User login
Atypical vascular lesions (AVLs) of the breast are rare cutaneous vascular proliferations that present as erythematous, violaceous, or flesh-colored papules, patches, or plaques in women who have undergone radiation treatment for breast carcinoma.1,2 These lesions most commonly develop in the irradiated area within 3 to 6 years following radiation treatment.3
Various terms have been used to describe AVLs in the literature, including atypical hemangiomas, benign lymphangiomatous papules, benign lymphangioendotheliomas, lymphangioma circumscriptum, and acquired progressive lymphangiomas, suggesting benign behavior.4-10 However, their identity as benign lesions has been a source of controversy, with some investigators proposing that AVLs may be a precursor lesion to postirradiation angiosarcoma.2 Research has addressed if there are markers that can predict AVL types that are more likely to develop into angiosarcomas.1 Although most clinicians treat AVLs with complete excision, there currently are no specific guidelines to direct this practice.
We report the case of a patient with a history of 1 AVL that was excised who developed 3 additional AVLs in the same breast over the course of 15 months.
Case Report
A 55-year-old woman with a history of obesity, hypertension, and infiltrating ductal carcinoma in situ of the right breast (grade 2, estrogen receptor and progesterone receptor positive) underwent a right breast lumpectomy and sentinel lymph node dissection. Three months later, she underwent re-excision for positive margins and started adjuvant hormonal therapy with tamoxifen. One month later, she began external beam radiation therapy and received a total dose of 6040 cGy over the course of 9 weeks (34 total treatments).
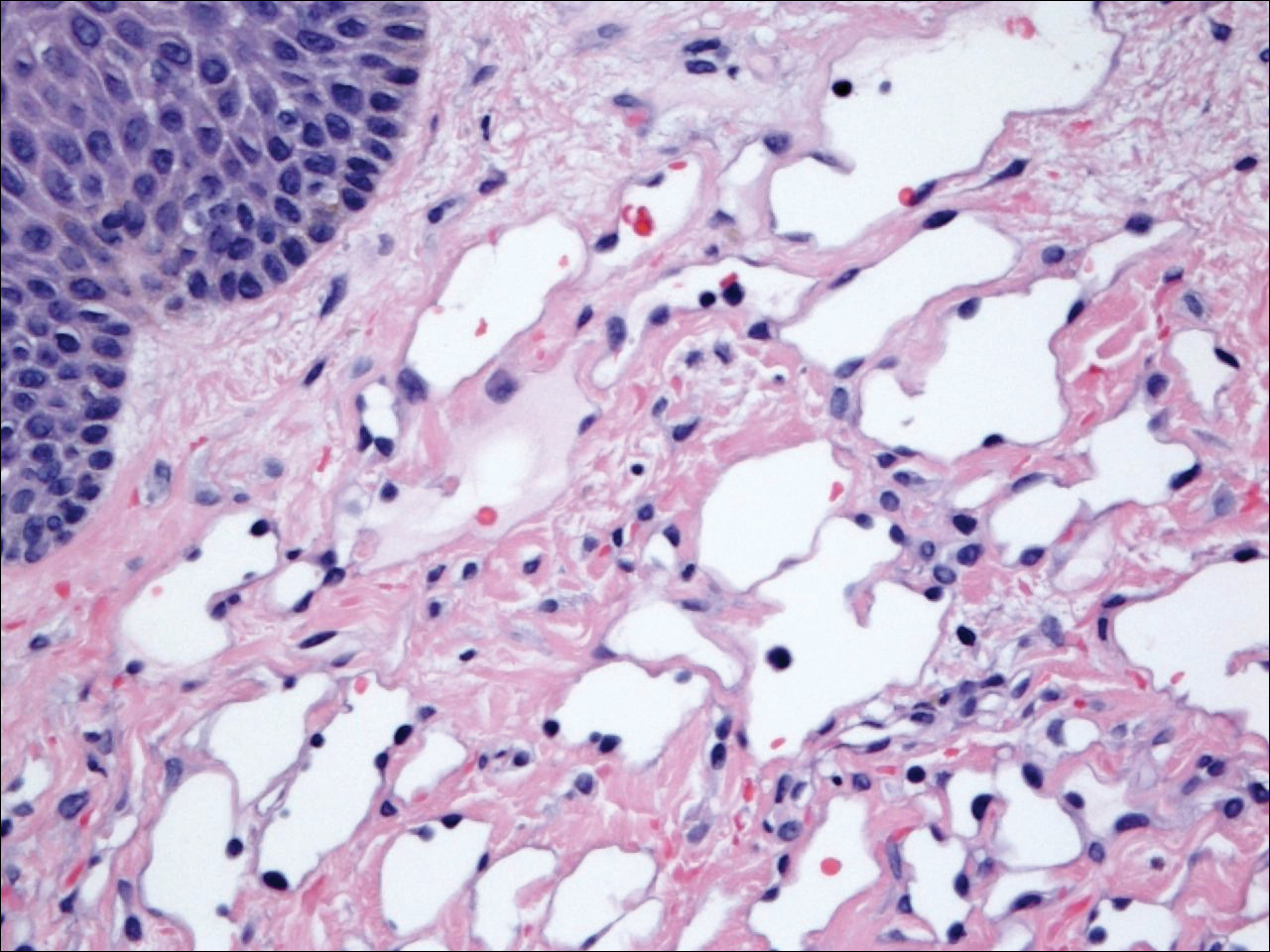
The patient presented to an outside dermatology clinic 2 years after completing external beam radiation therapy for evaluation of a new pink nodule on the right mid breast. The nodule was biopsied and discovered to be an AVL. Pathology showed an anastomosing proliferation of thin-walled vascular channels mainly located in the superficial dermis with notable endothelial nuclear atypia and hyperchromasia. There were several tiny foci with the beginnings of multilayering with prominent endothelial atypia (Figure 1). She underwent complete excision for this AVL with negative margins.
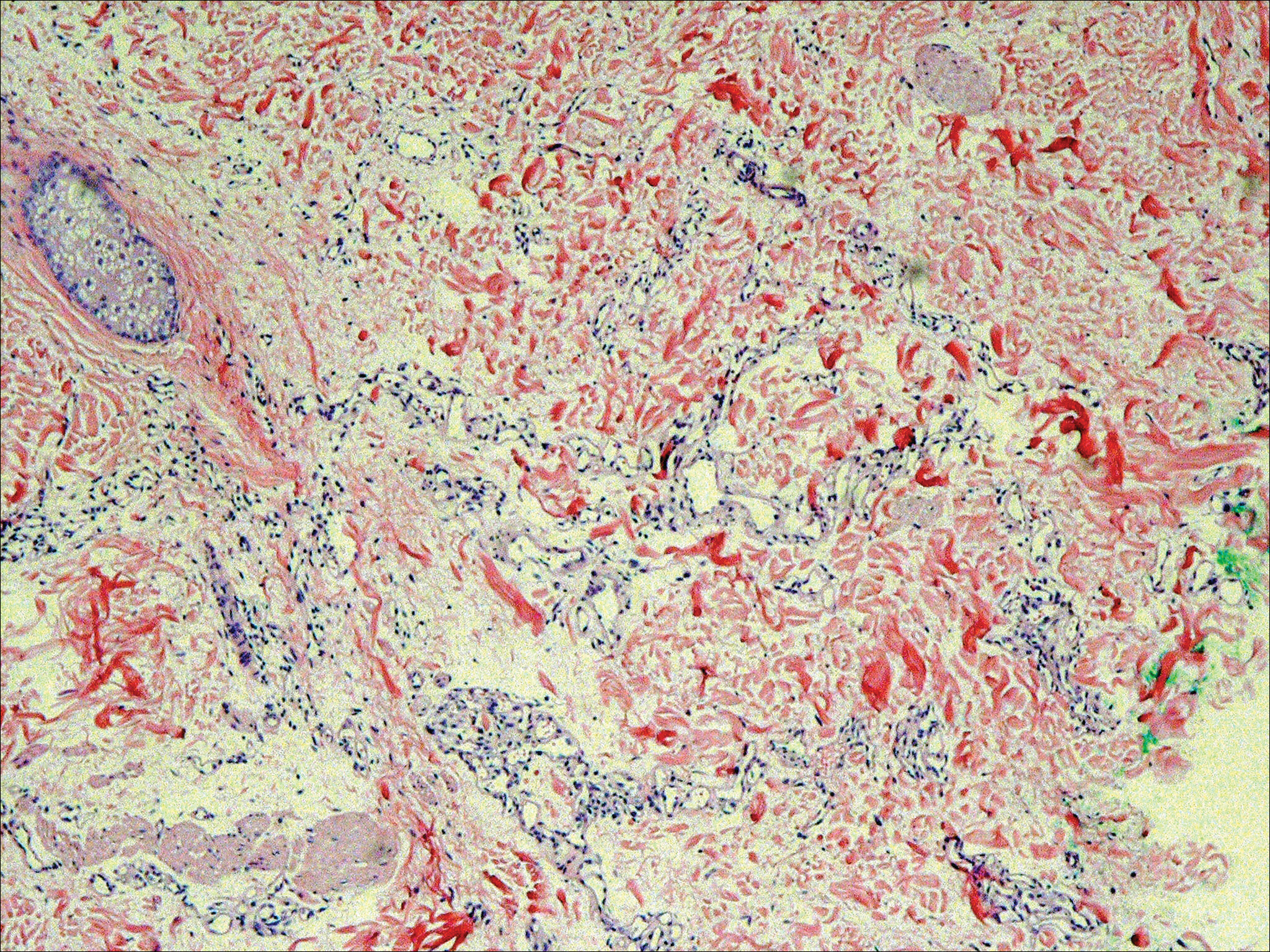
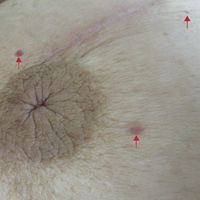
Six months after the initial AVL diagnosis, she presented to our dermatology clinic with another asymptomatic red bump on the right breast. On physical examination, a 4-mm firm, erythematous, well-circumscribed papule was noted on the medial aspect of the right breast along with a similar-appearing 4-mm papule on the right lateral aspect of the right breast (Figure 2). The patient was unsure of the duration of the second lesion but felt that it had been present at least as long as the other lesion. Both lesions clinically resembled typical capillary hemangiomas. A 6-mm punch biopsy of the right medial breast was performed and revealed enlarged vessels and capillaries in the upper dermis lined by endothelial cells with focal prominent nuclei without necrosis, overt atypia, mitosis, or tufting (Figure 3). Immunostaining was positive for CD34, factor VIII antigen, podoplanin (D2-40), and CD31, and negative for cytokeratin 7 and pankeratin. This staining was compatible with a lymphatic-type AVL.1 A diagnosis of AVL was made and complete excision with clear margins was performed. At the time of this excision, a biopsy of the right lateral breast was performed revealing thin-walled, dilated vascular channels in the superficial dermis with architecturally atypical angulated outlines, mild endothelial nuclear atypia, and hyperchromasia without endothelial multilayering. Clear margins were noted on the biopsy, but the patient subsequently declined re-excision of this third AVL.
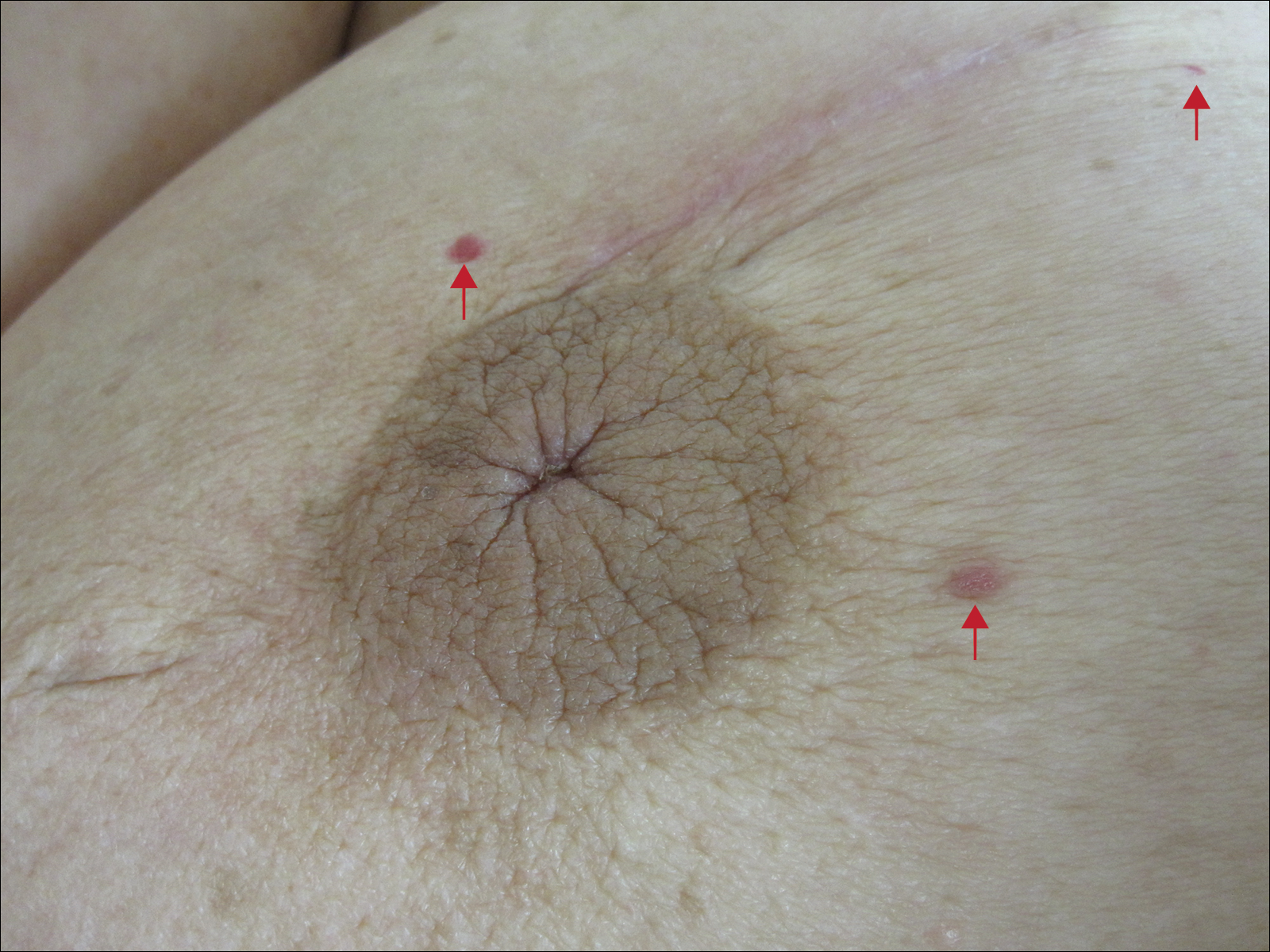
During a subsequent follow-up visit 9 months later, the patient was noted to have a 2-mm red, vascular-appearing papule on the right upper medial breast (Figure 2). A 6-mm biopsy was performed and revealed thin-walled vascular channels in the superficial dermis with endothelial nuclear atypia consistent with an AVL.
Comment
Fineberg and Rosen8 were the first to describe AVLs in their 1994 study of 4 women with cutaneous vascular proliferations that developed after radiation and chemotherapy for breast cancer. They concluded that these AVLs were benign lesions distinct from angiosarcomas.8 However, further research has challenged the benign nature of AVLs. In 2005, Brenn and Fletcher2 studied 42 women diagnosed with either angiosarcoma or atypical radiation-associated cutaneous vascular lesions. They suggested that AVLs resided on the same spectrum as angiosarcomas and that AVLs may be precursor lesions to angiosarcomas.2 Furthermore, Hildebrandt et al11 in 2001 and Di Tommaso and Fabbri12 in 2003 published case reports of individual patients who developed an angiosarcoma from a preexisting AVL.
The controversy continued when Patton et al1 published a study in 2008 in which 32 cases of AVLs were reviewed. In this study, 2 histologic types of AVLs were described: vascular type and lymphatic type. Vascular-type AVLs are characterized by irregularly dispersed, pericyte-invested, capillary-sized vessels within the papillary or reticular dermis that often are associated with extravasated erythrocytes or hemosiderin. On the other hand, lymphatic-type AVLs display thin-walled, variably anastomosing, lymphatic vessels lined by attenuated or slightly protuberant endothelial cells. These subtypes have been suggested based on the antigens known to be present in certain tissues, specifically vascular and lymphatic tissue. Despite these seemingly distinct histologies, 6 lesions classified as vascular type displayed some histologic overlap with the lymphatic-type AVLs. The authors concluded that the vascular type showed greater potential to develop into an angiosarcoma based on the degree of endothelial atypia.1
In 2011, Santi et al13 found that both AVLs and angiosarcomas share inactivation mutations in the tumor suppressor gene TP53, providing further evidence to suggest that AVLs may be precursors to angiosarcomas.
Although the malignant potential of AVLs remains questionable, research has shown that they do have a propensity to recur.3 In 2007, Gengler et al3 determined that 20% of patients with AVLs experienced recurrence after a biopsy or excision with varying margins; however, the group stated that these new vascular lesions might not be recurrences but rather entirely new lesions in the same irradiated field (field-effect phenomenon). Several other studies demonstrated that more than 30% of patients with 1 AVL developed more lesions within the same irradiated area.3,14-16 Despite the high rate of recurrence documented in the literature, only 5 of more than 100 diagnosed AVLs have progressed to angiosarcoma.1,3
Many differences can be noted when comparing the histology of AVLs versus angiosarcomas, though some are subtle (Table). Angiosarcomas display poorly circumscribed vascular infiltration into the subcutaneous tissue, multilayering of endothelial cells, prominent nucleoli, hemorrhage, mitoses, and notable aytpia. Atypical vascular lesions lack these features and tend to be wedge shaped and display chronic inflammation.8,15,17-19 Atypical vascular lesions show superficial localized growth without destruction of adjacent adnexa, display dilated vascular spaces, and exhibit large endothelial cells.5,6,8,14,15,19,20 However, there is overlap between AVLs and angiosarcomas that can make diagnosis difficult.2,14,16,17,19 Areas within or just outside of an angiosarcoma, especially in well-differentiated angiosarcomas, can appear histologically identical to AVLs, and multiple biopsies may be required for diagnosis.17,19,21
Conclusion
More research is needed in the arenas of classification, diagnosis, treatment, and follow-up recommendations for AVLs. In particular, more specific histologic markers may be needed to identify those AVLs that may progress to angiosarcomas. Although most AVLs are treated with excision, a consensus needs to be reached on adequate surgical margins. Lastly, due to the tendency of AVLs to recur coupled with their unknown malignant potential, recommendations are needed for consistent follow-up examinations.
- Patton KT, Deyrup AT, Weiss SW. Atypical vascular lesions after surgery and radiation of the breast: a clinicopathologic study of 32 cases analyzing histologic heterogeneity and association with angiosarcoma. Am J Surg Pathol. 2008;32:943-950.
- Brenn T, Fletcher CD. Radiation-associated cutaneous atypical vascular lesions and angiosarcoma: clinicopathologic analysis of 42 cases. Am J Surg Pathol. 2005;29:983-996.
- Gengler C, Coindre JM, Leroux A, et al. Vascular proliferations of the skin after radiation therapy for breast cancer: clinicopathologic analysis of a series in favor of a benign process; a study from the French sarcoma group. Cancer. 2007;109:1584-1598.
- Hoda SA, Cranor ML, Rosen PP. Hemangiomas of the breast with atypical histological features: further analysis of histological subtypes confirming their benign character. Am J Surg Pathol. 1992;16:553-560.
- Wagamon K, Ranchoff RE, Rosenberg AS, et al. Benign lymphangiomatous papules of the skin. J Am Acad Dermatol. 2005;52:912-913.
- Diaz-Cascajo C, Borghi S, Weyers W, et al. Benign lymphangiomatous papules of the skin following radiotherapy: a report of five new cases and review of the literature. Histopathology. 1999;35:319-327.
- Martín-González T, Sanz-Trelles A, Del Boz J, et al. Benign lymphangiomatous papules and plaques after radiotherapy [in Spanish]. Actas Dermosifiliogr. 2008;99:84-86.
- Fineberg S, Rosen PP. Cutaneous angiosarcoma and atypical vascular lesions of the skin and breast after radiation therapy for breast carcinoma. Am J Clin Pathol. 1994;102:757-763.
- Guillou L, Fletcher CD. Benign lymphangioendothelioma (acquired progressive lymphangioma): a lesion not to be confused with well-differentiated angiosarcoma and patch stage Kaposi’s sarcoma: clinicopathologic analysis of a series. Am J Surg Pathol. 2000;24:1047-1057.
- Rosso R, Gianelli U, Carnevali L. Acquired progressive lymphangioma of the skin following radiotherapy for breast carcinoma. J Cutan Pathol. 1995;22:164-167.
- Hildebrandt G, Mittag M, Gutz U, et al. Cutaneous breast angiosarcoma after conservative treatment of breast cancer. Eur J Dermatol. 2001;11:580-583.
- Di Tommaso L, Fabbri A. Cutaneous angiosarcoma arising after radiotherapy treatment of a breast carcinoma: description of a case and review of the literature [in Italian]. Pathologica. 2003;95:196-202.
- Santi R, Cetica V, Franchi A, et al. Tumour suppressor gene TP53 mutations in atypical vascular lesions of breast skin following radiotherapy. Histopathology. 2011;58:455-466.
- Requena L, Kutzner H, Mentzel T, et al. Benign vascular proliferations in irradiated skin. Am J Surg Pathol. 2002;26:328-337.
- Brodie C, Provenzano E. Vascular proliferations of the breast. Histopathology. 2008;52:30-44.
- Brenn T, Fletcher CD. Postradiation vascular proliferations: an increasing problem. Histopathology. 2006;48:106-114.
- Lucas DR. Angiosarcoma, radiation-associated angiosarcoma, and atypical vascular lesion. Arch Pathol Lab Med. 2009;133:1804-1809.
- Kardum-Skelin I, Jelić-Puskarić B, Pazur M, et al. A case report of breast angiosarcoma. Coll Antropol. 2010;34:645-648.
- Mattoch IW, Robbins JB, Kempson RL, et al. Post-radiotherapy vascular proliferations in mammary skin: a clinicopathologic study of 11 cases. J Am Acad Dermatol. 2007;57:126-133.
- Bodet D, Rodríguez-Cano L, Bartralot R, et al. Benign lymphangiomatous papules of the skin associated with ovarian fibroma. J Am Acad Dermatol. 2007;56(2 suppl):S41-S44.
- Losch A, Chilek KD, Zirwas MJ. Post-radiation atypical vascular proliferation mimicking angiosarcoma eight months following breast-conserving therapy for breast carcinoma. J Clin Aesthet Dermatol. 2011;4:47-48.
Atypical vascular lesions (AVLs) of the breast are rare cutaneous vascular proliferations that present as erythematous, violaceous, or flesh-colored papules, patches, or plaques in women who have undergone radiation treatment for breast carcinoma.1,2 These lesions most commonly develop in the irradiated area within 3 to 6 years following radiation treatment.3
Various terms have been used to describe AVLs in the literature, including atypical hemangiomas, benign lymphangiomatous papules, benign lymphangioendotheliomas, lymphangioma circumscriptum, and acquired progressive lymphangiomas, suggesting benign behavior.4-10 However, their identity as benign lesions has been a source of controversy, with some investigators proposing that AVLs may be a precursor lesion to postirradiation angiosarcoma.2 Research has addressed if there are markers that can predict AVL types that are more likely to develop into angiosarcomas.1 Although most clinicians treat AVLs with complete excision, there currently are no specific guidelines to direct this practice.
We report the case of a patient with a history of 1 AVL that was excised who developed 3 additional AVLs in the same breast over the course of 15 months.
Case Report
A 55-year-old woman with a history of obesity, hypertension, and infiltrating ductal carcinoma in situ of the right breast (grade 2, estrogen receptor and progesterone receptor positive) underwent a right breast lumpectomy and sentinel lymph node dissection. Three months later, she underwent re-excision for positive margins and started adjuvant hormonal therapy with tamoxifen. One month later, she began external beam radiation therapy and received a total dose of 6040 cGy over the course of 9 weeks (34 total treatments).
The patient presented to an outside dermatology clinic 2 years after completing external beam radiation therapy for evaluation of a new pink nodule on the right mid breast. The nodule was biopsied and discovered to be an AVL. Pathology showed an anastomosing proliferation of thin-walled vascular channels mainly located in the superficial dermis with notable endothelial nuclear atypia and hyperchromasia. There were several tiny foci with the beginnings of multilayering with prominent endothelial atypia (Figure 1). She underwent complete excision for this AVL with negative margins.

Six months after the initial AVL diagnosis, she presented to our dermatology clinic with another asymptomatic red bump on the right breast. On physical examination, a 4-mm firm, erythematous, well-circumscribed papule was noted on the medial aspect of the right breast along with a similar-appearing 4-mm papule on the right lateral aspect of the right breast (Figure 2). The patient was unsure of the duration of the second lesion but felt that it had been present at least as long as the other lesion. Both lesions clinically resembled typical capillary hemangiomas. A 6-mm punch biopsy of the right medial breast was performed and revealed enlarged vessels and capillaries in the upper dermis lined by endothelial cells with focal prominent nuclei without necrosis, overt atypia, mitosis, or tufting (Figure 3). Immunostaining was positive for CD34, factor VIII antigen, podoplanin (D2-40), and CD31, and negative for cytokeratin 7 and pankeratin. This staining was compatible with a lymphatic-type AVL.1 A diagnosis of AVL was made and complete excision with clear margins was performed. At the time of this excision, a biopsy of the right lateral breast was performed revealing thin-walled, dilated vascular channels in the superficial dermis with architecturally atypical angulated outlines, mild endothelial nuclear atypia, and hyperchromasia without endothelial multilayering. Clear margins were noted on the biopsy, but the patient subsequently declined re-excision of this third AVL.


During a subsequent follow-up visit 9 months later, the patient was noted to have a 2-mm red, vascular-appearing papule on the right upper medial breast (Figure 2). A 6-mm biopsy was performed and revealed thin-walled vascular channels in the superficial dermis with endothelial nuclear atypia consistent with an AVL.
Comment
Fineberg and Rosen8 were the first to describe AVLs in their 1994 study of 4 women with cutaneous vascular proliferations that developed after radiation and chemotherapy for breast cancer. They concluded that these AVLs were benign lesions distinct from angiosarcomas.8 However, further research has challenged the benign nature of AVLs. In 2005, Brenn and Fletcher2 studied 42 women diagnosed with either angiosarcoma or atypical radiation-associated cutaneous vascular lesions. They suggested that AVLs resided on the same spectrum as angiosarcomas and that AVLs may be precursor lesions to angiosarcomas.2 Furthermore, Hildebrandt et al11 in 2001 and Di Tommaso and Fabbri12 in 2003 published case reports of individual patients who developed an angiosarcoma from a preexisting AVL.
The controversy continued when Patton et al1 published a study in 2008 in which 32 cases of AVLs were reviewed. In this study, 2 histologic types of AVLs were described: vascular type and lymphatic type. Vascular-type AVLs are characterized by irregularly dispersed, pericyte-invested, capillary-sized vessels within the papillary or reticular dermis that often are associated with extravasated erythrocytes or hemosiderin. On the other hand, lymphatic-type AVLs display thin-walled, variably anastomosing, lymphatic vessels lined by attenuated or slightly protuberant endothelial cells. These subtypes have been suggested based on the antigens known to be present in certain tissues, specifically vascular and lymphatic tissue. Despite these seemingly distinct histologies, 6 lesions classified as vascular type displayed some histologic overlap with the lymphatic-type AVLs. The authors concluded that the vascular type showed greater potential to develop into an angiosarcoma based on the degree of endothelial atypia.1
In 2011, Santi et al13 found that both AVLs and angiosarcomas share inactivation mutations in the tumor suppressor gene TP53, providing further evidence to suggest that AVLs may be precursors to angiosarcomas.
Although the malignant potential of AVLs remains questionable, research has shown that they do have a propensity to recur.3 In 2007, Gengler et al3 determined that 20% of patients with AVLs experienced recurrence after a biopsy or excision with varying margins; however, the group stated that these new vascular lesions might not be recurrences but rather entirely new lesions in the same irradiated field (field-effect phenomenon). Several other studies demonstrated that more than 30% of patients with 1 AVL developed more lesions within the same irradiated area.3,14-16 Despite the high rate of recurrence documented in the literature, only 5 of more than 100 diagnosed AVLs have progressed to angiosarcoma.1,3
Many differences can be noted when comparing the histology of AVLs versus angiosarcomas, though some are subtle (Table). Angiosarcomas display poorly circumscribed vascular infiltration into the subcutaneous tissue, multilayering of endothelial cells, prominent nucleoli, hemorrhage, mitoses, and notable aytpia. Atypical vascular lesions lack these features and tend to be wedge shaped and display chronic inflammation.8,15,17-19 Atypical vascular lesions show superficial localized growth without destruction of adjacent adnexa, display dilated vascular spaces, and exhibit large endothelial cells.5,6,8,14,15,19,20 However, there is overlap between AVLs and angiosarcomas that can make diagnosis difficult.2,14,16,17,19 Areas within or just outside of an angiosarcoma, especially in well-differentiated angiosarcomas, can appear histologically identical to AVLs, and multiple biopsies may be required for diagnosis.17,19,21
Conclusion
More research is needed in the arenas of classification, diagnosis, treatment, and follow-up recommendations for AVLs. In particular, more specific histologic markers may be needed to identify those AVLs that may progress to angiosarcomas. Although most AVLs are treated with excision, a consensus needs to be reached on adequate surgical margins. Lastly, due to the tendency of AVLs to recur coupled with their unknown malignant potential, recommendations are needed for consistent follow-up examinations.
Atypical vascular lesions (AVLs) of the breast are rare cutaneous vascular proliferations that present as erythematous, violaceous, or flesh-colored papules, patches, or plaques in women who have undergone radiation treatment for breast carcinoma.1,2 These lesions most commonly develop in the irradiated area within 3 to 6 years following radiation treatment.3
Various terms have been used to describe AVLs in the literature, including atypical hemangiomas, benign lymphangiomatous papules, benign lymphangioendotheliomas, lymphangioma circumscriptum, and acquired progressive lymphangiomas, suggesting benign behavior.4-10 However, their identity as benign lesions has been a source of controversy, with some investigators proposing that AVLs may be a precursor lesion to postirradiation angiosarcoma.2 Research has addressed if there are markers that can predict AVL types that are more likely to develop into angiosarcomas.1 Although most clinicians treat AVLs with complete excision, there currently are no specific guidelines to direct this practice.
We report the case of a patient with a history of 1 AVL that was excised who developed 3 additional AVLs in the same breast over the course of 15 months.
Case Report
A 55-year-old woman with a history of obesity, hypertension, and infiltrating ductal carcinoma in situ of the right breast (grade 2, estrogen receptor and progesterone receptor positive) underwent a right breast lumpectomy and sentinel lymph node dissection. Three months later, she underwent re-excision for positive margins and started adjuvant hormonal therapy with tamoxifen. One month later, she began external beam radiation therapy and received a total dose of 6040 cGy over the course of 9 weeks (34 total treatments).
The patient presented to an outside dermatology clinic 2 years after completing external beam radiation therapy for evaluation of a new pink nodule on the right mid breast. The nodule was biopsied and discovered to be an AVL. Pathology showed an anastomosing proliferation of thin-walled vascular channels mainly located in the superficial dermis with notable endothelial nuclear atypia and hyperchromasia. There were several tiny foci with the beginnings of multilayering with prominent endothelial atypia (Figure 1). She underwent complete excision for this AVL with negative margins.

Six months after the initial AVL diagnosis, she presented to our dermatology clinic with another asymptomatic red bump on the right breast. On physical examination, a 4-mm firm, erythematous, well-circumscribed papule was noted on the medial aspect of the right breast along with a similar-appearing 4-mm papule on the right lateral aspect of the right breast (Figure 2). The patient was unsure of the duration of the second lesion but felt that it had been present at least as long as the other lesion. Both lesions clinically resembled typical capillary hemangiomas. A 6-mm punch biopsy of the right medial breast was performed and revealed enlarged vessels and capillaries in the upper dermis lined by endothelial cells with focal prominent nuclei without necrosis, overt atypia, mitosis, or tufting (Figure 3). Immunostaining was positive for CD34, factor VIII antigen, podoplanin (D2-40), and CD31, and negative for cytokeratin 7 and pankeratin. This staining was compatible with a lymphatic-type AVL.1 A diagnosis of AVL was made and complete excision with clear margins was performed. At the time of this excision, a biopsy of the right lateral breast was performed revealing thin-walled, dilated vascular channels in the superficial dermis with architecturally atypical angulated outlines, mild endothelial nuclear atypia, and hyperchromasia without endothelial multilayering. Clear margins were noted on the biopsy, but the patient subsequently declined re-excision of this third AVL.


During a subsequent follow-up visit 9 months later, the patient was noted to have a 2-mm red, vascular-appearing papule on the right upper medial breast (Figure 2). A 6-mm biopsy was performed and revealed thin-walled vascular channels in the superficial dermis with endothelial nuclear atypia consistent with an AVL.
Comment
Fineberg and Rosen8 were the first to describe AVLs in their 1994 study of 4 women with cutaneous vascular proliferations that developed after radiation and chemotherapy for breast cancer. They concluded that these AVLs were benign lesions distinct from angiosarcomas.8 However, further research has challenged the benign nature of AVLs. In 2005, Brenn and Fletcher2 studied 42 women diagnosed with either angiosarcoma or atypical radiation-associated cutaneous vascular lesions. They suggested that AVLs resided on the same spectrum as angiosarcomas and that AVLs may be precursor lesions to angiosarcomas.2 Furthermore, Hildebrandt et al11 in 2001 and Di Tommaso and Fabbri12 in 2003 published case reports of individual patients who developed an angiosarcoma from a preexisting AVL.
The controversy continued when Patton et al1 published a study in 2008 in which 32 cases of AVLs were reviewed. In this study, 2 histologic types of AVLs were described: vascular type and lymphatic type. Vascular-type AVLs are characterized by irregularly dispersed, pericyte-invested, capillary-sized vessels within the papillary or reticular dermis that often are associated with extravasated erythrocytes or hemosiderin. On the other hand, lymphatic-type AVLs display thin-walled, variably anastomosing, lymphatic vessels lined by attenuated or slightly protuberant endothelial cells. These subtypes have been suggested based on the antigens known to be present in certain tissues, specifically vascular and lymphatic tissue. Despite these seemingly distinct histologies, 6 lesions classified as vascular type displayed some histologic overlap with the lymphatic-type AVLs. The authors concluded that the vascular type showed greater potential to develop into an angiosarcoma based on the degree of endothelial atypia.1
In 2011, Santi et al13 found that both AVLs and angiosarcomas share inactivation mutations in the tumor suppressor gene TP53, providing further evidence to suggest that AVLs may be precursors to angiosarcomas.
Although the malignant potential of AVLs remains questionable, research has shown that they do have a propensity to recur.3 In 2007, Gengler et al3 determined that 20% of patients with AVLs experienced recurrence after a biopsy or excision with varying margins; however, the group stated that these new vascular lesions might not be recurrences but rather entirely new lesions in the same irradiated field (field-effect phenomenon). Several other studies demonstrated that more than 30% of patients with 1 AVL developed more lesions within the same irradiated area.3,14-16 Despite the high rate of recurrence documented in the literature, only 5 of more than 100 diagnosed AVLs have progressed to angiosarcoma.1,3
Many differences can be noted when comparing the histology of AVLs versus angiosarcomas, though some are subtle (Table). Angiosarcomas display poorly circumscribed vascular infiltration into the subcutaneous tissue, multilayering of endothelial cells, prominent nucleoli, hemorrhage, mitoses, and notable aytpia. Atypical vascular lesions lack these features and tend to be wedge shaped and display chronic inflammation.8,15,17-19 Atypical vascular lesions show superficial localized growth without destruction of adjacent adnexa, display dilated vascular spaces, and exhibit large endothelial cells.5,6,8,14,15,19,20 However, there is overlap between AVLs and angiosarcomas that can make diagnosis difficult.2,14,16,17,19 Areas within or just outside of an angiosarcoma, especially in well-differentiated angiosarcomas, can appear histologically identical to AVLs, and multiple biopsies may be required for diagnosis.17,19,21
Conclusion
More research is needed in the arenas of classification, diagnosis, treatment, and follow-up recommendations for AVLs. In particular, more specific histologic markers may be needed to identify those AVLs that may progress to angiosarcomas. Although most AVLs are treated with excision, a consensus needs to be reached on adequate surgical margins. Lastly, due to the tendency of AVLs to recur coupled with their unknown malignant potential, recommendations are needed for consistent follow-up examinations.
- Patton KT, Deyrup AT, Weiss SW. Atypical vascular lesions after surgery and radiation of the breast: a clinicopathologic study of 32 cases analyzing histologic heterogeneity and association with angiosarcoma. Am J Surg Pathol. 2008;32:943-950.
- Brenn T, Fletcher CD. Radiation-associated cutaneous atypical vascular lesions and angiosarcoma: clinicopathologic analysis of 42 cases. Am J Surg Pathol. 2005;29:983-996.
- Gengler C, Coindre JM, Leroux A, et al. Vascular proliferations of the skin after radiation therapy for breast cancer: clinicopathologic analysis of a series in favor of a benign process; a study from the French sarcoma group. Cancer. 2007;109:1584-1598.
- Hoda SA, Cranor ML, Rosen PP. Hemangiomas of the breast with atypical histological features: further analysis of histological subtypes confirming their benign character. Am J Surg Pathol. 1992;16:553-560.
- Wagamon K, Ranchoff RE, Rosenberg AS, et al. Benign lymphangiomatous papules of the skin. J Am Acad Dermatol. 2005;52:912-913.
- Diaz-Cascajo C, Borghi S, Weyers W, et al. Benign lymphangiomatous papules of the skin following radiotherapy: a report of five new cases and review of the literature. Histopathology. 1999;35:319-327.
- Martín-González T, Sanz-Trelles A, Del Boz J, et al. Benign lymphangiomatous papules and plaques after radiotherapy [in Spanish]. Actas Dermosifiliogr. 2008;99:84-86.
- Fineberg S, Rosen PP. Cutaneous angiosarcoma and atypical vascular lesions of the skin and breast after radiation therapy for breast carcinoma. Am J Clin Pathol. 1994;102:757-763.
- Guillou L, Fletcher CD. Benign lymphangioendothelioma (acquired progressive lymphangioma): a lesion not to be confused with well-differentiated angiosarcoma and patch stage Kaposi’s sarcoma: clinicopathologic analysis of a series. Am J Surg Pathol. 2000;24:1047-1057.
- Rosso R, Gianelli U, Carnevali L. Acquired progressive lymphangioma of the skin following radiotherapy for breast carcinoma. J Cutan Pathol. 1995;22:164-167.
- Hildebrandt G, Mittag M, Gutz U, et al. Cutaneous breast angiosarcoma after conservative treatment of breast cancer. Eur J Dermatol. 2001;11:580-583.
- Di Tommaso L, Fabbri A. Cutaneous angiosarcoma arising after radiotherapy treatment of a breast carcinoma: description of a case and review of the literature [in Italian]. Pathologica. 2003;95:196-202.
- Santi R, Cetica V, Franchi A, et al. Tumour suppressor gene TP53 mutations in atypical vascular lesions of breast skin following radiotherapy. Histopathology. 2011;58:455-466.
- Requena L, Kutzner H, Mentzel T, et al. Benign vascular proliferations in irradiated skin. Am J Surg Pathol. 2002;26:328-337.
- Brodie C, Provenzano E. Vascular proliferations of the breast. Histopathology. 2008;52:30-44.
- Brenn T, Fletcher CD. Postradiation vascular proliferations: an increasing problem. Histopathology. 2006;48:106-114.
- Lucas DR. Angiosarcoma, radiation-associated angiosarcoma, and atypical vascular lesion. Arch Pathol Lab Med. 2009;133:1804-1809.
- Kardum-Skelin I, Jelić-Puskarić B, Pazur M, et al. A case report of breast angiosarcoma. Coll Antropol. 2010;34:645-648.
- Mattoch IW, Robbins JB, Kempson RL, et al. Post-radiotherapy vascular proliferations in mammary skin: a clinicopathologic study of 11 cases. J Am Acad Dermatol. 2007;57:126-133.
- Bodet D, Rodríguez-Cano L, Bartralot R, et al. Benign lymphangiomatous papules of the skin associated with ovarian fibroma. J Am Acad Dermatol. 2007;56(2 suppl):S41-S44.
- Losch A, Chilek KD, Zirwas MJ. Post-radiation atypical vascular proliferation mimicking angiosarcoma eight months following breast-conserving therapy for breast carcinoma. J Clin Aesthet Dermatol. 2011;4:47-48.
- Patton KT, Deyrup AT, Weiss SW. Atypical vascular lesions after surgery and radiation of the breast: a clinicopathologic study of 32 cases analyzing histologic heterogeneity and association with angiosarcoma. Am J Surg Pathol. 2008;32:943-950.
- Brenn T, Fletcher CD. Radiation-associated cutaneous atypical vascular lesions and angiosarcoma: clinicopathologic analysis of 42 cases. Am J Surg Pathol. 2005;29:983-996.
- Gengler C, Coindre JM, Leroux A, et al. Vascular proliferations of the skin after radiation therapy for breast cancer: clinicopathologic analysis of a series in favor of a benign process; a study from the French sarcoma group. Cancer. 2007;109:1584-1598.
- Hoda SA, Cranor ML, Rosen PP. Hemangiomas of the breast with atypical histological features: further analysis of histological subtypes confirming their benign character. Am J Surg Pathol. 1992;16:553-560.
- Wagamon K, Ranchoff RE, Rosenberg AS, et al. Benign lymphangiomatous papules of the skin. J Am Acad Dermatol. 2005;52:912-913.
- Diaz-Cascajo C, Borghi S, Weyers W, et al. Benign lymphangiomatous papules of the skin following radiotherapy: a report of five new cases and review of the literature. Histopathology. 1999;35:319-327.
- Martín-González T, Sanz-Trelles A, Del Boz J, et al. Benign lymphangiomatous papules and plaques after radiotherapy [in Spanish]. Actas Dermosifiliogr. 2008;99:84-86.
- Fineberg S, Rosen PP. Cutaneous angiosarcoma and atypical vascular lesions of the skin and breast after radiation therapy for breast carcinoma. Am J Clin Pathol. 1994;102:757-763.
- Guillou L, Fletcher CD. Benign lymphangioendothelioma (acquired progressive lymphangioma): a lesion not to be confused with well-differentiated angiosarcoma and patch stage Kaposi’s sarcoma: clinicopathologic analysis of a series. Am J Surg Pathol. 2000;24:1047-1057.
- Rosso R, Gianelli U, Carnevali L. Acquired progressive lymphangioma of the skin following radiotherapy for breast carcinoma. J Cutan Pathol. 1995;22:164-167.
- Hildebrandt G, Mittag M, Gutz U, et al. Cutaneous breast angiosarcoma after conservative treatment of breast cancer. Eur J Dermatol. 2001;11:580-583.
- Di Tommaso L, Fabbri A. Cutaneous angiosarcoma arising after radiotherapy treatment of a breast carcinoma: description of a case and review of the literature [in Italian]. Pathologica. 2003;95:196-202.
- Santi R, Cetica V, Franchi A, et al. Tumour suppressor gene TP53 mutations in atypical vascular lesions of breast skin following radiotherapy. Histopathology. 2011;58:455-466.
- Requena L, Kutzner H, Mentzel T, et al. Benign vascular proliferations in irradiated skin. Am J Surg Pathol. 2002;26:328-337.
- Brodie C, Provenzano E. Vascular proliferations of the breast. Histopathology. 2008;52:30-44.
- Brenn T, Fletcher CD. Postradiation vascular proliferations: an increasing problem. Histopathology. 2006;48:106-114.
- Lucas DR. Angiosarcoma, radiation-associated angiosarcoma, and atypical vascular lesion. Arch Pathol Lab Med. 2009;133:1804-1809.
- Kardum-Skelin I, Jelić-Puskarić B, Pazur M, et al. A case report of breast angiosarcoma. Coll Antropol. 2010;34:645-648.
- Mattoch IW, Robbins JB, Kempson RL, et al. Post-radiotherapy vascular proliferations in mammary skin: a clinicopathologic study of 11 cases. J Am Acad Dermatol. 2007;57:126-133.
- Bodet D, Rodríguez-Cano L, Bartralot R, et al. Benign lymphangiomatous papules of the skin associated with ovarian fibroma. J Am Acad Dermatol. 2007;56(2 suppl):S41-S44.
- Losch A, Chilek KD, Zirwas MJ. Post-radiation atypical vascular proliferation mimicking angiosarcoma eight months following breast-conserving therapy for breast carcinoma. J Clin Aesthet Dermatol. 2011;4:47-48.
Practice Points
- Atypical vascular lesions (AVLs) of the breast can appear an average of 5 years following radiation therapy.
- Although the malignant potential of AVLs remains debatable, excision generally is recommended, as lesions tend to recur.