms nonthermal atmospheric plasma or cold atmospheric plasma. All English-language articles that described the use of NTAP as a treatment in pediatric populations or articles that described NTAP use in the treatment of common conditions in this patient group were included based on a review of the article titles and abstracts by 2 independent reviewers, followed by full-text review of relevant articles (M.G., C.L.). Any discrepancies in eligible articles were settled by a third independent researcher (M.V.). One hundred twenty studies were identified, and 95 were screened for inclusion; 9 studies met inclusion criteria and were summarized in this review.
Results
A total of 9 studies were included in this review: 3 describing the success of NTAP in pediatric populations6-8 and 6 describing the potential success of NTAP for dermatologic conditions commonly seen in children (Table).9-14

Studies Describing Success of NTAP—Three clinical reports described the efficacy of NTAP in pediatric dermatology. A case series from 2020 showed full clearance of warts in 100% of patients (n=5) with a 0% recurrence rate when NTAP treatment was applied for 2 minutes to each lesion during each treatment session with the electrode held 1 mm from the lesional surface.6 Each patient was followed up at 3 to 4 weeks, and treatment was repeated if lesions persisted. Patients reported no pain during the procedure, and no adverse effects were noted over the course of treatment.6 Second, a case report described full clearance of diaper dermatitis with no recurrence after 6 months following 6 treatments with NTAP in a 14-month-old girl.7 After treatment with econazole nitrate cream, oral antibiotics, and prednisone failed, CAP treatment was initiated. Each treatment lasted 15 minutes with 3-day time intervals between each of the 6 treatments. There were no adverse events or recurrence of rash at 6-month follow-up.7 A final case report described full clearance of molluscum contagiosum (MC), with no recurrence after 2 months following 4 treatments with NTAP in a 12-year-old boy.8 The patient had untreated MC on the face, neck, shoulder, and thighs. Lesions of the face were treated with CAP, while the other sites were treated with cantharidin using a 0.7% collodion-based solution. Four CAP treatments were performed at 1-month intervals, with CAP applied 1 mm from the lesional surfaces in a circular pattern for 2 minutes. At follow-up 2 months after the final treatment, the patient had no adverse effects and showed no pigmentary changes or scarring.8
Studies Describing the Potential Success of NTAP—Beyond these studies, limited research has been done on NTAP in pediatric populations. The Table summarizes 6 additional studies completed with promising treatment results for dermatologic conditions commonly seen in children: striae distensae, keloids, atopic dermatitis, psoriasis, inverse psoriasis, and acne vulgaris. Across all reports and studies, patients showed significant improvement in their dermatologic conditions following the use of NTAP technology with limited adverse effects reported (P<.05). Suwanchinda and Nararatwanchai9 studied the use of CAP for the treatment of striae distensae. They recruited 23 patients and treated half the body with CAP biweekly for 5 sessions; the other half was left untreated. At follow-up 30 days after the final treatment, striae distensae had improved for both patient and observer assessment scores.9 Another study performed by Suwanchinda and Nararatwanchai10 looked at the efficacy of CAP in treating keloids. They recruited 18 patients, and keloid scars were treated in halves—one half treated with CAP biweekly for 5 sessions and the other left untreated. At follow-up 30 days after the final treatment, keloids significantly improved in color, melanin, texture, and hemoglobin based on assessment by the Antera 3D imaging system (Miravex Limited)(P<.05).10
Kim et al11 studied the efficacy of CAP for the treatment of atopic dermatitis in 22 patients. Each patient had mild to moderate atopic dermatitis that had not been treated with topical agents or antibiotics for at least 2 weeks prior to beginning the study. Additionally, only patients with symmetric lesions—meaning only patients with lesions on both sides of the anatomical extremities—were included. Each patient then received CAP on 1 symmetric lesion and placebo on the other. Cold atmospheric plasma treatment was done 5 mm away from the lesion, and each treatment lasted for 5 minutes. Treatments were done at weeks 0, 1, and 2, with follow-up 4 weeks after the final treatment. The clinical severity of disease was assessed at weeks 0, 1, 2, and 4. Results showed that at week 4, the mean (SD) modified Atopic Dermatitis Antecubital Severity score decreased from 33.73 (21.21) at week 0 to 13.12 (15.92). Additionally, the pruritic visual analog scale showed significant improvement with treatment vs baseline (P≤.0001).11
Two studies examined how NTAP can be used in the treatment of psoriasis. First, Gareri et al12 used CAP to treat a psoriatic plaque in a 20-year-old woman. These plaques on the left hand previously had been unresponsive to topical psoriasis treatments. The patient received 2 treatments with CAP on days 0 and 3; at 14 days, the plaque completely resolved with an itch score of 0.12 Next, Zheng et al13 treated 2 patients with NTAP for inverse psoriasis. The first patient was a 26-year-old woman with plaques in the axilla and buttocks as well as inframammary lesions that failed to respond to treatment with topicals and vitamin D analogues. She received CAP treatments 2 to 3 times weekly for 5 total treatments with application to each region occurring 1 mm from the skin surface. The lesions completely resolved with no recurrence at 6 weeks. The second patient was a 38-year-old woman with inverse psoriasis in the axilla and groin; she received treatment every 3 days for 8 total treatments, which led to complete remission, with no recurrence noted at 1 month.13
Arisi et al14 used NTAP to treat acne vulgaris in 2 patients. The first patient was a 24-year-old man with moderate acne on the face that did not improve with topicals or oral antibiotics. The patient received 5 CAP treatments with no adverse events noted. The patient discontinued treatment on his own, but the number of lesions decreased after the fifth treatment. The second patient was a 21-year-old woman with moderate facial acne that failed to respond to treatment with topicals and oral tetracycline. The patient received 8 CAP treatments and experienced a reduction in the number of lesions during treatment. There were no adverse events, and improvement was maintained at 3-month follow-up.14
Comment
Although the use of NTAP in pediatric dermatology is scarcely described in the literature, the technology will certainly have applications in the future treatment of a wide variety of pediatric disorders. In addition to the clinical success shown in several studies,6-14 this technology has been shown to cause minimal damage to skin when application time is minimized. One study conducted on ex vivo skin showed that NTAP technology can safely be used for up to 2 minutes without major DNA damage.15 Through its diverse mechanisms of action, NTAP can induce modification of proteins and cell membranes in a noninvasive manner.2 In conditions with impaired barrier function, such as atopic and diaper dermatitis, studies in mouse models have shown improvement in lesions via upregulation of mesencephalic astrocyte-derived neurotrophic factor that contributes to decreased inflammation and cell apoptosis.16 Additionally, the generation of reactive oxygen and nitrogen species has been shown to decrease Staphylococcus aureus colonization to improve atopic dermatitis lesions in patients.11
Many other proposed benefits of NTAP in dermatologic disease also have been proposed. Nonthermal atmospheric plasma has been shown to increase messenger RNA expression of proinflammatory cytokines (IL-1, IL-6) and upregulate type III collagen production in early stages of wound healing.17 Furthermore, NTAP has been shown to stimulate nuclear factor erythroid 2–related pathways involved in antioxidant production in keratinocytes, further promoting wound healing.18 Additionally, CAP has been shown to increase expression of caspases and induce mitochondrial dysfunction that promotes cell death in different cancer cell lines.19 It is clear that the exact breadth of NTAP’s biochemical effects are unknown, but the current literature shows promise for its use in cutaneous healing and cancer treatment.
Beyond its diverse applications, treatment with NTAP yields a unique advantage to pharmacologic therapies in that there is no risk for medication interactions or risk for pharmacologic adverse effects. Cantharidin is not approved by the US Food and Drug Administration but commonly is used to treat MC. It is a blister beetle extract that causes a blister to form when applied to the skin. When orally ingested, the drug is toxic to the gastrointestinal tract and kidneys because of its phosphodiesterase inhibition, a feared complication in pediatric patients who may inadvertently ingest it during treatment.20 This utility extends beyond MC, such as the beneficial outcomes described by Suwanchinda and Nararatwanchai10 in using NTAP for keloid scars. Treatment with NTAP may replace triamcinolone injections, which are commonly associated with skin atrophy and ulceration. In addition, NTAP application to the skin has been reported to be relatively painless.5 Thus, NTAP maintains a distinct advantage over other commonly used nonpharmacologic treatment options, including curettage and cryosurgery. Curettage has widely been noted to be traumatic for the patient, may be more likely to leave a mark, and is prone to user error.20 Cryosurgery is a common form of treatment for MC because it is cost-effective and has good cosmetic results; however, it is more painful than cantharidin or anesthetized curettage.21 Treatment with NTAP is an emerging therapeutic tool with an expanding role in the treatment of dermatologic patients because it provides advantages over many standard therapies due to its minimal side-effect profile involving pain and nonpharmacologic nature.
Limitations of this report include exclusion of non–English-language articles and lack of control or comparison groups to standard therapies across studies. Additionally, reports of NTAP success occurred in many conditions that are self-limited and may have resolved on their own. Regardless, we aimed to summarize how NTAP currently is being used in pediatric populations and highlight its potential uses moving forward. Given its promising safety profile and painless nature, future clinical trials should prioritize the investigation of NTAP use in common pediatric dermatologic conditions to determine if they are equal or superior to current standards of care.
- Gan L, Zhang S, Poorun D, et al. Medical applications of nonthermal atmospheric pressure plasma in dermatology. J Dtsch Dermatol Ges. 2018;16:7-13. doi:https://doi.org/10.1111/ddg.13373
- Gay-Mimbrera J, García MC, Isla-Tejera B, et al. Clinical and biological principles of cold atmospheric plasma application in skin cancer. Adv Ther. 2016;33:894-909. doi:10.1007/s12325-016-0338-1. Published correction appears in Adv Ther. 2017;34:280. doi:10.1007/s12325-016-0437-z
- Zhai SY, Kong MG, Xia YM. Cold atmospheric plasma ameliorates skin diseases involving reactive oxygen/nitrogen species-mediated functions. Front Immunol. 2022;13:868386. doi:10.3389/fimmu.2022.868386
- Tan F, Wang Y, Zhang S, et al. Plasma dermatology: skin therapy using cold atmospheric plasma. Front Oncol. 2022;12:918484. doi:10.3389/fonc.2022.918484
- van Welzen A, Hoch M, Wahl P, et al. The response and tolerability of a novel cold atmospheric plasma wound dressing for the healing of split skin graft donor sites: a controlled pilot study. Skin Pharmacol Physiol. 2021;34:328-336. doi:10.1159/000517524
- Friedman PC, Fridman G, Fridman A. Using cold plasma to treat warts in children: a case series. Pediatr Dermatol. 2020;37:706-709. doi:10.1111/pde.14180
- Zhang C, Zhao J, Gao Y, et al. Cold atmospheric plasma treatment for diaper dermatitis: a case report [published online January 27, 2021]. Dermatol Ther. 2021;34:E14739. doi:10.1111/dth.14739
- Friedman PC, Fridman G, Fridman A. Cold atmospheric pressure plasma clears molluscum contagiosum. Exp Dermatol. 2023;32:562-563. doi:10.1111/exd.14695
- Suwanchinda A, Nararatwanchai T. The efficacy and safety of the innovative cold atmospheric-pressure plasma technology in the treatment of striae distensae: a randomized controlled trial. J Cosmet Dermatol. 2022;21:6805-6814. doi:10.1111/jocd.15458
- Suwanchinda A, Nararatwanchai T. Efficacy and safety of the innovative cold atmospheric-pressure plasma technology in the treatment of keloid: a randomized controlled trial. J Cosmet Dermatol. 2022;21:6788-6797. doi:10.1111/jocd.15397
- Kim YJ, Lim DJ, Lee MY, et al. Prospective, comparative clinical pilot study of cold atmospheric plasma device in the treatment of atopic dermatitis. Sci Rep. 2021;11:14461. doi:10.1038/s41598-021-93941-y
- Gareri C, Bennardo L, De Masi G. Use of a new cold plasma tool for psoriasis treatment: a case report. SAGE Open Med Case Rep. 2020;8:2050313X20922709. doi:10.1177/2050313X20922709
- Zheng L, Gao J, Cao Y, et al. Two case reports of inverse psoriasis treated with cold atmospheric plasma. Dermatol Ther. 2020;33:E14257. doi:10.1111/dth.14257
- Arisi M, Venturuzzo A, Gelmetti A, et al. Cold atmospheric plasma (CAP) as a promising therapeutic option for mild to moderate acne vulgaris: clinical and non-invasive evaluation of two cases. Clin Plasma Med. 2020;19-20:100110.
- Isbary G, Köritzer J, Mitra A, et al. Ex vivo human skin experiments for the evaluation of safety of new cold atmospheric plasma devices. Clin Plasma Med. 2013;1:36-44.
- Sun T, Zhang X, Hou C, et al. Cold plasma irradiation attenuates atopic dermatitis via enhancing HIF-1α-induced MANF transcription expression. Front Immunol. 2022;13:941219. doi:10.3389/fimmu.2022.941219
- Eggers B, Marciniak J, Memmert S, et al. The beneficial effect of cold atmospheric plasma on parameters of molecules and cell function involved in wound healing in human osteoblast-like cells in vitro. Odontology. 2020;108:607-616. doi:10.1007/s10266-020-00487-y
- Conway GE, He Z, Hutanu AL, et al. Cold atmospheric plasma induces accumulation of lysosomes and caspase-independent cell death in U373MG glioblastoma multiforme cells. Sci Rep. 2019;9:12891. doi:10.1038/s41598-019-49013-3
- Schmidt A, Dietrich S, Steuer A, et al. Non-thermal plasma activates human keratinocytes by stimulation of antioxidant and phase II pathways. J Biol Chem. 2015;290:6731-6750. doi:10.1074/jbc.M114.603555
- Silverberg NB. Pediatric molluscum contagiosum. Pediatr Drugs. 2003;5:505-511. doi:10.2165/00148581-200305080-00001
- Cotton DW, Cooper C, Barrett DF, et al. Severe atypical molluscum contagiosum infection in an immunocompromised host. Br J Dermatol. 1987;116:871-876. doi:10.1111/j.1365-2133.1987.tb04908.x
Nonthermal atmospheric plasma (NTAP)(or cold atmospheric plasma [CAP]) is a rapidly developing treatment modality for a wide range of dermatologic conditions. Plasma (or ionized gas) refers to a state of matter composed of electrons, protons, and neutral atoms that generate reactive oxygen and nitrogen species.1 Plasma previously was created using thermal energy, but recent advances have allowed the creation of plasma using atmospheric pressure and room temperature; thus, NTAP can be used without causing damage to living tissue through heat.1 Plasma technology varies greatly, but it generally can be classified as either direct or indirect therapy; direct therapy uses the human body as an electrode, whereas indirect therapy creates plasma through the interaction between 2 electrode devices.1,2 When used on the skin, important dose-dependent relationships have been observed, with CAP application longer than 2 minutes being associated with increased keratinocyte and fibroblast apoptosis.2 Thus, CAP can cause diverse changes to the skin depending on application time and methodology. At adequate yet low concentrations, plasma can promote fibroblast proliferation and upregulate genes involved in collagen and transforming growth factor synthesis.1 Additionally, the reactive oxygen and nitrogen species created by NTAP have been shown to inactivate microorganisms through the destruction of biofilms, lead to diminished immune cell infiltration and cytokine release in autoimmune dermatologic conditions, and exert antitumor properties through cellular DNA damage.1-3 In dermatology, these properties can be harvested to promote wound healing at low doses and the treatment of proliferative skin conditions at high doses.1
Because of its novelty, the safety profile of NTAP is still under investigation, but preliminary studies are promising and show no damage to the skin barrier when excessive plasma exposure is avoided.4 However, dose- and time-dependent damage to cells has been shown. As a result, the exact dose of plasma considered safe is highly variable depending on the vessel, technique, and user, and future clinical research is needed to guide this methodology.4 Additionally, CAP has been shown to cause little pain at the skin surface and may lead to decreased levels of pain in healing wound sites.5 Given this promising safety profile and minimal discomfort to patients, NTAP technology remains promising for use in pediatric dermatology, but there are limited data to characterize its potential use in this population. In this systematic review, we aimed to elucidate reported applications of NTAP for skin conditions in children and discuss the trajectory of this technology in the future of pediatric dermatology.
Methodology
A comprehensive literature review was conducted to identify studies evaluating NTAP technology in pediatric populations using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines. A search of PubMed, Embase, and Web of Science articles was conducted in April 2023 using the terms nonthermal atmospheric plasma or cold atmospheric plasma. All English-language articles that described the use of NTAP as a treatment in pediatric populations or articles that described NTAP use in the treatment of common conditions in this patient group were included based on a review of the article titles and abstracts by 2 independent reviewers, followed by full-text review of relevant articles (M.G., C.L.). Any discrepancies in eligible articles were settled by a third independent researcher (M.V.). One hundred twenty studies were identified, and 95 were screened for inclusion; 9 studies met inclusion criteria and were summarized in this review.
Results
A total of 9 studies were included in this review: 3 describing the success of NTAP in pediatric populations6-8 and 6 describing the potential success of NTAP for dermatologic conditions commonly seen in children (Table).9-14

Studies Describing Success of NTAP—Three clinical reports described the efficacy of NTAP in pediatric dermatology. A case series from 2020 showed full clearance of warts in 100% of patients (n=5) with a 0% recurrence rate when NTAP treatment was applied for 2 minutes to each lesion during each treatment session with the electrode held 1 mm from the lesional surface.6 Each patient was followed up at 3 to 4 weeks, and treatment was repeated if lesions persisted. Patients reported no pain during the procedure, and no adverse effects were noted over the course of treatment.6 Second, a case report described full clearance of diaper dermatitis with no recurrence after 6 months following 6 treatments with NTAP in a 14-month-old girl.7 After treatment with econazole nitrate cream, oral antibiotics, and prednisone failed, CAP treatment was initiated. Each treatment lasted 15 minutes with 3-day time intervals between each of the 6 treatments. There were no adverse events or recurrence of rash at 6-month follow-up.7 A final case report described full clearance of molluscum contagiosum (MC), with no recurrence after 2 months following 4 treatments with NTAP in a 12-year-old boy.8 The patient had untreated MC on the face, neck, shoulder, and thighs. Lesions of the face were treated with CAP, while the other sites were treated with cantharidin using a 0.7% collodion-based solution. Four CAP treatments were performed at 1-month intervals, with CAP applied 1 mm from the lesional surfaces in a circular pattern for 2 minutes. At follow-up 2 months after the final treatment, the patient had no adverse effects and showed no pigmentary changes or scarring.8
Studies Describing the Potential Success of NTAP—Beyond these studies, limited research has been done on NTAP in pediatric populations. The Table summarizes 6 additional studies completed with promising treatment results for dermatologic conditions commonly seen in children: striae distensae, keloids, atopic dermatitis, psoriasis, inverse psoriasis, and acne vulgaris. Across all reports and studies, patients showed significant improvement in their dermatologic conditions following the use of NTAP technology with limited adverse effects reported (P<.05). Suwanchinda and Nararatwanchai9 studied the use of CAP for the treatment of striae distensae. They recruited 23 patients and treated half the body with CAP biweekly for 5 sessions; the other half was left untreated. At follow-up 30 days after the final treatment, striae distensae had improved for both patient and observer assessment scores.9 Another study performed by Suwanchinda and Nararatwanchai10 looked at the efficacy of CAP in treating keloids. They recruited 18 patients, and keloid scars were treated in halves—one half treated with CAP biweekly for 5 sessions and the other left untreated. At follow-up 30 days after the final treatment, keloids significantly improved in color, melanin, texture, and hemoglobin based on assessment by the Antera 3D imaging system (Miravex Limited)(P<.05).10
Kim et al11 studied the efficacy of CAP for the treatment of atopic dermatitis in 22 patients. Each patient had mild to moderate atopic dermatitis that had not been treated with topical agents or antibiotics for at least 2 weeks prior to beginning the study. Additionally, only patients with symmetric lesions—meaning only patients with lesions on both sides of the anatomical extremities—were included. Each patient then received CAP on 1 symmetric lesion and placebo on the other. Cold atmospheric plasma treatment was done 5 mm away from the lesion, and each treatment lasted for 5 minutes. Treatments were done at weeks 0, 1, and 2, with follow-up 4 weeks after the final treatment. The clinical severity of disease was assessed at weeks 0, 1, 2, and 4. Results showed that at week 4, the mean (SD) modified Atopic Dermatitis Antecubital Severity score decreased from 33.73 (21.21) at week 0 to 13.12 (15.92). Additionally, the pruritic visual analog scale showed significant improvement with treatment vs baseline (P≤.0001).11
Two studies examined how NTAP can be used in the treatment of psoriasis. First, Gareri et al12 used CAP to treat a psoriatic plaque in a 20-year-old woman. These plaques on the left hand previously had been unresponsive to topical psoriasis treatments. The patient received 2 treatments with CAP on days 0 and 3; at 14 days, the plaque completely resolved with an itch score of 0.12 Next, Zheng et al13 treated 2 patients with NTAP for inverse psoriasis. The first patient was a 26-year-old woman with plaques in the axilla and buttocks as well as inframammary lesions that failed to respond to treatment with topicals and vitamin D analogues. She received CAP treatments 2 to 3 times weekly for 5 total treatments with application to each region occurring 1 mm from the skin surface. The lesions completely resolved with no recurrence at 6 weeks. The second patient was a 38-year-old woman with inverse psoriasis in the axilla and groin; she received treatment every 3 days for 8 total treatments, which led to complete remission, with no recurrence noted at 1 month.13
Arisi et al14 used NTAP to treat acne vulgaris in 2 patients. The first patient was a 24-year-old man with moderate acne on the face that did not improve with topicals or oral antibiotics. The patient received 5 CAP treatments with no adverse events noted. The patient discontinued treatment on his own, but the number of lesions decreased after the fifth treatment. The second patient was a 21-year-old woman with moderate facial acne that failed to respond to treatment with topicals and oral tetracycline. The patient received 8 CAP treatments and experienced a reduction in the number of lesions during treatment. There were no adverse events, and improvement was maintained at 3-month follow-up.14
Comment
Although the use of NTAP in pediatric dermatology is scarcely described in the literature, the technology will certainly have applications in the future treatment of a wide variety of pediatric disorders. In addition to the clinical success shown in several studies,6-14 this technology has been shown to cause minimal damage to skin when application time is minimized. One study conducted on ex vivo skin showed that NTAP technology can safely be used for up to 2 minutes without major DNA damage.15 Through its diverse mechanisms of action, NTAP can induce modification of proteins and cell membranes in a noninvasive manner.2 In conditions with impaired barrier function, such as atopic and diaper dermatitis, studies in mouse models have shown improvement in lesions via upregulation of mesencephalic astrocyte-derived neurotrophic factor that contributes to decreased inflammation and cell apoptosis.16 Additionally, the generation of reactive oxygen and nitrogen species has been shown to decrease Staphylococcus aureus colonization to improve atopic dermatitis lesions in patients.11
Many other proposed benefits of NTAP in dermatologic disease also have been proposed. Nonthermal atmospheric plasma has been shown to increase messenger RNA expression of proinflammatory cytokines (IL-1, IL-6) and upregulate type III collagen production in early stages of wound healing.17 Furthermore, NTAP has been shown to stimulate nuclear factor erythroid 2–related pathways involved in antioxidant production in keratinocytes, further promoting wound healing.18 Additionally, CAP has been shown to increase expression of caspases and induce mitochondrial dysfunction that promotes cell death in different cancer cell lines.19 It is clear that the exact breadth of NTAP’s biochemical effects are unknown, but the current literature shows promise for its use in cutaneous healing and cancer treatment.
Beyond its diverse applications, treatment with NTAP yields a unique advantage to pharmacologic therapies in that there is no risk for medication interactions or risk for pharmacologic adverse effects. Cantharidin is not approved by the US Food and Drug Administration but commonly is used to treat MC. It is a blister beetle extract that causes a blister to form when applied to the skin. When orally ingested, the drug is toxic to the gastrointestinal tract and kidneys because of its phosphodiesterase inhibition, a feared complication in pediatric patients who may inadvertently ingest it during treatment.20 This utility extends beyond MC, such as the beneficial outcomes described by Suwanchinda and Nararatwanchai10 in using NTAP for keloid scars. Treatment with NTAP may replace triamcinolone injections, which are commonly associated with skin atrophy and ulceration. In addition, NTAP application to the skin has been reported to be relatively painless.5 Thus, NTAP maintains a distinct advantage over other commonly used nonpharmacologic treatment options, including curettage and cryosurgery. Curettage has widely been noted to be traumatic for the patient, may be more likely to leave a mark, and is prone to user error.20 Cryosurgery is a common form of treatment for MC because it is cost-effective and has good cosmetic results; however, it is more painful than cantharidin or anesthetized curettage.21 Treatment with NTAP is an emerging therapeutic tool with an expanding role in the treatment of dermatologic patients because it provides advantages over many standard therapies due to its minimal side-effect profile involving pain and nonpharmacologic nature.
Limitations of this report include exclusion of non–English-language articles and lack of control or comparison groups to standard therapies across studies. Additionally, reports of NTAP success occurred in many conditions that are self-limited and may have resolved on their own. Regardless, we aimed to summarize how NTAP currently is being used in pediatric populations and highlight its potential uses moving forward. Given its promising safety profile and painless nature, future clinical trials should prioritize the investigation of NTAP use in common pediatric dermatologic conditions to determine if they are equal or superior to current standards of care.
Nonthermal atmospheric plasma (NTAP)(or cold atmospheric plasma [CAP]) is a rapidly developing treatment modality for a wide range of dermatologic conditions. Plasma (or ionized gas) refers to a state of matter composed of electrons, protons, and neutral atoms that generate reactive oxygen and nitrogen species.1 Plasma previously was created using thermal energy, but recent advances have allowed the creation of plasma using atmospheric pressure and room temperature; thus, NTAP can be used without causing damage to living tissue through heat.1 Plasma technology varies greatly, but it generally can be classified as either direct or indirect therapy; direct therapy uses the human body as an electrode, whereas indirect therapy creates plasma through the interaction between 2 electrode devices.1,2 When used on the skin, important dose-dependent relationships have been observed, with CAP application longer than 2 minutes being associated with increased keratinocyte and fibroblast apoptosis.2 Thus, CAP can cause diverse changes to the skin depending on application time and methodology. At adequate yet low concentrations, plasma can promote fibroblast proliferation and upregulate genes involved in collagen and transforming growth factor synthesis.1 Additionally, the reactive oxygen and nitrogen species created by NTAP have been shown to inactivate microorganisms through the destruction of biofilms, lead to diminished immune cell infiltration and cytokine release in autoimmune dermatologic conditions, and exert antitumor properties through cellular DNA damage.1-3 In dermatology, these properties can be harvested to promote wound healing at low doses and the treatment of proliferative skin conditions at high doses.1
Because of its novelty, the safety profile of NTAP is still under investigation, but preliminary studies are promising and show no damage to the skin barrier when excessive plasma exposure is avoided.4 However, dose- and time-dependent damage to cells has been shown. As a result, the exact dose of plasma considered safe is highly variable depending on the vessel, technique, and user, and future clinical research is needed to guide this methodology.4 Additionally, CAP has been shown to cause little pain at the skin surface and may lead to decreased levels of pain in healing wound sites.5 Given this promising safety profile and minimal discomfort to patients, NTAP technology remains promising for use in pediatric dermatology, but there are limited data to characterize its potential use in this population. In this systematic review, we aimed to elucidate reported applications of NTAP for skin conditions in children and discuss the trajectory of this technology in the future of pediatric dermatology.
Methodology
A comprehensive literature review was conducted to identify studies evaluating NTAP technology in pediatric populations using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines. A search of PubMed, Embase, and Web of Science articles was conducted in April 2023 using the terms nonthermal atmospheric plasma or cold atmospheric plasma. All English-language articles that described the use of NTAP as a treatment in pediatric populations or articles that described NTAP use in the treatment of common conditions in this patient group were included based on a review of the article titles and abstracts by 2 independent reviewers, followed by full-text review of relevant articles (M.G., C.L.). Any discrepancies in eligible articles were settled by a third independent researcher (M.V.). One hundred twenty studies were identified, and 95 were screened for inclusion; 9 studies met inclusion criteria and were summarized in this review.
Results
A total of 9 studies were included in this review: 3 describing the success of NTAP in pediatric populations6-8 and 6 describing the potential success of NTAP for dermatologic conditions commonly seen in children (Table).9-14

Studies Describing Success of NTAP—Three clinical reports described the efficacy of NTAP in pediatric dermatology. A case series from 2020 showed full clearance of warts in 100% of patients (n=5) with a 0% recurrence rate when NTAP treatment was applied for 2 minutes to each lesion during each treatment session with the electrode held 1 mm from the lesional surface.6 Each patient was followed up at 3 to 4 weeks, and treatment was repeated if lesions persisted. Patients reported no pain during the procedure, and no adverse effects were noted over the course of treatment.6 Second, a case report described full clearance of diaper dermatitis with no recurrence after 6 months following 6 treatments with NTAP in a 14-month-old girl.7 After treatment with econazole nitrate cream, oral antibiotics, and prednisone failed, CAP treatment was initiated. Each treatment lasted 15 minutes with 3-day time intervals between each of the 6 treatments. There were no adverse events or recurrence of rash at 6-month follow-up.7 A final case report described full clearance of molluscum contagiosum (MC), with no recurrence after 2 months following 4 treatments with NTAP in a 12-year-old boy.8 The patient had untreated MC on the face, neck, shoulder, and thighs. Lesions of the face were treated with CAP, while the other sites were treated with cantharidin using a 0.7% collodion-based solution. Four CAP treatments were performed at 1-month intervals, with CAP applied 1 mm from the lesional surfaces in a circular pattern for 2 minutes. At follow-up 2 months after the final treatment, the patient had no adverse effects and showed no pigmentary changes or scarring.8
Studies Describing the Potential Success of NTAP—Beyond these studies, limited research has been done on NTAP in pediatric populations. The Table summarizes 6 additional studies completed with promising treatment results for dermatologic conditions commonly seen in children: striae distensae, keloids, atopic dermatitis, psoriasis, inverse psoriasis, and acne vulgaris. Across all reports and studies, patients showed significant improvement in their dermatologic conditions following the use of NTAP technology with limited adverse effects reported (P<.05). Suwanchinda and Nararatwanchai9 studied the use of CAP for the treatment of striae distensae. They recruited 23 patients and treated half the body with CAP biweekly for 5 sessions; the other half was left untreated. At follow-up 30 days after the final treatment, striae distensae had improved for both patient and observer assessment scores.9 Another study performed by Suwanchinda and Nararatwanchai10 looked at the efficacy of CAP in treating keloids. They recruited 18 patients, and keloid scars were treated in halves—one half treated with CAP biweekly for 5 sessions and the other left untreated. At follow-up 30 days after the final treatment, keloids significantly improved in color, melanin, texture, and hemoglobin based on assessment by the Antera 3D imaging system (Miravex Limited)(P<.05).10
Kim et al11 studied the efficacy of CAP for the treatment of atopic dermatitis in 22 patients. Each patient had mild to moderate atopic dermatitis that had not been treated with topical agents or antibiotics for at least 2 weeks prior to beginning the study. Additionally, only patients with symmetric lesions—meaning only patients with lesions on both sides of the anatomical extremities—were included. Each patient then received CAP on 1 symmetric lesion and placebo on the other. Cold atmospheric plasma treatment was done 5 mm away from the lesion, and each treatment lasted for 5 minutes. Treatments were done at weeks 0, 1, and 2, with follow-up 4 weeks after the final treatment. The clinical severity of disease was assessed at weeks 0, 1, 2, and 4. Results showed that at week 4, the mean (SD) modified Atopic Dermatitis Antecubital Severity score decreased from 33.73 (21.21) at week 0 to 13.12 (15.92). Additionally, the pruritic visual analog scale showed significant improvement with treatment vs baseline (P≤.0001).11
Two studies examined how NTAP can be used in the treatment of psoriasis. First, Gareri et al12 used CAP to treat a psoriatic plaque in a 20-year-old woman. These plaques on the left hand previously had been unresponsive to topical psoriasis treatments. The patient received 2 treatments with CAP on days 0 and 3; at 14 days, the plaque completely resolved with an itch score of 0.12 Next, Zheng et al13 treated 2 patients with NTAP for inverse psoriasis. The first patient was a 26-year-old woman with plaques in the axilla and buttocks as well as inframammary lesions that failed to respond to treatment with topicals and vitamin D analogues. She received CAP treatments 2 to 3 times weekly for 5 total treatments with application to each region occurring 1 mm from the skin surface. The lesions completely resolved with no recurrence at 6 weeks. The second patient was a 38-year-old woman with inverse psoriasis in the axilla and groin; she received treatment every 3 days for 8 total treatments, which led to complete remission, with no recurrence noted at 1 month.13
Arisi et al14 used NTAP to treat acne vulgaris in 2 patients. The first patient was a 24-year-old man with moderate acne on the face that did not improve with topicals or oral antibiotics. The patient received 5 CAP treatments with no adverse events noted. The patient discontinued treatment on his own, but the number of lesions decreased after the fifth treatment. The second patient was a 21-year-old woman with moderate facial acne that failed to respond to treatment with topicals and oral tetracycline. The patient received 8 CAP treatments and experienced a reduction in the number of lesions during treatment. There were no adverse events, and improvement was maintained at 3-month follow-up.14
Comment
Although the use of NTAP in pediatric dermatology is scarcely described in the literature, the technology will certainly have applications in the future treatment of a wide variety of pediatric disorders. In addition to the clinical success shown in several studies,6-14 this technology has been shown to cause minimal damage to skin when application time is minimized. One study conducted on ex vivo skin showed that NTAP technology can safely be used for up to 2 minutes without major DNA damage.15 Through its diverse mechanisms of action, NTAP can induce modification of proteins and cell membranes in a noninvasive manner.2 In conditions with impaired barrier function, such as atopic and diaper dermatitis, studies in mouse models have shown improvement in lesions via upregulation of mesencephalic astrocyte-derived neurotrophic factor that contributes to decreased inflammation and cell apoptosis.16 Additionally, the generation of reactive oxygen and nitrogen species has been shown to decrease Staphylococcus aureus colonization to improve atopic dermatitis lesions in patients.11
Many other proposed benefits of NTAP in dermatologic disease also have been proposed. Nonthermal atmospheric plasma has been shown to increase messenger RNA expression of proinflammatory cytokines (IL-1, IL-6) and upregulate type III collagen production in early stages of wound healing.17 Furthermore, NTAP has been shown to stimulate nuclear factor erythroid 2–related pathways involved in antioxidant production in keratinocytes, further promoting wound healing.18 Additionally, CAP has been shown to increase expression of caspases and induce mitochondrial dysfunction that promotes cell death in different cancer cell lines.19 It is clear that the exact breadth of NTAP’s biochemical effects are unknown, but the current literature shows promise for its use in cutaneous healing and cancer treatment.
Beyond its diverse applications, treatment with NTAP yields a unique advantage to pharmacologic therapies in that there is no risk for medication interactions or risk for pharmacologic adverse effects. Cantharidin is not approved by the US Food and Drug Administration but commonly is used to treat MC. It is a blister beetle extract that causes a blister to form when applied to the skin. When orally ingested, the drug is toxic to the gastrointestinal tract and kidneys because of its phosphodiesterase inhibition, a feared complication in pediatric patients who may inadvertently ingest it during treatment.20 This utility extends beyond MC, such as the beneficial outcomes described by Suwanchinda and Nararatwanchai10 in using NTAP for keloid scars. Treatment with NTAP may replace triamcinolone injections, which are commonly associated with skin atrophy and ulceration. In addition, NTAP application to the skin has been reported to be relatively painless.5 Thus, NTAP maintains a distinct advantage over other commonly used nonpharmacologic treatment options, including curettage and cryosurgery. Curettage has widely been noted to be traumatic for the patient, may be more likely to leave a mark, and is prone to user error.20 Cryosurgery is a common form of treatment for MC because it is cost-effective and has good cosmetic results; however, it is more painful than cantharidin or anesthetized curettage.21 Treatment with NTAP is an emerging therapeutic tool with an expanding role in the treatment of dermatologic patients because it provides advantages over many standard therapies due to its minimal side-effect profile involving pain and nonpharmacologic nature.
Limitations of this report include exclusion of non–English-language articles and lack of control or comparison groups to standard therapies across studies. Additionally, reports of NTAP success occurred in many conditions that are self-limited and may have resolved on their own. Regardless, we aimed to summarize how NTAP currently is being used in pediatric populations and highlight its potential uses moving forward. Given its promising safety profile and painless nature, future clinical trials should prioritize the investigation of NTAP use in common pediatric dermatologic conditions to determine if they are equal or superior to current standards of care.
- Gan L, Zhang S, Poorun D, et al. Medical applications of nonthermal atmospheric pressure plasma in dermatology. J Dtsch Dermatol Ges. 2018;16:7-13. doi:https://doi.org/10.1111/ddg.13373
- Gay-Mimbrera J, García MC, Isla-Tejera B, et al. Clinical and biological principles of cold atmospheric plasma application in skin cancer. Adv Ther. 2016;33:894-909. doi:10.1007/s12325-016-0338-1. Published correction appears in Adv Ther. 2017;34:280. doi:10.1007/s12325-016-0437-z
- Zhai SY, Kong MG, Xia YM. Cold atmospheric plasma ameliorates skin diseases involving reactive oxygen/nitrogen species-mediated functions. Front Immunol. 2022;13:868386. doi:10.3389/fimmu.2022.868386
- Tan F, Wang Y, Zhang S, et al. Plasma dermatology: skin therapy using cold atmospheric plasma. Front Oncol. 2022;12:918484. doi:10.3389/fonc.2022.918484
- van Welzen A, Hoch M, Wahl P, et al. The response and tolerability of a novel cold atmospheric plasma wound dressing for the healing of split skin graft donor sites: a controlled pilot study. Skin Pharmacol Physiol. 2021;34:328-336. doi:10.1159/000517524
- Friedman PC, Fridman G, Fridman A. Using cold plasma to treat warts in children: a case series. Pediatr Dermatol. 2020;37:706-709. doi:10.1111/pde.14180
- Zhang C, Zhao J, Gao Y, et al. Cold atmospheric plasma treatment for diaper dermatitis: a case report [published online January 27, 2021]. Dermatol Ther. 2021;34:E14739. doi:10.1111/dth.14739
- Friedman PC, Fridman G, Fridman A. Cold atmospheric pressure plasma clears molluscum contagiosum. Exp Dermatol. 2023;32:562-563. doi:10.1111/exd.14695
- Suwanchinda A, Nararatwanchai T. The efficacy and safety of the innovative cold atmospheric-pressure plasma technology in the treatment of striae distensae: a randomized controlled trial. J Cosmet Dermatol. 2022;21:6805-6814. doi:10.1111/jocd.15458
- Suwanchinda A, Nararatwanchai T. Efficacy and safety of the innovative cold atmospheric-pressure plasma technology in the treatment of keloid: a randomized controlled trial. J Cosmet Dermatol. 2022;21:6788-6797. doi:10.1111/jocd.15397
- Kim YJ, Lim DJ, Lee MY, et al. Prospective, comparative clinical pilot study of cold atmospheric plasma device in the treatment of atopic dermatitis. Sci Rep. 2021;11:14461. doi:10.1038/s41598-021-93941-y
- Gareri C, Bennardo L, De Masi G. Use of a new cold plasma tool for psoriasis treatment: a case report. SAGE Open Med Case Rep. 2020;8:2050313X20922709. doi:10.1177/2050313X20922709
- Zheng L, Gao J, Cao Y, et al. Two case reports of inverse psoriasis treated with cold atmospheric plasma. Dermatol Ther. 2020;33:E14257. doi:10.1111/dth.14257
- Arisi M, Venturuzzo A, Gelmetti A, et al. Cold atmospheric plasma (CAP) as a promising therapeutic option for mild to moderate acne vulgaris: clinical and non-invasive evaluation of two cases. Clin Plasma Med. 2020;19-20:100110.
- Isbary G, Köritzer J, Mitra A, et al. Ex vivo human skin experiments for the evaluation of safety of new cold atmospheric plasma devices. Clin Plasma Med. 2013;1:36-44.
- Sun T, Zhang X, Hou C, et al. Cold plasma irradiation attenuates atopic dermatitis via enhancing HIF-1α-induced MANF transcription expression. Front Immunol. 2022;13:941219. doi:10.3389/fimmu.2022.941219
- Eggers B, Marciniak J, Memmert S, et al. The beneficial effect of cold atmospheric plasma on parameters of molecules and cell function involved in wound healing in human osteoblast-like cells in vitro. Odontology. 2020;108:607-616. doi:10.1007/s10266-020-00487-y
- Conway GE, He Z, Hutanu AL, et al. Cold atmospheric plasma induces accumulation of lysosomes and caspase-independent cell death in U373MG glioblastoma multiforme cells. Sci Rep. 2019;9:12891. doi:10.1038/s41598-019-49013-3
- Schmidt A, Dietrich S, Steuer A, et al. Non-thermal plasma activates human keratinocytes by stimulation of antioxidant and phase II pathways. J Biol Chem. 2015;290:6731-6750. doi:10.1074/jbc.M114.603555
- Silverberg NB. Pediatric molluscum contagiosum. Pediatr Drugs. 2003;5:505-511. doi:10.2165/00148581-200305080-00001
- Cotton DW, Cooper C, Barrett DF, et al. Severe atypical molluscum contagiosum infection in an immunocompromised host. Br J Dermatol. 1987;116:871-876. doi:10.1111/j.1365-2133.1987.tb04908.x
- Gan L, Zhang S, Poorun D, et al. Medical applications of nonthermal atmospheric pressure plasma in dermatology. J Dtsch Dermatol Ges. 2018;16:7-13. doi:https://doi.org/10.1111/ddg.13373
- Gay-Mimbrera J, García MC, Isla-Tejera B, et al. Clinical and biological principles of cold atmospheric plasma application in skin cancer. Adv Ther. 2016;33:894-909. doi:10.1007/s12325-016-0338-1. Published correction appears in Adv Ther. 2017;34:280. doi:10.1007/s12325-016-0437-z
- Zhai SY, Kong MG, Xia YM. Cold atmospheric plasma ameliorates skin diseases involving reactive oxygen/nitrogen species-mediated functions. Front Immunol. 2022;13:868386. doi:10.3389/fimmu.2022.868386
- Tan F, Wang Y, Zhang S, et al. Plasma dermatology: skin therapy using cold atmospheric plasma. Front Oncol. 2022;12:918484. doi:10.3389/fonc.2022.918484
- van Welzen A, Hoch M, Wahl P, et al. The response and tolerability of a novel cold atmospheric plasma wound dressing for the healing of split skin graft donor sites: a controlled pilot study. Skin Pharmacol Physiol. 2021;34:328-336. doi:10.1159/000517524
- Friedman PC, Fridman G, Fridman A. Using cold plasma to treat warts in children: a case series. Pediatr Dermatol. 2020;37:706-709. doi:10.1111/pde.14180
- Zhang C, Zhao J, Gao Y, et al. Cold atmospheric plasma treatment for diaper dermatitis: a case report [published online January 27, 2021]. Dermatol Ther. 2021;34:E14739. doi:10.1111/dth.14739
- Friedman PC, Fridman G, Fridman A. Cold atmospheric pressure plasma clears molluscum contagiosum. Exp Dermatol. 2023;32:562-563. doi:10.1111/exd.14695
- Suwanchinda A, Nararatwanchai T. The efficacy and safety of the innovative cold atmospheric-pressure plasma technology in the treatment of striae distensae: a randomized controlled trial. J Cosmet Dermatol. 2022;21:6805-6814. doi:10.1111/jocd.15458
- Suwanchinda A, Nararatwanchai T. Efficacy and safety of the innovative cold atmospheric-pressure plasma technology in the treatment of keloid: a randomized controlled trial. J Cosmet Dermatol. 2022;21:6788-6797. doi:10.1111/jocd.15397
- Kim YJ, Lim DJ, Lee MY, et al. Prospective, comparative clinical pilot study of cold atmospheric plasma device in the treatment of atopic dermatitis. Sci Rep. 2021;11:14461. doi:10.1038/s41598-021-93941-y
- Gareri C, Bennardo L, De Masi G. Use of a new cold plasma tool for psoriasis treatment: a case report. SAGE Open Med Case Rep. 2020;8:2050313X20922709. doi:10.1177/2050313X20922709
- Zheng L, Gao J, Cao Y, et al. Two case reports of inverse psoriasis treated with cold atmospheric plasma. Dermatol Ther. 2020;33:E14257. doi:10.1111/dth.14257
- Arisi M, Venturuzzo A, Gelmetti A, et al. Cold atmospheric plasma (CAP) as a promising therapeutic option for mild to moderate acne vulgaris: clinical and non-invasive evaluation of two cases. Clin Plasma Med. 2020;19-20:100110.
- Isbary G, Köritzer J, Mitra A, et al. Ex vivo human skin experiments for the evaluation of safety of new cold atmospheric plasma devices. Clin Plasma Med. 2013;1:36-44.
- Sun T, Zhang X, Hou C, et al. Cold plasma irradiation attenuates atopic dermatitis via enhancing HIF-1α-induced MANF transcription expression. Front Immunol. 2022;13:941219. doi:10.3389/fimmu.2022.941219
- Eggers B, Marciniak J, Memmert S, et al. The beneficial effect of cold atmospheric plasma on parameters of molecules and cell function involved in wound healing in human osteoblast-like cells in vitro. Odontology. 2020;108:607-616. doi:10.1007/s10266-020-00487-y
- Conway GE, He Z, Hutanu AL, et al. Cold atmospheric plasma induces accumulation of lysosomes and caspase-independent cell death in U373MG glioblastoma multiforme cells. Sci Rep. 2019;9:12891. doi:10.1038/s41598-019-49013-3
- Schmidt A, Dietrich S, Steuer A, et al. Non-thermal plasma activates human keratinocytes by stimulation of antioxidant and phase II pathways. J Biol Chem. 2015;290:6731-6750. doi:10.1074/jbc.M114.603555
- Silverberg NB. Pediatric molluscum contagiosum. Pediatr Drugs. 2003;5:505-511. doi:10.2165/00148581-200305080-00001
- Cotton DW, Cooper C, Barrett DF, et al. Severe atypical molluscum contagiosum infection in an immunocompromised host. Br J Dermatol. 1987;116:871-876. doi:10.1111/j.1365-2133.1987.tb04908.x
Practice Points
- Nonthermal atmospheric plasma (NTAP)(also known as cold atmospheric plasma) has been shown to cause minimal damage to skin when application time is minimized.
- Beyond its diverse applications, treatment with NTAP yields a unique advantage to pharmacologic therapies in that there is no risk for medication interactions or pharmacologic adverse effects.
- Although the use of NTAP in pediatric dermatology is scarcely described in the literature, the technology will certainly have applications in the future treatment of a wide variety of pediatric disorders.
Skin Cancer Education in the Medical School Curriculum
To the Editor:
Skin cancer represents a notable health care burden of rising incidence.1-3 Nondermatologist health care providers play a key role in skin cancer screening through the use of skin cancer examination (SCE)1,4; however, several factors including poor diagnostic accuracy, low confidence, and lack of training have contributed to limited use of the SCE by these providers.4,5 Therefore, it is important to identify and implement changes in the medical school curriculum that can facilitate improved use of SCE in clinical practice. We sought to examine factors in the medical school curriculum that influence skin cancer education.
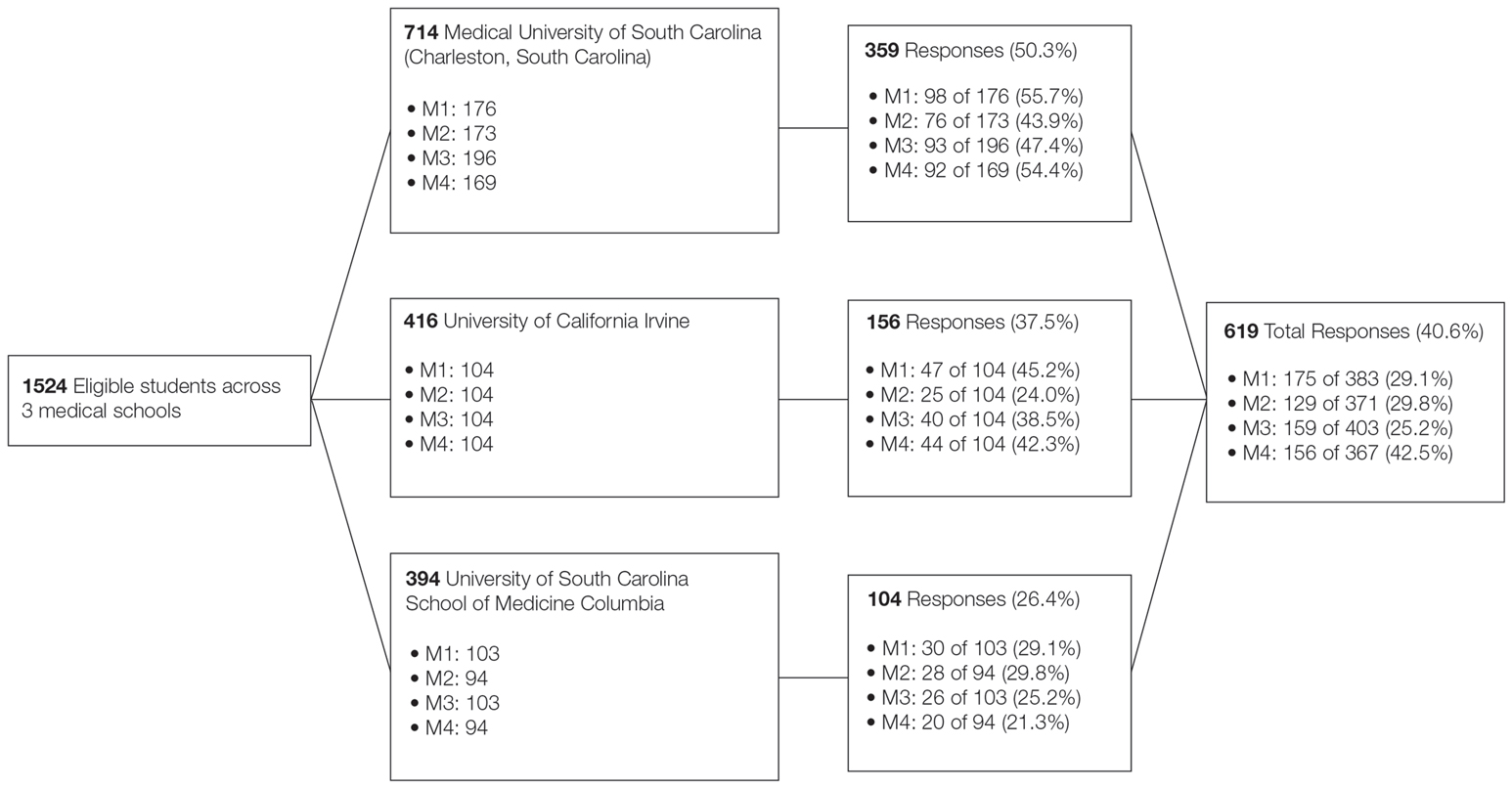
A voluntary electronic survey was distributed through class email and social media to all medical student classes at 4 medical schools (Figure). Responses were collected between March 2 and April 20, 2020. Survey items assessed demographics and curricular factors that influence skin cancer education.
Knowledge of the clinical features of melanoma was assessed by asking participants to correctly identify at least 5 of 6 pigmented lesions as concerning or not concerning for melanoma. Confidence in performing the SCE—the primary outcome—was measured by dichotomizing a 4-point Likert-type scale (“very confident” and “moderately confident” against “slightly confident” and “not at all confident”).
Logistic regression was used to examine curricular factors associated with confidence; descriptive statistics were used for remaining analyses. Analyses were performed using SAS 9.4 statistical software. Prior to analysis, responses from the University of South Carolina School of Medicine Greenville were excluded because the response rate was less than 20%.
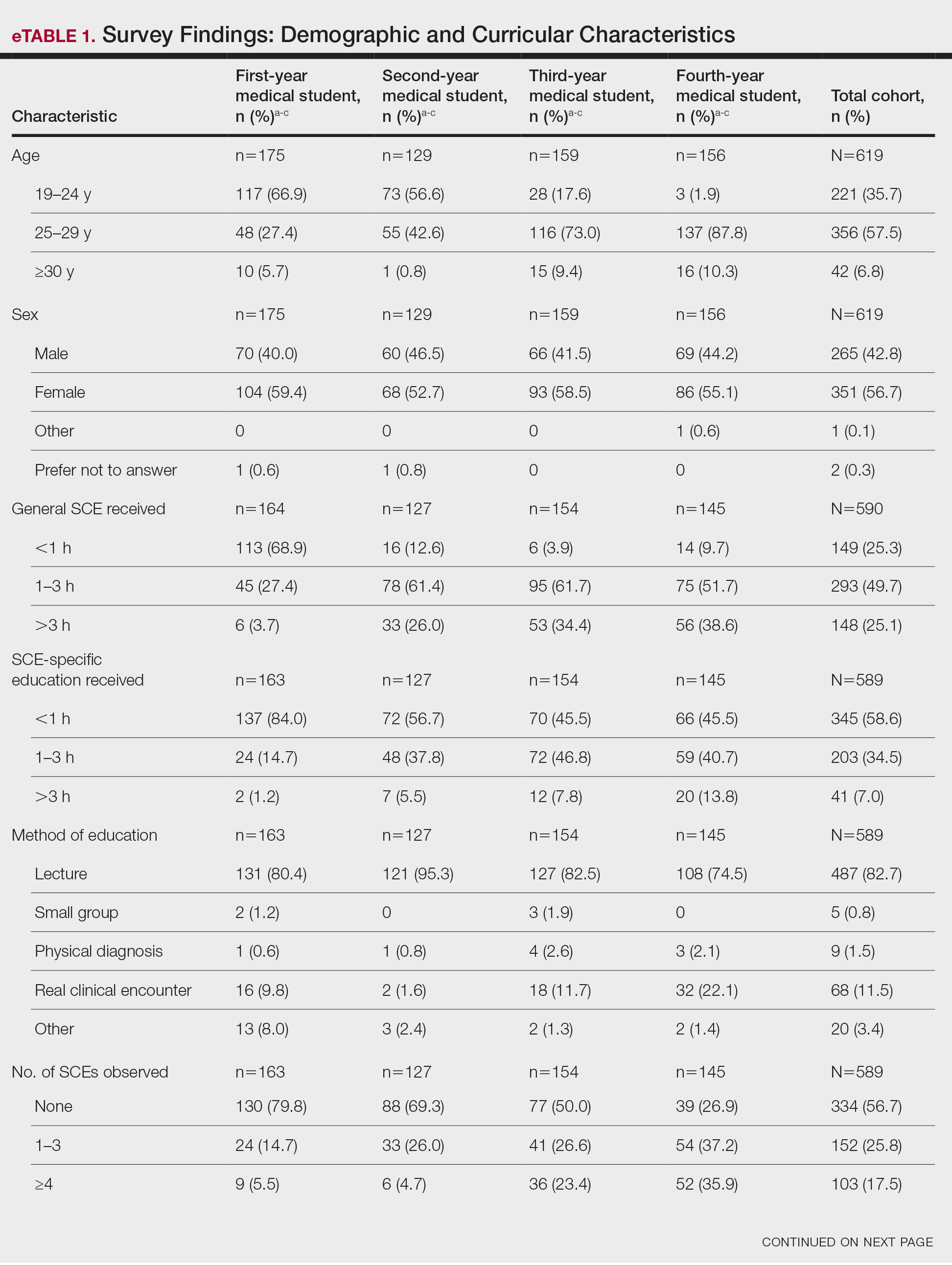
The survey was distributed to 1524 students; 619 (40.6%) answered at least 1 question, with a variable response rate to each item (eTable 1). Most respondents were female (351 [56.7%]); 438 (70.8%) were White.
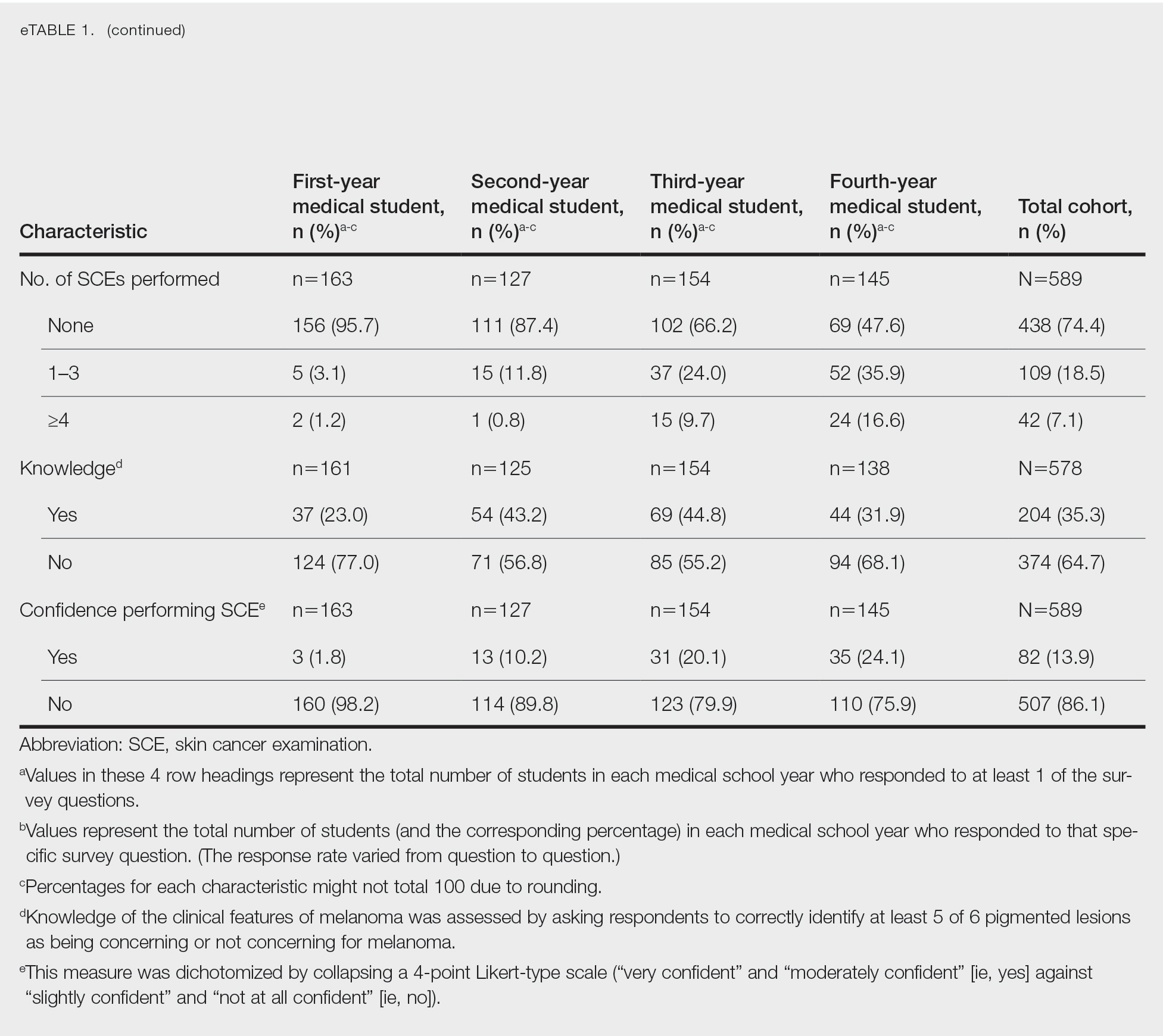
Most respondents said that they received 3 hours or less of general skin cancer (74.9%) or SCE-specific (93.0%) education by the end of their fourth year of medical training. Lecture was the most common method of instruction. Education was provided most often by dermatologists (48.6%), followed by general practice physicians (21.2%). Numerous (26.9%) fourth-year respondents reported that they had never observed SCE; even more (47.6%) had never performed SCE. Almost half of second- and third-year students (43.2% and 44.8%, respectively) considered themselves knowledgeable about the clinical features of melanoma, but only 31.9% of fourth-year students considered themselves knowledgeable.
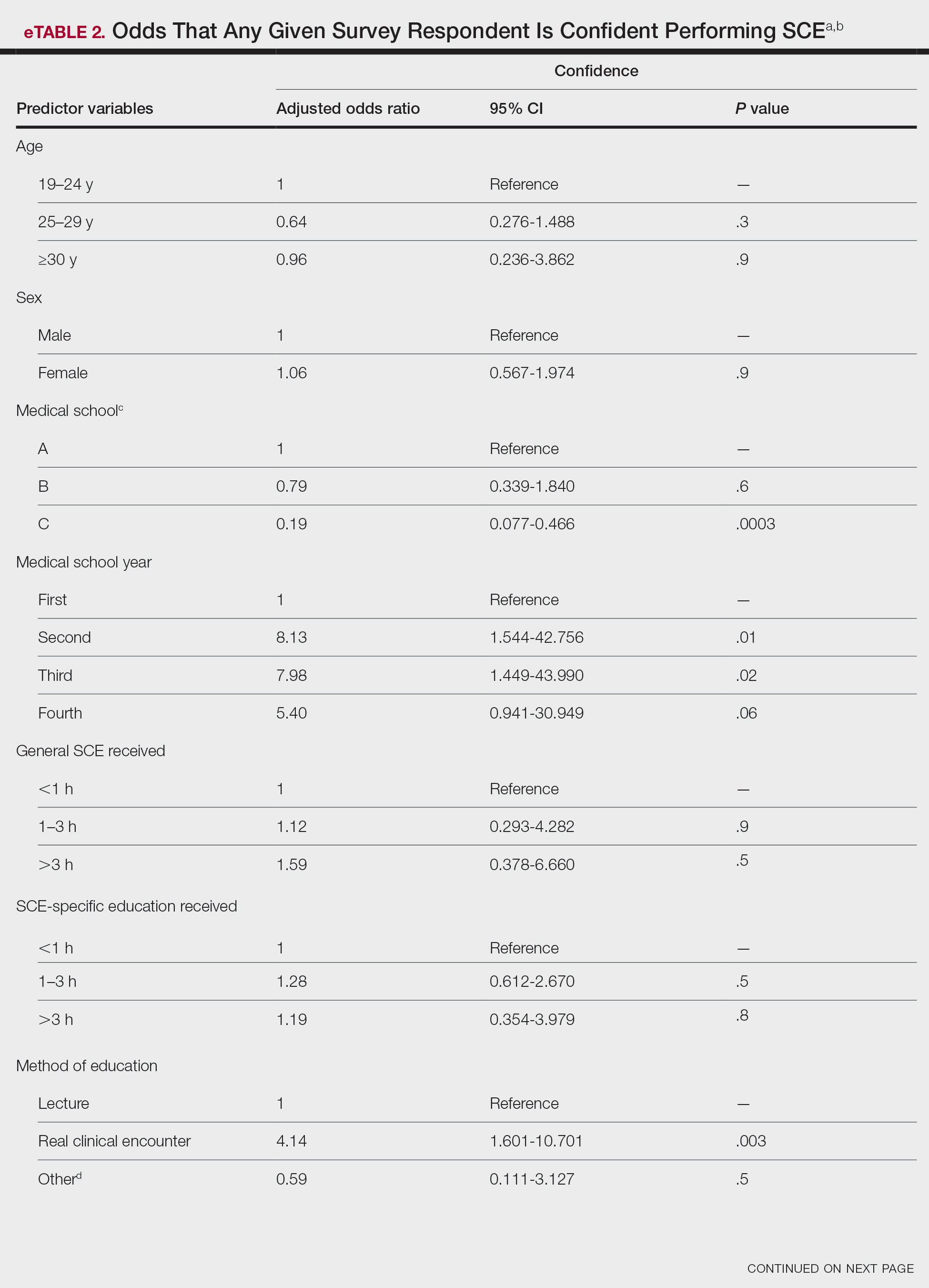
Only 24.1% of fourth-year students reported confidence performing SCE (eTable 1). Students who received most of their instruction through real clinical encounters were 4.14 times more likely to be confident performing SCE than students who had been given lecture-based learning. Students who performed 1 to 3 SCE or 4 or more SCE were 3.02 and 32.25 times, respectively, more likely to be confident than students who had never performed SCE (eTable 2).
Consistent with a recent study,6 our results reflect the discrepancy between the burden and education of skin cancer. This is especially demonstrated by our cohort’s low confidence in performing SCE, a metric associated with both intention to perform and actual performance of SCE in practice.4,5 We also observed a downward trend in knowledge among students who were about to enter residency, potentially indicating the need for longitudinal training.
Given curricular time constraints, it is essential that medical schools implement changes in learning that will have the greatest impact. Although our results strongly support the efficacy of hands-on clinical training, exposure to dermatology in the second half of medical school training is limited nationwide.6 Concentrated efforts to increase clinical exposure might help prepare future physicians in all specialties to combat the burden of this disease.
Limitations of our study include the potential for selection and recall biases. Although our survey spanned multiple institutions in different regions of the United States, results might not be universally representative.
Acknowledgments—We thank Dirk Elston, MD, and Amy Wahlquist, MS (both from Charleston, South Carolina), who helped facilitate the survey on which our research is based. We also acknowledge the assistance of Philip Carmon, MD (Columbia, South Carolina); Julie Flugel (Columbia, South Carolina); Algimantas Simpson, MD (Columbia, South Carolina); Nathan Jasperse, MD (Irvine, California); Jeremy Teruel, MD (Charleston, South Carolina); Alan Snyder, MD, MSCR (Charleston, South Carolina); John Bosland (Charleston, South Carolina); and Daniel Spangler (Greenville, South Carolina).
- Guy GP Jr, Machlin SR, Ekwueme DU, et al. Prevalence and costs of skin cancer treatment in the U.S., 2002–2006 and 2007-2011. Am J Prev Med. 2015;48:183-187. doi:10.1016/j.amepre.2014.08.036
- Paulson KG, Gupta D, Kim TS, et al. Age-specific incidence of melanoma in the United States. JAMA Dermatol. 2020;156:57-64. doi:10.1001/jamadermatol.2019.3353
- Lim HW, Collins SAB, Resneck JS Jr, et al. Contribution of health care factors to the burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:1151-1160.e21. doi:10.1016/j.jaad.2017.03.006
- Garg A, Wang J, Reddy SB, et al; Integrated Skin Exam Consortium. Curricular factors associated with medical students’ practice of the skin cancer examination: an educational enhancement initiative by the Integrated Skin Exam Consortium. JAMA Dermatol. 2014;150:850-855. doi:10.1001/jamadermatol.2013.8723
- Oliveria SA, Heneghan MK, Cushman LF, et al. Skin cancer screening by dermatologists, family practitioners, and internists: barriers and facilitating factors. Arch Dermatol. 2011;147:39-44. doi:10.1001/archdermatol.2010.414
- Cahn BA, Harper HE, Halverstam CP, et al. Current status of dermatologic education in US medical schools. JAMA Dermatol. 2020;156:468-470. doi:10.1001/jamadermatol.2020.0006
To the Editor:
Skin cancer represents a notable health care burden of rising incidence.1-3 Nondermatologist health care providers play a key role in skin cancer screening through the use of skin cancer examination (SCE)1,4; however, several factors including poor diagnostic accuracy, low confidence, and lack of training have contributed to limited use of the SCE by these providers.4,5 Therefore, it is important to identify and implement changes in the medical school curriculum that can facilitate improved use of SCE in clinical practice. We sought to examine factors in the medical school curriculum that influence skin cancer education.
A voluntary electronic survey was distributed through class email and social media to all medical student classes at 4 medical schools (Figure). Responses were collected between March 2 and April 20, 2020. Survey items assessed demographics and curricular factors that influence skin cancer education.
Knowledge of the clinical features of melanoma was assessed by asking participants to correctly identify at least 5 of 6 pigmented lesions as concerning or not concerning for melanoma. Confidence in performing the SCE—the primary outcome—was measured by dichotomizing a 4-point Likert-type scale (“very confident” and “moderately confident” against “slightly confident” and “not at all confident”).
Logistic regression was used to examine curricular factors associated with confidence; descriptive statistics were used for remaining analyses. Analyses were performed using SAS 9.4 statistical software. Prior to analysis, responses from the University of South Carolina School of Medicine Greenville were excluded because the response rate was less than 20%.
The survey was distributed to 1524 students; 619 (40.6%) answered at least 1 question, with a variable response rate to each item (eTable 1). Most respondents were female (351 [56.7%]); 438 (70.8%) were White.
Most respondents said that they received 3 hours or less of general skin cancer (74.9%) or SCE-specific (93.0%) education by the end of their fourth year of medical training. Lecture was the most common method of instruction. Education was provided most often by dermatologists (48.6%), followed by general practice physicians (21.2%). Numerous (26.9%) fourth-year respondents reported that they had never observed SCE; even more (47.6%) had never performed SCE. Almost half of second- and third-year students (43.2% and 44.8%, respectively) considered themselves knowledgeable about the clinical features of melanoma, but only 31.9% of fourth-year students considered themselves knowledgeable.
Only 24.1% of fourth-year students reported confidence performing SCE (eTable 1). Students who received most of their instruction through real clinical encounters were 4.14 times more likely to be confident performing SCE than students who had been given lecture-based learning. Students who performed 1 to 3 SCE or 4 or more SCE were 3.02 and 32.25 times, respectively, more likely to be confident than students who had never performed SCE (eTable 2).
Consistent with a recent study,6 our results reflect the discrepancy between the burden and education of skin cancer. This is especially demonstrated by our cohort’s low confidence in performing SCE, a metric associated with both intention to perform and actual performance of SCE in practice.4,5 We also observed a downward trend in knowledge among students who were about to enter residency, potentially indicating the need for longitudinal training.
Given curricular time constraints, it is essential that medical schools implement changes in learning that will have the greatest impact. Although our results strongly support the efficacy of hands-on clinical training, exposure to dermatology in the second half of medical school training is limited nationwide.6 Concentrated efforts to increase clinical exposure might help prepare future physicians in all specialties to combat the burden of this disease.
Limitations of our study include the potential for selection and recall biases. Although our survey spanned multiple institutions in different regions of the United States, results might not be universally representative.
Acknowledgments—We thank Dirk Elston, MD, and Amy Wahlquist, MS (both from Charleston, South Carolina), who helped facilitate the survey on which our research is based. We also acknowledge the assistance of Philip Carmon, MD (Columbia, South Carolina); Julie Flugel (Columbia, South Carolina); Algimantas Simpson, MD (Columbia, South Carolina); Nathan Jasperse, MD (Irvine, California); Jeremy Teruel, MD (Charleston, South Carolina); Alan Snyder, MD, MSCR (Charleston, South Carolina); John Bosland (Charleston, South Carolina); and Daniel Spangler (Greenville, South Carolina).
To the Editor:
Skin cancer represents a notable health care burden of rising incidence.1-3 Nondermatologist health care providers play a key role in skin cancer screening through the use of skin cancer examination (SCE)1,4; however, several factors including poor diagnostic accuracy, low confidence, and lack of training have contributed to limited use of the SCE by these providers.4,5 Therefore, it is important to identify and implement changes in the medical school curriculum that can facilitate improved use of SCE in clinical practice. We sought to examine factors in the medical school curriculum that influence skin cancer education.
A voluntary electronic survey was distributed through class email and social media to all medical student classes at 4 medical schools (Figure). Responses were collected between March 2 and April 20, 2020. Survey items assessed demographics and curricular factors that influence skin cancer education.
Knowledge of the clinical features of melanoma was assessed by asking participants to correctly identify at least 5 of 6 pigmented lesions as concerning or not concerning for melanoma. Confidence in performing the SCE—the primary outcome—was measured by dichotomizing a 4-point Likert-type scale (“very confident” and “moderately confident” against “slightly confident” and “not at all confident”).
Logistic regression was used to examine curricular factors associated with confidence; descriptive statistics were used for remaining analyses. Analyses were performed using SAS 9.4 statistical software. Prior to analysis, responses from the University of South Carolina School of Medicine Greenville were excluded because the response rate was less than 20%.
The survey was distributed to 1524 students; 619 (40.6%) answered at least 1 question, with a variable response rate to each item (eTable 1). Most respondents were female (351 [56.7%]); 438 (70.8%) were White.
Most respondents said that they received 3 hours or less of general skin cancer (74.9%) or SCE-specific (93.0%) education by the end of their fourth year of medical training. Lecture was the most common method of instruction. Education was provided most often by dermatologists (48.6%), followed by general practice physicians (21.2%). Numerous (26.9%) fourth-year respondents reported that they had never observed SCE; even more (47.6%) had never performed SCE. Almost half of second- and third-year students (43.2% and 44.8%, respectively) considered themselves knowledgeable about the clinical features of melanoma, but only 31.9% of fourth-year students considered themselves knowledgeable.
Only 24.1% of fourth-year students reported confidence performing SCE (eTable 1). Students who received most of their instruction through real clinical encounters were 4.14 times more likely to be confident performing SCE than students who had been given lecture-based learning. Students who performed 1 to 3 SCE or 4 or more SCE were 3.02 and 32.25 times, respectively, more likely to be confident than students who had never performed SCE (eTable 2).
Consistent with a recent study,6 our results reflect the discrepancy between the burden and education of skin cancer. This is especially demonstrated by our cohort’s low confidence in performing SCE, a metric associated with both intention to perform and actual performance of SCE in practice.4,5 We also observed a downward trend in knowledge among students who were about to enter residency, potentially indicating the need for longitudinal training.
Given curricular time constraints, it is essential that medical schools implement changes in learning that will have the greatest impact. Although our results strongly support the efficacy of hands-on clinical training, exposure to dermatology in the second half of medical school training is limited nationwide.6 Concentrated efforts to increase clinical exposure might help prepare future physicians in all specialties to combat the burden of this disease.
Limitations of our study include the potential for selection and recall biases. Although our survey spanned multiple institutions in different regions of the United States, results might not be universally representative.
Acknowledgments—We thank Dirk Elston, MD, and Amy Wahlquist, MS (both from Charleston, South Carolina), who helped facilitate the survey on which our research is based. We also acknowledge the assistance of Philip Carmon, MD (Columbia, South Carolina); Julie Flugel (Columbia, South Carolina); Algimantas Simpson, MD (Columbia, South Carolina); Nathan Jasperse, MD (Irvine, California); Jeremy Teruel, MD (Charleston, South Carolina); Alan Snyder, MD, MSCR (Charleston, South Carolina); John Bosland (Charleston, South Carolina); and Daniel Spangler (Greenville, South Carolina).
- Guy GP Jr, Machlin SR, Ekwueme DU, et al. Prevalence and costs of skin cancer treatment in the U.S., 2002–2006 and 2007-2011. Am J Prev Med. 2015;48:183-187. doi:10.1016/j.amepre.2014.08.036
- Paulson KG, Gupta D, Kim TS, et al. Age-specific incidence of melanoma in the United States. JAMA Dermatol. 2020;156:57-64. doi:10.1001/jamadermatol.2019.3353
- Lim HW, Collins SAB, Resneck JS Jr, et al. Contribution of health care factors to the burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:1151-1160.e21. doi:10.1016/j.jaad.2017.03.006
- Garg A, Wang J, Reddy SB, et al; Integrated Skin Exam Consortium. Curricular factors associated with medical students’ practice of the skin cancer examination: an educational enhancement initiative by the Integrated Skin Exam Consortium. JAMA Dermatol. 2014;150:850-855. doi:10.1001/jamadermatol.2013.8723
- Oliveria SA, Heneghan MK, Cushman LF, et al. Skin cancer screening by dermatologists, family practitioners, and internists: barriers and facilitating factors. Arch Dermatol. 2011;147:39-44. doi:10.1001/archdermatol.2010.414
- Cahn BA, Harper HE, Halverstam CP, et al. Current status of dermatologic education in US medical schools. JAMA Dermatol. 2020;156:468-470. doi:10.1001/jamadermatol.2020.0006
- Guy GP Jr, Machlin SR, Ekwueme DU, et al. Prevalence and costs of skin cancer treatment in the U.S., 2002–2006 and 2007-2011. Am J Prev Med. 2015;48:183-187. doi:10.1016/j.amepre.2014.08.036
- Paulson KG, Gupta D, Kim TS, et al. Age-specific incidence of melanoma in the United States. JAMA Dermatol. 2020;156:57-64. doi:10.1001/jamadermatol.2019.3353
- Lim HW, Collins SAB, Resneck JS Jr, et al. Contribution of health care factors to the burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:1151-1160.e21. doi:10.1016/j.jaad.2017.03.006
- Garg A, Wang J, Reddy SB, et al; Integrated Skin Exam Consortium. Curricular factors associated with medical students’ practice of the skin cancer examination: an educational enhancement initiative by the Integrated Skin Exam Consortium. JAMA Dermatol. 2014;150:850-855. doi:10.1001/jamadermatol.2013.8723
- Oliveria SA, Heneghan MK, Cushman LF, et al. Skin cancer screening by dermatologists, family practitioners, and internists: barriers and facilitating factors. Arch Dermatol. 2011;147:39-44. doi:10.1001/archdermatol.2010.414
- Cahn BA, Harper HE, Halverstam CP, et al. Current status of dermatologic education in US medical schools. JAMA Dermatol. 2020;156:468-470. doi:10.1001/jamadermatol.2020.0006
Practice Points
- Nondermatologist practitioners play a notable role in mitigating the health care burden of skin cancer by screening with the skin cancer examination.
- Exposure to the skin cancer examination should occur during medical school prior to graduates’ entering diverse specialties.
- Most medical students received relatively few hours of skin cancer education, and many never performed or even observed a skin cancer examination prior to graduating medical school.
- Increasing hands-on training and clinical exposure during medical school is imperative to adequately prepare future physicians.
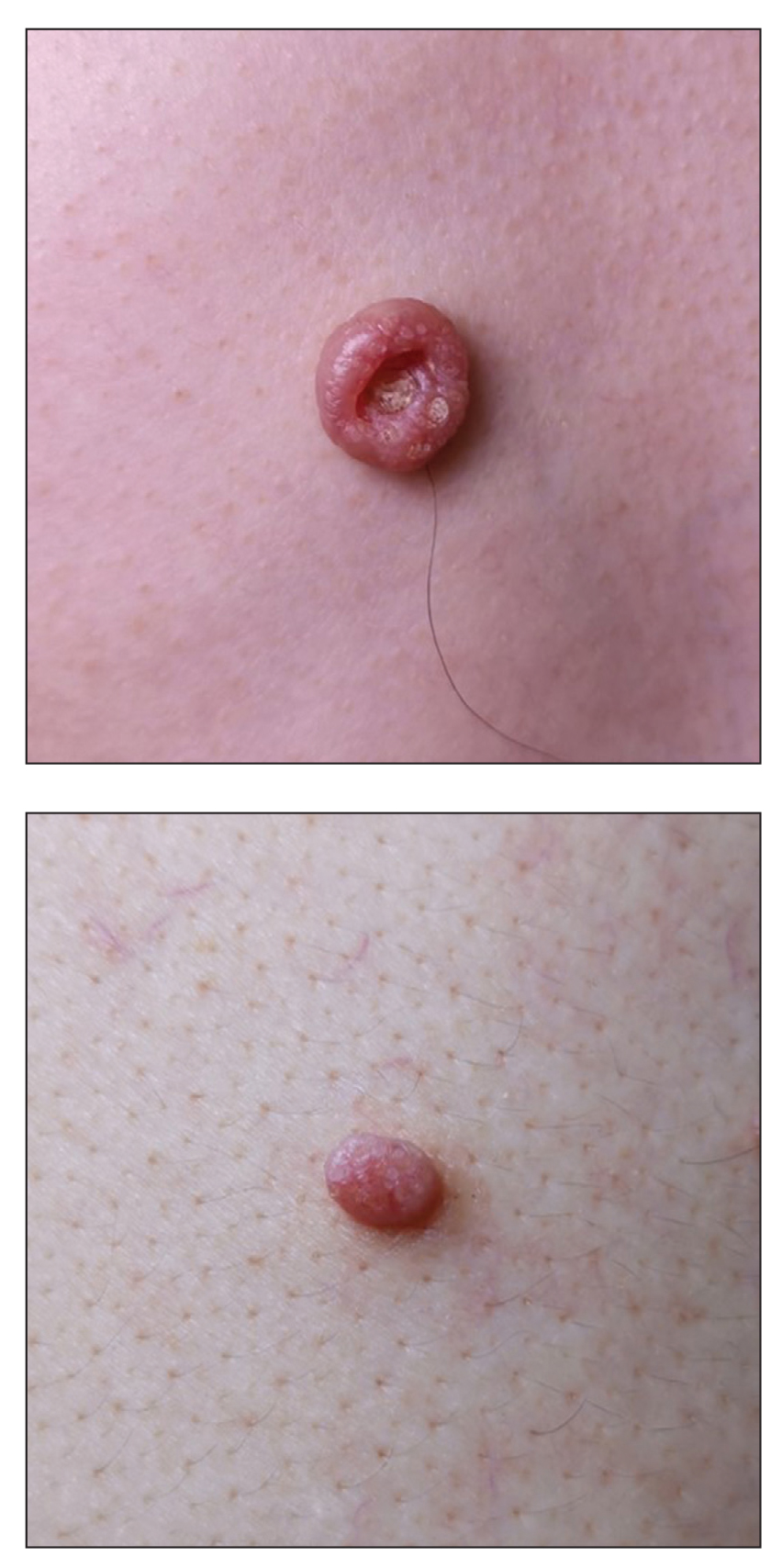
Umbilicated Neoplasm on the Chest
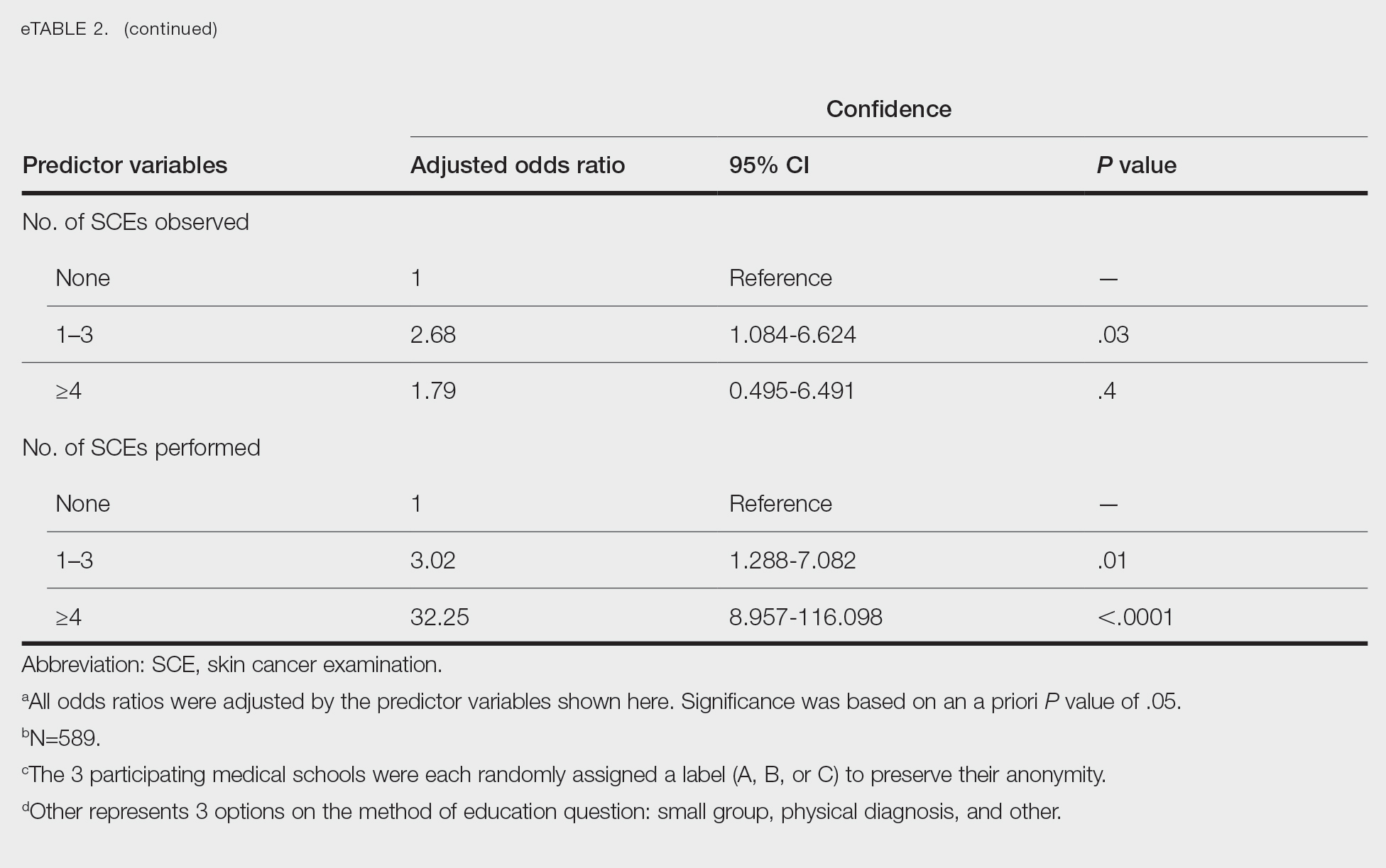
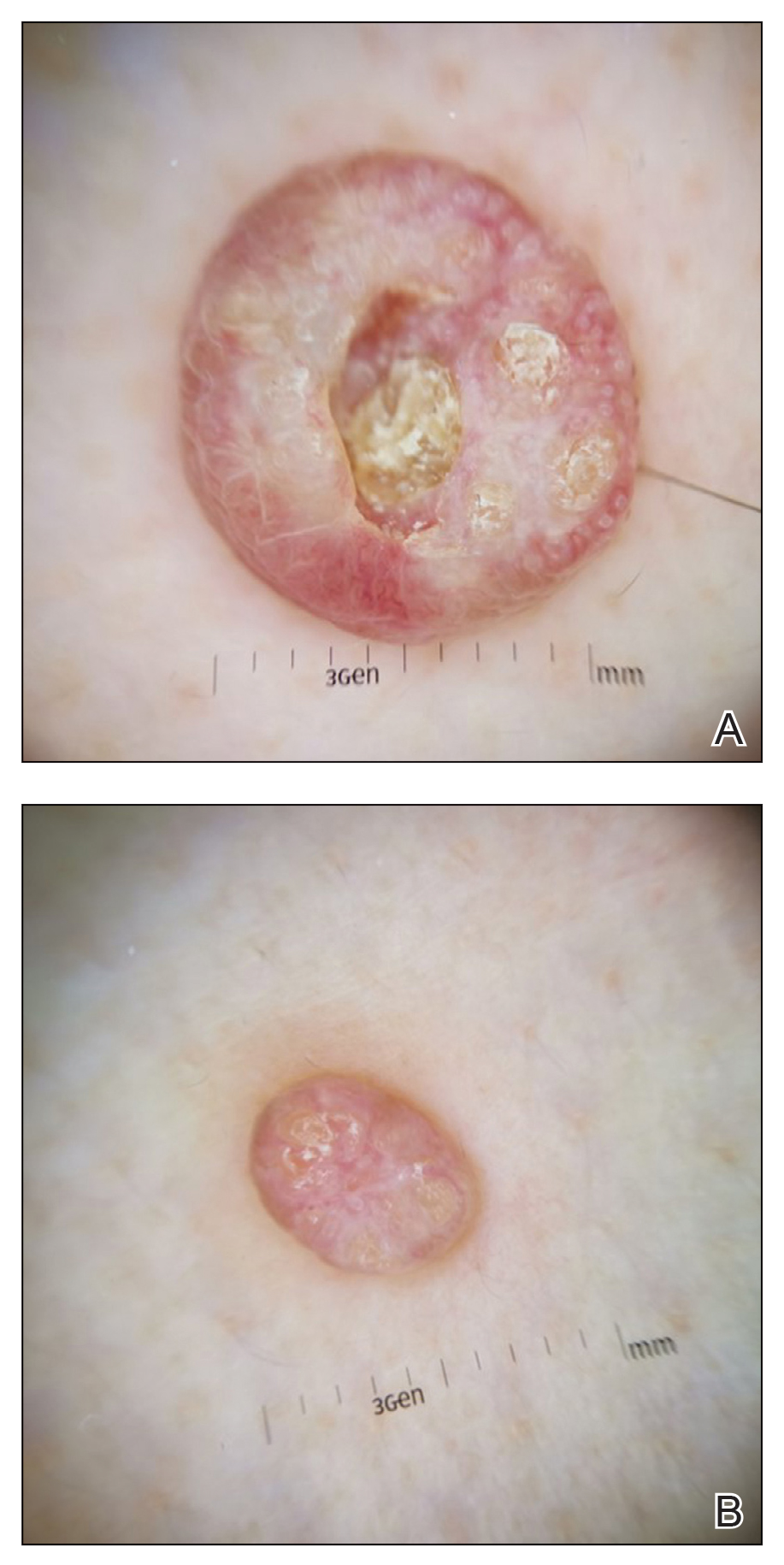
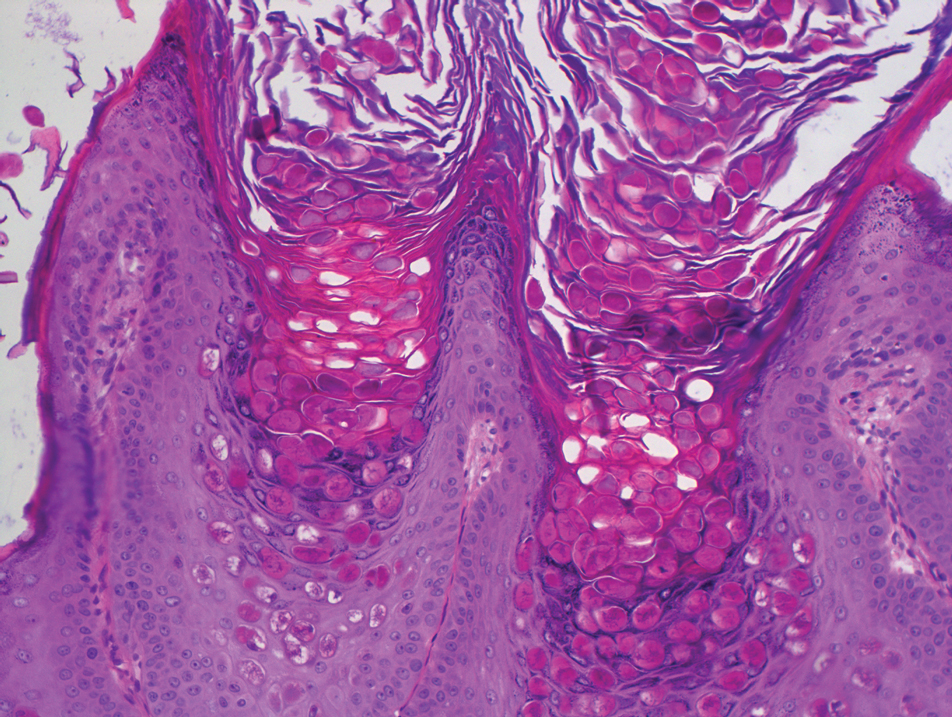
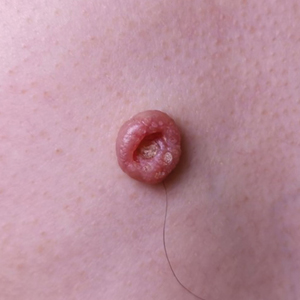
Dermoscopy showed polylobular, whitish yellow, amorphous structures at the center of the lesion surrounded by a crown of vessels (Figure 1). Histopathology revealed hyperplastic crateriform lesions containing large eosinophilic intracytoplasmic inclusion bodies within keratinocytes (Figure 2). At follow-up 2 weeks after the biopsy, the patient presented with approximately 20 more reddish papules of varying sizes on the abdomen and back that presented as dome-shaped papules and had a typical umbilicated center. The clinical manifestations, dermoscopy, and pathology findings were consistent with molluscum contagiosum (MC).
Molluscum contagiosum was first described in 1814. It is a benign cutaneous infectious disease caused by a double-stranded DNA virus of the poxvirus family. Molluscum contagiosum lesions usually manifest clinically as dome-shaped, flesh-colored or translucent, umbilicated papules measuring 1 to 5 mm in diameter that are commonly distributed over the face, trunk, and extremities and usually are self-limiting.1
Giant MC is rare and can be seen either in patients on immunosuppressive therapy or in those with diseases that can cause immunosuppression, such as human immunodeficiency virus, leukemia, atopic dermatitis, Wiskott-Aldrich syndrome, and sarcoidosis. In these instances, MC often is greater than 1 cm in diameter. Atypical variants may have an eczematous presentation or a lesion with secondary abscess formation and also can be spread widely over the body.2 Due to these atypical appearances and large dimensions in immunocompromised patients, other dermatologic diseases should be considered in the differential diagnosis, such as basal cell carcinoma, keratoacanthoma, squamous cell carcinoma, cutaneous horn, cutaneous cryptococcosis, histoplasmosis, and xanthomatosis.3
In our patient, the differential diagnosis included keratoacanthoma, which may present as a solitary, discrete, round to oval, flesh-colored, umbilicated nodule with a central keratin-filled crater and has a rapid clinical evolution, usually regressing within 4 to 6 months.
Squamous cell carcinoma may appear as scaly red patches, open sores, warts, or elevated growths with a central depression and may crust or bleed. Basal cell carcinoma typically may appear as a dome-shaped skin nodule with visible blood vessels or sometimes presents as a red patch similar to eczema. Xanthomatosis often appears as yellow to orange, mostly asymptomatic, supple patches or plaques, usually with sharp and distinctive edges.
Ancillary diagnostic modalities such as dermoscopy may be used to improve diagnostic accuracy. The best known capillaroscopic feature of MC is the peripheral crown of vessels in a radial distribution. A study of 258 MC lesions highlighted that crown and crown plus radial arrangements are the most common vascular structure patterns under dermoscopy. In addition, polylobular amorphous white structures in the center of the lesions tend to be a feature of larger MC papules.4 Histologically, MC shows lobulated crateriform lesions, thickening of the epidermis into the dermis, and the typical appearance of large eosinophilic intracytoplasmic inclusion bodies within keratinocytes.5
There are several treatment options available for MC. Common modalities include liquid nitrogen cryospray, curettage, and electrocauterization. In immunocompromised patients, MC lesions usually are resistant to ordinary therapy. The efficacy of topical agents such as imiquimod, which can induce high levels of IFN-α and other cytokines, has been demonstrated in these patients.6 Cidofovir, a nucleoside analog that has potent antiviral properties, also can be included as a therapeutic option.3 Our patient’s largest MC lesion was treated with surgical excision, the 2 large lesions on the left side of the chest with cryotherapy, and the other small lesions with curettage.
- Hanson D, Diven DG. Molluscum contagiosum. Dermatol Online J. 2003;9:2.
- Singh S, Swain M, Shukla S, et al. An unusual presentation of giant molluscum contagiosum diagnosed on cytology. Diagn Cytopathol. 2018;46:794-796.
- Mansur AT, Goktay F, Gunduz S, et al. Multiple giant molluscum contagiosum in a renal transplant recipient. Transpl Infect Dis. 2004;6:120-123.
- Ku SH, Cho EB, Park EJ, et al. Dermoscopic features of molluscum contagiosum based on white structures and their correlation with histopathological findings. Clin Exp Dermatol. 2015;40:208-210.
- Trčko K, Hošnjak L, Kušar B, et al. Clinical, histopathological, and virological evaluation of 203 patients with a clinical diagnosis of molluscum contagiosum [published online November 12, 2018]. Open Forum Infect Dis. 2018;5.
- Gardner LS, Ormond PJ. Treatment of multiple giant molluscum contagiosum in a renal transplant patient with imiquimod 5% cream. Clin Exp Dermatol. 2010;31:452-453.
Dermoscopy showed polylobular, whitish yellow, amorphous structures at the center of the lesion surrounded by a crown of vessels (Figure 1). Histopathology revealed hyperplastic crateriform lesions containing large eosinophilic intracytoplasmic inclusion bodies within keratinocytes (Figure 2). At follow-up 2 weeks after the biopsy, the patient presented with approximately 20 more reddish papules of varying sizes on the abdomen and back that presented as dome-shaped papules and had a typical umbilicated center. The clinical manifestations, dermoscopy, and pathology findings were consistent with molluscum contagiosum (MC).
Molluscum contagiosum was first described in 1814. It is a benign cutaneous infectious disease caused by a double-stranded DNA virus of the poxvirus family. Molluscum contagiosum lesions usually manifest clinically as dome-shaped, flesh-colored or translucent, umbilicated papules measuring 1 to 5 mm in diameter that are commonly distributed over the face, trunk, and extremities and usually are self-limiting.1
Giant MC is rare and can be seen either in patients on immunosuppressive therapy or in those with diseases that can cause immunosuppression, such as human immunodeficiency virus, leukemia, atopic dermatitis, Wiskott-Aldrich syndrome, and sarcoidosis. In these instances, MC often is greater than 1 cm in diameter. Atypical variants may have an eczematous presentation or a lesion with secondary abscess formation and also can be spread widely over the body.2 Due to these atypical appearances and large dimensions in immunocompromised patients, other dermatologic diseases should be considered in the differential diagnosis, such as basal cell carcinoma, keratoacanthoma, squamous cell carcinoma, cutaneous horn, cutaneous cryptococcosis, histoplasmosis, and xanthomatosis.3
In our patient, the differential diagnosis included keratoacanthoma, which may present as a solitary, discrete, round to oval, flesh-colored, umbilicated nodule with a central keratin-filled crater and has a rapid clinical evolution, usually regressing within 4 to 6 months.
Squamous cell carcinoma may appear as scaly red patches, open sores, warts, or elevated growths with a central depression and may crust or bleed. Basal cell carcinoma typically may appear as a dome-shaped skin nodule with visible blood vessels or sometimes presents as a red patch similar to eczema. Xanthomatosis often appears as yellow to orange, mostly asymptomatic, supple patches or plaques, usually with sharp and distinctive edges.
Ancillary diagnostic modalities such as dermoscopy may be used to improve diagnostic accuracy. The best known capillaroscopic feature of MC is the peripheral crown of vessels in a radial distribution. A study of 258 MC lesions highlighted that crown and crown plus radial arrangements are the most common vascular structure patterns under dermoscopy. In addition, polylobular amorphous white structures in the center of the lesions tend to be a feature of larger MC papules.4 Histologically, MC shows lobulated crateriform lesions, thickening of the epidermis into the dermis, and the typical appearance of large eosinophilic intracytoplasmic inclusion bodies within keratinocytes.5
There are several treatment options available for MC. Common modalities include liquid nitrogen cryospray, curettage, and electrocauterization. In immunocompromised patients, MC lesions usually are resistant to ordinary therapy. The efficacy of topical agents such as imiquimod, which can induce high levels of IFN-α and other cytokines, has been demonstrated in these patients.6 Cidofovir, a nucleoside analog that has potent antiviral properties, also can be included as a therapeutic option.3 Our patient’s largest MC lesion was treated with surgical excision, the 2 large lesions on the left side of the chest with cryotherapy, and the other small lesions with curettage.
Dermoscopy showed polylobular, whitish yellow, amorphous structures at the center of the lesion surrounded by a crown of vessels (Figure 1). Histopathology revealed hyperplastic crateriform lesions containing large eosinophilic intracytoplasmic inclusion bodies within keratinocytes (Figure 2). At follow-up 2 weeks after the biopsy, the patient presented with approximately 20 more reddish papules of varying sizes on the abdomen and back that presented as dome-shaped papules and had a typical umbilicated center. The clinical manifestations, dermoscopy, and pathology findings were consistent with molluscum contagiosum (MC).
Molluscum contagiosum was first described in 1814. It is a benign cutaneous infectious disease caused by a double-stranded DNA virus of the poxvirus family. Molluscum contagiosum lesions usually manifest clinically as dome-shaped, flesh-colored or translucent, umbilicated papules measuring 1 to 5 mm in diameter that are commonly distributed over the face, trunk, and extremities and usually are self-limiting.1
Giant MC is rare and can be seen either in patients on immunosuppressive therapy or in those with diseases that can cause immunosuppression, such as human immunodeficiency virus, leukemia, atopic dermatitis, Wiskott-Aldrich syndrome, and sarcoidosis. In these instances, MC often is greater than 1 cm in diameter. Atypical variants may have an eczematous presentation or a lesion with secondary abscess formation and also can be spread widely over the body.2 Due to these atypical appearances and large dimensions in immunocompromised patients, other dermatologic diseases should be considered in the differential diagnosis, such as basal cell carcinoma, keratoacanthoma, squamous cell carcinoma, cutaneous horn, cutaneous cryptococcosis, histoplasmosis, and xanthomatosis.3
In our patient, the differential diagnosis included keratoacanthoma, which may present as a solitary, discrete, round to oval, flesh-colored, umbilicated nodule with a central keratin-filled crater and has a rapid clinical evolution, usually regressing within 4 to 6 months.
Squamous cell carcinoma may appear as scaly red patches, open sores, warts, or elevated growths with a central depression and may crust or bleed. Basal cell carcinoma typically may appear as a dome-shaped skin nodule with visible blood vessels or sometimes presents as a red patch similar to eczema. Xanthomatosis often appears as yellow to orange, mostly asymptomatic, supple patches or plaques, usually with sharp and distinctive edges.
Ancillary diagnostic modalities such as dermoscopy may be used to improve diagnostic accuracy. The best known capillaroscopic feature of MC is the peripheral crown of vessels in a radial distribution. A study of 258 MC lesions highlighted that crown and crown plus radial arrangements are the most common vascular structure patterns under dermoscopy. In addition, polylobular amorphous white structures in the center of the lesions tend to be a feature of larger MC papules.4 Histologically, MC shows lobulated crateriform lesions, thickening of the epidermis into the dermis, and the typical appearance of large eosinophilic intracytoplasmic inclusion bodies within keratinocytes.5
There are several treatment options available for MC. Common modalities include liquid nitrogen cryospray, curettage, and electrocauterization. In immunocompromised patients, MC lesions usually are resistant to ordinary therapy. The efficacy of topical agents such as imiquimod, which can induce high levels of IFN-α and other cytokines, has been demonstrated in these patients.6 Cidofovir, a nucleoside analog that has potent antiviral properties, also can be included as a therapeutic option.3 Our patient’s largest MC lesion was treated with surgical excision, the 2 large lesions on the left side of the chest with cryotherapy, and the other small lesions with curettage.
- Hanson D, Diven DG. Molluscum contagiosum. Dermatol Online J. 2003;9:2.
- Singh S, Swain M, Shukla S, et al. An unusual presentation of giant molluscum contagiosum diagnosed on cytology. Diagn Cytopathol. 2018;46:794-796.
- Mansur AT, Goktay F, Gunduz S, et al. Multiple giant molluscum contagiosum in a renal transplant recipient. Transpl Infect Dis. 2004;6:120-123.
- Ku SH, Cho EB, Park EJ, et al. Dermoscopic features of molluscum contagiosum based on white structures and their correlation with histopathological findings. Clin Exp Dermatol. 2015;40:208-210.
- Trčko K, Hošnjak L, Kušar B, et al. Clinical, histopathological, and virological evaluation of 203 patients with a clinical diagnosis of molluscum contagiosum [published online November 12, 2018]. Open Forum Infect Dis. 2018;5.
- Gardner LS, Ormond PJ. Treatment of multiple giant molluscum contagiosum in a renal transplant patient with imiquimod 5% cream. Clin Exp Dermatol. 2010;31:452-453.
- Hanson D, Diven DG. Molluscum contagiosum. Dermatol Online J. 2003;9:2.
- Singh S, Swain M, Shukla S, et al. An unusual presentation of giant molluscum contagiosum diagnosed on cytology. Diagn Cytopathol. 2018;46:794-796.
- Mansur AT, Goktay F, Gunduz S, et al. Multiple giant molluscum contagiosum in a renal transplant recipient. Transpl Infect Dis. 2004;6:120-123.
- Ku SH, Cho EB, Park EJ, et al. Dermoscopic features of molluscum contagiosum based on white structures and their correlation with histopathological findings. Clin Exp Dermatol. 2015;40:208-210.
- Trčko K, Hošnjak L, Kušar B, et al. Clinical, histopathological, and virological evaluation of 203 patients with a clinical diagnosis of molluscum contagiosum [published online November 12, 2018]. Open Forum Infect Dis. 2018;5.
- Gardner LS, Ormond PJ. Treatment of multiple giant molluscum contagiosum in a renal transplant patient with imiquimod 5% cream. Clin Exp Dermatol. 2010;31:452-453.
A 49-year-old man presented with a slow-growing mass on the chest of 1 year’s duration. The neoplasm started as a small papule that gradually increased in size. The patient denied pain, itching, bleeding, or discharge. He had a history of end-stage renal disease with a kidney transplant 8 years prior. His medication history included long-term use of oral tacrolimus, mycophenolate mofetil, and prednisone. Physical examination revealed a yellowish red, exogenous, pedunculated neoplasm on the right side of the chest measuring 1 cm in diameter with an umbilicated center and keratotic material (top). There were 2 more yellowish red papules on the left side of the chest measuring 0.5 cm in diameter without an umbilicated center (bottom). Dermoscopy and a biopsy were performed.
Flesh-Colored Nodule With Underlying Sclerotic Plaque
The Diagnosis: Collision Tumor
Excisional biopsy and histopathological examination demonstrated a collision tumor composed of a benign intradermal melanocytic nevus, tumor of follicular infundibulum, and an underlying sclerosing epithelial neoplasm, with a differential diagnosis of desmoplastic trichoepithelioma, morpheaform basal cell carcinoma, and microcystic adnexal carcinoma (Figure).
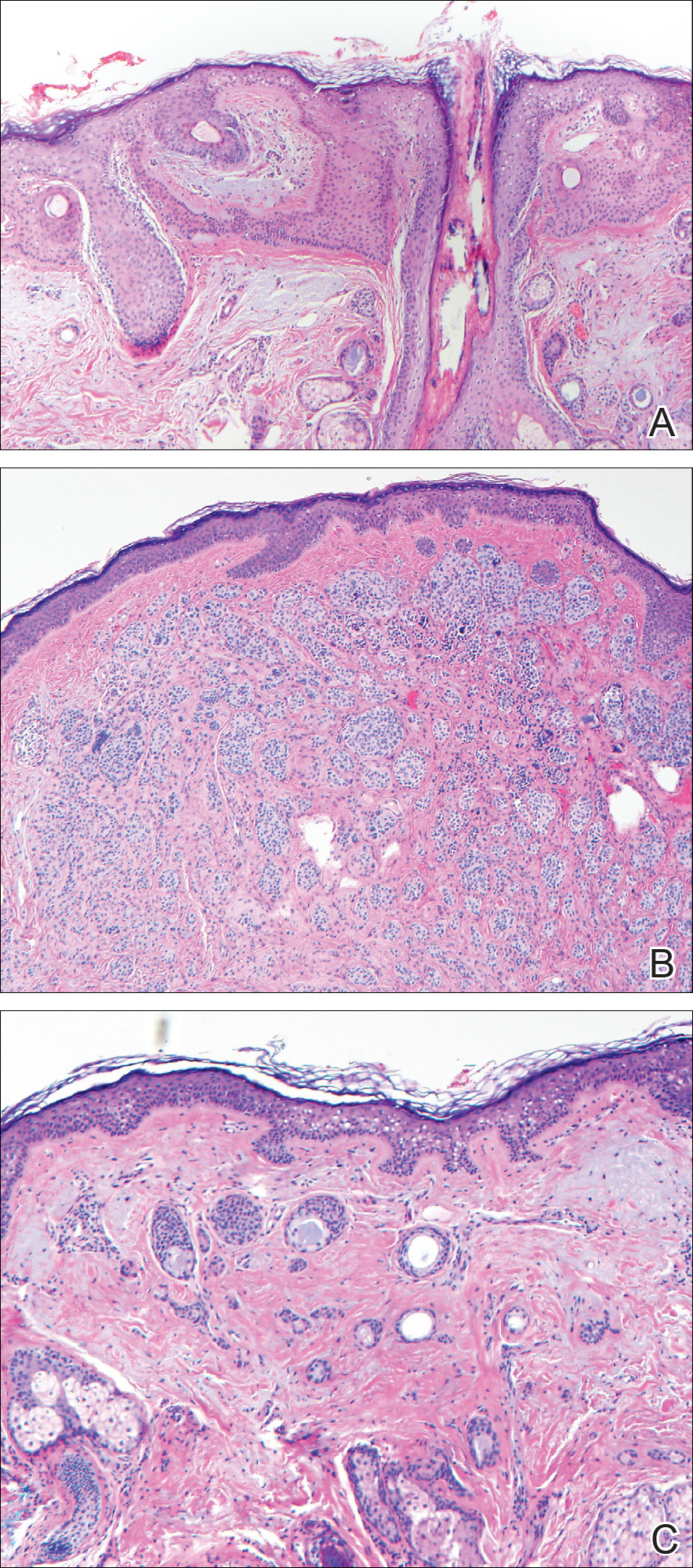
Common acquired melanocytic nevus presents clinically as a macule, papule, or nodule with smooth regular borders. The pigmented variant displays an evenly distributed pigment on the lesion. Intradermal melanocytic nevus often presents as a flesh-colored nodule, as in our case. Histopathologically, benign intradermal nevus typically is composed of a proliferation of melanocytes that exhibit dispersion as they go deeper in the dermis and maturation that manifests as melanocytes becoming smaller and more spindled in the deeper portions of the lesion.1 These 2 characteristics plus the bland cytology seen in the present case confirm the benign characteristic of this lesion (Figure, B).
In addition to the benign intradermal melanocytic nevus, an adjacent tumor of follicular infundibulum was noted. Tumor of follicular infundibulum is a rare adnexal tumor. It occurs frequently on the head and neck and shows some female predominance.2,3 Multiple lesions and eruptive lesions are rare forms that also have been reported.4 Histopathologically, the tumor demonstrates an epithelial plate that is present in the papillary dermis and is connected to the epidermis at multiple points with attachment to the follicular outer root sheath. Peripheral palisading is characteristically present above an eosinophilic basement membrane (Figure, A). Rare reports have documented sebaceous and eccrine differentiation.5,6
Tumor of follicular infundibulum has been reported to be associated with other tumors. Organoid nevus (nevus sebaceous), trichilemmal tumor, and fibroma have been reported to occur as a collision tumor with tumor of follicular infundibulum. An association with Cowden disease also has been described.7 Biopsies that represent partial samples should be interpreted cautiously, as step sections can reveal basal cell carcinoma.
The term sclerosing epithelial neoplasm describes tumors that share a paisley tielike epithelial pattern and sclerotic stroma. Small specimens often require clinicopathologic correlation (Figure, C). The differential diagnosis includes morpheaform basal cell carcinoma, desmoplastic trichoepithelioma, and microcystic adnexal carcinoma. A panel of stains using Ber-EP4, PHLDA1, cytokeratin 15, and cytokeratin 19 has been proposed to help differentiate these entities.8 CD34 and cytokeratin 20 also have been used with varying success in small specimens.9,10
- Ferringer T, Peckham S, Ko CJ, et al. Melanocytic neoplasms. In: Elston DM, Ferringer T, eds. Dermatopathology. 2nd ed. Philadelphia, PA: Elsevier Saunders; 2014:105-109.
- Headington JT. Tumors of the hair follicle. Am J Pathol. 1976;85:480-505.
- Davis DA, Cohen PR. Hair follicle nevus: case report and review of the literature. Pediatr Dermatol. 1996;13:135-138.
- Ikeda S, Kawada J, Yaguchi H, et al. A case of unilateral, systematized linear hair follicle nevi associated with epidermal nevus-like lesions. Dermatology. 2003;206:172-174.
- Mehregan AH. Hair follicle tumors of the skin. J Cutan Pathol. 1985;12:189-195.
- Mahalingam M, Bhawan J, Finn R, et al. Tumor of the follicular infundibulum with sebaceous differentiation. J Cutan Pathol. 2001;28:314-317.
- Cribier B, Grosshans E. Tumor of the follicular infundibulum: a clinicopathologic study. J Am Acad Dermatol. 1995;33:979-984.
- Sellheyer K, Nelson P, Kutzner H, et al. The immunohistochemical differential diagnosis of microcystic adnexal carcinoma, desmoplastic trichoepithelioma and morpheaform basal cell carcinoma using BerEP4 and stem cell markers. J Cutan Pathol. 2013;40:363-370.
- Abesamis-Cubillan E, El-Shabrawi-Caelen L, LeBoit PE. Merkel cells and sclerosing epithelial neoplasms. Am J Dermatopathol. 2000;22:311-315.
- Smith KJ, Williams J, Corbett D, et al. Microcystic adnexal carcinoma: an immunohistochemical study including markers of proliferation and apoptosis. Am J Surg Pathol. 2001;25:464-471.
The Diagnosis: Collision Tumor
Excisional biopsy and histopathological examination demonstrated a collision tumor composed of a benign intradermal melanocytic nevus, tumor of follicular infundibulum, and an underlying sclerosing epithelial neoplasm, with a differential diagnosis of desmoplastic trichoepithelioma, morpheaform basal cell carcinoma, and microcystic adnexal carcinoma (Figure).

Common acquired melanocytic nevus presents clinically as a macule, papule, or nodule with smooth regular borders. The pigmented variant displays an evenly distributed pigment on the lesion. Intradermal melanocytic nevus often presents as a flesh-colored nodule, as in our case. Histopathologically, benign intradermal nevus typically is composed of a proliferation of melanocytes that exhibit dispersion as they go deeper in the dermis and maturation that manifests as melanocytes becoming smaller and more spindled in the deeper portions of the lesion.1 These 2 characteristics plus the bland cytology seen in the present case confirm the benign characteristic of this lesion (Figure, B).
In addition to the benign intradermal melanocytic nevus, an adjacent tumor of follicular infundibulum was noted. Tumor of follicular infundibulum is a rare adnexal tumor. It occurs frequently on the head and neck and shows some female predominance.2,3 Multiple lesions and eruptive lesions are rare forms that also have been reported.4 Histopathologically, the tumor demonstrates an epithelial plate that is present in the papillary dermis and is connected to the epidermis at multiple points with attachment to the follicular outer root sheath. Peripheral palisading is characteristically present above an eosinophilic basement membrane (Figure, A). Rare reports have documented sebaceous and eccrine differentiation.5,6
Tumor of follicular infundibulum has been reported to be associated with other tumors. Organoid nevus (nevus sebaceous), trichilemmal tumor, and fibroma have been reported to occur as a collision tumor with tumor of follicular infundibulum. An association with Cowden disease also has been described.7 Biopsies that represent partial samples should be interpreted cautiously, as step sections can reveal basal cell carcinoma.
The term sclerosing epithelial neoplasm describes tumors that share a paisley tielike epithelial pattern and sclerotic stroma. Small specimens often require clinicopathologic correlation (Figure, C). The differential diagnosis includes morpheaform basal cell carcinoma, desmoplastic trichoepithelioma, and microcystic adnexal carcinoma. A panel of stains using Ber-EP4, PHLDA1, cytokeratin 15, and cytokeratin 19 has been proposed to help differentiate these entities.8 CD34 and cytokeratin 20 also have been used with varying success in small specimens.9,10
The Diagnosis: Collision Tumor
Excisional biopsy and histopathological examination demonstrated a collision tumor composed of a benign intradermal melanocytic nevus, tumor of follicular infundibulum, and an underlying sclerosing epithelial neoplasm, with a differential diagnosis of desmoplastic trichoepithelioma, morpheaform basal cell carcinoma, and microcystic adnexal carcinoma (Figure).

Common acquired melanocytic nevus presents clinically as a macule, papule, or nodule with smooth regular borders. The pigmented variant displays an evenly distributed pigment on the lesion. Intradermal melanocytic nevus often presents as a flesh-colored nodule, as in our case. Histopathologically, benign intradermal nevus typically is composed of a proliferation of melanocytes that exhibit dispersion as they go deeper in the dermis and maturation that manifests as melanocytes becoming smaller and more spindled in the deeper portions of the lesion.1 These 2 characteristics plus the bland cytology seen in the present case confirm the benign characteristic of this lesion (Figure, B).
In addition to the benign intradermal melanocytic nevus, an adjacent tumor of follicular infundibulum was noted. Tumor of follicular infundibulum is a rare adnexal tumor. It occurs frequently on the head and neck and shows some female predominance.2,3 Multiple lesions and eruptive lesions are rare forms that also have been reported.4 Histopathologically, the tumor demonstrates an epithelial plate that is present in the papillary dermis and is connected to the epidermis at multiple points with attachment to the follicular outer root sheath. Peripheral palisading is characteristically present above an eosinophilic basement membrane (Figure, A). Rare reports have documented sebaceous and eccrine differentiation.5,6
Tumor of follicular infundibulum has been reported to be associated with other tumors. Organoid nevus (nevus sebaceous), trichilemmal tumor, and fibroma have been reported to occur as a collision tumor with tumor of follicular infundibulum. An association with Cowden disease also has been described.7 Biopsies that represent partial samples should be interpreted cautiously, as step sections can reveal basal cell carcinoma.
The term sclerosing epithelial neoplasm describes tumors that share a paisley tielike epithelial pattern and sclerotic stroma. Small specimens often require clinicopathologic correlation (Figure, C). The differential diagnosis includes morpheaform basal cell carcinoma, desmoplastic trichoepithelioma, and microcystic adnexal carcinoma. A panel of stains using Ber-EP4, PHLDA1, cytokeratin 15, and cytokeratin 19 has been proposed to help differentiate these entities.8 CD34 and cytokeratin 20 also have been used with varying success in small specimens.9,10
- Ferringer T, Peckham S, Ko CJ, et al. Melanocytic neoplasms. In: Elston DM, Ferringer T, eds. Dermatopathology. 2nd ed. Philadelphia, PA: Elsevier Saunders; 2014:105-109.
- Headington JT. Tumors of the hair follicle. Am J Pathol. 1976;85:480-505.
- Davis DA, Cohen PR. Hair follicle nevus: case report and review of the literature. Pediatr Dermatol. 1996;13:135-138.
- Ikeda S, Kawada J, Yaguchi H, et al. A case of unilateral, systematized linear hair follicle nevi associated with epidermal nevus-like lesions. Dermatology. 2003;206:172-174.
- Mehregan AH. Hair follicle tumors of the skin. J Cutan Pathol. 1985;12:189-195.
- Mahalingam M, Bhawan J, Finn R, et al. Tumor of the follicular infundibulum with sebaceous differentiation. J Cutan Pathol. 2001;28:314-317.
- Cribier B, Grosshans E. Tumor of the follicular infundibulum: a clinicopathologic study. J Am Acad Dermatol. 1995;33:979-984.
- Sellheyer K, Nelson P, Kutzner H, et al. The immunohistochemical differential diagnosis of microcystic adnexal carcinoma, desmoplastic trichoepithelioma and morpheaform basal cell carcinoma using BerEP4 and stem cell markers. J Cutan Pathol. 2013;40:363-370.
- Abesamis-Cubillan E, El-Shabrawi-Caelen L, LeBoit PE. Merkel cells and sclerosing epithelial neoplasms. Am J Dermatopathol. 2000;22:311-315.
- Smith KJ, Williams J, Corbett D, et al. Microcystic adnexal carcinoma: an immunohistochemical study including markers of proliferation and apoptosis. Am J Surg Pathol. 2001;25:464-471.
- Ferringer T, Peckham S, Ko CJ, et al. Melanocytic neoplasms. In: Elston DM, Ferringer T, eds. Dermatopathology. 2nd ed. Philadelphia, PA: Elsevier Saunders; 2014:105-109.
- Headington JT. Tumors of the hair follicle. Am J Pathol. 1976;85:480-505.
- Davis DA, Cohen PR. Hair follicle nevus: case report and review of the literature. Pediatr Dermatol. 1996;13:135-138.
- Ikeda S, Kawada J, Yaguchi H, et al. A case of unilateral, systematized linear hair follicle nevi associated with epidermal nevus-like lesions. Dermatology. 2003;206:172-174.
- Mehregan AH. Hair follicle tumors of the skin. J Cutan Pathol. 1985;12:189-195.
- Mahalingam M, Bhawan J, Finn R, et al. Tumor of the follicular infundibulum with sebaceous differentiation. J Cutan Pathol. 2001;28:314-317.
- Cribier B, Grosshans E. Tumor of the follicular infundibulum: a clinicopathologic study. J Am Acad Dermatol. 1995;33:979-984.
- Sellheyer K, Nelson P, Kutzner H, et al. The immunohistochemical differential diagnosis of microcystic adnexal carcinoma, desmoplastic trichoepithelioma and morpheaform basal cell carcinoma using BerEP4 and stem cell markers. J Cutan Pathol. 2013;40:363-370.
- Abesamis-Cubillan E, El-Shabrawi-Caelen L, LeBoit PE. Merkel cells and sclerosing epithelial neoplasms. Am J Dermatopathol. 2000;22:311-315.
- Smith KJ, Williams J, Corbett D, et al. Microcystic adnexal carcinoma: an immunohistochemical study including markers of proliferation and apoptosis. Am J Surg Pathol. 2001;25:464-471.
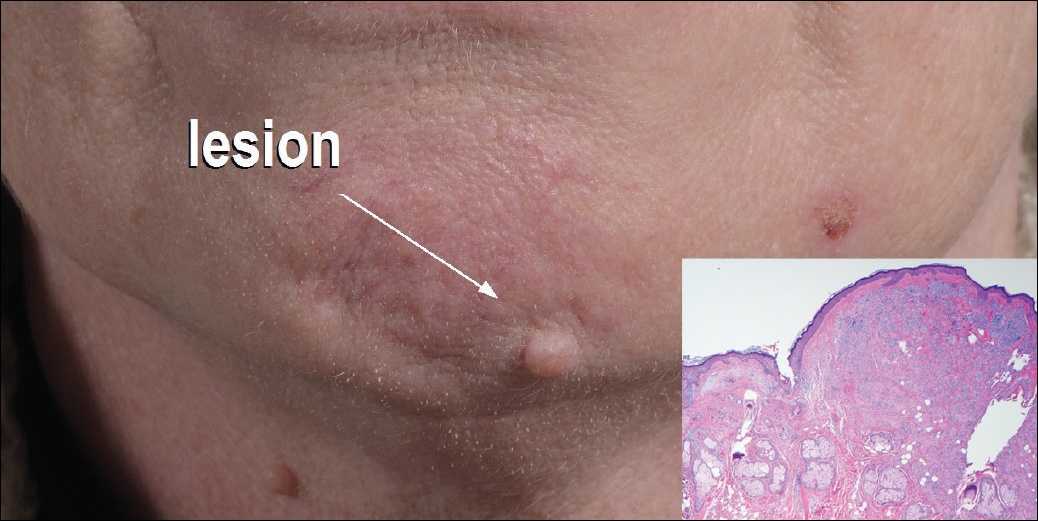
A 54-year-old man presented with a flesh-colored lesion on the chin. The nodule measured 0.6 cm in diameter. There was an underlying sclerotic plaque with indistinct borders.
Bluish Gray Hyperpigmentation on the Face and Neck
The Diagnosis: Erythema Dyschromicum Perstans
Erythema dyschromicum perstans (EDP), also referred to as ashy dermatosis, was first described by Ramirez1 in 1957 who labeled the patients los cenicientos (the ashen ones). It preferentially affects women in the second decade of life; however, patients of all ages can be affected, with reported cases occurring in children as young as 2 years of age.2 Most patients have Fitzpatrick skin type IV, mainly Amerindian, Hispanic South Asian, and Southwest Asian; however, there are cases reported worldwide.3 A genetic predisposition is proposed, as major histocompatibility complex genes associated with HLA-DR4⁎0407 are frequent in Mexican patients with ashy dermatosis and in the Amerindian population.4
The etiology of EDP is unknown. Various contributing factors have been reported including alimentary, occupational, and climatic factors,5,6 yet none have been conclusively demonstrated. High expression of CD36 (thrombospondin receptor not found in normal skin) in spinous and granular layers, CD94 (cytotoxic cell marker) in the basal cell layer and in the inflammatory dermal infiltrate,7 and focal keratinocytic expression of intercellular adhesion molecule I (CD54) in the active lesions of EDP, as well as the absence of these findings in normal skin, suggests an immunologic role in the development of the disease.8
Erythema dyschromicum perstans presents clinically with blue-gray hyperpigmented macules varying in size and shape and developing symmetrically in both sun-exposed and sun-protected areas of the face, neck, trunk, arms, and sometimes the dorsal hands (Figures 1 and 2). Notable sparing of the palms, soles, scalp, and mucous membranes occurs.
Occasionally, in the early active stage of the disease, elevated erythematous borders are noted surrounding the hyperpigmented macules. Eventually a hypopigmented halo develops after a prolonged duration of disease.9 The eruption typically is chronic and asymptomatic, though some cases may be pruritic.10
Histopathologically, the early lesions of EDP with an erythematous active border reveal lichenoid dermatitis with basal vacuolar change and occasional Civatte bodies. A mild to moderate perivascular lymphohistiocytic infiltrate admixed with melanophages can be seen in the papillary dermis (Figure 3). In older lesions, the inflammatory infiltrate is sparse, and pigment incontinence consistent with postinflammatory pigmentation is prominent, though melanophages extending deep into the reticular dermis may aid in distinguishing EDP from other causes of postinflammatory pigment alteration.7,11
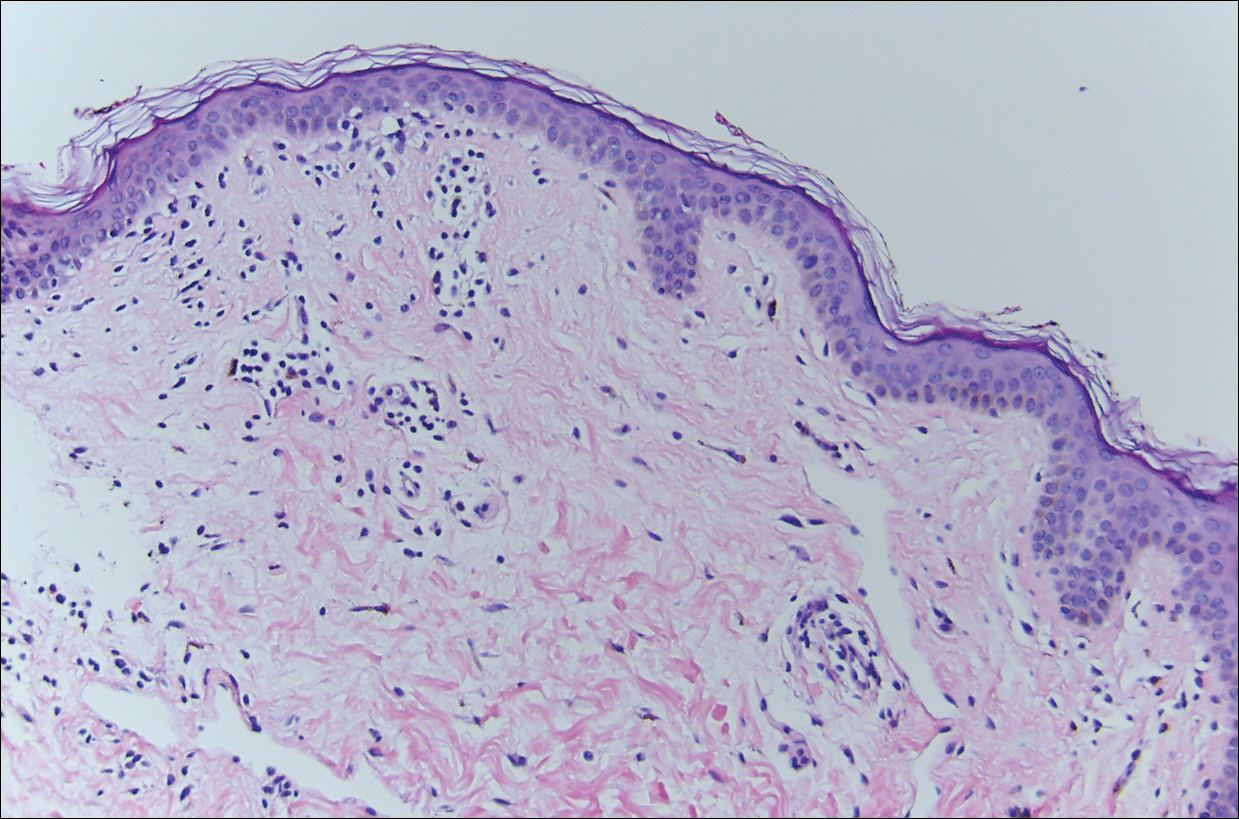
Erythema dyschromicum perstans and lichen planus pigmentosus (LPP) may be indistinguishable histopathologically and may both be variants of lichen planus actinicus. Lichen planus pigmentosus often differs from EDP in that it presents with brown-black macules and patches often on the face and flexural areas. A subset of cases of LPP also may have mucous membrane involvement. The erythematous border that characterizes the active lesion of EDP is characteristically absent in LPP. In addition, pruritus often is reported with LPP. Direct immunofluorescence is not a beneficial tool in distinguishing the entities.12
Other differential diagnoses of predominantly facial hyperpigmentation include a lichenoid drug eruption; drug-induced hyperpigmentation (deposition disorder); postinflammatory hyperpigmentation following atopic dermatitis; contact dermatitis or photosensitivity reaction; early pinta; and cutaneous findings of systemic diseases manifesting with diffuse hyperpigmentation such as lupus erythematosus, dermatomyositis, hemochromatosis, and Addison disease. A detailed history including medication use, thorough clinical examination, and careful histopathologic evaluation will help distinguish these conditions.
Chrysiasis is a rare bluish to slate gray discoloration of the skin that predominantly occurs in sun-exposed areas. It is caused by chronic use of gold salts, which have been used to treat rheumatoid arthritis. UV light may contribute to induce the uptake of gold and subsequently stimulate tyrosinase activity.13 Histologic features of chrysiasis include dermal and perivascular gold deposition within the macrophages and endothelial cells as well as extracellular granules. It demonstrates an orange-red birefringence on fluorescent microscopy.14,15
Minocycline-induced hyperpigmentation is a well-recognized side effect of this drug. It is dose dependent and appears as a blue-black pigmentation that most frequently affects the shins, ankles, and arms.16 Three distinct types were documented: abnormal discoloration of the skin that has been linked to deposition of pigmented metabolites of minocycline producing blue-black pigmentation at the site of scarring or prior inflammation (type 1); blue-gray pigmentation affecting normal skin, mainly the legs (type 2); and elevated levels of melanin on the sun-exposed areas producing dirty skin syndrome (type 3).17,18
Topical and systemic corticosteroids, UV light therapy, oral dapsone, griseofulvin, retinoids, and clofazimine are reported as treatment options for ashy dermatosis, though results typically are disappointing.7
- Ramirez CO. Los cenicientos: problema clinica. In: Memoria del Primer Congresso Centroamericano de Dermatologica, December 5-8, 1957. San Salvador, El Salvador; 1957:122-130.
- Lee SJ, Chung KY. Erythema dyschromicum perstans in early childhood. J Dermatol. 1999;26:119-121.
- Homez-Chacin, Barroso C. On the etiopathogenic of the erythema dyschromicum perstans: possibility of a melanosis neurocutaneous. Dermatol Venez. 1996;4:149-151.
- Correa MC, Memije EV, Vargas-Alarcon G, et al. HLA-DR association with the genetic susceptibility to develop ashy dermatosis in Mexican Mestizo patients [published online November 20, 2006]. J Am Acad Dermatol. 2007;56:617-620.
- Jablonska S. Ingestion of ammonium nitrate as a possible cause of erythema dyschromicum perstans (ashy dermatosis). Dermatologica. 1975;150:287-291.
- Stevenson JR, Miura M. Erythema dyschromicum perstans (ashy dermatosis). Arch Dermatol. 1966;94:196-199.
- Baranda L, Torres-Alvarez B, Cortes-Franco R, et al. Involvement of cell adhesion and activation molecules in the pathogenesis of erythema dyschromicum perstans (ashy dermatitis). the effect of clofazimine therapy. Arch Dermatol. 1997;133:325-329.
- Vasquez-Ochoa LA, Isaza-Guzman DM, Orozco-Mora B, et al. Immunopathologic study of erythema dyschromicum perstans (ashy dermatosis). Int J Dermatol. 2006;45:937-941.
- Convit J, Kerdel-Vegas F, Roderiguez G. Erythema dyschromicum perstans: a hiltherto undescribed skin disease. J Invest Dermatol. 1961;36:457-462.
- Ono S, Miyachi Y, Kabashima K. Ashy dermatosis with prior pruritic and scaling skin lesions. J Dermatol. 2012;39:1103-1104.
- Sanchez NP, Pathak MA, Sato SS, et al. Circumscribed dermal melaninoses: classification, light, histochemical, and electron microscopic studies on three patients with the erythema dyschromicum perstans type. Int J Dermatol. 1982;21:25-32.
- Vega ME, Waxtein L, Arenas R, et al. Ashy dermatosis and lichen planus pigmentosus: a clinicopathologic study of 31 cases. Int J Dermatol. 1992;31:90-94.
- Ahmed SV, Sajjan R. Chrysiasis: a gold "curse!" [published online May 21, 2009]. BMJ Case Rep. 2009;2009.
- Fiscus V, Hankinson A, Alweis R. Minocycline-induced hyperpigmentation. J Community Hosp Intern Med Perspect. 2014;4. doi:10.3402/jchimp.v4.24063.
- Cox AJ, Marich KW. Gold in the dermis following gold therapy for rheumatoid arthritis. Arch Dermatol. 1973;108:655-657.
- al-Talib RK, Wright DH, Theaker JM. Orange-red birefringence of gold particles in paraffin wax embedded sections: an aid to the diagnosis of chrysiasis. Histopathology. 1994;24:176-178.
- Meyer AJ, Nahass GT. Hyperpigmented patches on the dorsa of the feet. minocycline pigmentation. Arch Dermatol. 1995;131:1447-1450.
- Bayne-Poorman M, Shubrook J. Bluish pigmentation of face and sclera. J Fam Pract. 2010;59:519-522.
The Diagnosis: Erythema Dyschromicum Perstans
Erythema dyschromicum perstans (EDP), also referred to as ashy dermatosis, was first described by Ramirez1 in 1957 who labeled the patients los cenicientos (the ashen ones). It preferentially affects women in the second decade of life; however, patients of all ages can be affected, with reported cases occurring in children as young as 2 years of age.2 Most patients have Fitzpatrick skin type IV, mainly Amerindian, Hispanic South Asian, and Southwest Asian; however, there are cases reported worldwide.3 A genetic predisposition is proposed, as major histocompatibility complex genes associated with HLA-DR4⁎0407 are frequent in Mexican patients with ashy dermatosis and in the Amerindian population.4
The etiology of EDP is unknown. Various contributing factors have been reported including alimentary, occupational, and climatic factors,5,6 yet none have been conclusively demonstrated. High expression of CD36 (thrombospondin receptor not found in normal skin) in spinous and granular layers, CD94 (cytotoxic cell marker) in the basal cell layer and in the inflammatory dermal infiltrate,7 and focal keratinocytic expression of intercellular adhesion molecule I (CD54) in the active lesions of EDP, as well as the absence of these findings in normal skin, suggests an immunologic role in the development of the disease.8
Erythema dyschromicum perstans presents clinically with blue-gray hyperpigmented macules varying in size and shape and developing symmetrically in both sun-exposed and sun-protected areas of the face, neck, trunk, arms, and sometimes the dorsal hands (Figures 1 and 2). Notable sparing of the palms, soles, scalp, and mucous membranes occurs.


Occasionally, in the early active stage of the disease, elevated erythematous borders are noted surrounding the hyperpigmented macules. Eventually a hypopigmented halo develops after a prolonged duration of disease.9 The eruption typically is chronic and asymptomatic, though some cases may be pruritic.10
Histopathologically, the early lesions of EDP with an erythematous active border reveal lichenoid dermatitis with basal vacuolar change and occasional Civatte bodies. A mild to moderate perivascular lymphohistiocytic infiltrate admixed with melanophages can be seen in the papillary dermis (Figure 3). In older lesions, the inflammatory infiltrate is sparse, and pigment incontinence consistent with postinflammatory pigmentation is prominent, though melanophages extending deep into the reticular dermis may aid in distinguishing EDP from other causes of postinflammatory pigment alteration.7,11

Erythema dyschromicum perstans and lichen planus pigmentosus (LPP) may be indistinguishable histopathologically and may both be variants of lichen planus actinicus. Lichen planus pigmentosus often differs from EDP in that it presents with brown-black macules and patches often on the face and flexural areas. A subset of cases of LPP also may have mucous membrane involvement. The erythematous border that characterizes the active lesion of EDP is characteristically absent in LPP. In addition, pruritus often is reported with LPP. Direct immunofluorescence is not a beneficial tool in distinguishing the entities.12
Other differential diagnoses of predominantly facial hyperpigmentation include a lichenoid drug eruption; drug-induced hyperpigmentation (deposition disorder); postinflammatory hyperpigmentation following atopic dermatitis; contact dermatitis or photosensitivity reaction; early pinta; and cutaneous findings of systemic diseases manifesting with diffuse hyperpigmentation such as lupus erythematosus, dermatomyositis, hemochromatosis, and Addison disease. A detailed history including medication use, thorough clinical examination, and careful histopathologic evaluation will help distinguish these conditions.
Chrysiasis is a rare bluish to slate gray discoloration of the skin that predominantly occurs in sun-exposed areas. It is caused by chronic use of gold salts, which have been used to treat rheumatoid arthritis. UV light may contribute to induce the uptake of gold and subsequently stimulate tyrosinase activity.13 Histologic features of chrysiasis include dermal and perivascular gold deposition within the macrophages and endothelial cells as well as extracellular granules. It demonstrates an orange-red birefringence on fluorescent microscopy.14,15
Minocycline-induced hyperpigmentation is a well-recognized side effect of this drug. It is dose dependent and appears as a blue-black pigmentation that most frequently affects the shins, ankles, and arms.16 Three distinct types were documented: abnormal discoloration of the skin that has been linked to deposition of pigmented metabolites of minocycline producing blue-black pigmentation at the site of scarring or prior inflammation (type 1); blue-gray pigmentation affecting normal skin, mainly the legs (type 2); and elevated levels of melanin on the sun-exposed areas producing dirty skin syndrome (type 3).17,18
Topical and systemic corticosteroids, UV light therapy, oral dapsone, griseofulvin, retinoids, and clofazimine are reported as treatment options for ashy dermatosis, though results typically are disappointing.7
The Diagnosis: Erythema Dyschromicum Perstans
Erythema dyschromicum perstans (EDP), also referred to as ashy dermatosis, was first described by Ramirez1 in 1957 who labeled the patients los cenicientos (the ashen ones). It preferentially affects women in the second decade of life; however, patients of all ages can be affected, with reported cases occurring in children as young as 2 years of age.2 Most patients have Fitzpatrick skin type IV, mainly Amerindian, Hispanic South Asian, and Southwest Asian; however, there are cases reported worldwide.3 A genetic predisposition is proposed, as major histocompatibility complex genes associated with HLA-DR4⁎0407 are frequent in Mexican patients with ashy dermatosis and in the Amerindian population.4
The etiology of EDP is unknown. Various contributing factors have been reported including alimentary, occupational, and climatic factors,5,6 yet none have been conclusively demonstrated. High expression of CD36 (thrombospondin receptor not found in normal skin) in spinous and granular layers, CD94 (cytotoxic cell marker) in the basal cell layer and in the inflammatory dermal infiltrate,7 and focal keratinocytic expression of intercellular adhesion molecule I (CD54) in the active lesions of EDP, as well as the absence of these findings in normal skin, suggests an immunologic role in the development of the disease.8
Erythema dyschromicum perstans presents clinically with blue-gray hyperpigmented macules varying in size and shape and developing symmetrically in both sun-exposed and sun-protected areas of the face, neck, trunk, arms, and sometimes the dorsal hands (Figures 1 and 2). Notable sparing of the palms, soles, scalp, and mucous membranes occurs.


Occasionally, in the early active stage of the disease, elevated erythematous borders are noted surrounding the hyperpigmented macules. Eventually a hypopigmented halo develops after a prolonged duration of disease.9 The eruption typically is chronic and asymptomatic, though some cases may be pruritic.10
Histopathologically, the early lesions of EDP with an erythematous active border reveal lichenoid dermatitis with basal vacuolar change and occasional Civatte bodies. A mild to moderate perivascular lymphohistiocytic infiltrate admixed with melanophages can be seen in the papillary dermis (Figure 3). In older lesions, the inflammatory infiltrate is sparse, and pigment incontinence consistent with postinflammatory pigmentation is prominent, though melanophages extending deep into the reticular dermis may aid in distinguishing EDP from other causes of postinflammatory pigment alteration.7,11

Erythema dyschromicum perstans and lichen planus pigmentosus (LPP) may be indistinguishable histopathologically and may both be variants of lichen planus actinicus. Lichen planus pigmentosus often differs from EDP in that it presents with brown-black macules and patches often on the face and flexural areas. A subset of cases of LPP also may have mucous membrane involvement. The erythematous border that characterizes the active lesion of EDP is characteristically absent in LPP. In addition, pruritus often is reported with LPP. Direct immunofluorescence is not a beneficial tool in distinguishing the entities.12
Other differential diagnoses of predominantly facial hyperpigmentation include a lichenoid drug eruption; drug-induced hyperpigmentation (deposition disorder); postinflammatory hyperpigmentation following atopic dermatitis; contact dermatitis or photosensitivity reaction; early pinta; and cutaneous findings of systemic diseases manifesting with diffuse hyperpigmentation such as lupus erythematosus, dermatomyositis, hemochromatosis, and Addison disease. A detailed history including medication use, thorough clinical examination, and careful histopathologic evaluation will help distinguish these conditions.
Chrysiasis is a rare bluish to slate gray discoloration of the skin that predominantly occurs in sun-exposed areas. It is caused by chronic use of gold salts, which have been used to treat rheumatoid arthritis. UV light may contribute to induce the uptake of gold and subsequently stimulate tyrosinase activity.13 Histologic features of chrysiasis include dermal and perivascular gold deposition within the macrophages and endothelial cells as well as extracellular granules. It demonstrates an orange-red birefringence on fluorescent microscopy.14,15
Minocycline-induced hyperpigmentation is a well-recognized side effect of this drug. It is dose dependent and appears as a blue-black pigmentation that most frequently affects the shins, ankles, and arms.16 Three distinct types were documented: abnormal discoloration of the skin that has been linked to deposition of pigmented metabolites of minocycline producing blue-black pigmentation at the site of scarring or prior inflammation (type 1); blue-gray pigmentation affecting normal skin, mainly the legs (type 2); and elevated levels of melanin on the sun-exposed areas producing dirty skin syndrome (type 3).17,18
Topical and systemic corticosteroids, UV light therapy, oral dapsone, griseofulvin, retinoids, and clofazimine are reported as treatment options for ashy dermatosis, though results typically are disappointing.7
- Ramirez CO. Los cenicientos: problema clinica. In: Memoria del Primer Congresso Centroamericano de Dermatologica, December 5-8, 1957. San Salvador, El Salvador; 1957:122-130.
- Lee SJ, Chung KY. Erythema dyschromicum perstans in early childhood. J Dermatol. 1999;26:119-121.
- Homez-Chacin, Barroso C. On the etiopathogenic of the erythema dyschromicum perstans: possibility of a melanosis neurocutaneous. Dermatol Venez. 1996;4:149-151.
- Correa MC, Memije EV, Vargas-Alarcon G, et al. HLA-DR association with the genetic susceptibility to develop ashy dermatosis in Mexican Mestizo patients [published online November 20, 2006]. J Am Acad Dermatol. 2007;56:617-620.
- Jablonska S. Ingestion of ammonium nitrate as a possible cause of erythema dyschromicum perstans (ashy dermatosis). Dermatologica. 1975;150:287-291.
- Stevenson JR, Miura M. Erythema dyschromicum perstans (ashy dermatosis). Arch Dermatol. 1966;94:196-199.
- Baranda L, Torres-Alvarez B, Cortes-Franco R, et al. Involvement of cell adhesion and activation molecules in the pathogenesis of erythema dyschromicum perstans (ashy dermatitis). the effect of clofazimine therapy. Arch Dermatol. 1997;133:325-329.
- Vasquez-Ochoa LA, Isaza-Guzman DM, Orozco-Mora B, et al. Immunopathologic study of erythema dyschromicum perstans (ashy dermatosis). Int J Dermatol. 2006;45:937-941.
- Convit J, Kerdel-Vegas F, Roderiguez G. Erythema dyschromicum perstans: a hiltherto undescribed skin disease. J Invest Dermatol. 1961;36:457-462.
- Ono S, Miyachi Y, Kabashima K. Ashy dermatosis with prior pruritic and scaling skin lesions. J Dermatol. 2012;39:1103-1104.
- Sanchez NP, Pathak MA, Sato SS, et al. Circumscribed dermal melaninoses: classification, light, histochemical, and electron microscopic studies on three patients with the erythema dyschromicum perstans type. Int J Dermatol. 1982;21:25-32.
- Vega ME, Waxtein L, Arenas R, et al. Ashy dermatosis and lichen planus pigmentosus: a clinicopathologic study of 31 cases. Int J Dermatol. 1992;31:90-94.
- Ahmed SV, Sajjan R. Chrysiasis: a gold "curse!" [published online May 21, 2009]. BMJ Case Rep. 2009;2009.
- Fiscus V, Hankinson A, Alweis R. Minocycline-induced hyperpigmentation. J Community Hosp Intern Med Perspect. 2014;4. doi:10.3402/jchimp.v4.24063.
- Cox AJ, Marich KW. Gold in the dermis following gold therapy for rheumatoid arthritis. Arch Dermatol. 1973;108:655-657.
- al-Talib RK, Wright DH, Theaker JM. Orange-red birefringence of gold particles in paraffin wax embedded sections: an aid to the diagnosis of chrysiasis. Histopathology. 1994;24:176-178.
- Meyer AJ, Nahass GT. Hyperpigmented patches on the dorsa of the feet. minocycline pigmentation. Arch Dermatol. 1995;131:1447-1450.
- Bayne-Poorman M, Shubrook J. Bluish pigmentation of face and sclera. J Fam Pract. 2010;59:519-522.
- Ramirez CO. Los cenicientos: problema clinica. In: Memoria del Primer Congresso Centroamericano de Dermatologica, December 5-8, 1957. San Salvador, El Salvador; 1957:122-130.
- Lee SJ, Chung KY. Erythema dyschromicum perstans in early childhood. J Dermatol. 1999;26:119-121.
- Homez-Chacin, Barroso C. On the etiopathogenic of the erythema dyschromicum perstans: possibility of a melanosis neurocutaneous. Dermatol Venez. 1996;4:149-151.
- Correa MC, Memije EV, Vargas-Alarcon G, et al. HLA-DR association with the genetic susceptibility to develop ashy dermatosis in Mexican Mestizo patients [published online November 20, 2006]. J Am Acad Dermatol. 2007;56:617-620.
- Jablonska S. Ingestion of ammonium nitrate as a possible cause of erythema dyschromicum perstans (ashy dermatosis). Dermatologica. 1975;150:287-291.
- Stevenson JR, Miura M. Erythema dyschromicum perstans (ashy dermatosis). Arch Dermatol. 1966;94:196-199.
- Baranda L, Torres-Alvarez B, Cortes-Franco R, et al. Involvement of cell adhesion and activation molecules in the pathogenesis of erythema dyschromicum perstans (ashy dermatitis). the effect of clofazimine therapy. Arch Dermatol. 1997;133:325-329.
- Vasquez-Ochoa LA, Isaza-Guzman DM, Orozco-Mora B, et al. Immunopathologic study of erythema dyschromicum perstans (ashy dermatosis). Int J Dermatol. 2006;45:937-941.
- Convit J, Kerdel-Vegas F, Roderiguez G. Erythema dyschromicum perstans: a hiltherto undescribed skin disease. J Invest Dermatol. 1961;36:457-462.
- Ono S, Miyachi Y, Kabashima K. Ashy dermatosis with prior pruritic and scaling skin lesions. J Dermatol. 2012;39:1103-1104.
- Sanchez NP, Pathak MA, Sato SS, et al. Circumscribed dermal melaninoses: classification, light, histochemical, and electron microscopic studies on three patients with the erythema dyschromicum perstans type. Int J Dermatol. 1982;21:25-32.
- Vega ME, Waxtein L, Arenas R, et al. Ashy dermatosis and lichen planus pigmentosus: a clinicopathologic study of 31 cases. Int J Dermatol. 1992;31:90-94.
- Ahmed SV, Sajjan R. Chrysiasis: a gold "curse!" [published online May 21, 2009]. BMJ Case Rep. 2009;2009.
- Fiscus V, Hankinson A, Alweis R. Minocycline-induced hyperpigmentation. J Community Hosp Intern Med Perspect. 2014;4. doi:10.3402/jchimp.v4.24063.
- Cox AJ, Marich KW. Gold in the dermis following gold therapy for rheumatoid arthritis. Arch Dermatol. 1973;108:655-657.
- al-Talib RK, Wright DH, Theaker JM. Orange-red birefringence of gold particles in paraffin wax embedded sections: an aid to the diagnosis of chrysiasis. Histopathology. 1994;24:176-178.
- Meyer AJ, Nahass GT. Hyperpigmented patches on the dorsa of the feet. minocycline pigmentation. Arch Dermatol. 1995;131:1447-1450.
- Bayne-Poorman M, Shubrook J. Bluish pigmentation of face and sclera. J Fam Pract. 2010;59:519-522.
A middle-aged woman with Fitzpatrick skin type IV was evaluated for progressive hyperpigmentation of several months' duration involving the neck, jawline, both sides of the face, and forehead. The lesions were mildly pruritic. She denied contact with any new substance and there was no history of an eruption preceding the hyperpigmentation. Medical history included chronic anemia that was managed with iron supplementation. On physical examination, blue-gray nonscaly macules and patches were observed distributed symmetrically on the neck, jawline, sides of the face, and forehead. Microscopic examination of 2 shave biopsies revealed subtle vacuolar interface dermatitis with mild perivascular lymphocytic infiltrate and dermal melanophages (inset).