User login
The State of Skin of Color Centers in the United States: A Cross-Sectional Survey Study
Although individuals with skin of color (SoC) are expected to become at least half of the US population by the year 2044, there remains a paucity of education and exposure to treatment of patients with SoC at many dermatology residency programs across the country.1 One way to improve SoC education has been the formation of specialized clinics, centers, and programs. The first SoC center (SoCC) was established in 1999 at Mount Sinai–St. Luke’s Roosevelt in New York, New York2; since then, at least 13 additional formal SoCCs or SoC specialty clinics (SoCSCs) at US academic dermatology programs have been established.
Skin of color centers serve several important purposes: they improve dermatologic care in patients with SoC, increase research efforts focused on SoC dermatologic conditions, and educate dermatology resident and fellow trainees about SoC. Improving dermatologic care of patients with SoC in the United States is important in providing equitable health care and improving health disparities. Studies have shown that patient-physician racial and cultural concordance can positively impact patient care, increase patient trust and rapport, and improve patient-physician communication, and it can even influence patient decision-making to seek care.3,4 Unfortunately, even though the US population continues to diversify, the racial/ethnic backgrounds of dermatologists do not parallel this trend; Hispanic and Black physicians comprise 18.9% and 13.6% of the general population, respectively, but represent only 4.2% and 3.0% of dermatologists, respectively.5-7 This deficit is mirrored by resident and faculty representation, with Black and Latino representation ranging from 3% to 7%.8-10
Many SoCC’s engage in research focused on dermatologic conditions affecting patients with SoC, which is vital to improving the dermatologic care in this underserved population. Despite increasing recognition of the importance of SoC research, there remains a paucity of clinical trials and research specifically focused on or demonstrating equitable representation of SoC.11,12
The education and training of future dermatologists is another important area that can be improved by SoCCs. A 2008 study involving 63 chief residents showed that approximately half (52.4% [33/63]) of graduating dermatology residents reported receiving SoC-specific didactics, sessions, or lectures, and 30.2% (19/63) reported having a dedicated rotation where they gained specific experience treating patients with SoC.13 A later study in 2022 (N=125) found that 63.2% of graduating dermatology residents reported receiving SoC-specific didactics, sessions, or lectures, and only 11.2% reported having a dedicated rotation where they gained experience treating patients with SoC.14 These findings suggest that in the last 14 years, formal SoC education—specifically SoC clinical training—has not increased sufficiently.
We conducted a cross-sectional survey study to provide an in-depth analysis of SoCCs and SoCSCs in the United States, including their patient care focus, research, and program diversity.
Methods
We conducted an investigator-initiated, multicenter, cross-sectional survey study of all SoCCs in the United States and their respective academic residency programs. Fifteen formal SoCCs and/or SoCSCs were identified by dermatology program websites and an article by Tull et al2 on the state of ethnic skin centers. All programs and centers identified were associated with a dermatology residency program accredited by the Accreditation Council for Graduate Medical Education.
A 42-item questionnaire was sent via email to the directors of these centers and clinics with the intent to collect descriptive information about each of the SoCCs, the diversity of the faculty and residents of the associated dermatology department, current research and funding, diversity and inclusion initiatives, and trainee education from March through April 2020. Data were analyzed using Excel and SPSS statistical software to obtain descriptive statistics including the mean value numeric trends across programs.
This study underwent expedited review and was approved by the University of Southern California (Los Angeles, California) institutional review board (IRB #HS-20-00113). Patient consent was not applicable, as no information was collected about patients.
Results
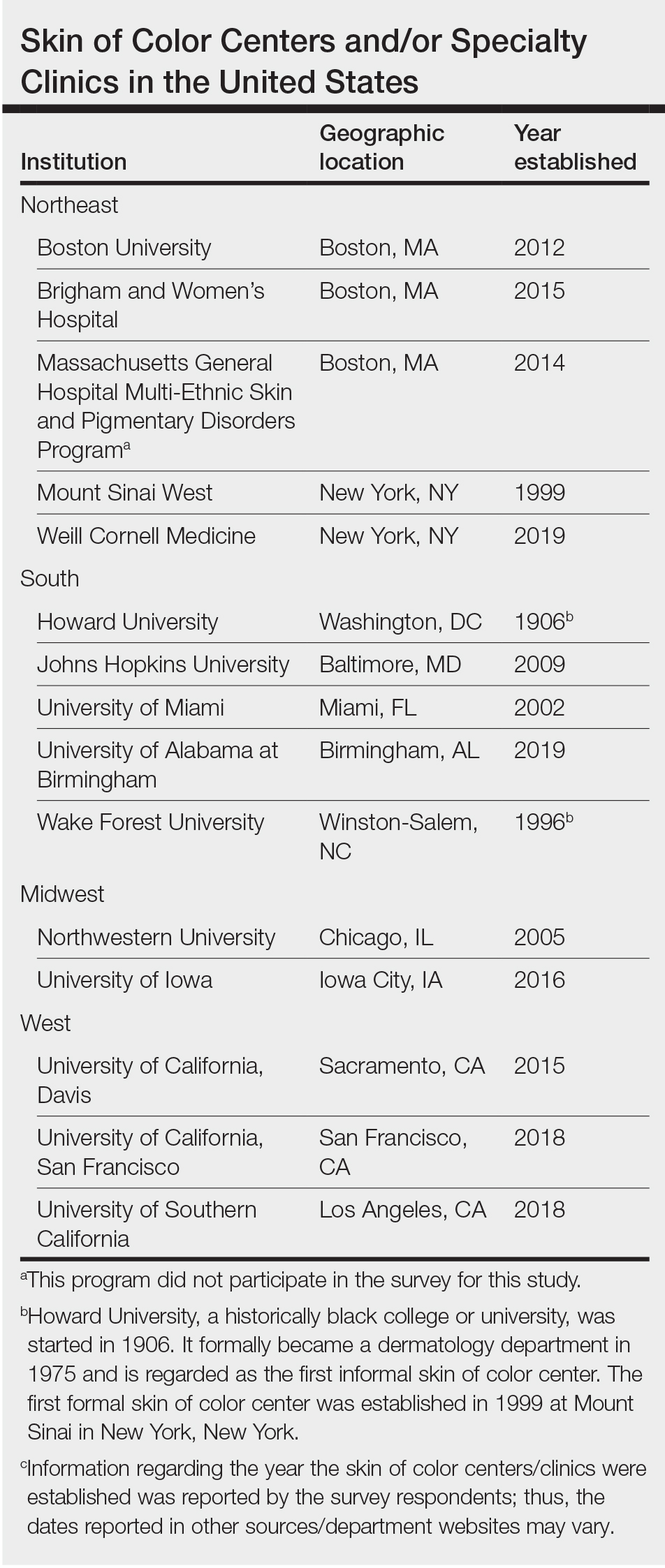
Fourteen directors from SoCCs/SoCSCs completed the questionnaire (93.3% response rate). Most centers were located in urban areas (12/14 [85.71%]), except for 2 in rural or suburban settings (Table). Most of the SoCCs/SoCSCs were located in the South (5/14 [35.71%]), followed by the Northeast (4/14 [28.57%]), West (3/14 [21.43%]), and Midwest (2/14 [14.29%])(Table). Six (42.86%) of the programs had a SoCSC, 3 (21.43%) had a formal SoCC, and 5 (35.71%) had both. Across all centers, the most common population seen and treated was Black/African American followed by Hispanic/Latino and Asian, respectively. The most commonly seen dermatologic conditions were acne, pigmentary disorders, alopecia, and atopic dermatitis (Figure). The most common cosmetic practice performed for patients with SoC was dermatosis papulosa nigra/seborrheic keratosis removal, followed by laser treatments, skin tag removal, chemical peels, and neuromodulator injections, respectively.
Faculty and Resident Demographics and Areas of Focus—The demographics and diversity of the dermatology faculty and residents at each individual institution also were assessed. The average number of full-time faculty at each institution was 19.4 (range, 2–48), while the average number of full-time faculty who identified as underrepresented in medicine (URiM) was 2.1 (range, 0–5). The average number of residents at each institution was 17.1 (range, 10–31), while the average number of URiM residents was 1.7 (range, 1–3).
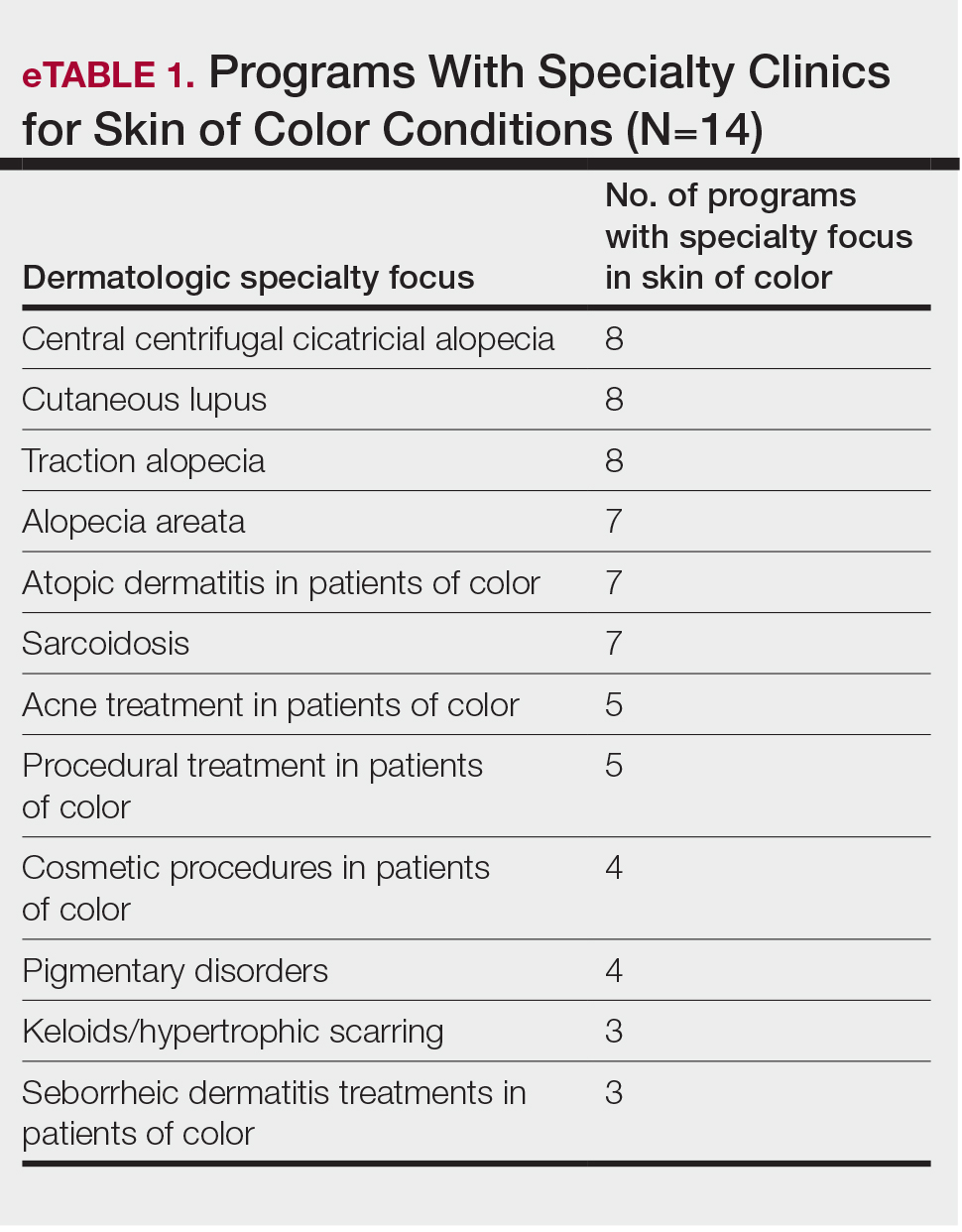
The average number of full-time faculty members at each SoCC was 1.6 (range, 1–4). The majority of program directors reported having other specialists in their department that also treated dermatologic conditions predominantly affecting patients with SoC (10/14 [71.43%]). The 3 most common areas of expertise were alopecia, including central centrifugal cicatricial alopecia (CCCA); cutaneous lupus; and traction alopecia (eTable 1).
Faculty SoC Research—Only a minority of programs had active clinical trials related to SoC (5/14 [35.71%]). Clinical research was the most common type of research being conducted (11/14 [78.57%]), followed by basic science/translational (4/14 [28.57%]) and epidemiologic research (2/14 [14.29%]). The most commonly investigated conditions for observational studies included CCCA, keloids/hypertrophic scarring, and atopic dermatitis (eTable 2). Only 8 of 14 programs had formal SoC research opportunities for residents (57.14%), while 9 had opportunities for medical students (64.29%).
Few institutions had internal funding (3/14 [21.43%]) or external funding (4/14 [28.57%]) for SoC research. Extramural fun ding sources included the Skin of Color Society, the Dermatology Foundation, and the Radiation Oncology Institute, as well as industry funding. No federal funding was received by any of the sites.
Skin of Color Education and Diversity Initiatives—All 14 programs had residents rotating through their SoCC and/or SoCSCs. The vast majority (12/14 [85.71%]) indicated resident exposure to clinical training at the SoCC and/or SoCSC during all 3 years of training. Residents at most of the programs spent 1 to 3 months rotating at the SoCC/SoCSC (6/14 [42.86%]). The other programs indicated residents spent 3 to 6 months (3/14 [21.43%]) or longer than 6 months (3/14 [21.4%]), and only 2 programs (14.29%) indicated that residents spent less than 1 month in the SoCC/SoCSC.
The majority of programs offered a SoC didactic curriculum for residents (10/14 [71.43%]), with an average of 3.3 SoC-related lectures per year (range, 0–5). Almost all programs (13/14 [92.86%]) invited SoC specialists from outside institutions as guest lecturers. Half of the programs (7/14 [50.0%]) used a SoC textbook for resident education. Only 3 programs (21.43%) offered at least 1 introductory SoC dermatology lecture as part of the preclinical medical student dermatology curriculum.
Home institution medical students were able to rotate at their respective SoCC/SoCSC at 11 of 14 institutions (78.57%), while visiting students were able to rotate at half of the programs (7/14 [50.0%]). At some programs, rotating at the SoCC/SoCSC was optional and was not formally integrated into the medical student rotation schedule for both home and visiting students (1/14 [7.14%] and 4/14 [28.57%], respectively). A majority of the programs (8/14 [57.14%]) offered scholarships and/or grants for home and/or visiting URiM students to help fund away rotations.
Despite their SoC focus, only half of the programs with SoCCs/SoCSCs had a formal committee focused on diversity and inclusion (7/14 [50.0%]) Additionally, only 5 of 14 (35.71%) programs had any URiM outreach programs with the medical school and/or the local community.
Comment
As the number of SoCCs/SoCSCs in the United States continues to grow, it is important to highlight their programmatic, research, and educational accomplishments to show the benefits of such programs, including their ability to increase access to culturally competent and inclusive care for diverse patient populations. One study found that nearly 92% of patients in the United States seen by dermatologists are White.15 Although studies have shown that Hispanic/Latino and Black patients are less likely to seek care from a dermatologist,16,17 there is no indication that these patients have a lesser need for such specialty care. Additionally, outcomes of common dermatologic conditions often are poorer in SoC populations.15 The dermatologists leading SoCCs/SoCSCs are actively working to reverse these trends, with Black and Hispanic/Latino patients representing the majority of their patients.
Faculty and Resident Demographics and Areas of Focus—Although there are increased diversity efforts in dermatology and the medical profession more broadly, there still is much work to be done. While individuals with SoC now comprise more than 35% of the US population, only 12% of dermatology residents and 6% of academic dermatology faculty identify as either Black or Hispanic/Latino.5,8,10 These numbers are even more discouraging when considering other URiM racial groups such as Pacific Islander/Native Hawaiians or Native American/American Indians who represent 0% and 0.1% of dermatology faculty, respectively.8,10 Academic programs with SoCCs/SoCSCs are working to create a space in which these discrepancies in representation can begin to be addressed. Compared to the national 6.8% rate of URiM faculty at academic institutions, those with SoCCs/SoCSCs report closer to 10% of faculty identifying as URiM.18 Moreover, almost all programs had faculty specialized in at least 1 condition that predominantly affects patients with SoC. This is of critical importance, as the conditions that most commonly affect SoC populations—such as CCCA, hidradenitis suppurativa, and cutaneous lupus—often are understudied, underfunded, underdiagnosed, and undertreated.19-22
Faculty SoC Research—An important step in narrowing the knowledge gap and improving health care disparities in patients with SoC is to increase SoC research and/or to increase the representation of patients with SoC in research studies. In a 2021 study, a PubMed search of articles indexed for MEDLINE using the terms race/ethnicity, dyschromia, atopic dermatitis, and acne was conducted to investigate publications pertaining to the top 3 most common chief concerns in patients with SoC. Only 1.6% of studies analyzed (N=74,941) had a specific focus on SoC.12 A similar study found that among the top 5 dermatology-focused research journals, only 3.4% of all research (N=11,003) on the top 3 most common chief concerns in patients with SOC was conducted in patients with SoC.23 Research efforts focused on dermatologic issues that affect patients with SoC are a priority at SoCCs/SoCSCs. In our study, all respondents indicated that they had at least 1 ongoing observational study; the most commonly studied conditions were CCCA, keloids/hypertrophic scarring, and atopic dermatitis, all of which are conditions that either occur in high frequency or primarily occur in SoC. Only 35.71% (5/14) of respondents had active clinical trials related to SoC, and only 21.43% (3/14) and 28.57% (4/14) had internal and external funding, respectively. Although research efforts are a priority at SoCCs/SoCSCs, our survey study highlights the continued paucity of formal clinical trials as well as funding for SoC-focused research. Improved research efforts for SoC must address these deficits in funding, academic support, and other resources.
It also is of great importance for institutions to provide support for trainees wanting to pursue SoC research. Encouragingly, more than half (57.14%) of SoCCs/SoCSCs have developed formal research opportunities for residents, and nearly 64.29% have formal opportunities for medical students. These efforts to provide early experiences in SoC research are especially impactful by cultivating interest in working with populations with SoC and hopefully inspiring future dermatologists to engage in further SoC research.
SoC Education and Diversity Initiatives—Although it is important to increase representation of URiM physicians in dermatology and to train more SoC specialists, it is imperative that all dermatologists feel comfortable recognizing and treating dermatologic conditions in patients of all skin tones and all racial/ethnic backgrounds; however, many studies suggest that residents not only lack formal didactics and education in SoC, but even more unsettling, they also lack confidence in treating SoC.13,24 However, one study showed that this can be changed; Mhlaba et al25 assessed a SoC curriculum for dermatology residents, and indeed all of the residents indicated that the curriculum improved their ability to treat SoC patients. This deficit in dermatology residency training is specifically addressed by SoCCs/SoCSCs. In our study, all respondents indicated that residents rotate through their centers. Moreover, our study found that most of the academic institutions with SoCCs/SoCSCs provide a SoC didactic curriculum for residents, and almost all of the programs invited SoC specialists to give guest lectures. This is in contrast to a 2022 study showing that 63.2% (N=125) of graduating dermatology residents reported receiving SoC-specific didactics, sessions, or lectures.14 These findings highlight the critical role that SoCCs/SoCSCs can provide in dermatology residency training.
Although SoCCs/SoCSCs have made considerable progress, there is still much room for improvement. Namely, only half of the respondents in our study indicated that their program has formally incorporated a SoC textbook into resident education (eTable 3). Representation of SoC in the textbooks that dermatology residents use is critically important because these images form the foundation of the morphologic aids of diagnosis. Numerous studies have analyzed popular dermatologic textbooks used by residency programs nationwide, finding the number of SoC images across dermatology textbooks ranging from 4% to 18%.26,27 The use of standard dermatology textbooks is not enough to train residents to be competent in diagnosing and treating patients with SoC. There should be a concerted effort across the field of dermatology to encourage the development of a SoC educational curriculum at every academic dermatology program, including SoC textbooks, Kodachromes, and online/electronic resources.
Efforts to increase diversity in dermatology and dermatologic training should start in medical school preclinical curriculums and medical student rotations. Although our survey did not assess current medical student curricula, the benefits of academic institutions with SoCCs/SoCSCs are highlighted by the ability for both home and visiting medical students to rotate through the centers and gain early exposure to SoC dermatology. Most of the programs even provide scholarships and/or grants for URiM students to help fund their rotations, which is of critical importance considering the mounting data that the financial burden of visiting rotations disproportionately affects URiM students.28
Study Limitations—Although we did an extensive search and believe to have correctly identified all 15 formal SoCCs/SoCSCs with a high response rate (93.3%), there are institutions that do not have formalized SoCCs/SoCSCs but are known to serve SoC populations. Likewise, there are private dermatology practices not associated with academic centers that have SoC specialists and positively contribute to SoC patient care, research, and education that were not included in this study. Additionally, the data for this study were collected in 2020 and analyzed in 2021, so it is possible that not all SoCCs, divisions, or clinics were included in this study, particularly if established after 2021.
Conclusion
As the United States continues to diversify, the proportion of patients with SoC will continue to grow, and it is imperative that this racial, ethnic, and cultural diversity is reflected in the dermatology workforce as well as research and training. The current deficits in medical training related to SoC populations and the importance for patients with SoC to find dermatologists who can appropriately treat them is well known.29 Skin of color centers/SoCSCs strive to increase access to care for patients with SoC, improve cultural competency, promote diversity among faculty and trainees, and encourage SoC research and education at all levels. We urge academic dermatology training programs to make SoC education, research, and patient care a departmental priority. Important first steps include departmental diversification at all levels, incorporating SoC into curricula for residents, providing and securing funding for SoC research, and supporting the establishment of more formal SoCCs and/or SoCSCs to help reduce dermatologic health care disparities among patients with SoC and improve health equity.
Appendix

- Colby SL, Jennifer JM. Projections of the size and composition of the U.S. population: 2014 to 2060. United States Census Bureau website. March 3, 2015. Accessed June 18, 2024. https://www.census.gov/library/publications/2015/demo/p25-1143.html
- Tull RZ, Kerby E, Subash JJ, et al. Ethnic skin centers in the United States: where are we in 2020? J Am Acad Dermatol. 2020;83:1757-1759. doi:10.1016/j.jaad.2020.03.054
- Shen MJ, Peterson EB, Costas-Muñiz R, et al. The effects of race and racial concordance on patient-physician communication: a systematic review of the literature. J Racial Ethn Health Disparities. 2018;5:117-140. doi:10.1007/s40615-017-0350-4
- Saha S, Beach MC. Impact of physician race on patient decision-making and ratings of physicians: a randomized experiment using video vignettes. J Gen Intern Med. 2020;35:1084-1091. doi:10.1007/s11606-020-05646-z
- Quick Facts: United States. US Census Bureau website. Accessed June 18, 2024. https://www.census.gov/quickfacts/fact/table/US/PST045221
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587. doi:10.1016/j.jaad.2015.10.044
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49. doi:10.1016/j.jisp.2017.07.001
- Association of American Medical Colleges. Table B5. number of active MD residents, by race/ethnicity (alone or in combination) and GME specialty. Accessed June 18, 2024. https://www.aamc.org/data-reports/students-residents/interactive-data/report-residents/2022/table-b5-md-residents-race-ethnicity-and-specialty
- Association of American Medical Colleges. Table B6. number of active DO residents, by race/ethnicity (alone or in combination) and GME specialty. Accessed June 18, 2024. https://www.aamc.org/data-reports/students-residents/interactive-data/report-residents/2022/table-b6-do-residents-race-ethnicity-and-specialty
- Association of American Medical Colleges. Table 16. U.S. medical school faculty by gender, race/ethnicity, and department, 2022. Accessed June 24, 2024. https://www.aamc.org/media/8456/download
- Chen V, Akhtar S, Zheng C, et al. Assessment of changes in diversity in dermatology clinical trials between 2010-2015 and 2015-2020: a systematic review. JAMA Dermatol. 2022;158:288-292. doi:10.1001/jamadermatol.2021.5596
- Montgomery SNB, Elbuluk N. A quantitative analysis of research publications focused on the top chief complaints in patients withskinof color. J Am Acad Dermatol. 2021;85:241-242. doi:10.1016/j.jaad.2020.08.031
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618. doi:10.1016/j.jaad.2008.06.024
- Ibraheim MK, Gupta R, Dao H, et al. Evaluating skin of color education in dermatology residency programs: data from a national survey. Clin Dermatol. 2022;40:228-233. doi:10.1016/j.clindermatol.2021.11.015
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii. doi:10.1016/j.det.2011.08.002
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291. doi:10.1001/jamadermatol.2018.3114
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 202;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Dlova NC, Salkey KS, Callender VD, et al. Central centrifugal cicatricial alopecia: new insights and a call for action. J Investig Dermatol Symp Proc. 2017;18:S54-S56. doi:10.1016/j.jisp.2017.01.004
- Okeke CAV, Perry JD, Simmonds FC, et al. Clinical trials and skin of color: the example of hidradenitis suppurativa. dermatology. 2022;238:180-184. doi:10.1159/000516467
- Robles J, Anim T, Wusu MH, et al. An Approach to Faculty Development for Underrepresented Minorities in Medicine. South Med J. 2021;114(9):579-582. doi:10.14423/SMJ.0000000000001290
- Serrano L, Ulschmid C, Szabo A, et al. Racial disparities of delay in diagnosis and dermatologic care for hidradenitis suppurativa. J Natl Med Assoc. 2022;114:613-616. doi:10.1016/j.jnma.2022.08.002
- Drenkard C, Lim SS. Update on lupus epidemiology: advancinghealth disparities research through the study of minority populations. Curr Opin Rheumatol. 2019;31:689-696. doi:10.1097/BOR.0000000000000646
- Militello M, Szeto MD, Presley CL, et al. A quantitative analysis of research publications focused on skin of color: representation in academic dermatology journals. J Am Acad Dermatol. 2021;85:E189-E192. doi:10.1016/j.jaad.2021.04.053
- Cline A, Winter RP, Kourosh S, et al. Multiethnic training in residency: a survey of dermatology residents. Cutis. 2020;105:310-313.
- Mhlaba JM, Pontes DS, Patterson SS, et al. Evaluation of a skin of color curriculum for dermatology residents. J Drugs Dermatol. 2021;20:786-789. doi:10.36849/JDD.6193
- Adelekun A, Onyekaba G, Lipoff JB. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2021;84:194-196. doi:10.1016/j.jaad.2020.04.084
- Harp T, Militello M, McCarver V, et al. Further analysis of skin of color representation in dermatology textbooks used by residents. J Am Acad Dermatol. 2022;87:E39-E41. doi:10.1016/j.jaad.2022.02.069
- Muzumdar S, Grant-Kels JM, Feng H. Strategies to improve medical student visiting rotations. Clin Dermatol. 2021;39:727-728. doi:10.1016/j.clindermatol.2020.11.001
- Gorbatenko-Roth K, Prose N, Kundu RV, et al. Assessment of Black patients’ perception of their dermatology care. JAMA Dermatol. 2019;155:1129-1134. doi:10.1001/jamadermatol.2019.2063
Although individuals with skin of color (SoC) are expected to become at least half of the US population by the year 2044, there remains a paucity of education and exposure to treatment of patients with SoC at many dermatology residency programs across the country.1 One way to improve SoC education has been the formation of specialized clinics, centers, and programs. The first SoC center (SoCC) was established in 1999 at Mount Sinai–St. Luke’s Roosevelt in New York, New York2; since then, at least 13 additional formal SoCCs or SoC specialty clinics (SoCSCs) at US academic dermatology programs have been established.
Skin of color centers serve several important purposes: they improve dermatologic care in patients with SoC, increase research efforts focused on SoC dermatologic conditions, and educate dermatology resident and fellow trainees about SoC. Improving dermatologic care of patients with SoC in the United States is important in providing equitable health care and improving health disparities. Studies have shown that patient-physician racial and cultural concordance can positively impact patient care, increase patient trust and rapport, and improve patient-physician communication, and it can even influence patient decision-making to seek care.3,4 Unfortunately, even though the US population continues to diversify, the racial/ethnic backgrounds of dermatologists do not parallel this trend; Hispanic and Black physicians comprise 18.9% and 13.6% of the general population, respectively, but represent only 4.2% and 3.0% of dermatologists, respectively.5-7 This deficit is mirrored by resident and faculty representation, with Black and Latino representation ranging from 3% to 7%.8-10
Many SoCC’s engage in research focused on dermatologic conditions affecting patients with SoC, which is vital to improving the dermatologic care in this underserved population. Despite increasing recognition of the importance of SoC research, there remains a paucity of clinical trials and research specifically focused on or demonstrating equitable representation of SoC.11,12
The education and training of future dermatologists is another important area that can be improved by SoCCs. A 2008 study involving 63 chief residents showed that approximately half (52.4% [33/63]) of graduating dermatology residents reported receiving SoC-specific didactics, sessions, or lectures, and 30.2% (19/63) reported having a dedicated rotation where they gained specific experience treating patients with SoC.13 A later study in 2022 (N=125) found that 63.2% of graduating dermatology residents reported receiving SoC-specific didactics, sessions, or lectures, and only 11.2% reported having a dedicated rotation where they gained experience treating patients with SoC.14 These findings suggest that in the last 14 years, formal SoC education—specifically SoC clinical training—has not increased sufficiently.
We conducted a cross-sectional survey study to provide an in-depth analysis of SoCCs and SoCSCs in the United States, including their patient care focus, research, and program diversity.
Methods
We conducted an investigator-initiated, multicenter, cross-sectional survey study of all SoCCs in the United States and their respective academic residency programs. Fifteen formal SoCCs and/or SoCSCs were identified by dermatology program websites and an article by Tull et al2 on the state of ethnic skin centers. All programs and centers identified were associated with a dermatology residency program accredited by the Accreditation Council for Graduate Medical Education.
A 42-item questionnaire was sent via email to the directors of these centers and clinics with the intent to collect descriptive information about each of the SoCCs, the diversity of the faculty and residents of the associated dermatology department, current research and funding, diversity and inclusion initiatives, and trainee education from March through April 2020. Data were analyzed using Excel and SPSS statistical software to obtain descriptive statistics including the mean value numeric trends across programs.
This study underwent expedited review and was approved by the University of Southern California (Los Angeles, California) institutional review board (IRB #HS-20-00113). Patient consent was not applicable, as no information was collected about patients.
Results
Fourteen directors from SoCCs/SoCSCs completed the questionnaire (93.3% response rate). Most centers were located in urban areas (12/14 [85.71%]), except for 2 in rural or suburban settings (Table). Most of the SoCCs/SoCSCs were located in the South (5/14 [35.71%]), followed by the Northeast (4/14 [28.57%]), West (3/14 [21.43%]), and Midwest (2/14 [14.29%])(Table). Six (42.86%) of the programs had a SoCSC, 3 (21.43%) had a formal SoCC, and 5 (35.71%) had both. Across all centers, the most common population seen and treated was Black/African American followed by Hispanic/Latino and Asian, respectively. The most commonly seen dermatologic conditions were acne, pigmentary disorders, alopecia, and atopic dermatitis (Figure). The most common cosmetic practice performed for patients with SoC was dermatosis papulosa nigra/seborrheic keratosis removal, followed by laser treatments, skin tag removal, chemical peels, and neuromodulator injections, respectively.

Faculty and Resident Demographics and Areas of Focus—The demographics and diversity of the dermatology faculty and residents at each individual institution also were assessed. The average number of full-time faculty at each institution was 19.4 (range, 2–48), while the average number of full-time faculty who identified as underrepresented in medicine (URiM) was 2.1 (range, 0–5). The average number of residents at each institution was 17.1 (range, 10–31), while the average number of URiM residents was 1.7 (range, 1–3).

The average number of full-time faculty members at each SoCC was 1.6 (range, 1–4). The majority of program directors reported having other specialists in their department that also treated dermatologic conditions predominantly affecting patients with SoC (10/14 [71.43%]). The 3 most common areas of expertise were alopecia, including central centrifugal cicatricial alopecia (CCCA); cutaneous lupus; and traction alopecia (eTable 1).
Faculty SoC Research—Only a minority of programs had active clinical trials related to SoC (5/14 [35.71%]). Clinical research was the most common type of research being conducted (11/14 [78.57%]), followed by basic science/translational (4/14 [28.57%]) and epidemiologic research (2/14 [14.29%]). The most commonly investigated conditions for observational studies included CCCA, keloids/hypertrophic scarring, and atopic dermatitis (eTable 2). Only 8 of 14 programs had formal SoC research opportunities for residents (57.14%), while 9 had opportunities for medical students (64.29%).
Few institutions had internal funding (3/14 [21.43%]) or external funding (4/14 [28.57%]) for SoC research. Extramural fun ding sources included the Skin of Color Society, the Dermatology Foundation, and the Radiation Oncology Institute, as well as industry funding. No federal funding was received by any of the sites.
Skin of Color Education and Diversity Initiatives—All 14 programs had residents rotating through their SoCC and/or SoCSCs. The vast majority (12/14 [85.71%]) indicated resident exposure to clinical training at the SoCC and/or SoCSC during all 3 years of training. Residents at most of the programs spent 1 to 3 months rotating at the SoCC/SoCSC (6/14 [42.86%]). The other programs indicated residents spent 3 to 6 months (3/14 [21.43%]) or longer than 6 months (3/14 [21.4%]), and only 2 programs (14.29%) indicated that residents spent less than 1 month in the SoCC/SoCSC.
The majority of programs offered a SoC didactic curriculum for residents (10/14 [71.43%]), with an average of 3.3 SoC-related lectures per year (range, 0–5). Almost all programs (13/14 [92.86%]) invited SoC specialists from outside institutions as guest lecturers. Half of the programs (7/14 [50.0%]) used a SoC textbook for resident education. Only 3 programs (21.43%) offered at least 1 introductory SoC dermatology lecture as part of the preclinical medical student dermatology curriculum.
Home institution medical students were able to rotate at their respective SoCC/SoCSC at 11 of 14 institutions (78.57%), while visiting students were able to rotate at half of the programs (7/14 [50.0%]). At some programs, rotating at the SoCC/SoCSC was optional and was not formally integrated into the medical student rotation schedule for both home and visiting students (1/14 [7.14%] and 4/14 [28.57%], respectively). A majority of the programs (8/14 [57.14%]) offered scholarships and/or grants for home and/or visiting URiM students to help fund away rotations.
Despite their SoC focus, only half of the programs with SoCCs/SoCSCs had a formal committee focused on diversity and inclusion (7/14 [50.0%]) Additionally, only 5 of 14 (35.71%) programs had any URiM outreach programs with the medical school and/or the local community.
Comment
As the number of SoCCs/SoCSCs in the United States continues to grow, it is important to highlight their programmatic, research, and educational accomplishments to show the benefits of such programs, including their ability to increase access to culturally competent and inclusive care for diverse patient populations. One study found that nearly 92% of patients in the United States seen by dermatologists are White.15 Although studies have shown that Hispanic/Latino and Black patients are less likely to seek care from a dermatologist,16,17 there is no indication that these patients have a lesser need for such specialty care. Additionally, outcomes of common dermatologic conditions often are poorer in SoC populations.15 The dermatologists leading SoCCs/SoCSCs are actively working to reverse these trends, with Black and Hispanic/Latino patients representing the majority of their patients.
Faculty and Resident Demographics and Areas of Focus—Although there are increased diversity efforts in dermatology and the medical profession more broadly, there still is much work to be done. While individuals with SoC now comprise more than 35% of the US population, only 12% of dermatology residents and 6% of academic dermatology faculty identify as either Black or Hispanic/Latino.5,8,10 These numbers are even more discouraging when considering other URiM racial groups such as Pacific Islander/Native Hawaiians or Native American/American Indians who represent 0% and 0.1% of dermatology faculty, respectively.8,10 Academic programs with SoCCs/SoCSCs are working to create a space in which these discrepancies in representation can begin to be addressed. Compared to the national 6.8% rate of URiM faculty at academic institutions, those with SoCCs/SoCSCs report closer to 10% of faculty identifying as URiM.18 Moreover, almost all programs had faculty specialized in at least 1 condition that predominantly affects patients with SoC. This is of critical importance, as the conditions that most commonly affect SoC populations—such as CCCA, hidradenitis suppurativa, and cutaneous lupus—often are understudied, underfunded, underdiagnosed, and undertreated.19-22
Faculty SoC Research—An important step in narrowing the knowledge gap and improving health care disparities in patients with SoC is to increase SoC research and/or to increase the representation of patients with SoC in research studies. In a 2021 study, a PubMed search of articles indexed for MEDLINE using the terms race/ethnicity, dyschromia, atopic dermatitis, and acne was conducted to investigate publications pertaining to the top 3 most common chief concerns in patients with SoC. Only 1.6% of studies analyzed (N=74,941) had a specific focus on SoC.12 A similar study found that among the top 5 dermatology-focused research journals, only 3.4% of all research (N=11,003) on the top 3 most common chief concerns in patients with SOC was conducted in patients with SoC.23 Research efforts focused on dermatologic issues that affect patients with SoC are a priority at SoCCs/SoCSCs. In our study, all respondents indicated that they had at least 1 ongoing observational study; the most commonly studied conditions were CCCA, keloids/hypertrophic scarring, and atopic dermatitis, all of which are conditions that either occur in high frequency or primarily occur in SoC. Only 35.71% (5/14) of respondents had active clinical trials related to SoC, and only 21.43% (3/14) and 28.57% (4/14) had internal and external funding, respectively. Although research efforts are a priority at SoCCs/SoCSCs, our survey study highlights the continued paucity of formal clinical trials as well as funding for SoC-focused research. Improved research efforts for SoC must address these deficits in funding, academic support, and other resources.
It also is of great importance for institutions to provide support for trainees wanting to pursue SoC research. Encouragingly, more than half (57.14%) of SoCCs/SoCSCs have developed formal research opportunities for residents, and nearly 64.29% have formal opportunities for medical students. These efforts to provide early experiences in SoC research are especially impactful by cultivating interest in working with populations with SoC and hopefully inspiring future dermatologists to engage in further SoC research.
SoC Education and Diversity Initiatives—Although it is important to increase representation of URiM physicians in dermatology and to train more SoC specialists, it is imperative that all dermatologists feel comfortable recognizing and treating dermatologic conditions in patients of all skin tones and all racial/ethnic backgrounds; however, many studies suggest that residents not only lack formal didactics and education in SoC, but even more unsettling, they also lack confidence in treating SoC.13,24 However, one study showed that this can be changed; Mhlaba et al25 assessed a SoC curriculum for dermatology residents, and indeed all of the residents indicated that the curriculum improved their ability to treat SoC patients. This deficit in dermatology residency training is specifically addressed by SoCCs/SoCSCs. In our study, all respondents indicated that residents rotate through their centers. Moreover, our study found that most of the academic institutions with SoCCs/SoCSCs provide a SoC didactic curriculum for residents, and almost all of the programs invited SoC specialists to give guest lectures. This is in contrast to a 2022 study showing that 63.2% (N=125) of graduating dermatology residents reported receiving SoC-specific didactics, sessions, or lectures.14 These findings highlight the critical role that SoCCs/SoCSCs can provide in dermatology residency training.
Although SoCCs/SoCSCs have made considerable progress, there is still much room for improvement. Namely, only half of the respondents in our study indicated that their program has formally incorporated a SoC textbook into resident education (eTable 3). Representation of SoC in the textbooks that dermatology residents use is critically important because these images form the foundation of the morphologic aids of diagnosis. Numerous studies have analyzed popular dermatologic textbooks used by residency programs nationwide, finding the number of SoC images across dermatology textbooks ranging from 4% to 18%.26,27 The use of standard dermatology textbooks is not enough to train residents to be competent in diagnosing and treating patients with SoC. There should be a concerted effort across the field of dermatology to encourage the development of a SoC educational curriculum at every academic dermatology program, including SoC textbooks, Kodachromes, and online/electronic resources.
Efforts to increase diversity in dermatology and dermatologic training should start in medical school preclinical curriculums and medical student rotations. Although our survey did not assess current medical student curricula, the benefits of academic institutions with SoCCs/SoCSCs are highlighted by the ability for both home and visiting medical students to rotate through the centers and gain early exposure to SoC dermatology. Most of the programs even provide scholarships and/or grants for URiM students to help fund their rotations, which is of critical importance considering the mounting data that the financial burden of visiting rotations disproportionately affects URiM students.28
Study Limitations—Although we did an extensive search and believe to have correctly identified all 15 formal SoCCs/SoCSCs with a high response rate (93.3%), there are institutions that do not have formalized SoCCs/SoCSCs but are known to serve SoC populations. Likewise, there are private dermatology practices not associated with academic centers that have SoC specialists and positively contribute to SoC patient care, research, and education that were not included in this study. Additionally, the data for this study were collected in 2020 and analyzed in 2021, so it is possible that not all SoCCs, divisions, or clinics were included in this study, particularly if established after 2021.
Conclusion
As the United States continues to diversify, the proportion of patients with SoC will continue to grow, and it is imperative that this racial, ethnic, and cultural diversity is reflected in the dermatology workforce as well as research and training. The current deficits in medical training related to SoC populations and the importance for patients with SoC to find dermatologists who can appropriately treat them is well known.29 Skin of color centers/SoCSCs strive to increase access to care for patients with SoC, improve cultural competency, promote diversity among faculty and trainees, and encourage SoC research and education at all levels. We urge academic dermatology training programs to make SoC education, research, and patient care a departmental priority. Important first steps include departmental diversification at all levels, incorporating SoC into curricula for residents, providing and securing funding for SoC research, and supporting the establishment of more formal SoCCs and/or SoCSCs to help reduce dermatologic health care disparities among patients with SoC and improve health equity.
Appendix



Although individuals with skin of color (SoC) are expected to become at least half of the US population by the year 2044, there remains a paucity of education and exposure to treatment of patients with SoC at many dermatology residency programs across the country.1 One way to improve SoC education has been the formation of specialized clinics, centers, and programs. The first SoC center (SoCC) was established in 1999 at Mount Sinai–St. Luke’s Roosevelt in New York, New York2; since then, at least 13 additional formal SoCCs or SoC specialty clinics (SoCSCs) at US academic dermatology programs have been established.
Skin of color centers serve several important purposes: they improve dermatologic care in patients with SoC, increase research efforts focused on SoC dermatologic conditions, and educate dermatology resident and fellow trainees about SoC. Improving dermatologic care of patients with SoC in the United States is important in providing equitable health care and improving health disparities. Studies have shown that patient-physician racial and cultural concordance can positively impact patient care, increase patient trust and rapport, and improve patient-physician communication, and it can even influence patient decision-making to seek care.3,4 Unfortunately, even though the US population continues to diversify, the racial/ethnic backgrounds of dermatologists do not parallel this trend; Hispanic and Black physicians comprise 18.9% and 13.6% of the general population, respectively, but represent only 4.2% and 3.0% of dermatologists, respectively.5-7 This deficit is mirrored by resident and faculty representation, with Black and Latino representation ranging from 3% to 7%.8-10
Many SoCC’s engage in research focused on dermatologic conditions affecting patients with SoC, which is vital to improving the dermatologic care in this underserved population. Despite increasing recognition of the importance of SoC research, there remains a paucity of clinical trials and research specifically focused on or demonstrating equitable representation of SoC.11,12
The education and training of future dermatologists is another important area that can be improved by SoCCs. A 2008 study involving 63 chief residents showed that approximately half (52.4% [33/63]) of graduating dermatology residents reported receiving SoC-specific didactics, sessions, or lectures, and 30.2% (19/63) reported having a dedicated rotation where they gained specific experience treating patients with SoC.13 A later study in 2022 (N=125) found that 63.2% of graduating dermatology residents reported receiving SoC-specific didactics, sessions, or lectures, and only 11.2% reported having a dedicated rotation where they gained experience treating patients with SoC.14 These findings suggest that in the last 14 years, formal SoC education—specifically SoC clinical training—has not increased sufficiently.
We conducted a cross-sectional survey study to provide an in-depth analysis of SoCCs and SoCSCs in the United States, including their patient care focus, research, and program diversity.
Methods
We conducted an investigator-initiated, multicenter, cross-sectional survey study of all SoCCs in the United States and their respective academic residency programs. Fifteen formal SoCCs and/or SoCSCs were identified by dermatology program websites and an article by Tull et al2 on the state of ethnic skin centers. All programs and centers identified were associated with a dermatology residency program accredited by the Accreditation Council for Graduate Medical Education.
A 42-item questionnaire was sent via email to the directors of these centers and clinics with the intent to collect descriptive information about each of the SoCCs, the diversity of the faculty and residents of the associated dermatology department, current research and funding, diversity and inclusion initiatives, and trainee education from March through April 2020. Data were analyzed using Excel and SPSS statistical software to obtain descriptive statistics including the mean value numeric trends across programs.
This study underwent expedited review and was approved by the University of Southern California (Los Angeles, California) institutional review board (IRB #HS-20-00113). Patient consent was not applicable, as no information was collected about patients.
Results
Fourteen directors from SoCCs/SoCSCs completed the questionnaire (93.3% response rate). Most centers were located in urban areas (12/14 [85.71%]), except for 2 in rural or suburban settings (Table). Most of the SoCCs/SoCSCs were located in the South (5/14 [35.71%]), followed by the Northeast (4/14 [28.57%]), West (3/14 [21.43%]), and Midwest (2/14 [14.29%])(Table). Six (42.86%) of the programs had a SoCSC, 3 (21.43%) had a formal SoCC, and 5 (35.71%) had both. Across all centers, the most common population seen and treated was Black/African American followed by Hispanic/Latino and Asian, respectively. The most commonly seen dermatologic conditions were acne, pigmentary disorders, alopecia, and atopic dermatitis (Figure). The most common cosmetic practice performed for patients with SoC was dermatosis papulosa nigra/seborrheic keratosis removal, followed by laser treatments, skin tag removal, chemical peels, and neuromodulator injections, respectively.

Faculty and Resident Demographics and Areas of Focus—The demographics and diversity of the dermatology faculty and residents at each individual institution also were assessed. The average number of full-time faculty at each institution was 19.4 (range, 2–48), while the average number of full-time faculty who identified as underrepresented in medicine (URiM) was 2.1 (range, 0–5). The average number of residents at each institution was 17.1 (range, 10–31), while the average number of URiM residents was 1.7 (range, 1–3).

The average number of full-time faculty members at each SoCC was 1.6 (range, 1–4). The majority of program directors reported having other specialists in their department that also treated dermatologic conditions predominantly affecting patients with SoC (10/14 [71.43%]). The 3 most common areas of expertise were alopecia, including central centrifugal cicatricial alopecia (CCCA); cutaneous lupus; and traction alopecia (eTable 1).
Faculty SoC Research—Only a minority of programs had active clinical trials related to SoC (5/14 [35.71%]). Clinical research was the most common type of research being conducted (11/14 [78.57%]), followed by basic science/translational (4/14 [28.57%]) and epidemiologic research (2/14 [14.29%]). The most commonly investigated conditions for observational studies included CCCA, keloids/hypertrophic scarring, and atopic dermatitis (eTable 2). Only 8 of 14 programs had formal SoC research opportunities for residents (57.14%), while 9 had opportunities for medical students (64.29%).
Few institutions had internal funding (3/14 [21.43%]) or external funding (4/14 [28.57%]) for SoC research. Extramural fun ding sources included the Skin of Color Society, the Dermatology Foundation, and the Radiation Oncology Institute, as well as industry funding. No federal funding was received by any of the sites.
Skin of Color Education and Diversity Initiatives—All 14 programs had residents rotating through their SoCC and/or SoCSCs. The vast majority (12/14 [85.71%]) indicated resident exposure to clinical training at the SoCC and/or SoCSC during all 3 years of training. Residents at most of the programs spent 1 to 3 months rotating at the SoCC/SoCSC (6/14 [42.86%]). The other programs indicated residents spent 3 to 6 months (3/14 [21.43%]) or longer than 6 months (3/14 [21.4%]), and only 2 programs (14.29%) indicated that residents spent less than 1 month in the SoCC/SoCSC.
The majority of programs offered a SoC didactic curriculum for residents (10/14 [71.43%]), with an average of 3.3 SoC-related lectures per year (range, 0–5). Almost all programs (13/14 [92.86%]) invited SoC specialists from outside institutions as guest lecturers. Half of the programs (7/14 [50.0%]) used a SoC textbook for resident education. Only 3 programs (21.43%) offered at least 1 introductory SoC dermatology lecture as part of the preclinical medical student dermatology curriculum.
Home institution medical students were able to rotate at their respective SoCC/SoCSC at 11 of 14 institutions (78.57%), while visiting students were able to rotate at half of the programs (7/14 [50.0%]). At some programs, rotating at the SoCC/SoCSC was optional and was not formally integrated into the medical student rotation schedule for both home and visiting students (1/14 [7.14%] and 4/14 [28.57%], respectively). A majority of the programs (8/14 [57.14%]) offered scholarships and/or grants for home and/or visiting URiM students to help fund away rotations.
Despite their SoC focus, only half of the programs with SoCCs/SoCSCs had a formal committee focused on diversity and inclusion (7/14 [50.0%]) Additionally, only 5 of 14 (35.71%) programs had any URiM outreach programs with the medical school and/or the local community.
Comment
As the number of SoCCs/SoCSCs in the United States continues to grow, it is important to highlight their programmatic, research, and educational accomplishments to show the benefits of such programs, including their ability to increase access to culturally competent and inclusive care for diverse patient populations. One study found that nearly 92% of patients in the United States seen by dermatologists are White.15 Although studies have shown that Hispanic/Latino and Black patients are less likely to seek care from a dermatologist,16,17 there is no indication that these patients have a lesser need for such specialty care. Additionally, outcomes of common dermatologic conditions often are poorer in SoC populations.15 The dermatologists leading SoCCs/SoCSCs are actively working to reverse these trends, with Black and Hispanic/Latino patients representing the majority of their patients.
Faculty and Resident Demographics and Areas of Focus—Although there are increased diversity efforts in dermatology and the medical profession more broadly, there still is much work to be done. While individuals with SoC now comprise more than 35% of the US population, only 12% of dermatology residents and 6% of academic dermatology faculty identify as either Black or Hispanic/Latino.5,8,10 These numbers are even more discouraging when considering other URiM racial groups such as Pacific Islander/Native Hawaiians or Native American/American Indians who represent 0% and 0.1% of dermatology faculty, respectively.8,10 Academic programs with SoCCs/SoCSCs are working to create a space in which these discrepancies in representation can begin to be addressed. Compared to the national 6.8% rate of URiM faculty at academic institutions, those with SoCCs/SoCSCs report closer to 10% of faculty identifying as URiM.18 Moreover, almost all programs had faculty specialized in at least 1 condition that predominantly affects patients with SoC. This is of critical importance, as the conditions that most commonly affect SoC populations—such as CCCA, hidradenitis suppurativa, and cutaneous lupus—often are understudied, underfunded, underdiagnosed, and undertreated.19-22
Faculty SoC Research—An important step in narrowing the knowledge gap and improving health care disparities in patients with SoC is to increase SoC research and/or to increase the representation of patients with SoC in research studies. In a 2021 study, a PubMed search of articles indexed for MEDLINE using the terms race/ethnicity, dyschromia, atopic dermatitis, and acne was conducted to investigate publications pertaining to the top 3 most common chief concerns in patients with SoC. Only 1.6% of studies analyzed (N=74,941) had a specific focus on SoC.12 A similar study found that among the top 5 dermatology-focused research journals, only 3.4% of all research (N=11,003) on the top 3 most common chief concerns in patients with SOC was conducted in patients with SoC.23 Research efforts focused on dermatologic issues that affect patients with SoC are a priority at SoCCs/SoCSCs. In our study, all respondents indicated that they had at least 1 ongoing observational study; the most commonly studied conditions were CCCA, keloids/hypertrophic scarring, and atopic dermatitis, all of which are conditions that either occur in high frequency or primarily occur in SoC. Only 35.71% (5/14) of respondents had active clinical trials related to SoC, and only 21.43% (3/14) and 28.57% (4/14) had internal and external funding, respectively. Although research efforts are a priority at SoCCs/SoCSCs, our survey study highlights the continued paucity of formal clinical trials as well as funding for SoC-focused research. Improved research efforts for SoC must address these deficits in funding, academic support, and other resources.
It also is of great importance for institutions to provide support for trainees wanting to pursue SoC research. Encouragingly, more than half (57.14%) of SoCCs/SoCSCs have developed formal research opportunities for residents, and nearly 64.29% have formal opportunities for medical students. These efforts to provide early experiences in SoC research are especially impactful by cultivating interest in working with populations with SoC and hopefully inspiring future dermatologists to engage in further SoC research.
SoC Education and Diversity Initiatives—Although it is important to increase representation of URiM physicians in dermatology and to train more SoC specialists, it is imperative that all dermatologists feel comfortable recognizing and treating dermatologic conditions in patients of all skin tones and all racial/ethnic backgrounds; however, many studies suggest that residents not only lack formal didactics and education in SoC, but even more unsettling, they also lack confidence in treating SoC.13,24 However, one study showed that this can be changed; Mhlaba et al25 assessed a SoC curriculum for dermatology residents, and indeed all of the residents indicated that the curriculum improved their ability to treat SoC patients. This deficit in dermatology residency training is specifically addressed by SoCCs/SoCSCs. In our study, all respondents indicated that residents rotate through their centers. Moreover, our study found that most of the academic institutions with SoCCs/SoCSCs provide a SoC didactic curriculum for residents, and almost all of the programs invited SoC specialists to give guest lectures. This is in contrast to a 2022 study showing that 63.2% (N=125) of graduating dermatology residents reported receiving SoC-specific didactics, sessions, or lectures.14 These findings highlight the critical role that SoCCs/SoCSCs can provide in dermatology residency training.
Although SoCCs/SoCSCs have made considerable progress, there is still much room for improvement. Namely, only half of the respondents in our study indicated that their program has formally incorporated a SoC textbook into resident education (eTable 3). Representation of SoC in the textbooks that dermatology residents use is critically important because these images form the foundation of the morphologic aids of diagnosis. Numerous studies have analyzed popular dermatologic textbooks used by residency programs nationwide, finding the number of SoC images across dermatology textbooks ranging from 4% to 18%.26,27 The use of standard dermatology textbooks is not enough to train residents to be competent in diagnosing and treating patients with SoC. There should be a concerted effort across the field of dermatology to encourage the development of a SoC educational curriculum at every academic dermatology program, including SoC textbooks, Kodachromes, and online/electronic resources.
Efforts to increase diversity in dermatology and dermatologic training should start in medical school preclinical curriculums and medical student rotations. Although our survey did not assess current medical student curricula, the benefits of academic institutions with SoCCs/SoCSCs are highlighted by the ability for both home and visiting medical students to rotate through the centers and gain early exposure to SoC dermatology. Most of the programs even provide scholarships and/or grants for URiM students to help fund their rotations, which is of critical importance considering the mounting data that the financial burden of visiting rotations disproportionately affects URiM students.28
Study Limitations—Although we did an extensive search and believe to have correctly identified all 15 formal SoCCs/SoCSCs with a high response rate (93.3%), there are institutions that do not have formalized SoCCs/SoCSCs but are known to serve SoC populations. Likewise, there are private dermatology practices not associated with academic centers that have SoC specialists and positively contribute to SoC patient care, research, and education that were not included in this study. Additionally, the data for this study were collected in 2020 and analyzed in 2021, so it is possible that not all SoCCs, divisions, or clinics were included in this study, particularly if established after 2021.
Conclusion
As the United States continues to diversify, the proportion of patients with SoC will continue to grow, and it is imperative that this racial, ethnic, and cultural diversity is reflected in the dermatology workforce as well as research and training. The current deficits in medical training related to SoC populations and the importance for patients with SoC to find dermatologists who can appropriately treat them is well known.29 Skin of color centers/SoCSCs strive to increase access to care for patients with SoC, improve cultural competency, promote diversity among faculty and trainees, and encourage SoC research and education at all levels. We urge academic dermatology training programs to make SoC education, research, and patient care a departmental priority. Important first steps include departmental diversification at all levels, incorporating SoC into curricula for residents, providing and securing funding for SoC research, and supporting the establishment of more formal SoCCs and/or SoCSCs to help reduce dermatologic health care disparities among patients with SoC and improve health equity.
Appendix



- Colby SL, Jennifer JM. Projections of the size and composition of the U.S. population: 2014 to 2060. United States Census Bureau website. March 3, 2015. Accessed June 18, 2024. https://www.census.gov/library/publications/2015/demo/p25-1143.html
- Tull RZ, Kerby E, Subash JJ, et al. Ethnic skin centers in the United States: where are we in 2020? J Am Acad Dermatol. 2020;83:1757-1759. doi:10.1016/j.jaad.2020.03.054
- Shen MJ, Peterson EB, Costas-Muñiz R, et al. The effects of race and racial concordance on patient-physician communication: a systematic review of the literature. J Racial Ethn Health Disparities. 2018;5:117-140. doi:10.1007/s40615-017-0350-4
- Saha S, Beach MC. Impact of physician race on patient decision-making and ratings of physicians: a randomized experiment using video vignettes. J Gen Intern Med. 2020;35:1084-1091. doi:10.1007/s11606-020-05646-z
- Quick Facts: United States. US Census Bureau website. Accessed June 18, 2024. https://www.census.gov/quickfacts/fact/table/US/PST045221
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587. doi:10.1016/j.jaad.2015.10.044
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49. doi:10.1016/j.jisp.2017.07.001
- Association of American Medical Colleges. Table B5. number of active MD residents, by race/ethnicity (alone or in combination) and GME specialty. Accessed June 18, 2024. https://www.aamc.org/data-reports/students-residents/interactive-data/report-residents/2022/table-b5-md-residents-race-ethnicity-and-specialty
- Association of American Medical Colleges. Table B6. number of active DO residents, by race/ethnicity (alone or in combination) and GME specialty. Accessed June 18, 2024. https://www.aamc.org/data-reports/students-residents/interactive-data/report-residents/2022/table-b6-do-residents-race-ethnicity-and-specialty
- Association of American Medical Colleges. Table 16. U.S. medical school faculty by gender, race/ethnicity, and department, 2022. Accessed June 24, 2024. https://www.aamc.org/media/8456/download
- Chen V, Akhtar S, Zheng C, et al. Assessment of changes in diversity in dermatology clinical trials between 2010-2015 and 2015-2020: a systematic review. JAMA Dermatol. 2022;158:288-292. doi:10.1001/jamadermatol.2021.5596
- Montgomery SNB, Elbuluk N. A quantitative analysis of research publications focused on the top chief complaints in patients withskinof color. J Am Acad Dermatol. 2021;85:241-242. doi:10.1016/j.jaad.2020.08.031
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618. doi:10.1016/j.jaad.2008.06.024
- Ibraheim MK, Gupta R, Dao H, et al. Evaluating skin of color education in dermatology residency programs: data from a national survey. Clin Dermatol. 2022;40:228-233. doi:10.1016/j.clindermatol.2021.11.015
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii. doi:10.1016/j.det.2011.08.002
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291. doi:10.1001/jamadermatol.2018.3114
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 202;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Dlova NC, Salkey KS, Callender VD, et al. Central centrifugal cicatricial alopecia: new insights and a call for action. J Investig Dermatol Symp Proc. 2017;18:S54-S56. doi:10.1016/j.jisp.2017.01.004
- Okeke CAV, Perry JD, Simmonds FC, et al. Clinical trials and skin of color: the example of hidradenitis suppurativa. dermatology. 2022;238:180-184. doi:10.1159/000516467
- Robles J, Anim T, Wusu MH, et al. An Approach to Faculty Development for Underrepresented Minorities in Medicine. South Med J. 2021;114(9):579-582. doi:10.14423/SMJ.0000000000001290
- Serrano L, Ulschmid C, Szabo A, et al. Racial disparities of delay in diagnosis and dermatologic care for hidradenitis suppurativa. J Natl Med Assoc. 2022;114:613-616. doi:10.1016/j.jnma.2022.08.002
- Drenkard C, Lim SS. Update on lupus epidemiology: advancinghealth disparities research through the study of minority populations. Curr Opin Rheumatol. 2019;31:689-696. doi:10.1097/BOR.0000000000000646
- Militello M, Szeto MD, Presley CL, et al. A quantitative analysis of research publications focused on skin of color: representation in academic dermatology journals. J Am Acad Dermatol. 2021;85:E189-E192. doi:10.1016/j.jaad.2021.04.053
- Cline A, Winter RP, Kourosh S, et al. Multiethnic training in residency: a survey of dermatology residents. Cutis. 2020;105:310-313.
- Mhlaba JM, Pontes DS, Patterson SS, et al. Evaluation of a skin of color curriculum for dermatology residents. J Drugs Dermatol. 2021;20:786-789. doi:10.36849/JDD.6193
- Adelekun A, Onyekaba G, Lipoff JB. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2021;84:194-196. doi:10.1016/j.jaad.2020.04.084
- Harp T, Militello M, McCarver V, et al. Further analysis of skin of color representation in dermatology textbooks used by residents. J Am Acad Dermatol. 2022;87:E39-E41. doi:10.1016/j.jaad.2022.02.069
- Muzumdar S, Grant-Kels JM, Feng H. Strategies to improve medical student visiting rotations. Clin Dermatol. 2021;39:727-728. doi:10.1016/j.clindermatol.2020.11.001
- Gorbatenko-Roth K, Prose N, Kundu RV, et al. Assessment of Black patients’ perception of their dermatology care. JAMA Dermatol. 2019;155:1129-1134. doi:10.1001/jamadermatol.2019.2063
- Colby SL, Jennifer JM. Projections of the size and composition of the U.S. population: 2014 to 2060. United States Census Bureau website. March 3, 2015. Accessed June 18, 2024. https://www.census.gov/library/publications/2015/demo/p25-1143.html
- Tull RZ, Kerby E, Subash JJ, et al. Ethnic skin centers in the United States: where are we in 2020? J Am Acad Dermatol. 2020;83:1757-1759. doi:10.1016/j.jaad.2020.03.054
- Shen MJ, Peterson EB, Costas-Muñiz R, et al. The effects of race and racial concordance on patient-physician communication: a systematic review of the literature. J Racial Ethn Health Disparities. 2018;5:117-140. doi:10.1007/s40615-017-0350-4
- Saha S, Beach MC. Impact of physician race on patient decision-making and ratings of physicians: a randomized experiment using video vignettes. J Gen Intern Med. 2020;35:1084-1091. doi:10.1007/s11606-020-05646-z
- Quick Facts: United States. US Census Bureau website. Accessed June 18, 2024. https://www.census.gov/quickfacts/fact/table/US/PST045221
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587. doi:10.1016/j.jaad.2015.10.044
- Van Voorhees AS, Enos CW. Diversity in dermatology residency programs. J Investig Dermatol Symp Proc. 2017;18:S46-S49. doi:10.1016/j.jisp.2017.07.001
- Association of American Medical Colleges. Table B5. number of active MD residents, by race/ethnicity (alone or in combination) and GME specialty. Accessed June 18, 2024. https://www.aamc.org/data-reports/students-residents/interactive-data/report-residents/2022/table-b5-md-residents-race-ethnicity-and-specialty
- Association of American Medical Colleges. Table B6. number of active DO residents, by race/ethnicity (alone or in combination) and GME specialty. Accessed June 18, 2024. https://www.aamc.org/data-reports/students-residents/interactive-data/report-residents/2022/table-b6-do-residents-race-ethnicity-and-specialty
- Association of American Medical Colleges. Table 16. U.S. medical school faculty by gender, race/ethnicity, and department, 2022. Accessed June 24, 2024. https://www.aamc.org/media/8456/download
- Chen V, Akhtar S, Zheng C, et al. Assessment of changes in diversity in dermatology clinical trials between 2010-2015 and 2015-2020: a systematic review. JAMA Dermatol. 2022;158:288-292. doi:10.1001/jamadermatol.2021.5596
- Montgomery SNB, Elbuluk N. A quantitative analysis of research publications focused on the top chief complaints in patients withskinof color. J Am Acad Dermatol. 2021;85:241-242. doi:10.1016/j.jaad.2020.08.031
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618. doi:10.1016/j.jaad.2008.06.024
- Ibraheim MK, Gupta R, Dao H, et al. Evaluating skin of color education in dermatology residency programs: data from a national survey. Clin Dermatol. 2022;40:228-233. doi:10.1016/j.clindermatol.2021.11.015
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii. doi:10.1016/j.det.2011.08.002
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291. doi:10.1001/jamadermatol.2018.3114
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 202;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Dlova NC, Salkey KS, Callender VD, et al. Central centrifugal cicatricial alopecia: new insights and a call for action. J Investig Dermatol Symp Proc. 2017;18:S54-S56. doi:10.1016/j.jisp.2017.01.004
- Okeke CAV, Perry JD, Simmonds FC, et al. Clinical trials and skin of color: the example of hidradenitis suppurativa. dermatology. 2022;238:180-184. doi:10.1159/000516467
- Robles J, Anim T, Wusu MH, et al. An Approach to Faculty Development for Underrepresented Minorities in Medicine. South Med J. 2021;114(9):579-582. doi:10.14423/SMJ.0000000000001290
- Serrano L, Ulschmid C, Szabo A, et al. Racial disparities of delay in diagnosis and dermatologic care for hidradenitis suppurativa. J Natl Med Assoc. 2022;114:613-616. doi:10.1016/j.jnma.2022.08.002
- Drenkard C, Lim SS. Update on lupus epidemiology: advancinghealth disparities research through the study of minority populations. Curr Opin Rheumatol. 2019;31:689-696. doi:10.1097/BOR.0000000000000646
- Militello M, Szeto MD, Presley CL, et al. A quantitative analysis of research publications focused on skin of color: representation in academic dermatology journals. J Am Acad Dermatol. 2021;85:E189-E192. doi:10.1016/j.jaad.2021.04.053
- Cline A, Winter RP, Kourosh S, et al. Multiethnic training in residency: a survey of dermatology residents. Cutis. 2020;105:310-313.
- Mhlaba JM, Pontes DS, Patterson SS, et al. Evaluation of a skin of color curriculum for dermatology residents. J Drugs Dermatol. 2021;20:786-789. doi:10.36849/JDD.6193
- Adelekun A, Onyekaba G, Lipoff JB. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2021;84:194-196. doi:10.1016/j.jaad.2020.04.084
- Harp T, Militello M, McCarver V, et al. Further analysis of skin of color representation in dermatology textbooks used by residents. J Am Acad Dermatol. 2022;87:E39-E41. doi:10.1016/j.jaad.2022.02.069
- Muzumdar S, Grant-Kels JM, Feng H. Strategies to improve medical student visiting rotations. Clin Dermatol. 2021;39:727-728. doi:10.1016/j.clindermatol.2020.11.001
- Gorbatenko-Roth K, Prose N, Kundu RV, et al. Assessment of Black patients’ perception of their dermatology care. JAMA Dermatol. 2019;155:1129-1134. doi:10.1001/jamadermatol.2019.2063
Practice Points
- Skin of color centers in the United States work to reverse the paucity of research, education, and training in skin of color dermatology and promote the diversification of residents and faculty.
- Skin of color centers expand access to culturally competent and inclusive care for diverse patient populations.
Skin Manifestations of Complex Regional Pain Syndrome
To the Editor:
Complex regional pain syndrome (CRPS) is a neurologic condition characterized by chronic pain and sensory changes, including allodynia and hyperalgesia, that usually affect the extremities.1,2 The syndrome is defined by the International Association for the Study of Pain (IASP) as a condition that appears regionally after an injury, with a variety of symptoms that often exceed the expected clinical course both in magnitude and duration, causing impairment of motor function and variable progression.3
Although CRPS most often is described following minor peripheral trauma, other precipitating causes include surgery and vascular events.4 Additional features of the condition include autonomic dysfunction, edema, and trophic changes.1 Symptoms of CRPS traditionally present in 3 stages, with notable skin changes most often documented in stages II and III.2
Skin changes are a known manifestation of the syndrome, but reports in the dermatologic literature are scarce. Qureshi and Friedman5 identified only 23 articles in the dermatology literature since 1990 in which skin changes in CRPS were described. We present a patient with a diagnosis of CRPS who developed hyperpigmentation and sclerotic changes, including skin thickening, induration, and skin tightening.
A middle-aged Black woman presented to dermatology for evaluation of progressive hyperpigmentation, hyperhidrosis, and sclerotic changes to the skin. Approximately 3 years prior, the patient was given a diagnosis of CRPS of the hands and feet. Pain symptoms started approximately 3 years prior to the onset of symptoms. Symptoms started in the left hand and eventually spread to the right arm, left leg, and subsequently to the right leg. The first dermatologic change the patient noticed was tightening of the skin in the affected area that led to decreased mobility, which improved over time—partly on its own and partly with physical therapy.
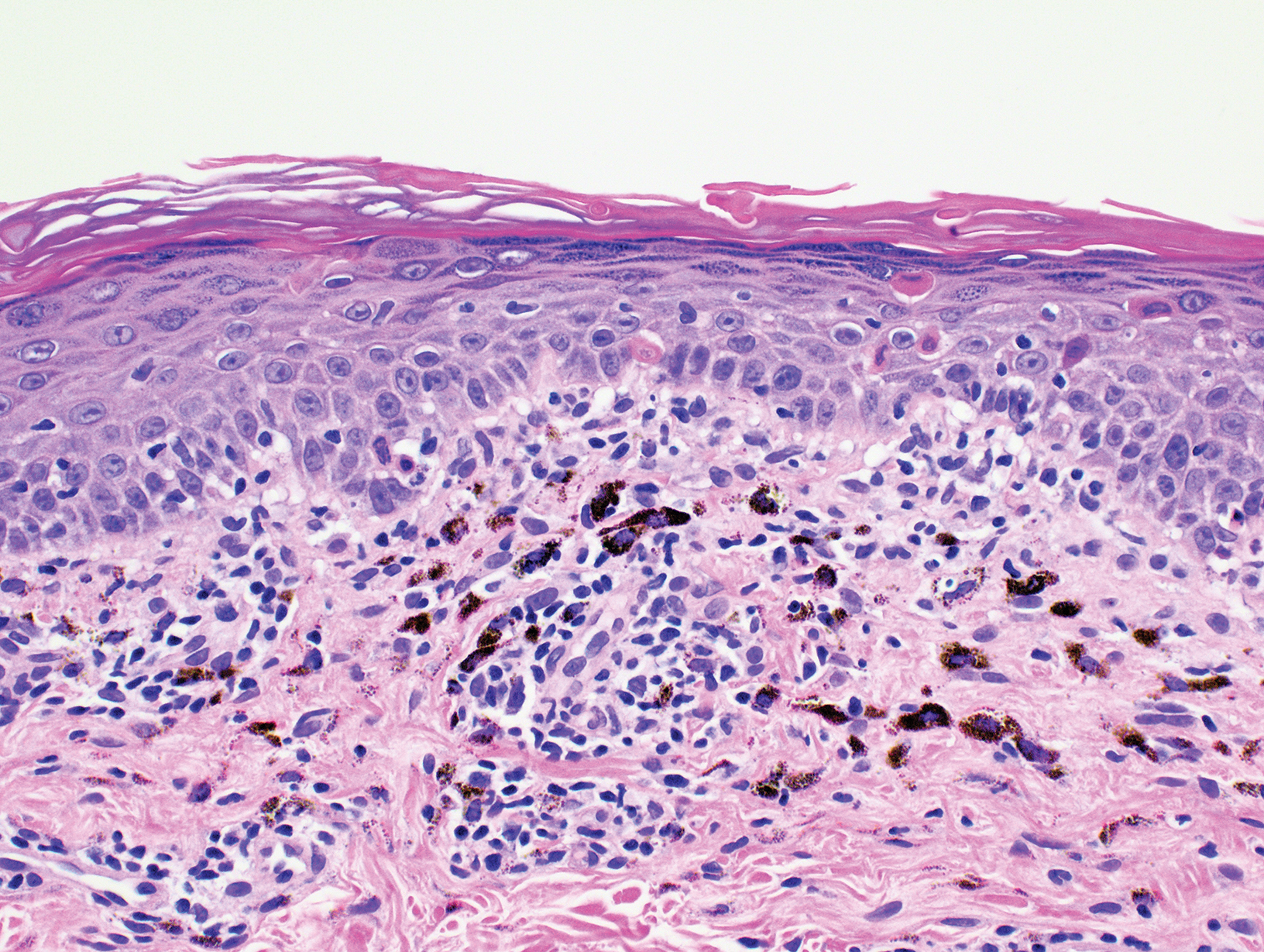
A biopsy performed by an outside dermatologist at the initial presentation demonstrated sclerodermalike changes, which were treated with creams but without improvement. Scleroderma was later ruled out by the same dermatologist. Skin tightening improved over time, with complete resolution approximately 1 year after the onset of symptoms.
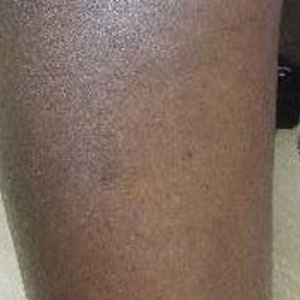
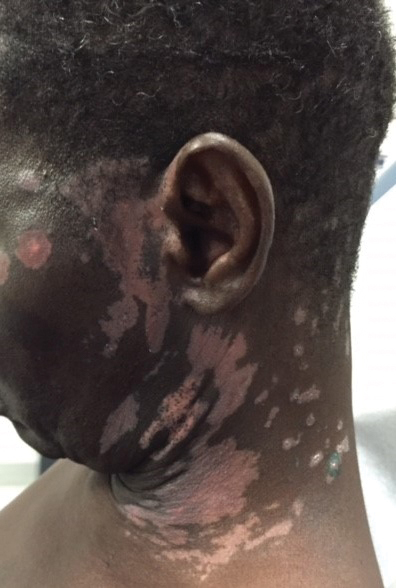
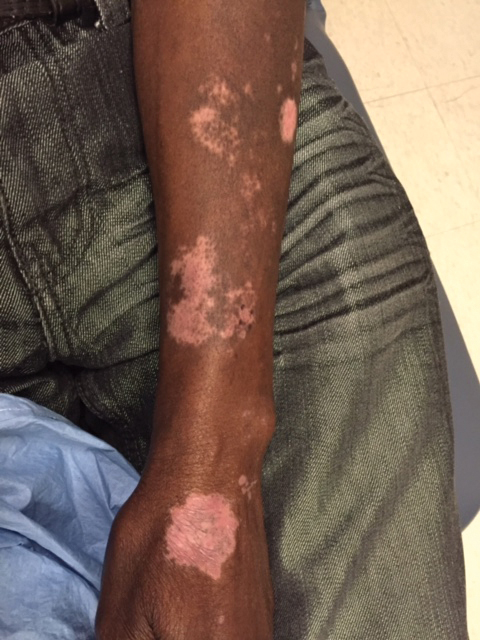
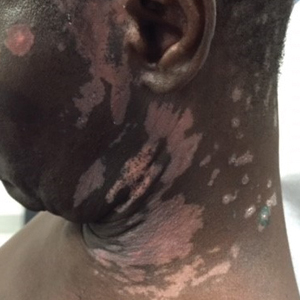
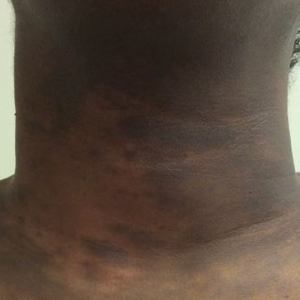
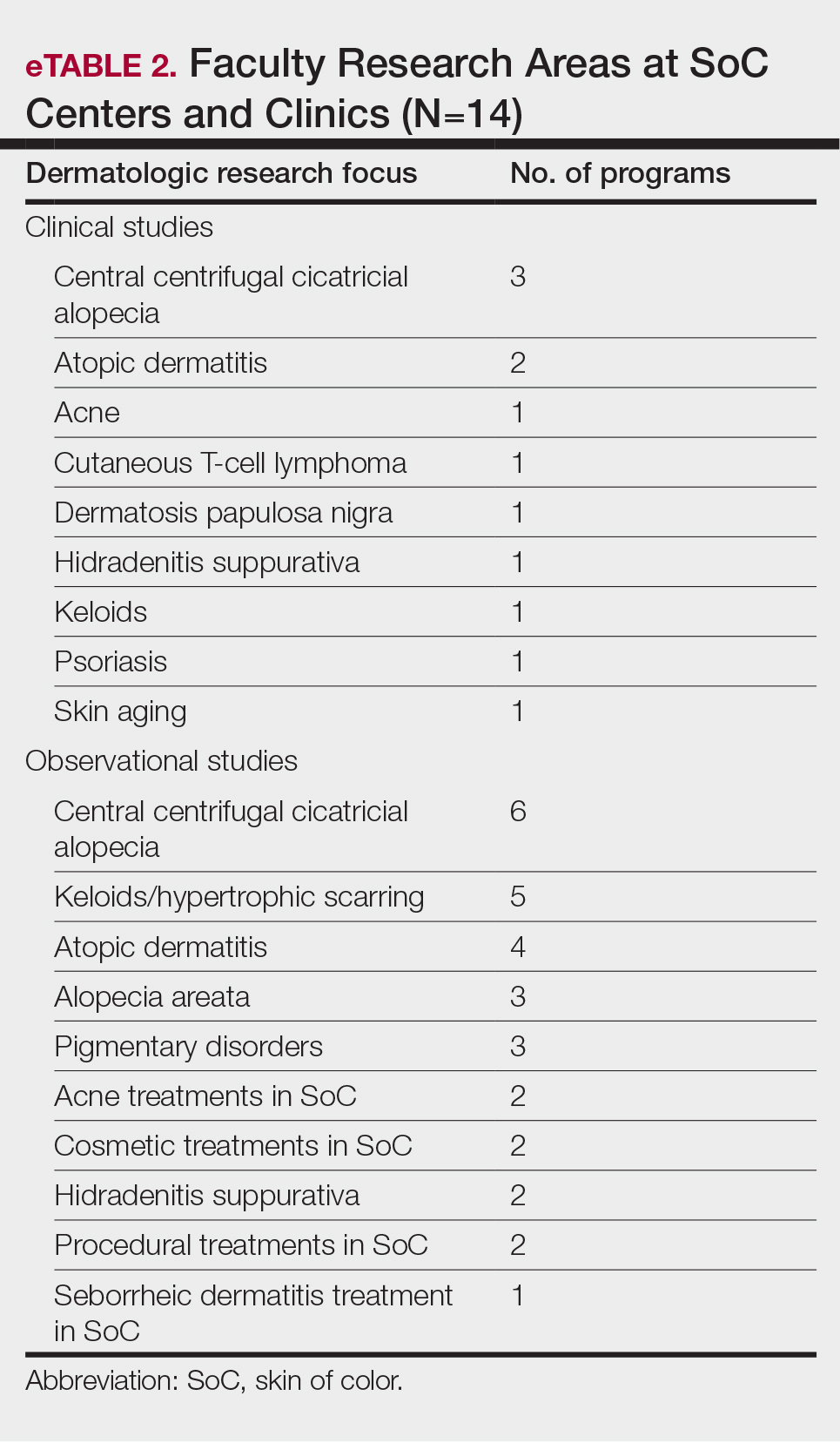
Upon presentation to our clinic, the patient reported continuing intermittent flares of CRPS; however, she said she was most concerned about diffuse hyperpigmentation, which spread to include the face, arms, abdomen, legs (Figure), and buttocks and persisted after skin tightening resolved.
To treat the hyperpigmentation, a decision was made to first focus on a localized area. Facial hyperpigmentation was chosen because it was of greatest concern to the patient. She was instructed to use azelaic acid gel 15% in the morning, tretinoin cream 0.05% at night, and sunscreen daily. The patient had mild improvement in hyperpigmentation after a 4-month period but has been inconsistent in follow-up. She continues to have intermittent flares of CRPS, which may interfere with her response to treatment. In addition to the aforementioned regimen of azelaic acid gel and tretinoin, she has continued to work with a pain specialist to better control the neurologic symptoms and pain associated with her CRPS.
Complex regional pain syndrome, a neurological condition characterized by chronic pain, affects women 3 times more often than men. The syndrome is more common in the fourth and fifth decades of life.1,2
There are 2 subtypes of CRPS. Type I (also known as reflex sympathetic dystrophy) is more common and occurs following minor trauma without peripheral nerve injury. Type II (otherwise known as causalgia) occurs following more notable trauma with injury to a peripheral nerve.1,6 Onset of symptoms most often is secondary to minor peripheral trauma. More common triggers include soft-tissue injury (40%); fractures and subsequent orthopedic surgery (25%); and visceral lesions, such as myocardial infarction and cerebral vascular accident (12%).5 Regardless of the inciting event, prolonged immobilization of a limb has been identified as an important predisposing factor. One study found that 47% of patients who received a diagnosis of CRPS previously underwent immobilization of the same limb.7
The pathogenesis of CRPS has not been fully elucidated. Possible explanations include central nervous system sensitization to thermal, mechanical, and pain stimuli; sympathetic dysfunction leading to vasomotor, pseudomotor, and trophic changes; and inflammatory cytokine release and microcirculatory dysfunction, causing tissue injury.1,2,6
The diagnosis of CRPS is a based on clinical findings. Using the Budapest Criteria established to define CRPS, a clinical diagnosis can be made when all of the following criteria are met: chronic continuing pain disproportionate to any inciting event; 1 or more reported symptoms from 3 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic; 1 or more sign at the time of evaluation in 2 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic.8 Dermatologic findings are a common presenting feature of CRPS and are included in the Budapest Criteria used for diagnosis. In a retrospective chart review (N=26), researchers found that vascular findings were the most common dermatologic manifestation of CRPS—edema in 58% of patients and erythema in 54%.9 Other common manifestations included dermatitis (35%), erythematous papules (23%), and cutaneous atrophy (23%). Hyperpigmentation, which was present in our patient, was seen in 8% of patients in the chart review.9
Complex regional pain syndrome progresses through 3 stages; dermatologic changes are present in each stage and are more severe in later stages. Stage I lasts 2 or 3 months and is characterized by onset of pain, usually burning type, accompanied by allodynia and hyperalgesia. Early vasomotor and pseudomotor changes, such as erythema and edema, may become apparent.1,2 Stage II lasts 3 to 6 months and is characterized by more severe edema and more obvious trophic changes. Functional limitations, such as limited range of motion and muscle weakness, begin to manifest. Stage III—the final and most severe stage—is characterized by obvious hair, skin, and nail changes, as well as functional limitations.1,2 The waxy thickened skin changes and hyperpigmentation observed in our patient are characteristic of stage III. Furthermore, our patient experienced decreased mobility and limited range of motion secondary to tightening of the skin, a characteristic motor change of late-stage CRPS. Although chronic pain and allodynia are the most common characteristics of CRPS, skin changes also can cause notable distress and early dermatologic manifestations can be a chief concern.
Dermatologic management is focused to address the specific skin changes of CRPS. However, traditional treatment of the common dermatologic findings of CRPS is difficult and often unsuccessful; instead, the most successful treatment of skin findings involves controlling the underlying CRPS.9 Current treatment options include removal of any nidus of tissue trauma, sympathetic neural blockade with a local anesthetic, spinal cord stimulation to interrupt dysregulated sympathetic innervation, and physiotherapy or occupational therapy to desensitize skin.1,10
Given the complexity of CRPS and the variability of its presentation, management of the syndrome and its associated dermatologic conditions often requires interdisciplinary care and coordination of multiple specialties. Dermatologists can play an important role in both identification of CRPS and co-management of affected patients. Early diagnosis of CRPS has been universally identified as a key prognostic factor. For that reason, dermatologists should be aware of CRPS and include the syndrome in the differential diagnosis when presented with severe cutaneous findings following trauma either with or without peripheral nerve damage, suggestive of CRPS.
- Sebastin SJ. Complex regional pain syndrome. Indian J Plast Surg. 2011;44:298-307. doi:10.4103/0970-0358.85351
- Kabani R, Brassard A. Dermatological findings in early detection of complex regional pain syndrome. JAMA Dermatol. 2014;150:640-642. doi:10.1001/jamadermatol.2013.7459
- Moseley L. What is complex regional pain syndrome – in plain English. International Association for the Study of Pain website. Published 2009. Accessed December 15, 2022. https://www.iasp-pain.org/publications/relief-news/article/what-is-complex-pain-syndrome-in-plain-english/
- Pak TJ, Martin GM, Magness JL, et al. Reflex sympathetic dystrophy. Review of 140 cases. Minn Med. 1970;53:507-512.
- Qureshi AA, Friedman AJ. Complex regional pain syndrome: what the dermatologist should know. J Drugs Dermatol. 2018;17:532-536.
- Gorodkin R. Complex regional pain syndrome. Rheumatology. 2016;55(suppl 1):i12.
- Araki E, Tanioka M, Miyachi Y, et al. A case of complex regional pain syndrome: an underdiagnosed condition in dermatology. Acta Derm Venereol. 2007;87:440-441. doi:10.2340/00015555-0281
- Pergolizzi JV, LeQuang JA, Nalamachu S, et al. The Budapest criteria for complex regional pain syndrome: the diagnostic challenge. Anaesthesiol Clin Sci Res. 2018;2:1-10. doi:10.35841/anesthesiology.2.1.1-10
- Sundaram S, Webster GF. Vascular diseases are the most common cutaneous manifestations of reflex sympathetic dystrophy. J Am Acad Dermatol. 2001;44:1050-1051. doi:10.1067/mjd.2001.114299
- Taylor RS, Van Buyten J-P, Buchser E. Spinal stimulation for complex regional pain syndrome: a systematic review of the clinical and cost-effectiveness literature and assessment of prognostic factors. Eur J Pain. 2006;10:91-101. doi:10.1016/j.ejpain.2005.02.004
To the Editor:
Complex regional pain syndrome (CRPS) is a neurologic condition characterized by chronic pain and sensory changes, including allodynia and hyperalgesia, that usually affect the extremities.1,2 The syndrome is defined by the International Association for the Study of Pain (IASP) as a condition that appears regionally after an injury, with a variety of symptoms that often exceed the expected clinical course both in magnitude and duration, causing impairment of motor function and variable progression.3
Although CRPS most often is described following minor peripheral trauma, other precipitating causes include surgery and vascular events.4 Additional features of the condition include autonomic dysfunction, edema, and trophic changes.1 Symptoms of CRPS traditionally present in 3 stages, with notable skin changes most often documented in stages II and III.2
Skin changes are a known manifestation of the syndrome, but reports in the dermatologic literature are scarce. Qureshi and Friedman5 identified only 23 articles in the dermatology literature since 1990 in which skin changes in CRPS were described. We present a patient with a diagnosis of CRPS who developed hyperpigmentation and sclerotic changes, including skin thickening, induration, and skin tightening.
A middle-aged Black woman presented to dermatology for evaluation of progressive hyperpigmentation, hyperhidrosis, and sclerotic changes to the skin. Approximately 3 years prior, the patient was given a diagnosis of CRPS of the hands and feet. Pain symptoms started approximately 3 years prior to the onset of symptoms. Symptoms started in the left hand and eventually spread to the right arm, left leg, and subsequently to the right leg. The first dermatologic change the patient noticed was tightening of the skin in the affected area that led to decreased mobility, which improved over time—partly on its own and partly with physical therapy.
A biopsy performed by an outside dermatologist at the initial presentation demonstrated sclerodermalike changes, which were treated with creams but without improvement. Scleroderma was later ruled out by the same dermatologist. Skin tightening improved over time, with complete resolution approximately 1 year after the onset of symptoms.
Upon presentation to our clinic, the patient reported continuing intermittent flares of CRPS; however, she said she was most concerned about diffuse hyperpigmentation, which spread to include the face, arms, abdomen, legs (Figure), and buttocks and persisted after skin tightening resolved.

To treat the hyperpigmentation, a decision was made to first focus on a localized area. Facial hyperpigmentation was chosen because it was of greatest concern to the patient. She was instructed to use azelaic acid gel 15% in the morning, tretinoin cream 0.05% at night, and sunscreen daily. The patient had mild improvement in hyperpigmentation after a 4-month period but has been inconsistent in follow-up. She continues to have intermittent flares of CRPS, which may interfere with her response to treatment. In addition to the aforementioned regimen of azelaic acid gel and tretinoin, she has continued to work with a pain specialist to better control the neurologic symptoms and pain associated with her CRPS.
Complex regional pain syndrome, a neurological condition characterized by chronic pain, affects women 3 times more often than men. The syndrome is more common in the fourth and fifth decades of life.1,2
There are 2 subtypes of CRPS. Type I (also known as reflex sympathetic dystrophy) is more common and occurs following minor trauma without peripheral nerve injury. Type II (otherwise known as causalgia) occurs following more notable trauma with injury to a peripheral nerve.1,6 Onset of symptoms most often is secondary to minor peripheral trauma. More common triggers include soft-tissue injury (40%); fractures and subsequent orthopedic surgery (25%); and visceral lesions, such as myocardial infarction and cerebral vascular accident (12%).5 Regardless of the inciting event, prolonged immobilization of a limb has been identified as an important predisposing factor. One study found that 47% of patients who received a diagnosis of CRPS previously underwent immobilization of the same limb.7
The pathogenesis of CRPS has not been fully elucidated. Possible explanations include central nervous system sensitization to thermal, mechanical, and pain stimuli; sympathetic dysfunction leading to vasomotor, pseudomotor, and trophic changes; and inflammatory cytokine release and microcirculatory dysfunction, causing tissue injury.1,2,6
The diagnosis of CRPS is a based on clinical findings. Using the Budapest Criteria established to define CRPS, a clinical diagnosis can be made when all of the following criteria are met: chronic continuing pain disproportionate to any inciting event; 1 or more reported symptoms from 3 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic; 1 or more sign at the time of evaluation in 2 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic.8 Dermatologic findings are a common presenting feature of CRPS and are included in the Budapest Criteria used for diagnosis. In a retrospective chart review (N=26), researchers found that vascular findings were the most common dermatologic manifestation of CRPS—edema in 58% of patients and erythema in 54%.9 Other common manifestations included dermatitis (35%), erythematous papules (23%), and cutaneous atrophy (23%). Hyperpigmentation, which was present in our patient, was seen in 8% of patients in the chart review.9
Complex regional pain syndrome progresses through 3 stages; dermatologic changes are present in each stage and are more severe in later stages. Stage I lasts 2 or 3 months and is characterized by onset of pain, usually burning type, accompanied by allodynia and hyperalgesia. Early vasomotor and pseudomotor changes, such as erythema and edema, may become apparent.1,2 Stage II lasts 3 to 6 months and is characterized by more severe edema and more obvious trophic changes. Functional limitations, such as limited range of motion and muscle weakness, begin to manifest. Stage III—the final and most severe stage—is characterized by obvious hair, skin, and nail changes, as well as functional limitations.1,2 The waxy thickened skin changes and hyperpigmentation observed in our patient are characteristic of stage III. Furthermore, our patient experienced decreased mobility and limited range of motion secondary to tightening of the skin, a characteristic motor change of late-stage CRPS. Although chronic pain and allodynia are the most common characteristics of CRPS, skin changes also can cause notable distress and early dermatologic manifestations can be a chief concern.
Dermatologic management is focused to address the specific skin changes of CRPS. However, traditional treatment of the common dermatologic findings of CRPS is difficult and often unsuccessful; instead, the most successful treatment of skin findings involves controlling the underlying CRPS.9 Current treatment options include removal of any nidus of tissue trauma, sympathetic neural blockade with a local anesthetic, spinal cord stimulation to interrupt dysregulated sympathetic innervation, and physiotherapy or occupational therapy to desensitize skin.1,10
Given the complexity of CRPS and the variability of its presentation, management of the syndrome and its associated dermatologic conditions often requires interdisciplinary care and coordination of multiple specialties. Dermatologists can play an important role in both identification of CRPS and co-management of affected patients. Early diagnosis of CRPS has been universally identified as a key prognostic factor. For that reason, dermatologists should be aware of CRPS and include the syndrome in the differential diagnosis when presented with severe cutaneous findings following trauma either with or without peripheral nerve damage, suggestive of CRPS.
To the Editor:
Complex regional pain syndrome (CRPS) is a neurologic condition characterized by chronic pain and sensory changes, including allodynia and hyperalgesia, that usually affect the extremities.1,2 The syndrome is defined by the International Association for the Study of Pain (IASP) as a condition that appears regionally after an injury, with a variety of symptoms that often exceed the expected clinical course both in magnitude and duration, causing impairment of motor function and variable progression.3
Although CRPS most often is described following minor peripheral trauma, other precipitating causes include surgery and vascular events.4 Additional features of the condition include autonomic dysfunction, edema, and trophic changes.1 Symptoms of CRPS traditionally present in 3 stages, with notable skin changes most often documented in stages II and III.2
Skin changes are a known manifestation of the syndrome, but reports in the dermatologic literature are scarce. Qureshi and Friedman5 identified only 23 articles in the dermatology literature since 1990 in which skin changes in CRPS were described. We present a patient with a diagnosis of CRPS who developed hyperpigmentation and sclerotic changes, including skin thickening, induration, and skin tightening.
A middle-aged Black woman presented to dermatology for evaluation of progressive hyperpigmentation, hyperhidrosis, and sclerotic changes to the skin. Approximately 3 years prior, the patient was given a diagnosis of CRPS of the hands and feet. Pain symptoms started approximately 3 years prior to the onset of symptoms. Symptoms started in the left hand and eventually spread to the right arm, left leg, and subsequently to the right leg. The first dermatologic change the patient noticed was tightening of the skin in the affected area that led to decreased mobility, which improved over time—partly on its own and partly with physical therapy.
A biopsy performed by an outside dermatologist at the initial presentation demonstrated sclerodermalike changes, which were treated with creams but without improvement. Scleroderma was later ruled out by the same dermatologist. Skin tightening improved over time, with complete resolution approximately 1 year after the onset of symptoms.
Upon presentation to our clinic, the patient reported continuing intermittent flares of CRPS; however, she said she was most concerned about diffuse hyperpigmentation, which spread to include the face, arms, abdomen, legs (Figure), and buttocks and persisted after skin tightening resolved.

To treat the hyperpigmentation, a decision was made to first focus on a localized area. Facial hyperpigmentation was chosen because it was of greatest concern to the patient. She was instructed to use azelaic acid gel 15% in the morning, tretinoin cream 0.05% at night, and sunscreen daily. The patient had mild improvement in hyperpigmentation after a 4-month period but has been inconsistent in follow-up. She continues to have intermittent flares of CRPS, which may interfere with her response to treatment. In addition to the aforementioned regimen of azelaic acid gel and tretinoin, she has continued to work with a pain specialist to better control the neurologic symptoms and pain associated with her CRPS.
Complex regional pain syndrome, a neurological condition characterized by chronic pain, affects women 3 times more often than men. The syndrome is more common in the fourth and fifth decades of life.1,2
There are 2 subtypes of CRPS. Type I (also known as reflex sympathetic dystrophy) is more common and occurs following minor trauma without peripheral nerve injury. Type II (otherwise known as causalgia) occurs following more notable trauma with injury to a peripheral nerve.1,6 Onset of symptoms most often is secondary to minor peripheral trauma. More common triggers include soft-tissue injury (40%); fractures and subsequent orthopedic surgery (25%); and visceral lesions, such as myocardial infarction and cerebral vascular accident (12%).5 Regardless of the inciting event, prolonged immobilization of a limb has been identified as an important predisposing factor. One study found that 47% of patients who received a diagnosis of CRPS previously underwent immobilization of the same limb.7
The pathogenesis of CRPS has not been fully elucidated. Possible explanations include central nervous system sensitization to thermal, mechanical, and pain stimuli; sympathetic dysfunction leading to vasomotor, pseudomotor, and trophic changes; and inflammatory cytokine release and microcirculatory dysfunction, causing tissue injury.1,2,6
The diagnosis of CRPS is a based on clinical findings. Using the Budapest Criteria established to define CRPS, a clinical diagnosis can be made when all of the following criteria are met: chronic continuing pain disproportionate to any inciting event; 1 or more reported symptoms from 3 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic; 1 or more sign at the time of evaluation in 2 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic.8 Dermatologic findings are a common presenting feature of CRPS and are included in the Budapest Criteria used for diagnosis. In a retrospective chart review (N=26), researchers found that vascular findings were the most common dermatologic manifestation of CRPS—edema in 58% of patients and erythema in 54%.9 Other common manifestations included dermatitis (35%), erythematous papules (23%), and cutaneous atrophy (23%). Hyperpigmentation, which was present in our patient, was seen in 8% of patients in the chart review.9
Complex regional pain syndrome progresses through 3 stages; dermatologic changes are present in each stage and are more severe in later stages. Stage I lasts 2 or 3 months and is characterized by onset of pain, usually burning type, accompanied by allodynia and hyperalgesia. Early vasomotor and pseudomotor changes, such as erythema and edema, may become apparent.1,2 Stage II lasts 3 to 6 months and is characterized by more severe edema and more obvious trophic changes. Functional limitations, such as limited range of motion and muscle weakness, begin to manifest. Stage III—the final and most severe stage—is characterized by obvious hair, skin, and nail changes, as well as functional limitations.1,2 The waxy thickened skin changes and hyperpigmentation observed in our patient are characteristic of stage III. Furthermore, our patient experienced decreased mobility and limited range of motion secondary to tightening of the skin, a characteristic motor change of late-stage CRPS. Although chronic pain and allodynia are the most common characteristics of CRPS, skin changes also can cause notable distress and early dermatologic manifestations can be a chief concern.
Dermatologic management is focused to address the specific skin changes of CRPS. However, traditional treatment of the common dermatologic findings of CRPS is difficult and often unsuccessful; instead, the most successful treatment of skin findings involves controlling the underlying CRPS.9 Current treatment options include removal of any nidus of tissue trauma, sympathetic neural blockade with a local anesthetic, spinal cord stimulation to interrupt dysregulated sympathetic innervation, and physiotherapy or occupational therapy to desensitize skin.1,10
Given the complexity of CRPS and the variability of its presentation, management of the syndrome and its associated dermatologic conditions often requires interdisciplinary care and coordination of multiple specialties. Dermatologists can play an important role in both identification of CRPS and co-management of affected patients. Early diagnosis of CRPS has been universally identified as a key prognostic factor. For that reason, dermatologists should be aware of CRPS and include the syndrome in the differential diagnosis when presented with severe cutaneous findings following trauma either with or without peripheral nerve damage, suggestive of CRPS.
- Sebastin SJ. Complex regional pain syndrome. Indian J Plast Surg. 2011;44:298-307. doi:10.4103/0970-0358.85351
- Kabani R, Brassard A. Dermatological findings in early detection of complex regional pain syndrome. JAMA Dermatol. 2014;150:640-642. doi:10.1001/jamadermatol.2013.7459
- Moseley L. What is complex regional pain syndrome – in plain English. International Association for the Study of Pain website. Published 2009. Accessed December 15, 2022. https://www.iasp-pain.org/publications/relief-news/article/what-is-complex-pain-syndrome-in-plain-english/
- Pak TJ, Martin GM, Magness JL, et al. Reflex sympathetic dystrophy. Review of 140 cases. Minn Med. 1970;53:507-512.
- Qureshi AA, Friedman AJ. Complex regional pain syndrome: what the dermatologist should know. J Drugs Dermatol. 2018;17:532-536.
- Gorodkin R. Complex regional pain syndrome. Rheumatology. 2016;55(suppl 1):i12.
- Araki E, Tanioka M, Miyachi Y, et al. A case of complex regional pain syndrome: an underdiagnosed condition in dermatology. Acta Derm Venereol. 2007;87:440-441. doi:10.2340/00015555-0281
- Pergolizzi JV, LeQuang JA, Nalamachu S, et al. The Budapest criteria for complex regional pain syndrome: the diagnostic challenge. Anaesthesiol Clin Sci Res. 2018;2:1-10. doi:10.35841/anesthesiology.2.1.1-10
- Sundaram S, Webster GF. Vascular diseases are the most common cutaneous manifestations of reflex sympathetic dystrophy. J Am Acad Dermatol. 2001;44:1050-1051. doi:10.1067/mjd.2001.114299
- Taylor RS, Van Buyten J-P, Buchser E. Spinal stimulation for complex regional pain syndrome: a systematic review of the clinical and cost-effectiveness literature and assessment of prognostic factors. Eur J Pain. 2006;10:91-101. doi:10.1016/j.ejpain.2005.02.004
- Sebastin SJ. Complex regional pain syndrome. Indian J Plast Surg. 2011;44:298-307. doi:10.4103/0970-0358.85351
- Kabani R, Brassard A. Dermatological findings in early detection of complex regional pain syndrome. JAMA Dermatol. 2014;150:640-642. doi:10.1001/jamadermatol.2013.7459
- Moseley L. What is complex regional pain syndrome – in plain English. International Association for the Study of Pain website. Published 2009. Accessed December 15, 2022. https://www.iasp-pain.org/publications/relief-news/article/what-is-complex-pain-syndrome-in-plain-english/
- Pak TJ, Martin GM, Magness JL, et al. Reflex sympathetic dystrophy. Review of 140 cases. Minn Med. 1970;53:507-512.
- Qureshi AA, Friedman AJ. Complex regional pain syndrome: what the dermatologist should know. J Drugs Dermatol. 2018;17:532-536.
- Gorodkin R. Complex regional pain syndrome. Rheumatology. 2016;55(suppl 1):i12.
- Araki E, Tanioka M, Miyachi Y, et al. A case of complex regional pain syndrome: an underdiagnosed condition in dermatology. Acta Derm Venereol. 2007;87:440-441. doi:10.2340/00015555-0281
- Pergolizzi JV, LeQuang JA, Nalamachu S, et al. The Budapest criteria for complex regional pain syndrome: the diagnostic challenge. Anaesthesiol Clin Sci Res. 2018;2:1-10. doi:10.35841/anesthesiology.2.1.1-10
- Sundaram S, Webster GF. Vascular diseases are the most common cutaneous manifestations of reflex sympathetic dystrophy. J Am Acad Dermatol. 2001;44:1050-1051. doi:10.1067/mjd.2001.114299
- Taylor RS, Van Buyten J-P, Buchser E. Spinal stimulation for complex regional pain syndrome: a systematic review of the clinical and cost-effectiveness literature and assessment of prognostic factors. Eur J Pain. 2006;10:91-101. doi:10.1016/j.ejpain.2005.02.004
PRACTICE POINTS
- Common dermatologic manifestations of complex regional pain syndrome (CRPS), which often are nonspecific and often the presenting symptoms of the syndrome, include allodynia, edema, erythema, hypopigmentation or hyperpigmentation, and petechiae.
- Diagnosis and management of CRPS are the most important steps in treating dermatologic manifestations of the syndrome.
Going Beyond Hydroquinone: Alternative Skin Lightening Agents
Disorders of hyperpigmentation—melasma, postinflammatory hyperpigmentation, lichen planus pigmentosus, erythema dyschromicum perstans, and pigmented contact dermatitis, among others—are common and challenging to treat. Although they can affect individuals of all skin types, they most commonly are seen in skin of color; in fact, dyspigmentation is one of the most common chief concerns for which individuals of color see a dermatologist.1,2
For many years, hydroquinone (HQ) was one of the main options available for use as a lightening agent. Although effective, it has the risk of causing irritant dermatitis, potentially leading to further dyspigmentation, in addition to the risk of ochronosis with long-term use. It remains an important and useful treatment for pigmentary disorders, but there are numerous other lightening agents that also can be considered in the treatment of disorders of hyperpigmentation.
Herein, we provide recommendations for traditional and newer non-HQ lightening agents that can be considered when treating disorders of hyperpigmentation.
Traditional Non-HQ Lightening Agents
Retinoids—Retinoids are topical vitamin A derivatives that have been used safely and effectively for decades in the treatment of pigmentary disorders. Retinoids have multiple mechanisms of action in improving pigmentation. In addition to impeding tyrosinase induction, they inhibit pigment transfer to keratinocytes and lead to accelerated pigment loss due to epidermal shedding.3 Over-the-counter formulations include retinol, retinaldehyde, and adapalene. Prescription formulations include tretinoin and tazarotene in different strengths and vehicle formulations.4
Glycolic Acid—Glycolic acid is derived from sugarcane and is considered an α-hydroxy acid that leads to rapid desquamation of pigmented keratinocytes.5 Glycolic acid can not only be used in chemical peels but also in topical creams. It is the most common α-hydroxy acid peel and is sometimes paired with HQ and other topical lightening agents for increased penetration. Glycolic acid peels are available in concentrations of 20% to 70% and can be used at various depths. When used incorrectly, it can cause redness, burning, and even skin discoloration; however, when used at the proper concentrations and depth according to Fitzpatrick skin type, there typically are no notable adverse effects, and clinical results are favorable.
Kojic Acid—Kojic acid is a natural metabolite derived from fungi and is widely used in Asian countries. It works by inhibiting the catecholase activity of tyrosinase6 and typically is available in concentrations of 1% to 4%. A study suggested that a concentration of 1% or less typically is safe to use for prolonged periods without adverse effects. Although not more effective than HQ as a monotherapy, kojic acid has been shown to haveimproved efficacy when used in combination with other lightening agents.7
Azelaic Acid—Azelaic acid works by inhibiting tyrosinase, mitochondrial oxidoreductase activation, and DNA synthesis. It preferentially targets heavily pigmented melanocytes and possesses anti-inflammatory and antibacterial properties.8 A 20% concentration of azelaic acid was compared to HQ 4% for the treatment of melasma, and results revealed that the liposomal form of azelaic acid was considerably more tolerable than HQ 4% and also more effective.9
Licorice Extracts—Licorice extracts have been safely used in several cosmeceutical skin lightening products.10 The main active compounds in licorice root are glabridin and liquiritin, which work to disperse melanin. These compounds often are used topically at concentrations of 10% to 40%. A study by Amer and Metwalli11 found that topical liquiritin produced a reduction of pigmentary intensity, with 80% of patients showing an excellent response, which was described as no difference between the previously pigmented area and the normal skin surrounding it.
Aloesin—Aloesin is a low-molecular-weight glycoprotein found in aloe vera plants. Its mechanism of action includes competitive inhibition of the dihydroxyphenylalanine oxidation site, resulting in the inhibition of tyrosinase.12 It often is combined with arbutin for an enhanced lightening effect.
Niacinamide—Niacinamide is a form of vitamin B3 that works by suppressing the transfer of melanosomes to keratinocytes.13 In addition to its skin lightening effects, it also is photoprotective and antimicrobial, and its tolerability and safety have led to its inclusion in many cosmeceutical and prescription products.14
Ascorbic Acid—Ascorbic acid affects the monopherase activity of tyrosinase, thus reducing the synthesis of melanin. It also serves as an antioxidant in the skin by preventing the production of free radicals that can induce melanogenesis.15 Although it tends to be well tolerated with a low adverse effect profile, its relative instability and varying permeability can present a challenge. It is less effective as a monotherapy, so it often is combined with other lightening ingredients for greater efficacy.
Corticosteroids—Topical corticosteroids are anti-inflammatory and impact melanogenesis, though the mechanism of action of the latter has not been fully elucidated.16,17 Low- to mid-potency topical steroids often are used in conjunction with skin lightening products to diminish irritation and decrease inflammation.18 However, prolonged use of corticosteroids can lead to cutaneous adverse effects such as striae, hypopigmentation, and acne, as well as systemic side effects if there is sufficient absorption over time.
Soybean Extracts—Soybean extracts contain serine protease inhibitors that reduce the transfer of melanosomes into keratinocytes by inhibiting the PAR-2 (protease-activated receptor 2) pathway.19,20
Ellagic Acid—Ellagic acid is found in common plants such as eucalyptus and strawberry as well as green tea.21 It works as an antioxidant and decreases melanogenesis through inhibition of tyrosinase activity.
Paper Mulberry—Paper mulberry extract comes from the roots of the Broussonetia papyrifera tree and functions by inhibiting tyrosinase activity. It is widely used in South Africa and Europe.22
Resveratrol—Resveratrol is an ingredient extracted from Morus alba L and functions as an antimelanogentic agent by directly inhibiting tyrosinase as well as transcriptional and posttranscriptional processing of tyrosinase.23 It also holds antiproliferative, anti-inflammatory, and antioxidant properties and has widely been used for antiaging and skin lightening purposes.24
Newer Non-HQ Lightening Agents
Silymarin—Silymarin (also known as milk thistle [Silybum marianum]), is a polyphenolic flavonoid that possesses anticarcinogenic, antioxidant, and anti-inflammatory properties. It prevents melanin production in a dose-dependent manner by inhibiting levodopa (L-dopa) oxidation activity of tyrosinase and also reduces the expression of tyrosinase protein.25 In combination with vitamins C and E and hexylresorcinol, silymarin has been found to reduce the effects of photodamage, brighten skin, improve evenness and lines, as well as improve global facial appearance.26
Malassezin—Malassezin is an indole produced by Malessezia furfur yeast and has recently been investigated for melanogenesis suppression. Grimes et al27 assessed the efficacy of topical malassezin in 7 patients with facial hyperpigmentation applied twice daily for 14 weeks. Punch biopsies were taken at weeks 0, 8, 14, and 22. Biopsies from weeks 8 and 14 demonstrated reduced epidermal melanin compared to baseline in all participants; however, at 22 weeks, biopsies showed no difference in melanin content compared to baseline, indicating a temporary process induced by the malassezin.27 More clinical studies are needed to investigate this further.
N-acetyl-glucosamine—N-acetyl-glucosamine is an aminosaccharide that inhibits the glycosylation of tyrosinase as well as its function in melanogenesis.28 It is synthesized and included in topical products for wound healing, rhytides, moisturization, and pigmentation disorders.
Topical Tranexamic Acid—Tranexamic acid traditionally has been used orally for the treatment of menorrhagia but also has been found to be beneficial as a therapy for hyperpigmentation and erythema. Tranexamic acid interferes with plasmin activity, thus indirectly inhibiting melanogenesis while also inhibiting angiogenesis by targeting vascular endothelial growth factor (VEGF) receptors.29 It also leads to an increase in the levels of β-endorphin and μ-opioid receptors as well as the expression of estrogen receptor β on the surface of mast cells.30 Its oral benefit led to the development of topical formulations, typically in 2% to 5% concentrations. It has proven particularly beneficial in the treatment of melasma due to its effects on improving pigmentation, erythema, and skin barrier function.31 Topical tranexamic acid has a relatively high safety profile, with minor side effects such as transient skin irritation and erythema being reported.32
Cysteamine—Cysteamine inhibits tyrosinase, peroxidase, and chelating copper ions necessary for melanogenesis. It has proven to be effective in treating melasma and chronic severe postinflammatory hyperpigmentation when used in a 5% cream formulation.33,34 Lima et al35 were the first to compare the effects of topical cysteamine to HQ in the treatment of facial melasma. They found that the mean reduction in modified Melasma Area and Severity Index score was 24% for cysteamine and 41% for HQ after 60 days. There were no severe adverse effects with either treatment group.35
Final Thoughts
Hydroquinone remains the gold standard for treatment of hyperpigmentation; however, its side-effect profile and risk of ochronosis with long-term use has ushered in various other safe and effective skin lightening agents that can be used as monotherapies or in combination with other lightening agents. Many of these products also can be used effectively with procedural treatments such as chemical peels, lasers, and microneedling for enhanced absorption and efficacy. As newer agents are developed, additional well-designed studies will be needed to determine their safety and efficacy in different skin types as well as their role in the treatment of pigmentary disorders.
- Woolery-Lloyd H, Kammer JN. Treatment of hyperpigmentation. Semin Cutan Med Surg. 2011;30:171-175. doi:10.1016/j.sder.2011.06.004
- Desai SR. Hyperpigmentation therapy: a review. J Clin Aesthet Dermatol. 2014;7:13-17.
- Kligman AM, Willis I. A new formula for depigmenting human skin. Arch Dermatol. 1975;111:40-48.
- Kligman AM, Grove GL, Hirose R, et al. Topical tretinoin for photoaged skin. J Am Acad Dermatol. 1986;15(4 pt 2):836-859. doi:10.1016/s0190-9622(86)70242-9
- Sharad J. Glycolic acid peel therapy—a current review. Clin Cosmet Investig Dermatol. 2013;6:281-288. doi:10.2147/CCID.S34029
- Nautiyal A, Wairkar S. Management of hyperpigmentation: current treatments and emerging therapies. Pigment Cell Melanoma Res. 2021;34:1000-1014. doi:10.1111/pcmr.12986
- Saeedi M, Eslamifar M, Khezri K. Kojic acid applications in cosmetic and pharmaceutical preparations. Biomed Pharmacother. 2019;110:582-593. doi:10.1016/j.biopha.2018.12.006
- Schulte BC, Wu W, Rosen T. Azelaic acid: evidence-based update on mechanism of action and clinical application. J Drugs Dermatol. 2015;14:964-968.
- Akl EM. Liposomal azelaic acid 20% cream vs hydroquinone 4% cream as adjuvant to oral tranexamic acid in melasma: a comparative study [published online April 7, 2021]. J Dermatol Treat. doi:10.1080/09546634.2021.1905765
- Holloway VL. Ethnic cosmetic products. Dermatol Clin. 2003;21:743-749. doi:10.1016/s0733-8635(03)00089-5
- Amer M, Metwalli M. Topical liquiritin improves melasma. Int J Dermatol. 2000;39:299-301. doi:10.1046/j.1365-4362.2000.00943.x
- Jones K, Hughes J, Hong M, et al. Modulation of melanogenesis by aloesin: a competitive inhibitor of tyrosinase. Pigment Cell Res. 2002;15:335-340. doi:10.1034/j.1600-0749.2002.02014.x
- Hakozaki T, Minwalla L, Zhuang J, et al. The effect of niacinamide on reducing cutaneous pigmentation and suppression of melanosome transfer. Br J Dermatol. 2002;147:20-31. doi:10.1046/j.1365-2133.2002.04834.x
- Wohlrab J, Kreft D. Niacinamide—mechanisms of action and its topical use in dermatology. Skin Pharmacol Physiol. 2014;27:311-315. doi:10.1159/000359974
- Fitzpatrick RE, Rostan EF. Double-blind, half-face study comparing topical vitamin C and vehicle for rejuvenation of photodamage. Dermatol Surg. 2002;28:231-236. doi:10.1046/j.1524-4725.2002.01129.x
- Mehta AB, Nadkarni NJ, Patil SP, et al. Topical corticosteroids in dermatology. Indian J Dermatol Venereol Leprol. 2016;82:371-378. doi:10.4103/0378-6323.178903
- Petit L, Piérard GE. Skin-lightening products revisited. Int J Cosmet Sci. 2003;25:169-181. doi:10.1046/j.1467-2494.2003.00182.x
- Kanwar AJ, Dhar S, Kaur S. Treatment of melasma with potent topical corticosteroids. Dermatol Basel Switz. 1994;188:170. doi:10.1159/000247129
- Paine C, Sharlow E, Liebel F, et al. An alternative approach to depigmentation by soybean extracts via inhibition of the PAR-2 pathway. J Invest Dermatol. 2001;116:587-595. doi:10.1046/j.1523-1747.2001.01291.x
- Seiberg M, Paine C, Sharlow E, et al. Inhibition of melanosome transfer results in skin lightening. J Invest Dermatol. 2000;115:162-167. doi:10.1046/j.1523-1747.2000.00035.x
- Shimogaki H, Tanaka Y, Tamai H, et al. In vitro and in vivo evaluation of ellagic acid on melanogenesis inhibition. Int J Cosmet Sci. 2000;22:291-303. doi:10.1046/j.1467-2494.2000.00023.x
- Rendon MI, Gaviria JI. Review of skin-lightening agents. Dermatol Surg. 2005;31(7 pt 2):886-889; discussion 889. doi:10.1111/j.1524-4725.2005.31736
- Na JI, Shin JW, Choi HR, et al. Resveratrol as a multifunctional topical hypopigmenting agent [published online February 22, 2019]. Int J Mol Sci. 2019;20:956. doi:10.3390/ijms20040956
- Ratz-Łyko A, Arct J. Resveratrol as an active ingredient for cosmetic and dermatological applications: a review. J Cosmet Laser Ther. 2019;21:84-90. doi:10.1080/14764172.2018.1469767
- Choo SJ, Ryoo IJ, Kim YH, et al. Silymarin inhibits melanin synthesis in melanocyte cells. J Pharm Pharmacol. 2009;61:663-667. doi:10.1211/jpp/61.05.0016
- Draelos ZD, Diaz I, Cohen A, et al. A novel skin brightening topical technology. J Cosmet Dermatol. 2020;19:3280-3285. doi:10.1111/jocd.13741
- Grimes P, Bhawan J, Howell M, et al. Histopathological changes induced by malassezin: a novel natural microbiome indole for treatment of facial hyperpigmentation. J Drugs Dermatol. 2022;21:141-145. doi:10.36849/jdd.6596
- Bissett DL. Glucosamine: an ingredient with skin and other benefits. J Cosmet Dermatol. 2006;5:309-315. doi:10.1111/j.1473-2165.2006.00277.x
- Zhu JW, Ni YJ, Tong XY, et al. Tranexamic acid inhibits angiogenesis and melanogenesis in vitro by targeting VEGF receptors. Int J Med Sci. 2020;17:903-911. doi:10.7150/ijms.44188
- Hiramoto K, Yamate Y, Sugiyama D, et al. Tranexamic acid inhibits the plasma and non-irradiated skin markers of photoaging induced by long-term UVA eye irradiation in female mice. Biomed Pharmacother. 2018;107:54-58. doi:10.1016/j.biopha.2018.07.146
- da Silva Souza ID, Lampe L, Winn D. New topical tranexamic acid derivative for the improvement of hyperpigmentation and inflammation in the sun-damaged skin. J Cosmet Dermatol. 2021;20:561-565. doi:10.1111/jocd.13545
- Kim HJ, Moon SH, Cho SH, et al. Efficacy and safety of tranexamic acid in melasma: a meta-analysis and systematic review. Acta Derm Venereol. 2017;97:776-781. doi:10.2340/00015555-2668
- Mathe N, Balogun M, Yoo J. A case report on the use of topical cysteamine 5% cream in the management of refractory postinflammatory hyperpigmentation (PIH) resistant to triple combination cream (hydroquinone, topical corticosteroids, and retinoids). J Cosmet Dermatol. 2021;20:204-206. doi:10.1111/jocd.13755
- Mansouri P, Farshi S, Hashemi Z, et al. Evaluation of the efficacy of cysteamine 5% cream in the treatment of epidermal melasma: a randomized double-blind placebo-controlled trial. Br J Dermatol. 2015;173:209-217. doi:10.1111/bjd.13424
- Lima PB, Dias JAF, Cassiano D, et al. A comparative study of topical 5% cysteamine versus 4% hydroquinone in the treatment of facial melasma in women. Int J Dermatol. 2020;59:1531-1536. doi:10.1111/ijd.15146
Disorders of hyperpigmentation—melasma, postinflammatory hyperpigmentation, lichen planus pigmentosus, erythema dyschromicum perstans, and pigmented contact dermatitis, among others—are common and challenging to treat. Although they can affect individuals of all skin types, they most commonly are seen in skin of color; in fact, dyspigmentation is one of the most common chief concerns for which individuals of color see a dermatologist.1,2
For many years, hydroquinone (HQ) was one of the main options available for use as a lightening agent. Although effective, it has the risk of causing irritant dermatitis, potentially leading to further dyspigmentation, in addition to the risk of ochronosis with long-term use. It remains an important and useful treatment for pigmentary disorders, but there are numerous other lightening agents that also can be considered in the treatment of disorders of hyperpigmentation.
Herein, we provide recommendations for traditional and newer non-HQ lightening agents that can be considered when treating disorders of hyperpigmentation.
Traditional Non-HQ Lightening Agents
Retinoids—Retinoids are topical vitamin A derivatives that have been used safely and effectively for decades in the treatment of pigmentary disorders. Retinoids have multiple mechanisms of action in improving pigmentation. In addition to impeding tyrosinase induction, they inhibit pigment transfer to keratinocytes and lead to accelerated pigment loss due to epidermal shedding.3 Over-the-counter formulations include retinol, retinaldehyde, and adapalene. Prescription formulations include tretinoin and tazarotene in different strengths and vehicle formulations.4
Glycolic Acid—Glycolic acid is derived from sugarcane and is considered an α-hydroxy acid that leads to rapid desquamation of pigmented keratinocytes.5 Glycolic acid can not only be used in chemical peels but also in topical creams. It is the most common α-hydroxy acid peel and is sometimes paired with HQ and other topical lightening agents for increased penetration. Glycolic acid peels are available in concentrations of 20% to 70% and can be used at various depths. When used incorrectly, it can cause redness, burning, and even skin discoloration; however, when used at the proper concentrations and depth according to Fitzpatrick skin type, there typically are no notable adverse effects, and clinical results are favorable.
Kojic Acid—Kojic acid is a natural metabolite derived from fungi and is widely used in Asian countries. It works by inhibiting the catecholase activity of tyrosinase6 and typically is available in concentrations of 1% to 4%. A study suggested that a concentration of 1% or less typically is safe to use for prolonged periods without adverse effects. Although not more effective than HQ as a monotherapy, kojic acid has been shown to haveimproved efficacy when used in combination with other lightening agents.7
Azelaic Acid—Azelaic acid works by inhibiting tyrosinase, mitochondrial oxidoreductase activation, and DNA synthesis. It preferentially targets heavily pigmented melanocytes and possesses anti-inflammatory and antibacterial properties.8 A 20% concentration of azelaic acid was compared to HQ 4% for the treatment of melasma, and results revealed that the liposomal form of azelaic acid was considerably more tolerable than HQ 4% and also more effective.9
Licorice Extracts—Licorice extracts have been safely used in several cosmeceutical skin lightening products.10 The main active compounds in licorice root are glabridin and liquiritin, which work to disperse melanin. These compounds often are used topically at concentrations of 10% to 40%. A study by Amer and Metwalli11 found that topical liquiritin produced a reduction of pigmentary intensity, with 80% of patients showing an excellent response, which was described as no difference between the previously pigmented area and the normal skin surrounding it.
Aloesin—Aloesin is a low-molecular-weight glycoprotein found in aloe vera plants. Its mechanism of action includes competitive inhibition of the dihydroxyphenylalanine oxidation site, resulting in the inhibition of tyrosinase.12 It often is combined with arbutin for an enhanced lightening effect.
Niacinamide—Niacinamide is a form of vitamin B3 that works by suppressing the transfer of melanosomes to keratinocytes.13 In addition to its skin lightening effects, it also is photoprotective and antimicrobial, and its tolerability and safety have led to its inclusion in many cosmeceutical and prescription products.14
Ascorbic Acid—Ascorbic acid affects the monopherase activity of tyrosinase, thus reducing the synthesis of melanin. It also serves as an antioxidant in the skin by preventing the production of free radicals that can induce melanogenesis.15 Although it tends to be well tolerated with a low adverse effect profile, its relative instability and varying permeability can present a challenge. It is less effective as a monotherapy, so it often is combined with other lightening ingredients for greater efficacy.
Corticosteroids—Topical corticosteroids are anti-inflammatory and impact melanogenesis, though the mechanism of action of the latter has not been fully elucidated.16,17 Low- to mid-potency topical steroids often are used in conjunction with skin lightening products to diminish irritation and decrease inflammation.18 However, prolonged use of corticosteroids can lead to cutaneous adverse effects such as striae, hypopigmentation, and acne, as well as systemic side effects if there is sufficient absorption over time.
Soybean Extracts—Soybean extracts contain serine protease inhibitors that reduce the transfer of melanosomes into keratinocytes by inhibiting the PAR-2 (protease-activated receptor 2) pathway.19,20
Ellagic Acid—Ellagic acid is found in common plants such as eucalyptus and strawberry as well as green tea.21 It works as an antioxidant and decreases melanogenesis through inhibition of tyrosinase activity.
Paper Mulberry—Paper mulberry extract comes from the roots of the Broussonetia papyrifera tree and functions by inhibiting tyrosinase activity. It is widely used in South Africa and Europe.22
Resveratrol—Resveratrol is an ingredient extracted from Morus alba L and functions as an antimelanogentic agent by directly inhibiting tyrosinase as well as transcriptional and posttranscriptional processing of tyrosinase.23 It also holds antiproliferative, anti-inflammatory, and antioxidant properties and has widely been used for antiaging and skin lightening purposes.24
Newer Non-HQ Lightening Agents
Silymarin—Silymarin (also known as milk thistle [Silybum marianum]), is a polyphenolic flavonoid that possesses anticarcinogenic, antioxidant, and anti-inflammatory properties. It prevents melanin production in a dose-dependent manner by inhibiting levodopa (L-dopa) oxidation activity of tyrosinase and also reduces the expression of tyrosinase protein.25 In combination with vitamins C and E and hexylresorcinol, silymarin has been found to reduce the effects of photodamage, brighten skin, improve evenness and lines, as well as improve global facial appearance.26
Malassezin—Malassezin is an indole produced by Malessezia furfur yeast and has recently been investigated for melanogenesis suppression. Grimes et al27 assessed the efficacy of topical malassezin in 7 patients with facial hyperpigmentation applied twice daily for 14 weeks. Punch biopsies were taken at weeks 0, 8, 14, and 22. Biopsies from weeks 8 and 14 demonstrated reduced epidermal melanin compared to baseline in all participants; however, at 22 weeks, biopsies showed no difference in melanin content compared to baseline, indicating a temporary process induced by the malassezin.27 More clinical studies are needed to investigate this further.
N-acetyl-glucosamine—N-acetyl-glucosamine is an aminosaccharide that inhibits the glycosylation of tyrosinase as well as its function in melanogenesis.28 It is synthesized and included in topical products for wound healing, rhytides, moisturization, and pigmentation disorders.
Topical Tranexamic Acid—Tranexamic acid traditionally has been used orally for the treatment of menorrhagia but also has been found to be beneficial as a therapy for hyperpigmentation and erythema. Tranexamic acid interferes with plasmin activity, thus indirectly inhibiting melanogenesis while also inhibiting angiogenesis by targeting vascular endothelial growth factor (VEGF) receptors.29 It also leads to an increase in the levels of β-endorphin and μ-opioid receptors as well as the expression of estrogen receptor β on the surface of mast cells.30 Its oral benefit led to the development of topical formulations, typically in 2% to 5% concentrations. It has proven particularly beneficial in the treatment of melasma due to its effects on improving pigmentation, erythema, and skin barrier function.31 Topical tranexamic acid has a relatively high safety profile, with minor side effects such as transient skin irritation and erythema being reported.32
Cysteamine—Cysteamine inhibits tyrosinase, peroxidase, and chelating copper ions necessary for melanogenesis. It has proven to be effective in treating melasma and chronic severe postinflammatory hyperpigmentation when used in a 5% cream formulation.33,34 Lima et al35 were the first to compare the effects of topical cysteamine to HQ in the treatment of facial melasma. They found that the mean reduction in modified Melasma Area and Severity Index score was 24% for cysteamine and 41% for HQ after 60 days. There were no severe adverse effects with either treatment group.35
Final Thoughts
Hydroquinone remains the gold standard for treatment of hyperpigmentation; however, its side-effect profile and risk of ochronosis with long-term use has ushered in various other safe and effective skin lightening agents that can be used as monotherapies or in combination with other lightening agents. Many of these products also can be used effectively with procedural treatments such as chemical peels, lasers, and microneedling for enhanced absorption and efficacy. As newer agents are developed, additional well-designed studies will be needed to determine their safety and efficacy in different skin types as well as their role in the treatment of pigmentary disorders.
Disorders of hyperpigmentation—melasma, postinflammatory hyperpigmentation, lichen planus pigmentosus, erythema dyschromicum perstans, and pigmented contact dermatitis, among others—are common and challenging to treat. Although they can affect individuals of all skin types, they most commonly are seen in skin of color; in fact, dyspigmentation is one of the most common chief concerns for which individuals of color see a dermatologist.1,2
For many years, hydroquinone (HQ) was one of the main options available for use as a lightening agent. Although effective, it has the risk of causing irritant dermatitis, potentially leading to further dyspigmentation, in addition to the risk of ochronosis with long-term use. It remains an important and useful treatment for pigmentary disorders, but there are numerous other lightening agents that also can be considered in the treatment of disorders of hyperpigmentation.
Herein, we provide recommendations for traditional and newer non-HQ lightening agents that can be considered when treating disorders of hyperpigmentation.
Traditional Non-HQ Lightening Agents
Retinoids—Retinoids are topical vitamin A derivatives that have been used safely and effectively for decades in the treatment of pigmentary disorders. Retinoids have multiple mechanisms of action in improving pigmentation. In addition to impeding tyrosinase induction, they inhibit pigment transfer to keratinocytes and lead to accelerated pigment loss due to epidermal shedding.3 Over-the-counter formulations include retinol, retinaldehyde, and adapalene. Prescription formulations include tretinoin and tazarotene in different strengths and vehicle formulations.4
Glycolic Acid—Glycolic acid is derived from sugarcane and is considered an α-hydroxy acid that leads to rapid desquamation of pigmented keratinocytes.5 Glycolic acid can not only be used in chemical peels but also in topical creams. It is the most common α-hydroxy acid peel and is sometimes paired with HQ and other topical lightening agents for increased penetration. Glycolic acid peels are available in concentrations of 20% to 70% and can be used at various depths. When used incorrectly, it can cause redness, burning, and even skin discoloration; however, when used at the proper concentrations and depth according to Fitzpatrick skin type, there typically are no notable adverse effects, and clinical results are favorable.
Kojic Acid—Kojic acid is a natural metabolite derived from fungi and is widely used in Asian countries. It works by inhibiting the catecholase activity of tyrosinase6 and typically is available in concentrations of 1% to 4%. A study suggested that a concentration of 1% or less typically is safe to use for prolonged periods without adverse effects. Although not more effective than HQ as a monotherapy, kojic acid has been shown to haveimproved efficacy when used in combination with other lightening agents.7
Azelaic Acid—Azelaic acid works by inhibiting tyrosinase, mitochondrial oxidoreductase activation, and DNA synthesis. It preferentially targets heavily pigmented melanocytes and possesses anti-inflammatory and antibacterial properties.8 A 20% concentration of azelaic acid was compared to HQ 4% for the treatment of melasma, and results revealed that the liposomal form of azelaic acid was considerably more tolerable than HQ 4% and also more effective.9
Licorice Extracts—Licorice extracts have been safely used in several cosmeceutical skin lightening products.10 The main active compounds in licorice root are glabridin and liquiritin, which work to disperse melanin. These compounds often are used topically at concentrations of 10% to 40%. A study by Amer and Metwalli11 found that topical liquiritin produced a reduction of pigmentary intensity, with 80% of patients showing an excellent response, which was described as no difference between the previously pigmented area and the normal skin surrounding it.
Aloesin—Aloesin is a low-molecular-weight glycoprotein found in aloe vera plants. Its mechanism of action includes competitive inhibition of the dihydroxyphenylalanine oxidation site, resulting in the inhibition of tyrosinase.12 It often is combined with arbutin for an enhanced lightening effect.
Niacinamide—Niacinamide is a form of vitamin B3 that works by suppressing the transfer of melanosomes to keratinocytes.13 In addition to its skin lightening effects, it also is photoprotective and antimicrobial, and its tolerability and safety have led to its inclusion in many cosmeceutical and prescription products.14
Ascorbic Acid—Ascorbic acid affects the monopherase activity of tyrosinase, thus reducing the synthesis of melanin. It also serves as an antioxidant in the skin by preventing the production of free radicals that can induce melanogenesis.15 Although it tends to be well tolerated with a low adverse effect profile, its relative instability and varying permeability can present a challenge. It is less effective as a monotherapy, so it often is combined with other lightening ingredients for greater efficacy.
Corticosteroids—Topical corticosteroids are anti-inflammatory and impact melanogenesis, though the mechanism of action of the latter has not been fully elucidated.16,17 Low- to mid-potency topical steroids often are used in conjunction with skin lightening products to diminish irritation and decrease inflammation.18 However, prolonged use of corticosteroids can lead to cutaneous adverse effects such as striae, hypopigmentation, and acne, as well as systemic side effects if there is sufficient absorption over time.
Soybean Extracts—Soybean extracts contain serine protease inhibitors that reduce the transfer of melanosomes into keratinocytes by inhibiting the PAR-2 (protease-activated receptor 2) pathway.19,20
Ellagic Acid—Ellagic acid is found in common plants such as eucalyptus and strawberry as well as green tea.21 It works as an antioxidant and decreases melanogenesis through inhibition of tyrosinase activity.
Paper Mulberry—Paper mulberry extract comes from the roots of the Broussonetia papyrifera tree and functions by inhibiting tyrosinase activity. It is widely used in South Africa and Europe.22
Resveratrol—Resveratrol is an ingredient extracted from Morus alba L and functions as an antimelanogentic agent by directly inhibiting tyrosinase as well as transcriptional and posttranscriptional processing of tyrosinase.23 It also holds antiproliferative, anti-inflammatory, and antioxidant properties and has widely been used for antiaging and skin lightening purposes.24
Newer Non-HQ Lightening Agents
Silymarin—Silymarin (also known as milk thistle [Silybum marianum]), is a polyphenolic flavonoid that possesses anticarcinogenic, antioxidant, and anti-inflammatory properties. It prevents melanin production in a dose-dependent manner by inhibiting levodopa (L-dopa) oxidation activity of tyrosinase and also reduces the expression of tyrosinase protein.25 In combination with vitamins C and E and hexylresorcinol, silymarin has been found to reduce the effects of photodamage, brighten skin, improve evenness and lines, as well as improve global facial appearance.26
Malassezin—Malassezin is an indole produced by Malessezia furfur yeast and has recently been investigated for melanogenesis suppression. Grimes et al27 assessed the efficacy of topical malassezin in 7 patients with facial hyperpigmentation applied twice daily for 14 weeks. Punch biopsies were taken at weeks 0, 8, 14, and 22. Biopsies from weeks 8 and 14 demonstrated reduced epidermal melanin compared to baseline in all participants; however, at 22 weeks, biopsies showed no difference in melanin content compared to baseline, indicating a temporary process induced by the malassezin.27 More clinical studies are needed to investigate this further.
N-acetyl-glucosamine—N-acetyl-glucosamine is an aminosaccharide that inhibits the glycosylation of tyrosinase as well as its function in melanogenesis.28 It is synthesized and included in topical products for wound healing, rhytides, moisturization, and pigmentation disorders.
Topical Tranexamic Acid—Tranexamic acid traditionally has been used orally for the treatment of menorrhagia but also has been found to be beneficial as a therapy for hyperpigmentation and erythema. Tranexamic acid interferes with plasmin activity, thus indirectly inhibiting melanogenesis while also inhibiting angiogenesis by targeting vascular endothelial growth factor (VEGF) receptors.29 It also leads to an increase in the levels of β-endorphin and μ-opioid receptors as well as the expression of estrogen receptor β on the surface of mast cells.30 Its oral benefit led to the development of topical formulations, typically in 2% to 5% concentrations. It has proven particularly beneficial in the treatment of melasma due to its effects on improving pigmentation, erythema, and skin barrier function.31 Topical tranexamic acid has a relatively high safety profile, with minor side effects such as transient skin irritation and erythema being reported.32
Cysteamine—Cysteamine inhibits tyrosinase, peroxidase, and chelating copper ions necessary for melanogenesis. It has proven to be effective in treating melasma and chronic severe postinflammatory hyperpigmentation when used in a 5% cream formulation.33,34 Lima et al35 were the first to compare the effects of topical cysteamine to HQ in the treatment of facial melasma. They found that the mean reduction in modified Melasma Area and Severity Index score was 24% for cysteamine and 41% for HQ after 60 days. There were no severe adverse effects with either treatment group.35
Final Thoughts
Hydroquinone remains the gold standard for treatment of hyperpigmentation; however, its side-effect profile and risk of ochronosis with long-term use has ushered in various other safe and effective skin lightening agents that can be used as monotherapies or in combination with other lightening agents. Many of these products also can be used effectively with procedural treatments such as chemical peels, lasers, and microneedling for enhanced absorption and efficacy. As newer agents are developed, additional well-designed studies will be needed to determine their safety and efficacy in different skin types as well as their role in the treatment of pigmentary disorders.
- Woolery-Lloyd H, Kammer JN. Treatment of hyperpigmentation. Semin Cutan Med Surg. 2011;30:171-175. doi:10.1016/j.sder.2011.06.004
- Desai SR. Hyperpigmentation therapy: a review. J Clin Aesthet Dermatol. 2014;7:13-17.
- Kligman AM, Willis I. A new formula for depigmenting human skin. Arch Dermatol. 1975;111:40-48.
- Kligman AM, Grove GL, Hirose R, et al. Topical tretinoin for photoaged skin. J Am Acad Dermatol. 1986;15(4 pt 2):836-859. doi:10.1016/s0190-9622(86)70242-9
- Sharad J. Glycolic acid peel therapy—a current review. Clin Cosmet Investig Dermatol. 2013;6:281-288. doi:10.2147/CCID.S34029
- Nautiyal A, Wairkar S. Management of hyperpigmentation: current treatments and emerging therapies. Pigment Cell Melanoma Res. 2021;34:1000-1014. doi:10.1111/pcmr.12986
- Saeedi M, Eslamifar M, Khezri K. Kojic acid applications in cosmetic and pharmaceutical preparations. Biomed Pharmacother. 2019;110:582-593. doi:10.1016/j.biopha.2018.12.006
- Schulte BC, Wu W, Rosen T. Azelaic acid: evidence-based update on mechanism of action and clinical application. J Drugs Dermatol. 2015;14:964-968.
- Akl EM. Liposomal azelaic acid 20% cream vs hydroquinone 4% cream as adjuvant to oral tranexamic acid in melasma: a comparative study [published online April 7, 2021]. J Dermatol Treat. doi:10.1080/09546634.2021.1905765
- Holloway VL. Ethnic cosmetic products. Dermatol Clin. 2003;21:743-749. doi:10.1016/s0733-8635(03)00089-5
- Amer M, Metwalli M. Topical liquiritin improves melasma. Int J Dermatol. 2000;39:299-301. doi:10.1046/j.1365-4362.2000.00943.x
- Jones K, Hughes J, Hong M, et al. Modulation of melanogenesis by aloesin: a competitive inhibitor of tyrosinase. Pigment Cell Res. 2002;15:335-340. doi:10.1034/j.1600-0749.2002.02014.x
- Hakozaki T, Minwalla L, Zhuang J, et al. The effect of niacinamide on reducing cutaneous pigmentation and suppression of melanosome transfer. Br J Dermatol. 2002;147:20-31. doi:10.1046/j.1365-2133.2002.04834.x
- Wohlrab J, Kreft D. Niacinamide—mechanisms of action and its topical use in dermatology. Skin Pharmacol Physiol. 2014;27:311-315. doi:10.1159/000359974
- Fitzpatrick RE, Rostan EF. Double-blind, half-face study comparing topical vitamin C and vehicle for rejuvenation of photodamage. Dermatol Surg. 2002;28:231-236. doi:10.1046/j.1524-4725.2002.01129.x
- Mehta AB, Nadkarni NJ, Patil SP, et al. Topical corticosteroids in dermatology. Indian J Dermatol Venereol Leprol. 2016;82:371-378. doi:10.4103/0378-6323.178903
- Petit L, Piérard GE. Skin-lightening products revisited. Int J Cosmet Sci. 2003;25:169-181. doi:10.1046/j.1467-2494.2003.00182.x
- Kanwar AJ, Dhar S, Kaur S. Treatment of melasma with potent topical corticosteroids. Dermatol Basel Switz. 1994;188:170. doi:10.1159/000247129
- Paine C, Sharlow E, Liebel F, et al. An alternative approach to depigmentation by soybean extracts via inhibition of the PAR-2 pathway. J Invest Dermatol. 2001;116:587-595. doi:10.1046/j.1523-1747.2001.01291.x
- Seiberg M, Paine C, Sharlow E, et al. Inhibition of melanosome transfer results in skin lightening. J Invest Dermatol. 2000;115:162-167. doi:10.1046/j.1523-1747.2000.00035.x
- Shimogaki H, Tanaka Y, Tamai H, et al. In vitro and in vivo evaluation of ellagic acid on melanogenesis inhibition. Int J Cosmet Sci. 2000;22:291-303. doi:10.1046/j.1467-2494.2000.00023.x
- Rendon MI, Gaviria JI. Review of skin-lightening agents. Dermatol Surg. 2005;31(7 pt 2):886-889; discussion 889. doi:10.1111/j.1524-4725.2005.31736
- Na JI, Shin JW, Choi HR, et al. Resveratrol as a multifunctional topical hypopigmenting agent [published online February 22, 2019]. Int J Mol Sci. 2019;20:956. doi:10.3390/ijms20040956
- Ratz-Łyko A, Arct J. Resveratrol as an active ingredient for cosmetic and dermatological applications: a review. J Cosmet Laser Ther. 2019;21:84-90. doi:10.1080/14764172.2018.1469767
- Choo SJ, Ryoo IJ, Kim YH, et al. Silymarin inhibits melanin synthesis in melanocyte cells. J Pharm Pharmacol. 2009;61:663-667. doi:10.1211/jpp/61.05.0016
- Draelos ZD, Diaz I, Cohen A, et al. A novel skin brightening topical technology. J Cosmet Dermatol. 2020;19:3280-3285. doi:10.1111/jocd.13741
- Grimes P, Bhawan J, Howell M, et al. Histopathological changes induced by malassezin: a novel natural microbiome indole for treatment of facial hyperpigmentation. J Drugs Dermatol. 2022;21:141-145. doi:10.36849/jdd.6596
- Bissett DL. Glucosamine: an ingredient with skin and other benefits. J Cosmet Dermatol. 2006;5:309-315. doi:10.1111/j.1473-2165.2006.00277.x
- Zhu JW, Ni YJ, Tong XY, et al. Tranexamic acid inhibits angiogenesis and melanogenesis in vitro by targeting VEGF receptors. Int J Med Sci. 2020;17:903-911. doi:10.7150/ijms.44188
- Hiramoto K, Yamate Y, Sugiyama D, et al. Tranexamic acid inhibits the plasma and non-irradiated skin markers of photoaging induced by long-term UVA eye irradiation in female mice. Biomed Pharmacother. 2018;107:54-58. doi:10.1016/j.biopha.2018.07.146
- da Silva Souza ID, Lampe L, Winn D. New topical tranexamic acid derivative for the improvement of hyperpigmentation and inflammation in the sun-damaged skin. J Cosmet Dermatol. 2021;20:561-565. doi:10.1111/jocd.13545
- Kim HJ, Moon SH, Cho SH, et al. Efficacy and safety of tranexamic acid in melasma: a meta-analysis and systematic review. Acta Derm Venereol. 2017;97:776-781. doi:10.2340/00015555-2668
- Mathe N, Balogun M, Yoo J. A case report on the use of topical cysteamine 5% cream in the management of refractory postinflammatory hyperpigmentation (PIH) resistant to triple combination cream (hydroquinone, topical corticosteroids, and retinoids). J Cosmet Dermatol. 2021;20:204-206. doi:10.1111/jocd.13755
- Mansouri P, Farshi S, Hashemi Z, et al. Evaluation of the efficacy of cysteamine 5% cream in the treatment of epidermal melasma: a randomized double-blind placebo-controlled trial. Br J Dermatol. 2015;173:209-217. doi:10.1111/bjd.13424
- Lima PB, Dias JAF, Cassiano D, et al. A comparative study of topical 5% cysteamine versus 4% hydroquinone in the treatment of facial melasma in women. Int J Dermatol. 2020;59:1531-1536. doi:10.1111/ijd.15146
- Woolery-Lloyd H, Kammer JN. Treatment of hyperpigmentation. Semin Cutan Med Surg. 2011;30:171-175. doi:10.1016/j.sder.2011.06.004
- Desai SR. Hyperpigmentation therapy: a review. J Clin Aesthet Dermatol. 2014;7:13-17.
- Kligman AM, Willis I. A new formula for depigmenting human skin. Arch Dermatol. 1975;111:40-48.
- Kligman AM, Grove GL, Hirose R, et al. Topical tretinoin for photoaged skin. J Am Acad Dermatol. 1986;15(4 pt 2):836-859. doi:10.1016/s0190-9622(86)70242-9
- Sharad J. Glycolic acid peel therapy—a current review. Clin Cosmet Investig Dermatol. 2013;6:281-288. doi:10.2147/CCID.S34029
- Nautiyal A, Wairkar S. Management of hyperpigmentation: current treatments and emerging therapies. Pigment Cell Melanoma Res. 2021;34:1000-1014. doi:10.1111/pcmr.12986
- Saeedi M, Eslamifar M, Khezri K. Kojic acid applications in cosmetic and pharmaceutical preparations. Biomed Pharmacother. 2019;110:582-593. doi:10.1016/j.biopha.2018.12.006
- Schulte BC, Wu W, Rosen T. Azelaic acid: evidence-based update on mechanism of action and clinical application. J Drugs Dermatol. 2015;14:964-968.
- Akl EM. Liposomal azelaic acid 20% cream vs hydroquinone 4% cream as adjuvant to oral tranexamic acid in melasma: a comparative study [published online April 7, 2021]. J Dermatol Treat. doi:10.1080/09546634.2021.1905765
- Holloway VL. Ethnic cosmetic products. Dermatol Clin. 2003;21:743-749. doi:10.1016/s0733-8635(03)00089-5
- Amer M, Metwalli M. Topical liquiritin improves melasma. Int J Dermatol. 2000;39:299-301. doi:10.1046/j.1365-4362.2000.00943.x
- Jones K, Hughes J, Hong M, et al. Modulation of melanogenesis by aloesin: a competitive inhibitor of tyrosinase. Pigment Cell Res. 2002;15:335-340. doi:10.1034/j.1600-0749.2002.02014.x
- Hakozaki T, Minwalla L, Zhuang J, et al. The effect of niacinamide on reducing cutaneous pigmentation and suppression of melanosome transfer. Br J Dermatol. 2002;147:20-31. doi:10.1046/j.1365-2133.2002.04834.x
- Wohlrab J, Kreft D. Niacinamide—mechanisms of action and its topical use in dermatology. Skin Pharmacol Physiol. 2014;27:311-315. doi:10.1159/000359974
- Fitzpatrick RE, Rostan EF. Double-blind, half-face study comparing topical vitamin C and vehicle for rejuvenation of photodamage. Dermatol Surg. 2002;28:231-236. doi:10.1046/j.1524-4725.2002.01129.x
- Mehta AB, Nadkarni NJ, Patil SP, et al. Topical corticosteroids in dermatology. Indian J Dermatol Venereol Leprol. 2016;82:371-378. doi:10.4103/0378-6323.178903
- Petit L, Piérard GE. Skin-lightening products revisited. Int J Cosmet Sci. 2003;25:169-181. doi:10.1046/j.1467-2494.2003.00182.x
- Kanwar AJ, Dhar S, Kaur S. Treatment of melasma with potent topical corticosteroids. Dermatol Basel Switz. 1994;188:170. doi:10.1159/000247129
- Paine C, Sharlow E, Liebel F, et al. An alternative approach to depigmentation by soybean extracts via inhibition of the PAR-2 pathway. J Invest Dermatol. 2001;116:587-595. doi:10.1046/j.1523-1747.2001.01291.x
- Seiberg M, Paine C, Sharlow E, et al. Inhibition of melanosome transfer results in skin lightening. J Invest Dermatol. 2000;115:162-167. doi:10.1046/j.1523-1747.2000.00035.x
- Shimogaki H, Tanaka Y, Tamai H, et al. In vitro and in vivo evaluation of ellagic acid on melanogenesis inhibition. Int J Cosmet Sci. 2000;22:291-303. doi:10.1046/j.1467-2494.2000.00023.x
- Rendon MI, Gaviria JI. Review of skin-lightening agents. Dermatol Surg. 2005;31(7 pt 2):886-889; discussion 889. doi:10.1111/j.1524-4725.2005.31736
- Na JI, Shin JW, Choi HR, et al. Resveratrol as a multifunctional topical hypopigmenting agent [published online February 22, 2019]. Int J Mol Sci. 2019;20:956. doi:10.3390/ijms20040956
- Ratz-Łyko A, Arct J. Resveratrol as an active ingredient for cosmetic and dermatological applications: a review. J Cosmet Laser Ther. 2019;21:84-90. doi:10.1080/14764172.2018.1469767
- Choo SJ, Ryoo IJ, Kim YH, et al. Silymarin inhibits melanin synthesis in melanocyte cells. J Pharm Pharmacol. 2009;61:663-667. doi:10.1211/jpp/61.05.0016
- Draelos ZD, Diaz I, Cohen A, et al. A novel skin brightening topical technology. J Cosmet Dermatol. 2020;19:3280-3285. doi:10.1111/jocd.13741
- Grimes P, Bhawan J, Howell M, et al. Histopathological changes induced by malassezin: a novel natural microbiome indole for treatment of facial hyperpigmentation. J Drugs Dermatol. 2022;21:141-145. doi:10.36849/jdd.6596
- Bissett DL. Glucosamine: an ingredient with skin and other benefits. J Cosmet Dermatol. 2006;5:309-315. doi:10.1111/j.1473-2165.2006.00277.x
- Zhu JW, Ni YJ, Tong XY, et al. Tranexamic acid inhibits angiogenesis and melanogenesis in vitro by targeting VEGF receptors. Int J Med Sci. 2020;17:903-911. doi:10.7150/ijms.44188
- Hiramoto K, Yamate Y, Sugiyama D, et al. Tranexamic acid inhibits the plasma and non-irradiated skin markers of photoaging induced by long-term UVA eye irradiation in female mice. Biomed Pharmacother. 2018;107:54-58. doi:10.1016/j.biopha.2018.07.146
- da Silva Souza ID, Lampe L, Winn D. New topical tranexamic acid derivative for the improvement of hyperpigmentation and inflammation in the sun-damaged skin. J Cosmet Dermatol. 2021;20:561-565. doi:10.1111/jocd.13545
- Kim HJ, Moon SH, Cho SH, et al. Efficacy and safety of tranexamic acid in melasma: a meta-analysis and systematic review. Acta Derm Venereol. 2017;97:776-781. doi:10.2340/00015555-2668
- Mathe N, Balogun M, Yoo J. A case report on the use of topical cysteamine 5% cream in the management of refractory postinflammatory hyperpigmentation (PIH) resistant to triple combination cream (hydroquinone, topical corticosteroids, and retinoids). J Cosmet Dermatol. 2021;20:204-206. doi:10.1111/jocd.13755
- Mansouri P, Farshi S, Hashemi Z, et al. Evaluation of the efficacy of cysteamine 5% cream in the treatment of epidermal melasma: a randomized double-blind placebo-controlled trial. Br J Dermatol. 2015;173:209-217. doi:10.1111/bjd.13424
- Lima PB, Dias JAF, Cassiano D, et al. A comparative study of topical 5% cysteamine versus 4% hydroquinone in the treatment of facial melasma in women. Int J Dermatol. 2020;59:1531-1536. doi:10.1111/ijd.15146
Telemedicine Alopecia Assessment: Highlighting Patients With Skin of Color
Practice Gap
In accordance with World Health Organization guidelines on social distancing to limit transmission of SARS-CoV-2, dermatologists have relied on teledermatology (TD) to develop novel adaptations of traditional workflows, optimize patient care, and limit in-person appointments during the COVID-19 pandemic. Pandemic-induced physical and emotional stress were anticipated to increase the incidence of dermatologic diseases with psychologic triggers.
The connection between hair loss and emotional stress is well documented for telogen effluvium and alopecia areata.1,2 As anticipated, dermatology visits increased during the COVID-19 pandemic for the diagnosis of alopecia1-4; a survey performed during the pandemic found that alopecia was one of the most common diagnoses dermatologists made through telehealth platforms.5
This article provides a practical guide for dermatology practitioners to efficiently and accurately assess alopecia by TD in all patients, with added considerations for skin of color patients.
Diagnostic Tools
The intersection of TD, as an effective mechanism for the diagnosis and treatment of dermatologic disorders, and the increase in alopecia observed during the COVID-19 pandemic prompted us to develop a workflow for conducting virtual scalp examinations. Seven dermatologists (A.M., A.A., O.A., N.E., V.C., C.M.B., S.C.T.) who are experts in hair disorders contributed to developing workflows to optimize the assessment of alopecia through a virtual scalp examination, with an emphasis on patients of color. These experts completed a 7-question survey (Table) detailing their approach to the virtual scalp examination. One author (B.N.W.) served as an independent reviewer and collated responses into the following workflows.
Telemedicine Previsit Workflow
Components of the previsit workflow include:
• Instruct patients to provide all laboratory values and biopsy reports before the appointment.
• Test for a stable Wi-Fi connection using a speed test (available at https://www.speedtest.net/). A speed of 10 megabits/second or more is required for high-quality video via TD.6
• Provide a handout illustrating the required photographs of the anterior hairline; the mid scalp, including vertex, bilateral parietal, and occipital scalp; and posterior hairline. Photographs should be uploaded 2 hours before the visit. Figures 1 and 2 are examples of photographs that should be requested.
• Request images with 2 or 3 different angles of the area of the scalp with the greatest involvement to help appreciate primary and secondary characteristics.
• Encourage patients to present with clean, recently shampooed, dried, and detangled natural hair, unless they have an itchy or flaky scalp.
• For concerns of scalp, hairline, eyebrow, or facial flaking and scaling, instruct the patient to avoid applying a moisturizer before the visit.
• Instruct the patient to remove false eyelashes, eyelash extensions, eyebrow pencil, hair camouflage, hair accessories, braids, extensions, weaves, twists, and other hairstyles so that the hair can be maneuvered to expose the scalp surface.
• Instruct the patient to have a comb, pic, or brush, or more than one of these implements, available during the visit.
Telemedicine Visit Workflow
Components of the visit workflow include:
• If a stable Wi-Fi connection cannot be established, switch to an audio-only visit to collect a pertinent history. Advise the patient that in-person follow-up must be scheduled.
• Confirm that (1) the patient is in a private setting where the scalp can be viewed and (2) lighting is positioned in front of the patient.
• Ensure that the patient’s hairline, full face, eyebrows, and eyelashes and, upon request, the vertex and posterior scalp, are completely visible.
• Initiate the virtual scalp examination by instructing the patient how to perform a hair pull test. Then, examine the pattern and distribution of hair loss alongside supplemental photographs.
• Instruct the patient to apply pressure with the fingertips throughout the scalp to help localize tenderness, which, in combination with the pattern of hair loss observed, might inform the diagnosis.
• Instruct the patient to scan the scalp with the fingertips for “bumps” to locate papules, pustules, and keloidal scars.
Diagnostic Pearls
Distribution of Alopecia—The experts noted that the pattern, distribution, and location of hair loss determined from the telemedicine alopecia assessment provided important clues to distinguish the type of alopecia.
Diagnostic clues for diffuse or generalized alopecia include:
• Either of these findings might be indicative of telogen effluvium or acquired trichorrhexis nodosa. Results of the hair pull test can help distinguish between these diagnoses.
• Recent stressful life events along with the presence of telogen hairs extracted during a hair pull test support the diagnosis of telogen effluvium.
• A history of external stress on the hair—thermal, traction, or chemical—along with broken hair shafts following the hair pull test support the diagnosis of acquired trichorrhexis nodosa.
Diagnostic clues for focal or patchy alopecia include:
• Alopecia areata generally presents as focal hair loss in an annular distribution; pruritus, erythema, and scale are absent.
• Seborrheic dermatitis can present as pruritic erythematous patches with scale distributed on the scalp and, in some cases, in the eyebrows, nasolabial folds, or paranasal skin.7 Some skin of color patients present with petaloid seborrheic dermatitis—pink or hypopigmented polycyclic coalescing rings with minimal scale.7,8
• Discoid lupus erythematosus, similar to seborrheic dermatitis, might present as pruritic, scaly, hypopigmented patches. However, in the experience of the experts, a more common presentation is tender erythematous patches of hair loss with central hypopigmentation and surrounding hyperpigmentation.
Diagnostic clues for vertex and mid scalp alopecia include:
• Androgenetic alopecia typically presents as a reduction of terminal hair density in the vertex and mid scalp regions (with widening through the midline part) and fine hair along the anterior hairline.9 Signs of concomitant hyperandrogenism, including facial hirsutism, acne, and obesity, might be observed.10
• Central centrifugal cicatricial alopecia typically affects the vertex and mid scalp with a shiny scalp appearance and follicular dropout.
Diagnostic clues for frontotemporal alopecia include:
• Frontal fibrosing alopecia (FFA) often presents with spared single terminal hairs (lonely hair sign).
• Traction alopecia commonly presents with the fringe hair sign.
Scalp Symptoms—The experts noted that the presence of symptoms (eg, pain, tenderness, pruritus) in conjunction with the pattern of hair loss might support the diagnosis of an inflammatory scarring alopecia.
When do symptoms raise suspicion of central centrifugal cicatricial alopecia?
• Suspected in the setting of vertex alopecia associated with tenderness, pain, or itching.
When do symptoms raise suspicion of FFA?
• Suspected when patients experience frontotemporal tenderness, pain, or burning associated with alopecia.
• The skin hue of the affected area might be lighter in color than, and contrast with, the darker hue of the photoaged upper forehead.11
• The lonely hair sign can aid in diagnosing FFA and distinguish it from the fringe sign of traction alopecia.
• Concurrent madarosis, flesh-colored papules on the cheeks, or lichen planus pigmentosus identified by visual inspection of the face confirms the diagnosis.9,12 Madarosis of the eyebrow was frequently cited by the experts as an associated symptom of FFA.
When do symptoms raise suspicion of lichen planopilaris?
• Suspected in the presence of pruritus, burning, tenderness, or pain associated with perifollicular erythema and scale in the setting of vertex and parietal alopecia.13
• Anagen hair release is observed during the hair pull test.11,14• The experts cited flesh-colored papules and lichen planus pigmentosus as frequently associated symptoms of lichen planopilaris.
Practice Implications
There are limitations to a virtual scalp examination—the inability to perform a scalp biopsy or administer certain treatments—but the consensus of the expert panel is that an initial alopecia assessment can be completed successfully utilizing TD. Although TD is not a replacement for an in-person dermatology visit, this technology has allowed for the diagnosis, treatment, and continuing care of many common dermatologic conditions without the patient needing to travel to the office.5
With the increased frequency of hair loss concerns documented over the last year and more patients seeking TD, it is imperative that dermatologists feel confident performing a virtual hair and scalp examination on all patients.1,3,4
- Kutlu Ö, Aktas¸ H, I·mren IG, et al. Short-term stress-related increasing cases of alopecia areata during the COVID-19 pandemic. J Dermatolog Treat. 2020;1. doi:10.1080/09546634.2020.1782820
- Cline A, Kazemi A, Moy J, et al. A surge in the incidence of telogen effluvium in minority predominant communities heavily impacted by COVID-19. J Am Acad Dermatol. 2021;84:773-775. doi:10.1016/j.jaad.2020.11.032
- Kutlu Ö, Metin A. Relative changes in the pattern of diseases presenting in dermatology outpatient clinic in the era of the COVID-19 pandemic. Dermatol Ther. 2020;33:e14096. doi:10.1111/dth.14096
- Tanacan E, Aksoy Sarac G, Emeksiz MAC, et al. Changing trends in dermatology practice during COVID-19 pandemic: a single tertiary center experience. Dermatol Ther. 2020;33:e14136. doi:10.1111/dth.14136
- Sharma A, Jindal V, Singla P, et al. Will teledermatology be the silver lining during and after COVID-19? Dermatol Ther. 2020;33:e13643. doi:10.1111/dth.13643
- Iscrupe L. How to receive virtual medical treatment while under quarantine. Allconnect website. Published March 26, 2020. Accessed December 9, 2021. https://www.allconnect.com/blog/online-doctor-visit-faq
- Elgash M, Dlova N, Ogunleye T, et al. Seborrheic dermatitis in skin of color: clinical considerations. J Drugs Dermatol. 2019;18:24-27.
- McLaurin CI. Annular facial dermatoses in blacks. Cutis. 1983;32:369-370, 384.
- Suchonwanit P, Hector CE, Bin Saif GA, McMichael AJ. Factors affecting the severity of central centrifugal cicatricial alopecia. Int J Dermatol. 2016;55:e338-343. doi:10.1111/ijd.13061
- Gabros S, Masood S. Central centrifugal cicatricial alopecia. StatPearls [Internet]. StatPearls Publishing; 2021. Updated July 20, 2021. Accessed December 9, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559187/
- Ross EK, Tan E, Shapiro J. Update on primary cicatricial alopecias. J Am Acad Dermatol. 2005;53:1-37. doi:10.1016/j.jaad.2004.06.015
- Cobos G, Kim RH, Meehan S, et al. Lichen planus pigmentosus and lichen planopilaris. Dermatol Online J. 2016;22:13030/qt7hp8n6dn.
- Lyakhovitsky A, Amichai B, Sizopoulou C, et al. A case series of 46 patients with lichen planopilaris: demographics, clinical evaluation, and treatment experience. J Dermatolog Treat. 2015;26:275-279. doi:10.3109/09546634.2014.933165
- Tan E, Martinka M, Ball N, et al. Primary cicatricial alopecias: clinicopathology of 112 cases. J Am Acad Dermatol. 2004;50:25-32. doi:10.1016/j.jaad.2003.04.001
Practice Gap
In accordance with World Health Organization guidelines on social distancing to limit transmission of SARS-CoV-2, dermatologists have relied on teledermatology (TD) to develop novel adaptations of traditional workflows, optimize patient care, and limit in-person appointments during the COVID-19 pandemic. Pandemic-induced physical and emotional stress were anticipated to increase the incidence of dermatologic diseases with psychologic triggers.
The connection between hair loss and emotional stress is well documented for telogen effluvium and alopecia areata.1,2 As anticipated, dermatology visits increased during the COVID-19 pandemic for the diagnosis of alopecia1-4; a survey performed during the pandemic found that alopecia was one of the most common diagnoses dermatologists made through telehealth platforms.5
This article provides a practical guide for dermatology practitioners to efficiently and accurately assess alopecia by TD in all patients, with added considerations for skin of color patients.
Diagnostic Tools
The intersection of TD, as an effective mechanism for the diagnosis and treatment of dermatologic disorders, and the increase in alopecia observed during the COVID-19 pandemic prompted us to develop a workflow for conducting virtual scalp examinations. Seven dermatologists (A.M., A.A., O.A., N.E., V.C., C.M.B., S.C.T.) who are experts in hair disorders contributed to developing workflows to optimize the assessment of alopecia through a virtual scalp examination, with an emphasis on patients of color. These experts completed a 7-question survey (Table) detailing their approach to the virtual scalp examination. One author (B.N.W.) served as an independent reviewer and collated responses into the following workflows.
Telemedicine Previsit Workflow
Components of the previsit workflow include:
• Instruct patients to provide all laboratory values and biopsy reports before the appointment.
• Test for a stable Wi-Fi connection using a speed test (available at https://www.speedtest.net/). A speed of 10 megabits/second or more is required for high-quality video via TD.6
• Provide a handout illustrating the required photographs of the anterior hairline; the mid scalp, including vertex, bilateral parietal, and occipital scalp; and posterior hairline. Photographs should be uploaded 2 hours before the visit. Figures 1 and 2 are examples of photographs that should be requested.
• Request images with 2 or 3 different angles of the area of the scalp with the greatest involvement to help appreciate primary and secondary characteristics.
• Encourage patients to present with clean, recently shampooed, dried, and detangled natural hair, unless they have an itchy or flaky scalp.
• For concerns of scalp, hairline, eyebrow, or facial flaking and scaling, instruct the patient to avoid applying a moisturizer before the visit.
• Instruct the patient to remove false eyelashes, eyelash extensions, eyebrow pencil, hair camouflage, hair accessories, braids, extensions, weaves, twists, and other hairstyles so that the hair can be maneuvered to expose the scalp surface.
• Instruct the patient to have a comb, pic, or brush, or more than one of these implements, available during the visit.
Telemedicine Visit Workflow
Components of the visit workflow include:
• If a stable Wi-Fi connection cannot be established, switch to an audio-only visit to collect a pertinent history. Advise the patient that in-person follow-up must be scheduled.
• Confirm that (1) the patient is in a private setting where the scalp can be viewed and (2) lighting is positioned in front of the patient.
• Ensure that the patient’s hairline, full face, eyebrows, and eyelashes and, upon request, the vertex and posterior scalp, are completely visible.
• Initiate the virtual scalp examination by instructing the patient how to perform a hair pull test. Then, examine the pattern and distribution of hair loss alongside supplemental photographs.
• Instruct the patient to apply pressure with the fingertips throughout the scalp to help localize tenderness, which, in combination with the pattern of hair loss observed, might inform the diagnosis.
• Instruct the patient to scan the scalp with the fingertips for “bumps” to locate papules, pustules, and keloidal scars.
Diagnostic Pearls
Distribution of Alopecia—The experts noted that the pattern, distribution, and location of hair loss determined from the telemedicine alopecia assessment provided important clues to distinguish the type of alopecia.
Diagnostic clues for diffuse or generalized alopecia include:
• Either of these findings might be indicative of telogen effluvium or acquired trichorrhexis nodosa. Results of the hair pull test can help distinguish between these diagnoses.
• Recent stressful life events along with the presence of telogen hairs extracted during a hair pull test support the diagnosis of telogen effluvium.
• A history of external stress on the hair—thermal, traction, or chemical—along with broken hair shafts following the hair pull test support the diagnosis of acquired trichorrhexis nodosa.
Diagnostic clues for focal or patchy alopecia include:
• Alopecia areata generally presents as focal hair loss in an annular distribution; pruritus, erythema, and scale are absent.
• Seborrheic dermatitis can present as pruritic erythematous patches with scale distributed on the scalp and, in some cases, in the eyebrows, nasolabial folds, or paranasal skin.7 Some skin of color patients present with petaloid seborrheic dermatitis—pink or hypopigmented polycyclic coalescing rings with minimal scale.7,8
• Discoid lupus erythematosus, similar to seborrheic dermatitis, might present as pruritic, scaly, hypopigmented patches. However, in the experience of the experts, a more common presentation is tender erythematous patches of hair loss with central hypopigmentation and surrounding hyperpigmentation.
Diagnostic clues for vertex and mid scalp alopecia include:
• Androgenetic alopecia typically presents as a reduction of terminal hair density in the vertex and mid scalp regions (with widening through the midline part) and fine hair along the anterior hairline.9 Signs of concomitant hyperandrogenism, including facial hirsutism, acne, and obesity, might be observed.10
• Central centrifugal cicatricial alopecia typically affects the vertex and mid scalp with a shiny scalp appearance and follicular dropout.
Diagnostic clues for frontotemporal alopecia include:
• Frontal fibrosing alopecia (FFA) often presents with spared single terminal hairs (lonely hair sign).
• Traction alopecia commonly presents with the fringe hair sign.
Scalp Symptoms—The experts noted that the presence of symptoms (eg, pain, tenderness, pruritus) in conjunction with the pattern of hair loss might support the diagnosis of an inflammatory scarring alopecia.
When do symptoms raise suspicion of central centrifugal cicatricial alopecia?
• Suspected in the setting of vertex alopecia associated with tenderness, pain, or itching.
When do symptoms raise suspicion of FFA?
• Suspected when patients experience frontotemporal tenderness, pain, or burning associated with alopecia.
• The skin hue of the affected area might be lighter in color than, and contrast with, the darker hue of the photoaged upper forehead.11
• The lonely hair sign can aid in diagnosing FFA and distinguish it from the fringe sign of traction alopecia.
• Concurrent madarosis, flesh-colored papules on the cheeks, or lichen planus pigmentosus identified by visual inspection of the face confirms the diagnosis.9,12 Madarosis of the eyebrow was frequently cited by the experts as an associated symptom of FFA.
When do symptoms raise suspicion of lichen planopilaris?
• Suspected in the presence of pruritus, burning, tenderness, or pain associated with perifollicular erythema and scale in the setting of vertex and parietal alopecia.13
• Anagen hair release is observed during the hair pull test.11,14• The experts cited flesh-colored papules and lichen planus pigmentosus as frequently associated symptoms of lichen planopilaris.
Practice Implications
There are limitations to a virtual scalp examination—the inability to perform a scalp biopsy or administer certain treatments—but the consensus of the expert panel is that an initial alopecia assessment can be completed successfully utilizing TD. Although TD is not a replacement for an in-person dermatology visit, this technology has allowed for the diagnosis, treatment, and continuing care of many common dermatologic conditions without the patient needing to travel to the office.5
With the increased frequency of hair loss concerns documented over the last year and more patients seeking TD, it is imperative that dermatologists feel confident performing a virtual hair and scalp examination on all patients.1,3,4
Practice Gap
In accordance with World Health Organization guidelines on social distancing to limit transmission of SARS-CoV-2, dermatologists have relied on teledermatology (TD) to develop novel adaptations of traditional workflows, optimize patient care, and limit in-person appointments during the COVID-19 pandemic. Pandemic-induced physical and emotional stress were anticipated to increase the incidence of dermatologic diseases with psychologic triggers.
The connection between hair loss and emotional stress is well documented for telogen effluvium and alopecia areata.1,2 As anticipated, dermatology visits increased during the COVID-19 pandemic for the diagnosis of alopecia1-4; a survey performed during the pandemic found that alopecia was one of the most common diagnoses dermatologists made through telehealth platforms.5
This article provides a practical guide for dermatology practitioners to efficiently and accurately assess alopecia by TD in all patients, with added considerations for skin of color patients.
Diagnostic Tools
The intersection of TD, as an effective mechanism for the diagnosis and treatment of dermatologic disorders, and the increase in alopecia observed during the COVID-19 pandemic prompted us to develop a workflow for conducting virtual scalp examinations. Seven dermatologists (A.M., A.A., O.A., N.E., V.C., C.M.B., S.C.T.) who are experts in hair disorders contributed to developing workflows to optimize the assessment of alopecia through a virtual scalp examination, with an emphasis on patients of color. These experts completed a 7-question survey (Table) detailing their approach to the virtual scalp examination. One author (B.N.W.) served as an independent reviewer and collated responses into the following workflows.
Telemedicine Previsit Workflow
Components of the previsit workflow include:
• Instruct patients to provide all laboratory values and biopsy reports before the appointment.
• Test for a stable Wi-Fi connection using a speed test (available at https://www.speedtest.net/). A speed of 10 megabits/second or more is required for high-quality video via TD.6
• Provide a handout illustrating the required photographs of the anterior hairline; the mid scalp, including vertex, bilateral parietal, and occipital scalp; and posterior hairline. Photographs should be uploaded 2 hours before the visit. Figures 1 and 2 are examples of photographs that should be requested.
• Request images with 2 or 3 different angles of the area of the scalp with the greatest involvement to help appreciate primary and secondary characteristics.
• Encourage patients to present with clean, recently shampooed, dried, and detangled natural hair, unless they have an itchy or flaky scalp.
• For concerns of scalp, hairline, eyebrow, or facial flaking and scaling, instruct the patient to avoid applying a moisturizer before the visit.
• Instruct the patient to remove false eyelashes, eyelash extensions, eyebrow pencil, hair camouflage, hair accessories, braids, extensions, weaves, twists, and other hairstyles so that the hair can be maneuvered to expose the scalp surface.
• Instruct the patient to have a comb, pic, or brush, or more than one of these implements, available during the visit.
Telemedicine Visit Workflow
Components of the visit workflow include:
• If a stable Wi-Fi connection cannot be established, switch to an audio-only visit to collect a pertinent history. Advise the patient that in-person follow-up must be scheduled.
• Confirm that (1) the patient is in a private setting where the scalp can be viewed and (2) lighting is positioned in front of the patient.
• Ensure that the patient’s hairline, full face, eyebrows, and eyelashes and, upon request, the vertex and posterior scalp, are completely visible.
• Initiate the virtual scalp examination by instructing the patient how to perform a hair pull test. Then, examine the pattern and distribution of hair loss alongside supplemental photographs.
• Instruct the patient to apply pressure with the fingertips throughout the scalp to help localize tenderness, which, in combination with the pattern of hair loss observed, might inform the diagnosis.
• Instruct the patient to scan the scalp with the fingertips for “bumps” to locate papules, pustules, and keloidal scars.
Diagnostic Pearls
Distribution of Alopecia—The experts noted that the pattern, distribution, and location of hair loss determined from the telemedicine alopecia assessment provided important clues to distinguish the type of alopecia.
Diagnostic clues for diffuse or generalized alopecia include:
• Either of these findings might be indicative of telogen effluvium or acquired trichorrhexis nodosa. Results of the hair pull test can help distinguish between these diagnoses.
• Recent stressful life events along with the presence of telogen hairs extracted during a hair pull test support the diagnosis of telogen effluvium.
• A history of external stress on the hair—thermal, traction, or chemical—along with broken hair shafts following the hair pull test support the diagnosis of acquired trichorrhexis nodosa.
Diagnostic clues for focal or patchy alopecia include:
• Alopecia areata generally presents as focal hair loss in an annular distribution; pruritus, erythema, and scale are absent.
• Seborrheic dermatitis can present as pruritic erythematous patches with scale distributed on the scalp and, in some cases, in the eyebrows, nasolabial folds, or paranasal skin.7 Some skin of color patients present with petaloid seborrheic dermatitis—pink or hypopigmented polycyclic coalescing rings with minimal scale.7,8
• Discoid lupus erythematosus, similar to seborrheic dermatitis, might present as pruritic, scaly, hypopigmented patches. However, in the experience of the experts, a more common presentation is tender erythematous patches of hair loss with central hypopigmentation and surrounding hyperpigmentation.
Diagnostic clues for vertex and mid scalp alopecia include:
• Androgenetic alopecia typically presents as a reduction of terminal hair density in the vertex and mid scalp regions (with widening through the midline part) and fine hair along the anterior hairline.9 Signs of concomitant hyperandrogenism, including facial hirsutism, acne, and obesity, might be observed.10
• Central centrifugal cicatricial alopecia typically affects the vertex and mid scalp with a shiny scalp appearance and follicular dropout.
Diagnostic clues for frontotemporal alopecia include:
• Frontal fibrosing alopecia (FFA) often presents with spared single terminal hairs (lonely hair sign).
• Traction alopecia commonly presents with the fringe hair sign.
Scalp Symptoms—The experts noted that the presence of symptoms (eg, pain, tenderness, pruritus) in conjunction with the pattern of hair loss might support the diagnosis of an inflammatory scarring alopecia.
When do symptoms raise suspicion of central centrifugal cicatricial alopecia?
• Suspected in the setting of vertex alopecia associated with tenderness, pain, or itching.
When do symptoms raise suspicion of FFA?
• Suspected when patients experience frontotemporal tenderness, pain, or burning associated with alopecia.
• The skin hue of the affected area might be lighter in color than, and contrast with, the darker hue of the photoaged upper forehead.11
• The lonely hair sign can aid in diagnosing FFA and distinguish it from the fringe sign of traction alopecia.
• Concurrent madarosis, flesh-colored papules on the cheeks, or lichen planus pigmentosus identified by visual inspection of the face confirms the diagnosis.9,12 Madarosis of the eyebrow was frequently cited by the experts as an associated symptom of FFA.
When do symptoms raise suspicion of lichen planopilaris?
• Suspected in the presence of pruritus, burning, tenderness, or pain associated with perifollicular erythema and scale in the setting of vertex and parietal alopecia.13
• Anagen hair release is observed during the hair pull test.11,14• The experts cited flesh-colored papules and lichen planus pigmentosus as frequently associated symptoms of lichen planopilaris.
Practice Implications
There are limitations to a virtual scalp examination—the inability to perform a scalp biopsy or administer certain treatments—but the consensus of the expert panel is that an initial alopecia assessment can be completed successfully utilizing TD. Although TD is not a replacement for an in-person dermatology visit, this technology has allowed for the diagnosis, treatment, and continuing care of many common dermatologic conditions without the patient needing to travel to the office.5
With the increased frequency of hair loss concerns documented over the last year and more patients seeking TD, it is imperative that dermatologists feel confident performing a virtual hair and scalp examination on all patients.1,3,4
- Kutlu Ö, Aktas¸ H, I·mren IG, et al. Short-term stress-related increasing cases of alopecia areata during the COVID-19 pandemic. J Dermatolog Treat. 2020;1. doi:10.1080/09546634.2020.1782820
- Cline A, Kazemi A, Moy J, et al. A surge in the incidence of telogen effluvium in minority predominant communities heavily impacted by COVID-19. J Am Acad Dermatol. 2021;84:773-775. doi:10.1016/j.jaad.2020.11.032
- Kutlu Ö, Metin A. Relative changes in the pattern of diseases presenting in dermatology outpatient clinic in the era of the COVID-19 pandemic. Dermatol Ther. 2020;33:e14096. doi:10.1111/dth.14096
- Tanacan E, Aksoy Sarac G, Emeksiz MAC, et al. Changing trends in dermatology practice during COVID-19 pandemic: a single tertiary center experience. Dermatol Ther. 2020;33:e14136. doi:10.1111/dth.14136
- Sharma A, Jindal V, Singla P, et al. Will teledermatology be the silver lining during and after COVID-19? Dermatol Ther. 2020;33:e13643. doi:10.1111/dth.13643
- Iscrupe L. How to receive virtual medical treatment while under quarantine. Allconnect website. Published March 26, 2020. Accessed December 9, 2021. https://www.allconnect.com/blog/online-doctor-visit-faq
- Elgash M, Dlova N, Ogunleye T, et al. Seborrheic dermatitis in skin of color: clinical considerations. J Drugs Dermatol. 2019;18:24-27.
- McLaurin CI. Annular facial dermatoses in blacks. Cutis. 1983;32:369-370, 384.
- Suchonwanit P, Hector CE, Bin Saif GA, McMichael AJ. Factors affecting the severity of central centrifugal cicatricial alopecia. Int J Dermatol. 2016;55:e338-343. doi:10.1111/ijd.13061
- Gabros S, Masood S. Central centrifugal cicatricial alopecia. StatPearls [Internet]. StatPearls Publishing; 2021. Updated July 20, 2021. Accessed December 9, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559187/
- Ross EK, Tan E, Shapiro J. Update on primary cicatricial alopecias. J Am Acad Dermatol. 2005;53:1-37. doi:10.1016/j.jaad.2004.06.015
- Cobos G, Kim RH, Meehan S, et al. Lichen planus pigmentosus and lichen planopilaris. Dermatol Online J. 2016;22:13030/qt7hp8n6dn.
- Lyakhovitsky A, Amichai B, Sizopoulou C, et al. A case series of 46 patients with lichen planopilaris: demographics, clinical evaluation, and treatment experience. J Dermatolog Treat. 2015;26:275-279. doi:10.3109/09546634.2014.933165
- Tan E, Martinka M, Ball N, et al. Primary cicatricial alopecias: clinicopathology of 112 cases. J Am Acad Dermatol. 2004;50:25-32. doi:10.1016/j.jaad.2003.04.001
- Kutlu Ö, Aktas¸ H, I·mren IG, et al. Short-term stress-related increasing cases of alopecia areata during the COVID-19 pandemic. J Dermatolog Treat. 2020;1. doi:10.1080/09546634.2020.1782820
- Cline A, Kazemi A, Moy J, et al. A surge in the incidence of telogen effluvium in minority predominant communities heavily impacted by COVID-19. J Am Acad Dermatol. 2021;84:773-775. doi:10.1016/j.jaad.2020.11.032
- Kutlu Ö, Metin A. Relative changes in the pattern of diseases presenting in dermatology outpatient clinic in the era of the COVID-19 pandemic. Dermatol Ther. 2020;33:e14096. doi:10.1111/dth.14096
- Tanacan E, Aksoy Sarac G, Emeksiz MAC, et al. Changing trends in dermatology practice during COVID-19 pandemic: a single tertiary center experience. Dermatol Ther. 2020;33:e14136. doi:10.1111/dth.14136
- Sharma A, Jindal V, Singla P, et al. Will teledermatology be the silver lining during and after COVID-19? Dermatol Ther. 2020;33:e13643. doi:10.1111/dth.13643
- Iscrupe L. How to receive virtual medical treatment while under quarantine. Allconnect website. Published March 26, 2020. Accessed December 9, 2021. https://www.allconnect.com/blog/online-doctor-visit-faq
- Elgash M, Dlova N, Ogunleye T, et al. Seborrheic dermatitis in skin of color: clinical considerations. J Drugs Dermatol. 2019;18:24-27.
- McLaurin CI. Annular facial dermatoses in blacks. Cutis. 1983;32:369-370, 384.
- Suchonwanit P, Hector CE, Bin Saif GA, McMichael AJ. Factors affecting the severity of central centrifugal cicatricial alopecia. Int J Dermatol. 2016;55:e338-343. doi:10.1111/ijd.13061
- Gabros S, Masood S. Central centrifugal cicatricial alopecia. StatPearls [Internet]. StatPearls Publishing; 2021. Updated July 20, 2021. Accessed December 9, 2021. https://www.ncbi.nlm.nih.gov/books/NBK559187/
- Ross EK, Tan E, Shapiro J. Update on primary cicatricial alopecias. J Am Acad Dermatol. 2005;53:1-37. doi:10.1016/j.jaad.2004.06.015
- Cobos G, Kim RH, Meehan S, et al. Lichen planus pigmentosus and lichen planopilaris. Dermatol Online J. 2016;22:13030/qt7hp8n6dn.
- Lyakhovitsky A, Amichai B, Sizopoulou C, et al. A case series of 46 patients with lichen planopilaris: demographics, clinical evaluation, and treatment experience. J Dermatolog Treat. 2015;26:275-279. doi:10.3109/09546634.2014.933165
- Tan E, Martinka M, Ball N, et al. Primary cicatricial alopecias: clinicopathology of 112 cases. J Am Acad Dermatol. 2004;50:25-32. doi:10.1016/j.jaad.2003.04.001
Photolichenoid Dermatitis: A Presenting Sign of Human Immunodeficiency Virus
Photolichenoid dermatitis is an uncommon eruptive dermatitis of variable clinical presentation. It has a histopathologic pattern of lichenoid inflammation and is best characterized as a photoallergic reaction.1 Photolichenoid dermatitis was first described in 1954 in association with the use of quinidine in the treatment of malaria.2 Subsequently, it has been associated with various medications, including trimethoprim-sulfamethoxazole, azithromycin, and nonsteroidal anti-inflammatory drugs.1,2 Photolichenoid dermatitis has been documented in patients with human immunodeficiency virus (HIV) with variable clinical presentations. Photolichenoid dermatitis in patients with HIV has been described both with and without an associated photosensitizing systemic agent, suggesting that HIV infection is an independent risk factor for the development of this eruption in patients with HIV.3-6
Case Report
A 62-year-old African man presented for evaluation of asymptomatic hypopigmented and depigmented patches in a photodistributed pattern. The eruption began the preceding summer when he noted a pink patch on the right side of the forehead. It progressed over 2 months to involve the face, ears, neck, and arms. His medical history was negative. The only medication he was taking was hydroxychloroquine, which was prescribed by another dermatologist when the patient first developed the eruption. The patient was unsure of the indication for the medication and admitted to poor compliance. A review of systems was negative. There was no personal or family history of autoimmune disease. A detailed sexual history and illicit drug history were not obtained. Physical examination revealed hypopigmented and depigmented patches, some with overlying erythema and collarettes of fine scale. The patches were photodistributed on the face, conchal bowls, neck, dorsal aspect of the hands, and extensor forearms (Figures 1 and 2). Macules of repigmentation were noted within some of the patches. There also were large hyperpigmented patches with peripheral hypopigmentation on the legs.
A punch biopsy taken from the left posterior neck revealed a patchy bandlike lymphocytic infiltrate in the superficial dermis with lymphocytes present at the dermoepidermal junction and scattered dyskeratotic keratinocytes extending into the mid spinous layer (Figure 3). Histopathologic findings were consistent with photolichenoid dermatitis.
Laboratory workup revealed a normal complete blood cell count and complete metabolic panel. Other negative results included antinuclear antibody, anti-Ro antibody, anti-La antibody, QuantiFERON-TB Gold, syphilis IgG antibody, and hepatitis B surface antigen and antibody. Positive results included hepatitis B antibody, hepatitis C antibody, and HIV-2 antibody. The patient denied overt symptoms suggestive of an immunocompromised status, including fever, chills, weight loss, or diarrhea. Initial treatment included mid-potency topical steroids with continued progression of the eruption. Following histopathologic and laboratory results indicating photolichenoid eruption, treatment with hydroxychloroquine 200 mg twice daily was resumed. The patient was counseled on the importance of sun protection and was referred to an infectious disease clinic for treatment of HIV. He was ultimately lost to follow-up before further laboratory workup was obtained. Therefore, his CD4+ T-cell count and viral load were not obtained.
Comment
Prevalence of Photosensitive Eruptions
Photodermatitis is an uncommon clinical manifestation of HIV occurring in approximately 5% of patients who are HIV positive.3 Photosensitive eruptions previously described in association with HIV include porphyria cutanea tarda, pseudoporphyria, chronic actinic dermatitis, granuloma annulare, photodistributed dyspigmentation, and lichenoid photodermatitis.7 These HIV-associated photosensitive eruptions have been found to disproportionally affect patients of African and Native American descent.5,7,8 Therefore, a new photodistributed eruption in a patient of African or Native American descent should prompt evaluation of possible underlying HIV infection.
Presenting Sign of HIV Infection
We report a case of photolichenoid dermatitis presenting with loss of pigmentation as a presenting sign of HIV. The patient had no known history of HIV or prior opportunistic infections and was not taking any medications at the time of onset or presentation to clinic. Similar cases of photodistributed depigmentation with lichenoid inflammation on histopathology occurring in patients with HIV have been previously described.4-6,9 In these cases, most patients were of African descent with previously diagnosed advanced HIV and CD4 counts of less than 50 cells/mL3. The additional clinical findings of lichenoid papules and plaques were noted in several of these cases.5,6
Exposure to Photosensitizing Drugs
Photodermatitis in patients with HIV often is attributed to exposure to a photosensitizing drug. Many reported cases are retrospective and identify a temporal association between the onset of photodermatitis following the initiation of a photosensitizing drug. The most commonly implicated drugs have included nonsteroidal anti-inflammatory drugs, trimethoprim-sulfamethoxazole, and azithromycin. Other potential offenders may include saquinavir, dapsone, ketoconazole, and efavirenz.3,5 In cases in which temporal association with a new medication could not be identified, the photodermatitis often has been presumed to be due to polypharmacy and the potential synergistic effect of multiple photosensitizing drugs.3,5-8
Advanced HIV
There are several reported cases of photodermatitis occurring in patients who were not exposed to systemic photosensitizers. These patients had advanced HIV, meeting criteria for AIDS with a CD4 count of less than 200 cells/mL3. The majority of patients had an even lower CD4 count of less than 50 cells/mL3. Clinical presentations have included photodistributed lichenoid papules and plaques as well as depigmented patches.4,5,8,10
Evaluating HIV as a Risk Factor for Photodermatitis
Discerning the validity of the correlation between photodermatitis and HIV is difficult, as all previously reported cases are case reports and small retrospective case series.
Conclusion
This case represents an uncommon presentation of photolichenoid dermatitis as the presenting sign of HIV infection.10 Although most reported cases of photodermatitis in HIV are attributed to photosensitizing drugs, we propose that HIV may be an independent risk factor for the development of photodermatitis. We recommend consideration of HIV testing in patients who present with photodistributed depigmenting eruptions, even in the absence of a photosensitizing drug, particularly in patients of African and Native American descent.
- Collazo MH, Sanchez JL, Figueroa LD. Defining lichenoid photodermatitis. Int J Dermatol. 2009;48:239-242.
- Wechsler HL. Dermatitis medicamentosa; a lichen-planus-like eruption due to quinidine. AMA Arch Derm Syphilol. 1954;69:741-744.
- Bilu D, Mamelak AJ, Nguyen RH, et al. Clinical and epidemiologic characterization of photosensitivity in HIV-positive individuals. Photodermatol Photoimmunol Photomed. 2004;20:175-183.
- Philips RC, Motaparthi K, Krishnan B, et al. HIV photodermatitis presenting with widespread vitiligo-like depigmentation. Dermatol Online J. 2012;18:6.
- Berger TG, Dhar A. Lichenoid photoeruptions in human immunodeficiency virus infection. Arch Dermatol. 1994;130:609-613.
- Tran K, Hartman R, Tzu J, et al. Photolichenoid plaques with associated vitiliginous pigmentary changes. Dermatol Online J. 2011;17:13.
- Gregory N, DeLeo VA. Clinical manifestations of photosensitivity in patients with human immunodeficiency virus infection. Arch Dermatol. 1994;130:630-633.
- Vin-Christian K, Epstein JH, Maurer TA, et al. Photosensitivity in HIV-infected individuals. J Dermatol. 2000;27:361-369.
- Kigonya C, Lutwama F, Colebunders R. Extensive hypopigmentation after starting antiretroviral treatment in a human immunodeficiency virus (HIV)-seropositive African woman. Int J Dermatol. 2008;47:102-103.
- Pardo RJ, Kerdel FA. Hypertrophic lichen planus and light sensitivity in an HIV-positive patient. Int J Dermatol. 1988;27:642-644.
Photolichenoid dermatitis is an uncommon eruptive dermatitis of variable clinical presentation. It has a histopathologic pattern of lichenoid inflammation and is best characterized as a photoallergic reaction.1 Photolichenoid dermatitis was first described in 1954 in association with the use of quinidine in the treatment of malaria.2 Subsequently, it has been associated with various medications, including trimethoprim-sulfamethoxazole, azithromycin, and nonsteroidal anti-inflammatory drugs.1,2 Photolichenoid dermatitis has been documented in patients with human immunodeficiency virus (HIV) with variable clinical presentations. Photolichenoid dermatitis in patients with HIV has been described both with and without an associated photosensitizing systemic agent, suggesting that HIV infection is an independent risk factor for the development of this eruption in patients with HIV.3-6
Case Report
A 62-year-old African man presented for evaluation of asymptomatic hypopigmented and depigmented patches in a photodistributed pattern. The eruption began the preceding summer when he noted a pink patch on the right side of the forehead. It progressed over 2 months to involve the face, ears, neck, and arms. His medical history was negative. The only medication he was taking was hydroxychloroquine, which was prescribed by another dermatologist when the patient first developed the eruption. The patient was unsure of the indication for the medication and admitted to poor compliance. A review of systems was negative. There was no personal or family history of autoimmune disease. A detailed sexual history and illicit drug history were not obtained. Physical examination revealed hypopigmented and depigmented patches, some with overlying erythema and collarettes of fine scale. The patches were photodistributed on the face, conchal bowls, neck, dorsal aspect of the hands, and extensor forearms (Figures 1 and 2). Macules of repigmentation were noted within some of the patches. There also were large hyperpigmented patches with peripheral hypopigmentation on the legs.
A punch biopsy taken from the left posterior neck revealed a patchy bandlike lymphocytic infiltrate in the superficial dermis with lymphocytes present at the dermoepidermal junction and scattered dyskeratotic keratinocytes extending into the mid spinous layer (Figure 3). Histopathologic findings were consistent with photolichenoid dermatitis.
Laboratory workup revealed a normal complete blood cell count and complete metabolic panel. Other negative results included antinuclear antibody, anti-Ro antibody, anti-La antibody, QuantiFERON-TB Gold, syphilis IgG antibody, and hepatitis B surface antigen and antibody. Positive results included hepatitis B antibody, hepatitis C antibody, and HIV-2 antibody. The patient denied overt symptoms suggestive of an immunocompromised status, including fever, chills, weight loss, or diarrhea. Initial treatment included mid-potency topical steroids with continued progression of the eruption. Following histopathologic and laboratory results indicating photolichenoid eruption, treatment with hydroxychloroquine 200 mg twice daily was resumed. The patient was counseled on the importance of sun protection and was referred to an infectious disease clinic for treatment of HIV. He was ultimately lost to follow-up before further laboratory workup was obtained. Therefore, his CD4+ T-cell count and viral load were not obtained.
Comment
Prevalence of Photosensitive Eruptions
Photodermatitis is an uncommon clinical manifestation of HIV occurring in approximately 5% of patients who are HIV positive.3 Photosensitive eruptions previously described in association with HIV include porphyria cutanea tarda, pseudoporphyria, chronic actinic dermatitis, granuloma annulare, photodistributed dyspigmentation, and lichenoid photodermatitis.7 These HIV-associated photosensitive eruptions have been found to disproportionally affect patients of African and Native American descent.5,7,8 Therefore, a new photodistributed eruption in a patient of African or Native American descent should prompt evaluation of possible underlying HIV infection.
Presenting Sign of HIV Infection
We report a case of photolichenoid dermatitis presenting with loss of pigmentation as a presenting sign of HIV. The patient had no known history of HIV or prior opportunistic infections and was not taking any medications at the time of onset or presentation to clinic. Similar cases of photodistributed depigmentation with lichenoid inflammation on histopathology occurring in patients with HIV have been previously described.4-6,9 In these cases, most patients were of African descent with previously diagnosed advanced HIV and CD4 counts of less than 50 cells/mL3. The additional clinical findings of lichenoid papules and plaques were noted in several of these cases.5,6
Exposure to Photosensitizing Drugs
Photodermatitis in patients with HIV often is attributed to exposure to a photosensitizing drug. Many reported cases are retrospective and identify a temporal association between the onset of photodermatitis following the initiation of a photosensitizing drug. The most commonly implicated drugs have included nonsteroidal anti-inflammatory drugs, trimethoprim-sulfamethoxazole, and azithromycin. Other potential offenders may include saquinavir, dapsone, ketoconazole, and efavirenz.3,5 In cases in which temporal association with a new medication could not be identified, the photodermatitis often has been presumed to be due to polypharmacy and the potential synergistic effect of multiple photosensitizing drugs.3,5-8
Advanced HIV
There are several reported cases of photodermatitis occurring in patients who were not exposed to systemic photosensitizers. These patients had advanced HIV, meeting criteria for AIDS with a CD4 count of less than 200 cells/mL3. The majority of patients had an even lower CD4 count of less than 50 cells/mL3. Clinical presentations have included photodistributed lichenoid papules and plaques as well as depigmented patches.4,5,8,10
Evaluating HIV as a Risk Factor for Photodermatitis
Discerning the validity of the correlation between photodermatitis and HIV is difficult, as all previously reported cases are case reports and small retrospective case series.
Conclusion
This case represents an uncommon presentation of photolichenoid dermatitis as the presenting sign of HIV infection.10 Although most reported cases of photodermatitis in HIV are attributed to photosensitizing drugs, we propose that HIV may be an independent risk factor for the development of photodermatitis. We recommend consideration of HIV testing in patients who present with photodistributed depigmenting eruptions, even in the absence of a photosensitizing drug, particularly in patients of African and Native American descent.
Photolichenoid dermatitis is an uncommon eruptive dermatitis of variable clinical presentation. It has a histopathologic pattern of lichenoid inflammation and is best characterized as a photoallergic reaction.1 Photolichenoid dermatitis was first described in 1954 in association with the use of quinidine in the treatment of malaria.2 Subsequently, it has been associated with various medications, including trimethoprim-sulfamethoxazole, azithromycin, and nonsteroidal anti-inflammatory drugs.1,2 Photolichenoid dermatitis has been documented in patients with human immunodeficiency virus (HIV) with variable clinical presentations. Photolichenoid dermatitis in patients with HIV has been described both with and without an associated photosensitizing systemic agent, suggesting that HIV infection is an independent risk factor for the development of this eruption in patients with HIV.3-6
Case Report
A 62-year-old African man presented for evaluation of asymptomatic hypopigmented and depigmented patches in a photodistributed pattern. The eruption began the preceding summer when he noted a pink patch on the right side of the forehead. It progressed over 2 months to involve the face, ears, neck, and arms. His medical history was negative. The only medication he was taking was hydroxychloroquine, which was prescribed by another dermatologist when the patient first developed the eruption. The patient was unsure of the indication for the medication and admitted to poor compliance. A review of systems was negative. There was no personal or family history of autoimmune disease. A detailed sexual history and illicit drug history were not obtained. Physical examination revealed hypopigmented and depigmented patches, some with overlying erythema and collarettes of fine scale. The patches were photodistributed on the face, conchal bowls, neck, dorsal aspect of the hands, and extensor forearms (Figures 1 and 2). Macules of repigmentation were noted within some of the patches. There also were large hyperpigmented patches with peripheral hypopigmentation on the legs.
A punch biopsy taken from the left posterior neck revealed a patchy bandlike lymphocytic infiltrate in the superficial dermis with lymphocytes present at the dermoepidermal junction and scattered dyskeratotic keratinocytes extending into the mid spinous layer (Figure 3). Histopathologic findings were consistent with photolichenoid dermatitis.
Laboratory workup revealed a normal complete blood cell count and complete metabolic panel. Other negative results included antinuclear antibody, anti-Ro antibody, anti-La antibody, QuantiFERON-TB Gold, syphilis IgG antibody, and hepatitis B surface antigen and antibody. Positive results included hepatitis B antibody, hepatitis C antibody, and HIV-2 antibody. The patient denied overt symptoms suggestive of an immunocompromised status, including fever, chills, weight loss, or diarrhea. Initial treatment included mid-potency topical steroids with continued progression of the eruption. Following histopathologic and laboratory results indicating photolichenoid eruption, treatment with hydroxychloroquine 200 mg twice daily was resumed. The patient was counseled on the importance of sun protection and was referred to an infectious disease clinic for treatment of HIV. He was ultimately lost to follow-up before further laboratory workup was obtained. Therefore, his CD4+ T-cell count and viral load were not obtained.
Comment
Prevalence of Photosensitive Eruptions
Photodermatitis is an uncommon clinical manifestation of HIV occurring in approximately 5% of patients who are HIV positive.3 Photosensitive eruptions previously described in association with HIV include porphyria cutanea tarda, pseudoporphyria, chronic actinic dermatitis, granuloma annulare, photodistributed dyspigmentation, and lichenoid photodermatitis.7 These HIV-associated photosensitive eruptions have been found to disproportionally affect patients of African and Native American descent.5,7,8 Therefore, a new photodistributed eruption in a patient of African or Native American descent should prompt evaluation of possible underlying HIV infection.
Presenting Sign of HIV Infection
We report a case of photolichenoid dermatitis presenting with loss of pigmentation as a presenting sign of HIV. The patient had no known history of HIV or prior opportunistic infections and was not taking any medications at the time of onset or presentation to clinic. Similar cases of photodistributed depigmentation with lichenoid inflammation on histopathology occurring in patients with HIV have been previously described.4-6,9 In these cases, most patients were of African descent with previously diagnosed advanced HIV and CD4 counts of less than 50 cells/mL3. The additional clinical findings of lichenoid papules and plaques were noted in several of these cases.5,6
Exposure to Photosensitizing Drugs
Photodermatitis in patients with HIV often is attributed to exposure to a photosensitizing drug. Many reported cases are retrospective and identify a temporal association between the onset of photodermatitis following the initiation of a photosensitizing drug. The most commonly implicated drugs have included nonsteroidal anti-inflammatory drugs, trimethoprim-sulfamethoxazole, and azithromycin. Other potential offenders may include saquinavir, dapsone, ketoconazole, and efavirenz.3,5 In cases in which temporal association with a new medication could not be identified, the photodermatitis often has been presumed to be due to polypharmacy and the potential synergistic effect of multiple photosensitizing drugs.3,5-8
Advanced HIV
There are several reported cases of photodermatitis occurring in patients who were not exposed to systemic photosensitizers. These patients had advanced HIV, meeting criteria for AIDS with a CD4 count of less than 200 cells/mL3. The majority of patients had an even lower CD4 count of less than 50 cells/mL3. Clinical presentations have included photodistributed lichenoid papules and plaques as well as depigmented patches.4,5,8,10
Evaluating HIV as a Risk Factor for Photodermatitis
Discerning the validity of the correlation between photodermatitis and HIV is difficult, as all previously reported cases are case reports and small retrospective case series.
Conclusion
This case represents an uncommon presentation of photolichenoid dermatitis as the presenting sign of HIV infection.10 Although most reported cases of photodermatitis in HIV are attributed to photosensitizing drugs, we propose that HIV may be an independent risk factor for the development of photodermatitis. We recommend consideration of HIV testing in patients who present with photodistributed depigmenting eruptions, even in the absence of a photosensitizing drug, particularly in patients of African and Native American descent.
- Collazo MH, Sanchez JL, Figueroa LD. Defining lichenoid photodermatitis. Int J Dermatol. 2009;48:239-242.
- Wechsler HL. Dermatitis medicamentosa; a lichen-planus-like eruption due to quinidine. AMA Arch Derm Syphilol. 1954;69:741-744.
- Bilu D, Mamelak AJ, Nguyen RH, et al. Clinical and epidemiologic characterization of photosensitivity in HIV-positive individuals. Photodermatol Photoimmunol Photomed. 2004;20:175-183.
- Philips RC, Motaparthi K, Krishnan B, et al. HIV photodermatitis presenting with widespread vitiligo-like depigmentation. Dermatol Online J. 2012;18:6.
- Berger TG, Dhar A. Lichenoid photoeruptions in human immunodeficiency virus infection. Arch Dermatol. 1994;130:609-613.
- Tran K, Hartman R, Tzu J, et al. Photolichenoid plaques with associated vitiliginous pigmentary changes. Dermatol Online J. 2011;17:13.
- Gregory N, DeLeo VA. Clinical manifestations of photosensitivity in patients with human immunodeficiency virus infection. Arch Dermatol. 1994;130:630-633.
- Vin-Christian K, Epstein JH, Maurer TA, et al. Photosensitivity in HIV-infected individuals. J Dermatol. 2000;27:361-369.
- Kigonya C, Lutwama F, Colebunders R. Extensive hypopigmentation after starting antiretroviral treatment in a human immunodeficiency virus (HIV)-seropositive African woman. Int J Dermatol. 2008;47:102-103.
- Pardo RJ, Kerdel FA. Hypertrophic lichen planus and light sensitivity in an HIV-positive patient. Int J Dermatol. 1988;27:642-644.
- Collazo MH, Sanchez JL, Figueroa LD. Defining lichenoid photodermatitis. Int J Dermatol. 2009;48:239-242.
- Wechsler HL. Dermatitis medicamentosa; a lichen-planus-like eruption due to quinidine. AMA Arch Derm Syphilol. 1954;69:741-744.
- Bilu D, Mamelak AJ, Nguyen RH, et al. Clinical and epidemiologic characterization of photosensitivity in HIV-positive individuals. Photodermatol Photoimmunol Photomed. 2004;20:175-183.
- Philips RC, Motaparthi K, Krishnan B, et al. HIV photodermatitis presenting with widespread vitiligo-like depigmentation. Dermatol Online J. 2012;18:6.
- Berger TG, Dhar A. Lichenoid photoeruptions in human immunodeficiency virus infection. Arch Dermatol. 1994;130:609-613.
- Tran K, Hartman R, Tzu J, et al. Photolichenoid plaques with associated vitiliginous pigmentary changes. Dermatol Online J. 2011;17:13.
- Gregory N, DeLeo VA. Clinical manifestations of photosensitivity in patients with human immunodeficiency virus infection. Arch Dermatol. 1994;130:630-633.
- Vin-Christian K, Epstein JH, Maurer TA, et al. Photosensitivity in HIV-infected individuals. J Dermatol. 2000;27:361-369.
- Kigonya C, Lutwama F, Colebunders R. Extensive hypopigmentation after starting antiretroviral treatment in a human immunodeficiency virus (HIV)-seropositive African woman. Int J Dermatol. 2008;47:102-103.
- Pardo RJ, Kerdel FA. Hypertrophic lichen planus and light sensitivity in an HIV-positive patient. Int J Dermatol. 1988;27:642-644.
Practice Points
- There are few reports in the literature of human immunodeficiency virus (HIV) presenting as a photolichenoid eruption.
- We report the case of a 62-year-old African man who presented with a new-onset photodistributed eruption and was subsequently diagnosed with HIV.
- This case supports testing for HIV in patients with a similar clinical presentation.
Frontal Fibrosing Alopecia: Cutaneous Associations in Women With Skin of Color
Frontal fibrosing alopecia (FFA) has been reported in association with lichen planus pigmentosus (LPP) and facial papules.1-3 Lichen planus pigmentosus is a variant of lichen planus that causes hyperpigmentation of the face, neck, and/or intertriginous areas that may be useful as a clinical indicator in the development of FFA.1 Facial papules in association with FFA are secondary to fibrosed vellus hairs.2,3 Currently, reports of concomitant FFA, LPP, and facial papules in women with skin of color are limited in the literature. This case series includes 5 women of color (Hispanic and black) who presented to our clinic with FFA and various cutaneous associations. A review of the current literature on cutaneous associations of FFA also is provided.
Case Reports
Patient 1
A 50-year-old Hispanic woman who was previously presumed to have melasma by an outside physician presented with pruritus of the scalp and eyebrows of 1 month’s duration. Physical examination revealed decreased frontal scalp hair density with perifollicular erythema and scale with thinning of the lateral eyebrows. Hyperpigmented coalesced macules (Figure 1A) and erythematous perifollicular papules were noted along the temples and on the perioral skin. Depressed forehead and temporal veins also were noted (Figure 1B). A biopsy of the scalp demonstrated perifollicular and perivascular lymphocytic inflammation and fibrosed hair follicles, and a biopsy of the perioral skin demonstrated perivascular lymphocytic inflammation with melanophages in the papillary dermis. A diagnosis of FFA with LPP was established with these biopsies.
Patient 2
A 61-year-old black woman presented with asymptomatic hair loss along the frontal hairline for an unknown duration. On physical examination the frontal scalp and lateral eyebrows demonstrated decreased hair density with loss of follicular ostia. Fine, flesh-colored, monomorphic papules were scattered along the forehead and temples, and ill-defined brown pigmentation was present along the forehead, temples, and cheeks. Biopsy of the frontal scalp demonstrated patchy lichenoid inflammation with decreased number of follicles with replacement by follicular scars, confirming the diagnosis of FFA.
Patient 3
A 47-year-old Hispanic woman presented with hair loss of the frontal scalp and bilateral eyebrows with associated burning of 2 years’ duration. Physical examination demonstrated recession of the frontotemporal hairline with scattered lone hairs and thinning of the eyebrows. Innumerable flesh-colored papules were present on the forehead and temples (Figure 2A). Glabellar and eyebrow erythema was noted (Figure 2B). Biopsy of the frontal scalp demonstrated decreased terminal anagen hair follicles with perifollicular lymphoid infiltrate and fibrosis, consistent with a diagnosis of FFA. The patient was started on oral hydroxychloroquine 400 mg once daily, and 3 months later hyperpigmentation of the forehead and perioral skin was noted. The patient reported that she had facial hyperpigmentation prior to starting hydroxychloroquine and declined a biopsy.
Patient 4
A 40-year-old black woman presented with brown pruritic macles of the face, neck, arms, and forearms of 4 years’ duration. She also reported hair loss on the frontal and occipital scalp, eyebrows, and arms. On physical examination, ill-defined brown macules and patches were noted on the neck (Figure 3), face, arms, and forearms. Decreased hair density was noted on the frontal and occipital scalp with follicular dropout and perifollicular hyperpigmentation. Biopsy of the scalp demonstrated perivascular lymphocytic inflammation with sparse anagen follicles and fibrous tracts, and biopsy of the neck revealed superficial perivascular inflammation with numerous melanophages in the upper dermis; these histopathologic findings were consistent with FFA and LPP, respectively.
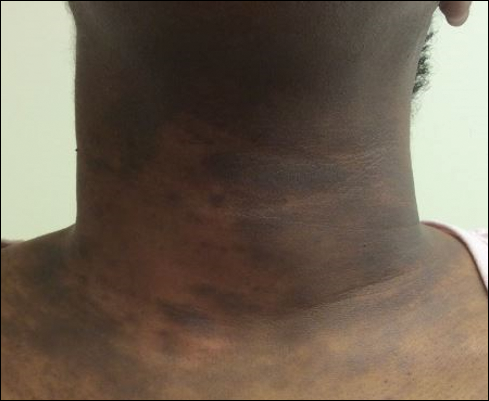
Patient 5
A 46-year-old black woman with history of hair loss presented with hyperpigmentation of the face and neck of 2 years’ duration. On physical examination decreased hair density of the frontal and vertex scalp and lateral eyebrows was noted. Flesh-colored papules were noted on the forehead and cheeks, and confluent dark brown patches were present on the temples and neck. Three punch biopsies were performed. Biopsy of the scalp revealed lymphocytic inflammation with surrounding fibroplasia with overlapping features of FFA and central centrifugal cicatricial alopecia (Figure 4). Biopsy of the neck revealed vacuolar interface dermatitis. Additionally, biopsy of a facial papule revealed lichenoid inflammation involving a vellus hair follicle. Clinical and histopathological correlation confirmed the diagnosis of FFA with LPP and facial papules.
Comment
Current understanding of FFA as a progressive, lymphocytic, scarring alopecia has expanded in recent years. Clinical observation suggests that the incidence of FFA is increasing4; however more epidemiologic data are needed. Frontal fibrosing alopecia presents clinically with symmetrical frontotemporal hair loss with lone hairs. Trichoscopy reveals perifollicular hyperkeratosis, perifollicular erythema, and follicular plugging in 72%, 66%, and 44% of cases, respectively.5 In one study (N=242), patients were classified into 3 clinical patterns of FFA: pattern I (linear) showed bandlike loss of frontal hair with normal density directly behind the hairline; pattern II (diffuse) showed loss of density behind the frontal hairline; and pattern III (double line) showed a pseudo–“fringe sign” appearance. The majority of patients were classified as either pattern I or II, with pattern II predicting a poorer prognosis.6
rontal fibrosing alopecia is increasingly recognized in men, with prevalence as high as 5%.1 Facial hair involvement, particularly of the upper lip and sideburns, is an important consideration in men.7 Most studies suggest that 80% to 90% of affected women are postmenopausal,8 though a case series presented by Dlova1 identified 27% of affected women as postmenopausal. The coexistence of premature menopause and hysterectomy in FFA patients suggests a hormonal contribution, but this association is still poorly understood.8 Epidemiologic data on ethnicity in FFA are sparse but suggest that white individuals are more likely to be affected. Frontal fibrosing alopecia also may be misdiagnosed as traction alopecia in Hispanic and black patients.8
It is prudent for physicians to assess for and recognize clinical clues to severe forms of FFA. A 2014 multicenter review of 355 patients identified 3 clinical entities that predicted more severe forms of FFA: eyelash loss (madarosis), loss of body hair, and facial papules.8 Madarosis occurs due to perifollicular inflammation and fibrosis of eyelash hair follicles. Similarly, perifollicular inflammation of body hair was present in 24% of patients (N=86), most commonly of the axillary and pubic hair. Facial papules form due to facial vellus hair inflammation and fibrosis and were identified in 14% of patients (N=49).8 These clinical findings may allow providers to predict more extensive clinical involvement of FFA.
Frontal fibrosing alopecia and LPP occur concomitantly in up 54% of patients, more commonly in darker-skinned patients.1,9,10 Lichen planus pigmentosus frequently occurs on the face and neck, most commonly in a diffuse pattern, though reticulated and macular patterns also have been identified.11 In some patients, LPP precedes the development of FFA and may be useful as a herald sign1; therefore, it is important for dermatologists to evaluate for signs of FFA when evaluating those with LPP. Thorough evaluation in patients with skin of color also is important because FFA may be misdiagnosed as traction alopecia.
Additional cutaneous associations of FFA include eyebrow loss, glabellar red dots, and prominent frontal veins. Eyebrow loss occurs secondary to fibrosis of eyebrow hair follicles and has been found in 40% to 80% of patients with FFA; it is thought to be associated with milder forms of FFA.8 Glabellar red dots correlate with histopathologic lymphocytic inflammation of vellus hair follicles.12 Additionally, frontal vein prominence has been described in FFA and is thought to be secondary to atrophy in this scarring process, perhaps worsened by local steroid treatments.13 Mucocutaneous lichen planus, rosacea, thyroid disease, vitiligo, and other autoimmune disorders also have been reported in patients with FFA.14
Conclusion
Concomitant FFA, LPP, and facial papules have been rarely reported and exemplify the spectrum of cutaneous associations with FFA, particularly in individuals with skin of color. Clinical variants and associations of FFA are broad, including predictors of poorer prognosis such as eyelash loss and vellus hair involvement seen as facial papules. Lichen planus pigmentosus is well described in association with FFA and may serve as a herald sign that frontal hair loss should not be mistaken for traction alopecia in early stages. Eyebrow loss is thought to represent milder disease. It is important for dermatologists to be aware of these findings to understand the breadth of this disease and for appropriate evaluation and management of patients with FFA.
- Dlova NC. Frontal fibrosing alopecia and lichen planus pigmentosus: is there a link? Br J Dermatol. 2013;168:439-432.
- Donati A, Molina L, Doche I, et al. Facial papules in frontal fibrosing alopecia: evidence of vellus follicle involvement. Arch Dermatol. 2011;147:1424-1427.
- Tan KT, Messenger AG. Frontal fibrosing alopecia: clinical presentations and prognosis. Br J Dermatol. 2009;160:75-79.
- Rudnicka L, Rakowska A. The increasing incidence of frontal fibrosing alopecia. in search of triggering factors. J Eur Acad Dermatol Venereol. 2017;31:1579-1580.
- Toledo-Pastrana T, Hernández MJ, Camacho Martínez FM. Perifollicular erythema as a trichoscopy sign of progression in frontal fibrosing alopecia. Int J Trichology. 2013;5:151-153.
- Moreno-Arrones OM, Saceda-Corralo D, Fonda-Pascual P, et al. Frontal fibrosing alopecia: clinical and prognostic classification. J Eur Acad Dermatol Venereol. 2017;31:1739-1745.
- Tolkachjov SN, Chaudhry HM, Camilleri MJ, et al. Frontal fibrosing alopecia among men: a clinicopathologic study of 7 cases. J Am Acad Dermatol. 2017;77:683-690.e2.
- Vañó-Galván S, Molina-Ruiz AM, Serrano-Falcón C, et al. Frontal fibrosing alopecia: a multicenter review of 355 patients. J Am Acad Dermatol. 2014;70:670-678.
- Berliner JG, McCalmont TH, Price VH, et al. Frontal fibrosing alopecia and lichen planus pigmentosus. J Am Acad Dermatol. 2014;71:E26-E27.
- Rao R, Sarda A, Khanna R, et al. Coexistence of frontal fibrosing alopecia with lichen planus pigmentosus. Int J Dermatol. 2014;53:622-624.
- Pirmez R, Duque-Estrada B, Donati A, et al. Clinical and dermoscopic features of lichen planus pigmentosus in 37 patients with frontal fibrosing alopecia. Br J Dermatol. 2016;175:1387-1390.
- Pirmez R, Donati A, Valente NS, et al. Glabellar red dots in frontal fibrosing alopecia: a further clinical sign of vellus follicle involvement. Br J Dermatol. 2014;170:745-746.
- Vañó-Galván S, Rodrigues-Barata AR, Urech M, et al. Depression of the frontal veins: a new clinical sign of frontal fibrosing alopecia. J Am Acad Dermatol. 2015;72:1087-1088.
- Pindado-Ortega C, Saceda-Corralo D, Buendía-Castaño D, et al. Frontal fibrosing alopecia and cutaneous comorbidities: a potential relationship with rosacea. J Am Acad Dermatol. 2018;78:596-597.e1.
Frontal fibrosing alopecia (FFA) has been reported in association with lichen planus pigmentosus (LPP) and facial papules.1-3 Lichen planus pigmentosus is a variant of lichen planus that causes hyperpigmentation of the face, neck, and/or intertriginous areas that may be useful as a clinical indicator in the development of FFA.1 Facial papules in association with FFA are secondary to fibrosed vellus hairs.2,3 Currently, reports of concomitant FFA, LPP, and facial papules in women with skin of color are limited in the literature. This case series includes 5 women of color (Hispanic and black) who presented to our clinic with FFA and various cutaneous associations. A review of the current literature on cutaneous associations of FFA also is provided.
Case Reports
Patient 1
A 50-year-old Hispanic woman who was previously presumed to have melasma by an outside physician presented with pruritus of the scalp and eyebrows of 1 month’s duration. Physical examination revealed decreased frontal scalp hair density with perifollicular erythema and scale with thinning of the lateral eyebrows. Hyperpigmented coalesced macules (Figure 1A) and erythematous perifollicular papules were noted along the temples and on the perioral skin. Depressed forehead and temporal veins also were noted (Figure 1B). A biopsy of the scalp demonstrated perifollicular and perivascular lymphocytic inflammation and fibrosed hair follicles, and a biopsy of the perioral skin demonstrated perivascular lymphocytic inflammation with melanophages in the papillary dermis. A diagnosis of FFA with LPP was established with these biopsies.

Patient 2
A 61-year-old black woman presented with asymptomatic hair loss along the frontal hairline for an unknown duration. On physical examination the frontal scalp and lateral eyebrows demonstrated decreased hair density with loss of follicular ostia. Fine, flesh-colored, monomorphic papules were scattered along the forehead and temples, and ill-defined brown pigmentation was present along the forehead, temples, and cheeks. Biopsy of the frontal scalp demonstrated patchy lichenoid inflammation with decreased number of follicles with replacement by follicular scars, confirming the diagnosis of FFA.
Patient 3
A 47-year-old Hispanic woman presented with hair loss of the frontal scalp and bilateral eyebrows with associated burning of 2 years’ duration. Physical examination demonstrated recession of the frontotemporal hairline with scattered lone hairs and thinning of the eyebrows. Innumerable flesh-colored papules were present on the forehead and temples (Figure 2A). Glabellar and eyebrow erythema was noted (Figure 2B). Biopsy of the frontal scalp demonstrated decreased terminal anagen hair follicles with perifollicular lymphoid infiltrate and fibrosis, consistent with a diagnosis of FFA. The patient was started on oral hydroxychloroquine 400 mg once daily, and 3 months later hyperpigmentation of the forehead and perioral skin was noted. The patient reported that she had facial hyperpigmentation prior to starting hydroxychloroquine and declined a biopsy.

Patient 4
A 40-year-old black woman presented with brown pruritic macles of the face, neck, arms, and forearms of 4 years’ duration. She also reported hair loss on the frontal and occipital scalp, eyebrows, and arms. On physical examination, ill-defined brown macules and patches were noted on the neck (Figure 3), face, arms, and forearms. Decreased hair density was noted on the frontal and occipital scalp with follicular dropout and perifollicular hyperpigmentation. Biopsy of the scalp demonstrated perivascular lymphocytic inflammation with sparse anagen follicles and fibrous tracts, and biopsy of the neck revealed superficial perivascular inflammation with numerous melanophages in the upper dermis; these histopathologic findings were consistent with FFA and LPP, respectively.

Patient 5
A 46-year-old black woman with history of hair loss presented with hyperpigmentation of the face and neck of 2 years’ duration. On physical examination decreased hair density of the frontal and vertex scalp and lateral eyebrows was noted. Flesh-colored papules were noted on the forehead and cheeks, and confluent dark brown patches were present on the temples and neck. Three punch biopsies were performed. Biopsy of the scalp revealed lymphocytic inflammation with surrounding fibroplasia with overlapping features of FFA and central centrifugal cicatricial alopecia (Figure 4). Biopsy of the neck revealed vacuolar interface dermatitis. Additionally, biopsy of a facial papule revealed lichenoid inflammation involving a vellus hair follicle. Clinical and histopathological correlation confirmed the diagnosis of FFA with LPP and facial papules.

Comment
Current understanding of FFA as a progressive, lymphocytic, scarring alopecia has expanded in recent years. Clinical observation suggests that the incidence of FFA is increasing4; however more epidemiologic data are needed. Frontal fibrosing alopecia presents clinically with symmetrical frontotemporal hair loss with lone hairs. Trichoscopy reveals perifollicular hyperkeratosis, perifollicular erythema, and follicular plugging in 72%, 66%, and 44% of cases, respectively.5 In one study (N=242), patients were classified into 3 clinical patterns of FFA: pattern I (linear) showed bandlike loss of frontal hair with normal density directly behind the hairline; pattern II (diffuse) showed loss of density behind the frontal hairline; and pattern III (double line) showed a pseudo–“fringe sign” appearance. The majority of patients were classified as either pattern I or II, with pattern II predicting a poorer prognosis.6
rontal fibrosing alopecia is increasingly recognized in men, with prevalence as high as 5%.1 Facial hair involvement, particularly of the upper lip and sideburns, is an important consideration in men.7 Most studies suggest that 80% to 90% of affected women are postmenopausal,8 though a case series presented by Dlova1 identified 27% of affected women as postmenopausal. The coexistence of premature menopause and hysterectomy in FFA patients suggests a hormonal contribution, but this association is still poorly understood.8 Epidemiologic data on ethnicity in FFA are sparse but suggest that white individuals are more likely to be affected. Frontal fibrosing alopecia also may be misdiagnosed as traction alopecia in Hispanic and black patients.8
It is prudent for physicians to assess for and recognize clinical clues to severe forms of FFA. A 2014 multicenter review of 355 patients identified 3 clinical entities that predicted more severe forms of FFA: eyelash loss (madarosis), loss of body hair, and facial papules.8 Madarosis occurs due to perifollicular inflammation and fibrosis of eyelash hair follicles. Similarly, perifollicular inflammation of body hair was present in 24% of patients (N=86), most commonly of the axillary and pubic hair. Facial papules form due to facial vellus hair inflammation and fibrosis and were identified in 14% of patients (N=49).8 These clinical findings may allow providers to predict more extensive clinical involvement of FFA.
Frontal fibrosing alopecia and LPP occur concomitantly in up 54% of patients, more commonly in darker-skinned patients.1,9,10 Lichen planus pigmentosus frequently occurs on the face and neck, most commonly in a diffuse pattern, though reticulated and macular patterns also have been identified.11 In some patients, LPP precedes the development of FFA and may be useful as a herald sign1; therefore, it is important for dermatologists to evaluate for signs of FFA when evaluating those with LPP. Thorough evaluation in patients with skin of color also is important because FFA may be misdiagnosed as traction alopecia.
Additional cutaneous associations of FFA include eyebrow loss, glabellar red dots, and prominent frontal veins. Eyebrow loss occurs secondary to fibrosis of eyebrow hair follicles and has been found in 40% to 80% of patients with FFA; it is thought to be associated with milder forms of FFA.8 Glabellar red dots correlate with histopathologic lymphocytic inflammation of vellus hair follicles.12 Additionally, frontal vein prominence has been described in FFA and is thought to be secondary to atrophy in this scarring process, perhaps worsened by local steroid treatments.13 Mucocutaneous lichen planus, rosacea, thyroid disease, vitiligo, and other autoimmune disorders also have been reported in patients with FFA.14
Conclusion
Concomitant FFA, LPP, and facial papules have been rarely reported and exemplify the spectrum of cutaneous associations with FFA, particularly in individuals with skin of color. Clinical variants and associations of FFA are broad, including predictors of poorer prognosis such as eyelash loss and vellus hair involvement seen as facial papules. Lichen planus pigmentosus is well described in association with FFA and may serve as a herald sign that frontal hair loss should not be mistaken for traction alopecia in early stages. Eyebrow loss is thought to represent milder disease. It is important for dermatologists to be aware of these findings to understand the breadth of this disease and for appropriate evaluation and management of patients with FFA.
Frontal fibrosing alopecia (FFA) has been reported in association with lichen planus pigmentosus (LPP) and facial papules.1-3 Lichen planus pigmentosus is a variant of lichen planus that causes hyperpigmentation of the face, neck, and/or intertriginous areas that may be useful as a clinical indicator in the development of FFA.1 Facial papules in association with FFA are secondary to fibrosed vellus hairs.2,3 Currently, reports of concomitant FFA, LPP, and facial papules in women with skin of color are limited in the literature. This case series includes 5 women of color (Hispanic and black) who presented to our clinic with FFA and various cutaneous associations. A review of the current literature on cutaneous associations of FFA also is provided.
Case Reports
Patient 1
A 50-year-old Hispanic woman who was previously presumed to have melasma by an outside physician presented with pruritus of the scalp and eyebrows of 1 month’s duration. Physical examination revealed decreased frontal scalp hair density with perifollicular erythema and scale with thinning of the lateral eyebrows. Hyperpigmented coalesced macules (Figure 1A) and erythematous perifollicular papules were noted along the temples and on the perioral skin. Depressed forehead and temporal veins also were noted (Figure 1B). A biopsy of the scalp demonstrated perifollicular and perivascular lymphocytic inflammation and fibrosed hair follicles, and a biopsy of the perioral skin demonstrated perivascular lymphocytic inflammation with melanophages in the papillary dermis. A diagnosis of FFA with LPP was established with these biopsies.

Patient 2
A 61-year-old black woman presented with asymptomatic hair loss along the frontal hairline for an unknown duration. On physical examination the frontal scalp and lateral eyebrows demonstrated decreased hair density with loss of follicular ostia. Fine, flesh-colored, monomorphic papules were scattered along the forehead and temples, and ill-defined brown pigmentation was present along the forehead, temples, and cheeks. Biopsy of the frontal scalp demonstrated patchy lichenoid inflammation with decreased number of follicles with replacement by follicular scars, confirming the diagnosis of FFA.
Patient 3
A 47-year-old Hispanic woman presented with hair loss of the frontal scalp and bilateral eyebrows with associated burning of 2 years’ duration. Physical examination demonstrated recession of the frontotemporal hairline with scattered lone hairs and thinning of the eyebrows. Innumerable flesh-colored papules were present on the forehead and temples (Figure 2A). Glabellar and eyebrow erythema was noted (Figure 2B). Biopsy of the frontal scalp demonstrated decreased terminal anagen hair follicles with perifollicular lymphoid infiltrate and fibrosis, consistent with a diagnosis of FFA. The patient was started on oral hydroxychloroquine 400 mg once daily, and 3 months later hyperpigmentation of the forehead and perioral skin was noted. The patient reported that she had facial hyperpigmentation prior to starting hydroxychloroquine and declined a biopsy.

Patient 4
A 40-year-old black woman presented with brown pruritic macles of the face, neck, arms, and forearms of 4 years’ duration. She also reported hair loss on the frontal and occipital scalp, eyebrows, and arms. On physical examination, ill-defined brown macules and patches were noted on the neck (Figure 3), face, arms, and forearms. Decreased hair density was noted on the frontal and occipital scalp with follicular dropout and perifollicular hyperpigmentation. Biopsy of the scalp demonstrated perivascular lymphocytic inflammation with sparse anagen follicles and fibrous tracts, and biopsy of the neck revealed superficial perivascular inflammation with numerous melanophages in the upper dermis; these histopathologic findings were consistent with FFA and LPP, respectively.

Patient 5
A 46-year-old black woman with history of hair loss presented with hyperpigmentation of the face and neck of 2 years’ duration. On physical examination decreased hair density of the frontal and vertex scalp and lateral eyebrows was noted. Flesh-colored papules were noted on the forehead and cheeks, and confluent dark brown patches were present on the temples and neck. Three punch biopsies were performed. Biopsy of the scalp revealed lymphocytic inflammation with surrounding fibroplasia with overlapping features of FFA and central centrifugal cicatricial alopecia (Figure 4). Biopsy of the neck revealed vacuolar interface dermatitis. Additionally, biopsy of a facial papule revealed lichenoid inflammation involving a vellus hair follicle. Clinical and histopathological correlation confirmed the diagnosis of FFA with LPP and facial papules.

Comment
Current understanding of FFA as a progressive, lymphocytic, scarring alopecia has expanded in recent years. Clinical observation suggests that the incidence of FFA is increasing4; however more epidemiologic data are needed. Frontal fibrosing alopecia presents clinically with symmetrical frontotemporal hair loss with lone hairs. Trichoscopy reveals perifollicular hyperkeratosis, perifollicular erythema, and follicular plugging in 72%, 66%, and 44% of cases, respectively.5 In one study (N=242), patients were classified into 3 clinical patterns of FFA: pattern I (linear) showed bandlike loss of frontal hair with normal density directly behind the hairline; pattern II (diffuse) showed loss of density behind the frontal hairline; and pattern III (double line) showed a pseudo–“fringe sign” appearance. The majority of patients were classified as either pattern I or II, with pattern II predicting a poorer prognosis.6
rontal fibrosing alopecia is increasingly recognized in men, with prevalence as high as 5%.1 Facial hair involvement, particularly of the upper lip and sideburns, is an important consideration in men.7 Most studies suggest that 80% to 90% of affected women are postmenopausal,8 though a case series presented by Dlova1 identified 27% of affected women as postmenopausal. The coexistence of premature menopause and hysterectomy in FFA patients suggests a hormonal contribution, but this association is still poorly understood.8 Epidemiologic data on ethnicity in FFA are sparse but suggest that white individuals are more likely to be affected. Frontal fibrosing alopecia also may be misdiagnosed as traction alopecia in Hispanic and black patients.8
It is prudent for physicians to assess for and recognize clinical clues to severe forms of FFA. A 2014 multicenter review of 355 patients identified 3 clinical entities that predicted more severe forms of FFA: eyelash loss (madarosis), loss of body hair, and facial papules.8 Madarosis occurs due to perifollicular inflammation and fibrosis of eyelash hair follicles. Similarly, perifollicular inflammation of body hair was present in 24% of patients (N=86), most commonly of the axillary and pubic hair. Facial papules form due to facial vellus hair inflammation and fibrosis and were identified in 14% of patients (N=49).8 These clinical findings may allow providers to predict more extensive clinical involvement of FFA.
Frontal fibrosing alopecia and LPP occur concomitantly in up 54% of patients, more commonly in darker-skinned patients.1,9,10 Lichen planus pigmentosus frequently occurs on the face and neck, most commonly in a diffuse pattern, though reticulated and macular patterns also have been identified.11 In some patients, LPP precedes the development of FFA and may be useful as a herald sign1; therefore, it is important for dermatologists to evaluate for signs of FFA when evaluating those with LPP. Thorough evaluation in patients with skin of color also is important because FFA may be misdiagnosed as traction alopecia.
Additional cutaneous associations of FFA include eyebrow loss, glabellar red dots, and prominent frontal veins. Eyebrow loss occurs secondary to fibrosis of eyebrow hair follicles and has been found in 40% to 80% of patients with FFA; it is thought to be associated with milder forms of FFA.8 Glabellar red dots correlate with histopathologic lymphocytic inflammation of vellus hair follicles.12 Additionally, frontal vein prominence has been described in FFA and is thought to be secondary to atrophy in this scarring process, perhaps worsened by local steroid treatments.13 Mucocutaneous lichen planus, rosacea, thyroid disease, vitiligo, and other autoimmune disorders also have been reported in patients with FFA.14
Conclusion
Concomitant FFA, LPP, and facial papules have been rarely reported and exemplify the spectrum of cutaneous associations with FFA, particularly in individuals with skin of color. Clinical variants and associations of FFA are broad, including predictors of poorer prognosis such as eyelash loss and vellus hair involvement seen as facial papules. Lichen planus pigmentosus is well described in association with FFA and may serve as a herald sign that frontal hair loss should not be mistaken for traction alopecia in early stages. Eyebrow loss is thought to represent milder disease. It is important for dermatologists to be aware of these findings to understand the breadth of this disease and for appropriate evaluation and management of patients with FFA.
- Dlova NC. Frontal fibrosing alopecia and lichen planus pigmentosus: is there a link? Br J Dermatol. 2013;168:439-432.
- Donati A, Molina L, Doche I, et al. Facial papules in frontal fibrosing alopecia: evidence of vellus follicle involvement. Arch Dermatol. 2011;147:1424-1427.
- Tan KT, Messenger AG. Frontal fibrosing alopecia: clinical presentations and prognosis. Br J Dermatol. 2009;160:75-79.
- Rudnicka L, Rakowska A. The increasing incidence of frontal fibrosing alopecia. in search of triggering factors. J Eur Acad Dermatol Venereol. 2017;31:1579-1580.
- Toledo-Pastrana T, Hernández MJ, Camacho Martínez FM. Perifollicular erythema as a trichoscopy sign of progression in frontal fibrosing alopecia. Int J Trichology. 2013;5:151-153.
- Moreno-Arrones OM, Saceda-Corralo D, Fonda-Pascual P, et al. Frontal fibrosing alopecia: clinical and prognostic classification. J Eur Acad Dermatol Venereol. 2017;31:1739-1745.
- Tolkachjov SN, Chaudhry HM, Camilleri MJ, et al. Frontal fibrosing alopecia among men: a clinicopathologic study of 7 cases. J Am Acad Dermatol. 2017;77:683-690.e2.
- Vañó-Galván S, Molina-Ruiz AM, Serrano-Falcón C, et al. Frontal fibrosing alopecia: a multicenter review of 355 patients. J Am Acad Dermatol. 2014;70:670-678.
- Berliner JG, McCalmont TH, Price VH, et al. Frontal fibrosing alopecia and lichen planus pigmentosus. J Am Acad Dermatol. 2014;71:E26-E27.
- Rao R, Sarda A, Khanna R, et al. Coexistence of frontal fibrosing alopecia with lichen planus pigmentosus. Int J Dermatol. 2014;53:622-624.
- Pirmez R, Duque-Estrada B, Donati A, et al. Clinical and dermoscopic features of lichen planus pigmentosus in 37 patients with frontal fibrosing alopecia. Br J Dermatol. 2016;175:1387-1390.
- Pirmez R, Donati A, Valente NS, et al. Glabellar red dots in frontal fibrosing alopecia: a further clinical sign of vellus follicle involvement. Br J Dermatol. 2014;170:745-746.
- Vañó-Galván S, Rodrigues-Barata AR, Urech M, et al. Depression of the frontal veins: a new clinical sign of frontal fibrosing alopecia. J Am Acad Dermatol. 2015;72:1087-1088.
- Pindado-Ortega C, Saceda-Corralo D, Buendía-Castaño D, et al. Frontal fibrosing alopecia and cutaneous comorbidities: a potential relationship with rosacea. J Am Acad Dermatol. 2018;78:596-597.e1.
- Dlova NC. Frontal fibrosing alopecia and lichen planus pigmentosus: is there a link? Br J Dermatol. 2013;168:439-432.
- Donati A, Molina L, Doche I, et al. Facial papules in frontal fibrosing alopecia: evidence of vellus follicle involvement. Arch Dermatol. 2011;147:1424-1427.
- Tan KT, Messenger AG. Frontal fibrosing alopecia: clinical presentations and prognosis. Br J Dermatol. 2009;160:75-79.
- Rudnicka L, Rakowska A. The increasing incidence of frontal fibrosing alopecia. in search of triggering factors. J Eur Acad Dermatol Venereol. 2017;31:1579-1580.
- Toledo-Pastrana T, Hernández MJ, Camacho Martínez FM. Perifollicular erythema as a trichoscopy sign of progression in frontal fibrosing alopecia. Int J Trichology. 2013;5:151-153.
- Moreno-Arrones OM, Saceda-Corralo D, Fonda-Pascual P, et al. Frontal fibrosing alopecia: clinical and prognostic classification. J Eur Acad Dermatol Venereol. 2017;31:1739-1745.
- Tolkachjov SN, Chaudhry HM, Camilleri MJ, et al. Frontal fibrosing alopecia among men: a clinicopathologic study of 7 cases. J Am Acad Dermatol. 2017;77:683-690.e2.
- Vañó-Galván S, Molina-Ruiz AM, Serrano-Falcón C, et al. Frontal fibrosing alopecia: a multicenter review of 355 patients. J Am Acad Dermatol. 2014;70:670-678.
- Berliner JG, McCalmont TH, Price VH, et al. Frontal fibrosing alopecia and lichen planus pigmentosus. J Am Acad Dermatol. 2014;71:E26-E27.
- Rao R, Sarda A, Khanna R, et al. Coexistence of frontal fibrosing alopecia with lichen planus pigmentosus. Int J Dermatol. 2014;53:622-624.
- Pirmez R, Duque-Estrada B, Donati A, et al. Clinical and dermoscopic features of lichen planus pigmentosus in 37 patients with frontal fibrosing alopecia. Br J Dermatol. 2016;175:1387-1390.
- Pirmez R, Donati A, Valente NS, et al. Glabellar red dots in frontal fibrosing alopecia: a further clinical sign of vellus follicle involvement. Br J Dermatol. 2014;170:745-746.
- Vañó-Galván S, Rodrigues-Barata AR, Urech M, et al. Depression of the frontal veins: a new clinical sign of frontal fibrosing alopecia. J Am Acad Dermatol. 2015;72:1087-1088.
- Pindado-Ortega C, Saceda-Corralo D, Buendía-Castaño D, et al. Frontal fibrosing alopecia and cutaneous comorbidities: a potential relationship with rosacea. J Am Acad Dermatol. 2018;78:596-597.e1.
Practice Points
- Frontal fibrosing alopecia (FFA) is associated with lichen planus pigmentosus, especially in patients with skin of color.
- Patients with FFA should be evaluated for additional cutaneous features including facial papules, glabellar red dots, and depressed frontal veins.