User login
Pediatric Warts: Update on Interventions
The definition of warts is variable, largely reflecting their manifold appearance, biologic potential, and public health concerns. One vernacular dictionary defines warts as:
Small, benign growths caused by a vital infection of the skin or mucous membrane. The virus infects the surface layer. The viruses that cause warts are members of the human papilloma virus (HPV) family. Warts are not cancerous but some strains of HPV, usually not associated with warts, have been linked with cancer formation. Warts are contagious from person to person and from one area of the body to another on the same person.1
The World Health Organization defines warts by their structural components as:
Human papillomavirus (HPV) is a small, non-enveloped deoxyribonucleic acid (DNA) virus that infects skin or mucosal cells. The circular, double-stranded viral genome is approximately 8-kb in length. The genome encodes for 6 early proteins responsible for virus replication and 2 late proteins, L1 and L2, which are the viral structural proteins.2
In pediatric and adolescent dermatology, warts often are defined by their location and morphology; for example, facial warts typically are flat, minimally hyperkeratotic, or filiform, wherein the base is narrow and the lesion is tall, growing at a 90° angle to the surface of the skin. On the arms and legs, warts usually present as round to oval papules with overlying thick hyperkeratosis and/or callosity.3,4 Common warts usually are flesh colored or lighter, and heavily pigmented lesions should be evaluated dermoscopically for a pigment network and biopsied when pigment is present.5
In this article, a successful paradigm for management of pediatric warts is provided with enhanced outcomes based on further insight into the disease course and patient selection.
Epidemiology of Pediatric Warts
There are more than 200 types of human papillomaviruses (HPV), with more than 100 oncogenic types. There is quite a bit of homology by species and genus that contributes to cross-immunity and similar behavior between certain types of HPV. The lifetime incidence of warts is very high. Approximately 30% of children develop a wart.6 A review of the 2007 National Health Interview Survey of 9417 children demonstrated a steady increase in prevalence of warts from 1 to 2 years of age to 7 to 8 years of age, with a peak at 9 to 10 years of age and a plateau at 11 to 17 years of age. Warts were most common in non-Hispanic white children and less common in black children.7 In an in-person survey of 12,370 individuals aged 18 to 74 years from 5 European countries, warts were the most common physician-diagnosed (27.3%) and self-reported (41.0%) dermatologic condition. Warts are more common in Northern countries (eg, Netherlands, Germany).8 Children with atopic dermatitis have a higher risk of developing warts and extracutaneous infections. In one study, children with warts and atopic dermatitis had a higher number of infections and food allergies and higher incidence of asthma and hay fever than either condition alone.9
Clinical Presentation of Warts
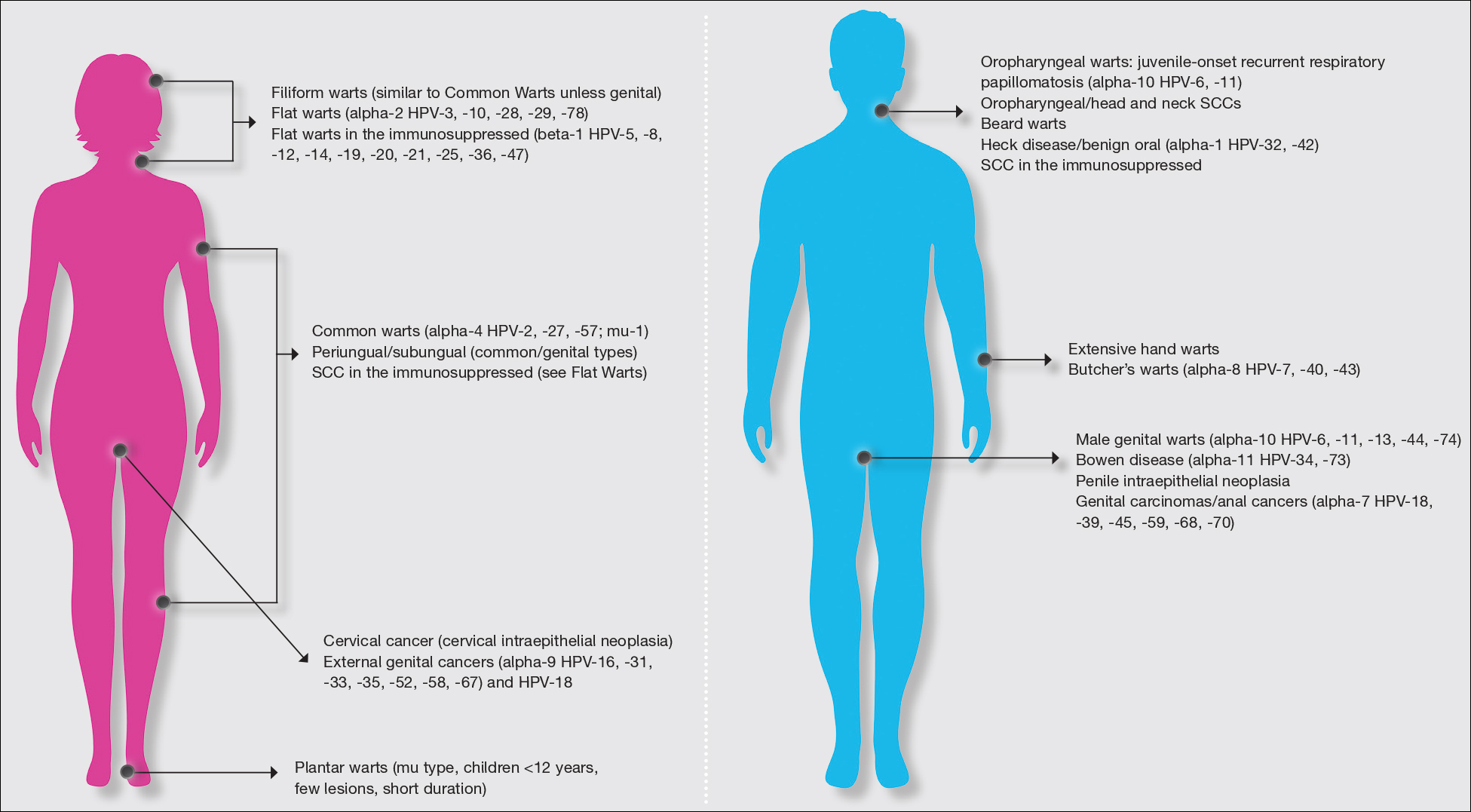
Warts usually present as common, palmoplantar, flat, or filiform in childhood, but variations by age are common (eFigure). The common and palmoplantar variants often are caused by HPV types 1 and 2.4,5 In infancy, vertically transmitted HPV infections can cause juvenile-onset respiratory papillomatosis or vertically transmitted condyloma. Juvenile-onset respiratory papillomatosis refers to upper respiratory papillomas that are difficult to eliminate and has been associated with exfoliated cervical cell testing with 18.1% (13/72) typed HPV-positive, which allows neonates to be exposed to HPV in the upper respiratory tract in utero.10
Vertically transmitted condyloma is a difficult topic. Much data supports the vertical transmission of condyloma as the leading cause of condyloma in small children; however, a reasonable amount of caution is needed in this patient population. In cases suspicious for sexual abuse as well as those presenting in children 4 years and older, formal household evaluation by a sexual abuse clinic and mandatory reporting is needed. Anywhere from 2.6% to 32% of cases of genital warts in children have been reported to be caused by sexual abuse.11-13 Therefore, most investigators have recommended careful review of the patient’s history and socioeconomic circumstances as well as a thorough physical examination. Mandatory reporting of suspected child sexual abuse is required in suspicious cases. Because HPV type 16 has been found in vertically transmitted cases, concern for long-term oncogenesis exists.11-13
Adolescents generally present with lesions on the hands and feet. Plantar warts often are caused by HPV types from the alpha genus. Subtypes noted in plantar warts include HPV types 1a, 2, 27, 57, and 65.14 By 15 years of age, genital HPV becomes a common adolescent infection, persisting into adulthood.15 When studied, genital HPV often is subclinical or latent and often is preventable through vaccination. High-risk oncogenic alpha-genus HPV types can immortalize human keratinocytes. When HPV types 11, 16, 18, and 31 are compared, HPV-18 has the highest oncogenic potential based on colony-stimulating potential.16 Vaccination with the 9-valent HPV vaccine is recommended in adolescence due to the concern for exposures to both low-potential (HPV types 6 and 11) and high-potential (HPV types 16 and 18) oncogenic HPV types. Data strongly support the benefit of 9-valent HPV vaccination in the prevention of sexually transmitted HPV in both males and females.17
Contagion of HPV is easy due to its excellent survival of fomites on surfaces, which generally is how warts are transferred in gym or pool settings where individuals who walk barefoot in changing rooms are almost twice as likely to contract plantar warts (odds ratio, 1.97 [95% CI, 1.39%-2.79%]).18 In another case series, walking barefoot, using a swimming pool, and having a household contact with warts were the leading risk factors for contraction of warts in children younger than 13 years.19 Children often transfer warts from site to site as well as to siblings and other close contacts. Skin-to-skin contact is responsible for sexual transmission of warts, and surface transmission occurs via fomites. Entry of the virus often occurs through small breaks in the skin. Other modes of transmission include orogenital.20
Therapeutic Options
Although the nuances of each available treatment for pediatric warts are beyond the scope of this article, the main core of therapy is 1 of 3 approaches: (1) observation, (2) over-the-counter salicylic acid therapy, and (3) in-office cryotherapy. Observation is an affirmed style of therapy for warts, as it is expected that two-thirds of warts will spontaneously resolve in 2 years and three-quarters will resolve in 3 years.4,5 Condyloma in children has been responsive to therapies such as cryotherapy and imiquimod,13 but spontaneous clearance in 5 years has been noted in 76% of children,21 which is linked to development of spontaneous immune response in most individuals.
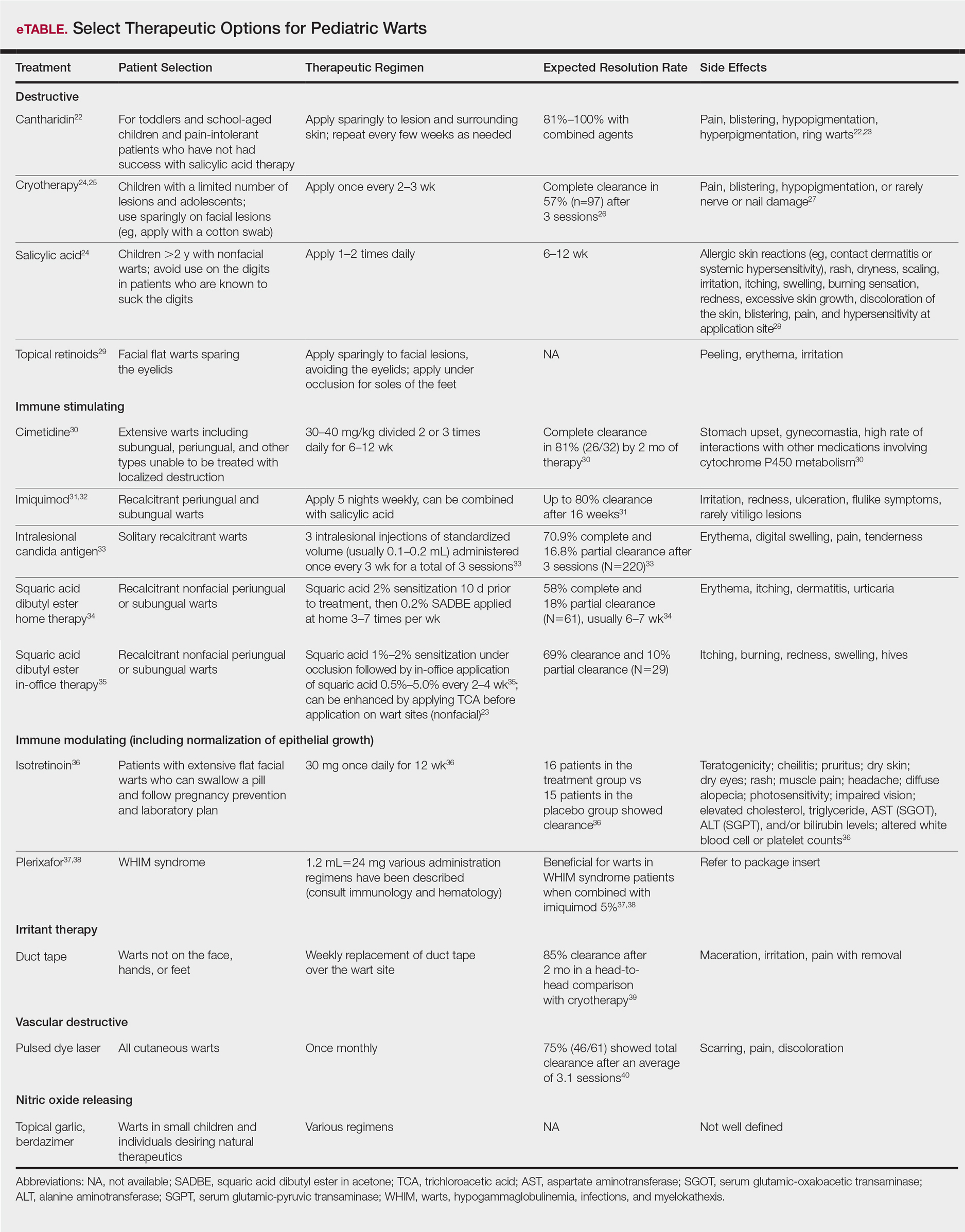
Therapies for pediatric warts are characterized according to 6 major categories: destructive; immune stimulating; immune modulating, including normalization of epithelial growth; irritant; vascular destructive; and nitric oxide releasing (eTable).
Destructive Therapies
Destructive therapies for warts often are implemented in cases of disfigurement, discomfort/pain, and/or spreading, as well as to control contagion. According to a 2001 Cochrane review, salicylic acid has the best evidence of all therapeutics for the clearance of warts compared to placebo.24 On the other hand, aggressive cryotherapy and combined salicylic acid and cryotherapy had the best evidence in their favor in a 2011 meta-analysis by Kwok et al.25 Both salicylic acid and cryotherapy are considered destructive therapies. A recent meta-analysis of cantharidin, another destructive therapy, showed that local cantharidin alone as well as in combination with salicylic acid and podophyllotoxin showed good efficacy for warts; however, increased caution should be exerted with the combination regimen in young children due to a potential increase in the side-effect profile (eg, severe blistering).22 Other destructive agents such as topical retinoids can only peel surface layers of the skin and therefore are limited to flat facial warts, which are not expected to have an extensive hyperkeratotic layer; however, with occlusion, agents such as adapalene gel 0.1% can be used even on plantar warts with some efficacy.29
Immune-Stimulating Therapies
Immune stimulants often are used to treat warts in children and adolescents who have many lesions, a prolonged disease course, disfigurement, and/or subungual localizations, as well as in those who have been treated with multiple destructive methods without success. Topical imiquimod and oral cimetidine are readily available, while squaric acid (at-home or in-office therapy) and intralesional candida antigen can be used in offices that carry these agents. Topical imiquimod has been reported to achieve success in genital warts in children,13 with good efficacy in recalcitrant, periungual, and subungual warts when used for up to 16 weeks.31 In one randomized clinical trial, imiquimod cream 5% combined with salicylic acid 15% was applied to warts for 6 to 10 hours for 5 consecutive days per week versus cryotherapy with liquid nitrogen every 2 weeks for a maximum of 3 months. At the end of the study period, 81.1% (30/37) of participants treated with imiquimod and salicylic acid showed clearance of their warts versus 67.3% (33/49) of those treated with cryotherapy.32
Oral cimetidine has been reported to be successful in treating recalcitrant warts in more than 80% of children when dosed at 30 to 40 mg/kg 3 times daily, requiring 6 to 12 weeks to achieve clearance. Side effects of oral cimetidine include many cytochrome P450 interactions; gynecomastia, which limits usage in teenaged males; and stomach upset.30
Treatment of recalcitrant pediatric warts with intralesional candida antigen has been associated with side effects consistent with delayed-type hypersensitivity reactions. Injections should be administered once monthly, with a minimum of 3 cycles if not effective and up to 6 cycles where partial efficacy is noted. In a retrospective review of 220 cases, 70.9% of children showed complete clearance and 16.8% had partial response.33 However, the treatment may be limited in children by fear of needles.
Squaric acid dibutyl ester is a universal allergen that is not mutagenic on Ames testing and causes milder allergy symptoms than the mutagenic dinitrochlorobenzene and less erythema and pruritus than diphencyclopropenone. Squaric acid dibutyl ester home therapy was evaluated in 61 children with at least one nonfacial wart.34 Application began with squaric acid dibutyl ester in acetone (SADBE) 2% sensitization on the arm followed by at-home application of SADBE 0.2% three to seven times weekly for a minimum of 2 months to determine benefit and for 3 to 4 months as needed; however, average response was 7 weeks. The average complete clearance was 58% and partial clearance was 18%. Side effects included erythema and mild itching as well as urticaria in one case.34 In-office SADBE also has been evaluated in children. In a case series that included 29 children sensitized with SADBE 1% to 2% under occlusion followed by once monthly application of SADBE 0.5% to 5.0% to their warts, 69% clearance and 10% partial clearance was noted after a little more than 4 months of treatment.35 One retrospective review compared combination SADBE, trichloroacetic acid (TCA), and cantharidin both alone and in combination as duos (eg, SADBE and TCA) or trios (SADBE, TCA, and cantharidin).23 Of the 74 children whose medical charts were reviewed, the addition of pretreatment of warts with TCA 50% prior to in-office sensitization and monthly in-office application of SADBE increased treatment response to 100% with an average 2.45 months of therapy, whereas no enhancement was noted with cantharidin. Therefore, it appears that there may be enhanced immune reactivity when TCA pretreatment of warts is performed.23
Immune-Modulating Therapies (Including Normalization of Epithelial Growth)
The most novel immunologic therapy for warts is plerixafor, an agent used to treat WHIM (warts, hypogammaglobulinemia, infections, and myelokathexis) syndrome, which has been linked to heterozygous gain of function mutations in the chemokine receptor CXCR4 (located on 2q22). In WHIM syndrome, the mutated CXCR4 is more sensitive to CXCL12 activation. Plerixafor is a selective reversible antagonist that blocks the capacity of the chemokine CXCL12 to sustain the permanent activation of CXCR4.37 Combination therapy with plerixafor and topical imiquimod has resulted in wart improvement in WHIM syndrome patients in a small series.38
Oral isotretinoin has been described to be efficacious over placebo at a dosage of 30 mg daily for 12 weeks and can be used in teenagers but requires standard monitoring.36
Irritant Therapies
Duct tape is a classic agent that produces maceration and irritation of warts. Application of duct tape over warts has been described in cycles of 6 days on, 1 day off with weekly repetition for a few months but usually not on the palms or soles due to difficulty maintaining occlusive tape in these locations over an extended period of time. In one trial, 85% (22/26) of duct tape–treated cases cleared versus 60% (15/25) of cryotherapy-treated cases over a 2-month maximum therapeutic period.39
Vascular Destructive Therapies
The pulsed dye laser is a classic modality that induces localized destruction of blood supply to warts in children. A case series of 61 children treated with the pulsed dye laser revealed 75% overall clearance in an average of 3.1 sessions. The usage of this therapy often is limited to institutions where the technology is readily available for usage.40
Nitric Oxide–Releasing Therapies
Nitric oxide release may increase local blood flow, thereby increasing immune response, or may have a primary mechanism of antimicrobial activity, which is why these agents have been investigated for wart treatment. Topical garlic has been described anecdotally as a therapy for thin childhood warts with the putative mechanism being nitric oxide release.42 A new investigational drug recently has had phase 2 data published. Berdazimer sodium plus carboxymethyl cellulose hydrogel has demonstrated benefit in adult warts, but data in children is lacking.41
Therapeutic Ladder for Childhood Warts
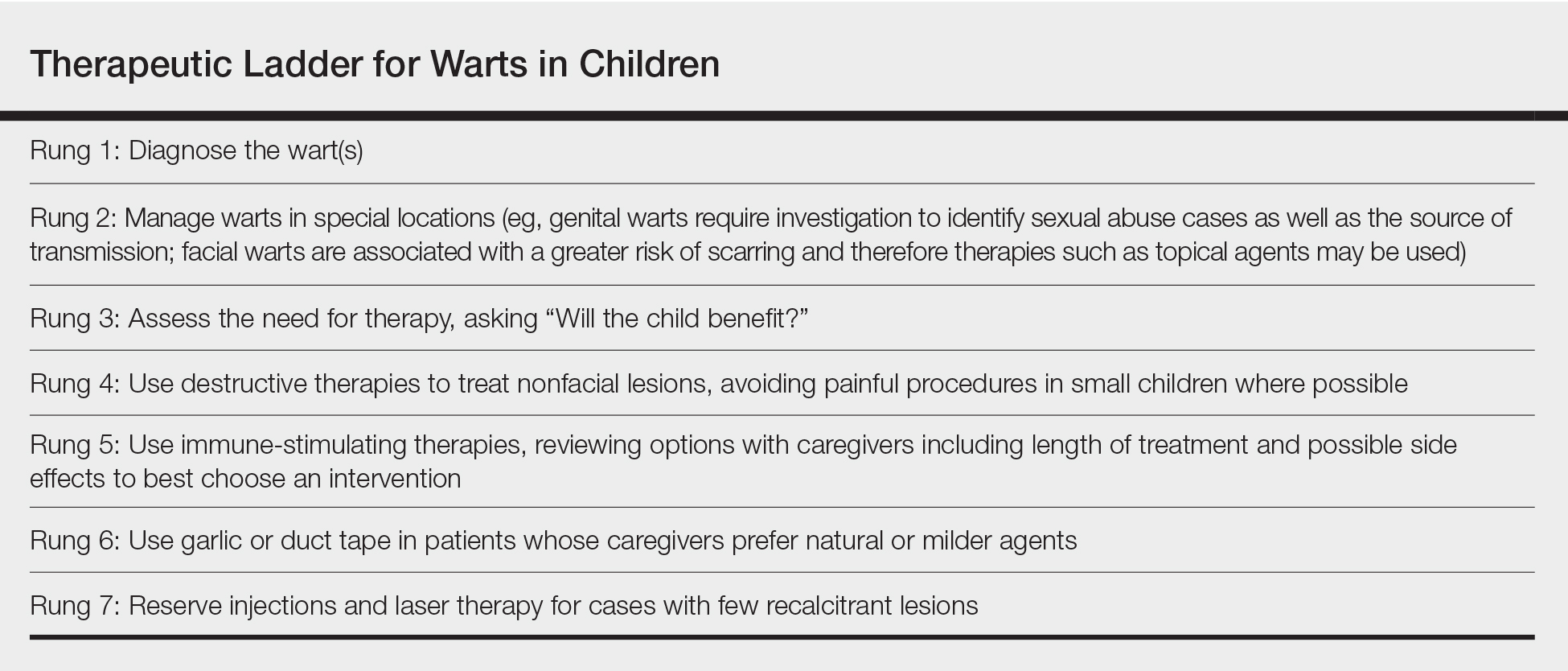
The therapeutic ladder (Table) for childhood warts starts with first doing no harm. Although many parents are disturbed by their child’s condition, the natural history of resolution is spontaneous and therefore no therapy is required in many cases. The child and his/her caregivers should be engaged to determine if he/she is emotionally disturbed or uncomfortable with their lesions and to address any fears and concerns that some children may experience (eg, contagion risk, pain with ambulation, ostracism). For example, children with hand warts may report that other children will not hold their hand while in line at school. Prominent facial lesions can be particularly problematic for children due to teasing and bullying.
Conclusion
Warts are a common infection in childhood caused by the ubiquitous HPV virus. Therapeutic options abound, but most cases are either ignored or treated with over-the-counter salicylic acid or in-office cryotherapy. The decision to employ alternative therapeutic options requires agreement by the child, his/her caregiver, and the treating physician and can be tailored to suit the desires and needs of the child. Whether or not therapy is offered, spontaneous clearance is frequently seen in common warts. On the other hand, genital warts are associated with later conversion to malignancies of the genital tract; therefore, encouragement of HPV vaccination is needed in the adolescent population to best ensure long-term genital health.
1. Warts. https://medical-dictionary.thefreedictionary.com/warts. Accessed November 30, 2018.
2. Human papillomavirus. WHO website. http://www.who.int/biologicals/areas/human_papillomavirus/en. Accessed December 3, 2018.
3. Silverberg NB. Human papillomavirus infections in children. Curr Opin Pediatr. 2004;16:402-409.
4. Silverberg NB. Warts and molluscum in children. Adv Dermatol. 2004;20:23-73.
5. Silverberg NB, McCuaig CC. Melanoma in childhood: changing our mind-set. Cutis. 2013;92:217-218.
6. Bruggink SC, Eekhof JA, Egberts PF, et al. Warts transmitted in families and schools: a prospective cohort. Pediatrics. 2013;131:928-934.
7. Silverberg JI, Silverberg NB. The U.S. prevalence of common warts in childhood: a population-based study. J Invest Dermatol. 2013;133:2788-2790.
8. Svensson A, Ofenloch RF, Bruze M, et al. Br J Dermatol. 2018;178:1111-1118.
9. Silverberg JI, Silverberg NB. Childhood atopic dermatitis and warts are associated with increased risk of infection: a US population-based study. J Allergy Clin Immunol. 2014;133:1041-1047.
10. Smith EM, Johnson SR, Cripe TP, et al. Perinatal vertical transmission of human papillomavirus and subsequent development of respiratory tract papillomatosis. Ann Otol Rhinol Laryngol. 1991;100:479-483.
11. Costa-Silva M, Azevedo F, Lisboa C. Anogenital warts in children: analysis of a cohort of 34 prepubertal children. Pediatr Dermatol. 2018;35:E325-E327.
12. Marcoux D, Nadeau K, McCuaig C, et al. Pediatric anogenital warts: a 7-year review of children referred to a tertiary-care hospital in Montreal, Canada. Pediatr Dermatol. 2006;23:199-207.
13. Stefanaki C, Barkas G, Valari M, et al. Condylomata acuminata in children. Pediatr Infect Dis J. 2012;31:422-424.
14. dePlanell-Mas E, Martinez-Garriga B, Zalacain AJ, et al. Human papillomaviruses genotyping in plantar warts. J Med Virol. 2017;89:902-907.
15. Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40:187-193.
16. Lace MJ, Anson JR, Klingelhutz AJ, et al. Human papillomavirus (HPV) type 18 induces extended growth in primary human cervical, tonsillar, or foreskin keratinocytes more effectively than other high-risk mucosal HPVs. J Virol. 2009;83:11784-11794.
17. Sudenga SL, Ingles DJ, Pierce Campbell CM, et al. Genital human papillomavirus infection progression to external genital lesions: the HIM study. Eur Urol. 2016;69:166-173.
18. Rigo MV, Martínez Campillo F, Verdú M, et al. Risk factors linked to the transmission of papilloma virus in the school environment [in Spanish]. Alicante, 1999. Aten Primaria. 2003;31:415-420.
19. Al-Mutairi N, AlKhalaf M. Mucocutaneous warts in children: clinical presentations, risk factors, and response to treatment. Acta Dermatovenerol Alp Pannonica Adriat. 2012;21:69-72.
20. Clarke J, Terry RM, Lacey CJ. A study to estimate the prevalence of upper respiratory tract papillomatosis in patients with genital warts. Int J STD AIDS. 1991;2:114-115.
21. Allen AL, Siegfried EC. The natural history of condyloma in children. J Am Acad Dermatol. 1998;39:951-955.
22. Vakharia PP, Chopra R, Silverberg NB, et al. Efficacy and safety of topical cantharidin treatment for molluscum contagiosum and warts: a systematic review. Am J Clin Dermatol. 2018;19:791-803.
23. Silverberg JI, Silverberg NB. Adjunctive trichloroacetic acid therapy enhances squaric acid response to verruca vulgaris. J Drugs Dermatol. 2012;11:1228-1230.
24. Gibbs S, Harvey I, Sterling JC, et al. Local treatments for cutaneous warts. Cochrane Database Syst Rev. 2001:CD001781.
25. Kwok CS, Holland R, Gibbs S. Efficacy of topical treatments for cutaneous warts: a meta-analysis and pooled analysis of randomized controlled trials. Br J Dermatol. 2011;165:233-246.
26. Allington HV. Liquid nitrogen in the treatment of skin diseases. Calif Med. 1950;72:153-155.
27. Caravati CM Jr, Wood BT, Richardson DR. Onychodystrophies secondary to liquid nitrogen cryotherapy. Arch Dermatol. 1969;100:441-442.
28. Duofilm [package insert]. Sligo, Ireland: Stiefel Laboratories (Ireland) Ltd; 2016.
29. Gupta R, Gupta S. Topical adapalene in the treatment of plantar warts: randomized comparative open trial in comparison with cryo-therapy. Indian J Dermatol. 2015;60:102.
30. Orlow SJ, Paller A. Cimetidine therapy for multiple viral warts in children. J Am Acad Dermatol. 1993;28(5 pt 1):794-796.
31. Micali G, Dall’Oglio F, Nasca MR. An open label evaluation of the efficacy of imiquimod 5% cream in the treatment of recalcitrant subungual and periungual cutaneous warts. J Dermatolog Treat. 2003;14:233-236.
32. Stefanaki C, Lagogiani I, Kouris A, et al. Cryotherapy versus imiquimod 5% cream combined with a keratolytic lotion in cutaneous warts in children: a randomized study. J Dermatolog Treat. 2016;27:80-82.
33. Muñoz Garza FZ, Roé Crespo E, Torres Pradilla M, et al. Intralesional Candida antigen immunotherapy for the treatment of recalcitrant and multiple warts in children. Pediatr Dermatol. 2015;32:797-801.
34. Silverberg NB, Lim JK, Paller AS, et al. Squaric acid immunotherapy for warts in children. J Am Acad Dermatol. 2000;42(5 pt 1):803-808.
35. Lee AN, Mallory SB. Contact immunotherapy with squaric acid dibutylester for the treatment of recalcitrant warts. J Am Acad Dermatol. 1999;41:595-599.
36. Olguin-García MG, Jurado-Santa Cruz F, Peralta-Pedrero ML, et al. A double-blind, randomized, placebo-controlled trial of oral isotretinoin in the treatment of recalcitrant facial flat warts. J Dermatolog Treat. 2015;26:78-82.
37. Badolato R, Donadieu J; WHIM Research Group. How I treat warts, hypogammaglobulinemia, infections, and myelokathexis syndrome. Blood. 2017;130:2491-2498.
38. McDermott DH, Liu Q, Velez D, et al. A phase 1 clinical trial of long-term, low-dose treatment of WHIM syndrome with the CXCR4 antagonist plerixafor. Blood. 2014;123:2308-2316.
39. Focht DR 3rd, Spicer C, Fairchok MP. The efficacy of duct tape vs cryotherapy in the treatment of verruca vulgaris (the common wart). Arch Pediatr Adolesc Med. 2002;156:971-974.
40. Sethuraman G, Richards KA, Hiremagalore RN, et al. Effectiveness of pulsed dye laser in the treatment of recalcitrant warts in children. Dermatol Surg. 2010;36:58-65.
41. Tyring SK, Rosen T, Berman B, et al. A phase 2 controlled study of SB206, a topical nitric oxide-releasing drug for extragenital wart treatment. J Drugs Dermatol. 2018;17:1100-1105.
42. Silverberg NB. Garlic cloves for verruca vulgaris. Pediatr Dermatol. 2002;19:183.
The definition of warts is variable, largely reflecting their manifold appearance, biologic potential, and public health concerns. One vernacular dictionary defines warts as:
Small, benign growths caused by a vital infection of the skin or mucous membrane. The virus infects the surface layer. The viruses that cause warts are members of the human papilloma virus (HPV) family. Warts are not cancerous but some strains of HPV, usually not associated with warts, have been linked with cancer formation. Warts are contagious from person to person and from one area of the body to another on the same person.1
The World Health Organization defines warts by their structural components as:
Human papillomavirus (HPV) is a small, non-enveloped deoxyribonucleic acid (DNA) virus that infects skin or mucosal cells. The circular, double-stranded viral genome is approximately 8-kb in length. The genome encodes for 6 early proteins responsible for virus replication and 2 late proteins, L1 and L2, which are the viral structural proteins.2
In pediatric and adolescent dermatology, warts often are defined by their location and morphology; for example, facial warts typically are flat, minimally hyperkeratotic, or filiform, wherein the base is narrow and the lesion is tall, growing at a 90° angle to the surface of the skin. On the arms and legs, warts usually present as round to oval papules with overlying thick hyperkeratosis and/or callosity.3,4 Common warts usually are flesh colored or lighter, and heavily pigmented lesions should be evaluated dermoscopically for a pigment network and biopsied when pigment is present.5
In this article, a successful paradigm for management of pediatric warts is provided with enhanced outcomes based on further insight into the disease course and patient selection.
Epidemiology of Pediatric Warts
There are more than 200 types of human papillomaviruses (HPV), with more than 100 oncogenic types. There is quite a bit of homology by species and genus that contributes to cross-immunity and similar behavior between certain types of HPV. The lifetime incidence of warts is very high. Approximately 30% of children develop a wart.6 A review of the 2007 National Health Interview Survey of 9417 children demonstrated a steady increase in prevalence of warts from 1 to 2 years of age to 7 to 8 years of age, with a peak at 9 to 10 years of age and a plateau at 11 to 17 years of age. Warts were most common in non-Hispanic white children and less common in black children.7 In an in-person survey of 12,370 individuals aged 18 to 74 years from 5 European countries, warts were the most common physician-diagnosed (27.3%) and self-reported (41.0%) dermatologic condition. Warts are more common in Northern countries (eg, Netherlands, Germany).8 Children with atopic dermatitis have a higher risk of developing warts and extracutaneous infections. In one study, children with warts and atopic dermatitis had a higher number of infections and food allergies and higher incidence of asthma and hay fever than either condition alone.9
Clinical Presentation of Warts
Warts usually present as common, palmoplantar, flat, or filiform in childhood, but variations by age are common (eFigure). The common and palmoplantar variants often are caused by HPV types 1 and 2.4,5 In infancy, vertically transmitted HPV infections can cause juvenile-onset respiratory papillomatosis or vertically transmitted condyloma. Juvenile-onset respiratory papillomatosis refers to upper respiratory papillomas that are difficult to eliminate and has been associated with exfoliated cervical cell testing with 18.1% (13/72) typed HPV-positive, which allows neonates to be exposed to HPV in the upper respiratory tract in utero.10
Vertically transmitted condyloma is a difficult topic. Much data supports the vertical transmission of condyloma as the leading cause of condyloma in small children; however, a reasonable amount of caution is needed in this patient population. In cases suspicious for sexual abuse as well as those presenting in children 4 years and older, formal household evaluation by a sexual abuse clinic and mandatory reporting is needed. Anywhere from 2.6% to 32% of cases of genital warts in children have been reported to be caused by sexual abuse.11-13 Therefore, most investigators have recommended careful review of the patient’s history and socioeconomic circumstances as well as a thorough physical examination. Mandatory reporting of suspected child sexual abuse is required in suspicious cases. Because HPV type 16 has been found in vertically transmitted cases, concern for long-term oncogenesis exists.11-13
Adolescents generally present with lesions on the hands and feet. Plantar warts often are caused by HPV types from the alpha genus. Subtypes noted in plantar warts include HPV types 1a, 2, 27, 57, and 65.14 By 15 years of age, genital HPV becomes a common adolescent infection, persisting into adulthood.15 When studied, genital HPV often is subclinical or latent and often is preventable through vaccination. High-risk oncogenic alpha-genus HPV types can immortalize human keratinocytes. When HPV types 11, 16, 18, and 31 are compared, HPV-18 has the highest oncogenic potential based on colony-stimulating potential.16 Vaccination with the 9-valent HPV vaccine is recommended in adolescence due to the concern for exposures to both low-potential (HPV types 6 and 11) and high-potential (HPV types 16 and 18) oncogenic HPV types. Data strongly support the benefit of 9-valent HPV vaccination in the prevention of sexually transmitted HPV in both males and females.17
Contagion of HPV is easy due to its excellent survival of fomites on surfaces, which generally is how warts are transferred in gym or pool settings where individuals who walk barefoot in changing rooms are almost twice as likely to contract plantar warts (odds ratio, 1.97 [95% CI, 1.39%-2.79%]).18 In another case series, walking barefoot, using a swimming pool, and having a household contact with warts were the leading risk factors for contraction of warts in children younger than 13 years.19 Children often transfer warts from site to site as well as to siblings and other close contacts. Skin-to-skin contact is responsible for sexual transmission of warts, and surface transmission occurs via fomites. Entry of the virus often occurs through small breaks in the skin. Other modes of transmission include orogenital.20
Therapeutic Options
Although the nuances of each available treatment for pediatric warts are beyond the scope of this article, the main core of therapy is 1 of 3 approaches: (1) observation, (2) over-the-counter salicylic acid therapy, and (3) in-office cryotherapy. Observation is an affirmed style of therapy for warts, as it is expected that two-thirds of warts will spontaneously resolve in 2 years and three-quarters will resolve in 3 years.4,5 Condyloma in children has been responsive to therapies such as cryotherapy and imiquimod,13 but spontaneous clearance in 5 years has been noted in 76% of children,21 which is linked to development of spontaneous immune response in most individuals.
Therapies for pediatric warts are characterized according to 6 major categories: destructive; immune stimulating; immune modulating, including normalization of epithelial growth; irritant; vascular destructive; and nitric oxide releasing (eTable).
Destructive Therapies
Destructive therapies for warts often are implemented in cases of disfigurement, discomfort/pain, and/or spreading, as well as to control contagion. According to a 2001 Cochrane review, salicylic acid has the best evidence of all therapeutics for the clearance of warts compared to placebo.24 On the other hand, aggressive cryotherapy and combined salicylic acid and cryotherapy had the best evidence in their favor in a 2011 meta-analysis by Kwok et al.25 Both salicylic acid and cryotherapy are considered destructive therapies. A recent meta-analysis of cantharidin, another destructive therapy, showed that local cantharidin alone as well as in combination with salicylic acid and podophyllotoxin showed good efficacy for warts; however, increased caution should be exerted with the combination regimen in young children due to a potential increase in the side-effect profile (eg, severe blistering).22 Other destructive agents such as topical retinoids can only peel surface layers of the skin and therefore are limited to flat facial warts, which are not expected to have an extensive hyperkeratotic layer; however, with occlusion, agents such as adapalene gel 0.1% can be used even on plantar warts with some efficacy.29
Immune-Stimulating Therapies
Immune stimulants often are used to treat warts in children and adolescents who have many lesions, a prolonged disease course, disfigurement, and/or subungual localizations, as well as in those who have been treated with multiple destructive methods without success. Topical imiquimod and oral cimetidine are readily available, while squaric acid (at-home or in-office therapy) and intralesional candida antigen can be used in offices that carry these agents. Topical imiquimod has been reported to achieve success in genital warts in children,13 with good efficacy in recalcitrant, periungual, and subungual warts when used for up to 16 weeks.31 In one randomized clinical trial, imiquimod cream 5% combined with salicylic acid 15% was applied to warts for 6 to 10 hours for 5 consecutive days per week versus cryotherapy with liquid nitrogen every 2 weeks for a maximum of 3 months. At the end of the study period, 81.1% (30/37) of participants treated with imiquimod and salicylic acid showed clearance of their warts versus 67.3% (33/49) of those treated with cryotherapy.32
Oral cimetidine has been reported to be successful in treating recalcitrant warts in more than 80% of children when dosed at 30 to 40 mg/kg 3 times daily, requiring 6 to 12 weeks to achieve clearance. Side effects of oral cimetidine include many cytochrome P450 interactions; gynecomastia, which limits usage in teenaged males; and stomach upset.30
Treatment of recalcitrant pediatric warts with intralesional candida antigen has been associated with side effects consistent with delayed-type hypersensitivity reactions. Injections should be administered once monthly, with a minimum of 3 cycles if not effective and up to 6 cycles where partial efficacy is noted. In a retrospective review of 220 cases, 70.9% of children showed complete clearance and 16.8% had partial response.33 However, the treatment may be limited in children by fear of needles.
Squaric acid dibutyl ester is a universal allergen that is not mutagenic on Ames testing and causes milder allergy symptoms than the mutagenic dinitrochlorobenzene and less erythema and pruritus than diphencyclopropenone. Squaric acid dibutyl ester home therapy was evaluated in 61 children with at least one nonfacial wart.34 Application began with squaric acid dibutyl ester in acetone (SADBE) 2% sensitization on the arm followed by at-home application of SADBE 0.2% three to seven times weekly for a minimum of 2 months to determine benefit and for 3 to 4 months as needed; however, average response was 7 weeks. The average complete clearance was 58% and partial clearance was 18%. Side effects included erythema and mild itching as well as urticaria in one case.34 In-office SADBE also has been evaluated in children. In a case series that included 29 children sensitized with SADBE 1% to 2% under occlusion followed by once monthly application of SADBE 0.5% to 5.0% to their warts, 69% clearance and 10% partial clearance was noted after a little more than 4 months of treatment.35 One retrospective review compared combination SADBE, trichloroacetic acid (TCA), and cantharidin both alone and in combination as duos (eg, SADBE and TCA) or trios (SADBE, TCA, and cantharidin).23 Of the 74 children whose medical charts were reviewed, the addition of pretreatment of warts with TCA 50% prior to in-office sensitization and monthly in-office application of SADBE increased treatment response to 100% with an average 2.45 months of therapy, whereas no enhancement was noted with cantharidin. Therefore, it appears that there may be enhanced immune reactivity when TCA pretreatment of warts is performed.23
Immune-Modulating Therapies (Including Normalization of Epithelial Growth)
The most novel immunologic therapy for warts is plerixafor, an agent used to treat WHIM (warts, hypogammaglobulinemia, infections, and myelokathexis) syndrome, which has been linked to heterozygous gain of function mutations in the chemokine receptor CXCR4 (located on 2q22). In WHIM syndrome, the mutated CXCR4 is more sensitive to CXCL12 activation. Plerixafor is a selective reversible antagonist that blocks the capacity of the chemokine CXCL12 to sustain the permanent activation of CXCR4.37 Combination therapy with plerixafor and topical imiquimod has resulted in wart improvement in WHIM syndrome patients in a small series.38
Oral isotretinoin has been described to be efficacious over placebo at a dosage of 30 mg daily for 12 weeks and can be used in teenagers but requires standard monitoring.36
Irritant Therapies
Duct tape is a classic agent that produces maceration and irritation of warts. Application of duct tape over warts has been described in cycles of 6 days on, 1 day off with weekly repetition for a few months but usually not on the palms or soles due to difficulty maintaining occlusive tape in these locations over an extended period of time. In one trial, 85% (22/26) of duct tape–treated cases cleared versus 60% (15/25) of cryotherapy-treated cases over a 2-month maximum therapeutic period.39
Vascular Destructive Therapies
The pulsed dye laser is a classic modality that induces localized destruction of blood supply to warts in children. A case series of 61 children treated with the pulsed dye laser revealed 75% overall clearance in an average of 3.1 sessions. The usage of this therapy often is limited to institutions where the technology is readily available for usage.40
Nitric Oxide–Releasing Therapies
Nitric oxide release may increase local blood flow, thereby increasing immune response, or may have a primary mechanism of antimicrobial activity, which is why these agents have been investigated for wart treatment. Topical garlic has been described anecdotally as a therapy for thin childhood warts with the putative mechanism being nitric oxide release.42 A new investigational drug recently has had phase 2 data published. Berdazimer sodium plus carboxymethyl cellulose hydrogel has demonstrated benefit in adult warts, but data in children is lacking.41
Therapeutic Ladder for Childhood Warts
The therapeutic ladder (Table) for childhood warts starts with first doing no harm. Although many parents are disturbed by their child’s condition, the natural history of resolution is spontaneous and therefore no therapy is required in many cases. The child and his/her caregivers should be engaged to determine if he/she is emotionally disturbed or uncomfortable with their lesions and to address any fears and concerns that some children may experience (eg, contagion risk, pain with ambulation, ostracism). For example, children with hand warts may report that other children will not hold their hand while in line at school. Prominent facial lesions can be particularly problematic for children due to teasing and bullying.
Conclusion
Warts are a common infection in childhood caused by the ubiquitous HPV virus. Therapeutic options abound, but most cases are either ignored or treated with over-the-counter salicylic acid or in-office cryotherapy. The decision to employ alternative therapeutic options requires agreement by the child, his/her caregiver, and the treating physician and can be tailored to suit the desires and needs of the child. Whether or not therapy is offered, spontaneous clearance is frequently seen in common warts. On the other hand, genital warts are associated with later conversion to malignancies of the genital tract; therefore, encouragement of HPV vaccination is needed in the adolescent population to best ensure long-term genital health.
The definition of warts is variable, largely reflecting their manifold appearance, biologic potential, and public health concerns. One vernacular dictionary defines warts as:
Small, benign growths caused by a vital infection of the skin or mucous membrane. The virus infects the surface layer. The viruses that cause warts are members of the human papilloma virus (HPV) family. Warts are not cancerous but some strains of HPV, usually not associated with warts, have been linked with cancer formation. Warts are contagious from person to person and from one area of the body to another on the same person.1
The World Health Organization defines warts by their structural components as:
Human papillomavirus (HPV) is a small, non-enveloped deoxyribonucleic acid (DNA) virus that infects skin or mucosal cells. The circular, double-stranded viral genome is approximately 8-kb in length. The genome encodes for 6 early proteins responsible for virus replication and 2 late proteins, L1 and L2, which are the viral structural proteins.2
In pediatric and adolescent dermatology, warts often are defined by their location and morphology; for example, facial warts typically are flat, minimally hyperkeratotic, or filiform, wherein the base is narrow and the lesion is tall, growing at a 90° angle to the surface of the skin. On the arms and legs, warts usually present as round to oval papules with overlying thick hyperkeratosis and/or callosity.3,4 Common warts usually are flesh colored or lighter, and heavily pigmented lesions should be evaluated dermoscopically for a pigment network and biopsied when pigment is present.5
In this article, a successful paradigm for management of pediatric warts is provided with enhanced outcomes based on further insight into the disease course and patient selection.
Epidemiology of Pediatric Warts
There are more than 200 types of human papillomaviruses (HPV), with more than 100 oncogenic types. There is quite a bit of homology by species and genus that contributes to cross-immunity and similar behavior between certain types of HPV. The lifetime incidence of warts is very high. Approximately 30% of children develop a wart.6 A review of the 2007 National Health Interview Survey of 9417 children demonstrated a steady increase in prevalence of warts from 1 to 2 years of age to 7 to 8 years of age, with a peak at 9 to 10 years of age and a plateau at 11 to 17 years of age. Warts were most common in non-Hispanic white children and less common in black children.7 In an in-person survey of 12,370 individuals aged 18 to 74 years from 5 European countries, warts were the most common physician-diagnosed (27.3%) and self-reported (41.0%) dermatologic condition. Warts are more common in Northern countries (eg, Netherlands, Germany).8 Children with atopic dermatitis have a higher risk of developing warts and extracutaneous infections. In one study, children with warts and atopic dermatitis had a higher number of infections and food allergies and higher incidence of asthma and hay fever than either condition alone.9
Clinical Presentation of Warts
Warts usually present as common, palmoplantar, flat, or filiform in childhood, but variations by age are common (eFigure). The common and palmoplantar variants often are caused by HPV types 1 and 2.4,5 In infancy, vertically transmitted HPV infections can cause juvenile-onset respiratory papillomatosis or vertically transmitted condyloma. Juvenile-onset respiratory papillomatosis refers to upper respiratory papillomas that are difficult to eliminate and has been associated with exfoliated cervical cell testing with 18.1% (13/72) typed HPV-positive, which allows neonates to be exposed to HPV in the upper respiratory tract in utero.10
Vertically transmitted condyloma is a difficult topic. Much data supports the vertical transmission of condyloma as the leading cause of condyloma in small children; however, a reasonable amount of caution is needed in this patient population. In cases suspicious for sexual abuse as well as those presenting in children 4 years and older, formal household evaluation by a sexual abuse clinic and mandatory reporting is needed. Anywhere from 2.6% to 32% of cases of genital warts in children have been reported to be caused by sexual abuse.11-13 Therefore, most investigators have recommended careful review of the patient’s history and socioeconomic circumstances as well as a thorough physical examination. Mandatory reporting of suspected child sexual abuse is required in suspicious cases. Because HPV type 16 has been found in vertically transmitted cases, concern for long-term oncogenesis exists.11-13
Adolescents generally present with lesions on the hands and feet. Plantar warts often are caused by HPV types from the alpha genus. Subtypes noted in plantar warts include HPV types 1a, 2, 27, 57, and 65.14 By 15 years of age, genital HPV becomes a common adolescent infection, persisting into adulthood.15 When studied, genital HPV often is subclinical or latent and often is preventable through vaccination. High-risk oncogenic alpha-genus HPV types can immortalize human keratinocytes. When HPV types 11, 16, 18, and 31 are compared, HPV-18 has the highest oncogenic potential based on colony-stimulating potential.16 Vaccination with the 9-valent HPV vaccine is recommended in adolescence due to the concern for exposures to both low-potential (HPV types 6 and 11) and high-potential (HPV types 16 and 18) oncogenic HPV types. Data strongly support the benefit of 9-valent HPV vaccination in the prevention of sexually transmitted HPV in both males and females.17
Contagion of HPV is easy due to its excellent survival of fomites on surfaces, which generally is how warts are transferred in gym or pool settings where individuals who walk barefoot in changing rooms are almost twice as likely to contract plantar warts (odds ratio, 1.97 [95% CI, 1.39%-2.79%]).18 In another case series, walking barefoot, using a swimming pool, and having a household contact with warts were the leading risk factors for contraction of warts in children younger than 13 years.19 Children often transfer warts from site to site as well as to siblings and other close contacts. Skin-to-skin contact is responsible for sexual transmission of warts, and surface transmission occurs via fomites. Entry of the virus often occurs through small breaks in the skin. Other modes of transmission include orogenital.20
Therapeutic Options
Although the nuances of each available treatment for pediatric warts are beyond the scope of this article, the main core of therapy is 1 of 3 approaches: (1) observation, (2) over-the-counter salicylic acid therapy, and (3) in-office cryotherapy. Observation is an affirmed style of therapy for warts, as it is expected that two-thirds of warts will spontaneously resolve in 2 years and three-quarters will resolve in 3 years.4,5 Condyloma in children has been responsive to therapies such as cryotherapy and imiquimod,13 but spontaneous clearance in 5 years has been noted in 76% of children,21 which is linked to development of spontaneous immune response in most individuals.
Therapies for pediatric warts are characterized according to 6 major categories: destructive; immune stimulating; immune modulating, including normalization of epithelial growth; irritant; vascular destructive; and nitric oxide releasing (eTable).
Destructive Therapies
Destructive therapies for warts often are implemented in cases of disfigurement, discomfort/pain, and/or spreading, as well as to control contagion. According to a 2001 Cochrane review, salicylic acid has the best evidence of all therapeutics for the clearance of warts compared to placebo.24 On the other hand, aggressive cryotherapy and combined salicylic acid and cryotherapy had the best evidence in their favor in a 2011 meta-analysis by Kwok et al.25 Both salicylic acid and cryotherapy are considered destructive therapies. A recent meta-analysis of cantharidin, another destructive therapy, showed that local cantharidin alone as well as in combination with salicylic acid and podophyllotoxin showed good efficacy for warts; however, increased caution should be exerted with the combination regimen in young children due to a potential increase in the side-effect profile (eg, severe blistering).22 Other destructive agents such as topical retinoids can only peel surface layers of the skin and therefore are limited to flat facial warts, which are not expected to have an extensive hyperkeratotic layer; however, with occlusion, agents such as adapalene gel 0.1% can be used even on plantar warts with some efficacy.29
Immune-Stimulating Therapies
Immune stimulants often are used to treat warts in children and adolescents who have many lesions, a prolonged disease course, disfigurement, and/or subungual localizations, as well as in those who have been treated with multiple destructive methods without success. Topical imiquimod and oral cimetidine are readily available, while squaric acid (at-home or in-office therapy) and intralesional candida antigen can be used in offices that carry these agents. Topical imiquimod has been reported to achieve success in genital warts in children,13 with good efficacy in recalcitrant, periungual, and subungual warts when used for up to 16 weeks.31 In one randomized clinical trial, imiquimod cream 5% combined with salicylic acid 15% was applied to warts for 6 to 10 hours for 5 consecutive days per week versus cryotherapy with liquid nitrogen every 2 weeks for a maximum of 3 months. At the end of the study period, 81.1% (30/37) of participants treated with imiquimod and salicylic acid showed clearance of their warts versus 67.3% (33/49) of those treated with cryotherapy.32
Oral cimetidine has been reported to be successful in treating recalcitrant warts in more than 80% of children when dosed at 30 to 40 mg/kg 3 times daily, requiring 6 to 12 weeks to achieve clearance. Side effects of oral cimetidine include many cytochrome P450 interactions; gynecomastia, which limits usage in teenaged males; and stomach upset.30
Treatment of recalcitrant pediatric warts with intralesional candida antigen has been associated with side effects consistent with delayed-type hypersensitivity reactions. Injections should be administered once monthly, with a minimum of 3 cycles if not effective and up to 6 cycles where partial efficacy is noted. In a retrospective review of 220 cases, 70.9% of children showed complete clearance and 16.8% had partial response.33 However, the treatment may be limited in children by fear of needles.
Squaric acid dibutyl ester is a universal allergen that is not mutagenic on Ames testing and causes milder allergy symptoms than the mutagenic dinitrochlorobenzene and less erythema and pruritus than diphencyclopropenone. Squaric acid dibutyl ester home therapy was evaluated in 61 children with at least one nonfacial wart.34 Application began with squaric acid dibutyl ester in acetone (SADBE) 2% sensitization on the arm followed by at-home application of SADBE 0.2% three to seven times weekly for a minimum of 2 months to determine benefit and for 3 to 4 months as needed; however, average response was 7 weeks. The average complete clearance was 58% and partial clearance was 18%. Side effects included erythema and mild itching as well as urticaria in one case.34 In-office SADBE also has been evaluated in children. In a case series that included 29 children sensitized with SADBE 1% to 2% under occlusion followed by once monthly application of SADBE 0.5% to 5.0% to their warts, 69% clearance and 10% partial clearance was noted after a little more than 4 months of treatment.35 One retrospective review compared combination SADBE, trichloroacetic acid (TCA), and cantharidin both alone and in combination as duos (eg, SADBE and TCA) or trios (SADBE, TCA, and cantharidin).23 Of the 74 children whose medical charts were reviewed, the addition of pretreatment of warts with TCA 50% prior to in-office sensitization and monthly in-office application of SADBE increased treatment response to 100% with an average 2.45 months of therapy, whereas no enhancement was noted with cantharidin. Therefore, it appears that there may be enhanced immune reactivity when TCA pretreatment of warts is performed.23
Immune-Modulating Therapies (Including Normalization of Epithelial Growth)
The most novel immunologic therapy for warts is plerixafor, an agent used to treat WHIM (warts, hypogammaglobulinemia, infections, and myelokathexis) syndrome, which has been linked to heterozygous gain of function mutations in the chemokine receptor CXCR4 (located on 2q22). In WHIM syndrome, the mutated CXCR4 is more sensitive to CXCL12 activation. Plerixafor is a selective reversible antagonist that blocks the capacity of the chemokine CXCL12 to sustain the permanent activation of CXCR4.37 Combination therapy with plerixafor and topical imiquimod has resulted in wart improvement in WHIM syndrome patients in a small series.38
Oral isotretinoin has been described to be efficacious over placebo at a dosage of 30 mg daily for 12 weeks and can be used in teenagers but requires standard monitoring.36
Irritant Therapies
Duct tape is a classic agent that produces maceration and irritation of warts. Application of duct tape over warts has been described in cycles of 6 days on, 1 day off with weekly repetition for a few months but usually not on the palms or soles due to difficulty maintaining occlusive tape in these locations over an extended period of time. In one trial, 85% (22/26) of duct tape–treated cases cleared versus 60% (15/25) of cryotherapy-treated cases over a 2-month maximum therapeutic period.39
Vascular Destructive Therapies
The pulsed dye laser is a classic modality that induces localized destruction of blood supply to warts in children. A case series of 61 children treated with the pulsed dye laser revealed 75% overall clearance in an average of 3.1 sessions. The usage of this therapy often is limited to institutions where the technology is readily available for usage.40
Nitric Oxide–Releasing Therapies
Nitric oxide release may increase local blood flow, thereby increasing immune response, or may have a primary mechanism of antimicrobial activity, which is why these agents have been investigated for wart treatment. Topical garlic has been described anecdotally as a therapy for thin childhood warts with the putative mechanism being nitric oxide release.42 A new investigational drug recently has had phase 2 data published. Berdazimer sodium plus carboxymethyl cellulose hydrogel has demonstrated benefit in adult warts, but data in children is lacking.41
Therapeutic Ladder for Childhood Warts
The therapeutic ladder (Table) for childhood warts starts with first doing no harm. Although many parents are disturbed by their child’s condition, the natural history of resolution is spontaneous and therefore no therapy is required in many cases. The child and his/her caregivers should be engaged to determine if he/she is emotionally disturbed or uncomfortable with their lesions and to address any fears and concerns that some children may experience (eg, contagion risk, pain with ambulation, ostracism). For example, children with hand warts may report that other children will not hold their hand while in line at school. Prominent facial lesions can be particularly problematic for children due to teasing and bullying.
Conclusion
Warts are a common infection in childhood caused by the ubiquitous HPV virus. Therapeutic options abound, but most cases are either ignored or treated with over-the-counter salicylic acid or in-office cryotherapy. The decision to employ alternative therapeutic options requires agreement by the child, his/her caregiver, and the treating physician and can be tailored to suit the desires and needs of the child. Whether or not therapy is offered, spontaneous clearance is frequently seen in common warts. On the other hand, genital warts are associated with later conversion to malignancies of the genital tract; therefore, encouragement of HPV vaccination is needed in the adolescent population to best ensure long-term genital health.
1. Warts. https://medical-dictionary.thefreedictionary.com/warts. Accessed November 30, 2018.
2. Human papillomavirus. WHO website. http://www.who.int/biologicals/areas/human_papillomavirus/en. Accessed December 3, 2018.
3. Silverberg NB. Human papillomavirus infections in children. Curr Opin Pediatr. 2004;16:402-409.
4. Silverberg NB. Warts and molluscum in children. Adv Dermatol. 2004;20:23-73.
5. Silverberg NB, McCuaig CC. Melanoma in childhood: changing our mind-set. Cutis. 2013;92:217-218.
6. Bruggink SC, Eekhof JA, Egberts PF, et al. Warts transmitted in families and schools: a prospective cohort. Pediatrics. 2013;131:928-934.
7. Silverberg JI, Silverberg NB. The U.S. prevalence of common warts in childhood: a population-based study. J Invest Dermatol. 2013;133:2788-2790.
8. Svensson A, Ofenloch RF, Bruze M, et al. Br J Dermatol. 2018;178:1111-1118.
9. Silverberg JI, Silverberg NB. Childhood atopic dermatitis and warts are associated with increased risk of infection: a US population-based study. J Allergy Clin Immunol. 2014;133:1041-1047.
10. Smith EM, Johnson SR, Cripe TP, et al. Perinatal vertical transmission of human papillomavirus and subsequent development of respiratory tract papillomatosis. Ann Otol Rhinol Laryngol. 1991;100:479-483.
11. Costa-Silva M, Azevedo F, Lisboa C. Anogenital warts in children: analysis of a cohort of 34 prepubertal children. Pediatr Dermatol. 2018;35:E325-E327.
12. Marcoux D, Nadeau K, McCuaig C, et al. Pediatric anogenital warts: a 7-year review of children referred to a tertiary-care hospital in Montreal, Canada. Pediatr Dermatol. 2006;23:199-207.
13. Stefanaki C, Barkas G, Valari M, et al. Condylomata acuminata in children. Pediatr Infect Dis J. 2012;31:422-424.
14. dePlanell-Mas E, Martinez-Garriga B, Zalacain AJ, et al. Human papillomaviruses genotyping in plantar warts. J Med Virol. 2017;89:902-907.
15. Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40:187-193.
16. Lace MJ, Anson JR, Klingelhutz AJ, et al. Human papillomavirus (HPV) type 18 induces extended growth in primary human cervical, tonsillar, or foreskin keratinocytes more effectively than other high-risk mucosal HPVs. J Virol. 2009;83:11784-11794.
17. Sudenga SL, Ingles DJ, Pierce Campbell CM, et al. Genital human papillomavirus infection progression to external genital lesions: the HIM study. Eur Urol. 2016;69:166-173.
18. Rigo MV, Martínez Campillo F, Verdú M, et al. Risk factors linked to the transmission of papilloma virus in the school environment [in Spanish]. Alicante, 1999. Aten Primaria. 2003;31:415-420.
19. Al-Mutairi N, AlKhalaf M. Mucocutaneous warts in children: clinical presentations, risk factors, and response to treatment. Acta Dermatovenerol Alp Pannonica Adriat. 2012;21:69-72.
20. Clarke J, Terry RM, Lacey CJ. A study to estimate the prevalence of upper respiratory tract papillomatosis in patients with genital warts. Int J STD AIDS. 1991;2:114-115.
21. Allen AL, Siegfried EC. The natural history of condyloma in children. J Am Acad Dermatol. 1998;39:951-955.
22. Vakharia PP, Chopra R, Silverberg NB, et al. Efficacy and safety of topical cantharidin treatment for molluscum contagiosum and warts: a systematic review. Am J Clin Dermatol. 2018;19:791-803.
23. Silverberg JI, Silverberg NB. Adjunctive trichloroacetic acid therapy enhances squaric acid response to verruca vulgaris. J Drugs Dermatol. 2012;11:1228-1230.
24. Gibbs S, Harvey I, Sterling JC, et al. Local treatments for cutaneous warts. Cochrane Database Syst Rev. 2001:CD001781.
25. Kwok CS, Holland R, Gibbs S. Efficacy of topical treatments for cutaneous warts: a meta-analysis and pooled analysis of randomized controlled trials. Br J Dermatol. 2011;165:233-246.
26. Allington HV. Liquid nitrogen in the treatment of skin diseases. Calif Med. 1950;72:153-155.
27. Caravati CM Jr, Wood BT, Richardson DR. Onychodystrophies secondary to liquid nitrogen cryotherapy. Arch Dermatol. 1969;100:441-442.
28. Duofilm [package insert]. Sligo, Ireland: Stiefel Laboratories (Ireland) Ltd; 2016.
29. Gupta R, Gupta S. Topical adapalene in the treatment of plantar warts: randomized comparative open trial in comparison with cryo-therapy. Indian J Dermatol. 2015;60:102.
30. Orlow SJ, Paller A. Cimetidine therapy for multiple viral warts in children. J Am Acad Dermatol. 1993;28(5 pt 1):794-796.
31. Micali G, Dall’Oglio F, Nasca MR. An open label evaluation of the efficacy of imiquimod 5% cream in the treatment of recalcitrant subungual and periungual cutaneous warts. J Dermatolog Treat. 2003;14:233-236.
32. Stefanaki C, Lagogiani I, Kouris A, et al. Cryotherapy versus imiquimod 5% cream combined with a keratolytic lotion in cutaneous warts in children: a randomized study. J Dermatolog Treat. 2016;27:80-82.
33. Muñoz Garza FZ, Roé Crespo E, Torres Pradilla M, et al. Intralesional Candida antigen immunotherapy for the treatment of recalcitrant and multiple warts in children. Pediatr Dermatol. 2015;32:797-801.
34. Silverberg NB, Lim JK, Paller AS, et al. Squaric acid immunotherapy for warts in children. J Am Acad Dermatol. 2000;42(5 pt 1):803-808.
35. Lee AN, Mallory SB. Contact immunotherapy with squaric acid dibutylester for the treatment of recalcitrant warts. J Am Acad Dermatol. 1999;41:595-599.
36. Olguin-García MG, Jurado-Santa Cruz F, Peralta-Pedrero ML, et al. A double-blind, randomized, placebo-controlled trial of oral isotretinoin in the treatment of recalcitrant facial flat warts. J Dermatolog Treat. 2015;26:78-82.
37. Badolato R, Donadieu J; WHIM Research Group. How I treat warts, hypogammaglobulinemia, infections, and myelokathexis syndrome. Blood. 2017;130:2491-2498.
38. McDermott DH, Liu Q, Velez D, et al. A phase 1 clinical trial of long-term, low-dose treatment of WHIM syndrome with the CXCR4 antagonist plerixafor. Blood. 2014;123:2308-2316.
39. Focht DR 3rd, Spicer C, Fairchok MP. The efficacy of duct tape vs cryotherapy in the treatment of verruca vulgaris (the common wart). Arch Pediatr Adolesc Med. 2002;156:971-974.
40. Sethuraman G, Richards KA, Hiremagalore RN, et al. Effectiveness of pulsed dye laser in the treatment of recalcitrant warts in children. Dermatol Surg. 2010;36:58-65.
41. Tyring SK, Rosen T, Berman B, et al. A phase 2 controlled study of SB206, a topical nitric oxide-releasing drug for extragenital wart treatment. J Drugs Dermatol. 2018;17:1100-1105.
42. Silverberg NB. Garlic cloves for verruca vulgaris. Pediatr Dermatol. 2002;19:183.
1. Warts. https://medical-dictionary.thefreedictionary.com/warts. Accessed November 30, 2018.
2. Human papillomavirus. WHO website. http://www.who.int/biologicals/areas/human_papillomavirus/en. Accessed December 3, 2018.
3. Silverberg NB. Human papillomavirus infections in children. Curr Opin Pediatr. 2004;16:402-409.
4. Silverberg NB. Warts and molluscum in children. Adv Dermatol. 2004;20:23-73.
5. Silverberg NB, McCuaig CC. Melanoma in childhood: changing our mind-set. Cutis. 2013;92:217-218.
6. Bruggink SC, Eekhof JA, Egberts PF, et al. Warts transmitted in families and schools: a prospective cohort. Pediatrics. 2013;131:928-934.
7. Silverberg JI, Silverberg NB. The U.S. prevalence of common warts in childhood: a population-based study. J Invest Dermatol. 2013;133:2788-2790.
8. Svensson A, Ofenloch RF, Bruze M, et al. Br J Dermatol. 2018;178:1111-1118.
9. Silverberg JI, Silverberg NB. Childhood atopic dermatitis and warts are associated with increased risk of infection: a US population-based study. J Allergy Clin Immunol. 2014;133:1041-1047.
10. Smith EM, Johnson SR, Cripe TP, et al. Perinatal vertical transmission of human papillomavirus and subsequent development of respiratory tract papillomatosis. Ann Otol Rhinol Laryngol. 1991;100:479-483.
11. Costa-Silva M, Azevedo F, Lisboa C. Anogenital warts in children: analysis of a cohort of 34 prepubertal children. Pediatr Dermatol. 2018;35:E325-E327.
12. Marcoux D, Nadeau K, McCuaig C, et al. Pediatric anogenital warts: a 7-year review of children referred to a tertiary-care hospital in Montreal, Canada. Pediatr Dermatol. 2006;23:199-207.
13. Stefanaki C, Barkas G, Valari M, et al. Condylomata acuminata in children. Pediatr Infect Dis J. 2012;31:422-424.
14. dePlanell-Mas E, Martinez-Garriga B, Zalacain AJ, et al. Human papillomaviruses genotyping in plantar warts. J Med Virol. 2017;89:902-907.
15. Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40:187-193.
16. Lace MJ, Anson JR, Klingelhutz AJ, et al. Human papillomavirus (HPV) type 18 induces extended growth in primary human cervical, tonsillar, or foreskin keratinocytes more effectively than other high-risk mucosal HPVs. J Virol. 2009;83:11784-11794.
17. Sudenga SL, Ingles DJ, Pierce Campbell CM, et al. Genital human papillomavirus infection progression to external genital lesions: the HIM study. Eur Urol. 2016;69:166-173.
18. Rigo MV, Martínez Campillo F, Verdú M, et al. Risk factors linked to the transmission of papilloma virus in the school environment [in Spanish]. Alicante, 1999. Aten Primaria. 2003;31:415-420.
19. Al-Mutairi N, AlKhalaf M. Mucocutaneous warts in children: clinical presentations, risk factors, and response to treatment. Acta Dermatovenerol Alp Pannonica Adriat. 2012;21:69-72.
20. Clarke J, Terry RM, Lacey CJ. A study to estimate the prevalence of upper respiratory tract papillomatosis in patients with genital warts. Int J STD AIDS. 1991;2:114-115.
21. Allen AL, Siegfried EC. The natural history of condyloma in children. J Am Acad Dermatol. 1998;39:951-955.
22. Vakharia PP, Chopra R, Silverberg NB, et al. Efficacy and safety of topical cantharidin treatment for molluscum contagiosum and warts: a systematic review. Am J Clin Dermatol. 2018;19:791-803.
23. Silverberg JI, Silverberg NB. Adjunctive trichloroacetic acid therapy enhances squaric acid response to verruca vulgaris. J Drugs Dermatol. 2012;11:1228-1230.
24. Gibbs S, Harvey I, Sterling JC, et al. Local treatments for cutaneous warts. Cochrane Database Syst Rev. 2001:CD001781.
25. Kwok CS, Holland R, Gibbs S. Efficacy of topical treatments for cutaneous warts: a meta-analysis and pooled analysis of randomized controlled trials. Br J Dermatol. 2011;165:233-246.
26. Allington HV. Liquid nitrogen in the treatment of skin diseases. Calif Med. 1950;72:153-155.
27. Caravati CM Jr, Wood BT, Richardson DR. Onychodystrophies secondary to liquid nitrogen cryotherapy. Arch Dermatol. 1969;100:441-442.
28. Duofilm [package insert]. Sligo, Ireland: Stiefel Laboratories (Ireland) Ltd; 2016.
29. Gupta R, Gupta S. Topical adapalene in the treatment of plantar warts: randomized comparative open trial in comparison with cryo-therapy. Indian J Dermatol. 2015;60:102.
30. Orlow SJ, Paller A. Cimetidine therapy for multiple viral warts in children. J Am Acad Dermatol. 1993;28(5 pt 1):794-796.
31. Micali G, Dall’Oglio F, Nasca MR. An open label evaluation of the efficacy of imiquimod 5% cream in the treatment of recalcitrant subungual and periungual cutaneous warts. J Dermatolog Treat. 2003;14:233-236.
32. Stefanaki C, Lagogiani I, Kouris A, et al. Cryotherapy versus imiquimod 5% cream combined with a keratolytic lotion in cutaneous warts in children: a randomized study. J Dermatolog Treat. 2016;27:80-82.
33. Muñoz Garza FZ, Roé Crespo E, Torres Pradilla M, et al. Intralesional Candida antigen immunotherapy for the treatment of recalcitrant and multiple warts in children. Pediatr Dermatol. 2015;32:797-801.
34. Silverberg NB, Lim JK, Paller AS, et al. Squaric acid immunotherapy for warts in children. J Am Acad Dermatol. 2000;42(5 pt 1):803-808.
35. Lee AN, Mallory SB. Contact immunotherapy with squaric acid dibutylester for the treatment of recalcitrant warts. J Am Acad Dermatol. 1999;41:595-599.
36. Olguin-García MG, Jurado-Santa Cruz F, Peralta-Pedrero ML, et al. A double-blind, randomized, placebo-controlled trial of oral isotretinoin in the treatment of recalcitrant facial flat warts. J Dermatolog Treat. 2015;26:78-82.
37. Badolato R, Donadieu J; WHIM Research Group. How I treat warts, hypogammaglobulinemia, infections, and myelokathexis syndrome. Blood. 2017;130:2491-2498.
38. McDermott DH, Liu Q, Velez D, et al. A phase 1 clinical trial of long-term, low-dose treatment of WHIM syndrome with the CXCR4 antagonist plerixafor. Blood. 2014;123:2308-2316.
39. Focht DR 3rd, Spicer C, Fairchok MP. The efficacy of duct tape vs cryotherapy in the treatment of verruca vulgaris (the common wart). Arch Pediatr Adolesc Med. 2002;156:971-974.
40. Sethuraman G, Richards KA, Hiremagalore RN, et al. Effectiveness of pulsed dye laser in the treatment of recalcitrant warts in children. Dermatol Surg. 2010;36:58-65.
41. Tyring SK, Rosen T, Berman B, et al. A phase 2 controlled study of SB206, a topical nitric oxide-releasing drug for extragenital wart treatment. J Drugs Dermatol. 2018;17:1100-1105.
42. Silverberg NB. Garlic cloves for verruca vulgaris. Pediatr Dermatol. 2002;19:183.
Practice Points
- Warts are caused by infection with the human papillomavirus.
- Warts are extremely common in all age groups, but risk factors and types of lesions vary by age and location of lesions.
- Therapies for pediatric warts are characterized according to 6 major categories: destructive; immune stimulating; immune modulating, including normalization of epithelial growth; vascular destructive; irritant; and nitric oxide releasing.
Blueberry Muffin Rash Secondary to Hereditary Spherocytosis
The term blueberry muffin rash historically was used to describe the cutaneous manifestations observed in congenital rubella. The term traditionally describes the result of a postnatal dermal extramedullary hematopoiesis. Today, TORCH (toxoplasmosis, other agents, rubella, cytomegalovirus, herpes) infections and plasma cell dyscrasias are all potential causes of extramedullary hematopoiesis. Herein, we present a unique case of a neonate born with a blueberry muffin rash secondary to extramedullary hematopoiesis induced by hereditary spherocytosis.
Case Report
The dermatology department was consulted to evaluate a 2-day-old male neonate born with a “rash.” The patient was born to a 34-year-old gravida 3, para 2, woman at 39 weeks’ gestation. The mother’s prenatal laboratory values were within reference range and ultrasounds were normal, and she was compliant with her prenatal care. She underwent a normal spontaneous vaginal delivery 3 hours after rupture of membranes without complication. The amniotic fluid and umbilical cord both were clear. There was no use of forceps or any other external aiding devices during the delivery. At the time of delivery, the consulting physician noted that the patient had “skin lesions from head to toe.”
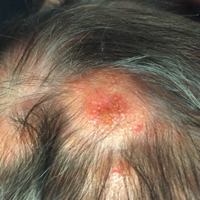
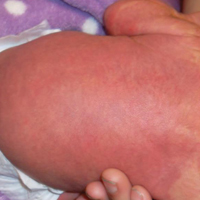
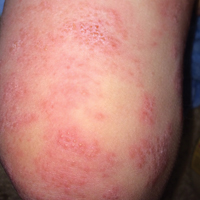
The patient’s parents reported that the rash did not seem to cause any discomfort for the patient. In the 24 hours after birth, the parents reported that the erythema seemed to slightly fade. Physical examination revealed many scattered erythematous to violaceous, nonblanching papulonodules affecting the scalp (Figure 1), face, arms, hands (Figure 2A), back (Figure 2B), buttocks, legs, and feet. Some of the papulonodules were soft while others were firm and indurated. Several lesions had a yellowish hue with some overlying crust. There was no mucosal, genital, or ocular involvement. No erosions, ulcerations, petechiae, ecchymoses, or hepatosplenomegaly were noted on examination.
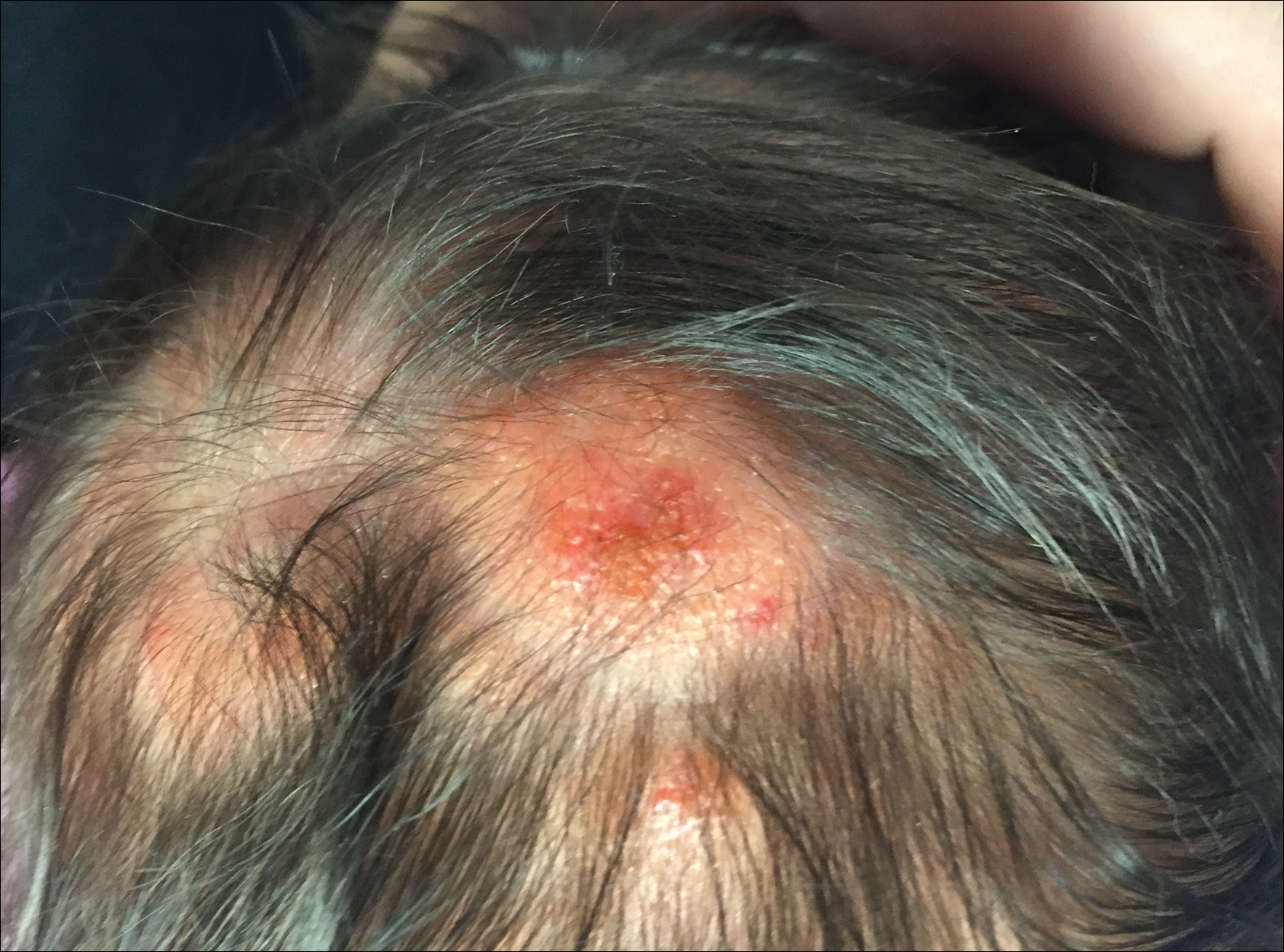

The patient was otherwise healthy with an Apgar score of 8/9 at 1 and 5 minutes. His birth weight, length, and head circumference were within normal limits. There was no evidence of ABO blood group or Rhesus factor incompatibility. His temperature, vital signs, laboratory values (including calcium level and TORCH titers, which included cytomegalovirus, rubella, toxoplasmosis, and herpes simplex virus), and review of systems all were within reference range. A bone survey of the skull, spine, ribs, arms, pelvis, legs, and feet was within normal limits.
The mother’s placenta was sent for pathology and revealed a lymphoplasmacytic chronic deciduitis and acute subchorionitis consistent with a nonspecific inflammatory response, unlikely to be from an infectious etiology.
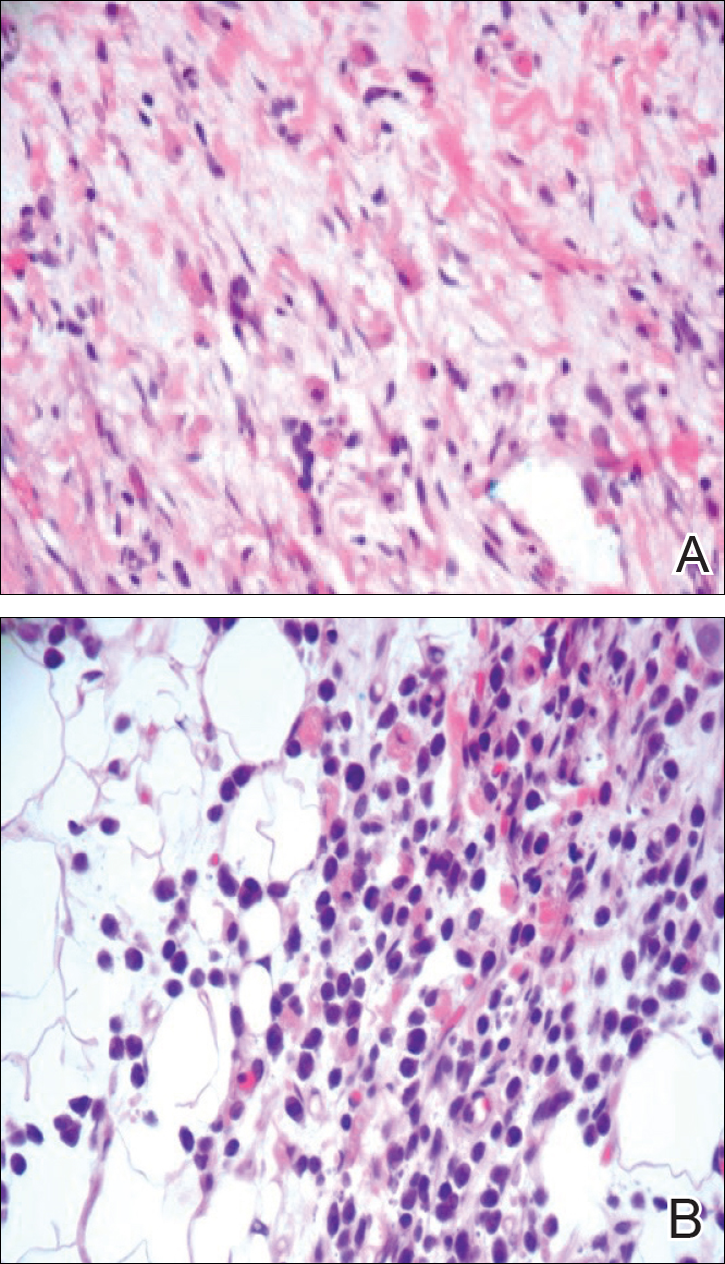
A 4-mm punch biopsy was taken from the left thigh and revealed a predominately lymphocytic infiltrate with rare eosinophils and erythrocyte precursors (Figure 3). Immunohistochemical staining was performed showing that the majority of the lymphocytes represented T lymphocytes, which stained positive for CD45 and CD3 and negative for S-100, CD1a, CD30, and CD117. There were scattered CD34+ cells, and scattered cells stained positive for myeloperoxidase. No significant CD20 immunoreactivity was noted. There were scattered eosinophils and rare normoblasts but no megakaryocytes. A complete blood cell count (CBC) with differential and reticulocyte count was within reference range.
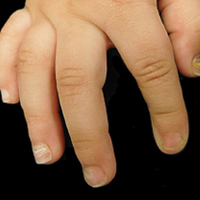
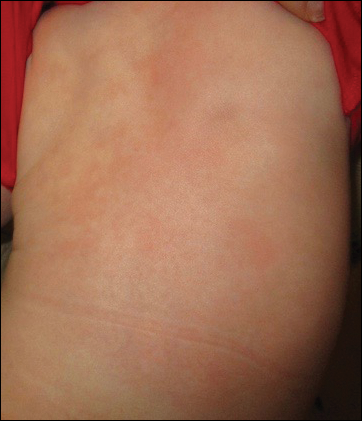
At 1-, 3-, 8-, 12-, and 28-week follow-up visits, the patient continued to grow and feed appropriately. No new lesions developed during this time, and the preexisting lesions continued to fade into slightly hyperpigmented patches without induration (Figure 4). At 6 months of age, a CBC performed at the time of an upper respiratory infection and otitis media revealed normocytic anemia with a hemoglobin level of 9.9 g/dL (reference range, 14.0–17.5 g/dL), a reticulocyte count of 0.8% (reference range, 0.5%–1.5%), and a lactate dehydrogenase level of 424 U/L (reference range, 100–200 U/L). All red blood cell (RBC) indices were within reference range. Flow cytometry, eosin-5-maleimide, and ektacytometry were performed with results consistent with mild hereditary spherocytosis.
Comment
Dermal extramedullary hematopoiesis is a normal component of embryologic development up until the fifth month of gestation.1 The term blueberry muffin rash typically is used to describe the cutaneous manifestations of extramedullary hematopoiesis, which commonly is caused by a TORCH infection or hematologic dyscrasia.2 It has been suggested that the term be expanded to include neoplastic processes (eg, neuroblastomas) and vascular processes (eg, multiple hemangiomas, blue rubber bleb nevus syndrome, glomangiomas, multifocal lymphangionendotheliomatosis), which although not associated with an extramedullary hematopoiesis, can clinically resemble a blueberry muffin rash.
Because of the potential for serious systemic complications, a cause must be sought for all newborns presenting with a blueberry muffin rash. Our patient’s lack of cardiovascular, otic, and ocular involvement combined with a negative TORCH screen and normal CBC strongly suggested against a TORCH infection. In addition, a normal bone survey and CBC, as well as a lack of petechiae, ecchymoses, and hepatosplenomegaly, were evidence against congenital leukemia.3 With the spontaneously resolving lesions and apparent clinical resolution, a bone marrow biopsy was not performed. The skin biopsy revealed negative staining for S-100 and CD1a, making the diagnosis of congenital self-healing reticulohistiocytosis unlikely. No panniculitis was noted and calcium levels were normal, ruling out subcutaneous fat necrosis of the newborn. The predominantly T-cell lymphocytic infiltrate demonstrated on skin biopsy led us to a differential diagnosis of aleukemic leukemia cutis versus idiopathic dermal extramedullary hematopoiesis; however, normocytic anemia was later identified when the patient’s hemoglobin level dropped to 9.9 g/dL. The abnormal eosin-5-maleimide and ektacytometry results unmasked a hereditary spherocytosis.
Hereditary spherocytosis typically is inherited in an autosomal-dominant manner and may be caused by mutations in ankyrin-1, band 3, spectrin, or protein 4.2 on the erythrocyte membrane. It is the third leading cause of hemolytic anemia in newborns and the leading cause of direct Coombs-negative hemolytic anemia requiring blood transfusion in neonates. It is most common in neonates of Northern European ancestry, affecting 1 in every 1000 to 2000 births.4 Presentation may range from asymptomatic to severe anemia with hydrops fetalis. Most neonates have an elevated mean corpuscular hemoglobin and low mean corpuscular volume. Acute illness may cause hemolytic or aplastic crises, possibly explaining our patient’s normocytic anemia discovered on a CBC during an episode of an upper respiratory infection and otitis media.
Treatment options for hereditary spherocytosis include phototherapy for jaundiced neonates, folate supplementation, packed erythrocyte transfusions for symptomatic anemia, and recombinant erythropoietin in neonates.4 Splenectomy is curative for the majority of patients and requires immunization against Streptococcus pneumoniae, Haemophilus influenzae type b, and Neisseria meningitidis several weeks preoperatively. Patients with symptomatic gallstones may be treated with cholecystectomy at the time of splenectomy or by laparoscopic cholecystectomy, endoscopic sphincterotomy, cholecystostomy, or extracorporeal cholecystolithotripsy.5
Although a PubMed search of articles indexed for MEDLINE using the terms dermal hematopoiesis, extramedullary hematopoiesis, hereditary spherocytosis, and blueberry muffin rash yielded only 1 other known case of blueberry muffin rash caused by hereditary spherocytosis,6 other case reports demonstrate extramedullary hematopoiesis in hereditary spherocytosis patients in locations other than the skin. Calhoun et al7 described a case of a 9-year-old boy with hereditary spherocytosis who presented with jaundice. Pathologic examination revealed a 5-cm suprarenal mass demonstrating extramedullary hematopoiesis.7 A case reported by Xiros et al8 described a 64-year-old man with a history of hereditary spherocytosis who presented with hemothorax from paravertebral extramedullary hematopoiesis. De Backer et al9 reported a case of a 60-year-old man diagnosed with hereditary spherocytosis after an abnormal CBC who was subsequently found to have paravertebral masses containing extramedullary hematopoiesis.
There is one known case of a blueberry muffin rash caused by hereditary spherocytosis.6 A female neonate was born at 38 weeks’ gestation with multiple petechiae and faint purpuric papules. Initial complications included intracranial ventricular hemorrhage, hyperbilirubinemia, and anemia requiring blood transfusions on the first day of life. TORCH titers were negative and a skin biopsy demonstrated a diffuse infiltrate of mature RBCs, normoblasts, and pronormoblasts in the reticular dermis. She was healthy until 3 months of age when she had several days of vomiting and diarrhea. Laboratory workup revealed a hematocrit level of 20.5% (reference range, 41%–50%); a reticulocyte count of 22.6% (reference range, 0.5%–1.5%); and a peripheral blood smear demonstrating polychromatophilia, anisocytosis, and spherocytosis. She was then diagnosed with hereditary spherocytosis.6
Hereditary spherocytosis is a known, albeit rare, cause of extramedullary hematopoiesis presenting as blueberry muffin rash. Patients with mild hereditary spherocytosis may have a compensated hemolysis without anemia or spherocytes on peripheral smear, which may explain the lack of severe hemolytic anemia or RBC-predominant pathology in our patient.5 Argyle and Zone6 proposed that severe hemolysis and hypoxia were the cause of extramedullary hematopoiesis in their patient. Because our patient did not experience a notable hemolytic episode until he had an upper respiratory infection and otitis media at 6 months of age, the pathophysiology is less clear; a compensated hemolytic process may underlie the extramedullary hematopoiesis and normal RBC indices.
Regardless of the precise cause of extramedullary hematopoiesis in our patient, this case of a T lymphocyte–dominant cutaneous infiltrate in a patient with mild hereditary spherocytosis is exceptionally rare and leads us to consider that perhaps there are causes of this pathology that are unknown to us.
- Zhang IH, Zane LT, Braun BS, et al. Congenital leukemia cutis with subsequent development of leukemia. J Am Acad Dermatol. 2006;54(2 suppl):S22–S27.
- Karmegaraj B, Vijayakumar S, Ramanathan R, et al. Extramedullary haematopoiesis resembling a blueberry muffin, in a neonate. BMJ Case Rep. pii: bcr2014208473. doi: 10.1136/bcr-2014-208473.
- Handler MZ, Schwartz RA. Neonatal leukaemia cutis. J Eur Acad Dermatol Venereol. 2015;29:1884-1889.
- Christensen RD, Yaish HM, Gallagher PG. A pediatrician’s practical guide to diagnosing and treating hereditary spherocytosis in neonates. Pediatrics. 2015;135:1107-1114.
- Perrotta S, Gallagher PG, Mohandas N. Hereditary spherocytosis. Lancet. 2008;372:1411-1426.
- Argyle JC, Zone JJ. Dermal erythropoiesis in a neonate. Arch Dermatol. 1981;117:492-494.
- Calhoun SK, Murphy RC, Shariati N, et al. Extramedullary hematopoiesis in a child with hereditary spherocytosis: an uncommon cause of an adrenal mass. Pediatr Radiol. 2001;31:879-881.
- Xiros N, Economopoulos T, Papageorgiou E, et al. Massive hemothorax due to intrathoracic extramedullary hematopoiesis in a patient with hereditary spherocytosis. Ann Hematol. 2001;80:38-40.
- De Backer AI, Zachée P, Vanschoubroeck IJ, et al. Extramedullary paraspinal hematopoiesis in hereditary spherocytosis. JBR-BTR. 2002;85:206-208.
The term blueberry muffin rash historically was used to describe the cutaneous manifestations observed in congenital rubella. The term traditionally describes the result of a postnatal dermal extramedullary hematopoiesis. Today, TORCH (toxoplasmosis, other agents, rubella, cytomegalovirus, herpes) infections and plasma cell dyscrasias are all potential causes of extramedullary hematopoiesis. Herein, we present a unique case of a neonate born with a blueberry muffin rash secondary to extramedullary hematopoiesis induced by hereditary spherocytosis.
Case Report
The dermatology department was consulted to evaluate a 2-day-old male neonate born with a “rash.” The patient was born to a 34-year-old gravida 3, para 2, woman at 39 weeks’ gestation. The mother’s prenatal laboratory values were within reference range and ultrasounds were normal, and she was compliant with her prenatal care. She underwent a normal spontaneous vaginal delivery 3 hours after rupture of membranes without complication. The amniotic fluid and umbilical cord both were clear. There was no use of forceps or any other external aiding devices during the delivery. At the time of delivery, the consulting physician noted that the patient had “skin lesions from head to toe.”
The patient’s parents reported that the rash did not seem to cause any discomfort for the patient. In the 24 hours after birth, the parents reported that the erythema seemed to slightly fade. Physical examination revealed many scattered erythematous to violaceous, nonblanching papulonodules affecting the scalp (Figure 1), face, arms, hands (Figure 2A), back (Figure 2B), buttocks, legs, and feet. Some of the papulonodules were soft while others were firm and indurated. Several lesions had a yellowish hue with some overlying crust. There was no mucosal, genital, or ocular involvement. No erosions, ulcerations, petechiae, ecchymoses, or hepatosplenomegaly were noted on examination.


The patient was otherwise healthy with an Apgar score of 8/9 at 1 and 5 minutes. His birth weight, length, and head circumference were within normal limits. There was no evidence of ABO blood group or Rhesus factor incompatibility. His temperature, vital signs, laboratory values (including calcium level and TORCH titers, which included cytomegalovirus, rubella, toxoplasmosis, and herpes simplex virus), and review of systems all were within reference range. A bone survey of the skull, spine, ribs, arms, pelvis, legs, and feet was within normal limits.
The mother’s placenta was sent for pathology and revealed a lymphoplasmacytic chronic deciduitis and acute subchorionitis consistent with a nonspecific inflammatory response, unlikely to be from an infectious etiology.
A 4-mm punch biopsy was taken from the left thigh and revealed a predominately lymphocytic infiltrate with rare eosinophils and erythrocyte precursors (Figure 3). Immunohistochemical staining was performed showing that the majority of the lymphocytes represented T lymphocytes, which stained positive for CD45 and CD3 and negative for S-100, CD1a, CD30, and CD117. There were scattered CD34+ cells, and scattered cells stained positive for myeloperoxidase. No significant CD20 immunoreactivity was noted. There were scattered eosinophils and rare normoblasts but no megakaryocytes. A complete blood cell count (CBC) with differential and reticulocyte count was within reference range.

At 1-, 3-, 8-, 12-, and 28-week follow-up visits, the patient continued to grow and feed appropriately. No new lesions developed during this time, and the preexisting lesions continued to fade into slightly hyperpigmented patches without induration (Figure 4). At 6 months of age, a CBC performed at the time of an upper respiratory infection and otitis media revealed normocytic anemia with a hemoglobin level of 9.9 g/dL (reference range, 14.0–17.5 g/dL), a reticulocyte count of 0.8% (reference range, 0.5%–1.5%), and a lactate dehydrogenase level of 424 U/L (reference range, 100–200 U/L). All red blood cell (RBC) indices were within reference range. Flow cytometry, eosin-5-maleimide, and ektacytometry were performed with results consistent with mild hereditary spherocytosis.

Comment
Dermal extramedullary hematopoiesis is a normal component of embryologic development up until the fifth month of gestation.1 The term blueberry muffin rash typically is used to describe the cutaneous manifestations of extramedullary hematopoiesis, which commonly is caused by a TORCH infection or hematologic dyscrasia.2 It has been suggested that the term be expanded to include neoplastic processes (eg, neuroblastomas) and vascular processes (eg, multiple hemangiomas, blue rubber bleb nevus syndrome, glomangiomas, multifocal lymphangionendotheliomatosis), which although not associated with an extramedullary hematopoiesis, can clinically resemble a blueberry muffin rash.
Because of the potential for serious systemic complications, a cause must be sought for all newborns presenting with a blueberry muffin rash. Our patient’s lack of cardiovascular, otic, and ocular involvement combined with a negative TORCH screen and normal CBC strongly suggested against a TORCH infection. In addition, a normal bone survey and CBC, as well as a lack of petechiae, ecchymoses, and hepatosplenomegaly, were evidence against congenital leukemia.3 With the spontaneously resolving lesions and apparent clinical resolution, a bone marrow biopsy was not performed. The skin biopsy revealed negative staining for S-100 and CD1a, making the diagnosis of congenital self-healing reticulohistiocytosis unlikely. No panniculitis was noted and calcium levels were normal, ruling out subcutaneous fat necrosis of the newborn. The predominantly T-cell lymphocytic infiltrate demonstrated on skin biopsy led us to a differential diagnosis of aleukemic leukemia cutis versus idiopathic dermal extramedullary hematopoiesis; however, normocytic anemia was later identified when the patient’s hemoglobin level dropped to 9.9 g/dL. The abnormal eosin-5-maleimide and ektacytometry results unmasked a hereditary spherocytosis.
Hereditary spherocytosis typically is inherited in an autosomal-dominant manner and may be caused by mutations in ankyrin-1, band 3, spectrin, or protein 4.2 on the erythrocyte membrane. It is the third leading cause of hemolytic anemia in newborns and the leading cause of direct Coombs-negative hemolytic anemia requiring blood transfusion in neonates. It is most common in neonates of Northern European ancestry, affecting 1 in every 1000 to 2000 births.4 Presentation may range from asymptomatic to severe anemia with hydrops fetalis. Most neonates have an elevated mean corpuscular hemoglobin and low mean corpuscular volume. Acute illness may cause hemolytic or aplastic crises, possibly explaining our patient’s normocytic anemia discovered on a CBC during an episode of an upper respiratory infection and otitis media.
Treatment options for hereditary spherocytosis include phototherapy for jaundiced neonates, folate supplementation, packed erythrocyte transfusions for symptomatic anemia, and recombinant erythropoietin in neonates.4 Splenectomy is curative for the majority of patients and requires immunization against Streptococcus pneumoniae, Haemophilus influenzae type b, and Neisseria meningitidis several weeks preoperatively. Patients with symptomatic gallstones may be treated with cholecystectomy at the time of splenectomy or by laparoscopic cholecystectomy, endoscopic sphincterotomy, cholecystostomy, or extracorporeal cholecystolithotripsy.5
Although a PubMed search of articles indexed for MEDLINE using the terms dermal hematopoiesis, extramedullary hematopoiesis, hereditary spherocytosis, and blueberry muffin rash yielded only 1 other known case of blueberry muffin rash caused by hereditary spherocytosis,6 other case reports demonstrate extramedullary hematopoiesis in hereditary spherocytosis patients in locations other than the skin. Calhoun et al7 described a case of a 9-year-old boy with hereditary spherocytosis who presented with jaundice. Pathologic examination revealed a 5-cm suprarenal mass demonstrating extramedullary hematopoiesis.7 A case reported by Xiros et al8 described a 64-year-old man with a history of hereditary spherocytosis who presented with hemothorax from paravertebral extramedullary hematopoiesis. De Backer et al9 reported a case of a 60-year-old man diagnosed with hereditary spherocytosis after an abnormal CBC who was subsequently found to have paravertebral masses containing extramedullary hematopoiesis.
There is one known case of a blueberry muffin rash caused by hereditary spherocytosis.6 A female neonate was born at 38 weeks’ gestation with multiple petechiae and faint purpuric papules. Initial complications included intracranial ventricular hemorrhage, hyperbilirubinemia, and anemia requiring blood transfusions on the first day of life. TORCH titers were negative and a skin biopsy demonstrated a diffuse infiltrate of mature RBCs, normoblasts, and pronormoblasts in the reticular dermis. She was healthy until 3 months of age when she had several days of vomiting and diarrhea. Laboratory workup revealed a hematocrit level of 20.5% (reference range, 41%–50%); a reticulocyte count of 22.6% (reference range, 0.5%–1.5%); and a peripheral blood smear demonstrating polychromatophilia, anisocytosis, and spherocytosis. She was then diagnosed with hereditary spherocytosis.6
Hereditary spherocytosis is a known, albeit rare, cause of extramedullary hematopoiesis presenting as blueberry muffin rash. Patients with mild hereditary spherocytosis may have a compensated hemolysis without anemia or spherocytes on peripheral smear, which may explain the lack of severe hemolytic anemia or RBC-predominant pathology in our patient.5 Argyle and Zone6 proposed that severe hemolysis and hypoxia were the cause of extramedullary hematopoiesis in their patient. Because our patient did not experience a notable hemolytic episode until he had an upper respiratory infection and otitis media at 6 months of age, the pathophysiology is less clear; a compensated hemolytic process may underlie the extramedullary hematopoiesis and normal RBC indices.
Regardless of the precise cause of extramedullary hematopoiesis in our patient, this case of a T lymphocyte–dominant cutaneous infiltrate in a patient with mild hereditary spherocytosis is exceptionally rare and leads us to consider that perhaps there are causes of this pathology that are unknown to us.
The term blueberry muffin rash historically was used to describe the cutaneous manifestations observed in congenital rubella. The term traditionally describes the result of a postnatal dermal extramedullary hematopoiesis. Today, TORCH (toxoplasmosis, other agents, rubella, cytomegalovirus, herpes) infections and plasma cell dyscrasias are all potential causes of extramedullary hematopoiesis. Herein, we present a unique case of a neonate born with a blueberry muffin rash secondary to extramedullary hematopoiesis induced by hereditary spherocytosis.
Case Report
The dermatology department was consulted to evaluate a 2-day-old male neonate born with a “rash.” The patient was born to a 34-year-old gravida 3, para 2, woman at 39 weeks’ gestation. The mother’s prenatal laboratory values were within reference range and ultrasounds were normal, and she was compliant with her prenatal care. She underwent a normal spontaneous vaginal delivery 3 hours after rupture of membranes without complication. The amniotic fluid and umbilical cord both were clear. There was no use of forceps or any other external aiding devices during the delivery. At the time of delivery, the consulting physician noted that the patient had “skin lesions from head to toe.”
The patient’s parents reported that the rash did not seem to cause any discomfort for the patient. In the 24 hours after birth, the parents reported that the erythema seemed to slightly fade. Physical examination revealed many scattered erythematous to violaceous, nonblanching papulonodules affecting the scalp (Figure 1), face, arms, hands (Figure 2A), back (Figure 2B), buttocks, legs, and feet. Some of the papulonodules were soft while others were firm and indurated. Several lesions had a yellowish hue with some overlying crust. There was no mucosal, genital, or ocular involvement. No erosions, ulcerations, petechiae, ecchymoses, or hepatosplenomegaly were noted on examination.


The patient was otherwise healthy with an Apgar score of 8/9 at 1 and 5 minutes. His birth weight, length, and head circumference were within normal limits. There was no evidence of ABO blood group or Rhesus factor incompatibility. His temperature, vital signs, laboratory values (including calcium level and TORCH titers, which included cytomegalovirus, rubella, toxoplasmosis, and herpes simplex virus), and review of systems all were within reference range. A bone survey of the skull, spine, ribs, arms, pelvis, legs, and feet was within normal limits.
The mother’s placenta was sent for pathology and revealed a lymphoplasmacytic chronic deciduitis and acute subchorionitis consistent with a nonspecific inflammatory response, unlikely to be from an infectious etiology.
A 4-mm punch biopsy was taken from the left thigh and revealed a predominately lymphocytic infiltrate with rare eosinophils and erythrocyte precursors (Figure 3). Immunohistochemical staining was performed showing that the majority of the lymphocytes represented T lymphocytes, which stained positive for CD45 and CD3 and negative for S-100, CD1a, CD30, and CD117. There were scattered CD34+ cells, and scattered cells stained positive for myeloperoxidase. No significant CD20 immunoreactivity was noted. There were scattered eosinophils and rare normoblasts but no megakaryocytes. A complete blood cell count (CBC) with differential and reticulocyte count was within reference range.

At 1-, 3-, 8-, 12-, and 28-week follow-up visits, the patient continued to grow and feed appropriately. No new lesions developed during this time, and the preexisting lesions continued to fade into slightly hyperpigmented patches without induration (Figure 4). At 6 months of age, a CBC performed at the time of an upper respiratory infection and otitis media revealed normocytic anemia with a hemoglobin level of 9.9 g/dL (reference range, 14.0–17.5 g/dL), a reticulocyte count of 0.8% (reference range, 0.5%–1.5%), and a lactate dehydrogenase level of 424 U/L (reference range, 100–200 U/L). All red blood cell (RBC) indices were within reference range. Flow cytometry, eosin-5-maleimide, and ektacytometry were performed with results consistent with mild hereditary spherocytosis.

Comment
Dermal extramedullary hematopoiesis is a normal component of embryologic development up until the fifth month of gestation.1 The term blueberry muffin rash typically is used to describe the cutaneous manifestations of extramedullary hematopoiesis, which commonly is caused by a TORCH infection or hematologic dyscrasia.2 It has been suggested that the term be expanded to include neoplastic processes (eg, neuroblastomas) and vascular processes (eg, multiple hemangiomas, blue rubber bleb nevus syndrome, glomangiomas, multifocal lymphangionendotheliomatosis), which although not associated with an extramedullary hematopoiesis, can clinically resemble a blueberry muffin rash.
Because of the potential for serious systemic complications, a cause must be sought for all newborns presenting with a blueberry muffin rash. Our patient’s lack of cardiovascular, otic, and ocular involvement combined with a negative TORCH screen and normal CBC strongly suggested against a TORCH infection. In addition, a normal bone survey and CBC, as well as a lack of petechiae, ecchymoses, and hepatosplenomegaly, were evidence against congenital leukemia.3 With the spontaneously resolving lesions and apparent clinical resolution, a bone marrow biopsy was not performed. The skin biopsy revealed negative staining for S-100 and CD1a, making the diagnosis of congenital self-healing reticulohistiocytosis unlikely. No panniculitis was noted and calcium levels were normal, ruling out subcutaneous fat necrosis of the newborn. The predominantly T-cell lymphocytic infiltrate demonstrated on skin biopsy led us to a differential diagnosis of aleukemic leukemia cutis versus idiopathic dermal extramedullary hematopoiesis; however, normocytic anemia was later identified when the patient’s hemoglobin level dropped to 9.9 g/dL. The abnormal eosin-5-maleimide and ektacytometry results unmasked a hereditary spherocytosis.
Hereditary spherocytosis typically is inherited in an autosomal-dominant manner and may be caused by mutations in ankyrin-1, band 3, spectrin, or protein 4.2 on the erythrocyte membrane. It is the third leading cause of hemolytic anemia in newborns and the leading cause of direct Coombs-negative hemolytic anemia requiring blood transfusion in neonates. It is most common in neonates of Northern European ancestry, affecting 1 in every 1000 to 2000 births.4 Presentation may range from asymptomatic to severe anemia with hydrops fetalis. Most neonates have an elevated mean corpuscular hemoglobin and low mean corpuscular volume. Acute illness may cause hemolytic or aplastic crises, possibly explaining our patient’s normocytic anemia discovered on a CBC during an episode of an upper respiratory infection and otitis media.
Treatment options for hereditary spherocytosis include phototherapy for jaundiced neonates, folate supplementation, packed erythrocyte transfusions for symptomatic anemia, and recombinant erythropoietin in neonates.4 Splenectomy is curative for the majority of patients and requires immunization against Streptococcus pneumoniae, Haemophilus influenzae type b, and Neisseria meningitidis several weeks preoperatively. Patients with symptomatic gallstones may be treated with cholecystectomy at the time of splenectomy or by laparoscopic cholecystectomy, endoscopic sphincterotomy, cholecystostomy, or extracorporeal cholecystolithotripsy.5
Although a PubMed search of articles indexed for MEDLINE using the terms dermal hematopoiesis, extramedullary hematopoiesis, hereditary spherocytosis, and blueberry muffin rash yielded only 1 other known case of blueberry muffin rash caused by hereditary spherocytosis,6 other case reports demonstrate extramedullary hematopoiesis in hereditary spherocytosis patients in locations other than the skin. Calhoun et al7 described a case of a 9-year-old boy with hereditary spherocytosis who presented with jaundice. Pathologic examination revealed a 5-cm suprarenal mass demonstrating extramedullary hematopoiesis.7 A case reported by Xiros et al8 described a 64-year-old man with a history of hereditary spherocytosis who presented with hemothorax from paravertebral extramedullary hematopoiesis. De Backer et al9 reported a case of a 60-year-old man diagnosed with hereditary spherocytosis after an abnormal CBC who was subsequently found to have paravertebral masses containing extramedullary hematopoiesis.
There is one known case of a blueberry muffin rash caused by hereditary spherocytosis.6 A female neonate was born at 38 weeks’ gestation with multiple petechiae and faint purpuric papules. Initial complications included intracranial ventricular hemorrhage, hyperbilirubinemia, and anemia requiring blood transfusions on the first day of life. TORCH titers were negative and a skin biopsy demonstrated a diffuse infiltrate of mature RBCs, normoblasts, and pronormoblasts in the reticular dermis. She was healthy until 3 months of age when she had several days of vomiting and diarrhea. Laboratory workup revealed a hematocrit level of 20.5% (reference range, 41%–50%); a reticulocyte count of 22.6% (reference range, 0.5%–1.5%); and a peripheral blood smear demonstrating polychromatophilia, anisocytosis, and spherocytosis. She was then diagnosed with hereditary spherocytosis.6
Hereditary spherocytosis is a known, albeit rare, cause of extramedullary hematopoiesis presenting as blueberry muffin rash. Patients with mild hereditary spherocytosis may have a compensated hemolysis without anemia or spherocytes on peripheral smear, which may explain the lack of severe hemolytic anemia or RBC-predominant pathology in our patient.5 Argyle and Zone6 proposed that severe hemolysis and hypoxia were the cause of extramedullary hematopoiesis in their patient. Because our patient did not experience a notable hemolytic episode until he had an upper respiratory infection and otitis media at 6 months of age, the pathophysiology is less clear; a compensated hemolytic process may underlie the extramedullary hematopoiesis and normal RBC indices.
Regardless of the precise cause of extramedullary hematopoiesis in our patient, this case of a T lymphocyte–dominant cutaneous infiltrate in a patient with mild hereditary spherocytosis is exceptionally rare and leads us to consider that perhaps there are causes of this pathology that are unknown to us.
- Zhang IH, Zane LT, Braun BS, et al. Congenital leukemia cutis with subsequent development of leukemia. J Am Acad Dermatol. 2006;54(2 suppl):S22–S27.
- Karmegaraj B, Vijayakumar S, Ramanathan R, et al. Extramedullary haematopoiesis resembling a blueberry muffin, in a neonate. BMJ Case Rep. pii: bcr2014208473. doi: 10.1136/bcr-2014-208473.
- Handler MZ, Schwartz RA. Neonatal leukaemia cutis. J Eur Acad Dermatol Venereol. 2015;29:1884-1889.
- Christensen RD, Yaish HM, Gallagher PG. A pediatrician’s practical guide to diagnosing and treating hereditary spherocytosis in neonates. Pediatrics. 2015;135:1107-1114.
- Perrotta S, Gallagher PG, Mohandas N. Hereditary spherocytosis. Lancet. 2008;372:1411-1426.
- Argyle JC, Zone JJ. Dermal erythropoiesis in a neonate. Arch Dermatol. 1981;117:492-494.
- Calhoun SK, Murphy RC, Shariati N, et al. Extramedullary hematopoiesis in a child with hereditary spherocytosis: an uncommon cause of an adrenal mass. Pediatr Radiol. 2001;31:879-881.
- Xiros N, Economopoulos T, Papageorgiou E, et al. Massive hemothorax due to intrathoracic extramedullary hematopoiesis in a patient with hereditary spherocytosis. Ann Hematol. 2001;80:38-40.
- De Backer AI, Zachée P, Vanschoubroeck IJ, et al. Extramedullary paraspinal hematopoiesis in hereditary spherocytosis. JBR-BTR. 2002;85:206-208.
- Zhang IH, Zane LT, Braun BS, et al. Congenital leukemia cutis with subsequent development of leukemia. J Am Acad Dermatol. 2006;54(2 suppl):S22–S27.
- Karmegaraj B, Vijayakumar S, Ramanathan R, et al. Extramedullary haematopoiesis resembling a blueberry muffin, in a neonate. BMJ Case Rep. pii: bcr2014208473. doi: 10.1136/bcr-2014-208473.
- Handler MZ, Schwartz RA. Neonatal leukaemia cutis. J Eur Acad Dermatol Venereol. 2015;29:1884-1889.
- Christensen RD, Yaish HM, Gallagher PG. A pediatrician’s practical guide to diagnosing and treating hereditary spherocytosis in neonates. Pediatrics. 2015;135:1107-1114.
- Perrotta S, Gallagher PG, Mohandas N. Hereditary spherocytosis. Lancet. 2008;372:1411-1426.
- Argyle JC, Zone JJ. Dermal erythropoiesis in a neonate. Arch Dermatol. 1981;117:492-494.
- Calhoun SK, Murphy RC, Shariati N, et al. Extramedullary hematopoiesis in a child with hereditary spherocytosis: an uncommon cause of an adrenal mass. Pediatr Radiol. 2001;31:879-881.
- Xiros N, Economopoulos T, Papageorgiou E, et al. Massive hemothorax due to intrathoracic extramedullary hematopoiesis in a patient with hereditary spherocytosis. Ann Hematol. 2001;80:38-40.
- De Backer AI, Zachée P, Vanschoubroeck IJ, et al. Extramedullary paraspinal hematopoiesis in hereditary spherocytosis. JBR-BTR. 2002;85:206-208.
Practice Points
- The term blueberry muffin rash is used to describe the clinical presentation of dermal extramedullary hematopoiesis. The common culprits of this rash include a TORCH (toxoplasmosis, other agents, rubella, cytomegalovirus, herpes) infection or hematologic dyscrasia.
- Because of the potential for serious systemic complications, a cause must be sought for all newborns presenting with a blueberry muffin rash.
- Hereditary spherocytosis typically is inherited in an autosomal-dominant manner and may be caused by mutations in ankyrin-1, band 3, spectrin, or protein 4.2 on the erythrocyte membrane. It is the third leading cause of hemolytic anemia in newborns and the leading cause of direct Coombs-negative hemolytic anemia requiring blood transfusion in neonates.
- Treatment options for hereditary spherocytosis include phototherapy for jaundiced neonates, folate supplementation, packed erythrocyte transfusions for symptomatic anemia, and recombinant erythropoietin in neonates.
Pediatric Periorificial Dermatitis
Perioral dermatitis is an acneform eruption presenting with erythematous papules, vesicles, and rarely pustules clustered around the orifices of the face. 1 Lesions may be found near the eyes, mouth, and nose but typically spare the vermilion border of the lips. 2 Nguyen and Eichenfield 3 preferred the term periorificial dermatitis (POD), which has since been adopted by others. 4 Patients may report pruritus, but there generally are no systemic symptoms unless patients have comorbid conditions such as atopic dermatitis. 5 Although this condition has been well examined in the literature on adults, data in the pediatric population are far more limited, consisting of case series and retrospective chart reviews. In 1979, Wilkinson et al 6 published a study of more than 200 patients with perioral dermatitis, but only 15 patients younger than 12 years were included.
Etiology
Although the exact pathogenesis of POD is unknown, a common denominator among many patients is prior exposure to topical corticosteroids.3,7-9 Periorificial dermatitis also has been linked to the use of systemic corticosteroids in pediatric patients.10 The exact relationship between steroid use and dermatitis is unknown; it may be related to a change in the flora of hair follicles and in particular an association with fusiform bacteria–rich conditions.11 Aside from steroid exposure, POD has been associated with the use of physical sunscreen in pediatric patients with dry skin,12 rosin in chewing gum,13 and inhaled corticosteroids in those with asthma.14 In one case, a 15-year-old adolescent girl developed POD and swelling of the lips after 2 years of playing a flute made of cocus wood.15,16
Epidemiology
Comorbidities and Family History
Goel et al17 (N=222) reported the following comorbidities associated with pediatric POD: atopic dermatitis (29.3%), asthma (14.9%), and allergies (9.9%). Steroid exposure was noted in 58.1% of patients.17 Similarly, Nguyen and Eichenfield3 (N=79) found that the most common comorbidities were atopic dermatitis (14%), keratosis pilaris (14%), viral infections (14%), acne (10%), and seborrheic dermatitis (10%). Family history of atopy was noted in 55% of patients and family history of rosacea was noted in 3%. In a case series of 11 pediatric patients, 3 (27%) had keratosis pilaris, 7 (64%) had a family history of atopy, and 2 (18%) had a family history of rosacea.8 Weston and Morelli9 found a much higher incidence of familial rosacea (20%) in 106 children with steroid rosacea.
Clinical Presentation
Periorificial dermatitis generally presents with small, pink- to flesh-colored papules in a perioral, periocular, and perinasal distribution. Although many patients are white, a particularly prominent variant has been noted in black children with papules that may be hyperpigmented.18 In a 2006 chart review in 79 pediatric POD patients aged 6 months to 18 years, Nguyen and Eichenfield3 reported that 92% (73/79) of patients presented for a facial rash with an average duration ranging from 2 weeks to 4 years.
Boeck et al19 described 7 pediatric patients with perioral dermatitis. Six (86%) patients had perioral lesions, and 6 (86%) had previously been treated with moderate- to high-potency topical corticosteroids. Skin prick tests were negative in 6 (86%) patients.19 In one case report, a 6-year-old boy did not present with the classic acneform lesions but rather sharply demarcated eczematous patches around the eyes, nose, and mouth. The rash began to fade after 2 weeks of using metronidazole gel 1%, and after 4 months he was only left with mild hyperpigmentation.4
Periorificial dermatitis was once thought to be a juvenile form of rosacea.5 In 1972, Savin et al8 described 11 pediatric patients with “rosacea-like” facial flushing, papules, pustules, and scaling over the cheeks, forehead, and chin. In some patients, the eyelids also were involved. At least 8 patients had been using potent topical corticosteroids and had noticed exacerbation of their skin lesions after stopping therapy.8
Variants of POD
Several other variants of POD have been described in pediatric patients including childhood granulomatous periorificial dermatitis (CGPD)(also known as facial Afro-Caribbean [childhood] eruption) and lupus miliaris disseminatus faciei. Childhood granulomatous periorificial dermatitis presents in prepubertal children as dome-shaped, red to yellow-brown, monomorphous papules around the eyes, nose, and mouth; there are no systemic findings.20,21 It occurs equally in males and females and is more commonly seen in dark-skinned patients. Childhood granulomatous periorificial dermatitis usually resolves within a few months to years but may be associated with blepharitis or conjunctivitis.20 Urbatsch et al20 analyzed extrafacial lesions in 8 patients (aged 2–12 years) with CGPD. Lesions were found on the trunk (38% [3/8]), neck (25% [2/8]), ears (25% [2/8]), extremities (50% [4/8]), labia majora (38% [3/8]), and abdomen (13% [1/8]). In addition, 2 (25% [2/8]) patients had blepharitis.20
Lupus miliaris disseminatus faciei, which occurs in adolescents and adults, commonly involves the eyelids and central areas of the face such as the nose and upper lips. Patients typically present with erythematous or flesh-colored papules.1
Diagnosis
Diagnosis of POD is made clinically based on the observation of papules (and sometimes pustules) around the orifices of the face, sparing the vermilion border, together with a lack of comedones.17 Laboratory tests are not useful.5 Biopsies rarely are performed, and the results mimic those of rosacea, demonstrating a perifollicular lymphohistiocytic infiltrate, epithelioid cells, and occasionally giant cells.5,22,23 Early papular lesions can show mild acanthosis, epidermal edema, and parakeratosis.23 Biopsies in patients with CGPD reveal noncaseating perifollicular granulomas.20
Treatment and Clinical Outcome
Although topical corticosteroids can improve facial lesions in pediatric POD, the eruption often rebounds when therapy is discontinued.1 One therapy frequently used in adults is oral tetracyclines; however, these agents must not be used in patients younger than 9 years due to potential dental staining.4 The standards are either topical metronidazole twice daily with clearance in 3 to 8 weeks or oral erythromycin.7
In the review conducted by Goel et al,17 treatment included azithromycin (44.6%), topical metronidazole (42.3%), sodium sulfacetamide lotion (35.6%), oral antibiotic monotherapy (15.3%), topical agent monotherapy (44.6%), and combined oral and topical agent therapy (40.1%). Of those patients who presented for a follow-up visit (59%), 72% of cases resolved and 10.7% showed some improvement. For those patients who returned for follow-up, the average duration until symptom resolution was approximately 4 months. The most common side effects were pigmentation changes (1.8%), worsening of symptoms (1.8%), gastrointestinal upset (0.9%), irritant dermatitis (0.9%), and xerosis (0.5%).17
Changes were made to the treatment plans for 16 patients, most often due to inadequate treatment response.17 Five patients treated with sodium sulfacetamide lotion also were started on oral azithromycin. Four patients treated with oral antibiotics were given a topical agent (metronidazole or sodium sulfacetamide lotion). Other modifications included replacing sodium sulfacetamide lotion with topical metronidazole and an oral antibiotic (azithromycin or doxycycline, n=3), adjusting the doses of oral or topical medications (n=2), adding tacrolimus (n=1), and replacing topical metronidazole with sodium sulfacetamide lotion (n=1). Of the patients who underwent a change in treatment plan, 5 experienced symptom recurrence, 4 had mild improvement, and 1 patient had no improvement. Six patients were lost to follow-up.17
In the study conducted by Nguyen and Eichenfield,3 follow-up visits occurred approximately 3 months after the first visit.
In the case series by Boeck et al,19 all patients were started on metronidazole gel 1% applied once daily for the first week, and then twice daily until the lesions resolved. All patients showed improvement after 4 to 6 weeks, and eventually the disease cleared between 3 and 6 months. All patients were still symptom free during a 2-year observation period.19
Manders and Lucky7 described 14 patients with POD (aged 9 months to 6.5 years). Eight patients used only metronidazole gel 0.75%, while 5 used the gel in combination with topical corticosteroids (21% [3/14]), oral erythromycin (7% [1/14]), or topical erythromycin (7% [1/14]); 1 patient remained on hydrocortisone 1% and cleared. Patients responded well within 1 to 8 weeks and were symptom free for up to 16 months. Mid- to high-potency steroids were discontinued in all patients.7
In some pediatric patients with CGPD, recovery occurs faster with the use of oral macrolides or tetracyclines, either alone or in combination with topical antibiotics or sulfur-based lotions.20 Extrafacial lesions associated with CGPD do not appear to negatively impact treatment response or duration of disease. In the review conducted by Urbatsch et al,20 7 of 8 (88%) CGPD patients with extrafacial lesions were treated with oral agents including erythromycin, hydroxychloroquine, cyclosporine, minocycline, and azithromycin. Most of these patients also were using topical agents such as triamcinolone acetonide, desonide, metronidazole, and erythromycin. The time to resolution ranged from several weeks to 6 months.20
Weston and Morelli9 described a treatment regimen for steroid rosacea. The study included data on 106 children (60 females, 46 males) who had been exposed to mostly class 7 low-potency agents. All patients were advised to immediately stop topical steroid therapy without gradual withdrawal and to begin oral erythromycin stearate 30 mg/kg daily in 2 doses per day for 4 weeks. Patients who were unable to tolerate erythromycin were advised to use topical clindamycin phosphate twice daily for 4 weeks (n=6). Eighty-six percent of patients showed resolution within 4 weeks, and 100% showed clearance by 8 weeks. Twenty-two percent of patients had clearance within 3 weeks. There was no difference in the duration until resolution for those who had used oral or topical antibiotics.9 A different study suggested that low-potency topical steroids can be used to control inflammation when weaning patients off of strong steroids.5
Differential Diagnosis
The differential diagnosis should include acne vulgaris, allergic contact dermatitis, irritant contact dermatitis, seborrheic dermatitis, impetigo, dermatophyte infection, rosacea, and angiofibromas.4
Acne vulgaris commonly is found in older adolescents, and unlike POD, it will present with open or closed comedones.2 In patients aged 1 to 7 years, acne is a reason to consider endocrine evaluation. Allergic contact dermatitis is extremely pruritic, and the lesions often are papulovesicular with active weeping or crusting. Patients with irritant contact dermatitis often report burning and pain, and papules and pustules typically are absent. A thorough history can help rule out allergic or irritant contact dermatitis. Seborrheic dermatitis presents with erythema and scaling of the scalp, eyebrows, and nasolabial folds; it tends to spare the perioral regions and also lacks papules.2 The lesions of impetigo typically have a yellow-brown exudate, which forms a honey-colored crust.24 Tinea faciei, unlike the other tinea infections, can have an extremely variable presentation. Lesions usually begin as scaly macules that develop raised borders with central hypopigmentation, but papules, vesicles, and crusts can be seen.25 Potassium hydroxide
Conclusion
Diagnosis of POD is clinical and rests upon the finding of erythematous papules on the face near the eyes, mouth, and nose. Extrafacial lesions also have been described, particularly in pediatric patients with CGPD. Many patients will report a history of atopic dermatitis and asthma. Therapy for POD includes both topical and systemic agents. For those with mild disease, topical metronidazole commonly is used. For patients requiring oral antibiotics, tetracyclines or macrolides can be prescribed based on the age of the patient. Many pediatric patients who begin with both oral and topical agents can later be maintained on topical therapy, sometimes with a low-dose oral antibiotic. Periorificial dermatitis has an excellent prognosis and most pediatric patients show marked improvement within weeks to months.
- Tempark T, Shwayder TA. Perioral dermatitis: a review of the condition with special attention to treatment options. Am J Clin Dermatol. 2014;15:101-113.
- McFarland SL, Polcari IC. Morphology-based diagnosis of acneiform eruptions. Pediatr Ann. 2015;44:E188-E193.
- Nguyen V, Eichenfield LF. Periorificial dermatitis in children and adolescents. J Am Acad Dermatol. 2006;55:781-785.
- Kihiczak GG, Cruz MA, Schwartz RA. Periorificial dermatitis in children: an update and description of a child with striking features. Int J Dermatol. 2009;48:304-306.
- Laude TA, Salvemini JN. Perioral dermatitis in children. Sem Cutan Med Surg. 1999;18:206-209.
- Wilkinson DS, Kirton V, Wilkinson JD. Perioral dermatitis: a 12-year review. Br J Dermatol. 1979;101:245-257.
- Manders SM, Lucky AW. Perioral dermatitis in childhood. J Am Acad Dermatol. 1992;27(5 pt 1):688-692.
- Savin JA, Alexander S, Marks R. A rosacea-like eruption of children. Br J Dermatol. 1972;87:425-429.
- Weston WL, Morelli JG. Steroid rosacea in prepubertal children. Arch Pediatr Adolesc Med. 2000;154:62-64.
- Clementson B, Smidt AC. Periorificial dermatitis due to systemic corticosteroids in children: report of two cases. Pediatr Dermatol. 2012;29:331-332.
- Takiwaki H, Tsuda H, Arase S, et al. Differences between intrafollicular microorganism profiles in perioral and seborrhoeic dermatitis. Clin Exp Dermatol. 2003;28:531-534.
- Abeck D, Geisenfelder B, Brandt O. Physical sunscreens with high sun protection factor may cause perioral dermatitis in children. J Dtsch Dermatol Ges. 2009;7:701-703.
- Satyawan I, Oranje AP, van Joost T. Perioral dermatitis in a child due to rosin in chewing gum. Contact Dermatitis. 1990;22:182-183.
- Dubus JC, Marguet C, Deschildre A, et al. Local side-effects of inhaled corticosteroids in asthmatic children: influence of drug, dose, age, and device. Allergy. 2001;56:944-948.
- Hausen BM, Bruhn G, Koenig WA. New hydroxyisoflavans as contact sensitizers in cocus wood Brya ebenus DC (Fabaceae). Contact Dermatitis. 1991;25:149-155.
- Dirschka T, Weber K, Tronnier H. Topical cosmetics and perioral dermatitis. J Dtsch Dermatol Ges. 2004;2:194-199.
- Goel NS, Burkhart CN, Morrell DS. Pediatric periorificial dermatitis: clinical course and treatment outcomes in 222 patients. Pediatr Dermatol. 2015;32:333-336.
- Cribier B, Lieber-Mbomeyo A, Lipsker D. Clinical and histological study of a case of facial Afro-Caribbean childhood eruption (FACE) [in French][published online July 23, 2008]. Ann Dermatol Venerol. 2008;135:663-667.
- Boeck K, Abeck D, Werfel S, et al. Perioral dermatitis in children—clinical presentation, pathogenesis-related factors and response to topical metronidazole. Dermatology. 1997;195:235-238.
- Urbatsch AJ, Frieden I, Williams ML, et al. Extrafacial and generalized granulomatous periorificial dermatitis. Arch Dermatol. 2002;138:1354-1358.
- Kroshinsky D, Glick SA. Pediatric rosacea. Dermatol Ther. 2006;19:196-201.
- Ramelet AA, Delacrétaz J. Histopathologic study of perioral dermatitis [in French]. Dermatologica. 1981;163:361-369.
- Ljubojevi´c S, Lipozenci´c J, Turci´c P. Perioral dermatitis. Acta Dermatovenerol Croat. 2008;16:96-100.
- Nichols RL, Florman S. Clinical presentations of soft-tissue infections and surgical site infections. Clin Infect Dis. 2001;33(suppl 2):S84-S93.
- Lin RL, Szepietowski JC, Schwartz RA. Tinea faciei, an often deceptive facial eruption. Int J Dermatol. 2004;43:437-440.
Perioral dermatitis is an acneform eruption presenting with erythematous papules, vesicles, and rarely pustules clustered around the orifices of the face. 1 Lesions may be found near the eyes, mouth, and nose but typically spare the vermilion border of the lips. 2 Nguyen and Eichenfield 3 preferred the term periorificial dermatitis (POD), which has since been adopted by others. 4 Patients may report pruritus, but there generally are no systemic symptoms unless patients have comorbid conditions such as atopic dermatitis. 5 Although this condition has been well examined in the literature on adults, data in the pediatric population are far more limited, consisting of case series and retrospective chart reviews. In 1979, Wilkinson et al 6 published a study of more than 200 patients with perioral dermatitis, but only 15 patients younger than 12 years were included.
Etiology
Although the exact pathogenesis of POD is unknown, a common denominator among many patients is prior exposure to topical corticosteroids.3,7-9 Periorificial dermatitis also has been linked to the use of systemic corticosteroids in pediatric patients.10 The exact relationship between steroid use and dermatitis is unknown; it may be related to a change in the flora of hair follicles and in particular an association with fusiform bacteria–rich conditions.11 Aside from steroid exposure, POD has been associated with the use of physical sunscreen in pediatric patients with dry skin,12 rosin in chewing gum,13 and inhaled corticosteroids in those with asthma.14 In one case, a 15-year-old adolescent girl developed POD and swelling of the lips after 2 years of playing a flute made of cocus wood.15,16
Epidemiology
Comorbidities and Family History
Goel et al17 (N=222) reported the following comorbidities associated with pediatric POD: atopic dermatitis (29.3%), asthma (14.9%), and allergies (9.9%). Steroid exposure was noted in 58.1% of patients.17 Similarly, Nguyen and Eichenfield3 (N=79) found that the most common comorbidities were atopic dermatitis (14%), keratosis pilaris (14%), viral infections (14%), acne (10%), and seborrheic dermatitis (10%). Family history of atopy was noted in 55% of patients and family history of rosacea was noted in 3%. In a case series of 11 pediatric patients, 3 (27%) had keratosis pilaris, 7 (64%) had a family history of atopy, and 2 (18%) had a family history of rosacea.8 Weston and Morelli9 found a much higher incidence of familial rosacea (20%) in 106 children with steroid rosacea.
Clinical Presentation
Periorificial dermatitis generally presents with small, pink- to flesh-colored papules in a perioral, periocular, and perinasal distribution. Although many patients are white, a particularly prominent variant has been noted in black children with papules that may be hyperpigmented.18 In a 2006 chart review in 79 pediatric POD patients aged 6 months to 18 years, Nguyen and Eichenfield3 reported that 92% (73/79) of patients presented for a facial rash with an average duration ranging from 2 weeks to 4 years.
Boeck et al19 described 7 pediatric patients with perioral dermatitis. Six (86%) patients had perioral lesions, and 6 (86%) had previously been treated with moderate- to high-potency topical corticosteroids. Skin prick tests were negative in 6 (86%) patients.19 In one case report, a 6-year-old boy did not present with the classic acneform lesions but rather sharply demarcated eczematous patches around the eyes, nose, and mouth. The rash began to fade after 2 weeks of using metronidazole gel 1%, and after 4 months he was only left with mild hyperpigmentation.4
Periorificial dermatitis was once thought to be a juvenile form of rosacea.5 In 1972, Savin et al8 described 11 pediatric patients with “rosacea-like” facial flushing, papules, pustules, and scaling over the cheeks, forehead, and chin. In some patients, the eyelids also were involved. At least 8 patients had been using potent topical corticosteroids and had noticed exacerbation of their skin lesions after stopping therapy.8
Variants of POD
Several other variants of POD have been described in pediatric patients including childhood granulomatous periorificial dermatitis (CGPD)(also known as facial Afro-Caribbean [childhood] eruption) and lupus miliaris disseminatus faciei. Childhood granulomatous periorificial dermatitis presents in prepubertal children as dome-shaped, red to yellow-brown, monomorphous papules around the eyes, nose, and mouth; there are no systemic findings.20,21 It occurs equally in males and females and is more commonly seen in dark-skinned patients. Childhood granulomatous periorificial dermatitis usually resolves within a few months to years but may be associated with blepharitis or conjunctivitis.20 Urbatsch et al20 analyzed extrafacial lesions in 8 patients (aged 2–12 years) with CGPD. Lesions were found on the trunk (38% [3/8]), neck (25% [2/8]), ears (25% [2/8]), extremities (50% [4/8]), labia majora (38% [3/8]), and abdomen (13% [1/8]). In addition, 2 (25% [2/8]) patients had blepharitis.20
Lupus miliaris disseminatus faciei, which occurs in adolescents and adults, commonly involves the eyelids and central areas of the face such as the nose and upper lips. Patients typically present with erythematous or flesh-colored papules.1
Diagnosis
Diagnosis of POD is made clinically based on the observation of papules (and sometimes pustules) around the orifices of the face, sparing the vermilion border, together with a lack of comedones.17 Laboratory tests are not useful.5 Biopsies rarely are performed, and the results mimic those of rosacea, demonstrating a perifollicular lymphohistiocytic infiltrate, epithelioid cells, and occasionally giant cells.5,22,23 Early papular lesions can show mild acanthosis, epidermal edema, and parakeratosis.23 Biopsies in patients with CGPD reveal noncaseating perifollicular granulomas.20
Treatment and Clinical Outcome
Although topical corticosteroids can improve facial lesions in pediatric POD, the eruption often rebounds when therapy is discontinued.1 One therapy frequently used in adults is oral tetracyclines; however, these agents must not be used in patients younger than 9 years due to potential dental staining.4 The standards are either topical metronidazole twice daily with clearance in 3 to 8 weeks or oral erythromycin.7
In the review conducted by Goel et al,17 treatment included azithromycin (44.6%), topical metronidazole (42.3%), sodium sulfacetamide lotion (35.6%), oral antibiotic monotherapy (15.3%), topical agent monotherapy (44.6%), and combined oral and topical agent therapy (40.1%). Of those patients who presented for a follow-up visit (59%), 72% of cases resolved and 10.7% showed some improvement. For those patients who returned for follow-up, the average duration until symptom resolution was approximately 4 months. The most common side effects were pigmentation changes (1.8%), worsening of symptoms (1.8%), gastrointestinal upset (0.9%), irritant dermatitis (0.9%), and xerosis (0.5%).17
Changes were made to the treatment plans for 16 patients, most often due to inadequate treatment response.17 Five patients treated with sodium sulfacetamide lotion also were started on oral azithromycin. Four patients treated with oral antibiotics were given a topical agent (metronidazole or sodium sulfacetamide lotion). Other modifications included replacing sodium sulfacetamide lotion with topical metronidazole and an oral antibiotic (azithromycin or doxycycline, n=3), adjusting the doses of oral or topical medications (n=2), adding tacrolimus (n=1), and replacing topical metronidazole with sodium sulfacetamide lotion (n=1). Of the patients who underwent a change in treatment plan, 5 experienced symptom recurrence, 4 had mild improvement, and 1 patient had no improvement. Six patients were lost to follow-up.17
In the study conducted by Nguyen and Eichenfield,3 follow-up visits occurred approximately 3 months after the first visit.
In the case series by Boeck et al,19 all patients were started on metronidazole gel 1% applied once daily for the first week, and then twice daily until the lesions resolved. All patients showed improvement after 4 to 6 weeks, and eventually the disease cleared between 3 and 6 months. All patients were still symptom free during a 2-year observation period.19
Manders and Lucky7 described 14 patients with POD (aged 9 months to 6.5 years). Eight patients used only metronidazole gel 0.75%, while 5 used the gel in combination with topical corticosteroids (21% [3/14]), oral erythromycin (7% [1/14]), or topical erythromycin (7% [1/14]); 1 patient remained on hydrocortisone 1% and cleared. Patients responded well within 1 to 8 weeks and were symptom free for up to 16 months. Mid- to high-potency steroids were discontinued in all patients.7
In some pediatric patients with CGPD, recovery occurs faster with the use of oral macrolides or tetracyclines, either alone or in combination with topical antibiotics or sulfur-based lotions.20 Extrafacial lesions associated with CGPD do not appear to negatively impact treatment response or duration of disease. In the review conducted by Urbatsch et al,20 7 of 8 (88%) CGPD patients with extrafacial lesions were treated with oral agents including erythromycin, hydroxychloroquine, cyclosporine, minocycline, and azithromycin. Most of these patients also were using topical agents such as triamcinolone acetonide, desonide, metronidazole, and erythromycin. The time to resolution ranged from several weeks to 6 months.20
Weston and Morelli9 described a treatment regimen for steroid rosacea. The study included data on 106 children (60 females, 46 males) who had been exposed to mostly class 7 low-potency agents. All patients were advised to immediately stop topical steroid therapy without gradual withdrawal and to begin oral erythromycin stearate 30 mg/kg daily in 2 doses per day for 4 weeks. Patients who were unable to tolerate erythromycin were advised to use topical clindamycin phosphate twice daily for 4 weeks (n=6). Eighty-six percent of patients showed resolution within 4 weeks, and 100% showed clearance by 8 weeks. Twenty-two percent of patients had clearance within 3 weeks. There was no difference in the duration until resolution for those who had used oral or topical antibiotics.9 A different study suggested that low-potency topical steroids can be used to control inflammation when weaning patients off of strong steroids.5
Differential Diagnosis
The differential diagnosis should include acne vulgaris, allergic contact dermatitis, irritant contact dermatitis, seborrheic dermatitis, impetigo, dermatophyte infection, rosacea, and angiofibromas.4
Acne vulgaris commonly is found in older adolescents, and unlike POD, it will present with open or closed comedones.2 In patients aged 1 to 7 years, acne is a reason to consider endocrine evaluation. Allergic contact dermatitis is extremely pruritic, and the lesions often are papulovesicular with active weeping or crusting. Patients with irritant contact dermatitis often report burning and pain, and papules and pustules typically are absent. A thorough history can help rule out allergic or irritant contact dermatitis. Seborrheic dermatitis presents with erythema and scaling of the scalp, eyebrows, and nasolabial folds; it tends to spare the perioral regions and also lacks papules.2 The lesions of impetigo typically have a yellow-brown exudate, which forms a honey-colored crust.24 Tinea faciei, unlike the other tinea infections, can have an extremely variable presentation. Lesions usually begin as scaly macules that develop raised borders with central hypopigmentation, but papules, vesicles, and crusts can be seen.25 Potassium hydroxide
Conclusion
Diagnosis of POD is clinical and rests upon the finding of erythematous papules on the face near the eyes, mouth, and nose. Extrafacial lesions also have been described, particularly in pediatric patients with CGPD. Many patients will report a history of atopic dermatitis and asthma. Therapy for POD includes both topical and systemic agents. For those with mild disease, topical metronidazole commonly is used. For patients requiring oral antibiotics, tetracyclines or macrolides can be prescribed based on the age of the patient. Many pediatric patients who begin with both oral and topical agents can later be maintained on topical therapy, sometimes with a low-dose oral antibiotic. Periorificial dermatitis has an excellent prognosis and most pediatric patients show marked improvement within weeks to months.
Perioral dermatitis is an acneform eruption presenting with erythematous papules, vesicles, and rarely pustules clustered around the orifices of the face. 1 Lesions may be found near the eyes, mouth, and nose but typically spare the vermilion border of the lips. 2 Nguyen and Eichenfield 3 preferred the term periorificial dermatitis (POD), which has since been adopted by others. 4 Patients may report pruritus, but there generally are no systemic symptoms unless patients have comorbid conditions such as atopic dermatitis. 5 Although this condition has been well examined in the literature on adults, data in the pediatric population are far more limited, consisting of case series and retrospective chart reviews. In 1979, Wilkinson et al 6 published a study of more than 200 patients with perioral dermatitis, but only 15 patients younger than 12 years were included.
Etiology
Although the exact pathogenesis of POD is unknown, a common denominator among many patients is prior exposure to topical corticosteroids.3,7-9 Periorificial dermatitis also has been linked to the use of systemic corticosteroids in pediatric patients.10 The exact relationship between steroid use and dermatitis is unknown; it may be related to a change in the flora of hair follicles and in particular an association with fusiform bacteria–rich conditions.11 Aside from steroid exposure, POD has been associated with the use of physical sunscreen in pediatric patients with dry skin,12 rosin in chewing gum,13 and inhaled corticosteroids in those with asthma.14 In one case, a 15-year-old adolescent girl developed POD and swelling of the lips after 2 years of playing a flute made of cocus wood.15,16
Epidemiology
Comorbidities and Family History
Goel et al17 (N=222) reported the following comorbidities associated with pediatric POD: atopic dermatitis (29.3%), asthma (14.9%), and allergies (9.9%). Steroid exposure was noted in 58.1% of patients.17 Similarly, Nguyen and Eichenfield3 (N=79) found that the most common comorbidities were atopic dermatitis (14%), keratosis pilaris (14%), viral infections (14%), acne (10%), and seborrheic dermatitis (10%). Family history of atopy was noted in 55% of patients and family history of rosacea was noted in 3%. In a case series of 11 pediatric patients, 3 (27%) had keratosis pilaris, 7 (64%) had a family history of atopy, and 2 (18%) had a family history of rosacea.8 Weston and Morelli9 found a much higher incidence of familial rosacea (20%) in 106 children with steroid rosacea.
Clinical Presentation
Periorificial dermatitis generally presents with small, pink- to flesh-colored papules in a perioral, periocular, and perinasal distribution. Although many patients are white, a particularly prominent variant has been noted in black children with papules that may be hyperpigmented.18 In a 2006 chart review in 79 pediatric POD patients aged 6 months to 18 years, Nguyen and Eichenfield3 reported that 92% (73/79) of patients presented for a facial rash with an average duration ranging from 2 weeks to 4 years.
Boeck et al19 described 7 pediatric patients with perioral dermatitis. Six (86%) patients had perioral lesions, and 6 (86%) had previously been treated with moderate- to high-potency topical corticosteroids. Skin prick tests were negative in 6 (86%) patients.19 In one case report, a 6-year-old boy did not present with the classic acneform lesions but rather sharply demarcated eczematous patches around the eyes, nose, and mouth. The rash began to fade after 2 weeks of using metronidazole gel 1%, and after 4 months he was only left with mild hyperpigmentation.4
Periorificial dermatitis was once thought to be a juvenile form of rosacea.5 In 1972, Savin et al8 described 11 pediatric patients with “rosacea-like” facial flushing, papules, pustules, and scaling over the cheeks, forehead, and chin. In some patients, the eyelids also were involved. At least 8 patients had been using potent topical corticosteroids and had noticed exacerbation of their skin lesions after stopping therapy.8
Variants of POD
Several other variants of POD have been described in pediatric patients including childhood granulomatous periorificial dermatitis (CGPD)(also known as facial Afro-Caribbean [childhood] eruption) and lupus miliaris disseminatus faciei. Childhood granulomatous periorificial dermatitis presents in prepubertal children as dome-shaped, red to yellow-brown, monomorphous papules around the eyes, nose, and mouth; there are no systemic findings.20,21 It occurs equally in males and females and is more commonly seen in dark-skinned patients. Childhood granulomatous periorificial dermatitis usually resolves within a few months to years but may be associated with blepharitis or conjunctivitis.20 Urbatsch et al20 analyzed extrafacial lesions in 8 patients (aged 2–12 years) with CGPD. Lesions were found on the trunk (38% [3/8]), neck (25% [2/8]), ears (25% [2/8]), extremities (50% [4/8]), labia majora (38% [3/8]), and abdomen (13% [1/8]). In addition, 2 (25% [2/8]) patients had blepharitis.20
Lupus miliaris disseminatus faciei, which occurs in adolescents and adults, commonly involves the eyelids and central areas of the face such as the nose and upper lips. Patients typically present with erythematous or flesh-colored papules.1
Diagnosis
Diagnosis of POD is made clinically based on the observation of papules (and sometimes pustules) around the orifices of the face, sparing the vermilion border, together with a lack of comedones.17 Laboratory tests are not useful.5 Biopsies rarely are performed, and the results mimic those of rosacea, demonstrating a perifollicular lymphohistiocytic infiltrate, epithelioid cells, and occasionally giant cells.5,22,23 Early papular lesions can show mild acanthosis, epidermal edema, and parakeratosis.23 Biopsies in patients with CGPD reveal noncaseating perifollicular granulomas.20
Treatment and Clinical Outcome
Although topical corticosteroids can improve facial lesions in pediatric POD, the eruption often rebounds when therapy is discontinued.1 One therapy frequently used in adults is oral tetracyclines; however, these agents must not be used in patients younger than 9 years due to potential dental staining.4 The standards are either topical metronidazole twice daily with clearance in 3 to 8 weeks or oral erythromycin.7
In the review conducted by Goel et al,17 treatment included azithromycin (44.6%), topical metronidazole (42.3%), sodium sulfacetamide lotion (35.6%), oral antibiotic monotherapy (15.3%), topical agent monotherapy (44.6%), and combined oral and topical agent therapy (40.1%). Of those patients who presented for a follow-up visit (59%), 72% of cases resolved and 10.7% showed some improvement. For those patients who returned for follow-up, the average duration until symptom resolution was approximately 4 months. The most common side effects were pigmentation changes (1.8%), worsening of symptoms (1.8%), gastrointestinal upset (0.9%), irritant dermatitis (0.9%), and xerosis (0.5%).17
Changes were made to the treatment plans for 16 patients, most often due to inadequate treatment response.17 Five patients treated with sodium sulfacetamide lotion also were started on oral azithromycin. Four patients treated with oral antibiotics were given a topical agent (metronidazole or sodium sulfacetamide lotion). Other modifications included replacing sodium sulfacetamide lotion with topical metronidazole and an oral antibiotic (azithromycin or doxycycline, n=3), adjusting the doses of oral or topical medications (n=2), adding tacrolimus (n=1), and replacing topical metronidazole with sodium sulfacetamide lotion (n=1). Of the patients who underwent a change in treatment plan, 5 experienced symptom recurrence, 4 had mild improvement, and 1 patient had no improvement. Six patients were lost to follow-up.17
In the study conducted by Nguyen and Eichenfield,3 follow-up visits occurred approximately 3 months after the first visit.
In the case series by Boeck et al,19 all patients were started on metronidazole gel 1% applied once daily for the first week, and then twice daily until the lesions resolved. All patients showed improvement after 4 to 6 weeks, and eventually the disease cleared between 3 and 6 months. All patients were still symptom free during a 2-year observation period.19
Manders and Lucky7 described 14 patients with POD (aged 9 months to 6.5 years). Eight patients used only metronidazole gel 0.75%, while 5 used the gel in combination with topical corticosteroids (21% [3/14]), oral erythromycin (7% [1/14]), or topical erythromycin (7% [1/14]); 1 patient remained on hydrocortisone 1% and cleared. Patients responded well within 1 to 8 weeks and were symptom free for up to 16 months. Mid- to high-potency steroids were discontinued in all patients.7
In some pediatric patients with CGPD, recovery occurs faster with the use of oral macrolides or tetracyclines, either alone or in combination with topical antibiotics or sulfur-based lotions.20 Extrafacial lesions associated with CGPD do not appear to negatively impact treatment response or duration of disease. In the review conducted by Urbatsch et al,20 7 of 8 (88%) CGPD patients with extrafacial lesions were treated with oral agents including erythromycin, hydroxychloroquine, cyclosporine, minocycline, and azithromycin. Most of these patients also were using topical agents such as triamcinolone acetonide, desonide, metronidazole, and erythromycin. The time to resolution ranged from several weeks to 6 months.20
Weston and Morelli9 described a treatment regimen for steroid rosacea. The study included data on 106 children (60 females, 46 males) who had been exposed to mostly class 7 low-potency agents. All patients were advised to immediately stop topical steroid therapy without gradual withdrawal and to begin oral erythromycin stearate 30 mg/kg daily in 2 doses per day for 4 weeks. Patients who were unable to tolerate erythromycin were advised to use topical clindamycin phosphate twice daily for 4 weeks (n=6). Eighty-six percent of patients showed resolution within 4 weeks, and 100% showed clearance by 8 weeks. Twenty-two percent of patients had clearance within 3 weeks. There was no difference in the duration until resolution for those who had used oral or topical antibiotics.9 A different study suggested that low-potency topical steroids can be used to control inflammation when weaning patients off of strong steroids.5
Differential Diagnosis
The differential diagnosis should include acne vulgaris, allergic contact dermatitis, irritant contact dermatitis, seborrheic dermatitis, impetigo, dermatophyte infection, rosacea, and angiofibromas.4
Acne vulgaris commonly is found in older adolescents, and unlike POD, it will present with open or closed comedones.2 In patients aged 1 to 7 years, acne is a reason to consider endocrine evaluation. Allergic contact dermatitis is extremely pruritic, and the lesions often are papulovesicular with active weeping or crusting. Patients with irritant contact dermatitis often report burning and pain, and papules and pustules typically are absent. A thorough history can help rule out allergic or irritant contact dermatitis. Seborrheic dermatitis presents with erythema and scaling of the scalp, eyebrows, and nasolabial folds; it tends to spare the perioral regions and also lacks papules.2 The lesions of impetigo typically have a yellow-brown exudate, which forms a honey-colored crust.24 Tinea faciei, unlike the other tinea infections, can have an extremely variable presentation. Lesions usually begin as scaly macules that develop raised borders with central hypopigmentation, but papules, vesicles, and crusts can be seen.25 Potassium hydroxide
Conclusion
Diagnosis of POD is clinical and rests upon the finding of erythematous papules on the face near the eyes, mouth, and nose. Extrafacial lesions also have been described, particularly in pediatric patients with CGPD. Many patients will report a history of atopic dermatitis and asthma. Therapy for POD includes both topical and systemic agents. For those with mild disease, topical metronidazole commonly is used. For patients requiring oral antibiotics, tetracyclines or macrolides can be prescribed based on the age of the patient. Many pediatric patients who begin with both oral and topical agents can later be maintained on topical therapy, sometimes with a low-dose oral antibiotic. Periorificial dermatitis has an excellent prognosis and most pediatric patients show marked improvement within weeks to months.
- Tempark T, Shwayder TA. Perioral dermatitis: a review of the condition with special attention to treatment options. Am J Clin Dermatol. 2014;15:101-113.
- McFarland SL, Polcari IC. Morphology-based diagnosis of acneiform eruptions. Pediatr Ann. 2015;44:E188-E193.
- Nguyen V, Eichenfield LF. Periorificial dermatitis in children and adolescents. J Am Acad Dermatol. 2006;55:781-785.
- Kihiczak GG, Cruz MA, Schwartz RA. Periorificial dermatitis in children: an update and description of a child with striking features. Int J Dermatol. 2009;48:304-306.
- Laude TA, Salvemini JN. Perioral dermatitis in children. Sem Cutan Med Surg. 1999;18:206-209.
- Wilkinson DS, Kirton V, Wilkinson JD. Perioral dermatitis: a 12-year review. Br J Dermatol. 1979;101:245-257.
- Manders SM, Lucky AW. Perioral dermatitis in childhood. J Am Acad Dermatol. 1992;27(5 pt 1):688-692.
- Savin JA, Alexander S, Marks R. A rosacea-like eruption of children. Br J Dermatol. 1972;87:425-429.
- Weston WL, Morelli JG. Steroid rosacea in prepubertal children. Arch Pediatr Adolesc Med. 2000;154:62-64.
- Clementson B, Smidt AC. Periorificial dermatitis due to systemic corticosteroids in children: report of two cases. Pediatr Dermatol. 2012;29:331-332.
- Takiwaki H, Tsuda H, Arase S, et al. Differences between intrafollicular microorganism profiles in perioral and seborrhoeic dermatitis. Clin Exp Dermatol. 2003;28:531-534.
- Abeck D, Geisenfelder B, Brandt O. Physical sunscreens with high sun protection factor may cause perioral dermatitis in children. J Dtsch Dermatol Ges. 2009;7:701-703.
- Satyawan I, Oranje AP, van Joost T. Perioral dermatitis in a child due to rosin in chewing gum. Contact Dermatitis. 1990;22:182-183.
- Dubus JC, Marguet C, Deschildre A, et al. Local side-effects of inhaled corticosteroids in asthmatic children: influence of drug, dose, age, and device. Allergy. 2001;56:944-948.
- Hausen BM, Bruhn G, Koenig WA. New hydroxyisoflavans as contact sensitizers in cocus wood Brya ebenus DC (Fabaceae). Contact Dermatitis. 1991;25:149-155.
- Dirschka T, Weber K, Tronnier H. Topical cosmetics and perioral dermatitis. J Dtsch Dermatol Ges. 2004;2:194-199.
- Goel NS, Burkhart CN, Morrell DS. Pediatric periorificial dermatitis: clinical course and treatment outcomes in 222 patients. Pediatr Dermatol. 2015;32:333-336.
- Cribier B, Lieber-Mbomeyo A, Lipsker D. Clinical and histological study of a case of facial Afro-Caribbean childhood eruption (FACE) [in French][published online July 23, 2008]. Ann Dermatol Venerol. 2008;135:663-667.
- Boeck K, Abeck D, Werfel S, et al. Perioral dermatitis in children—clinical presentation, pathogenesis-related factors and response to topical metronidazole. Dermatology. 1997;195:235-238.
- Urbatsch AJ, Frieden I, Williams ML, et al. Extrafacial and generalized granulomatous periorificial dermatitis. Arch Dermatol. 2002;138:1354-1358.
- Kroshinsky D, Glick SA. Pediatric rosacea. Dermatol Ther. 2006;19:196-201.
- Ramelet AA, Delacrétaz J. Histopathologic study of perioral dermatitis [in French]. Dermatologica. 1981;163:361-369.
- Ljubojevi´c S, Lipozenci´c J, Turci´c P. Perioral dermatitis. Acta Dermatovenerol Croat. 2008;16:96-100.
- Nichols RL, Florman S. Clinical presentations of soft-tissue infections and surgical site infections. Clin Infect Dis. 2001;33(suppl 2):S84-S93.
- Lin RL, Szepietowski JC, Schwartz RA. Tinea faciei, an often deceptive facial eruption. Int J Dermatol. 2004;43:437-440.
- Tempark T, Shwayder TA. Perioral dermatitis: a review of the condition with special attention to treatment options. Am J Clin Dermatol. 2014;15:101-113.
- McFarland SL, Polcari IC. Morphology-based diagnosis of acneiform eruptions. Pediatr Ann. 2015;44:E188-E193.
- Nguyen V, Eichenfield LF. Periorificial dermatitis in children and adolescents. J Am Acad Dermatol. 2006;55:781-785.
- Kihiczak GG, Cruz MA, Schwartz RA. Periorificial dermatitis in children: an update and description of a child with striking features. Int J Dermatol. 2009;48:304-306.
- Laude TA, Salvemini JN. Perioral dermatitis in children. Sem Cutan Med Surg. 1999;18:206-209.
- Wilkinson DS, Kirton V, Wilkinson JD. Perioral dermatitis: a 12-year review. Br J Dermatol. 1979;101:245-257.
- Manders SM, Lucky AW. Perioral dermatitis in childhood. J Am Acad Dermatol. 1992;27(5 pt 1):688-692.
- Savin JA, Alexander S, Marks R. A rosacea-like eruption of children. Br J Dermatol. 1972;87:425-429.
- Weston WL, Morelli JG. Steroid rosacea in prepubertal children. Arch Pediatr Adolesc Med. 2000;154:62-64.
- Clementson B, Smidt AC. Periorificial dermatitis due to systemic corticosteroids in children: report of two cases. Pediatr Dermatol. 2012;29:331-332.
- Takiwaki H, Tsuda H, Arase S, et al. Differences between intrafollicular microorganism profiles in perioral and seborrhoeic dermatitis. Clin Exp Dermatol. 2003;28:531-534.
- Abeck D, Geisenfelder B, Brandt O. Physical sunscreens with high sun protection factor may cause perioral dermatitis in children. J Dtsch Dermatol Ges. 2009;7:701-703.
- Satyawan I, Oranje AP, van Joost T. Perioral dermatitis in a child due to rosin in chewing gum. Contact Dermatitis. 1990;22:182-183.
- Dubus JC, Marguet C, Deschildre A, et al. Local side-effects of inhaled corticosteroids in asthmatic children: influence of drug, dose, age, and device. Allergy. 2001;56:944-948.
- Hausen BM, Bruhn G, Koenig WA. New hydroxyisoflavans as contact sensitizers in cocus wood Brya ebenus DC (Fabaceae). Contact Dermatitis. 1991;25:149-155.
- Dirschka T, Weber K, Tronnier H. Topical cosmetics and perioral dermatitis. J Dtsch Dermatol Ges. 2004;2:194-199.
- Goel NS, Burkhart CN, Morrell DS. Pediatric periorificial dermatitis: clinical course and treatment outcomes in 222 patients. Pediatr Dermatol. 2015;32:333-336.
- Cribier B, Lieber-Mbomeyo A, Lipsker D. Clinical and histological study of a case of facial Afro-Caribbean childhood eruption (FACE) [in French][published online July 23, 2008]. Ann Dermatol Venerol. 2008;135:663-667.
- Boeck K, Abeck D, Werfel S, et al. Perioral dermatitis in children—clinical presentation, pathogenesis-related factors and response to topical metronidazole. Dermatology. 1997;195:235-238.
- Urbatsch AJ, Frieden I, Williams ML, et al. Extrafacial and generalized granulomatous periorificial dermatitis. Arch Dermatol. 2002;138:1354-1358.
- Kroshinsky D, Glick SA. Pediatric rosacea. Dermatol Ther. 2006;19:196-201.
- Ramelet AA, Delacrétaz J. Histopathologic study of perioral dermatitis [in French]. Dermatologica. 1981;163:361-369.
- Ljubojevi´c S, Lipozenci´c J, Turci´c P. Perioral dermatitis. Acta Dermatovenerol Croat. 2008;16:96-100.
- Nichols RL, Florman S. Clinical presentations of soft-tissue infections and surgical site infections. Clin Infect Dis. 2001;33(suppl 2):S84-S93.
- Lin RL, Szepietowski JC, Schwartz RA. Tinea faciei, an often deceptive facial eruption. Int J Dermatol. 2004;43:437-440.
Practice Points
- Periorificial dermatitis (POD) affects young children and presents as flesh-colored papules around the mouth, nose, and even groin.
- Periorificial dermatitis has been associated with prior use of topical or inhaled steroids.
- Children with POD can be treated with oral erythromycin.
Concurrent Sturge-Weber Syndrome, Facial Infantile Hemangioma, and Cutis Marmorata Telangiectatica Congenita
Sturge-Weber syndrome (SWS) is a disease of dermatologic, neurologic, and ocular significance.1 The most distinctive manifestation is facial capillary malformation, commonly referred to as a port-wine stain or nevus flammeus. The dysregulated angiogenesis, caused by somatic mutations of the G protein subunit alpha Q gene, GNAQ, also affects the central nervous system.2 Seizures, intellectual disability, and glaucoma are common consequences.1 Not all port-wine stains are associated with SWS.3 Distribution in the ophthalmic dermatome is associated with increased risk for SWS, with 8% of patients with port-wine stains also having SWS.4 The disease is more serious when bilateral lesions are present.5 Diagnosis is clinical based on dermatologic, nervous system, and ophthalmologic findings.6 The disease is nonheritable because the mutation is found only in the somatic cell lines.2 The possibility of epigenetic influence on disease development has to be investigated. The treatment is aimed at managing complications, as there is no cure.7
Infantile hemangioma (IH) likewise represents a disruption in the process of vascular development but without the widespread consequences of SWS. The pathogenesis of hemangioma development has not been fully elucidated, with presence of GLUT1 (glucose transporter 1) protein implicated in lesions.4 Facial infantile hemangiomas have an incidence of approximately 5 in every 100 births, and the prevalence decreases with age. Most hemangiomas undergo growth followed by an involution process, with most lesions vanishing by 5 years of age.4 They typically are seen at 2 to 3 weeks of age, growing rapidly for the first 6 months, which is a contrast to the static nature of nevus flammeus. Infantile hemangiomas are regarded as sporadic, though autosomal-dominant inheritance patterns have been observed.4 Our patient demonstrated facial IH at birth, which is a rare and interesting finding suggesting that some epigenetic factors influenced this modification of the disease course in this patient.
Cutis marmorata telangiectatica congenita (CMTC) is a rare cutaneous vascular condition found in newborns. Its extraordinary infrequency is reflected in the fact that only 300 cases have been reported worldwide.8 At birth, CMTC manifests as a pinkish reticulated pattern all over the body mimicking cutis marmorata; however, unlike cutis marmorata, the lesions do not improve with warming.9 The lesions of CMTC gradually lighten as the patient ages.8 Limb asymmetry is the most common extravascular complication of CMTC and, similar to SWS, glaucoma also can occur.10 Cutis marmorata telangiectatica congenita has been known to occur simultaneously with SWS or IH, but the combination of all 3 conditions in our patient is unique. Due to the scarcity of cases, the pathophysiology and treatment is poorly understood, with appropriate monitoring for sequelae recommended.9
Case Report
The patient was born at 39 weeks’ gestation following an uncomplicated pregnancy and delivery. She weighed 2950 g, her length was 19 in, and her head circumference was 13.25 in, correlating to the 10th, 50th, and 25th percentiles, respectively. Her Apgar score was 8/9 at 1 and 5 minutes. Her parents were nonconsanguineous and in good health. The patient’s family lived in poverty, which led us to conjecture about the role that toxins played in the epigenetics of the patient and her family. It was the mother’s third pregnancy; both prior pregnancies resulted in healthy children. The patient was breastfed. No family history of heritable vascular disorders was reported.
On the first day of life during the newborn examination, dark red pigment changes were noticed under the nose and erythematous pigmentation over the whole body was observed (Figure). On examination, 2-toned reticular lesions identified as extensive nevus flammeus were found bilaterally over the distribution of the ophthalmic division of the trigeminal nerve. A separate erythematous plaque over the maxilla also was recognized. The pediatrician suspected SWS and facial IH. The patient was discharged after 3 days with a referral to pediatric dermatology, and appropriate follow-up with a pediatrician was scheduled. The patient returned for these appointments and the significance of SWS was explained to her parents. Consultation with pediatric dermatology at 2 weeks of age confirmed the diagnosis of SWS as well as facial IH.
Upon further follow-up with pediatric dermatology at 2 months of age, the patient received an additional diagnosis of CMTC. These exceedingly rare lesions were located over the back, trunk, arms, and legs. The patient’s parents were counseled about the management of these conditions and appropriate follow-up.
Comment
This case describes 3 different vascular malformations in the same patient. Cutis marmorata telangiectatica congenita is rare and yet is described in this patient along with 2 other notable endothelial abnormalities. The clinical interest of this case is heightened by the presence of CMTC.
The causative factor of SWS is a well-documented mutation of the GNAQ gene, but there is considerable variability in how it affects the patient. Unlike in SWS, no single factor can be attributed to the development of IH. This case shows that these 3 diseases are not mutually exclusive and can present with unusually severe features when they occur concomitantly. The embryologic basis of SWS traces its roots back to the first trimester during vascular development, wher
The severity of the SWS in our patient was highlighted by the extensive nevus flammeus. These lesions occurred over the face, trunk, arms, and legs. The port-wine stain with dermatomal distribution of the ophthalmic nerve was the most concerning feature regarding the development of neurologic complications in this patient. Although the developmental delays associated with SWS can be serious, early intervention is important and can improve long-term outcomes. The facial IH arising at birth was contrary to the typical presentation. All of these factors will be kept in mind as the patient progresses and patient-centered care is provided. Because this patient’s presentation differed from other patients with IH, we will be more vigilant in providing close follow-up and monitoring for other medical problems involving other organs (eg, the brain); for instance, we will monitor for seizures and developmental delay.
Conclusion
In our patient, a unique pattern of SWS, facial IH, and CMTC are described in a pediatric patient. Many disciplines are involved in the treatment. In the patient’s first days of life, extensive collaboration between pediatrics and dermatologists was pivotal, with ophthalmology, pathology, and radiology consultations at hand. This case highlights that several vascular malformations of different origins can occur in the same patient. Epigenetic along with genetic factors likely contributed to this fascinating presentation. The importance of parental education and maintaining appropriate follow-up for this patient is crucial for a favorable outcome.
- Sinawat S, Auvichayapat N, Auvichayapat P, et al. 12-year retrospective study of Sturge-weber syndrome and literature review. J Med Assoc Thail. 2014;97:742-750.
- Shirley MD, Tang H, Gallione CJ, et al. Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. N Engl J Med. 2013;368:1971-1979.
- Sudarsanam A, Ardern-Holmes SL. Sturge-Weber syndrome: from the past to the present [published online November 7, 2013]. Eur J Paediat Neurol. 2014;18:257-266.
- Paller AS, Mancini AJ. Hurwitz Clinical Pediatric Dermatology: A Textbook of Skin Disorders of Childhood and Adolescence. Philadelphia, PA: Elsevier Saunders; 2011.
- Sujansky E, Conradi S. Sturge-Weber syndrome: age of onset of seizures and glaucoma and the prognosis for affected children. J Child Neurol. 1995;10:49-58.
- Lo W, Marchuk DA, Ball KL, et al. Updates and future horizons on the understanding, diagnosis, and treatment of Sturge-Weber syndrome brain involvement. Dev Med Child Neurol. 2012;54:214-223.
- Comi AM. Update on Sturge-Weber syndrome: diagnosis, treatment, quantitative measures, and controversies. Lymphat Res Biol. 2007;5:257-264.
- Resende CI, Araujo C, Vieira AP, et al. Cutis marmorata telangiectatica congenital [published online October 17, 2013]. BMJ Case Rep. doi:10.1136/bcr-2013-200056.
- Levy R, Lam JM. Cutis marmorata telangiectatica congenita: a mimicker of a common disorder. CMAJ. 2011;183:E249-E251.
- Kienast AK, Hoeger PH. Cutis marmorata telangiectatica congenita: a prospective study of 27 cases and review of the literature with proposal of diagnostic criteria. Clin Exp Dermatol. 2009;34:319-323.
- Comi AM. Topical review: pathophysiology of Sturge-Weber syndrome. J Child Neurol. 2003;18:509-516.
Sturge-Weber syndrome (SWS) is a disease of dermatologic, neurologic, and ocular significance.1 The most distinctive manifestation is facial capillary malformation, commonly referred to as a port-wine stain or nevus flammeus. The dysregulated angiogenesis, caused by somatic mutations of the G protein subunit alpha Q gene, GNAQ, also affects the central nervous system.2 Seizures, intellectual disability, and glaucoma are common consequences.1 Not all port-wine stains are associated with SWS.3 Distribution in the ophthalmic dermatome is associated with increased risk for SWS, with 8% of patients with port-wine stains also having SWS.4 The disease is more serious when bilateral lesions are present.5 Diagnosis is clinical based on dermatologic, nervous system, and ophthalmologic findings.6 The disease is nonheritable because the mutation is found only in the somatic cell lines.2 The possibility of epigenetic influence on disease development has to be investigated. The treatment is aimed at managing complications, as there is no cure.7
Infantile hemangioma (IH) likewise represents a disruption in the process of vascular development but without the widespread consequences of SWS. The pathogenesis of hemangioma development has not been fully elucidated, with presence of GLUT1 (glucose transporter 1) protein implicated in lesions.4 Facial infantile hemangiomas have an incidence of approximately 5 in every 100 births, and the prevalence decreases with age. Most hemangiomas undergo growth followed by an involution process, with most lesions vanishing by 5 years of age.4 They typically are seen at 2 to 3 weeks of age, growing rapidly for the first 6 months, which is a contrast to the static nature of nevus flammeus. Infantile hemangiomas are regarded as sporadic, though autosomal-dominant inheritance patterns have been observed.4 Our patient demonstrated facial IH at birth, which is a rare and interesting finding suggesting that some epigenetic factors influenced this modification of the disease course in this patient.
Cutis marmorata telangiectatica congenita (CMTC) is a rare cutaneous vascular condition found in newborns. Its extraordinary infrequency is reflected in the fact that only 300 cases have been reported worldwide.8 At birth, CMTC manifests as a pinkish reticulated pattern all over the body mimicking cutis marmorata; however, unlike cutis marmorata, the lesions do not improve with warming.9 The lesions of CMTC gradually lighten as the patient ages.8 Limb asymmetry is the most common extravascular complication of CMTC and, similar to SWS, glaucoma also can occur.10 Cutis marmorata telangiectatica congenita has been known to occur simultaneously with SWS or IH, but the combination of all 3 conditions in our patient is unique. Due to the scarcity of cases, the pathophysiology and treatment is poorly understood, with appropriate monitoring for sequelae recommended.9
Case Report
The patient was born at 39 weeks’ gestation following an uncomplicated pregnancy and delivery. She weighed 2950 g, her length was 19 in, and her head circumference was 13.25 in, correlating to the 10th, 50th, and 25th percentiles, respectively. Her Apgar score was 8/9 at 1 and 5 minutes. Her parents were nonconsanguineous and in good health. The patient’s family lived in poverty, which led us to conjecture about the role that toxins played in the epigenetics of the patient and her family. It was the mother’s third pregnancy; both prior pregnancies resulted in healthy children. The patient was breastfed. No family history of heritable vascular disorders was reported.
On the first day of life during the newborn examination, dark red pigment changes were noticed under the nose and erythematous pigmentation over the whole body was observed (Figure). On examination, 2-toned reticular lesions identified as extensive nevus flammeus were found bilaterally over the distribution of the ophthalmic division of the trigeminal nerve. A separate erythematous plaque over the maxilla also was recognized. The pediatrician suspected SWS and facial IH. The patient was discharged after 3 days with a referral to pediatric dermatology, and appropriate follow-up with a pediatrician was scheduled. The patient returned for these appointments and the significance of SWS was explained to her parents. Consultation with pediatric dermatology at 2 weeks of age confirmed the diagnosis of SWS as well as facial IH.

Upon further follow-up with pediatric dermatology at 2 months of age, the patient received an additional diagnosis of CMTC. These exceedingly rare lesions were located over the back, trunk, arms, and legs. The patient’s parents were counseled about the management of these conditions and appropriate follow-up.
Comment
This case describes 3 different vascular malformations in the same patient. Cutis marmorata telangiectatica congenita is rare and yet is described in this patient along with 2 other notable endothelial abnormalities. The clinical interest of this case is heightened by the presence of CMTC.
The causative factor of SWS is a well-documented mutation of the GNAQ gene, but there is considerable variability in how it affects the patient. Unlike in SWS, no single factor can be attributed to the development of IH. This case shows that these 3 diseases are not mutually exclusive and can present with unusually severe features when they occur concomitantly. The embryologic basis of SWS traces its roots back to the first trimester during vascular development, wher
The severity of the SWS in our patient was highlighted by the extensive nevus flammeus. These lesions occurred over the face, trunk, arms, and legs. The port-wine stain with dermatomal distribution of the ophthalmic nerve was the most concerning feature regarding the development of neurologic complications in this patient. Although the developmental delays associated with SWS can be serious, early intervention is important and can improve long-term outcomes. The facial IH arising at birth was contrary to the typical presentation. All of these factors will be kept in mind as the patient progresses and patient-centered care is provided. Because this patient’s presentation differed from other patients with IH, we will be more vigilant in providing close follow-up and monitoring for other medical problems involving other organs (eg, the brain); for instance, we will monitor for seizures and developmental delay.
Conclusion
In our patient, a unique pattern of SWS, facial IH, and CMTC are described in a pediatric patient. Many disciplines are involved in the treatment. In the patient’s first days of life, extensive collaboration between pediatrics and dermatologists was pivotal, with ophthalmology, pathology, and radiology consultations at hand. This case highlights that several vascular malformations of different origins can occur in the same patient. Epigenetic along with genetic factors likely contributed to this fascinating presentation. The importance of parental education and maintaining appropriate follow-up for this patient is crucial for a favorable outcome.
Sturge-Weber syndrome (SWS) is a disease of dermatologic, neurologic, and ocular significance.1 The most distinctive manifestation is facial capillary malformation, commonly referred to as a port-wine stain or nevus flammeus. The dysregulated angiogenesis, caused by somatic mutations of the G protein subunit alpha Q gene, GNAQ, also affects the central nervous system.2 Seizures, intellectual disability, and glaucoma are common consequences.1 Not all port-wine stains are associated with SWS.3 Distribution in the ophthalmic dermatome is associated with increased risk for SWS, with 8% of patients with port-wine stains also having SWS.4 The disease is more serious when bilateral lesions are present.5 Diagnosis is clinical based on dermatologic, nervous system, and ophthalmologic findings.6 The disease is nonheritable because the mutation is found only in the somatic cell lines.2 The possibility of epigenetic influence on disease development has to be investigated. The treatment is aimed at managing complications, as there is no cure.7
Infantile hemangioma (IH) likewise represents a disruption in the process of vascular development but without the widespread consequences of SWS. The pathogenesis of hemangioma development has not been fully elucidated, with presence of GLUT1 (glucose transporter 1) protein implicated in lesions.4 Facial infantile hemangiomas have an incidence of approximately 5 in every 100 births, and the prevalence decreases with age. Most hemangiomas undergo growth followed by an involution process, with most lesions vanishing by 5 years of age.4 They typically are seen at 2 to 3 weeks of age, growing rapidly for the first 6 months, which is a contrast to the static nature of nevus flammeus. Infantile hemangiomas are regarded as sporadic, though autosomal-dominant inheritance patterns have been observed.4 Our patient demonstrated facial IH at birth, which is a rare and interesting finding suggesting that some epigenetic factors influenced this modification of the disease course in this patient.
Cutis marmorata telangiectatica congenita (CMTC) is a rare cutaneous vascular condition found in newborns. Its extraordinary infrequency is reflected in the fact that only 300 cases have been reported worldwide.8 At birth, CMTC manifests as a pinkish reticulated pattern all over the body mimicking cutis marmorata; however, unlike cutis marmorata, the lesions do not improve with warming.9 The lesions of CMTC gradually lighten as the patient ages.8 Limb asymmetry is the most common extravascular complication of CMTC and, similar to SWS, glaucoma also can occur.10 Cutis marmorata telangiectatica congenita has been known to occur simultaneously with SWS or IH, but the combination of all 3 conditions in our patient is unique. Due to the scarcity of cases, the pathophysiology and treatment is poorly understood, with appropriate monitoring for sequelae recommended.9
Case Report
The patient was born at 39 weeks’ gestation following an uncomplicated pregnancy and delivery. She weighed 2950 g, her length was 19 in, and her head circumference was 13.25 in, correlating to the 10th, 50th, and 25th percentiles, respectively. Her Apgar score was 8/9 at 1 and 5 minutes. Her parents were nonconsanguineous and in good health. The patient’s family lived in poverty, which led us to conjecture about the role that toxins played in the epigenetics of the patient and her family. It was the mother’s third pregnancy; both prior pregnancies resulted in healthy children. The patient was breastfed. No family history of heritable vascular disorders was reported.
On the first day of life during the newborn examination, dark red pigment changes were noticed under the nose and erythematous pigmentation over the whole body was observed (Figure). On examination, 2-toned reticular lesions identified as extensive nevus flammeus were found bilaterally over the distribution of the ophthalmic division of the trigeminal nerve. A separate erythematous plaque over the maxilla also was recognized. The pediatrician suspected SWS and facial IH. The patient was discharged after 3 days with a referral to pediatric dermatology, and appropriate follow-up with a pediatrician was scheduled. The patient returned for these appointments and the significance of SWS was explained to her parents. Consultation with pediatric dermatology at 2 weeks of age confirmed the diagnosis of SWS as well as facial IH.

Upon further follow-up with pediatric dermatology at 2 months of age, the patient received an additional diagnosis of CMTC. These exceedingly rare lesions were located over the back, trunk, arms, and legs. The patient’s parents were counseled about the management of these conditions and appropriate follow-up.
Comment
This case describes 3 different vascular malformations in the same patient. Cutis marmorata telangiectatica congenita is rare and yet is described in this patient along with 2 other notable endothelial abnormalities. The clinical interest of this case is heightened by the presence of CMTC.
The causative factor of SWS is a well-documented mutation of the GNAQ gene, but there is considerable variability in how it affects the patient. Unlike in SWS, no single factor can be attributed to the development of IH. This case shows that these 3 diseases are not mutually exclusive and can present with unusually severe features when they occur concomitantly. The embryologic basis of SWS traces its roots back to the first trimester during vascular development, wher
The severity of the SWS in our patient was highlighted by the extensive nevus flammeus. These lesions occurred over the face, trunk, arms, and legs. The port-wine stain with dermatomal distribution of the ophthalmic nerve was the most concerning feature regarding the development of neurologic complications in this patient. Although the developmental delays associated with SWS can be serious, early intervention is important and can improve long-term outcomes. The facial IH arising at birth was contrary to the typical presentation. All of these factors will be kept in mind as the patient progresses and patient-centered care is provided. Because this patient’s presentation differed from other patients with IH, we will be more vigilant in providing close follow-up and monitoring for other medical problems involving other organs (eg, the brain); for instance, we will monitor for seizures and developmental delay.
Conclusion
In our patient, a unique pattern of SWS, facial IH, and CMTC are described in a pediatric patient. Many disciplines are involved in the treatment. In the patient’s first days of life, extensive collaboration between pediatrics and dermatologists was pivotal, with ophthalmology, pathology, and radiology consultations at hand. This case highlights that several vascular malformations of different origins can occur in the same patient. Epigenetic along with genetic factors likely contributed to this fascinating presentation. The importance of parental education and maintaining appropriate follow-up for this patient is crucial for a favorable outcome.
- Sinawat S, Auvichayapat N, Auvichayapat P, et al. 12-year retrospective study of Sturge-weber syndrome and literature review. J Med Assoc Thail. 2014;97:742-750.
- Shirley MD, Tang H, Gallione CJ, et al. Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. N Engl J Med. 2013;368:1971-1979.
- Sudarsanam A, Ardern-Holmes SL. Sturge-Weber syndrome: from the past to the present [published online November 7, 2013]. Eur J Paediat Neurol. 2014;18:257-266.
- Paller AS, Mancini AJ. Hurwitz Clinical Pediatric Dermatology: A Textbook of Skin Disorders of Childhood and Adolescence. Philadelphia, PA: Elsevier Saunders; 2011.
- Sujansky E, Conradi S. Sturge-Weber syndrome: age of onset of seizures and glaucoma and the prognosis for affected children. J Child Neurol. 1995;10:49-58.
- Lo W, Marchuk DA, Ball KL, et al. Updates and future horizons on the understanding, diagnosis, and treatment of Sturge-Weber syndrome brain involvement. Dev Med Child Neurol. 2012;54:214-223.
- Comi AM. Update on Sturge-Weber syndrome: diagnosis, treatment, quantitative measures, and controversies. Lymphat Res Biol. 2007;5:257-264.
- Resende CI, Araujo C, Vieira AP, et al. Cutis marmorata telangiectatica congenital [published online October 17, 2013]. BMJ Case Rep. doi:10.1136/bcr-2013-200056.
- Levy R, Lam JM. Cutis marmorata telangiectatica congenita: a mimicker of a common disorder. CMAJ. 2011;183:E249-E251.
- Kienast AK, Hoeger PH. Cutis marmorata telangiectatica congenita: a prospective study of 27 cases and review of the literature with proposal of diagnostic criteria. Clin Exp Dermatol. 2009;34:319-323.
- Comi AM. Topical review: pathophysiology of Sturge-Weber syndrome. J Child Neurol. 2003;18:509-516.
- Sinawat S, Auvichayapat N, Auvichayapat P, et al. 12-year retrospective study of Sturge-weber syndrome and literature review. J Med Assoc Thail. 2014;97:742-750.
- Shirley MD, Tang H, Gallione CJ, et al. Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. N Engl J Med. 2013;368:1971-1979.
- Sudarsanam A, Ardern-Holmes SL. Sturge-Weber syndrome: from the past to the present [published online November 7, 2013]. Eur J Paediat Neurol. 2014;18:257-266.
- Paller AS, Mancini AJ. Hurwitz Clinical Pediatric Dermatology: A Textbook of Skin Disorders of Childhood and Adolescence. Philadelphia, PA: Elsevier Saunders; 2011.
- Sujansky E, Conradi S. Sturge-Weber syndrome: age of onset of seizures and glaucoma and the prognosis for affected children. J Child Neurol. 1995;10:49-58.
- Lo W, Marchuk DA, Ball KL, et al. Updates and future horizons on the understanding, diagnosis, and treatment of Sturge-Weber syndrome brain involvement. Dev Med Child Neurol. 2012;54:214-223.
- Comi AM. Update on Sturge-Weber syndrome: diagnosis, treatment, quantitative measures, and controversies. Lymphat Res Biol. 2007;5:257-264.
- Resende CI, Araujo C, Vieira AP, et al. Cutis marmorata telangiectatica congenital [published online October 17, 2013]. BMJ Case Rep. doi:10.1136/bcr-2013-200056.
- Levy R, Lam JM. Cutis marmorata telangiectatica congenita: a mimicker of a common disorder. CMAJ. 2011;183:E249-E251.
- Kienast AK, Hoeger PH. Cutis marmorata telangiectatica congenita: a prospective study of 27 cases and review of the literature with proposal of diagnostic criteria. Clin Exp Dermatol. 2009;34:319-323.
- Comi AM. Topical review: pathophysiology of Sturge-Weber syndrome. J Child Neurol. 2003;18:509-516.
Practice Points
- This case highlights that several vascular malformations of different origins can occur in the same patient.
- Epigenetic factors along with genetic factors can lead to development of complex vascular conditions.
- Close collaborations of different medical specialties is necessary to make an accurate diagnosis and to follow up to achieve optimal long-term outcomes for patients with complex medical conditions.
Herpes Zoster Following Varicella Vaccination in Children
Varicella-zoster virus (VZV) causes varicella as a primary infection. It is a highly contagious disease characterized by a widespread papulovesicular eruption with fever and malaise.1,2 After the primary infection, the virus remains latent within the sensory dorsal root ganglia and can reactivate as herpes zoster (HZ).1-5 Herpes zoster is characterized by unilateral radicular pain and a vesicular rash in a dermatomal pattern.1,2 It is most common in adults, especially elderly and immunocompromised patients, but rarely occurs in children. Herpes zoster is most often seen in individuals previously infected with VZV, but it also has occurred in individuals without known varicella infection,1-17 possibly because these individuals had a prior subclinical VZV infection.
A live attenuated VZV vaccine was created after isolation of the virus from a child in Japan.2 Since the introduction of the vaccine in 1995 in the United States, the incidence of VZV and HZ has declined.5 Herpes zoster rates after vaccination vary from 14 to 19 per 100,000 individuals.3,5 Breakthrough disease with the wild-type strain does occur in vaccinated children, but vaccine-strain HZ also has been reported.1-5 The risk for HZ caused by reactivated VZV vaccine in healthy children is unknown. We present a case of HZ in an otherwise healthy 19-month-old boy with no known varicella exposure who received the VZV vaccine at 13 months of age.
Case Report
An otherwise healthy 19-month-old boy presented to the dermatology clinic with a rash that began 2 days prior on the right groin and spread to the right leg. The patient’s mother denied that the child had been febrile and noted that the rash did not appear to bother him in any way. The patient was up-to-date on his vaccinations and received the first dose of the varicella series 6 months prior to presentation. He had no personal history of varicella, no exposure to sick contacts with varicella, and no known exposure to the virus. He was otherwise completely healthy with no signs or symptoms of immunocompromise.
Physical examination revealed grouped vesicles on an erythematous base on the right thigh, right sacrum, and lower abdomen that did not cross the midline (Figure). There were no other pertinent physical examination findings. The eruption was most consistent with HZ but concern remained for herpes simplex virus (HSV) or impetigo. A bacterial culture and polymerase chain reaction assay for VZV and HSV from skin swabs was ordered. The patient was prescribed acyclovir 20 mg/kg every 6 hours for 5 days. Laboratory testing revealed a positive result for VZV on polymerase chain reaction and a negative result for HSV. The majority of the patient’s lesions had crusted after 2 days of treatment with acyclovir, and the rash had nearly resolved 1 week after presentation. Subsequent evaluation with a complete blood cell count with differential and basic metabolic profile was normal. Levels of IgG, IgA, and IgM also were normal; IgE was slightly elevated.
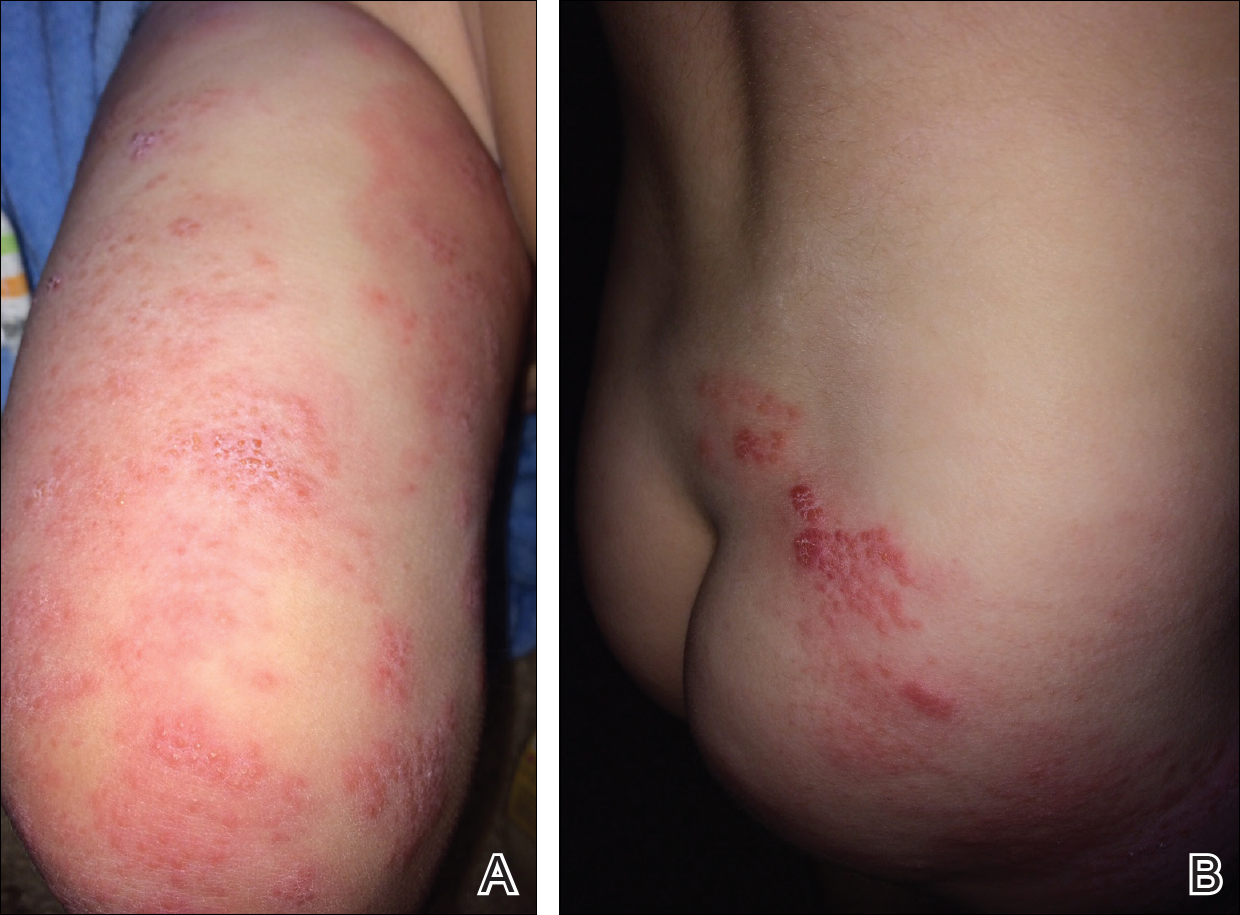
Comment
Herpes zoster in children is an uncommon clinical entity. Most children with HZ are immunocompromised, have a history of varicella, or were exposed to varicella during gestation.8 With the introduction of the live VZV vaccine, the incidence of HZ has declined, but reactivation of the live vaccine leading to HZ infection is possible. The vaccine is 90% effective, and breakthrough varicella has been reported in 15% to 20% of vaccinated patients.1-17 The cause of HZ in vaccinated children is unclear due to the potential for either wild-type or vaccine-strain VZV to induce HZ.
Twenty-two cases of HZ in healthy children after vaccination were identified with a PubMed search of articles indexed for MEDLINE using the search terms herpes zoster infection after vaccination and herpes zoster infection AND immunocompetent AND vaccination in separate searches for all English-language studies (Table). The search was limited to immunocompetent children and adolescents who were 18 years or younger with no history of varicella or exposure to varicella during gestation.
The mean age for HZ infection was 5.3 years. The average time between vaccination and HZ infection was 3.3 years. There was a spread of dermatomal patterns with cases in the first division of the trigeminal nerve, cervical, thoracic, lumbar, and sacral distributions. Of the 22 cases of HZ we reviewed, 16 underwent genotype testing to determine the source of the infection. The Oka vaccine strain virus was identified in 8 (50%) cases, while wild-type virus was found in 8 (50%) cases.1,2,4,5,7,8,10,11,13,14,16 Twelve cases were treated with acyclovir.2,3,5,6,9-12,14-17 The method of delivery, either oral or intravenous, and the length of treatment depended on the severity of the disease. Patients with meningoencephalitis and HZ ophthalmicus received intravenous acyclovir more often and also had a longer course of acyclovir compared to those individuals with involvement limited to the skin.
This review found HZ occurs from reactivation of wild-type or Oka vaccine-strain VZV in immunocompetent children.1-17 It shows that subclinical varicella infection is not the only explanation for HZ in a healthy vaccinated child. It is currently not clear why some healthy children experience HZ from vaccine-strain VZV. When HZ presents in a vaccinated immunocompetent child without a history of varicella infection or exposure, the possibility for vaccine strain–induced HZ should be considered.
- . Herpes zoster in three healthy children immunized with varicella vaccine (Oka/Biken); the causative virus differed from vaccine strain on PCR analysis of the IV variable region (R5) and of a PstI-site region. Br J Dermatol. 1997;137:255-258.
- Uebe B, Sauerbrei A, Burdach S, et al. Herpes zoster by reactivated vaccine varicella zoster virus in a healthy child [published online June 25, 2002]. Eur J Pediatr. 2002;161:442-444.
- Obieta MP, Jacinto SS. Herpes zoster after varicella vaccination in a healthy young child. Int J Dermatol. 2008;47:640-641.
- Ota K, Kim V, Lavi S, et al. Vaccine-strain varicella zoster virus causing recurrent herpes zoster in an immunocompetent 2-year-old. Pediatr Infect Dis J. 2008;27:847-848.
- Liang GL, Heidelberg KA, Jacobson RM, et al. Herpes zoster after varicella vaccination. J Am Acad Dermatol. 1998;38:761-763.
- Matsubara K, Nigami H, Harigaya H, et al. Herpes zoster in a normal child after varicella vaccination. Acta Paediatr Jpn. 1995;37:648-650.
- Kohl S, Rapp J, Larussa P, et al. Natural varicella-zoster virus reactivation shortly after varicella immunization in a child. Pediatr Infect Dis J. 1999;18:1112-1113.
- Feder HM Jr, Hoss DM. Herpes zoster in otherwise healthy children. Pediatr Infect Dis J. 2004;23:451-457; quiz 458-460.
- Binder NR, Holland GN, Hosea S, et al. Herpes zoster ophthalmicus in an otherwise-healthy child. J AAPOS. 2005;9:597-598.
- Levin MJ, DeBiasi RL, Bostik V, et al. Herpes zoster with skin lesions and meningitis caused by 2 different genotypes of the Oka varicella-zoster virus vaccine. J Infect Dis. 2008;198:1444-1447.
- Iyer S, Mittal MK, Hodinka RL. Herpes zoster and meningitis resulting from reactivation of varicella vaccine virus in an immunocompetent child. Ann Emerg Med. 2009;53:792-795.
- Lin P, Yoon MK, Chiu CS. Herpes zoster keratouveitis and inflammatory ocular hypertension 8 years after varicella vaccination. Ocul Immunol Inflamm. 2009;17:33-35.
- Chouliaras G, Spoulou V, Quinlivan M, et al. Vaccine-associated herpes zoster ophthalmicus [correction of opthalmicus] and encephalitis in an immunocompetent child [published online March 1, 2010]. Pediatrics. 2010;125:E969-E972.
- Han JY, Hanson DC, Way SS. Herpes zoster and meningitis due to reactivation of varicella vaccine virus in an immunocompetent child. Pediatr Infect Dis J. 2011;30:266-268.
- Ryu WY, Kim NY, Kwon YH, et al. Herpes zoster ophthalmicus with isolated trochlear nerve palsy in an otherwise healthy 13-year-old girl. J AAPOS. 2014;18:193-195.
- Iwasaki S, Motokura K, Honda Y, et al. Vaccine-strain herpes zoster found in the trigeminal nerve area in a healthy child: a case report [published online November 3, 2016]. J Clin Virol. 2016;85:44-47.
- Peterson N, Goodman S, Peterson M, et al. Herpes zoster in children. Cutis. 2016;98:94-95.
Varicella-zoster virus (VZV) causes varicella as a primary infection. It is a highly contagious disease characterized by a widespread papulovesicular eruption with fever and malaise.1,2 After the primary infection, the virus remains latent within the sensory dorsal root ganglia and can reactivate as herpes zoster (HZ).1-5 Herpes zoster is characterized by unilateral radicular pain and a vesicular rash in a dermatomal pattern.1,2 It is most common in adults, especially elderly and immunocompromised patients, but rarely occurs in children. Herpes zoster is most often seen in individuals previously infected with VZV, but it also has occurred in individuals without known varicella infection,1-17 possibly because these individuals had a prior subclinical VZV infection.
A live attenuated VZV vaccine was created after isolation of the virus from a child in Japan.2 Since the introduction of the vaccine in 1995 in the United States, the incidence of VZV and HZ has declined.5 Herpes zoster rates after vaccination vary from 14 to 19 per 100,000 individuals.3,5 Breakthrough disease with the wild-type strain does occur in vaccinated children, but vaccine-strain HZ also has been reported.1-5 The risk for HZ caused by reactivated VZV vaccine in healthy children is unknown. We present a case of HZ in an otherwise healthy 19-month-old boy with no known varicella exposure who received the VZV vaccine at 13 months of age.
Case Report
An otherwise healthy 19-month-old boy presented to the dermatology clinic with a rash that began 2 days prior on the right groin and spread to the right leg. The patient’s mother denied that the child had been febrile and noted that the rash did not appear to bother him in any way. The patient was up-to-date on his vaccinations and received the first dose of the varicella series 6 months prior to presentation. He had no personal history of varicella, no exposure to sick contacts with varicella, and no known exposure to the virus. He was otherwise completely healthy with no signs or symptoms of immunocompromise.
Physical examination revealed grouped vesicles on an erythematous base on the right thigh, right sacrum, and lower abdomen that did not cross the midline (Figure). There were no other pertinent physical examination findings. The eruption was most consistent with HZ but concern remained for herpes simplex virus (HSV) or impetigo. A bacterial culture and polymerase chain reaction assay for VZV and HSV from skin swabs was ordered. The patient was prescribed acyclovir 20 mg/kg every 6 hours for 5 days. Laboratory testing revealed a positive result for VZV on polymerase chain reaction and a negative result for HSV. The majority of the patient’s lesions had crusted after 2 days of treatment with acyclovir, and the rash had nearly resolved 1 week after presentation. Subsequent evaluation with a complete blood cell count with differential and basic metabolic profile was normal. Levels of IgG, IgA, and IgM also were normal; IgE was slightly elevated.

Comment
Herpes zoster in children is an uncommon clinical entity. Most children with HZ are immunocompromised, have a history of varicella, or were exposed to varicella during gestation.8 With the introduction of the live VZV vaccine, the incidence of HZ has declined, but reactivation of the live vaccine leading to HZ infection is possible. The vaccine is 90% effective, and breakthrough varicella has been reported in 15% to 20% of vaccinated patients.1-17 The cause of HZ in vaccinated children is unclear due to the potential for either wild-type or vaccine-strain VZV to induce HZ.
Twenty-two cases of HZ in healthy children after vaccination were identified with a PubMed search of articles indexed for MEDLINE using the search terms herpes zoster infection after vaccination and herpes zoster infection AND immunocompetent AND vaccination in separate searches for all English-language studies (Table). The search was limited to immunocompetent children and adolescents who were 18 years or younger with no history of varicella or exposure to varicella during gestation.

The mean age for HZ infection was 5.3 years. The average time between vaccination and HZ infection was 3.3 years. There was a spread of dermatomal patterns with cases in the first division of the trigeminal nerve, cervical, thoracic, lumbar, and sacral distributions. Of the 22 cases of HZ we reviewed, 16 underwent genotype testing to determine the source of the infection. The Oka vaccine strain virus was identified in 8 (50%) cases, while wild-type virus was found in 8 (50%) cases.1,2,4,5,7,8,10,11,13,14,16 Twelve cases were treated with acyclovir.2,3,5,6,9-12,14-17 The method of delivery, either oral or intravenous, and the length of treatment depended on the severity of the disease. Patients with meningoencephalitis and HZ ophthalmicus received intravenous acyclovir more often and also had a longer course of acyclovir compared to those individuals with involvement limited to the skin.
This review found HZ occurs from reactivation of wild-type or Oka vaccine-strain VZV in immunocompetent children.1-17 It shows that subclinical varicella infection is not the only explanation for HZ in a healthy vaccinated child. It is currently not clear why some healthy children experience HZ from vaccine-strain VZV. When HZ presents in a vaccinated immunocompetent child without a history of varicella infection or exposure, the possibility for vaccine strain–induced HZ should be considered.
Varicella-zoster virus (VZV) causes varicella as a primary infection. It is a highly contagious disease characterized by a widespread papulovesicular eruption with fever and malaise.1,2 After the primary infection, the virus remains latent within the sensory dorsal root ganglia and can reactivate as herpes zoster (HZ).1-5 Herpes zoster is characterized by unilateral radicular pain and a vesicular rash in a dermatomal pattern.1,2 It is most common in adults, especially elderly and immunocompromised patients, but rarely occurs in children. Herpes zoster is most often seen in individuals previously infected with VZV, but it also has occurred in individuals without known varicella infection,1-17 possibly because these individuals had a prior subclinical VZV infection.
A live attenuated VZV vaccine was created after isolation of the virus from a child in Japan.2 Since the introduction of the vaccine in 1995 in the United States, the incidence of VZV and HZ has declined.5 Herpes zoster rates after vaccination vary from 14 to 19 per 100,000 individuals.3,5 Breakthrough disease with the wild-type strain does occur in vaccinated children, but vaccine-strain HZ also has been reported.1-5 The risk for HZ caused by reactivated VZV vaccine in healthy children is unknown. We present a case of HZ in an otherwise healthy 19-month-old boy with no known varicella exposure who received the VZV vaccine at 13 months of age.
Case Report
An otherwise healthy 19-month-old boy presented to the dermatology clinic with a rash that began 2 days prior on the right groin and spread to the right leg. The patient’s mother denied that the child had been febrile and noted that the rash did not appear to bother him in any way. The patient was up-to-date on his vaccinations and received the first dose of the varicella series 6 months prior to presentation. He had no personal history of varicella, no exposure to sick contacts with varicella, and no known exposure to the virus. He was otherwise completely healthy with no signs or symptoms of immunocompromise.
Physical examination revealed grouped vesicles on an erythematous base on the right thigh, right sacrum, and lower abdomen that did not cross the midline (Figure). There were no other pertinent physical examination findings. The eruption was most consistent with HZ but concern remained for herpes simplex virus (HSV) or impetigo. A bacterial culture and polymerase chain reaction assay for VZV and HSV from skin swabs was ordered. The patient was prescribed acyclovir 20 mg/kg every 6 hours for 5 days. Laboratory testing revealed a positive result for VZV on polymerase chain reaction and a negative result for HSV. The majority of the patient’s lesions had crusted after 2 days of treatment with acyclovir, and the rash had nearly resolved 1 week after presentation. Subsequent evaluation with a complete blood cell count with differential and basic metabolic profile was normal. Levels of IgG, IgA, and IgM also were normal; IgE was slightly elevated.

Comment
Herpes zoster in children is an uncommon clinical entity. Most children with HZ are immunocompromised, have a history of varicella, or were exposed to varicella during gestation.8 With the introduction of the live VZV vaccine, the incidence of HZ has declined, but reactivation of the live vaccine leading to HZ infection is possible. The vaccine is 90% effective, and breakthrough varicella has been reported in 15% to 20% of vaccinated patients.1-17 The cause of HZ in vaccinated children is unclear due to the potential for either wild-type or vaccine-strain VZV to induce HZ.
Twenty-two cases of HZ in healthy children after vaccination were identified with a PubMed search of articles indexed for MEDLINE using the search terms herpes zoster infection after vaccination and herpes zoster infection AND immunocompetent AND vaccination in separate searches for all English-language studies (Table). The search was limited to immunocompetent children and adolescents who were 18 years or younger with no history of varicella or exposure to varicella during gestation.

The mean age for HZ infection was 5.3 years. The average time between vaccination and HZ infection was 3.3 years. There was a spread of dermatomal patterns with cases in the first division of the trigeminal nerve, cervical, thoracic, lumbar, and sacral distributions. Of the 22 cases of HZ we reviewed, 16 underwent genotype testing to determine the source of the infection. The Oka vaccine strain virus was identified in 8 (50%) cases, while wild-type virus was found in 8 (50%) cases.1,2,4,5,7,8,10,11,13,14,16 Twelve cases were treated with acyclovir.2,3,5,6,9-12,14-17 The method of delivery, either oral or intravenous, and the length of treatment depended on the severity of the disease. Patients with meningoencephalitis and HZ ophthalmicus received intravenous acyclovir more often and also had a longer course of acyclovir compared to those individuals with involvement limited to the skin.
This review found HZ occurs from reactivation of wild-type or Oka vaccine-strain VZV in immunocompetent children.1-17 It shows that subclinical varicella infection is not the only explanation for HZ in a healthy vaccinated child. It is currently not clear why some healthy children experience HZ from vaccine-strain VZV. When HZ presents in a vaccinated immunocompetent child without a history of varicella infection or exposure, the possibility for vaccine strain–induced HZ should be considered.
- . Herpes zoster in three healthy children immunized with varicella vaccine (Oka/Biken); the causative virus differed from vaccine strain on PCR analysis of the IV variable region (R5) and of a PstI-site region. Br J Dermatol. 1997;137:255-258.
- Uebe B, Sauerbrei A, Burdach S, et al. Herpes zoster by reactivated vaccine varicella zoster virus in a healthy child [published online June 25, 2002]. Eur J Pediatr. 2002;161:442-444.
- Obieta MP, Jacinto SS. Herpes zoster after varicella vaccination in a healthy young child. Int J Dermatol. 2008;47:640-641.
- Ota K, Kim V, Lavi S, et al. Vaccine-strain varicella zoster virus causing recurrent herpes zoster in an immunocompetent 2-year-old. Pediatr Infect Dis J. 2008;27:847-848.
- Liang GL, Heidelberg KA, Jacobson RM, et al. Herpes zoster after varicella vaccination. J Am Acad Dermatol. 1998;38:761-763.
- Matsubara K, Nigami H, Harigaya H, et al. Herpes zoster in a normal child after varicella vaccination. Acta Paediatr Jpn. 1995;37:648-650.
- Kohl S, Rapp J, Larussa P, et al. Natural varicella-zoster virus reactivation shortly after varicella immunization in a child. Pediatr Infect Dis J. 1999;18:1112-1113.
- Feder HM Jr, Hoss DM. Herpes zoster in otherwise healthy children. Pediatr Infect Dis J. 2004;23:451-457; quiz 458-460.
- Binder NR, Holland GN, Hosea S, et al. Herpes zoster ophthalmicus in an otherwise-healthy child. J AAPOS. 2005;9:597-598.
- Levin MJ, DeBiasi RL, Bostik V, et al. Herpes zoster with skin lesions and meningitis caused by 2 different genotypes of the Oka varicella-zoster virus vaccine. J Infect Dis. 2008;198:1444-1447.
- Iyer S, Mittal MK, Hodinka RL. Herpes zoster and meningitis resulting from reactivation of varicella vaccine virus in an immunocompetent child. Ann Emerg Med. 2009;53:792-795.
- Lin P, Yoon MK, Chiu CS. Herpes zoster keratouveitis and inflammatory ocular hypertension 8 years after varicella vaccination. Ocul Immunol Inflamm. 2009;17:33-35.
- Chouliaras G, Spoulou V, Quinlivan M, et al. Vaccine-associated herpes zoster ophthalmicus [correction of opthalmicus] and encephalitis in an immunocompetent child [published online March 1, 2010]. Pediatrics. 2010;125:E969-E972.
- Han JY, Hanson DC, Way SS. Herpes zoster and meningitis due to reactivation of varicella vaccine virus in an immunocompetent child. Pediatr Infect Dis J. 2011;30:266-268.
- Ryu WY, Kim NY, Kwon YH, et al. Herpes zoster ophthalmicus with isolated trochlear nerve palsy in an otherwise healthy 13-year-old girl. J AAPOS. 2014;18:193-195.
- Iwasaki S, Motokura K, Honda Y, et al. Vaccine-strain herpes zoster found in the trigeminal nerve area in a healthy child: a case report [published online November 3, 2016]. J Clin Virol. 2016;85:44-47.
- Peterson N, Goodman S, Peterson M, et al. Herpes zoster in children. Cutis. 2016;98:94-95.
- . Herpes zoster in three healthy children immunized with varicella vaccine (Oka/Biken); the causative virus differed from vaccine strain on PCR analysis of the IV variable region (R5) and of a PstI-site region. Br J Dermatol. 1997;137:255-258.
- Uebe B, Sauerbrei A, Burdach S, et al. Herpes zoster by reactivated vaccine varicella zoster virus in a healthy child [published online June 25, 2002]. Eur J Pediatr. 2002;161:442-444.
- Obieta MP, Jacinto SS. Herpes zoster after varicella vaccination in a healthy young child. Int J Dermatol. 2008;47:640-641.
- Ota K, Kim V, Lavi S, et al. Vaccine-strain varicella zoster virus causing recurrent herpes zoster in an immunocompetent 2-year-old. Pediatr Infect Dis J. 2008;27:847-848.
- Liang GL, Heidelberg KA, Jacobson RM, et al. Herpes zoster after varicella vaccination. J Am Acad Dermatol. 1998;38:761-763.
- Matsubara K, Nigami H, Harigaya H, et al. Herpes zoster in a normal child after varicella vaccination. Acta Paediatr Jpn. 1995;37:648-650.
- Kohl S, Rapp J, Larussa P, et al. Natural varicella-zoster virus reactivation shortly after varicella immunization in a child. Pediatr Infect Dis J. 1999;18:1112-1113.
- Feder HM Jr, Hoss DM. Herpes zoster in otherwise healthy children. Pediatr Infect Dis J. 2004;23:451-457; quiz 458-460.
- Binder NR, Holland GN, Hosea S, et al. Herpes zoster ophthalmicus in an otherwise-healthy child. J AAPOS. 2005;9:597-598.
- Levin MJ, DeBiasi RL, Bostik V, et al. Herpes zoster with skin lesions and meningitis caused by 2 different genotypes of the Oka varicella-zoster virus vaccine. J Infect Dis. 2008;198:1444-1447.
- Iyer S, Mittal MK, Hodinka RL. Herpes zoster and meningitis resulting from reactivation of varicella vaccine virus in an immunocompetent child. Ann Emerg Med. 2009;53:792-795.
- Lin P, Yoon MK, Chiu CS. Herpes zoster keratouveitis and inflammatory ocular hypertension 8 years after varicella vaccination. Ocul Immunol Inflamm. 2009;17:33-35.
- Chouliaras G, Spoulou V, Quinlivan M, et al. Vaccine-associated herpes zoster ophthalmicus [correction of opthalmicus] and encephalitis in an immunocompetent child [published online March 1, 2010]. Pediatrics. 2010;125:E969-E972.
- Han JY, Hanson DC, Way SS. Herpes zoster and meningitis due to reactivation of varicella vaccine virus in an immunocompetent child. Pediatr Infect Dis J. 2011;30:266-268.
- Ryu WY, Kim NY, Kwon YH, et al. Herpes zoster ophthalmicus with isolated trochlear nerve palsy in an otherwise healthy 13-year-old girl. J AAPOS. 2014;18:193-195.
- Iwasaki S, Motokura K, Honda Y, et al. Vaccine-strain herpes zoster found in the trigeminal nerve area in a healthy child: a case report [published online November 3, 2016]. J Clin Virol. 2016;85:44-47.
- Peterson N, Goodman S, Peterson M, et al. Herpes zoster in children. Cutis. 2016;98:94-95.
Practice Points
- Most children with herpes zoster are immunocompromised, have a history of varicella, or were exposed to varicella in utero.
- Herpes zoster has been reported in immunocompetent children due to either wild-type or vaccine-strain varicella-zoster virus.
Shedding Light on Onychomadesis
Onychomadesis is an acute, noninflammatory, painless, proximal separation of the nail plate from the nail matrix. It occurs due to an abrupt stoppage of nail production by matrix cells, producing temporary cessation of nail growth with or without subsequent complete shedding of nails.1-10 Onychomadesis has a wide spectrum of clinical presentations ranging from mild transverse ridges of the nail plate (Beau lines) to complete nail shedding.4,11 Onychomadesis may be related to systemic and dermatologic diseases, drugs (eg, chemotherapeutic agents, anticonvulsants, lithium, retinoids), nail trauma, fever, or infection,5 and a connection between onychomadesis and hand-foot-and-mouth disease (HFMD) was first described by Clementz et al12 following outbreaks in Europe, Asia, and the United States.
Epidemiology
Onychomadesis has been observed in children of all ages including neonates. Neonatal onychomadesis is thought to be related to perinatal stressors and birth trauma, with possible exacerbation by superimposed candidiasis.10 Depending on the underlying cause, there may be involvement of a single nail or multiple nails. Nag et al1 noted that onychomadesis was most commonly observed in nails of the middle finger (73.7%), followed by the thumb (63.2%) and ring finger (52.6%). Fingernails are more commonly involved than toenails.1
Clementz et al12 first proposed the association between onychomadesis and HFMD in 2000. Patients with a history of HFMD were found to be 14 times more likely to develop onychomadesis (relative risk, 14; 95% confidence interval, 4.57-42.86).4 A common pathogen for HFMD is coxsackievirus A6 (CVA6),13,14 but the mechanism of onychomadesis in HFMD remains unclear.5,7,13 Outbreaks of HFMD have been reported in Spain, Finland, Japan, Thailand, the United States, Singapore, and China.15 During an outbreak of HFMD in Taiwan, the incidence of onychomadesis following CVA6 infection was 37% (48/130) compared to 5% (7/145) in cases with non-CVA6 causative strains.16 There also have been observed differences in the prevalence of onychomadesis by age: a 55% (18/33) occurrence rate was noted in the youngest age group (range, 9–23 months), 30% (8/27) in the middle age group (range, 24–32 months), and 4% (1/28) in the oldest age group (range, 33–42 months), with an average of 4 nails shed per case.17 A study in Spain also found a high occurrence of onychomadesis in a nursery setting, with 92% (11/12) of onychomadesis cases preceded by HFMD 2 months prior.18
Etiology
Local trauma to the nail bed is the most common cause of single-digit onychomadesis.4 Multiple-digit involvement suggests a systemic etiology such as fever, erythroderma, and Kawasaki disease; use of drugs (eg, chemotherapeutic agents, anticonvulsants, lithium, retinoids); and viral infections such as HFMD and varicella at the infantile age (Table).5,9,19 Most drug-related nail changes are the outcome of acute toxicity to the proliferating nail matrix epithelium. If onychomadesis affects all nails at the same level, the patient’s history of medication use and other treatments taken 2 to 3 weeks prior to the appearance of the nail findings should be evaluated. Chemotherapeutic agents produce nail changes in a high proportion of patients, which often are related to drug dosage. These effects also are reproducible with re-administration of the drug.20 Onychomadesis also has been reported as a possible side effect of anticonvulsants such as valproic acid (VPA).21 One study evaluating the link between VPA and onychomadesis indicated that nail changes may be due to a disturbance of zinc metabolism.22 However, the pathomechanism of onychomadesis associated with VPA treatment remains unclear.21 Onychomadesis also has developed after an allergic drug reaction to oral penicillin V after treatment of a sore throat in a 23-month-old child.23
Nail involvement has been reported in 10% of cases of inflammatory conditions such as lichen planus21; however, it may be more common but underrecognized and underreported. Grover et al9 indicated that lichen planus–induced severe inflammation in the matrix of the nail unit leading to a temporary growth arrest was the possible mechanism leading to nail shedding. Prompt systemic and intramatricial steroid treatment of lichen planus is required to avoid potential scarring of the nail matrix and permanent damage.9
Onychomadesis also has been reported following varicella infection (chickenpox). Podder et al19 reported the case of a 7-year-old girl who had recovered from a varicella infection 5 weeks prior and presented with onychomadesis of the right index fingernail with all other fingernails and toenails appearing normal. Kocak and Koçak5 reported onychomadesis in 2 sisters with varicella infection. There are few reported cases, so it is still unclear whether varicella infection is an inciting factor.19
One of the most studied viral infections linked to onychomadesis is HFMD, which is a common viral infection that mostly affects children younger than 10 years.1 The precise mechanism of onychomadesis for these viral infection events remains unclear.7,10,13 Several theories have been delineated, including nail matrix arrest from fever occurring during HFMD.6 However, this cause is unlikely, as fevers are typically low grade and present only for a few hours.4,6,13 Direct inflammation spreading from skin lesions of HFMD around the nails or maceration associated with finger blisters could cause onychomadesis.1,5,7 Haneke24 hypothesized that nail shedding may be the consequence of vesicles localized in the periungual tissue, but studies have shown incidence without prior lesions on the fingers and no relationship between nail matrix arrest and severity of HFMD.5,6,13 Bettoli et al25 reported that inflammation secondary to viral infection around the nail matrix might be induced directly by viruses or indirectly by virus-specific immunocomplexes and consequent distal embolism. Osterback et al14 used reverse transcription–polymerase chain reaction to detect CVA6 in fragmented nails from 2 children and 1 parent following an HFMD episode, suggesting that virus replication could damage the nail matrix, resulting in onychomadesis. Cabrerizo et al18 also suggested that virus replication directly damages the nail matrix based on the presence of CVA6 in shed nails. Because fingernails with onychomadesis are not always of the fingers affected by HFMD, an indirect effect of viral infection on the nail matrix is more plausible.8 Additional studies are needed to clarify the virus-associated mechanism of nail matrix arrest.6 Finally, frequent washing of hands15 resulting in maceration, Candida infection, and allergic contact dermatitis2 may be possible causes. It is unclear if onychomadesis following HFMD is related to viral replication, inflammation, or intensive hygienic measures, and further investigation is needed.2,15
Clinical Characteristics
The ventral floor is the site of the germinal matrix and is responsible for 90% of nail production. As a result, more of the nail plate substance is produced proximally, leading to a natural convex curvature from the proximal to distal nail.11 Beau lines are transverse ridging of the nail plates.6 Onychomadesis may be viewed as a more severe form of Beau lines, with complete separation and possible shedding of the nail plate (Figure).3,4 In both cases, an insult to the nail matrix is followed by recovery and production of the nail plate at the nail matrix.4 In Beau lines, slowing or disruption of cell growth from the proximal matrix results in a thinner nail plate, leading to transverse depressions. Onychomadesis has a similar pathophysiology but is associated with a complete halt in the nail plate production.3
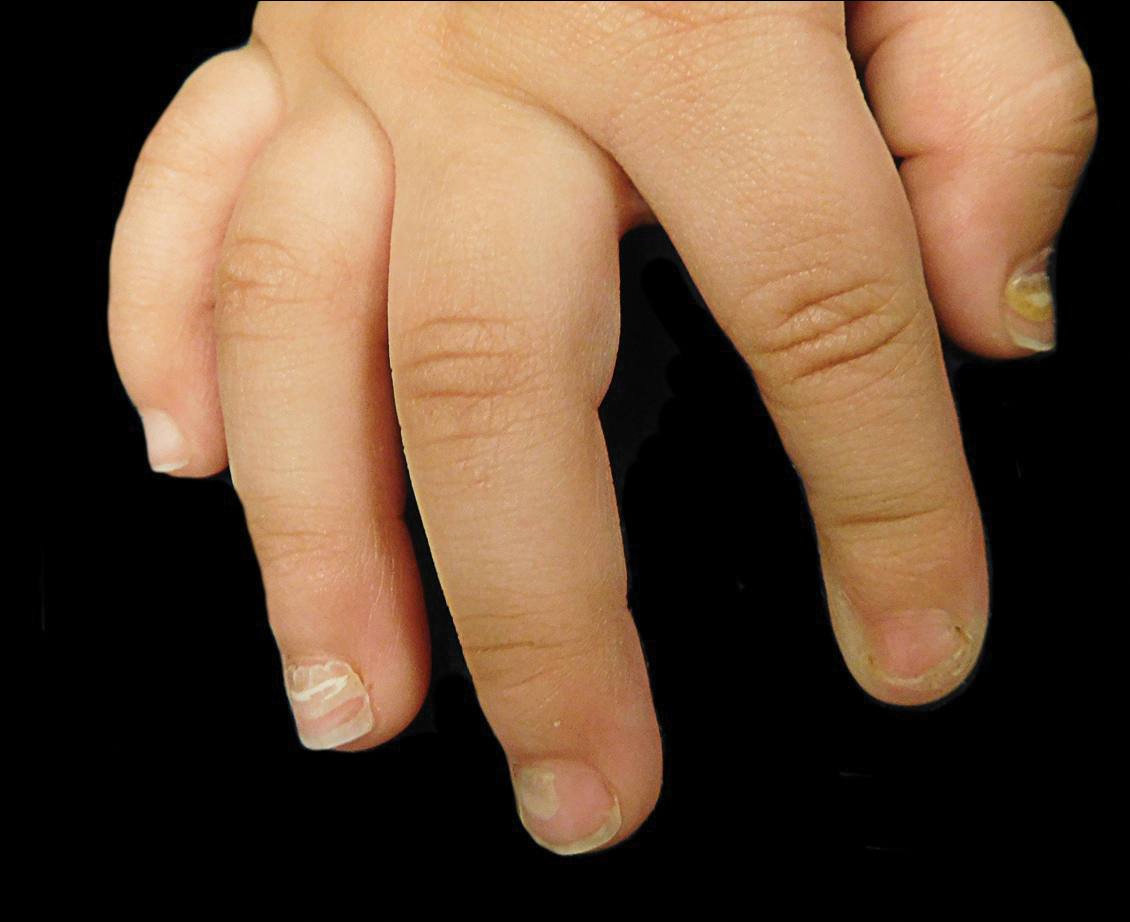
Diagnosis
The diagnosis of onychomadesis is made clinically.3,10 Distinct nail changes can be detected by inspection and palpation of the nail plate,3,11 which allows for differentiation between Beau lines and complete nail shedding. Additionally, any signs of nail trauma need to be noted, as well as pain, swelling, or pruritus, as these symptoms also can guide in determining the etiology of the nail dystrophy. Ultrasonography can confirm the diagnosis, as the defect can be identified beneath the proximal nail fold.3,26 When it occurs after HFMD or varicella, onychomadesis tends to present in 28 to 40 days following infection.4,6,10 Physicians should consider underlying associations. A review of viral illnesses within 1 to 2 months prior to development of nail changes often will identify the causative disease.4 Each patient should be evaluated for recent nail trauma; medications; viral infection; and autoimmune, systemic, and inflammatory diseases.
Treatment
Onychomadesis typically is mild and self-limited.4,10 There is no specific treatment,10 but a conservative approach to management is recommended. Treatment of any underlying medical conditions or discontinuation of an offending medication may help to prevent recurrent onychomadesis.3 Supportive care along with protection of the nail bed by maintaining short nails and using adhesive bandages over the affected nails to avoid snagging the nail or ripping off the partially attached nails is recommended.4 In some cases, onychomadesis has been treated with topical application of urea cream 40% under occlusion27 or halcinonide cream 0.1% under occlusion for 5 to 6 days,28 but these treatments have not been universally effective.3 External use of basic fibroblast growth factor to stimulate new regrowth of the nail plate has been advocated.3 It is important to reassure patients that as long as the underlying causes are eliminated and the nail matrix has not been permanently scarred, the nails should grow back within 12 weeks or sooner in children. Thus, typically only reassurance and counseling of parents/guardians is required for onychomadesis in children.1,2 However, the nails may be dystrophic or fail to regrow if there is poor peripheral circulation or permanent nail matrix damage.
Conclusion
Fortunately, onychomadesis is self-limited. Physicians should look for underlying causes of onychomadesis, including a history of viral infections such as HFMD and varicella as well as systemic diseases and use of medications. As long as any underlying disorder or condition has been resolved, spontaneous regrowth of healthy nails usually but not always occurs within 12 weeks or sooner in children.
- Nag SS, Dutta A, Mandal RK. Delayed cutaneous findings of hand, foot, and mouth disease. Indian Pediatr. 2016;53:42-44.
- Tan ZH, Koh MJ. Nail shedding following hand, foot and mouth disease. Arch Dis Child. 2013;98:665.
- Braswell MA, Daniel CR, Brodell RT. Beau lines, onychomadesis, and retronychia: a unifying hypothesis. J Am Acad Dermatol. 2015;73:849-855.
- Clark CM, Silverberg NB, Weinberg JM. What is your diagnosis? onychomadesis following hand-foot-and-mouth disease. Cutis. 2015;95:312, 319-320.
- Kocak AY, Koçak O. Onychomadesis in two sisters induced by varicella infection. Pediatr Dermatol. 2013;30:E108-E109.
- Shin JY, Cho BK, Park HJ. A clinical study of nail changes occurring secondary to hand-foot-mouth disease: onychomadesis and Beau’s lines. Ann Dermatol. 2014;26:280-283.
- Shikuma E, Endo Y, Fujisawa A, et al. Onychomadesis developed only on the nails having cutaneous lesions of severe hand-foot-mouth disease. Case Rep Dermatol Med. 2011;2011:324193.
- Kim EJ, Park HS, Yoon HS, et al. Four cases of onychomadesis after hand-foot-mouth disease. Ann Dermatol. 2014;26:777-778.
- Grover C, Vohra S. Onychomadesis with lichen planus: an under-recognized manifestation. Indian J Dermatol. 2015;60:420.
- Chu DH, Rubin AI. Diagnosis and management of nail disorders. In: Holland K, ed. The Pediatric Clinics of North America. Vol 61. Philadelphia, PA: Elsevier; 2014:301-302.
- Kowalewski C, Schwartz RA. Components, growth, and composition of the nail. In: Demis D, ed. Clinical Dermatology. Philadelphia, PA: Lippincott-Raven; 1998.
- Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11.
- Scarfì F, Arunachalam M, Galeone M, et al. An uncommon onychomadesis in adults. Int J Dermatol. 2014;53:1392-1394.
- Osterback R, Vuorinen T, Linna M, et al. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis. 2009;15:1485-1488.
- Yan X, Zhang ZZ, Yang ZH, et al. Clinical and etiological characteristics of atypical hand-foot-and-mouth disease in children from Chongqing, China: a retrospective study [published online November 26, 2015]. Biomed Res Int. 2015;2015:802046.
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis. 2011;11:346.
- Guimbao J, Rodrigo P, Alberto MJ, et al. Onychomadesis outbreak linked to hand, foot, and mouth disease, Spain, July 2008. Euro Surveill. 2010;15:19663.
- Cabrerizo M, De Miguel T, Armada A, et al. Onychomadesis after a hand, foot, and mouth disease outbreak in Spain, 2009. Epidemiol Infect. 2010;138:1775-1778.
- Podder I, Das A, Gharami RC. Onychomadesis following varicella infection: is it a mere co-incidence? Indian J Dermatol. 2015;60:626-627.
- Piraccini BM, Iorizzo M, Tosti A. Drug-induced nail abnormalities. Am J Clin Dermatol. 2003;4:31-37.
- Poretti A, Lips U, Belvedere M, et al. Onychomadesis: a rare side-effect of valproic acid medication? Pediatr Dermatol. 2009;26:749-750.
- Grech V, Vella C. Generalized onycholoysis associated with sodium valproate therapy. Eur Neurol. 1999;42:64-65.
- Shah RK, Uddin M, Fatunde OJ. Onychomadesis secondary to penicillin allergy in a child. J Pediatr. 2012;161:166.
- Haneke E. Onychomadesis and hand, foot and mouth disease—is there a connection? Euro Surveill. 2010;15(37).
- Bettoli V, Zauli S, Toni G, et al. Onychomadesis following hand, foot, and mouth disease: a case report from Italy and review of the literature. Int J Dermatol. 2013;52:728-730.
- Wortsman X, Wortsman J, Guerrero R, et al. Anatomical changes in retronychia and onychomadesis detected using ultrasound. Dermatol Surg. 2010;36:1615-1620.
- Fleming CJ, Hunt MJ, Barnetson RS. Mycosis fungoides with onychomadesis. Br J Dermatol. 1996;135:1012-1013.
- Mishra D, Singh G, Pandey SS. Possible carbamazepine-induced reversible onychomadesis. Int J Dermatol. 1989;28:460-461.
Onychomadesis is an acute, noninflammatory, painless, proximal separation of the nail plate from the nail matrix. It occurs due to an abrupt stoppage of nail production by matrix cells, producing temporary cessation of nail growth with or without subsequent complete shedding of nails.1-10 Onychomadesis has a wide spectrum of clinical presentations ranging from mild transverse ridges of the nail plate (Beau lines) to complete nail shedding.4,11 Onychomadesis may be related to systemic and dermatologic diseases, drugs (eg, chemotherapeutic agents, anticonvulsants, lithium, retinoids), nail trauma, fever, or infection,5 and a connection between onychomadesis and hand-foot-and-mouth disease (HFMD) was first described by Clementz et al12 following outbreaks in Europe, Asia, and the United States.
Epidemiology
Onychomadesis has been observed in children of all ages including neonates. Neonatal onychomadesis is thought to be related to perinatal stressors and birth trauma, with possible exacerbation by superimposed candidiasis.10 Depending on the underlying cause, there may be involvement of a single nail or multiple nails. Nag et al1 noted that onychomadesis was most commonly observed in nails of the middle finger (73.7%), followed by the thumb (63.2%) and ring finger (52.6%). Fingernails are more commonly involved than toenails.1
Clementz et al12 first proposed the association between onychomadesis and HFMD in 2000. Patients with a history of HFMD were found to be 14 times more likely to develop onychomadesis (relative risk, 14; 95% confidence interval, 4.57-42.86).4 A common pathogen for HFMD is coxsackievirus A6 (CVA6),13,14 but the mechanism of onychomadesis in HFMD remains unclear.5,7,13 Outbreaks of HFMD have been reported in Spain, Finland, Japan, Thailand, the United States, Singapore, and China.15 During an outbreak of HFMD in Taiwan, the incidence of onychomadesis following CVA6 infection was 37% (48/130) compared to 5% (7/145) in cases with non-CVA6 causative strains.16 There also have been observed differences in the prevalence of onychomadesis by age: a 55% (18/33) occurrence rate was noted in the youngest age group (range, 9–23 months), 30% (8/27) in the middle age group (range, 24–32 months), and 4% (1/28) in the oldest age group (range, 33–42 months), with an average of 4 nails shed per case.17 A study in Spain also found a high occurrence of onychomadesis in a nursery setting, with 92% (11/12) of onychomadesis cases preceded by HFMD 2 months prior.18
Etiology
Local trauma to the nail bed is the most common cause of single-digit onychomadesis.4 Multiple-digit involvement suggests a systemic etiology such as fever, erythroderma, and Kawasaki disease; use of drugs (eg, chemotherapeutic agents, anticonvulsants, lithium, retinoids); and viral infections such as HFMD and varicella at the infantile age (Table).5,9,19 Most drug-related nail changes are the outcome of acute toxicity to the proliferating nail matrix epithelium. If onychomadesis affects all nails at the same level, the patient’s history of medication use and other treatments taken 2 to 3 weeks prior to the appearance of the nail findings should be evaluated. Chemotherapeutic agents produce nail changes in a high proportion of patients, which often are related to drug dosage. These effects also are reproducible with re-administration of the drug.20 Onychomadesis also has been reported as a possible side effect of anticonvulsants such as valproic acid (VPA).21 One study evaluating the link between VPA and onychomadesis indicated that nail changes may be due to a disturbance of zinc metabolism.22 However, the pathomechanism of onychomadesis associated with VPA treatment remains unclear.21 Onychomadesis also has developed after an allergic drug reaction to oral penicillin V after treatment of a sore throat in a 23-month-old child.23
Nail involvement has been reported in 10% of cases of inflammatory conditions such as lichen planus21; however, it may be more common but underrecognized and underreported. Grover et al9 indicated that lichen planus–induced severe inflammation in the matrix of the nail unit leading to a temporary growth arrest was the possible mechanism leading to nail shedding. Prompt systemic and intramatricial steroid treatment of lichen planus is required to avoid potential scarring of the nail matrix and permanent damage.9
Onychomadesis also has been reported following varicella infection (chickenpox). Podder et al19 reported the case of a 7-year-old girl who had recovered from a varicella infection 5 weeks prior and presented with onychomadesis of the right index fingernail with all other fingernails and toenails appearing normal. Kocak and Koçak5 reported onychomadesis in 2 sisters with varicella infection. There are few reported cases, so it is still unclear whether varicella infection is an inciting factor.19
One of the most studied viral infections linked to onychomadesis is HFMD, which is a common viral infection that mostly affects children younger than 10 years.1 The precise mechanism of onychomadesis for these viral infection events remains unclear.7,10,13 Several theories have been delineated, including nail matrix arrest from fever occurring during HFMD.6 However, this cause is unlikely, as fevers are typically low grade and present only for a few hours.4,6,13 Direct inflammation spreading from skin lesions of HFMD around the nails or maceration associated with finger blisters could cause onychomadesis.1,5,7 Haneke24 hypothesized that nail shedding may be the consequence of vesicles localized in the periungual tissue, but studies have shown incidence without prior lesions on the fingers and no relationship between nail matrix arrest and severity of HFMD.5,6,13 Bettoli et al25 reported that inflammation secondary to viral infection around the nail matrix might be induced directly by viruses or indirectly by virus-specific immunocomplexes and consequent distal embolism. Osterback et al14 used reverse transcription–polymerase chain reaction to detect CVA6 in fragmented nails from 2 children and 1 parent following an HFMD episode, suggesting that virus replication could damage the nail matrix, resulting in onychomadesis. Cabrerizo et al18 also suggested that virus replication directly damages the nail matrix based on the presence of CVA6 in shed nails. Because fingernails with onychomadesis are not always of the fingers affected by HFMD, an indirect effect of viral infection on the nail matrix is more plausible.8 Additional studies are needed to clarify the virus-associated mechanism of nail matrix arrest.6 Finally, frequent washing of hands15 resulting in maceration, Candida infection, and allergic contact dermatitis2 may be possible causes. It is unclear if onychomadesis following HFMD is related to viral replication, inflammation, or intensive hygienic measures, and further investigation is needed.2,15
Clinical Characteristics
The ventral floor is the site of the germinal matrix and is responsible for 90% of nail production. As a result, more of the nail plate substance is produced proximally, leading to a natural convex curvature from the proximal to distal nail.11 Beau lines are transverse ridging of the nail plates.6 Onychomadesis may be viewed as a more severe form of Beau lines, with complete separation and possible shedding of the nail plate (Figure).3,4 In both cases, an insult to the nail matrix is followed by recovery and production of the nail plate at the nail matrix.4 In Beau lines, slowing or disruption of cell growth from the proximal matrix results in a thinner nail plate, leading to transverse depressions. Onychomadesis has a similar pathophysiology but is associated with a complete halt in the nail plate production.3

Diagnosis
The diagnosis of onychomadesis is made clinically.3,10 Distinct nail changes can be detected by inspection and palpation of the nail plate,3,11 which allows for differentiation between Beau lines and complete nail shedding. Additionally, any signs of nail trauma need to be noted, as well as pain, swelling, or pruritus, as these symptoms also can guide in determining the etiology of the nail dystrophy. Ultrasonography can confirm the diagnosis, as the defect can be identified beneath the proximal nail fold.3,26 When it occurs after HFMD or varicella, onychomadesis tends to present in 28 to 40 days following infection.4,6,10 Physicians should consider underlying associations. A review of viral illnesses within 1 to 2 months prior to development of nail changes often will identify the causative disease.4 Each patient should be evaluated for recent nail trauma; medications; viral infection; and autoimmune, systemic, and inflammatory diseases.
Treatment
Onychomadesis typically is mild and self-limited.4,10 There is no specific treatment,10 but a conservative approach to management is recommended. Treatment of any underlying medical conditions or discontinuation of an offending medication may help to prevent recurrent onychomadesis.3 Supportive care along with protection of the nail bed by maintaining short nails and using adhesive bandages over the affected nails to avoid snagging the nail or ripping off the partially attached nails is recommended.4 In some cases, onychomadesis has been treated with topical application of urea cream 40% under occlusion27 or halcinonide cream 0.1% under occlusion for 5 to 6 days,28 but these treatments have not been universally effective.3 External use of basic fibroblast growth factor to stimulate new regrowth of the nail plate has been advocated.3 It is important to reassure patients that as long as the underlying causes are eliminated and the nail matrix has not been permanently scarred, the nails should grow back within 12 weeks or sooner in children. Thus, typically only reassurance and counseling of parents/guardians is required for onychomadesis in children.1,2 However, the nails may be dystrophic or fail to regrow if there is poor peripheral circulation or permanent nail matrix damage.
Conclusion
Fortunately, onychomadesis is self-limited. Physicians should look for underlying causes of onychomadesis, including a history of viral infections such as HFMD and varicella as well as systemic diseases and use of medications. As long as any underlying disorder or condition has been resolved, spontaneous regrowth of healthy nails usually but not always occurs within 12 weeks or sooner in children.
Onychomadesis is an acute, noninflammatory, painless, proximal separation of the nail plate from the nail matrix. It occurs due to an abrupt stoppage of nail production by matrix cells, producing temporary cessation of nail growth with or without subsequent complete shedding of nails.1-10 Onychomadesis has a wide spectrum of clinical presentations ranging from mild transverse ridges of the nail plate (Beau lines) to complete nail shedding.4,11 Onychomadesis may be related to systemic and dermatologic diseases, drugs (eg, chemotherapeutic agents, anticonvulsants, lithium, retinoids), nail trauma, fever, or infection,5 and a connection between onychomadesis and hand-foot-and-mouth disease (HFMD) was first described by Clementz et al12 following outbreaks in Europe, Asia, and the United States.
Epidemiology
Onychomadesis has been observed in children of all ages including neonates. Neonatal onychomadesis is thought to be related to perinatal stressors and birth trauma, with possible exacerbation by superimposed candidiasis.10 Depending on the underlying cause, there may be involvement of a single nail or multiple nails. Nag et al1 noted that onychomadesis was most commonly observed in nails of the middle finger (73.7%), followed by the thumb (63.2%) and ring finger (52.6%). Fingernails are more commonly involved than toenails.1
Clementz et al12 first proposed the association between onychomadesis and HFMD in 2000. Patients with a history of HFMD were found to be 14 times more likely to develop onychomadesis (relative risk, 14; 95% confidence interval, 4.57-42.86).4 A common pathogen for HFMD is coxsackievirus A6 (CVA6),13,14 but the mechanism of onychomadesis in HFMD remains unclear.5,7,13 Outbreaks of HFMD have been reported in Spain, Finland, Japan, Thailand, the United States, Singapore, and China.15 During an outbreak of HFMD in Taiwan, the incidence of onychomadesis following CVA6 infection was 37% (48/130) compared to 5% (7/145) in cases with non-CVA6 causative strains.16 There also have been observed differences in the prevalence of onychomadesis by age: a 55% (18/33) occurrence rate was noted in the youngest age group (range, 9–23 months), 30% (8/27) in the middle age group (range, 24–32 months), and 4% (1/28) in the oldest age group (range, 33–42 months), with an average of 4 nails shed per case.17 A study in Spain also found a high occurrence of onychomadesis in a nursery setting, with 92% (11/12) of onychomadesis cases preceded by HFMD 2 months prior.18
Etiology
Local trauma to the nail bed is the most common cause of single-digit onychomadesis.4 Multiple-digit involvement suggests a systemic etiology such as fever, erythroderma, and Kawasaki disease; use of drugs (eg, chemotherapeutic agents, anticonvulsants, lithium, retinoids); and viral infections such as HFMD and varicella at the infantile age (Table).5,9,19 Most drug-related nail changes are the outcome of acute toxicity to the proliferating nail matrix epithelium. If onychomadesis affects all nails at the same level, the patient’s history of medication use and other treatments taken 2 to 3 weeks prior to the appearance of the nail findings should be evaluated. Chemotherapeutic agents produce nail changes in a high proportion of patients, which often are related to drug dosage. These effects also are reproducible with re-administration of the drug.20 Onychomadesis also has been reported as a possible side effect of anticonvulsants such as valproic acid (VPA).21 One study evaluating the link between VPA and onychomadesis indicated that nail changes may be due to a disturbance of zinc metabolism.22 However, the pathomechanism of onychomadesis associated with VPA treatment remains unclear.21 Onychomadesis also has developed after an allergic drug reaction to oral penicillin V after treatment of a sore throat in a 23-month-old child.23
Nail involvement has been reported in 10% of cases of inflammatory conditions such as lichen planus21; however, it may be more common but underrecognized and underreported. Grover et al9 indicated that lichen planus–induced severe inflammation in the matrix of the nail unit leading to a temporary growth arrest was the possible mechanism leading to nail shedding. Prompt systemic and intramatricial steroid treatment of lichen planus is required to avoid potential scarring of the nail matrix and permanent damage.9
Onychomadesis also has been reported following varicella infection (chickenpox). Podder et al19 reported the case of a 7-year-old girl who had recovered from a varicella infection 5 weeks prior and presented with onychomadesis of the right index fingernail with all other fingernails and toenails appearing normal. Kocak and Koçak5 reported onychomadesis in 2 sisters with varicella infection. There are few reported cases, so it is still unclear whether varicella infection is an inciting factor.19
One of the most studied viral infections linked to onychomadesis is HFMD, which is a common viral infection that mostly affects children younger than 10 years.1 The precise mechanism of onychomadesis for these viral infection events remains unclear.7,10,13 Several theories have been delineated, including nail matrix arrest from fever occurring during HFMD.6 However, this cause is unlikely, as fevers are typically low grade and present only for a few hours.4,6,13 Direct inflammation spreading from skin lesions of HFMD around the nails or maceration associated with finger blisters could cause onychomadesis.1,5,7 Haneke24 hypothesized that nail shedding may be the consequence of vesicles localized in the periungual tissue, but studies have shown incidence without prior lesions on the fingers and no relationship between nail matrix arrest and severity of HFMD.5,6,13 Bettoli et al25 reported that inflammation secondary to viral infection around the nail matrix might be induced directly by viruses or indirectly by virus-specific immunocomplexes and consequent distal embolism. Osterback et al14 used reverse transcription–polymerase chain reaction to detect CVA6 in fragmented nails from 2 children and 1 parent following an HFMD episode, suggesting that virus replication could damage the nail matrix, resulting in onychomadesis. Cabrerizo et al18 also suggested that virus replication directly damages the nail matrix based on the presence of CVA6 in shed nails. Because fingernails with onychomadesis are not always of the fingers affected by HFMD, an indirect effect of viral infection on the nail matrix is more plausible.8 Additional studies are needed to clarify the virus-associated mechanism of nail matrix arrest.6 Finally, frequent washing of hands15 resulting in maceration, Candida infection, and allergic contact dermatitis2 may be possible causes. It is unclear if onychomadesis following HFMD is related to viral replication, inflammation, or intensive hygienic measures, and further investigation is needed.2,15
Clinical Characteristics
The ventral floor is the site of the germinal matrix and is responsible for 90% of nail production. As a result, more of the nail plate substance is produced proximally, leading to a natural convex curvature from the proximal to distal nail.11 Beau lines are transverse ridging of the nail plates.6 Onychomadesis may be viewed as a more severe form of Beau lines, with complete separation and possible shedding of the nail plate (Figure).3,4 In both cases, an insult to the nail matrix is followed by recovery and production of the nail plate at the nail matrix.4 In Beau lines, slowing or disruption of cell growth from the proximal matrix results in a thinner nail plate, leading to transverse depressions. Onychomadesis has a similar pathophysiology but is associated with a complete halt in the nail plate production.3

Diagnosis
The diagnosis of onychomadesis is made clinically.3,10 Distinct nail changes can be detected by inspection and palpation of the nail plate,3,11 which allows for differentiation between Beau lines and complete nail shedding. Additionally, any signs of nail trauma need to be noted, as well as pain, swelling, or pruritus, as these symptoms also can guide in determining the etiology of the nail dystrophy. Ultrasonography can confirm the diagnosis, as the defect can be identified beneath the proximal nail fold.3,26 When it occurs after HFMD or varicella, onychomadesis tends to present in 28 to 40 days following infection.4,6,10 Physicians should consider underlying associations. A review of viral illnesses within 1 to 2 months prior to development of nail changes often will identify the causative disease.4 Each patient should be evaluated for recent nail trauma; medications; viral infection; and autoimmune, systemic, and inflammatory diseases.
Treatment
Onychomadesis typically is mild and self-limited.4,10 There is no specific treatment,10 but a conservative approach to management is recommended. Treatment of any underlying medical conditions or discontinuation of an offending medication may help to prevent recurrent onychomadesis.3 Supportive care along with protection of the nail bed by maintaining short nails and using adhesive bandages over the affected nails to avoid snagging the nail or ripping off the partially attached nails is recommended.4 In some cases, onychomadesis has been treated with topical application of urea cream 40% under occlusion27 or halcinonide cream 0.1% under occlusion for 5 to 6 days,28 but these treatments have not been universally effective.3 External use of basic fibroblast growth factor to stimulate new regrowth of the nail plate has been advocated.3 It is important to reassure patients that as long as the underlying causes are eliminated and the nail matrix has not been permanently scarred, the nails should grow back within 12 weeks or sooner in children. Thus, typically only reassurance and counseling of parents/guardians is required for onychomadesis in children.1,2 However, the nails may be dystrophic or fail to regrow if there is poor peripheral circulation or permanent nail matrix damage.
Conclusion
Fortunately, onychomadesis is self-limited. Physicians should look for underlying causes of onychomadesis, including a history of viral infections such as HFMD and varicella as well as systemic diseases and use of medications. As long as any underlying disorder or condition has been resolved, spontaneous regrowth of healthy nails usually but not always occurs within 12 weeks or sooner in children.
- Nag SS, Dutta A, Mandal RK. Delayed cutaneous findings of hand, foot, and mouth disease. Indian Pediatr. 2016;53:42-44.
- Tan ZH, Koh MJ. Nail shedding following hand, foot and mouth disease. Arch Dis Child. 2013;98:665.
- Braswell MA, Daniel CR, Brodell RT. Beau lines, onychomadesis, and retronychia: a unifying hypothesis. J Am Acad Dermatol. 2015;73:849-855.
- Clark CM, Silverberg NB, Weinberg JM. What is your diagnosis? onychomadesis following hand-foot-and-mouth disease. Cutis. 2015;95:312, 319-320.
- Kocak AY, Koçak O. Onychomadesis in two sisters induced by varicella infection. Pediatr Dermatol. 2013;30:E108-E109.
- Shin JY, Cho BK, Park HJ. A clinical study of nail changes occurring secondary to hand-foot-mouth disease: onychomadesis and Beau’s lines. Ann Dermatol. 2014;26:280-283.
- Shikuma E, Endo Y, Fujisawa A, et al. Onychomadesis developed only on the nails having cutaneous lesions of severe hand-foot-mouth disease. Case Rep Dermatol Med. 2011;2011:324193.
- Kim EJ, Park HS, Yoon HS, et al. Four cases of onychomadesis after hand-foot-mouth disease. Ann Dermatol. 2014;26:777-778.
- Grover C, Vohra S. Onychomadesis with lichen planus: an under-recognized manifestation. Indian J Dermatol. 2015;60:420.
- Chu DH, Rubin AI. Diagnosis and management of nail disorders. In: Holland K, ed. The Pediatric Clinics of North America. Vol 61. Philadelphia, PA: Elsevier; 2014:301-302.
- Kowalewski C, Schwartz RA. Components, growth, and composition of the nail. In: Demis D, ed. Clinical Dermatology. Philadelphia, PA: Lippincott-Raven; 1998.
- Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11.
- Scarfì F, Arunachalam M, Galeone M, et al. An uncommon onychomadesis in adults. Int J Dermatol. 2014;53:1392-1394.
- Osterback R, Vuorinen T, Linna M, et al. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis. 2009;15:1485-1488.
- Yan X, Zhang ZZ, Yang ZH, et al. Clinical and etiological characteristics of atypical hand-foot-and-mouth disease in children from Chongqing, China: a retrospective study [published online November 26, 2015]. Biomed Res Int. 2015;2015:802046.
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis. 2011;11:346.
- Guimbao J, Rodrigo P, Alberto MJ, et al. Onychomadesis outbreak linked to hand, foot, and mouth disease, Spain, July 2008. Euro Surveill. 2010;15:19663.
- Cabrerizo M, De Miguel T, Armada A, et al. Onychomadesis after a hand, foot, and mouth disease outbreak in Spain, 2009. Epidemiol Infect. 2010;138:1775-1778.
- Podder I, Das A, Gharami RC. Onychomadesis following varicella infection: is it a mere co-incidence? Indian J Dermatol. 2015;60:626-627.
- Piraccini BM, Iorizzo M, Tosti A. Drug-induced nail abnormalities. Am J Clin Dermatol. 2003;4:31-37.
- Poretti A, Lips U, Belvedere M, et al. Onychomadesis: a rare side-effect of valproic acid medication? Pediatr Dermatol. 2009;26:749-750.
- Grech V, Vella C. Generalized onycholoysis associated with sodium valproate therapy. Eur Neurol. 1999;42:64-65.
- Shah RK, Uddin M, Fatunde OJ. Onychomadesis secondary to penicillin allergy in a child. J Pediatr. 2012;161:166.
- Haneke E. Onychomadesis and hand, foot and mouth disease—is there a connection? Euro Surveill. 2010;15(37).
- Bettoli V, Zauli S, Toni G, et al. Onychomadesis following hand, foot, and mouth disease: a case report from Italy and review of the literature. Int J Dermatol. 2013;52:728-730.
- Wortsman X, Wortsman J, Guerrero R, et al. Anatomical changes in retronychia and onychomadesis detected using ultrasound. Dermatol Surg. 2010;36:1615-1620.
- Fleming CJ, Hunt MJ, Barnetson RS. Mycosis fungoides with onychomadesis. Br J Dermatol. 1996;135:1012-1013.
- Mishra D, Singh G, Pandey SS. Possible carbamazepine-induced reversible onychomadesis. Int J Dermatol. 1989;28:460-461.
- Nag SS, Dutta A, Mandal RK. Delayed cutaneous findings of hand, foot, and mouth disease. Indian Pediatr. 2016;53:42-44.
- Tan ZH, Koh MJ. Nail shedding following hand, foot and mouth disease. Arch Dis Child. 2013;98:665.
- Braswell MA, Daniel CR, Brodell RT. Beau lines, onychomadesis, and retronychia: a unifying hypothesis. J Am Acad Dermatol. 2015;73:849-855.
- Clark CM, Silverberg NB, Weinberg JM. What is your diagnosis? onychomadesis following hand-foot-and-mouth disease. Cutis. 2015;95:312, 319-320.
- Kocak AY, Koçak O. Onychomadesis in two sisters induced by varicella infection. Pediatr Dermatol. 2013;30:E108-E109.
- Shin JY, Cho BK, Park HJ. A clinical study of nail changes occurring secondary to hand-foot-mouth disease: onychomadesis and Beau’s lines. Ann Dermatol. 2014;26:280-283.
- Shikuma E, Endo Y, Fujisawa A, et al. Onychomadesis developed only on the nails having cutaneous lesions of severe hand-foot-mouth disease. Case Rep Dermatol Med. 2011;2011:324193.
- Kim EJ, Park HS, Yoon HS, et al. Four cases of onychomadesis after hand-foot-mouth disease. Ann Dermatol. 2014;26:777-778.
- Grover C, Vohra S. Onychomadesis with lichen planus: an under-recognized manifestation. Indian J Dermatol. 2015;60:420.
- Chu DH, Rubin AI. Diagnosis and management of nail disorders. In: Holland K, ed. The Pediatric Clinics of North America. Vol 61. Philadelphia, PA: Elsevier; 2014:301-302.
- Kowalewski C, Schwartz RA. Components, growth, and composition of the nail. In: Demis D, ed. Clinical Dermatology. Philadelphia, PA: Lippincott-Raven; 1998.
- Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11.
- Scarfì F, Arunachalam M, Galeone M, et al. An uncommon onychomadesis in adults. Int J Dermatol. 2014;53:1392-1394.
- Osterback R, Vuorinen T, Linna M, et al. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis. 2009;15:1485-1488.
- Yan X, Zhang ZZ, Yang ZH, et al. Clinical and etiological characteristics of atypical hand-foot-and-mouth disease in children from Chongqing, China: a retrospective study [published online November 26, 2015]. Biomed Res Int. 2015;2015:802046.
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis. 2011;11:346.
- Guimbao J, Rodrigo P, Alberto MJ, et al. Onychomadesis outbreak linked to hand, foot, and mouth disease, Spain, July 2008. Euro Surveill. 2010;15:19663.
- Cabrerizo M, De Miguel T, Armada A, et al. Onychomadesis after a hand, foot, and mouth disease outbreak in Spain, 2009. Epidemiol Infect. 2010;138:1775-1778.
- Podder I, Das A, Gharami RC. Onychomadesis following varicella infection: is it a mere co-incidence? Indian J Dermatol. 2015;60:626-627.
- Piraccini BM, Iorizzo M, Tosti A. Drug-induced nail abnormalities. Am J Clin Dermatol. 2003;4:31-37.
- Poretti A, Lips U, Belvedere M, et al. Onychomadesis: a rare side-effect of valproic acid medication? Pediatr Dermatol. 2009;26:749-750.
- Grech V, Vella C. Generalized onycholoysis associated with sodium valproate therapy. Eur Neurol. 1999;42:64-65.
- Shah RK, Uddin M, Fatunde OJ. Onychomadesis secondary to penicillin allergy in a child. J Pediatr. 2012;161:166.
- Haneke E. Onychomadesis and hand, foot and mouth disease—is there a connection? Euro Surveill. 2010;15(37).
- Bettoli V, Zauli S, Toni G, et al. Onychomadesis following hand, foot, and mouth disease: a case report from Italy and review of the literature. Int J Dermatol. 2013;52:728-730.
- Wortsman X, Wortsman J, Guerrero R, et al. Anatomical changes in retronychia and onychomadesis detected using ultrasound. Dermatol Surg. 2010;36:1615-1620.
- Fleming CJ, Hunt MJ, Barnetson RS. Mycosis fungoides with onychomadesis. Br J Dermatol. 1996;135:1012-1013.
- Mishra D, Singh G, Pandey SS. Possible carbamazepine-induced reversible onychomadesis. Int J Dermatol. 1989;28:460-461.
Practice Points
- Onychomadesis in a child may be a cutaneous sign of systemic disease.
- In childhood, onychomadesis is sometimes linked with hand-foot-and-mouth disease.
- Spontaneous nail regrowth usually occurs within 12 weeks but may occur faster in children.
Herpes Zoster in Children
Herpes zoster (HZ) is commonly seen in immunocompromised patients but is quite uncommon in immunocompetent children. Pediatric cases have been attributed to 1 of 3 primary exposures: intrauterine exposure to the varicella-zoster virus (VZV), postuterine exposure to wild-type VZV, or exposure due to vaccination with the live-attenuated strain of the virus.1
We report a case of HZ in an immunocompetent pediatric patient soon after routine VZV vaccination. We also review the literature on the incidence, clinical characteristics, and diagnostic aids for pediatric cases of HZ.
Case Report
A 15-month-old boy who was previously healthy presented with a red vesicular rash on the right upper cheek of 3 days’ duration. The patient was otherwise asymptomatic and had no constitutional symptoms. The patient’s mother reported an uncomplicated pregnancy and delivery with no history of maternal VZV infection. There was no known exposure to other individuals with VZV or a history of a similar rash. The patient was up-to-date on his immunizations, which included the VZV vaccine at 12 months of age.
Physical examination revealed vesicles and pustules with an erythematous base on the right zygoma extending to the right lateral canthus and upper eyelid in a dermatomal distribution (Figure). No lesions were present on any other area of the body. One group of vesicles was ruptured with a polyester-tipped applicator and submitted for polymerase chain reaction (PCR) analysis for suspected VZV infection. An ophthalmology evaluation revealed no ocular involvement.
Although no complications were noted on the ophthalmologist’s initial examination or at the follow-up visit, 1 month later the patient’s father noted a “cloudy change” to the right eye. The patient had several subsequent evaluations with ophthalmologists and was treated for HZ ophthalmicus with acyclovir over the following 10 months. The patient’s mother reported eventual clearance of the eye findings without permanent visual sequelae. She stated that the PCR results documenting VZV positivity were extremely helpful for the ophthalmologist in establishing a diagnosis and treatment plan.
Comment
Varicella-zoster virus is 1 of 8 viruses in the Herpesviridae family known to infect humans. It is known to cause 2 distinct disease states: varicella (chickenpox) due to a primary infection from the virus, and HZ (shingles) caused by a reactivation of the latent virus in the dorsal root ganglion, which then travels the neural pathway and manifests cutaneously along 1 to 2 dermatomes.1 This recurrence is possible in infants, children, and adults via 1 of 3 routes of exposure.
The overall incidence of HZ is lower in children compared to adults, and the risk dramatically increases in individuals older than 50 years. Evidence also shows that exposure to VZV before 1 year of age increases the lifetime risk for HZ.2,3 Children aged 1 to 18 years who were evaluated for HZ demonstrated a decreased incidence among those who were vaccinated versus those who were not.4,5 Interestingly, there was an increased incidence of HZ among children aged 1 to 2 years who had been vaccinated. Based on PCR analysis, it was noted that HZ was attributed to the vaccine-related strain of VZV in 92% of 1- to 2-year-old patients.4
There is some concern that the varicella vaccination program implemented in 1995 has led to increased rates of HZ. The literature presents mixed reports. Some studies showed an overall increased incidence of HZ,6,7 and 2 other studies showed no increase in the incidence of HZ.4,8 More recent studies have demonstrated that vaccination may have a protective effect against HZ.4-6,9 In a 2013 study in which HZ samples were tested by PCR analysis to determine the strains of VZV that were responsible for an HZ outbreak in children aged 1 to 18 years, the HZ incidence was 48 per 100,000 person-years in patients who were vaccinated versus 230 per 100,000 person-years in patients who were not vaccinated.Among the subset of patients who were vaccinated (n=118), 52% of the HZ lesions were from the wild-type strain.4
Clinical Characteristics
The typical presentation of HZ includes grouped vesicles or small bullae on an erythematous base that occur unilaterally within the distribution of a cranial or spinal sensory nerve, occasionally with overflow into the dermatomes above and below, typically without crossing the midline.8 The most frequent distributions in descending order are thoracic, cranial (mostly trigeminal), lumbar, and sacral. Pain in the dermatome may never occur, may precede, may occur during, or may even occur after the eruption. The initial presentation involves papules and plaques that develop blisters within hours of their development. Lesions continue to appear for several days and may coalesce. The lesions may become hemorrhagic, necrotic, or bullous, with or without adenopathy. Rarely, there can be pain without the associated skin eruption (zoster sine herpete). Lesions tend to crust by days 7 to 10.8
Herpes zoster typically affects children to a lesser extent than adults. The disease state often is milder in children with a decreased likelihood of postherpetic neuralgia.10 However, there are documented cases of severe sequelae secondary to zoster infection in pediatric patients, including but not limited to disseminated HZ,8 HZ ophthalmicus,11,12 Ramsay Hunt syndrome,8 and chronic encephalitis.8 In the adult population, ocular involvement will present in 33% to 50% of cases that involve the ophthalmic branch of the trigeminal nerve without clinical involvement of the nasociliary branch of the ophthalmic nerve. Involvement of the nasociliary branch will lead to ocular pathology in an estimated 76% to 100% of adult cases.13,14 It is unknown if this rate is the same in the pediatric population, but it highlights the importance of educating patients and/or guardians about possible complications. It also demonstrates the importance of including HZ in the differential diagnosis for pediatric patients presenting with papular or vesicular skin eruptions, particularly in the area of the ophthalmic branch of the trigeminal nerve.
Diagnosis
Herpes zoster usually is diagnosed based on its clinical presentation. Human herpesvirus 1 or 2 also may present with similar lesions and should be included in the differential diagnosis. To confirm a clinical diagnosis, additional testing may be done. A Tzanck smear historically has been the least expensive and most rapid test. Scrapings can be taken from the base of a vesicle, stained, and examined for multinucleated giant cells; however, a Tzanck smear cannot help in distinguishing herpes simplex virus from VZV. Direct fluorescent antibody testing and viral culture are less rapid but are standard tests that may help with the diagnosis. Direct fluorescent antibody testing can have a high false-negative rate, and viral cultures typically take 2 weeks for completion. These tests have largely been replaced by PCR analysis. Polymerase chain reaction has been the most sensitive test developed yet. With recent advances, real-time PCR, which can be performed within 1 hour in small hospital laboratories,15 has become more readily available and much more rapid than standard PCR. Further PCR testing can differentiate between the 2 possible infective strains (wild-type vs vaccine related).16 Real-time PCR is now commonly used as the first-line ancillary diagnostic test after physical examination.17
Conclusion
Although uncommon, HZ does occur in immunocompetent children and should be included in the differential diagnosis in children with vesicular lesions. Herpes zoster is a reactivation of VZV and initial exposure may be from the wild-type or vaccine-related strains. Clinicians must be vigilant in their evaluation of vesicular lesions in children even without known varicella exposure. Polymerase chain reaction testing can be helpful to distinguish between herpes simplex lesions and VZV. Polymerase chain reaction testing also may be of benefit to determine the strain of VZV infection.
- Myers MG, Seward JF, LaRussa PS. Varicella-zoster virus. In: Kliegman RM, Behrman RE, Jenson HB, et al, eds. Nelson Textbook of Pediatrics. 19th ed. Philadelphia, PA: Saunders; 2011:1104-1105.
- Terada K, Kawano S, Yoshihiro K, et al. Varicella-zoster virus (VZV) reactivation is related to the low response of VZV-specific immunity after chickenpox in infancy. J Infect Dis. 1994;169:650-652.
- Takayama N, Yamada H, Kaku H, et al. Herpes zoster in immunocompetent and immunocompromised Japanese children. Pediatr Int. 2000;42:275-279.
- Weinmann S, Chun C, Schmid DS, et al. Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005-2009. J Infect Dis. 2013;208:1859-1868.
- Civen R, Lopez AS, Zhang J, et al. Varicella outbreak epidemiology in an active surveillance site, 1995–2005. J Infect Dis. 2008;197(suppl 2):S114-S119.
- Russell ML, Dover DC, Simmonds KA, et al. Shingles in Alberta: before and after publicly funded varicella vaccination. Vaccine. 2014;32:6319-6324.
- Goldman GS, King PG. Review of the United States universal varicella vaccination program: herpes zoster incidence rates, cost-effectiveness, and vaccine efficacy based primarily on the Antelope Valley Varicella Active Surveillance Project data. Vaccine. 2013;31:1680-1694.
- Arikawa J, Asahi T, Au WY, et al. Zoster (shingles, herpes zoster). In: James WD, Berger TG, Elston DM, eds. Andrews’ Diseases of the Skin. 11th ed. Philadelphia, PA: Saunders/Elsevier; 2011:372-376.
- Guris D, Jumaan AO, Mascola L, et al. Changing varicella epidemiology in active surveillance sites–United States, 1995-2005. J Infect Dis. 2008;197(suppl 2):S71-S75.
- Petursson G, Helgason S, Gudmundsson S, et al. Herpes zoster in children and adolescents. Pediatr Infect Dis J. 1998;17:905-908.
- Oladokun RE, Olomukoro CN, Owa AB. Disseminated herpes zoster ophthalmicus in an immunocompetent 8-year-old boy. Clin Pract. 2013;3:e16.
- Lewkonia IK, Jackson AA. Infantile herpes zoster after intrauterine exposure to varicella. Br Med J. 1973;3:149.
- Zaal MJ, Völker-Dieben HJ, D’Amaro J. Prognostic value of Hutchinson’s sign in acute herpes zoster ophthalmicus. Graefes Arch Clin Exp Ophthalmol. 2003;241:187-191.
- Harding SP, Lipton JR, Wells JC. Natural history of herpes zoster ophthalmicus: predictors of postherpetic neuralgia and ocular involvement. Br J Ophthalmol. 1987;71:353-358.
- Higashimoto Y, Ihira M, Ohta A, et al. Discriminating between varicella-zoster virus vaccine and wild-type strains by loop-mediated isothermal amplification. J Clin Microbiol. 2008;46:2665-2670.
- Harbecke R, Oxman MN, Arnold, et al. A real-time PCR assay to identify and discriminate among wild-type and vaccine strains of varicella-zoster virus and herpes simplex virus in clinical specimens, and comparison with the clinical diagnoses. J Med Virol. 2009;81:1310-1322.
- Albrecht MA. Diagnosis of varicella-zoster infection. UpToDate website. http://www.uptodate.com/contents/diagnosis-of-varicella-zoster-virus-infection. Updated July 6, 2015. Accessed July 19, 2016.
Herpes zoster (HZ) is commonly seen in immunocompromised patients but is quite uncommon in immunocompetent children. Pediatric cases have been attributed to 1 of 3 primary exposures: intrauterine exposure to the varicella-zoster virus (VZV), postuterine exposure to wild-type VZV, or exposure due to vaccination with the live-attenuated strain of the virus.1
We report a case of HZ in an immunocompetent pediatric patient soon after routine VZV vaccination. We also review the literature on the incidence, clinical characteristics, and diagnostic aids for pediatric cases of HZ.
Case Report
A 15-month-old boy who was previously healthy presented with a red vesicular rash on the right upper cheek of 3 days’ duration. The patient was otherwise asymptomatic and had no constitutional symptoms. The patient’s mother reported an uncomplicated pregnancy and delivery with no history of maternal VZV infection. There was no known exposure to other individuals with VZV or a history of a similar rash. The patient was up-to-date on his immunizations, which included the VZV vaccine at 12 months of age.
Physical examination revealed vesicles and pustules with an erythematous base on the right zygoma extending to the right lateral canthus and upper eyelid in a dermatomal distribution (Figure). No lesions were present on any other area of the body. One group of vesicles was ruptured with a polyester-tipped applicator and submitted for polymerase chain reaction (PCR) analysis for suspected VZV infection. An ophthalmology evaluation revealed no ocular involvement.
Although no complications were noted on the ophthalmologist’s initial examination or at the follow-up visit, 1 month later the patient’s father noted a “cloudy change” to the right eye. The patient had several subsequent evaluations with ophthalmologists and was treated for HZ ophthalmicus with acyclovir over the following 10 months. The patient’s mother reported eventual clearance of the eye findings without permanent visual sequelae. She stated that the PCR results documenting VZV positivity were extremely helpful for the ophthalmologist in establishing a diagnosis and treatment plan.
Comment
Varicella-zoster virus is 1 of 8 viruses in the Herpesviridae family known to infect humans. It is known to cause 2 distinct disease states: varicella (chickenpox) due to a primary infection from the virus, and HZ (shingles) caused by a reactivation of the latent virus in the dorsal root ganglion, which then travels the neural pathway and manifests cutaneously along 1 to 2 dermatomes.1 This recurrence is possible in infants, children, and adults via 1 of 3 routes of exposure.
The overall incidence of HZ is lower in children compared to adults, and the risk dramatically increases in individuals older than 50 years. Evidence also shows that exposure to VZV before 1 year of age increases the lifetime risk for HZ.2,3 Children aged 1 to 18 years who were evaluated for HZ demonstrated a decreased incidence among those who were vaccinated versus those who were not.4,5 Interestingly, there was an increased incidence of HZ among children aged 1 to 2 years who had been vaccinated. Based on PCR analysis, it was noted that HZ was attributed to the vaccine-related strain of VZV in 92% of 1- to 2-year-old patients.4
There is some concern that the varicella vaccination program implemented in 1995 has led to increased rates of HZ. The literature presents mixed reports. Some studies showed an overall increased incidence of HZ,6,7 and 2 other studies showed no increase in the incidence of HZ.4,8 More recent studies have demonstrated that vaccination may have a protective effect against HZ.4-6,9 In a 2013 study in which HZ samples were tested by PCR analysis to determine the strains of VZV that were responsible for an HZ outbreak in children aged 1 to 18 years, the HZ incidence was 48 per 100,000 person-years in patients who were vaccinated versus 230 per 100,000 person-years in patients who were not vaccinated.Among the subset of patients who were vaccinated (n=118), 52% of the HZ lesions were from the wild-type strain.4
Clinical Characteristics
The typical presentation of HZ includes grouped vesicles or small bullae on an erythematous base that occur unilaterally within the distribution of a cranial or spinal sensory nerve, occasionally with overflow into the dermatomes above and below, typically without crossing the midline.8 The most frequent distributions in descending order are thoracic, cranial (mostly trigeminal), lumbar, and sacral. Pain in the dermatome may never occur, may precede, may occur during, or may even occur after the eruption. The initial presentation involves papules and plaques that develop blisters within hours of their development. Lesions continue to appear for several days and may coalesce. The lesions may become hemorrhagic, necrotic, or bullous, with or without adenopathy. Rarely, there can be pain without the associated skin eruption (zoster sine herpete). Lesions tend to crust by days 7 to 10.8
Herpes zoster typically affects children to a lesser extent than adults. The disease state often is milder in children with a decreased likelihood of postherpetic neuralgia.10 However, there are documented cases of severe sequelae secondary to zoster infection in pediatric patients, including but not limited to disseminated HZ,8 HZ ophthalmicus,11,12 Ramsay Hunt syndrome,8 and chronic encephalitis.8 In the adult population, ocular involvement will present in 33% to 50% of cases that involve the ophthalmic branch of the trigeminal nerve without clinical involvement of the nasociliary branch of the ophthalmic nerve. Involvement of the nasociliary branch will lead to ocular pathology in an estimated 76% to 100% of adult cases.13,14 It is unknown if this rate is the same in the pediatric population, but it highlights the importance of educating patients and/or guardians about possible complications. It also demonstrates the importance of including HZ in the differential diagnosis for pediatric patients presenting with papular or vesicular skin eruptions, particularly in the area of the ophthalmic branch of the trigeminal nerve.
Diagnosis
Herpes zoster usually is diagnosed based on its clinical presentation. Human herpesvirus 1 or 2 also may present with similar lesions and should be included in the differential diagnosis. To confirm a clinical diagnosis, additional testing may be done. A Tzanck smear historically has been the least expensive and most rapid test. Scrapings can be taken from the base of a vesicle, stained, and examined for multinucleated giant cells; however, a Tzanck smear cannot help in distinguishing herpes simplex virus from VZV. Direct fluorescent antibody testing and viral culture are less rapid but are standard tests that may help with the diagnosis. Direct fluorescent antibody testing can have a high false-negative rate, and viral cultures typically take 2 weeks for completion. These tests have largely been replaced by PCR analysis. Polymerase chain reaction has been the most sensitive test developed yet. With recent advances, real-time PCR, which can be performed within 1 hour in small hospital laboratories,15 has become more readily available and much more rapid than standard PCR. Further PCR testing can differentiate between the 2 possible infective strains (wild-type vs vaccine related).16 Real-time PCR is now commonly used as the first-line ancillary diagnostic test after physical examination.17
Conclusion
Although uncommon, HZ does occur in immunocompetent children and should be included in the differential diagnosis in children with vesicular lesions. Herpes zoster is a reactivation of VZV and initial exposure may be from the wild-type or vaccine-related strains. Clinicians must be vigilant in their evaluation of vesicular lesions in children even without known varicella exposure. Polymerase chain reaction testing can be helpful to distinguish between herpes simplex lesions and VZV. Polymerase chain reaction testing also may be of benefit to determine the strain of VZV infection.
Herpes zoster (HZ) is commonly seen in immunocompromised patients but is quite uncommon in immunocompetent children. Pediatric cases have been attributed to 1 of 3 primary exposures: intrauterine exposure to the varicella-zoster virus (VZV), postuterine exposure to wild-type VZV, or exposure due to vaccination with the live-attenuated strain of the virus.1
We report a case of HZ in an immunocompetent pediatric patient soon after routine VZV vaccination. We also review the literature on the incidence, clinical characteristics, and diagnostic aids for pediatric cases of HZ.
Case Report
A 15-month-old boy who was previously healthy presented with a red vesicular rash on the right upper cheek of 3 days’ duration. The patient was otherwise asymptomatic and had no constitutional symptoms. The patient’s mother reported an uncomplicated pregnancy and delivery with no history of maternal VZV infection. There was no known exposure to other individuals with VZV or a history of a similar rash. The patient was up-to-date on his immunizations, which included the VZV vaccine at 12 months of age.
Physical examination revealed vesicles and pustules with an erythematous base on the right zygoma extending to the right lateral canthus and upper eyelid in a dermatomal distribution (Figure). No lesions were present on any other area of the body. One group of vesicles was ruptured with a polyester-tipped applicator and submitted for polymerase chain reaction (PCR) analysis for suspected VZV infection. An ophthalmology evaluation revealed no ocular involvement.
Although no complications were noted on the ophthalmologist’s initial examination or at the follow-up visit, 1 month later the patient’s father noted a “cloudy change” to the right eye. The patient had several subsequent evaluations with ophthalmologists and was treated for HZ ophthalmicus with acyclovir over the following 10 months. The patient’s mother reported eventual clearance of the eye findings without permanent visual sequelae. She stated that the PCR results documenting VZV positivity were extremely helpful for the ophthalmologist in establishing a diagnosis and treatment plan.
Comment
Varicella-zoster virus is 1 of 8 viruses in the Herpesviridae family known to infect humans. It is known to cause 2 distinct disease states: varicella (chickenpox) due to a primary infection from the virus, and HZ (shingles) caused by a reactivation of the latent virus in the dorsal root ganglion, which then travels the neural pathway and manifests cutaneously along 1 to 2 dermatomes.1 This recurrence is possible in infants, children, and adults via 1 of 3 routes of exposure.
The overall incidence of HZ is lower in children compared to adults, and the risk dramatically increases in individuals older than 50 years. Evidence also shows that exposure to VZV before 1 year of age increases the lifetime risk for HZ.2,3 Children aged 1 to 18 years who were evaluated for HZ demonstrated a decreased incidence among those who were vaccinated versus those who were not.4,5 Interestingly, there was an increased incidence of HZ among children aged 1 to 2 years who had been vaccinated. Based on PCR analysis, it was noted that HZ was attributed to the vaccine-related strain of VZV in 92% of 1- to 2-year-old patients.4
There is some concern that the varicella vaccination program implemented in 1995 has led to increased rates of HZ. The literature presents mixed reports. Some studies showed an overall increased incidence of HZ,6,7 and 2 other studies showed no increase in the incidence of HZ.4,8 More recent studies have demonstrated that vaccination may have a protective effect against HZ.4-6,9 In a 2013 study in which HZ samples were tested by PCR analysis to determine the strains of VZV that were responsible for an HZ outbreak in children aged 1 to 18 years, the HZ incidence was 48 per 100,000 person-years in patients who were vaccinated versus 230 per 100,000 person-years in patients who were not vaccinated.Among the subset of patients who were vaccinated (n=118), 52% of the HZ lesions were from the wild-type strain.4
Clinical Characteristics
The typical presentation of HZ includes grouped vesicles or small bullae on an erythematous base that occur unilaterally within the distribution of a cranial or spinal sensory nerve, occasionally with overflow into the dermatomes above and below, typically without crossing the midline.8 The most frequent distributions in descending order are thoracic, cranial (mostly trigeminal), lumbar, and sacral. Pain in the dermatome may never occur, may precede, may occur during, or may even occur after the eruption. The initial presentation involves papules and plaques that develop blisters within hours of their development. Lesions continue to appear for several days and may coalesce. The lesions may become hemorrhagic, necrotic, or bullous, with or without adenopathy. Rarely, there can be pain without the associated skin eruption (zoster sine herpete). Lesions tend to crust by days 7 to 10.8
Herpes zoster typically affects children to a lesser extent than adults. The disease state often is milder in children with a decreased likelihood of postherpetic neuralgia.10 However, there are documented cases of severe sequelae secondary to zoster infection in pediatric patients, including but not limited to disseminated HZ,8 HZ ophthalmicus,11,12 Ramsay Hunt syndrome,8 and chronic encephalitis.8 In the adult population, ocular involvement will present in 33% to 50% of cases that involve the ophthalmic branch of the trigeminal nerve without clinical involvement of the nasociliary branch of the ophthalmic nerve. Involvement of the nasociliary branch will lead to ocular pathology in an estimated 76% to 100% of adult cases.13,14 It is unknown if this rate is the same in the pediatric population, but it highlights the importance of educating patients and/or guardians about possible complications. It also demonstrates the importance of including HZ in the differential diagnosis for pediatric patients presenting with papular or vesicular skin eruptions, particularly in the area of the ophthalmic branch of the trigeminal nerve.
Diagnosis
Herpes zoster usually is diagnosed based on its clinical presentation. Human herpesvirus 1 or 2 also may present with similar lesions and should be included in the differential diagnosis. To confirm a clinical diagnosis, additional testing may be done. A Tzanck smear historically has been the least expensive and most rapid test. Scrapings can be taken from the base of a vesicle, stained, and examined for multinucleated giant cells; however, a Tzanck smear cannot help in distinguishing herpes simplex virus from VZV. Direct fluorescent antibody testing and viral culture are less rapid but are standard tests that may help with the diagnosis. Direct fluorescent antibody testing can have a high false-negative rate, and viral cultures typically take 2 weeks for completion. These tests have largely been replaced by PCR analysis. Polymerase chain reaction has been the most sensitive test developed yet. With recent advances, real-time PCR, which can be performed within 1 hour in small hospital laboratories,15 has become more readily available and much more rapid than standard PCR. Further PCR testing can differentiate between the 2 possible infective strains (wild-type vs vaccine related).16 Real-time PCR is now commonly used as the first-line ancillary diagnostic test after physical examination.17
Conclusion
Although uncommon, HZ does occur in immunocompetent children and should be included in the differential diagnosis in children with vesicular lesions. Herpes zoster is a reactivation of VZV and initial exposure may be from the wild-type or vaccine-related strains. Clinicians must be vigilant in their evaluation of vesicular lesions in children even without known varicella exposure. Polymerase chain reaction testing can be helpful to distinguish between herpes simplex lesions and VZV. Polymerase chain reaction testing also may be of benefit to determine the strain of VZV infection.
- Myers MG, Seward JF, LaRussa PS. Varicella-zoster virus. In: Kliegman RM, Behrman RE, Jenson HB, et al, eds. Nelson Textbook of Pediatrics. 19th ed. Philadelphia, PA: Saunders; 2011:1104-1105.
- Terada K, Kawano S, Yoshihiro K, et al. Varicella-zoster virus (VZV) reactivation is related to the low response of VZV-specific immunity after chickenpox in infancy. J Infect Dis. 1994;169:650-652.
- Takayama N, Yamada H, Kaku H, et al. Herpes zoster in immunocompetent and immunocompromised Japanese children. Pediatr Int. 2000;42:275-279.
- Weinmann S, Chun C, Schmid DS, et al. Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005-2009. J Infect Dis. 2013;208:1859-1868.
- Civen R, Lopez AS, Zhang J, et al. Varicella outbreak epidemiology in an active surveillance site, 1995–2005. J Infect Dis. 2008;197(suppl 2):S114-S119.
- Russell ML, Dover DC, Simmonds KA, et al. Shingles in Alberta: before and after publicly funded varicella vaccination. Vaccine. 2014;32:6319-6324.
- Goldman GS, King PG. Review of the United States universal varicella vaccination program: herpes zoster incidence rates, cost-effectiveness, and vaccine efficacy based primarily on the Antelope Valley Varicella Active Surveillance Project data. Vaccine. 2013;31:1680-1694.
- Arikawa J, Asahi T, Au WY, et al. Zoster (shingles, herpes zoster). In: James WD, Berger TG, Elston DM, eds. Andrews’ Diseases of the Skin. 11th ed. Philadelphia, PA: Saunders/Elsevier; 2011:372-376.
- Guris D, Jumaan AO, Mascola L, et al. Changing varicella epidemiology in active surveillance sites–United States, 1995-2005. J Infect Dis. 2008;197(suppl 2):S71-S75.
- Petursson G, Helgason S, Gudmundsson S, et al. Herpes zoster in children and adolescents. Pediatr Infect Dis J. 1998;17:905-908.
- Oladokun RE, Olomukoro CN, Owa AB. Disseminated herpes zoster ophthalmicus in an immunocompetent 8-year-old boy. Clin Pract. 2013;3:e16.
- Lewkonia IK, Jackson AA. Infantile herpes zoster after intrauterine exposure to varicella. Br Med J. 1973;3:149.
- Zaal MJ, Völker-Dieben HJ, D’Amaro J. Prognostic value of Hutchinson’s sign in acute herpes zoster ophthalmicus. Graefes Arch Clin Exp Ophthalmol. 2003;241:187-191.
- Harding SP, Lipton JR, Wells JC. Natural history of herpes zoster ophthalmicus: predictors of postherpetic neuralgia and ocular involvement. Br J Ophthalmol. 1987;71:353-358.
- Higashimoto Y, Ihira M, Ohta A, et al. Discriminating between varicella-zoster virus vaccine and wild-type strains by loop-mediated isothermal amplification. J Clin Microbiol. 2008;46:2665-2670.
- Harbecke R, Oxman MN, Arnold, et al. A real-time PCR assay to identify and discriminate among wild-type and vaccine strains of varicella-zoster virus and herpes simplex virus in clinical specimens, and comparison with the clinical diagnoses. J Med Virol. 2009;81:1310-1322.
- Albrecht MA. Diagnosis of varicella-zoster infection. UpToDate website. http://www.uptodate.com/contents/diagnosis-of-varicella-zoster-virus-infection. Updated July 6, 2015. Accessed July 19, 2016.
- Myers MG, Seward JF, LaRussa PS. Varicella-zoster virus. In: Kliegman RM, Behrman RE, Jenson HB, et al, eds. Nelson Textbook of Pediatrics. 19th ed. Philadelphia, PA: Saunders; 2011:1104-1105.
- Terada K, Kawano S, Yoshihiro K, et al. Varicella-zoster virus (VZV) reactivation is related to the low response of VZV-specific immunity after chickenpox in infancy. J Infect Dis. 1994;169:650-652.
- Takayama N, Yamada H, Kaku H, et al. Herpes zoster in immunocompetent and immunocompromised Japanese children. Pediatr Int. 2000;42:275-279.
- Weinmann S, Chun C, Schmid DS, et al. Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005-2009. J Infect Dis. 2013;208:1859-1868.
- Civen R, Lopez AS, Zhang J, et al. Varicella outbreak epidemiology in an active surveillance site, 1995–2005. J Infect Dis. 2008;197(suppl 2):S114-S119.
- Russell ML, Dover DC, Simmonds KA, et al. Shingles in Alberta: before and after publicly funded varicella vaccination. Vaccine. 2014;32:6319-6324.
- Goldman GS, King PG. Review of the United States universal varicella vaccination program: herpes zoster incidence rates, cost-effectiveness, and vaccine efficacy based primarily on the Antelope Valley Varicella Active Surveillance Project data. Vaccine. 2013;31:1680-1694.
- Arikawa J, Asahi T, Au WY, et al. Zoster (shingles, herpes zoster). In: James WD, Berger TG, Elston DM, eds. Andrews’ Diseases of the Skin. 11th ed. Philadelphia, PA: Saunders/Elsevier; 2011:372-376.
- Guris D, Jumaan AO, Mascola L, et al. Changing varicella epidemiology in active surveillance sites–United States, 1995-2005. J Infect Dis. 2008;197(suppl 2):S71-S75.
- Petursson G, Helgason S, Gudmundsson S, et al. Herpes zoster in children and adolescents. Pediatr Infect Dis J. 1998;17:905-908.
- Oladokun RE, Olomukoro CN, Owa AB. Disseminated herpes zoster ophthalmicus in an immunocompetent 8-year-old boy. Clin Pract. 2013;3:e16.
- Lewkonia IK, Jackson AA. Infantile herpes zoster after intrauterine exposure to varicella. Br Med J. 1973;3:149.
- Zaal MJ, Völker-Dieben HJ, D’Amaro J. Prognostic value of Hutchinson’s sign in acute herpes zoster ophthalmicus. Graefes Arch Clin Exp Ophthalmol. 2003;241:187-191.
- Harding SP, Lipton JR, Wells JC. Natural history of herpes zoster ophthalmicus: predictors of postherpetic neuralgia and ocular involvement. Br J Ophthalmol. 1987;71:353-358.
- Higashimoto Y, Ihira M, Ohta A, et al. Discriminating between varicella-zoster virus vaccine and wild-type strains by loop-mediated isothermal amplification. J Clin Microbiol. 2008;46:2665-2670.
- Harbecke R, Oxman MN, Arnold, et al. A real-time PCR assay to identify and discriminate among wild-type and vaccine strains of varicella-zoster virus and herpes simplex virus in clinical specimens, and comparison with the clinical diagnoses. J Med Virol. 2009;81:1310-1322.
- Albrecht MA. Diagnosis of varicella-zoster infection. UpToDate website. http://www.uptodate.com/contents/diagnosis-of-varicella-zoster-virus-infection. Updated July 6, 2015. Accessed July 19, 2016.
Practice Points
- Herpes zoster (HZ) should be included in the differential diagnosis for children presenting with vesicular lesions in a dermatomal distribution and a history of varicella exposure.
- Clinical diagnosis of HZ and herpes simplex virus can be aided by the use of viral polymerase chain reaction testing.
- Children with HZ should be monitored for the same possible complications as adults.
Pediatric Rosacea
Rosacea is a chronic skin disease characterized by flushing, erythema, telangiectasia, papules, and pustules in the central face region.1 It most often affects middle-aged women (age range, 30–50 years).2 Rosacea is rare in the pediatric population, especially before puberty.3 There are 3 subtypes of pediatric rosacea: vascular, papulopustular, and ocular. Phymatous/rhinophymatous rosacea is only seen in the adult population.3 Recommendations for the management of pediatric rosacea heavily rely on data from retrospective chart reviews and case series.
Etiology of Pediatric Rosacea
Rosacea is thought to be a consequence of vasomotor instability in both adults and children. A family history of rosacea is sometimes reported in patients with pediatric rosacea.4 Patients often are sensitive to heat, sunlight, topical corticosteroids, spicy foods, hot liquids, and certain soaps and cleansers.1,3,4 In a review of the literature by Vemuri et al,5 the various reported triggers of rosacea include harsh climates that damage the blood vessels and dermal connective tissue, defects in the endothelium and dermal matrix, perivascular inflammation, orally ingested chemicals, changes in the flora of the hair follicles, excessive antimicrobial peptides, and the presence of free radicals. Overall, it is unclear which of these factors are triggers of pediatric rosacea.
The molecular basis of rosacea has been elucidated. It is well known that rosacea patients have higher levels of cathelicidins in the facial skin. Furthermore, they appear to have different processed forms of cathelicidin peptides compared to adults without rosacea, possibly due to changes in posttranslational processing.6 One such peptide, cathelicidin LL-37, also has been implicated in atopic dermatitis7 and psoriasis.8 Its role in rosacea appears to be multifaceted. Cathelicidin LL-37 helps to attract neutrophils, monocytes, and T lymphocytes, and also has antimicrobial properties; therefore, it plays a role in both the innate and adaptive immune systems.9 Cathelicidin LL-37 also has been implicated in inducing angiogenesis10 and suppressing dermal fibroblasts.11
Muto et al12 found that there is an increased number of mast cells in the dermis of patients with rosacea. Mast cells contribute to vasodilation, angiogenesis, and the recruitment of other inflammatory cells.12 Importantly, human mast cells are a source of cathelicidins including cathelicidin LL-37; these proteins play a vital role in the antimicrobial capabilities of mast cells.13
Clinical Presentation and Comorbidities
Vascular rosacea presents with characteristic flushing and erythema, which lasts more than a few minutes as compared to physiologic erythema,1 and sometimes telangiectasia is seen.3 The cheeks, chin, and nasolabial folds are most commonly involved.2 In papulopustular rosacea, papules and pustules are seen overlying the erythema.1,3 Open and closed comedones also have been documented in case reports but are not commonly seen.2 Pediatric rosacea often begins with flushing of the face and then progresses to the development of papules and pustules.4
Ocular rosacea can occur with or without cutaneous findings. In a retrospective study of 20 pediatric patients (aged 1–15 years), 11 (55%) patients had both ocular and cutaneous rosacea, 3 (15%) only had ocular symptoms, and 6 (30%) only had cutaneous symptoms. The most common form of rosacea in this study was papulopustular rosacea.14 Ocular symptoms often are bilateral15 and can include blepharitis, conjunctival injection, recurrent chalazion, conjunctivitis,2 and less commonly corneal ulceration and scarring.16 Patients also may report photophobia or a foreign body sensation.17 Importantly, ocular symptoms often precede the cutaneous symptoms and can delay the diagnosis of rosacea,14,16,18 as these symptoms often are misdiagnosed as viral or bacterial infections.15 Fortunately, ocular disease responds well to treatment if diagnosed early.
Weston and Morelli19 conducted a retrospective study of 106 children (46 males; 60 females) 13 years and younger with steroid rosacea; 29 children were younger than 3 years. A family history of rosacea was present in 20% of participants, and prior use of class 7 steroids was reported in 54%, whereas only 3% had used class 1 topical steroids. Ninety-eight participants had perinasal involvement, 94 had perioral involvement, and 44 had periorbital involvement of the lower eyelids.19
Rosacea fulminans (also known as pyoderma faciale) is a rare acute-onset eruption typically found in young women in their 20s and 30s.20 Rosacea fulminans is characterized by papules, pustules, nodules, cysts, draining sinuses, communicating sinus tracts, and less commonly comedones.20,21 The skin can appear erythematous, cyanotic, or dull red.21 Most of the lesions are found on the face, particularly on the forehead, cheeks, nose, and chin,21 but lesions on the chest and back have been documented in adult patients.20 In an examination of prior case series, most patients were otherwise healthy. There are case reports documenting rosacea fulminans in teenagers,20 but the youngest patient recorded was an otherwise healthy 3-year-old girl who developed a sudden onset of erythematous papules, pustules, cysts, and purulent discharging sinuses on the cheeks that spread to the chin, perioral, and paranasal areas.21
Differential Diagnosis
Rosacea is rare in children, so other papulopustular disorders must be ruled out, including acne vulgaris, periorificial/perioral dermatitis, sarcoidosis, systemic lupus erythematosus, steroid-induced rosacea, ataxia telangiectasia, and demodicosis.
Acne vulgaris commonly presents in older adolescents and teenagers with open and closed comedones, inflammatory papules, and pustules.2 Intense facial flushing and telangiectasia usually is not seen.
In perioral dermatitis, skin lesions often are clustered around the mouth, nose, and eyes. Typically there are no telangiectases or ocular complications.3 Facial flushing and telangiectases are uncommon, except in steroid-induced perioral dermatitis.2
The cutaneous findings of sarcoidosis include red-brown papules on the face and lips, and patients also may have ocular involvement such as uveitis and iritis.3 However, there are typically other systemic findings such as pulmonary symptoms, weight loss, fatigue, lethargy, fever, and erythema nodosum.2,3 Chest radiograph findings (eg, bilateral hilar lym-phadenopathy), ophthalmologic examination, and laboratory data (eg, elevated alkaline phosphate and/or elevated angiotensin-converting enzyme) can help confirm or rule out the diagnosis of sarcoidosis.2,3
Unlike systemic lupus erythematosus, patients with rosacea will have involvement of sun-protected areas of the skin. Patients with systemic lupus erythematosus typically report arthralgia and severe photosensitivity and will have elevated antinuclear antibody titers. Skin biopsies and immunofluorescence can help confirm the diagnosis.3 Importantly, some patients with rosacea will have a positive lupus band test.22,23
Steroid-induced rosacea typically occurs 2 weeks after discontinuing therapy with topical fluorinated glucocorticosteroids.24 Children present with monomorphic papules, pustules, and telangiectases4 on the eyelids and lateral face as opposed to the central face regions.24
Ataxia telangiectasia can present with telangiectases, skin atrophy, café au lait spots, and premature graying.25 A 15-year-old adolescent girl with ataxia telangiectasia presented with granulomatous acne rosacea that improved after 4 weeks of treatment with isotretinoin 0.5 mg/kg daily. The lesions cleared almost completely after 5 months.25
Demodicosis is a disorder of the pilosebaceous units caused by the human Demodex mite.26 It typically involves the periorificial regions in adults and the elderly population. Patients can present with fine, white-yellow, scaly changes of the sebaceous hair follicles, with minimal erythema and inflammation. Papules and pustules also can be present.26
Diagnosis and Histopathology
Because rosacea is rare in children, it is important to thoroughly evaluate other possible diagnoses. The diagnosis of pediatric rosacea is clinical and biopsies are rarely performed. Laboratory tests such as cultures generally are not useful.
Marks and Harcourt-Webster27 reviewed the biopsies of 108 adult patients with rosacea. The biopsies of patients with predominantly erythema and telangiectasia showed evidence of vascular dilatation with a perivascular infiltrate composed predominantly of lymphocytes and 39 specimens that were compared to controls showed more solar elastosis. Biopsies of papular rosacea contained inflammatory infiltrates in the upper and mid dermis composed primarily of lymphocytes and histiocytes. In some patients, neutrophils, plasma cells, and giant cells also were observed. Hair follicle abnormalities were present in 20% of the biopsies, with 19% showing evidence of the Demodex mite. Vascular dilatation also was common. Overall, common findings included lymphohistiocytic infiltrates around the blood vessels of the upper dermis, dilated vessels, edema, elastosis, and disorganization of connective tissue in the upper dermis.
Helm et al28 reviewed histopathologic patterns from 53 patients with granulomatous rosacea. Findings included a mixed lymphohistiocytic infiltrate (predominantly lymphocytic in 40% of patients and predominantly histiocytic with occasional giant cells in 34% of patients), epithelioid granulomas (11% of patients), and epithelioid granulomas with caseation necrosis (11% of patients).
The histopathology of rosacea fulminans is characterized by dense perivascular and periadnexal infiltrates composed of granulocytes, eosinophils, and epithelioid granulomas, as well as panniculitis.20
Treatment and Clinical Outcomes
Certain lifestyle recommendations are integral components of disease management, including avoidance of triggers such as extreme temperatures, hot drinks, spicy food, and topical agents that could be irritating (especially topical corticosteroids).29 Patients should be advised to use daily sunscreen containing physical blockers such as titanium dioxide or zinc oxide. Teenagers should avoid the use of cosmetics and makeup, especially products containing sodium lauryl sulfate, menthol, and camphor. Daily use of emollients can help some patients.29
There are both topical and systemic therapies available for pediatric rosacea; however, most of the data are based on the use of these treatments in the adult population. Patients with mild to moderate disease often can be managed using topical agents. Metronidazole (0.75% cream, 1% gel, or 0.75% lotion) has been studied extensively in adult patients, and when used once daily for 12 weeks, it has been able to control moderate to severe disease.30,31 In one study conducted in adult patients, topical metronidazole was able to maintain remission in adults who had previously been treated with a combination of oral tetracycline and metronidazole gel.31 Sodium sulfacetamide 10%–sulfur 5% (cleanser or lotion) has been successful in adult patients and often is used in combination with other therapies such as topical metronidazole.32-34 Azelaic acid cream 20%,35 benzoyl peroxide (wash or gel),29 topical clindamycin,36 topical erythromycin,29,37 tacrolimus ointment 0.1%,38 and tretinoin cream also have been studied in adults.3,39 Several of these topical agents can cause irritation on application (eg, metronidazole, sulfur-based agents, azelaic acid, benzoyl peroxide, erythromycin, tretinoin).3
The use of systemic treatments in pediatric patients is heavily based on case reports and case series.2,14,16,40 Therapies have included tetracycline (500 mg twice daily tapered to 250 mg daily),29 minocycline (50–100 mg twice daily), doxycycline (50–100 mg twice daily or 4 times daily), erythromycin (30–50 mg/kg daily), clarithromycin (15 mg/kg twice daily for 4 weeks and then daily for 4 weeks), and azithromycin (5–10 mg/kg daily).3 Tetracycline antibiotics should not be used in children 8 years or younger.
In a case series by Drolet and Paller,2 an 11-year-old girl was treated with tetracycline 500 mg (later tapered to 250 mg daily) and metronidazole gel 0.75%, both used twice daily. Previously, she had not responded to topical steroids, tretinoin cream 0.05%, benzoyl peroxide 5%, or systemic prednisone. After 6 weeks of treatment, the pustules and chalazion had resolved and she had only minimal erythema of the skin and conjunctiva. Sixteen months after the start of treatment, a regimen of tetracycline 250 mg daily and metronidazole gel resulted in disease clearance on the face.2
A 9-year-old girl with concurrent systemic lupus erythematosus was treated with tetracycline 250 mg and topical erythromycin 2%, both used twice daily.2 After 4 weeks her face was clear. Four months later she developed new telangiectases and topical erythromycin was replaced with topical metronidazole. Eventually the dose of tetracycline was reduced to 250 mg daily.2
An 11-year-old boy with likely granulomatous rosacea was treated with erythromycin 250 mg 4 times daily, alclometasone dipropionate cream 0.05% twice daily, and topical clindamycin twice daily.2 Marked improvement was noticed after 3 weeks of treatment. Metronidazole gel 0.75% was added and 3 months later the patient’s face was clear, without evidence of scarring. The dose of erythromycin was later reduced to 500 mg daily, and eventually the patient experienced clearance with the use of metronidazole gel daily.2
In another case series, 4 female patients (age range, 4–12 years) were treated with systemic erythromycin 20 mg/kg daily (ocular involvement only) or doxycycline 2.2 mg/kg daily used in two 12-year-old patients with ocular and cutaneous involvement for at least 12 months. All 4 patients showed considerable improvement within 1 month and remained free of disease throughout a mean follow-up period of 25.5 months.40
As evidenced by these case reports, there is a wide array of treatments that have been used for pediatric rosacea. Although there are no formal evidence-based guidelines, there are certain considerations that must be taken into account when choosing treatment plans. Doxycycline and minocycline are known to cause less gastrointestinal upset than tetracycline with similar efficacy.41 Importantly, the tetracyclines are contraindicated in children younger than 9 years, as they can cause teeth staining and possibly affect skeletal growth.3,4 When used in older children (age range, 9–12 years), patients must be advised not to take their medication with calcium or antacids.3 Clarithromycin and azithromycin tend to have fewer gastrointestinal side effects than erythromycin. Erythromycin and other macrolides can be used in children of all ages and in patients who are allergic to tetracyclines.3
Children with mild ocular symptoms often can control their disease with bacitracin and topical ocular antibiotics such as erythromycin.2,15 For patients who require systemic antibiotics, various tetracyclines and macrolides have been used with success.2,14-16,40
Adults with rosacea fulminans can require treatment with isotretinoin, oral antibiotics, and topical or even systemic corticosteroids.42 The 3-year-old girl with rosacea fulminans initially was treated with oral erythromycin (250 mg 4 times daily), oral prednisolone (0.5 mg/kg daily tapered over 2 weeks), fluocinolone acetonide cream 0.025%, and warm compresses with only moderate improvement.21 She was then started on oral isotretinoin (0.75 mg/kg daily) and within 4 weeks marked improvement was noted. After 8 weeks, the lesions had disappeared completely with only a few pitted scars remaining. Isotretinoin was continued for 24 weeks. One year after completion of treatment, she was still disease free.21
Weston and Morelli19 recommended the following treatment regimen for children with steroid rosacea: abrupt cessation of topical steroid use (as opposed to gradual withdrawal) and initiation of oral erythromycin stearate (30 mg/kg daily) in 2 daily doses for 4 weeks. Children who were unable to tolerate erythromycin (n=6) were told to use topical clindamycin phosphate twice daily for 4 weeks. Within 3 weeks 22% of patients had resolution, while 86% had resolution within 4 weeks. All of the patients cleared within 8 weeks. Importantly, there was no significant difference in duration of time until clearance between children who used the oral antibiotic or topical antibiotic.19
Conclusion
We know that the skin of rosacea patients contains higher levels of cathelicidins, which have been implicated in amplifying and contributing to the inflammatory response in several ways. Mast cells, which are a source of cathelicidins, also are increased in the skin of these patients. Children can present with vascular rosacea (characterized by flushing, erythema, and/or telangiectasia), papulopustular rosacea, or ocular rosacea. Common ocular symptoms include blepharitis, conjunctivitis, and recurrent chalazion. It is important to refer pediatric rosacea patients with ocular symptoms to an ophthalmologist to prevent ocular sequelae.
Rosacea is a clinical diagnosis but biopsy can be performed to rule out other diagnoses. Treatment consists of lifestyle modifications such as avoiding known triggers and the use of topical and/or oral agents. Common topical therapies include metronidazole and erythromycin. Systemic antibiotics include tetracycline, doxycycline, minocycline, azithromycin, and erythromycin. Some children are able to taper systemic agents and maintain disease control with topical therapy, while others may need to continue a low-dose antibiotic. Although flares can be controlled within weeks to months, rosacea is a chronic disorder and childhood rosacea tends to persist into adulthood.
- Crawford GH, Pelle MT, James WD. Rosacea: i. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341; quiz 342-324.
- Drolet B, Paller AS. Childhood rosacea. Pediatr Dermatol. 1992;9:22-26.
- Kroshinsky D, Glick SA. Pediatric rosacea. Dermatol Ther. 2006;19:196-201.
- Lacz NL, Schwartz RA. Rosacea in the pediatric population. Cutis. 2004;74:99-103.
- Vemuri RC, Gundamaraju R, Sekaran SD, et al. Major pathophysiological correlations of rosacea: a complete clinical appraisal. Int J Med Sci. 2015;12:387-396.
- Yamasaki K, Di Nardo A, Bardan A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975-980.
- Ong PY, Ohtake T, Brandt C, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151-1160.
- Lande R, Gregorio J, Facchinetti V, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564-569.
- De Y, Chen Q, Schmidt AP, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069-1074.
- Koczulla R, von Degenfeld G, Kupatt C, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111:1665-1672.
- Park HJ, Cho DH, Kim HJ, et al. Collagen synthesis is suppressed in dermal fibroblasts by the human antimicrobial peptide LL-37. J Invest Dermatol. 2009;129:843-850.
- Muto Y, Wang Z, Vanderberghe M, et al. Mast cells are key mediators of cathelicidin-initiated skin inflammation in rosacea. J Invest Dermatol. 2014;134:2728-2736.
- Di Nardo A, Vitiello A, Gallo RL. Cutting edge: mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J Immunol. 2003;170:2274-2278.
- Leoni S, Mesplie N, Aitali F, et al. Metronidazole: alternative treatment for ocular and cutaneous rosacea in the pediatric population [in French]. J Fr Ophthalmol. 2011;34:703-710.
- Nazir SA, Murphy S, Siatkowski RM, et al. Ocular rosacea in childhood. Am J Ophthalmol. 2004;137:138-144.
- Miguel AI, Salgado MB, Lisboa MS, et al. Pediatric ocular rosacea: 2 cases. Eur J Ophthalmol. 2012;22:664-666.
- Stone DU, Chodosh J. Ocular rosacea: an update on pathogenesis and therapy. Curr Opin Ophthalmol. 2004;15:499-502.
- Mavrakanas N, Schutz JS, Dosso AA. Pediatric ocular rosacea [published online March 22, 2010]. J Pediatr Ophthalmol Strabismus. 2010;47:117-120.
- Weston WL, Morelli JG. Steroid rosacea in prepubertal children. Arch Pediatr Adolesc Med. 2000;154:62-64.
- Plewig G, Jansen T, Kligman AM. Pyoderma faciale. a review and report of 20 additional cases: is it rosacea? Arch Dermatol. 1992;128:1611-1617.
- Firooz A, Firoozabadi MR, Dowlati Y. Rosacea fulminans (pyoderma faciale): successful treatment of a 3-year-old girl with oral isotretinoin. Int J Dermatol. 2001;40:203-205.
- Baart de la Faille H, Baart de la F-K. Immunofluorescent studies of the skin in rosacea. Dermatologica. 1969;139:49-54.
- Wilkin JK. Rosacea. Int J Dermatol. 1983;22:393-400.
- Franco HL, Weston WL. Steroid rosacea in children. Pediatrics. 1979;64:36-38.
- Cantarutti N, Claps A, Angelino G, et al. Multi-drugs resistant acne rosacea in a child affected by ataxia-telangiectasia: successful treatment with isotretinoin. Ital J Pediatr. 2015;41:23.
- Chen W, Plewig G. Human demodicosis: revisit and a proposed classification. Br J Dermatol. 2014;170:1219-1225.
- Marks R, Harcourt-Webster J. Histopathology of rosacea. Arch Dermatol. 1969;100:683-691.
- Helm KF, Menz J, Gibson LE, et al. A clinical and histopathologic study of granulomatous rosacea. J Am Acad Dermatol. 1991;25(6, pt 1):1038-1043.
- Pelle MT, Crawford GH, James WD. Rosacea: II. therapy. J Am Acad Dermatol. 2004;51:499-512; quiz 513-494.
- Dahl MV, Jarratt M, Kaplan D, et al. Once-daily topical metronidazole cream formulations in the treatment of the papules and pustules of rosacea. J Am Acad Dermatol. 2001;45:723-730.
- Dahl MV, Katz HI, Krueger GG, et al. Topical metronidazole maintains remissions of rosacea. Arch Dermatol. 1998;134:679-683.
- Trumbore MW, Goldstein JA, Gurge RM. Treatment of papulopustular rosacea with sodium sulfacetamide 10%/sulfur 5% emollient foam. J Drugs Dermatol. 2009;8:299-304.
- Del Rosso JQ. Evaluating the role of topical therapies in the management of rosacea: focus on combination sodium sulfacetamide and sulfur formulations. Cutis. 2004;73(1 suppl):29-33.
- Del Rosso JQ. A status report on the medical management of rosacea: focus on topical therapies. Cutis. 2002;70:271-275.
- Maddin S. A comparison of topical azelaic acid 20% cream and topical metronidazole 0.75% cream in the treatment of patients with papulopustular rosacea. J Am Acad Dermatol. 1999;40(6, pt 1):961-965.
- Wilkin JK, DeWitt S. Treatment of rosacea: topical clindamycin versus oral tetracycline. Int J Dermatol. 1993;32:65-67.
- Mills OH Jr, Kligman AM. Letter: topically applied erythromycin in rosacea. Arch Dermatol. 1976;112:553-554.
- Hengge UR. Off-label indications for topical tacrolimus [in German]. Hautarzt. 2013;64:752-756.
- Ertl GA, Levine N, Kligman AM. A comparison of the efficacy of topical tretinoin and low-dose oral isotretinoin in rosacea. Arch Dermatol. 1994;130:319-324.
- Cetinkaya A, Akova YA. Pediatric ocular acne rosacea: long-term treatment with systemic antibiotics. Am J Ophthalmol. 2006;142:816-821.
- Maibach H. Second-generation tetracyclines, a dermatologic overview: clinical uses and pharmacology. Cutis. 1991;48:411-417.
- Jansen T, Plewig G, Kligman AM. Diagnosis and treatment of rosacea fulminans. Dermatology. 1994;188:251-254.
Rosacea is a chronic skin disease characterized by flushing, erythema, telangiectasia, papules, and pustules in the central face region.1 It most often affects middle-aged women (age range, 30–50 years).2 Rosacea is rare in the pediatric population, especially before puberty.3 There are 3 subtypes of pediatric rosacea: vascular, papulopustular, and ocular. Phymatous/rhinophymatous rosacea is only seen in the adult population.3 Recommendations for the management of pediatric rosacea heavily rely on data from retrospective chart reviews and case series.
Etiology of Pediatric Rosacea
Rosacea is thought to be a consequence of vasomotor instability in both adults and children. A family history of rosacea is sometimes reported in patients with pediatric rosacea.4 Patients often are sensitive to heat, sunlight, topical corticosteroids, spicy foods, hot liquids, and certain soaps and cleansers.1,3,4 In a review of the literature by Vemuri et al,5 the various reported triggers of rosacea include harsh climates that damage the blood vessels and dermal connective tissue, defects in the endothelium and dermal matrix, perivascular inflammation, orally ingested chemicals, changes in the flora of the hair follicles, excessive antimicrobial peptides, and the presence of free radicals. Overall, it is unclear which of these factors are triggers of pediatric rosacea.
The molecular basis of rosacea has been elucidated. It is well known that rosacea patients have higher levels of cathelicidins in the facial skin. Furthermore, they appear to have different processed forms of cathelicidin peptides compared to adults without rosacea, possibly due to changes in posttranslational processing.6 One such peptide, cathelicidin LL-37, also has been implicated in atopic dermatitis7 and psoriasis.8 Its role in rosacea appears to be multifaceted. Cathelicidin LL-37 helps to attract neutrophils, monocytes, and T lymphocytes, and also has antimicrobial properties; therefore, it plays a role in both the innate and adaptive immune systems.9 Cathelicidin LL-37 also has been implicated in inducing angiogenesis10 and suppressing dermal fibroblasts.11
Muto et al12 found that there is an increased number of mast cells in the dermis of patients with rosacea. Mast cells contribute to vasodilation, angiogenesis, and the recruitment of other inflammatory cells.12 Importantly, human mast cells are a source of cathelicidins including cathelicidin LL-37; these proteins play a vital role in the antimicrobial capabilities of mast cells.13
Clinical Presentation and Comorbidities
Vascular rosacea presents with characteristic flushing and erythema, which lasts more than a few minutes as compared to physiologic erythema,1 and sometimes telangiectasia is seen.3 The cheeks, chin, and nasolabial folds are most commonly involved.2 In papulopustular rosacea, papules and pustules are seen overlying the erythema.1,3 Open and closed comedones also have been documented in case reports but are not commonly seen.2 Pediatric rosacea often begins with flushing of the face and then progresses to the development of papules and pustules.4
Ocular rosacea can occur with or without cutaneous findings. In a retrospective study of 20 pediatric patients (aged 1–15 years), 11 (55%) patients had both ocular and cutaneous rosacea, 3 (15%) only had ocular symptoms, and 6 (30%) only had cutaneous symptoms. The most common form of rosacea in this study was papulopustular rosacea.14 Ocular symptoms often are bilateral15 and can include blepharitis, conjunctival injection, recurrent chalazion, conjunctivitis,2 and less commonly corneal ulceration and scarring.16 Patients also may report photophobia or a foreign body sensation.17 Importantly, ocular symptoms often precede the cutaneous symptoms and can delay the diagnosis of rosacea,14,16,18 as these symptoms often are misdiagnosed as viral or bacterial infections.15 Fortunately, ocular disease responds well to treatment if diagnosed early.
Weston and Morelli19 conducted a retrospective study of 106 children (46 males; 60 females) 13 years and younger with steroid rosacea; 29 children were younger than 3 years. A family history of rosacea was present in 20% of participants, and prior use of class 7 steroids was reported in 54%, whereas only 3% had used class 1 topical steroids. Ninety-eight participants had perinasal involvement, 94 had perioral involvement, and 44 had periorbital involvement of the lower eyelids.19
Rosacea fulminans (also known as pyoderma faciale) is a rare acute-onset eruption typically found in young women in their 20s and 30s.20 Rosacea fulminans is characterized by papules, pustules, nodules, cysts, draining sinuses, communicating sinus tracts, and less commonly comedones.20,21 The skin can appear erythematous, cyanotic, or dull red.21 Most of the lesions are found on the face, particularly on the forehead, cheeks, nose, and chin,21 but lesions on the chest and back have been documented in adult patients.20 In an examination of prior case series, most patients were otherwise healthy. There are case reports documenting rosacea fulminans in teenagers,20 but the youngest patient recorded was an otherwise healthy 3-year-old girl who developed a sudden onset of erythematous papules, pustules, cysts, and purulent discharging sinuses on the cheeks that spread to the chin, perioral, and paranasal areas.21
Differential Diagnosis
Rosacea is rare in children, so other papulopustular disorders must be ruled out, including acne vulgaris, periorificial/perioral dermatitis, sarcoidosis, systemic lupus erythematosus, steroid-induced rosacea, ataxia telangiectasia, and demodicosis.
Acne vulgaris commonly presents in older adolescents and teenagers with open and closed comedones, inflammatory papules, and pustules.2 Intense facial flushing and telangiectasia usually is not seen.
In perioral dermatitis, skin lesions often are clustered around the mouth, nose, and eyes. Typically there are no telangiectases or ocular complications.3 Facial flushing and telangiectases are uncommon, except in steroid-induced perioral dermatitis.2
The cutaneous findings of sarcoidosis include red-brown papules on the face and lips, and patients also may have ocular involvement such as uveitis and iritis.3 However, there are typically other systemic findings such as pulmonary symptoms, weight loss, fatigue, lethargy, fever, and erythema nodosum.2,3 Chest radiograph findings (eg, bilateral hilar lym-phadenopathy), ophthalmologic examination, and laboratory data (eg, elevated alkaline phosphate and/or elevated angiotensin-converting enzyme) can help confirm or rule out the diagnosis of sarcoidosis.2,3
Unlike systemic lupus erythematosus, patients with rosacea will have involvement of sun-protected areas of the skin. Patients with systemic lupus erythematosus typically report arthralgia and severe photosensitivity and will have elevated antinuclear antibody titers. Skin biopsies and immunofluorescence can help confirm the diagnosis.3 Importantly, some patients with rosacea will have a positive lupus band test.22,23
Steroid-induced rosacea typically occurs 2 weeks after discontinuing therapy with topical fluorinated glucocorticosteroids.24 Children present with monomorphic papules, pustules, and telangiectases4 on the eyelids and lateral face as opposed to the central face regions.24
Ataxia telangiectasia can present with telangiectases, skin atrophy, café au lait spots, and premature graying.25 A 15-year-old adolescent girl with ataxia telangiectasia presented with granulomatous acne rosacea that improved after 4 weeks of treatment with isotretinoin 0.5 mg/kg daily. The lesions cleared almost completely after 5 months.25
Demodicosis is a disorder of the pilosebaceous units caused by the human Demodex mite.26 It typically involves the periorificial regions in adults and the elderly population. Patients can present with fine, white-yellow, scaly changes of the sebaceous hair follicles, with minimal erythema and inflammation. Papules and pustules also can be present.26
Diagnosis and Histopathology
Because rosacea is rare in children, it is important to thoroughly evaluate other possible diagnoses. The diagnosis of pediatric rosacea is clinical and biopsies are rarely performed. Laboratory tests such as cultures generally are not useful.
Marks and Harcourt-Webster27 reviewed the biopsies of 108 adult patients with rosacea. The biopsies of patients with predominantly erythema and telangiectasia showed evidence of vascular dilatation with a perivascular infiltrate composed predominantly of lymphocytes and 39 specimens that were compared to controls showed more solar elastosis. Biopsies of papular rosacea contained inflammatory infiltrates in the upper and mid dermis composed primarily of lymphocytes and histiocytes. In some patients, neutrophils, plasma cells, and giant cells also were observed. Hair follicle abnormalities were present in 20% of the biopsies, with 19% showing evidence of the Demodex mite. Vascular dilatation also was common. Overall, common findings included lymphohistiocytic infiltrates around the blood vessels of the upper dermis, dilated vessels, edema, elastosis, and disorganization of connective tissue in the upper dermis.
Helm et al28 reviewed histopathologic patterns from 53 patients with granulomatous rosacea. Findings included a mixed lymphohistiocytic infiltrate (predominantly lymphocytic in 40% of patients and predominantly histiocytic with occasional giant cells in 34% of patients), epithelioid granulomas (11% of patients), and epithelioid granulomas with caseation necrosis (11% of patients).
The histopathology of rosacea fulminans is characterized by dense perivascular and periadnexal infiltrates composed of granulocytes, eosinophils, and epithelioid granulomas, as well as panniculitis.20
Treatment and Clinical Outcomes
Certain lifestyle recommendations are integral components of disease management, including avoidance of triggers such as extreme temperatures, hot drinks, spicy food, and topical agents that could be irritating (especially topical corticosteroids).29 Patients should be advised to use daily sunscreen containing physical blockers such as titanium dioxide or zinc oxide. Teenagers should avoid the use of cosmetics and makeup, especially products containing sodium lauryl sulfate, menthol, and camphor. Daily use of emollients can help some patients.29
There are both topical and systemic therapies available for pediatric rosacea; however, most of the data are based on the use of these treatments in the adult population. Patients with mild to moderate disease often can be managed using topical agents. Metronidazole (0.75% cream, 1% gel, or 0.75% lotion) has been studied extensively in adult patients, and when used once daily for 12 weeks, it has been able to control moderate to severe disease.30,31 In one study conducted in adult patients, topical metronidazole was able to maintain remission in adults who had previously been treated with a combination of oral tetracycline and metronidazole gel.31 Sodium sulfacetamide 10%–sulfur 5% (cleanser or lotion) has been successful in adult patients and often is used in combination with other therapies such as topical metronidazole.32-34 Azelaic acid cream 20%,35 benzoyl peroxide (wash or gel),29 topical clindamycin,36 topical erythromycin,29,37 tacrolimus ointment 0.1%,38 and tretinoin cream also have been studied in adults.3,39 Several of these topical agents can cause irritation on application (eg, metronidazole, sulfur-based agents, azelaic acid, benzoyl peroxide, erythromycin, tretinoin).3
The use of systemic treatments in pediatric patients is heavily based on case reports and case series.2,14,16,40 Therapies have included tetracycline (500 mg twice daily tapered to 250 mg daily),29 minocycline (50–100 mg twice daily), doxycycline (50–100 mg twice daily or 4 times daily), erythromycin (30–50 mg/kg daily), clarithromycin (15 mg/kg twice daily for 4 weeks and then daily for 4 weeks), and azithromycin (5–10 mg/kg daily).3 Tetracycline antibiotics should not be used in children 8 years or younger.
In a case series by Drolet and Paller,2 an 11-year-old girl was treated with tetracycline 500 mg (later tapered to 250 mg daily) and metronidazole gel 0.75%, both used twice daily. Previously, she had not responded to topical steroids, tretinoin cream 0.05%, benzoyl peroxide 5%, or systemic prednisone. After 6 weeks of treatment, the pustules and chalazion had resolved and she had only minimal erythema of the skin and conjunctiva. Sixteen months after the start of treatment, a regimen of tetracycline 250 mg daily and metronidazole gel resulted in disease clearance on the face.2
A 9-year-old girl with concurrent systemic lupus erythematosus was treated with tetracycline 250 mg and topical erythromycin 2%, both used twice daily.2 After 4 weeks her face was clear. Four months later she developed new telangiectases and topical erythromycin was replaced with topical metronidazole. Eventually the dose of tetracycline was reduced to 250 mg daily.2
An 11-year-old boy with likely granulomatous rosacea was treated with erythromycin 250 mg 4 times daily, alclometasone dipropionate cream 0.05% twice daily, and topical clindamycin twice daily.2 Marked improvement was noticed after 3 weeks of treatment. Metronidazole gel 0.75% was added and 3 months later the patient’s face was clear, without evidence of scarring. The dose of erythromycin was later reduced to 500 mg daily, and eventually the patient experienced clearance with the use of metronidazole gel daily.2
In another case series, 4 female patients (age range, 4–12 years) were treated with systemic erythromycin 20 mg/kg daily (ocular involvement only) or doxycycline 2.2 mg/kg daily used in two 12-year-old patients with ocular and cutaneous involvement for at least 12 months. All 4 patients showed considerable improvement within 1 month and remained free of disease throughout a mean follow-up period of 25.5 months.40
As evidenced by these case reports, there is a wide array of treatments that have been used for pediatric rosacea. Although there are no formal evidence-based guidelines, there are certain considerations that must be taken into account when choosing treatment plans. Doxycycline and minocycline are known to cause less gastrointestinal upset than tetracycline with similar efficacy.41 Importantly, the tetracyclines are contraindicated in children younger than 9 years, as they can cause teeth staining and possibly affect skeletal growth.3,4 When used in older children (age range, 9–12 years), patients must be advised not to take their medication with calcium or antacids.3 Clarithromycin and azithromycin tend to have fewer gastrointestinal side effects than erythromycin. Erythromycin and other macrolides can be used in children of all ages and in patients who are allergic to tetracyclines.3
Children with mild ocular symptoms often can control their disease with bacitracin and topical ocular antibiotics such as erythromycin.2,15 For patients who require systemic antibiotics, various tetracyclines and macrolides have been used with success.2,14-16,40
Adults with rosacea fulminans can require treatment with isotretinoin, oral antibiotics, and topical or even systemic corticosteroids.42 The 3-year-old girl with rosacea fulminans initially was treated with oral erythromycin (250 mg 4 times daily), oral prednisolone (0.5 mg/kg daily tapered over 2 weeks), fluocinolone acetonide cream 0.025%, and warm compresses with only moderate improvement.21 She was then started on oral isotretinoin (0.75 mg/kg daily) and within 4 weeks marked improvement was noted. After 8 weeks, the lesions had disappeared completely with only a few pitted scars remaining. Isotretinoin was continued for 24 weeks. One year after completion of treatment, she was still disease free.21
Weston and Morelli19 recommended the following treatment regimen for children with steroid rosacea: abrupt cessation of topical steroid use (as opposed to gradual withdrawal) and initiation of oral erythromycin stearate (30 mg/kg daily) in 2 daily doses for 4 weeks. Children who were unable to tolerate erythromycin (n=6) were told to use topical clindamycin phosphate twice daily for 4 weeks. Within 3 weeks 22% of patients had resolution, while 86% had resolution within 4 weeks. All of the patients cleared within 8 weeks. Importantly, there was no significant difference in duration of time until clearance between children who used the oral antibiotic or topical antibiotic.19
Conclusion
We know that the skin of rosacea patients contains higher levels of cathelicidins, which have been implicated in amplifying and contributing to the inflammatory response in several ways. Mast cells, which are a source of cathelicidins, also are increased in the skin of these patients. Children can present with vascular rosacea (characterized by flushing, erythema, and/or telangiectasia), papulopustular rosacea, or ocular rosacea. Common ocular symptoms include blepharitis, conjunctivitis, and recurrent chalazion. It is important to refer pediatric rosacea patients with ocular symptoms to an ophthalmologist to prevent ocular sequelae.
Rosacea is a clinical diagnosis but biopsy can be performed to rule out other diagnoses. Treatment consists of lifestyle modifications such as avoiding known triggers and the use of topical and/or oral agents. Common topical therapies include metronidazole and erythromycin. Systemic antibiotics include tetracycline, doxycycline, minocycline, azithromycin, and erythromycin. Some children are able to taper systemic agents and maintain disease control with topical therapy, while others may need to continue a low-dose antibiotic. Although flares can be controlled within weeks to months, rosacea is a chronic disorder and childhood rosacea tends to persist into adulthood.
Rosacea is a chronic skin disease characterized by flushing, erythema, telangiectasia, papules, and pustules in the central face region.1 It most often affects middle-aged women (age range, 30–50 years).2 Rosacea is rare in the pediatric population, especially before puberty.3 There are 3 subtypes of pediatric rosacea: vascular, papulopustular, and ocular. Phymatous/rhinophymatous rosacea is only seen in the adult population.3 Recommendations for the management of pediatric rosacea heavily rely on data from retrospective chart reviews and case series.
Etiology of Pediatric Rosacea
Rosacea is thought to be a consequence of vasomotor instability in both adults and children. A family history of rosacea is sometimes reported in patients with pediatric rosacea.4 Patients often are sensitive to heat, sunlight, topical corticosteroids, spicy foods, hot liquids, and certain soaps and cleansers.1,3,4 In a review of the literature by Vemuri et al,5 the various reported triggers of rosacea include harsh climates that damage the blood vessels and dermal connective tissue, defects in the endothelium and dermal matrix, perivascular inflammation, orally ingested chemicals, changes in the flora of the hair follicles, excessive antimicrobial peptides, and the presence of free radicals. Overall, it is unclear which of these factors are triggers of pediatric rosacea.
The molecular basis of rosacea has been elucidated. It is well known that rosacea patients have higher levels of cathelicidins in the facial skin. Furthermore, they appear to have different processed forms of cathelicidin peptides compared to adults without rosacea, possibly due to changes in posttranslational processing.6 One such peptide, cathelicidin LL-37, also has been implicated in atopic dermatitis7 and psoriasis.8 Its role in rosacea appears to be multifaceted. Cathelicidin LL-37 helps to attract neutrophils, monocytes, and T lymphocytes, and also has antimicrobial properties; therefore, it plays a role in both the innate and adaptive immune systems.9 Cathelicidin LL-37 also has been implicated in inducing angiogenesis10 and suppressing dermal fibroblasts.11
Muto et al12 found that there is an increased number of mast cells in the dermis of patients with rosacea. Mast cells contribute to vasodilation, angiogenesis, and the recruitment of other inflammatory cells.12 Importantly, human mast cells are a source of cathelicidins including cathelicidin LL-37; these proteins play a vital role in the antimicrobial capabilities of mast cells.13
Clinical Presentation and Comorbidities
Vascular rosacea presents with characteristic flushing and erythema, which lasts more than a few minutes as compared to physiologic erythema,1 and sometimes telangiectasia is seen.3 The cheeks, chin, and nasolabial folds are most commonly involved.2 In papulopustular rosacea, papules and pustules are seen overlying the erythema.1,3 Open and closed comedones also have been documented in case reports but are not commonly seen.2 Pediatric rosacea often begins with flushing of the face and then progresses to the development of papules and pustules.4
Ocular rosacea can occur with or without cutaneous findings. In a retrospective study of 20 pediatric patients (aged 1–15 years), 11 (55%) patients had both ocular and cutaneous rosacea, 3 (15%) only had ocular symptoms, and 6 (30%) only had cutaneous symptoms. The most common form of rosacea in this study was papulopustular rosacea.14 Ocular symptoms often are bilateral15 and can include blepharitis, conjunctival injection, recurrent chalazion, conjunctivitis,2 and less commonly corneal ulceration and scarring.16 Patients also may report photophobia or a foreign body sensation.17 Importantly, ocular symptoms often precede the cutaneous symptoms and can delay the diagnosis of rosacea,14,16,18 as these symptoms often are misdiagnosed as viral or bacterial infections.15 Fortunately, ocular disease responds well to treatment if diagnosed early.
Weston and Morelli19 conducted a retrospective study of 106 children (46 males; 60 females) 13 years and younger with steroid rosacea; 29 children were younger than 3 years. A family history of rosacea was present in 20% of participants, and prior use of class 7 steroids was reported in 54%, whereas only 3% had used class 1 topical steroids. Ninety-eight participants had perinasal involvement, 94 had perioral involvement, and 44 had periorbital involvement of the lower eyelids.19
Rosacea fulminans (also known as pyoderma faciale) is a rare acute-onset eruption typically found in young women in their 20s and 30s.20 Rosacea fulminans is characterized by papules, pustules, nodules, cysts, draining sinuses, communicating sinus tracts, and less commonly comedones.20,21 The skin can appear erythematous, cyanotic, or dull red.21 Most of the lesions are found on the face, particularly on the forehead, cheeks, nose, and chin,21 but lesions on the chest and back have been documented in adult patients.20 In an examination of prior case series, most patients were otherwise healthy. There are case reports documenting rosacea fulminans in teenagers,20 but the youngest patient recorded was an otherwise healthy 3-year-old girl who developed a sudden onset of erythematous papules, pustules, cysts, and purulent discharging sinuses on the cheeks that spread to the chin, perioral, and paranasal areas.21
Differential Diagnosis
Rosacea is rare in children, so other papulopustular disorders must be ruled out, including acne vulgaris, periorificial/perioral dermatitis, sarcoidosis, systemic lupus erythematosus, steroid-induced rosacea, ataxia telangiectasia, and demodicosis.
Acne vulgaris commonly presents in older adolescents and teenagers with open and closed comedones, inflammatory papules, and pustules.2 Intense facial flushing and telangiectasia usually is not seen.
In perioral dermatitis, skin lesions often are clustered around the mouth, nose, and eyes. Typically there are no telangiectases or ocular complications.3 Facial flushing and telangiectases are uncommon, except in steroid-induced perioral dermatitis.2
The cutaneous findings of sarcoidosis include red-brown papules on the face and lips, and patients also may have ocular involvement such as uveitis and iritis.3 However, there are typically other systemic findings such as pulmonary symptoms, weight loss, fatigue, lethargy, fever, and erythema nodosum.2,3 Chest radiograph findings (eg, bilateral hilar lym-phadenopathy), ophthalmologic examination, and laboratory data (eg, elevated alkaline phosphate and/or elevated angiotensin-converting enzyme) can help confirm or rule out the diagnosis of sarcoidosis.2,3
Unlike systemic lupus erythematosus, patients with rosacea will have involvement of sun-protected areas of the skin. Patients with systemic lupus erythematosus typically report arthralgia and severe photosensitivity and will have elevated antinuclear antibody titers. Skin biopsies and immunofluorescence can help confirm the diagnosis.3 Importantly, some patients with rosacea will have a positive lupus band test.22,23
Steroid-induced rosacea typically occurs 2 weeks after discontinuing therapy with topical fluorinated glucocorticosteroids.24 Children present with monomorphic papules, pustules, and telangiectases4 on the eyelids and lateral face as opposed to the central face regions.24
Ataxia telangiectasia can present with telangiectases, skin atrophy, café au lait spots, and premature graying.25 A 15-year-old adolescent girl with ataxia telangiectasia presented with granulomatous acne rosacea that improved after 4 weeks of treatment with isotretinoin 0.5 mg/kg daily. The lesions cleared almost completely after 5 months.25
Demodicosis is a disorder of the pilosebaceous units caused by the human Demodex mite.26 It typically involves the periorificial regions in adults and the elderly population. Patients can present with fine, white-yellow, scaly changes of the sebaceous hair follicles, with minimal erythema and inflammation. Papules and pustules also can be present.26
Diagnosis and Histopathology
Because rosacea is rare in children, it is important to thoroughly evaluate other possible diagnoses. The diagnosis of pediatric rosacea is clinical and biopsies are rarely performed. Laboratory tests such as cultures generally are not useful.
Marks and Harcourt-Webster27 reviewed the biopsies of 108 adult patients with rosacea. The biopsies of patients with predominantly erythema and telangiectasia showed evidence of vascular dilatation with a perivascular infiltrate composed predominantly of lymphocytes and 39 specimens that were compared to controls showed more solar elastosis. Biopsies of papular rosacea contained inflammatory infiltrates in the upper and mid dermis composed primarily of lymphocytes and histiocytes. In some patients, neutrophils, plasma cells, and giant cells also were observed. Hair follicle abnormalities were present in 20% of the biopsies, with 19% showing evidence of the Demodex mite. Vascular dilatation also was common. Overall, common findings included lymphohistiocytic infiltrates around the blood vessels of the upper dermis, dilated vessels, edema, elastosis, and disorganization of connective tissue in the upper dermis.
Helm et al28 reviewed histopathologic patterns from 53 patients with granulomatous rosacea. Findings included a mixed lymphohistiocytic infiltrate (predominantly lymphocytic in 40% of patients and predominantly histiocytic with occasional giant cells in 34% of patients), epithelioid granulomas (11% of patients), and epithelioid granulomas with caseation necrosis (11% of patients).
The histopathology of rosacea fulminans is characterized by dense perivascular and periadnexal infiltrates composed of granulocytes, eosinophils, and epithelioid granulomas, as well as panniculitis.20
Treatment and Clinical Outcomes
Certain lifestyle recommendations are integral components of disease management, including avoidance of triggers such as extreme temperatures, hot drinks, spicy food, and topical agents that could be irritating (especially topical corticosteroids).29 Patients should be advised to use daily sunscreen containing physical blockers such as titanium dioxide or zinc oxide. Teenagers should avoid the use of cosmetics and makeup, especially products containing sodium lauryl sulfate, menthol, and camphor. Daily use of emollients can help some patients.29
There are both topical and systemic therapies available for pediatric rosacea; however, most of the data are based on the use of these treatments in the adult population. Patients with mild to moderate disease often can be managed using topical agents. Metronidazole (0.75% cream, 1% gel, or 0.75% lotion) has been studied extensively in adult patients, and when used once daily for 12 weeks, it has been able to control moderate to severe disease.30,31 In one study conducted in adult patients, topical metronidazole was able to maintain remission in adults who had previously been treated with a combination of oral tetracycline and metronidazole gel.31 Sodium sulfacetamide 10%–sulfur 5% (cleanser or lotion) has been successful in adult patients and often is used in combination with other therapies such as topical metronidazole.32-34 Azelaic acid cream 20%,35 benzoyl peroxide (wash or gel),29 topical clindamycin,36 topical erythromycin,29,37 tacrolimus ointment 0.1%,38 and tretinoin cream also have been studied in adults.3,39 Several of these topical agents can cause irritation on application (eg, metronidazole, sulfur-based agents, azelaic acid, benzoyl peroxide, erythromycin, tretinoin).3
The use of systemic treatments in pediatric patients is heavily based on case reports and case series.2,14,16,40 Therapies have included tetracycline (500 mg twice daily tapered to 250 mg daily),29 minocycline (50–100 mg twice daily), doxycycline (50–100 mg twice daily or 4 times daily), erythromycin (30–50 mg/kg daily), clarithromycin (15 mg/kg twice daily for 4 weeks and then daily for 4 weeks), and azithromycin (5–10 mg/kg daily).3 Tetracycline antibiotics should not be used in children 8 years or younger.
In a case series by Drolet and Paller,2 an 11-year-old girl was treated with tetracycline 500 mg (later tapered to 250 mg daily) and metronidazole gel 0.75%, both used twice daily. Previously, she had not responded to topical steroids, tretinoin cream 0.05%, benzoyl peroxide 5%, or systemic prednisone. After 6 weeks of treatment, the pustules and chalazion had resolved and she had only minimal erythema of the skin and conjunctiva. Sixteen months after the start of treatment, a regimen of tetracycline 250 mg daily and metronidazole gel resulted in disease clearance on the face.2
A 9-year-old girl with concurrent systemic lupus erythematosus was treated with tetracycline 250 mg and topical erythromycin 2%, both used twice daily.2 After 4 weeks her face was clear. Four months later she developed new telangiectases and topical erythromycin was replaced with topical metronidazole. Eventually the dose of tetracycline was reduced to 250 mg daily.2
An 11-year-old boy with likely granulomatous rosacea was treated with erythromycin 250 mg 4 times daily, alclometasone dipropionate cream 0.05% twice daily, and topical clindamycin twice daily.2 Marked improvement was noticed after 3 weeks of treatment. Metronidazole gel 0.75% was added and 3 months later the patient’s face was clear, without evidence of scarring. The dose of erythromycin was later reduced to 500 mg daily, and eventually the patient experienced clearance with the use of metronidazole gel daily.2
In another case series, 4 female patients (age range, 4–12 years) were treated with systemic erythromycin 20 mg/kg daily (ocular involvement only) or doxycycline 2.2 mg/kg daily used in two 12-year-old patients with ocular and cutaneous involvement for at least 12 months. All 4 patients showed considerable improvement within 1 month and remained free of disease throughout a mean follow-up period of 25.5 months.40
As evidenced by these case reports, there is a wide array of treatments that have been used for pediatric rosacea. Although there are no formal evidence-based guidelines, there are certain considerations that must be taken into account when choosing treatment plans. Doxycycline and minocycline are known to cause less gastrointestinal upset than tetracycline with similar efficacy.41 Importantly, the tetracyclines are contraindicated in children younger than 9 years, as they can cause teeth staining and possibly affect skeletal growth.3,4 When used in older children (age range, 9–12 years), patients must be advised not to take their medication with calcium or antacids.3 Clarithromycin and azithromycin tend to have fewer gastrointestinal side effects than erythromycin. Erythromycin and other macrolides can be used in children of all ages and in patients who are allergic to tetracyclines.3
Children with mild ocular symptoms often can control their disease with bacitracin and topical ocular antibiotics such as erythromycin.2,15 For patients who require systemic antibiotics, various tetracyclines and macrolides have been used with success.2,14-16,40
Adults with rosacea fulminans can require treatment with isotretinoin, oral antibiotics, and topical or even systemic corticosteroids.42 The 3-year-old girl with rosacea fulminans initially was treated with oral erythromycin (250 mg 4 times daily), oral prednisolone (0.5 mg/kg daily tapered over 2 weeks), fluocinolone acetonide cream 0.025%, and warm compresses with only moderate improvement.21 She was then started on oral isotretinoin (0.75 mg/kg daily) and within 4 weeks marked improvement was noted. After 8 weeks, the lesions had disappeared completely with only a few pitted scars remaining. Isotretinoin was continued for 24 weeks. One year after completion of treatment, she was still disease free.21
Weston and Morelli19 recommended the following treatment regimen for children with steroid rosacea: abrupt cessation of topical steroid use (as opposed to gradual withdrawal) and initiation of oral erythromycin stearate (30 mg/kg daily) in 2 daily doses for 4 weeks. Children who were unable to tolerate erythromycin (n=6) were told to use topical clindamycin phosphate twice daily for 4 weeks. Within 3 weeks 22% of patients had resolution, while 86% had resolution within 4 weeks. All of the patients cleared within 8 weeks. Importantly, there was no significant difference in duration of time until clearance between children who used the oral antibiotic or topical antibiotic.19
Conclusion
We know that the skin of rosacea patients contains higher levels of cathelicidins, which have been implicated in amplifying and contributing to the inflammatory response in several ways. Mast cells, which are a source of cathelicidins, also are increased in the skin of these patients. Children can present with vascular rosacea (characterized by flushing, erythema, and/or telangiectasia), papulopustular rosacea, or ocular rosacea. Common ocular symptoms include blepharitis, conjunctivitis, and recurrent chalazion. It is important to refer pediatric rosacea patients with ocular symptoms to an ophthalmologist to prevent ocular sequelae.
Rosacea is a clinical diagnosis but biopsy can be performed to rule out other diagnoses. Treatment consists of lifestyle modifications such as avoiding known triggers and the use of topical and/or oral agents. Common topical therapies include metronidazole and erythromycin. Systemic antibiotics include tetracycline, doxycycline, minocycline, azithromycin, and erythromycin. Some children are able to taper systemic agents and maintain disease control with topical therapy, while others may need to continue a low-dose antibiotic. Although flares can be controlled within weeks to months, rosacea is a chronic disorder and childhood rosacea tends to persist into adulthood.
- Crawford GH, Pelle MT, James WD. Rosacea: i. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341; quiz 342-324.
- Drolet B, Paller AS. Childhood rosacea. Pediatr Dermatol. 1992;9:22-26.
- Kroshinsky D, Glick SA. Pediatric rosacea. Dermatol Ther. 2006;19:196-201.
- Lacz NL, Schwartz RA. Rosacea in the pediatric population. Cutis. 2004;74:99-103.
- Vemuri RC, Gundamaraju R, Sekaran SD, et al. Major pathophysiological correlations of rosacea: a complete clinical appraisal. Int J Med Sci. 2015;12:387-396.
- Yamasaki K, Di Nardo A, Bardan A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975-980.
- Ong PY, Ohtake T, Brandt C, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151-1160.
- Lande R, Gregorio J, Facchinetti V, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564-569.
- De Y, Chen Q, Schmidt AP, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069-1074.
- Koczulla R, von Degenfeld G, Kupatt C, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111:1665-1672.
- Park HJ, Cho DH, Kim HJ, et al. Collagen synthesis is suppressed in dermal fibroblasts by the human antimicrobial peptide LL-37. J Invest Dermatol. 2009;129:843-850.
- Muto Y, Wang Z, Vanderberghe M, et al. Mast cells are key mediators of cathelicidin-initiated skin inflammation in rosacea. J Invest Dermatol. 2014;134:2728-2736.
- Di Nardo A, Vitiello A, Gallo RL. Cutting edge: mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J Immunol. 2003;170:2274-2278.
- Leoni S, Mesplie N, Aitali F, et al. Metronidazole: alternative treatment for ocular and cutaneous rosacea in the pediatric population [in French]. J Fr Ophthalmol. 2011;34:703-710.
- Nazir SA, Murphy S, Siatkowski RM, et al. Ocular rosacea in childhood. Am J Ophthalmol. 2004;137:138-144.
- Miguel AI, Salgado MB, Lisboa MS, et al. Pediatric ocular rosacea: 2 cases. Eur J Ophthalmol. 2012;22:664-666.
- Stone DU, Chodosh J. Ocular rosacea: an update on pathogenesis and therapy. Curr Opin Ophthalmol. 2004;15:499-502.
- Mavrakanas N, Schutz JS, Dosso AA. Pediatric ocular rosacea [published online March 22, 2010]. J Pediatr Ophthalmol Strabismus. 2010;47:117-120.
- Weston WL, Morelli JG. Steroid rosacea in prepubertal children. Arch Pediatr Adolesc Med. 2000;154:62-64.
- Plewig G, Jansen T, Kligman AM. Pyoderma faciale. a review and report of 20 additional cases: is it rosacea? Arch Dermatol. 1992;128:1611-1617.
- Firooz A, Firoozabadi MR, Dowlati Y. Rosacea fulminans (pyoderma faciale): successful treatment of a 3-year-old girl with oral isotretinoin. Int J Dermatol. 2001;40:203-205.
- Baart de la Faille H, Baart de la F-K. Immunofluorescent studies of the skin in rosacea. Dermatologica. 1969;139:49-54.
- Wilkin JK. Rosacea. Int J Dermatol. 1983;22:393-400.
- Franco HL, Weston WL. Steroid rosacea in children. Pediatrics. 1979;64:36-38.
- Cantarutti N, Claps A, Angelino G, et al. Multi-drugs resistant acne rosacea in a child affected by ataxia-telangiectasia: successful treatment with isotretinoin. Ital J Pediatr. 2015;41:23.
- Chen W, Plewig G. Human demodicosis: revisit and a proposed classification. Br J Dermatol. 2014;170:1219-1225.
- Marks R, Harcourt-Webster J. Histopathology of rosacea. Arch Dermatol. 1969;100:683-691.
- Helm KF, Menz J, Gibson LE, et al. A clinical and histopathologic study of granulomatous rosacea. J Am Acad Dermatol. 1991;25(6, pt 1):1038-1043.
- Pelle MT, Crawford GH, James WD. Rosacea: II. therapy. J Am Acad Dermatol. 2004;51:499-512; quiz 513-494.
- Dahl MV, Jarratt M, Kaplan D, et al. Once-daily topical metronidazole cream formulations in the treatment of the papules and pustules of rosacea. J Am Acad Dermatol. 2001;45:723-730.
- Dahl MV, Katz HI, Krueger GG, et al. Topical metronidazole maintains remissions of rosacea. Arch Dermatol. 1998;134:679-683.
- Trumbore MW, Goldstein JA, Gurge RM. Treatment of papulopustular rosacea with sodium sulfacetamide 10%/sulfur 5% emollient foam. J Drugs Dermatol. 2009;8:299-304.
- Del Rosso JQ. Evaluating the role of topical therapies in the management of rosacea: focus on combination sodium sulfacetamide and sulfur formulations. Cutis. 2004;73(1 suppl):29-33.
- Del Rosso JQ. A status report on the medical management of rosacea: focus on topical therapies. Cutis. 2002;70:271-275.
- Maddin S. A comparison of topical azelaic acid 20% cream and topical metronidazole 0.75% cream in the treatment of patients with papulopustular rosacea. J Am Acad Dermatol. 1999;40(6, pt 1):961-965.
- Wilkin JK, DeWitt S. Treatment of rosacea: topical clindamycin versus oral tetracycline. Int J Dermatol. 1993;32:65-67.
- Mills OH Jr, Kligman AM. Letter: topically applied erythromycin in rosacea. Arch Dermatol. 1976;112:553-554.
- Hengge UR. Off-label indications for topical tacrolimus [in German]. Hautarzt. 2013;64:752-756.
- Ertl GA, Levine N, Kligman AM. A comparison of the efficacy of topical tretinoin and low-dose oral isotretinoin in rosacea. Arch Dermatol. 1994;130:319-324.
- Cetinkaya A, Akova YA. Pediatric ocular acne rosacea: long-term treatment with systemic antibiotics. Am J Ophthalmol. 2006;142:816-821.
- Maibach H. Second-generation tetracyclines, a dermatologic overview: clinical uses and pharmacology. Cutis. 1991;48:411-417.
- Jansen T, Plewig G, Kligman AM. Diagnosis and treatment of rosacea fulminans. Dermatology. 1994;188:251-254.
- Crawford GH, Pelle MT, James WD. Rosacea: i. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341; quiz 342-324.
- Drolet B, Paller AS. Childhood rosacea. Pediatr Dermatol. 1992;9:22-26.
- Kroshinsky D, Glick SA. Pediatric rosacea. Dermatol Ther. 2006;19:196-201.
- Lacz NL, Schwartz RA. Rosacea in the pediatric population. Cutis. 2004;74:99-103.
- Vemuri RC, Gundamaraju R, Sekaran SD, et al. Major pathophysiological correlations of rosacea: a complete clinical appraisal. Int J Med Sci. 2015;12:387-396.
- Yamasaki K, Di Nardo A, Bardan A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975-980.
- Ong PY, Ohtake T, Brandt C, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151-1160.
- Lande R, Gregorio J, Facchinetti V, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564-569.
- De Y, Chen Q, Schmidt AP, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069-1074.
- Koczulla R, von Degenfeld G, Kupatt C, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111:1665-1672.
- Park HJ, Cho DH, Kim HJ, et al. Collagen synthesis is suppressed in dermal fibroblasts by the human antimicrobial peptide LL-37. J Invest Dermatol. 2009;129:843-850.
- Muto Y, Wang Z, Vanderberghe M, et al. Mast cells are key mediators of cathelicidin-initiated skin inflammation in rosacea. J Invest Dermatol. 2014;134:2728-2736.
- Di Nardo A, Vitiello A, Gallo RL. Cutting edge: mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J Immunol. 2003;170:2274-2278.
- Leoni S, Mesplie N, Aitali F, et al. Metronidazole: alternative treatment for ocular and cutaneous rosacea in the pediatric population [in French]. J Fr Ophthalmol. 2011;34:703-710.
- Nazir SA, Murphy S, Siatkowski RM, et al. Ocular rosacea in childhood. Am J Ophthalmol. 2004;137:138-144.
- Miguel AI, Salgado MB, Lisboa MS, et al. Pediatric ocular rosacea: 2 cases. Eur J Ophthalmol. 2012;22:664-666.
- Stone DU, Chodosh J. Ocular rosacea: an update on pathogenesis and therapy. Curr Opin Ophthalmol. 2004;15:499-502.
- Mavrakanas N, Schutz JS, Dosso AA. Pediatric ocular rosacea [published online March 22, 2010]. J Pediatr Ophthalmol Strabismus. 2010;47:117-120.
- Weston WL, Morelli JG. Steroid rosacea in prepubertal children. Arch Pediatr Adolesc Med. 2000;154:62-64.
- Plewig G, Jansen T, Kligman AM. Pyoderma faciale. a review and report of 20 additional cases: is it rosacea? Arch Dermatol. 1992;128:1611-1617.
- Firooz A, Firoozabadi MR, Dowlati Y. Rosacea fulminans (pyoderma faciale): successful treatment of a 3-year-old girl with oral isotretinoin. Int J Dermatol. 2001;40:203-205.
- Baart de la Faille H, Baart de la F-K. Immunofluorescent studies of the skin in rosacea. Dermatologica. 1969;139:49-54.
- Wilkin JK. Rosacea. Int J Dermatol. 1983;22:393-400.
- Franco HL, Weston WL. Steroid rosacea in children. Pediatrics. 1979;64:36-38.
- Cantarutti N, Claps A, Angelino G, et al. Multi-drugs resistant acne rosacea in a child affected by ataxia-telangiectasia: successful treatment with isotretinoin. Ital J Pediatr. 2015;41:23.
- Chen W, Plewig G. Human demodicosis: revisit and a proposed classification. Br J Dermatol. 2014;170:1219-1225.
- Marks R, Harcourt-Webster J. Histopathology of rosacea. Arch Dermatol. 1969;100:683-691.
- Helm KF, Menz J, Gibson LE, et al. A clinical and histopathologic study of granulomatous rosacea. J Am Acad Dermatol. 1991;25(6, pt 1):1038-1043.
- Pelle MT, Crawford GH, James WD. Rosacea: II. therapy. J Am Acad Dermatol. 2004;51:499-512; quiz 513-494.
- Dahl MV, Jarratt M, Kaplan D, et al. Once-daily topical metronidazole cream formulations in the treatment of the papules and pustules of rosacea. J Am Acad Dermatol. 2001;45:723-730.
- Dahl MV, Katz HI, Krueger GG, et al. Topical metronidazole maintains remissions of rosacea. Arch Dermatol. 1998;134:679-683.
- Trumbore MW, Goldstein JA, Gurge RM. Treatment of papulopustular rosacea with sodium sulfacetamide 10%/sulfur 5% emollient foam. J Drugs Dermatol. 2009;8:299-304.
- Del Rosso JQ. Evaluating the role of topical therapies in the management of rosacea: focus on combination sodium sulfacetamide and sulfur formulations. Cutis. 2004;73(1 suppl):29-33.
- Del Rosso JQ. A status report on the medical management of rosacea: focus on topical therapies. Cutis. 2002;70:271-275.
- Maddin S. A comparison of topical azelaic acid 20% cream and topical metronidazole 0.75% cream in the treatment of patients with papulopustular rosacea. J Am Acad Dermatol. 1999;40(6, pt 1):961-965.
- Wilkin JK, DeWitt S. Treatment of rosacea: topical clindamycin versus oral tetracycline. Int J Dermatol. 1993;32:65-67.
- Mills OH Jr, Kligman AM. Letter: topically applied erythromycin in rosacea. Arch Dermatol. 1976;112:553-554.
- Hengge UR. Off-label indications for topical tacrolimus [in German]. Hautarzt. 2013;64:752-756.
- Ertl GA, Levine N, Kligman AM. A comparison of the efficacy of topical tretinoin and low-dose oral isotretinoin in rosacea. Arch Dermatol. 1994;130:319-324.
- Cetinkaya A, Akova YA. Pediatric ocular acne rosacea: long-term treatment with systemic antibiotics. Am J Ophthalmol. 2006;142:816-821.
- Maibach H. Second-generation tetracyclines, a dermatologic overview: clinical uses and pharmacology. Cutis. 1991;48:411-417.
- Jansen T, Plewig G, Kligman AM. Diagnosis and treatment of rosacea fulminans. Dermatology. 1994;188:251-254.
Practice Points
- Although rosacea is largely a diagnosis of adults, it also can begin in childhood and adolescence.
- Ocular rosacea and papulopustular disease are common clinical findings in younger patients.
- Usage of topical metronidazole and age-appropriate oral antibiotics are the mainstay of management.
A Practical Overview of Pediatric Atopic Dermatitis, Part 3: Differential Diagnosis, Comorbidities, and Measurement of Disease Burden
In parts 1 and 2 of this series on atopic dermatitis (AD),1,2 the current putative pathogenesis, scoring systems for severity grading, and epidemiology were reviewed. Part 3 reviews the differential diagnosis, with an emphasis on the difficulty of differentiation from some rare but notable illnesses, as well as the recently expanding data on comorbidities that identify AD as a multisystem disorder with widespread health implications for the patient.
Differential Diagnosis for Pediatric AD
The differential diagnosis for pediatric AD includes chronic dermatoses (eg, seborrheic dermatitis, psoriasis), congenital disorders (eg, Netherton syndrome), malignant diseases (eg, cutaneous T-cell lymphoma [CTCL]), immunodeficiencies, infections, and metabolic disorders.3 Netherton syndrome must be ruled out to prevent extensive drug absorption when treating with topical calcineurin inhibitors (TCIs).4 Due to the presence of bamboo hairs in these patients, a hair mount may aid in the diagnosis of Netherton syndrome. Misdiagnosis of CTCL as AD may complicate the analysis of safety data on TCIs.4,5 Multiple skin biopsies are essential in cases of suspected CTCL to provide an accurate diagnosis. Biopsy can be considered in AD cases with changing and/or unusual morphology, erythrodermic skin changes, and disease that is poorly responsive to multiple therapeutic modalities.
Comorbidities in Pediatric AD
Psychosocial Comorbidities
Pediatric AD often takes a psychological toll on patients as well as household members. Almost half of children with AD are reported to have a severely impaired quality of life (QOL).6 Contributing factors include fatigue, sleep disturbance, activity restriction (eg, inability to participate in sports), and depression.7
Chamlin et al8 developed the Childhood Atopic Dermatitis Impact Scale (CADIS), a 45-item instrument (refined from a 62-item prototype), to measure QOL in young children with AD and their family members. Responses were evaluated with consideration of 5 domains: symptoms and activity limitations/behaviors in children, as well as family/social function, sleep, and emotions in parents. The top 12 factors that parents found most bothersome about AD included itching/scratching, child’s pain/discomfort, sleep issues, embarrassment or worry about appearance, child’s fussiness/irritability/crying/unhappiness, helplessness/can’t control it/predict it, worry about skin infection, dryness of skin/nonsmooth skin, skin bleeding, worry about damage/scars, stares/comments of strangers and other children, and rashes/redness of skin/discoloration. Parents were asked to respond to items about their emotional health and social functioning, such as “My child’s skin condition has strained my relationship with my spouse or partner,” “My child’s skin condition makes me feel sad or depressed,” and “I am bothered by the reaction of strangers to this skin condition.”8
Kiebert et al9 found that AD patients had lower scores on the Short Form-36 Health Survey’s vitality, social functioning, and mental health subscales compared to individuals in the general population. The authors noted that anxiety in AD patients is of particular concern, as stress has been found to trigger the itch-scratch cycle, potentially setting off AD flare-ups.9 Family impact of AD is aggravated by disease severity. Sleeplessness, relationship stress, and time management can all cause family problems in patients with AD.8
In a survey of 3775 older teenagers aged 18 to 19 years (80% response rate out of 4774 prospective participants), 9.7% of participants reported having current AD.10 Suicidal ideation was higher in those with current AD than those without AD (15.5% vs 9.1%). The prevalence of suicidal ideation rose to 23.8% in those with both AD and itch. Diagnosis of AD (as determined through participant responses to the question, ‘‘Do you have, or have you had eczema?’’) was associated with mental health problems in 16.0% of those with AD compared to 10.1% of those without AD, with an especially reduced likelihood of romantic relationships for adolescent boys with AD, as measured using the Strength and Difficulties Questionnaire, which measures 4 problem domains and assesses presence of mental health issues in the past 6 months, and the Hopkins Symptom Checklist 10, which uses 10 questions to measure anxiety and depression symptoms in the past week.10
Dalgard et al11 assessed whether the psychological burden of AD persists in adulthood in an international, multicenter, observational, cross-sectional study conducted in 13 European countries. Each dermatology clinic recruited 250 consecutive adult outpatients to complete a questionnaire along with a control group of 125 hospital employees without skin disease from the same institution but from different departments. The study included a total of 4994 participants (3635 patients and 1359 controls). Clinical depression and anxiety were present in 10.1% and 17.6% of patients, respectively, versus 4.3% and 11.1% of controls, respectively. The prevalence of depression and anxiety was highest in patients with leg ulcers, hand eczema, psoriasis, and AD.11 This study demonstrated that the psychological comorbidities of childhood conditions such as AD may persist into adulthood.
Lymphoma
In a systematic review of the literature and a separate meta-analysis, Legendre et al12 identified a slight increase in lymphoma among AD patients, with an uncertain but potential increase associated with topical corticosteroid application. This finding is similar to trends seen in other systemic inflammatory conditions that involve the skin, such as psoriasis, and is felt to relate to long-term inflammation.
Obesity
Obesity has been associated with a greater risk for moderate to severe AD in children.13,14
Infections
Children with AD are at a higher risk for cutaneous infections and generalization of these infections. The leading infections would be with Staphylococcus aureus, but group A streptococci infections do occur. Herpes simplex virus, vaccinia virus or Kaposi varicelliform eruption (KVE), molluscum with or without dermatitis, and fungal infections occur less commonly but with greater morbidity, largely due to the impaired barrier and some innate reduction in cutaneous immunity.15
Atopic dermatitis in children also is associated with a higher prevalence of extracutaneous infections such as influenza, pneumonia, urinary tract infections, varicella-zoster virus, recurrent ear infections, sinus infections, sore throat, and head or chest colds.16 Children with AD and warts (human papillomavirus infection) have an even greater risk for these comorbidities.17 Warts and molluscum infections may become more extensive in children with AD.18 Generalization of herpetic infections occurs more easily in AD patients due to the impaired skin barrier, which includes generalized skin surface extension of herpes simplex virus type 1, varicella-zoster virus, and historically smallpox. A similar clinical appearance of generalized vesiculopustular lesions with fever can be seen when coxsackievirus A6 infections occur in AD patients; these conditions are called eczema herpeticum due to herpes simplex virus, KVE due to varicella-zoster virus and smallpox, and eczema coxsackium due to coxsackievirus A6,19 though some authors refer to all of these as KVE.20 These generalized viral illnesses overlying AD often result in fever, malaise, pain, and life-threatening skin denudation with risk for dehydration and superinfection with S aureus.7,18 It has been shown that the occurrence of eczema herpeticum in AD is associated with and may be caused by an inability to induce human β-defensin 2 and 3 as well as cathelicidin.21
Staphylococcus aureus colonization has been noted in 90% to 100% of AD cases, which can be associated with a higher eczema area and severity index score.22-24 The role of S aureus in AD includes flare triggering through release of superantigens, leading to IL-31–induced pruritis.25 Recurrent infection with either methicillin-sensitive or methicillin-resistant S aureus has been noted in AD.18,26 Skin infections also occur in AD and appear as erosions and pustules, and coinfection with Streptococcus and Staphylococcus does occur; therefore, cultures often are needed to determine the type of bacteria present on the skin in severe cases and when infection is suspected.27 Perianal bacterial dermatitis is a variant of infected AD occurring in the anal/groin area that is associated with S aureus and/or streptococcal superinfection in which topical corticosteroids and topical anti-infectives can be used. In some severe cases, oral antibiotics may be needed.28
Injury/Hyperactivity
Children aged 0 to 5 years with AD carry an increased risk for injuries requiring medical attention, with association in part due to attention deficit disorder, depression, and anxiety. Antihistamines are believed to aggravate this issue by promoting daytime somnolence29; however, pruritus-induced sleep disturbances in AD also may be responsible for daytime somnolence.30
Contact Allergy and Sensitization
Children with AD may become sensitized to environmental allergens through delayed-type hypersensitivity. The presumed mechanism is that these agents include ingredients added into applied medicaments and application occurs over an impaired skin barrier allowing for absorption and greater risk of antigen presentation. Approximately 50% of children with difficult-to-control AD will react to 1 or more epicutaneous allergens, and patch testing can be performed to identify relevant allergens that can improve skin severity.7 Severe dermatitis and id generalized hypersensitivity reactions in patients with AD and nickel allergic contact dermatitis have been described and may aggravate underlying AD.31
Family Burden of AD
Parents or caregivers of children with moderate and severe AD spend nearly 3 hours a day caring for their child’s skin and experience QOL impairments including lack of sleep and/or privacy, often due to cosleeping; treatment-related financial expenditures; and feelings of hopelessness, guilt, and depression.7
Steroid Phobia
Steroid phobia is the fear of topical application of corticosteroids resulting in systemic side effects including unrealistic fears (eg, fear that the child will develop muscles such as an anabolic steroid user) as well as realistic but statistically low-risk fears (eg, fear of systemic absorption). These fears often result in underutilization of prescribed topical corticosteroid therapies and undertreatment of children with AD.32,33
Financial Burden
The cost of AD can be high in the United States, with adult data demonstrating costs ranging from $371 to $489 per person.34 The last published cost data for pediatric AD was from 2003, with an average cost of $219 per year.35 Costs include time lost from work, household purchases (eg, skin care products), and co-pays for visits and medication, with an estimated average expenditure per person (SE) of $601.06 ($137.26) annually in 2012.36 The cost of ambulatory care and emergency department visits for AD in children in the United States in 1993 was estimated at $364 million.37-39 In 2002, Ellis et al40 estimated the overall cost of AD to be between $900 million and $3.8 billion in the United States (1997-1998) based on projections from claims, prescriptions, and comorbidities reported to a private insurer and Medicaid. Ellis et al41 further determined that topical tacrolimus was similar in cost to high-potency corticosteroids.
Pediatric AD often progresses to adult hand eczema and leads to further morbidity, especially in health care workers.42 Kemp43 reviewed the cost of AD in children and concluded that AD was a condition with major handicap with personal, financial, and social effects. A cost review of studies conducted in 163,700 children with AD showed that costs related to AD totaled $316.7 million per year. The author concluded that there were substantial psychosocial and financial stresses associated with pediatric AD but no clear path to potential reduction in related costs.43
Sleep Disturbances
Sleep disturbances are common in pediatric AD patients. Pruritus usually is exacerbated at bedtime due to reduced humidity and lack of distractions to prevent scratching. Sleep deprivation has a substantial impact on both the patient and his/her household. Parental frustration increases with sleep disturbance.18,44 Sleep deprivation is associated with greater severity, both because it is one of the most difficult aspects of illness and because the associated pruritus makes for greater damage done to the skin through injurious scratching.
Sleep disturbances also may interfere with growth and overnight release of growth hormones.18,44 This latter issue can result in reduced linear growth velocity. Furthermore, sleep deprivation can cause increased risk of accidents and poor school performance.18,44,45
Many children do not outgrow AD. In adults, AD-associated sleep deprivation has been shown to have an association with fatigue, regular daytime sleepiness, and regular insomnia, correlating to number of sick days, doctor visits, and poorer overall health status.45
Inadequate Disease Control
Inadequate disease control has been described by Eichenfeld46 as an important issue in AD at this time. Untreated, undertreated, and improperly treated AD are important issues affecting long-term AD care. He further cited steroid phobia as a contributor to undertreatment.46 Fleischer47 has cited the black box warning present on TCIs as a further deterrent to adequate therapeutic control in our current therapeutic paradigm. Undertreatment may result in uncontrolled disease activity, impaired QOL, infections, and sleep disturbances. The role of undertreatment as a driver of the atopic march is unknown.
Conclusion
Atopic dermatitis is a multisystem disorder that has wide-reaching comorbidities and may mimic a variety of skin conditions. The topic of comorbidities is new and emerging and bears further review to define risk factors, prevention strategies, and long-term monitoring requirements.
- Silverberg NB. A practical overview of pediatric atopic dermatitis, part 1: epidemiology and pathogenesis. Cutis. 2016;97:267-271.
- Silverberg NB. A practical overview of pediatric atopic dermatitis, part 2: triggers and grading. Cutis. 2016;97:326-329.
- Siegfried EC, Hebert AA. Diagnosis of atopic dermatitis: mimics, overlaps, and complications. J Clin Med. 2015;4:884-917.
- Allen A, Siegfried E, Silverman R, et al. Significant absorption of topical tacrolimus in 3 patients with Netherton syndrome. Arch Dermatol. 2001;137:747-750.
- Carr WW. Topical calcineurin inhibitors for atopic dermatitis: review and treatment recommendations. Paediatr Drugs. 2013;15:303-310.
- Chamlin SL, Lai JS, Cella D, et al. Childhood Atopic Dermatitis Impact Scale: reliability, discriminative and concurrent validity, and responsiveness. Arch Dermatol. 2007;143:768-772.
- Tollefson MM, Bruckner AL. Atopic dermatitis: skin-directed management. Pediatrics. 2014;134:E1735-E1744.
- Chamlin SL, Cella D, Frieden IJ, et al. Development of the Childhood Atopic Dermatitis Impact Scale: initial validation of a quality-of-life measure for young children with atopic dermatitis and their families. J Invest Dermatol. 2005;125:1106-1111.
- Kiebert G, Sorensen SV, Revicki D, et al. Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol. 2002;41:151-158.
- Halvorsen JA, Lien L, Dalgard F, et al. Suicidal ideation, mental health problems, and social function in adolescents with eczema: a population-based study. J Invest Dermatol. 2014;134:1847-1854.
- Dalgard FJ, Gieler U, Tomas-Aragones L, et al. The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Invest Dermatol. 2015;135:984-991.
- Legendre L, Barnetche T, Mazereeuw-Hautier J, et al. Risk of lymphoma in patients with atopic dermatitis and the role of topical treatment: a systematic review and meta-analysis. J Am Acad Dermatol. 2015;72:992-1002.
- Koutroulis I, Magnelli L, Gaughan J, et al. Atopic dermatitis is more severe in children over the age of two who have an increased body mass index. Acta Paediatr. 2015;104:713-717.
- Silverberg JI, Becker L, Kwasny M, et al. Central obesity and high blood pressure in pediatric patients with atopic dermatitis. JAMA Dermatol. 2015;151:144-152.
- De D, Kanwar AJ, Handa S. Comparative efficacy of Hanifin and Rajka’s criteria and the UK working party’s diagnostic criteria in diagnosis of atopic dermatitis in a hospital setting in North India. J Eur Acad Dermatol Venereol. 2006;20:853-859.
- Silverberg JI, Silverberg NB. Childhood atopic dermatitis and warts are associated with increased risk of infection: a US population-based study [published online October 4, 2013]. J Allergy Clin Immunol. 2014;133:1041-1047.
- Silverberg J, Garg N, Silverberg NB. New developments in comorbidities of atopic dermatitis. Cutis. 2014;93:222-224.
- Kiken DA, Silverberg NB. Atopic dermatitis in children, part 1: epidemiology, clinical features, and complications. Cutis. 2006;78:241-247.
- Mathes EF, Oza V, Frieden IJ, et al. “Eczema coxsackium” and unusual cutaneous findings in an enterovirus outbreak. Pediatrics. 2013;132:E149-E157.
- Vora RV, Pilani AP, Jivani NB, et al. Kaposi varicelliform eruption. Indian Dermatol Online J. 2015;6:364-366.
- Hata TR, Kotol P, Boguniewicz M, et al. History of eczema herpeticum is associated with the inability to induce human β-defensin (HBD)-2, HBD-3 and cathelicidin in the skin of patients with atopic dermatitis. Br J Dermatol. 2010;163:659-661.
- Abeck D, Mempel M. Staphylococcus aureus colonization in atopic dermatitis and its therapeutic implications. Br J Dermatol. 1998;139:13-16.
- Leyden JJ, Marples RR, Kligman AM. Staphylococcus aureus in the lesions of atopic dermatitis. Br J Dermatol. 1974;90:525-530.
- Lipnharski C, d’Azevedo PA, Quinto VP, et al. Colonization by S. aureus increases the EASI and the number of appointments by patients with atopic dermatitis: cohort with 93 patients. An Bras Dermatol. 2013;88:518-521.
- Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
- Groner A, Laing-Grayman D, Silverberg NB. Outpatient pediatric community-acquired methicillin-resistant Staphylococcus aureus: a polymorphous clinical disease. Cutis. 2008;81:115-122.
- Sugarman JL, Hersh AL, Okamura T, et al. A retrospective review of streptococcal infections in pediatric atopic dermatitis. Pediatr Dermatol. 2011;28:230-234.
- Heath C, Desai N, Silverberg NB. Recent microbiological shifts in perianal bacterial dermatitis: Staphylococcus aureus predominance. Pediatr Dermatol. 2009;26:696-700.
- Garg N, Silverberg JI. Association between childhood allergic disease, psychological comorbidity, and injury requiring medical attention. Ann Allergy Asthma Immunol. 2014;112:525-532.
- Lavery MJ, Stull C, Kinney MO, et al. Nocturnal pruritus: the battle for a peaceful night’s sleep. Int J Mol Sci. 2016;17:E425.
- Silverberg NB, Licht J, Friedler S, et al. Nickel contact hypersensitivity in children. Pediatr Dermatol. 2002;19:110-113.
- Aubert-Wastiaux H, Moret L, Le Rhun A, et al. Topical corticosteroid phobia in atopic dermatitis: a study of its nature, origins and frequency. Br J Dermatol. 2011;165:808-814.
- Kojima R, Fujiwara T, Matsuda A, et al. Factors associated with steroid phobia in caregivers of children with atopic dermatitis. Pediatr Dermatol. 2013;30:29-35.
- Silverberg JI. Health care utilization, patient costs, and access to care in US adults with eczema: a population-based study. JAMA Dermatol. 2015;151:743-752.
- Weinmann S, Kamtsiuris P, Henke KD, et al. The costs of atopy and asthma in children: assessment of direct costs and their determinants in a birth cohort. Pediatr Allergy Immunol. 2003;14:18-26.
- Fivenson D, Arnold RJ, Kaniecki DJ, et al. The effect of atopic dermatitis on total burden of illness and quality of life on adults and children in a large managed care organization. J Manag Care Pharm. 2002;8:333-342.
- Verboom P, Hakkaart-Van L, Sturkenboom M, et al. The cost of atopic dermatitis in the Netherlands: an international comparison. Br J Dermatol. 2002;147:716-724.
- Lapidus CS, Schwarz DF, Honig PJ. Atopic dermatitis in children: who cares? who pays? J Am Acad Dermatol. 1993;28:699-703.
- Carroll CL, Balkrishnan R, Feldman SR, et al. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22:192-199.
- Ellis CN, Drake LA, Prendergast MM, et al. Cost of atopic dermatitis and eczema in the United States. J Am Acad Dermatol. 2002;46:361-370.
- Ellis CN, Prendergast MM, Tokar M, et al. Quantifying costs associated with atopic dermatitis. J Manag Care Pharm. 2003;9:278.
- Lee SW, Cheong SH, Byun JY, et al. Occupational hand eczema among nursing staffs in Korea: self-reported hand eczema and contact sensitization of hospital nursing staffs. J Dermatol. 2013;40:182-187.
- Kemp AS. Cost of illness of atopic dermatitis in children: a societal perspective. Pharmacoeconomics. 2003;21:105-113.
- Munro DD. Topical corticosteroid therapy and its effect on the hypothalamic-pituitary-adrenal axis. Dermatologica. 1976;152:173-180.
- Silverberg JI, Garg NK, Paller AS, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135:56-66.
- Eichenfield LF. Improving outcomes in atopic dermatitis. for advances in dermatology. Dermatology Focus. 2015;34:1-6.
- Fleischer AB Jr. Black box warning for topical calcineurin inhibitors and the death of common sense. Dermatol Online J. 2006;12:2.
In parts 1 and 2 of this series on atopic dermatitis (AD),1,2 the current putative pathogenesis, scoring systems for severity grading, and epidemiology were reviewed. Part 3 reviews the differential diagnosis, with an emphasis on the difficulty of differentiation from some rare but notable illnesses, as well as the recently expanding data on comorbidities that identify AD as a multisystem disorder with widespread health implications for the patient.
Differential Diagnosis for Pediatric AD
The differential diagnosis for pediatric AD includes chronic dermatoses (eg, seborrheic dermatitis, psoriasis), congenital disorders (eg, Netherton syndrome), malignant diseases (eg, cutaneous T-cell lymphoma [CTCL]), immunodeficiencies, infections, and metabolic disorders.3 Netherton syndrome must be ruled out to prevent extensive drug absorption when treating with topical calcineurin inhibitors (TCIs).4 Due to the presence of bamboo hairs in these patients, a hair mount may aid in the diagnosis of Netherton syndrome. Misdiagnosis of CTCL as AD may complicate the analysis of safety data on TCIs.4,5 Multiple skin biopsies are essential in cases of suspected CTCL to provide an accurate diagnosis. Biopsy can be considered in AD cases with changing and/or unusual morphology, erythrodermic skin changes, and disease that is poorly responsive to multiple therapeutic modalities.
Comorbidities in Pediatric AD
Psychosocial Comorbidities
Pediatric AD often takes a psychological toll on patients as well as household members. Almost half of children with AD are reported to have a severely impaired quality of life (QOL).6 Contributing factors include fatigue, sleep disturbance, activity restriction (eg, inability to participate in sports), and depression.7
Chamlin et al8 developed the Childhood Atopic Dermatitis Impact Scale (CADIS), a 45-item instrument (refined from a 62-item prototype), to measure QOL in young children with AD and their family members. Responses were evaluated with consideration of 5 domains: symptoms and activity limitations/behaviors in children, as well as family/social function, sleep, and emotions in parents. The top 12 factors that parents found most bothersome about AD included itching/scratching, child’s pain/discomfort, sleep issues, embarrassment or worry about appearance, child’s fussiness/irritability/crying/unhappiness, helplessness/can’t control it/predict it, worry about skin infection, dryness of skin/nonsmooth skin, skin bleeding, worry about damage/scars, stares/comments of strangers and other children, and rashes/redness of skin/discoloration. Parents were asked to respond to items about their emotional health and social functioning, such as “My child’s skin condition has strained my relationship with my spouse or partner,” “My child’s skin condition makes me feel sad or depressed,” and “I am bothered by the reaction of strangers to this skin condition.”8
Kiebert et al9 found that AD patients had lower scores on the Short Form-36 Health Survey’s vitality, social functioning, and mental health subscales compared to individuals in the general population. The authors noted that anxiety in AD patients is of particular concern, as stress has been found to trigger the itch-scratch cycle, potentially setting off AD flare-ups.9 Family impact of AD is aggravated by disease severity. Sleeplessness, relationship stress, and time management can all cause family problems in patients with AD.8
In a survey of 3775 older teenagers aged 18 to 19 years (80% response rate out of 4774 prospective participants), 9.7% of participants reported having current AD.10 Suicidal ideation was higher in those with current AD than those without AD (15.5% vs 9.1%). The prevalence of suicidal ideation rose to 23.8% in those with both AD and itch. Diagnosis of AD (as determined through participant responses to the question, ‘‘Do you have, or have you had eczema?’’) was associated with mental health problems in 16.0% of those with AD compared to 10.1% of those without AD, with an especially reduced likelihood of romantic relationships for adolescent boys with AD, as measured using the Strength and Difficulties Questionnaire, which measures 4 problem domains and assesses presence of mental health issues in the past 6 months, and the Hopkins Symptom Checklist 10, which uses 10 questions to measure anxiety and depression symptoms in the past week.10
Dalgard et al11 assessed whether the psychological burden of AD persists in adulthood in an international, multicenter, observational, cross-sectional study conducted in 13 European countries. Each dermatology clinic recruited 250 consecutive adult outpatients to complete a questionnaire along with a control group of 125 hospital employees without skin disease from the same institution but from different departments. The study included a total of 4994 participants (3635 patients and 1359 controls). Clinical depression and anxiety were present in 10.1% and 17.6% of patients, respectively, versus 4.3% and 11.1% of controls, respectively. The prevalence of depression and anxiety was highest in patients with leg ulcers, hand eczema, psoriasis, and AD.11 This study demonstrated that the psychological comorbidities of childhood conditions such as AD may persist into adulthood.
Lymphoma
In a systematic review of the literature and a separate meta-analysis, Legendre et al12 identified a slight increase in lymphoma among AD patients, with an uncertain but potential increase associated with topical corticosteroid application. This finding is similar to trends seen in other systemic inflammatory conditions that involve the skin, such as psoriasis, and is felt to relate to long-term inflammation.
Obesity
Obesity has been associated with a greater risk for moderate to severe AD in children.13,14
Infections
Children with AD are at a higher risk for cutaneous infections and generalization of these infections. The leading infections would be with Staphylococcus aureus, but group A streptococci infections do occur. Herpes simplex virus, vaccinia virus or Kaposi varicelliform eruption (KVE), molluscum with or without dermatitis, and fungal infections occur less commonly but with greater morbidity, largely due to the impaired barrier and some innate reduction in cutaneous immunity.15
Atopic dermatitis in children also is associated with a higher prevalence of extracutaneous infections such as influenza, pneumonia, urinary tract infections, varicella-zoster virus, recurrent ear infections, sinus infections, sore throat, and head or chest colds.16 Children with AD and warts (human papillomavirus infection) have an even greater risk for these comorbidities.17 Warts and molluscum infections may become more extensive in children with AD.18 Generalization of herpetic infections occurs more easily in AD patients due to the impaired skin barrier, which includes generalized skin surface extension of herpes simplex virus type 1, varicella-zoster virus, and historically smallpox. A similar clinical appearance of generalized vesiculopustular lesions with fever can be seen when coxsackievirus A6 infections occur in AD patients; these conditions are called eczema herpeticum due to herpes simplex virus, KVE due to varicella-zoster virus and smallpox, and eczema coxsackium due to coxsackievirus A6,19 though some authors refer to all of these as KVE.20 These generalized viral illnesses overlying AD often result in fever, malaise, pain, and life-threatening skin denudation with risk for dehydration and superinfection with S aureus.7,18 It has been shown that the occurrence of eczema herpeticum in AD is associated with and may be caused by an inability to induce human β-defensin 2 and 3 as well as cathelicidin.21
Staphylococcus aureus colonization has been noted in 90% to 100% of AD cases, which can be associated with a higher eczema area and severity index score.22-24 The role of S aureus in AD includes flare triggering through release of superantigens, leading to IL-31–induced pruritis.25 Recurrent infection with either methicillin-sensitive or methicillin-resistant S aureus has been noted in AD.18,26 Skin infections also occur in AD and appear as erosions and pustules, and coinfection with Streptococcus and Staphylococcus does occur; therefore, cultures often are needed to determine the type of bacteria present on the skin in severe cases and when infection is suspected.27 Perianal bacterial dermatitis is a variant of infected AD occurring in the anal/groin area that is associated with S aureus and/or streptococcal superinfection in which topical corticosteroids and topical anti-infectives can be used. In some severe cases, oral antibiotics may be needed.28
Injury/Hyperactivity
Children aged 0 to 5 years with AD carry an increased risk for injuries requiring medical attention, with association in part due to attention deficit disorder, depression, and anxiety. Antihistamines are believed to aggravate this issue by promoting daytime somnolence29; however, pruritus-induced sleep disturbances in AD also may be responsible for daytime somnolence.30
Contact Allergy and Sensitization
Children with AD may become sensitized to environmental allergens through delayed-type hypersensitivity. The presumed mechanism is that these agents include ingredients added into applied medicaments and application occurs over an impaired skin barrier allowing for absorption and greater risk of antigen presentation. Approximately 50% of children with difficult-to-control AD will react to 1 or more epicutaneous allergens, and patch testing can be performed to identify relevant allergens that can improve skin severity.7 Severe dermatitis and id generalized hypersensitivity reactions in patients with AD and nickel allergic contact dermatitis have been described and may aggravate underlying AD.31
Family Burden of AD
Parents or caregivers of children with moderate and severe AD spend nearly 3 hours a day caring for their child’s skin and experience QOL impairments including lack of sleep and/or privacy, often due to cosleeping; treatment-related financial expenditures; and feelings of hopelessness, guilt, and depression.7
Steroid Phobia
Steroid phobia is the fear of topical application of corticosteroids resulting in systemic side effects including unrealistic fears (eg, fear that the child will develop muscles such as an anabolic steroid user) as well as realistic but statistically low-risk fears (eg, fear of systemic absorption). These fears often result in underutilization of prescribed topical corticosteroid therapies and undertreatment of children with AD.32,33
Financial Burden
The cost of AD can be high in the United States, with adult data demonstrating costs ranging from $371 to $489 per person.34 The last published cost data for pediatric AD was from 2003, with an average cost of $219 per year.35 Costs include time lost from work, household purchases (eg, skin care products), and co-pays for visits and medication, with an estimated average expenditure per person (SE) of $601.06 ($137.26) annually in 2012.36 The cost of ambulatory care and emergency department visits for AD in children in the United States in 1993 was estimated at $364 million.37-39 In 2002, Ellis et al40 estimated the overall cost of AD to be between $900 million and $3.8 billion in the United States (1997-1998) based on projections from claims, prescriptions, and comorbidities reported to a private insurer and Medicaid. Ellis et al41 further determined that topical tacrolimus was similar in cost to high-potency corticosteroids.
Pediatric AD often progresses to adult hand eczema and leads to further morbidity, especially in health care workers.42 Kemp43 reviewed the cost of AD in children and concluded that AD was a condition with major handicap with personal, financial, and social effects. A cost review of studies conducted in 163,700 children with AD showed that costs related to AD totaled $316.7 million per year. The author concluded that there were substantial psychosocial and financial stresses associated with pediatric AD but no clear path to potential reduction in related costs.43
Sleep Disturbances
Sleep disturbances are common in pediatric AD patients. Pruritus usually is exacerbated at bedtime due to reduced humidity and lack of distractions to prevent scratching. Sleep deprivation has a substantial impact on both the patient and his/her household. Parental frustration increases with sleep disturbance.18,44 Sleep deprivation is associated with greater severity, both because it is one of the most difficult aspects of illness and because the associated pruritus makes for greater damage done to the skin through injurious scratching.
Sleep disturbances also may interfere with growth and overnight release of growth hormones.18,44 This latter issue can result in reduced linear growth velocity. Furthermore, sleep deprivation can cause increased risk of accidents and poor school performance.18,44,45
Many children do not outgrow AD. In adults, AD-associated sleep deprivation has been shown to have an association with fatigue, regular daytime sleepiness, and regular insomnia, correlating to number of sick days, doctor visits, and poorer overall health status.45
Inadequate Disease Control
Inadequate disease control has been described by Eichenfeld46 as an important issue in AD at this time. Untreated, undertreated, and improperly treated AD are important issues affecting long-term AD care. He further cited steroid phobia as a contributor to undertreatment.46 Fleischer47 has cited the black box warning present on TCIs as a further deterrent to adequate therapeutic control in our current therapeutic paradigm. Undertreatment may result in uncontrolled disease activity, impaired QOL, infections, and sleep disturbances. The role of undertreatment as a driver of the atopic march is unknown.
Conclusion
Atopic dermatitis is a multisystem disorder that has wide-reaching comorbidities and may mimic a variety of skin conditions. The topic of comorbidities is new and emerging and bears further review to define risk factors, prevention strategies, and long-term monitoring requirements.
In parts 1 and 2 of this series on atopic dermatitis (AD),1,2 the current putative pathogenesis, scoring systems for severity grading, and epidemiology were reviewed. Part 3 reviews the differential diagnosis, with an emphasis on the difficulty of differentiation from some rare but notable illnesses, as well as the recently expanding data on comorbidities that identify AD as a multisystem disorder with widespread health implications for the patient.
Differential Diagnosis for Pediatric AD
The differential diagnosis for pediatric AD includes chronic dermatoses (eg, seborrheic dermatitis, psoriasis), congenital disorders (eg, Netherton syndrome), malignant diseases (eg, cutaneous T-cell lymphoma [CTCL]), immunodeficiencies, infections, and metabolic disorders.3 Netherton syndrome must be ruled out to prevent extensive drug absorption when treating with topical calcineurin inhibitors (TCIs).4 Due to the presence of bamboo hairs in these patients, a hair mount may aid in the diagnosis of Netherton syndrome. Misdiagnosis of CTCL as AD may complicate the analysis of safety data on TCIs.4,5 Multiple skin biopsies are essential in cases of suspected CTCL to provide an accurate diagnosis. Biopsy can be considered in AD cases with changing and/or unusual morphology, erythrodermic skin changes, and disease that is poorly responsive to multiple therapeutic modalities.
Comorbidities in Pediatric AD
Psychosocial Comorbidities
Pediatric AD often takes a psychological toll on patients as well as household members. Almost half of children with AD are reported to have a severely impaired quality of life (QOL).6 Contributing factors include fatigue, sleep disturbance, activity restriction (eg, inability to participate in sports), and depression.7
Chamlin et al8 developed the Childhood Atopic Dermatitis Impact Scale (CADIS), a 45-item instrument (refined from a 62-item prototype), to measure QOL in young children with AD and their family members. Responses were evaluated with consideration of 5 domains: symptoms and activity limitations/behaviors in children, as well as family/social function, sleep, and emotions in parents. The top 12 factors that parents found most bothersome about AD included itching/scratching, child’s pain/discomfort, sleep issues, embarrassment or worry about appearance, child’s fussiness/irritability/crying/unhappiness, helplessness/can’t control it/predict it, worry about skin infection, dryness of skin/nonsmooth skin, skin bleeding, worry about damage/scars, stares/comments of strangers and other children, and rashes/redness of skin/discoloration. Parents were asked to respond to items about their emotional health and social functioning, such as “My child’s skin condition has strained my relationship with my spouse or partner,” “My child’s skin condition makes me feel sad or depressed,” and “I am bothered by the reaction of strangers to this skin condition.”8
Kiebert et al9 found that AD patients had lower scores on the Short Form-36 Health Survey’s vitality, social functioning, and mental health subscales compared to individuals in the general population. The authors noted that anxiety in AD patients is of particular concern, as stress has been found to trigger the itch-scratch cycle, potentially setting off AD flare-ups.9 Family impact of AD is aggravated by disease severity. Sleeplessness, relationship stress, and time management can all cause family problems in patients with AD.8
In a survey of 3775 older teenagers aged 18 to 19 years (80% response rate out of 4774 prospective participants), 9.7% of participants reported having current AD.10 Suicidal ideation was higher in those with current AD than those without AD (15.5% vs 9.1%). The prevalence of suicidal ideation rose to 23.8% in those with both AD and itch. Diagnosis of AD (as determined through participant responses to the question, ‘‘Do you have, or have you had eczema?’’) was associated with mental health problems in 16.0% of those with AD compared to 10.1% of those without AD, with an especially reduced likelihood of romantic relationships for adolescent boys with AD, as measured using the Strength and Difficulties Questionnaire, which measures 4 problem domains and assesses presence of mental health issues in the past 6 months, and the Hopkins Symptom Checklist 10, which uses 10 questions to measure anxiety and depression symptoms in the past week.10
Dalgard et al11 assessed whether the psychological burden of AD persists in adulthood in an international, multicenter, observational, cross-sectional study conducted in 13 European countries. Each dermatology clinic recruited 250 consecutive adult outpatients to complete a questionnaire along with a control group of 125 hospital employees without skin disease from the same institution but from different departments. The study included a total of 4994 participants (3635 patients and 1359 controls). Clinical depression and anxiety were present in 10.1% and 17.6% of patients, respectively, versus 4.3% and 11.1% of controls, respectively. The prevalence of depression and anxiety was highest in patients with leg ulcers, hand eczema, psoriasis, and AD.11 This study demonstrated that the psychological comorbidities of childhood conditions such as AD may persist into adulthood.
Lymphoma
In a systematic review of the literature and a separate meta-analysis, Legendre et al12 identified a slight increase in lymphoma among AD patients, with an uncertain but potential increase associated with topical corticosteroid application. This finding is similar to trends seen in other systemic inflammatory conditions that involve the skin, such as psoriasis, and is felt to relate to long-term inflammation.
Obesity
Obesity has been associated with a greater risk for moderate to severe AD in children.13,14
Infections
Children with AD are at a higher risk for cutaneous infections and generalization of these infections. The leading infections would be with Staphylococcus aureus, but group A streptococci infections do occur. Herpes simplex virus, vaccinia virus or Kaposi varicelliform eruption (KVE), molluscum with or without dermatitis, and fungal infections occur less commonly but with greater morbidity, largely due to the impaired barrier and some innate reduction in cutaneous immunity.15
Atopic dermatitis in children also is associated with a higher prevalence of extracutaneous infections such as influenza, pneumonia, urinary tract infections, varicella-zoster virus, recurrent ear infections, sinus infections, sore throat, and head or chest colds.16 Children with AD and warts (human papillomavirus infection) have an even greater risk for these comorbidities.17 Warts and molluscum infections may become more extensive in children with AD.18 Generalization of herpetic infections occurs more easily in AD patients due to the impaired skin barrier, which includes generalized skin surface extension of herpes simplex virus type 1, varicella-zoster virus, and historically smallpox. A similar clinical appearance of generalized vesiculopustular lesions with fever can be seen when coxsackievirus A6 infections occur in AD patients; these conditions are called eczema herpeticum due to herpes simplex virus, KVE due to varicella-zoster virus and smallpox, and eczema coxsackium due to coxsackievirus A6,19 though some authors refer to all of these as KVE.20 These generalized viral illnesses overlying AD often result in fever, malaise, pain, and life-threatening skin denudation with risk for dehydration and superinfection with S aureus.7,18 It has been shown that the occurrence of eczema herpeticum in AD is associated with and may be caused by an inability to induce human β-defensin 2 and 3 as well as cathelicidin.21
Staphylococcus aureus colonization has been noted in 90% to 100% of AD cases, which can be associated with a higher eczema area and severity index score.22-24 The role of S aureus in AD includes flare triggering through release of superantigens, leading to IL-31–induced pruritis.25 Recurrent infection with either methicillin-sensitive or methicillin-resistant S aureus has been noted in AD.18,26 Skin infections also occur in AD and appear as erosions and pustules, and coinfection with Streptococcus and Staphylococcus does occur; therefore, cultures often are needed to determine the type of bacteria present on the skin in severe cases and when infection is suspected.27 Perianal bacterial dermatitis is a variant of infected AD occurring in the anal/groin area that is associated with S aureus and/or streptococcal superinfection in which topical corticosteroids and topical anti-infectives can be used. In some severe cases, oral antibiotics may be needed.28
Injury/Hyperactivity
Children aged 0 to 5 years with AD carry an increased risk for injuries requiring medical attention, with association in part due to attention deficit disorder, depression, and anxiety. Antihistamines are believed to aggravate this issue by promoting daytime somnolence29; however, pruritus-induced sleep disturbances in AD also may be responsible for daytime somnolence.30
Contact Allergy and Sensitization
Children with AD may become sensitized to environmental allergens through delayed-type hypersensitivity. The presumed mechanism is that these agents include ingredients added into applied medicaments and application occurs over an impaired skin barrier allowing for absorption and greater risk of antigen presentation. Approximately 50% of children with difficult-to-control AD will react to 1 or more epicutaneous allergens, and patch testing can be performed to identify relevant allergens that can improve skin severity.7 Severe dermatitis and id generalized hypersensitivity reactions in patients with AD and nickel allergic contact dermatitis have been described and may aggravate underlying AD.31
Family Burden of AD
Parents or caregivers of children with moderate and severe AD spend nearly 3 hours a day caring for their child’s skin and experience QOL impairments including lack of sleep and/or privacy, often due to cosleeping; treatment-related financial expenditures; and feelings of hopelessness, guilt, and depression.7
Steroid Phobia
Steroid phobia is the fear of topical application of corticosteroids resulting in systemic side effects including unrealistic fears (eg, fear that the child will develop muscles such as an anabolic steroid user) as well as realistic but statistically low-risk fears (eg, fear of systemic absorption). These fears often result in underutilization of prescribed topical corticosteroid therapies and undertreatment of children with AD.32,33
Financial Burden
The cost of AD can be high in the United States, with adult data demonstrating costs ranging from $371 to $489 per person.34 The last published cost data for pediatric AD was from 2003, with an average cost of $219 per year.35 Costs include time lost from work, household purchases (eg, skin care products), and co-pays for visits and medication, with an estimated average expenditure per person (SE) of $601.06 ($137.26) annually in 2012.36 The cost of ambulatory care and emergency department visits for AD in children in the United States in 1993 was estimated at $364 million.37-39 In 2002, Ellis et al40 estimated the overall cost of AD to be between $900 million and $3.8 billion in the United States (1997-1998) based on projections from claims, prescriptions, and comorbidities reported to a private insurer and Medicaid. Ellis et al41 further determined that topical tacrolimus was similar in cost to high-potency corticosteroids.
Pediatric AD often progresses to adult hand eczema and leads to further morbidity, especially in health care workers.42 Kemp43 reviewed the cost of AD in children and concluded that AD was a condition with major handicap with personal, financial, and social effects. A cost review of studies conducted in 163,700 children with AD showed that costs related to AD totaled $316.7 million per year. The author concluded that there were substantial psychosocial and financial stresses associated with pediatric AD but no clear path to potential reduction in related costs.43
Sleep Disturbances
Sleep disturbances are common in pediatric AD patients. Pruritus usually is exacerbated at bedtime due to reduced humidity and lack of distractions to prevent scratching. Sleep deprivation has a substantial impact on both the patient and his/her household. Parental frustration increases with sleep disturbance.18,44 Sleep deprivation is associated with greater severity, both because it is one of the most difficult aspects of illness and because the associated pruritus makes for greater damage done to the skin through injurious scratching.
Sleep disturbances also may interfere with growth and overnight release of growth hormones.18,44 This latter issue can result in reduced linear growth velocity. Furthermore, sleep deprivation can cause increased risk of accidents and poor school performance.18,44,45
Many children do not outgrow AD. In adults, AD-associated sleep deprivation has been shown to have an association with fatigue, regular daytime sleepiness, and regular insomnia, correlating to number of sick days, doctor visits, and poorer overall health status.45
Inadequate Disease Control
Inadequate disease control has been described by Eichenfeld46 as an important issue in AD at this time. Untreated, undertreated, and improperly treated AD are important issues affecting long-term AD care. He further cited steroid phobia as a contributor to undertreatment.46 Fleischer47 has cited the black box warning present on TCIs as a further deterrent to adequate therapeutic control in our current therapeutic paradigm. Undertreatment may result in uncontrolled disease activity, impaired QOL, infections, and sleep disturbances. The role of undertreatment as a driver of the atopic march is unknown.
Conclusion
Atopic dermatitis is a multisystem disorder that has wide-reaching comorbidities and may mimic a variety of skin conditions. The topic of comorbidities is new and emerging and bears further review to define risk factors, prevention strategies, and long-term monitoring requirements.
- Silverberg NB. A practical overview of pediatric atopic dermatitis, part 1: epidemiology and pathogenesis. Cutis. 2016;97:267-271.
- Silverberg NB. A practical overview of pediatric atopic dermatitis, part 2: triggers and grading. Cutis. 2016;97:326-329.
- Siegfried EC, Hebert AA. Diagnosis of atopic dermatitis: mimics, overlaps, and complications. J Clin Med. 2015;4:884-917.
- Allen A, Siegfried E, Silverman R, et al. Significant absorption of topical tacrolimus in 3 patients with Netherton syndrome. Arch Dermatol. 2001;137:747-750.
- Carr WW. Topical calcineurin inhibitors for atopic dermatitis: review and treatment recommendations. Paediatr Drugs. 2013;15:303-310.
- Chamlin SL, Lai JS, Cella D, et al. Childhood Atopic Dermatitis Impact Scale: reliability, discriminative and concurrent validity, and responsiveness. Arch Dermatol. 2007;143:768-772.
- Tollefson MM, Bruckner AL. Atopic dermatitis: skin-directed management. Pediatrics. 2014;134:E1735-E1744.
- Chamlin SL, Cella D, Frieden IJ, et al. Development of the Childhood Atopic Dermatitis Impact Scale: initial validation of a quality-of-life measure for young children with atopic dermatitis and their families. J Invest Dermatol. 2005;125:1106-1111.
- Kiebert G, Sorensen SV, Revicki D, et al. Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol. 2002;41:151-158.
- Halvorsen JA, Lien L, Dalgard F, et al. Suicidal ideation, mental health problems, and social function in adolescents with eczema: a population-based study. J Invest Dermatol. 2014;134:1847-1854.
- Dalgard FJ, Gieler U, Tomas-Aragones L, et al. The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Invest Dermatol. 2015;135:984-991.
- Legendre L, Barnetche T, Mazereeuw-Hautier J, et al. Risk of lymphoma in patients with atopic dermatitis and the role of topical treatment: a systematic review and meta-analysis. J Am Acad Dermatol. 2015;72:992-1002.
- Koutroulis I, Magnelli L, Gaughan J, et al. Atopic dermatitis is more severe in children over the age of two who have an increased body mass index. Acta Paediatr. 2015;104:713-717.
- Silverberg JI, Becker L, Kwasny M, et al. Central obesity and high blood pressure in pediatric patients with atopic dermatitis. JAMA Dermatol. 2015;151:144-152.
- De D, Kanwar AJ, Handa S. Comparative efficacy of Hanifin and Rajka’s criteria and the UK working party’s diagnostic criteria in diagnosis of atopic dermatitis in a hospital setting in North India. J Eur Acad Dermatol Venereol. 2006;20:853-859.
- Silverberg JI, Silverberg NB. Childhood atopic dermatitis and warts are associated with increased risk of infection: a US population-based study [published online October 4, 2013]. J Allergy Clin Immunol. 2014;133:1041-1047.
- Silverberg J, Garg N, Silverberg NB. New developments in comorbidities of atopic dermatitis. Cutis. 2014;93:222-224.
- Kiken DA, Silverberg NB. Atopic dermatitis in children, part 1: epidemiology, clinical features, and complications. Cutis. 2006;78:241-247.
- Mathes EF, Oza V, Frieden IJ, et al. “Eczema coxsackium” and unusual cutaneous findings in an enterovirus outbreak. Pediatrics. 2013;132:E149-E157.
- Vora RV, Pilani AP, Jivani NB, et al. Kaposi varicelliform eruption. Indian Dermatol Online J. 2015;6:364-366.
- Hata TR, Kotol P, Boguniewicz M, et al. History of eczema herpeticum is associated with the inability to induce human β-defensin (HBD)-2, HBD-3 and cathelicidin in the skin of patients with atopic dermatitis. Br J Dermatol. 2010;163:659-661.
- Abeck D, Mempel M. Staphylococcus aureus colonization in atopic dermatitis and its therapeutic implications. Br J Dermatol. 1998;139:13-16.
- Leyden JJ, Marples RR, Kligman AM. Staphylococcus aureus in the lesions of atopic dermatitis. Br J Dermatol. 1974;90:525-530.
- Lipnharski C, d’Azevedo PA, Quinto VP, et al. Colonization by S. aureus increases the EASI and the number of appointments by patients with atopic dermatitis: cohort with 93 patients. An Bras Dermatol. 2013;88:518-521.
- Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
- Groner A, Laing-Grayman D, Silverberg NB. Outpatient pediatric community-acquired methicillin-resistant Staphylococcus aureus: a polymorphous clinical disease. Cutis. 2008;81:115-122.
- Sugarman JL, Hersh AL, Okamura T, et al. A retrospective review of streptococcal infections in pediatric atopic dermatitis. Pediatr Dermatol. 2011;28:230-234.
- Heath C, Desai N, Silverberg NB. Recent microbiological shifts in perianal bacterial dermatitis: Staphylococcus aureus predominance. Pediatr Dermatol. 2009;26:696-700.
- Garg N, Silverberg JI. Association between childhood allergic disease, psychological comorbidity, and injury requiring medical attention. Ann Allergy Asthma Immunol. 2014;112:525-532.
- Lavery MJ, Stull C, Kinney MO, et al. Nocturnal pruritus: the battle for a peaceful night’s sleep. Int J Mol Sci. 2016;17:E425.
- Silverberg NB, Licht J, Friedler S, et al. Nickel contact hypersensitivity in children. Pediatr Dermatol. 2002;19:110-113.
- Aubert-Wastiaux H, Moret L, Le Rhun A, et al. Topical corticosteroid phobia in atopic dermatitis: a study of its nature, origins and frequency. Br J Dermatol. 2011;165:808-814.
- Kojima R, Fujiwara T, Matsuda A, et al. Factors associated with steroid phobia in caregivers of children with atopic dermatitis. Pediatr Dermatol. 2013;30:29-35.
- Silverberg JI. Health care utilization, patient costs, and access to care in US adults with eczema: a population-based study. JAMA Dermatol. 2015;151:743-752.
- Weinmann S, Kamtsiuris P, Henke KD, et al. The costs of atopy and asthma in children: assessment of direct costs and their determinants in a birth cohort. Pediatr Allergy Immunol. 2003;14:18-26.
- Fivenson D, Arnold RJ, Kaniecki DJ, et al. The effect of atopic dermatitis on total burden of illness and quality of life on adults and children in a large managed care organization. J Manag Care Pharm. 2002;8:333-342.
- Verboom P, Hakkaart-Van L, Sturkenboom M, et al. The cost of atopic dermatitis in the Netherlands: an international comparison. Br J Dermatol. 2002;147:716-724.
- Lapidus CS, Schwarz DF, Honig PJ. Atopic dermatitis in children: who cares? who pays? J Am Acad Dermatol. 1993;28:699-703.
- Carroll CL, Balkrishnan R, Feldman SR, et al. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22:192-199.
- Ellis CN, Drake LA, Prendergast MM, et al. Cost of atopic dermatitis and eczema in the United States. J Am Acad Dermatol. 2002;46:361-370.
- Ellis CN, Prendergast MM, Tokar M, et al. Quantifying costs associated with atopic dermatitis. J Manag Care Pharm. 2003;9:278.
- Lee SW, Cheong SH, Byun JY, et al. Occupational hand eczema among nursing staffs in Korea: self-reported hand eczema and contact sensitization of hospital nursing staffs. J Dermatol. 2013;40:182-187.
- Kemp AS. Cost of illness of atopic dermatitis in children: a societal perspective. Pharmacoeconomics. 2003;21:105-113.
- Munro DD. Topical corticosteroid therapy and its effect on the hypothalamic-pituitary-adrenal axis. Dermatologica. 1976;152:173-180.
- Silverberg JI, Garg NK, Paller AS, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135:56-66.
- Eichenfield LF. Improving outcomes in atopic dermatitis. for advances in dermatology. Dermatology Focus. 2015;34:1-6.
- Fleischer AB Jr. Black box warning for topical calcineurin inhibitors and the death of common sense. Dermatol Online J. 2006;12:2.
- Silverberg NB. A practical overview of pediatric atopic dermatitis, part 1: epidemiology and pathogenesis. Cutis. 2016;97:267-271.
- Silverberg NB. A practical overview of pediatric atopic dermatitis, part 2: triggers and grading. Cutis. 2016;97:326-329.
- Siegfried EC, Hebert AA. Diagnosis of atopic dermatitis: mimics, overlaps, and complications. J Clin Med. 2015;4:884-917.
- Allen A, Siegfried E, Silverman R, et al. Significant absorption of topical tacrolimus in 3 patients with Netherton syndrome. Arch Dermatol. 2001;137:747-750.
- Carr WW. Topical calcineurin inhibitors for atopic dermatitis: review and treatment recommendations. Paediatr Drugs. 2013;15:303-310.
- Chamlin SL, Lai JS, Cella D, et al. Childhood Atopic Dermatitis Impact Scale: reliability, discriminative and concurrent validity, and responsiveness. Arch Dermatol. 2007;143:768-772.
- Tollefson MM, Bruckner AL. Atopic dermatitis: skin-directed management. Pediatrics. 2014;134:E1735-E1744.
- Chamlin SL, Cella D, Frieden IJ, et al. Development of the Childhood Atopic Dermatitis Impact Scale: initial validation of a quality-of-life measure for young children with atopic dermatitis and their families. J Invest Dermatol. 2005;125:1106-1111.
- Kiebert G, Sorensen SV, Revicki D, et al. Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol. 2002;41:151-158.
- Halvorsen JA, Lien L, Dalgard F, et al. Suicidal ideation, mental health problems, and social function in adolescents with eczema: a population-based study. J Invest Dermatol. 2014;134:1847-1854.
- Dalgard FJ, Gieler U, Tomas-Aragones L, et al. The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Invest Dermatol. 2015;135:984-991.
- Legendre L, Barnetche T, Mazereeuw-Hautier J, et al. Risk of lymphoma in patients with atopic dermatitis and the role of topical treatment: a systematic review and meta-analysis. J Am Acad Dermatol. 2015;72:992-1002.
- Koutroulis I, Magnelli L, Gaughan J, et al. Atopic dermatitis is more severe in children over the age of two who have an increased body mass index. Acta Paediatr. 2015;104:713-717.
- Silverberg JI, Becker L, Kwasny M, et al. Central obesity and high blood pressure in pediatric patients with atopic dermatitis. JAMA Dermatol. 2015;151:144-152.
- De D, Kanwar AJ, Handa S. Comparative efficacy of Hanifin and Rajka’s criteria and the UK working party’s diagnostic criteria in diagnosis of atopic dermatitis in a hospital setting in North India. J Eur Acad Dermatol Venereol. 2006;20:853-859.
- Silverberg JI, Silverberg NB. Childhood atopic dermatitis and warts are associated with increased risk of infection: a US population-based study [published online October 4, 2013]. J Allergy Clin Immunol. 2014;133:1041-1047.
- Silverberg J, Garg N, Silverberg NB. New developments in comorbidities of atopic dermatitis. Cutis. 2014;93:222-224.
- Kiken DA, Silverberg NB. Atopic dermatitis in children, part 1: epidemiology, clinical features, and complications. Cutis. 2006;78:241-247.
- Mathes EF, Oza V, Frieden IJ, et al. “Eczema coxsackium” and unusual cutaneous findings in an enterovirus outbreak. Pediatrics. 2013;132:E149-E157.
- Vora RV, Pilani AP, Jivani NB, et al. Kaposi varicelliform eruption. Indian Dermatol Online J. 2015;6:364-366.
- Hata TR, Kotol P, Boguniewicz M, et al. History of eczema herpeticum is associated with the inability to induce human β-defensin (HBD)-2, HBD-3 and cathelicidin in the skin of patients with atopic dermatitis. Br J Dermatol. 2010;163:659-661.
- Abeck D, Mempel M. Staphylococcus aureus colonization in atopic dermatitis and its therapeutic implications. Br J Dermatol. 1998;139:13-16.
- Leyden JJ, Marples RR, Kligman AM. Staphylococcus aureus in the lesions of atopic dermatitis. Br J Dermatol. 1974;90:525-530.
- Lipnharski C, d’Azevedo PA, Quinto VP, et al. Colonization by S. aureus increases the EASI and the number of appointments by patients with atopic dermatitis: cohort with 93 patients. An Bras Dermatol. 2013;88:518-521.
- Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
- Groner A, Laing-Grayman D, Silverberg NB. Outpatient pediatric community-acquired methicillin-resistant Staphylococcus aureus: a polymorphous clinical disease. Cutis. 2008;81:115-122.
- Sugarman JL, Hersh AL, Okamura T, et al. A retrospective review of streptococcal infections in pediatric atopic dermatitis. Pediatr Dermatol. 2011;28:230-234.
- Heath C, Desai N, Silverberg NB. Recent microbiological shifts in perianal bacterial dermatitis: Staphylococcus aureus predominance. Pediatr Dermatol. 2009;26:696-700.
- Garg N, Silverberg JI. Association between childhood allergic disease, psychological comorbidity, and injury requiring medical attention. Ann Allergy Asthma Immunol. 2014;112:525-532.
- Lavery MJ, Stull C, Kinney MO, et al. Nocturnal pruritus: the battle for a peaceful night’s sleep. Int J Mol Sci. 2016;17:E425.
- Silverberg NB, Licht J, Friedler S, et al. Nickel contact hypersensitivity in children. Pediatr Dermatol. 2002;19:110-113.
- Aubert-Wastiaux H, Moret L, Le Rhun A, et al. Topical corticosteroid phobia in atopic dermatitis: a study of its nature, origins and frequency. Br J Dermatol. 2011;165:808-814.
- Kojima R, Fujiwara T, Matsuda A, et al. Factors associated with steroid phobia in caregivers of children with atopic dermatitis. Pediatr Dermatol. 2013;30:29-35.
- Silverberg JI. Health care utilization, patient costs, and access to care in US adults with eczema: a population-based study. JAMA Dermatol. 2015;151:743-752.
- Weinmann S, Kamtsiuris P, Henke KD, et al. The costs of atopy and asthma in children: assessment of direct costs and their determinants in a birth cohort. Pediatr Allergy Immunol. 2003;14:18-26.
- Fivenson D, Arnold RJ, Kaniecki DJ, et al. The effect of atopic dermatitis on total burden of illness and quality of life on adults and children in a large managed care organization. J Manag Care Pharm. 2002;8:333-342.
- Verboom P, Hakkaart-Van L, Sturkenboom M, et al. The cost of atopic dermatitis in the Netherlands: an international comparison. Br J Dermatol. 2002;147:716-724.
- Lapidus CS, Schwarz DF, Honig PJ. Atopic dermatitis in children: who cares? who pays? J Am Acad Dermatol. 1993;28:699-703.
- Carroll CL, Balkrishnan R, Feldman SR, et al. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22:192-199.
- Ellis CN, Drake LA, Prendergast MM, et al. Cost of atopic dermatitis and eczema in the United States. J Am Acad Dermatol. 2002;46:361-370.
- Ellis CN, Prendergast MM, Tokar M, et al. Quantifying costs associated with atopic dermatitis. J Manag Care Pharm. 2003;9:278.
- Lee SW, Cheong SH, Byun JY, et al. Occupational hand eczema among nursing staffs in Korea: self-reported hand eczema and contact sensitization of hospital nursing staffs. J Dermatol. 2013;40:182-187.
- Kemp AS. Cost of illness of atopic dermatitis in children: a societal perspective. Pharmacoeconomics. 2003;21:105-113.
- Munro DD. Topical corticosteroid therapy and its effect on the hypothalamic-pituitary-adrenal axis. Dermatologica. 1976;152:173-180.
- Silverberg JI, Garg NK, Paller AS, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135:56-66.
- Eichenfield LF. Improving outcomes in atopic dermatitis. for advances in dermatology. Dermatology Focus. 2015;34:1-6.
- Fleischer AB Jr. Black box warning for topical calcineurin inhibitors and the death of common sense. Dermatol Online J. 2006;12:2.
Practice Points
- Atopic dermatitis (AD) has a variety of comorbidities including psychosocial disorders, obesity, and infection.
- A variety of skin conditions can mimic AD.
- Atopic dermatitis can be complicated by coinfections.
A Practical Overview of Pediatric Atopic Dermatitis, Part 2: Triggers and Grading
Atopic dermatitis (AD) may be triggered by viral infections, food allergens, weather, and other causes, and it may trigger an inflammatory progression known as the atopic march. This article reviews research on triggers of pediatric AD so that dermatologists may discuss trigger avoidance with patients and guardians. Other factors affecting AD development include genetics and hygiene. Grading of AD also is discussed.
The Atopic March
The persistence of AD in untreated skin can trigger an inflammatory progression called the atopic march in which food and environmental allergies as well as asthma may occur progressively due to ongoing inflammatory triggering.1 In a study of asthma and food allergy reporting and management in public schools in Chicago, Illinois, food allergies were seen in 9.3% of asthmatic students (n=18,000), and 40.1% of food allergic students (n=4000) had asthma.2 An observational study by Flohr et al3 in London, England, included 619 exclusively breastfed infants who were recruited at 3 months of age. The investigators determined that food sensitization was unrelated to the presence of filaggrin mutations, type of eczema (flexural vs nonflexural), and transepidermal water loss but was associated with AD severity as determined by SCORAD (SCORing Atopic Dermatitis), a composite score of AD that includes pruritus as a factor in severity. Other AD associations included 3 leading food allergens: eggs, milk, and peanuts. No association with cod, wheat, or sesame allergy was noted. The investigators concluded that AD and AD severity were the leading skin-related risk factors for food allergies and therefore food allergy development in breastfed infants was probably mediated by cutaneous antigen-presenting cells.3
The skin has been documented to react to contact with known food allergens4 and is known to be a route of allergic sensitization to allergens such as fragrance in patients with AD.5,6 Two phenotypes of eczema that have been associated with asthma development are severe AD disease and multiple environmental allergies, supporting the theory of the atopic march.7 There also is evidence that release of danger-associated proteins from an impaired barrier also may trigger asthma.8 An analysis of the 2007 National Survey of Children’s Health, a population-based study of91,642 children aged 0 to 17 years, showed that children with AD had a higher prevalence of comorbid asthma (25.1% vs 12.3%), hay fever (34.4% vs 14.3%), and food allergies (15.1% vs 3.6%) compared to children without AD.9 A recent article provided detailed information on how food and diet interplay with AD.10
Triggers of Disease Flares
Triggers are the leading source of AD flare initiation, and avoidance of triggers is an important mechanism by which patients can control disease activity. Despite the best skin care and trigger avoidance, disease flares occur, sometimes due to ongoing inflammation and other times due to inability to prevent flares such as heat and humidity. A survey of patients with AD in Spain identified the following triggers: cosmetic products, clothing, mites, detergents/soaps, and temperature changes.11 In childhood, wool also is a known trigger of AD.12 Viral infections including respiratory syncytial virus may trigger the first onset of AD.13 Patients with AD may become allergic to fragrance and metals causing disease exacerbation on exposure.14,15 Food allergens contribute to approximately 40% of cases of AD in infancy but are not the cause of AD. The best evidence for improvement of AD with food allergen avoidance exists for egg white allergy.16 Food avoidance programs should be developed in conjunction with an allergist, as it is no longer advised in many cases to completely withdraw foods; therefore, an allergist has to assess the level of allergic severity and the risk-benefit ratio of food avoidance or introduction.17 Emotional stressors, heat, and humidity, as well as indoor heating in the winter months, can cause AD flares.18
A study by Silverberg et al19 provided evidence of climate influences on the US prevalence of childhood eczema using a merged analysis of the 2007 National Survey of Children’s Health and the 2006-2007 National Climate Data Center and Weather Service. Results showed that eczema prevalence was significantly lower when associated with higher annual relative humidity (P=.01), UV index (P<.0001), and highest-quartile air temperature (P=.002).19 The Pediatric Eczema Elective Registry also showed that warm, humid, and high-sun-exposure climates are associated with poorly controlled eczema in affected patients.20 The association of eczema with latitude as well as its negative association with mean annual outdoor temperature has been described by Weiland et al21 in the ISAAC (International Study of Asthma and Allergies in Childhood) study. Long airplane flights in low humidity can trigger eczema in adults. Climate has been postulated to affect eczema through alterations in filaggrin and skin barrier function.22 Indoor temperature and humidity regulation may be used adjunctively for daily flare prevention.
Genetics and AD
Of 762 infants in a birth cohort with a parent with atopy in Cincinnati, Ohio, 39% developed eczema by the age of 3 years. Single nucleotide polymorphisms of IL-4Rα 175 V and CD14-159 C/T were linked to greater eczema risk at 2 to 3 years of age.23 Monozygotic twins have a concordance rate of 0.72 to 0.86 versus 0.21 to 0.23 in dizygotic twins, demonstrating a strong genetic component in the development of AD.24 Linkage to AD has been positively made to the epidermal differentiation complex on human chromosome 1q21, which contains the genes for filaggrin and other proteins such as loricrin. Other genes linked to AD include the serine protease inhibitor SPINK5 (serine peptidase inhibitor, Kazal type 5) implicated in Netherton syndrome (triad of ichthyosis linearis circumflexa, bamboo hair, and atopic disorders); RANTES (regulated on activation, normal T-expressed, and secreted), which has been associated with severity of AD; IL-4; and IL-13.5,25,26
The Hygiene Hypothesis
Atopic dermatitis is more common in wealthy developed countries, leading some to believe that hygiene and relative reduction in illness via vaccination have contributed to the rise of AD prevalence in developed nations.13,27 There currently is evidence demonstrating that wild-type varicella infection confers long-standing protection against AD and mediates reduced total IgE and peripheral blood lymphocytes.27
Grading of AD
Grading of AD is a subject of controversy, as there currently are no uniform grading scales.28 A recent outcomes group attempted to determine the best scale for disease monitoring. Schmitt et al29 presented the Harmonizing Outcome Measures for Eczema (HOME) roadmap, which was intended to determine a core outcome set for eczema; however, because these outcome measurements have not yet been standardized, only the eczema assessment and severity index (EASI) scoring system meets criteria for standardization. In clinical practice, physicians often assign mild, moderate, or severe labeling based on their general sense of the disease extent using an investigator global assessment score.28
The EASI score is a well-validated composite score of AD severity based on 4 body regions: (1) head and neck, (2) trunk (including genital area), (3) upper limbs, and (4) lower limbs (including buttocks). The total area of involvement in each region is graded on a scale of 0 to 6, and AD severity is graded as a composite of 4 parameters (ranked on a scale of 0–3), including redness (erythema, inflammation), thickness (induration, papulation, swelling [acute eczema]), scratching (excoriation), and lichenification (prurigo nodules [chronic eczema]). The surface area of each region relative to body size is used as a multiplying factor, resulting in the following severity strata: 0=clear; 0.1–1.0=almost clear; 1.1–7.0=mild; 7.1–21.0=moderate; 21.1–50.0=severe; 50.1–72.0=very severe (κ=0.75).30-32 The six area, six sign AD (SASSAD) score32,33 is a similar score without adjustment for body surface area by region.34
An older, now less frequently used eczema score is the SCORAD, which addressed surface area by rule of nines and severity of 6 features—redness, swelling, oozing/crusting, scratch marks, skin thickening (lichenification), dryness (assessed in an area with no inflammation)—by region on a scale of 0 to 3. A subjective symptom parameter for itching and sleeplessness helped highlight that these comorbidities are important in gauging disease activity and impact on a child’s life.35
Natural History of AD
The clinical dogma has been that AD would improve with age, with reduction at grade school entry and perhaps full disappearance in adulthood; however, 3 recent surveys have suggested otherwise. The ISAAC group has found prevalence of AD in wealthy developed countries among children aged 6 to 7 years to be at a consistent increase.36 A US-based survey from the National Health Interview Survey showed a 1-year prevalence of 10.2% of active AD in adults and 9.8% when occupational dermatitis was excluded.37 Halvorsen et al38 demonstrated that eczema prevalence is 9.7% in individuals aged 18 to 19 years.
A prospective trial of eighth graders followed from 1995 to 2010 demonstrated that AD persisted in 50% at school age. Persistent eczema into adulthood was associated with early-onset childhood allergic rhinitis and hand eczema.39 In a cohort of hand eczema patients (N=368), 28% had AD and 39% had an atopic illness.40 An association with allergic contact dermatitis and increased IgE to Malassezia furfur was further associated.41
Conclusion
The role of triggers and allergens in disease activity in AD is an important consideration in children with AD and requires ongoing consideration with age and varied exposures. Understanding the grading of AD is important in evaluating clinical trial data. The natural history of AD has changed, which is important for the practitioner to note when counseling patients and guardians.
- Li M. Current evidence of epidermal barrier dysfunction and thymic stromal lymphopoietin in the atopic march. Eur Respir Rev. 2014;23:292-298.
- Gupta RS, Rivkina V, DeSantiago-Cardenas L, et al. Asthma and food allergy management in Chicago public schools. Pediatrics. 2014;134:729-736.
- Flohr C, Perkin M, Logan K, et al. Atopic dermatitis and disease severity are the main risk factors for food sensitization in exclusively breastfed infants. J Invest Dermatol. 2014;134:345-350.
- Silverberg NB. Food, glorious food. Cutis. 2011;87:267-268.
- De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132:949-963.
- Thyssen JP, McFadden JP, Kimber I. The multiple factors affecting the association between atopic dermatitis and contact sensitization. Allergy. 2014;69:28-36.
- Amat F, Saint-Pierre P, Bourrat E, et al. Early-onset atopic dermatitis in children: which are the phenotypes at risk of asthma? results from the ORCA Cohort. PLoS One. 2015;10:e0131369.
- Demehri S, Morimoto M, Holtzman MJ, et al. Skin-derived TSLP triggers progression from epidermal-barrier defects to asthma. PLoS Biol. 2009;7:e1000067.
- Silverberg JI, Simpson EL. Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatr Allergy Immunol. 2013;24:476-486.
- Silverberg NB, Lee-Wong M, Yosipovitch G. Diet and atopic dermatitis. Cutis. 2016;97:227-232.
- Ortiz de Frutos FJ, Torrelo A, de Lucas R, et al. Patient perspectives on triggers, adherence to medical recommendations, and disease control in atopic dermatitis: the DATOP study. Actas Dermosifiliogr. 2014;105:487-496.
- Ricci G, Patrizi A, Bellini F, et al. Use of textiles in atopic dermatitis: care of atopic dermatitis. Curr Probl Dermatol. 2006;33:127-143.
- Welliver RC, Wong DT, Sun M, et al. The development of respiratory syncytial virus-specific IgE and the release of histamine in nasopharyngeal secretions after infection. N Engl J Med. 1981;305:841-846.
- Aquino M, Fonacier L. The role of contact dermatitis in patients with atopic dermatitis. J Allergy Clin Immunol Pract. 2014;2:382-387.
- Brod BA, Treat JR, Rothe MJ, et al. Allergic contact dermatitis: kids are not just little people. Clin Dermatol. 2015;33:605-612.
- Martorell A, Alonso E, Boné J, et al. Position document: IgE-mediated allergy to egg protein. Allergol Immunopathol (Madr). 2013;41:320-336.
- Sicherer SH. Early introduction of peanut to infants at high allergic risk can reduce peanut allergy at age 5 years [published online September 17, 2015]. Evid Based Med. 2015;20:204.
- Kiken DA, Silverberg NB. Atopic dermatitis in children, part 1: epidemiology, clinical features, and complications. Cutis. 2006;78:241-247.
- Silverberg JI, Hanifin J, Simpson EL. Climatic factors are associated with childhood eczema prevalence in the United States. J Invest Dermatol. 2013;133:1752-1759.
- Sargen MR, Hoffstad O, Margolis DJ. Warm, humid, and high sun exposure climates are associated with poorly controlled eczema: PEER (Pediatric Eczema Elective Registry) cohort, 2004-2012. J Invest Dermatol. 2014;134:51-57.
- Weiland SK, Hüsing A, Strachan DP, et al. Climate and the prevalence of symptoms of asthma, allergic rhinitis, and atopic eczema in children. Occup Environ Med. 2004;61:609-615.
- Langan SM, Irvine AD. Childhood eczema and the importance of the physical environment. J Invest Dermatol. 2013;133:1706-1709.
- Biagini Myers JM, Wang N, LeMasters GK, et al. Genetic and environmental risk factors for childhood eczema development and allergic sensitization in the CCAAPS cohort. J Invest Dermatol. 2010;130:430-437.
- Brown SJ, McLean WH. Eczema genetics: current state of knowledge and future goals. J Invest Dermatol. 2009;129:543-552.
- Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320-322.
- Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
- Silverberg JI, Norowitz KB, Kleiman E, et al. Association between varicella zoster virus infection and atopic dermatitis in early and late childhood: a case-control study. J Allergy Clin Immunol. 2010;126:300-305.
- Futamura M, Leshem YA, Thomas KS, et al. A systematic review of Investigator Global Assessment (IGA) in atopic dermatitis (AD) trials: many options, no standards. J Am Acad Dermatol. 2016;74:288-294.
- Schmitt J, Apfelbacher C, Spuls PI, et al. The Harmonizing Outcome Measures for Eczema (HOME) roadmap: a methodological framework to develop core sets of outcome measurements in dermatology. J Invest Dermatol. 2015;135:24-30.
- Hanifin JM, Thurston M, Omoto M, et al. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol. 2001;10:11-18.
- Leshem YA, Hajar T, Hanifin JM, et al. What the Eczema Area and Severity Index score tells us about the severity of atopic dermatitis: an interpretability study. Br J Dermatol. 2015;172:1353-1357.
- Barbier N, Paul C, Luger T, et al. Validation of the Eczema Area and Severity Index for atopic dermatitis in a cohort of 1550 patients from the pimecrolimus cream 1% randomized controlled clinical trials programme. Br J Dermatol. 2004;150:96-102.
- Berth-Jones J. Six area, six sign atopic dermatitis (SASSAD) severity score: a simple system for monitoring disease activity in atopic dermatitis. Br J Dermatol. 1996;135(suppl 48):25-30.
- Zhao CY, Tran AQ, Lazo-Dizon JP, et al. A pilot comparison study of four clinician-rated atopic dermatitis severity scales. Br J Dermatol. 2015;173:488-497.
- Kunz B, Oranje AP, Labrèze L, et al. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology. 1997;195:10-19.
- Williams H, Stewart A, von Mutius E, et al. Is eczema really on the increase worldwide? J Allergy Clin Immunol. 2008;121:947-954.
- Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132:1132-1138.
- Halvorsen JA, Lien L, Dalgard F, et al. Suicidal ideation, mental health problems, and social function in adolescents with eczema: a population-based study. J Invest Dermatol. 2014;134:1847-1854.
- Mortz CG, Andersen KE, Dellgren C, et al. Atopic dermatitis from adolescence to adulthood in the TOACS cohort: prevalence, persistence, and comorbidities. Allergy. 2015;70:836-845.
- Rystedt I. Atopic background in patients with occupational hand eczema. Contact Dermatitis. 1985;12:247-254.
- Mortz CG, Andersen KE, Dellgren C, et al. Atopic dermatitis from adolescence to adulthood in the TOACS cohort: prevalence, persistence and comorbidities. Allergy. 2015;70:836-845.
Atopic dermatitis (AD) may be triggered by viral infections, food allergens, weather, and other causes, and it may trigger an inflammatory progression known as the atopic march. This article reviews research on triggers of pediatric AD so that dermatologists may discuss trigger avoidance with patients and guardians. Other factors affecting AD development include genetics and hygiene. Grading of AD also is discussed.
The Atopic March
The persistence of AD in untreated skin can trigger an inflammatory progression called the atopic march in which food and environmental allergies as well as asthma may occur progressively due to ongoing inflammatory triggering.1 In a study of asthma and food allergy reporting and management in public schools in Chicago, Illinois, food allergies were seen in 9.3% of asthmatic students (n=18,000), and 40.1% of food allergic students (n=4000) had asthma.2 An observational study by Flohr et al3 in London, England, included 619 exclusively breastfed infants who were recruited at 3 months of age. The investigators determined that food sensitization was unrelated to the presence of filaggrin mutations, type of eczema (flexural vs nonflexural), and transepidermal water loss but was associated with AD severity as determined by SCORAD (SCORing Atopic Dermatitis), a composite score of AD that includes pruritus as a factor in severity. Other AD associations included 3 leading food allergens: eggs, milk, and peanuts. No association with cod, wheat, or sesame allergy was noted. The investigators concluded that AD and AD severity were the leading skin-related risk factors for food allergies and therefore food allergy development in breastfed infants was probably mediated by cutaneous antigen-presenting cells.3
The skin has been documented to react to contact with known food allergens4 and is known to be a route of allergic sensitization to allergens such as fragrance in patients with AD.5,6 Two phenotypes of eczema that have been associated with asthma development are severe AD disease and multiple environmental allergies, supporting the theory of the atopic march.7 There also is evidence that release of danger-associated proteins from an impaired barrier also may trigger asthma.8 An analysis of the 2007 National Survey of Children’s Health, a population-based study of91,642 children aged 0 to 17 years, showed that children with AD had a higher prevalence of comorbid asthma (25.1% vs 12.3%), hay fever (34.4% vs 14.3%), and food allergies (15.1% vs 3.6%) compared to children without AD.9 A recent article provided detailed information on how food and diet interplay with AD.10
Triggers of Disease Flares
Triggers are the leading source of AD flare initiation, and avoidance of triggers is an important mechanism by which patients can control disease activity. Despite the best skin care and trigger avoidance, disease flares occur, sometimes due to ongoing inflammation and other times due to inability to prevent flares such as heat and humidity. A survey of patients with AD in Spain identified the following triggers: cosmetic products, clothing, mites, detergents/soaps, and temperature changes.11 In childhood, wool also is a known trigger of AD.12 Viral infections including respiratory syncytial virus may trigger the first onset of AD.13 Patients with AD may become allergic to fragrance and metals causing disease exacerbation on exposure.14,15 Food allergens contribute to approximately 40% of cases of AD in infancy but are not the cause of AD. The best evidence for improvement of AD with food allergen avoidance exists for egg white allergy.16 Food avoidance programs should be developed in conjunction with an allergist, as it is no longer advised in many cases to completely withdraw foods; therefore, an allergist has to assess the level of allergic severity and the risk-benefit ratio of food avoidance or introduction.17 Emotional stressors, heat, and humidity, as well as indoor heating in the winter months, can cause AD flares.18
A study by Silverberg et al19 provided evidence of climate influences on the US prevalence of childhood eczema using a merged analysis of the 2007 National Survey of Children’s Health and the 2006-2007 National Climate Data Center and Weather Service. Results showed that eczema prevalence was significantly lower when associated with higher annual relative humidity (P=.01), UV index (P<.0001), and highest-quartile air temperature (P=.002).19 The Pediatric Eczema Elective Registry also showed that warm, humid, and high-sun-exposure climates are associated with poorly controlled eczema in affected patients.20 The association of eczema with latitude as well as its negative association with mean annual outdoor temperature has been described by Weiland et al21 in the ISAAC (International Study of Asthma and Allergies in Childhood) study. Long airplane flights in low humidity can trigger eczema in adults. Climate has been postulated to affect eczema through alterations in filaggrin and skin barrier function.22 Indoor temperature and humidity regulation may be used adjunctively for daily flare prevention.
Genetics and AD
Of 762 infants in a birth cohort with a parent with atopy in Cincinnati, Ohio, 39% developed eczema by the age of 3 years. Single nucleotide polymorphisms of IL-4Rα 175 V and CD14-159 C/T were linked to greater eczema risk at 2 to 3 years of age.23 Monozygotic twins have a concordance rate of 0.72 to 0.86 versus 0.21 to 0.23 in dizygotic twins, demonstrating a strong genetic component in the development of AD.24 Linkage to AD has been positively made to the epidermal differentiation complex on human chromosome 1q21, which contains the genes for filaggrin and other proteins such as loricrin. Other genes linked to AD include the serine protease inhibitor SPINK5 (serine peptidase inhibitor, Kazal type 5) implicated in Netherton syndrome (triad of ichthyosis linearis circumflexa, bamboo hair, and atopic disorders); RANTES (regulated on activation, normal T-expressed, and secreted), which has been associated with severity of AD; IL-4; and IL-13.5,25,26
The Hygiene Hypothesis
Atopic dermatitis is more common in wealthy developed countries, leading some to believe that hygiene and relative reduction in illness via vaccination have contributed to the rise of AD prevalence in developed nations.13,27 There currently is evidence demonstrating that wild-type varicella infection confers long-standing protection against AD and mediates reduced total IgE and peripheral blood lymphocytes.27
Grading of AD
Grading of AD is a subject of controversy, as there currently are no uniform grading scales.28 A recent outcomes group attempted to determine the best scale for disease monitoring. Schmitt et al29 presented the Harmonizing Outcome Measures for Eczema (HOME) roadmap, which was intended to determine a core outcome set for eczema; however, because these outcome measurements have not yet been standardized, only the eczema assessment and severity index (EASI) scoring system meets criteria for standardization. In clinical practice, physicians often assign mild, moderate, or severe labeling based on their general sense of the disease extent using an investigator global assessment score.28
The EASI score is a well-validated composite score of AD severity based on 4 body regions: (1) head and neck, (2) trunk (including genital area), (3) upper limbs, and (4) lower limbs (including buttocks). The total area of involvement in each region is graded on a scale of 0 to 6, and AD severity is graded as a composite of 4 parameters (ranked on a scale of 0–3), including redness (erythema, inflammation), thickness (induration, papulation, swelling [acute eczema]), scratching (excoriation), and lichenification (prurigo nodules [chronic eczema]). The surface area of each region relative to body size is used as a multiplying factor, resulting in the following severity strata: 0=clear; 0.1–1.0=almost clear; 1.1–7.0=mild; 7.1–21.0=moderate; 21.1–50.0=severe; 50.1–72.0=very severe (κ=0.75).30-32 The six area, six sign AD (SASSAD) score32,33 is a similar score without adjustment for body surface area by region.34
An older, now less frequently used eczema score is the SCORAD, which addressed surface area by rule of nines and severity of 6 features—redness, swelling, oozing/crusting, scratch marks, skin thickening (lichenification), dryness (assessed in an area with no inflammation)—by region on a scale of 0 to 3. A subjective symptom parameter for itching and sleeplessness helped highlight that these comorbidities are important in gauging disease activity and impact on a child’s life.35
Natural History of AD
The clinical dogma has been that AD would improve with age, with reduction at grade school entry and perhaps full disappearance in adulthood; however, 3 recent surveys have suggested otherwise. The ISAAC group has found prevalence of AD in wealthy developed countries among children aged 6 to 7 years to be at a consistent increase.36 A US-based survey from the National Health Interview Survey showed a 1-year prevalence of 10.2% of active AD in adults and 9.8% when occupational dermatitis was excluded.37 Halvorsen et al38 demonstrated that eczema prevalence is 9.7% in individuals aged 18 to 19 years.
A prospective trial of eighth graders followed from 1995 to 2010 demonstrated that AD persisted in 50% at school age. Persistent eczema into adulthood was associated with early-onset childhood allergic rhinitis and hand eczema.39 In a cohort of hand eczema patients (N=368), 28% had AD and 39% had an atopic illness.40 An association with allergic contact dermatitis and increased IgE to Malassezia furfur was further associated.41
Conclusion
The role of triggers and allergens in disease activity in AD is an important consideration in children with AD and requires ongoing consideration with age and varied exposures. Understanding the grading of AD is important in evaluating clinical trial data. The natural history of AD has changed, which is important for the practitioner to note when counseling patients and guardians.
Atopic dermatitis (AD) may be triggered by viral infections, food allergens, weather, and other causes, and it may trigger an inflammatory progression known as the atopic march. This article reviews research on triggers of pediatric AD so that dermatologists may discuss trigger avoidance with patients and guardians. Other factors affecting AD development include genetics and hygiene. Grading of AD also is discussed.
The Atopic March
The persistence of AD in untreated skin can trigger an inflammatory progression called the atopic march in which food and environmental allergies as well as asthma may occur progressively due to ongoing inflammatory triggering.1 In a study of asthma and food allergy reporting and management in public schools in Chicago, Illinois, food allergies were seen in 9.3% of asthmatic students (n=18,000), and 40.1% of food allergic students (n=4000) had asthma.2 An observational study by Flohr et al3 in London, England, included 619 exclusively breastfed infants who were recruited at 3 months of age. The investigators determined that food sensitization was unrelated to the presence of filaggrin mutations, type of eczema (flexural vs nonflexural), and transepidermal water loss but was associated with AD severity as determined by SCORAD (SCORing Atopic Dermatitis), a composite score of AD that includes pruritus as a factor in severity. Other AD associations included 3 leading food allergens: eggs, milk, and peanuts. No association with cod, wheat, or sesame allergy was noted. The investigators concluded that AD and AD severity were the leading skin-related risk factors for food allergies and therefore food allergy development in breastfed infants was probably mediated by cutaneous antigen-presenting cells.3
The skin has been documented to react to contact with known food allergens4 and is known to be a route of allergic sensitization to allergens such as fragrance in patients with AD.5,6 Two phenotypes of eczema that have been associated with asthma development are severe AD disease and multiple environmental allergies, supporting the theory of the atopic march.7 There also is evidence that release of danger-associated proteins from an impaired barrier also may trigger asthma.8 An analysis of the 2007 National Survey of Children’s Health, a population-based study of91,642 children aged 0 to 17 years, showed that children with AD had a higher prevalence of comorbid asthma (25.1% vs 12.3%), hay fever (34.4% vs 14.3%), and food allergies (15.1% vs 3.6%) compared to children without AD.9 A recent article provided detailed information on how food and diet interplay with AD.10
Triggers of Disease Flares
Triggers are the leading source of AD flare initiation, and avoidance of triggers is an important mechanism by which patients can control disease activity. Despite the best skin care and trigger avoidance, disease flares occur, sometimes due to ongoing inflammation and other times due to inability to prevent flares such as heat and humidity. A survey of patients with AD in Spain identified the following triggers: cosmetic products, clothing, mites, detergents/soaps, and temperature changes.11 In childhood, wool also is a known trigger of AD.12 Viral infections including respiratory syncytial virus may trigger the first onset of AD.13 Patients with AD may become allergic to fragrance and metals causing disease exacerbation on exposure.14,15 Food allergens contribute to approximately 40% of cases of AD in infancy but are not the cause of AD. The best evidence for improvement of AD with food allergen avoidance exists for egg white allergy.16 Food avoidance programs should be developed in conjunction with an allergist, as it is no longer advised in many cases to completely withdraw foods; therefore, an allergist has to assess the level of allergic severity and the risk-benefit ratio of food avoidance or introduction.17 Emotional stressors, heat, and humidity, as well as indoor heating in the winter months, can cause AD flares.18
A study by Silverberg et al19 provided evidence of climate influences on the US prevalence of childhood eczema using a merged analysis of the 2007 National Survey of Children’s Health and the 2006-2007 National Climate Data Center and Weather Service. Results showed that eczema prevalence was significantly lower when associated with higher annual relative humidity (P=.01), UV index (P<.0001), and highest-quartile air temperature (P=.002).19 The Pediatric Eczema Elective Registry also showed that warm, humid, and high-sun-exposure climates are associated with poorly controlled eczema in affected patients.20 The association of eczema with latitude as well as its negative association with mean annual outdoor temperature has been described by Weiland et al21 in the ISAAC (International Study of Asthma and Allergies in Childhood) study. Long airplane flights in low humidity can trigger eczema in adults. Climate has been postulated to affect eczema through alterations in filaggrin and skin barrier function.22 Indoor temperature and humidity regulation may be used adjunctively for daily flare prevention.
Genetics and AD
Of 762 infants in a birth cohort with a parent with atopy in Cincinnati, Ohio, 39% developed eczema by the age of 3 years. Single nucleotide polymorphisms of IL-4Rα 175 V and CD14-159 C/T were linked to greater eczema risk at 2 to 3 years of age.23 Monozygotic twins have a concordance rate of 0.72 to 0.86 versus 0.21 to 0.23 in dizygotic twins, demonstrating a strong genetic component in the development of AD.24 Linkage to AD has been positively made to the epidermal differentiation complex on human chromosome 1q21, which contains the genes for filaggrin and other proteins such as loricrin. Other genes linked to AD include the serine protease inhibitor SPINK5 (serine peptidase inhibitor, Kazal type 5) implicated in Netherton syndrome (triad of ichthyosis linearis circumflexa, bamboo hair, and atopic disorders); RANTES (regulated on activation, normal T-expressed, and secreted), which has been associated with severity of AD; IL-4; and IL-13.5,25,26
The Hygiene Hypothesis
Atopic dermatitis is more common in wealthy developed countries, leading some to believe that hygiene and relative reduction in illness via vaccination have contributed to the rise of AD prevalence in developed nations.13,27 There currently is evidence demonstrating that wild-type varicella infection confers long-standing protection against AD and mediates reduced total IgE and peripheral blood lymphocytes.27
Grading of AD
Grading of AD is a subject of controversy, as there currently are no uniform grading scales.28 A recent outcomes group attempted to determine the best scale for disease monitoring. Schmitt et al29 presented the Harmonizing Outcome Measures for Eczema (HOME) roadmap, which was intended to determine a core outcome set for eczema; however, because these outcome measurements have not yet been standardized, only the eczema assessment and severity index (EASI) scoring system meets criteria for standardization. In clinical practice, physicians often assign mild, moderate, or severe labeling based on their general sense of the disease extent using an investigator global assessment score.28
The EASI score is a well-validated composite score of AD severity based on 4 body regions: (1) head and neck, (2) trunk (including genital area), (3) upper limbs, and (4) lower limbs (including buttocks). The total area of involvement in each region is graded on a scale of 0 to 6, and AD severity is graded as a composite of 4 parameters (ranked on a scale of 0–3), including redness (erythema, inflammation), thickness (induration, papulation, swelling [acute eczema]), scratching (excoriation), and lichenification (prurigo nodules [chronic eczema]). The surface area of each region relative to body size is used as a multiplying factor, resulting in the following severity strata: 0=clear; 0.1–1.0=almost clear; 1.1–7.0=mild; 7.1–21.0=moderate; 21.1–50.0=severe; 50.1–72.0=very severe (κ=0.75).30-32 The six area, six sign AD (SASSAD) score32,33 is a similar score without adjustment for body surface area by region.34
An older, now less frequently used eczema score is the SCORAD, which addressed surface area by rule of nines and severity of 6 features—redness, swelling, oozing/crusting, scratch marks, skin thickening (lichenification), dryness (assessed in an area with no inflammation)—by region on a scale of 0 to 3. A subjective symptom parameter for itching and sleeplessness helped highlight that these comorbidities are important in gauging disease activity and impact on a child’s life.35
Natural History of AD
The clinical dogma has been that AD would improve with age, with reduction at grade school entry and perhaps full disappearance in adulthood; however, 3 recent surveys have suggested otherwise. The ISAAC group has found prevalence of AD in wealthy developed countries among children aged 6 to 7 years to be at a consistent increase.36 A US-based survey from the National Health Interview Survey showed a 1-year prevalence of 10.2% of active AD in adults and 9.8% when occupational dermatitis was excluded.37 Halvorsen et al38 demonstrated that eczema prevalence is 9.7% in individuals aged 18 to 19 years.
A prospective trial of eighth graders followed from 1995 to 2010 demonstrated that AD persisted in 50% at school age. Persistent eczema into adulthood was associated with early-onset childhood allergic rhinitis and hand eczema.39 In a cohort of hand eczema patients (N=368), 28% had AD and 39% had an atopic illness.40 An association with allergic contact dermatitis and increased IgE to Malassezia furfur was further associated.41
Conclusion
The role of triggers and allergens in disease activity in AD is an important consideration in children with AD and requires ongoing consideration with age and varied exposures. Understanding the grading of AD is important in evaluating clinical trial data. The natural history of AD has changed, which is important for the practitioner to note when counseling patients and guardians.
- Li M. Current evidence of epidermal barrier dysfunction and thymic stromal lymphopoietin in the atopic march. Eur Respir Rev. 2014;23:292-298.
- Gupta RS, Rivkina V, DeSantiago-Cardenas L, et al. Asthma and food allergy management in Chicago public schools. Pediatrics. 2014;134:729-736.
- Flohr C, Perkin M, Logan K, et al. Atopic dermatitis and disease severity are the main risk factors for food sensitization in exclusively breastfed infants. J Invest Dermatol. 2014;134:345-350.
- Silverberg NB. Food, glorious food. Cutis. 2011;87:267-268.
- De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132:949-963.
- Thyssen JP, McFadden JP, Kimber I. The multiple factors affecting the association between atopic dermatitis and contact sensitization. Allergy. 2014;69:28-36.
- Amat F, Saint-Pierre P, Bourrat E, et al. Early-onset atopic dermatitis in children: which are the phenotypes at risk of asthma? results from the ORCA Cohort. PLoS One. 2015;10:e0131369.
- Demehri S, Morimoto M, Holtzman MJ, et al. Skin-derived TSLP triggers progression from epidermal-barrier defects to asthma. PLoS Biol. 2009;7:e1000067.
- Silverberg JI, Simpson EL. Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatr Allergy Immunol. 2013;24:476-486.
- Silverberg NB, Lee-Wong M, Yosipovitch G. Diet and atopic dermatitis. Cutis. 2016;97:227-232.
- Ortiz de Frutos FJ, Torrelo A, de Lucas R, et al. Patient perspectives on triggers, adherence to medical recommendations, and disease control in atopic dermatitis: the DATOP study. Actas Dermosifiliogr. 2014;105:487-496.
- Ricci G, Patrizi A, Bellini F, et al. Use of textiles in atopic dermatitis: care of atopic dermatitis. Curr Probl Dermatol. 2006;33:127-143.
- Welliver RC, Wong DT, Sun M, et al. The development of respiratory syncytial virus-specific IgE and the release of histamine in nasopharyngeal secretions after infection. N Engl J Med. 1981;305:841-846.
- Aquino M, Fonacier L. The role of contact dermatitis in patients with atopic dermatitis. J Allergy Clin Immunol Pract. 2014;2:382-387.
- Brod BA, Treat JR, Rothe MJ, et al. Allergic contact dermatitis: kids are not just little people. Clin Dermatol. 2015;33:605-612.
- Martorell A, Alonso E, Boné J, et al. Position document: IgE-mediated allergy to egg protein. Allergol Immunopathol (Madr). 2013;41:320-336.
- Sicherer SH. Early introduction of peanut to infants at high allergic risk can reduce peanut allergy at age 5 years [published online September 17, 2015]. Evid Based Med. 2015;20:204.
- Kiken DA, Silverberg NB. Atopic dermatitis in children, part 1: epidemiology, clinical features, and complications. Cutis. 2006;78:241-247.
- Silverberg JI, Hanifin J, Simpson EL. Climatic factors are associated with childhood eczema prevalence in the United States. J Invest Dermatol. 2013;133:1752-1759.
- Sargen MR, Hoffstad O, Margolis DJ. Warm, humid, and high sun exposure climates are associated with poorly controlled eczema: PEER (Pediatric Eczema Elective Registry) cohort, 2004-2012. J Invest Dermatol. 2014;134:51-57.
- Weiland SK, Hüsing A, Strachan DP, et al. Climate and the prevalence of symptoms of asthma, allergic rhinitis, and atopic eczema in children. Occup Environ Med. 2004;61:609-615.
- Langan SM, Irvine AD. Childhood eczema and the importance of the physical environment. J Invest Dermatol. 2013;133:1706-1709.
- Biagini Myers JM, Wang N, LeMasters GK, et al. Genetic and environmental risk factors for childhood eczema development and allergic sensitization in the CCAAPS cohort. J Invest Dermatol. 2010;130:430-437.
- Brown SJ, McLean WH. Eczema genetics: current state of knowledge and future goals. J Invest Dermatol. 2009;129:543-552.
- Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320-322.
- Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
- Silverberg JI, Norowitz KB, Kleiman E, et al. Association between varicella zoster virus infection and atopic dermatitis in early and late childhood: a case-control study. J Allergy Clin Immunol. 2010;126:300-305.
- Futamura M, Leshem YA, Thomas KS, et al. A systematic review of Investigator Global Assessment (IGA) in atopic dermatitis (AD) trials: many options, no standards. J Am Acad Dermatol. 2016;74:288-294.
- Schmitt J, Apfelbacher C, Spuls PI, et al. The Harmonizing Outcome Measures for Eczema (HOME) roadmap: a methodological framework to develop core sets of outcome measurements in dermatology. J Invest Dermatol. 2015;135:24-30.
- Hanifin JM, Thurston M, Omoto M, et al. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol. 2001;10:11-18.
- Leshem YA, Hajar T, Hanifin JM, et al. What the Eczema Area and Severity Index score tells us about the severity of atopic dermatitis: an interpretability study. Br J Dermatol. 2015;172:1353-1357.
- Barbier N, Paul C, Luger T, et al. Validation of the Eczema Area and Severity Index for atopic dermatitis in a cohort of 1550 patients from the pimecrolimus cream 1% randomized controlled clinical trials programme. Br J Dermatol. 2004;150:96-102.
- Berth-Jones J. Six area, six sign atopic dermatitis (SASSAD) severity score: a simple system for monitoring disease activity in atopic dermatitis. Br J Dermatol. 1996;135(suppl 48):25-30.
- Zhao CY, Tran AQ, Lazo-Dizon JP, et al. A pilot comparison study of four clinician-rated atopic dermatitis severity scales. Br J Dermatol. 2015;173:488-497.
- Kunz B, Oranje AP, Labrèze L, et al. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology. 1997;195:10-19.
- Williams H, Stewart A, von Mutius E, et al. Is eczema really on the increase worldwide? J Allergy Clin Immunol. 2008;121:947-954.
- Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132:1132-1138.
- Halvorsen JA, Lien L, Dalgard F, et al. Suicidal ideation, mental health problems, and social function in adolescents with eczema: a population-based study. J Invest Dermatol. 2014;134:1847-1854.
- Mortz CG, Andersen KE, Dellgren C, et al. Atopic dermatitis from adolescence to adulthood in the TOACS cohort: prevalence, persistence, and comorbidities. Allergy. 2015;70:836-845.
- Rystedt I. Atopic background in patients with occupational hand eczema. Contact Dermatitis. 1985;12:247-254.
- Mortz CG, Andersen KE, Dellgren C, et al. Atopic dermatitis from adolescence to adulthood in the TOACS cohort: prevalence, persistence and comorbidities. Allergy. 2015;70:836-845.
- Li M. Current evidence of epidermal barrier dysfunction and thymic stromal lymphopoietin in the atopic march. Eur Respir Rev. 2014;23:292-298.
- Gupta RS, Rivkina V, DeSantiago-Cardenas L, et al. Asthma and food allergy management in Chicago public schools. Pediatrics. 2014;134:729-736.
- Flohr C, Perkin M, Logan K, et al. Atopic dermatitis and disease severity are the main risk factors for food sensitization in exclusively breastfed infants. J Invest Dermatol. 2014;134:345-350.
- Silverberg NB. Food, glorious food. Cutis. 2011;87:267-268.
- De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132:949-963.
- Thyssen JP, McFadden JP, Kimber I. The multiple factors affecting the association between atopic dermatitis and contact sensitization. Allergy. 2014;69:28-36.
- Amat F, Saint-Pierre P, Bourrat E, et al. Early-onset atopic dermatitis in children: which are the phenotypes at risk of asthma? results from the ORCA Cohort. PLoS One. 2015;10:e0131369.
- Demehri S, Morimoto M, Holtzman MJ, et al. Skin-derived TSLP triggers progression from epidermal-barrier defects to asthma. PLoS Biol. 2009;7:e1000067.
- Silverberg JI, Simpson EL. Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatr Allergy Immunol. 2013;24:476-486.
- Silverberg NB, Lee-Wong M, Yosipovitch G. Diet and atopic dermatitis. Cutis. 2016;97:227-232.
- Ortiz de Frutos FJ, Torrelo A, de Lucas R, et al. Patient perspectives on triggers, adherence to medical recommendations, and disease control in atopic dermatitis: the DATOP study. Actas Dermosifiliogr. 2014;105:487-496.
- Ricci G, Patrizi A, Bellini F, et al. Use of textiles in atopic dermatitis: care of atopic dermatitis. Curr Probl Dermatol. 2006;33:127-143.
- Welliver RC, Wong DT, Sun M, et al. The development of respiratory syncytial virus-specific IgE and the release of histamine in nasopharyngeal secretions after infection. N Engl J Med. 1981;305:841-846.
- Aquino M, Fonacier L. The role of contact dermatitis in patients with atopic dermatitis. J Allergy Clin Immunol Pract. 2014;2:382-387.
- Brod BA, Treat JR, Rothe MJ, et al. Allergic contact dermatitis: kids are not just little people. Clin Dermatol. 2015;33:605-612.
- Martorell A, Alonso E, Boné J, et al. Position document: IgE-mediated allergy to egg protein. Allergol Immunopathol (Madr). 2013;41:320-336.
- Sicherer SH. Early introduction of peanut to infants at high allergic risk can reduce peanut allergy at age 5 years [published online September 17, 2015]. Evid Based Med. 2015;20:204.
- Kiken DA, Silverberg NB. Atopic dermatitis in children, part 1: epidemiology, clinical features, and complications. Cutis. 2006;78:241-247.
- Silverberg JI, Hanifin J, Simpson EL. Climatic factors are associated with childhood eczema prevalence in the United States. J Invest Dermatol. 2013;133:1752-1759.
- Sargen MR, Hoffstad O, Margolis DJ. Warm, humid, and high sun exposure climates are associated with poorly controlled eczema: PEER (Pediatric Eczema Elective Registry) cohort, 2004-2012. J Invest Dermatol. 2014;134:51-57.
- Weiland SK, Hüsing A, Strachan DP, et al. Climate and the prevalence of symptoms of asthma, allergic rhinitis, and atopic eczema in children. Occup Environ Med. 2004;61:609-615.
- Langan SM, Irvine AD. Childhood eczema and the importance of the physical environment. J Invest Dermatol. 2013;133:1706-1709.
- Biagini Myers JM, Wang N, LeMasters GK, et al. Genetic and environmental risk factors for childhood eczema development and allergic sensitization in the CCAAPS cohort. J Invest Dermatol. 2010;130:430-437.
- Brown SJ, McLean WH. Eczema genetics: current state of knowledge and future goals. J Invest Dermatol. 2009;129:543-552.
- Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320-322.
- Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
- Silverberg JI, Norowitz KB, Kleiman E, et al. Association between varicella zoster virus infection and atopic dermatitis in early and late childhood: a case-control study. J Allergy Clin Immunol. 2010;126:300-305.
- Futamura M, Leshem YA, Thomas KS, et al. A systematic review of Investigator Global Assessment (IGA) in atopic dermatitis (AD) trials: many options, no standards. J Am Acad Dermatol. 2016;74:288-294.
- Schmitt J, Apfelbacher C, Spuls PI, et al. The Harmonizing Outcome Measures for Eczema (HOME) roadmap: a methodological framework to develop core sets of outcome measurements in dermatology. J Invest Dermatol. 2015;135:24-30.
- Hanifin JM, Thurston M, Omoto M, et al. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol. 2001;10:11-18.
- Leshem YA, Hajar T, Hanifin JM, et al. What the Eczema Area and Severity Index score tells us about the severity of atopic dermatitis: an interpretability study. Br J Dermatol. 2015;172:1353-1357.
- Barbier N, Paul C, Luger T, et al. Validation of the Eczema Area and Severity Index for atopic dermatitis in a cohort of 1550 patients from the pimecrolimus cream 1% randomized controlled clinical trials programme. Br J Dermatol. 2004;150:96-102.
- Berth-Jones J. Six area, six sign atopic dermatitis (SASSAD) severity score: a simple system for monitoring disease activity in atopic dermatitis. Br J Dermatol. 1996;135(suppl 48):25-30.
- Zhao CY, Tran AQ, Lazo-Dizon JP, et al. A pilot comparison study of four clinician-rated atopic dermatitis severity scales. Br J Dermatol. 2015;173:488-497.
- Kunz B, Oranje AP, Labrèze L, et al. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology. 1997;195:10-19.
- Williams H, Stewart A, von Mutius E, et al. Is eczema really on the increase worldwide? J Allergy Clin Immunol. 2008;121:947-954.
- Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132:1132-1138.
- Halvorsen JA, Lien L, Dalgard F, et al. Suicidal ideation, mental health problems, and social function in adolescents with eczema: a population-based study. J Invest Dermatol. 2014;134:1847-1854.
- Mortz CG, Andersen KE, Dellgren C, et al. Atopic dermatitis from adolescence to adulthood in the TOACS cohort: prevalence, persistence, and comorbidities. Allergy. 2015;70:836-845.
- Rystedt I. Atopic background in patients with occupational hand eczema. Contact Dermatitis. 1985;12:247-254.
- Mortz CG, Andersen KE, Dellgren C, et al. Atopic dermatitis from adolescence to adulthood in the TOACS cohort: prevalence, persistence and comorbidities. Allergy. 2015;70:836-845.
Practice Points
- Atopic dermatitis (AD) can be triggered by viral infections, weather, and food allergens.
- The scoring of AD is largely used experimentally and includes the eczema assessment and severity index; the SCORAD (SCORing Atopic Dermatitis); and the six area, six sign AD (SASSAD) scores.
- There is a strong genetic contribution to the development of AD.
- Children with AD may have persistent disease into adulthood in half of cases.