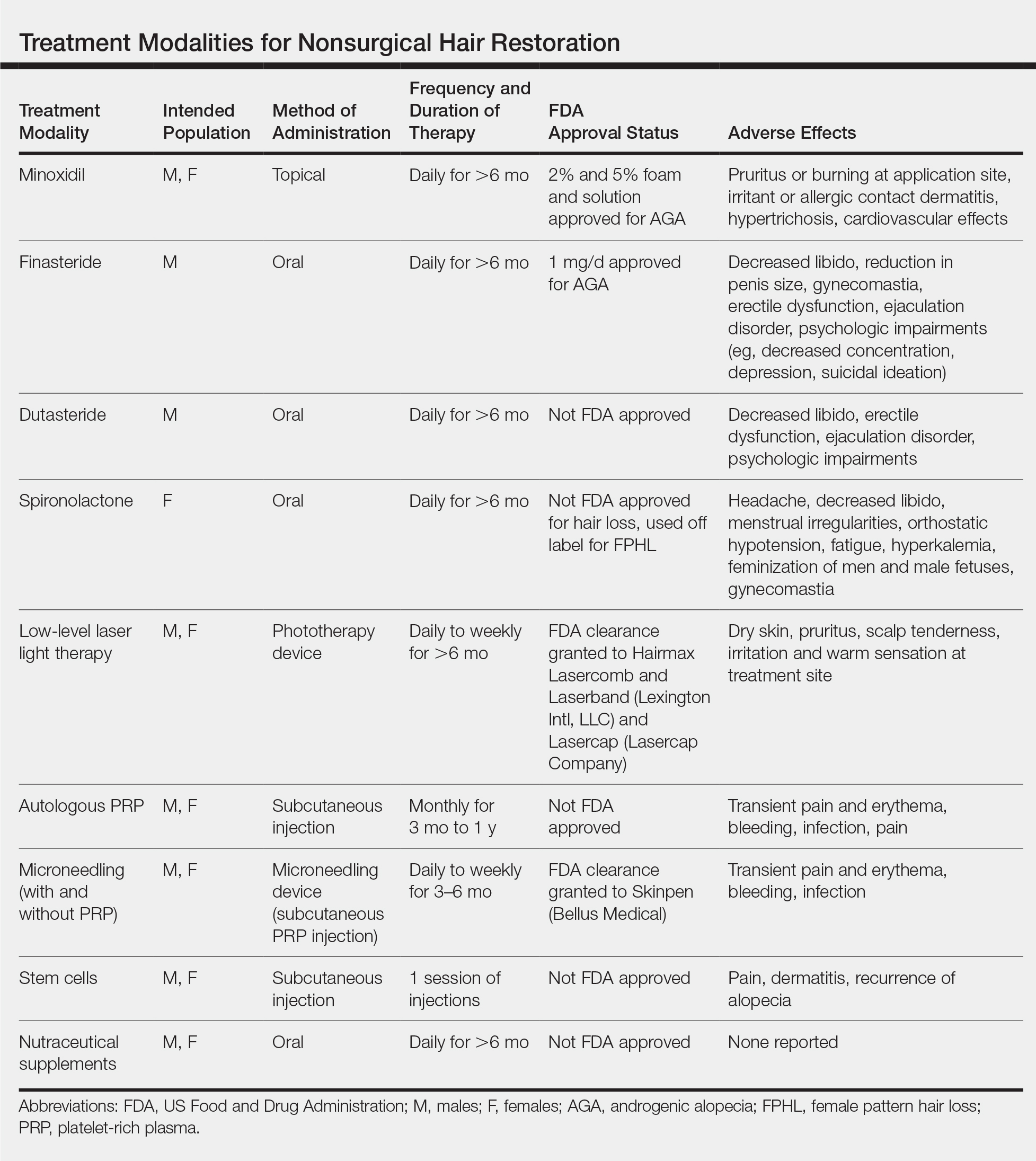
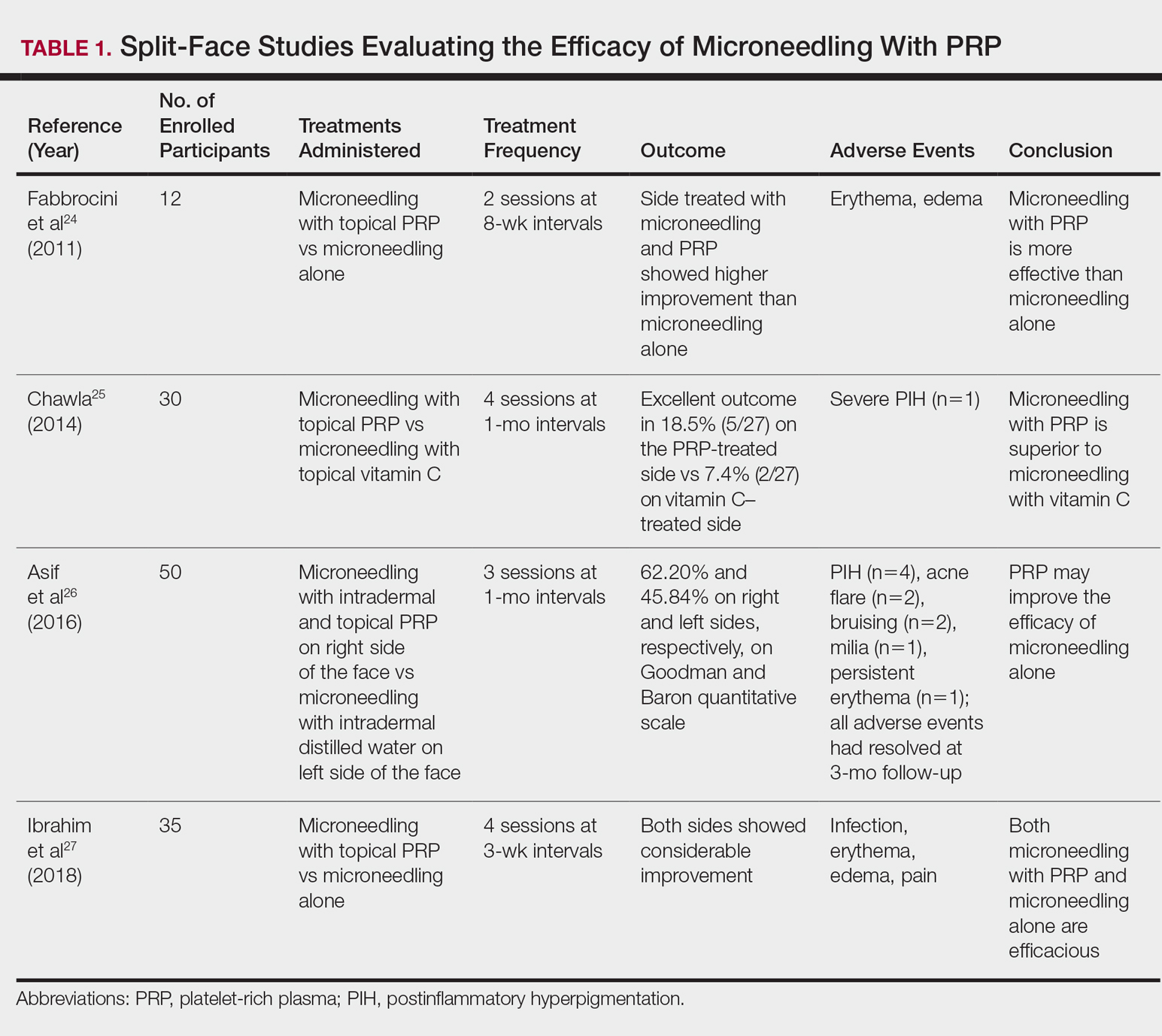
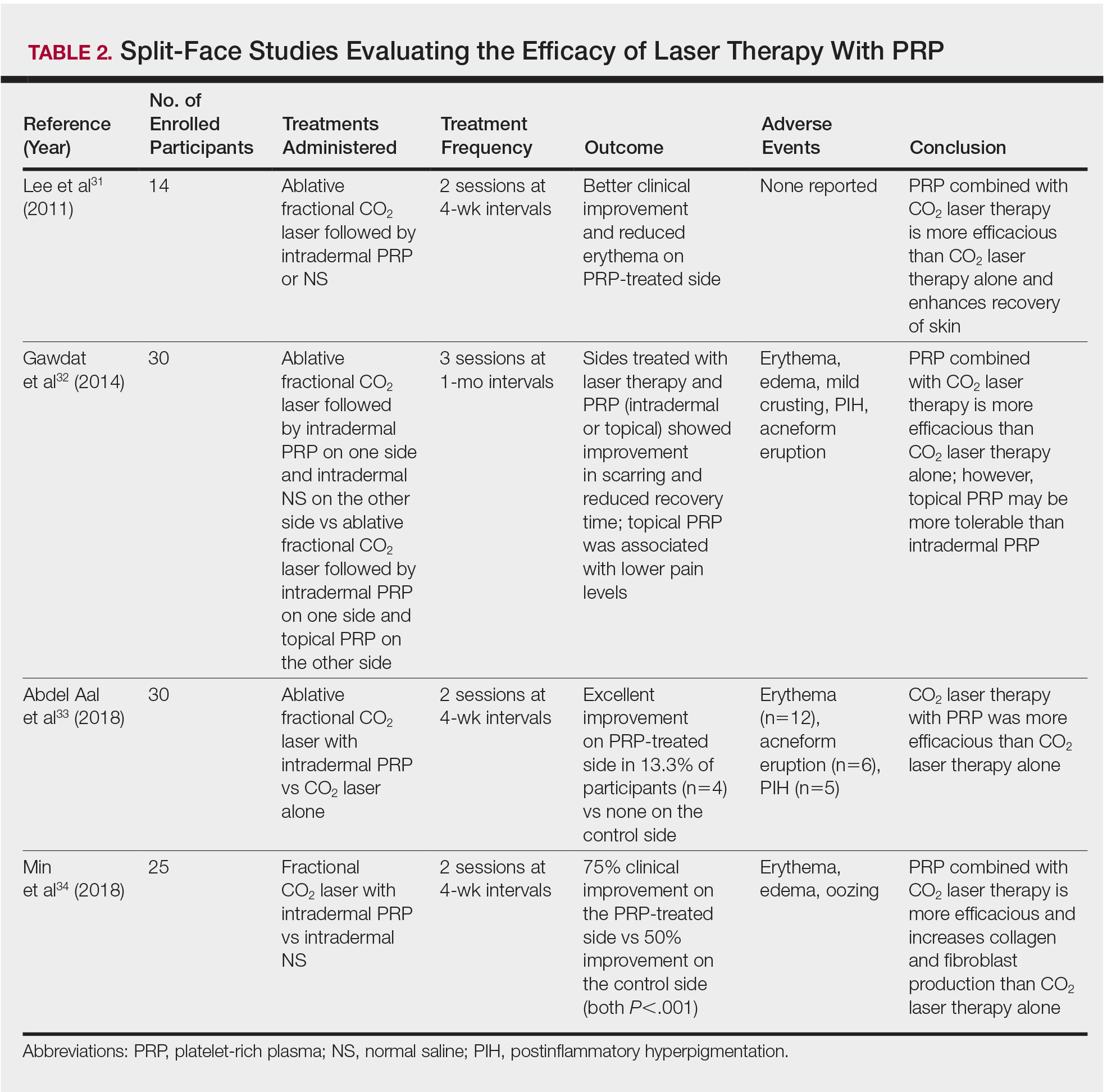
User login
Postirradiation Pseudosclerodermatous Panniculitis: A Rare Complication of Megavoltage External Beam Radiotherapy
To the Editor:
Postirradiation pseudosclerodermatous panniculitis (PIPP) is a rarely reported complication of megavoltage external beam radiotherapy that was first identified in 1993 by Winkelmann et al.1 The condition presents as an erythematous or hyperpigmented indurated plaque at a site of prior radiotherapy. Lesions caused by PIPP most commonly arise several months after treatment, although they may emerge up to 17 years following exposure.2 Herein, we report a rare case of a patient with PIPP occurring on the leg who previously had been treated for Kaposi sarcoma.
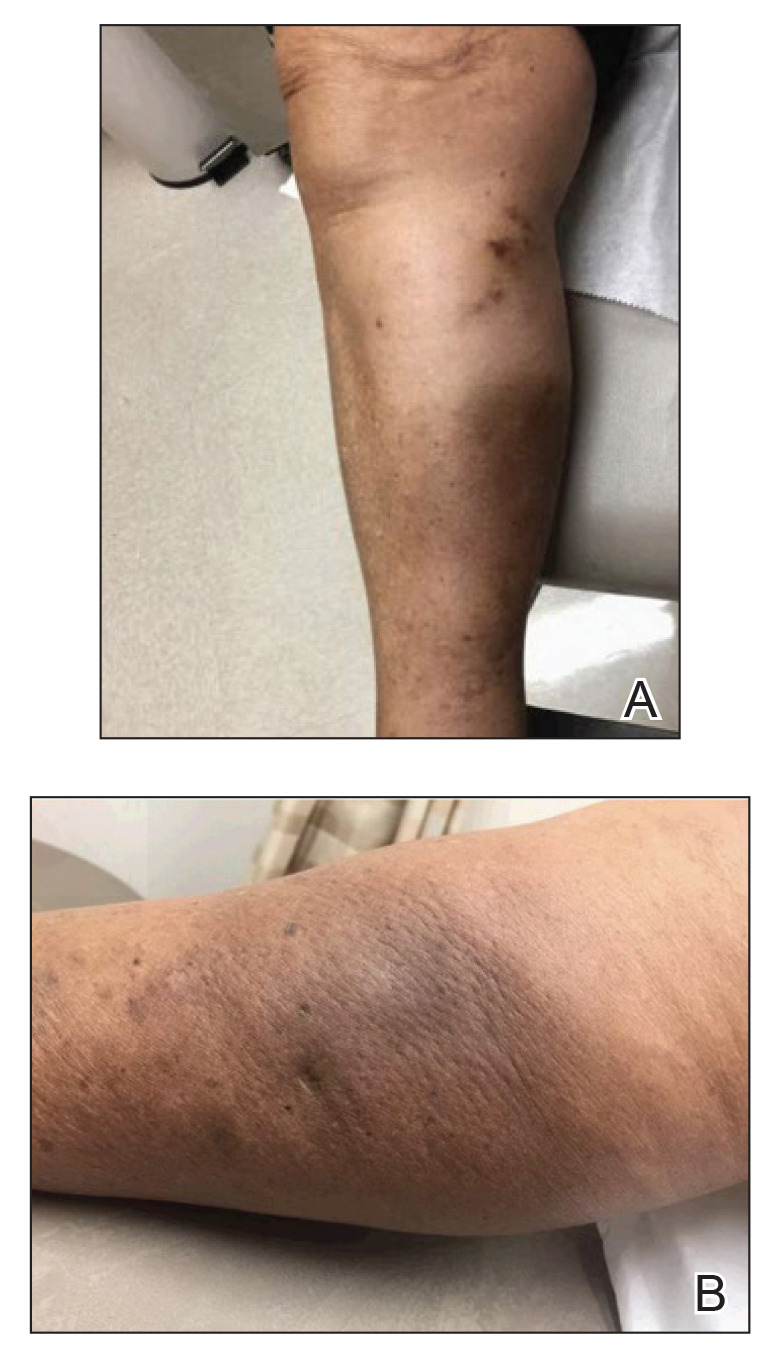
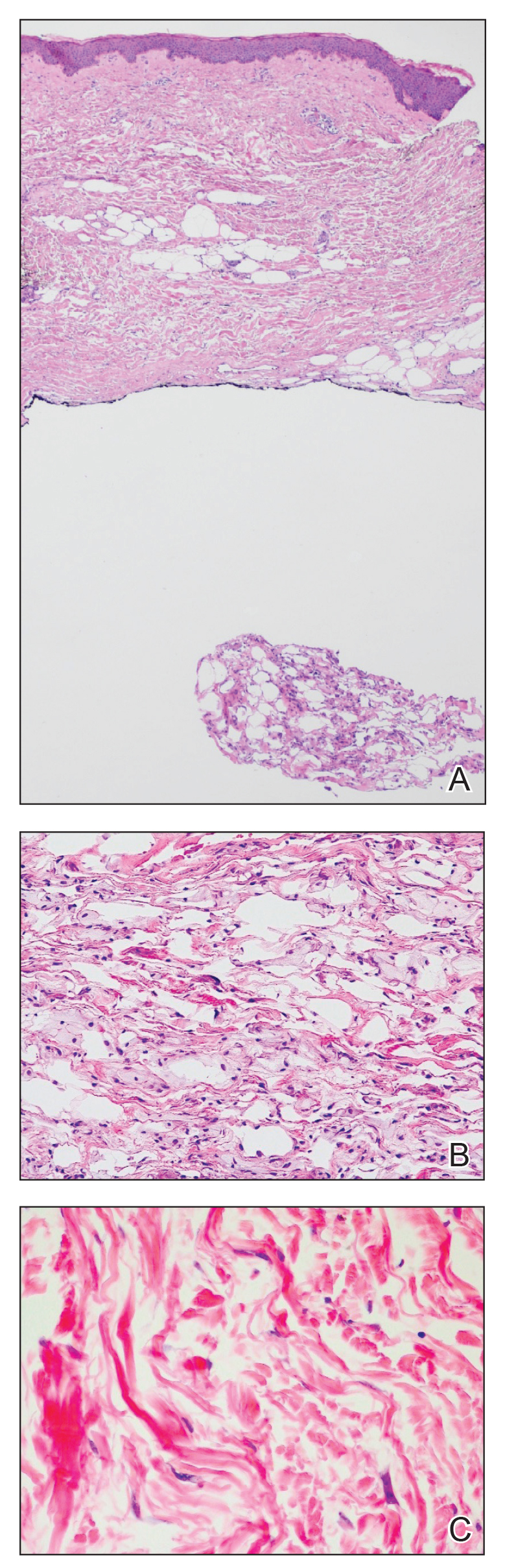
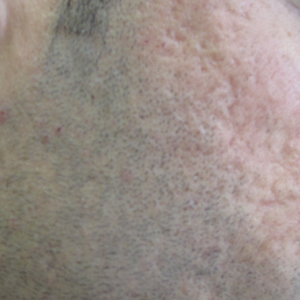
An 84-year-old woman presented with a tender plaque on the right lower leg of 2 months’ duration. Her medical history was remarkable for Kaposi sarcoma, with multiple sites on the body treated with megavoltage external beam radiotherapy during the prior 4 years. The most recent treatment occurred 8 months prior to presentation, at which time she had undergone radiotherapy for lesions on the posterior lower right leg. Physical examination demonstrated a hyperpigmented and indurated plaque at the treatment site (Figure 1). Skin biopsy results showed a mildly sclerotic dermis with atypical radiation fibroblasts scattered interstitially between collagen bundles, and a lobular panniculitis with degenerated adipocytes and foamy histiocytes (Figure 2). Hyalinized dermal vessels also were present. Based on the constellation of these biopsy findings, a diagnosis of PIPP was made.
The diagnosis of PIPP is challenging and invariably requires histologic examination. Clinically, the differential diagnosis includes cutaneous metastasis of the primary neoplasm, cellulitis, lipodermatosclerosis, morphea, and chronic radiation dermatitis.
Histologically, PIPP is characterized by a lobular panniculitis without vasculitis. Typical findings include the presence of centrilobular necrotic adipocytes along with a foamy histiocytic infiltrate containing lipophagic granulomas at the periphery of the fat lobules. Septal thickening and sclerosis around fat lobules also have been described, and dermal changes associated with chronic radiation dermatitis, such as papillary dermal sclerosis, endothelial swelling, vascular hyaline arteriosclerosis, and atypical star-shaped radiation fibroblasts, may be present.2 Features of radiation-induced vasculopathy commonly are seen, although the appearance of these features varies over time. Intimal injury and mural thrombosis can develop within 5 years of radiation therapy, fibrosis of the vessel wall can occur within 10 years of radiation therapy, and atherosclerosis and periarterial fibrosis can appear within 20 years of radiation therapy.2,3 The histologic findings in our patient showed characteristic dermal findings seen in radiation dermatitis in addition to a lobular panniculitis with foamy histiocytes and mild vessel damage.
In contrast, lipodermatosclerosis is a septal and lobular panniculitis with septal fibrosis. Membranocystic fat necrosis is present, characterized by fat microcysts lined by feathery eosinophilic material. Stasis changes in the dermis and epidermis are accompanied by a mild perivascular lymphocytic infiltrate.
Patients with traumatic panniculitis, which also may enter the clinical differential diagnosis of PIPP, often demonstrate nonspecific histologic changes. Early lesions show a perivascular infiltrate of lymphocytes and macrophages. Evolving lesions show variably sized fat microcysts surrounded by histiocytes, in addition to possible calcifications and a foreign-body giant cell reaction. A fibrous capsule may develop, surrounding the fat necrosis to form a mobile encapsulated lipoma. Late lesions frequently demonstrate lipomembranous changes and calcium deposits.4
To date, nearly all cases of PIPP in the literature have been described in breast cancer patients.1,2,5,6 However, Sandoval et al7 reported a case of PIPP occurring in the leg of a patient after radiotherapy for a soft tissue sarcoma. Similar to our patient, this patient presented with a painful, dully erythematous, indurated plaque, although her symptoms arose 5 years after radiotherapy.
Megavoltage external beam radiotherapy has become a widely used modality in the treatment of various cancers. As such, PIPP may represent an underdiagnosed condition with potential cases remaining unidentified when the clinical differential diagnosis does not lead to biopsy. Effective therapies have yet to be widely reported, and our patient failed to experience notable improvement with either topical or intralesional corticosteroids. Further studies are needed in order to address this knowledge gap.
- Winkelmann RK, Grado GL, Quimby SR, et al. Pseudosclerodermatous panniculitis after irradiation: an unusual complication of megavoltage treatment of breast carcinoma. Mayo Clin Proc. 1993;68:122-127.
- Pielasinski U, Machan S, Camacho D, et al. Postirradiation pseudosclerodermatous panniculitis: three new cases with additional histopathologic features supporting the radiotherapy etiology. Am J Dermatopathol. 2013;35:129-134.
- Butler MJ, Lane RH, Webster JH. Irradiation injury to large arteries. Br J Surg. 1980;67:341-343. Moreno A, Marcoval J, Peyri J. Traumatic panniculitis. Dermatol Clin. 2008;26:481-483.
- Shirsat HS, Walsh NM, McDonald LJ, et al. Postirradiation pseudosclerodermatous panniculitis with involvement of breast parenchyma: a dramatic example of a rare entity and a pitfall in diagnosis. J Cutan Pathol. 2016;43:444-450.
- Carrasco L, Moreno C, Pastor MA, et al. Postirradiation pseudosclerodermatous panniculitis. Am J Dermatopathol. 2001;23:283-287.
- Sandoval M, Giesen L, Cataldo K, et al. Postirradiation pseudosclerodermatous panniculitis of the leg: report of a case and review of the literature. Am J Dermatopathol. 2015;37:587-589.
To the Editor:
Postirradiation pseudosclerodermatous panniculitis (PIPP) is a rarely reported complication of megavoltage external beam radiotherapy that was first identified in 1993 by Winkelmann et al.1 The condition presents as an erythematous or hyperpigmented indurated plaque at a site of prior radiotherapy. Lesions caused by PIPP most commonly arise several months after treatment, although they may emerge up to 17 years following exposure.2 Herein, we report a rare case of a patient with PIPP occurring on the leg who previously had been treated for Kaposi sarcoma.
An 84-year-old woman presented with a tender plaque on the right lower leg of 2 months’ duration. Her medical history was remarkable for Kaposi sarcoma, with multiple sites on the body treated with megavoltage external beam radiotherapy during the prior 4 years. The most recent treatment occurred 8 months prior to presentation, at which time she had undergone radiotherapy for lesions on the posterior lower right leg. Physical examination demonstrated a hyperpigmented and indurated plaque at the treatment site (Figure 1). Skin biopsy results showed a mildly sclerotic dermis with atypical radiation fibroblasts scattered interstitially between collagen bundles, and a lobular panniculitis with degenerated adipocytes and foamy histiocytes (Figure 2). Hyalinized dermal vessels also were present. Based on the constellation of these biopsy findings, a diagnosis of PIPP was made.
The diagnosis of PIPP is challenging and invariably requires histologic examination. Clinically, the differential diagnosis includes cutaneous metastasis of the primary neoplasm, cellulitis, lipodermatosclerosis, morphea, and chronic radiation dermatitis.
Histologically, PIPP is characterized by a lobular panniculitis without vasculitis. Typical findings include the presence of centrilobular necrotic adipocytes along with a foamy histiocytic infiltrate containing lipophagic granulomas at the periphery of the fat lobules. Septal thickening and sclerosis around fat lobules also have been described, and dermal changes associated with chronic radiation dermatitis, such as papillary dermal sclerosis, endothelial swelling, vascular hyaline arteriosclerosis, and atypical star-shaped radiation fibroblasts, may be present.2 Features of radiation-induced vasculopathy commonly are seen, although the appearance of these features varies over time. Intimal injury and mural thrombosis can develop within 5 years of radiation therapy, fibrosis of the vessel wall can occur within 10 years of radiation therapy, and atherosclerosis and periarterial fibrosis can appear within 20 years of radiation therapy.2,3 The histologic findings in our patient showed characteristic dermal findings seen in radiation dermatitis in addition to a lobular panniculitis with foamy histiocytes and mild vessel damage.
In contrast, lipodermatosclerosis is a septal and lobular panniculitis with septal fibrosis. Membranocystic fat necrosis is present, characterized by fat microcysts lined by feathery eosinophilic material. Stasis changes in the dermis and epidermis are accompanied by a mild perivascular lymphocytic infiltrate.
Patients with traumatic panniculitis, which also may enter the clinical differential diagnosis of PIPP, often demonstrate nonspecific histologic changes. Early lesions show a perivascular infiltrate of lymphocytes and macrophages. Evolving lesions show variably sized fat microcysts surrounded by histiocytes, in addition to possible calcifications and a foreign-body giant cell reaction. A fibrous capsule may develop, surrounding the fat necrosis to form a mobile encapsulated lipoma. Late lesions frequently demonstrate lipomembranous changes and calcium deposits.4
To date, nearly all cases of PIPP in the literature have been described in breast cancer patients.1,2,5,6 However, Sandoval et al7 reported a case of PIPP occurring in the leg of a patient after radiotherapy for a soft tissue sarcoma. Similar to our patient, this patient presented with a painful, dully erythematous, indurated plaque, although her symptoms arose 5 years after radiotherapy.
Megavoltage external beam radiotherapy has become a widely used modality in the treatment of various cancers. As such, PIPP may represent an underdiagnosed condition with potential cases remaining unidentified when the clinical differential diagnosis does not lead to biopsy. Effective therapies have yet to be widely reported, and our patient failed to experience notable improvement with either topical or intralesional corticosteroids. Further studies are needed in order to address this knowledge gap.
To the Editor:
Postirradiation pseudosclerodermatous panniculitis (PIPP) is a rarely reported complication of megavoltage external beam radiotherapy that was first identified in 1993 by Winkelmann et al.1 The condition presents as an erythematous or hyperpigmented indurated plaque at a site of prior radiotherapy. Lesions caused by PIPP most commonly arise several months after treatment, although they may emerge up to 17 years following exposure.2 Herein, we report a rare case of a patient with PIPP occurring on the leg who previously had been treated for Kaposi sarcoma.
An 84-year-old woman presented with a tender plaque on the right lower leg of 2 months’ duration. Her medical history was remarkable for Kaposi sarcoma, with multiple sites on the body treated with megavoltage external beam radiotherapy during the prior 4 years. The most recent treatment occurred 8 months prior to presentation, at which time she had undergone radiotherapy for lesions on the posterior lower right leg. Physical examination demonstrated a hyperpigmented and indurated plaque at the treatment site (Figure 1). Skin biopsy results showed a mildly sclerotic dermis with atypical radiation fibroblasts scattered interstitially between collagen bundles, and a lobular panniculitis with degenerated adipocytes and foamy histiocytes (Figure 2). Hyalinized dermal vessels also were present. Based on the constellation of these biopsy findings, a diagnosis of PIPP was made.
The diagnosis of PIPP is challenging and invariably requires histologic examination. Clinically, the differential diagnosis includes cutaneous metastasis of the primary neoplasm, cellulitis, lipodermatosclerosis, morphea, and chronic radiation dermatitis.
Histologically, PIPP is characterized by a lobular panniculitis without vasculitis. Typical findings include the presence of centrilobular necrotic adipocytes along with a foamy histiocytic infiltrate containing lipophagic granulomas at the periphery of the fat lobules. Septal thickening and sclerosis around fat lobules also have been described, and dermal changes associated with chronic radiation dermatitis, such as papillary dermal sclerosis, endothelial swelling, vascular hyaline arteriosclerosis, and atypical star-shaped radiation fibroblasts, may be present.2 Features of radiation-induced vasculopathy commonly are seen, although the appearance of these features varies over time. Intimal injury and mural thrombosis can develop within 5 years of radiation therapy, fibrosis of the vessel wall can occur within 10 years of radiation therapy, and atherosclerosis and periarterial fibrosis can appear within 20 years of radiation therapy.2,3 The histologic findings in our patient showed characteristic dermal findings seen in radiation dermatitis in addition to a lobular panniculitis with foamy histiocytes and mild vessel damage.
In contrast, lipodermatosclerosis is a septal and lobular panniculitis with septal fibrosis. Membranocystic fat necrosis is present, characterized by fat microcysts lined by feathery eosinophilic material. Stasis changes in the dermis and epidermis are accompanied by a mild perivascular lymphocytic infiltrate.
Patients with traumatic panniculitis, which also may enter the clinical differential diagnosis of PIPP, often demonstrate nonspecific histologic changes. Early lesions show a perivascular infiltrate of lymphocytes and macrophages. Evolving lesions show variably sized fat microcysts surrounded by histiocytes, in addition to possible calcifications and a foreign-body giant cell reaction. A fibrous capsule may develop, surrounding the fat necrosis to form a mobile encapsulated lipoma. Late lesions frequently demonstrate lipomembranous changes and calcium deposits.4
To date, nearly all cases of PIPP in the literature have been described in breast cancer patients.1,2,5,6 However, Sandoval et al7 reported a case of PIPP occurring in the leg of a patient after radiotherapy for a soft tissue sarcoma. Similar to our patient, this patient presented with a painful, dully erythematous, indurated plaque, although her symptoms arose 5 years after radiotherapy.
Megavoltage external beam radiotherapy has become a widely used modality in the treatment of various cancers. As such, PIPP may represent an underdiagnosed condition with potential cases remaining unidentified when the clinical differential diagnosis does not lead to biopsy. Effective therapies have yet to be widely reported, and our patient failed to experience notable improvement with either topical or intralesional corticosteroids. Further studies are needed in order to address this knowledge gap.
- Winkelmann RK, Grado GL, Quimby SR, et al. Pseudosclerodermatous panniculitis after irradiation: an unusual complication of megavoltage treatment of breast carcinoma. Mayo Clin Proc. 1993;68:122-127.
- Pielasinski U, Machan S, Camacho D, et al. Postirradiation pseudosclerodermatous panniculitis: three new cases with additional histopathologic features supporting the radiotherapy etiology. Am J Dermatopathol. 2013;35:129-134.
- Butler MJ, Lane RH, Webster JH. Irradiation injury to large arteries. Br J Surg. 1980;67:341-343. Moreno A, Marcoval J, Peyri J. Traumatic panniculitis. Dermatol Clin. 2008;26:481-483.
- Shirsat HS, Walsh NM, McDonald LJ, et al. Postirradiation pseudosclerodermatous panniculitis with involvement of breast parenchyma: a dramatic example of a rare entity and a pitfall in diagnosis. J Cutan Pathol. 2016;43:444-450.
- Carrasco L, Moreno C, Pastor MA, et al. Postirradiation pseudosclerodermatous panniculitis. Am J Dermatopathol. 2001;23:283-287.
- Sandoval M, Giesen L, Cataldo K, et al. Postirradiation pseudosclerodermatous panniculitis of the leg: report of a case and review of the literature. Am J Dermatopathol. 2015;37:587-589.
- Winkelmann RK, Grado GL, Quimby SR, et al. Pseudosclerodermatous panniculitis after irradiation: an unusual complication of megavoltage treatment of breast carcinoma. Mayo Clin Proc. 1993;68:122-127.
- Pielasinski U, Machan S, Camacho D, et al. Postirradiation pseudosclerodermatous panniculitis: three new cases with additional histopathologic features supporting the radiotherapy etiology. Am J Dermatopathol. 2013;35:129-134.
- Butler MJ, Lane RH, Webster JH. Irradiation injury to large arteries. Br J Surg. 1980;67:341-343. Moreno A, Marcoval J, Peyri J. Traumatic panniculitis. Dermatol Clin. 2008;26:481-483.
- Shirsat HS, Walsh NM, McDonald LJ, et al. Postirradiation pseudosclerodermatous panniculitis with involvement of breast parenchyma: a dramatic example of a rare entity and a pitfall in diagnosis. J Cutan Pathol. 2016;43:444-450.
- Carrasco L, Moreno C, Pastor MA, et al. Postirradiation pseudosclerodermatous panniculitis. Am J Dermatopathol. 2001;23:283-287.
- Sandoval M, Giesen L, Cataldo K, et al. Postirradiation pseudosclerodermatous panniculitis of the leg: report of a case and review of the literature. Am J Dermatopathol. 2015;37:587-589.
Practice Points
- Postirradiation pseudosclerodermatous panniculitis presents as an erythematous or indurated plaque at a site of prior radiotherapy.
- This rare entity may be underreported and requires biopsy for accurate diagnosis.
The Role of Vitamins and Supplements on Skin Appearance
As the largest and most exposed organ in the body, the skin experiences trauma from both extrinsic and intrinsic aging factors, resulting in loss of elasticity, increased laxity, wrinkling, and rough-textured appearance.1 Chronologically aged skin appears dry, thin, and finely wrinkled; photoaged skin appears leathery with coarse wrinkles and uneven pigmentation.2 In recent years, numerous systemic nutrients have been proposed to improve skin appearance. This article reviews the efficacy of these vitamins and supplements.
Carotenoids
Carotenoids are a group of lipophilic molecules derived from vitamin A.3,4 Ingestion of carotenoids may play a role in photoprotection against UV radiation (UVR) by acting as acceptors of reactive oxygen species.4-6 Stahl et al7 investigated lycopene’s usefulness in protection against UVR-induced erythema. Over 10 weeks, 9 volunteers received 40 g of tomato paste containing 16 mg daily of lycopene while 10 controls received placebo. A solar simulator was used to induce erythema of the skin at weeks 0, 4, and 10. At week 10, erythema formation was 40% lower in the lycopene group compared to controls (P=.02).7
In another study assessing the photoprotective effects of a novel nutritional and phytonutrient blend of carotenoids, 36 women with Fitzpatrick skin types I and II were treated for 8 weeks.8 Presupplementation, UVR-induced erythema, and skin carotenoid concentrations were determined along with facial skin attributes and characteristics. Results showed protection against UVR-induced skin damage, with reductions in erythema at 3 minimal erythema doses (MEDs)(P=.01). Additionally, significant improvements were noted in facial skin elasticity, radiance, and overall appearance (all P<.05).8
In 2013, Meinke et al9 conducted an 8-week, double-blind, placebo-controlled study on 24 volunteers whose diets were supplemented with moderate amounts of carotenoids, including lutein, beta-carotene, and lycopene. Utilizing novel techniques to measure the skin’s ability to scavenge free radicals, they discovered that dietary carotenoids provided notable protection against stress-induced radical formation and increased baseline radical scavenging activity of the skin by 34%. The authors concluded that dietary supplementation could avoid premature skin aging.9
Vitamins C and E
Vitamin C is an essential vitamin that must be obtained through dietary sources.10 It functions as a free radical scavenger and is a necessary cofactor for the synthesis and stabilization of collagen.
A study evaluated the effect of UVR-induced oxidative stress and the association with vitamin C supplementation among 20 white patients with Fitzpatrick skin types II or III.11 The volunteers were treated with UVR on two 1-cm sites on the buttock. Six punch biopsies of these sites and 2 control biopsies from nonexposed skin were taken. Volunteers took vitamin C supplements (500 mg) for 8 weeks, and the exposure and biopsy were repeated. Researchers concluded that supplementation with vitamin C had no effect on the MED, with identical concentrations at baseline and after 8 weeks of supplementation. Additionally, there was no evidence that vitamin C affects UVR-induced oxidative stress.11
In 2007, Cosgrove et al12 conducted a study to assess the associations between nutrient intake and skin aging in more than 4000 women aged 40 to 74 years. Higher dietary vitamin C intakes were associated with a significantly lower likelihood of senile xerosis and wrinkled appearance (P<.009).12
Vitamin E is a lipid-soluble, membrane-bound vitamin, and its most active form is α-tocopherol.11,13 Vitamin E functions as an antioxidant and protects cellular membranes from lipid peroxidation by free radicals.13-15 Once oxidized, vitamin E can be regenerated to its reduced form by vitamin C.11 Their synergistic effects on skin protection have been studied extensively. A double-blind, placebo-controlled study of 10 patients compared 2 g of vitamin C combined with 1000 IU of vitamin E vs placebo.16 The patients’ skin reaction before and after 8 days of treatment were assessed by determination of MED and the cutaneous blood flow of skin irradiated with UV light. Results showed that the median MED of those taking vitamins increased from 80 to 96.5 mJ/cm2 (P<.01) and decreased for the placebo group. Investigators concluded that the combination of vitamins C and E reduces the sunburn reaction and leads to a reduction in the sequelae of UV-induced skin damage.16 A prospective, randomized, placebo-controlled study by Fuchs and Kern17 replicated these findings, also concluding that combinations of vitamins C and E provide improved photoprotective effects than either vitamin alone.
Vitamin D
Vitamin D is a fat-soluble vitamin obtained through dietary intake and exposure to UV light.3,18,19 Precursors of vitamin D require interaction with UV light for conversion into active forms. The highest concentrations of 7-dehydrocholesterol are found in keratinocytes in the basal cell and spinous cell layers of the skin where they are protected from UV light by melanin. As such, individuals with higher melanin content in their skin require more exposure to UV light to produce the same levels of vitamin D as those with less melanin,20 leading to a high rate of vitamin D deficiency in dark-skinned individuals. Because of their prodifferentiating and antiproliferative effects, vitamin D analogs have been very effective in the treatment of psoriasis.20,21 Vitamin D deficiency also has been implicated in the pathogenesis of vitiligo. A systematic review and meta-analysis conducted in 2016 found that a significant relationship existed between low 25-hydroxyvitamin D levels and vitiligo (P<.01), but no causal relationship could be established.22
A 2017 double-blind, placebo-controlled study performed by Scott et al23 aimed to elucidate the relationship between vitamin D concentrations and sunburn. Twenty adults received either placebo or high-dose vitamin D3 (200,000 IU) 1 hour after experimental sunburn induced by an erythemogenic dose of UVR. Investigators measured participants’ concentrations of the proinflammatory mediators tumor necrosis factor α and nitric oxide synthase via skin biopsy 48 hours later. Patients in the experimental group were found to have significantly reduced expression of both tumor necrosis factor α (P=.04) and nitric oxide synthase (P=.02). Additionally, participants with significantly higher vitamin D3 levels following supplementation (P=.007) demonstrated increased skin expression of the anti-inflammatory marker arginase-1 (P=.005) as well as a persistent reduction in skin redness (P=.02). Investigators concluded that vitamin D plays a large role in skin homeostasis and implicated vitamin D’s upregulation of arginase-1 as a potent mechanism of its anti-inflammatory effects.23
Collagen
As humans age, the density of collagen in the dermis decreases, leading to sagging and wrinkling of skin.24 Oral supplementation of collagen has been examined for its dermatologic benefits, primarily increasing the thickness and density of collagen in the dermal layer. In 2014, Proksch et al25 performed a double-blind, placebo-controlled trial in which 69 women were randomized to receive 2.5 or 5 g of collagen peptides or placebo for 8 weeks. Both treatment groups demonstrated improvements in skin elasticity as well as improved skin moisture and decreased skin evaporation; however, changes in the latter 2 qualities failed to reach statistical significance.25
The results of this study were replicated by Asserin et al.26 One hundred six female patients were randomly assigned to receive 10 g of collagen peptides or placebo daily for 8 weeks. The collagen group demonstrated significantly improved skin hydration (P=.003) and increased density of collagen in the dermis (P=.007) relative to placebo.26
In another randomized, double-blind, placebo-controlled study, 71 women consumed a 20-mL beverage containing either 3000 mg of collagen peptides or placebo for 12 weeks.27 Participants in the treatment group demonstrated significant decreases in periorbital wrinkles (P<.05) and enhanced facial skin moisture (P<.001) and elasticity (P<.001) after 12 weeks. Researchers concluded that oral supplementation with collagen peptides holds promise as a natural supplement to provide cutaneous antiaging properties.27
Ceramides
Ceramides are lipids composed of a sphingoid base conjugated to a fatty acid and serve as the main component of the stratum corneum of the skin. Ceramides are crucial for the maintenance of skin barrier integrity and for preventing transepidermal water loss.28 In a 3-month study of 51 women with dry skin, Guillou et al29 showed that a ceramide wheat extract capsule significantly increased corneometry measurements of skin hydration on the arms (P<.001) and the legs (P=.012) compared to placebo.
Mixed Supplements
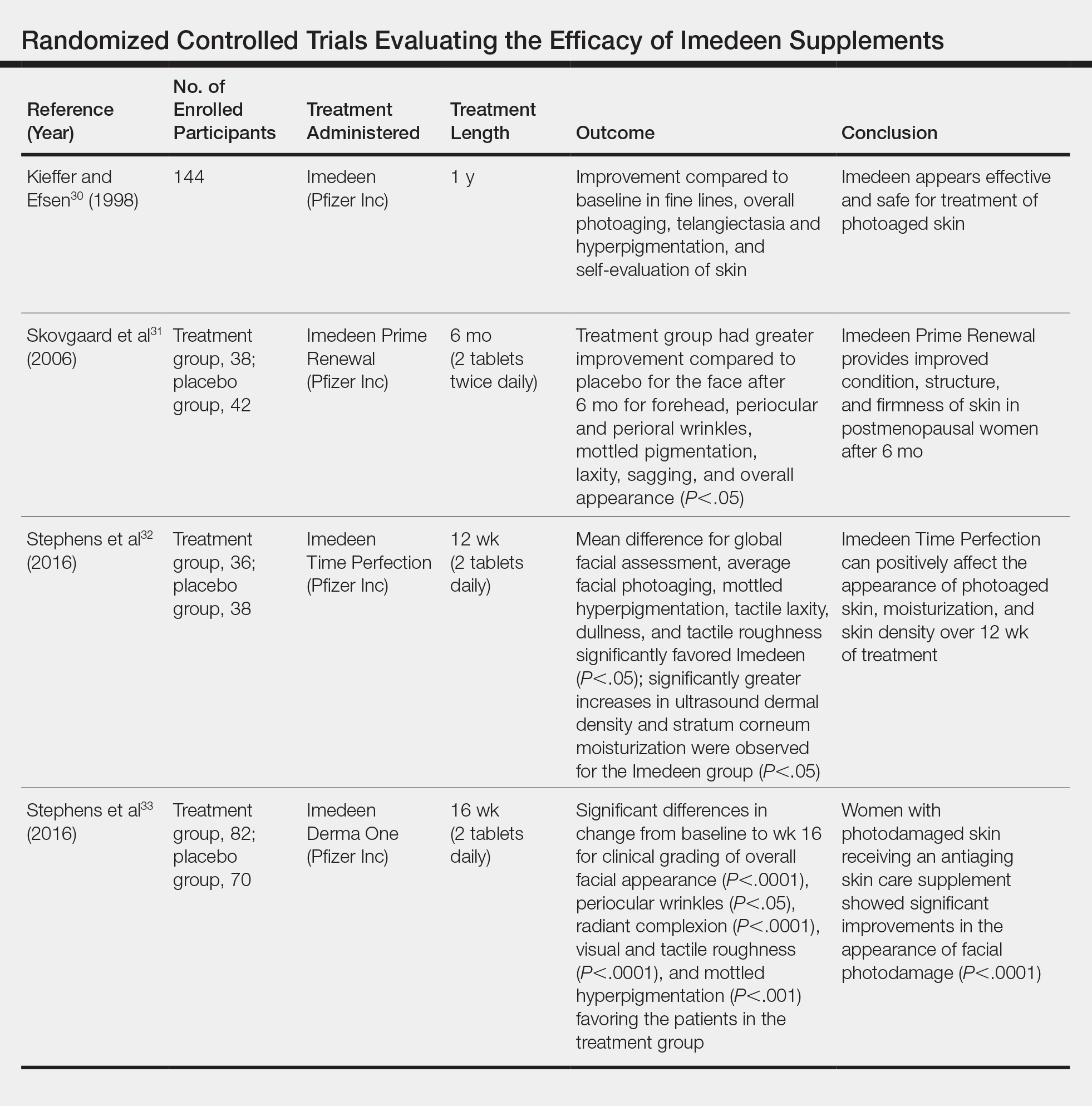
The discovery that nutritional contents can affect skin appearance has energized the development of combination supplements containing multiple vitamins and micronutrients. Imedeen is a biomarine complex and antioxidant supplement with several different formulations, including Prime Renewal, Time Perfection, and Derma One (Pfizer Inc). The ingredients include a combination of a biomarine complex (blend of fish proteins and polysaccharides), lycopene, grape seed extract, vitamin C, vitamin E, and zinc. Several trials have been conducted to assess the efficacy of the supplements on improving the appearance of photodamaged and aged skin (Table).
A placebo-controlled, randomized study of 144 participants conducted by Kieffer and Efsen30 assessed the efficacy of Imedeen supplements over 12 months. The trial included a 3-month placebo-controlled study and 9-month uncontrolled continuation. Imedeen’s efficacy was measured using clinical evaluation, transepidermal water loss, self-evaluation, and photograph evaluation. After 1 year of treatment, improvement occurred in photograph evaluation of fine lines, overall photoaging, telangiectasia and hyperpigmentation, and self-evaluation of skin condition.30 Additional double-blind, placebo-controlled, randomized studies assessing the efficacy of Imedeen have shown increased dermal and epidermal thickness, improvement of stratum corneum moisturization, and improved overall facial complexion.31-33
Several combined supplements containing collagen peptide as the main ingredient have been created for use in skin care. Collagen is found in the extracellular matrix of the dermis and is responsible for the resiliency and strength of skin.34,35 Damage to the dermis can occur with prolonged UV light exposure and is seen histologically as disorganized collagen fibrils and grossly as wrinkles and photoaged skin.35,36
A study assessed the effect of BioCell Collagen (BioCell Technology, LLC), a supplement containing type II collagen, on skin aging.37 Twenty-six women underwent baseline visual assessments of their skin before taking 2 tablets of the supplement daily. Twelve weeks of supplementation led to significant reduction in global lines and wrinkles (13.2%; P=.028) as well as skin dryness and scaling (76%; P=.002). Assessment of collagen content at 6 weeks revealed a significant increase from baseline (6.3%; P=.002), though the difference after 12 weeks was not significant (3.5%; P=.134). The authors concluded that although preliminary data suggested that BioCell Collagen may reduce visible signs of aging, a controlled study was necessary to verify this finding.37
A single-blind, case-controlled study assessed a similar supplement, Celergen, that contained marine collagen peptides.38 Forty-one adults took 2 capsules each day for 60 days. Assessment of their skin physiology was conducted at the enrollment visit, 2 months later, and after the treatment period ended. Skin elasticity, transepidermal water loss, epidermal and dermal thickness, and density were measured. Investigators found that Celergen administration significantly enhanced skin elasticity and sebum production (P<.0001) but did not influence cutaneous moisture. The dermal thickness and homogenous distribution of collagen fibers were enhanced in 11 patients while properties of the epidermis remained unchanged. The study determined that supplementation remarkably improved skin elasticity, sebum production, and dermal ultrasonic markers.38
A double-blind, randomized, placebo-controlled study assessed a collagen- and antioxidant-containing supplement, Gold Collagen Forte, on skin properties.39 The treatment and placebo groups each consisted of 60 patients who consumed 1 bottle (50 mL) of the product each day for 90 days. Patients completed a self-assessment of their skin regarding photoaging, focusing on the crow’s-feet area and nasolabial folds, while skin elasticity was assessed with the SkinLab USB elasticity module. Results showed a significant increase in skin elasticity (+7.5%; P≤.001). Self-assessment results showed improvements in both the treatment and placebo groups, and investigators concluded that Gold Collagen Forte may have photoprotective effects and help improve skin health.39
Safety
Although trials have demonstrated vitamin supplementation to be safe and effective for skin enhancement, it is important to consider potential vitamin toxicities. High doses of vitamin C supplementation have been shown to cause damage via lipid peroxidation.40 In a study assessing if high levels of beta-carotene and vitamin E were associated with a lower risk for lung cancer, data showed that these supplements may actually have harmful effects.40,41 Additionally, consumption of high-dose dietary supplements has been associated with an increased risk for severe medical events, including disability and death among adolescents and young adults.42
Conclusion
Numerous trials have indicated that the use of systemic vitamins can have beneficial effects on the protection and appearance of skin. Photodamage from UV light–induced erythema can be decreased by carotenoids and vitamins C and E. Similarly, supplements that combine multiple nutrients with collagen have been shown to improve the appearance of aging skin by decreasing the prominence of wrinkles. Given the growing number of products and advertisements that exist in the supplement marketplace, it is crucial for clinicians to ground their recommendations to patients in the scientific data of robust studies.
- Zhang S, Duan E. Fighting against skin aging: the way from bench to bedside. Cell Transplant. 2018;27:729-738.
- Rittié L, Fisher GJ. Natural and sun-induced aging of human skin. Cold Spring Harb Perspect Med. 2015;5:a015370.
- Draelos ZD. Nutrition and enhancing youthful-appearing skin. Clin Dermatol. 2010;28:400-408.
- Anunciato TP, da Rocha Filho PA. Carotenoids and polyphenols in nutricosmetics, nutraceuticals, and cosmeceuticals. J Cosmet Dermatol. 2012;11:51-54.
- Stahl W, Heinrich U, Jungmann H, et al. Carotenoids and carotenoids plus vitamin E protect against ultraviolet light-induced erythema in humans. Am J Clin Nutr. 2000;71:795-798.
- Anstey AV. Systemic photoprotection with alpha-tocopherol (vitamin E) and beta-carotene. Clin Exp Dermatol. 2002;27:170-176.
- Stahl W, Heinrich U, Wiseman S, et al. Dietary tomato paste protects against ultraviolet light-induced erythema in humans. J Nutr. 2001;131:1449-1451.
- Wood SM, Mastaloudis AF, Hester SN, et al. Protective effects of a novel nutritional and phytonutrient blend on ultraviolet radiation-induced skin damage and inflammatory response through aging defense mechanisms. J Cosmet Dermatol. 2017;16:491-499.
- Meinke MC, Friedrich A, Tscherch K, et al. Influence of dietary carotenoids on radical scavenging capacity of the skin and skin lipids. Eur J Pharm Biopharm. 2013;84:365-373.
- Manela-Azulay M, Bagatin E. Cosmeceuticals vitamins. Clin Dermatol. 2009;27:469-474.
- McArdle F, Rhodes LE, Parslew R, et al. UVR-induced oxidative stress in human skin in vivo: effects of oral vitamin C supplementation. Free Radic Biol Med. 2002;33:1355-1362.
- Cosgrove MC, Franco OH, Granger SP, et al. Dietary nutrient intakes and skin-aging appearance among middle-aged American women. Am J Clin Nutr. 2007;86:1225-1231.
- Thiele JJ, Ekanayake-Mudiyanselage S. Vitamin E in human skin: organ-specific physiology and considerations for its use in dermatology. Mol Aspects Med. 2007;28:646-667.
- Schagen SK, Zampeli VA, Makrantonaki E, et al. Discovering the link between nutrition and skin aging. Dermatoendocrinol. 2012;4:298-307.
- Chan AC. Partners in defense, vitamin E and vitamin C. Can J Physiol Pharmacol. 1993;71:725-731.
- Eberlein-Konig B, Placzek M, Przybilla B. Protective effect against sunburn of combined systemic ascorbic acid (vitamin C) and d-alpha-tocopherol (vitamin E). J Am Acad Dermatol. 1998;38:45-48.
- Fuchs J, Kern H. Modulation of UV-light-induced skin inflammation by D-alpha-tocopherol and L-ascorbic acid: a clinical study using solar simulated radiation. Free Radic Biol Med. 1998;25:1006-1012.
- Shahriari M, Kerr PE, Slade K, et al. Vitamin D and the skin. Clin Dermatol. 2010;28:663-668.
- Soleymani T, Hung T, Soung J. The role of vitamin D in psoriasis: a review. Int J Dermatol. 2015;54:383-392.
- Lehmann B, Querings K, Reichrath J. Vitamin D and skin: new aspects for dermatology. Exp Dermatol. 2004;13(suppl 4):11-15.
- Kannan S, Lim HW. Photoprotection and vitamin D: a review. Photodermatol Photoimmunol Photomed. 2014;30:137-145.
- Upala S, Sanguankeo A. Low 25-hydroxyvitamin D levels are associated with vitiligo: a systematic review and meta-analysis. Photodermatol Photoimmunol Photomed. 2016;32:181-190.
- Scott JF, Das LM, Ahsanuddin S, et al. Oral vitamin D rapidly attenuates inflammation from sunburn: an interventional study. J Invest Dermatol. 2017;137:2078-2086.
- Varani J, Dame MK, Rittie L, et al. Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am J Pathol. 2006;168:1861-1868.
- Proksch E, Segger D, Degwert J, et al. Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: a double-blind, placebo-controlled study. Skin Pharmacol Physiol. 2014;27:47-55.
- Asserin J, Lati E, Shioya T, et al. The effect of oral collagen peptide supplementation on skin moisture and the dermal collagen network: evidence from an ex vivo model and randomized, placebo-controlled clinical trials. J Cosmet Dermatol. 2015;14:291-301.
- Koizumi S, Inoue N, Shimizu M, et al. Effects of dietary supplementation with fish scales-derived collagen peptides on skin parameters and condition: a randomized, placebo-controlled, double-blind study. Int J Peptide Res Ther. 2018;24:397-402.
- Vollmer DL, West VA, Lephart ED. Enhancing skin health: by oral administration of natural compounds and minerals with implications to the dermal microbiome. Int J Mol Sci. 2018;19. doi:10.3390/ijms19103059.
- Guillou S, Ghabri S, Jannot C, et al. The moisturizing effect of a wheat extract food supplement on women’s skin: a randomized, double-blind placebo-controlled trial. Int J Cosmet Sci. 2011;33:138-143.
- Kieffer ME, Efsen J. Imedeen in the treatment of photoaged skin: an efficacy and safety trial over 12 months. J Eur Acad Dermatol Venereol. 1998;11:129-136.
- Skovgaard GR, Jensen AS, Sigler ML. Effect of a novel dietary supplement on skin aging in post-menopausal women. Eur J Clin Nutr. 2006;60:1201-1206.
- Stephens TJ, Sigler ML, Herndon JH Jr, et al. A placebo-controlled, double-blind clinical trial to evaluate the efficacy of Imedeen(®) Time Perfection(®) for improving the appearance of photodamaged skin. Clin Cosmet Investig Dermatol. 2016;9:63-70.
- Stephens TJ, Sigler ML, Hino PD, et al. A randomized, double-blind, placebo-controlled clinical trial evaluating an oral anti-aging skin care supplement for treating photodamaged skin. J Clin Aesthet Dermatol. 2016;9:25-32.
- El-Domyati M, Attia S, Saleh F, et al. Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp Dermatol. 2002;11:398-405.
- Fisher GJ, Wang ZQ, Datta SC, et al. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337:1419-1428.
- Kang MC, Yumnam S, Kim SY. Oral intake of collagen peptide attenuates ultraviolet B irradiation-induced skin dehydration in vivo by regulating hyaluronic acid synthesis. Int J Mol Sci. 2018;19. doi:10.3390/ijms19113551.
- Schwartz SR, Park J. Ingestion of BioCell Collagen(®), a novel hydrolyzed chicken sternal cartilage extract; enhanced blood microcirculation and reduced facial aging signs. Clin Interv Aging. 2012;7:267-273.
- De Luca C, Mikhal’chik EV, Suprun MV, et al. Skin antiageing and systemic redox effects of supplementation with marine collagen peptides and plant-derived antioxidants: a single-blind case-control clinical study. Oxid Med Cell Longev. 2016;2016:4389410.
- Genovese L, Corbo A, Sibilla S. An insight into the changes in skin texture and properties following dietary intervention with a nutricosmeceutical containing a blend of collagen bioactive peptides and antioxidants. Skin Pharmacol Physiol. 2017;30:146-158.
- Hamishehkar H, Ranjdoost F, Asgharian P, et al. Vitamins, are they safe? Adv Pharm Bull. 2016;6:467-477.
- Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330:1029-1035.
- Or F, Yongjoo K, Simms J, et al. Taking stock of dietary supplements’ harmful effects on children, adolescents, and young adults [published online June 3, 2019]. J Adolesc Health. S1054-139X(19)30163-6. doi:10.1016/j.jadohealth.2019.03.005.
As the largest and most exposed organ in the body, the skin experiences trauma from both extrinsic and intrinsic aging factors, resulting in loss of elasticity, increased laxity, wrinkling, and rough-textured appearance.1 Chronologically aged skin appears dry, thin, and finely wrinkled; photoaged skin appears leathery with coarse wrinkles and uneven pigmentation.2 In recent years, numerous systemic nutrients have been proposed to improve skin appearance. This article reviews the efficacy of these vitamins and supplements.
Carotenoids
Carotenoids are a group of lipophilic molecules derived from vitamin A.3,4 Ingestion of carotenoids may play a role in photoprotection against UV radiation (UVR) by acting as acceptors of reactive oxygen species.4-6 Stahl et al7 investigated lycopene’s usefulness in protection against UVR-induced erythema. Over 10 weeks, 9 volunteers received 40 g of tomato paste containing 16 mg daily of lycopene while 10 controls received placebo. A solar simulator was used to induce erythema of the skin at weeks 0, 4, and 10. At week 10, erythema formation was 40% lower in the lycopene group compared to controls (P=.02).7
In another study assessing the photoprotective effects of a novel nutritional and phytonutrient blend of carotenoids, 36 women with Fitzpatrick skin types I and II were treated for 8 weeks.8 Presupplementation, UVR-induced erythema, and skin carotenoid concentrations were determined along with facial skin attributes and characteristics. Results showed protection against UVR-induced skin damage, with reductions in erythema at 3 minimal erythema doses (MEDs)(P=.01). Additionally, significant improvements were noted in facial skin elasticity, radiance, and overall appearance (all P<.05).8
In 2013, Meinke et al9 conducted an 8-week, double-blind, placebo-controlled study on 24 volunteers whose diets were supplemented with moderate amounts of carotenoids, including lutein, beta-carotene, and lycopene. Utilizing novel techniques to measure the skin’s ability to scavenge free radicals, they discovered that dietary carotenoids provided notable protection against stress-induced radical formation and increased baseline radical scavenging activity of the skin by 34%. The authors concluded that dietary supplementation could avoid premature skin aging.9
Vitamins C and E
Vitamin C is an essential vitamin that must be obtained through dietary sources.10 It functions as a free radical scavenger and is a necessary cofactor for the synthesis and stabilization of collagen.
A study evaluated the effect of UVR-induced oxidative stress and the association with vitamin C supplementation among 20 white patients with Fitzpatrick skin types II or III.11 The volunteers were treated with UVR on two 1-cm sites on the buttock. Six punch biopsies of these sites and 2 control biopsies from nonexposed skin were taken. Volunteers took vitamin C supplements (500 mg) for 8 weeks, and the exposure and biopsy were repeated. Researchers concluded that supplementation with vitamin C had no effect on the MED, with identical concentrations at baseline and after 8 weeks of supplementation. Additionally, there was no evidence that vitamin C affects UVR-induced oxidative stress.11
In 2007, Cosgrove et al12 conducted a study to assess the associations between nutrient intake and skin aging in more than 4000 women aged 40 to 74 years. Higher dietary vitamin C intakes were associated with a significantly lower likelihood of senile xerosis and wrinkled appearance (P<.009).12
Vitamin E is a lipid-soluble, membrane-bound vitamin, and its most active form is α-tocopherol.11,13 Vitamin E functions as an antioxidant and protects cellular membranes from lipid peroxidation by free radicals.13-15 Once oxidized, vitamin E can be regenerated to its reduced form by vitamin C.11 Their synergistic effects on skin protection have been studied extensively. A double-blind, placebo-controlled study of 10 patients compared 2 g of vitamin C combined with 1000 IU of vitamin E vs placebo.16 The patients’ skin reaction before and after 8 days of treatment were assessed by determination of MED and the cutaneous blood flow of skin irradiated with UV light. Results showed that the median MED of those taking vitamins increased from 80 to 96.5 mJ/cm2 (P<.01) and decreased for the placebo group. Investigators concluded that the combination of vitamins C and E reduces the sunburn reaction and leads to a reduction in the sequelae of UV-induced skin damage.16 A prospective, randomized, placebo-controlled study by Fuchs and Kern17 replicated these findings, also concluding that combinations of vitamins C and E provide improved photoprotective effects than either vitamin alone.
Vitamin D
Vitamin D is a fat-soluble vitamin obtained through dietary intake and exposure to UV light.3,18,19 Precursors of vitamin D require interaction with UV light for conversion into active forms. The highest concentrations of 7-dehydrocholesterol are found in keratinocytes in the basal cell and spinous cell layers of the skin where they are protected from UV light by melanin. As such, individuals with higher melanin content in their skin require more exposure to UV light to produce the same levels of vitamin D as those with less melanin,20 leading to a high rate of vitamin D deficiency in dark-skinned individuals. Because of their prodifferentiating and antiproliferative effects, vitamin D analogs have been very effective in the treatment of psoriasis.20,21 Vitamin D deficiency also has been implicated in the pathogenesis of vitiligo. A systematic review and meta-analysis conducted in 2016 found that a significant relationship existed between low 25-hydroxyvitamin D levels and vitiligo (P<.01), but no causal relationship could be established.22
A 2017 double-blind, placebo-controlled study performed by Scott et al23 aimed to elucidate the relationship between vitamin D concentrations and sunburn. Twenty adults received either placebo or high-dose vitamin D3 (200,000 IU) 1 hour after experimental sunburn induced by an erythemogenic dose of UVR. Investigators measured participants’ concentrations of the proinflammatory mediators tumor necrosis factor α and nitric oxide synthase via skin biopsy 48 hours later. Patients in the experimental group were found to have significantly reduced expression of both tumor necrosis factor α (P=.04) and nitric oxide synthase (P=.02). Additionally, participants with significantly higher vitamin D3 levels following supplementation (P=.007) demonstrated increased skin expression of the anti-inflammatory marker arginase-1 (P=.005) as well as a persistent reduction in skin redness (P=.02). Investigators concluded that vitamin D plays a large role in skin homeostasis and implicated vitamin D’s upregulation of arginase-1 as a potent mechanism of its anti-inflammatory effects.23
Collagen
As humans age, the density of collagen in the dermis decreases, leading to sagging and wrinkling of skin.24 Oral supplementation of collagen has been examined for its dermatologic benefits, primarily increasing the thickness and density of collagen in the dermal layer. In 2014, Proksch et al25 performed a double-blind, placebo-controlled trial in which 69 women were randomized to receive 2.5 or 5 g of collagen peptides or placebo for 8 weeks. Both treatment groups demonstrated improvements in skin elasticity as well as improved skin moisture and decreased skin evaporation; however, changes in the latter 2 qualities failed to reach statistical significance.25
The results of this study were replicated by Asserin et al.26 One hundred six female patients were randomly assigned to receive 10 g of collagen peptides or placebo daily for 8 weeks. The collagen group demonstrated significantly improved skin hydration (P=.003) and increased density of collagen in the dermis (P=.007) relative to placebo.26
In another randomized, double-blind, placebo-controlled study, 71 women consumed a 20-mL beverage containing either 3000 mg of collagen peptides or placebo for 12 weeks.27 Participants in the treatment group demonstrated significant decreases in periorbital wrinkles (P<.05) and enhanced facial skin moisture (P<.001) and elasticity (P<.001) after 12 weeks. Researchers concluded that oral supplementation with collagen peptides holds promise as a natural supplement to provide cutaneous antiaging properties.27
Ceramides
Ceramides are lipids composed of a sphingoid base conjugated to a fatty acid and serve as the main component of the stratum corneum of the skin. Ceramides are crucial for the maintenance of skin barrier integrity and for preventing transepidermal water loss.28 In a 3-month study of 51 women with dry skin, Guillou et al29 showed that a ceramide wheat extract capsule significantly increased corneometry measurements of skin hydration on the arms (P<.001) and the legs (P=.012) compared to placebo.
Mixed Supplements
The discovery that nutritional contents can affect skin appearance has energized the development of combination supplements containing multiple vitamins and micronutrients. Imedeen is a biomarine complex and antioxidant supplement with several different formulations, including Prime Renewal, Time Perfection, and Derma One (Pfizer Inc). The ingredients include a combination of a biomarine complex (blend of fish proteins and polysaccharides), lycopene, grape seed extract, vitamin C, vitamin E, and zinc. Several trials have been conducted to assess the efficacy of the supplements on improving the appearance of photodamaged and aged skin (Table).
A placebo-controlled, randomized study of 144 participants conducted by Kieffer and Efsen30 assessed the efficacy of Imedeen supplements over 12 months. The trial included a 3-month placebo-controlled study and 9-month uncontrolled continuation. Imedeen’s efficacy was measured using clinical evaluation, transepidermal water loss, self-evaluation, and photograph evaluation. After 1 year of treatment, improvement occurred in photograph evaluation of fine lines, overall photoaging, telangiectasia and hyperpigmentation, and self-evaluation of skin condition.30 Additional double-blind, placebo-controlled, randomized studies assessing the efficacy of Imedeen have shown increased dermal and epidermal thickness, improvement of stratum corneum moisturization, and improved overall facial complexion.31-33
Several combined supplements containing collagen peptide as the main ingredient have been created for use in skin care. Collagen is found in the extracellular matrix of the dermis and is responsible for the resiliency and strength of skin.34,35 Damage to the dermis can occur with prolonged UV light exposure and is seen histologically as disorganized collagen fibrils and grossly as wrinkles and photoaged skin.35,36
A study assessed the effect of BioCell Collagen (BioCell Technology, LLC), a supplement containing type II collagen, on skin aging.37 Twenty-six women underwent baseline visual assessments of their skin before taking 2 tablets of the supplement daily. Twelve weeks of supplementation led to significant reduction in global lines and wrinkles (13.2%; P=.028) as well as skin dryness and scaling (76%; P=.002). Assessment of collagen content at 6 weeks revealed a significant increase from baseline (6.3%; P=.002), though the difference after 12 weeks was not significant (3.5%; P=.134). The authors concluded that although preliminary data suggested that BioCell Collagen may reduce visible signs of aging, a controlled study was necessary to verify this finding.37
A single-blind, case-controlled study assessed a similar supplement, Celergen, that contained marine collagen peptides.38 Forty-one adults took 2 capsules each day for 60 days. Assessment of their skin physiology was conducted at the enrollment visit, 2 months later, and after the treatment period ended. Skin elasticity, transepidermal water loss, epidermal and dermal thickness, and density were measured. Investigators found that Celergen administration significantly enhanced skin elasticity and sebum production (P<.0001) but did not influence cutaneous moisture. The dermal thickness and homogenous distribution of collagen fibers were enhanced in 11 patients while properties of the epidermis remained unchanged. The study determined that supplementation remarkably improved skin elasticity, sebum production, and dermal ultrasonic markers.38
A double-blind, randomized, placebo-controlled study assessed a collagen- and antioxidant-containing supplement, Gold Collagen Forte, on skin properties.39 The treatment and placebo groups each consisted of 60 patients who consumed 1 bottle (50 mL) of the product each day for 90 days. Patients completed a self-assessment of their skin regarding photoaging, focusing on the crow’s-feet area and nasolabial folds, while skin elasticity was assessed with the SkinLab USB elasticity module. Results showed a significant increase in skin elasticity (+7.5%; P≤.001). Self-assessment results showed improvements in both the treatment and placebo groups, and investigators concluded that Gold Collagen Forte may have photoprotective effects and help improve skin health.39
Safety
Although trials have demonstrated vitamin supplementation to be safe and effective for skin enhancement, it is important to consider potential vitamin toxicities. High doses of vitamin C supplementation have been shown to cause damage via lipid peroxidation.40 In a study assessing if high levels of beta-carotene and vitamin E were associated with a lower risk for lung cancer, data showed that these supplements may actually have harmful effects.40,41 Additionally, consumption of high-dose dietary supplements has been associated with an increased risk for severe medical events, including disability and death among adolescents and young adults.42
Conclusion
Numerous trials have indicated that the use of systemic vitamins can have beneficial effects on the protection and appearance of skin. Photodamage from UV light–induced erythema can be decreased by carotenoids and vitamins C and E. Similarly, supplements that combine multiple nutrients with collagen have been shown to improve the appearance of aging skin by decreasing the prominence of wrinkles. Given the growing number of products and advertisements that exist in the supplement marketplace, it is crucial for clinicians to ground their recommendations to patients in the scientific data of robust studies.
As the largest and most exposed organ in the body, the skin experiences trauma from both extrinsic and intrinsic aging factors, resulting in loss of elasticity, increased laxity, wrinkling, and rough-textured appearance.1 Chronologically aged skin appears dry, thin, and finely wrinkled; photoaged skin appears leathery with coarse wrinkles and uneven pigmentation.2 In recent years, numerous systemic nutrients have been proposed to improve skin appearance. This article reviews the efficacy of these vitamins and supplements.
Carotenoids
Carotenoids are a group of lipophilic molecules derived from vitamin A.3,4 Ingestion of carotenoids may play a role in photoprotection against UV radiation (UVR) by acting as acceptors of reactive oxygen species.4-6 Stahl et al7 investigated lycopene’s usefulness in protection against UVR-induced erythema. Over 10 weeks, 9 volunteers received 40 g of tomato paste containing 16 mg daily of lycopene while 10 controls received placebo. A solar simulator was used to induce erythema of the skin at weeks 0, 4, and 10. At week 10, erythema formation was 40% lower in the lycopene group compared to controls (P=.02).7
In another study assessing the photoprotective effects of a novel nutritional and phytonutrient blend of carotenoids, 36 women with Fitzpatrick skin types I and II were treated for 8 weeks.8 Presupplementation, UVR-induced erythema, and skin carotenoid concentrations were determined along with facial skin attributes and characteristics. Results showed protection against UVR-induced skin damage, with reductions in erythema at 3 minimal erythema doses (MEDs)(P=.01). Additionally, significant improvements were noted in facial skin elasticity, radiance, and overall appearance (all P<.05).8
In 2013, Meinke et al9 conducted an 8-week, double-blind, placebo-controlled study on 24 volunteers whose diets were supplemented with moderate amounts of carotenoids, including lutein, beta-carotene, and lycopene. Utilizing novel techniques to measure the skin’s ability to scavenge free radicals, they discovered that dietary carotenoids provided notable protection against stress-induced radical formation and increased baseline radical scavenging activity of the skin by 34%. The authors concluded that dietary supplementation could avoid premature skin aging.9
Vitamins C and E
Vitamin C is an essential vitamin that must be obtained through dietary sources.10 It functions as a free radical scavenger and is a necessary cofactor for the synthesis and stabilization of collagen.
A study evaluated the effect of UVR-induced oxidative stress and the association with vitamin C supplementation among 20 white patients with Fitzpatrick skin types II or III.11 The volunteers were treated with UVR on two 1-cm sites on the buttock. Six punch biopsies of these sites and 2 control biopsies from nonexposed skin were taken. Volunteers took vitamin C supplements (500 mg) for 8 weeks, and the exposure and biopsy were repeated. Researchers concluded that supplementation with vitamin C had no effect on the MED, with identical concentrations at baseline and after 8 weeks of supplementation. Additionally, there was no evidence that vitamin C affects UVR-induced oxidative stress.11
In 2007, Cosgrove et al12 conducted a study to assess the associations between nutrient intake and skin aging in more than 4000 women aged 40 to 74 years. Higher dietary vitamin C intakes were associated with a significantly lower likelihood of senile xerosis and wrinkled appearance (P<.009).12
Vitamin E is a lipid-soluble, membrane-bound vitamin, and its most active form is α-tocopherol.11,13 Vitamin E functions as an antioxidant and protects cellular membranes from lipid peroxidation by free radicals.13-15 Once oxidized, vitamin E can be regenerated to its reduced form by vitamin C.11 Their synergistic effects on skin protection have been studied extensively. A double-blind, placebo-controlled study of 10 patients compared 2 g of vitamin C combined with 1000 IU of vitamin E vs placebo.16 The patients’ skin reaction before and after 8 days of treatment were assessed by determination of MED and the cutaneous blood flow of skin irradiated with UV light. Results showed that the median MED of those taking vitamins increased from 80 to 96.5 mJ/cm2 (P<.01) and decreased for the placebo group. Investigators concluded that the combination of vitamins C and E reduces the sunburn reaction and leads to a reduction in the sequelae of UV-induced skin damage.16 A prospective, randomized, placebo-controlled study by Fuchs and Kern17 replicated these findings, also concluding that combinations of vitamins C and E provide improved photoprotective effects than either vitamin alone.
Vitamin D
Vitamin D is a fat-soluble vitamin obtained through dietary intake and exposure to UV light.3,18,19 Precursors of vitamin D require interaction with UV light for conversion into active forms. The highest concentrations of 7-dehydrocholesterol are found in keratinocytes in the basal cell and spinous cell layers of the skin where they are protected from UV light by melanin. As such, individuals with higher melanin content in their skin require more exposure to UV light to produce the same levels of vitamin D as those with less melanin,20 leading to a high rate of vitamin D deficiency in dark-skinned individuals. Because of their prodifferentiating and antiproliferative effects, vitamin D analogs have been very effective in the treatment of psoriasis.20,21 Vitamin D deficiency also has been implicated in the pathogenesis of vitiligo. A systematic review and meta-analysis conducted in 2016 found that a significant relationship existed between low 25-hydroxyvitamin D levels and vitiligo (P<.01), but no causal relationship could be established.22
A 2017 double-blind, placebo-controlled study performed by Scott et al23 aimed to elucidate the relationship between vitamin D concentrations and sunburn. Twenty adults received either placebo or high-dose vitamin D3 (200,000 IU) 1 hour after experimental sunburn induced by an erythemogenic dose of UVR. Investigators measured participants’ concentrations of the proinflammatory mediators tumor necrosis factor α and nitric oxide synthase via skin biopsy 48 hours later. Patients in the experimental group were found to have significantly reduced expression of both tumor necrosis factor α (P=.04) and nitric oxide synthase (P=.02). Additionally, participants with significantly higher vitamin D3 levels following supplementation (P=.007) demonstrated increased skin expression of the anti-inflammatory marker arginase-1 (P=.005) as well as a persistent reduction in skin redness (P=.02). Investigators concluded that vitamin D plays a large role in skin homeostasis and implicated vitamin D’s upregulation of arginase-1 as a potent mechanism of its anti-inflammatory effects.23
Collagen
As humans age, the density of collagen in the dermis decreases, leading to sagging and wrinkling of skin.24 Oral supplementation of collagen has been examined for its dermatologic benefits, primarily increasing the thickness and density of collagen in the dermal layer. In 2014, Proksch et al25 performed a double-blind, placebo-controlled trial in which 69 women were randomized to receive 2.5 or 5 g of collagen peptides or placebo for 8 weeks. Both treatment groups demonstrated improvements in skin elasticity as well as improved skin moisture and decreased skin evaporation; however, changes in the latter 2 qualities failed to reach statistical significance.25
The results of this study were replicated by Asserin et al.26 One hundred six female patients were randomly assigned to receive 10 g of collagen peptides or placebo daily for 8 weeks. The collagen group demonstrated significantly improved skin hydration (P=.003) and increased density of collagen in the dermis (P=.007) relative to placebo.26
In another randomized, double-blind, placebo-controlled study, 71 women consumed a 20-mL beverage containing either 3000 mg of collagen peptides or placebo for 12 weeks.27 Participants in the treatment group demonstrated significant decreases in periorbital wrinkles (P<.05) and enhanced facial skin moisture (P<.001) and elasticity (P<.001) after 12 weeks. Researchers concluded that oral supplementation with collagen peptides holds promise as a natural supplement to provide cutaneous antiaging properties.27
Ceramides
Ceramides are lipids composed of a sphingoid base conjugated to a fatty acid and serve as the main component of the stratum corneum of the skin. Ceramides are crucial for the maintenance of skin barrier integrity and for preventing transepidermal water loss.28 In a 3-month study of 51 women with dry skin, Guillou et al29 showed that a ceramide wheat extract capsule significantly increased corneometry measurements of skin hydration on the arms (P<.001) and the legs (P=.012) compared to placebo.
Mixed Supplements
The discovery that nutritional contents can affect skin appearance has energized the development of combination supplements containing multiple vitamins and micronutrients. Imedeen is a biomarine complex and antioxidant supplement with several different formulations, including Prime Renewal, Time Perfection, and Derma One (Pfizer Inc). The ingredients include a combination of a biomarine complex (blend of fish proteins and polysaccharides), lycopene, grape seed extract, vitamin C, vitamin E, and zinc. Several trials have been conducted to assess the efficacy of the supplements on improving the appearance of photodamaged and aged skin (Table).
A placebo-controlled, randomized study of 144 participants conducted by Kieffer and Efsen30 assessed the efficacy of Imedeen supplements over 12 months. The trial included a 3-month placebo-controlled study and 9-month uncontrolled continuation. Imedeen’s efficacy was measured using clinical evaluation, transepidermal water loss, self-evaluation, and photograph evaluation. After 1 year of treatment, improvement occurred in photograph evaluation of fine lines, overall photoaging, telangiectasia and hyperpigmentation, and self-evaluation of skin condition.30 Additional double-blind, placebo-controlled, randomized studies assessing the efficacy of Imedeen have shown increased dermal and epidermal thickness, improvement of stratum corneum moisturization, and improved overall facial complexion.31-33
Several combined supplements containing collagen peptide as the main ingredient have been created for use in skin care. Collagen is found in the extracellular matrix of the dermis and is responsible for the resiliency and strength of skin.34,35 Damage to the dermis can occur with prolonged UV light exposure and is seen histologically as disorganized collagen fibrils and grossly as wrinkles and photoaged skin.35,36
A study assessed the effect of BioCell Collagen (BioCell Technology, LLC), a supplement containing type II collagen, on skin aging.37 Twenty-six women underwent baseline visual assessments of their skin before taking 2 tablets of the supplement daily. Twelve weeks of supplementation led to significant reduction in global lines and wrinkles (13.2%; P=.028) as well as skin dryness and scaling (76%; P=.002). Assessment of collagen content at 6 weeks revealed a significant increase from baseline (6.3%; P=.002), though the difference after 12 weeks was not significant (3.5%; P=.134). The authors concluded that although preliminary data suggested that BioCell Collagen may reduce visible signs of aging, a controlled study was necessary to verify this finding.37
A single-blind, case-controlled study assessed a similar supplement, Celergen, that contained marine collagen peptides.38 Forty-one adults took 2 capsules each day for 60 days. Assessment of their skin physiology was conducted at the enrollment visit, 2 months later, and after the treatment period ended. Skin elasticity, transepidermal water loss, epidermal and dermal thickness, and density were measured. Investigators found that Celergen administration significantly enhanced skin elasticity and sebum production (P<.0001) but did not influence cutaneous moisture. The dermal thickness and homogenous distribution of collagen fibers were enhanced in 11 patients while properties of the epidermis remained unchanged. The study determined that supplementation remarkably improved skin elasticity, sebum production, and dermal ultrasonic markers.38
A double-blind, randomized, placebo-controlled study assessed a collagen- and antioxidant-containing supplement, Gold Collagen Forte, on skin properties.39 The treatment and placebo groups each consisted of 60 patients who consumed 1 bottle (50 mL) of the product each day for 90 days. Patients completed a self-assessment of their skin regarding photoaging, focusing on the crow’s-feet area and nasolabial folds, while skin elasticity was assessed with the SkinLab USB elasticity module. Results showed a significant increase in skin elasticity (+7.5%; P≤.001). Self-assessment results showed improvements in both the treatment and placebo groups, and investigators concluded that Gold Collagen Forte may have photoprotective effects and help improve skin health.39
Safety
Although trials have demonstrated vitamin supplementation to be safe and effective for skin enhancement, it is important to consider potential vitamin toxicities. High doses of vitamin C supplementation have been shown to cause damage via lipid peroxidation.40 In a study assessing if high levels of beta-carotene and vitamin E were associated with a lower risk for lung cancer, data showed that these supplements may actually have harmful effects.40,41 Additionally, consumption of high-dose dietary supplements has been associated with an increased risk for severe medical events, including disability and death among adolescents and young adults.42
Conclusion
Numerous trials have indicated that the use of systemic vitamins can have beneficial effects on the protection and appearance of skin. Photodamage from UV light–induced erythema can be decreased by carotenoids and vitamins C and E. Similarly, supplements that combine multiple nutrients with collagen have been shown to improve the appearance of aging skin by decreasing the prominence of wrinkles. Given the growing number of products and advertisements that exist in the supplement marketplace, it is crucial for clinicians to ground their recommendations to patients in the scientific data of robust studies.
- Zhang S, Duan E. Fighting against skin aging: the way from bench to bedside. Cell Transplant. 2018;27:729-738.
- Rittié L, Fisher GJ. Natural and sun-induced aging of human skin. Cold Spring Harb Perspect Med. 2015;5:a015370.
- Draelos ZD. Nutrition and enhancing youthful-appearing skin. Clin Dermatol. 2010;28:400-408.
- Anunciato TP, da Rocha Filho PA. Carotenoids and polyphenols in nutricosmetics, nutraceuticals, and cosmeceuticals. J Cosmet Dermatol. 2012;11:51-54.
- Stahl W, Heinrich U, Jungmann H, et al. Carotenoids and carotenoids plus vitamin E protect against ultraviolet light-induced erythema in humans. Am J Clin Nutr. 2000;71:795-798.
- Anstey AV. Systemic photoprotection with alpha-tocopherol (vitamin E) and beta-carotene. Clin Exp Dermatol. 2002;27:170-176.
- Stahl W, Heinrich U, Wiseman S, et al. Dietary tomato paste protects against ultraviolet light-induced erythema in humans. J Nutr. 2001;131:1449-1451.
- Wood SM, Mastaloudis AF, Hester SN, et al. Protective effects of a novel nutritional and phytonutrient blend on ultraviolet radiation-induced skin damage and inflammatory response through aging defense mechanisms. J Cosmet Dermatol. 2017;16:491-499.
- Meinke MC, Friedrich A, Tscherch K, et al. Influence of dietary carotenoids on radical scavenging capacity of the skin and skin lipids. Eur J Pharm Biopharm. 2013;84:365-373.
- Manela-Azulay M, Bagatin E. Cosmeceuticals vitamins. Clin Dermatol. 2009;27:469-474.
- McArdle F, Rhodes LE, Parslew R, et al. UVR-induced oxidative stress in human skin in vivo: effects of oral vitamin C supplementation. Free Radic Biol Med. 2002;33:1355-1362.
- Cosgrove MC, Franco OH, Granger SP, et al. Dietary nutrient intakes and skin-aging appearance among middle-aged American women. Am J Clin Nutr. 2007;86:1225-1231.
- Thiele JJ, Ekanayake-Mudiyanselage S. Vitamin E in human skin: organ-specific physiology and considerations for its use in dermatology. Mol Aspects Med. 2007;28:646-667.
- Schagen SK, Zampeli VA, Makrantonaki E, et al. Discovering the link between nutrition and skin aging. Dermatoendocrinol. 2012;4:298-307.
- Chan AC. Partners in defense, vitamin E and vitamin C. Can J Physiol Pharmacol. 1993;71:725-731.
- Eberlein-Konig B, Placzek M, Przybilla B. Protective effect against sunburn of combined systemic ascorbic acid (vitamin C) and d-alpha-tocopherol (vitamin E). J Am Acad Dermatol. 1998;38:45-48.
- Fuchs J, Kern H. Modulation of UV-light-induced skin inflammation by D-alpha-tocopherol and L-ascorbic acid: a clinical study using solar simulated radiation. Free Radic Biol Med. 1998;25:1006-1012.
- Shahriari M, Kerr PE, Slade K, et al. Vitamin D and the skin. Clin Dermatol. 2010;28:663-668.
- Soleymani T, Hung T, Soung J. The role of vitamin D in psoriasis: a review. Int J Dermatol. 2015;54:383-392.
- Lehmann B, Querings K, Reichrath J. Vitamin D and skin: new aspects for dermatology. Exp Dermatol. 2004;13(suppl 4):11-15.
- Kannan S, Lim HW. Photoprotection and vitamin D: a review. Photodermatol Photoimmunol Photomed. 2014;30:137-145.
- Upala S, Sanguankeo A. Low 25-hydroxyvitamin D levels are associated with vitiligo: a systematic review and meta-analysis. Photodermatol Photoimmunol Photomed. 2016;32:181-190.
- Scott JF, Das LM, Ahsanuddin S, et al. Oral vitamin D rapidly attenuates inflammation from sunburn: an interventional study. J Invest Dermatol. 2017;137:2078-2086.
- Varani J, Dame MK, Rittie L, et al. Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am J Pathol. 2006;168:1861-1868.
- Proksch E, Segger D, Degwert J, et al. Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: a double-blind, placebo-controlled study. Skin Pharmacol Physiol. 2014;27:47-55.
- Asserin J, Lati E, Shioya T, et al. The effect of oral collagen peptide supplementation on skin moisture and the dermal collagen network: evidence from an ex vivo model and randomized, placebo-controlled clinical trials. J Cosmet Dermatol. 2015;14:291-301.
- Koizumi S, Inoue N, Shimizu M, et al. Effects of dietary supplementation with fish scales-derived collagen peptides on skin parameters and condition: a randomized, placebo-controlled, double-blind study. Int J Peptide Res Ther. 2018;24:397-402.
- Vollmer DL, West VA, Lephart ED. Enhancing skin health: by oral administration of natural compounds and minerals with implications to the dermal microbiome. Int J Mol Sci. 2018;19. doi:10.3390/ijms19103059.
- Guillou S, Ghabri S, Jannot C, et al. The moisturizing effect of a wheat extract food supplement on women’s skin: a randomized, double-blind placebo-controlled trial. Int J Cosmet Sci. 2011;33:138-143.
- Kieffer ME, Efsen J. Imedeen in the treatment of photoaged skin: an efficacy and safety trial over 12 months. J Eur Acad Dermatol Venereol. 1998;11:129-136.
- Skovgaard GR, Jensen AS, Sigler ML. Effect of a novel dietary supplement on skin aging in post-menopausal women. Eur J Clin Nutr. 2006;60:1201-1206.
- Stephens TJ, Sigler ML, Herndon JH Jr, et al. A placebo-controlled, double-blind clinical trial to evaluate the efficacy of Imedeen(®) Time Perfection(®) for improving the appearance of photodamaged skin. Clin Cosmet Investig Dermatol. 2016;9:63-70.
- Stephens TJ, Sigler ML, Hino PD, et al. A randomized, double-blind, placebo-controlled clinical trial evaluating an oral anti-aging skin care supplement for treating photodamaged skin. J Clin Aesthet Dermatol. 2016;9:25-32.
- El-Domyati M, Attia S, Saleh F, et al. Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp Dermatol. 2002;11:398-405.
- Fisher GJ, Wang ZQ, Datta SC, et al. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337:1419-1428.
- Kang MC, Yumnam S, Kim SY. Oral intake of collagen peptide attenuates ultraviolet B irradiation-induced skin dehydration in vivo by regulating hyaluronic acid synthesis. Int J Mol Sci. 2018;19. doi:10.3390/ijms19113551.
- Schwartz SR, Park J. Ingestion of BioCell Collagen(®), a novel hydrolyzed chicken sternal cartilage extract; enhanced blood microcirculation and reduced facial aging signs. Clin Interv Aging. 2012;7:267-273.
- De Luca C, Mikhal’chik EV, Suprun MV, et al. Skin antiageing and systemic redox effects of supplementation with marine collagen peptides and plant-derived antioxidants: a single-blind case-control clinical study. Oxid Med Cell Longev. 2016;2016:4389410.
- Genovese L, Corbo A, Sibilla S. An insight into the changes in skin texture and properties following dietary intervention with a nutricosmeceutical containing a blend of collagen bioactive peptides and antioxidants. Skin Pharmacol Physiol. 2017;30:146-158.
- Hamishehkar H, Ranjdoost F, Asgharian P, et al. Vitamins, are they safe? Adv Pharm Bull. 2016;6:467-477.
- Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330:1029-1035.
- Or F, Yongjoo K, Simms J, et al. Taking stock of dietary supplements’ harmful effects on children, adolescents, and young adults [published online June 3, 2019]. J Adolesc Health. S1054-139X(19)30163-6. doi:10.1016/j.jadohealth.2019.03.005.
- Zhang S, Duan E. Fighting against skin aging: the way from bench to bedside. Cell Transplant. 2018;27:729-738.
- Rittié L, Fisher GJ. Natural and sun-induced aging of human skin. Cold Spring Harb Perspect Med. 2015;5:a015370.
- Draelos ZD. Nutrition and enhancing youthful-appearing skin. Clin Dermatol. 2010;28:400-408.
- Anunciato TP, da Rocha Filho PA. Carotenoids and polyphenols in nutricosmetics, nutraceuticals, and cosmeceuticals. J Cosmet Dermatol. 2012;11:51-54.
- Stahl W, Heinrich U, Jungmann H, et al. Carotenoids and carotenoids plus vitamin E protect against ultraviolet light-induced erythema in humans. Am J Clin Nutr. 2000;71:795-798.
- Anstey AV. Systemic photoprotection with alpha-tocopherol (vitamin E) and beta-carotene. Clin Exp Dermatol. 2002;27:170-176.
- Stahl W, Heinrich U, Wiseman S, et al. Dietary tomato paste protects against ultraviolet light-induced erythema in humans. J Nutr. 2001;131:1449-1451.
- Wood SM, Mastaloudis AF, Hester SN, et al. Protective effects of a novel nutritional and phytonutrient blend on ultraviolet radiation-induced skin damage and inflammatory response through aging defense mechanisms. J Cosmet Dermatol. 2017;16:491-499.
- Meinke MC, Friedrich A, Tscherch K, et al. Influence of dietary carotenoids on radical scavenging capacity of the skin and skin lipids. Eur J Pharm Biopharm. 2013;84:365-373.
- Manela-Azulay M, Bagatin E. Cosmeceuticals vitamins. Clin Dermatol. 2009;27:469-474.
- McArdle F, Rhodes LE, Parslew R, et al. UVR-induced oxidative stress in human skin in vivo: effects of oral vitamin C supplementation. Free Radic Biol Med. 2002;33:1355-1362.
- Cosgrove MC, Franco OH, Granger SP, et al. Dietary nutrient intakes and skin-aging appearance among middle-aged American women. Am J Clin Nutr. 2007;86:1225-1231.
- Thiele JJ, Ekanayake-Mudiyanselage S. Vitamin E in human skin: organ-specific physiology and considerations for its use in dermatology. Mol Aspects Med. 2007;28:646-667.
- Schagen SK, Zampeli VA, Makrantonaki E, et al. Discovering the link between nutrition and skin aging. Dermatoendocrinol. 2012;4:298-307.
- Chan AC. Partners in defense, vitamin E and vitamin C. Can J Physiol Pharmacol. 1993;71:725-731.
- Eberlein-Konig B, Placzek M, Przybilla B. Protective effect against sunburn of combined systemic ascorbic acid (vitamin C) and d-alpha-tocopherol (vitamin E). J Am Acad Dermatol. 1998;38:45-48.
- Fuchs J, Kern H. Modulation of UV-light-induced skin inflammation by D-alpha-tocopherol and L-ascorbic acid: a clinical study using solar simulated radiation. Free Radic Biol Med. 1998;25:1006-1012.
- Shahriari M, Kerr PE, Slade K, et al. Vitamin D and the skin. Clin Dermatol. 2010;28:663-668.
- Soleymani T, Hung T, Soung J. The role of vitamin D in psoriasis: a review. Int J Dermatol. 2015;54:383-392.
- Lehmann B, Querings K, Reichrath J. Vitamin D and skin: new aspects for dermatology. Exp Dermatol. 2004;13(suppl 4):11-15.
- Kannan S, Lim HW. Photoprotection and vitamin D: a review. Photodermatol Photoimmunol Photomed. 2014;30:137-145.
- Upala S, Sanguankeo A. Low 25-hydroxyvitamin D levels are associated with vitiligo: a systematic review and meta-analysis. Photodermatol Photoimmunol Photomed. 2016;32:181-190.
- Scott JF, Das LM, Ahsanuddin S, et al. Oral vitamin D rapidly attenuates inflammation from sunburn: an interventional study. J Invest Dermatol. 2017;137:2078-2086.
- Varani J, Dame MK, Rittie L, et al. Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am J Pathol. 2006;168:1861-1868.
- Proksch E, Segger D, Degwert J, et al. Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: a double-blind, placebo-controlled study. Skin Pharmacol Physiol. 2014;27:47-55.
- Asserin J, Lati E, Shioya T, et al. The effect of oral collagen peptide supplementation on skin moisture and the dermal collagen network: evidence from an ex vivo model and randomized, placebo-controlled clinical trials. J Cosmet Dermatol. 2015;14:291-301.
- Koizumi S, Inoue N, Shimizu M, et al. Effects of dietary supplementation with fish scales-derived collagen peptides on skin parameters and condition: a randomized, placebo-controlled, double-blind study. Int J Peptide Res Ther. 2018;24:397-402.
- Vollmer DL, West VA, Lephart ED. Enhancing skin health: by oral administration of natural compounds and minerals with implications to the dermal microbiome. Int J Mol Sci. 2018;19. doi:10.3390/ijms19103059.
- Guillou S, Ghabri S, Jannot C, et al. The moisturizing effect of a wheat extract food supplement on women’s skin: a randomized, double-blind placebo-controlled trial. Int J Cosmet Sci. 2011;33:138-143.
- Kieffer ME, Efsen J. Imedeen in the treatment of photoaged skin: an efficacy and safety trial over 12 months. J Eur Acad Dermatol Venereol. 1998;11:129-136.
- Skovgaard GR, Jensen AS, Sigler ML. Effect of a novel dietary supplement on skin aging in post-menopausal women. Eur J Clin Nutr. 2006;60:1201-1206.
- Stephens TJ, Sigler ML, Herndon JH Jr, et al. A placebo-controlled, double-blind clinical trial to evaluate the efficacy of Imedeen(®) Time Perfection(®) for improving the appearance of photodamaged skin. Clin Cosmet Investig Dermatol. 2016;9:63-70.
- Stephens TJ, Sigler ML, Hino PD, et al. A randomized, double-blind, placebo-controlled clinical trial evaluating an oral anti-aging skin care supplement for treating photodamaged skin. J Clin Aesthet Dermatol. 2016;9:25-32.
- El-Domyati M, Attia S, Saleh F, et al. Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp Dermatol. 2002;11:398-405.
- Fisher GJ, Wang ZQ, Datta SC, et al. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337:1419-1428.
- Kang MC, Yumnam S, Kim SY. Oral intake of collagen peptide attenuates ultraviolet B irradiation-induced skin dehydration in vivo by regulating hyaluronic acid synthesis. Int J Mol Sci. 2018;19. doi:10.3390/ijms19113551.
- Schwartz SR, Park J. Ingestion of BioCell Collagen(®), a novel hydrolyzed chicken sternal cartilage extract; enhanced blood microcirculation and reduced facial aging signs. Clin Interv Aging. 2012;7:267-273.
- De Luca C, Mikhal’chik EV, Suprun MV, et al. Skin antiageing and systemic redox effects of supplementation with marine collagen peptides and plant-derived antioxidants: a single-blind case-control clinical study. Oxid Med Cell Longev. 2016;2016:4389410.
- Genovese L, Corbo A, Sibilla S. An insight into the changes in skin texture and properties following dietary intervention with a nutricosmeceutical containing a blend of collagen bioactive peptides and antioxidants. Skin Pharmacol Physiol. 2017;30:146-158.
- Hamishehkar H, Ranjdoost F, Asgharian P, et al. Vitamins, are they safe? Adv Pharm Bull. 2016;6:467-477.
- Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330:1029-1035.
- Or F, Yongjoo K, Simms J, et al. Taking stock of dietary supplements’ harmful effects on children, adolescents, and young adults [published online June 3, 2019]. J Adolesc Health. S1054-139X(19)30163-6. doi:10.1016/j.jadohealth.2019.03.005.
Practice Points
- Multiple vitamins and supplements have demonstrated evidence in improving skin appearance.
- Carotenoids, along with vitamins C and E, have been shown to protect skin from UV-induced photodamage, while supplements containing collagen decrease the appearance of wrinkles.
Nonsurgical Hair Restoration Treatment
Hair plays an important role in identity, self-perception, and psychosocial functioning. Hair loss can be a devastating experience that decreases self-esteem and feelings of personal attractiveness while also leading to depression and anxiety.1,2 Although increasingly popular, surgical hair restoration, including hair transplantation, is costly and carries considerable risk.
Results of nonsurgical hair restoration are not immediate and may not be as dramatic; however, they do not carry the risks or recovery associated with surgical options. Treatments such as sex steroid hormone and biologic response modifiers have been used to inhibit hair miniaturization and stabilize hair loss in cases of androgenic alopecia (AGA).3 Currently, minoxidil and finasteride are the only US Food and Drug Administration (FDA)–approved medications for the treatment of hair loss; however, other nonsurgical treatment options have gained popularity, including dutasteride, spironolactone, low-level laser therapy (LLLT), platelet-rich plasma (PRP), microneedling, stem cells, and nutraceutical supplements. We provide an overview of these treatment options to help dermatologists select appropriate therapies for the treatment of alopecia (Table).
Minoxidil
Minoxidil has been known to improve hair growth for more than 40 years. Oral minoxidil was first introduced for hypertension in the 1970s with a common adverse effect of hypertrichosis; the 2% solution was marketed for AGA shortly thereafter in 1986.4 Minoxidil is a biologic response modifier that is thought to promote hair growth through vasodilation and stimulation of hair follicles into the growth phase.5 In animal studies, topical minoxidil has been shown to shorten telogen, prolong anagen, and increase hair follicle size.6,7 More recently, topical minoxidil was shown to have anti-inflammatory effects by downregulating IL-1, which may confer an additional role in combatting alopecia.8
Minoxidil is FDA approved for treatment of AGA in men and women and often is used as first-line therapy.9 In 3 separate meta-analyses of topical minoxidil, it was shown to be more effective than placebo for treating AGA in men and women, with a notable increase in target area hair growth.10 A study of 777 male patients treated with topical minoxidil 2% found that 45% subjectively experienced new hair growth.11 However, results may vary, and research indicates that higher concentrations are more effective. In a randomized, double-blind, placebo-controlled trial of 381 women with female pattern hair loss (FPHL), minoxidil solution 2% was found to be superior to placebo after 48 weeks, with average changes in nonvellus hair counts of 20.7/cm2 in the minoxidil group vs 9.4/cm2 in the placebo group.12 In a separate meta-analysis, minoxidil solution 5% demonstrated superiority to both the 2% formulation and placebo with a mean change in nonvellus hair counts of 26.0/cm2.13
Minoxidil also has demonstrated promising benefits in preventing chemotherapy-induced alopecia. Although oncologists most often use the scalp cooling method to prevent hair loss by decreasing perfusion and uptake of cytotoxic agents, cost may be prohibitive, as it is often not reimbursable by insurance companies.14,15 On the other hand, minoxidil is easily procured over-the-counter and has been successfully used to decrease the duration of alopecia caused by chemotherapeutic agents such as fluorouracil, doxorubicin, and cyclophosphamide, as well as endocrine therapies used to treat breast cancer in women.16-18 Minoxidil also has been used off label to treat other forms of alopecia, including alopecia areata, telogen effluvium, eyebrow hypotrichosis, and monilethrix; however, there is inconclusive evidence for its efficacy.5,13,19
Compared to other nonsurgical treatments for hair loss, a meta-analysis found that minoxidil was associated with the highest rate of adverse effects (AEs).16,17 Potential side effects include pruritus or burning at the application site; irritant or allergic contact dermatitis; hypertrichosis; and cardiovascular effects, which may be due to the vasodilatory mechanism of action of minoxidil.20 One randomized double-blind study found that while topical minoxidil did not affect blood pressure, it increased heart rate by 3 to 5 beats per minute, caused considerable increases in left ventricular end-diastolic volume, an increase in cardiac output (by 0.751 min-1), and an increase in left ventricular mass (by 5 g m-2). The authors concluded that short-term use is safe in healthy individuals, but providers should ask about history of coronary artery disease to avoid potential cardiac side effects.21
Patients also should be advised that at least 6 months of minoxidil therapy may be necessary.11 Furthermore, measurable hair changes may disappear within 3 months if the patient chooses to discontinue treatment.22 Finally, providers must consider patient perception of improvement and hair growth while on this medication. In one study, although investigator assessments of hair growth and hair count were increased with the use of minoxidil solution 5% compared to placebo, differences in patient assessment of hair growth were not significant at 48 weeks.22 Therefore, dermatologists should address patient expectations and consider additional treatments if necessary.
Finasteride
Finasteride is an oral medication that is FDA approved at a dose of 1 mg daily for the treatment of AGA in men. It competitively inhibits the type I and type II 5α-reductase enzymes, with a strong affinity for the type II enzyme, thereby inhibiting the conversion of testosterone to dihydrotestosterone (DHT), the potent androgen responsible for terminal hair follicle miniaturization and transformation of terminal hair into vellus hair.21,23
Finasteride has demonstrated efficacy and high tolerability in large-scale, placebo-controlled, randomized trials with only rare complications of sexual dysfunction, supporting its status as a first-line agent.24,25 One study found that in a population of 3177 Japanese men, an overall increase in hair growth was seen in 87.1% of men receiving oral finasteride 1 mg daily, with AEs such as decreased libido occurring in only 0.7% of patients.26 However, postmarketing studies described more severe complications in men taking finasteride to treat AGA or benign prostatic hyperplasia, even after the discontinuation of medication, described as postfinasteride syndrome.27,28 These side effects include decreased libido, reduction in penis size, gynecomastia, erectile dysfunction, and ejaculation disorder, in addition to psychologic impairments, including decreased concentration, depression, and suicidal ideation, presumably due to the role of 5α-reductase interacting with the γ-aminobutyric acid (GABAA) receptor within the central nervous system.29 The incidence of persistent erectile dysfunction was reported to be as low as 1.4% in a study assessing 11,909 men prescribed up to 5 mg once daily of finasteride to treat benign prostatic hyperplasia and AGA. The incidence was higher in patients using higher doses of finasteride and longer treatment courses as well as in patients with prostate disease.29 These potential side effects should be discussed with male patients prior to prescribing finasteride.
Finasteride is not FDA approved for use in women and is considered category X in pregnancy due to animal studies that demonstrated external genital abnormalities in male fetuses exposed to type II 5α-reductase inhibitors.30 Despite this potential teratogenicity, finasteride is prescribed off label to treat FPHL and hirsutism. A meta-analysis of 2683 women participating in 65 studies found that finasteride, when used at dosages of 0.5 to 5 mg daily, may improve FPHL and frontal fibrosing alopecia after 6 to 12 months.30 However, available studies have used varying treatment methods, yielding differing results. For example, one randomized trial of 137 postmenopausal women with FPHL and normal androgen levels found no benefit with 1 mg daily31; however, another trial of 87 women with normal levels of androgens found that 5 mg daily of finasteride showed significant improvements in hair quantity and thickness after 12 months (P<.01).32 Further studies are needed to assess the appropriate female population that may benefit from use of finasteride. Premenopausal women interested in this therapy should be counseled about the risk of teratogenicity, as well as potential breast tenderness, loss of libido, and menstrual irregularities.33 Furthermore, finasteride use in women may pose a theoretical risk of breast cancer, as DHT inhibition results in conversion of excess testosterone to estrogen, thereby altering the estrogen to androgen ratio.34
Dutasteride
Dutasteride is 100-times more potent than finasteride as an inhibitor of type I 5α-reductase enzyme and 3-times more potent as an inhibitor of type I 5α-reductase enzyme.35 Therefore, it has been hypothesized that dutasteride may be more effective than finasteride for restoring hair loss, though it is not yet FDA approved for this indication.
Research evaluating the efficacy of dutasteride is emerging. Randomized controlled trials in men with AGA are promising and suggest reversed hair miniaturization.36 One randomized trial of 153 men found that dutasteride 0.5 mg daily was superior to placebo for the treatment of hair loss, as evidenced by an increase in hair counts in dutasteride patients (12.2/cm2) compared to controls (4.7/cm2). Furthermore, 0.5-mg dutasteride resulted in significantly increased new hair growth after 24 weeks compared to a placebo control (23/cm2 vs 4/cm2; P<.05).37
Dutasteride also is now being used off label to treat FPHL. Little evidence-based research exists regarding the use of dutasteride in women, though 1 case report described successful treatment of FPHL after 6 months of treatment with 0.5 mg daily of dutasteride in a 46-year-old woman who showed only minimal improvement on oral finasteride.38
The side-effect profile is similar to finasteride, and research in the urologic literature demonstrated that the rate of AEs is comparable between the 2 drugs, with reports of sexual side effects occurring in 11% of patients taking dutasteride 0.5 mg daily vs 14% of patients taking finasteride 5 mg daily.39 In the dermatologic literature, there was no statistically significant difference between the rate of AEs, specifically sexual AEs, in patients taking dutasteride 0.5 mg daily vs finasteride 1 mg daily.36 Safety of dutasteride in women is not well established. The side-effect profile described for finasteride, including the risk of potential fetal anomalies, should be discussed with women receiving dutasteride therapy.
Spironolactone
Although topical minoxidil is still considered first-line therapy for women experiencing hair loss, spironolactone is growing in popularity as an off-label treatment of FPHL, though it is not FDA approved for this indication. Spironolactone is a synthetic steroid that has been used as a potassium-sparing diuretic for more than 60 years. Its primary metabolite, canrenone, competitively inhibits aldosterone.37 It is FDA approved for the treatment of essential hypertension (25–100 mg), congestive heart failure (25 mg), diuretic-induced hypokalemia (25–100 mg), and primary hyperaldosteronism (100–400 mg).37,40 Spironolactone was serendipitously discovered to treat hirsutism, acne, and seborrhea associated with polycystic ovary syndrome.41
Androgens are well studied in male pattern hair loss, and their role in FPHL is now becoming evident, with new research supporting the role of spironolactone as a useful antiandrogen.42,43 An Australian open-label trial randomized 80 women with biopsy-proven FPHL to receive either spironolactone 200 mg daily or cyproterone acetate, an antiandrogen used abroad, including in European countries, in conjunction with an oral contraceptive pill for premenopausal women.42 Spironolactone was found to be as effective as the alternate regimen, with 44% of patients experiencing hair regrowth, 44% experiencing no progression of hair loss, and only 12% experiencing continued hair loss.44 Spironolactone used in combination with minoxidil has been shown to demonstrate greater efficacy when compared to spironolactone alone.45 One observational study of 100 women with FPHL found that once-daily capsules of minoxidil 0.25 mg combined with once daily spironolactone 25 mg was a safe and effective treatment of FPHL.44 Spironolactone also is considered safe and effective to treat FPHL in postmenopausal women by inhibiting the relative androgen excess.46
The starting dose for spironolactone usually is 25 mg twice daily and increased by 50 mg daily up to 200 mg daily as tolerated. Furthermore, results should be monitored for at least 6 months to assess efficacy accurately.47 Side effects include headache, decreased libido, menstrual irregularities, orthostatic hypotension, fatigue, and hyperkalemia. Although hyperkalemia is a known side effect of spironolactone, one study of 974 male and female participants receiving spironolactone found that only 0.72% of participants experienced mild hyperkalemia (5.1–6.0 mEq/L) with no patients experiencing moderate or severe hyperkalemia. Regardless, providers may consider checking potassium levels within 4 to 8 weeks of initiating treatment with spironolactone.48 Other potential AEs include gynecomastia and feminization; therefore, it is not recommended for use in men.42 Oral contraception is recommended to prevent pregnancy in premenopausal women, as spironolactone may cause feminization of the male fetus. Because of the antiandrogenic and progestogenic effects of spironolactone, there has been a theoretical concern for risk of inducing breast cancer, especially in postmenopausal women. However, a study conducted in the United Kingdom of more than 1 million female patients older than 55 years found that there was no increased risk of breast cancer in postmenopausal women.49
Low-Level Laser Light Therapy
Low-level laser light therapy has been used to reduce pain, treat edema, and promote would healing for almost 50 years and is now one of the few FDA-cleared devices to treat alopecia. Low-level laser light therapy uses red beam or near-infrared nonthermal lasers at a wavelength of 600 to 1000 nm and from 5 to 500 mW. The exact mechanism of hair growth stimulation is not known; however, it is believed that LLLT accelerates mitosis, stimulates hair follicle stem cells to activate follicular keratinocytes, and alters cellular metabolism by inhibiting nitric oxide from cytochrome c oxidase.50
Trials evaluating the efficacy of LLLT laser combs for the treatment of AGA have demonstrated notable improvements in hair density. For example, one sham device–controlled, double-blind clinical trial randomized 334 men and women to treatment with either an FDA-cleared laser comb vs sham devices.51 The treatment devices were used 3 times weekly for 26 weeks. Hair counts for those treated with the 7-, 9-, and 12-beam LLLT laser combs were significantly higher than the sham after 26 weeks (P<.05), without any serious AEs being reported.51 Another study in men with AGA proved similarly efficacious results using at-home LLLT therapy of 655 nm to the scalp every other day for 16 weeks (60 treatments).52 However, a 24-week randomized, double-blind, sham device–controlled, multicenter trial evaluating the LLLT helmet (combining 650-nm laser with 630- and 660-nm light-emitting diodes) among male and female patients with AGA failed to show promising results. Although mean (SD) hair thickness (12.6 [9.4] in LLLT group vs 3.9 [7.3] in control group [P=.01]) and hair density (17.2 [12.1] in LLLT group vs –2.1 [18.3] in control group [P=.003]) increased significantly, there was no significant difference in subject assessment of global appearance between the 2 groups.53
Low-level laser light therapy devices are available both for use at home and in office, with 650- to 900-nm wavelengths at 5 mW being the recommended dose for men and women.51 With regard to AEs, the safety profile for LLLT is relatively favorable. Adverse events can include dry skin, pruritus, scalp tenderness, irritation, and a warm sensation at the treatment site.52
Platelet-Rich Plasma
Originally used in the orthopedic literature to stimulate collagen growth, PRP has since been used in dermatology to promote hair regrowth by releasing platelet-derived growth factors, vascular endothelial growth factor, epidermal growth factor, insulinlike growth factor, and fibroblast growth factors to stimulate vascularization to the dermal papillary cells.54,55 Platelet-rich plasma is derived from the supernatant of centrifuged whole blood and then injected in the dermis of the scalp to stimulate hair growth.
Although use of PRP is not approved or cleared by the FDA for treatment of hair loss, several studies have demonstrated the efficacy of autologous PRP use for treating AGA.56 One pilot study of 19 male and female participants given a total of 5 PRP injections monthly for 3 months and subsequently at months 4 and 7 found a statistically significant improvement in mean hair density, hair diameter, and terminal-vellus hair ratio at 1-year follow-up (P<.05). Furthermore, histomorphometric evaluation demonstrated a decrease in perivascular inflammatory infiltrate.57 On the other hand, 2 separate studies failed to show statistically significant improvements in hair growth after use of PRP.58,59 Varying levels of success may be due in part to lack of a standard protocol for performing PRP injections. Studies comparing efficacy of different PRP administration regimens are emerging. A trial of 40 men and women found that subdermal PRP injections administered 3 times per month with booster injections administered 3 months later was more effective than other injection regimens, including once monthly injections.58,59 Activators such as collagen, thrombin, 10% calcium chloride, and calcium gluconate may be added to the PRP serum to promote further growth factor secretion upon platelet activation.60 However, different means of activation are used in different trials, potentially leading to varying results in clinical trials, with no one proven superior method.61-63 The main drawback of PRP use is that there is no consensus regarding exact concentration, utility of activators, dosing parameters, depth of injection, or frequency of sessions.60 Transient pain and erythema are the most common side effects of PRP injections, with no major AEs reported in the literature.64
Microneedling
Microneedling is a minimally invasive procedure that uses needles to puncture the stratum corneum of the skin.65 It was first used cosmetically more than 20 years ago due to its ability to increase collagen and elastin formation.51 Since its discovery, microneedling has been used to reduce the appearance of scars; augment transdermal drug delivery; and treat active acne vulgaris, melasma, hyperhidrosis, and alopecia.65 Although there are numerous at-home and professional microneedling devices on the market, only one device has been FDA cleared thus far.
Microneedling is proposed to increase hair regrowth by triggering the wound healing response, which ultimately augments the release of platelet-derived and epidermal growth factors while also activating the hair bulge.66 Treatment often is performed with a roller instrument that uses needles 0.5- to 2.5-mm long. Topical anesthetic cream may be applied prior to treatment.67 The treated area is then washed and an antibiotic ointment is applied.55 Management regimens typically require daily to weekly treatments with a total of 12 to 28 weeks to demonstrate an effect.
Microneedling has demonstrated efficacy in the treatment of hair loss, especially when combined with minoxidil. One study randomized 68 patients to undergo microneedling with minoxidil solution 5% twice daily compared to a control group of minoxidil solution 5% twice daily alone. After 12 weeks, patients treated with microneedling and minoxidil had significantly higher hair counts than the control group (P<.05).68 It is speculated that microneedling increases penetration of topical medications, including minoxidil across the skin barrier, thereby enhancing absorption of large molecules.66
Topical PRP has been used synergistically to augment the effects of microneedling. A trial randomized 93 patients with alopecia to receive minoxidil solution 5% alone, minoxidil solution 5% plus PRP, or microneedling with PRP.69 Hair growth was appreciated in 26 of 31 patients treated with microneedling and PRP compared to 10 of 31 and 17 of 31 in the other 2 groups, respectively. However, when hair growth occurred in the minoxidil-treated group, it occurred faster, with changes in hair growth at 12 weeks compared to 26 weeks in the microneedling group.69 When evaluating the efficacy of microneedling and PRP, it must be noted that there is no established leading protocol for treating hair loss, which may affect the success of the treatment.
The reported side-effect profile for microneedling and PRP injections has been favorable without any major AEs noted in clinical trials.56,64,70 The possibility of bleeding, pain, erythema, and infection should be discussed with the patient nonetheless. More severe side effects such as allergic granulomatous reactions have been reported in the literature with the use of microneedling for facial rejuvenation.71
Stem Cells
Stem cell hair therapy is a new and promising area of research with the potential to treat alopecia. Although not yet FDA approved for this indication, human umbilical cord blood–derived mesenchymal stem cells (HUCB-MSCs) have received particular attention due to their proposed ability to promote tissue differentiation and repair, to replace aged and damaged hair cells, and to promote secretion of multiple growth factors.72 More recently, HUCB-MSCs have been shown to successfully differentiate into human hair follicles in vitro after 3 weeks of cell culture, establishing a method for high-speed and high-purity hair follicle cell differentiation with the hope of future injections to affected areas with hair loss.73 Another study found that HUCB-MSCs enhanced growth of human follicular stem cells in vitro; the authors proposed an altered Wnt/β‐catenin and JAK/STAT pathway was responsible for improved growth of hair follicular cells.74
Although umbilical cord blood is replete with the most rapidly dividing stem cells, autologous stem cells derived from the hair follicle or mononuclear cells also may be used to treat alopecia. One recent study randomized 40 patients with AGA and alopecia areata to receive 1 session of either autologous hair follicle or mononuclear cell–derived stem cell injections to the scalp.75 Mononuclear cells were acquired from the upper iliac crest bone marrow of patients who were treated with granulocyte colony-stimulating factor 3 days prior to the procedure. Follicular stem cells were taken from 4-mm punch biopsies of the unaffected scalp. After 6 months, there was a notable improvement in hair growth confirmed by immunostaining and dermoscopy, without a significant difference between the forms of autologous stem cell source. Of note, 45% of study patients with alopecia areata showed recurrence of disease at 1-year follow-up. The most common AEs were scalp dermatitis in 20% of participants. Participants who underwent bone marrow biopsy experienced bone pain, hematoma, and granulocyte colony-stimulating factor–induced fatigue and chills.75
Furthermore, the cost of stem cell therapy may be prohibitive. Therefore, although stem cell therapy is a novel and promising treatment for hair loss, future research is necessary to establish safety, efficacy, best practices, and accessibility.
Supplements
Patients failing routine treatments for alopecia may turn to holistic therapies. Nutrafol (Nutraceutical Wellness Inc), a novel nutraceutical product, is one such option that has been described for its anti-inflammatory, adaptogenic, antioxidant, and DHT-inhibiting properties. This supplement is not FDA approved or cleared, and large-scale clinical trials are lacking; however, one randomized controlled trial of 40 women with self-reported hair loss found a statistically significant increase in the number of terminal and vellus hair based on phototrichograms performed after 90 and 180 days (P=.009), with no AEs reported. This study, however, was limited by a small sample size.76
Lamdapil (ISDIN) is another oral supplement being investigated for hair loss. It contains L-cystine amino acids; zinc; vitamins B3, B5, B6; biotin; and the plant extract Serenoa repens.71Serenoa repens has reported activity inhibiting the enzyme 5α-reductase with the other vitamins, and amino acids are thought to maintain keratin and collagen growth in normal hair.77 One randomized trial investigated use of Lamdapil capsules in a total of 70 patients, which included men with AGA and women experiencing telogen effluvium. For men, the anagen-telogen ratio increased in the Lamdapil-treated group by 23.4%, indicating that more hair was in the growing phase compared to placebo (P<.05). Women with telogen effluvium experienced a significantly greater improvement in the hair-pull test compared to placebo (P<.05).77
Marine-derived nutraceutical substances also have been investigated for their role in treating hair loss. Viviscal, originally marketed under the name Hairgain, is one such supplement, which was shown to significantly reduce hair shedding at 3 and 6 months in a group of 96 premenopausal women diagnosed with subclinical hair thinning (P<.05). Additionally, phototrichogram images demonstrated a statistically significant increase in the mean velluslike hair diameter at 6 months compared to baseline.78
Although nutraceutical products are not first-line therapy for hair loss, dermatologists may recommend these treatments in patients refusing prescription medications, specifically requesting a natural treatment, or in addition to a first-line agent such as minoxidil. It must be noted, however, that both supplements are new, and there is need for further investigation on their efficacy, safety, and dosing, as neither is FDA regulated.
Conclusion
Hair loss affects millions of Americans each year and has detrimental effects on self-esteem and psychosocial functioning. Nonsurgical treatment options will undoubtedly continue to intrigue patients, as they are often less costly and do not carry risks associated with surgery. Minoxidil, finasteride, and LLLT remain staples of therapy, with the strongest evidence supporting their safety and efficacy. Numerous other treatment options are emerging, including PRP, microneedling, mesenchymal and autologous stem cell therapy, and oral supplements, though further research must be conducted to establish dosing, safety, and best practices. Physicians must discuss patient preference and anticipated length of treatment when discussing alopecia treatment to maximize patient satisfaction.
- Saed S, Ibrahim O, Bergfeld WF. Hair camouflage: a comprehensive review. Int J Womens Dermatol. 2016;2:122-127.
- Alfonso M, Richter-Appelt H, Tosti A, et al. The psychosocial impact of hair loss among men: a multinational European study. Curr Med Res Opin. 2005;21:1829-1836.
- Konior RJ. Complications in hair-restoration surgery. Facial Plast Surg Clin North Am. 2013;21:505-520.
- Manabe M, Tsuboi R, Itami S, et al. Guidelines for the diagnosis and treatment of male-pattern and female-pattern hair loss, 2017 version [published online June 4, 2018]. J Dermatol. 2018;45:1031-1043.
- Gupta AK, Mays RR, Dotzert MS, et al. Efficacy of non-surgical treatments for androgenetic alopecia: a systematic review and network meta-analysis. J Eur Acad Dermatol Venereol. 2018;32:2112-2125.
- Mehta PK, Mamdani B, Shansky RM, et al. Severe hypertension. treatment with minoxidil. JAMA. 1975;233:249-252.
- Zappacosta AR. Reversal of baldness in patient receiving minoxidil for hypertension. N Engl J Med. 1980;303:1480-1481.
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-194.
- Mori O, Uno H. The effect of topical minoxidil on hair follicular cycles of rats. J Dermatol. 1990;17:276-281.
- Pekmezci E, Turkoglu M, Gokalp H, et al. Minoxidil downregulates interleukin-1 alpha gene expression in HaCaT cells. Int J Trichol. 2018;10:108-112.
- Roenigk HH Jr, Pepper E, Kuruvilla S. Topical minoxidil therapy for hereditary male pattern alopecia. Cutis. 1987;39:337-342.
- Lucky AW, Piacquadio DJ, Ditre CM, et al. A randomized, placebo-controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss. J Am Acad Dermatol. 2004;50:541-553.
- Adil A, Godwin M. The effectiveness of treatments for androgenetic alopecia: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:136-141.e135.
- Nangia J, Wang T, Osborne C, et al. Effect of a scalp cooling device on alopecia in women undergoing chemotherapy for breast cancer: the SCALP randomized clinical trial. JAMA. 2017;317:596-605.
- Rugo HS, Melin SA, Voigt J. Scalp cooling with adjuvant/neoadjuvant chemotherapy for breast cancer and the risk of scalp metastases: systematic review and meta-analysis. Breast Cancer Res Treat. 2017;163:199-205.
- Duvic M, Lemak NA, Valero V, et al. A randomized trial of minoxidil in chemotherapy-induced alopecia. J Am Acad Dermatol. 1996;35:74-78.
- Yeager CE, Olsen EA. Treatment of chemotherapy-induced alopecia. Dermatol Ther. 2011;24:432-442.
- Freites-Martinez A, Shapiro J, Chan D, et al. Endocrine therapy-induced alopecia in patients with breast cancer. JAMA Dermatol. 2018;154:670-675.
- Gupta AK, Foley KA. 5% minoxidil: treatment for female pattern hair loss. Skin Ther Lett. 2014;19:5-7.
- Stoehr JR, Choi JN, Colavincenzo M, et al. Off-label use of topical minoxidil in alopecia: a review. Am J Clin Dermatol. 2019;20:237-250.
- Leenen FH, Smith DL, Unger WP. Topical minoxidil: cardiac effects in bald man. Br J Clin Pharmacol. 1988;26:481-485.
- Rossi A, Cantisani C, Melis L, et al. Minoxidil use in dermatology, side effects and recent patents. Recent Pat Inflamm Allergy Drug Discov. 2012;6:130-136.
- Rittmaster RS. Finasteride. N Engl J Med. 1994;330:120-125.
- Sawaya ME. Purification of androgen receptors in human sebocytes and hair. J Invest Dermatol. 1992;98(6 suppl):92S-96S.
- Sawaya ME, Shalita AR. Androgen receptor polymorphisms (CAG repeat lengths) in androgenetic alopecia, hirsutism, and acne. J Cutan Med Surg. 1998;3:9-15.
- Sato A, Takeda A. Evaluation of efficacy and safety of finasteride 1 mg in 3177 Japanese men with androgenetic alopecia [published online October 10, 2011]. J Dermatol. 2012;39:27-32.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998;39(4, pt 1):578-589.
- Kiguradze T, Temps WH, Yarnold PR, et al. Persistent erectile dysfunction in men exposed to the 5α-reductase inhibitors, finasteride, or dutasteride. PeerJ. 2017;5:E3020.
- Tsuboi R, Itami S, Inui S, et al. Guidelines for the management of androgenetic alopecia (2010). J Dermatol. 2012;39:113-120.
- Hu AC, Chapman LW, Mesinkovska NA. The efficacy and use of finasteride in women: a systematic review. Int J Dermatol. 2019;58:759-776.
- Price VH, Roberts JL, Hordinsky M, et al. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J Am Acad Dermatol. 2000;43(5, pt 1):768-776.
- Yeon JH, Jung JY, Choi JW, et al. 5 mg/day finasteride treatment for normoandrogenic Asian women with female pattern hair loss. J Eur Acad Dermatol Venereol. 2011;25:211-214.
- Oliveira-Soares R, André MC, Peres-Correia M. Adverse effects with finasteride 5 mg/day for patterned hair loss in premenopausal women. Int J Trichol. 2018;10:48-50.
- Kelly Y, Blanco A, Tosti A. Androgenetic alopecia: an update of treatment options. Drugs. 2016;76:1349-1364.
- Motofei IG, Rowland DL, Baconi DL, et al. Androgenetic alopecia; drug safety and therapeutic strategies [published online January 24, 2018]. Expert Opin Drug Saf. 2018;17:407-412.
- Shanshanwal SJ, Dhurat RS. Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: a randomized controlled open-label, evaluator-blinded study. Indian J Dermatol Venereol Leprol. 2017;83:47-54.
- Eun HC, Kwon OS, Yeon JH, et al. Efficacy, safety, and tolerability of dutasteride 0.5 mg once daily in male patients with male pattern hair loss: a randomized, double-blind, placebo-controlled, phase III study. J Am Acad Dermatol. 2010;63:252-258.
- Olszewska M, Rudnicka L. Effective treatment of female androgenic alopecia with dutasteride. J Drugs Dermatol. 2005;4:637-640.
- Nickel JC. Comparison of clinical trials with finasteride and dutasteride. Rev Urol. 2004;6(suppl 9):S31-S39.
- Olsen EA, Hordinsky M, Whiting D, et al. The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. J Am Acad Dermatol. 2006;55:1014-1023.
- Gómez R, Núñez L, Caballero R, et al. Spironolactone and its main metabolite canrenoic acid block hKv1.5, Kv4.3 and Kv7.1 + minK channels. Br J Pharmacol. 2005;146:146-161.
- Huffman DH, Kampmann JP, Hignite CE, et al. Gynecomastia induced in normal males by spironolactone. Clin Pharmacol Ther. 1978;24:465-473.
- Sinclair R, Patel M, Dawson TL Jr, et al. Hair loss in women: medical and cosmetic approaches to increase scalp hair fullness. Br J Dermatol. 2011;165(suppl 3):12-18.
- Sinclair R, Wewerinke M, Jolley D. Treatment of female pattern hair loss with oral antiandrogens. Br J Dermatol. 2005;152:466-473.
- Brough KR, Torgerson RR. Hormonal therapy in female pattern hair loss. Int J Womens Dermatol. 2017;3:53-57.
- Fabbrocini G, Cantelli M, Masarà A, et al. Female pattern hair loss: a clinical, pathophysiologic, and therapeutic review. Int J Womens Dermatol. 2018;4:203-211.
- Sinclair RD. Female pattern hair loss: a pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. Int J Dermatol. 2018;57:104-109.
- Camacho-Martinez FM. Hair loss in women. Semin Cutan Med Surg. 2009;28:19-32.
- Mackenzie IS, Macdonald TM, Thompson A, et al. Spironolactone and risk of incident breast cancer in women older than 55 years: retrospective, matched cohort study. BMJ. 2012;345:E4447.
- Farivar S, Malekshahabi T, Shiari R. Biological effects of low level laser therapy. J Laser Med Sci. 2014;5:58-62.
- Jimenez JJ, Wikramanayake TC, Bergfeld W, et al. Efficacy and safety of a low-level laser device in the treatment of male and female pattern hair loss: a multicenter, randomized, sham device-controlled, double-blind study. Am J Clin Dermatol. 2014;15:115-127.
- Lanzafame RJ, Blanche RR, Bodian AB, et al. The growth of human scalp hair mediated by visible red light laser and LED sources in males. Lasers Surg Med. 2013;45:487-495.
- Kim H, Choi JW, Kim JY, et al. Low-level light therapy for androgenetic alopecia: a 24-week, randomized, double-blind, sham device-controlled multicenter trial. Dermatol Surg. 2013;39:1177-1183.
- Banga AK. Transdermal and Intradermal Delivery of Therapeutic Agents: Application of Physical Technologies. New York, NY: CRC Press; 2011.
- Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichol. 2013;5:6-11.
- Jha AK, Vinay K, Zeeshan M, et al. Platelet-rich plasma and microneedling improves hair growth in patients of androgenetic alopecia when used as an adjuvant to minoxidil [published online January 28, 2019]. J Cosmet Dermatol. doi:10.1111/jocd.12864.
- Anitua E, Pino A, Martinez N, et al. The effect of plasma rich in growth factors on pattern hair loss: a pilot study. Dermatol Surg. 2017;43:658-670.
- Puig CJ, Reese R, Peters M. Double-blind, placebo-controlled pilot study on the use of platelet-rich plasma in women with female androgenetic alopecia. Dermatol Surg. 2016;42:1243-1247.
- Mapar MA, Shahriari S, Haghighizadeh MH. Efficacy of platelet-rich plasma in the treatment of androgenetic (male-patterned) alopecia: a pilot randomized controlled trial. J Cosmet Laser Ther. 2016;18:452-455.
- Maria-Angeliki G, Alexandros-Efstratios K, Dimitris R, et al. Platelet-rich plasma as a potential treatment for noncicatricial alopecias. Int J Trichol. 2015;7:54-63.
- Gkini MA, Kouskoukis AE, Tripsianis G, et al. Study of platelet-rich plasma injections in the treatment of androgenetic alopecia through an one-year period. J Cutan Aesthet Surg. 2014;7:213-219.
- Landesberg R, Roy M, Glickman RS. Quantification of growth factor levels using a simplified method of platelet-rich plasma gel preparation. J Oral Maxillofac Surg. 2000;58:297-300; discussion 300-301.
- Weibrich G, Kleis WK, Hafner G. Growth factor levels in the platelet-rich plasma produced by 2 different methods: curasan-type PRP kit versus PCCS PRP system. Int J Oral Maxillofac Implants. 2002;17:184-190.
- Alves R, Grimalt R. Randomized placebo-controlled, double-blind, half-head study to assess the efficacy of platelet-rich plasma on the treatment of androgenetic alopecia. Dermatol Surg. 2016;42:491-497.
- Hou A, Cohen B, Haimovic A, et al. Microneedling: a comprehensive review. Dermatol Surg. 2017;43:321-339.
- Singh A, Yadav S. Microneedling: advances and widening horizons. Indian Dermatol Online J. 2016;7:244-254.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study. J Cosmet Dermatol. 2016;15:434-443.
- Kumar MK, Inamadar AC, Palit A. A randomized controlled single-observer blinded study to determine the efficacy of topical minoxidil plus microneedling versus topical minoxidil alone in the treatment of androgenetic alopecia. J Cutan Aesthet Surg. 2018;11:211-216.
- Hausauer AK, Jones DH. Evaluating the efficacy of different platelet-rich plasma regimens for management of androgenetic alopecia: a single-center, blinded, randomized clinical trial. Dermatol Surg. 2018;44:1191-1200.
- Kang JS, Zheng Z, Choi MJ, et al. The effect of CD34+ cell-containing autologous platelet-rich plasma injection on pattern hair loss: a preliminary study. J Eur Acad Dermatol Venereol. 2014;28:72-79.
- Soltani-Arabshahi R, Wong JW, Duffy KL, et al. Facial allergic granulomatous reaction and systemic hypersensitivity associated with microneedle therapy for skin rejuvenation: adverse reactions with microneedle therapy. JAMA Dermatol. 2014;150:68-72.
- Bak DH, Choi MJ, Kim SR, et al. Human umbilical cord blood mesenchymal stem cells engineered to overexpress growth factors accelerate outcomes in hair growth. Korean J Physiol Pharmacol. 2018;22:555-566.
- Bu ZY, Wu LM, Yu XH, et al. Isolation and characterization of in vitro culture of hair follicle cells differentiated from umbilical cord blood mesenchymal stem cells. Exp Ther Med. 2017;14:303-307.
- Kim JE, Oh JH, Woo YJ, et al. Effects of mesenchymal stem cell therapy on alopecia areata in cellular and hair follicle organ culture models [published online October 29, 2018]. Exp Dermatol. doi:10.1111/exd.13812.
- Elmaadawi IH, Mohamed BM, Ibrahim ZAS, et al. Stem cell therapy as a novel therapeutic intervention for resistant cases of alopecia areata and androgenetic alopecia [published online March 6, 2018]. J Dermatolog Treat. 2018;29:431-440.
- Ablon G, Kogan S. A six-month, randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of a nutraceutical supplement for promoting hair growth in women with self-perceived thinning hair. J Drugs Dermatol. 2018;17:558-565.
- Narda M, Aladren S, Cestone E, et al. Efficacy and safety of a food supplement containing L-cystine, Serenoa repens extract and biotin for hair loss in healthy males and females. a prospective, randomized, double-blinded, controlled clinical trial. J Cosmo Trichol. 2017;3. doi:10.4172/2471-9323.1000127.
- Glynis A. A double-blind, placebo-controlled study evaluating the efficacy of an oral supplement in women with self-perceived thinning hair. J Clin Aesthet Dermatol. 2012;5:28-34.
Hair plays an important role in identity, self-perception, and psychosocial functioning. Hair loss can be a devastating experience that decreases self-esteem and feelings of personal attractiveness while also leading to depression and anxiety.1,2 Although increasingly popular, surgical hair restoration, including hair transplantation, is costly and carries considerable risk.
Results of nonsurgical hair restoration are not immediate and may not be as dramatic; however, they do not carry the risks or recovery associated with surgical options. Treatments such as sex steroid hormone and biologic response modifiers have been used to inhibit hair miniaturization and stabilize hair loss in cases of androgenic alopecia (AGA).3 Currently, minoxidil and finasteride are the only US Food and Drug Administration (FDA)–approved medications for the treatment of hair loss; however, other nonsurgical treatment options have gained popularity, including dutasteride, spironolactone, low-level laser therapy (LLLT), platelet-rich plasma (PRP), microneedling, stem cells, and nutraceutical supplements. We provide an overview of these treatment options to help dermatologists select appropriate therapies for the treatment of alopecia (Table).
Minoxidil
Minoxidil has been known to improve hair growth for more than 40 years. Oral minoxidil was first introduced for hypertension in the 1970s with a common adverse effect of hypertrichosis; the 2% solution was marketed for AGA shortly thereafter in 1986.4 Minoxidil is a biologic response modifier that is thought to promote hair growth through vasodilation and stimulation of hair follicles into the growth phase.5 In animal studies, topical minoxidil has been shown to shorten telogen, prolong anagen, and increase hair follicle size.6,7 More recently, topical minoxidil was shown to have anti-inflammatory effects by downregulating IL-1, which may confer an additional role in combatting alopecia.8
Minoxidil is FDA approved for treatment of AGA in men and women and often is used as first-line therapy.9 In 3 separate meta-analyses of topical minoxidil, it was shown to be more effective than placebo for treating AGA in men and women, with a notable increase in target area hair growth.10 A study of 777 male patients treated with topical minoxidil 2% found that 45% subjectively experienced new hair growth.11 However, results may vary, and research indicates that higher concentrations are more effective. In a randomized, double-blind, placebo-controlled trial of 381 women with female pattern hair loss (FPHL), minoxidil solution 2% was found to be superior to placebo after 48 weeks, with average changes in nonvellus hair counts of 20.7/cm2 in the minoxidil group vs 9.4/cm2 in the placebo group.12 In a separate meta-analysis, minoxidil solution 5% demonstrated superiority to both the 2% formulation and placebo with a mean change in nonvellus hair counts of 26.0/cm2.13
Minoxidil also has demonstrated promising benefits in preventing chemotherapy-induced alopecia. Although oncologists most often use the scalp cooling method to prevent hair loss by decreasing perfusion and uptake of cytotoxic agents, cost may be prohibitive, as it is often not reimbursable by insurance companies.14,15 On the other hand, minoxidil is easily procured over-the-counter and has been successfully used to decrease the duration of alopecia caused by chemotherapeutic agents such as fluorouracil, doxorubicin, and cyclophosphamide, as well as endocrine therapies used to treat breast cancer in women.16-18 Minoxidil also has been used off label to treat other forms of alopecia, including alopecia areata, telogen effluvium, eyebrow hypotrichosis, and monilethrix; however, there is inconclusive evidence for its efficacy.5,13,19
Compared to other nonsurgical treatments for hair loss, a meta-analysis found that minoxidil was associated with the highest rate of adverse effects (AEs).16,17 Potential side effects include pruritus or burning at the application site; irritant or allergic contact dermatitis; hypertrichosis; and cardiovascular effects, which may be due to the vasodilatory mechanism of action of minoxidil.20 One randomized double-blind study found that while topical minoxidil did not affect blood pressure, it increased heart rate by 3 to 5 beats per minute, caused considerable increases in left ventricular end-diastolic volume, an increase in cardiac output (by 0.751 min-1), and an increase in left ventricular mass (by 5 g m-2). The authors concluded that short-term use is safe in healthy individuals, but providers should ask about history of coronary artery disease to avoid potential cardiac side effects.21
Patients also should be advised that at least 6 months of minoxidil therapy may be necessary.11 Furthermore, measurable hair changes may disappear within 3 months if the patient chooses to discontinue treatment.22 Finally, providers must consider patient perception of improvement and hair growth while on this medication. In one study, although investigator assessments of hair growth and hair count were increased with the use of minoxidil solution 5% compared to placebo, differences in patient assessment of hair growth were not significant at 48 weeks.22 Therefore, dermatologists should address patient expectations and consider additional treatments if necessary.
Finasteride
Finasteride is an oral medication that is FDA approved at a dose of 1 mg daily for the treatment of AGA in men. It competitively inhibits the type I and type II 5α-reductase enzymes, with a strong affinity for the type II enzyme, thereby inhibiting the conversion of testosterone to dihydrotestosterone (DHT), the potent androgen responsible for terminal hair follicle miniaturization and transformation of terminal hair into vellus hair.21,23
Finasteride has demonstrated efficacy and high tolerability in large-scale, placebo-controlled, randomized trials with only rare complications of sexual dysfunction, supporting its status as a first-line agent.24,25 One study found that in a population of 3177 Japanese men, an overall increase in hair growth was seen in 87.1% of men receiving oral finasteride 1 mg daily, with AEs such as decreased libido occurring in only 0.7% of patients.26 However, postmarketing studies described more severe complications in men taking finasteride to treat AGA or benign prostatic hyperplasia, even after the discontinuation of medication, described as postfinasteride syndrome.27,28 These side effects include decreased libido, reduction in penis size, gynecomastia, erectile dysfunction, and ejaculation disorder, in addition to psychologic impairments, including decreased concentration, depression, and suicidal ideation, presumably due to the role of 5α-reductase interacting with the γ-aminobutyric acid (GABAA) receptor within the central nervous system.29 The incidence of persistent erectile dysfunction was reported to be as low as 1.4% in a study assessing 11,909 men prescribed up to 5 mg once daily of finasteride to treat benign prostatic hyperplasia and AGA. The incidence was higher in patients using higher doses of finasteride and longer treatment courses as well as in patients with prostate disease.29 These potential side effects should be discussed with male patients prior to prescribing finasteride.
Finasteride is not FDA approved for use in women and is considered category X in pregnancy due to animal studies that demonstrated external genital abnormalities in male fetuses exposed to type II 5α-reductase inhibitors.30 Despite this potential teratogenicity, finasteride is prescribed off label to treat FPHL and hirsutism. A meta-analysis of 2683 women participating in 65 studies found that finasteride, when used at dosages of 0.5 to 5 mg daily, may improve FPHL and frontal fibrosing alopecia after 6 to 12 months.30 However, available studies have used varying treatment methods, yielding differing results. For example, one randomized trial of 137 postmenopausal women with FPHL and normal androgen levels found no benefit with 1 mg daily31; however, another trial of 87 women with normal levels of androgens found that 5 mg daily of finasteride showed significant improvements in hair quantity and thickness after 12 months (P<.01).32 Further studies are needed to assess the appropriate female population that may benefit from use of finasteride. Premenopausal women interested in this therapy should be counseled about the risk of teratogenicity, as well as potential breast tenderness, loss of libido, and menstrual irregularities.33 Furthermore, finasteride use in women may pose a theoretical risk of breast cancer, as DHT inhibition results in conversion of excess testosterone to estrogen, thereby altering the estrogen to androgen ratio.34
Dutasteride
Dutasteride is 100-times more potent than finasteride as an inhibitor of type I 5α-reductase enzyme and 3-times more potent as an inhibitor of type I 5α-reductase enzyme.35 Therefore, it has been hypothesized that dutasteride may be more effective than finasteride for restoring hair loss, though it is not yet FDA approved for this indication.
Research evaluating the efficacy of dutasteride is emerging. Randomized controlled trials in men with AGA are promising and suggest reversed hair miniaturization.36 One randomized trial of 153 men found that dutasteride 0.5 mg daily was superior to placebo for the treatment of hair loss, as evidenced by an increase in hair counts in dutasteride patients (12.2/cm2) compared to controls (4.7/cm2). Furthermore, 0.5-mg dutasteride resulted in significantly increased new hair growth after 24 weeks compared to a placebo control (23/cm2 vs 4/cm2; P<.05).37
Dutasteride also is now being used off label to treat FPHL. Little evidence-based research exists regarding the use of dutasteride in women, though 1 case report described successful treatment of FPHL after 6 months of treatment with 0.5 mg daily of dutasteride in a 46-year-old woman who showed only minimal improvement on oral finasteride.38
The side-effect profile is similar to finasteride, and research in the urologic literature demonstrated that the rate of AEs is comparable between the 2 drugs, with reports of sexual side effects occurring in 11% of patients taking dutasteride 0.5 mg daily vs 14% of patients taking finasteride 5 mg daily.39 In the dermatologic literature, there was no statistically significant difference between the rate of AEs, specifically sexual AEs, in patients taking dutasteride 0.5 mg daily vs finasteride 1 mg daily.36 Safety of dutasteride in women is not well established. The side-effect profile described for finasteride, including the risk of potential fetal anomalies, should be discussed with women receiving dutasteride therapy.
Spironolactone
Although topical minoxidil is still considered first-line therapy for women experiencing hair loss, spironolactone is growing in popularity as an off-label treatment of FPHL, though it is not FDA approved for this indication. Spironolactone is a synthetic steroid that has been used as a potassium-sparing diuretic for more than 60 years. Its primary metabolite, canrenone, competitively inhibits aldosterone.37 It is FDA approved for the treatment of essential hypertension (25–100 mg), congestive heart failure (25 mg), diuretic-induced hypokalemia (25–100 mg), and primary hyperaldosteronism (100–400 mg).37,40 Spironolactone was serendipitously discovered to treat hirsutism, acne, and seborrhea associated with polycystic ovary syndrome.41
Androgens are well studied in male pattern hair loss, and their role in FPHL is now becoming evident, with new research supporting the role of spironolactone as a useful antiandrogen.42,43 An Australian open-label trial randomized 80 women with biopsy-proven FPHL to receive either spironolactone 200 mg daily or cyproterone acetate, an antiandrogen used abroad, including in European countries, in conjunction with an oral contraceptive pill for premenopausal women.42 Spironolactone was found to be as effective as the alternate regimen, with 44% of patients experiencing hair regrowth, 44% experiencing no progression of hair loss, and only 12% experiencing continued hair loss.44 Spironolactone used in combination with minoxidil has been shown to demonstrate greater efficacy when compared to spironolactone alone.45 One observational study of 100 women with FPHL found that once-daily capsules of minoxidil 0.25 mg combined with once daily spironolactone 25 mg was a safe and effective treatment of FPHL.44 Spironolactone also is considered safe and effective to treat FPHL in postmenopausal women by inhibiting the relative androgen excess.46
The starting dose for spironolactone usually is 25 mg twice daily and increased by 50 mg daily up to 200 mg daily as tolerated. Furthermore, results should be monitored for at least 6 months to assess efficacy accurately.47 Side effects include headache, decreased libido, menstrual irregularities, orthostatic hypotension, fatigue, and hyperkalemia. Although hyperkalemia is a known side effect of spironolactone, one study of 974 male and female participants receiving spironolactone found that only 0.72% of participants experienced mild hyperkalemia (5.1–6.0 mEq/L) with no patients experiencing moderate or severe hyperkalemia. Regardless, providers may consider checking potassium levels within 4 to 8 weeks of initiating treatment with spironolactone.48 Other potential AEs include gynecomastia and feminization; therefore, it is not recommended for use in men.42 Oral contraception is recommended to prevent pregnancy in premenopausal women, as spironolactone may cause feminization of the male fetus. Because of the antiandrogenic and progestogenic effects of spironolactone, there has been a theoretical concern for risk of inducing breast cancer, especially in postmenopausal women. However, a study conducted in the United Kingdom of more than 1 million female patients older than 55 years found that there was no increased risk of breast cancer in postmenopausal women.49
Low-Level Laser Light Therapy
Low-level laser light therapy has been used to reduce pain, treat edema, and promote would healing for almost 50 years and is now one of the few FDA-cleared devices to treat alopecia. Low-level laser light therapy uses red beam or near-infrared nonthermal lasers at a wavelength of 600 to 1000 nm and from 5 to 500 mW. The exact mechanism of hair growth stimulation is not known; however, it is believed that LLLT accelerates mitosis, stimulates hair follicle stem cells to activate follicular keratinocytes, and alters cellular metabolism by inhibiting nitric oxide from cytochrome c oxidase.50
Trials evaluating the efficacy of LLLT laser combs for the treatment of AGA have demonstrated notable improvements in hair density. For example, one sham device–controlled, double-blind clinical trial randomized 334 men and women to treatment with either an FDA-cleared laser comb vs sham devices.51 The treatment devices were used 3 times weekly for 26 weeks. Hair counts for those treated with the 7-, 9-, and 12-beam LLLT laser combs were significantly higher than the sham after 26 weeks (P<.05), without any serious AEs being reported.51 Another study in men with AGA proved similarly efficacious results using at-home LLLT therapy of 655 nm to the scalp every other day for 16 weeks (60 treatments).52 However, a 24-week randomized, double-blind, sham device–controlled, multicenter trial evaluating the LLLT helmet (combining 650-nm laser with 630- and 660-nm light-emitting diodes) among male and female patients with AGA failed to show promising results. Although mean (SD) hair thickness (12.6 [9.4] in LLLT group vs 3.9 [7.3] in control group [P=.01]) and hair density (17.2 [12.1] in LLLT group vs –2.1 [18.3] in control group [P=.003]) increased significantly, there was no significant difference in subject assessment of global appearance between the 2 groups.53
Low-level laser light therapy devices are available both for use at home and in office, with 650- to 900-nm wavelengths at 5 mW being the recommended dose for men and women.51 With regard to AEs, the safety profile for LLLT is relatively favorable. Adverse events can include dry skin, pruritus, scalp tenderness, irritation, and a warm sensation at the treatment site.52
Platelet-Rich Plasma
Originally used in the orthopedic literature to stimulate collagen growth, PRP has since been used in dermatology to promote hair regrowth by releasing platelet-derived growth factors, vascular endothelial growth factor, epidermal growth factor, insulinlike growth factor, and fibroblast growth factors to stimulate vascularization to the dermal papillary cells.54,55 Platelet-rich plasma is derived from the supernatant of centrifuged whole blood and then injected in the dermis of the scalp to stimulate hair growth.
Although use of PRP is not approved or cleared by the FDA for treatment of hair loss, several studies have demonstrated the efficacy of autologous PRP use for treating AGA.56 One pilot study of 19 male and female participants given a total of 5 PRP injections monthly for 3 months and subsequently at months 4 and 7 found a statistically significant improvement in mean hair density, hair diameter, and terminal-vellus hair ratio at 1-year follow-up (P<.05). Furthermore, histomorphometric evaluation demonstrated a decrease in perivascular inflammatory infiltrate.57 On the other hand, 2 separate studies failed to show statistically significant improvements in hair growth after use of PRP.58,59 Varying levels of success may be due in part to lack of a standard protocol for performing PRP injections. Studies comparing efficacy of different PRP administration regimens are emerging. A trial of 40 men and women found that subdermal PRP injections administered 3 times per month with booster injections administered 3 months later was more effective than other injection regimens, including once monthly injections.58,59 Activators such as collagen, thrombin, 10% calcium chloride, and calcium gluconate may be added to the PRP serum to promote further growth factor secretion upon platelet activation.60 However, different means of activation are used in different trials, potentially leading to varying results in clinical trials, with no one proven superior method.61-63 The main drawback of PRP use is that there is no consensus regarding exact concentration, utility of activators, dosing parameters, depth of injection, or frequency of sessions.60 Transient pain and erythema are the most common side effects of PRP injections, with no major AEs reported in the literature.64
Microneedling
Microneedling is a minimally invasive procedure that uses needles to puncture the stratum corneum of the skin.65 It was first used cosmetically more than 20 years ago due to its ability to increase collagen and elastin formation.51 Since its discovery, microneedling has been used to reduce the appearance of scars; augment transdermal drug delivery; and treat active acne vulgaris, melasma, hyperhidrosis, and alopecia.65 Although there are numerous at-home and professional microneedling devices on the market, only one device has been FDA cleared thus far.
Microneedling is proposed to increase hair regrowth by triggering the wound healing response, which ultimately augments the release of platelet-derived and epidermal growth factors while also activating the hair bulge.66 Treatment often is performed with a roller instrument that uses needles 0.5- to 2.5-mm long. Topical anesthetic cream may be applied prior to treatment.67 The treated area is then washed and an antibiotic ointment is applied.55 Management regimens typically require daily to weekly treatments with a total of 12 to 28 weeks to demonstrate an effect.
Microneedling has demonstrated efficacy in the treatment of hair loss, especially when combined with minoxidil. One study randomized 68 patients to undergo microneedling with minoxidil solution 5% twice daily compared to a control group of minoxidil solution 5% twice daily alone. After 12 weeks, patients treated with microneedling and minoxidil had significantly higher hair counts than the control group (P<.05).68 It is speculated that microneedling increases penetration of topical medications, including minoxidil across the skin barrier, thereby enhancing absorption of large molecules.66
Topical PRP has been used synergistically to augment the effects of microneedling. A trial randomized 93 patients with alopecia to receive minoxidil solution 5% alone, minoxidil solution 5% plus PRP, or microneedling with PRP.69 Hair growth was appreciated in 26 of 31 patients treated with microneedling and PRP compared to 10 of 31 and 17 of 31 in the other 2 groups, respectively. However, when hair growth occurred in the minoxidil-treated group, it occurred faster, with changes in hair growth at 12 weeks compared to 26 weeks in the microneedling group.69 When evaluating the efficacy of microneedling and PRP, it must be noted that there is no established leading protocol for treating hair loss, which may affect the success of the treatment.
The reported side-effect profile for microneedling and PRP injections has been favorable without any major AEs noted in clinical trials.56,64,70 The possibility of bleeding, pain, erythema, and infection should be discussed with the patient nonetheless. More severe side effects such as allergic granulomatous reactions have been reported in the literature with the use of microneedling for facial rejuvenation.71
Stem Cells
Stem cell hair therapy is a new and promising area of research with the potential to treat alopecia. Although not yet FDA approved for this indication, human umbilical cord blood–derived mesenchymal stem cells (HUCB-MSCs) have received particular attention due to their proposed ability to promote tissue differentiation and repair, to replace aged and damaged hair cells, and to promote secretion of multiple growth factors.72 More recently, HUCB-MSCs have been shown to successfully differentiate into human hair follicles in vitro after 3 weeks of cell culture, establishing a method for high-speed and high-purity hair follicle cell differentiation with the hope of future injections to affected areas with hair loss.73 Another study found that HUCB-MSCs enhanced growth of human follicular stem cells in vitro; the authors proposed an altered Wnt/β‐catenin and JAK/STAT pathway was responsible for improved growth of hair follicular cells.74
Although umbilical cord blood is replete with the most rapidly dividing stem cells, autologous stem cells derived from the hair follicle or mononuclear cells also may be used to treat alopecia. One recent study randomized 40 patients with AGA and alopecia areata to receive 1 session of either autologous hair follicle or mononuclear cell–derived stem cell injections to the scalp.75 Mononuclear cells were acquired from the upper iliac crest bone marrow of patients who were treated with granulocyte colony-stimulating factor 3 days prior to the procedure. Follicular stem cells were taken from 4-mm punch biopsies of the unaffected scalp. After 6 months, there was a notable improvement in hair growth confirmed by immunostaining and dermoscopy, without a significant difference between the forms of autologous stem cell source. Of note, 45% of study patients with alopecia areata showed recurrence of disease at 1-year follow-up. The most common AEs were scalp dermatitis in 20% of participants. Participants who underwent bone marrow biopsy experienced bone pain, hematoma, and granulocyte colony-stimulating factor–induced fatigue and chills.75
Furthermore, the cost of stem cell therapy may be prohibitive. Therefore, although stem cell therapy is a novel and promising treatment for hair loss, future research is necessary to establish safety, efficacy, best practices, and accessibility.
Supplements
Patients failing routine treatments for alopecia may turn to holistic therapies. Nutrafol (Nutraceutical Wellness Inc), a novel nutraceutical product, is one such option that has been described for its anti-inflammatory, adaptogenic, antioxidant, and DHT-inhibiting properties. This supplement is not FDA approved or cleared, and large-scale clinical trials are lacking; however, one randomized controlled trial of 40 women with self-reported hair loss found a statistically significant increase in the number of terminal and vellus hair based on phototrichograms performed after 90 and 180 days (P=.009), with no AEs reported. This study, however, was limited by a small sample size.76
Lamdapil (ISDIN) is another oral supplement being investigated for hair loss. It contains L-cystine amino acids; zinc; vitamins B3, B5, B6; biotin; and the plant extract Serenoa repens.71Serenoa repens has reported activity inhibiting the enzyme 5α-reductase with the other vitamins, and amino acids are thought to maintain keratin and collagen growth in normal hair.77 One randomized trial investigated use of Lamdapil capsules in a total of 70 patients, which included men with AGA and women experiencing telogen effluvium. For men, the anagen-telogen ratio increased in the Lamdapil-treated group by 23.4%, indicating that more hair was in the growing phase compared to placebo (P<.05). Women with telogen effluvium experienced a significantly greater improvement in the hair-pull test compared to placebo (P<.05).77
Marine-derived nutraceutical substances also have been investigated for their role in treating hair loss. Viviscal, originally marketed under the name Hairgain, is one such supplement, which was shown to significantly reduce hair shedding at 3 and 6 months in a group of 96 premenopausal women diagnosed with subclinical hair thinning (P<.05). Additionally, phototrichogram images demonstrated a statistically significant increase in the mean velluslike hair diameter at 6 months compared to baseline.78
Although nutraceutical products are not first-line therapy for hair loss, dermatologists may recommend these treatments in patients refusing prescription medications, specifically requesting a natural treatment, or in addition to a first-line agent such as minoxidil. It must be noted, however, that both supplements are new, and there is need for further investigation on their efficacy, safety, and dosing, as neither is FDA regulated.
Conclusion
Hair loss affects millions of Americans each year and has detrimental effects on self-esteem and psychosocial functioning. Nonsurgical treatment options will undoubtedly continue to intrigue patients, as they are often less costly and do not carry risks associated with surgery. Minoxidil, finasteride, and LLLT remain staples of therapy, with the strongest evidence supporting their safety and efficacy. Numerous other treatment options are emerging, including PRP, microneedling, mesenchymal and autologous stem cell therapy, and oral supplements, though further research must be conducted to establish dosing, safety, and best practices. Physicians must discuss patient preference and anticipated length of treatment when discussing alopecia treatment to maximize patient satisfaction.
Hair plays an important role in identity, self-perception, and psychosocial functioning. Hair loss can be a devastating experience that decreases self-esteem and feelings of personal attractiveness while also leading to depression and anxiety.1,2 Although increasingly popular, surgical hair restoration, including hair transplantation, is costly and carries considerable risk.
Results of nonsurgical hair restoration are not immediate and may not be as dramatic; however, they do not carry the risks or recovery associated with surgical options. Treatments such as sex steroid hormone and biologic response modifiers have been used to inhibit hair miniaturization and stabilize hair loss in cases of androgenic alopecia (AGA).3 Currently, minoxidil and finasteride are the only US Food and Drug Administration (FDA)–approved medications for the treatment of hair loss; however, other nonsurgical treatment options have gained popularity, including dutasteride, spironolactone, low-level laser therapy (LLLT), platelet-rich plasma (PRP), microneedling, stem cells, and nutraceutical supplements. We provide an overview of these treatment options to help dermatologists select appropriate therapies for the treatment of alopecia (Table).
Minoxidil
Minoxidil has been known to improve hair growth for more than 40 years. Oral minoxidil was first introduced for hypertension in the 1970s with a common adverse effect of hypertrichosis; the 2% solution was marketed for AGA shortly thereafter in 1986.4 Minoxidil is a biologic response modifier that is thought to promote hair growth through vasodilation and stimulation of hair follicles into the growth phase.5 In animal studies, topical minoxidil has been shown to shorten telogen, prolong anagen, and increase hair follicle size.6,7 More recently, topical minoxidil was shown to have anti-inflammatory effects by downregulating IL-1, which may confer an additional role in combatting alopecia.8
Minoxidil is FDA approved for treatment of AGA in men and women and often is used as first-line therapy.9 In 3 separate meta-analyses of topical minoxidil, it was shown to be more effective than placebo for treating AGA in men and women, with a notable increase in target area hair growth.10 A study of 777 male patients treated with topical minoxidil 2% found that 45% subjectively experienced new hair growth.11 However, results may vary, and research indicates that higher concentrations are more effective. In a randomized, double-blind, placebo-controlled trial of 381 women with female pattern hair loss (FPHL), minoxidil solution 2% was found to be superior to placebo after 48 weeks, with average changes in nonvellus hair counts of 20.7/cm2 in the minoxidil group vs 9.4/cm2 in the placebo group.12 In a separate meta-analysis, minoxidil solution 5% demonstrated superiority to both the 2% formulation and placebo with a mean change in nonvellus hair counts of 26.0/cm2.13
Minoxidil also has demonstrated promising benefits in preventing chemotherapy-induced alopecia. Although oncologists most often use the scalp cooling method to prevent hair loss by decreasing perfusion and uptake of cytotoxic agents, cost may be prohibitive, as it is often not reimbursable by insurance companies.14,15 On the other hand, minoxidil is easily procured over-the-counter and has been successfully used to decrease the duration of alopecia caused by chemotherapeutic agents such as fluorouracil, doxorubicin, and cyclophosphamide, as well as endocrine therapies used to treat breast cancer in women.16-18 Minoxidil also has been used off label to treat other forms of alopecia, including alopecia areata, telogen effluvium, eyebrow hypotrichosis, and monilethrix; however, there is inconclusive evidence for its efficacy.5,13,19
Compared to other nonsurgical treatments for hair loss, a meta-analysis found that minoxidil was associated with the highest rate of adverse effects (AEs).16,17 Potential side effects include pruritus or burning at the application site; irritant or allergic contact dermatitis; hypertrichosis; and cardiovascular effects, which may be due to the vasodilatory mechanism of action of minoxidil.20 One randomized double-blind study found that while topical minoxidil did not affect blood pressure, it increased heart rate by 3 to 5 beats per minute, caused considerable increases in left ventricular end-diastolic volume, an increase in cardiac output (by 0.751 min-1), and an increase in left ventricular mass (by 5 g m-2). The authors concluded that short-term use is safe in healthy individuals, but providers should ask about history of coronary artery disease to avoid potential cardiac side effects.21
Patients also should be advised that at least 6 months of minoxidil therapy may be necessary.11 Furthermore, measurable hair changes may disappear within 3 months if the patient chooses to discontinue treatment.22 Finally, providers must consider patient perception of improvement and hair growth while on this medication. In one study, although investigator assessments of hair growth and hair count were increased with the use of minoxidil solution 5% compared to placebo, differences in patient assessment of hair growth were not significant at 48 weeks.22 Therefore, dermatologists should address patient expectations and consider additional treatments if necessary.
Finasteride
Finasteride is an oral medication that is FDA approved at a dose of 1 mg daily for the treatment of AGA in men. It competitively inhibits the type I and type II 5α-reductase enzymes, with a strong affinity for the type II enzyme, thereby inhibiting the conversion of testosterone to dihydrotestosterone (DHT), the potent androgen responsible for terminal hair follicle miniaturization and transformation of terminal hair into vellus hair.21,23
Finasteride has demonstrated efficacy and high tolerability in large-scale, placebo-controlled, randomized trials with only rare complications of sexual dysfunction, supporting its status as a first-line agent.24,25 One study found that in a population of 3177 Japanese men, an overall increase in hair growth was seen in 87.1% of men receiving oral finasteride 1 mg daily, with AEs such as decreased libido occurring in only 0.7% of patients.26 However, postmarketing studies described more severe complications in men taking finasteride to treat AGA or benign prostatic hyperplasia, even after the discontinuation of medication, described as postfinasteride syndrome.27,28 These side effects include decreased libido, reduction in penis size, gynecomastia, erectile dysfunction, and ejaculation disorder, in addition to psychologic impairments, including decreased concentration, depression, and suicidal ideation, presumably due to the role of 5α-reductase interacting with the γ-aminobutyric acid (GABAA) receptor within the central nervous system.29 The incidence of persistent erectile dysfunction was reported to be as low as 1.4% in a study assessing 11,909 men prescribed up to 5 mg once daily of finasteride to treat benign prostatic hyperplasia and AGA. The incidence was higher in patients using higher doses of finasteride and longer treatment courses as well as in patients with prostate disease.29 These potential side effects should be discussed with male patients prior to prescribing finasteride.
Finasteride is not FDA approved for use in women and is considered category X in pregnancy due to animal studies that demonstrated external genital abnormalities in male fetuses exposed to type II 5α-reductase inhibitors.30 Despite this potential teratogenicity, finasteride is prescribed off label to treat FPHL and hirsutism. A meta-analysis of 2683 women participating in 65 studies found that finasteride, when used at dosages of 0.5 to 5 mg daily, may improve FPHL and frontal fibrosing alopecia after 6 to 12 months.30 However, available studies have used varying treatment methods, yielding differing results. For example, one randomized trial of 137 postmenopausal women with FPHL and normal androgen levels found no benefit with 1 mg daily31; however, another trial of 87 women with normal levels of androgens found that 5 mg daily of finasteride showed significant improvements in hair quantity and thickness after 12 months (P<.01).32 Further studies are needed to assess the appropriate female population that may benefit from use of finasteride. Premenopausal women interested in this therapy should be counseled about the risk of teratogenicity, as well as potential breast tenderness, loss of libido, and menstrual irregularities.33 Furthermore, finasteride use in women may pose a theoretical risk of breast cancer, as DHT inhibition results in conversion of excess testosterone to estrogen, thereby altering the estrogen to androgen ratio.34
Dutasteride
Dutasteride is 100-times more potent than finasteride as an inhibitor of type I 5α-reductase enzyme and 3-times more potent as an inhibitor of type I 5α-reductase enzyme.35 Therefore, it has been hypothesized that dutasteride may be more effective than finasteride for restoring hair loss, though it is not yet FDA approved for this indication.
Research evaluating the efficacy of dutasteride is emerging. Randomized controlled trials in men with AGA are promising and suggest reversed hair miniaturization.36 One randomized trial of 153 men found that dutasteride 0.5 mg daily was superior to placebo for the treatment of hair loss, as evidenced by an increase in hair counts in dutasteride patients (12.2/cm2) compared to controls (4.7/cm2). Furthermore, 0.5-mg dutasteride resulted in significantly increased new hair growth after 24 weeks compared to a placebo control (23/cm2 vs 4/cm2; P<.05).37
Dutasteride also is now being used off label to treat FPHL. Little evidence-based research exists regarding the use of dutasteride in women, though 1 case report described successful treatment of FPHL after 6 months of treatment with 0.5 mg daily of dutasteride in a 46-year-old woman who showed only minimal improvement on oral finasteride.38
The side-effect profile is similar to finasteride, and research in the urologic literature demonstrated that the rate of AEs is comparable between the 2 drugs, with reports of sexual side effects occurring in 11% of patients taking dutasteride 0.5 mg daily vs 14% of patients taking finasteride 5 mg daily.39 In the dermatologic literature, there was no statistically significant difference between the rate of AEs, specifically sexual AEs, in patients taking dutasteride 0.5 mg daily vs finasteride 1 mg daily.36 Safety of dutasteride in women is not well established. The side-effect profile described for finasteride, including the risk of potential fetal anomalies, should be discussed with women receiving dutasteride therapy.
Spironolactone
Although topical minoxidil is still considered first-line therapy for women experiencing hair loss, spironolactone is growing in popularity as an off-label treatment of FPHL, though it is not FDA approved for this indication. Spironolactone is a synthetic steroid that has been used as a potassium-sparing diuretic for more than 60 years. Its primary metabolite, canrenone, competitively inhibits aldosterone.37 It is FDA approved for the treatment of essential hypertension (25–100 mg), congestive heart failure (25 mg), diuretic-induced hypokalemia (25–100 mg), and primary hyperaldosteronism (100–400 mg).37,40 Spironolactone was serendipitously discovered to treat hirsutism, acne, and seborrhea associated with polycystic ovary syndrome.41
Androgens are well studied in male pattern hair loss, and their role in FPHL is now becoming evident, with new research supporting the role of spironolactone as a useful antiandrogen.42,43 An Australian open-label trial randomized 80 women with biopsy-proven FPHL to receive either spironolactone 200 mg daily or cyproterone acetate, an antiandrogen used abroad, including in European countries, in conjunction with an oral contraceptive pill for premenopausal women.42 Spironolactone was found to be as effective as the alternate regimen, with 44% of patients experiencing hair regrowth, 44% experiencing no progression of hair loss, and only 12% experiencing continued hair loss.44 Spironolactone used in combination with minoxidil has been shown to demonstrate greater efficacy when compared to spironolactone alone.45 One observational study of 100 women with FPHL found that once-daily capsules of minoxidil 0.25 mg combined with once daily spironolactone 25 mg was a safe and effective treatment of FPHL.44 Spironolactone also is considered safe and effective to treat FPHL in postmenopausal women by inhibiting the relative androgen excess.46
The starting dose for spironolactone usually is 25 mg twice daily and increased by 50 mg daily up to 200 mg daily as tolerated. Furthermore, results should be monitored for at least 6 months to assess efficacy accurately.47 Side effects include headache, decreased libido, menstrual irregularities, orthostatic hypotension, fatigue, and hyperkalemia. Although hyperkalemia is a known side effect of spironolactone, one study of 974 male and female participants receiving spironolactone found that only 0.72% of participants experienced mild hyperkalemia (5.1–6.0 mEq/L) with no patients experiencing moderate or severe hyperkalemia. Regardless, providers may consider checking potassium levels within 4 to 8 weeks of initiating treatment with spironolactone.48 Other potential AEs include gynecomastia and feminization; therefore, it is not recommended for use in men.42 Oral contraception is recommended to prevent pregnancy in premenopausal women, as spironolactone may cause feminization of the male fetus. Because of the antiandrogenic and progestogenic effects of spironolactone, there has been a theoretical concern for risk of inducing breast cancer, especially in postmenopausal women. However, a study conducted in the United Kingdom of more than 1 million female patients older than 55 years found that there was no increased risk of breast cancer in postmenopausal women.49
Low-Level Laser Light Therapy
Low-level laser light therapy has been used to reduce pain, treat edema, and promote would healing for almost 50 years and is now one of the few FDA-cleared devices to treat alopecia. Low-level laser light therapy uses red beam or near-infrared nonthermal lasers at a wavelength of 600 to 1000 nm and from 5 to 500 mW. The exact mechanism of hair growth stimulation is not known; however, it is believed that LLLT accelerates mitosis, stimulates hair follicle stem cells to activate follicular keratinocytes, and alters cellular metabolism by inhibiting nitric oxide from cytochrome c oxidase.50
Trials evaluating the efficacy of LLLT laser combs for the treatment of AGA have demonstrated notable improvements in hair density. For example, one sham device–controlled, double-blind clinical trial randomized 334 men and women to treatment with either an FDA-cleared laser comb vs sham devices.51 The treatment devices were used 3 times weekly for 26 weeks. Hair counts for those treated with the 7-, 9-, and 12-beam LLLT laser combs were significantly higher than the sham after 26 weeks (P<.05), without any serious AEs being reported.51 Another study in men with AGA proved similarly efficacious results using at-home LLLT therapy of 655 nm to the scalp every other day for 16 weeks (60 treatments).52 However, a 24-week randomized, double-blind, sham device–controlled, multicenter trial evaluating the LLLT helmet (combining 650-nm laser with 630- and 660-nm light-emitting diodes) among male and female patients with AGA failed to show promising results. Although mean (SD) hair thickness (12.6 [9.4] in LLLT group vs 3.9 [7.3] in control group [P=.01]) and hair density (17.2 [12.1] in LLLT group vs –2.1 [18.3] in control group [P=.003]) increased significantly, there was no significant difference in subject assessment of global appearance between the 2 groups.53
Low-level laser light therapy devices are available both for use at home and in office, with 650- to 900-nm wavelengths at 5 mW being the recommended dose for men and women.51 With regard to AEs, the safety profile for LLLT is relatively favorable. Adverse events can include dry skin, pruritus, scalp tenderness, irritation, and a warm sensation at the treatment site.52
Platelet-Rich Plasma
Originally used in the orthopedic literature to stimulate collagen growth, PRP has since been used in dermatology to promote hair regrowth by releasing platelet-derived growth factors, vascular endothelial growth factor, epidermal growth factor, insulinlike growth factor, and fibroblast growth factors to stimulate vascularization to the dermal papillary cells.54,55 Platelet-rich plasma is derived from the supernatant of centrifuged whole blood and then injected in the dermis of the scalp to stimulate hair growth.
Although use of PRP is not approved or cleared by the FDA for treatment of hair loss, several studies have demonstrated the efficacy of autologous PRP use for treating AGA.56 One pilot study of 19 male and female participants given a total of 5 PRP injections monthly for 3 months and subsequently at months 4 and 7 found a statistically significant improvement in mean hair density, hair diameter, and terminal-vellus hair ratio at 1-year follow-up (P<.05). Furthermore, histomorphometric evaluation demonstrated a decrease in perivascular inflammatory infiltrate.57 On the other hand, 2 separate studies failed to show statistically significant improvements in hair growth after use of PRP.58,59 Varying levels of success may be due in part to lack of a standard protocol for performing PRP injections. Studies comparing efficacy of different PRP administration regimens are emerging. A trial of 40 men and women found that subdermal PRP injections administered 3 times per month with booster injections administered 3 months later was more effective than other injection regimens, including once monthly injections.58,59 Activators such as collagen, thrombin, 10% calcium chloride, and calcium gluconate may be added to the PRP serum to promote further growth factor secretion upon platelet activation.60 However, different means of activation are used in different trials, potentially leading to varying results in clinical trials, with no one proven superior method.61-63 The main drawback of PRP use is that there is no consensus regarding exact concentration, utility of activators, dosing parameters, depth of injection, or frequency of sessions.60 Transient pain and erythema are the most common side effects of PRP injections, with no major AEs reported in the literature.64
Microneedling
Microneedling is a minimally invasive procedure that uses needles to puncture the stratum corneum of the skin.65 It was first used cosmetically more than 20 years ago due to its ability to increase collagen and elastin formation.51 Since its discovery, microneedling has been used to reduce the appearance of scars; augment transdermal drug delivery; and treat active acne vulgaris, melasma, hyperhidrosis, and alopecia.65 Although there are numerous at-home and professional microneedling devices on the market, only one device has been FDA cleared thus far.
Microneedling is proposed to increase hair regrowth by triggering the wound healing response, which ultimately augments the release of platelet-derived and epidermal growth factors while also activating the hair bulge.66 Treatment often is performed with a roller instrument that uses needles 0.5- to 2.5-mm long. Topical anesthetic cream may be applied prior to treatment.67 The treated area is then washed and an antibiotic ointment is applied.55 Management regimens typically require daily to weekly treatments with a total of 12 to 28 weeks to demonstrate an effect.
Microneedling has demonstrated efficacy in the treatment of hair loss, especially when combined with minoxidil. One study randomized 68 patients to undergo microneedling with minoxidil solution 5% twice daily compared to a control group of minoxidil solution 5% twice daily alone. After 12 weeks, patients treated with microneedling and minoxidil had significantly higher hair counts than the control group (P<.05).68 It is speculated that microneedling increases penetration of topical medications, including minoxidil across the skin barrier, thereby enhancing absorption of large molecules.66
Topical PRP has been used synergistically to augment the effects of microneedling. A trial randomized 93 patients with alopecia to receive minoxidil solution 5% alone, minoxidil solution 5% plus PRP, or microneedling with PRP.69 Hair growth was appreciated in 26 of 31 patients treated with microneedling and PRP compared to 10 of 31 and 17 of 31 in the other 2 groups, respectively. However, when hair growth occurred in the minoxidil-treated group, it occurred faster, with changes in hair growth at 12 weeks compared to 26 weeks in the microneedling group.69 When evaluating the efficacy of microneedling and PRP, it must be noted that there is no established leading protocol for treating hair loss, which may affect the success of the treatment.
The reported side-effect profile for microneedling and PRP injections has been favorable without any major AEs noted in clinical trials.56,64,70 The possibility of bleeding, pain, erythema, and infection should be discussed with the patient nonetheless. More severe side effects such as allergic granulomatous reactions have been reported in the literature with the use of microneedling for facial rejuvenation.71
Stem Cells
Stem cell hair therapy is a new and promising area of research with the potential to treat alopecia. Although not yet FDA approved for this indication, human umbilical cord blood–derived mesenchymal stem cells (HUCB-MSCs) have received particular attention due to their proposed ability to promote tissue differentiation and repair, to replace aged and damaged hair cells, and to promote secretion of multiple growth factors.72 More recently, HUCB-MSCs have been shown to successfully differentiate into human hair follicles in vitro after 3 weeks of cell culture, establishing a method for high-speed and high-purity hair follicle cell differentiation with the hope of future injections to affected areas with hair loss.73 Another study found that HUCB-MSCs enhanced growth of human follicular stem cells in vitro; the authors proposed an altered Wnt/β‐catenin and JAK/STAT pathway was responsible for improved growth of hair follicular cells.74
Although umbilical cord blood is replete with the most rapidly dividing stem cells, autologous stem cells derived from the hair follicle or mononuclear cells also may be used to treat alopecia. One recent study randomized 40 patients with AGA and alopecia areata to receive 1 session of either autologous hair follicle or mononuclear cell–derived stem cell injections to the scalp.75 Mononuclear cells were acquired from the upper iliac crest bone marrow of patients who were treated with granulocyte colony-stimulating factor 3 days prior to the procedure. Follicular stem cells were taken from 4-mm punch biopsies of the unaffected scalp. After 6 months, there was a notable improvement in hair growth confirmed by immunostaining and dermoscopy, without a significant difference between the forms of autologous stem cell source. Of note, 45% of study patients with alopecia areata showed recurrence of disease at 1-year follow-up. The most common AEs were scalp dermatitis in 20% of participants. Participants who underwent bone marrow biopsy experienced bone pain, hematoma, and granulocyte colony-stimulating factor–induced fatigue and chills.75
Furthermore, the cost of stem cell therapy may be prohibitive. Therefore, although stem cell therapy is a novel and promising treatment for hair loss, future research is necessary to establish safety, efficacy, best practices, and accessibility.
Supplements
Patients failing routine treatments for alopecia may turn to holistic therapies. Nutrafol (Nutraceutical Wellness Inc), a novel nutraceutical product, is one such option that has been described for its anti-inflammatory, adaptogenic, antioxidant, and DHT-inhibiting properties. This supplement is not FDA approved or cleared, and large-scale clinical trials are lacking; however, one randomized controlled trial of 40 women with self-reported hair loss found a statistically significant increase in the number of terminal and vellus hair based on phototrichograms performed after 90 and 180 days (P=.009), with no AEs reported. This study, however, was limited by a small sample size.76
Lamdapil (ISDIN) is another oral supplement being investigated for hair loss. It contains L-cystine amino acids; zinc; vitamins B3, B5, B6; biotin; and the plant extract Serenoa repens.71Serenoa repens has reported activity inhibiting the enzyme 5α-reductase with the other vitamins, and amino acids are thought to maintain keratin and collagen growth in normal hair.77 One randomized trial investigated use of Lamdapil capsules in a total of 70 patients, which included men with AGA and women experiencing telogen effluvium. For men, the anagen-telogen ratio increased in the Lamdapil-treated group by 23.4%, indicating that more hair was in the growing phase compared to placebo (P<.05). Women with telogen effluvium experienced a significantly greater improvement in the hair-pull test compared to placebo (P<.05).77
Marine-derived nutraceutical substances also have been investigated for their role in treating hair loss. Viviscal, originally marketed under the name Hairgain, is one such supplement, which was shown to significantly reduce hair shedding at 3 and 6 months in a group of 96 premenopausal women diagnosed with subclinical hair thinning (P<.05). Additionally, phototrichogram images demonstrated a statistically significant increase in the mean velluslike hair diameter at 6 months compared to baseline.78
Although nutraceutical products are not first-line therapy for hair loss, dermatologists may recommend these treatments in patients refusing prescription medications, specifically requesting a natural treatment, or in addition to a first-line agent such as minoxidil. It must be noted, however, that both supplements are new, and there is need for further investigation on their efficacy, safety, and dosing, as neither is FDA regulated.
Conclusion
Hair loss affects millions of Americans each year and has detrimental effects on self-esteem and psychosocial functioning. Nonsurgical treatment options will undoubtedly continue to intrigue patients, as they are often less costly and do not carry risks associated with surgery. Minoxidil, finasteride, and LLLT remain staples of therapy, with the strongest evidence supporting their safety and efficacy. Numerous other treatment options are emerging, including PRP, microneedling, mesenchymal and autologous stem cell therapy, and oral supplements, though further research must be conducted to establish dosing, safety, and best practices. Physicians must discuss patient preference and anticipated length of treatment when discussing alopecia treatment to maximize patient satisfaction.
- Saed S, Ibrahim O, Bergfeld WF. Hair camouflage: a comprehensive review. Int J Womens Dermatol. 2016;2:122-127.
- Alfonso M, Richter-Appelt H, Tosti A, et al. The psychosocial impact of hair loss among men: a multinational European study. Curr Med Res Opin. 2005;21:1829-1836.
- Konior RJ. Complications in hair-restoration surgery. Facial Plast Surg Clin North Am. 2013;21:505-520.
- Manabe M, Tsuboi R, Itami S, et al. Guidelines for the diagnosis and treatment of male-pattern and female-pattern hair loss, 2017 version [published online June 4, 2018]. J Dermatol. 2018;45:1031-1043.
- Gupta AK, Mays RR, Dotzert MS, et al. Efficacy of non-surgical treatments for androgenetic alopecia: a systematic review and network meta-analysis. J Eur Acad Dermatol Venereol. 2018;32:2112-2125.
- Mehta PK, Mamdani B, Shansky RM, et al. Severe hypertension. treatment with minoxidil. JAMA. 1975;233:249-252.
- Zappacosta AR. Reversal of baldness in patient receiving minoxidil for hypertension. N Engl J Med. 1980;303:1480-1481.
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-194.
- Mori O, Uno H. The effect of topical minoxidil on hair follicular cycles of rats. J Dermatol. 1990;17:276-281.
- Pekmezci E, Turkoglu M, Gokalp H, et al. Minoxidil downregulates interleukin-1 alpha gene expression in HaCaT cells. Int J Trichol. 2018;10:108-112.
- Roenigk HH Jr, Pepper E, Kuruvilla S. Topical minoxidil therapy for hereditary male pattern alopecia. Cutis. 1987;39:337-342.
- Lucky AW, Piacquadio DJ, Ditre CM, et al. A randomized, placebo-controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss. J Am Acad Dermatol. 2004;50:541-553.
- Adil A, Godwin M. The effectiveness of treatments for androgenetic alopecia: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:136-141.e135.
- Nangia J, Wang T, Osborne C, et al. Effect of a scalp cooling device on alopecia in women undergoing chemotherapy for breast cancer: the SCALP randomized clinical trial. JAMA. 2017;317:596-605.
- Rugo HS, Melin SA, Voigt J. Scalp cooling with adjuvant/neoadjuvant chemotherapy for breast cancer and the risk of scalp metastases: systematic review and meta-analysis. Breast Cancer Res Treat. 2017;163:199-205.
- Duvic M, Lemak NA, Valero V, et al. A randomized trial of minoxidil in chemotherapy-induced alopecia. J Am Acad Dermatol. 1996;35:74-78.
- Yeager CE, Olsen EA. Treatment of chemotherapy-induced alopecia. Dermatol Ther. 2011;24:432-442.
- Freites-Martinez A, Shapiro J, Chan D, et al. Endocrine therapy-induced alopecia in patients with breast cancer. JAMA Dermatol. 2018;154:670-675.
- Gupta AK, Foley KA. 5% minoxidil: treatment for female pattern hair loss. Skin Ther Lett. 2014;19:5-7.
- Stoehr JR, Choi JN, Colavincenzo M, et al. Off-label use of topical minoxidil in alopecia: a review. Am J Clin Dermatol. 2019;20:237-250.
- Leenen FH, Smith DL, Unger WP. Topical minoxidil: cardiac effects in bald man. Br J Clin Pharmacol. 1988;26:481-485.
- Rossi A, Cantisani C, Melis L, et al. Minoxidil use in dermatology, side effects and recent patents. Recent Pat Inflamm Allergy Drug Discov. 2012;6:130-136.
- Rittmaster RS. Finasteride. N Engl J Med. 1994;330:120-125.
- Sawaya ME. Purification of androgen receptors in human sebocytes and hair. J Invest Dermatol. 1992;98(6 suppl):92S-96S.
- Sawaya ME, Shalita AR. Androgen receptor polymorphisms (CAG repeat lengths) in androgenetic alopecia, hirsutism, and acne. J Cutan Med Surg. 1998;3:9-15.
- Sato A, Takeda A. Evaluation of efficacy and safety of finasteride 1 mg in 3177 Japanese men with androgenetic alopecia [published online October 10, 2011]. J Dermatol. 2012;39:27-32.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998;39(4, pt 1):578-589.
- Kiguradze T, Temps WH, Yarnold PR, et al. Persistent erectile dysfunction in men exposed to the 5α-reductase inhibitors, finasteride, or dutasteride. PeerJ. 2017;5:E3020.
- Tsuboi R, Itami S, Inui S, et al. Guidelines for the management of androgenetic alopecia (2010). J Dermatol. 2012;39:113-120.
- Hu AC, Chapman LW, Mesinkovska NA. The efficacy and use of finasteride in women: a systematic review. Int J Dermatol. 2019;58:759-776.
- Price VH, Roberts JL, Hordinsky M, et al. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J Am Acad Dermatol. 2000;43(5, pt 1):768-776.
- Yeon JH, Jung JY, Choi JW, et al. 5 mg/day finasteride treatment for normoandrogenic Asian women with female pattern hair loss. J Eur Acad Dermatol Venereol. 2011;25:211-214.
- Oliveira-Soares R, André MC, Peres-Correia M. Adverse effects with finasteride 5 mg/day for patterned hair loss in premenopausal women. Int J Trichol. 2018;10:48-50.
- Kelly Y, Blanco A, Tosti A. Androgenetic alopecia: an update of treatment options. Drugs. 2016;76:1349-1364.
- Motofei IG, Rowland DL, Baconi DL, et al. Androgenetic alopecia; drug safety and therapeutic strategies [published online January 24, 2018]. Expert Opin Drug Saf. 2018;17:407-412.
- Shanshanwal SJ, Dhurat RS. Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: a randomized controlled open-label, evaluator-blinded study. Indian J Dermatol Venereol Leprol. 2017;83:47-54.
- Eun HC, Kwon OS, Yeon JH, et al. Efficacy, safety, and tolerability of dutasteride 0.5 mg once daily in male patients with male pattern hair loss: a randomized, double-blind, placebo-controlled, phase III study. J Am Acad Dermatol. 2010;63:252-258.
- Olszewska M, Rudnicka L. Effective treatment of female androgenic alopecia with dutasteride. J Drugs Dermatol. 2005;4:637-640.
- Nickel JC. Comparison of clinical trials with finasteride and dutasteride. Rev Urol. 2004;6(suppl 9):S31-S39.
- Olsen EA, Hordinsky M, Whiting D, et al. The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. J Am Acad Dermatol. 2006;55:1014-1023.
- Gómez R, Núñez L, Caballero R, et al. Spironolactone and its main metabolite canrenoic acid block hKv1.5, Kv4.3 and Kv7.1 + minK channels. Br J Pharmacol. 2005;146:146-161.
- Huffman DH, Kampmann JP, Hignite CE, et al. Gynecomastia induced in normal males by spironolactone. Clin Pharmacol Ther. 1978;24:465-473.
- Sinclair R, Patel M, Dawson TL Jr, et al. Hair loss in women: medical and cosmetic approaches to increase scalp hair fullness. Br J Dermatol. 2011;165(suppl 3):12-18.
- Sinclair R, Wewerinke M, Jolley D. Treatment of female pattern hair loss with oral antiandrogens. Br J Dermatol. 2005;152:466-473.
- Brough KR, Torgerson RR. Hormonal therapy in female pattern hair loss. Int J Womens Dermatol. 2017;3:53-57.
- Fabbrocini G, Cantelli M, Masarà A, et al. Female pattern hair loss: a clinical, pathophysiologic, and therapeutic review. Int J Womens Dermatol. 2018;4:203-211.
- Sinclair RD. Female pattern hair loss: a pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. Int J Dermatol. 2018;57:104-109.
- Camacho-Martinez FM. Hair loss in women. Semin Cutan Med Surg. 2009;28:19-32.
- Mackenzie IS, Macdonald TM, Thompson A, et al. Spironolactone and risk of incident breast cancer in women older than 55 years: retrospective, matched cohort study. BMJ. 2012;345:E4447.
- Farivar S, Malekshahabi T, Shiari R. Biological effects of low level laser therapy. J Laser Med Sci. 2014;5:58-62.
- Jimenez JJ, Wikramanayake TC, Bergfeld W, et al. Efficacy and safety of a low-level laser device in the treatment of male and female pattern hair loss: a multicenter, randomized, sham device-controlled, double-blind study. Am J Clin Dermatol. 2014;15:115-127.
- Lanzafame RJ, Blanche RR, Bodian AB, et al. The growth of human scalp hair mediated by visible red light laser and LED sources in males. Lasers Surg Med. 2013;45:487-495.
- Kim H, Choi JW, Kim JY, et al. Low-level light therapy for androgenetic alopecia: a 24-week, randomized, double-blind, sham device-controlled multicenter trial. Dermatol Surg. 2013;39:1177-1183.
- Banga AK. Transdermal and Intradermal Delivery of Therapeutic Agents: Application of Physical Technologies. New York, NY: CRC Press; 2011.
- Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichol. 2013;5:6-11.
- Jha AK, Vinay K, Zeeshan M, et al. Platelet-rich plasma and microneedling improves hair growth in patients of androgenetic alopecia when used as an adjuvant to minoxidil [published online January 28, 2019]. J Cosmet Dermatol. doi:10.1111/jocd.12864.
- Anitua E, Pino A, Martinez N, et al. The effect of plasma rich in growth factors on pattern hair loss: a pilot study. Dermatol Surg. 2017;43:658-670.
- Puig CJ, Reese R, Peters M. Double-blind, placebo-controlled pilot study on the use of platelet-rich plasma in women with female androgenetic alopecia. Dermatol Surg. 2016;42:1243-1247.
- Mapar MA, Shahriari S, Haghighizadeh MH. Efficacy of platelet-rich plasma in the treatment of androgenetic (male-patterned) alopecia: a pilot randomized controlled trial. J Cosmet Laser Ther. 2016;18:452-455.
- Maria-Angeliki G, Alexandros-Efstratios K, Dimitris R, et al. Platelet-rich plasma as a potential treatment for noncicatricial alopecias. Int J Trichol. 2015;7:54-63.
- Gkini MA, Kouskoukis AE, Tripsianis G, et al. Study of platelet-rich plasma injections in the treatment of androgenetic alopecia through an one-year period. J Cutan Aesthet Surg. 2014;7:213-219.
- Landesberg R, Roy M, Glickman RS. Quantification of growth factor levels using a simplified method of platelet-rich plasma gel preparation. J Oral Maxillofac Surg. 2000;58:297-300; discussion 300-301.
- Weibrich G, Kleis WK, Hafner G. Growth factor levels in the platelet-rich plasma produced by 2 different methods: curasan-type PRP kit versus PCCS PRP system. Int J Oral Maxillofac Implants. 2002;17:184-190.
- Alves R, Grimalt R. Randomized placebo-controlled, double-blind, half-head study to assess the efficacy of platelet-rich plasma on the treatment of androgenetic alopecia. Dermatol Surg. 2016;42:491-497.
- Hou A, Cohen B, Haimovic A, et al. Microneedling: a comprehensive review. Dermatol Surg. 2017;43:321-339.
- Singh A, Yadav S. Microneedling: advances and widening horizons. Indian Dermatol Online J. 2016;7:244-254.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study. J Cosmet Dermatol. 2016;15:434-443.
- Kumar MK, Inamadar AC, Palit A. A randomized controlled single-observer blinded study to determine the efficacy of topical minoxidil plus microneedling versus topical minoxidil alone in the treatment of androgenetic alopecia. J Cutan Aesthet Surg. 2018;11:211-216.
- Hausauer AK, Jones DH. Evaluating the efficacy of different platelet-rich plasma regimens for management of androgenetic alopecia: a single-center, blinded, randomized clinical trial. Dermatol Surg. 2018;44:1191-1200.
- Kang JS, Zheng Z, Choi MJ, et al. The effect of CD34+ cell-containing autologous platelet-rich plasma injection on pattern hair loss: a preliminary study. J Eur Acad Dermatol Venereol. 2014;28:72-79.
- Soltani-Arabshahi R, Wong JW, Duffy KL, et al. Facial allergic granulomatous reaction and systemic hypersensitivity associated with microneedle therapy for skin rejuvenation: adverse reactions with microneedle therapy. JAMA Dermatol. 2014;150:68-72.
- Bak DH, Choi MJ, Kim SR, et al. Human umbilical cord blood mesenchymal stem cells engineered to overexpress growth factors accelerate outcomes in hair growth. Korean J Physiol Pharmacol. 2018;22:555-566.
- Bu ZY, Wu LM, Yu XH, et al. Isolation and characterization of in vitro culture of hair follicle cells differentiated from umbilical cord blood mesenchymal stem cells. Exp Ther Med. 2017;14:303-307.
- Kim JE, Oh JH, Woo YJ, et al. Effects of mesenchymal stem cell therapy on alopecia areata in cellular and hair follicle organ culture models [published online October 29, 2018]. Exp Dermatol. doi:10.1111/exd.13812.
- Elmaadawi IH, Mohamed BM, Ibrahim ZAS, et al. Stem cell therapy as a novel therapeutic intervention for resistant cases of alopecia areata and androgenetic alopecia [published online March 6, 2018]. J Dermatolog Treat. 2018;29:431-440.
- Ablon G, Kogan S. A six-month, randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of a nutraceutical supplement for promoting hair growth in women with self-perceived thinning hair. J Drugs Dermatol. 2018;17:558-565.
- Narda M, Aladren S, Cestone E, et al. Efficacy and safety of a food supplement containing L-cystine, Serenoa repens extract and biotin for hair loss in healthy males and females. a prospective, randomized, double-blinded, controlled clinical trial. J Cosmo Trichol. 2017;3. doi:10.4172/2471-9323.1000127.
- Glynis A. A double-blind, placebo-controlled study evaluating the efficacy of an oral supplement in women with self-perceived thinning hair. J Clin Aesthet Dermatol. 2012;5:28-34.
- Saed S, Ibrahim O, Bergfeld WF. Hair camouflage: a comprehensive review. Int J Womens Dermatol. 2016;2:122-127.
- Alfonso M, Richter-Appelt H, Tosti A, et al. The psychosocial impact of hair loss among men: a multinational European study. Curr Med Res Opin. 2005;21:1829-1836.
- Konior RJ. Complications in hair-restoration surgery. Facial Plast Surg Clin North Am. 2013;21:505-520.
- Manabe M, Tsuboi R, Itami S, et al. Guidelines for the diagnosis and treatment of male-pattern and female-pattern hair loss, 2017 version [published online June 4, 2018]. J Dermatol. 2018;45:1031-1043.
- Gupta AK, Mays RR, Dotzert MS, et al. Efficacy of non-surgical treatments for androgenetic alopecia: a systematic review and network meta-analysis. J Eur Acad Dermatol Venereol. 2018;32:2112-2125.
- Mehta PK, Mamdani B, Shansky RM, et al. Severe hypertension. treatment with minoxidil. JAMA. 1975;233:249-252.
- Zappacosta AR. Reversal of baldness in patient receiving minoxidil for hypertension. N Engl J Med. 1980;303:1480-1481.
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-194.
- Mori O, Uno H. The effect of topical minoxidil on hair follicular cycles of rats. J Dermatol. 1990;17:276-281.
- Pekmezci E, Turkoglu M, Gokalp H, et al. Minoxidil downregulates interleukin-1 alpha gene expression in HaCaT cells. Int J Trichol. 2018;10:108-112.
- Roenigk HH Jr, Pepper E, Kuruvilla S. Topical minoxidil therapy for hereditary male pattern alopecia. Cutis. 1987;39:337-342.
- Lucky AW, Piacquadio DJ, Ditre CM, et al. A randomized, placebo-controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss. J Am Acad Dermatol. 2004;50:541-553.
- Adil A, Godwin M. The effectiveness of treatments for androgenetic alopecia: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:136-141.e135.
- Nangia J, Wang T, Osborne C, et al. Effect of a scalp cooling device on alopecia in women undergoing chemotherapy for breast cancer: the SCALP randomized clinical trial. JAMA. 2017;317:596-605.
- Rugo HS, Melin SA, Voigt J. Scalp cooling with adjuvant/neoadjuvant chemotherapy for breast cancer and the risk of scalp metastases: systematic review and meta-analysis. Breast Cancer Res Treat. 2017;163:199-205.
- Duvic M, Lemak NA, Valero V, et al. A randomized trial of minoxidil in chemotherapy-induced alopecia. J Am Acad Dermatol. 1996;35:74-78.
- Yeager CE, Olsen EA. Treatment of chemotherapy-induced alopecia. Dermatol Ther. 2011;24:432-442.
- Freites-Martinez A, Shapiro J, Chan D, et al. Endocrine therapy-induced alopecia in patients with breast cancer. JAMA Dermatol. 2018;154:670-675.
- Gupta AK, Foley KA. 5% minoxidil: treatment for female pattern hair loss. Skin Ther Lett. 2014;19:5-7.
- Stoehr JR, Choi JN, Colavincenzo M, et al. Off-label use of topical minoxidil in alopecia: a review. Am J Clin Dermatol. 2019;20:237-250.
- Leenen FH, Smith DL, Unger WP. Topical minoxidil: cardiac effects in bald man. Br J Clin Pharmacol. 1988;26:481-485.
- Rossi A, Cantisani C, Melis L, et al. Minoxidil use in dermatology, side effects and recent patents. Recent Pat Inflamm Allergy Drug Discov. 2012;6:130-136.
- Rittmaster RS. Finasteride. N Engl J Med. 1994;330:120-125.
- Sawaya ME. Purification of androgen receptors in human sebocytes and hair. J Invest Dermatol. 1992;98(6 suppl):92S-96S.
- Sawaya ME, Shalita AR. Androgen receptor polymorphisms (CAG repeat lengths) in androgenetic alopecia, hirsutism, and acne. J Cutan Med Surg. 1998;3:9-15.
- Sato A, Takeda A. Evaluation of efficacy and safety of finasteride 1 mg in 3177 Japanese men with androgenetic alopecia [published online October 10, 2011]. J Dermatol. 2012;39:27-32.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998;39(4, pt 1):578-589.
- Kiguradze T, Temps WH, Yarnold PR, et al. Persistent erectile dysfunction in men exposed to the 5α-reductase inhibitors, finasteride, or dutasteride. PeerJ. 2017;5:E3020.
- Tsuboi R, Itami S, Inui S, et al. Guidelines for the management of androgenetic alopecia (2010). J Dermatol. 2012;39:113-120.
- Hu AC, Chapman LW, Mesinkovska NA. The efficacy and use of finasteride in women: a systematic review. Int J Dermatol. 2019;58:759-776.
- Price VH, Roberts JL, Hordinsky M, et al. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J Am Acad Dermatol. 2000;43(5, pt 1):768-776.
- Yeon JH, Jung JY, Choi JW, et al. 5 mg/day finasteride treatment for normoandrogenic Asian women with female pattern hair loss. J Eur Acad Dermatol Venereol. 2011;25:211-214.
- Oliveira-Soares R, André MC, Peres-Correia M. Adverse effects with finasteride 5 mg/day for patterned hair loss in premenopausal women. Int J Trichol. 2018;10:48-50.
- Kelly Y, Blanco A, Tosti A. Androgenetic alopecia: an update of treatment options. Drugs. 2016;76:1349-1364.
- Motofei IG, Rowland DL, Baconi DL, et al. Androgenetic alopecia; drug safety and therapeutic strategies [published online January 24, 2018]. Expert Opin Drug Saf. 2018;17:407-412.
- Shanshanwal SJ, Dhurat RS. Superiority of dutasteride over finasteride in hair regrowth and reversal of miniaturization in men with androgenetic alopecia: a randomized controlled open-label, evaluator-blinded study. Indian J Dermatol Venereol Leprol. 2017;83:47-54.
- Eun HC, Kwon OS, Yeon JH, et al. Efficacy, safety, and tolerability of dutasteride 0.5 mg once daily in male patients with male pattern hair loss: a randomized, double-blind, placebo-controlled, phase III study. J Am Acad Dermatol. 2010;63:252-258.
- Olszewska M, Rudnicka L. Effective treatment of female androgenic alopecia with dutasteride. J Drugs Dermatol. 2005;4:637-640.
- Nickel JC. Comparison of clinical trials with finasteride and dutasteride. Rev Urol. 2004;6(suppl 9):S31-S39.
- Olsen EA, Hordinsky M, Whiting D, et al. The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. J Am Acad Dermatol. 2006;55:1014-1023.
- Gómez R, Núñez L, Caballero R, et al. Spironolactone and its main metabolite canrenoic acid block hKv1.5, Kv4.3 and Kv7.1 + minK channels. Br J Pharmacol. 2005;146:146-161.
- Huffman DH, Kampmann JP, Hignite CE, et al. Gynecomastia induced in normal males by spironolactone. Clin Pharmacol Ther. 1978;24:465-473.
- Sinclair R, Patel M, Dawson TL Jr, et al. Hair loss in women: medical and cosmetic approaches to increase scalp hair fullness. Br J Dermatol. 2011;165(suppl 3):12-18.
- Sinclair R, Wewerinke M, Jolley D. Treatment of female pattern hair loss with oral antiandrogens. Br J Dermatol. 2005;152:466-473.
- Brough KR, Torgerson RR. Hormonal therapy in female pattern hair loss. Int J Womens Dermatol. 2017;3:53-57.
- Fabbrocini G, Cantelli M, Masarà A, et al. Female pattern hair loss: a clinical, pathophysiologic, and therapeutic review. Int J Womens Dermatol. 2018;4:203-211.
- Sinclair RD. Female pattern hair loss: a pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. Int J Dermatol. 2018;57:104-109.
- Camacho-Martinez FM. Hair loss in women. Semin Cutan Med Surg. 2009;28:19-32.
- Mackenzie IS, Macdonald TM, Thompson A, et al. Spironolactone and risk of incident breast cancer in women older than 55 years: retrospective, matched cohort study. BMJ. 2012;345:E4447.
- Farivar S, Malekshahabi T, Shiari R. Biological effects of low level laser therapy. J Laser Med Sci. 2014;5:58-62.
- Jimenez JJ, Wikramanayake TC, Bergfeld W, et al. Efficacy and safety of a low-level laser device in the treatment of male and female pattern hair loss: a multicenter, randomized, sham device-controlled, double-blind study. Am J Clin Dermatol. 2014;15:115-127.
- Lanzafame RJ, Blanche RR, Bodian AB, et al. The growth of human scalp hair mediated by visible red light laser and LED sources in males. Lasers Surg Med. 2013;45:487-495.
- Kim H, Choi JW, Kim JY, et al. Low-level light therapy for androgenetic alopecia: a 24-week, randomized, double-blind, sham device-controlled multicenter trial. Dermatol Surg. 2013;39:1177-1183.
- Banga AK. Transdermal and Intradermal Delivery of Therapeutic Agents: Application of Physical Technologies. New York, NY: CRC Press; 2011.
- Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichol. 2013;5:6-11.
- Jha AK, Vinay K, Zeeshan M, et al. Platelet-rich plasma and microneedling improves hair growth in patients of androgenetic alopecia when used as an adjuvant to minoxidil [published online January 28, 2019]. J Cosmet Dermatol. doi:10.1111/jocd.12864.
- Anitua E, Pino A, Martinez N, et al. The effect of plasma rich in growth factors on pattern hair loss: a pilot study. Dermatol Surg. 2017;43:658-670.
- Puig CJ, Reese R, Peters M. Double-blind, placebo-controlled pilot study on the use of platelet-rich plasma in women with female androgenetic alopecia. Dermatol Surg. 2016;42:1243-1247.
- Mapar MA, Shahriari S, Haghighizadeh MH. Efficacy of platelet-rich plasma in the treatment of androgenetic (male-patterned) alopecia: a pilot randomized controlled trial. J Cosmet Laser Ther. 2016;18:452-455.
- Maria-Angeliki G, Alexandros-Efstratios K, Dimitris R, et al. Platelet-rich plasma as a potential treatment for noncicatricial alopecias. Int J Trichol. 2015;7:54-63.
- Gkini MA, Kouskoukis AE, Tripsianis G, et al. Study of platelet-rich plasma injections in the treatment of androgenetic alopecia through an one-year period. J Cutan Aesthet Surg. 2014;7:213-219.
- Landesberg R, Roy M, Glickman RS. Quantification of growth factor levels using a simplified method of platelet-rich plasma gel preparation. J Oral Maxillofac Surg. 2000;58:297-300; discussion 300-301.
- Weibrich G, Kleis WK, Hafner G. Growth factor levels in the platelet-rich plasma produced by 2 different methods: curasan-type PRP kit versus PCCS PRP system. Int J Oral Maxillofac Implants. 2002;17:184-190.
- Alves R, Grimalt R. Randomized placebo-controlled, double-blind, half-head study to assess the efficacy of platelet-rich plasma on the treatment of androgenetic alopecia. Dermatol Surg. 2016;42:491-497.
- Hou A, Cohen B, Haimovic A, et al. Microneedling: a comprehensive review. Dermatol Surg. 2017;43:321-339.
- Singh A, Yadav S. Microneedling: advances and widening horizons. Indian Dermatol Online J. 2016;7:244-254.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study. J Cosmet Dermatol. 2016;15:434-443.
- Kumar MK, Inamadar AC, Palit A. A randomized controlled single-observer blinded study to determine the efficacy of topical minoxidil plus microneedling versus topical minoxidil alone in the treatment of androgenetic alopecia. J Cutan Aesthet Surg. 2018;11:211-216.
- Hausauer AK, Jones DH. Evaluating the efficacy of different platelet-rich plasma regimens for management of androgenetic alopecia: a single-center, blinded, randomized clinical trial. Dermatol Surg. 2018;44:1191-1200.
- Kang JS, Zheng Z, Choi MJ, et al. The effect of CD34+ cell-containing autologous platelet-rich plasma injection on pattern hair loss: a preliminary study. J Eur Acad Dermatol Venereol. 2014;28:72-79.
- Soltani-Arabshahi R, Wong JW, Duffy KL, et al. Facial allergic granulomatous reaction and systemic hypersensitivity associated with microneedle therapy for skin rejuvenation: adverse reactions with microneedle therapy. JAMA Dermatol. 2014;150:68-72.
- Bak DH, Choi MJ, Kim SR, et al. Human umbilical cord blood mesenchymal stem cells engineered to overexpress growth factors accelerate outcomes in hair growth. Korean J Physiol Pharmacol. 2018;22:555-566.
- Bu ZY, Wu LM, Yu XH, et al. Isolation and characterization of in vitro culture of hair follicle cells differentiated from umbilical cord blood mesenchymal stem cells. Exp Ther Med. 2017;14:303-307.
- Kim JE, Oh JH, Woo YJ, et al. Effects of mesenchymal stem cell therapy on alopecia areata in cellular and hair follicle organ culture models [published online October 29, 2018]. Exp Dermatol. doi:10.1111/exd.13812.
- Elmaadawi IH, Mohamed BM, Ibrahim ZAS, et al. Stem cell therapy as a novel therapeutic intervention for resistant cases of alopecia areata and androgenetic alopecia [published online March 6, 2018]. J Dermatolog Treat. 2018;29:431-440.
- Ablon G, Kogan S. A six-month, randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of a nutraceutical supplement for promoting hair growth in women with self-perceived thinning hair. J Drugs Dermatol. 2018;17:558-565.
- Narda M, Aladren S, Cestone E, et al. Efficacy and safety of a food supplement containing L-cystine, Serenoa repens extract and biotin for hair loss in healthy males and females. a prospective, randomized, double-blinded, controlled clinical trial. J Cosmo Trichol. 2017;3. doi:10.4172/2471-9323.1000127.
- Glynis A. A double-blind, placebo-controlled study evaluating the efficacy of an oral supplement in women with self-perceived thinning hair. J Clin Aesthet Dermatol. 2012;5:28-34.
Practice Points
- Hair loss is a common phenomenon in both men and women and can seriously impact psychosocial functioning.
- There are numerous US Food and Drug Administration–approved and off-label nonsurgical treatment options for alopecia. Dermatologists should be well versed in these treatment modalities and the associated sideeffect profiles to select the appropriate therapy for each patient.
The Role of Diet in Preventing Photoaging and Treating Common Skin Conditions
The connection between diet and physical beauty has been an area of increasing interest in popular culture as well as in the scientific community. Numerous supplements, plant derivatives, and antioxidants have been proposed to help improve skin conditions and prevent signs of aging.1 Clinical and basic research has played an important role in confirming or debunking these claims, leading to new insight into oral supplements that may play a role in improving signs of photoaging, as well as symptoms of common skin diseases such as acne vulgaris (AV), atopic dermatitis (AD), and psoriasis. This article reviews some of the vitamins, supplements, and antioxidants that have been studied in the improvement of these conditions.
Photoaging
Recently, there has been increased interest among researchers in the role of antioxidants in combatting photoaging. The main determinants of photoaging are chronic sunlight exposure and melanin density. Photoaging presentation includes deep rhytides, pigmentary changes, dryness, loss of skin tone, leathery appearance, and actinic purpura.2-4
Beta-carotene is a fat-soluble derivative of vitamin A, which has retinol activity and has an inhibitory effect on free radicals. It has been used to decrease the effect of UV light on the skin as well as to treat erythropoietic porphyria.5-7 One study evaluated the efficacy of low-dose and high-dose beta-carotene in improving facial rhytides and elasticity in a cohort of 30 women older than 50 years.8 Participants were given 30 or 90 mg of beta-carotene once daily for 90 days, and the final results were compared to baseline. Those who received the 30-mg dose showed improvements in facial rhytides and elasticity, increased type I procollagen messenger RNA levels, decreased UV-induced thymine dimer staining, and decreased 8-hydroxy-2′-deoxyguanosine staining. The lower dose of beta-carotene was found to prevent photoaging and was superior to the higher dose, which actually significantly decreased the minimal erythema dose (indicating a deleterious effect)(P=.025).8
Another study compared the role of a 25-mg carotenoid supplement vs a combination of carotenoid and vitamin E (335 mg [500 IU] RRR-α-tocopherol) supplements in preventing erythema development on the back.9 Using a blue light solar stimulator for illumination, erythema on the dorsal back skin was significantly reduced after week 8 (P<.01). The erythema was lower in the combination group than the carotenoid group alone, but the difference was not statistically significant. Furthermore, after 12 weeks, yellowing of the skin was observed in both groups, especially the skin of the palms and face.9
Collagen peptides also have been used in the prevention and repair of photoaging. Proksch et al10 conducted a double-blind, placebo-controlled trial to investigate the role of collagen peptides on skin elasticity in 69 women aged 35 to 55 years. At 4 weeks, oral supplementation of collagen hydrolysate (2.5 g once daily or 5 g once daily for 8 weeks) showed significant (P<.05) improvement of skin elasticity in both the low-dose and high-dose groups in women older than 50 years; however, collagen peptides did not lead to statistically significant improvement in skin hydration or transepidermal water loss. No known side effects were reported; thus, collagen peptides may be both efficacious and safe in improving signs of photoaging in elderly patients.10 Thus, these studies have shown potentially positive effects of beta-carotene, vitamin E, and collagen peptides in improving the signs of photoaging.
Acne Vulgaris
Acne vulgaris is a common dermatologic condition seen in the western hemisphere, with 40 to 50 million affected individuals in the United States annually.11,12 A landmark study that examined 1200 Kitavans from Papua New Guinea and 115 Aché individuals from a hunter-gatherer community in Paraguay found no cases of AV in either group.12 These findings have led to the speculation that AV may be associated with environmental factors, particularly the Western diet.
An investigator-blinded randomized clinical trial (RCT) explored the role of a low-glycemic diet compared to a carbohydrate-dense diet on improvement of AV lesions after 12 weeks.13 The results yielded a significant decrease in lesions in the low-glycemic group (mean [SEM], −23.5 [−3.9]) vs the control group (−12.0 [−3.0])(P=.03). Furthermore, the results indicated a significant decrease in weight (P<.001) and body mass index (P=.001) with an improvement in insulin sensitivity in the low-glycemic group vs the control group.13 Kwon et al14 conducted a similar investigator-blinded parallel study with 32 participants receiving either a low-glycemic diet or continuing their normal diet for 10 weeks. Participants in the low-glycemic group demonstrated a significant reduction in mean noninflammatory lesions (−27.6% [P=.04]) and mean inflammatory lesions (−70.9% [P<.05]). Histologic image analysis showed a significant decrease in the mean (SEM) area of sebaceous glands in the low-glycemic group (0.32 [0.03] mm2) compared to baseline (0.24 [0.03] mm2)(P=.03). At 10 weeks, immunohistochemical specimens showed reduction in IL-8 (P=.03) and sterol regulatory element-binding protein 1 (P=.03), which regulates the synthesis of lipids.14 Thus, both studies concluded that a reduction in glycemic load may improve acne overall.13,14
Another study attempted to investigate the role of additional dietary supplements in improving acne. A double-blinded RCT explored the efficacy of omega-3 fatty acids or γ-linoleic acid compared to a control group in improving mild to moderate AV lesions through clinical and histological evaluations.15 The 10-week prospective study included 45 patients who were allocated to 3 matched groups and randomized to 3 treatment arms. They were given omega-3 fatty acids (1000 mg each of eicosapentaenoic acid and docosahexaenoic acid) or γ-linoleic acid (borage oil with 400 mg of γ-linoleic acid) or no intervention. After treatment completion, patients in both treatment groups showed significant reduction in mean inflammatory acne lesions, mean noninflammatory acne lesions, and mean acne severity (all P<.05), while the control group showed no significant reduction in acne lesions or acne severity. Furthermore, hematoxylin and eosin and IL-8 immunohistochemical staining of biopsies from the affected areas showed significant reduction of inflammation in both treatment groups (P<.05) but not in the control group. Therefore, the authors concluded that both omega-3 fatty acids and γ-linoleic acid could be used as adjuvant therapies in AV treatment.15
Atopic Dermatitis
The prevalence of atopic dermatitis (AD) in children ranges from approximately 9% to 18% across the United States.16 Pyridoxine, or vitamin B6, is an important water-soluble vitamin and a cofactor for numerous biochemical processes including carbohydrate and amino acid metabolism pathways and glucocorticoid receptor regulation.17,18 However, a double-blinded, placebo-controlled RCT failed to show efficacy of once-daily pyridoxine hydrochloride 50 mg in improving erythema, itching, or nocturnal sleep disturbance associated with AD in a cohort of 48 children. The investigators concluded that pyridoxine supplementation cannot be recommended to improve the symptoms of AD in children.19
Zinc is an essential nutrient that functions as an important cofactor in cell metabolism and growth pathways.20 One study showed that intracellular erythrocyte zinc levels were significantly lower in AD patients compared to healthy controls (P<.001); however, there was no observed difference in serum zinc levels (P=.148). Furthermore, greater disease severity as determined by the SCORing Atopic Dermatitis (SCORAD) index was negatively correlated with erythrocyte zinc levels (r=−0.791; P<.001).21 Kim et al22 investigated hair zinc levels and the efficacy of oral zinc supplementation in children with mild to moderate AD. Mean (SD) hair zinc levels were lower in the AD group compared to the control group (113.10 [33.6] μg vs 130.90 [36.63] μg [P=.012]). Of 41 AD patients with low zinc levels, 22 were allocated to group A, which received oral zinc oxide 12 mg for 8 weeks, and 19 were allocated to group B, which did not receive any supplementation over the same period. Groups A and B also received oral antihistamines and topical moisturizers. Mean (SD) zinc levels increased significantly in group A from 96.36 (21.05) μg to 131.81 (27.45) μg (P<.001). Furthermore, relative to group B, group A showed significantly greater improvements in eczema area and severity index (P=.044), transepidermal water loss (P=.015), and visual analog scale for pruritus (P<.001) at the end of 8 weeks. The authors concluded that oral zinc supplementation might be an effective adjunctive therapy for AD patients with low hair zinc levels.22
Researchers also have explored the efficacy of fat-soluble vitamins D and E in treating AD. Vitamin D is thought to downregulate IgE-mediated skin reactions and decrease adverse effects of UV light on the skin.23,24 A double-blind, placebo-controlled trial randomized 45 patients with AD to 4 groups: vitamins D and E placebos (n=11), 1600 IU vitamin D3 plus vitamin E placebo (n=12), 600 IU vitamin E (synthetic all-rac-α-tocopherol) plus vitamin D placebo (n=11), and 1600 IU vitamin D3 plus 600 IU vitamin E (synthetic all-rac-α-tocopherol)(n=11).25 After 60 days, the SCORAD index was reduced by 28.9% in the placebo group, 34.8% in the vitamin D3 group, 35.7% in the vitamin E group, and 64.3% in the combined vitamins D and E group (P=.004). Furthermore, prior to intervention, a negative correlation was demonstrated between plasma α-tocopherol concentration and the SCORAD index (r=−.33; P=.025).25 Thus, supplementing vitamins D and E may play a beneficial role in the treatment of AD.
Other emerging studies are investigating the role of the gut microbiome in various pathologies. Prebiotics may alter the gut microbiome and are thought to play a role in reducing intestinal inflammation.26 One randomized, placebo-controlled, parallel study examined the effect of prebiotic oligosaccharide supplementation on the development of AD in at-risk children, defined as having a biological parent with a history of asthma, allergic rhinitis, or AD.27 At 6-month follow-up, 10 infants (9.8%)(95% CI, 5.4%-17.1%) in the intervention group (n=102) and 24 infants (23.1%)(95% CI, 16.0%-32.1%) in the placebo group (n=104) had developed AD. The authors postulated that the prebiotic oligosaccharides might play a role in immune modulation by altering bowel flora and preventing the development of AD in infancy.27
Notably, a 2012 Cochrane review evaluated 11 studies of dietary supplements as possible treatment options for AD. The authors concluded that the evidence was minimal to support the regular use of dietary supplements, especially due to their high cost as well as the possibility that high levels of certain vitamins (eg, vitamin D) may cause long-term complications.26
Psoriasis
Psoriasis is an autoimmune skin condition that has an annual prevalence ranging from approximately 1% to 9% in adults residing in Western countries.28,29 Some have argued that due to decreased bacterial diversity and increased bacterial growth in the small bowel, psoriatic patients are exposed to higher levels of bacterial peptidoglycans and endotoxins.30 To combat the absorption of these substances in psoriasis patients
The effects of very long chain fatty acids also have been examined. A 4-month, double-blind, multicenter RCT compared the effects of daily supplementation with 6 g of either omega-3 fatty acids or omega-6 fatty acids in patients with mild to moderate plaque psoriasis.31 Psoriasis area and severity index scores and patient subjective scores did not change significantly in either group; however, scaling was reduced in both groups (P<.01). The group receiving omega-3 fatty acids had decreased cellular infiltration (P<.01), and the group receiving omega-6 fatty acids had decreased desquamation and redness (P<.05). In the omega-6 group, there was a significant correlation between clinical improvement (decrease in clinical score) and increase in serum eicosapentaenoic acid (r=−0.34; P<.05) and total omega-3 fatty acids (r=−0.36; P<.05). Overall, the authors concluded that supplementation with omega-3 fatty acids (fish oil) was no better than omega-6 fatty acids (corn oil) for treatment of psoriasis.31
Some dermatologists have advocated for the use of oral vitamin D supplementation as an adjunctive treatment of psoriasis, given that it is inexpensive and also may play a role in reducing the risk for cancer and cardiovascular events.32 One study evaluated the level of 25-hydroxy vitamin D in 43 psoriasis patients compared to 43 healthy controls. Mean (SD) vitamin D levels were significantly lower in psoriasis patients (13.3 [6.9]) compared to controls (22.4 [18.4])(P=.004).33 A cross-sectional study similarly found significantly higher rates of vitamin D deficiency (25-hydroxy vitamin D <20 ng/mL) in psoriatic patients (57.8%) compared to patients with rheumatoid arthritis (37.5%) and healthy controls (29.7%)(P<.001). Interestingly, during winter the prevalence of vitamin D deficiency increased to 80.9%, 41.3%, and 30.3% in the 3 groups, respectively; however, no significant correlation was seen between psoriasis severity, as measured by psoriasis area and severity index, and serum vitamin D levels.34 Although vitamin D deficiency may be more prevalent among patients with psoriasis, data regarding the efficacy of treating psoriasis with oral vitamin D supplementation is still lacking.
Conclusion
Our understanding of the link between diet and dermatologic conditions continues to evolve. Recent data for several dietary supplements and therapies showed promising results in repairing signs of photoaging, as well as treating AV, AD, and psoriasis. As patients seek these adjunctive therapies, it is important for physicians to be well informed on the benefits and risks to appropriately counsel patients.
Globally, physicians advocate for a low-glycemic diet rich in fruits and vegetables. Furthermore, the cosmetic diet can be enhanced by the consumption of dietary supplements such as beta-carotene, collagen peptides, zinc, and fat-soluble vitamins such as vitamins D and E. However, prospective RCTs are needed to further investigate the role of these dietary elements in treating and improving dermatologic conditions.
- Khanna R, Shifrin N, Nektalova T, et al. Diet and dermatology: Google search results for acne, psoriasis, and eczema. Cutis. 2018;102:44, 46-48.
- Yaar M, Eller MS, Gilchrest BA. Fifty years of skin aging. J Invest Dermatol Symp Proc. 2002;7:51-58.
- Helfrich YR, Sachs DL, Voorhees JJ. Overview of skin aging and photoaging. Dermatol Nurs. 2008;20:177-183.
- Pandel R, Poljšak B, Godic A, et al. Skin photoaging and the role of antioxidants in its prevention. ISRN Dermatol. 2013;2013:930164.
- Mathews-Roth MM, Pathak MA, Fitzpatrick T, et al. Beta carotene therapy for erythropoietic protoporphyria and other photosensitivity diseases. Arch Dermatol. 1977;113:1229-1232.
- Myriam M, Sabatier M, Steiling H, et al. Skin bioavailability of dietary vitamin E, carotenoids, polyphenols, vitamin C, zinc and selenium. Br J Nutr. 2006;96:227-238.
- Cho S. The role of functional foods in cutaneous anti-aging. J Lifestyle Med. 2014;4:8-16.
- Cho S, Lee DH, Won CH, et al. Differential effects of low-dose and high-dose beta-carotene supplementation on the signs of photoaging and type I procollagen gene expression in human skin in vivo. Dermatology. 2010;221:160-171.
- Stahl W, Heinrich U, Jungmann H, et al. Carotenoids and carotenoids plus vitamin E protect against ultraviolet light–induced erythema in humans. Am J Clin Nutr. 2000;71:795-798.
- Proksch E, Segger D, Degwert J, et al. Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: a double-blind, placebo-controlled study. Skin Pharmacol Physiol. 2014;27:47-55.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39(2, pt 3):S34-S37.
- Cordain L, Lindeberg S, Hurtado M, et al. Acne vulgaris: a disease of Western civilization. Arch Dermatol. 2002;138:1584-1590.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Jung JY, Kwon HH, Hong JS, et al. Effect of dietary supplementation with omega-3 fatty acid and gamma-linolenic acid on acne vulgaris: a randomised, double-blind, controlled trial. Acta Derm Venereol. 2014;94:521-526.
- Shaw TE, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67-73.
- Merrill AH Jr, Henderson JM. Diseases associated with defects in vitamin B6 metabolism or utilization. Annu Rev Nutr. 1987;7:137-156.
- Allgood VE, Powell-Oliver FE, Cidlowski JA. The influence of vitamin B6 on the structure and function of the glucocorticoid receptor. Ann N Y Acad Sci. 1990;585:452-465.
- Mabin D, Hollis S, Lockwood J, et al. Pyridoxine in atopic dermatitis. Br J Dermatol. 1995;133:764-767.
- Maywald M, Rink L. Zinc homeostasis and immunosenescence. J Trace Elem Med Biol. 2015;29:24-30.
- Karabacak E, Aydin E, Kutlu A, et al. Erythrocyte zinc level in patients with atopic dermatitis and its relation to SCORAD index. Postepy Dermatol Alergol. 2016;33:349-352.
- Kim JE, Yoo SR, Jeong MG, et al. Hair zinc levels and the efficacy of oral zinc supplementation in children with atopic dermatitis. Acta Derm Venereol. 2014;94:558-562.
- De Haes P, Garmyn M, Verstuyf A, et al. 1, 25-Dihydroxyvitamin D3 and analogues protect primary human keratinocytes against UVB-induced DNA damage. J Photochem Photobiol B. 2005;78:141-148.
- Katayama I, Minatohara K, Yokozeki H, et al. Topical vitamin D3 downregulates IgE-mediated murine biphasic cutaneous reactions. Int Arch Allergy Immunol. 1996;111:71-76.
- Javanbakht MH, Keshavarz SA, Djalali M, et al. Randomized controlled trial using vitamins E and D supplementation in atopic dermatitis. J Dermatol Treat. 2011;22:144-150.
- Bath-Hextall FJ, Jenkinson C, Humphreys R, et al. Dietary supplements for established atopic eczema. Cochrane Database Syst Rev. 2012:CD005205.
- Moro G, Arslanoglu S, Stahl B, et al. A mixture of prebiotic oligosaccharides reduces the incidence of atopic dermatitis during the first six months of age. Arch Dis Child. 2006;91:814-819.
- Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: psoriasis comorbidities and preferred systemic agents. J Am Acad Dermatol. 2019;80:27-40.
- Ely PH. Is psoriasis a bowel disease? successful treatment with bile acids and bioflavonoids suggest it is. Clin Dermatol. 2018;36:376-389.
- Soyland E, Funk J, Rajka G, et al. Effect of dietary supplementation with very-long-chain n-3 fatty acids in patients with psoriasis. N Engl J Med. 1993;328:1812-1816.
- Kamangar F, Koo J, Heller M, et al. Oral vitamin D, still a viable treatment option for psoriasis. J Dermatol Treat. 2013;24:261-267.
- Chandrashekar L, Kumarit GK, Rajappa M, et al. 25-hydroxy vitamin D and ischaemia-modified albumin levels in psoriasis and their association with disease severity. Br J Biomed Sci. 2015;72:56-60.
- Gisondi P, Rossini M, Di Cesare A, et al. Vitamin D status in patients with chronic plaque psoriasis. Br J Dermatol. 2012;166:505-510.
The connection between diet and physical beauty has been an area of increasing interest in popular culture as well as in the scientific community. Numerous supplements, plant derivatives, and antioxidants have been proposed to help improve skin conditions and prevent signs of aging.1 Clinical and basic research has played an important role in confirming or debunking these claims, leading to new insight into oral supplements that may play a role in improving signs of photoaging, as well as symptoms of common skin diseases such as acne vulgaris (AV), atopic dermatitis (AD), and psoriasis. This article reviews some of the vitamins, supplements, and antioxidants that have been studied in the improvement of these conditions.
Photoaging
Recently, there has been increased interest among researchers in the role of antioxidants in combatting photoaging. The main determinants of photoaging are chronic sunlight exposure and melanin density. Photoaging presentation includes deep rhytides, pigmentary changes, dryness, loss of skin tone, leathery appearance, and actinic purpura.2-4
Beta-carotene is a fat-soluble derivative of vitamin A, which has retinol activity and has an inhibitory effect on free radicals. It has been used to decrease the effect of UV light on the skin as well as to treat erythropoietic porphyria.5-7 One study evaluated the efficacy of low-dose and high-dose beta-carotene in improving facial rhytides and elasticity in a cohort of 30 women older than 50 years.8 Participants were given 30 or 90 mg of beta-carotene once daily for 90 days, and the final results were compared to baseline. Those who received the 30-mg dose showed improvements in facial rhytides and elasticity, increased type I procollagen messenger RNA levels, decreased UV-induced thymine dimer staining, and decreased 8-hydroxy-2′-deoxyguanosine staining. The lower dose of beta-carotene was found to prevent photoaging and was superior to the higher dose, which actually significantly decreased the minimal erythema dose (indicating a deleterious effect)(P=.025).8
Another study compared the role of a 25-mg carotenoid supplement vs a combination of carotenoid and vitamin E (335 mg [500 IU] RRR-α-tocopherol) supplements in preventing erythema development on the back.9 Using a blue light solar stimulator for illumination, erythema on the dorsal back skin was significantly reduced after week 8 (P<.01). The erythema was lower in the combination group than the carotenoid group alone, but the difference was not statistically significant. Furthermore, after 12 weeks, yellowing of the skin was observed in both groups, especially the skin of the palms and face.9
Collagen peptides also have been used in the prevention and repair of photoaging. Proksch et al10 conducted a double-blind, placebo-controlled trial to investigate the role of collagen peptides on skin elasticity in 69 women aged 35 to 55 years. At 4 weeks, oral supplementation of collagen hydrolysate (2.5 g once daily or 5 g once daily for 8 weeks) showed significant (P<.05) improvement of skin elasticity in both the low-dose and high-dose groups in women older than 50 years; however, collagen peptides did not lead to statistically significant improvement in skin hydration or transepidermal water loss. No known side effects were reported; thus, collagen peptides may be both efficacious and safe in improving signs of photoaging in elderly patients.10 Thus, these studies have shown potentially positive effects of beta-carotene, vitamin E, and collagen peptides in improving the signs of photoaging.
Acne Vulgaris
Acne vulgaris is a common dermatologic condition seen in the western hemisphere, with 40 to 50 million affected individuals in the United States annually.11,12 A landmark study that examined 1200 Kitavans from Papua New Guinea and 115 Aché individuals from a hunter-gatherer community in Paraguay found no cases of AV in either group.12 These findings have led to the speculation that AV may be associated with environmental factors, particularly the Western diet.
An investigator-blinded randomized clinical trial (RCT) explored the role of a low-glycemic diet compared to a carbohydrate-dense diet on improvement of AV lesions after 12 weeks.13 The results yielded a significant decrease in lesions in the low-glycemic group (mean [SEM], −23.5 [−3.9]) vs the control group (−12.0 [−3.0])(P=.03). Furthermore, the results indicated a significant decrease in weight (P<.001) and body mass index (P=.001) with an improvement in insulin sensitivity in the low-glycemic group vs the control group.13 Kwon et al14 conducted a similar investigator-blinded parallel study with 32 participants receiving either a low-glycemic diet or continuing their normal diet for 10 weeks. Participants in the low-glycemic group demonstrated a significant reduction in mean noninflammatory lesions (−27.6% [P=.04]) and mean inflammatory lesions (−70.9% [P<.05]). Histologic image analysis showed a significant decrease in the mean (SEM) area of sebaceous glands in the low-glycemic group (0.32 [0.03] mm2) compared to baseline (0.24 [0.03] mm2)(P=.03). At 10 weeks, immunohistochemical specimens showed reduction in IL-8 (P=.03) and sterol regulatory element-binding protein 1 (P=.03), which regulates the synthesis of lipids.14 Thus, both studies concluded that a reduction in glycemic load may improve acne overall.13,14
Another study attempted to investigate the role of additional dietary supplements in improving acne. A double-blinded RCT explored the efficacy of omega-3 fatty acids or γ-linoleic acid compared to a control group in improving mild to moderate AV lesions through clinical and histological evaluations.15 The 10-week prospective study included 45 patients who were allocated to 3 matched groups and randomized to 3 treatment arms. They were given omega-3 fatty acids (1000 mg each of eicosapentaenoic acid and docosahexaenoic acid) or γ-linoleic acid (borage oil with 400 mg of γ-linoleic acid) or no intervention. After treatment completion, patients in both treatment groups showed significant reduction in mean inflammatory acne lesions, mean noninflammatory acne lesions, and mean acne severity (all P<.05), while the control group showed no significant reduction in acne lesions or acne severity. Furthermore, hematoxylin and eosin and IL-8 immunohistochemical staining of biopsies from the affected areas showed significant reduction of inflammation in both treatment groups (P<.05) but not in the control group. Therefore, the authors concluded that both omega-3 fatty acids and γ-linoleic acid could be used as adjuvant therapies in AV treatment.15
Atopic Dermatitis
The prevalence of atopic dermatitis (AD) in children ranges from approximately 9% to 18% across the United States.16 Pyridoxine, or vitamin B6, is an important water-soluble vitamin and a cofactor for numerous biochemical processes including carbohydrate and amino acid metabolism pathways and glucocorticoid receptor regulation.17,18 However, a double-blinded, placebo-controlled RCT failed to show efficacy of once-daily pyridoxine hydrochloride 50 mg in improving erythema, itching, or nocturnal sleep disturbance associated with AD in a cohort of 48 children. The investigators concluded that pyridoxine supplementation cannot be recommended to improve the symptoms of AD in children.19
Zinc is an essential nutrient that functions as an important cofactor in cell metabolism and growth pathways.20 One study showed that intracellular erythrocyte zinc levels were significantly lower in AD patients compared to healthy controls (P<.001); however, there was no observed difference in serum zinc levels (P=.148). Furthermore, greater disease severity as determined by the SCORing Atopic Dermatitis (SCORAD) index was negatively correlated with erythrocyte zinc levels (r=−0.791; P<.001).21 Kim et al22 investigated hair zinc levels and the efficacy of oral zinc supplementation in children with mild to moderate AD. Mean (SD) hair zinc levels were lower in the AD group compared to the control group (113.10 [33.6] μg vs 130.90 [36.63] μg [P=.012]). Of 41 AD patients with low zinc levels, 22 were allocated to group A, which received oral zinc oxide 12 mg for 8 weeks, and 19 were allocated to group B, which did not receive any supplementation over the same period. Groups A and B also received oral antihistamines and topical moisturizers. Mean (SD) zinc levels increased significantly in group A from 96.36 (21.05) μg to 131.81 (27.45) μg (P<.001). Furthermore, relative to group B, group A showed significantly greater improvements in eczema area and severity index (P=.044), transepidermal water loss (P=.015), and visual analog scale for pruritus (P<.001) at the end of 8 weeks. The authors concluded that oral zinc supplementation might be an effective adjunctive therapy for AD patients with low hair zinc levels.22
Researchers also have explored the efficacy of fat-soluble vitamins D and E in treating AD. Vitamin D is thought to downregulate IgE-mediated skin reactions and decrease adverse effects of UV light on the skin.23,24 A double-blind, placebo-controlled trial randomized 45 patients with AD to 4 groups: vitamins D and E placebos (n=11), 1600 IU vitamin D3 plus vitamin E placebo (n=12), 600 IU vitamin E (synthetic all-rac-α-tocopherol) plus vitamin D placebo (n=11), and 1600 IU vitamin D3 plus 600 IU vitamin E (synthetic all-rac-α-tocopherol)(n=11).25 After 60 days, the SCORAD index was reduced by 28.9% in the placebo group, 34.8% in the vitamin D3 group, 35.7% in the vitamin E group, and 64.3% in the combined vitamins D and E group (P=.004). Furthermore, prior to intervention, a negative correlation was demonstrated between plasma α-tocopherol concentration and the SCORAD index (r=−.33; P=.025).25 Thus, supplementing vitamins D and E may play a beneficial role in the treatment of AD.
Other emerging studies are investigating the role of the gut microbiome in various pathologies. Prebiotics may alter the gut microbiome and are thought to play a role in reducing intestinal inflammation.26 One randomized, placebo-controlled, parallel study examined the effect of prebiotic oligosaccharide supplementation on the development of AD in at-risk children, defined as having a biological parent with a history of asthma, allergic rhinitis, or AD.27 At 6-month follow-up, 10 infants (9.8%)(95% CI, 5.4%-17.1%) in the intervention group (n=102) and 24 infants (23.1%)(95% CI, 16.0%-32.1%) in the placebo group (n=104) had developed AD. The authors postulated that the prebiotic oligosaccharides might play a role in immune modulation by altering bowel flora and preventing the development of AD in infancy.27
Notably, a 2012 Cochrane review evaluated 11 studies of dietary supplements as possible treatment options for AD. The authors concluded that the evidence was minimal to support the regular use of dietary supplements, especially due to their high cost as well as the possibility that high levels of certain vitamins (eg, vitamin D) may cause long-term complications.26
Psoriasis
Psoriasis is an autoimmune skin condition that has an annual prevalence ranging from approximately 1% to 9% in adults residing in Western countries.28,29 Some have argued that due to decreased bacterial diversity and increased bacterial growth in the small bowel, psoriatic patients are exposed to higher levels of bacterial peptidoglycans and endotoxins.30 To combat the absorption of these substances in psoriasis patients
The effects of very long chain fatty acids also have been examined. A 4-month, double-blind, multicenter RCT compared the effects of daily supplementation with 6 g of either omega-3 fatty acids or omega-6 fatty acids in patients with mild to moderate plaque psoriasis.31 Psoriasis area and severity index scores and patient subjective scores did not change significantly in either group; however, scaling was reduced in both groups (P<.01). The group receiving omega-3 fatty acids had decreased cellular infiltration (P<.01), and the group receiving omega-6 fatty acids had decreased desquamation and redness (P<.05). In the omega-6 group, there was a significant correlation between clinical improvement (decrease in clinical score) and increase in serum eicosapentaenoic acid (r=−0.34; P<.05) and total omega-3 fatty acids (r=−0.36; P<.05). Overall, the authors concluded that supplementation with omega-3 fatty acids (fish oil) was no better than omega-6 fatty acids (corn oil) for treatment of psoriasis.31
Some dermatologists have advocated for the use of oral vitamin D supplementation as an adjunctive treatment of psoriasis, given that it is inexpensive and also may play a role in reducing the risk for cancer and cardiovascular events.32 One study evaluated the level of 25-hydroxy vitamin D in 43 psoriasis patients compared to 43 healthy controls. Mean (SD) vitamin D levels were significantly lower in psoriasis patients (13.3 [6.9]) compared to controls (22.4 [18.4])(P=.004).33 A cross-sectional study similarly found significantly higher rates of vitamin D deficiency (25-hydroxy vitamin D <20 ng/mL) in psoriatic patients (57.8%) compared to patients with rheumatoid arthritis (37.5%) and healthy controls (29.7%)(P<.001). Interestingly, during winter the prevalence of vitamin D deficiency increased to 80.9%, 41.3%, and 30.3% in the 3 groups, respectively; however, no significant correlation was seen between psoriasis severity, as measured by psoriasis area and severity index, and serum vitamin D levels.34 Although vitamin D deficiency may be more prevalent among patients with psoriasis, data regarding the efficacy of treating psoriasis with oral vitamin D supplementation is still lacking.
Conclusion
Our understanding of the link between diet and dermatologic conditions continues to evolve. Recent data for several dietary supplements and therapies showed promising results in repairing signs of photoaging, as well as treating AV, AD, and psoriasis. As patients seek these adjunctive therapies, it is important for physicians to be well informed on the benefits and risks to appropriately counsel patients.
Globally, physicians advocate for a low-glycemic diet rich in fruits and vegetables. Furthermore, the cosmetic diet can be enhanced by the consumption of dietary supplements such as beta-carotene, collagen peptides, zinc, and fat-soluble vitamins such as vitamins D and E. However, prospective RCTs are needed to further investigate the role of these dietary elements in treating and improving dermatologic conditions.
The connection between diet and physical beauty has been an area of increasing interest in popular culture as well as in the scientific community. Numerous supplements, plant derivatives, and antioxidants have been proposed to help improve skin conditions and prevent signs of aging.1 Clinical and basic research has played an important role in confirming or debunking these claims, leading to new insight into oral supplements that may play a role in improving signs of photoaging, as well as symptoms of common skin diseases such as acne vulgaris (AV), atopic dermatitis (AD), and psoriasis. This article reviews some of the vitamins, supplements, and antioxidants that have been studied in the improvement of these conditions.
Photoaging
Recently, there has been increased interest among researchers in the role of antioxidants in combatting photoaging. The main determinants of photoaging are chronic sunlight exposure and melanin density. Photoaging presentation includes deep rhytides, pigmentary changes, dryness, loss of skin tone, leathery appearance, and actinic purpura.2-4
Beta-carotene is a fat-soluble derivative of vitamin A, which has retinol activity and has an inhibitory effect on free radicals. It has been used to decrease the effect of UV light on the skin as well as to treat erythropoietic porphyria.5-7 One study evaluated the efficacy of low-dose and high-dose beta-carotene in improving facial rhytides and elasticity in a cohort of 30 women older than 50 years.8 Participants were given 30 or 90 mg of beta-carotene once daily for 90 days, and the final results were compared to baseline. Those who received the 30-mg dose showed improvements in facial rhytides and elasticity, increased type I procollagen messenger RNA levels, decreased UV-induced thymine dimer staining, and decreased 8-hydroxy-2′-deoxyguanosine staining. The lower dose of beta-carotene was found to prevent photoaging and was superior to the higher dose, which actually significantly decreased the minimal erythema dose (indicating a deleterious effect)(P=.025).8
Another study compared the role of a 25-mg carotenoid supplement vs a combination of carotenoid and vitamin E (335 mg [500 IU] RRR-α-tocopherol) supplements in preventing erythema development on the back.9 Using a blue light solar stimulator for illumination, erythema on the dorsal back skin was significantly reduced after week 8 (P<.01). The erythema was lower in the combination group than the carotenoid group alone, but the difference was not statistically significant. Furthermore, after 12 weeks, yellowing of the skin was observed in both groups, especially the skin of the palms and face.9
Collagen peptides also have been used in the prevention and repair of photoaging. Proksch et al10 conducted a double-blind, placebo-controlled trial to investigate the role of collagen peptides on skin elasticity in 69 women aged 35 to 55 years. At 4 weeks, oral supplementation of collagen hydrolysate (2.5 g once daily or 5 g once daily for 8 weeks) showed significant (P<.05) improvement of skin elasticity in both the low-dose and high-dose groups in women older than 50 years; however, collagen peptides did not lead to statistically significant improvement in skin hydration or transepidermal water loss. No known side effects were reported; thus, collagen peptides may be both efficacious and safe in improving signs of photoaging in elderly patients.10 Thus, these studies have shown potentially positive effects of beta-carotene, vitamin E, and collagen peptides in improving the signs of photoaging.
Acne Vulgaris
Acne vulgaris is a common dermatologic condition seen in the western hemisphere, with 40 to 50 million affected individuals in the United States annually.11,12 A landmark study that examined 1200 Kitavans from Papua New Guinea and 115 Aché individuals from a hunter-gatherer community in Paraguay found no cases of AV in either group.12 These findings have led to the speculation that AV may be associated with environmental factors, particularly the Western diet.
An investigator-blinded randomized clinical trial (RCT) explored the role of a low-glycemic diet compared to a carbohydrate-dense diet on improvement of AV lesions after 12 weeks.13 The results yielded a significant decrease in lesions in the low-glycemic group (mean [SEM], −23.5 [−3.9]) vs the control group (−12.0 [−3.0])(P=.03). Furthermore, the results indicated a significant decrease in weight (P<.001) and body mass index (P=.001) with an improvement in insulin sensitivity in the low-glycemic group vs the control group.13 Kwon et al14 conducted a similar investigator-blinded parallel study with 32 participants receiving either a low-glycemic diet or continuing their normal diet for 10 weeks. Participants in the low-glycemic group demonstrated a significant reduction in mean noninflammatory lesions (−27.6% [P=.04]) and mean inflammatory lesions (−70.9% [P<.05]). Histologic image analysis showed a significant decrease in the mean (SEM) area of sebaceous glands in the low-glycemic group (0.32 [0.03] mm2) compared to baseline (0.24 [0.03] mm2)(P=.03). At 10 weeks, immunohistochemical specimens showed reduction in IL-8 (P=.03) and sterol regulatory element-binding protein 1 (P=.03), which regulates the synthesis of lipids.14 Thus, both studies concluded that a reduction in glycemic load may improve acne overall.13,14
Another study attempted to investigate the role of additional dietary supplements in improving acne. A double-blinded RCT explored the efficacy of omega-3 fatty acids or γ-linoleic acid compared to a control group in improving mild to moderate AV lesions through clinical and histological evaluations.15 The 10-week prospective study included 45 patients who were allocated to 3 matched groups and randomized to 3 treatment arms. They were given omega-3 fatty acids (1000 mg each of eicosapentaenoic acid and docosahexaenoic acid) or γ-linoleic acid (borage oil with 400 mg of γ-linoleic acid) or no intervention. After treatment completion, patients in both treatment groups showed significant reduction in mean inflammatory acne lesions, mean noninflammatory acne lesions, and mean acne severity (all P<.05), while the control group showed no significant reduction in acne lesions or acne severity. Furthermore, hematoxylin and eosin and IL-8 immunohistochemical staining of biopsies from the affected areas showed significant reduction of inflammation in both treatment groups (P<.05) but not in the control group. Therefore, the authors concluded that both omega-3 fatty acids and γ-linoleic acid could be used as adjuvant therapies in AV treatment.15
Atopic Dermatitis
The prevalence of atopic dermatitis (AD) in children ranges from approximately 9% to 18% across the United States.16 Pyridoxine, or vitamin B6, is an important water-soluble vitamin and a cofactor for numerous biochemical processes including carbohydrate and amino acid metabolism pathways and glucocorticoid receptor regulation.17,18 However, a double-blinded, placebo-controlled RCT failed to show efficacy of once-daily pyridoxine hydrochloride 50 mg in improving erythema, itching, or nocturnal sleep disturbance associated with AD in a cohort of 48 children. The investigators concluded that pyridoxine supplementation cannot be recommended to improve the symptoms of AD in children.19
Zinc is an essential nutrient that functions as an important cofactor in cell metabolism and growth pathways.20 One study showed that intracellular erythrocyte zinc levels were significantly lower in AD patients compared to healthy controls (P<.001); however, there was no observed difference in serum zinc levels (P=.148). Furthermore, greater disease severity as determined by the SCORing Atopic Dermatitis (SCORAD) index was negatively correlated with erythrocyte zinc levels (r=−0.791; P<.001).21 Kim et al22 investigated hair zinc levels and the efficacy of oral zinc supplementation in children with mild to moderate AD. Mean (SD) hair zinc levels were lower in the AD group compared to the control group (113.10 [33.6] μg vs 130.90 [36.63] μg [P=.012]). Of 41 AD patients with low zinc levels, 22 were allocated to group A, which received oral zinc oxide 12 mg for 8 weeks, and 19 were allocated to group B, which did not receive any supplementation over the same period. Groups A and B also received oral antihistamines and topical moisturizers. Mean (SD) zinc levels increased significantly in group A from 96.36 (21.05) μg to 131.81 (27.45) μg (P<.001). Furthermore, relative to group B, group A showed significantly greater improvements in eczema area and severity index (P=.044), transepidermal water loss (P=.015), and visual analog scale for pruritus (P<.001) at the end of 8 weeks. The authors concluded that oral zinc supplementation might be an effective adjunctive therapy for AD patients with low hair zinc levels.22
Researchers also have explored the efficacy of fat-soluble vitamins D and E in treating AD. Vitamin D is thought to downregulate IgE-mediated skin reactions and decrease adverse effects of UV light on the skin.23,24 A double-blind, placebo-controlled trial randomized 45 patients with AD to 4 groups: vitamins D and E placebos (n=11), 1600 IU vitamin D3 plus vitamin E placebo (n=12), 600 IU vitamin E (synthetic all-rac-α-tocopherol) plus vitamin D placebo (n=11), and 1600 IU vitamin D3 plus 600 IU vitamin E (synthetic all-rac-α-tocopherol)(n=11).25 After 60 days, the SCORAD index was reduced by 28.9% in the placebo group, 34.8% in the vitamin D3 group, 35.7% in the vitamin E group, and 64.3% in the combined vitamins D and E group (P=.004). Furthermore, prior to intervention, a negative correlation was demonstrated between plasma α-tocopherol concentration and the SCORAD index (r=−.33; P=.025).25 Thus, supplementing vitamins D and E may play a beneficial role in the treatment of AD.
Other emerging studies are investigating the role of the gut microbiome in various pathologies. Prebiotics may alter the gut microbiome and are thought to play a role in reducing intestinal inflammation.26 One randomized, placebo-controlled, parallel study examined the effect of prebiotic oligosaccharide supplementation on the development of AD in at-risk children, defined as having a biological parent with a history of asthma, allergic rhinitis, or AD.27 At 6-month follow-up, 10 infants (9.8%)(95% CI, 5.4%-17.1%) in the intervention group (n=102) and 24 infants (23.1%)(95% CI, 16.0%-32.1%) in the placebo group (n=104) had developed AD. The authors postulated that the prebiotic oligosaccharides might play a role in immune modulation by altering bowel flora and preventing the development of AD in infancy.27
Notably, a 2012 Cochrane review evaluated 11 studies of dietary supplements as possible treatment options for AD. The authors concluded that the evidence was minimal to support the regular use of dietary supplements, especially due to their high cost as well as the possibility that high levels of certain vitamins (eg, vitamin D) may cause long-term complications.26
Psoriasis
Psoriasis is an autoimmune skin condition that has an annual prevalence ranging from approximately 1% to 9% in adults residing in Western countries.28,29 Some have argued that due to decreased bacterial diversity and increased bacterial growth in the small bowel, psoriatic patients are exposed to higher levels of bacterial peptidoglycans and endotoxins.30 To combat the absorption of these substances in psoriasis patients
The effects of very long chain fatty acids also have been examined. A 4-month, double-blind, multicenter RCT compared the effects of daily supplementation with 6 g of either omega-3 fatty acids or omega-6 fatty acids in patients with mild to moderate plaque psoriasis.31 Psoriasis area and severity index scores and patient subjective scores did not change significantly in either group; however, scaling was reduced in both groups (P<.01). The group receiving omega-3 fatty acids had decreased cellular infiltration (P<.01), and the group receiving omega-6 fatty acids had decreased desquamation and redness (P<.05). In the omega-6 group, there was a significant correlation between clinical improvement (decrease in clinical score) and increase in serum eicosapentaenoic acid (r=−0.34; P<.05) and total omega-3 fatty acids (r=−0.36; P<.05). Overall, the authors concluded that supplementation with omega-3 fatty acids (fish oil) was no better than omega-6 fatty acids (corn oil) for treatment of psoriasis.31
Some dermatologists have advocated for the use of oral vitamin D supplementation as an adjunctive treatment of psoriasis, given that it is inexpensive and also may play a role in reducing the risk for cancer and cardiovascular events.32 One study evaluated the level of 25-hydroxy vitamin D in 43 psoriasis patients compared to 43 healthy controls. Mean (SD) vitamin D levels were significantly lower in psoriasis patients (13.3 [6.9]) compared to controls (22.4 [18.4])(P=.004).33 A cross-sectional study similarly found significantly higher rates of vitamin D deficiency (25-hydroxy vitamin D <20 ng/mL) in psoriatic patients (57.8%) compared to patients with rheumatoid arthritis (37.5%) and healthy controls (29.7%)(P<.001). Interestingly, during winter the prevalence of vitamin D deficiency increased to 80.9%, 41.3%, and 30.3% in the 3 groups, respectively; however, no significant correlation was seen between psoriasis severity, as measured by psoriasis area and severity index, and serum vitamin D levels.34 Although vitamin D deficiency may be more prevalent among patients with psoriasis, data regarding the efficacy of treating psoriasis with oral vitamin D supplementation is still lacking.
Conclusion
Our understanding of the link between diet and dermatologic conditions continues to evolve. Recent data for several dietary supplements and therapies showed promising results in repairing signs of photoaging, as well as treating AV, AD, and psoriasis. As patients seek these adjunctive therapies, it is important for physicians to be well informed on the benefits and risks to appropriately counsel patients.
Globally, physicians advocate for a low-glycemic diet rich in fruits and vegetables. Furthermore, the cosmetic diet can be enhanced by the consumption of dietary supplements such as beta-carotene, collagen peptides, zinc, and fat-soluble vitamins such as vitamins D and E. However, prospective RCTs are needed to further investigate the role of these dietary elements in treating and improving dermatologic conditions.
- Khanna R, Shifrin N, Nektalova T, et al. Diet and dermatology: Google search results for acne, psoriasis, and eczema. Cutis. 2018;102:44, 46-48.
- Yaar M, Eller MS, Gilchrest BA. Fifty years of skin aging. J Invest Dermatol Symp Proc. 2002;7:51-58.
- Helfrich YR, Sachs DL, Voorhees JJ. Overview of skin aging and photoaging. Dermatol Nurs. 2008;20:177-183.
- Pandel R, Poljšak B, Godic A, et al. Skin photoaging and the role of antioxidants in its prevention. ISRN Dermatol. 2013;2013:930164.
- Mathews-Roth MM, Pathak MA, Fitzpatrick T, et al. Beta carotene therapy for erythropoietic protoporphyria and other photosensitivity diseases. Arch Dermatol. 1977;113:1229-1232.
- Myriam M, Sabatier M, Steiling H, et al. Skin bioavailability of dietary vitamin E, carotenoids, polyphenols, vitamin C, zinc and selenium. Br J Nutr. 2006;96:227-238.
- Cho S. The role of functional foods in cutaneous anti-aging. J Lifestyle Med. 2014;4:8-16.
- Cho S, Lee DH, Won CH, et al. Differential effects of low-dose and high-dose beta-carotene supplementation on the signs of photoaging and type I procollagen gene expression in human skin in vivo. Dermatology. 2010;221:160-171.
- Stahl W, Heinrich U, Jungmann H, et al. Carotenoids and carotenoids plus vitamin E protect against ultraviolet light–induced erythema in humans. Am J Clin Nutr. 2000;71:795-798.
- Proksch E, Segger D, Degwert J, et al. Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: a double-blind, placebo-controlled study. Skin Pharmacol Physiol. 2014;27:47-55.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39(2, pt 3):S34-S37.
- Cordain L, Lindeberg S, Hurtado M, et al. Acne vulgaris: a disease of Western civilization. Arch Dermatol. 2002;138:1584-1590.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Jung JY, Kwon HH, Hong JS, et al. Effect of dietary supplementation with omega-3 fatty acid and gamma-linolenic acid on acne vulgaris: a randomised, double-blind, controlled trial. Acta Derm Venereol. 2014;94:521-526.
- Shaw TE, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67-73.
- Merrill AH Jr, Henderson JM. Diseases associated with defects in vitamin B6 metabolism or utilization. Annu Rev Nutr. 1987;7:137-156.
- Allgood VE, Powell-Oliver FE, Cidlowski JA. The influence of vitamin B6 on the structure and function of the glucocorticoid receptor. Ann N Y Acad Sci. 1990;585:452-465.
- Mabin D, Hollis S, Lockwood J, et al. Pyridoxine in atopic dermatitis. Br J Dermatol. 1995;133:764-767.
- Maywald M, Rink L. Zinc homeostasis and immunosenescence. J Trace Elem Med Biol. 2015;29:24-30.
- Karabacak E, Aydin E, Kutlu A, et al. Erythrocyte zinc level in patients with atopic dermatitis and its relation to SCORAD index. Postepy Dermatol Alergol. 2016;33:349-352.
- Kim JE, Yoo SR, Jeong MG, et al. Hair zinc levels and the efficacy of oral zinc supplementation in children with atopic dermatitis. Acta Derm Venereol. 2014;94:558-562.
- De Haes P, Garmyn M, Verstuyf A, et al. 1, 25-Dihydroxyvitamin D3 and analogues protect primary human keratinocytes against UVB-induced DNA damage. J Photochem Photobiol B. 2005;78:141-148.
- Katayama I, Minatohara K, Yokozeki H, et al. Topical vitamin D3 downregulates IgE-mediated murine biphasic cutaneous reactions. Int Arch Allergy Immunol. 1996;111:71-76.
- Javanbakht MH, Keshavarz SA, Djalali M, et al. Randomized controlled trial using vitamins E and D supplementation in atopic dermatitis. J Dermatol Treat. 2011;22:144-150.
- Bath-Hextall FJ, Jenkinson C, Humphreys R, et al. Dietary supplements for established atopic eczema. Cochrane Database Syst Rev. 2012:CD005205.
- Moro G, Arslanoglu S, Stahl B, et al. A mixture of prebiotic oligosaccharides reduces the incidence of atopic dermatitis during the first six months of age. Arch Dis Child. 2006;91:814-819.
- Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: psoriasis comorbidities and preferred systemic agents. J Am Acad Dermatol. 2019;80:27-40.
- Ely PH. Is psoriasis a bowel disease? successful treatment with bile acids and bioflavonoids suggest it is. Clin Dermatol. 2018;36:376-389.
- Soyland E, Funk J, Rajka G, et al. Effect of dietary supplementation with very-long-chain n-3 fatty acids in patients with psoriasis. N Engl J Med. 1993;328:1812-1816.
- Kamangar F, Koo J, Heller M, et al. Oral vitamin D, still a viable treatment option for psoriasis. J Dermatol Treat. 2013;24:261-267.
- Chandrashekar L, Kumarit GK, Rajappa M, et al. 25-hydroxy vitamin D and ischaemia-modified albumin levels in psoriasis and their association with disease severity. Br J Biomed Sci. 2015;72:56-60.
- Gisondi P, Rossini M, Di Cesare A, et al. Vitamin D status in patients with chronic plaque psoriasis. Br J Dermatol. 2012;166:505-510.
- Khanna R, Shifrin N, Nektalova T, et al. Diet and dermatology: Google search results for acne, psoriasis, and eczema. Cutis. 2018;102:44, 46-48.
- Yaar M, Eller MS, Gilchrest BA. Fifty years of skin aging. J Invest Dermatol Symp Proc. 2002;7:51-58.
- Helfrich YR, Sachs DL, Voorhees JJ. Overview of skin aging and photoaging. Dermatol Nurs. 2008;20:177-183.
- Pandel R, Poljšak B, Godic A, et al. Skin photoaging and the role of antioxidants in its prevention. ISRN Dermatol. 2013;2013:930164.
- Mathews-Roth MM, Pathak MA, Fitzpatrick T, et al. Beta carotene therapy for erythropoietic protoporphyria and other photosensitivity diseases. Arch Dermatol. 1977;113:1229-1232.
- Myriam M, Sabatier M, Steiling H, et al. Skin bioavailability of dietary vitamin E, carotenoids, polyphenols, vitamin C, zinc and selenium. Br J Nutr. 2006;96:227-238.
- Cho S. The role of functional foods in cutaneous anti-aging. J Lifestyle Med. 2014;4:8-16.
- Cho S, Lee DH, Won CH, et al. Differential effects of low-dose and high-dose beta-carotene supplementation on the signs of photoaging and type I procollagen gene expression in human skin in vivo. Dermatology. 2010;221:160-171.
- Stahl W, Heinrich U, Jungmann H, et al. Carotenoids and carotenoids plus vitamin E protect against ultraviolet light–induced erythema in humans. Am J Clin Nutr. 2000;71:795-798.
- Proksch E, Segger D, Degwert J, et al. Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: a double-blind, placebo-controlled study. Skin Pharmacol Physiol. 2014;27:47-55.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39(2, pt 3):S34-S37.
- Cordain L, Lindeberg S, Hurtado M, et al. Acne vulgaris: a disease of Western civilization. Arch Dermatol. 2002;138:1584-1590.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Jung JY, Kwon HH, Hong JS, et al. Effect of dietary supplementation with omega-3 fatty acid and gamma-linolenic acid on acne vulgaris: a randomised, double-blind, controlled trial. Acta Derm Venereol. 2014;94:521-526.
- Shaw TE, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67-73.
- Merrill AH Jr, Henderson JM. Diseases associated with defects in vitamin B6 metabolism or utilization. Annu Rev Nutr. 1987;7:137-156.
- Allgood VE, Powell-Oliver FE, Cidlowski JA. The influence of vitamin B6 on the structure and function of the glucocorticoid receptor. Ann N Y Acad Sci. 1990;585:452-465.
- Mabin D, Hollis S, Lockwood J, et al. Pyridoxine in atopic dermatitis. Br J Dermatol. 1995;133:764-767.
- Maywald M, Rink L. Zinc homeostasis and immunosenescence. J Trace Elem Med Biol. 2015;29:24-30.
- Karabacak E, Aydin E, Kutlu A, et al. Erythrocyte zinc level in patients with atopic dermatitis and its relation to SCORAD index. Postepy Dermatol Alergol. 2016;33:349-352.
- Kim JE, Yoo SR, Jeong MG, et al. Hair zinc levels and the efficacy of oral zinc supplementation in children with atopic dermatitis. Acta Derm Venereol. 2014;94:558-562.
- De Haes P, Garmyn M, Verstuyf A, et al. 1, 25-Dihydroxyvitamin D3 and analogues protect primary human keratinocytes against UVB-induced DNA damage. J Photochem Photobiol B. 2005;78:141-148.
- Katayama I, Minatohara K, Yokozeki H, et al. Topical vitamin D3 downregulates IgE-mediated murine biphasic cutaneous reactions. Int Arch Allergy Immunol. 1996;111:71-76.
- Javanbakht MH, Keshavarz SA, Djalali M, et al. Randomized controlled trial using vitamins E and D supplementation in atopic dermatitis. J Dermatol Treat. 2011;22:144-150.
- Bath-Hextall FJ, Jenkinson C, Humphreys R, et al. Dietary supplements for established atopic eczema. Cochrane Database Syst Rev. 2012:CD005205.
- Moro G, Arslanoglu S, Stahl B, et al. A mixture of prebiotic oligosaccharides reduces the incidence of atopic dermatitis during the first six months of age. Arch Dis Child. 2006;91:814-819.
- Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: psoriasis comorbidities and preferred systemic agents. J Am Acad Dermatol. 2019;80:27-40.
- Ely PH. Is psoriasis a bowel disease? successful treatment with bile acids and bioflavonoids suggest it is. Clin Dermatol. 2018;36:376-389.
- Soyland E, Funk J, Rajka G, et al. Effect of dietary supplementation with very-long-chain n-3 fatty acids in patients with psoriasis. N Engl J Med. 1993;328:1812-1816.
- Kamangar F, Koo J, Heller M, et al. Oral vitamin D, still a viable treatment option for psoriasis. J Dermatol Treat. 2013;24:261-267.
- Chandrashekar L, Kumarit GK, Rajappa M, et al. 25-hydroxy vitamin D and ischaemia-modified albumin levels in psoriasis and their association with disease severity. Br J Biomed Sci. 2015;72:56-60.
- Gisondi P, Rossini M, Di Cesare A, et al. Vitamin D status in patients with chronic plaque psoriasis. Br J Dermatol. 2012;166:505-510.
Practice Points
- Growing evidence indicates that diet plays a role in overall skin health as well as the pathophysiology of several common cutaneous diseases.
- Broadly, we advocate for a low-glycemic diet that is rich in fruits and vegetables. In addition, dietary supplements of beta-carotene, collagen peptides, zinc, and fat-soluble vitamins (eg, vitamins D and E) have shown promising results in various conditions.
Noninvasive Vaginal Rejuvenation
Vaginal rejuvenation encompasses a group of procedures that alter the vaginal anatomy to improve cosmesis or achieve more pleasurable sexual intercourse. External vaginal procedures are defined as those performed on the female genitalia outside of the vaginal introitus, with major structures including the labia majora, mons pubis, labia minora, clitoral hood, clitoral glans, and vaginal vestibule. Internal vaginal procedures are defined as those performed within the vagina, extending from the vaginal introitus to the cervix.
The prevalence of elective vaginal rejuvenation procedures has increased in recent years, a trend that may be attributed to greater exposure through the media, including reality television and pornography. In a survey of 482 women undergoing labiaplasty, nearly all had heard about rejuvenation procedures within the last 2.2 years, and 78% had received their information through the media.1 Additionally, genital self-image can have a considerable effect on a woman’s sexual behavior and relationships. Genital dissatisfaction has been associated with decreased sexual activity, whereas positive genital self-image correlates with increased sexual desire and less sexual distress or depression.2,3
Currently, the 2 primary applications of noninvasive vaginal rejuvenation are vaginal laxity and genitourinary syndrome of menopause (GSM). Vaginal laxity occurs in premenopausal or postmenopausal women and is caused by aging, childbearing, or hormonal imbalances. These factors can lead to decreased friction within the vagina during intercourse, which in turn can decrease sexual pleasure. Genitourinary syndrome of menopause, previously known as vulvovaginal atrophy, encompasses genital (eg, dryness, burning, irritation), sexual (eg, lack of lubrication, discomfort or pain, impaired function), and urinary (eg, urgency, dysuria, recurrent urinary tract infections) symptoms of menopause.4
Noninvasive procedures are designed to apply ablative or nonablative energy to the vaginal mucosa to tighten a lax upper vagina, also known as a wide vagina.5 A wide vagina has been defined as a widened vaginal diameter that interferes with sexual function and sensation.6 Decreased sexual sensation also may result from fibrosis or scarring of the vaginal mucosa after prior vaginal surgery, episiotomy, or tears during childbirth.7 The objective of rejuvenation procedures to treat the vaginal mucosa is to create increased frictional forces that may lead to increased sexual sensation.8 Although there are numerous reports of heightened sexual satisfaction after reduction of the vaginal diameter, a formal link between sexual pleasure and vaginal laxity has yet to be established.8,9 At present, there are no US Food and Drug Administration (FDA)–approved energy-based devices to treat urinary incontinence or sexual function, and the FDA recently issued an alert cautioning patients on the current lack of safety and efficacy regulations.10
In this article we review the safety and efficacy data behind lasers and radiofrequency (RF) devices used in noninvasive vaginal rejuvenation procedures.
Lasers
CO2 Laser
The infrared CO2 laser utilizes 10,600-nm energy to target and vaporize water molecules within the target tissue. This thermal heating extends to the dermal collagen, which stimulates inflammatory pathways and neocollagenesis.11 The depth of penetration ranges from 20 to 125 μm.12 Zerbinati et al13 demonstrated the histologic and ultrastructural effects of a fractional CO2 laser on atrophic vaginal mucosa. Comparing pretreatment and posttreatment mucosal biopsies in 5 postmenopausal women, the investigators found that fractional CO2 laser treatment caused increased epithelial thickness, vascularity, and fibroblast activity, which led to augmented synthesis of collagen and ground substance proteins.13
New devices seek to translate these histologic improvements to the aesthetic appearance and function of female genitalia. The MonaLisa Touch (Cynosure), a new fractional CO2 laser specifically designed for treatment of the vaginal mucosa, uses dermal optical thermolysis (DOT) therapy to apply energy in a noncontinuous mode at 200-μm dots. Salvatore et al14 examined the use of this device in a noncontrolled study of 50 patients with GSM, with each patient undergoing 3 treatment sessions at monthly intervals. Intravaginal treatments were performed at the following settings: DOT (microablative zone) power of 30 W, dwell time of 1000 μs, DOT spacing of 1000 μm, and SmartStack parameter of 1 to 3. The investigators used the Vaginal Health Index (VHI) to objectively assess vaginal elasticity, secretions, pH, mucosa integrity, and moisture. Total VHI scores significantly improved between baseline and 1 month following the final treatment (mean score [SD], 13.1 [2.5] vs 23.1 [1.9]; P<.0001). There were no significant adverse events, and 84% of patients reported being satisfied with their outcome; however, the study lacked a comparison or control group, raising the possibility of placebo effect.14
Other noncontrolled series have corroborated the benefits of CO2 laser in GSM patients.15,16 In one of the largest studies to date, Filippini et al17 reviewed the outcomes of 386 menopausal women treated for GSM. Patients underwent 3 intravaginal laser sessions with the MonaLisa Touch. Intravaginal treatments were performed at a DOT power of 40 W, dwell time of 1000 μs, DOT spacing of 1000 μm, and SmartStack of 2. For the vulva, the DOT power was reduced to 30 W, dwell time of 1000 μs, DOT spacing of 1000 μm, and SmartStack of 1. Two months after the final treatment session, patients completed a nonvalidated questionnaire about their symptoms, with improved dryness reported in 60% of patients, improved burning in 56%, improved dyspareunia in 49%, improved itch in 56%, improved soreness in 73%, and improved vaginal introitus pain in 49%. Although most patients did not experience discomfort with the procedure, a minority noted a burning sensation (11%), bother with handpiece movement (6%), or vulvar pain (5%).17
Recently, Cruz et al18 performed one of the first randomized, double-blind, placebo-controlled trials comparing fractional CO2 laser therapy, topical estrogen therapy, and the combination of both treatments in patients with GSM. Forty-five women were included in the study, and validated assessments were performed at baseline and weeks 8 and 20. Intravaginal treatments were performed at a DOT power of 30 W, dwell time of 1000 μs, DOT spacing of 1000 μm, and SmartStack of 2. Importantly, the study incorporated placebo laser treatments (with the power adjusted to 0.0 W) in the topical estrogen group, thereby decreasing result bias. There was a significant increase in VHI scores from baseline to week 8 (P<.05) and week 20 (P<.01) in all study arms. At week 20, the laser group and laser plus estrogen group showed significant improvements in reported dyspareunia, burning, and dryness, whereas the estrogen arm only reported improvements in dryness (all values P<.05).18
Erbium-Doped YAG Laser
The erbium-doped YAG (Er:YAG) laser is an ablative laser emitting light at 2940 nm. This wavelength provides an absorption coefficient for water 16 times greater than the CO2 laser, leading to decreased penetration depth of 1 to 3 μm and reduced damage to the surrounding tissues.19,20 As such, the Er:YAG laser results in milder postoperative discomfort and faster overall healing times.21
In a noncontrolled study of vaginal relaxation syndrome, Lee22 used an Er:YAG laser fitted with Petit Lady (Lutronic) 90° and 360° vaginal scanning scopes. Thirty patients were divided into 2 groups and were treated with 4 sessions at weekly intervals. In group A, the first 2 sessions were performed with the 360° scope, and the last 2 sessions with the 90° scope in multiple micropulse mode (3 multishots; pulse width of 250 μs; 1.7 J delivered per shot). Group B was treated with the 90° scope in all 4 sessions in multiple micropulse mode (same parameters as group A), and during the last 2 sessions patients were additionally treated with 2 passes per session with the 360° scope (long-pulsed mode; pulse width of 1000 μs; 3.7 J delivered per shot). Perineometer measurements taken 2 months after the final treatment showed that the combined patient population experienced significant increases in both maximal vaginal pressure (P<.01) and average vaginal pressure (P<.05). Roughly 76% of patients’ partners noted improved vaginal tightening, and 70% of patients reported being satisfied with their treatment outcome. Histologic specimens taken at baseline and 2 months postprocedure showed evidence of thicker and more cellular epithelia along with more compact lamina propria with denser connective tissue. The sessions were well tolerated, with patients reporting a nonpainful heating sensation in the vagina during treatment. Three patients from the combined patient population experienced a mild burning sensation and vaginal ecchymoses, which lasted 24 to 48 hours following treatment and resolved spontaneously. There was no control group and no reports of major or long-term adverse events.22
Investigations also have shown the benefit of Er:YAG in the treatment of GSM.23,24 In a study by Gambacciani et al,24 patients treated with the Er:YAG laser FotonaSmooth (Fotona) every 30 days for 3 months reported significant improvements in vaginal dryness and dyspareunia (P<.01), which lasted up to 6 months posttreatment, though there was no placebo group comparator. Similar results were seen by Gaspar et al23 using 3 treatments at 3-week intervals, with results sustained up to 18 months after the final session.
Radiofrequency Devices
Radiofrequency devices emit focused electromagnetic waves that heat underlying tissues without targeting melanin. The release of thermal energy induces collagen contraction, neocollagenesis, and neovascularization, all of which aid in restoring the elasticity and moisture of the vaginal mucosa.25 Devices also may be equipped with cooling probes and reverse-heating gradients to protect the surface mucosa while deeper tissues are heated.
Millheiser et al26 performed a noncontrolled pilot study in 24 women with vaginal laxity using the Viveve System (Viveve), a cryogen-cooled monopolar RF device. Participants underwent a single 30-minute session (energy ranging from 75–90 J/cm2) during which the mucosal surface of the vaginal introitus (excluding the urethra) was treated with pulses at 0.5-cm overlapping intervals. Follow-up assessments were completed at 1, 3, and 6 months posttreatment. Self-reported vaginal tightness improved in 67% of participants at 1-month posttreatment and in 87% of participants at 6 months posttreatment (P<.001). There were no adverse events reported.26 Sekiguchi et al27 reported similar benefits lasting up to 12 months after a single 26-minute session at 90 J/cm2.
A prospective, randomized, placebo-controlled clinical trial using the Viveve system was recently completed by Krychman et al.28 Participants (N=186) were randomized to receive a single session of active treatment (90 J/cm2) or placebo treatment (1 J/cm2). In both groups, the vaginal introitus was treated with pulses at 0.5 cm in overlapping intervals, with the entire area (excluding the urethra) treated 5 times up to a total of 110 pulses. The primary end point was the proportion of randomized participants reporting no vaginal laxity at 6 months postin-tervention, which was assessed using the Vaginal Laxity Questionnaire. A grade of no vaginal laxity was achieved by 43.5% of participants in the active treatment group and 19.6% of participants in the sham group (P=.002). Overall numbers of treatment-emergent adverse events were comparable between the 2 groups, with the most commonly reported being vaginal discharge (2.6% in the active treatment group vs 3.5% in the sham group). There were no serious adverse events reported in the active treatment group.28
ThermiVa (ThermiGen, LLC), a unipolar RF device, was evaluated by Alinsod29 in the treatment of orgasmic dysfunction. The noncontrolled study included 25 women with self-reported difficulty achieving orgasm during intercourse, each of whom underwent 3 treatment sessions at 1-month intervals. Of the 25 enrolled women, 19 (76%) reported an average reduction in time to orgasm of at least 50%. All anorgasmic patients (n=10) at baseline reported renewed ability to achieve orgasms. Two (8%) patients failed to achieve a significant benefit from the treatments. Of note, the study did not include a control group, and specific data on the durability of beneficial effects was lacking.29
The Ultra Femme 360 (BLT Industries Inc), a monopolar RF device, was evaluated by Lalji and Lozanova30 in a noncontrolled study of 27 women with mild to moderate vaginal laxity and urinary incontinence. Participants underwent 3 treatment sessions at weekly intervals. Vaginal laxity was assessed by a subjective vulvovaginal laxity questionnaire, and data were collected before the first treatment and at 1-month follow-up. All 27 participants reported improvements in vaginal laxity, with the average grade (SD) increasing from very loose (2.19 [1.08]) to moderately tight (5.74 [0.76]; P<.05) on the questionnaire’s 7-point scale. The trial did not include a control group.30
Conclusion
With growing patient interest in vaginal rejuvenation, clinicians are increasingly incorporating a variety of procedures into their practice. Although long-term data on the safety and efficacy of these treatments has yet to be established, current evidence indicates that fractional ablative lasers and RF devices can improve vaginal laxity, sexual sensation, and symptoms of GSM.
To date, major complications have not been reported, but the FDA has advocated caution until regulatory approval is achieved.10 Concerns exist over the limited number of robust clinical trials as well as the prevalence of advertising campaigns that promise wide-ranging improvements without sufficient evidence. Definitive statements on medical or cosmetic indications will undoubtedly require more thorough investigation. At this time, the safety profile of these devices appears to be favorable, and high rates of patient satisfaction have been reported. As such, noninvasive vaginal rejuvenation procedures may represent a valuable addition to the cosmetic landscape.
- Koning M, Zeijlmans IA, Bouman TK, et al. Female attitudes regarding labia minora appearance and reduction with consideration of media influence. Aesthet Surg J. 2009;29:65-71.
- Rowen TS, Gaither TW, Shindel AW, et al. Characteristics of genital dissatisfaction among a nationally representative sample of U.S. women. J Sex Med. 2018;15:698-704.
- Berman L, Berman J, Miles M, et al. Genital self-image as a component of sexual health: relationship between genital self-image, female sexual function, and quality of life measures. J Sex Marital Ther. 2003;29(suppl 1):11-21.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21:1063-1068.
- Goodman MP, Placik OJ, Benson RH 3rd, et al. A large multicenter outcome study of female genital plastic surgery. J Sex Med. 2010;7(4 pt 1):1565-1577.
- Ostrzenski A. Vaginal rugation rejuvenation (restoration): a new surgical technique for an acquired sensation of wide/smooth vagina. Gynecol Obstet Invest. 2012;73:48-52.
- Singh A, Swift S, Khullar V, et al. Laser vaginal rejuvenation: not ready for prime time. Int Urogynecol J. 2015;26:163-164.
- Iglesia CB, Yurteri-Kaplan L, Alinsod R. Female genital cosmetic surgery: a review of techniques and outcomes. Int Urogynecol J. 2013;24:1997-2009.
- Dobbeleir JM, Landuyt KV, Monstrey SJ. Aesthetic surgery of the female genitalia. Semin Plast Surg. 2011;25:130-141.
- US Food and Drug Administration. FDA warns against use of energy-based devices to perform vaginal ‘rejuvenation’ or vaginal cosmetic procedures: FDA safety communication. July 30, 2018. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Accessed September 10, 2018.
- Patil UA, Dhami LD. Overview of lasers. Indian J Plast Surg. 2008;41(suppl):S101-S113.
- Qureshi AA, Tenenbaum MM, Myckatyn TM. Nonsurgical vulvovaginal rejuvenation with radiofrequency and laser devices: a literature review and comprehensive update for aesthetic surgeons. Aesthet Surg J. 2018;38:302-311.
- Zerbinati N, Serati M, Origoni M, et al. Microscopic and ultrastructural modifications of postmenopausal atrophic vaginal mucosa after fractional carbon dioxide laser treatment. Lasers Med Sci. 2015;30:429-436.
- Salvatore S, Nappi RE, Zerbinati N, et al. A 12-week treatment with fractional CO2 laser for vulvovaginal atrophy: a pilot study. Climacteric. 2014;17:363-369.
- Eder SE. Early effect of fractional CO2 laser treatment in post-menopausal women with vaginal atrophy. Laser Ther. 2018;27:41-47.
- Perino A, Calligaro A, Forlani F, et al. Vulvo-vaginal atrophy: a new treatment modality using thermo-ablative fractional CO2 laser. Maturitas. 2015;80:296-301.
- Filippini M, Del Duca E, Negosanti F, et al. Fractional CO2 laser: from skin rejuvenation to vulvo-vaginal reshaping. Photomed Laser Surg. 2017;35:171-175.
- Cruz VL, Steiner ML, Pompei LM, et al. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause. 2018;25:21-28.
- Preissig J, Hamilton K, Markus R. Current laser resurfacing technologies: a review that delves beneath the surface. Semin Plast Surg. 2012;26:109-116.
- Kaushik SB, Alexis AF. Nonablative fractional laser resurfacing in skin of color: evidence-based review. J Clin Aesthet Dermatol. 2017;10:51-67.
- Alexiades-Armenakas MR, Dover JS, Arndt KA. Fractional laser skin resurfacing. J Drugs Dermatol. 2012;11:1274-1287.
- Lee MS. Treatment of vaginal relaxation syndrome with an erbium:YAG laser using 90 degrees and 360 degrees scanning scopes: a pilot study & short-term results. Laser Ther. 2014;23:129-138.
- Gaspar A, Brandi H, Gomez V, et al. Efficacy of erbium:YAG laser treatment compared to topical estriol treatment for symptoms of genitourinary syndrome of menopause. Lasers Surg Med. 2017;49:160-168.
- Gambacciani M, Levancini M, Cervigni M. Vaginal erbium laser: the second-generation thermotherapy for the genitourinary syndrome of menopause. Climacteric. 2015;18:757-763.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy based therapeutics for genitourinary syndrome of menopause: consensus and controversies. Lasers Surg Med. 2017;49:137-159.
- Millheiser LS, Pauls RN, Herbst SJ, et al. Radiofrequency treatment of vaginal laxity after vaginal delivery: nonsurgical vaginal tightening. J Sex Med. 2010;7:3088-3095.
- Sekiguchi Y, Utsugisawa Y, Azekosi Y, et al. Laxity of the vaginal introitus after childbirth: nonsurgical outpatient procedure for vaginal tissue restoration and improved sexual satisfaction using low-energy radiofrequency thermal therapy. J Womens Health (Larchmt). 2013;22:775-781.
- Krychman M, Rowan CG, Allan BB, et al. Effect of single-treatment, surface-cooled radiofrequency therapy on vaginal laxity and female sexual function: the VIVEVE I randomized controlled trial. J Sex Med. 2017;14:215-225.
- Alinsod RM. Transcutaneous temperature controlled radiofrequency for orgasmic dysfunction. Lasers Surg Med. 2016;48:641-645.
- Lalji S, Lozanova P. Evaluation of the safety and efficacy of a monopolar nonablative radiofrequency device for the improvement of vulvo-vaginal laxity and urinary incontinence. J Cosmet Dermatol. 2017;16:230-234.
Vaginal rejuvenation encompasses a group of procedures that alter the vaginal anatomy to improve cosmesis or achieve more pleasurable sexual intercourse. External vaginal procedures are defined as those performed on the female genitalia outside of the vaginal introitus, with major structures including the labia majora, mons pubis, labia minora, clitoral hood, clitoral glans, and vaginal vestibule. Internal vaginal procedures are defined as those performed within the vagina, extending from the vaginal introitus to the cervix.
The prevalence of elective vaginal rejuvenation procedures has increased in recent years, a trend that may be attributed to greater exposure through the media, including reality television and pornography. In a survey of 482 women undergoing labiaplasty, nearly all had heard about rejuvenation procedures within the last 2.2 years, and 78% had received their information through the media.1 Additionally, genital self-image can have a considerable effect on a woman’s sexual behavior and relationships. Genital dissatisfaction has been associated with decreased sexual activity, whereas positive genital self-image correlates with increased sexual desire and less sexual distress or depression.2,3
Currently, the 2 primary applications of noninvasive vaginal rejuvenation are vaginal laxity and genitourinary syndrome of menopause (GSM). Vaginal laxity occurs in premenopausal or postmenopausal women and is caused by aging, childbearing, or hormonal imbalances. These factors can lead to decreased friction within the vagina during intercourse, which in turn can decrease sexual pleasure. Genitourinary syndrome of menopause, previously known as vulvovaginal atrophy, encompasses genital (eg, dryness, burning, irritation), sexual (eg, lack of lubrication, discomfort or pain, impaired function), and urinary (eg, urgency, dysuria, recurrent urinary tract infections) symptoms of menopause.4
Noninvasive procedures are designed to apply ablative or nonablative energy to the vaginal mucosa to tighten a lax upper vagina, also known as a wide vagina.5 A wide vagina has been defined as a widened vaginal diameter that interferes with sexual function and sensation.6 Decreased sexual sensation also may result from fibrosis or scarring of the vaginal mucosa after prior vaginal surgery, episiotomy, or tears during childbirth.7 The objective of rejuvenation procedures to treat the vaginal mucosa is to create increased frictional forces that may lead to increased sexual sensation.8 Although there are numerous reports of heightened sexual satisfaction after reduction of the vaginal diameter, a formal link between sexual pleasure and vaginal laxity has yet to be established.8,9 At present, there are no US Food and Drug Administration (FDA)–approved energy-based devices to treat urinary incontinence or sexual function, and the FDA recently issued an alert cautioning patients on the current lack of safety and efficacy regulations.10
In this article we review the safety and efficacy data behind lasers and radiofrequency (RF) devices used in noninvasive vaginal rejuvenation procedures.
Lasers
CO2 Laser
The infrared CO2 laser utilizes 10,600-nm energy to target and vaporize water molecules within the target tissue. This thermal heating extends to the dermal collagen, which stimulates inflammatory pathways and neocollagenesis.11 The depth of penetration ranges from 20 to 125 μm.12 Zerbinati et al13 demonstrated the histologic and ultrastructural effects of a fractional CO2 laser on atrophic vaginal mucosa. Comparing pretreatment and posttreatment mucosal biopsies in 5 postmenopausal women, the investigators found that fractional CO2 laser treatment caused increased epithelial thickness, vascularity, and fibroblast activity, which led to augmented synthesis of collagen and ground substance proteins.13
New devices seek to translate these histologic improvements to the aesthetic appearance and function of female genitalia. The MonaLisa Touch (Cynosure), a new fractional CO2 laser specifically designed for treatment of the vaginal mucosa, uses dermal optical thermolysis (DOT) therapy to apply energy in a noncontinuous mode at 200-μm dots. Salvatore et al14 examined the use of this device in a noncontrolled study of 50 patients with GSM, with each patient undergoing 3 treatment sessions at monthly intervals. Intravaginal treatments were performed at the following settings: DOT (microablative zone) power of 30 W, dwell time of 1000 μs, DOT spacing of 1000 μm, and SmartStack parameter of 1 to 3. The investigators used the Vaginal Health Index (VHI) to objectively assess vaginal elasticity, secretions, pH, mucosa integrity, and moisture. Total VHI scores significantly improved between baseline and 1 month following the final treatment (mean score [SD], 13.1 [2.5] vs 23.1 [1.9]; P<.0001). There were no significant adverse events, and 84% of patients reported being satisfied with their outcome; however, the study lacked a comparison or control group, raising the possibility of placebo effect.14
Other noncontrolled series have corroborated the benefits of CO2 laser in GSM patients.15,16 In one of the largest studies to date, Filippini et al17 reviewed the outcomes of 386 menopausal women treated for GSM. Patients underwent 3 intravaginal laser sessions with the MonaLisa Touch. Intravaginal treatments were performed at a DOT power of 40 W, dwell time of 1000 μs, DOT spacing of 1000 μm, and SmartStack of 2. For the vulva, the DOT power was reduced to 30 W, dwell time of 1000 μs, DOT spacing of 1000 μm, and SmartStack of 1. Two months after the final treatment session, patients completed a nonvalidated questionnaire about their symptoms, with improved dryness reported in 60% of patients, improved burning in 56%, improved dyspareunia in 49%, improved itch in 56%, improved soreness in 73%, and improved vaginal introitus pain in 49%. Although most patients did not experience discomfort with the procedure, a minority noted a burning sensation (11%), bother with handpiece movement (6%), or vulvar pain (5%).17
Recently, Cruz et al18 performed one of the first randomized, double-blind, placebo-controlled trials comparing fractional CO2 laser therapy, topical estrogen therapy, and the combination of both treatments in patients with GSM. Forty-five women were included in the study, and validated assessments were performed at baseline and weeks 8 and 20. Intravaginal treatments were performed at a DOT power of 30 W, dwell time of 1000 μs, DOT spacing of 1000 μm, and SmartStack of 2. Importantly, the study incorporated placebo laser treatments (with the power adjusted to 0.0 W) in the topical estrogen group, thereby decreasing result bias. There was a significant increase in VHI scores from baseline to week 8 (P<.05) and week 20 (P<.01) in all study arms. At week 20, the laser group and laser plus estrogen group showed significant improvements in reported dyspareunia, burning, and dryness, whereas the estrogen arm only reported improvements in dryness (all values P<.05).18
Erbium-Doped YAG Laser
The erbium-doped YAG (Er:YAG) laser is an ablative laser emitting light at 2940 nm. This wavelength provides an absorption coefficient for water 16 times greater than the CO2 laser, leading to decreased penetration depth of 1 to 3 μm and reduced damage to the surrounding tissues.19,20 As such, the Er:YAG laser results in milder postoperative discomfort and faster overall healing times.21
In a noncontrolled study of vaginal relaxation syndrome, Lee22 used an Er:YAG laser fitted with Petit Lady (Lutronic) 90° and 360° vaginal scanning scopes. Thirty patients were divided into 2 groups and were treated with 4 sessions at weekly intervals. In group A, the first 2 sessions were performed with the 360° scope, and the last 2 sessions with the 90° scope in multiple micropulse mode (3 multishots; pulse width of 250 μs; 1.7 J delivered per shot). Group B was treated with the 90° scope in all 4 sessions in multiple micropulse mode (same parameters as group A), and during the last 2 sessions patients were additionally treated with 2 passes per session with the 360° scope (long-pulsed mode; pulse width of 1000 μs; 3.7 J delivered per shot). Perineometer measurements taken 2 months after the final treatment showed that the combined patient population experienced significant increases in both maximal vaginal pressure (P<.01) and average vaginal pressure (P<.05). Roughly 76% of patients’ partners noted improved vaginal tightening, and 70% of patients reported being satisfied with their treatment outcome. Histologic specimens taken at baseline and 2 months postprocedure showed evidence of thicker and more cellular epithelia along with more compact lamina propria with denser connective tissue. The sessions were well tolerated, with patients reporting a nonpainful heating sensation in the vagina during treatment. Three patients from the combined patient population experienced a mild burning sensation and vaginal ecchymoses, which lasted 24 to 48 hours following treatment and resolved spontaneously. There was no control group and no reports of major or long-term adverse events.22
Investigations also have shown the benefit of Er:YAG in the treatment of GSM.23,24 In a study by Gambacciani et al,24 patients treated with the Er:YAG laser FotonaSmooth (Fotona) every 30 days for 3 months reported significant improvements in vaginal dryness and dyspareunia (P<.01), which lasted up to 6 months posttreatment, though there was no placebo group comparator. Similar results were seen by Gaspar et al23 using 3 treatments at 3-week intervals, with results sustained up to 18 months after the final session.
Radiofrequency Devices
Radiofrequency devices emit focused electromagnetic waves that heat underlying tissues without targeting melanin. The release of thermal energy induces collagen contraction, neocollagenesis, and neovascularization, all of which aid in restoring the elasticity and moisture of the vaginal mucosa.25 Devices also may be equipped with cooling probes and reverse-heating gradients to protect the surface mucosa while deeper tissues are heated.
Millheiser et al26 performed a noncontrolled pilot study in 24 women with vaginal laxity using the Viveve System (Viveve), a cryogen-cooled monopolar RF device. Participants underwent a single 30-minute session (energy ranging from 75–90 J/cm2) during which the mucosal surface of the vaginal introitus (excluding the urethra) was treated with pulses at 0.5-cm overlapping intervals. Follow-up assessments were completed at 1, 3, and 6 months posttreatment. Self-reported vaginal tightness improved in 67% of participants at 1-month posttreatment and in 87% of participants at 6 months posttreatment (P<.001). There were no adverse events reported.26 Sekiguchi et al27 reported similar benefits lasting up to 12 months after a single 26-minute session at 90 J/cm2.
A prospective, randomized, placebo-controlled clinical trial using the Viveve system was recently completed by Krychman et al.28 Participants (N=186) were randomized to receive a single session of active treatment (90 J/cm2) or placebo treatment (1 J/cm2). In both groups, the vaginal introitus was treated with pulses at 0.5 cm in overlapping intervals, with the entire area (excluding the urethra) treated 5 times up to a total of 110 pulses. The primary end point was the proportion of randomized participants reporting no vaginal laxity at 6 months postin-tervention, which was assessed using the Vaginal Laxity Questionnaire. A grade of no vaginal laxity was achieved by 43.5% of participants in the active treatment group and 19.6% of participants in the sham group (P=.002). Overall numbers of treatment-emergent adverse events were comparable between the 2 groups, with the most commonly reported being vaginal discharge (2.6% in the active treatment group vs 3.5% in the sham group). There were no serious adverse events reported in the active treatment group.28
ThermiVa (ThermiGen, LLC), a unipolar RF device, was evaluated by Alinsod29 in the treatment of orgasmic dysfunction. The noncontrolled study included 25 women with self-reported difficulty achieving orgasm during intercourse, each of whom underwent 3 treatment sessions at 1-month intervals. Of the 25 enrolled women, 19 (76%) reported an average reduction in time to orgasm of at least 50%. All anorgasmic patients (n=10) at baseline reported renewed ability to achieve orgasms. Two (8%) patients failed to achieve a significant benefit from the treatments. Of note, the study did not include a control group, and specific data on the durability of beneficial effects was lacking.29
The Ultra Femme 360 (BLT Industries Inc), a monopolar RF device, was evaluated by Lalji and Lozanova30 in a noncontrolled study of 27 women with mild to moderate vaginal laxity and urinary incontinence. Participants underwent 3 treatment sessions at weekly intervals. Vaginal laxity was assessed by a subjective vulvovaginal laxity questionnaire, and data were collected before the first treatment and at 1-month follow-up. All 27 participants reported improvements in vaginal laxity, with the average grade (SD) increasing from very loose (2.19 [1.08]) to moderately tight (5.74 [0.76]; P<.05) on the questionnaire’s 7-point scale. The trial did not include a control group.30
Conclusion
With growing patient interest in vaginal rejuvenation, clinicians are increasingly incorporating a variety of procedures into their practice. Although long-term data on the safety and efficacy of these treatments has yet to be established, current evidence indicates that fractional ablative lasers and RF devices can improve vaginal laxity, sexual sensation, and symptoms of GSM.
To date, major complications have not been reported, but the FDA has advocated caution until regulatory approval is achieved.10 Concerns exist over the limited number of robust clinical trials as well as the prevalence of advertising campaigns that promise wide-ranging improvements without sufficient evidence. Definitive statements on medical or cosmetic indications will undoubtedly require more thorough investigation. At this time, the safety profile of these devices appears to be favorable, and high rates of patient satisfaction have been reported. As such, noninvasive vaginal rejuvenation procedures may represent a valuable addition to the cosmetic landscape.
Vaginal rejuvenation encompasses a group of procedures that alter the vaginal anatomy to improve cosmesis or achieve more pleasurable sexual intercourse. External vaginal procedures are defined as those performed on the female genitalia outside of the vaginal introitus, with major structures including the labia majora, mons pubis, labia minora, clitoral hood, clitoral glans, and vaginal vestibule. Internal vaginal procedures are defined as those performed within the vagina, extending from the vaginal introitus to the cervix.
The prevalence of elective vaginal rejuvenation procedures has increased in recent years, a trend that may be attributed to greater exposure through the media, including reality television and pornography. In a survey of 482 women undergoing labiaplasty, nearly all had heard about rejuvenation procedures within the last 2.2 years, and 78% had received their information through the media.1 Additionally, genital self-image can have a considerable effect on a woman’s sexual behavior and relationships. Genital dissatisfaction has been associated with decreased sexual activity, whereas positive genital self-image correlates with increased sexual desire and less sexual distress or depression.2,3
Currently, the 2 primary applications of noninvasive vaginal rejuvenation are vaginal laxity and genitourinary syndrome of menopause (GSM). Vaginal laxity occurs in premenopausal or postmenopausal women and is caused by aging, childbearing, or hormonal imbalances. These factors can lead to decreased friction within the vagina during intercourse, which in turn can decrease sexual pleasure. Genitourinary syndrome of menopause, previously known as vulvovaginal atrophy, encompasses genital (eg, dryness, burning, irritation), sexual (eg, lack of lubrication, discomfort or pain, impaired function), and urinary (eg, urgency, dysuria, recurrent urinary tract infections) symptoms of menopause.4
Noninvasive procedures are designed to apply ablative or nonablative energy to the vaginal mucosa to tighten a lax upper vagina, also known as a wide vagina.5 A wide vagina has been defined as a widened vaginal diameter that interferes with sexual function and sensation.6 Decreased sexual sensation also may result from fibrosis or scarring of the vaginal mucosa after prior vaginal surgery, episiotomy, or tears during childbirth.7 The objective of rejuvenation procedures to treat the vaginal mucosa is to create increased frictional forces that may lead to increased sexual sensation.8 Although there are numerous reports of heightened sexual satisfaction after reduction of the vaginal diameter, a formal link between sexual pleasure and vaginal laxity has yet to be established.8,9 At present, there are no US Food and Drug Administration (FDA)–approved energy-based devices to treat urinary incontinence or sexual function, and the FDA recently issued an alert cautioning patients on the current lack of safety and efficacy regulations.10
In this article we review the safety and efficacy data behind lasers and radiofrequency (RF) devices used in noninvasive vaginal rejuvenation procedures.
Lasers
CO2 Laser
The infrared CO2 laser utilizes 10,600-nm energy to target and vaporize water molecules within the target tissue. This thermal heating extends to the dermal collagen, which stimulates inflammatory pathways and neocollagenesis.11 The depth of penetration ranges from 20 to 125 μm.12 Zerbinati et al13 demonstrated the histologic and ultrastructural effects of a fractional CO2 laser on atrophic vaginal mucosa. Comparing pretreatment and posttreatment mucosal biopsies in 5 postmenopausal women, the investigators found that fractional CO2 laser treatment caused increased epithelial thickness, vascularity, and fibroblast activity, which led to augmented synthesis of collagen and ground substance proteins.13
New devices seek to translate these histologic improvements to the aesthetic appearance and function of female genitalia. The MonaLisa Touch (Cynosure), a new fractional CO2 laser specifically designed for treatment of the vaginal mucosa, uses dermal optical thermolysis (DOT) therapy to apply energy in a noncontinuous mode at 200-μm dots. Salvatore et al14 examined the use of this device in a noncontrolled study of 50 patients with GSM, with each patient undergoing 3 treatment sessions at monthly intervals. Intravaginal treatments were performed at the following settings: DOT (microablative zone) power of 30 W, dwell time of 1000 μs, DOT spacing of 1000 μm, and SmartStack parameter of 1 to 3. The investigators used the Vaginal Health Index (VHI) to objectively assess vaginal elasticity, secretions, pH, mucosa integrity, and moisture. Total VHI scores significantly improved between baseline and 1 month following the final treatment (mean score [SD], 13.1 [2.5] vs 23.1 [1.9]; P<.0001). There were no significant adverse events, and 84% of patients reported being satisfied with their outcome; however, the study lacked a comparison or control group, raising the possibility of placebo effect.14
Other noncontrolled series have corroborated the benefits of CO2 laser in GSM patients.15,16 In one of the largest studies to date, Filippini et al17 reviewed the outcomes of 386 menopausal women treated for GSM. Patients underwent 3 intravaginal laser sessions with the MonaLisa Touch. Intravaginal treatments were performed at a DOT power of 40 W, dwell time of 1000 μs, DOT spacing of 1000 μm, and SmartStack of 2. For the vulva, the DOT power was reduced to 30 W, dwell time of 1000 μs, DOT spacing of 1000 μm, and SmartStack of 1. Two months after the final treatment session, patients completed a nonvalidated questionnaire about their symptoms, with improved dryness reported in 60% of patients, improved burning in 56%, improved dyspareunia in 49%, improved itch in 56%, improved soreness in 73%, and improved vaginal introitus pain in 49%. Although most patients did not experience discomfort with the procedure, a minority noted a burning sensation (11%), bother with handpiece movement (6%), or vulvar pain (5%).17
Recently, Cruz et al18 performed one of the first randomized, double-blind, placebo-controlled trials comparing fractional CO2 laser therapy, topical estrogen therapy, and the combination of both treatments in patients with GSM. Forty-five women were included in the study, and validated assessments were performed at baseline and weeks 8 and 20. Intravaginal treatments were performed at a DOT power of 30 W, dwell time of 1000 μs, DOT spacing of 1000 μm, and SmartStack of 2. Importantly, the study incorporated placebo laser treatments (with the power adjusted to 0.0 W) in the topical estrogen group, thereby decreasing result bias. There was a significant increase in VHI scores from baseline to week 8 (P<.05) and week 20 (P<.01) in all study arms. At week 20, the laser group and laser plus estrogen group showed significant improvements in reported dyspareunia, burning, and dryness, whereas the estrogen arm only reported improvements in dryness (all values P<.05).18
Erbium-Doped YAG Laser
The erbium-doped YAG (Er:YAG) laser is an ablative laser emitting light at 2940 nm. This wavelength provides an absorption coefficient for water 16 times greater than the CO2 laser, leading to decreased penetration depth of 1 to 3 μm and reduced damage to the surrounding tissues.19,20 As such, the Er:YAG laser results in milder postoperative discomfort and faster overall healing times.21
In a noncontrolled study of vaginal relaxation syndrome, Lee22 used an Er:YAG laser fitted with Petit Lady (Lutronic) 90° and 360° vaginal scanning scopes. Thirty patients were divided into 2 groups and were treated with 4 sessions at weekly intervals. In group A, the first 2 sessions were performed with the 360° scope, and the last 2 sessions with the 90° scope in multiple micropulse mode (3 multishots; pulse width of 250 μs; 1.7 J delivered per shot). Group B was treated with the 90° scope in all 4 sessions in multiple micropulse mode (same parameters as group A), and during the last 2 sessions patients were additionally treated with 2 passes per session with the 360° scope (long-pulsed mode; pulse width of 1000 μs; 3.7 J delivered per shot). Perineometer measurements taken 2 months after the final treatment showed that the combined patient population experienced significant increases in both maximal vaginal pressure (P<.01) and average vaginal pressure (P<.05). Roughly 76% of patients’ partners noted improved vaginal tightening, and 70% of patients reported being satisfied with their treatment outcome. Histologic specimens taken at baseline and 2 months postprocedure showed evidence of thicker and more cellular epithelia along with more compact lamina propria with denser connective tissue. The sessions were well tolerated, with patients reporting a nonpainful heating sensation in the vagina during treatment. Three patients from the combined patient population experienced a mild burning sensation and vaginal ecchymoses, which lasted 24 to 48 hours following treatment and resolved spontaneously. There was no control group and no reports of major or long-term adverse events.22
Investigations also have shown the benefit of Er:YAG in the treatment of GSM.23,24 In a study by Gambacciani et al,24 patients treated with the Er:YAG laser FotonaSmooth (Fotona) every 30 days for 3 months reported significant improvements in vaginal dryness and dyspareunia (P<.01), which lasted up to 6 months posttreatment, though there was no placebo group comparator. Similar results were seen by Gaspar et al23 using 3 treatments at 3-week intervals, with results sustained up to 18 months after the final session.
Radiofrequency Devices
Radiofrequency devices emit focused electromagnetic waves that heat underlying tissues without targeting melanin. The release of thermal energy induces collagen contraction, neocollagenesis, and neovascularization, all of which aid in restoring the elasticity and moisture of the vaginal mucosa.25 Devices also may be equipped with cooling probes and reverse-heating gradients to protect the surface mucosa while deeper tissues are heated.
Millheiser et al26 performed a noncontrolled pilot study in 24 women with vaginal laxity using the Viveve System (Viveve), a cryogen-cooled monopolar RF device. Participants underwent a single 30-minute session (energy ranging from 75–90 J/cm2) during which the mucosal surface of the vaginal introitus (excluding the urethra) was treated with pulses at 0.5-cm overlapping intervals. Follow-up assessments were completed at 1, 3, and 6 months posttreatment. Self-reported vaginal tightness improved in 67% of participants at 1-month posttreatment and in 87% of participants at 6 months posttreatment (P<.001). There were no adverse events reported.26 Sekiguchi et al27 reported similar benefits lasting up to 12 months after a single 26-minute session at 90 J/cm2.
A prospective, randomized, placebo-controlled clinical trial using the Viveve system was recently completed by Krychman et al.28 Participants (N=186) were randomized to receive a single session of active treatment (90 J/cm2) or placebo treatment (1 J/cm2). In both groups, the vaginal introitus was treated with pulses at 0.5 cm in overlapping intervals, with the entire area (excluding the urethra) treated 5 times up to a total of 110 pulses. The primary end point was the proportion of randomized participants reporting no vaginal laxity at 6 months postin-tervention, which was assessed using the Vaginal Laxity Questionnaire. A grade of no vaginal laxity was achieved by 43.5% of participants in the active treatment group and 19.6% of participants in the sham group (P=.002). Overall numbers of treatment-emergent adverse events were comparable between the 2 groups, with the most commonly reported being vaginal discharge (2.6% in the active treatment group vs 3.5% in the sham group). There were no serious adverse events reported in the active treatment group.28
ThermiVa (ThermiGen, LLC), a unipolar RF device, was evaluated by Alinsod29 in the treatment of orgasmic dysfunction. The noncontrolled study included 25 women with self-reported difficulty achieving orgasm during intercourse, each of whom underwent 3 treatment sessions at 1-month intervals. Of the 25 enrolled women, 19 (76%) reported an average reduction in time to orgasm of at least 50%. All anorgasmic patients (n=10) at baseline reported renewed ability to achieve orgasms. Two (8%) patients failed to achieve a significant benefit from the treatments. Of note, the study did not include a control group, and specific data on the durability of beneficial effects was lacking.29
The Ultra Femme 360 (BLT Industries Inc), a monopolar RF device, was evaluated by Lalji and Lozanova30 in a noncontrolled study of 27 women with mild to moderate vaginal laxity and urinary incontinence. Participants underwent 3 treatment sessions at weekly intervals. Vaginal laxity was assessed by a subjective vulvovaginal laxity questionnaire, and data were collected before the first treatment and at 1-month follow-up. All 27 participants reported improvements in vaginal laxity, with the average grade (SD) increasing from very loose (2.19 [1.08]) to moderately tight (5.74 [0.76]; P<.05) on the questionnaire’s 7-point scale. The trial did not include a control group.30
Conclusion
With growing patient interest in vaginal rejuvenation, clinicians are increasingly incorporating a variety of procedures into their practice. Although long-term data on the safety and efficacy of these treatments has yet to be established, current evidence indicates that fractional ablative lasers and RF devices can improve vaginal laxity, sexual sensation, and symptoms of GSM.
To date, major complications have not been reported, but the FDA has advocated caution until regulatory approval is achieved.10 Concerns exist over the limited number of robust clinical trials as well as the prevalence of advertising campaigns that promise wide-ranging improvements without sufficient evidence. Definitive statements on medical or cosmetic indications will undoubtedly require more thorough investigation. At this time, the safety profile of these devices appears to be favorable, and high rates of patient satisfaction have been reported. As such, noninvasive vaginal rejuvenation procedures may represent a valuable addition to the cosmetic landscape.
- Koning M, Zeijlmans IA, Bouman TK, et al. Female attitudes regarding labia minora appearance and reduction with consideration of media influence. Aesthet Surg J. 2009;29:65-71.
- Rowen TS, Gaither TW, Shindel AW, et al. Characteristics of genital dissatisfaction among a nationally representative sample of U.S. women. J Sex Med. 2018;15:698-704.
- Berman L, Berman J, Miles M, et al. Genital self-image as a component of sexual health: relationship between genital self-image, female sexual function, and quality of life measures. J Sex Marital Ther. 2003;29(suppl 1):11-21.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21:1063-1068.
- Goodman MP, Placik OJ, Benson RH 3rd, et al. A large multicenter outcome study of female genital plastic surgery. J Sex Med. 2010;7(4 pt 1):1565-1577.
- Ostrzenski A. Vaginal rugation rejuvenation (restoration): a new surgical technique for an acquired sensation of wide/smooth vagina. Gynecol Obstet Invest. 2012;73:48-52.
- Singh A, Swift S, Khullar V, et al. Laser vaginal rejuvenation: not ready for prime time. Int Urogynecol J. 2015;26:163-164.
- Iglesia CB, Yurteri-Kaplan L, Alinsod R. Female genital cosmetic surgery: a review of techniques and outcomes. Int Urogynecol J. 2013;24:1997-2009.
- Dobbeleir JM, Landuyt KV, Monstrey SJ. Aesthetic surgery of the female genitalia. Semin Plast Surg. 2011;25:130-141.
- US Food and Drug Administration. FDA warns against use of energy-based devices to perform vaginal ‘rejuvenation’ or vaginal cosmetic procedures: FDA safety communication. July 30, 2018. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Accessed September 10, 2018.
- Patil UA, Dhami LD. Overview of lasers. Indian J Plast Surg. 2008;41(suppl):S101-S113.
- Qureshi AA, Tenenbaum MM, Myckatyn TM. Nonsurgical vulvovaginal rejuvenation with radiofrequency and laser devices: a literature review and comprehensive update for aesthetic surgeons. Aesthet Surg J. 2018;38:302-311.
- Zerbinati N, Serati M, Origoni M, et al. Microscopic and ultrastructural modifications of postmenopausal atrophic vaginal mucosa after fractional carbon dioxide laser treatment. Lasers Med Sci. 2015;30:429-436.
- Salvatore S, Nappi RE, Zerbinati N, et al. A 12-week treatment with fractional CO2 laser for vulvovaginal atrophy: a pilot study. Climacteric. 2014;17:363-369.
- Eder SE. Early effect of fractional CO2 laser treatment in post-menopausal women with vaginal atrophy. Laser Ther. 2018;27:41-47.
- Perino A, Calligaro A, Forlani F, et al. Vulvo-vaginal atrophy: a new treatment modality using thermo-ablative fractional CO2 laser. Maturitas. 2015;80:296-301.
- Filippini M, Del Duca E, Negosanti F, et al. Fractional CO2 laser: from skin rejuvenation to vulvo-vaginal reshaping. Photomed Laser Surg. 2017;35:171-175.
- Cruz VL, Steiner ML, Pompei LM, et al. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause. 2018;25:21-28.
- Preissig J, Hamilton K, Markus R. Current laser resurfacing technologies: a review that delves beneath the surface. Semin Plast Surg. 2012;26:109-116.
- Kaushik SB, Alexis AF. Nonablative fractional laser resurfacing in skin of color: evidence-based review. J Clin Aesthet Dermatol. 2017;10:51-67.
- Alexiades-Armenakas MR, Dover JS, Arndt KA. Fractional laser skin resurfacing. J Drugs Dermatol. 2012;11:1274-1287.
- Lee MS. Treatment of vaginal relaxation syndrome with an erbium:YAG laser using 90 degrees and 360 degrees scanning scopes: a pilot study & short-term results. Laser Ther. 2014;23:129-138.
- Gaspar A, Brandi H, Gomez V, et al. Efficacy of erbium:YAG laser treatment compared to topical estriol treatment for symptoms of genitourinary syndrome of menopause. Lasers Surg Med. 2017;49:160-168.
- Gambacciani M, Levancini M, Cervigni M. Vaginal erbium laser: the second-generation thermotherapy for the genitourinary syndrome of menopause. Climacteric. 2015;18:757-763.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy based therapeutics for genitourinary syndrome of menopause: consensus and controversies. Lasers Surg Med. 2017;49:137-159.
- Millheiser LS, Pauls RN, Herbst SJ, et al. Radiofrequency treatment of vaginal laxity after vaginal delivery: nonsurgical vaginal tightening. J Sex Med. 2010;7:3088-3095.
- Sekiguchi Y, Utsugisawa Y, Azekosi Y, et al. Laxity of the vaginal introitus after childbirth: nonsurgical outpatient procedure for vaginal tissue restoration and improved sexual satisfaction using low-energy radiofrequency thermal therapy. J Womens Health (Larchmt). 2013;22:775-781.
- Krychman M, Rowan CG, Allan BB, et al. Effect of single-treatment, surface-cooled radiofrequency therapy on vaginal laxity and female sexual function: the VIVEVE I randomized controlled trial. J Sex Med. 2017;14:215-225.
- Alinsod RM. Transcutaneous temperature controlled radiofrequency for orgasmic dysfunction. Lasers Surg Med. 2016;48:641-645.
- Lalji S, Lozanova P. Evaluation of the safety and efficacy of a monopolar nonablative radiofrequency device for the improvement of vulvo-vaginal laxity and urinary incontinence. J Cosmet Dermatol. 2017;16:230-234.
- Koning M, Zeijlmans IA, Bouman TK, et al. Female attitudes regarding labia minora appearance and reduction with consideration of media influence. Aesthet Surg J. 2009;29:65-71.
- Rowen TS, Gaither TW, Shindel AW, et al. Characteristics of genital dissatisfaction among a nationally representative sample of U.S. women. J Sex Med. 2018;15:698-704.
- Berman L, Berman J, Miles M, et al. Genital self-image as a component of sexual health: relationship between genital self-image, female sexual function, and quality of life measures. J Sex Marital Ther. 2003;29(suppl 1):11-21.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21:1063-1068.
- Goodman MP, Placik OJ, Benson RH 3rd, et al. A large multicenter outcome study of female genital plastic surgery. J Sex Med. 2010;7(4 pt 1):1565-1577.
- Ostrzenski A. Vaginal rugation rejuvenation (restoration): a new surgical technique for an acquired sensation of wide/smooth vagina. Gynecol Obstet Invest. 2012;73:48-52.
- Singh A, Swift S, Khullar V, et al. Laser vaginal rejuvenation: not ready for prime time. Int Urogynecol J. 2015;26:163-164.
- Iglesia CB, Yurteri-Kaplan L, Alinsod R. Female genital cosmetic surgery: a review of techniques and outcomes. Int Urogynecol J. 2013;24:1997-2009.
- Dobbeleir JM, Landuyt KV, Monstrey SJ. Aesthetic surgery of the female genitalia. Semin Plast Surg. 2011;25:130-141.
- US Food and Drug Administration. FDA warns against use of energy-based devices to perform vaginal ‘rejuvenation’ or vaginal cosmetic procedures: FDA safety communication. July 30, 2018. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Accessed September 10, 2018.
- Patil UA, Dhami LD. Overview of lasers. Indian J Plast Surg. 2008;41(suppl):S101-S113.
- Qureshi AA, Tenenbaum MM, Myckatyn TM. Nonsurgical vulvovaginal rejuvenation with radiofrequency and laser devices: a literature review and comprehensive update for aesthetic surgeons. Aesthet Surg J. 2018;38:302-311.
- Zerbinati N, Serati M, Origoni M, et al. Microscopic and ultrastructural modifications of postmenopausal atrophic vaginal mucosa after fractional carbon dioxide laser treatment. Lasers Med Sci. 2015;30:429-436.
- Salvatore S, Nappi RE, Zerbinati N, et al. A 12-week treatment with fractional CO2 laser for vulvovaginal atrophy: a pilot study. Climacteric. 2014;17:363-369.
- Eder SE. Early effect of fractional CO2 laser treatment in post-menopausal women with vaginal atrophy. Laser Ther. 2018;27:41-47.
- Perino A, Calligaro A, Forlani F, et al. Vulvo-vaginal atrophy: a new treatment modality using thermo-ablative fractional CO2 laser. Maturitas. 2015;80:296-301.
- Filippini M, Del Duca E, Negosanti F, et al. Fractional CO2 laser: from skin rejuvenation to vulvo-vaginal reshaping. Photomed Laser Surg. 2017;35:171-175.
- Cruz VL, Steiner ML, Pompei LM, et al. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause. 2018;25:21-28.
- Preissig J, Hamilton K, Markus R. Current laser resurfacing technologies: a review that delves beneath the surface. Semin Plast Surg. 2012;26:109-116.
- Kaushik SB, Alexis AF. Nonablative fractional laser resurfacing in skin of color: evidence-based review. J Clin Aesthet Dermatol. 2017;10:51-67.
- Alexiades-Armenakas MR, Dover JS, Arndt KA. Fractional laser skin resurfacing. J Drugs Dermatol. 2012;11:1274-1287.
- Lee MS. Treatment of vaginal relaxation syndrome with an erbium:YAG laser using 90 degrees and 360 degrees scanning scopes: a pilot study & short-term results. Laser Ther. 2014;23:129-138.
- Gaspar A, Brandi H, Gomez V, et al. Efficacy of erbium:YAG laser treatment compared to topical estriol treatment for symptoms of genitourinary syndrome of menopause. Lasers Surg Med. 2017;49:160-168.
- Gambacciani M, Levancini M, Cervigni M. Vaginal erbium laser: the second-generation thermotherapy for the genitourinary syndrome of menopause. Climacteric. 2015;18:757-763.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy based therapeutics for genitourinary syndrome of menopause: consensus and controversies. Lasers Surg Med. 2017;49:137-159.
- Millheiser LS, Pauls RN, Herbst SJ, et al. Radiofrequency treatment of vaginal laxity after vaginal delivery: nonsurgical vaginal tightening. J Sex Med. 2010;7:3088-3095.
- Sekiguchi Y, Utsugisawa Y, Azekosi Y, et al. Laxity of the vaginal introitus after childbirth: nonsurgical outpatient procedure for vaginal tissue restoration and improved sexual satisfaction using low-energy radiofrequency thermal therapy. J Womens Health (Larchmt). 2013;22:775-781.
- Krychman M, Rowan CG, Allan BB, et al. Effect of single-treatment, surface-cooled radiofrequency therapy on vaginal laxity and female sexual function: the VIVEVE I randomized controlled trial. J Sex Med. 2017;14:215-225.
- Alinsod RM. Transcutaneous temperature controlled radiofrequency for orgasmic dysfunction. Lasers Surg Med. 2016;48:641-645.
- Lalji S, Lozanova P. Evaluation of the safety and efficacy of a monopolar nonablative radiofrequency device for the improvement of vulvo-vaginal laxity and urinary incontinence. J Cosmet Dermatol. 2017;16:230-234.
Practice Points
- Noninvasive vaginal rejuvenation represents a growing area of cosmetic dermatology.
- Radiofrequency and ablative laser devices have demonstrated promising results in treating vaginal laxity and genitourinary syndrome of menopause, but US Food and Drug Administration approval has yet to be obtained.
Update on Acne Scar Treatment
Acne vulgaris is prevalent in the general population, with 40 to 50 million affected individuals in the United States.1 Severe inflammation and injury can lead to disfiguring scarring, which has a considerable impact on quality of life.2 Numerous therapeutic options for acne scarring are available, including microneedling with and without platelet-rich plasma (PRP), lasers, chemical peels, and dermal fillers, with different modalities suited to individual patients and scar characteristics. This article reviews updates in treatment options for acne scarring.
Microneedling
Microneedling, also known as percutaneous collagen induction or collagen induction therapy, has been utilized for more than 2 decades.3 Dermatologic indications for microneedling include skin rejuvenation,4-6 atrophic acne scarring,7-9 and androgenic alopecia.10,11 Microneedling also has been used to enhance skin penetration of topically applied drugs.12-15 Fernandes16 described percutaneous collagen induction as the skin’s natural response to injury. Microneedling creates small wounds as fine needles puncture the epidermis and dermis, resulting in a cascade of growth factors that lead to tissue proliferation, regeneration, and a collagen remodeling phase that can last for several months.8,16
Microneedling has gained popularity in the treatment of acne scarring.7 Alam et al9 conducted a split-face randomized clinical trial (RCT) to evaluate acne scarring after 3 microneedling sessions performed at 2-week intervals. Twenty participants with acne scarring on both sides of the face were enrolled in the study and one side of the face was randomized for treatment. Participants had at least two 5×5-cm areas of acne scarring graded as 2 (moderately atrophic scars) to 4 (hyperplastic or papular scars) on the quantitative Global Acne Scarring Classification system. A roller device with a 1.0-mm depth was used on participants with fine, less sebaceous skin and a 2.0-mm device for all others. Two blinded investigators assessed acne scars at baseline and at 3 and 6 months after treatment. Scar improvement was measured using the quantitative Goodman and Baron scale, which provides a score according to type and number of scars.17 Mean scar scores were significantly reduced at 6 months compared to baseline on the treatment side (P=.03) but not the control side. Participants experienced minimal pain associated with microneedling therapy, rated 1.08 of 10, and adverse effects were limited to mild transient erythema and edema.9 Several other clinical trials have demonstrated clinical improvements with microneedling.18-20
The benefits of microneedling also have been observed on a histologic level. One group of investigators explored the effects of microneedling on dermal collagen in the treatment of various atrophic acne scars in 10 participants.7 After 6 treatment sessions performed at 2-week intervals, dermal collagen was assessed via punch biopsy. A roller device with a needle depth of 1.5 mm was used for all patients. At 1 month after treatment compared to baseline, mean (SD) levels of type I collagen were significantly increased (67.1% [4.2%] vs 70.4% [5.4%]; P=.01) as well as at 3 months after treatment compared to baseline for type III collagen (61.4% [3.6%] vs 74.3% [7.4%]; P=.01), type VII collagen (15.2% [2.1%] vs 21.3% [1.2%]; P=.03), and newly synthesized collagen (14.5% [5.8%] vs 19.5% [3.2%]; P=.02). Total elastin levels were significantly decreased at 3 months after treatment compared to baseline (51.3% [6.7%] vs 46.9% [4.3%]; P=.04). Adverse effects were limited to transient erythema and edema.7
Microneedling With Platelet-Rich Plasma
Microneedling has been combined with platelet-rich plasma (PRP) in the treatment of atrophic acne scars.21 In addition to inducing new collagen synthesis, microneedling aids in the absorption of PRP, an autologous concentrate of platelets that is obtained through peripheral venipuncture. The concentrate is centrifuged into 3 layers: (1) platelet-poor plasma, (2) PRP, and (3) erythrocytes.22 Platelet-rich plasma contains growth factors such as platelet-derived growth factor, transforming growth factor (TGF), and vascular endothelial growth factor, as well as cell adhesion molecules.22,23 The application of PRP is thought to result in upregulated protein synthesis, greater collagen remodeling, and accelerated wound healing.21
Several studies have shown that the addition of PRP to microneedling can improve treatment outcome (Table 1).24-27 Severity of acne scarring can be improved, such as reduced scar depth, by using both modalities synergistically (Figure).24 Asif et al26 compared microneedling with PRP to microneedling with distilled water in the treatment of 50 patients with atrophic acne scars graded 2 to 4 (mild to severe acne scarring) on the Goodman’s Qualitative classification and equal Goodman’s Qualitative and Quantitative scores on both halves of the face.17,28 The right side of the face was treated with a 1.5-mm microneedling roller with intradermal and topical PRP, while the left side was treated with distilled water (placebo) delivered intradermally. Patients underwent 3 treatment sessions at 1-month intervals. The area treated with microneedling and PRP showed a 62.20% improvement from baseline after 3 treatments, while the placebo-treated area showed a 45.84% improvement on the Goodman and Baron quantitative scale.26
Chawla25 compared microneedling with topical PRP to microneedling with topical vitamin C in a split-face study of 30 participants with atrophic acne scarring graded 2 to 4 on the Goodman and Baron scale. A 1.5-mm roller device was used. Patients underwent 4 treatment sessions at 1-month intervals, and treatment efficacy was evaluated using the qualitative Goodman and Baron scale.28 Participants experienced positive outcomes overall with both treatments. Notably, 18.5% (5/27) on the microneedling with PRP side demonstrated excellent response compared to 7.4% (2/27) on the microneedling with vitamin C side.25
Laser Treatment
Laser skin resurfacing has shown to be efficacious in the treatment of both acne vulgaris and acne scarring. Various lasers have been utilized, including nonfractional CO2 and erbium-doped:YAG (Er:YAG) lasers, as well as ablative fractional lasers (AFLs) and nonablative fractional lasers (NAFLs).29
One retrospective study of 58 patients compared the use of 2 resurfacing lasers—10,600-nm nonfractional CO2 and 2940-nm Er:YAG—and 2 fractional lasers—1550-nm NAFL and 10,600-nm AFL—in the treatment of atrophic acne scars.29 A retrospective photographic analysis was performed by 6 blinded dermatologists to evaluate clinical improvement on a scale of 0 (no improvement) to 10 (excellent improvement). The mean improvement scores of the CO2, Er:YAG, AFL, and NAFL groups were 6.0, 5.8, 2.2, and 5.2, respectively, and the mean number of treatments was 1.6, 1.1, 4.0, and 3.4, respectively. Thus, patients in the fractional laser groups required more treatments; however, those in the resurfacing laser groups had longer recovery times, pain, erythema, and postinflammatory hyperpigmentation. The investigators concluded that 3 consecutive AFL treatments could be as effective as a single resurfacing treatment with lower risk for complications.29
A split-face RCT compared the use of the fractional Er:YAG laser on one side of the face to microneedling with a 2.0-mm needle on the other side for treatment of atrophic acne scars.30 Thirty patients underwent 5 treatments at 1-month intervals. At 3-month follow-up, the areas treated with the Er:YAG laser showed 70% improvement from baseline compared to 30% improvement in the areas treated with microneedling (P<.001). Histologically, the Er:YAG laser showed a higher increase in dermal collagen than microneedling (P<.001). Furthermore, the Er:YAG laser yielded significantly lower pain scores (P<.001); however, patients reported higher rates of erythema, swelling, superficial crusting, and total downtime.30
Lasers With PRP
More recent studies have examined the use of laser therapy in addition to PRP for the treatment of acne scars (Table 2).31-34 Abdel Aal et al33 examined the use of the ablative fractional CO2 laser with and without intradermal PRP in a split-face study of 30 participants with various types of acne scarring (ie, boxcar, ice pick, and rolling scars). Participants underwent 2 treatments at 4-week intervals. Evaluations were performed by 2 blinded dermatologists 6 months after the final laser treatment using the qualitative Goodman and Baron scale.28 Both treatments yielded improvement in scarring, but the PRP-treated side showed shorter durations of postprocedure erythema (P=.0052) as well as higher patient satisfaction scores (P<.001) than laser therapy alone.33
In another split-face study, Gawdat et al32 examined combination treatment with the ablative fractional CO2 laser and PRP in 30 participants with atrophic acne scars graded 2 to 4 on the qualitative Goodman and Baron scale.28 Participants were randomized to 2 different treatment groups: In group 1, half of the face was treated with the fractional CO2 laser and intradermal PRP, while the other half was treated with fractional CO2 laser and intradermal saline. In group 2, half of the face was treated with fractional CO2 laser and intradermal PRP, while the other half was treated with fractional CO2 laser and topical PRP. All patients underwent 3 treatment sessions at 1-month intervals with assessment occurring a 6-month follow-up using the qualitative Goodman and Baron Scale.28 In all participants, areas treated with the combined laser and PRP showed significant improvement in scarring (P=.03) and reduced recovery time (P=.02) compared to areas treated with laser therapy only. Patients receiving intradermal or topical PRP showed no statistically significant differences in improvement of scarring or recovery time; however, areas treated with topical PRP had significantly lower pain levels (P=.005).32
Lee et al31 conducted a split-face study of 14 patients with moderate to severe acne scarring treated with an ablative fractional CO2 laser followed by intradermal PRP or intradermal normal saline injections. Patients underwent 2 treatment sessions at 4-week intervals. Photographs taken at baseline and 4 months posttreatment were evaluated by 2 blinded dermatologists for clinical improvement using a quartile grading system. Erythema was assessed using a skin color measuring device. A blinded dermatologist assessed patients for adverse events. At 4-month follow-up, mean (SD) clinical improvement on the side receiving intradermal PRP was significantly better than the control side (2.7 [0.7] vs 2.3 [0.5]; P=.03). Erythema on posttreatment day 4 was significantly less on the side treated with PRP (P=.01). No adverse events were reported.31
Another split-face study compared the use of intradermal PRP to intradermal normal saline following fractional CO2 laser treatment.34 Twenty-five participants with moderate to severe acne scars completed 2 treatment sessions at 4-week intervals. Additionally, skin biopsies were collected to evaluate collagen production using immunohistochemistry, quantitative polymerase chain reaction, and western blot techniques. Experimental fibroblasts and keratinocytes were isolated and cultured. The cultures were irradiated with a fractional CO2 laser and treated with PRP or platelet-poor plasma. Cultures were evaluated at 30 minutes, 24 hours, and 48 hours. Participants reported 75% improvement of acne scarring from baseline in the side treated with PRP compared to 50% improvement of acne scarring from baseline in the control group (P<.001). On days 7 and 84, participants reported greater improvement on the side treated with PRP (P=.03 and P=.02, respectively). On day 28, skin biopsy evaluation yielded higher levels of TGF-β1 (P=.02), TGF-β3 (P=.004), c-myc (P=.004), type I collagen (P=.03), and type III collagen (P=.03) on the PRP-treated side compared to the control side. Transforming growth factor β increases collagen and fibroblast production, while c-myc leads to cell cycle progression.35-37 Similarly, TGF-β1, TGF-β3, types I andIII collagen, and p-Akt were increased in all cultures treated with PRP and platelet-poor plasma in a dose-dependent manner.34 p-Akt is thought to regulate wound healing38; however, PRP-treated keratinocytes yielded increased epidermal growth factor receptor and decreased keratin-16 at 48 hours, which suggests PRP plays a role in increasing epithelization and reducing laser-induced keratinocyte damage.39 Adverse effects (eg, erythema, edema, oozing) were less frequent in the PRP-treated side.34
Chemical Peels
Chemical peels are widely used in the treatment of acne scarring.40 Peels improve scarring through destruction of the epidermal and/or dermal layers, leading to skin exfoliation, rejuvenation, and remodeling. Superficial peeling agents, which extend to the dermoepidermal junction, include resorcinol, tretinoin, glycolic acid, lactic acid, salicylic acid, and trichloroacetic acid (TCA) 10% to 35%.41 Medium-depth peeling agents extend to the upper reticular dermis and include phenol, TCA 35% to 50%, and Jessner solution (resorcinol, lactic acid, and salicylic acid in ethanol) followed by TCA 35%.41 Finally, the effects of deep peeling agents reach the mid reticular dermis and include the Baker-Gordon or Litton phenol formulas.41 Deep peels are associated with higher rates of adverse outcomes including infection, dyschromia, and scarring.41,42
An RCT was performed to evaluate the use of a deep phenol 60% peel compared to microneedling with a 1.5-mm roller device plus a TCA 20% peel in the treatment of atrophic acne scars.43 Twenty-four patients were randomly and evenly assigned to both treatment groups. The phenol group underwent a single treatment session, while the microneedling plus TCA group underwent 4 treatment sessions at 6-week intervals. Both groups were instructed to use daily topical tretinoin and hydroquinone 2% in the 2 weeks prior to treatment. Posttreatment results were evaluated using a quartile grading scale. Scarring improved from baseline by 75.12% (P<.001) in the phenol group and 69.43% (P<.001) in the microneedling plus TCA group, with no significant difference between groups. Adverse effects in the phenol group included erythema and hyperpigmentation, while adverse events in the microneedling plus TCA group included transient pain, edema, erythema, and desquamation.43
Another study compared the use of a TCA 15% peel with microneedling to PRP with microneedling and microneedling alone in the treatment of atrophic acne scars.44 Twenty-four patients were randomly assigned to the 3 treatment groups (8 to each group) and underwent 6 treatment sessions with 2-week intervals. A roller device with a 1.5-mm needle was used for microneedling. Microneedling plus TCA and microneedling plus PRP were significantly more effective than microneedling alone (P=.011 and P=.015, respectively); however, the TCA 15% peel with microneedling resulted in the largest increase in epidermal thickening. The investigators concluded that combined use of a TCA 15% peel and microneedling was the most effective in treating atrophic acne scarring.44
Dermal Fillers
Dermal or subcutaneous fillers are used to increase volume in depressed scars and stimulate the skin’s natural production.45 Tissue augmentation methods commonly are used for larger rolling acne scars. Options for filler materials include autologous fat, bovine, or human collagen derivatives; hyaluronic acid; and polymethyl methacrylate microspheres with collagen.45 Newer fillers are formulated with lidocaine to decrease pain associated with the procedure.46 Hyaluronic acid fillers provide natural volume correction and have limited potential to elicit an immune response due to their derivation from bacterial fermentation. Fillers using polymethyl methacrylate microspheres with collagen are permanent and effective, which may lead to reduced patient costs; however, they often are not a first choice for treatment.45,46 Furthermore, if dermal fillers consist of bovine collagen, it is necessary to perform skin testing for allergy prior to use. Autologous fat transfer also has become popular for treatment of acne scarring, especially because there is no risk of allergic reaction, as the patient’s own fat is used for correction.46 However, this method requires a high degree of skill, and results are unpredictable, generally lasting from 6 months to several years.
Therapies on the horizon include autologous cell therapy. A multicenter, double-blinded, placebo-controlled RCT examined the use of an autologous fibroblast filler in the treatment of bilateral, depressed, and distensible acne scars that were graded as moderate to severe.47 Autologous fat fibroblasts were harvested from full-thickness postauricular punch biopsies. In this split-face study, 99 participants were treated with an intradermal autologous fibroblast filler on one cheek and a protein-free cell-culture medium on the contralateral cheek. Participants received an average of 5.9 mL of both autologous fat fibroblasts and cell-culture medium over 3 treatment sessions at 2-week intervals. The autologous fat fibroblasts were associated with greater improvement compared to cell-culture medium based on participant (43% vs 18%), evaluator (59% vs 42%), and independent photographic viewer’s assessment.47
Conclusion
Acne scarring is a burden affecting millions of Americans. It often has a negative impact on quality of life and can lead to low self-esteem in patients. Numerous trials have indicated that microneedling is beneficial in the treatment of acne scarring, and emerging evidence indicates that the addition of PRP provides measurable benefits. Similarly, the addition of PRP to laser therapy may reduce recovery time as well as the commonly associated adverse events of erythema and pain. Chemical peels provide the advantage of being easily and efficiently performed in the office setting. Finally, the wide range of available dermal fillers can be tailored to treat specific types of acne scars. Autologous dermal fillers recently have been used and show promising benefits. It is important to consider desired outcome, cost, and adverse events when discussing therapeutic options for acne scarring with patients. The numerous therapeutic options warrant further research and well-designed RCTs to ensure optimal patient outcomes.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39(2, pt 3):S34-S37.
- Yazici K, Baz K, Yazici AE, et al. Disease-specific quality of life is associated with anxiety and depression in patients with acne. J Eur Acad Dermatol Venereol. 2004;18:435-439.
- Orentreich DS, Orentreich N. Subcutaneous incisionless (subcision) surgery for the correction of depressed scars and wrinkles. Dermatol Surg. 1995;21:543-549.
- Fabbrocini G, De Padova M, De Vita V, et al. Periorbital wrinkles treatment using collagen induction therapy. Surg Cosmet Dermatol. 2009;1:106-111.
- Fabbrocini G, De Vita V, Pastore F, et al. Collagen induction therapy for the treatment of upper lip wrinkles. J Dermatol Treat. 2012;23:144-152.
- Fabbrocini G, De Vita V, Di Costanzo L, et al. Skin needling in the treatment of the aging neck. Skinmed. 2011;9:347-351.
- El-Domyati M, Barakat M, Awad S, et al. Microneedling therapy for atrophic acne scars: an objective evaluation. J Clin Aesthet Dermatol. 2015;8:36-42.
- Fabbrocini G, Fardella N, Monfrecola A, et al. Acne scarring treatment using skin needling. Clin Exp Dermatol. 2009;34:874-879.
- Alam M, Han S, Pongprutthipan M, et al. Efficacy of a needling device for the treatment of acne scars: a randomized clinical trial. JAMA Dermatol. 2014;150:844-849.
- Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichology. 2013;5:6-11.
- Dhurat R, Mathapati S. Response to microneedling treatment in men with androgenetic alopecia who failed to respond to conventional therapy. Indian J Dermatol. 2015;60:260-263.
- Fabbrocini G, De Vita V, Fardella N, et al. Skin needling to enhance depigmenting serum penetration in the treatment of melasma [published online April 7, 2011]. Plast Surg Int. 2011;2011:158241.
- Bariya SH, Gohel MC, Mehta TA, et al. Microneedles: an emerging transdermal drug delivery system. J Pharm Pharmacol. 2012;64:11-29.
- Fabbrocini G, De Vita V, Izzo R, et al. The use of skin needling for the delivery of a eutectic mixture of local anesthetics. G Ital Dermatol Venereol. 2014;149:581-585.
- De Vita V. How to choose among the multiple options to enhance the penetration of topically applied methyl aminolevulinate prior to photodynamic therapy [published online February 22, 2018]. Photodiagnosis Photodyn Ther. doi:10.1016/j.pdpdt.2018.02.014.
- Fernandes D. Minimally invasive percutaneous collagen induction. Oral Maxillofac Surg Clin North Am. 2005;17:51-63.
- Goodman GJ, Baron JA. Postacne scarring—a quantitative global scarring grading system. J Cosmet Dermatol. 2006;5:48-52.
- Majid I. Microneedling therapy in atrophic facial scars: an objective assessment. J Cutan Aesthet Surg. 2009;2:26-30.
- Dogra S, Yadav S, Sarangal R. Microneedling for acne scars in Asian skin type: an effective low cost treatment modality. J Cosmet Dermatol. 2014;13:180-187.
- Fabbrocini G, De Vita V, Monfrecola A, et al. Percutaneous collagen induction: an effective and safe treatment for post-acne scarring in different skin phototypes. J Dermatol Treat. 2014;25:147-152.
- Hashim PW, Levy Z, Cohen JL, et al. Microneedling therapy with and without platelet-rich plasma. Cutis. 2017;99:239-242.
- Wang HL, Avila G. Platelet rich plasma: myth or reality? Eur J Dent. 2007;1:192-194.
- Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62:489-496.
- Fabbrocini G, De Vita V, Pastore F, et al. Combined use of skin needling and platelet-rich plasma in acne scarring treatment. Cosmet Dermatol. 2011;24:177-183.
- Chawla S. Split face comparative study of microneedling with PRP versus microneedling with vitamin C in treating atrophic post acne scars. J Cutan Aesthet Surg. 2014;7:209-212.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study. J Cosmet Dermatol. 2016;15:434-443.
- Ibrahim MK, Ibrahim SM, Salem AM. Skin microneedling plus platelet-rich plasma versus skin microneedling alone in the treatment of atrophic post acne scars: a split face comparative study. J Dermatolog Treat. 2018;29:281-286.
- Goodman GJ, Baron JA. Postacne scarring: a qualitative global scarring grading system. Dermatol Surg. 2006;32:1458-1466.
- You H, Kim D, Yoon E, et al. Comparison of four different lasers for acne scars: resurfacing and fractional lasers. J Plast Reconstr Aesthet Surg. 2016;69:E87-E95.
- Osman MA, Shokeir HA, Fawzy MM. Fractional erbium-doped yttrium aluminum garnet laser versus microneedling in treatment of atrophic acne scars: a randomized split-face clinical study. Dermatol Surg. 2017;43(suppl 1):S47-S56.
- Lee JW, Kim BJ, Kim MN, et al. The efficacy of autologous platelet rich plasma combined with ablative carbon dioxide fractional resurfacing for acne scars: a simultaneous split-face trial. Dermatol Surg. 2011;37:931-938.
- Gawdat HI, Hegazy RA, Fawzy MM, et al. Autologous platelet rich plasma: topical versus intradermal after fractional ablative carbon dioxide laser treatment of atrophic acne scars. Dermatol Surg. 2014;40:152-161.
- Abdel Aal AM, Ibrahim IM, Sami NA, et al. Evaluation of autologous platelet rich plasma plus ablative carbon dioxide fractional laser in the treatment of acne scars. J Cosmet Laser Ther. 2018;20:106-113.
- Min S, Yoon JY, Park SY, et al. Combination of platelet rich plasma in fractional carbon dioxide laser treatment increased clinical efficacy of for acne scar by enhancement of collagen production and modulation of laser-induced inflammation. Lasers Surg Med. 2018;50:302-310.
- Roberts AB, Sporn MB, Assoian RK, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986;83:4167-4171.
- Schmidt EV. The role of c-myc in cellular growth control. Oncogene. 1999;18:2988-2996.
- Varga J, Rosenbloom J, Jimenez SA. Transforming growth factor beta (TGF beta) causes a persistent increase in steady-state amounts of type I and type III collagen and fibronectin mRNAs in normal human dermal fibroblasts. Biochem J. 1987;247:597-604.
- Chen J, Somanath PR, Razorenova O, et al. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med. 2005;11:1188-1196.
- Repertinger SK, Campagnaro E, Fuhrman J, et al. EGFR enhances early healing after cutaneous incisional wounding. J Invest Dermatol. 2004;123:982-989.
- Landau M. Chemical peels. Clin Dermatol. 2008;26:200-208.
- Drake LA, Dinehart SM, Goltz RW, et al. Guidelines of care for chemical peeling. J Am Acad Dermatol. 1995;33:497-503.
- Meaike JD, Agrawal N, Chang D, et al. Noninvasive facial rejuvenation. part 3: physician-directed-lasers, chemical peels, and other noninvasive modalities. Semin Plast Surg. 2016;30:143-150.
- Leheta TM, Abdel Hay RM, El Garem YF. Deep peeling using phenol versus percutaneous collagen induction combined with trichloroacetic acid 20% in atrophic post-acne scars; a randomized controlled trial.J Dermatol Treat. 2014;25:130-136.
- El-Domyati M, Abdel-Wahab H, Hossam A. Microneedling combined with platelet-rich plasma or trichloroacetic acid peeling for management of acne scarring: a split-face clinical and histologic comparison.J Cosmet Dermatol. 2018;17:73-83.
- Hession MT, Graber EM. Atrophic acne scarring: a review of treatment options. J Clin Aesthet Dermatol. 2015;8:50-58.
- Dayan SH, Bassichis BA. Facial dermal fillers: selection of appropriate products and techniques. Aesthet Surg J. 2008;28:335-347.
- Munavalli GS, Smith S, Maslowski JM, et al. Successful treatment of depressed, distensible acne scars using autologous fibroblasts: a multi-site, prospective, double blind, placebo-controlled clinical trial. Dermatol Surg. 2013;39:1226-1236.
Acne vulgaris is prevalent in the general population, with 40 to 50 million affected individuals in the United States.1 Severe inflammation and injury can lead to disfiguring scarring, which has a considerable impact on quality of life.2 Numerous therapeutic options for acne scarring are available, including microneedling with and without platelet-rich plasma (PRP), lasers, chemical peels, and dermal fillers, with different modalities suited to individual patients and scar characteristics. This article reviews updates in treatment options for acne scarring.
Microneedling
Microneedling, also known as percutaneous collagen induction or collagen induction therapy, has been utilized for more than 2 decades.3 Dermatologic indications for microneedling include skin rejuvenation,4-6 atrophic acne scarring,7-9 and androgenic alopecia.10,11 Microneedling also has been used to enhance skin penetration of topically applied drugs.12-15 Fernandes16 described percutaneous collagen induction as the skin’s natural response to injury. Microneedling creates small wounds as fine needles puncture the epidermis and dermis, resulting in a cascade of growth factors that lead to tissue proliferation, regeneration, and a collagen remodeling phase that can last for several months.8,16
Microneedling has gained popularity in the treatment of acne scarring.7 Alam et al9 conducted a split-face randomized clinical trial (RCT) to evaluate acne scarring after 3 microneedling sessions performed at 2-week intervals. Twenty participants with acne scarring on both sides of the face were enrolled in the study and one side of the face was randomized for treatment. Participants had at least two 5×5-cm areas of acne scarring graded as 2 (moderately atrophic scars) to 4 (hyperplastic or papular scars) on the quantitative Global Acne Scarring Classification system. A roller device with a 1.0-mm depth was used on participants with fine, less sebaceous skin and a 2.0-mm device for all others. Two blinded investigators assessed acne scars at baseline and at 3 and 6 months after treatment. Scar improvement was measured using the quantitative Goodman and Baron scale, which provides a score according to type and number of scars.17 Mean scar scores were significantly reduced at 6 months compared to baseline on the treatment side (P=.03) but not the control side. Participants experienced minimal pain associated with microneedling therapy, rated 1.08 of 10, and adverse effects were limited to mild transient erythema and edema.9 Several other clinical trials have demonstrated clinical improvements with microneedling.18-20
The benefits of microneedling also have been observed on a histologic level. One group of investigators explored the effects of microneedling on dermal collagen in the treatment of various atrophic acne scars in 10 participants.7 After 6 treatment sessions performed at 2-week intervals, dermal collagen was assessed via punch biopsy. A roller device with a needle depth of 1.5 mm was used for all patients. At 1 month after treatment compared to baseline, mean (SD) levels of type I collagen were significantly increased (67.1% [4.2%] vs 70.4% [5.4%]; P=.01) as well as at 3 months after treatment compared to baseline for type III collagen (61.4% [3.6%] vs 74.3% [7.4%]; P=.01), type VII collagen (15.2% [2.1%] vs 21.3% [1.2%]; P=.03), and newly synthesized collagen (14.5% [5.8%] vs 19.5% [3.2%]; P=.02). Total elastin levels were significantly decreased at 3 months after treatment compared to baseline (51.3% [6.7%] vs 46.9% [4.3%]; P=.04). Adverse effects were limited to transient erythema and edema.7
Microneedling With Platelet-Rich Plasma
Microneedling has been combined with platelet-rich plasma (PRP) in the treatment of atrophic acne scars.21 In addition to inducing new collagen synthesis, microneedling aids in the absorption of PRP, an autologous concentrate of platelets that is obtained through peripheral venipuncture. The concentrate is centrifuged into 3 layers: (1) platelet-poor plasma, (2) PRP, and (3) erythrocytes.22 Platelet-rich plasma contains growth factors such as platelet-derived growth factor, transforming growth factor (TGF), and vascular endothelial growth factor, as well as cell adhesion molecules.22,23 The application of PRP is thought to result in upregulated protein synthesis, greater collagen remodeling, and accelerated wound healing.21
Several studies have shown that the addition of PRP to microneedling can improve treatment outcome (Table 1).24-27 Severity of acne scarring can be improved, such as reduced scar depth, by using both modalities synergistically (Figure).24 Asif et al26 compared microneedling with PRP to microneedling with distilled water in the treatment of 50 patients with atrophic acne scars graded 2 to 4 (mild to severe acne scarring) on the Goodman’s Qualitative classification and equal Goodman’s Qualitative and Quantitative scores on both halves of the face.17,28 The right side of the face was treated with a 1.5-mm microneedling roller with intradermal and topical PRP, while the left side was treated with distilled water (placebo) delivered intradermally. Patients underwent 3 treatment sessions at 1-month intervals. The area treated with microneedling and PRP showed a 62.20% improvement from baseline after 3 treatments, while the placebo-treated area showed a 45.84% improvement on the Goodman and Baron quantitative scale.26

Chawla25 compared microneedling with topical PRP to microneedling with topical vitamin C in a split-face study of 30 participants with atrophic acne scarring graded 2 to 4 on the Goodman and Baron scale. A 1.5-mm roller device was used. Patients underwent 4 treatment sessions at 1-month intervals, and treatment efficacy was evaluated using the qualitative Goodman and Baron scale.28 Participants experienced positive outcomes overall with both treatments. Notably, 18.5% (5/27) on the microneedling with PRP side demonstrated excellent response compared to 7.4% (2/27) on the microneedling with vitamin C side.25
Laser Treatment
Laser skin resurfacing has shown to be efficacious in the treatment of both acne vulgaris and acne scarring. Various lasers have been utilized, including nonfractional CO2 and erbium-doped:YAG (Er:YAG) lasers, as well as ablative fractional lasers (AFLs) and nonablative fractional lasers (NAFLs).29
One retrospective study of 58 patients compared the use of 2 resurfacing lasers—10,600-nm nonfractional CO2 and 2940-nm Er:YAG—and 2 fractional lasers—1550-nm NAFL and 10,600-nm AFL—in the treatment of atrophic acne scars.29 A retrospective photographic analysis was performed by 6 blinded dermatologists to evaluate clinical improvement on a scale of 0 (no improvement) to 10 (excellent improvement). The mean improvement scores of the CO2, Er:YAG, AFL, and NAFL groups were 6.0, 5.8, 2.2, and 5.2, respectively, and the mean number of treatments was 1.6, 1.1, 4.0, and 3.4, respectively. Thus, patients in the fractional laser groups required more treatments; however, those in the resurfacing laser groups had longer recovery times, pain, erythema, and postinflammatory hyperpigmentation. The investigators concluded that 3 consecutive AFL treatments could be as effective as a single resurfacing treatment with lower risk for complications.29
A split-face RCT compared the use of the fractional Er:YAG laser on one side of the face to microneedling with a 2.0-mm needle on the other side for treatment of atrophic acne scars.30 Thirty patients underwent 5 treatments at 1-month intervals. At 3-month follow-up, the areas treated with the Er:YAG laser showed 70% improvement from baseline compared to 30% improvement in the areas treated with microneedling (P<.001). Histologically, the Er:YAG laser showed a higher increase in dermal collagen than microneedling (P<.001). Furthermore, the Er:YAG laser yielded significantly lower pain scores (P<.001); however, patients reported higher rates of erythema, swelling, superficial crusting, and total downtime.30
Lasers With PRP
More recent studies have examined the use of laser therapy in addition to PRP for the treatment of acne scars (Table 2).31-34 Abdel Aal et al33 examined the use of the ablative fractional CO2 laser with and without intradermal PRP in a split-face study of 30 participants with various types of acne scarring (ie, boxcar, ice pick, and rolling scars). Participants underwent 2 treatments at 4-week intervals. Evaluations were performed by 2 blinded dermatologists 6 months after the final laser treatment using the qualitative Goodman and Baron scale.28 Both treatments yielded improvement in scarring, but the PRP-treated side showed shorter durations of postprocedure erythema (P=.0052) as well as higher patient satisfaction scores (P<.001) than laser therapy alone.33
In another split-face study, Gawdat et al32 examined combination treatment with the ablative fractional CO2 laser and PRP in 30 participants with atrophic acne scars graded 2 to 4 on the qualitative Goodman and Baron scale.28 Participants were randomized to 2 different treatment groups: In group 1, half of the face was treated with the fractional CO2 laser and intradermal PRP, while the other half was treated with fractional CO2 laser and intradermal saline. In group 2, half of the face was treated with fractional CO2 laser and intradermal PRP, while the other half was treated with fractional CO2 laser and topical PRP. All patients underwent 3 treatment sessions at 1-month intervals with assessment occurring a 6-month follow-up using the qualitative Goodman and Baron Scale.28 In all participants, areas treated with the combined laser and PRP showed significant improvement in scarring (P=.03) and reduced recovery time (P=.02) compared to areas treated with laser therapy only. Patients receiving intradermal or topical PRP showed no statistically significant differences in improvement of scarring or recovery time; however, areas treated with topical PRP had significantly lower pain levels (P=.005).32
Lee et al31 conducted a split-face study of 14 patients with moderate to severe acne scarring treated with an ablative fractional CO2 laser followed by intradermal PRP or intradermal normal saline injections. Patients underwent 2 treatment sessions at 4-week intervals. Photographs taken at baseline and 4 months posttreatment were evaluated by 2 blinded dermatologists for clinical improvement using a quartile grading system. Erythema was assessed using a skin color measuring device. A blinded dermatologist assessed patients for adverse events. At 4-month follow-up, mean (SD) clinical improvement on the side receiving intradermal PRP was significantly better than the control side (2.7 [0.7] vs 2.3 [0.5]; P=.03). Erythema on posttreatment day 4 was significantly less on the side treated with PRP (P=.01). No adverse events were reported.31
Another split-face study compared the use of intradermal PRP to intradermal normal saline following fractional CO2 laser treatment.34 Twenty-five participants with moderate to severe acne scars completed 2 treatment sessions at 4-week intervals. Additionally, skin biopsies were collected to evaluate collagen production using immunohistochemistry, quantitative polymerase chain reaction, and western blot techniques. Experimental fibroblasts and keratinocytes were isolated and cultured. The cultures were irradiated with a fractional CO2 laser and treated with PRP or platelet-poor plasma. Cultures were evaluated at 30 minutes, 24 hours, and 48 hours. Participants reported 75% improvement of acne scarring from baseline in the side treated with PRP compared to 50% improvement of acne scarring from baseline in the control group (P<.001). On days 7 and 84, participants reported greater improvement on the side treated with PRP (P=.03 and P=.02, respectively). On day 28, skin biopsy evaluation yielded higher levels of TGF-β1 (P=.02), TGF-β3 (P=.004), c-myc (P=.004), type I collagen (P=.03), and type III collagen (P=.03) on the PRP-treated side compared to the control side. Transforming growth factor β increases collagen and fibroblast production, while c-myc leads to cell cycle progression.35-37 Similarly, TGF-β1, TGF-β3, types I andIII collagen, and p-Akt were increased in all cultures treated with PRP and platelet-poor plasma in a dose-dependent manner.34 p-Akt is thought to regulate wound healing38; however, PRP-treated keratinocytes yielded increased epidermal growth factor receptor and decreased keratin-16 at 48 hours, which suggests PRP plays a role in increasing epithelization and reducing laser-induced keratinocyte damage.39 Adverse effects (eg, erythema, edema, oozing) were less frequent in the PRP-treated side.34
Chemical Peels
Chemical peels are widely used in the treatment of acne scarring.40 Peels improve scarring through destruction of the epidermal and/or dermal layers, leading to skin exfoliation, rejuvenation, and remodeling. Superficial peeling agents, which extend to the dermoepidermal junction, include resorcinol, tretinoin, glycolic acid, lactic acid, salicylic acid, and trichloroacetic acid (TCA) 10% to 35%.41 Medium-depth peeling agents extend to the upper reticular dermis and include phenol, TCA 35% to 50%, and Jessner solution (resorcinol, lactic acid, and salicylic acid in ethanol) followed by TCA 35%.41 Finally, the effects of deep peeling agents reach the mid reticular dermis and include the Baker-Gordon or Litton phenol formulas.41 Deep peels are associated with higher rates of adverse outcomes including infection, dyschromia, and scarring.41,42
An RCT was performed to evaluate the use of a deep phenol 60% peel compared to microneedling with a 1.5-mm roller device plus a TCA 20% peel in the treatment of atrophic acne scars.43 Twenty-four patients were randomly and evenly assigned to both treatment groups. The phenol group underwent a single treatment session, while the microneedling plus TCA group underwent 4 treatment sessions at 6-week intervals. Both groups were instructed to use daily topical tretinoin and hydroquinone 2% in the 2 weeks prior to treatment. Posttreatment results were evaluated using a quartile grading scale. Scarring improved from baseline by 75.12% (P<.001) in the phenol group and 69.43% (P<.001) in the microneedling plus TCA group, with no significant difference between groups. Adverse effects in the phenol group included erythema and hyperpigmentation, while adverse events in the microneedling plus TCA group included transient pain, edema, erythema, and desquamation.43
Another study compared the use of a TCA 15% peel with microneedling to PRP with microneedling and microneedling alone in the treatment of atrophic acne scars.44 Twenty-four patients were randomly assigned to the 3 treatment groups (8 to each group) and underwent 6 treatment sessions with 2-week intervals. A roller device with a 1.5-mm needle was used for microneedling. Microneedling plus TCA and microneedling plus PRP were significantly more effective than microneedling alone (P=.011 and P=.015, respectively); however, the TCA 15% peel with microneedling resulted in the largest increase in epidermal thickening. The investigators concluded that combined use of a TCA 15% peel and microneedling was the most effective in treating atrophic acne scarring.44
Dermal Fillers
Dermal or subcutaneous fillers are used to increase volume in depressed scars and stimulate the skin’s natural production.45 Tissue augmentation methods commonly are used for larger rolling acne scars. Options for filler materials include autologous fat, bovine, or human collagen derivatives; hyaluronic acid; and polymethyl methacrylate microspheres with collagen.45 Newer fillers are formulated with lidocaine to decrease pain associated with the procedure.46 Hyaluronic acid fillers provide natural volume correction and have limited potential to elicit an immune response due to their derivation from bacterial fermentation. Fillers using polymethyl methacrylate microspheres with collagen are permanent and effective, which may lead to reduced patient costs; however, they often are not a first choice for treatment.45,46 Furthermore, if dermal fillers consist of bovine collagen, it is necessary to perform skin testing for allergy prior to use. Autologous fat transfer also has become popular for treatment of acne scarring, especially because there is no risk of allergic reaction, as the patient’s own fat is used for correction.46 However, this method requires a high degree of skill, and results are unpredictable, generally lasting from 6 months to several years.
Therapies on the horizon include autologous cell therapy. A multicenter, double-blinded, placebo-controlled RCT examined the use of an autologous fibroblast filler in the treatment of bilateral, depressed, and distensible acne scars that were graded as moderate to severe.47 Autologous fat fibroblasts were harvested from full-thickness postauricular punch biopsies. In this split-face study, 99 participants were treated with an intradermal autologous fibroblast filler on one cheek and a protein-free cell-culture medium on the contralateral cheek. Participants received an average of 5.9 mL of both autologous fat fibroblasts and cell-culture medium over 3 treatment sessions at 2-week intervals. The autologous fat fibroblasts were associated with greater improvement compared to cell-culture medium based on participant (43% vs 18%), evaluator (59% vs 42%), and independent photographic viewer’s assessment.47
Conclusion
Acne scarring is a burden affecting millions of Americans. It often has a negative impact on quality of life and can lead to low self-esteem in patients. Numerous trials have indicated that microneedling is beneficial in the treatment of acne scarring, and emerging evidence indicates that the addition of PRP provides measurable benefits. Similarly, the addition of PRP to laser therapy may reduce recovery time as well as the commonly associated adverse events of erythema and pain. Chemical peels provide the advantage of being easily and efficiently performed in the office setting. Finally, the wide range of available dermal fillers can be tailored to treat specific types of acne scars. Autologous dermal fillers recently have been used and show promising benefits. It is important to consider desired outcome, cost, and adverse events when discussing therapeutic options for acne scarring with patients. The numerous therapeutic options warrant further research and well-designed RCTs to ensure optimal patient outcomes.
Acne vulgaris is prevalent in the general population, with 40 to 50 million affected individuals in the United States.1 Severe inflammation and injury can lead to disfiguring scarring, which has a considerable impact on quality of life.2 Numerous therapeutic options for acne scarring are available, including microneedling with and without platelet-rich plasma (PRP), lasers, chemical peels, and dermal fillers, with different modalities suited to individual patients and scar characteristics. This article reviews updates in treatment options for acne scarring.
Microneedling
Microneedling, also known as percutaneous collagen induction or collagen induction therapy, has been utilized for more than 2 decades.3 Dermatologic indications for microneedling include skin rejuvenation,4-6 atrophic acne scarring,7-9 and androgenic alopecia.10,11 Microneedling also has been used to enhance skin penetration of topically applied drugs.12-15 Fernandes16 described percutaneous collagen induction as the skin’s natural response to injury. Microneedling creates small wounds as fine needles puncture the epidermis and dermis, resulting in a cascade of growth factors that lead to tissue proliferation, regeneration, and a collagen remodeling phase that can last for several months.8,16
Microneedling has gained popularity in the treatment of acne scarring.7 Alam et al9 conducted a split-face randomized clinical trial (RCT) to evaluate acne scarring after 3 microneedling sessions performed at 2-week intervals. Twenty participants with acne scarring on both sides of the face were enrolled in the study and one side of the face was randomized for treatment. Participants had at least two 5×5-cm areas of acne scarring graded as 2 (moderately atrophic scars) to 4 (hyperplastic or papular scars) on the quantitative Global Acne Scarring Classification system. A roller device with a 1.0-mm depth was used on participants with fine, less sebaceous skin and a 2.0-mm device for all others. Two blinded investigators assessed acne scars at baseline and at 3 and 6 months after treatment. Scar improvement was measured using the quantitative Goodman and Baron scale, which provides a score according to type and number of scars.17 Mean scar scores were significantly reduced at 6 months compared to baseline on the treatment side (P=.03) but not the control side. Participants experienced minimal pain associated with microneedling therapy, rated 1.08 of 10, and adverse effects were limited to mild transient erythema and edema.9 Several other clinical trials have demonstrated clinical improvements with microneedling.18-20
The benefits of microneedling also have been observed on a histologic level. One group of investigators explored the effects of microneedling on dermal collagen in the treatment of various atrophic acne scars in 10 participants.7 After 6 treatment sessions performed at 2-week intervals, dermal collagen was assessed via punch biopsy. A roller device with a needle depth of 1.5 mm was used for all patients. At 1 month after treatment compared to baseline, mean (SD) levels of type I collagen were significantly increased (67.1% [4.2%] vs 70.4% [5.4%]; P=.01) as well as at 3 months after treatment compared to baseline for type III collagen (61.4% [3.6%] vs 74.3% [7.4%]; P=.01), type VII collagen (15.2% [2.1%] vs 21.3% [1.2%]; P=.03), and newly synthesized collagen (14.5% [5.8%] vs 19.5% [3.2%]; P=.02). Total elastin levels were significantly decreased at 3 months after treatment compared to baseline (51.3% [6.7%] vs 46.9% [4.3%]; P=.04). Adverse effects were limited to transient erythema and edema.7
Microneedling With Platelet-Rich Plasma
Microneedling has been combined with platelet-rich plasma (PRP) in the treatment of atrophic acne scars.21 In addition to inducing new collagen synthesis, microneedling aids in the absorption of PRP, an autologous concentrate of platelets that is obtained through peripheral venipuncture. The concentrate is centrifuged into 3 layers: (1) platelet-poor plasma, (2) PRP, and (3) erythrocytes.22 Platelet-rich plasma contains growth factors such as platelet-derived growth factor, transforming growth factor (TGF), and vascular endothelial growth factor, as well as cell adhesion molecules.22,23 The application of PRP is thought to result in upregulated protein synthesis, greater collagen remodeling, and accelerated wound healing.21
Several studies have shown that the addition of PRP to microneedling can improve treatment outcome (Table 1).24-27 Severity of acne scarring can be improved, such as reduced scar depth, by using both modalities synergistically (Figure).24 Asif et al26 compared microneedling with PRP to microneedling with distilled water in the treatment of 50 patients with atrophic acne scars graded 2 to 4 (mild to severe acne scarring) on the Goodman’s Qualitative classification and equal Goodman’s Qualitative and Quantitative scores on both halves of the face.17,28 The right side of the face was treated with a 1.5-mm microneedling roller with intradermal and topical PRP, while the left side was treated with distilled water (placebo) delivered intradermally. Patients underwent 3 treatment sessions at 1-month intervals. The area treated with microneedling and PRP showed a 62.20% improvement from baseline after 3 treatments, while the placebo-treated area showed a 45.84% improvement on the Goodman and Baron quantitative scale.26

Chawla25 compared microneedling with topical PRP to microneedling with topical vitamin C in a split-face study of 30 participants with atrophic acne scarring graded 2 to 4 on the Goodman and Baron scale. A 1.5-mm roller device was used. Patients underwent 4 treatment sessions at 1-month intervals, and treatment efficacy was evaluated using the qualitative Goodman and Baron scale.28 Participants experienced positive outcomes overall with both treatments. Notably, 18.5% (5/27) on the microneedling with PRP side demonstrated excellent response compared to 7.4% (2/27) on the microneedling with vitamin C side.25
Laser Treatment
Laser skin resurfacing has shown to be efficacious in the treatment of both acne vulgaris and acne scarring. Various lasers have been utilized, including nonfractional CO2 and erbium-doped:YAG (Er:YAG) lasers, as well as ablative fractional lasers (AFLs) and nonablative fractional lasers (NAFLs).29
One retrospective study of 58 patients compared the use of 2 resurfacing lasers—10,600-nm nonfractional CO2 and 2940-nm Er:YAG—and 2 fractional lasers—1550-nm NAFL and 10,600-nm AFL—in the treatment of atrophic acne scars.29 A retrospective photographic analysis was performed by 6 blinded dermatologists to evaluate clinical improvement on a scale of 0 (no improvement) to 10 (excellent improvement). The mean improvement scores of the CO2, Er:YAG, AFL, and NAFL groups were 6.0, 5.8, 2.2, and 5.2, respectively, and the mean number of treatments was 1.6, 1.1, 4.0, and 3.4, respectively. Thus, patients in the fractional laser groups required more treatments; however, those in the resurfacing laser groups had longer recovery times, pain, erythema, and postinflammatory hyperpigmentation. The investigators concluded that 3 consecutive AFL treatments could be as effective as a single resurfacing treatment with lower risk for complications.29
A split-face RCT compared the use of the fractional Er:YAG laser on one side of the face to microneedling with a 2.0-mm needle on the other side for treatment of atrophic acne scars.30 Thirty patients underwent 5 treatments at 1-month intervals. At 3-month follow-up, the areas treated with the Er:YAG laser showed 70% improvement from baseline compared to 30% improvement in the areas treated with microneedling (P<.001). Histologically, the Er:YAG laser showed a higher increase in dermal collagen than microneedling (P<.001). Furthermore, the Er:YAG laser yielded significantly lower pain scores (P<.001); however, patients reported higher rates of erythema, swelling, superficial crusting, and total downtime.30
Lasers With PRP
More recent studies have examined the use of laser therapy in addition to PRP for the treatment of acne scars (Table 2).31-34 Abdel Aal et al33 examined the use of the ablative fractional CO2 laser with and without intradermal PRP in a split-face study of 30 participants with various types of acne scarring (ie, boxcar, ice pick, and rolling scars). Participants underwent 2 treatments at 4-week intervals. Evaluations were performed by 2 blinded dermatologists 6 months after the final laser treatment using the qualitative Goodman and Baron scale.28 Both treatments yielded improvement in scarring, but the PRP-treated side showed shorter durations of postprocedure erythema (P=.0052) as well as higher patient satisfaction scores (P<.001) than laser therapy alone.33
In another split-face study, Gawdat et al32 examined combination treatment with the ablative fractional CO2 laser and PRP in 30 participants with atrophic acne scars graded 2 to 4 on the qualitative Goodman and Baron scale.28 Participants were randomized to 2 different treatment groups: In group 1, half of the face was treated with the fractional CO2 laser and intradermal PRP, while the other half was treated with fractional CO2 laser and intradermal saline. In group 2, half of the face was treated with fractional CO2 laser and intradermal PRP, while the other half was treated with fractional CO2 laser and topical PRP. All patients underwent 3 treatment sessions at 1-month intervals with assessment occurring a 6-month follow-up using the qualitative Goodman and Baron Scale.28 In all participants, areas treated with the combined laser and PRP showed significant improvement in scarring (P=.03) and reduced recovery time (P=.02) compared to areas treated with laser therapy only. Patients receiving intradermal or topical PRP showed no statistically significant differences in improvement of scarring or recovery time; however, areas treated with topical PRP had significantly lower pain levels (P=.005).32
Lee et al31 conducted a split-face study of 14 patients with moderate to severe acne scarring treated with an ablative fractional CO2 laser followed by intradermal PRP or intradermal normal saline injections. Patients underwent 2 treatment sessions at 4-week intervals. Photographs taken at baseline and 4 months posttreatment were evaluated by 2 blinded dermatologists for clinical improvement using a quartile grading system. Erythema was assessed using a skin color measuring device. A blinded dermatologist assessed patients for adverse events. At 4-month follow-up, mean (SD) clinical improvement on the side receiving intradermal PRP was significantly better than the control side (2.7 [0.7] vs 2.3 [0.5]; P=.03). Erythema on posttreatment day 4 was significantly less on the side treated with PRP (P=.01). No adverse events were reported.31
Another split-face study compared the use of intradermal PRP to intradermal normal saline following fractional CO2 laser treatment.34 Twenty-five participants with moderate to severe acne scars completed 2 treatment sessions at 4-week intervals. Additionally, skin biopsies were collected to evaluate collagen production using immunohistochemistry, quantitative polymerase chain reaction, and western blot techniques. Experimental fibroblasts and keratinocytes were isolated and cultured. The cultures were irradiated with a fractional CO2 laser and treated with PRP or platelet-poor plasma. Cultures were evaluated at 30 minutes, 24 hours, and 48 hours. Participants reported 75% improvement of acne scarring from baseline in the side treated with PRP compared to 50% improvement of acne scarring from baseline in the control group (P<.001). On days 7 and 84, participants reported greater improvement on the side treated with PRP (P=.03 and P=.02, respectively). On day 28, skin biopsy evaluation yielded higher levels of TGF-β1 (P=.02), TGF-β3 (P=.004), c-myc (P=.004), type I collagen (P=.03), and type III collagen (P=.03) on the PRP-treated side compared to the control side. Transforming growth factor β increases collagen and fibroblast production, while c-myc leads to cell cycle progression.35-37 Similarly, TGF-β1, TGF-β3, types I andIII collagen, and p-Akt were increased in all cultures treated with PRP and platelet-poor plasma in a dose-dependent manner.34 p-Akt is thought to regulate wound healing38; however, PRP-treated keratinocytes yielded increased epidermal growth factor receptor and decreased keratin-16 at 48 hours, which suggests PRP plays a role in increasing epithelization and reducing laser-induced keratinocyte damage.39 Adverse effects (eg, erythema, edema, oozing) were less frequent in the PRP-treated side.34
Chemical Peels
Chemical peels are widely used in the treatment of acne scarring.40 Peels improve scarring through destruction of the epidermal and/or dermal layers, leading to skin exfoliation, rejuvenation, and remodeling. Superficial peeling agents, which extend to the dermoepidermal junction, include resorcinol, tretinoin, glycolic acid, lactic acid, salicylic acid, and trichloroacetic acid (TCA) 10% to 35%.41 Medium-depth peeling agents extend to the upper reticular dermis and include phenol, TCA 35% to 50%, and Jessner solution (resorcinol, lactic acid, and salicylic acid in ethanol) followed by TCA 35%.41 Finally, the effects of deep peeling agents reach the mid reticular dermis and include the Baker-Gordon or Litton phenol formulas.41 Deep peels are associated with higher rates of adverse outcomes including infection, dyschromia, and scarring.41,42
An RCT was performed to evaluate the use of a deep phenol 60% peel compared to microneedling with a 1.5-mm roller device plus a TCA 20% peel in the treatment of atrophic acne scars.43 Twenty-four patients were randomly and evenly assigned to both treatment groups. The phenol group underwent a single treatment session, while the microneedling plus TCA group underwent 4 treatment sessions at 6-week intervals. Both groups were instructed to use daily topical tretinoin and hydroquinone 2% in the 2 weeks prior to treatment. Posttreatment results were evaluated using a quartile grading scale. Scarring improved from baseline by 75.12% (P<.001) in the phenol group and 69.43% (P<.001) in the microneedling plus TCA group, with no significant difference between groups. Adverse effects in the phenol group included erythema and hyperpigmentation, while adverse events in the microneedling plus TCA group included transient pain, edema, erythema, and desquamation.43
Another study compared the use of a TCA 15% peel with microneedling to PRP with microneedling and microneedling alone in the treatment of atrophic acne scars.44 Twenty-four patients were randomly assigned to the 3 treatment groups (8 to each group) and underwent 6 treatment sessions with 2-week intervals. A roller device with a 1.5-mm needle was used for microneedling. Microneedling plus TCA and microneedling plus PRP were significantly more effective than microneedling alone (P=.011 and P=.015, respectively); however, the TCA 15% peel with microneedling resulted in the largest increase in epidermal thickening. The investigators concluded that combined use of a TCA 15% peel and microneedling was the most effective in treating atrophic acne scarring.44
Dermal Fillers
Dermal or subcutaneous fillers are used to increase volume in depressed scars and stimulate the skin’s natural production.45 Tissue augmentation methods commonly are used for larger rolling acne scars. Options for filler materials include autologous fat, bovine, or human collagen derivatives; hyaluronic acid; and polymethyl methacrylate microspheres with collagen.45 Newer fillers are formulated with lidocaine to decrease pain associated with the procedure.46 Hyaluronic acid fillers provide natural volume correction and have limited potential to elicit an immune response due to their derivation from bacterial fermentation. Fillers using polymethyl methacrylate microspheres with collagen are permanent and effective, which may lead to reduced patient costs; however, they often are not a first choice for treatment.45,46 Furthermore, if dermal fillers consist of bovine collagen, it is necessary to perform skin testing for allergy prior to use. Autologous fat transfer also has become popular for treatment of acne scarring, especially because there is no risk of allergic reaction, as the patient’s own fat is used for correction.46 However, this method requires a high degree of skill, and results are unpredictable, generally lasting from 6 months to several years.
Therapies on the horizon include autologous cell therapy. A multicenter, double-blinded, placebo-controlled RCT examined the use of an autologous fibroblast filler in the treatment of bilateral, depressed, and distensible acne scars that were graded as moderate to severe.47 Autologous fat fibroblasts were harvested from full-thickness postauricular punch biopsies. In this split-face study, 99 participants were treated with an intradermal autologous fibroblast filler on one cheek and a protein-free cell-culture medium on the contralateral cheek. Participants received an average of 5.9 mL of both autologous fat fibroblasts and cell-culture medium over 3 treatment sessions at 2-week intervals. The autologous fat fibroblasts were associated with greater improvement compared to cell-culture medium based on participant (43% vs 18%), evaluator (59% vs 42%), and independent photographic viewer’s assessment.47
Conclusion
Acne scarring is a burden affecting millions of Americans. It often has a negative impact on quality of life and can lead to low self-esteem in patients. Numerous trials have indicated that microneedling is beneficial in the treatment of acne scarring, and emerging evidence indicates that the addition of PRP provides measurable benefits. Similarly, the addition of PRP to laser therapy may reduce recovery time as well as the commonly associated adverse events of erythema and pain. Chemical peels provide the advantage of being easily and efficiently performed in the office setting. Finally, the wide range of available dermal fillers can be tailored to treat specific types of acne scars. Autologous dermal fillers recently have been used and show promising benefits. It is important to consider desired outcome, cost, and adverse events when discussing therapeutic options for acne scarring with patients. The numerous therapeutic options warrant further research and well-designed RCTs to ensure optimal patient outcomes.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39(2, pt 3):S34-S37.
- Yazici K, Baz K, Yazici AE, et al. Disease-specific quality of life is associated with anxiety and depression in patients with acne. J Eur Acad Dermatol Venereol. 2004;18:435-439.
- Orentreich DS, Orentreich N. Subcutaneous incisionless (subcision) surgery for the correction of depressed scars and wrinkles. Dermatol Surg. 1995;21:543-549.
- Fabbrocini G, De Padova M, De Vita V, et al. Periorbital wrinkles treatment using collagen induction therapy. Surg Cosmet Dermatol. 2009;1:106-111.
- Fabbrocini G, De Vita V, Pastore F, et al. Collagen induction therapy for the treatment of upper lip wrinkles. J Dermatol Treat. 2012;23:144-152.
- Fabbrocini G, De Vita V, Di Costanzo L, et al. Skin needling in the treatment of the aging neck. Skinmed. 2011;9:347-351.
- El-Domyati M, Barakat M, Awad S, et al. Microneedling therapy for atrophic acne scars: an objective evaluation. J Clin Aesthet Dermatol. 2015;8:36-42.
- Fabbrocini G, Fardella N, Monfrecola A, et al. Acne scarring treatment using skin needling. Clin Exp Dermatol. 2009;34:874-879.
- Alam M, Han S, Pongprutthipan M, et al. Efficacy of a needling device for the treatment of acne scars: a randomized clinical trial. JAMA Dermatol. 2014;150:844-849.
- Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichology. 2013;5:6-11.
- Dhurat R, Mathapati S. Response to microneedling treatment in men with androgenetic alopecia who failed to respond to conventional therapy. Indian J Dermatol. 2015;60:260-263.
- Fabbrocini G, De Vita V, Fardella N, et al. Skin needling to enhance depigmenting serum penetration in the treatment of melasma [published online April 7, 2011]. Plast Surg Int. 2011;2011:158241.
- Bariya SH, Gohel MC, Mehta TA, et al. Microneedles: an emerging transdermal drug delivery system. J Pharm Pharmacol. 2012;64:11-29.
- Fabbrocini G, De Vita V, Izzo R, et al. The use of skin needling for the delivery of a eutectic mixture of local anesthetics. G Ital Dermatol Venereol. 2014;149:581-585.
- De Vita V. How to choose among the multiple options to enhance the penetration of topically applied methyl aminolevulinate prior to photodynamic therapy [published online February 22, 2018]. Photodiagnosis Photodyn Ther. doi:10.1016/j.pdpdt.2018.02.014.
- Fernandes D. Minimally invasive percutaneous collagen induction. Oral Maxillofac Surg Clin North Am. 2005;17:51-63.
- Goodman GJ, Baron JA. Postacne scarring—a quantitative global scarring grading system. J Cosmet Dermatol. 2006;5:48-52.
- Majid I. Microneedling therapy in atrophic facial scars: an objective assessment. J Cutan Aesthet Surg. 2009;2:26-30.
- Dogra S, Yadav S, Sarangal R. Microneedling for acne scars in Asian skin type: an effective low cost treatment modality. J Cosmet Dermatol. 2014;13:180-187.
- Fabbrocini G, De Vita V, Monfrecola A, et al. Percutaneous collagen induction: an effective and safe treatment for post-acne scarring in different skin phototypes. J Dermatol Treat. 2014;25:147-152.
- Hashim PW, Levy Z, Cohen JL, et al. Microneedling therapy with and without platelet-rich plasma. Cutis. 2017;99:239-242.
- Wang HL, Avila G. Platelet rich plasma: myth or reality? Eur J Dent. 2007;1:192-194.
- Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62:489-496.
- Fabbrocini G, De Vita V, Pastore F, et al. Combined use of skin needling and platelet-rich plasma in acne scarring treatment. Cosmet Dermatol. 2011;24:177-183.
- Chawla S. Split face comparative study of microneedling with PRP versus microneedling with vitamin C in treating atrophic post acne scars. J Cutan Aesthet Surg. 2014;7:209-212.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study. J Cosmet Dermatol. 2016;15:434-443.
- Ibrahim MK, Ibrahim SM, Salem AM. Skin microneedling plus platelet-rich plasma versus skin microneedling alone in the treatment of atrophic post acne scars: a split face comparative study. J Dermatolog Treat. 2018;29:281-286.
- Goodman GJ, Baron JA. Postacne scarring: a qualitative global scarring grading system. Dermatol Surg. 2006;32:1458-1466.
- You H, Kim D, Yoon E, et al. Comparison of four different lasers for acne scars: resurfacing and fractional lasers. J Plast Reconstr Aesthet Surg. 2016;69:E87-E95.
- Osman MA, Shokeir HA, Fawzy MM. Fractional erbium-doped yttrium aluminum garnet laser versus microneedling in treatment of atrophic acne scars: a randomized split-face clinical study. Dermatol Surg. 2017;43(suppl 1):S47-S56.
- Lee JW, Kim BJ, Kim MN, et al. The efficacy of autologous platelet rich plasma combined with ablative carbon dioxide fractional resurfacing for acne scars: a simultaneous split-face trial. Dermatol Surg. 2011;37:931-938.
- Gawdat HI, Hegazy RA, Fawzy MM, et al. Autologous platelet rich plasma: topical versus intradermal after fractional ablative carbon dioxide laser treatment of atrophic acne scars. Dermatol Surg. 2014;40:152-161.
- Abdel Aal AM, Ibrahim IM, Sami NA, et al. Evaluation of autologous platelet rich plasma plus ablative carbon dioxide fractional laser in the treatment of acne scars. J Cosmet Laser Ther. 2018;20:106-113.
- Min S, Yoon JY, Park SY, et al. Combination of platelet rich plasma in fractional carbon dioxide laser treatment increased clinical efficacy of for acne scar by enhancement of collagen production and modulation of laser-induced inflammation. Lasers Surg Med. 2018;50:302-310.
- Roberts AB, Sporn MB, Assoian RK, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986;83:4167-4171.
- Schmidt EV. The role of c-myc in cellular growth control. Oncogene. 1999;18:2988-2996.
- Varga J, Rosenbloom J, Jimenez SA. Transforming growth factor beta (TGF beta) causes a persistent increase in steady-state amounts of type I and type III collagen and fibronectin mRNAs in normal human dermal fibroblasts. Biochem J. 1987;247:597-604.
- Chen J, Somanath PR, Razorenova O, et al. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med. 2005;11:1188-1196.
- Repertinger SK, Campagnaro E, Fuhrman J, et al. EGFR enhances early healing after cutaneous incisional wounding. J Invest Dermatol. 2004;123:982-989.
- Landau M. Chemical peels. Clin Dermatol. 2008;26:200-208.
- Drake LA, Dinehart SM, Goltz RW, et al. Guidelines of care for chemical peeling. J Am Acad Dermatol. 1995;33:497-503.
- Meaike JD, Agrawal N, Chang D, et al. Noninvasive facial rejuvenation. part 3: physician-directed-lasers, chemical peels, and other noninvasive modalities. Semin Plast Surg. 2016;30:143-150.
- Leheta TM, Abdel Hay RM, El Garem YF. Deep peeling using phenol versus percutaneous collagen induction combined with trichloroacetic acid 20% in atrophic post-acne scars; a randomized controlled trial.J Dermatol Treat. 2014;25:130-136.
- El-Domyati M, Abdel-Wahab H, Hossam A. Microneedling combined with platelet-rich plasma or trichloroacetic acid peeling for management of acne scarring: a split-face clinical and histologic comparison.J Cosmet Dermatol. 2018;17:73-83.
- Hession MT, Graber EM. Atrophic acne scarring: a review of treatment options. J Clin Aesthet Dermatol. 2015;8:50-58.
- Dayan SH, Bassichis BA. Facial dermal fillers: selection of appropriate products and techniques. Aesthet Surg J. 2008;28:335-347.
- Munavalli GS, Smith S, Maslowski JM, et al. Successful treatment of depressed, distensible acne scars using autologous fibroblasts: a multi-site, prospective, double blind, placebo-controlled clinical trial. Dermatol Surg. 2013;39:1226-1236.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39(2, pt 3):S34-S37.
- Yazici K, Baz K, Yazici AE, et al. Disease-specific quality of life is associated with anxiety and depression in patients with acne. J Eur Acad Dermatol Venereol. 2004;18:435-439.
- Orentreich DS, Orentreich N. Subcutaneous incisionless (subcision) surgery for the correction of depressed scars and wrinkles. Dermatol Surg. 1995;21:543-549.
- Fabbrocini G, De Padova M, De Vita V, et al. Periorbital wrinkles treatment using collagen induction therapy. Surg Cosmet Dermatol. 2009;1:106-111.
- Fabbrocini G, De Vita V, Pastore F, et al. Collagen induction therapy for the treatment of upper lip wrinkles. J Dermatol Treat. 2012;23:144-152.
- Fabbrocini G, De Vita V, Di Costanzo L, et al. Skin needling in the treatment of the aging neck. Skinmed. 2011;9:347-351.
- El-Domyati M, Barakat M, Awad S, et al. Microneedling therapy for atrophic acne scars: an objective evaluation. J Clin Aesthet Dermatol. 2015;8:36-42.
- Fabbrocini G, Fardella N, Monfrecola A, et al. Acne scarring treatment using skin needling. Clin Exp Dermatol. 2009;34:874-879.
- Alam M, Han S, Pongprutthipan M, et al. Efficacy of a needling device for the treatment of acne scars: a randomized clinical trial. JAMA Dermatol. 2014;150:844-849.
- Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichology. 2013;5:6-11.
- Dhurat R, Mathapati S. Response to microneedling treatment in men with androgenetic alopecia who failed to respond to conventional therapy. Indian J Dermatol. 2015;60:260-263.
- Fabbrocini G, De Vita V, Fardella N, et al. Skin needling to enhance depigmenting serum penetration in the treatment of melasma [published online April 7, 2011]. Plast Surg Int. 2011;2011:158241.
- Bariya SH, Gohel MC, Mehta TA, et al. Microneedles: an emerging transdermal drug delivery system. J Pharm Pharmacol. 2012;64:11-29.
- Fabbrocini G, De Vita V, Izzo R, et al. The use of skin needling for the delivery of a eutectic mixture of local anesthetics. G Ital Dermatol Venereol. 2014;149:581-585.
- De Vita V. How to choose among the multiple options to enhance the penetration of topically applied methyl aminolevulinate prior to photodynamic therapy [published online February 22, 2018]. Photodiagnosis Photodyn Ther. doi:10.1016/j.pdpdt.2018.02.014.
- Fernandes D. Minimally invasive percutaneous collagen induction. Oral Maxillofac Surg Clin North Am. 2005;17:51-63.
- Goodman GJ, Baron JA. Postacne scarring—a quantitative global scarring grading system. J Cosmet Dermatol. 2006;5:48-52.
- Majid I. Microneedling therapy in atrophic facial scars: an objective assessment. J Cutan Aesthet Surg. 2009;2:26-30.
- Dogra S, Yadav S, Sarangal R. Microneedling for acne scars in Asian skin type: an effective low cost treatment modality. J Cosmet Dermatol. 2014;13:180-187.
- Fabbrocini G, De Vita V, Monfrecola A, et al. Percutaneous collagen induction: an effective and safe treatment for post-acne scarring in different skin phototypes. J Dermatol Treat. 2014;25:147-152.
- Hashim PW, Levy Z, Cohen JL, et al. Microneedling therapy with and without platelet-rich plasma. Cutis. 2017;99:239-242.
- Wang HL, Avila G. Platelet rich plasma: myth or reality? Eur J Dent. 2007;1:192-194.
- Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62:489-496.
- Fabbrocini G, De Vita V, Pastore F, et al. Combined use of skin needling and platelet-rich plasma in acne scarring treatment. Cosmet Dermatol. 2011;24:177-183.
- Chawla S. Split face comparative study of microneedling with PRP versus microneedling with vitamin C in treating atrophic post acne scars. J Cutan Aesthet Surg. 2014;7:209-212.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study. J Cosmet Dermatol. 2016;15:434-443.
- Ibrahim MK, Ibrahim SM, Salem AM. Skin microneedling plus platelet-rich plasma versus skin microneedling alone in the treatment of atrophic post acne scars: a split face comparative study. J Dermatolog Treat. 2018;29:281-286.
- Goodman GJ, Baron JA. Postacne scarring: a qualitative global scarring grading system. Dermatol Surg. 2006;32:1458-1466.
- You H, Kim D, Yoon E, et al. Comparison of four different lasers for acne scars: resurfacing and fractional lasers. J Plast Reconstr Aesthet Surg. 2016;69:E87-E95.
- Osman MA, Shokeir HA, Fawzy MM. Fractional erbium-doped yttrium aluminum garnet laser versus microneedling in treatment of atrophic acne scars: a randomized split-face clinical study. Dermatol Surg. 2017;43(suppl 1):S47-S56.
- Lee JW, Kim BJ, Kim MN, et al. The efficacy of autologous platelet rich plasma combined with ablative carbon dioxide fractional resurfacing for acne scars: a simultaneous split-face trial. Dermatol Surg. 2011;37:931-938.
- Gawdat HI, Hegazy RA, Fawzy MM, et al. Autologous platelet rich plasma: topical versus intradermal after fractional ablative carbon dioxide laser treatment of atrophic acne scars. Dermatol Surg. 2014;40:152-161.
- Abdel Aal AM, Ibrahim IM, Sami NA, et al. Evaluation of autologous platelet rich plasma plus ablative carbon dioxide fractional laser in the treatment of acne scars. J Cosmet Laser Ther. 2018;20:106-113.
- Min S, Yoon JY, Park SY, et al. Combination of platelet rich plasma in fractional carbon dioxide laser treatment increased clinical efficacy of for acne scar by enhancement of collagen production and modulation of laser-induced inflammation. Lasers Surg Med. 2018;50:302-310.
- Roberts AB, Sporn MB, Assoian RK, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986;83:4167-4171.
- Schmidt EV. The role of c-myc in cellular growth control. Oncogene. 1999;18:2988-2996.
- Varga J, Rosenbloom J, Jimenez SA. Transforming growth factor beta (TGF beta) causes a persistent increase in steady-state amounts of type I and type III collagen and fibronectin mRNAs in normal human dermal fibroblasts. Biochem J. 1987;247:597-604.
- Chen J, Somanath PR, Razorenova O, et al. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med. 2005;11:1188-1196.
- Repertinger SK, Campagnaro E, Fuhrman J, et al. EGFR enhances early healing after cutaneous incisional wounding. J Invest Dermatol. 2004;123:982-989.
- Landau M. Chemical peels. Clin Dermatol. 2008;26:200-208.
- Drake LA, Dinehart SM, Goltz RW, et al. Guidelines of care for chemical peeling. J Am Acad Dermatol. 1995;33:497-503.
- Meaike JD, Agrawal N, Chang D, et al. Noninvasive facial rejuvenation. part 3: physician-directed-lasers, chemical peels, and other noninvasive modalities. Semin Plast Surg. 2016;30:143-150.
- Leheta TM, Abdel Hay RM, El Garem YF. Deep peeling using phenol versus percutaneous collagen induction combined with trichloroacetic acid 20% in atrophic post-acne scars; a randomized controlled trial.J Dermatol Treat. 2014;25:130-136.
- El-Domyati M, Abdel-Wahab H, Hossam A. Microneedling combined with platelet-rich plasma or trichloroacetic acid peeling for management of acne scarring: a split-face clinical and histologic comparison.J Cosmet Dermatol. 2018;17:73-83.
- Hession MT, Graber EM. Atrophic acne scarring: a review of treatment options. J Clin Aesthet Dermatol. 2015;8:50-58.
- Dayan SH, Bassichis BA. Facial dermal fillers: selection of appropriate products and techniques. Aesthet Surg J. 2008;28:335-347.
- Munavalli GS, Smith S, Maslowski JM, et al. Successful treatment of depressed, distensible acne scars using autologous fibroblasts: a multi-site, prospective, double blind, placebo-controlled clinical trial. Dermatol Surg. 2013;39:1226-1236.
Practice Points
- Acne scarring affects millions of Americans and can lead to poor psychological sequelae such as low self-esteem.
- Multiple modalities for acne scarring treatment exist including microneedling, lasers, chemical peels, and dermal fillers.
- Consider patient-desired outcome, cost, and adverse events when choosing a specific treatment modality.
Regenerative Medicine in Cosmetic Dermatology
Regenerative medicine encompasses innovative therapies that allow the body to repair or regenerate aging cells, tissues, and organs. The skin is a particularly attractive organ for the application of novel regenerative therapies due to its easy accessibility. Among these therapies, stem cells and platelet-rich plasma (PRP) have garnered interest based on their therapeutic potential in scar reduction, antiaging effects, and treatment of alopecia.
Stem cells possess the cardinal features of self-renewal and plasticity. Self-renewal refers to symmetric cell division generating daughter cells identical to the parent cell.1 Plasticity is the ability to generate cell types other than the germ line or tissue lineage from which stem cells derive.2 Stem cells can be categorized according to their differentiation potential. Totipotent stem cells may develop into any primary germ cell layer (ectoderm, mesoderm, endoderm) of the embryo, as well as extraembryonic tissue such as the trophoblast, which gives rise to the placenta. Pluripotent stem cells such as embryonic stem cells have the capacity to differentiate into any derivative of the 3 germ cell layers but have lost their ability to differentiate into the trophoblast.3 Adults lack totipotent or pluripotent cells; they have multipotent or unipotent cells. Multipotent stem cells are able to differentiate into multiple cell types from similar lineages; mesenchymal stem cells (MSCs), for example, can differentiate into adipogenic, osteogenic, chondrogenic, and myogenic cells.4 Unipotent stem cells have the lowest differentiation potential and can only self-regenerate. Herein, we review stem cell sources and their therapeutic potential in aesthetic dermatology.
Multipotent Stem Cells
Multipotent stem cells derived from the bone marrow, umbilical cord, adipose tissue, dermis, or hair follicle bulge have various clinical applications in dermatology. Stem cells from these sources are primarily utilized in an autologous manner in which they are processed outside the body and reintroduced into the donor. Autologous multipotent hematopoietic bone marrow cells were first successfully used for the treatment of chronic wounds and show promise for the treatment of atrophic scars.5,6 However, due to the invasive nature of extracting bone marrow stem cells and their declining number with age, other sources of multipotent stem cells have fallen into favor.
Umbilical cord blood is a source of multipotent hematopoietic stem cells for which surgical intervention is not necessary because they are retrieved after umbilical cord clamping.7 Advantages of sourcing stem cells from umbilical cord blood includes high regenerative power compared to a newborn’s skin and low immunogenicity given that the newborn is immunologically immature.8
Another popular source for autologous stem cells is adipose tissue due to its ease of accessibility and relative abundance. Given that adipose tissue–derived stem cells (ASCs) are capable of differentiating into adipocytes that help maintain volume over time, they are being used for midface contouring, lip augmentation, facial rejuvenation, facial scarring, lipodystrophy, penile girth enhancement, and vaginal augmentation. Adipose tissue–derived stem cells also are capable of differentiating into other types of tissue, including cartilage and bone. Thus, they have been successfully harnessed in the treatment of patients affected by systemic sclerosis and Parry-Romberg syndrome as well in the functional and aesthetic reconstruction of various military combat–related deformities.9,10
Adipose tissue–derived stem cells are commonly harvested from lipoaspirate of the abdomen and are combined with supportive mechanical scaffolds such as hydrogels. Lipoaspirate itself can serve as a scaffold for ASCs. Accordingly, ASCs also are being utilized as a scaffold for autologous fat transfer procedures in an effort to increase the viability of transplanted donor tissue, a process known as cell-assisted lipotransfer (CAL). In CAL, a fraction of the aspirated fat is processed for isolation of ASCs, which are then recombined with the remainder of the aspirated fat prior to grafting.11 However, there is conflicting evidence as to whether CAL leads to improved graft success relative to conventional autologous fat transfer.12,13
The skin also serves as an easily accessible and abundant autologous source of stem cells. A subtype of dermal fibroblasts has been proven to have multipotent potential.14,15 These dermal fibroblasts are harvested from one area of the skin using punch biopsy and are processed and reinjected into another desired area of the skin.16 Autologous human fibroblasts have proven to be effective for the treatment of wrinkles, rhytides, and acne scars.17 In June 2011, the US Food and Drug Administration approved azficel-T, an autologous cellular product created by harvesting fibroblasts from a patient’s own postauricular skin, culture-expanding them in vitro for 3 months, and reinjecting the cells into the desired area of dermis in a series of treatments. This product was the first personalized cell therapy approved by the US Food and Drug Administration for aesthetic uses, specifically for the improvement of nasolabial fold wrinkles.18
In adults, hair follicles contain an area known as the bulge, which is a site rich in epithelial and melanocytic stem cells. Bulge stem cells have the ability to reproduce the interfollicular epidermis, hair follicle structures, and sebaceous glands, and they have been used to construct entirely new hair follicles in an artificial in vivo system.19 Sugiyama-Nakagiri et al20 demonstrated that an entire hair follicle epithelium and interfollicular epidermis can be regenerated using cultured bulge stem cells. The cultured bulge stem cells were mixed with dermal papilla cells from neonatal rat vibrissae and engrafted into a silicone chamber implanted on the backs of severe combined immune deficient (SCID) mice. The grafts exhibited tufts of hair as well as a complete interfollicular epidermis at 4 weeks after transplantation.20 Thus, these bulge stem cells have the potential to treat male androgenic alopecia and female pattern hair loss. Bulge stem cells also have been shown to accelerate wound healing.21 Additionally, autologous melanocytic stem cells located at the hair follicle bulge are effective for treating vitiligo and are being investigated for the treatment of hair graying.22
Induced Pluripotent Stem Cells
Given the ethical concerns that surround the procurement and use of embryonic stem cells, efforts have been made to retrieve pluripotent stem cells from adults. A major breakthrough occurred in 2006 when researchers altered the genes of specialized adult mouse cells to cause dedifferentiation and the return to an embryoniclike stem cell state.23 Mouse somatic cells were reprogrammed through the activation of a combination of transcription factors. The resulting cells were termed induced pluripotent stem cells (iPSCs) and have since been recreated in human cell lines. The discovery of iPSCs precipitated a translational science revolution. Physician-scientists sought ways to apply the reprogrammed cells to the pathophysiology of obscure diseases, examination of drug targets, and regeneration of human tissue.24 Tissue regeneration via induced naïve somatic cells has shown promise as a future method to treat neurologic, cardiovascular, and ophthalmologic diseases.25
As the technology of cultivating and identifying optimal sources of iPSCs continues to advance, stem cell–based treatments have evolved as leading prospects in the field of biogerontology.26-29 Although much of the research in antiaging medicine has utilized iPSCs to reprogram cell senescence, the altering of iPSCs at a cellular level also allows for the stimulation of collagen synthesis. This potential for collagen generation may have direct applicability in dermatologic practice, particularly for aesthetic treatments.
Much of the research into iPSC-derived collagen has focused on genodermatoses. Itoh et al30 examined the creation of collagen through iPSCs to identify possible treatments for recessive dystrophic epidermolysis bullosa (DEB). Recessive DEB is characterized by mutations in the COL7A1 gene, which encodes type VII collagen, a basement membrane protein and component of the anchoring fibrils essential for skin integrity.31 Itoh et al30 began with source cells obtained from a skin biopsy. The cells were dedifferentiated to iPSCs and then induced into dermal fibroblasts according to the methods established in prior studies of embryonic stem cells, namely with the use of ascorbic acid and transforming growth factor b. The newly formed fibroblasts were determined to be functional based on their ability to synthesize mature type VII collagen.30 Once the viability of the iPSC-derived fibroblasts was confirmed in vitro, the cells were further tested through combination with human keratinocytes on SCID mice. The human keratinocytes grew together with the iPSC-derived fibroblasts, producing type VII collagen in the basement membrane zone and creating an epidermis with the normal markers.30 Similarly, Robbins et al32 utilized SCID mice to successfully demonstrate that the transfection of keratinocytes from patients with junctional epidermolysis bullosa into SCID mice produced phenotypically normal skin.
Sebastiano et al33 combined the concepts of iPSCs and genome editing in another study of recessive DEB. The investigators first cultured iPSCs from biopsies of affected patients. After deriving iPSCs and correcting their mutation via adenovirus-associated viral gene editing, the COL7A1 mutation-free cells were differentiated into keratinocytes. These iPSC-derived keratinocytes were subsequently grafted onto mice, which led to the production of wild-type collagen VII and a stratified epidermis. Despite this successful outcome, the grafts of iPSC-derived epidermis did not survive longer than 1 month.33
One of the many obstacles facing the practical use of stem cells is their successful incorporation into human tissue. A possible solution was uncovered by Zhang et al34 who examined iPSC-derived MSCs. Mesenchymal stem cells communicate via paracrine mechanisms, whereby exosomes containing RNA and proteins are released to potentiate a regenerative effect.35 Zhang et al34 found that injecting exosomes from human iPSC-derived MSCs into the wound sites of rats stimulated the production of type I collagen, type III collagen, and elastin. The wound sites demonstrated accelerated closure, narrower scar widths, and increased collagen maturity.
Understanding the role that local environment plays in stem cell differentiation, Xu et al36 aimed to create an extracellular scaffold to induce fibroblast behavior from iPSCs. The authors engineered a framework similar to the normal extracellular membrane using proteoglycans, glycosaminoglycans, fibrinogen, and connective tissue growth factor. The iPSCs were then applied to the scaffolding, which led to successful fibroblast differentiation and type I collagen synthesis.36 This use of local biosignaling cues holds important ramifications for controlling the fate of stem cells that have been introduced into a new environment.
Although the application of iPSCs in clinical dermatology has yet to be achieved, progress in the field is moving at a rapid pace. Several logistical elements require further mastery before therapeutics can be delivered. These areas include the optimal environment for iPSC differentiation, methods for maximization of graft survival, and different modes of transplanting iPSC-derived cells into patients. In cosmetic practice, success will depend on intradermal injections of collagen-producing iPSC-derived cells that possess long-term proliferative potential. Current research in mice models has demonstrated viability up to 16 weeks after intradermal injection of such cells.37
Plant Stem Cells
In discussing the dermatologic applications of stem cell technology, clinicians should be aware of the plant stem cell products that have become a popular cosmeceutical trend. Companies advertise plant cells as a natural source of regenerative cells that can induce rejuvenation in human skin; however, there are no significant data to indicate that plant stem cells encourage or activate cellular growth in humans. Indeed, for stem cells to differentiate and produce viable components, the cells must first be incorporated as living components in the host tissue. Because plant stem cells do not survive in human tissue and plant cell cytokines fail to interact with the receptors on human cells, their current value in cosmeceuticals may be overstated.
Platelet-Rich Plasma
Platelet-rich plasma also is commonly associated with stem cell therapy, as PRP is known to potentiate stem cell proliferation, migration, and differentiation. However, PRP does not contain stem cells and is instead autologous plasma concentrated with platelets. In fact, platelets cannot even be classified as cells given that they lack a nucleus; platelets are considered cell fragments. The regenerative potential of PRP can be attributed to the growth factors released from platelets, which play an important role in tissue regeneration and repair. Platelet-rich plasma currently is being used in dermatology for skin rejuvenation (reduction of wrinkles and furrows) and treatment of acne scars.38 There also is evidence supporting the effectiveness of PRP for alopecia and wound therapy, as growth factors play a vital role in hair growth and wound healing.38 Apart from the use of PRP on its own, it can be used as a supplement to enhance the effects of antiaging procedures such as microneedling.39
Future Directions
Multipotent stem cells and iPSCs discussed herein provide much promise in the field of regenerative dermatology. They are increasingly accessible and circumvent the use of ethically questionable embryonic stem cells. Although there is a general consensus on the great potential of stem cells for treating aesthetic skin conditions, high-quality randomized controlled trials remain scarce within the literature. Recognizing and optimizing these opportunities remains the next step in the future delivery of evidence-based care in regenerative dermatology.
- Thomas ED, Lochte HL, Lu WC, et al. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957;257:491-496.
- Ogliari KS, Marinowic D, Brum DE, et al. Stem cells in dermatology. An Bras Dermatol. 2014;89:286-291.
- Xu C, Inokuma MS, Denham J, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971-974.
- Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211-228.
- Badiavas EV, Falanga V. Treatment of chronic wounds with bone marrow-derived cells. Arch Dermatol. 2003;139:510-516.
- Ibrahim ZA, Eltatawy RA, Ghaly NR, et al. Autologous bone marrow stem cells in atrophic acne scars: a pilot study. J Dermatolog Treat. 2015;26:260-265.
- Broxmeyer HE, Douglas GW, Hangoc G, et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci U S A. 1989;86:3828-3832.
- Gluckman E, Rocha V, Boyer-Chammard A, et al. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. N Engl J Med. 1997;337:373-381.
- Valerio IL, Sabino JM, Dearth CL. Plastic surgery challenges in war wounded II: regenerative medicine. Adv Wound Care (New Rochelle). 2016;5:412-419.
- Vescarelli E, D’Amici S, Onesti MG, et al. Adipose-derived stem cell: an innovative therapeutic approach in systemic sclerosis and Parry-Romberg syndrome. CellR4. 2014;2:E791-E797.
- Yoshimura K, Sato K, Aoi N, et al. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008;32:48-55.
- Grabin S, Antes G, Stark GB, et al. Cell-assisted lipotransfer: a critical appraisal of the evidence. Dtsch Arztebl Int. 2015;112:255.
- Zhou Y, Wang J, Li H, et al. Efficacy and safety of cell-assisted lipotransfer: a systematic review and meta-analysis. Plast Reconstr Surg. 2016;137:E44-E57.
- Toma JG, Akhavan M, Fernandes KJL, et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778-784.
- Toma JG, McKenzie IA, Bagli D, et al. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005;23:727-737.
- Homicz MR, Watson D. Review of injectable materials for soft tissue augmentation. Facial Plast Surg. 2004;20:21-29.
- Kumar S, Mahajan BB, Kaur S, et al. Autologous therapies in dermatology. J Clin Aesthet Dermatol. 2014;7:38-45.
- Schmidt C. FDA approves first cell therapy for wrinkle-free visage. Nat Biotech. 2011;29:674-675.
- Gentile P, Scioli MG, Bielli A, et al. Stem cells from human hair follicles: first mechanical isolation for immediate autologous clinical use in androgenetic alopecia and hair loss. Stem Cell Investig. 2017;4:58.
- Sugiyama-Nakagiri Y, Akiyama M, Shimizu H. Hair follicle stem cell-targeted gene transfer and reconstitution system. Gene Ther. 2006;13:732-737.
- Heidari F, Yari A, Rasoolijazi H, et al. Bulge hair follicle stem cells accelerate cutaneous wound healing in rats. Wounds. 2016;28:132-141.
- Lee JH, Fisher DE. Melanocyte stem cells as potential therapeutics in skin disorders. Expert Opin Biol Ther. 2014;14:1-11.
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676.
- Singh VK, Kalsan M, Kumar N, et al. Induced pluripotent stem cells: applications in regenerative medicine, disease modeling, and drug discovery. Front Cell Dev Biol. 2015;3:2.
- Aoi T. 10th anniversary of iPS cells: The challenges that lie ahead. J Biochem. 2016;160:121-129.
- Lowry WE, Plath K. The many ways to make an iPS cell. Nat Biotechnol. 2008;26:1246-1248.
- Kim K, Doi A, Wen B, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285-290.
- Gafni O, Weinberger L, Mansour AA, et al. Derivation of novel human ground state naive pluripotent stem cells. Nature. 2013;504:282-286.
- Pareja-Galeano H, Sanchis-Gomar F, Pérez LM, et al. IPSCs-based anti-aging therapies: Recent discoveries and future challenges. Ageing Res Rev. 2016;27:37-41.
- Itoh M, Umegaki-Arao N, Guo Z, et al. Generation of 3D skin equivalents fully reconstituted from human induced pluripotent stem cells (iPSCs). PLoS One. 2013;8:e77673.
- Nyström A, Velati D, Mittapalli VR, et al. Collagen VII plays a dual role in wound healing. J Clin Invest. 2013;123:3498-3509.
- Robbins PB, Lin Q, Goodnough JB, et al. In vivo restoration of laminin 5 β3 expression and function in junctional epidermolysis bullosa. Proc Natl Acad Sci. 2001;98:5193-5198.
- Sebastiano V, Zhen HH, Haddad B, et al. Human COL7A1-corrected induced pluripotent stem cells for the treatment of recessive dystrophic epidermolysis bullosa. Sci Transl Med. 2014;6:264ra163.
- Zhang J, Guan J, Niu X, et al. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13:49.
- Pap E, Pállinger É, Pásztói M, et al. Highlights of a new type of intercellular communication: microvesicle-based information transfer. Inflamm Res. 2009;58:1-8.
- Xu R, Taskin MB, Rubert M, et al. hiPS-MSCs differentiation towards fibroblasts on a 3D ECM mimicking scaffold. Sci Rep. 2015;5:8480.
- Wenzel D, Bayerl J, Nyström A, et al. Genetically corrected iPSCs as cell therapy for recessive dystrophic epidermolysis bullosa. Sci Transl Med. 2014;6:264ra165.
- Bednarska K, Kieszek R, Domagała P, et al. The use of platelet-rich-plasma in aesthetic and regenerative medicine. MEDtube Science. 2015;2:8-15.
- Hashim PW, Levy Z, Cohen JL, et al. Microneedling therapy with and without platelet-rich plasma. Cutis. 2017;99:239-242.
Regenerative medicine encompasses innovative therapies that allow the body to repair or regenerate aging cells, tissues, and organs. The skin is a particularly attractive organ for the application of novel regenerative therapies due to its easy accessibility. Among these therapies, stem cells and platelet-rich plasma (PRP) have garnered interest based on their therapeutic potential in scar reduction, antiaging effects, and treatment of alopecia.
Stem cells possess the cardinal features of self-renewal and plasticity. Self-renewal refers to symmetric cell division generating daughter cells identical to the parent cell.1 Plasticity is the ability to generate cell types other than the germ line or tissue lineage from which stem cells derive.2 Stem cells can be categorized according to their differentiation potential. Totipotent stem cells may develop into any primary germ cell layer (ectoderm, mesoderm, endoderm) of the embryo, as well as extraembryonic tissue such as the trophoblast, which gives rise to the placenta. Pluripotent stem cells such as embryonic stem cells have the capacity to differentiate into any derivative of the 3 germ cell layers but have lost their ability to differentiate into the trophoblast.3 Adults lack totipotent or pluripotent cells; they have multipotent or unipotent cells. Multipotent stem cells are able to differentiate into multiple cell types from similar lineages; mesenchymal stem cells (MSCs), for example, can differentiate into adipogenic, osteogenic, chondrogenic, and myogenic cells.4 Unipotent stem cells have the lowest differentiation potential and can only self-regenerate. Herein, we review stem cell sources and their therapeutic potential in aesthetic dermatology.
Multipotent Stem Cells
Multipotent stem cells derived from the bone marrow, umbilical cord, adipose tissue, dermis, or hair follicle bulge have various clinical applications in dermatology. Stem cells from these sources are primarily utilized in an autologous manner in which they are processed outside the body and reintroduced into the donor. Autologous multipotent hematopoietic bone marrow cells were first successfully used for the treatment of chronic wounds and show promise for the treatment of atrophic scars.5,6 However, due to the invasive nature of extracting bone marrow stem cells and their declining number with age, other sources of multipotent stem cells have fallen into favor.
Umbilical cord blood is a source of multipotent hematopoietic stem cells for which surgical intervention is not necessary because they are retrieved after umbilical cord clamping.7 Advantages of sourcing stem cells from umbilical cord blood includes high regenerative power compared to a newborn’s skin and low immunogenicity given that the newborn is immunologically immature.8
Another popular source for autologous stem cells is adipose tissue due to its ease of accessibility and relative abundance. Given that adipose tissue–derived stem cells (ASCs) are capable of differentiating into adipocytes that help maintain volume over time, they are being used for midface contouring, lip augmentation, facial rejuvenation, facial scarring, lipodystrophy, penile girth enhancement, and vaginal augmentation. Adipose tissue–derived stem cells also are capable of differentiating into other types of tissue, including cartilage and bone. Thus, they have been successfully harnessed in the treatment of patients affected by systemic sclerosis and Parry-Romberg syndrome as well in the functional and aesthetic reconstruction of various military combat–related deformities.9,10
Adipose tissue–derived stem cells are commonly harvested from lipoaspirate of the abdomen and are combined with supportive mechanical scaffolds such as hydrogels. Lipoaspirate itself can serve as a scaffold for ASCs. Accordingly, ASCs also are being utilized as a scaffold for autologous fat transfer procedures in an effort to increase the viability of transplanted donor tissue, a process known as cell-assisted lipotransfer (CAL). In CAL, a fraction of the aspirated fat is processed for isolation of ASCs, which are then recombined with the remainder of the aspirated fat prior to grafting.11 However, there is conflicting evidence as to whether CAL leads to improved graft success relative to conventional autologous fat transfer.12,13
The skin also serves as an easily accessible and abundant autologous source of stem cells. A subtype of dermal fibroblasts has been proven to have multipotent potential.14,15 These dermal fibroblasts are harvested from one area of the skin using punch biopsy and are processed and reinjected into another desired area of the skin.16 Autologous human fibroblasts have proven to be effective for the treatment of wrinkles, rhytides, and acne scars.17 In June 2011, the US Food and Drug Administration approved azficel-T, an autologous cellular product created by harvesting fibroblasts from a patient’s own postauricular skin, culture-expanding them in vitro for 3 months, and reinjecting the cells into the desired area of dermis in a series of treatments. This product was the first personalized cell therapy approved by the US Food and Drug Administration for aesthetic uses, specifically for the improvement of nasolabial fold wrinkles.18
In adults, hair follicles contain an area known as the bulge, which is a site rich in epithelial and melanocytic stem cells. Bulge stem cells have the ability to reproduce the interfollicular epidermis, hair follicle structures, and sebaceous glands, and they have been used to construct entirely new hair follicles in an artificial in vivo system.19 Sugiyama-Nakagiri et al20 demonstrated that an entire hair follicle epithelium and interfollicular epidermis can be regenerated using cultured bulge stem cells. The cultured bulge stem cells were mixed with dermal papilla cells from neonatal rat vibrissae and engrafted into a silicone chamber implanted on the backs of severe combined immune deficient (SCID) mice. The grafts exhibited tufts of hair as well as a complete interfollicular epidermis at 4 weeks after transplantation.20 Thus, these bulge stem cells have the potential to treat male androgenic alopecia and female pattern hair loss. Bulge stem cells also have been shown to accelerate wound healing.21 Additionally, autologous melanocytic stem cells located at the hair follicle bulge are effective for treating vitiligo and are being investigated for the treatment of hair graying.22
Induced Pluripotent Stem Cells
Given the ethical concerns that surround the procurement and use of embryonic stem cells, efforts have been made to retrieve pluripotent stem cells from adults. A major breakthrough occurred in 2006 when researchers altered the genes of specialized adult mouse cells to cause dedifferentiation and the return to an embryoniclike stem cell state.23 Mouse somatic cells were reprogrammed through the activation of a combination of transcription factors. The resulting cells were termed induced pluripotent stem cells (iPSCs) and have since been recreated in human cell lines. The discovery of iPSCs precipitated a translational science revolution. Physician-scientists sought ways to apply the reprogrammed cells to the pathophysiology of obscure diseases, examination of drug targets, and regeneration of human tissue.24 Tissue regeneration via induced naïve somatic cells has shown promise as a future method to treat neurologic, cardiovascular, and ophthalmologic diseases.25
As the technology of cultivating and identifying optimal sources of iPSCs continues to advance, stem cell–based treatments have evolved as leading prospects in the field of biogerontology.26-29 Although much of the research in antiaging medicine has utilized iPSCs to reprogram cell senescence, the altering of iPSCs at a cellular level also allows for the stimulation of collagen synthesis. This potential for collagen generation may have direct applicability in dermatologic practice, particularly for aesthetic treatments.
Much of the research into iPSC-derived collagen has focused on genodermatoses. Itoh et al30 examined the creation of collagen through iPSCs to identify possible treatments for recessive dystrophic epidermolysis bullosa (DEB). Recessive DEB is characterized by mutations in the COL7A1 gene, which encodes type VII collagen, a basement membrane protein and component of the anchoring fibrils essential for skin integrity.31 Itoh et al30 began with source cells obtained from a skin biopsy. The cells were dedifferentiated to iPSCs and then induced into dermal fibroblasts according to the methods established in prior studies of embryonic stem cells, namely with the use of ascorbic acid and transforming growth factor b. The newly formed fibroblasts were determined to be functional based on their ability to synthesize mature type VII collagen.30 Once the viability of the iPSC-derived fibroblasts was confirmed in vitro, the cells were further tested through combination with human keratinocytes on SCID mice. The human keratinocytes grew together with the iPSC-derived fibroblasts, producing type VII collagen in the basement membrane zone and creating an epidermis with the normal markers.30 Similarly, Robbins et al32 utilized SCID mice to successfully demonstrate that the transfection of keratinocytes from patients with junctional epidermolysis bullosa into SCID mice produced phenotypically normal skin.
Sebastiano et al33 combined the concepts of iPSCs and genome editing in another study of recessive DEB. The investigators first cultured iPSCs from biopsies of affected patients. After deriving iPSCs and correcting their mutation via adenovirus-associated viral gene editing, the COL7A1 mutation-free cells were differentiated into keratinocytes. These iPSC-derived keratinocytes were subsequently grafted onto mice, which led to the production of wild-type collagen VII and a stratified epidermis. Despite this successful outcome, the grafts of iPSC-derived epidermis did not survive longer than 1 month.33
One of the many obstacles facing the practical use of stem cells is their successful incorporation into human tissue. A possible solution was uncovered by Zhang et al34 who examined iPSC-derived MSCs. Mesenchymal stem cells communicate via paracrine mechanisms, whereby exosomes containing RNA and proteins are released to potentiate a regenerative effect.35 Zhang et al34 found that injecting exosomes from human iPSC-derived MSCs into the wound sites of rats stimulated the production of type I collagen, type III collagen, and elastin. The wound sites demonstrated accelerated closure, narrower scar widths, and increased collagen maturity.
Understanding the role that local environment plays in stem cell differentiation, Xu et al36 aimed to create an extracellular scaffold to induce fibroblast behavior from iPSCs. The authors engineered a framework similar to the normal extracellular membrane using proteoglycans, glycosaminoglycans, fibrinogen, and connective tissue growth factor. The iPSCs were then applied to the scaffolding, which led to successful fibroblast differentiation and type I collagen synthesis.36 This use of local biosignaling cues holds important ramifications for controlling the fate of stem cells that have been introduced into a new environment.
Although the application of iPSCs in clinical dermatology has yet to be achieved, progress in the field is moving at a rapid pace. Several logistical elements require further mastery before therapeutics can be delivered. These areas include the optimal environment for iPSC differentiation, methods for maximization of graft survival, and different modes of transplanting iPSC-derived cells into patients. In cosmetic practice, success will depend on intradermal injections of collagen-producing iPSC-derived cells that possess long-term proliferative potential. Current research in mice models has demonstrated viability up to 16 weeks after intradermal injection of such cells.37
Plant Stem Cells
In discussing the dermatologic applications of stem cell technology, clinicians should be aware of the plant stem cell products that have become a popular cosmeceutical trend. Companies advertise plant cells as a natural source of regenerative cells that can induce rejuvenation in human skin; however, there are no significant data to indicate that plant stem cells encourage or activate cellular growth in humans. Indeed, for stem cells to differentiate and produce viable components, the cells must first be incorporated as living components in the host tissue. Because plant stem cells do not survive in human tissue and plant cell cytokines fail to interact with the receptors on human cells, their current value in cosmeceuticals may be overstated.
Platelet-Rich Plasma
Platelet-rich plasma also is commonly associated with stem cell therapy, as PRP is known to potentiate stem cell proliferation, migration, and differentiation. However, PRP does not contain stem cells and is instead autologous plasma concentrated with platelets. In fact, platelets cannot even be classified as cells given that they lack a nucleus; platelets are considered cell fragments. The regenerative potential of PRP can be attributed to the growth factors released from platelets, which play an important role in tissue regeneration and repair. Platelet-rich plasma currently is being used in dermatology for skin rejuvenation (reduction of wrinkles and furrows) and treatment of acne scars.38 There also is evidence supporting the effectiveness of PRP for alopecia and wound therapy, as growth factors play a vital role in hair growth and wound healing.38 Apart from the use of PRP on its own, it can be used as a supplement to enhance the effects of antiaging procedures such as microneedling.39
Future Directions
Multipotent stem cells and iPSCs discussed herein provide much promise in the field of regenerative dermatology. They are increasingly accessible and circumvent the use of ethically questionable embryonic stem cells. Although there is a general consensus on the great potential of stem cells for treating aesthetic skin conditions, high-quality randomized controlled trials remain scarce within the literature. Recognizing and optimizing these opportunities remains the next step in the future delivery of evidence-based care in regenerative dermatology.
Regenerative medicine encompasses innovative therapies that allow the body to repair or regenerate aging cells, tissues, and organs. The skin is a particularly attractive organ for the application of novel regenerative therapies due to its easy accessibility. Among these therapies, stem cells and platelet-rich plasma (PRP) have garnered interest based on their therapeutic potential in scar reduction, antiaging effects, and treatment of alopecia.
Stem cells possess the cardinal features of self-renewal and plasticity. Self-renewal refers to symmetric cell division generating daughter cells identical to the parent cell.1 Plasticity is the ability to generate cell types other than the germ line or tissue lineage from which stem cells derive.2 Stem cells can be categorized according to their differentiation potential. Totipotent stem cells may develop into any primary germ cell layer (ectoderm, mesoderm, endoderm) of the embryo, as well as extraembryonic tissue such as the trophoblast, which gives rise to the placenta. Pluripotent stem cells such as embryonic stem cells have the capacity to differentiate into any derivative of the 3 germ cell layers but have lost their ability to differentiate into the trophoblast.3 Adults lack totipotent or pluripotent cells; they have multipotent or unipotent cells. Multipotent stem cells are able to differentiate into multiple cell types from similar lineages; mesenchymal stem cells (MSCs), for example, can differentiate into adipogenic, osteogenic, chondrogenic, and myogenic cells.4 Unipotent stem cells have the lowest differentiation potential and can only self-regenerate. Herein, we review stem cell sources and their therapeutic potential in aesthetic dermatology.
Multipotent Stem Cells
Multipotent stem cells derived from the bone marrow, umbilical cord, adipose tissue, dermis, or hair follicle bulge have various clinical applications in dermatology. Stem cells from these sources are primarily utilized in an autologous manner in which they are processed outside the body and reintroduced into the donor. Autologous multipotent hematopoietic bone marrow cells were first successfully used for the treatment of chronic wounds and show promise for the treatment of atrophic scars.5,6 However, due to the invasive nature of extracting bone marrow stem cells and their declining number with age, other sources of multipotent stem cells have fallen into favor.
Umbilical cord blood is a source of multipotent hematopoietic stem cells for which surgical intervention is not necessary because they are retrieved after umbilical cord clamping.7 Advantages of sourcing stem cells from umbilical cord blood includes high regenerative power compared to a newborn’s skin and low immunogenicity given that the newborn is immunologically immature.8
Another popular source for autologous stem cells is adipose tissue due to its ease of accessibility and relative abundance. Given that adipose tissue–derived stem cells (ASCs) are capable of differentiating into adipocytes that help maintain volume over time, they are being used for midface contouring, lip augmentation, facial rejuvenation, facial scarring, lipodystrophy, penile girth enhancement, and vaginal augmentation. Adipose tissue–derived stem cells also are capable of differentiating into other types of tissue, including cartilage and bone. Thus, they have been successfully harnessed in the treatment of patients affected by systemic sclerosis and Parry-Romberg syndrome as well in the functional and aesthetic reconstruction of various military combat–related deformities.9,10
Adipose tissue–derived stem cells are commonly harvested from lipoaspirate of the abdomen and are combined with supportive mechanical scaffolds such as hydrogels. Lipoaspirate itself can serve as a scaffold for ASCs. Accordingly, ASCs also are being utilized as a scaffold for autologous fat transfer procedures in an effort to increase the viability of transplanted donor tissue, a process known as cell-assisted lipotransfer (CAL). In CAL, a fraction of the aspirated fat is processed for isolation of ASCs, which are then recombined with the remainder of the aspirated fat prior to grafting.11 However, there is conflicting evidence as to whether CAL leads to improved graft success relative to conventional autologous fat transfer.12,13
The skin also serves as an easily accessible and abundant autologous source of stem cells. A subtype of dermal fibroblasts has been proven to have multipotent potential.14,15 These dermal fibroblasts are harvested from one area of the skin using punch biopsy and are processed and reinjected into another desired area of the skin.16 Autologous human fibroblasts have proven to be effective for the treatment of wrinkles, rhytides, and acne scars.17 In June 2011, the US Food and Drug Administration approved azficel-T, an autologous cellular product created by harvesting fibroblasts from a patient’s own postauricular skin, culture-expanding them in vitro for 3 months, and reinjecting the cells into the desired area of dermis in a series of treatments. This product was the first personalized cell therapy approved by the US Food and Drug Administration for aesthetic uses, specifically for the improvement of nasolabial fold wrinkles.18
In adults, hair follicles contain an area known as the bulge, which is a site rich in epithelial and melanocytic stem cells. Bulge stem cells have the ability to reproduce the interfollicular epidermis, hair follicle structures, and sebaceous glands, and they have been used to construct entirely new hair follicles in an artificial in vivo system.19 Sugiyama-Nakagiri et al20 demonstrated that an entire hair follicle epithelium and interfollicular epidermis can be regenerated using cultured bulge stem cells. The cultured bulge stem cells were mixed with dermal papilla cells from neonatal rat vibrissae and engrafted into a silicone chamber implanted on the backs of severe combined immune deficient (SCID) mice. The grafts exhibited tufts of hair as well as a complete interfollicular epidermis at 4 weeks after transplantation.20 Thus, these bulge stem cells have the potential to treat male androgenic alopecia and female pattern hair loss. Bulge stem cells also have been shown to accelerate wound healing.21 Additionally, autologous melanocytic stem cells located at the hair follicle bulge are effective for treating vitiligo and are being investigated for the treatment of hair graying.22
Induced Pluripotent Stem Cells
Given the ethical concerns that surround the procurement and use of embryonic stem cells, efforts have been made to retrieve pluripotent stem cells from adults. A major breakthrough occurred in 2006 when researchers altered the genes of specialized adult mouse cells to cause dedifferentiation and the return to an embryoniclike stem cell state.23 Mouse somatic cells were reprogrammed through the activation of a combination of transcription factors. The resulting cells were termed induced pluripotent stem cells (iPSCs) and have since been recreated in human cell lines. The discovery of iPSCs precipitated a translational science revolution. Physician-scientists sought ways to apply the reprogrammed cells to the pathophysiology of obscure diseases, examination of drug targets, and regeneration of human tissue.24 Tissue regeneration via induced naïve somatic cells has shown promise as a future method to treat neurologic, cardiovascular, and ophthalmologic diseases.25
As the technology of cultivating and identifying optimal sources of iPSCs continues to advance, stem cell–based treatments have evolved as leading prospects in the field of biogerontology.26-29 Although much of the research in antiaging medicine has utilized iPSCs to reprogram cell senescence, the altering of iPSCs at a cellular level also allows for the stimulation of collagen synthesis. This potential for collagen generation may have direct applicability in dermatologic practice, particularly for aesthetic treatments.
Much of the research into iPSC-derived collagen has focused on genodermatoses. Itoh et al30 examined the creation of collagen through iPSCs to identify possible treatments for recessive dystrophic epidermolysis bullosa (DEB). Recessive DEB is characterized by mutations in the COL7A1 gene, which encodes type VII collagen, a basement membrane protein and component of the anchoring fibrils essential for skin integrity.31 Itoh et al30 began with source cells obtained from a skin biopsy. The cells were dedifferentiated to iPSCs and then induced into dermal fibroblasts according to the methods established in prior studies of embryonic stem cells, namely with the use of ascorbic acid and transforming growth factor b. The newly formed fibroblasts were determined to be functional based on their ability to synthesize mature type VII collagen.30 Once the viability of the iPSC-derived fibroblasts was confirmed in vitro, the cells were further tested through combination with human keratinocytes on SCID mice. The human keratinocytes grew together with the iPSC-derived fibroblasts, producing type VII collagen in the basement membrane zone and creating an epidermis with the normal markers.30 Similarly, Robbins et al32 utilized SCID mice to successfully demonstrate that the transfection of keratinocytes from patients with junctional epidermolysis bullosa into SCID mice produced phenotypically normal skin.
Sebastiano et al33 combined the concepts of iPSCs and genome editing in another study of recessive DEB. The investigators first cultured iPSCs from biopsies of affected patients. After deriving iPSCs and correcting their mutation via adenovirus-associated viral gene editing, the COL7A1 mutation-free cells were differentiated into keratinocytes. These iPSC-derived keratinocytes were subsequently grafted onto mice, which led to the production of wild-type collagen VII and a stratified epidermis. Despite this successful outcome, the grafts of iPSC-derived epidermis did not survive longer than 1 month.33
One of the many obstacles facing the practical use of stem cells is their successful incorporation into human tissue. A possible solution was uncovered by Zhang et al34 who examined iPSC-derived MSCs. Mesenchymal stem cells communicate via paracrine mechanisms, whereby exosomes containing RNA and proteins are released to potentiate a regenerative effect.35 Zhang et al34 found that injecting exosomes from human iPSC-derived MSCs into the wound sites of rats stimulated the production of type I collagen, type III collagen, and elastin. The wound sites demonstrated accelerated closure, narrower scar widths, and increased collagen maturity.
Understanding the role that local environment plays in stem cell differentiation, Xu et al36 aimed to create an extracellular scaffold to induce fibroblast behavior from iPSCs. The authors engineered a framework similar to the normal extracellular membrane using proteoglycans, glycosaminoglycans, fibrinogen, and connective tissue growth factor. The iPSCs were then applied to the scaffolding, which led to successful fibroblast differentiation and type I collagen synthesis.36 This use of local biosignaling cues holds important ramifications for controlling the fate of stem cells that have been introduced into a new environment.
Although the application of iPSCs in clinical dermatology has yet to be achieved, progress in the field is moving at a rapid pace. Several logistical elements require further mastery before therapeutics can be delivered. These areas include the optimal environment for iPSC differentiation, methods for maximization of graft survival, and different modes of transplanting iPSC-derived cells into patients. In cosmetic practice, success will depend on intradermal injections of collagen-producing iPSC-derived cells that possess long-term proliferative potential. Current research in mice models has demonstrated viability up to 16 weeks after intradermal injection of such cells.37
Plant Stem Cells
In discussing the dermatologic applications of stem cell technology, clinicians should be aware of the plant stem cell products that have become a popular cosmeceutical trend. Companies advertise plant cells as a natural source of regenerative cells that can induce rejuvenation in human skin; however, there are no significant data to indicate that plant stem cells encourage or activate cellular growth in humans. Indeed, for stem cells to differentiate and produce viable components, the cells must first be incorporated as living components in the host tissue. Because plant stem cells do not survive in human tissue and plant cell cytokines fail to interact with the receptors on human cells, their current value in cosmeceuticals may be overstated.
Platelet-Rich Plasma
Platelet-rich plasma also is commonly associated with stem cell therapy, as PRP is known to potentiate stem cell proliferation, migration, and differentiation. However, PRP does not contain stem cells and is instead autologous plasma concentrated with platelets. In fact, platelets cannot even be classified as cells given that they lack a nucleus; platelets are considered cell fragments. The regenerative potential of PRP can be attributed to the growth factors released from platelets, which play an important role in tissue regeneration and repair. Platelet-rich plasma currently is being used in dermatology for skin rejuvenation (reduction of wrinkles and furrows) and treatment of acne scars.38 There also is evidence supporting the effectiveness of PRP for alopecia and wound therapy, as growth factors play a vital role in hair growth and wound healing.38 Apart from the use of PRP on its own, it can be used as a supplement to enhance the effects of antiaging procedures such as microneedling.39
Future Directions
Multipotent stem cells and iPSCs discussed herein provide much promise in the field of regenerative dermatology. They are increasingly accessible and circumvent the use of ethically questionable embryonic stem cells. Although there is a general consensus on the great potential of stem cells for treating aesthetic skin conditions, high-quality randomized controlled trials remain scarce within the literature. Recognizing and optimizing these opportunities remains the next step in the future delivery of evidence-based care in regenerative dermatology.
- Thomas ED, Lochte HL, Lu WC, et al. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957;257:491-496.
- Ogliari KS, Marinowic D, Brum DE, et al. Stem cells in dermatology. An Bras Dermatol. 2014;89:286-291.
- Xu C, Inokuma MS, Denham J, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971-974.
- Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211-228.
- Badiavas EV, Falanga V. Treatment of chronic wounds with bone marrow-derived cells. Arch Dermatol. 2003;139:510-516.
- Ibrahim ZA, Eltatawy RA, Ghaly NR, et al. Autologous bone marrow stem cells in atrophic acne scars: a pilot study. J Dermatolog Treat. 2015;26:260-265.
- Broxmeyer HE, Douglas GW, Hangoc G, et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci U S A. 1989;86:3828-3832.
- Gluckman E, Rocha V, Boyer-Chammard A, et al. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. N Engl J Med. 1997;337:373-381.
- Valerio IL, Sabino JM, Dearth CL. Plastic surgery challenges in war wounded II: regenerative medicine. Adv Wound Care (New Rochelle). 2016;5:412-419.
- Vescarelli E, D’Amici S, Onesti MG, et al. Adipose-derived stem cell: an innovative therapeutic approach in systemic sclerosis and Parry-Romberg syndrome. CellR4. 2014;2:E791-E797.
- Yoshimura K, Sato K, Aoi N, et al. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008;32:48-55.
- Grabin S, Antes G, Stark GB, et al. Cell-assisted lipotransfer: a critical appraisal of the evidence. Dtsch Arztebl Int. 2015;112:255.
- Zhou Y, Wang J, Li H, et al. Efficacy and safety of cell-assisted lipotransfer: a systematic review and meta-analysis. Plast Reconstr Surg. 2016;137:E44-E57.
- Toma JG, Akhavan M, Fernandes KJL, et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778-784.
- Toma JG, McKenzie IA, Bagli D, et al. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005;23:727-737.
- Homicz MR, Watson D. Review of injectable materials for soft tissue augmentation. Facial Plast Surg. 2004;20:21-29.
- Kumar S, Mahajan BB, Kaur S, et al. Autologous therapies in dermatology. J Clin Aesthet Dermatol. 2014;7:38-45.
- Schmidt C. FDA approves first cell therapy for wrinkle-free visage. Nat Biotech. 2011;29:674-675.
- Gentile P, Scioli MG, Bielli A, et al. Stem cells from human hair follicles: first mechanical isolation for immediate autologous clinical use in androgenetic alopecia and hair loss. Stem Cell Investig. 2017;4:58.
- Sugiyama-Nakagiri Y, Akiyama M, Shimizu H. Hair follicle stem cell-targeted gene transfer and reconstitution system. Gene Ther. 2006;13:732-737.
- Heidari F, Yari A, Rasoolijazi H, et al. Bulge hair follicle stem cells accelerate cutaneous wound healing in rats. Wounds. 2016;28:132-141.
- Lee JH, Fisher DE. Melanocyte stem cells as potential therapeutics in skin disorders. Expert Opin Biol Ther. 2014;14:1-11.
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676.
- Singh VK, Kalsan M, Kumar N, et al. Induced pluripotent stem cells: applications in regenerative medicine, disease modeling, and drug discovery. Front Cell Dev Biol. 2015;3:2.
- Aoi T. 10th anniversary of iPS cells: The challenges that lie ahead. J Biochem. 2016;160:121-129.
- Lowry WE, Plath K. The many ways to make an iPS cell. Nat Biotechnol. 2008;26:1246-1248.
- Kim K, Doi A, Wen B, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285-290.
- Gafni O, Weinberger L, Mansour AA, et al. Derivation of novel human ground state naive pluripotent stem cells. Nature. 2013;504:282-286.
- Pareja-Galeano H, Sanchis-Gomar F, Pérez LM, et al. IPSCs-based anti-aging therapies: Recent discoveries and future challenges. Ageing Res Rev. 2016;27:37-41.
- Itoh M, Umegaki-Arao N, Guo Z, et al. Generation of 3D skin equivalents fully reconstituted from human induced pluripotent stem cells (iPSCs). PLoS One. 2013;8:e77673.
- Nyström A, Velati D, Mittapalli VR, et al. Collagen VII plays a dual role in wound healing. J Clin Invest. 2013;123:3498-3509.
- Robbins PB, Lin Q, Goodnough JB, et al. In vivo restoration of laminin 5 β3 expression and function in junctional epidermolysis bullosa. Proc Natl Acad Sci. 2001;98:5193-5198.
- Sebastiano V, Zhen HH, Haddad B, et al. Human COL7A1-corrected induced pluripotent stem cells for the treatment of recessive dystrophic epidermolysis bullosa. Sci Transl Med. 2014;6:264ra163.
- Zhang J, Guan J, Niu X, et al. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13:49.
- Pap E, Pállinger É, Pásztói M, et al. Highlights of a new type of intercellular communication: microvesicle-based information transfer. Inflamm Res. 2009;58:1-8.
- Xu R, Taskin MB, Rubert M, et al. hiPS-MSCs differentiation towards fibroblasts on a 3D ECM mimicking scaffold. Sci Rep. 2015;5:8480.
- Wenzel D, Bayerl J, Nyström A, et al. Genetically corrected iPSCs as cell therapy for recessive dystrophic epidermolysis bullosa. Sci Transl Med. 2014;6:264ra165.
- Bednarska K, Kieszek R, Domagała P, et al. The use of platelet-rich-plasma in aesthetic and regenerative medicine. MEDtube Science. 2015;2:8-15.
- Hashim PW, Levy Z, Cohen JL, et al. Microneedling therapy with and without platelet-rich plasma. Cutis. 2017;99:239-242.
- Thomas ED, Lochte HL, Lu WC, et al. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957;257:491-496.
- Ogliari KS, Marinowic D, Brum DE, et al. Stem cells in dermatology. An Bras Dermatol. 2014;89:286-291.
- Xu C, Inokuma MS, Denham J, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971-974.
- Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211-228.
- Badiavas EV, Falanga V. Treatment of chronic wounds with bone marrow-derived cells. Arch Dermatol. 2003;139:510-516.
- Ibrahim ZA, Eltatawy RA, Ghaly NR, et al. Autologous bone marrow stem cells in atrophic acne scars: a pilot study. J Dermatolog Treat. 2015;26:260-265.
- Broxmeyer HE, Douglas GW, Hangoc G, et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci U S A. 1989;86:3828-3832.
- Gluckman E, Rocha V, Boyer-Chammard A, et al. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. N Engl J Med. 1997;337:373-381.
- Valerio IL, Sabino JM, Dearth CL. Plastic surgery challenges in war wounded II: regenerative medicine. Adv Wound Care (New Rochelle). 2016;5:412-419.
- Vescarelli E, D’Amici S, Onesti MG, et al. Adipose-derived stem cell: an innovative therapeutic approach in systemic sclerosis and Parry-Romberg syndrome. CellR4. 2014;2:E791-E797.
- Yoshimura K, Sato K, Aoi N, et al. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008;32:48-55.
- Grabin S, Antes G, Stark GB, et al. Cell-assisted lipotransfer: a critical appraisal of the evidence. Dtsch Arztebl Int. 2015;112:255.
- Zhou Y, Wang J, Li H, et al. Efficacy and safety of cell-assisted lipotransfer: a systematic review and meta-analysis. Plast Reconstr Surg. 2016;137:E44-E57.
- Toma JG, Akhavan M, Fernandes KJL, et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778-784.
- Toma JG, McKenzie IA, Bagli D, et al. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005;23:727-737.
- Homicz MR, Watson D. Review of injectable materials for soft tissue augmentation. Facial Plast Surg. 2004;20:21-29.
- Kumar S, Mahajan BB, Kaur S, et al. Autologous therapies in dermatology. J Clin Aesthet Dermatol. 2014;7:38-45.
- Schmidt C. FDA approves first cell therapy for wrinkle-free visage. Nat Biotech. 2011;29:674-675.
- Gentile P, Scioli MG, Bielli A, et al. Stem cells from human hair follicles: first mechanical isolation for immediate autologous clinical use in androgenetic alopecia and hair loss. Stem Cell Investig. 2017;4:58.
- Sugiyama-Nakagiri Y, Akiyama M, Shimizu H. Hair follicle stem cell-targeted gene transfer and reconstitution system. Gene Ther. 2006;13:732-737.
- Heidari F, Yari A, Rasoolijazi H, et al. Bulge hair follicle stem cells accelerate cutaneous wound healing in rats. Wounds. 2016;28:132-141.
- Lee JH, Fisher DE. Melanocyte stem cells as potential therapeutics in skin disorders. Expert Opin Biol Ther. 2014;14:1-11.
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676.
- Singh VK, Kalsan M, Kumar N, et al. Induced pluripotent stem cells: applications in regenerative medicine, disease modeling, and drug discovery. Front Cell Dev Biol. 2015;3:2.
- Aoi T. 10th anniversary of iPS cells: The challenges that lie ahead. J Biochem. 2016;160:121-129.
- Lowry WE, Plath K. The many ways to make an iPS cell. Nat Biotechnol. 2008;26:1246-1248.
- Kim K, Doi A, Wen B, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285-290.
- Gafni O, Weinberger L, Mansour AA, et al. Derivation of novel human ground state naive pluripotent stem cells. Nature. 2013;504:282-286.
- Pareja-Galeano H, Sanchis-Gomar F, Pérez LM, et al. IPSCs-based anti-aging therapies: Recent discoveries and future challenges. Ageing Res Rev. 2016;27:37-41.
- Itoh M, Umegaki-Arao N, Guo Z, et al. Generation of 3D skin equivalents fully reconstituted from human induced pluripotent stem cells (iPSCs). PLoS One. 2013;8:e77673.
- Nyström A, Velati D, Mittapalli VR, et al. Collagen VII plays a dual role in wound healing. J Clin Invest. 2013;123:3498-3509.
- Robbins PB, Lin Q, Goodnough JB, et al. In vivo restoration of laminin 5 β3 expression and function in junctional epidermolysis bullosa. Proc Natl Acad Sci. 2001;98:5193-5198.
- Sebastiano V, Zhen HH, Haddad B, et al. Human COL7A1-corrected induced pluripotent stem cells for the treatment of recessive dystrophic epidermolysis bullosa. Sci Transl Med. 2014;6:264ra163.
- Zhang J, Guan J, Niu X, et al. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13:49.
- Pap E, Pállinger É, Pásztói M, et al. Highlights of a new type of intercellular communication: microvesicle-based information transfer. Inflamm Res. 2009;58:1-8.
- Xu R, Taskin MB, Rubert M, et al. hiPS-MSCs differentiation towards fibroblasts on a 3D ECM mimicking scaffold. Sci Rep. 2015;5:8480.
- Wenzel D, Bayerl J, Nyström A, et al. Genetically corrected iPSCs as cell therapy for recessive dystrophic epidermolysis bullosa. Sci Transl Med. 2014;6:264ra165.
- Bednarska K, Kieszek R, Domagała P, et al. The use of platelet-rich-plasma in aesthetic and regenerative medicine. MEDtube Science. 2015;2:8-15.
- Hashim PW, Levy Z, Cohen JL, et al. Microneedling therapy with and without platelet-rich plasma. Cutis. 2017;99:239-242.
Practice Points
- Multipotent stem cells derived from the bone marrow, umbilical cord, adipose tissue, dermis, and hair follicle bulge show promise in tissue regeneration for various dermatologic conditions and aesthetic applications.
- Induced pluripotent stem cells, progenitor cells that result from the dedifferentiation of specialized adult cells, have potential for collagen generation.
Ideals of Facial Beauty
Several concepts of ideal aesthetic measurements can be traced back to ancient Greek and European Renaissance art. In examining canons of beauty, these classical ideals often are compared to modern-day standards, allowing clinicians to delineate the parameters of an attractive facial appearance and facilitate the planning of cosmetic procedures.
Given the growing number of available cosmetic interventions, dermatologists have a powerful ability to modify facial proportions; however, changes to individual structures should be made with a mindful approach to improving overall facial harmony. This article reviews the established parameters of facial beauty to assist the clinician in enhancing cosmetic outcomes.
Canons of Facial Aesthetics
Horizontal Thirds
In his writings on human anatomy, Leonardo da Vinci described dividing the face into equal thirds (Figure 1). The upper third measures from the trichion (the midline point of the normal hairline) to the glabella (the smooth prominence between the eyebrows). The middle third measures from the glabella to the subnasale (the midline point where the nasal septum meets the upper lip). The lower third measures from the subnasale to the menton (the most inferior point of the chin).1
Although the validity of the canon is intended to apply across race and gender, these proportions may vary by ethnicity (Table). In white individuals, the middle third of the face tends to be shorter than the upper and lower thirds.2 This same relationship has been observed in black males.3 In Chinese females, the upper third commonly is shorter than the middle and lower thirds, correlating with a less prominent forehead. In contrast, black females tend to have a relatively longer upper third.4
The relationship between modern perceptions of attractiveness and the neoclassical norm of equal thirds remains a topic of interest. Milutinovic et al1 examined facial thirds in white female celebrities from beauty and fashion magazines and compared them to a group of anonymous white females from the general population. The group of anonymous females showed statistically significant (P<.05) differences between the sizes of the 3 facial segments, whereas the group of celebrity faces demonstrated uniformity between the facial thirds.1
The lower face can itself be divided into thirds, with the upper third measured from the subnasale to the stomion (the midline point of the oral fissure when the lips are closed), and the lower two-thirds measured from the stomion to the menton (Figure 1). Mommaerts and Moerenhout5 examined photographs of 105 attractive celebrity faces and compared their proportions to those of classical sculptures of gods and goddesses (antique faces). The authors identified an upper one-third to lower two-thirds ratio of 69.8% in celebrity females and 69.1% in celebrity males; these ratios were not significantly different from the 72.4% seen in antique females and 73.1% in antique males. The authors concluded that a 30% upper lip to 70% lower lip-chin proportion may be the most appropriate to describe contemporary standards.5
Vertical Fifths
In the vertical dimension, the neoclassical canon of facial proportions divides the face into equal fifths (Figure 2).6 The 2 most lateral fifths are measured from the lateral helix of each ear to the exocanthus of each eye. The eye fissure lengths (measured between the endocanthion and exocanthion of each eye) represent one-fifth. The middle fifth is measured between the medial canthi of both eyes (endocanthion to endocanthion). This distance is equal to the width of the nose, as measured between both alae. Finally, the width of the mouth represents 1.5-times the width of the nose. These ratios of the vertical fifths apply to both males and females.6
Anthropometric studies have examined deviations from the neoclassical canon according to ethnicity. Wang et al7 compared the measurements of North American white and Han Chinese patients to these standards. White patients demonstrated a greater ratio of mouth width to nose width relative to the canon. In contrast, Han Chinese patients demonstrated a relatively wider nose and narrower mouth.7
In black individuals, it has been observed that the dimensions of most facial segments correspond to the neoclassical standards; however, nose width is relatively wider in black individuals relative to the canon as well as relative to white individuals.8
Milutinovic et al1 also compared vertical fifths between white celebrities and anonymous females. In the anonymous female group, statistically significant (P<.05) variations were found between the sizes of the different facial components. In contrast, the celebrity female group showed balance between the widths of vertical fifths.1
Lips
In the lower facial third, the lips represent a key element of attractiveness. Recently, lip augmentation, aimed at creating fuller and plumper lips, has dominated the popular culture and social media landscape.9 Although the aesthetic ideal of lips continues to evolve over time, recent studies have aimed at quantifying modern notions of attractive lip appearance.
Popenko et al10 examined lip measurements using computer-generated images of white women with different variations of lip sizes and lower face proportions. Computer-generated faces were graded on attractiveness by more than 400 individuals from focus groups. An upper lip to lower lip ratio of 1:2 was judged to be the most attractive, while a ratio of 2:1 was judged to be the least attractive. Results also showed that the surface area of the most attractive lips comprised roughly 10% of the lower third of the face.10
Penna et al11 analyzed various parameters of the lips and lower facial third using photographs of 176 white males and females that were judged on attractiveness by 250 volunteer evaluators. Faces were graded on a scale from 1 (absolutely attractive) to 7 (absolutely unattractive). Attractive males and females (grades 1 and 2) both demonstrated an average ratio of upper vermilion height to nose-mouth distance (measured from the subnasalae to the lower edge of the upper vermilion border) of 0.28, which was significantly greater than the average ratio observed in less attractive individuals (grades 6 or 7)(P<.05). In addition, attractive males and females demonstrated a ratio of upper vermilion height to nose-chin distance (measured from the subnasalae to the menton) of 0.09, which again was larger than the average ratio seen in less attractive individuals. Figure 3 demonstrates an aesthetic ideal of the lips derived from these 2 studies, though consideration should be given to the fact that these studies were based in white populations.
Golden Ratio
The golden ratio, also known as Phi, can be observed in nature, art, and architecture. Approximately equal to 1.618, the golden ratio also has been identified as a possible marker of beauty in the human face and has garnered attention in the lay press. The ratio has been applied to several proportions and structures in the face, such as the ratio of mouth width to nose width or the ratio of tooth height to tooth width, with investigation providing varying levels of validation about whether these ratios truly correlate with perceptions of beauty.12 Swift and Remington13 advocated for application of the golden ratio toward a comprehensive set of facial proportions. Marquardt14 used the golden ratio to create a 3-dimensional representation of an idealized face, known as the golden decagon mask. Although the golden ratio and the golden decagon mask have been proposed as analytic tools, their utility in clinical practice may be limited. Firstly, due to its popularity in the lay press, the golden ratio has been inconsistently applied to a wide range of facial ratios, which may undermine confidence in its representation as truth rather than coincidence. Secondly, although some authors have found validity of the golden decagon mask in representing unified ratios of attractiveness, others have asserted that it characterizes a masculinized white female and fails to account for ethnic differences.15-19
Age-Related Changes
In addition to the facial proportions guided by genetics, several changes occur with increased age. Over the course of a lifetime, predictable patterns emerge in the dimensions of the skin, soft tissue, and bone. These alterations in structural proportions may ultimately lead to an unevenness in facial aesthetics.
In skeletal structure, gradual bone resorption and expansion causes a reduction in facial height as well as an increase in facial width and depth.20 Fat atrophy and hypertrophy affect soft tissue proportions, visualized as hollowing at the temples, cheeks, and around the eyes, along with fullness in the submental region and jowls.21 Finally, decreases in skin elasticity and collagen exacerbate the appearance of rhytides and sagging. In older patients who desire a more youthful appearance, various applications of dermal fillers, fat grafting, liposuction, and skin tightening techniques can help to mitigate these changes.
Conclusion
Improving facial aesthetics relies on an understanding of the norms of facial proportions. Although cosmetic interventions commonly are advertised or described based on a single anatomical unit, it is important to appreciate the relationships between facial structures. Most notably, clinicians should be mindful of facial ratios when considering the introduction of filler materials or implants. Augmentation procedures at the temples, zygomatic arch, jaw, chin, and lips all have the possibility to alter facial ratios. Changes should therefore be considered in the context of improving overall facial harmony, with the clinician remaining cognizant of the ideal vertical and horizontal divisions of the face. Understanding such concepts and communicating them to patients can help in appropriately addressing all target areas, thereby leading to greater patient satisfaction.
- Milutinovic J, Zelic K, Nedeljkovic N. Evaluation of facial beauty using anthropometric proportions. ScientificWorldJournal. 2014;2014:428250. doi:10.1155/2014/428250.
- Farkas LG, Hreczko TA, Kolar JC, et al. Vertical and horizontal proportions of the face in young-adult North-American Caucasians: revision of neoclassical canons. Plast Reconstr Surg. 1985;75:328-338.
- Porter JP. The average African American male face: an anthropometric analysis. Arch Facial Plast Surg. 2004;6:78-81.
- Porter JP, Olson KL. Anthropometric facial analysis of the African American woman. Arch Facial Plast Surg. 2001;3:191-197.
- Mommaerts MY, Moerenhout BA. Ideal proportions in full face front view, contemporary versus antique. J Craniomaxillofac Surg. 2011;39:107-110.
- Vegter F, Hage JJ. Clinical anthropometry and canons of the face in historical perspective. Plast Reconstr Surg. 2000;106:1090-1096.
- Wang D, Qian G, Zhang M, et al. Differences in horizontal, neoclassical facial canons in Chinese (Han) and North American Caucasian populations. Aesthetic Plast Surg. 1997;21:265-269.
- Farkas LG, Forrest CR, Litsas L. Revision of neoclassical facial canons in young adult Afro-Americans. Aesthetic Plast Surg. 2000;24:179-184.
- Coleman GG, Lindauer SJ, Tüfekçi E, et al. Influence of chin prominence on esthetic lip profile preferences. Am J Orthod Dentofacial Orthop. 2007;132:36-42.
- Popenko NA, Tripathi PB, Devcic Z, et al. A quantitative approach to determining the ideal female lip aesthetic and its effect on facial attractiveness. JAMA Facial Plast Surg. 2017;19:261-267.
- Penna V, Fricke A, Iblher N, et al. The attractive lip: a photomorphometric analysis. J Plast Reconstr Aesthet Surg. 2015;68:920-929.
- Prokopakis EP, Vlastos IM, Picavet VA, et al. The golden ratio in facial symmetry. Rhinology. 2013;51:18-21.
- Swift A, Remington K. BeautiPHIcationTM: a global approach to facial beauty. Clin Plast Surg. 2011;38:247-277.
- Marquardt SR. Dr. Stephen R. Marquardt on the Golden Decagon and human facial beauty. interview by Dr. Gottlieb. J Clin Orthod. 2002;36:339-347.
- Veerala G, Gandikota CS, Yadagiri PK, et al. Marquardt’s facial Golden Decagon mask and its fitness with South Indian facial traits. J Clin Diagn Res. 2016;10:ZC49-ZC52.
- Holland E. Marquardt’s Phi mask: pitfalls of relying on fashion models and the golden ratio to describe a beautiful face. Aesthetic Plast Surg. 2008;32:200-208.
- Alam MK, Mohd Noor NF, Basri R, et al. Multiracial facial golden ratio and evaluation of facial appearance. PLoS One. 2015;10:e0142914.
- Kim YH. Easy facial analysis using the facial golden mask. J Craniofac Surg. 2007;18:643-649.
- Bashour M. An objective system for measuring facial attractiveness. Plast Reconstr Surg. 2006;118:757-774; discussion 775-776.
- Bartlett SP, Grossman R, Whitaker LA. Age-related changes of the craniofacial skeleton: an anthropometric and histologic analysis. Plast Reconstr Surg. 1992;90:592-600.
- Donofrio LM. Fat distribution: a morphologic study of the aging face. Dermatol Surg. 2000;26:1107-1112.
Several concepts of ideal aesthetic measurements can be traced back to ancient Greek and European Renaissance art. In examining canons of beauty, these classical ideals often are compared to modern-day standards, allowing clinicians to delineate the parameters of an attractive facial appearance and facilitate the planning of cosmetic procedures.
Given the growing number of available cosmetic interventions, dermatologists have a powerful ability to modify facial proportions; however, changes to individual structures should be made with a mindful approach to improving overall facial harmony. This article reviews the established parameters of facial beauty to assist the clinician in enhancing cosmetic outcomes.
Canons of Facial Aesthetics
Horizontal Thirds
In his writings on human anatomy, Leonardo da Vinci described dividing the face into equal thirds (Figure 1). The upper third measures from the trichion (the midline point of the normal hairline) to the glabella (the smooth prominence between the eyebrows). The middle third measures from the glabella to the subnasale (the midline point where the nasal septum meets the upper lip). The lower third measures from the subnasale to the menton (the most inferior point of the chin).1
Although the validity of the canon is intended to apply across race and gender, these proportions may vary by ethnicity (Table). In white individuals, the middle third of the face tends to be shorter than the upper and lower thirds.2 This same relationship has been observed in black males.3 In Chinese females, the upper third commonly is shorter than the middle and lower thirds, correlating with a less prominent forehead. In contrast, black females tend to have a relatively longer upper third.4
The relationship between modern perceptions of attractiveness and the neoclassical norm of equal thirds remains a topic of interest. Milutinovic et al1 examined facial thirds in white female celebrities from beauty and fashion magazines and compared them to a group of anonymous white females from the general population. The group of anonymous females showed statistically significant (P<.05) differences between the sizes of the 3 facial segments, whereas the group of celebrity faces demonstrated uniformity between the facial thirds.1
The lower face can itself be divided into thirds, with the upper third measured from the subnasale to the stomion (the midline point of the oral fissure when the lips are closed), and the lower two-thirds measured from the stomion to the menton (Figure 1). Mommaerts and Moerenhout5 examined photographs of 105 attractive celebrity faces and compared their proportions to those of classical sculptures of gods and goddesses (antique faces). The authors identified an upper one-third to lower two-thirds ratio of 69.8% in celebrity females and 69.1% in celebrity males; these ratios were not significantly different from the 72.4% seen in antique females and 73.1% in antique males. The authors concluded that a 30% upper lip to 70% lower lip-chin proportion may be the most appropriate to describe contemporary standards.5

Vertical Fifths
In the vertical dimension, the neoclassical canon of facial proportions divides the face into equal fifths (Figure 2).6 The 2 most lateral fifths are measured from the lateral helix of each ear to the exocanthus of each eye. The eye fissure lengths (measured between the endocanthion and exocanthion of each eye) represent one-fifth. The middle fifth is measured between the medial canthi of both eyes (endocanthion to endocanthion). This distance is equal to the width of the nose, as measured between both alae. Finally, the width of the mouth represents 1.5-times the width of the nose. These ratios of the vertical fifths apply to both males and females.6

Anthropometric studies have examined deviations from the neoclassical canon according to ethnicity. Wang et al7 compared the measurements of North American white and Han Chinese patients to these standards. White patients demonstrated a greater ratio of mouth width to nose width relative to the canon. In contrast, Han Chinese patients demonstrated a relatively wider nose and narrower mouth.7
In black individuals, it has been observed that the dimensions of most facial segments correspond to the neoclassical standards; however, nose width is relatively wider in black individuals relative to the canon as well as relative to white individuals.8
Milutinovic et al1 also compared vertical fifths between white celebrities and anonymous females. In the anonymous female group, statistically significant (P<.05) variations were found between the sizes of the different facial components. In contrast, the celebrity female group showed balance between the widths of vertical fifths.1
Lips
In the lower facial third, the lips represent a key element of attractiveness. Recently, lip augmentation, aimed at creating fuller and plumper lips, has dominated the popular culture and social media landscape.9 Although the aesthetic ideal of lips continues to evolve over time, recent studies have aimed at quantifying modern notions of attractive lip appearance.
Popenko et al10 examined lip measurements using computer-generated images of white women with different variations of lip sizes and lower face proportions. Computer-generated faces were graded on attractiveness by more than 400 individuals from focus groups. An upper lip to lower lip ratio of 1:2 was judged to be the most attractive, while a ratio of 2:1 was judged to be the least attractive. Results also showed that the surface area of the most attractive lips comprised roughly 10% of the lower third of the face.10
Penna et al11 analyzed various parameters of the lips and lower facial third using photographs of 176 white males and females that were judged on attractiveness by 250 volunteer evaluators. Faces were graded on a scale from 1 (absolutely attractive) to 7 (absolutely unattractive). Attractive males and females (grades 1 and 2) both demonstrated an average ratio of upper vermilion height to nose-mouth distance (measured from the subnasalae to the lower edge of the upper vermilion border) of 0.28, which was significantly greater than the average ratio observed in less attractive individuals (grades 6 or 7)(P<.05). In addition, attractive males and females demonstrated a ratio of upper vermilion height to nose-chin distance (measured from the subnasalae to the menton) of 0.09, which again was larger than the average ratio seen in less attractive individuals. Figure 3 demonstrates an aesthetic ideal of the lips derived from these 2 studies, though consideration should be given to the fact that these studies were based in white populations.

Golden Ratio
The golden ratio, also known as Phi, can be observed in nature, art, and architecture. Approximately equal to 1.618, the golden ratio also has been identified as a possible marker of beauty in the human face and has garnered attention in the lay press. The ratio has been applied to several proportions and structures in the face, such as the ratio of mouth width to nose width or the ratio of tooth height to tooth width, with investigation providing varying levels of validation about whether these ratios truly correlate with perceptions of beauty.12 Swift and Remington13 advocated for application of the golden ratio toward a comprehensive set of facial proportions. Marquardt14 used the golden ratio to create a 3-dimensional representation of an idealized face, known as the golden decagon mask. Although the golden ratio and the golden decagon mask have been proposed as analytic tools, their utility in clinical practice may be limited. Firstly, due to its popularity in the lay press, the golden ratio has been inconsistently applied to a wide range of facial ratios, which may undermine confidence in its representation as truth rather than coincidence. Secondly, although some authors have found validity of the golden decagon mask in representing unified ratios of attractiveness, others have asserted that it characterizes a masculinized white female and fails to account for ethnic differences.15-19
Age-Related Changes
In addition to the facial proportions guided by genetics, several changes occur with increased age. Over the course of a lifetime, predictable patterns emerge in the dimensions of the skin, soft tissue, and bone. These alterations in structural proportions may ultimately lead to an unevenness in facial aesthetics.
In skeletal structure, gradual bone resorption and expansion causes a reduction in facial height as well as an increase in facial width and depth.20 Fat atrophy and hypertrophy affect soft tissue proportions, visualized as hollowing at the temples, cheeks, and around the eyes, along with fullness in the submental region and jowls.21 Finally, decreases in skin elasticity and collagen exacerbate the appearance of rhytides and sagging. In older patients who desire a more youthful appearance, various applications of dermal fillers, fat grafting, liposuction, and skin tightening techniques can help to mitigate these changes.
Conclusion
Improving facial aesthetics relies on an understanding of the norms of facial proportions. Although cosmetic interventions commonly are advertised or described based on a single anatomical unit, it is important to appreciate the relationships between facial structures. Most notably, clinicians should be mindful of facial ratios when considering the introduction of filler materials or implants. Augmentation procedures at the temples, zygomatic arch, jaw, chin, and lips all have the possibility to alter facial ratios. Changes should therefore be considered in the context of improving overall facial harmony, with the clinician remaining cognizant of the ideal vertical and horizontal divisions of the face. Understanding such concepts and communicating them to patients can help in appropriately addressing all target areas, thereby leading to greater patient satisfaction.
Several concepts of ideal aesthetic measurements can be traced back to ancient Greek and European Renaissance art. In examining canons of beauty, these classical ideals often are compared to modern-day standards, allowing clinicians to delineate the parameters of an attractive facial appearance and facilitate the planning of cosmetic procedures.
Given the growing number of available cosmetic interventions, dermatologists have a powerful ability to modify facial proportions; however, changes to individual structures should be made with a mindful approach to improving overall facial harmony. This article reviews the established parameters of facial beauty to assist the clinician in enhancing cosmetic outcomes.
Canons of Facial Aesthetics
Horizontal Thirds
In his writings on human anatomy, Leonardo da Vinci described dividing the face into equal thirds (Figure 1). The upper third measures from the trichion (the midline point of the normal hairline) to the glabella (the smooth prominence between the eyebrows). The middle third measures from the glabella to the subnasale (the midline point where the nasal septum meets the upper lip). The lower third measures from the subnasale to the menton (the most inferior point of the chin).1
Although the validity of the canon is intended to apply across race and gender, these proportions may vary by ethnicity (Table). In white individuals, the middle third of the face tends to be shorter than the upper and lower thirds.2 This same relationship has been observed in black males.3 In Chinese females, the upper third commonly is shorter than the middle and lower thirds, correlating with a less prominent forehead. In contrast, black females tend to have a relatively longer upper third.4
The relationship between modern perceptions of attractiveness and the neoclassical norm of equal thirds remains a topic of interest. Milutinovic et al1 examined facial thirds in white female celebrities from beauty and fashion magazines and compared them to a group of anonymous white females from the general population. The group of anonymous females showed statistically significant (P<.05) differences between the sizes of the 3 facial segments, whereas the group of celebrity faces demonstrated uniformity between the facial thirds.1
The lower face can itself be divided into thirds, with the upper third measured from the subnasale to the stomion (the midline point of the oral fissure when the lips are closed), and the lower two-thirds measured from the stomion to the menton (Figure 1). Mommaerts and Moerenhout5 examined photographs of 105 attractive celebrity faces and compared their proportions to those of classical sculptures of gods and goddesses (antique faces). The authors identified an upper one-third to lower two-thirds ratio of 69.8% in celebrity females and 69.1% in celebrity males; these ratios were not significantly different from the 72.4% seen in antique females and 73.1% in antique males. The authors concluded that a 30% upper lip to 70% lower lip-chin proportion may be the most appropriate to describe contemporary standards.5

Vertical Fifths
In the vertical dimension, the neoclassical canon of facial proportions divides the face into equal fifths (Figure 2).6 The 2 most lateral fifths are measured from the lateral helix of each ear to the exocanthus of each eye. The eye fissure lengths (measured between the endocanthion and exocanthion of each eye) represent one-fifth. The middle fifth is measured between the medial canthi of both eyes (endocanthion to endocanthion). This distance is equal to the width of the nose, as measured between both alae. Finally, the width of the mouth represents 1.5-times the width of the nose. These ratios of the vertical fifths apply to both males and females.6

Anthropometric studies have examined deviations from the neoclassical canon according to ethnicity. Wang et al7 compared the measurements of North American white and Han Chinese patients to these standards. White patients demonstrated a greater ratio of mouth width to nose width relative to the canon. In contrast, Han Chinese patients demonstrated a relatively wider nose and narrower mouth.7
In black individuals, it has been observed that the dimensions of most facial segments correspond to the neoclassical standards; however, nose width is relatively wider in black individuals relative to the canon as well as relative to white individuals.8
Milutinovic et al1 also compared vertical fifths between white celebrities and anonymous females. In the anonymous female group, statistically significant (P<.05) variations were found between the sizes of the different facial components. In contrast, the celebrity female group showed balance between the widths of vertical fifths.1
Lips
In the lower facial third, the lips represent a key element of attractiveness. Recently, lip augmentation, aimed at creating fuller and plumper lips, has dominated the popular culture and social media landscape.9 Although the aesthetic ideal of lips continues to evolve over time, recent studies have aimed at quantifying modern notions of attractive lip appearance.
Popenko et al10 examined lip measurements using computer-generated images of white women with different variations of lip sizes and lower face proportions. Computer-generated faces were graded on attractiveness by more than 400 individuals from focus groups. An upper lip to lower lip ratio of 1:2 was judged to be the most attractive, while a ratio of 2:1 was judged to be the least attractive. Results also showed that the surface area of the most attractive lips comprised roughly 10% of the lower third of the face.10
Penna et al11 analyzed various parameters of the lips and lower facial third using photographs of 176 white males and females that were judged on attractiveness by 250 volunteer evaluators. Faces were graded on a scale from 1 (absolutely attractive) to 7 (absolutely unattractive). Attractive males and females (grades 1 and 2) both demonstrated an average ratio of upper vermilion height to nose-mouth distance (measured from the subnasalae to the lower edge of the upper vermilion border) of 0.28, which was significantly greater than the average ratio observed in less attractive individuals (grades 6 or 7)(P<.05). In addition, attractive males and females demonstrated a ratio of upper vermilion height to nose-chin distance (measured from the subnasalae to the menton) of 0.09, which again was larger than the average ratio seen in less attractive individuals. Figure 3 demonstrates an aesthetic ideal of the lips derived from these 2 studies, though consideration should be given to the fact that these studies were based in white populations.

Golden Ratio
The golden ratio, also known as Phi, can be observed in nature, art, and architecture. Approximately equal to 1.618, the golden ratio also has been identified as a possible marker of beauty in the human face and has garnered attention in the lay press. The ratio has been applied to several proportions and structures in the face, such as the ratio of mouth width to nose width or the ratio of tooth height to tooth width, with investigation providing varying levels of validation about whether these ratios truly correlate with perceptions of beauty.12 Swift and Remington13 advocated for application of the golden ratio toward a comprehensive set of facial proportions. Marquardt14 used the golden ratio to create a 3-dimensional representation of an idealized face, known as the golden decagon mask. Although the golden ratio and the golden decagon mask have been proposed as analytic tools, their utility in clinical practice may be limited. Firstly, due to its popularity in the lay press, the golden ratio has been inconsistently applied to a wide range of facial ratios, which may undermine confidence in its representation as truth rather than coincidence. Secondly, although some authors have found validity of the golden decagon mask in representing unified ratios of attractiveness, others have asserted that it characterizes a masculinized white female and fails to account for ethnic differences.15-19
Age-Related Changes
In addition to the facial proportions guided by genetics, several changes occur with increased age. Over the course of a lifetime, predictable patterns emerge in the dimensions of the skin, soft tissue, and bone. These alterations in structural proportions may ultimately lead to an unevenness in facial aesthetics.
In skeletal structure, gradual bone resorption and expansion causes a reduction in facial height as well as an increase in facial width and depth.20 Fat atrophy and hypertrophy affect soft tissue proportions, visualized as hollowing at the temples, cheeks, and around the eyes, along with fullness in the submental region and jowls.21 Finally, decreases in skin elasticity and collagen exacerbate the appearance of rhytides and sagging. In older patients who desire a more youthful appearance, various applications of dermal fillers, fat grafting, liposuction, and skin tightening techniques can help to mitigate these changes.
Conclusion
Improving facial aesthetics relies on an understanding of the norms of facial proportions. Although cosmetic interventions commonly are advertised or described based on a single anatomical unit, it is important to appreciate the relationships between facial structures. Most notably, clinicians should be mindful of facial ratios when considering the introduction of filler materials or implants. Augmentation procedures at the temples, zygomatic arch, jaw, chin, and lips all have the possibility to alter facial ratios. Changes should therefore be considered in the context of improving overall facial harmony, with the clinician remaining cognizant of the ideal vertical and horizontal divisions of the face. Understanding such concepts and communicating them to patients can help in appropriately addressing all target areas, thereby leading to greater patient satisfaction.
- Milutinovic J, Zelic K, Nedeljkovic N. Evaluation of facial beauty using anthropometric proportions. ScientificWorldJournal. 2014;2014:428250. doi:10.1155/2014/428250.
- Farkas LG, Hreczko TA, Kolar JC, et al. Vertical and horizontal proportions of the face in young-adult North-American Caucasians: revision of neoclassical canons. Plast Reconstr Surg. 1985;75:328-338.
- Porter JP. The average African American male face: an anthropometric analysis. Arch Facial Plast Surg. 2004;6:78-81.
- Porter JP, Olson KL. Anthropometric facial analysis of the African American woman. Arch Facial Plast Surg. 2001;3:191-197.
- Mommaerts MY, Moerenhout BA. Ideal proportions in full face front view, contemporary versus antique. J Craniomaxillofac Surg. 2011;39:107-110.
- Vegter F, Hage JJ. Clinical anthropometry and canons of the face in historical perspective. Plast Reconstr Surg. 2000;106:1090-1096.
- Wang D, Qian G, Zhang M, et al. Differences in horizontal, neoclassical facial canons in Chinese (Han) and North American Caucasian populations. Aesthetic Plast Surg. 1997;21:265-269.
- Farkas LG, Forrest CR, Litsas L. Revision of neoclassical facial canons in young adult Afro-Americans. Aesthetic Plast Surg. 2000;24:179-184.
- Coleman GG, Lindauer SJ, Tüfekçi E, et al. Influence of chin prominence on esthetic lip profile preferences. Am J Orthod Dentofacial Orthop. 2007;132:36-42.
- Popenko NA, Tripathi PB, Devcic Z, et al. A quantitative approach to determining the ideal female lip aesthetic and its effect on facial attractiveness. JAMA Facial Plast Surg. 2017;19:261-267.
- Penna V, Fricke A, Iblher N, et al. The attractive lip: a photomorphometric analysis. J Plast Reconstr Aesthet Surg. 2015;68:920-929.
- Prokopakis EP, Vlastos IM, Picavet VA, et al. The golden ratio in facial symmetry. Rhinology. 2013;51:18-21.
- Swift A, Remington K. BeautiPHIcationTM: a global approach to facial beauty. Clin Plast Surg. 2011;38:247-277.
- Marquardt SR. Dr. Stephen R. Marquardt on the Golden Decagon and human facial beauty. interview by Dr. Gottlieb. J Clin Orthod. 2002;36:339-347.
- Veerala G, Gandikota CS, Yadagiri PK, et al. Marquardt’s facial Golden Decagon mask and its fitness with South Indian facial traits. J Clin Diagn Res. 2016;10:ZC49-ZC52.
- Holland E. Marquardt’s Phi mask: pitfalls of relying on fashion models and the golden ratio to describe a beautiful face. Aesthetic Plast Surg. 2008;32:200-208.
- Alam MK, Mohd Noor NF, Basri R, et al. Multiracial facial golden ratio and evaluation of facial appearance. PLoS One. 2015;10:e0142914.
- Kim YH. Easy facial analysis using the facial golden mask. J Craniofac Surg. 2007;18:643-649.
- Bashour M. An objective system for measuring facial attractiveness. Plast Reconstr Surg. 2006;118:757-774; discussion 775-776.
- Bartlett SP, Grossman R, Whitaker LA. Age-related changes of the craniofacial skeleton: an anthropometric and histologic analysis. Plast Reconstr Surg. 1992;90:592-600.
- Donofrio LM. Fat distribution: a morphologic study of the aging face. Dermatol Surg. 2000;26:1107-1112.
- Milutinovic J, Zelic K, Nedeljkovic N. Evaluation of facial beauty using anthropometric proportions. ScientificWorldJournal. 2014;2014:428250. doi:10.1155/2014/428250.
- Farkas LG, Hreczko TA, Kolar JC, et al. Vertical and horizontal proportions of the face in young-adult North-American Caucasians: revision of neoclassical canons. Plast Reconstr Surg. 1985;75:328-338.
- Porter JP. The average African American male face: an anthropometric analysis. Arch Facial Plast Surg. 2004;6:78-81.
- Porter JP, Olson KL. Anthropometric facial analysis of the African American woman. Arch Facial Plast Surg. 2001;3:191-197.
- Mommaerts MY, Moerenhout BA. Ideal proportions in full face front view, contemporary versus antique. J Craniomaxillofac Surg. 2011;39:107-110.
- Vegter F, Hage JJ. Clinical anthropometry and canons of the face in historical perspective. Plast Reconstr Surg. 2000;106:1090-1096.
- Wang D, Qian G, Zhang M, et al. Differences in horizontal, neoclassical facial canons in Chinese (Han) and North American Caucasian populations. Aesthetic Plast Surg. 1997;21:265-269.
- Farkas LG, Forrest CR, Litsas L. Revision of neoclassical facial canons in young adult Afro-Americans. Aesthetic Plast Surg. 2000;24:179-184.
- Coleman GG, Lindauer SJ, Tüfekçi E, et al. Influence of chin prominence on esthetic lip profile preferences. Am J Orthod Dentofacial Orthop. 2007;132:36-42.
- Popenko NA, Tripathi PB, Devcic Z, et al. A quantitative approach to determining the ideal female lip aesthetic and its effect on facial attractiveness. JAMA Facial Plast Surg. 2017;19:261-267.
- Penna V, Fricke A, Iblher N, et al. The attractive lip: a photomorphometric analysis. J Plast Reconstr Aesthet Surg. 2015;68:920-929.
- Prokopakis EP, Vlastos IM, Picavet VA, et al. The golden ratio in facial symmetry. Rhinology. 2013;51:18-21.
- Swift A, Remington K. BeautiPHIcationTM: a global approach to facial beauty. Clin Plast Surg. 2011;38:247-277.
- Marquardt SR. Dr. Stephen R. Marquardt on the Golden Decagon and human facial beauty. interview by Dr. Gottlieb. J Clin Orthod. 2002;36:339-347.
- Veerala G, Gandikota CS, Yadagiri PK, et al. Marquardt’s facial Golden Decagon mask and its fitness with South Indian facial traits. J Clin Diagn Res. 2016;10:ZC49-ZC52.
- Holland E. Marquardt’s Phi mask: pitfalls of relying on fashion models and the golden ratio to describe a beautiful face. Aesthetic Plast Surg. 2008;32:200-208.
- Alam MK, Mohd Noor NF, Basri R, et al. Multiracial facial golden ratio and evaluation of facial appearance. PLoS One. 2015;10:e0142914.
- Kim YH. Easy facial analysis using the facial golden mask. J Craniofac Surg. 2007;18:643-649.
- Bashour M. An objective system for measuring facial attractiveness. Plast Reconstr Surg. 2006;118:757-774; discussion 775-776.
- Bartlett SP, Grossman R, Whitaker LA. Age-related changes of the craniofacial skeleton: an anthropometric and histologic analysis. Plast Reconstr Surg. 1992;90:592-600.
- Donofrio LM. Fat distribution: a morphologic study of the aging face. Dermatol Surg. 2000;26:1107-1112.
Practice Points
- Canons of ideal facial dimensions have existed since antiquity and remain relevant in modern times.
- Horizontal and vertical anatomical ratios can provide a useful framework for cosmetic interventions.
- To maximize aesthetic results, alterations to individual cosmetic units should be made with thoughtful consideration of overall facial harmony.
Topical Cannabinoids in Dermatology
The prevalence of topical cannabinoids has risen sharply in recent years. Commercial advertisers promote their usage as a safe means to treat a multitude of skin disorders, including atopic dermatitis (AD), psoriasis, and acne. Topical compounds have garnered interest in laboratory studies, but the purchase of commercial formulations is limited to over-the-counter products from unregulated suppliers. In this article, we review the scientific evidence behind topical cannabinoids and evaluate their role in clinical dermatology.
Background
Cannabis is designated as a Schedule I drug, according to the Controlled Substances Act of 1970. This listing is given to substances with no therapeutic value and a high potential for abuse. However, as of 2017, 29 states and the District of Columbia have laws legalizing cannabis in some capacity. These regulations typically apply to medicinal use, though several states have now legalized recreational use.
Cannabinoids represent a broad class of chemical compounds derived from the cannabis plant. Originally, this class only comprised phytocannabinoids, cannabinoids produced by the cannabis plant. Tetrahydrocannabinol (THC) is the most well-known phytocannabinoid and leads to the psychoactive effects typically associated with cannabis use. Later investigation led to the discovery of endocannabinoids, cannabinoids that are naturally produced by human and animal bodies, as well as synthetic cannabinoids.1 Cannabidiol is a phytocannabinoid that has been investigated in neurologic and anti-inflammatory conditions.2-4
Cannabinoids act as agonists on 2 principal receptors— cannabinoid receptor type 1 (CB1) and cannabinoid receptor type 2 (CB2)—which are both G protein–coupled receptors (Figure).5 Both have distinct distributions throughout different organ systems, to which cannabinoids (eg, THC, cannabidiol, endocannabinoids) show differential binding.6,7 Importantly, the expression of CB1 and CB2 has been identified on sensory nerve fibers, inflammatory cells, and adnexal structures of human skin.8 Based on these associations, topical application of cannabinoids has become a modality of interest for dermatological disorders. These formulations aim to influence cutaneous morphology without producing psychoactive effects.
Topical Cannabinoids in Inflammatory Disorders
Atopic dermatitis has emerged as an active area of investigation for cannabinoid receptors and topical agonists (Table 1). In an animal model, Kim et al9 examined the effects of CB1 agonism on skin inflammation. Mice treated with topical CB1 agonists showed greater recovery of epidermal barrier function in acutely abrogated skin relative to those treated with a vehicle preparation. In addition, agonism of CB1 led to significant (P<.001) decreases in skin fold thickness among models of acute and chronic skin inflammation.9
Nam et al10 also examined the role of topical CB1 agonists in mice with induced AD-like symptoms. Relative to treatment with vehicle, CB1 agonists significantly reduced the recruitment of mast cells (P<.01) and lowered the blood concentration of histamine (P<.05). Given the noted decrease in the release of inflammatory mediators, the authors speculated that topical agonsim of CB1 may prove useful in several conditions related to mast cell activation, such as AD, contact dermatitis, and psoriasis.10
The anti-inflammatory properties of topical THC were evaluated by Gaffal et al.11 In a mouse model of allergic contact dermatitis, mice treated with topical THC showed decreases in myeloid immune cell infiltration, with these beneficial effects existing even in mice with deficient CB1 and CB2 receptors. These results support a potentially wide anti-inflammatory activity of topical THC.11
Topical Cannabinoids in Pain Management
The effects of smoked cannabis in treating pain have undergone thorough investigation over recent years. Benefits have been noted in treating neuropathic pain, particularly in human immunodeficiency virus–associated sensory neuropathy.12-15 Smoked cannabis also may provide value as a synergistic therapy with opioids, thereby allowing for lower opioid doses.16
In contrast, research into the relationship between topical application of cannabinoids and nociception remains in preliminary stages (Table 2). In a mouse model, Dogrul et al17 assessed the topical antinociceptive potential of a mixed CB1-CB2 agonist. Results showed significant (P<.01) and dose-dependent antinociceptive effects relative to treatment with a vehicle.17 In a related study, Yesilyurt et al18 evaluated whether a mixed CB1-CB2 agonist could enhance the antinociceptive effects of topical opioids. Among mice treated with the combination of a cannabinoid agonist and topical morphine, a significantly (P<.05) greater analgesic effect was demonstrated relative to topical morphine alone.18
Studies in humans have been far more limited. Phan et al19 conducted a small, nonrandomized, open-label trial of a topical cannabinoid cream in patients with facial postherpetic neuralgia. Of 8 patients treated, 5 noted a mean pain reduction of 87.8%. No comparison vehicle was used. Based on this narrow study design, it is difficult to extrapolate these positive results to a broader patient population.19
Commercial Products
Although preliminary models with topical cannabinoids have shown potential, large-scale clinical trials in humans have yet to be performed. Despite this lack of investigation, commercial formulations of topical cannabinoids are available to dermatology patients. These formulations are nonstandardized, and no safety data exists regarding their use. Topical cannabinoids on the market may contain various amounts of active ingredient and may be combined with a range of other compounds.
In dermatology offices, it is not uncommon for patients to express an intention to use topical cannabinoid products following their planned treatment or procedure. Patients also have been known to use topical cannabinoid products prior to dermatologic procedures, sometimes in place of an approved topical anesthetic, without consulting the physician performing the procedure. With interventions that lead to active areas of wound healing, the application of such products may increase the risk for contamination and infection. Therefore, patients should be counseled that the use of commercial topical cannabinoids could jeopardize the success of their planned procedure, put them at risk for infection, and possibly lead to systemic absorption and/or changes in wound-healing capacities.
Conclusion
Based on the results from recent animal models, cannabinoids may have a role in future treatment algorithms for several inflammatory conditions. However, current efficacy and safety data are almost entirely limited to preliminary animal studies in rodents. In addition, the formulation of topical cannabinoid products is nonstandardized and poorly regulated. As such, the present evidence does not support the use of topical cannabinoids in dermatology practices. Dermatologists should ask patients about the use of any cannabinoid products as part of a treatment program, especially given the unsubstantiated claims often made by unscrupulous advertisers. This issue highlights the need for further research and regulation.
- Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389-462.
- Giacoppo S, Galuppo M, Pollastro F, et al. A new formulation of cannabidiol in cream shows therapeutic effects in a mouse model of experimental autoimmune encephalomyelitis. Daru. 2015;23:48.
- Hammell DC, Zhang LP, Ma F, et al. Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur J Pain. 2016;20:936-948.
- Schicho R, Storr M. Topical and systemic cannabidiol improves trinitrobenzene sulfonic acid colitis in mice. Pharmacology. 2012;89:149-155.
- Howlett AC, Barth F, Bonner TI, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161-202.
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199-215.
- Svizenska I, Dubovy P, Sulcova A. Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous system structures—a short review. Pharmacol Biochem Behav. 2008;90:501-511.
- Stander S, Schmelz M, Metze D, et al. Distribution of cannabinoid receptor 1 (CB1) and 2 (CB2) on sensory nerve fibers and adnexal structures in human skin. J Dermatol Sci. 2005;38:177-188.
- Kim HJ, Kim B, Park BM, et al. Topical cannabinoid receptor 1 agonist attenuates the cutaneous inflammatory responses in oxazolone-induced atopic dermatitis model. Int J Dermatol. 2015;54:E401-E408.
- Nam G, Jeong SK, Park BM, et al. Selective cannabinoid receptor-1 agonists regulate mast cell activation in an oxazolone-induced atopic dermatitis model. Ann Dermatol. 2016;28:22-29.
- Gaffal E, Cron M, Glodde N, et al. Anti-inflammatory activity of topical THC in DNFB-mediated mouse allergic contact dermatitis independent of CB1 and CB2 receptors. Allergy. 2013;68:994-1000.
- Abrams DI, Jay CA, Shade SB, et al. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68:515-521.
- Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2009;34:672-680.
- Wilsey B, Marcotte T, Deutsch R, et al. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain. 2013;14:136-148.
- Wilsey B, Marcotte T, Tsodikov A, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain. 2008;9:506-521.
- Abrams DI, Couey P, Shade SB, et al. Cannabinoid-opioid interaction in chronic pain. Clin Pharmacol Ther. 2011;90:844-851.
- Dogrul A, Gul H, Akar A, et al. Topical cannabinoid antinociception: synergy with spinal sites. Pain. 2003;105:11-16.
- Yesilyurt O, Dogrul A, Gul H, et al. Topical cannabinoid enhances topical morphine antinociception. Pain. 2003;105:303-308.
- Phan NQ, Siepmann D, Gralow I, et al. Adjuvant topical therapy with a cannabinoid receptor agonist in facial postherpetic neuralgia. J Dtsch Dermatol Ges. 2010;8:88-91.
The prevalence of topical cannabinoids has risen sharply in recent years. Commercial advertisers promote their usage as a safe means to treat a multitude of skin disorders, including atopic dermatitis (AD), psoriasis, and acne. Topical compounds have garnered interest in laboratory studies, but the purchase of commercial formulations is limited to over-the-counter products from unregulated suppliers. In this article, we review the scientific evidence behind topical cannabinoids and evaluate their role in clinical dermatology.
Background
Cannabis is designated as a Schedule I drug, according to the Controlled Substances Act of 1970. This listing is given to substances with no therapeutic value and a high potential for abuse. However, as of 2017, 29 states and the District of Columbia have laws legalizing cannabis in some capacity. These regulations typically apply to medicinal use, though several states have now legalized recreational use.
Cannabinoids represent a broad class of chemical compounds derived from the cannabis plant. Originally, this class only comprised phytocannabinoids, cannabinoids produced by the cannabis plant. Tetrahydrocannabinol (THC) is the most well-known phytocannabinoid and leads to the psychoactive effects typically associated with cannabis use. Later investigation led to the discovery of endocannabinoids, cannabinoids that are naturally produced by human and animal bodies, as well as synthetic cannabinoids.1 Cannabidiol is a phytocannabinoid that has been investigated in neurologic and anti-inflammatory conditions.2-4
Cannabinoids act as agonists on 2 principal receptors— cannabinoid receptor type 1 (CB1) and cannabinoid receptor type 2 (CB2)—which are both G protein–coupled receptors (Figure).5 Both have distinct distributions throughout different organ systems, to which cannabinoids (eg, THC, cannabidiol, endocannabinoids) show differential binding.6,7 Importantly, the expression of CB1 and CB2 has been identified on sensory nerve fibers, inflammatory cells, and adnexal structures of human skin.8 Based on these associations, topical application of cannabinoids has become a modality of interest for dermatological disorders. These formulations aim to influence cutaneous morphology without producing psychoactive effects.

Topical Cannabinoids in Inflammatory Disorders
Atopic dermatitis has emerged as an active area of investigation for cannabinoid receptors and topical agonists (Table 1). In an animal model, Kim et al9 examined the effects of CB1 agonism on skin inflammation. Mice treated with topical CB1 agonists showed greater recovery of epidermal barrier function in acutely abrogated skin relative to those treated with a vehicle preparation. In addition, agonism of CB1 led to significant (P<.001) decreases in skin fold thickness among models of acute and chronic skin inflammation.9
Nam et al10 also examined the role of topical CB1 agonists in mice with induced AD-like symptoms. Relative to treatment with vehicle, CB1 agonists significantly reduced the recruitment of mast cells (P<.01) and lowered the blood concentration of histamine (P<.05). Given the noted decrease in the release of inflammatory mediators, the authors speculated that topical agonsim of CB1 may prove useful in several conditions related to mast cell activation, such as AD, contact dermatitis, and psoriasis.10
The anti-inflammatory properties of topical THC were evaluated by Gaffal et al.11 In a mouse model of allergic contact dermatitis, mice treated with topical THC showed decreases in myeloid immune cell infiltration, with these beneficial effects existing even in mice with deficient CB1 and CB2 receptors. These results support a potentially wide anti-inflammatory activity of topical THC.11
Topical Cannabinoids in Pain Management
The effects of smoked cannabis in treating pain have undergone thorough investigation over recent years. Benefits have been noted in treating neuropathic pain, particularly in human immunodeficiency virus–associated sensory neuropathy.12-15 Smoked cannabis also may provide value as a synergistic therapy with opioids, thereby allowing for lower opioid doses.16
In contrast, research into the relationship between topical application of cannabinoids and nociception remains in preliminary stages (Table 2). In a mouse model, Dogrul et al17 assessed the topical antinociceptive potential of a mixed CB1-CB2 agonist. Results showed significant (P<.01) and dose-dependent antinociceptive effects relative to treatment with a vehicle.17 In a related study, Yesilyurt et al18 evaluated whether a mixed CB1-CB2 agonist could enhance the antinociceptive effects of topical opioids. Among mice treated with the combination of a cannabinoid agonist and topical morphine, a significantly (P<.05) greater analgesic effect was demonstrated relative to topical morphine alone.18
Studies in humans have been far more limited. Phan et al19 conducted a small, nonrandomized, open-label trial of a topical cannabinoid cream in patients with facial postherpetic neuralgia. Of 8 patients treated, 5 noted a mean pain reduction of 87.8%. No comparison vehicle was used. Based on this narrow study design, it is difficult to extrapolate these positive results to a broader patient population.19
Commercial Products
Although preliminary models with topical cannabinoids have shown potential, large-scale clinical trials in humans have yet to be performed. Despite this lack of investigation, commercial formulations of topical cannabinoids are available to dermatology patients. These formulations are nonstandardized, and no safety data exists regarding their use. Topical cannabinoids on the market may contain various amounts of active ingredient and may be combined with a range of other compounds.
In dermatology offices, it is not uncommon for patients to express an intention to use topical cannabinoid products following their planned treatment or procedure. Patients also have been known to use topical cannabinoid products prior to dermatologic procedures, sometimes in place of an approved topical anesthetic, without consulting the physician performing the procedure. With interventions that lead to active areas of wound healing, the application of such products may increase the risk for contamination and infection. Therefore, patients should be counseled that the use of commercial topical cannabinoids could jeopardize the success of their planned procedure, put them at risk for infection, and possibly lead to systemic absorption and/or changes in wound-healing capacities.
Conclusion
Based on the results from recent animal models, cannabinoids may have a role in future treatment algorithms for several inflammatory conditions. However, current efficacy and safety data are almost entirely limited to preliminary animal studies in rodents. In addition, the formulation of topical cannabinoid products is nonstandardized and poorly regulated. As such, the present evidence does not support the use of topical cannabinoids in dermatology practices. Dermatologists should ask patients about the use of any cannabinoid products as part of a treatment program, especially given the unsubstantiated claims often made by unscrupulous advertisers. This issue highlights the need for further research and regulation.
The prevalence of topical cannabinoids has risen sharply in recent years. Commercial advertisers promote their usage as a safe means to treat a multitude of skin disorders, including atopic dermatitis (AD), psoriasis, and acne. Topical compounds have garnered interest in laboratory studies, but the purchase of commercial formulations is limited to over-the-counter products from unregulated suppliers. In this article, we review the scientific evidence behind topical cannabinoids and evaluate their role in clinical dermatology.
Background
Cannabis is designated as a Schedule I drug, according to the Controlled Substances Act of 1970. This listing is given to substances with no therapeutic value and a high potential for abuse. However, as of 2017, 29 states and the District of Columbia have laws legalizing cannabis in some capacity. These regulations typically apply to medicinal use, though several states have now legalized recreational use.
Cannabinoids represent a broad class of chemical compounds derived from the cannabis plant. Originally, this class only comprised phytocannabinoids, cannabinoids produced by the cannabis plant. Tetrahydrocannabinol (THC) is the most well-known phytocannabinoid and leads to the psychoactive effects typically associated with cannabis use. Later investigation led to the discovery of endocannabinoids, cannabinoids that are naturally produced by human and animal bodies, as well as synthetic cannabinoids.1 Cannabidiol is a phytocannabinoid that has been investigated in neurologic and anti-inflammatory conditions.2-4
Cannabinoids act as agonists on 2 principal receptors— cannabinoid receptor type 1 (CB1) and cannabinoid receptor type 2 (CB2)—which are both G protein–coupled receptors (Figure).5 Both have distinct distributions throughout different organ systems, to which cannabinoids (eg, THC, cannabidiol, endocannabinoids) show differential binding.6,7 Importantly, the expression of CB1 and CB2 has been identified on sensory nerve fibers, inflammatory cells, and adnexal structures of human skin.8 Based on these associations, topical application of cannabinoids has become a modality of interest for dermatological disorders. These formulations aim to influence cutaneous morphology without producing psychoactive effects.

Topical Cannabinoids in Inflammatory Disorders
Atopic dermatitis has emerged as an active area of investigation for cannabinoid receptors and topical agonists (Table 1). In an animal model, Kim et al9 examined the effects of CB1 agonism on skin inflammation. Mice treated with topical CB1 agonists showed greater recovery of epidermal barrier function in acutely abrogated skin relative to those treated with a vehicle preparation. In addition, agonism of CB1 led to significant (P<.001) decreases in skin fold thickness among models of acute and chronic skin inflammation.9
Nam et al10 also examined the role of topical CB1 agonists in mice with induced AD-like symptoms. Relative to treatment with vehicle, CB1 agonists significantly reduced the recruitment of mast cells (P<.01) and lowered the blood concentration of histamine (P<.05). Given the noted decrease in the release of inflammatory mediators, the authors speculated that topical agonsim of CB1 may prove useful in several conditions related to mast cell activation, such as AD, contact dermatitis, and psoriasis.10
The anti-inflammatory properties of topical THC were evaluated by Gaffal et al.11 In a mouse model of allergic contact dermatitis, mice treated with topical THC showed decreases in myeloid immune cell infiltration, with these beneficial effects existing even in mice with deficient CB1 and CB2 receptors. These results support a potentially wide anti-inflammatory activity of topical THC.11
Topical Cannabinoids in Pain Management
The effects of smoked cannabis in treating pain have undergone thorough investigation over recent years. Benefits have been noted in treating neuropathic pain, particularly in human immunodeficiency virus–associated sensory neuropathy.12-15 Smoked cannabis also may provide value as a synergistic therapy with opioids, thereby allowing for lower opioid doses.16
In contrast, research into the relationship between topical application of cannabinoids and nociception remains in preliminary stages (Table 2). In a mouse model, Dogrul et al17 assessed the topical antinociceptive potential of a mixed CB1-CB2 agonist. Results showed significant (P<.01) and dose-dependent antinociceptive effects relative to treatment with a vehicle.17 In a related study, Yesilyurt et al18 evaluated whether a mixed CB1-CB2 agonist could enhance the antinociceptive effects of topical opioids. Among mice treated with the combination of a cannabinoid agonist and topical morphine, a significantly (P<.05) greater analgesic effect was demonstrated relative to topical morphine alone.18
Studies in humans have been far more limited. Phan et al19 conducted a small, nonrandomized, open-label trial of a topical cannabinoid cream in patients with facial postherpetic neuralgia. Of 8 patients treated, 5 noted a mean pain reduction of 87.8%. No comparison vehicle was used. Based on this narrow study design, it is difficult to extrapolate these positive results to a broader patient population.19
Commercial Products
Although preliminary models with topical cannabinoids have shown potential, large-scale clinical trials in humans have yet to be performed. Despite this lack of investigation, commercial formulations of topical cannabinoids are available to dermatology patients. These formulations are nonstandardized, and no safety data exists regarding their use. Topical cannabinoids on the market may contain various amounts of active ingredient and may be combined with a range of other compounds.
In dermatology offices, it is not uncommon for patients to express an intention to use topical cannabinoid products following their planned treatment or procedure. Patients also have been known to use topical cannabinoid products prior to dermatologic procedures, sometimes in place of an approved topical anesthetic, without consulting the physician performing the procedure. With interventions that lead to active areas of wound healing, the application of such products may increase the risk for contamination and infection. Therefore, patients should be counseled that the use of commercial topical cannabinoids could jeopardize the success of their planned procedure, put them at risk for infection, and possibly lead to systemic absorption and/or changes in wound-healing capacities.
Conclusion
Based on the results from recent animal models, cannabinoids may have a role in future treatment algorithms for several inflammatory conditions. However, current efficacy and safety data are almost entirely limited to preliminary animal studies in rodents. In addition, the formulation of topical cannabinoid products is nonstandardized and poorly regulated. As such, the present evidence does not support the use of topical cannabinoids in dermatology practices. Dermatologists should ask patients about the use of any cannabinoid products as part of a treatment program, especially given the unsubstantiated claims often made by unscrupulous advertisers. This issue highlights the need for further research and regulation.
- Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389-462.
- Giacoppo S, Galuppo M, Pollastro F, et al. A new formulation of cannabidiol in cream shows therapeutic effects in a mouse model of experimental autoimmune encephalomyelitis. Daru. 2015;23:48.
- Hammell DC, Zhang LP, Ma F, et al. Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur J Pain. 2016;20:936-948.
- Schicho R, Storr M. Topical and systemic cannabidiol improves trinitrobenzene sulfonic acid colitis in mice. Pharmacology. 2012;89:149-155.
- Howlett AC, Barth F, Bonner TI, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161-202.
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199-215.
- Svizenska I, Dubovy P, Sulcova A. Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous system structures—a short review. Pharmacol Biochem Behav. 2008;90:501-511.
- Stander S, Schmelz M, Metze D, et al. Distribution of cannabinoid receptor 1 (CB1) and 2 (CB2) on sensory nerve fibers and adnexal structures in human skin. J Dermatol Sci. 2005;38:177-188.
- Kim HJ, Kim B, Park BM, et al. Topical cannabinoid receptor 1 agonist attenuates the cutaneous inflammatory responses in oxazolone-induced atopic dermatitis model. Int J Dermatol. 2015;54:E401-E408.
- Nam G, Jeong SK, Park BM, et al. Selective cannabinoid receptor-1 agonists regulate mast cell activation in an oxazolone-induced atopic dermatitis model. Ann Dermatol. 2016;28:22-29.
- Gaffal E, Cron M, Glodde N, et al. Anti-inflammatory activity of topical THC in DNFB-mediated mouse allergic contact dermatitis independent of CB1 and CB2 receptors. Allergy. 2013;68:994-1000.
- Abrams DI, Jay CA, Shade SB, et al. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68:515-521.
- Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2009;34:672-680.
- Wilsey B, Marcotte T, Deutsch R, et al. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain. 2013;14:136-148.
- Wilsey B, Marcotte T, Tsodikov A, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain. 2008;9:506-521.
- Abrams DI, Couey P, Shade SB, et al. Cannabinoid-opioid interaction in chronic pain. Clin Pharmacol Ther. 2011;90:844-851.
- Dogrul A, Gul H, Akar A, et al. Topical cannabinoid antinociception: synergy with spinal sites. Pain. 2003;105:11-16.
- Yesilyurt O, Dogrul A, Gul H, et al. Topical cannabinoid enhances topical morphine antinociception. Pain. 2003;105:303-308.
- Phan NQ, Siepmann D, Gralow I, et al. Adjuvant topical therapy with a cannabinoid receptor agonist in facial postherpetic neuralgia. J Dtsch Dermatol Ges. 2010;8:88-91.
- Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389-462.
- Giacoppo S, Galuppo M, Pollastro F, et al. A new formulation of cannabidiol in cream shows therapeutic effects in a mouse model of experimental autoimmune encephalomyelitis. Daru. 2015;23:48.
- Hammell DC, Zhang LP, Ma F, et al. Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur J Pain. 2016;20:936-948.
- Schicho R, Storr M. Topical and systemic cannabidiol improves trinitrobenzene sulfonic acid colitis in mice. Pharmacology. 2012;89:149-155.
- Howlett AC, Barth F, Bonner TI, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161-202.
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199-215.
- Svizenska I, Dubovy P, Sulcova A. Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous system structures—a short review. Pharmacol Biochem Behav. 2008;90:501-511.
- Stander S, Schmelz M, Metze D, et al. Distribution of cannabinoid receptor 1 (CB1) and 2 (CB2) on sensory nerve fibers and adnexal structures in human skin. J Dermatol Sci. 2005;38:177-188.
- Kim HJ, Kim B, Park BM, et al. Topical cannabinoid receptor 1 agonist attenuates the cutaneous inflammatory responses in oxazolone-induced atopic dermatitis model. Int J Dermatol. 2015;54:E401-E408.
- Nam G, Jeong SK, Park BM, et al. Selective cannabinoid receptor-1 agonists regulate mast cell activation in an oxazolone-induced atopic dermatitis model. Ann Dermatol. 2016;28:22-29.
- Gaffal E, Cron M, Glodde N, et al. Anti-inflammatory activity of topical THC in DNFB-mediated mouse allergic contact dermatitis independent of CB1 and CB2 receptors. Allergy. 2013;68:994-1000.
- Abrams DI, Jay CA, Shade SB, et al. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68:515-521.
- Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2009;34:672-680.
- Wilsey B, Marcotte T, Deutsch R, et al. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain. 2013;14:136-148.
- Wilsey B, Marcotte T, Tsodikov A, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain. 2008;9:506-521.
- Abrams DI, Couey P, Shade SB, et al. Cannabinoid-opioid interaction in chronic pain. Clin Pharmacol Ther. 2011;90:844-851.
- Dogrul A, Gul H, Akar A, et al. Topical cannabinoid antinociception: synergy with spinal sites. Pain. 2003;105:11-16.
- Yesilyurt O, Dogrul A, Gul H, et al. Topical cannabinoid enhances topical morphine antinociception. Pain. 2003;105:303-308.
- Phan NQ, Siepmann D, Gralow I, et al. Adjuvant topical therapy with a cannabinoid receptor agonist in facial postherpetic neuralgia. J Dtsch Dermatol Ges. 2010;8:88-91.
Practice Points
- Topical cannabinoids are advertised by companies as treatment options for numerous dermatologic conditions.
- Despite promising data in rodent models, there have been no rigorous studies to date confirming efficacy or safety in humans.
- Dermatologists should therefore inquire with patients about the use of any topical cannabinoid products, especially around the time of planned procedures, as they may affect treatment outcomes.
Local Anesthetics in Cosmetic Dermatology
Local anesthesia is a central component of successful interventions in cosmetic dermatology. The number of anesthetic medications and administration techniques has grown in recent years as outpatient cosmetic procedures continue to expand. Pain is a common barrier to cosmetic procedures, and alleviating the fear of painful interventions is critical to patient satisfaction and future visits. To accommodate a multitude of cosmetic interventions, it is important for clinicians to be well versed in applications of topical and regional anesthesia. In this article, we review pain management strategies for use in cosmetic practice.
Local Anesthetics
The sensation of pain is carried to the central nervous system by unmyelinated C nerve fibers. Local anesthetics (LAs) act by blocking fast voltage-gated sodium channels in the cell membrane of the nerve, thereby inhibiting downstream propagation of an action potential and the transmission of painful stimuli.1 The chemical structure of LAs is fundamental to their mechanism of action and metabolism. Local anesthetics contain a lipophilic aromatic group, an intermediate chain, and a hydrophilic amine group. Broadly, agents are classified as amides or esters depending on the chemical group attached to the intermediate chain.2 Amides (eg, lidocaine, bupivacaine, articaine, mepivacaine, prilocaine, levobupivacaine) are metabolized by the hepatic system; esters (eg, procaine, proparacaine, benzocaine, chlorprocaine, tetracaine, cocaine) are metabolized by plasma cholinesterase, which produces para-aminobenzoic acid, a potentially dangerous metabolite that has been implicated in allergic reactions.3
Lidocaine is the most prevalent LA used in dermatology practices. Importantly, lidocaine is a class IB antiarrhythmic agent used in cardiology to treat ventricular arrhythmias.4 As an anesthetic, a maximum dose of 4.5 mg/kg can be administered, increasing to 7.0 mg/kg when mixed with epinephrine; with higher doses, there is a risk for central nervous system and cardiovascular toxicity.5 Initial symptoms of lidocaine toxicity include dizziness, tinnitus, circumoral paresthesia, blurred vision, and a metallic taste in the mouth.6 Systemic absorption of topical anesthetics is heightened across mucosal membranes, and care should be taken when applying over large surface areas.
Allergic reactions to LAs may be local or less frequently systemic. It is important to note that LAs tend to show cross-reactivity within their class rather than across different classes.7 Reactions can be classified as type I or type IV. Type I (IgE-mediated) reactions evolve in minutes to hours, affecting the skin and possibly leading to respiratory and circulatory collapse. Delayed reactions to LAs have increased in recent years, with type IV contact allergy most frequently found in connection with benzocaine and lidocaine.8
Topical Anesthesia
Topical anesthetics are effective and easy to use and are particularly valuable in patients with needle phobia. In certain cases, these medications may be applied by the patient prior to arrival, thereby reducing visit time. Topical agents act on nerve fibers running through the dermis; therefore, efficacy is dependent on successful penetration through the stratum corneum and viable epidermis. To enhance absorption, agents may be applied under an occlusive dressing.
Topical anesthetics are most commonly used for injectable fillers, ablative and nonablative laser resurfacing, laser hair removal, and tattoo removal. The eutectic mixture of 2.5% lidocaine and 2.5% prilocaine as well as topical 4% or 5% lidocaine are the most commonly used US Food and Drug Administration–approved products for topical anesthesia. In addition, several compounded pharmacy products are available.
After 60 minutes of application of the eutectic mixture of 2.5% lidocaine and 2.5% prilocaine, a 3-mm depth of analgesia is reached, and after 120 minutes, a 4.5-mm depth is reached.9 It elicits a biphasic vascular response of vasoconstriction and blanching followed by vasodilation and erythema.10 Most adverse events are mild and transient, but allergic contact dermatitis and contact urticaria have been reported.11-13 In older children and adults, the maximum application area is 200 cm2, with a maximum dose of 20 g used for no longer than 4 hours.
The 4% or 5% lidocaine cream uses a liposomal delivery system, which is designed to improve cutaneous penetration and has been shown to provide longer durations of anesthesia than nonliposomal lidocaine preparations.14 Application should be performed 30 to 60 minutes prior to a procedure. In a study comparing the eutectic mixture of 2.5% lidocaine and 2.5% prilocaine versus lidocaine cream 5% for pain control during laser hair removal with a 1064-nm Nd:YAG laser, no significant differences were found.15 The maximum application area is 100 cm2 in children weighing less than 20 kg. A study of healthy adults demonstrated safety with the use of 30 to 60 g of occluded liposomal lidocaine cream 4%.16
In addition to US Food and Drug Administration–approved products, several compounded pharmacy products are available for topical anesthesia. These formulations include benzocaine-lidocaine-tetracaine gel, tetracaine-adrenaline-cocaine solution, and lidocaine-epinephrine-tetracaine solution. A triple-anesthetic gel, benzocaine-lidocaine-tetracaine is widely used in cosmetic practice. The product has been shown to provide adequate anesthesia for laser resurfacing after 20 minutes without occlusion.17 Of note, compounded anesthetics lack standardization, and different pharmacies may follow their own individual protocols.
Regional Anesthesia
Regional nerve blockade is a useful option for more widespread or complex interventions. Using regional nerve blockade, effective analgesia can be delivered to a target area while avoiding the toxicity and pain associated with numerous anesthetic infiltrations. In addition, there is no distortion of the tissue architecture, allowing for improved visual evaluation during the procedure. Recently, hyaluronic acid fillers have been compounded with lidocaine as a means of reducing procedural pain.
Blocks for Dermal Fillers
Forehead
For dermal filler injections of the glabellar and frontalis lines, anesthesia of the forehead may be desired. The supraorbital and supratrochlear nerves supply this area. The supraorbital nerve can be injected at the supraorbital notch, which is measured roughly 2.7 cm from the glabella. The orbital rim should be palpated with the nondominant hand, and 1 to 2 mL of anesthetic should be injected just below the rim (Figure 1). The supratrochlear nerve is located roughly 1.7 cm from the midline and can be similarly injected under the orbital rim with 1 to 2 mL of anesthetic (Figure 1).
Lateral Temple Region
Anesthesia of the zygomaticotemporal nerve can be used to reduce pain from dermal filler injections of the lateral canthal and temporal areas. The nerve is identified by first palpating the zygomaticofrontal suture. A long needle is then inserted posteriorly, immediately behind the concave surface of the lateral orbital rim, and 1 to 2 mL of anesthetic is injected (Figure 1).
Malar Region
Blockade of the zygomaticofacial nerve is commonly performed in conjunction with the zygomaticotemporal nerve and provides anesthesia to the malar region for cheek augmentation procedures. To identify the target area, the junction of the lateral and inferior orbital rim should be palpated. With the needle placed just lateral to this point, 1 to 2 mL of anesthetic is injected (Figure 1).
Blocks for Perioral Fillers
Upper Lips/Nasolabial Folds
Bilateral blockade of the infraorbital nerves provides anesthesia to the upper lip and nasolabial folds prior to filler injections. The infraorbital nerve can be targeted via an intraoral route where it exits the maxilla at the infraorbital foramen. The nerve is anesthetized by palpating the infraorbital ridge and injecting 3 to 5 mL of anesthetic roughly 1 cm below this point on the vertical axis of the midpupillary line (Figure 1). The external nasal nerve, thought to be a branch of cranial nerve V, also may be targeted if there is inadequate anesthesia from the infraorbital block. This nerve is reached by injecting at the osseocartilaginous junction of the nasal bones (Figure 1).
Lower Lips
Blockade of the mental nerve provides anesthesia to the lower lips for augmentation procedures. The mental nerve can be targeted on each side at the mental foramen, which is located below the root of the lower second premolar. Aiming roughly 1 cm below the gumline, 3 to 5 mL of anesthetic is injected intraorally (Figure 1). A transcutaneous approach toward the same target also is possible, though this technique risks visible bruising. Alternatively, the upper or lower lips can be anesthetized using 4 to 5 submucosal injections at evenly spaced intervals between the canine teeth.18
Blocks for Palmoplantar Hyperhidrosis
The treatment of palmoplantar hyperhidrosis benefits from regional blocks. Botulinum toxin has been well established as an effective therapy for the condition.19-21 Given the sensitivity of palmoplantar sites, it is valuable to achieve effective analgesia of the region prior to dermal injections of botulinum toxin.
Wrists
Sensory innervation of the palm is provided by the median, ulnar, and radial nerves (Figure 2A).
The ulnar nerve is anesthetized between the ulnar artery and the flexor carpi ulnaris muscle. The artery is identified by palpation, and special care should be taken to avoid intra-arterial injection. The needle is directed toward the radial styloid, and 3 to 5 mL of anesthetic is injected roughly 1 cm proximal to the wrist crease (Figure 2B).
Anesthesia of the radial nerve can be considered a field block given the numerous small branches that supply the hand. These branches are reached by injecting anesthetic roughly 2 to 3 cm proximal to the radial styloid with the needle aimed medially and extending the injection dorsally (Figure 2B). A total of 4 to 6 mL of anesthetic is used.
Ankles
An ankle block provides anesthesia to the dorsal and plantar surfaces of the foot.22 The region is supplied by the superficial peroneal nerve, deep peroneal nerve, sural nerve, saphenous nerve, and branches of the posterior tibial nerve (Figure 3A).
To anesthetize the deep peroneal nerve, the extensor hallucis longus tendon is first identified on the anterior surface of the ankle through dorsiflexion of the toes; the dorsalis pedis artery runs in close proximity. The injection should be placed lateral to the tendon and artery (Figure 3B). The needle should be inserted until bone is reached, withdrawn slightly, and then 3 to 5 mL of anesthetic should be injected. To block the saphenous nerve, the needle can then be directed superficially toward the medial malleolus, and 3 to 5 mL should be injected in a subcutaneous wheal (Figure 3C). To block the superficial peroneal nerve, the needle should then be directed toward the lateral malleolus, and 3 to 5 mL should be injected in a subcutaneous wheal (Figure 3C).
The posterior tibial nerve is located posterior to the medial malleolus. The dorsalis pedis artery can be palpated near this location. The needle should be inserted posterior to the artery, extending until bone is reached (Figure 3C). The needle is then withdrawn slightly, and 3 to 5 mL of anesthetic is injected. Finally, the sural nerve is anesthetized between the Achilles tendon and the lateral malleolus, using 5 mL of anesthetic to raise a subcutaneous wheal (Figure 3C).
Conclusion
Proper pain management is integral to ensuring a positive experience for cosmetic patients. Enhanced knowledge of local anesthetic techniques allows the clinician to provide for a variety of procedural indications and patient preferences. As anesthetic strategies are continually evolving, it is important for practitioners to remain informed of these developments.
- Scholz A. Mechanisms of (local) anaesthetics on voltage-gated sodium and other ion channels. Br J Anaesth. 2002;89:52-61.
- Auletta MJ. Local anesthesia for dermatologic surgery. Semin Dermatol. 1994;13:35-42.
- Park KK, Sharon VR. A review of local anesthetics: minimizing risk and side effects in cutaneous surgery. Dermatol Surg. 2017;43:173-187.
- Reiz S, Nath S. Cardiotoxicity of local anaesthetic agents. Br J Anaesth. 1986;58:736-746.
- Klein JA, Kassarjdian N. Lidocaine toxicity with tumescent liposuction. a case report of probable drug interactions. Dermatol Surg. 1997;23:1169-1174.
- Minkis K, Whittington A, Alam M. Dermatologic surgery emergencies: complications caused by systemic reactions, high-energy systems, and trauma. J Am Acad Dermatol. 2016;75:265-284.
- Morais-Almeida M, Gaspar A, Marinho S, et al. Allergy to local anesthetics of the amide group with tolerance to procaine. Allergy. 2003;58:827-828.
- To D, Kossintseva I, de Gannes G. Lidocaine contact allergy is becoming more prevalent. Dermatol Surg. 2014;40:1367-1372.
- Wahlgren CF, Quiding H. Depth of cutaneous analgesia after application of a eutectic mixture of the local anesthetics lidocaine and prilocaine (EMLA cream). J Am Acad Dermatol. 2000;42:584-588.
- Bjerring P, Andersen PH, Arendt-Nielsen L. Vascular response of human skin after analgesia with EMLA cream. Br J Anaesth. 1989;63:655-660.
- Ismail F, Goldsmith PC. EMLA cream-induced allergic contact dermatitis in a child with thalassaemia major. Contact Dermatitis. 2005;52:111.
- Thakur BK, Murali MR. EMLA cream-induced allergic contact dermatitis: a role for prilocaine as an immunogen. J Allergy Clin Immunol. 1995;95:776-778.
- Waton J, Boulanger A, Trechot PH, et al. Contact urticaria from EMLA cream. Contact Dermatitis. 2004;51:284-287.
- Bucalo BD, Mirikitani EJ, Moy RL. Comparison of skin anesthetic effect of liposomal lidocaine, nonliposomal lidocaine, and EMLA using 30-minute application time. Dermatol Surg. 1998;24:537-541.
- Guardiano RA, Norwood CW. Direct comparison of EMLA versus lidocaine for pain control in Nd:YAG 1,064 nm laser hair removal. Dermatol Surg. 2005;31:396-398.
- Nestor MS. Safety of occluded 4% liposomal lidocaine cream. J Drugs Dermatol. 2006;5:618-620.
- Oni G, Rasko Y, Kenkel J. Topical lidocaine enhanced by laser pretreatment: a safe and effective method of analgesia for facial rejuvenation. Aesthet Surg J. 2013;33:854-861.
- Niamtu J 3rd. Simple technique for lip and nasolabial fold anesthesia for injectable fillers. Dermatol Surg. 2005;31:1330-1332.
- Naumann M, Flachenecker P, Brocker EB, et al. Botulinum toxin for palmar hyperhidrosis. Lancet. 1997;349:252.
- Naumann M, Hofmann U, Bergmann I, et al. Focal hyperhidrosis: effective treatment with intracutaneous botulinum toxin. Arch Dermatol. 1998;134:301-304.
- Shelley WB, Talanin NY, Shelley ED. Botulinum toxin therapy for palmar hyperhidrosis. J Am Acad Dermatol. 1998;38(2, pt 1):227-229.
- Davies T, Karanovic S, Shergill B. Essential regional nerve blocks for the dermatologist: part 2. Clin Exp Dermatol. 2014;39:861-867.
Local anesthesia is a central component of successful interventions in cosmetic dermatology. The number of anesthetic medications and administration techniques has grown in recent years as outpatient cosmetic procedures continue to expand. Pain is a common barrier to cosmetic procedures, and alleviating the fear of painful interventions is critical to patient satisfaction and future visits. To accommodate a multitude of cosmetic interventions, it is important for clinicians to be well versed in applications of topical and regional anesthesia. In this article, we review pain management strategies for use in cosmetic practice.
Local Anesthetics
The sensation of pain is carried to the central nervous system by unmyelinated C nerve fibers. Local anesthetics (LAs) act by blocking fast voltage-gated sodium channels in the cell membrane of the nerve, thereby inhibiting downstream propagation of an action potential and the transmission of painful stimuli.1 The chemical structure of LAs is fundamental to their mechanism of action and metabolism. Local anesthetics contain a lipophilic aromatic group, an intermediate chain, and a hydrophilic amine group. Broadly, agents are classified as amides or esters depending on the chemical group attached to the intermediate chain.2 Amides (eg, lidocaine, bupivacaine, articaine, mepivacaine, prilocaine, levobupivacaine) are metabolized by the hepatic system; esters (eg, procaine, proparacaine, benzocaine, chlorprocaine, tetracaine, cocaine) are metabolized by plasma cholinesterase, which produces para-aminobenzoic acid, a potentially dangerous metabolite that has been implicated in allergic reactions.3
Lidocaine is the most prevalent LA used in dermatology practices. Importantly, lidocaine is a class IB antiarrhythmic agent used in cardiology to treat ventricular arrhythmias.4 As an anesthetic, a maximum dose of 4.5 mg/kg can be administered, increasing to 7.0 mg/kg when mixed with epinephrine; with higher doses, there is a risk for central nervous system and cardiovascular toxicity.5 Initial symptoms of lidocaine toxicity include dizziness, tinnitus, circumoral paresthesia, blurred vision, and a metallic taste in the mouth.6 Systemic absorption of topical anesthetics is heightened across mucosal membranes, and care should be taken when applying over large surface areas.
Allergic reactions to LAs may be local or less frequently systemic. It is important to note that LAs tend to show cross-reactivity within their class rather than across different classes.7 Reactions can be classified as type I or type IV. Type I (IgE-mediated) reactions evolve in minutes to hours, affecting the skin and possibly leading to respiratory and circulatory collapse. Delayed reactions to LAs have increased in recent years, with type IV contact allergy most frequently found in connection with benzocaine and lidocaine.8
Topical Anesthesia
Topical anesthetics are effective and easy to use and are particularly valuable in patients with needle phobia. In certain cases, these medications may be applied by the patient prior to arrival, thereby reducing visit time. Topical agents act on nerve fibers running through the dermis; therefore, efficacy is dependent on successful penetration through the stratum corneum and viable epidermis. To enhance absorption, agents may be applied under an occlusive dressing.
Topical anesthetics are most commonly used for injectable fillers, ablative and nonablative laser resurfacing, laser hair removal, and tattoo removal. The eutectic mixture of 2.5% lidocaine and 2.5% prilocaine as well as topical 4% or 5% lidocaine are the most commonly used US Food and Drug Administration–approved products for topical anesthesia. In addition, several compounded pharmacy products are available.
After 60 minutes of application of the eutectic mixture of 2.5% lidocaine and 2.5% prilocaine, a 3-mm depth of analgesia is reached, and after 120 minutes, a 4.5-mm depth is reached.9 It elicits a biphasic vascular response of vasoconstriction and blanching followed by vasodilation and erythema.10 Most adverse events are mild and transient, but allergic contact dermatitis and contact urticaria have been reported.11-13 In older children and adults, the maximum application area is 200 cm2, with a maximum dose of 20 g used for no longer than 4 hours.
The 4% or 5% lidocaine cream uses a liposomal delivery system, which is designed to improve cutaneous penetration and has been shown to provide longer durations of anesthesia than nonliposomal lidocaine preparations.14 Application should be performed 30 to 60 minutes prior to a procedure. In a study comparing the eutectic mixture of 2.5% lidocaine and 2.5% prilocaine versus lidocaine cream 5% for pain control during laser hair removal with a 1064-nm Nd:YAG laser, no significant differences were found.15 The maximum application area is 100 cm2 in children weighing less than 20 kg. A study of healthy adults demonstrated safety with the use of 30 to 60 g of occluded liposomal lidocaine cream 4%.16
In addition to US Food and Drug Administration–approved products, several compounded pharmacy products are available for topical anesthesia. These formulations include benzocaine-lidocaine-tetracaine gel, tetracaine-adrenaline-cocaine solution, and lidocaine-epinephrine-tetracaine solution. A triple-anesthetic gel, benzocaine-lidocaine-tetracaine is widely used in cosmetic practice. The product has been shown to provide adequate anesthesia for laser resurfacing after 20 minutes without occlusion.17 Of note, compounded anesthetics lack standardization, and different pharmacies may follow their own individual protocols.
Regional Anesthesia
Regional nerve blockade is a useful option for more widespread or complex interventions. Using regional nerve blockade, effective analgesia can be delivered to a target area while avoiding the toxicity and pain associated with numerous anesthetic infiltrations. In addition, there is no distortion of the tissue architecture, allowing for improved visual evaluation during the procedure. Recently, hyaluronic acid fillers have been compounded with lidocaine as a means of reducing procedural pain.
Blocks for Dermal Fillers
Forehead
For dermal filler injections of the glabellar and frontalis lines, anesthesia of the forehead may be desired. The supraorbital and supratrochlear nerves supply this area. The supraorbital nerve can be injected at the supraorbital notch, which is measured roughly 2.7 cm from the glabella. The orbital rim should be palpated with the nondominant hand, and 1 to 2 mL of anesthetic should be injected just below the rim (Figure 1). The supratrochlear nerve is located roughly 1.7 cm from the midline and can be similarly injected under the orbital rim with 1 to 2 mL of anesthetic (Figure 1).
Lateral Temple Region
Anesthesia of the zygomaticotemporal nerve can be used to reduce pain from dermal filler injections of the lateral canthal and temporal areas. The nerve is identified by first palpating the zygomaticofrontal suture. A long needle is then inserted posteriorly, immediately behind the concave surface of the lateral orbital rim, and 1 to 2 mL of anesthetic is injected (Figure 1).
Malar Region
Blockade of the zygomaticofacial nerve is commonly performed in conjunction with the zygomaticotemporal nerve and provides anesthesia to the malar region for cheek augmentation procedures. To identify the target area, the junction of the lateral and inferior orbital rim should be palpated. With the needle placed just lateral to this point, 1 to 2 mL of anesthetic is injected (Figure 1).

Blocks for Perioral Fillers
Upper Lips/Nasolabial Folds
Bilateral blockade of the infraorbital nerves provides anesthesia to the upper lip and nasolabial folds prior to filler injections. The infraorbital nerve can be targeted via an intraoral route where it exits the maxilla at the infraorbital foramen. The nerve is anesthetized by palpating the infraorbital ridge and injecting 3 to 5 mL of anesthetic roughly 1 cm below this point on the vertical axis of the midpupillary line (Figure 1). The external nasal nerve, thought to be a branch of cranial nerve V, also may be targeted if there is inadequate anesthesia from the infraorbital block. This nerve is reached by injecting at the osseocartilaginous junction of the nasal bones (Figure 1).
Lower Lips
Blockade of the mental nerve provides anesthesia to the lower lips for augmentation procedures. The mental nerve can be targeted on each side at the mental foramen, which is located below the root of the lower second premolar. Aiming roughly 1 cm below the gumline, 3 to 5 mL of anesthetic is injected intraorally (Figure 1). A transcutaneous approach toward the same target also is possible, though this technique risks visible bruising. Alternatively, the upper or lower lips can be anesthetized using 4 to 5 submucosal injections at evenly spaced intervals between the canine teeth.18
Blocks for Palmoplantar Hyperhidrosis
The treatment of palmoplantar hyperhidrosis benefits from regional blocks. Botulinum toxin has been well established as an effective therapy for the condition.19-21 Given the sensitivity of palmoplantar sites, it is valuable to achieve effective analgesia of the region prior to dermal injections of botulinum toxin.
Wrists
Sensory innervation of the palm is provided by the median, ulnar, and radial nerves (Figure 2A).
The ulnar nerve is anesthetized between the ulnar artery and the flexor carpi ulnaris muscle. The artery is identified by palpation, and special care should be taken to avoid intra-arterial injection. The needle is directed toward the radial styloid, and 3 to 5 mL of anesthetic is injected roughly 1 cm proximal to the wrist crease (Figure 2B).
Anesthesia of the radial nerve can be considered a field block given the numerous small branches that supply the hand. These branches are reached by injecting anesthetic roughly 2 to 3 cm proximal to the radial styloid with the needle aimed medially and extending the injection dorsally (Figure 2B). A total of 4 to 6 mL of anesthetic is used.

Ankles
An ankle block provides anesthesia to the dorsal and plantar surfaces of the foot.22 The region is supplied by the superficial peroneal nerve, deep peroneal nerve, sural nerve, saphenous nerve, and branches of the posterior tibial nerve (Figure 3A).
To anesthetize the deep peroneal nerve, the extensor hallucis longus tendon is first identified on the anterior surface of the ankle through dorsiflexion of the toes; the dorsalis pedis artery runs in close proximity. The injection should be placed lateral to the tendon and artery (Figure 3B). The needle should be inserted until bone is reached, withdrawn slightly, and then 3 to 5 mL of anesthetic should be injected. To block the saphenous nerve, the needle can then be directed superficially toward the medial malleolus, and 3 to 5 mL should be injected in a subcutaneous wheal (Figure 3C). To block the superficial peroneal nerve, the needle should then be directed toward the lateral malleolus, and 3 to 5 mL should be injected in a subcutaneous wheal (Figure 3C).
The posterior tibial nerve is located posterior to the medial malleolus. The dorsalis pedis artery can be palpated near this location. The needle should be inserted posterior to the artery, extending until bone is reached (Figure 3C). The needle is then withdrawn slightly, and 3 to 5 mL of anesthetic is injected. Finally, the sural nerve is anesthetized between the Achilles tendon and the lateral malleolus, using 5 mL of anesthetic to raise a subcutaneous wheal (Figure 3C).

Conclusion
Proper pain management is integral to ensuring a positive experience for cosmetic patients. Enhanced knowledge of local anesthetic techniques allows the clinician to provide for a variety of procedural indications and patient preferences. As anesthetic strategies are continually evolving, it is important for practitioners to remain informed of these developments.
Local anesthesia is a central component of successful interventions in cosmetic dermatology. The number of anesthetic medications and administration techniques has grown in recent years as outpatient cosmetic procedures continue to expand. Pain is a common barrier to cosmetic procedures, and alleviating the fear of painful interventions is critical to patient satisfaction and future visits. To accommodate a multitude of cosmetic interventions, it is important for clinicians to be well versed in applications of topical and regional anesthesia. In this article, we review pain management strategies for use in cosmetic practice.
Local Anesthetics
The sensation of pain is carried to the central nervous system by unmyelinated C nerve fibers. Local anesthetics (LAs) act by blocking fast voltage-gated sodium channels in the cell membrane of the nerve, thereby inhibiting downstream propagation of an action potential and the transmission of painful stimuli.1 The chemical structure of LAs is fundamental to their mechanism of action and metabolism. Local anesthetics contain a lipophilic aromatic group, an intermediate chain, and a hydrophilic amine group. Broadly, agents are classified as amides or esters depending on the chemical group attached to the intermediate chain.2 Amides (eg, lidocaine, bupivacaine, articaine, mepivacaine, prilocaine, levobupivacaine) are metabolized by the hepatic system; esters (eg, procaine, proparacaine, benzocaine, chlorprocaine, tetracaine, cocaine) are metabolized by plasma cholinesterase, which produces para-aminobenzoic acid, a potentially dangerous metabolite that has been implicated in allergic reactions.3
Lidocaine is the most prevalent LA used in dermatology practices. Importantly, lidocaine is a class IB antiarrhythmic agent used in cardiology to treat ventricular arrhythmias.4 As an anesthetic, a maximum dose of 4.5 mg/kg can be administered, increasing to 7.0 mg/kg when mixed with epinephrine; with higher doses, there is a risk for central nervous system and cardiovascular toxicity.5 Initial symptoms of lidocaine toxicity include dizziness, tinnitus, circumoral paresthesia, blurred vision, and a metallic taste in the mouth.6 Systemic absorption of topical anesthetics is heightened across mucosal membranes, and care should be taken when applying over large surface areas.
Allergic reactions to LAs may be local or less frequently systemic. It is important to note that LAs tend to show cross-reactivity within their class rather than across different classes.7 Reactions can be classified as type I or type IV. Type I (IgE-mediated) reactions evolve in minutes to hours, affecting the skin and possibly leading to respiratory and circulatory collapse. Delayed reactions to LAs have increased in recent years, with type IV contact allergy most frequently found in connection with benzocaine and lidocaine.8
Topical Anesthesia
Topical anesthetics are effective and easy to use and are particularly valuable in patients with needle phobia. In certain cases, these medications may be applied by the patient prior to arrival, thereby reducing visit time. Topical agents act on nerve fibers running through the dermis; therefore, efficacy is dependent on successful penetration through the stratum corneum and viable epidermis. To enhance absorption, agents may be applied under an occlusive dressing.
Topical anesthetics are most commonly used for injectable fillers, ablative and nonablative laser resurfacing, laser hair removal, and tattoo removal. The eutectic mixture of 2.5% lidocaine and 2.5% prilocaine as well as topical 4% or 5% lidocaine are the most commonly used US Food and Drug Administration–approved products for topical anesthesia. In addition, several compounded pharmacy products are available.
After 60 minutes of application of the eutectic mixture of 2.5% lidocaine and 2.5% prilocaine, a 3-mm depth of analgesia is reached, and after 120 minutes, a 4.5-mm depth is reached.9 It elicits a biphasic vascular response of vasoconstriction and blanching followed by vasodilation and erythema.10 Most adverse events are mild and transient, but allergic contact dermatitis and contact urticaria have been reported.11-13 In older children and adults, the maximum application area is 200 cm2, with a maximum dose of 20 g used for no longer than 4 hours.
The 4% or 5% lidocaine cream uses a liposomal delivery system, which is designed to improve cutaneous penetration and has been shown to provide longer durations of anesthesia than nonliposomal lidocaine preparations.14 Application should be performed 30 to 60 minutes prior to a procedure. In a study comparing the eutectic mixture of 2.5% lidocaine and 2.5% prilocaine versus lidocaine cream 5% for pain control during laser hair removal with a 1064-nm Nd:YAG laser, no significant differences were found.15 The maximum application area is 100 cm2 in children weighing less than 20 kg. A study of healthy adults demonstrated safety with the use of 30 to 60 g of occluded liposomal lidocaine cream 4%.16
In addition to US Food and Drug Administration–approved products, several compounded pharmacy products are available for topical anesthesia. These formulations include benzocaine-lidocaine-tetracaine gel, tetracaine-adrenaline-cocaine solution, and lidocaine-epinephrine-tetracaine solution. A triple-anesthetic gel, benzocaine-lidocaine-tetracaine is widely used in cosmetic practice. The product has been shown to provide adequate anesthesia for laser resurfacing after 20 minutes without occlusion.17 Of note, compounded anesthetics lack standardization, and different pharmacies may follow their own individual protocols.
Regional Anesthesia
Regional nerve blockade is a useful option for more widespread or complex interventions. Using regional nerve blockade, effective analgesia can be delivered to a target area while avoiding the toxicity and pain associated with numerous anesthetic infiltrations. In addition, there is no distortion of the tissue architecture, allowing for improved visual evaluation during the procedure. Recently, hyaluronic acid fillers have been compounded with lidocaine as a means of reducing procedural pain.
Blocks for Dermal Fillers
Forehead
For dermal filler injections of the glabellar and frontalis lines, anesthesia of the forehead may be desired. The supraorbital and supratrochlear nerves supply this area. The supraorbital nerve can be injected at the supraorbital notch, which is measured roughly 2.7 cm from the glabella. The orbital rim should be palpated with the nondominant hand, and 1 to 2 mL of anesthetic should be injected just below the rim (Figure 1). The supratrochlear nerve is located roughly 1.7 cm from the midline and can be similarly injected under the orbital rim with 1 to 2 mL of anesthetic (Figure 1).
Lateral Temple Region
Anesthesia of the zygomaticotemporal nerve can be used to reduce pain from dermal filler injections of the lateral canthal and temporal areas. The nerve is identified by first palpating the zygomaticofrontal suture. A long needle is then inserted posteriorly, immediately behind the concave surface of the lateral orbital rim, and 1 to 2 mL of anesthetic is injected (Figure 1).
Malar Region
Blockade of the zygomaticofacial nerve is commonly performed in conjunction with the zygomaticotemporal nerve and provides anesthesia to the malar region for cheek augmentation procedures. To identify the target area, the junction of the lateral and inferior orbital rim should be palpated. With the needle placed just lateral to this point, 1 to 2 mL of anesthetic is injected (Figure 1).

Blocks for Perioral Fillers
Upper Lips/Nasolabial Folds
Bilateral blockade of the infraorbital nerves provides anesthesia to the upper lip and nasolabial folds prior to filler injections. The infraorbital nerve can be targeted via an intraoral route where it exits the maxilla at the infraorbital foramen. The nerve is anesthetized by palpating the infraorbital ridge and injecting 3 to 5 mL of anesthetic roughly 1 cm below this point on the vertical axis of the midpupillary line (Figure 1). The external nasal nerve, thought to be a branch of cranial nerve V, also may be targeted if there is inadequate anesthesia from the infraorbital block. This nerve is reached by injecting at the osseocartilaginous junction of the nasal bones (Figure 1).
Lower Lips
Blockade of the mental nerve provides anesthesia to the lower lips for augmentation procedures. The mental nerve can be targeted on each side at the mental foramen, which is located below the root of the lower second premolar. Aiming roughly 1 cm below the gumline, 3 to 5 mL of anesthetic is injected intraorally (Figure 1). A transcutaneous approach toward the same target also is possible, though this technique risks visible bruising. Alternatively, the upper or lower lips can be anesthetized using 4 to 5 submucosal injections at evenly spaced intervals between the canine teeth.18
Blocks for Palmoplantar Hyperhidrosis
The treatment of palmoplantar hyperhidrosis benefits from regional blocks. Botulinum toxin has been well established as an effective therapy for the condition.19-21 Given the sensitivity of palmoplantar sites, it is valuable to achieve effective analgesia of the region prior to dermal injections of botulinum toxin.
Wrists
Sensory innervation of the palm is provided by the median, ulnar, and radial nerves (Figure 2A).
The ulnar nerve is anesthetized between the ulnar artery and the flexor carpi ulnaris muscle. The artery is identified by palpation, and special care should be taken to avoid intra-arterial injection. The needle is directed toward the radial styloid, and 3 to 5 mL of anesthetic is injected roughly 1 cm proximal to the wrist crease (Figure 2B).
Anesthesia of the radial nerve can be considered a field block given the numerous small branches that supply the hand. These branches are reached by injecting anesthetic roughly 2 to 3 cm proximal to the radial styloid with the needle aimed medially and extending the injection dorsally (Figure 2B). A total of 4 to 6 mL of anesthetic is used.

Ankles
An ankle block provides anesthesia to the dorsal and plantar surfaces of the foot.22 The region is supplied by the superficial peroneal nerve, deep peroneal nerve, sural nerve, saphenous nerve, and branches of the posterior tibial nerve (Figure 3A).
To anesthetize the deep peroneal nerve, the extensor hallucis longus tendon is first identified on the anterior surface of the ankle through dorsiflexion of the toes; the dorsalis pedis artery runs in close proximity. The injection should be placed lateral to the tendon and artery (Figure 3B). The needle should be inserted until bone is reached, withdrawn slightly, and then 3 to 5 mL of anesthetic should be injected. To block the saphenous nerve, the needle can then be directed superficially toward the medial malleolus, and 3 to 5 mL should be injected in a subcutaneous wheal (Figure 3C). To block the superficial peroneal nerve, the needle should then be directed toward the lateral malleolus, and 3 to 5 mL should be injected in a subcutaneous wheal (Figure 3C).
The posterior tibial nerve is located posterior to the medial malleolus. The dorsalis pedis artery can be palpated near this location. The needle should be inserted posterior to the artery, extending until bone is reached (Figure 3C). The needle is then withdrawn slightly, and 3 to 5 mL of anesthetic is injected. Finally, the sural nerve is anesthetized between the Achilles tendon and the lateral malleolus, using 5 mL of anesthetic to raise a subcutaneous wheal (Figure 3C).

Conclusion
Proper pain management is integral to ensuring a positive experience for cosmetic patients. Enhanced knowledge of local anesthetic techniques allows the clinician to provide for a variety of procedural indications and patient preferences. As anesthetic strategies are continually evolving, it is important for practitioners to remain informed of these developments.
- Scholz A. Mechanisms of (local) anaesthetics on voltage-gated sodium and other ion channels. Br J Anaesth. 2002;89:52-61.
- Auletta MJ. Local anesthesia for dermatologic surgery. Semin Dermatol. 1994;13:35-42.
- Park KK, Sharon VR. A review of local anesthetics: minimizing risk and side effects in cutaneous surgery. Dermatol Surg. 2017;43:173-187.
- Reiz S, Nath S. Cardiotoxicity of local anaesthetic agents. Br J Anaesth. 1986;58:736-746.
- Klein JA, Kassarjdian N. Lidocaine toxicity with tumescent liposuction. a case report of probable drug interactions. Dermatol Surg. 1997;23:1169-1174.
- Minkis K, Whittington A, Alam M. Dermatologic surgery emergencies: complications caused by systemic reactions, high-energy systems, and trauma. J Am Acad Dermatol. 2016;75:265-284.
- Morais-Almeida M, Gaspar A, Marinho S, et al. Allergy to local anesthetics of the amide group with tolerance to procaine. Allergy. 2003;58:827-828.
- To D, Kossintseva I, de Gannes G. Lidocaine contact allergy is becoming more prevalent. Dermatol Surg. 2014;40:1367-1372.
- Wahlgren CF, Quiding H. Depth of cutaneous analgesia after application of a eutectic mixture of the local anesthetics lidocaine and prilocaine (EMLA cream). J Am Acad Dermatol. 2000;42:584-588.
- Bjerring P, Andersen PH, Arendt-Nielsen L. Vascular response of human skin after analgesia with EMLA cream. Br J Anaesth. 1989;63:655-660.
- Ismail F, Goldsmith PC. EMLA cream-induced allergic contact dermatitis in a child with thalassaemia major. Contact Dermatitis. 2005;52:111.
- Thakur BK, Murali MR. EMLA cream-induced allergic contact dermatitis: a role for prilocaine as an immunogen. J Allergy Clin Immunol. 1995;95:776-778.
- Waton J, Boulanger A, Trechot PH, et al. Contact urticaria from EMLA cream. Contact Dermatitis. 2004;51:284-287.
- Bucalo BD, Mirikitani EJ, Moy RL. Comparison of skin anesthetic effect of liposomal lidocaine, nonliposomal lidocaine, and EMLA using 30-minute application time. Dermatol Surg. 1998;24:537-541.
- Guardiano RA, Norwood CW. Direct comparison of EMLA versus lidocaine for pain control in Nd:YAG 1,064 nm laser hair removal. Dermatol Surg. 2005;31:396-398.
- Nestor MS. Safety of occluded 4% liposomal lidocaine cream. J Drugs Dermatol. 2006;5:618-620.
- Oni G, Rasko Y, Kenkel J. Topical lidocaine enhanced by laser pretreatment: a safe and effective method of analgesia for facial rejuvenation. Aesthet Surg J. 2013;33:854-861.
- Niamtu J 3rd. Simple technique for lip and nasolabial fold anesthesia for injectable fillers. Dermatol Surg. 2005;31:1330-1332.
- Naumann M, Flachenecker P, Brocker EB, et al. Botulinum toxin for palmar hyperhidrosis. Lancet. 1997;349:252.
- Naumann M, Hofmann U, Bergmann I, et al. Focal hyperhidrosis: effective treatment with intracutaneous botulinum toxin. Arch Dermatol. 1998;134:301-304.
- Shelley WB, Talanin NY, Shelley ED. Botulinum toxin therapy for palmar hyperhidrosis. J Am Acad Dermatol. 1998;38(2, pt 1):227-229.
- Davies T, Karanovic S, Shergill B. Essential regional nerve blocks for the dermatologist: part 2. Clin Exp Dermatol. 2014;39:861-867.
- Scholz A. Mechanisms of (local) anaesthetics on voltage-gated sodium and other ion channels. Br J Anaesth. 2002;89:52-61.
- Auletta MJ. Local anesthesia for dermatologic surgery. Semin Dermatol. 1994;13:35-42.
- Park KK, Sharon VR. A review of local anesthetics: minimizing risk and side effects in cutaneous surgery. Dermatol Surg. 2017;43:173-187.
- Reiz S, Nath S. Cardiotoxicity of local anaesthetic agents. Br J Anaesth. 1986;58:736-746.
- Klein JA, Kassarjdian N. Lidocaine toxicity with tumescent liposuction. a case report of probable drug interactions. Dermatol Surg. 1997;23:1169-1174.
- Minkis K, Whittington A, Alam M. Dermatologic surgery emergencies: complications caused by systemic reactions, high-energy systems, and trauma. J Am Acad Dermatol. 2016;75:265-284.
- Morais-Almeida M, Gaspar A, Marinho S, et al. Allergy to local anesthetics of the amide group with tolerance to procaine. Allergy. 2003;58:827-828.
- To D, Kossintseva I, de Gannes G. Lidocaine contact allergy is becoming more prevalent. Dermatol Surg. 2014;40:1367-1372.
- Wahlgren CF, Quiding H. Depth of cutaneous analgesia after application of a eutectic mixture of the local anesthetics lidocaine and prilocaine (EMLA cream). J Am Acad Dermatol. 2000;42:584-588.
- Bjerring P, Andersen PH, Arendt-Nielsen L. Vascular response of human skin after analgesia with EMLA cream. Br J Anaesth. 1989;63:655-660.
- Ismail F, Goldsmith PC. EMLA cream-induced allergic contact dermatitis in a child with thalassaemia major. Contact Dermatitis. 2005;52:111.
- Thakur BK, Murali MR. EMLA cream-induced allergic contact dermatitis: a role for prilocaine as an immunogen. J Allergy Clin Immunol. 1995;95:776-778.
- Waton J, Boulanger A, Trechot PH, et al. Contact urticaria from EMLA cream. Contact Dermatitis. 2004;51:284-287.
- Bucalo BD, Mirikitani EJ, Moy RL. Comparison of skin anesthetic effect of liposomal lidocaine, nonliposomal lidocaine, and EMLA using 30-minute application time. Dermatol Surg. 1998;24:537-541.
- Guardiano RA, Norwood CW. Direct comparison of EMLA versus lidocaine for pain control in Nd:YAG 1,064 nm laser hair removal. Dermatol Surg. 2005;31:396-398.
- Nestor MS. Safety of occluded 4% liposomal lidocaine cream. J Drugs Dermatol. 2006;5:618-620.
- Oni G, Rasko Y, Kenkel J. Topical lidocaine enhanced by laser pretreatment: a safe and effective method of analgesia for facial rejuvenation. Aesthet Surg J. 2013;33:854-861.
- Niamtu J 3rd. Simple technique for lip and nasolabial fold anesthesia for injectable fillers. Dermatol Surg. 2005;31:1330-1332.
- Naumann M, Flachenecker P, Brocker EB, et al. Botulinum toxin for palmar hyperhidrosis. Lancet. 1997;349:252.
- Naumann M, Hofmann U, Bergmann I, et al. Focal hyperhidrosis: effective treatment with intracutaneous botulinum toxin. Arch Dermatol. 1998;134:301-304.
- Shelley WB, Talanin NY, Shelley ED. Botulinum toxin therapy for palmar hyperhidrosis. J Am Acad Dermatol. 1998;38(2, pt 1):227-229.
- Davies T, Karanovic S, Shergill B. Essential regional nerve blocks for the dermatologist: part 2. Clin Exp Dermatol. 2014;39:861-867.
Practice Points
- The proper delivery of local anesthesia is integral to successful cosmetic interventions.
- Regional nerve blocks can provide effective analgesia while reducing the number of injections and preserving the architecture of the cosmetic field.