User login
American College of Rheumatology (ACR): Annual Scientific Meeting
ACR: Study challenges protein citrullination as a central cause of RA
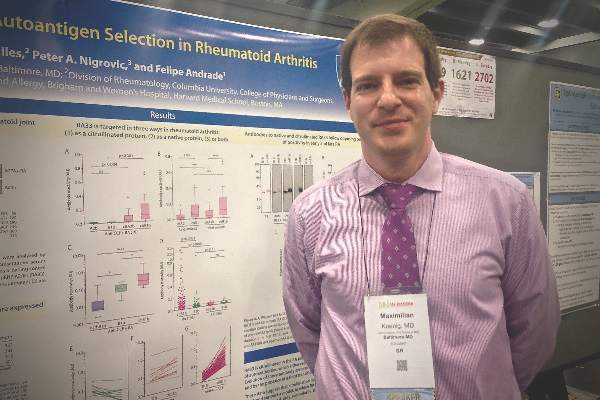
SAN FRANCISCO – Autoantibodies in patients with rheumatoid arthritis target both the native and citrullinated forms of the RA33 autoantigen, challenging the idea that protein citrullination underlies loss of tolerance in this disease, Dr. Maximilian Konig said at the annual meeting of the American College of Rheumatology.
“I think the important thing is that this makes us rethink how RA actually starts,” Dr. Konig said in an interview. “We identified three different antibody groups that clinically behave very differently. We identified a group of patients that has cross-reactive antibodies against RA33, and they seem to be the ones with the highest and the most rapid disease progression. Maybe identifying these patients early and targeting them more would help bring a subset of patients with the most advanced and aggressive disease under better control.”
Signs and symptoms are often inconclusive early in the course of RA, leading to the search for reliable biomarkers that can hasten diagnosis and treatment. Anti-citrullinated protein antibodies (ACPAs) are one hallmark of RA, and protein citrullination has been seen as central to autoimmunity in its pathogenesis, said Dr. Konig, a postdoctoral fellow in rheumatology at Johns Hopkins University, Baltimore. But patients also have autoantibodies against native or unmodified proteins, including calpastatin, Fc-gamma, peptidylarginine deiminase type 4, and heterogeneous nuclear ribonucleoprotein A2/B1 (also known as RA33), which has been correlated with clinical disease activity, radiographic evidence of bone resorption, C-reactive protein levels, and erythrocyte sedimentation rate in a few previous studies (Pediatr Int. 2009 Apr;51[2]:188-92 and J Immunol Res. 2014;2014:516593).
Dr. Konig and his coinvestigators contended that current models of RA do not adequately account for autoimmunity against native proteins. To explore that idea, they tested sera from 196 patients from the ESCAPE RA (Evaluation of Subclinical Cardiovascular Disease and Predictors of Events in Rheumatoid Arthritis) cohort study and from 56 healthy controls. They used quantitative ELISA to identify autoantibodies, and performed immunoblotting and immunoprecipitation to test antibody specificity. They also used immunoblotting and mass spectrometry to study synovial fluid from the patients.
The assays identified citrullinated RA33 in the joints of RA patients, and revealed distinct autoantibodies that targeted native and citrullinated RA33. Furthermore, this single antibody system seemed to change with disease duration, Dr. Konig said. Autoantibody against native RA33 was almost exclusively found in samples from patients in early-stage disease, whereas patients with long-established RA had much higher levels of anti-citrullinated RA33 antibodies. The switch from a predominance of anti-RA33 to anti-citrullinated RA33 autoantibodies seemed to happen about a decade into the disease course, Dr. Konig added. “These data suggest that citrullination may not be required to break tolerance to RA33, and support a model in which RA autoantigens are initially targeted as a native protein, and only later become targets of a citrulline-specific response,” he said.
Notably, immunoprecipitation, immunoblotting, and competitive assays all confirmed a third type of autoantibody that cross-reacted against both RA33 and citrullinated RA33. The “RA33 [protein] is targeted by patient sera in three ways: only as a native protein, both as a native and as a citrullinated protein, and only as a citrullinated protein,” Dr. Konig concluded. He and his associates are exploring the clinical implications of the finding, particularly because patients with the cross-reactive anti-RA33 autoantibodies seem to have the most rapidly progressive and severe disease, he added. The presence of these antibodies might one day help identify a patient who needs especially aggressive monitoring and treatment, he concluded.
Dr. Konig had no disclosures.*
*This story was updated 12/3/2015.
SAN FRANCISCO – Autoantibodies in patients with rheumatoid arthritis target both the native and citrullinated forms of the RA33 autoantigen, challenging the idea that protein citrullination underlies loss of tolerance in this disease, Dr. Maximilian Konig said at the annual meeting of the American College of Rheumatology.
“I think the important thing is that this makes us rethink how RA actually starts,” Dr. Konig said in an interview. “We identified three different antibody groups that clinically behave very differently. We identified a group of patients that has cross-reactive antibodies against RA33, and they seem to be the ones with the highest and the most rapid disease progression. Maybe identifying these patients early and targeting them more would help bring a subset of patients with the most advanced and aggressive disease under better control.”
Signs and symptoms are often inconclusive early in the course of RA, leading to the search for reliable biomarkers that can hasten diagnosis and treatment. Anti-citrullinated protein antibodies (ACPAs) are one hallmark of RA, and protein citrullination has been seen as central to autoimmunity in its pathogenesis, said Dr. Konig, a postdoctoral fellow in rheumatology at Johns Hopkins University, Baltimore. But patients also have autoantibodies against native or unmodified proteins, including calpastatin, Fc-gamma, peptidylarginine deiminase type 4, and heterogeneous nuclear ribonucleoprotein A2/B1 (also known as RA33), which has been correlated with clinical disease activity, radiographic evidence of bone resorption, C-reactive protein levels, and erythrocyte sedimentation rate in a few previous studies (Pediatr Int. 2009 Apr;51[2]:188-92 and J Immunol Res. 2014;2014:516593).
Dr. Konig and his coinvestigators contended that current models of RA do not adequately account for autoimmunity against native proteins. To explore that idea, they tested sera from 196 patients from the ESCAPE RA (Evaluation of Subclinical Cardiovascular Disease and Predictors of Events in Rheumatoid Arthritis) cohort study and from 56 healthy controls. They used quantitative ELISA to identify autoantibodies, and performed immunoblotting and immunoprecipitation to test antibody specificity. They also used immunoblotting and mass spectrometry to study synovial fluid from the patients.
The assays identified citrullinated RA33 in the joints of RA patients, and revealed distinct autoantibodies that targeted native and citrullinated RA33. Furthermore, this single antibody system seemed to change with disease duration, Dr. Konig said. Autoantibody against native RA33 was almost exclusively found in samples from patients in early-stage disease, whereas patients with long-established RA had much higher levels of anti-citrullinated RA33 antibodies. The switch from a predominance of anti-RA33 to anti-citrullinated RA33 autoantibodies seemed to happen about a decade into the disease course, Dr. Konig added. “These data suggest that citrullination may not be required to break tolerance to RA33, and support a model in which RA autoantigens are initially targeted as a native protein, and only later become targets of a citrulline-specific response,” he said.
Notably, immunoprecipitation, immunoblotting, and competitive assays all confirmed a third type of autoantibody that cross-reacted against both RA33 and citrullinated RA33. The “RA33 [protein] is targeted by patient sera in three ways: only as a native protein, both as a native and as a citrullinated protein, and only as a citrullinated protein,” Dr. Konig concluded. He and his associates are exploring the clinical implications of the finding, particularly because patients with the cross-reactive anti-RA33 autoantibodies seem to have the most rapidly progressive and severe disease, he added. The presence of these antibodies might one day help identify a patient who needs especially aggressive monitoring and treatment, he concluded.
Dr. Konig had no disclosures.*
*This story was updated 12/3/2015.
SAN FRANCISCO – Autoantibodies in patients with rheumatoid arthritis target both the native and citrullinated forms of the RA33 autoantigen, challenging the idea that protein citrullination underlies loss of tolerance in this disease, Dr. Maximilian Konig said at the annual meeting of the American College of Rheumatology.
“I think the important thing is that this makes us rethink how RA actually starts,” Dr. Konig said in an interview. “We identified three different antibody groups that clinically behave very differently. We identified a group of patients that has cross-reactive antibodies against RA33, and they seem to be the ones with the highest and the most rapid disease progression. Maybe identifying these patients early and targeting them more would help bring a subset of patients with the most advanced and aggressive disease under better control.”
Signs and symptoms are often inconclusive early in the course of RA, leading to the search for reliable biomarkers that can hasten diagnosis and treatment. Anti-citrullinated protein antibodies (ACPAs) are one hallmark of RA, and protein citrullination has been seen as central to autoimmunity in its pathogenesis, said Dr. Konig, a postdoctoral fellow in rheumatology at Johns Hopkins University, Baltimore. But patients also have autoantibodies against native or unmodified proteins, including calpastatin, Fc-gamma, peptidylarginine deiminase type 4, and heterogeneous nuclear ribonucleoprotein A2/B1 (also known as RA33), which has been correlated with clinical disease activity, radiographic evidence of bone resorption, C-reactive protein levels, and erythrocyte sedimentation rate in a few previous studies (Pediatr Int. 2009 Apr;51[2]:188-92 and J Immunol Res. 2014;2014:516593).
Dr. Konig and his coinvestigators contended that current models of RA do not adequately account for autoimmunity against native proteins. To explore that idea, they tested sera from 196 patients from the ESCAPE RA (Evaluation of Subclinical Cardiovascular Disease and Predictors of Events in Rheumatoid Arthritis) cohort study and from 56 healthy controls. They used quantitative ELISA to identify autoantibodies, and performed immunoblotting and immunoprecipitation to test antibody specificity. They also used immunoblotting and mass spectrometry to study synovial fluid from the patients.
The assays identified citrullinated RA33 in the joints of RA patients, and revealed distinct autoantibodies that targeted native and citrullinated RA33. Furthermore, this single antibody system seemed to change with disease duration, Dr. Konig said. Autoantibody against native RA33 was almost exclusively found in samples from patients in early-stage disease, whereas patients with long-established RA had much higher levels of anti-citrullinated RA33 antibodies. The switch from a predominance of anti-RA33 to anti-citrullinated RA33 autoantibodies seemed to happen about a decade into the disease course, Dr. Konig added. “These data suggest that citrullination may not be required to break tolerance to RA33, and support a model in which RA autoantigens are initially targeted as a native protein, and only later become targets of a citrulline-specific response,” he said.
Notably, immunoprecipitation, immunoblotting, and competitive assays all confirmed a third type of autoantibody that cross-reacted against both RA33 and citrullinated RA33. The “RA33 [protein] is targeted by patient sera in three ways: only as a native protein, both as a native and as a citrullinated protein, and only as a citrullinated protein,” Dr. Konig concluded. He and his associates are exploring the clinical implications of the finding, particularly because patients with the cross-reactive anti-RA33 autoantibodies seem to have the most rapidly progressive and severe disease, he added. The presence of these antibodies might one day help identify a patient who needs especially aggressive monitoring and treatment, he concluded.
Dr. Konig had no disclosures.*
*This story was updated 12/3/2015.
AT THE ACR ANNUAL MEETING
Key clinical point: Patterns of antibody activity against the RA33 autoantigen challenge the idea that citrullination is central to the pathology of rheumatoid arthritis.
Major finding: Laboratory analyses revealed three distinct groups of autoantibodies targeting native RA33, citrullinated RA33, or both forms of the autoantigen.
Data source: Assays of serum and synovial fluid samples from 56 controls and 196 participants from the ESCAPE RA (Evaluation of Subclinical Cardiovascular Disease and Predictors of Events in Rheumatoid Arthritis) cohort study.
Disclosures: Dr. Konig had no disclosures.*
ACR: Cardiovascular risk factors in psoriatic diseases are common, often go untreated
SAN FRANCISCO – Despite their frequent contact with the health care system, patients with psoriasis and psoriatic arthritis often receive no treatment for major cardiovascular risk factors, according to two large multicenter studies.
“We identified a gap in quality of care in terms of the primary prevention of cardiovascular risk factors in psoriatic arthritis and psoriasis. The next step will be to develop strategies to increase awareness and implement treatment recommendations among primary care physicians, dermatologists, and rheumatologists,” Dr. Lihi Eder of the University of Toronto said in an interview at the annual meeting of the American College of Rheumatology.
Psoriatic and cardiovascular diseases share an inflammatory etiology and often co-occur. In past studies, patients with psoriasis and psoriatic arthritis were about 50% more likely than average to have dyslipidemia and ischemic heart disease, and about 80%-90% more likely than usual to have hypertension and diabetes, Dr. Eder said.
She and her associates studied dyslipidemia and hypertension among 1,327 patients with psoriatic arthritis and 927 patients with psoriasis at eight sites in Canada, the United States, and Israel as part of the International Psoriasis & Arthritis Research Team (IPART). Based on medical and laboratory reports and self-reported data, the investigators assessed these comorbidities and whether treatment adhered to cholesterol and hypertension guidelines from the American College of Cardiology and the American Heart Association (Circulation. 2014 Jul 1;129:S1-45), and the Eighth Joint National Committee (JAMA. 2014 Feb 5;311[5]:507-20), respectively.
More than 80% of patients in the cohort had at least one modifiable cardiovascular risk factor, Dr. Eder said. While 6% had ischemic heart disease, 45% had hypertension, 71% had dyslipidemia, 13% had diabetes, 54% had central obesity, and 17% were current smokers. Furthermore, close to half of patients who had been diagnosed with hypertension had uncontrolled high blood pressure, and 57% were not receiving antihypertensive medications. Likewise, 58% of patients with dyslipidemia met criteria for statins, but only a third of these patients were receiving them.
Undertreatment was associated with having psoriatic arthritis or severe psoriasis and with having a high school or lower level of education, Dr. Eder added. “You have to remember that this study was conducted among specialists – these are supposed to be experts in the field,” she said. “If the treatment adherence is relatively low in these centers, then I would expect that for patients who are being followed in centers that do not specialize in psoriatic disease, adherence would be even lower.”
The second study detected significantly higher rates of cardiovascular risk factors among patients with psoriatic diseases, compared with controls from the Health Improvement Network, a medical records database that covers more than 9 million individuals in the United Kingdom. Patients with psoriatic arthritis or severe psoriasis were significantly more likely than were controls to develop hypertension, hyperlipidemia, obesity, or diabetes, with odds ratios ranging from 1.22 to 1.78, reported Dr. Kashif A. Jafri, who led the study while he was an internal medicine resident at the University of Pennsylvania in Philadelphia.
But despite their disproportionate risk, patients were treated at about the same rate as controls, Dr. Jafri said. About 15% of individuals with hypertension received no treatment, 30%-40% with hyperlipidemia went untreated, and nearly 60% with diabetes received no documented therapy. “The absence of a significant difference in receipt of appropriate therapy among the groups reflects a need for more careful attention to the management of cardiovascular risk factors in patients with inflammatory diseases,” Dr. Jafri emphasized. Because these risk factors can be successfully treated, it is “critical” to educate primary care providers about the need to do so, he said.
Rheumatologists also should periodically discuss cardiovascular risk factors with their patients as part of routine care, Dr. Jafri advised. “Although there are obviously time constraints during each office visit, this is a topic that dramatically influences the morbidity and mortality of our patient population, and rheumatologists have the unique ability to address this issue in the context of their long-term relationships with their patients,” he said.
Dr. Jafri is now a fellow in rheumatology at the University of California, San Francisco. His was supported by an Ephraim P. Engleman Endowed Resident Research Preceptorship Award from the Rheumatology Research Foundation. IPART is sponsored by the Krembil Foundation and the Canadian Institutes of Health Research. Dr. Jafri and Dr. Eder had no disclosures.
SAN FRANCISCO – Despite their frequent contact with the health care system, patients with psoriasis and psoriatic arthritis often receive no treatment for major cardiovascular risk factors, according to two large multicenter studies.
“We identified a gap in quality of care in terms of the primary prevention of cardiovascular risk factors in psoriatic arthritis and psoriasis. The next step will be to develop strategies to increase awareness and implement treatment recommendations among primary care physicians, dermatologists, and rheumatologists,” Dr. Lihi Eder of the University of Toronto said in an interview at the annual meeting of the American College of Rheumatology.
Psoriatic and cardiovascular diseases share an inflammatory etiology and often co-occur. In past studies, patients with psoriasis and psoriatic arthritis were about 50% more likely than average to have dyslipidemia and ischemic heart disease, and about 80%-90% more likely than usual to have hypertension and diabetes, Dr. Eder said.
She and her associates studied dyslipidemia and hypertension among 1,327 patients with psoriatic arthritis and 927 patients with psoriasis at eight sites in Canada, the United States, and Israel as part of the International Psoriasis & Arthritis Research Team (IPART). Based on medical and laboratory reports and self-reported data, the investigators assessed these comorbidities and whether treatment adhered to cholesterol and hypertension guidelines from the American College of Cardiology and the American Heart Association (Circulation. 2014 Jul 1;129:S1-45), and the Eighth Joint National Committee (JAMA. 2014 Feb 5;311[5]:507-20), respectively.
More than 80% of patients in the cohort had at least one modifiable cardiovascular risk factor, Dr. Eder said. While 6% had ischemic heart disease, 45% had hypertension, 71% had dyslipidemia, 13% had diabetes, 54% had central obesity, and 17% were current smokers. Furthermore, close to half of patients who had been diagnosed with hypertension had uncontrolled high blood pressure, and 57% were not receiving antihypertensive medications. Likewise, 58% of patients with dyslipidemia met criteria for statins, but only a third of these patients were receiving them.
Undertreatment was associated with having psoriatic arthritis or severe psoriasis and with having a high school or lower level of education, Dr. Eder added. “You have to remember that this study was conducted among specialists – these are supposed to be experts in the field,” she said. “If the treatment adherence is relatively low in these centers, then I would expect that for patients who are being followed in centers that do not specialize in psoriatic disease, adherence would be even lower.”
The second study detected significantly higher rates of cardiovascular risk factors among patients with psoriatic diseases, compared with controls from the Health Improvement Network, a medical records database that covers more than 9 million individuals in the United Kingdom. Patients with psoriatic arthritis or severe psoriasis were significantly more likely than were controls to develop hypertension, hyperlipidemia, obesity, or diabetes, with odds ratios ranging from 1.22 to 1.78, reported Dr. Kashif A. Jafri, who led the study while he was an internal medicine resident at the University of Pennsylvania in Philadelphia.
But despite their disproportionate risk, patients were treated at about the same rate as controls, Dr. Jafri said. About 15% of individuals with hypertension received no treatment, 30%-40% with hyperlipidemia went untreated, and nearly 60% with diabetes received no documented therapy. “The absence of a significant difference in receipt of appropriate therapy among the groups reflects a need for more careful attention to the management of cardiovascular risk factors in patients with inflammatory diseases,” Dr. Jafri emphasized. Because these risk factors can be successfully treated, it is “critical” to educate primary care providers about the need to do so, he said.
Rheumatologists also should periodically discuss cardiovascular risk factors with their patients as part of routine care, Dr. Jafri advised. “Although there are obviously time constraints during each office visit, this is a topic that dramatically influences the morbidity and mortality of our patient population, and rheumatologists have the unique ability to address this issue in the context of their long-term relationships with their patients,” he said.
Dr. Jafri is now a fellow in rheumatology at the University of California, San Francisco. His was supported by an Ephraim P. Engleman Endowed Resident Research Preceptorship Award from the Rheumatology Research Foundation. IPART is sponsored by the Krembil Foundation and the Canadian Institutes of Health Research. Dr. Jafri and Dr. Eder had no disclosures.
SAN FRANCISCO – Despite their frequent contact with the health care system, patients with psoriasis and psoriatic arthritis often receive no treatment for major cardiovascular risk factors, according to two large multicenter studies.
“We identified a gap in quality of care in terms of the primary prevention of cardiovascular risk factors in psoriatic arthritis and psoriasis. The next step will be to develop strategies to increase awareness and implement treatment recommendations among primary care physicians, dermatologists, and rheumatologists,” Dr. Lihi Eder of the University of Toronto said in an interview at the annual meeting of the American College of Rheumatology.
Psoriatic and cardiovascular diseases share an inflammatory etiology and often co-occur. In past studies, patients with psoriasis and psoriatic arthritis were about 50% more likely than average to have dyslipidemia and ischemic heart disease, and about 80%-90% more likely than usual to have hypertension and diabetes, Dr. Eder said.
She and her associates studied dyslipidemia and hypertension among 1,327 patients with psoriatic arthritis and 927 patients with psoriasis at eight sites in Canada, the United States, and Israel as part of the International Psoriasis & Arthritis Research Team (IPART). Based on medical and laboratory reports and self-reported data, the investigators assessed these comorbidities and whether treatment adhered to cholesterol and hypertension guidelines from the American College of Cardiology and the American Heart Association (Circulation. 2014 Jul 1;129:S1-45), and the Eighth Joint National Committee (JAMA. 2014 Feb 5;311[5]:507-20), respectively.
More than 80% of patients in the cohort had at least one modifiable cardiovascular risk factor, Dr. Eder said. While 6% had ischemic heart disease, 45% had hypertension, 71% had dyslipidemia, 13% had diabetes, 54% had central obesity, and 17% were current smokers. Furthermore, close to half of patients who had been diagnosed with hypertension had uncontrolled high blood pressure, and 57% were not receiving antihypertensive medications. Likewise, 58% of patients with dyslipidemia met criteria for statins, but only a third of these patients were receiving them.
Undertreatment was associated with having psoriatic arthritis or severe psoriasis and with having a high school or lower level of education, Dr. Eder added. “You have to remember that this study was conducted among specialists – these are supposed to be experts in the field,” she said. “If the treatment adherence is relatively low in these centers, then I would expect that for patients who are being followed in centers that do not specialize in psoriatic disease, adherence would be even lower.”
The second study detected significantly higher rates of cardiovascular risk factors among patients with psoriatic diseases, compared with controls from the Health Improvement Network, a medical records database that covers more than 9 million individuals in the United Kingdom. Patients with psoriatic arthritis or severe psoriasis were significantly more likely than were controls to develop hypertension, hyperlipidemia, obesity, or diabetes, with odds ratios ranging from 1.22 to 1.78, reported Dr. Kashif A. Jafri, who led the study while he was an internal medicine resident at the University of Pennsylvania in Philadelphia.
But despite their disproportionate risk, patients were treated at about the same rate as controls, Dr. Jafri said. About 15% of individuals with hypertension received no treatment, 30%-40% with hyperlipidemia went untreated, and nearly 60% with diabetes received no documented therapy. “The absence of a significant difference in receipt of appropriate therapy among the groups reflects a need for more careful attention to the management of cardiovascular risk factors in patients with inflammatory diseases,” Dr. Jafri emphasized. Because these risk factors can be successfully treated, it is “critical” to educate primary care providers about the need to do so, he said.
Rheumatologists also should periodically discuss cardiovascular risk factors with their patients as part of routine care, Dr. Jafri advised. “Although there are obviously time constraints during each office visit, this is a topic that dramatically influences the morbidity and mortality of our patient population, and rheumatologists have the unique ability to address this issue in the context of their long-term relationships with their patients,” he said.
Dr. Jafri is now a fellow in rheumatology at the University of California, San Francisco. His was supported by an Ephraim P. Engleman Endowed Resident Research Preceptorship Award from the Rheumatology Research Foundation. IPART is sponsored by the Krembil Foundation and the Canadian Institutes of Health Research. Dr. Jafri and Dr. Eder had no disclosures.
AT THE ACR ANNUAL MEETING
Key clinical point: Patients with psoriatic diseases have high rates of modifiable cardiovascular risk factors that often go untreated, based on two large studies.
Major finding: These risk factors were untreated about one-third to one-half of the time.
Data source: The first study included 1,327 patients with psoriatic arthritis and 927 patients with psoriasis identified through the International Psoriasis & Arthritis Research Team (IPART). The second study analyzed data from The Health Improvement Network, including 211,832 patients with psoriatic disease and more than 1.3 million controls.
Disclosures: The IPART is sponsored by the Krembil Foundation and the Canadian Institutes of Health Research. The second study was supported by an Ephraim P. Engleman Endowed Resident Research Preceptorship Award from the Rheumatology Research Foundation. Dr. Eder and Dr. Jafri had no disclosures.
ACR: Filgotinib cleared phase IIb trials for rheumatoid arthritis
SAN FRANCISCO – Filgotinib monotherapy yielded a 72% week 12 ACR 20 response for patients with rheumatoid arthritis and a 79% response when combined with methotrexate, according to two phase IIb dose-finding studies reported at the annual meeting of the American College of Rheumatology.
The Janus 1 kinase (JAK1) inhibitor caused dose-dependent increases in hemoglobin levels in both trials, and a few patients also had “substantial increases in creatinine, with some indication of a dose response,” said Dr. Arthur Kavanaugh, professor of clinical medicine and director of the Center for Innovative Therapy at the University of California, San Diego. But the overall safety profile for filgotinib was “attractive. We can see that this is an effective therapy,” he added.
Filgotinib, the first selective JAK1 inhibitor in development for rheumatoid arthritis, entered double-blind phase IIb trials in 2013. The DARWIN 1 trial compared filgotinib and a stable methotrexate dose with methotrexate and placebo in 594 patients with rheumatoid arthritis. The DARWIN 2 study compared filgotinib monotherapy with placebo in 283 patients who had not responded adequately to methotrexate. Patients in both trials were usually in their mid-fifties, had been diagnosed 7-10 years earlier, and had baseline 28-joint Disease Activity Score using C-reactive protein (DAS28-CRP) scores ranging from 6.0 to 6.2. The primary endpoint in both studies was the proportion of patients who achieved an ACR 20 response by week 12, said Dr. Rene R. Westhovens of KU Leuven (Belgium). He reported the results of DARWIN 1.
The combination regimen statistically outperformed methotrexate plus placebo at filgotinib doses of 100 mg once per day (week 12 ACR 20, 64% vs. 44%; P =.044), 200 mg once per day (69%; P less than .01), and 100 mg twice per day (79%; P less than .0001), Dr. Westhovens said. Filgotinib monotherapy also significantly outperformed placebo in terms of week 12 ACR 20, at all three doses tested – 50 mg once a day (67% vs. 29% for placebo), 100 mg once a day (66%), and 200 mg once a day (72%; all P less than .0001), according to Dr. Kavanaugh. “You might take away that perhaps the higher dose group got there faster. That should be the focus of future studies,” he said.
All three doses of filgotinib, given alone, also significantly outperformed placebo on several other endpoints, including ACR 50, ACR-N, DAS28-CRP, the Clinical Disease Activity Index, and the Simple Disease Activity Index, Dr. Kavanaugh added. By week 12, 24%-45% of patients who received filgotinib achieved low disease activity (DAS28-CRP of 3.2 or less), compared with 14% of the placebo group. Furthermore, after just 1 week of 100 mg or 200 mg of filgotinib per day, patients significantly improved on the ACR-N and the DAS28-CRP, compared with the placebo group.
Rates of serious adverse events were similar for the filgotinib and comparison groups in both trials, Dr. Kavanaugh and Dr. Westhovens reported. Notably, filgotinib was not associated with declines in natural killer cells or other forms of lymphopenia. Neutrophil counts declined slightly, compared with placebo, through 24 weeks, but did not seem to increase the risk of serious infections, although there was one case of cutaneous herpes zoster, Dr. Kavanaugh said. Likewise, rises in creatinine were usually mild and clinically insignificant, he added. “The reason for the rise in creatinine is unclear. It might be mechanistic, but whether we will see differences among the different [JAK] compounds remains to be elucidated,” he said.
Both DARWIN 1 and DARWIN 2 also reported dose-dependent rises in hemoglobin levels. Patients tended to have “small” increases in blood lipid levels, but the effects on HDL cholesterol were greater than for LDL cholesterol, Dr. Westhovens said.
For DARWIN 1, Dr. Westhovens reported financial support from Galapagos, the maker of filgotinib, and from Roche, Bristol-Myers Squibb, and Janssen. His senior author and three coauthors also reported financial relationships with Galapagos, and the other five coauthors had no disclosures. For DARWIN 2, Dr. Kavanaugh, his senior author, and three coauthors reported financial support from Galapagos, and five coauthors had no disclosures.
SAN FRANCISCO – Filgotinib monotherapy yielded a 72% week 12 ACR 20 response for patients with rheumatoid arthritis and a 79% response when combined with methotrexate, according to two phase IIb dose-finding studies reported at the annual meeting of the American College of Rheumatology.
The Janus 1 kinase (JAK1) inhibitor caused dose-dependent increases in hemoglobin levels in both trials, and a few patients also had “substantial increases in creatinine, with some indication of a dose response,” said Dr. Arthur Kavanaugh, professor of clinical medicine and director of the Center for Innovative Therapy at the University of California, San Diego. But the overall safety profile for filgotinib was “attractive. We can see that this is an effective therapy,” he added.
Filgotinib, the first selective JAK1 inhibitor in development for rheumatoid arthritis, entered double-blind phase IIb trials in 2013. The DARWIN 1 trial compared filgotinib and a stable methotrexate dose with methotrexate and placebo in 594 patients with rheumatoid arthritis. The DARWIN 2 study compared filgotinib monotherapy with placebo in 283 patients who had not responded adequately to methotrexate. Patients in both trials were usually in their mid-fifties, had been diagnosed 7-10 years earlier, and had baseline 28-joint Disease Activity Score using C-reactive protein (DAS28-CRP) scores ranging from 6.0 to 6.2. The primary endpoint in both studies was the proportion of patients who achieved an ACR 20 response by week 12, said Dr. Rene R. Westhovens of KU Leuven (Belgium). He reported the results of DARWIN 1.
The combination regimen statistically outperformed methotrexate plus placebo at filgotinib doses of 100 mg once per day (week 12 ACR 20, 64% vs. 44%; P =.044), 200 mg once per day (69%; P less than .01), and 100 mg twice per day (79%; P less than .0001), Dr. Westhovens said. Filgotinib monotherapy also significantly outperformed placebo in terms of week 12 ACR 20, at all three doses tested – 50 mg once a day (67% vs. 29% for placebo), 100 mg once a day (66%), and 200 mg once a day (72%; all P less than .0001), according to Dr. Kavanaugh. “You might take away that perhaps the higher dose group got there faster. That should be the focus of future studies,” he said.
All three doses of filgotinib, given alone, also significantly outperformed placebo on several other endpoints, including ACR 50, ACR-N, DAS28-CRP, the Clinical Disease Activity Index, and the Simple Disease Activity Index, Dr. Kavanaugh added. By week 12, 24%-45% of patients who received filgotinib achieved low disease activity (DAS28-CRP of 3.2 or less), compared with 14% of the placebo group. Furthermore, after just 1 week of 100 mg or 200 mg of filgotinib per day, patients significantly improved on the ACR-N and the DAS28-CRP, compared with the placebo group.
Rates of serious adverse events were similar for the filgotinib and comparison groups in both trials, Dr. Kavanaugh and Dr. Westhovens reported. Notably, filgotinib was not associated with declines in natural killer cells or other forms of lymphopenia. Neutrophil counts declined slightly, compared with placebo, through 24 weeks, but did not seem to increase the risk of serious infections, although there was one case of cutaneous herpes zoster, Dr. Kavanaugh said. Likewise, rises in creatinine were usually mild and clinically insignificant, he added. “The reason for the rise in creatinine is unclear. It might be mechanistic, but whether we will see differences among the different [JAK] compounds remains to be elucidated,” he said.
Both DARWIN 1 and DARWIN 2 also reported dose-dependent rises in hemoglobin levels. Patients tended to have “small” increases in blood lipid levels, but the effects on HDL cholesterol were greater than for LDL cholesterol, Dr. Westhovens said.
For DARWIN 1, Dr. Westhovens reported financial support from Galapagos, the maker of filgotinib, and from Roche, Bristol-Myers Squibb, and Janssen. His senior author and three coauthors also reported financial relationships with Galapagos, and the other five coauthors had no disclosures. For DARWIN 2, Dr. Kavanaugh, his senior author, and three coauthors reported financial support from Galapagos, and five coauthors had no disclosures.
SAN FRANCISCO – Filgotinib monotherapy yielded a 72% week 12 ACR 20 response for patients with rheumatoid arthritis and a 79% response when combined with methotrexate, according to two phase IIb dose-finding studies reported at the annual meeting of the American College of Rheumatology.
The Janus 1 kinase (JAK1) inhibitor caused dose-dependent increases in hemoglobin levels in both trials, and a few patients also had “substantial increases in creatinine, with some indication of a dose response,” said Dr. Arthur Kavanaugh, professor of clinical medicine and director of the Center for Innovative Therapy at the University of California, San Diego. But the overall safety profile for filgotinib was “attractive. We can see that this is an effective therapy,” he added.
Filgotinib, the first selective JAK1 inhibitor in development for rheumatoid arthritis, entered double-blind phase IIb trials in 2013. The DARWIN 1 trial compared filgotinib and a stable methotrexate dose with methotrexate and placebo in 594 patients with rheumatoid arthritis. The DARWIN 2 study compared filgotinib monotherapy with placebo in 283 patients who had not responded adequately to methotrexate. Patients in both trials were usually in their mid-fifties, had been diagnosed 7-10 years earlier, and had baseline 28-joint Disease Activity Score using C-reactive protein (DAS28-CRP) scores ranging from 6.0 to 6.2. The primary endpoint in both studies was the proportion of patients who achieved an ACR 20 response by week 12, said Dr. Rene R. Westhovens of KU Leuven (Belgium). He reported the results of DARWIN 1.
The combination regimen statistically outperformed methotrexate plus placebo at filgotinib doses of 100 mg once per day (week 12 ACR 20, 64% vs. 44%; P =.044), 200 mg once per day (69%; P less than .01), and 100 mg twice per day (79%; P less than .0001), Dr. Westhovens said. Filgotinib monotherapy also significantly outperformed placebo in terms of week 12 ACR 20, at all three doses tested – 50 mg once a day (67% vs. 29% for placebo), 100 mg once a day (66%), and 200 mg once a day (72%; all P less than .0001), according to Dr. Kavanaugh. “You might take away that perhaps the higher dose group got there faster. That should be the focus of future studies,” he said.
All three doses of filgotinib, given alone, also significantly outperformed placebo on several other endpoints, including ACR 50, ACR-N, DAS28-CRP, the Clinical Disease Activity Index, and the Simple Disease Activity Index, Dr. Kavanaugh added. By week 12, 24%-45% of patients who received filgotinib achieved low disease activity (DAS28-CRP of 3.2 or less), compared with 14% of the placebo group. Furthermore, after just 1 week of 100 mg or 200 mg of filgotinib per day, patients significantly improved on the ACR-N and the DAS28-CRP, compared with the placebo group.
Rates of serious adverse events were similar for the filgotinib and comparison groups in both trials, Dr. Kavanaugh and Dr. Westhovens reported. Notably, filgotinib was not associated with declines in natural killer cells or other forms of lymphopenia. Neutrophil counts declined slightly, compared with placebo, through 24 weeks, but did not seem to increase the risk of serious infections, although there was one case of cutaneous herpes zoster, Dr. Kavanaugh said. Likewise, rises in creatinine were usually mild and clinically insignificant, he added. “The reason for the rise in creatinine is unclear. It might be mechanistic, but whether we will see differences among the different [JAK] compounds remains to be elucidated,” he said.
Both DARWIN 1 and DARWIN 2 also reported dose-dependent rises in hemoglobin levels. Patients tended to have “small” increases in blood lipid levels, but the effects on HDL cholesterol were greater than for LDL cholesterol, Dr. Westhovens said.
For DARWIN 1, Dr. Westhovens reported financial support from Galapagos, the maker of filgotinib, and from Roche, Bristol-Myers Squibb, and Janssen. His senior author and three coauthors also reported financial relationships with Galapagos, and the other five coauthors had no disclosures. For DARWIN 2, Dr. Kavanaugh, his senior author, and three coauthors reported financial support from Galapagos, and five coauthors had no disclosures.
AT THE ACR ANNUAL MEETING
Key clinical point: Filgotinib appeared effective and safe as monotherapy or in combination with methotrexate in two phase IIb trials of patients with rheumatoid arthritis.
Major finding: The highest week 12 ACR 20 response for filgotinib with methotrexate was 79% at 100 mg twice daily versus 44% for methotrexate with placebo (P less than .0001). For filgotinib monotherapy, week 12 ACR 20 responses were 67% at 50 mg once daily, 66% at 100 mg once daily, and 72% at 200 mg once daily, versus 29% for placebo (all P values less than .0001).
Data source: The DARWIN 1 trial compared filgotinib plus methotrexate and methotrexate plus placebo in 594 patients. DARWIN 2 compared filgotinib monotherapy with placebo in 283 patients who had responded inadequately to methotrexate.
Disclosures: For DARWIN 1, Dr. Westhovens reported financial support from Galapagos, the maker of filgotinib, and from Roche, Bristol-Myers Squibb, and Janssen. His senior author and three coauthors also reported financial relationships with Galapagos, and the other five coauthors had no disclosures. For DARWIN 2, Dr. Kavanaugh, his senior author, and three coauthors reported financial support from Galapagos, and five coauthors had no disclosures.
ACR: Don’t be fooled by contaminated synovial fluid
SAN FRANCISCO – Hold off on surgery in patients with presumed septic arthritis if they’re not otherwise too sick and their cultures don’t grow out a pathogenic organism within 48 hours.
The reason is because those patients are likely to have synovial fluid that was contaminated during collection, not a true joint infection.
The advice comes from investigators at Beth Israel Deaconess Medical Center, Boston, who compared 425 monoarticular septic arthritis cases with 25 cases that turned out to be false positives due to synovial fluid contamination; most of the false positives got antibiotics, and three (12%) had joint operations that they did not need.
“Rushing off to the operating room isn’t” always warranted. “You can suspect contamination if patients have milder disease manifestations and cultures grow late,” said investigator Dr. Robert H. Shmerling, clinical chief of Beth Israel’s division of rheumatology.
The findings help determine when – and when not – to be aggressive with patients who present with what looks to be septic arthritis. “No one’s ever really looked at this before,” he said at the annual meeting of the American College of Rheumatology.
“These are very different sorts of patients. Look at the full range of clinical characteristics and lab values, not just the synovial fluid tap. If contamination is suspected, you can wait until the cultures come back or possibly do serial taps before going to the operating room,” said coinvestigator Clara Zhu, a medical student at Boston University.
Patients with true joint infections had higher mean peripheral polymorphonuclear neutrophil percentages (78% vs. 68% in false positives) and synovial fluid polymorphonuclear cell percentages (88% vs. 74% in false positives). True cases also had substantially higher mean synovial fluid white blood cell counts (88,000 vs. 29,000).
Unlike true cases, contaminated synovial fluid took about 4 days to grow out a positive culture, and the most common organisms by far were coagulase-negative staphylococci, typically normal skin bacteria.
Patients with contaminated fluid also tended to be older (71 vs. 59 years), with fewer prior admissions. They were far less likely to have had recent joint procedures and histories of septic arthritis but were more likely to have synovial fluid crystals, as in gout. False positives also left the hospital sooner (7 vs. 11 days) and were less likely to be readmitted within 2 months. They were also less likely to present with fever (19% vs. 37%) but not significantly so.
This “study suggests that contaminated synovial fluid is found in up to 6% of patients with suspected septic arthritis and positive synovial fluid or synovial biopsy cultures. We recommend a conservative approach for patients with ... mild disease manifestations and no growth of pathogenic organisms within the first 48 hours,” the investigators concluded.
The authors have no disclosures, and there was no outside funding for the work.
SAN FRANCISCO – Hold off on surgery in patients with presumed septic arthritis if they’re not otherwise too sick and their cultures don’t grow out a pathogenic organism within 48 hours.
The reason is because those patients are likely to have synovial fluid that was contaminated during collection, not a true joint infection.
The advice comes from investigators at Beth Israel Deaconess Medical Center, Boston, who compared 425 monoarticular septic arthritis cases with 25 cases that turned out to be false positives due to synovial fluid contamination; most of the false positives got antibiotics, and three (12%) had joint operations that they did not need.
“Rushing off to the operating room isn’t” always warranted. “You can suspect contamination if patients have milder disease manifestations and cultures grow late,” said investigator Dr. Robert H. Shmerling, clinical chief of Beth Israel’s division of rheumatology.
The findings help determine when – and when not – to be aggressive with patients who present with what looks to be septic arthritis. “No one’s ever really looked at this before,” he said at the annual meeting of the American College of Rheumatology.
“These are very different sorts of patients. Look at the full range of clinical characteristics and lab values, not just the synovial fluid tap. If contamination is suspected, you can wait until the cultures come back or possibly do serial taps before going to the operating room,” said coinvestigator Clara Zhu, a medical student at Boston University.
Patients with true joint infections had higher mean peripheral polymorphonuclear neutrophil percentages (78% vs. 68% in false positives) and synovial fluid polymorphonuclear cell percentages (88% vs. 74% in false positives). True cases also had substantially higher mean synovial fluid white blood cell counts (88,000 vs. 29,000).
Unlike true cases, contaminated synovial fluid took about 4 days to grow out a positive culture, and the most common organisms by far were coagulase-negative staphylococci, typically normal skin bacteria.
Patients with contaminated fluid also tended to be older (71 vs. 59 years), with fewer prior admissions. They were far less likely to have had recent joint procedures and histories of septic arthritis but were more likely to have synovial fluid crystals, as in gout. False positives also left the hospital sooner (7 vs. 11 days) and were less likely to be readmitted within 2 months. They were also less likely to present with fever (19% vs. 37%) but not significantly so.
This “study suggests that contaminated synovial fluid is found in up to 6% of patients with suspected septic arthritis and positive synovial fluid or synovial biopsy cultures. We recommend a conservative approach for patients with ... mild disease manifestations and no growth of pathogenic organisms within the first 48 hours,” the investigators concluded.
The authors have no disclosures, and there was no outside funding for the work.
SAN FRANCISCO – Hold off on surgery in patients with presumed septic arthritis if they’re not otherwise too sick and their cultures don’t grow out a pathogenic organism within 48 hours.
The reason is because those patients are likely to have synovial fluid that was contaminated during collection, not a true joint infection.
The advice comes from investigators at Beth Israel Deaconess Medical Center, Boston, who compared 425 monoarticular septic arthritis cases with 25 cases that turned out to be false positives due to synovial fluid contamination; most of the false positives got antibiotics, and three (12%) had joint operations that they did not need.
“Rushing off to the operating room isn’t” always warranted. “You can suspect contamination if patients have milder disease manifestations and cultures grow late,” said investigator Dr. Robert H. Shmerling, clinical chief of Beth Israel’s division of rheumatology.
The findings help determine when – and when not – to be aggressive with patients who present with what looks to be septic arthritis. “No one’s ever really looked at this before,” he said at the annual meeting of the American College of Rheumatology.
“These are very different sorts of patients. Look at the full range of clinical characteristics and lab values, not just the synovial fluid tap. If contamination is suspected, you can wait until the cultures come back or possibly do serial taps before going to the operating room,” said coinvestigator Clara Zhu, a medical student at Boston University.
Patients with true joint infections had higher mean peripheral polymorphonuclear neutrophil percentages (78% vs. 68% in false positives) and synovial fluid polymorphonuclear cell percentages (88% vs. 74% in false positives). True cases also had substantially higher mean synovial fluid white blood cell counts (88,000 vs. 29,000).
Unlike true cases, contaminated synovial fluid took about 4 days to grow out a positive culture, and the most common organisms by far were coagulase-negative staphylococci, typically normal skin bacteria.
Patients with contaminated fluid also tended to be older (71 vs. 59 years), with fewer prior admissions. They were far less likely to have had recent joint procedures and histories of septic arthritis but were more likely to have synovial fluid crystals, as in gout. False positives also left the hospital sooner (7 vs. 11 days) and were less likely to be readmitted within 2 months. They were also less likely to present with fever (19% vs. 37%) but not significantly so.
This “study suggests that contaminated synovial fluid is found in up to 6% of patients with suspected septic arthritis and positive synovial fluid or synovial biopsy cultures. We recommend a conservative approach for patients with ... mild disease manifestations and no growth of pathogenic organisms within the first 48 hours,” the investigators concluded.
The authors have no disclosures, and there was no outside funding for the work.
AT THE ACR ANNUAL MEETING
Key clinical point: It’s probably not really septic arthritis if patients have mild disease manifestations and slow-growing synovial fluid cultures.
Major finding: True cases of septic arthritis had substantially higher mean synovial fluid white blood cell counts than did false-positive cases (88,000 vs. 29,000).
Data source: Review of 450 patients with presumed septic arthritis.
Disclosures: The authors have no disclosures, and there was no outside funding for the work.
ACR: Don’t stop TNFis during rheumatoid arthritis pregnancy
SAN FRANCISCO – It might be best to keep women with rheumatoid arthritis on their tumor necrosis factor blockers during pregnancy, according to German investigators.
They found that women are likely to flare without them and need more prednisolone, which is associated with preterm birth and other problems, while an increasing body of evidence suggests that tumor necrosis factor inhibitors (TNFis) are relatively safe during pregnancy.
“We should” rethink discontinuing TNFis during pregnancy, as recommended in some quarters. “We do not want women to flare during pregnancy,” said investigator Dr. Rebecca Fischer-Betz of the department of rheumatology at Heinrich Heine University in Düsseldorf.
She and her colleagues compared birth outcomes in 18 rheumatoid arthritis (RA) patients who discontinued TNFi treatment shortly after they got pregnant against those of 24 women with RA who were never exposed to a TNFi because, in general, they had less severe disease.
Twelve of the women (75%) in the TNFi group flared, versus four women (17%) in the control group. Although patients in both groups started with a mean 28-joint Disease Activity Score using C-reactive protein (DAS28-CRP) below 3.0, women in the TNFi group had a rise in activity to a mean of about 3.5 in the second trimester, while disease activity in control patients remained stable.
Compared with controls, women who stopped TNFis were also far more likely to flare (odds ratio, 10.0; 95% confidence interval, 2.3-42.8; P = .002), even after adjusting for age, DAS28-CRP at conception, rheumatoid factor and cyclic citrullinated peptide status, and other potential confounders. They also relied more heavily on prednisolone, taking, for example, a mean dose of 13 mg in the second trimester versus 8 mg in the control group. Perhaps not surprisingly, the mean duration of pregnancy was 37 weeks in the TNFi group, with six women (33%) delivering at or before 37 weeks; women in the control group delivered, on average, at 39 weeks, with four (17%) delivering at or before week 37.
The investigators found that the risk of preterm birth increased with every cumulative milligram of prednisolone (OR, 1.08; 95% CI, 1.02-1.15; P less than .01).
“Women with RA who discontinue TNFis at conception face a high risk for flares during pregnancy, independently of known risk factors like seropositivity. Flares are usually treated with prednisolone. We found a dose-dependent, significant increased risk for preterm birth associated with prednisolone. In this era of treat-to-target management of RA, our paradigm for RA pregnancy management may need adjusting. By controlling RA activity with medications considered relatively safe in pregnancy, we may be able to improve both the pregnancy experience and pregnancy outcomes,” the investigators concluded.
The women were 33 years old on average, and all had live births; four early miscarriages were excluded from analysis. All the pregnancies were planned, with methotrexate discontinued at least 3 months before conception. There was no statistical difference in the rate of seropositivity between the groups, “which is interesting because we know seropositivity is a risk factor for staying active during pregnancy,” Dr. Fischer-Betz said.
Two boys born to women who took TNFis had minor malformations, one with nasal bone aplasia, retrognathia, and hydronephrosis, and the other with hypospadias. Both of their mothers had taken etanercept (Enbrel) in the first trimester. There was one malformation in the control group, a girl born with hydronephrosis.
There was no outside funding for the work, and the investigators have no disclosures.
SAN FRANCISCO – It might be best to keep women with rheumatoid arthritis on their tumor necrosis factor blockers during pregnancy, according to German investigators.
They found that women are likely to flare without them and need more prednisolone, which is associated with preterm birth and other problems, while an increasing body of evidence suggests that tumor necrosis factor inhibitors (TNFis) are relatively safe during pregnancy.
“We should” rethink discontinuing TNFis during pregnancy, as recommended in some quarters. “We do not want women to flare during pregnancy,” said investigator Dr. Rebecca Fischer-Betz of the department of rheumatology at Heinrich Heine University in Düsseldorf.
She and her colleagues compared birth outcomes in 18 rheumatoid arthritis (RA) patients who discontinued TNFi treatment shortly after they got pregnant against those of 24 women with RA who were never exposed to a TNFi because, in general, they had less severe disease.
Twelve of the women (75%) in the TNFi group flared, versus four women (17%) in the control group. Although patients in both groups started with a mean 28-joint Disease Activity Score using C-reactive protein (DAS28-CRP) below 3.0, women in the TNFi group had a rise in activity to a mean of about 3.5 in the second trimester, while disease activity in control patients remained stable.
Compared with controls, women who stopped TNFis were also far more likely to flare (odds ratio, 10.0; 95% confidence interval, 2.3-42.8; P = .002), even after adjusting for age, DAS28-CRP at conception, rheumatoid factor and cyclic citrullinated peptide status, and other potential confounders. They also relied more heavily on prednisolone, taking, for example, a mean dose of 13 mg in the second trimester versus 8 mg in the control group. Perhaps not surprisingly, the mean duration of pregnancy was 37 weeks in the TNFi group, with six women (33%) delivering at or before 37 weeks; women in the control group delivered, on average, at 39 weeks, with four (17%) delivering at or before week 37.
The investigators found that the risk of preterm birth increased with every cumulative milligram of prednisolone (OR, 1.08; 95% CI, 1.02-1.15; P less than .01).
“Women with RA who discontinue TNFis at conception face a high risk for flares during pregnancy, independently of known risk factors like seropositivity. Flares are usually treated with prednisolone. We found a dose-dependent, significant increased risk for preterm birth associated with prednisolone. In this era of treat-to-target management of RA, our paradigm for RA pregnancy management may need adjusting. By controlling RA activity with medications considered relatively safe in pregnancy, we may be able to improve both the pregnancy experience and pregnancy outcomes,” the investigators concluded.
The women were 33 years old on average, and all had live births; four early miscarriages were excluded from analysis. All the pregnancies were planned, with methotrexate discontinued at least 3 months before conception. There was no statistical difference in the rate of seropositivity between the groups, “which is interesting because we know seropositivity is a risk factor for staying active during pregnancy,” Dr. Fischer-Betz said.
Two boys born to women who took TNFis had minor malformations, one with nasal bone aplasia, retrognathia, and hydronephrosis, and the other with hypospadias. Both of their mothers had taken etanercept (Enbrel) in the first trimester. There was one malformation in the control group, a girl born with hydronephrosis.
There was no outside funding for the work, and the investigators have no disclosures.
SAN FRANCISCO – It might be best to keep women with rheumatoid arthritis on their tumor necrosis factor blockers during pregnancy, according to German investigators.
They found that women are likely to flare without them and need more prednisolone, which is associated with preterm birth and other problems, while an increasing body of evidence suggests that tumor necrosis factor inhibitors (TNFis) are relatively safe during pregnancy.
“We should” rethink discontinuing TNFis during pregnancy, as recommended in some quarters. “We do not want women to flare during pregnancy,” said investigator Dr. Rebecca Fischer-Betz of the department of rheumatology at Heinrich Heine University in Düsseldorf.
She and her colleagues compared birth outcomes in 18 rheumatoid arthritis (RA) patients who discontinued TNFi treatment shortly after they got pregnant against those of 24 women with RA who were never exposed to a TNFi because, in general, they had less severe disease.
Twelve of the women (75%) in the TNFi group flared, versus four women (17%) in the control group. Although patients in both groups started with a mean 28-joint Disease Activity Score using C-reactive protein (DAS28-CRP) below 3.0, women in the TNFi group had a rise in activity to a mean of about 3.5 in the second trimester, while disease activity in control patients remained stable.
Compared with controls, women who stopped TNFis were also far more likely to flare (odds ratio, 10.0; 95% confidence interval, 2.3-42.8; P = .002), even after adjusting for age, DAS28-CRP at conception, rheumatoid factor and cyclic citrullinated peptide status, and other potential confounders. They also relied more heavily on prednisolone, taking, for example, a mean dose of 13 mg in the second trimester versus 8 mg in the control group. Perhaps not surprisingly, the mean duration of pregnancy was 37 weeks in the TNFi group, with six women (33%) delivering at or before 37 weeks; women in the control group delivered, on average, at 39 weeks, with four (17%) delivering at or before week 37.
The investigators found that the risk of preterm birth increased with every cumulative milligram of prednisolone (OR, 1.08; 95% CI, 1.02-1.15; P less than .01).
“Women with RA who discontinue TNFis at conception face a high risk for flares during pregnancy, independently of known risk factors like seropositivity. Flares are usually treated with prednisolone. We found a dose-dependent, significant increased risk for preterm birth associated with prednisolone. In this era of treat-to-target management of RA, our paradigm for RA pregnancy management may need adjusting. By controlling RA activity with medications considered relatively safe in pregnancy, we may be able to improve both the pregnancy experience and pregnancy outcomes,” the investigators concluded.
The women were 33 years old on average, and all had live births; four early miscarriages were excluded from analysis. All the pregnancies were planned, with methotrexate discontinued at least 3 months before conception. There was no statistical difference in the rate of seropositivity between the groups, “which is interesting because we know seropositivity is a risk factor for staying active during pregnancy,” Dr. Fischer-Betz said.
Two boys born to women who took TNFis had minor malformations, one with nasal bone aplasia, retrognathia, and hydronephrosis, and the other with hypospadias. Both of their mothers had taken etanercept (Enbrel) in the first trimester. There was one malformation in the control group, a girl born with hydronephrosis.
There was no outside funding for the work, and the investigators have no disclosures.
AT THE ACR ANNUAL MEETING
Key clinical point: Stopping a TNFi during pregnancy comes at the cost of increased reliance on prednisolone.
Major finding: Compared with controls, women who stopped a TNFi during pregnancy were far more likely to flare (OR, 10.0; 95% CI, 2.3-42.8; P = .002).
Data source: Birth outcomes in 42 women with rheumatoid arthritis.
Disclosures: There was no outside funding for the work, and the investigators have no disclosures.
ACR: Rheumatologists aren’t giving methotrexate a fair shot
SAN FRANCISCO – Oral methotrexate is frequently underdosed, given for an inadequate length of time, and rarely switched to subcutaneous formulations before rheumatologists move on to biologics, according to an analysis of claims data from 35,640 rheumatoid arthritis patients.
“There’re some major concerns here. Methotrexate is the anchor drug for rheumatoid arthritis, the best drug we have. More appropriate [use] could lead to better control” and “produce significant cost savings,” said investigator Dr. James O’Dell, chief of the division of rheumatology at the University of Nebraska Medical Center, Omaha.
When patients don’t fully respond to lower doses, the ground rules for oral methotrexate include escalation up to 25 mg or a switch to subcutaneous formulations, which have better bioavailability. Those moves should be considered before turning to biologics, Dr. O’Dell explained at the American College of Rheumatology annual meeting.
Rheumatologists, by and large, aren’t playing by those rules, according to the analysis. “We need to own these data because the majority of patients, over three-quarters, were treated by rheumatologists. We are not doing a great job,” Dr. ODell said.
The claims data came from Symphony Health Solutions, which captures about 92% of prescriptions written in the United States. The 35,640 rheumatoid arthritis patients in the study were started on methotrexate in 2009 and followed through 2014; 15,599 (43.8%) didn’t need anything else and stayed on oral methotrexate alone throughout the study period.
Prescribers, however, gave up on oral methotrexate at a mean dose of 15.3 mg and moved 17,528 patients (49%) straight to a biologic without giving subcutaneous methotrexate a shot. They did that after a median of less than 6 months, and within 3 months in more than 40% of patients.
Just 2,513 patients (7%) moved on to subcutaneous methotrexate when their oral formulation wasn’t enough. That’s all most of them needed; 1,802 (72%) remained on subcutaneous methotrexate alone for the remainder of the study period. The rest moved on to a biologic, but after a median of almost a year, not a few months. When their time on oral and subcutaneous methotrexate was included, their median time to a biologic was more than 2 years.
The investigators checked to see if things improved for patients who started on oral methotrexate in 2012. “The answer was no. We didn’t do better,” Dr. O’Dell said.
He didn’t speculate on why methotrexate is underused in the United States.
Claims data can’t address why patients were switched from methotrexate and other issues, but such nuances “don’t even begin to explain the doses and the timing of switch that we saw here,” he said.
Dr. O’Dell is an adviser for AbbVie, Lilly, Coherus, Bristol-Myers Squibb, Antares, and Medac. Other investigators disclosed relationships with those or other companies.
SAN FRANCISCO – Oral methotrexate is frequently underdosed, given for an inadequate length of time, and rarely switched to subcutaneous formulations before rheumatologists move on to biologics, according to an analysis of claims data from 35,640 rheumatoid arthritis patients.
“There’re some major concerns here. Methotrexate is the anchor drug for rheumatoid arthritis, the best drug we have. More appropriate [use] could lead to better control” and “produce significant cost savings,” said investigator Dr. James O’Dell, chief of the division of rheumatology at the University of Nebraska Medical Center, Omaha.
When patients don’t fully respond to lower doses, the ground rules for oral methotrexate include escalation up to 25 mg or a switch to subcutaneous formulations, which have better bioavailability. Those moves should be considered before turning to biologics, Dr. O’Dell explained at the American College of Rheumatology annual meeting.
Rheumatologists, by and large, aren’t playing by those rules, according to the analysis. “We need to own these data because the majority of patients, over three-quarters, were treated by rheumatologists. We are not doing a great job,” Dr. ODell said.
The claims data came from Symphony Health Solutions, which captures about 92% of prescriptions written in the United States. The 35,640 rheumatoid arthritis patients in the study were started on methotrexate in 2009 and followed through 2014; 15,599 (43.8%) didn’t need anything else and stayed on oral methotrexate alone throughout the study period.
Prescribers, however, gave up on oral methotrexate at a mean dose of 15.3 mg and moved 17,528 patients (49%) straight to a biologic without giving subcutaneous methotrexate a shot. They did that after a median of less than 6 months, and within 3 months in more than 40% of patients.
Just 2,513 patients (7%) moved on to subcutaneous methotrexate when their oral formulation wasn’t enough. That’s all most of them needed; 1,802 (72%) remained on subcutaneous methotrexate alone for the remainder of the study period. The rest moved on to a biologic, but after a median of almost a year, not a few months. When their time on oral and subcutaneous methotrexate was included, their median time to a biologic was more than 2 years.
The investigators checked to see if things improved for patients who started on oral methotrexate in 2012. “The answer was no. We didn’t do better,” Dr. O’Dell said.
He didn’t speculate on why methotrexate is underused in the United States.
Claims data can’t address why patients were switched from methotrexate and other issues, but such nuances “don’t even begin to explain the doses and the timing of switch that we saw here,” he said.
Dr. O’Dell is an adviser for AbbVie, Lilly, Coherus, Bristol-Myers Squibb, Antares, and Medac. Other investigators disclosed relationships with those or other companies.
SAN FRANCISCO – Oral methotrexate is frequently underdosed, given for an inadequate length of time, and rarely switched to subcutaneous formulations before rheumatologists move on to biologics, according to an analysis of claims data from 35,640 rheumatoid arthritis patients.
“There’re some major concerns here. Methotrexate is the anchor drug for rheumatoid arthritis, the best drug we have. More appropriate [use] could lead to better control” and “produce significant cost savings,” said investigator Dr. James O’Dell, chief of the division of rheumatology at the University of Nebraska Medical Center, Omaha.
When patients don’t fully respond to lower doses, the ground rules for oral methotrexate include escalation up to 25 mg or a switch to subcutaneous formulations, which have better bioavailability. Those moves should be considered before turning to biologics, Dr. O’Dell explained at the American College of Rheumatology annual meeting.
Rheumatologists, by and large, aren’t playing by those rules, according to the analysis. “We need to own these data because the majority of patients, over three-quarters, were treated by rheumatologists. We are not doing a great job,” Dr. ODell said.
The claims data came from Symphony Health Solutions, which captures about 92% of prescriptions written in the United States. The 35,640 rheumatoid arthritis patients in the study were started on methotrexate in 2009 and followed through 2014; 15,599 (43.8%) didn’t need anything else and stayed on oral methotrexate alone throughout the study period.
Prescribers, however, gave up on oral methotrexate at a mean dose of 15.3 mg and moved 17,528 patients (49%) straight to a biologic without giving subcutaneous methotrexate a shot. They did that after a median of less than 6 months, and within 3 months in more than 40% of patients.
Just 2,513 patients (7%) moved on to subcutaneous methotrexate when their oral formulation wasn’t enough. That’s all most of them needed; 1,802 (72%) remained on subcutaneous methotrexate alone for the remainder of the study period. The rest moved on to a biologic, but after a median of almost a year, not a few months. When their time on oral and subcutaneous methotrexate was included, their median time to a biologic was more than 2 years.
The investigators checked to see if things improved for patients who started on oral methotrexate in 2012. “The answer was no. We didn’t do better,” Dr. O’Dell said.
He didn’t speculate on why methotrexate is underused in the United States.
Claims data can’t address why patients were switched from methotrexate and other issues, but such nuances “don’t even begin to explain the doses and the timing of switch that we saw here,” he said.
Dr. O’Dell is an adviser for AbbVie, Lilly, Coherus, Bristol-Myers Squibb, Antares, and Medac. Other investigators disclosed relationships with those or other companies.
AT THE ACR ANNUAL MEETING
Key clinical point: Give methotrexate a chance in rheumatoid arthritis; don’t be too quick with the biologics.
Major finding: Prescribers gave up on oral methotrexate at a mean dose of 15.3 mg, and moved 49% of patients straight to a biologic without first trying subcutaneous methotrexate.
Data source: Claims data from 35,640 rheumatoid arthritis patients.
Disclosures: The presenter is an adviser for AbbVie, Lilly, Coherus, Bristol-Myers Squibb, Antares, and Medac. Other investigators disclosed relationships with those or other companies.
ACR: Years of TNF blockers did not increase risk of lymphoma in RA
SAN FRANCISCO – Rheumatoid arthritis conferred a doubling of the risk of lymphoma when compared against the general population in a large Swedish registry study, regardless of the patients’ experience with biological disease-modifying antirheumatic drugs.
But patients who took biological disease-modifying antirheumatic drugs (bDMARDs) had a sixfold greater risk of natural killer or T-cell lymphoma than did the general population, and that association was about four times stronger than for patients who had never taken biologics, reported Dr. Karin Hellgren, who led the study at the Karolinska Institute in Stockholm. That finding in particular shows the need to keep studying the links between bDMARDs and specific lymphoma subtypes, he said.
Severe rheumatoid arthritis seems to strongly increase the risk of lymphoma (Arthritis Rheum. 2006;54[3]:692-701), but researchers have debated whether the reason relates to bDMARDs or RA itself. Clinical trials have produced conflicting results; observational studies have reported no overall link between bDMARDs and lymphoma, but have raised questions about long-term exposure, the effects of individual agents, and lymphoma subtypes, Dr. Hellgren said at the annual meeting of the American College of Rheumatology.
To delve deeper into these issues, he and his associates compared 15 years of data for 13,240 RA patients on bDMARDs from the Swedish Biologics (ARTIS), Patient, and Cancer Registers and a national cohort of 46,568 bDMARD-naive patients. The researchers also compared both groups with 458,846 age- and gender-matched adults from the Swedish Population Register, following individuals until the end of 2012 or until lymphoma diagnosis, death, emigration, or bDMARD initiation, in the case of the naive patients.
Overall, patients with RA averaged one diagnosis of lymphoma per 1,000 population, compared with 0.5 cases per 1,000 for the overall population of Sweden, Dr. Hellgren said. In terms of absolute numbers, there were 241 cases of lymphoma among bDMARD-naive patients, 1,413 cases in the general population, and 69 cases among patients on bDMARDs, including 68 who were taking TNF inhibitors. The average age of the latter group of patients was 57 years. They had been diagnosed with RA at about age 50, had a mean 28-joint Disease Activity Score score of 5.3, and averaged 5.9 years of exposure to TNF inhibitors. Their risk of any type of malignant lymphoma was about 20% higher than for bDMARD-naive patients, but the difference was insignificant overall and in subgroups stratified by gender, age, and year starting treatment. Likewise, although patients were at greater risk of lymphoma if they had been on bDMARDs for 5-15 years (hazard ratio, 1.9) than for less time (HRs, about 1.0), there was no significant difference in risk compared with bDMARD-naive patients.
“There also were no statistically significant differences between drugs,” including infliximab (Remicade), etanercept (Enbrel), and adalimumab (Humira), Dr. Hellgren said. “In terms of the newer TNF inhibitors and other biologics, data are still too scarce to evaluate,” he added. Both exposed and bDMARD-naive patients were at especially high risk of Hodgkin lymphoma and diffuse large B-cell lymphoma, compared with the general population, but the strongest association of all was between bDMARD exposure and natural killer or T-cell lymphoma (HR, 6.0; 95% CI, 2.7-13.3). “The distribution of lymphoma subtypes warrants further assessment,” Dr. Hellgren concluded.
The ARTIS registry is funded by AbbVie, Bristol-Myers Squibb, Roche, Merck, Pfizer, Sobi, Lilly, and UCB. Dr. Hellgren had no disclosures. One coauthor reported financial relationships with AstraZeneca, Pfizer, UCB, Roche, Merck, Bristol-Myers Squibb, and AbbVie.
SAN FRANCISCO – Rheumatoid arthritis conferred a doubling of the risk of lymphoma when compared against the general population in a large Swedish registry study, regardless of the patients’ experience with biological disease-modifying antirheumatic drugs.
But patients who took biological disease-modifying antirheumatic drugs (bDMARDs) had a sixfold greater risk of natural killer or T-cell lymphoma than did the general population, and that association was about four times stronger than for patients who had never taken biologics, reported Dr. Karin Hellgren, who led the study at the Karolinska Institute in Stockholm. That finding in particular shows the need to keep studying the links between bDMARDs and specific lymphoma subtypes, he said.
Severe rheumatoid arthritis seems to strongly increase the risk of lymphoma (Arthritis Rheum. 2006;54[3]:692-701), but researchers have debated whether the reason relates to bDMARDs or RA itself. Clinical trials have produced conflicting results; observational studies have reported no overall link between bDMARDs and lymphoma, but have raised questions about long-term exposure, the effects of individual agents, and lymphoma subtypes, Dr. Hellgren said at the annual meeting of the American College of Rheumatology.
To delve deeper into these issues, he and his associates compared 15 years of data for 13,240 RA patients on bDMARDs from the Swedish Biologics (ARTIS), Patient, and Cancer Registers and a national cohort of 46,568 bDMARD-naive patients. The researchers also compared both groups with 458,846 age- and gender-matched adults from the Swedish Population Register, following individuals until the end of 2012 or until lymphoma diagnosis, death, emigration, or bDMARD initiation, in the case of the naive patients.
Overall, patients with RA averaged one diagnosis of lymphoma per 1,000 population, compared with 0.5 cases per 1,000 for the overall population of Sweden, Dr. Hellgren said. In terms of absolute numbers, there were 241 cases of lymphoma among bDMARD-naive patients, 1,413 cases in the general population, and 69 cases among patients on bDMARDs, including 68 who were taking TNF inhibitors. The average age of the latter group of patients was 57 years. They had been diagnosed with RA at about age 50, had a mean 28-joint Disease Activity Score score of 5.3, and averaged 5.9 years of exposure to TNF inhibitors. Their risk of any type of malignant lymphoma was about 20% higher than for bDMARD-naive patients, but the difference was insignificant overall and in subgroups stratified by gender, age, and year starting treatment. Likewise, although patients were at greater risk of lymphoma if they had been on bDMARDs for 5-15 years (hazard ratio, 1.9) than for less time (HRs, about 1.0), there was no significant difference in risk compared with bDMARD-naive patients.
“There also were no statistically significant differences between drugs,” including infliximab (Remicade), etanercept (Enbrel), and adalimumab (Humira), Dr. Hellgren said. “In terms of the newer TNF inhibitors and other biologics, data are still too scarce to evaluate,” he added. Both exposed and bDMARD-naive patients were at especially high risk of Hodgkin lymphoma and diffuse large B-cell lymphoma, compared with the general population, but the strongest association of all was between bDMARD exposure and natural killer or T-cell lymphoma (HR, 6.0; 95% CI, 2.7-13.3). “The distribution of lymphoma subtypes warrants further assessment,” Dr. Hellgren concluded.
The ARTIS registry is funded by AbbVie, Bristol-Myers Squibb, Roche, Merck, Pfizer, Sobi, Lilly, and UCB. Dr. Hellgren had no disclosures. One coauthor reported financial relationships with AstraZeneca, Pfizer, UCB, Roche, Merck, Bristol-Myers Squibb, and AbbVie.
SAN FRANCISCO – Rheumatoid arthritis conferred a doubling of the risk of lymphoma when compared against the general population in a large Swedish registry study, regardless of the patients’ experience with biological disease-modifying antirheumatic drugs.
But patients who took biological disease-modifying antirheumatic drugs (bDMARDs) had a sixfold greater risk of natural killer or T-cell lymphoma than did the general population, and that association was about four times stronger than for patients who had never taken biologics, reported Dr. Karin Hellgren, who led the study at the Karolinska Institute in Stockholm. That finding in particular shows the need to keep studying the links between bDMARDs and specific lymphoma subtypes, he said.
Severe rheumatoid arthritis seems to strongly increase the risk of lymphoma (Arthritis Rheum. 2006;54[3]:692-701), but researchers have debated whether the reason relates to bDMARDs or RA itself. Clinical trials have produced conflicting results; observational studies have reported no overall link between bDMARDs and lymphoma, but have raised questions about long-term exposure, the effects of individual agents, and lymphoma subtypes, Dr. Hellgren said at the annual meeting of the American College of Rheumatology.
To delve deeper into these issues, he and his associates compared 15 years of data for 13,240 RA patients on bDMARDs from the Swedish Biologics (ARTIS), Patient, and Cancer Registers and a national cohort of 46,568 bDMARD-naive patients. The researchers also compared both groups with 458,846 age- and gender-matched adults from the Swedish Population Register, following individuals until the end of 2012 or until lymphoma diagnosis, death, emigration, or bDMARD initiation, in the case of the naive patients.
Overall, patients with RA averaged one diagnosis of lymphoma per 1,000 population, compared with 0.5 cases per 1,000 for the overall population of Sweden, Dr. Hellgren said. In terms of absolute numbers, there were 241 cases of lymphoma among bDMARD-naive patients, 1,413 cases in the general population, and 69 cases among patients on bDMARDs, including 68 who were taking TNF inhibitors. The average age of the latter group of patients was 57 years. They had been diagnosed with RA at about age 50, had a mean 28-joint Disease Activity Score score of 5.3, and averaged 5.9 years of exposure to TNF inhibitors. Their risk of any type of malignant lymphoma was about 20% higher than for bDMARD-naive patients, but the difference was insignificant overall and in subgroups stratified by gender, age, and year starting treatment. Likewise, although patients were at greater risk of lymphoma if they had been on bDMARDs for 5-15 years (hazard ratio, 1.9) than for less time (HRs, about 1.0), there was no significant difference in risk compared with bDMARD-naive patients.
“There also were no statistically significant differences between drugs,” including infliximab (Remicade), etanercept (Enbrel), and adalimumab (Humira), Dr. Hellgren said. “In terms of the newer TNF inhibitors and other biologics, data are still too scarce to evaluate,” he added. Both exposed and bDMARD-naive patients were at especially high risk of Hodgkin lymphoma and diffuse large B-cell lymphoma, compared with the general population, but the strongest association of all was between bDMARD exposure and natural killer or T-cell lymphoma (HR, 6.0; 95% CI, 2.7-13.3). “The distribution of lymphoma subtypes warrants further assessment,” Dr. Hellgren concluded.
The ARTIS registry is funded by AbbVie, Bristol-Myers Squibb, Roche, Merck, Pfizer, Sobi, Lilly, and UCB. Dr. Hellgren had no disclosures. One coauthor reported financial relationships with AstraZeneca, Pfizer, UCB, Roche, Merck, Bristol-Myers Squibb, and AbbVie.
AT THE ACR ANNUAL MEETING
Key clinical point: Exposure to biological disease-modifying antirheumatic drugs did not increase the overall risk of lymphoma among patients with rheumatoid arthritis.
Major finding: Patients were at greater risk of lymphoma if they had been on bDMARDs for 5-15 years (hazard ratio, 1.9) than for less time (HRs, about 1.0), but there was no significant difference in risk compared with bDMARD-naive patients. However, patients on bDMARDs had about a fourfold greater risk of NK/T-cell lymphoma compared with bDMARD-naive patients.
Data source: A matched registry analysis of 13,240 patients from the Swedish Biologics (ARTIS), Patient, and Cancer Registers; 46,568 bio-naive patients; and 458,846 members of the general population.
Disclosures: The ARTIS registry is funded by AbbVie, Bristol-Myers Squibb, Roche, Merck, Pfizer, Sobi, Lilly, and UCB. Dr. Hellgren had no disclosures. One coauthor reported financial relationships with AstraZeneca, Pfizer, UCB, Roche, Merck, Bristol-Myers Squibb, and AbbVie.
ACR: Infection risk with DMARDs doesn’t change with age of RA onset
SAN FRANCISCO – Biologics do not pose an increased risk of infection in patients with elderly-onset rheumatoid arthritis over the age of 60 years because their time to first infection and time to multiple infections were not different from people of the same age who developed the disease at a younger age in a large, prospective, longitudinal, observational study .
The data also suggest that noncytotoxic disease-modifying antirheumatic drugs (DMARDs) and non-TNF biologics may have a protective effect against infection in elderly patients with RA regardless of time of disease onset when compared with methotrexate monotherapy.
“Elderly-onset RA [EORA] patients are less likely to be treated with biologics than [are] patients with younger-onset RA [YORA]. This difference may be related to concerns about the risk of serious infections in elderly patients versus younger ones. There are only two papers on this subject in the literature, and now our study shows no increased risk. These findings support providing similar treatment for elderly versus younger-onset RA patients,” lead author Sofia Pedro of the National Data Bank for Rheumatic Diseases, Wichita, Kan., said at the annual meeting of the American College of Rheumatology.
The study was based on people with RA included in the National Data Bank for Rheumatic Diseases followed from 1998 through 2014. Patients filled out questionnaires on demographics, clinical factors, medications, and infections. A total of 1,865 EORA patients (onset older than 60 years) were matched 1:3 with 5,595 YORA patients (onset younger than 60 years) based on age, sex, and year of study entry.
At baseline, all patients were older than 60 years. The median age was between 65 and 70 years.
Serious infections were those that required hospitalization or intravenous antibiotics or caused death. Serious infectious were validated from hospital, physician, and death records. Overall, a total of 1,196 serious infections were reported during the study: 204 (11%) in the EORA group and 992 (18%) in YORA patients. Pneumonia, skin, and sepsis were the most common infections in both groups. Pneumonia was reported in 55% of EORA patients and 66% of the YORA group; skin infections, in 22% vs. 14%, respectively; and sepsis in 16% vs. 17%, respectively. The rate of self-reported prior infections was 4% in EORA patients and 7% in YORA patients (P less than .01). Overall, EORA patients were no quicker to have a first infection or multiple infections than were YORA patients after adjustments for Comorbidity Index, Health Assessment Questionnaire (HAQ), headache, pain, education, ethnicity, prednisone use, and urban versus rural setting.
Treatments were grouped according to noncytotoxic DMARDS; cytotoxic DMARDs; anti-TNF biologics; and non-TNF biologics. Methotrexate monotherapy was the referent. The hazard ratios for time to first infection and time to multiple infections were 0.72 and 0.82, respectively, for non-cytotoxic DMARDs and 0.43 and 0.48, respectively, for non-TNF biologics, all of which were statistically significant after adjustment for confounders.
EORA patients, compared with YORA patients, had greater exposure to prednisone and fewer prior serious infections, shorter disease duration (4.4 years vs. 20.9 years, respectively), lower Comorbidity Index, and more favorable disease markers. YORA patients had greater exposure to TNF biologics.
YORA patients had slightly higher incidence rates across all age groups, except between the ages of 60-70 years, when more infections were reported in EORA patients. The risk for multiple infections was greater in patients aged 80 years and older, but Ms. Pedro noted that covariates, such as HAQ, Comorbidity Index, prior serious infection, and exposure to prednisone, were associated with increased risk.
Ms. Pedro had no financial disclosures.
SAN FRANCISCO – Biologics do not pose an increased risk of infection in patients with elderly-onset rheumatoid arthritis over the age of 60 years because their time to first infection and time to multiple infections were not different from people of the same age who developed the disease at a younger age in a large, prospective, longitudinal, observational study .
The data also suggest that noncytotoxic disease-modifying antirheumatic drugs (DMARDs) and non-TNF biologics may have a protective effect against infection in elderly patients with RA regardless of time of disease onset when compared with methotrexate monotherapy.
“Elderly-onset RA [EORA] patients are less likely to be treated with biologics than [are] patients with younger-onset RA [YORA]. This difference may be related to concerns about the risk of serious infections in elderly patients versus younger ones. There are only two papers on this subject in the literature, and now our study shows no increased risk. These findings support providing similar treatment for elderly versus younger-onset RA patients,” lead author Sofia Pedro of the National Data Bank for Rheumatic Diseases, Wichita, Kan., said at the annual meeting of the American College of Rheumatology.
The study was based on people with RA included in the National Data Bank for Rheumatic Diseases followed from 1998 through 2014. Patients filled out questionnaires on demographics, clinical factors, medications, and infections. A total of 1,865 EORA patients (onset older than 60 years) were matched 1:3 with 5,595 YORA patients (onset younger than 60 years) based on age, sex, and year of study entry.
At baseline, all patients were older than 60 years. The median age was between 65 and 70 years.
Serious infections were those that required hospitalization or intravenous antibiotics or caused death. Serious infectious were validated from hospital, physician, and death records. Overall, a total of 1,196 serious infections were reported during the study: 204 (11%) in the EORA group and 992 (18%) in YORA patients. Pneumonia, skin, and sepsis were the most common infections in both groups. Pneumonia was reported in 55% of EORA patients and 66% of the YORA group; skin infections, in 22% vs. 14%, respectively; and sepsis in 16% vs. 17%, respectively. The rate of self-reported prior infections was 4% in EORA patients and 7% in YORA patients (P less than .01). Overall, EORA patients were no quicker to have a first infection or multiple infections than were YORA patients after adjustments for Comorbidity Index, Health Assessment Questionnaire (HAQ), headache, pain, education, ethnicity, prednisone use, and urban versus rural setting.
Treatments were grouped according to noncytotoxic DMARDS; cytotoxic DMARDs; anti-TNF biologics; and non-TNF biologics. Methotrexate monotherapy was the referent. The hazard ratios for time to first infection and time to multiple infections were 0.72 and 0.82, respectively, for non-cytotoxic DMARDs and 0.43 and 0.48, respectively, for non-TNF biologics, all of which were statistically significant after adjustment for confounders.
EORA patients, compared with YORA patients, had greater exposure to prednisone and fewer prior serious infections, shorter disease duration (4.4 years vs. 20.9 years, respectively), lower Comorbidity Index, and more favorable disease markers. YORA patients had greater exposure to TNF biologics.
YORA patients had slightly higher incidence rates across all age groups, except between the ages of 60-70 years, when more infections were reported in EORA patients. The risk for multiple infections was greater in patients aged 80 years and older, but Ms. Pedro noted that covariates, such as HAQ, Comorbidity Index, prior serious infection, and exposure to prednisone, were associated with increased risk.
Ms. Pedro had no financial disclosures.
SAN FRANCISCO – Biologics do not pose an increased risk of infection in patients with elderly-onset rheumatoid arthritis over the age of 60 years because their time to first infection and time to multiple infections were not different from people of the same age who developed the disease at a younger age in a large, prospective, longitudinal, observational study .
The data also suggest that noncytotoxic disease-modifying antirheumatic drugs (DMARDs) and non-TNF biologics may have a protective effect against infection in elderly patients with RA regardless of time of disease onset when compared with methotrexate monotherapy.
“Elderly-onset RA [EORA] patients are less likely to be treated with biologics than [are] patients with younger-onset RA [YORA]. This difference may be related to concerns about the risk of serious infections in elderly patients versus younger ones. There are only two papers on this subject in the literature, and now our study shows no increased risk. These findings support providing similar treatment for elderly versus younger-onset RA patients,” lead author Sofia Pedro of the National Data Bank for Rheumatic Diseases, Wichita, Kan., said at the annual meeting of the American College of Rheumatology.
The study was based on people with RA included in the National Data Bank for Rheumatic Diseases followed from 1998 through 2014. Patients filled out questionnaires on demographics, clinical factors, medications, and infections. A total of 1,865 EORA patients (onset older than 60 years) were matched 1:3 with 5,595 YORA patients (onset younger than 60 years) based on age, sex, and year of study entry.
At baseline, all patients were older than 60 years. The median age was between 65 and 70 years.
Serious infections were those that required hospitalization or intravenous antibiotics or caused death. Serious infectious were validated from hospital, physician, and death records. Overall, a total of 1,196 serious infections were reported during the study: 204 (11%) in the EORA group and 992 (18%) in YORA patients. Pneumonia, skin, and sepsis were the most common infections in both groups. Pneumonia was reported in 55% of EORA patients and 66% of the YORA group; skin infections, in 22% vs. 14%, respectively; and sepsis in 16% vs. 17%, respectively. The rate of self-reported prior infections was 4% in EORA patients and 7% in YORA patients (P less than .01). Overall, EORA patients were no quicker to have a first infection or multiple infections than were YORA patients after adjustments for Comorbidity Index, Health Assessment Questionnaire (HAQ), headache, pain, education, ethnicity, prednisone use, and urban versus rural setting.
Treatments were grouped according to noncytotoxic DMARDS; cytotoxic DMARDs; anti-TNF biologics; and non-TNF biologics. Methotrexate monotherapy was the referent. The hazard ratios for time to first infection and time to multiple infections were 0.72 and 0.82, respectively, for non-cytotoxic DMARDs and 0.43 and 0.48, respectively, for non-TNF biologics, all of which were statistically significant after adjustment for confounders.
EORA patients, compared with YORA patients, had greater exposure to prednisone and fewer prior serious infections, shorter disease duration (4.4 years vs. 20.9 years, respectively), lower Comorbidity Index, and more favorable disease markers. YORA patients had greater exposure to TNF biologics.
YORA patients had slightly higher incidence rates across all age groups, except between the ages of 60-70 years, when more infections were reported in EORA patients. The risk for multiple infections was greater in patients aged 80 years and older, but Ms. Pedro noted that covariates, such as HAQ, Comorbidity Index, prior serious infection, and exposure to prednisone, were associated with increased risk.
Ms. Pedro had no financial disclosures.
AT THE ACR ANNUAL MEETING
Key clinical point: Patients with older onset RA can be safely treated with biologics.
Major finding: There was no significant difference in serious infections between older onset and younger onset RA patients.
Data source: A prospective, longitudinal observational study of 7,460 patients with RA followed during 1998-2014 in the National Data Bank for Rheumatic Diseases.
Disclosures: The presenter had no financial disclosures.
State laws, regulatory concerns complicate biosimilars landscape
SAN FRANCISCO – A growing number of states are passing laws that set the parameters for how originator biologics may be replaced with biosimilars, and not all of them are helpful to physicians trying to maintain some knowledge and control over how prescriptions for biologics are dispensed, according to speakers at the annual meeting of the American College of Rheumatology.
These laws – now passed by a total of 19 states and Puerto Rico, including 12 in 2015 – address a requirement built into the Biologics Price Competition and Innovation Act of 2009 (part of the Affordable Care Act) that introduced the term “interchangeability” into the biosimilars approval pathway, stating that an approved biosimilar “may be substituted for the reference product without the intervention of the health care provider who prescribed the reference product.” The law also gives 1 year of exclusive marketing rights to the first biosimilar approved as being interchangeable with the reference product.
Although regulations on how the Food and Drug Administration will deem a biosimilar to be interchangeable have yet to be drafted, it’s important for physicians to play an active role in shaping the laws that address interchangeability, said Dr. J. Eugene Huffstutter, a rheumatologist in private practice in Hixson, Tenn., and clinical assistant professor of medicine at the University of Tennessee, Chattanooga.
Dr. Huffstutter encouraged rheumatologists and other physicians to work with their local state medical associations to contact their state legislators to explain to them the need for strict laws on how biosimilars can be dispensed. He suggested that good state legislation on biosimilars should contain provisions stating that:
• Only FDA-approved interchangeable biosimilars can be substituted.
• No substitution can be made with “dispense as written” prescription.
• The prescribing physician must be notified of substitution within 1-5 days by electronic record or fax.
• The pharmacy must maintain a record of the dispensed drug.
Organizations including the ACR, the Coalition of State Rheumatology Organizations, patient groups, electronic prescribers, state medical societies, and pharmaceutical companies (for the most part) support strong state laws on biosimilars, while the pharmacy lobby and insurance companies have opposed them, Dr. Huffstutter said.
The 19 states with biosimilars laws include California, Colorado, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Louisiana, Massachusetts, New Jersey, North Carolina, North Dakota, Oregon, Tennessee, Texas, Utah, Virginia, and Washington, along with Puerto Rico. However, the laws in Oregon and Virginia will sunset in 2016. Another seven states have 2015 or current session legislation on biosimilar substitution: Hawaii, Maryland, Michigan, Mississippi, Oklahoma, Pennsylvania, and Vermont. States with bills that failed or were not acted upon at adjournment include Arizona, Arkansas, Nevada, and Rhode Island, according to information provided to Dr. Huffstutter by the Coalition.
State laws may differ on substitution notification, he noted. In Tennessee, the law (H.B. 572) states that notification should occur in “a reasonable period of time” instead of a defined period. “What may be reasonable for one person may not be reasonable for another. I really think 5 days is the outside time, or 5 business days, because if you’re thinking about some of the biosimilars that are coming down the pike, they’ll be given on a weekly basis, so you’ll at least know before [patients] get their second shot what they’re receiving,” Dr. Huffstutter noted.
Idaho is unique in that it impaneled a board of pharmacy regulation on biosimilar substitution rather than a separate law. It states that “A pharmacist may substitute an interchangeable biosimilar product for a prescribed biological product if the biosimilar has been determined by the FDA to be interchangeable and published in the Purple Book; the prescriber does not indicate by any means that the prescribed biological product must be dispensed; and the name of the drug and the manufacturer or the NDC [National Drug Code] number is documented in the patient medical record.”
The situation may become trickier as more biosimilars are approved, noted Dr. Huffstutter, who is a member of the ACR’s Government Affairs Committee and is a liaison to the Committee on Rheumatologic Care. It could potentially be a problem when a patient is taking a biosimilar for a particular originator biologic and then other biosimilars for that originator join the marketplace. A patient could potentially be switched from one biosimilar to another when a prescription is filled if the company marketing the second biosimilar happens to win a competitive bid with an insurance company, he said.
The filgrastim biosimilar called Zarxio is the only biosimilar currently approved in the United States, but the FDA has received two applications for biosimilars for inflammatory diseases: one from Celltrion for infliximab biosimilar Remsima (August 2014) and one from Sandoz for an etanercept biosimilar (October 2015). In addition to two infliximab biosimilars that have been approved in other countries, one etanercept biosimilar that has been approved in South Korea, and one adalimumab biosimilar approved in India, there are many others in development to treat inflammatory diseases as of July 2015, including 12 for adalimumab, 9 for etanercept, 5 for infliximab, 2 for tocilizumab, and 7 for rituximab, according to Dr. Jonathan Kay, professor of medicine and director of clinical research in the division of rheumatology at the University of Massachusetts, Worcester.
Unresolved regulatory concerns
In separate presentations at the meeting, Dr. Kay described many of the nuances that define biosimilars, which go by different terminology in other countries, such as “follow-on biologic” in Japan, “subsequent-entry biological” in Canada, and “similar biological medicinal product” in the European Union. They are defined as a legitimate copy of an off-patent biopharmaceutical that has undergone rigorous analytical comparison and clinical assessment, in comparison to its reference product, and are approved by a regulatory agency according to a specific pathway for biosimilar evaluation. These differ from biomimetics, which are developed as a replica of a biopharmaceutical but have not been developed, assessed, or approved according to regulatory guidelines for biosimilars.
The FDA takes a “totality of the evidence” approach to establish biosimilarity. The biosimilar pathway for approval is different from the originator pathway by relying more on analytical and preclinical studies and less on the clinical pharmacology and clinical trial data to support biosimilarity.
However, the agency has not yet published final guidance for all steps in demonstrating biosimilarity, particularly for clinical pharmacology data. Draft guidance says the biosimilar must be analyzed and assigned to an assessment grade: not similar, similar, highly similar, and highly similar with fingerprintlike similarity.
During production, both originator and biosimilar biopharmaceuticals can have protein-folding variants, misfolding, aggregation, enzymatic cleavage, and degradation that can lead to inactivation of the protein or increased immunogenicity. Even so, most biosimilars are not identical to the originator because of post-translational modifications, but they do not matter as long as they are not clinically meaningful. Even originator products can drift over time because small changes in manufacturing processes can lead to gradual changes in the molecule, Dr. Kay said.
Extrapolation of indications from the originator to a biosimilar is another area of concern, Dr. Kay said. The FDA will consider the extrapolation of data from a clinical trial of a biosimilar conducted in one disease to support approval for additional indications for which the originator product is already licensed, but not across indications with different mechanisms of action, such as between rheumatoid arthritis and non-Hodgkin’s lymphoma for rituximab. But for which inflammatory diseases should a biosimilar be studied to provide adequate information for extrapolation of indications? Dr. Kay asked. This situation brings about questions of whether dermatologists would be comfortable using a biosimilar that has been studied for rheumatoid arthritis to treat psoriasis or gastroenterologists using a drug with data only from psoriatic arthritis to treat Crohn’s disease or ulcerative colitis.
Both the Coalition of State Rheumatology Organizations and the ACR oppose extrapolation of indications from the clinical trial data of a biosimilar in one disease to support approval of additional indications for which the originator product is already licensed, but Dr. Kay thought that “extrapolation of indications makes perfect sense” because it won’t be possible to review a biosimilar for indications not already approved for the originator and it’s natural to extrapolate to already approved indications once the protein is shown to function nearly identically to the originator.
Ideally, the nomenclature system for biosimilars should clearly identify each product to improve pharmacovigilance and to differentiate between products that have not been determined to be interchangeable, Dr. Kay said. In August, the FDA announced draft guidance proposing that all biopharmaceutical products (originator and biosimilars) would have a nonproprietary name that includes a suffix of four lowercase letters that is devoid of meaning. For example, all products that share a core name such as replicamab would be named replicamab-cznm, replicamab-hixf, and so on, Dr. Kay said. Some people have voiced concern that it would be better for names to have some meaning, such as using an abbreviated name for the developing company along with the nonproprietary name of the originator product, but others noted that method could be problematic when companies merge or are acquired by others.
Dr. Huffstutter disclosed relationships with Janssen, UCB, Lilly, Pfizer, Genentech, Bristol-Myers Squibb, and Celgene. Dr. Kay has received grant and research support from many pharmaceutical companies and has served as a consultant or on an advisory board for many pharmaceutical companies, including those developing biosimilars.
SAN FRANCISCO – A growing number of states are passing laws that set the parameters for how originator biologics may be replaced with biosimilars, and not all of them are helpful to physicians trying to maintain some knowledge and control over how prescriptions for biologics are dispensed, according to speakers at the annual meeting of the American College of Rheumatology.
These laws – now passed by a total of 19 states and Puerto Rico, including 12 in 2015 – address a requirement built into the Biologics Price Competition and Innovation Act of 2009 (part of the Affordable Care Act) that introduced the term “interchangeability” into the biosimilars approval pathway, stating that an approved biosimilar “may be substituted for the reference product without the intervention of the health care provider who prescribed the reference product.” The law also gives 1 year of exclusive marketing rights to the first biosimilar approved as being interchangeable with the reference product.
Although regulations on how the Food and Drug Administration will deem a biosimilar to be interchangeable have yet to be drafted, it’s important for physicians to play an active role in shaping the laws that address interchangeability, said Dr. J. Eugene Huffstutter, a rheumatologist in private practice in Hixson, Tenn., and clinical assistant professor of medicine at the University of Tennessee, Chattanooga.
Dr. Huffstutter encouraged rheumatologists and other physicians to work with their local state medical associations to contact their state legislators to explain to them the need for strict laws on how biosimilars can be dispensed. He suggested that good state legislation on biosimilars should contain provisions stating that:
• Only FDA-approved interchangeable biosimilars can be substituted.
• No substitution can be made with “dispense as written” prescription.
• The prescribing physician must be notified of substitution within 1-5 days by electronic record or fax.
• The pharmacy must maintain a record of the dispensed drug.
Organizations including the ACR, the Coalition of State Rheumatology Organizations, patient groups, electronic prescribers, state medical societies, and pharmaceutical companies (for the most part) support strong state laws on biosimilars, while the pharmacy lobby and insurance companies have opposed them, Dr. Huffstutter said.
The 19 states with biosimilars laws include California, Colorado, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Louisiana, Massachusetts, New Jersey, North Carolina, North Dakota, Oregon, Tennessee, Texas, Utah, Virginia, and Washington, along with Puerto Rico. However, the laws in Oregon and Virginia will sunset in 2016. Another seven states have 2015 or current session legislation on biosimilar substitution: Hawaii, Maryland, Michigan, Mississippi, Oklahoma, Pennsylvania, and Vermont. States with bills that failed or were not acted upon at adjournment include Arizona, Arkansas, Nevada, and Rhode Island, according to information provided to Dr. Huffstutter by the Coalition.
State laws may differ on substitution notification, he noted. In Tennessee, the law (H.B. 572) states that notification should occur in “a reasonable period of time” instead of a defined period. “What may be reasonable for one person may not be reasonable for another. I really think 5 days is the outside time, or 5 business days, because if you’re thinking about some of the biosimilars that are coming down the pike, they’ll be given on a weekly basis, so you’ll at least know before [patients] get their second shot what they’re receiving,” Dr. Huffstutter noted.
Idaho is unique in that it impaneled a board of pharmacy regulation on biosimilar substitution rather than a separate law. It states that “A pharmacist may substitute an interchangeable biosimilar product for a prescribed biological product if the biosimilar has been determined by the FDA to be interchangeable and published in the Purple Book; the prescriber does not indicate by any means that the prescribed biological product must be dispensed; and the name of the drug and the manufacturer or the NDC [National Drug Code] number is documented in the patient medical record.”
The situation may become trickier as more biosimilars are approved, noted Dr. Huffstutter, who is a member of the ACR’s Government Affairs Committee and is a liaison to the Committee on Rheumatologic Care. It could potentially be a problem when a patient is taking a biosimilar for a particular originator biologic and then other biosimilars for that originator join the marketplace. A patient could potentially be switched from one biosimilar to another when a prescription is filled if the company marketing the second biosimilar happens to win a competitive bid with an insurance company, he said.
The filgrastim biosimilar called Zarxio is the only biosimilar currently approved in the United States, but the FDA has received two applications for biosimilars for inflammatory diseases: one from Celltrion for infliximab biosimilar Remsima (August 2014) and one from Sandoz for an etanercept biosimilar (October 2015). In addition to two infliximab biosimilars that have been approved in other countries, one etanercept biosimilar that has been approved in South Korea, and one adalimumab biosimilar approved in India, there are many others in development to treat inflammatory diseases as of July 2015, including 12 for adalimumab, 9 for etanercept, 5 for infliximab, 2 for tocilizumab, and 7 for rituximab, according to Dr. Jonathan Kay, professor of medicine and director of clinical research in the division of rheumatology at the University of Massachusetts, Worcester.
Unresolved regulatory concerns
In separate presentations at the meeting, Dr. Kay described many of the nuances that define biosimilars, which go by different terminology in other countries, such as “follow-on biologic” in Japan, “subsequent-entry biological” in Canada, and “similar biological medicinal product” in the European Union. They are defined as a legitimate copy of an off-patent biopharmaceutical that has undergone rigorous analytical comparison and clinical assessment, in comparison to its reference product, and are approved by a regulatory agency according to a specific pathway for biosimilar evaluation. These differ from biomimetics, which are developed as a replica of a biopharmaceutical but have not been developed, assessed, or approved according to regulatory guidelines for biosimilars.
The FDA takes a “totality of the evidence” approach to establish biosimilarity. The biosimilar pathway for approval is different from the originator pathway by relying more on analytical and preclinical studies and less on the clinical pharmacology and clinical trial data to support biosimilarity.
However, the agency has not yet published final guidance for all steps in demonstrating biosimilarity, particularly for clinical pharmacology data. Draft guidance says the biosimilar must be analyzed and assigned to an assessment grade: not similar, similar, highly similar, and highly similar with fingerprintlike similarity.
During production, both originator and biosimilar biopharmaceuticals can have protein-folding variants, misfolding, aggregation, enzymatic cleavage, and degradation that can lead to inactivation of the protein or increased immunogenicity. Even so, most biosimilars are not identical to the originator because of post-translational modifications, but they do not matter as long as they are not clinically meaningful. Even originator products can drift over time because small changes in manufacturing processes can lead to gradual changes in the molecule, Dr. Kay said.
Extrapolation of indications from the originator to a biosimilar is another area of concern, Dr. Kay said. The FDA will consider the extrapolation of data from a clinical trial of a biosimilar conducted in one disease to support approval for additional indications for which the originator product is already licensed, but not across indications with different mechanisms of action, such as between rheumatoid arthritis and non-Hodgkin’s lymphoma for rituximab. But for which inflammatory diseases should a biosimilar be studied to provide adequate information for extrapolation of indications? Dr. Kay asked. This situation brings about questions of whether dermatologists would be comfortable using a biosimilar that has been studied for rheumatoid arthritis to treat psoriasis or gastroenterologists using a drug with data only from psoriatic arthritis to treat Crohn’s disease or ulcerative colitis.
Both the Coalition of State Rheumatology Organizations and the ACR oppose extrapolation of indications from the clinical trial data of a biosimilar in one disease to support approval of additional indications for which the originator product is already licensed, but Dr. Kay thought that “extrapolation of indications makes perfect sense” because it won’t be possible to review a biosimilar for indications not already approved for the originator and it’s natural to extrapolate to already approved indications once the protein is shown to function nearly identically to the originator.
Ideally, the nomenclature system for biosimilars should clearly identify each product to improve pharmacovigilance and to differentiate between products that have not been determined to be interchangeable, Dr. Kay said. In August, the FDA announced draft guidance proposing that all biopharmaceutical products (originator and biosimilars) would have a nonproprietary name that includes a suffix of four lowercase letters that is devoid of meaning. For example, all products that share a core name such as replicamab would be named replicamab-cznm, replicamab-hixf, and so on, Dr. Kay said. Some people have voiced concern that it would be better for names to have some meaning, such as using an abbreviated name for the developing company along with the nonproprietary name of the originator product, but others noted that method could be problematic when companies merge or are acquired by others.
Dr. Huffstutter disclosed relationships with Janssen, UCB, Lilly, Pfizer, Genentech, Bristol-Myers Squibb, and Celgene. Dr. Kay has received grant and research support from many pharmaceutical companies and has served as a consultant or on an advisory board for many pharmaceutical companies, including those developing biosimilars.
SAN FRANCISCO – A growing number of states are passing laws that set the parameters for how originator biologics may be replaced with biosimilars, and not all of them are helpful to physicians trying to maintain some knowledge and control over how prescriptions for biologics are dispensed, according to speakers at the annual meeting of the American College of Rheumatology.
These laws – now passed by a total of 19 states and Puerto Rico, including 12 in 2015 – address a requirement built into the Biologics Price Competition and Innovation Act of 2009 (part of the Affordable Care Act) that introduced the term “interchangeability” into the biosimilars approval pathway, stating that an approved biosimilar “may be substituted for the reference product without the intervention of the health care provider who prescribed the reference product.” The law also gives 1 year of exclusive marketing rights to the first biosimilar approved as being interchangeable with the reference product.
Although regulations on how the Food and Drug Administration will deem a biosimilar to be interchangeable have yet to be drafted, it’s important for physicians to play an active role in shaping the laws that address interchangeability, said Dr. J. Eugene Huffstutter, a rheumatologist in private practice in Hixson, Tenn., and clinical assistant professor of medicine at the University of Tennessee, Chattanooga.
Dr. Huffstutter encouraged rheumatologists and other physicians to work with their local state medical associations to contact their state legislators to explain to them the need for strict laws on how biosimilars can be dispensed. He suggested that good state legislation on biosimilars should contain provisions stating that:
• Only FDA-approved interchangeable biosimilars can be substituted.
• No substitution can be made with “dispense as written” prescription.
• The prescribing physician must be notified of substitution within 1-5 days by electronic record or fax.
• The pharmacy must maintain a record of the dispensed drug.
Organizations including the ACR, the Coalition of State Rheumatology Organizations, patient groups, electronic prescribers, state medical societies, and pharmaceutical companies (for the most part) support strong state laws on biosimilars, while the pharmacy lobby and insurance companies have opposed them, Dr. Huffstutter said.
The 19 states with biosimilars laws include California, Colorado, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Louisiana, Massachusetts, New Jersey, North Carolina, North Dakota, Oregon, Tennessee, Texas, Utah, Virginia, and Washington, along with Puerto Rico. However, the laws in Oregon and Virginia will sunset in 2016. Another seven states have 2015 or current session legislation on biosimilar substitution: Hawaii, Maryland, Michigan, Mississippi, Oklahoma, Pennsylvania, and Vermont. States with bills that failed or were not acted upon at adjournment include Arizona, Arkansas, Nevada, and Rhode Island, according to information provided to Dr. Huffstutter by the Coalition.
State laws may differ on substitution notification, he noted. In Tennessee, the law (H.B. 572) states that notification should occur in “a reasonable period of time” instead of a defined period. “What may be reasonable for one person may not be reasonable for another. I really think 5 days is the outside time, or 5 business days, because if you’re thinking about some of the biosimilars that are coming down the pike, they’ll be given on a weekly basis, so you’ll at least know before [patients] get their second shot what they’re receiving,” Dr. Huffstutter noted.
Idaho is unique in that it impaneled a board of pharmacy regulation on biosimilar substitution rather than a separate law. It states that “A pharmacist may substitute an interchangeable biosimilar product for a prescribed biological product if the biosimilar has been determined by the FDA to be interchangeable and published in the Purple Book; the prescriber does not indicate by any means that the prescribed biological product must be dispensed; and the name of the drug and the manufacturer or the NDC [National Drug Code] number is documented in the patient medical record.”
The situation may become trickier as more biosimilars are approved, noted Dr. Huffstutter, who is a member of the ACR’s Government Affairs Committee and is a liaison to the Committee on Rheumatologic Care. It could potentially be a problem when a patient is taking a biosimilar for a particular originator biologic and then other biosimilars for that originator join the marketplace. A patient could potentially be switched from one biosimilar to another when a prescription is filled if the company marketing the second biosimilar happens to win a competitive bid with an insurance company, he said.
The filgrastim biosimilar called Zarxio is the only biosimilar currently approved in the United States, but the FDA has received two applications for biosimilars for inflammatory diseases: one from Celltrion for infliximab biosimilar Remsima (August 2014) and one from Sandoz for an etanercept biosimilar (October 2015). In addition to two infliximab biosimilars that have been approved in other countries, one etanercept biosimilar that has been approved in South Korea, and one adalimumab biosimilar approved in India, there are many others in development to treat inflammatory diseases as of July 2015, including 12 for adalimumab, 9 for etanercept, 5 for infliximab, 2 for tocilizumab, and 7 for rituximab, according to Dr. Jonathan Kay, professor of medicine and director of clinical research in the division of rheumatology at the University of Massachusetts, Worcester.
Unresolved regulatory concerns
In separate presentations at the meeting, Dr. Kay described many of the nuances that define biosimilars, which go by different terminology in other countries, such as “follow-on biologic” in Japan, “subsequent-entry biological” in Canada, and “similar biological medicinal product” in the European Union. They are defined as a legitimate copy of an off-patent biopharmaceutical that has undergone rigorous analytical comparison and clinical assessment, in comparison to its reference product, and are approved by a regulatory agency according to a specific pathway for biosimilar evaluation. These differ from biomimetics, which are developed as a replica of a biopharmaceutical but have not been developed, assessed, or approved according to regulatory guidelines for biosimilars.
The FDA takes a “totality of the evidence” approach to establish biosimilarity. The biosimilar pathway for approval is different from the originator pathway by relying more on analytical and preclinical studies and less on the clinical pharmacology and clinical trial data to support biosimilarity.
However, the agency has not yet published final guidance for all steps in demonstrating biosimilarity, particularly for clinical pharmacology data. Draft guidance says the biosimilar must be analyzed and assigned to an assessment grade: not similar, similar, highly similar, and highly similar with fingerprintlike similarity.
During production, both originator and biosimilar biopharmaceuticals can have protein-folding variants, misfolding, aggregation, enzymatic cleavage, and degradation that can lead to inactivation of the protein or increased immunogenicity. Even so, most biosimilars are not identical to the originator because of post-translational modifications, but they do not matter as long as they are not clinically meaningful. Even originator products can drift over time because small changes in manufacturing processes can lead to gradual changes in the molecule, Dr. Kay said.
Extrapolation of indications from the originator to a biosimilar is another area of concern, Dr. Kay said. The FDA will consider the extrapolation of data from a clinical trial of a biosimilar conducted in one disease to support approval for additional indications for which the originator product is already licensed, but not across indications with different mechanisms of action, such as between rheumatoid arthritis and non-Hodgkin’s lymphoma for rituximab. But for which inflammatory diseases should a biosimilar be studied to provide adequate information for extrapolation of indications? Dr. Kay asked. This situation brings about questions of whether dermatologists would be comfortable using a biosimilar that has been studied for rheumatoid arthritis to treat psoriasis or gastroenterologists using a drug with data only from psoriatic arthritis to treat Crohn’s disease or ulcerative colitis.
Both the Coalition of State Rheumatology Organizations and the ACR oppose extrapolation of indications from the clinical trial data of a biosimilar in one disease to support approval of additional indications for which the originator product is already licensed, but Dr. Kay thought that “extrapolation of indications makes perfect sense” because it won’t be possible to review a biosimilar for indications not already approved for the originator and it’s natural to extrapolate to already approved indications once the protein is shown to function nearly identically to the originator.
Ideally, the nomenclature system for biosimilars should clearly identify each product to improve pharmacovigilance and to differentiate between products that have not been determined to be interchangeable, Dr. Kay said. In August, the FDA announced draft guidance proposing that all biopharmaceutical products (originator and biosimilars) would have a nonproprietary name that includes a suffix of four lowercase letters that is devoid of meaning. For example, all products that share a core name such as replicamab would be named replicamab-cznm, replicamab-hixf, and so on, Dr. Kay said. Some people have voiced concern that it would be better for names to have some meaning, such as using an abbreviated name for the developing company along with the nonproprietary name of the originator product, but others noted that method could be problematic when companies merge or are acquired by others.
Dr. Huffstutter disclosed relationships with Janssen, UCB, Lilly, Pfizer, Genentech, Bristol-Myers Squibb, and Celgene. Dr. Kay has received grant and research support from many pharmaceutical companies and has served as a consultant or on an advisory board for many pharmaceutical companies, including those developing biosimilars.
EXPERT ANALYSIS FROM THE ACR ANNUAL MEETING
ACR: Don’t give pneumococcal vaccine to CAPS, Behçet’s patients
SAN FRANCISCO – Pneumococcal vaccines can trigger severe local and systemic inflammatory reactions in patients with cryopyrin-associated periodic syndromes (CAPS), Behçet’s disease, and possibly other autoinflammatory disorders, according to a report from the annual meeting of the American College of Rheumatology.
The European League Against Rheumatism currently recommends pneumococcal vaccine in patients with inflammatory rheumatic diseases, and the Centers for Disease Control and Prevention recommends the vaccine in patients treated with immunosuppressive drugs.
“Careful consideration is warranted when implicating” these “guidelines in these patient populations. The risk of coming down with pneumococcal disease in these patients has to be balanced against the risk of severe reactions. We have here a 100% risk of unusually severe reactions in CAPS and Behçet’s patients with one shot, so we and others find it unethical to continue giving this vaccine to these patients. We no longer do this,” said investigator Dr. Ulrich Walker, a rheumatologist at Basel University in Switzerland and a member of the steering committee for the Ilaris registry, a Novartis database of CAPS patients treated with the company’s biologic canakinumab (Ilaris).
He and his colleagues reported on seven consecutive CAPS patients vaccinated with pneumococcal polysaccharide or conjugate vaccines according to current guidelines. Dr. Walker noted, however, that the Ilaris registry now has at least 14 CAPS patients who ran into serious trouble after receiving the vaccine.
“This is definitely real, and it’s not an adjuvant problem because not all pneumococcal vaccines contain adjuvants.” Instead, vaccine antigens trigger a flare. “The same thing happens with Behçet’s, where we’ve seen clear evidence of the triggering of underlying disease because patients had folliculitis around the vaccination site,” said Dr. Walker, who’s also published on that problem (Rheumatology [Oxford]. 2012 Apr;51[4]:761-2).
Within a few hours of vaccination, all seven patients developed severe, local injection site reactions and fever. Most received antibiotics, and two had to be hospitalized for systemic reactions. Patients ranged in age from 7 to 52 years old.
One patient, a 43-year-old woman, developed a severe headache associated with neck stiffness and photophobia. A florid red rash covered much of the arm where she got the shot. She was admitted to the hospital that evening with a presumed diagnosis of viral meningitis. Lumbar puncture revealed elevated white cells, normal glucose, and elevated protein. She was hospitalized for 18 days.
The reactions occurred with pneumococcal vaccines from three different companies and in patients with different CAPS phenotypes. Four vaccine reactions occurred in temporal association with concomitant coinjections of canakinumab; two reactions were separated by 15 days from the last canakinumab dose; and one reaction occurred in a patient who had never been exposed to canakinumab. Some patients had been vaccinated with a variety of other vaccines without any problem, so “this is something specific to pneumococcal vaccines,” Dr. Walker said.
Symptoms resolved in all the patients in 3-18 days.
The rapid onset, severity, and systemic nature of the reactions suggest that the vaccine triggers tau-like receptor activity, with subsequent inflammasome hyperactivation. “Patients with CAPS already have inflammasome constitutively turned on, so when you activate it even further you come down with a fever. The same thing happens with Behçet’s, which is also thought to be a multigenic disease associated with aberrant inflammasome activation,” Dr. Walker said.
The findings were so strong that the investigators are now testing low-dose pneumococcal vaccine for diagnostic screening before genetic testing for CAPs, Behçet’s, and some other autoimmune diseases. “A similar problem has been described in patients with mevalonate kinase deficiency,” Dr. Walker noted.
Novartis is worried the problem will be associated with canakinumab, but it doesn’t seem to be. “We had a lot of discussion about this and decided that we need to bring this out. Patients need to be aware of this,” said Dr. Jasmin Kümmerle-Deschner, a pediatrician at the University Hospital of Tübingen, Germany, and also a member of the Ilaris registry steering committee.
Dr. Walker disclosed financial relationships with Novartis, as did Dr. Kümmerle-Deschner, who also reported involvement with Sobi.
SAN FRANCISCO – Pneumococcal vaccines can trigger severe local and systemic inflammatory reactions in patients with cryopyrin-associated periodic syndromes (CAPS), Behçet’s disease, and possibly other autoinflammatory disorders, according to a report from the annual meeting of the American College of Rheumatology.
The European League Against Rheumatism currently recommends pneumococcal vaccine in patients with inflammatory rheumatic diseases, and the Centers for Disease Control and Prevention recommends the vaccine in patients treated with immunosuppressive drugs.
“Careful consideration is warranted when implicating” these “guidelines in these patient populations. The risk of coming down with pneumococcal disease in these patients has to be balanced against the risk of severe reactions. We have here a 100% risk of unusually severe reactions in CAPS and Behçet’s patients with one shot, so we and others find it unethical to continue giving this vaccine to these patients. We no longer do this,” said investigator Dr. Ulrich Walker, a rheumatologist at Basel University in Switzerland and a member of the steering committee for the Ilaris registry, a Novartis database of CAPS patients treated with the company’s biologic canakinumab (Ilaris).
He and his colleagues reported on seven consecutive CAPS patients vaccinated with pneumococcal polysaccharide or conjugate vaccines according to current guidelines. Dr. Walker noted, however, that the Ilaris registry now has at least 14 CAPS patients who ran into serious trouble after receiving the vaccine.
“This is definitely real, and it’s not an adjuvant problem because not all pneumococcal vaccines contain adjuvants.” Instead, vaccine antigens trigger a flare. “The same thing happens with Behçet’s, where we’ve seen clear evidence of the triggering of underlying disease because patients had folliculitis around the vaccination site,” said Dr. Walker, who’s also published on that problem (Rheumatology [Oxford]. 2012 Apr;51[4]:761-2).
Within a few hours of vaccination, all seven patients developed severe, local injection site reactions and fever. Most received antibiotics, and two had to be hospitalized for systemic reactions. Patients ranged in age from 7 to 52 years old.
One patient, a 43-year-old woman, developed a severe headache associated with neck stiffness and photophobia. A florid red rash covered much of the arm where she got the shot. She was admitted to the hospital that evening with a presumed diagnosis of viral meningitis. Lumbar puncture revealed elevated white cells, normal glucose, and elevated protein. She was hospitalized for 18 days.
The reactions occurred with pneumococcal vaccines from three different companies and in patients with different CAPS phenotypes. Four vaccine reactions occurred in temporal association with concomitant coinjections of canakinumab; two reactions were separated by 15 days from the last canakinumab dose; and one reaction occurred in a patient who had never been exposed to canakinumab. Some patients had been vaccinated with a variety of other vaccines without any problem, so “this is something specific to pneumococcal vaccines,” Dr. Walker said.
Symptoms resolved in all the patients in 3-18 days.
The rapid onset, severity, and systemic nature of the reactions suggest that the vaccine triggers tau-like receptor activity, with subsequent inflammasome hyperactivation. “Patients with CAPS already have inflammasome constitutively turned on, so when you activate it even further you come down with a fever. The same thing happens with Behçet’s, which is also thought to be a multigenic disease associated with aberrant inflammasome activation,” Dr. Walker said.
The findings were so strong that the investigators are now testing low-dose pneumococcal vaccine for diagnostic screening before genetic testing for CAPs, Behçet’s, and some other autoimmune diseases. “A similar problem has been described in patients with mevalonate kinase deficiency,” Dr. Walker noted.
Novartis is worried the problem will be associated with canakinumab, but it doesn’t seem to be. “We had a lot of discussion about this and decided that we need to bring this out. Patients need to be aware of this,” said Dr. Jasmin Kümmerle-Deschner, a pediatrician at the University Hospital of Tübingen, Germany, and also a member of the Ilaris registry steering committee.
Dr. Walker disclosed financial relationships with Novartis, as did Dr. Kümmerle-Deschner, who also reported involvement with Sobi.
SAN FRANCISCO – Pneumococcal vaccines can trigger severe local and systemic inflammatory reactions in patients with cryopyrin-associated periodic syndromes (CAPS), Behçet’s disease, and possibly other autoinflammatory disorders, according to a report from the annual meeting of the American College of Rheumatology.
The European League Against Rheumatism currently recommends pneumococcal vaccine in patients with inflammatory rheumatic diseases, and the Centers for Disease Control and Prevention recommends the vaccine in patients treated with immunosuppressive drugs.
“Careful consideration is warranted when implicating” these “guidelines in these patient populations. The risk of coming down with pneumococcal disease in these patients has to be balanced against the risk of severe reactions. We have here a 100% risk of unusually severe reactions in CAPS and Behçet’s patients with one shot, so we and others find it unethical to continue giving this vaccine to these patients. We no longer do this,” said investigator Dr. Ulrich Walker, a rheumatologist at Basel University in Switzerland and a member of the steering committee for the Ilaris registry, a Novartis database of CAPS patients treated with the company’s biologic canakinumab (Ilaris).
He and his colleagues reported on seven consecutive CAPS patients vaccinated with pneumococcal polysaccharide or conjugate vaccines according to current guidelines. Dr. Walker noted, however, that the Ilaris registry now has at least 14 CAPS patients who ran into serious trouble after receiving the vaccine.
“This is definitely real, and it’s not an adjuvant problem because not all pneumococcal vaccines contain adjuvants.” Instead, vaccine antigens trigger a flare. “The same thing happens with Behçet’s, where we’ve seen clear evidence of the triggering of underlying disease because patients had folliculitis around the vaccination site,” said Dr. Walker, who’s also published on that problem (Rheumatology [Oxford]. 2012 Apr;51[4]:761-2).
Within a few hours of vaccination, all seven patients developed severe, local injection site reactions and fever. Most received antibiotics, and two had to be hospitalized for systemic reactions. Patients ranged in age from 7 to 52 years old.
One patient, a 43-year-old woman, developed a severe headache associated with neck stiffness and photophobia. A florid red rash covered much of the arm where she got the shot. She was admitted to the hospital that evening with a presumed diagnosis of viral meningitis. Lumbar puncture revealed elevated white cells, normal glucose, and elevated protein. She was hospitalized for 18 days.
The reactions occurred with pneumococcal vaccines from three different companies and in patients with different CAPS phenotypes. Four vaccine reactions occurred in temporal association with concomitant coinjections of canakinumab; two reactions were separated by 15 days from the last canakinumab dose; and one reaction occurred in a patient who had never been exposed to canakinumab. Some patients had been vaccinated with a variety of other vaccines without any problem, so “this is something specific to pneumococcal vaccines,” Dr. Walker said.
Symptoms resolved in all the patients in 3-18 days.
The rapid onset, severity, and systemic nature of the reactions suggest that the vaccine triggers tau-like receptor activity, with subsequent inflammasome hyperactivation. “Patients with CAPS already have inflammasome constitutively turned on, so when you activate it even further you come down with a fever. The same thing happens with Behçet’s, which is also thought to be a multigenic disease associated with aberrant inflammasome activation,” Dr. Walker said.
The findings were so strong that the investigators are now testing low-dose pneumococcal vaccine for diagnostic screening before genetic testing for CAPs, Behçet’s, and some other autoimmune diseases. “A similar problem has been described in patients with mevalonate kinase deficiency,” Dr. Walker noted.
Novartis is worried the problem will be associated with canakinumab, but it doesn’t seem to be. “We had a lot of discussion about this and decided that we need to bring this out. Patients need to be aware of this,” said Dr. Jasmin Kümmerle-Deschner, a pediatrician at the University Hospital of Tübingen, Germany, and also a member of the Ilaris registry steering committee.
Dr. Walker disclosed financial relationships with Novartis, as did Dr. Kümmerle-Deschner, who also reported involvement with Sobi.
AT THE ACR ANNUAL MEETING
Key clinical point: Pneumococcal vaccines trigger severe local and systemic inflammatory reactions in patients with CAPS, Behçet’s disease, and perhaps other autoinflammatory disorders.
Major finding: Pneumococcal vaccine triggered disease flares in 100% of the CAPS and Behçet’s disease patients who received it.
Data source: Small case series of patients vaccinated against pneumococcal disease.
Disclosures: The investigators disclosed relationships with Novartis and Sobi.