User login
Bigfoot, Bermuda Triangle, ‘No Lido With Epi’?
“Fingers, toes, ears, and nose are places where epinephrine never goes,” Thomas Ehlers, DPM, wrote in Podiatry Today. “That is an adage I heard during podiatry school, my clerkships, and from various attendings throughout my training.”
But as Dr. Ehlers added, epinephrine gets a bad rap. The catchy admonition “has been proven a myth time and time again.”
So Although medical trainees across multiple disciplines are taught to fear the practice, citing the potential for gangrene, its reputation for harm is not supported by the evidence.
Lack of Feeling Doesn’t Care About Your Facts
The debate surfaced anew in response to a recent column by Kenny Lin, MD, MPH, family physician and associate director of the Lancaster General Hospital Family Medicine Residency, in Lancaster, Pennsylvania, about the rather pedestrian topic of why he no longer performs surgery to correct ingrown toenails. Dr. Lin’s admission that he used to do the procedure with a combination of epinephrine and lidocaine turned into a major focus of the comments — many of them harshly critical of the practice:
“Epinephrine is not an appropriate drug to use for podiatry or use in any peripheral area. Gangrene?” one commenter posted.
“Leave epi out of lidocaine to fingers, toes, nose, and ear lobes,” another wrote.
“No lido with epi, whether or not it is contraindicated, because: If there’s any adverse outcome, a lawyer will find plenty of references saying it was contraindicated,” a reader chimed in.
Other commenters disagreed, with one saying, “Please, folks, don’t show that you trained 50 years ago and haven’t changed practice since…”
For Dr. Lin, the response was surprising given what he believes to be the lack of evidence supporting the purported dangers.
“When I think about this, it’s something that was taught to me during residency — that they should not be used on certain areas,” Dr. Lin said. “But since then, studies have been published looking at thousands of cases of people using epinephrine with lidocaine and haven’t found any cases of necrosis.”
Many doctors, like Dr. Lin, say they were cautioned against this in their training. Others don’t remember exactly where they’ve heard it but recognize the idea has a nebulous hold on practice.
Combining epinephrine with lidocaine helps make the numbing last longer, stops bleeding, and reduces the use of lidocaine required, all of which improve the chances of an effective and comfortable intervention for the patient, Dr. Lin said. The approach also reduces the use of tourniquets, which come with their own risks including nerve injury.
However, in areas with limited circulation, this vasoconstrictive effect may be more pronounced, potentially leading to complications for patients with complicating factors.
Clinicians who regularly use the combination of epinephrine and lidocaine for surgery do concede that it can pose certain hazards and considerations in areas without robust blood flow.
But the literature largely points to its safety.
In 2001, California-based plastic and reconstructive surgeon Keith Denkler, MD, published a deep dive on the topic starting in the 19th century, including a review of Index Medicus from 1880 to 1966, a computer review of the National Library of Medicine database from 1966 to 2000, and major textbooks from 1900 to 2000.
He found a total of 48 cases of digital gangrene — but most involved the use of cocaine or procaine. Of the 48 cases, 21 involved the use of epinephrine, and 17 used an unknown concentration based on manual dilution.
“Multiple other concurrent conditions (hot soaks, tight tourniquets, and infection) existed in these case reports, making it difficult to determine the exact cause of the tissue insult,” Dr. Denkler wrote.
In a 2010 retrospective review in the Journal of the American Society of Plastic Surgeons, authors examined 1111 cases involving digital and hand surgery. Of the 611 patients who received injections of 1% lidocaine with epinephrine, none experienced digital necrosis.
Another review from 2003 touted the combination’s safety, in hopes to “help dispel the myth that epinephrine has no place in podiatric anesthesia.” But authors noted limitations of use, including “known sensitivity, thyrotoxicosis, and use of either tricyclic antidepressants or monoamine oxidase inhibitors.”
James Christina, DPM, executive director and CEO of the American Podiatric Medical Association, echoed that sentiment. He said he regularly used the combination to correct bunions, hammer toes, and ingrown toenails over his 20 years of practicing but acknowledged the technique is not appropriate for all such patients.
“There’s always been caution when using epinephrine with local anesthetic,” Dr. Christina told this news organization. “You need a healthy patient with normal circulation and no other complications; someone without vascular compromise.”
Marie Hanna, MD, MEHP, chief of regional anesthesia and acute pain management at Johns Hopkins University, Baltimore, counts herself among the cautious. Citing Principles of Office Anesthesia: Part I. Infiltrative Anesthesia, Dr. Hanna said epinephrine should never be used in digital and penile blocks or in skin flaps with marginal viability.
“It is perfectly fine in certain areas, like the wrist or the arm,” Dr. Hanna said. “But specifically for use in end organs like nose, fingers, ears, toes — all of these with tenuous blood supply — it is not good practice.”
The divide among doctors comes down to theoretical concern, rather than empirical basis, said Rebecca Johnson, MD, chair of the American Society of Anesthesiologists committee on Regional Anesthesia and Acute Pain Medicine and a faculty member at Mayo Clinic, in Rochester, Minnesota.
“It’s just one of those myths we have in practice,” she said.
And legally, Dr. Johnson noted, the mere existence of a myth can be enough of a deterrent for medical practitioners: “Like anything, when you’re trying to do the right thing, if a complication would occur for another reason, you’d want to make sure a jury of your peers didn’t bring up that myth.”
The sources in this story reported no relevant financial conflicts of interest.
A version of this article appeared on Medscape.com.
“Fingers, toes, ears, and nose are places where epinephrine never goes,” Thomas Ehlers, DPM, wrote in Podiatry Today. “That is an adage I heard during podiatry school, my clerkships, and from various attendings throughout my training.”
But as Dr. Ehlers added, epinephrine gets a bad rap. The catchy admonition “has been proven a myth time and time again.”
So Although medical trainees across multiple disciplines are taught to fear the practice, citing the potential for gangrene, its reputation for harm is not supported by the evidence.
Lack of Feeling Doesn’t Care About Your Facts
The debate surfaced anew in response to a recent column by Kenny Lin, MD, MPH, family physician and associate director of the Lancaster General Hospital Family Medicine Residency, in Lancaster, Pennsylvania, about the rather pedestrian topic of why he no longer performs surgery to correct ingrown toenails. Dr. Lin’s admission that he used to do the procedure with a combination of epinephrine and lidocaine turned into a major focus of the comments — many of them harshly critical of the practice:
“Epinephrine is not an appropriate drug to use for podiatry or use in any peripheral area. Gangrene?” one commenter posted.
“Leave epi out of lidocaine to fingers, toes, nose, and ear lobes,” another wrote.
“No lido with epi, whether or not it is contraindicated, because: If there’s any adverse outcome, a lawyer will find plenty of references saying it was contraindicated,” a reader chimed in.
Other commenters disagreed, with one saying, “Please, folks, don’t show that you trained 50 years ago and haven’t changed practice since…”
For Dr. Lin, the response was surprising given what he believes to be the lack of evidence supporting the purported dangers.
“When I think about this, it’s something that was taught to me during residency — that they should not be used on certain areas,” Dr. Lin said. “But since then, studies have been published looking at thousands of cases of people using epinephrine with lidocaine and haven’t found any cases of necrosis.”
Many doctors, like Dr. Lin, say they were cautioned against this in their training. Others don’t remember exactly where they’ve heard it but recognize the idea has a nebulous hold on practice.
Combining epinephrine with lidocaine helps make the numbing last longer, stops bleeding, and reduces the use of lidocaine required, all of which improve the chances of an effective and comfortable intervention for the patient, Dr. Lin said. The approach also reduces the use of tourniquets, which come with their own risks including nerve injury.
However, in areas with limited circulation, this vasoconstrictive effect may be more pronounced, potentially leading to complications for patients with complicating factors.
Clinicians who regularly use the combination of epinephrine and lidocaine for surgery do concede that it can pose certain hazards and considerations in areas without robust blood flow.
But the literature largely points to its safety.
In 2001, California-based plastic and reconstructive surgeon Keith Denkler, MD, published a deep dive on the topic starting in the 19th century, including a review of Index Medicus from 1880 to 1966, a computer review of the National Library of Medicine database from 1966 to 2000, and major textbooks from 1900 to 2000.
He found a total of 48 cases of digital gangrene — but most involved the use of cocaine or procaine. Of the 48 cases, 21 involved the use of epinephrine, and 17 used an unknown concentration based on manual dilution.
“Multiple other concurrent conditions (hot soaks, tight tourniquets, and infection) existed in these case reports, making it difficult to determine the exact cause of the tissue insult,” Dr. Denkler wrote.
In a 2010 retrospective review in the Journal of the American Society of Plastic Surgeons, authors examined 1111 cases involving digital and hand surgery. Of the 611 patients who received injections of 1% lidocaine with epinephrine, none experienced digital necrosis.
Another review from 2003 touted the combination’s safety, in hopes to “help dispel the myth that epinephrine has no place in podiatric anesthesia.” But authors noted limitations of use, including “known sensitivity, thyrotoxicosis, and use of either tricyclic antidepressants or monoamine oxidase inhibitors.”
James Christina, DPM, executive director and CEO of the American Podiatric Medical Association, echoed that sentiment. He said he regularly used the combination to correct bunions, hammer toes, and ingrown toenails over his 20 years of practicing but acknowledged the technique is not appropriate for all such patients.
“There’s always been caution when using epinephrine with local anesthetic,” Dr. Christina told this news organization. “You need a healthy patient with normal circulation and no other complications; someone without vascular compromise.”
Marie Hanna, MD, MEHP, chief of regional anesthesia and acute pain management at Johns Hopkins University, Baltimore, counts herself among the cautious. Citing Principles of Office Anesthesia: Part I. Infiltrative Anesthesia, Dr. Hanna said epinephrine should never be used in digital and penile blocks or in skin flaps with marginal viability.
“It is perfectly fine in certain areas, like the wrist or the arm,” Dr. Hanna said. “But specifically for use in end organs like nose, fingers, ears, toes — all of these with tenuous blood supply — it is not good practice.”
The divide among doctors comes down to theoretical concern, rather than empirical basis, said Rebecca Johnson, MD, chair of the American Society of Anesthesiologists committee on Regional Anesthesia and Acute Pain Medicine and a faculty member at Mayo Clinic, in Rochester, Minnesota.
“It’s just one of those myths we have in practice,” she said.
And legally, Dr. Johnson noted, the mere existence of a myth can be enough of a deterrent for medical practitioners: “Like anything, when you’re trying to do the right thing, if a complication would occur for another reason, you’d want to make sure a jury of your peers didn’t bring up that myth.”
The sources in this story reported no relevant financial conflicts of interest.
A version of this article appeared on Medscape.com.
“Fingers, toes, ears, and nose are places where epinephrine never goes,” Thomas Ehlers, DPM, wrote in Podiatry Today. “That is an adage I heard during podiatry school, my clerkships, and from various attendings throughout my training.”
But as Dr. Ehlers added, epinephrine gets a bad rap. The catchy admonition “has been proven a myth time and time again.”
So Although medical trainees across multiple disciplines are taught to fear the practice, citing the potential for gangrene, its reputation for harm is not supported by the evidence.
Lack of Feeling Doesn’t Care About Your Facts
The debate surfaced anew in response to a recent column by Kenny Lin, MD, MPH, family physician and associate director of the Lancaster General Hospital Family Medicine Residency, in Lancaster, Pennsylvania, about the rather pedestrian topic of why he no longer performs surgery to correct ingrown toenails. Dr. Lin’s admission that he used to do the procedure with a combination of epinephrine and lidocaine turned into a major focus of the comments — many of them harshly critical of the practice:
“Epinephrine is not an appropriate drug to use for podiatry or use in any peripheral area. Gangrene?” one commenter posted.
“Leave epi out of lidocaine to fingers, toes, nose, and ear lobes,” another wrote.
“No lido with epi, whether or not it is contraindicated, because: If there’s any adverse outcome, a lawyer will find plenty of references saying it was contraindicated,” a reader chimed in.
Other commenters disagreed, with one saying, “Please, folks, don’t show that you trained 50 years ago and haven’t changed practice since…”
For Dr. Lin, the response was surprising given what he believes to be the lack of evidence supporting the purported dangers.
“When I think about this, it’s something that was taught to me during residency — that they should not be used on certain areas,” Dr. Lin said. “But since then, studies have been published looking at thousands of cases of people using epinephrine with lidocaine and haven’t found any cases of necrosis.”
Many doctors, like Dr. Lin, say they were cautioned against this in their training. Others don’t remember exactly where they’ve heard it but recognize the idea has a nebulous hold on practice.
Combining epinephrine with lidocaine helps make the numbing last longer, stops bleeding, and reduces the use of lidocaine required, all of which improve the chances of an effective and comfortable intervention for the patient, Dr. Lin said. The approach also reduces the use of tourniquets, which come with their own risks including nerve injury.
However, in areas with limited circulation, this vasoconstrictive effect may be more pronounced, potentially leading to complications for patients with complicating factors.
Clinicians who regularly use the combination of epinephrine and lidocaine for surgery do concede that it can pose certain hazards and considerations in areas without robust blood flow.
But the literature largely points to its safety.
In 2001, California-based plastic and reconstructive surgeon Keith Denkler, MD, published a deep dive on the topic starting in the 19th century, including a review of Index Medicus from 1880 to 1966, a computer review of the National Library of Medicine database from 1966 to 2000, and major textbooks from 1900 to 2000.
He found a total of 48 cases of digital gangrene — but most involved the use of cocaine or procaine. Of the 48 cases, 21 involved the use of epinephrine, and 17 used an unknown concentration based on manual dilution.
“Multiple other concurrent conditions (hot soaks, tight tourniquets, and infection) existed in these case reports, making it difficult to determine the exact cause of the tissue insult,” Dr. Denkler wrote.
In a 2010 retrospective review in the Journal of the American Society of Plastic Surgeons, authors examined 1111 cases involving digital and hand surgery. Of the 611 patients who received injections of 1% lidocaine with epinephrine, none experienced digital necrosis.
Another review from 2003 touted the combination’s safety, in hopes to “help dispel the myth that epinephrine has no place in podiatric anesthesia.” But authors noted limitations of use, including “known sensitivity, thyrotoxicosis, and use of either tricyclic antidepressants or monoamine oxidase inhibitors.”
James Christina, DPM, executive director and CEO of the American Podiatric Medical Association, echoed that sentiment. He said he regularly used the combination to correct bunions, hammer toes, and ingrown toenails over his 20 years of practicing but acknowledged the technique is not appropriate for all such patients.
“There’s always been caution when using epinephrine with local anesthetic,” Dr. Christina told this news organization. “You need a healthy patient with normal circulation and no other complications; someone without vascular compromise.”
Marie Hanna, MD, MEHP, chief of regional anesthesia and acute pain management at Johns Hopkins University, Baltimore, counts herself among the cautious. Citing Principles of Office Anesthesia: Part I. Infiltrative Anesthesia, Dr. Hanna said epinephrine should never be used in digital and penile blocks or in skin flaps with marginal viability.
“It is perfectly fine in certain areas, like the wrist or the arm,” Dr. Hanna said. “But specifically for use in end organs like nose, fingers, ears, toes — all of these with tenuous blood supply — it is not good practice.”
The divide among doctors comes down to theoretical concern, rather than empirical basis, said Rebecca Johnson, MD, chair of the American Society of Anesthesiologists committee on Regional Anesthesia and Acute Pain Medicine and a faculty member at Mayo Clinic, in Rochester, Minnesota.
“It’s just one of those myths we have in practice,” she said.
And legally, Dr. Johnson noted, the mere existence of a myth can be enough of a deterrent for medical practitioners: “Like anything, when you’re trying to do the right thing, if a complication would occur for another reason, you’d want to make sure a jury of your peers didn’t bring up that myth.”
The sources in this story reported no relevant financial conflicts of interest.
A version of this article appeared on Medscape.com.
Surgery Shows Longer-Term Benefits for Dupuytren Contracture
Dupuytren contracture can be treated with three invasive methods, but new data from a randomized controlled trial show better 2-year success rates for surgery than for needle fasciotomy and collagenase injection, despite retreatments.
The common hereditary disorder affects the palmar fascia in middle-aged and older people, more often men. The disease typically affects the ring and little fingers and they may curl toward the palm. The disease can’t be cured, but can be eased.
Findings of the study, led by Mikko Petteri Räisänen, MD, with the Department of Orthopedics, Traumatology and Hand Surgery, Kuopio University Hospital, Kuopio, and Tampere University, Tampere, both in Finland, were published online in Annals of Internal Medicine.
Initially, Outcomes Similar
Initially, in the multisite, randomized controlled, outcome assessor–blinded, superiority trial, the outcomes were similar among the treatments, the authors write, but at 2 years only the surgery group maintained the success rate.
The primary outcome was more than 50% contracture release and patients reaching the patient-acceptable symptom state. Secondary outcomes included hand function, pain, patient satisfaction, quality of life, finger flexion, residual contracture angle, risk for retreatment, and serious adverse events.
A total of 292 (97%) and 284 (94%) patients completed the 3-month and 2-year follow ups, respectively.
Success rates at 3 months were similar: 71% (95% CI, 62%-80%) for surgery; 73% (95% CI, 64%-82%) for needle fasciotomy; and 73% (95% CI, 64%-82%) for collagenase injection.
At 2 Years, Surgery Superior
At 2 years, however, surgery had superior success rates. Surgery success rates vs needle fasciotomy were 78% vs 50% (adjusted risk difference, 0.30; 95% CI, 0.17-0.43).
Surgery success rates vs collagenase injection were 78% vs 65% (aRD, 0.13; 95% CI, 0.01-0.26).
“Secondary analyses paralleled with the primary analysis,” the authors write.
Patients may choose surgery despite initial morbidity which includes potential time off work and higher costs than the other options if the long-term outcome is better, the authors write.
“Collagenase is likely a viable alternative to needle fasciotomy only if its costs are substantially reduced,” the authors write.
A strength of the study is its generalizability, as researchers recruited patients in a setting with universal healthcare where few people seek care outside public hospitals.
Another strength of the trial is that the blinded outcome assessors measured the contracture angles with the participant’s hand covered by a rubber glove and patients were instructed not to reveal their treatment group to the assessor.
Some Physicians Offer Noninvasive Treatments First
Family physician Shannon Scott, DO, medical director of the Midwestern University Multispecialty Clinic in Scottsdale, Arizona, treats many patients with the contracture.
In her practice, patients come to her seeking noninvasive options first. But if they are not satisfied with their hand function after noninvasive treatments such as osteopathic manipulative treatment, physical therapy, and a home exercise program, the next steps are the choices compared in the study. The findings of this randomized controlled trial, she says, will help her in counseling patients choosing among those options.
“What’s important for me as a family physician to understand is more about the path that led to this decision” to seek more invasive treatment and whether the patients in the study had first completed a course of noninvasive care, Dr. Scott says.
The condition, especially in the population most affected — older adults — can greatly affect activities of daily living, she noted. Patients may also often have other conditions contributing to the symptoms of Dupuytren contracture in the neck, arm, or shoulder, for instance, that limit range of motion or cause pain. Addressing those symptoms noninvasively may help relieve the contracture, she says.
Asking patients about their goals is essential, Dr. Scott says. “What patients are looking for is function and the definition for one patient may be different than the level of function for another. Many patients get to a desired level of function with nonsurgical options first.”
A First for the Comparison
Dawn LaPorte, MD, a hand surgeon at Johns Hopkins Medicine in Baltimore, Maryland, who also was not part of the study, says although surgery was thought to have better long-term success rates, this is the first time the data have been able to show that at 2 years.
She added that the results are particularly striking because the endpoint was a 50% release when surgeons hope for a complete release. Even with the 50% release outcome at 2 years, surgery had better success.
She noted that the authors plan to look at outcomes at 5 and 10 years, but, she says, “the fact that surgery is already significantly better at 2 years really says a lot.”
Treatments Have Tradeoffs
She says the conclusions may change the discussions physicians have with patients.
Collagenase injections are an office procedure, and there’s no anesthesia. “There’s usually no lost time from work, and they can use their hand pretty normally the following day,” Dr. LaPorte says. One downside, compared with surgery, is that there may be a more frequent recurrence rate. Patients may have a skin tear that usually heals over a couple of weeks, she added.
Additionally, “the collagenase drug is very expensive,” she notes, so preapproval is important so that the patient doesn’t have to pay out of pocket.
Needle fasciotomy can also be done in the office without anesthesia. There’s less time off work than with surgery.
“With both that and the injection, they should see release of the contracture right away,” Dr. LaPorte says, but the concern is a quicker recurrence rate.
While surgery isn’t a cure, she says, and there is a lower recurrence rate, it typically means time off work, anesthesia, and an incision to heal, and may mean postoperative therapy.
The study was funded by the Research Council of Finland. Disclosures are available with the full text.
Dr. LaPorte and Dr. Scott report no relevant financial relationships.
Dupuytren contracture can be treated with three invasive methods, but new data from a randomized controlled trial show better 2-year success rates for surgery than for needle fasciotomy and collagenase injection, despite retreatments.
The common hereditary disorder affects the palmar fascia in middle-aged and older people, more often men. The disease typically affects the ring and little fingers and they may curl toward the palm. The disease can’t be cured, but can be eased.
Findings of the study, led by Mikko Petteri Räisänen, MD, with the Department of Orthopedics, Traumatology and Hand Surgery, Kuopio University Hospital, Kuopio, and Tampere University, Tampere, both in Finland, were published online in Annals of Internal Medicine.
Initially, Outcomes Similar
Initially, in the multisite, randomized controlled, outcome assessor–blinded, superiority trial, the outcomes were similar among the treatments, the authors write, but at 2 years only the surgery group maintained the success rate.
The primary outcome was more than 50% contracture release and patients reaching the patient-acceptable symptom state. Secondary outcomes included hand function, pain, patient satisfaction, quality of life, finger flexion, residual contracture angle, risk for retreatment, and serious adverse events.
A total of 292 (97%) and 284 (94%) patients completed the 3-month and 2-year follow ups, respectively.
Success rates at 3 months were similar: 71% (95% CI, 62%-80%) for surgery; 73% (95% CI, 64%-82%) for needle fasciotomy; and 73% (95% CI, 64%-82%) for collagenase injection.
At 2 Years, Surgery Superior
At 2 years, however, surgery had superior success rates. Surgery success rates vs needle fasciotomy were 78% vs 50% (adjusted risk difference, 0.30; 95% CI, 0.17-0.43).
Surgery success rates vs collagenase injection were 78% vs 65% (aRD, 0.13; 95% CI, 0.01-0.26).
“Secondary analyses paralleled with the primary analysis,” the authors write.
Patients may choose surgery despite initial morbidity which includes potential time off work and higher costs than the other options if the long-term outcome is better, the authors write.
“Collagenase is likely a viable alternative to needle fasciotomy only if its costs are substantially reduced,” the authors write.
A strength of the study is its generalizability, as researchers recruited patients in a setting with universal healthcare where few people seek care outside public hospitals.
Another strength of the trial is that the blinded outcome assessors measured the contracture angles with the participant’s hand covered by a rubber glove and patients were instructed not to reveal their treatment group to the assessor.
Some Physicians Offer Noninvasive Treatments First
Family physician Shannon Scott, DO, medical director of the Midwestern University Multispecialty Clinic in Scottsdale, Arizona, treats many patients with the contracture.
In her practice, patients come to her seeking noninvasive options first. But if they are not satisfied with their hand function after noninvasive treatments such as osteopathic manipulative treatment, physical therapy, and a home exercise program, the next steps are the choices compared in the study. The findings of this randomized controlled trial, she says, will help her in counseling patients choosing among those options.
“What’s important for me as a family physician to understand is more about the path that led to this decision” to seek more invasive treatment and whether the patients in the study had first completed a course of noninvasive care, Dr. Scott says.
The condition, especially in the population most affected — older adults — can greatly affect activities of daily living, she noted. Patients may also often have other conditions contributing to the symptoms of Dupuytren contracture in the neck, arm, or shoulder, for instance, that limit range of motion or cause pain. Addressing those symptoms noninvasively may help relieve the contracture, she says.
Asking patients about their goals is essential, Dr. Scott says. “What patients are looking for is function and the definition for one patient may be different than the level of function for another. Many patients get to a desired level of function with nonsurgical options first.”
A First for the Comparison
Dawn LaPorte, MD, a hand surgeon at Johns Hopkins Medicine in Baltimore, Maryland, who also was not part of the study, says although surgery was thought to have better long-term success rates, this is the first time the data have been able to show that at 2 years.
She added that the results are particularly striking because the endpoint was a 50% release when surgeons hope for a complete release. Even with the 50% release outcome at 2 years, surgery had better success.
She noted that the authors plan to look at outcomes at 5 and 10 years, but, she says, “the fact that surgery is already significantly better at 2 years really says a lot.”
Treatments Have Tradeoffs
She says the conclusions may change the discussions physicians have with patients.
Collagenase injections are an office procedure, and there’s no anesthesia. “There’s usually no lost time from work, and they can use their hand pretty normally the following day,” Dr. LaPorte says. One downside, compared with surgery, is that there may be a more frequent recurrence rate. Patients may have a skin tear that usually heals over a couple of weeks, she added.
Additionally, “the collagenase drug is very expensive,” she notes, so preapproval is important so that the patient doesn’t have to pay out of pocket.
Needle fasciotomy can also be done in the office without anesthesia. There’s less time off work than with surgery.
“With both that and the injection, they should see release of the contracture right away,” Dr. LaPorte says, but the concern is a quicker recurrence rate.
While surgery isn’t a cure, she says, and there is a lower recurrence rate, it typically means time off work, anesthesia, and an incision to heal, and may mean postoperative therapy.
The study was funded by the Research Council of Finland. Disclosures are available with the full text.
Dr. LaPorte and Dr. Scott report no relevant financial relationships.
Dupuytren contracture can be treated with three invasive methods, but new data from a randomized controlled trial show better 2-year success rates for surgery than for needle fasciotomy and collagenase injection, despite retreatments.
The common hereditary disorder affects the palmar fascia in middle-aged and older people, more often men. The disease typically affects the ring and little fingers and they may curl toward the palm. The disease can’t be cured, but can be eased.
Findings of the study, led by Mikko Petteri Räisänen, MD, with the Department of Orthopedics, Traumatology and Hand Surgery, Kuopio University Hospital, Kuopio, and Tampere University, Tampere, both in Finland, were published online in Annals of Internal Medicine.
Initially, Outcomes Similar
Initially, in the multisite, randomized controlled, outcome assessor–blinded, superiority trial, the outcomes were similar among the treatments, the authors write, but at 2 years only the surgery group maintained the success rate.
The primary outcome was more than 50% contracture release and patients reaching the patient-acceptable symptom state. Secondary outcomes included hand function, pain, patient satisfaction, quality of life, finger flexion, residual contracture angle, risk for retreatment, and serious adverse events.
A total of 292 (97%) and 284 (94%) patients completed the 3-month and 2-year follow ups, respectively.
Success rates at 3 months were similar: 71% (95% CI, 62%-80%) for surgery; 73% (95% CI, 64%-82%) for needle fasciotomy; and 73% (95% CI, 64%-82%) for collagenase injection.
At 2 Years, Surgery Superior
At 2 years, however, surgery had superior success rates. Surgery success rates vs needle fasciotomy were 78% vs 50% (adjusted risk difference, 0.30; 95% CI, 0.17-0.43).
Surgery success rates vs collagenase injection were 78% vs 65% (aRD, 0.13; 95% CI, 0.01-0.26).
“Secondary analyses paralleled with the primary analysis,” the authors write.
Patients may choose surgery despite initial morbidity which includes potential time off work and higher costs than the other options if the long-term outcome is better, the authors write.
“Collagenase is likely a viable alternative to needle fasciotomy only if its costs are substantially reduced,” the authors write.
A strength of the study is its generalizability, as researchers recruited patients in a setting with universal healthcare where few people seek care outside public hospitals.
Another strength of the trial is that the blinded outcome assessors measured the contracture angles with the participant’s hand covered by a rubber glove and patients were instructed not to reveal their treatment group to the assessor.
Some Physicians Offer Noninvasive Treatments First
Family physician Shannon Scott, DO, medical director of the Midwestern University Multispecialty Clinic in Scottsdale, Arizona, treats many patients with the contracture.
In her practice, patients come to her seeking noninvasive options first. But if they are not satisfied with their hand function after noninvasive treatments such as osteopathic manipulative treatment, physical therapy, and a home exercise program, the next steps are the choices compared in the study. The findings of this randomized controlled trial, she says, will help her in counseling patients choosing among those options.
“What’s important for me as a family physician to understand is more about the path that led to this decision” to seek more invasive treatment and whether the patients in the study had first completed a course of noninvasive care, Dr. Scott says.
The condition, especially in the population most affected — older adults — can greatly affect activities of daily living, she noted. Patients may also often have other conditions contributing to the symptoms of Dupuytren contracture in the neck, arm, or shoulder, for instance, that limit range of motion or cause pain. Addressing those symptoms noninvasively may help relieve the contracture, she says.
Asking patients about their goals is essential, Dr. Scott says. “What patients are looking for is function and the definition for one patient may be different than the level of function for another. Many patients get to a desired level of function with nonsurgical options first.”
A First for the Comparison
Dawn LaPorte, MD, a hand surgeon at Johns Hopkins Medicine in Baltimore, Maryland, who also was not part of the study, says although surgery was thought to have better long-term success rates, this is the first time the data have been able to show that at 2 years.
She added that the results are particularly striking because the endpoint was a 50% release when surgeons hope for a complete release. Even with the 50% release outcome at 2 years, surgery had better success.
She noted that the authors plan to look at outcomes at 5 and 10 years, but, she says, “the fact that surgery is already significantly better at 2 years really says a lot.”
Treatments Have Tradeoffs
She says the conclusions may change the discussions physicians have with patients.
Collagenase injections are an office procedure, and there’s no anesthesia. “There’s usually no lost time from work, and they can use their hand pretty normally the following day,” Dr. LaPorte says. One downside, compared with surgery, is that there may be a more frequent recurrence rate. Patients may have a skin tear that usually heals over a couple of weeks, she added.
Additionally, “the collagenase drug is very expensive,” she notes, so preapproval is important so that the patient doesn’t have to pay out of pocket.
Needle fasciotomy can also be done in the office without anesthesia. There’s less time off work than with surgery.
“With both that and the injection, they should see release of the contracture right away,” Dr. LaPorte says, but the concern is a quicker recurrence rate.
While surgery isn’t a cure, she says, and there is a lower recurrence rate, it typically means time off work, anesthesia, and an incision to heal, and may mean postoperative therapy.
The study was funded by the Research Council of Finland. Disclosures are available with the full text.
Dr. LaPorte and Dr. Scott report no relevant financial relationships.
FROM ANNALS OF INTERNAL MEDICINE
New ESC guidelines for cutting CV risk in noncardiac surgery
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2022
Add AFib to noncardiac surgery risk evaluation: New support
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Analysis: Surgery may not be better than casting for some wrist fractures
There are around 100,000 adult distal radius fractures in the United Kingdom each year. Current National Health Service guidelines in the United Kingdom recommend using K-wires to stabilize wrist fractures when closed reduction is possible or there is no involvement of the articular surface. This is in contrast to fractures that require open reduction and internal fixation with a plate and screws to align the joint articular surface.
As a result, the use of K-wires for surgical fixation has been increasing since 2010 with a comparable decrease of the use of plates and screws.
Even though fixation with wires can provide reliable functional outcomes for patients after reduction of a displaced wrist fracture, surgery still carries risks for the patient and adds an additional expense. A well-molded plaster cast is a safer and cheaper intervention, but it is unclear if it could provide the same functional outcome as pinning.
Therefore, researchers in the United Kingdom conducted a multicenter, randomized trial among 36 hospitals within the NHS as part of the Distal Radius Acute Fracture Fixation Trial 2 (DRAFFT2). Investigators randomly assigned 500 patients aged 16 years and older with dorsally displaced distal radius fractures to manipulation followed by a molded cast or manipulation followed by surgical fixation with K-wires plus a cast.
The study was published online in BMJ.
At 1 year, there were no significant differences between the groups in Patient-Rated Wrist Evaluation (PRWE) scores centered on pain and function.
In an interview, Matthew Costa, PhD, professor of orthopaedic trauma at the University of Oxford (England) and the study’s lead author, said, “If a closed reduction of the fracture can be achieved, clinicians may consider the application of a molded plaster cast as a safe and cost-effective alternative to surgical fixation.”
However, in referencing the data his group published, he did find one thing surprising: “One in eight patients treated with a molded cast required later surgery for loss of fracture position in the first 6 weeks after their injury.”
Dr. Costa added, “This was indeed the key bit of information that patients need when making their decision about surgery. Initial feedback from our patient and public involvement group is that they would be happy to take this chance given that seven out of eight patients didn’t need any form of surgical fixation.”
Philip Blazar, MD, chief of the hand and upper extremity service, Brigham and Women’s Faulkner Hospital in Boston, commended the U.K. authors on completing a challenging randomized controlled trial.
Speaking to this news organization, Dr. Blazer observed a critical difference between U.K. and U.S. guidelines. “It is important to remember that a sizable number of these patients had surgery,” said Dr. Blazar, who was not involved with the study. “They had pins inserted under an anesthetic, and would not have [had] surgery compared to current practice as recommended by many authorities, including the American Academy of Orthopedic Surgeon’s Clinical Practice Guidelines on Distal Radius Fractures.”
Like Dr. Costa, Dr. Blazar expressed concerns about the secondary surgeries in the study group: “27% of patients had a second surgery: 13% in the first 6 weeks after manipulation for loss of reduction, and the remaining 14% had carpal tunnel releases, tendon transfers, tenolysis, and/or capsulectomy for limited range of motion.”
In addition, Dr. Blazar is worried that, although recovery is generally considered to be only 12 months for these type of injuries – the duration of follow-up time in the DRAFFT2 study – “the probable outcome is that in the second 12 months after the injury, there will continue to be more of these types of surgeries.”
Dr. Costa agreed that close follow-up is warranted, “It does suggest that patients treated in a molded cast do need to be followed up carefully to spot those that do need later surgery.”
Still, for Dr. Blazar, the largest takeaway of the study is that, “At 12 months, disability scores between these two groups are not different, but the group treated nonsurgically had 10 times the number of secondary surgeries (27% vs. 2%-3%).”
Moving forward, Dr. Blazar would like to see more specific indications for who would benefit from pinning. He told this news organization, “The greatest limitation is that this study provides no information on which patients with distal radius fractures where reduction is indicated would benefit from surgery. Looking at the details of this study, all patients with displaced fractures from age 16 to the elderly were treated as one indication. My impression is that most surgeons operate on patients taking into account radiographic and patient factors such as age, hand dominance, occupation, overall medical health, and activity level.”
The DRAFFT2 study was funded by the U.K. National Institute for Health Research Health Technology Assessment Programme and was supported by NIHR Oxford Biomedical Research Centre. Dr. Blazar and Dr. Costa have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
There are around 100,000 adult distal radius fractures in the United Kingdom each year. Current National Health Service guidelines in the United Kingdom recommend using K-wires to stabilize wrist fractures when closed reduction is possible or there is no involvement of the articular surface. This is in contrast to fractures that require open reduction and internal fixation with a plate and screws to align the joint articular surface.
As a result, the use of K-wires for surgical fixation has been increasing since 2010 with a comparable decrease of the use of plates and screws.
Even though fixation with wires can provide reliable functional outcomes for patients after reduction of a displaced wrist fracture, surgery still carries risks for the patient and adds an additional expense. A well-molded plaster cast is a safer and cheaper intervention, but it is unclear if it could provide the same functional outcome as pinning.
Therefore, researchers in the United Kingdom conducted a multicenter, randomized trial among 36 hospitals within the NHS as part of the Distal Radius Acute Fracture Fixation Trial 2 (DRAFFT2). Investigators randomly assigned 500 patients aged 16 years and older with dorsally displaced distal radius fractures to manipulation followed by a molded cast or manipulation followed by surgical fixation with K-wires plus a cast.
The study was published online in BMJ.
At 1 year, there were no significant differences between the groups in Patient-Rated Wrist Evaluation (PRWE) scores centered on pain and function.
In an interview, Matthew Costa, PhD, professor of orthopaedic trauma at the University of Oxford (England) and the study’s lead author, said, “If a closed reduction of the fracture can be achieved, clinicians may consider the application of a molded plaster cast as a safe and cost-effective alternative to surgical fixation.”
However, in referencing the data his group published, he did find one thing surprising: “One in eight patients treated with a molded cast required later surgery for loss of fracture position in the first 6 weeks after their injury.”
Dr. Costa added, “This was indeed the key bit of information that patients need when making their decision about surgery. Initial feedback from our patient and public involvement group is that they would be happy to take this chance given that seven out of eight patients didn’t need any form of surgical fixation.”
Philip Blazar, MD, chief of the hand and upper extremity service, Brigham and Women’s Faulkner Hospital in Boston, commended the U.K. authors on completing a challenging randomized controlled trial.
Speaking to this news organization, Dr. Blazer observed a critical difference between U.K. and U.S. guidelines. “It is important to remember that a sizable number of these patients had surgery,” said Dr. Blazar, who was not involved with the study. “They had pins inserted under an anesthetic, and would not have [had] surgery compared to current practice as recommended by many authorities, including the American Academy of Orthopedic Surgeon’s Clinical Practice Guidelines on Distal Radius Fractures.”
Like Dr. Costa, Dr. Blazar expressed concerns about the secondary surgeries in the study group: “27% of patients had a second surgery: 13% in the first 6 weeks after manipulation for loss of reduction, and the remaining 14% had carpal tunnel releases, tendon transfers, tenolysis, and/or capsulectomy for limited range of motion.”
In addition, Dr. Blazar is worried that, although recovery is generally considered to be only 12 months for these type of injuries – the duration of follow-up time in the DRAFFT2 study – “the probable outcome is that in the second 12 months after the injury, there will continue to be more of these types of surgeries.”
Dr. Costa agreed that close follow-up is warranted, “It does suggest that patients treated in a molded cast do need to be followed up carefully to spot those that do need later surgery.”
Still, for Dr. Blazar, the largest takeaway of the study is that, “At 12 months, disability scores between these two groups are not different, but the group treated nonsurgically had 10 times the number of secondary surgeries (27% vs. 2%-3%).”
Moving forward, Dr. Blazar would like to see more specific indications for who would benefit from pinning. He told this news organization, “The greatest limitation is that this study provides no information on which patients with distal radius fractures where reduction is indicated would benefit from surgery. Looking at the details of this study, all patients with displaced fractures from age 16 to the elderly were treated as one indication. My impression is that most surgeons operate on patients taking into account radiographic and patient factors such as age, hand dominance, occupation, overall medical health, and activity level.”
The DRAFFT2 study was funded by the U.K. National Institute for Health Research Health Technology Assessment Programme and was supported by NIHR Oxford Biomedical Research Centre. Dr. Blazar and Dr. Costa have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
There are around 100,000 adult distal radius fractures in the United Kingdom each year. Current National Health Service guidelines in the United Kingdom recommend using K-wires to stabilize wrist fractures when closed reduction is possible or there is no involvement of the articular surface. This is in contrast to fractures that require open reduction and internal fixation with a plate and screws to align the joint articular surface.
As a result, the use of K-wires for surgical fixation has been increasing since 2010 with a comparable decrease of the use of plates and screws.
Even though fixation with wires can provide reliable functional outcomes for patients after reduction of a displaced wrist fracture, surgery still carries risks for the patient and adds an additional expense. A well-molded plaster cast is a safer and cheaper intervention, but it is unclear if it could provide the same functional outcome as pinning.
Therefore, researchers in the United Kingdom conducted a multicenter, randomized trial among 36 hospitals within the NHS as part of the Distal Radius Acute Fracture Fixation Trial 2 (DRAFFT2). Investigators randomly assigned 500 patients aged 16 years and older with dorsally displaced distal radius fractures to manipulation followed by a molded cast or manipulation followed by surgical fixation with K-wires plus a cast.
The study was published online in BMJ.
At 1 year, there were no significant differences between the groups in Patient-Rated Wrist Evaluation (PRWE) scores centered on pain and function.
In an interview, Matthew Costa, PhD, professor of orthopaedic trauma at the University of Oxford (England) and the study’s lead author, said, “If a closed reduction of the fracture can be achieved, clinicians may consider the application of a molded plaster cast as a safe and cost-effective alternative to surgical fixation.”
However, in referencing the data his group published, he did find one thing surprising: “One in eight patients treated with a molded cast required later surgery for loss of fracture position in the first 6 weeks after their injury.”
Dr. Costa added, “This was indeed the key bit of information that patients need when making their decision about surgery. Initial feedback from our patient and public involvement group is that they would be happy to take this chance given that seven out of eight patients didn’t need any form of surgical fixation.”
Philip Blazar, MD, chief of the hand and upper extremity service, Brigham and Women’s Faulkner Hospital in Boston, commended the U.K. authors on completing a challenging randomized controlled trial.
Speaking to this news organization, Dr. Blazer observed a critical difference between U.K. and U.S. guidelines. “It is important to remember that a sizable number of these patients had surgery,” said Dr. Blazar, who was not involved with the study. “They had pins inserted under an anesthetic, and would not have [had] surgery compared to current practice as recommended by many authorities, including the American Academy of Orthopedic Surgeon’s Clinical Practice Guidelines on Distal Radius Fractures.”
Like Dr. Costa, Dr. Blazar expressed concerns about the secondary surgeries in the study group: “27% of patients had a second surgery: 13% in the first 6 weeks after manipulation for loss of reduction, and the remaining 14% had carpal tunnel releases, tendon transfers, tenolysis, and/or capsulectomy for limited range of motion.”
In addition, Dr. Blazar is worried that, although recovery is generally considered to be only 12 months for these type of injuries – the duration of follow-up time in the DRAFFT2 study – “the probable outcome is that in the second 12 months after the injury, there will continue to be more of these types of surgeries.”
Dr. Costa agreed that close follow-up is warranted, “It does suggest that patients treated in a molded cast do need to be followed up carefully to spot those that do need later surgery.”
Still, for Dr. Blazar, the largest takeaway of the study is that, “At 12 months, disability scores between these two groups are not different, but the group treated nonsurgically had 10 times the number of secondary surgeries (27% vs. 2%-3%).”
Moving forward, Dr. Blazar would like to see more specific indications for who would benefit from pinning. He told this news organization, “The greatest limitation is that this study provides no information on which patients with distal radius fractures where reduction is indicated would benefit from surgery. Looking at the details of this study, all patients with displaced fractures from age 16 to the elderly were treated as one indication. My impression is that most surgeons operate on patients taking into account radiographic and patient factors such as age, hand dominance, occupation, overall medical health, and activity level.”
The DRAFFT2 study was funded by the U.K. National Institute for Health Research Health Technology Assessment Programme and was supported by NIHR Oxford Biomedical Research Centre. Dr. Blazar and Dr. Costa have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM BMJ
Standardized protocol guides therapies to reduce VTE after arthroplasty
A simple tool to guide choice of antithrombotic therapy following total joint arthroplasty led to a reduction in pulmonary embolism (PE) after being introduced systemwide, according to a prospectively tracked evaluation of a large patient cohort. The results of the study were reported in an abstract scheduled for release at the annual meeting of the American Academy of Orthopaedic Surgeons. The meeting was canceled because of COVID-19.
“We developed a simplified scoring system for evaluating risk of thromboembolism and guiding prophylaxis that led to a significant reduction in events across a large integrated health care system,” reported James Wylie, MD, associate medical director for hip and knee preservation and orthopedic research at Intermountain Healthcare, Salt Lake City, Utah.
The goal of the methodology was to create a uniform and evidence-based approach to risk assessment in order to guide selection of appropriate venous thromboembolism (VTE) prophylaxis. The tool takes into account the need to individualize antithrombotic drugs for risk of both VTE and for bleeding.
“VTE is a major threat following total joint replacement, but not all patients require anticoagulants. Recent evidence supports a shift to aspirin for low-risk patients,” explained Dr. Wylie in an interview.
The risk tool assigns points for such factors as history of VTE, older age, history of coronary artery disease, history of cancer, and increased body mass index. There are two possible ratings to guide strategies. Those with standard risk are candidates for 81 mg of aspirin twice daily. Those with high risk are candidates for 2.5 mg of apixaban, also administered twice daily. Custom dosing of warfarin is an alternative for the latter group. Regardless of strategy, prophylaxis is administered for 30 days following arthroplasty
“The risk score is calculated automatically, because you have to click a box in the electronic medical record for all of those factors as part of admission orders,” Dr. Wylie said.
The protocol was introduced in July 2017 and adoption was tracked prospectively over 18 months. In an evaluable cohort of 20,284 patients, PE rates in the 71% of patients adherent to the protocol were compared with the 29% who were not.
Over the observation period, the rates of PE were 0.34% and 0.62% (P = .004) for those adherent and nonadherent, respectively. The rate of unplanned readmissions and death, which were secondary outcomes, were both numerically lower in the group treated by adherent surgeons, but the differences did not reach statistical significance.
Adoption of the protocol by surgeons did increase over the course of the observation period, and this correlated with a decrease in unplanned readmissions. Bleeding-related readmission was a rare event in this analysis and did not significantly increase over time, according to Dr. Wylie.
The risk assessment tool, developed by a multispecialty team at Intermountain Healthcare, was based on a review of hundreds of published papers and guidelines, according to Nathan Momberger, MD, who is the associate medical director of total joint replacement at Intermountain and was a coauthor on this study. A member of the team that developed the risk assessment tool, Dr. Momberger noted that new risk score was developed at a time when clinicians have been moving quickly away from warfarin to direct oral anticoagulants.
“None of our surgeons were using the same VTE prophylaxis when we started this project,” Dr. Momberger said. This was a motivation for developing a systemwide approach. In the 22 participating hospitals, there were 50 surgeons performing total knee arthroplasty and 40 surgeons were performing total hip surgery at the time the new protocol was introduced.
Further analyses will provide a more detailed analysis of the effect of the protocol on other thrombotic events, including deep vein thrombosis, and on cost. Since these data were analyzed, protocol adoption has increased and now exceeds 80%, according to Dr. Wylie.
Although a standardized approach to VTE prophylaxis following total joint arthroplasty is attractive, the ideal strategy remains controversial, according to Sunny Parikh, MD, an orthopedic surgeon affiliated with Colchester (England) General Hospital.
As a coauthor of a recent study that quantified symptomatic VTE rates at his and a neighboring hospital over a 3-year period (BMC Musculoskelet Disord. 2020;21:95), Dr. Parikh reported that VTE rates did not reach zero even with a prolonged course of the low-molecular-weight heparin enoxaparin.
At 90 days, the symptomatic VTE rate was only 0.3% for total knee arthroplasty but reached 1.2% for total hip arthroplasty.
“At the time of this study we were using enoxaparin for 28 days following total hip replacements and for 14 days following total knee replacements,” Dr. Parikh reported. Since this study, his institution has switched to a regimen recommended by the U.K.’s National Institute for Health and Clinical Excellence (NICE).
Under the NICE guidelines, VTE prophylaxis for total hip arthroplasty is 40 mg enoxaparin once daily for 14 days followed by 75 mg aspirin for another 14 days, according to Dr. Parikh. For total knee arthroplasty, the standard regimen is 75 mg aspirin for 14 days.
For those who might not be best managed with the standard approach, “there is no clear guideline.” Rather, in patients with renal or liver impairment, “we discuss the case with the hematology team to adjust the doses,” Dr. Parikh reported.
The advantage of a standardized approach applied to all or most patients is that is eliminates disparities, but Dr. Parikh agreed that risk-adjusted prophylaxis might be warranted for optimal outcomes.
Dr. Wylie reported a financial relationship with Arthrex.
A simple tool to guide choice of antithrombotic therapy following total joint arthroplasty led to a reduction in pulmonary embolism (PE) after being introduced systemwide, according to a prospectively tracked evaluation of a large patient cohort. The results of the study were reported in an abstract scheduled for release at the annual meeting of the American Academy of Orthopaedic Surgeons. The meeting was canceled because of COVID-19.
“We developed a simplified scoring system for evaluating risk of thromboembolism and guiding prophylaxis that led to a significant reduction in events across a large integrated health care system,” reported James Wylie, MD, associate medical director for hip and knee preservation and orthopedic research at Intermountain Healthcare, Salt Lake City, Utah.
The goal of the methodology was to create a uniform and evidence-based approach to risk assessment in order to guide selection of appropriate venous thromboembolism (VTE) prophylaxis. The tool takes into account the need to individualize antithrombotic drugs for risk of both VTE and for bleeding.
“VTE is a major threat following total joint replacement, but not all patients require anticoagulants. Recent evidence supports a shift to aspirin for low-risk patients,” explained Dr. Wylie in an interview.
The risk tool assigns points for such factors as history of VTE, older age, history of coronary artery disease, history of cancer, and increased body mass index. There are two possible ratings to guide strategies. Those with standard risk are candidates for 81 mg of aspirin twice daily. Those with high risk are candidates for 2.5 mg of apixaban, also administered twice daily. Custom dosing of warfarin is an alternative for the latter group. Regardless of strategy, prophylaxis is administered for 30 days following arthroplasty
“The risk score is calculated automatically, because you have to click a box in the electronic medical record for all of those factors as part of admission orders,” Dr. Wylie said.
The protocol was introduced in July 2017 and adoption was tracked prospectively over 18 months. In an evaluable cohort of 20,284 patients, PE rates in the 71% of patients adherent to the protocol were compared with the 29% who were not.
Over the observation period, the rates of PE were 0.34% and 0.62% (P = .004) for those adherent and nonadherent, respectively. The rate of unplanned readmissions and death, which were secondary outcomes, were both numerically lower in the group treated by adherent surgeons, but the differences did not reach statistical significance.
Adoption of the protocol by surgeons did increase over the course of the observation period, and this correlated with a decrease in unplanned readmissions. Bleeding-related readmission was a rare event in this analysis and did not significantly increase over time, according to Dr. Wylie.
The risk assessment tool, developed by a multispecialty team at Intermountain Healthcare, was based on a review of hundreds of published papers and guidelines, according to Nathan Momberger, MD, who is the associate medical director of total joint replacement at Intermountain and was a coauthor on this study. A member of the team that developed the risk assessment tool, Dr. Momberger noted that new risk score was developed at a time when clinicians have been moving quickly away from warfarin to direct oral anticoagulants.
“None of our surgeons were using the same VTE prophylaxis when we started this project,” Dr. Momberger said. This was a motivation for developing a systemwide approach. In the 22 participating hospitals, there were 50 surgeons performing total knee arthroplasty and 40 surgeons were performing total hip surgery at the time the new protocol was introduced.
Further analyses will provide a more detailed analysis of the effect of the protocol on other thrombotic events, including deep vein thrombosis, and on cost. Since these data were analyzed, protocol adoption has increased and now exceeds 80%, according to Dr. Wylie.
Although a standardized approach to VTE prophylaxis following total joint arthroplasty is attractive, the ideal strategy remains controversial, according to Sunny Parikh, MD, an orthopedic surgeon affiliated with Colchester (England) General Hospital.
As a coauthor of a recent study that quantified symptomatic VTE rates at his and a neighboring hospital over a 3-year period (BMC Musculoskelet Disord. 2020;21:95), Dr. Parikh reported that VTE rates did not reach zero even with a prolonged course of the low-molecular-weight heparin enoxaparin.
At 90 days, the symptomatic VTE rate was only 0.3% for total knee arthroplasty but reached 1.2% for total hip arthroplasty.
“At the time of this study we were using enoxaparin for 28 days following total hip replacements and for 14 days following total knee replacements,” Dr. Parikh reported. Since this study, his institution has switched to a regimen recommended by the U.K.’s National Institute for Health and Clinical Excellence (NICE).
Under the NICE guidelines, VTE prophylaxis for total hip arthroplasty is 40 mg enoxaparin once daily for 14 days followed by 75 mg aspirin for another 14 days, according to Dr. Parikh. For total knee arthroplasty, the standard regimen is 75 mg aspirin for 14 days.
For those who might not be best managed with the standard approach, “there is no clear guideline.” Rather, in patients with renal or liver impairment, “we discuss the case with the hematology team to adjust the doses,” Dr. Parikh reported.
The advantage of a standardized approach applied to all or most patients is that is eliminates disparities, but Dr. Parikh agreed that risk-adjusted prophylaxis might be warranted for optimal outcomes.
Dr. Wylie reported a financial relationship with Arthrex.
A simple tool to guide choice of antithrombotic therapy following total joint arthroplasty led to a reduction in pulmonary embolism (PE) after being introduced systemwide, according to a prospectively tracked evaluation of a large patient cohort. The results of the study were reported in an abstract scheduled for release at the annual meeting of the American Academy of Orthopaedic Surgeons. The meeting was canceled because of COVID-19.
“We developed a simplified scoring system for evaluating risk of thromboembolism and guiding prophylaxis that led to a significant reduction in events across a large integrated health care system,” reported James Wylie, MD, associate medical director for hip and knee preservation and orthopedic research at Intermountain Healthcare, Salt Lake City, Utah.
The goal of the methodology was to create a uniform and evidence-based approach to risk assessment in order to guide selection of appropriate venous thromboembolism (VTE) prophylaxis. The tool takes into account the need to individualize antithrombotic drugs for risk of both VTE and for bleeding.
“VTE is a major threat following total joint replacement, but not all patients require anticoagulants. Recent evidence supports a shift to aspirin for low-risk patients,” explained Dr. Wylie in an interview.
The risk tool assigns points for such factors as history of VTE, older age, history of coronary artery disease, history of cancer, and increased body mass index. There are two possible ratings to guide strategies. Those with standard risk are candidates for 81 mg of aspirin twice daily. Those with high risk are candidates for 2.5 mg of apixaban, also administered twice daily. Custom dosing of warfarin is an alternative for the latter group. Regardless of strategy, prophylaxis is administered for 30 days following arthroplasty
“The risk score is calculated automatically, because you have to click a box in the electronic medical record for all of those factors as part of admission orders,” Dr. Wylie said.
The protocol was introduced in July 2017 and adoption was tracked prospectively over 18 months. In an evaluable cohort of 20,284 patients, PE rates in the 71% of patients adherent to the protocol were compared with the 29% who were not.
Over the observation period, the rates of PE were 0.34% and 0.62% (P = .004) for those adherent and nonadherent, respectively. The rate of unplanned readmissions and death, which were secondary outcomes, were both numerically lower in the group treated by adherent surgeons, but the differences did not reach statistical significance.
Adoption of the protocol by surgeons did increase over the course of the observation period, and this correlated with a decrease in unplanned readmissions. Bleeding-related readmission was a rare event in this analysis and did not significantly increase over time, according to Dr. Wylie.
The risk assessment tool, developed by a multispecialty team at Intermountain Healthcare, was based on a review of hundreds of published papers and guidelines, according to Nathan Momberger, MD, who is the associate medical director of total joint replacement at Intermountain and was a coauthor on this study. A member of the team that developed the risk assessment tool, Dr. Momberger noted that new risk score was developed at a time when clinicians have been moving quickly away from warfarin to direct oral anticoagulants.
“None of our surgeons were using the same VTE prophylaxis when we started this project,” Dr. Momberger said. This was a motivation for developing a systemwide approach. In the 22 participating hospitals, there were 50 surgeons performing total knee arthroplasty and 40 surgeons were performing total hip surgery at the time the new protocol was introduced.
Further analyses will provide a more detailed analysis of the effect of the protocol on other thrombotic events, including deep vein thrombosis, and on cost. Since these data were analyzed, protocol adoption has increased and now exceeds 80%, according to Dr. Wylie.
Although a standardized approach to VTE prophylaxis following total joint arthroplasty is attractive, the ideal strategy remains controversial, according to Sunny Parikh, MD, an orthopedic surgeon affiliated with Colchester (England) General Hospital.
As a coauthor of a recent study that quantified symptomatic VTE rates at his and a neighboring hospital over a 3-year period (BMC Musculoskelet Disord. 2020;21:95), Dr. Parikh reported that VTE rates did not reach zero even with a prolonged course of the low-molecular-weight heparin enoxaparin.
At 90 days, the symptomatic VTE rate was only 0.3% for total knee arthroplasty but reached 1.2% for total hip arthroplasty.
“At the time of this study we were using enoxaparin for 28 days following total hip replacements and for 14 days following total knee replacements,” Dr. Parikh reported. Since this study, his institution has switched to a regimen recommended by the U.K.’s National Institute for Health and Clinical Excellence (NICE).
Under the NICE guidelines, VTE prophylaxis for total hip arthroplasty is 40 mg enoxaparin once daily for 14 days followed by 75 mg aspirin for another 14 days, according to Dr. Parikh. For total knee arthroplasty, the standard regimen is 75 mg aspirin for 14 days.
For those who might not be best managed with the standard approach, “there is no clear guideline.” Rather, in patients with renal or liver impairment, “we discuss the case with the hematology team to adjust the doses,” Dr. Parikh reported.
The advantage of a standardized approach applied to all or most patients is that is eliminates disparities, but Dr. Parikh agreed that risk-adjusted prophylaxis might be warranted for optimal outcomes.
Dr. Wylie reported a financial relationship with Arthrex.
REPORTING FROM AAOS 2020
Fragility Fractures: Diagnosis and Treatment
ABSTRACT
Fragility fractures are estimated to affect 3 million people annually in the United States. As they are associated with a significant mortality rate, the prevention of these fractures should be a priority for orthopedists. At-risk patients include the elderly and those with thyroid disease, diabetes, hypertension, and heart disease. Osteoporosis is diagnosed by the presence of a fragility fracture or by dual-energy x-ray absorptiometry (DXA) in the absence of a fragility fracture. In 2011, the United States Preventive Services Task Force (USPSTF) recommended that all women ≥65 years should be screened for osteoporosis by DXA. Women <65 years with a 10-year fracture risk =/> than that of a 65-year-old white woman should also be screened for osteoporosis. Lifestyle changes, such as calcium and vitamin D supplementation, exercise, and smoking cessation, are non-pharmacologic treatment options. The National Osteoporosis Foundation recommends treating osteoporosis with pharmacotherapy in patients with a high risk for fracture (T score <–2.5) or history of fragility fracture. Understanding risk factors and eliminating medications known to cause decreased BMD are vital to prevention and will be necessary to limit these fractures and their associated expenses in the future.
Continue to: Fragility fractures are caused by...
Fragility fractures are caused by falls from standing height or repetitive physiological loads.1 With the growing aging population in the United States, it is estimated that 3 million people will be affected by fragility fractures yearly.2 In the setting of osseous insufficiency, fractures that are typically associated with high-energy trauma are encountered in patients who simply trip over a parking lot curb or fall off their bike. After surgery, the severe disruption of patients’ lives continues with a prolonged rehabilitation period.
Fragility fractures are not only traumatizing for patients; they are also associated with significantly increased mortality. A study by Gosch and colleagues found that 70.6% of patients died during the normal follow-up period, and 29.4% of patients died within the first year of suffering a fracture.3 Also, the mean life expectancy post-fragility fracture was only 527 days.3 Diagnosis and treatment of osteoporosis is imperative to prevent fragility fractures before they occur.
RISK FACTORS AND CAUSES
The incidence of fragility fractures increases in patients with comorbidities such as thyroid disease, diabetes, hypertension, and heart disease.4 Hyperthyroidism and treated hypothyroidism cause an imbalance between osteoblast and osteoclast activity, resulting in osteoporosis.5 A thyroid-stimulating hormone level < 0.1 increases the risk of vertebral and non-vertebral fractures by a factor of 4.5 and 3.2 mIU/L respectively.4 Patients with diabetes also have an increased risk of fragility fractures, which is due to impaired healing capabilities, especially that of bone healing. Approximately 2 million people are affected by type 1 diabetes in the United States, and 20% of those patients will develop osteoporosis.6
Hypertension and osteoporosis are 2 diseases that occur often in the elderly. Common etiological factors believed to cause both hypertension and osteoporosis are low calcium intake, high consumption of salt, and vitamin D and vitamin K deficiency. Also, hypertension treated with loop diuretics has been found to cause negative effects on bone and increase the risk of osteoporosis.7 The only antihypertensive medications that preserve bone mineral density (BMD) and reduce fracture risk are thiazide diuretics.7 Lastly, an association between coronary artery disease and osteoporosis has been hypothesized. The link is not completely understood, but it is believed that oxidative stress and inflammation are the culprits in both diseases.8 In contrast to previous hypotheses, Sosa and colleagues found an independent association between beta blockers and fragility fractures.9 The idea that beta blockers and fragility fractures are linked is still controversial and needs more study. Unlike beta blockers, statins provide a protective effect on bone. They increase BMD and reduce fracture risk by inhibiting osteoclastogenesis.10
In addition to loop diuretics and beta blockers, inhaled glucocorticoids, oral glucocorticoids, proton pump inhibitors (PPIs), H2 receptor antagonists, and anticonvulsants decrease bone density and increase the incidence of fragility fractures.11 Chronic glucocorticoid therapy is the most common cause of secondary osteoporosis. Osteoblasts and osteocytes undergo apoptosis in the presence of glucocorticoids.12 Patients on glucocorticoid therapy have an increased risk of fracture, even with higher BMD values.13 Bone changes that occur while a patient is taking glucocorticoids may not be detected during BMD testing. Therefore, a high level of suspicion of osteoporosis in patients on long-term glucocorticoids is imperative.
Proton pump inhibitors are among the most prescribed medications in the world; they reduce bone resorption, increasing the risk of fracture.14 Proton pump inhibitors and H2 receptor antagonists are hypothesized to cause malabsorption of calcium and indirectly cause osteoporosis. The risk of osteoporosis increases with the length of PPI treatment.15 However, exposure lasting <7 years does not increase the risk of fracture.16 It is recommended that patients on long-term PPIs be referred for BMD testing.
An association between anticonvulsants and osteoporosis has been found in observational studies. The mechanism of this association is not yet fully understood, but it is believed that exacerbation of vitamin D deficiency leads to increased bone metabolism.17 Gastrointestinal (GI) calcium absorption also decreases with anticonvulsant use. Prolonged antiepileptic therapy and high-dose therapy rapidly decrease BMD. Primidone, carbamazepine, phenobarbital, and phenytoin are the drugs most often associated with decreased BMD. Osteoporosis and fragility fracture in these patients can be prevented with calcium, vitamin D, and the bisphosphonate risedronate. These medications have been shown to improve BMD by 69%.18
Continue to: DIAGNOSIS...
DIAGNOSIS
Osteoporosis is diagnosed by the presence of a fragility fracture or by dual-energy x-ray absorptiometry (DXA) in the absence of a fragility fracture.19 Measurements of the femoral neck by DXA are used to diagnose osteoporosis, although DXA can also be used to measure the bone density of the spine and peripheral skeleton.20
The World Health Organization developed a set of T score criteria to diagnose osteoporosis in postmenopausal women (Table 1). A T score >-1 is normal, <-1 but >-2.5 signifies osteopenia, <-2.5 is osteoporosis, and <-2.5 with fragility fracture is severe osteoporosis.19 The Z score, not the T score, should be used to assess osteoporosis in premenopausal women, men <50 years, and children (Table 2). The Z score is calculated by comparing the patient’s BMD with the mean BMD of their peers of a similar age, race, and gender.19 Z scores <-2.0 indicate low BMD for chronological age. A Z score > -2.0 is considered within the expected range for age.20 Bone mineral density testing is the rate- limiting step to starting osteoporosis treatment.21 Without testing, treatment of osteoporosis is very unlikely.
Table 1. T Score Criteria
T score | Diagnosis |
> -1.0 | Normal |
-1.0 to -2.5 | Osteopenia |
< -2.5 | Osteoporosis |
< -2.5 with fragility fracture | Severe osteoporosis |
Table 2. Z Score Criteria
Z score | Diagnosis |
> -2.0 | Normal BMD for age |
< -2.0 | Low BMD for age |
The World Health Organization also developed a tool to predict fracture risk. The Fracture Risk Assessment Tool uses fracture history in addition to other risk factors to predict a patient’s 10-year risk of major fracture.22 Risk factors used to assess fracture risk include age, sex, weight, height, previous fracture, parental hip fracture history, current smoker, glucocorticoid use, rheumatoid arthritis, secondary osteoporosis, excessive alcohol use, and femoral neck BMD.
In 2011, the United States Preventive Services Task Force (USPSTF) recommended that all women ≥65 years should be screened for osteoporosis by DXA. Women <65 years with a 10-year fracture risk =/> than that of a 65-year-old white woman should also be screened for osteoporosis. These recommendations are different for men. It was concluded that the evidence was insufficient to support osteoporosis screening in men.23 As of April 2017, Centers for Medicare and Medicaid Services current reimbursement rates for DXA scans are, on average, $123.10 in the hospital setting and $41.63 in the office setting. The axial DXA CPT code is 77080.
Continue to: TREATMENT...
TREATMENT
NONPHARMACOLOGIC
Patients with mild osteoporosis may be treated first non-pharmacologically. Lifestyle changes such as calcium and vitamin D supplementation, exercise, and smoking cessation are non-pharmacologic treatment options. Calcium carbonate and calcium citrate are common supplements. Calcium carbonate is 40% elemental calcium, whereas calcium citrate supplements are only 21% elemental calcium. Calcium supplements are best absorbed when taken with food.24 The recommended daily total calcium intake is 1200 mg.25 Only 500 to 600 milligrams of calcium can be absorbed by the GI tract at a time. Therefore, calcium supplements should be taken at least 4 to 5 hours apart.24Patients should also be counseled that calcium supplements may cause GI side effects such as bloating and constipation. To reduce side effects, patients can slowly increase the dose of calcium to a therapeutic level.
Vitamin D supplementation works best in conjunction with calcium supplementation. Vitamin D functions to regulate calcium absorption in the intestine and stimulate bone resorption and maintain the serum calcium concentration. The National Osteoporosis Foundation recommends 800 to 1000 international units of vitamin D daily.24 Lifestyle changes may be sufficient to stop the progression of osteoporosis in its early stages. Once osteoporosis becomes severe enough, pharmacotherapy is needed to stop further bone destruction and improve BMD.
PHARMACOLOGIC
After an initial fragility fracture, the risk of additional ones increases significantly, making treatment of osteoporosis essential. The National Osteoporosis Foundation recommends treating osteoporosis with pharmacotherapy in patients with a high risk of fracture (T score <-2.5) or history of fragility fracture.26 Bisphosphonates inhibit bone resorption and are considered the first-line therapy for postmenopausal women with osteoporosis. A common side effect of oral bisphosphonates is GI toxicity. Patients are advised to avoid lying down for at least 30 minutes after medication administration to avoid esophageal irritation. Oral bisphosphonates should also be taken in the morning on an empty stomach with at least 8 ounces of water. Recurrent bisphosphonate use should be avoided in patients with chronic kidney disease. Oral alendronate and risedronate are typically discontinued after 5 years of use.27 Long-term bisphosphonate use may cause an increased risk of fragility fracture due to oversuppression of bone turnover. To avoid this risk, bisphosphonate “drug holidays” are an option. Bisphosphonates accumulate over time, creating reservoirs. Even after therapy is stopped, patients continue to have therapeutic effects for 2 to 5 years.28
Bisphosphonates are available in both oral and intravenous forms. Alendronate is available in doses of 10 mg and 70 mg for daily and weekly administration, respectively. Both are available in tablet form, but the 70 mg weekly dose is also available in a dissolvable formulation. Alendronate is available in a reduced dose for osteoporosis prevention. Alendronate dosing for osteoporosis prevention is 5 mg daily or 35 mg weekly. Risedronate is dosed as 5 mg daily, 35 mg weekly, or 150 mg monthly. Intravenous bisphosphonates are indicated when oral bisphosphonates are not tolerated, only after vitamin D has been assessed and is within the normal range. Zoledronic acid is administered as a 15-minute infusion once a year.
Teriparatide (Forteo; PTH-1-34) is available for glucocorticoid-induced osteoporosis, postmenopausal women, and men with severe osteoporosis. It is indicated for patients in whom bisphosphonate treatment has failed or those who do not tolerate bisphosphonates. Teriparatide is a synthetic parathyroid hormone (PTH) that acts as an anabolic agent, stimulating bone formation, maturation, and remodeling.29 In addition to its application as a bone-building hormone, teriparatide has gained popularity for various off-label uses. These include accelerated osteosynthesis, stress fracture healing, and in the nonoperative treatment of osteoarthritis.29 Parathyroid hormone has been shown to stimulate the maturation, proliferation, and maintenance of osteoblast progenitor cells. More recently, PTH has been shown to regulate chondrocyte signaling, as well as differentiation and maturation. Further study on the chondroregenerative potential of PTH has demonstrated its efficacy as a novel disease-modifying agent in the treatment of osteoarthritis.29 Teriparatide is administered as a daily subcutaneous injection. The United States dosing is 600 mcg/2.4 mL. Adverse effects such as orthostatic hypotension and osteosarcoma may occur. BMD testing should be performed 1 to 2 years after initiation of teriparatide and every 2 years thereafter.26
Abaloparatide (Tymlos), a human parathyroid hormone, is another treatment option for postmenopausal women at risk of osteoporotic fracture. In a study comparing the efficacy of abaloparatide and teriparatide, treatment with abaloparatide was found to induce higher BMD levels in a time frame of 12 months. The BMD differences could be attributed to many factors, such as an enhanced net anabolic effect or a reduced osteoblast expression. Furthermore, the risk of developing new vertebral and nonvertebral fractures decreased in the abaloparatide group compared with the placebo group over a period of 18 months.30
Continue to: The recommended daily dose for abaloparatide...
The recommended daily dose for abaloparatide is 80 mcg via subcutaneous injection with calcium and vitamin D supplements.31 Adverse reactions were consistent between abaloparatide and teriparatide, and included hypercalcemia, hypercalciuria, and orthostatic hypotension.30 The use of parathyroid analogs for >2 years is not recommended due to the risk of osteosarcoma.
Denosumab (Prolia) is a monoclonal antibody that stops osteoclastogenesis by blocking the binding of RANKL to RANK.31 It is indicated for patients intolerant to bisphosphonates or with impaired kidney function. Prolia is administered subcutaneously in 60 mg doses every 6 months in men and postmenopausal women with osteoporosis. Prolia is contraindicated in patients with hypersensitivity to any component of the medication, pregnancy, and hypocalcemia.
Selective estrogen receptor modulators (SERMs), such as raloxifene and tamoxifen, can treat osteoporosis effectively in postmenopausal women. Raloxifene is considered the SERM of choice due to the availability of more robust safety and efficacy data. Raloxifene increases BMD while decreasing bone resorption and bone turnover.32 It is also used to reduce breast cancer risk; however, it increases the risk of thromboembolic events and hot flashes. Tamoxifen is not typically used to treat osteoporosis, but women treated for breast cancer with tamoxifen receive some bone protection.
Lastly, calcitonin and strontium ranelate are also options to treat osteoporosis. However, both calcitonin and strontium ranelate have weak effects on BMD. Calcitonin only transiently inhibits osteoclast activity.33 Therefore, medications like bisphosphonates, teriparatide, denosumab, and SERMs are preferred.
A summary of medications used to treat osteoporosis can be found in Table 3.
Table 3. Overview of Common Medications Used in the Treatment and Prevention of Osteoporosis
Medication | Indication | Dosing |
Calcium supplementation | Mild osteoporosis | 1200 mg oral/d |
Vitamin D supplementation | Mild osteoporosis | 800 to 1000 IU oral/d |
Alendronate | Postmenopausal osteoporosis
Osteoporosis prevention | 10 mg oral/d 70 mg oral/wk
5 mg/d 35 mg/wk |
Risedronate | Postmenopausal osteoporosis | 5 mg oral/d 35 mg oral/wk 150 mg oral/mo |
Teriparatide (Forteo) | Glucocorticoid-inducted osteoporosis, postmenopausal osteoporosis, men with severe osteoporosis | 600 mcg/2.4 mL subcutaneous/d |
Abaloparatide (Tymlos) | Postmenopausal osteoporosis | 80 mcg subcutaneous/d |
Denosumab (Prolia) | Patients intolerant to bisphosphonates; patients with impaired kidney function. | 60 mg subcutaneous every 6 mo |
Raloxifene | Postmenopausal osteoporosis | 60 mg oral/d |
Tamoxifen | Postmenopausal osteoporosis | 20 mg oral/d |
Calcitonin | Postmenopausal osteoporosis | 100 units intramuscular or subcutaneous/d 200 units (1 spray) intranasal/d |
Strontium ranelate | Postmenopausal osteoporosis Severe osteoporosis in men | 2 g/d dissolved in water, prior to bedtime Not recommended in CrCl <30 mL/min |
Abbreviation: CrCl, creatinine clearance.
CONCLUSION
With a growing aging population, the prevalence of osteoporosis is expected to increase. By 2025, experts estimate that there will be 2 million fractures yearly, costing the United States upwards of $25 billion.34,35 This estimate does not include the cost of lost productivity or disability, which will likely cost billions more.34,35 Understanding risk factors and eliminating medications known to cause decreased BMD are vital. Obtaining a BMD measurement is the rate-limiting step for treatment initiation. Without an appropriate diagnosis, treatment is unlikely. As providers, it us our responsibility to maintain a high level of suspicion of osteoporosis in the elderly and promptly diagnose and treat them.
- Dietz SO, Hofmann A, Rommens PM. Haemorrhage in fragility fractures of the pelvis. Eur J Trauma Emerg Surg. 2015;41:363-367. doi: 10.1007/s00068-014-0452-1
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465-475. doi: 10.1359/jbmr.061113.
- Gosch M, Hoffmann-Weltin Y, Roth T, Blauth M, Nicholas JA, Kammerlander C. Orthogeriatric co-management improves the outcome of long-term care residents with fragility fractures. Arch Orthop Trauma Surg. 2016; 136(10):1403-1409. doi: 10.1007/s00402-016-2543-4.
- Maccagnano G, Notarnicola A, Pesce V, Mudoni S, Tafuri S, Moretti B. The prevalence of fragility fractures in a population of a region of southern Italy affected by thyroid disorders. BioMed Res Int. 2016. doi: 10.1155/2016/6017165.
- Mosekilde L, Eriksen EF, Charles P. Effects of thyroid hormones on bone and mineral metabolism. Endocrinol Metab Clin North Am. 1990;19(1):35-63. doi: 10.1016/S0889-8529(18)30338-4.
- Liporace FA, Breitbart EA, Yoon RS, Doyle E, Paglia DM, Lin S. The effect of locally delivered recombinant human bone morphogenic protein-2 with hydroxyapatite/tri-calcium phosphate on the biomechanical properties of bone in diabetes-related osteoporosis. J Orthop Traumatol.2015;16(2):151-159. doi: 10.1007/s10195-014-0327-6.
- Ilic K, Obradovic N, Vujasinovic-Stupar N. The relationship among hypertension, antihypertensive medications, and osteoporosis: a narrative review. Calcif. Tissue Int. 2013;92(3):217-227. doi: 10.1007/s00223-012-9671-9.
- Yesil Y, Ulger, Z, Halil M, et al. Coexistence of osteoporosis (OP) and coronary artery disease (CAD) in the elderly: it is not just a by chance event. Arch Gerontol Geriatr. 2012;54(3):473-476. doi: 10.1016/j.archger.2011.06.007.
- Sosa M, Saavedra P, de Tejada MJG, et al, GIUMO Cooperative Group. Beta-blocker use is associated with fragility fractures in postmenopausal women with coronary heart disease. Aging Clin Exp Res.2011;23(3):112-117. doi: 10.3275/7041.
- An T, Hao J, Li R, Yang M, Cheng G, Zou M. Efficacy of statins for osteoporosis: a systematic review and met-analysis. Osteoporos Int. 2017;28(1):47-57. doi: 10.1007/s00198-016-3844-8.
- Munson JC, Bynum JP, Bell J, et al. Patterns of prescription drug use before and after fragility fracture. JAMA Intern Med. 2016;176(10):1531-1538. doi: 10.1001/jamainternmed.2016.4814.
- Saag KG, Agnesdei D, Hans D, et al. Trabecular bone score in patients with chronic glucocorticoid therapy-induced osteoporosis treated with alendronate or teriparatide. Arthritis Rheumatol. 2016;68(9):2122-2128. doi: 10.1002/art.39726.
- Chuang MH, Chuang TL, Koo M, Wang YF. Trabecular bone score reflects trabecular microarchitecture deterioration and fragility fracture in female adult patients receiving glucocorticoid therapy: A pre-post controlled study. BioMed Res Int. 2017. doi: 10.1155/2017/4210217.
- Andersen BN, Johansen PB, Abrahamsen B. Proton pump inhibitors and osteoporosis. Curr Opin Rheumatol. 2016;28(4):420-425. doi: 10.1097/BOR.0000000000000291.
- Jacob L, Hadji P, Kostev K. The use of proton pump inhibitors is positively associated with osteoporosis in postmenopausal women in Germany. Climacteric. 2016; 19(5):478-481. doi: 10.1080/13697137.2016.1200549.
- Targownik LE, Lix LM, Metge CJ, Prior HJ, Leung S, Leslie WD. Use of proton pump inhibitors and risk of osteoporosis-related fracture. Can Med Assoc J. 2008;179:319-326. doi: 10.1503/cmaj.071330.
- Lee RH, Lyles KH, Colon-Emeric C. A review of the effect of anticonvulsant medications on bone mineral density and fracture risk. Am J Geriatr Pharmacother. 2010;8(1):34-46. doi: 10.1016/j.amjopharm.2010.02.003.
- Arora E, Singh H, Gupta YK. Impact of antiepileptic drugs on bone health: Need for monitoring, treatment, and prevention. J Family Med Prim Care. 2016;5(2):248-253. doi: 10.4103/2249-4863.192338.
- Maghraoui AE, Roux C. DXA scanning in clinical practice. Q J Med. 2008;101(8):605-617. doi: 10.1093/qjmed/hcn022.
- Watts NB, Lewiecki EM, Miller PD, Baim S. National osteoporosis foundation 2008 clinician’s guide to prevention and treatment of osteoporosis and the world health organization fracture risk assessment tool (FRAX): What they mean to the bone densiometrist and bone technologist. J Clin Densitom. 2008;11(4):473-477. doi: 10.1016/j.jocd.2008.04.003.
- MacLean C, Newberry S, Maglione M, et al. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med. 2007;148(3):197-213. doi: 10.7326/0003-4819-148-3-200802050-00198.
- Beaton DE, Vidmar M, Pitzul KB, et al. Addition of a fracture risk assessment to a coordinator’s role improved treatment rates within 6 months of screening in a fragility fracture screening program. J Am Geriatr Soc. 2017; 28(3):863-869. doi: 10.1007/s00198-016-3794-1.
- U.S. Preventative Services Task Force. Screening for osteoporosis. Ann Intern Med. 2011;154(5):356-364. doi: 10.7326/0003-4819-154-5-201103010-00307.
- Sunyecz JA. The use of calcium and vitamin D in the management of osteoporosis. Ther Clin Risk Manag. 2008;4(4):827-836.
- Eastell, R. (1998). Treatment of postmenopausal osteoporosis. N Engl J Med. 1998;338:736-746. doi: 10.1056/NEJM199803123381107.
- Cosman F, de Beur SJ, LeBoff MS, et al, National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2381. doi: 10.1007/s00198-014-2794-2.
- Black DM, Schartz AV, Ensrud KE, et al, doi:10.1001/jama.296.24.2927.
- Schmidt GA, Horner KE, McDanel DL, Ross MB, Moores KG. Risks and benefits of long-term bisphosphonate therapy. Am J Health Syst Pharm. 2010;67(12):994-1001. doi: 10.2146/ajhp090506.
- Kraenzlin, ME, Meier C. Parathyroid hormone analogues in the treatment of osteoporosis. Nat Rev Endocrinol. 2011;7(11):647-656. doi: 10.1038/nrendo.2011.108.
- Miller P, Hattersley G, Riis B, et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis. JAMA. 2016;316(7):722-733. doi: 10.1001/jama.2016.11136.
- TYMLOSTM [prescribing information]. Waltham, MA: Radius Health, Inc; 2017.
- Tetsunaga T, Tetsunaga T, Nishida K, et al. Denosumab and alendronate treatment in patients with back pain due to fresh osteoporotic vertebral fractures. J Orthop Sci. 2017;22(2):230-236. doi: 10.1016/j.jos.2016.11.017.
- Recker, RR, Mitlak BH, Ni X, Krege JH. Long-term raloxifene for postmenopausal osteoporosis. Curr Med Res Opin. 2011;27(9):1755-1761. doi: 10.1185/03007995.2011.606312.
- Yildirim K, Gureser G, Karatay S, et al. Comparison of the effects of alendronate, risedronate and calcitonin treatment in postmenopausal osteoporosis. J Back Musculoskelet Rehabil.2005;18(3/4):85-89. doi: 10.3233/BMR-2005-183-405.
- Christensen L, Iqbal S, Macarios D, Badamgarav E, Harley C. Cost of fractures commonly associated with osteoporosis in a managed-care population. J Med Econ. 2010;13(2):302-313. doi: 10.3111/13696998.2010.488969.
ABSTRACT
Fragility fractures are estimated to affect 3 million people annually in the United States. As they are associated with a significant mortality rate, the prevention of these fractures should be a priority for orthopedists. At-risk patients include the elderly and those with thyroid disease, diabetes, hypertension, and heart disease. Osteoporosis is diagnosed by the presence of a fragility fracture or by dual-energy x-ray absorptiometry (DXA) in the absence of a fragility fracture. In 2011, the United States Preventive Services Task Force (USPSTF) recommended that all women ≥65 years should be screened for osteoporosis by DXA. Women <65 years with a 10-year fracture risk =/> than that of a 65-year-old white woman should also be screened for osteoporosis. Lifestyle changes, such as calcium and vitamin D supplementation, exercise, and smoking cessation, are non-pharmacologic treatment options. The National Osteoporosis Foundation recommends treating osteoporosis with pharmacotherapy in patients with a high risk for fracture (T score <–2.5) or history of fragility fracture. Understanding risk factors and eliminating medications known to cause decreased BMD are vital to prevention and will be necessary to limit these fractures and their associated expenses in the future.
Continue to: Fragility fractures are caused by...
Fragility fractures are caused by falls from standing height or repetitive physiological loads.1 With the growing aging population in the United States, it is estimated that 3 million people will be affected by fragility fractures yearly.2 In the setting of osseous insufficiency, fractures that are typically associated with high-energy trauma are encountered in patients who simply trip over a parking lot curb or fall off their bike. After surgery, the severe disruption of patients’ lives continues with a prolonged rehabilitation period.
Fragility fractures are not only traumatizing for patients; they are also associated with significantly increased mortality. A study by Gosch and colleagues found that 70.6% of patients died during the normal follow-up period, and 29.4% of patients died within the first year of suffering a fracture.3 Also, the mean life expectancy post-fragility fracture was only 527 days.3 Diagnosis and treatment of osteoporosis is imperative to prevent fragility fractures before they occur.
RISK FACTORS AND CAUSES
The incidence of fragility fractures increases in patients with comorbidities such as thyroid disease, diabetes, hypertension, and heart disease.4 Hyperthyroidism and treated hypothyroidism cause an imbalance between osteoblast and osteoclast activity, resulting in osteoporosis.5 A thyroid-stimulating hormone level < 0.1 increases the risk of vertebral and non-vertebral fractures by a factor of 4.5 and 3.2 mIU/L respectively.4 Patients with diabetes also have an increased risk of fragility fractures, which is due to impaired healing capabilities, especially that of bone healing. Approximately 2 million people are affected by type 1 diabetes in the United States, and 20% of those patients will develop osteoporosis.6
Hypertension and osteoporosis are 2 diseases that occur often in the elderly. Common etiological factors believed to cause both hypertension and osteoporosis are low calcium intake, high consumption of salt, and vitamin D and vitamin K deficiency. Also, hypertension treated with loop diuretics has been found to cause negative effects on bone and increase the risk of osteoporosis.7 The only antihypertensive medications that preserve bone mineral density (BMD) and reduce fracture risk are thiazide diuretics.7 Lastly, an association between coronary artery disease and osteoporosis has been hypothesized. The link is not completely understood, but it is believed that oxidative stress and inflammation are the culprits in both diseases.8 In contrast to previous hypotheses, Sosa and colleagues found an independent association between beta blockers and fragility fractures.9 The idea that beta blockers and fragility fractures are linked is still controversial and needs more study. Unlike beta blockers, statins provide a protective effect on bone. They increase BMD and reduce fracture risk by inhibiting osteoclastogenesis.10
In addition to loop diuretics and beta blockers, inhaled glucocorticoids, oral glucocorticoids, proton pump inhibitors (PPIs), H2 receptor antagonists, and anticonvulsants decrease bone density and increase the incidence of fragility fractures.11 Chronic glucocorticoid therapy is the most common cause of secondary osteoporosis. Osteoblasts and osteocytes undergo apoptosis in the presence of glucocorticoids.12 Patients on glucocorticoid therapy have an increased risk of fracture, even with higher BMD values.13 Bone changes that occur while a patient is taking glucocorticoids may not be detected during BMD testing. Therefore, a high level of suspicion of osteoporosis in patients on long-term glucocorticoids is imperative.
Proton pump inhibitors are among the most prescribed medications in the world; they reduce bone resorption, increasing the risk of fracture.14 Proton pump inhibitors and H2 receptor antagonists are hypothesized to cause malabsorption of calcium and indirectly cause osteoporosis. The risk of osteoporosis increases with the length of PPI treatment.15 However, exposure lasting <7 years does not increase the risk of fracture.16 It is recommended that patients on long-term PPIs be referred for BMD testing.
An association between anticonvulsants and osteoporosis has been found in observational studies. The mechanism of this association is not yet fully understood, but it is believed that exacerbation of vitamin D deficiency leads to increased bone metabolism.17 Gastrointestinal (GI) calcium absorption also decreases with anticonvulsant use. Prolonged antiepileptic therapy and high-dose therapy rapidly decrease BMD. Primidone, carbamazepine, phenobarbital, and phenytoin are the drugs most often associated with decreased BMD. Osteoporosis and fragility fracture in these patients can be prevented with calcium, vitamin D, and the bisphosphonate risedronate. These medications have been shown to improve BMD by 69%.18
Continue to: DIAGNOSIS...
DIAGNOSIS
Osteoporosis is diagnosed by the presence of a fragility fracture or by dual-energy x-ray absorptiometry (DXA) in the absence of a fragility fracture.19 Measurements of the femoral neck by DXA are used to diagnose osteoporosis, although DXA can also be used to measure the bone density of the spine and peripheral skeleton.20
The World Health Organization developed a set of T score criteria to diagnose osteoporosis in postmenopausal women (Table 1). A T score >-1 is normal, <-1 but >-2.5 signifies osteopenia, <-2.5 is osteoporosis, and <-2.5 with fragility fracture is severe osteoporosis.19 The Z score, not the T score, should be used to assess osteoporosis in premenopausal women, men <50 years, and children (Table 2). The Z score is calculated by comparing the patient’s BMD with the mean BMD of their peers of a similar age, race, and gender.19 Z scores <-2.0 indicate low BMD for chronological age. A Z score > -2.0 is considered within the expected range for age.20 Bone mineral density testing is the rate- limiting step to starting osteoporosis treatment.21 Without testing, treatment of osteoporosis is very unlikely.
Table 1. T Score Criteria
T score | Diagnosis |
> -1.0 | Normal |
-1.0 to -2.5 | Osteopenia |
< -2.5 | Osteoporosis |
< -2.5 with fragility fracture | Severe osteoporosis |
Table 2. Z Score Criteria
Z score | Diagnosis |
> -2.0 | Normal BMD for age |
< -2.0 | Low BMD for age |
The World Health Organization also developed a tool to predict fracture risk. The Fracture Risk Assessment Tool uses fracture history in addition to other risk factors to predict a patient’s 10-year risk of major fracture.22 Risk factors used to assess fracture risk include age, sex, weight, height, previous fracture, parental hip fracture history, current smoker, glucocorticoid use, rheumatoid arthritis, secondary osteoporosis, excessive alcohol use, and femoral neck BMD.
In 2011, the United States Preventive Services Task Force (USPSTF) recommended that all women ≥65 years should be screened for osteoporosis by DXA. Women <65 years with a 10-year fracture risk =/> than that of a 65-year-old white woman should also be screened for osteoporosis. These recommendations are different for men. It was concluded that the evidence was insufficient to support osteoporosis screening in men.23 As of April 2017, Centers for Medicare and Medicaid Services current reimbursement rates for DXA scans are, on average, $123.10 in the hospital setting and $41.63 in the office setting. The axial DXA CPT code is 77080.
Continue to: TREATMENT...
TREATMENT
NONPHARMACOLOGIC
Patients with mild osteoporosis may be treated first non-pharmacologically. Lifestyle changes such as calcium and vitamin D supplementation, exercise, and smoking cessation are non-pharmacologic treatment options. Calcium carbonate and calcium citrate are common supplements. Calcium carbonate is 40% elemental calcium, whereas calcium citrate supplements are only 21% elemental calcium. Calcium supplements are best absorbed when taken with food.24 The recommended daily total calcium intake is 1200 mg.25 Only 500 to 600 milligrams of calcium can be absorbed by the GI tract at a time. Therefore, calcium supplements should be taken at least 4 to 5 hours apart.24Patients should also be counseled that calcium supplements may cause GI side effects such as bloating and constipation. To reduce side effects, patients can slowly increase the dose of calcium to a therapeutic level.
Vitamin D supplementation works best in conjunction with calcium supplementation. Vitamin D functions to regulate calcium absorption in the intestine and stimulate bone resorption and maintain the serum calcium concentration. The National Osteoporosis Foundation recommends 800 to 1000 international units of vitamin D daily.24 Lifestyle changes may be sufficient to stop the progression of osteoporosis in its early stages. Once osteoporosis becomes severe enough, pharmacotherapy is needed to stop further bone destruction and improve BMD.
PHARMACOLOGIC
After an initial fragility fracture, the risk of additional ones increases significantly, making treatment of osteoporosis essential. The National Osteoporosis Foundation recommends treating osteoporosis with pharmacotherapy in patients with a high risk of fracture (T score <-2.5) or history of fragility fracture.26 Bisphosphonates inhibit bone resorption and are considered the first-line therapy for postmenopausal women with osteoporosis. A common side effect of oral bisphosphonates is GI toxicity. Patients are advised to avoid lying down for at least 30 minutes after medication administration to avoid esophageal irritation. Oral bisphosphonates should also be taken in the morning on an empty stomach with at least 8 ounces of water. Recurrent bisphosphonate use should be avoided in patients with chronic kidney disease. Oral alendronate and risedronate are typically discontinued after 5 years of use.27 Long-term bisphosphonate use may cause an increased risk of fragility fracture due to oversuppression of bone turnover. To avoid this risk, bisphosphonate “drug holidays” are an option. Bisphosphonates accumulate over time, creating reservoirs. Even after therapy is stopped, patients continue to have therapeutic effects for 2 to 5 years.28
Bisphosphonates are available in both oral and intravenous forms. Alendronate is available in doses of 10 mg and 70 mg for daily and weekly administration, respectively. Both are available in tablet form, but the 70 mg weekly dose is also available in a dissolvable formulation. Alendronate is available in a reduced dose for osteoporosis prevention. Alendronate dosing for osteoporosis prevention is 5 mg daily or 35 mg weekly. Risedronate is dosed as 5 mg daily, 35 mg weekly, or 150 mg monthly. Intravenous bisphosphonates are indicated when oral bisphosphonates are not tolerated, only after vitamin D has been assessed and is within the normal range. Zoledronic acid is administered as a 15-minute infusion once a year.
Teriparatide (Forteo; PTH-1-34) is available for glucocorticoid-induced osteoporosis, postmenopausal women, and men with severe osteoporosis. It is indicated for patients in whom bisphosphonate treatment has failed or those who do not tolerate bisphosphonates. Teriparatide is a synthetic parathyroid hormone (PTH) that acts as an anabolic agent, stimulating bone formation, maturation, and remodeling.29 In addition to its application as a bone-building hormone, teriparatide has gained popularity for various off-label uses. These include accelerated osteosynthesis, stress fracture healing, and in the nonoperative treatment of osteoarthritis.29 Parathyroid hormone has been shown to stimulate the maturation, proliferation, and maintenance of osteoblast progenitor cells. More recently, PTH has been shown to regulate chondrocyte signaling, as well as differentiation and maturation. Further study on the chondroregenerative potential of PTH has demonstrated its efficacy as a novel disease-modifying agent in the treatment of osteoarthritis.29 Teriparatide is administered as a daily subcutaneous injection. The United States dosing is 600 mcg/2.4 mL. Adverse effects such as orthostatic hypotension and osteosarcoma may occur. BMD testing should be performed 1 to 2 years after initiation of teriparatide and every 2 years thereafter.26
Abaloparatide (Tymlos), a human parathyroid hormone, is another treatment option for postmenopausal women at risk of osteoporotic fracture. In a study comparing the efficacy of abaloparatide and teriparatide, treatment with abaloparatide was found to induce higher BMD levels in a time frame of 12 months. The BMD differences could be attributed to many factors, such as an enhanced net anabolic effect or a reduced osteoblast expression. Furthermore, the risk of developing new vertebral and nonvertebral fractures decreased in the abaloparatide group compared with the placebo group over a period of 18 months.30
Continue to: The recommended daily dose for abaloparatide...
The recommended daily dose for abaloparatide is 80 mcg via subcutaneous injection with calcium and vitamin D supplements.31 Adverse reactions were consistent between abaloparatide and teriparatide, and included hypercalcemia, hypercalciuria, and orthostatic hypotension.30 The use of parathyroid analogs for >2 years is not recommended due to the risk of osteosarcoma.
Denosumab (Prolia) is a monoclonal antibody that stops osteoclastogenesis by blocking the binding of RANKL to RANK.31 It is indicated for patients intolerant to bisphosphonates or with impaired kidney function. Prolia is administered subcutaneously in 60 mg doses every 6 months in men and postmenopausal women with osteoporosis. Prolia is contraindicated in patients with hypersensitivity to any component of the medication, pregnancy, and hypocalcemia.
Selective estrogen receptor modulators (SERMs), such as raloxifene and tamoxifen, can treat osteoporosis effectively in postmenopausal women. Raloxifene is considered the SERM of choice due to the availability of more robust safety and efficacy data. Raloxifene increases BMD while decreasing bone resorption and bone turnover.32 It is also used to reduce breast cancer risk; however, it increases the risk of thromboembolic events and hot flashes. Tamoxifen is not typically used to treat osteoporosis, but women treated for breast cancer with tamoxifen receive some bone protection.
Lastly, calcitonin and strontium ranelate are also options to treat osteoporosis. However, both calcitonin and strontium ranelate have weak effects on BMD. Calcitonin only transiently inhibits osteoclast activity.33 Therefore, medications like bisphosphonates, teriparatide, denosumab, and SERMs are preferred.
A summary of medications used to treat osteoporosis can be found in Table 3.
Table 3. Overview of Common Medications Used in the Treatment and Prevention of Osteoporosis
Medication | Indication | Dosing |
Calcium supplementation | Mild osteoporosis | 1200 mg oral/d |
Vitamin D supplementation | Mild osteoporosis | 800 to 1000 IU oral/d |
Alendronate | Postmenopausal osteoporosis
Osteoporosis prevention | 10 mg oral/d 70 mg oral/wk
5 mg/d 35 mg/wk |
Risedronate | Postmenopausal osteoporosis | 5 mg oral/d 35 mg oral/wk 150 mg oral/mo |
Teriparatide (Forteo) | Glucocorticoid-inducted osteoporosis, postmenopausal osteoporosis, men with severe osteoporosis | 600 mcg/2.4 mL subcutaneous/d |
Abaloparatide (Tymlos) | Postmenopausal osteoporosis | 80 mcg subcutaneous/d |
Denosumab (Prolia) | Patients intolerant to bisphosphonates; patients with impaired kidney function. | 60 mg subcutaneous every 6 mo |
Raloxifene | Postmenopausal osteoporosis | 60 mg oral/d |
Tamoxifen | Postmenopausal osteoporosis | 20 mg oral/d |
Calcitonin | Postmenopausal osteoporosis | 100 units intramuscular or subcutaneous/d 200 units (1 spray) intranasal/d |
Strontium ranelate | Postmenopausal osteoporosis Severe osteoporosis in men | 2 g/d dissolved in water, prior to bedtime Not recommended in CrCl <30 mL/min |
Abbreviation: CrCl, creatinine clearance.
CONCLUSION
With a growing aging population, the prevalence of osteoporosis is expected to increase. By 2025, experts estimate that there will be 2 million fractures yearly, costing the United States upwards of $25 billion.34,35 This estimate does not include the cost of lost productivity or disability, which will likely cost billions more.34,35 Understanding risk factors and eliminating medications known to cause decreased BMD are vital. Obtaining a BMD measurement is the rate-limiting step for treatment initiation. Without an appropriate diagnosis, treatment is unlikely. As providers, it us our responsibility to maintain a high level of suspicion of osteoporosis in the elderly and promptly diagnose and treat them.
ABSTRACT
Fragility fractures are estimated to affect 3 million people annually in the United States. As they are associated with a significant mortality rate, the prevention of these fractures should be a priority for orthopedists. At-risk patients include the elderly and those with thyroid disease, diabetes, hypertension, and heart disease. Osteoporosis is diagnosed by the presence of a fragility fracture or by dual-energy x-ray absorptiometry (DXA) in the absence of a fragility fracture. In 2011, the United States Preventive Services Task Force (USPSTF) recommended that all women ≥65 years should be screened for osteoporosis by DXA. Women <65 years with a 10-year fracture risk =/> than that of a 65-year-old white woman should also be screened for osteoporosis. Lifestyle changes, such as calcium and vitamin D supplementation, exercise, and smoking cessation, are non-pharmacologic treatment options. The National Osteoporosis Foundation recommends treating osteoporosis with pharmacotherapy in patients with a high risk for fracture (T score <–2.5) or history of fragility fracture. Understanding risk factors and eliminating medications known to cause decreased BMD are vital to prevention and will be necessary to limit these fractures and their associated expenses in the future.
Continue to: Fragility fractures are caused by...
Fragility fractures are caused by falls from standing height or repetitive physiological loads.1 With the growing aging population in the United States, it is estimated that 3 million people will be affected by fragility fractures yearly.2 In the setting of osseous insufficiency, fractures that are typically associated with high-energy trauma are encountered in patients who simply trip over a parking lot curb or fall off their bike. After surgery, the severe disruption of patients’ lives continues with a prolonged rehabilitation period.
Fragility fractures are not only traumatizing for patients; they are also associated with significantly increased mortality. A study by Gosch and colleagues found that 70.6% of patients died during the normal follow-up period, and 29.4% of patients died within the first year of suffering a fracture.3 Also, the mean life expectancy post-fragility fracture was only 527 days.3 Diagnosis and treatment of osteoporosis is imperative to prevent fragility fractures before they occur.
RISK FACTORS AND CAUSES
The incidence of fragility fractures increases in patients with comorbidities such as thyroid disease, diabetes, hypertension, and heart disease.4 Hyperthyroidism and treated hypothyroidism cause an imbalance between osteoblast and osteoclast activity, resulting in osteoporosis.5 A thyroid-stimulating hormone level < 0.1 increases the risk of vertebral and non-vertebral fractures by a factor of 4.5 and 3.2 mIU/L respectively.4 Patients with diabetes also have an increased risk of fragility fractures, which is due to impaired healing capabilities, especially that of bone healing. Approximately 2 million people are affected by type 1 diabetes in the United States, and 20% of those patients will develop osteoporosis.6
Hypertension and osteoporosis are 2 diseases that occur often in the elderly. Common etiological factors believed to cause both hypertension and osteoporosis are low calcium intake, high consumption of salt, and vitamin D and vitamin K deficiency. Also, hypertension treated with loop diuretics has been found to cause negative effects on bone and increase the risk of osteoporosis.7 The only antihypertensive medications that preserve bone mineral density (BMD) and reduce fracture risk are thiazide diuretics.7 Lastly, an association between coronary artery disease and osteoporosis has been hypothesized. The link is not completely understood, but it is believed that oxidative stress and inflammation are the culprits in both diseases.8 In contrast to previous hypotheses, Sosa and colleagues found an independent association between beta blockers and fragility fractures.9 The idea that beta blockers and fragility fractures are linked is still controversial and needs more study. Unlike beta blockers, statins provide a protective effect on bone. They increase BMD and reduce fracture risk by inhibiting osteoclastogenesis.10
In addition to loop diuretics and beta blockers, inhaled glucocorticoids, oral glucocorticoids, proton pump inhibitors (PPIs), H2 receptor antagonists, and anticonvulsants decrease bone density and increase the incidence of fragility fractures.11 Chronic glucocorticoid therapy is the most common cause of secondary osteoporosis. Osteoblasts and osteocytes undergo apoptosis in the presence of glucocorticoids.12 Patients on glucocorticoid therapy have an increased risk of fracture, even with higher BMD values.13 Bone changes that occur while a patient is taking glucocorticoids may not be detected during BMD testing. Therefore, a high level of suspicion of osteoporosis in patients on long-term glucocorticoids is imperative.
Proton pump inhibitors are among the most prescribed medications in the world; they reduce bone resorption, increasing the risk of fracture.14 Proton pump inhibitors and H2 receptor antagonists are hypothesized to cause malabsorption of calcium and indirectly cause osteoporosis. The risk of osteoporosis increases with the length of PPI treatment.15 However, exposure lasting <7 years does not increase the risk of fracture.16 It is recommended that patients on long-term PPIs be referred for BMD testing.
An association between anticonvulsants and osteoporosis has been found in observational studies. The mechanism of this association is not yet fully understood, but it is believed that exacerbation of vitamin D deficiency leads to increased bone metabolism.17 Gastrointestinal (GI) calcium absorption also decreases with anticonvulsant use. Prolonged antiepileptic therapy and high-dose therapy rapidly decrease BMD. Primidone, carbamazepine, phenobarbital, and phenytoin are the drugs most often associated with decreased BMD. Osteoporosis and fragility fracture in these patients can be prevented with calcium, vitamin D, and the bisphosphonate risedronate. These medications have been shown to improve BMD by 69%.18
Continue to: DIAGNOSIS...
DIAGNOSIS
Osteoporosis is diagnosed by the presence of a fragility fracture or by dual-energy x-ray absorptiometry (DXA) in the absence of a fragility fracture.19 Measurements of the femoral neck by DXA are used to diagnose osteoporosis, although DXA can also be used to measure the bone density of the spine and peripheral skeleton.20
The World Health Organization developed a set of T score criteria to diagnose osteoporosis in postmenopausal women (Table 1). A T score >-1 is normal, <-1 but >-2.5 signifies osteopenia, <-2.5 is osteoporosis, and <-2.5 with fragility fracture is severe osteoporosis.19 The Z score, not the T score, should be used to assess osteoporosis in premenopausal women, men <50 years, and children (Table 2). The Z score is calculated by comparing the patient’s BMD with the mean BMD of their peers of a similar age, race, and gender.19 Z scores <-2.0 indicate low BMD for chronological age. A Z score > -2.0 is considered within the expected range for age.20 Bone mineral density testing is the rate- limiting step to starting osteoporosis treatment.21 Without testing, treatment of osteoporosis is very unlikely.
Table 1. T Score Criteria
T score | Diagnosis |
> -1.0 | Normal |
-1.0 to -2.5 | Osteopenia |
< -2.5 | Osteoporosis |
< -2.5 with fragility fracture | Severe osteoporosis |
Table 2. Z Score Criteria
Z score | Diagnosis |
> -2.0 | Normal BMD for age |
< -2.0 | Low BMD for age |
The World Health Organization also developed a tool to predict fracture risk. The Fracture Risk Assessment Tool uses fracture history in addition to other risk factors to predict a patient’s 10-year risk of major fracture.22 Risk factors used to assess fracture risk include age, sex, weight, height, previous fracture, parental hip fracture history, current smoker, glucocorticoid use, rheumatoid arthritis, secondary osteoporosis, excessive alcohol use, and femoral neck BMD.
In 2011, the United States Preventive Services Task Force (USPSTF) recommended that all women ≥65 years should be screened for osteoporosis by DXA. Women <65 years with a 10-year fracture risk =/> than that of a 65-year-old white woman should also be screened for osteoporosis. These recommendations are different for men. It was concluded that the evidence was insufficient to support osteoporosis screening in men.23 As of April 2017, Centers for Medicare and Medicaid Services current reimbursement rates for DXA scans are, on average, $123.10 in the hospital setting and $41.63 in the office setting. The axial DXA CPT code is 77080.
Continue to: TREATMENT...
TREATMENT
NONPHARMACOLOGIC
Patients with mild osteoporosis may be treated first non-pharmacologically. Lifestyle changes such as calcium and vitamin D supplementation, exercise, and smoking cessation are non-pharmacologic treatment options. Calcium carbonate and calcium citrate are common supplements. Calcium carbonate is 40% elemental calcium, whereas calcium citrate supplements are only 21% elemental calcium. Calcium supplements are best absorbed when taken with food.24 The recommended daily total calcium intake is 1200 mg.25 Only 500 to 600 milligrams of calcium can be absorbed by the GI tract at a time. Therefore, calcium supplements should be taken at least 4 to 5 hours apart.24Patients should also be counseled that calcium supplements may cause GI side effects such as bloating and constipation. To reduce side effects, patients can slowly increase the dose of calcium to a therapeutic level.
Vitamin D supplementation works best in conjunction with calcium supplementation. Vitamin D functions to regulate calcium absorption in the intestine and stimulate bone resorption and maintain the serum calcium concentration. The National Osteoporosis Foundation recommends 800 to 1000 international units of vitamin D daily.24 Lifestyle changes may be sufficient to stop the progression of osteoporosis in its early stages. Once osteoporosis becomes severe enough, pharmacotherapy is needed to stop further bone destruction and improve BMD.
PHARMACOLOGIC
After an initial fragility fracture, the risk of additional ones increases significantly, making treatment of osteoporosis essential. The National Osteoporosis Foundation recommends treating osteoporosis with pharmacotherapy in patients with a high risk of fracture (T score <-2.5) or history of fragility fracture.26 Bisphosphonates inhibit bone resorption and are considered the first-line therapy for postmenopausal women with osteoporosis. A common side effect of oral bisphosphonates is GI toxicity. Patients are advised to avoid lying down for at least 30 minutes after medication administration to avoid esophageal irritation. Oral bisphosphonates should also be taken in the morning on an empty stomach with at least 8 ounces of water. Recurrent bisphosphonate use should be avoided in patients with chronic kidney disease. Oral alendronate and risedronate are typically discontinued after 5 years of use.27 Long-term bisphosphonate use may cause an increased risk of fragility fracture due to oversuppression of bone turnover. To avoid this risk, bisphosphonate “drug holidays” are an option. Bisphosphonates accumulate over time, creating reservoirs. Even after therapy is stopped, patients continue to have therapeutic effects for 2 to 5 years.28
Bisphosphonates are available in both oral and intravenous forms. Alendronate is available in doses of 10 mg and 70 mg for daily and weekly administration, respectively. Both are available in tablet form, but the 70 mg weekly dose is also available in a dissolvable formulation. Alendronate is available in a reduced dose for osteoporosis prevention. Alendronate dosing for osteoporosis prevention is 5 mg daily or 35 mg weekly. Risedronate is dosed as 5 mg daily, 35 mg weekly, or 150 mg monthly. Intravenous bisphosphonates are indicated when oral bisphosphonates are not tolerated, only after vitamin D has been assessed and is within the normal range. Zoledronic acid is administered as a 15-minute infusion once a year.
Teriparatide (Forteo; PTH-1-34) is available for glucocorticoid-induced osteoporosis, postmenopausal women, and men with severe osteoporosis. It is indicated for patients in whom bisphosphonate treatment has failed or those who do not tolerate bisphosphonates. Teriparatide is a synthetic parathyroid hormone (PTH) that acts as an anabolic agent, stimulating bone formation, maturation, and remodeling.29 In addition to its application as a bone-building hormone, teriparatide has gained popularity for various off-label uses. These include accelerated osteosynthesis, stress fracture healing, and in the nonoperative treatment of osteoarthritis.29 Parathyroid hormone has been shown to stimulate the maturation, proliferation, and maintenance of osteoblast progenitor cells. More recently, PTH has been shown to regulate chondrocyte signaling, as well as differentiation and maturation. Further study on the chondroregenerative potential of PTH has demonstrated its efficacy as a novel disease-modifying agent in the treatment of osteoarthritis.29 Teriparatide is administered as a daily subcutaneous injection. The United States dosing is 600 mcg/2.4 mL. Adverse effects such as orthostatic hypotension and osteosarcoma may occur. BMD testing should be performed 1 to 2 years after initiation of teriparatide and every 2 years thereafter.26
Abaloparatide (Tymlos), a human parathyroid hormone, is another treatment option for postmenopausal women at risk of osteoporotic fracture. In a study comparing the efficacy of abaloparatide and teriparatide, treatment with abaloparatide was found to induce higher BMD levels in a time frame of 12 months. The BMD differences could be attributed to many factors, such as an enhanced net anabolic effect or a reduced osteoblast expression. Furthermore, the risk of developing new vertebral and nonvertebral fractures decreased in the abaloparatide group compared with the placebo group over a period of 18 months.30
Continue to: The recommended daily dose for abaloparatide...
The recommended daily dose for abaloparatide is 80 mcg via subcutaneous injection with calcium and vitamin D supplements.31 Adverse reactions were consistent between abaloparatide and teriparatide, and included hypercalcemia, hypercalciuria, and orthostatic hypotension.30 The use of parathyroid analogs for >2 years is not recommended due to the risk of osteosarcoma.
Denosumab (Prolia) is a monoclonal antibody that stops osteoclastogenesis by blocking the binding of RANKL to RANK.31 It is indicated for patients intolerant to bisphosphonates or with impaired kidney function. Prolia is administered subcutaneously in 60 mg doses every 6 months in men and postmenopausal women with osteoporosis. Prolia is contraindicated in patients with hypersensitivity to any component of the medication, pregnancy, and hypocalcemia.
Selective estrogen receptor modulators (SERMs), such as raloxifene and tamoxifen, can treat osteoporosis effectively in postmenopausal women. Raloxifene is considered the SERM of choice due to the availability of more robust safety and efficacy data. Raloxifene increases BMD while decreasing bone resorption and bone turnover.32 It is also used to reduce breast cancer risk; however, it increases the risk of thromboembolic events and hot flashes. Tamoxifen is not typically used to treat osteoporosis, but women treated for breast cancer with tamoxifen receive some bone protection.
Lastly, calcitonin and strontium ranelate are also options to treat osteoporosis. However, both calcitonin and strontium ranelate have weak effects on BMD. Calcitonin only transiently inhibits osteoclast activity.33 Therefore, medications like bisphosphonates, teriparatide, denosumab, and SERMs are preferred.
A summary of medications used to treat osteoporosis can be found in Table 3.
Table 3. Overview of Common Medications Used in the Treatment and Prevention of Osteoporosis
Medication | Indication | Dosing |
Calcium supplementation | Mild osteoporosis | 1200 mg oral/d |
Vitamin D supplementation | Mild osteoporosis | 800 to 1000 IU oral/d |
Alendronate | Postmenopausal osteoporosis
Osteoporosis prevention | 10 mg oral/d 70 mg oral/wk
5 mg/d 35 mg/wk |
Risedronate | Postmenopausal osteoporosis | 5 mg oral/d 35 mg oral/wk 150 mg oral/mo |
Teriparatide (Forteo) | Glucocorticoid-inducted osteoporosis, postmenopausal osteoporosis, men with severe osteoporosis | 600 mcg/2.4 mL subcutaneous/d |
Abaloparatide (Tymlos) | Postmenopausal osteoporosis | 80 mcg subcutaneous/d |
Denosumab (Prolia) | Patients intolerant to bisphosphonates; patients with impaired kidney function. | 60 mg subcutaneous every 6 mo |
Raloxifene | Postmenopausal osteoporosis | 60 mg oral/d |
Tamoxifen | Postmenopausal osteoporosis | 20 mg oral/d |
Calcitonin | Postmenopausal osteoporosis | 100 units intramuscular or subcutaneous/d 200 units (1 spray) intranasal/d |
Strontium ranelate | Postmenopausal osteoporosis Severe osteoporosis in men | 2 g/d dissolved in water, prior to bedtime Not recommended in CrCl <30 mL/min |
Abbreviation: CrCl, creatinine clearance.
CONCLUSION
With a growing aging population, the prevalence of osteoporosis is expected to increase. By 2025, experts estimate that there will be 2 million fractures yearly, costing the United States upwards of $25 billion.34,35 This estimate does not include the cost of lost productivity or disability, which will likely cost billions more.34,35 Understanding risk factors and eliminating medications known to cause decreased BMD are vital. Obtaining a BMD measurement is the rate-limiting step for treatment initiation. Without an appropriate diagnosis, treatment is unlikely. As providers, it us our responsibility to maintain a high level of suspicion of osteoporosis in the elderly and promptly diagnose and treat them.
- Dietz SO, Hofmann A, Rommens PM. Haemorrhage in fragility fractures of the pelvis. Eur J Trauma Emerg Surg. 2015;41:363-367. doi: 10.1007/s00068-014-0452-1
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465-475. doi: 10.1359/jbmr.061113.
- Gosch M, Hoffmann-Weltin Y, Roth T, Blauth M, Nicholas JA, Kammerlander C. Orthogeriatric co-management improves the outcome of long-term care residents with fragility fractures. Arch Orthop Trauma Surg. 2016; 136(10):1403-1409. doi: 10.1007/s00402-016-2543-4.
- Maccagnano G, Notarnicola A, Pesce V, Mudoni S, Tafuri S, Moretti B. The prevalence of fragility fractures in a population of a region of southern Italy affected by thyroid disorders. BioMed Res Int. 2016. doi: 10.1155/2016/6017165.
- Mosekilde L, Eriksen EF, Charles P. Effects of thyroid hormones on bone and mineral metabolism. Endocrinol Metab Clin North Am. 1990;19(1):35-63. doi: 10.1016/S0889-8529(18)30338-4.
- Liporace FA, Breitbart EA, Yoon RS, Doyle E, Paglia DM, Lin S. The effect of locally delivered recombinant human bone morphogenic protein-2 with hydroxyapatite/tri-calcium phosphate on the biomechanical properties of bone in diabetes-related osteoporosis. J Orthop Traumatol.2015;16(2):151-159. doi: 10.1007/s10195-014-0327-6.
- Ilic K, Obradovic N, Vujasinovic-Stupar N. The relationship among hypertension, antihypertensive medications, and osteoporosis: a narrative review. Calcif. Tissue Int. 2013;92(3):217-227. doi: 10.1007/s00223-012-9671-9.
- Yesil Y, Ulger, Z, Halil M, et al. Coexistence of osteoporosis (OP) and coronary artery disease (CAD) in the elderly: it is not just a by chance event. Arch Gerontol Geriatr. 2012;54(3):473-476. doi: 10.1016/j.archger.2011.06.007.
- Sosa M, Saavedra P, de Tejada MJG, et al, GIUMO Cooperative Group. Beta-blocker use is associated with fragility fractures in postmenopausal women with coronary heart disease. Aging Clin Exp Res.2011;23(3):112-117. doi: 10.3275/7041.
- An T, Hao J, Li R, Yang M, Cheng G, Zou M. Efficacy of statins for osteoporosis: a systematic review and met-analysis. Osteoporos Int. 2017;28(1):47-57. doi: 10.1007/s00198-016-3844-8.
- Munson JC, Bynum JP, Bell J, et al. Patterns of prescription drug use before and after fragility fracture. JAMA Intern Med. 2016;176(10):1531-1538. doi: 10.1001/jamainternmed.2016.4814.
- Saag KG, Agnesdei D, Hans D, et al. Trabecular bone score in patients with chronic glucocorticoid therapy-induced osteoporosis treated with alendronate or teriparatide. Arthritis Rheumatol. 2016;68(9):2122-2128. doi: 10.1002/art.39726.
- Chuang MH, Chuang TL, Koo M, Wang YF. Trabecular bone score reflects trabecular microarchitecture deterioration and fragility fracture in female adult patients receiving glucocorticoid therapy: A pre-post controlled study. BioMed Res Int. 2017. doi: 10.1155/2017/4210217.
- Andersen BN, Johansen PB, Abrahamsen B. Proton pump inhibitors and osteoporosis. Curr Opin Rheumatol. 2016;28(4):420-425. doi: 10.1097/BOR.0000000000000291.
- Jacob L, Hadji P, Kostev K. The use of proton pump inhibitors is positively associated with osteoporosis in postmenopausal women in Germany. Climacteric. 2016; 19(5):478-481. doi: 10.1080/13697137.2016.1200549.
- Targownik LE, Lix LM, Metge CJ, Prior HJ, Leung S, Leslie WD. Use of proton pump inhibitors and risk of osteoporosis-related fracture. Can Med Assoc J. 2008;179:319-326. doi: 10.1503/cmaj.071330.
- Lee RH, Lyles KH, Colon-Emeric C. A review of the effect of anticonvulsant medications on bone mineral density and fracture risk. Am J Geriatr Pharmacother. 2010;8(1):34-46. doi: 10.1016/j.amjopharm.2010.02.003.
- Arora E, Singh H, Gupta YK. Impact of antiepileptic drugs on bone health: Need for monitoring, treatment, and prevention. J Family Med Prim Care. 2016;5(2):248-253. doi: 10.4103/2249-4863.192338.
- Maghraoui AE, Roux C. DXA scanning in clinical practice. Q J Med. 2008;101(8):605-617. doi: 10.1093/qjmed/hcn022.
- Watts NB, Lewiecki EM, Miller PD, Baim S. National osteoporosis foundation 2008 clinician’s guide to prevention and treatment of osteoporosis and the world health organization fracture risk assessment tool (FRAX): What they mean to the bone densiometrist and bone technologist. J Clin Densitom. 2008;11(4):473-477. doi: 10.1016/j.jocd.2008.04.003.
- MacLean C, Newberry S, Maglione M, et al. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med. 2007;148(3):197-213. doi: 10.7326/0003-4819-148-3-200802050-00198.
- Beaton DE, Vidmar M, Pitzul KB, et al. Addition of a fracture risk assessment to a coordinator’s role improved treatment rates within 6 months of screening in a fragility fracture screening program. J Am Geriatr Soc. 2017; 28(3):863-869. doi: 10.1007/s00198-016-3794-1.
- U.S. Preventative Services Task Force. Screening for osteoporosis. Ann Intern Med. 2011;154(5):356-364. doi: 10.7326/0003-4819-154-5-201103010-00307.
- Sunyecz JA. The use of calcium and vitamin D in the management of osteoporosis. Ther Clin Risk Manag. 2008;4(4):827-836.
- Eastell, R. (1998). Treatment of postmenopausal osteoporosis. N Engl J Med. 1998;338:736-746. doi: 10.1056/NEJM199803123381107.
- Cosman F, de Beur SJ, LeBoff MS, et al, National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2381. doi: 10.1007/s00198-014-2794-2.
- Black DM, Schartz AV, Ensrud KE, et al, doi:10.1001/jama.296.24.2927.
- Schmidt GA, Horner KE, McDanel DL, Ross MB, Moores KG. Risks and benefits of long-term bisphosphonate therapy. Am J Health Syst Pharm. 2010;67(12):994-1001. doi: 10.2146/ajhp090506.
- Kraenzlin, ME, Meier C. Parathyroid hormone analogues in the treatment of osteoporosis. Nat Rev Endocrinol. 2011;7(11):647-656. doi: 10.1038/nrendo.2011.108.
- Miller P, Hattersley G, Riis B, et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis. JAMA. 2016;316(7):722-733. doi: 10.1001/jama.2016.11136.
- TYMLOSTM [prescribing information]. Waltham, MA: Radius Health, Inc; 2017.
- Tetsunaga T, Tetsunaga T, Nishida K, et al. Denosumab and alendronate treatment in patients with back pain due to fresh osteoporotic vertebral fractures. J Orthop Sci. 2017;22(2):230-236. doi: 10.1016/j.jos.2016.11.017.
- Recker, RR, Mitlak BH, Ni X, Krege JH. Long-term raloxifene for postmenopausal osteoporosis. Curr Med Res Opin. 2011;27(9):1755-1761. doi: 10.1185/03007995.2011.606312.
- Yildirim K, Gureser G, Karatay S, et al. Comparison of the effects of alendronate, risedronate and calcitonin treatment in postmenopausal osteoporosis. J Back Musculoskelet Rehabil.2005;18(3/4):85-89. doi: 10.3233/BMR-2005-183-405.
- Christensen L, Iqbal S, Macarios D, Badamgarav E, Harley C. Cost of fractures commonly associated with osteoporosis in a managed-care population. J Med Econ. 2010;13(2):302-313. doi: 10.3111/13696998.2010.488969.
- Dietz SO, Hofmann A, Rommens PM. Haemorrhage in fragility fractures of the pelvis. Eur J Trauma Emerg Surg. 2015;41:363-367. doi: 10.1007/s00068-014-0452-1
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465-475. doi: 10.1359/jbmr.061113.
- Gosch M, Hoffmann-Weltin Y, Roth T, Blauth M, Nicholas JA, Kammerlander C. Orthogeriatric co-management improves the outcome of long-term care residents with fragility fractures. Arch Orthop Trauma Surg. 2016; 136(10):1403-1409. doi: 10.1007/s00402-016-2543-4.
- Maccagnano G, Notarnicola A, Pesce V, Mudoni S, Tafuri S, Moretti B. The prevalence of fragility fractures in a population of a region of southern Italy affected by thyroid disorders. BioMed Res Int. 2016. doi: 10.1155/2016/6017165.
- Mosekilde L, Eriksen EF, Charles P. Effects of thyroid hormones on bone and mineral metabolism. Endocrinol Metab Clin North Am. 1990;19(1):35-63. doi: 10.1016/S0889-8529(18)30338-4.
- Liporace FA, Breitbart EA, Yoon RS, Doyle E, Paglia DM, Lin S. The effect of locally delivered recombinant human bone morphogenic protein-2 with hydroxyapatite/tri-calcium phosphate on the biomechanical properties of bone in diabetes-related osteoporosis. J Orthop Traumatol.2015;16(2):151-159. doi: 10.1007/s10195-014-0327-6.
- Ilic K, Obradovic N, Vujasinovic-Stupar N. The relationship among hypertension, antihypertensive medications, and osteoporosis: a narrative review. Calcif. Tissue Int. 2013;92(3):217-227. doi: 10.1007/s00223-012-9671-9.
- Yesil Y, Ulger, Z, Halil M, et al. Coexistence of osteoporosis (OP) and coronary artery disease (CAD) in the elderly: it is not just a by chance event. Arch Gerontol Geriatr. 2012;54(3):473-476. doi: 10.1016/j.archger.2011.06.007.
- Sosa M, Saavedra P, de Tejada MJG, et al, GIUMO Cooperative Group. Beta-blocker use is associated with fragility fractures in postmenopausal women with coronary heart disease. Aging Clin Exp Res.2011;23(3):112-117. doi: 10.3275/7041.
- An T, Hao J, Li R, Yang M, Cheng G, Zou M. Efficacy of statins for osteoporosis: a systematic review and met-analysis. Osteoporos Int. 2017;28(1):47-57. doi: 10.1007/s00198-016-3844-8.
- Munson JC, Bynum JP, Bell J, et al. Patterns of prescription drug use before and after fragility fracture. JAMA Intern Med. 2016;176(10):1531-1538. doi: 10.1001/jamainternmed.2016.4814.
- Saag KG, Agnesdei D, Hans D, et al. Trabecular bone score in patients with chronic glucocorticoid therapy-induced osteoporosis treated with alendronate or teriparatide. Arthritis Rheumatol. 2016;68(9):2122-2128. doi: 10.1002/art.39726.
- Chuang MH, Chuang TL, Koo M, Wang YF. Trabecular bone score reflects trabecular microarchitecture deterioration and fragility fracture in female adult patients receiving glucocorticoid therapy: A pre-post controlled study. BioMed Res Int. 2017. doi: 10.1155/2017/4210217.
- Andersen BN, Johansen PB, Abrahamsen B. Proton pump inhibitors and osteoporosis. Curr Opin Rheumatol. 2016;28(4):420-425. doi: 10.1097/BOR.0000000000000291.
- Jacob L, Hadji P, Kostev K. The use of proton pump inhibitors is positively associated with osteoporosis in postmenopausal women in Germany. Climacteric. 2016; 19(5):478-481. doi: 10.1080/13697137.2016.1200549.
- Targownik LE, Lix LM, Metge CJ, Prior HJ, Leung S, Leslie WD. Use of proton pump inhibitors and risk of osteoporosis-related fracture. Can Med Assoc J. 2008;179:319-326. doi: 10.1503/cmaj.071330.
- Lee RH, Lyles KH, Colon-Emeric C. A review of the effect of anticonvulsant medications on bone mineral density and fracture risk. Am J Geriatr Pharmacother. 2010;8(1):34-46. doi: 10.1016/j.amjopharm.2010.02.003.
- Arora E, Singh H, Gupta YK. Impact of antiepileptic drugs on bone health: Need for monitoring, treatment, and prevention. J Family Med Prim Care. 2016;5(2):248-253. doi: 10.4103/2249-4863.192338.
- Maghraoui AE, Roux C. DXA scanning in clinical practice. Q J Med. 2008;101(8):605-617. doi: 10.1093/qjmed/hcn022.
- Watts NB, Lewiecki EM, Miller PD, Baim S. National osteoporosis foundation 2008 clinician’s guide to prevention and treatment of osteoporosis and the world health organization fracture risk assessment tool (FRAX): What they mean to the bone densiometrist and bone technologist. J Clin Densitom. 2008;11(4):473-477. doi: 10.1016/j.jocd.2008.04.003.
- MacLean C, Newberry S, Maglione M, et al. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med. 2007;148(3):197-213. doi: 10.7326/0003-4819-148-3-200802050-00198.
- Beaton DE, Vidmar M, Pitzul KB, et al. Addition of a fracture risk assessment to a coordinator’s role improved treatment rates within 6 months of screening in a fragility fracture screening program. J Am Geriatr Soc. 2017; 28(3):863-869. doi: 10.1007/s00198-016-3794-1.
- U.S. Preventative Services Task Force. Screening for osteoporosis. Ann Intern Med. 2011;154(5):356-364. doi: 10.7326/0003-4819-154-5-201103010-00307.
- Sunyecz JA. The use of calcium and vitamin D in the management of osteoporosis. Ther Clin Risk Manag. 2008;4(4):827-836.
- Eastell, R. (1998). Treatment of postmenopausal osteoporosis. N Engl J Med. 1998;338:736-746. doi: 10.1056/NEJM199803123381107.
- Cosman F, de Beur SJ, LeBoff MS, et al, National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2381. doi: 10.1007/s00198-014-2794-2.
- Black DM, Schartz AV, Ensrud KE, et al, doi:10.1001/jama.296.24.2927.
- Schmidt GA, Horner KE, McDanel DL, Ross MB, Moores KG. Risks and benefits of long-term bisphosphonate therapy. Am J Health Syst Pharm. 2010;67(12):994-1001. doi: 10.2146/ajhp090506.
- Kraenzlin, ME, Meier C. Parathyroid hormone analogues in the treatment of osteoporosis. Nat Rev Endocrinol. 2011;7(11):647-656. doi: 10.1038/nrendo.2011.108.
- Miller P, Hattersley G, Riis B, et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis. JAMA. 2016;316(7):722-733. doi: 10.1001/jama.2016.11136.
- TYMLOSTM [prescribing information]. Waltham, MA: Radius Health, Inc; 2017.
- Tetsunaga T, Tetsunaga T, Nishida K, et al. Denosumab and alendronate treatment in patients with back pain due to fresh osteoporotic vertebral fractures. J Orthop Sci. 2017;22(2):230-236. doi: 10.1016/j.jos.2016.11.017.
- Recker, RR, Mitlak BH, Ni X, Krege JH. Long-term raloxifene for postmenopausal osteoporosis. Curr Med Res Opin. 2011;27(9):1755-1761. doi: 10.1185/03007995.2011.606312.
- Yildirim K, Gureser G, Karatay S, et al. Comparison of the effects of alendronate, risedronate and calcitonin treatment in postmenopausal osteoporosis. J Back Musculoskelet Rehabil.2005;18(3/4):85-89. doi: 10.3233/BMR-2005-183-405.
- Christensen L, Iqbal S, Macarios D, Badamgarav E, Harley C. Cost of fractures commonly associated with osteoporosis in a managed-care population. J Med Econ. 2010;13(2):302-313. doi: 10.3111/13696998.2010.488969.
TAKE-HOME POINTS
- 3 million people sustain fragility fractures annually, and nearly 30% die within a year of the fracture.
- The incidence of fragility fractures increases in patients with comorbidities such as thyroid disease, diabetes, hypertension, and heart disease.
- The World Health Organization has developed a set of T-core criteria to diagnose osteoporosis in postmenopausal women: a score >–1 is normal; <–1 but >–2.5 signifies osteopenia; <–2.5 denotes osteoporosis; and <–2.5 with fragility fracture indicates severe osteoporosis.
- The Z score, not the T score, should be used to assess osteoporosis in premenopausal women, men <50 years, and children. The Z score is calculated by comparing the patient’s BMD with the mean BMD of their peers of a similar age, race, and gender. Z scores <–2.0 indicate low BMD for chronological age. A Z score > –2.0 is considered within the expected range for age.
- After an initial fragility fracture, the risk for additional ones increases significantly, making treatment of osteoporosis essential. The National Osteoporosis Foundation recommends treating osteoporosis with pharmacotherapy in patients with a high risk for fracture (T score <–2.5) or history of fragility fracture.26
Upper Extremity Injuries in Soccer
ABSTRACT
Upper limb injuries in soccer represent only a marginal portion of injuries, however this is mainly true for outfield players. Goalkeepers are reported to have up to 5 times more upper extremity injuries, many of them requiring substantial time-loss for treatment and rehabilitation. The most common upper extremity injury locations are the shoulder/clavicle followed by the hand/finger/thumb, elbow, wrist, forearm, and upper arm. The mechanism of injury, presentation, physical examination, and imaging features all play a significant role in reaching the correct diagnosis. Taking to consideration the position the player plays and his demands will also enable tailoring the optimal treatment plan that allows timely and safe return to play. This article discusses common upper extremity injuries observed in soccer players, focusing on proper diagnosis and optimal management.
Continue to: Upper limb injuries in association with soccer...
Upper limb injuries in association with soccer have been reported to represent only 3% of all time-loss injuries in professional soccer players1. However, they are considered an increasing problem in recent years2-4 and have been reported in high proportions in children under the age of 15 years.5 Some of the reasons for the increase in upper extremity injuries may be explained by modern soccer tactics that have been characterized by high speed, pressing, and marking.2 Furthermore, upper extremity injuries may still be underestimated in soccer, mainly because outfield players are sometimes able to train and play even when they suffer from an upper extremity injury.
Unsurprisingly, upper extremity injuries are reported to be up to 5 times more common in goalkeepers than in outfield players,1,2 reaching a high rate of up to 18% of all injuries among professional goalkeepers. The usage of upper extremities to stop the ball and repeated reaching to the ball and landing on the ground with changing upper extremity positions are some of the contributors to the increased upper extremity injury risk in goalkeepers.
Following 57 male professional European soccer teams from 16 countries between the years 2001 and 2011, Ekstrand and colleagues1 showed that 90% of upper extremity injuries are traumatic, and only 10% are related to overuse. They also reported that the most common upper extremity injury location is the shoulder/clavicle (56%), followed by the hand/finger/thumb (24%), elbow (10%), wrist (5%), forearm (4%), and upper arm (1%). Specifically, the 6 most common injuries are acromioclavicular joint (ACJ) sprain (13%), shoulder dislocation (12%), hand metacarpal fracture (8%), shoulder rotator cuff tendinopathy (6%), hand phalanx fracture (6%), and shoulder ACJ dislocation (5%).
This article will discuss common upper extremity injuries observed in soccer players, focusing on proper diagnosis and optimal management.
Continue to: THE SHOULDER...
THE SHOULDER
The majority of upper extremity injuries in professional soccer players are shoulder injuries.1,2,4 Almost a third of these injuries (28%) are considered severe, preventing participation in training and matches for 28 days or more.6Ekstrand and colleagues1 reported that shoulder dislocation represents the most severe upper extremity injury with a mean of 41 days of absence from soccer. When considering the position of the player, they further demonstrated that absence from full training and matches is twice as long for goalkeepers as for outfield players, which reflects the importance of shoulder function for goalkeepers.
In terms of the mechanism of shoulder instability injuries in soccer players, more than half (56%) of these injuries occur with a high-energy mechanism in the recognized position of combined humeral abduction and external rotation against a force of external rotation and horizontal extension.3 However, almost a quarter (24%) occur with a mechanism of varied upper extremity position and low-energy trauma, and 20% of injuries are either a low energy injury with little or no contact or gradual onset. These unique characteristics of shoulder instability injuries in soccer players should be accounted for during training and may imply that current training programs are suboptimal for the prevention of upper extremity injuries and shoulder injuries. Ejnisman and colleagues2 reported on the development of a Fédération Internationale de Football Association (FIFA) 11+ shoulder injury prevention program for soccer goalkeepers as one of the ways to promote training programs that address the risk of shoulder injuries.
Reporting on the management of severe shoulder injuries requiring surgery in 25 professional soccer players in England, between 2007 and 2011, Hart and Funk3 found that the majority of subjects (88%) reported a dislocation as a feature of their presentation. Twenty-one (84%) subjects were diagnosed with labral injuries, of which 7 had an associated Hill-Sachs lesion. Two (8%) subjects were diagnosed with rotator cuff tears requiring repair, and 2 (8%) subjects had a combination of rotator cuff and labral injury repair. All patients underwent arthroscopic repair, except for 5 who had a Latarjet coracoid transfer. Post-surgery, all players were able to return to unrestricted participation in soccer at a mean of 11.4 weeks, with no significant difference between goalkeepers and outfield players and no recurrences at a mean of 91 weeks’ follow-up.
Up to one-third of shoulder instability injuries in soccer players are reported to be recurrences,1,3 which emphasizes the need to carefully assess soccer players before clearing them to return to play. These data raise the controversy over the treatment of first time shoulder dislocators and may support early surgical intervention.7-9 In terms of the preferred surgical intervention in these cases, Balg and Boileau10 suggested a simple scoring system based on factors derived from a preoperative questionnaire, physical examination, and anteroposterior radiographs to help distinguish between patients who will benefit from an arthroscopic anterior stabilization using suture anchors and those who will require a bony procedure (open or arthroscopic). Cerciello and colleagues11 reported excellent results for bony stabilization (modified Latarjet) in a population of 26 soccer players (28 shoulders) affected by chronic anterior instability. Only 1 player did not return to soccer, and 18 players (20 shoulders, 71%) returned to the same level. One re-dislocation was noted in a goalkeeper 74 months after surgery.
An injury to the ACJ has been previously reported to be the most prevalent type of shoulder injury in contact sports.12In soccer, injury to the ACJ is responsible for 18% of upper extremity injuries, and the majority (72%) are sprains.1Interestingly, but unsurprisingly, implications of such an injury differ significantly between goalkeepers and outfield players with up to 3 times longer required absence periods for goalkeepers vs outfield players sustaining the same injury.
ACJ injury is commonly the result of a direct fall on the shoulder with the arm adducted or extended. Six grades of ACJ injuries have been described and distinguished by the injured anatomical structure (acromioclavicular ligaments and coracoclavicular ligaments) and the direction and magnitude of clavicular dislocation.13,14 Presentation will usually include anterior shoulder pain, a noticeable swelling or change in morphology of the lateral end of the clavicle (mainly in dislocation types), and sharp pain provoked by palpation of the ACJ. Radiographic imaging will confirm the diagnosis and help with identifying the specific grade/type of injury.
Decision making and management of acute ACJ injury should be based on the type/grade of injury. Nonoperative treatment is recommended for types I and II, and most athletes have a successful outcome with a full return to play.12Types IV, V, and VI are treated early with operative intervention, mostly due to the morbidity associated with prolonged dislocation of the joint and subsequent soft tissue damage.12 Treatment of type III injury remains controversial. Pereira-Graterol and colleagues15 reported the effectiveness of clavicular hook plate (DePuy Synthes) in the surgical stabilization of grade III ACJ dislocation in 11 professional soccer players. At a mean follow-up of 4 years, they showed excellent functional results with full shoulder range of motion at 5 weeks and latest return to soccer at 6 months. The hook plate was removed after 16 weeks in 10 patients in whom no apparent complication was observed.
Continue to: THE ELBOW...
THE ELBOW
Ekstrand and colleagues1 reported that 10% of all upper extremity injuries in professional soccer players are elbow injuries, of which only 19% are considered severe injuries that require more than 28 days of absence from playing soccer. The most common elbow injuries in their cohort were elbow medial collateral ligament (MCL) sprain and olecranon bursitis.
Elbow MCL is the primary constraint of the elbow joint to valgus stress, and MCL sprain occurs when the elbow is subjected to a valgus, or laterally directed force, which distracts the medial side of the elbow, exceeding the tensile properties of the MCL.16 A thorough physical examination that includes valgus stress tests through the arc of elbow flexion and extension to elicit a possible subjective feeling of apprehension, instability, or localized pain is essential for optimal evaluation and treatment.16,17 Imaging studies (X-ray and stress X-rays, dynamic ultrasound, computed tomography [CT], magnetic resonance imaging [MRI], and MR arthrography) have a role in further establishing the diagnosis and identifying possible additional associated injuries.16 The treatment plan should be specifically tailored to the individual athlete, depending on his demands and the degree of MCL injury. In soccer, which is a non-throwing shoulder sport, nonoperative treatment should be the preferred initial treatment in most cases. Ekstrand and colleagues1 showed that this injury requires a mean of 4 days of absence from soccer for outfield players and a mean of 21 days of absence from soccer for goalkeepers, thereby indicating more severe sprains and cautious return to soccer in goalkeepers. Athletes who fail nonoperative treatment are candidates for MCL reconstruction.16
The olecranon bursa is a synovium-lined sac that facilitates gliding between the olecranon and overlying skin. Olecranon bursitis is characterized by accumulation of fluid in the bursa with or without inflammation. The fluid can be serous, sanguineous, or purulent depending on the etiology.18 In soccer, traumatic etiology is common, but infection secondary to cuts or scratches of the skin around the elbow or previous therapeutic injections around the elbow should always be ruled out. Local pain, swelling, warmth, and redness are usually the presenting symptoms. Aseptic olecranon bursitis may be managed non-surgically with ‘‘benign neglect’’ and avoidance of pressure to the area, non-steroidal anti-inflammatory drugs, needle aspiration, corticosteroid injection, compression dressings, and/or padded splinting; whereas acute septic bursitis requires needle aspiration for diagnosis, appropriate oral or intravenous antibiotics directed toward the offending organism, and, when clinically indicated, surgical evacuation/excision of the bursa.18 When treating this condition with cortisone injection, possible complications, such as skin atrophy, secondary infection, and chronic local pain, have been reported and should be considered.19
Severe elbow injuries in professional athletes in general,20-22 and soccer players specifically, are elbow subluxations/dislocations and elbow fracture. The mechanism of injury is usually contact injury with an opponent player or a fall on the palm with the arm extended. Posterolateral is the most common type of elbow dislocation. Elbow dislocations are further classified into simple (no associated fractures) and complex (associated with fractures) categories.22 Simple dislocations are usually treated with early mobilization after closed reduction; it is associated with a low risk for re-dislocation and with generally good results. The complex type of elbow fracture dislocation is more difficult to treat, has higher complication and re-dislocation rates, and requires operative treatment in most cases compared with simple dislocation.22 The “terrible triad” of the elbow (posterior elbow dislocation, radial head fracture, and coronoid fracture) represents a specific complex elbow dislocation scenario that is difficult to manage because of conflicting aims of ensuring elbow stability while maintaining early range of motion.22
Isolated fracture around the elbow should be treated based on known principles of fracture management: mechanism of injury, fracture patterns, fracture displacement, intra-articular involvement, soft tissue condition, and associated injuries.
Continue to: THE WRIST...
THE WRIST
Ekstrand and colleagues1 reported that 5% of all upper extremity injuries in their cohort of professional soccer players are wrist injuries, of which, only 2% are considered severe injuries that require >28 days of absence from playing soccer. The more common wrist injuries in soccer, which is considered a high-impact sport, are fractures (distal radius, scaphoid, capitate), and less reported injuries are dislocations (lunate, perilunate) and ligamentous injuries or tears (scapholunate ligament).23
Distal radius fractures in high-impact sports, like soccer, usually occur as a result of a fall on an out-stretched hand and will usually be more comminuted, displaced, and intra-articular compared with low-impact sports.23 All these aforementioned characteristics usually indicate surgical management of open reduction and internal fixation, which will allow for rapid start of rehabilitation and return to play.
Scaphoid fracture is the most common carpal bone fracture and presents unique challenges in terms of diagnosis and optimal treatment24 in professional athletes. A typical injury scenario would be a player falling on an outstretched hand and sustaining a scaphoid fracture during a match or training session. The player may acknowledge some wrist pain but will often continue to play with minimal or no limitation. As wrist pain and swelling become more evident after the match/training session, the player will seek medical evaluation.24 A complete wrist and upper extremity examination should be performed in addition to a specific assessment, which includes palpation of the distal scaphoid pole at the distal wrist flexion crease, palpation of the scaphoid waist through the wrist snuff box, and palpation dorsally just distal to the Lister tubercle at the scapholunate joint. Any wrist injury that results in decreased range of motion, snuff box swelling, and scaphoid tenderness should be further evaluated with imaging. Plain radiographs with special scaphoid views are the initial preferred imaging studies, but occult fracture will require an additional study such as a bone scan, CT, or MRI. Several studies have validated the benefit of MRI and the fact that it may outweigh the costs associated with lost productivity from unnecessary cast immobilization, especially in elite athletes.23-25Casting the patient with a nondisplaced scaphoid waist fracture has been the traditional treatment; however, stiffness, weakness, and deconditioning that can occur with long-term casting required for scaphoid fractures are significant impairments for the professional athlete and usually end the player’s season. Surgical treatment, which was traditionally indicated for displaced or proximal pole fractures, is currently also considered for non-displaced scaphoid waist fractures in professional athletes. This treatment allows for a rapid return to the rehabilitation of the extremity and possible early return to professional sport. In view of the known complications (eg, malunion, nonunion, and avascular necrosis), return to soccer can be considered when imaging confirms advanced healing, which some consider as at least 50% of bone fracture bridging on CT scan, no pain, excellent motion, and at least 80% of normal grip strength.24 Outfield players can return to play with a protective cast or brace until full healing is observed on imaging.
Continue to: THE HAND/FINGERS/THUMB...
THE HAND/FINGERS/THUMB
Almost a quarter of upper extremity injuries in professional soccer players were reported to involve the hand, fingers, and thumb. A quarter of them were classified as severe injuries requiring >28 days of absence from playing soccer.1Specifically, hand metacarpal and phalanx fractures are the most common reported injuries in sports in general,26 and in soccer,1 and account for 14% of all upper extremity injuries1 in professional soccer players. Goalkeepers require a functional hand to play, whereas an outfielder can play with protection on the injured area; thus, the period of absence from soccer in these injuries is significantly different between goalkeepers and outfielders with more than 4 times longer absence from soccer for a goalkeeper compared with an outfielder. The fifth ray has been shown to be the most frequently fractured ray in the hand in soccer with 51.7% of all hand fractures reported.26 The common mechanism is a full hit on the hand, and a direct hit from the ball is another possible mechanism in goalkeepers.
In general, the diagnosis of hand injuries requires evaluation of the mechanism of injury and injury symptoms, proper and comprehensive physical examination of the whole extremity, and prompt imaging. In most cases, plain radiographs in several projections will suffice for the diagnosis of obvious fractures, but CT scan is an additional modality that allows for improved appreciation of occult or complex and comminuted fracture patterns. MRI or ultrasound can be used additionally whenever associated soft tissue injury is suspected. Optimal management of the hand is based on the specific characteristics of the fractures, which include location, direction of the fracture line, presence of comminution, displacement, articular involvement, and associated soft tissue injury. Nondisplaced extra-articular fractures often can be treated with buddy taping or splinting, whereas intra-articular fractures often require surgical treatment. Displaced fractures of the hand have a tendency to angulate volarly because of attachments of the interosseous muscles. Marginal fractures or avulsion fractures involving the metacarpals or phalanges can be sentinels of serious associated soft tissue injuries.27
Phalangeal fractures can potentially affect the function of the entire hand; therefore, no malrotation is acceptable for phalangeal fractures because they can lead to overlap and malalignment of the digit. Displaced or malrotated fractures should be reduced either by closed or open techniques. Acceptable reduction is <6 mm of shortening, <15° of angulation, and no rotational deformity.27,28 Nondisplaced phalangeal fractures can be treated nonoperatively with buddy taping and splinting with good results.27 Interphalangeal (IP) dislocations can be reduced on the sidelines and then taped or splinted. Any injury with a force significant enough to cause joint dislocation indicates further evaluation for associated fractures and ligamentous injury or tear. The proximal interphalangeal (PIP) joint is the most common IP joint dislocation and is usually a dorsal dislocation. Reduction is often achieved by traction and flexion of the middle phalanx,27 followed by splinting of the finger with the PIP in 30° of flexion or an extension block splint.29 Successful reduction with no associated intra-articular fractures involving more than a third of the joint can be further managed nonoperatively with the splint, allowing 2 to 4 weeks for the volar plate, joint capsule, and collateral ligaments to heal. Additional 2 to 4 weeks of splinting with buddy taping to the adjacent finger is usually recommended.29
The “Mallet finger” injury can be observed in goalkeepers and is caused by a flexion force on the tip of the finger while the distal interphalangeal (DIP) joint is extended. This force results in tearing of the extensor tendon or an avulsion fracture at the tendinous attachment on the dorsal lip of the distal phalangeal base. The classic mechanism of injury is an extended finger struck on the tip by a ball. Physical examination will indicate loss of DIP joint active extension, and the joint rests in an abnormally flexed position. Treatment typically consists of splinting the DIP joint in extension for 6 to 8 weeks. Operative treatment is reserved for severe injuries or fractures involving greater than one-third of the articular surface of the DIP joint or with failed nonoperative treatment.27
Metacarpal fractures can be subdivided into distal, metacarpal neck, metacarpal shaft, and metacarpal base fractures. Metacarpal shaft fractures raise a specific concern regarding rotation, because even a small degree of rotation can create a substantial degree of deformity at the fingertip. This concern must be addressed during evaluation of the player. Fractures of the metacarpal base most commonly involve the fourth and fifth metacarpals and are often reduced easily but have a tendency to re-subluxate, which may indicate operative treatment. Most fractures of the metacarpals are low energy and result in simple fracture patterns that can be treated nonoperatively. Open reduction is reserved for high-energy trauma, fractures with excessive angulation, or multiple fractures.27
Continue to: An important subgroup of metacarpal injuries...
An important subgroup of metacarpal injuries involves the base of the thumb. These injuries result from an axial load applied to the thumb. The most common injury is the “Bennett fracture,” which is an intra-articular fracture or dislocation involving the base of the first metacarpal. Bennett fractures are unstable fractures; unless properly recognized and treated, this intra-articular fracture subluxation may result in an unstable arthritic first carpometacarpal joint. These fractures are most commonly treated with closed or open reduction combined with internal fixation.27 “Rolando fractures” are similar in location and etiology but are comminuted and usually require operative treatment.27, 29
Another common hand injury found in soccer goalkeepers and involving the base of the thumb is disruption of the ulnar collateral ligament (UCL) of the first metacarpophalangeal (MCP) joint as a result of an acute radial or valgus stress on the thumb. Known as “gamekeeper’s thumb” or “skier’s thumb,” this injury can occur in the form of an avulsion fracture, an isolated ligament tear, or combined fracture and ligament rupture. On examination, swelling and tenderness over the thumb UCL are observed. A MCP joint stress test should be performed by gently applying a radially directed force to the thumb while stabilizing the metacarpal bone at both 0° and 30° at the MCP joint. Increased laxity, a soft or nonexistent end point, and gaping of the joint, as compared with the contralateral side, will indicate this injury.29 Radiographs may show a small avulsion fracture fragment at the ulnar aspect of the base of the first metacarpal and at the attachment of the UCL. A Stener lesion is an abnormality that occurs when the thumb adductor muscle aponeurosis interposes between the 2 ends of the ruptured UCL, preventing UCL healing by immobilization alone. Ultrasound and MRI are additional imaging modalities that can assist with the diagnosis of a Stener lesion. The presence of a Stener lesion is a prime indication for surgical intervention. A nondisplaced fracture or isolated ligament injury with no evidence of a Stener lesion can be treated nonoperatively with splinting of the thumb and may lead to healing and restoration of stability. However, in professional players, surgical repair is often times preferred.27
CONCLUSION
Upper extremity injuries are less common injuries among soccer players, but their prevalence is on the rise in recent years. Modern playing tactics and the increase in participation in soccer in younger age groups may be 2 contributing factors to this rise. Given the characteristics of their unique playing role and specific demands, the risk for upper extremity injuries among goalkeepers is significantly higher than that in outfielders and will usually result in a long absence period from soccer before they return to play. A thorough understanding of the mechanism of injury, players’ complaints and presentation, osseous and soft tissue involvement based on a systematic physical examination, imaging features, and treatment options is important for the optimal care of the players. Prompt and accurate diagnosis and appropriate management are essential for improved outcomes and timely return to play.
1. Ekstrand J, Hagglund M, Tornqvist H, et al. Upper extremity injuries in male elite football players. Knee Surg Sports Traumatol Arthrosc. 2013;21(7):1626-1632. doi:10.1007/s00167-012-2164-6.
2. Ejnisman B, Barbosa G, Andreoli CV, et al. Shoulder injuries in soccer goalkeepers: Review and development of a FIFA 11+ shoulder injury prevention program. Open Access J Sports Med. 2016;7:75-80. doi:10.2147/OAJSM.S97917.
3. Hart D, Funk L. Serious shoulder injuries in professional soccer: Return to participation after surgery. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):2123-2129. doi:10.1007/s00167-013-2796-1.
4. Longo UG, Loppini M, Berton A, Martinelli N, Maffulli N, Denaro V. Shoulder injuries in soccer players. Clin Cases Miner Bone Metab. 2012;9(3):138-141.
5. Faude O, Rossler R, Junge A. Football injuries in children and adolescent players: Are there clues for prevention? Sports Med. 2013;43(9):819-837. doi:10.1007/s40279-013-0061-x.
6. Ekstrand J, Hagglund M, Walden M. Injury incidence and injury patterns in professional football: The UEFA injury study. Br J Sports Med. 2011;45(7):553-558. doi:10.1136/bjsm.2009.060582.
7. Boone JL, Arciero RA. First-time anterior shoulder dislocations: Has the standard changed? Br J Sports Med. 2010;44(5):355-360. doi:10.1136/bjsm.2009.062596.
8. Handoll HH, Almaiyah MA, Rangan A. Surgical versus non-surgical treatment for acute anterior shoulder dislocation. Cochrane Database Syst Rev. 2004;(1):CD004325.
9. Kirkley A, Werstine R, Ratjek A, Griffin S. Prospective randomized clinical trial comparing the effectiveness of immediate arthroscopic stabilization versus immobilization and rehabilitation in first traumatic anterior dislocations of the shoulder: Long-term evaluation. Arthroscopy. 2005;21(1):55-63.
10. Balg F, Boileau P. The instability severity index score. A simple pre-operative score to select patients for arthroscopic or open shoulder stabilisation. J Bone Joint Surg Br. 2007;89(11):1470-1477.
11. Cerciello S, Edwards TB, Walch G. Chronic anterior glenohumeral instability in soccer players: Results for a series of 28 shoulders treated with the latarjet procedure. J Orthop Traumatol. 2012;13(4):197-202. doi:10.1007/s10195-012-0201-3.
12. Bishop JY, Kaeding C. Treatment of the acute traumatic acromioclavicular separation. Sports Med Arthrosc Rev. 2006;14(4):237-245. doi:10.1097/01.jsa.0000212330.32969.6e.
13. de Putter CE, van Beeck EF, Burdorf A, et al. Increase in upper extremity fractures in young male soccer players in the netherlands, 1998-2009. Scand J Med Sci Sports. 2015;25(4):462-466. doi:10.1111/sms.12287.
14. Rockwood CJ, Williams G, Young D. Disorders of the acromioclavicular joint. In: Rockwood CJ, Matsen FA III, eds. The Shoulder. 2nd ed. Philadelphia: WB Saunders; 1998:483-553.
15. Pereira-Graterol E, Alvarez-Diaz P, Seijas R, Ares O, Cusco X, Cugat R. Treatment and evolution of grade III acromioclavicular dislocations in soccer players. Knee Surg Sports Traumatol Arthrosc. 2013;21(7):1633-1635. doi:10.1007/s00167-012-2186-0.
16. Rahman RK, Levine WN, Ahmad CS. Elbow medial collateral ligament injuries. Curr Rev Musculoskelet Med. 2008;1(3-4):197-204. doi:10.1007/s12178-008-9026-3.
17. Redler LH, Watling JP, Ahmad CS. Physical examination of the throwing athlete's elbow. Am J Orthop. 2015;44(1):13-18.
18. Sayegh ET, Strauch RJ. Treatment of olecranon bursitis: A systematic review. Arch Orthop Trauma Surg. 2014;134(11):1517-1536. doi:10.1007/s00402-014-2088-3.
19. Weinstein PS, Canoso JJ, Wohlgethan JR. Long-term follow-up of corticosteroid injection for traumatic olecranon bursitis. Ann Rheum Dis. 1984;43(1):44-46.
20. Carlisle JC, Goldfarb CA, Mall N, Powell JW, Matava MJ. Upper extremity injuries in the national football league: Part II: Elbow, forearm, and wrist injuries. Am J Sports Med. 2008;36(10):1945-1952. doi:10.1177/0363546508318198.
21. Dizdarevic I, Low S, Currie DW, Comstock RD, Hammoud S, Atanda A Jr. Epidemiology of elbow dislocations in high school athletes. Am J Sports Med. 2016;44(1):202-208. doi:10.1177/0363546515610527.
22. Saati AZ, McKee MD. Fracture-dislocation of the elbow: Diagnosis, treatment, and prognosis. Hand Clin. 2004;20(4):405-414.
23. Bancroft LW. Wrist injuries: A comparison between high- and low-impact sports. Radiol Clin North Am. 2013;51(2):299-311. doi:10.1016/j.rcl.2012.09.017.
24. Belsky MR, Leibman MI, Ruchelsman DE. Scaphoid fracture in the elite athlete. Hand Clin. 2012;28(3):78, vii. doi:10.1016/j.hcl.2012.05.005.
25. Mallee W, Doornberg JN, Ring D, van Dijk CN, Maas M, Goslings JC. Comparison of CT and MRI for diagnosis of suspected scaphoid fractures. J Bone Joint Surg Am. 2011;93(1):20-28. doi:10.2106/JBJS.I.01523.
26. Aitken S, Court-Brown CM. The epidemiology of sports-related fractures of the hand. Injury. 2008;39(12):1377-1383. doi:10.1016/j.injury.2008.04.012.
27. Peterson JJ, Bancroft LW. Injuries of the fingers and thumb in the athlete. Clin Sports Med. 2006;25(3):viii.
28. Walsh JJ 4th. Fractures of the hand and carpal navicular bone in athletes. South Med J. 2004;97(8):762-765.
29. Hong E. Hand injuries in sports medicine. Prim Care. 2005;32(1):91-103.
ABSTRACT
Upper limb injuries in soccer represent only a marginal portion of injuries, however this is mainly true for outfield players. Goalkeepers are reported to have up to 5 times more upper extremity injuries, many of them requiring substantial time-loss for treatment and rehabilitation. The most common upper extremity injury locations are the shoulder/clavicle followed by the hand/finger/thumb, elbow, wrist, forearm, and upper arm. The mechanism of injury, presentation, physical examination, and imaging features all play a significant role in reaching the correct diagnosis. Taking to consideration the position the player plays and his demands will also enable tailoring the optimal treatment plan that allows timely and safe return to play. This article discusses common upper extremity injuries observed in soccer players, focusing on proper diagnosis and optimal management.
Continue to: Upper limb injuries in association with soccer...
Upper limb injuries in association with soccer have been reported to represent only 3% of all time-loss injuries in professional soccer players1. However, they are considered an increasing problem in recent years2-4 and have been reported in high proportions in children under the age of 15 years.5 Some of the reasons for the increase in upper extremity injuries may be explained by modern soccer tactics that have been characterized by high speed, pressing, and marking.2 Furthermore, upper extremity injuries may still be underestimated in soccer, mainly because outfield players are sometimes able to train and play even when they suffer from an upper extremity injury.
Unsurprisingly, upper extremity injuries are reported to be up to 5 times more common in goalkeepers than in outfield players,1,2 reaching a high rate of up to 18% of all injuries among professional goalkeepers. The usage of upper extremities to stop the ball and repeated reaching to the ball and landing on the ground with changing upper extremity positions are some of the contributors to the increased upper extremity injury risk in goalkeepers.
Following 57 male professional European soccer teams from 16 countries between the years 2001 and 2011, Ekstrand and colleagues1 showed that 90% of upper extremity injuries are traumatic, and only 10% are related to overuse. They also reported that the most common upper extremity injury location is the shoulder/clavicle (56%), followed by the hand/finger/thumb (24%), elbow (10%), wrist (5%), forearm (4%), and upper arm (1%). Specifically, the 6 most common injuries are acromioclavicular joint (ACJ) sprain (13%), shoulder dislocation (12%), hand metacarpal fracture (8%), shoulder rotator cuff tendinopathy (6%), hand phalanx fracture (6%), and shoulder ACJ dislocation (5%).
This article will discuss common upper extremity injuries observed in soccer players, focusing on proper diagnosis and optimal management.
Continue to: THE SHOULDER...
THE SHOULDER
The majority of upper extremity injuries in professional soccer players are shoulder injuries.1,2,4 Almost a third of these injuries (28%) are considered severe, preventing participation in training and matches for 28 days or more.6Ekstrand and colleagues1 reported that shoulder dislocation represents the most severe upper extremity injury with a mean of 41 days of absence from soccer. When considering the position of the player, they further demonstrated that absence from full training and matches is twice as long for goalkeepers as for outfield players, which reflects the importance of shoulder function for goalkeepers.
In terms of the mechanism of shoulder instability injuries in soccer players, more than half (56%) of these injuries occur with a high-energy mechanism in the recognized position of combined humeral abduction and external rotation against a force of external rotation and horizontal extension.3 However, almost a quarter (24%) occur with a mechanism of varied upper extremity position and low-energy trauma, and 20% of injuries are either a low energy injury with little or no contact or gradual onset. These unique characteristics of shoulder instability injuries in soccer players should be accounted for during training and may imply that current training programs are suboptimal for the prevention of upper extremity injuries and shoulder injuries. Ejnisman and colleagues2 reported on the development of a Fédération Internationale de Football Association (FIFA) 11+ shoulder injury prevention program for soccer goalkeepers as one of the ways to promote training programs that address the risk of shoulder injuries.
Reporting on the management of severe shoulder injuries requiring surgery in 25 professional soccer players in England, between 2007 and 2011, Hart and Funk3 found that the majority of subjects (88%) reported a dislocation as a feature of their presentation. Twenty-one (84%) subjects were diagnosed with labral injuries, of which 7 had an associated Hill-Sachs lesion. Two (8%) subjects were diagnosed with rotator cuff tears requiring repair, and 2 (8%) subjects had a combination of rotator cuff and labral injury repair. All patients underwent arthroscopic repair, except for 5 who had a Latarjet coracoid transfer. Post-surgery, all players were able to return to unrestricted participation in soccer at a mean of 11.4 weeks, with no significant difference between goalkeepers and outfield players and no recurrences at a mean of 91 weeks’ follow-up.
Up to one-third of shoulder instability injuries in soccer players are reported to be recurrences,1,3 which emphasizes the need to carefully assess soccer players before clearing them to return to play. These data raise the controversy over the treatment of first time shoulder dislocators and may support early surgical intervention.7-9 In terms of the preferred surgical intervention in these cases, Balg and Boileau10 suggested a simple scoring system based on factors derived from a preoperative questionnaire, physical examination, and anteroposterior radiographs to help distinguish between patients who will benefit from an arthroscopic anterior stabilization using suture anchors and those who will require a bony procedure (open or arthroscopic). Cerciello and colleagues11 reported excellent results for bony stabilization (modified Latarjet) in a population of 26 soccer players (28 shoulders) affected by chronic anterior instability. Only 1 player did not return to soccer, and 18 players (20 shoulders, 71%) returned to the same level. One re-dislocation was noted in a goalkeeper 74 months after surgery.
An injury to the ACJ has been previously reported to be the most prevalent type of shoulder injury in contact sports.12In soccer, injury to the ACJ is responsible for 18% of upper extremity injuries, and the majority (72%) are sprains.1Interestingly, but unsurprisingly, implications of such an injury differ significantly between goalkeepers and outfield players with up to 3 times longer required absence periods for goalkeepers vs outfield players sustaining the same injury.
ACJ injury is commonly the result of a direct fall on the shoulder with the arm adducted or extended. Six grades of ACJ injuries have been described and distinguished by the injured anatomical structure (acromioclavicular ligaments and coracoclavicular ligaments) and the direction and magnitude of clavicular dislocation.13,14 Presentation will usually include anterior shoulder pain, a noticeable swelling or change in morphology of the lateral end of the clavicle (mainly in dislocation types), and sharp pain provoked by palpation of the ACJ. Radiographic imaging will confirm the diagnosis and help with identifying the specific grade/type of injury.
Decision making and management of acute ACJ injury should be based on the type/grade of injury. Nonoperative treatment is recommended for types I and II, and most athletes have a successful outcome with a full return to play.12Types IV, V, and VI are treated early with operative intervention, mostly due to the morbidity associated with prolonged dislocation of the joint and subsequent soft tissue damage.12 Treatment of type III injury remains controversial. Pereira-Graterol and colleagues15 reported the effectiveness of clavicular hook plate (DePuy Synthes) in the surgical stabilization of grade III ACJ dislocation in 11 professional soccer players. At a mean follow-up of 4 years, they showed excellent functional results with full shoulder range of motion at 5 weeks and latest return to soccer at 6 months. The hook plate was removed after 16 weeks in 10 patients in whom no apparent complication was observed.
Continue to: THE ELBOW...
THE ELBOW
Ekstrand and colleagues1 reported that 10% of all upper extremity injuries in professional soccer players are elbow injuries, of which only 19% are considered severe injuries that require more than 28 days of absence from playing soccer. The most common elbow injuries in their cohort were elbow medial collateral ligament (MCL) sprain and olecranon bursitis.
Elbow MCL is the primary constraint of the elbow joint to valgus stress, and MCL sprain occurs when the elbow is subjected to a valgus, or laterally directed force, which distracts the medial side of the elbow, exceeding the tensile properties of the MCL.16 A thorough physical examination that includes valgus stress tests through the arc of elbow flexion and extension to elicit a possible subjective feeling of apprehension, instability, or localized pain is essential for optimal evaluation and treatment.16,17 Imaging studies (X-ray and stress X-rays, dynamic ultrasound, computed tomography [CT], magnetic resonance imaging [MRI], and MR arthrography) have a role in further establishing the diagnosis and identifying possible additional associated injuries.16 The treatment plan should be specifically tailored to the individual athlete, depending on his demands and the degree of MCL injury. In soccer, which is a non-throwing shoulder sport, nonoperative treatment should be the preferred initial treatment in most cases. Ekstrand and colleagues1 showed that this injury requires a mean of 4 days of absence from soccer for outfield players and a mean of 21 days of absence from soccer for goalkeepers, thereby indicating more severe sprains and cautious return to soccer in goalkeepers. Athletes who fail nonoperative treatment are candidates for MCL reconstruction.16
The olecranon bursa is a synovium-lined sac that facilitates gliding between the olecranon and overlying skin. Olecranon bursitis is characterized by accumulation of fluid in the bursa with or without inflammation. The fluid can be serous, sanguineous, or purulent depending on the etiology.18 In soccer, traumatic etiology is common, but infection secondary to cuts or scratches of the skin around the elbow or previous therapeutic injections around the elbow should always be ruled out. Local pain, swelling, warmth, and redness are usually the presenting symptoms. Aseptic olecranon bursitis may be managed non-surgically with ‘‘benign neglect’’ and avoidance of pressure to the area, non-steroidal anti-inflammatory drugs, needle aspiration, corticosteroid injection, compression dressings, and/or padded splinting; whereas acute septic bursitis requires needle aspiration for diagnosis, appropriate oral or intravenous antibiotics directed toward the offending organism, and, when clinically indicated, surgical evacuation/excision of the bursa.18 When treating this condition with cortisone injection, possible complications, such as skin atrophy, secondary infection, and chronic local pain, have been reported and should be considered.19
Severe elbow injuries in professional athletes in general,20-22 and soccer players specifically, are elbow subluxations/dislocations and elbow fracture. The mechanism of injury is usually contact injury with an opponent player or a fall on the palm with the arm extended. Posterolateral is the most common type of elbow dislocation. Elbow dislocations are further classified into simple (no associated fractures) and complex (associated with fractures) categories.22 Simple dislocations are usually treated with early mobilization after closed reduction; it is associated with a low risk for re-dislocation and with generally good results. The complex type of elbow fracture dislocation is more difficult to treat, has higher complication and re-dislocation rates, and requires operative treatment in most cases compared with simple dislocation.22 The “terrible triad” of the elbow (posterior elbow dislocation, radial head fracture, and coronoid fracture) represents a specific complex elbow dislocation scenario that is difficult to manage because of conflicting aims of ensuring elbow stability while maintaining early range of motion.22
Isolated fracture around the elbow should be treated based on known principles of fracture management: mechanism of injury, fracture patterns, fracture displacement, intra-articular involvement, soft tissue condition, and associated injuries.
Continue to: THE WRIST...
THE WRIST
Ekstrand and colleagues1 reported that 5% of all upper extremity injuries in their cohort of professional soccer players are wrist injuries, of which, only 2% are considered severe injuries that require >28 days of absence from playing soccer. The more common wrist injuries in soccer, which is considered a high-impact sport, are fractures (distal radius, scaphoid, capitate), and less reported injuries are dislocations (lunate, perilunate) and ligamentous injuries or tears (scapholunate ligament).23
Distal radius fractures in high-impact sports, like soccer, usually occur as a result of a fall on an out-stretched hand and will usually be more comminuted, displaced, and intra-articular compared with low-impact sports.23 All these aforementioned characteristics usually indicate surgical management of open reduction and internal fixation, which will allow for rapid start of rehabilitation and return to play.
Scaphoid fracture is the most common carpal bone fracture and presents unique challenges in terms of diagnosis and optimal treatment24 in professional athletes. A typical injury scenario would be a player falling on an outstretched hand and sustaining a scaphoid fracture during a match or training session. The player may acknowledge some wrist pain but will often continue to play with minimal or no limitation. As wrist pain and swelling become more evident after the match/training session, the player will seek medical evaluation.24 A complete wrist and upper extremity examination should be performed in addition to a specific assessment, which includes palpation of the distal scaphoid pole at the distal wrist flexion crease, palpation of the scaphoid waist through the wrist snuff box, and palpation dorsally just distal to the Lister tubercle at the scapholunate joint. Any wrist injury that results in decreased range of motion, snuff box swelling, and scaphoid tenderness should be further evaluated with imaging. Plain radiographs with special scaphoid views are the initial preferred imaging studies, but occult fracture will require an additional study such as a bone scan, CT, or MRI. Several studies have validated the benefit of MRI and the fact that it may outweigh the costs associated with lost productivity from unnecessary cast immobilization, especially in elite athletes.23-25Casting the patient with a nondisplaced scaphoid waist fracture has been the traditional treatment; however, stiffness, weakness, and deconditioning that can occur with long-term casting required for scaphoid fractures are significant impairments for the professional athlete and usually end the player’s season. Surgical treatment, which was traditionally indicated for displaced or proximal pole fractures, is currently also considered for non-displaced scaphoid waist fractures in professional athletes. This treatment allows for a rapid return to the rehabilitation of the extremity and possible early return to professional sport. In view of the known complications (eg, malunion, nonunion, and avascular necrosis), return to soccer can be considered when imaging confirms advanced healing, which some consider as at least 50% of bone fracture bridging on CT scan, no pain, excellent motion, and at least 80% of normal grip strength.24 Outfield players can return to play with a protective cast or brace until full healing is observed on imaging.
Continue to: THE HAND/FINGERS/THUMB...
THE HAND/FINGERS/THUMB
Almost a quarter of upper extremity injuries in professional soccer players were reported to involve the hand, fingers, and thumb. A quarter of them were classified as severe injuries requiring >28 days of absence from playing soccer.1Specifically, hand metacarpal and phalanx fractures are the most common reported injuries in sports in general,26 and in soccer,1 and account for 14% of all upper extremity injuries1 in professional soccer players. Goalkeepers require a functional hand to play, whereas an outfielder can play with protection on the injured area; thus, the period of absence from soccer in these injuries is significantly different between goalkeepers and outfielders with more than 4 times longer absence from soccer for a goalkeeper compared with an outfielder. The fifth ray has been shown to be the most frequently fractured ray in the hand in soccer with 51.7% of all hand fractures reported.26 The common mechanism is a full hit on the hand, and a direct hit from the ball is another possible mechanism in goalkeepers.
In general, the diagnosis of hand injuries requires evaluation of the mechanism of injury and injury symptoms, proper and comprehensive physical examination of the whole extremity, and prompt imaging. In most cases, plain radiographs in several projections will suffice for the diagnosis of obvious fractures, but CT scan is an additional modality that allows for improved appreciation of occult or complex and comminuted fracture patterns. MRI or ultrasound can be used additionally whenever associated soft tissue injury is suspected. Optimal management of the hand is based on the specific characteristics of the fractures, which include location, direction of the fracture line, presence of comminution, displacement, articular involvement, and associated soft tissue injury. Nondisplaced extra-articular fractures often can be treated with buddy taping or splinting, whereas intra-articular fractures often require surgical treatment. Displaced fractures of the hand have a tendency to angulate volarly because of attachments of the interosseous muscles. Marginal fractures or avulsion fractures involving the metacarpals or phalanges can be sentinels of serious associated soft tissue injuries.27
Phalangeal fractures can potentially affect the function of the entire hand; therefore, no malrotation is acceptable for phalangeal fractures because they can lead to overlap and malalignment of the digit. Displaced or malrotated fractures should be reduced either by closed or open techniques. Acceptable reduction is <6 mm of shortening, <15° of angulation, and no rotational deformity.27,28 Nondisplaced phalangeal fractures can be treated nonoperatively with buddy taping and splinting with good results.27 Interphalangeal (IP) dislocations can be reduced on the sidelines and then taped or splinted. Any injury with a force significant enough to cause joint dislocation indicates further evaluation for associated fractures and ligamentous injury or tear. The proximal interphalangeal (PIP) joint is the most common IP joint dislocation and is usually a dorsal dislocation. Reduction is often achieved by traction and flexion of the middle phalanx,27 followed by splinting of the finger with the PIP in 30° of flexion or an extension block splint.29 Successful reduction with no associated intra-articular fractures involving more than a third of the joint can be further managed nonoperatively with the splint, allowing 2 to 4 weeks for the volar plate, joint capsule, and collateral ligaments to heal. Additional 2 to 4 weeks of splinting with buddy taping to the adjacent finger is usually recommended.29
The “Mallet finger” injury can be observed in goalkeepers and is caused by a flexion force on the tip of the finger while the distal interphalangeal (DIP) joint is extended. This force results in tearing of the extensor tendon or an avulsion fracture at the tendinous attachment on the dorsal lip of the distal phalangeal base. The classic mechanism of injury is an extended finger struck on the tip by a ball. Physical examination will indicate loss of DIP joint active extension, and the joint rests in an abnormally flexed position. Treatment typically consists of splinting the DIP joint in extension for 6 to 8 weeks. Operative treatment is reserved for severe injuries or fractures involving greater than one-third of the articular surface of the DIP joint or with failed nonoperative treatment.27
Metacarpal fractures can be subdivided into distal, metacarpal neck, metacarpal shaft, and metacarpal base fractures. Metacarpal shaft fractures raise a specific concern regarding rotation, because even a small degree of rotation can create a substantial degree of deformity at the fingertip. This concern must be addressed during evaluation of the player. Fractures of the metacarpal base most commonly involve the fourth and fifth metacarpals and are often reduced easily but have a tendency to re-subluxate, which may indicate operative treatment. Most fractures of the metacarpals are low energy and result in simple fracture patterns that can be treated nonoperatively. Open reduction is reserved for high-energy trauma, fractures with excessive angulation, or multiple fractures.27
Continue to: An important subgroup of metacarpal injuries...
An important subgroup of metacarpal injuries involves the base of the thumb. These injuries result from an axial load applied to the thumb. The most common injury is the “Bennett fracture,” which is an intra-articular fracture or dislocation involving the base of the first metacarpal. Bennett fractures are unstable fractures; unless properly recognized and treated, this intra-articular fracture subluxation may result in an unstable arthritic first carpometacarpal joint. These fractures are most commonly treated with closed or open reduction combined with internal fixation.27 “Rolando fractures” are similar in location and etiology but are comminuted and usually require operative treatment.27, 29
Another common hand injury found in soccer goalkeepers and involving the base of the thumb is disruption of the ulnar collateral ligament (UCL) of the first metacarpophalangeal (MCP) joint as a result of an acute radial or valgus stress on the thumb. Known as “gamekeeper’s thumb” or “skier’s thumb,” this injury can occur in the form of an avulsion fracture, an isolated ligament tear, or combined fracture and ligament rupture. On examination, swelling and tenderness over the thumb UCL are observed. A MCP joint stress test should be performed by gently applying a radially directed force to the thumb while stabilizing the metacarpal bone at both 0° and 30° at the MCP joint. Increased laxity, a soft or nonexistent end point, and gaping of the joint, as compared with the contralateral side, will indicate this injury.29 Radiographs may show a small avulsion fracture fragment at the ulnar aspect of the base of the first metacarpal and at the attachment of the UCL. A Stener lesion is an abnormality that occurs when the thumb adductor muscle aponeurosis interposes between the 2 ends of the ruptured UCL, preventing UCL healing by immobilization alone. Ultrasound and MRI are additional imaging modalities that can assist with the diagnosis of a Stener lesion. The presence of a Stener lesion is a prime indication for surgical intervention. A nondisplaced fracture or isolated ligament injury with no evidence of a Stener lesion can be treated nonoperatively with splinting of the thumb and may lead to healing and restoration of stability. However, in professional players, surgical repair is often times preferred.27
CONCLUSION
Upper extremity injuries are less common injuries among soccer players, but their prevalence is on the rise in recent years. Modern playing tactics and the increase in participation in soccer in younger age groups may be 2 contributing factors to this rise. Given the characteristics of their unique playing role and specific demands, the risk for upper extremity injuries among goalkeepers is significantly higher than that in outfielders and will usually result in a long absence period from soccer before they return to play. A thorough understanding of the mechanism of injury, players’ complaints and presentation, osseous and soft tissue involvement based on a systematic physical examination, imaging features, and treatment options is important for the optimal care of the players. Prompt and accurate diagnosis and appropriate management are essential for improved outcomes and timely return to play.
ABSTRACT
Upper limb injuries in soccer represent only a marginal portion of injuries, however this is mainly true for outfield players. Goalkeepers are reported to have up to 5 times more upper extremity injuries, many of them requiring substantial time-loss for treatment and rehabilitation. The most common upper extremity injury locations are the shoulder/clavicle followed by the hand/finger/thumb, elbow, wrist, forearm, and upper arm. The mechanism of injury, presentation, physical examination, and imaging features all play a significant role in reaching the correct diagnosis. Taking to consideration the position the player plays and his demands will also enable tailoring the optimal treatment plan that allows timely and safe return to play. This article discusses common upper extremity injuries observed in soccer players, focusing on proper diagnosis and optimal management.
Continue to: Upper limb injuries in association with soccer...
Upper limb injuries in association with soccer have been reported to represent only 3% of all time-loss injuries in professional soccer players1. However, they are considered an increasing problem in recent years2-4 and have been reported in high proportions in children under the age of 15 years.5 Some of the reasons for the increase in upper extremity injuries may be explained by modern soccer tactics that have been characterized by high speed, pressing, and marking.2 Furthermore, upper extremity injuries may still be underestimated in soccer, mainly because outfield players are sometimes able to train and play even when they suffer from an upper extremity injury.
Unsurprisingly, upper extremity injuries are reported to be up to 5 times more common in goalkeepers than in outfield players,1,2 reaching a high rate of up to 18% of all injuries among professional goalkeepers. The usage of upper extremities to stop the ball and repeated reaching to the ball and landing on the ground with changing upper extremity positions are some of the contributors to the increased upper extremity injury risk in goalkeepers.
Following 57 male professional European soccer teams from 16 countries between the years 2001 and 2011, Ekstrand and colleagues1 showed that 90% of upper extremity injuries are traumatic, and only 10% are related to overuse. They also reported that the most common upper extremity injury location is the shoulder/clavicle (56%), followed by the hand/finger/thumb (24%), elbow (10%), wrist (5%), forearm (4%), and upper arm (1%). Specifically, the 6 most common injuries are acromioclavicular joint (ACJ) sprain (13%), shoulder dislocation (12%), hand metacarpal fracture (8%), shoulder rotator cuff tendinopathy (6%), hand phalanx fracture (6%), and shoulder ACJ dislocation (5%).
This article will discuss common upper extremity injuries observed in soccer players, focusing on proper diagnosis and optimal management.
Continue to: THE SHOULDER...
THE SHOULDER
The majority of upper extremity injuries in professional soccer players are shoulder injuries.1,2,4 Almost a third of these injuries (28%) are considered severe, preventing participation in training and matches for 28 days or more.6Ekstrand and colleagues1 reported that shoulder dislocation represents the most severe upper extremity injury with a mean of 41 days of absence from soccer. When considering the position of the player, they further demonstrated that absence from full training and matches is twice as long for goalkeepers as for outfield players, which reflects the importance of shoulder function for goalkeepers.
In terms of the mechanism of shoulder instability injuries in soccer players, more than half (56%) of these injuries occur with a high-energy mechanism in the recognized position of combined humeral abduction and external rotation against a force of external rotation and horizontal extension.3 However, almost a quarter (24%) occur with a mechanism of varied upper extremity position and low-energy trauma, and 20% of injuries are either a low energy injury with little or no contact or gradual onset. These unique characteristics of shoulder instability injuries in soccer players should be accounted for during training and may imply that current training programs are suboptimal for the prevention of upper extremity injuries and shoulder injuries. Ejnisman and colleagues2 reported on the development of a Fédération Internationale de Football Association (FIFA) 11+ shoulder injury prevention program for soccer goalkeepers as one of the ways to promote training programs that address the risk of shoulder injuries.
Reporting on the management of severe shoulder injuries requiring surgery in 25 professional soccer players in England, between 2007 and 2011, Hart and Funk3 found that the majority of subjects (88%) reported a dislocation as a feature of their presentation. Twenty-one (84%) subjects were diagnosed with labral injuries, of which 7 had an associated Hill-Sachs lesion. Two (8%) subjects were diagnosed with rotator cuff tears requiring repair, and 2 (8%) subjects had a combination of rotator cuff and labral injury repair. All patients underwent arthroscopic repair, except for 5 who had a Latarjet coracoid transfer. Post-surgery, all players were able to return to unrestricted participation in soccer at a mean of 11.4 weeks, with no significant difference between goalkeepers and outfield players and no recurrences at a mean of 91 weeks’ follow-up.
Up to one-third of shoulder instability injuries in soccer players are reported to be recurrences,1,3 which emphasizes the need to carefully assess soccer players before clearing them to return to play. These data raise the controversy over the treatment of first time shoulder dislocators and may support early surgical intervention.7-9 In terms of the preferred surgical intervention in these cases, Balg and Boileau10 suggested a simple scoring system based on factors derived from a preoperative questionnaire, physical examination, and anteroposterior radiographs to help distinguish between patients who will benefit from an arthroscopic anterior stabilization using suture anchors and those who will require a bony procedure (open or arthroscopic). Cerciello and colleagues11 reported excellent results for bony stabilization (modified Latarjet) in a population of 26 soccer players (28 shoulders) affected by chronic anterior instability. Only 1 player did not return to soccer, and 18 players (20 shoulders, 71%) returned to the same level. One re-dislocation was noted in a goalkeeper 74 months after surgery.
An injury to the ACJ has been previously reported to be the most prevalent type of shoulder injury in contact sports.12In soccer, injury to the ACJ is responsible for 18% of upper extremity injuries, and the majority (72%) are sprains.1Interestingly, but unsurprisingly, implications of such an injury differ significantly between goalkeepers and outfield players with up to 3 times longer required absence periods for goalkeepers vs outfield players sustaining the same injury.
ACJ injury is commonly the result of a direct fall on the shoulder with the arm adducted or extended. Six grades of ACJ injuries have been described and distinguished by the injured anatomical structure (acromioclavicular ligaments and coracoclavicular ligaments) and the direction and magnitude of clavicular dislocation.13,14 Presentation will usually include anterior shoulder pain, a noticeable swelling or change in morphology of the lateral end of the clavicle (mainly in dislocation types), and sharp pain provoked by palpation of the ACJ. Radiographic imaging will confirm the diagnosis and help with identifying the specific grade/type of injury.
Decision making and management of acute ACJ injury should be based on the type/grade of injury. Nonoperative treatment is recommended for types I and II, and most athletes have a successful outcome with a full return to play.12Types IV, V, and VI are treated early with operative intervention, mostly due to the morbidity associated with prolonged dislocation of the joint and subsequent soft tissue damage.12 Treatment of type III injury remains controversial. Pereira-Graterol and colleagues15 reported the effectiveness of clavicular hook plate (DePuy Synthes) in the surgical stabilization of grade III ACJ dislocation in 11 professional soccer players. At a mean follow-up of 4 years, they showed excellent functional results with full shoulder range of motion at 5 weeks and latest return to soccer at 6 months. The hook plate was removed after 16 weeks in 10 patients in whom no apparent complication was observed.
Continue to: THE ELBOW...
THE ELBOW
Ekstrand and colleagues1 reported that 10% of all upper extremity injuries in professional soccer players are elbow injuries, of which only 19% are considered severe injuries that require more than 28 days of absence from playing soccer. The most common elbow injuries in their cohort were elbow medial collateral ligament (MCL) sprain and olecranon bursitis.
Elbow MCL is the primary constraint of the elbow joint to valgus stress, and MCL sprain occurs when the elbow is subjected to a valgus, or laterally directed force, which distracts the medial side of the elbow, exceeding the tensile properties of the MCL.16 A thorough physical examination that includes valgus stress tests through the arc of elbow flexion and extension to elicit a possible subjective feeling of apprehension, instability, or localized pain is essential for optimal evaluation and treatment.16,17 Imaging studies (X-ray and stress X-rays, dynamic ultrasound, computed tomography [CT], magnetic resonance imaging [MRI], and MR arthrography) have a role in further establishing the diagnosis and identifying possible additional associated injuries.16 The treatment plan should be specifically tailored to the individual athlete, depending on his demands and the degree of MCL injury. In soccer, which is a non-throwing shoulder sport, nonoperative treatment should be the preferred initial treatment in most cases. Ekstrand and colleagues1 showed that this injury requires a mean of 4 days of absence from soccer for outfield players and a mean of 21 days of absence from soccer for goalkeepers, thereby indicating more severe sprains and cautious return to soccer in goalkeepers. Athletes who fail nonoperative treatment are candidates for MCL reconstruction.16
The olecranon bursa is a synovium-lined sac that facilitates gliding between the olecranon and overlying skin. Olecranon bursitis is characterized by accumulation of fluid in the bursa with or without inflammation. The fluid can be serous, sanguineous, or purulent depending on the etiology.18 In soccer, traumatic etiology is common, but infection secondary to cuts or scratches of the skin around the elbow or previous therapeutic injections around the elbow should always be ruled out. Local pain, swelling, warmth, and redness are usually the presenting symptoms. Aseptic olecranon bursitis may be managed non-surgically with ‘‘benign neglect’’ and avoidance of pressure to the area, non-steroidal anti-inflammatory drugs, needle aspiration, corticosteroid injection, compression dressings, and/or padded splinting; whereas acute septic bursitis requires needle aspiration for diagnosis, appropriate oral or intravenous antibiotics directed toward the offending organism, and, when clinically indicated, surgical evacuation/excision of the bursa.18 When treating this condition with cortisone injection, possible complications, such as skin atrophy, secondary infection, and chronic local pain, have been reported and should be considered.19
Severe elbow injuries in professional athletes in general,20-22 and soccer players specifically, are elbow subluxations/dislocations and elbow fracture. The mechanism of injury is usually contact injury with an opponent player or a fall on the palm with the arm extended. Posterolateral is the most common type of elbow dislocation. Elbow dislocations are further classified into simple (no associated fractures) and complex (associated with fractures) categories.22 Simple dislocations are usually treated with early mobilization after closed reduction; it is associated with a low risk for re-dislocation and with generally good results. The complex type of elbow fracture dislocation is more difficult to treat, has higher complication and re-dislocation rates, and requires operative treatment in most cases compared with simple dislocation.22 The “terrible triad” of the elbow (posterior elbow dislocation, radial head fracture, and coronoid fracture) represents a specific complex elbow dislocation scenario that is difficult to manage because of conflicting aims of ensuring elbow stability while maintaining early range of motion.22
Isolated fracture around the elbow should be treated based on known principles of fracture management: mechanism of injury, fracture patterns, fracture displacement, intra-articular involvement, soft tissue condition, and associated injuries.
Continue to: THE WRIST...
THE WRIST
Ekstrand and colleagues1 reported that 5% of all upper extremity injuries in their cohort of professional soccer players are wrist injuries, of which, only 2% are considered severe injuries that require >28 days of absence from playing soccer. The more common wrist injuries in soccer, which is considered a high-impact sport, are fractures (distal radius, scaphoid, capitate), and less reported injuries are dislocations (lunate, perilunate) and ligamentous injuries or tears (scapholunate ligament).23
Distal radius fractures in high-impact sports, like soccer, usually occur as a result of a fall on an out-stretched hand and will usually be more comminuted, displaced, and intra-articular compared with low-impact sports.23 All these aforementioned characteristics usually indicate surgical management of open reduction and internal fixation, which will allow for rapid start of rehabilitation and return to play.
Scaphoid fracture is the most common carpal bone fracture and presents unique challenges in terms of diagnosis and optimal treatment24 in professional athletes. A typical injury scenario would be a player falling on an outstretched hand and sustaining a scaphoid fracture during a match or training session. The player may acknowledge some wrist pain but will often continue to play with minimal or no limitation. As wrist pain and swelling become more evident after the match/training session, the player will seek medical evaluation.24 A complete wrist and upper extremity examination should be performed in addition to a specific assessment, which includes palpation of the distal scaphoid pole at the distal wrist flexion crease, palpation of the scaphoid waist through the wrist snuff box, and palpation dorsally just distal to the Lister tubercle at the scapholunate joint. Any wrist injury that results in decreased range of motion, snuff box swelling, and scaphoid tenderness should be further evaluated with imaging. Plain radiographs with special scaphoid views are the initial preferred imaging studies, but occult fracture will require an additional study such as a bone scan, CT, or MRI. Several studies have validated the benefit of MRI and the fact that it may outweigh the costs associated with lost productivity from unnecessary cast immobilization, especially in elite athletes.23-25Casting the patient with a nondisplaced scaphoid waist fracture has been the traditional treatment; however, stiffness, weakness, and deconditioning that can occur with long-term casting required for scaphoid fractures are significant impairments for the professional athlete and usually end the player’s season. Surgical treatment, which was traditionally indicated for displaced or proximal pole fractures, is currently also considered for non-displaced scaphoid waist fractures in professional athletes. This treatment allows for a rapid return to the rehabilitation of the extremity and possible early return to professional sport. In view of the known complications (eg, malunion, nonunion, and avascular necrosis), return to soccer can be considered when imaging confirms advanced healing, which some consider as at least 50% of bone fracture bridging on CT scan, no pain, excellent motion, and at least 80% of normal grip strength.24 Outfield players can return to play with a protective cast or brace until full healing is observed on imaging.
Continue to: THE HAND/FINGERS/THUMB...
THE HAND/FINGERS/THUMB
Almost a quarter of upper extremity injuries in professional soccer players were reported to involve the hand, fingers, and thumb. A quarter of them were classified as severe injuries requiring >28 days of absence from playing soccer.1Specifically, hand metacarpal and phalanx fractures are the most common reported injuries in sports in general,26 and in soccer,1 and account for 14% of all upper extremity injuries1 in professional soccer players. Goalkeepers require a functional hand to play, whereas an outfielder can play with protection on the injured area; thus, the period of absence from soccer in these injuries is significantly different between goalkeepers and outfielders with more than 4 times longer absence from soccer for a goalkeeper compared with an outfielder. The fifth ray has been shown to be the most frequently fractured ray in the hand in soccer with 51.7% of all hand fractures reported.26 The common mechanism is a full hit on the hand, and a direct hit from the ball is another possible mechanism in goalkeepers.
In general, the diagnosis of hand injuries requires evaluation of the mechanism of injury and injury symptoms, proper and comprehensive physical examination of the whole extremity, and prompt imaging. In most cases, plain radiographs in several projections will suffice for the diagnosis of obvious fractures, but CT scan is an additional modality that allows for improved appreciation of occult or complex and comminuted fracture patterns. MRI or ultrasound can be used additionally whenever associated soft tissue injury is suspected. Optimal management of the hand is based on the specific characteristics of the fractures, which include location, direction of the fracture line, presence of comminution, displacement, articular involvement, and associated soft tissue injury. Nondisplaced extra-articular fractures often can be treated with buddy taping or splinting, whereas intra-articular fractures often require surgical treatment. Displaced fractures of the hand have a tendency to angulate volarly because of attachments of the interosseous muscles. Marginal fractures or avulsion fractures involving the metacarpals or phalanges can be sentinels of serious associated soft tissue injuries.27
Phalangeal fractures can potentially affect the function of the entire hand; therefore, no malrotation is acceptable for phalangeal fractures because they can lead to overlap and malalignment of the digit. Displaced or malrotated fractures should be reduced either by closed or open techniques. Acceptable reduction is <6 mm of shortening, <15° of angulation, and no rotational deformity.27,28 Nondisplaced phalangeal fractures can be treated nonoperatively with buddy taping and splinting with good results.27 Interphalangeal (IP) dislocations can be reduced on the sidelines and then taped or splinted. Any injury with a force significant enough to cause joint dislocation indicates further evaluation for associated fractures and ligamentous injury or tear. The proximal interphalangeal (PIP) joint is the most common IP joint dislocation and is usually a dorsal dislocation. Reduction is often achieved by traction and flexion of the middle phalanx,27 followed by splinting of the finger with the PIP in 30° of flexion or an extension block splint.29 Successful reduction with no associated intra-articular fractures involving more than a third of the joint can be further managed nonoperatively with the splint, allowing 2 to 4 weeks for the volar plate, joint capsule, and collateral ligaments to heal. Additional 2 to 4 weeks of splinting with buddy taping to the adjacent finger is usually recommended.29
The “Mallet finger” injury can be observed in goalkeepers and is caused by a flexion force on the tip of the finger while the distal interphalangeal (DIP) joint is extended. This force results in tearing of the extensor tendon or an avulsion fracture at the tendinous attachment on the dorsal lip of the distal phalangeal base. The classic mechanism of injury is an extended finger struck on the tip by a ball. Physical examination will indicate loss of DIP joint active extension, and the joint rests in an abnormally flexed position. Treatment typically consists of splinting the DIP joint in extension for 6 to 8 weeks. Operative treatment is reserved for severe injuries or fractures involving greater than one-third of the articular surface of the DIP joint or with failed nonoperative treatment.27
Metacarpal fractures can be subdivided into distal, metacarpal neck, metacarpal shaft, and metacarpal base fractures. Metacarpal shaft fractures raise a specific concern regarding rotation, because even a small degree of rotation can create a substantial degree of deformity at the fingertip. This concern must be addressed during evaluation of the player. Fractures of the metacarpal base most commonly involve the fourth and fifth metacarpals and are often reduced easily but have a tendency to re-subluxate, which may indicate operative treatment. Most fractures of the metacarpals are low energy and result in simple fracture patterns that can be treated nonoperatively. Open reduction is reserved for high-energy trauma, fractures with excessive angulation, or multiple fractures.27
Continue to: An important subgroup of metacarpal injuries...
An important subgroup of metacarpal injuries involves the base of the thumb. These injuries result from an axial load applied to the thumb. The most common injury is the “Bennett fracture,” which is an intra-articular fracture or dislocation involving the base of the first metacarpal. Bennett fractures are unstable fractures; unless properly recognized and treated, this intra-articular fracture subluxation may result in an unstable arthritic first carpometacarpal joint. These fractures are most commonly treated with closed or open reduction combined with internal fixation.27 “Rolando fractures” are similar in location and etiology but are comminuted and usually require operative treatment.27, 29
Another common hand injury found in soccer goalkeepers and involving the base of the thumb is disruption of the ulnar collateral ligament (UCL) of the first metacarpophalangeal (MCP) joint as a result of an acute radial or valgus stress on the thumb. Known as “gamekeeper’s thumb” or “skier’s thumb,” this injury can occur in the form of an avulsion fracture, an isolated ligament tear, or combined fracture and ligament rupture. On examination, swelling and tenderness over the thumb UCL are observed. A MCP joint stress test should be performed by gently applying a radially directed force to the thumb while stabilizing the metacarpal bone at both 0° and 30° at the MCP joint. Increased laxity, a soft or nonexistent end point, and gaping of the joint, as compared with the contralateral side, will indicate this injury.29 Radiographs may show a small avulsion fracture fragment at the ulnar aspect of the base of the first metacarpal and at the attachment of the UCL. A Stener lesion is an abnormality that occurs when the thumb adductor muscle aponeurosis interposes between the 2 ends of the ruptured UCL, preventing UCL healing by immobilization alone. Ultrasound and MRI are additional imaging modalities that can assist with the diagnosis of a Stener lesion. The presence of a Stener lesion is a prime indication for surgical intervention. A nondisplaced fracture or isolated ligament injury with no evidence of a Stener lesion can be treated nonoperatively with splinting of the thumb and may lead to healing and restoration of stability. However, in professional players, surgical repair is often times preferred.27
CONCLUSION
Upper extremity injuries are less common injuries among soccer players, but their prevalence is on the rise in recent years. Modern playing tactics and the increase in participation in soccer in younger age groups may be 2 contributing factors to this rise. Given the characteristics of their unique playing role and specific demands, the risk for upper extremity injuries among goalkeepers is significantly higher than that in outfielders and will usually result in a long absence period from soccer before they return to play. A thorough understanding of the mechanism of injury, players’ complaints and presentation, osseous and soft tissue involvement based on a systematic physical examination, imaging features, and treatment options is important for the optimal care of the players. Prompt and accurate diagnosis and appropriate management are essential for improved outcomes and timely return to play.
1. Ekstrand J, Hagglund M, Tornqvist H, et al. Upper extremity injuries in male elite football players. Knee Surg Sports Traumatol Arthrosc. 2013;21(7):1626-1632. doi:10.1007/s00167-012-2164-6.
2. Ejnisman B, Barbosa G, Andreoli CV, et al. Shoulder injuries in soccer goalkeepers: Review and development of a FIFA 11+ shoulder injury prevention program. Open Access J Sports Med. 2016;7:75-80. doi:10.2147/OAJSM.S97917.
3. Hart D, Funk L. Serious shoulder injuries in professional soccer: Return to participation after surgery. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):2123-2129. doi:10.1007/s00167-013-2796-1.
4. Longo UG, Loppini M, Berton A, Martinelli N, Maffulli N, Denaro V. Shoulder injuries in soccer players. Clin Cases Miner Bone Metab. 2012;9(3):138-141.
5. Faude O, Rossler R, Junge A. Football injuries in children and adolescent players: Are there clues for prevention? Sports Med. 2013;43(9):819-837. doi:10.1007/s40279-013-0061-x.
6. Ekstrand J, Hagglund M, Walden M. Injury incidence and injury patterns in professional football: The UEFA injury study. Br J Sports Med. 2011;45(7):553-558. doi:10.1136/bjsm.2009.060582.
7. Boone JL, Arciero RA. First-time anterior shoulder dislocations: Has the standard changed? Br J Sports Med. 2010;44(5):355-360. doi:10.1136/bjsm.2009.062596.
8. Handoll HH, Almaiyah MA, Rangan A. Surgical versus non-surgical treatment for acute anterior shoulder dislocation. Cochrane Database Syst Rev. 2004;(1):CD004325.
9. Kirkley A, Werstine R, Ratjek A, Griffin S. Prospective randomized clinical trial comparing the effectiveness of immediate arthroscopic stabilization versus immobilization and rehabilitation in first traumatic anterior dislocations of the shoulder: Long-term evaluation. Arthroscopy. 2005;21(1):55-63.
10. Balg F, Boileau P. The instability severity index score. A simple pre-operative score to select patients for arthroscopic or open shoulder stabilisation. J Bone Joint Surg Br. 2007;89(11):1470-1477.
11. Cerciello S, Edwards TB, Walch G. Chronic anterior glenohumeral instability in soccer players: Results for a series of 28 shoulders treated with the latarjet procedure. J Orthop Traumatol. 2012;13(4):197-202. doi:10.1007/s10195-012-0201-3.
12. Bishop JY, Kaeding C. Treatment of the acute traumatic acromioclavicular separation. Sports Med Arthrosc Rev. 2006;14(4):237-245. doi:10.1097/01.jsa.0000212330.32969.6e.
13. de Putter CE, van Beeck EF, Burdorf A, et al. Increase in upper extremity fractures in young male soccer players in the netherlands, 1998-2009. Scand J Med Sci Sports. 2015;25(4):462-466. doi:10.1111/sms.12287.
14. Rockwood CJ, Williams G, Young D. Disorders of the acromioclavicular joint. In: Rockwood CJ, Matsen FA III, eds. The Shoulder. 2nd ed. Philadelphia: WB Saunders; 1998:483-553.
15. Pereira-Graterol E, Alvarez-Diaz P, Seijas R, Ares O, Cusco X, Cugat R. Treatment and evolution of grade III acromioclavicular dislocations in soccer players. Knee Surg Sports Traumatol Arthrosc. 2013;21(7):1633-1635. doi:10.1007/s00167-012-2186-0.
16. Rahman RK, Levine WN, Ahmad CS. Elbow medial collateral ligament injuries. Curr Rev Musculoskelet Med. 2008;1(3-4):197-204. doi:10.1007/s12178-008-9026-3.
17. Redler LH, Watling JP, Ahmad CS. Physical examination of the throwing athlete's elbow. Am J Orthop. 2015;44(1):13-18.
18. Sayegh ET, Strauch RJ. Treatment of olecranon bursitis: A systematic review. Arch Orthop Trauma Surg. 2014;134(11):1517-1536. doi:10.1007/s00402-014-2088-3.
19. Weinstein PS, Canoso JJ, Wohlgethan JR. Long-term follow-up of corticosteroid injection for traumatic olecranon bursitis. Ann Rheum Dis. 1984;43(1):44-46.
20. Carlisle JC, Goldfarb CA, Mall N, Powell JW, Matava MJ. Upper extremity injuries in the national football league: Part II: Elbow, forearm, and wrist injuries. Am J Sports Med. 2008;36(10):1945-1952. doi:10.1177/0363546508318198.
21. Dizdarevic I, Low S, Currie DW, Comstock RD, Hammoud S, Atanda A Jr. Epidemiology of elbow dislocations in high school athletes. Am J Sports Med. 2016;44(1):202-208. doi:10.1177/0363546515610527.
22. Saati AZ, McKee MD. Fracture-dislocation of the elbow: Diagnosis, treatment, and prognosis. Hand Clin. 2004;20(4):405-414.
23. Bancroft LW. Wrist injuries: A comparison between high- and low-impact sports. Radiol Clin North Am. 2013;51(2):299-311. doi:10.1016/j.rcl.2012.09.017.
24. Belsky MR, Leibman MI, Ruchelsman DE. Scaphoid fracture in the elite athlete. Hand Clin. 2012;28(3):78, vii. doi:10.1016/j.hcl.2012.05.005.
25. Mallee W, Doornberg JN, Ring D, van Dijk CN, Maas M, Goslings JC. Comparison of CT and MRI for diagnosis of suspected scaphoid fractures. J Bone Joint Surg Am. 2011;93(1):20-28. doi:10.2106/JBJS.I.01523.
26. Aitken S, Court-Brown CM. The epidemiology of sports-related fractures of the hand. Injury. 2008;39(12):1377-1383. doi:10.1016/j.injury.2008.04.012.
27. Peterson JJ, Bancroft LW. Injuries of the fingers and thumb in the athlete. Clin Sports Med. 2006;25(3):viii.
28. Walsh JJ 4th. Fractures of the hand and carpal navicular bone in athletes. South Med J. 2004;97(8):762-765.
29. Hong E. Hand injuries in sports medicine. Prim Care. 2005;32(1):91-103.
1. Ekstrand J, Hagglund M, Tornqvist H, et al. Upper extremity injuries in male elite football players. Knee Surg Sports Traumatol Arthrosc. 2013;21(7):1626-1632. doi:10.1007/s00167-012-2164-6.
2. Ejnisman B, Barbosa G, Andreoli CV, et al. Shoulder injuries in soccer goalkeepers: Review and development of a FIFA 11+ shoulder injury prevention program. Open Access J Sports Med. 2016;7:75-80. doi:10.2147/OAJSM.S97917.
3. Hart D, Funk L. Serious shoulder injuries in professional soccer: Return to participation after surgery. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):2123-2129. doi:10.1007/s00167-013-2796-1.
4. Longo UG, Loppini M, Berton A, Martinelli N, Maffulli N, Denaro V. Shoulder injuries in soccer players. Clin Cases Miner Bone Metab. 2012;9(3):138-141.
5. Faude O, Rossler R, Junge A. Football injuries in children and adolescent players: Are there clues for prevention? Sports Med. 2013;43(9):819-837. doi:10.1007/s40279-013-0061-x.
6. Ekstrand J, Hagglund M, Walden M. Injury incidence and injury patterns in professional football: The UEFA injury study. Br J Sports Med. 2011;45(7):553-558. doi:10.1136/bjsm.2009.060582.
7. Boone JL, Arciero RA. First-time anterior shoulder dislocations: Has the standard changed? Br J Sports Med. 2010;44(5):355-360. doi:10.1136/bjsm.2009.062596.
8. Handoll HH, Almaiyah MA, Rangan A. Surgical versus non-surgical treatment for acute anterior shoulder dislocation. Cochrane Database Syst Rev. 2004;(1):CD004325.
9. Kirkley A, Werstine R, Ratjek A, Griffin S. Prospective randomized clinical trial comparing the effectiveness of immediate arthroscopic stabilization versus immobilization and rehabilitation in first traumatic anterior dislocations of the shoulder: Long-term evaluation. Arthroscopy. 2005;21(1):55-63.
10. Balg F, Boileau P. The instability severity index score. A simple pre-operative score to select patients for arthroscopic or open shoulder stabilisation. J Bone Joint Surg Br. 2007;89(11):1470-1477.
11. Cerciello S, Edwards TB, Walch G. Chronic anterior glenohumeral instability in soccer players: Results for a series of 28 shoulders treated with the latarjet procedure. J Orthop Traumatol. 2012;13(4):197-202. doi:10.1007/s10195-012-0201-3.
12. Bishop JY, Kaeding C. Treatment of the acute traumatic acromioclavicular separation. Sports Med Arthrosc Rev. 2006;14(4):237-245. doi:10.1097/01.jsa.0000212330.32969.6e.
13. de Putter CE, van Beeck EF, Burdorf A, et al. Increase in upper extremity fractures in young male soccer players in the netherlands, 1998-2009. Scand J Med Sci Sports. 2015;25(4):462-466. doi:10.1111/sms.12287.
14. Rockwood CJ, Williams G, Young D. Disorders of the acromioclavicular joint. In: Rockwood CJ, Matsen FA III, eds. The Shoulder. 2nd ed. Philadelphia: WB Saunders; 1998:483-553.
15. Pereira-Graterol E, Alvarez-Diaz P, Seijas R, Ares O, Cusco X, Cugat R. Treatment and evolution of grade III acromioclavicular dislocations in soccer players. Knee Surg Sports Traumatol Arthrosc. 2013;21(7):1633-1635. doi:10.1007/s00167-012-2186-0.
16. Rahman RK, Levine WN, Ahmad CS. Elbow medial collateral ligament injuries. Curr Rev Musculoskelet Med. 2008;1(3-4):197-204. doi:10.1007/s12178-008-9026-3.
17. Redler LH, Watling JP, Ahmad CS. Physical examination of the throwing athlete's elbow. Am J Orthop. 2015;44(1):13-18.
18. Sayegh ET, Strauch RJ. Treatment of olecranon bursitis: A systematic review. Arch Orthop Trauma Surg. 2014;134(11):1517-1536. doi:10.1007/s00402-014-2088-3.
19. Weinstein PS, Canoso JJ, Wohlgethan JR. Long-term follow-up of corticosteroid injection for traumatic olecranon bursitis. Ann Rheum Dis. 1984;43(1):44-46.
20. Carlisle JC, Goldfarb CA, Mall N, Powell JW, Matava MJ. Upper extremity injuries in the national football league: Part II: Elbow, forearm, and wrist injuries. Am J Sports Med. 2008;36(10):1945-1952. doi:10.1177/0363546508318198.
21. Dizdarevic I, Low S, Currie DW, Comstock RD, Hammoud S, Atanda A Jr. Epidemiology of elbow dislocations in high school athletes. Am J Sports Med. 2016;44(1):202-208. doi:10.1177/0363546515610527.
22. Saati AZ, McKee MD. Fracture-dislocation of the elbow: Diagnosis, treatment, and prognosis. Hand Clin. 2004;20(4):405-414.
23. Bancroft LW. Wrist injuries: A comparison between high- and low-impact sports. Radiol Clin North Am. 2013;51(2):299-311. doi:10.1016/j.rcl.2012.09.017.
24. Belsky MR, Leibman MI, Ruchelsman DE. Scaphoid fracture in the elite athlete. Hand Clin. 2012;28(3):78, vii. doi:10.1016/j.hcl.2012.05.005.
25. Mallee W, Doornberg JN, Ring D, van Dijk CN, Maas M, Goslings JC. Comparison of CT and MRI for diagnosis of suspected scaphoid fractures. J Bone Joint Surg Am. 2011;93(1):20-28. doi:10.2106/JBJS.I.01523.
26. Aitken S, Court-Brown CM. The epidemiology of sports-related fractures of the hand. Injury. 2008;39(12):1377-1383. doi:10.1016/j.injury.2008.04.012.
27. Peterson JJ, Bancroft LW. Injuries of the fingers and thumb in the athlete. Clin Sports Med. 2006;25(3):viii.
28. Walsh JJ 4th. Fractures of the hand and carpal navicular bone in athletes. South Med J. 2004;97(8):762-765.
29. Hong E. Hand injuries in sports medicine. Prim Care. 2005;32(1):91-103.
TAKE-HOME POINTS
- Upper extremity injuries in soccer are not common, however they can reach up to 18% of all injuries in professional goalkeepers.
- Common injury locations in the upper extremity in soccer are the shoulder/clavicle, hand/finger/thumb, the elbow, and the wrist and most of these injuries are traumatic injuries.
- Mechanism of injury, players’ complaints and presentation, physical examination, and imaging features are all important for a proper evaluation and optimal management.
- Position of play is an important consideration in the management of upper extremity injuries in soccer. Outfield players may be able to return to play before a complete resolution of their injury, with protective accessories.
- Prompt and accurate diagnosis and appropriate management are essential for improved outcomes and timely return to play.
Screw Fixation Without Bone Grafting for Delayed Unions and Nonunions of Minimally Displaced Scaphoids
ABSTRACT
Delayed unions and nonunions of the scaphoid are most often treated by open reduction and internal fixation with bone grafting. We sought to evaluate a large consecutive series of nondisplaced or minimally displaced scaphoid nonunions and delayed unions treated by a compression screw without bone grafting by 2 fellowship trained hand surgeons. A total of 23 patients (19 males, 4 females) were identified who had fractures located at the distal third (2), the waist (18), and the proximal third (3). Of the 23 patients, 19 had a complete follow-up (mean follow-up period, 5.2 months) with evidence of radiographic union. There were no radiographic signs of arthrosis, osteonecrosis of the scaphoid, hardware-related complications, or reported revision surgeries. In conclusion, nonunions and delayed unions in nondisplaced or minimally displaced scaphoids without carpal malalignment can be successfully treated using compression screw fixation without bone grafting.
Continued to: Scaphoid nonunions or delayed unions with displacement...
Scaphoid nonunions or delayed unions with displacement, humpback deformities, or dorsal intercalated segmental instability deformities require open exposure with reduction of the fracture and autogenous bone grafting (structural or nonstructural and vascularized or nonvascularized).1,2 However, in the absence of displacement or deformity, compression and internal fixation without bone grafting may be sufficient to achieve union.
Several reports have described the use of internal fixation alone in the management of scaphoid nonunions with both minimal and extensive bone loss.3-7 These studies have shown that screw fixation alone affords less morbidity to the patient while allowing high rates of union.
Previous reports of internal fixation alone included limited numbers of patients for review. Therefore, we aim to review a large consecutive series of scaphoid delayed unions and nonunions without osteonecrosis or deformity managed by only internal fixation. Our hypothesis is that drilling combined with compression and rigid stabilization would allow for bony union in these cases
MATERIALS AND METHODS
After Institutional Review Board approval was obtained, a retrospective review of prospectively collected data was performed on consecutive patients with a delayed union or nonunion of the scaphoid. All injuries had failed conservative treatment of casting for at least 12 weeks and ultrasound stimulation, and were subsequently treated by compression screw fixation by 1 of 2 fellowship trained hand surgeons. The database comprised the data of patients who presented to a single, Level 1 trauma center between 2000 and 2012.
Delayed unions and nonunions were defined as a lack of radiographic trabecular bridging and pain on clinical examination at 3 and 6 months, respectively. All fractures were nondisplaced or minimally displaced (<2 mm), and patients with carpal malalignment or humpback deformity (based on scapholunate angle on plain radiographs) were excluded. Clinical outcome measures included evidence of radiographic union, revision surgery, pain, and reported complications.
Continue to: Inclusion criteria were all patients who sustained...
Inclusion criteria were all patients who sustained a minimally displaced scaphoid fracture and were treated conservatively with casting for at least 12 weeks and ultrasound stimulation, and progressed to delayed unions or nonunions.
Patients younger than age 18 years or with radiographic evidence of arthrosis or humpback deformity were excluded. Any fracture with >2 mm of gapping on original injury radiographs was not considered as minimally displaced and was also excluded. Furthermore, patients with a previous ipsilateral scaphoid injury or hand surgery were also excluded.
Compression screw placement was recorded as being either central or eccentric based on Trumble and colleagues’8 criteria. Posteroanterior (PA), lateral, and scaphoid view radiographs were reviewed by the first author (DS) and the treating hand surgeon (AS). Central screw placement was substantiated if the screw was in the middle third of the proximal pole in all 3 views.
The final set of postoperative radiographs was reviewed for unions. Union was defined as bridging trabeculation with near or complete obliteration of the fracture line on PA, lateral, and scaphoid radiographic views. Computerized tomography (CT) was performed at the discretion of the treating surgeon, and its use was not required if there was near obliteration of the fracture line on the 3-view radiographs and in the absence of patient-reported pain. Patients with bone loss or sclerosis were included as long as no deformity existed.
After surgical intervention, a short-arm cast was applied for 6 weeks, followed by a wrist splint for 4 to 8 weeks depending on patient comfort.
Continue to: SURGICAL TECHNIQUE...
SURGICAL TECHNIQUE
Either a 1-cm to 2-cm transverse incision distal to Lister’s tubercle or a longitudinal incision just ulnar was utilized. The extensor pollicis longus was identified and retracted. A longitudinal or an L-shaped capsulotomy was made to identify the proximal pole of the scaphoid. With the wrist flexed, a guide wire was inserted down the central axis of the scaphoid and confirmed by fluoroscopy. The measurement was made off the guidewire and 4 to 6 mm was subtracted. The scaphoid was then drilled, and the variable pitch compression screw (Acutrak Headless Compression Screw, Acumed) was inserted. Compression and position of the screw were confirmed by fluoroscopy before closure.
RESULTS
A total of 23 patients (19 males, 4 females) with acute scaphoid fractures who were treated conservatively with casting for at least 12 weeks and ultrasound stimulation, and progressed to delayed unions or nonunions were identified in this study. The ages of the patients ranged from 19 to 50 years. Of the 23 patients, 6 were smokers. The majority of patients sustained fractures in the scaphoid waist (18 patients) (Figure 1). Two patients had distal third fractures, and 3 had proximal third fractures.
The average time from the sustained injury to the surgical intervention was 8.2 months (range, 3.1-27.6 months). There were no patients with delayed diagnoses. Three fractures were identified as delayed unions with failure of union and pain after 3 months of conservative treatment, whereas the other 20 were identified as nonunions with at least 6 months of failed conservative treatment.
Of the 23 patients, 21 were found to have centrally placed variable compression screws based on Trumble and colleagues’8 criteria. Of the 23 patients, 19 had a complete follow-up course with radiographs at 6 months after surgery. All of these 19 patients had evidence of radiographic union defined as bridging trabeculation with near or complete obliteration of the fracture line on PA, lateral, and scaphoid radiographic views (Figure 2). Of the 6 smokers, 5 progressed to radiographic union and 1 patient had <6 months of postoperative return visits and could not be contacted. At the final clinic visit, all of the 19 patients denied wrist pain on direct palpation over the scaphoid tubercle, and no complications were reported. There were no repeat or revision surgical interventions.
Four patients had limited follow-up with <6 months of postoperative return visits. Their final set of radiographs did not demonstrate complete bridging trabeculation. One patient who moved away from the area was lost to follow-up but was contacted. The patient stated that he had a pain-free wrist with no further surgical interventions on his scaphoid. The other 3 patients could not be contacted.
DISCUSSION
The management of scaphoid nonunions and delayed unions has dramatically evolved over the past 20 years.1,3-8 Historically, semi-rigid stabilization using Kirschner wires and casting afforded a 77% union rate in these cases.9 More recently, several authors have reported that stabilization without bone grafting can predictably unite scaphoid nonunions. Treating patients with uncomplicated scaphoid nonunions and delayed unions by internal fixation alone may be all that is required to achieve union.
The definitions of a scaphoid nonunion and delayed union are complex. The exact time when a scaphoid fracture heals varies between patients.2,5,10 However, the majority of hand surgeons believe that failure to see clear signs of healing (in waist fractures) after 3 months from the injury would suggest a failure to heal and a “delayed” union, whereas failure after 6 months from the injury and without clear signs of healing indicate a nonunion.5,6,10,11 Any resorption at the fracture site suggests that the fracture will not heal by continued immobilization alone and will require surgery.10
Continue to: Hand surgeons have several surgical options...
Hand surgeons have several surgical options when managing scaphoid injuries. Mahmoud and Koptan4 used a volar approach to percutaneously deliver a headless compression screw into 27 nonunions. Postoperative CT scans demonstrated fracture union in all 27 patients, and no patient underwent revision surgery. Interestingly, 14 of their patients had extensive preoperative resorption (but no deformity) of >5 mm.
Although volar percutaneous approaches for internal fixation have been cited to provide high rates of union and high patient satisfaction in acute scaphoid fracture fixation, this study utilized a dorsal approach. Both Wozasek and Moser12 and Haddad and Goddard13 reported excellent results and high union rates using a volar approach in consecutive acute scaphoid fractures. Despite these results, there are concerns that using a volar approach may damage the scaphotrapezial joint and may be prone to eccentric placement of compression screws.8,14
Slade and colleagues3 did utilize the dorsal approach with arthroscopic assistance to deliver a compression screw into scaphoid nonunions in 15 consecutive patients without any evidence of deformity, sclerosis, or resorption. Similar to our investigation, they treated patients with both delayed unions and nonunions. CT scans were used to confirm unions in all their patients. Using a dorsal approach, Yassaee and Yang15 treated 9 consecutive patients using a compression screw without bone grafting for both delayed and nonunion scaphoid injuries. Other authors have used both volar and dorsal approaches in 12 consecutive delayed and nonunion scaphoid injuries and found that 11 of the 12 injuries progressed to unions.6
Although these authors and several others advocate the use of CT scans to assess unions, our investigation used bridging trabeculation obliteration of the fracture line on 3 standard radiographic views to confirm unions in addition to the absence of pain clinically.16,17 CT scans expose the patient to increased radiation that, in our experience, does not alter the postoperative clinical course.18 If there is clear evidence of bridged callus and no pain on physical examination, a CT scan performed to reconfirm the union affords little benefit to clinical management.19
Continue to: All these previous studies have demonstrated...
All these previous studies have demonstrated excellent union rates but using a limited series of patients. We reviewed a large number of consecutive patients with scaphoid delayed unions and nonunions treated by screw fixation without bone grafting. Our hospital is a safety net institution for a large urban catchment area and had complete radiographic and clinical data for 19 of our 23 patients. One patient was contacted by telephone and he reported no pain and no revision surgical interventions.
The limitations of this study include not only its retrospective design but also its limited secondary outcome measures. However, our primary outcomes of union, pain, and complications are of utmost importance to clinicians and patients alike. Similar to other authors, we used radiographs to confirm unions. Although bridging trabeculation in radiographs has been demonstrated as soon as 1 month after the injury, there may be problems with interobserver reliability.4,13,15,20,21
Patients being lost to follow-up is not uncommon in the orthopedic trauma literature and can influence results.22,23 It is speculative to infer that the 3 patients who did not complete a follow-up course did not return because their pain had mitigated.
CONCLUSION
Like several fractures, the lack of stability and the absence of micro-motion are believed to contribute to fibrous nonunions in scaphoid fractures.13 This study provides a large consecutive cohort of patients with minimally displaced scaphoid delayed unions and nonunions that were successfully treated by rigid internal fixation without bone grafting. These results confirm previous reports that bone grafting is not required to provide predictable unions for the majority of scaphoid nonunions.
This paper will be judged for the Resident Writer’s Award.
1. Trumble TE, Salas P, Barthel T, Robert KQ 3rd. Management of scaphoid nonunions. J Am Acad Orthop Surg. 2003;11(6):380-391. doi:10.1016/j.jhsa.2012.03.002.
2. Munk B, Larsen CF. Bone grafting the scaphoid nonunion: a systematic review of 147 publications including 5,246 cases of scaphoid nonunion. Acta Orthop Scand. 2004;75(5):618-629. doi:10.1080/00016470410001529.
3. Slade JF 3rd, Geissler WB, Gutow AP, Merrell GA. Percutaneous internal fixation of selected scaphoid nonunions with an arthroscopically assisted dorsal approach. J Bone Joint Surg Am. 2003;85-A Suppl 4:20-32.
4. Mahmoud M, Koptan W. Percutaneous screw fixation without bone grafting for established scaphoid nonunion with substantial bone loss. J Bone Joint Surg Br. 2011;93(7):932-936. doi:10.1302/0301-620X.93B7.25418.
5. Inaparthy PK, Nicholl JE. Treatment of delayed/nonunion of scaphoid waist with Synthes cannulated scaphoid screw and bone graft. Hand N Y N. 2008;3(4):292-296. doi:10.1007/s11552-008-9112-4.
6. Capo JT, Shamian B, Rizzo M. Percutaneous screw fixation without bone grafting of scaphoid non-union. Isr Med Assoc J. 2012;14(12):729-732.
7. Kim JK, Kim JO, Lee SY. Volar percutaneous screw fixation for scaphoid waist delayed union. Clin Orthop Relat Res. 2010;468(4):1066-1071. doi:10.1007/s11999-009-1032-2.
8. Trumble TE, Clarke T, Kreder HJ. Non-union of the scaphoid. Treatment with cannulated screws compared with treatment with Herbert screws. J Bone Joint Surg Am. 1996;78(12):1829-1837.
9. Cosio MQ, Camp RA. Percutaneous pinning of symptomatic scaphoid nonunions. J Hand Surg. 1986;11(3):350-355. doi:10.1016/S0363-5023(86)80141-1.
10. Steinmann SP, Adams JE. Scaphoid fractures and nonunions: diagnosis and treatment. J Orthop Sci. 2006;11(4):424-431. doi:10.1007/s00776-006-1025-x.
11. Zarezadeh A, Moezi M, Rastegar S, Motififard M, Foladi A, Daneshpajouhnejad P. Scaphoid nonunion fracture and results of the modified Matti-Russe technique. Adv Biomed Res. 2015;4:39. doi:10.4103/2277-9175.151248.
12. Wozasek GE, Moser KD. Percutaneous screw fixation for fractures of the scaphoid. J Bone Joint Surg Br. 1991;73(1):138-142. doi:10.3928/01477447-20170509-04.
13. Haddad FS, Goddard NJ. Acute percutaneous scaphoid fixation. A pilot study. J Bone Joint Surg Br. 1998;80(1):95-99. doi:10.1302/0301-620X.80B1.8076.
14. Yip HSF, Wu WC, Chang RYP, So TYC. Percutaneous cannulated screw fixation of acute scaphoid waist fracture. J Hand Surg Br. 2002;27(1):42-46. doi:10.1054/jhsb.2001.0690.
15. Yassaee F, Yang SS. Mini-incision fixation of nondisplaced scaphoid fracture nonunions. J Hand Surg. 2008;33(7):1116-1120. doi:10.1016/j.jhsa.2008.03.004.
16. Slade JF 3rd, Gillon T. Retrospective review of 234 scaphoid fractures and nonunions treated with arthroscopy for union and complications. Scand J Surg. 2008;97(4):280-289. doi:10.1177/145749690809700402
17. Geoghegan JM, Woodruff MJ, Bhatia R, et al. Undisplaced scaphoid waist fractures: is 4 weeks’ immobilisation in a below-elbow cast sufficient if a week 4 CT scan suggests fracture union? J Hand Surg Eur Vol. 2009;34(5):631-637. doi:10.1177/1753193409105189.
18. Biswas D, Bible JE, Bohan M, Simpson AK, Whang PG, Grauer JN. Radiation exposure from musculoskeletal computerized tomographic scans. J Bone Joint Surg Am. 2009;91(8):1882-1889. doi:10.2106/JBJS.H.01199.
19. Dias JJ, Taylor M, Thompson J, Brenkel IJ, Gregg PJ. Radiographic signs of union of scaphoid fractures. An analysis of inter-observer agreement and reproducibility. J Bone Joint Surg Br. 1988;70(2):299-301. doi:10.1302/0301-620X.70B2.3346310.
20. Martus JE, Bedi A, Jebson PJL. Cannulated variable pitch compression screw fixation of scaphoid fractures using a limited dorsal approach. Tech Hand Up Extrem Surg. 2005;9(4):202-206. doi:10.1097/01.bth.0000191422.26565.25.
21. Clay NR, Dias JJ, Costigan PS, Gregg PJ, Barton NJ. Need the thumb be immobilised in scaphoid fractures? A randomised prospective trial. J Bone Joint Surg Br. 1991;73(5):828-832. doi:10.1302/0301-620X.73B5.1894676.
22. Zelle BA, Bhandari M, Sanchez AI, Probst C, Pape HC. Loss of follow-up in orthopaedic trauma: is 80% follow-up still acceptable? J Orthop Trauma. 2013;27(3):177-181. doi:10.1097/BOT.0b013e31825cf367.
23. Sprague S, Leece P, Bhandari M, et al. Limiting loss to follow-up in a multicenter randomized trial in orthopedic surgery. Control Clin Trials. 2003;24(6):719-725. doi:10.1016/j.cct.2003.08.012.
ABSTRACT
Delayed unions and nonunions of the scaphoid are most often treated by open reduction and internal fixation with bone grafting. We sought to evaluate a large consecutive series of nondisplaced or minimally displaced scaphoid nonunions and delayed unions treated by a compression screw without bone grafting by 2 fellowship trained hand surgeons. A total of 23 patients (19 males, 4 females) were identified who had fractures located at the distal third (2), the waist (18), and the proximal third (3). Of the 23 patients, 19 had a complete follow-up (mean follow-up period, 5.2 months) with evidence of radiographic union. There were no radiographic signs of arthrosis, osteonecrosis of the scaphoid, hardware-related complications, or reported revision surgeries. In conclusion, nonunions and delayed unions in nondisplaced or minimally displaced scaphoids without carpal malalignment can be successfully treated using compression screw fixation without bone grafting.
Continued to: Scaphoid nonunions or delayed unions with displacement...
Scaphoid nonunions or delayed unions with displacement, humpback deformities, or dorsal intercalated segmental instability deformities require open exposure with reduction of the fracture and autogenous bone grafting (structural or nonstructural and vascularized or nonvascularized).1,2 However, in the absence of displacement or deformity, compression and internal fixation without bone grafting may be sufficient to achieve union.
Several reports have described the use of internal fixation alone in the management of scaphoid nonunions with both minimal and extensive bone loss.3-7 These studies have shown that screw fixation alone affords less morbidity to the patient while allowing high rates of union.
Previous reports of internal fixation alone included limited numbers of patients for review. Therefore, we aim to review a large consecutive series of scaphoid delayed unions and nonunions without osteonecrosis or deformity managed by only internal fixation. Our hypothesis is that drilling combined with compression and rigid stabilization would allow for bony union in these cases
MATERIALS AND METHODS
After Institutional Review Board approval was obtained, a retrospective review of prospectively collected data was performed on consecutive patients with a delayed union or nonunion of the scaphoid. All injuries had failed conservative treatment of casting for at least 12 weeks and ultrasound stimulation, and were subsequently treated by compression screw fixation by 1 of 2 fellowship trained hand surgeons. The database comprised the data of patients who presented to a single, Level 1 trauma center between 2000 and 2012.
Delayed unions and nonunions were defined as a lack of radiographic trabecular bridging and pain on clinical examination at 3 and 6 months, respectively. All fractures were nondisplaced or minimally displaced (<2 mm), and patients with carpal malalignment or humpback deformity (based on scapholunate angle on plain radiographs) were excluded. Clinical outcome measures included evidence of radiographic union, revision surgery, pain, and reported complications.
Continue to: Inclusion criteria were all patients who sustained...
Inclusion criteria were all patients who sustained a minimally displaced scaphoid fracture and were treated conservatively with casting for at least 12 weeks and ultrasound stimulation, and progressed to delayed unions or nonunions.
Patients younger than age 18 years or with radiographic evidence of arthrosis or humpback deformity were excluded. Any fracture with >2 mm of gapping on original injury radiographs was not considered as minimally displaced and was also excluded. Furthermore, patients with a previous ipsilateral scaphoid injury or hand surgery were also excluded.
Compression screw placement was recorded as being either central or eccentric based on Trumble and colleagues’8 criteria. Posteroanterior (PA), lateral, and scaphoid view radiographs were reviewed by the first author (DS) and the treating hand surgeon (AS). Central screw placement was substantiated if the screw was in the middle third of the proximal pole in all 3 views.
The final set of postoperative radiographs was reviewed for unions. Union was defined as bridging trabeculation with near or complete obliteration of the fracture line on PA, lateral, and scaphoid radiographic views. Computerized tomography (CT) was performed at the discretion of the treating surgeon, and its use was not required if there was near obliteration of the fracture line on the 3-view radiographs and in the absence of patient-reported pain. Patients with bone loss or sclerosis were included as long as no deformity existed.
After surgical intervention, a short-arm cast was applied for 6 weeks, followed by a wrist splint for 4 to 8 weeks depending on patient comfort.
Continue to: SURGICAL TECHNIQUE...
SURGICAL TECHNIQUE
Either a 1-cm to 2-cm transverse incision distal to Lister’s tubercle or a longitudinal incision just ulnar was utilized. The extensor pollicis longus was identified and retracted. A longitudinal or an L-shaped capsulotomy was made to identify the proximal pole of the scaphoid. With the wrist flexed, a guide wire was inserted down the central axis of the scaphoid and confirmed by fluoroscopy. The measurement was made off the guidewire and 4 to 6 mm was subtracted. The scaphoid was then drilled, and the variable pitch compression screw (Acutrak Headless Compression Screw, Acumed) was inserted. Compression and position of the screw were confirmed by fluoroscopy before closure.
RESULTS
A total of 23 patients (19 males, 4 females) with acute scaphoid fractures who were treated conservatively with casting for at least 12 weeks and ultrasound stimulation, and progressed to delayed unions or nonunions were identified in this study. The ages of the patients ranged from 19 to 50 years. Of the 23 patients, 6 were smokers. The majority of patients sustained fractures in the scaphoid waist (18 patients) (Figure 1). Two patients had distal third fractures, and 3 had proximal third fractures.
The average time from the sustained injury to the surgical intervention was 8.2 months (range, 3.1-27.6 months). There were no patients with delayed diagnoses. Three fractures were identified as delayed unions with failure of union and pain after 3 months of conservative treatment, whereas the other 20 were identified as nonunions with at least 6 months of failed conservative treatment.

Of the 23 patients, 21 were found to have centrally placed variable compression screws based on Trumble and colleagues’8 criteria. Of the 23 patients, 19 had a complete follow-up course with radiographs at 6 months after surgery. All of these 19 patients had evidence of radiographic union defined as bridging trabeculation with near or complete obliteration of the fracture line on PA, lateral, and scaphoid radiographic views (Figure 2). Of the 6 smokers, 5 progressed to radiographic union and 1 patient had <6 months of postoperative return visits and could not be contacted. At the final clinic visit, all of the 19 patients denied wrist pain on direct palpation over the scaphoid tubercle, and no complications were reported. There were no repeat or revision surgical interventions.
Four patients had limited follow-up with <6 months of postoperative return visits. Their final set of radiographs did not demonstrate complete bridging trabeculation. One patient who moved away from the area was lost to follow-up but was contacted. The patient stated that he had a pain-free wrist with no further surgical interventions on his scaphoid. The other 3 patients could not be contacted.

DISCUSSION
The management of scaphoid nonunions and delayed unions has dramatically evolved over the past 20 years.1,3-8 Historically, semi-rigid stabilization using Kirschner wires and casting afforded a 77% union rate in these cases.9 More recently, several authors have reported that stabilization without bone grafting can predictably unite scaphoid nonunions. Treating patients with uncomplicated scaphoid nonunions and delayed unions by internal fixation alone may be all that is required to achieve union.
The definitions of a scaphoid nonunion and delayed union are complex. The exact time when a scaphoid fracture heals varies between patients.2,5,10 However, the majority of hand surgeons believe that failure to see clear signs of healing (in waist fractures) after 3 months from the injury would suggest a failure to heal and a “delayed” union, whereas failure after 6 months from the injury and without clear signs of healing indicate a nonunion.5,6,10,11 Any resorption at the fracture site suggests that the fracture will not heal by continued immobilization alone and will require surgery.10
Continue to: Hand surgeons have several surgical options...
Hand surgeons have several surgical options when managing scaphoid injuries. Mahmoud and Koptan4 used a volar approach to percutaneously deliver a headless compression screw into 27 nonunions. Postoperative CT scans demonstrated fracture union in all 27 patients, and no patient underwent revision surgery. Interestingly, 14 of their patients had extensive preoperative resorption (but no deformity) of >5 mm.
Although volar percutaneous approaches for internal fixation have been cited to provide high rates of union and high patient satisfaction in acute scaphoid fracture fixation, this study utilized a dorsal approach. Both Wozasek and Moser12 and Haddad and Goddard13 reported excellent results and high union rates using a volar approach in consecutive acute scaphoid fractures. Despite these results, there are concerns that using a volar approach may damage the scaphotrapezial joint and may be prone to eccentric placement of compression screws.8,14
Slade and colleagues3 did utilize the dorsal approach with arthroscopic assistance to deliver a compression screw into scaphoid nonunions in 15 consecutive patients without any evidence of deformity, sclerosis, or resorption. Similar to our investigation, they treated patients with both delayed unions and nonunions. CT scans were used to confirm unions in all their patients. Using a dorsal approach, Yassaee and Yang15 treated 9 consecutive patients using a compression screw without bone grafting for both delayed and nonunion scaphoid injuries. Other authors have used both volar and dorsal approaches in 12 consecutive delayed and nonunion scaphoid injuries and found that 11 of the 12 injuries progressed to unions.6
Although these authors and several others advocate the use of CT scans to assess unions, our investigation used bridging trabeculation obliteration of the fracture line on 3 standard radiographic views to confirm unions in addition to the absence of pain clinically.16,17 CT scans expose the patient to increased radiation that, in our experience, does not alter the postoperative clinical course.18 If there is clear evidence of bridged callus and no pain on physical examination, a CT scan performed to reconfirm the union affords little benefit to clinical management.19
Continue to: All these previous studies have demonstrated...
All these previous studies have demonstrated excellent union rates but using a limited series of patients. We reviewed a large number of consecutive patients with scaphoid delayed unions and nonunions treated by screw fixation without bone grafting. Our hospital is a safety net institution for a large urban catchment area and had complete radiographic and clinical data for 19 of our 23 patients. One patient was contacted by telephone and he reported no pain and no revision surgical interventions.
The limitations of this study include not only its retrospective design but also its limited secondary outcome measures. However, our primary outcomes of union, pain, and complications are of utmost importance to clinicians and patients alike. Similar to other authors, we used radiographs to confirm unions. Although bridging trabeculation in radiographs has been demonstrated as soon as 1 month after the injury, there may be problems with interobserver reliability.4,13,15,20,21
Patients being lost to follow-up is not uncommon in the orthopedic trauma literature and can influence results.22,23 It is speculative to infer that the 3 patients who did not complete a follow-up course did not return because their pain had mitigated.
CONCLUSION
Like several fractures, the lack of stability and the absence of micro-motion are believed to contribute to fibrous nonunions in scaphoid fractures.13 This study provides a large consecutive cohort of patients with minimally displaced scaphoid delayed unions and nonunions that were successfully treated by rigid internal fixation without bone grafting. These results confirm previous reports that bone grafting is not required to provide predictable unions for the majority of scaphoid nonunions.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Delayed unions and nonunions of the scaphoid are most often treated by open reduction and internal fixation with bone grafting. We sought to evaluate a large consecutive series of nondisplaced or minimally displaced scaphoid nonunions and delayed unions treated by a compression screw without bone grafting by 2 fellowship trained hand surgeons. A total of 23 patients (19 males, 4 females) were identified who had fractures located at the distal third (2), the waist (18), and the proximal third (3). Of the 23 patients, 19 had a complete follow-up (mean follow-up period, 5.2 months) with evidence of radiographic union. There were no radiographic signs of arthrosis, osteonecrosis of the scaphoid, hardware-related complications, or reported revision surgeries. In conclusion, nonunions and delayed unions in nondisplaced or minimally displaced scaphoids without carpal malalignment can be successfully treated using compression screw fixation without bone grafting.
Continued to: Scaphoid nonunions or delayed unions with displacement...
Scaphoid nonunions or delayed unions with displacement, humpback deformities, or dorsal intercalated segmental instability deformities require open exposure with reduction of the fracture and autogenous bone grafting (structural or nonstructural and vascularized or nonvascularized).1,2 However, in the absence of displacement or deformity, compression and internal fixation without bone grafting may be sufficient to achieve union.
Several reports have described the use of internal fixation alone in the management of scaphoid nonunions with both minimal and extensive bone loss.3-7 These studies have shown that screw fixation alone affords less morbidity to the patient while allowing high rates of union.
Previous reports of internal fixation alone included limited numbers of patients for review. Therefore, we aim to review a large consecutive series of scaphoid delayed unions and nonunions without osteonecrosis or deformity managed by only internal fixation. Our hypothesis is that drilling combined with compression and rigid stabilization would allow for bony union in these cases
MATERIALS AND METHODS
After Institutional Review Board approval was obtained, a retrospective review of prospectively collected data was performed on consecutive patients with a delayed union or nonunion of the scaphoid. All injuries had failed conservative treatment of casting for at least 12 weeks and ultrasound stimulation, and were subsequently treated by compression screw fixation by 1 of 2 fellowship trained hand surgeons. The database comprised the data of patients who presented to a single, Level 1 trauma center between 2000 and 2012.
Delayed unions and nonunions were defined as a lack of radiographic trabecular bridging and pain on clinical examination at 3 and 6 months, respectively. All fractures were nondisplaced or minimally displaced (<2 mm), and patients with carpal malalignment or humpback deformity (based on scapholunate angle on plain radiographs) were excluded. Clinical outcome measures included evidence of radiographic union, revision surgery, pain, and reported complications.
Continue to: Inclusion criteria were all patients who sustained...
Inclusion criteria were all patients who sustained a minimally displaced scaphoid fracture and were treated conservatively with casting for at least 12 weeks and ultrasound stimulation, and progressed to delayed unions or nonunions.
Patients younger than age 18 years or with radiographic evidence of arthrosis or humpback deformity were excluded. Any fracture with >2 mm of gapping on original injury radiographs was not considered as minimally displaced and was also excluded. Furthermore, patients with a previous ipsilateral scaphoid injury or hand surgery were also excluded.
Compression screw placement was recorded as being either central or eccentric based on Trumble and colleagues’8 criteria. Posteroanterior (PA), lateral, and scaphoid view radiographs were reviewed by the first author (DS) and the treating hand surgeon (AS). Central screw placement was substantiated if the screw was in the middle third of the proximal pole in all 3 views.
The final set of postoperative radiographs was reviewed for unions. Union was defined as bridging trabeculation with near or complete obliteration of the fracture line on PA, lateral, and scaphoid radiographic views. Computerized tomography (CT) was performed at the discretion of the treating surgeon, and its use was not required if there was near obliteration of the fracture line on the 3-view radiographs and in the absence of patient-reported pain. Patients with bone loss or sclerosis were included as long as no deformity existed.
After surgical intervention, a short-arm cast was applied for 6 weeks, followed by a wrist splint for 4 to 8 weeks depending on patient comfort.
Continue to: SURGICAL TECHNIQUE...
SURGICAL TECHNIQUE
Either a 1-cm to 2-cm transverse incision distal to Lister’s tubercle or a longitudinal incision just ulnar was utilized. The extensor pollicis longus was identified and retracted. A longitudinal or an L-shaped capsulotomy was made to identify the proximal pole of the scaphoid. With the wrist flexed, a guide wire was inserted down the central axis of the scaphoid and confirmed by fluoroscopy. The measurement was made off the guidewire and 4 to 6 mm was subtracted. The scaphoid was then drilled, and the variable pitch compression screw (Acutrak Headless Compression Screw, Acumed) was inserted. Compression and position of the screw were confirmed by fluoroscopy before closure.
RESULTS
A total of 23 patients (19 males, 4 females) with acute scaphoid fractures who were treated conservatively with casting for at least 12 weeks and ultrasound stimulation, and progressed to delayed unions or nonunions were identified in this study. The ages of the patients ranged from 19 to 50 years. Of the 23 patients, 6 were smokers. The majority of patients sustained fractures in the scaphoid waist (18 patients) (Figure 1). Two patients had distal third fractures, and 3 had proximal third fractures.
The average time from the sustained injury to the surgical intervention was 8.2 months (range, 3.1-27.6 months). There were no patients with delayed diagnoses. Three fractures were identified as delayed unions with failure of union and pain after 3 months of conservative treatment, whereas the other 20 were identified as nonunions with at least 6 months of failed conservative treatment.

Of the 23 patients, 21 were found to have centrally placed variable compression screws based on Trumble and colleagues’8 criteria. Of the 23 patients, 19 had a complete follow-up course with radiographs at 6 months after surgery. All of these 19 patients had evidence of radiographic union defined as bridging trabeculation with near or complete obliteration of the fracture line on PA, lateral, and scaphoid radiographic views (Figure 2). Of the 6 smokers, 5 progressed to radiographic union and 1 patient had <6 months of postoperative return visits and could not be contacted. At the final clinic visit, all of the 19 patients denied wrist pain on direct palpation over the scaphoid tubercle, and no complications were reported. There were no repeat or revision surgical interventions.
Four patients had limited follow-up with <6 months of postoperative return visits. Their final set of radiographs did not demonstrate complete bridging trabeculation. One patient who moved away from the area was lost to follow-up but was contacted. The patient stated that he had a pain-free wrist with no further surgical interventions on his scaphoid. The other 3 patients could not be contacted.

DISCUSSION
The management of scaphoid nonunions and delayed unions has dramatically evolved over the past 20 years.1,3-8 Historically, semi-rigid stabilization using Kirschner wires and casting afforded a 77% union rate in these cases.9 More recently, several authors have reported that stabilization without bone grafting can predictably unite scaphoid nonunions. Treating patients with uncomplicated scaphoid nonunions and delayed unions by internal fixation alone may be all that is required to achieve union.
The definitions of a scaphoid nonunion and delayed union are complex. The exact time when a scaphoid fracture heals varies between patients.2,5,10 However, the majority of hand surgeons believe that failure to see clear signs of healing (in waist fractures) after 3 months from the injury would suggest a failure to heal and a “delayed” union, whereas failure after 6 months from the injury and without clear signs of healing indicate a nonunion.5,6,10,11 Any resorption at the fracture site suggests that the fracture will not heal by continued immobilization alone and will require surgery.10
Continue to: Hand surgeons have several surgical options...
Hand surgeons have several surgical options when managing scaphoid injuries. Mahmoud and Koptan4 used a volar approach to percutaneously deliver a headless compression screw into 27 nonunions. Postoperative CT scans demonstrated fracture union in all 27 patients, and no patient underwent revision surgery. Interestingly, 14 of their patients had extensive preoperative resorption (but no deformity) of >5 mm.
Although volar percutaneous approaches for internal fixation have been cited to provide high rates of union and high patient satisfaction in acute scaphoid fracture fixation, this study utilized a dorsal approach. Both Wozasek and Moser12 and Haddad and Goddard13 reported excellent results and high union rates using a volar approach in consecutive acute scaphoid fractures. Despite these results, there are concerns that using a volar approach may damage the scaphotrapezial joint and may be prone to eccentric placement of compression screws.8,14
Slade and colleagues3 did utilize the dorsal approach with arthroscopic assistance to deliver a compression screw into scaphoid nonunions in 15 consecutive patients without any evidence of deformity, sclerosis, or resorption. Similar to our investigation, they treated patients with both delayed unions and nonunions. CT scans were used to confirm unions in all their patients. Using a dorsal approach, Yassaee and Yang15 treated 9 consecutive patients using a compression screw without bone grafting for both delayed and nonunion scaphoid injuries. Other authors have used both volar and dorsal approaches in 12 consecutive delayed and nonunion scaphoid injuries and found that 11 of the 12 injuries progressed to unions.6
Although these authors and several others advocate the use of CT scans to assess unions, our investigation used bridging trabeculation obliteration of the fracture line on 3 standard radiographic views to confirm unions in addition to the absence of pain clinically.16,17 CT scans expose the patient to increased radiation that, in our experience, does not alter the postoperative clinical course.18 If there is clear evidence of bridged callus and no pain on physical examination, a CT scan performed to reconfirm the union affords little benefit to clinical management.19
Continue to: All these previous studies have demonstrated...
All these previous studies have demonstrated excellent union rates but using a limited series of patients. We reviewed a large number of consecutive patients with scaphoid delayed unions and nonunions treated by screw fixation without bone grafting. Our hospital is a safety net institution for a large urban catchment area and had complete radiographic and clinical data for 19 of our 23 patients. One patient was contacted by telephone and he reported no pain and no revision surgical interventions.
The limitations of this study include not only its retrospective design but also its limited secondary outcome measures. However, our primary outcomes of union, pain, and complications are of utmost importance to clinicians and patients alike. Similar to other authors, we used radiographs to confirm unions. Although bridging trabeculation in radiographs has been demonstrated as soon as 1 month after the injury, there may be problems with interobserver reliability.4,13,15,20,21
Patients being lost to follow-up is not uncommon in the orthopedic trauma literature and can influence results.22,23 It is speculative to infer that the 3 patients who did not complete a follow-up course did not return because their pain had mitigated.
CONCLUSION
Like several fractures, the lack of stability and the absence of micro-motion are believed to contribute to fibrous nonunions in scaphoid fractures.13 This study provides a large consecutive cohort of patients with minimally displaced scaphoid delayed unions and nonunions that were successfully treated by rigid internal fixation without bone grafting. These results confirm previous reports that bone grafting is not required to provide predictable unions for the majority of scaphoid nonunions.
This paper will be judged for the Resident Writer’s Award.
1. Trumble TE, Salas P, Barthel T, Robert KQ 3rd. Management of scaphoid nonunions. J Am Acad Orthop Surg. 2003;11(6):380-391. doi:10.1016/j.jhsa.2012.03.002.
2. Munk B, Larsen CF. Bone grafting the scaphoid nonunion: a systematic review of 147 publications including 5,246 cases of scaphoid nonunion. Acta Orthop Scand. 2004;75(5):618-629. doi:10.1080/00016470410001529.
3. Slade JF 3rd, Geissler WB, Gutow AP, Merrell GA. Percutaneous internal fixation of selected scaphoid nonunions with an arthroscopically assisted dorsal approach. J Bone Joint Surg Am. 2003;85-A Suppl 4:20-32.
4. Mahmoud M, Koptan W. Percutaneous screw fixation without bone grafting for established scaphoid nonunion with substantial bone loss. J Bone Joint Surg Br. 2011;93(7):932-936. doi:10.1302/0301-620X.93B7.25418.
5. Inaparthy PK, Nicholl JE. Treatment of delayed/nonunion of scaphoid waist with Synthes cannulated scaphoid screw and bone graft. Hand N Y N. 2008;3(4):292-296. doi:10.1007/s11552-008-9112-4.
6. Capo JT, Shamian B, Rizzo M. Percutaneous screw fixation without bone grafting of scaphoid non-union. Isr Med Assoc J. 2012;14(12):729-732.
7. Kim JK, Kim JO, Lee SY. Volar percutaneous screw fixation for scaphoid waist delayed union. Clin Orthop Relat Res. 2010;468(4):1066-1071. doi:10.1007/s11999-009-1032-2.
8. Trumble TE, Clarke T, Kreder HJ. Non-union of the scaphoid. Treatment with cannulated screws compared with treatment with Herbert screws. J Bone Joint Surg Am. 1996;78(12):1829-1837.
9. Cosio MQ, Camp RA. Percutaneous pinning of symptomatic scaphoid nonunions. J Hand Surg. 1986;11(3):350-355. doi:10.1016/S0363-5023(86)80141-1.
10. Steinmann SP, Adams JE. Scaphoid fractures and nonunions: diagnosis and treatment. J Orthop Sci. 2006;11(4):424-431. doi:10.1007/s00776-006-1025-x.
11. Zarezadeh A, Moezi M, Rastegar S, Motififard M, Foladi A, Daneshpajouhnejad P. Scaphoid nonunion fracture and results of the modified Matti-Russe technique. Adv Biomed Res. 2015;4:39. doi:10.4103/2277-9175.151248.
12. Wozasek GE, Moser KD. Percutaneous screw fixation for fractures of the scaphoid. J Bone Joint Surg Br. 1991;73(1):138-142. doi:10.3928/01477447-20170509-04.
13. Haddad FS, Goddard NJ. Acute percutaneous scaphoid fixation. A pilot study. J Bone Joint Surg Br. 1998;80(1):95-99. doi:10.1302/0301-620X.80B1.8076.
14. Yip HSF, Wu WC, Chang RYP, So TYC. Percutaneous cannulated screw fixation of acute scaphoid waist fracture. J Hand Surg Br. 2002;27(1):42-46. doi:10.1054/jhsb.2001.0690.
15. Yassaee F, Yang SS. Mini-incision fixation of nondisplaced scaphoid fracture nonunions. J Hand Surg. 2008;33(7):1116-1120. doi:10.1016/j.jhsa.2008.03.004.
16. Slade JF 3rd, Gillon T. Retrospective review of 234 scaphoid fractures and nonunions treated with arthroscopy for union and complications. Scand J Surg. 2008;97(4):280-289. doi:10.1177/145749690809700402
17. Geoghegan JM, Woodruff MJ, Bhatia R, et al. Undisplaced scaphoid waist fractures: is 4 weeks’ immobilisation in a below-elbow cast sufficient if a week 4 CT scan suggests fracture union? J Hand Surg Eur Vol. 2009;34(5):631-637. doi:10.1177/1753193409105189.
18. Biswas D, Bible JE, Bohan M, Simpson AK, Whang PG, Grauer JN. Radiation exposure from musculoskeletal computerized tomographic scans. J Bone Joint Surg Am. 2009;91(8):1882-1889. doi:10.2106/JBJS.H.01199.
19. Dias JJ, Taylor M, Thompson J, Brenkel IJ, Gregg PJ. Radiographic signs of union of scaphoid fractures. An analysis of inter-observer agreement and reproducibility. J Bone Joint Surg Br. 1988;70(2):299-301. doi:10.1302/0301-620X.70B2.3346310.
20. Martus JE, Bedi A, Jebson PJL. Cannulated variable pitch compression screw fixation of scaphoid fractures using a limited dorsal approach. Tech Hand Up Extrem Surg. 2005;9(4):202-206. doi:10.1097/01.bth.0000191422.26565.25.
21. Clay NR, Dias JJ, Costigan PS, Gregg PJ, Barton NJ. Need the thumb be immobilised in scaphoid fractures? A randomised prospective trial. J Bone Joint Surg Br. 1991;73(5):828-832. doi:10.1302/0301-620X.73B5.1894676.
22. Zelle BA, Bhandari M, Sanchez AI, Probst C, Pape HC. Loss of follow-up in orthopaedic trauma: is 80% follow-up still acceptable? J Orthop Trauma. 2013;27(3):177-181. doi:10.1097/BOT.0b013e31825cf367.
23. Sprague S, Leece P, Bhandari M, et al. Limiting loss to follow-up in a multicenter randomized trial in orthopedic surgery. Control Clin Trials. 2003;24(6):719-725. doi:10.1016/j.cct.2003.08.012.
1. Trumble TE, Salas P, Barthel T, Robert KQ 3rd. Management of scaphoid nonunions. J Am Acad Orthop Surg. 2003;11(6):380-391. doi:10.1016/j.jhsa.2012.03.002.
2. Munk B, Larsen CF. Bone grafting the scaphoid nonunion: a systematic review of 147 publications including 5,246 cases of scaphoid nonunion. Acta Orthop Scand. 2004;75(5):618-629. doi:10.1080/00016470410001529.
3. Slade JF 3rd, Geissler WB, Gutow AP, Merrell GA. Percutaneous internal fixation of selected scaphoid nonunions with an arthroscopically assisted dorsal approach. J Bone Joint Surg Am. 2003;85-A Suppl 4:20-32.
4. Mahmoud M, Koptan W. Percutaneous screw fixation without bone grafting for established scaphoid nonunion with substantial bone loss. J Bone Joint Surg Br. 2011;93(7):932-936. doi:10.1302/0301-620X.93B7.25418.
5. Inaparthy PK, Nicholl JE. Treatment of delayed/nonunion of scaphoid waist with Synthes cannulated scaphoid screw and bone graft. Hand N Y N. 2008;3(4):292-296. doi:10.1007/s11552-008-9112-4.
6. Capo JT, Shamian B, Rizzo M. Percutaneous screw fixation without bone grafting of scaphoid non-union. Isr Med Assoc J. 2012;14(12):729-732.
7. Kim JK, Kim JO, Lee SY. Volar percutaneous screw fixation for scaphoid waist delayed union. Clin Orthop Relat Res. 2010;468(4):1066-1071. doi:10.1007/s11999-009-1032-2.
8. Trumble TE, Clarke T, Kreder HJ. Non-union of the scaphoid. Treatment with cannulated screws compared with treatment with Herbert screws. J Bone Joint Surg Am. 1996;78(12):1829-1837.
9. Cosio MQ, Camp RA. Percutaneous pinning of symptomatic scaphoid nonunions. J Hand Surg. 1986;11(3):350-355. doi:10.1016/S0363-5023(86)80141-1.
10. Steinmann SP, Adams JE. Scaphoid fractures and nonunions: diagnosis and treatment. J Orthop Sci. 2006;11(4):424-431. doi:10.1007/s00776-006-1025-x.
11. Zarezadeh A, Moezi M, Rastegar S, Motififard M, Foladi A, Daneshpajouhnejad P. Scaphoid nonunion fracture and results of the modified Matti-Russe technique. Adv Biomed Res. 2015;4:39. doi:10.4103/2277-9175.151248.
12. Wozasek GE, Moser KD. Percutaneous screw fixation for fractures of the scaphoid. J Bone Joint Surg Br. 1991;73(1):138-142. doi:10.3928/01477447-20170509-04.
13. Haddad FS, Goddard NJ. Acute percutaneous scaphoid fixation. A pilot study. J Bone Joint Surg Br. 1998;80(1):95-99. doi:10.1302/0301-620X.80B1.8076.
14. Yip HSF, Wu WC, Chang RYP, So TYC. Percutaneous cannulated screw fixation of acute scaphoid waist fracture. J Hand Surg Br. 2002;27(1):42-46. doi:10.1054/jhsb.2001.0690.
15. Yassaee F, Yang SS. Mini-incision fixation of nondisplaced scaphoid fracture nonunions. J Hand Surg. 2008;33(7):1116-1120. doi:10.1016/j.jhsa.2008.03.004.
16. Slade JF 3rd, Gillon T. Retrospective review of 234 scaphoid fractures and nonunions treated with arthroscopy for union and complications. Scand J Surg. 2008;97(4):280-289. doi:10.1177/145749690809700402
17. Geoghegan JM, Woodruff MJ, Bhatia R, et al. Undisplaced scaphoid waist fractures: is 4 weeks’ immobilisation in a below-elbow cast sufficient if a week 4 CT scan suggests fracture union? J Hand Surg Eur Vol. 2009;34(5):631-637. doi:10.1177/1753193409105189.
18. Biswas D, Bible JE, Bohan M, Simpson AK, Whang PG, Grauer JN. Radiation exposure from musculoskeletal computerized tomographic scans. J Bone Joint Surg Am. 2009;91(8):1882-1889. doi:10.2106/JBJS.H.01199.
19. Dias JJ, Taylor M, Thompson J, Brenkel IJ, Gregg PJ. Radiographic signs of union of scaphoid fractures. An analysis of inter-observer agreement and reproducibility. J Bone Joint Surg Br. 1988;70(2):299-301. doi:10.1302/0301-620X.70B2.3346310.
20. Martus JE, Bedi A, Jebson PJL. Cannulated variable pitch compression screw fixation of scaphoid fractures using a limited dorsal approach. Tech Hand Up Extrem Surg. 2005;9(4):202-206. doi:10.1097/01.bth.0000191422.26565.25.
21. Clay NR, Dias JJ, Costigan PS, Gregg PJ, Barton NJ. Need the thumb be immobilised in scaphoid fractures? A randomised prospective trial. J Bone Joint Surg Br. 1991;73(5):828-832. doi:10.1302/0301-620X.73B5.1894676.
22. Zelle BA, Bhandari M, Sanchez AI, Probst C, Pape HC. Loss of follow-up in orthopaedic trauma: is 80% follow-up still acceptable? J Orthop Trauma. 2013;27(3):177-181. doi:10.1097/BOT.0b013e31825cf367.
23. Sprague S, Leece P, Bhandari M, et al. Limiting loss to follow-up in a multicenter randomized trial in orthopedic surgery. Control Clin Trials. 2003;24(6):719-725. doi:10.1016/j.cct.2003.08.012.
TAKE-HOME POINTS
- Scaphoid nonunions can occur in minimally displaced fractures.
- If there is no deformity of the scaphoid delayed or nonunion, then a percutaneous screw fixation without bone grafting can reliably lead to bony union.
- Not all scaphoid delayed unions and nonunions require bone grafting.
Volumetric Considerations for Valving Long-Arm Casts: The Utility of the Cast Spacer
ABSTRACT
Fiberglass casts are frequently valved to accommodate swelling following injury or surgery. The use of cast spacers has been recommended to bridge this gap between pressure reduction and cast strength, but no studies have assessed their effect on cast pressure.
We applied 30 long-arm fiberglass casts to adult volunteers, divided between a univalve group and a bivalve group. A pediatric blood pressure bladder was applied under the cast to simulate soft tissue swelling. Valved casts were secured using an elastic wrap, 10-mm cast spacer, or 15-mm cast spacer. Measurements of cast pressure and circumference were performed at each stage and compared on the basis of type of valve and securement.
Our results indicated that cast univalving resulted in an approximately 60% reduction in cast pressures, with a 75% reduction seen in the bivalve group. The addition of cast spacers resulted in significant pressure reductions for both valving groups. The univalve group secured with a 10-mm cast spacer produced reductions in cast pressure similar to those of the elastic-wrapped bivalve cast, both with the cast padding intact and with it released.
The use of cast spacers results in significant cast pressure reductions, regardless of valving technique. A univalved cast secured with a cast spacer can produce decreases in cast pressures similar to those seen with an elastic-wrapped bivalved cast, and it is a viable option for reducing cast pressure without compromising cast structural integrity with a bivalve technique.
Continue to: Complications following closed reduction...
Complications following closed reduction and casting of pediatric forearm fractures are rare, but they do occur. Arguably the most devastating of these complications is the risk of developing compartment syndrome or Volkmann contracture secondary to injury-associated swelling under a circumferential cast.1-4 The peak in swelling can develop from 4 to 24 hours following the initial cast application,5 and as such, medical providers may not be able to identify it early because most children are discharged following closed reductions. For this reason, many providers implement prophylactic measures to minimize pressure-related complications.
A popular method for reducing pressure accumulation within a cast is to valve, or cut, the cast. Previous investigations have shown that cast valving results in significant reductions in cast pressure.2,6-9 Bivalving a circumferential cast results in significantly greater reductions in cast pressure when compared with univalve techniques;6,7,9 however, bivalving has also been shown to result in significant impairment in the structural integrity of the cast.10 An additional method to facilitate cast pressure reduction without impairing the structural integrity of the cast that accompanies a bivalve is to incorporate a cast spacer with a univalve technique to hold the split cast open.11 Although this method is commonly used in clinical practice, its ability to mitigate cast pressures has not previously been investigated.
The goal of this study is to investigate the influence of incorporating cast spacers with valved long-arm casts. We hypothesized that cast spacers would provide a greater pressure reduction for both univalved and bivalved casts when compared with the use of an elastic wrap. Additionally, we proposed that by incorporating a cast spacer with a univalved cast, we could attain pressure reduction equivalent to that of a bivalved cast secured with an elastic wrap.
MATERIALS AND METHODS
Upon receiving approval from the Institutional Review Board, experimental testing began with the application of 30 total casts performed on uninjured adult human volunteers. Pressure readings were provided with the use of a bladder from a pediatric blood pressure cuff (Welch Allyn Inc), as previously described.6 The bladder was placed on the volar aspect of the volunteer’s forearm, held in place with a 3-in diameter cotton stockinet (3M). Cotton cast padding (Webril-Kendall) was applied, 3 in wide and 2 layers thick, and a long-arm cast was applied, 2 layers thick with 3-in wide fiberglass casting material (Scotchcast Plus Casting Tape; 3M).
Once the cast was applied and allowed to set, the blood pressure bladder was inflated to 100 mm Hg. After inflation, forearm cast circumference was measured at 2 set points, assessed at points 2 cm distal to the elbow flexor crease and 10 cm distal to the previous point (Figure 1). Using these data, we calculated estimated cast volume using the volumetric equation for a frustum. Following this point, casts were split into 2 experimental groups, univalve or bivalve, with 15 casts comprising each group. The univalve group consisted of a single cut along the dorsum of the extremity, and the bivalve group incorporated a second cut to the volar extremity. Cast valving was performed using an oscillating cast saw (Cast Vac; Stryker Instruments), with care taken to ensure the continuity of the underlying cast padding.
Continue to: Following valving, casts were secured via...
Following valving, casts were secured via 3 separate techniques: overwrap with a 3-in elastic wrap (Econo Wrap; Vitality Medical), application of two 10-mm and 15-mm cast spacers (CastWedge; DM Systems) (Figure 2). After securement, cast pressures were recorded, and circumference measurements were performed at the 2 previously identified points. The cast padding was then cut at the valve site and secured via the 3 listed techniques. Cast pressure and circumference measurements were performed at set time points (Figure 3). Changes in cast pressure were recorded in terms of the amount of change from the initial cast placement to account for differences in the size of volunteers’ forearms. Volumetric calculations were performed only for the spacer subgroups owing to the added material in the elastic wrap group. Estimated cast volume was calculated using the equation for volume of a frustum (Figure 4).
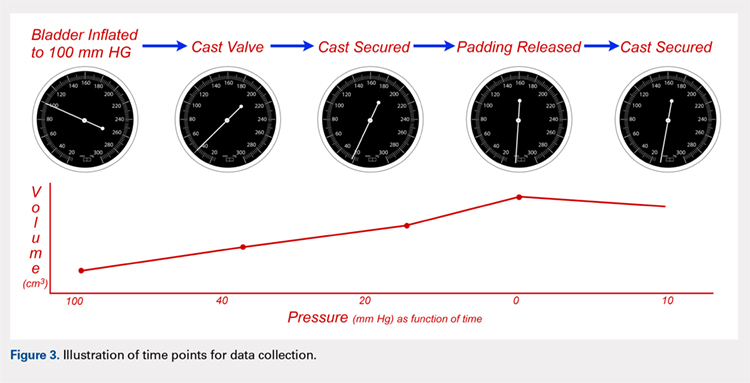
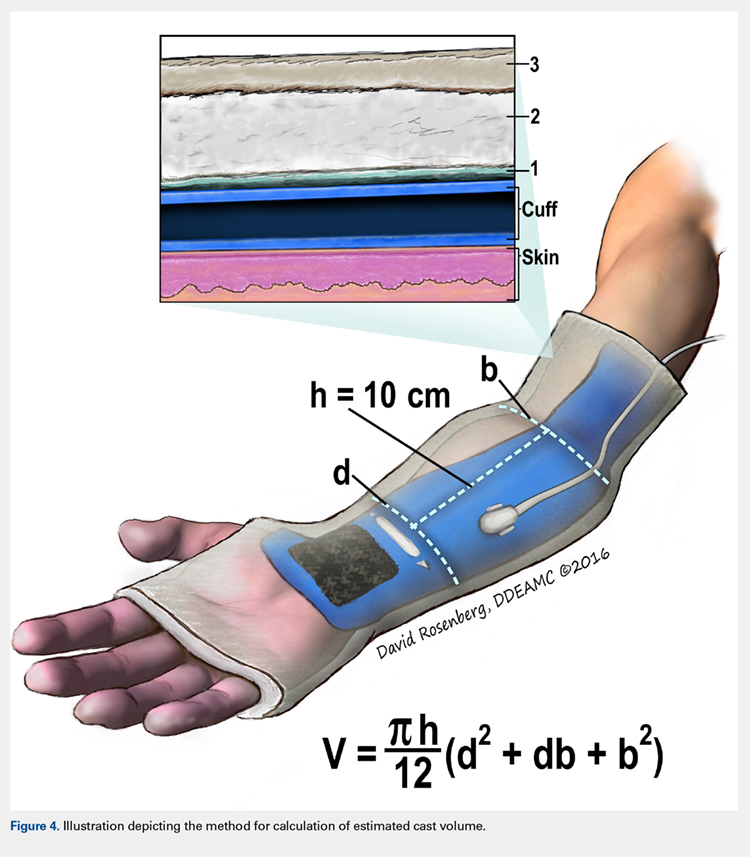
We used a 2-cast type (univalve and bivalve) by 4 securement subgroups (initial, elastic wrap, 10-mm spacer, and 15-mm spacer) design, with cast type serving as a between-subject measure and securement serving as a within-subject variable. An a priori power analysis showed that a minimum sample size of 15 subjects per condition should provide sufficient power of .80 and alpha set at .05, for a total of 30 casts. Statistical analyses were performed using IBM SPSS Statistics software version 21 (IBM). Experimental groups were analyzed using mixed-design analysis of variance (ANOVA). Post hoc comparisons between valving groups and cast securement were performed using Scheffe’s test to control for type II errors. Change in cast volume between the initial cast and cast spacers groups was compared using paired Student’s t tests. Statistical significance was predetermined as P < .05.
RESULTS
A summary of collected data for cast pressure and volume is detailed in Table 1, subdividing the variables on the basis of cast type and type of securement. Recorded pressures of the different subgroups are depicted in Figures 5 and 6 according to type of securement (initial, elastic wrap, 10-mm spacer, or 15-mm spacer). Results of the mixed-design ANOVA demonstrated significant differences between the initial cast pressure and univalve and bivalve groups (P < .05). There was a main effect for bivalve having lower pressure overall (F [1, 1)] = 3321.51, P < .001). There was also a main effect indicating that pressure was different for each type of securement (elastic wrap, 10-mm spacer, 15-mm spacer) (F [1, 28] = 538.54, P <. 01). Post hoc testing confirmed pressure decreased significantly, in descending order from elastic wrap, to 10-mm spacers, to 15-mm spacers (P < .05).
Table 1. Cumulative Data for Two Casting groups at Each Timepoint
Cast | Pressure | Standard Deviation | Volume |
Univalve |
|
|
|
Initial | 100 | --- | 2654.3 |
Elastic Wrap | 39.47 | 3.33 | --- |
10-mm Spacer | 23.93 | 2.73 | 2708.23 |
15-mm Spacer | 18.87 | 2.94 | 2734.86 |
Padding and Elastic Wrap | 20.93 | 2.91 | --- |
Padding and 10-mm Spacer | 15.46 | 2.19 | 2733.24 |
Padding and 15-mm Spacer | 0 | --- | 2819.27 |
Bivalve |
|
|
|
Initial | 100 | --- | 2839.3 |
Elastic Wrap | 25.9 | 3.17 | --- |
10-mm Spacer | 16.53 | 2.32 | 3203.13 |
15-mm Spacer | 13.6 | 2.74 | 3380.32 |
Padding and Elastic Wrap | 12.67 | 1.95 | --- |
Padding and 10-mm Spacer | 0 | --- | 3296.55 |
Padding and 15- mm Spacer | 0 | --- | 3438.67 |
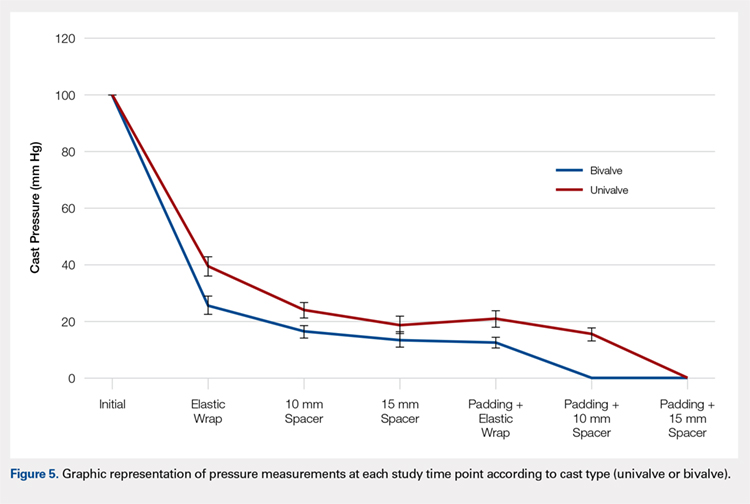
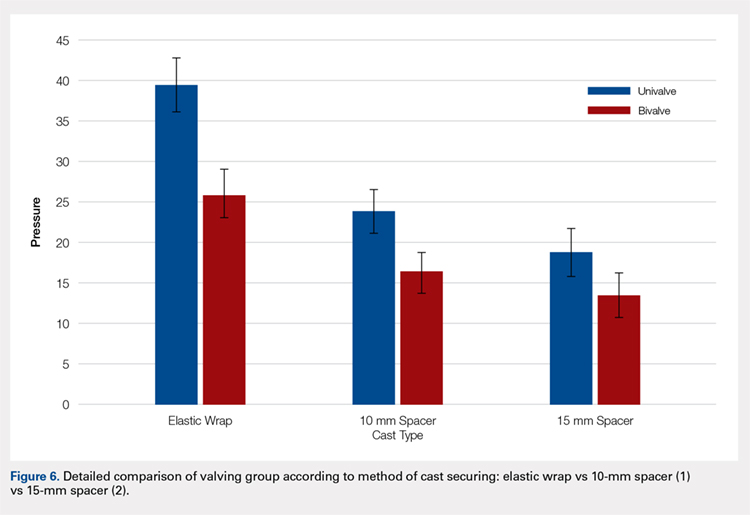
Continue to: Table 2...
The summary of volumetric changes is listed in Table 2. The decrease in pressure correlated with an associated increase in cast volume, as demonstrated in Figure 7. The degree of increase in cast volume was more pronounced in the bivalve group (P < .001). The volume increased in the 15-mm group compared with the 10-mm group for both groups (P < .001) and increased for each spacer group with the release of the underlying padding (P < .05).
Table 2. Volumetric Data
Cast | Average Volumetric change (cm3) | Standard Deviation |
Univalve |
|
|
10-mm Spacer | 175.6 | 65.4 |
15-mm Spacer | 269.4 | 73.3 |
Padding and 10-mm Spacer | 202.3 | 62.5 |
Padding and 15-mm Spacer | 294.1 | 66.9 |
Bivalve |
|
|
10-mm Spacer | 363.7 | 67.2 |
15-mm Spacer | 540.9 | 85.7 |
Padding and 10-mm Spacer | 457.2 | 97.9 |
Padding and 15-mm Spacer | 599.3 | 84.2 |
Analysis of the planned comparisons demonstrated no significant difference between the bivalve with elastic wrap and univalve with 10-mm spacer subgroups (t [28] = 1.85, P = .075, d = .68). In comparing the bivalve with elastic wrap group with the univalve and 15-mm spacer subgroup, the univalve group showed significantly lower pressures [t [28] = 6.32, P < .001, d = .2.31).
DISCUSSION
Valving of circumferential casting is a well-established technique to minimize potential pressure-related complications. Previous studies have demonstrated that univalving techniques produce a 65% reduction in cast pressure, whereas bivalving produces an 80% decrease.6,7,9 Our results showed comparable pressure reductions of 75% with bivalving and 60% with univalving. The type of cast padding has been shown to have a significant effect on the cast pressure, favoring lower pressures with cotton padding over synthetic and waterproof padding, which, when released, can provide an additional 10% pressure reduction.6,7
Although bivalving techniques are superior in pressure reduction, the reduction comes at the cost of the cast’s structural integrity. Crickard and colleagues10 performed a biomechanical assessment of the structural integrity by 3-point bending of casts following univalve and bivalve compared with an intact cast. The authors found that valving resulted in a significant decrease in the casts’ bending stiffness and load to failure, with bivalved casts demonstrating a significantly lower load to failure than univalved casts. One technique that has been used to enhance the pressure reduction in univalved casting techniques is the application of a cast spacer. Rang and colleagues11 recommended this technique as part of a graded cast-splitting approach for the treatment of children’s fractures. This technique was applied to fractures with only modest anticipated swelling, which accounted for approximately 95% of casts applied in their children’s hospital. Our results support the use of cast spacers, demonstrating significant reduction in cast pressure in both univalve and bivalve techniques. Additionally, we found that a univalved cast with a 10-mm cast spacer provided pressure reduction similar to that of a bivalved cast.
The theory behind the application of cast spacers is that a split fiberglass cast will not remain open unless held in position.11 Holding the cast open is less of a restraint to pressure reduction in bivalving techniques, because the split cast no longer has the contralateral intact hinge point to resist cast opening, demonstrated in the compromise in structural integrity seen with this technique.10 By maintaining the split cast in an opened position, the effective volume of the cast is increased, which allows for the reduction in cast pressure. This is demonstrated in our results indicating an increase in estimated cast volume with an associated incremental reduction in cast pressure with the application of incrementally sized cast spacers. Although this technique does have the potential for skin irritation caused by cast expansion, as well as local swelling at the cast window location, it is a cost-effective treatment method compared with overwrapping a bivalved cast, $1.55 for 1 cast spacer vs an estimated $200 for a forearm cast application.
This study is not without its limitations. Our model does not account for the soft tissue injury associated with forearm fractures. However, by using human volunteers, we were able to include the viscoelastic properties that are omitted with nonliving models, and our results do align with those of previous investigations regarding pressure change following valving. We did not incorporate a 3-point molding technique commonly used with reduction and casting of acute forearm fractures, owing to the lack of a standardized method for applying the mold to healthy volunteers. Although molding is necessary for most fractures in which valving is considered, we believe our data still provide valuable information. Additionally, valving of circumferential casts has not been shown, prospectively, to result in a reduction of cast-related compartment syndrome, maintenance of reduction, or need for surgery.12,13 However, these results are reflective of reliable patients who completed the requisite follow-up care necessary for inclusion in a randomized controlled trial and may be applicable to unreliable patients or patient situations, a setting in which the compromise in cast structural integrity may be unacceptable.
CONCLUSION
We demonstrated that incorporating cast spacers into valved long-arm casts provides pressure reduction comparable to that achieved with the use of an elastic wrap. The addition of a 10-mm cast spacer to a univalved long-arm cast provides pressure reduction equivalent to that of a bivalved cast secured with an elastic wrap. A univalved cast secured with a cast spacer is a viable option for treatment of displaced pediatric forearm fractures, without compromising the cast’s structural integrity as required with bivalved techniques.
This paper will be judged for the Resident Writer’s Award.
- Halanski M, Noonan KJ. Cast and splint immobilization: complications. J Am Acad Orthop Surg. 2008;16(1):30-40.
- Zaino CJ, Patel MR, Arief MS, Pivec R. The effectiveness of bivalving, cast spreading, and webril cutting to reduce cast pressure in a fiberglass short arm cast. J Bone Joint Surg Am. 2015;97(5):374-380. doi:10.2106/JBJS.N.00579.
- Rodriguez-Merchan EC. Pediatric fractures of the forearm. Clin Orthop Relat Res. 2005;(432):65-72.
- von Volkmann R. Ischaemic muscle paralyses and contractures. Clin Orthop Relat Res. 1967;50:5-56. doi:10.1097/BLO.0b013e318032561f.
- Patrick JH, Levack B. A study of pressures beneath forearm plasters. Injury. 1981;13(1):37-41.
- Roberts A, Shaw KA, Boomsma SE, Cameron CD. Effect of casting material on the cast pressure after sequential cast splitting. J Pediatr Orthop. 2017;37(1):74-77. doi:10.1097/BPO.0000000000000574.
- Garfin SR, Mubarak SJ, Evans KL, Hargens AR, Akeson WH. Quantification of intracompartmental pressure and volume under plaster casts. J Bone Joint Surg Am. 1981;63(3):449-453.
- Capo JT, Renard RL, Moulton MJ, et al. How is forearm compliance affected by various circumferential dressings? Clin Orthop Relat Res. 2014 472(10):3228-3234. doi:10.1007/s11999-014-3747-y.
- Bingold AC. On splitting plasters. A useful analogy. J Bone Joint Surg Br. 1979;61-b(3):294-295.
- Crickard CV, Riccio AI, Carney JR, Anderson TD. Analysis and comparison of the biomechanical properties of univalved and bivalved cast models. J Pediatr Orthop.2011;31(1):39-43. doi:10.1097/BPO.0b013e318202c446.
- Rang M, Wenger DR, Pring ME. Rang's Children's Fractures. 3rd ed. Wenger DR, Rang M, eds. Philadelphia, PA: Lippincott Williams & Wilkins; 2005.
- Schulte D, Habernig S, Zuzak T, et al. Forearm fractures in children: split opinions about splitting the cast. Eur J Pediatr Surg. 2014;24(2):163-167. doi:10.1055/s-0033-1341412.
- Bae DS, Valim C, Connell P, Brustowicz KA, Waters PM. Bivalved versus circumferential cast immobilization for displaced forearm fractures: a randomized clinical trial to assess efficacy and safety. J Pediatr Orthop. 2017;37(4):239-246 doi:10.1097/BPO.0000000000000655.
ABSTRACT
Fiberglass casts are frequently valved to accommodate swelling following injury or surgery. The use of cast spacers has been recommended to bridge this gap between pressure reduction and cast strength, but no studies have assessed their effect on cast pressure.
We applied 30 long-arm fiberglass casts to adult volunteers, divided between a univalve group and a bivalve group. A pediatric blood pressure bladder was applied under the cast to simulate soft tissue swelling. Valved casts were secured using an elastic wrap, 10-mm cast spacer, or 15-mm cast spacer. Measurements of cast pressure and circumference were performed at each stage and compared on the basis of type of valve and securement.
Our results indicated that cast univalving resulted in an approximately 60% reduction in cast pressures, with a 75% reduction seen in the bivalve group. The addition of cast spacers resulted in significant pressure reductions for both valving groups. The univalve group secured with a 10-mm cast spacer produced reductions in cast pressure similar to those of the elastic-wrapped bivalve cast, both with the cast padding intact and with it released.
The use of cast spacers results in significant cast pressure reductions, regardless of valving technique. A univalved cast secured with a cast spacer can produce decreases in cast pressures similar to those seen with an elastic-wrapped bivalved cast, and it is a viable option for reducing cast pressure without compromising cast structural integrity with a bivalve technique.
Continue to: Complications following closed reduction...
Complications following closed reduction and casting of pediatric forearm fractures are rare, but they do occur. Arguably the most devastating of these complications is the risk of developing compartment syndrome or Volkmann contracture secondary to injury-associated swelling under a circumferential cast.1-4 The peak in swelling can develop from 4 to 24 hours following the initial cast application,5 and as such, medical providers may not be able to identify it early because most children are discharged following closed reductions. For this reason, many providers implement prophylactic measures to minimize pressure-related complications.
A popular method for reducing pressure accumulation within a cast is to valve, or cut, the cast. Previous investigations have shown that cast valving results in significant reductions in cast pressure.2,6-9 Bivalving a circumferential cast results in significantly greater reductions in cast pressure when compared with univalve techniques;6,7,9 however, bivalving has also been shown to result in significant impairment in the structural integrity of the cast.10 An additional method to facilitate cast pressure reduction without impairing the structural integrity of the cast that accompanies a bivalve is to incorporate a cast spacer with a univalve technique to hold the split cast open.11 Although this method is commonly used in clinical practice, its ability to mitigate cast pressures has not previously been investigated.
The goal of this study is to investigate the influence of incorporating cast spacers with valved long-arm casts. We hypothesized that cast spacers would provide a greater pressure reduction for both univalved and bivalved casts when compared with the use of an elastic wrap. Additionally, we proposed that by incorporating a cast spacer with a univalved cast, we could attain pressure reduction equivalent to that of a bivalved cast secured with an elastic wrap.
MATERIALS AND METHODS
Upon receiving approval from the Institutional Review Board, experimental testing began with the application of 30 total casts performed on uninjured adult human volunteers. Pressure readings were provided with the use of a bladder from a pediatric blood pressure cuff (Welch Allyn Inc), as previously described.6 The bladder was placed on the volar aspect of the volunteer’s forearm, held in place with a 3-in diameter cotton stockinet (3M). Cotton cast padding (Webril-Kendall) was applied, 3 in wide and 2 layers thick, and a long-arm cast was applied, 2 layers thick with 3-in wide fiberglass casting material (Scotchcast Plus Casting Tape; 3M).
Once the cast was applied and allowed to set, the blood pressure bladder was inflated to 100 mm Hg. After inflation, forearm cast circumference was measured at 2 set points, assessed at points 2 cm distal to the elbow flexor crease and 10 cm distal to the previous point (Figure 1). Using these data, we calculated estimated cast volume using the volumetric equation for a frustum. Following this point, casts were split into 2 experimental groups, univalve or bivalve, with 15 casts comprising each group. The univalve group consisted of a single cut along the dorsum of the extremity, and the bivalve group incorporated a second cut to the volar extremity. Cast valving was performed using an oscillating cast saw (Cast Vac; Stryker Instruments), with care taken to ensure the continuity of the underlying cast padding.

Continue to: Following valving, casts were secured via...
Following valving, casts were secured via 3 separate techniques: overwrap with a 3-in elastic wrap (Econo Wrap; Vitality Medical), application of two 10-mm and 15-mm cast spacers (CastWedge; DM Systems) (Figure 2). After securement, cast pressures were recorded, and circumference measurements were performed at the 2 previously identified points. The cast padding was then cut at the valve site and secured via the 3 listed techniques. Cast pressure and circumference measurements were performed at set time points (Figure 3). Changes in cast pressure were recorded in terms of the amount of change from the initial cast placement to account for differences in the size of volunteers’ forearms. Volumetric calculations were performed only for the spacer subgroups owing to the added material in the elastic wrap group. Estimated cast volume was calculated using the equation for volume of a frustum (Figure 4).



We used a 2-cast type (univalve and bivalve) by 4 securement subgroups (initial, elastic wrap, 10-mm spacer, and 15-mm spacer) design, with cast type serving as a between-subject measure and securement serving as a within-subject variable. An a priori power analysis showed that a minimum sample size of 15 subjects per condition should provide sufficient power of .80 and alpha set at .05, for a total of 30 casts. Statistical analyses were performed using IBM SPSS Statistics software version 21 (IBM). Experimental groups were analyzed using mixed-design analysis of variance (ANOVA). Post hoc comparisons between valving groups and cast securement were performed using Scheffe’s test to control for type II errors. Change in cast volume between the initial cast and cast spacers groups was compared using paired Student’s t tests. Statistical significance was predetermined as P < .05.
RESULTS
A summary of collected data for cast pressure and volume is detailed in Table 1, subdividing the variables on the basis of cast type and type of securement. Recorded pressures of the different subgroups are depicted in Figures 5 and 6 according to type of securement (initial, elastic wrap, 10-mm spacer, or 15-mm spacer). Results of the mixed-design ANOVA demonstrated significant differences between the initial cast pressure and univalve and bivalve groups (P < .05). There was a main effect for bivalve having lower pressure overall (F [1, 1)] = 3321.51, P < .001). There was also a main effect indicating that pressure was different for each type of securement (elastic wrap, 10-mm spacer, 15-mm spacer) (F [1, 28] = 538.54, P <. 01). Post hoc testing confirmed pressure decreased significantly, in descending order from elastic wrap, to 10-mm spacers, to 15-mm spacers (P < .05).
Table 1. Cumulative Data for Two Casting groups at Each Timepoint
Cast | Pressure | Standard Deviation | Volume |
Univalve |
|
|
|
Initial | 100 | --- | 2654.3 |
Elastic Wrap | 39.47 | 3.33 | --- |
10-mm Spacer | 23.93 | 2.73 | 2708.23 |
15-mm Spacer | 18.87 | 2.94 | 2734.86 |
Padding and Elastic Wrap | 20.93 | 2.91 | --- |
Padding and 10-mm Spacer | 15.46 | 2.19 | 2733.24 |
Padding and 15-mm Spacer | 0 | --- | 2819.27 |
Bivalve |
|
|
|
Initial | 100 | --- | 2839.3 |
Elastic Wrap | 25.9 | 3.17 | --- |
10-mm Spacer | 16.53 | 2.32 | 3203.13 |
15-mm Spacer | 13.6 | 2.74 | 3380.32 |
Padding and Elastic Wrap | 12.67 | 1.95 | --- |
Padding and 10-mm Spacer | 0 | --- | 3296.55 |
Padding and 15- mm Spacer | 0 | --- | 3438.67 |


Continue to: Table 2...
The summary of volumetric changes is listed in Table 2. The decrease in pressure correlated with an associated increase in cast volume, as demonstrated in Figure 7. The degree of increase in cast volume was more pronounced in the bivalve group (P < .001). The volume increased in the 15-mm group compared with the 10-mm group for both groups (P < .001) and increased for each spacer group with the release of the underlying padding (P < .05).
Table 2. Volumetric Data
Cast | Average Volumetric change (cm3) | Standard Deviation |
Univalve |
|
|
10-mm Spacer | 175.6 | 65.4 |
15-mm Spacer | 269.4 | 73.3 |
Padding and 10-mm Spacer | 202.3 | 62.5 |
Padding and 15-mm Spacer | 294.1 | 66.9 |
Bivalve |
|
|
10-mm Spacer | 363.7 | 67.2 |
15-mm Spacer | 540.9 | 85.7 |
Padding and 10-mm Spacer | 457.2 | 97.9 |
Padding and 15-mm Spacer | 599.3 | 84.2 |

Analysis of the planned comparisons demonstrated no significant difference between the bivalve with elastic wrap and univalve with 10-mm spacer subgroups (t [28] = 1.85, P = .075, d = .68). In comparing the bivalve with elastic wrap group with the univalve and 15-mm spacer subgroup, the univalve group showed significantly lower pressures [t [28] = 6.32, P < .001, d = .2.31).
DISCUSSION
Valving of circumferential casting is a well-established technique to minimize potential pressure-related complications. Previous studies have demonstrated that univalving techniques produce a 65% reduction in cast pressure, whereas bivalving produces an 80% decrease.6,7,9 Our results showed comparable pressure reductions of 75% with bivalving and 60% with univalving. The type of cast padding has been shown to have a significant effect on the cast pressure, favoring lower pressures with cotton padding over synthetic and waterproof padding, which, when released, can provide an additional 10% pressure reduction.6,7
Although bivalving techniques are superior in pressure reduction, the reduction comes at the cost of the cast’s structural integrity. Crickard and colleagues10 performed a biomechanical assessment of the structural integrity by 3-point bending of casts following univalve and bivalve compared with an intact cast. The authors found that valving resulted in a significant decrease in the casts’ bending stiffness and load to failure, with bivalved casts demonstrating a significantly lower load to failure than univalved casts. One technique that has been used to enhance the pressure reduction in univalved casting techniques is the application of a cast spacer. Rang and colleagues11 recommended this technique as part of a graded cast-splitting approach for the treatment of children’s fractures. This technique was applied to fractures with only modest anticipated swelling, which accounted for approximately 95% of casts applied in their children’s hospital. Our results support the use of cast spacers, demonstrating significant reduction in cast pressure in both univalve and bivalve techniques. Additionally, we found that a univalved cast with a 10-mm cast spacer provided pressure reduction similar to that of a bivalved cast.
The theory behind the application of cast spacers is that a split fiberglass cast will not remain open unless held in position.11 Holding the cast open is less of a restraint to pressure reduction in bivalving techniques, because the split cast no longer has the contralateral intact hinge point to resist cast opening, demonstrated in the compromise in structural integrity seen with this technique.10 By maintaining the split cast in an opened position, the effective volume of the cast is increased, which allows for the reduction in cast pressure. This is demonstrated in our results indicating an increase in estimated cast volume with an associated incremental reduction in cast pressure with the application of incrementally sized cast spacers. Although this technique does have the potential for skin irritation caused by cast expansion, as well as local swelling at the cast window location, it is a cost-effective treatment method compared with overwrapping a bivalved cast, $1.55 for 1 cast spacer vs an estimated $200 for a forearm cast application.
This study is not without its limitations. Our model does not account for the soft tissue injury associated with forearm fractures. However, by using human volunteers, we were able to include the viscoelastic properties that are omitted with nonliving models, and our results do align with those of previous investigations regarding pressure change following valving. We did not incorporate a 3-point molding technique commonly used with reduction and casting of acute forearm fractures, owing to the lack of a standardized method for applying the mold to healthy volunteers. Although molding is necessary for most fractures in which valving is considered, we believe our data still provide valuable information. Additionally, valving of circumferential casts has not been shown, prospectively, to result in a reduction of cast-related compartment syndrome, maintenance of reduction, or need for surgery.12,13 However, these results are reflective of reliable patients who completed the requisite follow-up care necessary for inclusion in a randomized controlled trial and may be applicable to unreliable patients or patient situations, a setting in which the compromise in cast structural integrity may be unacceptable.
CONCLUSION
We demonstrated that incorporating cast spacers into valved long-arm casts provides pressure reduction comparable to that achieved with the use of an elastic wrap. The addition of a 10-mm cast spacer to a univalved long-arm cast provides pressure reduction equivalent to that of a bivalved cast secured with an elastic wrap. A univalved cast secured with a cast spacer is a viable option for treatment of displaced pediatric forearm fractures, without compromising the cast’s structural integrity as required with bivalved techniques.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Fiberglass casts are frequently valved to accommodate swelling following injury or surgery. The use of cast spacers has been recommended to bridge this gap between pressure reduction and cast strength, but no studies have assessed their effect on cast pressure.
We applied 30 long-arm fiberglass casts to adult volunteers, divided between a univalve group and a bivalve group. A pediatric blood pressure bladder was applied under the cast to simulate soft tissue swelling. Valved casts were secured using an elastic wrap, 10-mm cast spacer, or 15-mm cast spacer. Measurements of cast pressure and circumference were performed at each stage and compared on the basis of type of valve and securement.
Our results indicated that cast univalving resulted in an approximately 60% reduction in cast pressures, with a 75% reduction seen in the bivalve group. The addition of cast spacers resulted in significant pressure reductions for both valving groups. The univalve group secured with a 10-mm cast spacer produced reductions in cast pressure similar to those of the elastic-wrapped bivalve cast, both with the cast padding intact and with it released.
The use of cast spacers results in significant cast pressure reductions, regardless of valving technique. A univalved cast secured with a cast spacer can produce decreases in cast pressures similar to those seen with an elastic-wrapped bivalved cast, and it is a viable option for reducing cast pressure without compromising cast structural integrity with a bivalve technique.
Continue to: Complications following closed reduction...
Complications following closed reduction and casting of pediatric forearm fractures are rare, but they do occur. Arguably the most devastating of these complications is the risk of developing compartment syndrome or Volkmann contracture secondary to injury-associated swelling under a circumferential cast.1-4 The peak in swelling can develop from 4 to 24 hours following the initial cast application,5 and as such, medical providers may not be able to identify it early because most children are discharged following closed reductions. For this reason, many providers implement prophylactic measures to minimize pressure-related complications.
A popular method for reducing pressure accumulation within a cast is to valve, or cut, the cast. Previous investigations have shown that cast valving results in significant reductions in cast pressure.2,6-9 Bivalving a circumferential cast results in significantly greater reductions in cast pressure when compared with univalve techniques;6,7,9 however, bivalving has also been shown to result in significant impairment in the structural integrity of the cast.10 An additional method to facilitate cast pressure reduction without impairing the structural integrity of the cast that accompanies a bivalve is to incorporate a cast spacer with a univalve technique to hold the split cast open.11 Although this method is commonly used in clinical practice, its ability to mitigate cast pressures has not previously been investigated.
The goal of this study is to investigate the influence of incorporating cast spacers with valved long-arm casts. We hypothesized that cast spacers would provide a greater pressure reduction for both univalved and bivalved casts when compared with the use of an elastic wrap. Additionally, we proposed that by incorporating a cast spacer with a univalved cast, we could attain pressure reduction equivalent to that of a bivalved cast secured with an elastic wrap.
MATERIALS AND METHODS
Upon receiving approval from the Institutional Review Board, experimental testing began with the application of 30 total casts performed on uninjured adult human volunteers. Pressure readings were provided with the use of a bladder from a pediatric blood pressure cuff (Welch Allyn Inc), as previously described.6 The bladder was placed on the volar aspect of the volunteer’s forearm, held in place with a 3-in diameter cotton stockinet (3M). Cotton cast padding (Webril-Kendall) was applied, 3 in wide and 2 layers thick, and a long-arm cast was applied, 2 layers thick with 3-in wide fiberglass casting material (Scotchcast Plus Casting Tape; 3M).
Once the cast was applied and allowed to set, the blood pressure bladder was inflated to 100 mm Hg. After inflation, forearm cast circumference was measured at 2 set points, assessed at points 2 cm distal to the elbow flexor crease and 10 cm distal to the previous point (Figure 1). Using these data, we calculated estimated cast volume using the volumetric equation for a frustum. Following this point, casts were split into 2 experimental groups, univalve or bivalve, with 15 casts comprising each group. The univalve group consisted of a single cut along the dorsum of the extremity, and the bivalve group incorporated a second cut to the volar extremity. Cast valving was performed using an oscillating cast saw (Cast Vac; Stryker Instruments), with care taken to ensure the continuity of the underlying cast padding.

Continue to: Following valving, casts were secured via...
Following valving, casts were secured via 3 separate techniques: overwrap with a 3-in elastic wrap (Econo Wrap; Vitality Medical), application of two 10-mm and 15-mm cast spacers (CastWedge; DM Systems) (Figure 2). After securement, cast pressures were recorded, and circumference measurements were performed at the 2 previously identified points. The cast padding was then cut at the valve site and secured via the 3 listed techniques. Cast pressure and circumference measurements were performed at set time points (Figure 3). Changes in cast pressure were recorded in terms of the amount of change from the initial cast placement to account for differences in the size of volunteers’ forearms. Volumetric calculations were performed only for the spacer subgroups owing to the added material in the elastic wrap group. Estimated cast volume was calculated using the equation for volume of a frustum (Figure 4).



We used a 2-cast type (univalve and bivalve) by 4 securement subgroups (initial, elastic wrap, 10-mm spacer, and 15-mm spacer) design, with cast type serving as a between-subject measure and securement serving as a within-subject variable. An a priori power analysis showed that a minimum sample size of 15 subjects per condition should provide sufficient power of .80 and alpha set at .05, for a total of 30 casts. Statistical analyses were performed using IBM SPSS Statistics software version 21 (IBM). Experimental groups were analyzed using mixed-design analysis of variance (ANOVA). Post hoc comparisons between valving groups and cast securement were performed using Scheffe’s test to control for type II errors. Change in cast volume between the initial cast and cast spacers groups was compared using paired Student’s t tests. Statistical significance was predetermined as P < .05.
RESULTS
A summary of collected data for cast pressure and volume is detailed in Table 1, subdividing the variables on the basis of cast type and type of securement. Recorded pressures of the different subgroups are depicted in Figures 5 and 6 according to type of securement (initial, elastic wrap, 10-mm spacer, or 15-mm spacer). Results of the mixed-design ANOVA demonstrated significant differences between the initial cast pressure and univalve and bivalve groups (P < .05). There was a main effect for bivalve having lower pressure overall (F [1, 1)] = 3321.51, P < .001). There was also a main effect indicating that pressure was different for each type of securement (elastic wrap, 10-mm spacer, 15-mm spacer) (F [1, 28] = 538.54, P <. 01). Post hoc testing confirmed pressure decreased significantly, in descending order from elastic wrap, to 10-mm spacers, to 15-mm spacers (P < .05).
Table 1. Cumulative Data for Two Casting groups at Each Timepoint
Cast | Pressure | Standard Deviation | Volume |
Univalve |
|
|
|
Initial | 100 | --- | 2654.3 |
Elastic Wrap | 39.47 | 3.33 | --- |
10-mm Spacer | 23.93 | 2.73 | 2708.23 |
15-mm Spacer | 18.87 | 2.94 | 2734.86 |
Padding and Elastic Wrap | 20.93 | 2.91 | --- |
Padding and 10-mm Spacer | 15.46 | 2.19 | 2733.24 |
Padding and 15-mm Spacer | 0 | --- | 2819.27 |
Bivalve |
|
|
|
Initial | 100 | --- | 2839.3 |
Elastic Wrap | 25.9 | 3.17 | --- |
10-mm Spacer | 16.53 | 2.32 | 3203.13 |
15-mm Spacer | 13.6 | 2.74 | 3380.32 |
Padding and Elastic Wrap | 12.67 | 1.95 | --- |
Padding and 10-mm Spacer | 0 | --- | 3296.55 |
Padding and 15- mm Spacer | 0 | --- | 3438.67 |


Continue to: Table 2...
The summary of volumetric changes is listed in Table 2. The decrease in pressure correlated with an associated increase in cast volume, as demonstrated in Figure 7. The degree of increase in cast volume was more pronounced in the bivalve group (P < .001). The volume increased in the 15-mm group compared with the 10-mm group for both groups (P < .001) and increased for each spacer group with the release of the underlying padding (P < .05).
Table 2. Volumetric Data
Cast | Average Volumetric change (cm3) | Standard Deviation |
Univalve |
|
|
10-mm Spacer | 175.6 | 65.4 |
15-mm Spacer | 269.4 | 73.3 |
Padding and 10-mm Spacer | 202.3 | 62.5 |
Padding and 15-mm Spacer | 294.1 | 66.9 |
Bivalve |
|
|
10-mm Spacer | 363.7 | 67.2 |
15-mm Spacer | 540.9 | 85.7 |
Padding and 10-mm Spacer | 457.2 | 97.9 |
Padding and 15-mm Spacer | 599.3 | 84.2 |

Analysis of the planned comparisons demonstrated no significant difference between the bivalve with elastic wrap and univalve with 10-mm spacer subgroups (t [28] = 1.85, P = .075, d = .68). In comparing the bivalve with elastic wrap group with the univalve and 15-mm spacer subgroup, the univalve group showed significantly lower pressures [t [28] = 6.32, P < .001, d = .2.31).
DISCUSSION
Valving of circumferential casting is a well-established technique to minimize potential pressure-related complications. Previous studies have demonstrated that univalving techniques produce a 65% reduction in cast pressure, whereas bivalving produces an 80% decrease.6,7,9 Our results showed comparable pressure reductions of 75% with bivalving and 60% with univalving. The type of cast padding has been shown to have a significant effect on the cast pressure, favoring lower pressures with cotton padding over synthetic and waterproof padding, which, when released, can provide an additional 10% pressure reduction.6,7
Although bivalving techniques are superior in pressure reduction, the reduction comes at the cost of the cast’s structural integrity. Crickard and colleagues10 performed a biomechanical assessment of the structural integrity by 3-point bending of casts following univalve and bivalve compared with an intact cast. The authors found that valving resulted in a significant decrease in the casts’ bending stiffness and load to failure, with bivalved casts demonstrating a significantly lower load to failure than univalved casts. One technique that has been used to enhance the pressure reduction in univalved casting techniques is the application of a cast spacer. Rang and colleagues11 recommended this technique as part of a graded cast-splitting approach for the treatment of children’s fractures. This technique was applied to fractures with only modest anticipated swelling, which accounted for approximately 95% of casts applied in their children’s hospital. Our results support the use of cast spacers, demonstrating significant reduction in cast pressure in both univalve and bivalve techniques. Additionally, we found that a univalved cast with a 10-mm cast spacer provided pressure reduction similar to that of a bivalved cast.
The theory behind the application of cast spacers is that a split fiberglass cast will not remain open unless held in position.11 Holding the cast open is less of a restraint to pressure reduction in bivalving techniques, because the split cast no longer has the contralateral intact hinge point to resist cast opening, demonstrated in the compromise in structural integrity seen with this technique.10 By maintaining the split cast in an opened position, the effective volume of the cast is increased, which allows for the reduction in cast pressure. This is demonstrated in our results indicating an increase in estimated cast volume with an associated incremental reduction in cast pressure with the application of incrementally sized cast spacers. Although this technique does have the potential for skin irritation caused by cast expansion, as well as local swelling at the cast window location, it is a cost-effective treatment method compared with overwrapping a bivalved cast, $1.55 for 1 cast spacer vs an estimated $200 for a forearm cast application.
This study is not without its limitations. Our model does not account for the soft tissue injury associated with forearm fractures. However, by using human volunteers, we were able to include the viscoelastic properties that are omitted with nonliving models, and our results do align with those of previous investigations regarding pressure change following valving. We did not incorporate a 3-point molding technique commonly used with reduction and casting of acute forearm fractures, owing to the lack of a standardized method for applying the mold to healthy volunteers. Although molding is necessary for most fractures in which valving is considered, we believe our data still provide valuable information. Additionally, valving of circumferential casts has not been shown, prospectively, to result in a reduction of cast-related compartment syndrome, maintenance of reduction, or need for surgery.12,13 However, these results are reflective of reliable patients who completed the requisite follow-up care necessary for inclusion in a randomized controlled trial and may be applicable to unreliable patients or patient situations, a setting in which the compromise in cast structural integrity may be unacceptable.
CONCLUSION
We demonstrated that incorporating cast spacers into valved long-arm casts provides pressure reduction comparable to that achieved with the use of an elastic wrap. The addition of a 10-mm cast spacer to a univalved long-arm cast provides pressure reduction equivalent to that of a bivalved cast secured with an elastic wrap. A univalved cast secured with a cast spacer is a viable option for treatment of displaced pediatric forearm fractures, without compromising the cast’s structural integrity as required with bivalved techniques.
This paper will be judged for the Resident Writer’s Award.
- Halanski M, Noonan KJ. Cast and splint immobilization: complications. J Am Acad Orthop Surg. 2008;16(1):30-40.
- Zaino CJ, Patel MR, Arief MS, Pivec R. The effectiveness of bivalving, cast spreading, and webril cutting to reduce cast pressure in a fiberglass short arm cast. J Bone Joint Surg Am. 2015;97(5):374-380. doi:10.2106/JBJS.N.00579.
- Rodriguez-Merchan EC. Pediatric fractures of the forearm. Clin Orthop Relat Res. 2005;(432):65-72.
- von Volkmann R. Ischaemic muscle paralyses and contractures. Clin Orthop Relat Res. 1967;50:5-56. doi:10.1097/BLO.0b013e318032561f.
- Patrick JH, Levack B. A study of pressures beneath forearm plasters. Injury. 1981;13(1):37-41.
- Roberts A, Shaw KA, Boomsma SE, Cameron CD. Effect of casting material on the cast pressure after sequential cast splitting. J Pediatr Orthop. 2017;37(1):74-77. doi:10.1097/BPO.0000000000000574.
- Garfin SR, Mubarak SJ, Evans KL, Hargens AR, Akeson WH. Quantification of intracompartmental pressure and volume under plaster casts. J Bone Joint Surg Am. 1981;63(3):449-453.
- Capo JT, Renard RL, Moulton MJ, et al. How is forearm compliance affected by various circumferential dressings? Clin Orthop Relat Res. 2014 472(10):3228-3234. doi:10.1007/s11999-014-3747-y.
- Bingold AC. On splitting plasters. A useful analogy. J Bone Joint Surg Br. 1979;61-b(3):294-295.
- Crickard CV, Riccio AI, Carney JR, Anderson TD. Analysis and comparison of the biomechanical properties of univalved and bivalved cast models. J Pediatr Orthop.2011;31(1):39-43. doi:10.1097/BPO.0b013e318202c446.
- Rang M, Wenger DR, Pring ME. Rang's Children's Fractures. 3rd ed. Wenger DR, Rang M, eds. Philadelphia, PA: Lippincott Williams & Wilkins; 2005.
- Schulte D, Habernig S, Zuzak T, et al. Forearm fractures in children: split opinions about splitting the cast. Eur J Pediatr Surg. 2014;24(2):163-167. doi:10.1055/s-0033-1341412.
- Bae DS, Valim C, Connell P, Brustowicz KA, Waters PM. Bivalved versus circumferential cast immobilization for displaced forearm fractures: a randomized clinical trial to assess efficacy and safety. J Pediatr Orthop. 2017;37(4):239-246 doi:10.1097/BPO.0000000000000655.
- Halanski M, Noonan KJ. Cast and splint immobilization: complications. J Am Acad Orthop Surg. 2008;16(1):30-40.
- Zaino CJ, Patel MR, Arief MS, Pivec R. The effectiveness of bivalving, cast spreading, and webril cutting to reduce cast pressure in a fiberglass short arm cast. J Bone Joint Surg Am. 2015;97(5):374-380. doi:10.2106/JBJS.N.00579.
- Rodriguez-Merchan EC. Pediatric fractures of the forearm. Clin Orthop Relat Res. 2005;(432):65-72.
- von Volkmann R. Ischaemic muscle paralyses and contractures. Clin Orthop Relat Res. 1967;50:5-56. doi:10.1097/BLO.0b013e318032561f.
- Patrick JH, Levack B. A study of pressures beneath forearm plasters. Injury. 1981;13(1):37-41.
- Roberts A, Shaw KA, Boomsma SE, Cameron CD. Effect of casting material on the cast pressure after sequential cast splitting. J Pediatr Orthop. 2017;37(1):74-77. doi:10.1097/BPO.0000000000000574.
- Garfin SR, Mubarak SJ, Evans KL, Hargens AR, Akeson WH. Quantification of intracompartmental pressure and volume under plaster casts. J Bone Joint Surg Am. 1981;63(3):449-453.
- Capo JT, Renard RL, Moulton MJ, et al. How is forearm compliance affected by various circumferential dressings? Clin Orthop Relat Res. 2014 472(10):3228-3234. doi:10.1007/s11999-014-3747-y.
- Bingold AC. On splitting plasters. A useful analogy. J Bone Joint Surg Br. 1979;61-b(3):294-295.
- Crickard CV, Riccio AI, Carney JR, Anderson TD. Analysis and comparison of the biomechanical properties of univalved and bivalved cast models. J Pediatr Orthop.2011;31(1):39-43. doi:10.1097/BPO.0b013e318202c446.
- Rang M, Wenger DR, Pring ME. Rang's Children's Fractures. 3rd ed. Wenger DR, Rang M, eds. Philadelphia, PA: Lippincott Williams & Wilkins; 2005.
- Schulte D, Habernig S, Zuzak T, et al. Forearm fractures in children: split opinions about splitting the cast. Eur J Pediatr Surg. 2014;24(2):163-167. doi:10.1055/s-0033-1341412.
- Bae DS, Valim C, Connell P, Brustowicz KA, Waters PM. Bivalved versus circumferential cast immobilization for displaced forearm fractures: a randomized clinical trial to assess efficacy and safety. J Pediatr Orthop. 2017;37(4):239-246 doi:10.1097/BPO.0000000000000655.
TAKE-HOME POINTS
- Valving a long-arm cast results in decreased cast pressures.
- Univalving can produce a 60% reduction in cast pressure.
- Bivalving produces a 75% reduction in cast pressure.
- Release of the underlying cast padding produces an additional pressure reduction.
- Adding a cast spacer to a univalved cast obtains similar pressure reduction to a bivalved cast.



