User login
Centers for Disease Control and Prevention (CDC): Advisory Committee on Immunization Practices (ACIP)
ACIP votes to drop preference for LAIV in healthy children aged 2-8 years
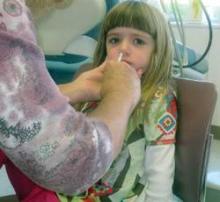
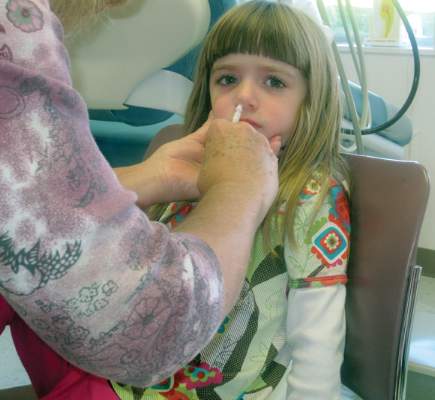
For the next influenza season, there will no longer be a preference for using the nasal spray influenza vaccine over the flu shot for healthy children aged 2-8 years, based on data from recent influenza seasons.
In a 14-0 vote, with one abstention, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) supported the following revision to the influenza vaccine recommendations, for the 2015-2016 influenza season: “For healthy children aged 2 through 8 years who have no contraindications or precautions, either LAIV [live attenuated influenza vaccine] or IIV [inactivated influenza vaccine] is an appropriate option. No preference is expressed for LAIV or IIV or any person aged 2 though 49 years for whom either vaccine is appropriate.”
The recommendation that all people aged 6 months and older should receive the influenza vaccine every year remains unchanged.
Currently, the recommendation is that when “immediately available, LAIV should be used for healthy children aged 2 through 8 years who have no contraindications or precautions,” which was approved in June 2014. This was based on evidence during several influenza seasons that LAIV provided better protection than the IIV for children in this age group. The decision to make the change regarding preference “was made based on new data from more recent seasons, which have not confirmed superior effectiveness of LAIV observed in earlier studies,” according to a statement issued by the CDC following the meeting.
For the next influenza season, there will no longer be a preference for using the nasal spray influenza vaccine over the flu shot for healthy children aged 2-8 years, based on data from recent influenza seasons.
In a 14-0 vote, with one abstention, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) supported the following revision to the influenza vaccine recommendations, for the 2015-2016 influenza season: “For healthy children aged 2 through 8 years who have no contraindications or precautions, either LAIV [live attenuated influenza vaccine] or IIV [inactivated influenza vaccine] is an appropriate option. No preference is expressed for LAIV or IIV or any person aged 2 though 49 years for whom either vaccine is appropriate.”
The recommendation that all people aged 6 months and older should receive the influenza vaccine every year remains unchanged.
Currently, the recommendation is that when “immediately available, LAIV should be used for healthy children aged 2 through 8 years who have no contraindications or precautions,” which was approved in June 2014. This was based on evidence during several influenza seasons that LAIV provided better protection than the IIV for children in this age group. The decision to make the change regarding preference “was made based on new data from more recent seasons, which have not confirmed superior effectiveness of LAIV observed in earlier studies,” according to a statement issued by the CDC following the meeting.
For the next influenza season, there will no longer be a preference for using the nasal spray influenza vaccine over the flu shot for healthy children aged 2-8 years, based on data from recent influenza seasons.
In a 14-0 vote, with one abstention, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) supported the following revision to the influenza vaccine recommendations, for the 2015-2016 influenza season: “For healthy children aged 2 through 8 years who have no contraindications or precautions, either LAIV [live attenuated influenza vaccine] or IIV [inactivated influenza vaccine] is an appropriate option. No preference is expressed for LAIV or IIV or any person aged 2 though 49 years for whom either vaccine is appropriate.”
The recommendation that all people aged 6 months and older should receive the influenza vaccine every year remains unchanged.
Currently, the recommendation is that when “immediately available, LAIV should be used for healthy children aged 2 through 8 years who have no contraindications or precautions,” which was approved in June 2014. This was based on evidence during several influenza seasons that LAIV provided better protection than the IIV for children in this age group. The decision to make the change regarding preference “was made based on new data from more recent seasons, which have not confirmed superior effectiveness of LAIV observed in earlier studies,” according to a statement issued by the CDC following the meeting.
FROM AN ACIP MEETING
ACIP Recommends Meningococcal B Vaccine During College Outbreaks
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices unanimously recommended serogroup B meningococcal (MenB) vaccination for several groups at increased risk for serogroup B disease, including students during outbreaks at college campuses, but is not addressing broader use of these vaccines in adolescents and college students until June 2015.
At a meeting of the Advisory Committee on Immunization Practices (ACIP) on Feb. 26, the panel voted 15-0 to recommend vaccination for the following groups of people over age 10 years at increased risk of serogroup B meningococcal disease: those with persistent complement deficiencies; anatomic or functional asplenia, including people with sickle cell; microbiologists routinely exposed to Neisseria meningitidis isolates; and individuals, such as college students, at increased risk during an outbreak of serogroup B meningococcal disease.
First-year college students living in residence halls were not discussed at this meeting. The ACIP work group on meningococcal vaccines also has been reviewing the available data and evidence for broader use of MenB vaccines in adolescents and college students separately, Jessica MacNeil of the CDC’s National Center for Immunization and Respiratory Diseases said at the meeting. Recommendations for more universal use of MenB vaccination in adolescents and college students will be discussed and voted on at the next ACIP meeting in June 2015.
Two vaccines are now licensed by the Food and Drug Administration for preventing meningococcal disease caused by N. meningitidis serogroup B in people aged 10-25 years in the United States: Trumenba (Pfizer), licensed in October 2014, and Bexsero (Novartis Vaccines and Diagnostics), in January 2015. Bexsero is already licensed in more than 30 countries for people aged 2 months and older. The currently recommended meningococcal vaccines cover four of the five main serogroups of N. meningitidis bacteria that cause meningococcal disease (A, C, W, and Y): they are the meningococcal polysaccharide vaccine (Menomune) and the meningococcal conjugate vaccine (Menactra and Menveo).
Based on the available data and evidence reviewed, Ms. MacNeil said that the work group supported routine vaccination of people at increased risk of meningococcal disease, based on the risk of disease in those groups and because they are included in the current recommendation for the meningitis A, C, W, Y conjugate vaccines. In addition, “there is demonstrated immune response to MenB in the general adolescent population … and there are no theoretical safety concerns for persons over 25 years of age, from vaccination, as compared to persons 10-25 years of age.”
Ms. MacNeil referred to the serogroup B clusters and outbreaks during the past several years on U.S. college campuses, noting that, in two of the recent outbreaks, “students were estimated to be at 200- to 1,400-fold increased risk for meningococcal disease during the outbreak period.”
In the first two months of 2015, there have already been two outbreaks of serogroup B meningococcal disease at U.S. college campuses: two cases at Providence College in Rhode Island, where students are being vaccinated with Trumenba; and four cases at the University of Oregon, including one death, where a mass vaccination campaign is being planned – the first doses are scheduled to be administered on March 2.
The threshold for vaccination during serogroup B outbreaks in institutional settings has been two cases in a population of under 5,000 people and three cases in a population of 5,000 or more people. Providence has 4,500 students and the University of Oregon has 25,000 students, Ms. MacNeil said. About 98% of the target population at Providence College has received one dose so far, she added.
During the public comments segment of the meeting, consumer advocates and parents of children who had died of meningitis B questioned why the discussion for routine use in college students and adolescents was being delayed, when the two vaccines had been approved.
“We think it would benefit even more if students came to campus protected,” said one of the public speakers, Dr. Mary Ferris, director of student health at the University of California, Santa Barbara, which had an outbreak of serogroup B disease in 2013. In November 2013, four cases at UCSB were diagnosed within 10 days, including a freshman athlete who survived but had two limbs amputated, she added.
Even one case in a college setting has a major impact on the community, Dr. Ferris said.
In response to the outbreak, more than 17,000 students at UCSB received the vaccine, which was not yet approved but was made available through an FDA investigational new drug application.
ACIP is composed of medical and public health experts who develop recommendations on how to use vaccines to control diseases in the United States. One of the members said that he received research funding from MedImmune; the other members had no disclosures.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices unanimously recommended serogroup B meningococcal (MenB) vaccination for several groups at increased risk for serogroup B disease, including students during outbreaks at college campuses, but is not addressing broader use of these vaccines in adolescents and college students until June 2015.
At a meeting of the Advisory Committee on Immunization Practices (ACIP) on Feb. 26, the panel voted 15-0 to recommend vaccination for the following groups of people over age 10 years at increased risk of serogroup B meningococcal disease: those with persistent complement deficiencies; anatomic or functional asplenia, including people with sickle cell; microbiologists routinely exposed to Neisseria meningitidis isolates; and individuals, such as college students, at increased risk during an outbreak of serogroup B meningococcal disease.
First-year college students living in residence halls were not discussed at this meeting. The ACIP work group on meningococcal vaccines also has been reviewing the available data and evidence for broader use of MenB vaccines in adolescents and college students separately, Jessica MacNeil of the CDC’s National Center for Immunization and Respiratory Diseases said at the meeting. Recommendations for more universal use of MenB vaccination in adolescents and college students will be discussed and voted on at the next ACIP meeting in June 2015.
Two vaccines are now licensed by the Food and Drug Administration for preventing meningococcal disease caused by N. meningitidis serogroup B in people aged 10-25 years in the United States: Trumenba (Pfizer), licensed in October 2014, and Bexsero (Novartis Vaccines and Diagnostics), in January 2015. Bexsero is already licensed in more than 30 countries for people aged 2 months and older. The currently recommended meningococcal vaccines cover four of the five main serogroups of N. meningitidis bacteria that cause meningococcal disease (A, C, W, and Y): they are the meningococcal polysaccharide vaccine (Menomune) and the meningococcal conjugate vaccine (Menactra and Menveo).
Based on the available data and evidence reviewed, Ms. MacNeil said that the work group supported routine vaccination of people at increased risk of meningococcal disease, based on the risk of disease in those groups and because they are included in the current recommendation for the meningitis A, C, W, Y conjugate vaccines. In addition, “there is demonstrated immune response to MenB in the general adolescent population … and there are no theoretical safety concerns for persons over 25 years of age, from vaccination, as compared to persons 10-25 years of age.”
Ms. MacNeil referred to the serogroup B clusters and outbreaks during the past several years on U.S. college campuses, noting that, in two of the recent outbreaks, “students were estimated to be at 200- to 1,400-fold increased risk for meningococcal disease during the outbreak period.”
In the first two months of 2015, there have already been two outbreaks of serogroup B meningococcal disease at U.S. college campuses: two cases at Providence College in Rhode Island, where students are being vaccinated with Trumenba; and four cases at the University of Oregon, including one death, where a mass vaccination campaign is being planned – the first doses are scheduled to be administered on March 2.
The threshold for vaccination during serogroup B outbreaks in institutional settings has been two cases in a population of under 5,000 people and three cases in a population of 5,000 or more people. Providence has 4,500 students and the University of Oregon has 25,000 students, Ms. MacNeil said. About 98% of the target population at Providence College has received one dose so far, she added.
During the public comments segment of the meeting, consumer advocates and parents of children who had died of meningitis B questioned why the discussion for routine use in college students and adolescents was being delayed, when the two vaccines had been approved.
“We think it would benefit even more if students came to campus protected,” said one of the public speakers, Dr. Mary Ferris, director of student health at the University of California, Santa Barbara, which had an outbreak of serogroup B disease in 2013. In November 2013, four cases at UCSB were diagnosed within 10 days, including a freshman athlete who survived but had two limbs amputated, she added.
Even one case in a college setting has a major impact on the community, Dr. Ferris said.
In response to the outbreak, more than 17,000 students at UCSB received the vaccine, which was not yet approved but was made available through an FDA investigational new drug application.
ACIP is composed of medical and public health experts who develop recommendations on how to use vaccines to control diseases in the United States. One of the members said that he received research funding from MedImmune; the other members had no disclosures.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices unanimously recommended serogroup B meningococcal (MenB) vaccination for several groups at increased risk for serogroup B disease, including students during outbreaks at college campuses, but is not addressing broader use of these vaccines in adolescents and college students until June 2015.
At a meeting of the Advisory Committee on Immunization Practices (ACIP) on Feb. 26, the panel voted 15-0 to recommend vaccination for the following groups of people over age 10 years at increased risk of serogroup B meningococcal disease: those with persistent complement deficiencies; anatomic or functional asplenia, including people with sickle cell; microbiologists routinely exposed to Neisseria meningitidis isolates; and individuals, such as college students, at increased risk during an outbreak of serogroup B meningococcal disease.
First-year college students living in residence halls were not discussed at this meeting. The ACIP work group on meningococcal vaccines also has been reviewing the available data and evidence for broader use of MenB vaccines in adolescents and college students separately, Jessica MacNeil of the CDC’s National Center for Immunization and Respiratory Diseases said at the meeting. Recommendations for more universal use of MenB vaccination in adolescents and college students will be discussed and voted on at the next ACIP meeting in June 2015.
Two vaccines are now licensed by the Food and Drug Administration for preventing meningococcal disease caused by N. meningitidis serogroup B in people aged 10-25 years in the United States: Trumenba (Pfizer), licensed in October 2014, and Bexsero (Novartis Vaccines and Diagnostics), in January 2015. Bexsero is already licensed in more than 30 countries for people aged 2 months and older. The currently recommended meningococcal vaccines cover four of the five main serogroups of N. meningitidis bacteria that cause meningococcal disease (A, C, W, and Y): they are the meningococcal polysaccharide vaccine (Menomune) and the meningococcal conjugate vaccine (Menactra and Menveo).
Based on the available data and evidence reviewed, Ms. MacNeil said that the work group supported routine vaccination of people at increased risk of meningococcal disease, based on the risk of disease in those groups and because they are included in the current recommendation for the meningitis A, C, W, Y conjugate vaccines. In addition, “there is demonstrated immune response to MenB in the general adolescent population … and there are no theoretical safety concerns for persons over 25 years of age, from vaccination, as compared to persons 10-25 years of age.”
Ms. MacNeil referred to the serogroup B clusters and outbreaks during the past several years on U.S. college campuses, noting that, in two of the recent outbreaks, “students were estimated to be at 200- to 1,400-fold increased risk for meningococcal disease during the outbreak period.”
In the first two months of 2015, there have already been two outbreaks of serogroup B meningococcal disease at U.S. college campuses: two cases at Providence College in Rhode Island, where students are being vaccinated with Trumenba; and four cases at the University of Oregon, including one death, where a mass vaccination campaign is being planned – the first doses are scheduled to be administered on March 2.
The threshold for vaccination during serogroup B outbreaks in institutional settings has been two cases in a population of under 5,000 people and three cases in a population of 5,000 or more people. Providence has 4,500 students and the University of Oregon has 25,000 students, Ms. MacNeil said. About 98% of the target population at Providence College has received one dose so far, she added.
During the public comments segment of the meeting, consumer advocates and parents of children who had died of meningitis B questioned why the discussion for routine use in college students and adolescents was being delayed, when the two vaccines had been approved.
“We think it would benefit even more if students came to campus protected,” said one of the public speakers, Dr. Mary Ferris, director of student health at the University of California, Santa Barbara, which had an outbreak of serogroup B disease in 2013. In November 2013, four cases at UCSB were diagnosed within 10 days, including a freshman athlete who survived but had two limbs amputated, she added.
Even one case in a college setting has a major impact on the community, Dr. Ferris said.
In response to the outbreak, more than 17,000 students at UCSB received the vaccine, which was not yet approved but was made available through an FDA investigational new drug application.
ACIP is composed of medical and public health experts who develop recommendations on how to use vaccines to control diseases in the United States. One of the members said that he received research funding from MedImmune; the other members had no disclosures.
FROM AN ACIP MEETING
ACIP recommends meningococcal B vaccine during college outbreaks
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices unanimously recommended serogroup B meningococcal (MenB) vaccination for several groups at increased risk for serogroup B disease, including students during outbreaks at college campuses, but is not addressing broader use of these vaccines in adolescents and college students until June 2015.
At a meeting of the Advisory Committee on Immunization Practices (ACIP) on Feb. 26, the panel voted 15-0 to recommend vaccination for the following groups of people over age 10 years at increased risk of serogroup B meningococcal disease: those with persistent complement deficiencies; anatomic or functional asplenia, including people with sickle cell; microbiologists routinely exposed to Neisseria meningitidis isolates; and individuals, such as college students, at increased risk during an outbreak of serogroup B meningococcal disease.
First-year college students living in residence halls were not discussed at this meeting. The ACIP work group on meningococcal vaccines also has been reviewing the available data and evidence for broader use of MenB vaccines in adolescents and college students separately, Jessica MacNeil of the CDC’s National Center for Immunization and Respiratory Diseases said at the meeting. Recommendations for more universal use of MenB vaccination in adolescents and college students will be discussed and voted on at the next ACIP meeting in June 2015.
Two vaccines are now licensed by the Food and Drug Administration for preventing meningococcal disease caused by N. meningitidis serogroup B in people aged 10-25 years in the United States: Trumenba (Pfizer), licensed in October 2014, and Bexsero (Novartis Vaccines and Diagnostics), in January 2015. Bexsero is already licensed in more than 30 countries for people aged 2 months and older. The currently recommended meningococcal vaccines cover four of the five main serogroups of N. meningitidis bacteria that cause meningococcal disease (A, C, W, and Y): they are the meningococcal polysaccharide vaccine (Menomune) and the meningococcal conjugate vaccine (Menactra and Menveo).
Based on the available data and evidence reviewed, Ms. MacNeil said that the work group supported routine vaccination of people at increased risk of meningococcal disease, based on the risk of disease in those groups and because they are included in the current recommendation for the meningitis A, C, W, Y conjugate vaccines. In addition, “there is demonstrated immune response to MenB in the general adolescent population … and there are no theoretical safety concerns for persons over 25 years of age, from vaccination, as compared to persons 10-25 years of age.”
Ms. MacNeil referred to the serogroup B clusters and outbreaks during the past several years on U.S. college campuses, noting that, in two of the recent outbreaks, “students were estimated to be at 200- to 1,400-fold increased risk for meningococcal disease during the outbreak period.”
In the first two months of 2015, there have already been two outbreaks of serogroup B meningococcal disease at U.S. college campuses: two cases at Providence College in Rhode Island, where students are being vaccinated with Trumenba; and four cases at the University of Oregon, including one death, where a mass vaccination campaign is being planned – the first doses are scheduled to be administered on March 2.
The threshold for vaccination during serogroup B outbreaks in institutional settings has been two cases in a population of under 5,000 people and three cases in a population of 5,000 or more people. Providence has 4,500 students and the University of Oregon has 25,000 students, Ms. MacNeil said. About 98% of the target population at Providence College has received one dose so far, she added.
During the public comments segment of the meeting, consumer advocates and parents of children who had died of meningitis B questioned why the discussion for routine use in college students and adolescents was being delayed, when the two vaccines had been approved.
“We think it would benefit even more if students came to campus protected,” said one of the public speakers, Dr. Mary Ferris, director of student health at the University of California, Santa Barbara, which had an outbreak of serogroup B disease in 2013. In November 2013, four cases at UCSB were diagnosed within 10 days, including a freshman athlete who survived but had two limbs amputated, she added.
Even one case in a college setting has a major impact on the community, Dr. Ferris said.
In response to the outbreak, more than 17,000 students at UCSB received the vaccine, which was not yet approved but was made available through an FDA investigational new drug application.
ACIP is composed of medical and public health experts who develop recommendations on how to use vaccines to control diseases in the United States. One of the members said that he received research funding from MedImmune; the other members had no disclosures.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices unanimously recommended serogroup B meningococcal (MenB) vaccination for several groups at increased risk for serogroup B disease, including students during outbreaks at college campuses, but is not addressing broader use of these vaccines in adolescents and college students until June 2015.
At a meeting of the Advisory Committee on Immunization Practices (ACIP) on Feb. 26, the panel voted 15-0 to recommend vaccination for the following groups of people over age 10 years at increased risk of serogroup B meningococcal disease: those with persistent complement deficiencies; anatomic or functional asplenia, including people with sickle cell; microbiologists routinely exposed to Neisseria meningitidis isolates; and individuals, such as college students, at increased risk during an outbreak of serogroup B meningococcal disease.
First-year college students living in residence halls were not discussed at this meeting. The ACIP work group on meningococcal vaccines also has been reviewing the available data and evidence for broader use of MenB vaccines in adolescents and college students separately, Jessica MacNeil of the CDC’s National Center for Immunization and Respiratory Diseases said at the meeting. Recommendations for more universal use of MenB vaccination in adolescents and college students will be discussed and voted on at the next ACIP meeting in June 2015.
Two vaccines are now licensed by the Food and Drug Administration for preventing meningococcal disease caused by N. meningitidis serogroup B in people aged 10-25 years in the United States: Trumenba (Pfizer), licensed in October 2014, and Bexsero (Novartis Vaccines and Diagnostics), in January 2015. Bexsero is already licensed in more than 30 countries for people aged 2 months and older. The currently recommended meningococcal vaccines cover four of the five main serogroups of N. meningitidis bacteria that cause meningococcal disease (A, C, W, and Y): they are the meningococcal polysaccharide vaccine (Menomune) and the meningococcal conjugate vaccine (Menactra and Menveo).
Based on the available data and evidence reviewed, Ms. MacNeil said that the work group supported routine vaccination of people at increased risk of meningococcal disease, based on the risk of disease in those groups and because they are included in the current recommendation for the meningitis A, C, W, Y conjugate vaccines. In addition, “there is demonstrated immune response to MenB in the general adolescent population … and there are no theoretical safety concerns for persons over 25 years of age, from vaccination, as compared to persons 10-25 years of age.”
Ms. MacNeil referred to the serogroup B clusters and outbreaks during the past several years on U.S. college campuses, noting that, in two of the recent outbreaks, “students were estimated to be at 200- to 1,400-fold increased risk for meningococcal disease during the outbreak period.”
In the first two months of 2015, there have already been two outbreaks of serogroup B meningococcal disease at U.S. college campuses: two cases at Providence College in Rhode Island, where students are being vaccinated with Trumenba; and four cases at the University of Oregon, including one death, where a mass vaccination campaign is being planned – the first doses are scheduled to be administered on March 2.
The threshold for vaccination during serogroup B outbreaks in institutional settings has been two cases in a population of under 5,000 people and three cases in a population of 5,000 or more people. Providence has 4,500 students and the University of Oregon has 25,000 students, Ms. MacNeil said. About 98% of the target population at Providence College has received one dose so far, she added.
During the public comments segment of the meeting, consumer advocates and parents of children who had died of meningitis B questioned why the discussion for routine use in college students and adolescents was being delayed, when the two vaccines had been approved.
“We think it would benefit even more if students came to campus protected,” said one of the public speakers, Dr. Mary Ferris, director of student health at the University of California, Santa Barbara, which had an outbreak of serogroup B disease in 2013. In November 2013, four cases at UCSB were diagnosed within 10 days, including a freshman athlete who survived but had two limbs amputated, she added.
Even one case in a college setting has a major impact on the community, Dr. Ferris said.
In response to the outbreak, more than 17,000 students at UCSB received the vaccine, which was not yet approved but was made available through an FDA investigational new drug application.
ACIP is composed of medical and public health experts who develop recommendations on how to use vaccines to control diseases in the United States. One of the members said that he received research funding from MedImmune; the other members had no disclosures.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices unanimously recommended serogroup B meningococcal (MenB) vaccination for several groups at increased risk for serogroup B disease, including students during outbreaks at college campuses, but is not addressing broader use of these vaccines in adolescents and college students until June 2015.
At a meeting of the Advisory Committee on Immunization Practices (ACIP) on Feb. 26, the panel voted 15-0 to recommend vaccination for the following groups of people over age 10 years at increased risk of serogroup B meningococcal disease: those with persistent complement deficiencies; anatomic or functional asplenia, including people with sickle cell; microbiologists routinely exposed to Neisseria meningitidis isolates; and individuals, such as college students, at increased risk during an outbreak of serogroup B meningococcal disease.
First-year college students living in residence halls were not discussed at this meeting. The ACIP work group on meningococcal vaccines also has been reviewing the available data and evidence for broader use of MenB vaccines in adolescents and college students separately, Jessica MacNeil of the CDC’s National Center for Immunization and Respiratory Diseases said at the meeting. Recommendations for more universal use of MenB vaccination in adolescents and college students will be discussed and voted on at the next ACIP meeting in June 2015.
Two vaccines are now licensed by the Food and Drug Administration for preventing meningococcal disease caused by N. meningitidis serogroup B in people aged 10-25 years in the United States: Trumenba (Pfizer), licensed in October 2014, and Bexsero (Novartis Vaccines and Diagnostics), in January 2015. Bexsero is already licensed in more than 30 countries for people aged 2 months and older. The currently recommended meningococcal vaccines cover four of the five main serogroups of N. meningitidis bacteria that cause meningococcal disease (A, C, W, and Y): they are the meningococcal polysaccharide vaccine (Menomune) and the meningococcal conjugate vaccine (Menactra and Menveo).
Based on the available data and evidence reviewed, Ms. MacNeil said that the work group supported routine vaccination of people at increased risk of meningococcal disease, based on the risk of disease in those groups and because they are included in the current recommendation for the meningitis A, C, W, Y conjugate vaccines. In addition, “there is demonstrated immune response to MenB in the general adolescent population … and there are no theoretical safety concerns for persons over 25 years of age, from vaccination, as compared to persons 10-25 years of age.”
Ms. MacNeil referred to the serogroup B clusters and outbreaks during the past several years on U.S. college campuses, noting that, in two of the recent outbreaks, “students were estimated to be at 200- to 1,400-fold increased risk for meningococcal disease during the outbreak period.”
In the first two months of 2015, there have already been two outbreaks of serogroup B meningococcal disease at U.S. college campuses: two cases at Providence College in Rhode Island, where students are being vaccinated with Trumenba; and four cases at the University of Oregon, including one death, where a mass vaccination campaign is being planned – the first doses are scheduled to be administered on March 2.
The threshold for vaccination during serogroup B outbreaks in institutional settings has been two cases in a population of under 5,000 people and three cases in a population of 5,000 or more people. Providence has 4,500 students and the University of Oregon has 25,000 students, Ms. MacNeil said. About 98% of the target population at Providence College has received one dose so far, she added.
During the public comments segment of the meeting, consumer advocates and parents of children who had died of meningitis B questioned why the discussion for routine use in college students and adolescents was being delayed, when the two vaccines had been approved.
“We think it would benefit even more if students came to campus protected,” said one of the public speakers, Dr. Mary Ferris, director of student health at the University of California, Santa Barbara, which had an outbreak of serogroup B disease in 2013. In November 2013, four cases at UCSB were diagnosed within 10 days, including a freshman athlete who survived but had two limbs amputated, she added.
Even one case in a college setting has a major impact on the community, Dr. Ferris said.
In response to the outbreak, more than 17,000 students at UCSB received the vaccine, which was not yet approved but was made available through an FDA investigational new drug application.
ACIP is composed of medical and public health experts who develop recommendations on how to use vaccines to control diseases in the United States. One of the members said that he received research funding from MedImmune; the other members had no disclosures.
FROM AN ACIP MEETING