User login
Novel Psoriasis Therapies and Patient Outcomes, Part 3: Systemic Medications
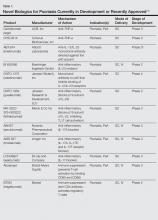
Evolving knowledge of the underlying pathogenesis of psoriasis has afforded the development of a broad spectrum of nonbiologic systemic medications with therapeutic potential in moderate to severe psoriasis and psoriatic arthritis (PsA). The targets for these medications are antagonists of proinflammatory mediators such as tyrosine kinase, protein kinase, Janus kinase (JAK), p38α mitogen-activated protein (MAP) kinase, phosphodiesterase 4 (PDE-4), calcineurin, Rho-associated kinase (ROCK) 2, cytochrome P450 26 (CYP26), and purine nucleoside phosphorylase (PNP); agonists of anti-inflammatory mediators such as sphingosine-1-phosphate receptor 1 (S1P1) and adeno-sine A3 receptor; and myriad other mechanisms. A brief introduction to some of these therapies presently in phase 2 and phase 3 clinical trials are presented (Table).1
Masitinib (Tyrosine Kinase Inhibitor)
Masitinib (formerly known as AB1010)(AB Sciences) is a tyrosine kinase inhibitor that is purported to decrease inflammation by inhibiting stem cell factor receptor (c-kit) and consequently limiting mast cell degranulation.2 A randomized, placebo-controlled, double-blind phase 3 study evaluating its efficacy as an oral formulation was completed, but the results were not available at the time of publication (registered at www.clinicaltrials.gov with the identifier NCT01045577).
Ponesimod (S1P1 Receptor Agonist)
Ponesimod (formerly known as ACT-128800)(Actelion Pharmaceuticals US, Inc) is an orally formulated S1P1 receptor agonist. Sphingosine-1-phosphate receptor 1 is necessary for lymphoid chemotaxis.3 A randomized, placebo-controlled, double-blind phase 2 trial of 326 patients demonstrated 75% improvement in psoriasis area and severity index (PASI) score at week 16 in 13.4%, 46.0%, and 48.1% of participants receiving placebo, ponesimod 20 mg, and ponesimod 40 mg, respectively.4 At week 28, PASI 75 scores for participants transitioned from ponesimod 40 mg to placebo or ponesimod 20 mg to placebo and those maintained on ponesimod 20 mg and 40 mg were 40.4%, 42.2%, 77.4%, and 71.4%, respectively. This study demonstrated benefit of treatment with ponesimod versus placebo with increased efficacy using maintenance therapy.4
Sotrastaurin (Protein Kinase C Inhibitor)
Sotrastaurin (formerly known as AEB071)(Novartis AG) is an oral medication that inhibitsprotein kinase C, thereby limiting CD28-induced activation of T cells. Furthermore, it increases forkhead box P3 expression, which is important, as proinflammatory IL-17 production is stimulated in regulatory T cells with lost forkhead box P3 expression.5 In a study of 32 patients who received placebo or sotrastaurin 25, 100, 200, or 300 mg twice daily for 2 weeks, the mean PASI score was reduced by 69% for the 300-mg group versus 5.3% for placebo.6 A randomized, placebo-controlled, double-blind phase 2 study was completed for patients with moderate to severe psoriasis, but the results were not available at the time of publication (NCT00885196).
Alitretinoin (Retinoid)
Alitretinoin (9-cis-retinoic acid; Stiefel, a GSK company) is an oral retinoid with purported promise for patients with palmoplantar pustular psoriasis recalcitrant to conventional therapies. In one study, 7 participants with palmoplantar psoriasis received alitretinoin 30 mg daily for 12 weeks with 60% to 90% clinical improvement noted using the visual analog scale and palmoplantar pustular PASI to assess response.7 A randomized, placebo-controlled, double-blind phase 2 trial of alitretinoin assessing the success of alitretinoin in patients with palmoplantar psoriasis recalcitrant to topical treatments was completed, but the results were not available at the time of publication (NCT01245140).
Apo805K1 (Unknown Mechanism of Action)
Apo805K1 (ApoPharma Inc) is an oral agent whose mechanism of action has not been disclosed. A randomized, placebo-controlled, double-blind phase 2 trial in patients with moderate to severe psoriasis with a treatment duration of 14 days has been completed. For 12 weeks there was a daily dosing regimen of Apo805K1 10, 30, 60, or 100 mg or placebo, with 12 patients in each treatment group. The proportion of patients achieving PASI 75 was 16.7%, 0%, 0%, 8.3%, and 16.7%, respectively (P=.1975). The number of participants with adverse events reported ranged between 4 to 7, with the greatest number of adverse events reported in the 30-mg subset.8 It may be of interest to repeat the study with a larger sample size.
ASP015K (JAK 1 and JAK 3 Inhibitor)
ASP015K (Astellas Pharma US, Inc) is the first of the JAK inhibitors that will be discussed in this section. Janus kinase inhibitors represent a group of tyrosine kinases that regulate cytokine-mediated signaling pathways through the activation of signal transducer and activator of transcription proteins via phosphorylation in the cytoplasm, which in turn control the transcription of genes that generate inflammation. ASP015K and tofacitinib (formerly known as CP-690550)(Pfizer, Inc) inhibit JAK 1 and JAK 3, whereas GSK2586184 (GlaxoSmithKline) inhibits JAK 1 and baricitinib (formerly known as LY3009104 or INCB28050)(Eli Lilly and Company) inhibits JAK 1 and JAK 2.9 In a 6-week, dose-escalation phase 2 trial in patients with moderate to severe psoriasis, ASP015K showed a dose-dependent decline in PASI, psoriasis static global assessment, and body surface area.10
BMS-582949 (p38α MAP Kinase Inhibitor)
BMS-582949 (Bristol-Myers Squibb Company) is an oral p38α MAP kinase inhibitor.11 Along with c-Jun N-terminal kinase and extracellular signal-regulated protein kinases 1 and 2, MAP kinase plays a role in the pathogenesis of psoriasis.12 A randomized, placebo-controlled, double-blind, 12-week phase 2a study of placebo versus BMS-582949 dosed at 10, 30, and 100 mg has been completed, but the results were not available at the time of publication (NCT00399906).
Apremilast (PDE-4 Inhibitor)
Apremilast (formerly known as CC-10004)(Celgene Corporation) is an oral PDE-4 inhibitor that acts to inhibit the degradation of cyclic adenosine monophosphate. It is approved by the US Food and Drug Administration for moderate to severe plaque psoriasis13 and is a particularly good treatment for patients with recalcitrant disease.14 Several studies on apremilast have been published, including a phase 2 trial of 204 participants receiving placebo or apremilast 20 mg or 40 mg twice daily with American College of Rheumatology (ACR) 20% improvement response of 11.8%, 35.8%, and 43.5%, respectively.15 The phase 3 PALACE 1, 2, 3, and 4 trials also demonstrated therapeutic efficacy.16 In PALACE 1, 504 patients receiving placebo or apremilast 20 mg and 30 mg twice daily had an ACR20 of 19%, 31%, and 40%, respectively. In the PALACE 4 study, ACR20 was achieved by 58% of participants at week 52, but the ACR50 and ACR70 rates were less impressive.16
Lestaurtinib (Multikinase Inhibitor)
Lestaurtinib (formerly known as CEP-701)(Teva Pharmaceutical Industries) is an oral multikinase inhibitor for which a 12-week, nonrandomized, dose-escalation phase 2 study was completed, but the results were not available at the time of publication (NCT00236119).
CF101 (Adenosine A3 Receptor Agonist)
CF101 (IB-MECA; Can-Fite BioPharma Ltd) is an oral adenosine A3 receptor agonist. Adenosine A3 is a G protein–coupled receptor that has an anti-inflammatory role, which lends itself to the treatment of inflammatory conditions such as rheumatoid arthritis.17 In a randomized, placebo-controlled, double-blind phase 2 trial of 75 patients who received placebo or CF101 1, 2, or 4 mg twice daily for 12 weeks, PASI 50 or greater was reported in 35.3% of participants in the 2-mg group, which was statistically significant at weeks 8 (P=.047) and 12 (P=.031).18 A randomized, placebo-controlled, double-blind, 16-week phase 2/phase 3 trial in patients with moderate to severe psoriasis treated with CF101 2 mg twice daily versus placebo is ongoing but not recruiting participants (NCT01265667).
Tofacitinib (JAK 1 and JAK 3 Inhibitor)
Tofacitinib is a JAK 1 and JAK 3 inhibitor that is available in both topical and oral formulations. Many studies have been published on tofacitinib, including a dose-ranging phase 2b trial of 197 patients randomized to placebo or tofacitinib 2, 5, or 15 mg daily in which 2.0%, 25.0% (P<.001), 40.8% (P<.0001), and 66.7% (P<.0001) of participants in each treatment group, respectively, achieved PASI 75 at week 12.19 Another 12-week phase 2b trial in patients with moderate to severe psoriasis explored the effectiveness of oral formulations of tofacitinib based on body location, namely the head and neck, arms, trunk, and legs.20 Designed with twice-daily placebo and tofacitinib 2-, 5-, and 15-mg treatment arms, the target plaque severity score demonstrated a statistically significant dose-responsive improvement in each of the 4 anatomic regions (P<.01).
Two randomized, double-blinded, placebo-controlled phase 3 trials of tofacitinib have been completed. One trial compared the safety and effectiveness of tofacitinib and etanercept in patients with moderate to severe psoriasis.21 The 329 participants were randomized to treatment with oral tofacitinib 5 mg or 10 mg twice daily with placebo subcutaneous (SQ) injections twice weekly; oral placebo twice daily with etanercept 50-mg SQ injections twice weekly; or oral placebo twice daily with placebo SQ injection twice weekly. At week 12, PASI 75 was achieved in 39.51%, 63.64%, 58.81%, and 5.61% of participants in each treatment group, respectively. Thus, tofacitinib 10 mg and etanercept 50 mg were the first and second best performers in this study design.21
In the other phase 3 trial, the efficacy and safety of treatment, treatment withdrawal, and subsequent resumption of treatment with tofacitinib was examined in 290 patients who received either tofacitinib 5 mg or 10 mg or placebo twice daily for 24 weeks.22 In the withdrawal period, the percentage of patients who maintained a PASI 75 response in the tofacitinib 5-mg group was 56.2% versus 23.3% in the placebo group at week 16 (P<.0008). In the 10-mg group, 62.3% of participants maintained PASI 75 at week 16 versus 26.1% in the placebo group (P<.0001). During the re-treatment period for those who showed a greater than 50% reduction in week 24 PASI during treatment withdrawal (N=102), 50.0% of the tofacitinib 5-mg group showed PASI 75 versus 31.58% of the placebo group at week 16. In the tofacitinib 10-mg group, PASI 75 was seen in 0% versus 50.85% in the placebo group. Of note, only 5 participants who demonstrated a greater than 50% reduction in week 24 PASI response following withdrawal of therapy were in the tofacitinib 5- or 10-mg groups, as the rest were in the placebo group.22
FP187 (Fumaric Acid Ester)
FP187 (Forward Pharma A/S) is an oral fumaric acid ester whose underlying mechanism is thought to be elevation of glutathione levels that decreases the amount of inflammatory cytokines by blocking the translocation of nuclear factor κB. Other fumaric acid esters such as monoethyl fumarate and dimethyl fumarate have been used in Europe for the management of psoriasis.23 A phase 2 study of the safety and effectiveness of FP187 for moderate to severe psoriasis has been completed, but the results were not available at the time of publication (NCT01230138). A phase 3 trial examining the efficacy of FP187 in managing moderate to severe psoriasis was not yet open for participant recruitment at the time of publication (NCT01815723).
Doxercalciferol (Vitamin D2 Analogue)
Doxercalciferol (1α-hydroxyvitamin D2; Genzyme Corporation, a Sanofi company) is an oral prodrug of vitamin D.24 Topical preparations of vitamin D analogues approved by the US Food and Drug Administration such as calcipotriene are mainstays in psoriasis management. Doxercalciferol has been used for other therapeutic applications such as decreasing elevated parathyroid hormone levels.25 Research has demonstrated that CYP24A1 can extrahepatically regulate the activation of vitamin D prodrugs in cutaneous tissues.26 In a phase 2 study examining the efficacy and safety of doxercalciferol in patients with moderate to severe psoriasis, 111 patients were randomized to placebo or doxercalciferol 2.5, 5, or 7.5 mg daily for 24 weeks (NCT00601107). At week 12, PASI 50 was similar regardless of the treatment administered, reported at 20.0%, 20.0%, 17.9%, and 20.0%, respectively (P=1.000).27
GSK2586184 (JAK 1 Inhibitor)
GSK2586184 is an oral JAK 1 inhibitor. A placebo-controlled, double-blind, dose-dependent phase 2 study of GSK2586184 in patients with chronic plaque psoriasis who were randomized to placebo or GSK2586184 100, 200, and 400 mg twice daily for 84 days has been completed, but the results were not available at the time of publication (NCT01782664). Of note, despite clinical promise, due to potential adverse effects of the medication that included a possible interaction with statin medication, the manufacturer decided against pursuing GSK2586184 as a treatment in the management of psoriasis.28
Voclosporin (Calcineurin Inhibitor)
Voclosporin (formerly known as ISA247)(Aurinia Pharmaceuticals Inc) is an oral calcineurin inhibitor that is purported to be as effective as cyclospor-ine A with less associated toxicity. A 12-week randomized, placebo-controlled, double-blind phase 2 trial demonstrated PASI 75 in 0%, 18.2%, and 66.7% of 201 participants receiving placebo, voclospo-rin 0.5 mg/kg, and voclosporin 1.5 mg/kg twice daily, respectively (P<.0001). Elevated serum creatinine levels within the high range of normal were noted in the 1.5-mg/kg group.29 A 12-week phase 3 study of 451 patients randomized to placebo or voclospo-rin 0.2-, 0.3-, or 0.4-mg/kg treatment groups similarly demonstrated a dose-responsive PASI 75 of 4%, 16%, 25%, and 47%, respectively, that was maintained at week 24. Mild to moderate decreases in glomerular filtration rates were noted in 8 patients in the 0.3- and 0.4-mg/kg subsets.30 Another phase 2 study of voclosporin in patients with moderate to severe psoriasis showed improvements according to the psoriasis disability index and dermatology life quality index.31
Three randomized, placebo-controlled, double-blind phase 3 trials have been completed for voclosporin, but the results were not available at the time of publication. The phase 3 ESSENCE trial compared voclosporin to placebo and ciclosporin controls (NCT00408187). Two phase 3 trials known as the SPIRIT trials—one a 12-week study (NCT00244842) and the other a 36-week extension trial (NCT00258713)—compared the efficacy of placebo to voclosporin administered at 0.2-, 0.3-, and 0.4-mg/kg doses.
KD025 (ROCK 2 Inhibitor)
KD025 (Kadmon Corporation, LLC) is an oral ROCK 2 inhibitor. The inhibition of ROCK in the ras homolog gene family, member A/ROCK pathway has been targeted therapeutically for pulmonary arterial hypertension,32 glaucoma,33 and many other uses. A phase 2a study assessing the tolerability and safety profile of KD025 in patients with moderate to severe psoriasis was completed, but the results were not available at the time of publication (NCT02106195).
LAS41008 (Fumaric Acid Ester)
LAS41008 (Almirall, S.A.) is purported to be an oral dimethyl fumarate that inhibits endothelial cell proliferation, migration, and differentiation.34 A phase 3 trial comparing the safety and effectiveness of LAS41008, LASW1835 (an active comparator), and placebo in patients with moderate to severe psoriasis is ongoing but not recruiting participants (NCT01726933).
LEO 22811 (Anti-inflammatory)
LEO 22811 (LEO Pharma) is an oral anti-inflammatory agent for the treatment of psoriasis. Two clinical trials have been completed, the results of which have not yet been published. A phase 1 trial assessing the tolerability and safety profile of LEO 22811 (NCT00833872) and a phase 2 proof-of-concept study assessing dose response of LEO 22811 versus placebo (NCT01116895) were completed, but the results were not available at the time of publication.
Baricitinib (JAK 1 and JAK 2 Inhibitor)
As noted above, baricitinib (LY3009104 or INCB28050) is a JAK 1 and JAK 2 inhibitor. A phase 2b trial examining dose response for LY3009104 or baricitinib administration in patients with moderate to severe psoriasis has been completed, but the results were not available at the time of publication (NCT01490632).
Talarozole (CYP26 Inhibitor)
Talarozole (formerly known as R115866)(GlaxoSmithKline) is a selective oral CYP26 inhibitor that could serve to regulate the degradation of all-trans retinoic acid in the management of conditions such as acne and psoriasis. One phase 2 nonrandomized, open-label study (NCT00725348) and another phase 2 randomized, placebo-controlled, double-blind, dose-dependent trial examining the effectiveness and safety profile of 12-week talarozole treatment in patients with severe plaque psoriasis have been completed, but the results were not available at the time of publication (NCT00716144).
R3421 or BCX-4208 (PNP Inhibitor)
R3421 or BCX-4208 (BioCryst Pharmaceuticals, Inc/Hoffman–La Roche) is an oral PNP inhibitor that may help regulate apoptosis of T cells and B cells.35 A randomized, placebo-controlled, double-blind, dose-dependent phase 2 trial in patients with moderate to severe psoriasis has been completed, but the results were not available at the time of publication (NCT00504270).
RWJ-445380 (Cathepsin S Inhibitor)
RWJ-445380 (Alza Corporation, DE) is an oral cathepsin S inhibitor. Cathepsin S is produced by antigen-presenting cells and activates CD4+ T cells via presentation of antigen by class II major histocompatibility complex.36 A randomized, placebo-controlled, double-blind, dose-dependent phase 2 trial evaluating the tolerability, safety, pharmacodynamics, and pharmacokinetics of RWJ-445380 in patients with plaque psoriasis has been completed, but the results were not available at the time of publication (NCT00396422).
SRT2104 (Sirtuin 1 Activator)
SRT2104 (GlaxoSmithKline) is a selective oral NAD+-dependent deacetylase sirtuin 1 activator. Sirtuins help regulate apoptosis, inflammation, and other important cellular mechanisms.37 A randomized, open-label phase 1 trial examining drug bioavailability (NCT01702493) and a randomized, placebo-controlled, double-blind, dose-dependent phase 2 trial studying the efficacy, tolerability, and safety of SRT2104 in patients with moderate to severe psoriasis have been completed (NCT01154101), but the results were not available at the time of publication.
VB-201 (Oxidized Phospholipid)
VB-201 (VBL Therapeutics) is an orally administered oxidized phospholipid analogue with anti-inflammatory effects.38 Oxidized phospholipids are another element in the intricate spectrum of inflammatory mediators. A randomized, placebo-controlled, double-blind, dose-dependent phase 2 trial of VB-201 20 mg or 80 mg daily in patients with moderate to severe psoriasis was completed, but the results were not available at the time of publication (NCT01001468). Another randomized, placebo-controlled, double-blind, dose-dependent phase 2 trial of VB-201 80 mg and 160 mg twice daily in patients with moderate to severe psoriasis has been completed, but the results were not available at the time of publication (NCT01837420).
Conclusion
We are living in an exciting time in the treatment of psoriasis and PsA, with promising novel therapies afforded by the discovery of new therapeutic targets. In this 3-part series, we have reviewed topical, systemic, and biologic therapies that currently are in phase 2 through phase 4 clinical trials or have recently been approved by the US Food and Drug Administration for the management of psoriasis and PsA. These treatments offer patients and their caregivers the prospect of more targeted therapeutic regimens that offer enhanced clinical outcomes with more favorable side-effect profiles. As clinicians and researchers build upon this knowledge in the years to come, we can offer psoriasis patients an increasingly diverse and powerful therapeutic armamentarium.
1. Lu PD, Mazza JM. Research pipeline III: biologic therapies. In: Weinberg JM, Lebwohl M, eds. Advances in Psoriasis. New York, NY: Springer; 2014:227-242.
2. Humbert M, de Blay F, Garcia G, et al. Masitinib, a c-kit/PDGF receptor tyrosine kinase inhibitor, improves disease control in severe corticosteroid-dependent asthmatics. Allergy. 2009;64:1194-1201.
3. Krause A, Brossard P, D’Ambrosio D, et al. Population pharmacokinetics and pharmacodynamics of ponesimod, a selective S1P1 receptor modulator. J Pharmacokinet Pharmacodyn. 2014;41:261-278.
4. Vaclavkova A, Chimenti S, Arenberger P, et al. Oral ponesimod in patients with chronic plaque psoriasis: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet. 2014;384:2036-2045.
5. He X, Koenen HJ, Smeets RL, et al. Targeting PKC in human T cells using sotrastaurin (AEB071) preserves regulatory T cells and prevents IL-17 production. J Invest Dermatol. 2014;134:975-983.
6. Skvara H, Dawid M, Kleyn E, et al. The PKC inhibitor AEB071 may be a therapeutic option for psoriasis. J Clin Invest. 2008;118:3151-3159.
7. Irla N, Navarini AA, Yawalkar N. Alitretinoin abrogates innate inflammation in palmoplantar pustular psoriasis. Br J Dermatol. 2012;167:1170-1174.
8. Study to evaluate Apo805K1 in subjects with moderate to severe chronic plaque psoriasis (NCT01483924). https://clinicaltrials.gov/ct2/results?term=NCT01483924&Search=Search. Updated February 9, 2015. Accessed June 23, 2015.
9. Kofoed K, Skov L, Zachariae C. New drugs and treatment targets in psoriasis. Acta Derm Venereol. 2015;95:133-139.
10. Gooderham M. Small molecules: an overview of emerging therapeutic options in the treatment of psoriasis. Skin Therapy Lett. 2013;18:1-4.
11. Liu C, Lin J, Wrobleski ST, et al. Discovery of 4-(5-(cyclopropylcarbamoyl)-2-methylphenylamino)-5-methyl-N-propylpyrrolo[1,2-f][1,2,4]triazine-6-carboxamide (BMS-582949), a clinical p38a MAP kinase inhibitor for the treatment of inflammatory diseases. J Med Chem. 2010;53:6629-6639.
12. Mavropoulos A, Rigopoulou EI, Liaskos C, et al. The role of p38 MAPK in the aetiopathogenesis of psoriasis and psoriatic arthritis. Clin Dev Immunol. 2013;2013:569751.
13. Oral OTEZLA® (apremilast) approved by the U.S. Food and Drug Administration for the treatment of patients with moderate to severe plaque psoriasis [news release]. Summit, NJ: Celegene Corporation; September 23, 2014. http://ir.celgene.com/releasedetail.cfm?releaseid=872240. Accessed June 23, 2015.
14. Gottlieb AB, Matheson RT, Menter A, et al. Efficacy, tolerability, and pharmacodynamics of apremilast in recalcitrant plaque psoriasis: a phase II open-label study. J Drugs Dermatol. 2013;12:888-897.
15. Moustafa F, Feldman SR. A review of phosphodiesterase-inhibition and the potential role for phosphodiesterase 4-inhibitors in clinical dermatology. Dermatol Online J. 2014;20:22608.
16. Huynh D, Kavanaugh A. Psoriatic arthritis: current therapy and future approaches. Rheumatology (Oxford). 2015;54:20-28.
17. Fishman P, Bar-Yehuda S, Liang BT, et al. Pharmacological and therapeutic effects of A3 adenosine receptor agonists. Drug Discov Today. 2012;17:359-366.
18. David M, Akerman L, Ziv M, et al. Treatment of plaque-type psoriasis with oral CF101: data from an exploratory randomized phase 2 clinical trial. J Eur Acad Dermatol Venereol. 2012;26:361-367.
19. Papp KA, Menter A, Strober B, et al. Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: a phase 2b randomized placebo-controlled dose-ranging study. Br J Dermatol. 2012;167:668-677.
20. Menter A, Papp KA, Tan H, et al. Efficacy of tofacitinib, an oral Janus kinase inhibitor, on clinical signs of moderate-to-severe plaque psoriasis in different body regions. J Drugs Dermatol. 2014;13:252-256.
21. A phase 3, multi site, randomized, double blind, placebo controlled study of the efficacy and safety comparing CP-690,550 and etanercept in subjects with moderate to severe chronic plaque psoriasis (NCT01241591). https://clinicaltrials.gov/ct2/show/NCT01241591?term=01241591&rank=1. Updated May 8, 2014. Accessed June 16, 2015.
22. A study to evaluate the effects and safety of treatment, treatment withdrawal, followed by re-treatment with CP-690,550 in subjects with moderate to severe chronic plaque psoriasis (NCT01186744). https://clinicaltrials.gov/ct2/show/NCT01186744?term=01186744&rank=1. Updated May 14, 2014. Accessed June 16, 2015.
23. Rostami Yazdi M, Mrowietz U. Fumaric acid esters. Clin Dermatol. 2008;26:522-526.
24. Kubodera N. A new look at the most successful prodrugs for active vitamin D (D hormone): alfacalcidol and doxercalciferol. Molecules. 2009;14:3869-3880.
25. Brown AJ. Therapeutic uses of vitamin D analogues. Am J Kidney Dis. 2001;38(5 suppl 5):S3-S19.
26. Masuda S, Strugnell SA, Knutson JC, et al. Evidence for the activation of 1alpha-hydroxyvitamin D2 by 25-hydroxyvitamin D-24-hydroxylase: delineation of pathways involving 1alpha,24-dihydroxyvitamin D2 and 1alpha,25-dihydroxyvitamin D2. Biochim Biophys Acta. 2006;1761:221-234.
27. A study to evaluate the safety and effectiveness of doxercalciferol capsules in participants with moderate to severe psoriasis (NCT00601107). https://clinicaltrials.gov/ct2/show/NCT00601107?term=00601107&rank=1. Updated April 2, 2014. Accessed June 16, 2015.
28. GSK discloses good efficacy in psoriasis with GSK2856184 [news release]. Mechelen, Belgium: Galapagos NV; November 6, 2014. http://www.globenewswire.com/news-release/2014/11/06/680358/10106744/en/GSK-discloses-good-efficacy-in-psoriasis-with-GSK2856184.html. Accessed June 24, 2015.
29. Bissonnette R, Papp K, Poulin Y, et al. A randomized, multicenter, double-blind, placebo-controlled phase 2 trial of ISA247 in patients with chronic plaque psoriasis. J Am Acad Dermatol. 2006;54:472-478.
30. Papp K, Bissonnette R, Rosoph L, et al. Efficacy of ISA247 in plaque psoriasis: a randomised, multicentre, double-blind, placebo-controlled phase III study. Lancet. 2008;371:1337-1342.
31. Gupta AK, Langley RG, Lynde C, et al. ISA247: quality of life results from a phase II, randomized, placebo-controlled study. J Cutan Med Surg. 2008;12:268-275.
32. Antoniu SA. Targeting RhoA/ROCK pathway in pulmonary arterial hypertension. Expert Opin Ther Targets. 2012;16:355-363.
33. Challa P, Arnold JJ. Rho-kinase inhibitors offer a new approach in the treatment of glaucoma. Expert Opin Investig Drugs. 2014;23:81-95.
34. National Institute for Health Research Horizon Scanning Centre. Dimethyl fumarate for plaque psoriasis. http://www.hsc.nihr.ac.uk/files/downloads/2228/2526.163fbff8.Dimethylfumarate_Nov13.pdf. Published November 2013. Accessed June 15, 2015.
35. Bantia S, Parker C, Upshaw R, et al. Potent orally bioavailable purine nucleoside phosphorylase inhibitor BCX-4208 induces apoptosis in B- and T-lymphocytes—a novel treatment approach for autoimmune diseases, organ transplantation and hematologic malignancies. Int Immunopharmacol. 2010;10:784-790.
36. García-Pérez ME, Stevanovic T, Poubelle PE. New therapies under development for psoriasis treatment. Curr Opin Pediatr. 2013;25:480-487.
37. Villalba JM, Alcaín FJ. Sirtuin activators and inhibitors. Biofactors. 2012;38:349-359.
38. Feige E, Mendel I, George J, et al. Modified phospholipids as anti-inflammatory compounds. Curr Opin Lipidol. 2010;21:525-529.
Evolving knowledge of the underlying pathogenesis of psoriasis has afforded the development of a broad spectrum of nonbiologic systemic medications with therapeutic potential in moderate to severe psoriasis and psoriatic arthritis (PsA). The targets for these medications are antagonists of proinflammatory mediators such as tyrosine kinase, protein kinase, Janus kinase (JAK), p38α mitogen-activated protein (MAP) kinase, phosphodiesterase 4 (PDE-4), calcineurin, Rho-associated kinase (ROCK) 2, cytochrome P450 26 (CYP26), and purine nucleoside phosphorylase (PNP); agonists of anti-inflammatory mediators such as sphingosine-1-phosphate receptor 1 (S1P1) and adeno-sine A3 receptor; and myriad other mechanisms. A brief introduction to some of these therapies presently in phase 2 and phase 3 clinical trials are presented (Table).1
Masitinib (Tyrosine Kinase Inhibitor)
Masitinib (formerly known as AB1010)(AB Sciences) is a tyrosine kinase inhibitor that is purported to decrease inflammation by inhibiting stem cell factor receptor (c-kit) and consequently limiting mast cell degranulation.2 A randomized, placebo-controlled, double-blind phase 3 study evaluating its efficacy as an oral formulation was completed, but the results were not available at the time of publication (registered at www.clinicaltrials.gov with the identifier NCT01045577).
Ponesimod (S1P1 Receptor Agonist)
Ponesimod (formerly known as ACT-128800)(Actelion Pharmaceuticals US, Inc) is an orally formulated S1P1 receptor agonist. Sphingosine-1-phosphate receptor 1 is necessary for lymphoid chemotaxis.3 A randomized, placebo-controlled, double-blind phase 2 trial of 326 patients demonstrated 75% improvement in psoriasis area and severity index (PASI) score at week 16 in 13.4%, 46.0%, and 48.1% of participants receiving placebo, ponesimod 20 mg, and ponesimod 40 mg, respectively.4 At week 28, PASI 75 scores for participants transitioned from ponesimod 40 mg to placebo or ponesimod 20 mg to placebo and those maintained on ponesimod 20 mg and 40 mg were 40.4%, 42.2%, 77.4%, and 71.4%, respectively. This study demonstrated benefit of treatment with ponesimod versus placebo with increased efficacy using maintenance therapy.4
Sotrastaurin (Protein Kinase C Inhibitor)
Sotrastaurin (formerly known as AEB071)(Novartis AG) is an oral medication that inhibitsprotein kinase C, thereby limiting CD28-induced activation of T cells. Furthermore, it increases forkhead box P3 expression, which is important, as proinflammatory IL-17 production is stimulated in regulatory T cells with lost forkhead box P3 expression.5 In a study of 32 patients who received placebo or sotrastaurin 25, 100, 200, or 300 mg twice daily for 2 weeks, the mean PASI score was reduced by 69% for the 300-mg group versus 5.3% for placebo.6 A randomized, placebo-controlled, double-blind phase 2 study was completed for patients with moderate to severe psoriasis, but the results were not available at the time of publication (NCT00885196).
Alitretinoin (Retinoid)
Alitretinoin (9-cis-retinoic acid; Stiefel, a GSK company) is an oral retinoid with purported promise for patients with palmoplantar pustular psoriasis recalcitrant to conventional therapies. In one study, 7 participants with palmoplantar psoriasis received alitretinoin 30 mg daily for 12 weeks with 60% to 90% clinical improvement noted using the visual analog scale and palmoplantar pustular PASI to assess response.7 A randomized, placebo-controlled, double-blind phase 2 trial of alitretinoin assessing the success of alitretinoin in patients with palmoplantar psoriasis recalcitrant to topical treatments was completed, but the results were not available at the time of publication (NCT01245140).
Apo805K1 (Unknown Mechanism of Action)
Apo805K1 (ApoPharma Inc) is an oral agent whose mechanism of action has not been disclosed. A randomized, placebo-controlled, double-blind phase 2 trial in patients with moderate to severe psoriasis with a treatment duration of 14 days has been completed. For 12 weeks there was a daily dosing regimen of Apo805K1 10, 30, 60, or 100 mg or placebo, with 12 patients in each treatment group. The proportion of patients achieving PASI 75 was 16.7%, 0%, 0%, 8.3%, and 16.7%, respectively (P=.1975). The number of participants with adverse events reported ranged between 4 to 7, with the greatest number of adverse events reported in the 30-mg subset.8 It may be of interest to repeat the study with a larger sample size.
ASP015K (JAK 1 and JAK 3 Inhibitor)
ASP015K (Astellas Pharma US, Inc) is the first of the JAK inhibitors that will be discussed in this section. Janus kinase inhibitors represent a group of tyrosine kinases that regulate cytokine-mediated signaling pathways through the activation of signal transducer and activator of transcription proteins via phosphorylation in the cytoplasm, which in turn control the transcription of genes that generate inflammation. ASP015K and tofacitinib (formerly known as CP-690550)(Pfizer, Inc) inhibit JAK 1 and JAK 3, whereas GSK2586184 (GlaxoSmithKline) inhibits JAK 1 and baricitinib (formerly known as LY3009104 or INCB28050)(Eli Lilly and Company) inhibits JAK 1 and JAK 2.9 In a 6-week, dose-escalation phase 2 trial in patients with moderate to severe psoriasis, ASP015K showed a dose-dependent decline in PASI, psoriasis static global assessment, and body surface area.10
BMS-582949 (p38α MAP Kinase Inhibitor)
BMS-582949 (Bristol-Myers Squibb Company) is an oral p38α MAP kinase inhibitor.11 Along with c-Jun N-terminal kinase and extracellular signal-regulated protein kinases 1 and 2, MAP kinase plays a role in the pathogenesis of psoriasis.12 A randomized, placebo-controlled, double-blind, 12-week phase 2a study of placebo versus BMS-582949 dosed at 10, 30, and 100 mg has been completed, but the results were not available at the time of publication (NCT00399906).
Apremilast (PDE-4 Inhibitor)
Apremilast (formerly known as CC-10004)(Celgene Corporation) is an oral PDE-4 inhibitor that acts to inhibit the degradation of cyclic adenosine monophosphate. It is approved by the US Food and Drug Administration for moderate to severe plaque psoriasis13 and is a particularly good treatment for patients with recalcitrant disease.14 Several studies on apremilast have been published, including a phase 2 trial of 204 participants receiving placebo or apremilast 20 mg or 40 mg twice daily with American College of Rheumatology (ACR) 20% improvement response of 11.8%, 35.8%, and 43.5%, respectively.15 The phase 3 PALACE 1, 2, 3, and 4 trials also demonstrated therapeutic efficacy.16 In PALACE 1, 504 patients receiving placebo or apremilast 20 mg and 30 mg twice daily had an ACR20 of 19%, 31%, and 40%, respectively. In the PALACE 4 study, ACR20 was achieved by 58% of participants at week 52, but the ACR50 and ACR70 rates were less impressive.16
Lestaurtinib (Multikinase Inhibitor)
Lestaurtinib (formerly known as CEP-701)(Teva Pharmaceutical Industries) is an oral multikinase inhibitor for which a 12-week, nonrandomized, dose-escalation phase 2 study was completed, but the results were not available at the time of publication (NCT00236119).
CF101 (Adenosine A3 Receptor Agonist)
CF101 (IB-MECA; Can-Fite BioPharma Ltd) is an oral adenosine A3 receptor agonist. Adenosine A3 is a G protein–coupled receptor that has an anti-inflammatory role, which lends itself to the treatment of inflammatory conditions such as rheumatoid arthritis.17 In a randomized, placebo-controlled, double-blind phase 2 trial of 75 patients who received placebo or CF101 1, 2, or 4 mg twice daily for 12 weeks, PASI 50 or greater was reported in 35.3% of participants in the 2-mg group, which was statistically significant at weeks 8 (P=.047) and 12 (P=.031).18 A randomized, placebo-controlled, double-blind, 16-week phase 2/phase 3 trial in patients with moderate to severe psoriasis treated with CF101 2 mg twice daily versus placebo is ongoing but not recruiting participants (NCT01265667).
Tofacitinib (JAK 1 and JAK 3 Inhibitor)
Tofacitinib is a JAK 1 and JAK 3 inhibitor that is available in both topical and oral formulations. Many studies have been published on tofacitinib, including a dose-ranging phase 2b trial of 197 patients randomized to placebo or tofacitinib 2, 5, or 15 mg daily in which 2.0%, 25.0% (P<.001), 40.8% (P<.0001), and 66.7% (P<.0001) of participants in each treatment group, respectively, achieved PASI 75 at week 12.19 Another 12-week phase 2b trial in patients with moderate to severe psoriasis explored the effectiveness of oral formulations of tofacitinib based on body location, namely the head and neck, arms, trunk, and legs.20 Designed with twice-daily placebo and tofacitinib 2-, 5-, and 15-mg treatment arms, the target plaque severity score demonstrated a statistically significant dose-responsive improvement in each of the 4 anatomic regions (P<.01).
Two randomized, double-blinded, placebo-controlled phase 3 trials of tofacitinib have been completed. One trial compared the safety and effectiveness of tofacitinib and etanercept in patients with moderate to severe psoriasis.21 The 329 participants were randomized to treatment with oral tofacitinib 5 mg or 10 mg twice daily with placebo subcutaneous (SQ) injections twice weekly; oral placebo twice daily with etanercept 50-mg SQ injections twice weekly; or oral placebo twice daily with placebo SQ injection twice weekly. At week 12, PASI 75 was achieved in 39.51%, 63.64%, 58.81%, and 5.61% of participants in each treatment group, respectively. Thus, tofacitinib 10 mg and etanercept 50 mg were the first and second best performers in this study design.21
In the other phase 3 trial, the efficacy and safety of treatment, treatment withdrawal, and subsequent resumption of treatment with tofacitinib was examined in 290 patients who received either tofacitinib 5 mg or 10 mg or placebo twice daily for 24 weeks.22 In the withdrawal period, the percentage of patients who maintained a PASI 75 response in the tofacitinib 5-mg group was 56.2% versus 23.3% in the placebo group at week 16 (P<.0008). In the 10-mg group, 62.3% of participants maintained PASI 75 at week 16 versus 26.1% in the placebo group (P<.0001). During the re-treatment period for those who showed a greater than 50% reduction in week 24 PASI during treatment withdrawal (N=102), 50.0% of the tofacitinib 5-mg group showed PASI 75 versus 31.58% of the placebo group at week 16. In the tofacitinib 10-mg group, PASI 75 was seen in 0% versus 50.85% in the placebo group. Of note, only 5 participants who demonstrated a greater than 50% reduction in week 24 PASI response following withdrawal of therapy were in the tofacitinib 5- or 10-mg groups, as the rest were in the placebo group.22
FP187 (Fumaric Acid Ester)
FP187 (Forward Pharma A/S) is an oral fumaric acid ester whose underlying mechanism is thought to be elevation of glutathione levels that decreases the amount of inflammatory cytokines by blocking the translocation of nuclear factor κB. Other fumaric acid esters such as monoethyl fumarate and dimethyl fumarate have been used in Europe for the management of psoriasis.23 A phase 2 study of the safety and effectiveness of FP187 for moderate to severe psoriasis has been completed, but the results were not available at the time of publication (NCT01230138). A phase 3 trial examining the efficacy of FP187 in managing moderate to severe psoriasis was not yet open for participant recruitment at the time of publication (NCT01815723).
Doxercalciferol (Vitamin D2 Analogue)
Doxercalciferol (1α-hydroxyvitamin D2; Genzyme Corporation, a Sanofi company) is an oral prodrug of vitamin D.24 Topical preparations of vitamin D analogues approved by the US Food and Drug Administration such as calcipotriene are mainstays in psoriasis management. Doxercalciferol has been used for other therapeutic applications such as decreasing elevated parathyroid hormone levels.25 Research has demonstrated that CYP24A1 can extrahepatically regulate the activation of vitamin D prodrugs in cutaneous tissues.26 In a phase 2 study examining the efficacy and safety of doxercalciferol in patients with moderate to severe psoriasis, 111 patients were randomized to placebo or doxercalciferol 2.5, 5, or 7.5 mg daily for 24 weeks (NCT00601107). At week 12, PASI 50 was similar regardless of the treatment administered, reported at 20.0%, 20.0%, 17.9%, and 20.0%, respectively (P=1.000).27
GSK2586184 (JAK 1 Inhibitor)
GSK2586184 is an oral JAK 1 inhibitor. A placebo-controlled, double-blind, dose-dependent phase 2 study of GSK2586184 in patients with chronic plaque psoriasis who were randomized to placebo or GSK2586184 100, 200, and 400 mg twice daily for 84 days has been completed, but the results were not available at the time of publication (NCT01782664). Of note, despite clinical promise, due to potential adverse effects of the medication that included a possible interaction with statin medication, the manufacturer decided against pursuing GSK2586184 as a treatment in the management of psoriasis.28
Voclosporin (Calcineurin Inhibitor)
Voclosporin (formerly known as ISA247)(Aurinia Pharmaceuticals Inc) is an oral calcineurin inhibitor that is purported to be as effective as cyclospor-ine A with less associated toxicity. A 12-week randomized, placebo-controlled, double-blind phase 2 trial demonstrated PASI 75 in 0%, 18.2%, and 66.7% of 201 participants receiving placebo, voclospo-rin 0.5 mg/kg, and voclosporin 1.5 mg/kg twice daily, respectively (P<.0001). Elevated serum creatinine levels within the high range of normal were noted in the 1.5-mg/kg group.29 A 12-week phase 3 study of 451 patients randomized to placebo or voclospo-rin 0.2-, 0.3-, or 0.4-mg/kg treatment groups similarly demonstrated a dose-responsive PASI 75 of 4%, 16%, 25%, and 47%, respectively, that was maintained at week 24. Mild to moderate decreases in glomerular filtration rates were noted in 8 patients in the 0.3- and 0.4-mg/kg subsets.30 Another phase 2 study of voclosporin in patients with moderate to severe psoriasis showed improvements according to the psoriasis disability index and dermatology life quality index.31
Three randomized, placebo-controlled, double-blind phase 3 trials have been completed for voclosporin, but the results were not available at the time of publication. The phase 3 ESSENCE trial compared voclosporin to placebo and ciclosporin controls (NCT00408187). Two phase 3 trials known as the SPIRIT trials—one a 12-week study (NCT00244842) and the other a 36-week extension trial (NCT00258713)—compared the efficacy of placebo to voclosporin administered at 0.2-, 0.3-, and 0.4-mg/kg doses.
KD025 (ROCK 2 Inhibitor)
KD025 (Kadmon Corporation, LLC) is an oral ROCK 2 inhibitor. The inhibition of ROCK in the ras homolog gene family, member A/ROCK pathway has been targeted therapeutically for pulmonary arterial hypertension,32 glaucoma,33 and many other uses. A phase 2a study assessing the tolerability and safety profile of KD025 in patients with moderate to severe psoriasis was completed, but the results were not available at the time of publication (NCT02106195).
LAS41008 (Fumaric Acid Ester)
LAS41008 (Almirall, S.A.) is purported to be an oral dimethyl fumarate that inhibits endothelial cell proliferation, migration, and differentiation.34 A phase 3 trial comparing the safety and effectiveness of LAS41008, LASW1835 (an active comparator), and placebo in patients with moderate to severe psoriasis is ongoing but not recruiting participants (NCT01726933).
LEO 22811 (Anti-inflammatory)
LEO 22811 (LEO Pharma) is an oral anti-inflammatory agent for the treatment of psoriasis. Two clinical trials have been completed, the results of which have not yet been published. A phase 1 trial assessing the tolerability and safety profile of LEO 22811 (NCT00833872) and a phase 2 proof-of-concept study assessing dose response of LEO 22811 versus placebo (NCT01116895) were completed, but the results were not available at the time of publication.
Baricitinib (JAK 1 and JAK 2 Inhibitor)
As noted above, baricitinib (LY3009104 or INCB28050) is a JAK 1 and JAK 2 inhibitor. A phase 2b trial examining dose response for LY3009104 or baricitinib administration in patients with moderate to severe psoriasis has been completed, but the results were not available at the time of publication (NCT01490632).
Talarozole (CYP26 Inhibitor)
Talarozole (formerly known as R115866)(GlaxoSmithKline) is a selective oral CYP26 inhibitor that could serve to regulate the degradation of all-trans retinoic acid in the management of conditions such as acne and psoriasis. One phase 2 nonrandomized, open-label study (NCT00725348) and another phase 2 randomized, placebo-controlled, double-blind, dose-dependent trial examining the effectiveness and safety profile of 12-week talarozole treatment in patients with severe plaque psoriasis have been completed, but the results were not available at the time of publication (NCT00716144).
R3421 or BCX-4208 (PNP Inhibitor)
R3421 or BCX-4208 (BioCryst Pharmaceuticals, Inc/Hoffman–La Roche) is an oral PNP inhibitor that may help regulate apoptosis of T cells and B cells.35 A randomized, placebo-controlled, double-blind, dose-dependent phase 2 trial in patients with moderate to severe psoriasis has been completed, but the results were not available at the time of publication (NCT00504270).
RWJ-445380 (Cathepsin S Inhibitor)
RWJ-445380 (Alza Corporation, DE) is an oral cathepsin S inhibitor. Cathepsin S is produced by antigen-presenting cells and activates CD4+ T cells via presentation of antigen by class II major histocompatibility complex.36 A randomized, placebo-controlled, double-blind, dose-dependent phase 2 trial evaluating the tolerability, safety, pharmacodynamics, and pharmacokinetics of RWJ-445380 in patients with plaque psoriasis has been completed, but the results were not available at the time of publication (NCT00396422).
SRT2104 (Sirtuin 1 Activator)
SRT2104 (GlaxoSmithKline) is a selective oral NAD+-dependent deacetylase sirtuin 1 activator. Sirtuins help regulate apoptosis, inflammation, and other important cellular mechanisms.37 A randomized, open-label phase 1 trial examining drug bioavailability (NCT01702493) and a randomized, placebo-controlled, double-blind, dose-dependent phase 2 trial studying the efficacy, tolerability, and safety of SRT2104 in patients with moderate to severe psoriasis have been completed (NCT01154101), but the results were not available at the time of publication.
VB-201 (Oxidized Phospholipid)
VB-201 (VBL Therapeutics) is an orally administered oxidized phospholipid analogue with anti-inflammatory effects.38 Oxidized phospholipids are another element in the intricate spectrum of inflammatory mediators. A randomized, placebo-controlled, double-blind, dose-dependent phase 2 trial of VB-201 20 mg or 80 mg daily in patients with moderate to severe psoriasis was completed, but the results were not available at the time of publication (NCT01001468). Another randomized, placebo-controlled, double-blind, dose-dependent phase 2 trial of VB-201 80 mg and 160 mg twice daily in patients with moderate to severe psoriasis has been completed, but the results were not available at the time of publication (NCT01837420).
Conclusion
We are living in an exciting time in the treatment of psoriasis and PsA, with promising novel therapies afforded by the discovery of new therapeutic targets. In this 3-part series, we have reviewed topical, systemic, and biologic therapies that currently are in phase 2 through phase 4 clinical trials or have recently been approved by the US Food and Drug Administration for the management of psoriasis and PsA. These treatments offer patients and their caregivers the prospect of more targeted therapeutic regimens that offer enhanced clinical outcomes with more favorable side-effect profiles. As clinicians and researchers build upon this knowledge in the years to come, we can offer psoriasis patients an increasingly diverse and powerful therapeutic armamentarium.
Evolving knowledge of the underlying pathogenesis of psoriasis has afforded the development of a broad spectrum of nonbiologic systemic medications with therapeutic potential in moderate to severe psoriasis and psoriatic arthritis (PsA). The targets for these medications are antagonists of proinflammatory mediators such as tyrosine kinase, protein kinase, Janus kinase (JAK), p38α mitogen-activated protein (MAP) kinase, phosphodiesterase 4 (PDE-4), calcineurin, Rho-associated kinase (ROCK) 2, cytochrome P450 26 (CYP26), and purine nucleoside phosphorylase (PNP); agonists of anti-inflammatory mediators such as sphingosine-1-phosphate receptor 1 (S1P1) and adeno-sine A3 receptor; and myriad other mechanisms. A brief introduction to some of these therapies presently in phase 2 and phase 3 clinical trials are presented (Table).1
Masitinib (Tyrosine Kinase Inhibitor)
Masitinib (formerly known as AB1010)(AB Sciences) is a tyrosine kinase inhibitor that is purported to decrease inflammation by inhibiting stem cell factor receptor (c-kit) and consequently limiting mast cell degranulation.2 A randomized, placebo-controlled, double-blind phase 3 study evaluating its efficacy as an oral formulation was completed, but the results were not available at the time of publication (registered at www.clinicaltrials.gov with the identifier NCT01045577).
Ponesimod (S1P1 Receptor Agonist)
Ponesimod (formerly known as ACT-128800)(Actelion Pharmaceuticals US, Inc) is an orally formulated S1P1 receptor agonist. Sphingosine-1-phosphate receptor 1 is necessary for lymphoid chemotaxis.3 A randomized, placebo-controlled, double-blind phase 2 trial of 326 patients demonstrated 75% improvement in psoriasis area and severity index (PASI) score at week 16 in 13.4%, 46.0%, and 48.1% of participants receiving placebo, ponesimod 20 mg, and ponesimod 40 mg, respectively.4 At week 28, PASI 75 scores for participants transitioned from ponesimod 40 mg to placebo or ponesimod 20 mg to placebo and those maintained on ponesimod 20 mg and 40 mg were 40.4%, 42.2%, 77.4%, and 71.4%, respectively. This study demonstrated benefit of treatment with ponesimod versus placebo with increased efficacy using maintenance therapy.4
Sotrastaurin (Protein Kinase C Inhibitor)
Sotrastaurin (formerly known as AEB071)(Novartis AG) is an oral medication that inhibitsprotein kinase C, thereby limiting CD28-induced activation of T cells. Furthermore, it increases forkhead box P3 expression, which is important, as proinflammatory IL-17 production is stimulated in regulatory T cells with lost forkhead box P3 expression.5 In a study of 32 patients who received placebo or sotrastaurin 25, 100, 200, or 300 mg twice daily for 2 weeks, the mean PASI score was reduced by 69% for the 300-mg group versus 5.3% for placebo.6 A randomized, placebo-controlled, double-blind phase 2 study was completed for patients with moderate to severe psoriasis, but the results were not available at the time of publication (NCT00885196).
Alitretinoin (Retinoid)
Alitretinoin (9-cis-retinoic acid; Stiefel, a GSK company) is an oral retinoid with purported promise for patients with palmoplantar pustular psoriasis recalcitrant to conventional therapies. In one study, 7 participants with palmoplantar psoriasis received alitretinoin 30 mg daily for 12 weeks with 60% to 90% clinical improvement noted using the visual analog scale and palmoplantar pustular PASI to assess response.7 A randomized, placebo-controlled, double-blind phase 2 trial of alitretinoin assessing the success of alitretinoin in patients with palmoplantar psoriasis recalcitrant to topical treatments was completed, but the results were not available at the time of publication (NCT01245140).
Apo805K1 (Unknown Mechanism of Action)
Apo805K1 (ApoPharma Inc) is an oral agent whose mechanism of action has not been disclosed. A randomized, placebo-controlled, double-blind phase 2 trial in patients with moderate to severe psoriasis with a treatment duration of 14 days has been completed. For 12 weeks there was a daily dosing regimen of Apo805K1 10, 30, 60, or 100 mg or placebo, with 12 patients in each treatment group. The proportion of patients achieving PASI 75 was 16.7%, 0%, 0%, 8.3%, and 16.7%, respectively (P=.1975). The number of participants with adverse events reported ranged between 4 to 7, with the greatest number of adverse events reported in the 30-mg subset.8 It may be of interest to repeat the study with a larger sample size.
ASP015K (JAK 1 and JAK 3 Inhibitor)
ASP015K (Astellas Pharma US, Inc) is the first of the JAK inhibitors that will be discussed in this section. Janus kinase inhibitors represent a group of tyrosine kinases that regulate cytokine-mediated signaling pathways through the activation of signal transducer and activator of transcription proteins via phosphorylation in the cytoplasm, which in turn control the transcription of genes that generate inflammation. ASP015K and tofacitinib (formerly known as CP-690550)(Pfizer, Inc) inhibit JAK 1 and JAK 3, whereas GSK2586184 (GlaxoSmithKline) inhibits JAK 1 and baricitinib (formerly known as LY3009104 or INCB28050)(Eli Lilly and Company) inhibits JAK 1 and JAK 2.9 In a 6-week, dose-escalation phase 2 trial in patients with moderate to severe psoriasis, ASP015K showed a dose-dependent decline in PASI, psoriasis static global assessment, and body surface area.10
BMS-582949 (p38α MAP Kinase Inhibitor)
BMS-582949 (Bristol-Myers Squibb Company) is an oral p38α MAP kinase inhibitor.11 Along with c-Jun N-terminal kinase and extracellular signal-regulated protein kinases 1 and 2, MAP kinase plays a role in the pathogenesis of psoriasis.12 A randomized, placebo-controlled, double-blind, 12-week phase 2a study of placebo versus BMS-582949 dosed at 10, 30, and 100 mg has been completed, but the results were not available at the time of publication (NCT00399906).
Apremilast (PDE-4 Inhibitor)
Apremilast (formerly known as CC-10004)(Celgene Corporation) is an oral PDE-4 inhibitor that acts to inhibit the degradation of cyclic adenosine monophosphate. It is approved by the US Food and Drug Administration for moderate to severe plaque psoriasis13 and is a particularly good treatment for patients with recalcitrant disease.14 Several studies on apremilast have been published, including a phase 2 trial of 204 participants receiving placebo or apremilast 20 mg or 40 mg twice daily with American College of Rheumatology (ACR) 20% improvement response of 11.8%, 35.8%, and 43.5%, respectively.15 The phase 3 PALACE 1, 2, 3, and 4 trials also demonstrated therapeutic efficacy.16 In PALACE 1, 504 patients receiving placebo or apremilast 20 mg and 30 mg twice daily had an ACR20 of 19%, 31%, and 40%, respectively. In the PALACE 4 study, ACR20 was achieved by 58% of participants at week 52, but the ACR50 and ACR70 rates were less impressive.16
Lestaurtinib (Multikinase Inhibitor)
Lestaurtinib (formerly known as CEP-701)(Teva Pharmaceutical Industries) is an oral multikinase inhibitor for which a 12-week, nonrandomized, dose-escalation phase 2 study was completed, but the results were not available at the time of publication (NCT00236119).
CF101 (Adenosine A3 Receptor Agonist)
CF101 (IB-MECA; Can-Fite BioPharma Ltd) is an oral adenosine A3 receptor agonist. Adenosine A3 is a G protein–coupled receptor that has an anti-inflammatory role, which lends itself to the treatment of inflammatory conditions such as rheumatoid arthritis.17 In a randomized, placebo-controlled, double-blind phase 2 trial of 75 patients who received placebo or CF101 1, 2, or 4 mg twice daily for 12 weeks, PASI 50 or greater was reported in 35.3% of participants in the 2-mg group, which was statistically significant at weeks 8 (P=.047) and 12 (P=.031).18 A randomized, placebo-controlled, double-blind, 16-week phase 2/phase 3 trial in patients with moderate to severe psoriasis treated with CF101 2 mg twice daily versus placebo is ongoing but not recruiting participants (NCT01265667).
Tofacitinib (JAK 1 and JAK 3 Inhibitor)
Tofacitinib is a JAK 1 and JAK 3 inhibitor that is available in both topical and oral formulations. Many studies have been published on tofacitinib, including a dose-ranging phase 2b trial of 197 patients randomized to placebo or tofacitinib 2, 5, or 15 mg daily in which 2.0%, 25.0% (P<.001), 40.8% (P<.0001), and 66.7% (P<.0001) of participants in each treatment group, respectively, achieved PASI 75 at week 12.19 Another 12-week phase 2b trial in patients with moderate to severe psoriasis explored the effectiveness of oral formulations of tofacitinib based on body location, namely the head and neck, arms, trunk, and legs.20 Designed with twice-daily placebo and tofacitinib 2-, 5-, and 15-mg treatment arms, the target plaque severity score demonstrated a statistically significant dose-responsive improvement in each of the 4 anatomic regions (P<.01).
Two randomized, double-blinded, placebo-controlled phase 3 trials of tofacitinib have been completed. One trial compared the safety and effectiveness of tofacitinib and etanercept in patients with moderate to severe psoriasis.21 The 329 participants were randomized to treatment with oral tofacitinib 5 mg or 10 mg twice daily with placebo subcutaneous (SQ) injections twice weekly; oral placebo twice daily with etanercept 50-mg SQ injections twice weekly; or oral placebo twice daily with placebo SQ injection twice weekly. At week 12, PASI 75 was achieved in 39.51%, 63.64%, 58.81%, and 5.61% of participants in each treatment group, respectively. Thus, tofacitinib 10 mg and etanercept 50 mg were the first and second best performers in this study design.21
In the other phase 3 trial, the efficacy and safety of treatment, treatment withdrawal, and subsequent resumption of treatment with tofacitinib was examined in 290 patients who received either tofacitinib 5 mg or 10 mg or placebo twice daily for 24 weeks.22 In the withdrawal period, the percentage of patients who maintained a PASI 75 response in the tofacitinib 5-mg group was 56.2% versus 23.3% in the placebo group at week 16 (P<.0008). In the 10-mg group, 62.3% of participants maintained PASI 75 at week 16 versus 26.1% in the placebo group (P<.0001). During the re-treatment period for those who showed a greater than 50% reduction in week 24 PASI during treatment withdrawal (N=102), 50.0% of the tofacitinib 5-mg group showed PASI 75 versus 31.58% of the placebo group at week 16. In the tofacitinib 10-mg group, PASI 75 was seen in 0% versus 50.85% in the placebo group. Of note, only 5 participants who demonstrated a greater than 50% reduction in week 24 PASI response following withdrawal of therapy were in the tofacitinib 5- or 10-mg groups, as the rest were in the placebo group.22
FP187 (Fumaric Acid Ester)
FP187 (Forward Pharma A/S) is an oral fumaric acid ester whose underlying mechanism is thought to be elevation of glutathione levels that decreases the amount of inflammatory cytokines by blocking the translocation of nuclear factor κB. Other fumaric acid esters such as monoethyl fumarate and dimethyl fumarate have been used in Europe for the management of psoriasis.23 A phase 2 study of the safety and effectiveness of FP187 for moderate to severe psoriasis has been completed, but the results were not available at the time of publication (NCT01230138). A phase 3 trial examining the efficacy of FP187 in managing moderate to severe psoriasis was not yet open for participant recruitment at the time of publication (NCT01815723).
Doxercalciferol (Vitamin D2 Analogue)
Doxercalciferol (1α-hydroxyvitamin D2; Genzyme Corporation, a Sanofi company) is an oral prodrug of vitamin D.24 Topical preparations of vitamin D analogues approved by the US Food and Drug Administration such as calcipotriene are mainstays in psoriasis management. Doxercalciferol has been used for other therapeutic applications such as decreasing elevated parathyroid hormone levels.25 Research has demonstrated that CYP24A1 can extrahepatically regulate the activation of vitamin D prodrugs in cutaneous tissues.26 In a phase 2 study examining the efficacy and safety of doxercalciferol in patients with moderate to severe psoriasis, 111 patients were randomized to placebo or doxercalciferol 2.5, 5, or 7.5 mg daily for 24 weeks (NCT00601107). At week 12, PASI 50 was similar regardless of the treatment administered, reported at 20.0%, 20.0%, 17.9%, and 20.0%, respectively (P=1.000).27
GSK2586184 (JAK 1 Inhibitor)
GSK2586184 is an oral JAK 1 inhibitor. A placebo-controlled, double-blind, dose-dependent phase 2 study of GSK2586184 in patients with chronic plaque psoriasis who were randomized to placebo or GSK2586184 100, 200, and 400 mg twice daily for 84 days has been completed, but the results were not available at the time of publication (NCT01782664). Of note, despite clinical promise, due to potential adverse effects of the medication that included a possible interaction with statin medication, the manufacturer decided against pursuing GSK2586184 as a treatment in the management of psoriasis.28
Voclosporin (Calcineurin Inhibitor)
Voclosporin (formerly known as ISA247)(Aurinia Pharmaceuticals Inc) is an oral calcineurin inhibitor that is purported to be as effective as cyclospor-ine A with less associated toxicity. A 12-week randomized, placebo-controlled, double-blind phase 2 trial demonstrated PASI 75 in 0%, 18.2%, and 66.7% of 201 participants receiving placebo, voclospo-rin 0.5 mg/kg, and voclosporin 1.5 mg/kg twice daily, respectively (P<.0001). Elevated serum creatinine levels within the high range of normal were noted in the 1.5-mg/kg group.29 A 12-week phase 3 study of 451 patients randomized to placebo or voclospo-rin 0.2-, 0.3-, or 0.4-mg/kg treatment groups similarly demonstrated a dose-responsive PASI 75 of 4%, 16%, 25%, and 47%, respectively, that was maintained at week 24. Mild to moderate decreases in glomerular filtration rates were noted in 8 patients in the 0.3- and 0.4-mg/kg subsets.30 Another phase 2 study of voclosporin in patients with moderate to severe psoriasis showed improvements according to the psoriasis disability index and dermatology life quality index.31
Three randomized, placebo-controlled, double-blind phase 3 trials have been completed for voclosporin, but the results were not available at the time of publication. The phase 3 ESSENCE trial compared voclosporin to placebo and ciclosporin controls (NCT00408187). Two phase 3 trials known as the SPIRIT trials—one a 12-week study (NCT00244842) and the other a 36-week extension trial (NCT00258713)—compared the efficacy of placebo to voclosporin administered at 0.2-, 0.3-, and 0.4-mg/kg doses.
KD025 (ROCK 2 Inhibitor)
KD025 (Kadmon Corporation, LLC) is an oral ROCK 2 inhibitor. The inhibition of ROCK in the ras homolog gene family, member A/ROCK pathway has been targeted therapeutically for pulmonary arterial hypertension,32 glaucoma,33 and many other uses. A phase 2a study assessing the tolerability and safety profile of KD025 in patients with moderate to severe psoriasis was completed, but the results were not available at the time of publication (NCT02106195).
LAS41008 (Fumaric Acid Ester)
LAS41008 (Almirall, S.A.) is purported to be an oral dimethyl fumarate that inhibits endothelial cell proliferation, migration, and differentiation.34 A phase 3 trial comparing the safety and effectiveness of LAS41008, LASW1835 (an active comparator), and placebo in patients with moderate to severe psoriasis is ongoing but not recruiting participants (NCT01726933).
LEO 22811 (Anti-inflammatory)
LEO 22811 (LEO Pharma) is an oral anti-inflammatory agent for the treatment of psoriasis. Two clinical trials have been completed, the results of which have not yet been published. A phase 1 trial assessing the tolerability and safety profile of LEO 22811 (NCT00833872) and a phase 2 proof-of-concept study assessing dose response of LEO 22811 versus placebo (NCT01116895) were completed, but the results were not available at the time of publication.
Baricitinib (JAK 1 and JAK 2 Inhibitor)
As noted above, baricitinib (LY3009104 or INCB28050) is a JAK 1 and JAK 2 inhibitor. A phase 2b trial examining dose response for LY3009104 or baricitinib administration in patients with moderate to severe psoriasis has been completed, but the results were not available at the time of publication (NCT01490632).
Talarozole (CYP26 Inhibitor)
Talarozole (formerly known as R115866)(GlaxoSmithKline) is a selective oral CYP26 inhibitor that could serve to regulate the degradation of all-trans retinoic acid in the management of conditions such as acne and psoriasis. One phase 2 nonrandomized, open-label study (NCT00725348) and another phase 2 randomized, placebo-controlled, double-blind, dose-dependent trial examining the effectiveness and safety profile of 12-week talarozole treatment in patients with severe plaque psoriasis have been completed, but the results were not available at the time of publication (NCT00716144).
R3421 or BCX-4208 (PNP Inhibitor)
R3421 or BCX-4208 (BioCryst Pharmaceuticals, Inc/Hoffman–La Roche) is an oral PNP inhibitor that may help regulate apoptosis of T cells and B cells.35 A randomized, placebo-controlled, double-blind, dose-dependent phase 2 trial in patients with moderate to severe psoriasis has been completed, but the results were not available at the time of publication (NCT00504270).
RWJ-445380 (Cathepsin S Inhibitor)
RWJ-445380 (Alza Corporation, DE) is an oral cathepsin S inhibitor. Cathepsin S is produced by antigen-presenting cells and activates CD4+ T cells via presentation of antigen by class II major histocompatibility complex.36 A randomized, placebo-controlled, double-blind, dose-dependent phase 2 trial evaluating the tolerability, safety, pharmacodynamics, and pharmacokinetics of RWJ-445380 in patients with plaque psoriasis has been completed, but the results were not available at the time of publication (NCT00396422).
SRT2104 (Sirtuin 1 Activator)
SRT2104 (GlaxoSmithKline) is a selective oral NAD+-dependent deacetylase sirtuin 1 activator. Sirtuins help regulate apoptosis, inflammation, and other important cellular mechanisms.37 A randomized, open-label phase 1 trial examining drug bioavailability (NCT01702493) and a randomized, placebo-controlled, double-blind, dose-dependent phase 2 trial studying the efficacy, tolerability, and safety of SRT2104 in patients with moderate to severe psoriasis have been completed (NCT01154101), but the results were not available at the time of publication.
VB-201 (Oxidized Phospholipid)
VB-201 (VBL Therapeutics) is an orally administered oxidized phospholipid analogue with anti-inflammatory effects.38 Oxidized phospholipids are another element in the intricate spectrum of inflammatory mediators. A randomized, placebo-controlled, double-blind, dose-dependent phase 2 trial of VB-201 20 mg or 80 mg daily in patients with moderate to severe psoriasis was completed, but the results were not available at the time of publication (NCT01001468). Another randomized, placebo-controlled, double-blind, dose-dependent phase 2 trial of VB-201 80 mg and 160 mg twice daily in patients with moderate to severe psoriasis has been completed, but the results were not available at the time of publication (NCT01837420).
Conclusion
We are living in an exciting time in the treatment of psoriasis and PsA, with promising novel therapies afforded by the discovery of new therapeutic targets. In this 3-part series, we have reviewed topical, systemic, and biologic therapies that currently are in phase 2 through phase 4 clinical trials or have recently been approved by the US Food and Drug Administration for the management of psoriasis and PsA. These treatments offer patients and their caregivers the prospect of more targeted therapeutic regimens that offer enhanced clinical outcomes with more favorable side-effect profiles. As clinicians and researchers build upon this knowledge in the years to come, we can offer psoriasis patients an increasingly diverse and powerful therapeutic armamentarium.
1. Lu PD, Mazza JM. Research pipeline III: biologic therapies. In: Weinberg JM, Lebwohl M, eds. Advances in Psoriasis. New York, NY: Springer; 2014:227-242.
2. Humbert M, de Blay F, Garcia G, et al. Masitinib, a c-kit/PDGF receptor tyrosine kinase inhibitor, improves disease control in severe corticosteroid-dependent asthmatics. Allergy. 2009;64:1194-1201.
3. Krause A, Brossard P, D’Ambrosio D, et al. Population pharmacokinetics and pharmacodynamics of ponesimod, a selective S1P1 receptor modulator. J Pharmacokinet Pharmacodyn. 2014;41:261-278.
4. Vaclavkova A, Chimenti S, Arenberger P, et al. Oral ponesimod in patients with chronic plaque psoriasis: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet. 2014;384:2036-2045.
5. He X, Koenen HJ, Smeets RL, et al. Targeting PKC in human T cells using sotrastaurin (AEB071) preserves regulatory T cells and prevents IL-17 production. J Invest Dermatol. 2014;134:975-983.
6. Skvara H, Dawid M, Kleyn E, et al. The PKC inhibitor AEB071 may be a therapeutic option for psoriasis. J Clin Invest. 2008;118:3151-3159.
7. Irla N, Navarini AA, Yawalkar N. Alitretinoin abrogates innate inflammation in palmoplantar pustular psoriasis. Br J Dermatol. 2012;167:1170-1174.
8. Study to evaluate Apo805K1 in subjects with moderate to severe chronic plaque psoriasis (NCT01483924). https://clinicaltrials.gov/ct2/results?term=NCT01483924&Search=Search. Updated February 9, 2015. Accessed June 23, 2015.
9. Kofoed K, Skov L, Zachariae C. New drugs and treatment targets in psoriasis. Acta Derm Venereol. 2015;95:133-139.
10. Gooderham M. Small molecules: an overview of emerging therapeutic options in the treatment of psoriasis. Skin Therapy Lett. 2013;18:1-4.
11. Liu C, Lin J, Wrobleski ST, et al. Discovery of 4-(5-(cyclopropylcarbamoyl)-2-methylphenylamino)-5-methyl-N-propylpyrrolo[1,2-f][1,2,4]triazine-6-carboxamide (BMS-582949), a clinical p38a MAP kinase inhibitor for the treatment of inflammatory diseases. J Med Chem. 2010;53:6629-6639.
12. Mavropoulos A, Rigopoulou EI, Liaskos C, et al. The role of p38 MAPK in the aetiopathogenesis of psoriasis and psoriatic arthritis. Clin Dev Immunol. 2013;2013:569751.
13. Oral OTEZLA® (apremilast) approved by the U.S. Food and Drug Administration for the treatment of patients with moderate to severe plaque psoriasis [news release]. Summit, NJ: Celegene Corporation; September 23, 2014. http://ir.celgene.com/releasedetail.cfm?releaseid=872240. Accessed June 23, 2015.
14. Gottlieb AB, Matheson RT, Menter A, et al. Efficacy, tolerability, and pharmacodynamics of apremilast in recalcitrant plaque psoriasis: a phase II open-label study. J Drugs Dermatol. 2013;12:888-897.
15. Moustafa F, Feldman SR. A review of phosphodiesterase-inhibition and the potential role for phosphodiesterase 4-inhibitors in clinical dermatology. Dermatol Online J. 2014;20:22608.
16. Huynh D, Kavanaugh A. Psoriatic arthritis: current therapy and future approaches. Rheumatology (Oxford). 2015;54:20-28.
17. Fishman P, Bar-Yehuda S, Liang BT, et al. Pharmacological and therapeutic effects of A3 adenosine receptor agonists. Drug Discov Today. 2012;17:359-366.
18. David M, Akerman L, Ziv M, et al. Treatment of plaque-type psoriasis with oral CF101: data from an exploratory randomized phase 2 clinical trial. J Eur Acad Dermatol Venereol. 2012;26:361-367.
19. Papp KA, Menter A, Strober B, et al. Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: a phase 2b randomized placebo-controlled dose-ranging study. Br J Dermatol. 2012;167:668-677.
20. Menter A, Papp KA, Tan H, et al. Efficacy of tofacitinib, an oral Janus kinase inhibitor, on clinical signs of moderate-to-severe plaque psoriasis in different body regions. J Drugs Dermatol. 2014;13:252-256.
21. A phase 3, multi site, randomized, double blind, placebo controlled study of the efficacy and safety comparing CP-690,550 and etanercept in subjects with moderate to severe chronic plaque psoriasis (NCT01241591). https://clinicaltrials.gov/ct2/show/NCT01241591?term=01241591&rank=1. Updated May 8, 2014. Accessed June 16, 2015.
22. A study to evaluate the effects and safety of treatment, treatment withdrawal, followed by re-treatment with CP-690,550 in subjects with moderate to severe chronic plaque psoriasis (NCT01186744). https://clinicaltrials.gov/ct2/show/NCT01186744?term=01186744&rank=1. Updated May 14, 2014. Accessed June 16, 2015.
23. Rostami Yazdi M, Mrowietz U. Fumaric acid esters. Clin Dermatol. 2008;26:522-526.
24. Kubodera N. A new look at the most successful prodrugs for active vitamin D (D hormone): alfacalcidol and doxercalciferol. Molecules. 2009;14:3869-3880.
25. Brown AJ. Therapeutic uses of vitamin D analogues. Am J Kidney Dis. 2001;38(5 suppl 5):S3-S19.
26. Masuda S, Strugnell SA, Knutson JC, et al. Evidence for the activation of 1alpha-hydroxyvitamin D2 by 25-hydroxyvitamin D-24-hydroxylase: delineation of pathways involving 1alpha,24-dihydroxyvitamin D2 and 1alpha,25-dihydroxyvitamin D2. Biochim Biophys Acta. 2006;1761:221-234.
27. A study to evaluate the safety and effectiveness of doxercalciferol capsules in participants with moderate to severe psoriasis (NCT00601107). https://clinicaltrials.gov/ct2/show/NCT00601107?term=00601107&rank=1. Updated April 2, 2014. Accessed June 16, 2015.
28. GSK discloses good efficacy in psoriasis with GSK2856184 [news release]. Mechelen, Belgium: Galapagos NV; November 6, 2014. http://www.globenewswire.com/news-release/2014/11/06/680358/10106744/en/GSK-discloses-good-efficacy-in-psoriasis-with-GSK2856184.html. Accessed June 24, 2015.
29. Bissonnette R, Papp K, Poulin Y, et al. A randomized, multicenter, double-blind, placebo-controlled phase 2 trial of ISA247 in patients with chronic plaque psoriasis. J Am Acad Dermatol. 2006;54:472-478.
30. Papp K, Bissonnette R, Rosoph L, et al. Efficacy of ISA247 in plaque psoriasis: a randomised, multicentre, double-blind, placebo-controlled phase III study. Lancet. 2008;371:1337-1342.
31. Gupta AK, Langley RG, Lynde C, et al. ISA247: quality of life results from a phase II, randomized, placebo-controlled study. J Cutan Med Surg. 2008;12:268-275.
32. Antoniu SA. Targeting RhoA/ROCK pathway in pulmonary arterial hypertension. Expert Opin Ther Targets. 2012;16:355-363.
33. Challa P, Arnold JJ. Rho-kinase inhibitors offer a new approach in the treatment of glaucoma. Expert Opin Investig Drugs. 2014;23:81-95.
34. National Institute for Health Research Horizon Scanning Centre. Dimethyl fumarate for plaque psoriasis. http://www.hsc.nihr.ac.uk/files/downloads/2228/2526.163fbff8.Dimethylfumarate_Nov13.pdf. Published November 2013. Accessed June 15, 2015.
35. Bantia S, Parker C, Upshaw R, et al. Potent orally bioavailable purine nucleoside phosphorylase inhibitor BCX-4208 induces apoptosis in B- and T-lymphocytes—a novel treatment approach for autoimmune diseases, organ transplantation and hematologic malignancies. Int Immunopharmacol. 2010;10:784-790.
36. García-Pérez ME, Stevanovic T, Poubelle PE. New therapies under development for psoriasis treatment. Curr Opin Pediatr. 2013;25:480-487.
37. Villalba JM, Alcaín FJ. Sirtuin activators and inhibitors. Biofactors. 2012;38:349-359.
38. Feige E, Mendel I, George J, et al. Modified phospholipids as anti-inflammatory compounds. Curr Opin Lipidol. 2010;21:525-529.
1. Lu PD, Mazza JM. Research pipeline III: biologic therapies. In: Weinberg JM, Lebwohl M, eds. Advances in Psoriasis. New York, NY: Springer; 2014:227-242.
2. Humbert M, de Blay F, Garcia G, et al. Masitinib, a c-kit/PDGF receptor tyrosine kinase inhibitor, improves disease control in severe corticosteroid-dependent asthmatics. Allergy. 2009;64:1194-1201.
3. Krause A, Brossard P, D’Ambrosio D, et al. Population pharmacokinetics and pharmacodynamics of ponesimod, a selective S1P1 receptor modulator. J Pharmacokinet Pharmacodyn. 2014;41:261-278.
4. Vaclavkova A, Chimenti S, Arenberger P, et al. Oral ponesimod in patients with chronic plaque psoriasis: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet. 2014;384:2036-2045.
5. He X, Koenen HJ, Smeets RL, et al. Targeting PKC in human T cells using sotrastaurin (AEB071) preserves regulatory T cells and prevents IL-17 production. J Invest Dermatol. 2014;134:975-983.
6. Skvara H, Dawid M, Kleyn E, et al. The PKC inhibitor AEB071 may be a therapeutic option for psoriasis. J Clin Invest. 2008;118:3151-3159.
7. Irla N, Navarini AA, Yawalkar N. Alitretinoin abrogates innate inflammation in palmoplantar pustular psoriasis. Br J Dermatol. 2012;167:1170-1174.
8. Study to evaluate Apo805K1 in subjects with moderate to severe chronic plaque psoriasis (NCT01483924). https://clinicaltrials.gov/ct2/results?term=NCT01483924&Search=Search. Updated February 9, 2015. Accessed June 23, 2015.
9. Kofoed K, Skov L, Zachariae C. New drugs and treatment targets in psoriasis. Acta Derm Venereol. 2015;95:133-139.
10. Gooderham M. Small molecules: an overview of emerging therapeutic options in the treatment of psoriasis. Skin Therapy Lett. 2013;18:1-4.
11. Liu C, Lin J, Wrobleski ST, et al. Discovery of 4-(5-(cyclopropylcarbamoyl)-2-methylphenylamino)-5-methyl-N-propylpyrrolo[1,2-f][1,2,4]triazine-6-carboxamide (BMS-582949), a clinical p38a MAP kinase inhibitor for the treatment of inflammatory diseases. J Med Chem. 2010;53:6629-6639.
12. Mavropoulos A, Rigopoulou EI, Liaskos C, et al. The role of p38 MAPK in the aetiopathogenesis of psoriasis and psoriatic arthritis. Clin Dev Immunol. 2013;2013:569751.
13. Oral OTEZLA® (apremilast) approved by the U.S. Food and Drug Administration for the treatment of patients with moderate to severe plaque psoriasis [news release]. Summit, NJ: Celegene Corporation; September 23, 2014. http://ir.celgene.com/releasedetail.cfm?releaseid=872240. Accessed June 23, 2015.
14. Gottlieb AB, Matheson RT, Menter A, et al. Efficacy, tolerability, and pharmacodynamics of apremilast in recalcitrant plaque psoriasis: a phase II open-label study. J Drugs Dermatol. 2013;12:888-897.
15. Moustafa F, Feldman SR. A review of phosphodiesterase-inhibition and the potential role for phosphodiesterase 4-inhibitors in clinical dermatology. Dermatol Online J. 2014;20:22608.
16. Huynh D, Kavanaugh A. Psoriatic arthritis: current therapy and future approaches. Rheumatology (Oxford). 2015;54:20-28.
17. Fishman P, Bar-Yehuda S, Liang BT, et al. Pharmacological and therapeutic effects of A3 adenosine receptor agonists. Drug Discov Today. 2012;17:359-366.
18. David M, Akerman L, Ziv M, et al. Treatment of plaque-type psoriasis with oral CF101: data from an exploratory randomized phase 2 clinical trial. J Eur Acad Dermatol Venereol. 2012;26:361-367.
19. Papp KA, Menter A, Strober B, et al. Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: a phase 2b randomized placebo-controlled dose-ranging study. Br J Dermatol. 2012;167:668-677.
20. Menter A, Papp KA, Tan H, et al. Efficacy of tofacitinib, an oral Janus kinase inhibitor, on clinical signs of moderate-to-severe plaque psoriasis in different body regions. J Drugs Dermatol. 2014;13:252-256.
21. A phase 3, multi site, randomized, double blind, placebo controlled study of the efficacy and safety comparing CP-690,550 and etanercept in subjects with moderate to severe chronic plaque psoriasis (NCT01241591). https://clinicaltrials.gov/ct2/show/NCT01241591?term=01241591&rank=1. Updated May 8, 2014. Accessed June 16, 2015.
22. A study to evaluate the effects and safety of treatment, treatment withdrawal, followed by re-treatment with CP-690,550 in subjects with moderate to severe chronic plaque psoriasis (NCT01186744). https://clinicaltrials.gov/ct2/show/NCT01186744?term=01186744&rank=1. Updated May 14, 2014. Accessed June 16, 2015.
23. Rostami Yazdi M, Mrowietz U. Fumaric acid esters. Clin Dermatol. 2008;26:522-526.
24. Kubodera N. A new look at the most successful prodrugs for active vitamin D (D hormone): alfacalcidol and doxercalciferol. Molecules. 2009;14:3869-3880.
25. Brown AJ. Therapeutic uses of vitamin D analogues. Am J Kidney Dis. 2001;38(5 suppl 5):S3-S19.
26. Masuda S, Strugnell SA, Knutson JC, et al. Evidence for the activation of 1alpha-hydroxyvitamin D2 by 25-hydroxyvitamin D-24-hydroxylase: delineation of pathways involving 1alpha,24-dihydroxyvitamin D2 and 1alpha,25-dihydroxyvitamin D2. Biochim Biophys Acta. 2006;1761:221-234.
27. A study to evaluate the safety and effectiveness of doxercalciferol capsules in participants with moderate to severe psoriasis (NCT00601107). https://clinicaltrials.gov/ct2/show/NCT00601107?term=00601107&rank=1. Updated April 2, 2014. Accessed June 16, 2015.
28. GSK discloses good efficacy in psoriasis with GSK2856184 [news release]. Mechelen, Belgium: Galapagos NV; November 6, 2014. http://www.globenewswire.com/news-release/2014/11/06/680358/10106744/en/GSK-discloses-good-efficacy-in-psoriasis-with-GSK2856184.html. Accessed June 24, 2015.
29. Bissonnette R, Papp K, Poulin Y, et al. A randomized, multicenter, double-blind, placebo-controlled phase 2 trial of ISA247 in patients with chronic plaque psoriasis. J Am Acad Dermatol. 2006;54:472-478.
30. Papp K, Bissonnette R, Rosoph L, et al. Efficacy of ISA247 in plaque psoriasis: a randomised, multicentre, double-blind, placebo-controlled phase III study. Lancet. 2008;371:1337-1342.
31. Gupta AK, Langley RG, Lynde C, et al. ISA247: quality of life results from a phase II, randomized, placebo-controlled study. J Cutan Med Surg. 2008;12:268-275.
32. Antoniu SA. Targeting RhoA/ROCK pathway in pulmonary arterial hypertension. Expert Opin Ther Targets. 2012;16:355-363.
33. Challa P, Arnold JJ. Rho-kinase inhibitors offer a new approach in the treatment of glaucoma. Expert Opin Investig Drugs. 2014;23:81-95.
34. National Institute for Health Research Horizon Scanning Centre. Dimethyl fumarate for plaque psoriasis. http://www.hsc.nihr.ac.uk/files/downloads/2228/2526.163fbff8.Dimethylfumarate_Nov13.pdf. Published November 2013. Accessed June 15, 2015.
35. Bantia S, Parker C, Upshaw R, et al. Potent orally bioavailable purine nucleoside phosphorylase inhibitor BCX-4208 induces apoptosis in B- and T-lymphocytes—a novel treatment approach for autoimmune diseases, organ transplantation and hematologic malignancies. Int Immunopharmacol. 2010;10:784-790.
36. García-Pérez ME, Stevanovic T, Poubelle PE. New therapies under development for psoriasis treatment. Curr Opin Pediatr. 2013;25:480-487.
37. Villalba JM, Alcaín FJ. Sirtuin activators and inhibitors. Biofactors. 2012;38:349-359.
38. Feige E, Mendel I, George J, et al. Modified phospholipids as anti-inflammatory compounds. Curr Opin Lipidol. 2010;21:525-529.
Practice Points
- A wide spectrum of promising nonbiologic systemic medications presently are in development or were recently approved for the management of moderate to severe psoriasis and psoriatic arthritis (PsA).
- Systemic medications broaden the therapeutic armamentarium for patients with psoriasis and PsA, offering targeted therapeutic regimens that afford enhanced clinical outcomes with favorable side-effect profiles.
Novel Psoriasis Therapies and Patient Outcomes, Part 2: Biologic Treatments
Biologic agents that currently are in use for the management of moderate to severe psoriasis and psoriatic arthritis (PsA) include the anti–tumor necrosis factor (TNF) α monoclonal antibodies adalimumab, etanercept, and infliximab1; however, additional TNF-α inhibitors as well as drugs targeting other pathways presently are in the pipeline. Novel biologic treatments currently in phase 2 through phase 4 clinical trials, including those that have recently been approved by the US Food and Drug Administration (FDA), are discussed in this article, and a summary is provided in Table 1.
Tumor Necrosis Factor α Inhibitors
Certolizumab Pegol
Certolizumab pegol (CZP; UCB, Inc), a pegylated TNF-α inhibitor, is unique in that it does not possess a fragment crystallizable (Fc) region and consequently does not trigger complement activation. The drug is presently FDA approved for active PsA, rheumatoid arthritis, and ankylosing spondylitis. One phase 2 study reported psoriasis area severity index (PASI) scores of 75 in 83% (48/58) of participants who received CZP 400 mg at week 0 and every other week until week 10 (P<.001 vs placebo).3 In a 24-week phase 3 study (known as RAPID-PsA), 409 participants were randomized into 3 study arms: (1) CZP 400 mg every 4 weeks; (2) CZP 200 mg every 2 weeks; (3) placebo every 2 weeks.4 Of note, 20% of participants had previously received a TNF inhibitor. The study demonstrated improvements in participant-reported outcomes with use of CZP regardless of prior TNF inhibitor use.4
CHS-0214
CHS-0214 (Coherus BioSciences, Inc) is a TNF-α inhibitor and etanercept biosimilar that has entered into a 48-week multicenter phase 3 trial (known as RaPsOdy) for patients with chronic plaque psoriasis. The purpose of the study is to compare PASI scores for CHS-0214 and etanercept to evaluate immunogenicity, safety, and effectiveness over a 12-week period.5 Comparable pharmacokinetics were established in an earlier study.6
Inhibition of the IL-12/IL-23 Pathway
IL-12 and IL-23 are cytokines with prostaglan-din E2–mediated production by dendritic cells that share structural (eg, the p40 subunit) and functional similarities (eg, IFN-γ production). However, each has distinct characteristics. IL-12 aids in naive CD4+ T-cell differentiation, while IL-23 induces IL-17 production by CD4+ memory T cells. IL-17 triggers a proinflammatory chemokine cascade and produces IL-1, IL-6, nitric oxide synthase 2, and TNF-α.7
Briakinumab (ABT-874)
Briakinumab (formerly known as ABT-874)(Abbott Laboratories) is a human monoclonal antibody that inhibits the p40 subunit of IL-12 and IL-23. In a phase 3 trial of 350 participants with moderate to severe psoriasis, week 12 PASI 75 scores were achieved in 80.6% of participants who received briakinumab versus 39.6% of those who received etanercept and 6.9% of those who received placebo.8 In a 52-week phase 3 trial of 317 participants with moderate to severe psoriasis, PASI 75 scores were observed in 81.8% of participants who received briakinumab versus 39.9% of those who received methotrexate.9 In another 52-week phase 3 trial of 1465 participants with moderate to severe psoriasis, clinical benefit was reported at 12 weeks in 75.9% of participants for Dermatology Life Quality Index, and 64.8% and 54.1% for psoriasis- and PsA-related pain scores, respectively.10 However, ABT-874 was withdrawn by the manufacturer as of 2011 due to concerns regarding adverse cardiovascular events.9
BI 655066
BI 655066 (Boehringer Ingelheim GmbH) is a human monoclonal antibody that targets the p19 subunit of IL-23. A phase 1 study of the pharmacokinetics and pharmacodynamics of intravenous (IV) versus subcutaneous (SC) administration of BI 655066 as well as its safety and effectiveness versus placebo recently was completed (NCT01577550), but the results were not available at the time of publication. A phase 2 study comparing 3 dosing regimens of BI 655066 versus ustekinumab is ongoing but not actively recruiting patients at the time of publi-cation (NCT02054481).
Ustekinumab (CNTO 1275)
Ustekinumab (formerly known as CNTO 1275)(Janssen Biotech, Inc) is a human monoclonal antibody that inhibits the p40 subunit of IL-12 and IL-23. It was FDA approved for treatment of moderate to severe plaque psoriasis in September 200911 and PsA in September 201312 for adult patients 18 years or older. One phase 3 trial (known as ACCEPT) compared the effectiveness of ustekinumab versus etanercept in 903 participants with moderate to severe psoriasis at 67 centers worldwide.13 Participants were randomly assigned to receive SC injections of either 45 mg or 90 mg of ustekinumab (at weeks 0 and 4) or high-dose etanercept (50 mg twice weekly for 12 weeks). At week 12, PASI 75 was noted in 67.5% of participants who received 45 mg of ustekinumab and 73.8% of participants who received 90 mg compared to 56.8% of those who received etanercept (P=.01 and P<.001, respectively). In participants who showed no response to etanercept, PASI 75 was achieved in 48.9% within 12 weeks after crossover to ustekinumab. One or more adverse events (AEs) occurred through week 12 in 66.0% of the 45-mg ustekinumab group, 69.2% of the 90-mg group, and 70.0% of the etanercept group; serious AEs were noted in 1.9%, 1.2%, and 1.2%, respectively.13 A 5-year follow-up study of 3117 participants reported an incidence of AEs with ustekinumab that was comparable to other biologics, with malignancy and mortality rates comparable to age-matched controls.14
In a phase 3, multicenter, double-blind, placebo-controlled trial (know as PSUMMIT I), 615 adults with active PsA who had not previously been treated with TNF inhibitors were randomly assigned to placebo, 45 mg of ustekinumab, or 90 mg of ustekinumab. At week 24, more participants receiving ustekinumab 90 mg achieved 20%, 50%, and 70% improvement in American College of Rheumatology (ACR) criteria (49.5%, 27.9%, and 14.2%, respectively) and PASI 75 (62.4%) versus the placebo group (22.8%, 8.7%, 2.4%, and 11%, respectively).15 In a phase 3, multicenter, placebo-controlled trial (known as PSUMMIT 2), 312 adult participants with active PsA who had formerly been treated with conventional therapies and/or TNF inhibitors were randomized to receive placebo (at weeks 0, 4, and 16 with crossover to 45 mg of ustekinumab at weeks 24, 28, and 40) or ustekinumab (45 mg or 90 mg at weeks 0, 4, and every 12 weeks).16 For participants with less than 5% improvement, there was an early escape clinical trial design with placebo to 45 mg of ustekinumab, 45 mg of ustekinumab to 90 mg, and 90 mg of ustekinumab maintained at the same dose. The ACR20 was 43.8% for the ustekinumab group versus 20.2% for the placebo group (P<.001).16
A phase 3, multicenter, randomized, double-blind, placebo-controlled study (known as CADMUS) evaluated the efficacy and safety of ustekinumab in the treatment of adolescents (age range, 12–18 years) with moderate to severe plaque-type psoriasis.17 The primary outcome of the study was the percentage of participants achieving a physician global assessment (PGA) score of cleared (0) or minimal (1) at week 12. One hundred ten participants started and completed the first period in the study (ie, controlled period [weeks 0–12]) and were randomized into 3 groups: placebo (SC injections at weeks 0 and 4), ustekinumab half-standard dose, and ustekinumab standard dose. At week 12, 101 participants started and completed the second period in the study (weeks 12–60). The placebo group received either ustekinumab half-standard dose or ustekinumab standard dose at weeks 12 and 16, then once every 12 weeks with the last dose at week 40, and the ustekinumab half-standard and standard dose groups received the respective doses every 12 weeks with the last dose at week 40. At week 12, PGA scores of 0 or 1 were reported in 5.4% of the placebo group, 67.6% of the ustekinumab half-standard dose group, and 69.4% of the ustekinumab standard dose group (P<.001), and PASI 75 was achieved in 10.8%, 78.4%, and 80.6%, respectively (P<.001).17
A phase 4 study (known as TRANSIT) assessed the safety and efficacy of ustekinumab in participants with plaque psoriasis who had a suboptimal response to methotrexate.18 Participants in the first treatment group received either 45 mg (weight, ≤100 kg) of ustekinumab at weeks 0, 4, and then every 12 weeks until week 40, or 90 mg (weight, >100 kg) in 2 SC injections after immediate discontinuation of methotrexate. The second treatment group followed the same dosing regimen with gradual withdrawal of methotrexate therapy. Adverse events were reported in 61.1% and 64.5% of participants in groups 1 and 2, respectively. In group 1, PASI 75 was observed in 58.1% of participants (95% confi-dence interval [CI], 51.9%-64.3%) at week 12 and 76.3% (95% CI, 70.8%-81.9%) at week 52. In group 2, PASI 75 was observed in 62.2% of participants (95% CI, 56.0%-68.3%) at week 12 and 76.9% (95% CI, 71.4%-82.5%) at week 52.18
In another study that assessed the efficacy and safety of ustekinumab in 24 participants with moderate to severe palmoplantar psoriasis, 37.5% of participants achieved a palmar/plantar PGA score of 0 or 1 at week 16.19 A phase 3, multicenter, randomized, double-blind, placebo-controlled study of the safety and effectiveness of ustekinumab in 615 PsA participants showed ACR20 response in 49.5% of the ustekinumab 90-mg group, 42.4% of the ustekinumab 45-mg group, and 22.8% of the placebo group (P<.001).20
A phase 1 study was performed to assess gene expression in the following: (1) IFN-γ modulation in the IL-12 pathway; (2) IL-23 pathway with ustekinumab (45 mg for those weighing <100 kg and 90 mg for ≤100 kg administered SC on day 1 and at weeks 4 and 16); and (3) IL-17 pathway with etanercept (50 mg administered SC twice weekly for 12 weeks, then once weekly for 4 weeks).21 The change in gene expression from baseline in the IL-12 pathway with ustekinumab achieved statistical significance by week 1 (P=.016) with increasing levels of gene expression through week 16 (P=.000184). The change in gene expression from baseline in the IL-23 pathway with ustekinumab achieved statistical significance by week 2 (P=.010) with increasing levels of gene expression through week 16 (P=.000215). The results were less powerful for etanercept, with a change in gene expression from baseline in the IL-17 pathway increasing through week 4 (P=.053) and decreasing by week 16 (P=.098).21
Several clinical trials are underway and are currently recruiting participants (Table 2).
Guselkumab (CNTO 1959)
Guselkumab (formerly known as CNTO 1959)(Janssen Research & Development, LLC) is a human monoclonal antibody targeting the p19 subunit of IL-23. In a double-blind, randomized study of 24 participants receiving 1 dose of CNTO 1959 at 10 mg, 30 mg, 100 mg, or 300 mg versus placebo, a PASI 75 of 50% for the 10-mg subset, 60% for the 30- and 100-mg group, and 100% for the 300-mg group was achieved as opposed to 0% in the placebo group at 12 weeks.22 The rate of AEs was 65% in the CNTO 1959 treatment arm versus 50% in the placebo group at 24 weeks. Furthermore, decreased serum IL-17A titers and gene expression for psoriasis was demonstrated as well as decreased thickness of the epidermis and less dendritic and T-cell expression for the CNTO 1959 study population histologically.22 Results of a phase 2 trial in 293 participants who received CNTO 1959, adalimumab, or placebo indicated PASI 75 at 16 weeks for 81% of the CNTO 1959 50-mg group versus 71% of the adalimumab group, with serious AEs in 3% of participants treated with CNTO 1959 versus 5% treated with adalimumab.23
Tildrakizumab (MK-3222/SCH 900222)
Tildrakizumab (formerly known as MK-3222/SCH 900222)(Merck & Co Inc) is a monoclonal antibody that also targets the p19 subunit of IL-23. Results of a phase 2b trial were promising. This study reported on 355 participants who received placebo versus MK-3222 5 mg, 25 mg, 100 mg, or 200 mg, with PASI 75 scores of 4.4%, 33%, 64%, 66%, and 74%, respectively, noted at 16 weeks.24 A 64-week phase 3 study currently is underway to assess the long-term benefit and safety of MK-3222, but it is not recruiting participants (NCT01722331).
Inhibition of the IL-17 Pathway
The T helper 17 cells (TH17) produce IL-17, a cytokine mediating inflammation that is implicated in psoriasis. Two products target IL-17A, while another targets the IL-17 receptor.25
Secukinumab (AIN457)
Secukinumab (formerly known as AIN457)(Novartis Pharmaceutical Corporation) was FDA approved for treatment of moderate to severe psoriasis in adult patients who are candidates for systemic therapy or phototherapy in January 2015.26 Secukinumab is a human monoclonal antibody that inhibits IL-17A. There are many clinical trials underway including a phase 2 extension study (NCT01132612). Many phase 3 studies also are underway evaluating the effectiveness and safety of AIN457 in patients with psoriasis resistant to TNF inhibitors (NCT01961609); its usability and tolerability (NCT01555125), including 2-year extension studies (NCT01640951; NCT01544595); its effectiveness as opposed to ustekinumab (NCT02074982); effectiveness using an autoinjector (NCT01636687); and the PASI 90 in HLA-Cw6–positive and HLA-Cw6–negative patients with moderate to severe psoriasis (known as SUPREME)(NCT02394561).
Other phase 3 trials are being undertaken in patients with moderate to severe palmoplantar psoriasis (NCT01806597; NCT02008890); moderate to severe nail psoriasis (known as TRANSFIGURE)(NCT01807520); moderate to severe scalp psoriasis (NCT02267135); and PsA (NCT01989468; NCT02294227; NCT01892436), including a 5-year study for PsA (known as FUTURE 2)(NCT01752634).
Other studies that are completed with pending results include a phase 1 trial to evaluate its mechanism of action in vivo by studying the spread of AIN457 in tissue as assessed by open flow microperfusion (NCT01539213), a phase 2 trial of the clinical effectiveness of AIN457 at 12 months and biomarker changes (NCT01537432), as well as phase 3 trials of the clinical efficacy of various dosing regimens (known as SCULPTURE)(NCT01406938); safety and effectiveness at 1 year (known as ERASURE)(NCT01365455); and the effectiveness, tolerability, and safety of AIN457 over 2 years in PsA (known as FUTURE 1)(NCT01392326).
In a 56-week phase 2 clinical trial of 100 participants, the PASI scores at 12 weeks and percentage of participants without relapse up to 56 weeks were evaluated.27 There were 4 arms in the study: (1) AIN457 3 mg/kg (day 1) then placebo (days 15 and 29); (2) AIN457 10 mg/kg (day 1) then placebo (days 15 and 29); (3) AIN457 10 mg/kg (days 1, 15, and 29); and (4) placebo (days 1, 15, and 29), with AIN457 and the placebo administered IV. The mean (standard deviation) change from baseline for PASI scores for these respective groups was -12.46 (7.668), -13.35 (6.195), -18.02 (6.792), and -4.18 (4.698), respectively. At week 56, the percentage of participants without a relapse at any point during the study was 12.5%, 22.2%, and 27.8%, respectively.27
In a phase 2 study of 404 participants, PASI 75 scores were assessed at 12 weeks with the SC administration of AIN457 in participants with moderate to severe psoriasis at 3 dosing regimens: (1) a single dose of 150 mg (week 1), (2) monthly doses of 150 mg (weeks 1, 5, and 9), (3) early loading doses of 150 mg (weeks 1, 2, 3, 5, and 9), as compared to placebo. At 12 weeks, PASI 75 scores were 7%, 58%, 72%, and 1%, respectively.28
The phase 3 STATURE trial assessed the safety and effectiveness of SC and IV AIN457 in moderate to severe psoriasis in partial AIN457 nonresponders.29 Nonresponders were participants who demonstrated a PASI score of 50% or more but less than 75%. Participants in this study design who received SC AIN457 demonstrated a PASI 75 of 66.7%, with a 2011 investigator global assessment score of 0 (clear) or 1 (almost clear) in 66.7%. In those receiving IV AIN457, the PASI 75 was 90.5%, with a 2011 investigator global assessment score of 0 or 1 in 33.3%.29
In a 52-week phase 3 efficacy trial (known as FIXTURE), 1306 participants received 1 dose of AIN457 300 mg or 150 mg weekly for 5 weeks, then every 4 weeks; 12 weeks of etanercept 50 mg twice weekly, then once weekly; or placebo. The PASI 75 was 77.1% for AIN457 300 mg, 67.0% for AIN457 150 mg, 44.0% for etanercept, and 4.9% for placebo (P<.001).30 In a 52-week efficacy and safety trial (known as ERASURE), 738 participants received 1 dose of AIN457 300 mg or 150 mg weekly for 5 weeks, then every 4 weeks, versus placebo. The PASI 75 was 65.3% for AIN457 300 mg, 51.2% for AIN457 150 mg, and 2.4% for placebo (P<.001). There was a comparable incidence of infection among participants with AIN457 and etanercept, which was greater than placebo.30
Brodalumab (AMG 827)
Brodalumab (formerly known as AMG 827)(Amgen Inc) is a human monoclonal antibody that targets the IL-17A receptor. In a phase 1 randomized trial, 25 participants received either IV brodalumab 700 mg, SC brodalumab 350 mg or 140 mg, or placebo.31 Results demonstrated improvement in PASI score that correlated with increased dosage of brodalumab as well as decreased psoriasis gene expression and decreased thickness of the epidermis in participants receiving the 700-mg IV or 350-mg SC doses. In a phase 2 trial, 198 participants received either brodalumab 280 mg at week 0, then every 4 weeks for 8 weeks, or brodalumab 210 mg, 140 mg, 70 mg, or placebo at week 0, then every 2 weeks for 10 weeks. At week 12, PASI 75 was observed in 82% and 77% of the 210-mg and 140-mg groups, respectively, with no benefit noted in the placebo group (P<.001).31 In a phase 3 trial, 661 participants received brodalumab 210 mg or 140 mg or placebo. At week 12, PASI 75 was observed in 83% of the 210-mg group versus 60% of the 140-mg group; PASI 100 was observed in 42% of the 210-mg group versus 23% of the 140-mg group.32
Ixekizumab (LY2439821)
Ixekizumab (formerly known as LY2439821)(Eli Lilly and Company) is a human monoclonal antibody that targets IL-17A. In a phase 2 double-blind, placebo-controlled trial, 142 participants with chronic moderate to severe plaque psoriasis were randomized to receive 10-mg, 25-mg, 75-mg, or 150-mg SC injections of ixekizumab or placebo at 0, 2, 4, 8, 12, and 16 weeks. At week 12, the percentage of participants with a 75% reduction in PASI score was significantly greater with ixekizumab (150 mg [82.1%], 75 mg [82.8%], and 25 mg [76.7%]), except the 10-mg group, than with placebo (7.7%)(P<.001 for each comparison).33
Inhibition of T-Cell Activation in Antigen-Presenting Cells
Abatacept
Abatacept (Bristol-Myers Squibb) is a fusion protein designed to inhibit T-cell activation by binding receptors for CD80 and CD86 in antigen-presenting cells.34 A phase 1 study of 43 participants demonstrated improved PGA scores of 50% in 46% of psoriasis participants who were treated with abatacept, indicating a dose-responsive association with abatacept in psoriasis patients refractory to other therapies.35 In a 6-month, phase 2, multicenter, randomized, double-blind, placebo-controlled trial, 170 participants with PsA were randomized to receive placebo or abatacept at doses of 3 mg/kg, 10 mg/kg, or 30/10 mg/kg (2 initial doses of 30 mg/kg followed by 10 mg/kg).36 At day 169, ACR20 was observed in 19%, 33%, 48%, and 42% of the placebo and abatacept 3 mg/kg, 10 mg/kg, and 30/10 mg/kg groups, respectively. Compared with placebo, improvements were significantly higher for the abatacept 10-mg/kg (P=.006) and 30/10-mg/kg (P=.022) groups but not for the 3-mg/kg group (P=.121). The authors concluded that abatacept 10 mg/kg could be an appropriate dosing regimen for PsA, as is presently used in the FDA-approved management of rheumatoid arthritis.36 At the time of publication, a phase 3 trial evaluating the efficacy and safety of abatacept SC injection in adults with active PsA was ongoing but was not actively recruiting participants (NCT01860976).
Activation of Regulatory T Cells
Tregalizumab (BT061)
Tregalizumab (formerly known as BT061)(Biotest) is a human monoclonal antibody that activates regulatory T cells. A phase 2, randomized, placebo-controlled, double-blind, multicenter, multiple-dose, cohort study with escalating doses evaluating the safety and efficacy of BT061 in patients with moderate to severe chronic plaque psoriasis was completed, but the results were not available at the time of publication (NCT01072383).
Inhibition of Toll-like Receptors 7, 8, and 9
IMO-8400
IMO-8400 (Idera Pharmaceuticals) is unique in that it treats psoriasis by targeting toll-like receptors (TLRs) 7, 8, and 9.37 In phase 1 studies, IMO-8400 was well tolerated when administered to a maximum of 0.6 mg/kg.38 An 18-week, phase 2, randomized, double-blind, placebo-controlled, dose-ranging study evaluating the safety and tolerability of different dose levels—0.075 mg/kg, 0.15 mg/kg, and 0.3 mg/kg—of IMO-8400 versus placebo in patients with moderate to severe plaque psoriasis was completed, but the results were not available at the time of publication (NCT01899729).
Inhibition of Granulocyte-Macrophage Colony-Stimulating Factor
Namilumab (MT203)
Namilumab (formerly known as MT203)(Takeda Pharmaceutical Company Limited) is a granulocyte-macrophage colony-stimulating factor inhibitor. At the time of publication, participants were actively being recruited for a phase 2, multicenter, randomized, double-blind, placebo-controlled, dose-finding and proof-of-concept study to assess the efficacy, safety, and tolerability of namilumab at 4 different SC doses—300 mg, 160 mg, 100 mg, and 40 mg at baseline with half the dose on days 15, 43, and 71 for each of the 4 treatment arms—versus placebo in patients with moderate to severe chronic plaque psoriasis (NCT02129777).
Conclusion
Novel biologic treatments promise exciting new therapeutic avenues for psoriasis and PsA. Although biologics currently are in use for treatment of psoriasis and PsA in the form of TNF-α inhibitors, other drugs currently in phase 2 through phase 4 clinical trials aim to target other pathways underlying the pathogenesis of psoriasis and PsA, including inhibition of the IL-12/IL-23 pathway; inhibition of the IL-17 pathway; inhibition of T-cell activation in antigen-presenting cells; activation of regulatory T cells; inhibition of TLR-7, TLR-8, and TLR-9; and inhibition of granulocyte-macrophage colony-stimulating factor. These novel therapies offer hope for more targeted treatment strategies for patients with psoriasis and/or PsA.
1. Lee S, Coleman CI, Limone B, et al. Biologic and nonbiologic systemic agents and phototherapy for treatment of chronic plaque psoriasis. Rockville, MD: Agency for Healthcare Research and Quality; 2012.
2. Nagler AR, Weinberg JM. Research pipeline III: biologic therapies. In: Weinberg JM, Lebwohl M, eds. Advances in Psoriasis. New York, NY: Springer; 2014:243-251.
3. Reich K, Ortonne JP, Gottlieb AB, et al. Successful treatment of moderate to severe plaque psoriasis with the PEGylated Fab’ certolizumab pegol: results of a phase II randomized, placebo-controlled trial with a re-treatment extension. Br J Dermatol. 2012;167:180-190.
4. Gladman D, Fleischmann R, Coteur G, et al. Effect of certolizumab pegol on multiple facets of psoriatic arthritis as reported by patients: 24-week patient-reported outcome results of a phase III, multicenter study. Arthritis Care Res (Hoboken). 2014;66:1085-1092.
5. Coherus announces initiation of Phase 3 trial of CHS-0214 (investigational etanercept biosimilar) in chronic plaque psoriasis (RaPsOdy) [press release]. Redwood City, CA: Coherus BioSciences, Inc; July 16, 2014.
6. Coherus announces CHS-0214 (proposed etanercept biosimilar) meets primary endpoint in pivotal pharmacokinetic clinical study [press release]. Redwood City, CA: Coherus BioSciences, Inc; October 28, 2013.
7. Tang C, Chen S, Qian H, et al. Interleukin-23: as a drug target for autoimmune inflammatory diseases. Immunology. 2012;135:112-124.
8. Strober BE, Crowley JJ, Yamauchi PS, et al. Efficacy and safety results from a phase III, randomized controlled trial comparing the safety and efficacy of briakinumab with etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. Br J Dermatol. 2011;165:661-668.
9. Reich K, Langley RG, Papp KA, et al. A 52-week trial comparing briakinumab with methotrexate in patients with psoriasis. N Engl J Med. 2011;365:1586-1596.
10. Papp KA, Sundaram M, Bao Y, et al. Effects of briakinumab treatment for moderate to severe psoriasis on health-related quality of life and work productivity and activity impairment: results from a randomized phase III study. J Eur Acad Dermatol Venereol. 2014;28:790-798.
11. Stelara (ustekinumab) receives FDA approval to treat active psoriatic arthritis. first and only anti-IL-12/23 treatment approved for adult patients living with psoriatic arthritis [press release]. Horsham, PA: Johnson & Johnson; September 23, 2013.
12. FDA approves new drug to treat psoriasis [press release]. Silver Spring, MD: US Food and Drug Administration; April 17, 2013.
13. Griffiths CE, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362:118-128.
14. Papp KA, Griffiths CE, Gordon K, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013;168:844-854.
15. McInnes IB, Kavanaugh A, Gottlieb AB, et al. Ustekinumab in patients with active psoriatic arthritis: results of the phase 3, multicenter, double-blind, placebo-controlled PSUMMIT I study. Ann Rheum Dis. 2012;71(suppl):S107-S148.
16. Ritchlin C, Rahman P, Kavanaugh A, et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial [published online ahead of print Jan 30, 2014]. Ann Rheum Dis. 2014;73:990-999.
17. A study of the safety and efficacy of ustekinumab in adolescent patients with psoriasis (CADMUS)(NCT01090427). https://clinicaltrials.gov/ct2/show/NCT01090427?term=NCT01090427&rank=1. Updated January 16, 2015. Accessed April 16, 2015.
18. A safety and efficacy study of ustekinumab in patients with plaque psoriasis who have had an inadequate response to methotrexate (NCT01059773). https://clinicaltrials.gov/ct2/show/NCT01059773?term=NCT01059773&rank=1. Updated November 13, 2014. Accessed April 16, 2015.
19. Efficacy and safety of ustekinumab in patients with moderate to severe palmar plantar psoriasis (PPP)(NCT01090063). https://clinicaltrials.gov/ct2/show/NCT01090063?term=NCT01090063&rank=1. Updated January 31, 2013. Accessed April 16, 2015.
20. A study of the safety and effectiveness of ustekinumab in patients with psoriatic arthritis (NCT01009086). https://clinicaltrials.gov/ct2/show/NCT01009086?term=NCT01009086&rank=1. Updated February 11, 2015. Accessed April 16, 2015.
21. A study to assess the effect of ustekinumab (Stelara) and etanercept (Enbrel) in participants with moderate to severe psoriasis (MK-0000-206)(NCT01276847). https://clinicaltrials.gov/ct2/show/NCT01276847?term=NCT01276847&rank=1. Updated January 13, 2015. Accessed April 16, 2015.
22. Sofen H, Smith S, Matheson RT, et al. Guselkumab (an IL-23-specific mAb) demonstrates clinical and molecular response in patients with moderate-to-severe psoriasis. J Allergy Clin Immunol. 2014;133:1032-1040.
23. Callis Duffin K, Wasfi Y, Shen YK, et al. A phase 2, multicenter, randomized, placebo- and active-comparator-controlled, dose-ranging trial to evaluate Guselkumab for the treatment of patients with moderate-to-severe plaque-type psoriasis (X-PLORE). Poster presented at: 72nd Annual Meeting of the American Academy of Dermatology; March 21-25, 2014; Denver, CO.
24. Papp K. Monoclonal antibody MK-3222 and chronic plaque psoriasis: phase 2b. Paper presented at: 71st Annual Meeting of the American Academy of Dermatology; March 1-5, 2013; Miami, FL.
25. Huynh D, Kavanaugh A. Psoriatic arthritis: current therapy and future approaches. Rheumatology (Oxford). 2015;54:20-28.
26. FDA approves new psoriasis drug Cosentyx [press release]. Silver Spring, MD: US Food and Drug Administration; January 21, 2015.
27. Multiple-loading dose regimen study in patients with chronic plaque-type psoriasis (NCT00805480). https://clinicaltrials.gov/ct2/show/NCT00805480?term
=NCT00805480&rank=1. Updated January 28, 2015. Accessed April 16, 2015.
28. AIN457 regimen finding study in patients with moderate to severe psoriasis (NCT00941031). https://clinicaltrials.gov/ct2/show/NCT00941031?term=NCT00941031&rank=1. Updated March 23, 2015. Accessed April 16, 2015.
29. Efficacy and safety of intravenous and subcutaneous secukinumab in moderate to severe chronic plaque-type psoriasis (STATURE)(NCT014712944). https://clinical trials.gov/ct2/show/NCT01412944?term=efficacy+and+safety+of+intravenous+and+subcutaneoussecukinumab&rank=1. Updated March 17, 2015. Accessed April 16, 2015.
30. Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. 2014;371:326-338.
31. Coimbra S, Figueiredo A, Santos-Silva A. Brodalumab: an evidence-based review of its potential in the treatment of moderate-to-severe psoriasis. Core Evid. 2014;9:89-97.
32. Leavitt M. New biologic clears psoriasis in 42 percent of patients. National Psoriasis Foundation Web site. https://www.psoriasis.org/advance/new-biologic-clears-psoriasis-in-42-percent-of-patients. Accessed April 10, 2015.
33. Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
34. Herrero-Beaumont G, Martínez Calatrava MJ, Castañeda S. Abatacept mechanism of action: concordance with its clinical profile. Rheumatol Clin. 2012;8:78-83.
35. Abrams JR, Lebwohl MG, Guzzo CA, et al. CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris. J Clin Invest. 1999;103:1243-1252.
36. Mease P, Genovese MC, Gladstein G, et al. Abatacept in the treatment of patients with psoriatic arthritis: results of a six-month, multicenter, randomized, double-blind, placebo-controlled, phase II trial. Arthritis Rheum. 2011;63:939-948.
37. Suárez-Fariñas M, Arbeit R, Jiang W, et al. Suppression of molecular inflammatory pathways by Toll-like receptor 7, 8, and 9 antagonists in a model of IL-23-induced skin inflammation. PLoS One. 2013;8:e84634.
38. A 12-week dose-ranging trial in patients with moderate to severe plaque psoriasis (8400-201)(NCT01899729). https://clinicaltrials.gov/ct2/show/NCT01899729. Updated October 16, 2014. Accessed April 27, 2015.
Biologic agents that currently are in use for the management of moderate to severe psoriasis and psoriatic arthritis (PsA) include the anti–tumor necrosis factor (TNF) α monoclonal antibodies adalimumab, etanercept, and infliximab1; however, additional TNF-α inhibitors as well as drugs targeting other pathways presently are in the pipeline. Novel biologic treatments currently in phase 2 through phase 4 clinical trials, including those that have recently been approved by the US Food and Drug Administration (FDA), are discussed in this article, and a summary is provided in Table 1.
Tumor Necrosis Factor α Inhibitors
Certolizumab Pegol
Certolizumab pegol (CZP; UCB, Inc), a pegylated TNF-α inhibitor, is unique in that it does not possess a fragment crystallizable (Fc) region and consequently does not trigger complement activation. The drug is presently FDA approved for active PsA, rheumatoid arthritis, and ankylosing spondylitis. One phase 2 study reported psoriasis area severity index (PASI) scores of 75 in 83% (48/58) of participants who received CZP 400 mg at week 0 and every other week until week 10 (P<.001 vs placebo).3 In a 24-week phase 3 study (known as RAPID-PsA), 409 participants were randomized into 3 study arms: (1) CZP 400 mg every 4 weeks; (2) CZP 200 mg every 2 weeks; (3) placebo every 2 weeks.4 Of note, 20% of participants had previously received a TNF inhibitor. The study demonstrated improvements in participant-reported outcomes with use of CZP regardless of prior TNF inhibitor use.4
CHS-0214
CHS-0214 (Coherus BioSciences, Inc) is a TNF-α inhibitor and etanercept biosimilar that has entered into a 48-week multicenter phase 3 trial (known as RaPsOdy) for patients with chronic plaque psoriasis. The purpose of the study is to compare PASI scores for CHS-0214 and etanercept to evaluate immunogenicity, safety, and effectiveness over a 12-week period.5 Comparable pharmacokinetics were established in an earlier study.6
Inhibition of the IL-12/IL-23 Pathway
IL-12 and IL-23 are cytokines with prostaglan-din E2–mediated production by dendritic cells that share structural (eg, the p40 subunit) and functional similarities (eg, IFN-γ production). However, each has distinct characteristics. IL-12 aids in naive CD4+ T-cell differentiation, while IL-23 induces IL-17 production by CD4+ memory T cells. IL-17 triggers a proinflammatory chemokine cascade and produces IL-1, IL-6, nitric oxide synthase 2, and TNF-α.7
Briakinumab (ABT-874)
Briakinumab (formerly known as ABT-874)(Abbott Laboratories) is a human monoclonal antibody that inhibits the p40 subunit of IL-12 and IL-23. In a phase 3 trial of 350 participants with moderate to severe psoriasis, week 12 PASI 75 scores were achieved in 80.6% of participants who received briakinumab versus 39.6% of those who received etanercept and 6.9% of those who received placebo.8 In a 52-week phase 3 trial of 317 participants with moderate to severe psoriasis, PASI 75 scores were observed in 81.8% of participants who received briakinumab versus 39.9% of those who received methotrexate.9 In another 52-week phase 3 trial of 1465 participants with moderate to severe psoriasis, clinical benefit was reported at 12 weeks in 75.9% of participants for Dermatology Life Quality Index, and 64.8% and 54.1% for psoriasis- and PsA-related pain scores, respectively.10 However, ABT-874 was withdrawn by the manufacturer as of 2011 due to concerns regarding adverse cardiovascular events.9
BI 655066
BI 655066 (Boehringer Ingelheim GmbH) is a human monoclonal antibody that targets the p19 subunit of IL-23. A phase 1 study of the pharmacokinetics and pharmacodynamics of intravenous (IV) versus subcutaneous (SC) administration of BI 655066 as well as its safety and effectiveness versus placebo recently was completed (NCT01577550), but the results were not available at the time of publication. A phase 2 study comparing 3 dosing regimens of BI 655066 versus ustekinumab is ongoing but not actively recruiting patients at the time of publi-cation (NCT02054481).
Ustekinumab (CNTO 1275)
Ustekinumab (formerly known as CNTO 1275)(Janssen Biotech, Inc) is a human monoclonal antibody that inhibits the p40 subunit of IL-12 and IL-23. It was FDA approved for treatment of moderate to severe plaque psoriasis in September 200911 and PsA in September 201312 for adult patients 18 years or older. One phase 3 trial (known as ACCEPT) compared the effectiveness of ustekinumab versus etanercept in 903 participants with moderate to severe psoriasis at 67 centers worldwide.13 Participants were randomly assigned to receive SC injections of either 45 mg or 90 mg of ustekinumab (at weeks 0 and 4) or high-dose etanercept (50 mg twice weekly for 12 weeks). At week 12, PASI 75 was noted in 67.5% of participants who received 45 mg of ustekinumab and 73.8% of participants who received 90 mg compared to 56.8% of those who received etanercept (P=.01 and P<.001, respectively). In participants who showed no response to etanercept, PASI 75 was achieved in 48.9% within 12 weeks after crossover to ustekinumab. One or more adverse events (AEs) occurred through week 12 in 66.0% of the 45-mg ustekinumab group, 69.2% of the 90-mg group, and 70.0% of the etanercept group; serious AEs were noted in 1.9%, 1.2%, and 1.2%, respectively.13 A 5-year follow-up study of 3117 participants reported an incidence of AEs with ustekinumab that was comparable to other biologics, with malignancy and mortality rates comparable to age-matched controls.14
In a phase 3, multicenter, double-blind, placebo-controlled trial (know as PSUMMIT I), 615 adults with active PsA who had not previously been treated with TNF inhibitors were randomly assigned to placebo, 45 mg of ustekinumab, or 90 mg of ustekinumab. At week 24, more participants receiving ustekinumab 90 mg achieved 20%, 50%, and 70% improvement in American College of Rheumatology (ACR) criteria (49.5%, 27.9%, and 14.2%, respectively) and PASI 75 (62.4%) versus the placebo group (22.8%, 8.7%, 2.4%, and 11%, respectively).15 In a phase 3, multicenter, placebo-controlled trial (known as PSUMMIT 2), 312 adult participants with active PsA who had formerly been treated with conventional therapies and/or TNF inhibitors were randomized to receive placebo (at weeks 0, 4, and 16 with crossover to 45 mg of ustekinumab at weeks 24, 28, and 40) or ustekinumab (45 mg or 90 mg at weeks 0, 4, and every 12 weeks).16 For participants with less than 5% improvement, there was an early escape clinical trial design with placebo to 45 mg of ustekinumab, 45 mg of ustekinumab to 90 mg, and 90 mg of ustekinumab maintained at the same dose. The ACR20 was 43.8% for the ustekinumab group versus 20.2% for the placebo group (P<.001).16
A phase 3, multicenter, randomized, double-blind, placebo-controlled study (known as CADMUS) evaluated the efficacy and safety of ustekinumab in the treatment of adolescents (age range, 12–18 years) with moderate to severe plaque-type psoriasis.17 The primary outcome of the study was the percentage of participants achieving a physician global assessment (PGA) score of cleared (0) or minimal (1) at week 12. One hundred ten participants started and completed the first period in the study (ie, controlled period [weeks 0–12]) and were randomized into 3 groups: placebo (SC injections at weeks 0 and 4), ustekinumab half-standard dose, and ustekinumab standard dose. At week 12, 101 participants started and completed the second period in the study (weeks 12–60). The placebo group received either ustekinumab half-standard dose or ustekinumab standard dose at weeks 12 and 16, then once every 12 weeks with the last dose at week 40, and the ustekinumab half-standard and standard dose groups received the respective doses every 12 weeks with the last dose at week 40. At week 12, PGA scores of 0 or 1 were reported in 5.4% of the placebo group, 67.6% of the ustekinumab half-standard dose group, and 69.4% of the ustekinumab standard dose group (P<.001), and PASI 75 was achieved in 10.8%, 78.4%, and 80.6%, respectively (P<.001).17
A phase 4 study (known as TRANSIT) assessed the safety and efficacy of ustekinumab in participants with plaque psoriasis who had a suboptimal response to methotrexate.18 Participants in the first treatment group received either 45 mg (weight, ≤100 kg) of ustekinumab at weeks 0, 4, and then every 12 weeks until week 40, or 90 mg (weight, >100 kg) in 2 SC injections after immediate discontinuation of methotrexate. The second treatment group followed the same dosing regimen with gradual withdrawal of methotrexate therapy. Adverse events were reported in 61.1% and 64.5% of participants in groups 1 and 2, respectively. In group 1, PASI 75 was observed in 58.1% of participants (95% confi-dence interval [CI], 51.9%-64.3%) at week 12 and 76.3% (95% CI, 70.8%-81.9%) at week 52. In group 2, PASI 75 was observed in 62.2% of participants (95% CI, 56.0%-68.3%) at week 12 and 76.9% (95% CI, 71.4%-82.5%) at week 52.18
In another study that assessed the efficacy and safety of ustekinumab in 24 participants with moderate to severe palmoplantar psoriasis, 37.5% of participants achieved a palmar/plantar PGA score of 0 or 1 at week 16.19 A phase 3, multicenter, randomized, double-blind, placebo-controlled study of the safety and effectiveness of ustekinumab in 615 PsA participants showed ACR20 response in 49.5% of the ustekinumab 90-mg group, 42.4% of the ustekinumab 45-mg group, and 22.8% of the placebo group (P<.001).20
A phase 1 study was performed to assess gene expression in the following: (1) IFN-γ modulation in the IL-12 pathway; (2) IL-23 pathway with ustekinumab (45 mg for those weighing <100 kg and 90 mg for ≤100 kg administered SC on day 1 and at weeks 4 and 16); and (3) IL-17 pathway with etanercept (50 mg administered SC twice weekly for 12 weeks, then once weekly for 4 weeks).21 The change in gene expression from baseline in the IL-12 pathway with ustekinumab achieved statistical significance by week 1 (P=.016) with increasing levels of gene expression through week 16 (P=.000184). The change in gene expression from baseline in the IL-23 pathway with ustekinumab achieved statistical significance by week 2 (P=.010) with increasing levels of gene expression through week 16 (P=.000215). The results were less powerful for etanercept, with a change in gene expression from baseline in the IL-17 pathway increasing through week 4 (P=.053) and decreasing by week 16 (P=.098).21
Several clinical trials are underway and are currently recruiting participants (Table 2).
Guselkumab (CNTO 1959)
Guselkumab (formerly known as CNTO 1959)(Janssen Research & Development, LLC) is a human monoclonal antibody targeting the p19 subunit of IL-23. In a double-blind, randomized study of 24 participants receiving 1 dose of CNTO 1959 at 10 mg, 30 mg, 100 mg, or 300 mg versus placebo, a PASI 75 of 50% for the 10-mg subset, 60% for the 30- and 100-mg group, and 100% for the 300-mg group was achieved as opposed to 0% in the placebo group at 12 weeks.22 The rate of AEs was 65% in the CNTO 1959 treatment arm versus 50% in the placebo group at 24 weeks. Furthermore, decreased serum IL-17A titers and gene expression for psoriasis was demonstrated as well as decreased thickness of the epidermis and less dendritic and T-cell expression for the CNTO 1959 study population histologically.22 Results of a phase 2 trial in 293 participants who received CNTO 1959, adalimumab, or placebo indicated PASI 75 at 16 weeks for 81% of the CNTO 1959 50-mg group versus 71% of the adalimumab group, with serious AEs in 3% of participants treated with CNTO 1959 versus 5% treated with adalimumab.23
Tildrakizumab (MK-3222/SCH 900222)
Tildrakizumab (formerly known as MK-3222/SCH 900222)(Merck & Co Inc) is a monoclonal antibody that also targets the p19 subunit of IL-23. Results of a phase 2b trial were promising. This study reported on 355 participants who received placebo versus MK-3222 5 mg, 25 mg, 100 mg, or 200 mg, with PASI 75 scores of 4.4%, 33%, 64%, 66%, and 74%, respectively, noted at 16 weeks.24 A 64-week phase 3 study currently is underway to assess the long-term benefit and safety of MK-3222, but it is not recruiting participants (NCT01722331).
Inhibition of the IL-17 Pathway
The T helper 17 cells (TH17) produce IL-17, a cytokine mediating inflammation that is implicated in psoriasis. Two products target IL-17A, while another targets the IL-17 receptor.25
Secukinumab (AIN457)
Secukinumab (formerly known as AIN457)(Novartis Pharmaceutical Corporation) was FDA approved for treatment of moderate to severe psoriasis in adult patients who are candidates for systemic therapy or phototherapy in January 2015.26 Secukinumab is a human monoclonal antibody that inhibits IL-17A. There are many clinical trials underway including a phase 2 extension study (NCT01132612). Many phase 3 studies also are underway evaluating the effectiveness and safety of AIN457 in patients with psoriasis resistant to TNF inhibitors (NCT01961609); its usability and tolerability (NCT01555125), including 2-year extension studies (NCT01640951; NCT01544595); its effectiveness as opposed to ustekinumab (NCT02074982); effectiveness using an autoinjector (NCT01636687); and the PASI 90 in HLA-Cw6–positive and HLA-Cw6–negative patients with moderate to severe psoriasis (known as SUPREME)(NCT02394561).
Other phase 3 trials are being undertaken in patients with moderate to severe palmoplantar psoriasis (NCT01806597; NCT02008890); moderate to severe nail psoriasis (known as TRANSFIGURE)(NCT01807520); moderate to severe scalp psoriasis (NCT02267135); and PsA (NCT01989468; NCT02294227; NCT01892436), including a 5-year study for PsA (known as FUTURE 2)(NCT01752634).
Other studies that are completed with pending results include a phase 1 trial to evaluate its mechanism of action in vivo by studying the spread of AIN457 in tissue as assessed by open flow microperfusion (NCT01539213), a phase 2 trial of the clinical effectiveness of AIN457 at 12 months and biomarker changes (NCT01537432), as well as phase 3 trials of the clinical efficacy of various dosing regimens (known as SCULPTURE)(NCT01406938); safety and effectiveness at 1 year (known as ERASURE)(NCT01365455); and the effectiveness, tolerability, and safety of AIN457 over 2 years in PsA (known as FUTURE 1)(NCT01392326).
In a 56-week phase 2 clinical trial of 100 participants, the PASI scores at 12 weeks and percentage of participants without relapse up to 56 weeks were evaluated.27 There were 4 arms in the study: (1) AIN457 3 mg/kg (day 1) then placebo (days 15 and 29); (2) AIN457 10 mg/kg (day 1) then placebo (days 15 and 29); (3) AIN457 10 mg/kg (days 1, 15, and 29); and (4) placebo (days 1, 15, and 29), with AIN457 and the placebo administered IV. The mean (standard deviation) change from baseline for PASI scores for these respective groups was -12.46 (7.668), -13.35 (6.195), -18.02 (6.792), and -4.18 (4.698), respectively. At week 56, the percentage of participants without a relapse at any point during the study was 12.5%, 22.2%, and 27.8%, respectively.27
In a phase 2 study of 404 participants, PASI 75 scores were assessed at 12 weeks with the SC administration of AIN457 in participants with moderate to severe psoriasis at 3 dosing regimens: (1) a single dose of 150 mg (week 1), (2) monthly doses of 150 mg (weeks 1, 5, and 9), (3) early loading doses of 150 mg (weeks 1, 2, 3, 5, and 9), as compared to placebo. At 12 weeks, PASI 75 scores were 7%, 58%, 72%, and 1%, respectively.28
The phase 3 STATURE trial assessed the safety and effectiveness of SC and IV AIN457 in moderate to severe psoriasis in partial AIN457 nonresponders.29 Nonresponders were participants who demonstrated a PASI score of 50% or more but less than 75%. Participants in this study design who received SC AIN457 demonstrated a PASI 75 of 66.7%, with a 2011 investigator global assessment score of 0 (clear) or 1 (almost clear) in 66.7%. In those receiving IV AIN457, the PASI 75 was 90.5%, with a 2011 investigator global assessment score of 0 or 1 in 33.3%.29
In a 52-week phase 3 efficacy trial (known as FIXTURE), 1306 participants received 1 dose of AIN457 300 mg or 150 mg weekly for 5 weeks, then every 4 weeks; 12 weeks of etanercept 50 mg twice weekly, then once weekly; or placebo. The PASI 75 was 77.1% for AIN457 300 mg, 67.0% for AIN457 150 mg, 44.0% for etanercept, and 4.9% for placebo (P<.001).30 In a 52-week efficacy and safety trial (known as ERASURE), 738 participants received 1 dose of AIN457 300 mg or 150 mg weekly for 5 weeks, then every 4 weeks, versus placebo. The PASI 75 was 65.3% for AIN457 300 mg, 51.2% for AIN457 150 mg, and 2.4% for placebo (P<.001). There was a comparable incidence of infection among participants with AIN457 and etanercept, which was greater than placebo.30
Brodalumab (AMG 827)
Brodalumab (formerly known as AMG 827)(Amgen Inc) is a human monoclonal antibody that targets the IL-17A receptor. In a phase 1 randomized trial, 25 participants received either IV brodalumab 700 mg, SC brodalumab 350 mg or 140 mg, or placebo.31 Results demonstrated improvement in PASI score that correlated with increased dosage of brodalumab as well as decreased psoriasis gene expression and decreased thickness of the epidermis in participants receiving the 700-mg IV or 350-mg SC doses. In a phase 2 trial, 198 participants received either brodalumab 280 mg at week 0, then every 4 weeks for 8 weeks, or brodalumab 210 mg, 140 mg, 70 mg, or placebo at week 0, then every 2 weeks for 10 weeks. At week 12, PASI 75 was observed in 82% and 77% of the 210-mg and 140-mg groups, respectively, with no benefit noted in the placebo group (P<.001).31 In a phase 3 trial, 661 participants received brodalumab 210 mg or 140 mg or placebo. At week 12, PASI 75 was observed in 83% of the 210-mg group versus 60% of the 140-mg group; PASI 100 was observed in 42% of the 210-mg group versus 23% of the 140-mg group.32
Ixekizumab (LY2439821)
Ixekizumab (formerly known as LY2439821)(Eli Lilly and Company) is a human monoclonal antibody that targets IL-17A. In a phase 2 double-blind, placebo-controlled trial, 142 participants with chronic moderate to severe plaque psoriasis were randomized to receive 10-mg, 25-mg, 75-mg, or 150-mg SC injections of ixekizumab or placebo at 0, 2, 4, 8, 12, and 16 weeks. At week 12, the percentage of participants with a 75% reduction in PASI score was significantly greater with ixekizumab (150 mg [82.1%], 75 mg [82.8%], and 25 mg [76.7%]), except the 10-mg group, than with placebo (7.7%)(P<.001 for each comparison).33
Inhibition of T-Cell Activation in Antigen-Presenting Cells
Abatacept
Abatacept (Bristol-Myers Squibb) is a fusion protein designed to inhibit T-cell activation by binding receptors for CD80 and CD86 in antigen-presenting cells.34 A phase 1 study of 43 participants demonstrated improved PGA scores of 50% in 46% of psoriasis participants who were treated with abatacept, indicating a dose-responsive association with abatacept in psoriasis patients refractory to other therapies.35 In a 6-month, phase 2, multicenter, randomized, double-blind, placebo-controlled trial, 170 participants with PsA were randomized to receive placebo or abatacept at doses of 3 mg/kg, 10 mg/kg, or 30/10 mg/kg (2 initial doses of 30 mg/kg followed by 10 mg/kg).36 At day 169, ACR20 was observed in 19%, 33%, 48%, and 42% of the placebo and abatacept 3 mg/kg, 10 mg/kg, and 30/10 mg/kg groups, respectively. Compared with placebo, improvements were significantly higher for the abatacept 10-mg/kg (P=.006) and 30/10-mg/kg (P=.022) groups but not for the 3-mg/kg group (P=.121). The authors concluded that abatacept 10 mg/kg could be an appropriate dosing regimen for PsA, as is presently used in the FDA-approved management of rheumatoid arthritis.36 At the time of publication, a phase 3 trial evaluating the efficacy and safety of abatacept SC injection in adults with active PsA was ongoing but was not actively recruiting participants (NCT01860976).
Activation of Regulatory T Cells
Tregalizumab (BT061)
Tregalizumab (formerly known as BT061)(Biotest) is a human monoclonal antibody that activates regulatory T cells. A phase 2, randomized, placebo-controlled, double-blind, multicenter, multiple-dose, cohort study with escalating doses evaluating the safety and efficacy of BT061 in patients with moderate to severe chronic plaque psoriasis was completed, but the results were not available at the time of publication (NCT01072383).
Inhibition of Toll-like Receptors 7, 8, and 9
IMO-8400
IMO-8400 (Idera Pharmaceuticals) is unique in that it treats psoriasis by targeting toll-like receptors (TLRs) 7, 8, and 9.37 In phase 1 studies, IMO-8400 was well tolerated when administered to a maximum of 0.6 mg/kg.38 An 18-week, phase 2, randomized, double-blind, placebo-controlled, dose-ranging study evaluating the safety and tolerability of different dose levels—0.075 mg/kg, 0.15 mg/kg, and 0.3 mg/kg—of IMO-8400 versus placebo in patients with moderate to severe plaque psoriasis was completed, but the results were not available at the time of publication (NCT01899729).
Inhibition of Granulocyte-Macrophage Colony-Stimulating Factor
Namilumab (MT203)
Namilumab (formerly known as MT203)(Takeda Pharmaceutical Company Limited) is a granulocyte-macrophage colony-stimulating factor inhibitor. At the time of publication, participants were actively being recruited for a phase 2, multicenter, randomized, double-blind, placebo-controlled, dose-finding and proof-of-concept study to assess the efficacy, safety, and tolerability of namilumab at 4 different SC doses—300 mg, 160 mg, 100 mg, and 40 mg at baseline with half the dose on days 15, 43, and 71 for each of the 4 treatment arms—versus placebo in patients with moderate to severe chronic plaque psoriasis (NCT02129777).
Conclusion
Novel biologic treatments promise exciting new therapeutic avenues for psoriasis and PsA. Although biologics currently are in use for treatment of psoriasis and PsA in the form of TNF-α inhibitors, other drugs currently in phase 2 through phase 4 clinical trials aim to target other pathways underlying the pathogenesis of psoriasis and PsA, including inhibition of the IL-12/IL-23 pathway; inhibition of the IL-17 pathway; inhibition of T-cell activation in antigen-presenting cells; activation of regulatory T cells; inhibition of TLR-7, TLR-8, and TLR-9; and inhibition of granulocyte-macrophage colony-stimulating factor. These novel therapies offer hope for more targeted treatment strategies for patients with psoriasis and/or PsA.
Biologic agents that currently are in use for the management of moderate to severe psoriasis and psoriatic arthritis (PsA) include the anti–tumor necrosis factor (TNF) α monoclonal antibodies adalimumab, etanercept, and infliximab1; however, additional TNF-α inhibitors as well as drugs targeting other pathways presently are in the pipeline. Novel biologic treatments currently in phase 2 through phase 4 clinical trials, including those that have recently been approved by the US Food and Drug Administration (FDA), are discussed in this article, and a summary is provided in Table 1.
Tumor Necrosis Factor α Inhibitors
Certolizumab Pegol
Certolizumab pegol (CZP; UCB, Inc), a pegylated TNF-α inhibitor, is unique in that it does not possess a fragment crystallizable (Fc) region and consequently does not trigger complement activation. The drug is presently FDA approved for active PsA, rheumatoid arthritis, and ankylosing spondylitis. One phase 2 study reported psoriasis area severity index (PASI) scores of 75 in 83% (48/58) of participants who received CZP 400 mg at week 0 and every other week until week 10 (P<.001 vs placebo).3 In a 24-week phase 3 study (known as RAPID-PsA), 409 participants were randomized into 3 study arms: (1) CZP 400 mg every 4 weeks; (2) CZP 200 mg every 2 weeks; (3) placebo every 2 weeks.4 Of note, 20% of participants had previously received a TNF inhibitor. The study demonstrated improvements in participant-reported outcomes with use of CZP regardless of prior TNF inhibitor use.4
CHS-0214
CHS-0214 (Coherus BioSciences, Inc) is a TNF-α inhibitor and etanercept biosimilar that has entered into a 48-week multicenter phase 3 trial (known as RaPsOdy) for patients with chronic plaque psoriasis. The purpose of the study is to compare PASI scores for CHS-0214 and etanercept to evaluate immunogenicity, safety, and effectiveness over a 12-week period.5 Comparable pharmacokinetics were established in an earlier study.6
Inhibition of the IL-12/IL-23 Pathway
IL-12 and IL-23 are cytokines with prostaglan-din E2–mediated production by dendritic cells that share structural (eg, the p40 subunit) and functional similarities (eg, IFN-γ production). However, each has distinct characteristics. IL-12 aids in naive CD4+ T-cell differentiation, while IL-23 induces IL-17 production by CD4+ memory T cells. IL-17 triggers a proinflammatory chemokine cascade and produces IL-1, IL-6, nitric oxide synthase 2, and TNF-α.7
Briakinumab (ABT-874)
Briakinumab (formerly known as ABT-874)(Abbott Laboratories) is a human monoclonal antibody that inhibits the p40 subunit of IL-12 and IL-23. In a phase 3 trial of 350 participants with moderate to severe psoriasis, week 12 PASI 75 scores were achieved in 80.6% of participants who received briakinumab versus 39.6% of those who received etanercept and 6.9% of those who received placebo.8 In a 52-week phase 3 trial of 317 participants with moderate to severe psoriasis, PASI 75 scores were observed in 81.8% of participants who received briakinumab versus 39.9% of those who received methotrexate.9 In another 52-week phase 3 trial of 1465 participants with moderate to severe psoriasis, clinical benefit was reported at 12 weeks in 75.9% of participants for Dermatology Life Quality Index, and 64.8% and 54.1% for psoriasis- and PsA-related pain scores, respectively.10 However, ABT-874 was withdrawn by the manufacturer as of 2011 due to concerns regarding adverse cardiovascular events.9
BI 655066
BI 655066 (Boehringer Ingelheim GmbH) is a human monoclonal antibody that targets the p19 subunit of IL-23. A phase 1 study of the pharmacokinetics and pharmacodynamics of intravenous (IV) versus subcutaneous (SC) administration of BI 655066 as well as its safety and effectiveness versus placebo recently was completed (NCT01577550), but the results were not available at the time of publication. A phase 2 study comparing 3 dosing regimens of BI 655066 versus ustekinumab is ongoing but not actively recruiting patients at the time of publi-cation (NCT02054481).
Ustekinumab (CNTO 1275)
Ustekinumab (formerly known as CNTO 1275)(Janssen Biotech, Inc) is a human monoclonal antibody that inhibits the p40 subunit of IL-12 and IL-23. It was FDA approved for treatment of moderate to severe plaque psoriasis in September 200911 and PsA in September 201312 for adult patients 18 years or older. One phase 3 trial (known as ACCEPT) compared the effectiveness of ustekinumab versus etanercept in 903 participants with moderate to severe psoriasis at 67 centers worldwide.13 Participants were randomly assigned to receive SC injections of either 45 mg or 90 mg of ustekinumab (at weeks 0 and 4) or high-dose etanercept (50 mg twice weekly for 12 weeks). At week 12, PASI 75 was noted in 67.5% of participants who received 45 mg of ustekinumab and 73.8% of participants who received 90 mg compared to 56.8% of those who received etanercept (P=.01 and P<.001, respectively). In participants who showed no response to etanercept, PASI 75 was achieved in 48.9% within 12 weeks after crossover to ustekinumab. One or more adverse events (AEs) occurred through week 12 in 66.0% of the 45-mg ustekinumab group, 69.2% of the 90-mg group, and 70.0% of the etanercept group; serious AEs were noted in 1.9%, 1.2%, and 1.2%, respectively.13 A 5-year follow-up study of 3117 participants reported an incidence of AEs with ustekinumab that was comparable to other biologics, with malignancy and mortality rates comparable to age-matched controls.14
In a phase 3, multicenter, double-blind, placebo-controlled trial (know as PSUMMIT I), 615 adults with active PsA who had not previously been treated with TNF inhibitors were randomly assigned to placebo, 45 mg of ustekinumab, or 90 mg of ustekinumab. At week 24, more participants receiving ustekinumab 90 mg achieved 20%, 50%, and 70% improvement in American College of Rheumatology (ACR) criteria (49.5%, 27.9%, and 14.2%, respectively) and PASI 75 (62.4%) versus the placebo group (22.8%, 8.7%, 2.4%, and 11%, respectively).15 In a phase 3, multicenter, placebo-controlled trial (known as PSUMMIT 2), 312 adult participants with active PsA who had formerly been treated with conventional therapies and/or TNF inhibitors were randomized to receive placebo (at weeks 0, 4, and 16 with crossover to 45 mg of ustekinumab at weeks 24, 28, and 40) or ustekinumab (45 mg or 90 mg at weeks 0, 4, and every 12 weeks).16 For participants with less than 5% improvement, there was an early escape clinical trial design with placebo to 45 mg of ustekinumab, 45 mg of ustekinumab to 90 mg, and 90 mg of ustekinumab maintained at the same dose. The ACR20 was 43.8% for the ustekinumab group versus 20.2% for the placebo group (P<.001).16
A phase 3, multicenter, randomized, double-blind, placebo-controlled study (known as CADMUS) evaluated the efficacy and safety of ustekinumab in the treatment of adolescents (age range, 12–18 years) with moderate to severe plaque-type psoriasis.17 The primary outcome of the study was the percentage of participants achieving a physician global assessment (PGA) score of cleared (0) or minimal (1) at week 12. One hundred ten participants started and completed the first period in the study (ie, controlled period [weeks 0–12]) and were randomized into 3 groups: placebo (SC injections at weeks 0 and 4), ustekinumab half-standard dose, and ustekinumab standard dose. At week 12, 101 participants started and completed the second period in the study (weeks 12–60). The placebo group received either ustekinumab half-standard dose or ustekinumab standard dose at weeks 12 and 16, then once every 12 weeks with the last dose at week 40, and the ustekinumab half-standard and standard dose groups received the respective doses every 12 weeks with the last dose at week 40. At week 12, PGA scores of 0 or 1 were reported in 5.4% of the placebo group, 67.6% of the ustekinumab half-standard dose group, and 69.4% of the ustekinumab standard dose group (P<.001), and PASI 75 was achieved in 10.8%, 78.4%, and 80.6%, respectively (P<.001).17
A phase 4 study (known as TRANSIT) assessed the safety and efficacy of ustekinumab in participants with plaque psoriasis who had a suboptimal response to methotrexate.18 Participants in the first treatment group received either 45 mg (weight, ≤100 kg) of ustekinumab at weeks 0, 4, and then every 12 weeks until week 40, or 90 mg (weight, >100 kg) in 2 SC injections after immediate discontinuation of methotrexate. The second treatment group followed the same dosing regimen with gradual withdrawal of methotrexate therapy. Adverse events were reported in 61.1% and 64.5% of participants in groups 1 and 2, respectively. In group 1, PASI 75 was observed in 58.1% of participants (95% confi-dence interval [CI], 51.9%-64.3%) at week 12 and 76.3% (95% CI, 70.8%-81.9%) at week 52. In group 2, PASI 75 was observed in 62.2% of participants (95% CI, 56.0%-68.3%) at week 12 and 76.9% (95% CI, 71.4%-82.5%) at week 52.18
In another study that assessed the efficacy and safety of ustekinumab in 24 participants with moderate to severe palmoplantar psoriasis, 37.5% of participants achieved a palmar/plantar PGA score of 0 or 1 at week 16.19 A phase 3, multicenter, randomized, double-blind, placebo-controlled study of the safety and effectiveness of ustekinumab in 615 PsA participants showed ACR20 response in 49.5% of the ustekinumab 90-mg group, 42.4% of the ustekinumab 45-mg group, and 22.8% of the placebo group (P<.001).20
A phase 1 study was performed to assess gene expression in the following: (1) IFN-γ modulation in the IL-12 pathway; (2) IL-23 pathway with ustekinumab (45 mg for those weighing <100 kg and 90 mg for ≤100 kg administered SC on day 1 and at weeks 4 and 16); and (3) IL-17 pathway with etanercept (50 mg administered SC twice weekly for 12 weeks, then once weekly for 4 weeks).21 The change in gene expression from baseline in the IL-12 pathway with ustekinumab achieved statistical significance by week 1 (P=.016) with increasing levels of gene expression through week 16 (P=.000184). The change in gene expression from baseline in the IL-23 pathway with ustekinumab achieved statistical significance by week 2 (P=.010) with increasing levels of gene expression through week 16 (P=.000215). The results were less powerful for etanercept, with a change in gene expression from baseline in the IL-17 pathway increasing through week 4 (P=.053) and decreasing by week 16 (P=.098).21
Several clinical trials are underway and are currently recruiting participants (Table 2).
Guselkumab (CNTO 1959)
Guselkumab (formerly known as CNTO 1959)(Janssen Research & Development, LLC) is a human monoclonal antibody targeting the p19 subunit of IL-23. In a double-blind, randomized study of 24 participants receiving 1 dose of CNTO 1959 at 10 mg, 30 mg, 100 mg, or 300 mg versus placebo, a PASI 75 of 50% for the 10-mg subset, 60% for the 30- and 100-mg group, and 100% for the 300-mg group was achieved as opposed to 0% in the placebo group at 12 weeks.22 The rate of AEs was 65% in the CNTO 1959 treatment arm versus 50% in the placebo group at 24 weeks. Furthermore, decreased serum IL-17A titers and gene expression for psoriasis was demonstrated as well as decreased thickness of the epidermis and less dendritic and T-cell expression for the CNTO 1959 study population histologically.22 Results of a phase 2 trial in 293 participants who received CNTO 1959, adalimumab, or placebo indicated PASI 75 at 16 weeks for 81% of the CNTO 1959 50-mg group versus 71% of the adalimumab group, with serious AEs in 3% of participants treated with CNTO 1959 versus 5% treated with adalimumab.23
Tildrakizumab (MK-3222/SCH 900222)
Tildrakizumab (formerly known as MK-3222/SCH 900222)(Merck & Co Inc) is a monoclonal antibody that also targets the p19 subunit of IL-23. Results of a phase 2b trial were promising. This study reported on 355 participants who received placebo versus MK-3222 5 mg, 25 mg, 100 mg, or 200 mg, with PASI 75 scores of 4.4%, 33%, 64%, 66%, and 74%, respectively, noted at 16 weeks.24 A 64-week phase 3 study currently is underway to assess the long-term benefit and safety of MK-3222, but it is not recruiting participants (NCT01722331).
Inhibition of the IL-17 Pathway
The T helper 17 cells (TH17) produce IL-17, a cytokine mediating inflammation that is implicated in psoriasis. Two products target IL-17A, while another targets the IL-17 receptor.25
Secukinumab (AIN457)
Secukinumab (formerly known as AIN457)(Novartis Pharmaceutical Corporation) was FDA approved for treatment of moderate to severe psoriasis in adult patients who are candidates for systemic therapy or phototherapy in January 2015.26 Secukinumab is a human monoclonal antibody that inhibits IL-17A. There are many clinical trials underway including a phase 2 extension study (NCT01132612). Many phase 3 studies also are underway evaluating the effectiveness and safety of AIN457 in patients with psoriasis resistant to TNF inhibitors (NCT01961609); its usability and tolerability (NCT01555125), including 2-year extension studies (NCT01640951; NCT01544595); its effectiveness as opposed to ustekinumab (NCT02074982); effectiveness using an autoinjector (NCT01636687); and the PASI 90 in HLA-Cw6–positive and HLA-Cw6–negative patients with moderate to severe psoriasis (known as SUPREME)(NCT02394561).
Other phase 3 trials are being undertaken in patients with moderate to severe palmoplantar psoriasis (NCT01806597; NCT02008890); moderate to severe nail psoriasis (known as TRANSFIGURE)(NCT01807520); moderate to severe scalp psoriasis (NCT02267135); and PsA (NCT01989468; NCT02294227; NCT01892436), including a 5-year study for PsA (known as FUTURE 2)(NCT01752634).
Other studies that are completed with pending results include a phase 1 trial to evaluate its mechanism of action in vivo by studying the spread of AIN457 in tissue as assessed by open flow microperfusion (NCT01539213), a phase 2 trial of the clinical effectiveness of AIN457 at 12 months and biomarker changes (NCT01537432), as well as phase 3 trials of the clinical efficacy of various dosing regimens (known as SCULPTURE)(NCT01406938); safety and effectiveness at 1 year (known as ERASURE)(NCT01365455); and the effectiveness, tolerability, and safety of AIN457 over 2 years in PsA (known as FUTURE 1)(NCT01392326).
In a 56-week phase 2 clinical trial of 100 participants, the PASI scores at 12 weeks and percentage of participants without relapse up to 56 weeks were evaluated.27 There were 4 arms in the study: (1) AIN457 3 mg/kg (day 1) then placebo (days 15 and 29); (2) AIN457 10 mg/kg (day 1) then placebo (days 15 and 29); (3) AIN457 10 mg/kg (days 1, 15, and 29); and (4) placebo (days 1, 15, and 29), with AIN457 and the placebo administered IV. The mean (standard deviation) change from baseline for PASI scores for these respective groups was -12.46 (7.668), -13.35 (6.195), -18.02 (6.792), and -4.18 (4.698), respectively. At week 56, the percentage of participants without a relapse at any point during the study was 12.5%, 22.2%, and 27.8%, respectively.27
In a phase 2 study of 404 participants, PASI 75 scores were assessed at 12 weeks with the SC administration of AIN457 in participants with moderate to severe psoriasis at 3 dosing regimens: (1) a single dose of 150 mg (week 1), (2) monthly doses of 150 mg (weeks 1, 5, and 9), (3) early loading doses of 150 mg (weeks 1, 2, 3, 5, and 9), as compared to placebo. At 12 weeks, PASI 75 scores were 7%, 58%, 72%, and 1%, respectively.28
The phase 3 STATURE trial assessed the safety and effectiveness of SC and IV AIN457 in moderate to severe psoriasis in partial AIN457 nonresponders.29 Nonresponders were participants who demonstrated a PASI score of 50% or more but less than 75%. Participants in this study design who received SC AIN457 demonstrated a PASI 75 of 66.7%, with a 2011 investigator global assessment score of 0 (clear) or 1 (almost clear) in 66.7%. In those receiving IV AIN457, the PASI 75 was 90.5%, with a 2011 investigator global assessment score of 0 or 1 in 33.3%.29
In a 52-week phase 3 efficacy trial (known as FIXTURE), 1306 participants received 1 dose of AIN457 300 mg or 150 mg weekly for 5 weeks, then every 4 weeks; 12 weeks of etanercept 50 mg twice weekly, then once weekly; or placebo. The PASI 75 was 77.1% for AIN457 300 mg, 67.0% for AIN457 150 mg, 44.0% for etanercept, and 4.9% for placebo (P<.001).30 In a 52-week efficacy and safety trial (known as ERASURE), 738 participants received 1 dose of AIN457 300 mg or 150 mg weekly for 5 weeks, then every 4 weeks, versus placebo. The PASI 75 was 65.3% for AIN457 300 mg, 51.2% for AIN457 150 mg, and 2.4% for placebo (P<.001). There was a comparable incidence of infection among participants with AIN457 and etanercept, which was greater than placebo.30
Brodalumab (AMG 827)
Brodalumab (formerly known as AMG 827)(Amgen Inc) is a human monoclonal antibody that targets the IL-17A receptor. In a phase 1 randomized trial, 25 participants received either IV brodalumab 700 mg, SC brodalumab 350 mg or 140 mg, or placebo.31 Results demonstrated improvement in PASI score that correlated with increased dosage of brodalumab as well as decreased psoriasis gene expression and decreased thickness of the epidermis in participants receiving the 700-mg IV or 350-mg SC doses. In a phase 2 trial, 198 participants received either brodalumab 280 mg at week 0, then every 4 weeks for 8 weeks, or brodalumab 210 mg, 140 mg, 70 mg, or placebo at week 0, then every 2 weeks for 10 weeks. At week 12, PASI 75 was observed in 82% and 77% of the 210-mg and 140-mg groups, respectively, with no benefit noted in the placebo group (P<.001).31 In a phase 3 trial, 661 participants received brodalumab 210 mg or 140 mg or placebo. At week 12, PASI 75 was observed in 83% of the 210-mg group versus 60% of the 140-mg group; PASI 100 was observed in 42% of the 210-mg group versus 23% of the 140-mg group.32
Ixekizumab (LY2439821)
Ixekizumab (formerly known as LY2439821)(Eli Lilly and Company) is a human monoclonal antibody that targets IL-17A. In a phase 2 double-blind, placebo-controlled trial, 142 participants with chronic moderate to severe plaque psoriasis were randomized to receive 10-mg, 25-mg, 75-mg, or 150-mg SC injections of ixekizumab or placebo at 0, 2, 4, 8, 12, and 16 weeks. At week 12, the percentage of participants with a 75% reduction in PASI score was significantly greater with ixekizumab (150 mg [82.1%], 75 mg [82.8%], and 25 mg [76.7%]), except the 10-mg group, than with placebo (7.7%)(P<.001 for each comparison).33
Inhibition of T-Cell Activation in Antigen-Presenting Cells
Abatacept
Abatacept (Bristol-Myers Squibb) is a fusion protein designed to inhibit T-cell activation by binding receptors for CD80 and CD86 in antigen-presenting cells.34 A phase 1 study of 43 participants demonstrated improved PGA scores of 50% in 46% of psoriasis participants who were treated with abatacept, indicating a dose-responsive association with abatacept in psoriasis patients refractory to other therapies.35 In a 6-month, phase 2, multicenter, randomized, double-blind, placebo-controlled trial, 170 participants with PsA were randomized to receive placebo or abatacept at doses of 3 mg/kg, 10 mg/kg, or 30/10 mg/kg (2 initial doses of 30 mg/kg followed by 10 mg/kg).36 At day 169, ACR20 was observed in 19%, 33%, 48%, and 42% of the placebo and abatacept 3 mg/kg, 10 mg/kg, and 30/10 mg/kg groups, respectively. Compared with placebo, improvements were significantly higher for the abatacept 10-mg/kg (P=.006) and 30/10-mg/kg (P=.022) groups but not for the 3-mg/kg group (P=.121). The authors concluded that abatacept 10 mg/kg could be an appropriate dosing regimen for PsA, as is presently used in the FDA-approved management of rheumatoid arthritis.36 At the time of publication, a phase 3 trial evaluating the efficacy and safety of abatacept SC injection in adults with active PsA was ongoing but was not actively recruiting participants (NCT01860976).
Activation of Regulatory T Cells
Tregalizumab (BT061)
Tregalizumab (formerly known as BT061)(Biotest) is a human monoclonal antibody that activates regulatory T cells. A phase 2, randomized, placebo-controlled, double-blind, multicenter, multiple-dose, cohort study with escalating doses evaluating the safety and efficacy of BT061 in patients with moderate to severe chronic plaque psoriasis was completed, but the results were not available at the time of publication (NCT01072383).
Inhibition of Toll-like Receptors 7, 8, and 9
IMO-8400
IMO-8400 (Idera Pharmaceuticals) is unique in that it treats psoriasis by targeting toll-like receptors (TLRs) 7, 8, and 9.37 In phase 1 studies, IMO-8400 was well tolerated when administered to a maximum of 0.6 mg/kg.38 An 18-week, phase 2, randomized, double-blind, placebo-controlled, dose-ranging study evaluating the safety and tolerability of different dose levels—0.075 mg/kg, 0.15 mg/kg, and 0.3 mg/kg—of IMO-8400 versus placebo in patients with moderate to severe plaque psoriasis was completed, but the results were not available at the time of publication (NCT01899729).
Inhibition of Granulocyte-Macrophage Colony-Stimulating Factor
Namilumab (MT203)
Namilumab (formerly known as MT203)(Takeda Pharmaceutical Company Limited) is a granulocyte-macrophage colony-stimulating factor inhibitor. At the time of publication, participants were actively being recruited for a phase 2, multicenter, randomized, double-blind, placebo-controlled, dose-finding and proof-of-concept study to assess the efficacy, safety, and tolerability of namilumab at 4 different SC doses—300 mg, 160 mg, 100 mg, and 40 mg at baseline with half the dose on days 15, 43, and 71 for each of the 4 treatment arms—versus placebo in patients with moderate to severe chronic plaque psoriasis (NCT02129777).
Conclusion
Novel biologic treatments promise exciting new therapeutic avenues for psoriasis and PsA. Although biologics currently are in use for treatment of psoriasis and PsA in the form of TNF-α inhibitors, other drugs currently in phase 2 through phase 4 clinical trials aim to target other pathways underlying the pathogenesis of psoriasis and PsA, including inhibition of the IL-12/IL-23 pathway; inhibition of the IL-17 pathway; inhibition of T-cell activation in antigen-presenting cells; activation of regulatory T cells; inhibition of TLR-7, TLR-8, and TLR-9; and inhibition of granulocyte-macrophage colony-stimulating factor. These novel therapies offer hope for more targeted treatment strategies for patients with psoriasis and/or PsA.
1. Lee S, Coleman CI, Limone B, et al. Biologic and nonbiologic systemic agents and phototherapy for treatment of chronic plaque psoriasis. Rockville, MD: Agency for Healthcare Research and Quality; 2012.
2. Nagler AR, Weinberg JM. Research pipeline III: biologic therapies. In: Weinberg JM, Lebwohl M, eds. Advances in Psoriasis. New York, NY: Springer; 2014:243-251.
3. Reich K, Ortonne JP, Gottlieb AB, et al. Successful treatment of moderate to severe plaque psoriasis with the PEGylated Fab’ certolizumab pegol: results of a phase II randomized, placebo-controlled trial with a re-treatment extension. Br J Dermatol. 2012;167:180-190.
4. Gladman D, Fleischmann R, Coteur G, et al. Effect of certolizumab pegol on multiple facets of psoriatic arthritis as reported by patients: 24-week patient-reported outcome results of a phase III, multicenter study. Arthritis Care Res (Hoboken). 2014;66:1085-1092.
5. Coherus announces initiation of Phase 3 trial of CHS-0214 (investigational etanercept biosimilar) in chronic plaque psoriasis (RaPsOdy) [press release]. Redwood City, CA: Coherus BioSciences, Inc; July 16, 2014.
6. Coherus announces CHS-0214 (proposed etanercept biosimilar) meets primary endpoint in pivotal pharmacokinetic clinical study [press release]. Redwood City, CA: Coherus BioSciences, Inc; October 28, 2013.
7. Tang C, Chen S, Qian H, et al. Interleukin-23: as a drug target for autoimmune inflammatory diseases. Immunology. 2012;135:112-124.
8. Strober BE, Crowley JJ, Yamauchi PS, et al. Efficacy and safety results from a phase III, randomized controlled trial comparing the safety and efficacy of briakinumab with etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. Br J Dermatol. 2011;165:661-668.
9. Reich K, Langley RG, Papp KA, et al. A 52-week trial comparing briakinumab with methotrexate in patients with psoriasis. N Engl J Med. 2011;365:1586-1596.
10. Papp KA, Sundaram M, Bao Y, et al. Effects of briakinumab treatment for moderate to severe psoriasis on health-related quality of life and work productivity and activity impairment: results from a randomized phase III study. J Eur Acad Dermatol Venereol. 2014;28:790-798.
11. Stelara (ustekinumab) receives FDA approval to treat active psoriatic arthritis. first and only anti-IL-12/23 treatment approved for adult patients living with psoriatic arthritis [press release]. Horsham, PA: Johnson & Johnson; September 23, 2013.
12. FDA approves new drug to treat psoriasis [press release]. Silver Spring, MD: US Food and Drug Administration; April 17, 2013.
13. Griffiths CE, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362:118-128.
14. Papp KA, Griffiths CE, Gordon K, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013;168:844-854.
15. McInnes IB, Kavanaugh A, Gottlieb AB, et al. Ustekinumab in patients with active psoriatic arthritis: results of the phase 3, multicenter, double-blind, placebo-controlled PSUMMIT I study. Ann Rheum Dis. 2012;71(suppl):S107-S148.
16. Ritchlin C, Rahman P, Kavanaugh A, et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial [published online ahead of print Jan 30, 2014]. Ann Rheum Dis. 2014;73:990-999.
17. A study of the safety and efficacy of ustekinumab in adolescent patients with psoriasis (CADMUS)(NCT01090427). https://clinicaltrials.gov/ct2/show/NCT01090427?term=NCT01090427&rank=1. Updated January 16, 2015. Accessed April 16, 2015.
18. A safety and efficacy study of ustekinumab in patients with plaque psoriasis who have had an inadequate response to methotrexate (NCT01059773). https://clinicaltrials.gov/ct2/show/NCT01059773?term=NCT01059773&rank=1. Updated November 13, 2014. Accessed April 16, 2015.
19. Efficacy and safety of ustekinumab in patients with moderate to severe palmar plantar psoriasis (PPP)(NCT01090063). https://clinicaltrials.gov/ct2/show/NCT01090063?term=NCT01090063&rank=1. Updated January 31, 2013. Accessed April 16, 2015.
20. A study of the safety and effectiveness of ustekinumab in patients with psoriatic arthritis (NCT01009086). https://clinicaltrials.gov/ct2/show/NCT01009086?term=NCT01009086&rank=1. Updated February 11, 2015. Accessed April 16, 2015.
21. A study to assess the effect of ustekinumab (Stelara) and etanercept (Enbrel) in participants with moderate to severe psoriasis (MK-0000-206)(NCT01276847). https://clinicaltrials.gov/ct2/show/NCT01276847?term=NCT01276847&rank=1. Updated January 13, 2015. Accessed April 16, 2015.
22. Sofen H, Smith S, Matheson RT, et al. Guselkumab (an IL-23-specific mAb) demonstrates clinical and molecular response in patients with moderate-to-severe psoriasis. J Allergy Clin Immunol. 2014;133:1032-1040.
23. Callis Duffin K, Wasfi Y, Shen YK, et al. A phase 2, multicenter, randomized, placebo- and active-comparator-controlled, dose-ranging trial to evaluate Guselkumab for the treatment of patients with moderate-to-severe plaque-type psoriasis (X-PLORE). Poster presented at: 72nd Annual Meeting of the American Academy of Dermatology; March 21-25, 2014; Denver, CO.
24. Papp K. Monoclonal antibody MK-3222 and chronic plaque psoriasis: phase 2b. Paper presented at: 71st Annual Meeting of the American Academy of Dermatology; March 1-5, 2013; Miami, FL.
25. Huynh D, Kavanaugh A. Psoriatic arthritis: current therapy and future approaches. Rheumatology (Oxford). 2015;54:20-28.
26. FDA approves new psoriasis drug Cosentyx [press release]. Silver Spring, MD: US Food and Drug Administration; January 21, 2015.
27. Multiple-loading dose regimen study in patients with chronic plaque-type psoriasis (NCT00805480). https://clinicaltrials.gov/ct2/show/NCT00805480?term
=NCT00805480&rank=1. Updated January 28, 2015. Accessed April 16, 2015.
28. AIN457 regimen finding study in patients with moderate to severe psoriasis (NCT00941031). https://clinicaltrials.gov/ct2/show/NCT00941031?term=NCT00941031&rank=1. Updated March 23, 2015. Accessed April 16, 2015.
29. Efficacy and safety of intravenous and subcutaneous secukinumab in moderate to severe chronic plaque-type psoriasis (STATURE)(NCT014712944). https://clinical trials.gov/ct2/show/NCT01412944?term=efficacy+and+safety+of+intravenous+and+subcutaneoussecukinumab&rank=1. Updated March 17, 2015. Accessed April 16, 2015.
30. Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. 2014;371:326-338.
31. Coimbra S, Figueiredo A, Santos-Silva A. Brodalumab: an evidence-based review of its potential in the treatment of moderate-to-severe psoriasis. Core Evid. 2014;9:89-97.
32. Leavitt M. New biologic clears psoriasis in 42 percent of patients. National Psoriasis Foundation Web site. https://www.psoriasis.org/advance/new-biologic-clears-psoriasis-in-42-percent-of-patients. Accessed April 10, 2015.
33. Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
34. Herrero-Beaumont G, Martínez Calatrava MJ, Castañeda S. Abatacept mechanism of action: concordance with its clinical profile. Rheumatol Clin. 2012;8:78-83.
35. Abrams JR, Lebwohl MG, Guzzo CA, et al. CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris. J Clin Invest. 1999;103:1243-1252.
36. Mease P, Genovese MC, Gladstein G, et al. Abatacept in the treatment of patients with psoriatic arthritis: results of a six-month, multicenter, randomized, double-blind, placebo-controlled, phase II trial. Arthritis Rheum. 2011;63:939-948.
37. Suárez-Fariñas M, Arbeit R, Jiang W, et al. Suppression of molecular inflammatory pathways by Toll-like receptor 7, 8, and 9 antagonists in a model of IL-23-induced skin inflammation. PLoS One. 2013;8:e84634.
38. A 12-week dose-ranging trial in patients with moderate to severe plaque psoriasis (8400-201)(NCT01899729). https://clinicaltrials.gov/ct2/show/NCT01899729. Updated October 16, 2014. Accessed April 27, 2015.
1. Lee S, Coleman CI, Limone B, et al. Biologic and nonbiologic systemic agents and phototherapy for treatment of chronic plaque psoriasis. Rockville, MD: Agency for Healthcare Research and Quality; 2012.
2. Nagler AR, Weinberg JM. Research pipeline III: biologic therapies. In: Weinberg JM, Lebwohl M, eds. Advances in Psoriasis. New York, NY: Springer; 2014:243-251.
3. Reich K, Ortonne JP, Gottlieb AB, et al. Successful treatment of moderate to severe plaque psoriasis with the PEGylated Fab’ certolizumab pegol: results of a phase II randomized, placebo-controlled trial with a re-treatment extension. Br J Dermatol. 2012;167:180-190.
4. Gladman D, Fleischmann R, Coteur G, et al. Effect of certolizumab pegol on multiple facets of psoriatic arthritis as reported by patients: 24-week patient-reported outcome results of a phase III, multicenter study. Arthritis Care Res (Hoboken). 2014;66:1085-1092.
5. Coherus announces initiation of Phase 3 trial of CHS-0214 (investigational etanercept biosimilar) in chronic plaque psoriasis (RaPsOdy) [press release]. Redwood City, CA: Coherus BioSciences, Inc; July 16, 2014.
6. Coherus announces CHS-0214 (proposed etanercept biosimilar) meets primary endpoint in pivotal pharmacokinetic clinical study [press release]. Redwood City, CA: Coherus BioSciences, Inc; October 28, 2013.
7. Tang C, Chen S, Qian H, et al. Interleukin-23: as a drug target for autoimmune inflammatory diseases. Immunology. 2012;135:112-124.
8. Strober BE, Crowley JJ, Yamauchi PS, et al. Efficacy and safety results from a phase III, randomized controlled trial comparing the safety and efficacy of briakinumab with etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. Br J Dermatol. 2011;165:661-668.
9. Reich K, Langley RG, Papp KA, et al. A 52-week trial comparing briakinumab with methotrexate in patients with psoriasis. N Engl J Med. 2011;365:1586-1596.
10. Papp KA, Sundaram M, Bao Y, et al. Effects of briakinumab treatment for moderate to severe psoriasis on health-related quality of life and work productivity and activity impairment: results from a randomized phase III study. J Eur Acad Dermatol Venereol. 2014;28:790-798.
11. Stelara (ustekinumab) receives FDA approval to treat active psoriatic arthritis. first and only anti-IL-12/23 treatment approved for adult patients living with psoriatic arthritis [press release]. Horsham, PA: Johnson & Johnson; September 23, 2013.
12. FDA approves new drug to treat psoriasis [press release]. Silver Spring, MD: US Food and Drug Administration; April 17, 2013.
13. Griffiths CE, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362:118-128.
14. Papp KA, Griffiths CE, Gordon K, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013;168:844-854.
15. McInnes IB, Kavanaugh A, Gottlieb AB, et al. Ustekinumab in patients with active psoriatic arthritis: results of the phase 3, multicenter, double-blind, placebo-controlled PSUMMIT I study. Ann Rheum Dis. 2012;71(suppl):S107-S148.
16. Ritchlin C, Rahman P, Kavanaugh A, et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial [published online ahead of print Jan 30, 2014]. Ann Rheum Dis. 2014;73:990-999.
17. A study of the safety and efficacy of ustekinumab in adolescent patients with psoriasis (CADMUS)(NCT01090427). https://clinicaltrials.gov/ct2/show/NCT01090427?term=NCT01090427&rank=1. Updated January 16, 2015. Accessed April 16, 2015.
18. A safety and efficacy study of ustekinumab in patients with plaque psoriasis who have had an inadequate response to methotrexate (NCT01059773). https://clinicaltrials.gov/ct2/show/NCT01059773?term=NCT01059773&rank=1. Updated November 13, 2014. Accessed April 16, 2015.
19. Efficacy and safety of ustekinumab in patients with moderate to severe palmar plantar psoriasis (PPP)(NCT01090063). https://clinicaltrials.gov/ct2/show/NCT01090063?term=NCT01090063&rank=1. Updated January 31, 2013. Accessed April 16, 2015.
20. A study of the safety and effectiveness of ustekinumab in patients with psoriatic arthritis (NCT01009086). https://clinicaltrials.gov/ct2/show/NCT01009086?term=NCT01009086&rank=1. Updated February 11, 2015. Accessed April 16, 2015.
21. A study to assess the effect of ustekinumab (Stelara) and etanercept (Enbrel) in participants with moderate to severe psoriasis (MK-0000-206)(NCT01276847). https://clinicaltrials.gov/ct2/show/NCT01276847?term=NCT01276847&rank=1. Updated January 13, 2015. Accessed April 16, 2015.
22. Sofen H, Smith S, Matheson RT, et al. Guselkumab (an IL-23-specific mAb) demonstrates clinical and molecular response in patients with moderate-to-severe psoriasis. J Allergy Clin Immunol. 2014;133:1032-1040.
23. Callis Duffin K, Wasfi Y, Shen YK, et al. A phase 2, multicenter, randomized, placebo- and active-comparator-controlled, dose-ranging trial to evaluate Guselkumab for the treatment of patients with moderate-to-severe plaque-type psoriasis (X-PLORE). Poster presented at: 72nd Annual Meeting of the American Academy of Dermatology; March 21-25, 2014; Denver, CO.
24. Papp K. Monoclonal antibody MK-3222 and chronic plaque psoriasis: phase 2b. Paper presented at: 71st Annual Meeting of the American Academy of Dermatology; March 1-5, 2013; Miami, FL.
25. Huynh D, Kavanaugh A. Psoriatic arthritis: current therapy and future approaches. Rheumatology (Oxford). 2015;54:20-28.
26. FDA approves new psoriasis drug Cosentyx [press release]. Silver Spring, MD: US Food and Drug Administration; January 21, 2015.
27. Multiple-loading dose regimen study in patients with chronic plaque-type psoriasis (NCT00805480). https://clinicaltrials.gov/ct2/show/NCT00805480?term
=NCT00805480&rank=1. Updated January 28, 2015. Accessed April 16, 2015.
28. AIN457 regimen finding study in patients with moderate to severe psoriasis (NCT00941031). https://clinicaltrials.gov/ct2/show/NCT00941031?term=NCT00941031&rank=1. Updated March 23, 2015. Accessed April 16, 2015.
29. Efficacy and safety of intravenous and subcutaneous secukinumab in moderate to severe chronic plaque-type psoriasis (STATURE)(NCT014712944). https://clinical trials.gov/ct2/show/NCT01412944?term=efficacy+and+safety+of+intravenous+and+subcutaneoussecukinumab&rank=1. Updated March 17, 2015. Accessed April 16, 2015.
30. Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. 2014;371:326-338.
31. Coimbra S, Figueiredo A, Santos-Silva A. Brodalumab: an evidence-based review of its potential in the treatment of moderate-to-severe psoriasis. Core Evid. 2014;9:89-97.
32. Leavitt M. New biologic clears psoriasis in 42 percent of patients. National Psoriasis Foundation Web site. https://www.psoriasis.org/advance/new-biologic-clears-psoriasis-in-42-percent-of-patients. Accessed April 10, 2015.
33. Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
34. Herrero-Beaumont G, Martínez Calatrava MJ, Castañeda S. Abatacept mechanism of action: concordance with its clinical profile. Rheumatol Clin. 2012;8:78-83.
35. Abrams JR, Lebwohl MG, Guzzo CA, et al. CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris. J Clin Invest. 1999;103:1243-1252.
36. Mease P, Genovese MC, Gladstein G, et al. Abatacept in the treatment of patients with psoriatic arthritis: results of a six-month, multicenter, randomized, double-blind, placebo-controlled, phase II trial. Arthritis Rheum. 2011;63:939-948.
37. Suárez-Fariñas M, Arbeit R, Jiang W, et al. Suppression of molecular inflammatory pathways by Toll-like receptor 7, 8, and 9 antagonists in a model of IL-23-induced skin inflammation. PLoS One. 2013;8:e84634.
38. A 12-week dose-ranging trial in patients with moderate to severe plaque psoriasis (8400-201)(NCT01899729). https://clinicaltrials.gov/ct2/show/NCT01899729. Updated October 16, 2014. Accessed April 27, 2015.
Practice Points
- Novel biologic treatments promise exciting new therapeutic avenues for psoriasis and psoriatic arthritis (PsA).
- Although biologics currently in use for treatment of psoriasis and PsA are in the form of tumor necrosis factor α inhibitors, other drugs in phase 2 through phase 4 clinical trials aim to target alternative pathways underlying the pathogenesis of these disorders, including IL-12/IL-23 inhibition, IL-17 inhibition, inhibition of T-cell activation in antigen-presenting cells, regulatory T-cell activation, toll-like receptor inhibition, and granulocyte-macrophage colony-stimulating factor inhibition.
- New approaches to the management of psoriasis and PsA offer patients hope for more targeted treatment regimens.
Novel Psoriasis Therapies and Patient Outcomes, Part 1: Topical Medications
Topical therapies are a mainstay in the management of patients with mild to moderate psoriasis (Figure). Presently, US Food and Drug Administration–approved topical medications that are commercially available for use in patients with psoriasis include corticosteroids, vitamin D3 analogues, calcineurin inhibitors, retinoids, anthralin, and tar-based formulations.1 In recent years, research has furthered our understanding of the molecular mechanisms underlying the pathogenesis of psoriasis and has afforded the development of more targeted therapies. Novel topical medications currently in phase 2 and phase 3 clinical trials are discussed in this article, and a summary is provided in the Table.
AN2728 (Phosphodiesterase 4 Inhibitor)
AN2728 (Anacor Pharmaceuticals, Inc) is a phosphodiesterase 4 inhibitor that blocks the inactivation of cyclic adenosine monophosphate, resulting in decreased production of inflammatory cytokines (eg, IL-6, IL-12, IL-23, tumor necrosis factor α [TNF-α]).2,3 In a randomized, double-blind, phase 2 clinical trial (N=35), 40% of patients treated with AN2728 ointment 5% reported improvement of more than 2 points in overall target plaque severity score versus 6% of patients treated with vehicle. In another randomized, double-blind, dose-response trial of 145 patients, those treated with AN2728 ointment 2% twice daily reported a 60% improvement versus 40% improvement in those treated with AN2728 ointment 0.5% once daily.3 In total, 3 phase 1 trials (registered at www.clinicaltrials.gov with the identifiers NCT01258088, NCT00762658, NCT00763204) and 4 phase 2 trials (NCT01029405, NCT00755196, NCT00759161, NCT01300052) have been completed; results were not available at the time of publication.
AS101 (Integrin Inhibitor)
AS101 (BioMAS Ltd), or ammonium trichloro (dioxoethylene-o,o') tellurate, acts as stimulator of regulatory T cells and a redox modulator inhibiting the leukocyte integrins α4β1 and α4β7 that enable CD4+ T-cell and macrophage extravasation; it also limits expression of the inflammatory cytokines IL-6 and IL-17.4 A randomized, placebo-controlled, double-blind, phase 2 study evaluating the efficacy of AS101 cream 4% twice daily for 12 weeks was withdrawn prior to enrollment (NCT00788424).
Tofacitinib (Janus Kinase 1 and 3 Inhibitor)
Tofacitinib (formerly known as CP-690,550)(Pfizer Inc) is a selective Janus kinase (Jak) 1 and Jak3 inhibitor that limits expression of cytokines that promote inflammation (eg, IFN-γ) and inhibits helper T cells (TH17) by downregulating expression of the IL-23 receptor. Epidermal keratinocyte proliferation in psoriasis is activated by TH17 cells that release IL-17 as well as TH1 cells that release IFN-γ and tumor necrosis factor. A phase 2a trial showed statistically significant improvement from baseline in the target plaque severity score for tofacitinib ointment 2% (least squares mean, −54.4%) versus vehicle (least squares mean, −41.5%).5 Two other phase 2 trials (NCT01246583, NCT00678561) assessing the efficacy, safety, tolerability, and pharmacokinetics of tofacitinib ointment in patients with mild to moderate psoriasis have been completed; results were not available at the time of publication. A phase 2b study that compared 2 dose strengths of tofacitinib ointment—10 mg/g and 20 mg/g—versus placebo over a 12-week period also was completed (NCT01831466); results were not available at the time of publication.
CT327 (Tyrosine Kinase Inhibitor)
CT327 (Creabilis SA) is a tyrosine kinase A (TrkA) inhibitor that affords a novel perspective in the treatment of pruritus by shifting the focus to sensory neurons. In a phase 2b study of 160 patients, a 60% change in the visual analog scale was noted at 8 weeks in the treatment group versus 21% in the placebo group.6 Two other phase 2 studies have been completed, one with a cream formulation of pegylated K252a (NCT00995969) and another with an ointment formulation (NCT01465282); results were not available at the time of publication.
DPS-101 (Vitamin D Analogue)
DPS-101 (Dermipsor Ltd) is a combination of calcipotriol and niacinamide. Calcipotriol is a vitamin D3 analogue that increases IL-10 expression while decreasing IL-8 expression.7 It curbs epidermal keratinocyte proliferation by limiting the expression of polo-like kinase 2 and early growth response-1.8 It also may induce keratinocyte apoptosis.9 Niacinamide is the amide of vitamin B3 and inhibits proinflammatory cytokines such as TNF-α, IL-1β, IL-6, and IL-8.10 In a dose-response phase 2b trial of 168 patients, DPS-101 demonstrated better results than either calcipotriol or niacinamide alone.11
IDP-118 (Proprietary Steroid and Retinoid Combination)
IDP-118 (Valeant Pharmaceuticals International, Inc) is a combination of halobetasol propionate (HP) 0.01% (a topical corticosteroid) and tazar-otene 0.045% (a selective topical retinoid) in a lotion formulation. In isolation, tazarotene is as effective as a mid to highly potent corticosteroid, but irritation may limit its tolerability. The use of combination treatments of mid to highly potent corticosteroids and tazarotene has shown enhanced tolerability and therapeutic efficacy.12 Ongoing studies include a phase 1 trial and a phase 2 trial to evaluate low- and high-strength preparations of IDP-118, respectively (NCT01670513). Another phase 2 trial evaluating the efficacy and safety of IDP-118 lotion (HP 0.01% and tazarotene 0.045%) versus IDP-118 monad HP 0.01% lotion, IDP-118 monad tazar-otene 0.045% lotion, and placebo has been completed (NCT02045277); results were not available at the time of publication.
Ruxolitinib (Jak1 and Jak2 Inhibitor)
Ruxolitinib (formerly known as INCB18424)(Incyte Corporation) is a selective Jak1 and Jak2 inhibitor. A phase 2 trial of ruxolitinib showed a 53% decline in the score for mean total lesions in patients treated with ruxolitinib phosphate cream 1% (P=.033) versus 54% in those treated with ruxolitinib phosphate cream 1.5% (P=.056) and 32% in those treated with placebo.13 Three other phase 2 studies (NCT00617994, NCT00820950, NCT00778700) have been completed; results were not available at the time of publication.
LAS41004 (Proprietary Steroid and Retinoid Combination)
LAS41004 (Almirall, SA) is an ointment containing the corticosteroid betamethasone dipropionate and the retinoid bexarotene that is being evaluated for treatment of mild to moderate psoriasis. Five phase 2 studies (NCT01119339, NCT01283698, NCT01360944, NCT02111499, NCT01462643) have been completed; results were not available at the time of publication. A randomized, double-blind, phase 2a study (NCT02180464) with a left-right design assessing clinical response to LAS41004 versus control in patients with mild to moderate psoriasis was actively recruiting at the time of publication.
LEO 80190 (Vitamin D3 Analogue and Steroid Combination)
LEO 80190 (LEO Pharma) is a combination of the vitamin D3 analogue calcipotriol and the corticosteroid hydrocortisone. It was developed as a treatment for sensitive areas such as the face and intertriginous regions. A randomized, investigator-blind, phase 3 trial (NCT00640822) of LEO 80190 ointment versus tacalcitol ointment and placebo once daily for 8 weeks demonstrated controlled disease of the face in 56.8% (183/322) of patients in the LEO 80190 group, 46.4% (147/317) in the tacalcitol group, and 36.3% (37/102) in the placebo group.14 Another phase 2 study (NCT00704262) and 2 phase 3 studies (NCT00691002, NCT01007591) have been completed; results were not available at the time of publication.
LEO 90100 (Vitamin D Analogue and Steroid Combination)
LEO 90100 (LEO Pharma) contains the vitamin D3 analogue calcipotriol and the corticosteroid betamethasone. Three phase 2 studies (NCT01347255, NCT01536886, NCT01536938) and a phase 3 study (NCT01866163) examining the efficacy and safety of various vehicles and formulations of LEO 90100 have been completed; results were not available at the time of publication. Another phase 3 study (NCT02132936) is ongoing but not recruiting participants. Other completed studies whose results were not yet available include a phase 1 pharmacodynamic study (NCT01946386), a phase 1 study that used patch testing to assess the degree of skin irritation and sensitization associated with LEO 90100 (NCT01935869), and a phase 2 study examining the impact of LEO 90100 on calcium metabolism and the hypothalamic-pituitary-adrenal axis (NCT01600222).
M518101 (Vitamin D Analogue)
M518101 (Maruho Co, Ltd) is a novel topical vitamin D3 analogue. Phase 1 (NCT01844973) and phase 2 (NCT01301157, NCT00884169) trials evaluating the safety, pharmacokinetics, and efficacy of M518101 have been completed; results were not available at the time of publication. A phase 3 study (NCT01989429) assessing the safety and therapeutic efficacy of M518101 according to changes in the modified psoriasis area and severity index over an 8-week treatment period also has been completed; results were not yet available. Three phase 3 studies assessing the safety and therapeutic efficacy of M518101 are ongoing: one is currently closed to recruitment (NCT01908595) and 2 are actively recruiting participants at the time of publication (NCT01878461, NCT01873677).
MOL4239 and MOL4249 (Phosphorylated Signal Transducer and Activator of Transcription 3 Inhibitors)
MOL4239 (Moleculin, LLC) is a novel topical agent for use in mild to moderate psoriasis that acts via phosphorylated signal transducer and activator of transcription 3 (p-STAT3) inhibition.15 The p-STAT3 protein has increased expression in psoriasis.16 A phase 2 trial of MOL4239 ointment (NCT01826201) has been completed, showing a greater mean (standard deviation) change in the psoriasis severity score in lesions treated at 28 days with MOL4239 ointment 10% (−1.9 [1.45]) versus lesions treated with placebo ointment (−1.5 [1.87]).17
MOL4249 (Moleculin, LLC) is more potent than MOL4239 with better lipid solubility. In the MOL4249 subset of a placebo-controlled, double-blind, phase 2a study of 16 patients with mild to moderate psoriasis, 10% (1/10) of patients experienced complete clearance of psoriatic plaques, 30% (3/10) of patients experienced 75% or greater improvement, and 50% (5/10) of patients experienced 50% or greater improvement compared to 17% (1/6) in the placebo group. Currently, a phase 2a contralateral study, a phase 2b psoriasis area and severity index trial, and a phase 3 pivotal trial are planned, according to the manufacturer.18
MQX-5902 (Dihydrofolate Reductase Inhibitor)
MQX-5902 (MediQuest Therapeutics) is a topical preparation of methotrexate for the treatment of fingernail psoriasis. Methotrexate is a dihydrofolate reductase inhibitor and antimetabolite that inhibits folic acid metabolism, thereby disrupting DNA synthesis.19 A phase 2b dose-ranging trial (NCT00666354) was designed to assess the therapeutic efficacy and safety of MQX-5902 delivered via a proprietary drug delivery formulation in fingernail psoriasis; the outcome of this trial was not available at the time of publication.
PH-10 (Xanthine Dye)
PH-10 (Provectus Biopharmaceuticals, Inc) is a topical aqueous hydrogel derived from rose bengal disodium that may be beneficial in treating skin conditions such as atopic dermatitis and mild to moderate psoriasis. Rose bengal disodium is a hydrophilic xanthine dye with diagnostic utility in ophthalmology and gastroenterology as well as projected use as a melanoma treatment as demonstrated in phase 1 and phase 2 clinical trials of PV-10 (Provectus Biopharmaceuticals, Inc).20 Two phase 2 studies assessing the safety and therapeutic efficacy of PH-10 in psoriasis (NCT01247818, NCT00941278) have been completed; results were not available at the time of publication.
STF115469 (Vitamin D Analogue)
STF115469 (GlaxoSmithKline) is a calcipotriene foam. At the time of publication, a randomized, placebo-controlled, double-blind, phase 3 trial (NCT01582932) of this vitamin D3 analogue with a projected enrollment of 180 participants was actively recruiting patients aged 2 to 11 years with mild to moderate plaque psoriasis to study the efficacy, safety, and tolerability of STF115469, as well as its pharmacokinetics and pharmacodynamics.
WBI-1001 (Proprietary Product)
WBI-1001 (Welichem Biotech Inc), or 2-isopropyl-5-[(E)-2-phenylethenyl] benzene-1, 3-diol, is a novel proprietary agent that inhibits proinflammatory cytokines (eg, IFN-γ, TNF-α). A randomized, placebo-controlled, double-blind, phase 1 trial (NCT00830817) assessing the efficacy, safety, tolerability, and pharmacokinetics of WBI-1001 has been completed; results were not available at the time of publication. Another randomized, placebo-controlled, double-blind, phase 2 trial (NCT01098721) evaluating its efficacy and safety according to the physician’s global assessment demonstrated a therapeutic benefit of 62.8% in patients treated with WBI-1001 cream 1% versus 13.0% in those treated with a placebo after a 12-week treatment period (P<.0001).21 WBI-1001 may offer a novel approach in the treatment of mild to moderate psoriasis.
Conclusion
Enhanced knowledge of the underlying pathogeneses of psoriasis and psoriatic arthritis has identified new therapeutic targets and enabled the development of exciting novel treatments for these conditions. The topical agents currently in phase 2 and phase 3 clinical trials show promise in enhancing the way physicians treat psoriasis. There is hope for more individualized treatment regimens with improved tolerability and better safety profiles with increased therapeutic efficacy. As our understanding of the molecular underpinnings of psoriasis continues to deepen, it will afford the development of even more innovative therapeutics for use in the management of psoriasis.
1. Mason A, Mason J, Cork M, et al. Topical treatments for chronic plaque psoriasis: an abridged Cochrane systematic review. J Am Acad Dermatol. 2013;69:799-807.
2. Nazarian R, Weinberg JM. AN-2728, a PDE4 inhibitor for the potential topical treatment of psoriasis and atopic dermatitis. Curr Opin Investig Drugs. 2009;10:1236-1242.
3. Moustafa F, Feldman SR. A review of phosphodiesterase-inhibition and the potential role for phosphodiesterase 4-inhibitors in clinical dermatology. Dermatol Online J. 2014;20:22608.
4. Halpert G, Sredni B. The effect of the novel tellurium compound AS101 on autoimmune diseases. Autoimmun Rev. 2014;13:1230-1235.
5. Ports WC, Khan S, Lan S, et al. A randomized phase 2a efficacy and safety trial of the topical Janus kinase inhibitor tofacitinib in the treatment of chronic plaque psoriasis. Br J Dermatol. 2013;169:137-145.
6. Yosipovitch G, Roblin D, Traversa S, et al. A novel topical targeted anti-pruritic treatment in phase 2b development for chronic pruritus. Paper presented at: 72nd Annual Meeting of the American Academy of Dermatology; March 21-25, 2014; Denver, CO.
7. Kang S, Yi S, Griffiths CE, et al. Calcipotriene-induced improvement in psoriasis is associated with reduced interleukin-8 and increased interleukin-10 levels within lesions. Br J Dermatol. 1998;138:77-83.
8. Kristl J, Slanc P, Krasna M, et al. Calcipotriol affects keratinocyte proliferation by decreasing expression of early growth response-1 and polo-like kinase-2. Pharm Res. 2008;25:521-529.
9. Tiberio R, Bozzo C, Pertusi G, et al. Calcipotriol induces apoptosis in psoriatic keratinocytes. Clin Exp Dermatol. 2009;34:e972-e974.
10. Luger T, Seite S, Humbert P, et al. Recommendations for adjunctive basic skin care in patients with psoriasis. Eur J Dermatol. 2014;24:194-200.
11. Dermipsor reports good results in DPS-101 Phase IIb study for plaque psoriasis [press release]. Evaluate Web site. http://www.evaluategroup.com/Universal/View.aspx?type=Story&id=250042. Published October 15, 2007. Accessed February 13, 2015.
12. Rivera AM, Hsu S. Topical halobetasol propionate in the treatment of plaque psoriasis: a review. Am J Clin Dermatol. 2005;6:311-316.
13. Punwani N, Scherle P, Flores R, et al. Preliminary clinical activity of a topical JAK1/2 inhibitor in the treatment of psoriasis. J Am Acad Dermatol. 2012;67:658-664.
14. Efficacy and safety of calcipotriol plus hydrocortisone ointment compared with tacalcitol ointment in patients with psoriasis on the face and skin folds (NCT00640822). https://clinicaltrials.gov/ct2/show/results/NCT00640822?term=NCT00640822&rank=1. Updated October 21, 2013. Accessed May 30, 2014.
15. Product candidates: targeting p-STAT3 for improved psoriasis treatment. Moleculin Web site. http://moleculin.com/product-candidates/mol4239. Accessed February 13, 2015.
16. Chowdhari S, Saini N. hsa-miR-4516 mediated downregulation of STAT3/CDK6/UBE2N plays a role in PUVA induced apoptosis in keratinocytes. J Cell Physiol. 2014;229:1630-1638.
17. Paired psoriasis lesion, comparative, study to evaluate MOL4239 in psoriasis (NCT01826201). https://clinicaltrials.gov/ct2/show/results/NCT01826201?term=NCT01826201&rank=1§=X01256#all. Updated December 22, 2014. Accessed February 25, 2015.
18. Clinical development pipeline. Moleculin Web site. http://moleculin.com/clinical-trials/psoriasis-trials. Accessed February 13, 2015.
19. de la Brassinne M, Nikkels A. Psoriasis: state of the art 2013. Acta Clin Belg. 2013;68:433-441.
20. Ross MI. Intralesional therapy with PV-10 (Rose Bengal) for in-transit melanoma. J Surg Oncol. 2014;109:314-319.
21. Bissonnette R, Bolduc C, Maari C, et al. Efficacy and safety of topical WBI-1001 in patients with mild to moderate psoriasis: results from a randomized, double-blind placebo-controlled, phase II trial. J Eur Acad Dermatol Venereol. 2012;26:1516-1521.
Topical therapies are a mainstay in the management of patients with mild to moderate psoriasis (Figure). Presently, US Food and Drug Administration–approved topical medications that are commercially available for use in patients with psoriasis include corticosteroids, vitamin D3 analogues, calcineurin inhibitors, retinoids, anthralin, and tar-based formulations.1 In recent years, research has furthered our understanding of the molecular mechanisms underlying the pathogenesis of psoriasis and has afforded the development of more targeted therapies. Novel topical medications currently in phase 2 and phase 3 clinical trials are discussed in this article, and a summary is provided in the Table.
AN2728 (Phosphodiesterase 4 Inhibitor)
AN2728 (Anacor Pharmaceuticals, Inc) is a phosphodiesterase 4 inhibitor that blocks the inactivation of cyclic adenosine monophosphate, resulting in decreased production of inflammatory cytokines (eg, IL-6, IL-12, IL-23, tumor necrosis factor α [TNF-α]).2,3 In a randomized, double-blind, phase 2 clinical trial (N=35), 40% of patients treated with AN2728 ointment 5% reported improvement of more than 2 points in overall target plaque severity score versus 6% of patients treated with vehicle. In another randomized, double-blind, dose-response trial of 145 patients, those treated with AN2728 ointment 2% twice daily reported a 60% improvement versus 40% improvement in those treated with AN2728 ointment 0.5% once daily.3 In total, 3 phase 1 trials (registered at www.clinicaltrials.gov with the identifiers NCT01258088, NCT00762658, NCT00763204) and 4 phase 2 trials (NCT01029405, NCT00755196, NCT00759161, NCT01300052) have been completed; results were not available at the time of publication.
AS101 (Integrin Inhibitor)
AS101 (BioMAS Ltd), or ammonium trichloro (dioxoethylene-o,o') tellurate, acts as stimulator of regulatory T cells and a redox modulator inhibiting the leukocyte integrins α4β1 and α4β7 that enable CD4+ T-cell and macrophage extravasation; it also limits expression of the inflammatory cytokines IL-6 and IL-17.4 A randomized, placebo-controlled, double-blind, phase 2 study evaluating the efficacy of AS101 cream 4% twice daily for 12 weeks was withdrawn prior to enrollment (NCT00788424).
Tofacitinib (Janus Kinase 1 and 3 Inhibitor)
Tofacitinib (formerly known as CP-690,550)(Pfizer Inc) is a selective Janus kinase (Jak) 1 and Jak3 inhibitor that limits expression of cytokines that promote inflammation (eg, IFN-γ) and inhibits helper T cells (TH17) by downregulating expression of the IL-23 receptor. Epidermal keratinocyte proliferation in psoriasis is activated by TH17 cells that release IL-17 as well as TH1 cells that release IFN-γ and tumor necrosis factor. A phase 2a trial showed statistically significant improvement from baseline in the target plaque severity score for tofacitinib ointment 2% (least squares mean, −54.4%) versus vehicle (least squares mean, −41.5%).5 Two other phase 2 trials (NCT01246583, NCT00678561) assessing the efficacy, safety, tolerability, and pharmacokinetics of tofacitinib ointment in patients with mild to moderate psoriasis have been completed; results were not available at the time of publication. A phase 2b study that compared 2 dose strengths of tofacitinib ointment—10 mg/g and 20 mg/g—versus placebo over a 12-week period also was completed (NCT01831466); results were not available at the time of publication.
CT327 (Tyrosine Kinase Inhibitor)
CT327 (Creabilis SA) is a tyrosine kinase A (TrkA) inhibitor that affords a novel perspective in the treatment of pruritus by shifting the focus to sensory neurons. In a phase 2b study of 160 patients, a 60% change in the visual analog scale was noted at 8 weeks in the treatment group versus 21% in the placebo group.6 Two other phase 2 studies have been completed, one with a cream formulation of pegylated K252a (NCT00995969) and another with an ointment formulation (NCT01465282); results were not available at the time of publication.
DPS-101 (Vitamin D Analogue)
DPS-101 (Dermipsor Ltd) is a combination of calcipotriol and niacinamide. Calcipotriol is a vitamin D3 analogue that increases IL-10 expression while decreasing IL-8 expression.7 It curbs epidermal keratinocyte proliferation by limiting the expression of polo-like kinase 2 and early growth response-1.8 It also may induce keratinocyte apoptosis.9 Niacinamide is the amide of vitamin B3 and inhibits proinflammatory cytokines such as TNF-α, IL-1β, IL-6, and IL-8.10 In a dose-response phase 2b trial of 168 patients, DPS-101 demonstrated better results than either calcipotriol or niacinamide alone.11
IDP-118 (Proprietary Steroid and Retinoid Combination)
IDP-118 (Valeant Pharmaceuticals International, Inc) is a combination of halobetasol propionate (HP) 0.01% (a topical corticosteroid) and tazar-otene 0.045% (a selective topical retinoid) in a lotion formulation. In isolation, tazarotene is as effective as a mid to highly potent corticosteroid, but irritation may limit its tolerability. The use of combination treatments of mid to highly potent corticosteroids and tazarotene has shown enhanced tolerability and therapeutic efficacy.12 Ongoing studies include a phase 1 trial and a phase 2 trial to evaluate low- and high-strength preparations of IDP-118, respectively (NCT01670513). Another phase 2 trial evaluating the efficacy and safety of IDP-118 lotion (HP 0.01% and tazarotene 0.045%) versus IDP-118 monad HP 0.01% lotion, IDP-118 monad tazar-otene 0.045% lotion, and placebo has been completed (NCT02045277); results were not available at the time of publication.
Ruxolitinib (Jak1 and Jak2 Inhibitor)
Ruxolitinib (formerly known as INCB18424)(Incyte Corporation) is a selective Jak1 and Jak2 inhibitor. A phase 2 trial of ruxolitinib showed a 53% decline in the score for mean total lesions in patients treated with ruxolitinib phosphate cream 1% (P=.033) versus 54% in those treated with ruxolitinib phosphate cream 1.5% (P=.056) and 32% in those treated with placebo.13 Three other phase 2 studies (NCT00617994, NCT00820950, NCT00778700) have been completed; results were not available at the time of publication.
LAS41004 (Proprietary Steroid and Retinoid Combination)
LAS41004 (Almirall, SA) is an ointment containing the corticosteroid betamethasone dipropionate and the retinoid bexarotene that is being evaluated for treatment of mild to moderate psoriasis. Five phase 2 studies (NCT01119339, NCT01283698, NCT01360944, NCT02111499, NCT01462643) have been completed; results were not available at the time of publication. A randomized, double-blind, phase 2a study (NCT02180464) with a left-right design assessing clinical response to LAS41004 versus control in patients with mild to moderate psoriasis was actively recruiting at the time of publication.
LEO 80190 (Vitamin D3 Analogue and Steroid Combination)
LEO 80190 (LEO Pharma) is a combination of the vitamin D3 analogue calcipotriol and the corticosteroid hydrocortisone. It was developed as a treatment for sensitive areas such as the face and intertriginous regions. A randomized, investigator-blind, phase 3 trial (NCT00640822) of LEO 80190 ointment versus tacalcitol ointment and placebo once daily for 8 weeks demonstrated controlled disease of the face in 56.8% (183/322) of patients in the LEO 80190 group, 46.4% (147/317) in the tacalcitol group, and 36.3% (37/102) in the placebo group.14 Another phase 2 study (NCT00704262) and 2 phase 3 studies (NCT00691002, NCT01007591) have been completed; results were not available at the time of publication.
LEO 90100 (Vitamin D Analogue and Steroid Combination)
LEO 90100 (LEO Pharma) contains the vitamin D3 analogue calcipotriol and the corticosteroid betamethasone. Three phase 2 studies (NCT01347255, NCT01536886, NCT01536938) and a phase 3 study (NCT01866163) examining the efficacy and safety of various vehicles and formulations of LEO 90100 have been completed; results were not available at the time of publication. Another phase 3 study (NCT02132936) is ongoing but not recruiting participants. Other completed studies whose results were not yet available include a phase 1 pharmacodynamic study (NCT01946386), a phase 1 study that used patch testing to assess the degree of skin irritation and sensitization associated with LEO 90100 (NCT01935869), and a phase 2 study examining the impact of LEO 90100 on calcium metabolism and the hypothalamic-pituitary-adrenal axis (NCT01600222).
M518101 (Vitamin D Analogue)
M518101 (Maruho Co, Ltd) is a novel topical vitamin D3 analogue. Phase 1 (NCT01844973) and phase 2 (NCT01301157, NCT00884169) trials evaluating the safety, pharmacokinetics, and efficacy of M518101 have been completed; results were not available at the time of publication. A phase 3 study (NCT01989429) assessing the safety and therapeutic efficacy of M518101 according to changes in the modified psoriasis area and severity index over an 8-week treatment period also has been completed; results were not yet available. Three phase 3 studies assessing the safety and therapeutic efficacy of M518101 are ongoing: one is currently closed to recruitment (NCT01908595) and 2 are actively recruiting participants at the time of publication (NCT01878461, NCT01873677).
MOL4239 and MOL4249 (Phosphorylated Signal Transducer and Activator of Transcription 3 Inhibitors)
MOL4239 (Moleculin, LLC) is a novel topical agent for use in mild to moderate psoriasis that acts via phosphorylated signal transducer and activator of transcription 3 (p-STAT3) inhibition.15 The p-STAT3 protein has increased expression in psoriasis.16 A phase 2 trial of MOL4239 ointment (NCT01826201) has been completed, showing a greater mean (standard deviation) change in the psoriasis severity score in lesions treated at 28 days with MOL4239 ointment 10% (−1.9 [1.45]) versus lesions treated with placebo ointment (−1.5 [1.87]).17
MOL4249 (Moleculin, LLC) is more potent than MOL4239 with better lipid solubility. In the MOL4249 subset of a placebo-controlled, double-blind, phase 2a study of 16 patients with mild to moderate psoriasis, 10% (1/10) of patients experienced complete clearance of psoriatic plaques, 30% (3/10) of patients experienced 75% or greater improvement, and 50% (5/10) of patients experienced 50% or greater improvement compared to 17% (1/6) in the placebo group. Currently, a phase 2a contralateral study, a phase 2b psoriasis area and severity index trial, and a phase 3 pivotal trial are planned, according to the manufacturer.18
MQX-5902 (Dihydrofolate Reductase Inhibitor)
MQX-5902 (MediQuest Therapeutics) is a topical preparation of methotrexate for the treatment of fingernail psoriasis. Methotrexate is a dihydrofolate reductase inhibitor and antimetabolite that inhibits folic acid metabolism, thereby disrupting DNA synthesis.19 A phase 2b dose-ranging trial (NCT00666354) was designed to assess the therapeutic efficacy and safety of MQX-5902 delivered via a proprietary drug delivery formulation in fingernail psoriasis; the outcome of this trial was not available at the time of publication.
PH-10 (Xanthine Dye)
PH-10 (Provectus Biopharmaceuticals, Inc) is a topical aqueous hydrogel derived from rose bengal disodium that may be beneficial in treating skin conditions such as atopic dermatitis and mild to moderate psoriasis. Rose bengal disodium is a hydrophilic xanthine dye with diagnostic utility in ophthalmology and gastroenterology as well as projected use as a melanoma treatment as demonstrated in phase 1 and phase 2 clinical trials of PV-10 (Provectus Biopharmaceuticals, Inc).20 Two phase 2 studies assessing the safety and therapeutic efficacy of PH-10 in psoriasis (NCT01247818, NCT00941278) have been completed; results were not available at the time of publication.
STF115469 (Vitamin D Analogue)
STF115469 (GlaxoSmithKline) is a calcipotriene foam. At the time of publication, a randomized, placebo-controlled, double-blind, phase 3 trial (NCT01582932) of this vitamin D3 analogue with a projected enrollment of 180 participants was actively recruiting patients aged 2 to 11 years with mild to moderate plaque psoriasis to study the efficacy, safety, and tolerability of STF115469, as well as its pharmacokinetics and pharmacodynamics.
WBI-1001 (Proprietary Product)
WBI-1001 (Welichem Biotech Inc), or 2-isopropyl-5-[(E)-2-phenylethenyl] benzene-1, 3-diol, is a novel proprietary agent that inhibits proinflammatory cytokines (eg, IFN-γ, TNF-α). A randomized, placebo-controlled, double-blind, phase 1 trial (NCT00830817) assessing the efficacy, safety, tolerability, and pharmacokinetics of WBI-1001 has been completed; results were not available at the time of publication. Another randomized, placebo-controlled, double-blind, phase 2 trial (NCT01098721) evaluating its efficacy and safety according to the physician’s global assessment demonstrated a therapeutic benefit of 62.8% in patients treated with WBI-1001 cream 1% versus 13.0% in those treated with a placebo after a 12-week treatment period (P<.0001).21 WBI-1001 may offer a novel approach in the treatment of mild to moderate psoriasis.
Conclusion
Enhanced knowledge of the underlying pathogeneses of psoriasis and psoriatic arthritis has identified new therapeutic targets and enabled the development of exciting novel treatments for these conditions. The topical agents currently in phase 2 and phase 3 clinical trials show promise in enhancing the way physicians treat psoriasis. There is hope for more individualized treatment regimens with improved tolerability and better safety profiles with increased therapeutic efficacy. As our understanding of the molecular underpinnings of psoriasis continues to deepen, it will afford the development of even more innovative therapeutics for use in the management of psoriasis.
Topical therapies are a mainstay in the management of patients with mild to moderate psoriasis (Figure). Presently, US Food and Drug Administration–approved topical medications that are commercially available for use in patients with psoriasis include corticosteroids, vitamin D3 analogues, calcineurin inhibitors, retinoids, anthralin, and tar-based formulations.1 In recent years, research has furthered our understanding of the molecular mechanisms underlying the pathogenesis of psoriasis and has afforded the development of more targeted therapies. Novel topical medications currently in phase 2 and phase 3 clinical trials are discussed in this article, and a summary is provided in the Table.
AN2728 (Phosphodiesterase 4 Inhibitor)
AN2728 (Anacor Pharmaceuticals, Inc) is a phosphodiesterase 4 inhibitor that blocks the inactivation of cyclic adenosine monophosphate, resulting in decreased production of inflammatory cytokines (eg, IL-6, IL-12, IL-23, tumor necrosis factor α [TNF-α]).2,3 In a randomized, double-blind, phase 2 clinical trial (N=35), 40% of patients treated with AN2728 ointment 5% reported improvement of more than 2 points in overall target plaque severity score versus 6% of patients treated with vehicle. In another randomized, double-blind, dose-response trial of 145 patients, those treated with AN2728 ointment 2% twice daily reported a 60% improvement versus 40% improvement in those treated with AN2728 ointment 0.5% once daily.3 In total, 3 phase 1 trials (registered at www.clinicaltrials.gov with the identifiers NCT01258088, NCT00762658, NCT00763204) and 4 phase 2 trials (NCT01029405, NCT00755196, NCT00759161, NCT01300052) have been completed; results were not available at the time of publication.
AS101 (Integrin Inhibitor)
AS101 (BioMAS Ltd), or ammonium trichloro (dioxoethylene-o,o') tellurate, acts as stimulator of regulatory T cells and a redox modulator inhibiting the leukocyte integrins α4β1 and α4β7 that enable CD4+ T-cell and macrophage extravasation; it also limits expression of the inflammatory cytokines IL-6 and IL-17.4 A randomized, placebo-controlled, double-blind, phase 2 study evaluating the efficacy of AS101 cream 4% twice daily for 12 weeks was withdrawn prior to enrollment (NCT00788424).
Tofacitinib (Janus Kinase 1 and 3 Inhibitor)
Tofacitinib (formerly known as CP-690,550)(Pfizer Inc) is a selective Janus kinase (Jak) 1 and Jak3 inhibitor that limits expression of cytokines that promote inflammation (eg, IFN-γ) and inhibits helper T cells (TH17) by downregulating expression of the IL-23 receptor. Epidermal keratinocyte proliferation in psoriasis is activated by TH17 cells that release IL-17 as well as TH1 cells that release IFN-γ and tumor necrosis factor. A phase 2a trial showed statistically significant improvement from baseline in the target plaque severity score for tofacitinib ointment 2% (least squares mean, −54.4%) versus vehicle (least squares mean, −41.5%).5 Two other phase 2 trials (NCT01246583, NCT00678561) assessing the efficacy, safety, tolerability, and pharmacokinetics of tofacitinib ointment in patients with mild to moderate psoriasis have been completed; results were not available at the time of publication. A phase 2b study that compared 2 dose strengths of tofacitinib ointment—10 mg/g and 20 mg/g—versus placebo over a 12-week period also was completed (NCT01831466); results were not available at the time of publication.
CT327 (Tyrosine Kinase Inhibitor)
CT327 (Creabilis SA) is a tyrosine kinase A (TrkA) inhibitor that affords a novel perspective in the treatment of pruritus by shifting the focus to sensory neurons. In a phase 2b study of 160 patients, a 60% change in the visual analog scale was noted at 8 weeks in the treatment group versus 21% in the placebo group.6 Two other phase 2 studies have been completed, one with a cream formulation of pegylated K252a (NCT00995969) and another with an ointment formulation (NCT01465282); results were not available at the time of publication.
DPS-101 (Vitamin D Analogue)
DPS-101 (Dermipsor Ltd) is a combination of calcipotriol and niacinamide. Calcipotriol is a vitamin D3 analogue that increases IL-10 expression while decreasing IL-8 expression.7 It curbs epidermal keratinocyte proliferation by limiting the expression of polo-like kinase 2 and early growth response-1.8 It also may induce keratinocyte apoptosis.9 Niacinamide is the amide of vitamin B3 and inhibits proinflammatory cytokines such as TNF-α, IL-1β, IL-6, and IL-8.10 In a dose-response phase 2b trial of 168 patients, DPS-101 demonstrated better results than either calcipotriol or niacinamide alone.11
IDP-118 (Proprietary Steroid and Retinoid Combination)
IDP-118 (Valeant Pharmaceuticals International, Inc) is a combination of halobetasol propionate (HP) 0.01% (a topical corticosteroid) and tazar-otene 0.045% (a selective topical retinoid) in a lotion formulation. In isolation, tazarotene is as effective as a mid to highly potent corticosteroid, but irritation may limit its tolerability. The use of combination treatments of mid to highly potent corticosteroids and tazarotene has shown enhanced tolerability and therapeutic efficacy.12 Ongoing studies include a phase 1 trial and a phase 2 trial to evaluate low- and high-strength preparations of IDP-118, respectively (NCT01670513). Another phase 2 trial evaluating the efficacy and safety of IDP-118 lotion (HP 0.01% and tazarotene 0.045%) versus IDP-118 monad HP 0.01% lotion, IDP-118 monad tazar-otene 0.045% lotion, and placebo has been completed (NCT02045277); results were not available at the time of publication.
Ruxolitinib (Jak1 and Jak2 Inhibitor)
Ruxolitinib (formerly known as INCB18424)(Incyte Corporation) is a selective Jak1 and Jak2 inhibitor. A phase 2 trial of ruxolitinib showed a 53% decline in the score for mean total lesions in patients treated with ruxolitinib phosphate cream 1% (P=.033) versus 54% in those treated with ruxolitinib phosphate cream 1.5% (P=.056) and 32% in those treated with placebo.13 Three other phase 2 studies (NCT00617994, NCT00820950, NCT00778700) have been completed; results were not available at the time of publication.
LAS41004 (Proprietary Steroid and Retinoid Combination)
LAS41004 (Almirall, SA) is an ointment containing the corticosteroid betamethasone dipropionate and the retinoid bexarotene that is being evaluated for treatment of mild to moderate psoriasis. Five phase 2 studies (NCT01119339, NCT01283698, NCT01360944, NCT02111499, NCT01462643) have been completed; results were not available at the time of publication. A randomized, double-blind, phase 2a study (NCT02180464) with a left-right design assessing clinical response to LAS41004 versus control in patients with mild to moderate psoriasis was actively recruiting at the time of publication.
LEO 80190 (Vitamin D3 Analogue and Steroid Combination)
LEO 80190 (LEO Pharma) is a combination of the vitamin D3 analogue calcipotriol and the corticosteroid hydrocortisone. It was developed as a treatment for sensitive areas such as the face and intertriginous regions. A randomized, investigator-blind, phase 3 trial (NCT00640822) of LEO 80190 ointment versus tacalcitol ointment and placebo once daily for 8 weeks demonstrated controlled disease of the face in 56.8% (183/322) of patients in the LEO 80190 group, 46.4% (147/317) in the tacalcitol group, and 36.3% (37/102) in the placebo group.14 Another phase 2 study (NCT00704262) and 2 phase 3 studies (NCT00691002, NCT01007591) have been completed; results were not available at the time of publication.
LEO 90100 (Vitamin D Analogue and Steroid Combination)
LEO 90100 (LEO Pharma) contains the vitamin D3 analogue calcipotriol and the corticosteroid betamethasone. Three phase 2 studies (NCT01347255, NCT01536886, NCT01536938) and a phase 3 study (NCT01866163) examining the efficacy and safety of various vehicles and formulations of LEO 90100 have been completed; results were not available at the time of publication. Another phase 3 study (NCT02132936) is ongoing but not recruiting participants. Other completed studies whose results were not yet available include a phase 1 pharmacodynamic study (NCT01946386), a phase 1 study that used patch testing to assess the degree of skin irritation and sensitization associated with LEO 90100 (NCT01935869), and a phase 2 study examining the impact of LEO 90100 on calcium metabolism and the hypothalamic-pituitary-adrenal axis (NCT01600222).
M518101 (Vitamin D Analogue)
M518101 (Maruho Co, Ltd) is a novel topical vitamin D3 analogue. Phase 1 (NCT01844973) and phase 2 (NCT01301157, NCT00884169) trials evaluating the safety, pharmacokinetics, and efficacy of M518101 have been completed; results were not available at the time of publication. A phase 3 study (NCT01989429) assessing the safety and therapeutic efficacy of M518101 according to changes in the modified psoriasis area and severity index over an 8-week treatment period also has been completed; results were not yet available. Three phase 3 studies assessing the safety and therapeutic efficacy of M518101 are ongoing: one is currently closed to recruitment (NCT01908595) and 2 are actively recruiting participants at the time of publication (NCT01878461, NCT01873677).
MOL4239 and MOL4249 (Phosphorylated Signal Transducer and Activator of Transcription 3 Inhibitors)
MOL4239 (Moleculin, LLC) is a novel topical agent for use in mild to moderate psoriasis that acts via phosphorylated signal transducer and activator of transcription 3 (p-STAT3) inhibition.15 The p-STAT3 protein has increased expression in psoriasis.16 A phase 2 trial of MOL4239 ointment (NCT01826201) has been completed, showing a greater mean (standard deviation) change in the psoriasis severity score in lesions treated at 28 days with MOL4239 ointment 10% (−1.9 [1.45]) versus lesions treated with placebo ointment (−1.5 [1.87]).17
MOL4249 (Moleculin, LLC) is more potent than MOL4239 with better lipid solubility. In the MOL4249 subset of a placebo-controlled, double-blind, phase 2a study of 16 patients with mild to moderate psoriasis, 10% (1/10) of patients experienced complete clearance of psoriatic plaques, 30% (3/10) of patients experienced 75% or greater improvement, and 50% (5/10) of patients experienced 50% or greater improvement compared to 17% (1/6) in the placebo group. Currently, a phase 2a contralateral study, a phase 2b psoriasis area and severity index trial, and a phase 3 pivotal trial are planned, according to the manufacturer.18
MQX-5902 (Dihydrofolate Reductase Inhibitor)
MQX-5902 (MediQuest Therapeutics) is a topical preparation of methotrexate for the treatment of fingernail psoriasis. Methotrexate is a dihydrofolate reductase inhibitor and antimetabolite that inhibits folic acid metabolism, thereby disrupting DNA synthesis.19 A phase 2b dose-ranging trial (NCT00666354) was designed to assess the therapeutic efficacy and safety of MQX-5902 delivered via a proprietary drug delivery formulation in fingernail psoriasis; the outcome of this trial was not available at the time of publication.
PH-10 (Xanthine Dye)
PH-10 (Provectus Biopharmaceuticals, Inc) is a topical aqueous hydrogel derived from rose bengal disodium that may be beneficial in treating skin conditions such as atopic dermatitis and mild to moderate psoriasis. Rose bengal disodium is a hydrophilic xanthine dye with diagnostic utility in ophthalmology and gastroenterology as well as projected use as a melanoma treatment as demonstrated in phase 1 and phase 2 clinical trials of PV-10 (Provectus Biopharmaceuticals, Inc).20 Two phase 2 studies assessing the safety and therapeutic efficacy of PH-10 in psoriasis (NCT01247818, NCT00941278) have been completed; results were not available at the time of publication.
STF115469 (Vitamin D Analogue)
STF115469 (GlaxoSmithKline) is a calcipotriene foam. At the time of publication, a randomized, placebo-controlled, double-blind, phase 3 trial (NCT01582932) of this vitamin D3 analogue with a projected enrollment of 180 participants was actively recruiting patients aged 2 to 11 years with mild to moderate plaque psoriasis to study the efficacy, safety, and tolerability of STF115469, as well as its pharmacokinetics and pharmacodynamics.
WBI-1001 (Proprietary Product)
WBI-1001 (Welichem Biotech Inc), or 2-isopropyl-5-[(E)-2-phenylethenyl] benzene-1, 3-diol, is a novel proprietary agent that inhibits proinflammatory cytokines (eg, IFN-γ, TNF-α). A randomized, placebo-controlled, double-blind, phase 1 trial (NCT00830817) assessing the efficacy, safety, tolerability, and pharmacokinetics of WBI-1001 has been completed; results were not available at the time of publication. Another randomized, placebo-controlled, double-blind, phase 2 trial (NCT01098721) evaluating its efficacy and safety according to the physician’s global assessment demonstrated a therapeutic benefit of 62.8% in patients treated with WBI-1001 cream 1% versus 13.0% in those treated with a placebo after a 12-week treatment period (P<.0001).21 WBI-1001 may offer a novel approach in the treatment of mild to moderate psoriasis.
Conclusion
Enhanced knowledge of the underlying pathogeneses of psoriasis and psoriatic arthritis has identified new therapeutic targets and enabled the development of exciting novel treatments for these conditions. The topical agents currently in phase 2 and phase 3 clinical trials show promise in enhancing the way physicians treat psoriasis. There is hope for more individualized treatment regimens with improved tolerability and better safety profiles with increased therapeutic efficacy. As our understanding of the molecular underpinnings of psoriasis continues to deepen, it will afford the development of even more innovative therapeutics for use in the management of psoriasis.
1. Mason A, Mason J, Cork M, et al. Topical treatments for chronic plaque psoriasis: an abridged Cochrane systematic review. J Am Acad Dermatol. 2013;69:799-807.
2. Nazarian R, Weinberg JM. AN-2728, a PDE4 inhibitor for the potential topical treatment of psoriasis and atopic dermatitis. Curr Opin Investig Drugs. 2009;10:1236-1242.
3. Moustafa F, Feldman SR. A review of phosphodiesterase-inhibition and the potential role for phosphodiesterase 4-inhibitors in clinical dermatology. Dermatol Online J. 2014;20:22608.
4. Halpert G, Sredni B. The effect of the novel tellurium compound AS101 on autoimmune diseases. Autoimmun Rev. 2014;13:1230-1235.
5. Ports WC, Khan S, Lan S, et al. A randomized phase 2a efficacy and safety trial of the topical Janus kinase inhibitor tofacitinib in the treatment of chronic plaque psoriasis. Br J Dermatol. 2013;169:137-145.
6. Yosipovitch G, Roblin D, Traversa S, et al. A novel topical targeted anti-pruritic treatment in phase 2b development for chronic pruritus. Paper presented at: 72nd Annual Meeting of the American Academy of Dermatology; March 21-25, 2014; Denver, CO.
7. Kang S, Yi S, Griffiths CE, et al. Calcipotriene-induced improvement in psoriasis is associated with reduced interleukin-8 and increased interleukin-10 levels within lesions. Br J Dermatol. 1998;138:77-83.
8. Kristl J, Slanc P, Krasna M, et al. Calcipotriol affects keratinocyte proliferation by decreasing expression of early growth response-1 and polo-like kinase-2. Pharm Res. 2008;25:521-529.
9. Tiberio R, Bozzo C, Pertusi G, et al. Calcipotriol induces apoptosis in psoriatic keratinocytes. Clin Exp Dermatol. 2009;34:e972-e974.
10. Luger T, Seite S, Humbert P, et al. Recommendations for adjunctive basic skin care in patients with psoriasis. Eur J Dermatol. 2014;24:194-200.
11. Dermipsor reports good results in DPS-101 Phase IIb study for plaque psoriasis [press release]. Evaluate Web site. http://www.evaluategroup.com/Universal/View.aspx?type=Story&id=250042. Published October 15, 2007. Accessed February 13, 2015.
12. Rivera AM, Hsu S. Topical halobetasol propionate in the treatment of plaque psoriasis: a review. Am J Clin Dermatol. 2005;6:311-316.
13. Punwani N, Scherle P, Flores R, et al. Preliminary clinical activity of a topical JAK1/2 inhibitor in the treatment of psoriasis. J Am Acad Dermatol. 2012;67:658-664.
14. Efficacy and safety of calcipotriol plus hydrocortisone ointment compared with tacalcitol ointment in patients with psoriasis on the face and skin folds (NCT00640822). https://clinicaltrials.gov/ct2/show/results/NCT00640822?term=NCT00640822&rank=1. Updated October 21, 2013. Accessed May 30, 2014.
15. Product candidates: targeting p-STAT3 for improved psoriasis treatment. Moleculin Web site. http://moleculin.com/product-candidates/mol4239. Accessed February 13, 2015.
16. Chowdhari S, Saini N. hsa-miR-4516 mediated downregulation of STAT3/CDK6/UBE2N plays a role in PUVA induced apoptosis in keratinocytes. J Cell Physiol. 2014;229:1630-1638.
17. Paired psoriasis lesion, comparative, study to evaluate MOL4239 in psoriasis (NCT01826201). https://clinicaltrials.gov/ct2/show/results/NCT01826201?term=NCT01826201&rank=1§=X01256#all. Updated December 22, 2014. Accessed February 25, 2015.
18. Clinical development pipeline. Moleculin Web site. http://moleculin.com/clinical-trials/psoriasis-trials. Accessed February 13, 2015.
19. de la Brassinne M, Nikkels A. Psoriasis: state of the art 2013. Acta Clin Belg. 2013;68:433-441.
20. Ross MI. Intralesional therapy with PV-10 (Rose Bengal) for in-transit melanoma. J Surg Oncol. 2014;109:314-319.
21. Bissonnette R, Bolduc C, Maari C, et al. Efficacy and safety of topical WBI-1001 in patients with mild to moderate psoriasis: results from a randomized, double-blind placebo-controlled, phase II trial. J Eur Acad Dermatol Venereol. 2012;26:1516-1521.
1. Mason A, Mason J, Cork M, et al. Topical treatments for chronic plaque psoriasis: an abridged Cochrane systematic review. J Am Acad Dermatol. 2013;69:799-807.
2. Nazarian R, Weinberg JM. AN-2728, a PDE4 inhibitor for the potential topical treatment of psoriasis and atopic dermatitis. Curr Opin Investig Drugs. 2009;10:1236-1242.
3. Moustafa F, Feldman SR. A review of phosphodiesterase-inhibition and the potential role for phosphodiesterase 4-inhibitors in clinical dermatology. Dermatol Online J. 2014;20:22608.
4. Halpert G, Sredni B. The effect of the novel tellurium compound AS101 on autoimmune diseases. Autoimmun Rev. 2014;13:1230-1235.
5. Ports WC, Khan S, Lan S, et al. A randomized phase 2a efficacy and safety trial of the topical Janus kinase inhibitor tofacitinib in the treatment of chronic plaque psoriasis. Br J Dermatol. 2013;169:137-145.
6. Yosipovitch G, Roblin D, Traversa S, et al. A novel topical targeted anti-pruritic treatment in phase 2b development for chronic pruritus. Paper presented at: 72nd Annual Meeting of the American Academy of Dermatology; March 21-25, 2014; Denver, CO.
7. Kang S, Yi S, Griffiths CE, et al. Calcipotriene-induced improvement in psoriasis is associated with reduced interleukin-8 and increased interleukin-10 levels within lesions. Br J Dermatol. 1998;138:77-83.
8. Kristl J, Slanc P, Krasna M, et al. Calcipotriol affects keratinocyte proliferation by decreasing expression of early growth response-1 and polo-like kinase-2. Pharm Res. 2008;25:521-529.
9. Tiberio R, Bozzo C, Pertusi G, et al. Calcipotriol induces apoptosis in psoriatic keratinocytes. Clin Exp Dermatol. 2009;34:e972-e974.
10. Luger T, Seite S, Humbert P, et al. Recommendations for adjunctive basic skin care in patients with psoriasis. Eur J Dermatol. 2014;24:194-200.
11. Dermipsor reports good results in DPS-101 Phase IIb study for plaque psoriasis [press release]. Evaluate Web site. http://www.evaluategroup.com/Universal/View.aspx?type=Story&id=250042. Published October 15, 2007. Accessed February 13, 2015.
12. Rivera AM, Hsu S. Topical halobetasol propionate in the treatment of plaque psoriasis: a review. Am J Clin Dermatol. 2005;6:311-316.
13. Punwani N, Scherle P, Flores R, et al. Preliminary clinical activity of a topical JAK1/2 inhibitor in the treatment of psoriasis. J Am Acad Dermatol. 2012;67:658-664.
14. Efficacy and safety of calcipotriol plus hydrocortisone ointment compared with tacalcitol ointment in patients with psoriasis on the face and skin folds (NCT00640822). https://clinicaltrials.gov/ct2/show/results/NCT00640822?term=NCT00640822&rank=1. Updated October 21, 2013. Accessed May 30, 2014.
15. Product candidates: targeting p-STAT3 for improved psoriasis treatment. Moleculin Web site. http://moleculin.com/product-candidates/mol4239. Accessed February 13, 2015.
16. Chowdhari S, Saini N. hsa-miR-4516 mediated downregulation of STAT3/CDK6/UBE2N plays a role in PUVA induced apoptosis in keratinocytes. J Cell Physiol. 2014;229:1630-1638.
17. Paired psoriasis lesion, comparative, study to evaluate MOL4239 in psoriasis (NCT01826201). https://clinicaltrials.gov/ct2/show/results/NCT01826201?term=NCT01826201&rank=1§=X01256#all. Updated December 22, 2014. Accessed February 25, 2015.
18. Clinical development pipeline. Moleculin Web site. http://moleculin.com/clinical-trials/psoriasis-trials. Accessed February 13, 2015.
19. de la Brassinne M, Nikkels A. Psoriasis: state of the art 2013. Acta Clin Belg. 2013;68:433-441.
20. Ross MI. Intralesional therapy with PV-10 (Rose Bengal) for in-transit melanoma. J Surg Oncol. 2014;109:314-319.
21. Bissonnette R, Bolduc C, Maari C, et al. Efficacy and safety of topical WBI-1001 in patients with mild to moderate psoriasis: results from a randomized, double-blind placebo-controlled, phase II trial. J Eur Acad Dermatol Venereol. 2012;26:1516-1521.
Practice Points
- Topical therapies are the cornerstone of treating patients with mild to moderate psoriasis. Commercially available medications approved by the US Food and Drug Administration for use in patients with psoriasis include corticosteroids, vitamin D3 analogues, calcineurin inhibitors, retinoids, anthralin, and tar-based formulations.
- Recent developments in our understanding of inflammatory mediators and the underlying pathogenesis of psoriasis have revealed new potential therapeutic targets, leading to the discovery of many promising treatments.
- Novel topical therapies currently in phase 2 and phase 3 clinical trials for patients with mild to moderate psoriasis may offer hope to patients who have reported a suboptimal therapeutic response to conventional treatments.