User login
Opioids and Opioid‐Related Adverse Events
Recent reports have drawn attention to the high and increasing rates of opioid prescribing and overdose‐related deaths in the United States.[1, 2, 3, 4, 5, 6, 7, 8, 9] These studies have focused on community‐based and emergency department prescribing, leaving prescribing practices in the inpatient setting unexamined. Given that pain is a frequent complaint in hospitalized patients, and that the Joint Commission mandates assessing pain as a vital sign, hospitalization is potentially a time of heightened use of such medications and could significantly contribute to nosocomial complications and subsequent outpatient use.[10] Variation in prescribing practices, unrelated to patient characteristics, could be a marker of inappropriate prescribing practices and poor quality of care.
Using a large, nationally representative cohort of admissions from July 2009 to June 2010, we sought to determine patterns and predictors of opioid utilization in nonsurgical admissions to US medical centers, hospital variation in use, and the association between hospital‐level use and the risk of opioid‐related adverse events. We hypothesized that hospitals with higher rates of opioid use would have an increased risk of an opioid‐related adverse event per patient exposed.
METHODS
Setting and Patients
We conducted a retrospective cohort study using data from 286 US nonfederal acute‐care facilities contributing to the database maintained by Premier (Premier Healthcare Solutions, Inc., Charlotte, NC). This database, created to measure healthcare utilization and quality of care, is drawn from voluntarily participating hospitals and contains data on approximately 1 in every 4 discharges nationwide.[11] Participating hospitals are similar in geographic distribution and metropolitan (urban/rural) status to hospitals nationwide, although large, nonteaching hospitals are slightly overrepresented in Premier. The database contains patient demographics, International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) codes, hospital demographics, and a date‐stamped log of all charges during the course of each hospitalization, including diagnostic tests, therapeutic treatments, and medications with dose and route of administration. The study was approved by the institutional review board at Beth Israel Deaconess Medical Center and granted a waiver of informed consent.
We studied a cohort of all adult nonsurgical admissions to participating hospitals from July 1, 2009, through June 30, 2010. We chose to study nonsurgical admissions, as patients undergoing surgical procedures have a clear indication for, and almost always receive, opioid pain medications. We defined a nonsurgical admission as an admission in which there were no charges for operating‐room procedures (including labor and delivery) and the attending of record was not a surgeon. We excluded admissions with unknown gender, since this is a key demographic variable, and admissions with a length of stay greater than 365 days, as these admissions are not representative of the typical admission to an acute‐care hospital. At the hospital level, we excluded hospitals contributing <100 admissions owing to resultant lack of precision in corresponding hospital prescribing rates, and hospitals that did not prescribe the full range of opioid medications (these hospitals had charges for codeine only), as these facilities seemed likely to have unusual limitations on prescribing or incomplete data capture.
Opioid Exposure
We defined opioid exposure as presence of 1 charge for an opioid medication during the admission. Opioid medications included morphine, hydrocodone, hydromorphone, oxycodone, fentanyl, meperidine, methadone, codeine, tramadol, buprenorphine, levorphanol, oxymorphone, pentazocine, propoxyphene, tapentadol, butorphanol, dezocine, and nalbuphine. We grouped the last 9 into an other category owing to infrequent use and/or differing characteristics from the main opioid drug types, such as synthetic, semisynthetic, and partial agonist qualities.
Severe Opioid‐Related Adverse Events
We defined severe opioid‐related adverse events as either naloxone exposure or an opioid‐related adverse drug event diagnosis code. Naloxone use in an adult patient exposed to opioids is one of the Institute for Healthcare Improvement's Trigger Tools for identifying adverse drug events[12] and previously has been demonstrated to have high positive predictive value for a confirmed adverse drug event.[13] We defined naloxone exposure as presence of 1 charge for naloxone. We excluded charges on hospital day 1 to focus on nosocomial events. We defined opioid‐related adverse drug events using ICD‐9‐CM diagnosis codes for poisoning by opioids (overdose, wrong substance given, or taken in error; ICD‐9‐CM 965.02, 965.09, E850.1, E850.2) and drugs causing adverse effects in therapeutic use (ICD‐9‐CM E935.1, E935.2), as specified in prior analyses by the Agency for Healthcare Research and Quality (AHRQ).[14, 15] To avoid capturing adverse events associated with outpatient use, we required the ICD‐9‐CM code to be qualified as not present on admission using the present on admission indicator required by the Centers for Medicare and Medicaid Services for all discharge diagnosis codes since 2008.[16]
Covariates of Interest
We were interested in the relationship between both patient and hospital characteristics and opioid exposure. Patient characteristics of interest included (1) demographic variables, such as age, sex, race (self‐reported by patients at the time of admission), marital status, and payer; (2) whether or not the patient spent any time in the intensive care unit (ICU); (3) comorbidities, identified via ICD‐9‐CM secondary diagnosis codes and diagnosis‐related groups using Healthcare Cost and Utilization Project Comorbidity Software, version 3.7, based on the work of Elixhauser et al[17, 18]; (4) primary ICD‐9‐CM discharge diagnosis groupings, selected based on hypothesized associations with receipt of opioids, and based on the Clinical Classifications Software (CCS)a diagnosis and procedure categorization scheme maintained by the AHRQ, and defined in the Appendix[19]; (5) and nonoperating‐room‐based procedures potentially necessitating opioids during the admission, selected from the 50 most common ICD‐9‐CM procedure codes in our cohort and grouped as cardiovascular procedures (catheterization and insertion of vascular stents), gastrointestinal procedures (upper and lower endoscopy), and mechanical ventilation, further defined in the Appendix. Hospital characteristics of interest included number of beds, population served (urban vs rural), teaching status, and US census region (Northeast, Midwest, South, West).
Statistical Analysis
We calculated the percent of admissions with exposure to any opioid and the percent exposed to each opioid, along with the total number of different opioid medications used during each admission. We also calculated the percent of admissions with parenteral administration and the percent of admissions with oral administration, among those exposed to the individual categories, and in aggregate. Because medications after discharge were unavailable in Premier's dataset, we report the percent of patients with a charge for opioids on the day of discharge.
We determined the daily dose of an opioid by taking the sum of the doses for that opioid charged on a given day. The average daily dose of an opioid was determined by taking the sum of the daily doses and dividing by the number of days on which 1 dose was charged. To facilitate comparison, all opioids, with the exception of those for which standard equivalences are unavailable (tramadol, other opioid category, oral fentanyl, epidural route for all), were converted to oral morphine equivalents using a standard equivalence conversion table.[20, 21] We excluded from our dosage calculations those charges for which standard morphine equivalence was unavailable, or for which dosage was missing. We also excluded from our dosage calculations any dose that was >3 standard deviations (SD) above the mean dose for that opioid, as such extreme values seemed physiologically implausible and more likely to be a data entry error that could lead to significant overestimation of the mean for that opioid.
All multivariable models used a generalized estimating equation (GEE) via the genmod procedure in SAS, with a Poisson distribution error term and a log link, controlling for repeated patient admissions with an autoregressive correlation structure.
To identify independent predictors of opioid receipt, we used a GEE model of opioid receipt where all patient and hospital characteristics listed in Table 1 were included as independent variables.
| Patient characteristics, N=1,139,419 | N | % |
|---|---|---|
| ||
| Age group, y | ||
| 1824 | 37,464 | 3 |
| 2534 | 66,541 | 6 |
| 3544 | 102,701 | 9 |
| 4554 | 174,830 | 15 |
| 5564 | 192,570 | 17 |
| 6574 | 196,407 | 17 |
| 75+ | 368,906 | 32 |
| Sex | ||
| Male | 527,062 | 46 |
| Female | 612,357 | 54 |
| Race | ||
| White | 711,993 | 62 |
| Black | 176,993 | 16 |
| Hispanic | 54,406 | 5 |
| Other | 196,027 | 17 |
| Marital status | ||
| Married | 427,648 | 38 |
| Single | 586,343 | 51 |
| Unknown/other | 125,428 | 11 |
| Primary insurance | ||
| Private/commercial | 269,725 | 24 |
| Medicare traditional | 502,301 | 44 |
| Medicare managed care | 126,344 | 11 |
| Medicaid | 125,025 | 11 |
| Self‐pay/other | 116,024 | 10 |
| ICU care | ||
| No | 1,023,027 | 90 |
| Yes | 116,392 | 10 |
| Comorbidities | ||
| AIDS | 5724 | 1 |
| Alcohol abuse | 79,633 | 7 |
| Deficiency anemias | 213,437 | 19 |
| RA/collagen vascular disease | 35,210 | 3 |
| Chronic blood‐loss anemia | 10,860 | 1 |
| CHF | 190,085 | 17 |
| Chronic pulmonary disease | 285,954 | 25 |
| Coagulopathy | 48,513 | 4 |
| Depression | 145,553 | 13 |
| DM without chronic complications | 270,087 | 24 |
| DM with chronic complications | 70,732 | 6 |
| Drug abuse | 66,886 | 6 |
| Hypertension | 696,299 | 61 |
| Hypothyroidism | 146,136 | 13 |
| Liver disease | 38,130 | 3 |
| Lymphoma | 14,032 | 1 |
| Fluid and electrolyte disorders | 326,576 | 29 |
| Metastatic cancer | 33,435 | 3 |
| Other neurological disorders | 124,195 | 11 |
| Obesity | 118,915 | 10 |
| Paralysis | 38,584 | 3 |
| PVD | 77,334 | 7 |
| Psychoses | 101,856 | 9 |
| Pulmonary circulation disease | 52,106 | 5 |
| Renal failure | 175,398 | 15 |
| Solid tumor without metastasis | 29,594 | 3 |
| Peptic ulcer disease excluding bleeding | 536 | 0 |
| Valvular disease | 86,616 | 8 |
| Weight loss | 45,132 | 4 |
| Primary discharge diagnoses | ||
| Cancer | 19,168 | 2 |
| Musculoskeletal injuries | 16,798 | 1 |
| Pain‐related diagnosesb | 101,533 | 9 |
| Alcohol‐related disorders | 16,777 | 1 |
| Substance‐related disorders | 13,697 | 1 |
| Psychiatric disorders | 41,153 | 4 |
| Mood disorders | 28,761 | 3 |
| Schizophrenia and other psychotic disorders | 12,392 | 1 |
| Procedures | ||
| Cardiovascular procedures | 59,901 | 5 |
| GI procedures | 31,224 | 3 |
| Mechanical ventilation | 7853 | 1 |
| Hospital characteristics, N=286 | ||
| Number of beds | ||
| <200 | 103 | 36 |
| 201300 | 63 | 22 |
| 301500 | 81 | 28 |
| >500 | 39 | 14 |
| Population served | ||
| Urban | 225 | 79 |
| Rural | 61 | 21 |
| Teaching status | ||
| Nonteaching | 207 | 72 |
| Teaching | 79 | 28 |
| US Census region | ||
| Northeast | 47 | 16 |
| Midwest | 63 | 22 |
| South | 115 | 40 |
| West | 61 | 21 |
To assess hospital variation in opioid prescribing after adjusting for patient characteristics, we used a GEE model of opioid receipt, controlling for all patient characteristics listed in Table 1. We then took the mean of the predicted probabilities of opioid receipt for the patients within each hospital in our cohort to derive the hospital prescribing rate adjusted for patient characteristics. We report the mean, SD, and range of the prescribing rates for the hospitals in our cohort before and after adjustment for patient characteristics.
To assess whether patients admitted to hospitals with higher rates of opioid prescribing have higher relative risk of severe opioid‐related adverse events, we stratified hospitals into opioid‐prescribing rate quartiles and compared the rates of opioid‐related adverse eventsboth overall and among opioid exposedbetween quartiles. To adjust for patient characteristics, we used a GEE model in which severe opioid‐related adverse event (yes/no) was the dependent variable and hospital‐prescribing rate quartile and all patient characteristics in Table 1 were independent variables. We also performed a sensitivity analysis in which we assessed the association between hospital prescribing‐rate quartile and the individual components of our composite outcome. Our results were qualitatively unchanged using this approach, and only the results of our main analysis are presented.
All analyses were carried out using SAS software, version 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
Patient Admission Characteristics
There were 3,190,934 adult admissions to 300 acute‐care hospitals during our study period. After excluding admissions with a length of stay >365 days (n=25), missing patient sex (n=17), and charges for operating‐room procedures or a surgical attending of record (n=2,018,553), 1,172,339 admissions were available for analysis. There were 12 hospitals with incomplete opioid‐prescribing data (n=32,794) and 2 hospitals that contributed <100 admissions each (n=126), leaving 1,139,419 admissions in 286 hospitals in our analytic cohort. The median age of the cohort was 64 years (interquartile range, 4979 years), and 527,062 (46%) were men. Table 1 shows the characteristics of the admissions in the cohort.
Rate, Route, and Dose of Opioid Exposures
Overall, there were 576,373 (51%) admissions with charges for opioid medications. Among those exposed, 244,760 (43%) had charges for multiple opioids during the admission; 172,090 (30%) had charges for 2 different opioids; and 72,670 (13%) had charges for 3 different opioids. Table 2 shows the percent exposed to each opioid, the percent of exposed with parenteral and oral routes of administration, and the mean daily dose received in oral morphine equivalents.
| Exposed | Parenteral Administration | Oral Administration | Dose Received, in Oral Morphine Equivalents | |||||
|---|---|---|---|---|---|---|---|---|
| N | %a | N | %b | N | %b | Mean | SDc | |
| ||||||||
| All opioids | 576,373 | 51 | 378,771 | 66 | 371,796 | 65 | 68 | 185 |
| Morphine | 224,811 | 20 | 209,040 | 93 | 21,645 | 10 | 40 | 121 |
| Hydrocodone | 162,558 | 14 | 0 | 0 | 160,941 | 99 | 14 | 12 |
| Hydromorphone | 146,236 | 13 | 137,936 | 94 | 16,052 | 11 | 113 | 274 |
| Oxycodone | 126,733 | 11 | 0 | 0 | 125,033 | 99 | 26 | 37 |
| Fentanyl | 105,052 | 9 | 103,113 | 98 | 641 | 1 | 64 | 75 |
| Tramadol | 35,570 | 3 | 0 | 0 | 35,570 | 100 | ||
| Meperidine | 24,850 | 2 | 24,398 | 98 | 515 | 2 | 36 | 34 |
| Methadone | 15,302 | 1 | 370 | 2 | 14,781 | 97 | 337 | 384 |
| Codeine | 22,818 | 2 | 178 | 1 | 22,183 | 97 | 9 | 15 |
| Other | 45,469 | 4 | 5821 | 13 | 39,618 | 87 | ||
Among the medications/routes for which conversion to morphine equivalents was possible, dosage was missing in 39,728 out of 2,294,673 opioid charges (2%). The average daily dose received in oral morphine equivalents was 68 mg. A total dose of 50 mg per day was received in 39% of exposed, and a total dose of 100 mg per day was received in 23% of exposed. Among those exposed, 52% (26% of overall admissions) had charges for opioids on the day of discharge.
Rates of Opioid Use by Patient and Hospital Characteristics
Table 3 reports the association between admission characteristics and opioid use. Use was highest in patients between the ages of 25 and 54 years. Although use declined with age, 44% of admissions age 65 years had charges for opioid medication. After adjustment for patient demographics, comorbidities, and hospital characteristics, opioid use was more common in females than males, those age 2554 years compared with those older and younger, those of Caucasian race compared with non‐Caucasian race, and those with Medicare or Medicaid primary insurance. Among the primary discharge diagnoses, patients with musculoskeletal injuries, various specific and nonspecific pain‐related diagnoses, and cancer were significantly more likely to receive opioids than patients without these diagnoses, whereas patients with alcohol‐related disorders and psychiatric disorders were significantly less likely to receive opioids than patients without these diagnoses. Patients admitted to hospitals in the Midwest, South, and West were significantly more likely to receive opioid medications than patients in the Northeast.
| Exposed, N=576,373 | Unexposed, N=563,046 | % Exposed | Adjusted RRa | 95% CI | |
|---|---|---|---|---|---|
| |||||
| Patient characteristics | |||||
| Age group, y | |||||
| 1824 | 17,360 | 20,104 | 46 | (ref) | |
| 2534 | 37,793 | 28,748 | 57 | 1.17 | 1.16‐1.19 |
| 3544 | 60,712 | 41,989 | 59 | 1.16 | 1.15‐1.17 |
| 4554 | 103,798 | 71,032 | 59 | 1.11 | 1.09‐1.12 |
| 5564 | 108,256 | 84,314 | 56 | 1.00 | 0.98‐1.01 |
| 6574 | 98,110 | 98,297 | 50 | 0.84 | 0.83‐0.85 |
| 75+ | 150,344 | 218,562 | 41 | 0.71 | 0.70‐0.72 |
| Sex | |||||
| Male | 255,315 | 271,747 | 48 | (ref) | |
| Female | 321,058 | 291,299 | 52 | 1.11 | 1.10‐1.11 |
| Race | |||||
| White | 365,107 | 346,886 | 51 | (ref) | |
| Black | 92,013 | 84,980 | 52 | 0.93 | 0.92‐0.93 |
| Hispanic | 27,592 | 26,814 | 51 | 0.94 | 0.93‐0.94 |
| Other | 91,661 | 104,366 | 47 | 0.93 | 0.92‐0.93 |
| Marital status | |||||
| Married | 222,912 | 204,736 | 52 | (ref) | |
| Single | 297,742 | 288,601 | 51 | 1.00 | 0.99‐1.01 |
| Unknown/other | 55,719 | 69,709 | 44 | 0.94 | 0.93‐0.95 |
| Primary insurance | |||||
| Private/commercial | 143,954 | 125,771 | 53 | (ref) | |
| Medicare traditional | 236,114 | 266,187 | 47 | 1.10 | 1.09‐1.10 |
| Medicare managed care | 59,104 | 67,240 | 47 | 1.11 | 1.11‐1.12 |
| Medicaid | 73,583 | 51,442 | 59 | 1.13 | 1.12‐1.13 |
| Self‐pay/other | 63,618 | 52,406 | 55 | 1.03 | 1.02‐1.04 |
| ICU care | |||||
| No | 510,654 | 512,373 | 50 | (ref) | |
| Yes | 65,719 | 50,673 | 56 | 1.02 | 1.01‐1.03 |
| Comorbiditiesb | |||||
| AIDS | 3655 | 2069 | 64 | 1.09 | 1.07‐1.12 |
| Alcohol abuse | 35,112 | 44,521 | 44 | 0.92 | 0.91‐0.93 |
| Deficiency anemias | 115,842 | 97,595 | 54 | 1.08 | 1.08‐1.09 |
| RA/collagen vascular disease | 22,519 | 12,691 | 64 | 1.22 | 1.21‐1.23 |
| Chronic blood‐loss anemia | 6444 | 4416 | 59 | 1.04 | 1.02‐1.05 |
| CHF | 88,895 | 101,190 | 47 | 0.99 | 0.98‐0.99 |
| Chronic pulmonary disease | 153,667 | 132,287 | 54 | 1.08 | 1.08‐1.08 |
| Coagulopathy | 25,802 | 22,711 | 53 | 1.03 | 1.02‐1.04 |
| Depression | 83,051 | 62,502 | 57 | 1.08 | 1.08‐1.09 |
| DM without chronic complications | 136,184 | 133,903 | 50 | 0.99 | 0.99‐0.99 |
| DM with chronic complications | 38,696 | 32,036 | 55 | 1.04 | 1.03‐1.05 |
| Drug abuse | 37,202 | 29,684 | 56 | 1.14 | 1.13‐1.15 |
| Hypertension | 344,718 | 351,581 | 50 | 0.98 | 0.97‐0.98 |
| Hypothyroidism | 70,786 | 75,350 | 48 | 0.99 | 0.99‐0.99 |
| Liver disease | 24,067 | 14,063 | 63 | 1.15 | 1.14‐1.16 |
| Lymphoma | 7727 | 6305 | 55 | 1.16 | 1.14‐1.17 |
| Fluid and electrolyte disorders | 168,814 | 157,762 | 52 | 1.04 | 1.03‐1.04 |
| Metastatic cancer | 23,920 | 9515 | 72 | 1.40 | 1.39‐1.42 |
| Other neurological disorders | 51,091 | 73,104 | 41 | 0.87 | 0.86‐0.87 |
| Obesity | 69,584 | 49,331 | 59 | 1.05 | 1.04‐1.05 |
| Paralysis | 17,497 | 21,087 | 45 | 0.97 | 0.96‐0.98 |
| PVD | 42,176 | 35,158 | 55 | 1.11 | 1.11‐1.12 |
| Psychoses | 38,638 | 63,218 | 38 | 0.91 | 0.90‐0.92 |
| Pulmonary circulation disease | 26,656 | 25,450 | 51 | 1.05 | 1.04‐1.06 |
| Renal failure | 86,565 | 88,833 | 49 | 1.01 | 1.01‐1.02 |
| Solid tumor without metastasis | 16,258 | 13,336 | 55 | 1.14 | 1.13‐1.15 |
| Peptic ulcer disease excluding bleeding | 376 | 160 | 70 | 1.12 | 1.07‐1.18 |
| Valvular disease | 38,396 | 48,220 | 44 | 0.93 | 0.92‐0.94 |
| Weight loss | 25,724 | 19,408 | 57 | 1.09 | 1.08‐1.10 |
| Primary discharge diagnosesb | |||||
| Cancer | 13,986 | 5182 | 73 | 1.20 | 1.19‐1.21 |
| Musculoskeletal injuries | 14,638 | 2160 | 87 | 2.02 | 2.002.04 |
| Pain‐related diagnosesc | 64,656 | 36,877 | 64 | 1.20 | 1.20‐1.21 |
| Alcohol‐related disorders | 3425 | 13,352 | 20 | 0.46 | 0.44‐0.47 |
| Substance‐related disorders | 8680 | 5017 | 63 | 1.03 | 1.01‐1.04 |
| Psychiatric disorders | 7253 | 33,900 | 18 | 0.37 | 0.36‐0.38 |
| Mood disorders | 5943 | 22,818 | 21 | ||
| Schizophrenia and other psychotic disorders | 1310 | 11,082 | 11 | ||
| Proceduresb | |||||
| Cardiovascular procedures | 50,997 | 8904 | 85 | 1.80 | 1.79‐1.81 |
| GI procedures | 27,206 | 4018 | 87 | 1.70 | 1.69‐1.71 |
| Mechanical ventilation | 5341 | 2512 | 68 | 1.37 | 1.34‐1.39 |
| Hospital characteristics | |||||
| Number of beds | |||||
| <200 | 100,900 | 88,439 | 53 | (ref) | |
| 201300 | 104,213 | 99,995 | 51 | 0.95 | 0.95‐0.96 |
| 301500 | 215,340 | 209,104 | 51 | 0.94 | 0.94‐0.95 |
| >500 | 155,920 | 165,508 | 49 | 0.96 | 0.95‐0.96 |
| Population served | |||||
| Urban | 511,727 | 506,803 | 50 | (ref) | |
| Rural | 64,646 | 56,243 | 53 | 0.98 | 0.97‐0.99 |
| Teaching status | |||||
| Nonteaching | 366,623 | 343,581 | 52 | (ref) | |
| Teaching | 209,750 | 219,465 | 49 | 1.00 | 0.99‐1.01 |
| US Census region | |||||
| Northeast | 99,377 | 149,446 | 40 | (ref) | |
| Midwest | 123,194 | 120,322 | 51 | 1.26 | (1.25‐1.27) |
| South | 251,624 | 213,029 | 54 | 1.33 | (1.33‐1.34) |
| West | 102,178 | 80,249 | 56 | 1.37 | (1.36‐1.38) |
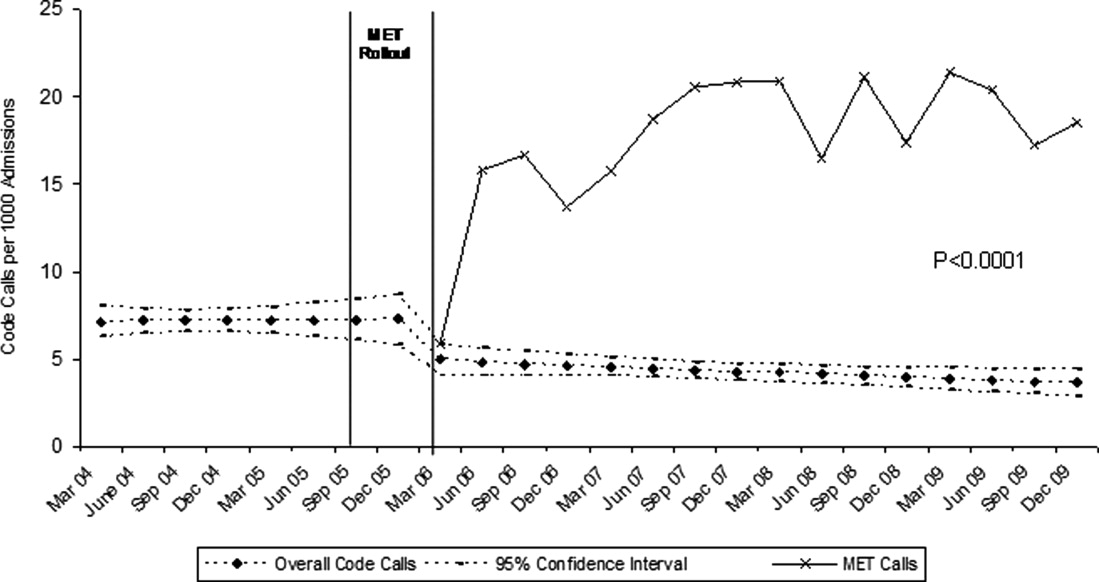
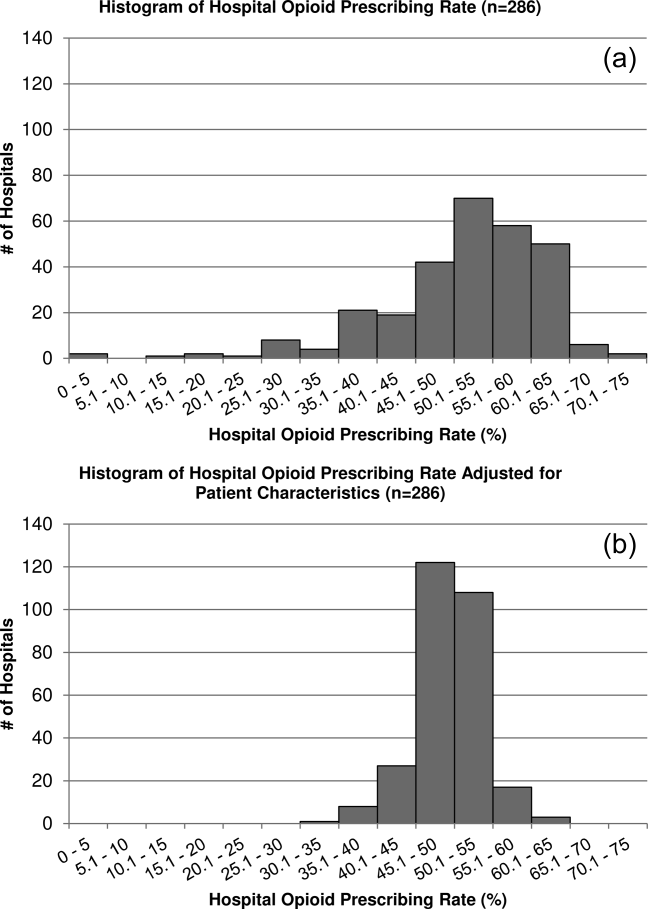
Variation in Opioid Prescribing
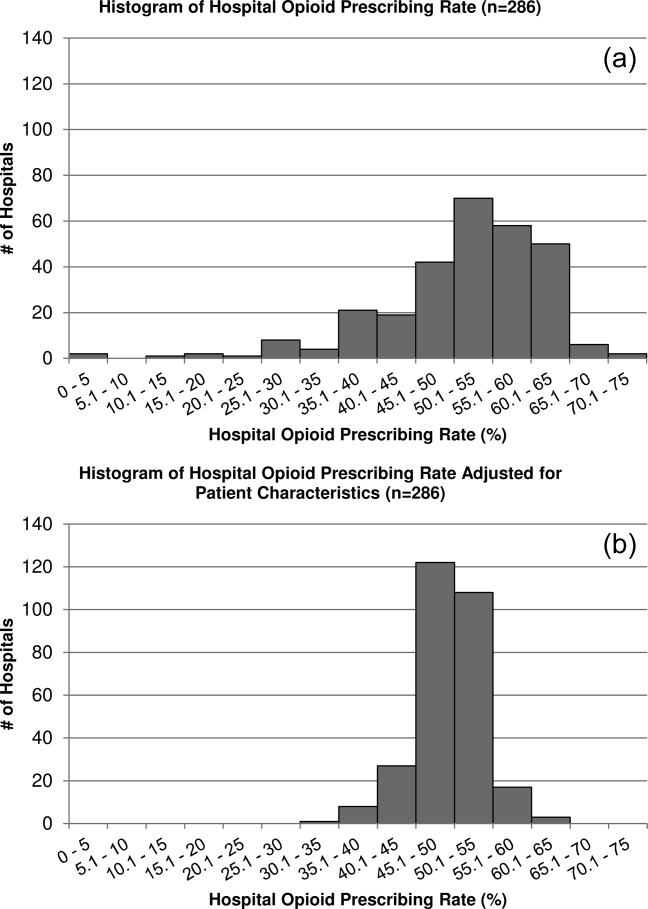
Figure 1 shows the histograms of hospital opioid prescribing rate for the 286 hospitals in our cohort (A) before and (B) after adjustment for patient characteristics. The observed rates ranged from 5% in the lowest‐prescribing hospital to 72% in the highest‐prescribing hospital, with a mean (SD) of 51% (10%). After adjusting for patient characteristics, the adjusted opioid‐prescribing rates ranged from 33% to 64%, with a mean (SD) of 50% (4%).
Severe Opioid‐Related Adverse Events
Among admissions with opioid exposure (n=576,373), naloxone use occurred in 2345 (0.41%) and opioid‐related adverse drug events in 1174 (0.20%), for a total of 3441 (0.60%) severe opioid‐related adverse events (some patients experienced both). Table 4 reports the opioid exposure and severe opioid‐related adverse‐event rates within hospital opioid‐prescribing rate quartiles, along with the adjusted association between the hospital opioid‐prescribing rate quartile and severe opioid‐related adverse events. After adjusting for patient characteristics, the relative risk of a severe opioid‐related adverse event was significantly greater in hospitals with higher opioid‐prescribing rates, both overall and among opioid exposed.
| Quartile | No. of Patients | Opioid Exposed, n (%) | Opioid‐Related Adverse Events, n (%) | Adjusted RR in All Patients, RR (95% CI), N=1,139,419a | Adjusted RR in Opioid Exposed, RR (95% CI), N=576,373a |
|---|---|---|---|---|---|
| |||||
| 1 | 349,747 | 132,824 (38) | 719 (0.21) | (ref) | (ref) |
| 2 | 266,652 | 134,590 (50) | 729 (0.27) | 1.31 (1.17‐1.45) | 1.07 (0.96‐1.18) |
| 3 | 251,042 | 139,770 (56) | 922 (0.37) | 1.72 (1.56‐1.90) | 1.31 (1.19‐1.44) |
| 4 | 271,978 | 169,189 (62) | 1071 (0.39) | 1.73 (1.57‐1.90) | 1.23 (1.12‐1.35) |
DISCUSSION
In this analysis of a large cohort of hospitalized nonsurgical patients, we found that more than half of all patients received opioids, with 43% of those exposed receiving multiple opioids during their admission and 52% receiving opioids on the day of discharge. Considerable hospital variation in opioid use was evident, and this was not fully explained by patient characteristics. Severe opioid‐related adverse events occurred more frequently at hospitals with higher opioid‐prescribing rates, and the relative risk of a severe adverse event per patient prescribed opioids was also higher in these hospitals. To our knowledge, this is the first study to describe the scope of opioid utilization and the relationship between utilization and severe opioid‐related adverse events in a sample of nonsurgical patients in US acute‐care facilities.
Our use of naloxone charges and opioid‐specific ICD‐9‐CM coding to define an opioid‐related adverse event was intended to capture only the most severe opioid‐related adverse events. We chose to focus on these events in our analysis to maximize the specificity of our outcome definition and thereby minimize confounding in our observed associations. The rate of less‐severe opioid‐related adverse events, such as nausea, constipation, and pruritis, is likely much higher and not captured in our outcome definition. Prior analyses have found variable rates of opioid‐related adverse events of approximately 1.8% to 13.6% of exposed patients.[22, 23, 24] However, these analyses focused on surgical patients and included less‐severe events. To our knowledge, ours is the first analysis of severe opioid‐related adverse events in nonsurgical patients.
Our finding that severe opioid‐related adverse events increase as opioid prescribing increases is consistent with that which has been demonstrated in the community setting, where rates of opioid‐related adverse events and mortality are higher in communities with higher levels of opioid prescribing.[2, 8, 25] This finding is expected, as greater use of a class of medications with known side effects would be expected to result in a higher overall rate of adverse events. More concerning, however, is the fact that this relationship persists when focusing exclusively on opioid‐exposed patients. Among similar patients receiving opioids at different hospitals, those hospitalized in facilities with higher opioid‐prescribing rates have higher rates of severe opioid‐related adverse events. This suggests that hospitals that use opioids more frequently do not do so more safely. Rather, the increased overall prescribing rates are associated with heightened risk for a serious adverse event per patient exposed and may reflect unsafe prescribing practices.
Furthermore, our results demonstrate both regional and hospital variation in use of opioids not fully explained by patient characteristics, similar to that which has been demonstrated for other drugs and heathcare services.[26, 27, 28, 29, 30] The implications of these findings are limited by our lack of information on pain severity or prior outpatient treatment, and our resultant inability to evaluate the appropriateness of opioid use in this analysis. Additionally, although we controlled for a large number of patient and hospital characteristics, there could be other significant predictors of use not accounted for in our analysis. However, it seems unlikely that differential pain severity or patient characteristics between patients in different regions of the country could fully explain a 37% relative difference in prescribing between the lowest‐ and highest‐prescribing regions, after accounting for the 44 patient‐level variables in our models. Whereas variation in use unrelated to patient factors could represent inappropriate prescribing practices, it could also be a marker of uncertainty regarding what constitutes appropriate prescribing and high‐quality care in this realm. Although guidelines advocate for standard pain assessments and a step‐up approach to treatment,[31, 32, 33] the lack of objective measures of pain severity and lack of evidence‐based recommendations on the use of opioids for noncancer pain[34] will almost certainly lead to persistent variation in opioid prescribing despite guideline‐driven care.
Nonetheless, our findings suggest that opportunities exist to make opioid prescribing safer in hospitalized patients. Studies aimed at elucidating the source of regional and hospital variation are necessary. Additionally, efforts should focus on identifying patient and prescribing characteristics associated with heightened risk of opioid‐related adverse events. Prior studies have demonstrated that the risks of opioid medications increase with increasing age of the patient.[35, 36] Although opioid use in our cohort declined with age, 44% of admissions age 65 years had charges for opioid medications. Studies in outpatients have also demonstrated that the risks of opioid overdose and overdose‐related death increase with dose.[5, 7] One study demonstrated a 3.7‐fold increased risk of overdose at doses of 5099 mg/day in oral morphine equivalents, and an 8.9‐fold increased risk at doses of 100 mg/day, compared with doses of 20 mg/day.[7] The prevalence of high dose exposure observed in our cohort, coupled with the older age of hospitalized patients, suggests potential targets for promoting safer use in hospitalized patients through interventions such as computerized decision support and enhanced monitoring in those at highest risk.
Because medications after discharge were unavailable in our dataset, the percentage of patients given a prescription for opioid medication on discharge is unknown. However, given that opioids are often tapered rather than abruptly discontinued, our finding that 26% of hospitalized nonsurgical patients received opioids on the day of discharge suggests that a substantial proportion of patients may be discharged with a prescription for opioid medication. Given the possibility of coexistent outpatient opioid prescriptions, these findings draw attention to the importance of assuring development and streamlined accessibility of data from state prescription drug monitoring programs and suggest that increased attention should be paid to the role that inpatient opioid prescribing plays in the increased rates of chronic opioid use and overdose‐related deaths in the United States.
There are additional limitations to our analysis. First, although the database used for this analysis captures a large proportion of admissions to US acute‐care facilities and is similar in composition, it is possible that participating medical centers differ from nonparticipating medical centers in ways that could be associated with opioid prescribing. Additionally, although Premier performs extensive validation and correction processes to assure the quality of their data, there is still likely to be a small amount of random error in the database, which could particularly impact dosage calculations. The lack of preadmission medications in our database precluded identification of the proportion of patients newly started on opioid medications. Lastly, it is possible that the hospital prescribing‐rate quartile is associated with patient characteristics unaccounted for in our analysis, and, therefore, the possibility of residual confounding still exists.
In conclusion, the majority of hospitalized nonsurgical patients are exposed to opioid medications during the course of their hospitalizations, often at high doses. More than half of those exposed are still receiving these medications on the day of discharge. We found hospital and regional variation in opioid use that was not fully explained by patient characteristics, and higher levels of hospital use were associated with higher risk of severe opioid‐related adverse events in opioid‐exposed patients. Further research is necessary to investigate the appropriateness of opioid use in this patient population, the sources of variation in use, and the predictors of opioid‐related adverse events in hospitalized patients to allow development of interventions to make hospital use safer.
Disclosures
Disclosures: Dr. Herzig had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Herzig was funded by grant no. K23AG042459 from the National Institute on Aging. Dr. Marcantonio was funded by grant nos. P01AG031720, R01AG030618, R03AG028189, and K24AG035075 from the National Institute on Aging. The funding organization had no involvement in any aspect of the study, including design, conduct, and reporting of the study. None of the authors have any conflicts of interest to disclose.
- . A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363(21):1981–1985.
- , , . Increasing deaths from opioid analgesics in the United States. Pharmacoepidemiol Drug Saf. 2006;15(9):618–627.
- , , , . Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA. 2008;299(1):70–78.
- , , , . Trends in medical use and abuse of opioid analgesics. JAMA. 2000;283(13):1710–1714.
- , , , et al. Association between opioid prescribing patterns and opioid overdose‐related deaths. JAMA. 2011;305(13):1315–1321.
- , , , et al. Prescription opioid mortality trends in New York City, 1990–2006: examining the emergence of an epidemic. Drug Alcohol Depend. 2013;132(1‐2):53–62.
- , , , et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85–92.
- , , , et al. Relationship of opioid prescription sales and overdoses, North Carolina. Drug Alcohol Depend. 2013;132(1‐2):81–86.
- Deaths from prescription opioids soar in New York. BMJ. 2013;346:f921.
- , , . Mortality in elderly dementia patients treated with risperidone. J Clin Psychopharmacol. 2006;26(6):566–570.
- , , , et al. Medication reconciliation: a practical tool to reduce the risk of medication errors. J Crit Care. 2003;18(4):201–205.
- , , . Adverse drug event trigger tool: a practical methodology for measuring medication related harm. Qual Saf Health Care. 2003;12(3):194–200.
- , , , , . Improvement in the detection of adverse drug events by the use of electronic health and prescription records: an evaluation of two trigger tools. Eur J Clin Pharmacol. 2013;69(2):255–259.
- , . Adverse Drug Events in U.S. Hospitals, 2004. Rockville, MD: Agency for Healthcare Research and Quality; April 2007. Statistical Brief 29.
- , , . Medication‐Related Adverse Outcomes in U.S. Hospitals and Emergency Departments, 2008. Rockville, MD: Agency for Healthcare Research and Quality; April 2011. Statistical Brief 109.
- US Centers for Medicare and Medicaid Services. Hospital‐Acquired Conditions (Present on Admission Indicator). Available at: http://www.cms.hhs.gov/HospitalAcqCond/. Accessed August 16, 2011. Updated September 20, 2012.
- , , . Fracture risk associated with the use of morphine and opiates. J Intern Med. 2006;260(1):76–87.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
- Clinical Classifications Software, Healthcare Cost and Utilization Project (HCUP). Rockville, MD; Agency for Healthcare Research and Quality; December 2009.
- , , , , . Clinical application of opioid equianalgesic data. Clin J Pain. 2003;19(5)286–297.
- , , , , , . Choosing the unit of measurement counts: the use of oral morphine equivalents in studies of opioid consumption is a useful addition to defined daily doses. Palliat Med. 2011;25(7):725–732.
- , , , . Cost and quality implications of opioid‐based postsurgical pain control using administrative claims data from a large health system: opioid‐related adverse events and their impact on clinical and economic outcomes. Pharmacotherapy. 2013;33(4):383–391.
- , , , et al. Opioid‐related adverse drug events in surgical hospitalizations: impact on costs and length of stay. Ann Pharmacother. 2007;41(3):400–406.
- , , , et al. Cost of opioid‐related adverse drug events in surgical patients. J Pain Symptom Manage. 2003;25(3):276–283.
- , . Opioid analgesics and rates of fatal drug poisoning in the United States. Am J Prev Med. 2006;31(6):506–511.
- , , , et al. Geographic variation in the treatment of acute myocardial infarction: the Cooperative Cardiovascular Project. JAMA. 1999;281(7):627–633.
- , , , et al; GUSTO‐1 Investigators. Regional variation across the United States in the management of acute myocardial infarction. Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries. N Engl J Med. 1995;333(9):565–572.
- , , . Predictors of broad‐spectrum antibiotic prescribing for acute respiratory tract infections in adult primary care. JAMA. 2003;289(6):719–725.
- , , . Geographic variation in Medicare drug spending. N Engl J Med. 2010;363(5):405–409.
- , , . Geographic variation in outpatient antibiotic prescribing among older adults. Arch Intern Med. 2012;172(19):1465–1471.
- , , , et al. Clinical policy: critical issues in the prescribing of opioids for adult patients in the emergency department. Ann Emerg Med. 2012;60(4):499–525.
- The Joint Commission. Facts About Pain Management. Available at: http://www.jointcommission.org/pain_management/. Accessed July 23, 2012.
- The Joint Commission. Sentinel Event Alert: Safe Use of Opioids in Hospitals. Published August 8, 2012. Available at: http://www.jointcommission.org/assets/1/18/SEA_49_opioids_8_2_12_final.pdf. Accessed March 4, 2013.
- , , , , . Research gaps on use of opioids for chronic noncancer pain: findings from a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10(2):147–159.
- , , , , , . Side effects of opioids during short‐term administration: effect of age, gender, and race. Clin Pharmacol Ther. 2003;74(2):102–112.
- , , , . Postoperative day one: a high‐risk period for respiratory events. Am J Surg. 2005;190(5):752–756.
Recent reports have drawn attention to the high and increasing rates of opioid prescribing and overdose‐related deaths in the United States.[1, 2, 3, 4, 5, 6, 7, 8, 9] These studies have focused on community‐based and emergency department prescribing, leaving prescribing practices in the inpatient setting unexamined. Given that pain is a frequent complaint in hospitalized patients, and that the Joint Commission mandates assessing pain as a vital sign, hospitalization is potentially a time of heightened use of such medications and could significantly contribute to nosocomial complications and subsequent outpatient use.[10] Variation in prescribing practices, unrelated to patient characteristics, could be a marker of inappropriate prescribing practices and poor quality of care.
Using a large, nationally representative cohort of admissions from July 2009 to June 2010, we sought to determine patterns and predictors of opioid utilization in nonsurgical admissions to US medical centers, hospital variation in use, and the association between hospital‐level use and the risk of opioid‐related adverse events. We hypothesized that hospitals with higher rates of opioid use would have an increased risk of an opioid‐related adverse event per patient exposed.
METHODS
Setting and Patients
We conducted a retrospective cohort study using data from 286 US nonfederal acute‐care facilities contributing to the database maintained by Premier (Premier Healthcare Solutions, Inc., Charlotte, NC). This database, created to measure healthcare utilization and quality of care, is drawn from voluntarily participating hospitals and contains data on approximately 1 in every 4 discharges nationwide.[11] Participating hospitals are similar in geographic distribution and metropolitan (urban/rural) status to hospitals nationwide, although large, nonteaching hospitals are slightly overrepresented in Premier. The database contains patient demographics, International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) codes, hospital demographics, and a date‐stamped log of all charges during the course of each hospitalization, including diagnostic tests, therapeutic treatments, and medications with dose and route of administration. The study was approved by the institutional review board at Beth Israel Deaconess Medical Center and granted a waiver of informed consent.
We studied a cohort of all adult nonsurgical admissions to participating hospitals from July 1, 2009, through June 30, 2010. We chose to study nonsurgical admissions, as patients undergoing surgical procedures have a clear indication for, and almost always receive, opioid pain medications. We defined a nonsurgical admission as an admission in which there were no charges for operating‐room procedures (including labor and delivery) and the attending of record was not a surgeon. We excluded admissions with unknown gender, since this is a key demographic variable, and admissions with a length of stay greater than 365 days, as these admissions are not representative of the typical admission to an acute‐care hospital. At the hospital level, we excluded hospitals contributing <100 admissions owing to resultant lack of precision in corresponding hospital prescribing rates, and hospitals that did not prescribe the full range of opioid medications (these hospitals had charges for codeine only), as these facilities seemed likely to have unusual limitations on prescribing or incomplete data capture.
Opioid Exposure
We defined opioid exposure as presence of 1 charge for an opioid medication during the admission. Opioid medications included morphine, hydrocodone, hydromorphone, oxycodone, fentanyl, meperidine, methadone, codeine, tramadol, buprenorphine, levorphanol, oxymorphone, pentazocine, propoxyphene, tapentadol, butorphanol, dezocine, and nalbuphine. We grouped the last 9 into an other category owing to infrequent use and/or differing characteristics from the main opioid drug types, such as synthetic, semisynthetic, and partial agonist qualities.
Severe Opioid‐Related Adverse Events
We defined severe opioid‐related adverse events as either naloxone exposure or an opioid‐related adverse drug event diagnosis code. Naloxone use in an adult patient exposed to opioids is one of the Institute for Healthcare Improvement's Trigger Tools for identifying adverse drug events[12] and previously has been demonstrated to have high positive predictive value for a confirmed adverse drug event.[13] We defined naloxone exposure as presence of 1 charge for naloxone. We excluded charges on hospital day 1 to focus on nosocomial events. We defined opioid‐related adverse drug events using ICD‐9‐CM diagnosis codes for poisoning by opioids (overdose, wrong substance given, or taken in error; ICD‐9‐CM 965.02, 965.09, E850.1, E850.2) and drugs causing adverse effects in therapeutic use (ICD‐9‐CM E935.1, E935.2), as specified in prior analyses by the Agency for Healthcare Research and Quality (AHRQ).[14, 15] To avoid capturing adverse events associated with outpatient use, we required the ICD‐9‐CM code to be qualified as not present on admission using the present on admission indicator required by the Centers for Medicare and Medicaid Services for all discharge diagnosis codes since 2008.[16]
Covariates of Interest
We were interested in the relationship between both patient and hospital characteristics and opioid exposure. Patient characteristics of interest included (1) demographic variables, such as age, sex, race (self‐reported by patients at the time of admission), marital status, and payer; (2) whether or not the patient spent any time in the intensive care unit (ICU); (3) comorbidities, identified via ICD‐9‐CM secondary diagnosis codes and diagnosis‐related groups using Healthcare Cost and Utilization Project Comorbidity Software, version 3.7, based on the work of Elixhauser et al[17, 18]; (4) primary ICD‐9‐CM discharge diagnosis groupings, selected based on hypothesized associations with receipt of opioids, and based on the Clinical Classifications Software (CCS)a diagnosis and procedure categorization scheme maintained by the AHRQ, and defined in the Appendix[19]; (5) and nonoperating‐room‐based procedures potentially necessitating opioids during the admission, selected from the 50 most common ICD‐9‐CM procedure codes in our cohort and grouped as cardiovascular procedures (catheterization and insertion of vascular stents), gastrointestinal procedures (upper and lower endoscopy), and mechanical ventilation, further defined in the Appendix. Hospital characteristics of interest included number of beds, population served (urban vs rural), teaching status, and US census region (Northeast, Midwest, South, West).
Statistical Analysis
We calculated the percent of admissions with exposure to any opioid and the percent exposed to each opioid, along with the total number of different opioid medications used during each admission. We also calculated the percent of admissions with parenteral administration and the percent of admissions with oral administration, among those exposed to the individual categories, and in aggregate. Because medications after discharge were unavailable in Premier's dataset, we report the percent of patients with a charge for opioids on the day of discharge.
We determined the daily dose of an opioid by taking the sum of the doses for that opioid charged on a given day. The average daily dose of an opioid was determined by taking the sum of the daily doses and dividing by the number of days on which 1 dose was charged. To facilitate comparison, all opioids, with the exception of those for which standard equivalences are unavailable (tramadol, other opioid category, oral fentanyl, epidural route for all), were converted to oral morphine equivalents using a standard equivalence conversion table.[20, 21] We excluded from our dosage calculations those charges for which standard morphine equivalence was unavailable, or for which dosage was missing. We also excluded from our dosage calculations any dose that was >3 standard deviations (SD) above the mean dose for that opioid, as such extreme values seemed physiologically implausible and more likely to be a data entry error that could lead to significant overestimation of the mean for that opioid.
All multivariable models used a generalized estimating equation (GEE) via the genmod procedure in SAS, with a Poisson distribution error term and a log link, controlling for repeated patient admissions with an autoregressive correlation structure.
To identify independent predictors of opioid receipt, we used a GEE model of opioid receipt where all patient and hospital characteristics listed in Table 1 were included as independent variables.
| Patient characteristics, N=1,139,419 | N | % |
|---|---|---|
| ||
| Age group, y | ||
| 1824 | 37,464 | 3 |
| 2534 | 66,541 | 6 |
| 3544 | 102,701 | 9 |
| 4554 | 174,830 | 15 |
| 5564 | 192,570 | 17 |
| 6574 | 196,407 | 17 |
| 75+ | 368,906 | 32 |
| Sex | ||
| Male | 527,062 | 46 |
| Female | 612,357 | 54 |
| Race | ||
| White | 711,993 | 62 |
| Black | 176,993 | 16 |
| Hispanic | 54,406 | 5 |
| Other | 196,027 | 17 |
| Marital status | ||
| Married | 427,648 | 38 |
| Single | 586,343 | 51 |
| Unknown/other | 125,428 | 11 |
| Primary insurance | ||
| Private/commercial | 269,725 | 24 |
| Medicare traditional | 502,301 | 44 |
| Medicare managed care | 126,344 | 11 |
| Medicaid | 125,025 | 11 |
| Self‐pay/other | 116,024 | 10 |
| ICU care | ||
| No | 1,023,027 | 90 |
| Yes | 116,392 | 10 |
| Comorbidities | ||
| AIDS | 5724 | 1 |
| Alcohol abuse | 79,633 | 7 |
| Deficiency anemias | 213,437 | 19 |
| RA/collagen vascular disease | 35,210 | 3 |
| Chronic blood‐loss anemia | 10,860 | 1 |
| CHF | 190,085 | 17 |
| Chronic pulmonary disease | 285,954 | 25 |
| Coagulopathy | 48,513 | 4 |
| Depression | 145,553 | 13 |
| DM without chronic complications | 270,087 | 24 |
| DM with chronic complications | 70,732 | 6 |
| Drug abuse | 66,886 | 6 |
| Hypertension | 696,299 | 61 |
| Hypothyroidism | 146,136 | 13 |
| Liver disease | 38,130 | 3 |
| Lymphoma | 14,032 | 1 |
| Fluid and electrolyte disorders | 326,576 | 29 |
| Metastatic cancer | 33,435 | 3 |
| Other neurological disorders | 124,195 | 11 |
| Obesity | 118,915 | 10 |
| Paralysis | 38,584 | 3 |
| PVD | 77,334 | 7 |
| Psychoses | 101,856 | 9 |
| Pulmonary circulation disease | 52,106 | 5 |
| Renal failure | 175,398 | 15 |
| Solid tumor without metastasis | 29,594 | 3 |
| Peptic ulcer disease excluding bleeding | 536 | 0 |
| Valvular disease | 86,616 | 8 |
| Weight loss | 45,132 | 4 |
| Primary discharge diagnoses | ||
| Cancer | 19,168 | 2 |
| Musculoskeletal injuries | 16,798 | 1 |
| Pain‐related diagnosesb | 101,533 | 9 |
| Alcohol‐related disorders | 16,777 | 1 |
| Substance‐related disorders | 13,697 | 1 |
| Psychiatric disorders | 41,153 | 4 |
| Mood disorders | 28,761 | 3 |
| Schizophrenia and other psychotic disorders | 12,392 | 1 |
| Procedures | ||
| Cardiovascular procedures | 59,901 | 5 |
| GI procedures | 31,224 | 3 |
| Mechanical ventilation | 7853 | 1 |
| Hospital characteristics, N=286 | ||
| Number of beds | ||
| <200 | 103 | 36 |
| 201300 | 63 | 22 |
| 301500 | 81 | 28 |
| >500 | 39 | 14 |
| Population served | ||
| Urban | 225 | 79 |
| Rural | 61 | 21 |
| Teaching status | ||
| Nonteaching | 207 | 72 |
| Teaching | 79 | 28 |
| US Census region | ||
| Northeast | 47 | 16 |
| Midwest | 63 | 22 |
| South | 115 | 40 |
| West | 61 | 21 |
To assess hospital variation in opioid prescribing after adjusting for patient characteristics, we used a GEE model of opioid receipt, controlling for all patient characteristics listed in Table 1. We then took the mean of the predicted probabilities of opioid receipt for the patients within each hospital in our cohort to derive the hospital prescribing rate adjusted for patient characteristics. We report the mean, SD, and range of the prescribing rates for the hospitals in our cohort before and after adjustment for patient characteristics.
To assess whether patients admitted to hospitals with higher rates of opioid prescribing have higher relative risk of severe opioid‐related adverse events, we stratified hospitals into opioid‐prescribing rate quartiles and compared the rates of opioid‐related adverse eventsboth overall and among opioid exposedbetween quartiles. To adjust for patient characteristics, we used a GEE model in which severe opioid‐related adverse event (yes/no) was the dependent variable and hospital‐prescribing rate quartile and all patient characteristics in Table 1 were independent variables. We also performed a sensitivity analysis in which we assessed the association between hospital prescribing‐rate quartile and the individual components of our composite outcome. Our results were qualitatively unchanged using this approach, and only the results of our main analysis are presented.
All analyses were carried out using SAS software, version 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
Patient Admission Characteristics
There were 3,190,934 adult admissions to 300 acute‐care hospitals during our study period. After excluding admissions with a length of stay >365 days (n=25), missing patient sex (n=17), and charges for operating‐room procedures or a surgical attending of record (n=2,018,553), 1,172,339 admissions were available for analysis. There were 12 hospitals with incomplete opioid‐prescribing data (n=32,794) and 2 hospitals that contributed <100 admissions each (n=126), leaving 1,139,419 admissions in 286 hospitals in our analytic cohort. The median age of the cohort was 64 years (interquartile range, 4979 years), and 527,062 (46%) were men. Table 1 shows the characteristics of the admissions in the cohort.
Rate, Route, and Dose of Opioid Exposures
Overall, there were 576,373 (51%) admissions with charges for opioid medications. Among those exposed, 244,760 (43%) had charges for multiple opioids during the admission; 172,090 (30%) had charges for 2 different opioids; and 72,670 (13%) had charges for 3 different opioids. Table 2 shows the percent exposed to each opioid, the percent of exposed with parenteral and oral routes of administration, and the mean daily dose received in oral morphine equivalents.
| Exposed | Parenteral Administration | Oral Administration | Dose Received, in Oral Morphine Equivalents | |||||
|---|---|---|---|---|---|---|---|---|
| N | %a | N | %b | N | %b | Mean | SDc | |
| ||||||||
| All opioids | 576,373 | 51 | 378,771 | 66 | 371,796 | 65 | 68 | 185 |
| Morphine | 224,811 | 20 | 209,040 | 93 | 21,645 | 10 | 40 | 121 |
| Hydrocodone | 162,558 | 14 | 0 | 0 | 160,941 | 99 | 14 | 12 |
| Hydromorphone | 146,236 | 13 | 137,936 | 94 | 16,052 | 11 | 113 | 274 |
| Oxycodone | 126,733 | 11 | 0 | 0 | 125,033 | 99 | 26 | 37 |
| Fentanyl | 105,052 | 9 | 103,113 | 98 | 641 | 1 | 64 | 75 |
| Tramadol | 35,570 | 3 | 0 | 0 | 35,570 | 100 | ||
| Meperidine | 24,850 | 2 | 24,398 | 98 | 515 | 2 | 36 | 34 |
| Methadone | 15,302 | 1 | 370 | 2 | 14,781 | 97 | 337 | 384 |
| Codeine | 22,818 | 2 | 178 | 1 | 22,183 | 97 | 9 | 15 |
| Other | 45,469 | 4 | 5821 | 13 | 39,618 | 87 | ||
Among the medications/routes for which conversion to morphine equivalents was possible, dosage was missing in 39,728 out of 2,294,673 opioid charges (2%). The average daily dose received in oral morphine equivalents was 68 mg. A total dose of 50 mg per day was received in 39% of exposed, and a total dose of 100 mg per day was received in 23% of exposed. Among those exposed, 52% (26% of overall admissions) had charges for opioids on the day of discharge.
Rates of Opioid Use by Patient and Hospital Characteristics
Table 3 reports the association between admission characteristics and opioid use. Use was highest in patients between the ages of 25 and 54 years. Although use declined with age, 44% of admissions age 65 years had charges for opioid medication. After adjustment for patient demographics, comorbidities, and hospital characteristics, opioid use was more common in females than males, those age 2554 years compared with those older and younger, those of Caucasian race compared with non‐Caucasian race, and those with Medicare or Medicaid primary insurance. Among the primary discharge diagnoses, patients with musculoskeletal injuries, various specific and nonspecific pain‐related diagnoses, and cancer were significantly more likely to receive opioids than patients without these diagnoses, whereas patients with alcohol‐related disorders and psychiatric disorders were significantly less likely to receive opioids than patients without these diagnoses. Patients admitted to hospitals in the Midwest, South, and West were significantly more likely to receive opioid medications than patients in the Northeast.
| Exposed, N=576,373 | Unexposed, N=563,046 | % Exposed | Adjusted RRa | 95% CI | |
|---|---|---|---|---|---|
| |||||
| Patient characteristics | |||||
| Age group, y | |||||
| 1824 | 17,360 | 20,104 | 46 | (ref) | |
| 2534 | 37,793 | 28,748 | 57 | 1.17 | 1.16‐1.19 |
| 3544 | 60,712 | 41,989 | 59 | 1.16 | 1.15‐1.17 |
| 4554 | 103,798 | 71,032 | 59 | 1.11 | 1.09‐1.12 |
| 5564 | 108,256 | 84,314 | 56 | 1.00 | 0.98‐1.01 |
| 6574 | 98,110 | 98,297 | 50 | 0.84 | 0.83‐0.85 |
| 75+ | 150,344 | 218,562 | 41 | 0.71 | 0.70‐0.72 |
| Sex | |||||
| Male | 255,315 | 271,747 | 48 | (ref) | |
| Female | 321,058 | 291,299 | 52 | 1.11 | 1.10‐1.11 |
| Race | |||||
| White | 365,107 | 346,886 | 51 | (ref) | |
| Black | 92,013 | 84,980 | 52 | 0.93 | 0.92‐0.93 |
| Hispanic | 27,592 | 26,814 | 51 | 0.94 | 0.93‐0.94 |
| Other | 91,661 | 104,366 | 47 | 0.93 | 0.92‐0.93 |
| Marital status | |||||
| Married | 222,912 | 204,736 | 52 | (ref) | |
| Single | 297,742 | 288,601 | 51 | 1.00 | 0.99‐1.01 |
| Unknown/other | 55,719 | 69,709 | 44 | 0.94 | 0.93‐0.95 |
| Primary insurance | |||||
| Private/commercial | 143,954 | 125,771 | 53 | (ref) | |
| Medicare traditional | 236,114 | 266,187 | 47 | 1.10 | 1.09‐1.10 |
| Medicare managed care | 59,104 | 67,240 | 47 | 1.11 | 1.11‐1.12 |
| Medicaid | 73,583 | 51,442 | 59 | 1.13 | 1.12‐1.13 |
| Self‐pay/other | 63,618 | 52,406 | 55 | 1.03 | 1.02‐1.04 |
| ICU care | |||||
| No | 510,654 | 512,373 | 50 | (ref) | |
| Yes | 65,719 | 50,673 | 56 | 1.02 | 1.01‐1.03 |
| Comorbiditiesb | |||||
| AIDS | 3655 | 2069 | 64 | 1.09 | 1.07‐1.12 |
| Alcohol abuse | 35,112 | 44,521 | 44 | 0.92 | 0.91‐0.93 |
| Deficiency anemias | 115,842 | 97,595 | 54 | 1.08 | 1.08‐1.09 |
| RA/collagen vascular disease | 22,519 | 12,691 | 64 | 1.22 | 1.21‐1.23 |
| Chronic blood‐loss anemia | 6444 | 4416 | 59 | 1.04 | 1.02‐1.05 |
| CHF | 88,895 | 101,190 | 47 | 0.99 | 0.98‐0.99 |
| Chronic pulmonary disease | 153,667 | 132,287 | 54 | 1.08 | 1.08‐1.08 |
| Coagulopathy | 25,802 | 22,711 | 53 | 1.03 | 1.02‐1.04 |
| Depression | 83,051 | 62,502 | 57 | 1.08 | 1.08‐1.09 |
| DM without chronic complications | 136,184 | 133,903 | 50 | 0.99 | 0.99‐0.99 |
| DM with chronic complications | 38,696 | 32,036 | 55 | 1.04 | 1.03‐1.05 |
| Drug abuse | 37,202 | 29,684 | 56 | 1.14 | 1.13‐1.15 |
| Hypertension | 344,718 | 351,581 | 50 | 0.98 | 0.97‐0.98 |
| Hypothyroidism | 70,786 | 75,350 | 48 | 0.99 | 0.99‐0.99 |
| Liver disease | 24,067 | 14,063 | 63 | 1.15 | 1.14‐1.16 |
| Lymphoma | 7727 | 6305 | 55 | 1.16 | 1.14‐1.17 |
| Fluid and electrolyte disorders | 168,814 | 157,762 | 52 | 1.04 | 1.03‐1.04 |
| Metastatic cancer | 23,920 | 9515 | 72 | 1.40 | 1.39‐1.42 |
| Other neurological disorders | 51,091 | 73,104 | 41 | 0.87 | 0.86‐0.87 |
| Obesity | 69,584 | 49,331 | 59 | 1.05 | 1.04‐1.05 |
| Paralysis | 17,497 | 21,087 | 45 | 0.97 | 0.96‐0.98 |
| PVD | 42,176 | 35,158 | 55 | 1.11 | 1.11‐1.12 |
| Psychoses | 38,638 | 63,218 | 38 | 0.91 | 0.90‐0.92 |
| Pulmonary circulation disease | 26,656 | 25,450 | 51 | 1.05 | 1.04‐1.06 |
| Renal failure | 86,565 | 88,833 | 49 | 1.01 | 1.01‐1.02 |
| Solid tumor without metastasis | 16,258 | 13,336 | 55 | 1.14 | 1.13‐1.15 |
| Peptic ulcer disease excluding bleeding | 376 | 160 | 70 | 1.12 | 1.07‐1.18 |
| Valvular disease | 38,396 | 48,220 | 44 | 0.93 | 0.92‐0.94 |
| Weight loss | 25,724 | 19,408 | 57 | 1.09 | 1.08‐1.10 |
| Primary discharge diagnosesb | |||||
| Cancer | 13,986 | 5182 | 73 | 1.20 | 1.19‐1.21 |
| Musculoskeletal injuries | 14,638 | 2160 | 87 | 2.02 | 2.002.04 |
| Pain‐related diagnosesc | 64,656 | 36,877 | 64 | 1.20 | 1.20‐1.21 |
| Alcohol‐related disorders | 3425 | 13,352 | 20 | 0.46 | 0.44‐0.47 |
| Substance‐related disorders | 8680 | 5017 | 63 | 1.03 | 1.01‐1.04 |
| Psychiatric disorders | 7253 | 33,900 | 18 | 0.37 | 0.36‐0.38 |
| Mood disorders | 5943 | 22,818 | 21 | ||
| Schizophrenia and other psychotic disorders | 1310 | 11,082 | 11 | ||
| Proceduresb | |||||
| Cardiovascular procedures | 50,997 | 8904 | 85 | 1.80 | 1.79‐1.81 |
| GI procedures | 27,206 | 4018 | 87 | 1.70 | 1.69‐1.71 |
| Mechanical ventilation | 5341 | 2512 | 68 | 1.37 | 1.34‐1.39 |
| Hospital characteristics | |||||
| Number of beds | |||||
| <200 | 100,900 | 88,439 | 53 | (ref) | |
| 201300 | 104,213 | 99,995 | 51 | 0.95 | 0.95‐0.96 |
| 301500 | 215,340 | 209,104 | 51 | 0.94 | 0.94‐0.95 |
| >500 | 155,920 | 165,508 | 49 | 0.96 | 0.95‐0.96 |
| Population served | |||||
| Urban | 511,727 | 506,803 | 50 | (ref) | |
| Rural | 64,646 | 56,243 | 53 | 0.98 | 0.97‐0.99 |
| Teaching status | |||||
| Nonteaching | 366,623 | 343,581 | 52 | (ref) | |
| Teaching | 209,750 | 219,465 | 49 | 1.00 | 0.99‐1.01 |
| US Census region | |||||
| Northeast | 99,377 | 149,446 | 40 | (ref) | |
| Midwest | 123,194 | 120,322 | 51 | 1.26 | (1.25‐1.27) |
| South | 251,624 | 213,029 | 54 | 1.33 | (1.33‐1.34) |
| West | 102,178 | 80,249 | 56 | 1.37 | (1.36‐1.38) |
Variation in Opioid Prescribing
Figure 1 shows the histograms of hospital opioid prescribing rate for the 286 hospitals in our cohort (A) before and (B) after adjustment for patient characteristics. The observed rates ranged from 5% in the lowest‐prescribing hospital to 72% in the highest‐prescribing hospital, with a mean (SD) of 51% (10%). After adjusting for patient characteristics, the adjusted opioid‐prescribing rates ranged from 33% to 64%, with a mean (SD) of 50% (4%).

Severe Opioid‐Related Adverse Events
Among admissions with opioid exposure (n=576,373), naloxone use occurred in 2345 (0.41%) and opioid‐related adverse drug events in 1174 (0.20%), for a total of 3441 (0.60%) severe opioid‐related adverse events (some patients experienced both). Table 4 reports the opioid exposure and severe opioid‐related adverse‐event rates within hospital opioid‐prescribing rate quartiles, along with the adjusted association between the hospital opioid‐prescribing rate quartile and severe opioid‐related adverse events. After adjusting for patient characteristics, the relative risk of a severe opioid‐related adverse event was significantly greater in hospitals with higher opioid‐prescribing rates, both overall and among opioid exposed.
| Quartile | No. of Patients | Opioid Exposed, n (%) | Opioid‐Related Adverse Events, n (%) | Adjusted RR in All Patients, RR (95% CI), N=1,139,419a | Adjusted RR in Opioid Exposed, RR (95% CI), N=576,373a |
|---|---|---|---|---|---|
| |||||
| 1 | 349,747 | 132,824 (38) | 719 (0.21) | (ref) | (ref) |
| 2 | 266,652 | 134,590 (50) | 729 (0.27) | 1.31 (1.17‐1.45) | 1.07 (0.96‐1.18) |
| 3 | 251,042 | 139,770 (56) | 922 (0.37) | 1.72 (1.56‐1.90) | 1.31 (1.19‐1.44) |
| 4 | 271,978 | 169,189 (62) | 1071 (0.39) | 1.73 (1.57‐1.90) | 1.23 (1.12‐1.35) |
DISCUSSION
In this analysis of a large cohort of hospitalized nonsurgical patients, we found that more than half of all patients received opioids, with 43% of those exposed receiving multiple opioids during their admission and 52% receiving opioids on the day of discharge. Considerable hospital variation in opioid use was evident, and this was not fully explained by patient characteristics. Severe opioid‐related adverse events occurred more frequently at hospitals with higher opioid‐prescribing rates, and the relative risk of a severe adverse event per patient prescribed opioids was also higher in these hospitals. To our knowledge, this is the first study to describe the scope of opioid utilization and the relationship between utilization and severe opioid‐related adverse events in a sample of nonsurgical patients in US acute‐care facilities.
Our use of naloxone charges and opioid‐specific ICD‐9‐CM coding to define an opioid‐related adverse event was intended to capture only the most severe opioid‐related adverse events. We chose to focus on these events in our analysis to maximize the specificity of our outcome definition and thereby minimize confounding in our observed associations. The rate of less‐severe opioid‐related adverse events, such as nausea, constipation, and pruritis, is likely much higher and not captured in our outcome definition. Prior analyses have found variable rates of opioid‐related adverse events of approximately 1.8% to 13.6% of exposed patients.[22, 23, 24] However, these analyses focused on surgical patients and included less‐severe events. To our knowledge, ours is the first analysis of severe opioid‐related adverse events in nonsurgical patients.
Our finding that severe opioid‐related adverse events increase as opioid prescribing increases is consistent with that which has been demonstrated in the community setting, where rates of opioid‐related adverse events and mortality are higher in communities with higher levels of opioid prescribing.[2, 8, 25] This finding is expected, as greater use of a class of medications with known side effects would be expected to result in a higher overall rate of adverse events. More concerning, however, is the fact that this relationship persists when focusing exclusively on opioid‐exposed patients. Among similar patients receiving opioids at different hospitals, those hospitalized in facilities with higher opioid‐prescribing rates have higher rates of severe opioid‐related adverse events. This suggests that hospitals that use opioids more frequently do not do so more safely. Rather, the increased overall prescribing rates are associated with heightened risk for a serious adverse event per patient exposed and may reflect unsafe prescribing practices.
Furthermore, our results demonstrate both regional and hospital variation in use of opioids not fully explained by patient characteristics, similar to that which has been demonstrated for other drugs and heathcare services.[26, 27, 28, 29, 30] The implications of these findings are limited by our lack of information on pain severity or prior outpatient treatment, and our resultant inability to evaluate the appropriateness of opioid use in this analysis. Additionally, although we controlled for a large number of patient and hospital characteristics, there could be other significant predictors of use not accounted for in our analysis. However, it seems unlikely that differential pain severity or patient characteristics between patients in different regions of the country could fully explain a 37% relative difference in prescribing between the lowest‐ and highest‐prescribing regions, after accounting for the 44 patient‐level variables in our models. Whereas variation in use unrelated to patient factors could represent inappropriate prescribing practices, it could also be a marker of uncertainty regarding what constitutes appropriate prescribing and high‐quality care in this realm. Although guidelines advocate for standard pain assessments and a step‐up approach to treatment,[31, 32, 33] the lack of objective measures of pain severity and lack of evidence‐based recommendations on the use of opioids for noncancer pain[34] will almost certainly lead to persistent variation in opioid prescribing despite guideline‐driven care.
Nonetheless, our findings suggest that opportunities exist to make opioid prescribing safer in hospitalized patients. Studies aimed at elucidating the source of regional and hospital variation are necessary. Additionally, efforts should focus on identifying patient and prescribing characteristics associated with heightened risk of opioid‐related adverse events. Prior studies have demonstrated that the risks of opioid medications increase with increasing age of the patient.[35, 36] Although opioid use in our cohort declined with age, 44% of admissions age 65 years had charges for opioid medications. Studies in outpatients have also demonstrated that the risks of opioid overdose and overdose‐related death increase with dose.[5, 7] One study demonstrated a 3.7‐fold increased risk of overdose at doses of 5099 mg/day in oral morphine equivalents, and an 8.9‐fold increased risk at doses of 100 mg/day, compared with doses of 20 mg/day.[7] The prevalence of high dose exposure observed in our cohort, coupled with the older age of hospitalized patients, suggests potential targets for promoting safer use in hospitalized patients through interventions such as computerized decision support and enhanced monitoring in those at highest risk.
Because medications after discharge were unavailable in our dataset, the percentage of patients given a prescription for opioid medication on discharge is unknown. However, given that opioids are often tapered rather than abruptly discontinued, our finding that 26% of hospitalized nonsurgical patients received opioids on the day of discharge suggests that a substantial proportion of patients may be discharged with a prescription for opioid medication. Given the possibility of coexistent outpatient opioid prescriptions, these findings draw attention to the importance of assuring development and streamlined accessibility of data from state prescription drug monitoring programs and suggest that increased attention should be paid to the role that inpatient opioid prescribing plays in the increased rates of chronic opioid use and overdose‐related deaths in the United States.
There are additional limitations to our analysis. First, although the database used for this analysis captures a large proportion of admissions to US acute‐care facilities and is similar in composition, it is possible that participating medical centers differ from nonparticipating medical centers in ways that could be associated with opioid prescribing. Additionally, although Premier performs extensive validation and correction processes to assure the quality of their data, there is still likely to be a small amount of random error in the database, which could particularly impact dosage calculations. The lack of preadmission medications in our database precluded identification of the proportion of patients newly started on opioid medications. Lastly, it is possible that the hospital prescribing‐rate quartile is associated with patient characteristics unaccounted for in our analysis, and, therefore, the possibility of residual confounding still exists.
In conclusion, the majority of hospitalized nonsurgical patients are exposed to opioid medications during the course of their hospitalizations, often at high doses. More than half of those exposed are still receiving these medications on the day of discharge. We found hospital and regional variation in opioid use that was not fully explained by patient characteristics, and higher levels of hospital use were associated with higher risk of severe opioid‐related adverse events in opioid‐exposed patients. Further research is necessary to investigate the appropriateness of opioid use in this patient population, the sources of variation in use, and the predictors of opioid‐related adverse events in hospitalized patients to allow development of interventions to make hospital use safer.
Disclosures
Disclosures: Dr. Herzig had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Herzig was funded by grant no. K23AG042459 from the National Institute on Aging. Dr. Marcantonio was funded by grant nos. P01AG031720, R01AG030618, R03AG028189, and K24AG035075 from the National Institute on Aging. The funding organization had no involvement in any aspect of the study, including design, conduct, and reporting of the study. None of the authors have any conflicts of interest to disclose.
Recent reports have drawn attention to the high and increasing rates of opioid prescribing and overdose‐related deaths in the United States.[1, 2, 3, 4, 5, 6, 7, 8, 9] These studies have focused on community‐based and emergency department prescribing, leaving prescribing practices in the inpatient setting unexamined. Given that pain is a frequent complaint in hospitalized patients, and that the Joint Commission mandates assessing pain as a vital sign, hospitalization is potentially a time of heightened use of such medications and could significantly contribute to nosocomial complications and subsequent outpatient use.[10] Variation in prescribing practices, unrelated to patient characteristics, could be a marker of inappropriate prescribing practices and poor quality of care.
Using a large, nationally representative cohort of admissions from July 2009 to June 2010, we sought to determine patterns and predictors of opioid utilization in nonsurgical admissions to US medical centers, hospital variation in use, and the association between hospital‐level use and the risk of opioid‐related adverse events. We hypothesized that hospitals with higher rates of opioid use would have an increased risk of an opioid‐related adverse event per patient exposed.
METHODS
Setting and Patients
We conducted a retrospective cohort study using data from 286 US nonfederal acute‐care facilities contributing to the database maintained by Premier (Premier Healthcare Solutions, Inc., Charlotte, NC). This database, created to measure healthcare utilization and quality of care, is drawn from voluntarily participating hospitals and contains data on approximately 1 in every 4 discharges nationwide.[11] Participating hospitals are similar in geographic distribution and metropolitan (urban/rural) status to hospitals nationwide, although large, nonteaching hospitals are slightly overrepresented in Premier. The database contains patient demographics, International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) codes, hospital demographics, and a date‐stamped log of all charges during the course of each hospitalization, including diagnostic tests, therapeutic treatments, and medications with dose and route of administration. The study was approved by the institutional review board at Beth Israel Deaconess Medical Center and granted a waiver of informed consent.
We studied a cohort of all adult nonsurgical admissions to participating hospitals from July 1, 2009, through June 30, 2010. We chose to study nonsurgical admissions, as patients undergoing surgical procedures have a clear indication for, and almost always receive, opioid pain medications. We defined a nonsurgical admission as an admission in which there were no charges for operating‐room procedures (including labor and delivery) and the attending of record was not a surgeon. We excluded admissions with unknown gender, since this is a key demographic variable, and admissions with a length of stay greater than 365 days, as these admissions are not representative of the typical admission to an acute‐care hospital. At the hospital level, we excluded hospitals contributing <100 admissions owing to resultant lack of precision in corresponding hospital prescribing rates, and hospitals that did not prescribe the full range of opioid medications (these hospitals had charges for codeine only), as these facilities seemed likely to have unusual limitations on prescribing or incomplete data capture.
Opioid Exposure
We defined opioid exposure as presence of 1 charge for an opioid medication during the admission. Opioid medications included morphine, hydrocodone, hydromorphone, oxycodone, fentanyl, meperidine, methadone, codeine, tramadol, buprenorphine, levorphanol, oxymorphone, pentazocine, propoxyphene, tapentadol, butorphanol, dezocine, and nalbuphine. We grouped the last 9 into an other category owing to infrequent use and/or differing characteristics from the main opioid drug types, such as synthetic, semisynthetic, and partial agonist qualities.
Severe Opioid‐Related Adverse Events
We defined severe opioid‐related adverse events as either naloxone exposure or an opioid‐related adverse drug event diagnosis code. Naloxone use in an adult patient exposed to opioids is one of the Institute for Healthcare Improvement's Trigger Tools for identifying adverse drug events[12] and previously has been demonstrated to have high positive predictive value for a confirmed adverse drug event.[13] We defined naloxone exposure as presence of 1 charge for naloxone. We excluded charges on hospital day 1 to focus on nosocomial events. We defined opioid‐related adverse drug events using ICD‐9‐CM diagnosis codes for poisoning by opioids (overdose, wrong substance given, or taken in error; ICD‐9‐CM 965.02, 965.09, E850.1, E850.2) and drugs causing adverse effects in therapeutic use (ICD‐9‐CM E935.1, E935.2), as specified in prior analyses by the Agency for Healthcare Research and Quality (AHRQ).[14, 15] To avoid capturing adverse events associated with outpatient use, we required the ICD‐9‐CM code to be qualified as not present on admission using the present on admission indicator required by the Centers for Medicare and Medicaid Services for all discharge diagnosis codes since 2008.[16]
Covariates of Interest
We were interested in the relationship between both patient and hospital characteristics and opioid exposure. Patient characteristics of interest included (1) demographic variables, such as age, sex, race (self‐reported by patients at the time of admission), marital status, and payer; (2) whether or not the patient spent any time in the intensive care unit (ICU); (3) comorbidities, identified via ICD‐9‐CM secondary diagnosis codes and diagnosis‐related groups using Healthcare Cost and Utilization Project Comorbidity Software, version 3.7, based on the work of Elixhauser et al[17, 18]; (4) primary ICD‐9‐CM discharge diagnosis groupings, selected based on hypothesized associations with receipt of opioids, and based on the Clinical Classifications Software (CCS)a diagnosis and procedure categorization scheme maintained by the AHRQ, and defined in the Appendix[19]; (5) and nonoperating‐room‐based procedures potentially necessitating opioids during the admission, selected from the 50 most common ICD‐9‐CM procedure codes in our cohort and grouped as cardiovascular procedures (catheterization and insertion of vascular stents), gastrointestinal procedures (upper and lower endoscopy), and mechanical ventilation, further defined in the Appendix. Hospital characteristics of interest included number of beds, population served (urban vs rural), teaching status, and US census region (Northeast, Midwest, South, West).
Statistical Analysis
We calculated the percent of admissions with exposure to any opioid and the percent exposed to each opioid, along with the total number of different opioid medications used during each admission. We also calculated the percent of admissions with parenteral administration and the percent of admissions with oral administration, among those exposed to the individual categories, and in aggregate. Because medications after discharge were unavailable in Premier's dataset, we report the percent of patients with a charge for opioids on the day of discharge.
We determined the daily dose of an opioid by taking the sum of the doses for that opioid charged on a given day. The average daily dose of an opioid was determined by taking the sum of the daily doses and dividing by the number of days on which 1 dose was charged. To facilitate comparison, all opioids, with the exception of those for which standard equivalences are unavailable (tramadol, other opioid category, oral fentanyl, epidural route for all), were converted to oral morphine equivalents using a standard equivalence conversion table.[20, 21] We excluded from our dosage calculations those charges for which standard morphine equivalence was unavailable, or for which dosage was missing. We also excluded from our dosage calculations any dose that was >3 standard deviations (SD) above the mean dose for that opioid, as such extreme values seemed physiologically implausible and more likely to be a data entry error that could lead to significant overestimation of the mean for that opioid.
All multivariable models used a generalized estimating equation (GEE) via the genmod procedure in SAS, with a Poisson distribution error term and a log link, controlling for repeated patient admissions with an autoregressive correlation structure.
To identify independent predictors of opioid receipt, we used a GEE model of opioid receipt where all patient and hospital characteristics listed in Table 1 were included as independent variables.
| Patient characteristics, N=1,139,419 | N | % |
|---|---|---|
| ||
| Age group, y | ||
| 1824 | 37,464 | 3 |
| 2534 | 66,541 | 6 |
| 3544 | 102,701 | 9 |
| 4554 | 174,830 | 15 |
| 5564 | 192,570 | 17 |
| 6574 | 196,407 | 17 |
| 75+ | 368,906 | 32 |
| Sex | ||
| Male | 527,062 | 46 |
| Female | 612,357 | 54 |
| Race | ||
| White | 711,993 | 62 |
| Black | 176,993 | 16 |
| Hispanic | 54,406 | 5 |
| Other | 196,027 | 17 |
| Marital status | ||
| Married | 427,648 | 38 |
| Single | 586,343 | 51 |
| Unknown/other | 125,428 | 11 |
| Primary insurance | ||
| Private/commercial | 269,725 | 24 |
| Medicare traditional | 502,301 | 44 |
| Medicare managed care | 126,344 | 11 |
| Medicaid | 125,025 | 11 |
| Self‐pay/other | 116,024 | 10 |
| ICU care | ||
| No | 1,023,027 | 90 |
| Yes | 116,392 | 10 |
| Comorbidities | ||
| AIDS | 5724 | 1 |
| Alcohol abuse | 79,633 | 7 |
| Deficiency anemias | 213,437 | 19 |
| RA/collagen vascular disease | 35,210 | 3 |
| Chronic blood‐loss anemia | 10,860 | 1 |
| CHF | 190,085 | 17 |
| Chronic pulmonary disease | 285,954 | 25 |
| Coagulopathy | 48,513 | 4 |
| Depression | 145,553 | 13 |
| DM without chronic complications | 270,087 | 24 |
| DM with chronic complications | 70,732 | 6 |
| Drug abuse | 66,886 | 6 |
| Hypertension | 696,299 | 61 |
| Hypothyroidism | 146,136 | 13 |
| Liver disease | 38,130 | 3 |
| Lymphoma | 14,032 | 1 |
| Fluid and electrolyte disorders | 326,576 | 29 |
| Metastatic cancer | 33,435 | 3 |
| Other neurological disorders | 124,195 | 11 |
| Obesity | 118,915 | 10 |
| Paralysis | 38,584 | 3 |
| PVD | 77,334 | 7 |
| Psychoses | 101,856 | 9 |
| Pulmonary circulation disease | 52,106 | 5 |
| Renal failure | 175,398 | 15 |
| Solid tumor without metastasis | 29,594 | 3 |
| Peptic ulcer disease excluding bleeding | 536 | 0 |
| Valvular disease | 86,616 | 8 |
| Weight loss | 45,132 | 4 |
| Primary discharge diagnoses | ||
| Cancer | 19,168 | 2 |
| Musculoskeletal injuries | 16,798 | 1 |
| Pain‐related diagnosesb | 101,533 | 9 |
| Alcohol‐related disorders | 16,777 | 1 |
| Substance‐related disorders | 13,697 | 1 |
| Psychiatric disorders | 41,153 | 4 |
| Mood disorders | 28,761 | 3 |
| Schizophrenia and other psychotic disorders | 12,392 | 1 |
| Procedures | ||
| Cardiovascular procedures | 59,901 | 5 |
| GI procedures | 31,224 | 3 |
| Mechanical ventilation | 7853 | 1 |
| Hospital characteristics, N=286 | ||
| Number of beds | ||
| <200 | 103 | 36 |
| 201300 | 63 | 22 |
| 301500 | 81 | 28 |
| >500 | 39 | 14 |
| Population served | ||
| Urban | 225 | 79 |
| Rural | 61 | 21 |
| Teaching status | ||
| Nonteaching | 207 | 72 |
| Teaching | 79 | 28 |
| US Census region | ||
| Northeast | 47 | 16 |
| Midwest | 63 | 22 |
| South | 115 | 40 |
| West | 61 | 21 |
To assess hospital variation in opioid prescribing after adjusting for patient characteristics, we used a GEE model of opioid receipt, controlling for all patient characteristics listed in Table 1. We then took the mean of the predicted probabilities of opioid receipt for the patients within each hospital in our cohort to derive the hospital prescribing rate adjusted for patient characteristics. We report the mean, SD, and range of the prescribing rates for the hospitals in our cohort before and after adjustment for patient characteristics.
To assess whether patients admitted to hospitals with higher rates of opioid prescribing have higher relative risk of severe opioid‐related adverse events, we stratified hospitals into opioid‐prescribing rate quartiles and compared the rates of opioid‐related adverse eventsboth overall and among opioid exposedbetween quartiles. To adjust for patient characteristics, we used a GEE model in which severe opioid‐related adverse event (yes/no) was the dependent variable and hospital‐prescribing rate quartile and all patient characteristics in Table 1 were independent variables. We also performed a sensitivity analysis in which we assessed the association between hospital prescribing‐rate quartile and the individual components of our composite outcome. Our results were qualitatively unchanged using this approach, and only the results of our main analysis are presented.
All analyses were carried out using SAS software, version 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
Patient Admission Characteristics
There were 3,190,934 adult admissions to 300 acute‐care hospitals during our study period. After excluding admissions with a length of stay >365 days (n=25), missing patient sex (n=17), and charges for operating‐room procedures or a surgical attending of record (n=2,018,553), 1,172,339 admissions were available for analysis. There were 12 hospitals with incomplete opioid‐prescribing data (n=32,794) and 2 hospitals that contributed <100 admissions each (n=126), leaving 1,139,419 admissions in 286 hospitals in our analytic cohort. The median age of the cohort was 64 years (interquartile range, 4979 years), and 527,062 (46%) were men. Table 1 shows the characteristics of the admissions in the cohort.
Rate, Route, and Dose of Opioid Exposures
Overall, there were 576,373 (51%) admissions with charges for opioid medications. Among those exposed, 244,760 (43%) had charges for multiple opioids during the admission; 172,090 (30%) had charges for 2 different opioids; and 72,670 (13%) had charges for 3 different opioids. Table 2 shows the percent exposed to each opioid, the percent of exposed with parenteral and oral routes of administration, and the mean daily dose received in oral morphine equivalents.
| Exposed | Parenteral Administration | Oral Administration | Dose Received, in Oral Morphine Equivalents | |||||
|---|---|---|---|---|---|---|---|---|
| N | %a | N | %b | N | %b | Mean | SDc | |
| ||||||||
| All opioids | 576,373 | 51 | 378,771 | 66 | 371,796 | 65 | 68 | 185 |
| Morphine | 224,811 | 20 | 209,040 | 93 | 21,645 | 10 | 40 | 121 |
| Hydrocodone | 162,558 | 14 | 0 | 0 | 160,941 | 99 | 14 | 12 |
| Hydromorphone | 146,236 | 13 | 137,936 | 94 | 16,052 | 11 | 113 | 274 |
| Oxycodone | 126,733 | 11 | 0 | 0 | 125,033 | 99 | 26 | 37 |
| Fentanyl | 105,052 | 9 | 103,113 | 98 | 641 | 1 | 64 | 75 |
| Tramadol | 35,570 | 3 | 0 | 0 | 35,570 | 100 | ||
| Meperidine | 24,850 | 2 | 24,398 | 98 | 515 | 2 | 36 | 34 |
| Methadone | 15,302 | 1 | 370 | 2 | 14,781 | 97 | 337 | 384 |
| Codeine | 22,818 | 2 | 178 | 1 | 22,183 | 97 | 9 | 15 |
| Other | 45,469 | 4 | 5821 | 13 | 39,618 | 87 | ||
Among the medications/routes for which conversion to morphine equivalents was possible, dosage was missing in 39,728 out of 2,294,673 opioid charges (2%). The average daily dose received in oral morphine equivalents was 68 mg. A total dose of 50 mg per day was received in 39% of exposed, and a total dose of 100 mg per day was received in 23% of exposed. Among those exposed, 52% (26% of overall admissions) had charges for opioids on the day of discharge.
Rates of Opioid Use by Patient and Hospital Characteristics
Table 3 reports the association between admission characteristics and opioid use. Use was highest in patients between the ages of 25 and 54 years. Although use declined with age, 44% of admissions age 65 years had charges for opioid medication. After adjustment for patient demographics, comorbidities, and hospital characteristics, opioid use was more common in females than males, those age 2554 years compared with those older and younger, those of Caucasian race compared with non‐Caucasian race, and those with Medicare or Medicaid primary insurance. Among the primary discharge diagnoses, patients with musculoskeletal injuries, various specific and nonspecific pain‐related diagnoses, and cancer were significantly more likely to receive opioids than patients without these diagnoses, whereas patients with alcohol‐related disorders and psychiatric disorders were significantly less likely to receive opioids than patients without these diagnoses. Patients admitted to hospitals in the Midwest, South, and West were significantly more likely to receive opioid medications than patients in the Northeast.
| Exposed, N=576,373 | Unexposed, N=563,046 | % Exposed | Adjusted RRa | 95% CI | |
|---|---|---|---|---|---|
| |||||
| Patient characteristics | |||||
| Age group, y | |||||
| 1824 | 17,360 | 20,104 | 46 | (ref) | |
| 2534 | 37,793 | 28,748 | 57 | 1.17 | 1.16‐1.19 |
| 3544 | 60,712 | 41,989 | 59 | 1.16 | 1.15‐1.17 |
| 4554 | 103,798 | 71,032 | 59 | 1.11 | 1.09‐1.12 |
| 5564 | 108,256 | 84,314 | 56 | 1.00 | 0.98‐1.01 |
| 6574 | 98,110 | 98,297 | 50 | 0.84 | 0.83‐0.85 |
| 75+ | 150,344 | 218,562 | 41 | 0.71 | 0.70‐0.72 |
| Sex | |||||
| Male | 255,315 | 271,747 | 48 | (ref) | |
| Female | 321,058 | 291,299 | 52 | 1.11 | 1.10‐1.11 |
| Race | |||||
| White | 365,107 | 346,886 | 51 | (ref) | |
| Black | 92,013 | 84,980 | 52 | 0.93 | 0.92‐0.93 |
| Hispanic | 27,592 | 26,814 | 51 | 0.94 | 0.93‐0.94 |
| Other | 91,661 | 104,366 | 47 | 0.93 | 0.92‐0.93 |
| Marital status | |||||
| Married | 222,912 | 204,736 | 52 | (ref) | |
| Single | 297,742 | 288,601 | 51 | 1.00 | 0.99‐1.01 |
| Unknown/other | 55,719 | 69,709 | 44 | 0.94 | 0.93‐0.95 |
| Primary insurance | |||||
| Private/commercial | 143,954 | 125,771 | 53 | (ref) | |
| Medicare traditional | 236,114 | 266,187 | 47 | 1.10 | 1.09‐1.10 |
| Medicare managed care | 59,104 | 67,240 | 47 | 1.11 | 1.11‐1.12 |
| Medicaid | 73,583 | 51,442 | 59 | 1.13 | 1.12‐1.13 |
| Self‐pay/other | 63,618 | 52,406 | 55 | 1.03 | 1.02‐1.04 |
| ICU care | |||||
| No | 510,654 | 512,373 | 50 | (ref) | |
| Yes | 65,719 | 50,673 | 56 | 1.02 | 1.01‐1.03 |
| Comorbiditiesb | |||||
| AIDS | 3655 | 2069 | 64 | 1.09 | 1.07‐1.12 |
| Alcohol abuse | 35,112 | 44,521 | 44 | 0.92 | 0.91‐0.93 |
| Deficiency anemias | 115,842 | 97,595 | 54 | 1.08 | 1.08‐1.09 |
| RA/collagen vascular disease | 22,519 | 12,691 | 64 | 1.22 | 1.21‐1.23 |
| Chronic blood‐loss anemia | 6444 | 4416 | 59 | 1.04 | 1.02‐1.05 |
| CHF | 88,895 | 101,190 | 47 | 0.99 | 0.98‐0.99 |
| Chronic pulmonary disease | 153,667 | 132,287 | 54 | 1.08 | 1.08‐1.08 |
| Coagulopathy | 25,802 | 22,711 | 53 | 1.03 | 1.02‐1.04 |
| Depression | 83,051 | 62,502 | 57 | 1.08 | 1.08‐1.09 |
| DM without chronic complications | 136,184 | 133,903 | 50 | 0.99 | 0.99‐0.99 |
| DM with chronic complications | 38,696 | 32,036 | 55 | 1.04 | 1.03‐1.05 |
| Drug abuse | 37,202 | 29,684 | 56 | 1.14 | 1.13‐1.15 |
| Hypertension | 344,718 | 351,581 | 50 | 0.98 | 0.97‐0.98 |
| Hypothyroidism | 70,786 | 75,350 | 48 | 0.99 | 0.99‐0.99 |
| Liver disease | 24,067 | 14,063 | 63 | 1.15 | 1.14‐1.16 |
| Lymphoma | 7727 | 6305 | 55 | 1.16 | 1.14‐1.17 |
| Fluid and electrolyte disorders | 168,814 | 157,762 | 52 | 1.04 | 1.03‐1.04 |
| Metastatic cancer | 23,920 | 9515 | 72 | 1.40 | 1.39‐1.42 |
| Other neurological disorders | 51,091 | 73,104 | 41 | 0.87 | 0.86‐0.87 |
| Obesity | 69,584 | 49,331 | 59 | 1.05 | 1.04‐1.05 |
| Paralysis | 17,497 | 21,087 | 45 | 0.97 | 0.96‐0.98 |
| PVD | 42,176 | 35,158 | 55 | 1.11 | 1.11‐1.12 |
| Psychoses | 38,638 | 63,218 | 38 | 0.91 | 0.90‐0.92 |
| Pulmonary circulation disease | 26,656 | 25,450 | 51 | 1.05 | 1.04‐1.06 |
| Renal failure | 86,565 | 88,833 | 49 | 1.01 | 1.01‐1.02 |
| Solid tumor without metastasis | 16,258 | 13,336 | 55 | 1.14 | 1.13‐1.15 |
| Peptic ulcer disease excluding bleeding | 376 | 160 | 70 | 1.12 | 1.07‐1.18 |
| Valvular disease | 38,396 | 48,220 | 44 | 0.93 | 0.92‐0.94 |
| Weight loss | 25,724 | 19,408 | 57 | 1.09 | 1.08‐1.10 |
| Primary discharge diagnosesb | |||||
| Cancer | 13,986 | 5182 | 73 | 1.20 | 1.19‐1.21 |
| Musculoskeletal injuries | 14,638 | 2160 | 87 | 2.02 | 2.002.04 |
| Pain‐related diagnosesc | 64,656 | 36,877 | 64 | 1.20 | 1.20‐1.21 |
| Alcohol‐related disorders | 3425 | 13,352 | 20 | 0.46 | 0.44‐0.47 |
| Substance‐related disorders | 8680 | 5017 | 63 | 1.03 | 1.01‐1.04 |
| Psychiatric disorders | 7253 | 33,900 | 18 | 0.37 | 0.36‐0.38 |
| Mood disorders | 5943 | 22,818 | 21 | ||
| Schizophrenia and other psychotic disorders | 1310 | 11,082 | 11 | ||
| Proceduresb | |||||
| Cardiovascular procedures | 50,997 | 8904 | 85 | 1.80 | 1.79‐1.81 |
| GI procedures | 27,206 | 4018 | 87 | 1.70 | 1.69‐1.71 |
| Mechanical ventilation | 5341 | 2512 | 68 | 1.37 | 1.34‐1.39 |
| Hospital characteristics | |||||
| Number of beds | |||||
| <200 | 100,900 | 88,439 | 53 | (ref) | |
| 201300 | 104,213 | 99,995 | 51 | 0.95 | 0.95‐0.96 |
| 301500 | 215,340 | 209,104 | 51 | 0.94 | 0.94‐0.95 |
| >500 | 155,920 | 165,508 | 49 | 0.96 | 0.95‐0.96 |
| Population served | |||||
| Urban | 511,727 | 506,803 | 50 | (ref) | |
| Rural | 64,646 | 56,243 | 53 | 0.98 | 0.97‐0.99 |
| Teaching status | |||||
| Nonteaching | 366,623 | 343,581 | 52 | (ref) | |
| Teaching | 209,750 | 219,465 | 49 | 1.00 | 0.99‐1.01 |
| US Census region | |||||
| Northeast | 99,377 | 149,446 | 40 | (ref) | |
| Midwest | 123,194 | 120,322 | 51 | 1.26 | (1.25‐1.27) |
| South | 251,624 | 213,029 | 54 | 1.33 | (1.33‐1.34) |
| West | 102,178 | 80,249 | 56 | 1.37 | (1.36‐1.38) |
Variation in Opioid Prescribing
Figure 1 shows the histograms of hospital opioid prescribing rate for the 286 hospitals in our cohort (A) before and (B) after adjustment for patient characteristics. The observed rates ranged from 5% in the lowest‐prescribing hospital to 72% in the highest‐prescribing hospital, with a mean (SD) of 51% (10%). After adjusting for patient characteristics, the adjusted opioid‐prescribing rates ranged from 33% to 64%, with a mean (SD) of 50% (4%).

Severe Opioid‐Related Adverse Events
Among admissions with opioid exposure (n=576,373), naloxone use occurred in 2345 (0.41%) and opioid‐related adverse drug events in 1174 (0.20%), for a total of 3441 (0.60%) severe opioid‐related adverse events (some patients experienced both). Table 4 reports the opioid exposure and severe opioid‐related adverse‐event rates within hospital opioid‐prescribing rate quartiles, along with the adjusted association between the hospital opioid‐prescribing rate quartile and severe opioid‐related adverse events. After adjusting for patient characteristics, the relative risk of a severe opioid‐related adverse event was significantly greater in hospitals with higher opioid‐prescribing rates, both overall and among opioid exposed.
| Quartile | No. of Patients | Opioid Exposed, n (%) | Opioid‐Related Adverse Events, n (%) | Adjusted RR in All Patients, RR (95% CI), N=1,139,419a | Adjusted RR in Opioid Exposed, RR (95% CI), N=576,373a |
|---|---|---|---|---|---|
| |||||
| 1 | 349,747 | 132,824 (38) | 719 (0.21) | (ref) | (ref) |
| 2 | 266,652 | 134,590 (50) | 729 (0.27) | 1.31 (1.17‐1.45) | 1.07 (0.96‐1.18) |
| 3 | 251,042 | 139,770 (56) | 922 (0.37) | 1.72 (1.56‐1.90) | 1.31 (1.19‐1.44) |
| 4 | 271,978 | 169,189 (62) | 1071 (0.39) | 1.73 (1.57‐1.90) | 1.23 (1.12‐1.35) |
DISCUSSION
In this analysis of a large cohort of hospitalized nonsurgical patients, we found that more than half of all patients received opioids, with 43% of those exposed receiving multiple opioids during their admission and 52% receiving opioids on the day of discharge. Considerable hospital variation in opioid use was evident, and this was not fully explained by patient characteristics. Severe opioid‐related adverse events occurred more frequently at hospitals with higher opioid‐prescribing rates, and the relative risk of a severe adverse event per patient prescribed opioids was also higher in these hospitals. To our knowledge, this is the first study to describe the scope of opioid utilization and the relationship between utilization and severe opioid‐related adverse events in a sample of nonsurgical patients in US acute‐care facilities.
Our use of naloxone charges and opioid‐specific ICD‐9‐CM coding to define an opioid‐related adverse event was intended to capture only the most severe opioid‐related adverse events. We chose to focus on these events in our analysis to maximize the specificity of our outcome definition and thereby minimize confounding in our observed associations. The rate of less‐severe opioid‐related adverse events, such as nausea, constipation, and pruritis, is likely much higher and not captured in our outcome definition. Prior analyses have found variable rates of opioid‐related adverse events of approximately 1.8% to 13.6% of exposed patients.[22, 23, 24] However, these analyses focused on surgical patients and included less‐severe events. To our knowledge, ours is the first analysis of severe opioid‐related adverse events in nonsurgical patients.
Our finding that severe opioid‐related adverse events increase as opioid prescribing increases is consistent with that which has been demonstrated in the community setting, where rates of opioid‐related adverse events and mortality are higher in communities with higher levels of opioid prescribing.[2, 8, 25] This finding is expected, as greater use of a class of medications with known side effects would be expected to result in a higher overall rate of adverse events. More concerning, however, is the fact that this relationship persists when focusing exclusively on opioid‐exposed patients. Among similar patients receiving opioids at different hospitals, those hospitalized in facilities with higher opioid‐prescribing rates have higher rates of severe opioid‐related adverse events. This suggests that hospitals that use opioids more frequently do not do so more safely. Rather, the increased overall prescribing rates are associated with heightened risk for a serious adverse event per patient exposed and may reflect unsafe prescribing practices.
Furthermore, our results demonstrate both regional and hospital variation in use of opioids not fully explained by patient characteristics, similar to that which has been demonstrated for other drugs and heathcare services.[26, 27, 28, 29, 30] The implications of these findings are limited by our lack of information on pain severity or prior outpatient treatment, and our resultant inability to evaluate the appropriateness of opioid use in this analysis. Additionally, although we controlled for a large number of patient and hospital characteristics, there could be other significant predictors of use not accounted for in our analysis. However, it seems unlikely that differential pain severity or patient characteristics between patients in different regions of the country could fully explain a 37% relative difference in prescribing between the lowest‐ and highest‐prescribing regions, after accounting for the 44 patient‐level variables in our models. Whereas variation in use unrelated to patient factors could represent inappropriate prescribing practices, it could also be a marker of uncertainty regarding what constitutes appropriate prescribing and high‐quality care in this realm. Although guidelines advocate for standard pain assessments and a step‐up approach to treatment,[31, 32, 33] the lack of objective measures of pain severity and lack of evidence‐based recommendations on the use of opioids for noncancer pain[34] will almost certainly lead to persistent variation in opioid prescribing despite guideline‐driven care.
Nonetheless, our findings suggest that opportunities exist to make opioid prescribing safer in hospitalized patients. Studies aimed at elucidating the source of regional and hospital variation are necessary. Additionally, efforts should focus on identifying patient and prescribing characteristics associated with heightened risk of opioid‐related adverse events. Prior studies have demonstrated that the risks of opioid medications increase with increasing age of the patient.[35, 36] Although opioid use in our cohort declined with age, 44% of admissions age 65 years had charges for opioid medications. Studies in outpatients have also demonstrated that the risks of opioid overdose and overdose‐related death increase with dose.[5, 7] One study demonstrated a 3.7‐fold increased risk of overdose at doses of 5099 mg/day in oral morphine equivalents, and an 8.9‐fold increased risk at doses of 100 mg/day, compared with doses of 20 mg/day.[7] The prevalence of high dose exposure observed in our cohort, coupled with the older age of hospitalized patients, suggests potential targets for promoting safer use in hospitalized patients through interventions such as computerized decision support and enhanced monitoring in those at highest risk.
Because medications after discharge were unavailable in our dataset, the percentage of patients given a prescription for opioid medication on discharge is unknown. However, given that opioids are often tapered rather than abruptly discontinued, our finding that 26% of hospitalized nonsurgical patients received opioids on the day of discharge suggests that a substantial proportion of patients may be discharged with a prescription for opioid medication. Given the possibility of coexistent outpatient opioid prescriptions, these findings draw attention to the importance of assuring development and streamlined accessibility of data from state prescription drug monitoring programs and suggest that increased attention should be paid to the role that inpatient opioid prescribing plays in the increased rates of chronic opioid use and overdose‐related deaths in the United States.
There are additional limitations to our analysis. First, although the database used for this analysis captures a large proportion of admissions to US acute‐care facilities and is similar in composition, it is possible that participating medical centers differ from nonparticipating medical centers in ways that could be associated with opioid prescribing. Additionally, although Premier performs extensive validation and correction processes to assure the quality of their data, there is still likely to be a small amount of random error in the database, which could particularly impact dosage calculations. The lack of preadmission medications in our database precluded identification of the proportion of patients newly started on opioid medications. Lastly, it is possible that the hospital prescribing‐rate quartile is associated with patient characteristics unaccounted for in our analysis, and, therefore, the possibility of residual confounding still exists.
In conclusion, the majority of hospitalized nonsurgical patients are exposed to opioid medications during the course of their hospitalizations, often at high doses. More than half of those exposed are still receiving these medications on the day of discharge. We found hospital and regional variation in opioid use that was not fully explained by patient characteristics, and higher levels of hospital use were associated with higher risk of severe opioid‐related adverse events in opioid‐exposed patients. Further research is necessary to investigate the appropriateness of opioid use in this patient population, the sources of variation in use, and the predictors of opioid‐related adverse events in hospitalized patients to allow development of interventions to make hospital use safer.
Disclosures
Disclosures: Dr. Herzig had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Herzig was funded by grant no. K23AG042459 from the National Institute on Aging. Dr. Marcantonio was funded by grant nos. P01AG031720, R01AG030618, R03AG028189, and K24AG035075 from the National Institute on Aging. The funding organization had no involvement in any aspect of the study, including design, conduct, and reporting of the study. None of the authors have any conflicts of interest to disclose.
- . A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363(21):1981–1985.
- , , . Increasing deaths from opioid analgesics in the United States. Pharmacoepidemiol Drug Saf. 2006;15(9):618–627.
- , , , . Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA. 2008;299(1):70–78.
- , , , . Trends in medical use and abuse of opioid analgesics. JAMA. 2000;283(13):1710–1714.
- , , , et al. Association between opioid prescribing patterns and opioid overdose‐related deaths. JAMA. 2011;305(13):1315–1321.
- , , , et al. Prescription opioid mortality trends in New York City, 1990–2006: examining the emergence of an epidemic. Drug Alcohol Depend. 2013;132(1‐2):53–62.
- , , , et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85–92.
- , , , et al. Relationship of opioid prescription sales and overdoses, North Carolina. Drug Alcohol Depend. 2013;132(1‐2):81–86.
- Deaths from prescription opioids soar in New York. BMJ. 2013;346:f921.
- , , . Mortality in elderly dementia patients treated with risperidone. J Clin Psychopharmacol. 2006;26(6):566–570.
- , , , et al. Medication reconciliation: a practical tool to reduce the risk of medication errors. J Crit Care. 2003;18(4):201–205.
- , , . Adverse drug event trigger tool: a practical methodology for measuring medication related harm. Qual Saf Health Care. 2003;12(3):194–200.
- , , , , . Improvement in the detection of adverse drug events by the use of electronic health and prescription records: an evaluation of two trigger tools. Eur J Clin Pharmacol. 2013;69(2):255–259.
- , . Adverse Drug Events in U.S. Hospitals, 2004. Rockville, MD: Agency for Healthcare Research and Quality; April 2007. Statistical Brief 29.
- , , . Medication‐Related Adverse Outcomes in U.S. Hospitals and Emergency Departments, 2008. Rockville, MD: Agency for Healthcare Research and Quality; April 2011. Statistical Brief 109.
- US Centers for Medicare and Medicaid Services. Hospital‐Acquired Conditions (Present on Admission Indicator). Available at: http://www.cms.hhs.gov/HospitalAcqCond/. Accessed August 16, 2011. Updated September 20, 2012.
- , , . Fracture risk associated with the use of morphine and opiates. J Intern Med. 2006;260(1):76–87.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
- Clinical Classifications Software, Healthcare Cost and Utilization Project (HCUP). Rockville, MD; Agency for Healthcare Research and Quality; December 2009.
- , , , , . Clinical application of opioid equianalgesic data. Clin J Pain. 2003;19(5)286–297.
- , , , , , . Choosing the unit of measurement counts: the use of oral morphine equivalents in studies of opioid consumption is a useful addition to defined daily doses. Palliat Med. 2011;25(7):725–732.
- , , , . Cost and quality implications of opioid‐based postsurgical pain control using administrative claims data from a large health system: opioid‐related adverse events and their impact on clinical and economic outcomes. Pharmacotherapy. 2013;33(4):383–391.
- , , , et al. Opioid‐related adverse drug events in surgical hospitalizations: impact on costs and length of stay. Ann Pharmacother. 2007;41(3):400–406.
- , , , et al. Cost of opioid‐related adverse drug events in surgical patients. J Pain Symptom Manage. 2003;25(3):276–283.
- , . Opioid analgesics and rates of fatal drug poisoning in the United States. Am J Prev Med. 2006;31(6):506–511.
- , , , et al. Geographic variation in the treatment of acute myocardial infarction: the Cooperative Cardiovascular Project. JAMA. 1999;281(7):627–633.
- , , , et al; GUSTO‐1 Investigators. Regional variation across the United States in the management of acute myocardial infarction. Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries. N Engl J Med. 1995;333(9):565–572.
- , , . Predictors of broad‐spectrum antibiotic prescribing for acute respiratory tract infections in adult primary care. JAMA. 2003;289(6):719–725.
- , , . Geographic variation in Medicare drug spending. N Engl J Med. 2010;363(5):405–409.
- , , . Geographic variation in outpatient antibiotic prescribing among older adults. Arch Intern Med. 2012;172(19):1465–1471.
- , , , et al. Clinical policy: critical issues in the prescribing of opioids for adult patients in the emergency department. Ann Emerg Med. 2012;60(4):499–525.
- The Joint Commission. Facts About Pain Management. Available at: http://www.jointcommission.org/pain_management/. Accessed July 23, 2012.
- The Joint Commission. Sentinel Event Alert: Safe Use of Opioids in Hospitals. Published August 8, 2012. Available at: http://www.jointcommission.org/assets/1/18/SEA_49_opioids_8_2_12_final.pdf. Accessed March 4, 2013.
- , , , , . Research gaps on use of opioids for chronic noncancer pain: findings from a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10(2):147–159.
- , , , , , . Side effects of opioids during short‐term administration: effect of age, gender, and race. Clin Pharmacol Ther. 2003;74(2):102–112.
- , , , . Postoperative day one: a high‐risk period for respiratory events. Am J Surg. 2005;190(5):752–756.
- . A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363(21):1981–1985.
- , , . Increasing deaths from opioid analgesics in the United States. Pharmacoepidemiol Drug Saf. 2006;15(9):618–627.
- , , , . Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA. 2008;299(1):70–78.
- , , , . Trends in medical use and abuse of opioid analgesics. JAMA. 2000;283(13):1710–1714.
- , , , et al. Association between opioid prescribing patterns and opioid overdose‐related deaths. JAMA. 2011;305(13):1315–1321.
- , , , et al. Prescription opioid mortality trends in New York City, 1990–2006: examining the emergence of an epidemic. Drug Alcohol Depend. 2013;132(1‐2):53–62.
- , , , et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85–92.
- , , , et al. Relationship of opioid prescription sales and overdoses, North Carolina. Drug Alcohol Depend. 2013;132(1‐2):81–86.
- Deaths from prescription opioids soar in New York. BMJ. 2013;346:f921.
- , , . Mortality in elderly dementia patients treated with risperidone. J Clin Psychopharmacol. 2006;26(6):566–570.
- , , , et al. Medication reconciliation: a practical tool to reduce the risk of medication errors. J Crit Care. 2003;18(4):201–205.
- , , . Adverse drug event trigger tool: a practical methodology for measuring medication related harm. Qual Saf Health Care. 2003;12(3):194–200.
- , , , , . Improvement in the detection of adverse drug events by the use of electronic health and prescription records: an evaluation of two trigger tools. Eur J Clin Pharmacol. 2013;69(2):255–259.
- , . Adverse Drug Events in U.S. Hospitals, 2004. Rockville, MD: Agency for Healthcare Research and Quality; April 2007. Statistical Brief 29.
- , , . Medication‐Related Adverse Outcomes in U.S. Hospitals and Emergency Departments, 2008. Rockville, MD: Agency for Healthcare Research and Quality; April 2011. Statistical Brief 109.
- US Centers for Medicare and Medicaid Services. Hospital‐Acquired Conditions (Present on Admission Indicator). Available at: http://www.cms.hhs.gov/HospitalAcqCond/. Accessed August 16, 2011. Updated September 20, 2012.
- , , . Fracture risk associated with the use of morphine and opiates. J Intern Med. 2006;260(1):76–87.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
- Clinical Classifications Software, Healthcare Cost and Utilization Project (HCUP). Rockville, MD; Agency for Healthcare Research and Quality; December 2009.
- , , , , . Clinical application of opioid equianalgesic data. Clin J Pain. 2003;19(5)286–297.
- , , , , , . Choosing the unit of measurement counts: the use of oral morphine equivalents in studies of opioid consumption is a useful addition to defined daily doses. Palliat Med. 2011;25(7):725–732.
- , , , . Cost and quality implications of opioid‐based postsurgical pain control using administrative claims data from a large health system: opioid‐related adverse events and their impact on clinical and economic outcomes. Pharmacotherapy. 2013;33(4):383–391.
- , , , et al. Opioid‐related adverse drug events in surgical hospitalizations: impact on costs and length of stay. Ann Pharmacother. 2007;41(3):400–406.
- , , , et al. Cost of opioid‐related adverse drug events in surgical patients. J Pain Symptom Manage. 2003;25(3):276–283.
- , . Opioid analgesics and rates of fatal drug poisoning in the United States. Am J Prev Med. 2006;31(6):506–511.
- , , , et al. Geographic variation in the treatment of acute myocardial infarction: the Cooperative Cardiovascular Project. JAMA. 1999;281(7):627–633.
- , , , et al; GUSTO‐1 Investigators. Regional variation across the United States in the management of acute myocardial infarction. Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries. N Engl J Med. 1995;333(9):565–572.
- , , . Predictors of broad‐spectrum antibiotic prescribing for acute respiratory tract infections in adult primary care. JAMA. 2003;289(6):719–725.
- , , . Geographic variation in Medicare drug spending. N Engl J Med. 2010;363(5):405–409.
- , , . Geographic variation in outpatient antibiotic prescribing among older adults. Arch Intern Med. 2012;172(19):1465–1471.
- , , , et al. Clinical policy: critical issues in the prescribing of opioids for adult patients in the emergency department. Ann Emerg Med. 2012;60(4):499–525.
- The Joint Commission. Facts About Pain Management. Available at: http://www.jointcommission.org/pain_management/. Accessed July 23, 2012.
- The Joint Commission. Sentinel Event Alert: Safe Use of Opioids in Hospitals. Published August 8, 2012. Available at: http://www.jointcommission.org/assets/1/18/SEA_49_opioids_8_2_12_final.pdf. Accessed March 4, 2013.
- , , , , . Research gaps on use of opioids for chronic noncancer pain: findings from a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10(2):147–159.
- , , , , , . Side effects of opioids during short‐term administration: effect of age, gender, and race. Clin Pharmacol Ther. 2003;74(2):102–112.
- , , , . Postoperative day one: a high‐risk period for respiratory events. Am J Surg. 2005;190(5):752–756.
© 2013 Society of Hospital Medicine
Stress Testing Effect on ED Visits
More than 9 million people visit the emergency department (ED) annually for evaluation of acute chest pain.[1, 2] Most of these patients are placed on observation status while being assessed for an acute coronary syndrome (ACS). Traditionally, serial cardiac enzymes and absence of changes suggestive of ischemia on electrocardiogram rule out ACS. Patients are then stratified based on their presentation and risk factors. However, healthcare providers are not comfortable discharging even low‐risk patients without further testing.[3] Routine treadmill stress testing is usually performed, often complimented by an imaging modality. A negative stress test before discharge reassures both the physician and the patient that the chest pain is not caused by an obstructive coronary lesion.
Patients with chest pain who have been discharged from the ED after ruling out an ACS are frequently readmitted for chest pain within 1 year.[4] It is unclear whether stress testing can prevent these readmissions by preventing return to the ED or by influencing the decision of ED physicians to admit patients for observation.[5, 6, 7] Even if stress testing can reduce ED visits or readmissions, it is not known whether the savings from preventing these visits can offset the initial cost of stress testing. The purpose of this study was to examine the impact of stress testing on readmission for chest pain, and to determine whether stress testing can reduce overall costs.
METHODS
Study Population
The hospital's billing database was used to obtain the data. Inclusion criteria included age 18 years or older with index hospitalization between January 2007and July 2009 with International Classification of Diseases, 9th Revision admitting diagnoses of chest pain (786.5), chest pain NOSnot otherwise specified (786.50), chest pain NECnot elsewhere classified (786.59) or angina pectoris (413.9). All eligible patients were admitted under observation status. Although observation patients are technically outpatients, they are cared for by inpatient physicians on inpatient units and are otherwise indistinguishable from inpatients. Patients with a discharge diagnosis of acute myocardial infarction at index admission were excluded. Also, patients who had a chest pain admission or an outpatient stress test within the previous 12 months of index admission were excluded.
Data Collection and Outcomes
All data were extracted electronically from the hospital's billing database. For each patient we noted age, sex, race, insurance status, and cardiovascular comorbidities (current smoker, congestive heart failure, valvular disease, pulmonary/circulatory disorders, peripheral vascular disease, obesity, diabetes mellitus, and hypertension). For each admission we ascertained whether or not any type of stress test was performed. We obtained ED and hospitalization costs for chest pain visits within 12 months of index admission from the hospital's cost accounting system. We also obtained corresponding physician charges as well as collection rate from the health system's clinical decision support system.
The primary outcome was the rate of ED visits and readmissions for chest pain within 1 year of the index visit. Secondary outcomes included total annual hospitalization and ED costs. Total annual costs were calculated by summing index costs and follow‐up costs for subsequent ED visits and readmissions.
Statistical Analysis
Fisher exact (categorical) and unpaired t tests/Wilcoxon rank sum (continuous) tests were used to compare the baseline characteristics of patients who received a stress test at index admission to those who did not. To address possible confounding by indication (allocation bias), the association between stress testing and various outcomes was quantified using multivariable logistic (ED visits and readmissions) or linear regression (costs).[8, 9] In addition, we developed a propensity model using conditional logistic regression and matched patients on propensity score using 1:1 greedy matching algorithm with a caliper tolerance of 0.05.[10, 11] For cost analyses, the annual collection rate was applied to all physician charges, and these were added to hospital or ED costs to obtain the total cost of each visit. The average cost of ED visits or readmissions for each group was calculated by dividing the total ED or readmission cost by the number of ED visits and readmissions, respectively. Physician charges were unavailable for approximately one‐third (1487/5163 or 29%) of all hospitalizations; missing charges were estimated using mean imputation, and sensitivity analyses were conducted to ensure consistency of inferences between full (imputed) and restricted models.[12, 13, 14] Stata/MP 12.1 for Windows (StataCorp, College Station, TX) was used for all analyses.
RESULTS
A total of 3315 patients admitted with chest pain during the study period met the inclusion criteria. Of these, 2376 (71.7%) had a stress test on index admission. Table 1 describes the baseline characteristics of the study population. Receipt of a stress test during index admission was positively associated with white race, private insurance, and number of cardiac comorbidities. The propensity model included these covariates as well as study year, age (80+ vs younger), sex, and smoking status. The C statistic, which quantifies the model's ability to discriminate subjects who received a stress test from those who did not, was 0.63 (95% confidence interval [CI]: 0.61 to 0.65). Of patients who returned to the ED, we were able to find propensity matches for 69% to create a matched sample of 1776 patients. Of patients who were readmitted, we were able to find matches for 83% to create a matched sample of 186 patients.
| Total, N=3315 | Stress Test Original Admission, N=2376 | No Stress Test, N=939 | P Valuea | |
|---|---|---|---|---|
| ||||
| Age, y, mean/SD | 57.5/13.9 | 57.2/12.8 | 58.2/16.2 | 0.10 |
| Male, n (%) | 1505 (45.4) | 1080 (45.5) | 425 (45.3) | 0.94 |
| Race, n (%) | <0.001 | |||
| White | 2082 (62.8) | 1552 (65.3) | 530 (56.4) | |
| Black | 345 (10.4) | 239 (10.1) | 106 (11.3) | |
| Hispanic | 585 (17.7) | 381 (16.0) | 204 (21.7) | |
| Other | 303 (9.1) | 204 (8.6) | 99 (10.5) | |
| Private insurance, n (%) | 1469 (44.3) | 1176 (49.5) | 293 (31.2) | <0.001 |
| No. of cardiovascular comorbidities, mean/SDb | 0.68/0.78 | 0.70/0.78 | 0.64/0.77 | 0.04 |
| Smoker, n (%) | 335 (10.1) | 249 (10.5) | 86 (9.2) | 0.28 |
| Return for chest pain, n (%) | 256 (7.7) | 148 (6.2) | 108 (11.5) | <0.001 |
| All cause return, n (%) | 1279 (38.6) | 819 (34.5) | 460 (49.0) | <0.001 |
| Median time to next chest pain visit, d (25th, 75th percentile) | 69 (6, 180) | 67 (5, 190) | 71 (9, 172) | 0.86 |
| Median time to all cause return, d (25th, 75th percentile) | 92 (27, 198) | 108 (33, 207) | 67 (20, 175) | <0.001 |
| Admitted upon first return for chest pain, n (%) | 112 (43.8) | 62 (41.9) | 50 (46.3) | 0.53 |
Subsequent ED Visits for Chest Pain
Within 1 year, 1279 (38.6%) of all patients returned to the ED, and 256 (7.7%) returned at least once for chest pain. Patients who had a stress test at index admission were less likely to return to ED for chest pain, compared to those who did not get a stress test at admission (6.2% vs 11.5%; P<0.001). The median time to the first subsequent ED visit for any complaint was greater among patients who had a stress test at index admission (108 days vs 67 days, P<0.001), but no effect was noted on time to return for chest complaint (67 days vs 71 days, P=0.86).
In a multivariable model, return to the ED for chest pain was positively associated with self‐reported nonwhite race, insurance with Medicare or Medicaid, and earlier year of index admission (Table 2). Return ED visit was negatively associated with stress testing at index admission (adjusted odds ratio [OR]: 0.5, 95% CI: 0.4 to 0.7; propensity‐matched analysis OR: 0.6, 95% CI: 0.5 to 0.9).
| Variable | Odds Ratio | 95% CI |
|---|---|---|
| ||
| Stress test | 0.5 | 0.4 0.7 |
| Age >80 years | 1.0 | 0.6 1.6 |
| Gender | ||
| Female | 1.0 | |
| Male | 1.0 | 0.8 1.3 |
| Race/ethnicity | ||
| White | 1.0 | |
| Hispanic | 1.6 | 1.2 2.3 |
| Black | 1.6 | 1.1 2.4 |
| Other | 2.3 | 1.6 3.5 |
| 1 Cardiac comorbiditya | 1.1 | 0.8 1.4 |
| Medicare/Medicaid | 1.5 | 1.1 2.0 |
| Year of index admission | ||
| 2007 | 1.0 | |
| 2008 | 0.8 | 0.6 1.1 |
| 2009 | 0.5 | 0.4 0.7 |
| Smoking | 1.4 | 0.9 2.1 |
Subsequent Readmissions for Chest Pain
Of the 256 patients who returned to the ED for chest pain, 112 (43.8%) were readmitted during the first return visit. There was no statistically significant difference in the proportion admitted from the ED by prior stress test status. In a multivariable model, readmission after returning to the ED for chest pain was positively associated with cardiac comorbidities and earlier year of index admission (Table 3). The decision to readmit was not significantly associated with prior stress testing (adjusted OR: 0.8, 95% CI: 0.5 to 1.4; propensity‐matched analysis OR: 0.8, 95% CI: 0.4 to 1.4).
| Variable | Odds Ratio | 95% CI |
|---|---|---|
| ||
| Stress test | 0.8 | 0.5 1.4 |
| Age >80 years | 1.0 | 0.4 2.6 |
| Gender | ||
| Female | 1.0 | |
| Male | 1.0 | 0.6 1.7 |
| Race/ethnicity | ||
| White | 1.0 | |
| Hispanic | 1.3 | 0.6 2.5 |
| Black | 0.6 | 0.2 1.4 |
| Other | 4.5 | 1.9 10.6 |
| 1 Cardiac comorbiditya | 1.8 | 1.0 3.4 |
| Medicare/Medicaid | 1.3 | 0.7 2.4 |
| Year of index admission | ||
| 2007 | 1.0 | |
| 2008 | 0.6 | 0.4 1.2 |
| 2009 | 0.2 | 0.1 0.5 |
| Smoker | 0.3 | 0.1 0.8 |
Cost Analysis
The average multivariable‐adjusted cost (hospital+physician costs) for a patient at index chest pain admission was $3462 if a stress test was performed compared to $2374 without a stress test (+$1088, 95% CI: $972 to $1203). In the propensity‐matched sample the difference was +$1211(95% CI: $1084 to $1338). There were 155 occasions on which a patient returned to the ED for chest pain but was not readmitted. The average per‐visit cost did not differ based on prior stress test status in the overall sample ($763 if stress testing done previously vs $722 if not [+$41, 95% CI: $43 to+$125]) or in the propensity‐matched sample ($787 if stress testing was done vs $744 if not [$43, 95% CI: $54 to +$140]). Because ED visits were less frequent among patients who had a stress test at index admission, the average annual cost of ED visits was significantly lower for this group ($32 vs $52; $20, 95% CI: $36 to $4) or ($42 vs $54; $12 (95% CI: $32 to +$8) in the propensity‐matched sample. For the 117 occasions on which a patient returned with chest pain and was readmitted, the average cost per readmission also did not differ based on whether a stress test was performed at index admission or not ($2912 vs $2806, P=0.85). Again, because readmissions were less common after stress testing, the average cost of readmissions was lower for patients with stress tests than for those without ($88 vs $180; $92, 95% CI: $176 to $8) or $137 vs $194 ( $57, 95% CI: $161 to $47) in the propensity‐matched sample. The total cost of all visits (index, ED, and readmissions) was higher for patients who had a stress test at index admission than for those who did not ($3582 vs $2606; +$975, 95% CI: $829 to $1122) or ($3833 vs $2690; +$1142, 95% CI: $970, $1315) in the propensity‐matched sample.
DISCUSSION
In this retrospective cohort study of patients admitted with low‐risk chest pain, we found that a majority (>70%) underwent stress testing prior to discharge. Within 1 year approximately 8% returned to the ED with chest pain. Stress testing at index admission was associated with 40% reduction in the odds of subsequent ED visits for chest pain; however, once in the ED, having a previous stress test did not significantly affect the decision to admit. Despite the reduction in readmission rates, the overall hospital costsincluding cost of index admission, subsequent ED visits, and readmissionswere higher for patients who had a stress test at index admission.
Two other studies have evaluated the impact of stress testing on return ED visits.[5, 6] In a cohort of 1195 low‐risk chest pain patients at a tertiary center in New York, patients who underwent stress testing were less likely to return to the ED for chest pain within 3 months compared to those who did not get a stress test (10% vs 15%, P<0.001).[5] In contrast, another prospective study of 692 low‐risk chest pain patients found no difference in return ED visits between patients who were evaluated versus those who were not evaluated for underlying coronary artery disease at index admission by stress testing or cardiac catheterization (39% vs 40%; P=0.85).[6] In this study, the lack of difference may have been due to the population sampled, which had high rates of return in both groups. In our study, we also found that having a previous stress test does not significantly impact the decision to admit the patient. This was consistent with the results of another prospective cohort study of low‐risk chest pain patients presenting to the ED.[7]
Previous studies offer conflicting interpretations of the cost implications of stress testing in this population. Based on studies conducted in the 1990s that showed that mandatory stress testing in the ED was cost‐effective compared to hospital admission,[15, 16] the most recent scientific statement by the American Heart Association recommends stress testing for all low‐risk chest pain patients.[17] However, more recent studies have questioned the value of diagnostic testing beyond serial electrocardiograms and cardiac enzymes in low‐risk patients.[18, 19, 20, 21, 22] In a study done at our institution among patients admitted with low‐risk chest pain, the rate of positive stress tests was noted to be extremely low, and patients had a benign course; at 30 days the rates of major cardiovascular events was as low as 0.3%.[19] Other studies also showed no difference in outcomes among patients who received inpatient, outpatient, or no stress testing.[21, 22]
These studies have generally been limited to the initial hospitalization period. Our study extends these findings in terms of resource utilization to the year following hospitalization. This is important because physicians might order stress tests to reassure patients or themselves that the pain is noncardiac, with the hope that this will decrease subsequent ED visits or readmissions. In our study, stress tests did reduce both ED visits and readmissions, but the index cost of hospitalization was so much higher with stress testing that the reduced readmissions did not offset the initial costs. Because stress tests have not been shown to change cardiovascular outcomes but did increase costs, it may be time to reevaluate the need for any kind of inpatient stress testing in these patients.
Our study has several limitations. The retrospective nature of the study subjects it to confounding. We adjusted for demographics, insurance, and comorbidities, but other unmeasured elements of the patients' presentation might have affected stress test ordering and subsequent return to the ED. In addition, we relied on administrative data, and comorbidities may not have been documented completely. During the follow‐up period, we did not take into account patients who presented to the EDs of other hospitals or those who might have died. Because there is only one other hospital in our city, and it does not perform angioplasties, it is unlikely that we missed many infarctions this way, but we may not have included all ED visits. Similarly, we included only costs accrued within our healthcare system. If patients presented to outside facilities for testing or treatment, we were unable to capture it. It is possible that patients who did not undergo initial stress testing may have been more likely to have subsequent testing at outside facilities, which would have reduced the difference in cost that we observed. However, given the magnitude of this difference, it is unlikely that including outside costs would have completely eliminated the difference. The data in our study were collected over a 3‐year period. Secular trends in the healthcare system over that time could potentially have affected our results. To reduce this bias, we included the year of the study in the propensity model. Also, the study was performed at a single hospital, and the results might not be generalizable to other institutions. Ours is a large independent academic medical center serving both a tertiary and a community role. Therefore, the population it serves would appear to be representative of the general population having chest pain without ACS.
Finally, we did not collect data on the results of stress tests. It is probable that the decision to admit a patient is modified by the results of a previous test, and this was not explored in our analysis. Presumably, patients with positive tests would be more likely, and those with negative tests less likely, to be admitted than patients who had no previous test. Previous studies have shown that among low‐risk chest pain patients, the rate of abnormal stress tests is <15%, and among these only a minority (0.6%0.7%) can benefit from revascularization.[19, 20] Therefore, testing should result in a lower rate of readmissions overall, which is what we observed in this study. Once patients reached the ED, however, the decision to admit was not associated with having a previous stress test. This could be due to a high rate of positive tests among patients who came to the ED, or a lack of discrimination by ED physicians. Although our study design could not distinguish between these 2 possibilities, studies have shown that fear of litigation and aversion to risk play an important role in this decision,[23, 24] and it is possible that these considerations override the results of previous stress tests, which cannot categorically rule out current ischemia.
In an era of rising healthcare costs and limited resources, the care of low‐risk chest pain is an attractive target for cost‐reduction strategies. Low‐risk chest pain accounts for 1.8 % of all admissions, at an average annual cost of $3.4 billion in the United States,[25] so figuring out how to prevent such admissions has important economic implications. Although stress testing did keep patients from returning to the ED, it did not affect the ED physicians' decisions to admit. We found that stress testing does decrease subsequent resource utilization, but not enough to offset the initial cost of testing. Thus, stress testing does not appear to be a cost‐effective means to reduce readmissions.
Disclosures: Jaya Mallidi and Michael Rothberg had full access to all of the data in the study and take full responsibility for the integrity of the data and accuracy of the analysis. The authors report no conflicts of interest.
More than 9 million people visit the emergency department (ED) annually for evaluation of acute chest pain.[1, 2] Most of these patients are placed on observation status while being assessed for an acute coronary syndrome (ACS). Traditionally, serial cardiac enzymes and absence of changes suggestive of ischemia on electrocardiogram rule out ACS. Patients are then stratified based on their presentation and risk factors. However, healthcare providers are not comfortable discharging even low‐risk patients without further testing.[3] Routine treadmill stress testing is usually performed, often complimented by an imaging modality. A negative stress test before discharge reassures both the physician and the patient that the chest pain is not caused by an obstructive coronary lesion.
Patients with chest pain who have been discharged from the ED after ruling out an ACS are frequently readmitted for chest pain within 1 year.[4] It is unclear whether stress testing can prevent these readmissions by preventing return to the ED or by influencing the decision of ED physicians to admit patients for observation.[5, 6, 7] Even if stress testing can reduce ED visits or readmissions, it is not known whether the savings from preventing these visits can offset the initial cost of stress testing. The purpose of this study was to examine the impact of stress testing on readmission for chest pain, and to determine whether stress testing can reduce overall costs.
METHODS
Study Population
The hospital's billing database was used to obtain the data. Inclusion criteria included age 18 years or older with index hospitalization between January 2007and July 2009 with International Classification of Diseases, 9th Revision admitting diagnoses of chest pain (786.5), chest pain NOSnot otherwise specified (786.50), chest pain NECnot elsewhere classified (786.59) or angina pectoris (413.9). All eligible patients were admitted under observation status. Although observation patients are technically outpatients, they are cared for by inpatient physicians on inpatient units and are otherwise indistinguishable from inpatients. Patients with a discharge diagnosis of acute myocardial infarction at index admission were excluded. Also, patients who had a chest pain admission or an outpatient stress test within the previous 12 months of index admission were excluded.
Data Collection and Outcomes
All data were extracted electronically from the hospital's billing database. For each patient we noted age, sex, race, insurance status, and cardiovascular comorbidities (current smoker, congestive heart failure, valvular disease, pulmonary/circulatory disorders, peripheral vascular disease, obesity, diabetes mellitus, and hypertension). For each admission we ascertained whether or not any type of stress test was performed. We obtained ED and hospitalization costs for chest pain visits within 12 months of index admission from the hospital's cost accounting system. We also obtained corresponding physician charges as well as collection rate from the health system's clinical decision support system.
The primary outcome was the rate of ED visits and readmissions for chest pain within 1 year of the index visit. Secondary outcomes included total annual hospitalization and ED costs. Total annual costs were calculated by summing index costs and follow‐up costs for subsequent ED visits and readmissions.
Statistical Analysis
Fisher exact (categorical) and unpaired t tests/Wilcoxon rank sum (continuous) tests were used to compare the baseline characteristics of patients who received a stress test at index admission to those who did not. To address possible confounding by indication (allocation bias), the association between stress testing and various outcomes was quantified using multivariable logistic (ED visits and readmissions) or linear regression (costs).[8, 9] In addition, we developed a propensity model using conditional logistic regression and matched patients on propensity score using 1:1 greedy matching algorithm with a caliper tolerance of 0.05.[10, 11] For cost analyses, the annual collection rate was applied to all physician charges, and these were added to hospital or ED costs to obtain the total cost of each visit. The average cost of ED visits or readmissions for each group was calculated by dividing the total ED or readmission cost by the number of ED visits and readmissions, respectively. Physician charges were unavailable for approximately one‐third (1487/5163 or 29%) of all hospitalizations; missing charges were estimated using mean imputation, and sensitivity analyses were conducted to ensure consistency of inferences between full (imputed) and restricted models.[12, 13, 14] Stata/MP 12.1 for Windows (StataCorp, College Station, TX) was used for all analyses.
RESULTS
A total of 3315 patients admitted with chest pain during the study period met the inclusion criteria. Of these, 2376 (71.7%) had a stress test on index admission. Table 1 describes the baseline characteristics of the study population. Receipt of a stress test during index admission was positively associated with white race, private insurance, and number of cardiac comorbidities. The propensity model included these covariates as well as study year, age (80+ vs younger), sex, and smoking status. The C statistic, which quantifies the model's ability to discriminate subjects who received a stress test from those who did not, was 0.63 (95% confidence interval [CI]: 0.61 to 0.65). Of patients who returned to the ED, we were able to find propensity matches for 69% to create a matched sample of 1776 patients. Of patients who were readmitted, we were able to find matches for 83% to create a matched sample of 186 patients.
| Total, N=3315 | Stress Test Original Admission, N=2376 | No Stress Test, N=939 | P Valuea | |
|---|---|---|---|---|
| ||||
| Age, y, mean/SD | 57.5/13.9 | 57.2/12.8 | 58.2/16.2 | 0.10 |
| Male, n (%) | 1505 (45.4) | 1080 (45.5) | 425 (45.3) | 0.94 |
| Race, n (%) | <0.001 | |||
| White | 2082 (62.8) | 1552 (65.3) | 530 (56.4) | |
| Black | 345 (10.4) | 239 (10.1) | 106 (11.3) | |
| Hispanic | 585 (17.7) | 381 (16.0) | 204 (21.7) | |
| Other | 303 (9.1) | 204 (8.6) | 99 (10.5) | |
| Private insurance, n (%) | 1469 (44.3) | 1176 (49.5) | 293 (31.2) | <0.001 |
| No. of cardiovascular comorbidities, mean/SDb | 0.68/0.78 | 0.70/0.78 | 0.64/0.77 | 0.04 |
| Smoker, n (%) | 335 (10.1) | 249 (10.5) | 86 (9.2) | 0.28 |
| Return for chest pain, n (%) | 256 (7.7) | 148 (6.2) | 108 (11.5) | <0.001 |
| All cause return, n (%) | 1279 (38.6) | 819 (34.5) | 460 (49.0) | <0.001 |
| Median time to next chest pain visit, d (25th, 75th percentile) | 69 (6, 180) | 67 (5, 190) | 71 (9, 172) | 0.86 |
| Median time to all cause return, d (25th, 75th percentile) | 92 (27, 198) | 108 (33, 207) | 67 (20, 175) | <0.001 |
| Admitted upon first return for chest pain, n (%) | 112 (43.8) | 62 (41.9) | 50 (46.3) | 0.53 |
Subsequent ED Visits for Chest Pain
Within 1 year, 1279 (38.6%) of all patients returned to the ED, and 256 (7.7%) returned at least once for chest pain. Patients who had a stress test at index admission were less likely to return to ED for chest pain, compared to those who did not get a stress test at admission (6.2% vs 11.5%; P<0.001). The median time to the first subsequent ED visit for any complaint was greater among patients who had a stress test at index admission (108 days vs 67 days, P<0.001), but no effect was noted on time to return for chest complaint (67 days vs 71 days, P=0.86).
In a multivariable model, return to the ED for chest pain was positively associated with self‐reported nonwhite race, insurance with Medicare or Medicaid, and earlier year of index admission (Table 2). Return ED visit was negatively associated with stress testing at index admission (adjusted odds ratio [OR]: 0.5, 95% CI: 0.4 to 0.7; propensity‐matched analysis OR: 0.6, 95% CI: 0.5 to 0.9).
| Variable | Odds Ratio | 95% CI |
|---|---|---|
| ||
| Stress test | 0.5 | 0.4 0.7 |
| Age >80 years | 1.0 | 0.6 1.6 |
| Gender | ||
| Female | 1.0 | |
| Male | 1.0 | 0.8 1.3 |
| Race/ethnicity | ||
| White | 1.0 | |
| Hispanic | 1.6 | 1.2 2.3 |
| Black | 1.6 | 1.1 2.4 |
| Other | 2.3 | 1.6 3.5 |
| 1 Cardiac comorbiditya | 1.1 | 0.8 1.4 |
| Medicare/Medicaid | 1.5 | 1.1 2.0 |
| Year of index admission | ||
| 2007 | 1.0 | |
| 2008 | 0.8 | 0.6 1.1 |
| 2009 | 0.5 | 0.4 0.7 |
| Smoking | 1.4 | 0.9 2.1 |
Subsequent Readmissions for Chest Pain
Of the 256 patients who returned to the ED for chest pain, 112 (43.8%) were readmitted during the first return visit. There was no statistically significant difference in the proportion admitted from the ED by prior stress test status. In a multivariable model, readmission after returning to the ED for chest pain was positively associated with cardiac comorbidities and earlier year of index admission (Table 3). The decision to readmit was not significantly associated with prior stress testing (adjusted OR: 0.8, 95% CI: 0.5 to 1.4; propensity‐matched analysis OR: 0.8, 95% CI: 0.4 to 1.4).
| Variable | Odds Ratio | 95% CI |
|---|---|---|
| ||
| Stress test | 0.8 | 0.5 1.4 |
| Age >80 years | 1.0 | 0.4 2.6 |
| Gender | ||
| Female | 1.0 | |
| Male | 1.0 | 0.6 1.7 |
| Race/ethnicity | ||
| White | 1.0 | |
| Hispanic | 1.3 | 0.6 2.5 |
| Black | 0.6 | 0.2 1.4 |
| Other | 4.5 | 1.9 10.6 |
| 1 Cardiac comorbiditya | 1.8 | 1.0 3.4 |
| Medicare/Medicaid | 1.3 | 0.7 2.4 |
| Year of index admission | ||
| 2007 | 1.0 | |
| 2008 | 0.6 | 0.4 1.2 |
| 2009 | 0.2 | 0.1 0.5 |
| Smoker | 0.3 | 0.1 0.8 |
Cost Analysis
The average multivariable‐adjusted cost (hospital+physician costs) for a patient at index chest pain admission was $3462 if a stress test was performed compared to $2374 without a stress test (+$1088, 95% CI: $972 to $1203). In the propensity‐matched sample the difference was +$1211(95% CI: $1084 to $1338). There were 155 occasions on which a patient returned to the ED for chest pain but was not readmitted. The average per‐visit cost did not differ based on prior stress test status in the overall sample ($763 if stress testing done previously vs $722 if not [+$41, 95% CI: $43 to+$125]) or in the propensity‐matched sample ($787 if stress testing was done vs $744 if not [$43, 95% CI: $54 to +$140]). Because ED visits were less frequent among patients who had a stress test at index admission, the average annual cost of ED visits was significantly lower for this group ($32 vs $52; $20, 95% CI: $36 to $4) or ($42 vs $54; $12 (95% CI: $32 to +$8) in the propensity‐matched sample. For the 117 occasions on which a patient returned with chest pain and was readmitted, the average cost per readmission also did not differ based on whether a stress test was performed at index admission or not ($2912 vs $2806, P=0.85). Again, because readmissions were less common after stress testing, the average cost of readmissions was lower for patients with stress tests than for those without ($88 vs $180; $92, 95% CI: $176 to $8) or $137 vs $194 ( $57, 95% CI: $161 to $47) in the propensity‐matched sample. The total cost of all visits (index, ED, and readmissions) was higher for patients who had a stress test at index admission than for those who did not ($3582 vs $2606; +$975, 95% CI: $829 to $1122) or ($3833 vs $2690; +$1142, 95% CI: $970, $1315) in the propensity‐matched sample.
DISCUSSION
In this retrospective cohort study of patients admitted with low‐risk chest pain, we found that a majority (>70%) underwent stress testing prior to discharge. Within 1 year approximately 8% returned to the ED with chest pain. Stress testing at index admission was associated with 40% reduction in the odds of subsequent ED visits for chest pain; however, once in the ED, having a previous stress test did not significantly affect the decision to admit. Despite the reduction in readmission rates, the overall hospital costsincluding cost of index admission, subsequent ED visits, and readmissionswere higher for patients who had a stress test at index admission.
Two other studies have evaluated the impact of stress testing on return ED visits.[5, 6] In a cohort of 1195 low‐risk chest pain patients at a tertiary center in New York, patients who underwent stress testing were less likely to return to the ED for chest pain within 3 months compared to those who did not get a stress test (10% vs 15%, P<0.001).[5] In contrast, another prospective study of 692 low‐risk chest pain patients found no difference in return ED visits between patients who were evaluated versus those who were not evaluated for underlying coronary artery disease at index admission by stress testing or cardiac catheterization (39% vs 40%; P=0.85).[6] In this study, the lack of difference may have been due to the population sampled, which had high rates of return in both groups. In our study, we also found that having a previous stress test does not significantly impact the decision to admit the patient. This was consistent with the results of another prospective cohort study of low‐risk chest pain patients presenting to the ED.[7]
Previous studies offer conflicting interpretations of the cost implications of stress testing in this population. Based on studies conducted in the 1990s that showed that mandatory stress testing in the ED was cost‐effective compared to hospital admission,[15, 16] the most recent scientific statement by the American Heart Association recommends stress testing for all low‐risk chest pain patients.[17] However, more recent studies have questioned the value of diagnostic testing beyond serial electrocardiograms and cardiac enzymes in low‐risk patients.[18, 19, 20, 21, 22] In a study done at our institution among patients admitted with low‐risk chest pain, the rate of positive stress tests was noted to be extremely low, and patients had a benign course; at 30 days the rates of major cardiovascular events was as low as 0.3%.[19] Other studies also showed no difference in outcomes among patients who received inpatient, outpatient, or no stress testing.[21, 22]
These studies have generally been limited to the initial hospitalization period. Our study extends these findings in terms of resource utilization to the year following hospitalization. This is important because physicians might order stress tests to reassure patients or themselves that the pain is noncardiac, with the hope that this will decrease subsequent ED visits or readmissions. In our study, stress tests did reduce both ED visits and readmissions, but the index cost of hospitalization was so much higher with stress testing that the reduced readmissions did not offset the initial costs. Because stress tests have not been shown to change cardiovascular outcomes but did increase costs, it may be time to reevaluate the need for any kind of inpatient stress testing in these patients.
Our study has several limitations. The retrospective nature of the study subjects it to confounding. We adjusted for demographics, insurance, and comorbidities, but other unmeasured elements of the patients' presentation might have affected stress test ordering and subsequent return to the ED. In addition, we relied on administrative data, and comorbidities may not have been documented completely. During the follow‐up period, we did not take into account patients who presented to the EDs of other hospitals or those who might have died. Because there is only one other hospital in our city, and it does not perform angioplasties, it is unlikely that we missed many infarctions this way, but we may not have included all ED visits. Similarly, we included only costs accrued within our healthcare system. If patients presented to outside facilities for testing or treatment, we were unable to capture it. It is possible that patients who did not undergo initial stress testing may have been more likely to have subsequent testing at outside facilities, which would have reduced the difference in cost that we observed. However, given the magnitude of this difference, it is unlikely that including outside costs would have completely eliminated the difference. The data in our study were collected over a 3‐year period. Secular trends in the healthcare system over that time could potentially have affected our results. To reduce this bias, we included the year of the study in the propensity model. Also, the study was performed at a single hospital, and the results might not be generalizable to other institutions. Ours is a large independent academic medical center serving both a tertiary and a community role. Therefore, the population it serves would appear to be representative of the general population having chest pain without ACS.
Finally, we did not collect data on the results of stress tests. It is probable that the decision to admit a patient is modified by the results of a previous test, and this was not explored in our analysis. Presumably, patients with positive tests would be more likely, and those with negative tests less likely, to be admitted than patients who had no previous test. Previous studies have shown that among low‐risk chest pain patients, the rate of abnormal stress tests is <15%, and among these only a minority (0.6%0.7%) can benefit from revascularization.[19, 20] Therefore, testing should result in a lower rate of readmissions overall, which is what we observed in this study. Once patients reached the ED, however, the decision to admit was not associated with having a previous stress test. This could be due to a high rate of positive tests among patients who came to the ED, or a lack of discrimination by ED physicians. Although our study design could not distinguish between these 2 possibilities, studies have shown that fear of litigation and aversion to risk play an important role in this decision,[23, 24] and it is possible that these considerations override the results of previous stress tests, which cannot categorically rule out current ischemia.
In an era of rising healthcare costs and limited resources, the care of low‐risk chest pain is an attractive target for cost‐reduction strategies. Low‐risk chest pain accounts for 1.8 % of all admissions, at an average annual cost of $3.4 billion in the United States,[25] so figuring out how to prevent such admissions has important economic implications. Although stress testing did keep patients from returning to the ED, it did not affect the ED physicians' decisions to admit. We found that stress testing does decrease subsequent resource utilization, but not enough to offset the initial cost of testing. Thus, stress testing does not appear to be a cost‐effective means to reduce readmissions.
Disclosures: Jaya Mallidi and Michael Rothberg had full access to all of the data in the study and take full responsibility for the integrity of the data and accuracy of the analysis. The authors report no conflicts of interest.
More than 9 million people visit the emergency department (ED) annually for evaluation of acute chest pain.[1, 2] Most of these patients are placed on observation status while being assessed for an acute coronary syndrome (ACS). Traditionally, serial cardiac enzymes and absence of changes suggestive of ischemia on electrocardiogram rule out ACS. Patients are then stratified based on their presentation and risk factors. However, healthcare providers are not comfortable discharging even low‐risk patients without further testing.[3] Routine treadmill stress testing is usually performed, often complimented by an imaging modality. A negative stress test before discharge reassures both the physician and the patient that the chest pain is not caused by an obstructive coronary lesion.
Patients with chest pain who have been discharged from the ED after ruling out an ACS are frequently readmitted for chest pain within 1 year.[4] It is unclear whether stress testing can prevent these readmissions by preventing return to the ED or by influencing the decision of ED physicians to admit patients for observation.[5, 6, 7] Even if stress testing can reduce ED visits or readmissions, it is not known whether the savings from preventing these visits can offset the initial cost of stress testing. The purpose of this study was to examine the impact of stress testing on readmission for chest pain, and to determine whether stress testing can reduce overall costs.
METHODS
Study Population
The hospital's billing database was used to obtain the data. Inclusion criteria included age 18 years or older with index hospitalization between January 2007and July 2009 with International Classification of Diseases, 9th Revision admitting diagnoses of chest pain (786.5), chest pain NOSnot otherwise specified (786.50), chest pain NECnot elsewhere classified (786.59) or angina pectoris (413.9). All eligible patients were admitted under observation status. Although observation patients are technically outpatients, they are cared for by inpatient physicians on inpatient units and are otherwise indistinguishable from inpatients. Patients with a discharge diagnosis of acute myocardial infarction at index admission were excluded. Also, patients who had a chest pain admission or an outpatient stress test within the previous 12 months of index admission were excluded.
Data Collection and Outcomes
All data were extracted electronically from the hospital's billing database. For each patient we noted age, sex, race, insurance status, and cardiovascular comorbidities (current smoker, congestive heart failure, valvular disease, pulmonary/circulatory disorders, peripheral vascular disease, obesity, diabetes mellitus, and hypertension). For each admission we ascertained whether or not any type of stress test was performed. We obtained ED and hospitalization costs for chest pain visits within 12 months of index admission from the hospital's cost accounting system. We also obtained corresponding physician charges as well as collection rate from the health system's clinical decision support system.
The primary outcome was the rate of ED visits and readmissions for chest pain within 1 year of the index visit. Secondary outcomes included total annual hospitalization and ED costs. Total annual costs were calculated by summing index costs and follow‐up costs for subsequent ED visits and readmissions.
Statistical Analysis
Fisher exact (categorical) and unpaired t tests/Wilcoxon rank sum (continuous) tests were used to compare the baseline characteristics of patients who received a stress test at index admission to those who did not. To address possible confounding by indication (allocation bias), the association between stress testing and various outcomes was quantified using multivariable logistic (ED visits and readmissions) or linear regression (costs).[8, 9] In addition, we developed a propensity model using conditional logistic regression and matched patients on propensity score using 1:1 greedy matching algorithm with a caliper tolerance of 0.05.[10, 11] For cost analyses, the annual collection rate was applied to all physician charges, and these were added to hospital or ED costs to obtain the total cost of each visit. The average cost of ED visits or readmissions for each group was calculated by dividing the total ED or readmission cost by the number of ED visits and readmissions, respectively. Physician charges were unavailable for approximately one‐third (1487/5163 or 29%) of all hospitalizations; missing charges were estimated using mean imputation, and sensitivity analyses were conducted to ensure consistency of inferences between full (imputed) and restricted models.[12, 13, 14] Stata/MP 12.1 for Windows (StataCorp, College Station, TX) was used for all analyses.
RESULTS
A total of 3315 patients admitted with chest pain during the study period met the inclusion criteria. Of these, 2376 (71.7%) had a stress test on index admission. Table 1 describes the baseline characteristics of the study population. Receipt of a stress test during index admission was positively associated with white race, private insurance, and number of cardiac comorbidities. The propensity model included these covariates as well as study year, age (80+ vs younger), sex, and smoking status. The C statistic, which quantifies the model's ability to discriminate subjects who received a stress test from those who did not, was 0.63 (95% confidence interval [CI]: 0.61 to 0.65). Of patients who returned to the ED, we were able to find propensity matches for 69% to create a matched sample of 1776 patients. Of patients who were readmitted, we were able to find matches for 83% to create a matched sample of 186 patients.
| Total, N=3315 | Stress Test Original Admission, N=2376 | No Stress Test, N=939 | P Valuea | |
|---|---|---|---|---|
| ||||
| Age, y, mean/SD | 57.5/13.9 | 57.2/12.8 | 58.2/16.2 | 0.10 |
| Male, n (%) | 1505 (45.4) | 1080 (45.5) | 425 (45.3) | 0.94 |
| Race, n (%) | <0.001 | |||
| White | 2082 (62.8) | 1552 (65.3) | 530 (56.4) | |
| Black | 345 (10.4) | 239 (10.1) | 106 (11.3) | |
| Hispanic | 585 (17.7) | 381 (16.0) | 204 (21.7) | |
| Other | 303 (9.1) | 204 (8.6) | 99 (10.5) | |
| Private insurance, n (%) | 1469 (44.3) | 1176 (49.5) | 293 (31.2) | <0.001 |
| No. of cardiovascular comorbidities, mean/SDb | 0.68/0.78 | 0.70/0.78 | 0.64/0.77 | 0.04 |
| Smoker, n (%) | 335 (10.1) | 249 (10.5) | 86 (9.2) | 0.28 |
| Return for chest pain, n (%) | 256 (7.7) | 148 (6.2) | 108 (11.5) | <0.001 |
| All cause return, n (%) | 1279 (38.6) | 819 (34.5) | 460 (49.0) | <0.001 |
| Median time to next chest pain visit, d (25th, 75th percentile) | 69 (6, 180) | 67 (5, 190) | 71 (9, 172) | 0.86 |
| Median time to all cause return, d (25th, 75th percentile) | 92 (27, 198) | 108 (33, 207) | 67 (20, 175) | <0.001 |
| Admitted upon first return for chest pain, n (%) | 112 (43.8) | 62 (41.9) | 50 (46.3) | 0.53 |
Subsequent ED Visits for Chest Pain
Within 1 year, 1279 (38.6%) of all patients returned to the ED, and 256 (7.7%) returned at least once for chest pain. Patients who had a stress test at index admission were less likely to return to ED for chest pain, compared to those who did not get a stress test at admission (6.2% vs 11.5%; P<0.001). The median time to the first subsequent ED visit for any complaint was greater among patients who had a stress test at index admission (108 days vs 67 days, P<0.001), but no effect was noted on time to return for chest complaint (67 days vs 71 days, P=0.86).
In a multivariable model, return to the ED for chest pain was positively associated with self‐reported nonwhite race, insurance with Medicare or Medicaid, and earlier year of index admission (Table 2). Return ED visit was negatively associated with stress testing at index admission (adjusted odds ratio [OR]: 0.5, 95% CI: 0.4 to 0.7; propensity‐matched analysis OR: 0.6, 95% CI: 0.5 to 0.9).
| Variable | Odds Ratio | 95% CI |
|---|---|---|
| ||
| Stress test | 0.5 | 0.4 0.7 |
| Age >80 years | 1.0 | 0.6 1.6 |
| Gender | ||
| Female | 1.0 | |
| Male | 1.0 | 0.8 1.3 |
| Race/ethnicity | ||
| White | 1.0 | |
| Hispanic | 1.6 | 1.2 2.3 |
| Black | 1.6 | 1.1 2.4 |
| Other | 2.3 | 1.6 3.5 |
| 1 Cardiac comorbiditya | 1.1 | 0.8 1.4 |
| Medicare/Medicaid | 1.5 | 1.1 2.0 |
| Year of index admission | ||
| 2007 | 1.0 | |
| 2008 | 0.8 | 0.6 1.1 |
| 2009 | 0.5 | 0.4 0.7 |
| Smoking | 1.4 | 0.9 2.1 |
Subsequent Readmissions for Chest Pain
Of the 256 patients who returned to the ED for chest pain, 112 (43.8%) were readmitted during the first return visit. There was no statistically significant difference in the proportion admitted from the ED by prior stress test status. In a multivariable model, readmission after returning to the ED for chest pain was positively associated with cardiac comorbidities and earlier year of index admission (Table 3). The decision to readmit was not significantly associated with prior stress testing (adjusted OR: 0.8, 95% CI: 0.5 to 1.4; propensity‐matched analysis OR: 0.8, 95% CI: 0.4 to 1.4).
| Variable | Odds Ratio | 95% CI |
|---|---|---|
| ||
| Stress test | 0.8 | 0.5 1.4 |
| Age >80 years | 1.0 | 0.4 2.6 |
| Gender | ||
| Female | 1.0 | |
| Male | 1.0 | 0.6 1.7 |
| Race/ethnicity | ||
| White | 1.0 | |
| Hispanic | 1.3 | 0.6 2.5 |
| Black | 0.6 | 0.2 1.4 |
| Other | 4.5 | 1.9 10.6 |
| 1 Cardiac comorbiditya | 1.8 | 1.0 3.4 |
| Medicare/Medicaid | 1.3 | 0.7 2.4 |
| Year of index admission | ||
| 2007 | 1.0 | |
| 2008 | 0.6 | 0.4 1.2 |
| 2009 | 0.2 | 0.1 0.5 |
| Smoker | 0.3 | 0.1 0.8 |
Cost Analysis
The average multivariable‐adjusted cost (hospital+physician costs) for a patient at index chest pain admission was $3462 if a stress test was performed compared to $2374 without a stress test (+$1088, 95% CI: $972 to $1203). In the propensity‐matched sample the difference was +$1211(95% CI: $1084 to $1338). There were 155 occasions on which a patient returned to the ED for chest pain but was not readmitted. The average per‐visit cost did not differ based on prior stress test status in the overall sample ($763 if stress testing done previously vs $722 if not [+$41, 95% CI: $43 to+$125]) or in the propensity‐matched sample ($787 if stress testing was done vs $744 if not [$43, 95% CI: $54 to +$140]). Because ED visits were less frequent among patients who had a stress test at index admission, the average annual cost of ED visits was significantly lower for this group ($32 vs $52; $20, 95% CI: $36 to $4) or ($42 vs $54; $12 (95% CI: $32 to +$8) in the propensity‐matched sample. For the 117 occasions on which a patient returned with chest pain and was readmitted, the average cost per readmission also did not differ based on whether a stress test was performed at index admission or not ($2912 vs $2806, P=0.85). Again, because readmissions were less common after stress testing, the average cost of readmissions was lower for patients with stress tests than for those without ($88 vs $180; $92, 95% CI: $176 to $8) or $137 vs $194 ( $57, 95% CI: $161 to $47) in the propensity‐matched sample. The total cost of all visits (index, ED, and readmissions) was higher for patients who had a stress test at index admission than for those who did not ($3582 vs $2606; +$975, 95% CI: $829 to $1122) or ($3833 vs $2690; +$1142, 95% CI: $970, $1315) in the propensity‐matched sample.
DISCUSSION
In this retrospective cohort study of patients admitted with low‐risk chest pain, we found that a majority (>70%) underwent stress testing prior to discharge. Within 1 year approximately 8% returned to the ED with chest pain. Stress testing at index admission was associated with 40% reduction in the odds of subsequent ED visits for chest pain; however, once in the ED, having a previous stress test did not significantly affect the decision to admit. Despite the reduction in readmission rates, the overall hospital costsincluding cost of index admission, subsequent ED visits, and readmissionswere higher for patients who had a stress test at index admission.
Two other studies have evaluated the impact of stress testing on return ED visits.[5, 6] In a cohort of 1195 low‐risk chest pain patients at a tertiary center in New York, patients who underwent stress testing were less likely to return to the ED for chest pain within 3 months compared to those who did not get a stress test (10% vs 15%, P<0.001).[5] In contrast, another prospective study of 692 low‐risk chest pain patients found no difference in return ED visits between patients who were evaluated versus those who were not evaluated for underlying coronary artery disease at index admission by stress testing or cardiac catheterization (39% vs 40%; P=0.85).[6] In this study, the lack of difference may have been due to the population sampled, which had high rates of return in both groups. In our study, we also found that having a previous stress test does not significantly impact the decision to admit the patient. This was consistent with the results of another prospective cohort study of low‐risk chest pain patients presenting to the ED.[7]
Previous studies offer conflicting interpretations of the cost implications of stress testing in this population. Based on studies conducted in the 1990s that showed that mandatory stress testing in the ED was cost‐effective compared to hospital admission,[15, 16] the most recent scientific statement by the American Heart Association recommends stress testing for all low‐risk chest pain patients.[17] However, more recent studies have questioned the value of diagnostic testing beyond serial electrocardiograms and cardiac enzymes in low‐risk patients.[18, 19, 20, 21, 22] In a study done at our institution among patients admitted with low‐risk chest pain, the rate of positive stress tests was noted to be extremely low, and patients had a benign course; at 30 days the rates of major cardiovascular events was as low as 0.3%.[19] Other studies also showed no difference in outcomes among patients who received inpatient, outpatient, or no stress testing.[21, 22]
These studies have generally been limited to the initial hospitalization period. Our study extends these findings in terms of resource utilization to the year following hospitalization. This is important because physicians might order stress tests to reassure patients or themselves that the pain is noncardiac, with the hope that this will decrease subsequent ED visits or readmissions. In our study, stress tests did reduce both ED visits and readmissions, but the index cost of hospitalization was so much higher with stress testing that the reduced readmissions did not offset the initial costs. Because stress tests have not been shown to change cardiovascular outcomes but did increase costs, it may be time to reevaluate the need for any kind of inpatient stress testing in these patients.
Our study has several limitations. The retrospective nature of the study subjects it to confounding. We adjusted for demographics, insurance, and comorbidities, but other unmeasured elements of the patients' presentation might have affected stress test ordering and subsequent return to the ED. In addition, we relied on administrative data, and comorbidities may not have been documented completely. During the follow‐up period, we did not take into account patients who presented to the EDs of other hospitals or those who might have died. Because there is only one other hospital in our city, and it does not perform angioplasties, it is unlikely that we missed many infarctions this way, but we may not have included all ED visits. Similarly, we included only costs accrued within our healthcare system. If patients presented to outside facilities for testing or treatment, we were unable to capture it. It is possible that patients who did not undergo initial stress testing may have been more likely to have subsequent testing at outside facilities, which would have reduced the difference in cost that we observed. However, given the magnitude of this difference, it is unlikely that including outside costs would have completely eliminated the difference. The data in our study were collected over a 3‐year period. Secular trends in the healthcare system over that time could potentially have affected our results. To reduce this bias, we included the year of the study in the propensity model. Also, the study was performed at a single hospital, and the results might not be generalizable to other institutions. Ours is a large independent academic medical center serving both a tertiary and a community role. Therefore, the population it serves would appear to be representative of the general population having chest pain without ACS.
Finally, we did not collect data on the results of stress tests. It is probable that the decision to admit a patient is modified by the results of a previous test, and this was not explored in our analysis. Presumably, patients with positive tests would be more likely, and those with negative tests less likely, to be admitted than patients who had no previous test. Previous studies have shown that among low‐risk chest pain patients, the rate of abnormal stress tests is <15%, and among these only a minority (0.6%0.7%) can benefit from revascularization.[19, 20] Therefore, testing should result in a lower rate of readmissions overall, which is what we observed in this study. Once patients reached the ED, however, the decision to admit was not associated with having a previous stress test. This could be due to a high rate of positive tests among patients who came to the ED, or a lack of discrimination by ED physicians. Although our study design could not distinguish between these 2 possibilities, studies have shown that fear of litigation and aversion to risk play an important role in this decision,[23, 24] and it is possible that these considerations override the results of previous stress tests, which cannot categorically rule out current ischemia.
In an era of rising healthcare costs and limited resources, the care of low‐risk chest pain is an attractive target for cost‐reduction strategies. Low‐risk chest pain accounts for 1.8 % of all admissions, at an average annual cost of $3.4 billion in the United States,[25] so figuring out how to prevent such admissions has important economic implications. Although stress testing did keep patients from returning to the ED, it did not affect the ED physicians' decisions to admit. We found that stress testing does decrease subsequent resource utilization, but not enough to offset the initial cost of testing. Thus, stress testing does not appear to be a cost‐effective means to reduce readmissions.
Disclosures: Jaya Mallidi and Michael Rothberg had full access to all of the data in the study and take full responsibility for the integrity of the data and accuracy of the analysis. The authors report no conflicts of interest.
© 2013 Society of Hospital Medicine
Acute Respiratory Failure Epidemiology
Acute respiratory failure (ARF), a common and serious complication in hospitalized patients, may be caused by several conditions including pneumonia, chronic obstructive pulmonary disease (COPD), adult respiratory distress syndrome (ARDS), and congestive heart failure (CHF). Although ARF is conventionally defined by an arterial oxygen tension of <60 mm Hg, an arterial carbon dioxide tension of >45 mm Hg, or both, these thresholds serve as a guide to be used in combination with history and clinical assessment of the patient.[1, 2] Supplemental oxygen and treatment of the underlying cause is the mainstay of therapy for ARF, but in severe cases patients are treated with invasive mechanical ventilation (IMV) or noninvasive ventilation (NIV). ARF is the most frequent reason for admission to the intensive care unit (ICU)[3, 4] and has an in‐hospital mortality rate of 33% to 37% among those who require IMV.[5, 6] The majority of epidemiologic studies of ARF have been limited to patients requiring mechanical ventilation or those admitted to the ICU, and information about the characteristics and outcomes of patients across the full spectrum of severity is much more limited.[5, 7, 8, 9, 10, 11] General improvements in the management of underlying conditions, implementation of more effective ventilation strategies,[12, 13] and increasing use of NIV[14, 15] may have led to better outcomes for patients with ARF, yet empirical evidence of a change in the adjusted mortality rate over time is lacking.
The objective of this study was to provide a broad characterization of the epidemiology of ARF among adults hospitalized in the United States using a large nationally representative database. We sought to evaluate whether incidence, mortality, cost, or ventilation practice associated with ARF in the United States changed over the period of 2001 to 2009.
METHODS
Data Source
We utilized data from the Nationwide Inpatient Sample (NIS) of the Health Care Cost and Utilization Project,[16] which is a 20% stratified probability sample of all US acute‐care hospitals each year. These data are drawn from a sampling frame that contains close to 95% of all discharges in the United States, with the hospital discharge record as the unit of analysis. The NIS has been used to study trends in many different diagnoses.[17, 18, 19] The database contains demographic information, payer information, principal and secondary diagnoses, cost, discharge disposition, and death during hospitalization. It also contains information on hospital characteristics including ownership, size, teaching status, and geographic region.
Definitions
We included patients 18 years old discharged between 2001 and 2009 with a primary or secondary diagnosis of ARF. We identified cases of ARF using diagnostic codes (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD‐9‐CM]) previously used in studies of acute organ dysfunction in sepsis (518.81, 518.82, 518.84, 518.4, 799.1, 786.09).[17, 20, 21] To define ARDS we relied on ICD‐9‐CM codes (518.4, 518.82, 518.5, 786.09) used in prior studies that showed good sensitivity and specificity.[22, 23] The use of ventilatory support was identified using the ICD‐9‐CM procedure codes[24] (93.90, 93.70, 93.71, 93.76). Comorbidities were classified using the Agency for Healthcare Research and Quality's (Rockville, MD) Healthcare Cost and Utilization Project's (HCUP) Comorbidity Software version 3.103.5.[25]
Outcomes
The primary outcomes included the annual number of hospitalizations, population incidence, hospital mortality, and costs of care. Secondary outcomes included length of stay, most common diagnoses associated with ARF, disposition at discharge, and use and type of ventilatory support.
Analysis
We estimated the number of hospitalizations with a diagnosis of ARF/year, and we calculated the weighted frequencies following HCUP‐NIS recommendations using SAS/STAT survey procedures. Using population estimates for the years 2001 to 2009 from the US Census Bureau, we employed direct standardization to calculate age‐, gender‐, and race‐adjusted population incidence and mortality rates of ARF per 100,000 population. Hospital mortality was defined as the ratio of ARF hospitalizations ending in death divided by total number of ARF hospitalizations. Mechanical ventilation rates and rates of selected comorbidities were similarly defined.
We employed indirect standardization to adjust hospital mortality rates for age, sex, race/ethnicity, comorbidities, and hospital characteristics using logistic regression models from 2001 to predict hospital mortality for 2002 to 2009. We used linear regression models to test whether the slope of year was significant for trends in outcomes overtime. Costs were calculated using hospital‐specific cost‐to‐charge ratios when available and a weighted group average at the state level for remaining hospitals. We converted all costs to 2009 US dollars using the Consumer Price Index. Costs and lengths of stay were not normally distributed, so we calculated weighted geometric means (the average of all logarithmic values), then converted back to a base‐10 number. Using a Taylor series expansion, we then calculated standard errors. All analyses were performed using SAS version 9.2 (SAS Institute, Inc., Cary, NC).
The Baystate Medical Center institutional review board determined that the project did not constitute human subjects research.
RESULTS
Hospitalization Trends
The number of hospitalizations with an ARF diagnosis code increased at an average annual rate of 11.3% from 1,007,549 (standard deviation [SD] = 19,268) in 2001 to 1,917,910 (SD = 47,558) in 2009. More than two‐thirds of ARF admissions were associated with medical, rather than surgical, conditions (69.5% in 2001 and 71.2% in 2009). The median age, racial make‐up, and gender did not change significantly. Over the study period we observed an increase in ARF‐related hospitalizations in large, urban, teaching hospitals and in hospitals located in the Midwest (Table 1).
| 2001 | 2003 | 2005 | 2007 | 2009 | |
|---|---|---|---|---|---|
| |||||
| Patient characteristics | |||||
| All, N (SD) | 1,007,549 (19,268) | 1,184,928 (25,542) | 1,288,594 (30,493) | 1,480,270 (32,002) | 1,917,910 (47,558) |
| Age, mean (SE), y | 66.6 (0.2) | 66.0 (0.2) | 66.1 (0.2) | 65.8 (0.2) | 65.8 (0.2) |
| Age group, % | |||||
| 1844 | 11.5 | 12.0 | 11.5 | 11.6 | 10.9 |
| 4564* | 26.7 | 28.9 | 29.6 | 30.7 | 31.7 |
| 6584* | 50.2 | 47.8 | 47.0 | 45.7 | 45.3 |
| 85+ | 11.5 | 11.4 | 11.9 | 12.0 | 12.1 |
| Male* | 48.1 | 48.2 | 48.6 | 49.3 | 49.2 |
| Race | |||||
| White | 75.8 | 71.9 | 76.5 | 71.8 | 73.4 |
| Black | 12.7 | 13.6 | 11.2 | 14.2 | 12.5 |
| Hispanic | 7.2 | 9.8 | 7.7 | 8.5 | 7.8 |
| Other | 4.2 | 4.7 | 4.7 | 5.5 | 6.3 |
| Primary ARF | 20.7 | 20.9 | 25.9 | 26.1 | 19.9 |
| Secondary ARF | 79.3 | 79.1 | 74.1 | 73.9 | 80.1 |
| Medical* | 69.5 | 69.1 | 69.9 | 70.2 | 71.2 |
| Surgical* | 30.5 | 30.8 | 30.1 | 29.8 | 28.8 |
| Hospital characteristics, % | |||||
| Number of beds | |||||
| Small | 10.0 | 10.1 | 10.5 | 10.8 | 11.3 |
| Medium | 25.2 | 25.3 | 24.6 | 24.0 | 22.7 |
| Large | 64.7 | 64.6 | 64.9 | 65.2 | 66.0 |
| Region | |||||
| South* | 18.5 | 18.5 | 17.6 | 17.0 | 16.3 |
| Midwest | 21.4 | 22.0 | 23.6 | 23.2 | 23.5 |
| Northeast | 42.6 | 41.7 | 41.4 | 42.2 | 42.1 |
| West* | 17.5 | 17.8 | 17.3 | 17.6 | 18.1 |
| Hospital type | |||||
| Rural | 13.6 | 13.0 | 11.8 | 11.0 | 10.8 |
| Urban nonteaching | 45.5 | 44.5 | 50.1 | 45.3 | 45.7 |
| Urban teaching | 40.9 | 42.5 | 38.1 | 43.7 | 43.6 |
| Patient outcomes | |||||
| Ventilation strategy | |||||
| IMV* | 48.5 | 48.4 | 47.5 | 46.5 | 42.1 |
| NIV* | 3.8 | 5.3 | 6.9 | 9.4 | 10.1 |
| IMV or NIV | 50.9 | 51.7 | 52.1 | 52.9 | 49.7 |
| Disposition | |||||
| Home/home healthcare* | 42.1 | 43.8 | 42.8 | 43.4 | 45.7 |
| Transfer to acute care | 5.2 | 4.7 | 4.6 | 4.6 | 4.4 |
| Nursing facility* | 24.4 | 24.9 | 27.4 | 28.6 | 29.0 |
| Other | 0.7 | 0.8 | 0.9 | 0.9 | 1.0 |
| Adjusted mortality, % (SE)* | 27.6 (0.3) | 26.4 (0.4) | 24.9 (0.4) | 22.7 (0.4) | 20.6 (0.3) |
| Adjusted mean, LOS/case, d (SE)* | 7.8 (0.1) | 7.9 (0.1) | 7.7 (0.1) | 7.5 (0.1) | 7.1 (0.1) |
| Adjusted mean cost/case, 2009 US$, (SE) | 15,818 (251) | 16,981 (419) | 17,236 (411) | 16,941 (436) | 15,987 (402) |
After adjusting for age and sex, the population incidence of ARF increased from 502 (standard error [SE] = 10) cases per 100,000 in 2001 to 784 (SE = 19) cases per 100,000 in 2009 (a 56% increase, P < 0.0001). Hispanics had the lowest rates of ARF, with both black and white groups having similar rates (Table 2).
| 2001 | 2003 | 2005 | 2007 | 2009 | |
|---|---|---|---|---|---|
| |||||
| All* | 502 (10) | 569 (12) | 595 (14) | 627 (14) | 784 (19) |
| Age group | |||||
| 1844* | 107 (3) | 130 (4) | 137 (4) | 153 (5) | 189 (6) |
| 4564* | 422 (9) | 500 (12) | 521 (13) | 580 (14) | 739 (19) |
| 6584* | 1697 (35) | 1863 (42) | 1950 (50) | 2066 (46) | 2578 (69) |
| 85+ | 3449 (86) | 3792 (106) | 3981 (120) | 3429 (97) | 4163 (123) |
| Sex | |||||
| Male* | 491 (10) | 553 (13) | 582 (14) | 629 (14) | 782 (20) |
| Female* | 512 (10) | 583 (12) | 607 (15) | 625 (13) | 786 (19) |
| Race/ethnicity | |||||
| White* | 398 (11) | 427 (12) | 466 (16) | 450 (13) | 699 (21) |
| Black* | 423 (27) | 513 (33) | 432 (26) | 574 (38) | 738 (37) |
| Hispanic* | 247 (24) | 381 (42) | 307 (27) | 353 (34) | 478 (42) |
| Other* | 268 (20) | 342 (29) | 347 (26) | 424 (29) | 713 (77) |
| In‐hospital mortality | 140 (3) | 148 (3) | 146 (3) | 140 (3) | 154 (4) |
The most common etiologies of ARF among medical patients were pneumonia, CHF, ARDS, COPD exacerbation, and sepsis. Over the 9‐year study, the proportion of cases secondary to pneumonia and sepsis rose significantly: from 39% to 46% and 13% to 21%, respectively (Figure 1).
Mortality and Other Outcomes
The number of in‐hospital deaths related to ARF increased from 277,407 deaths in 2001 to 381,155 in 2009 (a 37% increase, P < 0.001). Standardized to the population, deaths increased from 140 in 2001 to 154 cases per 100,000 in 2009 (a 10% increase, P = 0.027). Despite slightly increasing mortality rates at a population level, adjusted in‐hospital mortality improved from 27.6% in 2001 to 20.6% in 2009 (P < 0.001). Mortality declined for both IMV and NIV patients from 35.3% in 2001 to 30.2% in 2009 and from 23.5% to 19%, respectively, but increased for those who required both NIV and IMV (from 26.9% in 2001 to 28% in 2009).
Adjusted hospital length of stay decreased from 7.8 days per patient in 2001 to 7.1 days in 2009 (P < 0.001), with a concomitant increase in discharges to nursing facilities, from 24% in 2001 to 29% in 2009. There was no linear trend in adjusted cost per case, with $15,818 in 2001 and $15,987 in 2009 (in 2009 US dollars) (Table 1).
Ventilation Practices
Overall, 50.9% patients received ventilatory support (NIV or IMV or both) in 2001 and 49.7% in 2009 (P= 0.25). The use of NIV increased from 3.8% to 10.1% (P < 0.001), a 169% increase, whereas the utilization of IMV decreased from 48.5% in 2001 to 42.1% in 2009 (P for trend < 0.0001), a 13% decrease. Uses of both NIV and IMV during hospitalization were seen in 1.4% of cases in 2001 and 2.5% of cases in 2009.
2009 Data Analysis
In 2009 the 1,917,910 hospitalizations with ARF resulted in 381,155 (SD = 8965) deaths and a total inpatient cost of $54 billion. The most common etiologies in patients over 65 years old were pneumonia, CHF, COPD, ARDS, and sepsis. In patients younger than 45 years the most frequent diagnoses were pneumonia, ARDS, sepsis, asthma, drug ingestion, and trauma. Stratified analysis by gender and by age groups showed that mortality rates among men were higher than for women and were highest in patients older than 85 years (Table 3).
| Disease | Total | Age <45 Years | 4565 Years | 6584 Years | 85+ Years | Male | Female |
|---|---|---|---|---|---|---|---|
| |||||||
| Medical | |||||||
| Total, N (%) | 1,364,624 (71.2) | 144,715 (10.6) | 416,922 (30.6) | 615,009 (45.1) | 187,977 (13.8) | 647,894 (47.5) | 716,635 (52.5) |
| Pneumonia, %* | 46.1 | 41.7 | 42.8 | 46.9 | 54.3 | 48.8 | 43.7 |
| CHF, %* | 36.6 | 10.4 | 27.3 | 43.6 | 54.8 | 35.0 | 38.1 |
| ARDS, %* | 16.1 | 22.9 | 16.2 | 14.5 | 15.9 | 15.5 | 16.7 |
| Sepsis, %* | 21.2 | 18.1 | 21.3 | 21.3 | 23.1 | 22.8 | 19.8 |
| COPD, %* | 25.4 | 4.2 | 25.6 | 32.3 | 18.3 | 25.0 | 25.7 |
| AMI, %* | 9.0 | 2.6 | 7.1 | 10.5 | 13.3 | 9.3 | 8.8 |
| Asthma, %* | 9.2 | 18.1 | 11.6 | 6.7 | 5.4 | 6.2 | 12.0 |
| Stroke, %* | 4.8 | 2.3 | 4.1 | 5.5 | 6.0 | 5.0 | 4.7 |
| Trauma or burns, %* | 3.4 | 5.4 | 2.9 | 3.0 | 4.1 | 4.3 | 2.5 |
| Cardiorespiratory arrest, %* | 4.1 | 3.9 | 4.4 | 4.1 | 3.8 | 4.6 | 3.7 |
| Drug, %* | 3.7 | 16.6 | 5.1 | 0.8 | 0.3 | 3.8 | 3.6 |
| IMV, %* | 37.7 | 54.6 | 43.7 | 33.5 | 24.8 | 41.1 | 34.5 |
| NIV, %* | 11.9 | 7.1 | 11.5 | 13.0 | 12.7 | 11.4 | 12.3 |
| In‐hospital mortality (CI) | 22 (21.322.7) | 12.9 (11.913.9) | 18.5 (17.619.4) | 23.9 (23.024.9) | 31.8 (30.633.1) | 24.2 (23.325.1) | 20.9 (20.121.7) |
| Surgical | |||||||
| Total, N (%) | 552971 (28.8) | 64983 (11.8) | 190225 (34.4) | 254336 (46) | 43426 (7.9) | 295660 (53.5) | 257287 (46.5) |
| Pneumonia, %* | 34.9 | 33.0 | 34.0 | 35.0 | 40.5 | 37.1 | 32.2 |
| CHF, %* | 27.2 | 8.9 | 21.7 | 33.3 | 42.6 | 26.7 | 27.7 |
| ARDS, %* | 45.5 | 51.5 | 45.2 | 44.7 | 42.7 | 45.0 | 46.1 |
| Sepsis, %* | 25.1 | 22.8 | 25.4 | 25.2 | 26.1 | 25.4 | 24.7 |
| COPD, %* | 8.2 | 1.1 | 7.4 | 10.8 | 7.5 | 8.3 | 8.1 |
| AMI, %* | 16.9 | 4.9 | 17.0 | 19.8 | 17.9 | 19.1 | 14.4 |
| Asthma, %* | 6.1 | 7.6 | 7.2 | 5.4 | 3.6 | 4.1 | 8.5 |
| Stroke, %* | 8.9 | 6.6 | 9.2 | 9.4 | 7.2 | 8.9 | 8.8 |
| Trauma or burns, %* | 12.2 | 26.5 | 9.6 | 9.2 | 20.3 | 13.8 | 10.4 |
| Cardiorespiratory arrest, %* | 5.5 | 4.4 | 6.0 | 5.4 | 5.2 | 6.1 | 4.7 |
| Drug, %* | 0.5 | 1.3 | 0.7 | 0.2 | 0.2 | 0.4 | 0.6 |
| IMV, %* | 52.9 | 57.1 | 54.3 | 51.3 | 50.0 | 54.5 | 51.0 |
| NIV, %* | 5.8 | 3.5 | 5.5 | 6.4 | 6.4 | 5.6 | 6.0 |
| In‐hospital mortality, % (CI) | 18.6 (17.819.5) | 10.7 (9.312.0) | 15.5 (14.216.8) | 20.8 (19.821.9) | 29.4 (27.831.1) | 19.0 (18.219.8) | 18.3 (17.319.2) |
When we examined ventilation practices among medical patients we found that patients older than 85 years, when compared to patients younger than 45 years, were less likely to be treated with IMV (25% vs 55%) and more likely to be treated with NIV (12.7% vs 7%). At the same time, the average cost per case was lowest among patients 85 years and older, and hospital costs per case fell sharply after age 70 years. Costs were considerably higher for those who did not survive during hospitalization, particularly for patients younger than 45 years (Figure 2).
DISCUSSION
In this large population‐based study, we found that the number of hospitalizations associated with a diagnosis of ARF almost doubled over a 9‐year period. In 2009 there were nearly 2 million hospitalizations with ARF in the United States, resulting in approximately 380,000 deaths and inpatient costs of over $54 billion. The population‐adjusted ARF hospitalization rates increased in all age groups, and patients 85 years and older had the highest age‐specific hospitalization rate. Although overall rates of mechanical ventilation (NIV or IMV) remained stable over the 9‐year period, there was an important shift away from IMV (which decreased from 48% in 2001 to 42% in 2009) toward NIV (which increased from 4% in 2001 to 10% in 2009). Overall, there was a significant increase in the number of total deaths despite a decline in adjusted in‐hospital mortality rates. In‐hospital mortality rates decreased for all cases of ARF regardless of ventilation choice.
The findings of this study mirror results of others that have shown that although the incidence of critical care illnesses like sepsis[17, 20, 21, 26] and acute renal failure[27] has increased over the last decade, in‐hospital mortality rates have decreased.[20, 21, 28] Our results also compliment the results of a recent study that looked at hospitalizations for noncardiogenic ARF, which observed a 3.7‐fold increase in the number of cases and a steady decline in case fatality.[11]
Most prior studies addressing the incidence of ARF have included only patients receiving mechanical ventilation. In 1994, the estimated number of cases of ARF requiring IMV was 329,766,[9] which increased to 790,257 in 2005.[10] In our study we found that in 2009, the number of patients with ARF hospitalizations with IMV increased to 806,538. The increase in the overall number of cases with ARF was mainly driven by a surge in cases of sepsis and pneumonia. Our findings are consistent with national trends over time in noncardiogenic ARF[11] and in conditions that predispose patients to ARF such as sepsis[17, 20, 28] and acute renal failure.[27] As the number of claims for ARF doubled and the number of deaths increased, we found that adjusted hospital mortality improved from 27.6% in 2001 to 20.6% in 2009. This decline in hospital mortality was observed among all patients groups, regardless of ventilation choice. The decline in overall case fatality is consistent with prior findings in noncardiogenic ARF,[11] sepsis,[17, 28] and CHF.[29]
There are a number of potential explanations for the reduction in mortality observed over the study period, including improvements in hospital management of the underlying conditions leading to ARF, an increase in the proportion of patients being treated with NIV,[30] and advances in the care of critically ill patients such as the use of low‐tidal volume ventilation.[31, 32] Another contributor may be an increase in the proportion of discharges to nursing facilities, although this change in discharge disposition cannot fully explain our findings. For example, from 2007 to 2009, mortality decreased by 2 percentage points, and nursing home discharges increased by only 0.4 percentage points. Growth and aging of the US population only partially explain the increase we observed in the incidence of ARF, as age‐ and sex‐adjusted population rates increased by 56% from 2001 to 2009. In addition, the NIS captures data on hospital discharges and not individual patients; thus, a patient may have had multiple admissions. Over the last decade adoption of a more intensive practice style has been associated with improved in‐hospital mortality,[33, 34] and although these patients may be living longer they may have multiple readmissions.[35, 36]
We also observed that older patients were less likely to be treated with IMV, had a higher mortality rate, and less expensive care. These results are consistent with other studies and suggest that the intensity of treatment decreases with increasing age, and decisions to withhold or withdraw life‐supporting treatments are more frequent in the elderly.[26, 37] Prior research has shown that severity of illness is more important than age on patients' prognosis,[38, 39] and aggressive treatment strategies are not less cost‐effective when provided to older patients.[40]
Another important finding of this study is the marked increase in the use of NIV paired with a modest reduction in the use of IMV in the treatment of patients with ARF. This finding adds to evidence from other studies, which have similarly reported a dramatic increase in the use of NIV and a decrease in the use of IMV in patients with COPD as well as in ARF of other etiologies.[30, 41]
Our work has several limitations. First, we identified ARF based on ICD‐9‐CM codes and therefore cannot exclude disease misclassification. We did not find any studies in the literature addressing the accuracy and the completeness of ARF coding. However, we employed the same codes used to define ARF as has been used to define organ dysfunction in studies of severe sepsis,[17, 20] and the ICD‐9‐CM codes that we used to identify cases of ARDS have been used in prior studies.[11, 22, 23] Another limitation is that it is not clear to what extent the trends we observed may be due to changes over time in documentation and coding practices. Although this should be considered given the additional reimbursement associated with the diagnosis of ARF, our observation that rates of assisted ventilation have remained almost flat over the 9‐year period of the study suggest that would not wholly account for the rise in ARF. Second, because we did not have access to physiological data such as results of blood gas testing, we could not determine whether the threshold for applying the diagnosis of ARF or for delivering ventilatory support has changed over time. Third, for the purpose of this study we employed a broad definition of ARF, not limiting cases to those requiring mechanical ventilation, and this led to a more heterogeneous cohort including less severe cases of ARF. However, this is not dissimilar to the heterogeneity in disease severity observed among patients who receive a diagnosis of heart failure or acute renal failure. Fourth, survivors of ARF remain at high risk of death in the months after hospitalization,[42] but we assessed only in‐hospital mortality. It is possible that although in‐hospital mortality has improved, 30‐day mortality remained stable. Finally, as the NIS contains only discharge‐level data, we could not distinguish between patients admitted for ARF from those who developed ARF (potentially iatrogenic) after admission.
In summary, over the period of 2001 to 2009, there was a large increase in the number of patients given a diagnosis of ARF and a concomitant reduction in inpatient mortality. Although rates of mechanical ventilation remained relatively constant, there was a significant shift toward greater use of NIV at the expense of IMV.
Disclosures
Dr. Stefan is supported by KM1 CA156726 from the National Cancer Institute (NCI) and by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through grant UL1 RR025752. The work on this study was supported by a Charlton grant from Tufts University School of Medicine. Dr. Lindenauer and Dr. Pekow are supported by 1R18HL108810‐01 from the National Heart, Lung, and Blood Institute (NHLBI). The content of this publication is solely the responsibility of the authors and does not represent the official views of the NIH, NHLBI, or NCI.
All authors have read and approved the manuscript and none of them have any potential conflicts of interest to report.
Dr. Stefan had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Conception and design: Mihaela S. Stefan, Penelope S. Pekow, Michael B. Rothberg, Jay Steingrub, Peter K. Lindenauer; analysis and interpretation: Meng‐Shiou Shieh, Mihaela S. Stefan, Penelope S. Pekow, Michael B. Rothberg, Tara Lagu, Peter K. Lindenauer; drafting the manuscript for important intellectual content: Mihaela S. Stefan, Penelope S. Pekow, Michael B. Rothberg, Jay Steingrub, Tara Lagu, and Peter K. Lindenauer.
- , . Goldman's Cecil Medicine. 24th ed. Amsterdam, the Netherlands: Elsevier Inc.; 2012.
- , . Textbook of Respiratory Medicine. 5th ed. Philadelphia, PA: Saunders; 2010.
- , , . Epidemiology and outcome of acute respiratory failure in intensive care unit patients. Crit Care Med. 2003;31(4 suppl):S296–S299.
- , , , et al. Epidemiology of critical care syndromes, organ failures, and life‐support interventions in a suburban US community. Chest. 2011;140(6):1447–1455.
- , , , , . The changing epidemiology of mechanical ventilation: a population‐based study. J Intensive Care Med. 2006;21(3):173–182.
- , , , , . Mechanical ventilation in Ontario, 1992–2000: incidence, survival, and hospital bed utilization of noncardiac surgery adult patients. Crit Care Med. 2004;32(7):1504–1509.
- . Contributions to the epidemiology of acute respiratory failure. Crit Care. 2003;7(4):288–290.
- , , , et al. Incidence, severity, and mortality of acute respiratory failure in Berlin, Germany. Am J Respir Crit Care Med. 1995;151(4):1121–1125.
- . Acute respiratory failure in the United States: incidence and 31‐day survival. Chest. 2000;118(4):1100–1105.
- , , , , , . The epidemiology of mechanical ventilation use in the United States. Crit Care Med. 2010;38(10):1947–1953.
- , , , . Trends in the incidence of noncardiogenic acute respiratory failure: the role of race. Crit Care Med. 2012;40(5):1532–1538.
- , , , , . Secular trends in nosocomial infections and mortality associated with noninvasive ventilation in patients with exacerbation of COPD and pulmonary edema. JAMA. 2003;290(22):2985–2991.
- , , , et al. Association of noninvasive ventilation with nosocomial infections and survival in critically ill patients. JAMA. 2000;284(18):2361–2367.
- , , , , . Noninvasive versus conventional mechanical ventilation. An epidemiologic survey. Am J Respir Crit Care Med. 2001;163(4):874–880.
- , , , , , . Does noninvasive ventilation reduce the ICU nosocomial infection risk? A prospective clinical survey. Intensive Care Med. 1999;25(6):567–573.
- Heathcare Cost and Utilization Project (HCUP). Overview of the Nationwide Inpatient Sample. Available at: http://www.hcup‐us.ahrq.gov/nisoverview.jsp. Accessed December 6, 2011.
- , , , , , . Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2011;40(3):754–761.
- , , , , . Association of diagnostic coding with trends in hospitalizations and mortality of patients with pneumonia, 2003–2009. JAMA. 2012;307(13):1405–1413.
- , , , , . Little evidence of correlation between growth in health care spending and reduced mortality. Health Aff (Millwood). 2010;29(8):1523–1531.
- , , , . The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554.
- , , , . Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35(5):1244–1250.
- , , . Risk factors for ARDS in the United States: analysis of the 1993 National Mortality Followback Study. Chest. 2001;119(4):1179–1184.
- , , , , , . Acute respiratory distress syndrome: estimated incidence and mortality rate in a 5 million‐person population base. Crit Care. 1998;2(1):29–34.
- , , . Validity of procedure codes in International Classification of Diseases, 9th Revision, Clinical Modification administrative data. Med Care. 2004;42(8):801–809.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
- , . Epidemiology of sepsis: an update. Crit Care Med. 2001;29(7 suppl):S109–S116.
- , , , , , . Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol. 2006;1(1):43–51.
- , , , . Facing the challenge: decreasing case fatality rates in severe sepsis despite increasing hospitalizations. Crit Care Med. 2005;33(11):2555–2562.
- , , , . National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries,1998–2008. JAMA. 2011;306(15):1669–1678.
- , , , et al. Outcomes of noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease in the United States, 1998–2008. Am J Respir Crit Care Med. 2011;185(2):152–159.
- , , , et al. A trial of goal‐oriented hemodynamic therapy in critically ill patients. SvO2 Collaborative Group. N Engl J Med. 1995;333(16):1025–1032.
- , . Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury. N Engl J Med. 2000;343(11):813; author reply 813–814.
- , , . Short‐ and long‐term survival of nonsurgical intensive care patients and its relation to diagnosis, severity of disease, age and comorbidities. Curr Aging Sci. 2009;2(3):240–248.
- , , , , , . The impact of COPD on management and outcomes of patients hospitalized with acute myocardial infarction—a ten‐year retrospective observational study. Chest. 2012;141(6):1441–1448.
- . The paradox of health. N Engl J Med. 1988;318(7):414–418.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , , , et al. Outcomes and cost‐effectiveness of ventilator support and aggressive care for patients with acute respiratory failure due to pneumonia or acute respiratory distress syndrome. Am J Med. 2000;109(8):614–620.
- , , , et al. Older age, aggressiveness of care, and survival for seriously ill, hospitalized adults. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. Ann Intern Med. 1999;131(10):721–728.
- , , , et al. Patient age and decisions to withhold life‐sustaining treatments from seriously ill, hospitalized adults. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatment. Ann Intern Med. 1999;130(2):116–125.
- , , , et al. Are aggressive treatment strategies less cost‐effective for older patients? The case of ventilator support and aggressive care for patients with acute respiratory failure. J Am Geriatr Soc. 2001;49(4):382–390.
- , . Utilization of non‐invasive ventilation in patients with acute respiratory failure from 2000–2009: a population‐based study. Am J Respir Crit Care Med. 2012;185:A6488.
- , , , et al. One‐year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348(8):683–693.
Acute respiratory failure (ARF), a common and serious complication in hospitalized patients, may be caused by several conditions including pneumonia, chronic obstructive pulmonary disease (COPD), adult respiratory distress syndrome (ARDS), and congestive heart failure (CHF). Although ARF is conventionally defined by an arterial oxygen tension of <60 mm Hg, an arterial carbon dioxide tension of >45 mm Hg, or both, these thresholds serve as a guide to be used in combination with history and clinical assessment of the patient.[1, 2] Supplemental oxygen and treatment of the underlying cause is the mainstay of therapy for ARF, but in severe cases patients are treated with invasive mechanical ventilation (IMV) or noninvasive ventilation (NIV). ARF is the most frequent reason for admission to the intensive care unit (ICU)[3, 4] and has an in‐hospital mortality rate of 33% to 37% among those who require IMV.[5, 6] The majority of epidemiologic studies of ARF have been limited to patients requiring mechanical ventilation or those admitted to the ICU, and information about the characteristics and outcomes of patients across the full spectrum of severity is much more limited.[5, 7, 8, 9, 10, 11] General improvements in the management of underlying conditions, implementation of more effective ventilation strategies,[12, 13] and increasing use of NIV[14, 15] may have led to better outcomes for patients with ARF, yet empirical evidence of a change in the adjusted mortality rate over time is lacking.
The objective of this study was to provide a broad characterization of the epidemiology of ARF among adults hospitalized in the United States using a large nationally representative database. We sought to evaluate whether incidence, mortality, cost, or ventilation practice associated with ARF in the United States changed over the period of 2001 to 2009.
METHODS
Data Source
We utilized data from the Nationwide Inpatient Sample (NIS) of the Health Care Cost and Utilization Project,[16] which is a 20% stratified probability sample of all US acute‐care hospitals each year. These data are drawn from a sampling frame that contains close to 95% of all discharges in the United States, with the hospital discharge record as the unit of analysis. The NIS has been used to study trends in many different diagnoses.[17, 18, 19] The database contains demographic information, payer information, principal and secondary diagnoses, cost, discharge disposition, and death during hospitalization. It also contains information on hospital characteristics including ownership, size, teaching status, and geographic region.
Definitions
We included patients 18 years old discharged between 2001 and 2009 with a primary or secondary diagnosis of ARF. We identified cases of ARF using diagnostic codes (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD‐9‐CM]) previously used in studies of acute organ dysfunction in sepsis (518.81, 518.82, 518.84, 518.4, 799.1, 786.09).[17, 20, 21] To define ARDS we relied on ICD‐9‐CM codes (518.4, 518.82, 518.5, 786.09) used in prior studies that showed good sensitivity and specificity.[22, 23] The use of ventilatory support was identified using the ICD‐9‐CM procedure codes[24] (93.90, 93.70, 93.71, 93.76). Comorbidities were classified using the Agency for Healthcare Research and Quality's (Rockville, MD) Healthcare Cost and Utilization Project's (HCUP) Comorbidity Software version 3.103.5.[25]
Outcomes
The primary outcomes included the annual number of hospitalizations, population incidence, hospital mortality, and costs of care. Secondary outcomes included length of stay, most common diagnoses associated with ARF, disposition at discharge, and use and type of ventilatory support.
Analysis
We estimated the number of hospitalizations with a diagnosis of ARF/year, and we calculated the weighted frequencies following HCUP‐NIS recommendations using SAS/STAT survey procedures. Using population estimates for the years 2001 to 2009 from the US Census Bureau, we employed direct standardization to calculate age‐, gender‐, and race‐adjusted population incidence and mortality rates of ARF per 100,000 population. Hospital mortality was defined as the ratio of ARF hospitalizations ending in death divided by total number of ARF hospitalizations. Mechanical ventilation rates and rates of selected comorbidities were similarly defined.
We employed indirect standardization to adjust hospital mortality rates for age, sex, race/ethnicity, comorbidities, and hospital characteristics using logistic regression models from 2001 to predict hospital mortality for 2002 to 2009. We used linear regression models to test whether the slope of year was significant for trends in outcomes overtime. Costs were calculated using hospital‐specific cost‐to‐charge ratios when available and a weighted group average at the state level for remaining hospitals. We converted all costs to 2009 US dollars using the Consumer Price Index. Costs and lengths of stay were not normally distributed, so we calculated weighted geometric means (the average of all logarithmic values), then converted back to a base‐10 number. Using a Taylor series expansion, we then calculated standard errors. All analyses were performed using SAS version 9.2 (SAS Institute, Inc., Cary, NC).
The Baystate Medical Center institutional review board determined that the project did not constitute human subjects research.
RESULTS
Hospitalization Trends
The number of hospitalizations with an ARF diagnosis code increased at an average annual rate of 11.3% from 1,007,549 (standard deviation [SD] = 19,268) in 2001 to 1,917,910 (SD = 47,558) in 2009. More than two‐thirds of ARF admissions were associated with medical, rather than surgical, conditions (69.5% in 2001 and 71.2% in 2009). The median age, racial make‐up, and gender did not change significantly. Over the study period we observed an increase in ARF‐related hospitalizations in large, urban, teaching hospitals and in hospitals located in the Midwest (Table 1).
| 2001 | 2003 | 2005 | 2007 | 2009 | |
|---|---|---|---|---|---|
| |||||
| Patient characteristics | |||||
| All, N (SD) | 1,007,549 (19,268) | 1,184,928 (25,542) | 1,288,594 (30,493) | 1,480,270 (32,002) | 1,917,910 (47,558) |
| Age, mean (SE), y | 66.6 (0.2) | 66.0 (0.2) | 66.1 (0.2) | 65.8 (0.2) | 65.8 (0.2) |
| Age group, % | |||||
| 1844 | 11.5 | 12.0 | 11.5 | 11.6 | 10.9 |
| 4564* | 26.7 | 28.9 | 29.6 | 30.7 | 31.7 |
| 6584* | 50.2 | 47.8 | 47.0 | 45.7 | 45.3 |
| 85+ | 11.5 | 11.4 | 11.9 | 12.0 | 12.1 |
| Male* | 48.1 | 48.2 | 48.6 | 49.3 | 49.2 |
| Race | |||||
| White | 75.8 | 71.9 | 76.5 | 71.8 | 73.4 |
| Black | 12.7 | 13.6 | 11.2 | 14.2 | 12.5 |
| Hispanic | 7.2 | 9.8 | 7.7 | 8.5 | 7.8 |
| Other | 4.2 | 4.7 | 4.7 | 5.5 | 6.3 |
| Primary ARF | 20.7 | 20.9 | 25.9 | 26.1 | 19.9 |
| Secondary ARF | 79.3 | 79.1 | 74.1 | 73.9 | 80.1 |
| Medical* | 69.5 | 69.1 | 69.9 | 70.2 | 71.2 |
| Surgical* | 30.5 | 30.8 | 30.1 | 29.8 | 28.8 |
| Hospital characteristics, % | |||||
| Number of beds | |||||
| Small | 10.0 | 10.1 | 10.5 | 10.8 | 11.3 |
| Medium | 25.2 | 25.3 | 24.6 | 24.0 | 22.7 |
| Large | 64.7 | 64.6 | 64.9 | 65.2 | 66.0 |
| Region | |||||
| South* | 18.5 | 18.5 | 17.6 | 17.0 | 16.3 |
| Midwest | 21.4 | 22.0 | 23.6 | 23.2 | 23.5 |
| Northeast | 42.6 | 41.7 | 41.4 | 42.2 | 42.1 |
| West* | 17.5 | 17.8 | 17.3 | 17.6 | 18.1 |
| Hospital type | |||||
| Rural | 13.6 | 13.0 | 11.8 | 11.0 | 10.8 |
| Urban nonteaching | 45.5 | 44.5 | 50.1 | 45.3 | 45.7 |
| Urban teaching | 40.9 | 42.5 | 38.1 | 43.7 | 43.6 |
| Patient outcomes | |||||
| Ventilation strategy | |||||
| IMV* | 48.5 | 48.4 | 47.5 | 46.5 | 42.1 |
| NIV* | 3.8 | 5.3 | 6.9 | 9.4 | 10.1 |
| IMV or NIV | 50.9 | 51.7 | 52.1 | 52.9 | 49.7 |
| Disposition | |||||
| Home/home healthcare* | 42.1 | 43.8 | 42.8 | 43.4 | 45.7 |
| Transfer to acute care | 5.2 | 4.7 | 4.6 | 4.6 | 4.4 |
| Nursing facility* | 24.4 | 24.9 | 27.4 | 28.6 | 29.0 |
| Other | 0.7 | 0.8 | 0.9 | 0.9 | 1.0 |
| Adjusted mortality, % (SE)* | 27.6 (0.3) | 26.4 (0.4) | 24.9 (0.4) | 22.7 (0.4) | 20.6 (0.3) |
| Adjusted mean, LOS/case, d (SE)* | 7.8 (0.1) | 7.9 (0.1) | 7.7 (0.1) | 7.5 (0.1) | 7.1 (0.1) |
| Adjusted mean cost/case, 2009 US$, (SE) | 15,818 (251) | 16,981 (419) | 17,236 (411) | 16,941 (436) | 15,987 (402) |
After adjusting for age and sex, the population incidence of ARF increased from 502 (standard error [SE] = 10) cases per 100,000 in 2001 to 784 (SE = 19) cases per 100,000 in 2009 (a 56% increase, P < 0.0001). Hispanics had the lowest rates of ARF, with both black and white groups having similar rates (Table 2).
| 2001 | 2003 | 2005 | 2007 | 2009 | |
|---|---|---|---|---|---|
| |||||
| All* | 502 (10) | 569 (12) | 595 (14) | 627 (14) | 784 (19) |
| Age group | |||||
| 1844* | 107 (3) | 130 (4) | 137 (4) | 153 (5) | 189 (6) |
| 4564* | 422 (9) | 500 (12) | 521 (13) | 580 (14) | 739 (19) |
| 6584* | 1697 (35) | 1863 (42) | 1950 (50) | 2066 (46) | 2578 (69) |
| 85+ | 3449 (86) | 3792 (106) | 3981 (120) | 3429 (97) | 4163 (123) |
| Sex | |||||
| Male* | 491 (10) | 553 (13) | 582 (14) | 629 (14) | 782 (20) |
| Female* | 512 (10) | 583 (12) | 607 (15) | 625 (13) | 786 (19) |
| Race/ethnicity | |||||
| White* | 398 (11) | 427 (12) | 466 (16) | 450 (13) | 699 (21) |
| Black* | 423 (27) | 513 (33) | 432 (26) | 574 (38) | 738 (37) |
| Hispanic* | 247 (24) | 381 (42) | 307 (27) | 353 (34) | 478 (42) |
| Other* | 268 (20) | 342 (29) | 347 (26) | 424 (29) | 713 (77) |
| In‐hospital mortality | 140 (3) | 148 (3) | 146 (3) | 140 (3) | 154 (4) |
The most common etiologies of ARF among medical patients were pneumonia, CHF, ARDS, COPD exacerbation, and sepsis. Over the 9‐year study, the proportion of cases secondary to pneumonia and sepsis rose significantly: from 39% to 46% and 13% to 21%, respectively (Figure 1).

Mortality and Other Outcomes
The number of in‐hospital deaths related to ARF increased from 277,407 deaths in 2001 to 381,155 in 2009 (a 37% increase, P < 0.001). Standardized to the population, deaths increased from 140 in 2001 to 154 cases per 100,000 in 2009 (a 10% increase, P = 0.027). Despite slightly increasing mortality rates at a population level, adjusted in‐hospital mortality improved from 27.6% in 2001 to 20.6% in 2009 (P < 0.001). Mortality declined for both IMV and NIV patients from 35.3% in 2001 to 30.2% in 2009 and from 23.5% to 19%, respectively, but increased for those who required both NIV and IMV (from 26.9% in 2001 to 28% in 2009).
Adjusted hospital length of stay decreased from 7.8 days per patient in 2001 to 7.1 days in 2009 (P < 0.001), with a concomitant increase in discharges to nursing facilities, from 24% in 2001 to 29% in 2009. There was no linear trend in adjusted cost per case, with $15,818 in 2001 and $15,987 in 2009 (in 2009 US dollars) (Table 1).
Ventilation Practices
Overall, 50.9% patients received ventilatory support (NIV or IMV or both) in 2001 and 49.7% in 2009 (P= 0.25). The use of NIV increased from 3.8% to 10.1% (P < 0.001), a 169% increase, whereas the utilization of IMV decreased from 48.5% in 2001 to 42.1% in 2009 (P for trend < 0.0001), a 13% decrease. Uses of both NIV and IMV during hospitalization were seen in 1.4% of cases in 2001 and 2.5% of cases in 2009.
2009 Data Analysis
In 2009 the 1,917,910 hospitalizations with ARF resulted in 381,155 (SD = 8965) deaths and a total inpatient cost of $54 billion. The most common etiologies in patients over 65 years old were pneumonia, CHF, COPD, ARDS, and sepsis. In patients younger than 45 years the most frequent diagnoses were pneumonia, ARDS, sepsis, asthma, drug ingestion, and trauma. Stratified analysis by gender and by age groups showed that mortality rates among men were higher than for women and were highest in patients older than 85 years (Table 3).
| Disease | Total | Age <45 Years | 4565 Years | 6584 Years | 85+ Years | Male | Female |
|---|---|---|---|---|---|---|---|
| |||||||
| Medical | |||||||
| Total, N (%) | 1,364,624 (71.2) | 144,715 (10.6) | 416,922 (30.6) | 615,009 (45.1) | 187,977 (13.8) | 647,894 (47.5) | 716,635 (52.5) |
| Pneumonia, %* | 46.1 | 41.7 | 42.8 | 46.9 | 54.3 | 48.8 | 43.7 |
| CHF, %* | 36.6 | 10.4 | 27.3 | 43.6 | 54.8 | 35.0 | 38.1 |
| ARDS, %* | 16.1 | 22.9 | 16.2 | 14.5 | 15.9 | 15.5 | 16.7 |
| Sepsis, %* | 21.2 | 18.1 | 21.3 | 21.3 | 23.1 | 22.8 | 19.8 |
| COPD, %* | 25.4 | 4.2 | 25.6 | 32.3 | 18.3 | 25.0 | 25.7 |
| AMI, %* | 9.0 | 2.6 | 7.1 | 10.5 | 13.3 | 9.3 | 8.8 |
| Asthma, %* | 9.2 | 18.1 | 11.6 | 6.7 | 5.4 | 6.2 | 12.0 |
| Stroke, %* | 4.8 | 2.3 | 4.1 | 5.5 | 6.0 | 5.0 | 4.7 |
| Trauma or burns, %* | 3.4 | 5.4 | 2.9 | 3.0 | 4.1 | 4.3 | 2.5 |
| Cardiorespiratory arrest, %* | 4.1 | 3.9 | 4.4 | 4.1 | 3.8 | 4.6 | 3.7 |
| Drug, %* | 3.7 | 16.6 | 5.1 | 0.8 | 0.3 | 3.8 | 3.6 |
| IMV, %* | 37.7 | 54.6 | 43.7 | 33.5 | 24.8 | 41.1 | 34.5 |
| NIV, %* | 11.9 | 7.1 | 11.5 | 13.0 | 12.7 | 11.4 | 12.3 |
| In‐hospital mortality (CI) | 22 (21.322.7) | 12.9 (11.913.9) | 18.5 (17.619.4) | 23.9 (23.024.9) | 31.8 (30.633.1) | 24.2 (23.325.1) | 20.9 (20.121.7) |
| Surgical | |||||||
| Total, N (%) | 552971 (28.8) | 64983 (11.8) | 190225 (34.4) | 254336 (46) | 43426 (7.9) | 295660 (53.5) | 257287 (46.5) |
| Pneumonia, %* | 34.9 | 33.0 | 34.0 | 35.0 | 40.5 | 37.1 | 32.2 |
| CHF, %* | 27.2 | 8.9 | 21.7 | 33.3 | 42.6 | 26.7 | 27.7 |
| ARDS, %* | 45.5 | 51.5 | 45.2 | 44.7 | 42.7 | 45.0 | 46.1 |
| Sepsis, %* | 25.1 | 22.8 | 25.4 | 25.2 | 26.1 | 25.4 | 24.7 |
| COPD, %* | 8.2 | 1.1 | 7.4 | 10.8 | 7.5 | 8.3 | 8.1 |
| AMI, %* | 16.9 | 4.9 | 17.0 | 19.8 | 17.9 | 19.1 | 14.4 |
| Asthma, %* | 6.1 | 7.6 | 7.2 | 5.4 | 3.6 | 4.1 | 8.5 |
| Stroke, %* | 8.9 | 6.6 | 9.2 | 9.4 | 7.2 | 8.9 | 8.8 |
| Trauma or burns, %* | 12.2 | 26.5 | 9.6 | 9.2 | 20.3 | 13.8 | 10.4 |
| Cardiorespiratory arrest, %* | 5.5 | 4.4 | 6.0 | 5.4 | 5.2 | 6.1 | 4.7 |
| Drug, %* | 0.5 | 1.3 | 0.7 | 0.2 | 0.2 | 0.4 | 0.6 |
| IMV, %* | 52.9 | 57.1 | 54.3 | 51.3 | 50.0 | 54.5 | 51.0 |
| NIV, %* | 5.8 | 3.5 | 5.5 | 6.4 | 6.4 | 5.6 | 6.0 |
| In‐hospital mortality, % (CI) | 18.6 (17.819.5) | 10.7 (9.312.0) | 15.5 (14.216.8) | 20.8 (19.821.9) | 29.4 (27.831.1) | 19.0 (18.219.8) | 18.3 (17.319.2) |
When we examined ventilation practices among medical patients we found that patients older than 85 years, when compared to patients younger than 45 years, were less likely to be treated with IMV (25% vs 55%) and more likely to be treated with NIV (12.7% vs 7%). At the same time, the average cost per case was lowest among patients 85 years and older, and hospital costs per case fell sharply after age 70 years. Costs were considerably higher for those who did not survive during hospitalization, particularly for patients younger than 45 years (Figure 2).

DISCUSSION
In this large population‐based study, we found that the number of hospitalizations associated with a diagnosis of ARF almost doubled over a 9‐year period. In 2009 there were nearly 2 million hospitalizations with ARF in the United States, resulting in approximately 380,000 deaths and inpatient costs of over $54 billion. The population‐adjusted ARF hospitalization rates increased in all age groups, and patients 85 years and older had the highest age‐specific hospitalization rate. Although overall rates of mechanical ventilation (NIV or IMV) remained stable over the 9‐year period, there was an important shift away from IMV (which decreased from 48% in 2001 to 42% in 2009) toward NIV (which increased from 4% in 2001 to 10% in 2009). Overall, there was a significant increase in the number of total deaths despite a decline in adjusted in‐hospital mortality rates. In‐hospital mortality rates decreased for all cases of ARF regardless of ventilation choice.
The findings of this study mirror results of others that have shown that although the incidence of critical care illnesses like sepsis[17, 20, 21, 26] and acute renal failure[27] has increased over the last decade, in‐hospital mortality rates have decreased.[20, 21, 28] Our results also compliment the results of a recent study that looked at hospitalizations for noncardiogenic ARF, which observed a 3.7‐fold increase in the number of cases and a steady decline in case fatality.[11]
Most prior studies addressing the incidence of ARF have included only patients receiving mechanical ventilation. In 1994, the estimated number of cases of ARF requiring IMV was 329,766,[9] which increased to 790,257 in 2005.[10] In our study we found that in 2009, the number of patients with ARF hospitalizations with IMV increased to 806,538. The increase in the overall number of cases with ARF was mainly driven by a surge in cases of sepsis and pneumonia. Our findings are consistent with national trends over time in noncardiogenic ARF[11] and in conditions that predispose patients to ARF such as sepsis[17, 20, 28] and acute renal failure.[27] As the number of claims for ARF doubled and the number of deaths increased, we found that adjusted hospital mortality improved from 27.6% in 2001 to 20.6% in 2009. This decline in hospital mortality was observed among all patients groups, regardless of ventilation choice. The decline in overall case fatality is consistent with prior findings in noncardiogenic ARF,[11] sepsis,[17, 28] and CHF.[29]
There are a number of potential explanations for the reduction in mortality observed over the study period, including improvements in hospital management of the underlying conditions leading to ARF, an increase in the proportion of patients being treated with NIV,[30] and advances in the care of critically ill patients such as the use of low‐tidal volume ventilation.[31, 32] Another contributor may be an increase in the proportion of discharges to nursing facilities, although this change in discharge disposition cannot fully explain our findings. For example, from 2007 to 2009, mortality decreased by 2 percentage points, and nursing home discharges increased by only 0.4 percentage points. Growth and aging of the US population only partially explain the increase we observed in the incidence of ARF, as age‐ and sex‐adjusted population rates increased by 56% from 2001 to 2009. In addition, the NIS captures data on hospital discharges and not individual patients; thus, a patient may have had multiple admissions. Over the last decade adoption of a more intensive practice style has been associated with improved in‐hospital mortality,[33, 34] and although these patients may be living longer they may have multiple readmissions.[35, 36]
We also observed that older patients were less likely to be treated with IMV, had a higher mortality rate, and less expensive care. These results are consistent with other studies and suggest that the intensity of treatment decreases with increasing age, and decisions to withhold or withdraw life‐supporting treatments are more frequent in the elderly.[26, 37] Prior research has shown that severity of illness is more important than age on patients' prognosis,[38, 39] and aggressive treatment strategies are not less cost‐effective when provided to older patients.[40]
Another important finding of this study is the marked increase in the use of NIV paired with a modest reduction in the use of IMV in the treatment of patients with ARF. This finding adds to evidence from other studies, which have similarly reported a dramatic increase in the use of NIV and a decrease in the use of IMV in patients with COPD as well as in ARF of other etiologies.[30, 41]
Our work has several limitations. First, we identified ARF based on ICD‐9‐CM codes and therefore cannot exclude disease misclassification. We did not find any studies in the literature addressing the accuracy and the completeness of ARF coding. However, we employed the same codes used to define ARF as has been used to define organ dysfunction in studies of severe sepsis,[17, 20] and the ICD‐9‐CM codes that we used to identify cases of ARDS have been used in prior studies.[11, 22, 23] Another limitation is that it is not clear to what extent the trends we observed may be due to changes over time in documentation and coding practices. Although this should be considered given the additional reimbursement associated with the diagnosis of ARF, our observation that rates of assisted ventilation have remained almost flat over the 9‐year period of the study suggest that would not wholly account for the rise in ARF. Second, because we did not have access to physiological data such as results of blood gas testing, we could not determine whether the threshold for applying the diagnosis of ARF or for delivering ventilatory support has changed over time. Third, for the purpose of this study we employed a broad definition of ARF, not limiting cases to those requiring mechanical ventilation, and this led to a more heterogeneous cohort including less severe cases of ARF. However, this is not dissimilar to the heterogeneity in disease severity observed among patients who receive a diagnosis of heart failure or acute renal failure. Fourth, survivors of ARF remain at high risk of death in the months after hospitalization,[42] but we assessed only in‐hospital mortality. It is possible that although in‐hospital mortality has improved, 30‐day mortality remained stable. Finally, as the NIS contains only discharge‐level data, we could not distinguish between patients admitted for ARF from those who developed ARF (potentially iatrogenic) after admission.
In summary, over the period of 2001 to 2009, there was a large increase in the number of patients given a diagnosis of ARF and a concomitant reduction in inpatient mortality. Although rates of mechanical ventilation remained relatively constant, there was a significant shift toward greater use of NIV at the expense of IMV.
Disclosures
Dr. Stefan is supported by KM1 CA156726 from the National Cancer Institute (NCI) and by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through grant UL1 RR025752. The work on this study was supported by a Charlton grant from Tufts University School of Medicine. Dr. Lindenauer and Dr. Pekow are supported by 1R18HL108810‐01 from the National Heart, Lung, and Blood Institute (NHLBI). The content of this publication is solely the responsibility of the authors and does not represent the official views of the NIH, NHLBI, or NCI.
All authors have read and approved the manuscript and none of them have any potential conflicts of interest to report.
Dr. Stefan had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Conception and design: Mihaela S. Stefan, Penelope S. Pekow, Michael B. Rothberg, Jay Steingrub, Peter K. Lindenauer; analysis and interpretation: Meng‐Shiou Shieh, Mihaela S. Stefan, Penelope S. Pekow, Michael B. Rothberg, Tara Lagu, Peter K. Lindenauer; drafting the manuscript for important intellectual content: Mihaela S. Stefan, Penelope S. Pekow, Michael B. Rothberg, Jay Steingrub, Tara Lagu, and Peter K. Lindenauer.
Acute respiratory failure (ARF), a common and serious complication in hospitalized patients, may be caused by several conditions including pneumonia, chronic obstructive pulmonary disease (COPD), adult respiratory distress syndrome (ARDS), and congestive heart failure (CHF). Although ARF is conventionally defined by an arterial oxygen tension of <60 mm Hg, an arterial carbon dioxide tension of >45 mm Hg, or both, these thresholds serve as a guide to be used in combination with history and clinical assessment of the patient.[1, 2] Supplemental oxygen and treatment of the underlying cause is the mainstay of therapy for ARF, but in severe cases patients are treated with invasive mechanical ventilation (IMV) or noninvasive ventilation (NIV). ARF is the most frequent reason for admission to the intensive care unit (ICU)[3, 4] and has an in‐hospital mortality rate of 33% to 37% among those who require IMV.[5, 6] The majority of epidemiologic studies of ARF have been limited to patients requiring mechanical ventilation or those admitted to the ICU, and information about the characteristics and outcomes of patients across the full spectrum of severity is much more limited.[5, 7, 8, 9, 10, 11] General improvements in the management of underlying conditions, implementation of more effective ventilation strategies,[12, 13] and increasing use of NIV[14, 15] may have led to better outcomes for patients with ARF, yet empirical evidence of a change in the adjusted mortality rate over time is lacking.
The objective of this study was to provide a broad characterization of the epidemiology of ARF among adults hospitalized in the United States using a large nationally representative database. We sought to evaluate whether incidence, mortality, cost, or ventilation practice associated with ARF in the United States changed over the period of 2001 to 2009.
METHODS
Data Source
We utilized data from the Nationwide Inpatient Sample (NIS) of the Health Care Cost and Utilization Project,[16] which is a 20% stratified probability sample of all US acute‐care hospitals each year. These data are drawn from a sampling frame that contains close to 95% of all discharges in the United States, with the hospital discharge record as the unit of analysis. The NIS has been used to study trends in many different diagnoses.[17, 18, 19] The database contains demographic information, payer information, principal and secondary diagnoses, cost, discharge disposition, and death during hospitalization. It also contains information on hospital characteristics including ownership, size, teaching status, and geographic region.
Definitions
We included patients 18 years old discharged between 2001 and 2009 with a primary or secondary diagnosis of ARF. We identified cases of ARF using diagnostic codes (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD‐9‐CM]) previously used in studies of acute organ dysfunction in sepsis (518.81, 518.82, 518.84, 518.4, 799.1, 786.09).[17, 20, 21] To define ARDS we relied on ICD‐9‐CM codes (518.4, 518.82, 518.5, 786.09) used in prior studies that showed good sensitivity and specificity.[22, 23] The use of ventilatory support was identified using the ICD‐9‐CM procedure codes[24] (93.90, 93.70, 93.71, 93.76). Comorbidities were classified using the Agency for Healthcare Research and Quality's (Rockville, MD) Healthcare Cost and Utilization Project's (HCUP) Comorbidity Software version 3.103.5.[25]
Outcomes
The primary outcomes included the annual number of hospitalizations, population incidence, hospital mortality, and costs of care. Secondary outcomes included length of stay, most common diagnoses associated with ARF, disposition at discharge, and use and type of ventilatory support.
Analysis
We estimated the number of hospitalizations with a diagnosis of ARF/year, and we calculated the weighted frequencies following HCUP‐NIS recommendations using SAS/STAT survey procedures. Using population estimates for the years 2001 to 2009 from the US Census Bureau, we employed direct standardization to calculate age‐, gender‐, and race‐adjusted population incidence and mortality rates of ARF per 100,000 population. Hospital mortality was defined as the ratio of ARF hospitalizations ending in death divided by total number of ARF hospitalizations. Mechanical ventilation rates and rates of selected comorbidities were similarly defined.
We employed indirect standardization to adjust hospital mortality rates for age, sex, race/ethnicity, comorbidities, and hospital characteristics using logistic regression models from 2001 to predict hospital mortality for 2002 to 2009. We used linear regression models to test whether the slope of year was significant for trends in outcomes overtime. Costs were calculated using hospital‐specific cost‐to‐charge ratios when available and a weighted group average at the state level for remaining hospitals. We converted all costs to 2009 US dollars using the Consumer Price Index. Costs and lengths of stay were not normally distributed, so we calculated weighted geometric means (the average of all logarithmic values), then converted back to a base‐10 number. Using a Taylor series expansion, we then calculated standard errors. All analyses were performed using SAS version 9.2 (SAS Institute, Inc., Cary, NC).
The Baystate Medical Center institutional review board determined that the project did not constitute human subjects research.
RESULTS
Hospitalization Trends
The number of hospitalizations with an ARF diagnosis code increased at an average annual rate of 11.3% from 1,007,549 (standard deviation [SD] = 19,268) in 2001 to 1,917,910 (SD = 47,558) in 2009. More than two‐thirds of ARF admissions were associated with medical, rather than surgical, conditions (69.5% in 2001 and 71.2% in 2009). The median age, racial make‐up, and gender did not change significantly. Over the study period we observed an increase in ARF‐related hospitalizations in large, urban, teaching hospitals and in hospitals located in the Midwest (Table 1).
| 2001 | 2003 | 2005 | 2007 | 2009 | |
|---|---|---|---|---|---|
| |||||
| Patient characteristics | |||||
| All, N (SD) | 1,007,549 (19,268) | 1,184,928 (25,542) | 1,288,594 (30,493) | 1,480,270 (32,002) | 1,917,910 (47,558) |
| Age, mean (SE), y | 66.6 (0.2) | 66.0 (0.2) | 66.1 (0.2) | 65.8 (0.2) | 65.8 (0.2) |
| Age group, % | |||||
| 1844 | 11.5 | 12.0 | 11.5 | 11.6 | 10.9 |
| 4564* | 26.7 | 28.9 | 29.6 | 30.7 | 31.7 |
| 6584* | 50.2 | 47.8 | 47.0 | 45.7 | 45.3 |
| 85+ | 11.5 | 11.4 | 11.9 | 12.0 | 12.1 |
| Male* | 48.1 | 48.2 | 48.6 | 49.3 | 49.2 |
| Race | |||||
| White | 75.8 | 71.9 | 76.5 | 71.8 | 73.4 |
| Black | 12.7 | 13.6 | 11.2 | 14.2 | 12.5 |
| Hispanic | 7.2 | 9.8 | 7.7 | 8.5 | 7.8 |
| Other | 4.2 | 4.7 | 4.7 | 5.5 | 6.3 |
| Primary ARF | 20.7 | 20.9 | 25.9 | 26.1 | 19.9 |
| Secondary ARF | 79.3 | 79.1 | 74.1 | 73.9 | 80.1 |
| Medical* | 69.5 | 69.1 | 69.9 | 70.2 | 71.2 |
| Surgical* | 30.5 | 30.8 | 30.1 | 29.8 | 28.8 |
| Hospital characteristics, % | |||||
| Number of beds | |||||
| Small | 10.0 | 10.1 | 10.5 | 10.8 | 11.3 |
| Medium | 25.2 | 25.3 | 24.6 | 24.0 | 22.7 |
| Large | 64.7 | 64.6 | 64.9 | 65.2 | 66.0 |
| Region | |||||
| South* | 18.5 | 18.5 | 17.6 | 17.0 | 16.3 |
| Midwest | 21.4 | 22.0 | 23.6 | 23.2 | 23.5 |
| Northeast | 42.6 | 41.7 | 41.4 | 42.2 | 42.1 |
| West* | 17.5 | 17.8 | 17.3 | 17.6 | 18.1 |
| Hospital type | |||||
| Rural | 13.6 | 13.0 | 11.8 | 11.0 | 10.8 |
| Urban nonteaching | 45.5 | 44.5 | 50.1 | 45.3 | 45.7 |
| Urban teaching | 40.9 | 42.5 | 38.1 | 43.7 | 43.6 |
| Patient outcomes | |||||
| Ventilation strategy | |||||
| IMV* | 48.5 | 48.4 | 47.5 | 46.5 | 42.1 |
| NIV* | 3.8 | 5.3 | 6.9 | 9.4 | 10.1 |
| IMV or NIV | 50.9 | 51.7 | 52.1 | 52.9 | 49.7 |
| Disposition | |||||
| Home/home healthcare* | 42.1 | 43.8 | 42.8 | 43.4 | 45.7 |
| Transfer to acute care | 5.2 | 4.7 | 4.6 | 4.6 | 4.4 |
| Nursing facility* | 24.4 | 24.9 | 27.4 | 28.6 | 29.0 |
| Other | 0.7 | 0.8 | 0.9 | 0.9 | 1.0 |
| Adjusted mortality, % (SE)* | 27.6 (0.3) | 26.4 (0.4) | 24.9 (0.4) | 22.7 (0.4) | 20.6 (0.3) |
| Adjusted mean, LOS/case, d (SE)* | 7.8 (0.1) | 7.9 (0.1) | 7.7 (0.1) | 7.5 (0.1) | 7.1 (0.1) |
| Adjusted mean cost/case, 2009 US$, (SE) | 15,818 (251) | 16,981 (419) | 17,236 (411) | 16,941 (436) | 15,987 (402) |
After adjusting for age and sex, the population incidence of ARF increased from 502 (standard error [SE] = 10) cases per 100,000 in 2001 to 784 (SE = 19) cases per 100,000 in 2009 (a 56% increase, P < 0.0001). Hispanics had the lowest rates of ARF, with both black and white groups having similar rates (Table 2).
| 2001 | 2003 | 2005 | 2007 | 2009 | |
|---|---|---|---|---|---|
| |||||
| All* | 502 (10) | 569 (12) | 595 (14) | 627 (14) | 784 (19) |
| Age group | |||||
| 1844* | 107 (3) | 130 (4) | 137 (4) | 153 (5) | 189 (6) |
| 4564* | 422 (9) | 500 (12) | 521 (13) | 580 (14) | 739 (19) |
| 6584* | 1697 (35) | 1863 (42) | 1950 (50) | 2066 (46) | 2578 (69) |
| 85+ | 3449 (86) | 3792 (106) | 3981 (120) | 3429 (97) | 4163 (123) |
| Sex | |||||
| Male* | 491 (10) | 553 (13) | 582 (14) | 629 (14) | 782 (20) |
| Female* | 512 (10) | 583 (12) | 607 (15) | 625 (13) | 786 (19) |
| Race/ethnicity | |||||
| White* | 398 (11) | 427 (12) | 466 (16) | 450 (13) | 699 (21) |
| Black* | 423 (27) | 513 (33) | 432 (26) | 574 (38) | 738 (37) |
| Hispanic* | 247 (24) | 381 (42) | 307 (27) | 353 (34) | 478 (42) |
| Other* | 268 (20) | 342 (29) | 347 (26) | 424 (29) | 713 (77) |
| In‐hospital mortality | 140 (3) | 148 (3) | 146 (3) | 140 (3) | 154 (4) |
The most common etiologies of ARF among medical patients were pneumonia, CHF, ARDS, COPD exacerbation, and sepsis. Over the 9‐year study, the proportion of cases secondary to pneumonia and sepsis rose significantly: from 39% to 46% and 13% to 21%, respectively (Figure 1).

Mortality and Other Outcomes
The number of in‐hospital deaths related to ARF increased from 277,407 deaths in 2001 to 381,155 in 2009 (a 37% increase, P < 0.001). Standardized to the population, deaths increased from 140 in 2001 to 154 cases per 100,000 in 2009 (a 10% increase, P = 0.027). Despite slightly increasing mortality rates at a population level, adjusted in‐hospital mortality improved from 27.6% in 2001 to 20.6% in 2009 (P < 0.001). Mortality declined for both IMV and NIV patients from 35.3% in 2001 to 30.2% in 2009 and from 23.5% to 19%, respectively, but increased for those who required both NIV and IMV (from 26.9% in 2001 to 28% in 2009).
Adjusted hospital length of stay decreased from 7.8 days per patient in 2001 to 7.1 days in 2009 (P < 0.001), with a concomitant increase in discharges to nursing facilities, from 24% in 2001 to 29% in 2009. There was no linear trend in adjusted cost per case, with $15,818 in 2001 and $15,987 in 2009 (in 2009 US dollars) (Table 1).
Ventilation Practices
Overall, 50.9% patients received ventilatory support (NIV or IMV or both) in 2001 and 49.7% in 2009 (P= 0.25). The use of NIV increased from 3.8% to 10.1% (P < 0.001), a 169% increase, whereas the utilization of IMV decreased from 48.5% in 2001 to 42.1% in 2009 (P for trend < 0.0001), a 13% decrease. Uses of both NIV and IMV during hospitalization were seen in 1.4% of cases in 2001 and 2.5% of cases in 2009.
2009 Data Analysis
In 2009 the 1,917,910 hospitalizations with ARF resulted in 381,155 (SD = 8965) deaths and a total inpatient cost of $54 billion. The most common etiologies in patients over 65 years old were pneumonia, CHF, COPD, ARDS, and sepsis. In patients younger than 45 years the most frequent diagnoses were pneumonia, ARDS, sepsis, asthma, drug ingestion, and trauma. Stratified analysis by gender and by age groups showed that mortality rates among men were higher than for women and were highest in patients older than 85 years (Table 3).
| Disease | Total | Age <45 Years | 4565 Years | 6584 Years | 85+ Years | Male | Female |
|---|---|---|---|---|---|---|---|
| |||||||
| Medical | |||||||
| Total, N (%) | 1,364,624 (71.2) | 144,715 (10.6) | 416,922 (30.6) | 615,009 (45.1) | 187,977 (13.8) | 647,894 (47.5) | 716,635 (52.5) |
| Pneumonia, %* | 46.1 | 41.7 | 42.8 | 46.9 | 54.3 | 48.8 | 43.7 |
| CHF, %* | 36.6 | 10.4 | 27.3 | 43.6 | 54.8 | 35.0 | 38.1 |
| ARDS, %* | 16.1 | 22.9 | 16.2 | 14.5 | 15.9 | 15.5 | 16.7 |
| Sepsis, %* | 21.2 | 18.1 | 21.3 | 21.3 | 23.1 | 22.8 | 19.8 |
| COPD, %* | 25.4 | 4.2 | 25.6 | 32.3 | 18.3 | 25.0 | 25.7 |
| AMI, %* | 9.0 | 2.6 | 7.1 | 10.5 | 13.3 | 9.3 | 8.8 |
| Asthma, %* | 9.2 | 18.1 | 11.6 | 6.7 | 5.4 | 6.2 | 12.0 |
| Stroke, %* | 4.8 | 2.3 | 4.1 | 5.5 | 6.0 | 5.0 | 4.7 |
| Trauma or burns, %* | 3.4 | 5.4 | 2.9 | 3.0 | 4.1 | 4.3 | 2.5 |
| Cardiorespiratory arrest, %* | 4.1 | 3.9 | 4.4 | 4.1 | 3.8 | 4.6 | 3.7 |
| Drug, %* | 3.7 | 16.6 | 5.1 | 0.8 | 0.3 | 3.8 | 3.6 |
| IMV, %* | 37.7 | 54.6 | 43.7 | 33.5 | 24.8 | 41.1 | 34.5 |
| NIV, %* | 11.9 | 7.1 | 11.5 | 13.0 | 12.7 | 11.4 | 12.3 |
| In‐hospital mortality (CI) | 22 (21.322.7) | 12.9 (11.913.9) | 18.5 (17.619.4) | 23.9 (23.024.9) | 31.8 (30.633.1) | 24.2 (23.325.1) | 20.9 (20.121.7) |
| Surgical | |||||||
| Total, N (%) | 552971 (28.8) | 64983 (11.8) | 190225 (34.4) | 254336 (46) | 43426 (7.9) | 295660 (53.5) | 257287 (46.5) |
| Pneumonia, %* | 34.9 | 33.0 | 34.0 | 35.0 | 40.5 | 37.1 | 32.2 |
| CHF, %* | 27.2 | 8.9 | 21.7 | 33.3 | 42.6 | 26.7 | 27.7 |
| ARDS, %* | 45.5 | 51.5 | 45.2 | 44.7 | 42.7 | 45.0 | 46.1 |
| Sepsis, %* | 25.1 | 22.8 | 25.4 | 25.2 | 26.1 | 25.4 | 24.7 |
| COPD, %* | 8.2 | 1.1 | 7.4 | 10.8 | 7.5 | 8.3 | 8.1 |
| AMI, %* | 16.9 | 4.9 | 17.0 | 19.8 | 17.9 | 19.1 | 14.4 |
| Asthma, %* | 6.1 | 7.6 | 7.2 | 5.4 | 3.6 | 4.1 | 8.5 |
| Stroke, %* | 8.9 | 6.6 | 9.2 | 9.4 | 7.2 | 8.9 | 8.8 |
| Trauma or burns, %* | 12.2 | 26.5 | 9.6 | 9.2 | 20.3 | 13.8 | 10.4 |
| Cardiorespiratory arrest, %* | 5.5 | 4.4 | 6.0 | 5.4 | 5.2 | 6.1 | 4.7 |
| Drug, %* | 0.5 | 1.3 | 0.7 | 0.2 | 0.2 | 0.4 | 0.6 |
| IMV, %* | 52.9 | 57.1 | 54.3 | 51.3 | 50.0 | 54.5 | 51.0 |
| NIV, %* | 5.8 | 3.5 | 5.5 | 6.4 | 6.4 | 5.6 | 6.0 |
| In‐hospital mortality, % (CI) | 18.6 (17.819.5) | 10.7 (9.312.0) | 15.5 (14.216.8) | 20.8 (19.821.9) | 29.4 (27.831.1) | 19.0 (18.219.8) | 18.3 (17.319.2) |
When we examined ventilation practices among medical patients we found that patients older than 85 years, when compared to patients younger than 45 years, were less likely to be treated with IMV (25% vs 55%) and more likely to be treated with NIV (12.7% vs 7%). At the same time, the average cost per case was lowest among patients 85 years and older, and hospital costs per case fell sharply after age 70 years. Costs were considerably higher for those who did not survive during hospitalization, particularly for patients younger than 45 years (Figure 2).

DISCUSSION
In this large population‐based study, we found that the number of hospitalizations associated with a diagnosis of ARF almost doubled over a 9‐year period. In 2009 there were nearly 2 million hospitalizations with ARF in the United States, resulting in approximately 380,000 deaths and inpatient costs of over $54 billion. The population‐adjusted ARF hospitalization rates increased in all age groups, and patients 85 years and older had the highest age‐specific hospitalization rate. Although overall rates of mechanical ventilation (NIV or IMV) remained stable over the 9‐year period, there was an important shift away from IMV (which decreased from 48% in 2001 to 42% in 2009) toward NIV (which increased from 4% in 2001 to 10% in 2009). Overall, there was a significant increase in the number of total deaths despite a decline in adjusted in‐hospital mortality rates. In‐hospital mortality rates decreased for all cases of ARF regardless of ventilation choice.
The findings of this study mirror results of others that have shown that although the incidence of critical care illnesses like sepsis[17, 20, 21, 26] and acute renal failure[27] has increased over the last decade, in‐hospital mortality rates have decreased.[20, 21, 28] Our results also compliment the results of a recent study that looked at hospitalizations for noncardiogenic ARF, which observed a 3.7‐fold increase in the number of cases and a steady decline in case fatality.[11]
Most prior studies addressing the incidence of ARF have included only patients receiving mechanical ventilation. In 1994, the estimated number of cases of ARF requiring IMV was 329,766,[9] which increased to 790,257 in 2005.[10] In our study we found that in 2009, the number of patients with ARF hospitalizations with IMV increased to 806,538. The increase in the overall number of cases with ARF was mainly driven by a surge in cases of sepsis and pneumonia. Our findings are consistent with national trends over time in noncardiogenic ARF[11] and in conditions that predispose patients to ARF such as sepsis[17, 20, 28] and acute renal failure.[27] As the number of claims for ARF doubled and the number of deaths increased, we found that adjusted hospital mortality improved from 27.6% in 2001 to 20.6% in 2009. This decline in hospital mortality was observed among all patients groups, regardless of ventilation choice. The decline in overall case fatality is consistent with prior findings in noncardiogenic ARF,[11] sepsis,[17, 28] and CHF.[29]
There are a number of potential explanations for the reduction in mortality observed over the study period, including improvements in hospital management of the underlying conditions leading to ARF, an increase in the proportion of patients being treated with NIV,[30] and advances in the care of critically ill patients such as the use of low‐tidal volume ventilation.[31, 32] Another contributor may be an increase in the proportion of discharges to nursing facilities, although this change in discharge disposition cannot fully explain our findings. For example, from 2007 to 2009, mortality decreased by 2 percentage points, and nursing home discharges increased by only 0.4 percentage points. Growth and aging of the US population only partially explain the increase we observed in the incidence of ARF, as age‐ and sex‐adjusted population rates increased by 56% from 2001 to 2009. In addition, the NIS captures data on hospital discharges and not individual patients; thus, a patient may have had multiple admissions. Over the last decade adoption of a more intensive practice style has been associated with improved in‐hospital mortality,[33, 34] and although these patients may be living longer they may have multiple readmissions.[35, 36]
We also observed that older patients were less likely to be treated with IMV, had a higher mortality rate, and less expensive care. These results are consistent with other studies and suggest that the intensity of treatment decreases with increasing age, and decisions to withhold or withdraw life‐supporting treatments are more frequent in the elderly.[26, 37] Prior research has shown that severity of illness is more important than age on patients' prognosis,[38, 39] and aggressive treatment strategies are not less cost‐effective when provided to older patients.[40]
Another important finding of this study is the marked increase in the use of NIV paired with a modest reduction in the use of IMV in the treatment of patients with ARF. This finding adds to evidence from other studies, which have similarly reported a dramatic increase in the use of NIV and a decrease in the use of IMV in patients with COPD as well as in ARF of other etiologies.[30, 41]
Our work has several limitations. First, we identified ARF based on ICD‐9‐CM codes and therefore cannot exclude disease misclassification. We did not find any studies in the literature addressing the accuracy and the completeness of ARF coding. However, we employed the same codes used to define ARF as has been used to define organ dysfunction in studies of severe sepsis,[17, 20] and the ICD‐9‐CM codes that we used to identify cases of ARDS have been used in prior studies.[11, 22, 23] Another limitation is that it is not clear to what extent the trends we observed may be due to changes over time in documentation and coding practices. Although this should be considered given the additional reimbursement associated with the diagnosis of ARF, our observation that rates of assisted ventilation have remained almost flat over the 9‐year period of the study suggest that would not wholly account for the rise in ARF. Second, because we did not have access to physiological data such as results of blood gas testing, we could not determine whether the threshold for applying the diagnosis of ARF or for delivering ventilatory support has changed over time. Third, for the purpose of this study we employed a broad definition of ARF, not limiting cases to those requiring mechanical ventilation, and this led to a more heterogeneous cohort including less severe cases of ARF. However, this is not dissimilar to the heterogeneity in disease severity observed among patients who receive a diagnosis of heart failure or acute renal failure. Fourth, survivors of ARF remain at high risk of death in the months after hospitalization,[42] but we assessed only in‐hospital mortality. It is possible that although in‐hospital mortality has improved, 30‐day mortality remained stable. Finally, as the NIS contains only discharge‐level data, we could not distinguish between patients admitted for ARF from those who developed ARF (potentially iatrogenic) after admission.
In summary, over the period of 2001 to 2009, there was a large increase in the number of patients given a diagnosis of ARF and a concomitant reduction in inpatient mortality. Although rates of mechanical ventilation remained relatively constant, there was a significant shift toward greater use of NIV at the expense of IMV.
Disclosures
Dr. Stefan is supported by KM1 CA156726 from the National Cancer Institute (NCI) and by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through grant UL1 RR025752. The work on this study was supported by a Charlton grant from Tufts University School of Medicine. Dr. Lindenauer and Dr. Pekow are supported by 1R18HL108810‐01 from the National Heart, Lung, and Blood Institute (NHLBI). The content of this publication is solely the responsibility of the authors and does not represent the official views of the NIH, NHLBI, or NCI.
All authors have read and approved the manuscript and none of them have any potential conflicts of interest to report.
Dr. Stefan had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Conception and design: Mihaela S. Stefan, Penelope S. Pekow, Michael B. Rothberg, Jay Steingrub, Peter K. Lindenauer; analysis and interpretation: Meng‐Shiou Shieh, Mihaela S. Stefan, Penelope S. Pekow, Michael B. Rothberg, Tara Lagu, Peter K. Lindenauer; drafting the manuscript for important intellectual content: Mihaela S. Stefan, Penelope S. Pekow, Michael B. Rothberg, Jay Steingrub, Tara Lagu, and Peter K. Lindenauer.
- , . Goldman's Cecil Medicine. 24th ed. Amsterdam, the Netherlands: Elsevier Inc.; 2012.
- , . Textbook of Respiratory Medicine. 5th ed. Philadelphia, PA: Saunders; 2010.
- , , . Epidemiology and outcome of acute respiratory failure in intensive care unit patients. Crit Care Med. 2003;31(4 suppl):S296–S299.
- , , , et al. Epidemiology of critical care syndromes, organ failures, and life‐support interventions in a suburban US community. Chest. 2011;140(6):1447–1455.
- , , , , . The changing epidemiology of mechanical ventilation: a population‐based study. J Intensive Care Med. 2006;21(3):173–182.
- , , , , . Mechanical ventilation in Ontario, 1992–2000: incidence, survival, and hospital bed utilization of noncardiac surgery adult patients. Crit Care Med. 2004;32(7):1504–1509.
- . Contributions to the epidemiology of acute respiratory failure. Crit Care. 2003;7(4):288–290.
- , , , et al. Incidence, severity, and mortality of acute respiratory failure in Berlin, Germany. Am J Respir Crit Care Med. 1995;151(4):1121–1125.
- . Acute respiratory failure in the United States: incidence and 31‐day survival. Chest. 2000;118(4):1100–1105.
- , , , , , . The epidemiology of mechanical ventilation use in the United States. Crit Care Med. 2010;38(10):1947–1953.
- , , , . Trends in the incidence of noncardiogenic acute respiratory failure: the role of race. Crit Care Med. 2012;40(5):1532–1538.
- , , , , . Secular trends in nosocomial infections and mortality associated with noninvasive ventilation in patients with exacerbation of COPD and pulmonary edema. JAMA. 2003;290(22):2985–2991.
- , , , et al. Association of noninvasive ventilation with nosocomial infections and survival in critically ill patients. JAMA. 2000;284(18):2361–2367.
- , , , , . Noninvasive versus conventional mechanical ventilation. An epidemiologic survey. Am J Respir Crit Care Med. 2001;163(4):874–880.
- , , , , , . Does noninvasive ventilation reduce the ICU nosocomial infection risk? A prospective clinical survey. Intensive Care Med. 1999;25(6):567–573.
- Heathcare Cost and Utilization Project (HCUP). Overview of the Nationwide Inpatient Sample. Available at: http://www.hcup‐us.ahrq.gov/nisoverview.jsp. Accessed December 6, 2011.
- , , , , , . Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2011;40(3):754–761.
- , , , , . Association of diagnostic coding with trends in hospitalizations and mortality of patients with pneumonia, 2003–2009. JAMA. 2012;307(13):1405–1413.
- , , , , . Little evidence of correlation between growth in health care spending and reduced mortality. Health Aff (Millwood). 2010;29(8):1523–1531.
- , , , . The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554.
- , , , . Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35(5):1244–1250.
- , , . Risk factors for ARDS in the United States: analysis of the 1993 National Mortality Followback Study. Chest. 2001;119(4):1179–1184.
- , , , , , . Acute respiratory distress syndrome: estimated incidence and mortality rate in a 5 million‐person population base. Crit Care. 1998;2(1):29–34.
- , , . Validity of procedure codes in International Classification of Diseases, 9th Revision, Clinical Modification administrative data. Med Care. 2004;42(8):801–809.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
- , . Epidemiology of sepsis: an update. Crit Care Med. 2001;29(7 suppl):S109–S116.
- , , , , , . Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol. 2006;1(1):43–51.
- , , , . Facing the challenge: decreasing case fatality rates in severe sepsis despite increasing hospitalizations. Crit Care Med. 2005;33(11):2555–2562.
- , , , . National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries,1998–2008. JAMA. 2011;306(15):1669–1678.
- , , , et al. Outcomes of noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease in the United States, 1998–2008. Am J Respir Crit Care Med. 2011;185(2):152–159.
- , , , et al. A trial of goal‐oriented hemodynamic therapy in critically ill patients. SvO2 Collaborative Group. N Engl J Med. 1995;333(16):1025–1032.
- , . Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury. N Engl J Med. 2000;343(11):813; author reply 813–814.
- , , . Short‐ and long‐term survival of nonsurgical intensive care patients and its relation to diagnosis, severity of disease, age and comorbidities. Curr Aging Sci. 2009;2(3):240–248.
- , , , , , . The impact of COPD on management and outcomes of patients hospitalized with acute myocardial infarction—a ten‐year retrospective observational study. Chest. 2012;141(6):1441–1448.
- . The paradox of health. N Engl J Med. 1988;318(7):414–418.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , , , et al. Outcomes and cost‐effectiveness of ventilator support and aggressive care for patients with acute respiratory failure due to pneumonia or acute respiratory distress syndrome. Am J Med. 2000;109(8):614–620.
- , , , et al. Older age, aggressiveness of care, and survival for seriously ill, hospitalized adults. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. Ann Intern Med. 1999;131(10):721–728.
- , , , et al. Patient age and decisions to withhold life‐sustaining treatments from seriously ill, hospitalized adults. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatment. Ann Intern Med. 1999;130(2):116–125.
- , , , et al. Are aggressive treatment strategies less cost‐effective for older patients? The case of ventilator support and aggressive care for patients with acute respiratory failure. J Am Geriatr Soc. 2001;49(4):382–390.
- , . Utilization of non‐invasive ventilation in patients with acute respiratory failure from 2000–2009: a population‐based study. Am J Respir Crit Care Med. 2012;185:A6488.
- , , , et al. One‐year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348(8):683–693.
- , . Goldman's Cecil Medicine. 24th ed. Amsterdam, the Netherlands: Elsevier Inc.; 2012.
- , . Textbook of Respiratory Medicine. 5th ed. Philadelphia, PA: Saunders; 2010.
- , , . Epidemiology and outcome of acute respiratory failure in intensive care unit patients. Crit Care Med. 2003;31(4 suppl):S296–S299.
- , , , et al. Epidemiology of critical care syndromes, organ failures, and life‐support interventions in a suburban US community. Chest. 2011;140(6):1447–1455.
- , , , , . The changing epidemiology of mechanical ventilation: a population‐based study. J Intensive Care Med. 2006;21(3):173–182.
- , , , , . Mechanical ventilation in Ontario, 1992–2000: incidence, survival, and hospital bed utilization of noncardiac surgery adult patients. Crit Care Med. 2004;32(7):1504–1509.
- . Contributions to the epidemiology of acute respiratory failure. Crit Care. 2003;7(4):288–290.
- , , , et al. Incidence, severity, and mortality of acute respiratory failure in Berlin, Germany. Am J Respir Crit Care Med. 1995;151(4):1121–1125.
- . Acute respiratory failure in the United States: incidence and 31‐day survival. Chest. 2000;118(4):1100–1105.
- , , , , , . The epidemiology of mechanical ventilation use in the United States. Crit Care Med. 2010;38(10):1947–1953.
- , , , . Trends in the incidence of noncardiogenic acute respiratory failure: the role of race. Crit Care Med. 2012;40(5):1532–1538.
- , , , , . Secular trends in nosocomial infections and mortality associated with noninvasive ventilation in patients with exacerbation of COPD and pulmonary edema. JAMA. 2003;290(22):2985–2991.
- , , , et al. Association of noninvasive ventilation with nosocomial infections and survival in critically ill patients. JAMA. 2000;284(18):2361–2367.
- , , , , . Noninvasive versus conventional mechanical ventilation. An epidemiologic survey. Am J Respir Crit Care Med. 2001;163(4):874–880.
- , , , , , . Does noninvasive ventilation reduce the ICU nosocomial infection risk? A prospective clinical survey. Intensive Care Med. 1999;25(6):567–573.
- Heathcare Cost and Utilization Project (HCUP). Overview of the Nationwide Inpatient Sample. Available at: http://www.hcup‐us.ahrq.gov/nisoverview.jsp. Accessed December 6, 2011.
- , , , , , . Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2011;40(3):754–761.
- , , , , . Association of diagnostic coding with trends in hospitalizations and mortality of patients with pneumonia, 2003–2009. JAMA. 2012;307(13):1405–1413.
- , , , , . Little evidence of correlation between growth in health care spending and reduced mortality. Health Aff (Millwood). 2010;29(8):1523–1531.
- , , , . The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554.
- , , , . Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35(5):1244–1250.
- , , . Risk factors for ARDS in the United States: analysis of the 1993 National Mortality Followback Study. Chest. 2001;119(4):1179–1184.
- , , , , , . Acute respiratory distress syndrome: estimated incidence and mortality rate in a 5 million‐person population base. Crit Care. 1998;2(1):29–34.
- , , . Validity of procedure codes in International Classification of Diseases, 9th Revision, Clinical Modification administrative data. Med Care. 2004;42(8):801–809.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
- , . Epidemiology of sepsis: an update. Crit Care Med. 2001;29(7 suppl):S109–S116.
- , , , , , . Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol. 2006;1(1):43–51.
- , , , . Facing the challenge: decreasing case fatality rates in severe sepsis despite increasing hospitalizations. Crit Care Med. 2005;33(11):2555–2562.
- , , , . National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries,1998–2008. JAMA. 2011;306(15):1669–1678.
- , , , et al. Outcomes of noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease in the United States, 1998–2008. Am J Respir Crit Care Med. 2011;185(2):152–159.
- , , , et al. A trial of goal‐oriented hemodynamic therapy in critically ill patients. SvO2 Collaborative Group. N Engl J Med. 1995;333(16):1025–1032.
- , . Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury. N Engl J Med. 2000;343(11):813; author reply 813–814.
- , , . Short‐ and long‐term survival of nonsurgical intensive care patients and its relation to diagnosis, severity of disease, age and comorbidities. Curr Aging Sci. 2009;2(3):240–248.
- , , , , , . The impact of COPD on management and outcomes of patients hospitalized with acute myocardial infarction—a ten‐year retrospective observational study. Chest. 2012;141(6):1441–1448.
- . The paradox of health. N Engl J Med. 1988;318(7):414–418.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , , , et al. Outcomes and cost‐effectiveness of ventilator support and aggressive care for patients with acute respiratory failure due to pneumonia or acute respiratory distress syndrome. Am J Med. 2000;109(8):614–620.
- , , , et al. Older age, aggressiveness of care, and survival for seriously ill, hospitalized adults. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. Ann Intern Med. 1999;131(10):721–728.
- , , , et al. Patient age and decisions to withhold life‐sustaining treatments from seriously ill, hospitalized adults. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatment. Ann Intern Med. 1999;130(2):116–125.
- , , , et al. Are aggressive treatment strategies less cost‐effective for older patients? The case of ventilator support and aggressive care for patients with acute respiratory failure. J Am Geriatr Soc. 2001;49(4):382–390.
- , . Utilization of non‐invasive ventilation in patients with acute respiratory failure from 2000–2009: a population‐based study. Am J Respir Crit Care Med. 2012;185:A6488.
- , , , et al. One‐year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348(8):683–693.
Copyright © 2012 Society of Hospital Medicine
LMW vs UF Heparin
Venous thromboembolism (VTE) is a major source of morbidity and mortality for hospitalized patients, with as many as 16% of high‐risk medical patients developing VTE during their hospital stay.1, 2 Pharmacologic prophylaxis with subcutaneous heparin reduces the risk of VTE by approximately 50%,3, 4 and guidelines produced by the American College of Chest Physicians (ACCP) recommend thromboprophylaxis for patients at moderate‐to‐high risk of VTE with either low‐molecular‐weight heparin (LMWH) or unfractionated heparin (UFH).2 UFH is less expensive per dose, but meta‐analyses have suggested that UFH may be either less effective than LMWH3 or more likely to cause complications, such as bleeding5 or heparin‐induced thrombocytopenia (HIT).6 Others have argued that the efficacy and risk of bleeding with UFH and LMWH are similar.7, 8 In either case, there are few head‐to‐head studies of LMWH and UFH in medical patients and they tend to be small. In the most recent meta‐analysis, which included fewer than 4500 patients, several different low‐molecular‐weight heparins were assessed together, and the observed rate of deep vein thrombosis (DVT) with UFH was high (5.4%), with evidence suggesting publication bias.3
Given the current Joint Commission requirement9 that all medical patients either receive VTE prophylaxis or have documented a reason not to, the implications related to choosing one form of VTE prophylaxis over another are substantial on a national scale. In order to compare the effectiveness of UFH and LMWH in routine practice among hospitalized medical patients, we conducted a retrospective cohort study in a national sample of hospitals and compared the risk of VTE, bleeding, HIT, and death associated with each treatment.
METHODS
Setting and Patients
We conducted a retrospective cohort study of patients discharged between January 1, 2004 and June 30, 2005 from 333 acute care facilities in the United States that participated in Premier's Perspective, a database we have described previously.10 Compared to US hospitals as a whole, Perspective hospitals are more likely to be located in the South and in urban areas. Perspective contains the following data elements: sociodemographic information, International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) diagnosis and procedure codes, as well as a list of all billed items with a date of service, including diagnostic tests, medications, and other treatments. Hospitals' characteristics include size, region, setting, and teaching status. The Institutional Review Board at Baystate Medical Center granted permission to conduct the study (#132280‐1).
We included general medical patients aged 18 years whose ICD‐9‐CM primary diagnosis code (congestive heart failure, stroke, pneumonia, and urinary tract infection) placed them at moderate‐to‐high risk of VTE according to the ACCP recommendations,2 and who received daily prophylactic dosages of either LMWH (40 mg daily) or UFH (10,00015,000 units daily) initiated by hospital day 2 and continued to discharge or until the patient developed a VTE or a complication attributable to heparin. Patients were included so long as they missed no more than 1 day of prophylaxis or had no more than 1 unusual dose recorded. Patients who switched between heparin types were included and analyzed according to their initial therapy. Patients who received any other regimen were excluded. We also excluded patients who received warfarin on hospital day 1 or 2, because they would not be considered candidates for heparin prophylaxis, and patients whose length of stay was 2 days, because the value of VTE prophylaxis in such cases is unknown.
Data Elements
For each patient, we extracted age, gender, race, and insurance status, principal diagnosis, comorbidities, and specialty of the attending physician. Comorbidities were identified from ICD‐9‐CM secondary diagnosis codes and Diagnosis Related Groups using Healthcare Cost and Utilization Project Comorbidity Software, version 3.1, based on the work of Elixhauser and colleagues.11 We also identified additional risk factors for VTE using a combination of ICD‐9‐CM codes and specific charges. These included cancer, chemotherapy/radiation, prior VTE, use of estrogens and estrogen modulators, inflammatory bowel disease, nephrotic syndrome, myeloproliferative disorders, smoking, central venous catheter, inherited or acquired thrombophilia, mechanical ventilation, urinary catheter, decubitus ulcer, 3‐hydroxy‐3‐methylglutaryl coenzyme A (HMG‐CoA) reductase inhibitors, restraints, and varicose veins. Hospitals were categorized by region (Northeast, South, Midwest, or West), bed size, setting (urban vs rural), and teaching status.
Outcome Variables
We defined hospital‐acquired VTE as a secondary diagnosis of VTE (ICD‐9‐CM diagnoses 453.4, 453.40, 453.41, 453.42, 453.8, 453.9, 415.1, 415.11, 415.19), combined with a diagnostic test for VTE (lower extremity ultrasound, venography, computed tomography (CT) angiogram, ventilation‐perfusion scan, or pulmonary angiogram) after hospital day 2, followed by treatment for VTE (intravenous unfractionated heparin, >60 mg of enoxaparin, 7500 mg of dalteparin, or placement of an inferior vena cava filter) for at least 50% of the remaining hospital days or until initiation of warfarin or appearance of a complication (eg, transfusion or treatment for heparin‐induced thrombocytopenia). We chose this definition to differentiate hospital‐acquired VTE from VTE present on admission.12 In addition, patients who were readmitted within 30 days of discharge with a primary diagnosis of VTE were also considered to have hospital‐acquired VTE.
We also assessed complications of VTE prophylaxis. Major bleeding was defined as the receipt of 2 or more units of packed red blood cells on a single day or a secondary diagnosis of intracranial bleeding. Because there was no ICD‐9‐CM code for HIT, we assessed codes for all thrombocytopenia, as well as secondary thrombocytopenia. Definite HIT was defined as an ICD‐9‐CM code for thrombocytopenia, together with discontinuation of heparin and initiation of treatment with argatroban. A definite complication was defined as HIT or evidence of major bleeding coupled with discontinuation of heparin. Finally, we evaluated all‐cause in‐hospital mortality and total hospital costs.
Statistical Analysis
We computed summary statistics using frequencies and percents for categorical variables, and means, medians, and standard deviations and interquartile range for continuous variables. Associations of prophylaxis type with patient and hospital characteristics and outcomes were assessed using chi‐square tests or Fisher's exact test for categorical variables, and z‐tests or Wilcoxon tests for continuous variables.
We developed a propensity model for treatment with UFH as the outcome; the model included patient characteristics, early treatments, comorbidities, risk factors for VTE, physician specialty, and selected interaction terms. We then developed a series of multivariable models to evaluate the impact of heparin choice on the risk of VTE, complications of treatment, mortality, and total cost. Generalized estimating equation models with a logit link were used to assess the association between the choice of heparin and the risk of VTE, and of complications and mortality, while adjusting for the effects of within‐hospital correlation; identity link models were used for analyses of cost. Costs were trimmed at 3 standard deviations above the mean, and natural log‐transformed values were modeled due to extreme positive skew.
Unadjusted and covariate‐adjusted models were evaluated with and without adjustments for propensity score. In addition, since the hospital was the single strongest predictor of treatment, we developed grouped treatment models, in which a patient's actual treatment was replaced by a probability equal to the proportion of prophylaxed patients receiving UFH at that hospital. This adaptation of instrumental variable analysis uses the hospital as the instrument, and attempts to assess whether patients treated at a hospital which uses UFH more frequently have outcomes that differ from those of patients treated at hospitals which use LMWH more frequently, while adjusting for other patient, physician, and hospital variables. By relying on treatment at the hospital level, this method reduces the opportunity for selection bias at the patient level.
Finally, in order to exclude the possibility that our surrogate bleeding outcome was due to transfusion practices at hospitals that use a particular form of heparin, we compared the hospital rates of transfusion of 2 or more units of packed red cells to the hospital rates of prophylaxis with UFH in a larger dataset of the same hospitals. This set included patients with congestive heart failure, stroke, pneumonia, and urinary tract infection who did not receive daily prophylaxis, as well as patients admitted for chronic obstructive pulmonary disease (COPD) or acute myocardial infarction, and patients who received either warfarin or a treatment dose of heparin in the first 2 hospital days. We also compared the transfusion rates at hospitals that used unfractionated heparin in 80% of patients to hospitals that used LMWH in 80%. All analyses were performed using SAS version 9.1 (SAS Institute Inc, Cary, NC).
RESULTS
Our final sample included 32,104 patients who received prophylaxis at 333 hospitals (see Supporting Information, e‐Figure, in the online version of this article). Patient characteristics appear in Table 1. Most patients (66%) were over age 65; 59% were female and 61% were white. The most common primary diagnoses were pneumonia (40%) and congestive heart failure (25%). Additional risk factors for thromboembolism included cancer (13%), paralysis (8%), or diabetes (35%). Most patients' attending physicians were either internists (61%) or family practitioners (14%). Almost half of the patients were cared for at hospitals in the South (46%).
| Total | UFH | LMWH | ||
|---|---|---|---|---|
| 32,104 (100) | 14,591 (45.4) | 17,513 (54.6) | ||
| N (%) | N (%) | N (%) | P | |
| ||||
| Demographics | ||||
| Age | 0.0002 | |||
| 1849 | 4,061 (12.7) | 1,950 (13.4) | 2,111 (12.1) | |
| 5064 | 6,962 (21.7) | 3,225 (22.1) | 3,737 (21.3) | |
| 6579 | 10,909 (34.0) | 4,921 (33.7) | 5,988 (34.2) | |
| 80+ | 10,172 (31.7) | 4,495 (30.8) | 5,677 (32.4) | |
| Sex | 0.0071 | |||
| Male | 13,234 (41.2) | 6,133 (42.0) | 7,101 (40.5) | |
| Female | 18,870 (58.8) | 8,458 (58.0) | 10,412 (59.5) | |
| Race/ethnicity | <0.0001 | |||
| White | 19,489 (60.7) | 8,063 (55.3) | 11,426 (65.2) | |
| Black | 7,429 (23.1) | 4,101 (28.1) | 3,328 (19.0) | |
| Hispanic | 1,304 (4.1) | 591 (4.1) | 713 (4.1) | |
| Other | 3,882 (12.1) | 1,836 (12.6) | 2,046 (11.7) | |
| Primary diagnosis | <0.0001 | |||
| Pneumonia | 12,768 (39.8) | 5,354 (36.7) | 7,414 (42.3) | |
| Sepsis* | 1,217 (3.8) | 562 (3.9) | 655 (3.7) | |
| Respiratory failure* | 2,017 (6.3) | 814 (5.6) | 1,203 (6.9) | |
| Heart failure | 8,157 (25.4) | 3,825 (26.2) | 4,332 (24.7) | |
| Stroke | 4,416 (13.8) | 2,295 (15.7) | 2,121 (12.1) | |
| Urinary tract infection | 3,529 (11.0) | 1,741 (11.9) | 1,788 (10.2) | |
| Attending specialty | <0.0001 | |||
| Internist | 19,511 (60.8) | 8,945 (61.3) | 10,566 (60.3) | |
| General practice/Family medicine | 4,326 (13.5) | 1,964 (13.5) | 2,362 (13.5) | |
| Cardiologist | 1,606 (5.0) | 730 (5.0) | 876 (5.0) | |
| Pulmonologist | 2,179 (6.8) | 854 (5.9) | 1,325 (7.6) | |
| Nephrology | 583 (1.8) | 380 (2.6) | 203 (1.2) | |
| Critical care/Intensivist | 150 (0.5) | 93 (0.6) | 57 (0.3) | |
| Other | 3,749 (11.7) | 1,625 (11.1) | 2,124 (12.1) | |
| Insurance | <0.0001 | |||
| Medicare traditional | 20,281 (63.2) | 8,929 (61.2) | 11,352 (64.8) | |
| Medicare managed care | 1,737 (5.4) | 826 (5.7) | 911 (5.2) | |
| Medicaid | 2,629 (8.2) | 1,401 (9.6) | 1,228 (7.0) | |
| Private | 5,967 (18.6) | 2,830 (19.4) | 3,137 (17.9) | |
| Self‐pay/uninsured/other | 1,490 (4.6) | 605 (4.1) | 885 (5.1) | |
| Risk factors for VTE | ||||
| Admit from skilled nursing facility | 476 (1.5) | 277 (1.9) | 199 (1.1) | <0.0001 |
| Paralysis | 2,608 (8.1) | 1,317 (9.0) | 1,291 (7.4) | <0.0001 |
| Restraints | 417 (1.3) | 147 (1.0) | 270 (1.5) | <0.0001 |
| Decubitus ulcer | 1,190 (3.7) | 631 (4.3) | 559 (3.2) | <0.0001 |
| Cancer | 4,154 (12.9) | 1,858 (12.7) | 2,296 (13.1) | 0.3171 |
| Chemotherapy | 86 (0.3) | 41 (0.3) | 45 (0.3) | 0.6781 |
| Prior venous thromboembolism | 494 (1.5) | 202 (1.4) | 292 (1.7) | 0.0403 |
| Pregnancy | 1 (0) | 1 (0) | 0 (0) | 0.2733 |
| Estrogens | 438 (1.4) | 143 (1.0) | 295 (1.7) | <0.0001 |
| Estrogen modulators | 246 (0.8) | 80 (0.5) | 166 (0.9) | <0.0001 |
| Congestive heart failure | 3,107 (9.7) | 1,438 (9.9) | 1,669 (9.5) | 0.3263 |
| Respiratory failure | 2,210 (6.9) | 1,037 (7.1) | 1,173 (6.7) | 0.1493 |
| Inflammatory bowel disease | 108 (0.3) | 41 (0.3) | 67 (0.4) | 0.1176 |
| Nephrotic syndrome | 92 (0.3) | 50 (0.3) | 42 (0.2) | 0.0860 |
| Myeloproliferative disorder | 198 (0.6) | 68 (0.5) | 130 (0.7) | 0.0016 |
| Obesity | 2,973 (9.3) | 1,211 (8.3) | 1,762 (10.1) | <0.0001 |
| Smoking | 4,476 (13.9) | 1,887 (12.9) | 2,589 (14.8) | <0.0001 |
| Varicose veins | 19 (0.1) | 6 (0) | 13 (0.1) | 0.2245 |
| Central line | 1,070 (3.3) | 502 (3.4) | 568 (3.2) | 0.3271 |
| Inherited or acquired thrombophilia | 16 (0) | 9 (0.1) | 7 (0) | 0.3855 |
| Diabetes | 11,136 (34.7) | 5,157 (35.3) | 5,979 (34.1) | 0.0241 |
| Procedures associated with VTE or bleed | ||||
| Mechanical ventilation | 2,282 (7.1) | 1,111 (7.6) | 1,171 (6.7) | 0.0013 |
| Urinary catheter | 4,496 (14.0) | 1,545 (10.6) | 2,951 (16.9) | <0.0001 |
| Aspirin | 12,865 (40.1) | 6,101 (41.8) | 6,764 (38.6) | <0.0001 |
| Clopidogrel | 4,575 (14.3) | 2,087 (14.3) | 2,488 (14.2) | 0.8050 |
| Non‐steroidal anti‐inflammatory drugs | 2,147 (6.7) | 867 (5.9) | 1,280 (7.3) | <0.0001 |
| Steroids | 7,938 (24.7) | 3,136 (21.5) | 4,802 (27.4) | <0.0001 |
| Statins | 7,376 (23.0) | 3,462 (23.7) | 3,914 (22.3) | 0.0035 |
| Comorbidities | ||||
| AIDS | 124 (0.4) | 73 (0.5) | 51 (0.3) | 0.0026 |
| Alcohol abuse | 1,048 (3.3) | 523 (3.6) | 525 (3.0) | 0.0032 |
| Deficiency anemia | 7,010 (21.8) | 3,228 (22.1) | 3,782 (21.6) | 0.2543 |
| Rheumatoid arthritis/collagen vas | 967 (3.0) | 426 (2.9) | 541 (3.1) | 0.3762 |
| Chronic blood loss anemia | 177 (0.6) | 79 (0.5) | 98 (0.6) | 0.8269 |
| Chronic pulmonary disease | 12,418 (38.7) | 5,314 (36.4) | 7,104 (40.6) | <0.0001 |
| Depression | 3,334 (10.4) | 1433 (9.8) | 1901 (10.9) | 0.0025 |
| Drug abuse | 694 (2.2) | 412 (2.8) | 282 (1.6) | <0.0001 |
| Hypertension | 16,979 (52.9) | 7,658 (52.5) | 9,321 (53.2) | 0.1866 |
| Hypothyroidism | 4,016 (12.5) | 1,716 (11.8) | 2,300 (13.1) | 0.0002 |
| Liver disease | 453 (1.4) | 227 (1.6) | 226 (1.3) | 0.0448 |
| Other neurological disorders | 4,682 (14.6) | 2,202 (15.1) | 2,480 (14.2) | 0.0187 |
| Peripheral vascular disease | 2,134 (6.6) | 980 (6.7) | 1,154 (6.6) | 0.6490 |
| Psychoses | 1,295 (4.0) | 574 (3.9) | 721 (4.1) | 0.4066 |
| Pulmonary circulation disease | 1,034 (3.2) | 442 (3.0) | 592 (3.4) | 0.0760 |
| Renal failure | 2,794 (8.7) | 1,636 (11.2) | 1,158 (6.6) | 0.0000 |
| Peptic ulcer disease with bleeding | 563 (1.8) | 232 (1.6) | 331 (1.9) | 0.0414 |
| Valvular disease | 2,079 (6.5) | 899 (6.2) | 1,180 (6.7) | 0.0366 |
| Weight loss | 1,231 (3.8) | 556 (3.8) | 675 (3.9) | 0.8391 |
| Other prophylaxis | ||||
| Intermittent pneumatic compression | 1,003 (3.1) | 456 (3.1) | 547 (3.1) | 0.9926 |
| Mechanical prophylaxis | 1,281 (4.0) | 524 (3.6) | 757 (4.3) | 0.0009 |
Fifty‐five percent of patients received LMWH and the remainder received UFH; 1274 (4%) patients switched type of heparin during their stay. The proportion of patients receiving LMWH at an individual hospital varied from 0% to 100% with a u‐shaped distribution, with almost one‐third of hospitals prescribing one treatment or the other exclusively (Figure 1). Similarly, the proportion of an individual physician's patients who received prophylaxis with UFH (vs LMWH) varied from 0% to 100% (Figure 1), with 51% prescribing LMWH exclusively and 31% prescribing UFH exclusively. Compared to patients who received UFH, patients receiving LMWH were older and were more likely to be white, female, and to have pneumonia. By far the biggest difference between the groups was the hospitals at which they received their care (see Supporting Information, e‐Table, in the online version of this article). Patients receiving LMWH were much more likely to be from smaller, rural, non‐teaching hospitals in the South or the West. There were also numerous small differences in comorbidities and individual VTE risk factors between the 2 groups. The only large difference was that patients with a secondary diagnosis of renal failure (for which LMWH is not US Food and Drug Administration [FDA] approved) were almost twice as likely to receive UFH.
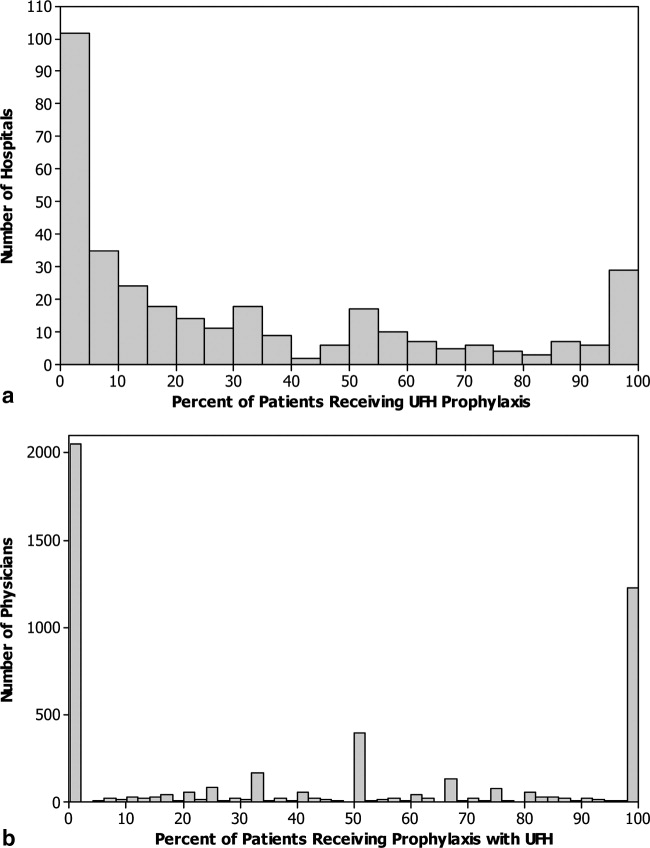
We identified 163 (0.51%) episodes of VTE (Table 2). Compared to patients receiving UFH, those receiving standard LMWH had similar unadjusted rates of VTE (0.53% vs 0.48%; P = 0.54), major bleeding (0.77% vs 0.76%; P = 0.88), thrombocytopenia (1.9% vs 2.0%; P = 0.48), definite HIT (n = 1 vs n = 3; P = 0.34), and mortality (2.8% vs 3.1%; P = 0.07). Definite complications of prophylaxis (HIT or major bleed combined with the discontinuation of heparin) were more common among patients receiving UFH (0.2% vs 0.1%; P = 0.022). Patients treated with UFH had longer unadjusted lengths of stay (P < 0.0001) and higher unadjusted costs (P < 0.0001).
| Total | UFH | LMWH | P | |
|---|---|---|---|---|
| 32,104 (100) | 14,591 (45.4) | 17,513 (54.6) | ||
| n (%) | n (%) | n (%) | ||
| ||||
| Venous thromboembolism | 163 (0.5) | 78 (0.5) | 85 (0.5) | 0.54 |
| Heparin‐induced thrombocytopenia | 4 (0) | 3 (0) | 1 (0) | 0.34* |
| Any major bleeding | 246 (0.8) | 113 (0.8) | 133 (0.8) | 0.88 |
| Transfusion with 2 units of packed red blood cells | 218 (0.7) | 97 (0.7) | 121 (0.7) | 0.78 |
| Intracranial hemorrhage | 30 (0.1) | 17 (0.1) | 13 (0.1) | 0.22 |
| Complication resulting in stopping heparin | 44 (0.1) | 28 (0.2) | 16 (0.1) | 0.02 |
| In‐hospital mortality | 944 (2.9) | 456 (3.1) | 488 (2.8) | 0.07 |
| LOS in days; mean (SD) | 6.2 (5.9) | 6.4 (6.2) | 6.0 (5.6) | <0.001 |
| Median (IQR) | 5 (37) | 5 (37) | 5 (37) | |
| Cost in USD; median (IQR) | 5873 (41718982) | 6007 (41779456) | 5774 (41658660) | <0.001 |
A propensity model for UFH treatment based upon patient characteristics and treatments was not strongly predictive of treatment (c = 0.58) and propensity matching failed to balance many of the patient characteristics. However, hospital alone, ignoring patient characteristics was strongly predictive (c = 0.91) of treatment.
In a model adjusting only for clustering within hospitals, patients treated with UFH had an odds ratio (OR) for VTE of 1.08 (95% confidence interval [CI] 0.79 to 1.49) compared to patients receiving LMWH (Figure 2). Adjustment for propensity for UFH and other covariates attenuated the effect of LMWH (OR 1.04, 95% CI 0.76 to 1.43). When individual patients were assigned a probability of treatment with UFH equal to the hospital rate where they received care, UFH use was associated with a nonsignificant change in the odds of VTE (OR 1.14, 95% CI 0.72 to 1.81).
Adjusted for clustering within hospital only, patients treated with UFH had an odds ratio for major bleed of 1.38 (95% CI 1.00 to 1.91) compared to patients receiving LMWH (Figure 3). Adjustment for propensity for UFH and other covariates gave similar results (OR 1.34, 95% CI 0.97 to 1.84). When individual patients were assigned a probability of treatment with UFH equal to the hospital rate where they received care, UFH treatment was associated with a nonsignificant increase in the odds of major bleed (OR 1.64, 95% CI 0.50 to 5.33). When we compared the rate of transfusion across hospitals, including 576,231 additional patients who were excluded from the original analyses because they did not receive daily prophylaxis or had a diagnosis of myocardial infarction or COPD, there was a slight negative correlation between transfusion rates and use of UFH (Spearman Correlation Coefficient 0.03; P = 0.61). Hospitals that used primarily UFH had a transfusion rate of 0.60% versus 0.76% at hospitals using primarily LMWH (P = 0.54), indicating that the increased risk of major bleeding associated with UFH was not confounded by local transfusion practices.
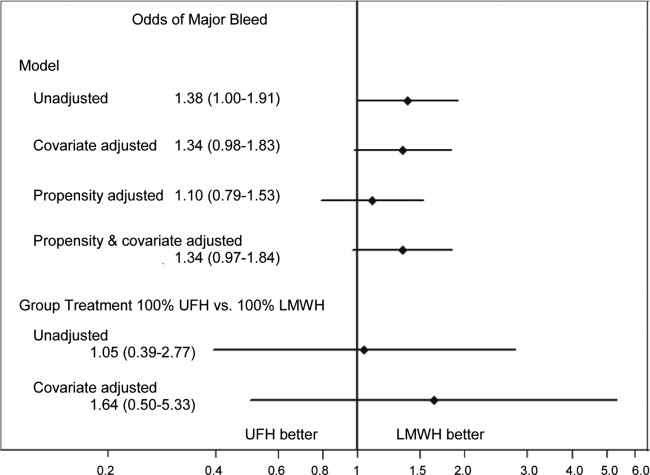
Adjusted for clustering only, patients treated with UFH had an odds ratio for definite complication of 2.35 (95% CI 1.17 to 4.72) compared to those treated with LMWH. Adjustment for propensity and covariates accentuated the association (OR 2.84, 95% CI 1.43 to 5.66). When assigned a probability of treatment with UFH equal to the hospital rate where they received care, UFH treatment was associated with an increase in the risk of definite complication (OR 2.79, 95% CI 1.00 to 7.74).
Adjusted for clustering only, patients treated with UFH had higher costs than those treated with LMWH (cost ratio 1.07, 95% CI 1.05 to 1.09). Adjustment for propensity for UFH and other covariates attenuated the association (cost ratio 1.02, 95% CI 1.00 to 1.03). Finally, when individual patients were assigned a probability of initial treatment with UFH equal to the hospital rate where they received care, treatment with UFH was associated with a nonsignificant change in the relative cost (cost ratio 0.97, 95% CI 0.90 to 1.05).
DISCUSSION
In this retrospective cohort study, we found that low‐molecular‐weight heparin and unfractionated heparin were associated with similar rates of VTE in moderate‐to‐high risk medical patients. However, unfractionated heparin was associated with a small, but higher risk of complications, even after adjustment. There were no statistical differences in rates of heparin‐induced thrombocytopenia, but this complication was exceedingly rare. Finally, overall costs associated with both treatments were similar.
A number of industry‐funded studies have compared LMWH to UFH in randomized clinical trials. These trials have generally been small and used endpoints of uncertain significance, such as asymptomatic deep vein thrombosis assessed by ultrasound. At least 3 meta‐analyses of these trials have been published. Each used different inclusion criteria. The only one to find an efficacy benefit to LMWH over UFH was heavily influenced by the inclusion of a number of studies of stroke patients.3 In that study, LMWH reduced VTE by approximately one‐third relative to UFH. The other 2 analyses found smaller reductions in DVT and pulmonary embolism (PE), and these results were not statistically significant.5, 8 Similarly, 1 analysis5 found a reduction in major bleeding events with LMWH versus UFH, whereas the other 2 studies found smaller reductions which were not statistically significant. The assessment of major bleeding is further complicated by differences in the definition of major bleeding across studies. Using a standard definition of 2 units of packed red blood cells transfused in 1 day to denote major bleeding, we found an associated reduction in bleeding with LMWH that was similar to that observed in the meta‐analyses. Moreover, patients receiving UFH were twice as likely to have a complication that resulted in stopping the prophylaxis, although these complications were overall quite rare. Lastly, there are no cost comparisons based on randomized trials. Several comparisons based on modeling have favored LMWH. One assumed that 3% of patients receiving UFH would develop HIT;13 something we did not observe. At least 3 additional analyses,1416 all funded by the manufacturer of enoxaparin, assumed that LMWH was both more effective and safer than UFH. We found that adjusted costs were similar or slightly lower with UFH than LMWH.
Our study has a number of limitations. First, its observational design makes it vulnerable to selection bias. We attempted to overcome this with rigorous multivariable adjustment, including the propensity for treatment and by using an adaptation of the instrumental variable approach. This method is of particular interest because individual hospitals were strongly predictive of choice of heparin. Still, we cannot exclude the possibility of residual confounding, especially if other outcomes, such as transfusion decisions, were also tied to specific hospital practices. Second, our study used administrative data, and therefore we could not directly adjust for certain differences which may exist between patients who received LMWH and those who received UFH. However, we did adjust for many classic risk factors for VTE. More importantly, it seems that the chance of being treated with a particular form of heparin depends more on the hospital where one receives care than on any combination of patient characteristics. Thus, apart from renal failure, for which we adjusted, it seems unlikely that there were major differences in unmeasured physiological confounders. Third, we limited our analysis to patients who received standard dosing of either type of heparin. We did this to bolster the validity of our findings, but they may not apply to unconventional dosing often observed in clinical practice. Fourth, we measured only outcomes that occurred in the hospital or that prompted a return to the hospital. VTEs which were diagnosed and treated in ambulatory care were not included. While this may have led us to underestimate the true risk of VTE, we have little reason to believe that the choice of whether to admit a patient with VTE is influenced by the original choice of VTE prophylaxis. Finally, our study was conducted before the introduction of generic LMWH, which would be expected to reduce costs associated with LMWH prophylaxis.
VTE prophylaxis for medical patients has emerged as a major focus for quality improvement initiatives. As a result, a significant proportion of general medical patients receive some form of chemoprophylaxis during their hospital stay. Small differences in efficacy or safety of different forms of prophylaxis multiplied by millions of admissions each year can have profound effects on the health of hospitalized patients. Similarly, differences in cost could also have a substantial impact on the healthcare system. We found no difference in efficacy or cost, but treatment with LMWH was less likely to be associated with subsequent transfusion of 2 or more units of packed red blood cells, a surrogate marker for bleeding. In addition, LMWH is more convenient since it can be dosed once daily, and for that reason may be more acceptable to patients. For these reasons, LMWH may be the drug of choice for inpatient prophylaxis of general medical patients. In situations where the cost of the medication itself is important, UFH represents an equally effective alternative.
Acknowledgements
All authors have contributed sufficiently to this study and have provided written permission to be named in the manuscript. No other persons have made substantial contributions to this manuscript. Michael B. Rothberg is the guarantor of the entire manuscript.
Disclosures: This study was supported by a Clinical Scientist Development Award from the Doris Duke Charitable Foundation. The funding source had no role in the study design, analysis, or interpretation of the data. Dr Rothberg served for 1 day as a consultant to Novartis Pharma about an influenza vaccine model. Sandoz, a division of Novartis, was recently granted approval to manufacture a generic form of low‐molecular‐weight heparin. None of the other authors have any conflicts of interest.
- ,,, et al.A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group.N Engl J Med.1999;341:793–800.
- ,,, et al.Prevention of venous thromboembolism: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition).Chest.2008;133:381S–453S.
- ,,,,.Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta‐analysis of randomized controlled trials.Arch Intern Med.2007;167:1476–1486.
- ,,,,.Meta‐analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients.Ann Intern Med.2007;146:278–288.
- ,,, et al.Prevention of venous thromboembolism in internal medicine with unfractionated or low‐molecular‐weight heparins: a meta‐analysis of randomised clinical trials.Thromb Haemost.2000;83:14–19.
- ,,.Risk for heparin‐induced thrombocytopenia with unfractionated and low‐molecular‐weight heparin thromboprophylaxis: a meta‐analysis.Blood.2005;106:2710–2715.
- ,.A safety analysis of thromboprophylaxis in acute medical illness.Thromb Haemost.2003;89:590–591.
- ,,,,.How complete is the evidence for thromboembolism prophylaxis in general medicine patients? A meta‐analysis of randomized controlled trials.J Hosp Med.2009;4:289–297.
- The Joint Commission on the Accreditation of Healthcare Organizations. Venous Thromboembolism (VTE) Core Measure Set. Available at: http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/VTE.htm. Accessed June 1,2009.
- ,,, et al.Potentially inappropriate medication use in hospitalized elders.J Hosp Med.2008;3:91–102.
- ,,,.Comorbidity measures for use with administrative data.Med Care.1998;36:8–27.
- ,,,,,.Identification of in‐hospital complications from claims data. Is it valid?Med Care.2000;38:785–795.
- ,,,.Cost utility of substituting enoxaparin for unfractionated heparin for prophylaxis of venous thrombosis in the hospitalized medical patient.J Hosp Med.2006;1:168–176.
- ,,,.Cost effectiveness of thromboprophylaxis with a low‐molecular‐weight heparin versus unfractionated heparin in acutely ill medical inpatients.Am J Manag Care.2004;10:632–642.
- ,,,.Comparison of the two‐year outcomes and costs of prophylaxis in medical patients at risk of venous thromboembolism.Thromb Haemost.2008;100:810–820.
- ,,, et al.Cost effectiveness of enoxaparin as prophylaxis against venous thromboembolic complications in acutely ill medical inpatients: modelling study from the hospital perspective in Germany.Pharmacoeconomics.2006;24:571–591.
Venous thromboembolism (VTE) is a major source of morbidity and mortality for hospitalized patients, with as many as 16% of high‐risk medical patients developing VTE during their hospital stay.1, 2 Pharmacologic prophylaxis with subcutaneous heparin reduces the risk of VTE by approximately 50%,3, 4 and guidelines produced by the American College of Chest Physicians (ACCP) recommend thromboprophylaxis for patients at moderate‐to‐high risk of VTE with either low‐molecular‐weight heparin (LMWH) or unfractionated heparin (UFH).2 UFH is less expensive per dose, but meta‐analyses have suggested that UFH may be either less effective than LMWH3 or more likely to cause complications, such as bleeding5 or heparin‐induced thrombocytopenia (HIT).6 Others have argued that the efficacy and risk of bleeding with UFH and LMWH are similar.7, 8 In either case, there are few head‐to‐head studies of LMWH and UFH in medical patients and they tend to be small. In the most recent meta‐analysis, which included fewer than 4500 patients, several different low‐molecular‐weight heparins were assessed together, and the observed rate of deep vein thrombosis (DVT) with UFH was high (5.4%), with evidence suggesting publication bias.3
Given the current Joint Commission requirement9 that all medical patients either receive VTE prophylaxis or have documented a reason not to, the implications related to choosing one form of VTE prophylaxis over another are substantial on a national scale. In order to compare the effectiveness of UFH and LMWH in routine practice among hospitalized medical patients, we conducted a retrospective cohort study in a national sample of hospitals and compared the risk of VTE, bleeding, HIT, and death associated with each treatment.
METHODS
Setting and Patients
We conducted a retrospective cohort study of patients discharged between January 1, 2004 and June 30, 2005 from 333 acute care facilities in the United States that participated in Premier's Perspective, a database we have described previously.10 Compared to US hospitals as a whole, Perspective hospitals are more likely to be located in the South and in urban areas. Perspective contains the following data elements: sociodemographic information, International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) diagnosis and procedure codes, as well as a list of all billed items with a date of service, including diagnostic tests, medications, and other treatments. Hospitals' characteristics include size, region, setting, and teaching status. The Institutional Review Board at Baystate Medical Center granted permission to conduct the study (#132280‐1).
We included general medical patients aged 18 years whose ICD‐9‐CM primary diagnosis code (congestive heart failure, stroke, pneumonia, and urinary tract infection) placed them at moderate‐to‐high risk of VTE according to the ACCP recommendations,2 and who received daily prophylactic dosages of either LMWH (40 mg daily) or UFH (10,00015,000 units daily) initiated by hospital day 2 and continued to discharge or until the patient developed a VTE or a complication attributable to heparin. Patients were included so long as they missed no more than 1 day of prophylaxis or had no more than 1 unusual dose recorded. Patients who switched between heparin types were included and analyzed according to their initial therapy. Patients who received any other regimen were excluded. We also excluded patients who received warfarin on hospital day 1 or 2, because they would not be considered candidates for heparin prophylaxis, and patients whose length of stay was 2 days, because the value of VTE prophylaxis in such cases is unknown.
Data Elements
For each patient, we extracted age, gender, race, and insurance status, principal diagnosis, comorbidities, and specialty of the attending physician. Comorbidities were identified from ICD‐9‐CM secondary diagnosis codes and Diagnosis Related Groups using Healthcare Cost and Utilization Project Comorbidity Software, version 3.1, based on the work of Elixhauser and colleagues.11 We also identified additional risk factors for VTE using a combination of ICD‐9‐CM codes and specific charges. These included cancer, chemotherapy/radiation, prior VTE, use of estrogens and estrogen modulators, inflammatory bowel disease, nephrotic syndrome, myeloproliferative disorders, smoking, central venous catheter, inherited or acquired thrombophilia, mechanical ventilation, urinary catheter, decubitus ulcer, 3‐hydroxy‐3‐methylglutaryl coenzyme A (HMG‐CoA) reductase inhibitors, restraints, and varicose veins. Hospitals were categorized by region (Northeast, South, Midwest, or West), bed size, setting (urban vs rural), and teaching status.
Outcome Variables
We defined hospital‐acquired VTE as a secondary diagnosis of VTE (ICD‐9‐CM diagnoses 453.4, 453.40, 453.41, 453.42, 453.8, 453.9, 415.1, 415.11, 415.19), combined with a diagnostic test for VTE (lower extremity ultrasound, venography, computed tomography (CT) angiogram, ventilation‐perfusion scan, or pulmonary angiogram) after hospital day 2, followed by treatment for VTE (intravenous unfractionated heparin, >60 mg of enoxaparin, 7500 mg of dalteparin, or placement of an inferior vena cava filter) for at least 50% of the remaining hospital days or until initiation of warfarin or appearance of a complication (eg, transfusion or treatment for heparin‐induced thrombocytopenia). We chose this definition to differentiate hospital‐acquired VTE from VTE present on admission.12 In addition, patients who were readmitted within 30 days of discharge with a primary diagnosis of VTE were also considered to have hospital‐acquired VTE.
We also assessed complications of VTE prophylaxis. Major bleeding was defined as the receipt of 2 or more units of packed red blood cells on a single day or a secondary diagnosis of intracranial bleeding. Because there was no ICD‐9‐CM code for HIT, we assessed codes for all thrombocytopenia, as well as secondary thrombocytopenia. Definite HIT was defined as an ICD‐9‐CM code for thrombocytopenia, together with discontinuation of heparin and initiation of treatment with argatroban. A definite complication was defined as HIT or evidence of major bleeding coupled with discontinuation of heparin. Finally, we evaluated all‐cause in‐hospital mortality and total hospital costs.
Statistical Analysis
We computed summary statistics using frequencies and percents for categorical variables, and means, medians, and standard deviations and interquartile range for continuous variables. Associations of prophylaxis type with patient and hospital characteristics and outcomes were assessed using chi‐square tests or Fisher's exact test for categorical variables, and z‐tests or Wilcoxon tests for continuous variables.
We developed a propensity model for treatment with UFH as the outcome; the model included patient characteristics, early treatments, comorbidities, risk factors for VTE, physician specialty, and selected interaction terms. We then developed a series of multivariable models to evaluate the impact of heparin choice on the risk of VTE, complications of treatment, mortality, and total cost. Generalized estimating equation models with a logit link were used to assess the association between the choice of heparin and the risk of VTE, and of complications and mortality, while adjusting for the effects of within‐hospital correlation; identity link models were used for analyses of cost. Costs were trimmed at 3 standard deviations above the mean, and natural log‐transformed values were modeled due to extreme positive skew.
Unadjusted and covariate‐adjusted models were evaluated with and without adjustments for propensity score. In addition, since the hospital was the single strongest predictor of treatment, we developed grouped treatment models, in which a patient's actual treatment was replaced by a probability equal to the proportion of prophylaxed patients receiving UFH at that hospital. This adaptation of instrumental variable analysis uses the hospital as the instrument, and attempts to assess whether patients treated at a hospital which uses UFH more frequently have outcomes that differ from those of patients treated at hospitals which use LMWH more frequently, while adjusting for other patient, physician, and hospital variables. By relying on treatment at the hospital level, this method reduces the opportunity for selection bias at the patient level.
Finally, in order to exclude the possibility that our surrogate bleeding outcome was due to transfusion practices at hospitals that use a particular form of heparin, we compared the hospital rates of transfusion of 2 or more units of packed red cells to the hospital rates of prophylaxis with UFH in a larger dataset of the same hospitals. This set included patients with congestive heart failure, stroke, pneumonia, and urinary tract infection who did not receive daily prophylaxis, as well as patients admitted for chronic obstructive pulmonary disease (COPD) or acute myocardial infarction, and patients who received either warfarin or a treatment dose of heparin in the first 2 hospital days. We also compared the transfusion rates at hospitals that used unfractionated heparin in 80% of patients to hospitals that used LMWH in 80%. All analyses were performed using SAS version 9.1 (SAS Institute Inc, Cary, NC).
RESULTS
Our final sample included 32,104 patients who received prophylaxis at 333 hospitals (see Supporting Information, e‐Figure, in the online version of this article). Patient characteristics appear in Table 1. Most patients (66%) were over age 65; 59% were female and 61% were white. The most common primary diagnoses were pneumonia (40%) and congestive heart failure (25%). Additional risk factors for thromboembolism included cancer (13%), paralysis (8%), or diabetes (35%). Most patients' attending physicians were either internists (61%) or family practitioners (14%). Almost half of the patients were cared for at hospitals in the South (46%).
| Total | UFH | LMWH | ||
|---|---|---|---|---|
| 32,104 (100) | 14,591 (45.4) | 17,513 (54.6) | ||
| N (%) | N (%) | N (%) | P | |
| ||||
| Demographics | ||||
| Age | 0.0002 | |||
| 1849 | 4,061 (12.7) | 1,950 (13.4) | 2,111 (12.1) | |
| 5064 | 6,962 (21.7) | 3,225 (22.1) | 3,737 (21.3) | |
| 6579 | 10,909 (34.0) | 4,921 (33.7) | 5,988 (34.2) | |
| 80+ | 10,172 (31.7) | 4,495 (30.8) | 5,677 (32.4) | |
| Sex | 0.0071 | |||
| Male | 13,234 (41.2) | 6,133 (42.0) | 7,101 (40.5) | |
| Female | 18,870 (58.8) | 8,458 (58.0) | 10,412 (59.5) | |
| Race/ethnicity | <0.0001 | |||
| White | 19,489 (60.7) | 8,063 (55.3) | 11,426 (65.2) | |
| Black | 7,429 (23.1) | 4,101 (28.1) | 3,328 (19.0) | |
| Hispanic | 1,304 (4.1) | 591 (4.1) | 713 (4.1) | |
| Other | 3,882 (12.1) | 1,836 (12.6) | 2,046 (11.7) | |
| Primary diagnosis | <0.0001 | |||
| Pneumonia | 12,768 (39.8) | 5,354 (36.7) | 7,414 (42.3) | |
| Sepsis* | 1,217 (3.8) | 562 (3.9) | 655 (3.7) | |
| Respiratory failure* | 2,017 (6.3) | 814 (5.6) | 1,203 (6.9) | |
| Heart failure | 8,157 (25.4) | 3,825 (26.2) | 4,332 (24.7) | |
| Stroke | 4,416 (13.8) | 2,295 (15.7) | 2,121 (12.1) | |
| Urinary tract infection | 3,529 (11.0) | 1,741 (11.9) | 1,788 (10.2) | |
| Attending specialty | <0.0001 | |||
| Internist | 19,511 (60.8) | 8,945 (61.3) | 10,566 (60.3) | |
| General practice/Family medicine | 4,326 (13.5) | 1,964 (13.5) | 2,362 (13.5) | |
| Cardiologist | 1,606 (5.0) | 730 (5.0) | 876 (5.0) | |
| Pulmonologist | 2,179 (6.8) | 854 (5.9) | 1,325 (7.6) | |
| Nephrology | 583 (1.8) | 380 (2.6) | 203 (1.2) | |
| Critical care/Intensivist | 150 (0.5) | 93 (0.6) | 57 (0.3) | |
| Other | 3,749 (11.7) | 1,625 (11.1) | 2,124 (12.1) | |
| Insurance | <0.0001 | |||
| Medicare traditional | 20,281 (63.2) | 8,929 (61.2) | 11,352 (64.8) | |
| Medicare managed care | 1,737 (5.4) | 826 (5.7) | 911 (5.2) | |
| Medicaid | 2,629 (8.2) | 1,401 (9.6) | 1,228 (7.0) | |
| Private | 5,967 (18.6) | 2,830 (19.4) | 3,137 (17.9) | |
| Self‐pay/uninsured/other | 1,490 (4.6) | 605 (4.1) | 885 (5.1) | |
| Risk factors for VTE | ||||
| Admit from skilled nursing facility | 476 (1.5) | 277 (1.9) | 199 (1.1) | <0.0001 |
| Paralysis | 2,608 (8.1) | 1,317 (9.0) | 1,291 (7.4) | <0.0001 |
| Restraints | 417 (1.3) | 147 (1.0) | 270 (1.5) | <0.0001 |
| Decubitus ulcer | 1,190 (3.7) | 631 (4.3) | 559 (3.2) | <0.0001 |
| Cancer | 4,154 (12.9) | 1,858 (12.7) | 2,296 (13.1) | 0.3171 |
| Chemotherapy | 86 (0.3) | 41 (0.3) | 45 (0.3) | 0.6781 |
| Prior venous thromboembolism | 494 (1.5) | 202 (1.4) | 292 (1.7) | 0.0403 |
| Pregnancy | 1 (0) | 1 (0) | 0 (0) | 0.2733 |
| Estrogens | 438 (1.4) | 143 (1.0) | 295 (1.7) | <0.0001 |
| Estrogen modulators | 246 (0.8) | 80 (0.5) | 166 (0.9) | <0.0001 |
| Congestive heart failure | 3,107 (9.7) | 1,438 (9.9) | 1,669 (9.5) | 0.3263 |
| Respiratory failure | 2,210 (6.9) | 1,037 (7.1) | 1,173 (6.7) | 0.1493 |
| Inflammatory bowel disease | 108 (0.3) | 41 (0.3) | 67 (0.4) | 0.1176 |
| Nephrotic syndrome | 92 (0.3) | 50 (0.3) | 42 (0.2) | 0.0860 |
| Myeloproliferative disorder | 198 (0.6) | 68 (0.5) | 130 (0.7) | 0.0016 |
| Obesity | 2,973 (9.3) | 1,211 (8.3) | 1,762 (10.1) | <0.0001 |
| Smoking | 4,476 (13.9) | 1,887 (12.9) | 2,589 (14.8) | <0.0001 |
| Varicose veins | 19 (0.1) | 6 (0) | 13 (0.1) | 0.2245 |
| Central line | 1,070 (3.3) | 502 (3.4) | 568 (3.2) | 0.3271 |
| Inherited or acquired thrombophilia | 16 (0) | 9 (0.1) | 7 (0) | 0.3855 |
| Diabetes | 11,136 (34.7) | 5,157 (35.3) | 5,979 (34.1) | 0.0241 |
| Procedures associated with VTE or bleed | ||||
| Mechanical ventilation | 2,282 (7.1) | 1,111 (7.6) | 1,171 (6.7) | 0.0013 |
| Urinary catheter | 4,496 (14.0) | 1,545 (10.6) | 2,951 (16.9) | <0.0001 |
| Aspirin | 12,865 (40.1) | 6,101 (41.8) | 6,764 (38.6) | <0.0001 |
| Clopidogrel | 4,575 (14.3) | 2,087 (14.3) | 2,488 (14.2) | 0.8050 |
| Non‐steroidal anti‐inflammatory drugs | 2,147 (6.7) | 867 (5.9) | 1,280 (7.3) | <0.0001 |
| Steroids | 7,938 (24.7) | 3,136 (21.5) | 4,802 (27.4) | <0.0001 |
| Statins | 7,376 (23.0) | 3,462 (23.7) | 3,914 (22.3) | 0.0035 |
| Comorbidities | ||||
| AIDS | 124 (0.4) | 73 (0.5) | 51 (0.3) | 0.0026 |
| Alcohol abuse | 1,048 (3.3) | 523 (3.6) | 525 (3.0) | 0.0032 |
| Deficiency anemia | 7,010 (21.8) | 3,228 (22.1) | 3,782 (21.6) | 0.2543 |
| Rheumatoid arthritis/collagen vas | 967 (3.0) | 426 (2.9) | 541 (3.1) | 0.3762 |
| Chronic blood loss anemia | 177 (0.6) | 79 (0.5) | 98 (0.6) | 0.8269 |
| Chronic pulmonary disease | 12,418 (38.7) | 5,314 (36.4) | 7,104 (40.6) | <0.0001 |
| Depression | 3,334 (10.4) | 1433 (9.8) | 1901 (10.9) | 0.0025 |
| Drug abuse | 694 (2.2) | 412 (2.8) | 282 (1.6) | <0.0001 |
| Hypertension | 16,979 (52.9) | 7,658 (52.5) | 9,321 (53.2) | 0.1866 |
| Hypothyroidism | 4,016 (12.5) | 1,716 (11.8) | 2,300 (13.1) | 0.0002 |
| Liver disease | 453 (1.4) | 227 (1.6) | 226 (1.3) | 0.0448 |
| Other neurological disorders | 4,682 (14.6) | 2,202 (15.1) | 2,480 (14.2) | 0.0187 |
| Peripheral vascular disease | 2,134 (6.6) | 980 (6.7) | 1,154 (6.6) | 0.6490 |
| Psychoses | 1,295 (4.0) | 574 (3.9) | 721 (4.1) | 0.4066 |
| Pulmonary circulation disease | 1,034 (3.2) | 442 (3.0) | 592 (3.4) | 0.0760 |
| Renal failure | 2,794 (8.7) | 1,636 (11.2) | 1,158 (6.6) | 0.0000 |
| Peptic ulcer disease with bleeding | 563 (1.8) | 232 (1.6) | 331 (1.9) | 0.0414 |
| Valvular disease | 2,079 (6.5) | 899 (6.2) | 1,180 (6.7) | 0.0366 |
| Weight loss | 1,231 (3.8) | 556 (3.8) | 675 (3.9) | 0.8391 |
| Other prophylaxis | ||||
| Intermittent pneumatic compression | 1,003 (3.1) | 456 (3.1) | 547 (3.1) | 0.9926 |
| Mechanical prophylaxis | 1,281 (4.0) | 524 (3.6) | 757 (4.3) | 0.0009 |
Fifty‐five percent of patients received LMWH and the remainder received UFH; 1274 (4%) patients switched type of heparin during their stay. The proportion of patients receiving LMWH at an individual hospital varied from 0% to 100% with a u‐shaped distribution, with almost one‐third of hospitals prescribing one treatment or the other exclusively (Figure 1). Similarly, the proportion of an individual physician's patients who received prophylaxis with UFH (vs LMWH) varied from 0% to 100% (Figure 1), with 51% prescribing LMWH exclusively and 31% prescribing UFH exclusively. Compared to patients who received UFH, patients receiving LMWH were older and were more likely to be white, female, and to have pneumonia. By far the biggest difference between the groups was the hospitals at which they received their care (see Supporting Information, e‐Table, in the online version of this article). Patients receiving LMWH were much more likely to be from smaller, rural, non‐teaching hospitals in the South or the West. There were also numerous small differences in comorbidities and individual VTE risk factors between the 2 groups. The only large difference was that patients with a secondary diagnosis of renal failure (for which LMWH is not US Food and Drug Administration [FDA] approved) were almost twice as likely to receive UFH.

We identified 163 (0.51%) episodes of VTE (Table 2). Compared to patients receiving UFH, those receiving standard LMWH had similar unadjusted rates of VTE (0.53% vs 0.48%; P = 0.54), major bleeding (0.77% vs 0.76%; P = 0.88), thrombocytopenia (1.9% vs 2.0%; P = 0.48), definite HIT (n = 1 vs n = 3; P = 0.34), and mortality (2.8% vs 3.1%; P = 0.07). Definite complications of prophylaxis (HIT or major bleed combined with the discontinuation of heparin) were more common among patients receiving UFH (0.2% vs 0.1%; P = 0.022). Patients treated with UFH had longer unadjusted lengths of stay (P < 0.0001) and higher unadjusted costs (P < 0.0001).
| Total | UFH | LMWH | P | |
|---|---|---|---|---|
| 32,104 (100) | 14,591 (45.4) | 17,513 (54.6) | ||
| n (%) | n (%) | n (%) | ||
| ||||
| Venous thromboembolism | 163 (0.5) | 78 (0.5) | 85 (0.5) | 0.54 |
| Heparin‐induced thrombocytopenia | 4 (0) | 3 (0) | 1 (0) | 0.34* |
| Any major bleeding | 246 (0.8) | 113 (0.8) | 133 (0.8) | 0.88 |
| Transfusion with 2 units of packed red blood cells | 218 (0.7) | 97 (0.7) | 121 (0.7) | 0.78 |
| Intracranial hemorrhage | 30 (0.1) | 17 (0.1) | 13 (0.1) | 0.22 |
| Complication resulting in stopping heparin | 44 (0.1) | 28 (0.2) | 16 (0.1) | 0.02 |
| In‐hospital mortality | 944 (2.9) | 456 (3.1) | 488 (2.8) | 0.07 |
| LOS in days; mean (SD) | 6.2 (5.9) | 6.4 (6.2) | 6.0 (5.6) | <0.001 |
| Median (IQR) | 5 (37) | 5 (37) | 5 (37) | |
| Cost in USD; median (IQR) | 5873 (41718982) | 6007 (41779456) | 5774 (41658660) | <0.001 |
A propensity model for UFH treatment based upon patient characteristics and treatments was not strongly predictive of treatment (c = 0.58) and propensity matching failed to balance many of the patient characteristics. However, hospital alone, ignoring patient characteristics was strongly predictive (c = 0.91) of treatment.
In a model adjusting only for clustering within hospitals, patients treated with UFH had an odds ratio (OR) for VTE of 1.08 (95% confidence interval [CI] 0.79 to 1.49) compared to patients receiving LMWH (Figure 2). Adjustment for propensity for UFH and other covariates attenuated the effect of LMWH (OR 1.04, 95% CI 0.76 to 1.43). When individual patients were assigned a probability of treatment with UFH equal to the hospital rate where they received care, UFH use was associated with a nonsignificant change in the odds of VTE (OR 1.14, 95% CI 0.72 to 1.81).

Adjusted for clustering within hospital only, patients treated with UFH had an odds ratio for major bleed of 1.38 (95% CI 1.00 to 1.91) compared to patients receiving LMWH (Figure 3). Adjustment for propensity for UFH and other covariates gave similar results (OR 1.34, 95% CI 0.97 to 1.84). When individual patients were assigned a probability of treatment with UFH equal to the hospital rate where they received care, UFH treatment was associated with a nonsignificant increase in the odds of major bleed (OR 1.64, 95% CI 0.50 to 5.33). When we compared the rate of transfusion across hospitals, including 576,231 additional patients who were excluded from the original analyses because they did not receive daily prophylaxis or had a diagnosis of myocardial infarction or COPD, there was a slight negative correlation between transfusion rates and use of UFH (Spearman Correlation Coefficient 0.03; P = 0.61). Hospitals that used primarily UFH had a transfusion rate of 0.60% versus 0.76% at hospitals using primarily LMWH (P = 0.54), indicating that the increased risk of major bleeding associated with UFH was not confounded by local transfusion practices.

Adjusted for clustering only, patients treated with UFH had an odds ratio for definite complication of 2.35 (95% CI 1.17 to 4.72) compared to those treated with LMWH. Adjustment for propensity and covariates accentuated the association (OR 2.84, 95% CI 1.43 to 5.66). When assigned a probability of treatment with UFH equal to the hospital rate where they received care, UFH treatment was associated with an increase in the risk of definite complication (OR 2.79, 95% CI 1.00 to 7.74).
Adjusted for clustering only, patients treated with UFH had higher costs than those treated with LMWH (cost ratio 1.07, 95% CI 1.05 to 1.09). Adjustment for propensity for UFH and other covariates attenuated the association (cost ratio 1.02, 95% CI 1.00 to 1.03). Finally, when individual patients were assigned a probability of initial treatment with UFH equal to the hospital rate where they received care, treatment with UFH was associated with a nonsignificant change in the relative cost (cost ratio 0.97, 95% CI 0.90 to 1.05).
DISCUSSION
In this retrospective cohort study, we found that low‐molecular‐weight heparin and unfractionated heparin were associated with similar rates of VTE in moderate‐to‐high risk medical patients. However, unfractionated heparin was associated with a small, but higher risk of complications, even after adjustment. There were no statistical differences in rates of heparin‐induced thrombocytopenia, but this complication was exceedingly rare. Finally, overall costs associated with both treatments were similar.
A number of industry‐funded studies have compared LMWH to UFH in randomized clinical trials. These trials have generally been small and used endpoints of uncertain significance, such as asymptomatic deep vein thrombosis assessed by ultrasound. At least 3 meta‐analyses of these trials have been published. Each used different inclusion criteria. The only one to find an efficacy benefit to LMWH over UFH was heavily influenced by the inclusion of a number of studies of stroke patients.3 In that study, LMWH reduced VTE by approximately one‐third relative to UFH. The other 2 analyses found smaller reductions in DVT and pulmonary embolism (PE), and these results were not statistically significant.5, 8 Similarly, 1 analysis5 found a reduction in major bleeding events with LMWH versus UFH, whereas the other 2 studies found smaller reductions which were not statistically significant. The assessment of major bleeding is further complicated by differences in the definition of major bleeding across studies. Using a standard definition of 2 units of packed red blood cells transfused in 1 day to denote major bleeding, we found an associated reduction in bleeding with LMWH that was similar to that observed in the meta‐analyses. Moreover, patients receiving UFH were twice as likely to have a complication that resulted in stopping the prophylaxis, although these complications were overall quite rare. Lastly, there are no cost comparisons based on randomized trials. Several comparisons based on modeling have favored LMWH. One assumed that 3% of patients receiving UFH would develop HIT;13 something we did not observe. At least 3 additional analyses,1416 all funded by the manufacturer of enoxaparin, assumed that LMWH was both more effective and safer than UFH. We found that adjusted costs were similar or slightly lower with UFH than LMWH.
Our study has a number of limitations. First, its observational design makes it vulnerable to selection bias. We attempted to overcome this with rigorous multivariable adjustment, including the propensity for treatment and by using an adaptation of the instrumental variable approach. This method is of particular interest because individual hospitals were strongly predictive of choice of heparin. Still, we cannot exclude the possibility of residual confounding, especially if other outcomes, such as transfusion decisions, were also tied to specific hospital practices. Second, our study used administrative data, and therefore we could not directly adjust for certain differences which may exist between patients who received LMWH and those who received UFH. However, we did adjust for many classic risk factors for VTE. More importantly, it seems that the chance of being treated with a particular form of heparin depends more on the hospital where one receives care than on any combination of patient characteristics. Thus, apart from renal failure, for which we adjusted, it seems unlikely that there were major differences in unmeasured physiological confounders. Third, we limited our analysis to patients who received standard dosing of either type of heparin. We did this to bolster the validity of our findings, but they may not apply to unconventional dosing often observed in clinical practice. Fourth, we measured only outcomes that occurred in the hospital or that prompted a return to the hospital. VTEs which were diagnosed and treated in ambulatory care were not included. While this may have led us to underestimate the true risk of VTE, we have little reason to believe that the choice of whether to admit a patient with VTE is influenced by the original choice of VTE prophylaxis. Finally, our study was conducted before the introduction of generic LMWH, which would be expected to reduce costs associated with LMWH prophylaxis.
VTE prophylaxis for medical patients has emerged as a major focus for quality improvement initiatives. As a result, a significant proportion of general medical patients receive some form of chemoprophylaxis during their hospital stay. Small differences in efficacy or safety of different forms of prophylaxis multiplied by millions of admissions each year can have profound effects on the health of hospitalized patients. Similarly, differences in cost could also have a substantial impact on the healthcare system. We found no difference in efficacy or cost, but treatment with LMWH was less likely to be associated with subsequent transfusion of 2 or more units of packed red blood cells, a surrogate marker for bleeding. In addition, LMWH is more convenient since it can be dosed once daily, and for that reason may be more acceptable to patients. For these reasons, LMWH may be the drug of choice for inpatient prophylaxis of general medical patients. In situations where the cost of the medication itself is important, UFH represents an equally effective alternative.
Acknowledgements
All authors have contributed sufficiently to this study and have provided written permission to be named in the manuscript. No other persons have made substantial contributions to this manuscript. Michael B. Rothberg is the guarantor of the entire manuscript.
Disclosures: This study was supported by a Clinical Scientist Development Award from the Doris Duke Charitable Foundation. The funding source had no role in the study design, analysis, or interpretation of the data. Dr Rothberg served for 1 day as a consultant to Novartis Pharma about an influenza vaccine model. Sandoz, a division of Novartis, was recently granted approval to manufacture a generic form of low‐molecular‐weight heparin. None of the other authors have any conflicts of interest.
Venous thromboembolism (VTE) is a major source of morbidity and mortality for hospitalized patients, with as many as 16% of high‐risk medical patients developing VTE during their hospital stay.1, 2 Pharmacologic prophylaxis with subcutaneous heparin reduces the risk of VTE by approximately 50%,3, 4 and guidelines produced by the American College of Chest Physicians (ACCP) recommend thromboprophylaxis for patients at moderate‐to‐high risk of VTE with either low‐molecular‐weight heparin (LMWH) or unfractionated heparin (UFH).2 UFH is less expensive per dose, but meta‐analyses have suggested that UFH may be either less effective than LMWH3 or more likely to cause complications, such as bleeding5 or heparin‐induced thrombocytopenia (HIT).6 Others have argued that the efficacy and risk of bleeding with UFH and LMWH are similar.7, 8 In either case, there are few head‐to‐head studies of LMWH and UFH in medical patients and they tend to be small. In the most recent meta‐analysis, which included fewer than 4500 patients, several different low‐molecular‐weight heparins were assessed together, and the observed rate of deep vein thrombosis (DVT) with UFH was high (5.4%), with evidence suggesting publication bias.3
Given the current Joint Commission requirement9 that all medical patients either receive VTE prophylaxis or have documented a reason not to, the implications related to choosing one form of VTE prophylaxis over another are substantial on a national scale. In order to compare the effectiveness of UFH and LMWH in routine practice among hospitalized medical patients, we conducted a retrospective cohort study in a national sample of hospitals and compared the risk of VTE, bleeding, HIT, and death associated with each treatment.
METHODS
Setting and Patients
We conducted a retrospective cohort study of patients discharged between January 1, 2004 and June 30, 2005 from 333 acute care facilities in the United States that participated in Premier's Perspective, a database we have described previously.10 Compared to US hospitals as a whole, Perspective hospitals are more likely to be located in the South and in urban areas. Perspective contains the following data elements: sociodemographic information, International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) diagnosis and procedure codes, as well as a list of all billed items with a date of service, including diagnostic tests, medications, and other treatments. Hospitals' characteristics include size, region, setting, and teaching status. The Institutional Review Board at Baystate Medical Center granted permission to conduct the study (#132280‐1).
We included general medical patients aged 18 years whose ICD‐9‐CM primary diagnosis code (congestive heart failure, stroke, pneumonia, and urinary tract infection) placed them at moderate‐to‐high risk of VTE according to the ACCP recommendations,2 and who received daily prophylactic dosages of either LMWH (40 mg daily) or UFH (10,00015,000 units daily) initiated by hospital day 2 and continued to discharge or until the patient developed a VTE or a complication attributable to heparin. Patients were included so long as they missed no more than 1 day of prophylaxis or had no more than 1 unusual dose recorded. Patients who switched between heparin types were included and analyzed according to their initial therapy. Patients who received any other regimen were excluded. We also excluded patients who received warfarin on hospital day 1 or 2, because they would not be considered candidates for heparin prophylaxis, and patients whose length of stay was 2 days, because the value of VTE prophylaxis in such cases is unknown.
Data Elements
For each patient, we extracted age, gender, race, and insurance status, principal diagnosis, comorbidities, and specialty of the attending physician. Comorbidities were identified from ICD‐9‐CM secondary diagnosis codes and Diagnosis Related Groups using Healthcare Cost and Utilization Project Comorbidity Software, version 3.1, based on the work of Elixhauser and colleagues.11 We also identified additional risk factors for VTE using a combination of ICD‐9‐CM codes and specific charges. These included cancer, chemotherapy/radiation, prior VTE, use of estrogens and estrogen modulators, inflammatory bowel disease, nephrotic syndrome, myeloproliferative disorders, smoking, central venous catheter, inherited or acquired thrombophilia, mechanical ventilation, urinary catheter, decubitus ulcer, 3‐hydroxy‐3‐methylglutaryl coenzyme A (HMG‐CoA) reductase inhibitors, restraints, and varicose veins. Hospitals were categorized by region (Northeast, South, Midwest, or West), bed size, setting (urban vs rural), and teaching status.
Outcome Variables
We defined hospital‐acquired VTE as a secondary diagnosis of VTE (ICD‐9‐CM diagnoses 453.4, 453.40, 453.41, 453.42, 453.8, 453.9, 415.1, 415.11, 415.19), combined with a diagnostic test for VTE (lower extremity ultrasound, venography, computed tomography (CT) angiogram, ventilation‐perfusion scan, or pulmonary angiogram) after hospital day 2, followed by treatment for VTE (intravenous unfractionated heparin, >60 mg of enoxaparin, 7500 mg of dalteparin, or placement of an inferior vena cava filter) for at least 50% of the remaining hospital days or until initiation of warfarin or appearance of a complication (eg, transfusion or treatment for heparin‐induced thrombocytopenia). We chose this definition to differentiate hospital‐acquired VTE from VTE present on admission.12 In addition, patients who were readmitted within 30 days of discharge with a primary diagnosis of VTE were also considered to have hospital‐acquired VTE.
We also assessed complications of VTE prophylaxis. Major bleeding was defined as the receipt of 2 or more units of packed red blood cells on a single day or a secondary diagnosis of intracranial bleeding. Because there was no ICD‐9‐CM code for HIT, we assessed codes for all thrombocytopenia, as well as secondary thrombocytopenia. Definite HIT was defined as an ICD‐9‐CM code for thrombocytopenia, together with discontinuation of heparin and initiation of treatment with argatroban. A definite complication was defined as HIT or evidence of major bleeding coupled with discontinuation of heparin. Finally, we evaluated all‐cause in‐hospital mortality and total hospital costs.
Statistical Analysis
We computed summary statistics using frequencies and percents for categorical variables, and means, medians, and standard deviations and interquartile range for continuous variables. Associations of prophylaxis type with patient and hospital characteristics and outcomes were assessed using chi‐square tests or Fisher's exact test for categorical variables, and z‐tests or Wilcoxon tests for continuous variables.
We developed a propensity model for treatment with UFH as the outcome; the model included patient characteristics, early treatments, comorbidities, risk factors for VTE, physician specialty, and selected interaction terms. We then developed a series of multivariable models to evaluate the impact of heparin choice on the risk of VTE, complications of treatment, mortality, and total cost. Generalized estimating equation models with a logit link were used to assess the association between the choice of heparin and the risk of VTE, and of complications and mortality, while adjusting for the effects of within‐hospital correlation; identity link models were used for analyses of cost. Costs were trimmed at 3 standard deviations above the mean, and natural log‐transformed values were modeled due to extreme positive skew.
Unadjusted and covariate‐adjusted models were evaluated with and without adjustments for propensity score. In addition, since the hospital was the single strongest predictor of treatment, we developed grouped treatment models, in which a patient's actual treatment was replaced by a probability equal to the proportion of prophylaxed patients receiving UFH at that hospital. This adaptation of instrumental variable analysis uses the hospital as the instrument, and attempts to assess whether patients treated at a hospital which uses UFH more frequently have outcomes that differ from those of patients treated at hospitals which use LMWH more frequently, while adjusting for other patient, physician, and hospital variables. By relying on treatment at the hospital level, this method reduces the opportunity for selection bias at the patient level.
Finally, in order to exclude the possibility that our surrogate bleeding outcome was due to transfusion practices at hospitals that use a particular form of heparin, we compared the hospital rates of transfusion of 2 or more units of packed red cells to the hospital rates of prophylaxis with UFH in a larger dataset of the same hospitals. This set included patients with congestive heart failure, stroke, pneumonia, and urinary tract infection who did not receive daily prophylaxis, as well as patients admitted for chronic obstructive pulmonary disease (COPD) or acute myocardial infarction, and patients who received either warfarin or a treatment dose of heparin in the first 2 hospital days. We also compared the transfusion rates at hospitals that used unfractionated heparin in 80% of patients to hospitals that used LMWH in 80%. All analyses were performed using SAS version 9.1 (SAS Institute Inc, Cary, NC).
RESULTS
Our final sample included 32,104 patients who received prophylaxis at 333 hospitals (see Supporting Information, e‐Figure, in the online version of this article). Patient characteristics appear in Table 1. Most patients (66%) were over age 65; 59% were female and 61% were white. The most common primary diagnoses were pneumonia (40%) and congestive heart failure (25%). Additional risk factors for thromboembolism included cancer (13%), paralysis (8%), or diabetes (35%). Most patients' attending physicians were either internists (61%) or family practitioners (14%). Almost half of the patients were cared for at hospitals in the South (46%).
| Total | UFH | LMWH | ||
|---|---|---|---|---|
| 32,104 (100) | 14,591 (45.4) | 17,513 (54.6) | ||
| N (%) | N (%) | N (%) | P | |
| ||||
| Demographics | ||||
| Age | 0.0002 | |||
| 1849 | 4,061 (12.7) | 1,950 (13.4) | 2,111 (12.1) | |
| 5064 | 6,962 (21.7) | 3,225 (22.1) | 3,737 (21.3) | |
| 6579 | 10,909 (34.0) | 4,921 (33.7) | 5,988 (34.2) | |
| 80+ | 10,172 (31.7) | 4,495 (30.8) | 5,677 (32.4) | |
| Sex | 0.0071 | |||
| Male | 13,234 (41.2) | 6,133 (42.0) | 7,101 (40.5) | |
| Female | 18,870 (58.8) | 8,458 (58.0) | 10,412 (59.5) | |
| Race/ethnicity | <0.0001 | |||
| White | 19,489 (60.7) | 8,063 (55.3) | 11,426 (65.2) | |
| Black | 7,429 (23.1) | 4,101 (28.1) | 3,328 (19.0) | |
| Hispanic | 1,304 (4.1) | 591 (4.1) | 713 (4.1) | |
| Other | 3,882 (12.1) | 1,836 (12.6) | 2,046 (11.7) | |
| Primary diagnosis | <0.0001 | |||
| Pneumonia | 12,768 (39.8) | 5,354 (36.7) | 7,414 (42.3) | |
| Sepsis* | 1,217 (3.8) | 562 (3.9) | 655 (3.7) | |
| Respiratory failure* | 2,017 (6.3) | 814 (5.6) | 1,203 (6.9) | |
| Heart failure | 8,157 (25.4) | 3,825 (26.2) | 4,332 (24.7) | |
| Stroke | 4,416 (13.8) | 2,295 (15.7) | 2,121 (12.1) | |
| Urinary tract infection | 3,529 (11.0) | 1,741 (11.9) | 1,788 (10.2) | |
| Attending specialty | <0.0001 | |||
| Internist | 19,511 (60.8) | 8,945 (61.3) | 10,566 (60.3) | |
| General practice/Family medicine | 4,326 (13.5) | 1,964 (13.5) | 2,362 (13.5) | |
| Cardiologist | 1,606 (5.0) | 730 (5.0) | 876 (5.0) | |
| Pulmonologist | 2,179 (6.8) | 854 (5.9) | 1,325 (7.6) | |
| Nephrology | 583 (1.8) | 380 (2.6) | 203 (1.2) | |
| Critical care/Intensivist | 150 (0.5) | 93 (0.6) | 57 (0.3) | |
| Other | 3,749 (11.7) | 1,625 (11.1) | 2,124 (12.1) | |
| Insurance | <0.0001 | |||
| Medicare traditional | 20,281 (63.2) | 8,929 (61.2) | 11,352 (64.8) | |
| Medicare managed care | 1,737 (5.4) | 826 (5.7) | 911 (5.2) | |
| Medicaid | 2,629 (8.2) | 1,401 (9.6) | 1,228 (7.0) | |
| Private | 5,967 (18.6) | 2,830 (19.4) | 3,137 (17.9) | |
| Self‐pay/uninsured/other | 1,490 (4.6) | 605 (4.1) | 885 (5.1) | |
| Risk factors for VTE | ||||
| Admit from skilled nursing facility | 476 (1.5) | 277 (1.9) | 199 (1.1) | <0.0001 |
| Paralysis | 2,608 (8.1) | 1,317 (9.0) | 1,291 (7.4) | <0.0001 |
| Restraints | 417 (1.3) | 147 (1.0) | 270 (1.5) | <0.0001 |
| Decubitus ulcer | 1,190 (3.7) | 631 (4.3) | 559 (3.2) | <0.0001 |
| Cancer | 4,154 (12.9) | 1,858 (12.7) | 2,296 (13.1) | 0.3171 |
| Chemotherapy | 86 (0.3) | 41 (0.3) | 45 (0.3) | 0.6781 |
| Prior venous thromboembolism | 494 (1.5) | 202 (1.4) | 292 (1.7) | 0.0403 |
| Pregnancy | 1 (0) | 1 (0) | 0 (0) | 0.2733 |
| Estrogens | 438 (1.4) | 143 (1.0) | 295 (1.7) | <0.0001 |
| Estrogen modulators | 246 (0.8) | 80 (0.5) | 166 (0.9) | <0.0001 |
| Congestive heart failure | 3,107 (9.7) | 1,438 (9.9) | 1,669 (9.5) | 0.3263 |
| Respiratory failure | 2,210 (6.9) | 1,037 (7.1) | 1,173 (6.7) | 0.1493 |
| Inflammatory bowel disease | 108 (0.3) | 41 (0.3) | 67 (0.4) | 0.1176 |
| Nephrotic syndrome | 92 (0.3) | 50 (0.3) | 42 (0.2) | 0.0860 |
| Myeloproliferative disorder | 198 (0.6) | 68 (0.5) | 130 (0.7) | 0.0016 |
| Obesity | 2,973 (9.3) | 1,211 (8.3) | 1,762 (10.1) | <0.0001 |
| Smoking | 4,476 (13.9) | 1,887 (12.9) | 2,589 (14.8) | <0.0001 |
| Varicose veins | 19 (0.1) | 6 (0) | 13 (0.1) | 0.2245 |
| Central line | 1,070 (3.3) | 502 (3.4) | 568 (3.2) | 0.3271 |
| Inherited or acquired thrombophilia | 16 (0) | 9 (0.1) | 7 (0) | 0.3855 |
| Diabetes | 11,136 (34.7) | 5,157 (35.3) | 5,979 (34.1) | 0.0241 |
| Procedures associated with VTE or bleed | ||||
| Mechanical ventilation | 2,282 (7.1) | 1,111 (7.6) | 1,171 (6.7) | 0.0013 |
| Urinary catheter | 4,496 (14.0) | 1,545 (10.6) | 2,951 (16.9) | <0.0001 |
| Aspirin | 12,865 (40.1) | 6,101 (41.8) | 6,764 (38.6) | <0.0001 |
| Clopidogrel | 4,575 (14.3) | 2,087 (14.3) | 2,488 (14.2) | 0.8050 |
| Non‐steroidal anti‐inflammatory drugs | 2,147 (6.7) | 867 (5.9) | 1,280 (7.3) | <0.0001 |
| Steroids | 7,938 (24.7) | 3,136 (21.5) | 4,802 (27.4) | <0.0001 |
| Statins | 7,376 (23.0) | 3,462 (23.7) | 3,914 (22.3) | 0.0035 |
| Comorbidities | ||||
| AIDS | 124 (0.4) | 73 (0.5) | 51 (0.3) | 0.0026 |
| Alcohol abuse | 1,048 (3.3) | 523 (3.6) | 525 (3.0) | 0.0032 |
| Deficiency anemia | 7,010 (21.8) | 3,228 (22.1) | 3,782 (21.6) | 0.2543 |
| Rheumatoid arthritis/collagen vas | 967 (3.0) | 426 (2.9) | 541 (3.1) | 0.3762 |
| Chronic blood loss anemia | 177 (0.6) | 79 (0.5) | 98 (0.6) | 0.8269 |
| Chronic pulmonary disease | 12,418 (38.7) | 5,314 (36.4) | 7,104 (40.6) | <0.0001 |
| Depression | 3,334 (10.4) | 1433 (9.8) | 1901 (10.9) | 0.0025 |
| Drug abuse | 694 (2.2) | 412 (2.8) | 282 (1.6) | <0.0001 |
| Hypertension | 16,979 (52.9) | 7,658 (52.5) | 9,321 (53.2) | 0.1866 |
| Hypothyroidism | 4,016 (12.5) | 1,716 (11.8) | 2,300 (13.1) | 0.0002 |
| Liver disease | 453 (1.4) | 227 (1.6) | 226 (1.3) | 0.0448 |
| Other neurological disorders | 4,682 (14.6) | 2,202 (15.1) | 2,480 (14.2) | 0.0187 |
| Peripheral vascular disease | 2,134 (6.6) | 980 (6.7) | 1,154 (6.6) | 0.6490 |
| Psychoses | 1,295 (4.0) | 574 (3.9) | 721 (4.1) | 0.4066 |
| Pulmonary circulation disease | 1,034 (3.2) | 442 (3.0) | 592 (3.4) | 0.0760 |
| Renal failure | 2,794 (8.7) | 1,636 (11.2) | 1,158 (6.6) | 0.0000 |
| Peptic ulcer disease with bleeding | 563 (1.8) | 232 (1.6) | 331 (1.9) | 0.0414 |
| Valvular disease | 2,079 (6.5) | 899 (6.2) | 1,180 (6.7) | 0.0366 |
| Weight loss | 1,231 (3.8) | 556 (3.8) | 675 (3.9) | 0.8391 |
| Other prophylaxis | ||||
| Intermittent pneumatic compression | 1,003 (3.1) | 456 (3.1) | 547 (3.1) | 0.9926 |
| Mechanical prophylaxis | 1,281 (4.0) | 524 (3.6) | 757 (4.3) | 0.0009 |
Fifty‐five percent of patients received LMWH and the remainder received UFH; 1274 (4%) patients switched type of heparin during their stay. The proportion of patients receiving LMWH at an individual hospital varied from 0% to 100% with a u‐shaped distribution, with almost one‐third of hospitals prescribing one treatment or the other exclusively (Figure 1). Similarly, the proportion of an individual physician's patients who received prophylaxis with UFH (vs LMWH) varied from 0% to 100% (Figure 1), with 51% prescribing LMWH exclusively and 31% prescribing UFH exclusively. Compared to patients who received UFH, patients receiving LMWH were older and were more likely to be white, female, and to have pneumonia. By far the biggest difference between the groups was the hospitals at which they received their care (see Supporting Information, e‐Table, in the online version of this article). Patients receiving LMWH were much more likely to be from smaller, rural, non‐teaching hospitals in the South or the West. There were also numerous small differences in comorbidities and individual VTE risk factors between the 2 groups. The only large difference was that patients with a secondary diagnosis of renal failure (for which LMWH is not US Food and Drug Administration [FDA] approved) were almost twice as likely to receive UFH.

We identified 163 (0.51%) episodes of VTE (Table 2). Compared to patients receiving UFH, those receiving standard LMWH had similar unadjusted rates of VTE (0.53% vs 0.48%; P = 0.54), major bleeding (0.77% vs 0.76%; P = 0.88), thrombocytopenia (1.9% vs 2.0%; P = 0.48), definite HIT (n = 1 vs n = 3; P = 0.34), and mortality (2.8% vs 3.1%; P = 0.07). Definite complications of prophylaxis (HIT or major bleed combined with the discontinuation of heparin) were more common among patients receiving UFH (0.2% vs 0.1%; P = 0.022). Patients treated with UFH had longer unadjusted lengths of stay (P < 0.0001) and higher unadjusted costs (P < 0.0001).
| Total | UFH | LMWH | P | |
|---|---|---|---|---|
| 32,104 (100) | 14,591 (45.4) | 17,513 (54.6) | ||
| n (%) | n (%) | n (%) | ||
| ||||
| Venous thromboembolism | 163 (0.5) | 78 (0.5) | 85 (0.5) | 0.54 |
| Heparin‐induced thrombocytopenia | 4 (0) | 3 (0) | 1 (0) | 0.34* |
| Any major bleeding | 246 (0.8) | 113 (0.8) | 133 (0.8) | 0.88 |
| Transfusion with 2 units of packed red blood cells | 218 (0.7) | 97 (0.7) | 121 (0.7) | 0.78 |
| Intracranial hemorrhage | 30 (0.1) | 17 (0.1) | 13 (0.1) | 0.22 |
| Complication resulting in stopping heparin | 44 (0.1) | 28 (0.2) | 16 (0.1) | 0.02 |
| In‐hospital mortality | 944 (2.9) | 456 (3.1) | 488 (2.8) | 0.07 |
| LOS in days; mean (SD) | 6.2 (5.9) | 6.4 (6.2) | 6.0 (5.6) | <0.001 |
| Median (IQR) | 5 (37) | 5 (37) | 5 (37) | |
| Cost in USD; median (IQR) | 5873 (41718982) | 6007 (41779456) | 5774 (41658660) | <0.001 |
A propensity model for UFH treatment based upon patient characteristics and treatments was not strongly predictive of treatment (c = 0.58) and propensity matching failed to balance many of the patient characteristics. However, hospital alone, ignoring patient characteristics was strongly predictive (c = 0.91) of treatment.
In a model adjusting only for clustering within hospitals, patients treated with UFH had an odds ratio (OR) for VTE of 1.08 (95% confidence interval [CI] 0.79 to 1.49) compared to patients receiving LMWH (Figure 2). Adjustment for propensity for UFH and other covariates attenuated the effect of LMWH (OR 1.04, 95% CI 0.76 to 1.43). When individual patients were assigned a probability of treatment with UFH equal to the hospital rate where they received care, UFH use was associated with a nonsignificant change in the odds of VTE (OR 1.14, 95% CI 0.72 to 1.81).

Adjusted for clustering within hospital only, patients treated with UFH had an odds ratio for major bleed of 1.38 (95% CI 1.00 to 1.91) compared to patients receiving LMWH (Figure 3). Adjustment for propensity for UFH and other covariates gave similar results (OR 1.34, 95% CI 0.97 to 1.84). When individual patients were assigned a probability of treatment with UFH equal to the hospital rate where they received care, UFH treatment was associated with a nonsignificant increase in the odds of major bleed (OR 1.64, 95% CI 0.50 to 5.33). When we compared the rate of transfusion across hospitals, including 576,231 additional patients who were excluded from the original analyses because they did not receive daily prophylaxis or had a diagnosis of myocardial infarction or COPD, there was a slight negative correlation between transfusion rates and use of UFH (Spearman Correlation Coefficient 0.03; P = 0.61). Hospitals that used primarily UFH had a transfusion rate of 0.60% versus 0.76% at hospitals using primarily LMWH (P = 0.54), indicating that the increased risk of major bleeding associated with UFH was not confounded by local transfusion practices.

Adjusted for clustering only, patients treated with UFH had an odds ratio for definite complication of 2.35 (95% CI 1.17 to 4.72) compared to those treated with LMWH. Adjustment for propensity and covariates accentuated the association (OR 2.84, 95% CI 1.43 to 5.66). When assigned a probability of treatment with UFH equal to the hospital rate where they received care, UFH treatment was associated with an increase in the risk of definite complication (OR 2.79, 95% CI 1.00 to 7.74).
Adjusted for clustering only, patients treated with UFH had higher costs than those treated with LMWH (cost ratio 1.07, 95% CI 1.05 to 1.09). Adjustment for propensity for UFH and other covariates attenuated the association (cost ratio 1.02, 95% CI 1.00 to 1.03). Finally, when individual patients were assigned a probability of initial treatment with UFH equal to the hospital rate where they received care, treatment with UFH was associated with a nonsignificant change in the relative cost (cost ratio 0.97, 95% CI 0.90 to 1.05).
DISCUSSION
In this retrospective cohort study, we found that low‐molecular‐weight heparin and unfractionated heparin were associated with similar rates of VTE in moderate‐to‐high risk medical patients. However, unfractionated heparin was associated with a small, but higher risk of complications, even after adjustment. There were no statistical differences in rates of heparin‐induced thrombocytopenia, but this complication was exceedingly rare. Finally, overall costs associated with both treatments were similar.
A number of industry‐funded studies have compared LMWH to UFH in randomized clinical trials. These trials have generally been small and used endpoints of uncertain significance, such as asymptomatic deep vein thrombosis assessed by ultrasound. At least 3 meta‐analyses of these trials have been published. Each used different inclusion criteria. The only one to find an efficacy benefit to LMWH over UFH was heavily influenced by the inclusion of a number of studies of stroke patients.3 In that study, LMWH reduced VTE by approximately one‐third relative to UFH. The other 2 analyses found smaller reductions in DVT and pulmonary embolism (PE), and these results were not statistically significant.5, 8 Similarly, 1 analysis5 found a reduction in major bleeding events with LMWH versus UFH, whereas the other 2 studies found smaller reductions which were not statistically significant. The assessment of major bleeding is further complicated by differences in the definition of major bleeding across studies. Using a standard definition of 2 units of packed red blood cells transfused in 1 day to denote major bleeding, we found an associated reduction in bleeding with LMWH that was similar to that observed in the meta‐analyses. Moreover, patients receiving UFH were twice as likely to have a complication that resulted in stopping the prophylaxis, although these complications were overall quite rare. Lastly, there are no cost comparisons based on randomized trials. Several comparisons based on modeling have favored LMWH. One assumed that 3% of patients receiving UFH would develop HIT;13 something we did not observe. At least 3 additional analyses,1416 all funded by the manufacturer of enoxaparin, assumed that LMWH was both more effective and safer than UFH. We found that adjusted costs were similar or slightly lower with UFH than LMWH.
Our study has a number of limitations. First, its observational design makes it vulnerable to selection bias. We attempted to overcome this with rigorous multivariable adjustment, including the propensity for treatment and by using an adaptation of the instrumental variable approach. This method is of particular interest because individual hospitals were strongly predictive of choice of heparin. Still, we cannot exclude the possibility of residual confounding, especially if other outcomes, such as transfusion decisions, were also tied to specific hospital practices. Second, our study used administrative data, and therefore we could not directly adjust for certain differences which may exist between patients who received LMWH and those who received UFH. However, we did adjust for many classic risk factors for VTE. More importantly, it seems that the chance of being treated with a particular form of heparin depends more on the hospital where one receives care than on any combination of patient characteristics. Thus, apart from renal failure, for which we adjusted, it seems unlikely that there were major differences in unmeasured physiological confounders. Third, we limited our analysis to patients who received standard dosing of either type of heparin. We did this to bolster the validity of our findings, but they may not apply to unconventional dosing often observed in clinical practice. Fourth, we measured only outcomes that occurred in the hospital or that prompted a return to the hospital. VTEs which were diagnosed and treated in ambulatory care were not included. While this may have led us to underestimate the true risk of VTE, we have little reason to believe that the choice of whether to admit a patient with VTE is influenced by the original choice of VTE prophylaxis. Finally, our study was conducted before the introduction of generic LMWH, which would be expected to reduce costs associated with LMWH prophylaxis.
VTE prophylaxis for medical patients has emerged as a major focus for quality improvement initiatives. As a result, a significant proportion of general medical patients receive some form of chemoprophylaxis during their hospital stay. Small differences in efficacy or safety of different forms of prophylaxis multiplied by millions of admissions each year can have profound effects on the health of hospitalized patients. Similarly, differences in cost could also have a substantial impact on the healthcare system. We found no difference in efficacy or cost, but treatment with LMWH was less likely to be associated with subsequent transfusion of 2 or more units of packed red blood cells, a surrogate marker for bleeding. In addition, LMWH is more convenient since it can be dosed once daily, and for that reason may be more acceptable to patients. For these reasons, LMWH may be the drug of choice for inpatient prophylaxis of general medical patients. In situations where the cost of the medication itself is important, UFH represents an equally effective alternative.
Acknowledgements
All authors have contributed sufficiently to this study and have provided written permission to be named in the manuscript. No other persons have made substantial contributions to this manuscript. Michael B. Rothberg is the guarantor of the entire manuscript.
Disclosures: This study was supported by a Clinical Scientist Development Award from the Doris Duke Charitable Foundation. The funding source had no role in the study design, analysis, or interpretation of the data. Dr Rothberg served for 1 day as a consultant to Novartis Pharma about an influenza vaccine model. Sandoz, a division of Novartis, was recently granted approval to manufacture a generic form of low‐molecular‐weight heparin. None of the other authors have any conflicts of interest.
- ,,, et al.A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group.N Engl J Med.1999;341:793–800.
- ,,, et al.Prevention of venous thromboembolism: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition).Chest.2008;133:381S–453S.
- ,,,,.Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta‐analysis of randomized controlled trials.Arch Intern Med.2007;167:1476–1486.
- ,,,,.Meta‐analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients.Ann Intern Med.2007;146:278–288.
- ,,, et al.Prevention of venous thromboembolism in internal medicine with unfractionated or low‐molecular‐weight heparins: a meta‐analysis of randomised clinical trials.Thromb Haemost.2000;83:14–19.
- ,,.Risk for heparin‐induced thrombocytopenia with unfractionated and low‐molecular‐weight heparin thromboprophylaxis: a meta‐analysis.Blood.2005;106:2710–2715.
- ,.A safety analysis of thromboprophylaxis in acute medical illness.Thromb Haemost.2003;89:590–591.
- ,,,,.How complete is the evidence for thromboembolism prophylaxis in general medicine patients? A meta‐analysis of randomized controlled trials.J Hosp Med.2009;4:289–297.
- The Joint Commission on the Accreditation of Healthcare Organizations. Venous Thromboembolism (VTE) Core Measure Set. Available at: http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/VTE.htm. Accessed June 1,2009.
- ,,, et al.Potentially inappropriate medication use in hospitalized elders.J Hosp Med.2008;3:91–102.
- ,,,.Comorbidity measures for use with administrative data.Med Care.1998;36:8–27.
- ,,,,,.Identification of in‐hospital complications from claims data. Is it valid?Med Care.2000;38:785–795.
- ,,,.Cost utility of substituting enoxaparin for unfractionated heparin for prophylaxis of venous thrombosis in the hospitalized medical patient.J Hosp Med.2006;1:168–176.
- ,,,.Cost effectiveness of thromboprophylaxis with a low‐molecular‐weight heparin versus unfractionated heparin in acutely ill medical inpatients.Am J Manag Care.2004;10:632–642.
- ,,,.Comparison of the two‐year outcomes and costs of prophylaxis in medical patients at risk of venous thromboembolism.Thromb Haemost.2008;100:810–820.
- ,,, et al.Cost effectiveness of enoxaparin as prophylaxis against venous thromboembolic complications in acutely ill medical inpatients: modelling study from the hospital perspective in Germany.Pharmacoeconomics.2006;24:571–591.
- ,,, et al.A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group.N Engl J Med.1999;341:793–800.
- ,,, et al.Prevention of venous thromboembolism: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition).Chest.2008;133:381S–453S.
- ,,,,.Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta‐analysis of randomized controlled trials.Arch Intern Med.2007;167:1476–1486.
- ,,,,.Meta‐analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients.Ann Intern Med.2007;146:278–288.
- ,,, et al.Prevention of venous thromboembolism in internal medicine with unfractionated or low‐molecular‐weight heparins: a meta‐analysis of randomised clinical trials.Thromb Haemost.2000;83:14–19.
- ,,.Risk for heparin‐induced thrombocytopenia with unfractionated and low‐molecular‐weight heparin thromboprophylaxis: a meta‐analysis.Blood.2005;106:2710–2715.
- ,.A safety analysis of thromboprophylaxis in acute medical illness.Thromb Haemost.2003;89:590–591.
- ,,,,.How complete is the evidence for thromboembolism prophylaxis in general medicine patients? A meta‐analysis of randomized controlled trials.J Hosp Med.2009;4:289–297.
- The Joint Commission on the Accreditation of Healthcare Organizations. Venous Thromboembolism (VTE) Core Measure Set. Available at: http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/VTE.htm. Accessed June 1,2009.
- ,,, et al.Potentially inappropriate medication use in hospitalized elders.J Hosp Med.2008;3:91–102.
- ,,,.Comorbidity measures for use with administrative data.Med Care.1998;36:8–27.
- ,,,,,.Identification of in‐hospital complications from claims data. Is it valid?Med Care.2000;38:785–795.
- ,,,.Cost utility of substituting enoxaparin for unfractionated heparin for prophylaxis of venous thrombosis in the hospitalized medical patient.J Hosp Med.2006;1:168–176.
- ,,,.Cost effectiveness of thromboprophylaxis with a low‐molecular‐weight heparin versus unfractionated heparin in acutely ill medical inpatients.Am J Manag Care.2004;10:632–642.
- ,,,.Comparison of the two‐year outcomes and costs of prophylaxis in medical patients at risk of venous thromboembolism.Thromb Haemost.2008;100:810–820.
- ,,, et al.Cost effectiveness of enoxaparin as prophylaxis against venous thromboembolic complications in acutely ill medical inpatients: modelling study from the hospital perspective in Germany.Pharmacoeconomics.2006;24:571–591.
Copyright © 2012 Society of Hospital Medicine
PCP Referral
Over the past decade, research has demonstrated a value gap in US healthcare, characterized by rapidly rising costs and substandard quality.1, 2 Public reporting of hospital performance data is one of several strategies promoted to help address these deficiencies. To this end, a number of hospital rating services have created Web sites aimed at healthcare consumers.3 These services provide information about multiple aspects of healthcare quality, which in theory might be used by patients when deciding where to seek medical care.
Despite the increasing availability of publicly reported quality data comparing doctors and hospitals, a 2008 survey found that only 14% of Americans have seen and used such information in the past year, a decrease from 2006 (36%).4 A similar study in 2007 found that after seeking input from family and friends, patients generally rely on their primary care physician (PCP) to assist them to make decisions about where to have elective surgery.5 Surprisingly, almost nothing is known about how publicly reported data is used, if at all, by PCPs in the referral of patients to hospitals.
The physician is an important intermediary in the buying process for many healthcare services.6 Tertiary care hospitals depend on physician referrals for much of their patient volume.7 Until the emergence of the hospitalist model of care, most primary care physicians cared for their own hospitalized patients, and thus hospital referral decisions were largely driven by the PCP's admitting privileges. However, following the rapid expansion of the hospitalist movement,8, 9 there has been a sharp decrease in the number of PCPs who provide direct patient care for their hospitalized patients.8 As a result, PCPs may now have more choice in regards to hospital referrals for general medical conditions. Potential factors influencing a PCP's referral decisions might include familiarity with the hospital, care quality, patient convenience, satisfaction with the hospital, or hospital reputation.
Studies of cardiac surgery report cards in New York9 and Pennsylvania,10 conducted in the mid‐1990s, found that cardiologists did not use publicly reported mortality data in referral decisions, nor did they share it with patients. Over the past 2 decades, public reporting has grown exponentially, and now includes many measures of structure, processes, and outcomes for almost all US hospitals, available for free over the Internet. The growth of the patient safety movement and mandated public reporting might also have affected physicians' views about publicly reported quality data. We surveyed primary care physicians to determine the extent to which they use information about hospital quality in their referral decisions for community‐acquired pneumonia, and to identify other factors that might influence referral decisions.
METHODS
We obtained an e‐mail list of primary care physicians from the medical staff offices of all area hospitals within a 10‐mile radius of Springfield, MA (Baystate Medical Center, Holyoke Medical Center, and Mercy Medical Center). Baystate Medical Center is a 659‐bed academic medical center and Level 1 trauma center, while Holyoke and Mercy Medical Center are both 180‐bed acute care hospitals. Physicians were contacted via e‐mail from June through September of 2009, and asked to participate in an anonymous, 10‐minute, online survey accessible through an Internet link (SurveyMonkey.com) about factors influencing a primary care physician's hospital referral choice for a patient with pneumonia. To facilitate participation, we sent 2 follow‐up e‐mail reminders, and respondents who completed the entire survey received a $15 gift card. The study was approved by the institutional review board of Baystate Medical Center and closed to participation on September 23, 2009.
We created the online survey based on previous research7 and approximately 10 key informant interviews. The survey (see Supporting Information, Appendix, in the online version of this article) contained 13 demographic questions and 10 questions based on a case study of pneumonia (Figure 1). The instrument was pilot tested for clarity with a small group of primary care physicians at the author's institution and subsequently modified. We chose pneumonia because it is a common reason for a PCP to make an urgent hospital referral,11 and because there is a well‐established set of quality measures that are publicly reported.12 Unlike elective surgery, for which patients might research hospitals or surgeons on their own, patients with pneumonia would likely rely on their PCP to recommend a hospital for urgent referral. In contrast, PCPs know they will refer a number of pneumonia patients to hospitals each year and therefore might have an interest in comparing the publicly reported quality measures for local hospitals.

Respondents were shown the case study and asked to refer the hypothetical patient to 1 of 4 area hospitals. Respondents were asked to rate (on a 3‐point scale: not at all, somewhat, or very) the importance of the following factors in their referral decision: waiting time in the emergency room, distance traveled by the patient, experience of other patients, severity of patient's illness, patient's insurance, hospital's reputation among other physicians and partners, admitting privileges with a specific hospital, admitting arrangements with a hospitalist group, familiarity with the hospital, availability of subspecialists, quality of subspecialists, nursing quality, nursing staffing ratios, hospital's case volume for pneumonia, publicly available quality measures, patient preference, distance from your practice, shared electronic record system, and quality of hospital discharge summaries. Next, we measured provider's awareness of publicly reported hospital quality data and whether they used such data in referring patients or choosing their own medical care. Specifically, we asked about familiarity with the following 4 Web sites: Massachusetts Quality and Cost (a state‐specific Web site produced by the Massachusetts Executive Office of Health and Human Services)13; Hospital Compare (a Web site developed and maintained by Centers for Medicare and Medicaid Services [CMS] and the Department of Health and Human Services)14; Leapfrog Group (a private, nonprofit organization)15; and Health Grades (a private, for‐profit company).16
We then asked participants to rate the importance of the following performance measures when judging a hospital's performance: antibiotics within 6 hours of arrival to the hospital, appropriate initial antibiotic, blood culture drawn before antibiotics given, smoking cessation advice/counseling, oxygenation assessment, risk‐adjusted mortality, intensive care unit staffing, influenza vaccination, pneumococcal vaccination, Leapfrog's never events,15 volume, Leapfrog safe practices score, cost, computerized physician order entry system, Magnet status,17 and U.S. News & World Report's Best Hospitals designation.18 Lastly, we asked participants to state, using a 3‐point scale (agree, disagree, neutral), their level of agreement that the following factors, adapted from Schneider and Epstein,10 represented limitations of public reporting: 1) risk‐adjusted methods are inadequate to compare hospitals fairly; 2) mortality rates are an incomplete indication of the quality of a hospital's care; 3) hospitals can manipulate the data; and 4) ratings are inaccurate for hospitals with small caseloads.
Factors associated with physicians' knowledge of publicly reported data were analyzed with bivariate analysis. Since all factors are categorical, chi‐square analysis was used for bivariate analysis. No factor had a P value <0.2 on bivariate analysis, thus multiple logistic regression was not performed.
RESULTS
Of 194 primary care physicians who received invitations, 92 responded (response rate of 47%). See Table 1 for respondents' characteristics. All age groups were represented; most were male and between 3554 years of age. Respondents were evenly divided between those who owned their own practices (54%) and those working for a health system (46%). Ninety‐three percent of PCPs maintained admitting privileges (45% to more than 1 hospital), but only 20% continued to admit their own patients. When asked where they would send a hypothetical pneumonia patient, only 4% of PCPs chose a hospital to which they had never had admitting privileges.
| Variable | No. (%) of Respondents |
|---|---|
| Age | |
| 2534 | 5 (5) |
| 3544 | 27 (29) |
| 4554 | 24 (26) |
| >55 | 36 (39) |
| Gender | |
| Male | 65 (71) |
| Female | 27 (29) |
| Years out of medical school | |
| <6 | 6 (7) |
| 610 | 9 (10) |
| 1115 | 17 (18) |
| >15 | 60 (65) |
| % Patients seen who are covered by | |
| Medicaid: Mean (SD) | 28 (26) |
| Medicare: Mean (SD) | 31 (18) |
| Private: Mean (SD) | 40 (25) |
| Number of time doing patient care: Mean (SD) | 85 (23) |
| Number of patients admitted/sent to hospital/mo | |
| <6 | 40 (47) |
| 610 | 25 (29) |
| 1120 | 12 (14) |
| >20 | 8 (9) |
| Practice type | |
| Solo | 13 (15) |
| Single specialty group | 36 (42) |
| Multi‐specialty group | 36 (42) |
| Practice ownership | |
| Independent | 45 (54) |
| Health system | 38 (46) |
| Currently admits own patients | |
| Yes | 17 (20) |
| No | 66 (80) |
| Current hospital admitting privileges | |
| A | 63 (76) |
| B | 41 (49) |
| C | 3 (4) |
| D | 12 (14) |
| None | 6 (7) |
| Other | 2 (2) |
Physician's ratings of the importance of various factors in their referral decision are shown in Figure 2. The following factors were most often considered very important: familiarity with the hospital (70%), patient preference (62%), and admitting arrangements with a hospitalist group (62%). In contrast, only 18% of physicians viewed publicly available hospital quality measures as very important when making a referral decision. Factors most often rated not at all important to participants' decisions were patient insurance (48%), hospital's case volume for pneumonia (48%), and publicly available quality measures (42%).
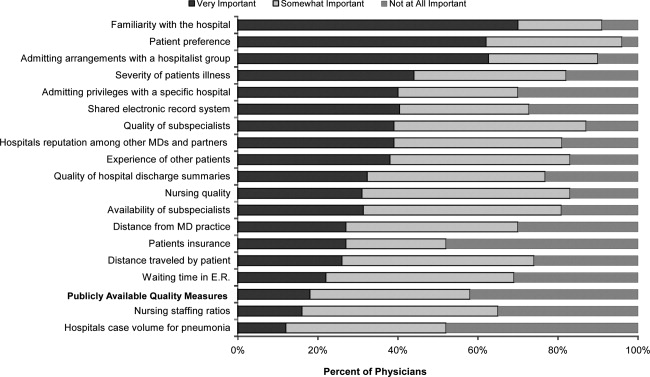
Of the 61% who were aware of Web sites that report hospital quality, most (52%) were familiar with Massachusetts Quality and Cost, while few (27%) were familiar with Hospital Compare. None of the physicians we surveyed reported having used publicly reported quality information when making a referral decision or having discussed such data with their patients. However, 49% stated that publicly reported performance data was somewhat and 10% very important to decisions regarding the medical care they receive. None of the demographic characteristics that we assessed (including age, gender, or years out of medical school) were associated with awareness of publicly reported data in bivariate analyses.
Respondents' ratings of specific quality measures appear in Figure 3. PCPs most often identified the following factors as being very important when judging hospital quality: percent of pneumonia patients given initial antibiotics within 6 hours after arrival (66%), percent of pneumonia patients given the most appropriate initial antibiotic (63%), and percent of pneumonia patients whose initial emergency room (ER) blood culture was performed prior to the administration of the first hospital dose of antibiotics (51%). The factors most often rated not at all important included: U.S. News & World Report's Best Hospitals designation (57%), Magnet Status (42%), and computer physician order entry system (40%).
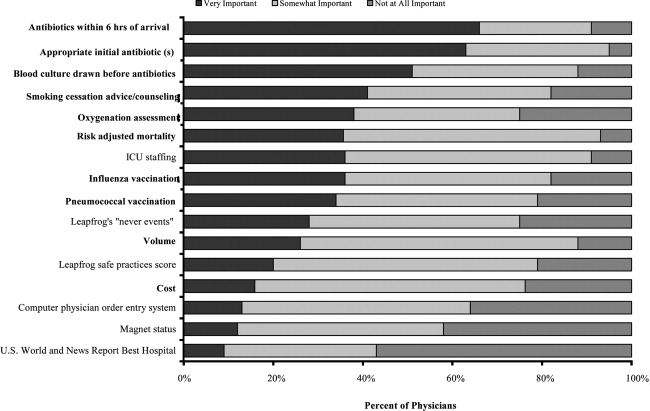
When asked about limitations of publicly reported performance data, 42% agreed that risk‐adjusted methods were inadequate to compare hospitals fairly, 76% agreed that mortality rates were an incomplete indication of the quality of hospitals care, 62% agreed that hospitals could manipulate the data, and 72% agreed that the ratings were inaccurate for hospitals with small caseloads.
DISCUSSION
In 2003, the Hospital Quality Alliance began a voluntary public reporting program of hospital performance measures, for pneumonia, acute myocardial infarction, and congestive heart failure, that was intended to encourage quality improvement activity by hospitals, and to provide patients and referring physicians with information to make better‐informed choices.19 These data are now easily available to the public through a free Web site (
Despite their lack of familiarity with Hospital Compare, it was the quality measures that are reported by Hospital Compare that they identified as the best indicators of hospital quality: appropriate initial antibiotic, antibiotics within 6 hours, and blood cultures performed prior to the administration of antibiotics. In fact, the 5 measures most often cited as very important to judging hospital quality were all measures reported on Hospital Compare.
As the US healthcare system becomes increasingly complex and costly, there is a growing interest in providing patients with physician and hospital performance data to help them select the provider.21 It is postulated that if patients took a more active role in choosing healthcare providers, and were forced to assume greater financial responsibility, then consumerism will force improvements in quality of care while maintaining or even lowering costs.21 However, studies demonstrate that most patients are unaware of performance data and, if they are aware, still value familiarity over quality ratings.4 Moreover, patients rely on the knowledge of their primary care physician to guide them.5
This is the first study we are aware of that examines how primary care physicians use publicly reported quality data in hospital referral decisions. Studies from more than a decade ago found that publicly reported data had minimal impact on referral decisions from cardiologists to cardiac surgeons. A survey of Pennsylvania's cardiologists and cardiac surgeons showed that although 82% were aware of risk‐adjusted mortality rates published for surgeons, only 10% of cardiologists reported these to be very important when evaluating the performance of a cardiothoracic surgeon. Furthermore, 87% of cardiologists stated that mortality and case volume information reported on cardiac surgeons had minimal or no influence on their referral practices.10 In 1997, a survey of cardiologists in New York found that only 38% of respondents reported that risk‐adjusted outcome data had affected their referrals to surgeons very much or somewhat.9 In addition, most authors conclude that public reporting has had little or no effect on market share.22 Despite growth in the number of measures and improved accessibility, our physicians were even less likely to be aware of, or use, publicly reported data than physicians a decade earlier.
Of course, even if public reporting does not influence referral patterns, it could still improve healthcare quality in several ways. First, feedback about performance may focus quality improvement activities in specific areas that represent gaps in care.10 This could take the form of an appeal to professionalism,23 or the desire to preserve one's reputation by not appearing on a list of poor performers.24 Second, hospitals' desire to appear on lists of high performers, such as U.S. News & World Report's hospital rankings, for marketing purposes, might stimulate improvement activities.10 Finally, publicly reported measures could form the basis for pay‐for‐performance incentives that further speed improvement.25
Our study has several limitations. First, our sample size was small and restricted to 1 region of 1 state, and may not be representative of either the state or nation as a whole. Still, our area has a high level of Internet use, and several local hospitals have been at the vanguard of the quality movement, generally scoring above both state and national averages on Hospital Compare. In addition, Massachusetts has made substantial efforts to promote its own public reporting program, and half the surveyed physicians reported being aware of the Massachusetts Quality and Cost Web site. The fact that not a single area physician surveyed used publicly reported data when making referral decisions is sobering. We believe it is unlikely that other areas of the country will have a substantially higher rate of use. Similarly, our response rate was under 50%. Physicians who did not take the survey may have differed in important ways from those who did. Nevertheless, our sample included a broad range of physician ages, practice types, and affiliations. It seems unlikely that those who did not respond would be more inclined to use publicly reported data than those who did. Second, we assessed decision‐making around a single medical condition. Physicians may have used publicly reported data for other decisions. However, the condition we chose was both urgent (as opposed to emergent) and possesses a robust set of publicly reported quality measures. If physicians do not use publicly reported data for this decision, it seems unlikely they would use it for conditions that have fewer reliable measures (eg, gall bladder surgery) or where the choice of hospital is generally made in an ambulance (eg, myocardial infarction). Finally, the low awareness of public reporting made it difficult for some physicians to answer some of the questions regarding publicly reported hospital quality data because they were unfamiliar with the language utilized by the Web sites (eg, magnet status, Leapfrog never events). It is possible that our results may have been altered slightly if a glossary had been provided.
Despite these limitations, our study suggests that more than 6 years after the launch of the Hospital Quality Alliance, primary care physicians do not appear to make use of these data when choosing a hospital for their patients suffering from pneumonia. Instead, they rely on familiarity with a hospital and past relationships. Even though a majority of the physicians surveyed no longer admitted their own patients, they continue to send patients to hospitals where they had privileges. This finding is not surprising, as physicians also cling to familiar therapies, and may be reluctant to prescribe a new medication or perform an unfamiliar procedure, even if it is indicated. Such reliance on familiarity may make physicians feel comfortable, but does not always result in the best care for patients. Acquiring familiarity, however, requires time and effort, something that physicians generally have in short supply; and while there are plenty of industry representatives to overcome physicians' hesitancy to prescribe new treatments, there are no analogous agents to educate physicians about public reporting or to help them overcome hesitancy about trying a new hospital.
Suspicion about the validity of public reporting may also play a role in the physicians' reported behavior. In past studies of cardiac report cards, cardiologists were most concerned that risk adjustment methods were inadequate (77%) and that mortality rates were an incomplete indicator of the quality of surgical care (74%). They were less concerned about manipulation of data (52%) or small caseloads (15%).10 Our physicians were also concerned that mortality rates were an incomplete measure of quality (76%) but less concerned about risk adjustment (42%), perhaps because many structure and process measures are not subject to risk adjustment. In contrast, they were somewhat more concerned that hospitals could manipulate the data (62%), which again may reflect process measures versus mortality statistics. Other reasons for not using the data may include a lack of awareness of the data or how to access it, or a belief that hospitals do not vary in quality.
Interestingly, even though most respondents were not aware of Hospital Compare, they found the information presented there to best reflect the overall hospital quality. Also, while respondents indicated that they did not use publicly reported data when referring patients, almost half of PCPs reported that publicly reported performance data was at least somewhat important in choosing their own medical care. Thus, although public reporting appears not to have reached its full potential, some publicly reported quality measures have clearly entered the consciousness of PCPs. In contrast, other highly touted measures such as computerized physician order entry systems were not appreciated, and popular designations such as U.S. News & World Report's Best Hospitals were least valued, even though 1 area hospital carries this designation. One conclusion might be that CMS should abandon Hospital Compare since neither patients4 nor providers use it. However, public reporting may improve quality in other ways. Moreover, physicians appear interested in the data even if they are not aware of it. Therefore, given the large investment by CMS and individual hospitals in collecting the data required for Hospital Compare, CMS might consider making greater efforts to increase primary care physician awareness of the Hospital Compare Web site. At the same time, high‐performing hospitals may want to communicate their performance scores to local PCPs as part of their marketing strategy. Future studies could assess whether such practices affect physician referral decisions and subsequent market share of high‐performing hospitals.
Acknowledgements
The authors of this study thank Jane Garb for her help with statistical analysis.
- Centers for Medicare and Medicaid Services. National Health Care Expenditures Data.2010. Available at: http://www.2.cms.gov/NationalHealthExpendData/25_NHE_Fact_Sheet.asp. Accessed April 22,year="2010"2010.
- ,,, et al.The quality of health care delivered to adults in the United States.N Engl J Med.2003;348(26):2635–2645.
- ,. The State‐of‐the‐Art of Online Hospital Public Reporting: a Review of Fifty‐One Websites. 2005. Available at: http://www.delmarvafoundation.org/newsAndPublications/reports/documents/WebSummariesFinal9.2.04.pdf. Accessed February 24,2012.
- . Kaiser Family Foundation. 2008 Update on Consumers' Views of Patient Safety and Quality Information. 2010. Available at: http://www.kff.org/kaiserpolls/upload/7819.pdf. Accessed April 20,2010.
- ,,.Choosing where to have major surgery: who makes the decision?Arch Surg.2007;142(3):242–246.
- ,,, et al.Resolving the gatekeeper conundrum: what patients value in primary care and referrals to specialists.JAMA.1999;282(3):261–266.
- ,,,.How physicians make referrals.J Health Care Mark.1993;13(2):6–17.
- ,,,.Growth in the care of older patients by hospitalists in the United States.N Engl J Med.2009;360(11):1102–1112.
- ,,,DeBuono BA. Public release of cardiac surgery outcomes data in New York: what do New York state cardiologists think of it?Am Heart J.1997;134(6):1120–1128.
- ,.Influence of cardiac‐surgery performance reports on referral practices and access to care. A survey of cardiovascular specialists.N Engl J Med.1996;335(4):251–256.
- ,,,,.Primary care summary of the British Thoracic Society Guidelines for the management of community acquired pneumonia in adults: 2009 update. Endorsed by the Royal College of General Practitioners and the Primary Care Respiratory Society UK.Prim Care Respir J.2010;19(1):21–27.
- Hospital Quality Alliance Quality Measures.2010. Available at: http://www.hospitalqualityalliance.org/hospitalqualityalliance/qualitymeasures/qualitymeasures.html. Accessed April 25,year="2010"2010.
- Massachusetts Executive Office of Health and Human Services. Massachusetts Executive Quality and Cost.2010. Available at: http://www.mass.gov/healthcareqc. Accessed February 24,year="2012"2012.
- Centers for Medicare and Medicaid Services. Hospital Compare.2010. Available at: http://www.hospitalcompare.hhs.gov. Accessed April 19,year="2010"2010.
- The Leapfrog Group for Patient Safety.2010. Available at: http://www.leapfroggroup.org/. Accessed April 23,year="2010"2010.
- Health Grades. 2010. Available at: http://www.healthgrades.com. Accessed April 19,2010.
- American Nurses Credentialing Center. Magnet Recognition Program. 2010. Available at: http://www.nursecredentialing.org/Magnet.aspx. Accessed April 15,2010.
- U.S. News 353(3):265–274.
- . US ads push patients to shop for hospitals. USA Today. May 20, 2008. Available at: http://www.usatoday.com/news/health/2008‐05‐20‐Hospitalads_N.htm. Accessed February 24, 2012.
- ,,.How do elderly patients decide where to go for major surgery? Telephone interview survey.BMJ.2005;331(7520):821.
- ,,, et al.Public reporting of cardiac surgery performance: part 1—history, rationale, consequences.Ann Thorac Surg.2011;92(3 suppl):S2–S11.
- ,,.Public reporting of hospital quality: recommendations to benefit patients and hospitals.J Hosp Med.2009;4(9):541–545.
- ,,, et al.When things go wrong: the impact of being a statistical outlier in publicly reported coronary artery bypass graft surgery mortality data.Am J Med Qual.2008;23(2):90–95.
- ,,, et al.Public reporting and pay for performance in hospital quality improvement.N Engl J Med.2007;356(5):486–496.
Over the past decade, research has demonstrated a value gap in US healthcare, characterized by rapidly rising costs and substandard quality.1, 2 Public reporting of hospital performance data is one of several strategies promoted to help address these deficiencies. To this end, a number of hospital rating services have created Web sites aimed at healthcare consumers.3 These services provide information about multiple aspects of healthcare quality, which in theory might be used by patients when deciding where to seek medical care.
Despite the increasing availability of publicly reported quality data comparing doctors and hospitals, a 2008 survey found that only 14% of Americans have seen and used such information in the past year, a decrease from 2006 (36%).4 A similar study in 2007 found that after seeking input from family and friends, patients generally rely on their primary care physician (PCP) to assist them to make decisions about where to have elective surgery.5 Surprisingly, almost nothing is known about how publicly reported data is used, if at all, by PCPs in the referral of patients to hospitals.
The physician is an important intermediary in the buying process for many healthcare services.6 Tertiary care hospitals depend on physician referrals for much of their patient volume.7 Until the emergence of the hospitalist model of care, most primary care physicians cared for their own hospitalized patients, and thus hospital referral decisions were largely driven by the PCP's admitting privileges. However, following the rapid expansion of the hospitalist movement,8, 9 there has been a sharp decrease in the number of PCPs who provide direct patient care for their hospitalized patients.8 As a result, PCPs may now have more choice in regards to hospital referrals for general medical conditions. Potential factors influencing a PCP's referral decisions might include familiarity with the hospital, care quality, patient convenience, satisfaction with the hospital, or hospital reputation.
Studies of cardiac surgery report cards in New York9 and Pennsylvania,10 conducted in the mid‐1990s, found that cardiologists did not use publicly reported mortality data in referral decisions, nor did they share it with patients. Over the past 2 decades, public reporting has grown exponentially, and now includes many measures of structure, processes, and outcomes for almost all US hospitals, available for free over the Internet. The growth of the patient safety movement and mandated public reporting might also have affected physicians' views about publicly reported quality data. We surveyed primary care physicians to determine the extent to which they use information about hospital quality in their referral decisions for community‐acquired pneumonia, and to identify other factors that might influence referral decisions.
METHODS
We obtained an e‐mail list of primary care physicians from the medical staff offices of all area hospitals within a 10‐mile radius of Springfield, MA (Baystate Medical Center, Holyoke Medical Center, and Mercy Medical Center). Baystate Medical Center is a 659‐bed academic medical center and Level 1 trauma center, while Holyoke and Mercy Medical Center are both 180‐bed acute care hospitals. Physicians were contacted via e‐mail from June through September of 2009, and asked to participate in an anonymous, 10‐minute, online survey accessible through an Internet link (SurveyMonkey.com) about factors influencing a primary care physician's hospital referral choice for a patient with pneumonia. To facilitate participation, we sent 2 follow‐up e‐mail reminders, and respondents who completed the entire survey received a $15 gift card. The study was approved by the institutional review board of Baystate Medical Center and closed to participation on September 23, 2009.
We created the online survey based on previous research7 and approximately 10 key informant interviews. The survey (see Supporting Information, Appendix, in the online version of this article) contained 13 demographic questions and 10 questions based on a case study of pneumonia (Figure 1). The instrument was pilot tested for clarity with a small group of primary care physicians at the author's institution and subsequently modified. We chose pneumonia because it is a common reason for a PCP to make an urgent hospital referral,11 and because there is a well‐established set of quality measures that are publicly reported.12 Unlike elective surgery, for which patients might research hospitals or surgeons on their own, patients with pneumonia would likely rely on their PCP to recommend a hospital for urgent referral. In contrast, PCPs know they will refer a number of pneumonia patients to hospitals each year and therefore might have an interest in comparing the publicly reported quality measures for local hospitals.

Respondents were shown the case study and asked to refer the hypothetical patient to 1 of 4 area hospitals. Respondents were asked to rate (on a 3‐point scale: not at all, somewhat, or very) the importance of the following factors in their referral decision: waiting time in the emergency room, distance traveled by the patient, experience of other patients, severity of patient's illness, patient's insurance, hospital's reputation among other physicians and partners, admitting privileges with a specific hospital, admitting arrangements with a hospitalist group, familiarity with the hospital, availability of subspecialists, quality of subspecialists, nursing quality, nursing staffing ratios, hospital's case volume for pneumonia, publicly available quality measures, patient preference, distance from your practice, shared electronic record system, and quality of hospital discharge summaries. Next, we measured provider's awareness of publicly reported hospital quality data and whether they used such data in referring patients or choosing their own medical care. Specifically, we asked about familiarity with the following 4 Web sites: Massachusetts Quality and Cost (a state‐specific Web site produced by the Massachusetts Executive Office of Health and Human Services)13; Hospital Compare (a Web site developed and maintained by Centers for Medicare and Medicaid Services [CMS] and the Department of Health and Human Services)14; Leapfrog Group (a private, nonprofit organization)15; and Health Grades (a private, for‐profit company).16
We then asked participants to rate the importance of the following performance measures when judging a hospital's performance: antibiotics within 6 hours of arrival to the hospital, appropriate initial antibiotic, blood culture drawn before antibiotics given, smoking cessation advice/counseling, oxygenation assessment, risk‐adjusted mortality, intensive care unit staffing, influenza vaccination, pneumococcal vaccination, Leapfrog's never events,15 volume, Leapfrog safe practices score, cost, computerized physician order entry system, Magnet status,17 and U.S. News & World Report's Best Hospitals designation.18 Lastly, we asked participants to state, using a 3‐point scale (agree, disagree, neutral), their level of agreement that the following factors, adapted from Schneider and Epstein,10 represented limitations of public reporting: 1) risk‐adjusted methods are inadequate to compare hospitals fairly; 2) mortality rates are an incomplete indication of the quality of a hospital's care; 3) hospitals can manipulate the data; and 4) ratings are inaccurate for hospitals with small caseloads.
Factors associated with physicians' knowledge of publicly reported data were analyzed with bivariate analysis. Since all factors are categorical, chi‐square analysis was used for bivariate analysis. No factor had a P value <0.2 on bivariate analysis, thus multiple logistic regression was not performed.
RESULTS
Of 194 primary care physicians who received invitations, 92 responded (response rate of 47%). See Table 1 for respondents' characteristics. All age groups were represented; most were male and between 3554 years of age. Respondents were evenly divided between those who owned their own practices (54%) and those working for a health system (46%). Ninety‐three percent of PCPs maintained admitting privileges (45% to more than 1 hospital), but only 20% continued to admit their own patients. When asked where they would send a hypothetical pneumonia patient, only 4% of PCPs chose a hospital to which they had never had admitting privileges.
| Variable | No. (%) of Respondents |
|---|---|
| Age | |
| 2534 | 5 (5) |
| 3544 | 27 (29) |
| 4554 | 24 (26) |
| >55 | 36 (39) |
| Gender | |
| Male | 65 (71) |
| Female | 27 (29) |
| Years out of medical school | |
| <6 | 6 (7) |
| 610 | 9 (10) |
| 1115 | 17 (18) |
| >15 | 60 (65) |
| % Patients seen who are covered by | |
| Medicaid: Mean (SD) | 28 (26) |
| Medicare: Mean (SD) | 31 (18) |
| Private: Mean (SD) | 40 (25) |
| Number of time doing patient care: Mean (SD) | 85 (23) |
| Number of patients admitted/sent to hospital/mo | |
| <6 | 40 (47) |
| 610 | 25 (29) |
| 1120 | 12 (14) |
| >20 | 8 (9) |
| Practice type | |
| Solo | 13 (15) |
| Single specialty group | 36 (42) |
| Multi‐specialty group | 36 (42) |
| Practice ownership | |
| Independent | 45 (54) |
| Health system | 38 (46) |
| Currently admits own patients | |
| Yes | 17 (20) |
| No | 66 (80) |
| Current hospital admitting privileges | |
| A | 63 (76) |
| B | 41 (49) |
| C | 3 (4) |
| D | 12 (14) |
| None | 6 (7) |
| Other | 2 (2) |
Physician's ratings of the importance of various factors in their referral decision are shown in Figure 2. The following factors were most often considered very important: familiarity with the hospital (70%), patient preference (62%), and admitting arrangements with a hospitalist group (62%). In contrast, only 18% of physicians viewed publicly available hospital quality measures as very important when making a referral decision. Factors most often rated not at all important to participants' decisions were patient insurance (48%), hospital's case volume for pneumonia (48%), and publicly available quality measures (42%).

Of the 61% who were aware of Web sites that report hospital quality, most (52%) were familiar with Massachusetts Quality and Cost, while few (27%) were familiar with Hospital Compare. None of the physicians we surveyed reported having used publicly reported quality information when making a referral decision or having discussed such data with their patients. However, 49% stated that publicly reported performance data was somewhat and 10% very important to decisions regarding the medical care they receive. None of the demographic characteristics that we assessed (including age, gender, or years out of medical school) were associated with awareness of publicly reported data in bivariate analyses.
Respondents' ratings of specific quality measures appear in Figure 3. PCPs most often identified the following factors as being very important when judging hospital quality: percent of pneumonia patients given initial antibiotics within 6 hours after arrival (66%), percent of pneumonia patients given the most appropriate initial antibiotic (63%), and percent of pneumonia patients whose initial emergency room (ER) blood culture was performed prior to the administration of the first hospital dose of antibiotics (51%). The factors most often rated not at all important included: U.S. News & World Report's Best Hospitals designation (57%), Magnet Status (42%), and computer physician order entry system (40%).

When asked about limitations of publicly reported performance data, 42% agreed that risk‐adjusted methods were inadequate to compare hospitals fairly, 76% agreed that mortality rates were an incomplete indication of the quality of hospitals care, 62% agreed that hospitals could manipulate the data, and 72% agreed that the ratings were inaccurate for hospitals with small caseloads.
DISCUSSION
In 2003, the Hospital Quality Alliance began a voluntary public reporting program of hospital performance measures, for pneumonia, acute myocardial infarction, and congestive heart failure, that was intended to encourage quality improvement activity by hospitals, and to provide patients and referring physicians with information to make better‐informed choices.19 These data are now easily available to the public through a free Web site (
Despite their lack of familiarity with Hospital Compare, it was the quality measures that are reported by Hospital Compare that they identified as the best indicators of hospital quality: appropriate initial antibiotic, antibiotics within 6 hours, and blood cultures performed prior to the administration of antibiotics. In fact, the 5 measures most often cited as very important to judging hospital quality were all measures reported on Hospital Compare.
As the US healthcare system becomes increasingly complex and costly, there is a growing interest in providing patients with physician and hospital performance data to help them select the provider.21 It is postulated that if patients took a more active role in choosing healthcare providers, and were forced to assume greater financial responsibility, then consumerism will force improvements in quality of care while maintaining or even lowering costs.21 However, studies demonstrate that most patients are unaware of performance data and, if they are aware, still value familiarity over quality ratings.4 Moreover, patients rely on the knowledge of their primary care physician to guide them.5
This is the first study we are aware of that examines how primary care physicians use publicly reported quality data in hospital referral decisions. Studies from more than a decade ago found that publicly reported data had minimal impact on referral decisions from cardiologists to cardiac surgeons. A survey of Pennsylvania's cardiologists and cardiac surgeons showed that although 82% were aware of risk‐adjusted mortality rates published for surgeons, only 10% of cardiologists reported these to be very important when evaluating the performance of a cardiothoracic surgeon. Furthermore, 87% of cardiologists stated that mortality and case volume information reported on cardiac surgeons had minimal or no influence on their referral practices.10 In 1997, a survey of cardiologists in New York found that only 38% of respondents reported that risk‐adjusted outcome data had affected their referrals to surgeons very much or somewhat.9 In addition, most authors conclude that public reporting has had little or no effect on market share.22 Despite growth in the number of measures and improved accessibility, our physicians were even less likely to be aware of, or use, publicly reported data than physicians a decade earlier.
Of course, even if public reporting does not influence referral patterns, it could still improve healthcare quality in several ways. First, feedback about performance may focus quality improvement activities in specific areas that represent gaps in care.10 This could take the form of an appeal to professionalism,23 or the desire to preserve one's reputation by not appearing on a list of poor performers.24 Second, hospitals' desire to appear on lists of high performers, such as U.S. News & World Report's hospital rankings, for marketing purposes, might stimulate improvement activities.10 Finally, publicly reported measures could form the basis for pay‐for‐performance incentives that further speed improvement.25
Our study has several limitations. First, our sample size was small and restricted to 1 region of 1 state, and may not be representative of either the state or nation as a whole. Still, our area has a high level of Internet use, and several local hospitals have been at the vanguard of the quality movement, generally scoring above both state and national averages on Hospital Compare. In addition, Massachusetts has made substantial efforts to promote its own public reporting program, and half the surveyed physicians reported being aware of the Massachusetts Quality and Cost Web site. The fact that not a single area physician surveyed used publicly reported data when making referral decisions is sobering. We believe it is unlikely that other areas of the country will have a substantially higher rate of use. Similarly, our response rate was under 50%. Physicians who did not take the survey may have differed in important ways from those who did. Nevertheless, our sample included a broad range of physician ages, practice types, and affiliations. It seems unlikely that those who did not respond would be more inclined to use publicly reported data than those who did. Second, we assessed decision‐making around a single medical condition. Physicians may have used publicly reported data for other decisions. However, the condition we chose was both urgent (as opposed to emergent) and possesses a robust set of publicly reported quality measures. If physicians do not use publicly reported data for this decision, it seems unlikely they would use it for conditions that have fewer reliable measures (eg, gall bladder surgery) or where the choice of hospital is generally made in an ambulance (eg, myocardial infarction). Finally, the low awareness of public reporting made it difficult for some physicians to answer some of the questions regarding publicly reported hospital quality data because they were unfamiliar with the language utilized by the Web sites (eg, magnet status, Leapfrog never events). It is possible that our results may have been altered slightly if a glossary had been provided.
Despite these limitations, our study suggests that more than 6 years after the launch of the Hospital Quality Alliance, primary care physicians do not appear to make use of these data when choosing a hospital for their patients suffering from pneumonia. Instead, they rely on familiarity with a hospital and past relationships. Even though a majority of the physicians surveyed no longer admitted their own patients, they continue to send patients to hospitals where they had privileges. This finding is not surprising, as physicians also cling to familiar therapies, and may be reluctant to prescribe a new medication or perform an unfamiliar procedure, even if it is indicated. Such reliance on familiarity may make physicians feel comfortable, but does not always result in the best care for patients. Acquiring familiarity, however, requires time and effort, something that physicians generally have in short supply; and while there are plenty of industry representatives to overcome physicians' hesitancy to prescribe new treatments, there are no analogous agents to educate physicians about public reporting or to help them overcome hesitancy about trying a new hospital.
Suspicion about the validity of public reporting may also play a role in the physicians' reported behavior. In past studies of cardiac report cards, cardiologists were most concerned that risk adjustment methods were inadequate (77%) and that mortality rates were an incomplete indicator of the quality of surgical care (74%). They were less concerned about manipulation of data (52%) or small caseloads (15%).10 Our physicians were also concerned that mortality rates were an incomplete measure of quality (76%) but less concerned about risk adjustment (42%), perhaps because many structure and process measures are not subject to risk adjustment. In contrast, they were somewhat more concerned that hospitals could manipulate the data (62%), which again may reflect process measures versus mortality statistics. Other reasons for not using the data may include a lack of awareness of the data or how to access it, or a belief that hospitals do not vary in quality.
Interestingly, even though most respondents were not aware of Hospital Compare, they found the information presented there to best reflect the overall hospital quality. Also, while respondents indicated that they did not use publicly reported data when referring patients, almost half of PCPs reported that publicly reported performance data was at least somewhat important in choosing their own medical care. Thus, although public reporting appears not to have reached its full potential, some publicly reported quality measures have clearly entered the consciousness of PCPs. In contrast, other highly touted measures such as computerized physician order entry systems were not appreciated, and popular designations such as U.S. News & World Report's Best Hospitals were least valued, even though 1 area hospital carries this designation. One conclusion might be that CMS should abandon Hospital Compare since neither patients4 nor providers use it. However, public reporting may improve quality in other ways. Moreover, physicians appear interested in the data even if they are not aware of it. Therefore, given the large investment by CMS and individual hospitals in collecting the data required for Hospital Compare, CMS might consider making greater efforts to increase primary care physician awareness of the Hospital Compare Web site. At the same time, high‐performing hospitals may want to communicate their performance scores to local PCPs as part of their marketing strategy. Future studies could assess whether such practices affect physician referral decisions and subsequent market share of high‐performing hospitals.
Acknowledgements
The authors of this study thank Jane Garb for her help with statistical analysis.
Over the past decade, research has demonstrated a value gap in US healthcare, characterized by rapidly rising costs and substandard quality.1, 2 Public reporting of hospital performance data is one of several strategies promoted to help address these deficiencies. To this end, a number of hospital rating services have created Web sites aimed at healthcare consumers.3 These services provide information about multiple aspects of healthcare quality, which in theory might be used by patients when deciding where to seek medical care.
Despite the increasing availability of publicly reported quality data comparing doctors and hospitals, a 2008 survey found that only 14% of Americans have seen and used such information in the past year, a decrease from 2006 (36%).4 A similar study in 2007 found that after seeking input from family and friends, patients generally rely on their primary care physician (PCP) to assist them to make decisions about where to have elective surgery.5 Surprisingly, almost nothing is known about how publicly reported data is used, if at all, by PCPs in the referral of patients to hospitals.
The physician is an important intermediary in the buying process for many healthcare services.6 Tertiary care hospitals depend on physician referrals for much of their patient volume.7 Until the emergence of the hospitalist model of care, most primary care physicians cared for their own hospitalized patients, and thus hospital referral decisions were largely driven by the PCP's admitting privileges. However, following the rapid expansion of the hospitalist movement,8, 9 there has been a sharp decrease in the number of PCPs who provide direct patient care for their hospitalized patients.8 As a result, PCPs may now have more choice in regards to hospital referrals for general medical conditions. Potential factors influencing a PCP's referral decisions might include familiarity with the hospital, care quality, patient convenience, satisfaction with the hospital, or hospital reputation.
Studies of cardiac surgery report cards in New York9 and Pennsylvania,10 conducted in the mid‐1990s, found that cardiologists did not use publicly reported mortality data in referral decisions, nor did they share it with patients. Over the past 2 decades, public reporting has grown exponentially, and now includes many measures of structure, processes, and outcomes for almost all US hospitals, available for free over the Internet. The growth of the patient safety movement and mandated public reporting might also have affected physicians' views about publicly reported quality data. We surveyed primary care physicians to determine the extent to which they use information about hospital quality in their referral decisions for community‐acquired pneumonia, and to identify other factors that might influence referral decisions.
METHODS
We obtained an e‐mail list of primary care physicians from the medical staff offices of all area hospitals within a 10‐mile radius of Springfield, MA (Baystate Medical Center, Holyoke Medical Center, and Mercy Medical Center). Baystate Medical Center is a 659‐bed academic medical center and Level 1 trauma center, while Holyoke and Mercy Medical Center are both 180‐bed acute care hospitals. Physicians were contacted via e‐mail from June through September of 2009, and asked to participate in an anonymous, 10‐minute, online survey accessible through an Internet link (SurveyMonkey.com) about factors influencing a primary care physician's hospital referral choice for a patient with pneumonia. To facilitate participation, we sent 2 follow‐up e‐mail reminders, and respondents who completed the entire survey received a $15 gift card. The study was approved by the institutional review board of Baystate Medical Center and closed to participation on September 23, 2009.
We created the online survey based on previous research7 and approximately 10 key informant interviews. The survey (see Supporting Information, Appendix, in the online version of this article) contained 13 demographic questions and 10 questions based on a case study of pneumonia (Figure 1). The instrument was pilot tested for clarity with a small group of primary care physicians at the author's institution and subsequently modified. We chose pneumonia because it is a common reason for a PCP to make an urgent hospital referral,11 and because there is a well‐established set of quality measures that are publicly reported.12 Unlike elective surgery, for which patients might research hospitals or surgeons on their own, patients with pneumonia would likely rely on their PCP to recommend a hospital for urgent referral. In contrast, PCPs know they will refer a number of pneumonia patients to hospitals each year and therefore might have an interest in comparing the publicly reported quality measures for local hospitals.

Respondents were shown the case study and asked to refer the hypothetical patient to 1 of 4 area hospitals. Respondents were asked to rate (on a 3‐point scale: not at all, somewhat, or very) the importance of the following factors in their referral decision: waiting time in the emergency room, distance traveled by the patient, experience of other patients, severity of patient's illness, patient's insurance, hospital's reputation among other physicians and partners, admitting privileges with a specific hospital, admitting arrangements with a hospitalist group, familiarity with the hospital, availability of subspecialists, quality of subspecialists, nursing quality, nursing staffing ratios, hospital's case volume for pneumonia, publicly available quality measures, patient preference, distance from your practice, shared electronic record system, and quality of hospital discharge summaries. Next, we measured provider's awareness of publicly reported hospital quality data and whether they used such data in referring patients or choosing their own medical care. Specifically, we asked about familiarity with the following 4 Web sites: Massachusetts Quality and Cost (a state‐specific Web site produced by the Massachusetts Executive Office of Health and Human Services)13; Hospital Compare (a Web site developed and maintained by Centers for Medicare and Medicaid Services [CMS] and the Department of Health and Human Services)14; Leapfrog Group (a private, nonprofit organization)15; and Health Grades (a private, for‐profit company).16
We then asked participants to rate the importance of the following performance measures when judging a hospital's performance: antibiotics within 6 hours of arrival to the hospital, appropriate initial antibiotic, blood culture drawn before antibiotics given, smoking cessation advice/counseling, oxygenation assessment, risk‐adjusted mortality, intensive care unit staffing, influenza vaccination, pneumococcal vaccination, Leapfrog's never events,15 volume, Leapfrog safe practices score, cost, computerized physician order entry system, Magnet status,17 and U.S. News & World Report's Best Hospitals designation.18 Lastly, we asked participants to state, using a 3‐point scale (agree, disagree, neutral), their level of agreement that the following factors, adapted from Schneider and Epstein,10 represented limitations of public reporting: 1) risk‐adjusted methods are inadequate to compare hospitals fairly; 2) mortality rates are an incomplete indication of the quality of a hospital's care; 3) hospitals can manipulate the data; and 4) ratings are inaccurate for hospitals with small caseloads.
Factors associated with physicians' knowledge of publicly reported data were analyzed with bivariate analysis. Since all factors are categorical, chi‐square analysis was used for bivariate analysis. No factor had a P value <0.2 on bivariate analysis, thus multiple logistic regression was not performed.
RESULTS
Of 194 primary care physicians who received invitations, 92 responded (response rate of 47%). See Table 1 for respondents' characteristics. All age groups were represented; most were male and between 3554 years of age. Respondents were evenly divided between those who owned their own practices (54%) and those working for a health system (46%). Ninety‐three percent of PCPs maintained admitting privileges (45% to more than 1 hospital), but only 20% continued to admit their own patients. When asked where they would send a hypothetical pneumonia patient, only 4% of PCPs chose a hospital to which they had never had admitting privileges.
| Variable | No. (%) of Respondents |
|---|---|
| Age | |
| 2534 | 5 (5) |
| 3544 | 27 (29) |
| 4554 | 24 (26) |
| >55 | 36 (39) |
| Gender | |
| Male | 65 (71) |
| Female | 27 (29) |
| Years out of medical school | |
| <6 | 6 (7) |
| 610 | 9 (10) |
| 1115 | 17 (18) |
| >15 | 60 (65) |
| % Patients seen who are covered by | |
| Medicaid: Mean (SD) | 28 (26) |
| Medicare: Mean (SD) | 31 (18) |
| Private: Mean (SD) | 40 (25) |
| Number of time doing patient care: Mean (SD) | 85 (23) |
| Number of patients admitted/sent to hospital/mo | |
| <6 | 40 (47) |
| 610 | 25 (29) |
| 1120 | 12 (14) |
| >20 | 8 (9) |
| Practice type | |
| Solo | 13 (15) |
| Single specialty group | 36 (42) |
| Multi‐specialty group | 36 (42) |
| Practice ownership | |
| Independent | 45 (54) |
| Health system | 38 (46) |
| Currently admits own patients | |
| Yes | 17 (20) |
| No | 66 (80) |
| Current hospital admitting privileges | |
| A | 63 (76) |
| B | 41 (49) |
| C | 3 (4) |
| D | 12 (14) |
| None | 6 (7) |
| Other | 2 (2) |
Physician's ratings of the importance of various factors in their referral decision are shown in Figure 2. The following factors were most often considered very important: familiarity with the hospital (70%), patient preference (62%), and admitting arrangements with a hospitalist group (62%). In contrast, only 18% of physicians viewed publicly available hospital quality measures as very important when making a referral decision. Factors most often rated not at all important to participants' decisions were patient insurance (48%), hospital's case volume for pneumonia (48%), and publicly available quality measures (42%).

Of the 61% who were aware of Web sites that report hospital quality, most (52%) were familiar with Massachusetts Quality and Cost, while few (27%) were familiar with Hospital Compare. None of the physicians we surveyed reported having used publicly reported quality information when making a referral decision or having discussed such data with their patients. However, 49% stated that publicly reported performance data was somewhat and 10% very important to decisions regarding the medical care they receive. None of the demographic characteristics that we assessed (including age, gender, or years out of medical school) were associated with awareness of publicly reported data in bivariate analyses.
Respondents' ratings of specific quality measures appear in Figure 3. PCPs most often identified the following factors as being very important when judging hospital quality: percent of pneumonia patients given initial antibiotics within 6 hours after arrival (66%), percent of pneumonia patients given the most appropriate initial antibiotic (63%), and percent of pneumonia patients whose initial emergency room (ER) blood culture was performed prior to the administration of the first hospital dose of antibiotics (51%). The factors most often rated not at all important included: U.S. News & World Report's Best Hospitals designation (57%), Magnet Status (42%), and computer physician order entry system (40%).

When asked about limitations of publicly reported performance data, 42% agreed that risk‐adjusted methods were inadequate to compare hospitals fairly, 76% agreed that mortality rates were an incomplete indication of the quality of hospitals care, 62% agreed that hospitals could manipulate the data, and 72% agreed that the ratings were inaccurate for hospitals with small caseloads.
DISCUSSION
In 2003, the Hospital Quality Alliance began a voluntary public reporting program of hospital performance measures, for pneumonia, acute myocardial infarction, and congestive heart failure, that was intended to encourage quality improvement activity by hospitals, and to provide patients and referring physicians with information to make better‐informed choices.19 These data are now easily available to the public through a free Web site (
Despite their lack of familiarity with Hospital Compare, it was the quality measures that are reported by Hospital Compare that they identified as the best indicators of hospital quality: appropriate initial antibiotic, antibiotics within 6 hours, and blood cultures performed prior to the administration of antibiotics. In fact, the 5 measures most often cited as very important to judging hospital quality were all measures reported on Hospital Compare.
As the US healthcare system becomes increasingly complex and costly, there is a growing interest in providing patients with physician and hospital performance data to help them select the provider.21 It is postulated that if patients took a more active role in choosing healthcare providers, and were forced to assume greater financial responsibility, then consumerism will force improvements in quality of care while maintaining or even lowering costs.21 However, studies demonstrate that most patients are unaware of performance data and, if they are aware, still value familiarity over quality ratings.4 Moreover, patients rely on the knowledge of their primary care physician to guide them.5
This is the first study we are aware of that examines how primary care physicians use publicly reported quality data in hospital referral decisions. Studies from more than a decade ago found that publicly reported data had minimal impact on referral decisions from cardiologists to cardiac surgeons. A survey of Pennsylvania's cardiologists and cardiac surgeons showed that although 82% were aware of risk‐adjusted mortality rates published for surgeons, only 10% of cardiologists reported these to be very important when evaluating the performance of a cardiothoracic surgeon. Furthermore, 87% of cardiologists stated that mortality and case volume information reported on cardiac surgeons had minimal or no influence on their referral practices.10 In 1997, a survey of cardiologists in New York found that only 38% of respondents reported that risk‐adjusted outcome data had affected their referrals to surgeons very much or somewhat.9 In addition, most authors conclude that public reporting has had little or no effect on market share.22 Despite growth in the number of measures and improved accessibility, our physicians were even less likely to be aware of, or use, publicly reported data than physicians a decade earlier.
Of course, even if public reporting does not influence referral patterns, it could still improve healthcare quality in several ways. First, feedback about performance may focus quality improvement activities in specific areas that represent gaps in care.10 This could take the form of an appeal to professionalism,23 or the desire to preserve one's reputation by not appearing on a list of poor performers.24 Second, hospitals' desire to appear on lists of high performers, such as U.S. News & World Report's hospital rankings, for marketing purposes, might stimulate improvement activities.10 Finally, publicly reported measures could form the basis for pay‐for‐performance incentives that further speed improvement.25
Our study has several limitations. First, our sample size was small and restricted to 1 region of 1 state, and may not be representative of either the state or nation as a whole. Still, our area has a high level of Internet use, and several local hospitals have been at the vanguard of the quality movement, generally scoring above both state and national averages on Hospital Compare. In addition, Massachusetts has made substantial efforts to promote its own public reporting program, and half the surveyed physicians reported being aware of the Massachusetts Quality and Cost Web site. The fact that not a single area physician surveyed used publicly reported data when making referral decisions is sobering. We believe it is unlikely that other areas of the country will have a substantially higher rate of use. Similarly, our response rate was under 50%. Physicians who did not take the survey may have differed in important ways from those who did. Nevertheless, our sample included a broad range of physician ages, practice types, and affiliations. It seems unlikely that those who did not respond would be more inclined to use publicly reported data than those who did. Second, we assessed decision‐making around a single medical condition. Physicians may have used publicly reported data for other decisions. However, the condition we chose was both urgent (as opposed to emergent) and possesses a robust set of publicly reported quality measures. If physicians do not use publicly reported data for this decision, it seems unlikely they would use it for conditions that have fewer reliable measures (eg, gall bladder surgery) or where the choice of hospital is generally made in an ambulance (eg, myocardial infarction). Finally, the low awareness of public reporting made it difficult for some physicians to answer some of the questions regarding publicly reported hospital quality data because they were unfamiliar with the language utilized by the Web sites (eg, magnet status, Leapfrog never events). It is possible that our results may have been altered slightly if a glossary had been provided.
Despite these limitations, our study suggests that more than 6 years after the launch of the Hospital Quality Alliance, primary care physicians do not appear to make use of these data when choosing a hospital for their patients suffering from pneumonia. Instead, they rely on familiarity with a hospital and past relationships. Even though a majority of the physicians surveyed no longer admitted their own patients, they continue to send patients to hospitals where they had privileges. This finding is not surprising, as physicians also cling to familiar therapies, and may be reluctant to prescribe a new medication or perform an unfamiliar procedure, even if it is indicated. Such reliance on familiarity may make physicians feel comfortable, but does not always result in the best care for patients. Acquiring familiarity, however, requires time and effort, something that physicians generally have in short supply; and while there are plenty of industry representatives to overcome physicians' hesitancy to prescribe new treatments, there are no analogous agents to educate physicians about public reporting or to help them overcome hesitancy about trying a new hospital.
Suspicion about the validity of public reporting may also play a role in the physicians' reported behavior. In past studies of cardiac report cards, cardiologists were most concerned that risk adjustment methods were inadequate (77%) and that mortality rates were an incomplete indicator of the quality of surgical care (74%). They were less concerned about manipulation of data (52%) or small caseloads (15%).10 Our physicians were also concerned that mortality rates were an incomplete measure of quality (76%) but less concerned about risk adjustment (42%), perhaps because many structure and process measures are not subject to risk adjustment. In contrast, they were somewhat more concerned that hospitals could manipulate the data (62%), which again may reflect process measures versus mortality statistics. Other reasons for not using the data may include a lack of awareness of the data or how to access it, or a belief that hospitals do not vary in quality.
Interestingly, even though most respondents were not aware of Hospital Compare, they found the information presented there to best reflect the overall hospital quality. Also, while respondents indicated that they did not use publicly reported data when referring patients, almost half of PCPs reported that publicly reported performance data was at least somewhat important in choosing their own medical care. Thus, although public reporting appears not to have reached its full potential, some publicly reported quality measures have clearly entered the consciousness of PCPs. In contrast, other highly touted measures such as computerized physician order entry systems were not appreciated, and popular designations such as U.S. News & World Report's Best Hospitals were least valued, even though 1 area hospital carries this designation. One conclusion might be that CMS should abandon Hospital Compare since neither patients4 nor providers use it. However, public reporting may improve quality in other ways. Moreover, physicians appear interested in the data even if they are not aware of it. Therefore, given the large investment by CMS and individual hospitals in collecting the data required for Hospital Compare, CMS might consider making greater efforts to increase primary care physician awareness of the Hospital Compare Web site. At the same time, high‐performing hospitals may want to communicate their performance scores to local PCPs as part of their marketing strategy. Future studies could assess whether such practices affect physician referral decisions and subsequent market share of high‐performing hospitals.
Acknowledgements
The authors of this study thank Jane Garb for her help with statistical analysis.
- Centers for Medicare and Medicaid Services. National Health Care Expenditures Data.2010. Available at: http://www.2.cms.gov/NationalHealthExpendData/25_NHE_Fact_Sheet.asp. Accessed April 22,year="2010"2010.
- ,,, et al.The quality of health care delivered to adults in the United States.N Engl J Med.2003;348(26):2635–2645.
- ,. The State‐of‐the‐Art of Online Hospital Public Reporting: a Review of Fifty‐One Websites. 2005. Available at: http://www.delmarvafoundation.org/newsAndPublications/reports/documents/WebSummariesFinal9.2.04.pdf. Accessed February 24,2012.
- . Kaiser Family Foundation. 2008 Update on Consumers' Views of Patient Safety and Quality Information. 2010. Available at: http://www.kff.org/kaiserpolls/upload/7819.pdf. Accessed April 20,2010.
- ,,.Choosing where to have major surgery: who makes the decision?Arch Surg.2007;142(3):242–246.
- ,,, et al.Resolving the gatekeeper conundrum: what patients value in primary care and referrals to specialists.JAMA.1999;282(3):261–266.
- ,,,.How physicians make referrals.J Health Care Mark.1993;13(2):6–17.
- ,,,.Growth in the care of older patients by hospitalists in the United States.N Engl J Med.2009;360(11):1102–1112.
- ,,,DeBuono BA. Public release of cardiac surgery outcomes data in New York: what do New York state cardiologists think of it?Am Heart J.1997;134(6):1120–1128.
- ,.Influence of cardiac‐surgery performance reports on referral practices and access to care. A survey of cardiovascular specialists.N Engl J Med.1996;335(4):251–256.
- ,,,,.Primary care summary of the British Thoracic Society Guidelines for the management of community acquired pneumonia in adults: 2009 update. Endorsed by the Royal College of General Practitioners and the Primary Care Respiratory Society UK.Prim Care Respir J.2010;19(1):21–27.
- Hospital Quality Alliance Quality Measures.2010. Available at: http://www.hospitalqualityalliance.org/hospitalqualityalliance/qualitymeasures/qualitymeasures.html. Accessed April 25,year="2010"2010.
- Massachusetts Executive Office of Health and Human Services. Massachusetts Executive Quality and Cost.2010. Available at: http://www.mass.gov/healthcareqc. Accessed February 24,year="2012"2012.
- Centers for Medicare and Medicaid Services. Hospital Compare.2010. Available at: http://www.hospitalcompare.hhs.gov. Accessed April 19,year="2010"2010.
- The Leapfrog Group for Patient Safety.2010. Available at: http://www.leapfroggroup.org/. Accessed April 23,year="2010"2010.
- Health Grades. 2010. Available at: http://www.healthgrades.com. Accessed April 19,2010.
- American Nurses Credentialing Center. Magnet Recognition Program. 2010. Available at: http://www.nursecredentialing.org/Magnet.aspx. Accessed April 15,2010.
- U.S. News 353(3):265–274.
- . US ads push patients to shop for hospitals. USA Today. May 20, 2008. Available at: http://www.usatoday.com/news/health/2008‐05‐20‐Hospitalads_N.htm. Accessed February 24, 2012.
- ,,.How do elderly patients decide where to go for major surgery? Telephone interview survey.BMJ.2005;331(7520):821.
- ,,, et al.Public reporting of cardiac surgery performance: part 1—history, rationale, consequences.Ann Thorac Surg.2011;92(3 suppl):S2–S11.
- ,,.Public reporting of hospital quality: recommendations to benefit patients and hospitals.J Hosp Med.2009;4(9):541–545.
- ,,, et al.When things go wrong: the impact of being a statistical outlier in publicly reported coronary artery bypass graft surgery mortality data.Am J Med Qual.2008;23(2):90–95.
- ,,, et al.Public reporting and pay for performance in hospital quality improvement.N Engl J Med.2007;356(5):486–496.
- Centers for Medicare and Medicaid Services. National Health Care Expenditures Data.2010. Available at: http://www.2.cms.gov/NationalHealthExpendData/25_NHE_Fact_Sheet.asp. Accessed April 22,year="2010"2010.
- ,,, et al.The quality of health care delivered to adults in the United States.N Engl J Med.2003;348(26):2635–2645.
- ,. The State‐of‐the‐Art of Online Hospital Public Reporting: a Review of Fifty‐One Websites. 2005. Available at: http://www.delmarvafoundation.org/newsAndPublications/reports/documents/WebSummariesFinal9.2.04.pdf. Accessed February 24,2012.
- . Kaiser Family Foundation. 2008 Update on Consumers' Views of Patient Safety and Quality Information. 2010. Available at: http://www.kff.org/kaiserpolls/upload/7819.pdf. Accessed April 20,2010.
- ,,.Choosing where to have major surgery: who makes the decision?Arch Surg.2007;142(3):242–246.
- ,,, et al.Resolving the gatekeeper conundrum: what patients value in primary care and referrals to specialists.JAMA.1999;282(3):261–266.
- ,,,.How physicians make referrals.J Health Care Mark.1993;13(2):6–17.
- ,,,.Growth in the care of older patients by hospitalists in the United States.N Engl J Med.2009;360(11):1102–1112.
- ,,,DeBuono BA. Public release of cardiac surgery outcomes data in New York: what do New York state cardiologists think of it?Am Heart J.1997;134(6):1120–1128.
- ,.Influence of cardiac‐surgery performance reports on referral practices and access to care. A survey of cardiovascular specialists.N Engl J Med.1996;335(4):251–256.
- ,,,,.Primary care summary of the British Thoracic Society Guidelines for the management of community acquired pneumonia in adults: 2009 update. Endorsed by the Royal College of General Practitioners and the Primary Care Respiratory Society UK.Prim Care Respir J.2010;19(1):21–27.
- Hospital Quality Alliance Quality Measures.2010. Available at: http://www.hospitalqualityalliance.org/hospitalqualityalliance/qualitymeasures/qualitymeasures.html. Accessed April 25,year="2010"2010.
- Massachusetts Executive Office of Health and Human Services. Massachusetts Executive Quality and Cost.2010. Available at: http://www.mass.gov/healthcareqc. Accessed February 24,year="2012"2012.
- Centers for Medicare and Medicaid Services. Hospital Compare.2010. Available at: http://www.hospitalcompare.hhs.gov. Accessed April 19,year="2010"2010.
- The Leapfrog Group for Patient Safety.2010. Available at: http://www.leapfroggroup.org/. Accessed April 23,year="2010"2010.
- Health Grades. 2010. Available at: http://www.healthgrades.com. Accessed April 19,2010.
- American Nurses Credentialing Center. Magnet Recognition Program. 2010. Available at: http://www.nursecredentialing.org/Magnet.aspx. Accessed April 15,2010.
- U.S. News 353(3):265–274.
- . US ads push patients to shop for hospitals. USA Today. May 20, 2008. Available at: http://www.usatoday.com/news/health/2008‐05‐20‐Hospitalads_N.htm. Accessed February 24, 2012.
- ,,.How do elderly patients decide where to go for major surgery? Telephone interview survey.BMJ.2005;331(7520):821.
- ,,, et al.Public reporting of cardiac surgery performance: part 1—history, rationale, consequences.Ann Thorac Surg.2011;92(3 suppl):S2–S11.
- ,,.Public reporting of hospital quality: recommendations to benefit patients and hospitals.J Hosp Med.2009;4(9):541–545.
- ,,, et al.When things go wrong: the impact of being a statistical outlier in publicly reported coronary artery bypass graft surgery mortality data.Am J Med Qual.2008;23(2):90–95.
- ,,, et al.Public reporting and pay for performance in hospital quality improvement.N Engl J Med.2007;356(5):486–496.
Copyright © 2012 Society of Hospital Medicine
Patient Satisfaction with Hospital Care
Over the past decade, hospital medicine has been the nation's fastest‐growing medical specialty. According to the American Hospital Association's (AHA) 2009 survey, 58% of United States (US) hospitals now have hospital medicine programs, and for hospitals with 200 or more beds, this figure is 89%.1 In 2009, the AHA estimated that the number of US hospitalists would increase to over 34,000 by 2011, over double that of the 16,000 present in 2005.1 Studies demonstrate that, compared to a system where primary care physicians provide inpatient care, the hospitalist model improves efficiency while maintaining at least equal patient outcomes.211 However, scant data exist as to the effects of hospitalists on patient satisfaction.12 Understanding how care models affect patient experience is vital in the current environment of healthcare reform and performance reporting, especially in light of the Centers for Medicare and Medicaid Services' (CMS) efforts to link the patient experience to reimbursement through value‐based purchasing.13 Value‐based purchasing is a strategy to encourage and reward excellence in healthcare delivery through differential reimbursement based on defined performance measures. As one part of value‐based purchasing, hospital reimbursement will be linked to patient‐experience measures, including patient ratings of their doctor's ability to communicate with them and other questions assessing patient satisfaction with their hospital stay.14
In the outpatient setting, trust is the variable most strongly associated with patient satisfaction.1518 In contrast to PCPs, who may develop relationships with patients over years, hospitalists often first meet a patient in the hospital and must engender trust quickly. In addition, hospitalists work in shifts and may not be responsible for the same patients each day. Since continuity is positively related to trust,19, 20 there is reason to believe satisfaction with hospitalist care might be lower than satisfaction with care provided by PCPs. We report on 8295 patients and 6 years experience with hospitalist programs at 3 hospitals. Based on the known link between continuity and patient satisfaction, we hypothesized that patient satisfaction would be lower with hospitalists than with primary care internists.
METHODS
Setting
Our study was conducted at 3 Western Massachusetts hospitals affiliated with Baystate Health, an integrated healthcare delivery system. These included 2 small community hospitals (<100 beds) and a 653‐bed tertiary care, academic teaching hospital. Hospitalist services were established at the tertiary care center in 2001 and at the community hospitals in 2004 and 2005; the programs have evolved over time. In addition, the tertiary care center has 3 different hospitalist groups: an academic group that is employed by the hospital and works with house staff, a hospitalist service that is owned by the hospital and cares for patients from specific outpatient practices, and one that is privately owned caring for patients from another group of practices. The community hospitals each have a single, hospital‐owned service. Primary care physicians also provide inpatient care at all 3 institutions, although their number has decreased over time as the hospitalist programs have grown. All hospitalist services varied in the number of consecutive days in a rounding cycle (degree of continuity), and which services had an admitting team (single initial physician encounter with a different rounding physician) versus a single physician being both the admitting and rounding physician. Consequently, continuity, as measured by the number of different physicians caring for an individual patient during 1 hospitalization, would be expected to vary depending on the type of hospitalist service and the length of stay. Likewise, patients admitted by their primary care physician's office may have been cared for by either their PCP or a practice colleague. All hospitalists and PCPs care for inpatients having similar hospital experiences, as all aspects of a patient's care (including the medical wards, nursing staff, discharge planners, and information systems) are identical, regardless of physician designation. The study was approved by Baystate Health System's Institutional Review Board.
Data Collection
Since February 2001, Baystate Health, in conjunction with Professional Research Consultants, Inc (PRC), has conducted scripted postdischarge patient satisfaction telephone interviews of random discharged adult medicine patients, with Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) questions added in January 2007. Approximately 50 surveys per quarter, per hospital floor, were conducted. Trained PRC staff assessed up to 115 variables encompassing the inpatient experience. We limited our analysis to those domains that reflected satisfaction with physician care, including satisfaction with physician care quality, physician communication, physician behavior, and pain management. The survey responses were scored, depending on question type, with: never, sometimes, usually, always (HCAHPS); or excellent, very good, good, fair, poor (PRC). Each score was converted to a numeric equivalent, with the highest score (4 or 5, depending on scale used) being best and 1 being worst. The specific questions are included in Supporting Appendix A in the online version of this article.
Additional patient information for respondents was extracted from the hospitals' billing database, using medical record numbers, and included age, gender, admission year, education level, language, illness severity, emergency room (ER) admission status, institution, and attending physician type (academic hospitalist [AH], hospital‐owned hospitalist [HOH], private hospitalist [PH], or primary care physician [PCP]). It was not possible to distinguish whether PCP patients were cared for by their own PCP or a colleague from the same practice.
Statistical Analysis
Patient satisfaction data were derived from survey responses of adult inpatients cared for by hospitalists or PCPs between January 1, 2003 and March 31, 2009. The primary outcome was patient‐reported satisfaction with physician care quality measured on a 5‐point Likert scale. In a secondary analysis, physician groups were compared on the proportion of responses that were excellent (a score of 5 on the Likert scale) and the proportion that were poor (a score of 1). Other secondary outcomes included patient satisfaction ratings of physician behavior, pain management, and communication. Averages and percent ranking excellent and poor were calculated for each hospitalist group and for PCPs. Other outcomes analyzed included average patient satisfaction with physician care quality, both over time and stratified by the presence or absence of having an established PCP prior to admission.
In view of the large sample size, Likert‐scale responses were analyzed as continuous outcomes. For unadjusted comparisons among hospitalist groups, t tests and 1‐way ANOVAs were conducted for the scales scores, while chi‐square tests were used for dichotomous outcomes. For multivariable analyses, multiple linear regression was used for continuous outcomes. For dichotomous outcomes, adjusted prevalence ratios were estimated using Poisson regression with robust standard errors.21 All multivariable models controlled for sex, marital status, illness severity, age group, ethnicity, length of stay, and emergency room admission. Observations with missing data were excluded from analyses. Differences in bivariable and multivariable analyses were considered significant at a critical test level of 5%. Prevalence ratios are reported with 95% confidence intervals. All analyses were conducted in Stata, version 11 (StataCorp, College Station, TX).
RESULTS
Of patients who were reached by telephone, 87% agreed to participate in the hospital survey. However, most patients could not be reached by phone; thus our estimated response rate, including those who could not be reached, was 27%. For the subset of patients interviewed using the HCAHPS protocol, the response rate was 40%. Our final sample included 8295 patients (3597 cared for by 59 hospitalists and 4698 by 288 PCPs) interviewed between 2003 and 2009. Three‐quarters of the patients were from the tertiary care center, whereas 17% and 8% were from each of the community hospitals (see Supporting Appendix B in the online version of this article). Patient characteristics appear in Table 1. Patients cared for by hospitalists were similar to those cared for by PCPs in terms of age, sex, marital status, education, and language, but hospitalist patients were more likely to have been admitted through the emergency department (93% vs 84%, P < 0.001) and less likely to be white (83% vs 85%, P = 0.01). Patients cared for by hospitalists also had higher average illness severity score (2.2 0.8 vs 2.0 0.8, P < 0.001), longer average LOS (4.3 4.3 vs 4.0 3.6, P < 0.001), and lower mean perceived health score (2.8 1.2 vs 3.0 1.2, P = 0.01).
| Characteristic | PCP N = 4698 | Hospitalist N = 3597 | P Value |
|---|---|---|---|
| |||
| Age (mean, SD) | 63.5 (16.6) | 63.7 (16.3) | 0.53 |
| Male sex (%) | 44.9 | 46.2 | 0.28 |
| White race (%) | 85.3 | 83.2 | 0.01 |
| Married (%) | 49.1 | 48.7 | 0.69 |
| English spoken at home (%) | 96.0 | 97.0 | 0.09 |
| At least some college education (%) | 47.1 | 43.7 | 0.22 |
| Admitted through the emergency department (%) | 84.3 | 92.5 | <0.001 |
| Average illness severity rating (mean, SD) | 2.0 (0.8) | 2.2 (0.8) | <0.001 |
| Average perceived health score (mean, SD) | 3.0 (1.2) | 2.8 (1.2) | 0.01 |
| Average length of stay (days) (mean, SD) | 4.0 (3.6) | 4.3 (4.3) | <0.001 |
| Discharged home (%) | 87.9 | 88.5 | 0.73 |
Unadjusted patient reported satisfaction with physician care quality was slightly greater for PCPs than hospitalists (4.25 vs 4.19, P = 0.009). After multivariable adjustment, the difference was attenuated but persisted (4.24 vs 4.20, P = 0.04). We found no statistical difference among the hospitals or the specific hospitalist groups in terms of satisfaction with overall physician care quality (Figure 1). There were no statistical differences in patient satisfaction ratings of hospitalist and PCPs for the subdomains of behavior, pain, and communication (Table 2). There were also no differences in the proportion of patients cared for by hospitalists or PCPs who rated their physicians in the highest satisfaction category (79% vs 81%, P = 0.17) or the lowest (5% vs 5%, P = 0.19). Among patients cared for by academic hospitalists, there was no difference in satisfaction rating between those patients who had a designated primary care physician in the outpatient setting and those who did not (4.22 0.94 vs 4.19 0.94, P = 0.97). Finally, satisfaction with both hospitalists and PCPs showed equivalent rates of improvement over time (Figure 2).
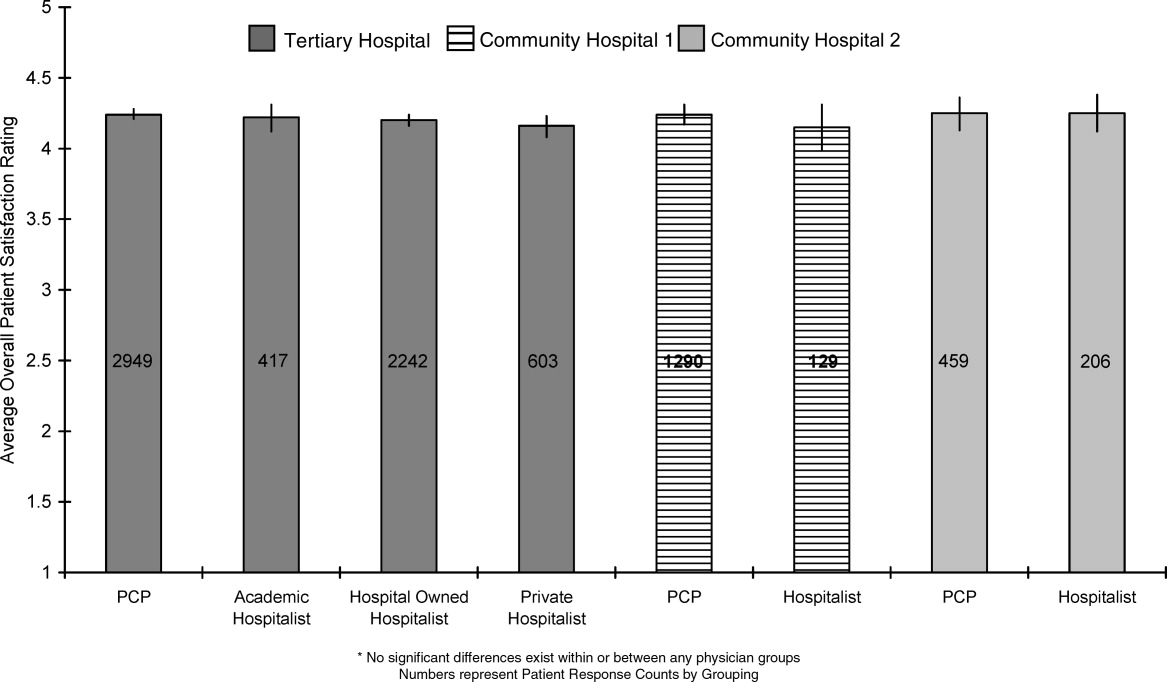
| PCP | Hospitalist | P Value | ||
|---|---|---|---|---|
| ||||
| Satisfaction | Overall, you would rate the quality of doctor care as:* | 4.24 [4.21, 4.27] | 4.20 [4.17, 4.23] | 0.04 |
| Behavior | Doctors treated you with courtesy/respect | 3.77 [3.73, 3.82] | 3.78 [3.73, 3.82] | 0.88 |
| Pain control | Pain management by hospital staff* | 4.11 [4.08, 4.14] | 4.09 [4.05, 4.12] | 0.35 |
| Pain well controlled | 3.55 [3.47, 3.63] | 3.48 [3.41, 3.55] | 0.23 | |
| Staff did everything to help with pain | 3.73 [3.66, 3.80] | 3.68 [3.62, 3.75] | 0.33 | |
| Communication skills | Doctors listened carefully to you | 3.66 [3.61, 3.72] | 3.67 [3.62, 3.72] | 0.83 |
| Doctors explained things in an understandable way | 3.60 [3.54, 3.66] | 3.61 [3.56, 3.67] | 0.73 | |
| Doctor's communication* | 4.02 [3.97, 4.07] | 3.98 [3.93, 4.03] | 0.27 | |
| Doctor discussed your anxiety/fears* | 4.00 [3.96, 4.03] | 3.97 [3.93, 4.01] | 0.26 | |
| Doctor involved you in decisions* | 4.00 [3.95, 4.06] | 3.98 [3.93, 4.03] | 0.49 | |
DISCUSSION
In this observational study of over 8200 patients cared for over 6 years by 347 physicians at 3 hospitals, we found that patient satisfaction with inpatient care provided by hospitalists and primary care doctors was almost identical. As we hypothesized, overall satisfaction with physician care quality, our primary outcome, was slightly greater with primary care doctors; however, the observed difference, 0.04 on a scale of 1 to 5, cannot be considered clinically significant. All patients were generally satisfied (4.2‐4.3 rating on 5‐point scale) with their inpatient care, and satisfaction scores increased over time. We also found no differences among the specific domains of satisfaction, including communication skills, pain control, and physician behavior. Finally, we found no significant difference in patient satisfaction with physician care quality among the different hospitalist services.
Previous studies of patient satisfaction conducted in the outpatient setting found that continuity of care was an important determinant of trust and, consequently, overall satisfaction.15, 16, 19, 20, 22 Because hospitalist models introduce discontinuity, they might be expected to undermine satisfaction. Surprisingly, few studies have addressed this issue. In a review of the hospitalist studies through 2002, Wachter and Goldman found 19 studies, 5 of which measured patient satisfaction.23 Three of these were conducted on teaching services and compared designated faculty hospitalists to traditional ward attendings, who rotated onto the inpatient services 1 to 2 months per year. Primary care doctors were excluded.2426 A fourth study provided a descriptive narrative of the development of the first hospitalist program in Minneapolis, Minnesota, and anecdotally noted no difference in patient satisfaction between the hospitalist and traditional model, but presented no data because the satisfaction surveys were not designed with publication in mind.27 The only study to actually assess whether patient satisfaction was greater with hospitalists or PCPs was an observational study by Davis et al., conducted in 1 rural hospital during the first year of its hospitalist program. In that study, 2 hospitalists were compared to 17 PCPs, and patient satisfaction surveys were available for approximately 44 patients managed by hospitalists and 168 patients managed by PCPs. Specific data were not reported, but it was noted that there was no statistical difference in satisfaction between those cared for by hospitalists versus PCPs.28 On the basis of these studies, Wachter and Goldman concluded that surveys of patients who were cared for by hospitalists show high levels of satisfaction, no lower than that of similar patients cared for by their own primary physicians.23 Wachter and Goldman's review has been highly cited, and we could find no subsequent studies addressing this issue. Our study provides the first real evidence to support this conclusion, including data from 59 hospitalists practicing in 5 separate hospitalist programs at 3 different hospitals.
Our finding that hospitalists maintain satisfaction despite a lack of continuity suggests that other aspects of care may be more important to patient satisfaction. Larson et al. found that physician ability to meet patient's information needs was positively associated with patient satisfaction.29 Similarly, Tarrant et al. found that patient's trust in a physician improved with increasing communication, interpersonal care, and knowledge of the patient. Interestingly, continuity, ie. the proportion of visits to the usual general practitioner (GP) or duration with the practice, did not correlate with trust.30 Finally, a systematic review of determinants of outpatient satisfaction found that continuity has a variable effect on satisfaction. Subjective continuity measures, such as whether patients saw their regular physician on the day they were surveyed, were consistently associated with patient satisfaction, however, quantitative measures including relationship duration were not.31
It is also possible that patients believe they value continuity more than they actually do. In 1 survey of inpatients with an established PCP yet cared for by a hospitalist, most agreed that patients receive better care and have more trust in physicians with whom they have long‐term relationships. Yet most also had positive opinions of their hospital care.32 Similarly, in a survey of over 2500 outpatients, 92% rated continuity as very important or important, but the majority was unwilling to expend substantial personal time (88%), defined as driving greater than 60 minutes, or money (82%), defined as spending an additional $20 to $40 a month, to maintain continuity with their PCP.33 Our study appears to confirm the lack of connection between continuity and satisfaction. Even those patients who valued continuity, as evidenced by having an established PCP, were as satisfied with hospitalist physician care as patients who had no established PCP.
Our study has several limitations. First, we report on outcomes of 3 institutions within a single healthcare system, within a limited geographic area. Although our sample included a wide range of patient demographics, hundreds of physicians, and multiple hospitalist models, it is possible that some hospitalist models may provide greater or lesser satisfaction than those we observed. Second, our study was observational, and thus subject to selection bias and confounding. Patients cared for by the hospitalists differed in a number of ways from those cared for by PCPs. We controlled for identifiable confounders such as illness severity, self‐perceived health, and admission through the emergency department, but the possibility exists that additional unidentified factors could have affected our results. It is possible other drivers of patient satisfaction, such as amenities, nursing, or food, could have influenced our findings. However, this is unlikely because all patient groups shared these components of hospital experience equally. Third, only a minority of patients could be reached for interview. This is typical for post‐hospitalization surveys, and our response rate of 40% for HCAHPS patients compared favorably to the 2010 HCAHPS national average of 33%.34 Still, the responses of those who could not be reached may have differed from those who were interviewed. Fourth, we identified hospitalists and PCPs by the attending of record, but we were unable to tell who provided care to the patient on any given day. Thus, we could not determine to what extent patients cared for by PCPs were actually seen by their own doctor, as opposed to an associated physician within the practice. Nevertheless, our results are representative of the care model provided by PCPs in the hospital. Similarly, we could not know or compare the number of different attending physicians each patient experienced during their hospitalization. Higher turnover of inpatient physicians may have affected patient satisfaction scores independent of attending physician designation. These are potentially important measures of relationship duration, yet whether duration affects patient satisfaction remains undecided.1618, 20, 28, 30, 32, 33 We assessed satisfaction using HCAHPS questions, in order to provide objective and meaningful comparisons across hospitals. The HCAHPS instrument, however, is intended to assess patient satisfaction with doctors in general, not with subgroups or individuals, and responses in our study were uniformly high. A more sensitive survey instrument may have yielded different results. Finally, it is possible that individual physicians may possess lower satisfaction scores than others, making the results not representative of hospitalist models as much as specific doctors' care quality. We think this is unlikely since surveys reached over 8000 patients, over 6 years, representing the care of 347 individual physicians. However, hospital medicine is a rapidly evolving field with many divergent organizational structures, and patient satisfaction is bound to fluctuate while there exists high variability in how care is provided.
Over the past decade, the hospitalist model has become one of the dominant models for care of medical inpatients. Compared to the traditional model in which PCPs provide inpatient care, the hospitalist model has a number of advantages, including continuous on‐site coverage for increasingly acute patients, specialization, and incentives aligned with the hospital to provide efficient, high‐quality care. One concern that remains, however, is that patients may not trust doctors they first meet in the hospital or may be dissatisfied with the lack of continuity from day to day. Our findings are reassuring in this regard. Although patients cared for by hospitalists were slightly less satisfied, the differences could not be considered clinically meaningful and should be outweighed by gains in quality and efficiency. Furthermore, hospitalists can expect to fare well under value‐based purchasing. Given the rapid ascension of hospital medicine programs, prospective comparisons of hospitalists and PCPs may no longer be feasible. Future research might employ survey instruments designed specifically to measure patient experience under hospitalist care in order to identify methods to maximize patient satisfaction within the hospitalist model.
Acknowledgements
Jane Garb, MS, Academic Affairs, Baystate Medical Center, contributed to the initial database management and statistical analysis. She received no financial compensation. Dr Adrianne Seiler has received written permission for acknowledgement from Ms Garb.
Dr Adrianne Seiler made substantial contributions to our manuscript's conception and design, data acquisition, analysis, and interpretation, manuscript drafting and critical revision, and administrative support. Dr Paul Visintainer made substantial contributions to our manuscript's data analysis and interpretation, manuscript critical revision, and statistical analysis. Michael Ehresman and Richard Brzostek made substantial contributions to our manuscript's data acquisition, manuscript critical revision, and administrative support. Dr Evan Benjamin made substantial contributions to our manuscript's conception and design, analysis and interpretation of data, manuscript drafting, and administrative support. Dr Winthrop Whitcomb made substantial contributions to our manuscript's data analysis and interpretation, and manuscript critical revision. Dr Michael Rothberg made substantial contributions to our manuscript's conception and design, data analysis and interpretation, manuscript critical revision, and supervision.
- American Hospital Association Annual Survey Database.Fiscal Year2009.
- ,,,,.Quality of care for patients hospitalized with heart failure: assessing the impact of hospitalists.Arch Intern Med.2002;162:1251–1256.
- ,,,,,.Outcomes of care by hospitalists, general internists, and family physicians.N Engl J Med.2007;357:2589–2600.
- ,,,.Comparison of hospitalists and nonhospitalists regarding core measures of pneumonia care.Am J Manag Care.2007;13:129–132.
- ,,,.Comparison of processes and outcomes of pneumonia care between hospitalists and community‐based primary care physicians.Mayo Clin Proc.2002;77:1053–1058.
- ,,,.Comparison of hospitalists and nonhospitalists in inpatient length of stay adjusting for patient and physician characteristics.J Gen Intern Med.2004;19:1127–1132.
- ,,.Comparison of practice patterns of hospitalists and community physicians in the care of patients with congestive heart failure.J Hosp Med.2008;3:35–41.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,,,,.The value of a hospitalist service: efficient care for the aging population?Chest.2001;119:580–589.
- ,,,,.Comparison of hospital costs and length of stay associated with general internists and hospitalist physicians at a community hospital.Am J Manag Care.2004;10:626–630.
- ,,,,.Hospitalist care and length of stay in patients requiring complex discharge planning and close clinical monitoring.Arch Intern Med.2007;167:1869–1874.
- ,.The impact of hospitalists on the cost and quality of inpatient care in the United States: a research synthesis.Med Care Res Rev.2005;62(4):379–406.
- US Department of Health and Human Services Medicare Hospital Value‐Based Purchasing Plan Development Issues Paper. 1st Public Listening Session January 17, 2007. Available at: https://www.cms. gov/AcuteInpatientPPS/downloads/hospital_VBP_plan_issues_paper. pdf. Accessed on May 26, 2011.
- Hospital Value‐Based Purchasing: Measure Explanations. Available at: http://www.healthcare.gov/news/factsheets/valuebasedpurchasing 04292011b.html. Accessed on May 26, 2011.
- ,,,,,.Linking primary care performance to outcomes of care.J Fam Pract.1998;47:213–220.
- ,.Interpersonal continuity of care and patient satisfaction: a critical review.Ann Fam Med.2004;2:445–451.
- ,.Does continuity of care improve patient outcomes?J Fam Pract.2004;53:974–980.
- ,,,.Continuity of care and other determinants of patient satisfaction with primary care.J Gen Intern Med.2005;20:226–233.
- ,,,,.Continuity of care and trust in one's physician: evidence from primary care in the United States and the United Kingdom.Fam Med.2001;33:22–27.
- ,,,,.Patients' trust in their physicians: effects of choice, continuity, and payment method.J Gen Intern Med.1998;13:681–686.
- ,.Alternatives for logistic regression in cross‐sectional studies: an empirical comparison of models that directly estimate the prevalence ratio.BMC Med Res Methodol.2003;3:21.
- ,,, et al.Continuity of outpatient medical care in elderly men. A randomized trial.JAMA.1984;252:2413–2417.
- ,.The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- ,,, et al.The effect of a hospitalist service with nurse discharge planner on patient care in an academic teaching hospital.Am J Med.2001;111(8):627–632.
- ,,, et al.Decreased length of stay, costs and mortality in a randomized trial of academic hospitalists.J Gen Intern Med.2001;16(suppl):S208.
- ,,,,.Reorganizing an academic medical service: impact on cost, quality, patient satisfaction, and education.JAMA.1998;279(19):1560–1565.
- .The Park Nicollet experience in establishing a hospitalist system.Ann Intern Med.1999;130:350–354.
- ,,,,,.Effects of hospitalists on cost, outcomes, and patient satisfaction in a rural health system.Am J Med.2000;108:621–626.
- ,,,.The relationship between meeting patients' information needs and their satisfaction with hospital care and general health status outcomes.Int J Qual Health Care.1996;8:447–456.
- ,,.Factors associated with patients' trust in their general practitioner: a cross‐sectional survey.Br J Gen Pract.2003;53:798–800.
- ,,.The relationship between continuity and patient satisfaction: a systematic review.Fam Pract.2010;27:171–178.
- ,,.How do patients view the role of the primary care physician in inpatient care?Dis Mon.2002;48:230–238.
- ,.Patient attitudes toward continuity of care.Arch Intern Med.2003;163:909–912.
- Summary of HCAHPS Survey Results. Available at: http://www. hcahpsonline.org/files/12–13‐10_Summary_of_HCAHPS_Survey_ Results_December_2010.pdf. Accessed on May 27,2011.
Over the past decade, hospital medicine has been the nation's fastest‐growing medical specialty. According to the American Hospital Association's (AHA) 2009 survey, 58% of United States (US) hospitals now have hospital medicine programs, and for hospitals with 200 or more beds, this figure is 89%.1 In 2009, the AHA estimated that the number of US hospitalists would increase to over 34,000 by 2011, over double that of the 16,000 present in 2005.1 Studies demonstrate that, compared to a system where primary care physicians provide inpatient care, the hospitalist model improves efficiency while maintaining at least equal patient outcomes.211 However, scant data exist as to the effects of hospitalists on patient satisfaction.12 Understanding how care models affect patient experience is vital in the current environment of healthcare reform and performance reporting, especially in light of the Centers for Medicare and Medicaid Services' (CMS) efforts to link the patient experience to reimbursement through value‐based purchasing.13 Value‐based purchasing is a strategy to encourage and reward excellence in healthcare delivery through differential reimbursement based on defined performance measures. As one part of value‐based purchasing, hospital reimbursement will be linked to patient‐experience measures, including patient ratings of their doctor's ability to communicate with them and other questions assessing patient satisfaction with their hospital stay.14
In the outpatient setting, trust is the variable most strongly associated with patient satisfaction.1518 In contrast to PCPs, who may develop relationships with patients over years, hospitalists often first meet a patient in the hospital and must engender trust quickly. In addition, hospitalists work in shifts and may not be responsible for the same patients each day. Since continuity is positively related to trust,19, 20 there is reason to believe satisfaction with hospitalist care might be lower than satisfaction with care provided by PCPs. We report on 8295 patients and 6 years experience with hospitalist programs at 3 hospitals. Based on the known link between continuity and patient satisfaction, we hypothesized that patient satisfaction would be lower with hospitalists than with primary care internists.
METHODS
Setting
Our study was conducted at 3 Western Massachusetts hospitals affiliated with Baystate Health, an integrated healthcare delivery system. These included 2 small community hospitals (<100 beds) and a 653‐bed tertiary care, academic teaching hospital. Hospitalist services were established at the tertiary care center in 2001 and at the community hospitals in 2004 and 2005; the programs have evolved over time. In addition, the tertiary care center has 3 different hospitalist groups: an academic group that is employed by the hospital and works with house staff, a hospitalist service that is owned by the hospital and cares for patients from specific outpatient practices, and one that is privately owned caring for patients from another group of practices. The community hospitals each have a single, hospital‐owned service. Primary care physicians also provide inpatient care at all 3 institutions, although their number has decreased over time as the hospitalist programs have grown. All hospitalist services varied in the number of consecutive days in a rounding cycle (degree of continuity), and which services had an admitting team (single initial physician encounter with a different rounding physician) versus a single physician being both the admitting and rounding physician. Consequently, continuity, as measured by the number of different physicians caring for an individual patient during 1 hospitalization, would be expected to vary depending on the type of hospitalist service and the length of stay. Likewise, patients admitted by their primary care physician's office may have been cared for by either their PCP or a practice colleague. All hospitalists and PCPs care for inpatients having similar hospital experiences, as all aspects of a patient's care (including the medical wards, nursing staff, discharge planners, and information systems) are identical, regardless of physician designation. The study was approved by Baystate Health System's Institutional Review Board.
Data Collection
Since February 2001, Baystate Health, in conjunction with Professional Research Consultants, Inc (PRC), has conducted scripted postdischarge patient satisfaction telephone interviews of random discharged adult medicine patients, with Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) questions added in January 2007. Approximately 50 surveys per quarter, per hospital floor, were conducted. Trained PRC staff assessed up to 115 variables encompassing the inpatient experience. We limited our analysis to those domains that reflected satisfaction with physician care, including satisfaction with physician care quality, physician communication, physician behavior, and pain management. The survey responses were scored, depending on question type, with: never, sometimes, usually, always (HCAHPS); or excellent, very good, good, fair, poor (PRC). Each score was converted to a numeric equivalent, with the highest score (4 or 5, depending on scale used) being best and 1 being worst. The specific questions are included in Supporting Appendix A in the online version of this article.
Additional patient information for respondents was extracted from the hospitals' billing database, using medical record numbers, and included age, gender, admission year, education level, language, illness severity, emergency room (ER) admission status, institution, and attending physician type (academic hospitalist [AH], hospital‐owned hospitalist [HOH], private hospitalist [PH], or primary care physician [PCP]). It was not possible to distinguish whether PCP patients were cared for by their own PCP or a colleague from the same practice.
Statistical Analysis
Patient satisfaction data were derived from survey responses of adult inpatients cared for by hospitalists or PCPs between January 1, 2003 and March 31, 2009. The primary outcome was patient‐reported satisfaction with physician care quality measured on a 5‐point Likert scale. In a secondary analysis, physician groups were compared on the proportion of responses that were excellent (a score of 5 on the Likert scale) and the proportion that were poor (a score of 1). Other secondary outcomes included patient satisfaction ratings of physician behavior, pain management, and communication. Averages and percent ranking excellent and poor were calculated for each hospitalist group and for PCPs. Other outcomes analyzed included average patient satisfaction with physician care quality, both over time and stratified by the presence or absence of having an established PCP prior to admission.
In view of the large sample size, Likert‐scale responses were analyzed as continuous outcomes. For unadjusted comparisons among hospitalist groups, t tests and 1‐way ANOVAs were conducted for the scales scores, while chi‐square tests were used for dichotomous outcomes. For multivariable analyses, multiple linear regression was used for continuous outcomes. For dichotomous outcomes, adjusted prevalence ratios were estimated using Poisson regression with robust standard errors.21 All multivariable models controlled for sex, marital status, illness severity, age group, ethnicity, length of stay, and emergency room admission. Observations with missing data were excluded from analyses. Differences in bivariable and multivariable analyses were considered significant at a critical test level of 5%. Prevalence ratios are reported with 95% confidence intervals. All analyses were conducted in Stata, version 11 (StataCorp, College Station, TX).
RESULTS
Of patients who were reached by telephone, 87% agreed to participate in the hospital survey. However, most patients could not be reached by phone; thus our estimated response rate, including those who could not be reached, was 27%. For the subset of patients interviewed using the HCAHPS protocol, the response rate was 40%. Our final sample included 8295 patients (3597 cared for by 59 hospitalists and 4698 by 288 PCPs) interviewed between 2003 and 2009. Three‐quarters of the patients were from the tertiary care center, whereas 17% and 8% were from each of the community hospitals (see Supporting Appendix B in the online version of this article). Patient characteristics appear in Table 1. Patients cared for by hospitalists were similar to those cared for by PCPs in terms of age, sex, marital status, education, and language, but hospitalist patients were more likely to have been admitted through the emergency department (93% vs 84%, P < 0.001) and less likely to be white (83% vs 85%, P = 0.01). Patients cared for by hospitalists also had higher average illness severity score (2.2 0.8 vs 2.0 0.8, P < 0.001), longer average LOS (4.3 4.3 vs 4.0 3.6, P < 0.001), and lower mean perceived health score (2.8 1.2 vs 3.0 1.2, P = 0.01).
| Characteristic | PCP N = 4698 | Hospitalist N = 3597 | P Value |
|---|---|---|---|
| |||
| Age (mean, SD) | 63.5 (16.6) | 63.7 (16.3) | 0.53 |
| Male sex (%) | 44.9 | 46.2 | 0.28 |
| White race (%) | 85.3 | 83.2 | 0.01 |
| Married (%) | 49.1 | 48.7 | 0.69 |
| English spoken at home (%) | 96.0 | 97.0 | 0.09 |
| At least some college education (%) | 47.1 | 43.7 | 0.22 |
| Admitted through the emergency department (%) | 84.3 | 92.5 | <0.001 |
| Average illness severity rating (mean, SD) | 2.0 (0.8) | 2.2 (0.8) | <0.001 |
| Average perceived health score (mean, SD) | 3.0 (1.2) | 2.8 (1.2) | 0.01 |
| Average length of stay (days) (mean, SD) | 4.0 (3.6) | 4.3 (4.3) | <0.001 |
| Discharged home (%) | 87.9 | 88.5 | 0.73 |
Unadjusted patient reported satisfaction with physician care quality was slightly greater for PCPs than hospitalists (4.25 vs 4.19, P = 0.009). After multivariable adjustment, the difference was attenuated but persisted (4.24 vs 4.20, P = 0.04). We found no statistical difference among the hospitals or the specific hospitalist groups in terms of satisfaction with overall physician care quality (Figure 1). There were no statistical differences in patient satisfaction ratings of hospitalist and PCPs for the subdomains of behavior, pain, and communication (Table 2). There were also no differences in the proportion of patients cared for by hospitalists or PCPs who rated their physicians in the highest satisfaction category (79% vs 81%, P = 0.17) or the lowest (5% vs 5%, P = 0.19). Among patients cared for by academic hospitalists, there was no difference in satisfaction rating between those patients who had a designated primary care physician in the outpatient setting and those who did not (4.22 0.94 vs 4.19 0.94, P = 0.97). Finally, satisfaction with both hospitalists and PCPs showed equivalent rates of improvement over time (Figure 2).


| PCP | Hospitalist | P Value | ||
|---|---|---|---|---|
| ||||
| Satisfaction | Overall, you would rate the quality of doctor care as:* | 4.24 [4.21, 4.27] | 4.20 [4.17, 4.23] | 0.04 |
| Behavior | Doctors treated you with courtesy/respect | 3.77 [3.73, 3.82] | 3.78 [3.73, 3.82] | 0.88 |
| Pain control | Pain management by hospital staff* | 4.11 [4.08, 4.14] | 4.09 [4.05, 4.12] | 0.35 |
| Pain well controlled | 3.55 [3.47, 3.63] | 3.48 [3.41, 3.55] | 0.23 | |
| Staff did everything to help with pain | 3.73 [3.66, 3.80] | 3.68 [3.62, 3.75] | 0.33 | |
| Communication skills | Doctors listened carefully to you | 3.66 [3.61, 3.72] | 3.67 [3.62, 3.72] | 0.83 |
| Doctors explained things in an understandable way | 3.60 [3.54, 3.66] | 3.61 [3.56, 3.67] | 0.73 | |
| Doctor's communication* | 4.02 [3.97, 4.07] | 3.98 [3.93, 4.03] | 0.27 | |
| Doctor discussed your anxiety/fears* | 4.00 [3.96, 4.03] | 3.97 [3.93, 4.01] | 0.26 | |
| Doctor involved you in decisions* | 4.00 [3.95, 4.06] | 3.98 [3.93, 4.03] | 0.49 | |
DISCUSSION
In this observational study of over 8200 patients cared for over 6 years by 347 physicians at 3 hospitals, we found that patient satisfaction with inpatient care provided by hospitalists and primary care doctors was almost identical. As we hypothesized, overall satisfaction with physician care quality, our primary outcome, was slightly greater with primary care doctors; however, the observed difference, 0.04 on a scale of 1 to 5, cannot be considered clinically significant. All patients were generally satisfied (4.2‐4.3 rating on 5‐point scale) with their inpatient care, and satisfaction scores increased over time. We also found no differences among the specific domains of satisfaction, including communication skills, pain control, and physician behavior. Finally, we found no significant difference in patient satisfaction with physician care quality among the different hospitalist services.
Previous studies of patient satisfaction conducted in the outpatient setting found that continuity of care was an important determinant of trust and, consequently, overall satisfaction.15, 16, 19, 20, 22 Because hospitalist models introduce discontinuity, they might be expected to undermine satisfaction. Surprisingly, few studies have addressed this issue. In a review of the hospitalist studies through 2002, Wachter and Goldman found 19 studies, 5 of which measured patient satisfaction.23 Three of these were conducted on teaching services and compared designated faculty hospitalists to traditional ward attendings, who rotated onto the inpatient services 1 to 2 months per year. Primary care doctors were excluded.2426 A fourth study provided a descriptive narrative of the development of the first hospitalist program in Minneapolis, Minnesota, and anecdotally noted no difference in patient satisfaction between the hospitalist and traditional model, but presented no data because the satisfaction surveys were not designed with publication in mind.27 The only study to actually assess whether patient satisfaction was greater with hospitalists or PCPs was an observational study by Davis et al., conducted in 1 rural hospital during the first year of its hospitalist program. In that study, 2 hospitalists were compared to 17 PCPs, and patient satisfaction surveys were available for approximately 44 patients managed by hospitalists and 168 patients managed by PCPs. Specific data were not reported, but it was noted that there was no statistical difference in satisfaction between those cared for by hospitalists versus PCPs.28 On the basis of these studies, Wachter and Goldman concluded that surveys of patients who were cared for by hospitalists show high levels of satisfaction, no lower than that of similar patients cared for by their own primary physicians.23 Wachter and Goldman's review has been highly cited, and we could find no subsequent studies addressing this issue. Our study provides the first real evidence to support this conclusion, including data from 59 hospitalists practicing in 5 separate hospitalist programs at 3 different hospitals.
Our finding that hospitalists maintain satisfaction despite a lack of continuity suggests that other aspects of care may be more important to patient satisfaction. Larson et al. found that physician ability to meet patient's information needs was positively associated with patient satisfaction.29 Similarly, Tarrant et al. found that patient's trust in a physician improved with increasing communication, interpersonal care, and knowledge of the patient. Interestingly, continuity, ie. the proportion of visits to the usual general practitioner (GP) or duration with the practice, did not correlate with trust.30 Finally, a systematic review of determinants of outpatient satisfaction found that continuity has a variable effect on satisfaction. Subjective continuity measures, such as whether patients saw their regular physician on the day they were surveyed, were consistently associated with patient satisfaction, however, quantitative measures including relationship duration were not.31
It is also possible that patients believe they value continuity more than they actually do. In 1 survey of inpatients with an established PCP yet cared for by a hospitalist, most agreed that patients receive better care and have more trust in physicians with whom they have long‐term relationships. Yet most also had positive opinions of their hospital care.32 Similarly, in a survey of over 2500 outpatients, 92% rated continuity as very important or important, but the majority was unwilling to expend substantial personal time (88%), defined as driving greater than 60 minutes, or money (82%), defined as spending an additional $20 to $40 a month, to maintain continuity with their PCP.33 Our study appears to confirm the lack of connection between continuity and satisfaction. Even those patients who valued continuity, as evidenced by having an established PCP, were as satisfied with hospitalist physician care as patients who had no established PCP.
Our study has several limitations. First, we report on outcomes of 3 institutions within a single healthcare system, within a limited geographic area. Although our sample included a wide range of patient demographics, hundreds of physicians, and multiple hospitalist models, it is possible that some hospitalist models may provide greater or lesser satisfaction than those we observed. Second, our study was observational, and thus subject to selection bias and confounding. Patients cared for by the hospitalists differed in a number of ways from those cared for by PCPs. We controlled for identifiable confounders such as illness severity, self‐perceived health, and admission through the emergency department, but the possibility exists that additional unidentified factors could have affected our results. It is possible other drivers of patient satisfaction, such as amenities, nursing, or food, could have influenced our findings. However, this is unlikely because all patient groups shared these components of hospital experience equally. Third, only a minority of patients could be reached for interview. This is typical for post‐hospitalization surveys, and our response rate of 40% for HCAHPS patients compared favorably to the 2010 HCAHPS national average of 33%.34 Still, the responses of those who could not be reached may have differed from those who were interviewed. Fourth, we identified hospitalists and PCPs by the attending of record, but we were unable to tell who provided care to the patient on any given day. Thus, we could not determine to what extent patients cared for by PCPs were actually seen by their own doctor, as opposed to an associated physician within the practice. Nevertheless, our results are representative of the care model provided by PCPs in the hospital. Similarly, we could not know or compare the number of different attending physicians each patient experienced during their hospitalization. Higher turnover of inpatient physicians may have affected patient satisfaction scores independent of attending physician designation. These are potentially important measures of relationship duration, yet whether duration affects patient satisfaction remains undecided.1618, 20, 28, 30, 32, 33 We assessed satisfaction using HCAHPS questions, in order to provide objective and meaningful comparisons across hospitals. The HCAHPS instrument, however, is intended to assess patient satisfaction with doctors in general, not with subgroups or individuals, and responses in our study were uniformly high. A more sensitive survey instrument may have yielded different results. Finally, it is possible that individual physicians may possess lower satisfaction scores than others, making the results not representative of hospitalist models as much as specific doctors' care quality. We think this is unlikely since surveys reached over 8000 patients, over 6 years, representing the care of 347 individual physicians. However, hospital medicine is a rapidly evolving field with many divergent organizational structures, and patient satisfaction is bound to fluctuate while there exists high variability in how care is provided.
Over the past decade, the hospitalist model has become one of the dominant models for care of medical inpatients. Compared to the traditional model in which PCPs provide inpatient care, the hospitalist model has a number of advantages, including continuous on‐site coverage for increasingly acute patients, specialization, and incentives aligned with the hospital to provide efficient, high‐quality care. One concern that remains, however, is that patients may not trust doctors they first meet in the hospital or may be dissatisfied with the lack of continuity from day to day. Our findings are reassuring in this regard. Although patients cared for by hospitalists were slightly less satisfied, the differences could not be considered clinically meaningful and should be outweighed by gains in quality and efficiency. Furthermore, hospitalists can expect to fare well under value‐based purchasing. Given the rapid ascension of hospital medicine programs, prospective comparisons of hospitalists and PCPs may no longer be feasible. Future research might employ survey instruments designed specifically to measure patient experience under hospitalist care in order to identify methods to maximize patient satisfaction within the hospitalist model.
Acknowledgements
Jane Garb, MS, Academic Affairs, Baystate Medical Center, contributed to the initial database management and statistical analysis. She received no financial compensation. Dr Adrianne Seiler has received written permission for acknowledgement from Ms Garb.
Dr Adrianne Seiler made substantial contributions to our manuscript's conception and design, data acquisition, analysis, and interpretation, manuscript drafting and critical revision, and administrative support. Dr Paul Visintainer made substantial contributions to our manuscript's data analysis and interpretation, manuscript critical revision, and statistical analysis. Michael Ehresman and Richard Brzostek made substantial contributions to our manuscript's data acquisition, manuscript critical revision, and administrative support. Dr Evan Benjamin made substantial contributions to our manuscript's conception and design, analysis and interpretation of data, manuscript drafting, and administrative support. Dr Winthrop Whitcomb made substantial contributions to our manuscript's data analysis and interpretation, and manuscript critical revision. Dr Michael Rothberg made substantial contributions to our manuscript's conception and design, data analysis and interpretation, manuscript critical revision, and supervision.
Over the past decade, hospital medicine has been the nation's fastest‐growing medical specialty. According to the American Hospital Association's (AHA) 2009 survey, 58% of United States (US) hospitals now have hospital medicine programs, and for hospitals with 200 or more beds, this figure is 89%.1 In 2009, the AHA estimated that the number of US hospitalists would increase to over 34,000 by 2011, over double that of the 16,000 present in 2005.1 Studies demonstrate that, compared to a system where primary care physicians provide inpatient care, the hospitalist model improves efficiency while maintaining at least equal patient outcomes.211 However, scant data exist as to the effects of hospitalists on patient satisfaction.12 Understanding how care models affect patient experience is vital in the current environment of healthcare reform and performance reporting, especially in light of the Centers for Medicare and Medicaid Services' (CMS) efforts to link the patient experience to reimbursement through value‐based purchasing.13 Value‐based purchasing is a strategy to encourage and reward excellence in healthcare delivery through differential reimbursement based on defined performance measures. As one part of value‐based purchasing, hospital reimbursement will be linked to patient‐experience measures, including patient ratings of their doctor's ability to communicate with them and other questions assessing patient satisfaction with their hospital stay.14
In the outpatient setting, trust is the variable most strongly associated with patient satisfaction.1518 In contrast to PCPs, who may develop relationships with patients over years, hospitalists often first meet a patient in the hospital and must engender trust quickly. In addition, hospitalists work in shifts and may not be responsible for the same patients each day. Since continuity is positively related to trust,19, 20 there is reason to believe satisfaction with hospitalist care might be lower than satisfaction with care provided by PCPs. We report on 8295 patients and 6 years experience with hospitalist programs at 3 hospitals. Based on the known link between continuity and patient satisfaction, we hypothesized that patient satisfaction would be lower with hospitalists than with primary care internists.
METHODS
Setting
Our study was conducted at 3 Western Massachusetts hospitals affiliated with Baystate Health, an integrated healthcare delivery system. These included 2 small community hospitals (<100 beds) and a 653‐bed tertiary care, academic teaching hospital. Hospitalist services were established at the tertiary care center in 2001 and at the community hospitals in 2004 and 2005; the programs have evolved over time. In addition, the tertiary care center has 3 different hospitalist groups: an academic group that is employed by the hospital and works with house staff, a hospitalist service that is owned by the hospital and cares for patients from specific outpatient practices, and one that is privately owned caring for patients from another group of practices. The community hospitals each have a single, hospital‐owned service. Primary care physicians also provide inpatient care at all 3 institutions, although their number has decreased over time as the hospitalist programs have grown. All hospitalist services varied in the number of consecutive days in a rounding cycle (degree of continuity), and which services had an admitting team (single initial physician encounter with a different rounding physician) versus a single physician being both the admitting and rounding physician. Consequently, continuity, as measured by the number of different physicians caring for an individual patient during 1 hospitalization, would be expected to vary depending on the type of hospitalist service and the length of stay. Likewise, patients admitted by their primary care physician's office may have been cared for by either their PCP or a practice colleague. All hospitalists and PCPs care for inpatients having similar hospital experiences, as all aspects of a patient's care (including the medical wards, nursing staff, discharge planners, and information systems) are identical, regardless of physician designation. The study was approved by Baystate Health System's Institutional Review Board.
Data Collection
Since February 2001, Baystate Health, in conjunction with Professional Research Consultants, Inc (PRC), has conducted scripted postdischarge patient satisfaction telephone interviews of random discharged adult medicine patients, with Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) questions added in January 2007. Approximately 50 surveys per quarter, per hospital floor, were conducted. Trained PRC staff assessed up to 115 variables encompassing the inpatient experience. We limited our analysis to those domains that reflected satisfaction with physician care, including satisfaction with physician care quality, physician communication, physician behavior, and pain management. The survey responses were scored, depending on question type, with: never, sometimes, usually, always (HCAHPS); or excellent, very good, good, fair, poor (PRC). Each score was converted to a numeric equivalent, with the highest score (4 or 5, depending on scale used) being best and 1 being worst. The specific questions are included in Supporting Appendix A in the online version of this article.
Additional patient information for respondents was extracted from the hospitals' billing database, using medical record numbers, and included age, gender, admission year, education level, language, illness severity, emergency room (ER) admission status, institution, and attending physician type (academic hospitalist [AH], hospital‐owned hospitalist [HOH], private hospitalist [PH], or primary care physician [PCP]). It was not possible to distinguish whether PCP patients were cared for by their own PCP or a colleague from the same practice.
Statistical Analysis
Patient satisfaction data were derived from survey responses of adult inpatients cared for by hospitalists or PCPs between January 1, 2003 and March 31, 2009. The primary outcome was patient‐reported satisfaction with physician care quality measured on a 5‐point Likert scale. In a secondary analysis, physician groups were compared on the proportion of responses that were excellent (a score of 5 on the Likert scale) and the proportion that were poor (a score of 1). Other secondary outcomes included patient satisfaction ratings of physician behavior, pain management, and communication. Averages and percent ranking excellent and poor were calculated for each hospitalist group and for PCPs. Other outcomes analyzed included average patient satisfaction with physician care quality, both over time and stratified by the presence or absence of having an established PCP prior to admission.
In view of the large sample size, Likert‐scale responses were analyzed as continuous outcomes. For unadjusted comparisons among hospitalist groups, t tests and 1‐way ANOVAs were conducted for the scales scores, while chi‐square tests were used for dichotomous outcomes. For multivariable analyses, multiple linear regression was used for continuous outcomes. For dichotomous outcomes, adjusted prevalence ratios were estimated using Poisson regression with robust standard errors.21 All multivariable models controlled for sex, marital status, illness severity, age group, ethnicity, length of stay, and emergency room admission. Observations with missing data were excluded from analyses. Differences in bivariable and multivariable analyses were considered significant at a critical test level of 5%. Prevalence ratios are reported with 95% confidence intervals. All analyses were conducted in Stata, version 11 (StataCorp, College Station, TX).
RESULTS
Of patients who were reached by telephone, 87% agreed to participate in the hospital survey. However, most patients could not be reached by phone; thus our estimated response rate, including those who could not be reached, was 27%. For the subset of patients interviewed using the HCAHPS protocol, the response rate was 40%. Our final sample included 8295 patients (3597 cared for by 59 hospitalists and 4698 by 288 PCPs) interviewed between 2003 and 2009. Three‐quarters of the patients were from the tertiary care center, whereas 17% and 8% were from each of the community hospitals (see Supporting Appendix B in the online version of this article). Patient characteristics appear in Table 1. Patients cared for by hospitalists were similar to those cared for by PCPs in terms of age, sex, marital status, education, and language, but hospitalist patients were more likely to have been admitted through the emergency department (93% vs 84%, P < 0.001) and less likely to be white (83% vs 85%, P = 0.01). Patients cared for by hospitalists also had higher average illness severity score (2.2 0.8 vs 2.0 0.8, P < 0.001), longer average LOS (4.3 4.3 vs 4.0 3.6, P < 0.001), and lower mean perceived health score (2.8 1.2 vs 3.0 1.2, P = 0.01).
| Characteristic | PCP N = 4698 | Hospitalist N = 3597 | P Value |
|---|---|---|---|
| |||
| Age (mean, SD) | 63.5 (16.6) | 63.7 (16.3) | 0.53 |
| Male sex (%) | 44.9 | 46.2 | 0.28 |
| White race (%) | 85.3 | 83.2 | 0.01 |
| Married (%) | 49.1 | 48.7 | 0.69 |
| English spoken at home (%) | 96.0 | 97.0 | 0.09 |
| At least some college education (%) | 47.1 | 43.7 | 0.22 |
| Admitted through the emergency department (%) | 84.3 | 92.5 | <0.001 |
| Average illness severity rating (mean, SD) | 2.0 (0.8) | 2.2 (0.8) | <0.001 |
| Average perceived health score (mean, SD) | 3.0 (1.2) | 2.8 (1.2) | 0.01 |
| Average length of stay (days) (mean, SD) | 4.0 (3.6) | 4.3 (4.3) | <0.001 |
| Discharged home (%) | 87.9 | 88.5 | 0.73 |
Unadjusted patient reported satisfaction with physician care quality was slightly greater for PCPs than hospitalists (4.25 vs 4.19, P = 0.009). After multivariable adjustment, the difference was attenuated but persisted (4.24 vs 4.20, P = 0.04). We found no statistical difference among the hospitals or the specific hospitalist groups in terms of satisfaction with overall physician care quality (Figure 1). There were no statistical differences in patient satisfaction ratings of hospitalist and PCPs for the subdomains of behavior, pain, and communication (Table 2). There were also no differences in the proportion of patients cared for by hospitalists or PCPs who rated their physicians in the highest satisfaction category (79% vs 81%, P = 0.17) or the lowest (5% vs 5%, P = 0.19). Among patients cared for by academic hospitalists, there was no difference in satisfaction rating between those patients who had a designated primary care physician in the outpatient setting and those who did not (4.22 0.94 vs 4.19 0.94, P = 0.97). Finally, satisfaction with both hospitalists and PCPs showed equivalent rates of improvement over time (Figure 2).


| PCP | Hospitalist | P Value | ||
|---|---|---|---|---|
| ||||
| Satisfaction | Overall, you would rate the quality of doctor care as:* | 4.24 [4.21, 4.27] | 4.20 [4.17, 4.23] | 0.04 |
| Behavior | Doctors treated you with courtesy/respect | 3.77 [3.73, 3.82] | 3.78 [3.73, 3.82] | 0.88 |
| Pain control | Pain management by hospital staff* | 4.11 [4.08, 4.14] | 4.09 [4.05, 4.12] | 0.35 |
| Pain well controlled | 3.55 [3.47, 3.63] | 3.48 [3.41, 3.55] | 0.23 | |
| Staff did everything to help with pain | 3.73 [3.66, 3.80] | 3.68 [3.62, 3.75] | 0.33 | |
| Communication skills | Doctors listened carefully to you | 3.66 [3.61, 3.72] | 3.67 [3.62, 3.72] | 0.83 |
| Doctors explained things in an understandable way | 3.60 [3.54, 3.66] | 3.61 [3.56, 3.67] | 0.73 | |
| Doctor's communication* | 4.02 [3.97, 4.07] | 3.98 [3.93, 4.03] | 0.27 | |
| Doctor discussed your anxiety/fears* | 4.00 [3.96, 4.03] | 3.97 [3.93, 4.01] | 0.26 | |
| Doctor involved you in decisions* | 4.00 [3.95, 4.06] | 3.98 [3.93, 4.03] | 0.49 | |
DISCUSSION
In this observational study of over 8200 patients cared for over 6 years by 347 physicians at 3 hospitals, we found that patient satisfaction with inpatient care provided by hospitalists and primary care doctors was almost identical. As we hypothesized, overall satisfaction with physician care quality, our primary outcome, was slightly greater with primary care doctors; however, the observed difference, 0.04 on a scale of 1 to 5, cannot be considered clinically significant. All patients were generally satisfied (4.2‐4.3 rating on 5‐point scale) with their inpatient care, and satisfaction scores increased over time. We also found no differences among the specific domains of satisfaction, including communication skills, pain control, and physician behavior. Finally, we found no significant difference in patient satisfaction with physician care quality among the different hospitalist services.
Previous studies of patient satisfaction conducted in the outpatient setting found that continuity of care was an important determinant of trust and, consequently, overall satisfaction.15, 16, 19, 20, 22 Because hospitalist models introduce discontinuity, they might be expected to undermine satisfaction. Surprisingly, few studies have addressed this issue. In a review of the hospitalist studies through 2002, Wachter and Goldman found 19 studies, 5 of which measured patient satisfaction.23 Three of these were conducted on teaching services and compared designated faculty hospitalists to traditional ward attendings, who rotated onto the inpatient services 1 to 2 months per year. Primary care doctors were excluded.2426 A fourth study provided a descriptive narrative of the development of the first hospitalist program in Minneapolis, Minnesota, and anecdotally noted no difference in patient satisfaction between the hospitalist and traditional model, but presented no data because the satisfaction surveys were not designed with publication in mind.27 The only study to actually assess whether patient satisfaction was greater with hospitalists or PCPs was an observational study by Davis et al., conducted in 1 rural hospital during the first year of its hospitalist program. In that study, 2 hospitalists were compared to 17 PCPs, and patient satisfaction surveys were available for approximately 44 patients managed by hospitalists and 168 patients managed by PCPs. Specific data were not reported, but it was noted that there was no statistical difference in satisfaction between those cared for by hospitalists versus PCPs.28 On the basis of these studies, Wachter and Goldman concluded that surveys of patients who were cared for by hospitalists show high levels of satisfaction, no lower than that of similar patients cared for by their own primary physicians.23 Wachter and Goldman's review has been highly cited, and we could find no subsequent studies addressing this issue. Our study provides the first real evidence to support this conclusion, including data from 59 hospitalists practicing in 5 separate hospitalist programs at 3 different hospitals.
Our finding that hospitalists maintain satisfaction despite a lack of continuity suggests that other aspects of care may be more important to patient satisfaction. Larson et al. found that physician ability to meet patient's information needs was positively associated with patient satisfaction.29 Similarly, Tarrant et al. found that patient's trust in a physician improved with increasing communication, interpersonal care, and knowledge of the patient. Interestingly, continuity, ie. the proportion of visits to the usual general practitioner (GP) or duration with the practice, did not correlate with trust.30 Finally, a systematic review of determinants of outpatient satisfaction found that continuity has a variable effect on satisfaction. Subjective continuity measures, such as whether patients saw their regular physician on the day they were surveyed, were consistently associated with patient satisfaction, however, quantitative measures including relationship duration were not.31
It is also possible that patients believe they value continuity more than they actually do. In 1 survey of inpatients with an established PCP yet cared for by a hospitalist, most agreed that patients receive better care and have more trust in physicians with whom they have long‐term relationships. Yet most also had positive opinions of their hospital care.32 Similarly, in a survey of over 2500 outpatients, 92% rated continuity as very important or important, but the majority was unwilling to expend substantial personal time (88%), defined as driving greater than 60 minutes, or money (82%), defined as spending an additional $20 to $40 a month, to maintain continuity with their PCP.33 Our study appears to confirm the lack of connection between continuity and satisfaction. Even those patients who valued continuity, as evidenced by having an established PCP, were as satisfied with hospitalist physician care as patients who had no established PCP.
Our study has several limitations. First, we report on outcomes of 3 institutions within a single healthcare system, within a limited geographic area. Although our sample included a wide range of patient demographics, hundreds of physicians, and multiple hospitalist models, it is possible that some hospitalist models may provide greater or lesser satisfaction than those we observed. Second, our study was observational, and thus subject to selection bias and confounding. Patients cared for by the hospitalists differed in a number of ways from those cared for by PCPs. We controlled for identifiable confounders such as illness severity, self‐perceived health, and admission through the emergency department, but the possibility exists that additional unidentified factors could have affected our results. It is possible other drivers of patient satisfaction, such as amenities, nursing, or food, could have influenced our findings. However, this is unlikely because all patient groups shared these components of hospital experience equally. Third, only a minority of patients could be reached for interview. This is typical for post‐hospitalization surveys, and our response rate of 40% for HCAHPS patients compared favorably to the 2010 HCAHPS national average of 33%.34 Still, the responses of those who could not be reached may have differed from those who were interviewed. Fourth, we identified hospitalists and PCPs by the attending of record, but we were unable to tell who provided care to the patient on any given day. Thus, we could not determine to what extent patients cared for by PCPs were actually seen by their own doctor, as opposed to an associated physician within the practice. Nevertheless, our results are representative of the care model provided by PCPs in the hospital. Similarly, we could not know or compare the number of different attending physicians each patient experienced during their hospitalization. Higher turnover of inpatient physicians may have affected patient satisfaction scores independent of attending physician designation. These are potentially important measures of relationship duration, yet whether duration affects patient satisfaction remains undecided.1618, 20, 28, 30, 32, 33 We assessed satisfaction using HCAHPS questions, in order to provide objective and meaningful comparisons across hospitals. The HCAHPS instrument, however, is intended to assess patient satisfaction with doctors in general, not with subgroups or individuals, and responses in our study were uniformly high. A more sensitive survey instrument may have yielded different results. Finally, it is possible that individual physicians may possess lower satisfaction scores than others, making the results not representative of hospitalist models as much as specific doctors' care quality. We think this is unlikely since surveys reached over 8000 patients, over 6 years, representing the care of 347 individual physicians. However, hospital medicine is a rapidly evolving field with many divergent organizational structures, and patient satisfaction is bound to fluctuate while there exists high variability in how care is provided.
Over the past decade, the hospitalist model has become one of the dominant models for care of medical inpatients. Compared to the traditional model in which PCPs provide inpatient care, the hospitalist model has a number of advantages, including continuous on‐site coverage for increasingly acute patients, specialization, and incentives aligned with the hospital to provide efficient, high‐quality care. One concern that remains, however, is that patients may not trust doctors they first meet in the hospital or may be dissatisfied with the lack of continuity from day to day. Our findings are reassuring in this regard. Although patients cared for by hospitalists were slightly less satisfied, the differences could not be considered clinically meaningful and should be outweighed by gains in quality and efficiency. Furthermore, hospitalists can expect to fare well under value‐based purchasing. Given the rapid ascension of hospital medicine programs, prospective comparisons of hospitalists and PCPs may no longer be feasible. Future research might employ survey instruments designed specifically to measure patient experience under hospitalist care in order to identify methods to maximize patient satisfaction within the hospitalist model.
Acknowledgements
Jane Garb, MS, Academic Affairs, Baystate Medical Center, contributed to the initial database management and statistical analysis. She received no financial compensation. Dr Adrianne Seiler has received written permission for acknowledgement from Ms Garb.
Dr Adrianne Seiler made substantial contributions to our manuscript's conception and design, data acquisition, analysis, and interpretation, manuscript drafting and critical revision, and administrative support. Dr Paul Visintainer made substantial contributions to our manuscript's data analysis and interpretation, manuscript critical revision, and statistical analysis. Michael Ehresman and Richard Brzostek made substantial contributions to our manuscript's data acquisition, manuscript critical revision, and administrative support. Dr Evan Benjamin made substantial contributions to our manuscript's conception and design, analysis and interpretation of data, manuscript drafting, and administrative support. Dr Winthrop Whitcomb made substantial contributions to our manuscript's data analysis and interpretation, and manuscript critical revision. Dr Michael Rothberg made substantial contributions to our manuscript's conception and design, data analysis and interpretation, manuscript critical revision, and supervision.
- American Hospital Association Annual Survey Database.Fiscal Year2009.
- ,,,,.Quality of care for patients hospitalized with heart failure: assessing the impact of hospitalists.Arch Intern Med.2002;162:1251–1256.
- ,,,,,.Outcomes of care by hospitalists, general internists, and family physicians.N Engl J Med.2007;357:2589–2600.
- ,,,.Comparison of hospitalists and nonhospitalists regarding core measures of pneumonia care.Am J Manag Care.2007;13:129–132.
- ,,,.Comparison of processes and outcomes of pneumonia care between hospitalists and community‐based primary care physicians.Mayo Clin Proc.2002;77:1053–1058.
- ,,,.Comparison of hospitalists and nonhospitalists in inpatient length of stay adjusting for patient and physician characteristics.J Gen Intern Med.2004;19:1127–1132.
- ,,.Comparison of practice patterns of hospitalists and community physicians in the care of patients with congestive heart failure.J Hosp Med.2008;3:35–41.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,,,,.The value of a hospitalist service: efficient care for the aging population?Chest.2001;119:580–589.
- ,,,,.Comparison of hospital costs and length of stay associated with general internists and hospitalist physicians at a community hospital.Am J Manag Care.2004;10:626–630.
- ,,,,.Hospitalist care and length of stay in patients requiring complex discharge planning and close clinical monitoring.Arch Intern Med.2007;167:1869–1874.
- ,.The impact of hospitalists on the cost and quality of inpatient care in the United States: a research synthesis.Med Care Res Rev.2005;62(4):379–406.
- US Department of Health and Human Services Medicare Hospital Value‐Based Purchasing Plan Development Issues Paper. 1st Public Listening Session January 17, 2007. Available at: https://www.cms. gov/AcuteInpatientPPS/downloads/hospital_VBP_plan_issues_paper. pdf. Accessed on May 26, 2011.
- Hospital Value‐Based Purchasing: Measure Explanations. Available at: http://www.healthcare.gov/news/factsheets/valuebasedpurchasing 04292011b.html. Accessed on May 26, 2011.
- ,,,,,.Linking primary care performance to outcomes of care.J Fam Pract.1998;47:213–220.
- ,.Interpersonal continuity of care and patient satisfaction: a critical review.Ann Fam Med.2004;2:445–451.
- ,.Does continuity of care improve patient outcomes?J Fam Pract.2004;53:974–980.
- ,,,.Continuity of care and other determinants of patient satisfaction with primary care.J Gen Intern Med.2005;20:226–233.
- ,,,,.Continuity of care and trust in one's physician: evidence from primary care in the United States and the United Kingdom.Fam Med.2001;33:22–27.
- ,,,,.Patients' trust in their physicians: effects of choice, continuity, and payment method.J Gen Intern Med.1998;13:681–686.
- ,.Alternatives for logistic regression in cross‐sectional studies: an empirical comparison of models that directly estimate the prevalence ratio.BMC Med Res Methodol.2003;3:21.
- ,,, et al.Continuity of outpatient medical care in elderly men. A randomized trial.JAMA.1984;252:2413–2417.
- ,.The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- ,,, et al.The effect of a hospitalist service with nurse discharge planner on patient care in an academic teaching hospital.Am J Med.2001;111(8):627–632.
- ,,, et al.Decreased length of stay, costs and mortality in a randomized trial of academic hospitalists.J Gen Intern Med.2001;16(suppl):S208.
- ,,,,.Reorganizing an academic medical service: impact on cost, quality, patient satisfaction, and education.JAMA.1998;279(19):1560–1565.
- .The Park Nicollet experience in establishing a hospitalist system.Ann Intern Med.1999;130:350–354.
- ,,,,,.Effects of hospitalists on cost, outcomes, and patient satisfaction in a rural health system.Am J Med.2000;108:621–626.
- ,,,.The relationship between meeting patients' information needs and their satisfaction with hospital care and general health status outcomes.Int J Qual Health Care.1996;8:447–456.
- ,,.Factors associated with patients' trust in their general practitioner: a cross‐sectional survey.Br J Gen Pract.2003;53:798–800.
- ,,.The relationship between continuity and patient satisfaction: a systematic review.Fam Pract.2010;27:171–178.
- ,,.How do patients view the role of the primary care physician in inpatient care?Dis Mon.2002;48:230–238.
- ,.Patient attitudes toward continuity of care.Arch Intern Med.2003;163:909–912.
- Summary of HCAHPS Survey Results. Available at: http://www. hcahpsonline.org/files/12–13‐10_Summary_of_HCAHPS_Survey_ Results_December_2010.pdf. Accessed on May 27,2011.
- American Hospital Association Annual Survey Database.Fiscal Year2009.
- ,,,,.Quality of care for patients hospitalized with heart failure: assessing the impact of hospitalists.Arch Intern Med.2002;162:1251–1256.
- ,,,,,.Outcomes of care by hospitalists, general internists, and family physicians.N Engl J Med.2007;357:2589–2600.
- ,,,.Comparison of hospitalists and nonhospitalists regarding core measures of pneumonia care.Am J Manag Care.2007;13:129–132.
- ,,,.Comparison of processes and outcomes of pneumonia care between hospitalists and community‐based primary care physicians.Mayo Clin Proc.2002;77:1053–1058.
- ,,,.Comparison of hospitalists and nonhospitalists in inpatient length of stay adjusting for patient and physician characteristics.J Gen Intern Med.2004;19:1127–1132.
- ,,.Comparison of practice patterns of hospitalists and community physicians in the care of patients with congestive heart failure.J Hosp Med.2008;3:35–41.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,,,,.The value of a hospitalist service: efficient care for the aging population?Chest.2001;119:580–589.
- ,,,,.Comparison of hospital costs and length of stay associated with general internists and hospitalist physicians at a community hospital.Am J Manag Care.2004;10:626–630.
- ,,,,.Hospitalist care and length of stay in patients requiring complex discharge planning and close clinical monitoring.Arch Intern Med.2007;167:1869–1874.
- ,.The impact of hospitalists on the cost and quality of inpatient care in the United States: a research synthesis.Med Care Res Rev.2005;62(4):379–406.
- US Department of Health and Human Services Medicare Hospital Value‐Based Purchasing Plan Development Issues Paper. 1st Public Listening Session January 17, 2007. Available at: https://www.cms. gov/AcuteInpatientPPS/downloads/hospital_VBP_plan_issues_paper. pdf. Accessed on May 26, 2011.
- Hospital Value‐Based Purchasing: Measure Explanations. Available at: http://www.healthcare.gov/news/factsheets/valuebasedpurchasing 04292011b.html. Accessed on May 26, 2011.
- ,,,,,.Linking primary care performance to outcomes of care.J Fam Pract.1998;47:213–220.
- ,.Interpersonal continuity of care and patient satisfaction: a critical review.Ann Fam Med.2004;2:445–451.
- ,.Does continuity of care improve patient outcomes?J Fam Pract.2004;53:974–980.
- ,,,.Continuity of care and other determinants of patient satisfaction with primary care.J Gen Intern Med.2005;20:226–233.
- ,,,,.Continuity of care and trust in one's physician: evidence from primary care in the United States and the United Kingdom.Fam Med.2001;33:22–27.
- ,,,,.Patients' trust in their physicians: effects of choice, continuity, and payment method.J Gen Intern Med.1998;13:681–686.
- ,.Alternatives for logistic regression in cross‐sectional studies: an empirical comparison of models that directly estimate the prevalence ratio.BMC Med Res Methodol.2003;3:21.
- ,,, et al.Continuity of outpatient medical care in elderly men. A randomized trial.JAMA.1984;252:2413–2417.
- ,.The hospitalist movement 5 years later.JAMA.2002;287:487–494.
- ,,, et al.The effect of a hospitalist service with nurse discharge planner on patient care in an academic teaching hospital.Am J Med.2001;111(8):627–632.
- ,,, et al.Decreased length of stay, costs and mortality in a randomized trial of academic hospitalists.J Gen Intern Med.2001;16(suppl):S208.
- ,,,,.Reorganizing an academic medical service: impact on cost, quality, patient satisfaction, and education.JAMA.1998;279(19):1560–1565.
- .The Park Nicollet experience in establishing a hospitalist system.Ann Intern Med.1999;130:350–354.
- ,,,,,.Effects of hospitalists on cost, outcomes, and patient satisfaction in a rural health system.Am J Med.2000;108:621–626.
- ,,,.The relationship between meeting patients' information needs and their satisfaction with hospital care and general health status outcomes.Int J Qual Health Care.1996;8:447–456.
- ,,.Factors associated with patients' trust in their general practitioner: a cross‐sectional survey.Br J Gen Pract.2003;53:798–800.
- ,,.The relationship between continuity and patient satisfaction: a systematic review.Fam Pract.2010;27:171–178.
- ,,.How do patients view the role of the primary care physician in inpatient care?Dis Mon.2002;48:230–238.
- ,.Patient attitudes toward continuity of care.Arch Intern Med.2003;163:909–912.
- Summary of HCAHPS Survey Results. Available at: http://www. hcahpsonline.org/files/12–13‐10_Summary_of_HCAHPS_Survey_ Results_December_2010.pdf. Accessed on May 27,2011.
Copyright © 2011 Society of Hospital Medicine
Implementing an RRT
In‐hospital cardiopulmonary arrests are often preceded by signs of clinical instability, such as changes in vital signs or mental status.1 Nearly 85% of patients who suffer from cardiopulmonary arrests have documented observations of deterioration in the 8 hours before arrest.2 A Medical Emergency Team (MET), sometimes known as Rapid Response Team (RRT), can rapidly assess and manage unstable patients, with the goal that early intervention will prevent adverse outcomes. In 2004, the Institute for Healthcare Improvement (IHI), as part of its 100,000 Lives Campaign, called for hospitals to implement rapid response systems as 1 of 6 strategies to reduce deaths in hospital.3 Since this recommendation, hundreds of hospitals in the United States have invested substantial financial and personnel resources to implement some form of a rapid response system, which is comprised of a varying array of healthcare providers who bring critical care expertise to the patient's bedside.4, 5
Despite the intuitive appeal of the approach, and early observational data which suggested that METs could reduce both codes and unexpected in‐hospital mortality,2, 6 the largest randomized controlled trial found that METs failed to reduce unplanned intensive care unit (ICU) admissions, cardiac arrests, or unexpected deaths.7 More recently, in a prospective observational cohort study at 1 US hospital, Chan et al. found that a nurse‐led RRT did not impact hospital‐wide code rates or mortality.4
The study of rapid response systems is further complicated by a lack of standard definition, and the many types of hospitals in which they may be implemented. In 2006, a consensus conference suggested that MET be used to refer to teams led by physicians (usually intensivists), and RRT be used for teams led by nurses.8 Many studies have been conducted at non‐US institutions, and follow‐up periods have generally been 1 year or less. We report on almost 4 years of experience with a hospitalist‐led MET implemented in a major US academic medical center, and examine the subsequent changes in code calls, cardiac arrests, deaths following cardiopulmonary resuscitation, and overall hospital mortality. Because the MET did not operate in the critical care units, and because cardiac arrest may occur without prior signs of deterioration, we hypothesized that implementation of the MET would correspond to a small drop in total code calls, no change in codes called inside of critical care units, no change in cardiac arrest outside of critical care, and a marked drop in other medical crises (mostly respiratory distress) outside critical care. We also hypothesized that there would be no change in the rate of fatal codes, because most deaths occur in patients who were found to be pulseless on arrival of the code team.
METHODS
Setting
Beginning in March 2006, Baystate Medical Center implemented an MET in accordance with the recommendations of the IHI. Baystate is a 670‐bed tertiary care referral center in Springfield, Massachusetts, and a major teaching hospital for Tufts University. Throughout the study period, the hospital had full‐time intensivists and >90% of medical patients were cared for by hospitalists with 24‐hour coverage. As a result, a medical patient's attending physician or corresponding coverage was usually on site. In order to promote acceptance of the team as well as to maximize continuity of care, we constructed our MET to include a critical care nurse, a respiratory therapist, intravenous therapist, and the patient's physician (either attending or resident). Baystate staff members carry alpha‐numeric pagers, so attendings could be alerted to the fact that the MET had been activated by means of a text page. In the event that the patient's physician could not respond, an ICU physician served as a backup team member. The MET was implemented initially in March of 2006 on 2 medical floors, and over a period of 3 months was gradually expanded to cover the entire hospital. For surgical patients, the MET was led by the attending surgeon or appropriate resident. Educational efforts, including meetings, e‐mails, and posters, targeted nurses in particular, but anyone could summon the MET by calling the activation number posted on all ward telephones. Nurses were encouraged to activate the system for any of the following: heart rate (<40 and >130 beats per minute), systolic blood pressure (<90 mmHg), respiratory rate (<8 or >24 per minute), oxygen saturation (<90% despite supplemental oxygen), altered mental status, or simply concern that something is wrong. The MET implementation oversight committee met biweekly and made adjustments to the team composition and protocols using rapid Plan Do Study Act (PDSA) cycles. A full description of the implementation process has been published elsewhere.9
In addition to the MET, Baystate has a separate code team which can be activated for cardiovascular arrests via a call to a designated phone extension, which activates a page to the code team members and an overhead announcement. Code team members include the ICU medical resident and intern, a critical care nurse, an anesthesiologist, a respiratory therapist, a staff nurse, and the house supervisor. In response to the overhead announcement, doctors, nurses and students in the vicinity often respond as well. Prior to implementation of the MET, a code blue was the only level of immediate response available.
Data and Outcomes
The nurse attending a code blue or code completes a report form which becomes part of the permanent medical record. A copy of the report is reviewed by the Division of Healthcare Quality and housed in the Critical Care administrative offices. For this study, we reviewed all code reports from January 2004 through December 2009. For each report, we extracted the following information: the date, location (inside or outside of a critical care unit), whether the patient had a pulse on arrival of the team, and whether the patient survived to discharge. All activations of the code system were included, regardless of the patient's code status (ie, even if the code was called in error) or the reason for the code call. Patients were then aggregated to calculate the rate of codes called per calendar quarter, as well as the rates of codes called in and out of critical care and the rates of two subsets of code calls, namely cardiac arrests and other medical crises (eg, respiratory arrest or seizures).
MET members were also required to collect data on the reason for the MET call, as well as the response time, time of day and unit, duration of the call, whether the physician was present, whether the patient was transferred to critical care, and whether the patient survived to discharge. In addition, we surveyed the nursing staff directly after the call, asking the following questions: 1) Did the team arrive promptly? 2) Were the critical care nurse and respiratory therapist efficient and respectful? 3) Did you feel the patient's needs were addressed appropriately? 4) Did you feel supported by the MET? and 5) Would you call the MET again?
Statistical Analysis
Quarterly event rates per 1000 admissions were calculated for each outcome. Event rates were compared using piecewise Poisson regression10 with robust standard errors.11 We excluded the 2 quarterly periods (2006 Q1 and Q2) during which the MET was implemented. A piecewise Poisson regression model was chosen to facilitate estimation of: 1) change in code calls from immediately before implementation to immediately after; and 2) temporal trends in code calls before and after implementation. Each model was built with 1 pre‐implementation intercept (December 2005), and 1 post‐implementation intercept (July 2006), as well as 2 slopes, with time coded negatively before the intervention (ie, 2, 1, 0), and positively after (ie, 0, 1, 2). Linear contrasts tested for differences in each parameter. A significant difference in intercepts suggests a post‐intervention decrease in code call rates; a significant, negative post‐intervention slope suggests continuing decline in code call rates. Statistical inferences were aided with visual plots of predicted incidence rates for each quarter in the observation period, with 95% confidence intervals (CI) for each quarterly rate estimated by the delta method.12 Alpha was specified at 0.05 and all significance tests were 2‐sided. Analyses were conducted in Stata 11.1 for Windows ( 2010, StataCorp LP, College Station, TX).
RESULTS
Implementation of the MET
The MET was introduced in the first and second quarters of 2006, with 2717 calls logged through the end of 2009 (out of 154,382 admissions). The rate of MET calls increased during the first 6 months of implementation from 5.95 per 1000 admissions in the first quarter of the intervention, to 15.8 calls per 1000 admissions in the second quarter. Call rates peaked in the first half of 2009, at 20.9 calls per 1000 admissions, leveling off to 17.9 calls per 1000 admissions in the last half of 2009 (Figure 1). Of calls with time recorded, 40% occurred on the day shift, 35% on the evening shift, and 25% on the night shift. The most common reason to call the MET was respiratory distress (33%), followed by cardiovascular instability (25%), and neurological abnormality (20%). In 15% of cases, concern about a patient's condition prompted the nurse to call. Calls came primarily from medical floors (75%) and surgical units (20%). The median response time was 4 minutes (interquartile range [IQR], 2.8 to 5.2 minutes) with no meaningful trend during the study period. The median call duration was 50 minutes (IQR, 38 to 72 minutes). Again, there was no trend over time. The most common interventions were arterial blood gas, fluid resuscitation, and electrocardiogram (see Supporting Web Appendix Table 1 in the online version of this article). A physician was present at 52% of the calls in the first year, which rose to 93% of calls in the final year. Approximately 25% of calls resulted in the patient being transferred to a critical care unit. Staff evaluations were overwhelmingly positive. Nurses rated the teams on the following points: whether the critical care nurse and respiratory therapist were efficient and respectful (mean rating 98%, SD 5.6%); promptness (98%, SD 5.6%); whether the patient's needs were addressed appropriately (mean 98%, SD 4.2%); whether the nurse felt supported by the MET (99.5%, SD 1.7%); and whether they would call the MET again (99.7%, SD 1.4%).

Effect of MET on Code Calls and Mortality
Between January 2004 and December of 2009, the hospital case mix index remained constant, and there were a total of 1202 codes called. The majority (62%) took place outside of critical care units. Linear contrasts of pre‐piecewise and post‐piecewise intercepts revealed that overall code calls declined significantly between pre‐implementation and post‐implementation of the MET from 7.30 (95% CI 5.81, 9.16) codes called per 1000 admissions to 4.21 (95% CI 3.42, 5.18) calls per 1000 admissions (Figure 1; also see Supporting Web Appendix Table 2 in the online version of this article). Outside of critical care, code calls declined from 4.70 (95% CI 3.92, 5.63) before the MET was implemented to 3.11 (95% CI 2.44, 3.97) afterwards (Figure 2); this was due primarily to a decrease in medical crises, which averaged 3.29 events per 1000 admissions (95% CI 2.70, 4.02) before implementation and decreased to 1.72 (95% CI 1.28, 2.31) afterwards, whereas cardiac arrests did not change significantly (Figure 3). Following implementation, code calls within critical care also declined significantly, from 2.59 events per 1000 admissions (95% CI 1.82, 3.69) before to 1.24 events per 1000 admissions (95% CI 0.94, 1.63) afterwards. The change in codes called within critical care was smaller, however, and included reductions in both cardiac arrests ( 0.84 events, P = 0.01) and medical crises ( 0.55, P = 0.08). There was no significant change in the rate of fatal codes per 1000 admissions ( +0.06, P = 0.65) (Figure 4). Overall hospital mortality remained steady at 22.0 deaths per 1000 admissions throughout the study period.
DISCUSSION
In this report, we detail the implementation of a novel hospitalist‐led medical emergency team at a large academic medical center over a period of 4 years. The team, which consisted of the patient's physician, a critical care nurse, a respiratory therapist, and an intravenous therapist, achieved full implementation within 6 months, was well received by the nursing staff, and was associated with a 42% decrease in code calls hospital‐wide. Most of the overall reduction was due to a reduction in codes called for medical crises outside of critical care, accompanied by a lesser reduction in codes called for cardiac arrests and medical crises within critical care units. There was no significant effect on the rate of cardiac arrest outside critical care. More importantly, there was no change in the rate of fatal codes or overall hospital mortality.
The idea of early intervention to prevent deterioration among hospitalized patients appeals to the concept that an ounce of prevention is worth a pound of cure. Like many other preventive interventions, rapid response systems have not always delivered on this promise. Since several early reports from Australia2 suggested that medical emergency teams could reduce not only cardiopulmonary arrests, but overall hospital mortality, there has been a rapid proliferation in their implementation, spurred on by the IHI's 100,000 Lives Campaign, which incorporated rapid response systems as one of 6 hospital‐wide interventions aimed at reducing harm and mortality.13 Subsequent randomized trials have both reproduced and refuted the early observational results. A ward‐randomized trial within 1 British hospital found a 50% reduction in hospital mortality for wards assigned to have an RRT,14 while a cluster randomized trial conducted at 23 Australian hospitals found no difference in rates of cardiac arrest or mortality between hospitals implementing METs and those continuing with usual care.7 Interestingly, in the Australian trial, the rates of cardiac arrest and mortality declined for both groups compared to historical controls, an important limitation to observational trials. Reports from single‐institution observational trials are also divided between those that found a reduction in mortality following implementation and those that did not. A recent meta‐analysis reported that there was too much heterogeneity among these trials to reach a conclusion about the benefits of rapid response systems.15
Our study adds to this literature in several ways. First, our MET design, which included the patient's physician (as opposed to an intensive care physician), was different from those previously studied. Including the patient's physician increases the team's knowledge of the patient and disease, and may improve physician acceptance of METs. In addition, our study provides 4 full years of follow‐up. Second, our rate of MET activation (18 calls/1000 admissions) was 2 to 3 times higher than that seen in most other studies,16 thus, the lack of mortality benefit was not likely the result of underuse. Third, our hospital employs a large number of hospitalists whose continuous presence might be expected to attenuate the benefits of an MET. Indeed, our initial rate of codes (7.5/1000 admissions) was similar to the post‐intervention rate in other studies.4 Nevertheless, the decrease in the overall rate of code calls following implementation of our MET was similar to that observed by others.17 Finally, our stratification of code calls inside critical care (where the MET was not deployed) and outside critical care, as well as the division of codes into cardiac arrest (where intervention is often unsuccessful) and other medical crises (primarily respiratory distress), gives further insight into how METs might work. As expected, we found that outside critical care only, codes called for medical crises declined, implying that the main effect of the MET was to provide early interventions for patients who were not likely to die anyway (eg, respiratory care for patients with respiratory distress or intravenous fluids for hypotensive patients). Instead of intervening to prevent death, MET may avoid emergent intubation by providing respiratory therapy and/or urgent intubation. In addition, it represents a less‐intense option for responding to nonlife‐threatening emergencies, such as seizures or syncope. As codes were no longer called for these types of crises, the rate of code calls necessarily fell. The reason that code calls declined inside critical care is less clear. It could be that patients transferred to critical care by the MET were less likely to code than those transferred before implementation, or the decline might be due to other factors that were not evaluated. Regardless, it is clear that the MET did not simply relocate codes to critical care units.
Our study has a number of limitations. First, it is an observational study and cannot account for other confounders relating to temporal trends in the hospital. However, our long time window allowed us to examine trends over several years. For 2 years prior to implementation of the MET, there was no decline at all in the rate of code calls, followed by an immediate and sustained drop after implementation. Other interventions, including ventilator‐associated pneumonia bundles, sepsis bundles, and advanced cardiac life support simulation training were also implemented at different times during the study period. However, the stark demarcation in code call rates coinciding with MET implementation makes it less likely that these other interventions were responsible for the observed decline. Second, our study was limited to a single institution and a single type of MET. Our findings may not apply to other types of institutions with different staffing arrangements or a different hospital culture, nor would they necessarily apply to different types of MET. Third, our nurse surveys were not collected anonymously, and this may have affected the nurses' responses. Finally, we did not collect physiological parameters on our patients, so we cannot state with certainty what the MET intervention accomplished.
Since initial studies suggested that METs could reduce hospital mortality rates, the Joint Commission has effectively mandated implementation of rapid response systems in all hospitals. Newer evidence, however, has been less convincing of mortality or other benefit. Our study adds to the literature in that we also did not find a mortality benefit. However, there were 2 clear benefits that we did identify. Our MET did appear to substantially reduce total numbers of code calls, particularly codes called for medical crises. Also, our nurses had a very positive response to the MET, which empowered them to get help for a patient when the patient's physician was unavailable or did not take their concerns seriously. Clearly, additional study is needed to better understand the effects of METs on mortality, codes, and other indicators of patient outcomes. However, in the current regulatory environment, such studies will be difficult to perform. Instead, additional studies can clarify which models deliver best outcomes and optimal use of our limited resources.
- ,,,,,.Recognising clinical instability in hospital patients before cardiac arrest or unplanned admission to intensive care. A pilot study in a tertiary‐care hospital.Med J Aust.1999;171:22–25.
- ,,, et al.Rates of in‐hospital arrests, deaths and intensive care admissions: the effect of a medical emergency team.Med J Aust.2000;173:236–240.
- ,,,.The 100,000 Lives Campaign: setting a goal and a deadline for improving health care quality.JAMA.2006;295:324–327.
- ,,,,,.Hospital‐wide code rates and mortality before and after implementation of a rapid response team.JAMA.2008;300:2506–2513.
- ,,,,.Rapid response teams: do they make a difference?Dimens Crit Care Nurs.2007;26:253–262.
- ,,,,,.Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study.BMJ.2002;324:387–390.
- ,,, et al.Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365:2091–2097.
- ,,, et al.Findings of the first consensus conference on medical emergency teams.Crit Care Med.2006;34:2463–2478.
- ,.Implementation of a rapid response team: a success story.Crit Care Nurse.2009;29:66–76.
- .Practical Biostatistical Methods.Belmont, CA:Wadsworth Publishing;1995.
- ,,,.Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models.New York:Springer Science + Business Media;2005.
- .A note on the delta method.Am Stat.1992;46:27–29.
- ,.The 100,000 Lives Campaign: crystallizing standards of care for hospitals.Health Aff.2005;24:1560–1570.
- ,,, et al.Introducing critical care outreach: a ward‐randomised trial of phased introduction in a general hospital.Intensive Care Med.2004;30:1398–404.
- ,,,,,.Rapid response systems: a systematic review.Crit Care Med.2007;35:1238–1243.
- ,,,,.Effects of rapid response systems on clinical outcomes: systematic review and meta‐analysis.J Hosp Med.2007;2:422–432.
- ,,,,.Rapid response teams: a systematic review and meta‐analysis.Arch Intern Med.2010;170:18–26.
In‐hospital cardiopulmonary arrests are often preceded by signs of clinical instability, such as changes in vital signs or mental status.1 Nearly 85% of patients who suffer from cardiopulmonary arrests have documented observations of deterioration in the 8 hours before arrest.2 A Medical Emergency Team (MET), sometimes known as Rapid Response Team (RRT), can rapidly assess and manage unstable patients, with the goal that early intervention will prevent adverse outcomes. In 2004, the Institute for Healthcare Improvement (IHI), as part of its 100,000 Lives Campaign, called for hospitals to implement rapid response systems as 1 of 6 strategies to reduce deaths in hospital.3 Since this recommendation, hundreds of hospitals in the United States have invested substantial financial and personnel resources to implement some form of a rapid response system, which is comprised of a varying array of healthcare providers who bring critical care expertise to the patient's bedside.4, 5
Despite the intuitive appeal of the approach, and early observational data which suggested that METs could reduce both codes and unexpected in‐hospital mortality,2, 6 the largest randomized controlled trial found that METs failed to reduce unplanned intensive care unit (ICU) admissions, cardiac arrests, or unexpected deaths.7 More recently, in a prospective observational cohort study at 1 US hospital, Chan et al. found that a nurse‐led RRT did not impact hospital‐wide code rates or mortality.4
The study of rapid response systems is further complicated by a lack of standard definition, and the many types of hospitals in which they may be implemented. In 2006, a consensus conference suggested that MET be used to refer to teams led by physicians (usually intensivists), and RRT be used for teams led by nurses.8 Many studies have been conducted at non‐US institutions, and follow‐up periods have generally been 1 year or less. We report on almost 4 years of experience with a hospitalist‐led MET implemented in a major US academic medical center, and examine the subsequent changes in code calls, cardiac arrests, deaths following cardiopulmonary resuscitation, and overall hospital mortality. Because the MET did not operate in the critical care units, and because cardiac arrest may occur without prior signs of deterioration, we hypothesized that implementation of the MET would correspond to a small drop in total code calls, no change in codes called inside of critical care units, no change in cardiac arrest outside of critical care, and a marked drop in other medical crises (mostly respiratory distress) outside critical care. We also hypothesized that there would be no change in the rate of fatal codes, because most deaths occur in patients who were found to be pulseless on arrival of the code team.
METHODS
Setting
Beginning in March 2006, Baystate Medical Center implemented an MET in accordance with the recommendations of the IHI. Baystate is a 670‐bed tertiary care referral center in Springfield, Massachusetts, and a major teaching hospital for Tufts University. Throughout the study period, the hospital had full‐time intensivists and >90% of medical patients were cared for by hospitalists with 24‐hour coverage. As a result, a medical patient's attending physician or corresponding coverage was usually on site. In order to promote acceptance of the team as well as to maximize continuity of care, we constructed our MET to include a critical care nurse, a respiratory therapist, intravenous therapist, and the patient's physician (either attending or resident). Baystate staff members carry alpha‐numeric pagers, so attendings could be alerted to the fact that the MET had been activated by means of a text page. In the event that the patient's physician could not respond, an ICU physician served as a backup team member. The MET was implemented initially in March of 2006 on 2 medical floors, and over a period of 3 months was gradually expanded to cover the entire hospital. For surgical patients, the MET was led by the attending surgeon or appropriate resident. Educational efforts, including meetings, e‐mails, and posters, targeted nurses in particular, but anyone could summon the MET by calling the activation number posted on all ward telephones. Nurses were encouraged to activate the system for any of the following: heart rate (<40 and >130 beats per minute), systolic blood pressure (<90 mmHg), respiratory rate (<8 or >24 per minute), oxygen saturation (<90% despite supplemental oxygen), altered mental status, or simply concern that something is wrong. The MET implementation oversight committee met biweekly and made adjustments to the team composition and protocols using rapid Plan Do Study Act (PDSA) cycles. A full description of the implementation process has been published elsewhere.9
In addition to the MET, Baystate has a separate code team which can be activated for cardiovascular arrests via a call to a designated phone extension, which activates a page to the code team members and an overhead announcement. Code team members include the ICU medical resident and intern, a critical care nurse, an anesthesiologist, a respiratory therapist, a staff nurse, and the house supervisor. In response to the overhead announcement, doctors, nurses and students in the vicinity often respond as well. Prior to implementation of the MET, a code blue was the only level of immediate response available.
Data and Outcomes
The nurse attending a code blue or code completes a report form which becomes part of the permanent medical record. A copy of the report is reviewed by the Division of Healthcare Quality and housed in the Critical Care administrative offices. For this study, we reviewed all code reports from January 2004 through December 2009. For each report, we extracted the following information: the date, location (inside or outside of a critical care unit), whether the patient had a pulse on arrival of the team, and whether the patient survived to discharge. All activations of the code system were included, regardless of the patient's code status (ie, even if the code was called in error) or the reason for the code call. Patients were then aggregated to calculate the rate of codes called per calendar quarter, as well as the rates of codes called in and out of critical care and the rates of two subsets of code calls, namely cardiac arrests and other medical crises (eg, respiratory arrest or seizures).
MET members were also required to collect data on the reason for the MET call, as well as the response time, time of day and unit, duration of the call, whether the physician was present, whether the patient was transferred to critical care, and whether the patient survived to discharge. In addition, we surveyed the nursing staff directly after the call, asking the following questions: 1) Did the team arrive promptly? 2) Were the critical care nurse and respiratory therapist efficient and respectful? 3) Did you feel the patient's needs were addressed appropriately? 4) Did you feel supported by the MET? and 5) Would you call the MET again?
Statistical Analysis
Quarterly event rates per 1000 admissions were calculated for each outcome. Event rates were compared using piecewise Poisson regression10 with robust standard errors.11 We excluded the 2 quarterly periods (2006 Q1 and Q2) during which the MET was implemented. A piecewise Poisson regression model was chosen to facilitate estimation of: 1) change in code calls from immediately before implementation to immediately after; and 2) temporal trends in code calls before and after implementation. Each model was built with 1 pre‐implementation intercept (December 2005), and 1 post‐implementation intercept (July 2006), as well as 2 slopes, with time coded negatively before the intervention (ie, 2, 1, 0), and positively after (ie, 0, 1, 2). Linear contrasts tested for differences in each parameter. A significant difference in intercepts suggests a post‐intervention decrease in code call rates; a significant, negative post‐intervention slope suggests continuing decline in code call rates. Statistical inferences were aided with visual plots of predicted incidence rates for each quarter in the observation period, with 95% confidence intervals (CI) for each quarterly rate estimated by the delta method.12 Alpha was specified at 0.05 and all significance tests were 2‐sided. Analyses were conducted in Stata 11.1 for Windows ( 2010, StataCorp LP, College Station, TX).
RESULTS
Implementation of the MET
The MET was introduced in the first and second quarters of 2006, with 2717 calls logged through the end of 2009 (out of 154,382 admissions). The rate of MET calls increased during the first 6 months of implementation from 5.95 per 1000 admissions in the first quarter of the intervention, to 15.8 calls per 1000 admissions in the second quarter. Call rates peaked in the first half of 2009, at 20.9 calls per 1000 admissions, leveling off to 17.9 calls per 1000 admissions in the last half of 2009 (Figure 1). Of calls with time recorded, 40% occurred on the day shift, 35% on the evening shift, and 25% on the night shift. The most common reason to call the MET was respiratory distress (33%), followed by cardiovascular instability (25%), and neurological abnormality (20%). In 15% of cases, concern about a patient's condition prompted the nurse to call. Calls came primarily from medical floors (75%) and surgical units (20%). The median response time was 4 minutes (interquartile range [IQR], 2.8 to 5.2 minutes) with no meaningful trend during the study period. The median call duration was 50 minutes (IQR, 38 to 72 minutes). Again, there was no trend over time. The most common interventions were arterial blood gas, fluid resuscitation, and electrocardiogram (see Supporting Web Appendix Table 1 in the online version of this article). A physician was present at 52% of the calls in the first year, which rose to 93% of calls in the final year. Approximately 25% of calls resulted in the patient being transferred to a critical care unit. Staff evaluations were overwhelmingly positive. Nurses rated the teams on the following points: whether the critical care nurse and respiratory therapist were efficient and respectful (mean rating 98%, SD 5.6%); promptness (98%, SD 5.6%); whether the patient's needs were addressed appropriately (mean 98%, SD 4.2%); whether the nurse felt supported by the MET (99.5%, SD 1.7%); and whether they would call the MET again (99.7%, SD 1.4%).

Effect of MET on Code Calls and Mortality
Between January 2004 and December of 2009, the hospital case mix index remained constant, and there were a total of 1202 codes called. The majority (62%) took place outside of critical care units. Linear contrasts of pre‐piecewise and post‐piecewise intercepts revealed that overall code calls declined significantly between pre‐implementation and post‐implementation of the MET from 7.30 (95% CI 5.81, 9.16) codes called per 1000 admissions to 4.21 (95% CI 3.42, 5.18) calls per 1000 admissions (Figure 1; also see Supporting Web Appendix Table 2 in the online version of this article). Outside of critical care, code calls declined from 4.70 (95% CI 3.92, 5.63) before the MET was implemented to 3.11 (95% CI 2.44, 3.97) afterwards (Figure 2); this was due primarily to a decrease in medical crises, which averaged 3.29 events per 1000 admissions (95% CI 2.70, 4.02) before implementation and decreased to 1.72 (95% CI 1.28, 2.31) afterwards, whereas cardiac arrests did not change significantly (Figure 3). Following implementation, code calls within critical care also declined significantly, from 2.59 events per 1000 admissions (95% CI 1.82, 3.69) before to 1.24 events per 1000 admissions (95% CI 0.94, 1.63) afterwards. The change in codes called within critical care was smaller, however, and included reductions in both cardiac arrests ( 0.84 events, P = 0.01) and medical crises ( 0.55, P = 0.08). There was no significant change in the rate of fatal codes per 1000 admissions ( +0.06, P = 0.65) (Figure 4). Overall hospital mortality remained steady at 22.0 deaths per 1000 admissions throughout the study period.



DISCUSSION
In this report, we detail the implementation of a novel hospitalist‐led medical emergency team at a large academic medical center over a period of 4 years. The team, which consisted of the patient's physician, a critical care nurse, a respiratory therapist, and an intravenous therapist, achieved full implementation within 6 months, was well received by the nursing staff, and was associated with a 42% decrease in code calls hospital‐wide. Most of the overall reduction was due to a reduction in codes called for medical crises outside of critical care, accompanied by a lesser reduction in codes called for cardiac arrests and medical crises within critical care units. There was no significant effect on the rate of cardiac arrest outside critical care. More importantly, there was no change in the rate of fatal codes or overall hospital mortality.
The idea of early intervention to prevent deterioration among hospitalized patients appeals to the concept that an ounce of prevention is worth a pound of cure. Like many other preventive interventions, rapid response systems have not always delivered on this promise. Since several early reports from Australia2 suggested that medical emergency teams could reduce not only cardiopulmonary arrests, but overall hospital mortality, there has been a rapid proliferation in their implementation, spurred on by the IHI's 100,000 Lives Campaign, which incorporated rapid response systems as one of 6 hospital‐wide interventions aimed at reducing harm and mortality.13 Subsequent randomized trials have both reproduced and refuted the early observational results. A ward‐randomized trial within 1 British hospital found a 50% reduction in hospital mortality for wards assigned to have an RRT,14 while a cluster randomized trial conducted at 23 Australian hospitals found no difference in rates of cardiac arrest or mortality between hospitals implementing METs and those continuing with usual care.7 Interestingly, in the Australian trial, the rates of cardiac arrest and mortality declined for both groups compared to historical controls, an important limitation to observational trials. Reports from single‐institution observational trials are also divided between those that found a reduction in mortality following implementation and those that did not. A recent meta‐analysis reported that there was too much heterogeneity among these trials to reach a conclusion about the benefits of rapid response systems.15
Our study adds to this literature in several ways. First, our MET design, which included the patient's physician (as opposed to an intensive care physician), was different from those previously studied. Including the patient's physician increases the team's knowledge of the patient and disease, and may improve physician acceptance of METs. In addition, our study provides 4 full years of follow‐up. Second, our rate of MET activation (18 calls/1000 admissions) was 2 to 3 times higher than that seen in most other studies,16 thus, the lack of mortality benefit was not likely the result of underuse. Third, our hospital employs a large number of hospitalists whose continuous presence might be expected to attenuate the benefits of an MET. Indeed, our initial rate of codes (7.5/1000 admissions) was similar to the post‐intervention rate in other studies.4 Nevertheless, the decrease in the overall rate of code calls following implementation of our MET was similar to that observed by others.17 Finally, our stratification of code calls inside critical care (where the MET was not deployed) and outside critical care, as well as the division of codes into cardiac arrest (where intervention is often unsuccessful) and other medical crises (primarily respiratory distress), gives further insight into how METs might work. As expected, we found that outside critical care only, codes called for medical crises declined, implying that the main effect of the MET was to provide early interventions for patients who were not likely to die anyway (eg, respiratory care for patients with respiratory distress or intravenous fluids for hypotensive patients). Instead of intervening to prevent death, MET may avoid emergent intubation by providing respiratory therapy and/or urgent intubation. In addition, it represents a less‐intense option for responding to nonlife‐threatening emergencies, such as seizures or syncope. As codes were no longer called for these types of crises, the rate of code calls necessarily fell. The reason that code calls declined inside critical care is less clear. It could be that patients transferred to critical care by the MET were less likely to code than those transferred before implementation, or the decline might be due to other factors that were not evaluated. Regardless, it is clear that the MET did not simply relocate codes to critical care units.
Our study has a number of limitations. First, it is an observational study and cannot account for other confounders relating to temporal trends in the hospital. However, our long time window allowed us to examine trends over several years. For 2 years prior to implementation of the MET, there was no decline at all in the rate of code calls, followed by an immediate and sustained drop after implementation. Other interventions, including ventilator‐associated pneumonia bundles, sepsis bundles, and advanced cardiac life support simulation training were also implemented at different times during the study period. However, the stark demarcation in code call rates coinciding with MET implementation makes it less likely that these other interventions were responsible for the observed decline. Second, our study was limited to a single institution and a single type of MET. Our findings may not apply to other types of institutions with different staffing arrangements or a different hospital culture, nor would they necessarily apply to different types of MET. Third, our nurse surveys were not collected anonymously, and this may have affected the nurses' responses. Finally, we did not collect physiological parameters on our patients, so we cannot state with certainty what the MET intervention accomplished.
Since initial studies suggested that METs could reduce hospital mortality rates, the Joint Commission has effectively mandated implementation of rapid response systems in all hospitals. Newer evidence, however, has been less convincing of mortality or other benefit. Our study adds to the literature in that we also did not find a mortality benefit. However, there were 2 clear benefits that we did identify. Our MET did appear to substantially reduce total numbers of code calls, particularly codes called for medical crises. Also, our nurses had a very positive response to the MET, which empowered them to get help for a patient when the patient's physician was unavailable or did not take their concerns seriously. Clearly, additional study is needed to better understand the effects of METs on mortality, codes, and other indicators of patient outcomes. However, in the current regulatory environment, such studies will be difficult to perform. Instead, additional studies can clarify which models deliver best outcomes and optimal use of our limited resources.
In‐hospital cardiopulmonary arrests are often preceded by signs of clinical instability, such as changes in vital signs or mental status.1 Nearly 85% of patients who suffer from cardiopulmonary arrests have documented observations of deterioration in the 8 hours before arrest.2 A Medical Emergency Team (MET), sometimes known as Rapid Response Team (RRT), can rapidly assess and manage unstable patients, with the goal that early intervention will prevent adverse outcomes. In 2004, the Institute for Healthcare Improvement (IHI), as part of its 100,000 Lives Campaign, called for hospitals to implement rapid response systems as 1 of 6 strategies to reduce deaths in hospital.3 Since this recommendation, hundreds of hospitals in the United States have invested substantial financial and personnel resources to implement some form of a rapid response system, which is comprised of a varying array of healthcare providers who bring critical care expertise to the patient's bedside.4, 5
Despite the intuitive appeal of the approach, and early observational data which suggested that METs could reduce both codes and unexpected in‐hospital mortality,2, 6 the largest randomized controlled trial found that METs failed to reduce unplanned intensive care unit (ICU) admissions, cardiac arrests, or unexpected deaths.7 More recently, in a prospective observational cohort study at 1 US hospital, Chan et al. found that a nurse‐led RRT did not impact hospital‐wide code rates or mortality.4
The study of rapid response systems is further complicated by a lack of standard definition, and the many types of hospitals in which they may be implemented. In 2006, a consensus conference suggested that MET be used to refer to teams led by physicians (usually intensivists), and RRT be used for teams led by nurses.8 Many studies have been conducted at non‐US institutions, and follow‐up periods have generally been 1 year or less. We report on almost 4 years of experience with a hospitalist‐led MET implemented in a major US academic medical center, and examine the subsequent changes in code calls, cardiac arrests, deaths following cardiopulmonary resuscitation, and overall hospital mortality. Because the MET did not operate in the critical care units, and because cardiac arrest may occur without prior signs of deterioration, we hypothesized that implementation of the MET would correspond to a small drop in total code calls, no change in codes called inside of critical care units, no change in cardiac arrest outside of critical care, and a marked drop in other medical crises (mostly respiratory distress) outside critical care. We also hypothesized that there would be no change in the rate of fatal codes, because most deaths occur in patients who were found to be pulseless on arrival of the code team.
METHODS
Setting
Beginning in March 2006, Baystate Medical Center implemented an MET in accordance with the recommendations of the IHI. Baystate is a 670‐bed tertiary care referral center in Springfield, Massachusetts, and a major teaching hospital for Tufts University. Throughout the study period, the hospital had full‐time intensivists and >90% of medical patients were cared for by hospitalists with 24‐hour coverage. As a result, a medical patient's attending physician or corresponding coverage was usually on site. In order to promote acceptance of the team as well as to maximize continuity of care, we constructed our MET to include a critical care nurse, a respiratory therapist, intravenous therapist, and the patient's physician (either attending or resident). Baystate staff members carry alpha‐numeric pagers, so attendings could be alerted to the fact that the MET had been activated by means of a text page. In the event that the patient's physician could not respond, an ICU physician served as a backup team member. The MET was implemented initially in March of 2006 on 2 medical floors, and over a period of 3 months was gradually expanded to cover the entire hospital. For surgical patients, the MET was led by the attending surgeon or appropriate resident. Educational efforts, including meetings, e‐mails, and posters, targeted nurses in particular, but anyone could summon the MET by calling the activation number posted on all ward telephones. Nurses were encouraged to activate the system for any of the following: heart rate (<40 and >130 beats per minute), systolic blood pressure (<90 mmHg), respiratory rate (<8 or >24 per minute), oxygen saturation (<90% despite supplemental oxygen), altered mental status, or simply concern that something is wrong. The MET implementation oversight committee met biweekly and made adjustments to the team composition and protocols using rapid Plan Do Study Act (PDSA) cycles. A full description of the implementation process has been published elsewhere.9
In addition to the MET, Baystate has a separate code team which can be activated for cardiovascular arrests via a call to a designated phone extension, which activates a page to the code team members and an overhead announcement. Code team members include the ICU medical resident and intern, a critical care nurse, an anesthesiologist, a respiratory therapist, a staff nurse, and the house supervisor. In response to the overhead announcement, doctors, nurses and students in the vicinity often respond as well. Prior to implementation of the MET, a code blue was the only level of immediate response available.
Data and Outcomes
The nurse attending a code blue or code completes a report form which becomes part of the permanent medical record. A copy of the report is reviewed by the Division of Healthcare Quality and housed in the Critical Care administrative offices. For this study, we reviewed all code reports from January 2004 through December 2009. For each report, we extracted the following information: the date, location (inside or outside of a critical care unit), whether the patient had a pulse on arrival of the team, and whether the patient survived to discharge. All activations of the code system were included, regardless of the patient's code status (ie, even if the code was called in error) or the reason for the code call. Patients were then aggregated to calculate the rate of codes called per calendar quarter, as well as the rates of codes called in and out of critical care and the rates of two subsets of code calls, namely cardiac arrests and other medical crises (eg, respiratory arrest or seizures).
MET members were also required to collect data on the reason for the MET call, as well as the response time, time of day and unit, duration of the call, whether the physician was present, whether the patient was transferred to critical care, and whether the patient survived to discharge. In addition, we surveyed the nursing staff directly after the call, asking the following questions: 1) Did the team arrive promptly? 2) Were the critical care nurse and respiratory therapist efficient and respectful? 3) Did you feel the patient's needs were addressed appropriately? 4) Did you feel supported by the MET? and 5) Would you call the MET again?
Statistical Analysis
Quarterly event rates per 1000 admissions were calculated for each outcome. Event rates were compared using piecewise Poisson regression10 with robust standard errors.11 We excluded the 2 quarterly periods (2006 Q1 and Q2) during which the MET was implemented. A piecewise Poisson regression model was chosen to facilitate estimation of: 1) change in code calls from immediately before implementation to immediately after; and 2) temporal trends in code calls before and after implementation. Each model was built with 1 pre‐implementation intercept (December 2005), and 1 post‐implementation intercept (July 2006), as well as 2 slopes, with time coded negatively before the intervention (ie, 2, 1, 0), and positively after (ie, 0, 1, 2). Linear contrasts tested for differences in each parameter. A significant difference in intercepts suggests a post‐intervention decrease in code call rates; a significant, negative post‐intervention slope suggests continuing decline in code call rates. Statistical inferences were aided with visual plots of predicted incidence rates for each quarter in the observation period, with 95% confidence intervals (CI) for each quarterly rate estimated by the delta method.12 Alpha was specified at 0.05 and all significance tests were 2‐sided. Analyses were conducted in Stata 11.1 for Windows ( 2010, StataCorp LP, College Station, TX).
RESULTS
Implementation of the MET
The MET was introduced in the first and second quarters of 2006, with 2717 calls logged through the end of 2009 (out of 154,382 admissions). The rate of MET calls increased during the first 6 months of implementation from 5.95 per 1000 admissions in the first quarter of the intervention, to 15.8 calls per 1000 admissions in the second quarter. Call rates peaked in the first half of 2009, at 20.9 calls per 1000 admissions, leveling off to 17.9 calls per 1000 admissions in the last half of 2009 (Figure 1). Of calls with time recorded, 40% occurred on the day shift, 35% on the evening shift, and 25% on the night shift. The most common reason to call the MET was respiratory distress (33%), followed by cardiovascular instability (25%), and neurological abnormality (20%). In 15% of cases, concern about a patient's condition prompted the nurse to call. Calls came primarily from medical floors (75%) and surgical units (20%). The median response time was 4 minutes (interquartile range [IQR], 2.8 to 5.2 minutes) with no meaningful trend during the study period. The median call duration was 50 minutes (IQR, 38 to 72 minutes). Again, there was no trend over time. The most common interventions were arterial blood gas, fluid resuscitation, and electrocardiogram (see Supporting Web Appendix Table 1 in the online version of this article). A physician was present at 52% of the calls in the first year, which rose to 93% of calls in the final year. Approximately 25% of calls resulted in the patient being transferred to a critical care unit. Staff evaluations were overwhelmingly positive. Nurses rated the teams on the following points: whether the critical care nurse and respiratory therapist were efficient and respectful (mean rating 98%, SD 5.6%); promptness (98%, SD 5.6%); whether the patient's needs were addressed appropriately (mean 98%, SD 4.2%); whether the nurse felt supported by the MET (99.5%, SD 1.7%); and whether they would call the MET again (99.7%, SD 1.4%).

Effect of MET on Code Calls and Mortality
Between January 2004 and December of 2009, the hospital case mix index remained constant, and there were a total of 1202 codes called. The majority (62%) took place outside of critical care units. Linear contrasts of pre‐piecewise and post‐piecewise intercepts revealed that overall code calls declined significantly between pre‐implementation and post‐implementation of the MET from 7.30 (95% CI 5.81, 9.16) codes called per 1000 admissions to 4.21 (95% CI 3.42, 5.18) calls per 1000 admissions (Figure 1; also see Supporting Web Appendix Table 2 in the online version of this article). Outside of critical care, code calls declined from 4.70 (95% CI 3.92, 5.63) before the MET was implemented to 3.11 (95% CI 2.44, 3.97) afterwards (Figure 2); this was due primarily to a decrease in medical crises, which averaged 3.29 events per 1000 admissions (95% CI 2.70, 4.02) before implementation and decreased to 1.72 (95% CI 1.28, 2.31) afterwards, whereas cardiac arrests did not change significantly (Figure 3). Following implementation, code calls within critical care also declined significantly, from 2.59 events per 1000 admissions (95% CI 1.82, 3.69) before to 1.24 events per 1000 admissions (95% CI 0.94, 1.63) afterwards. The change in codes called within critical care was smaller, however, and included reductions in both cardiac arrests ( 0.84 events, P = 0.01) and medical crises ( 0.55, P = 0.08). There was no significant change in the rate of fatal codes per 1000 admissions ( +0.06, P = 0.65) (Figure 4). Overall hospital mortality remained steady at 22.0 deaths per 1000 admissions throughout the study period.



DISCUSSION
In this report, we detail the implementation of a novel hospitalist‐led medical emergency team at a large academic medical center over a period of 4 years. The team, which consisted of the patient's physician, a critical care nurse, a respiratory therapist, and an intravenous therapist, achieved full implementation within 6 months, was well received by the nursing staff, and was associated with a 42% decrease in code calls hospital‐wide. Most of the overall reduction was due to a reduction in codes called for medical crises outside of critical care, accompanied by a lesser reduction in codes called for cardiac arrests and medical crises within critical care units. There was no significant effect on the rate of cardiac arrest outside critical care. More importantly, there was no change in the rate of fatal codes or overall hospital mortality.
The idea of early intervention to prevent deterioration among hospitalized patients appeals to the concept that an ounce of prevention is worth a pound of cure. Like many other preventive interventions, rapid response systems have not always delivered on this promise. Since several early reports from Australia2 suggested that medical emergency teams could reduce not only cardiopulmonary arrests, but overall hospital mortality, there has been a rapid proliferation in their implementation, spurred on by the IHI's 100,000 Lives Campaign, which incorporated rapid response systems as one of 6 hospital‐wide interventions aimed at reducing harm and mortality.13 Subsequent randomized trials have both reproduced and refuted the early observational results. A ward‐randomized trial within 1 British hospital found a 50% reduction in hospital mortality for wards assigned to have an RRT,14 while a cluster randomized trial conducted at 23 Australian hospitals found no difference in rates of cardiac arrest or mortality between hospitals implementing METs and those continuing with usual care.7 Interestingly, in the Australian trial, the rates of cardiac arrest and mortality declined for both groups compared to historical controls, an important limitation to observational trials. Reports from single‐institution observational trials are also divided between those that found a reduction in mortality following implementation and those that did not. A recent meta‐analysis reported that there was too much heterogeneity among these trials to reach a conclusion about the benefits of rapid response systems.15
Our study adds to this literature in several ways. First, our MET design, which included the patient's physician (as opposed to an intensive care physician), was different from those previously studied. Including the patient's physician increases the team's knowledge of the patient and disease, and may improve physician acceptance of METs. In addition, our study provides 4 full years of follow‐up. Second, our rate of MET activation (18 calls/1000 admissions) was 2 to 3 times higher than that seen in most other studies,16 thus, the lack of mortality benefit was not likely the result of underuse. Third, our hospital employs a large number of hospitalists whose continuous presence might be expected to attenuate the benefits of an MET. Indeed, our initial rate of codes (7.5/1000 admissions) was similar to the post‐intervention rate in other studies.4 Nevertheless, the decrease in the overall rate of code calls following implementation of our MET was similar to that observed by others.17 Finally, our stratification of code calls inside critical care (where the MET was not deployed) and outside critical care, as well as the division of codes into cardiac arrest (where intervention is often unsuccessful) and other medical crises (primarily respiratory distress), gives further insight into how METs might work. As expected, we found that outside critical care only, codes called for medical crises declined, implying that the main effect of the MET was to provide early interventions for patients who were not likely to die anyway (eg, respiratory care for patients with respiratory distress or intravenous fluids for hypotensive patients). Instead of intervening to prevent death, MET may avoid emergent intubation by providing respiratory therapy and/or urgent intubation. In addition, it represents a less‐intense option for responding to nonlife‐threatening emergencies, such as seizures or syncope. As codes were no longer called for these types of crises, the rate of code calls necessarily fell. The reason that code calls declined inside critical care is less clear. It could be that patients transferred to critical care by the MET were less likely to code than those transferred before implementation, or the decline might be due to other factors that were not evaluated. Regardless, it is clear that the MET did not simply relocate codes to critical care units.
Our study has a number of limitations. First, it is an observational study and cannot account for other confounders relating to temporal trends in the hospital. However, our long time window allowed us to examine trends over several years. For 2 years prior to implementation of the MET, there was no decline at all in the rate of code calls, followed by an immediate and sustained drop after implementation. Other interventions, including ventilator‐associated pneumonia bundles, sepsis bundles, and advanced cardiac life support simulation training were also implemented at different times during the study period. However, the stark demarcation in code call rates coinciding with MET implementation makes it less likely that these other interventions were responsible for the observed decline. Second, our study was limited to a single institution and a single type of MET. Our findings may not apply to other types of institutions with different staffing arrangements or a different hospital culture, nor would they necessarily apply to different types of MET. Third, our nurse surveys were not collected anonymously, and this may have affected the nurses' responses. Finally, we did not collect physiological parameters on our patients, so we cannot state with certainty what the MET intervention accomplished.
Since initial studies suggested that METs could reduce hospital mortality rates, the Joint Commission has effectively mandated implementation of rapid response systems in all hospitals. Newer evidence, however, has been less convincing of mortality or other benefit. Our study adds to the literature in that we also did not find a mortality benefit. However, there were 2 clear benefits that we did identify. Our MET did appear to substantially reduce total numbers of code calls, particularly codes called for medical crises. Also, our nurses had a very positive response to the MET, which empowered them to get help for a patient when the patient's physician was unavailable or did not take their concerns seriously. Clearly, additional study is needed to better understand the effects of METs on mortality, codes, and other indicators of patient outcomes. However, in the current regulatory environment, such studies will be difficult to perform. Instead, additional studies can clarify which models deliver best outcomes and optimal use of our limited resources.
- ,,,,,.Recognising clinical instability in hospital patients before cardiac arrest or unplanned admission to intensive care. A pilot study in a tertiary‐care hospital.Med J Aust.1999;171:22–25.
- ,,, et al.Rates of in‐hospital arrests, deaths and intensive care admissions: the effect of a medical emergency team.Med J Aust.2000;173:236–240.
- ,,,.The 100,000 Lives Campaign: setting a goal and a deadline for improving health care quality.JAMA.2006;295:324–327.
- ,,,,,.Hospital‐wide code rates and mortality before and after implementation of a rapid response team.JAMA.2008;300:2506–2513.
- ,,,,.Rapid response teams: do they make a difference?Dimens Crit Care Nurs.2007;26:253–262.
- ,,,,,.Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study.BMJ.2002;324:387–390.
- ,,, et al.Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365:2091–2097.
- ,,, et al.Findings of the first consensus conference on medical emergency teams.Crit Care Med.2006;34:2463–2478.
- ,.Implementation of a rapid response team: a success story.Crit Care Nurse.2009;29:66–76.
- .Practical Biostatistical Methods.Belmont, CA:Wadsworth Publishing;1995.
- ,,,.Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models.New York:Springer Science + Business Media;2005.
- .A note on the delta method.Am Stat.1992;46:27–29.
- ,.The 100,000 Lives Campaign: crystallizing standards of care for hospitals.Health Aff.2005;24:1560–1570.
- ,,, et al.Introducing critical care outreach: a ward‐randomised trial of phased introduction in a general hospital.Intensive Care Med.2004;30:1398–404.
- ,,,,,.Rapid response systems: a systematic review.Crit Care Med.2007;35:1238–1243.
- ,,,,.Effects of rapid response systems on clinical outcomes: systematic review and meta‐analysis.J Hosp Med.2007;2:422–432.
- ,,,,.Rapid response teams: a systematic review and meta‐analysis.Arch Intern Med.2010;170:18–26.
- ,,,,,.Recognising clinical instability in hospital patients before cardiac arrest or unplanned admission to intensive care. A pilot study in a tertiary‐care hospital.Med J Aust.1999;171:22–25.
- ,,, et al.Rates of in‐hospital arrests, deaths and intensive care admissions: the effect of a medical emergency team.Med J Aust.2000;173:236–240.
- ,,,.The 100,000 Lives Campaign: setting a goal and a deadline for improving health care quality.JAMA.2006;295:324–327.
- ,,,,,.Hospital‐wide code rates and mortality before and after implementation of a rapid response team.JAMA.2008;300:2506–2513.
- ,,,,.Rapid response teams: do they make a difference?Dimens Crit Care Nurs.2007;26:253–262.
- ,,,,,.Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study.BMJ.2002;324:387–390.
- ,,, et al.Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365:2091–2097.
- ,,, et al.Findings of the first consensus conference on medical emergency teams.Crit Care Med.2006;34:2463–2478.
- ,.Implementation of a rapid response team: a success story.Crit Care Nurse.2009;29:66–76.
- .Practical Biostatistical Methods.Belmont, CA:Wadsworth Publishing;1995.
- ,,,.Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models.New York:Springer Science + Business Media;2005.
- .A note on the delta method.Am Stat.1992;46:27–29.
- ,.The 100,000 Lives Campaign: crystallizing standards of care for hospitals.Health Aff.2005;24:1560–1570.
- ,,, et al.Introducing critical care outreach: a ward‐randomised trial of phased introduction in a general hospital.Intensive Care Med.2004;30:1398–404.
- ,,,,,.Rapid response systems: a systematic review.Crit Care Med.2007;35:1238–1243.
- ,,,,.Effects of rapid response systems on clinical outcomes: systematic review and meta‐analysis.J Hosp Med.2007;2:422–432.
- ,,,,.Rapid response teams: a systematic review and meta‐analysis.Arch Intern Med.2010;170:18–26.
Copyright © 2011 Society of Hospital Medicine
Risk Model for VTE
Venous thromboembolism (VTE) is a major source of morbidity and mortality for hospitalized patients. Among medical patients at the highest risk, as many as 15% can be expected to develop a VTE during their hospital stay1, 2; however, among general medical patients, the incidence of symptomatic VTE is less than 1%,1 and potentially as low as 0.3%.3 Thromboprophylaxis with subcutaneous heparin reduces the risk of VTE by approximately 50%,4 and is therefore recommended for medical patients at high risk. However, heparin also increases the risk of bleeding and thrombocytopenia and thus should be avoided for patients at low risk of VTE. Consequently, the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) recommends that all hospitalized medical patients receive a risk assessment for VTE.5
Certain disease states, including stroke, acute myocardial infarction, heart failure, respiratory disease, sepsis, and cancer, have been associated with increased risk for VTE, and, based on the inclusion criteria of several randomized trials, current American College of Chest Physicians (ACCP) guidelines recommend thromboprophylaxis for patients hospitalized with these diagnoses.2 However, evidence that these factors actually increase a patient's risk for VTE comes from studies of ambulatory patients and is often weak or conflicting. Existing risk‐stratification tools,6, 7 as well as the ACCP guidelines, have not been validated, and accordingly JCAHO does not specify how risk assessment should be conducted. In order to help clinicians better estimate the risk of VTE in medical patients and therefore to provide more targeted thromboprophylaxis, we examined a large cohort of patients with high‐risk diagnoses and created a risk stratification model.
Methods
Setting and Patients
We identified a retrospective cohort of patients discharged between January 1, 2004 and June 30, 2005 from 374 acute care facilities in the US that participated in Premier's Perspective, a database developed for measuring quality and healthcare utilization. Participating hospitals represent all regions of the US, and are generally similar in composition to US hospitals; however, in comparison to information contained in the American Hospital Association annual survey, Perspective hospitals are more likely to be located in the South and in urban areas. Available data elements include those derived from the uniform billing 04 form, such as sociodemographic information about each patient, their International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) diagnosis and procedure codes, as well as hospital and physician information. This information is supplemented with a date‐stamped log of all items and services billed to the patient or insurer, including diagnostic tests, medications, and other treatments. Permission to conduct the study was obtained from the Institutional Review Board at Baystate Medical Center.
We included all patients age 18 years at moderate‐to‐high risk of VTE according to the ACCP recommendations,8 based on a principal diagnosis of pneumonia, septicemia or respiratory failure with pneumonia, heart failure, chronic obstructive pulmonary disease (COPD), stroke, and urinary tract infection. Diagnoses were assessed using ICD‐9‐CM codes. Patients who were prescribed warfarin or therapeutic doses of heparin on hospital day 1 or 2, and those who received >1 therapeutic dose of heparin but otherwise did not fulfill criteria for VTE, were excluded because we could not evaluate whether they experienced a VTE event during hospitalization. We also excluded patients whose length of stay was <3 days, because our definition of hospital‐acquired VTE required treatment begun on day 3 or later, and those with an indication for anticoagulation other than VTE (eg, prosthetic cardiac valve or atrial fibrillation), because we could not reliably distinguish treatment for VTE from treatment of the underlying condition.
Risk Factors
For each patient, we extracted age, gender, race/ethnicity, and insurance status, principal diagnosis, comorbidities, and specialty of the attending physician. Comorbidities were identified from ICD‐9‐CM secondary diagnosis codes and Diagnosis Related Groups using Healthcare Cost and Utilization Project Comorbidity Software, version 3.1, based on the work of Elixhauser et al.9 We also assessed risk factors which have been previously linked to VTE: paralysis, cancer (metastatic, solid tumor, and lymphoma), chemotherapy/radiation, prior VTE, use of estrogens and estrogen modulators, inflammatory bowel disease, nephrotic syndrome, myeloproliferative disorders, obesity, smoking, central venous catheter, inherited or acquired thrombophilia, steroid use, mechanical ventilation, urinary catheter, decubitus ulcer, HMGco‐A reductase inhibitors, restraints, diabetes, varicose veins, and length‐of‐stay 6 days. These additional comorbidities were defined based on the presence of specific ICD‐9 codes, while use of HMG‐co‐A reductase inhibitors were identified from medication charge files. We also noted whether patients received anticoagulants, the dosages and days of administration, as well as intermittent pneumatic compression devices.
Identification of VTE
Because the presence of a secondary diagnosis of VTE in medical patients is not a reliable way of differentiating hospital‐acquired VTE from those present at the time of admission,10 subjects were considered to have experienced a hospital‐acquired VTE only if they underwent a diagnostic test for VTE (lower extremity ultrasound, venography, CT angiogram, ventilation‐perfusion scan, or pulmonary angiogram) on hospital day 3 or later, received treatment for VTE for at least 50% of the remaining hospital stay, or until initiation of warfarin or appearance of a complication (eg, transfusion or treatment for heparin‐induced thrombocytopenia) and were given a secondary diagnosis of VTE (ICD‐9 diagnoses 453.4, 453.40, 453.41, 453.42, 453.8, 453.9, 415.1, 415.11, 415.19). We considered the following to be treatments for VTE: intravenous unfractionated heparin, >60 mg of enoxaparin, 7500 mg of dalteparin, or placement of an inferior vena cava filter. In addition, patients who were readmitted within 30 days of discharge with a primary diagnosis of VTE were also considered to have developed a VTE as a complication of their previous hospital stay.
Statistical Analysis
Univariate predictors of VTE were assessed using chi‐square tests. We developed a multivariable logistic regression model for VTE on an 80% randomly selected subset of the eligible admissions (the derivation cohort) using all measured risk factors for VTE and selected interaction terms. Generalized estimating equations (GEE) models with a logit link (SAS PROC GENMOD) were used to account for the clustering of patients within hospitals. Initial models were stratified on VTE prophylaxis. Factors significant at P < 0.05 were retained. Parameter estimates derived from the model were used to compute individual VTE risk in the remaining 20% of the admissions (the validation cohort). Discrimination in the validation model was assessed by the c‐statistic, as well as the expected/observed ratio. Both cohorts were categorized by decile of risk, based on the probability distribution in the derivation cohort, and observed VTE events compared to those predicted by the model. All analyses were performed using the Statistical Analysis System (version 9.1, SAS Institute, Inc., Cary, NC).
Role of the Funding Source
This study was supported by a Clinical Scientist Development Award from the Doris Duke Charitable Foundation. The funding source had no role in the study design, analysis, or interpretation of the data.
Results
Our sample contained 242,738 patients, 194,198 (80%) assigned to the derivation set and 48,540 (20%) to the validation set. Patient characteristics were similar in both sets (Supporting Information Appendix Table 1). Most patients were over age 65, 59% were female, and 64% were white (Table 1). The most common primary diagnoses were pneumonia (33%) and congestive heart failure (19%). The most common comorbidities were hypertension (50%), diabetes (31%), chronic pulmonary disease (30%), and anemia (20%). Most patients were cared for by internists (54%) or family practitioners (21%), and 30% received some form of anticoagulant VTE prophylaxis (Table 2). Of patients with an ICD‐9 code for VTE during hospitalization, just over half lacked either diagnostic testing, treatment, or both, leaving 612 (0.25%) patients who fulfilled our criteria for VTE; an additional 440 (0.18%) were readmitted for VTE, for an overall incidence of 0.43%. Patients with a length of stay 6 days had an incidence of 0.79% vs 0.19% for patients with shorter stays.
| Total | No VTE | VTE | |||||
|---|---|---|---|---|---|---|---|
| Variable | N | % | N | % | N | % | P‐Value |
| Total | 242,738 | 100 | 241,686 | 100.0 | 1,052 | 100.0 | |
| Demographics | |||||||
| Age | 0.20 | ||||||
| 18‐49 | 31,065 | 12.8 | 30,952 | 12.8 | 113 | 10.7 | |
| 50‐64 | 51,309 | 21.1 | 51,083 | 21.1 | 226 | 21.5 | |
| 65‐74 | 51,230 | 21.1 | 50,993 | 21.1 | 237 | 22.5 | |
| 75+ | 109,134 | 45.0 | 108,658 | 45.0 | 476 | 45.2 | |
| Female | 142,910 | 58.9 | 142,330 | 58.9 | 580 | 55.1 | 0.01 |
| Race/ethnicity | 0.49 | ||||||
| White | 155,866 | 64.2 | 155,189 | 64.2 | 677 | 64.4 | |
| Black | 41,556 | 17.1 | 41,374 | 17.1 | 182 | 17.3 | |
| Hispanic | 9,809 | 4.0 | 9,776 | 4.0 | 33 | 3.1 | |
| Other | 35,507 | 14.6 | 35,347 | 14.6 | 160 | 15.2 | |
| Marital status | 0.28 | ||||||
| Married/life partner | 88,035 | 36.3 | 87,627 | 36.3 | 408 | 38.8 | |
| Single | 39,254 | 16.2 | 39,103 | 16.2 | 151 | 14.4 | |
| Separated/divorced | 23,492 | 9.7 | 23,394 | 9.7 | 98 | 9.3 | |
| Widowed | 58,669 | 24.2 | 58,426 | 24.2 | 243 | 23.1 | |
| Other | 33,288 | 13.7 | 33,136 | 13.7 | 152 | 14.4 | |
| Admission characteristics | |||||||
| Primary diagnosis | <0.001 | ||||||
| Community‐acquired pneumonia | 81,171 | 33.4 | 80,792 | 33.4 | 379 | 36.0 | |
| Septicemia | 7,643 | 3.2 | 7,568 | 3.1 | 75 | 7.1 | |
| Chronic obstructive pulmonary disease | 35,116 | 14.5 | 35,027 | 14.5 | 89 | 8.5 | |
| Respiratory failure | 7,098 | 2.9 | 7,012 | 2.9 | 86 | 8.2 | |
| Congestive heart failure | 46,503 | 19.2 | 46,336 | 19.2 | 167 | 15.9 | |
| Cardiovascular disease | 33,044 | 13.6 | 32,931 | 13.6 | 113 | 10.7 | |
| Urinary tract infection | 32,163 | 13.3 | 32,020 | 13.2 | 143 | 13.6 | |
| Insurance payer | 0.93 | ||||||
| Medicare traditional | 157,609 | 64.9 | 156,927 | 64.9 | 682 | 64.8 | |
| Medicare managed care | 10,649 | 4.4 | 10,597 | 4.4 | 52 | 4.9 | |
| Medicaid | 17,796 | 7.3 | 17,720 | 7.3 | 76 | 7.2 | |
| Private | 44,858 | 18.5 | 44,665 | 18.5 | 193 | 18.3 | |
| Self‐pay/uninsured/other | 11,826 | 4.9 | 11,777 | 4.9 | 49 | 4.7 | |
| Admitted from skilled nursing facility | 3,003 | 1.2 | 2,980 | 1.2 | 23 | 2.2 | 0.005 |
| Risk factors | |||||||
| Any VTE prophylaxis | 72,558 | 29.9 | 72,164 | 29.9 | 394 | 37.5 | <0.001 |
| Length of stay 6 days | 99,463 | 41.0 | 98,680 | 40.8 | 783 | 74.4 | <0.001 |
| Paralysis | 16,764 | 6.9 | 16,689 | 6.9 | 75 | 7.1 | 0.77 |
| Metastatic cancer | 5,013 | 2.1 | 4,928 | 2.0 | 85 | 8.1 | <0.001 |
| Solid tumor without metastasis | 25,127 | 10.4 | 24,995 | 10.3 | 132 | 12.5 | 0.02 |
| Lymphoma | 3,026 | 1.2 | 2,995 | 1.2 | 31 | 2.9 | <0.001 |
| Cancer chemotherapy/radiation | 1,254 | 0.5 | 1,231 | 0.5 | 23 | 2.2 | <0.001 |
| Prior venous thromboembolism | 2,945 | 1.2 | 2,926 | 1.2 | 19 | 1.8 | 0.08 |
| Estrogens | 4,819 | 2.0 | 4,807 | 2.0 | 12 | 1.1 | 0.05 |
| Estrogen modulators | 2,102 | 0.9 | 2,091 | 0.9 | 11 | 1.0 | 0.53 |
| Inflammatory bowel disease | 814 | 0.3 | 803 | 0.3 | 11 | 1.0 | <0.001 |
| Nephrotic syndrome | 520 | 0.2 | 517 | 0.2 | 3 | 0.3 | 0.62 |
| Myeloproliferative disorder | 1,983 | 0.8 | 1,973 | 0.8 | 10 | 1.0 | 0.63 |
| Obesity | 16,938 | 7.0 | 16,856 | 7.0 | 82 | 7.8 | 0.30 |
| Smoking | 35,386 | 14.6 | 35,284 | 14.6 | 102 | 9.7 | <0.001 |
| Central venous catheter | 14,754 | 6.1 | 14,525 | 6.0 | 229 | 21.8 | <0.001 |
| Inherited or acquired thrombophilia | 114 | 0.1 | 108 | 0.0 | 6 | 0.6 | <0.001 |
| Steroids | 82,606 | 34.0 | 82,185 | 34.0 | 421 | 40.0 | <0.001 |
| Mechanical ventilation | 13,347 | 5.5 | 13,167 | 5.4 | 180 | 17.1 | <0.001 |
| Urinary catheter | 39,080 | 16.1 | 38,816 | 16.1 | 264 | 25.1 | <0.001 |
| Decubitus ulcer | 6,829 | 2.8 | 6,776 | 2.8 | 53 | 5.0 | <0.001 |
| Statins use | 57,282 | 23.6 | 57,068 | 23.6 | 214 | 20.3 | 0.01 |
| Use of restraints | 5,970 | 2.5 | 5,914 | 2.4 | 56 | 5.3 | <0.001 |
| Diabetes mellitus | 75,103 | 30.9 | 74,799 | 30.9 | 304 | 28.9 | 0.15 |
| Varicose veins | 166 | 0.1 | 165 | 0.1 | 1 | 0.1 | 0.74 |
| Comorbidities | |||||||
| Hypertension | 120,606 | 49.7 | 120,126 | 49.7 | 480 | 45.6 | 0.008 |
| Congestive heart failure | 18,900 | 7.8 | 18,793 | 7.8 | 107 | 10.2 | 0.004 |
| Peripheral vascular disease | 16,705 | 6.9 | 16,639 | 6.9 | 66 | 6.3 | 0.43 |
| Valvular disease | 13,683 | 5.6 | 13,628 | 5.6 | 55 | 5.2 | 0.56 |
| Pulmonary circulation disease | 5,530 | 2.3 | 5,492 | 2.3 | 38 | 3.6 | 0.004 |
| Chronic pulmonary disease | 72,028 | 29.7 | 71,698 | 29.7 | 330 | 31.4 | 0.23 |
| Respiratory failure second diagnosis | 13,027 | 5.4 | 12,893 | 5.3 | 134 | 12.7 | <0.001 |
| Rheumatoid arthritis/collagen vascular disease | 7,090 | 2.9 | 7,050 | 2.9 | 40 | 3.8 | 0.09 |
| Deficiency anemias | 49,605 | 20.4 | 49,352 | 20.4 | 253 | 24.0 | 0.004 |
| Weight loss | 8,810 | 3.6 | 8,714 | 3.6 | 96 | 9.1 | <0.001 |
| Peptic ulcer disease bleeding | 4,736 | 2.0 | 4,723 | 2.0 | 13 | 1.2 | 0.09 |
| Chronic blood loss anemia | 2,354 | 1.0 | 2,338 | 1.0 | 16 | 1.5 | 0.07 |
| Hypothyroidism | 28,773 | 11.9 | 28,668 | 11.9 | 105 | 10.0 | 0.06 |
| Renal failure | 19,768 | 8.1 | 19,669 | 8.1 | 99 | 9.4 | 0.13 |
| Liver disease | 4,682 | 1.9 | 4,657 | 1.9 | 25 | 2.4 | 0.29 |
| Other neurological disorders | 33,094 | 13.6 | 32,905 | 13.6 | 189 | 18.0 | <0.001 |
| Psychoses | 9,330 | 3.8 | 9,283 | 3.8 | 47 | 4.5 | 0.29 |
| Depression | 25,561 | 10.5 | 25,442 | 10.5 | 119 | 11.3 | 0.41 |
| Alcohol abuse | 7,756 | 3.2 | 7,727 | 3.2 | 29 | 2.8 | 0.42 |
| Drug abuse | 4,336 | 1.8 | 4,318 | 1.8 | 18 | 1.7 | 0.85 |
| Acquired immune deficiency syndrome | 1,048 | 0.4 | 1,045 | 0.4 | 3 | 0.3 | 0.47 |
| Total | Derivation | Validation | |||||
|---|---|---|---|---|---|---|---|
| Variable | N | % | N | % | N | % | P‐Value |
| |||||||
| Total | 242,738 | 100 | 194,198 | 100 | 48,540 | 100 | |
| VTE prophylaxis | 0.97 | ||||||
| No prophylaxis | 170,180 | 70.1 | 136,153 | 70.1 | 34,027 | 70.1 | |
| Any prophylaxis | 72,558 | 29.9 | 58,045 | 29.9 | 14,513 | 29.9 | |
| Outcomes | |||||||
| ICD‐9 code for VTE | 1,304 | 0.5 | 1,025 | 0.5 | 279 | 0.6 | 0.21 |
| ICD‐9 code + diagnostic test | 989 | 0.4 | 777 | 0.4 | 212 | 0.4 | 0.26 |
| ICD‐9 code + diagnostic test + treatment for VTE | 612 | 0.3 | 471 | 0.2 | 141 | 0.3 | 0.06 |
| Readmission for VTE within 30 days | 446 | 0.2 | 363 | 0.2 | 83 | 0.2 | 0.46 |
| Total hospital‐acquired VTE | 1,052 | 0.4 | 829 | 0.4 | 223 | 0.5 | 0.33 |
| In‐hospital mortality | 8,019 | 3.3 | 6,403 | 3.3 | 1,616 | 3.3 | 0.72 |
| Any readmission within 30 days | 28,664 | 11.8 | 22,885 | 11.8 | 5,779 | 11.9 | 0.46 |
Risk factors for VTE
A large number of patient and hospital factors were associated with the development of VTE (Table 1). Due to the large sample size, even weak associations appear highly statistically significant. Compared to patients without VTE, those with VTE were more likely to have received VTE prophylaxis (37% vs 30%, P < 0.001). However, models of patients receiving prophylaxis and of patients not receiving prophylaxis produced similar odds ratios for the various risk factors (Supporting Information Appendix Table 2); therefore, the final model includes both patients who did, and did not, receive VTE prophylaxis. In the multivariable model (Supporting Information Appendix Table 3), age, length of stay, gender, primary diagnosis, cancer, inflammatory bowel disease, obesity, central venous catheter, inherited thrombophilia, steroid use, mechanical ventilation, active chemotherapy, and urinary catheters were all associated with VTE (Table 3). The strongest risk factors were length of stay 6 days (OR 3.22, 95% CI 2.73, 3.79), central venous catheter (OR 1.87, 95% CI 1.52, 2.29), inflammatory bowel disease (OR 3.11, 95% CI 1.59, 6.08), and inherited thrombophilia (OR 4.00, 95% CI 0.98, 16.40). In addition, there were important interactions between age and cancer; cancer was a strong risk factor among younger patients, but is not as strong a risk factor among older patients (OR compared to young patients without cancer was 4.62 (95% CI 2.72, 7.87) for those age 1849 years, and 3.64 (95% CI 2.52, 5.25) for those aged 5064 years).
| Risk Factor | OR | 95% CI |
|---|---|---|
| ||
| Any prophylaxis | 0.98 | (0.84, 1.14) |
| Female | 0.85 | (0.74, 0.98) |
| Length of stay 6 days | 3.22 | (2.73, 3.79) |
| Age* | ||
| 18‐49 years | 1 | Referent |
| 50‐64 years | 1.15 | (0.86, 1.56) |
| >65 years | 1.51 | (1.17, 1.96) |
| Primary diagnosis | ||
| Pneumonia | 1 | Referent |
| Chronic obstructive pulmonary disease | 0.57 | (0.44, 0.75) |
| Stroke | 0.84 | (0.66, 1.08) |
| Congestive heart failure | 0.86 | (0.70, 1.06) |
| Urinary tract infection | 1.19 | (0.95, 1.50) |
| Respiratory failure | 1.15 | (0.85, 1.55) |
| Septicemia | 1.11 | (0.82, 1.50) |
| Comorbidities | ||
| Inflammatory bowel disease | 3.11 | (1.59, 6.08) |
| Obesity | 1.28 | (0.99, 1.66) |
| Inherited thrombophilia | 4.00 | (0.98, 16.40) |
| Cancer | ||
| 18‐49 years | 4.62 | (2.72, 7.87) |
| 50‐64 years | 3.64 | (2.52, 5.25) |
| >65 years | 2.17 | (1.61, 2.92) |
| Treatments | ||
| Central venous catheter | 1.87 | (1.52, 2.29) |
| Mechanical ventilation | 1.61 | (1.27, 2.05) |
| Urinary catheter | 1.17 | (0.99, 1.38) |
| Chemotherapy | 1.71 | (1.03, 2.83) |
| Steroids | 1.22 | (1.04, 1.43) |
In the derivation set, the multivariable model produced deciles of mean predicted risk from 0.11% to 1.45%, while mean observed risk over the same deciles ranged from 0.12% to 1.42% (Figure 1). Within the validation cohort, the observed rate of VTE was 0.46% (223 cases among 48,543 subjects). The expected rate according to the model was 0.43% (expected/observed ratio: 0.93 [95% CI 0.82, 1.06]). Model discrimination measured by the c‐statistic in the validation set was 0.75 (95% CI 0.71, 0.78). The model produced deciles of mean predicted risk from 0.11% to 1.46%, with mean observed risk over the same deciles from 0.17% to 1.81%. Risk gradient was relatively flat across the first 6 deciles, began to rise at the seventh decile, and rose sharply in the highest one. Using a risk threshold of 1%, the model had a sensitivity of 28% and a specificity of 93%. In the validation set, this translated into a positive predictive value of 2.2% and a negative predictive value of 99.7%. Assuming that VTE prophylaxis has an efficacy of 50%, the number‐needed‐to‐treat to prevent one VTE among high‐risk patients (predicted risk >1%) would be 91. In contrast, providing prophylaxis to the entire validation sample would result in a number‐needed‐to‐treat of 435. Using a lower treatment threshold of 0.4% produced a positive predictive value of 1% and a negative predictive value of 99.8%. At this threshold, the model would detect 73% of patients with VTE and the number‐needed‐to‐treat to prevent one VTE would be 200.
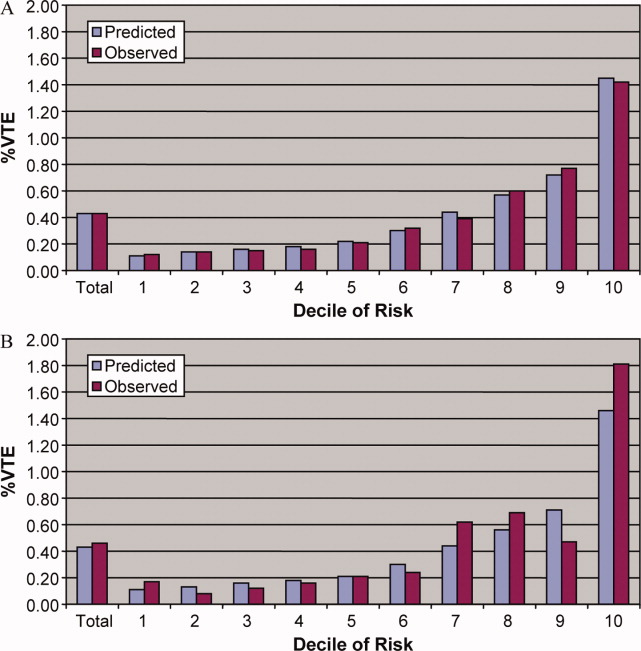
Discussion
In a representative sample of 243,000 hospitalized medical patients with at least one major risk factor for VTE, we found that symptomatic VTE was an uncommon event, occurring in approximately 1 in 231 patients. We identified a number of factors that were associated with an increased risk of VTE, but many previously cited risk factors did not show an association in multivariable models. In particular, patients with a primary diagnosis of COPD appeared not to share the same high risk of VTE as patients with the other diagnoses we examined, a finding reported by others.11 The risk model we developed accurately stratifies patients across a wide range of VTE probabilities, but even among those with the highest predicted rates, symptomatic VTE occurred in less than 2%.
VTE is often described as a frequent complication of hospitalization for medical illness and one of the most common potentially preventable causes of death. Indeed, rates of asymptomatic VTE have been demonstrated to be 3.7% to 26%.12 Although some of these might have fatal consequences, most are distal vein thromboses and their significance is unknown. In contrast, symptomatic events are uncommon, with previous estimates among general medical patients in observational studies in the range of 0.3%3 to 0.8%,12 similar to the rate observed in our study. Symptomatic event rates among control patients in landmark randomized trials have ranged from 0.86%13 to 2.3%,14 but these studies enrolled only very high‐risk patients with more extended hospitalizations, and may involve follow‐up periods of a month or more.
Because it is unlikely that our diagnostic algorithm was 100% sensitive, and because 30% of our patients received chemoprophylaxis, it is probable that we have underestimated the true rate of VTE in our sample. Among the patients who received prophylaxis, the observed rate of VTE was 0.54%. If we assume that prophylaxis is 50% effective, then had these patients not received prophylaxis, their rate of VTE would have been 1.08% (vs 0.39% among those patients who received no prophylaxis) and the overall rate of VTE for the sample would have been 0.60% (1.08 0.30 + 0.39 0.70). If we further assume that our algorithm was only 80% sensitive and 100% specific, the true underlying rate of symptomatic VTE could have been as high as 0.75%, still less than half that seen in randomized trials.
Prophylaxis with heparin has been shown to decrease the rate of both asymptomatic and symptomatic events, but because of the low prevalence, the number‐needed‐to‐treat to prevent one symptomatic pulmonary embolism has been estimated at 345, and prophylaxis has not been shown to affect all‐cause mortality.4, 15 At the same time, prophylaxis costs money, is uncomfortable, and carries a small risk of bleeding and heparin‐induced thrombocytopenia. Given the generally low incidence of symptomatic VTE, it therefore makes sense to reserve prophylaxis for patients at higher risk of thromboembolism.
To decide whether prophylaxis is appropriate for a given patient, it is necessary to quantify the patient's risk and then apply an appropriate threshold for treatment. The National Quality Forum (NQF) recommends,16 and JCAHO has adopted, that a clinician must evaluate each patient upon admission, and regularly thereafter, for the risk of developing DVT [deep vein thrombosis]/VTE. Until now, however, there has been no widely accepted, validated method to risk stratify medical patients. The ACCP recommendations cite just three studies of VTE risk factors in hospitalized medical patients.11, 17, 18 Together they examined 477 cases and 1197 controls, identifying congestive heart failure, pneumonia, cancer, and previous VTE as risk factors. Predictive models based on these factors17, 1921 have not been subjected to validation or have performed poorly.18 Acknowledging this lack of standardized risk assessment, JCAHO leaves the means of assessment to individual hospitals. A quality improvement guide published by the Agency for Healthcare Research and Quality goes one step further, stating that In a typical hospital, it is estimated that fewer than 5% of medical patients could be considered at low risk by most VTE risk stratification methods.22 The guide recommends near universal VTE prophylaxis.
In light of the JCAHO requirements, our model should be welcomed by hospitalists. Rather than assuming that all patients over 40 years of age are at high risk, our model will enable clinicians to risk stratify patients from a low of 0.1% to >1.4% (>10‐fold increase in risk). Moreover, the model was derived from more than 800 episodes of symptomatic VTE among almost 190,000 general medical patients and validated on almost 50,000 more. The observed patients were cared for in clinical practice at a nationally representative group of US hospitals, not in a highly selected clinical trial, increasing the generalizability of our findings. Finally, the model includes ten common risk factors that can easily be entered into decision support software or extracted automatically from the electronic medical record. Electronic reminder systems have already been shown to increase use of VTE prophylaxis, and prevent VTE, especially among cancer patients.23
A more challenging task is defining the appropriate risk threshold to initiate VTE prophylaxis. The Thromboembolic Risk Factors (THRIFT) Consensus Group classified patients according to risk of proximal DVT as low (<1%), moderate (1%‐10%), and high (>10%).21 They recommended heparin prophylaxis for all patients at moderate risk or higher. Although the patients included in our study all had a diagnosis that warranted prophylaxis according to the ACCP guidelines, using the THRIFT threshold for moderate‐to‐high risk, only 7% of our patients should have received prophylaxis. The recommendation not to offer heparin prophylaxis to patients with less than 1% chance of developing symptomatic VTE seems reasonable, given the large number‐needed‐to‐treat, but formal decision analyses should be conducted to better define this threshold. Many hospitalists, however, may feel uncomfortable using the 1% threshold, because our model failed to identify almost three out of four patients who ultimately experienced symptomatic VTE. At that threshold, it would seem that hospital‐acquired VTE is not a preventable complication in most medical patients, as others have pointed out.3, 24 Alternatively, if the threshold were lowered to 0.4%, our model could reduce the use of prophylaxis by 60%, while still identifying three‐fourths of all VTE cases. Further research is needed to know whether such a threshold is reasonable.
Our study has a number of important limitations. First, we relied on claims data, not chart review. We do not know for certain which patients experienced VTE, although our definition of VTE required diagnosis codes plus charges for both diagnosis and treatment. Moreover, our rates are similar to those observed in other trials where symptomatic events were confirmed. Second, about 30% of our patients received at least some VTE prophylaxis, and this may have prevented as many as half of the VTEs in that group. Without prophylaxis, rates might have been 20%30% higher. Similarly, we could not detect patients who were diagnosed after discharge but not admitted to hospital. While we believe this number to be small, it would again increase the rate slightly. Third, we could not assess certain clinical circumstances that are not associated with hospital charges or diagnosis codes, especially prolonged bed rest. Other risk factors, such as the urinary catheter, were probably surrogate markers for immobilization rather than true risk factors. Fourth, we included length of stay in our prediction model. We did this because most randomized trials of VTE prophylaxis included only patients with an expected length of stay 6 days. Physicians' estimates about probable length of stay may be less accurate than actual length of stay as a predictor of VTE. Moreover, the relationship may have been confounded if hospital‐acquired VTE led to longer lengths of stay. We think this unlikely since many of the events were discovered on readmission. Fifth, we studied only patients carrying high‐risk diagnoses, and therefore do not know the baseline risk for patients with less risky conditions, although it should be lower than what we observed. It seems probable that COPD, rather than being protective, as it appears in our model, actually represents the baseline risk for low‐risk diagnoses. It should be noted that we did include a number of other high‐risk diagnoses, such as cancer and inflammatory bowel disease, as secondary diagnoses. A larger, more inclusive study should be conducted to validate our model in other populations. Finally, we cannot know who died of undiagnosed VTE, either in the hospital or after discharge. Such an outcome would be important, but those events are likely to be rare, and VTE prophylaxis has not been shown to affect mortality.
VTE remains a daunting problem in hospitalized medical patients. Although VTE is responsible for a large number of hospital deaths each year, identifying patients at high risk for clinically important VTE is challenging, and may contribute to the persistently low rates of VTE prophylaxis seen in hospitals.25 Current efforts to treat nearly all patients are likely to lead to unnecessary cost, discomfort, and side effects. We present a simple logistic regression model that can easily identify patients at moderate‐to‐high risk (>1%) of developing symptomatic VTE. Future studies should focus on prospectively validating the model in a wider spectrum of medical illness, and better defining the appropriate risk cutoff for general prophylaxis.
Acknowledgements
The authors thank Aruna Priya, MS, for her help with some of the statistical analyses.
- ,,, et al.A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group.N Engl J Med.1999;341(11):793–800.
- ,,, et al.Prevention of venous thromboembolism: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th ed).Chest.2008;133(6 suppl):381S–453S.
- ,,.Thrombosis prophylaxis in hospitalised medical patients: does prophylaxis in all patients make sense?Neth J Med.2000;56(5):171–176.
- ,,,,.Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta‐analysis of randomized controlled trials.Arch Intern Med.2007;167(14):1476–1486.
- The Joint Commission on the Accreditation of Healthcare Organizations. Venous thromboembolism (VTE) core measure set. Available at: http://www. jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/VTE.htm. Accessed June 1,2009.
- ,,.Effective risk stratification of surgical and nonsurgical patients for venous thromboembolic disease.Semin Hematol.2001;38(2 suppl 5):12–19.
- ,,, et al.Assessment of venous thromboembolism risk and the benefits of thromboprophylaxis in medical patients.Thromb Haemost.2005;94(4):750–759.
- ,,, et al.Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126(3 suppl):338S–400S.
- ,,,.Comorbidity measures for use with administrative data.Med Care.1998;36(1):8–27.
- ,,,,,.Identification of in‐hospital complications from claims data. Is it valid?Med Care.2000;38(8):785–795.
- ,,, et al.Risk factors for venous thromboembolism in hospitalized patients with acute medical illness: analysis of the MEDENOX Study.Arch Intern Med.2004;164(9):963–968.
- ,,.The magnitude of an iatrogenic disorder: a systematic review of the incidence of venous thromboembolism for general medical inpatients.Thromb Haemost.2006;95(5):758–762.
- ,,,,,.Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients.Circulation.2004;110(7):874–879.
- .Randomised, controlled trial of low‐dose heparin for prevention of fatal pulmonary embolism in patients with infectious diseases. The Heparin Prophylaxis Study Group.Lancet.1996;347(9012):1357–1361.
- ,,,,.Meta‐analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients.Ann Intern Med.2007;146(4):278–288.
- National Quality Forum.National Voluntary Consensus Standards for Prevention and Care of Venous Thromboembolism: Policy, Preferred Practices, and Initial Performance Measures.Washington, DC;2006.
- ,,, et al.Risk factors for deep vein thrombosis in inpatients aged 65 and older: a case‐control multicenter study.J Am Geriatr Soc.2004;52(8):1299–1304.
- ,,.Risk factors for venous thrombosis in medical inpatients: validation of a thrombosis risk score.J Thromb Haemost.2004;2(12):2156–2161.
- ,,,,,.Venous thromboembolism prophylaxis and risk assessment in medical patients.Semin Thromb Hemost.1991;17(suppl 3):313–318.
- ,,, et al.A population‐based perspective of the hospital incidence and case‐fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study.Arch Intern Med.1991;151(5):933–938.
- Thromboembolic Risk Factors (THRIFT) Consensus Group.Risk of and prophylaxis for venous thromboembolism in hospital patients.BMJ.1992;305(6853):567–574.
- ,.Preventing Hospital‐Acquired Venous Thromboembolism: A Guide for Effective Quality Improvement. AHRQ Publication No. 08–0075.Rockville, MD:Agency for Healthcare Research and Quality;2008.
- ,,, et al.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352(10):969–977.
- ,,.Prophylaxis against venous thromboembolism.BMJ.1992;305(6862):1156.
- ,.Prevention of in‐hospital VTE: why can't we do better?Lancet.2008;371(9610):361–362.
Venous thromboembolism (VTE) is a major source of morbidity and mortality for hospitalized patients. Among medical patients at the highest risk, as many as 15% can be expected to develop a VTE during their hospital stay1, 2; however, among general medical patients, the incidence of symptomatic VTE is less than 1%,1 and potentially as low as 0.3%.3 Thromboprophylaxis with subcutaneous heparin reduces the risk of VTE by approximately 50%,4 and is therefore recommended for medical patients at high risk. However, heparin also increases the risk of bleeding and thrombocytopenia and thus should be avoided for patients at low risk of VTE. Consequently, the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) recommends that all hospitalized medical patients receive a risk assessment for VTE.5
Certain disease states, including stroke, acute myocardial infarction, heart failure, respiratory disease, sepsis, and cancer, have been associated with increased risk for VTE, and, based on the inclusion criteria of several randomized trials, current American College of Chest Physicians (ACCP) guidelines recommend thromboprophylaxis for patients hospitalized with these diagnoses.2 However, evidence that these factors actually increase a patient's risk for VTE comes from studies of ambulatory patients and is often weak or conflicting. Existing risk‐stratification tools,6, 7 as well as the ACCP guidelines, have not been validated, and accordingly JCAHO does not specify how risk assessment should be conducted. In order to help clinicians better estimate the risk of VTE in medical patients and therefore to provide more targeted thromboprophylaxis, we examined a large cohort of patients with high‐risk diagnoses and created a risk stratification model.
Methods
Setting and Patients
We identified a retrospective cohort of patients discharged between January 1, 2004 and June 30, 2005 from 374 acute care facilities in the US that participated in Premier's Perspective, a database developed for measuring quality and healthcare utilization. Participating hospitals represent all regions of the US, and are generally similar in composition to US hospitals; however, in comparison to information contained in the American Hospital Association annual survey, Perspective hospitals are more likely to be located in the South and in urban areas. Available data elements include those derived from the uniform billing 04 form, such as sociodemographic information about each patient, their International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) diagnosis and procedure codes, as well as hospital and physician information. This information is supplemented with a date‐stamped log of all items and services billed to the patient or insurer, including diagnostic tests, medications, and other treatments. Permission to conduct the study was obtained from the Institutional Review Board at Baystate Medical Center.
We included all patients age 18 years at moderate‐to‐high risk of VTE according to the ACCP recommendations,8 based on a principal diagnosis of pneumonia, septicemia or respiratory failure with pneumonia, heart failure, chronic obstructive pulmonary disease (COPD), stroke, and urinary tract infection. Diagnoses were assessed using ICD‐9‐CM codes. Patients who were prescribed warfarin or therapeutic doses of heparin on hospital day 1 or 2, and those who received >1 therapeutic dose of heparin but otherwise did not fulfill criteria for VTE, were excluded because we could not evaluate whether they experienced a VTE event during hospitalization. We also excluded patients whose length of stay was <3 days, because our definition of hospital‐acquired VTE required treatment begun on day 3 or later, and those with an indication for anticoagulation other than VTE (eg, prosthetic cardiac valve or atrial fibrillation), because we could not reliably distinguish treatment for VTE from treatment of the underlying condition.
Risk Factors
For each patient, we extracted age, gender, race/ethnicity, and insurance status, principal diagnosis, comorbidities, and specialty of the attending physician. Comorbidities were identified from ICD‐9‐CM secondary diagnosis codes and Diagnosis Related Groups using Healthcare Cost and Utilization Project Comorbidity Software, version 3.1, based on the work of Elixhauser et al.9 We also assessed risk factors which have been previously linked to VTE: paralysis, cancer (metastatic, solid tumor, and lymphoma), chemotherapy/radiation, prior VTE, use of estrogens and estrogen modulators, inflammatory bowel disease, nephrotic syndrome, myeloproliferative disorders, obesity, smoking, central venous catheter, inherited or acquired thrombophilia, steroid use, mechanical ventilation, urinary catheter, decubitus ulcer, HMGco‐A reductase inhibitors, restraints, diabetes, varicose veins, and length‐of‐stay 6 days. These additional comorbidities were defined based on the presence of specific ICD‐9 codes, while use of HMG‐co‐A reductase inhibitors were identified from medication charge files. We also noted whether patients received anticoagulants, the dosages and days of administration, as well as intermittent pneumatic compression devices.
Identification of VTE
Because the presence of a secondary diagnosis of VTE in medical patients is not a reliable way of differentiating hospital‐acquired VTE from those present at the time of admission,10 subjects were considered to have experienced a hospital‐acquired VTE only if they underwent a diagnostic test for VTE (lower extremity ultrasound, venography, CT angiogram, ventilation‐perfusion scan, or pulmonary angiogram) on hospital day 3 or later, received treatment for VTE for at least 50% of the remaining hospital stay, or until initiation of warfarin or appearance of a complication (eg, transfusion or treatment for heparin‐induced thrombocytopenia) and were given a secondary diagnosis of VTE (ICD‐9 diagnoses 453.4, 453.40, 453.41, 453.42, 453.8, 453.9, 415.1, 415.11, 415.19). We considered the following to be treatments for VTE: intravenous unfractionated heparin, >60 mg of enoxaparin, 7500 mg of dalteparin, or placement of an inferior vena cava filter. In addition, patients who were readmitted within 30 days of discharge with a primary diagnosis of VTE were also considered to have developed a VTE as a complication of their previous hospital stay.
Statistical Analysis
Univariate predictors of VTE were assessed using chi‐square tests. We developed a multivariable logistic regression model for VTE on an 80% randomly selected subset of the eligible admissions (the derivation cohort) using all measured risk factors for VTE and selected interaction terms. Generalized estimating equations (GEE) models with a logit link (SAS PROC GENMOD) were used to account for the clustering of patients within hospitals. Initial models were stratified on VTE prophylaxis. Factors significant at P < 0.05 were retained. Parameter estimates derived from the model were used to compute individual VTE risk in the remaining 20% of the admissions (the validation cohort). Discrimination in the validation model was assessed by the c‐statistic, as well as the expected/observed ratio. Both cohorts were categorized by decile of risk, based on the probability distribution in the derivation cohort, and observed VTE events compared to those predicted by the model. All analyses were performed using the Statistical Analysis System (version 9.1, SAS Institute, Inc., Cary, NC).
Role of the Funding Source
This study was supported by a Clinical Scientist Development Award from the Doris Duke Charitable Foundation. The funding source had no role in the study design, analysis, or interpretation of the data.
Results
Our sample contained 242,738 patients, 194,198 (80%) assigned to the derivation set and 48,540 (20%) to the validation set. Patient characteristics were similar in both sets (Supporting Information Appendix Table 1). Most patients were over age 65, 59% were female, and 64% were white (Table 1). The most common primary diagnoses were pneumonia (33%) and congestive heart failure (19%). The most common comorbidities were hypertension (50%), diabetes (31%), chronic pulmonary disease (30%), and anemia (20%). Most patients were cared for by internists (54%) or family practitioners (21%), and 30% received some form of anticoagulant VTE prophylaxis (Table 2). Of patients with an ICD‐9 code for VTE during hospitalization, just over half lacked either diagnostic testing, treatment, or both, leaving 612 (0.25%) patients who fulfilled our criteria for VTE; an additional 440 (0.18%) were readmitted for VTE, for an overall incidence of 0.43%. Patients with a length of stay 6 days had an incidence of 0.79% vs 0.19% for patients with shorter stays.
| Total | No VTE | VTE | |||||
|---|---|---|---|---|---|---|---|
| Variable | N | % | N | % | N | % | P‐Value |
| Total | 242,738 | 100 | 241,686 | 100.0 | 1,052 | 100.0 | |
| Demographics | |||||||
| Age | 0.20 | ||||||
| 18‐49 | 31,065 | 12.8 | 30,952 | 12.8 | 113 | 10.7 | |
| 50‐64 | 51,309 | 21.1 | 51,083 | 21.1 | 226 | 21.5 | |
| 65‐74 | 51,230 | 21.1 | 50,993 | 21.1 | 237 | 22.5 | |
| 75+ | 109,134 | 45.0 | 108,658 | 45.0 | 476 | 45.2 | |
| Female | 142,910 | 58.9 | 142,330 | 58.9 | 580 | 55.1 | 0.01 |
| Race/ethnicity | 0.49 | ||||||
| White | 155,866 | 64.2 | 155,189 | 64.2 | 677 | 64.4 | |
| Black | 41,556 | 17.1 | 41,374 | 17.1 | 182 | 17.3 | |
| Hispanic | 9,809 | 4.0 | 9,776 | 4.0 | 33 | 3.1 | |
| Other | 35,507 | 14.6 | 35,347 | 14.6 | 160 | 15.2 | |
| Marital status | 0.28 | ||||||
| Married/life partner | 88,035 | 36.3 | 87,627 | 36.3 | 408 | 38.8 | |
| Single | 39,254 | 16.2 | 39,103 | 16.2 | 151 | 14.4 | |
| Separated/divorced | 23,492 | 9.7 | 23,394 | 9.7 | 98 | 9.3 | |
| Widowed | 58,669 | 24.2 | 58,426 | 24.2 | 243 | 23.1 | |
| Other | 33,288 | 13.7 | 33,136 | 13.7 | 152 | 14.4 | |
| Admission characteristics | |||||||
| Primary diagnosis | <0.001 | ||||||
| Community‐acquired pneumonia | 81,171 | 33.4 | 80,792 | 33.4 | 379 | 36.0 | |
| Septicemia | 7,643 | 3.2 | 7,568 | 3.1 | 75 | 7.1 | |
| Chronic obstructive pulmonary disease | 35,116 | 14.5 | 35,027 | 14.5 | 89 | 8.5 | |
| Respiratory failure | 7,098 | 2.9 | 7,012 | 2.9 | 86 | 8.2 | |
| Congestive heart failure | 46,503 | 19.2 | 46,336 | 19.2 | 167 | 15.9 | |
| Cardiovascular disease | 33,044 | 13.6 | 32,931 | 13.6 | 113 | 10.7 | |
| Urinary tract infection | 32,163 | 13.3 | 32,020 | 13.2 | 143 | 13.6 | |
| Insurance payer | 0.93 | ||||||
| Medicare traditional | 157,609 | 64.9 | 156,927 | 64.9 | 682 | 64.8 | |
| Medicare managed care | 10,649 | 4.4 | 10,597 | 4.4 | 52 | 4.9 | |
| Medicaid | 17,796 | 7.3 | 17,720 | 7.3 | 76 | 7.2 | |
| Private | 44,858 | 18.5 | 44,665 | 18.5 | 193 | 18.3 | |
| Self‐pay/uninsured/other | 11,826 | 4.9 | 11,777 | 4.9 | 49 | 4.7 | |
| Admitted from skilled nursing facility | 3,003 | 1.2 | 2,980 | 1.2 | 23 | 2.2 | 0.005 |
| Risk factors | |||||||
| Any VTE prophylaxis | 72,558 | 29.9 | 72,164 | 29.9 | 394 | 37.5 | <0.001 |
| Length of stay 6 days | 99,463 | 41.0 | 98,680 | 40.8 | 783 | 74.4 | <0.001 |
| Paralysis | 16,764 | 6.9 | 16,689 | 6.9 | 75 | 7.1 | 0.77 |
| Metastatic cancer | 5,013 | 2.1 | 4,928 | 2.0 | 85 | 8.1 | <0.001 |
| Solid tumor without metastasis | 25,127 | 10.4 | 24,995 | 10.3 | 132 | 12.5 | 0.02 |
| Lymphoma | 3,026 | 1.2 | 2,995 | 1.2 | 31 | 2.9 | <0.001 |
| Cancer chemotherapy/radiation | 1,254 | 0.5 | 1,231 | 0.5 | 23 | 2.2 | <0.001 |
| Prior venous thromboembolism | 2,945 | 1.2 | 2,926 | 1.2 | 19 | 1.8 | 0.08 |
| Estrogens | 4,819 | 2.0 | 4,807 | 2.0 | 12 | 1.1 | 0.05 |
| Estrogen modulators | 2,102 | 0.9 | 2,091 | 0.9 | 11 | 1.0 | 0.53 |
| Inflammatory bowel disease | 814 | 0.3 | 803 | 0.3 | 11 | 1.0 | <0.001 |
| Nephrotic syndrome | 520 | 0.2 | 517 | 0.2 | 3 | 0.3 | 0.62 |
| Myeloproliferative disorder | 1,983 | 0.8 | 1,973 | 0.8 | 10 | 1.0 | 0.63 |
| Obesity | 16,938 | 7.0 | 16,856 | 7.0 | 82 | 7.8 | 0.30 |
| Smoking | 35,386 | 14.6 | 35,284 | 14.6 | 102 | 9.7 | <0.001 |
| Central venous catheter | 14,754 | 6.1 | 14,525 | 6.0 | 229 | 21.8 | <0.001 |
| Inherited or acquired thrombophilia | 114 | 0.1 | 108 | 0.0 | 6 | 0.6 | <0.001 |
| Steroids | 82,606 | 34.0 | 82,185 | 34.0 | 421 | 40.0 | <0.001 |
| Mechanical ventilation | 13,347 | 5.5 | 13,167 | 5.4 | 180 | 17.1 | <0.001 |
| Urinary catheter | 39,080 | 16.1 | 38,816 | 16.1 | 264 | 25.1 | <0.001 |
| Decubitus ulcer | 6,829 | 2.8 | 6,776 | 2.8 | 53 | 5.0 | <0.001 |
| Statins use | 57,282 | 23.6 | 57,068 | 23.6 | 214 | 20.3 | 0.01 |
| Use of restraints | 5,970 | 2.5 | 5,914 | 2.4 | 56 | 5.3 | <0.001 |
| Diabetes mellitus | 75,103 | 30.9 | 74,799 | 30.9 | 304 | 28.9 | 0.15 |
| Varicose veins | 166 | 0.1 | 165 | 0.1 | 1 | 0.1 | 0.74 |
| Comorbidities | |||||||
| Hypertension | 120,606 | 49.7 | 120,126 | 49.7 | 480 | 45.6 | 0.008 |
| Congestive heart failure | 18,900 | 7.8 | 18,793 | 7.8 | 107 | 10.2 | 0.004 |
| Peripheral vascular disease | 16,705 | 6.9 | 16,639 | 6.9 | 66 | 6.3 | 0.43 |
| Valvular disease | 13,683 | 5.6 | 13,628 | 5.6 | 55 | 5.2 | 0.56 |
| Pulmonary circulation disease | 5,530 | 2.3 | 5,492 | 2.3 | 38 | 3.6 | 0.004 |
| Chronic pulmonary disease | 72,028 | 29.7 | 71,698 | 29.7 | 330 | 31.4 | 0.23 |
| Respiratory failure second diagnosis | 13,027 | 5.4 | 12,893 | 5.3 | 134 | 12.7 | <0.001 |
| Rheumatoid arthritis/collagen vascular disease | 7,090 | 2.9 | 7,050 | 2.9 | 40 | 3.8 | 0.09 |
| Deficiency anemias | 49,605 | 20.4 | 49,352 | 20.4 | 253 | 24.0 | 0.004 |
| Weight loss | 8,810 | 3.6 | 8,714 | 3.6 | 96 | 9.1 | <0.001 |
| Peptic ulcer disease bleeding | 4,736 | 2.0 | 4,723 | 2.0 | 13 | 1.2 | 0.09 |
| Chronic blood loss anemia | 2,354 | 1.0 | 2,338 | 1.0 | 16 | 1.5 | 0.07 |
| Hypothyroidism | 28,773 | 11.9 | 28,668 | 11.9 | 105 | 10.0 | 0.06 |
| Renal failure | 19,768 | 8.1 | 19,669 | 8.1 | 99 | 9.4 | 0.13 |
| Liver disease | 4,682 | 1.9 | 4,657 | 1.9 | 25 | 2.4 | 0.29 |
| Other neurological disorders | 33,094 | 13.6 | 32,905 | 13.6 | 189 | 18.0 | <0.001 |
| Psychoses | 9,330 | 3.8 | 9,283 | 3.8 | 47 | 4.5 | 0.29 |
| Depression | 25,561 | 10.5 | 25,442 | 10.5 | 119 | 11.3 | 0.41 |
| Alcohol abuse | 7,756 | 3.2 | 7,727 | 3.2 | 29 | 2.8 | 0.42 |
| Drug abuse | 4,336 | 1.8 | 4,318 | 1.8 | 18 | 1.7 | 0.85 |
| Acquired immune deficiency syndrome | 1,048 | 0.4 | 1,045 | 0.4 | 3 | 0.3 | 0.47 |
| Total | Derivation | Validation | |||||
|---|---|---|---|---|---|---|---|
| Variable | N | % | N | % | N | % | P‐Value |
| |||||||
| Total | 242,738 | 100 | 194,198 | 100 | 48,540 | 100 | |
| VTE prophylaxis | 0.97 | ||||||
| No prophylaxis | 170,180 | 70.1 | 136,153 | 70.1 | 34,027 | 70.1 | |
| Any prophylaxis | 72,558 | 29.9 | 58,045 | 29.9 | 14,513 | 29.9 | |
| Outcomes | |||||||
| ICD‐9 code for VTE | 1,304 | 0.5 | 1,025 | 0.5 | 279 | 0.6 | 0.21 |
| ICD‐9 code + diagnostic test | 989 | 0.4 | 777 | 0.4 | 212 | 0.4 | 0.26 |
| ICD‐9 code + diagnostic test + treatment for VTE | 612 | 0.3 | 471 | 0.2 | 141 | 0.3 | 0.06 |
| Readmission for VTE within 30 days | 446 | 0.2 | 363 | 0.2 | 83 | 0.2 | 0.46 |
| Total hospital‐acquired VTE | 1,052 | 0.4 | 829 | 0.4 | 223 | 0.5 | 0.33 |
| In‐hospital mortality | 8,019 | 3.3 | 6,403 | 3.3 | 1,616 | 3.3 | 0.72 |
| Any readmission within 30 days | 28,664 | 11.8 | 22,885 | 11.8 | 5,779 | 11.9 | 0.46 |
Risk factors for VTE
A large number of patient and hospital factors were associated with the development of VTE (Table 1). Due to the large sample size, even weak associations appear highly statistically significant. Compared to patients without VTE, those with VTE were more likely to have received VTE prophylaxis (37% vs 30%, P < 0.001). However, models of patients receiving prophylaxis and of patients not receiving prophylaxis produced similar odds ratios for the various risk factors (Supporting Information Appendix Table 2); therefore, the final model includes both patients who did, and did not, receive VTE prophylaxis. In the multivariable model (Supporting Information Appendix Table 3), age, length of stay, gender, primary diagnosis, cancer, inflammatory bowel disease, obesity, central venous catheter, inherited thrombophilia, steroid use, mechanical ventilation, active chemotherapy, and urinary catheters were all associated with VTE (Table 3). The strongest risk factors were length of stay 6 days (OR 3.22, 95% CI 2.73, 3.79), central venous catheter (OR 1.87, 95% CI 1.52, 2.29), inflammatory bowel disease (OR 3.11, 95% CI 1.59, 6.08), and inherited thrombophilia (OR 4.00, 95% CI 0.98, 16.40). In addition, there were important interactions between age and cancer; cancer was a strong risk factor among younger patients, but is not as strong a risk factor among older patients (OR compared to young patients without cancer was 4.62 (95% CI 2.72, 7.87) for those age 1849 years, and 3.64 (95% CI 2.52, 5.25) for those aged 5064 years).
| Risk Factor | OR | 95% CI |
|---|---|---|
| ||
| Any prophylaxis | 0.98 | (0.84, 1.14) |
| Female | 0.85 | (0.74, 0.98) |
| Length of stay 6 days | 3.22 | (2.73, 3.79) |
| Age* | ||
| 18‐49 years | 1 | Referent |
| 50‐64 years | 1.15 | (0.86, 1.56) |
| >65 years | 1.51 | (1.17, 1.96) |
| Primary diagnosis | ||
| Pneumonia | 1 | Referent |
| Chronic obstructive pulmonary disease | 0.57 | (0.44, 0.75) |
| Stroke | 0.84 | (0.66, 1.08) |
| Congestive heart failure | 0.86 | (0.70, 1.06) |
| Urinary tract infection | 1.19 | (0.95, 1.50) |
| Respiratory failure | 1.15 | (0.85, 1.55) |
| Septicemia | 1.11 | (0.82, 1.50) |
| Comorbidities | ||
| Inflammatory bowel disease | 3.11 | (1.59, 6.08) |
| Obesity | 1.28 | (0.99, 1.66) |
| Inherited thrombophilia | 4.00 | (0.98, 16.40) |
| Cancer | ||
| 18‐49 years | 4.62 | (2.72, 7.87) |
| 50‐64 years | 3.64 | (2.52, 5.25) |
| >65 years | 2.17 | (1.61, 2.92) |
| Treatments | ||
| Central venous catheter | 1.87 | (1.52, 2.29) |
| Mechanical ventilation | 1.61 | (1.27, 2.05) |
| Urinary catheter | 1.17 | (0.99, 1.38) |
| Chemotherapy | 1.71 | (1.03, 2.83) |
| Steroids | 1.22 | (1.04, 1.43) |
In the derivation set, the multivariable model produced deciles of mean predicted risk from 0.11% to 1.45%, while mean observed risk over the same deciles ranged from 0.12% to 1.42% (Figure 1). Within the validation cohort, the observed rate of VTE was 0.46% (223 cases among 48,543 subjects). The expected rate according to the model was 0.43% (expected/observed ratio: 0.93 [95% CI 0.82, 1.06]). Model discrimination measured by the c‐statistic in the validation set was 0.75 (95% CI 0.71, 0.78). The model produced deciles of mean predicted risk from 0.11% to 1.46%, with mean observed risk over the same deciles from 0.17% to 1.81%. Risk gradient was relatively flat across the first 6 deciles, began to rise at the seventh decile, and rose sharply in the highest one. Using a risk threshold of 1%, the model had a sensitivity of 28% and a specificity of 93%. In the validation set, this translated into a positive predictive value of 2.2% and a negative predictive value of 99.7%. Assuming that VTE prophylaxis has an efficacy of 50%, the number‐needed‐to‐treat to prevent one VTE among high‐risk patients (predicted risk >1%) would be 91. In contrast, providing prophylaxis to the entire validation sample would result in a number‐needed‐to‐treat of 435. Using a lower treatment threshold of 0.4% produced a positive predictive value of 1% and a negative predictive value of 99.8%. At this threshold, the model would detect 73% of patients with VTE and the number‐needed‐to‐treat to prevent one VTE would be 200.

Discussion
In a representative sample of 243,000 hospitalized medical patients with at least one major risk factor for VTE, we found that symptomatic VTE was an uncommon event, occurring in approximately 1 in 231 patients. We identified a number of factors that were associated with an increased risk of VTE, but many previously cited risk factors did not show an association in multivariable models. In particular, patients with a primary diagnosis of COPD appeared not to share the same high risk of VTE as patients with the other diagnoses we examined, a finding reported by others.11 The risk model we developed accurately stratifies patients across a wide range of VTE probabilities, but even among those with the highest predicted rates, symptomatic VTE occurred in less than 2%.
VTE is often described as a frequent complication of hospitalization for medical illness and one of the most common potentially preventable causes of death. Indeed, rates of asymptomatic VTE have been demonstrated to be 3.7% to 26%.12 Although some of these might have fatal consequences, most are distal vein thromboses and their significance is unknown. In contrast, symptomatic events are uncommon, with previous estimates among general medical patients in observational studies in the range of 0.3%3 to 0.8%,12 similar to the rate observed in our study. Symptomatic event rates among control patients in landmark randomized trials have ranged from 0.86%13 to 2.3%,14 but these studies enrolled only very high‐risk patients with more extended hospitalizations, and may involve follow‐up periods of a month or more.
Because it is unlikely that our diagnostic algorithm was 100% sensitive, and because 30% of our patients received chemoprophylaxis, it is probable that we have underestimated the true rate of VTE in our sample. Among the patients who received prophylaxis, the observed rate of VTE was 0.54%. If we assume that prophylaxis is 50% effective, then had these patients not received prophylaxis, their rate of VTE would have been 1.08% (vs 0.39% among those patients who received no prophylaxis) and the overall rate of VTE for the sample would have been 0.60% (1.08 0.30 + 0.39 0.70). If we further assume that our algorithm was only 80% sensitive and 100% specific, the true underlying rate of symptomatic VTE could have been as high as 0.75%, still less than half that seen in randomized trials.
Prophylaxis with heparin has been shown to decrease the rate of both asymptomatic and symptomatic events, but because of the low prevalence, the number‐needed‐to‐treat to prevent one symptomatic pulmonary embolism has been estimated at 345, and prophylaxis has not been shown to affect all‐cause mortality.4, 15 At the same time, prophylaxis costs money, is uncomfortable, and carries a small risk of bleeding and heparin‐induced thrombocytopenia. Given the generally low incidence of symptomatic VTE, it therefore makes sense to reserve prophylaxis for patients at higher risk of thromboembolism.
To decide whether prophylaxis is appropriate for a given patient, it is necessary to quantify the patient's risk and then apply an appropriate threshold for treatment. The National Quality Forum (NQF) recommends,16 and JCAHO has adopted, that a clinician must evaluate each patient upon admission, and regularly thereafter, for the risk of developing DVT [deep vein thrombosis]/VTE. Until now, however, there has been no widely accepted, validated method to risk stratify medical patients. The ACCP recommendations cite just three studies of VTE risk factors in hospitalized medical patients.11, 17, 18 Together they examined 477 cases and 1197 controls, identifying congestive heart failure, pneumonia, cancer, and previous VTE as risk factors. Predictive models based on these factors17, 1921 have not been subjected to validation or have performed poorly.18 Acknowledging this lack of standardized risk assessment, JCAHO leaves the means of assessment to individual hospitals. A quality improvement guide published by the Agency for Healthcare Research and Quality goes one step further, stating that In a typical hospital, it is estimated that fewer than 5% of medical patients could be considered at low risk by most VTE risk stratification methods.22 The guide recommends near universal VTE prophylaxis.
In light of the JCAHO requirements, our model should be welcomed by hospitalists. Rather than assuming that all patients over 40 years of age are at high risk, our model will enable clinicians to risk stratify patients from a low of 0.1% to >1.4% (>10‐fold increase in risk). Moreover, the model was derived from more than 800 episodes of symptomatic VTE among almost 190,000 general medical patients and validated on almost 50,000 more. The observed patients were cared for in clinical practice at a nationally representative group of US hospitals, not in a highly selected clinical trial, increasing the generalizability of our findings. Finally, the model includes ten common risk factors that can easily be entered into decision support software or extracted automatically from the electronic medical record. Electronic reminder systems have already been shown to increase use of VTE prophylaxis, and prevent VTE, especially among cancer patients.23
A more challenging task is defining the appropriate risk threshold to initiate VTE prophylaxis. The Thromboembolic Risk Factors (THRIFT) Consensus Group classified patients according to risk of proximal DVT as low (<1%), moderate (1%‐10%), and high (>10%).21 They recommended heparin prophylaxis for all patients at moderate risk or higher. Although the patients included in our study all had a diagnosis that warranted prophylaxis according to the ACCP guidelines, using the THRIFT threshold for moderate‐to‐high risk, only 7% of our patients should have received prophylaxis. The recommendation not to offer heparin prophylaxis to patients with less than 1% chance of developing symptomatic VTE seems reasonable, given the large number‐needed‐to‐treat, but formal decision analyses should be conducted to better define this threshold. Many hospitalists, however, may feel uncomfortable using the 1% threshold, because our model failed to identify almost three out of four patients who ultimately experienced symptomatic VTE. At that threshold, it would seem that hospital‐acquired VTE is not a preventable complication in most medical patients, as others have pointed out.3, 24 Alternatively, if the threshold were lowered to 0.4%, our model could reduce the use of prophylaxis by 60%, while still identifying three‐fourths of all VTE cases. Further research is needed to know whether such a threshold is reasonable.
Our study has a number of important limitations. First, we relied on claims data, not chart review. We do not know for certain which patients experienced VTE, although our definition of VTE required diagnosis codes plus charges for both diagnosis and treatment. Moreover, our rates are similar to those observed in other trials where symptomatic events were confirmed. Second, about 30% of our patients received at least some VTE prophylaxis, and this may have prevented as many as half of the VTEs in that group. Without prophylaxis, rates might have been 20%30% higher. Similarly, we could not detect patients who were diagnosed after discharge but not admitted to hospital. While we believe this number to be small, it would again increase the rate slightly. Third, we could not assess certain clinical circumstances that are not associated with hospital charges or diagnosis codes, especially prolonged bed rest. Other risk factors, such as the urinary catheter, were probably surrogate markers for immobilization rather than true risk factors. Fourth, we included length of stay in our prediction model. We did this because most randomized trials of VTE prophylaxis included only patients with an expected length of stay 6 days. Physicians' estimates about probable length of stay may be less accurate than actual length of stay as a predictor of VTE. Moreover, the relationship may have been confounded if hospital‐acquired VTE led to longer lengths of stay. We think this unlikely since many of the events were discovered on readmission. Fifth, we studied only patients carrying high‐risk diagnoses, and therefore do not know the baseline risk for patients with less risky conditions, although it should be lower than what we observed. It seems probable that COPD, rather than being protective, as it appears in our model, actually represents the baseline risk for low‐risk diagnoses. It should be noted that we did include a number of other high‐risk diagnoses, such as cancer and inflammatory bowel disease, as secondary diagnoses. A larger, more inclusive study should be conducted to validate our model in other populations. Finally, we cannot know who died of undiagnosed VTE, either in the hospital or after discharge. Such an outcome would be important, but those events are likely to be rare, and VTE prophylaxis has not been shown to affect mortality.
VTE remains a daunting problem in hospitalized medical patients. Although VTE is responsible for a large number of hospital deaths each year, identifying patients at high risk for clinically important VTE is challenging, and may contribute to the persistently low rates of VTE prophylaxis seen in hospitals.25 Current efforts to treat nearly all patients are likely to lead to unnecessary cost, discomfort, and side effects. We present a simple logistic regression model that can easily identify patients at moderate‐to‐high risk (>1%) of developing symptomatic VTE. Future studies should focus on prospectively validating the model in a wider spectrum of medical illness, and better defining the appropriate risk cutoff for general prophylaxis.
Acknowledgements
The authors thank Aruna Priya, MS, for her help with some of the statistical analyses.
Venous thromboembolism (VTE) is a major source of morbidity and mortality for hospitalized patients. Among medical patients at the highest risk, as many as 15% can be expected to develop a VTE during their hospital stay1, 2; however, among general medical patients, the incidence of symptomatic VTE is less than 1%,1 and potentially as low as 0.3%.3 Thromboprophylaxis with subcutaneous heparin reduces the risk of VTE by approximately 50%,4 and is therefore recommended for medical patients at high risk. However, heparin also increases the risk of bleeding and thrombocytopenia and thus should be avoided for patients at low risk of VTE. Consequently, the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) recommends that all hospitalized medical patients receive a risk assessment for VTE.5
Certain disease states, including stroke, acute myocardial infarction, heart failure, respiratory disease, sepsis, and cancer, have been associated with increased risk for VTE, and, based on the inclusion criteria of several randomized trials, current American College of Chest Physicians (ACCP) guidelines recommend thromboprophylaxis for patients hospitalized with these diagnoses.2 However, evidence that these factors actually increase a patient's risk for VTE comes from studies of ambulatory patients and is often weak or conflicting. Existing risk‐stratification tools,6, 7 as well as the ACCP guidelines, have not been validated, and accordingly JCAHO does not specify how risk assessment should be conducted. In order to help clinicians better estimate the risk of VTE in medical patients and therefore to provide more targeted thromboprophylaxis, we examined a large cohort of patients with high‐risk diagnoses and created a risk stratification model.
Methods
Setting and Patients
We identified a retrospective cohort of patients discharged between January 1, 2004 and June 30, 2005 from 374 acute care facilities in the US that participated in Premier's Perspective, a database developed for measuring quality and healthcare utilization. Participating hospitals represent all regions of the US, and are generally similar in composition to US hospitals; however, in comparison to information contained in the American Hospital Association annual survey, Perspective hospitals are more likely to be located in the South and in urban areas. Available data elements include those derived from the uniform billing 04 form, such as sociodemographic information about each patient, their International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) diagnosis and procedure codes, as well as hospital and physician information. This information is supplemented with a date‐stamped log of all items and services billed to the patient or insurer, including diagnostic tests, medications, and other treatments. Permission to conduct the study was obtained from the Institutional Review Board at Baystate Medical Center.
We included all patients age 18 years at moderate‐to‐high risk of VTE according to the ACCP recommendations,8 based on a principal diagnosis of pneumonia, septicemia or respiratory failure with pneumonia, heart failure, chronic obstructive pulmonary disease (COPD), stroke, and urinary tract infection. Diagnoses were assessed using ICD‐9‐CM codes. Patients who were prescribed warfarin or therapeutic doses of heparin on hospital day 1 or 2, and those who received >1 therapeutic dose of heparin but otherwise did not fulfill criteria for VTE, were excluded because we could not evaluate whether they experienced a VTE event during hospitalization. We also excluded patients whose length of stay was <3 days, because our definition of hospital‐acquired VTE required treatment begun on day 3 or later, and those with an indication for anticoagulation other than VTE (eg, prosthetic cardiac valve or atrial fibrillation), because we could not reliably distinguish treatment for VTE from treatment of the underlying condition.
Risk Factors
For each patient, we extracted age, gender, race/ethnicity, and insurance status, principal diagnosis, comorbidities, and specialty of the attending physician. Comorbidities were identified from ICD‐9‐CM secondary diagnosis codes and Diagnosis Related Groups using Healthcare Cost and Utilization Project Comorbidity Software, version 3.1, based on the work of Elixhauser et al.9 We also assessed risk factors which have been previously linked to VTE: paralysis, cancer (metastatic, solid tumor, and lymphoma), chemotherapy/radiation, prior VTE, use of estrogens and estrogen modulators, inflammatory bowel disease, nephrotic syndrome, myeloproliferative disorders, obesity, smoking, central venous catheter, inherited or acquired thrombophilia, steroid use, mechanical ventilation, urinary catheter, decubitus ulcer, HMGco‐A reductase inhibitors, restraints, diabetes, varicose veins, and length‐of‐stay 6 days. These additional comorbidities were defined based on the presence of specific ICD‐9 codes, while use of HMG‐co‐A reductase inhibitors were identified from medication charge files. We also noted whether patients received anticoagulants, the dosages and days of administration, as well as intermittent pneumatic compression devices.
Identification of VTE
Because the presence of a secondary diagnosis of VTE in medical patients is not a reliable way of differentiating hospital‐acquired VTE from those present at the time of admission,10 subjects were considered to have experienced a hospital‐acquired VTE only if they underwent a diagnostic test for VTE (lower extremity ultrasound, venography, CT angiogram, ventilation‐perfusion scan, or pulmonary angiogram) on hospital day 3 or later, received treatment for VTE for at least 50% of the remaining hospital stay, or until initiation of warfarin or appearance of a complication (eg, transfusion or treatment for heparin‐induced thrombocytopenia) and were given a secondary diagnosis of VTE (ICD‐9 diagnoses 453.4, 453.40, 453.41, 453.42, 453.8, 453.9, 415.1, 415.11, 415.19). We considered the following to be treatments for VTE: intravenous unfractionated heparin, >60 mg of enoxaparin, 7500 mg of dalteparin, or placement of an inferior vena cava filter. In addition, patients who were readmitted within 30 days of discharge with a primary diagnosis of VTE were also considered to have developed a VTE as a complication of their previous hospital stay.
Statistical Analysis
Univariate predictors of VTE were assessed using chi‐square tests. We developed a multivariable logistic regression model for VTE on an 80% randomly selected subset of the eligible admissions (the derivation cohort) using all measured risk factors for VTE and selected interaction terms. Generalized estimating equations (GEE) models with a logit link (SAS PROC GENMOD) were used to account for the clustering of patients within hospitals. Initial models were stratified on VTE prophylaxis. Factors significant at P < 0.05 were retained. Parameter estimates derived from the model were used to compute individual VTE risk in the remaining 20% of the admissions (the validation cohort). Discrimination in the validation model was assessed by the c‐statistic, as well as the expected/observed ratio. Both cohorts were categorized by decile of risk, based on the probability distribution in the derivation cohort, and observed VTE events compared to those predicted by the model. All analyses were performed using the Statistical Analysis System (version 9.1, SAS Institute, Inc., Cary, NC).
Role of the Funding Source
This study was supported by a Clinical Scientist Development Award from the Doris Duke Charitable Foundation. The funding source had no role in the study design, analysis, or interpretation of the data.
Results
Our sample contained 242,738 patients, 194,198 (80%) assigned to the derivation set and 48,540 (20%) to the validation set. Patient characteristics were similar in both sets (Supporting Information Appendix Table 1). Most patients were over age 65, 59% were female, and 64% were white (Table 1). The most common primary diagnoses were pneumonia (33%) and congestive heart failure (19%). The most common comorbidities were hypertension (50%), diabetes (31%), chronic pulmonary disease (30%), and anemia (20%). Most patients were cared for by internists (54%) or family practitioners (21%), and 30% received some form of anticoagulant VTE prophylaxis (Table 2). Of patients with an ICD‐9 code for VTE during hospitalization, just over half lacked either diagnostic testing, treatment, or both, leaving 612 (0.25%) patients who fulfilled our criteria for VTE; an additional 440 (0.18%) were readmitted for VTE, for an overall incidence of 0.43%. Patients with a length of stay 6 days had an incidence of 0.79% vs 0.19% for patients with shorter stays.
| Total | No VTE | VTE | |||||
|---|---|---|---|---|---|---|---|
| Variable | N | % | N | % | N | % | P‐Value |
| Total | 242,738 | 100 | 241,686 | 100.0 | 1,052 | 100.0 | |
| Demographics | |||||||
| Age | 0.20 | ||||||
| 18‐49 | 31,065 | 12.8 | 30,952 | 12.8 | 113 | 10.7 | |
| 50‐64 | 51,309 | 21.1 | 51,083 | 21.1 | 226 | 21.5 | |
| 65‐74 | 51,230 | 21.1 | 50,993 | 21.1 | 237 | 22.5 | |
| 75+ | 109,134 | 45.0 | 108,658 | 45.0 | 476 | 45.2 | |
| Female | 142,910 | 58.9 | 142,330 | 58.9 | 580 | 55.1 | 0.01 |
| Race/ethnicity | 0.49 | ||||||
| White | 155,866 | 64.2 | 155,189 | 64.2 | 677 | 64.4 | |
| Black | 41,556 | 17.1 | 41,374 | 17.1 | 182 | 17.3 | |
| Hispanic | 9,809 | 4.0 | 9,776 | 4.0 | 33 | 3.1 | |
| Other | 35,507 | 14.6 | 35,347 | 14.6 | 160 | 15.2 | |
| Marital status | 0.28 | ||||||
| Married/life partner | 88,035 | 36.3 | 87,627 | 36.3 | 408 | 38.8 | |
| Single | 39,254 | 16.2 | 39,103 | 16.2 | 151 | 14.4 | |
| Separated/divorced | 23,492 | 9.7 | 23,394 | 9.7 | 98 | 9.3 | |
| Widowed | 58,669 | 24.2 | 58,426 | 24.2 | 243 | 23.1 | |
| Other | 33,288 | 13.7 | 33,136 | 13.7 | 152 | 14.4 | |
| Admission characteristics | |||||||
| Primary diagnosis | <0.001 | ||||||
| Community‐acquired pneumonia | 81,171 | 33.4 | 80,792 | 33.4 | 379 | 36.0 | |
| Septicemia | 7,643 | 3.2 | 7,568 | 3.1 | 75 | 7.1 | |
| Chronic obstructive pulmonary disease | 35,116 | 14.5 | 35,027 | 14.5 | 89 | 8.5 | |
| Respiratory failure | 7,098 | 2.9 | 7,012 | 2.9 | 86 | 8.2 | |
| Congestive heart failure | 46,503 | 19.2 | 46,336 | 19.2 | 167 | 15.9 | |
| Cardiovascular disease | 33,044 | 13.6 | 32,931 | 13.6 | 113 | 10.7 | |
| Urinary tract infection | 32,163 | 13.3 | 32,020 | 13.2 | 143 | 13.6 | |
| Insurance payer | 0.93 | ||||||
| Medicare traditional | 157,609 | 64.9 | 156,927 | 64.9 | 682 | 64.8 | |
| Medicare managed care | 10,649 | 4.4 | 10,597 | 4.4 | 52 | 4.9 | |
| Medicaid | 17,796 | 7.3 | 17,720 | 7.3 | 76 | 7.2 | |
| Private | 44,858 | 18.5 | 44,665 | 18.5 | 193 | 18.3 | |
| Self‐pay/uninsured/other | 11,826 | 4.9 | 11,777 | 4.9 | 49 | 4.7 | |
| Admitted from skilled nursing facility | 3,003 | 1.2 | 2,980 | 1.2 | 23 | 2.2 | 0.005 |
| Risk factors | |||||||
| Any VTE prophylaxis | 72,558 | 29.9 | 72,164 | 29.9 | 394 | 37.5 | <0.001 |
| Length of stay 6 days | 99,463 | 41.0 | 98,680 | 40.8 | 783 | 74.4 | <0.001 |
| Paralysis | 16,764 | 6.9 | 16,689 | 6.9 | 75 | 7.1 | 0.77 |
| Metastatic cancer | 5,013 | 2.1 | 4,928 | 2.0 | 85 | 8.1 | <0.001 |
| Solid tumor without metastasis | 25,127 | 10.4 | 24,995 | 10.3 | 132 | 12.5 | 0.02 |
| Lymphoma | 3,026 | 1.2 | 2,995 | 1.2 | 31 | 2.9 | <0.001 |
| Cancer chemotherapy/radiation | 1,254 | 0.5 | 1,231 | 0.5 | 23 | 2.2 | <0.001 |
| Prior venous thromboembolism | 2,945 | 1.2 | 2,926 | 1.2 | 19 | 1.8 | 0.08 |
| Estrogens | 4,819 | 2.0 | 4,807 | 2.0 | 12 | 1.1 | 0.05 |
| Estrogen modulators | 2,102 | 0.9 | 2,091 | 0.9 | 11 | 1.0 | 0.53 |
| Inflammatory bowel disease | 814 | 0.3 | 803 | 0.3 | 11 | 1.0 | <0.001 |
| Nephrotic syndrome | 520 | 0.2 | 517 | 0.2 | 3 | 0.3 | 0.62 |
| Myeloproliferative disorder | 1,983 | 0.8 | 1,973 | 0.8 | 10 | 1.0 | 0.63 |
| Obesity | 16,938 | 7.0 | 16,856 | 7.0 | 82 | 7.8 | 0.30 |
| Smoking | 35,386 | 14.6 | 35,284 | 14.6 | 102 | 9.7 | <0.001 |
| Central venous catheter | 14,754 | 6.1 | 14,525 | 6.0 | 229 | 21.8 | <0.001 |
| Inherited or acquired thrombophilia | 114 | 0.1 | 108 | 0.0 | 6 | 0.6 | <0.001 |
| Steroids | 82,606 | 34.0 | 82,185 | 34.0 | 421 | 40.0 | <0.001 |
| Mechanical ventilation | 13,347 | 5.5 | 13,167 | 5.4 | 180 | 17.1 | <0.001 |
| Urinary catheter | 39,080 | 16.1 | 38,816 | 16.1 | 264 | 25.1 | <0.001 |
| Decubitus ulcer | 6,829 | 2.8 | 6,776 | 2.8 | 53 | 5.0 | <0.001 |
| Statins use | 57,282 | 23.6 | 57,068 | 23.6 | 214 | 20.3 | 0.01 |
| Use of restraints | 5,970 | 2.5 | 5,914 | 2.4 | 56 | 5.3 | <0.001 |
| Diabetes mellitus | 75,103 | 30.9 | 74,799 | 30.9 | 304 | 28.9 | 0.15 |
| Varicose veins | 166 | 0.1 | 165 | 0.1 | 1 | 0.1 | 0.74 |
| Comorbidities | |||||||
| Hypertension | 120,606 | 49.7 | 120,126 | 49.7 | 480 | 45.6 | 0.008 |
| Congestive heart failure | 18,900 | 7.8 | 18,793 | 7.8 | 107 | 10.2 | 0.004 |
| Peripheral vascular disease | 16,705 | 6.9 | 16,639 | 6.9 | 66 | 6.3 | 0.43 |
| Valvular disease | 13,683 | 5.6 | 13,628 | 5.6 | 55 | 5.2 | 0.56 |
| Pulmonary circulation disease | 5,530 | 2.3 | 5,492 | 2.3 | 38 | 3.6 | 0.004 |
| Chronic pulmonary disease | 72,028 | 29.7 | 71,698 | 29.7 | 330 | 31.4 | 0.23 |
| Respiratory failure second diagnosis | 13,027 | 5.4 | 12,893 | 5.3 | 134 | 12.7 | <0.001 |
| Rheumatoid arthritis/collagen vascular disease | 7,090 | 2.9 | 7,050 | 2.9 | 40 | 3.8 | 0.09 |
| Deficiency anemias | 49,605 | 20.4 | 49,352 | 20.4 | 253 | 24.0 | 0.004 |
| Weight loss | 8,810 | 3.6 | 8,714 | 3.6 | 96 | 9.1 | <0.001 |
| Peptic ulcer disease bleeding | 4,736 | 2.0 | 4,723 | 2.0 | 13 | 1.2 | 0.09 |
| Chronic blood loss anemia | 2,354 | 1.0 | 2,338 | 1.0 | 16 | 1.5 | 0.07 |
| Hypothyroidism | 28,773 | 11.9 | 28,668 | 11.9 | 105 | 10.0 | 0.06 |
| Renal failure | 19,768 | 8.1 | 19,669 | 8.1 | 99 | 9.4 | 0.13 |
| Liver disease | 4,682 | 1.9 | 4,657 | 1.9 | 25 | 2.4 | 0.29 |
| Other neurological disorders | 33,094 | 13.6 | 32,905 | 13.6 | 189 | 18.0 | <0.001 |
| Psychoses | 9,330 | 3.8 | 9,283 | 3.8 | 47 | 4.5 | 0.29 |
| Depression | 25,561 | 10.5 | 25,442 | 10.5 | 119 | 11.3 | 0.41 |
| Alcohol abuse | 7,756 | 3.2 | 7,727 | 3.2 | 29 | 2.8 | 0.42 |
| Drug abuse | 4,336 | 1.8 | 4,318 | 1.8 | 18 | 1.7 | 0.85 |
| Acquired immune deficiency syndrome | 1,048 | 0.4 | 1,045 | 0.4 | 3 | 0.3 | 0.47 |
| Total | Derivation | Validation | |||||
|---|---|---|---|---|---|---|---|
| Variable | N | % | N | % | N | % | P‐Value |
| |||||||
| Total | 242,738 | 100 | 194,198 | 100 | 48,540 | 100 | |
| VTE prophylaxis | 0.97 | ||||||
| No prophylaxis | 170,180 | 70.1 | 136,153 | 70.1 | 34,027 | 70.1 | |
| Any prophylaxis | 72,558 | 29.9 | 58,045 | 29.9 | 14,513 | 29.9 | |
| Outcomes | |||||||
| ICD‐9 code for VTE | 1,304 | 0.5 | 1,025 | 0.5 | 279 | 0.6 | 0.21 |
| ICD‐9 code + diagnostic test | 989 | 0.4 | 777 | 0.4 | 212 | 0.4 | 0.26 |
| ICD‐9 code + diagnostic test + treatment for VTE | 612 | 0.3 | 471 | 0.2 | 141 | 0.3 | 0.06 |
| Readmission for VTE within 30 days | 446 | 0.2 | 363 | 0.2 | 83 | 0.2 | 0.46 |
| Total hospital‐acquired VTE | 1,052 | 0.4 | 829 | 0.4 | 223 | 0.5 | 0.33 |
| In‐hospital mortality | 8,019 | 3.3 | 6,403 | 3.3 | 1,616 | 3.3 | 0.72 |
| Any readmission within 30 days | 28,664 | 11.8 | 22,885 | 11.8 | 5,779 | 11.9 | 0.46 |
Risk factors for VTE
A large number of patient and hospital factors were associated with the development of VTE (Table 1). Due to the large sample size, even weak associations appear highly statistically significant. Compared to patients without VTE, those with VTE were more likely to have received VTE prophylaxis (37% vs 30%, P < 0.001). However, models of patients receiving prophylaxis and of patients not receiving prophylaxis produced similar odds ratios for the various risk factors (Supporting Information Appendix Table 2); therefore, the final model includes both patients who did, and did not, receive VTE prophylaxis. In the multivariable model (Supporting Information Appendix Table 3), age, length of stay, gender, primary diagnosis, cancer, inflammatory bowel disease, obesity, central venous catheter, inherited thrombophilia, steroid use, mechanical ventilation, active chemotherapy, and urinary catheters were all associated with VTE (Table 3). The strongest risk factors were length of stay 6 days (OR 3.22, 95% CI 2.73, 3.79), central venous catheter (OR 1.87, 95% CI 1.52, 2.29), inflammatory bowel disease (OR 3.11, 95% CI 1.59, 6.08), and inherited thrombophilia (OR 4.00, 95% CI 0.98, 16.40). In addition, there were important interactions between age and cancer; cancer was a strong risk factor among younger patients, but is not as strong a risk factor among older patients (OR compared to young patients without cancer was 4.62 (95% CI 2.72, 7.87) for those age 1849 years, and 3.64 (95% CI 2.52, 5.25) for those aged 5064 years).
| Risk Factor | OR | 95% CI |
|---|---|---|
| ||
| Any prophylaxis | 0.98 | (0.84, 1.14) |
| Female | 0.85 | (0.74, 0.98) |
| Length of stay 6 days | 3.22 | (2.73, 3.79) |
| Age* | ||
| 18‐49 years | 1 | Referent |
| 50‐64 years | 1.15 | (0.86, 1.56) |
| >65 years | 1.51 | (1.17, 1.96) |
| Primary diagnosis | ||
| Pneumonia | 1 | Referent |
| Chronic obstructive pulmonary disease | 0.57 | (0.44, 0.75) |
| Stroke | 0.84 | (0.66, 1.08) |
| Congestive heart failure | 0.86 | (0.70, 1.06) |
| Urinary tract infection | 1.19 | (0.95, 1.50) |
| Respiratory failure | 1.15 | (0.85, 1.55) |
| Septicemia | 1.11 | (0.82, 1.50) |
| Comorbidities | ||
| Inflammatory bowel disease | 3.11 | (1.59, 6.08) |
| Obesity | 1.28 | (0.99, 1.66) |
| Inherited thrombophilia | 4.00 | (0.98, 16.40) |
| Cancer | ||
| 18‐49 years | 4.62 | (2.72, 7.87) |
| 50‐64 years | 3.64 | (2.52, 5.25) |
| >65 years | 2.17 | (1.61, 2.92) |
| Treatments | ||
| Central venous catheter | 1.87 | (1.52, 2.29) |
| Mechanical ventilation | 1.61 | (1.27, 2.05) |
| Urinary catheter | 1.17 | (0.99, 1.38) |
| Chemotherapy | 1.71 | (1.03, 2.83) |
| Steroids | 1.22 | (1.04, 1.43) |
In the derivation set, the multivariable model produced deciles of mean predicted risk from 0.11% to 1.45%, while mean observed risk over the same deciles ranged from 0.12% to 1.42% (Figure 1). Within the validation cohort, the observed rate of VTE was 0.46% (223 cases among 48,543 subjects). The expected rate according to the model was 0.43% (expected/observed ratio: 0.93 [95% CI 0.82, 1.06]). Model discrimination measured by the c‐statistic in the validation set was 0.75 (95% CI 0.71, 0.78). The model produced deciles of mean predicted risk from 0.11% to 1.46%, with mean observed risk over the same deciles from 0.17% to 1.81%. Risk gradient was relatively flat across the first 6 deciles, began to rise at the seventh decile, and rose sharply in the highest one. Using a risk threshold of 1%, the model had a sensitivity of 28% and a specificity of 93%. In the validation set, this translated into a positive predictive value of 2.2% and a negative predictive value of 99.7%. Assuming that VTE prophylaxis has an efficacy of 50%, the number‐needed‐to‐treat to prevent one VTE among high‐risk patients (predicted risk >1%) would be 91. In contrast, providing prophylaxis to the entire validation sample would result in a number‐needed‐to‐treat of 435. Using a lower treatment threshold of 0.4% produced a positive predictive value of 1% and a negative predictive value of 99.8%. At this threshold, the model would detect 73% of patients with VTE and the number‐needed‐to‐treat to prevent one VTE would be 200.

Discussion
In a representative sample of 243,000 hospitalized medical patients with at least one major risk factor for VTE, we found that symptomatic VTE was an uncommon event, occurring in approximately 1 in 231 patients. We identified a number of factors that were associated with an increased risk of VTE, but many previously cited risk factors did not show an association in multivariable models. In particular, patients with a primary diagnosis of COPD appeared not to share the same high risk of VTE as patients with the other diagnoses we examined, a finding reported by others.11 The risk model we developed accurately stratifies patients across a wide range of VTE probabilities, but even among those with the highest predicted rates, symptomatic VTE occurred in less than 2%.
VTE is often described as a frequent complication of hospitalization for medical illness and one of the most common potentially preventable causes of death. Indeed, rates of asymptomatic VTE have been demonstrated to be 3.7% to 26%.12 Although some of these might have fatal consequences, most are distal vein thromboses and their significance is unknown. In contrast, symptomatic events are uncommon, with previous estimates among general medical patients in observational studies in the range of 0.3%3 to 0.8%,12 similar to the rate observed in our study. Symptomatic event rates among control patients in landmark randomized trials have ranged from 0.86%13 to 2.3%,14 but these studies enrolled only very high‐risk patients with more extended hospitalizations, and may involve follow‐up periods of a month or more.
Because it is unlikely that our diagnostic algorithm was 100% sensitive, and because 30% of our patients received chemoprophylaxis, it is probable that we have underestimated the true rate of VTE in our sample. Among the patients who received prophylaxis, the observed rate of VTE was 0.54%. If we assume that prophylaxis is 50% effective, then had these patients not received prophylaxis, their rate of VTE would have been 1.08% (vs 0.39% among those patients who received no prophylaxis) and the overall rate of VTE for the sample would have been 0.60% (1.08 0.30 + 0.39 0.70). If we further assume that our algorithm was only 80% sensitive and 100% specific, the true underlying rate of symptomatic VTE could have been as high as 0.75%, still less than half that seen in randomized trials.
Prophylaxis with heparin has been shown to decrease the rate of both asymptomatic and symptomatic events, but because of the low prevalence, the number‐needed‐to‐treat to prevent one symptomatic pulmonary embolism has been estimated at 345, and prophylaxis has not been shown to affect all‐cause mortality.4, 15 At the same time, prophylaxis costs money, is uncomfortable, and carries a small risk of bleeding and heparin‐induced thrombocytopenia. Given the generally low incidence of symptomatic VTE, it therefore makes sense to reserve prophylaxis for patients at higher risk of thromboembolism.
To decide whether prophylaxis is appropriate for a given patient, it is necessary to quantify the patient's risk and then apply an appropriate threshold for treatment. The National Quality Forum (NQF) recommends,16 and JCAHO has adopted, that a clinician must evaluate each patient upon admission, and regularly thereafter, for the risk of developing DVT [deep vein thrombosis]/VTE. Until now, however, there has been no widely accepted, validated method to risk stratify medical patients. The ACCP recommendations cite just three studies of VTE risk factors in hospitalized medical patients.11, 17, 18 Together they examined 477 cases and 1197 controls, identifying congestive heart failure, pneumonia, cancer, and previous VTE as risk factors. Predictive models based on these factors17, 1921 have not been subjected to validation or have performed poorly.18 Acknowledging this lack of standardized risk assessment, JCAHO leaves the means of assessment to individual hospitals. A quality improvement guide published by the Agency for Healthcare Research and Quality goes one step further, stating that In a typical hospital, it is estimated that fewer than 5% of medical patients could be considered at low risk by most VTE risk stratification methods.22 The guide recommends near universal VTE prophylaxis.
In light of the JCAHO requirements, our model should be welcomed by hospitalists. Rather than assuming that all patients over 40 years of age are at high risk, our model will enable clinicians to risk stratify patients from a low of 0.1% to >1.4% (>10‐fold increase in risk). Moreover, the model was derived from more than 800 episodes of symptomatic VTE among almost 190,000 general medical patients and validated on almost 50,000 more. The observed patients were cared for in clinical practice at a nationally representative group of US hospitals, not in a highly selected clinical trial, increasing the generalizability of our findings. Finally, the model includes ten common risk factors that can easily be entered into decision support software or extracted automatically from the electronic medical record. Electronic reminder systems have already been shown to increase use of VTE prophylaxis, and prevent VTE, especially among cancer patients.23
A more challenging task is defining the appropriate risk threshold to initiate VTE prophylaxis. The Thromboembolic Risk Factors (THRIFT) Consensus Group classified patients according to risk of proximal DVT as low (<1%), moderate (1%‐10%), and high (>10%).21 They recommended heparin prophylaxis for all patients at moderate risk or higher. Although the patients included in our study all had a diagnosis that warranted prophylaxis according to the ACCP guidelines, using the THRIFT threshold for moderate‐to‐high risk, only 7% of our patients should have received prophylaxis. The recommendation not to offer heparin prophylaxis to patients with less than 1% chance of developing symptomatic VTE seems reasonable, given the large number‐needed‐to‐treat, but formal decision analyses should be conducted to better define this threshold. Many hospitalists, however, may feel uncomfortable using the 1% threshold, because our model failed to identify almost three out of four patients who ultimately experienced symptomatic VTE. At that threshold, it would seem that hospital‐acquired VTE is not a preventable complication in most medical patients, as others have pointed out.3, 24 Alternatively, if the threshold were lowered to 0.4%, our model could reduce the use of prophylaxis by 60%, while still identifying three‐fourths of all VTE cases. Further research is needed to know whether such a threshold is reasonable.
Our study has a number of important limitations. First, we relied on claims data, not chart review. We do not know for certain which patients experienced VTE, although our definition of VTE required diagnosis codes plus charges for both diagnosis and treatment. Moreover, our rates are similar to those observed in other trials where symptomatic events were confirmed. Second, about 30% of our patients received at least some VTE prophylaxis, and this may have prevented as many as half of the VTEs in that group. Without prophylaxis, rates might have been 20%30% higher. Similarly, we could not detect patients who were diagnosed after discharge but not admitted to hospital. While we believe this number to be small, it would again increase the rate slightly. Third, we could not assess certain clinical circumstances that are not associated with hospital charges or diagnosis codes, especially prolonged bed rest. Other risk factors, such as the urinary catheter, were probably surrogate markers for immobilization rather than true risk factors. Fourth, we included length of stay in our prediction model. We did this because most randomized trials of VTE prophylaxis included only patients with an expected length of stay 6 days. Physicians' estimates about probable length of stay may be less accurate than actual length of stay as a predictor of VTE. Moreover, the relationship may have been confounded if hospital‐acquired VTE led to longer lengths of stay. We think this unlikely since many of the events were discovered on readmission. Fifth, we studied only patients carrying high‐risk diagnoses, and therefore do not know the baseline risk for patients with less risky conditions, although it should be lower than what we observed. It seems probable that COPD, rather than being protective, as it appears in our model, actually represents the baseline risk for low‐risk diagnoses. It should be noted that we did include a number of other high‐risk diagnoses, such as cancer and inflammatory bowel disease, as secondary diagnoses. A larger, more inclusive study should be conducted to validate our model in other populations. Finally, we cannot know who died of undiagnosed VTE, either in the hospital or after discharge. Such an outcome would be important, but those events are likely to be rare, and VTE prophylaxis has not been shown to affect mortality.
VTE remains a daunting problem in hospitalized medical patients. Although VTE is responsible for a large number of hospital deaths each year, identifying patients at high risk for clinically important VTE is challenging, and may contribute to the persistently low rates of VTE prophylaxis seen in hospitals.25 Current efforts to treat nearly all patients are likely to lead to unnecessary cost, discomfort, and side effects. We present a simple logistic regression model that can easily identify patients at moderate‐to‐high risk (>1%) of developing symptomatic VTE. Future studies should focus on prospectively validating the model in a wider spectrum of medical illness, and better defining the appropriate risk cutoff for general prophylaxis.
Acknowledgements
The authors thank Aruna Priya, MS, for her help with some of the statistical analyses.
- ,,, et al.A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group.N Engl J Med.1999;341(11):793–800.
- ,,, et al.Prevention of venous thromboembolism: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th ed).Chest.2008;133(6 suppl):381S–453S.
- ,,.Thrombosis prophylaxis in hospitalised medical patients: does prophylaxis in all patients make sense?Neth J Med.2000;56(5):171–176.
- ,,,,.Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta‐analysis of randomized controlled trials.Arch Intern Med.2007;167(14):1476–1486.
- The Joint Commission on the Accreditation of Healthcare Organizations. Venous thromboembolism (VTE) core measure set. Available at: http://www. jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/VTE.htm. Accessed June 1,2009.
- ,,.Effective risk stratification of surgical and nonsurgical patients for venous thromboembolic disease.Semin Hematol.2001;38(2 suppl 5):12–19.
- ,,, et al.Assessment of venous thromboembolism risk and the benefits of thromboprophylaxis in medical patients.Thromb Haemost.2005;94(4):750–759.
- ,,, et al.Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126(3 suppl):338S–400S.
- ,,,.Comorbidity measures for use with administrative data.Med Care.1998;36(1):8–27.
- ,,,,,.Identification of in‐hospital complications from claims data. Is it valid?Med Care.2000;38(8):785–795.
- ,,, et al.Risk factors for venous thromboembolism in hospitalized patients with acute medical illness: analysis of the MEDENOX Study.Arch Intern Med.2004;164(9):963–968.
- ,,.The magnitude of an iatrogenic disorder: a systematic review of the incidence of venous thromboembolism for general medical inpatients.Thromb Haemost.2006;95(5):758–762.
- ,,,,,.Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients.Circulation.2004;110(7):874–879.
- .Randomised, controlled trial of low‐dose heparin for prevention of fatal pulmonary embolism in patients with infectious diseases. The Heparin Prophylaxis Study Group.Lancet.1996;347(9012):1357–1361.
- ,,,,.Meta‐analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients.Ann Intern Med.2007;146(4):278–288.
- National Quality Forum.National Voluntary Consensus Standards for Prevention and Care of Venous Thromboembolism: Policy, Preferred Practices, and Initial Performance Measures.Washington, DC;2006.
- ,,, et al.Risk factors for deep vein thrombosis in inpatients aged 65 and older: a case‐control multicenter study.J Am Geriatr Soc.2004;52(8):1299–1304.
- ,,.Risk factors for venous thrombosis in medical inpatients: validation of a thrombosis risk score.J Thromb Haemost.2004;2(12):2156–2161.
- ,,,,,.Venous thromboembolism prophylaxis and risk assessment in medical patients.Semin Thromb Hemost.1991;17(suppl 3):313–318.
- ,,, et al.A population‐based perspective of the hospital incidence and case‐fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study.Arch Intern Med.1991;151(5):933–938.
- Thromboembolic Risk Factors (THRIFT) Consensus Group.Risk of and prophylaxis for venous thromboembolism in hospital patients.BMJ.1992;305(6853):567–574.
- ,.Preventing Hospital‐Acquired Venous Thromboembolism: A Guide for Effective Quality Improvement. AHRQ Publication No. 08–0075.Rockville, MD:Agency for Healthcare Research and Quality;2008.
- ,,, et al.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352(10):969–977.
- ,,.Prophylaxis against venous thromboembolism.BMJ.1992;305(6862):1156.
- ,.Prevention of in‐hospital VTE: why can't we do better?Lancet.2008;371(9610):361–362.
- ,,, et al.A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group.N Engl J Med.1999;341(11):793–800.
- ,,, et al.Prevention of venous thromboembolism: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th ed).Chest.2008;133(6 suppl):381S–453S.
- ,,.Thrombosis prophylaxis in hospitalised medical patients: does prophylaxis in all patients make sense?Neth J Med.2000;56(5):171–176.
- ,,,,.Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta‐analysis of randomized controlled trials.Arch Intern Med.2007;167(14):1476–1486.
- The Joint Commission on the Accreditation of Healthcare Organizations. Venous thromboembolism (VTE) core measure set. Available at: http://www. jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/VTE.htm. Accessed June 1,2009.
- ,,.Effective risk stratification of surgical and nonsurgical patients for venous thromboembolic disease.Semin Hematol.2001;38(2 suppl 5):12–19.
- ,,, et al.Assessment of venous thromboembolism risk and the benefits of thromboprophylaxis in medical patients.Thromb Haemost.2005;94(4):750–759.
- ,,, et al.Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126(3 suppl):338S–400S.
- ,,,.Comorbidity measures for use with administrative data.Med Care.1998;36(1):8–27.
- ,,,,,.Identification of in‐hospital complications from claims data. Is it valid?Med Care.2000;38(8):785–795.
- ,,, et al.Risk factors for venous thromboembolism in hospitalized patients with acute medical illness: analysis of the MEDENOX Study.Arch Intern Med.2004;164(9):963–968.
- ,,.The magnitude of an iatrogenic disorder: a systematic review of the incidence of venous thromboembolism for general medical inpatients.Thromb Haemost.2006;95(5):758–762.
- ,,,,,.Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients.Circulation.2004;110(7):874–879.
- .Randomised, controlled trial of low‐dose heparin for prevention of fatal pulmonary embolism in patients with infectious diseases. The Heparin Prophylaxis Study Group.Lancet.1996;347(9012):1357–1361.
- ,,,,.Meta‐analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients.Ann Intern Med.2007;146(4):278–288.
- National Quality Forum.National Voluntary Consensus Standards for Prevention and Care of Venous Thromboembolism: Policy, Preferred Practices, and Initial Performance Measures.Washington, DC;2006.
- ,,, et al.Risk factors for deep vein thrombosis in inpatients aged 65 and older: a case‐control multicenter study.J Am Geriatr Soc.2004;52(8):1299–1304.
- ,,.Risk factors for venous thrombosis in medical inpatients: validation of a thrombosis risk score.J Thromb Haemost.2004;2(12):2156–2161.
- ,,,,,.Venous thromboembolism prophylaxis and risk assessment in medical patients.Semin Thromb Hemost.1991;17(suppl 3):313–318.
- ,,, et al.A population‐based perspective of the hospital incidence and case‐fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study.Arch Intern Med.1991;151(5):933–938.
- Thromboembolic Risk Factors (THRIFT) Consensus Group.Risk of and prophylaxis for venous thromboembolism in hospital patients.BMJ.1992;305(6853):567–574.
- ,.Preventing Hospital‐Acquired Venous Thromboembolism: A Guide for Effective Quality Improvement. AHRQ Publication No. 08–0075.Rockville, MD:Agency for Healthcare Research and Quality;2008.
- ,,, et al.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352(10):969–977.
- ,,.Prophylaxis against venous thromboembolism.BMJ.1992;305(6862):1156.
- ,.Prevention of in‐hospital VTE: why can't we do better?Lancet.2008;371(9610):361–362.
Copyright © 2011 Society of Hospital Medicine
Macrolides and Quinolones for AECOPD
Acute exacerbations of chronic obstructive pulmonary disease (AECOPD) are responsible for more than 600,000 hospitalizations annually, resulting in direct costs of over $20 billion.1 Bacterial infections appear responsible for 50% of such exacerbations,25 and current COPD guidelines recommend treatment with antibiotics for patients with severe exacerbations or a change in sputum.1,69 These recommendations are based on a number of small randomized trials, most of which were conducted more than 20 years ago using narrow spectrum antibiotics that are no longer commonly used.10 Only 4 studies, totaling 321 subjects, included hospitalized patients, and most studies excluded patients who required steroids. Because no clinical trials have compared different antibiotic regimens for AECOPD, existing guidelines offer a range of treatment options, including amoxicillin‐clavulonate, macrolides, quinolones, cephalosporins, aminopenicillins, and tetracyclines.
Among hospitalized patients, macrolides and quinolones appear to be the most frequently prescribed antibiotics.11 Both are available in oral formulations, have excellent bioavailability, and are administered once daily. In addition to their antimicrobial activity, macrolides are believed to have antiinflammatory effects, which could be especially advantageous in AECOPD.1214 In trials of chronic bronchitis, however, fluoroquinolones have been shown to reduce the risk of recurrent exacerbation when compared to macrolides.15 The wide variation that has been observed in antibiotic selection for patients hospitalized for AECOPD suggests a high degree of uncertainty among clinicians about the benefits of different treatment options.11 Given the limited evidence from randomized trials, we sought to evaluate the comparative effectiveness of macrolides and quinolones among a large, representative sample of patients hospitalized with AECOPD.
Subjects and Methods
Setting and Subjects
We conducted a retrospective cohort study of all patients hospitalized between January 1 and December 31, 2001 for AECOPD at any 1 of 375 acute care facilities in the United States that participated in Premier's Perspective, a voluntary, fee‐supported database developed for measuring quality and healthcare utilization. Participating hospitals represent all geographical regions, and are primarily small‐sized to medium‐sized nonteaching hospitals located mostly in urban areas. In addition to the information contained in the standard hospital discharge file (Uniform Billing 92) such as patient age, International Classification of Disease, 9th Edition, Clinical Modification (ICD‐9‐CM) codes, the Perspective database includes a date‐stamped log of all billed items, including diagnostic tests, medications, and other treatments, as well as costs, for individual patients. The study was approved by the Institutional Review Board of Baystate Medical Center.
Patients were included if they had a primary diagnosis consistent with AECOPD (ICD‐9 codes 491.21 and 493.22) or a primary diagnosis of respiratory failure (ICD‐9 codes 518.81 and 518.84) paired with secondary diagnosis of AECOPD; they also had to receive at least 2 consecutive days of either a macrolide or a quinolone, started within 48 hours of hospitalization. Patients receiving both antibiotics were excluded, but patients who received additional antibiotics were included. To enhance the specificity of our diagnosis codes, we limited our study to patients age 40 years.16 Because mechanical ventilation initiated after hospital day 2 was an outcome measure, we excluded patients admitted directly to the intensive care unit. We also excluded: those with other bacterial infections, such as pneumonia or cellulitis, who might have another indication for antibiotics; those with a length of stay <2 days, because we could not ascertain whether they received a full course of antibiotics; patients with a secondary diagnosis of pulmonary embolism or pneumothorax; and those whose attending physicians were not internists, family practitioners, hospitalists, pulmonologists, or intensivists. For patients with more than 1 admission during the study period, we included only the first admission.
Data Elements
For each patient, we assessed age, gender, race, marital and insurance status, principal diagnosis, comorbidities, and specialty of the attending physician. Comorbidities were identified from ICD‐9 secondary diagnosis codes and Diagnosis Related Groups using Healthcare Cost and Utilization Project Comorbidity Software (version 3.1), based on the work of Elixhauser et al.17 In addition, to assess disease severity we recorded the presence of chronic pulmonary heart disease, the number of admissions for COPD during the 12 months prior to the index admission, and arterial blood gas testing.18,19 We also identified pharmacy or diagnostic charges for interventions that were recommended in current guidelines (beta‐adrenergic and anticholinergic bronchodilators, steroids, and noninvasive positive‐pressure ventilation); those that were not recommended or were of uncertain benefit (methylxanthine bronchodilators, spirometry/pulmonary function testing, mucolytic medications, chest physiotherapy, and sputum testing); and drugs that might be associated with severe exacerbations or end‐stage COPD (loop diuretics, morphine, and nutritional supplements).1,69 Hospitals were categorized by region (Northeast, South, Midwest, or West), bed size, setting (urban vs. rural), ownership, and teaching status.
Antibiotic Class and Outcome Variables
Our primary predictor variable was the antibiotic initiated during the first 2 hospital days and continued for at least 2 days, regardless of other antibiotics the patient may have received during the course of hospitalization. Because we anticipated low in‐hospital mortality, our primary outcome was a composite measure of treatment failure, defined as initiation of mechanical ventilation after hospital day 2, in‐hospital mortality, or readmission for COPD within 30 days of discharge.20 Secondary outcomes included hospital costs and length of stay, as well as allergic reactions identified by ICD‐9 code, and antibiotic‐associated diarrhea, defined as treatment with either metronidazole or oral vancomycin begun after hospital day 3.
Statistical Analysis
Summary statistics were computed using frequencies and percents for categorical variables; and means, medians, standard deviations, and interquartile ranges for continuous variables. Associations between antibiotic selection and patient and hospital characteristics were assessed using chi‐square tests for categorical variables and z‐tests for continuous variables.
We developed a series of multivariable models to evaluate the impact of initial antibiotic selection on the risk of treatment failure, length of stay, and total cost. In order to account for the effects of within‐hospital correlation, generalized estimating equation (GEE) models with a logit link were used to assess the effect of antibiotic selection on the risk of treatment failure, and identity link models were used for analyses of length of stay and cost. Unadjusted and covariate‐adjusted models for treatment failure were evaluated with and without adjustments for propensity score. A propensity score is the probability that a given patient would receive treatment with a macrolide, derived from a nonparsimonious model in which treatment with a macrolide was considered the outcome. The propensity model included all patient characteristics, other early treatments and tests, comorbidities, hospital and physician characteristics, and selected interaction terms.21 Length of stay and cost were trimmed at 3 standard deviations above the mean, and natural log‐transformed values were modeled due to extreme positive skew. In addition, we carried out matched analyses in which we compared the outcomes of patients who were treated with a macrolide to those with similar propensity scores (ie, with similar likelihood of receiving a macrolide) who received a quinolone.22
Finally, to reduce the threat of residual confounding by indication, which can occur if sicker patients are more likely to receive a particular antibiotic, we developed a grouped treatment model, in which all patients treated at the same hospital were assigned a probability of treatment with a macrolide equal to the overall treatment rate at that hospital.23 This is an adaptation of instrumental variable analysis, a well‐accepted technique in econometrics with growing use in health care.24,25 It attempts to assess whether patients treated at a hospital at which quinolones are used more frequently have better outcomes than patients treated at hospitals at which macrolides are used more frequently, while adjusting for other patient, physician, and hospital variables. It ignores the actual treatment the patient received, and instead substitutes the hospital's rate of macrolide use. By grouping treatment at the hospital level, this method greatly reduces the possibility of residual selection bias, unless hospitals that use a lot of macrolides have patients who differ in a consistent way from hospitals which use mostly quinolones.
All analyses were performed using SAS version 9.1 (SAS Institute, Inc., Cary, NC).
Results
Of 26,248 AECOPD patients treated with antibiotics, 19,608 patients met the inclusion criteria; of these, 6139 (31%) were treated initially with a macrolide; the median age was 70 years; 60% were female; and 78% were white. A total of 86% of patients had a primary diagnosis of obstructive chronic bronchitis with acute exacerbation, and 6% had respiratory failure. The most common comorbidities were hypertension, diabetes, and congestive heart failure. Twenty‐two percent had been admitted at least once in the preceding 12 months. Treatment failure occurred in 7.7% of patients, and 1.3% died in the hospital. Mean length of stay was 4.8 days. Hospital prescribing rates for macrolides varied from 0% to 100%, with a mean of 33% and an interquartile range of 14% to 46% (Supporting Appendix Figure 1).
Compared to patients receiving macrolides, those receiving quinolones were older, more likely to have respiratory failure, to be cared for by a pulmonologist, and to have an admission in the previous year (Table 1). They were also more likely to be treated with bronchodilators, methylxanthines, steroids, diuretics, and noninvasive positive pressure ventilation, and to have an arterial blood gas, but less likely to receive concomitant treatment with a cephalosporin (11% vs. 57%). With the exception of cephalosporin treatment, these differences were small, but due to the large sample were statistically significant. Comorbidities were similar in both groups. Patients in the quinolone group were also more likely to experience treatment failure (8.1% vs. 6.8%), death (1.5% vs. 1.0%), and antibiotic‐associated diarrhea (1.1% vs. 0.5%).
| Complete Cohort | Propensity‐matched Subsample | |||||
|---|---|---|---|---|---|---|
| Characteristic | Quinolone (n = 13469) | Macrolide (n = 6139) | P Value | Quinolone (n = 5610) | Macrolide (n = 5610) | P Value |
| ||||||
| Antibiotics received during hospitalization* [n (%)] | ||||||
| Macrolide | 264 (2) | 6139 (100) | 119 (2) | 5610 (100) | ||
| Quinolone | 13469 (100) | 459 (8) | 5610 (100) | 424 (8) | ||
| Cephalosporin | 1696 (13) | 3579 (59) | <0.001 | 726 (13) | 3305 (59) | <0.001 |
| Tetracycline | 231 (2) | 75 (2) | 0.01 | 101 (2) | 73 (2) | 0.06 |
| Other antibiotics | 397 (3) | 220 (4) | 0.02 | 166 (3) | 193 (3) | 0.03 |
| Age (years) (mean [SD]) | 69.1 (11.4) | 68.2 (11.8) | <0.001 | 68.6 (11.7) | 68.5 (11.7) | 0.58 |
| Male sex (n [%]) | 5447 (40) | 2440 (40) | 0.36 | 2207 (39) | 2196 (39) | 0.85 |
| Race/ethnic group (n [%]) | <0.001 | 0.44 | ||||
| White | 10454 (78) | 4758 (78) | 4359 (78) | 4368 (78) | ||
| Black | 1060 (8) | 540 (9) | 470 (8) | 455 (8) | ||
| Hispanic | 463 (3) | 144 (2) | 157 (3) | 134 (2) | ||
| Other | 1492 (11) | 697 (11) | 624 (11) | 653 (12) | ||
| Primary diagnosis (n [%]) | <0.001 | 0.78 | ||||
| Obstructive chronic bronchitis with acute exacerbation | 11650 (87) | 5298 (86) | 4884 (87) | 4860 (87) | ||
| Chronic obstructive asthma/asthma with COPD | 908 (7) | 569 (9) | 466 (8) | 486 (9) | ||
| Respiratory failure | 911 (7) | 272 (4) | 260 (5) | 264 (5) | ||
| Admissions in the prior year (n [%]) | <0.001 | 0.84 | ||||
| 0 | 9846 (73) | 4654 (76) | 4249 (76) | 4231 (75) | ||
| 1 | 1918 (14) | 816 (13) | 747 (13) | 750 (13) | ||
| 2+ | 1085 (8) | 445 (7) | 397 (7) | 420 (8) | ||
| Missing | 620 (5) | 224 (4) | 217 (4) | 209 (4) | ||
| Physician specialty (n [%]) | <0.001 | 0.84 | ||||
| Internal medicine/hospitalist | 7069 (53) | 3321 (54) | 3032 (54) | 3072 (55) | ||
| Family/general medicine | 3569 (27) | 2074 (34) | 1824 (33) | 1812 (32) | ||
| Pulmonologist | 2776 (21) | 727 (12) | 738 (13) | 711 (13) | ||
| Critical care/emntensivist | 55 (0) | 17 (0) | 16 (0) | 15 (0) | ||
| Tests on hospital day 1 or 2 (n [%]) | ||||||
| Arterial blood gas | 8084 (60) | 3377 (55) | <0.001 | 3195 (57) | 3129 (56) | 0.22 |
| Sputum test | 1741 (13) | 766 (13) | 0.39 | 20 (0) | 16 (0) | 0.62 |
| Medications/therapies on hospital day 1 or 2 (n [%]) | ||||||
| Short‐acting bronchodilators | 7555 (56) | 3242 (53) | <0.001 | 2969 (53) | 2820 (50) | 0.005 |
| Long‐acting beta‐2 agonists | 2068 (15) | 748 (12) | <0.001 | 704 (13) | 719 (13) | 0.69 |
| Methylxanthine bronchodilators | 3051 (23) | 1149 (19) | <0.001 | 1102 (20) | 1093 (20) | 0.85 |
| Steroids | 0.04 | 0.68 | ||||
| Intravenous | 11148 (83) | 4989 (81) | 4547 (81) | 4581 (82) | ||
| Oral | 772 (6) | 376 (6) | 334 (6) | 330 (6) | ||
| Severity indicators (n [%]) | ||||||
| Chronic pulmonary heart disease | 890 (7) | 401 (7) | 0.85 | 337 (6) | 368 (7) | 0.24 |
| Sleep apnea | 586 (4) | 234 (4) | 0.08 | 211 (4) | 218 (4) | 0.77 |
| Noninvasive positive pressure ventilation | 391 (3) | 128 (2) | <0.001 | 128 (2) | 114 (2) | 0.40 |
| Loop diuretics | 4838 (36) | 1971 (32) | <0.001 | 1884 (34) | 1862 (33) | 0.67 |
| Hospital characteristics (n [%]) | ||||||
| Staffed beds | <0.001 | 0.71 | ||||
| 6200 | 3483 (26) | 1688 (28) | 1610 (29) | 1586 (28) | ||
| 201300 | 3132 (23) | 1198 (20) | 1174 (21) | 1154 (21) | ||
| 301500 | 4265 (32) | 2047 (33) | 1809 (32) | 1867 (33) | ||
| 500+ | 2589 (19) | 1206 (20) | 1017 (18) | 1003 (18) | ||
| Hospital region (n [%]) | <0.001 | 0.65 | ||||
| South | 8562 (64) | 3270 (53) | 3212 (57) | 3160 (56) | ||
| Midwest | 2602 (19) | 1444 (24) | 1170 (21) | 1216 (22) | ||
| Northeast | 1163 (9) | 871 (14) | 687 (12) | 704 (13) | ||
| West | 1142 (9) | 554 (9) | 541 (10) | 530 (9) | ||
| Teaching hospital | <0.001 | 0.63 | ||||
| No | 12090 (90) | 5037 (82) | 4896 (87) | 4878 (87) | ||
| Yes | 1379 (10) | 1102 (18) | 714 (13) | 732 (13) | ||
| Comorbidities (n [%]) | ||||||
| Congestive heart failure | 2673 (20) | 1147 (19) | 0.06 | 1081 (19) | 1060 (19) | 0.63 |
| Metastatic cancer | 134 (1) | 27 (0) | <0.001 | 34 (1) | 38 (1) | 0.72 |
| Depression | 1419 (11) | 669 (11) | 0.45 | 598 (11) | 603 (11) | 0.90 |
| Deficiency anemias | 1155 (9) | 476 (8) | 0.05 | 426 (8) | 432 (8) | 0.86 |
| Solid tumor without metastasis | 1487 (11) | 586 (10) | 0.002 | 550 (10) | 552 (10) | 0.97 |
| Hypothyroidism | 1267 (9) | 527 (9) | 0.07 | 481 (9) | 482 (9) | 1.00 |
| Peripheral vascular disease | 821 (6) | 312 (5) | 0.005 | 287 (5) | 288 (5) | 1.00 |
| Paralysis | 165 (1) | 46 (1) | 0.003 | 49 (1) | 51 (1) | 0.92 |
| Obesity | 957 (7) | 435 (7) | 0.98 | 386 (7) | 398 (7) | 0.68 |
| Hypertension | 5793 (43) | 2688 (44) | 0.31 | 2474 (44) | 2468 (44) | 0.92 |
| Diabetes | 0.04 | 0.45 | ||||
| Without chronic complications | 2630 (20) | 1127 (18) | 1057 (19) | 1066 (19) | ||
| With chronic complications | 298 (2) | 116 (2) | 115 (2) | 97 (2) | ||
In the unadjusted analysis, compared to patients receiving quinolones, those treated with macrolides were less likely to experience treatment failure (OR, 0.83; 95% CI, 0.740.94) (Table 2). Adjusting for all patient, hospital, and physician covariates, including the propensity for treatment with macrolides, increased the OR to 0.89 and the results were no longer significant (95% CI, 0.781.01). Propensity matching successfully balanced all measured covariates except for the use of short‐acting bronchodilators and additional antibiotics (Table 1). In the propensity‐matched sample (Figure 1), quinolone‐treated patients were more likely to experience antibiotic‐associated diarrhea (1.2% vs. 0.6%; P = 0.0003) and late mechanical ventilation (1.3% vs. 0.8%; P = 0.02). There were no differences in adjusted cost or length of stay between the 2 groups. The results of the grouped treatment analysis, substituting the hospital's specific rate of macrolide use in place of the actual treatment that each patient received suggested that the 2 antibiotics were associated with similar rates of treatment failure. The OR for a 100% hospital rate of macrolide treatment vs. a 0% rate was 1.01 (95% CI, 0.751.35).
| Treatment Failure | Cost | LOS | ||||
|---|---|---|---|---|---|---|
| Models | OR | 95% CI | Ratio | 95% CI | Ratio | 95% CI |
| ||||||
| Unadjusted | 0.83 | 0.730.93 | 0.98 | 0.971.00 | 0.96 | 0.950.98 |
| Adjusted for propensity score only* | 0.89 | 0.791.01 | 1.00 | 0.981.01 | 0.98 | 0.971.00 |
| Adjusted for covariates | 0.87 | 0.770.99 | 1.00 | 0.991.02 | 0.99 | 0.971.00 |
| Adjusted for covariates and propensity score | 0.89 | 0.781.01 | 1.00 | 0.991.02 | 0.98 | 0.971.00 |
| Matched sample, unadjusted | 0.87 | 0.751.00 | 0.99 | 0.981.01 | 0.99 | 0.971.01 |
| Matched sample, adjusted for unbalanced variables | 0.87 | 0.751.01 | 1.00 | 0.981.02 | 0.99 | 0.971.01 |
| Grouped treatment model, unadjusted | 0.90 | 0.681.19 | 0.97 | 0.891.06 | 0.92 | 0.870.96 |
| Group treatment model, adjusted for covariates∥ | 1.01 | 0.751.35 | 0.96 | 0.881.05 | 0.96 | 0.911.00 |
Discussion
In this large observational study conducted at 375 hospitals, we took advantage of a natural experiment in which antibiotic prescribing patterns varied widely across hospitals to compare the effectiveness of 2 common antibiotic regimens for AECOPD. Treatment with macrolides and quinolones were associated with a similar risk of treatment failure, costs, and length of stay; however, patients treated with macrolides were less likely to experience late mechanical ventilation or treatment for antibiotic‐associated diarrhea.
Despite broad consensus in COPD guidelines that patients with severe acute exacerbations should receive antibiotics, there is little agreement about the preferred empiric agent. Controversy exists regarding antibiotics' comparative effectiveness, and even over which pathogens cause COPD exacerbations. Given the frequency of hospitalization for AECOPD, understanding the comparative effectiveness of treatments in this setting could have important implications for health outcomes and costs. Unfortunately, most antibiotic studies in AECOPD were conducted >20 years ago, using antibiotics that rarely appeared in our sample.26 Consequently, clinical practice guidelines offer conflicting recommendations. For example, the National Institute for Clinical Excellence recommends empirical treatment with an aminopenicillin, a macrolide, or a tetracycline,8 while the American Thoracic Society recommends amoxicillin‐clavulonate or a fluoroquinolone.9
As might be expected in light of so much uncertainty, we found wide variation in prescribing patterns across hospitals. Overall, approximately one‐third of patients received a macrolide and two‐thirds a quinolone. Both regimens provide adequate coverage of H. influenza, S. pneumoniae, and M. catarrhalis, and conform to at least 1 COPD guideline. Nevertheless, patients receiving macrolides often received a cephalosporin as well; this pattern of treatment suggests that antibiotic selection is likely to have been influenced more by guidelines for the treatment of community‐acquired pneumonia than COPD.
Previous studies comparing antibiotic effectiveness suffer from shortcomings that limit their application to patients hospitalized with AECOPD. First, they enrolled patients with chronic bronchitis, and included patients without obstructive lung disease, and most studies included patients as young as 18 years old. Second, many either did not include treatment with steroids or excluded patients receiving more than 10 mg of prednisone daily. Third, almost all enrolled only ambulatory patients.
While there are no studies comparing quinolones and macrolides in patients hospitalized for AECOPD, a meta‐analysis comparing quinolones, macrolides, and amoxicillin‐clavulonate identified 19 trials of ambulatory patients with chronic bronchitis. That study found that all 3 drugs had similar efficacy initially, but that quinolones resulted in the fewest relapses over a 26‐week period.27 Macrolides and quinolones had similar rates of adverse effects. In contrast, we did not find a difference in treatment failure, cost, or length of stay, but did find a higher rate of diarrhea associated with quinolones. Others have also documented an association between fluoroquinolones and C. difficile diarrhea.2830 This trend, first noted in 2001, is of particular concern because the fluoroquinolone‐resistant strains appear to be hypervirulent and have been associated with nosocomial epidemics.3134
Our study has several limitations. First, its observational design leaves open the possibility of selection bias. For this reason we analyzed our data in several ways, including using a grouped treatment approach, an adaptation of the instrumental variable technique, and accepted only those differences which were consistent across all models. Second, our study used claims data, and therefore we could not directly adjust for physiological measures of severity. However, the highly detailed nature of the data allowed us to adjust for numerous tests and treatments that reflected the clinician's assessment of the patient's severity, as well as the number of prior COPD admissions. Third, we cannot exclude the possibility that some patients may have had concurrent pneumonia without an ICD‐9 code. We think that the number would be small because reimbursement for pneumonia is generally higher than for COPD, so hospitals have an incentive to code pneumonia as the principal diagnosis when present. Finally, we compared initial antibiotics only. More than one‐quarter of our patients received an additional antibiotic before discharge. In particular, patients receiving macrolides were often prescribed a concomitant cephalosporin. We do not know to what extent these additional antibiotics may have affected the outcomes.
Despite the large number of patients hospitalized annually for AECOPD, there are no randomized trials comparing different antibiotics in this population. Studies comparing antibiotics in chronic bronchitis can offer little guidance, since they have primarily focused on proving equivalence between existing antibiotics and newer, more expensive formulations.35 Because many of the patients enrolled in such trials do not benefit from antibiotics at all, either because they do not have COPD or because their exacerbation is not caused by bacteria, it is relatively easy to prove equivalence. Given that AECOPD is one of the leading causes of hospitalization in the United States, large, randomized trials comparing the effectiveness of different antibiotics should be a high priority. In the meantime, macrolides (often given together with cephalosporins) and quinolones appear to be equally effective initial antibiotic choices; considering antibiotic‐associated diarrhea, macrolides appear to be the safer of the 2.
- ,,.Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease.Ann Intern Med.2001;134:595–599.
- ,,, et al.Infectious etiologies in acute exacerbation of COPD.Diagn Microbiol Infect Dis.2001;40:95–102.
- ,.Bacterial infections in patients requiring admission for an acute exacerbation of COPD; a 1‐year prospective study.Respir Med.2003;97:770–777.
- ,,, et al.Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease.Arch Intern Med.2005;165:891–897.
- ,.Infection in the pathogenesis and course of chronic obstructive pulmonary disease.N Engl J Med.2008;359:2355–2365.
- ,,, et al.Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary.Am J Respir Crit Care Med.2007;176:532–555.
- ,,, et al.Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2008 update—highlights for primary care.Can Respir J.2008;15(suppl A):1A–8A.
- Chronic obstructive pulmonary disease.National clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care.Thorax.2004;59(suppl 1):1–232.
- ,,, et al.Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper.Eur Respir J.2004;23:932–946.
- ,,,,.Antibiotics for exacerbations of chronic obstructive pulmonary disease.Cochrane Database Syst Rev.2006:CD004403.
- ,,,,,.Quality of care for patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease.Ann Intern Med.2006;144:894–903.
- ,,, et al.Modulation of neutrophil and inflammation markers in chronic obstructive pulmonary disease by short‐term azithromycin treatment.Eur J Pharmacol.2005;517:132–143.
- ,,, et al.Azithromycin modulates neutrophil function and circulating inflammatory mediators in healthy human subjects.Eur J Pharmacol.2002;450:277–289.
- ,,,,,.The effect of clarithromycin on inflammatory markers in chronic obstructive pulmonary disease: preliminary data.Ann Pharmacother.2004;38:1400–1405.
- ,,,.A comparison of gemifloxacin and clarithromycin in acute exacerbations of chronic bronchitis and long‐term clinical outcomes.Clin Ther.2002;24:639–652.
- ,,,.In‐hospital mortality following acute exacerbations of chronic obstructive pulmonary disease.Arch Intern Med.2003;163:1180–1186.
- ,,,.Comorbidity measures for use with administrative data.Med Care.1998;36:8–27.
- ,,, et al.Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments).Am J Respir Crit Care Med.1996;154:959–967.
- ,,.Mortality and mortality‐related factors after hospitalization for acute exacerbation of COPD.Chest.2003;124:459–467.
- ,,, et al.Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease.N Engl J Med.1999;340:1941–1947.
- .Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group.Stat Med.1998;17:2265–2281.
- .Reducing bias in a propensity score matched‐pair sample using greedy matching techniques. Proceedings of the Twenty‐Sixth Annual SAS Users Group International Conference. Cary, NC: SAS Institute;2001,214–216.
- ,,,.Modeling treatment effects on binary outcomes with grouped‐treatment variables and individual covariates.Am J Epidemiol.2002;156:753–760.
- ,,.Does more intensive treatment of acute myocardial infarction in the elderly reduce mortality? Analysis using instrumental variables. [see Comment].JAMA.1994;272:859–866.
- ,,,,,.Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods.JAMA.2007;297:278–285.
- ,,,.Antibiotics in chronic obstructive pulmonary disease exacerbations. A meta‐analysis.JAMA.1995;273:957–960.
- ,,,,.Macrolides, quinolones and amoxicillin/clavulanate for chronic bronchitis: a meta‐analysis.Eur Respir J.2007;29:1127–1137.
- ,,,,.Antimicrobial‐associated risk factors for Clostridium difficile infection.Clin Infect Dis.2008;46(suppl 1):S19–S31.
- ,,, et al.Short‐term and long‐term outcomes of moxifloxacin compared to standard antibiotic treatment in acute exacerbations of chronic bronchitis.Chest.2004;125:953–964.
- ,,,,.Oral gemifloxacin once daily for 5 days compared with sequential therapy with i.v. ceftriaxone/oral cefuroxime (maximum of 10 days) in the treatment of hospitalized patients with acute exacerbations of chronic bronchitis.Respir Med.2003;97:242–249.
- ,,, et al.A large outbreak of Clostridium difficile‐associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use.Infect Control Hosp Epidemiol.2005;26:273–280.
- ,,, et al.An epidemic, toxin gene‐variant strain of Clostridium difficile.N Engl J Med.2005;353:2433–2441.
- ,,, et al.Outbreak of Clostridium difficile infection in a long‐term care facility: association with gatifloxacin use.Clin Infect Dis.2004;38:640–645.
- ,,, et al.A predominantly clonal multi‐institutional outbreak of Clostridium difficile‐associated diarrhea with high morbidity and mortality.N Engl J Med.2005;353:2442–2449.
- ,.No more equivalence trials for antibiotics in exacerbations of COPD, please.Chest.2004;125:811–813.
Acute exacerbations of chronic obstructive pulmonary disease (AECOPD) are responsible for more than 600,000 hospitalizations annually, resulting in direct costs of over $20 billion.1 Bacterial infections appear responsible for 50% of such exacerbations,25 and current COPD guidelines recommend treatment with antibiotics for patients with severe exacerbations or a change in sputum.1,69 These recommendations are based on a number of small randomized trials, most of which were conducted more than 20 years ago using narrow spectrum antibiotics that are no longer commonly used.10 Only 4 studies, totaling 321 subjects, included hospitalized patients, and most studies excluded patients who required steroids. Because no clinical trials have compared different antibiotic regimens for AECOPD, existing guidelines offer a range of treatment options, including amoxicillin‐clavulonate, macrolides, quinolones, cephalosporins, aminopenicillins, and tetracyclines.
Among hospitalized patients, macrolides and quinolones appear to be the most frequently prescribed antibiotics.11 Both are available in oral formulations, have excellent bioavailability, and are administered once daily. In addition to their antimicrobial activity, macrolides are believed to have antiinflammatory effects, which could be especially advantageous in AECOPD.1214 In trials of chronic bronchitis, however, fluoroquinolones have been shown to reduce the risk of recurrent exacerbation when compared to macrolides.15 The wide variation that has been observed in antibiotic selection for patients hospitalized for AECOPD suggests a high degree of uncertainty among clinicians about the benefits of different treatment options.11 Given the limited evidence from randomized trials, we sought to evaluate the comparative effectiveness of macrolides and quinolones among a large, representative sample of patients hospitalized with AECOPD.
Subjects and Methods
Setting and Subjects
We conducted a retrospective cohort study of all patients hospitalized between January 1 and December 31, 2001 for AECOPD at any 1 of 375 acute care facilities in the United States that participated in Premier's Perspective, a voluntary, fee‐supported database developed for measuring quality and healthcare utilization. Participating hospitals represent all geographical regions, and are primarily small‐sized to medium‐sized nonteaching hospitals located mostly in urban areas. In addition to the information contained in the standard hospital discharge file (Uniform Billing 92) such as patient age, International Classification of Disease, 9th Edition, Clinical Modification (ICD‐9‐CM) codes, the Perspective database includes a date‐stamped log of all billed items, including diagnostic tests, medications, and other treatments, as well as costs, for individual patients. The study was approved by the Institutional Review Board of Baystate Medical Center.
Patients were included if they had a primary diagnosis consistent with AECOPD (ICD‐9 codes 491.21 and 493.22) or a primary diagnosis of respiratory failure (ICD‐9 codes 518.81 and 518.84) paired with secondary diagnosis of AECOPD; they also had to receive at least 2 consecutive days of either a macrolide or a quinolone, started within 48 hours of hospitalization. Patients receiving both antibiotics were excluded, but patients who received additional antibiotics were included. To enhance the specificity of our diagnosis codes, we limited our study to patients age 40 years.16 Because mechanical ventilation initiated after hospital day 2 was an outcome measure, we excluded patients admitted directly to the intensive care unit. We also excluded: those with other bacterial infections, such as pneumonia or cellulitis, who might have another indication for antibiotics; those with a length of stay <2 days, because we could not ascertain whether they received a full course of antibiotics; patients with a secondary diagnosis of pulmonary embolism or pneumothorax; and those whose attending physicians were not internists, family practitioners, hospitalists, pulmonologists, or intensivists. For patients with more than 1 admission during the study period, we included only the first admission.
Data Elements
For each patient, we assessed age, gender, race, marital and insurance status, principal diagnosis, comorbidities, and specialty of the attending physician. Comorbidities were identified from ICD‐9 secondary diagnosis codes and Diagnosis Related Groups using Healthcare Cost and Utilization Project Comorbidity Software (version 3.1), based on the work of Elixhauser et al.17 In addition, to assess disease severity we recorded the presence of chronic pulmonary heart disease, the number of admissions for COPD during the 12 months prior to the index admission, and arterial blood gas testing.18,19 We also identified pharmacy or diagnostic charges for interventions that were recommended in current guidelines (beta‐adrenergic and anticholinergic bronchodilators, steroids, and noninvasive positive‐pressure ventilation); those that were not recommended or were of uncertain benefit (methylxanthine bronchodilators, spirometry/pulmonary function testing, mucolytic medications, chest physiotherapy, and sputum testing); and drugs that might be associated with severe exacerbations or end‐stage COPD (loop diuretics, morphine, and nutritional supplements).1,69 Hospitals were categorized by region (Northeast, South, Midwest, or West), bed size, setting (urban vs. rural), ownership, and teaching status.
Antibiotic Class and Outcome Variables
Our primary predictor variable was the antibiotic initiated during the first 2 hospital days and continued for at least 2 days, regardless of other antibiotics the patient may have received during the course of hospitalization. Because we anticipated low in‐hospital mortality, our primary outcome was a composite measure of treatment failure, defined as initiation of mechanical ventilation after hospital day 2, in‐hospital mortality, or readmission for COPD within 30 days of discharge.20 Secondary outcomes included hospital costs and length of stay, as well as allergic reactions identified by ICD‐9 code, and antibiotic‐associated diarrhea, defined as treatment with either metronidazole or oral vancomycin begun after hospital day 3.
Statistical Analysis
Summary statistics were computed using frequencies and percents for categorical variables; and means, medians, standard deviations, and interquartile ranges for continuous variables. Associations between antibiotic selection and patient and hospital characteristics were assessed using chi‐square tests for categorical variables and z‐tests for continuous variables.
We developed a series of multivariable models to evaluate the impact of initial antibiotic selection on the risk of treatment failure, length of stay, and total cost. In order to account for the effects of within‐hospital correlation, generalized estimating equation (GEE) models with a logit link were used to assess the effect of antibiotic selection on the risk of treatment failure, and identity link models were used for analyses of length of stay and cost. Unadjusted and covariate‐adjusted models for treatment failure were evaluated with and without adjustments for propensity score. A propensity score is the probability that a given patient would receive treatment with a macrolide, derived from a nonparsimonious model in which treatment with a macrolide was considered the outcome. The propensity model included all patient characteristics, other early treatments and tests, comorbidities, hospital and physician characteristics, and selected interaction terms.21 Length of stay and cost were trimmed at 3 standard deviations above the mean, and natural log‐transformed values were modeled due to extreme positive skew. In addition, we carried out matched analyses in which we compared the outcomes of patients who were treated with a macrolide to those with similar propensity scores (ie, with similar likelihood of receiving a macrolide) who received a quinolone.22
Finally, to reduce the threat of residual confounding by indication, which can occur if sicker patients are more likely to receive a particular antibiotic, we developed a grouped treatment model, in which all patients treated at the same hospital were assigned a probability of treatment with a macrolide equal to the overall treatment rate at that hospital.23 This is an adaptation of instrumental variable analysis, a well‐accepted technique in econometrics with growing use in health care.24,25 It attempts to assess whether patients treated at a hospital at which quinolones are used more frequently have better outcomes than patients treated at hospitals at which macrolides are used more frequently, while adjusting for other patient, physician, and hospital variables. It ignores the actual treatment the patient received, and instead substitutes the hospital's rate of macrolide use. By grouping treatment at the hospital level, this method greatly reduces the possibility of residual selection bias, unless hospitals that use a lot of macrolides have patients who differ in a consistent way from hospitals which use mostly quinolones.
All analyses were performed using SAS version 9.1 (SAS Institute, Inc., Cary, NC).
Results
Of 26,248 AECOPD patients treated with antibiotics, 19,608 patients met the inclusion criteria; of these, 6139 (31%) were treated initially with a macrolide; the median age was 70 years; 60% were female; and 78% were white. A total of 86% of patients had a primary diagnosis of obstructive chronic bronchitis with acute exacerbation, and 6% had respiratory failure. The most common comorbidities were hypertension, diabetes, and congestive heart failure. Twenty‐two percent had been admitted at least once in the preceding 12 months. Treatment failure occurred in 7.7% of patients, and 1.3% died in the hospital. Mean length of stay was 4.8 days. Hospital prescribing rates for macrolides varied from 0% to 100%, with a mean of 33% and an interquartile range of 14% to 46% (Supporting Appendix Figure 1).
Compared to patients receiving macrolides, those receiving quinolones were older, more likely to have respiratory failure, to be cared for by a pulmonologist, and to have an admission in the previous year (Table 1). They were also more likely to be treated with bronchodilators, methylxanthines, steroids, diuretics, and noninvasive positive pressure ventilation, and to have an arterial blood gas, but less likely to receive concomitant treatment with a cephalosporin (11% vs. 57%). With the exception of cephalosporin treatment, these differences were small, but due to the large sample were statistically significant. Comorbidities were similar in both groups. Patients in the quinolone group were also more likely to experience treatment failure (8.1% vs. 6.8%), death (1.5% vs. 1.0%), and antibiotic‐associated diarrhea (1.1% vs. 0.5%).
| Complete Cohort | Propensity‐matched Subsample | |||||
|---|---|---|---|---|---|---|
| Characteristic | Quinolone (n = 13469) | Macrolide (n = 6139) | P Value | Quinolone (n = 5610) | Macrolide (n = 5610) | P Value |
| ||||||
| Antibiotics received during hospitalization* [n (%)] | ||||||
| Macrolide | 264 (2) | 6139 (100) | 119 (2) | 5610 (100) | ||
| Quinolone | 13469 (100) | 459 (8) | 5610 (100) | 424 (8) | ||
| Cephalosporin | 1696 (13) | 3579 (59) | <0.001 | 726 (13) | 3305 (59) | <0.001 |
| Tetracycline | 231 (2) | 75 (2) | 0.01 | 101 (2) | 73 (2) | 0.06 |
| Other antibiotics | 397 (3) | 220 (4) | 0.02 | 166 (3) | 193 (3) | 0.03 |
| Age (years) (mean [SD]) | 69.1 (11.4) | 68.2 (11.8) | <0.001 | 68.6 (11.7) | 68.5 (11.7) | 0.58 |
| Male sex (n [%]) | 5447 (40) | 2440 (40) | 0.36 | 2207 (39) | 2196 (39) | 0.85 |
| Race/ethnic group (n [%]) | <0.001 | 0.44 | ||||
| White | 10454 (78) | 4758 (78) | 4359 (78) | 4368 (78) | ||
| Black | 1060 (8) | 540 (9) | 470 (8) | 455 (8) | ||
| Hispanic | 463 (3) | 144 (2) | 157 (3) | 134 (2) | ||
| Other | 1492 (11) | 697 (11) | 624 (11) | 653 (12) | ||
| Primary diagnosis (n [%]) | <0.001 | 0.78 | ||||
| Obstructive chronic bronchitis with acute exacerbation | 11650 (87) | 5298 (86) | 4884 (87) | 4860 (87) | ||
| Chronic obstructive asthma/asthma with COPD | 908 (7) | 569 (9) | 466 (8) | 486 (9) | ||
| Respiratory failure | 911 (7) | 272 (4) | 260 (5) | 264 (5) | ||
| Admissions in the prior year (n [%]) | <0.001 | 0.84 | ||||
| 0 | 9846 (73) | 4654 (76) | 4249 (76) | 4231 (75) | ||
| 1 | 1918 (14) | 816 (13) | 747 (13) | 750 (13) | ||
| 2+ | 1085 (8) | 445 (7) | 397 (7) | 420 (8) | ||
| Missing | 620 (5) | 224 (4) | 217 (4) | 209 (4) | ||
| Physician specialty (n [%]) | <0.001 | 0.84 | ||||
| Internal medicine/hospitalist | 7069 (53) | 3321 (54) | 3032 (54) | 3072 (55) | ||
| Family/general medicine | 3569 (27) | 2074 (34) | 1824 (33) | 1812 (32) | ||
| Pulmonologist | 2776 (21) | 727 (12) | 738 (13) | 711 (13) | ||
| Critical care/emntensivist | 55 (0) | 17 (0) | 16 (0) | 15 (0) | ||
| Tests on hospital day 1 or 2 (n [%]) | ||||||
| Arterial blood gas | 8084 (60) | 3377 (55) | <0.001 | 3195 (57) | 3129 (56) | 0.22 |
| Sputum test | 1741 (13) | 766 (13) | 0.39 | 20 (0) | 16 (0) | 0.62 |
| Medications/therapies on hospital day 1 or 2 (n [%]) | ||||||
| Short‐acting bronchodilators | 7555 (56) | 3242 (53) | <0.001 | 2969 (53) | 2820 (50) | 0.005 |
| Long‐acting beta‐2 agonists | 2068 (15) | 748 (12) | <0.001 | 704 (13) | 719 (13) | 0.69 |
| Methylxanthine bronchodilators | 3051 (23) | 1149 (19) | <0.001 | 1102 (20) | 1093 (20) | 0.85 |
| Steroids | 0.04 | 0.68 | ||||
| Intravenous | 11148 (83) | 4989 (81) | 4547 (81) | 4581 (82) | ||
| Oral | 772 (6) | 376 (6) | 334 (6) | 330 (6) | ||
| Severity indicators (n [%]) | ||||||
| Chronic pulmonary heart disease | 890 (7) | 401 (7) | 0.85 | 337 (6) | 368 (7) | 0.24 |
| Sleep apnea | 586 (4) | 234 (4) | 0.08 | 211 (4) | 218 (4) | 0.77 |
| Noninvasive positive pressure ventilation | 391 (3) | 128 (2) | <0.001 | 128 (2) | 114 (2) | 0.40 |
| Loop diuretics | 4838 (36) | 1971 (32) | <0.001 | 1884 (34) | 1862 (33) | 0.67 |
| Hospital characteristics (n [%]) | ||||||
| Staffed beds | <0.001 | 0.71 | ||||
| 6200 | 3483 (26) | 1688 (28) | 1610 (29) | 1586 (28) | ||
| 201300 | 3132 (23) | 1198 (20) | 1174 (21) | 1154 (21) | ||
| 301500 | 4265 (32) | 2047 (33) | 1809 (32) | 1867 (33) | ||
| 500+ | 2589 (19) | 1206 (20) | 1017 (18) | 1003 (18) | ||
| Hospital region (n [%]) | <0.001 | 0.65 | ||||
| South | 8562 (64) | 3270 (53) | 3212 (57) | 3160 (56) | ||
| Midwest | 2602 (19) | 1444 (24) | 1170 (21) | 1216 (22) | ||
| Northeast | 1163 (9) | 871 (14) | 687 (12) | 704 (13) | ||
| West | 1142 (9) | 554 (9) | 541 (10) | 530 (9) | ||
| Teaching hospital | <0.001 | 0.63 | ||||
| No | 12090 (90) | 5037 (82) | 4896 (87) | 4878 (87) | ||
| Yes | 1379 (10) | 1102 (18) | 714 (13) | 732 (13) | ||
| Comorbidities (n [%]) | ||||||
| Congestive heart failure | 2673 (20) | 1147 (19) | 0.06 | 1081 (19) | 1060 (19) | 0.63 |
| Metastatic cancer | 134 (1) | 27 (0) | <0.001 | 34 (1) | 38 (1) | 0.72 |
| Depression | 1419 (11) | 669 (11) | 0.45 | 598 (11) | 603 (11) | 0.90 |
| Deficiency anemias | 1155 (9) | 476 (8) | 0.05 | 426 (8) | 432 (8) | 0.86 |
| Solid tumor without metastasis | 1487 (11) | 586 (10) | 0.002 | 550 (10) | 552 (10) | 0.97 |
| Hypothyroidism | 1267 (9) | 527 (9) | 0.07 | 481 (9) | 482 (9) | 1.00 |
| Peripheral vascular disease | 821 (6) | 312 (5) | 0.005 | 287 (5) | 288 (5) | 1.00 |
| Paralysis | 165 (1) | 46 (1) | 0.003 | 49 (1) | 51 (1) | 0.92 |
| Obesity | 957 (7) | 435 (7) | 0.98 | 386 (7) | 398 (7) | 0.68 |
| Hypertension | 5793 (43) | 2688 (44) | 0.31 | 2474 (44) | 2468 (44) | 0.92 |
| Diabetes | 0.04 | 0.45 | ||||
| Without chronic complications | 2630 (20) | 1127 (18) | 1057 (19) | 1066 (19) | ||
| With chronic complications | 298 (2) | 116 (2) | 115 (2) | 97 (2) | ||
In the unadjusted analysis, compared to patients receiving quinolones, those treated with macrolides were less likely to experience treatment failure (OR, 0.83; 95% CI, 0.740.94) (Table 2). Adjusting for all patient, hospital, and physician covariates, including the propensity for treatment with macrolides, increased the OR to 0.89 and the results were no longer significant (95% CI, 0.781.01). Propensity matching successfully balanced all measured covariates except for the use of short‐acting bronchodilators and additional antibiotics (Table 1). In the propensity‐matched sample (Figure 1), quinolone‐treated patients were more likely to experience antibiotic‐associated diarrhea (1.2% vs. 0.6%; P = 0.0003) and late mechanical ventilation (1.3% vs. 0.8%; P = 0.02). There were no differences in adjusted cost or length of stay between the 2 groups. The results of the grouped treatment analysis, substituting the hospital's specific rate of macrolide use in place of the actual treatment that each patient received suggested that the 2 antibiotics were associated with similar rates of treatment failure. The OR for a 100% hospital rate of macrolide treatment vs. a 0% rate was 1.01 (95% CI, 0.751.35).

| Treatment Failure | Cost | LOS | ||||
|---|---|---|---|---|---|---|
| Models | OR | 95% CI | Ratio | 95% CI | Ratio | 95% CI |
| ||||||
| Unadjusted | 0.83 | 0.730.93 | 0.98 | 0.971.00 | 0.96 | 0.950.98 |
| Adjusted for propensity score only* | 0.89 | 0.791.01 | 1.00 | 0.981.01 | 0.98 | 0.971.00 |
| Adjusted for covariates | 0.87 | 0.770.99 | 1.00 | 0.991.02 | 0.99 | 0.971.00 |
| Adjusted for covariates and propensity score | 0.89 | 0.781.01 | 1.00 | 0.991.02 | 0.98 | 0.971.00 |
| Matched sample, unadjusted | 0.87 | 0.751.00 | 0.99 | 0.981.01 | 0.99 | 0.971.01 |
| Matched sample, adjusted for unbalanced variables | 0.87 | 0.751.01 | 1.00 | 0.981.02 | 0.99 | 0.971.01 |
| Grouped treatment model, unadjusted | 0.90 | 0.681.19 | 0.97 | 0.891.06 | 0.92 | 0.870.96 |
| Group treatment model, adjusted for covariates∥ | 1.01 | 0.751.35 | 0.96 | 0.881.05 | 0.96 | 0.911.00 |
Discussion
In this large observational study conducted at 375 hospitals, we took advantage of a natural experiment in which antibiotic prescribing patterns varied widely across hospitals to compare the effectiveness of 2 common antibiotic regimens for AECOPD. Treatment with macrolides and quinolones were associated with a similar risk of treatment failure, costs, and length of stay; however, patients treated with macrolides were less likely to experience late mechanical ventilation or treatment for antibiotic‐associated diarrhea.
Despite broad consensus in COPD guidelines that patients with severe acute exacerbations should receive antibiotics, there is little agreement about the preferred empiric agent. Controversy exists regarding antibiotics' comparative effectiveness, and even over which pathogens cause COPD exacerbations. Given the frequency of hospitalization for AECOPD, understanding the comparative effectiveness of treatments in this setting could have important implications for health outcomes and costs. Unfortunately, most antibiotic studies in AECOPD were conducted >20 years ago, using antibiotics that rarely appeared in our sample.26 Consequently, clinical practice guidelines offer conflicting recommendations. For example, the National Institute for Clinical Excellence recommends empirical treatment with an aminopenicillin, a macrolide, or a tetracycline,8 while the American Thoracic Society recommends amoxicillin‐clavulonate or a fluoroquinolone.9
As might be expected in light of so much uncertainty, we found wide variation in prescribing patterns across hospitals. Overall, approximately one‐third of patients received a macrolide and two‐thirds a quinolone. Both regimens provide adequate coverage of H. influenza, S. pneumoniae, and M. catarrhalis, and conform to at least 1 COPD guideline. Nevertheless, patients receiving macrolides often received a cephalosporin as well; this pattern of treatment suggests that antibiotic selection is likely to have been influenced more by guidelines for the treatment of community‐acquired pneumonia than COPD.
Previous studies comparing antibiotic effectiveness suffer from shortcomings that limit their application to patients hospitalized with AECOPD. First, they enrolled patients with chronic bronchitis, and included patients without obstructive lung disease, and most studies included patients as young as 18 years old. Second, many either did not include treatment with steroids or excluded patients receiving more than 10 mg of prednisone daily. Third, almost all enrolled only ambulatory patients.
While there are no studies comparing quinolones and macrolides in patients hospitalized for AECOPD, a meta‐analysis comparing quinolones, macrolides, and amoxicillin‐clavulonate identified 19 trials of ambulatory patients with chronic bronchitis. That study found that all 3 drugs had similar efficacy initially, but that quinolones resulted in the fewest relapses over a 26‐week period.27 Macrolides and quinolones had similar rates of adverse effects. In contrast, we did not find a difference in treatment failure, cost, or length of stay, but did find a higher rate of diarrhea associated with quinolones. Others have also documented an association between fluoroquinolones and C. difficile diarrhea.2830 This trend, first noted in 2001, is of particular concern because the fluoroquinolone‐resistant strains appear to be hypervirulent and have been associated with nosocomial epidemics.3134
Our study has several limitations. First, its observational design leaves open the possibility of selection bias. For this reason we analyzed our data in several ways, including using a grouped treatment approach, an adaptation of the instrumental variable technique, and accepted only those differences which were consistent across all models. Second, our study used claims data, and therefore we could not directly adjust for physiological measures of severity. However, the highly detailed nature of the data allowed us to adjust for numerous tests and treatments that reflected the clinician's assessment of the patient's severity, as well as the number of prior COPD admissions. Third, we cannot exclude the possibility that some patients may have had concurrent pneumonia without an ICD‐9 code. We think that the number would be small because reimbursement for pneumonia is generally higher than for COPD, so hospitals have an incentive to code pneumonia as the principal diagnosis when present. Finally, we compared initial antibiotics only. More than one‐quarter of our patients received an additional antibiotic before discharge. In particular, patients receiving macrolides were often prescribed a concomitant cephalosporin. We do not know to what extent these additional antibiotics may have affected the outcomes.
Despite the large number of patients hospitalized annually for AECOPD, there are no randomized trials comparing different antibiotics in this population. Studies comparing antibiotics in chronic bronchitis can offer little guidance, since they have primarily focused on proving equivalence between existing antibiotics and newer, more expensive formulations.35 Because many of the patients enrolled in such trials do not benefit from antibiotics at all, either because they do not have COPD or because their exacerbation is not caused by bacteria, it is relatively easy to prove equivalence. Given that AECOPD is one of the leading causes of hospitalization in the United States, large, randomized trials comparing the effectiveness of different antibiotics should be a high priority. In the meantime, macrolides (often given together with cephalosporins) and quinolones appear to be equally effective initial antibiotic choices; considering antibiotic‐associated diarrhea, macrolides appear to be the safer of the 2.
Acute exacerbations of chronic obstructive pulmonary disease (AECOPD) are responsible for more than 600,000 hospitalizations annually, resulting in direct costs of over $20 billion.1 Bacterial infections appear responsible for 50% of such exacerbations,25 and current COPD guidelines recommend treatment with antibiotics for patients with severe exacerbations or a change in sputum.1,69 These recommendations are based on a number of small randomized trials, most of which were conducted more than 20 years ago using narrow spectrum antibiotics that are no longer commonly used.10 Only 4 studies, totaling 321 subjects, included hospitalized patients, and most studies excluded patients who required steroids. Because no clinical trials have compared different antibiotic regimens for AECOPD, existing guidelines offer a range of treatment options, including amoxicillin‐clavulonate, macrolides, quinolones, cephalosporins, aminopenicillins, and tetracyclines.
Among hospitalized patients, macrolides and quinolones appear to be the most frequently prescribed antibiotics.11 Both are available in oral formulations, have excellent bioavailability, and are administered once daily. In addition to their antimicrobial activity, macrolides are believed to have antiinflammatory effects, which could be especially advantageous in AECOPD.1214 In trials of chronic bronchitis, however, fluoroquinolones have been shown to reduce the risk of recurrent exacerbation when compared to macrolides.15 The wide variation that has been observed in antibiotic selection for patients hospitalized for AECOPD suggests a high degree of uncertainty among clinicians about the benefits of different treatment options.11 Given the limited evidence from randomized trials, we sought to evaluate the comparative effectiveness of macrolides and quinolones among a large, representative sample of patients hospitalized with AECOPD.
Subjects and Methods
Setting and Subjects
We conducted a retrospective cohort study of all patients hospitalized between January 1 and December 31, 2001 for AECOPD at any 1 of 375 acute care facilities in the United States that participated in Premier's Perspective, a voluntary, fee‐supported database developed for measuring quality and healthcare utilization. Participating hospitals represent all geographical regions, and are primarily small‐sized to medium‐sized nonteaching hospitals located mostly in urban areas. In addition to the information contained in the standard hospital discharge file (Uniform Billing 92) such as patient age, International Classification of Disease, 9th Edition, Clinical Modification (ICD‐9‐CM) codes, the Perspective database includes a date‐stamped log of all billed items, including diagnostic tests, medications, and other treatments, as well as costs, for individual patients. The study was approved by the Institutional Review Board of Baystate Medical Center.
Patients were included if they had a primary diagnosis consistent with AECOPD (ICD‐9 codes 491.21 and 493.22) or a primary diagnosis of respiratory failure (ICD‐9 codes 518.81 and 518.84) paired with secondary diagnosis of AECOPD; they also had to receive at least 2 consecutive days of either a macrolide or a quinolone, started within 48 hours of hospitalization. Patients receiving both antibiotics were excluded, but patients who received additional antibiotics were included. To enhance the specificity of our diagnosis codes, we limited our study to patients age 40 years.16 Because mechanical ventilation initiated after hospital day 2 was an outcome measure, we excluded patients admitted directly to the intensive care unit. We also excluded: those with other bacterial infections, such as pneumonia or cellulitis, who might have another indication for antibiotics; those with a length of stay <2 days, because we could not ascertain whether they received a full course of antibiotics; patients with a secondary diagnosis of pulmonary embolism or pneumothorax; and those whose attending physicians were not internists, family practitioners, hospitalists, pulmonologists, or intensivists. For patients with more than 1 admission during the study period, we included only the first admission.
Data Elements
For each patient, we assessed age, gender, race, marital and insurance status, principal diagnosis, comorbidities, and specialty of the attending physician. Comorbidities were identified from ICD‐9 secondary diagnosis codes and Diagnosis Related Groups using Healthcare Cost and Utilization Project Comorbidity Software (version 3.1), based on the work of Elixhauser et al.17 In addition, to assess disease severity we recorded the presence of chronic pulmonary heart disease, the number of admissions for COPD during the 12 months prior to the index admission, and arterial blood gas testing.18,19 We also identified pharmacy or diagnostic charges for interventions that were recommended in current guidelines (beta‐adrenergic and anticholinergic bronchodilators, steroids, and noninvasive positive‐pressure ventilation); those that were not recommended or were of uncertain benefit (methylxanthine bronchodilators, spirometry/pulmonary function testing, mucolytic medications, chest physiotherapy, and sputum testing); and drugs that might be associated with severe exacerbations or end‐stage COPD (loop diuretics, morphine, and nutritional supplements).1,69 Hospitals were categorized by region (Northeast, South, Midwest, or West), bed size, setting (urban vs. rural), ownership, and teaching status.
Antibiotic Class and Outcome Variables
Our primary predictor variable was the antibiotic initiated during the first 2 hospital days and continued for at least 2 days, regardless of other antibiotics the patient may have received during the course of hospitalization. Because we anticipated low in‐hospital mortality, our primary outcome was a composite measure of treatment failure, defined as initiation of mechanical ventilation after hospital day 2, in‐hospital mortality, or readmission for COPD within 30 days of discharge.20 Secondary outcomes included hospital costs and length of stay, as well as allergic reactions identified by ICD‐9 code, and antibiotic‐associated diarrhea, defined as treatment with either metronidazole or oral vancomycin begun after hospital day 3.
Statistical Analysis
Summary statistics were computed using frequencies and percents for categorical variables; and means, medians, standard deviations, and interquartile ranges for continuous variables. Associations between antibiotic selection and patient and hospital characteristics were assessed using chi‐square tests for categorical variables and z‐tests for continuous variables.
We developed a series of multivariable models to evaluate the impact of initial antibiotic selection on the risk of treatment failure, length of stay, and total cost. In order to account for the effects of within‐hospital correlation, generalized estimating equation (GEE) models with a logit link were used to assess the effect of antibiotic selection on the risk of treatment failure, and identity link models were used for analyses of length of stay and cost. Unadjusted and covariate‐adjusted models for treatment failure were evaluated with and without adjustments for propensity score. A propensity score is the probability that a given patient would receive treatment with a macrolide, derived from a nonparsimonious model in which treatment with a macrolide was considered the outcome. The propensity model included all patient characteristics, other early treatments and tests, comorbidities, hospital and physician characteristics, and selected interaction terms.21 Length of stay and cost were trimmed at 3 standard deviations above the mean, and natural log‐transformed values were modeled due to extreme positive skew. In addition, we carried out matched analyses in which we compared the outcomes of patients who were treated with a macrolide to those with similar propensity scores (ie, with similar likelihood of receiving a macrolide) who received a quinolone.22
Finally, to reduce the threat of residual confounding by indication, which can occur if sicker patients are more likely to receive a particular antibiotic, we developed a grouped treatment model, in which all patients treated at the same hospital were assigned a probability of treatment with a macrolide equal to the overall treatment rate at that hospital.23 This is an adaptation of instrumental variable analysis, a well‐accepted technique in econometrics with growing use in health care.24,25 It attempts to assess whether patients treated at a hospital at which quinolones are used more frequently have better outcomes than patients treated at hospitals at which macrolides are used more frequently, while adjusting for other patient, physician, and hospital variables. It ignores the actual treatment the patient received, and instead substitutes the hospital's rate of macrolide use. By grouping treatment at the hospital level, this method greatly reduces the possibility of residual selection bias, unless hospitals that use a lot of macrolides have patients who differ in a consistent way from hospitals which use mostly quinolones.
All analyses were performed using SAS version 9.1 (SAS Institute, Inc., Cary, NC).
Results
Of 26,248 AECOPD patients treated with antibiotics, 19,608 patients met the inclusion criteria; of these, 6139 (31%) were treated initially with a macrolide; the median age was 70 years; 60% were female; and 78% were white. A total of 86% of patients had a primary diagnosis of obstructive chronic bronchitis with acute exacerbation, and 6% had respiratory failure. The most common comorbidities were hypertension, diabetes, and congestive heart failure. Twenty‐two percent had been admitted at least once in the preceding 12 months. Treatment failure occurred in 7.7% of patients, and 1.3% died in the hospital. Mean length of stay was 4.8 days. Hospital prescribing rates for macrolides varied from 0% to 100%, with a mean of 33% and an interquartile range of 14% to 46% (Supporting Appendix Figure 1).
Compared to patients receiving macrolides, those receiving quinolones were older, more likely to have respiratory failure, to be cared for by a pulmonologist, and to have an admission in the previous year (Table 1). They were also more likely to be treated with bronchodilators, methylxanthines, steroids, diuretics, and noninvasive positive pressure ventilation, and to have an arterial blood gas, but less likely to receive concomitant treatment with a cephalosporin (11% vs. 57%). With the exception of cephalosporin treatment, these differences were small, but due to the large sample were statistically significant. Comorbidities were similar in both groups. Patients in the quinolone group were also more likely to experience treatment failure (8.1% vs. 6.8%), death (1.5% vs. 1.0%), and antibiotic‐associated diarrhea (1.1% vs. 0.5%).
| Complete Cohort | Propensity‐matched Subsample | |||||
|---|---|---|---|---|---|---|
| Characteristic | Quinolone (n = 13469) | Macrolide (n = 6139) | P Value | Quinolone (n = 5610) | Macrolide (n = 5610) | P Value |
| ||||||
| Antibiotics received during hospitalization* [n (%)] | ||||||
| Macrolide | 264 (2) | 6139 (100) | 119 (2) | 5610 (100) | ||
| Quinolone | 13469 (100) | 459 (8) | 5610 (100) | 424 (8) | ||
| Cephalosporin | 1696 (13) | 3579 (59) | <0.001 | 726 (13) | 3305 (59) | <0.001 |
| Tetracycline | 231 (2) | 75 (2) | 0.01 | 101 (2) | 73 (2) | 0.06 |
| Other antibiotics | 397 (3) | 220 (4) | 0.02 | 166 (3) | 193 (3) | 0.03 |
| Age (years) (mean [SD]) | 69.1 (11.4) | 68.2 (11.8) | <0.001 | 68.6 (11.7) | 68.5 (11.7) | 0.58 |
| Male sex (n [%]) | 5447 (40) | 2440 (40) | 0.36 | 2207 (39) | 2196 (39) | 0.85 |
| Race/ethnic group (n [%]) | <0.001 | 0.44 | ||||
| White | 10454 (78) | 4758 (78) | 4359 (78) | 4368 (78) | ||
| Black | 1060 (8) | 540 (9) | 470 (8) | 455 (8) | ||
| Hispanic | 463 (3) | 144 (2) | 157 (3) | 134 (2) | ||
| Other | 1492 (11) | 697 (11) | 624 (11) | 653 (12) | ||
| Primary diagnosis (n [%]) | <0.001 | 0.78 | ||||
| Obstructive chronic bronchitis with acute exacerbation | 11650 (87) | 5298 (86) | 4884 (87) | 4860 (87) | ||
| Chronic obstructive asthma/asthma with COPD | 908 (7) | 569 (9) | 466 (8) | 486 (9) | ||
| Respiratory failure | 911 (7) | 272 (4) | 260 (5) | 264 (5) | ||
| Admissions in the prior year (n [%]) | <0.001 | 0.84 | ||||
| 0 | 9846 (73) | 4654 (76) | 4249 (76) | 4231 (75) | ||
| 1 | 1918 (14) | 816 (13) | 747 (13) | 750 (13) | ||
| 2+ | 1085 (8) | 445 (7) | 397 (7) | 420 (8) | ||
| Missing | 620 (5) | 224 (4) | 217 (4) | 209 (4) | ||
| Physician specialty (n [%]) | <0.001 | 0.84 | ||||
| Internal medicine/hospitalist | 7069 (53) | 3321 (54) | 3032 (54) | 3072 (55) | ||
| Family/general medicine | 3569 (27) | 2074 (34) | 1824 (33) | 1812 (32) | ||
| Pulmonologist | 2776 (21) | 727 (12) | 738 (13) | 711 (13) | ||
| Critical care/emntensivist | 55 (0) | 17 (0) | 16 (0) | 15 (0) | ||
| Tests on hospital day 1 or 2 (n [%]) | ||||||
| Arterial blood gas | 8084 (60) | 3377 (55) | <0.001 | 3195 (57) | 3129 (56) | 0.22 |
| Sputum test | 1741 (13) | 766 (13) | 0.39 | 20 (0) | 16 (0) | 0.62 |
| Medications/therapies on hospital day 1 or 2 (n [%]) | ||||||
| Short‐acting bronchodilators | 7555 (56) | 3242 (53) | <0.001 | 2969 (53) | 2820 (50) | 0.005 |
| Long‐acting beta‐2 agonists | 2068 (15) | 748 (12) | <0.001 | 704 (13) | 719 (13) | 0.69 |
| Methylxanthine bronchodilators | 3051 (23) | 1149 (19) | <0.001 | 1102 (20) | 1093 (20) | 0.85 |
| Steroids | 0.04 | 0.68 | ||||
| Intravenous | 11148 (83) | 4989 (81) | 4547 (81) | 4581 (82) | ||
| Oral | 772 (6) | 376 (6) | 334 (6) | 330 (6) | ||
| Severity indicators (n [%]) | ||||||
| Chronic pulmonary heart disease | 890 (7) | 401 (7) | 0.85 | 337 (6) | 368 (7) | 0.24 |
| Sleep apnea | 586 (4) | 234 (4) | 0.08 | 211 (4) | 218 (4) | 0.77 |
| Noninvasive positive pressure ventilation | 391 (3) | 128 (2) | <0.001 | 128 (2) | 114 (2) | 0.40 |
| Loop diuretics | 4838 (36) | 1971 (32) | <0.001 | 1884 (34) | 1862 (33) | 0.67 |
| Hospital characteristics (n [%]) | ||||||
| Staffed beds | <0.001 | 0.71 | ||||
| 6200 | 3483 (26) | 1688 (28) | 1610 (29) | 1586 (28) | ||
| 201300 | 3132 (23) | 1198 (20) | 1174 (21) | 1154 (21) | ||
| 301500 | 4265 (32) | 2047 (33) | 1809 (32) | 1867 (33) | ||
| 500+ | 2589 (19) | 1206 (20) | 1017 (18) | 1003 (18) | ||
| Hospital region (n [%]) | <0.001 | 0.65 | ||||
| South | 8562 (64) | 3270 (53) | 3212 (57) | 3160 (56) | ||
| Midwest | 2602 (19) | 1444 (24) | 1170 (21) | 1216 (22) | ||
| Northeast | 1163 (9) | 871 (14) | 687 (12) | 704 (13) | ||
| West | 1142 (9) | 554 (9) | 541 (10) | 530 (9) | ||
| Teaching hospital | <0.001 | 0.63 | ||||
| No | 12090 (90) | 5037 (82) | 4896 (87) | 4878 (87) | ||
| Yes | 1379 (10) | 1102 (18) | 714 (13) | 732 (13) | ||
| Comorbidities (n [%]) | ||||||
| Congestive heart failure | 2673 (20) | 1147 (19) | 0.06 | 1081 (19) | 1060 (19) | 0.63 |
| Metastatic cancer | 134 (1) | 27 (0) | <0.001 | 34 (1) | 38 (1) | 0.72 |
| Depression | 1419 (11) | 669 (11) | 0.45 | 598 (11) | 603 (11) | 0.90 |
| Deficiency anemias | 1155 (9) | 476 (8) | 0.05 | 426 (8) | 432 (8) | 0.86 |
| Solid tumor without metastasis | 1487 (11) | 586 (10) | 0.002 | 550 (10) | 552 (10) | 0.97 |
| Hypothyroidism | 1267 (9) | 527 (9) | 0.07 | 481 (9) | 482 (9) | 1.00 |
| Peripheral vascular disease | 821 (6) | 312 (5) | 0.005 | 287 (5) | 288 (5) | 1.00 |
| Paralysis | 165 (1) | 46 (1) | 0.003 | 49 (1) | 51 (1) | 0.92 |
| Obesity | 957 (7) | 435 (7) | 0.98 | 386 (7) | 398 (7) | 0.68 |
| Hypertension | 5793 (43) | 2688 (44) | 0.31 | 2474 (44) | 2468 (44) | 0.92 |
| Diabetes | 0.04 | 0.45 | ||||
| Without chronic complications | 2630 (20) | 1127 (18) | 1057 (19) | 1066 (19) | ||
| With chronic complications | 298 (2) | 116 (2) | 115 (2) | 97 (2) | ||
In the unadjusted analysis, compared to patients receiving quinolones, those treated with macrolides were less likely to experience treatment failure (OR, 0.83; 95% CI, 0.740.94) (Table 2). Adjusting for all patient, hospital, and physician covariates, including the propensity for treatment with macrolides, increased the OR to 0.89 and the results were no longer significant (95% CI, 0.781.01). Propensity matching successfully balanced all measured covariates except for the use of short‐acting bronchodilators and additional antibiotics (Table 1). In the propensity‐matched sample (Figure 1), quinolone‐treated patients were more likely to experience antibiotic‐associated diarrhea (1.2% vs. 0.6%; P = 0.0003) and late mechanical ventilation (1.3% vs. 0.8%; P = 0.02). There were no differences in adjusted cost or length of stay between the 2 groups. The results of the grouped treatment analysis, substituting the hospital's specific rate of macrolide use in place of the actual treatment that each patient received suggested that the 2 antibiotics were associated with similar rates of treatment failure. The OR for a 100% hospital rate of macrolide treatment vs. a 0% rate was 1.01 (95% CI, 0.751.35).

| Treatment Failure | Cost | LOS | ||||
|---|---|---|---|---|---|---|
| Models | OR | 95% CI | Ratio | 95% CI | Ratio | 95% CI |
| ||||||
| Unadjusted | 0.83 | 0.730.93 | 0.98 | 0.971.00 | 0.96 | 0.950.98 |
| Adjusted for propensity score only* | 0.89 | 0.791.01 | 1.00 | 0.981.01 | 0.98 | 0.971.00 |
| Adjusted for covariates | 0.87 | 0.770.99 | 1.00 | 0.991.02 | 0.99 | 0.971.00 |
| Adjusted for covariates and propensity score | 0.89 | 0.781.01 | 1.00 | 0.991.02 | 0.98 | 0.971.00 |
| Matched sample, unadjusted | 0.87 | 0.751.00 | 0.99 | 0.981.01 | 0.99 | 0.971.01 |
| Matched sample, adjusted for unbalanced variables | 0.87 | 0.751.01 | 1.00 | 0.981.02 | 0.99 | 0.971.01 |
| Grouped treatment model, unadjusted | 0.90 | 0.681.19 | 0.97 | 0.891.06 | 0.92 | 0.870.96 |
| Group treatment model, adjusted for covariates∥ | 1.01 | 0.751.35 | 0.96 | 0.881.05 | 0.96 | 0.911.00 |
Discussion
In this large observational study conducted at 375 hospitals, we took advantage of a natural experiment in which antibiotic prescribing patterns varied widely across hospitals to compare the effectiveness of 2 common antibiotic regimens for AECOPD. Treatment with macrolides and quinolones were associated with a similar risk of treatment failure, costs, and length of stay; however, patients treated with macrolides were less likely to experience late mechanical ventilation or treatment for antibiotic‐associated diarrhea.
Despite broad consensus in COPD guidelines that patients with severe acute exacerbations should receive antibiotics, there is little agreement about the preferred empiric agent. Controversy exists regarding antibiotics' comparative effectiveness, and even over which pathogens cause COPD exacerbations. Given the frequency of hospitalization for AECOPD, understanding the comparative effectiveness of treatments in this setting could have important implications for health outcomes and costs. Unfortunately, most antibiotic studies in AECOPD were conducted >20 years ago, using antibiotics that rarely appeared in our sample.26 Consequently, clinical practice guidelines offer conflicting recommendations. For example, the National Institute for Clinical Excellence recommends empirical treatment with an aminopenicillin, a macrolide, or a tetracycline,8 while the American Thoracic Society recommends amoxicillin‐clavulonate or a fluoroquinolone.9
As might be expected in light of so much uncertainty, we found wide variation in prescribing patterns across hospitals. Overall, approximately one‐third of patients received a macrolide and two‐thirds a quinolone. Both regimens provide adequate coverage of H. influenza, S. pneumoniae, and M. catarrhalis, and conform to at least 1 COPD guideline. Nevertheless, patients receiving macrolides often received a cephalosporin as well; this pattern of treatment suggests that antibiotic selection is likely to have been influenced more by guidelines for the treatment of community‐acquired pneumonia than COPD.
Previous studies comparing antibiotic effectiveness suffer from shortcomings that limit their application to patients hospitalized with AECOPD. First, they enrolled patients with chronic bronchitis, and included patients without obstructive lung disease, and most studies included patients as young as 18 years old. Second, many either did not include treatment with steroids or excluded patients receiving more than 10 mg of prednisone daily. Third, almost all enrolled only ambulatory patients.
While there are no studies comparing quinolones and macrolides in patients hospitalized for AECOPD, a meta‐analysis comparing quinolones, macrolides, and amoxicillin‐clavulonate identified 19 trials of ambulatory patients with chronic bronchitis. That study found that all 3 drugs had similar efficacy initially, but that quinolones resulted in the fewest relapses over a 26‐week period.27 Macrolides and quinolones had similar rates of adverse effects. In contrast, we did not find a difference in treatment failure, cost, or length of stay, but did find a higher rate of diarrhea associated with quinolones. Others have also documented an association between fluoroquinolones and C. difficile diarrhea.2830 This trend, first noted in 2001, is of particular concern because the fluoroquinolone‐resistant strains appear to be hypervirulent and have been associated with nosocomial epidemics.3134
Our study has several limitations. First, its observational design leaves open the possibility of selection bias. For this reason we analyzed our data in several ways, including using a grouped treatment approach, an adaptation of the instrumental variable technique, and accepted only those differences which were consistent across all models. Second, our study used claims data, and therefore we could not directly adjust for physiological measures of severity. However, the highly detailed nature of the data allowed us to adjust for numerous tests and treatments that reflected the clinician's assessment of the patient's severity, as well as the number of prior COPD admissions. Third, we cannot exclude the possibility that some patients may have had concurrent pneumonia without an ICD‐9 code. We think that the number would be small because reimbursement for pneumonia is generally higher than for COPD, so hospitals have an incentive to code pneumonia as the principal diagnosis when present. Finally, we compared initial antibiotics only. More than one‐quarter of our patients received an additional antibiotic before discharge. In particular, patients receiving macrolides were often prescribed a concomitant cephalosporin. We do not know to what extent these additional antibiotics may have affected the outcomes.
Despite the large number of patients hospitalized annually for AECOPD, there are no randomized trials comparing different antibiotics in this population. Studies comparing antibiotics in chronic bronchitis can offer little guidance, since they have primarily focused on proving equivalence between existing antibiotics and newer, more expensive formulations.35 Because many of the patients enrolled in such trials do not benefit from antibiotics at all, either because they do not have COPD or because their exacerbation is not caused by bacteria, it is relatively easy to prove equivalence. Given that AECOPD is one of the leading causes of hospitalization in the United States, large, randomized trials comparing the effectiveness of different antibiotics should be a high priority. In the meantime, macrolides (often given together with cephalosporins) and quinolones appear to be equally effective initial antibiotic choices; considering antibiotic‐associated diarrhea, macrolides appear to be the safer of the 2.
- ,,.Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease.Ann Intern Med.2001;134:595–599.
- ,,, et al.Infectious etiologies in acute exacerbation of COPD.Diagn Microbiol Infect Dis.2001;40:95–102.
- ,.Bacterial infections in patients requiring admission for an acute exacerbation of COPD; a 1‐year prospective study.Respir Med.2003;97:770–777.
- ,,, et al.Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease.Arch Intern Med.2005;165:891–897.
- ,.Infection in the pathogenesis and course of chronic obstructive pulmonary disease.N Engl J Med.2008;359:2355–2365.
- ,,, et al.Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary.Am J Respir Crit Care Med.2007;176:532–555.
- ,,, et al.Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2008 update—highlights for primary care.Can Respir J.2008;15(suppl A):1A–8A.
- Chronic obstructive pulmonary disease.National clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care.Thorax.2004;59(suppl 1):1–232.
- ,,, et al.Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper.Eur Respir J.2004;23:932–946.
- ,,,,.Antibiotics for exacerbations of chronic obstructive pulmonary disease.Cochrane Database Syst Rev.2006:CD004403.
- ,,,,,.Quality of care for patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease.Ann Intern Med.2006;144:894–903.
- ,,, et al.Modulation of neutrophil and inflammation markers in chronic obstructive pulmonary disease by short‐term azithromycin treatment.Eur J Pharmacol.2005;517:132–143.
- ,,, et al.Azithromycin modulates neutrophil function and circulating inflammatory mediators in healthy human subjects.Eur J Pharmacol.2002;450:277–289.
- ,,,,,.The effect of clarithromycin on inflammatory markers in chronic obstructive pulmonary disease: preliminary data.Ann Pharmacother.2004;38:1400–1405.
- ,,,.A comparison of gemifloxacin and clarithromycin in acute exacerbations of chronic bronchitis and long‐term clinical outcomes.Clin Ther.2002;24:639–652.
- ,,,.In‐hospital mortality following acute exacerbations of chronic obstructive pulmonary disease.Arch Intern Med.2003;163:1180–1186.
- ,,,.Comorbidity measures for use with administrative data.Med Care.1998;36:8–27.
- ,,, et al.Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments).Am J Respir Crit Care Med.1996;154:959–967.
- ,,.Mortality and mortality‐related factors after hospitalization for acute exacerbation of COPD.Chest.2003;124:459–467.
- ,,, et al.Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease.N Engl J Med.1999;340:1941–1947.
- .Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group.Stat Med.1998;17:2265–2281.
- .Reducing bias in a propensity score matched‐pair sample using greedy matching techniques. Proceedings of the Twenty‐Sixth Annual SAS Users Group International Conference. Cary, NC: SAS Institute;2001,214–216.
- ,,,.Modeling treatment effects on binary outcomes with grouped‐treatment variables and individual covariates.Am J Epidemiol.2002;156:753–760.
- ,,.Does more intensive treatment of acute myocardial infarction in the elderly reduce mortality? Analysis using instrumental variables. [see Comment].JAMA.1994;272:859–866.
- ,,,,,.Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods.JAMA.2007;297:278–285.
- ,,,.Antibiotics in chronic obstructive pulmonary disease exacerbations. A meta‐analysis.JAMA.1995;273:957–960.
- ,,,,.Macrolides, quinolones and amoxicillin/clavulanate for chronic bronchitis: a meta‐analysis.Eur Respir J.2007;29:1127–1137.
- ,,,,.Antimicrobial‐associated risk factors for Clostridium difficile infection.Clin Infect Dis.2008;46(suppl 1):S19–S31.
- ,,, et al.Short‐term and long‐term outcomes of moxifloxacin compared to standard antibiotic treatment in acute exacerbations of chronic bronchitis.Chest.2004;125:953–964.
- ,,,,.Oral gemifloxacin once daily for 5 days compared with sequential therapy with i.v. ceftriaxone/oral cefuroxime (maximum of 10 days) in the treatment of hospitalized patients with acute exacerbations of chronic bronchitis.Respir Med.2003;97:242–249.
- ,,, et al.A large outbreak of Clostridium difficile‐associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use.Infect Control Hosp Epidemiol.2005;26:273–280.
- ,,, et al.An epidemic, toxin gene‐variant strain of Clostridium difficile.N Engl J Med.2005;353:2433–2441.
- ,,, et al.Outbreak of Clostridium difficile infection in a long‐term care facility: association with gatifloxacin use.Clin Infect Dis.2004;38:640–645.
- ,,, et al.A predominantly clonal multi‐institutional outbreak of Clostridium difficile‐associated diarrhea with high morbidity and mortality.N Engl J Med.2005;353:2442–2449.
- ,.No more equivalence trials for antibiotics in exacerbations of COPD, please.Chest.2004;125:811–813.
- ,,.Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease.Ann Intern Med.2001;134:595–599.
- ,,, et al.Infectious etiologies in acute exacerbation of COPD.Diagn Microbiol Infect Dis.2001;40:95–102.
- ,.Bacterial infections in patients requiring admission for an acute exacerbation of COPD; a 1‐year prospective study.Respir Med.2003;97:770–777.
- ,,, et al.Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease.Arch Intern Med.2005;165:891–897.
- ,.Infection in the pathogenesis and course of chronic obstructive pulmonary disease.N Engl J Med.2008;359:2355–2365.
- ,,, et al.Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary.Am J Respir Crit Care Med.2007;176:532–555.
- ,,, et al.Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2008 update—highlights for primary care.Can Respir J.2008;15(suppl A):1A–8A.
- Chronic obstructive pulmonary disease.National clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care.Thorax.2004;59(suppl 1):1–232.
- ,,, et al.Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper.Eur Respir J.2004;23:932–946.
- ,,,,.Antibiotics for exacerbations of chronic obstructive pulmonary disease.Cochrane Database Syst Rev.2006:CD004403.
- ,,,,,.Quality of care for patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease.Ann Intern Med.2006;144:894–903.
- ,,, et al.Modulation of neutrophil and inflammation markers in chronic obstructive pulmonary disease by short‐term azithromycin treatment.Eur J Pharmacol.2005;517:132–143.
- ,,, et al.Azithromycin modulates neutrophil function and circulating inflammatory mediators in healthy human subjects.Eur J Pharmacol.2002;450:277–289.
- ,,,,,.The effect of clarithromycin on inflammatory markers in chronic obstructive pulmonary disease: preliminary data.Ann Pharmacother.2004;38:1400–1405.
- ,,,.A comparison of gemifloxacin and clarithromycin in acute exacerbations of chronic bronchitis and long‐term clinical outcomes.Clin Ther.2002;24:639–652.
- ,,,.In‐hospital mortality following acute exacerbations of chronic obstructive pulmonary disease.Arch Intern Med.2003;163:1180–1186.
- ,,,.Comorbidity measures for use with administrative data.Med Care.1998;36:8–27.
- ,,, et al.Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments).Am J Respir Crit Care Med.1996;154:959–967.
- ,,.Mortality and mortality‐related factors after hospitalization for acute exacerbation of COPD.Chest.2003;124:459–467.
- ,,, et al.Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease.N Engl J Med.1999;340:1941–1947.
- .Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group.Stat Med.1998;17:2265–2281.
- .Reducing bias in a propensity score matched‐pair sample using greedy matching techniques. Proceedings of the Twenty‐Sixth Annual SAS Users Group International Conference. Cary, NC: SAS Institute;2001,214–216.
- ,,,.Modeling treatment effects on binary outcomes with grouped‐treatment variables and individual covariates.Am J Epidemiol.2002;156:753–760.
- ,,.Does more intensive treatment of acute myocardial infarction in the elderly reduce mortality? Analysis using instrumental variables. [see Comment].JAMA.1994;272:859–866.
- ,,,,,.Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods.JAMA.2007;297:278–285.
- ,,,.Antibiotics in chronic obstructive pulmonary disease exacerbations. A meta‐analysis.JAMA.1995;273:957–960.
- ,,,,.Macrolides, quinolones and amoxicillin/clavulanate for chronic bronchitis: a meta‐analysis.Eur Respir J.2007;29:1127–1137.
- ,,,,.Antimicrobial‐associated risk factors for Clostridium difficile infection.Clin Infect Dis.2008;46(suppl 1):S19–S31.
- ,,, et al.Short‐term and long‐term outcomes of moxifloxacin compared to standard antibiotic treatment in acute exacerbations of chronic bronchitis.Chest.2004;125:953–964.
- ,,,,.Oral gemifloxacin once daily for 5 days compared with sequential therapy with i.v. ceftriaxone/oral cefuroxime (maximum of 10 days) in the treatment of hospitalized patients with acute exacerbations of chronic bronchitis.Respir Med.2003;97:242–249.
- ,,, et al.A large outbreak of Clostridium difficile‐associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use.Infect Control Hosp Epidemiol.2005;26:273–280.
- ,,, et al.An epidemic, toxin gene‐variant strain of Clostridium difficile.N Engl J Med.2005;353:2433–2441.
- ,,, et al.Outbreak of Clostridium difficile infection in a long‐term care facility: association with gatifloxacin use.Clin Infect Dis.2004;38:640–645.
- ,,, et al.A predominantly clonal multi‐institutional outbreak of Clostridium difficile‐associated diarrhea with high morbidity and mortality.N Engl J Med.2005;353:2442–2449.
- ,.No more equivalence trials for antibiotics in exacerbations of COPD, please.Chest.2004;125:811–813.
Copyright © 2010 Society of Hospital Medicine
Reporting Hospital Quality
Acknowledging striking deficiencies in the quality and safety of healthcare, the Institute of Medicine, policy makers, and payors have called for transformation of the US healthcare system.1 Public reporting of hospital performance is one key strategy for accelerating improvement2 and may improve quality in several ways. First, feedback about performance relative to peers may stimulate quality improvement activities by appealing to professionalism. Second, the desire to preserve one's reputation by not appearing on a list of poor performers may be a powerful incentive. Finally, patients and referring providers could use reports to select high‐quality hospitals, thereby shifting care from low‐quality to high‐quality hospitals and stimulating quality improvement efforts to maintain or enhance market share.
Almost 20 years after New York and Pennsylvania began reporting cardiac surgery outcomes,3 the evidence that public reporting improves healthcare quality is equivocal.4 Moreover, stakeholders have embraced public reporting to differing degrees. Public reporting does lead to greater engagement in quality improvement activities,58 and additional financial incentives provide modest incremental benefits.9 Purchasers, too, are starting to pay attention.10 In New York State, payors appear to contract more with high‐quality surgeons and avoid poorly performing outliers.11 Some payors are creating tiered systems, assigning higher patient copayments for hospitals with poor quality metrics. These new systems have not been rigorously studied and should raise concern among hospitals.12
In contrast to hospitals and payors, patients have been slow to embrace public reporting. In a survey of coronary artery bypass graft (CABG) patients in Pennsylvania, only 2% said that public reporting of mortality rates affected their decision making.13 Eight years later, only 11% of patients sought information about hospitals before deciding on elective major surgery,14 although a majority of patients in both studies expressed interest in the information. It is not clear whether recent proliferation of information on the internet will change patient behavior, but to date public reporting appears not to effect market share.5, 15, 16
Barriers to patients' use of public reporting include difficulty accessing the information, lack of trust, information that is not salient, and data that are difficult to interpret.17 In the absence of consensus on what or how to report, a growing number of organizations, including state and federal government, accrediting bodies, private foundations, and for‐profit companies report a variety of measures relating to structure, processes, and outcomes. Although these sites purport to target consumers, they sometimes offer conflicting information18 and are not easily interpreted by lay readers.19
To realize the benefits of public reporting, and minimize the unintended consequences, rating systems must report salient information in a way that is comprehensible to patients and trusted by the doctors who advise them. At the same time, they should be fair to hospitals and offer useful data for quality improvement. We offer 10 recommendations for improving the public reporting of healthcare quality information: 5 describing what to report and 5 detailing how it should be reported (Figure 1). We also examine 3 leading performance reporting programs to see how well they implement these recommendations.

Recommendations to Make Data Salient for Patients
1. Prioritize Elective Procedures
Hospital quality is not uniform across conditions.2 For data to be salient, then, it should be disease‐specific and focus on common elective procedures, for which consumer choice is possible. Table 1 compares 3 popular reporting services. Hospital Compare, produced by the Center for Medicare Services (CMS, US Department of Health and Human Services, Washington, DC), provides process of care measures for 4 conditions, 3 of which are not elective. The fourth, surgical infection prevention, contains 5 measures3 related to perioperative antibiotics and 2 related to thromboembolism prophylaxisfor all surgical cases. Recently, more conditions have been added, but reports are limited to the number of cases and mean Medicare charge. By year 2011, however, Hospital Compare will offer many new measures, including rates of central line infection, ventilator‐associated pneumonia, and surgical site infection. HealthGrades, a private company, offers comparative mortality rates on over 30 diagnoses, of which 15 can be considered elective, at least some of the time. Only the Leapfrog group, an industry consortium, focuses exclusively on elective procedures, offering volume measures on 7 and outcome measures on 2.
| Rule | Hospital Compare | HealthGrades | Leapfrog | |||
|---|---|---|---|---|---|---|
| ||||||
| 1. Prioritize elective procedures | Yes | 22/28 at least partially elective | Yes | 15/31 at least partially elective | Yes | 7/8 elective |
| 2. Include quality of life and outcome data, if possible | Yes | Mortality for AMI and CHF | Yes | Mortality or complications* | Yes | Outcomes for CABG, PCI, and AVR |
| 3. Include standardized patient satisfaction and service measures | Yes | HCAHPS | No | No | ||
| 4. Offer composite measures that are weighted and evidence‐based | No | No | Specialty excellence award, not evidence‐based | No | ||
| 5. Costs comparisons should include patient prices | Yes | Average Medicare payment | Yes | Charges, health plan and Medicare costs available for a fee | No | |
| 6. Adjust outcomes for severity and risk | Yes | Methodology published on website | Yes | Methodology not public | Yes | Various methodologies published or referenced on website |
| 7. Identify differences not due to chance | Yes | Compares mortality to national mean | Yes | Compares mortality or complications to mean | Yes | Compares mortality to national mean |
| 8. Standardize reporting periods | October 2005 to September 2006 | 2004‐2006 | 12‐24 months, ending 12/31/07 or 6/30/08 | |||
| 9. Avoid use of nonvalidated administrative data | Yes | None used | No | Uses PSIs for safety rating | Yes | None used |
| 10. Utilization rates should be evidence‐based | No | Surgical case volume of Medicare patients | No | Includes Caesarian‐section rates | Yes | Some case volume rates are evidence‐based |
2. Include Quality of Life and Outcome Data
Outcomes are more valuable to patients than process measures, but the risk adjustment needed to compare outcomes requires considerable effort. So far, public reporting of risk‐adjusted outcomes has been limited almost exclusively to mortality. Yet a patient contemplating knee replacement surgery would find no meaningful difference in mortalitythere were only 510 deaths nationally in year 200620but might be interested in whether patients return to full mobility after surgery, and all patients should compare rates of nosocomial infections. For some low‐risk procedures, HealthGrades Inc. (Golden, CO) includes a composite measure of major complications, including complication of an orthopedic implant, stroke, cardiac arrest, excessive bleeding, and some types of infection; CMS will soon add rates of infection and readmission.
3. Include Measures of Patient Experience, Such as Satisfaction and Service Measures
Beyond outcomes, patients want to know about the experience of others.21 Satisfaction surveys should be standardized and made disease‐specific, since patients' experiences may differ between the cardiology suite and the delivery unit. Questions could address the attentiveness of the nursing staff, how well privacy was respected, how easy it was to deal with insurance issues, whether patients were promptly informed of test results, and whether the care team answered questions fully. Medicare has begun reporting patient satisfaction using the Hospital Consumer Assessment of Healthcare Providers (HCAHPS) survey on Hospital Compare, but the data are not disease‐specific and audit a very small number of patients from each institution. Other services are unlikely to perform their own surveys, as multiple surveys would prove burdensome. Social networking sites that allow patients to post their own personal reviews of hospitals and doctors offer an additional if less reliable dimension to traditional public reporting. Such sites are already transforming the market for other industries, such as travel.22
4. Offer Composite Measures That Are Weighted and Evidence‐Based
Interpreting multiple measures, some of which are more important than others, and some of which have better evidence than others, is difficult for health care providers and may be impossible for patients. Is it more important to get aspirin on arrival or at discharge? Also, how does a patient weigh a 1% difference in the number of heart attack patients who get aspirin on arrival against a 14% difference in those who are offered smoking cessation? Because patients may be overwhelmed by data,23 public reports should include evidence‐based, weighted measures of overall care for a given condition, with higher weights attached to those process measures most likely to have clinical benefit, and careful attention to visual representations that convey relative differences.19, 23 More sophisticated measures should be developed to guard against overuse. For example, while hospitals should be rewarded for providing vaccination, they should be penalized for vaccinating the same patient twice.
None of the services we examined provides weighted outcomes. Leapfrog (The Leapfrog Group, Washington, DC) offers a composite snapshot containing 9 pie charts, divided into 4 leaps. The 6 pies representing high‐risk procedures are of equal size, even though 2 of these, esophagectomy and pancreatic resection represent very rare surgeries, even at major medical centers. From a visual perspective, however, these are equivalent to having computerized physician order entry and full‐time intensive care unit staffing, which affect thousands more patients. Similarly, in determining pay‐for‐performance measures, CMS created a composite based on the total number of opportunities of all interventions, weighting all measures equally. Because no validated weighting measures exist, future research will be necessary to achieve this goal. Also, none of the evidence‐based measures contained safeguards against overtreatment.
5. Cost Comparisons Should Include Patient Prices
In an era of patient copayments and deductibles, consumers are increasingly aware of costs. For patients with very high deductible plans or no health insurance, hospital fees are a common cause of bankruptcy.24 Several public reporting agencies, including Hospital Compare and HealthGrades have incorporated Medicare costs into their reported measures, but these have little connection to what patients actually pay. Health sites aimed at consumers should publish the average patient copayment.
Recommendations to Ensure That Data Reflects Hospital Quality
6. Adjust Outcomes for Severity and Risk
Not all bypass operations are the same and not all patients are at equal risk. More difficult operations (eg, CABG for a patient with a previous bypass) will have more complications; similarly, patients with serious comorbidities will experience worse outcomes. Since hospitals which specialize in a procedure will attract complicated cases and higher risk patients, it is important to adjust outcomes to account for these differences. Otherwise, hospitals and surgeons may be discouraged from taking difficult cases. Outside of cardiac surgery, most risk adjustment systems use administrative claims data but vary dramatically in the numbers of variables considered and the underlying proprietary models, which are often criticized as being black boxes that yield discordant results.25 Thus, a hospital's mortality may appear below expected by 1 system and above expected by another. Instead, risk adjustment systems should include clinical data abstracted from patient records using standardized data definitions. Although costly to collect, clinical data offer more predictive information than do administrative data. For example, for heart failure patients undergoing CABG, the ejection fraction predicts mortality better than many stable comorbid diagnoses. A single transparent risk‐adjustment system should be recognized as the industry standard. The American College of Surgeons' standardized risk‐adjusted outcome reporting for the National Surgical Quality Improvement Program (NSQIP) is a good example of such an effort.
7. Identify Differences Not Due to Chance
As a result of random variation, during any period, some hospitals will appear better than average and others worse. Statistical tests should be employed to identify hospitals that differ from the mean, and to allow consumers to compare 2 hospitals directly, with appropriate caveats when the hospitals serve very different patient populations. Medicare's mortality rating system for myocardial infarction identifies only 17 hospitals in the nation as better than average and 7 as worse, out of 4,500 institutions. HealthGrades compares hospitals' actual mortality or complication rates to their predicted rates based on disease‐specific logistic regression models and reports whether the hospital is statistically better or worse than predicted. Hospitals are not compared directly to one another. Given the rarity of mortality in most procedures, other outcome measures will be necessary to distinguish among hospitals.26
8. Standardize Reporting Periods
In a world of continuous quality improvement, public reporting should represent a hospital's recent performance, but reporting periods also need to be long enough to provide a stable estimate of infrequent events, especially at low‐volume institutions. In contrast, the lag time between the end of the reporting period and public availability should be kept to a minimum. We found that reporting periods varied from 1 to 3 years, and did not always cover the same years for all conditions, even on the same website. Some data were 3 years old. Patients will have a hard time making decisions on data that is 1 year old, and hospitals will have little incentive to make improvements that will not be acknowledged for years.
9. Avoid Use of Nonvalidated Administrative Data
Administrative data collected for billing purposes, unlike most clinical data, are already in electronic format, and can inexpensively produce quality rankings using validated models.27 In contrast, screening tools, such as the Agency for Healthcare Research and Quality's patient safety indicators (PSIs), were designed to identify potential quality problems, such as postoperative deep vein thrombosis, for internal quality improvement. Cases identified by the PSI software require additional chart review,28, 29 and should not be used as quality indicators. Even so, HealthGrades reports PSIs and some insurers use them in pay‐for‐performance initiatives. Improvements in PSIs, including present‐on‐admission coding, may increase accuracy,30 but these measures need to be validated before they can be adopted for public reporting.
10. Utilization Rates Should Be Evidence‐Based
Although utilization rates for most procedures vary as much as 2‐fold by state or institution, there is little evidence for a best rate. Nevertheless, HealthGrades reports utilization rates for several obstetrical procedures. At present, there are no standards for these, and it is possible that utilization could be too low in some places. Further research is needed; until then, utilization should not purport to measure quality.
Discussion
The growing commitment to making hospital performance data public could transform the quality and safety of care in the US, introducing competition on quality and price and fostering informed consumer choice. To date, the promise of public reporting remains only partially fulfilled. Few hospitals have done more than comply with regulatory mandates and payer incentives, and consumers have failed to respond. To capture the full benefits of public reporting, we have made 10 recommendations to benefit patients and better engage hospitals. We suggest that reporting be patient‐centered, with an emphasis on making the data useful, meaningful, important, interpretable, and relevant. At the same time, hospitals, which are being judged on their performance, should have a level playing field, with measures that are timely, consistent, severity‐adjusted, evidence‐based, and which foster good clinical care. Of the 3 services we examined, Hospital Compare came closest to meeting these recommendations.
Although this blueprint for public reporting is easy to draft, it is challenging to implement. In particular, some of our suggestions, such as the one regarding risk adjustment, may not currently be feasible, because the complexity and cost of collecting clinical data, even in the era of electronic medical records, may be prohibitive. Until such data are readily available, it may be preferable to report nothing at all, rather than report data that are misleading. In the rush to make hospitals accountable, enthusiasm has often outstripped science,31 and several measures have had to be revised for unintended consequences.32
Any initiative to improve public reporting should have the buy‐in of all stakeholders, but particularly hospitals, which stand to benefit in several ways. By receiving regular feedback, they can focus on improving care, becoming better organizations. These improvements may be rewarded through direct compensation (pay‐for‐performance), decreased costs from complications, or increased market share. Hospitals will be more engaged if the data reflect actual quality, are adequately adjusted for severity, and acknowledge the role of chance. Otherwise, they will merely comply, or worse, look for opportunities to game the system. To succeed, public reporting needs to involve hospitals in establishing standards for reporting and validation, as well as auditing procedures to prevent fraud.33 The Hospital Quality Alliance (HQA, Washington, DC), a first step in this direction, at present has few measures. NSQIP (American College of Surgeons, Chicago, IL) is perhaps a better example of hospitals cooperating to set measurement standards to promote best‐practices. Public release of NSQIP data might accelerate progress. Alternatively, the National Quality Forum (NQF, Washington, DC) could expand its role from endorsing quality measures to include standardizing the way these measures are used in public reporting.
Still, if you build it, will they come? To date, public reporting has not been embraced by the public, despite its stated interest in the information. Several explanations could be offered. First, we may be presenting the wrong data. Process measures and mortality rates are important but represent abstract concepts for most patients. Surveys tell us that patients value most the experiences of other patients.14, 21 They want to know whether their pain will be controlled, whether the doctor will listen to them, whether the nurse will come when they call. The recent advent of the HCAHPS survey (AHRQ, Washington, DC) is another positive step. Stratifying the results by diagnosis and adding a few diagnosis‐specific questions would make HCAHPS even more valuable. Second, the data may not be readily available. Although most public reporting is done on the web, older patients who are deciding about hospitals may not have Internet access. Some reports are still proprietary, and cost could present another obstacle. Finally, even if freely‐available and patient‐centered, the results may not be interpretable by physicians, let alone patients.34
If public reporting is to succeed, it will require measures that better reflect patients' concerns. In order to collect the massive amounts of data required and present them in a timely fashion, better electronic record systems will be necessary. But these are no panacea; others have noted that the Department of Veterans Affairs, a leader in electronic records, still invests considerable time and money to review charts for NSQIP.35 Given the value that Americans place on transparency in other facets of their lives, it is clear that public reporting is here to stay. While much progress has been made over the past 5 years, additional research is needed to better measure quality from the patient's perspective, and to determine how this information can be used to help guide decision‐making, and to reward hospitals for offering the highest‐quality care.
Acknowledgements
The authors thank Kenneth Flax for his help with an earlier version of this manuscript.
- Committee on Quality of Health Care in America IoM.Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, DC:National Academy Press;2001.
- ,,,.Care in U.S. hospitals: the Hospital Quality Alliance program.N Engl J Med.2005;353(3):265–274.
- .Achieving and sustaining improved quality: lessons from New York state and cardiac surgery.Health Aff. 20022002;21(4):40–51.
- ,,,,.Systematic review: the evidence that publishing patient care performance data improves quality of care.Ann Intern Med.2008;148(2):111–123.
- ,,.Hospital performance reports: impact on quality, market share, and reputation.Health Aff (Millwood).2005;24(4):1150–1160.
- ,,.Does publicizing hospital performance stimulate quality improvement efforts?Health Aff (Millwood).2003;22(2):84–94.
- ,,,,.Improving the outcomes of coronary artery bypass surgery in New York State.JAMA.1994;271(10):761–766.
- ,,.Declines in hospital mortality associated with a regional initiative to measure hospital performance.Am J Med Qual.1997;12(2):103–112.
- ,,, et al.Public reporting and pay for performance in hospital quality improvement.N Engl J Med.2007;356(5):486–496.
- ,,,,,.Do quality report cards play a role in HMOs' contracting practices? Evidence from New York State.Health Serv Res.2000;35(1 Pt 2):319–332.
- ,,,.Quality of cardiac surgeons and managed care contracting practices.Health Serv Res.2002;37(5):1129–1144.
- ,,,,,.Using performance data to identify preferred hospitals.Health Serv Res.2007;42(6 Pt 1):2109–2119; discussion 2294–2323.
- ,.Use of public performance reports: a survey of patients undergoing cardiac surgery.JAMA.1998;279(20):1638–1642.
- ,,.How do elderly patients decide where to go for major surgery? Telephone interview survey.BMJ.2005;331(7520):821.
- ,,,,,.The effect of publicly reporting hospital performance on market share and risk‐adjusted mortality at high‐mortality hospitals.Med Care.2003;41(6):729–740.
- ,.The predictive accuracy of the New York State coronary artery bypass surgery report‐card system.Health Aff (Millwood).2006;25(3):844–855.
- ,.Publicly disclosed information about the quality of health care: response of the US public.Qual Saf Health Care.2001;10(2):96–103.
- ,,,,.Choosing the best hospital: the limitations of public reporting of hospital quality.Health Aff (Millwood).2008;27(6):1680–1687.
- ,.Will quality report cards help consumers?Health Aff (Millwood).1997;16(3):218–228.
- Agency for Healthcare Research and Quality. HCUPnet, Healthcare Cost and Utilization Project. Available at: http://hcupnet.ahrq.gov. Accessed January 2009.
- ,,.Recovering from cardiac surgery: what patients want you to know.Am J Crit Care.2002;11(4):333–343.
- Trip Advisor. Available at: http://www.tripadvisor.com. Accessed January 2009.
- ,,,,.Less is more in presenting quality information to consumers.Med Care Res Rev.2007;64(2):169–190.
- ,,,.MarketWatch: illness and injury as contributors to bankruptcy.Health Aff (Millwood)2005;(Suppl Web Exclusives):W5‐63–W5‐73.
- .The Lake Wobegon effect: when all the patients are sicker.Am J Med Qual.2006;21(6):365–366.
- ,,.Surgical mortality as an indicator of hospital quality: the problem with small sample size.JAMA.2004;292(7):847–851.
- ,,, et al.An administrative claims model suitable for profiling hospital performance based on 30‐day mortality rates among patients with heart failure.Circulation.2006;113(13):1693–1701.
- ,,,.Can administrative data be used to compare postoperative complication rates across hospitals?Med Care.2002;40(10):856–867.
- ,,,,.Impact of diagnosis‐timing indicators on measures of safety, comorbidity, and case mix groupings from administrative data sources.Med Care.2007;45(8):781–788.
- ,,,,.Do the AHRQ patient safety indicators flag conditions that are present at the time of hospital admission?Med Care.2008;46(5):516–522.
- ,,.The tension between needing to improve care and knowing how to do it.N Engl J Med.2007;357(6):608–613.
- ,,,.Public reporting of antibiotic timing in patients with pneumonia: lessons from a flawed performance measure.Ann Intern Med.2008;149(1):29–32.
- ,,.The GAAP in quality measurement and reporting.JAMA.2007;298(15):1800–1802.
- ,,,.Consumer competencies and the use of comparative quality information: it isn't just about literacy.Med Care Res Rev.2007;64(4):379–394.
- .Performance measurement in search of a path.N Engl J Med.2007;356(9):951–953.
Acknowledging striking deficiencies in the quality and safety of healthcare, the Institute of Medicine, policy makers, and payors have called for transformation of the US healthcare system.1 Public reporting of hospital performance is one key strategy for accelerating improvement2 and may improve quality in several ways. First, feedback about performance relative to peers may stimulate quality improvement activities by appealing to professionalism. Second, the desire to preserve one's reputation by not appearing on a list of poor performers may be a powerful incentive. Finally, patients and referring providers could use reports to select high‐quality hospitals, thereby shifting care from low‐quality to high‐quality hospitals and stimulating quality improvement efforts to maintain or enhance market share.
Almost 20 years after New York and Pennsylvania began reporting cardiac surgery outcomes,3 the evidence that public reporting improves healthcare quality is equivocal.4 Moreover, stakeholders have embraced public reporting to differing degrees. Public reporting does lead to greater engagement in quality improvement activities,58 and additional financial incentives provide modest incremental benefits.9 Purchasers, too, are starting to pay attention.10 In New York State, payors appear to contract more with high‐quality surgeons and avoid poorly performing outliers.11 Some payors are creating tiered systems, assigning higher patient copayments for hospitals with poor quality metrics. These new systems have not been rigorously studied and should raise concern among hospitals.12
In contrast to hospitals and payors, patients have been slow to embrace public reporting. In a survey of coronary artery bypass graft (CABG) patients in Pennsylvania, only 2% said that public reporting of mortality rates affected their decision making.13 Eight years later, only 11% of patients sought information about hospitals before deciding on elective major surgery,14 although a majority of patients in both studies expressed interest in the information. It is not clear whether recent proliferation of information on the internet will change patient behavior, but to date public reporting appears not to effect market share.5, 15, 16
Barriers to patients' use of public reporting include difficulty accessing the information, lack of trust, information that is not salient, and data that are difficult to interpret.17 In the absence of consensus on what or how to report, a growing number of organizations, including state and federal government, accrediting bodies, private foundations, and for‐profit companies report a variety of measures relating to structure, processes, and outcomes. Although these sites purport to target consumers, they sometimes offer conflicting information18 and are not easily interpreted by lay readers.19
To realize the benefits of public reporting, and minimize the unintended consequences, rating systems must report salient information in a way that is comprehensible to patients and trusted by the doctors who advise them. At the same time, they should be fair to hospitals and offer useful data for quality improvement. We offer 10 recommendations for improving the public reporting of healthcare quality information: 5 describing what to report and 5 detailing how it should be reported (Figure 1). We also examine 3 leading performance reporting programs to see how well they implement these recommendations.

Recommendations to Make Data Salient for Patients
1. Prioritize Elective Procedures
Hospital quality is not uniform across conditions.2 For data to be salient, then, it should be disease‐specific and focus on common elective procedures, for which consumer choice is possible. Table 1 compares 3 popular reporting services. Hospital Compare, produced by the Center for Medicare Services (CMS, US Department of Health and Human Services, Washington, DC), provides process of care measures for 4 conditions, 3 of which are not elective. The fourth, surgical infection prevention, contains 5 measures3 related to perioperative antibiotics and 2 related to thromboembolism prophylaxisfor all surgical cases. Recently, more conditions have been added, but reports are limited to the number of cases and mean Medicare charge. By year 2011, however, Hospital Compare will offer many new measures, including rates of central line infection, ventilator‐associated pneumonia, and surgical site infection. HealthGrades, a private company, offers comparative mortality rates on over 30 diagnoses, of which 15 can be considered elective, at least some of the time. Only the Leapfrog group, an industry consortium, focuses exclusively on elective procedures, offering volume measures on 7 and outcome measures on 2.
| Rule | Hospital Compare | HealthGrades | Leapfrog | |||
|---|---|---|---|---|---|---|
| ||||||
| 1. Prioritize elective procedures | Yes | 22/28 at least partially elective | Yes | 15/31 at least partially elective | Yes | 7/8 elective |
| 2. Include quality of life and outcome data, if possible | Yes | Mortality for AMI and CHF | Yes | Mortality or complications* | Yes | Outcomes for CABG, PCI, and AVR |
| 3. Include standardized patient satisfaction and service measures | Yes | HCAHPS | No | No | ||
| 4. Offer composite measures that are weighted and evidence‐based | No | No | Specialty excellence award, not evidence‐based | No | ||
| 5. Costs comparisons should include patient prices | Yes | Average Medicare payment | Yes | Charges, health plan and Medicare costs available for a fee | No | |
| 6. Adjust outcomes for severity and risk | Yes | Methodology published on website | Yes | Methodology not public | Yes | Various methodologies published or referenced on website |
| 7. Identify differences not due to chance | Yes | Compares mortality to national mean | Yes | Compares mortality or complications to mean | Yes | Compares mortality to national mean |
| 8. Standardize reporting periods | October 2005 to September 2006 | 2004‐2006 | 12‐24 months, ending 12/31/07 or 6/30/08 | |||
| 9. Avoid use of nonvalidated administrative data | Yes | None used | No | Uses PSIs for safety rating | Yes | None used |
| 10. Utilization rates should be evidence‐based | No | Surgical case volume of Medicare patients | No | Includes Caesarian‐section rates | Yes | Some case volume rates are evidence‐based |
2. Include Quality of Life and Outcome Data
Outcomes are more valuable to patients than process measures, but the risk adjustment needed to compare outcomes requires considerable effort. So far, public reporting of risk‐adjusted outcomes has been limited almost exclusively to mortality. Yet a patient contemplating knee replacement surgery would find no meaningful difference in mortalitythere were only 510 deaths nationally in year 200620but might be interested in whether patients return to full mobility after surgery, and all patients should compare rates of nosocomial infections. For some low‐risk procedures, HealthGrades Inc. (Golden, CO) includes a composite measure of major complications, including complication of an orthopedic implant, stroke, cardiac arrest, excessive bleeding, and some types of infection; CMS will soon add rates of infection and readmission.
3. Include Measures of Patient Experience, Such as Satisfaction and Service Measures
Beyond outcomes, patients want to know about the experience of others.21 Satisfaction surveys should be standardized and made disease‐specific, since patients' experiences may differ between the cardiology suite and the delivery unit. Questions could address the attentiveness of the nursing staff, how well privacy was respected, how easy it was to deal with insurance issues, whether patients were promptly informed of test results, and whether the care team answered questions fully. Medicare has begun reporting patient satisfaction using the Hospital Consumer Assessment of Healthcare Providers (HCAHPS) survey on Hospital Compare, but the data are not disease‐specific and audit a very small number of patients from each institution. Other services are unlikely to perform their own surveys, as multiple surveys would prove burdensome. Social networking sites that allow patients to post their own personal reviews of hospitals and doctors offer an additional if less reliable dimension to traditional public reporting. Such sites are already transforming the market for other industries, such as travel.22
4. Offer Composite Measures That Are Weighted and Evidence‐Based
Interpreting multiple measures, some of which are more important than others, and some of which have better evidence than others, is difficult for health care providers and may be impossible for patients. Is it more important to get aspirin on arrival or at discharge? Also, how does a patient weigh a 1% difference in the number of heart attack patients who get aspirin on arrival against a 14% difference in those who are offered smoking cessation? Because patients may be overwhelmed by data,23 public reports should include evidence‐based, weighted measures of overall care for a given condition, with higher weights attached to those process measures most likely to have clinical benefit, and careful attention to visual representations that convey relative differences.19, 23 More sophisticated measures should be developed to guard against overuse. For example, while hospitals should be rewarded for providing vaccination, they should be penalized for vaccinating the same patient twice.
None of the services we examined provides weighted outcomes. Leapfrog (The Leapfrog Group, Washington, DC) offers a composite snapshot containing 9 pie charts, divided into 4 leaps. The 6 pies representing high‐risk procedures are of equal size, even though 2 of these, esophagectomy and pancreatic resection represent very rare surgeries, even at major medical centers. From a visual perspective, however, these are equivalent to having computerized physician order entry and full‐time intensive care unit staffing, which affect thousands more patients. Similarly, in determining pay‐for‐performance measures, CMS created a composite based on the total number of opportunities of all interventions, weighting all measures equally. Because no validated weighting measures exist, future research will be necessary to achieve this goal. Also, none of the evidence‐based measures contained safeguards against overtreatment.
5. Cost Comparisons Should Include Patient Prices
In an era of patient copayments and deductibles, consumers are increasingly aware of costs. For patients with very high deductible plans or no health insurance, hospital fees are a common cause of bankruptcy.24 Several public reporting agencies, including Hospital Compare and HealthGrades have incorporated Medicare costs into their reported measures, but these have little connection to what patients actually pay. Health sites aimed at consumers should publish the average patient copayment.
Recommendations to Ensure That Data Reflects Hospital Quality
6. Adjust Outcomes for Severity and Risk
Not all bypass operations are the same and not all patients are at equal risk. More difficult operations (eg, CABG for a patient with a previous bypass) will have more complications; similarly, patients with serious comorbidities will experience worse outcomes. Since hospitals which specialize in a procedure will attract complicated cases and higher risk patients, it is important to adjust outcomes to account for these differences. Otherwise, hospitals and surgeons may be discouraged from taking difficult cases. Outside of cardiac surgery, most risk adjustment systems use administrative claims data but vary dramatically in the numbers of variables considered and the underlying proprietary models, which are often criticized as being black boxes that yield discordant results.25 Thus, a hospital's mortality may appear below expected by 1 system and above expected by another. Instead, risk adjustment systems should include clinical data abstracted from patient records using standardized data definitions. Although costly to collect, clinical data offer more predictive information than do administrative data. For example, for heart failure patients undergoing CABG, the ejection fraction predicts mortality better than many stable comorbid diagnoses. A single transparent risk‐adjustment system should be recognized as the industry standard. The American College of Surgeons' standardized risk‐adjusted outcome reporting for the National Surgical Quality Improvement Program (NSQIP) is a good example of such an effort.
7. Identify Differences Not Due to Chance
As a result of random variation, during any period, some hospitals will appear better than average and others worse. Statistical tests should be employed to identify hospitals that differ from the mean, and to allow consumers to compare 2 hospitals directly, with appropriate caveats when the hospitals serve very different patient populations. Medicare's mortality rating system for myocardial infarction identifies only 17 hospitals in the nation as better than average and 7 as worse, out of 4,500 institutions. HealthGrades compares hospitals' actual mortality or complication rates to their predicted rates based on disease‐specific logistic regression models and reports whether the hospital is statistically better or worse than predicted. Hospitals are not compared directly to one another. Given the rarity of mortality in most procedures, other outcome measures will be necessary to distinguish among hospitals.26
8. Standardize Reporting Periods
In a world of continuous quality improvement, public reporting should represent a hospital's recent performance, but reporting periods also need to be long enough to provide a stable estimate of infrequent events, especially at low‐volume institutions. In contrast, the lag time between the end of the reporting period and public availability should be kept to a minimum. We found that reporting periods varied from 1 to 3 years, and did not always cover the same years for all conditions, even on the same website. Some data were 3 years old. Patients will have a hard time making decisions on data that is 1 year old, and hospitals will have little incentive to make improvements that will not be acknowledged for years.
9. Avoid Use of Nonvalidated Administrative Data
Administrative data collected for billing purposes, unlike most clinical data, are already in electronic format, and can inexpensively produce quality rankings using validated models.27 In contrast, screening tools, such as the Agency for Healthcare Research and Quality's patient safety indicators (PSIs), were designed to identify potential quality problems, such as postoperative deep vein thrombosis, for internal quality improvement. Cases identified by the PSI software require additional chart review,28, 29 and should not be used as quality indicators. Even so, HealthGrades reports PSIs and some insurers use them in pay‐for‐performance initiatives. Improvements in PSIs, including present‐on‐admission coding, may increase accuracy,30 but these measures need to be validated before they can be adopted for public reporting.
10. Utilization Rates Should Be Evidence‐Based
Although utilization rates for most procedures vary as much as 2‐fold by state or institution, there is little evidence for a best rate. Nevertheless, HealthGrades reports utilization rates for several obstetrical procedures. At present, there are no standards for these, and it is possible that utilization could be too low in some places. Further research is needed; until then, utilization should not purport to measure quality.
Discussion
The growing commitment to making hospital performance data public could transform the quality and safety of care in the US, introducing competition on quality and price and fostering informed consumer choice. To date, the promise of public reporting remains only partially fulfilled. Few hospitals have done more than comply with regulatory mandates and payer incentives, and consumers have failed to respond. To capture the full benefits of public reporting, we have made 10 recommendations to benefit patients and better engage hospitals. We suggest that reporting be patient‐centered, with an emphasis on making the data useful, meaningful, important, interpretable, and relevant. At the same time, hospitals, which are being judged on their performance, should have a level playing field, with measures that are timely, consistent, severity‐adjusted, evidence‐based, and which foster good clinical care. Of the 3 services we examined, Hospital Compare came closest to meeting these recommendations.
Although this blueprint for public reporting is easy to draft, it is challenging to implement. In particular, some of our suggestions, such as the one regarding risk adjustment, may not currently be feasible, because the complexity and cost of collecting clinical data, even in the era of electronic medical records, may be prohibitive. Until such data are readily available, it may be preferable to report nothing at all, rather than report data that are misleading. In the rush to make hospitals accountable, enthusiasm has often outstripped science,31 and several measures have had to be revised for unintended consequences.32
Any initiative to improve public reporting should have the buy‐in of all stakeholders, but particularly hospitals, which stand to benefit in several ways. By receiving regular feedback, they can focus on improving care, becoming better organizations. These improvements may be rewarded through direct compensation (pay‐for‐performance), decreased costs from complications, or increased market share. Hospitals will be more engaged if the data reflect actual quality, are adequately adjusted for severity, and acknowledge the role of chance. Otherwise, they will merely comply, or worse, look for opportunities to game the system. To succeed, public reporting needs to involve hospitals in establishing standards for reporting and validation, as well as auditing procedures to prevent fraud.33 The Hospital Quality Alliance (HQA, Washington, DC), a first step in this direction, at present has few measures. NSQIP (American College of Surgeons, Chicago, IL) is perhaps a better example of hospitals cooperating to set measurement standards to promote best‐practices. Public release of NSQIP data might accelerate progress. Alternatively, the National Quality Forum (NQF, Washington, DC) could expand its role from endorsing quality measures to include standardizing the way these measures are used in public reporting.
Still, if you build it, will they come? To date, public reporting has not been embraced by the public, despite its stated interest in the information. Several explanations could be offered. First, we may be presenting the wrong data. Process measures and mortality rates are important but represent abstract concepts for most patients. Surveys tell us that patients value most the experiences of other patients.14, 21 They want to know whether their pain will be controlled, whether the doctor will listen to them, whether the nurse will come when they call. The recent advent of the HCAHPS survey (AHRQ, Washington, DC) is another positive step. Stratifying the results by diagnosis and adding a few diagnosis‐specific questions would make HCAHPS even more valuable. Second, the data may not be readily available. Although most public reporting is done on the web, older patients who are deciding about hospitals may not have Internet access. Some reports are still proprietary, and cost could present another obstacle. Finally, even if freely‐available and patient‐centered, the results may not be interpretable by physicians, let alone patients.34
If public reporting is to succeed, it will require measures that better reflect patients' concerns. In order to collect the massive amounts of data required and present them in a timely fashion, better electronic record systems will be necessary. But these are no panacea; others have noted that the Department of Veterans Affairs, a leader in electronic records, still invests considerable time and money to review charts for NSQIP.35 Given the value that Americans place on transparency in other facets of their lives, it is clear that public reporting is here to stay. While much progress has been made over the past 5 years, additional research is needed to better measure quality from the patient's perspective, and to determine how this information can be used to help guide decision‐making, and to reward hospitals for offering the highest‐quality care.
Acknowledgements
The authors thank Kenneth Flax for his help with an earlier version of this manuscript.
Acknowledging striking deficiencies in the quality and safety of healthcare, the Institute of Medicine, policy makers, and payors have called for transformation of the US healthcare system.1 Public reporting of hospital performance is one key strategy for accelerating improvement2 and may improve quality in several ways. First, feedback about performance relative to peers may stimulate quality improvement activities by appealing to professionalism. Second, the desire to preserve one's reputation by not appearing on a list of poor performers may be a powerful incentive. Finally, patients and referring providers could use reports to select high‐quality hospitals, thereby shifting care from low‐quality to high‐quality hospitals and stimulating quality improvement efforts to maintain or enhance market share.
Almost 20 years after New York and Pennsylvania began reporting cardiac surgery outcomes,3 the evidence that public reporting improves healthcare quality is equivocal.4 Moreover, stakeholders have embraced public reporting to differing degrees. Public reporting does lead to greater engagement in quality improvement activities,58 and additional financial incentives provide modest incremental benefits.9 Purchasers, too, are starting to pay attention.10 In New York State, payors appear to contract more with high‐quality surgeons and avoid poorly performing outliers.11 Some payors are creating tiered systems, assigning higher patient copayments for hospitals with poor quality metrics. These new systems have not been rigorously studied and should raise concern among hospitals.12
In contrast to hospitals and payors, patients have been slow to embrace public reporting. In a survey of coronary artery bypass graft (CABG) patients in Pennsylvania, only 2% said that public reporting of mortality rates affected their decision making.13 Eight years later, only 11% of patients sought information about hospitals before deciding on elective major surgery,14 although a majority of patients in both studies expressed interest in the information. It is not clear whether recent proliferation of information on the internet will change patient behavior, but to date public reporting appears not to effect market share.5, 15, 16
Barriers to patients' use of public reporting include difficulty accessing the information, lack of trust, information that is not salient, and data that are difficult to interpret.17 In the absence of consensus on what or how to report, a growing number of organizations, including state and federal government, accrediting bodies, private foundations, and for‐profit companies report a variety of measures relating to structure, processes, and outcomes. Although these sites purport to target consumers, they sometimes offer conflicting information18 and are not easily interpreted by lay readers.19
To realize the benefits of public reporting, and minimize the unintended consequences, rating systems must report salient information in a way that is comprehensible to patients and trusted by the doctors who advise them. At the same time, they should be fair to hospitals and offer useful data for quality improvement. We offer 10 recommendations for improving the public reporting of healthcare quality information: 5 describing what to report and 5 detailing how it should be reported (Figure 1). We also examine 3 leading performance reporting programs to see how well they implement these recommendations.

Recommendations to Make Data Salient for Patients
1. Prioritize Elective Procedures
Hospital quality is not uniform across conditions.2 For data to be salient, then, it should be disease‐specific and focus on common elective procedures, for which consumer choice is possible. Table 1 compares 3 popular reporting services. Hospital Compare, produced by the Center for Medicare Services (CMS, US Department of Health and Human Services, Washington, DC), provides process of care measures for 4 conditions, 3 of which are not elective. The fourth, surgical infection prevention, contains 5 measures3 related to perioperative antibiotics and 2 related to thromboembolism prophylaxisfor all surgical cases. Recently, more conditions have been added, but reports are limited to the number of cases and mean Medicare charge. By year 2011, however, Hospital Compare will offer many new measures, including rates of central line infection, ventilator‐associated pneumonia, and surgical site infection. HealthGrades, a private company, offers comparative mortality rates on over 30 diagnoses, of which 15 can be considered elective, at least some of the time. Only the Leapfrog group, an industry consortium, focuses exclusively on elective procedures, offering volume measures on 7 and outcome measures on 2.
| Rule | Hospital Compare | HealthGrades | Leapfrog | |||
|---|---|---|---|---|---|---|
| ||||||
| 1. Prioritize elective procedures | Yes | 22/28 at least partially elective | Yes | 15/31 at least partially elective | Yes | 7/8 elective |
| 2. Include quality of life and outcome data, if possible | Yes | Mortality for AMI and CHF | Yes | Mortality or complications* | Yes | Outcomes for CABG, PCI, and AVR |
| 3. Include standardized patient satisfaction and service measures | Yes | HCAHPS | No | No | ||
| 4. Offer composite measures that are weighted and evidence‐based | No | No | Specialty excellence award, not evidence‐based | No | ||
| 5. Costs comparisons should include patient prices | Yes | Average Medicare payment | Yes | Charges, health plan and Medicare costs available for a fee | No | |
| 6. Adjust outcomes for severity and risk | Yes | Methodology published on website | Yes | Methodology not public | Yes | Various methodologies published or referenced on website |
| 7. Identify differences not due to chance | Yes | Compares mortality to national mean | Yes | Compares mortality or complications to mean | Yes | Compares mortality to national mean |
| 8. Standardize reporting periods | October 2005 to September 2006 | 2004‐2006 | 12‐24 months, ending 12/31/07 or 6/30/08 | |||
| 9. Avoid use of nonvalidated administrative data | Yes | None used | No | Uses PSIs for safety rating | Yes | None used |
| 10. Utilization rates should be evidence‐based | No | Surgical case volume of Medicare patients | No | Includes Caesarian‐section rates | Yes | Some case volume rates are evidence‐based |
2. Include Quality of Life and Outcome Data
Outcomes are more valuable to patients than process measures, but the risk adjustment needed to compare outcomes requires considerable effort. So far, public reporting of risk‐adjusted outcomes has been limited almost exclusively to mortality. Yet a patient contemplating knee replacement surgery would find no meaningful difference in mortalitythere were only 510 deaths nationally in year 200620but might be interested in whether patients return to full mobility after surgery, and all patients should compare rates of nosocomial infections. For some low‐risk procedures, HealthGrades Inc. (Golden, CO) includes a composite measure of major complications, including complication of an orthopedic implant, stroke, cardiac arrest, excessive bleeding, and some types of infection; CMS will soon add rates of infection and readmission.
3. Include Measures of Patient Experience, Such as Satisfaction and Service Measures
Beyond outcomes, patients want to know about the experience of others.21 Satisfaction surveys should be standardized and made disease‐specific, since patients' experiences may differ between the cardiology suite and the delivery unit. Questions could address the attentiveness of the nursing staff, how well privacy was respected, how easy it was to deal with insurance issues, whether patients were promptly informed of test results, and whether the care team answered questions fully. Medicare has begun reporting patient satisfaction using the Hospital Consumer Assessment of Healthcare Providers (HCAHPS) survey on Hospital Compare, but the data are not disease‐specific and audit a very small number of patients from each institution. Other services are unlikely to perform their own surveys, as multiple surveys would prove burdensome. Social networking sites that allow patients to post their own personal reviews of hospitals and doctors offer an additional if less reliable dimension to traditional public reporting. Such sites are already transforming the market for other industries, such as travel.22
4. Offer Composite Measures That Are Weighted and Evidence‐Based
Interpreting multiple measures, some of which are more important than others, and some of which have better evidence than others, is difficult for health care providers and may be impossible for patients. Is it more important to get aspirin on arrival or at discharge? Also, how does a patient weigh a 1% difference in the number of heart attack patients who get aspirin on arrival against a 14% difference in those who are offered smoking cessation? Because patients may be overwhelmed by data,23 public reports should include evidence‐based, weighted measures of overall care for a given condition, with higher weights attached to those process measures most likely to have clinical benefit, and careful attention to visual representations that convey relative differences.19, 23 More sophisticated measures should be developed to guard against overuse. For example, while hospitals should be rewarded for providing vaccination, they should be penalized for vaccinating the same patient twice.
None of the services we examined provides weighted outcomes. Leapfrog (The Leapfrog Group, Washington, DC) offers a composite snapshot containing 9 pie charts, divided into 4 leaps. The 6 pies representing high‐risk procedures are of equal size, even though 2 of these, esophagectomy and pancreatic resection represent very rare surgeries, even at major medical centers. From a visual perspective, however, these are equivalent to having computerized physician order entry and full‐time intensive care unit staffing, which affect thousands more patients. Similarly, in determining pay‐for‐performance measures, CMS created a composite based on the total number of opportunities of all interventions, weighting all measures equally. Because no validated weighting measures exist, future research will be necessary to achieve this goal. Also, none of the evidence‐based measures contained safeguards against overtreatment.
5. Cost Comparisons Should Include Patient Prices
In an era of patient copayments and deductibles, consumers are increasingly aware of costs. For patients with very high deductible plans or no health insurance, hospital fees are a common cause of bankruptcy.24 Several public reporting agencies, including Hospital Compare and HealthGrades have incorporated Medicare costs into their reported measures, but these have little connection to what patients actually pay. Health sites aimed at consumers should publish the average patient copayment.
Recommendations to Ensure That Data Reflects Hospital Quality
6. Adjust Outcomes for Severity and Risk
Not all bypass operations are the same and not all patients are at equal risk. More difficult operations (eg, CABG for a patient with a previous bypass) will have more complications; similarly, patients with serious comorbidities will experience worse outcomes. Since hospitals which specialize in a procedure will attract complicated cases and higher risk patients, it is important to adjust outcomes to account for these differences. Otherwise, hospitals and surgeons may be discouraged from taking difficult cases. Outside of cardiac surgery, most risk adjustment systems use administrative claims data but vary dramatically in the numbers of variables considered and the underlying proprietary models, which are often criticized as being black boxes that yield discordant results.25 Thus, a hospital's mortality may appear below expected by 1 system and above expected by another. Instead, risk adjustment systems should include clinical data abstracted from patient records using standardized data definitions. Although costly to collect, clinical data offer more predictive information than do administrative data. For example, for heart failure patients undergoing CABG, the ejection fraction predicts mortality better than many stable comorbid diagnoses. A single transparent risk‐adjustment system should be recognized as the industry standard. The American College of Surgeons' standardized risk‐adjusted outcome reporting for the National Surgical Quality Improvement Program (NSQIP) is a good example of such an effort.
7. Identify Differences Not Due to Chance
As a result of random variation, during any period, some hospitals will appear better than average and others worse. Statistical tests should be employed to identify hospitals that differ from the mean, and to allow consumers to compare 2 hospitals directly, with appropriate caveats when the hospitals serve very different patient populations. Medicare's mortality rating system for myocardial infarction identifies only 17 hospitals in the nation as better than average and 7 as worse, out of 4,500 institutions. HealthGrades compares hospitals' actual mortality or complication rates to their predicted rates based on disease‐specific logistic regression models and reports whether the hospital is statistically better or worse than predicted. Hospitals are not compared directly to one another. Given the rarity of mortality in most procedures, other outcome measures will be necessary to distinguish among hospitals.26
8. Standardize Reporting Periods
In a world of continuous quality improvement, public reporting should represent a hospital's recent performance, but reporting periods also need to be long enough to provide a stable estimate of infrequent events, especially at low‐volume institutions. In contrast, the lag time between the end of the reporting period and public availability should be kept to a minimum. We found that reporting periods varied from 1 to 3 years, and did not always cover the same years for all conditions, even on the same website. Some data were 3 years old. Patients will have a hard time making decisions on data that is 1 year old, and hospitals will have little incentive to make improvements that will not be acknowledged for years.
9. Avoid Use of Nonvalidated Administrative Data
Administrative data collected for billing purposes, unlike most clinical data, are already in electronic format, and can inexpensively produce quality rankings using validated models.27 In contrast, screening tools, such as the Agency for Healthcare Research and Quality's patient safety indicators (PSIs), were designed to identify potential quality problems, such as postoperative deep vein thrombosis, for internal quality improvement. Cases identified by the PSI software require additional chart review,28, 29 and should not be used as quality indicators. Even so, HealthGrades reports PSIs and some insurers use them in pay‐for‐performance initiatives. Improvements in PSIs, including present‐on‐admission coding, may increase accuracy,30 but these measures need to be validated before they can be adopted for public reporting.
10. Utilization Rates Should Be Evidence‐Based
Although utilization rates for most procedures vary as much as 2‐fold by state or institution, there is little evidence for a best rate. Nevertheless, HealthGrades reports utilization rates for several obstetrical procedures. At present, there are no standards for these, and it is possible that utilization could be too low in some places. Further research is needed; until then, utilization should not purport to measure quality.
Discussion
The growing commitment to making hospital performance data public could transform the quality and safety of care in the US, introducing competition on quality and price and fostering informed consumer choice. To date, the promise of public reporting remains only partially fulfilled. Few hospitals have done more than comply with regulatory mandates and payer incentives, and consumers have failed to respond. To capture the full benefits of public reporting, we have made 10 recommendations to benefit patients and better engage hospitals. We suggest that reporting be patient‐centered, with an emphasis on making the data useful, meaningful, important, interpretable, and relevant. At the same time, hospitals, which are being judged on their performance, should have a level playing field, with measures that are timely, consistent, severity‐adjusted, evidence‐based, and which foster good clinical care. Of the 3 services we examined, Hospital Compare came closest to meeting these recommendations.
Although this blueprint for public reporting is easy to draft, it is challenging to implement. In particular, some of our suggestions, such as the one regarding risk adjustment, may not currently be feasible, because the complexity and cost of collecting clinical data, even in the era of electronic medical records, may be prohibitive. Until such data are readily available, it may be preferable to report nothing at all, rather than report data that are misleading. In the rush to make hospitals accountable, enthusiasm has often outstripped science,31 and several measures have had to be revised for unintended consequences.32
Any initiative to improve public reporting should have the buy‐in of all stakeholders, but particularly hospitals, which stand to benefit in several ways. By receiving regular feedback, they can focus on improving care, becoming better organizations. These improvements may be rewarded through direct compensation (pay‐for‐performance), decreased costs from complications, or increased market share. Hospitals will be more engaged if the data reflect actual quality, are adequately adjusted for severity, and acknowledge the role of chance. Otherwise, they will merely comply, or worse, look for opportunities to game the system. To succeed, public reporting needs to involve hospitals in establishing standards for reporting and validation, as well as auditing procedures to prevent fraud.33 The Hospital Quality Alliance (HQA, Washington, DC), a first step in this direction, at present has few measures. NSQIP (American College of Surgeons, Chicago, IL) is perhaps a better example of hospitals cooperating to set measurement standards to promote best‐practices. Public release of NSQIP data might accelerate progress. Alternatively, the National Quality Forum (NQF, Washington, DC) could expand its role from endorsing quality measures to include standardizing the way these measures are used in public reporting.
Still, if you build it, will they come? To date, public reporting has not been embraced by the public, despite its stated interest in the information. Several explanations could be offered. First, we may be presenting the wrong data. Process measures and mortality rates are important but represent abstract concepts for most patients. Surveys tell us that patients value most the experiences of other patients.14, 21 They want to know whether their pain will be controlled, whether the doctor will listen to them, whether the nurse will come when they call. The recent advent of the HCAHPS survey (AHRQ, Washington, DC) is another positive step. Stratifying the results by diagnosis and adding a few diagnosis‐specific questions would make HCAHPS even more valuable. Second, the data may not be readily available. Although most public reporting is done on the web, older patients who are deciding about hospitals may not have Internet access. Some reports are still proprietary, and cost could present another obstacle. Finally, even if freely‐available and patient‐centered, the results may not be interpretable by physicians, let alone patients.34
If public reporting is to succeed, it will require measures that better reflect patients' concerns. In order to collect the massive amounts of data required and present them in a timely fashion, better electronic record systems will be necessary. But these are no panacea; others have noted that the Department of Veterans Affairs, a leader in electronic records, still invests considerable time and money to review charts for NSQIP.35 Given the value that Americans place on transparency in other facets of their lives, it is clear that public reporting is here to stay. While much progress has been made over the past 5 years, additional research is needed to better measure quality from the patient's perspective, and to determine how this information can be used to help guide decision‐making, and to reward hospitals for offering the highest‐quality care.
Acknowledgements
The authors thank Kenneth Flax for his help with an earlier version of this manuscript.
- Committee on Quality of Health Care in America IoM.Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, DC:National Academy Press;2001.
- ,,,.Care in U.S. hospitals: the Hospital Quality Alliance program.N Engl J Med.2005;353(3):265–274.
- .Achieving and sustaining improved quality: lessons from New York state and cardiac surgery.Health Aff. 20022002;21(4):40–51.
- ,,,,.Systematic review: the evidence that publishing patient care performance data improves quality of care.Ann Intern Med.2008;148(2):111–123.
- ,,.Hospital performance reports: impact on quality, market share, and reputation.Health Aff (Millwood).2005;24(4):1150–1160.
- ,,.Does publicizing hospital performance stimulate quality improvement efforts?Health Aff (Millwood).2003;22(2):84–94.
- ,,,,.Improving the outcomes of coronary artery bypass surgery in New York State.JAMA.1994;271(10):761–766.
- ,,.Declines in hospital mortality associated with a regional initiative to measure hospital performance.Am J Med Qual.1997;12(2):103–112.
- ,,, et al.Public reporting and pay for performance in hospital quality improvement.N Engl J Med.2007;356(5):486–496.
- ,,,,,.Do quality report cards play a role in HMOs' contracting practices? Evidence from New York State.Health Serv Res.2000;35(1 Pt 2):319–332.
- ,,,.Quality of cardiac surgeons and managed care contracting practices.Health Serv Res.2002;37(5):1129–1144.
- ,,,,,.Using performance data to identify preferred hospitals.Health Serv Res.2007;42(6 Pt 1):2109–2119; discussion 2294–2323.
- ,.Use of public performance reports: a survey of patients undergoing cardiac surgery.JAMA.1998;279(20):1638–1642.
- ,,.How do elderly patients decide where to go for major surgery? Telephone interview survey.BMJ.2005;331(7520):821.
- ,,,,,.The effect of publicly reporting hospital performance on market share and risk‐adjusted mortality at high‐mortality hospitals.Med Care.2003;41(6):729–740.
- ,.The predictive accuracy of the New York State coronary artery bypass surgery report‐card system.Health Aff (Millwood).2006;25(3):844–855.
- ,.Publicly disclosed information about the quality of health care: response of the US public.Qual Saf Health Care.2001;10(2):96–103.
- ,,,,.Choosing the best hospital: the limitations of public reporting of hospital quality.Health Aff (Millwood).2008;27(6):1680–1687.
- ,.Will quality report cards help consumers?Health Aff (Millwood).1997;16(3):218–228.
- Agency for Healthcare Research and Quality. HCUPnet, Healthcare Cost and Utilization Project. Available at: http://hcupnet.ahrq.gov. Accessed January 2009.
- ,,.Recovering from cardiac surgery: what patients want you to know.Am J Crit Care.2002;11(4):333–343.
- Trip Advisor. Available at: http://www.tripadvisor.com. Accessed January 2009.
- ,,,,.Less is more in presenting quality information to consumers.Med Care Res Rev.2007;64(2):169–190.
- ,,,.MarketWatch: illness and injury as contributors to bankruptcy.Health Aff (Millwood)2005;(Suppl Web Exclusives):W5‐63–W5‐73.
- .The Lake Wobegon effect: when all the patients are sicker.Am J Med Qual.2006;21(6):365–366.
- ,,.Surgical mortality as an indicator of hospital quality: the problem with small sample size.JAMA.2004;292(7):847–851.
- ,,, et al.An administrative claims model suitable for profiling hospital performance based on 30‐day mortality rates among patients with heart failure.Circulation.2006;113(13):1693–1701.
- ,,,.Can administrative data be used to compare postoperative complication rates across hospitals?Med Care.2002;40(10):856–867.
- ,,,,.Impact of diagnosis‐timing indicators on measures of safety, comorbidity, and case mix groupings from administrative data sources.Med Care.2007;45(8):781–788.
- ,,,,.Do the AHRQ patient safety indicators flag conditions that are present at the time of hospital admission?Med Care.2008;46(5):516–522.
- ,,.The tension between needing to improve care and knowing how to do it.N Engl J Med.2007;357(6):608–613.
- ,,,.Public reporting of antibiotic timing in patients with pneumonia: lessons from a flawed performance measure.Ann Intern Med.2008;149(1):29–32.
- ,,.The GAAP in quality measurement and reporting.JAMA.2007;298(15):1800–1802.
- ,,,.Consumer competencies and the use of comparative quality information: it isn't just about literacy.Med Care Res Rev.2007;64(4):379–394.
- .Performance measurement in search of a path.N Engl J Med.2007;356(9):951–953.
- Committee on Quality of Health Care in America IoM.Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, DC:National Academy Press;2001.
- ,,,.Care in U.S. hospitals: the Hospital Quality Alliance program.N Engl J Med.2005;353(3):265–274.
- .Achieving and sustaining improved quality: lessons from New York state and cardiac surgery.Health Aff. 20022002;21(4):40–51.
- ,,,,.Systematic review: the evidence that publishing patient care performance data improves quality of care.Ann Intern Med.2008;148(2):111–123.
- ,,.Hospital performance reports: impact on quality, market share, and reputation.Health Aff (Millwood).2005;24(4):1150–1160.
- ,,.Does publicizing hospital performance stimulate quality improvement efforts?Health Aff (Millwood).2003;22(2):84–94.
- ,,,,.Improving the outcomes of coronary artery bypass surgery in New York State.JAMA.1994;271(10):761–766.
- ,,.Declines in hospital mortality associated with a regional initiative to measure hospital performance.Am J Med Qual.1997;12(2):103–112.
- ,,, et al.Public reporting and pay for performance in hospital quality improvement.N Engl J Med.2007;356(5):486–496.
- ,,,,,.Do quality report cards play a role in HMOs' contracting practices? Evidence from New York State.Health Serv Res.2000;35(1 Pt 2):319–332.
- ,,,.Quality of cardiac surgeons and managed care contracting practices.Health Serv Res.2002;37(5):1129–1144.
- ,,,,,.Using performance data to identify preferred hospitals.Health Serv Res.2007;42(6 Pt 1):2109–2119; discussion 2294–2323.
- ,.Use of public performance reports: a survey of patients undergoing cardiac surgery.JAMA.1998;279(20):1638–1642.
- ,,.How do elderly patients decide where to go for major surgery? Telephone interview survey.BMJ.2005;331(7520):821.
- ,,,,,.The effect of publicly reporting hospital performance on market share and risk‐adjusted mortality at high‐mortality hospitals.Med Care.2003;41(6):729–740.
- ,.The predictive accuracy of the New York State coronary artery bypass surgery report‐card system.Health Aff (Millwood).2006;25(3):844–855.
- ,.Publicly disclosed information about the quality of health care: response of the US public.Qual Saf Health Care.2001;10(2):96–103.
- ,,,,.Choosing the best hospital: the limitations of public reporting of hospital quality.Health Aff (Millwood).2008;27(6):1680–1687.
- ,.Will quality report cards help consumers?Health Aff (Millwood).1997;16(3):218–228.
- Agency for Healthcare Research and Quality. HCUPnet, Healthcare Cost and Utilization Project. Available at: http://hcupnet.ahrq.gov. Accessed January 2009.
- ,,.Recovering from cardiac surgery: what patients want you to know.Am J Crit Care.2002;11(4):333–343.
- Trip Advisor. Available at: http://www.tripadvisor.com. Accessed January 2009.
- ,,,,.Less is more in presenting quality information to consumers.Med Care Res Rev.2007;64(2):169–190.
- ,,,.MarketWatch: illness and injury as contributors to bankruptcy.Health Aff (Millwood)2005;(Suppl Web Exclusives):W5‐63–W5‐73.
- .The Lake Wobegon effect: when all the patients are sicker.Am J Med Qual.2006;21(6):365–366.
- ,,.Surgical mortality as an indicator of hospital quality: the problem with small sample size.JAMA.2004;292(7):847–851.
- ,,, et al.An administrative claims model suitable for profiling hospital performance based on 30‐day mortality rates among patients with heart failure.Circulation.2006;113(13):1693–1701.
- ,,,.Can administrative data be used to compare postoperative complication rates across hospitals?Med Care.2002;40(10):856–867.
- ,,,,.Impact of diagnosis‐timing indicators on measures of safety, comorbidity, and case mix groupings from administrative data sources.Med Care.2007;45(8):781–788.
- ,,,,.Do the AHRQ patient safety indicators flag conditions that are present at the time of hospital admission?Med Care.2008;46(5):516–522.
- ,,.The tension between needing to improve care and knowing how to do it.N Engl J Med.2007;357(6):608–613.
- ,,,.Public reporting of antibiotic timing in patients with pneumonia: lessons from a flawed performance measure.Ann Intern Med.2008;149(1):29–32.
- ,,.The GAAP in quality measurement and reporting.JAMA.2007;298(15):1800–1802.
- ,,,.Consumer competencies and the use of comparative quality information: it isn't just about literacy.Med Care Res Rev.2007;64(4):379–394.
- .Performance measurement in search of a path.N Engl J Med.2007;356(9):951–953.
Copyright © 2009 Society of Hospital Medicine